User login
Official news magazine of the Society of Hospital Medicine
Copyright by Society of Hospital Medicine or related companies. All rights reserved. ISSN 1553-085X
nav[contains(@class, 'nav-ce-stack nav-ce-stack__large-screen')]
header[@id='header']
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'main-prefix')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
div[contains(@class, 'view-medstat-quiz-listing-panes')]
div[contains(@class, 'pane-article-sidebar-latest-news')]
div[contains(@class, 'pane-pub-article-hospitalist')]


How should asymptomatic hypertension be managed in the hospital?
Case
A 62-year-old man with diabetes mellitus and hypertension presents with painful erythema of the left lower extremity and is admitted for purulent cellulitis. During the first evening of admission, he has increased left lower extremity pain and nursing reports a blood pressure of 188/96 mm Hg. He denies dyspnea, chest pain, visual changes, confusion, or severe headache.
Background
The prevalence of hypertension in the outpatient setting in the United States is estimated at 29% by the National Health and Nutrition Examination Survey.1 Hypertension generally is defined in the outpatient setting as an average blood pressure reading greater than or equal to140/90 mm Hg on two or more separate occasions.2 There is no consensus on the definition of hypertension in the inpatient setting; however, hypertensive urgency often is defined as a sustained blood pressure above the range of 180-200 mm Hg systolic and/or 110-120 mm Hg diastolic without target organ damage, and hypertensive emergency has a similar blood pressure range, but with evidence of target organ damage.3
The evidence
There are several clinical trials to suggest that the ambulatory treatment of chronic hypertension reduces the incidence of myocardial infarction, cerebrovascular accident (CVA), and heart failure7,8; however, these trials are difficult to extrapolate to acutely hospitalized patients.6 Overall, evidence on the appropriate management of asymptomatic hypertension in the inpatient setting is lacking.
Some evidence suggests we often are overly aggressive with intravenous antihypertensives without clinical indications in the inpatient setting.3,9,10 For example, Campbell et al. prospectively examined the use of intravenous hydralazine for the treatment of asymptomatic hypertension in 94 hospitalized patients.10 It was determined that in 90 of those patients, there was no clinical indication to use intravenous hydralazine and 17 patients experienced adverse events from hypotension, which included dizziness/light-headedness, syncope, and chest pain.10 They recommend against the use of intravenous hydralazine in the setting of asymptomatic hypertension because of its risk of adverse events with the rapid lowering of blood pressure.10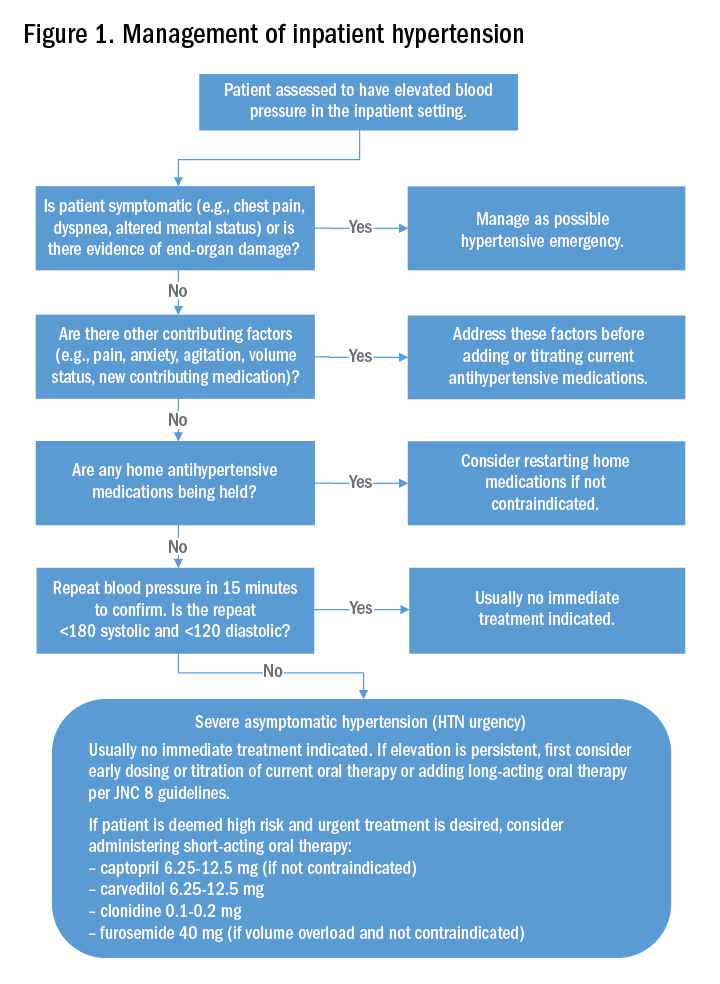
Weder and Erickson performed a retrospective review on the use of intravenous hydralazine and labetalol administration in 2,189 hospitalized patients.3 They found that only 3% of those patients had symptoms indicating the need for IV antihypertensives and that the length of stay was several days longer in those who had received IV antihypertensives.3
Other studies have examined the role of oral antihypertensives for management of asymptomatic hypertension in the inpatient setting. A systematic review and meta-analysis by Souza et al. assessed the use of oral pharmacotherapy for hypertensive urgency.11 Sixteen randomized clinical trials were reviewed and it was determined that angiotensin-converting enzyme inhibitors (ACE-I) had a superior effect in treating hypertensive urgencies.11 The most common side effect of using an ACE-I was a bad taste in patient’s mouths, and researchers did not observe side effects that were similar as those seen with the use of IV antihypertensives.11
Further, Jaker et al. performed a randomized, double-blind prospective study comparing a single dose of oral nifedipine with oral clonidine for the treatment of hypertensive urgency in 51 patients.12 Both the oral nifedipine and oral clonidine were extremely effective in reducing blood pressure fairly safely.12 However, the rapid lowering of blood pressure with oral nifedipine was concerning to Grossman et al.13 In their literature review of the side effects of oral and sublingual nifedipine, they found that it was one of the most common therapeutic interventions for hypertensive urgency or emergency.13 However, it was potentially dangerous because of the inconsistent blood pressure response after nifedipine administration, particularly with the sublingual form.13 CVAs, acute MIs, and even death were the reported adverse events with the use of oral and sublingual nifedipine.13 Because of that, the investigators recommend against the use of oral or sublingual nifedipine in hypertensive urgency or emergency and suggest using other oral antihypertensive agents instead.13
Typically, if the patient’s blood pressure remains elevated despite these efforts, no urgent treatment is indicated and we recommend close monitoring of the patient’s blood pressure during the hospitalization. If hypertension persists, the next best step would be to titrate a patient’s current oral antihypertensive therapy or to start a long-acting antihypertensive therapy per the JNC 8 (Eighth Joint National Committee) guidelines. It should be noted that, in those patients that are high risk, such as those with known coronary artery disease, heart failure, or prior hemorrhagic CVA, a short-acting oral antihypertensive such as captopril, carvedilol, clonidine, or furosemide should be considered.
Back to the case
The patient’s pain was treated with oral oxycodone. He received no oral or intravenous antihypertensive therapy, and the following morning, his blood pressure improved to 145/95 mm Hg. Based on our suggested approach in Fig. 1, the patient would require no acute treatment despite an improved but elevated blood pressure. We continued to monitor his blood pressure and despite adequate pain control, his blood pressure remain persistently elevated. Thus, per the JNC 8 guidelines, we started him on a long-acting antihypertensive, which improved his blood pressure to 123/78 at the time of discharge.
Bottom line
Management of asymptomatic hypertension in the hospital begins with addressing contributing factors, reviewing held home medications – and rarely – urgent oral pharmacotherapy.
Dr. Lippert is PGY-3 in the department of internal medicine at the University of Kentucky, Lexington. Dr. Bailey is associate professor of medicine at the University of Kentucky. Dr. Gray is associate professor of medicine at the University of Kentucky.
References
1. Yoon S, Fryar C, Carroll M. Hypertension prevalence and control among adults: United States, 2011-2014. 2015. Accessed Oct 2, 2017.
2. Poulter NR, Prabhakaran D, Caulfield M. Hypertension. Lancet. 2015;386(9995):801-12.
3. Weder AB, Erickson S. Treatment of hypertension in the inpatient setting: Use of intravenous labetalol and hydralazine. J Clin Hypertens. 2010;12(1):29-33.
4. Axon RN, Cousineau L, Egan BM. Prevalence and management of hypertension in the inpatient setting: A systematic review. J Hosp Med. 2011;6(7):417-22.
5. Weiss AJ, Elixhauser A. Overview of hospital stays in the United States, 2012. Statistical brief; 2014 Oct. Accessed Oct 2, 2017.
6. Weder AB. Treating acute hypertension in the hospital: a Lacuna in the guidelines. Hypertension. 2011;57(1):18-20.
7. Mancia G, Fagard R, Narkiewicz K, et al. 2013 ESH/ESC guidelines for the management of arterial hypertension: The Task Force for the management of arterial hypertension of the European Society of Hypertension and of the European Society of Cardiology. J Hypertens. 2013 Jul;31(7):1281-357.
8. James PA, Oparil S, Carter BL, et al. 2014 Evidence-based guideline for the management of high blood pressure in adults: Report from the panel members appointed to the Eighth Joint National Committee (JNC 8). JAMA. 2014;311(5):507-20.
9. Lipari M, Moser LR, Petrovitch EA, et al. As-needed intravenous antihypertensive therapy and blood pressure control. J Hosp Med. 2016;11(3):193-8.
10. Campbell P, Baker WL, Bendel SD, et al. Intravenous hydralazine for blood pressure management in the hospitalized patient: Its use is often unjustified. J Am Soc Hypertens. 2011;5(6):473-7.
11. Souza LM, Riera R, Saconato H, et al. Oral drugs for hypertensive urgencies: Systematic review and meta-analysis. Sao Paulo Med J. 2009;127(6):366-72.
12. Jaker M, Atkin S, Soto M, et al. Oral nifedipine vs. oral clonidine in the treatment of urgent hypertension. Arch Intern Med. 1989;149(2):260-5.
13. Grossman E, Messerli FH, Grodzicki T, et al. Should a moratorium be placed on sublingual nifedipine capsules given for hypertensive emergencies and pseudoemergencies? JAMA. 1996;276(16):1328-31.
14. Axon RN, Turner M, Buckley R. An update on inpatient hypertension management. Curr Cardiol Rep. 2015;17(11):94.
Additional Reading
1. Axon RN, Turner M, Buckley R. An update on inpatient hypertension management. Curr Cardiol Rep. 2015 Nov;17(11):94.
2. Herzog E, Frankenberger O, Aziz E, et al. A novel pathway for the management of hypertension for hospitalized patients. Crit Pathw Cardiol. 2007;6(4):150-60.
3. Sharma P, Shrestha A. Inpatient hypertension management. ACP Hospitalist. Aug 2014.
Quiz
Asymptomatic hypertension in the hospital
Hypertension is a common focus in the ambulatory setting because of its increased risk for cardiovascular events. Evidence for management in the inpatient setting is limited but does suggest a more conservative approach.
Question: A 75-year-old woman is hospitalized after sustaining a mechanical fall and subsequent right femoral neck fracture. She has a history of hypertension and hyperlipidemia for which she takes amlodipine and atorvastatin. Her blood pressure initially on admission is 170/102 mm Hg, and she is asymptomatic other than severe right hip pain. Her amlodipine and atorvastatin are resumed. Repeat blood pressures after resuming her amlodipine are still elevated with an average blood pressure reading of 168/98 mm Hg. Which of the following would be the next best step in treating this patient?
A. A one-time dose of intravenous hydralazine at 10 mg to reduce blood pressure by 25% over next several hours.
B. A one-time dose of oral clonidine at 0.1 mg to reduce blood pressure by 25% over next several hours.
C. Start a second daily antihypertensive with lisinopril 5 mg daily.
D. Address the patient’s pain.
The best answer is choice D. The patient’s hypertension is likely aggravated by her hip pain. Thus, the best course of action would be to address her pain.
Choice A is not the best answer as an intravenous antihypertensive is not indicated in this patient as she is asymptomatic and exhibiting no signs/symptoms of end-organ damage.
Choice B is not the best answer as by addressing her pain it is likely her blood pressure will improve. Urgent use of oral antihypertensives would not be indicated.
Choice C is not the best answer as patient has acute elevation of blood pressure in setting of a right femoral neck fracture and pain. Her blood pressure will likely improve after addressing her pain. However, if there is persistent blood pressure elevation, starting long-acting antihypertensive would be appropriate per JNC 8 guidelines.
Key Points
- Evidence for treatment of inpatient asymptomatic hypertension is lacking.
- The use of intravenous antihypertensives in the setting of inpatient asymptomatic hypertension is inappropriate and may be harmful.
- A conservative approach for inpatient asymptomatic hypertension should be employed by addressing contributing factors and reviewing held home antihypertensive medications prior to administering any oral antihypertensive pharmacotherapy.
Case
A 62-year-old man with diabetes mellitus and hypertension presents with painful erythema of the left lower extremity and is admitted for purulent cellulitis. During the first evening of admission, he has increased left lower extremity pain and nursing reports a blood pressure of 188/96 mm Hg. He denies dyspnea, chest pain, visual changes, confusion, or severe headache.
Background
The prevalence of hypertension in the outpatient setting in the United States is estimated at 29% by the National Health and Nutrition Examination Survey.1 Hypertension generally is defined in the outpatient setting as an average blood pressure reading greater than or equal to140/90 mm Hg on two or more separate occasions.2 There is no consensus on the definition of hypertension in the inpatient setting; however, hypertensive urgency often is defined as a sustained blood pressure above the range of 180-200 mm Hg systolic and/or 110-120 mm Hg diastolic without target organ damage, and hypertensive emergency has a similar blood pressure range, but with evidence of target organ damage.3
The evidence
There are several clinical trials to suggest that the ambulatory treatment of chronic hypertension reduces the incidence of myocardial infarction, cerebrovascular accident (CVA), and heart failure7,8; however, these trials are difficult to extrapolate to acutely hospitalized patients.6 Overall, evidence on the appropriate management of asymptomatic hypertension in the inpatient setting is lacking.
Some evidence suggests we often are overly aggressive with intravenous antihypertensives without clinical indications in the inpatient setting.3,9,10 For example, Campbell et al. prospectively examined the use of intravenous hydralazine for the treatment of asymptomatic hypertension in 94 hospitalized patients.10 It was determined that in 90 of those patients, there was no clinical indication to use intravenous hydralazine and 17 patients experienced adverse events from hypotension, which included dizziness/light-headedness, syncope, and chest pain.10 They recommend against the use of intravenous hydralazine in the setting of asymptomatic hypertension because of its risk of adverse events with the rapid lowering of blood pressure.10
Weder and Erickson performed a retrospective review on the use of intravenous hydralazine and labetalol administration in 2,189 hospitalized patients.3 They found that only 3% of those patients had symptoms indicating the need for IV antihypertensives and that the length of stay was several days longer in those who had received IV antihypertensives.3
Other studies have examined the role of oral antihypertensives for management of asymptomatic hypertension in the inpatient setting. A systematic review and meta-analysis by Souza et al. assessed the use of oral pharmacotherapy for hypertensive urgency.11 Sixteen randomized clinical trials were reviewed and it was determined that angiotensin-converting enzyme inhibitors (ACE-I) had a superior effect in treating hypertensive urgencies.11 The most common side effect of using an ACE-I was a bad taste in patient’s mouths, and researchers did not observe side effects that were similar as those seen with the use of IV antihypertensives.11
Further, Jaker et al. performed a randomized, double-blind prospective study comparing a single dose of oral nifedipine with oral clonidine for the treatment of hypertensive urgency in 51 patients.12 Both the oral nifedipine and oral clonidine were extremely effective in reducing blood pressure fairly safely.12 However, the rapid lowering of blood pressure with oral nifedipine was concerning to Grossman et al.13 In their literature review of the side effects of oral and sublingual nifedipine, they found that it was one of the most common therapeutic interventions for hypertensive urgency or emergency.13 However, it was potentially dangerous because of the inconsistent blood pressure response after nifedipine administration, particularly with the sublingual form.13 CVAs, acute MIs, and even death were the reported adverse events with the use of oral and sublingual nifedipine.13 Because of that, the investigators recommend against the use of oral or sublingual nifedipine in hypertensive urgency or emergency and suggest using other oral antihypertensive agents instead.13
Typically, if the patient’s blood pressure remains elevated despite these efforts, no urgent treatment is indicated and we recommend close monitoring of the patient’s blood pressure during the hospitalization. If hypertension persists, the next best step would be to titrate a patient’s current oral antihypertensive therapy or to start a long-acting antihypertensive therapy per the JNC 8 (Eighth Joint National Committee) guidelines. It should be noted that, in those patients that are high risk, such as those with known coronary artery disease, heart failure, or prior hemorrhagic CVA, a short-acting oral antihypertensive such as captopril, carvedilol, clonidine, or furosemide should be considered.
Back to the case
The patient’s pain was treated with oral oxycodone. He received no oral or intravenous antihypertensive therapy, and the following morning, his blood pressure improved to 145/95 mm Hg. Based on our suggested approach in Fig. 1, the patient would require no acute treatment despite an improved but elevated blood pressure. We continued to monitor his blood pressure and despite adequate pain control, his blood pressure remain persistently elevated. Thus, per the JNC 8 guidelines, we started him on a long-acting antihypertensive, which improved his blood pressure to 123/78 at the time of discharge.
Bottom line
Management of asymptomatic hypertension in the hospital begins with addressing contributing factors, reviewing held home medications – and rarely – urgent oral pharmacotherapy.
Dr. Lippert is PGY-3 in the department of internal medicine at the University of Kentucky, Lexington. Dr. Bailey is associate professor of medicine at the University of Kentucky. Dr. Gray is associate professor of medicine at the University of Kentucky.
References
1. Yoon S, Fryar C, Carroll M. Hypertension prevalence and control among adults: United States, 2011-2014. 2015. Accessed Oct 2, 2017.
2. Poulter NR, Prabhakaran D, Caulfield M. Hypertension. Lancet. 2015;386(9995):801-12.
3. Weder AB, Erickson S. Treatment of hypertension in the inpatient setting: Use of intravenous labetalol and hydralazine. J Clin Hypertens. 2010;12(1):29-33.
4. Axon RN, Cousineau L, Egan BM. Prevalence and management of hypertension in the inpatient setting: A systematic review. J Hosp Med. 2011;6(7):417-22.
5. Weiss AJ, Elixhauser A. Overview of hospital stays in the United States, 2012. Statistical brief; 2014 Oct. Accessed Oct 2, 2017.
6. Weder AB. Treating acute hypertension in the hospital: a Lacuna in the guidelines. Hypertension. 2011;57(1):18-20.
7. Mancia G, Fagard R, Narkiewicz K, et al. 2013 ESH/ESC guidelines for the management of arterial hypertension: The Task Force for the management of arterial hypertension of the European Society of Hypertension and of the European Society of Cardiology. J Hypertens. 2013 Jul;31(7):1281-357.
8. James PA, Oparil S, Carter BL, et al. 2014 Evidence-based guideline for the management of high blood pressure in adults: Report from the panel members appointed to the Eighth Joint National Committee (JNC 8). JAMA. 2014;311(5):507-20.
9. Lipari M, Moser LR, Petrovitch EA, et al. As-needed intravenous antihypertensive therapy and blood pressure control. J Hosp Med. 2016;11(3):193-8.
10. Campbell P, Baker WL, Bendel SD, et al. Intravenous hydralazine for blood pressure management in the hospitalized patient: Its use is often unjustified. J Am Soc Hypertens. 2011;5(6):473-7.
11. Souza LM, Riera R, Saconato H, et al. Oral drugs for hypertensive urgencies: Systematic review and meta-analysis. Sao Paulo Med J. 2009;127(6):366-72.
12. Jaker M, Atkin S, Soto M, et al. Oral nifedipine vs. oral clonidine in the treatment of urgent hypertension. Arch Intern Med. 1989;149(2):260-5.
13. Grossman E, Messerli FH, Grodzicki T, et al. Should a moratorium be placed on sublingual nifedipine capsules given for hypertensive emergencies and pseudoemergencies? JAMA. 1996;276(16):1328-31.
14. Axon RN, Turner M, Buckley R. An update on inpatient hypertension management. Curr Cardiol Rep. 2015;17(11):94.
Additional Reading
1. Axon RN, Turner M, Buckley R. An update on inpatient hypertension management. Curr Cardiol Rep. 2015 Nov;17(11):94.
2. Herzog E, Frankenberger O, Aziz E, et al. A novel pathway for the management of hypertension for hospitalized patients. Crit Pathw Cardiol. 2007;6(4):150-60.
3. Sharma P, Shrestha A. Inpatient hypertension management. ACP Hospitalist. Aug 2014.
Quiz
Asymptomatic hypertension in the hospital
Hypertension is a common focus in the ambulatory setting because of its increased risk for cardiovascular events. Evidence for management in the inpatient setting is limited but does suggest a more conservative approach.
Question: A 75-year-old woman is hospitalized after sustaining a mechanical fall and subsequent right femoral neck fracture. She has a history of hypertension and hyperlipidemia for which she takes amlodipine and atorvastatin. Her blood pressure initially on admission is 170/102 mm Hg, and she is asymptomatic other than severe right hip pain. Her amlodipine and atorvastatin are resumed. Repeat blood pressures after resuming her amlodipine are still elevated with an average blood pressure reading of 168/98 mm Hg. Which of the following would be the next best step in treating this patient?
A. A one-time dose of intravenous hydralazine at 10 mg to reduce blood pressure by 25% over next several hours.
B. A one-time dose of oral clonidine at 0.1 mg to reduce blood pressure by 25% over next several hours.
C. Start a second daily antihypertensive with lisinopril 5 mg daily.
D. Address the patient’s pain.
The best answer is choice D. The patient’s hypertension is likely aggravated by her hip pain. Thus, the best course of action would be to address her pain.
Choice A is not the best answer as an intravenous antihypertensive is not indicated in this patient as she is asymptomatic and exhibiting no signs/symptoms of end-organ damage.
Choice B is not the best answer as by addressing her pain it is likely her blood pressure will improve. Urgent use of oral antihypertensives would not be indicated.
Choice C is not the best answer as patient has acute elevation of blood pressure in setting of a right femoral neck fracture and pain. Her blood pressure will likely improve after addressing her pain. However, if there is persistent blood pressure elevation, starting long-acting antihypertensive would be appropriate per JNC 8 guidelines.
Key Points
- Evidence for treatment of inpatient asymptomatic hypertension is lacking.
- The use of intravenous antihypertensives in the setting of inpatient asymptomatic hypertension is inappropriate and may be harmful.
- A conservative approach for inpatient asymptomatic hypertension should be employed by addressing contributing factors and reviewing held home antihypertensive medications prior to administering any oral antihypertensive pharmacotherapy.
Case
A 62-year-old man with diabetes mellitus and hypertension presents with painful erythema of the left lower extremity and is admitted for purulent cellulitis. During the first evening of admission, he has increased left lower extremity pain and nursing reports a blood pressure of 188/96 mm Hg. He denies dyspnea, chest pain, visual changes, confusion, or severe headache.
Background
The prevalence of hypertension in the outpatient setting in the United States is estimated at 29% by the National Health and Nutrition Examination Survey.1 Hypertension generally is defined in the outpatient setting as an average blood pressure reading greater than or equal to140/90 mm Hg on two or more separate occasions.2 There is no consensus on the definition of hypertension in the inpatient setting; however, hypertensive urgency often is defined as a sustained blood pressure above the range of 180-200 mm Hg systolic and/or 110-120 mm Hg diastolic without target organ damage, and hypertensive emergency has a similar blood pressure range, but with evidence of target organ damage.3
The evidence
There are several clinical trials to suggest that the ambulatory treatment of chronic hypertension reduces the incidence of myocardial infarction, cerebrovascular accident (CVA), and heart failure7,8; however, these trials are difficult to extrapolate to acutely hospitalized patients.6 Overall, evidence on the appropriate management of asymptomatic hypertension in the inpatient setting is lacking.
Some evidence suggests we often are overly aggressive with intravenous antihypertensives without clinical indications in the inpatient setting.3,9,10 For example, Campbell et al. prospectively examined the use of intravenous hydralazine for the treatment of asymptomatic hypertension in 94 hospitalized patients.10 It was determined that in 90 of those patients, there was no clinical indication to use intravenous hydralazine and 17 patients experienced adverse events from hypotension, which included dizziness/light-headedness, syncope, and chest pain.10 They recommend against the use of intravenous hydralazine in the setting of asymptomatic hypertension because of its risk of adverse events with the rapid lowering of blood pressure.10
Weder and Erickson performed a retrospective review on the use of intravenous hydralazine and labetalol administration in 2,189 hospitalized patients.3 They found that only 3% of those patients had symptoms indicating the need for IV antihypertensives and that the length of stay was several days longer in those who had received IV antihypertensives.3
Other studies have examined the role of oral antihypertensives for management of asymptomatic hypertension in the inpatient setting. A systematic review and meta-analysis by Souza et al. assessed the use of oral pharmacotherapy for hypertensive urgency.11 Sixteen randomized clinical trials were reviewed and it was determined that angiotensin-converting enzyme inhibitors (ACE-I) had a superior effect in treating hypertensive urgencies.11 The most common side effect of using an ACE-I was a bad taste in patient’s mouths, and researchers did not observe side effects that were similar as those seen with the use of IV antihypertensives.11
Further, Jaker et al. performed a randomized, double-blind prospective study comparing a single dose of oral nifedipine with oral clonidine for the treatment of hypertensive urgency in 51 patients.12 Both the oral nifedipine and oral clonidine were extremely effective in reducing blood pressure fairly safely.12 However, the rapid lowering of blood pressure with oral nifedipine was concerning to Grossman et al.13 In their literature review of the side effects of oral and sublingual nifedipine, they found that it was one of the most common therapeutic interventions for hypertensive urgency or emergency.13 However, it was potentially dangerous because of the inconsistent blood pressure response after nifedipine administration, particularly with the sublingual form.13 CVAs, acute MIs, and even death were the reported adverse events with the use of oral and sublingual nifedipine.13 Because of that, the investigators recommend against the use of oral or sublingual nifedipine in hypertensive urgency or emergency and suggest using other oral antihypertensive agents instead.13
Typically, if the patient’s blood pressure remains elevated despite these efforts, no urgent treatment is indicated and we recommend close monitoring of the patient’s blood pressure during the hospitalization. If hypertension persists, the next best step would be to titrate a patient’s current oral antihypertensive therapy or to start a long-acting antihypertensive therapy per the JNC 8 (Eighth Joint National Committee) guidelines. It should be noted that, in those patients that are high risk, such as those with known coronary artery disease, heart failure, or prior hemorrhagic CVA, a short-acting oral antihypertensive such as captopril, carvedilol, clonidine, or furosemide should be considered.
Back to the case
The patient’s pain was treated with oral oxycodone. He received no oral or intravenous antihypertensive therapy, and the following morning, his blood pressure improved to 145/95 mm Hg. Based on our suggested approach in Fig. 1, the patient would require no acute treatment despite an improved but elevated blood pressure. We continued to monitor his blood pressure and despite adequate pain control, his blood pressure remain persistently elevated. Thus, per the JNC 8 guidelines, we started him on a long-acting antihypertensive, which improved his blood pressure to 123/78 at the time of discharge.
Bottom line
Management of asymptomatic hypertension in the hospital begins with addressing contributing factors, reviewing held home medications – and rarely – urgent oral pharmacotherapy.
Dr. Lippert is PGY-3 in the department of internal medicine at the University of Kentucky, Lexington. Dr. Bailey is associate professor of medicine at the University of Kentucky. Dr. Gray is associate professor of medicine at the University of Kentucky.
References
1. Yoon S, Fryar C, Carroll M. Hypertension prevalence and control among adults: United States, 2011-2014. 2015. Accessed Oct 2, 2017.
2. Poulter NR, Prabhakaran D, Caulfield M. Hypertension. Lancet. 2015;386(9995):801-12.
3. Weder AB, Erickson S. Treatment of hypertension in the inpatient setting: Use of intravenous labetalol and hydralazine. J Clin Hypertens. 2010;12(1):29-33.
4. Axon RN, Cousineau L, Egan BM. Prevalence and management of hypertension in the inpatient setting: A systematic review. J Hosp Med. 2011;6(7):417-22.
5. Weiss AJ, Elixhauser A. Overview of hospital stays in the United States, 2012. Statistical brief; 2014 Oct. Accessed Oct 2, 2017.
6. Weder AB. Treating acute hypertension in the hospital: a Lacuna in the guidelines. Hypertension. 2011;57(1):18-20.
7. Mancia G, Fagard R, Narkiewicz K, et al. 2013 ESH/ESC guidelines for the management of arterial hypertension: The Task Force for the management of arterial hypertension of the European Society of Hypertension and of the European Society of Cardiology. J Hypertens. 2013 Jul;31(7):1281-357.
8. James PA, Oparil S, Carter BL, et al. 2014 Evidence-based guideline for the management of high blood pressure in adults: Report from the panel members appointed to the Eighth Joint National Committee (JNC 8). JAMA. 2014;311(5):507-20.
9. Lipari M, Moser LR, Petrovitch EA, et al. As-needed intravenous antihypertensive therapy and blood pressure control. J Hosp Med. 2016;11(3):193-8.
10. Campbell P, Baker WL, Bendel SD, et al. Intravenous hydralazine for blood pressure management in the hospitalized patient: Its use is often unjustified. J Am Soc Hypertens. 2011;5(6):473-7.
11. Souza LM, Riera R, Saconato H, et al. Oral drugs for hypertensive urgencies: Systematic review and meta-analysis. Sao Paulo Med J. 2009;127(6):366-72.
12. Jaker M, Atkin S, Soto M, et al. Oral nifedipine vs. oral clonidine in the treatment of urgent hypertension. Arch Intern Med. 1989;149(2):260-5.
13. Grossman E, Messerli FH, Grodzicki T, et al. Should a moratorium be placed on sublingual nifedipine capsules given for hypertensive emergencies and pseudoemergencies? JAMA. 1996;276(16):1328-31.
14. Axon RN, Turner M, Buckley R. An update on inpatient hypertension management. Curr Cardiol Rep. 2015;17(11):94.
Additional Reading
1. Axon RN, Turner M, Buckley R. An update on inpatient hypertension management. Curr Cardiol Rep. 2015 Nov;17(11):94.
2. Herzog E, Frankenberger O, Aziz E, et al. A novel pathway for the management of hypertension for hospitalized patients. Crit Pathw Cardiol. 2007;6(4):150-60.
3. Sharma P, Shrestha A. Inpatient hypertension management. ACP Hospitalist. Aug 2014.
Quiz
Asymptomatic hypertension in the hospital
Hypertension is a common focus in the ambulatory setting because of its increased risk for cardiovascular events. Evidence for management in the inpatient setting is limited but does suggest a more conservative approach.
Question: A 75-year-old woman is hospitalized after sustaining a mechanical fall and subsequent right femoral neck fracture. She has a history of hypertension and hyperlipidemia for which she takes amlodipine and atorvastatin. Her blood pressure initially on admission is 170/102 mm Hg, and she is asymptomatic other than severe right hip pain. Her amlodipine and atorvastatin are resumed. Repeat blood pressures after resuming her amlodipine are still elevated with an average blood pressure reading of 168/98 mm Hg. Which of the following would be the next best step in treating this patient?
A. A one-time dose of intravenous hydralazine at 10 mg to reduce blood pressure by 25% over next several hours.
B. A one-time dose of oral clonidine at 0.1 mg to reduce blood pressure by 25% over next several hours.
C. Start a second daily antihypertensive with lisinopril 5 mg daily.
D. Address the patient’s pain.
The best answer is choice D. The patient’s hypertension is likely aggravated by her hip pain. Thus, the best course of action would be to address her pain.
Choice A is not the best answer as an intravenous antihypertensive is not indicated in this patient as she is asymptomatic and exhibiting no signs/symptoms of end-organ damage.
Choice B is not the best answer as by addressing her pain it is likely her blood pressure will improve. Urgent use of oral antihypertensives would not be indicated.
Choice C is not the best answer as patient has acute elevation of blood pressure in setting of a right femoral neck fracture and pain. Her blood pressure will likely improve after addressing her pain. However, if there is persistent blood pressure elevation, starting long-acting antihypertensive would be appropriate per JNC 8 guidelines.
Key Points
- Evidence for treatment of inpatient asymptomatic hypertension is lacking.
- The use of intravenous antihypertensives in the setting of inpatient asymptomatic hypertension is inappropriate and may be harmful.
- A conservative approach for inpatient asymptomatic hypertension should be employed by addressing contributing factors and reviewing held home antihypertensive medications prior to administering any oral antihypertensive pharmacotherapy.
Prompt palliative care cut hospital costs in pooled study
For adults with serious illness, consulting with a palliative care team within 3 days of hospital admission significantly reduced hospital costs, according to findings from a systematic review and meta-analysis.
In a pooled analysis of six cohort studies, average cost savings per admission were $3,237 (95% confidence interval, –$3,581 to −$2,893) overall, $4,251 for patients with cancer, and $2,105 for patients with other serious illnesses (all P values less than .001), reported Peter May, PhD, of Trinity College Dublin, and his associates.
In this latter group, prompt palliative care consultations saved more when patients had at least four comorbidities rather than two or fewer comorbidities, the reviewers wrote. The report was published in JAMA Internal Medicine.
About one in four Medicare beneficiaries dies in acute care hospitals, often after weeks of intensive, costly care that may not reflect personal wishes, according to an earlier study (JAMA. 2013;309:470-7). Economic studies have tried to pinpoint the cost savings of palliative care. These studies have found it important to consider both the clinical characteristics of patients and the amount of time between admission and palliative consultations, the reviewers noted. However, heterogeneity among older studies had precluded pooled analyses.
The six studies in this meta-analysis were identified by a search of Embase, PsycINFO, CENTRAL, PubMed, CINAHL, and EconLit databases for economic studies of hospital-based palliative care consultations. The studies were published between 2008 and 2017 and included 133,118 adults with cancer, chronic obstructive pulmonary disease, major organ failure, AIDS/HIV, or serious neurodegenerative disease. Patients tended to be in their 60s and were usually Medicare beneficiaries, although one study focused only on Medicaid enrollees. Forty-one percent of patients had a primary diagnosis of cancer, and 93% were discharged alive. Most also had at least two comorbidities. Only 3.6% received a palliative care consultation (range, 2.2% to 22.3%).
The link that they found between more comorbidities and greater cost savings “is the reverse of prior research that assumed that long-stay, high-cost hospitalized patients could not have their care trajectories affected by palliative care,” the researchers wrote. “Current palliative care provision in the United States is characterized by widespread understaffing. Our results suggest that acute care hospitals may be able to reduce costs for this population by increasing palliative care capacity to meet national guidelines.”
Dr. May received grant support from The Atlantic Philanthropies. The reviewers reported having no conflicts of interest.
SOURCE: May P et al. JAMA Intern Med. 2018 Apr 30. doi: 10.1001/jamainternmed.2018.0750.
For adults with serious illness, consulting with a palliative care team within 3 days of hospital admission significantly reduced hospital costs, according to findings from a systematic review and meta-analysis.
In a pooled analysis of six cohort studies, average cost savings per admission were $3,237 (95% confidence interval, –$3,581 to −$2,893) overall, $4,251 for patients with cancer, and $2,105 for patients with other serious illnesses (all P values less than .001), reported Peter May, PhD, of Trinity College Dublin, and his associates.
In this latter group, prompt palliative care consultations saved more when patients had at least four comorbidities rather than two or fewer comorbidities, the reviewers wrote. The report was published in JAMA Internal Medicine.
About one in four Medicare beneficiaries dies in acute care hospitals, often after weeks of intensive, costly care that may not reflect personal wishes, according to an earlier study (JAMA. 2013;309:470-7). Economic studies have tried to pinpoint the cost savings of palliative care. These studies have found it important to consider both the clinical characteristics of patients and the amount of time between admission and palliative consultations, the reviewers noted. However, heterogeneity among older studies had precluded pooled analyses.
The six studies in this meta-analysis were identified by a search of Embase, PsycINFO, CENTRAL, PubMed, CINAHL, and EconLit databases for economic studies of hospital-based palliative care consultations. The studies were published between 2008 and 2017 and included 133,118 adults with cancer, chronic obstructive pulmonary disease, major organ failure, AIDS/HIV, or serious neurodegenerative disease. Patients tended to be in their 60s and were usually Medicare beneficiaries, although one study focused only on Medicaid enrollees. Forty-one percent of patients had a primary diagnosis of cancer, and 93% were discharged alive. Most also had at least two comorbidities. Only 3.6% received a palliative care consultation (range, 2.2% to 22.3%).
The link that they found between more comorbidities and greater cost savings “is the reverse of prior research that assumed that long-stay, high-cost hospitalized patients could not have their care trajectories affected by palliative care,” the researchers wrote. “Current palliative care provision in the United States is characterized by widespread understaffing. Our results suggest that acute care hospitals may be able to reduce costs for this population by increasing palliative care capacity to meet national guidelines.”
Dr. May received grant support from The Atlantic Philanthropies. The reviewers reported having no conflicts of interest.
SOURCE: May P et al. JAMA Intern Med. 2018 Apr 30. doi: 10.1001/jamainternmed.2018.0750.
For adults with serious illness, consulting with a palliative care team within 3 days of hospital admission significantly reduced hospital costs, according to findings from a systematic review and meta-analysis.
In a pooled analysis of six cohort studies, average cost savings per admission were $3,237 (95% confidence interval, –$3,581 to −$2,893) overall, $4,251 for patients with cancer, and $2,105 for patients with other serious illnesses (all P values less than .001), reported Peter May, PhD, of Trinity College Dublin, and his associates.
In this latter group, prompt palliative care consultations saved more when patients had at least four comorbidities rather than two or fewer comorbidities, the reviewers wrote. The report was published in JAMA Internal Medicine.
About one in four Medicare beneficiaries dies in acute care hospitals, often after weeks of intensive, costly care that may not reflect personal wishes, according to an earlier study (JAMA. 2013;309:470-7). Economic studies have tried to pinpoint the cost savings of palliative care. These studies have found it important to consider both the clinical characteristics of patients and the amount of time between admission and palliative consultations, the reviewers noted. However, heterogeneity among older studies had precluded pooled analyses.
The six studies in this meta-analysis were identified by a search of Embase, PsycINFO, CENTRAL, PubMed, CINAHL, and EconLit databases for economic studies of hospital-based palliative care consultations. The studies were published between 2008 and 2017 and included 133,118 adults with cancer, chronic obstructive pulmonary disease, major organ failure, AIDS/HIV, or serious neurodegenerative disease. Patients tended to be in their 60s and were usually Medicare beneficiaries, although one study focused only on Medicaid enrollees. Forty-one percent of patients had a primary diagnosis of cancer, and 93% were discharged alive. Most also had at least two comorbidities. Only 3.6% received a palliative care consultation (range, 2.2% to 22.3%).
The link that they found between more comorbidities and greater cost savings “is the reverse of prior research that assumed that long-stay, high-cost hospitalized patients could not have their care trajectories affected by palliative care,” the researchers wrote. “Current palliative care provision in the United States is characterized by widespread understaffing. Our results suggest that acute care hospitals may be able to reduce costs for this population by increasing palliative care capacity to meet national guidelines.”
Dr. May received grant support from The Atlantic Philanthropies. The reviewers reported having no conflicts of interest.
SOURCE: May P et al. JAMA Intern Med. 2018 Apr 30. doi: 10.1001/jamainternmed.2018.0750.
FROM JAMA INTERNAL MEDICINE
Key clinical point:
Major finding: Average cost savings per admission were $3,237 overall, $4,251 for patients with cancer, and $2,105 for patients with other serious illnesses (all P-values less than .001).
Study details: Systematic review and meta-analysis of six cohort studies of 133,118 adults with cancer, chronic obstructive pulmonary disease, major organ failure, AIDS/HIV, or serious neurodegenerative disease.
Disclosures: Dr. May received grant support from The Atlantic Philanthropies. The reviewers reported having no conflicts of interest.
Source: May P et al. JAMA Intern Med. 2018 Apr 30. doi: 10.1001/jamainternmed.2018.0750.
Readmitted patients less likely to be “very satisfied” with index admission
Clinical question: Are patient perceptions of care during index hospitalization associated with likelihood of 30-day readmission?
Background: Hospital readmissions are costly (more than $40 billion annually) and common. Nearly one readmission in five is thought to be preventable. While many risk-prediction models exist, few incorporate patient perceptions during the index hospitalization or factors associated with patient experience.
Setting: Single-center academic medical center.
Synopsis: A total of 846 patients – admitted to one of two inpatient general medicine wards at Massachusetts General Hospital in Boston, and were English speaking, aged 18 years or older, and possessed the ability to complete a 20-item study questionnaire – were screened from January 2012 to January 2016. An interviewer-assisted questionnaire was coupled with structured medical records review. Among items assessed were demographic information, patient perceptions of health, satisfaction with inpatient care, confidence in ability to perform self-care and understanding of the care plan, presence of a caregiver, and patient-predicted likelihood of readmission. Of 846 enrolled patients, 201 were readmitted within 30 days. Readmitted patients were less likely to report being “very satisfied” with their overall care during the index admission, and less likely to report that physicians “always listened” to them during the index stay.
Bottom line: This is the first study to relate patients’ perceptions of their care during index hospitalization to the likelihood of readmission. Further investigation will be necessary to determine whether timely assessment of these perceptions can prompt effective intervention that improves likelihood of an enduringly successful transition home.
Citation: Carter J et al. The association between patient experience factors and likelihood of 30-day readmission: A prospective cohort study. BMJ Qual Saf. 2017 Nov 16. doi: 10.1136/bmjqs-2017-007184.
Dr. Helgerson is associate professor of medicine and section head, division of hospital medicine, University of Virginia.
Clinical question: Are patient perceptions of care during index hospitalization associated with likelihood of 30-day readmission?
Background: Hospital readmissions are costly (more than $40 billion annually) and common. Nearly one readmission in five is thought to be preventable. While many risk-prediction models exist, few incorporate patient perceptions during the index hospitalization or factors associated with patient experience.
Setting: Single-center academic medical center.
Synopsis: A total of 846 patients – admitted to one of two inpatient general medicine wards at Massachusetts General Hospital in Boston, and were English speaking, aged 18 years or older, and possessed the ability to complete a 20-item study questionnaire – were screened from January 2012 to January 2016. An interviewer-assisted questionnaire was coupled with structured medical records review. Among items assessed were demographic information, patient perceptions of health, satisfaction with inpatient care, confidence in ability to perform self-care and understanding of the care plan, presence of a caregiver, and patient-predicted likelihood of readmission. Of 846 enrolled patients, 201 were readmitted within 30 days. Readmitted patients were less likely to report being “very satisfied” with their overall care during the index admission, and less likely to report that physicians “always listened” to them during the index stay.
Bottom line: This is the first study to relate patients’ perceptions of their care during index hospitalization to the likelihood of readmission. Further investigation will be necessary to determine whether timely assessment of these perceptions can prompt effective intervention that improves likelihood of an enduringly successful transition home.
Citation: Carter J et al. The association between patient experience factors and likelihood of 30-day readmission: A prospective cohort study. BMJ Qual Saf. 2017 Nov 16. doi: 10.1136/bmjqs-2017-007184.
Dr. Helgerson is associate professor of medicine and section head, division of hospital medicine, University of Virginia.
Clinical question: Are patient perceptions of care during index hospitalization associated with likelihood of 30-day readmission?
Background: Hospital readmissions are costly (more than $40 billion annually) and common. Nearly one readmission in five is thought to be preventable. While many risk-prediction models exist, few incorporate patient perceptions during the index hospitalization or factors associated with patient experience.
Setting: Single-center academic medical center.
Synopsis: A total of 846 patients – admitted to one of two inpatient general medicine wards at Massachusetts General Hospital in Boston, and were English speaking, aged 18 years or older, and possessed the ability to complete a 20-item study questionnaire – were screened from January 2012 to January 2016. An interviewer-assisted questionnaire was coupled with structured medical records review. Among items assessed were demographic information, patient perceptions of health, satisfaction with inpatient care, confidence in ability to perform self-care and understanding of the care plan, presence of a caregiver, and patient-predicted likelihood of readmission. Of 846 enrolled patients, 201 were readmitted within 30 days. Readmitted patients were less likely to report being “very satisfied” with their overall care during the index admission, and less likely to report that physicians “always listened” to them during the index stay.
Bottom line: This is the first study to relate patients’ perceptions of their care during index hospitalization to the likelihood of readmission. Further investigation will be necessary to determine whether timely assessment of these perceptions can prompt effective intervention that improves likelihood of an enduringly successful transition home.
Citation: Carter J et al. The association between patient experience factors and likelihood of 30-day readmission: A prospective cohort study. BMJ Qual Saf. 2017 Nov 16. doi: 10.1136/bmjqs-2017-007184.
Dr. Helgerson is associate professor of medicine and section head, division of hospital medicine, University of Virginia.
Avoiding in-hospital acute kidney injury is a new imperative
NEW ORLEANS– Preventing acute kidney injury and its progression in hospitalized patients deserves to be a high priority – and now there is finally proof that it’s doable, Harold M. Szerlip, MD, declared at the annual meeting of the American College of Physicians.
The PrevAKI study, a recent randomized controlled clinical trial conducted by German investigators, has demonstrated that the use of renal biomarkers to identify patients at high risk for acute kidney injury (AKI) after major cardiac surgery and providing them with a range of internationally recommended supportive measures known as the KDIGO (Kidney Disease: Improving Global Outcomes) care bundle reduced the occurrence of moderate-to-severe AKI by 34% (Intensive Care Med. 2017 Nov;43[11]:1551-61).
The enthusiasm that greeted the PrevAKI trial findings is reflected in an editorial entitled, “AKI: the Myth of Inevitability is Finally Shattered,” by John A. Kellum, MD, professor of critical care medicine and director of the Center for Critical Care Nephrology at the University of Pittsburgh. Dr. Kellum noted that the renal biomarker-based approach to implementation of the KDIGO care bundle resulted in an attractively low number needed to treat (NNT) of only 6, whereas without biomarker-based enrichment of the target population, the NNT would have been more than 33.
“,” Dr. Kellum declared in the editorial (Nat Rev Nephrol. 2017 Mar;13[3]:140-1).
Indeed, another way to do it was recently demonstrated in the SALT-ED trial, in which 13,347 noncritically ill hospitalized patients requiring intravenous fluid administration were randomized to conventional saline or balanced crystalloids. The incidence of AKI and other major adverse kidney events was 4.7% in the balanced crystalloids group, for a significant 18% risk reduction relative to the 5.6% rate with saline (N Engl J Med. 2018 Mar 1;378[9]:819-28).
While that absolute 0.9% risk reduction might initially not sound like much, with 35 million people per year getting IV saline while in the hospital, it translates into 315,000 fewer major adverse kidney events as a result of a simple switch to balanced crystalloids, Dr. Szerlip observed.
The PrevAKI findings validate the concept of AKI ‘golden hours’ during which time potentially reversible early kidney injury detectable via renal biomarkers is occurring prior to the abrupt decline in kidney function measured by change in serum creatinine. “The problem with using change in creatinine to define AKI is the delay in diagnosis, which makes AKI more difficult to treat,” he explained.
The renal biomarkers utilized in PrevAKI were insulin-like growth factor binding protein-7 (IGFBP7) and tissue inhibitor of metalloproteinase-2 (TIMP-2), as incorporated in the commercially available urinary NephroCheck test, which was administered to study participants 4 hours after cardiopulmonary bypass. A test result of 0.3 or more identified a group at high risk for AKI for randomization to the KDIGO bundle or usual care. The KDIGO bundle consists of discontinuation of nephrotoxic agents when feasible, early optimization of fluid status, and maintenance of perfusion pressure.
Patients known to be at increased risk for in-hospital AKI include the elderly, those with diabetes, patients with heart failure or other conditions prone to volume contraction or overload, those undergoing major surgery, individuals with chronic kidney disease, and patients with sepsis.
Dr. Szerlip singled out as particularly nephrotoxic several drugs widely used in hospitalized patients, including the combination of vancomycin plus piperacillin-tazobactam, which in a recent metaanalysis was found to have a number needed to harm of 11 in terms of AKI in comparison to vancomycin monotherapy or vancomycin in combination with cefepime or carbapenem (Crit Care Med. 2018 Jan;46[1]:12-20). He was also critical of the American Society of Anesthesiologists practice parameter recommending that in-hospital pain management plans for surgical patients include continuous regimens of NSAIDs or COX-2 inhibitors as a means of combating the ongoing opioid epidemic.
“These are highly toxic drugs to the kidney and we shouldn’t be using them,” Dr. Szerlip said.
He reported receiving research grants from LaJolla, Bayer, Akebia, and BioPorto, serving on a speakers’ bureau for Astute Medical, and acting as a consultant to Zs Pharma, Amarin, and LaJolla.
NEW ORLEANS– Preventing acute kidney injury and its progression in hospitalized patients deserves to be a high priority – and now there is finally proof that it’s doable, Harold M. Szerlip, MD, declared at the annual meeting of the American College of Physicians.
The PrevAKI study, a recent randomized controlled clinical trial conducted by German investigators, has demonstrated that the use of renal biomarkers to identify patients at high risk for acute kidney injury (AKI) after major cardiac surgery and providing them with a range of internationally recommended supportive measures known as the KDIGO (Kidney Disease: Improving Global Outcomes) care bundle reduced the occurrence of moderate-to-severe AKI by 34% (Intensive Care Med. 2017 Nov;43[11]:1551-61).
The enthusiasm that greeted the PrevAKI trial findings is reflected in an editorial entitled, “AKI: the Myth of Inevitability is Finally Shattered,” by John A. Kellum, MD, professor of critical care medicine and director of the Center for Critical Care Nephrology at the University of Pittsburgh. Dr. Kellum noted that the renal biomarker-based approach to implementation of the KDIGO care bundle resulted in an attractively low number needed to treat (NNT) of only 6, whereas without biomarker-based enrichment of the target population, the NNT would have been more than 33.
“,” Dr. Kellum declared in the editorial (Nat Rev Nephrol. 2017 Mar;13[3]:140-1).
Indeed, another way to do it was recently demonstrated in the SALT-ED trial, in which 13,347 noncritically ill hospitalized patients requiring intravenous fluid administration were randomized to conventional saline or balanced crystalloids. The incidence of AKI and other major adverse kidney events was 4.7% in the balanced crystalloids group, for a significant 18% risk reduction relative to the 5.6% rate with saline (N Engl J Med. 2018 Mar 1;378[9]:819-28).
While that absolute 0.9% risk reduction might initially not sound like much, with 35 million people per year getting IV saline while in the hospital, it translates into 315,000 fewer major adverse kidney events as a result of a simple switch to balanced crystalloids, Dr. Szerlip observed.
The PrevAKI findings validate the concept of AKI ‘golden hours’ during which time potentially reversible early kidney injury detectable via renal biomarkers is occurring prior to the abrupt decline in kidney function measured by change in serum creatinine. “The problem with using change in creatinine to define AKI is the delay in diagnosis, which makes AKI more difficult to treat,” he explained.
The renal biomarkers utilized in PrevAKI were insulin-like growth factor binding protein-7 (IGFBP7) and tissue inhibitor of metalloproteinase-2 (TIMP-2), as incorporated in the commercially available urinary NephroCheck test, which was administered to study participants 4 hours after cardiopulmonary bypass. A test result of 0.3 or more identified a group at high risk for AKI for randomization to the KDIGO bundle or usual care. The KDIGO bundle consists of discontinuation of nephrotoxic agents when feasible, early optimization of fluid status, and maintenance of perfusion pressure.
Patients known to be at increased risk for in-hospital AKI include the elderly, those with diabetes, patients with heart failure or other conditions prone to volume contraction or overload, those undergoing major surgery, individuals with chronic kidney disease, and patients with sepsis.
Dr. Szerlip singled out as particularly nephrotoxic several drugs widely used in hospitalized patients, including the combination of vancomycin plus piperacillin-tazobactam, which in a recent metaanalysis was found to have a number needed to harm of 11 in terms of AKI in comparison to vancomycin monotherapy or vancomycin in combination with cefepime or carbapenem (Crit Care Med. 2018 Jan;46[1]:12-20). He was also critical of the American Society of Anesthesiologists practice parameter recommending that in-hospital pain management plans for surgical patients include continuous regimens of NSAIDs or COX-2 inhibitors as a means of combating the ongoing opioid epidemic.
“These are highly toxic drugs to the kidney and we shouldn’t be using them,” Dr. Szerlip said.
He reported receiving research grants from LaJolla, Bayer, Akebia, and BioPorto, serving on a speakers’ bureau for Astute Medical, and acting as a consultant to Zs Pharma, Amarin, and LaJolla.
NEW ORLEANS– Preventing acute kidney injury and its progression in hospitalized patients deserves to be a high priority – and now there is finally proof that it’s doable, Harold M. Szerlip, MD, declared at the annual meeting of the American College of Physicians.
The PrevAKI study, a recent randomized controlled clinical trial conducted by German investigators, has demonstrated that the use of renal biomarkers to identify patients at high risk for acute kidney injury (AKI) after major cardiac surgery and providing them with a range of internationally recommended supportive measures known as the KDIGO (Kidney Disease: Improving Global Outcomes) care bundle reduced the occurrence of moderate-to-severe AKI by 34% (Intensive Care Med. 2017 Nov;43[11]:1551-61).
The enthusiasm that greeted the PrevAKI trial findings is reflected in an editorial entitled, “AKI: the Myth of Inevitability is Finally Shattered,” by John A. Kellum, MD, professor of critical care medicine and director of the Center for Critical Care Nephrology at the University of Pittsburgh. Dr. Kellum noted that the renal biomarker-based approach to implementation of the KDIGO care bundle resulted in an attractively low number needed to treat (NNT) of only 6, whereas without biomarker-based enrichment of the target population, the NNT would have been more than 33.
“,” Dr. Kellum declared in the editorial (Nat Rev Nephrol. 2017 Mar;13[3]:140-1).
Indeed, another way to do it was recently demonstrated in the SALT-ED trial, in which 13,347 noncritically ill hospitalized patients requiring intravenous fluid administration were randomized to conventional saline or balanced crystalloids. The incidence of AKI and other major adverse kidney events was 4.7% in the balanced crystalloids group, for a significant 18% risk reduction relative to the 5.6% rate with saline (N Engl J Med. 2018 Mar 1;378[9]:819-28).
While that absolute 0.9% risk reduction might initially not sound like much, with 35 million people per year getting IV saline while in the hospital, it translates into 315,000 fewer major adverse kidney events as a result of a simple switch to balanced crystalloids, Dr. Szerlip observed.
The PrevAKI findings validate the concept of AKI ‘golden hours’ during which time potentially reversible early kidney injury detectable via renal biomarkers is occurring prior to the abrupt decline in kidney function measured by change in serum creatinine. “The problem with using change in creatinine to define AKI is the delay in diagnosis, which makes AKI more difficult to treat,” he explained.
The renal biomarkers utilized in PrevAKI were insulin-like growth factor binding protein-7 (IGFBP7) and tissue inhibitor of metalloproteinase-2 (TIMP-2), as incorporated in the commercially available urinary NephroCheck test, which was administered to study participants 4 hours after cardiopulmonary bypass. A test result of 0.3 or more identified a group at high risk for AKI for randomization to the KDIGO bundle or usual care. The KDIGO bundle consists of discontinuation of nephrotoxic agents when feasible, early optimization of fluid status, and maintenance of perfusion pressure.
Patients known to be at increased risk for in-hospital AKI include the elderly, those with diabetes, patients with heart failure or other conditions prone to volume contraction or overload, those undergoing major surgery, individuals with chronic kidney disease, and patients with sepsis.
Dr. Szerlip singled out as particularly nephrotoxic several drugs widely used in hospitalized patients, including the combination of vancomycin plus piperacillin-tazobactam, which in a recent metaanalysis was found to have a number needed to harm of 11 in terms of AKI in comparison to vancomycin monotherapy or vancomycin in combination with cefepime or carbapenem (Crit Care Med. 2018 Jan;46[1]:12-20). He was also critical of the American Society of Anesthesiologists practice parameter recommending that in-hospital pain management plans for surgical patients include continuous regimens of NSAIDs or COX-2 inhibitors as a means of combating the ongoing opioid epidemic.
“These are highly toxic drugs to the kidney and we shouldn’t be using them,” Dr. Szerlip said.
He reported receiving research grants from LaJolla, Bayer, Akebia, and BioPorto, serving on a speakers’ bureau for Astute Medical, and acting as a consultant to Zs Pharma, Amarin, and LaJolla.
EXPERT ANALYSIS FROM ACP INTERNAL MECICINE
CMS floats Medicare direct provider contracting
Under a direct provider contracting (DPC) arrangement, Medicare could pay physicians or physician groups a monthly fee to deliver a specific set of services to beneficiaries, who would gain greater access to the physicians. The physicians would be accountable for those Medicare patients’ costs and care quality.
CMS is looking at how to incorporate this concept into the Medicare ranks. On April 23, CMS issued a request for information (RFI) seeking input across a wide range of topics, including provider/state participation, beneficiary participation, payment, general model design, program integrity and beneficiary protection, and how such models would fit within the existing accountable care organization framework.
The RFI offered one possible vision on how a direct provider contracting model could work.
“Under a primary care–focused DPC model, CMS could enter into arrangements with primary care practices under which CMS would pay these participating practices a fixed per beneficiary per month (PBPM) payment to cover the primary care services the practice would be expected to furnish under the model, which may include office visits, certain office-based procedures, and other non–visit-based services covered under the physician fee schedule, and flexibility in how otherwise billable services are delivered,” the RFI states.
Physicians could also earn performance bonuses, depending on how the DPC is structured, through “performance-based incentives for total cost of care and quality.”
CMS noted it also “could test ways to reduce administrative burden though innovative changes to claims submission processes for services included in the PBPM payment under these models.”
The direct provider contracting idea grew out of a previous RFI issued in 2017 by CMS’s Center for Medicare and Medicaid Innovation to collect ideas on new ways to deliver patient-centered care. The agency released the more than 1,000 comments received from that request on the same day it issued the RFI on direct provider contracting.
In those comments, a number of physician groups offered support for a direct-contracting approach.
For example, the American Academy of Family Physicians wrote that it “sees continued growth and interest in family physicians adopting this practice model in all settings types, including rural and underserved communities.” And the AAFP suggested that the innovation center should work with DPC organizations to learn more about them.
The American College of Physicians reiterated its previous position that it “supports physician and patient choice of practice and delivery models that are accessible, ethical, and viable and that strengthen the patient-physician relationship.” But the ACP raised a number of issues that could impede access to care or result in lower quality care.
The American Medical Association offered support for “testing of models in which physicians have the ability to deliver more or different services to patients who need them and to be paid more for doing so.”
The AMA suggested that some of the models to be tested include allowing patients to contract directly with physicians, with Medicare paying its fee schedule rates and patients paying the difference; allowing patients to receive their care from DPC practices and get reimbursed by Medicare; or allowing “physicians to define a team of providers who will provide all of the treatment needed for an acute condition or management of a chronic condition, and then allowing patients who select the team to receive all of the services related to their condition from the team in return for a single predefined cost-sharing amount.”
Comments on the RFI are due May 25.
Under a direct provider contracting (DPC) arrangement, Medicare could pay physicians or physician groups a monthly fee to deliver a specific set of services to beneficiaries, who would gain greater access to the physicians. The physicians would be accountable for those Medicare patients’ costs and care quality.
CMS is looking at how to incorporate this concept into the Medicare ranks. On April 23, CMS issued a request for information (RFI) seeking input across a wide range of topics, including provider/state participation, beneficiary participation, payment, general model design, program integrity and beneficiary protection, and how such models would fit within the existing accountable care organization framework.
The RFI offered one possible vision on how a direct provider contracting model could work.
“Under a primary care–focused DPC model, CMS could enter into arrangements with primary care practices under which CMS would pay these participating practices a fixed per beneficiary per month (PBPM) payment to cover the primary care services the practice would be expected to furnish under the model, which may include office visits, certain office-based procedures, and other non–visit-based services covered under the physician fee schedule, and flexibility in how otherwise billable services are delivered,” the RFI states.
Physicians could also earn performance bonuses, depending on how the DPC is structured, through “performance-based incentives for total cost of care and quality.”
CMS noted it also “could test ways to reduce administrative burden though innovative changes to claims submission processes for services included in the PBPM payment under these models.”
The direct provider contracting idea grew out of a previous RFI issued in 2017 by CMS’s Center for Medicare and Medicaid Innovation to collect ideas on new ways to deliver patient-centered care. The agency released the more than 1,000 comments received from that request on the same day it issued the RFI on direct provider contracting.
In those comments, a number of physician groups offered support for a direct-contracting approach.
For example, the American Academy of Family Physicians wrote that it “sees continued growth and interest in family physicians adopting this practice model in all settings types, including rural and underserved communities.” And the AAFP suggested that the innovation center should work with DPC organizations to learn more about them.
The American College of Physicians reiterated its previous position that it “supports physician and patient choice of practice and delivery models that are accessible, ethical, and viable and that strengthen the patient-physician relationship.” But the ACP raised a number of issues that could impede access to care or result in lower quality care.
The American Medical Association offered support for “testing of models in which physicians have the ability to deliver more or different services to patients who need them and to be paid more for doing so.”
The AMA suggested that some of the models to be tested include allowing patients to contract directly with physicians, with Medicare paying its fee schedule rates and patients paying the difference; allowing patients to receive their care from DPC practices and get reimbursed by Medicare; or allowing “physicians to define a team of providers who will provide all of the treatment needed for an acute condition or management of a chronic condition, and then allowing patients who select the team to receive all of the services related to their condition from the team in return for a single predefined cost-sharing amount.”
Comments on the RFI are due May 25.
Under a direct provider contracting (DPC) arrangement, Medicare could pay physicians or physician groups a monthly fee to deliver a specific set of services to beneficiaries, who would gain greater access to the physicians. The physicians would be accountable for those Medicare patients’ costs and care quality.
CMS is looking at how to incorporate this concept into the Medicare ranks. On April 23, CMS issued a request for information (RFI) seeking input across a wide range of topics, including provider/state participation, beneficiary participation, payment, general model design, program integrity and beneficiary protection, and how such models would fit within the existing accountable care organization framework.
The RFI offered one possible vision on how a direct provider contracting model could work.
“Under a primary care–focused DPC model, CMS could enter into arrangements with primary care practices under which CMS would pay these participating practices a fixed per beneficiary per month (PBPM) payment to cover the primary care services the practice would be expected to furnish under the model, which may include office visits, certain office-based procedures, and other non–visit-based services covered under the physician fee schedule, and flexibility in how otherwise billable services are delivered,” the RFI states.
Physicians could also earn performance bonuses, depending on how the DPC is structured, through “performance-based incentives for total cost of care and quality.”
CMS noted it also “could test ways to reduce administrative burden though innovative changes to claims submission processes for services included in the PBPM payment under these models.”
The direct provider contracting idea grew out of a previous RFI issued in 2017 by CMS’s Center for Medicare and Medicaid Innovation to collect ideas on new ways to deliver patient-centered care. The agency released the more than 1,000 comments received from that request on the same day it issued the RFI on direct provider contracting.
In those comments, a number of physician groups offered support for a direct-contracting approach.
For example, the American Academy of Family Physicians wrote that it “sees continued growth and interest in family physicians adopting this practice model in all settings types, including rural and underserved communities.” And the AAFP suggested that the innovation center should work with DPC organizations to learn more about them.
The American College of Physicians reiterated its previous position that it “supports physician and patient choice of practice and delivery models that are accessible, ethical, and viable and that strengthen the patient-physician relationship.” But the ACP raised a number of issues that could impede access to care or result in lower quality care.
The American Medical Association offered support for “testing of models in which physicians have the ability to deliver more or different services to patients who need them and to be paid more for doing so.”
The AMA suggested that some of the models to be tested include allowing patients to contract directly with physicians, with Medicare paying its fee schedule rates and patients paying the difference; allowing patients to receive their care from DPC practices and get reimbursed by Medicare; or allowing “physicians to define a team of providers who will provide all of the treatment needed for an acute condition or management of a chronic condition, and then allowing patients who select the team to receive all of the services related to their condition from the team in return for a single predefined cost-sharing amount.”
Comments on the RFI are due May 25.
Noninvasive cardiac testing to rule out acute coronary syndromes provides no benefit in low-risk chest pain patients
Background: The 2014 American College of Cardiology/American Heart Association clinical guideline includes a recommendation for noninvasive testing (exercise test or coronary commuted tomographic angiography [CCTA]) in patients with chest pain but no evidence of ischemia. The ROMICAT-II (Rule Out Myocardial Ischemia/Infarction by Computer Assisted Tomography) trial randomized 1,000 ED patients with chest pain to undergo CCTA or usual care with unknown benefits and risks of this testing recommendation. The question of whether this testing may be omitted is addressed in secondary analysis.
Study design: Retrospective analysis of the ROMICAT-II trial data.
Setting: Emergency department.
Synopsis: As compared with 882 patients who underwent noninvasive testing, 118 patients in the ROMICAT-II usual care group who did not undergo noninvasive testing had shorter lengths of stay (20.3 vs. 27.9 hours; P less than .001), lower rates of diagnostic testing (P less than .001), angiography (2% vs. 11%; P less than .001), and lower costs ($2,261.50 vs. $2,584.30). There was no difference in percutaneous coronary intervention (2% vs. 5%; P = .15), coronary artery bypass surgery (0% vs. 1%; P = .61), major adverse cardiac events (MACE; 2% vs. 1%; P = .24), or return ED visits (5.8% vs. 2.8%; P = .08) during the 28-day follow-up period. These findings suggest noninvasive cardiac testing may be omitted in low- to intermediate-risk patients presenting with chest pain. However, this study was not designed or powered to address this question and patients were not randomized. These data may support hospitalists who choose not to order noninvasive testing in ED patients with chest pain.
Bottom line: In this secondary analysis of ROMICAT-II clinical trial data, patients who underwent clinical evaluation without noninvasive testing had shorter length of stay, less diagnostic testing, lower radiation exposure, and reduced costs with no difference in missed diagnosis of acute coronary syndromes, development of MACE, or return ED visits.
Citation: Reinhardt SW et al. Noninvasive cardiac testing vs. clinical evaluation alone in acute chest pain: A secondary analysis of the ROMICAT-II randomized clinical trial. JAMA Intern Med. 2018 Feb 1;178(2):212-9.
Dr. Moulder is assistant professor, University of Virginia Health System.
Background: The 2014 American College of Cardiology/American Heart Association clinical guideline includes a recommendation for noninvasive testing (exercise test or coronary commuted tomographic angiography [CCTA]) in patients with chest pain but no evidence of ischemia. The ROMICAT-II (Rule Out Myocardial Ischemia/Infarction by Computer Assisted Tomography) trial randomized 1,000 ED patients with chest pain to undergo CCTA or usual care with unknown benefits and risks of this testing recommendation. The question of whether this testing may be omitted is addressed in secondary analysis.
Study design: Retrospective analysis of the ROMICAT-II trial data.
Setting: Emergency department.
Synopsis: As compared with 882 patients who underwent noninvasive testing, 118 patients in the ROMICAT-II usual care group who did not undergo noninvasive testing had shorter lengths of stay (20.3 vs. 27.9 hours; P less than .001), lower rates of diagnostic testing (P less than .001), angiography (2% vs. 11%; P less than .001), and lower costs ($2,261.50 vs. $2,584.30). There was no difference in percutaneous coronary intervention (2% vs. 5%; P = .15), coronary artery bypass surgery (0% vs. 1%; P = .61), major adverse cardiac events (MACE; 2% vs. 1%; P = .24), or return ED visits (5.8% vs. 2.8%; P = .08) during the 28-day follow-up period. These findings suggest noninvasive cardiac testing may be omitted in low- to intermediate-risk patients presenting with chest pain. However, this study was not designed or powered to address this question and patients were not randomized. These data may support hospitalists who choose not to order noninvasive testing in ED patients with chest pain.
Bottom line: In this secondary analysis of ROMICAT-II clinical trial data, patients who underwent clinical evaluation without noninvasive testing had shorter length of stay, less diagnostic testing, lower radiation exposure, and reduced costs with no difference in missed diagnosis of acute coronary syndromes, development of MACE, or return ED visits.
Citation: Reinhardt SW et al. Noninvasive cardiac testing vs. clinical evaluation alone in acute chest pain: A secondary analysis of the ROMICAT-II randomized clinical trial. JAMA Intern Med. 2018 Feb 1;178(2):212-9.
Dr. Moulder is assistant professor, University of Virginia Health System.
Background: The 2014 American College of Cardiology/American Heart Association clinical guideline includes a recommendation for noninvasive testing (exercise test or coronary commuted tomographic angiography [CCTA]) in patients with chest pain but no evidence of ischemia. The ROMICAT-II (Rule Out Myocardial Ischemia/Infarction by Computer Assisted Tomography) trial randomized 1,000 ED patients with chest pain to undergo CCTA or usual care with unknown benefits and risks of this testing recommendation. The question of whether this testing may be omitted is addressed in secondary analysis.
Study design: Retrospective analysis of the ROMICAT-II trial data.
Setting: Emergency department.
Synopsis: As compared with 882 patients who underwent noninvasive testing, 118 patients in the ROMICAT-II usual care group who did not undergo noninvasive testing had shorter lengths of stay (20.3 vs. 27.9 hours; P less than .001), lower rates of diagnostic testing (P less than .001), angiography (2% vs. 11%; P less than .001), and lower costs ($2,261.50 vs. $2,584.30). There was no difference in percutaneous coronary intervention (2% vs. 5%; P = .15), coronary artery bypass surgery (0% vs. 1%; P = .61), major adverse cardiac events (MACE; 2% vs. 1%; P = .24), or return ED visits (5.8% vs. 2.8%; P = .08) during the 28-day follow-up period. These findings suggest noninvasive cardiac testing may be omitted in low- to intermediate-risk patients presenting with chest pain. However, this study was not designed or powered to address this question and patients were not randomized. These data may support hospitalists who choose not to order noninvasive testing in ED patients with chest pain.
Bottom line: In this secondary analysis of ROMICAT-II clinical trial data, patients who underwent clinical evaluation without noninvasive testing had shorter length of stay, less diagnostic testing, lower radiation exposure, and reduced costs with no difference in missed diagnosis of acute coronary syndromes, development of MACE, or return ED visits.
Citation: Reinhardt SW et al. Noninvasive cardiac testing vs. clinical evaluation alone in acute chest pain: A secondary analysis of the ROMICAT-II randomized clinical trial. JAMA Intern Med. 2018 Feb 1;178(2):212-9.
Dr. Moulder is assistant professor, University of Virginia Health System.
Female physicians face enduring wage gap
Male physicians make more money than female physicians, and that seems to be a rule with few exceptions. Among the 50 largest metro areas, there were none where women earn as much as men, according to a new survey by the medical social network Doximity.
The metro area that comes the closest is Las Vegas, where female physicians earned 20% less – that works out to $73,654 – than their male counterparts in 2017. Rochester, N.Y., had the smallest gap in terms of dollars ($68,758) and the second-smallest percent difference (21%), Doximity said in its 2018 Physician Compensation Report.
A quick look at the 2016 data shows that the wage gap between female and male physicians increased from 26.5% to 27.7% in 2017, going from more than $92,000 to $105,000. “Medicine is a highly trained field, and as such, one might expect the gender wage gap to be less prominent here than in other industries. However, the gap endures, despite the level of education required to practice medicine and market forces suggesting that this gap should shrink,” Doximity said.
Male physicians make more money than female physicians, and that seems to be a rule with few exceptions. Among the 50 largest metro areas, there were none where women earn as much as men, according to a new survey by the medical social network Doximity.
The metro area that comes the closest is Las Vegas, where female physicians earned 20% less – that works out to $73,654 – than their male counterparts in 2017. Rochester, N.Y., had the smallest gap in terms of dollars ($68,758) and the second-smallest percent difference (21%), Doximity said in its 2018 Physician Compensation Report.
A quick look at the 2016 data shows that the wage gap between female and male physicians increased from 26.5% to 27.7% in 2017, going from more than $92,000 to $105,000. “Medicine is a highly trained field, and as such, one might expect the gender wage gap to be less prominent here than in other industries. However, the gap endures, despite the level of education required to practice medicine and market forces suggesting that this gap should shrink,” Doximity said.
Male physicians make more money than female physicians, and that seems to be a rule with few exceptions. Among the 50 largest metro areas, there were none where women earn as much as men, according to a new survey by the medical social network Doximity.
The metro area that comes the closest is Las Vegas, where female physicians earned 20% less – that works out to $73,654 – than their male counterparts in 2017. Rochester, N.Y., had the smallest gap in terms of dollars ($68,758) and the second-smallest percent difference (21%), Doximity said in its 2018 Physician Compensation Report.
A quick look at the 2016 data shows that the wage gap between female and male physicians increased from 26.5% to 27.7% in 2017, going from more than $92,000 to $105,000. “Medicine is a highly trained field, and as such, one might expect the gender wage gap to be less prominent here than in other industries. However, the gap endures, despite the level of education required to practice medicine and market forces suggesting that this gap should shrink,” Doximity said.
Sepsis versus SIRS blood test shows high sensitivity
A molecular host response assay, called SeptiCyte Lab, holds promise as a tool to distinguish between sepsis and noninfectious systemic inflammation (SIRS), reported researchers in an industry-funded study.
Sepsis is a complex and hard-to-diagnose condition, noted two members of the editorial advisory board of CHEST Physician® in interviews. To make things more complicated, there’s not even a standard definition of sepsis, explained board member Nirmal S. Sharma, MD, of the University of South Florida, Tampa.
“Although newer sepsis definitions have been proposed, all of them have pitfalls and are not used universally. Additionally, the presence of inflammatory response leading to suspicion of sepsis can be due to a new infection or underlying disease processes, thus making it difficult to identify the possible cause,” said Dr. Sharma. “Culture-negative cases due to the use of antibiotics prior to suspicion/onset of sepsis can further muddle the picture. Finally, in certain subsets of patients, such as the immunocompromised and elderly, the signs of sepsis may be delayed due to inadequate/dampened immune response, thus making early diagnosis difficult.”
Blood testing can provide information about germs that are causing an infection, but “they often take several days, and we need to start the antibiotics before we have those results,” added Daniel R. Ouellette, MD, FCCP, the other board member interviewed.
The SeptiCyte Lab assay, which was approved by the FDA for use in diagnosing sepsis in 2017, was developed to help physicians distinguish sepsis from SIRS in patients during their first day of ICU treatment, noted the authors of the new study in the American Journal of Respiratory and Critical Care Medicine.
This new tool seems to overcome some of the obstacles encountered when other diagnostic methods are used to determine if a patient has sepsis.
Russell R. Miller III, MD, FCCM, and his colleagues performed their SeptiCyte Lab assay on patients’ blood samples; this involved real-time, reverse-transcription, quantitative polymerase chain reaction screening designed to analyze the relative expression levels of four genes. The testing procedure took approximately 6 hours from the draw of the blood sample, according to the study, which was recently published online.
comprising three members. Negative predictive values were at least 0.89, according to the researchers.
Overall, the findings show “good reliability,” wrote Dr. Miller of the Intermountain Medical Center in Murray, Utah, and the University of Utah, Salt Lake City, and his colleagues.
The test produced scores in four bands, with scores at or above 3.1 considered to be evidence of infection. Lower levels were considered to be evidence of noninfection.
Dr. Miller and his coauthors reported that 86% of patients unanimously considered to have sepsis had scores above 3.1. In contrast, only 30% of those considered to have SIRS had such high scores.
In addition, the study authors determined that the test was more reliable than were the clinical signs and laboratory variables that are commonly used to diagnose sepsis within 24 hours of arrival at the ICU.
Reaching a definitive sepsis diagnosis is challenging based on clinical signs alone, since various conditions mimic the signs of sepsis, noted Dr. Ouellette of Henry Ford Hospital and Wayne State University School of Medicine in Detroit.
In some cases, physicians simply assume that a patient has sepsis and begin antibiotics, he said, “but that’s not a free ride. Each [antibiotic] may produce side effects with consequences for patients. The other problem is that overuse of antibiotics leads to resistance.”
The study by Dr. Miller and his colleagues combined the results of three trials conducted from during 2011-2016 in the United States and the Netherlands in 447 subjects.
One trial analyzed the experiences of 198 consecutive subjects, all critically ill, who met various criteria. (They were part of a consortium trial of 7,500 patients). The second trial had 129 participants, and the third had 120. Of the total participants, 71% were white and 20% were black.
Inclusion of procalcitonin levels in the laboratory variables didn’t appear to make a significant difference. The study authors wrote that the test “differs from, and is complementary to that of procalcitonin. The latter test is cleared for predicting progression from severe sepsis to septic shock, for predicting 28-day mortality, and for managing antibiotic de-escalation.”
According to the researchers, differences in age, sex, and race/ethnicity did not significantly affect the test.
The study concludes by noting that “future studies are warranted to determine how host gene expression could most effectively be integrated into clinical decision making to ensure susceptible patients are accurately managed early in the course of disease.”
The test is “promising new technology, but I don’t think you could say it’s definitive,” noted Dr. Ouellette.“Like any test, it’s not perfect,” he explained. “That’s important because physicians wouldn’t want to guess wrong. We might err on the side of choosing to treat with antibiotics even in the face of a test that suggested they might not have infection.”
Immunexpress and the Australian Government funded the study. Fourteen authors disclosed being current or former employees of Immunexpress and/or shareholders; others reported receiving funding from the company via their institutions. Four authors declared having filed patent applications related to the study or to the diagnosis of community-acquired pneumonia upon ICU admission. Some authors reported various other disclosures.
Dr. Ouellette and Dr. Sharma said they did not have any disclosures.
SOURCE: Miller RR et al. Am J Respir Crit Care Med. 2018 Apr 6. doi: 10.1164/rccm.201712-2472OC.
A molecular host response assay, called SeptiCyte Lab, holds promise as a tool to distinguish between sepsis and noninfectious systemic inflammation (SIRS), reported researchers in an industry-funded study.
Sepsis is a complex and hard-to-diagnose condition, noted two members of the editorial advisory board of CHEST Physician® in interviews. To make things more complicated, there’s not even a standard definition of sepsis, explained board member Nirmal S. Sharma, MD, of the University of South Florida, Tampa.
“Although newer sepsis definitions have been proposed, all of them have pitfalls and are not used universally. Additionally, the presence of inflammatory response leading to suspicion of sepsis can be due to a new infection or underlying disease processes, thus making it difficult to identify the possible cause,” said Dr. Sharma. “Culture-negative cases due to the use of antibiotics prior to suspicion/onset of sepsis can further muddle the picture. Finally, in certain subsets of patients, such as the immunocompromised and elderly, the signs of sepsis may be delayed due to inadequate/dampened immune response, thus making early diagnosis difficult.”
Blood testing can provide information about germs that are causing an infection, but “they often take several days, and we need to start the antibiotics before we have those results,” added Daniel R. Ouellette, MD, FCCP, the other board member interviewed.
The SeptiCyte Lab assay, which was approved by the FDA for use in diagnosing sepsis in 2017, was developed to help physicians distinguish sepsis from SIRS in patients during their first day of ICU treatment, noted the authors of the new study in the American Journal of Respiratory and Critical Care Medicine.
This new tool seems to overcome some of the obstacles encountered when other diagnostic methods are used to determine if a patient has sepsis.
Russell R. Miller III, MD, FCCM, and his colleagues performed their SeptiCyte Lab assay on patients’ blood samples; this involved real-time, reverse-transcription, quantitative polymerase chain reaction screening designed to analyze the relative expression levels of four genes. The testing procedure took approximately 6 hours from the draw of the blood sample, according to the study, which was recently published online.
comprising three members. Negative predictive values were at least 0.89, according to the researchers.
Overall, the findings show “good reliability,” wrote Dr. Miller of the Intermountain Medical Center in Murray, Utah, and the University of Utah, Salt Lake City, and his colleagues.
The test produced scores in four bands, with scores at or above 3.1 considered to be evidence of infection. Lower levels were considered to be evidence of noninfection.
Dr. Miller and his coauthors reported that 86% of patients unanimously considered to have sepsis had scores above 3.1. In contrast, only 30% of those considered to have SIRS had such high scores.
In addition, the study authors determined that the test was more reliable than were the clinical signs and laboratory variables that are commonly used to diagnose sepsis within 24 hours of arrival at the ICU.
Reaching a definitive sepsis diagnosis is challenging based on clinical signs alone, since various conditions mimic the signs of sepsis, noted Dr. Ouellette of Henry Ford Hospital and Wayne State University School of Medicine in Detroit.
In some cases, physicians simply assume that a patient has sepsis and begin antibiotics, he said, “but that’s not a free ride. Each [antibiotic] may produce side effects with consequences for patients. The other problem is that overuse of antibiotics leads to resistance.”
The study by Dr. Miller and his colleagues combined the results of three trials conducted from during 2011-2016 in the United States and the Netherlands in 447 subjects.
One trial analyzed the experiences of 198 consecutive subjects, all critically ill, who met various criteria. (They were part of a consortium trial of 7,500 patients). The second trial had 129 participants, and the third had 120. Of the total participants, 71% were white and 20% were black.
Inclusion of procalcitonin levels in the laboratory variables didn’t appear to make a significant difference. The study authors wrote that the test “differs from, and is complementary to that of procalcitonin. The latter test is cleared for predicting progression from severe sepsis to septic shock, for predicting 28-day mortality, and for managing antibiotic de-escalation.”
According to the researchers, differences in age, sex, and race/ethnicity did not significantly affect the test.
The study concludes by noting that “future studies are warranted to determine how host gene expression could most effectively be integrated into clinical decision making to ensure susceptible patients are accurately managed early in the course of disease.”
The test is “promising new technology, but I don’t think you could say it’s definitive,” noted Dr. Ouellette.“Like any test, it’s not perfect,” he explained. “That’s important because physicians wouldn’t want to guess wrong. We might err on the side of choosing to treat with antibiotics even in the face of a test that suggested they might not have infection.”
Immunexpress and the Australian Government funded the study. Fourteen authors disclosed being current or former employees of Immunexpress and/or shareholders; others reported receiving funding from the company via their institutions. Four authors declared having filed patent applications related to the study or to the diagnosis of community-acquired pneumonia upon ICU admission. Some authors reported various other disclosures.
Dr. Ouellette and Dr. Sharma said they did not have any disclosures.
SOURCE: Miller RR et al. Am J Respir Crit Care Med. 2018 Apr 6. doi: 10.1164/rccm.201712-2472OC.
A molecular host response assay, called SeptiCyte Lab, holds promise as a tool to distinguish between sepsis and noninfectious systemic inflammation (SIRS), reported researchers in an industry-funded study.
Sepsis is a complex and hard-to-diagnose condition, noted two members of the editorial advisory board of CHEST Physician® in interviews. To make things more complicated, there’s not even a standard definition of sepsis, explained board member Nirmal S. Sharma, MD, of the University of South Florida, Tampa.
“Although newer sepsis definitions have been proposed, all of them have pitfalls and are not used universally. Additionally, the presence of inflammatory response leading to suspicion of sepsis can be due to a new infection or underlying disease processes, thus making it difficult to identify the possible cause,” said Dr. Sharma. “Culture-negative cases due to the use of antibiotics prior to suspicion/onset of sepsis can further muddle the picture. Finally, in certain subsets of patients, such as the immunocompromised and elderly, the signs of sepsis may be delayed due to inadequate/dampened immune response, thus making early diagnosis difficult.”
Blood testing can provide information about germs that are causing an infection, but “they often take several days, and we need to start the antibiotics before we have those results,” added Daniel R. Ouellette, MD, FCCP, the other board member interviewed.
The SeptiCyte Lab assay, which was approved by the FDA for use in diagnosing sepsis in 2017, was developed to help physicians distinguish sepsis from SIRS in patients during their first day of ICU treatment, noted the authors of the new study in the American Journal of Respiratory and Critical Care Medicine.
This new tool seems to overcome some of the obstacles encountered when other diagnostic methods are used to determine if a patient has sepsis.
Russell R. Miller III, MD, FCCM, and his colleagues performed their SeptiCyte Lab assay on patients’ blood samples; this involved real-time, reverse-transcription, quantitative polymerase chain reaction screening designed to analyze the relative expression levels of four genes. The testing procedure took approximately 6 hours from the draw of the blood sample, according to the study, which was recently published online.
comprising three members. Negative predictive values were at least 0.89, according to the researchers.
Overall, the findings show “good reliability,” wrote Dr. Miller of the Intermountain Medical Center in Murray, Utah, and the University of Utah, Salt Lake City, and his colleagues.
The test produced scores in four bands, with scores at or above 3.1 considered to be evidence of infection. Lower levels were considered to be evidence of noninfection.
Dr. Miller and his coauthors reported that 86% of patients unanimously considered to have sepsis had scores above 3.1. In contrast, only 30% of those considered to have SIRS had such high scores.
In addition, the study authors determined that the test was more reliable than were the clinical signs and laboratory variables that are commonly used to diagnose sepsis within 24 hours of arrival at the ICU.
Reaching a definitive sepsis diagnosis is challenging based on clinical signs alone, since various conditions mimic the signs of sepsis, noted Dr. Ouellette of Henry Ford Hospital and Wayne State University School of Medicine in Detroit.
In some cases, physicians simply assume that a patient has sepsis and begin antibiotics, he said, “but that’s not a free ride. Each [antibiotic] may produce side effects with consequences for patients. The other problem is that overuse of antibiotics leads to resistance.”
The study by Dr. Miller and his colleagues combined the results of three trials conducted from during 2011-2016 in the United States and the Netherlands in 447 subjects.
One trial analyzed the experiences of 198 consecutive subjects, all critically ill, who met various criteria. (They were part of a consortium trial of 7,500 patients). The second trial had 129 participants, and the third had 120. Of the total participants, 71% were white and 20% were black.
Inclusion of procalcitonin levels in the laboratory variables didn’t appear to make a significant difference. The study authors wrote that the test “differs from, and is complementary to that of procalcitonin. The latter test is cleared for predicting progression from severe sepsis to septic shock, for predicting 28-day mortality, and for managing antibiotic de-escalation.”
According to the researchers, differences in age, sex, and race/ethnicity did not significantly affect the test.
The study concludes by noting that “future studies are warranted to determine how host gene expression could most effectively be integrated into clinical decision making to ensure susceptible patients are accurately managed early in the course of disease.”
The test is “promising new technology, but I don’t think you could say it’s definitive,” noted Dr. Ouellette.“Like any test, it’s not perfect,” he explained. “That’s important because physicians wouldn’t want to guess wrong. We might err on the side of choosing to treat with antibiotics even in the face of a test that suggested they might not have infection.”
Immunexpress and the Australian Government funded the study. Fourteen authors disclosed being current or former employees of Immunexpress and/or shareholders; others reported receiving funding from the company via their institutions. Four authors declared having filed patent applications related to the study or to the diagnosis of community-acquired pneumonia upon ICU admission. Some authors reported various other disclosures.
Dr. Ouellette and Dr. Sharma said they did not have any disclosures.
SOURCE: Miller RR et al. Am J Respir Crit Care Med. 2018 Apr 6. doi: 10.1164/rccm.201712-2472OC.
FROM AJRCCM
Key clinical point: A blood test to distinguish sepsis from noninfectious systemic inflammation showed high sensitivity.
Major finding: The sensitivity at detecting sepsis was 0.97 in patients unanimously believed by expert panelists to have the condition.
Study details: Prospective, observational, noninterventional analysis of 447 critically ill patients in three trials.
Disclosures: Immunexpress and the Australian Government funded the study. Fourteen authors disclose they are current or were former employees of Immunexpress and/or shareholders, and others disclosed receiving funding from the company.
Source: Miller III RR et al. Am J Respir Crit Care Med. 2018 Apr 6. doi: 10.1164/rccm.201712-2472OC.
Don’t delay hip-fracture surgery
Background: Guidelines from the American College of Surgeons and Canadian Institute for Health recommend hip fracture surgery within 48 hours. However, a time-to-surgery threshold after which mortality and complications are increased has not been determined. This study aims to determine a time to surgery threshold for hip-fracture surgery.
Study design: Retrospective cohort trial.
Setting: 72 hospitals in Ontario, Ca., during April 1, 2009-March 31, 2014.
Synopsis: Of the 42,230 adult patients in this study, 14,174 (33.6%) received hip-fracture surgery within 24 hours of emergency department arrival. A matched patient analysis of early surgery (within 24 hours of ED arrival) vs. delayed surgery determined that patients undergoing early operation experienced lower 30-day mortality (5.8% vs 6.5%) and fewer complications (myocardial infarction, deep vein thrombosis, pulmonary embolism, and pneumonia). Major bleeding was not assessed as a complication. Also omitted from analysis were patients undergoing nonoperative hip-fracture management.
These findings suggest a time to surgery of 24 hours may represent a threshold defining higher risk. Two-thirds of patients in this study surpassed this threshold. Hospitalists seeing patients with hip fracture should balance time delay risks with the need for medical optimization.
Bottom line: Wait time greater than 24 hours for adults undergoing hip fracture surgery is associated with an increased risk of 30-day mortality and complications.
Citation: Pincus D et al. Association between wait time and 30-day mortality in adults undergoing hip fracture surgery. JAMA. 2017 Nov 28;318(20):1994-2003.
Dr. Moulder is assistant professor, University of Virginia Health System.
Background: Guidelines from the American College of Surgeons and Canadian Institute for Health recommend hip fracture surgery within 48 hours. However, a time-to-surgery threshold after which mortality and complications are increased has not been determined. This study aims to determine a time to surgery threshold for hip-fracture surgery.
Study design: Retrospective cohort trial.
Setting: 72 hospitals in Ontario, Ca., during April 1, 2009-March 31, 2014.
Synopsis: Of the 42,230 adult patients in this study, 14,174 (33.6%) received hip-fracture surgery within 24 hours of emergency department arrival. A matched patient analysis of early surgery (within 24 hours of ED arrival) vs. delayed surgery determined that patients undergoing early operation experienced lower 30-day mortality (5.8% vs 6.5%) and fewer complications (myocardial infarction, deep vein thrombosis, pulmonary embolism, and pneumonia). Major bleeding was not assessed as a complication. Also omitted from analysis were patients undergoing nonoperative hip-fracture management.
These findings suggest a time to surgery of 24 hours may represent a threshold defining higher risk. Two-thirds of patients in this study surpassed this threshold. Hospitalists seeing patients with hip fracture should balance time delay risks with the need for medical optimization.
Bottom line: Wait time greater than 24 hours for adults undergoing hip fracture surgery is associated with an increased risk of 30-day mortality and complications.
Citation: Pincus D et al. Association between wait time and 30-day mortality in adults undergoing hip fracture surgery. JAMA. 2017 Nov 28;318(20):1994-2003.
Dr. Moulder is assistant professor, University of Virginia Health System.
Background: Guidelines from the American College of Surgeons and Canadian Institute for Health recommend hip fracture surgery within 48 hours. However, a time-to-surgery threshold after which mortality and complications are increased has not been determined. This study aims to determine a time to surgery threshold for hip-fracture surgery.
Study design: Retrospective cohort trial.
Setting: 72 hospitals in Ontario, Ca., during April 1, 2009-March 31, 2014.
Synopsis: Of the 42,230 adult patients in this study, 14,174 (33.6%) received hip-fracture surgery within 24 hours of emergency department arrival. A matched patient analysis of early surgery (within 24 hours of ED arrival) vs. delayed surgery determined that patients undergoing early operation experienced lower 30-day mortality (5.8% vs 6.5%) and fewer complications (myocardial infarction, deep vein thrombosis, pulmonary embolism, and pneumonia). Major bleeding was not assessed as a complication. Also omitted from analysis were patients undergoing nonoperative hip-fracture management.
These findings suggest a time to surgery of 24 hours may represent a threshold defining higher risk. Two-thirds of patients in this study surpassed this threshold. Hospitalists seeing patients with hip fracture should balance time delay risks with the need for medical optimization.
Bottom line: Wait time greater than 24 hours for adults undergoing hip fracture surgery is associated with an increased risk of 30-day mortality and complications.
Citation: Pincus D et al. Association between wait time and 30-day mortality in adults undergoing hip fracture surgery. JAMA. 2017 Nov 28;318(20):1994-2003.
Dr. Moulder is assistant professor, University of Virginia Health System.
Lower risk of ICH with dabigatran as compared to warfarin in atrial fibrillation
Clinical question: Is dabigatran superior to warfarin with regards to the risk of intracranial hemorrhage and myocardial infarction in patients with atrial fibrillation?
Background: Several studies – including the RELY trial – have revealed a lower rate of intracranial hemorrhage with dabigatran, compared with warfarin, in patients with atrial fibrillation; however, few of these have been on populations generalizable to clinical practice. Furthermore, there are conflicting data on the association of dabigatran with higher rates of myocardial infarction in patients with atrial fibrillation versus warfarin.
Study design: Retrospective cohort study.
Setting: The Sentinel Program (national surveillance system) including 17 collaborating institutions and health care delivery systems.
Synopsis: In 25,289 propensity score–matched pairs of commercially insured patients aged 21 years or older starting dabigatran or warfarin therapy for atrial fibrillation, dabigatran was associated with a lower rate of intracranial hemorrhage (hazard ratio, 0.89; 95% confidence interval, 0.72-1.09), similar rates of ischemic stroke and extracranial hemorrhage, and a potentially higher rate of myocardial infarction (HR, 1.88; 95% CI, 1.22-2.90). However, the association between dabigatran use and myocardial infarction was smaller and not statistically significant in sensitivity analyses. Also, subgroup analyses demonstrated higher rates of gastrointestinal bleeding with dabigatran than did warfarin in patients aged 75 years or older and those with kidney dysfunction.
Although providing further reassuring evidence about bleeding risks – particularly intracranial hemorrhage – associated with dabigatran use, this observational study fails to clarify the association between dabigatran and myocardial infarction.
Bottom line: Dabigatran, compared with warfarin, in adults with atrial fibrillation is associated with a lower risk of intracranial hemorrhage and similar risk of ischemic stroke and extracranial hemorrhage.
Citation: Go AS et al. Outcomes of dabigatran and warfarin for atrial fibrillation in contemporary practice. Ann Intern Med. 2017 Dec.19;167:845-54.
Dr. Mehta is assistant professor, division of hospital medicine, University of Virginia.
Clinical question: Is dabigatran superior to warfarin with regards to the risk of intracranial hemorrhage and myocardial infarction in patients with atrial fibrillation?
Background: Several studies – including the RELY trial – have revealed a lower rate of intracranial hemorrhage with dabigatran, compared with warfarin, in patients with atrial fibrillation; however, few of these have been on populations generalizable to clinical practice. Furthermore, there are conflicting data on the association of dabigatran with higher rates of myocardial infarction in patients with atrial fibrillation versus warfarin.
Study design: Retrospective cohort study.
Setting: The Sentinel Program (national surveillance system) including 17 collaborating institutions and health care delivery systems.
Synopsis: In 25,289 propensity score–matched pairs of commercially insured patients aged 21 years or older starting dabigatran or warfarin therapy for atrial fibrillation, dabigatran was associated with a lower rate of intracranial hemorrhage (hazard ratio, 0.89; 95% confidence interval, 0.72-1.09), similar rates of ischemic stroke and extracranial hemorrhage, and a potentially higher rate of myocardial infarction (HR, 1.88; 95% CI, 1.22-2.90). However, the association between dabigatran use and myocardial infarction was smaller and not statistically significant in sensitivity analyses. Also, subgroup analyses demonstrated higher rates of gastrointestinal bleeding with dabigatran than did warfarin in patients aged 75 years or older and those with kidney dysfunction.
Although providing further reassuring evidence about bleeding risks – particularly intracranial hemorrhage – associated with dabigatran use, this observational study fails to clarify the association between dabigatran and myocardial infarction.
Bottom line: Dabigatran, compared with warfarin, in adults with atrial fibrillation is associated with a lower risk of intracranial hemorrhage and similar risk of ischemic stroke and extracranial hemorrhage.
Citation: Go AS et al. Outcomes of dabigatran and warfarin for atrial fibrillation in contemporary practice. Ann Intern Med. 2017 Dec.19;167:845-54.
Dr. Mehta is assistant professor, division of hospital medicine, University of Virginia.
Clinical question: Is dabigatran superior to warfarin with regards to the risk of intracranial hemorrhage and myocardial infarction in patients with atrial fibrillation?
Background: Several studies – including the RELY trial – have revealed a lower rate of intracranial hemorrhage with dabigatran, compared with warfarin, in patients with atrial fibrillation; however, few of these have been on populations generalizable to clinical practice. Furthermore, there are conflicting data on the association of dabigatran with higher rates of myocardial infarction in patients with atrial fibrillation versus warfarin.
Study design: Retrospective cohort study.
Setting: The Sentinel Program (national surveillance system) including 17 collaborating institutions and health care delivery systems.
Synopsis: In 25,289 propensity score–matched pairs of commercially insured patients aged 21 years or older starting dabigatran or warfarin therapy for atrial fibrillation, dabigatran was associated with a lower rate of intracranial hemorrhage (hazard ratio, 0.89; 95% confidence interval, 0.72-1.09), similar rates of ischemic stroke and extracranial hemorrhage, and a potentially higher rate of myocardial infarction (HR, 1.88; 95% CI, 1.22-2.90). However, the association between dabigatran use and myocardial infarction was smaller and not statistically significant in sensitivity analyses. Also, subgroup analyses demonstrated higher rates of gastrointestinal bleeding with dabigatran than did warfarin in patients aged 75 years or older and those with kidney dysfunction.
Although providing further reassuring evidence about bleeding risks – particularly intracranial hemorrhage – associated with dabigatran use, this observational study fails to clarify the association between dabigatran and myocardial infarction.
Bottom line: Dabigatran, compared with warfarin, in adults with atrial fibrillation is associated with a lower risk of intracranial hemorrhage and similar risk of ischemic stroke and extracranial hemorrhage.
Citation: Go AS et al. Outcomes of dabigatran and warfarin for atrial fibrillation in contemporary practice. Ann Intern Med. 2017 Dec.19;167:845-54.
Dr. Mehta is assistant professor, division of hospital medicine, University of Virginia.

















