User login
Wave, surge, or tsunami
Different COVID-19 models and predicting inpatient bed capacity
The COVID-19 pandemic is one of the defining moments in history for this generation’s health care leaders. In 2019, most of us wrongly assumed that this virus would be similar to the past viral epidemics and pandemics such as 2002 severe acute respiratory syndrome–CoV in Asia, 2009 H1N1 influenza in the United States, 2012 Middle East respiratory syndrome–CoV in Saudi Arabia, and 2014-2016 Ebola in West Africa. Moreover, we understood that the 50% fatality rate of Ebola, a single-stranded RNA virus, was deadly on the continent of Africa, but its transmission was through direct contact with blood or other bodily fluids. Hence, the infectivity of Ebola to the general public was lower than SARS-CoV-2, which is spread by respiratory droplets and contact routes in addition to being the virus that causes COVID-19.1 Many of us did not expect that SARS-CoV-2, a single-stranded RNA virus consisting of 32 kilobytes, would reach the shores of the United States from the Hubei province of China, the northern Lombardy region of Italy, or other initial hotspots. We could not imagine its effects would be so devastating from an economic and medical perspective. Until it did.
The first reported case of SARS-CoV-2 was on Jan. 20, 2020 in Snohomish County, Wash., and the first known death from COVID-19 occurred on Feb. 6, 2020 in Santa Clara County, Calif.2,3 Since then, the United States has lost over 135,000 people from COVID-19 with death(s) reported in every state and the highest number of overall deaths of any country in the world.4 At the beginning of 2020, at our institution, Wake Forest Baptist Health System in Winston-Salem, N.C., we began preparing for the wave, surge, or tsunami of inpatients that was coming. Plans were afoot to increase our staff, even perhaps by hiring out-of-state physicians and nurses if needed, and every possible bed was considered within the system. It was not an if, but rather a when, as to the arrival of COVID-19.
Epidemiologists and biostatisticians developed predictive COVID-19 models so that health care leaders could plan accordingly, especially those patients that required critical care or inpatient medical care. These predictive models have been used across the globe and can be categorized into three groups: Susceptible-Exposed-Infectious-Recovered, Agent-Based, and Curve Fitting Extrapolation.5 Our original predictions were based on the Institute for Health Metrics and Evaluation model from Washington state (Curve Fitting Extrapolation). It creates projections from COVID-19 mortality data and assumes a 3% infection rate. Other health systems in our region used the COVID-19 Hospital Impact Model for Epidemics–University of Pennsylvania model. It pins its suppositions on hospitalized COVID-19 patients, regional infection rates, and hospital market shares. Lastly, the agent-based mode, such as the Global Epidemic and Mobility Project, takes simulated populations and forecasts the spread of SARS-CoV-2 anchoring on the interplay of individuals and groups. The assumptions are created secondary to the interactions of people, time, health care interventions, and public health policies.
Based on these predictive simulations, health systems have spent countless hours of planning and have utilized resources for the anticipated needs related to beds, ventilators, supplies, and staffing. Frontline staff were retrained how to don and doff personal protective equipment. Our teams were ready if we saw a wave of 250, a surge of 500, or a tsunami of 750 COVID-19 inpatients. We were prepared to run into the fire fully knowing the personal risks and consequences.
But, as yet, the tsunami in North Carolina has never come. On April 21, 2020, the COVID-19 mortality data in North Carolina peaked at 34 deaths, with the total number of deaths standing at 1,510 as of July 13, 2020.6 A surge did not hit our institutional shores at Wake Forest Baptist Health. As we looked through the proverbial back window and hear about the tsunami in Houston, Texas, we are very thankful that the tsunami turned out to be a small wave so far in North Carolina. We are grateful that there were fewer deaths than expected. The dust is settling now and the question, spoken or unspoken, is: “How could we be so wrong with our predictions?”
Models have strengths and weaknesses and none are perfect.7 There is an old aphorism in statistics that is often attributed to George Box that says: “All models are wrong but some are useful.”8 Predictions and projections are good, but not perfect. Our measurements and tests should not only be accurate, but also be as precise as possible.9 Moreover, the assumptions we make should be on solid ground. Since the beginning of the pandemic, there may have been undercounts and delays in reporting. The assumptions of the effects of social distancing may have been inaccurate. Just as important, the lack of early testing in our pandemic and the relatively limited testing currently available provide challenges not only in attributing past deaths to COVID-19, but also with planning and public health measures. To be fair, the tsunami that turned out to be a small wave in North Carolina may be caused by the strong leadership from politicians, public health officials, and health system leaders for their stay-at-home decree and vigorous public health measures in our state.
Some of the health systems in the United States have created “reemergence plans” to care for those patients who have stayed at home for the past several months. Elective surgeries and procedures have begun in different regions of the United States and will likely continue reopening into the late summer. Nevertheless, challenges and opportunities continue to abound during these difficult times of COVID-19. The tsunamis or surges will continue to occur in the United States and the premature reopening of some of the public places and businesses have not helped our collective efforts. In addition, the personal costs have been and will be immeasurable. Many of us have lost loved ones, been laid off, or face mental health crises because of the social isolation and false news.
COVID-19 is here to stay and will be with us for the foreseeable future. Health care providers have been literally risking their lives to serve the public and we will continue to do so. Hitting the target of needed inpatient beds and critical care beds is critically important and is tough without accurate data. We simply have inadequate and unreliable data of COVID-19 incidence and prevalence rates in the communities that we serve. More available testing would allow frontline health care providers and health care leaders to match hospital demand to supply, at individual hospitals and within the health care system. Moreover, contact tracing capabilities would give us the opportunity to isolate individuals and extinguish population-based hotspots.
We may have seen the first wave, but other waves of COVID-19 in North Carolina are sure to come. Since the partial reopening of North Carolina on May 8, 2020, coupled with pockets of nonadherence to social distancing and mask wearing, we expect a second wave sooner rather than later. Interestingly, daily new lab-confirmed COVID-19 cases in North Carolina have been on the rise, with the highest one-day total occurring on June 12, 2020 with 1,768 cases reported.6 As a result, North Carolina Gov. Roy Cooper and Secretary of the North Carolina Department of Health and Human Services, Dr. Mandy Cohen, placed a temporary pause on the Phase 2 reopening plan and mandated masks in public on June 24, 2020. It is unclear whether these intermittent daily spikes in lab-confirmed COVID-19 cases are a foreshadowing of our next wave, surge, or tsunami, or just an anomaly. Only time will tell, but as Jim Kim, MD, PhD, has stated so well, there is still time for social distancing, contact tracing, testing, isolation, and treatment.10 There is still time for us, for our loved ones, for our hospital systems, and for our public health system.
Dr. Huang is the executive medical director and service line director of general medicine and hospital medicine within the Wake Forest Baptist Health System and associate professor of internal medicine at Wake Forest School of Medicine. Dr. Lippert is assistant professor of internal medicine at Wake Forest School of Medicine. Mr. Payne is the associate vice president of Wake Forest Baptist Health. He is responsible for engineering, facilities planning & design as well as environmental health and safety departments. Dr. Pariyadath is comedical director of the Patient Flow Operations Center which facilitates patient placement throughout the Wake Forest Baptist Health system. He is also the associate medical director for the adult emergency department. Dr. Sunkara is assistant professor of internal medicine at Wake Forest School of Medicine. He is the medical director for hospital medicine units and the newly established PUI unit.
Acknowledgments
The authors would like to thank Julie Freischlag, MD; Kevin High, MD, MS; Gary Rosenthal, MD; Wayne Meredith, MD;Russ Howerton, MD; Mike Waid, Andrea Fernandez, MD; Brian Hiestand, MD; the Wake Forest Baptist Health System COVID-19 task force, the Operations Center, and the countless frontline staff at all five hospitals within the Wake Forest Baptist Health System.
References
1. World Health Organization. Modes of transmission of virus causing COVID-19: Implications for IPC precaution recommendations. 2020 June 30. https://www.who.int/news-room/commentaries/detail/modes-of-transmission-of-virus-causing-covid-19-implications-for-ipc-precaution-recommendations.
2. Holshue et al. First case of 2019 novel coronavirus in the United States. N Engl J Med. 2020;382: 929-36.
3. Fuller T, Baker M. Coronavirus death in California came weeks before first known U.S. death. New York Times. 2020 Apr 22. https://www.nytimes.com/2020/04/22/us/coronavirus-first-united-states-death.html.
4. Johns Hopkins Coronavirus Resource Center. https://coronavirus.jhu.edu/us-map. Accessed 2020 May 28.
5. Michaud J et al. COVID-19 models: Can they tell us what we want to know? 2020 April 16. https://www.kff.org/coronavirus-policy-watch/covid-19-models.
6. Centers for Disease Control and Prevention. https://www.cdc.gov/coronavirus/2019-ncov/cases-updates/cases-in-us.html. Accessed 2020 June 30.
7. Jewell N et al. Caution warranted: Using the Institute for Health Metrics and Evaluation Model for predicting the course of the COVID-19 pandemic. Ann Intern Med. 2020;173:1-3.
8. Box G. Science and statistics. J Am Stat Assoc. 1972;71:791-9.
9. Shapiro DE. The interpretation of diagnostic tests. Stat Methods Med Res. 1999;8:113-34.
10. Kim J. It is not too late to go on the offense against the coronavirus. The New Yorker. 2020 Apr 20. https://www.newyorker.com/science/medical-dispatch/its-not-too-late-to-go-on-offense-against-the-coronavirus.
Different COVID-19 models and predicting inpatient bed capacity
Different COVID-19 models and predicting inpatient bed capacity
The COVID-19 pandemic is one of the defining moments in history for this generation’s health care leaders. In 2019, most of us wrongly assumed that this virus would be similar to the past viral epidemics and pandemics such as 2002 severe acute respiratory syndrome–CoV in Asia, 2009 H1N1 influenza in the United States, 2012 Middle East respiratory syndrome–CoV in Saudi Arabia, and 2014-2016 Ebola in West Africa. Moreover, we understood that the 50% fatality rate of Ebola, a single-stranded RNA virus, was deadly on the continent of Africa, but its transmission was through direct contact with blood or other bodily fluids. Hence, the infectivity of Ebola to the general public was lower than SARS-CoV-2, which is spread by respiratory droplets and contact routes in addition to being the virus that causes COVID-19.1 Many of us did not expect that SARS-CoV-2, a single-stranded RNA virus consisting of 32 kilobytes, would reach the shores of the United States from the Hubei province of China, the northern Lombardy region of Italy, or other initial hotspots. We could not imagine its effects would be so devastating from an economic and medical perspective. Until it did.
The first reported case of SARS-CoV-2 was on Jan. 20, 2020 in Snohomish County, Wash., and the first known death from COVID-19 occurred on Feb. 6, 2020 in Santa Clara County, Calif.2,3 Since then, the United States has lost over 135,000 people from COVID-19 with death(s) reported in every state and the highest number of overall deaths of any country in the world.4 At the beginning of 2020, at our institution, Wake Forest Baptist Health System in Winston-Salem, N.C., we began preparing for the wave, surge, or tsunami of inpatients that was coming. Plans were afoot to increase our staff, even perhaps by hiring out-of-state physicians and nurses if needed, and every possible bed was considered within the system. It was not an if, but rather a when, as to the arrival of COVID-19.
Epidemiologists and biostatisticians developed predictive COVID-19 models so that health care leaders could plan accordingly, especially those patients that required critical care or inpatient medical care. These predictive models have been used across the globe and can be categorized into three groups: Susceptible-Exposed-Infectious-Recovered, Agent-Based, and Curve Fitting Extrapolation.5 Our original predictions were based on the Institute for Health Metrics and Evaluation model from Washington state (Curve Fitting Extrapolation). It creates projections from COVID-19 mortality data and assumes a 3% infection rate. Other health systems in our region used the COVID-19 Hospital Impact Model for Epidemics–University of Pennsylvania model. It pins its suppositions on hospitalized COVID-19 patients, regional infection rates, and hospital market shares. Lastly, the agent-based mode, such as the Global Epidemic and Mobility Project, takes simulated populations and forecasts the spread of SARS-CoV-2 anchoring on the interplay of individuals and groups. The assumptions are created secondary to the interactions of people, time, health care interventions, and public health policies.
Based on these predictive simulations, health systems have spent countless hours of planning and have utilized resources for the anticipated needs related to beds, ventilators, supplies, and staffing. Frontline staff were retrained how to don and doff personal protective equipment. Our teams were ready if we saw a wave of 250, a surge of 500, or a tsunami of 750 COVID-19 inpatients. We were prepared to run into the fire fully knowing the personal risks and consequences.
But, as yet, the tsunami in North Carolina has never come. On April 21, 2020, the COVID-19 mortality data in North Carolina peaked at 34 deaths, with the total number of deaths standing at 1,510 as of July 13, 2020.6 A surge did not hit our institutional shores at Wake Forest Baptist Health. As we looked through the proverbial back window and hear about the tsunami in Houston, Texas, we are very thankful that the tsunami turned out to be a small wave so far in North Carolina. We are grateful that there were fewer deaths than expected. The dust is settling now and the question, spoken or unspoken, is: “How could we be so wrong with our predictions?”
Models have strengths and weaknesses and none are perfect.7 There is an old aphorism in statistics that is often attributed to George Box that says: “All models are wrong but some are useful.”8 Predictions and projections are good, but not perfect. Our measurements and tests should not only be accurate, but also be as precise as possible.9 Moreover, the assumptions we make should be on solid ground. Since the beginning of the pandemic, there may have been undercounts and delays in reporting. The assumptions of the effects of social distancing may have been inaccurate. Just as important, the lack of early testing in our pandemic and the relatively limited testing currently available provide challenges not only in attributing past deaths to COVID-19, but also with planning and public health measures. To be fair, the tsunami that turned out to be a small wave in North Carolina may be caused by the strong leadership from politicians, public health officials, and health system leaders for their stay-at-home decree and vigorous public health measures in our state.
Some of the health systems in the United States have created “reemergence plans” to care for those patients who have stayed at home for the past several months. Elective surgeries and procedures have begun in different regions of the United States and will likely continue reopening into the late summer. Nevertheless, challenges and opportunities continue to abound during these difficult times of COVID-19. The tsunamis or surges will continue to occur in the United States and the premature reopening of some of the public places and businesses have not helped our collective efforts. In addition, the personal costs have been and will be immeasurable. Many of us have lost loved ones, been laid off, or face mental health crises because of the social isolation and false news.
COVID-19 is here to stay and will be with us for the foreseeable future. Health care providers have been literally risking their lives to serve the public and we will continue to do so. Hitting the target of needed inpatient beds and critical care beds is critically important and is tough without accurate data. We simply have inadequate and unreliable data of COVID-19 incidence and prevalence rates in the communities that we serve. More available testing would allow frontline health care providers and health care leaders to match hospital demand to supply, at individual hospitals and within the health care system. Moreover, contact tracing capabilities would give us the opportunity to isolate individuals and extinguish population-based hotspots.
We may have seen the first wave, but other waves of COVID-19 in North Carolina are sure to come. Since the partial reopening of North Carolina on May 8, 2020, coupled with pockets of nonadherence to social distancing and mask wearing, we expect a second wave sooner rather than later. Interestingly, daily new lab-confirmed COVID-19 cases in North Carolina have been on the rise, with the highest one-day total occurring on June 12, 2020 with 1,768 cases reported.6 As a result, North Carolina Gov. Roy Cooper and Secretary of the North Carolina Department of Health and Human Services, Dr. Mandy Cohen, placed a temporary pause on the Phase 2 reopening plan and mandated masks in public on June 24, 2020. It is unclear whether these intermittent daily spikes in lab-confirmed COVID-19 cases are a foreshadowing of our next wave, surge, or tsunami, or just an anomaly. Only time will tell, but as Jim Kim, MD, PhD, has stated so well, there is still time for social distancing, contact tracing, testing, isolation, and treatment.10 There is still time for us, for our loved ones, for our hospital systems, and for our public health system.
Dr. Huang is the executive medical director and service line director of general medicine and hospital medicine within the Wake Forest Baptist Health System and associate professor of internal medicine at Wake Forest School of Medicine. Dr. Lippert is assistant professor of internal medicine at Wake Forest School of Medicine. Mr. Payne is the associate vice president of Wake Forest Baptist Health. He is responsible for engineering, facilities planning & design as well as environmental health and safety departments. Dr. Pariyadath is comedical director of the Patient Flow Operations Center which facilitates patient placement throughout the Wake Forest Baptist Health system. He is also the associate medical director for the adult emergency department. Dr. Sunkara is assistant professor of internal medicine at Wake Forest School of Medicine. He is the medical director for hospital medicine units and the newly established PUI unit.
Acknowledgments
The authors would like to thank Julie Freischlag, MD; Kevin High, MD, MS; Gary Rosenthal, MD; Wayne Meredith, MD;Russ Howerton, MD; Mike Waid, Andrea Fernandez, MD; Brian Hiestand, MD; the Wake Forest Baptist Health System COVID-19 task force, the Operations Center, and the countless frontline staff at all five hospitals within the Wake Forest Baptist Health System.
References
1. World Health Organization. Modes of transmission of virus causing COVID-19: Implications for IPC precaution recommendations. 2020 June 30. https://www.who.int/news-room/commentaries/detail/modes-of-transmission-of-virus-causing-covid-19-implications-for-ipc-precaution-recommendations.
2. Holshue et al. First case of 2019 novel coronavirus in the United States. N Engl J Med. 2020;382: 929-36.
3. Fuller T, Baker M. Coronavirus death in California came weeks before first known U.S. death. New York Times. 2020 Apr 22. https://www.nytimes.com/2020/04/22/us/coronavirus-first-united-states-death.html.
4. Johns Hopkins Coronavirus Resource Center. https://coronavirus.jhu.edu/us-map. Accessed 2020 May 28.
5. Michaud J et al. COVID-19 models: Can they tell us what we want to know? 2020 April 16. https://www.kff.org/coronavirus-policy-watch/covid-19-models.
6. Centers for Disease Control and Prevention. https://www.cdc.gov/coronavirus/2019-ncov/cases-updates/cases-in-us.html. Accessed 2020 June 30.
7. Jewell N et al. Caution warranted: Using the Institute for Health Metrics and Evaluation Model for predicting the course of the COVID-19 pandemic. Ann Intern Med. 2020;173:1-3.
8. Box G. Science and statistics. J Am Stat Assoc. 1972;71:791-9.
9. Shapiro DE. The interpretation of diagnostic tests. Stat Methods Med Res. 1999;8:113-34.
10. Kim J. It is not too late to go on the offense against the coronavirus. The New Yorker. 2020 Apr 20. https://www.newyorker.com/science/medical-dispatch/its-not-too-late-to-go-on-offense-against-the-coronavirus.
The COVID-19 pandemic is one of the defining moments in history for this generation’s health care leaders. In 2019, most of us wrongly assumed that this virus would be similar to the past viral epidemics and pandemics such as 2002 severe acute respiratory syndrome–CoV in Asia, 2009 H1N1 influenza in the United States, 2012 Middle East respiratory syndrome–CoV in Saudi Arabia, and 2014-2016 Ebola in West Africa. Moreover, we understood that the 50% fatality rate of Ebola, a single-stranded RNA virus, was deadly on the continent of Africa, but its transmission was through direct contact with blood or other bodily fluids. Hence, the infectivity of Ebola to the general public was lower than SARS-CoV-2, which is spread by respiratory droplets and contact routes in addition to being the virus that causes COVID-19.1 Many of us did not expect that SARS-CoV-2, a single-stranded RNA virus consisting of 32 kilobytes, would reach the shores of the United States from the Hubei province of China, the northern Lombardy region of Italy, or other initial hotspots. We could not imagine its effects would be so devastating from an economic and medical perspective. Until it did.
The first reported case of SARS-CoV-2 was on Jan. 20, 2020 in Snohomish County, Wash., and the first known death from COVID-19 occurred on Feb. 6, 2020 in Santa Clara County, Calif.2,3 Since then, the United States has lost over 135,000 people from COVID-19 with death(s) reported in every state and the highest number of overall deaths of any country in the world.4 At the beginning of 2020, at our institution, Wake Forest Baptist Health System in Winston-Salem, N.C., we began preparing for the wave, surge, or tsunami of inpatients that was coming. Plans were afoot to increase our staff, even perhaps by hiring out-of-state physicians and nurses if needed, and every possible bed was considered within the system. It was not an if, but rather a when, as to the arrival of COVID-19.
Epidemiologists and biostatisticians developed predictive COVID-19 models so that health care leaders could plan accordingly, especially those patients that required critical care or inpatient medical care. These predictive models have been used across the globe and can be categorized into three groups: Susceptible-Exposed-Infectious-Recovered, Agent-Based, and Curve Fitting Extrapolation.5 Our original predictions were based on the Institute for Health Metrics and Evaluation model from Washington state (Curve Fitting Extrapolation). It creates projections from COVID-19 mortality data and assumes a 3% infection rate. Other health systems in our region used the COVID-19 Hospital Impact Model for Epidemics–University of Pennsylvania model. It pins its suppositions on hospitalized COVID-19 patients, regional infection rates, and hospital market shares. Lastly, the agent-based mode, such as the Global Epidemic and Mobility Project, takes simulated populations and forecasts the spread of SARS-CoV-2 anchoring on the interplay of individuals and groups. The assumptions are created secondary to the interactions of people, time, health care interventions, and public health policies.
Based on these predictive simulations, health systems have spent countless hours of planning and have utilized resources for the anticipated needs related to beds, ventilators, supplies, and staffing. Frontline staff were retrained how to don and doff personal protective equipment. Our teams were ready if we saw a wave of 250, a surge of 500, or a tsunami of 750 COVID-19 inpatients. We were prepared to run into the fire fully knowing the personal risks and consequences.
But, as yet, the tsunami in North Carolina has never come. On April 21, 2020, the COVID-19 mortality data in North Carolina peaked at 34 deaths, with the total number of deaths standing at 1,510 as of July 13, 2020.6 A surge did not hit our institutional shores at Wake Forest Baptist Health. As we looked through the proverbial back window and hear about the tsunami in Houston, Texas, we are very thankful that the tsunami turned out to be a small wave so far in North Carolina. We are grateful that there were fewer deaths than expected. The dust is settling now and the question, spoken or unspoken, is: “How could we be so wrong with our predictions?”
Models have strengths and weaknesses and none are perfect.7 There is an old aphorism in statistics that is often attributed to George Box that says: “All models are wrong but some are useful.”8 Predictions and projections are good, but not perfect. Our measurements and tests should not only be accurate, but also be as precise as possible.9 Moreover, the assumptions we make should be on solid ground. Since the beginning of the pandemic, there may have been undercounts and delays in reporting. The assumptions of the effects of social distancing may have been inaccurate. Just as important, the lack of early testing in our pandemic and the relatively limited testing currently available provide challenges not only in attributing past deaths to COVID-19, but also with planning and public health measures. To be fair, the tsunami that turned out to be a small wave in North Carolina may be caused by the strong leadership from politicians, public health officials, and health system leaders for their stay-at-home decree and vigorous public health measures in our state.
Some of the health systems in the United States have created “reemergence plans” to care for those patients who have stayed at home for the past several months. Elective surgeries and procedures have begun in different regions of the United States and will likely continue reopening into the late summer. Nevertheless, challenges and opportunities continue to abound during these difficult times of COVID-19. The tsunamis or surges will continue to occur in the United States and the premature reopening of some of the public places and businesses have not helped our collective efforts. In addition, the personal costs have been and will be immeasurable. Many of us have lost loved ones, been laid off, or face mental health crises because of the social isolation and false news.
COVID-19 is here to stay and will be with us for the foreseeable future. Health care providers have been literally risking their lives to serve the public and we will continue to do so. Hitting the target of needed inpatient beds and critical care beds is critically important and is tough without accurate data. We simply have inadequate and unreliable data of COVID-19 incidence and prevalence rates in the communities that we serve. More available testing would allow frontline health care providers and health care leaders to match hospital demand to supply, at individual hospitals and within the health care system. Moreover, contact tracing capabilities would give us the opportunity to isolate individuals and extinguish population-based hotspots.
We may have seen the first wave, but other waves of COVID-19 in North Carolina are sure to come. Since the partial reopening of North Carolina on May 8, 2020, coupled with pockets of nonadherence to social distancing and mask wearing, we expect a second wave sooner rather than later. Interestingly, daily new lab-confirmed COVID-19 cases in North Carolina have been on the rise, with the highest one-day total occurring on June 12, 2020 with 1,768 cases reported.6 As a result, North Carolina Gov. Roy Cooper and Secretary of the North Carolina Department of Health and Human Services, Dr. Mandy Cohen, placed a temporary pause on the Phase 2 reopening plan and mandated masks in public on June 24, 2020. It is unclear whether these intermittent daily spikes in lab-confirmed COVID-19 cases are a foreshadowing of our next wave, surge, or tsunami, or just an anomaly. Only time will tell, but as Jim Kim, MD, PhD, has stated so well, there is still time for social distancing, contact tracing, testing, isolation, and treatment.10 There is still time for us, for our loved ones, for our hospital systems, and for our public health system.
Dr. Huang is the executive medical director and service line director of general medicine and hospital medicine within the Wake Forest Baptist Health System and associate professor of internal medicine at Wake Forest School of Medicine. Dr. Lippert is assistant professor of internal medicine at Wake Forest School of Medicine. Mr. Payne is the associate vice president of Wake Forest Baptist Health. He is responsible for engineering, facilities planning & design as well as environmental health and safety departments. Dr. Pariyadath is comedical director of the Patient Flow Operations Center which facilitates patient placement throughout the Wake Forest Baptist Health system. He is also the associate medical director for the adult emergency department. Dr. Sunkara is assistant professor of internal medicine at Wake Forest School of Medicine. He is the medical director for hospital medicine units and the newly established PUI unit.
Acknowledgments
The authors would like to thank Julie Freischlag, MD; Kevin High, MD, MS; Gary Rosenthal, MD; Wayne Meredith, MD;Russ Howerton, MD; Mike Waid, Andrea Fernandez, MD; Brian Hiestand, MD; the Wake Forest Baptist Health System COVID-19 task force, the Operations Center, and the countless frontline staff at all five hospitals within the Wake Forest Baptist Health System.
References
1. World Health Organization. Modes of transmission of virus causing COVID-19: Implications for IPC precaution recommendations. 2020 June 30. https://www.who.int/news-room/commentaries/detail/modes-of-transmission-of-virus-causing-covid-19-implications-for-ipc-precaution-recommendations.
2. Holshue et al. First case of 2019 novel coronavirus in the United States. N Engl J Med. 2020;382: 929-36.
3. Fuller T, Baker M. Coronavirus death in California came weeks before first known U.S. death. New York Times. 2020 Apr 22. https://www.nytimes.com/2020/04/22/us/coronavirus-first-united-states-death.html.
4. Johns Hopkins Coronavirus Resource Center. https://coronavirus.jhu.edu/us-map. Accessed 2020 May 28.
5. Michaud J et al. COVID-19 models: Can they tell us what we want to know? 2020 April 16. https://www.kff.org/coronavirus-policy-watch/covid-19-models.
6. Centers for Disease Control and Prevention. https://www.cdc.gov/coronavirus/2019-ncov/cases-updates/cases-in-us.html. Accessed 2020 June 30.
7. Jewell N et al. Caution warranted: Using the Institute for Health Metrics and Evaluation Model for predicting the course of the COVID-19 pandemic. Ann Intern Med. 2020;173:1-3.
8. Box G. Science and statistics. J Am Stat Assoc. 1972;71:791-9.
9. Shapiro DE. The interpretation of diagnostic tests. Stat Methods Med Res. 1999;8:113-34.
10. Kim J. It is not too late to go on the offense against the coronavirus. The New Yorker. 2020 Apr 20. https://www.newyorker.com/science/medical-dispatch/its-not-too-late-to-go-on-offense-against-the-coronavirus.
Doing things right vs. doing the right things
A framework for a COVID-19 Person Under Investigation unit
The current coronavirus disease 2019 (COVID-19) pandemic shocked the world with its rapid spread despite stringent containment efforts, and it continues to wreak havoc. The surrounding uncertainty due to the novelty of this virus has prompted significant investigation to determine proper containment, treatment, and eradication efforts.1,2 In addition, health care facilities are facing surge capacity issues and a shortage of resources resulting in lower quality care for patients and putting health care workers (HCWs) at risk for infection.3,4
While there is a lot of emerging clinical and basic science research in this area, there has been inconsistent guidance in regard to the containment and prevention of spread in health care systems. An initiative to minimize HCW exposure risk and to provide the highest quality care to patients was implemented by the Section of Hospital Medicine at our large academic medical center. We used a hospital medicine medical-surgical unit and converted it into a Person Under Investigation (PUI) unit for patients suspected of COVID-19.
Unit goals
- Deliver dedicated, comprehensive, and high-quality care to our PUI patients suspected of COVID-19.
- Minimize cross contamination with healthy patients on other hospital units.
- Provide clear and direct communications with our HCWs.
- Educate HCWs on optimal donning and doffing techniques.
- Minimize our HCW exposure risk.
- Efficiently use our personal protective equipment (PPE) supply.
Unit and team characteristics
We used a preexisting 24-bed hospital medicine medical-surgical unit with a dyad rounding model of an attending physician and advanced practice provider (APP). Other team members include a designated care coordinator (social worker/case manager), pharmacist, respiratory therapist, physical/occupational therapist, speech language pathologist, unit medical director, and nurse manager. A daily multidisciplinary huddle with all the team members was held to discuss the care of the PUI patients.
Administrative leadership
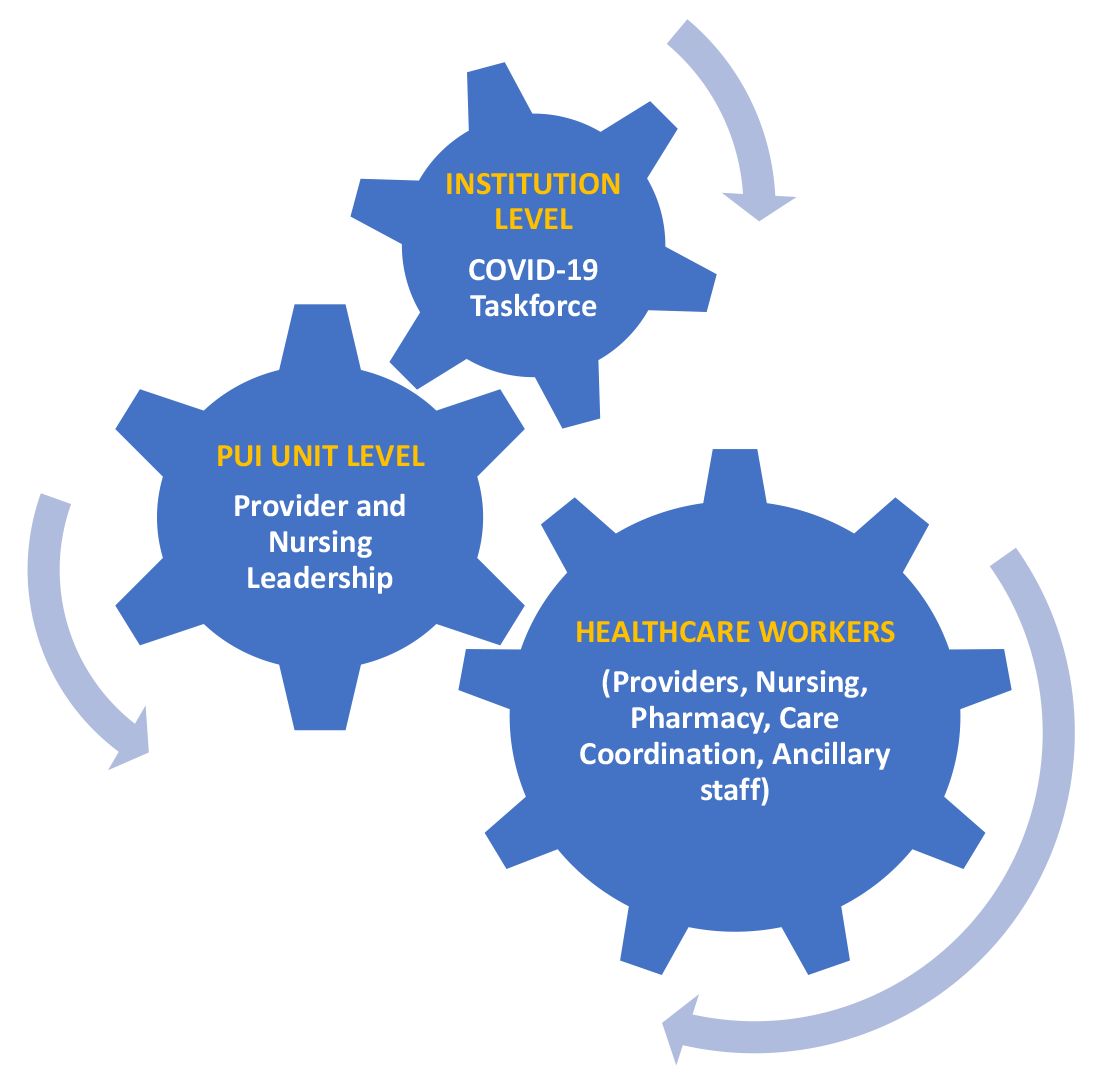
A COVID-19 task force composed of the medical director of clinical operations from the Section of Hospital Medicine, infectious disease, infection prevention, and several other important stakeholders conducted a daily conference call. This call allowed for the dissemination of information, including any treatment updates based on literature review or care processes. This information was then relayed to the HCWs following the meeting through the PUI unit medical director and nurse manager, who also facilitated feedback from the HCWs to the COVID-19 task force during the daily conference call. (See Figure 1.)
Patient flow
Hospital medicine was designated as the default service for all PUI patients suspected of COVID-19 and confirmed COVID-19 cases requiring hospitalization. These patients were admitted to this PUI unit directly from the emergency department (ED), or as transfers from outside institutions with assistance from our patient placement specialist team. Those patients admitted from our ED were tested for COVID-19 prior to arriving on the unit. Other suspected COVID-19 patients arriving as transfers from outside institutions were screened by the patient placement specialist team asking the following questions about the patient:
- “Has the patient had a fever or cough and been in contact with a laboratory-confirmed COVID-19 patient?”
- “Has the patient had a fever and cough?”
If the answer to either screening question was “yes,” then the patient was accepted to the PUI unit and tested upon arrival. Lastly, patients who were found to be COVID-19 positive at the outside institution, but who required transfer for other clinical reasons, were placed on this PUI unit as well.
Mechanisms to efficiently utilize PPE and mitigate HCW exposure risk
Our objectives are reducing the number of HCWs encountering PUI patients, reducing the number of encounters the HCWs have with PUI patients, and reducing the amount of time HCWs spent with PUI patients.
First, we maintained a log outside each patient’s room to track the details of staff encounters. Second, there was only one medical provider (either the attending physician or APP) assigned to each patient to limit personnel exposure. Third, we removed all learners (e.g. residents and students) from this unit. Fourth, we limited the number of entries into patient rooms to only critical staff directly involved in patient care (e.g. dietary and other ancillary staff were not allowed to enter the rooms) and provided updates to the patients by calling into the rooms. In addition, care coordination, pharmacy, and other staff members also utilized the same approach of calling into the room to speak with the patient regarding updates to minimize the duration of time spent in the room. Furthermore, our medical providers – with the help of the pharmacist and nursing – timed a patient’s medications to help reduce the number of entries into the room.
The medical providers also eliminated any unnecessary blood draws, imaging, and other procedures to minimize the number of encounters our HCWs had with the PUI. Lastly, the medical providers also avoided using any nebulizer treatments and noninvasive positive pressure ventilation to reduce any aerosol transmission of the virus. These measures not only helped to minimize our HCWs exposures, but also helped with the preservation of PPE.
Other efforts involved collaboration with infection prevention. They assisted with the training of our HCWs on proper PPE donning and doffing skills. This included watching a video and having an infection prevention specialist guide the HCWs throughout the entire process. We felt this was vital given the high amount of active failures with PPE use (up to 87%) reported in the literature.5 Furthermore, to ensure adequate mastery of these skills, infection prevention performed daily direct observation checks and provided real-time feedback to our HCWs.
Other things to consider for your PUI unit
There are several ideas that were not implemented in our PUI unit, but something to consider for your PUI unit, including:
- The use of elongated intravenous (IV) tubing, such that the IV poles and pumps were stationed outside the patient’s room, would be useful in reducing the amount of PPE required as well as HCW exposure to the patient.
- Having designated chest radiography, computed tomography, and magnetic resonance imaging scanners for these PUI patients to help minimize contamination with our non-PUI patients and to standardize the cleaning process.
- Supply our HCWs with designated scrubs at the beginning of their shifts, such that they can discard them at the end of their shifts for decontamination/sterilization purposes. This would help reduce HCWs fear of potentially exposing their families at home.
- Supply our HCWs with a designated place to stay, such as a hotel or other living quarters, to reduce HCWs fear of potentially exposing their families at home.
- Although we encouraged providers and staff to utilize designated phones to conduct patient history and review of systems information-gathering, to decrease the time spent in the room, the availability of more sophisticated audiovisual equipment could also improve the quality of the interview.
Conclusions
The increasing incidence in suspected COVID-19 patients has led to significant strain on health care systems of the world along with the associated economic and social crisis. Some health care facilities are facing surge capacity issues and inadequate resources, while others are facing a humanitarian crisis. Overall, we are all being affected by this pandemic, but are most concerned about its effects on our HCWs and our patients.
To address the concerns of low-quality care to our patients and anxiety levels among HCWs, we created this dedicated PUI unit in an effort to provide high-quality care for these suspected (and confirmed) COVID-19 patients and to maintain clear direct and constant communication with our HCWs.
Dr. Sunkara ([email protected]) is assistant professor of internal medicine at Wake Forest School of Medicine, Winston-Salem, N.C. He is the medical director for Hospital Medicine Units and the newly established PUI Unit, and is the corresponding author for this article. Dr. Lippert ([email protected]) is assistant professor of internal medicine at Wake Forest School of Medicine. Dr. Morris ([email protected]) is a PGY-3 internal medicine resident at Wake Forest School of Medicine. Dr. Huang ([email protected]) is associate professor of internal medicine at Wake Forest School of Medicine.
References
1. Food and Drug Administration. Recommendations for investigational COVID-19 convalescent plasma. 2020 Apr 8.
2. Fauci AS et al. Covid-19 – Navigating the uncharted. N Engl J Med. 2020 Feb 28. doi: 10.1056/NEJMe2002387. 3. Emanuel EJ et al. Fair allocation of scarce medical resources in the time of Covid-19. N Engl J Med. 2020 Mar 23. doi: 10.1056/NEJMsb2005114.
4. Li Ran et al. Risk factors of healthcare workers with corona virus disease 2019: A retrospective cohort study in a designated hospital of Wuhan in China. Clin Infect Dis. 2020 Mar 17. doi: 10.1093/cid/ciaa287.
5. Krein SL et al. Identification and characterization of failures in infectious agent transmission precaution practices in hospitals: A qualitative study. JAMA Intern Med. 2018;178(8):1016-57. doi: 10.1001/jamainternmed.2018.1898.
A framework for a COVID-19 Person Under Investigation unit
A framework for a COVID-19 Person Under Investigation unit
The current coronavirus disease 2019 (COVID-19) pandemic shocked the world with its rapid spread despite stringent containment efforts, and it continues to wreak havoc. The surrounding uncertainty due to the novelty of this virus has prompted significant investigation to determine proper containment, treatment, and eradication efforts.1,2 In addition, health care facilities are facing surge capacity issues and a shortage of resources resulting in lower quality care for patients and putting health care workers (HCWs) at risk for infection.3,4
While there is a lot of emerging clinical and basic science research in this area, there has been inconsistent guidance in regard to the containment and prevention of spread in health care systems. An initiative to minimize HCW exposure risk and to provide the highest quality care to patients was implemented by the Section of Hospital Medicine at our large academic medical center. We used a hospital medicine medical-surgical unit and converted it into a Person Under Investigation (PUI) unit for patients suspected of COVID-19.
Unit goals
- Deliver dedicated, comprehensive, and high-quality care to our PUI patients suspected of COVID-19.
- Minimize cross contamination with healthy patients on other hospital units.
- Provide clear and direct communications with our HCWs.
- Educate HCWs on optimal donning and doffing techniques.
- Minimize our HCW exposure risk.
- Efficiently use our personal protective equipment (PPE) supply.
Unit and team characteristics
We used a preexisting 24-bed hospital medicine medical-surgical unit with a dyad rounding model of an attending physician and advanced practice provider (APP). Other team members include a designated care coordinator (social worker/case manager), pharmacist, respiratory therapist, physical/occupational therapist, speech language pathologist, unit medical director, and nurse manager. A daily multidisciplinary huddle with all the team members was held to discuss the care of the PUI patients.
Administrative leadership
A COVID-19 task force composed of the medical director of clinical operations from the Section of Hospital Medicine, infectious disease, infection prevention, and several other important stakeholders conducted a daily conference call. This call allowed for the dissemination of information, including any treatment updates based on literature review or care processes. This information was then relayed to the HCWs following the meeting through the PUI unit medical director and nurse manager, who also facilitated feedback from the HCWs to the COVID-19 task force during the daily conference call. (See Figure 1.)

Patient flow
Hospital medicine was designated as the default service for all PUI patients suspected of COVID-19 and confirmed COVID-19 cases requiring hospitalization. These patients were admitted to this PUI unit directly from the emergency department (ED), or as transfers from outside institutions with assistance from our patient placement specialist team. Those patients admitted from our ED were tested for COVID-19 prior to arriving on the unit. Other suspected COVID-19 patients arriving as transfers from outside institutions were screened by the patient placement specialist team asking the following questions about the patient:
- “Has the patient had a fever or cough and been in contact with a laboratory-confirmed COVID-19 patient?”
- “Has the patient had a fever and cough?”
If the answer to either screening question was “yes,” then the patient was accepted to the PUI unit and tested upon arrival. Lastly, patients who were found to be COVID-19 positive at the outside institution, but who required transfer for other clinical reasons, were placed on this PUI unit as well.
Mechanisms to efficiently utilize PPE and mitigate HCW exposure risk
Our objectives are reducing the number of HCWs encountering PUI patients, reducing the number of encounters the HCWs have with PUI patients, and reducing the amount of time HCWs spent with PUI patients.
First, we maintained a log outside each patient’s room to track the details of staff encounters. Second, there was only one medical provider (either the attending physician or APP) assigned to each patient to limit personnel exposure. Third, we removed all learners (e.g. residents and students) from this unit. Fourth, we limited the number of entries into patient rooms to only critical staff directly involved in patient care (e.g. dietary and other ancillary staff were not allowed to enter the rooms) and provided updates to the patients by calling into the rooms. In addition, care coordination, pharmacy, and other staff members also utilized the same approach of calling into the room to speak with the patient regarding updates to minimize the duration of time spent in the room. Furthermore, our medical providers – with the help of the pharmacist and nursing – timed a patient’s medications to help reduce the number of entries into the room.
The medical providers also eliminated any unnecessary blood draws, imaging, and other procedures to minimize the number of encounters our HCWs had with the PUI. Lastly, the medical providers also avoided using any nebulizer treatments and noninvasive positive pressure ventilation to reduce any aerosol transmission of the virus. These measures not only helped to minimize our HCWs exposures, but also helped with the preservation of PPE.
Other efforts involved collaboration with infection prevention. They assisted with the training of our HCWs on proper PPE donning and doffing skills. This included watching a video and having an infection prevention specialist guide the HCWs throughout the entire process. We felt this was vital given the high amount of active failures with PPE use (up to 87%) reported in the literature.5 Furthermore, to ensure adequate mastery of these skills, infection prevention performed daily direct observation checks and provided real-time feedback to our HCWs.
Other things to consider for your PUI unit
There are several ideas that were not implemented in our PUI unit, but something to consider for your PUI unit, including:
- The use of elongated intravenous (IV) tubing, such that the IV poles and pumps were stationed outside the patient’s room, would be useful in reducing the amount of PPE required as well as HCW exposure to the patient.
- Having designated chest radiography, computed tomography, and magnetic resonance imaging scanners for these PUI patients to help minimize contamination with our non-PUI patients and to standardize the cleaning process.
- Supply our HCWs with designated scrubs at the beginning of their shifts, such that they can discard them at the end of their shifts for decontamination/sterilization purposes. This would help reduce HCWs fear of potentially exposing their families at home.
- Supply our HCWs with a designated place to stay, such as a hotel or other living quarters, to reduce HCWs fear of potentially exposing their families at home.
- Although we encouraged providers and staff to utilize designated phones to conduct patient history and review of systems information-gathering, to decrease the time spent in the room, the availability of more sophisticated audiovisual equipment could also improve the quality of the interview.
Conclusions
The increasing incidence in suspected COVID-19 patients has led to significant strain on health care systems of the world along with the associated economic and social crisis. Some health care facilities are facing surge capacity issues and inadequate resources, while others are facing a humanitarian crisis. Overall, we are all being affected by this pandemic, but are most concerned about its effects on our HCWs and our patients.
To address the concerns of low-quality care to our patients and anxiety levels among HCWs, we created this dedicated PUI unit in an effort to provide high-quality care for these suspected (and confirmed) COVID-19 patients and to maintain clear direct and constant communication with our HCWs.
Dr. Sunkara ([email protected]) is assistant professor of internal medicine at Wake Forest School of Medicine, Winston-Salem, N.C. He is the medical director for Hospital Medicine Units and the newly established PUI Unit, and is the corresponding author for this article. Dr. Lippert ([email protected]) is assistant professor of internal medicine at Wake Forest School of Medicine. Dr. Morris ([email protected]) is a PGY-3 internal medicine resident at Wake Forest School of Medicine. Dr. Huang ([email protected]) is associate professor of internal medicine at Wake Forest School of Medicine.
References
1. Food and Drug Administration. Recommendations for investigational COVID-19 convalescent plasma. 2020 Apr 8.
2. Fauci AS et al. Covid-19 – Navigating the uncharted. N Engl J Med. 2020 Feb 28. doi: 10.1056/NEJMe2002387. 3. Emanuel EJ et al. Fair allocation of scarce medical resources in the time of Covid-19. N Engl J Med. 2020 Mar 23. doi: 10.1056/NEJMsb2005114.
4. Li Ran et al. Risk factors of healthcare workers with corona virus disease 2019: A retrospective cohort study in a designated hospital of Wuhan in China. Clin Infect Dis. 2020 Mar 17. doi: 10.1093/cid/ciaa287.
5. Krein SL et al. Identification and characterization of failures in infectious agent transmission precaution practices in hospitals: A qualitative study. JAMA Intern Med. 2018;178(8):1016-57. doi: 10.1001/jamainternmed.2018.1898.
The current coronavirus disease 2019 (COVID-19) pandemic shocked the world with its rapid spread despite stringent containment efforts, and it continues to wreak havoc. The surrounding uncertainty due to the novelty of this virus has prompted significant investigation to determine proper containment, treatment, and eradication efforts.1,2 In addition, health care facilities are facing surge capacity issues and a shortage of resources resulting in lower quality care for patients and putting health care workers (HCWs) at risk for infection.3,4
While there is a lot of emerging clinical and basic science research in this area, there has been inconsistent guidance in regard to the containment and prevention of spread in health care systems. An initiative to minimize HCW exposure risk and to provide the highest quality care to patients was implemented by the Section of Hospital Medicine at our large academic medical center. We used a hospital medicine medical-surgical unit and converted it into a Person Under Investigation (PUI) unit for patients suspected of COVID-19.
Unit goals
- Deliver dedicated, comprehensive, and high-quality care to our PUI patients suspected of COVID-19.
- Minimize cross contamination with healthy patients on other hospital units.
- Provide clear and direct communications with our HCWs.
- Educate HCWs on optimal donning and doffing techniques.
- Minimize our HCW exposure risk.
- Efficiently use our personal protective equipment (PPE) supply.
Unit and team characteristics
We used a preexisting 24-bed hospital medicine medical-surgical unit with a dyad rounding model of an attending physician and advanced practice provider (APP). Other team members include a designated care coordinator (social worker/case manager), pharmacist, respiratory therapist, physical/occupational therapist, speech language pathologist, unit medical director, and nurse manager. A daily multidisciplinary huddle with all the team members was held to discuss the care of the PUI patients.
Administrative leadership
A COVID-19 task force composed of the medical director of clinical operations from the Section of Hospital Medicine, infectious disease, infection prevention, and several other important stakeholders conducted a daily conference call. This call allowed for the dissemination of information, including any treatment updates based on literature review or care processes. This information was then relayed to the HCWs following the meeting through the PUI unit medical director and nurse manager, who also facilitated feedback from the HCWs to the COVID-19 task force during the daily conference call. (See Figure 1.)

Patient flow
Hospital medicine was designated as the default service for all PUI patients suspected of COVID-19 and confirmed COVID-19 cases requiring hospitalization. These patients were admitted to this PUI unit directly from the emergency department (ED), or as transfers from outside institutions with assistance from our patient placement specialist team. Those patients admitted from our ED were tested for COVID-19 prior to arriving on the unit. Other suspected COVID-19 patients arriving as transfers from outside institutions were screened by the patient placement specialist team asking the following questions about the patient:
- “Has the patient had a fever or cough and been in contact with a laboratory-confirmed COVID-19 patient?”
- “Has the patient had a fever and cough?”
If the answer to either screening question was “yes,” then the patient was accepted to the PUI unit and tested upon arrival. Lastly, patients who were found to be COVID-19 positive at the outside institution, but who required transfer for other clinical reasons, were placed on this PUI unit as well.
Mechanisms to efficiently utilize PPE and mitigate HCW exposure risk
Our objectives are reducing the number of HCWs encountering PUI patients, reducing the number of encounters the HCWs have with PUI patients, and reducing the amount of time HCWs spent with PUI patients.
First, we maintained a log outside each patient’s room to track the details of staff encounters. Second, there was only one medical provider (either the attending physician or APP) assigned to each patient to limit personnel exposure. Third, we removed all learners (e.g. residents and students) from this unit. Fourth, we limited the number of entries into patient rooms to only critical staff directly involved in patient care (e.g. dietary and other ancillary staff were not allowed to enter the rooms) and provided updates to the patients by calling into the rooms. In addition, care coordination, pharmacy, and other staff members also utilized the same approach of calling into the room to speak with the patient regarding updates to minimize the duration of time spent in the room. Furthermore, our medical providers – with the help of the pharmacist and nursing – timed a patient’s medications to help reduce the number of entries into the room.
The medical providers also eliminated any unnecessary blood draws, imaging, and other procedures to minimize the number of encounters our HCWs had with the PUI. Lastly, the medical providers also avoided using any nebulizer treatments and noninvasive positive pressure ventilation to reduce any aerosol transmission of the virus. These measures not only helped to minimize our HCWs exposures, but also helped with the preservation of PPE.
Other efforts involved collaboration with infection prevention. They assisted with the training of our HCWs on proper PPE donning and doffing skills. This included watching a video and having an infection prevention specialist guide the HCWs throughout the entire process. We felt this was vital given the high amount of active failures with PPE use (up to 87%) reported in the literature.5 Furthermore, to ensure adequate mastery of these skills, infection prevention performed daily direct observation checks and provided real-time feedback to our HCWs.
Other things to consider for your PUI unit
There are several ideas that were not implemented in our PUI unit, but something to consider for your PUI unit, including:
- The use of elongated intravenous (IV) tubing, such that the IV poles and pumps were stationed outside the patient’s room, would be useful in reducing the amount of PPE required as well as HCW exposure to the patient.
- Having designated chest radiography, computed tomography, and magnetic resonance imaging scanners for these PUI patients to help minimize contamination with our non-PUI patients and to standardize the cleaning process.
- Supply our HCWs with designated scrubs at the beginning of their shifts, such that they can discard them at the end of their shifts for decontamination/sterilization purposes. This would help reduce HCWs fear of potentially exposing their families at home.
- Supply our HCWs with a designated place to stay, such as a hotel or other living quarters, to reduce HCWs fear of potentially exposing their families at home.
- Although we encouraged providers and staff to utilize designated phones to conduct patient history and review of systems information-gathering, to decrease the time spent in the room, the availability of more sophisticated audiovisual equipment could also improve the quality of the interview.
Conclusions
The increasing incidence in suspected COVID-19 patients has led to significant strain on health care systems of the world along with the associated economic and social crisis. Some health care facilities are facing surge capacity issues and inadequate resources, while others are facing a humanitarian crisis. Overall, we are all being affected by this pandemic, but are most concerned about its effects on our HCWs and our patients.
To address the concerns of low-quality care to our patients and anxiety levels among HCWs, we created this dedicated PUI unit in an effort to provide high-quality care for these suspected (and confirmed) COVID-19 patients and to maintain clear direct and constant communication with our HCWs.
Dr. Sunkara ([email protected]) is assistant professor of internal medicine at Wake Forest School of Medicine, Winston-Salem, N.C. He is the medical director for Hospital Medicine Units and the newly established PUI Unit, and is the corresponding author for this article. Dr. Lippert ([email protected]) is assistant professor of internal medicine at Wake Forest School of Medicine. Dr. Morris ([email protected]) is a PGY-3 internal medicine resident at Wake Forest School of Medicine. Dr. Huang ([email protected]) is associate professor of internal medicine at Wake Forest School of Medicine.
References
1. Food and Drug Administration. Recommendations for investigational COVID-19 convalescent plasma. 2020 Apr 8.
2. Fauci AS et al. Covid-19 – Navigating the uncharted. N Engl J Med. 2020 Feb 28. doi: 10.1056/NEJMe2002387. 3. Emanuel EJ et al. Fair allocation of scarce medical resources in the time of Covid-19. N Engl J Med. 2020 Mar 23. doi: 10.1056/NEJMsb2005114.
4. Li Ran et al. Risk factors of healthcare workers with corona virus disease 2019: A retrospective cohort study in a designated hospital of Wuhan in China. Clin Infect Dis. 2020 Mar 17. doi: 10.1093/cid/ciaa287.
5. Krein SL et al. Identification and characterization of failures in infectious agent transmission precaution practices in hospitals: A qualitative study. JAMA Intern Med. 2018;178(8):1016-57. doi: 10.1001/jamainternmed.2018.1898.
A woman, age 35, with new-onset ascites
A 35-year-old woman is admitted to the hospital with a 5-day history of abdominal distention and jaundice. She reports no history of fever, chills, night sweats, abdominal pain, nausea, vomiting, diarrhea, changes in urine color, change in stool color, weight loss, weight gain, or loss of appetite.
She is petite, with a body mass index of 19.4 kg/m2. She has no known history of medical conditions or surgery and is not taking any medications. Her family history is unremarkable, and she denies current or past tobacco, alcohol, or illicit drug use.
RECENT TRAVEL
She says that during a trip to Central America several months ago, she had suffered a seizure and was taken to a local hospital, where laboratory testing revealed elevated aspartate aminotransferase (AST) and alanine aminotransferase (ALT) levels. She says that the rest of the workup at that time was normal.
About 1 week after that incident, she returned home and saw her primary care physician, who ordered further testing, which showed mild hyperbilirubinemia and mild elevation of AST and ALT levels. Her physician attributed the elevations to atovaquone, which she had been taking for malaria prophylaxis, as repeat testing 2 weeks later showed improvement in AST and ALT levels.
The patient says she returned to her normal state of health until about 5 days ago, when she noticed jaundice and abdominal distention, but without abdominal pain, dark urine, or clay-colored stools. She became concerned and went to her local hospital. Testing there noted mild elevation of AST and ALT, as well as an elevated international normalized ratio (INR) and hyperbilirubinemia. Computed tomography of the abdomen and pelvis showed hepatomegaly with possible fatty liver. Because of these results, the patient was transferred to our institution for further evaluation.
EVALUATION AT OUR INSTITUTION
On examination at our institution, she is afebrile, and vital signs are within normal ranges. She has bilateral scleral icterus and diffuse jaundice, but no other skin finding such as rash or spider angioma. She has no lymphadenopathy. Her abdomen is distended, with tense ascites, and her liver is tender to palpation. The tip of the spleen is not palpable.
The cardiovascular examination reveals no murmurs, rubs, or gallops, but she has jugular venous distention and +2 pitting edema of both lower extremities.
On respiratory examination, there is dullness to percussion, with slight crackles on auscultation at the right lung base. The neurologic examination is normal.
Table 1 shows the results of initial laboratory testing.
1. Which study would provide the most information on the cause of ascites?
- Abdominal ultrasonography
- Abdominal paracentesis with ascitic fluid analysis
- Chest radiography
- Echocardiography
- Urine protein-to-creatinine ratio
Abdominal paracentesis with ascitic fluid analysis is the essential study for any patient with clinically apparent new-onset ascites.1–3 It is the study that provides the most information on the cause of ascites.
In our patient, abdominal paracentesis yields 1,000 mL of straw-colored ascitic fluid, and analysis shows 86 nucleated cells, 28 of which are polymorphonuclear cells, and 0 red blood cells, with negative Gram stain and culture. The ascitic albumin level is 0.85 g/dL, with an ascitic protein of 1.1 g/dL.
Abdominal ultrasonography shows a diffusely echogenic liver, no focal lesions, moderate ascites, normal portal vein flow, no intrahepatic or extrahepatic biliary duct dilation, normal kidney sizes, no hydronephrosis, and no intra-abdominal mass. Chest radiography is clear with no sign of consolidation, edema, or effusion. Echocardiography shows a normal left ventricular ejection fraction with no valvular disease or pericardial effusion. A random urine protein-creatinine ratio is normal at 0.1 (reference range < 0.2).
2. What is the most likely cause of her ascites based on the workup to this point?
- Cirrhosis
- Heart failure
- Nephrotic syndrome
- Portal vein thrombus
- Abdominal malignancy
- Malaria
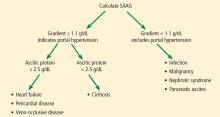
An initial approach to ascitic fluid analysis is to calculate the serum-ascites albumin gradient (SAAG). The SAAG is calculated as the serum albumin level minus the ascitic fluid albumin level.4,5 This is useful in determining the cause of the ascites (Figure 1).4,5 A gradient of 1.1 g/dL or higher indicates portal hypertension.4,5
Common causes of portal hypertension include cirrhosis, alcoholic hepatitis, heart failure, vascular occlusion syndromes (eg, Budd-Chiari syndrome, portal vein thrombosis), idiopathic portal fibrosis, and metastatic liver disease.5,6
If portal hypertension is present based on the SAAG, the next step is to review the ascitic protein level to help distinguish between a hepatic and a cardiac etiology of the ascites. An ascitic protein level less than 2.5 g/dL indicates a primary liver pathology (eg, cirrhosis). An ascitic protein level of 2.5 g/dL or greater typically indicates a cardiac condition (eg, heart failure, pericardial disease) with secondary congestive hepatopathy.5,6
If the SAAG is less than 1.1 g/dL, the ascites is likely not from portal hypertension. Typical causes of a low SAAG include infection, malignancy, pancreatic ascites, and nephrotic syndrome.5,6
In our patient, the SAAG is 1.35 g/dL (2.2 g/dL minus 0.85 g/dL), ie, elevated and due to portal hypertension. With an SAAG of 1.1 g/dL or greater and an ascitic fluid protein level less than 2.5 g/dL, as in our patient, the most likely cause is cirrhosis.
Heart failure is unlikely based on her normal brain natriuretic peptide level, an ascitic fluid protein level below 2.5 g/dL, and normal results on echocardiography. Nephrotic syndrome is also very unlikely based on the patient’s normal random urine protein-creatinine ratio. Portal vein thrombus and abdominal malignancy are essentially ruled out by the negative results of Doppler abdominal ultrasonography, with normal venous flow and no intra-abdominal mass and coupled with an elevated SAAG.
Although the patient has a history of travel, the incubation period for malaria would not fit the time frame of presentation. Also, she did not have typical malarial symptoms, her rapid malaria test was negative, and a peripheral blood smear for blood parasites was negative. It should be noted, however, that Plasmodium malariae infection classically presents with flulike symptoms and can resemble nephrotic syndrome, including peripheral edema, ascites, heavy proteinuria, hypoalbuminemia, and hyperlipidemia.7
3. In which patients is antibiotic prophylaxis against spontaneous bacterial peritonitis (SBP) appropriate?
- Any patient with cirrhosis
- Any patient with cirrhosis who is hospitalized
- Any patient with cirrhosis and an ascitic fluid protein level below 2.0 g/dL
- Any patient with cirrhosis and a history of SBP
Any patient with cirrhosis and a history of SBP should receive prophylactic antibiotics,8 as should any patient deemed at high risk of SBP. It is indicated in the following patients:
- Patients with cirrhosis and gastrointestinal bleeding9,10
- Patients with cirrhosis and a previous episode of SBP8
- Patients with cirrhosis and an ascitic fluid protein level less than 1.5 g/dL with either impaired renal function (creatinine ≥ 1.2 mg/dL, blood urea nitrogen level ≥ 25 mg/dL, or serum sodium ≤ 130 mmol/L) or liver failure (Child-Pugh score ≥ 9 and a bilirubin ≥ 3 mg/dL)9
- Patients with cirrhosis who are hospitalized for other reasons and have an ascitic protein level < 1.0 g/dL.9
Our patient has no signs or symptoms of gastrointestinal bleeding and no history of SBP. Her ascitic fluid protein level is 1.1 g/dL, and she has normal renal function. However, her Child-Pugh score is 12 (3 points for total bilirubin > 3 mg/dL, 3 points for serum albumin < 2.8 g/dL, 2 points for an INR 1.7 to 2.2, 3 points for moderate ascites, and 1 point for no encephalopathy), with a bilirubin of 17.0 mg/dL. Based on this, she is placed on antibiotic prophylaxis for SBP.
Our patient then undergoes an extensive workup for liver disease. Results of tests for toxins, autoimmune diseases, and inheritable diseases are all within normal limits. At this point, despite the patient’s reported negative alcohol history, our leading diagnosis is alcoholic hepatitis.
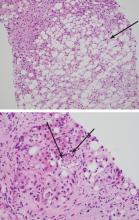
To confirm this diagnosis, she subsequently undergoes transjugular liver biopsy, considered the gold standard for the diagnosis of alcoholic hepatitis. During the procedure, the hepatic venous pressure gradient is measured at 18 mm Hg (reference range 1–5 mm Hg), suggestive of portal hypertension. The pathology study shows severe fatty change, active steatohepatitis with ballooning degeneration, easily identifiable Mallory-Denk bodies, and prominent neutrophilic infiltration, as well as extensive bridging fibrosis (Figure 2). These findings point to alcoholic hepatitis.
After the biopsy results, we speak with the patient further about her alcohol habits. At this point, she informs us that she has consumed significant amounts of alcohol since the age of 18 (6 to 12 alcoholic beverages per day, including beer and hard liquor). Therefore, based on this new information, on her jaundice and ascites, and on results of laboratory testing and biopsy, we confirmed our diagnosis of alcoholic hepatitis.
4. When is drug treatment appropriate for alcoholic hepatitis?
- Model for End-stage Liver Disease (MELD) score greater than 12
- MELD score greater than 15
- Maddrey Discriminant Function score greater than 25
- Maddrey Discriminant Function score greater than 32
- Glasgow score greater than 5
- Glasgow score greater than 7
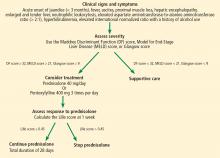
The best answer is a Maddrey Discriminant Function score greater than 32. A variety of scoring systems have been used to assess the severity of alcoholic hepatitis and to guide treatment, including the Maddrey Discriminant Function score, the MELD score, and the Glasgow score.11–16 They share similar laboratory values in their calculations, including prothrombin time (or INR) and total bilirubin.11–16 Typically, a Maddrey Discriminant Function score greater than 32, a Glasgow score of greater than 9, or a MELD score greater than 21 is used to determine whether pharmacologic treatment is indicated.11–16
The typical treatment is prednisolone or pentoxifylline.11,17–21 The Lille score is designed to help decide whether to stop corticosteroids after 1 week of administration due to lack of treatment response.22 It predicts mortality rates within 6 months; a score of 0.45 or less indicates a good prognosis, and corticosteroid therapy should continue for 28 days (Figure 3).22
Our patient’s discriminant function score is 50, her Glasgow score is 10, and her MELD score is 28; thus, she begins treatment with oral prednisolone. Her Lille score at 1 week is 0.119, indicating a good prognosis, and her corticosteroids are continued for a total of 28 days.
It should be highlighted that the most important treatment is abstinence from alcohol.11 Recent literature suggests that any benefit of prednisolone or pentoxifylline in terms of mortality rates is questionable,19–20 and there is evidence that giving both drugs simultaneously may improve mortality rates,11,21 but the evidence remains conflicting at this time.
ALCOHOLIC HEPATITIS
Alcoholic hepatitis is a clinical syndrome of jaundice and liver failure, often in the setting of heavy alcohol use for decades.11,12 The incidence is unknown, but the typical age of presentation is between 40 and 50.11,12 The chief sign is a rapid onset of jaundice (< 3 months); common signs and symptoms include fever, ascites, proximal muscle loss, and an enlarged, tender liver.12 Encephalopathy may be seen in severe alcoholic hepatitis.12
Our patient is 35 years old. She has jaundice with rapid onset, as well as ascites and a tender liver.
The diagnosis of alcoholic hepatitis must take into account the patient’s history, physical examination, and laboratory findings. Until proven otherwise, the diagnosis should be presumed in the following scenario: ascites and jaundice on examination (usually with a duration < 3 months); a history of heavy alcohol use; neutrophilic leukocytosis; an AST level that is elevated but below 300 U/L; an ALT level above the normal range but below 300 U/L; an AST-ALT ratio greater than 2; a total serum bilirubin level above 5 mg/dL; and an elevated INR.11,12 Liver biopsy is the gold standard for diagnosis. Though not routinely done because of risks associated with the procedure, it may help confirm the diagnosis if it is in question.
CASE CONCLUDED
We start our patient on oral prednisolone 40 mg daily for alcoholic hepatitis. Her symptoms and laboratory testing results including bilirubin improve. Her Lille score at 7 days indicates a good prognosis, prompting continuation of corticosteroid treatment for the full 28 days.
She is referred to an outpatient alcohol rehabilitation program and has remained sober as of the last outpatient note.
Alcoholic hepatitis is extremely difficult to diagnose, and no single blood test or imaging study confirms the diagnosis. The history, physical examination findings, and laboratory findings are crucial. If the diagnosis is still in doubt, liver biopsy may help confirm the diagnosis.
- Ruyon BA; AASLD Practice Guidelines Committee. Management of adult patients with ascites due to cirrhosis: an update. Hepatology 2009; 49(6):2087–2107. doi:10.1002/hep.22853
- Hoefs JC, Canawati HN, Sapico FL, Hopkins RR, Weiner J, Montgomerie JZ. Spontaneous bacterial peritonitis. Hepatology 1982; 2(4):399–407. pmid:7095741
- Ginès P, Cárdenas A, Arroyo V, Rodés J. Management of cirrhosis and ascites. N Engl J Med 2004; 350(16):1646–1654. doi:10.1056/NEJMra035021
- Runyon BA, Montano AA, Akriviadis EA, Antillon MR, Irving MA, McHutchison JG. The serum-ascites albumin gradient is superior to the exudate-transudate concept in the differential diagnosis of ascites. Ann Intern Med 1992; 117(3):215–220. pmid:1616215
- Hernaez R, Hamilton JP. Unexplained ascites. Clin Liver Dis 2016; 7(3):53–56. https://aasldpubs.onlinelibrary.wiley.com/doi/epdf/10.1002/cld.537
- Huang LL, Xia HH, Zhu SL. Ascitic fluid analysis in the differential diagnosis of ascites: focus on cirrhotic ascites. J Clin Transl Hepatol 2014; 2(1):58–64. doi:10.14218/JCTH.2013.00010
- Bartoloni A, Zammarchi L. Clinical aspects of uncomplicated and severe malaria. Mediterr J Hematol Infect Dis 2012; 4(1):e2012026. doi:10.4084/MJHID.2012.026
- Titó L, Rimola A, Ginès P, Llach J, Arroyo V, Rodés J. Recurrence of spontaneous bacterial peritonitis in cirrhosis: frequency and predictive factors. Hepatology 1988; 8(1):27–31. pmid:3257456
- Fernández J, Ruiz del Arbol L, Gómez C, et al. Norfloxacin vs ceftriaxone in the prophylaxis of infections in patients with advanced cirrhosis and hemorrhage. Gastroenterology 2006; 131(4):1049–1056. doi:10.1053/j.gastro.2006.07.010
- Runyon B; The American Association for the Study of Liver Diseases (AASLD). Management of adult patients with ascites due to cirrhosis: update 2012. https://www.aasld.org/sites/default/files/guideline_documents/141020_Guideline_Ascites_4UFb_2015.pdf. Accessed September 4, 2018.
- Sidhu SS, Goyal O, Kishore H, Sidhu S. New paradigms in management of alcoholic hepatitis: a review. Hepatol Int 2017; 11(3):255–267. doi:10.1007/s12072-017-9790-5
- Lucey MR, Mathurin P, Morgan TR. Alcoholic hepatitis. N Engl J Med 2009; 360(26):2758–2769. doi:10.1056/NEJMra0805786
- Maddrey WC, Boitnott JK, Bedine MS, Weber FL Jr, Mezey E, White RI Jr. Corticosteroid therapy of alcoholic hepatitis. Gastroenterology 1978; 75(2):193–199. pmid:352788
- Forrest EH, Evans CD, Stewart S, et al. Analysis of factors predictive of mortality in alcoholic hepatitis and derivation and validation of the Glasgow alcoholic hepatitis score. Gut 2005; 54(8):1174–1179. doi:10.1136/gut.2004.050781
- Dunn W, Jamil LH, Brown LS, et al. MELD accurately predicts mortality in patients with alcoholic hepatitis. Hepatology 2005; 41(2):353–358. doi:10.1002/hep.20503
- Sheth M, Riggs M, Patel T. Utility of the Mayo end-stage liver disease (MELD) score in assessing prognosis of patients with alcoholic hepatitis. BMC Gastroenterol 2002; 2:2. pmid:11835693
- Akriviadis E, Botla R, Briggs W, Han S, Reynolds T, Shakil O. Pentoxifylline improves short-term survival in severe acute alcoholic hepatitis: a double-blind, placebo-controlled trial. Gastroenterology 2000; 119(6):1637–1648. pmid:11113085
- Mathurin P, O’Grady J, Carithers RL, et al. Corticosteroids improve short-term survival in patients with severe alcoholic hepatitis: meta-analysis of individual patient data. Gut 2011; 60(2):255–260. doi:10.1136/gut.2010.224097
- Thursz MR, Richardson P, Allison M, et al; STOPAH Trial. Prednisolone or pentoxifylline for alcoholic hepatitis. N Engl J Med 2015; 372(17):1619–1628. doi:10.1056/NEJMoa1412278
- Thursz M, Forrest E, Roderick P, et al. The clinical effectiveness and cost-effectiveness of steroids or pentoxifylline for alcoholic hepatitis (STOPAH): a 2 × 2 factorial randomised controlled trial. Health Technol Assess 2015; 19(102):1–104. doi:10.3310/hta191020
- Lee YS, Kim HJ, Kim JH, et al. Treatment of severe alcoholic hepatitis with corticosteroid, pentoxifylline, or dual therapy: a systematic review and meta-analysis. J Clin Gastroenterol 2017; 51(4):364–377. doi:10.1097/MCG.0000000000000674
- Louvet A, Naveau S, Abdelnour M, et al. The Lille model: a new tool for therapeutic strategy in patients with severe alcoholic hepatitis treated with steroids. Hepatology 2007; 45(6):1348–1354. doi:10.1002/hep.21607
A 35-year-old woman is admitted to the hospital with a 5-day history of abdominal distention and jaundice. She reports no history of fever, chills, night sweats, abdominal pain, nausea, vomiting, diarrhea, changes in urine color, change in stool color, weight loss, weight gain, or loss of appetite.
She is petite, with a body mass index of 19.4 kg/m2. She has no known history of medical conditions or surgery and is not taking any medications. Her family history is unremarkable, and she denies current or past tobacco, alcohol, or illicit drug use.
RECENT TRAVEL
She says that during a trip to Central America several months ago, she had suffered a seizure and was taken to a local hospital, where laboratory testing revealed elevated aspartate aminotransferase (AST) and alanine aminotransferase (ALT) levels. She says that the rest of the workup at that time was normal.
About 1 week after that incident, she returned home and saw her primary care physician, who ordered further testing, which showed mild hyperbilirubinemia and mild elevation of AST and ALT levels. Her physician attributed the elevations to atovaquone, which she had been taking for malaria prophylaxis, as repeat testing 2 weeks later showed improvement in AST and ALT levels.
The patient says she returned to her normal state of health until about 5 days ago, when she noticed jaundice and abdominal distention, but without abdominal pain, dark urine, or clay-colored stools. She became concerned and went to her local hospital. Testing there noted mild elevation of AST and ALT, as well as an elevated international normalized ratio (INR) and hyperbilirubinemia. Computed tomography of the abdomen and pelvis showed hepatomegaly with possible fatty liver. Because of these results, the patient was transferred to our institution for further evaluation.
EVALUATION AT OUR INSTITUTION
On examination at our institution, she is afebrile, and vital signs are within normal ranges. She has bilateral scleral icterus and diffuse jaundice, but no other skin finding such as rash or spider angioma. She has no lymphadenopathy. Her abdomen is distended, with tense ascites, and her liver is tender to palpation. The tip of the spleen is not palpable.
The cardiovascular examination reveals no murmurs, rubs, or gallops, but she has jugular venous distention and +2 pitting edema of both lower extremities.
On respiratory examination, there is dullness to percussion, with slight crackles on auscultation at the right lung base. The neurologic examination is normal.
Table 1 shows the results of initial laboratory testing.
1. Which study would provide the most information on the cause of ascites?
- Abdominal ultrasonography
- Abdominal paracentesis with ascitic fluid analysis
- Chest radiography
- Echocardiography
- Urine protein-to-creatinine ratio
Abdominal paracentesis with ascitic fluid analysis is the essential study for any patient with clinically apparent new-onset ascites.1–3 It is the study that provides the most information on the cause of ascites.
In our patient, abdominal paracentesis yields 1,000 mL of straw-colored ascitic fluid, and analysis shows 86 nucleated cells, 28 of which are polymorphonuclear cells, and 0 red blood cells, with negative Gram stain and culture. The ascitic albumin level is 0.85 g/dL, with an ascitic protein of 1.1 g/dL.
Abdominal ultrasonography shows a diffusely echogenic liver, no focal lesions, moderate ascites, normal portal vein flow, no intrahepatic or extrahepatic biliary duct dilation, normal kidney sizes, no hydronephrosis, and no intra-abdominal mass. Chest radiography is clear with no sign of consolidation, edema, or effusion. Echocardiography shows a normal left ventricular ejection fraction with no valvular disease or pericardial effusion. A random urine protein-creatinine ratio is normal at 0.1 (reference range < 0.2).
2. What is the most likely cause of her ascites based on the workup to this point?
- Cirrhosis
- Heart failure
- Nephrotic syndrome
- Portal vein thrombus
- Abdominal malignancy
- Malaria
An initial approach to ascitic fluid analysis is to calculate the serum-ascites albumin gradient (SAAG). The SAAG is calculated as the serum albumin level minus the ascitic fluid albumin level.4,5 This is useful in determining the cause of the ascites (Figure 1).4,5 A gradient of 1.1 g/dL or higher indicates portal hypertension.4,5
Common causes of portal hypertension include cirrhosis, alcoholic hepatitis, heart failure, vascular occlusion syndromes (eg, Budd-Chiari syndrome, portal vein thrombosis), idiopathic portal fibrosis, and metastatic liver disease.5,6
If portal hypertension is present based on the SAAG, the next step is to review the ascitic protein level to help distinguish between a hepatic and a cardiac etiology of the ascites. An ascitic protein level less than 2.5 g/dL indicates a primary liver pathology (eg, cirrhosis). An ascitic protein level of 2.5 g/dL or greater typically indicates a cardiac condition (eg, heart failure, pericardial disease) with secondary congestive hepatopathy.5,6
If the SAAG is less than 1.1 g/dL, the ascites is likely not from portal hypertension. Typical causes of a low SAAG include infection, malignancy, pancreatic ascites, and nephrotic syndrome.5,6
In our patient, the SAAG is 1.35 g/dL (2.2 g/dL minus 0.85 g/dL), ie, elevated and due to portal hypertension. With an SAAG of 1.1 g/dL or greater and an ascitic fluid protein level less than 2.5 g/dL, as in our patient, the most likely cause is cirrhosis.
Heart failure is unlikely based on her normal brain natriuretic peptide level, an ascitic fluid protein level below 2.5 g/dL, and normal results on echocardiography. Nephrotic syndrome is also very unlikely based on the patient’s normal random urine protein-creatinine ratio. Portal vein thrombus and abdominal malignancy are essentially ruled out by the negative results of Doppler abdominal ultrasonography, with normal venous flow and no intra-abdominal mass and coupled with an elevated SAAG.
Although the patient has a history of travel, the incubation period for malaria would not fit the time frame of presentation. Also, she did not have typical malarial symptoms, her rapid malaria test was negative, and a peripheral blood smear for blood parasites was negative. It should be noted, however, that Plasmodium malariae infection classically presents with flulike symptoms and can resemble nephrotic syndrome, including peripheral edema, ascites, heavy proteinuria, hypoalbuminemia, and hyperlipidemia.7
3. In which patients is antibiotic prophylaxis against spontaneous bacterial peritonitis (SBP) appropriate?
- Any patient with cirrhosis
- Any patient with cirrhosis who is hospitalized
- Any patient with cirrhosis and an ascitic fluid protein level below 2.0 g/dL
- Any patient with cirrhosis and a history of SBP
Any patient with cirrhosis and a history of SBP should receive prophylactic antibiotics,8 as should any patient deemed at high risk of SBP. It is indicated in the following patients:
- Patients with cirrhosis and gastrointestinal bleeding9,10
- Patients with cirrhosis and a previous episode of SBP8
- Patients with cirrhosis and an ascitic fluid protein level less than 1.5 g/dL with either impaired renal function (creatinine ≥ 1.2 mg/dL, blood urea nitrogen level ≥ 25 mg/dL, or serum sodium ≤ 130 mmol/L) or liver failure (Child-Pugh score ≥ 9 and a bilirubin ≥ 3 mg/dL)9
- Patients with cirrhosis who are hospitalized for other reasons and have an ascitic protein level < 1.0 g/dL.9
Our patient has no signs or symptoms of gastrointestinal bleeding and no history of SBP. Her ascitic fluid protein level is 1.1 g/dL, and she has normal renal function. However, her Child-Pugh score is 12 (3 points for total bilirubin > 3 mg/dL, 3 points for serum albumin < 2.8 g/dL, 2 points for an INR 1.7 to 2.2, 3 points for moderate ascites, and 1 point for no encephalopathy), with a bilirubin of 17.0 mg/dL. Based on this, she is placed on antibiotic prophylaxis for SBP.
Our patient then undergoes an extensive workup for liver disease. Results of tests for toxins, autoimmune diseases, and inheritable diseases are all within normal limits. At this point, despite the patient’s reported negative alcohol history, our leading diagnosis is alcoholic hepatitis.
To confirm this diagnosis, she subsequently undergoes transjugular liver biopsy, considered the gold standard for the diagnosis of alcoholic hepatitis. During the procedure, the hepatic venous pressure gradient is measured at 18 mm Hg (reference range 1–5 mm Hg), suggestive of portal hypertension. The pathology study shows severe fatty change, active steatohepatitis with ballooning degeneration, easily identifiable Mallory-Denk bodies, and prominent neutrophilic infiltration, as well as extensive bridging fibrosis (Figure 2). These findings point to alcoholic hepatitis.
After the biopsy results, we speak with the patient further about her alcohol habits. At this point, she informs us that she has consumed significant amounts of alcohol since the age of 18 (6 to 12 alcoholic beverages per day, including beer and hard liquor). Therefore, based on this new information, on her jaundice and ascites, and on results of laboratory testing and biopsy, we confirmed our diagnosis of alcoholic hepatitis.
4. When is drug treatment appropriate for alcoholic hepatitis?
- Model for End-stage Liver Disease (MELD) score greater than 12
- MELD score greater than 15
- Maddrey Discriminant Function score greater than 25
- Maddrey Discriminant Function score greater than 32
- Glasgow score greater than 5
- Glasgow score greater than 7
The best answer is a Maddrey Discriminant Function score greater than 32. A variety of scoring systems have been used to assess the severity of alcoholic hepatitis and to guide treatment, including the Maddrey Discriminant Function score, the MELD score, and the Glasgow score.11–16 They share similar laboratory values in their calculations, including prothrombin time (or INR) and total bilirubin.11–16 Typically, a Maddrey Discriminant Function score greater than 32, a Glasgow score of greater than 9, or a MELD score greater than 21 is used to determine whether pharmacologic treatment is indicated.11–16
The typical treatment is prednisolone or pentoxifylline.11,17–21 The Lille score is designed to help decide whether to stop corticosteroids after 1 week of administration due to lack of treatment response.22 It predicts mortality rates within 6 months; a score of 0.45 or less indicates a good prognosis, and corticosteroid therapy should continue for 28 days (Figure 3).22
Our patient’s discriminant function score is 50, her Glasgow score is 10, and her MELD score is 28; thus, she begins treatment with oral prednisolone. Her Lille score at 1 week is 0.119, indicating a good prognosis, and her corticosteroids are continued for a total of 28 days.
It should be highlighted that the most important treatment is abstinence from alcohol.11 Recent literature suggests that any benefit of prednisolone or pentoxifylline in terms of mortality rates is questionable,19–20 and there is evidence that giving both drugs simultaneously may improve mortality rates,11,21 but the evidence remains conflicting at this time.
ALCOHOLIC HEPATITIS
Alcoholic hepatitis is a clinical syndrome of jaundice and liver failure, often in the setting of heavy alcohol use for decades.11,12 The incidence is unknown, but the typical age of presentation is between 40 and 50.11,12 The chief sign is a rapid onset of jaundice (< 3 months); common signs and symptoms include fever, ascites, proximal muscle loss, and an enlarged, tender liver.12 Encephalopathy may be seen in severe alcoholic hepatitis.12
Our patient is 35 years old. She has jaundice with rapid onset, as well as ascites and a tender liver.
The diagnosis of alcoholic hepatitis must take into account the patient’s history, physical examination, and laboratory findings. Until proven otherwise, the diagnosis should be presumed in the following scenario: ascites and jaundice on examination (usually with a duration < 3 months); a history of heavy alcohol use; neutrophilic leukocytosis; an AST level that is elevated but below 300 U/L; an ALT level above the normal range but below 300 U/L; an AST-ALT ratio greater than 2; a total serum bilirubin level above 5 mg/dL; and an elevated INR.11,12 Liver biopsy is the gold standard for diagnosis. Though not routinely done because of risks associated with the procedure, it may help confirm the diagnosis if it is in question.
CASE CONCLUDED
We start our patient on oral prednisolone 40 mg daily for alcoholic hepatitis. Her symptoms and laboratory testing results including bilirubin improve. Her Lille score at 7 days indicates a good prognosis, prompting continuation of corticosteroid treatment for the full 28 days.
She is referred to an outpatient alcohol rehabilitation program and has remained sober as of the last outpatient note.
Alcoholic hepatitis is extremely difficult to diagnose, and no single blood test or imaging study confirms the diagnosis. The history, physical examination findings, and laboratory findings are crucial. If the diagnosis is still in doubt, liver biopsy may help confirm the diagnosis.
A 35-year-old woman is admitted to the hospital with a 5-day history of abdominal distention and jaundice. She reports no history of fever, chills, night sweats, abdominal pain, nausea, vomiting, diarrhea, changes in urine color, change in stool color, weight loss, weight gain, or loss of appetite.
She is petite, with a body mass index of 19.4 kg/m2. She has no known history of medical conditions or surgery and is not taking any medications. Her family history is unremarkable, and she denies current or past tobacco, alcohol, or illicit drug use.
RECENT TRAVEL
She says that during a trip to Central America several months ago, she had suffered a seizure and was taken to a local hospital, where laboratory testing revealed elevated aspartate aminotransferase (AST) and alanine aminotransferase (ALT) levels. She says that the rest of the workup at that time was normal.
About 1 week after that incident, she returned home and saw her primary care physician, who ordered further testing, which showed mild hyperbilirubinemia and mild elevation of AST and ALT levels. Her physician attributed the elevations to atovaquone, which she had been taking for malaria prophylaxis, as repeat testing 2 weeks later showed improvement in AST and ALT levels.
The patient says she returned to her normal state of health until about 5 days ago, when she noticed jaundice and abdominal distention, but without abdominal pain, dark urine, or clay-colored stools. She became concerned and went to her local hospital. Testing there noted mild elevation of AST and ALT, as well as an elevated international normalized ratio (INR) and hyperbilirubinemia. Computed tomography of the abdomen and pelvis showed hepatomegaly with possible fatty liver. Because of these results, the patient was transferred to our institution for further evaluation.
EVALUATION AT OUR INSTITUTION
On examination at our institution, she is afebrile, and vital signs are within normal ranges. She has bilateral scleral icterus and diffuse jaundice, but no other skin finding such as rash or spider angioma. She has no lymphadenopathy. Her abdomen is distended, with tense ascites, and her liver is tender to palpation. The tip of the spleen is not palpable.
The cardiovascular examination reveals no murmurs, rubs, or gallops, but she has jugular venous distention and +2 pitting edema of both lower extremities.
On respiratory examination, there is dullness to percussion, with slight crackles on auscultation at the right lung base. The neurologic examination is normal.
Table 1 shows the results of initial laboratory testing.
1. Which study would provide the most information on the cause of ascites?
- Abdominal ultrasonography
- Abdominal paracentesis with ascitic fluid analysis
- Chest radiography
- Echocardiography
- Urine protein-to-creatinine ratio
Abdominal paracentesis with ascitic fluid analysis is the essential study for any patient with clinically apparent new-onset ascites.1–3 It is the study that provides the most information on the cause of ascites.
In our patient, abdominal paracentesis yields 1,000 mL of straw-colored ascitic fluid, and analysis shows 86 nucleated cells, 28 of which are polymorphonuclear cells, and 0 red blood cells, with negative Gram stain and culture. The ascitic albumin level is 0.85 g/dL, with an ascitic protein of 1.1 g/dL.
Abdominal ultrasonography shows a diffusely echogenic liver, no focal lesions, moderate ascites, normal portal vein flow, no intrahepatic or extrahepatic biliary duct dilation, normal kidney sizes, no hydronephrosis, and no intra-abdominal mass. Chest radiography is clear with no sign of consolidation, edema, or effusion. Echocardiography shows a normal left ventricular ejection fraction with no valvular disease or pericardial effusion. A random urine protein-creatinine ratio is normal at 0.1 (reference range < 0.2).
2. What is the most likely cause of her ascites based on the workup to this point?
- Cirrhosis
- Heart failure
- Nephrotic syndrome
- Portal vein thrombus
- Abdominal malignancy
- Malaria
An initial approach to ascitic fluid analysis is to calculate the serum-ascites albumin gradient (SAAG). The SAAG is calculated as the serum albumin level minus the ascitic fluid albumin level.4,5 This is useful in determining the cause of the ascites (Figure 1).4,5 A gradient of 1.1 g/dL or higher indicates portal hypertension.4,5
Common causes of portal hypertension include cirrhosis, alcoholic hepatitis, heart failure, vascular occlusion syndromes (eg, Budd-Chiari syndrome, portal vein thrombosis), idiopathic portal fibrosis, and metastatic liver disease.5,6
If portal hypertension is present based on the SAAG, the next step is to review the ascitic protein level to help distinguish between a hepatic and a cardiac etiology of the ascites. An ascitic protein level less than 2.5 g/dL indicates a primary liver pathology (eg, cirrhosis). An ascitic protein level of 2.5 g/dL or greater typically indicates a cardiac condition (eg, heart failure, pericardial disease) with secondary congestive hepatopathy.5,6
If the SAAG is less than 1.1 g/dL, the ascites is likely not from portal hypertension. Typical causes of a low SAAG include infection, malignancy, pancreatic ascites, and nephrotic syndrome.5,6
In our patient, the SAAG is 1.35 g/dL (2.2 g/dL minus 0.85 g/dL), ie, elevated and due to portal hypertension. With an SAAG of 1.1 g/dL or greater and an ascitic fluid protein level less than 2.5 g/dL, as in our patient, the most likely cause is cirrhosis.
Heart failure is unlikely based on her normal brain natriuretic peptide level, an ascitic fluid protein level below 2.5 g/dL, and normal results on echocardiography. Nephrotic syndrome is also very unlikely based on the patient’s normal random urine protein-creatinine ratio. Portal vein thrombus and abdominal malignancy are essentially ruled out by the negative results of Doppler abdominal ultrasonography, with normal venous flow and no intra-abdominal mass and coupled with an elevated SAAG.
Although the patient has a history of travel, the incubation period for malaria would not fit the time frame of presentation. Also, she did not have typical malarial symptoms, her rapid malaria test was negative, and a peripheral blood smear for blood parasites was negative. It should be noted, however, that Plasmodium malariae infection classically presents with flulike symptoms and can resemble nephrotic syndrome, including peripheral edema, ascites, heavy proteinuria, hypoalbuminemia, and hyperlipidemia.7
3. In which patients is antibiotic prophylaxis against spontaneous bacterial peritonitis (SBP) appropriate?
- Any patient with cirrhosis
- Any patient with cirrhosis who is hospitalized
- Any patient with cirrhosis and an ascitic fluid protein level below 2.0 g/dL
- Any patient with cirrhosis and a history of SBP
Any patient with cirrhosis and a history of SBP should receive prophylactic antibiotics,8 as should any patient deemed at high risk of SBP. It is indicated in the following patients:
- Patients with cirrhosis and gastrointestinal bleeding9,10
- Patients with cirrhosis and a previous episode of SBP8
- Patients with cirrhosis and an ascitic fluid protein level less than 1.5 g/dL with either impaired renal function (creatinine ≥ 1.2 mg/dL, blood urea nitrogen level ≥ 25 mg/dL, or serum sodium ≤ 130 mmol/L) or liver failure (Child-Pugh score ≥ 9 and a bilirubin ≥ 3 mg/dL)9
- Patients with cirrhosis who are hospitalized for other reasons and have an ascitic protein level < 1.0 g/dL.9
Our patient has no signs or symptoms of gastrointestinal bleeding and no history of SBP. Her ascitic fluid protein level is 1.1 g/dL, and she has normal renal function. However, her Child-Pugh score is 12 (3 points for total bilirubin > 3 mg/dL, 3 points for serum albumin < 2.8 g/dL, 2 points for an INR 1.7 to 2.2, 3 points for moderate ascites, and 1 point for no encephalopathy), with a bilirubin of 17.0 mg/dL. Based on this, she is placed on antibiotic prophylaxis for SBP.
Our patient then undergoes an extensive workup for liver disease. Results of tests for toxins, autoimmune diseases, and inheritable diseases are all within normal limits. At this point, despite the patient’s reported negative alcohol history, our leading diagnosis is alcoholic hepatitis.
To confirm this diagnosis, she subsequently undergoes transjugular liver biopsy, considered the gold standard for the diagnosis of alcoholic hepatitis. During the procedure, the hepatic venous pressure gradient is measured at 18 mm Hg (reference range 1–5 mm Hg), suggestive of portal hypertension. The pathology study shows severe fatty change, active steatohepatitis with ballooning degeneration, easily identifiable Mallory-Denk bodies, and prominent neutrophilic infiltration, as well as extensive bridging fibrosis (Figure 2). These findings point to alcoholic hepatitis.
After the biopsy results, we speak with the patient further about her alcohol habits. At this point, she informs us that she has consumed significant amounts of alcohol since the age of 18 (6 to 12 alcoholic beverages per day, including beer and hard liquor). Therefore, based on this new information, on her jaundice and ascites, and on results of laboratory testing and biopsy, we confirmed our diagnosis of alcoholic hepatitis.
4. When is drug treatment appropriate for alcoholic hepatitis?
- Model for End-stage Liver Disease (MELD) score greater than 12
- MELD score greater than 15
- Maddrey Discriminant Function score greater than 25
- Maddrey Discriminant Function score greater than 32
- Glasgow score greater than 5
- Glasgow score greater than 7
The best answer is a Maddrey Discriminant Function score greater than 32. A variety of scoring systems have been used to assess the severity of alcoholic hepatitis and to guide treatment, including the Maddrey Discriminant Function score, the MELD score, and the Glasgow score.11–16 They share similar laboratory values in their calculations, including prothrombin time (or INR) and total bilirubin.11–16 Typically, a Maddrey Discriminant Function score greater than 32, a Glasgow score of greater than 9, or a MELD score greater than 21 is used to determine whether pharmacologic treatment is indicated.11–16
The typical treatment is prednisolone or pentoxifylline.11,17–21 The Lille score is designed to help decide whether to stop corticosteroids after 1 week of administration due to lack of treatment response.22 It predicts mortality rates within 6 months; a score of 0.45 or less indicates a good prognosis, and corticosteroid therapy should continue for 28 days (Figure 3).22
Our patient’s discriminant function score is 50, her Glasgow score is 10, and her MELD score is 28; thus, she begins treatment with oral prednisolone. Her Lille score at 1 week is 0.119, indicating a good prognosis, and her corticosteroids are continued for a total of 28 days.
It should be highlighted that the most important treatment is abstinence from alcohol.11 Recent literature suggests that any benefit of prednisolone or pentoxifylline in terms of mortality rates is questionable,19–20 and there is evidence that giving both drugs simultaneously may improve mortality rates,11,21 but the evidence remains conflicting at this time.
ALCOHOLIC HEPATITIS
Alcoholic hepatitis is a clinical syndrome of jaundice and liver failure, often in the setting of heavy alcohol use for decades.11,12 The incidence is unknown, but the typical age of presentation is between 40 and 50.11,12 The chief sign is a rapid onset of jaundice (< 3 months); common signs and symptoms include fever, ascites, proximal muscle loss, and an enlarged, tender liver.12 Encephalopathy may be seen in severe alcoholic hepatitis.12
Our patient is 35 years old. She has jaundice with rapid onset, as well as ascites and a tender liver.
The diagnosis of alcoholic hepatitis must take into account the patient’s history, physical examination, and laboratory findings. Until proven otherwise, the diagnosis should be presumed in the following scenario: ascites and jaundice on examination (usually with a duration < 3 months); a history of heavy alcohol use; neutrophilic leukocytosis; an AST level that is elevated but below 300 U/L; an ALT level above the normal range but below 300 U/L; an AST-ALT ratio greater than 2; a total serum bilirubin level above 5 mg/dL; and an elevated INR.11,12 Liver biopsy is the gold standard for diagnosis. Though not routinely done because of risks associated with the procedure, it may help confirm the diagnosis if it is in question.
CASE CONCLUDED
We start our patient on oral prednisolone 40 mg daily for alcoholic hepatitis. Her symptoms and laboratory testing results including bilirubin improve. Her Lille score at 7 days indicates a good prognosis, prompting continuation of corticosteroid treatment for the full 28 days.
She is referred to an outpatient alcohol rehabilitation program and has remained sober as of the last outpatient note.
Alcoholic hepatitis is extremely difficult to diagnose, and no single blood test or imaging study confirms the diagnosis. The history, physical examination findings, and laboratory findings are crucial. If the diagnosis is still in doubt, liver biopsy may help confirm the diagnosis.
- Ruyon BA; AASLD Practice Guidelines Committee. Management of adult patients with ascites due to cirrhosis: an update. Hepatology 2009; 49(6):2087–2107. doi:10.1002/hep.22853
- Hoefs JC, Canawati HN, Sapico FL, Hopkins RR, Weiner J, Montgomerie JZ. Spontaneous bacterial peritonitis. Hepatology 1982; 2(4):399–407. pmid:7095741
- Ginès P, Cárdenas A, Arroyo V, Rodés J. Management of cirrhosis and ascites. N Engl J Med 2004; 350(16):1646–1654. doi:10.1056/NEJMra035021
- Runyon BA, Montano AA, Akriviadis EA, Antillon MR, Irving MA, McHutchison JG. The serum-ascites albumin gradient is superior to the exudate-transudate concept in the differential diagnosis of ascites. Ann Intern Med 1992; 117(3):215–220. pmid:1616215
- Hernaez R, Hamilton JP. Unexplained ascites. Clin Liver Dis 2016; 7(3):53–56. https://aasldpubs.onlinelibrary.wiley.com/doi/epdf/10.1002/cld.537
- Huang LL, Xia HH, Zhu SL. Ascitic fluid analysis in the differential diagnosis of ascites: focus on cirrhotic ascites. J Clin Transl Hepatol 2014; 2(1):58–64. doi:10.14218/JCTH.2013.00010
- Bartoloni A, Zammarchi L. Clinical aspects of uncomplicated and severe malaria. Mediterr J Hematol Infect Dis 2012; 4(1):e2012026. doi:10.4084/MJHID.2012.026
- Titó L, Rimola A, Ginès P, Llach J, Arroyo V, Rodés J. Recurrence of spontaneous bacterial peritonitis in cirrhosis: frequency and predictive factors. Hepatology 1988; 8(1):27–31. pmid:3257456
- Fernández J, Ruiz del Arbol L, Gómez C, et al. Norfloxacin vs ceftriaxone in the prophylaxis of infections in patients with advanced cirrhosis and hemorrhage. Gastroenterology 2006; 131(4):1049–1056. doi:10.1053/j.gastro.2006.07.010
- Runyon B; The American Association for the Study of Liver Diseases (AASLD). Management of adult patients with ascites due to cirrhosis: update 2012. https://www.aasld.org/sites/default/files/guideline_documents/141020_Guideline_Ascites_4UFb_2015.pdf. Accessed September 4, 2018.
- Sidhu SS, Goyal O, Kishore H, Sidhu S. New paradigms in management of alcoholic hepatitis: a review. Hepatol Int 2017; 11(3):255–267. doi:10.1007/s12072-017-9790-5
- Lucey MR, Mathurin P, Morgan TR. Alcoholic hepatitis. N Engl J Med 2009; 360(26):2758–2769. doi:10.1056/NEJMra0805786
- Maddrey WC, Boitnott JK, Bedine MS, Weber FL Jr, Mezey E, White RI Jr. Corticosteroid therapy of alcoholic hepatitis. Gastroenterology 1978; 75(2):193–199. pmid:352788
- Forrest EH, Evans CD, Stewart S, et al. Analysis of factors predictive of mortality in alcoholic hepatitis and derivation and validation of the Glasgow alcoholic hepatitis score. Gut 2005; 54(8):1174–1179. doi:10.1136/gut.2004.050781
- Dunn W, Jamil LH, Brown LS, et al. MELD accurately predicts mortality in patients with alcoholic hepatitis. Hepatology 2005; 41(2):353–358. doi:10.1002/hep.20503
- Sheth M, Riggs M, Patel T. Utility of the Mayo end-stage liver disease (MELD) score in assessing prognosis of patients with alcoholic hepatitis. BMC Gastroenterol 2002; 2:2. pmid:11835693
- Akriviadis E, Botla R, Briggs W, Han S, Reynolds T, Shakil O. Pentoxifylline improves short-term survival in severe acute alcoholic hepatitis: a double-blind, placebo-controlled trial. Gastroenterology 2000; 119(6):1637–1648. pmid:11113085
- Mathurin P, O’Grady J, Carithers RL, et al. Corticosteroids improve short-term survival in patients with severe alcoholic hepatitis: meta-analysis of individual patient data. Gut 2011; 60(2):255–260. doi:10.1136/gut.2010.224097
- Thursz MR, Richardson P, Allison M, et al; STOPAH Trial. Prednisolone or pentoxifylline for alcoholic hepatitis. N Engl J Med 2015; 372(17):1619–1628. doi:10.1056/NEJMoa1412278
- Thursz M, Forrest E, Roderick P, et al. The clinical effectiveness and cost-effectiveness of steroids or pentoxifylline for alcoholic hepatitis (STOPAH): a 2 × 2 factorial randomised controlled trial. Health Technol Assess 2015; 19(102):1–104. doi:10.3310/hta191020
- Lee YS, Kim HJ, Kim JH, et al. Treatment of severe alcoholic hepatitis with corticosteroid, pentoxifylline, or dual therapy: a systematic review and meta-analysis. J Clin Gastroenterol 2017; 51(4):364–377. doi:10.1097/MCG.0000000000000674
- Louvet A, Naveau S, Abdelnour M, et al. The Lille model: a new tool for therapeutic strategy in patients with severe alcoholic hepatitis treated with steroids. Hepatology 2007; 45(6):1348–1354. doi:10.1002/hep.21607
- Ruyon BA; AASLD Practice Guidelines Committee. Management of adult patients with ascites due to cirrhosis: an update. Hepatology 2009; 49(6):2087–2107. doi:10.1002/hep.22853
- Hoefs JC, Canawati HN, Sapico FL, Hopkins RR, Weiner J, Montgomerie JZ. Spontaneous bacterial peritonitis. Hepatology 1982; 2(4):399–407. pmid:7095741
- Ginès P, Cárdenas A, Arroyo V, Rodés J. Management of cirrhosis and ascites. N Engl J Med 2004; 350(16):1646–1654. doi:10.1056/NEJMra035021
- Runyon BA, Montano AA, Akriviadis EA, Antillon MR, Irving MA, McHutchison JG. The serum-ascites albumin gradient is superior to the exudate-transudate concept in the differential diagnosis of ascites. Ann Intern Med 1992; 117(3):215–220. pmid:1616215
- Hernaez R, Hamilton JP. Unexplained ascites. Clin Liver Dis 2016; 7(3):53–56. https://aasldpubs.onlinelibrary.wiley.com/doi/epdf/10.1002/cld.537
- Huang LL, Xia HH, Zhu SL. Ascitic fluid analysis in the differential diagnosis of ascites: focus on cirrhotic ascites. J Clin Transl Hepatol 2014; 2(1):58–64. doi:10.14218/JCTH.2013.00010
- Bartoloni A, Zammarchi L. Clinical aspects of uncomplicated and severe malaria. Mediterr J Hematol Infect Dis 2012; 4(1):e2012026. doi:10.4084/MJHID.2012.026
- Titó L, Rimola A, Ginès P, Llach J, Arroyo V, Rodés J. Recurrence of spontaneous bacterial peritonitis in cirrhosis: frequency and predictive factors. Hepatology 1988; 8(1):27–31. pmid:3257456
- Fernández J, Ruiz del Arbol L, Gómez C, et al. Norfloxacin vs ceftriaxone in the prophylaxis of infections in patients with advanced cirrhosis and hemorrhage. Gastroenterology 2006; 131(4):1049–1056. doi:10.1053/j.gastro.2006.07.010
- Runyon B; The American Association for the Study of Liver Diseases (AASLD). Management of adult patients with ascites due to cirrhosis: update 2012. https://www.aasld.org/sites/default/files/guideline_documents/141020_Guideline_Ascites_4UFb_2015.pdf. Accessed September 4, 2018.
- Sidhu SS, Goyal O, Kishore H, Sidhu S. New paradigms in management of alcoholic hepatitis: a review. Hepatol Int 2017; 11(3):255–267. doi:10.1007/s12072-017-9790-5
- Lucey MR, Mathurin P, Morgan TR. Alcoholic hepatitis. N Engl J Med 2009; 360(26):2758–2769. doi:10.1056/NEJMra0805786
- Maddrey WC, Boitnott JK, Bedine MS, Weber FL Jr, Mezey E, White RI Jr. Corticosteroid therapy of alcoholic hepatitis. Gastroenterology 1978; 75(2):193–199. pmid:352788
- Forrest EH, Evans CD, Stewart S, et al. Analysis of factors predictive of mortality in alcoholic hepatitis and derivation and validation of the Glasgow alcoholic hepatitis score. Gut 2005; 54(8):1174–1179. doi:10.1136/gut.2004.050781
- Dunn W, Jamil LH, Brown LS, et al. MELD accurately predicts mortality in patients with alcoholic hepatitis. Hepatology 2005; 41(2):353–358. doi:10.1002/hep.20503
- Sheth M, Riggs M, Patel T. Utility of the Mayo end-stage liver disease (MELD) score in assessing prognosis of patients with alcoholic hepatitis. BMC Gastroenterol 2002; 2:2. pmid:11835693
- Akriviadis E, Botla R, Briggs W, Han S, Reynolds T, Shakil O. Pentoxifylline improves short-term survival in severe acute alcoholic hepatitis: a double-blind, placebo-controlled trial. Gastroenterology 2000; 119(6):1637–1648. pmid:11113085
- Mathurin P, O’Grady J, Carithers RL, et al. Corticosteroids improve short-term survival in patients with severe alcoholic hepatitis: meta-analysis of individual patient data. Gut 2011; 60(2):255–260. doi:10.1136/gut.2010.224097
- Thursz MR, Richardson P, Allison M, et al; STOPAH Trial. Prednisolone or pentoxifylline for alcoholic hepatitis. N Engl J Med 2015; 372(17):1619–1628. doi:10.1056/NEJMoa1412278
- Thursz M, Forrest E, Roderick P, et al. The clinical effectiveness and cost-effectiveness of steroids or pentoxifylline for alcoholic hepatitis (STOPAH): a 2 × 2 factorial randomised controlled trial. Health Technol Assess 2015; 19(102):1–104. doi:10.3310/hta191020
- Lee YS, Kim HJ, Kim JH, et al. Treatment of severe alcoholic hepatitis with corticosteroid, pentoxifylline, or dual therapy: a systematic review and meta-analysis. J Clin Gastroenterol 2017; 51(4):364–377. doi:10.1097/MCG.0000000000000674
- Louvet A, Naveau S, Abdelnour M, et al. The Lille model: a new tool for therapeutic strategy in patients with severe alcoholic hepatitis treated with steroids. Hepatology 2007; 45(6):1348–1354. doi:10.1002/hep.21607
How should asymptomatic hypertension be managed in the hospital?
Case
A 62-year-old man with diabetes mellitus and hypertension presents with painful erythema of the left lower extremity and is admitted for purulent cellulitis. During the first evening of admission, he has increased left lower extremity pain and nursing reports a blood pressure of 188/96 mm Hg. He denies dyspnea, chest pain, visual changes, confusion, or severe headache.
Background
The prevalence of hypertension in the outpatient setting in the United States is estimated at 29% by the National Health and Nutrition Examination Survey.1 Hypertension generally is defined in the outpatient setting as an average blood pressure reading greater than or equal to140/90 mm Hg on two or more separate occasions.2 There is no consensus on the definition of hypertension in the inpatient setting; however, hypertensive urgency often is defined as a sustained blood pressure above the range of 180-200 mm Hg systolic and/or 110-120 mm Hg diastolic without target organ damage, and hypertensive emergency has a similar blood pressure range, but with evidence of target organ damage.3
The evidence
There are several clinical trials to suggest that the ambulatory treatment of chronic hypertension reduces the incidence of myocardial infarction, cerebrovascular accident (CVA), and heart failure7,8; however, these trials are difficult to extrapolate to acutely hospitalized patients.6 Overall, evidence on the appropriate management of asymptomatic hypertension in the inpatient setting is lacking.
Some evidence suggests we often are overly aggressive with intravenous antihypertensives without clinical indications in the inpatient setting.3,9,10 For example, Campbell et al. prospectively examined the use of intravenous hydralazine for the treatment of asymptomatic hypertension in 94 hospitalized patients.10 It was determined that in 90 of those patients, there was no clinical indication to use intravenous hydralazine and 17 patients experienced adverse events from hypotension, which included dizziness/light-headedness, syncope, and chest pain.10 They recommend against the use of intravenous hydralazine in the setting of asymptomatic hypertension because of its risk of adverse events with the rapid lowering of blood pressure.10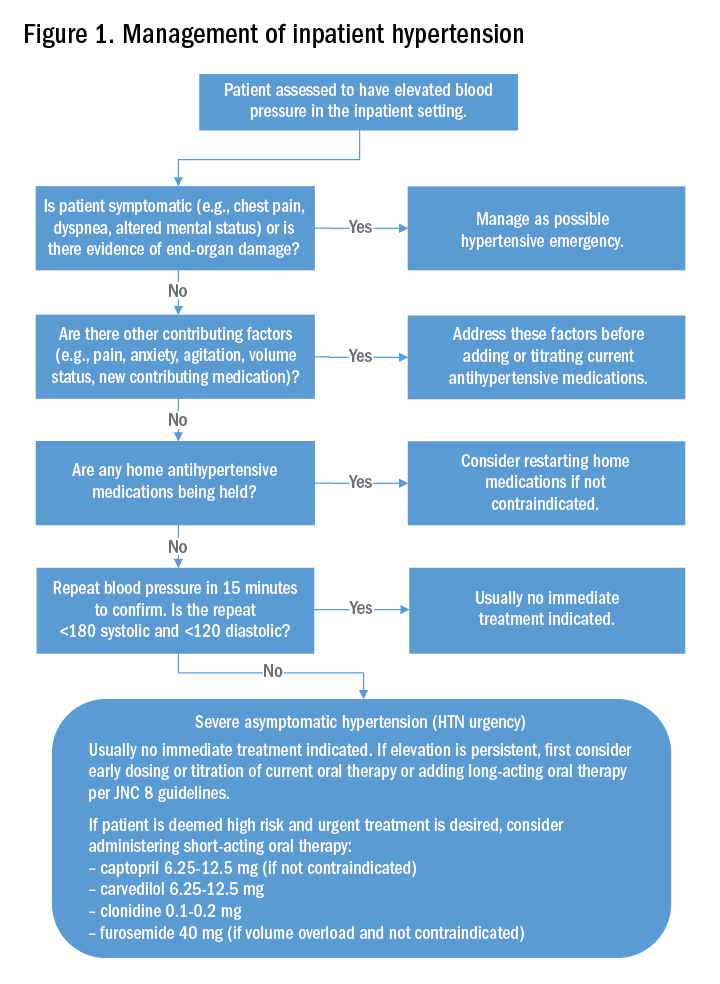
Weder and Erickson performed a retrospective review on the use of intravenous hydralazine and labetalol administration in 2,189 hospitalized patients.3 They found that only 3% of those patients had symptoms indicating the need for IV antihypertensives and that the length of stay was several days longer in those who had received IV antihypertensives.3
Other studies have examined the role of oral antihypertensives for management of asymptomatic hypertension in the inpatient setting. A systematic review and meta-analysis by Souza et al. assessed the use of oral pharmacotherapy for hypertensive urgency.11 Sixteen randomized clinical trials were reviewed and it was determined that angiotensin-converting enzyme inhibitors (ACE-I) had a superior effect in treating hypertensive urgencies.11 The most common side effect of using an ACE-I was a bad taste in patient’s mouths, and researchers did not observe side effects that were similar as those seen with the use of IV antihypertensives.11
Further, Jaker et al. performed a randomized, double-blind prospective study comparing a single dose of oral nifedipine with oral clonidine for the treatment of hypertensive urgency in 51 patients.12 Both the oral nifedipine and oral clonidine were extremely effective in reducing blood pressure fairly safely.12 However, the rapid lowering of blood pressure with oral nifedipine was concerning to Grossman et al.13 In their literature review of the side effects of oral and sublingual nifedipine, they found that it was one of the most common therapeutic interventions for hypertensive urgency or emergency.13 However, it was potentially dangerous because of the inconsistent blood pressure response after nifedipine administration, particularly with the sublingual form.13 CVAs, acute MIs, and even death were the reported adverse events with the use of oral and sublingual nifedipine.13 Because of that, the investigators recommend against the use of oral or sublingual nifedipine in hypertensive urgency or emergency and suggest using other oral antihypertensive agents instead.13
Typically, if the patient’s blood pressure remains elevated despite these efforts, no urgent treatment is indicated and we recommend close monitoring of the patient’s blood pressure during the hospitalization. If hypertension persists, the next best step would be to titrate a patient’s current oral antihypertensive therapy or to start a long-acting antihypertensive therapy per the JNC 8 (Eighth Joint National Committee) guidelines. It should be noted that, in those patients that are high risk, such as those with known coronary artery disease, heart failure, or prior hemorrhagic CVA, a short-acting oral antihypertensive such as captopril, carvedilol, clonidine, or furosemide should be considered.
Back to the case
The patient’s pain was treated with oral oxycodone. He received no oral or intravenous antihypertensive therapy, and the following morning, his blood pressure improved to 145/95 mm Hg. Based on our suggested approach in Fig. 1, the patient would require no acute treatment despite an improved but elevated blood pressure. We continued to monitor his blood pressure and despite adequate pain control, his blood pressure remain persistently elevated. Thus, per the JNC 8 guidelines, we started him on a long-acting antihypertensive, which improved his blood pressure to 123/78 at the time of discharge.
Bottom line
Management of asymptomatic hypertension in the hospital begins with addressing contributing factors, reviewing held home medications – and rarely – urgent oral pharmacotherapy.
Dr. Lippert is PGY-3 in the department of internal medicine at the University of Kentucky, Lexington. Dr. Bailey is associate professor of medicine at the University of Kentucky. Dr. Gray is associate professor of medicine at the University of Kentucky.
References
1. Yoon S, Fryar C, Carroll M. Hypertension prevalence and control among adults: United States, 2011-2014. 2015. Accessed Oct 2, 2017.
2. Poulter NR, Prabhakaran D, Caulfield M. Hypertension. Lancet. 2015;386(9995):801-12.
3. Weder AB, Erickson S. Treatment of hypertension in the inpatient setting: Use of intravenous labetalol and hydralazine. J Clin Hypertens. 2010;12(1):29-33.
4. Axon RN, Cousineau L, Egan BM. Prevalence and management of hypertension in the inpatient setting: A systematic review. J Hosp Med. 2011;6(7):417-22.
5. Weiss AJ, Elixhauser A. Overview of hospital stays in the United States, 2012. Statistical brief; 2014 Oct. Accessed Oct 2, 2017.
6. Weder AB. Treating acute hypertension in the hospital: a Lacuna in the guidelines. Hypertension. 2011;57(1):18-20.
7. Mancia G, Fagard R, Narkiewicz K, et al. 2013 ESH/ESC guidelines for the management of arterial hypertension: The Task Force for the management of arterial hypertension of the European Society of Hypertension and of the European Society of Cardiology. J Hypertens. 2013 Jul;31(7):1281-357.
8. James PA, Oparil S, Carter BL, et al. 2014 Evidence-based guideline for the management of high blood pressure in adults: Report from the panel members appointed to the Eighth Joint National Committee (JNC 8). JAMA. 2014;311(5):507-20.
9. Lipari M, Moser LR, Petrovitch EA, et al. As-needed intravenous antihypertensive therapy and blood pressure control. J Hosp Med. 2016;11(3):193-8.
10. Campbell P, Baker WL, Bendel SD, et al. Intravenous hydralazine for blood pressure management in the hospitalized patient: Its use is often unjustified. J Am Soc Hypertens. 2011;5(6):473-7.
11. Souza LM, Riera R, Saconato H, et al. Oral drugs for hypertensive urgencies: Systematic review and meta-analysis. Sao Paulo Med J. 2009;127(6):366-72.
12. Jaker M, Atkin S, Soto M, et al. Oral nifedipine vs. oral clonidine in the treatment of urgent hypertension. Arch Intern Med. 1989;149(2):260-5.
13. Grossman E, Messerli FH, Grodzicki T, et al. Should a moratorium be placed on sublingual nifedipine capsules given for hypertensive emergencies and pseudoemergencies? JAMA. 1996;276(16):1328-31.
14. Axon RN, Turner M, Buckley R. An update on inpatient hypertension management. Curr Cardiol Rep. 2015;17(11):94.
Additional Reading
1. Axon RN, Turner M, Buckley R. An update on inpatient hypertension management. Curr Cardiol Rep. 2015 Nov;17(11):94.
2. Herzog E, Frankenberger O, Aziz E, et al. A novel pathway for the management of hypertension for hospitalized patients. Crit Pathw Cardiol. 2007;6(4):150-60.
3. Sharma P, Shrestha A. Inpatient hypertension management. ACP Hospitalist. Aug 2014.
Quiz
Asymptomatic hypertension in the hospital
Hypertension is a common focus in the ambulatory setting because of its increased risk for cardiovascular events. Evidence for management in the inpatient setting is limited but does suggest a more conservative approach.
Question: A 75-year-old woman is hospitalized after sustaining a mechanical fall and subsequent right femoral neck fracture. She has a history of hypertension and hyperlipidemia for which she takes amlodipine and atorvastatin. Her blood pressure initially on admission is 170/102 mm Hg, and she is asymptomatic other than severe right hip pain. Her amlodipine and atorvastatin are resumed. Repeat blood pressures after resuming her amlodipine are still elevated with an average blood pressure reading of 168/98 mm Hg. Which of the following would be the next best step in treating this patient?
A. A one-time dose of intravenous hydralazine at 10 mg to reduce blood pressure by 25% over next several hours.
B. A one-time dose of oral clonidine at 0.1 mg to reduce blood pressure by 25% over next several hours.
C. Start a second daily antihypertensive with lisinopril 5 mg daily.
D. Address the patient’s pain.
The best answer is choice D. The patient’s hypertension is likely aggravated by her hip pain. Thus, the best course of action would be to address her pain.
Choice A is not the best answer as an intravenous antihypertensive is not indicated in this patient as she is asymptomatic and exhibiting no signs/symptoms of end-organ damage.
Choice B is not the best answer as by addressing her pain it is likely her blood pressure will improve. Urgent use of oral antihypertensives would not be indicated.
Choice C is not the best answer as patient has acute elevation of blood pressure in setting of a right femoral neck fracture and pain. Her blood pressure will likely improve after addressing her pain. However, if there is persistent blood pressure elevation, starting long-acting antihypertensive would be appropriate per JNC 8 guidelines.
Key Points
- Evidence for treatment of inpatient asymptomatic hypertension is lacking.
- The use of intravenous antihypertensives in the setting of inpatient asymptomatic hypertension is inappropriate and may be harmful.
- A conservative approach for inpatient asymptomatic hypertension should be employed by addressing contributing factors and reviewing held home antihypertensive medications prior to administering any oral antihypertensive pharmacotherapy.
Case
A 62-year-old man with diabetes mellitus and hypertension presents with painful erythema of the left lower extremity and is admitted for purulent cellulitis. During the first evening of admission, he has increased left lower extremity pain and nursing reports a blood pressure of 188/96 mm Hg. He denies dyspnea, chest pain, visual changes, confusion, or severe headache.
Background
The prevalence of hypertension in the outpatient setting in the United States is estimated at 29% by the National Health and Nutrition Examination Survey.1 Hypertension generally is defined in the outpatient setting as an average blood pressure reading greater than or equal to140/90 mm Hg on two or more separate occasions.2 There is no consensus on the definition of hypertension in the inpatient setting; however, hypertensive urgency often is defined as a sustained blood pressure above the range of 180-200 mm Hg systolic and/or 110-120 mm Hg diastolic without target organ damage, and hypertensive emergency has a similar blood pressure range, but with evidence of target organ damage.3
The evidence
There are several clinical trials to suggest that the ambulatory treatment of chronic hypertension reduces the incidence of myocardial infarction, cerebrovascular accident (CVA), and heart failure7,8; however, these trials are difficult to extrapolate to acutely hospitalized patients.6 Overall, evidence on the appropriate management of asymptomatic hypertension in the inpatient setting is lacking.
Some evidence suggests we often are overly aggressive with intravenous antihypertensives without clinical indications in the inpatient setting.3,9,10 For example, Campbell et al. prospectively examined the use of intravenous hydralazine for the treatment of asymptomatic hypertension in 94 hospitalized patients.10 It was determined that in 90 of those patients, there was no clinical indication to use intravenous hydralazine and 17 patients experienced adverse events from hypotension, which included dizziness/light-headedness, syncope, and chest pain.10 They recommend against the use of intravenous hydralazine in the setting of asymptomatic hypertension because of its risk of adverse events with the rapid lowering of blood pressure.10
Weder and Erickson performed a retrospective review on the use of intravenous hydralazine and labetalol administration in 2,189 hospitalized patients.3 They found that only 3% of those patients had symptoms indicating the need for IV antihypertensives and that the length of stay was several days longer in those who had received IV antihypertensives.3
Other studies have examined the role of oral antihypertensives for management of asymptomatic hypertension in the inpatient setting. A systematic review and meta-analysis by Souza et al. assessed the use of oral pharmacotherapy for hypertensive urgency.11 Sixteen randomized clinical trials were reviewed and it was determined that angiotensin-converting enzyme inhibitors (ACE-I) had a superior effect in treating hypertensive urgencies.11 The most common side effect of using an ACE-I was a bad taste in patient’s mouths, and researchers did not observe side effects that were similar as those seen with the use of IV antihypertensives.11
Further, Jaker et al. performed a randomized, double-blind prospective study comparing a single dose of oral nifedipine with oral clonidine for the treatment of hypertensive urgency in 51 patients.12 Both the oral nifedipine and oral clonidine were extremely effective in reducing blood pressure fairly safely.12 However, the rapid lowering of blood pressure with oral nifedipine was concerning to Grossman et al.13 In their literature review of the side effects of oral and sublingual nifedipine, they found that it was one of the most common therapeutic interventions for hypertensive urgency or emergency.13 However, it was potentially dangerous because of the inconsistent blood pressure response after nifedipine administration, particularly with the sublingual form.13 CVAs, acute MIs, and even death were the reported adverse events with the use of oral and sublingual nifedipine.13 Because of that, the investigators recommend against the use of oral or sublingual nifedipine in hypertensive urgency or emergency and suggest using other oral antihypertensive agents instead.13
Typically, if the patient’s blood pressure remains elevated despite these efforts, no urgent treatment is indicated and we recommend close monitoring of the patient’s blood pressure during the hospitalization. If hypertension persists, the next best step would be to titrate a patient’s current oral antihypertensive therapy or to start a long-acting antihypertensive therapy per the JNC 8 (Eighth Joint National Committee) guidelines. It should be noted that, in those patients that are high risk, such as those with known coronary artery disease, heart failure, or prior hemorrhagic CVA, a short-acting oral antihypertensive such as captopril, carvedilol, clonidine, or furosemide should be considered.
Back to the case
The patient’s pain was treated with oral oxycodone. He received no oral or intravenous antihypertensive therapy, and the following morning, his blood pressure improved to 145/95 mm Hg. Based on our suggested approach in Fig. 1, the patient would require no acute treatment despite an improved but elevated blood pressure. We continued to monitor his blood pressure and despite adequate pain control, his blood pressure remain persistently elevated. Thus, per the JNC 8 guidelines, we started him on a long-acting antihypertensive, which improved his blood pressure to 123/78 at the time of discharge.
Bottom line
Management of asymptomatic hypertension in the hospital begins with addressing contributing factors, reviewing held home medications – and rarely – urgent oral pharmacotherapy.
Dr. Lippert is PGY-3 in the department of internal medicine at the University of Kentucky, Lexington. Dr. Bailey is associate professor of medicine at the University of Kentucky. Dr. Gray is associate professor of medicine at the University of Kentucky.
References
1. Yoon S, Fryar C, Carroll M. Hypertension prevalence and control among adults: United States, 2011-2014. 2015. Accessed Oct 2, 2017.
2. Poulter NR, Prabhakaran D, Caulfield M. Hypertension. Lancet. 2015;386(9995):801-12.
3. Weder AB, Erickson S. Treatment of hypertension in the inpatient setting: Use of intravenous labetalol and hydralazine. J Clin Hypertens. 2010;12(1):29-33.
4. Axon RN, Cousineau L, Egan BM. Prevalence and management of hypertension in the inpatient setting: A systematic review. J Hosp Med. 2011;6(7):417-22.
5. Weiss AJ, Elixhauser A. Overview of hospital stays in the United States, 2012. Statistical brief; 2014 Oct. Accessed Oct 2, 2017.
6. Weder AB. Treating acute hypertension in the hospital: a Lacuna in the guidelines. Hypertension. 2011;57(1):18-20.
7. Mancia G, Fagard R, Narkiewicz K, et al. 2013 ESH/ESC guidelines for the management of arterial hypertension: The Task Force for the management of arterial hypertension of the European Society of Hypertension and of the European Society of Cardiology. J Hypertens. 2013 Jul;31(7):1281-357.
8. James PA, Oparil S, Carter BL, et al. 2014 Evidence-based guideline for the management of high blood pressure in adults: Report from the panel members appointed to the Eighth Joint National Committee (JNC 8). JAMA. 2014;311(5):507-20.
9. Lipari M, Moser LR, Petrovitch EA, et al. As-needed intravenous antihypertensive therapy and blood pressure control. J Hosp Med. 2016;11(3):193-8.
10. Campbell P, Baker WL, Bendel SD, et al. Intravenous hydralazine for blood pressure management in the hospitalized patient: Its use is often unjustified. J Am Soc Hypertens. 2011;5(6):473-7.
11. Souza LM, Riera R, Saconato H, et al. Oral drugs for hypertensive urgencies: Systematic review and meta-analysis. Sao Paulo Med J. 2009;127(6):366-72.
12. Jaker M, Atkin S, Soto M, et al. Oral nifedipine vs. oral clonidine in the treatment of urgent hypertension. Arch Intern Med. 1989;149(2):260-5.
13. Grossman E, Messerli FH, Grodzicki T, et al. Should a moratorium be placed on sublingual nifedipine capsules given for hypertensive emergencies and pseudoemergencies? JAMA. 1996;276(16):1328-31.
14. Axon RN, Turner M, Buckley R. An update on inpatient hypertension management. Curr Cardiol Rep. 2015;17(11):94.
Additional Reading
1. Axon RN, Turner M, Buckley R. An update on inpatient hypertension management. Curr Cardiol Rep. 2015 Nov;17(11):94.
2. Herzog E, Frankenberger O, Aziz E, et al. A novel pathway for the management of hypertension for hospitalized patients. Crit Pathw Cardiol. 2007;6(4):150-60.
3. Sharma P, Shrestha A. Inpatient hypertension management. ACP Hospitalist. Aug 2014.
Quiz
Asymptomatic hypertension in the hospital
Hypertension is a common focus in the ambulatory setting because of its increased risk for cardiovascular events. Evidence for management in the inpatient setting is limited but does suggest a more conservative approach.
Question: A 75-year-old woman is hospitalized after sustaining a mechanical fall and subsequent right femoral neck fracture. She has a history of hypertension and hyperlipidemia for which she takes amlodipine and atorvastatin. Her blood pressure initially on admission is 170/102 mm Hg, and she is asymptomatic other than severe right hip pain. Her amlodipine and atorvastatin are resumed. Repeat blood pressures after resuming her amlodipine are still elevated with an average blood pressure reading of 168/98 mm Hg. Which of the following would be the next best step in treating this patient?
A. A one-time dose of intravenous hydralazine at 10 mg to reduce blood pressure by 25% over next several hours.
B. A one-time dose of oral clonidine at 0.1 mg to reduce blood pressure by 25% over next several hours.
C. Start a second daily antihypertensive with lisinopril 5 mg daily.
D. Address the patient’s pain.
The best answer is choice D. The patient’s hypertension is likely aggravated by her hip pain. Thus, the best course of action would be to address her pain.
Choice A is not the best answer as an intravenous antihypertensive is not indicated in this patient as she is asymptomatic and exhibiting no signs/symptoms of end-organ damage.
Choice B is not the best answer as by addressing her pain it is likely her blood pressure will improve. Urgent use of oral antihypertensives would not be indicated.
Choice C is not the best answer as patient has acute elevation of blood pressure in setting of a right femoral neck fracture and pain. Her blood pressure will likely improve after addressing her pain. However, if there is persistent blood pressure elevation, starting long-acting antihypertensive would be appropriate per JNC 8 guidelines.
Key Points
- Evidence for treatment of inpatient asymptomatic hypertension is lacking.
- The use of intravenous antihypertensives in the setting of inpatient asymptomatic hypertension is inappropriate and may be harmful.
- A conservative approach for inpatient asymptomatic hypertension should be employed by addressing contributing factors and reviewing held home antihypertensive medications prior to administering any oral antihypertensive pharmacotherapy.
Case
A 62-year-old man with diabetes mellitus and hypertension presents with painful erythema of the left lower extremity and is admitted for purulent cellulitis. During the first evening of admission, he has increased left lower extremity pain and nursing reports a blood pressure of 188/96 mm Hg. He denies dyspnea, chest pain, visual changes, confusion, or severe headache.
Background
The prevalence of hypertension in the outpatient setting in the United States is estimated at 29% by the National Health and Nutrition Examination Survey.1 Hypertension generally is defined in the outpatient setting as an average blood pressure reading greater than or equal to140/90 mm Hg on two or more separate occasions.2 There is no consensus on the definition of hypertension in the inpatient setting; however, hypertensive urgency often is defined as a sustained blood pressure above the range of 180-200 mm Hg systolic and/or 110-120 mm Hg diastolic without target organ damage, and hypertensive emergency has a similar blood pressure range, but with evidence of target organ damage.3
The evidence
There are several clinical trials to suggest that the ambulatory treatment of chronic hypertension reduces the incidence of myocardial infarction, cerebrovascular accident (CVA), and heart failure7,8; however, these trials are difficult to extrapolate to acutely hospitalized patients.6 Overall, evidence on the appropriate management of asymptomatic hypertension in the inpatient setting is lacking.
Some evidence suggests we often are overly aggressive with intravenous antihypertensives without clinical indications in the inpatient setting.3,9,10 For example, Campbell et al. prospectively examined the use of intravenous hydralazine for the treatment of asymptomatic hypertension in 94 hospitalized patients.10 It was determined that in 90 of those patients, there was no clinical indication to use intravenous hydralazine and 17 patients experienced adverse events from hypotension, which included dizziness/light-headedness, syncope, and chest pain.10 They recommend against the use of intravenous hydralazine in the setting of asymptomatic hypertension because of its risk of adverse events with the rapid lowering of blood pressure.10
Weder and Erickson performed a retrospective review on the use of intravenous hydralazine and labetalol administration in 2,189 hospitalized patients.3 They found that only 3% of those patients had symptoms indicating the need for IV antihypertensives and that the length of stay was several days longer in those who had received IV antihypertensives.3
Other studies have examined the role of oral antihypertensives for management of asymptomatic hypertension in the inpatient setting. A systematic review and meta-analysis by Souza et al. assessed the use of oral pharmacotherapy for hypertensive urgency.11 Sixteen randomized clinical trials were reviewed and it was determined that angiotensin-converting enzyme inhibitors (ACE-I) had a superior effect in treating hypertensive urgencies.11 The most common side effect of using an ACE-I was a bad taste in patient’s mouths, and researchers did not observe side effects that were similar as those seen with the use of IV antihypertensives.11
Further, Jaker et al. performed a randomized, double-blind prospective study comparing a single dose of oral nifedipine with oral clonidine for the treatment of hypertensive urgency in 51 patients.12 Both the oral nifedipine and oral clonidine were extremely effective in reducing blood pressure fairly safely.12 However, the rapid lowering of blood pressure with oral nifedipine was concerning to Grossman et al.13 In their literature review of the side effects of oral and sublingual nifedipine, they found that it was one of the most common therapeutic interventions for hypertensive urgency or emergency.13 However, it was potentially dangerous because of the inconsistent blood pressure response after nifedipine administration, particularly with the sublingual form.13 CVAs, acute MIs, and even death were the reported adverse events with the use of oral and sublingual nifedipine.13 Because of that, the investigators recommend against the use of oral or sublingual nifedipine in hypertensive urgency or emergency and suggest using other oral antihypertensive agents instead.13
Typically, if the patient’s blood pressure remains elevated despite these efforts, no urgent treatment is indicated and we recommend close monitoring of the patient’s blood pressure during the hospitalization. If hypertension persists, the next best step would be to titrate a patient’s current oral antihypertensive therapy or to start a long-acting antihypertensive therapy per the JNC 8 (Eighth Joint National Committee) guidelines. It should be noted that, in those patients that are high risk, such as those with known coronary artery disease, heart failure, or prior hemorrhagic CVA, a short-acting oral antihypertensive such as captopril, carvedilol, clonidine, or furosemide should be considered.
Back to the case
The patient’s pain was treated with oral oxycodone. He received no oral or intravenous antihypertensive therapy, and the following morning, his blood pressure improved to 145/95 mm Hg. Based on our suggested approach in Fig. 1, the patient would require no acute treatment despite an improved but elevated blood pressure. We continued to monitor his blood pressure and despite adequate pain control, his blood pressure remain persistently elevated. Thus, per the JNC 8 guidelines, we started him on a long-acting antihypertensive, which improved his blood pressure to 123/78 at the time of discharge.
Bottom line
Management of asymptomatic hypertension in the hospital begins with addressing contributing factors, reviewing held home medications – and rarely – urgent oral pharmacotherapy.
Dr. Lippert is PGY-3 in the department of internal medicine at the University of Kentucky, Lexington. Dr. Bailey is associate professor of medicine at the University of Kentucky. Dr. Gray is associate professor of medicine at the University of Kentucky.
References
1. Yoon S, Fryar C, Carroll M. Hypertension prevalence and control among adults: United States, 2011-2014. 2015. Accessed Oct 2, 2017.
2. Poulter NR, Prabhakaran D, Caulfield M. Hypertension. Lancet. 2015;386(9995):801-12.
3. Weder AB, Erickson S. Treatment of hypertension in the inpatient setting: Use of intravenous labetalol and hydralazine. J Clin Hypertens. 2010;12(1):29-33.
4. Axon RN, Cousineau L, Egan BM. Prevalence and management of hypertension in the inpatient setting: A systematic review. J Hosp Med. 2011;6(7):417-22.
5. Weiss AJ, Elixhauser A. Overview of hospital stays in the United States, 2012. Statistical brief; 2014 Oct. Accessed Oct 2, 2017.
6. Weder AB. Treating acute hypertension in the hospital: a Lacuna in the guidelines. Hypertension. 2011;57(1):18-20.
7. Mancia G, Fagard R, Narkiewicz K, et al. 2013 ESH/ESC guidelines for the management of arterial hypertension: The Task Force for the management of arterial hypertension of the European Society of Hypertension and of the European Society of Cardiology. J Hypertens. 2013 Jul;31(7):1281-357.
8. James PA, Oparil S, Carter BL, et al. 2014 Evidence-based guideline for the management of high blood pressure in adults: Report from the panel members appointed to the Eighth Joint National Committee (JNC 8). JAMA. 2014;311(5):507-20.
9. Lipari M, Moser LR, Petrovitch EA, et al. As-needed intravenous antihypertensive therapy and blood pressure control. J Hosp Med. 2016;11(3):193-8.
10. Campbell P, Baker WL, Bendel SD, et al. Intravenous hydralazine for blood pressure management in the hospitalized patient: Its use is often unjustified. J Am Soc Hypertens. 2011;5(6):473-7.
11. Souza LM, Riera R, Saconato H, et al. Oral drugs for hypertensive urgencies: Systematic review and meta-analysis. Sao Paulo Med J. 2009;127(6):366-72.
12. Jaker M, Atkin S, Soto M, et al. Oral nifedipine vs. oral clonidine in the treatment of urgent hypertension. Arch Intern Med. 1989;149(2):260-5.
13. Grossman E, Messerli FH, Grodzicki T, et al. Should a moratorium be placed on sublingual nifedipine capsules given for hypertensive emergencies and pseudoemergencies? JAMA. 1996;276(16):1328-31.
14. Axon RN, Turner M, Buckley R. An update on inpatient hypertension management. Curr Cardiol Rep. 2015;17(11):94.
Additional Reading
1. Axon RN, Turner M, Buckley R. An update on inpatient hypertension management. Curr Cardiol Rep. 2015 Nov;17(11):94.
2. Herzog E, Frankenberger O, Aziz E, et al. A novel pathway for the management of hypertension for hospitalized patients. Crit Pathw Cardiol. 2007;6(4):150-60.
3. Sharma P, Shrestha A. Inpatient hypertension management. ACP Hospitalist. Aug 2014.
Quiz
Asymptomatic hypertension in the hospital
Hypertension is a common focus in the ambulatory setting because of its increased risk for cardiovascular events. Evidence for management in the inpatient setting is limited but does suggest a more conservative approach.
Question: A 75-year-old woman is hospitalized after sustaining a mechanical fall and subsequent right femoral neck fracture. She has a history of hypertension and hyperlipidemia for which she takes amlodipine and atorvastatin. Her blood pressure initially on admission is 170/102 mm Hg, and she is asymptomatic other than severe right hip pain. Her amlodipine and atorvastatin are resumed. Repeat blood pressures after resuming her amlodipine are still elevated with an average blood pressure reading of 168/98 mm Hg. Which of the following would be the next best step in treating this patient?
A. A one-time dose of intravenous hydralazine at 10 mg to reduce blood pressure by 25% over next several hours.
B. A one-time dose of oral clonidine at 0.1 mg to reduce blood pressure by 25% over next several hours.
C. Start a second daily antihypertensive with lisinopril 5 mg daily.
D. Address the patient’s pain.
The best answer is choice D. The patient’s hypertension is likely aggravated by her hip pain. Thus, the best course of action would be to address her pain.
Choice A is not the best answer as an intravenous antihypertensive is not indicated in this patient as she is asymptomatic and exhibiting no signs/symptoms of end-organ damage.
Choice B is not the best answer as by addressing her pain it is likely her blood pressure will improve. Urgent use of oral antihypertensives would not be indicated.
Choice C is not the best answer as patient has acute elevation of blood pressure in setting of a right femoral neck fracture and pain. Her blood pressure will likely improve after addressing her pain. However, if there is persistent blood pressure elevation, starting long-acting antihypertensive would be appropriate per JNC 8 guidelines.
Key Points
- Evidence for treatment of inpatient asymptomatic hypertension is lacking.
- The use of intravenous antihypertensives in the setting of inpatient asymptomatic hypertension is inappropriate and may be harmful.
- A conservative approach for inpatient asymptomatic hypertension should be employed by addressing contributing factors and reviewing held home antihypertensive medications prior to administering any oral antihypertensive pharmacotherapy.
Methemoglobinemia in an HIV patient
A 45-year-old man with known human immunodeficiency virus infection presented with a 5-day history of dyspnea. When his dyspnea had become symptomatic, he had restarted his home dapsone prophylaxis, but his dyspnea had progressively worsened, and his urine became dark.
Based on these test results, the patient’s dapsone was stopped and replaced with atovaquone. Intravenous infusion of methylene blue was started, with subsequent improvement of the hypoxia and cyanosis (Figure 1). His urine became green, but it returned to a normal color in a matter of hours. He was ultimately diagnosed with P jirovecii pneumonia and completed a course of atovaquone with total resolution of his symptoms.
THE MECHANISMS BEHIND METHEMOGLOBINEMIA
Heme iron is normally in the ferrous state (Fe2+), which allows for hemoglobin to carry oxygen and release it to tissues.1 Exposure to an oxidative stress can lead to methemoglobinemia from an increase in abnormal hemoglobin that contains iron in a ferric state (Fe3+).1,2
Methemoglobin reduces oxygen-carrying capacity in two ways: it is unable to carry oxygen, and its presence shifts the oxygen dissociation curve to the left, causing any remaining normal hemoglobin to be unable to release oxygen to the tissues.1,2
Causes of acquired methemoglobinemia include topical anesthetics (eg, benzocaine, lidocaine) and antibiotics (eg, dapsone).2,3 Signs and symptoms include cyanosis, headache, fatigue, dyspnea, lethargy, respiratory distress, and dark-colored urine.1,2
MANAGEMENT
Treatment consists of intravenous methylene blue, which reduces the hemoglobin from a ferric state to a ferrous state.1–4 Methylene blue is a water-soluble dye excreted primarily in the urine, and common side effects include dizziness, nausea, and green urine.5–7 The blue pigments from methylene blue combine with urobilin (a yellow pigment in the urine), producing a green color.7 This is not pathological and requires no treatment, as the urine returns to normal color after the body fully excretes the dye.5–7
If intravenous methylene blue fails to produce a response, other treatments to consider include hemodialysis, blood transfusion, exchange transfusion, and hyperbaric oxygen therapy.2
- Umbreit J. Methemoglobin—it’s not just blue: a concise review. Am J Hematol 2007; 82:134–144.
- Ash-Bernal R, Wise R, Wright SM. Acquired methemoglobinemia: a retrospective series of 138 cases at 2 teaching hospitals. Medicine (Baltimore) 2004; 83:265–273.
- Coleman MD, Coleman NA. Drug-induced methaemoglobinaemia. Treatment issues. Drug Saf 1996; 14:394–405.
- Sikka P, Bindra VK, Kapoor S, Jain V, Saxena KK. Blue cures blue but be cautious. J Pharm Bioallied Sci 2011; 3:543–545.
- Stratta P, Barbe MC. Images in clinical medicine. Green urine. N Engl J Med 2008; 358:e12.
- Miri-Aliabad G. Green urine secondary to methylene blue. Indian J Pediatr 2014; 81:1255–1256.
- Prakash S, Saini S, Mullick P, Pawar M. Green urine: a cause for concern? J Anaesthesiol Clin Pharmacol 2017; 33:128–130.
A 45-year-old man with known human immunodeficiency virus infection presented with a 5-day history of dyspnea. When his dyspnea had become symptomatic, he had restarted his home dapsone prophylaxis, but his dyspnea had progressively worsened, and his urine became dark.
Based on these test results, the patient’s dapsone was stopped and replaced with atovaquone. Intravenous infusion of methylene blue was started, with subsequent improvement of the hypoxia and cyanosis (Figure 1). His urine became green, but it returned to a normal color in a matter of hours. He was ultimately diagnosed with P jirovecii pneumonia and completed a course of atovaquone with total resolution of his symptoms.
THE MECHANISMS BEHIND METHEMOGLOBINEMIA
Heme iron is normally in the ferrous state (Fe2+), which allows for hemoglobin to carry oxygen and release it to tissues.1 Exposure to an oxidative stress can lead to methemoglobinemia from an increase in abnormal hemoglobin that contains iron in a ferric state (Fe3+).1,2
Methemoglobin reduces oxygen-carrying capacity in two ways: it is unable to carry oxygen, and its presence shifts the oxygen dissociation curve to the left, causing any remaining normal hemoglobin to be unable to release oxygen to the tissues.1,2
Causes of acquired methemoglobinemia include topical anesthetics (eg, benzocaine, lidocaine) and antibiotics (eg, dapsone).2,3 Signs and symptoms include cyanosis, headache, fatigue, dyspnea, lethargy, respiratory distress, and dark-colored urine.1,2
MANAGEMENT
Treatment consists of intravenous methylene blue, which reduces the hemoglobin from a ferric state to a ferrous state.1–4 Methylene blue is a water-soluble dye excreted primarily in the urine, and common side effects include dizziness, nausea, and green urine.5–7 The blue pigments from methylene blue combine with urobilin (a yellow pigment in the urine), producing a green color.7 This is not pathological and requires no treatment, as the urine returns to normal color after the body fully excretes the dye.5–7
If intravenous methylene blue fails to produce a response, other treatments to consider include hemodialysis, blood transfusion, exchange transfusion, and hyperbaric oxygen therapy.2
A 45-year-old man with known human immunodeficiency virus infection presented with a 5-day history of dyspnea. When his dyspnea had become symptomatic, he had restarted his home dapsone prophylaxis, but his dyspnea had progressively worsened, and his urine became dark.
Based on these test results, the patient’s dapsone was stopped and replaced with atovaquone. Intravenous infusion of methylene blue was started, with subsequent improvement of the hypoxia and cyanosis (Figure 1). His urine became green, but it returned to a normal color in a matter of hours. He was ultimately diagnosed with P jirovecii pneumonia and completed a course of atovaquone with total resolution of his symptoms.
THE MECHANISMS BEHIND METHEMOGLOBINEMIA
Heme iron is normally in the ferrous state (Fe2+), which allows for hemoglobin to carry oxygen and release it to tissues.1 Exposure to an oxidative stress can lead to methemoglobinemia from an increase in abnormal hemoglobin that contains iron in a ferric state (Fe3+).1,2
Methemoglobin reduces oxygen-carrying capacity in two ways: it is unable to carry oxygen, and its presence shifts the oxygen dissociation curve to the left, causing any remaining normal hemoglobin to be unable to release oxygen to the tissues.1,2
Causes of acquired methemoglobinemia include topical anesthetics (eg, benzocaine, lidocaine) and antibiotics (eg, dapsone).2,3 Signs and symptoms include cyanosis, headache, fatigue, dyspnea, lethargy, respiratory distress, and dark-colored urine.1,2
MANAGEMENT
Treatment consists of intravenous methylene blue, which reduces the hemoglobin from a ferric state to a ferrous state.1–4 Methylene blue is a water-soluble dye excreted primarily in the urine, and common side effects include dizziness, nausea, and green urine.5–7 The blue pigments from methylene blue combine with urobilin (a yellow pigment in the urine), producing a green color.7 This is not pathological and requires no treatment, as the urine returns to normal color after the body fully excretes the dye.5–7
If intravenous methylene blue fails to produce a response, other treatments to consider include hemodialysis, blood transfusion, exchange transfusion, and hyperbaric oxygen therapy.2
- Umbreit J. Methemoglobin—it’s not just blue: a concise review. Am J Hematol 2007; 82:134–144.
- Ash-Bernal R, Wise R, Wright SM. Acquired methemoglobinemia: a retrospective series of 138 cases at 2 teaching hospitals. Medicine (Baltimore) 2004; 83:265–273.
- Coleman MD, Coleman NA. Drug-induced methaemoglobinaemia. Treatment issues. Drug Saf 1996; 14:394–405.
- Sikka P, Bindra VK, Kapoor S, Jain V, Saxena KK. Blue cures blue but be cautious. J Pharm Bioallied Sci 2011; 3:543–545.
- Stratta P, Barbe MC. Images in clinical medicine. Green urine. N Engl J Med 2008; 358:e12.
- Miri-Aliabad G. Green urine secondary to methylene blue. Indian J Pediatr 2014; 81:1255–1256.
- Prakash S, Saini S, Mullick P, Pawar M. Green urine: a cause for concern? J Anaesthesiol Clin Pharmacol 2017; 33:128–130.
- Umbreit J. Methemoglobin—it’s not just blue: a concise review. Am J Hematol 2007; 82:134–144.
- Ash-Bernal R, Wise R, Wright SM. Acquired methemoglobinemia: a retrospective series of 138 cases at 2 teaching hospitals. Medicine (Baltimore) 2004; 83:265–273.
- Coleman MD, Coleman NA. Drug-induced methaemoglobinaemia. Treatment issues. Drug Saf 1996; 14:394–405.
- Sikka P, Bindra VK, Kapoor S, Jain V, Saxena KK. Blue cures blue but be cautious. J Pharm Bioallied Sci 2011; 3:543–545.
- Stratta P, Barbe MC. Images in clinical medicine. Green urine. N Engl J Med 2008; 358:e12.
- Miri-Aliabad G. Green urine secondary to methylene blue. Indian J Pediatr 2014; 81:1255–1256.
- Prakash S, Saini S, Mullick P, Pawar M. Green urine: a cause for concern? J Anaesthesiol Clin Pharmacol 2017; 33:128–130.