User login
In Case You Missed It: COVID
As COVID resurges, vaccinated Americans rage against holdouts
Outraged at vaccine-hesitant people, some are even calling for mandates requiring all Americans to get inoculated, arguing the holdouts are allowing the Delta coronavirus variant to gain traction and reverse the progress the United States was making against the virus.
“I am angry, I am resentful, and I think it’s a fair and appropriate response,” said Jonathan Hyman, a Berea, Ohio, attorney who blames the unvaccinated for the backslide in pandemic progress.
Mr. Hyman has been following the difficult guidelines health experts have been urging from the beginning. He has been masking up, avoiding large gatherings, postponing travel, and he signed up to receive the vaccine as soon as it was available.
“We have been responsible, I did everything I was supposed to do,” said Mr. Hyman, 48, who didn’t visit his parents for 18 months to keep them safe. “Yet here we are, 16, 17 months later, and it feels like we’re in the exact same place we were last summer, and it’s all because some people refuse to do the responsible things they were told to do.”
James Simmons, a retired South Florida high school finance teacher, is also angered by the vaccine holdouts, citing new spikes in COVID-19 infections, hospitalization rates, and deaths across the country – nearly all of which are among unvaccinated people.
“I can’t fathom the fact that people have seen over 600,000 Americans die from COVID, yet are resistant to a vaccine that provides direct protection for themselves and others,” said Mr. Simmons, 63, who received the shot early. “Their irresponsible decision is an affront to those of us who are vaccinated and still wear masks for the benefit of our society.”
Melissa Martin, an Atlanta resident who contracted a serious case of COVID-19 in September 2020, says it is “perplexing and frustrating” that so many Americans are refusing the vaccine. She believes the anger so many vaccinated people feel is tied to fear.
“I believe at the core of this anger is a fear of losing the ones we love,” said Ms. Martin, 55, who has been vaccinated, as has her fiancé, Shane McGeehin. “I was very angry last year after contracting COVID. The experience of having COVID was negative physically, emotionally, and socially.”
She recalled arguing with friends and relatives who downplayed how severe the virus was and who still refuse vaccination, despite seeing how COVID affected her.
“I am trying to understand why they feel the way they do,” she said, “but I would describe the emotions I have now towards those who do not get the vaccine as frustration, confusion, and disbelief.”
Leana Wen, MD, an emergency medicine doctor and public health policy professor at George Washington University, said such sentiments are common and justified.
“I understand that feeling of frustration and anger, because it is the unvaccinated who are setting back the progress that we’ve made [because of] the many sacrifices that many people have undergone,” said Dr. Wen, author of the newly published book “Lifelines: A Doctor’s Journey in the Fight for Public Health.”
“I think it is appropriate for the vaccinated to feel like they’re being punished right now,” she said. “We as a country had the opportunity to beat this virus – to return to prepandemic normal [life] and have our kids go back to school without worrying about coronavirus and our economy fully recovering. We came so close to achieving this, but we didn’t, and now COVID-19 is surging again. The vaccinated are having to pay the price for the choices that some have made to not end this pandemic.”
COVID rising, driving anger
The rising anger among vaccinated Americans comes as health officials are reporting huge spikes in new cases, hospitalizations, and deaths. Meanwhile, only about half of all Americans fully vaccinated, according to the Centers for Disease Control and Prevention.
Per Aug. 6 estimates from the CDC, the nation is averaging more than 100,000 new cases every day – the highest levels seen since February.
Southern states, with the lowest vaccination rates in the country, have been particularly hard-hit. Florida and Louisiana recently set 7-day records for new cases and hospitalizations, beating previous peaks last summer. Those two states, along with Mississippi, North Carolina, South Carolina, Tennessee, Kentucky, and Georgia, account for 41% of all new COVID-19 hospitalizations in the country, according to the CDC.
“It’s time to start blaming the unvaccinated folks, not the regular folks,” an angry Gov. Kay Ivey (R) of Alabama, told reporters. “It’s the unvaccinated folks that are letting us down.”
In response to the resurgence in cases, President Joe Biden has ordered new vaccine mandates for millions of federal workers.
California started requiring health care professionals to be vaccinated in August 2021, removing the option for unvaccinated employees to submit to regular testing.
New York City became the first in the country to require proof of vaccination for all workers and customers to enter restaurants, gyms, concert halls, movie theaters, and Broadway venues.
Nearly 60 major medical organizations, including the American Medical Association and the American Nurses Association, have called for mandatory vaccination of all health care workers.
Meanwhile, many businesses are requiring workers to be vaccinated before returning to offices and other workplaces. Colleges across the country are mandating the shots for students and staff. And some states and cities are also returning to mask mandates, including Hawaii; Louisiana; Washington, D.C.; San Francisco; and Los Angeles.
Experts say the 90 million unvaccinated Americans are most at risk from COVID and have helped the new Delta variant gain a foothold and spread, posing a risk of “breakthrough” cases even in vaccinated people.
Delta is more contagious and causes more severe disease than other known variants of the virus, according to the CDC. It is also more contagious than the viruses that cause Middle East respiratory syndrome, severe acute respiratory syndrome, Ebola, the common cold, flu, and smallpox
Calls for mandates grow
With Delta helping to drive new spikes in COVID cases, some vaccinated Americans argue that the federal government should be taking a harder line with holdouts. Others have even advocated withholding government stimulus checks or tax credits from vaccine refusers and cutting federal funding to states that don’t meet vaccine targets.
Eric Jaffe, a creative writer and producer from Florida who is vaccinated, said he would like to see government agencies and private businesses do more to put pressure on unvaccinated Americans to get the shot.
“In the interest of public safety, I believe the government and private businesses need to [make] life difficult for the unvaccinated,” said Mr. Jaffe, 29, whose parents both contracted the virus but recovered. “They should not be allowed to dine at restaurants, ride public transportation, attend concerts, or broadly be in spaces with large concentrations of people without passing a COVID test at the door.
“They’ll stand in long lines and be inconvenienced at every turn, while vaccinated people get to fly through security, TSA PreCheck-style. The holdouts at [this] point are beyond convincing. The vaccinated should be able to return to a level of normalcy, and the unvaccinated should face restrictions. Any other dynamic puts the stress on citizens who did the right thing.”
Elif Akcali, 49, who teaches engineering at the University of Florida, Gainesville, worries that the rights of people who refuse the vaccine are being put ahead of those of vaccinated people. She’s also concerned for people who face greater COVID risks, including health care workers and children too young to be inoculated.
“Each infection is an opportunity for the virus to evolve into a stronger version in itself,” said Ms. Akcali, who felt such a sense of relief when she received her vaccination that she teared up. “Each hospitalization is an unnecessary burden to health care workers and the system. Each death brings heartbreak to someone in their circle.”
Ed Berliner, an Emmy Award–winning broadcast journalist and Florida-based media specialist, blames social media for spreading misinformation that has taken root with unvaccinated Americans.
“When America rallied together to combat polio, there were two things we didn’t have. One was a lack of the sewer-dwelling, troll-infested social media, which has become the main source of news for the less intelligent and arrogant,” said Mr. Berliner, CEO of Entourage Media and host of The Man in the Arena, a talk show. “Second, children were dying across the country, and that made people sit up and take notice.”
Mr. Berliner, who knows two people who’ve died from COVID and who received the vaccine early, also believes too many political leaders are still fueling falsehoods that are giving unvaccinated Americans a license to refuse the shot.
“We are also here because governments and officials spend too little time being brutally honest, choosing instead to dance around issues with soft words,” he said. “The first words out of their mouths should have been: ‘What we are doing is trying to save lives. Help us save your life and that of everyone else.’ Would it have made a difference? We will never know.”
Shon Neyland, senior pastor at the Highland Christian Center church in Portland, Ore., said vaccine tensions have divided his congregation, with about half refusing the shot by his estimation. But he said it’s important to understand why some are making that choice, rather than rage at them and hammer home the benefits of the shot.
Many vaccine holdouts don’t trust the government or medical establishment or have bought into political arguments against the shot, he says. Some conservative evangelicals are also swayed by spiritual beliefs that COVID-19 is a sign of “biblical end-times prophesies” and the vaccine is “the mark of the beast.”
But he has tried to counter those beliefs and biases, arguing they are false and unfounded, urging members of his church to get the vaccine, and partnering with local health officials to run clinics to deliver it.
“I gently try to show them that the vaccine is for our own good and, in fact, is a blessing from God, and it’s up to us to accept the blessing [so] we can get back to somewhat of normalcy,” said Mr. Neyland, author of “The Courage to Stand: A New America.”
“I also believe that to get a vaccine this quick, this was nothing short of a miracle to turn the tide so quickly. Now, for us to resist, it would cause us to continue to suffer and lose lives. And you can’t turn away from the lives that have already been lost.”
Mr. Hyman fears we may not have seen the worst of the pandemic and that the Delta variant won’t be the last or most virulent mutation to emerge.
“The number of unvaccinated people is allowing this virus to continue circulating in the community,” he noted. “And while I have a tremendous amount of confidence that the vaccine protects me now from Delta, I have less confidence that it’s going to protect me from whatever [variant] comes next.
“So, I have a tremendous amount of concern for my own health and safety and welfare, and that of the people that I love. But I’m also concerned about what’s it going to do to businesses [and] the economy. Are we going to have more shutdowns if cases continue trending up? I’m very concerned as to what this could do [to] the country.”
A version of this article first appeared on WebMD.com.
Outraged at vaccine-hesitant people, some are even calling for mandates requiring all Americans to get inoculated, arguing the holdouts are allowing the Delta coronavirus variant to gain traction and reverse the progress the United States was making against the virus.
“I am angry, I am resentful, and I think it’s a fair and appropriate response,” said Jonathan Hyman, a Berea, Ohio, attorney who blames the unvaccinated for the backslide in pandemic progress.
Mr. Hyman has been following the difficult guidelines health experts have been urging from the beginning. He has been masking up, avoiding large gatherings, postponing travel, and he signed up to receive the vaccine as soon as it was available.
“We have been responsible, I did everything I was supposed to do,” said Mr. Hyman, 48, who didn’t visit his parents for 18 months to keep them safe. “Yet here we are, 16, 17 months later, and it feels like we’re in the exact same place we were last summer, and it’s all because some people refuse to do the responsible things they were told to do.”
James Simmons, a retired South Florida high school finance teacher, is also angered by the vaccine holdouts, citing new spikes in COVID-19 infections, hospitalization rates, and deaths across the country – nearly all of which are among unvaccinated people.
“I can’t fathom the fact that people have seen over 600,000 Americans die from COVID, yet are resistant to a vaccine that provides direct protection for themselves and others,” said Mr. Simmons, 63, who received the shot early. “Their irresponsible decision is an affront to those of us who are vaccinated and still wear masks for the benefit of our society.”
Melissa Martin, an Atlanta resident who contracted a serious case of COVID-19 in September 2020, says it is “perplexing and frustrating” that so many Americans are refusing the vaccine. She believes the anger so many vaccinated people feel is tied to fear.
“I believe at the core of this anger is a fear of losing the ones we love,” said Ms. Martin, 55, who has been vaccinated, as has her fiancé, Shane McGeehin. “I was very angry last year after contracting COVID. The experience of having COVID was negative physically, emotionally, and socially.”
She recalled arguing with friends and relatives who downplayed how severe the virus was and who still refuse vaccination, despite seeing how COVID affected her.
“I am trying to understand why they feel the way they do,” she said, “but I would describe the emotions I have now towards those who do not get the vaccine as frustration, confusion, and disbelief.”
Leana Wen, MD, an emergency medicine doctor and public health policy professor at George Washington University, said such sentiments are common and justified.
“I understand that feeling of frustration and anger, because it is the unvaccinated who are setting back the progress that we’ve made [because of] the many sacrifices that many people have undergone,” said Dr. Wen, author of the newly published book “Lifelines: A Doctor’s Journey in the Fight for Public Health.”
“I think it is appropriate for the vaccinated to feel like they’re being punished right now,” she said. “We as a country had the opportunity to beat this virus – to return to prepandemic normal [life] and have our kids go back to school without worrying about coronavirus and our economy fully recovering. We came so close to achieving this, but we didn’t, and now COVID-19 is surging again. The vaccinated are having to pay the price for the choices that some have made to not end this pandemic.”
COVID rising, driving anger
The rising anger among vaccinated Americans comes as health officials are reporting huge spikes in new cases, hospitalizations, and deaths. Meanwhile, only about half of all Americans fully vaccinated, according to the Centers for Disease Control and Prevention.
Per Aug. 6 estimates from the CDC, the nation is averaging more than 100,000 new cases every day – the highest levels seen since February.
Southern states, with the lowest vaccination rates in the country, have been particularly hard-hit. Florida and Louisiana recently set 7-day records for new cases and hospitalizations, beating previous peaks last summer. Those two states, along with Mississippi, North Carolina, South Carolina, Tennessee, Kentucky, and Georgia, account for 41% of all new COVID-19 hospitalizations in the country, according to the CDC.
“It’s time to start blaming the unvaccinated folks, not the regular folks,” an angry Gov. Kay Ivey (R) of Alabama, told reporters. “It’s the unvaccinated folks that are letting us down.”
In response to the resurgence in cases, President Joe Biden has ordered new vaccine mandates for millions of federal workers.
California started requiring health care professionals to be vaccinated in August 2021, removing the option for unvaccinated employees to submit to regular testing.
New York City became the first in the country to require proof of vaccination for all workers and customers to enter restaurants, gyms, concert halls, movie theaters, and Broadway venues.
Nearly 60 major medical organizations, including the American Medical Association and the American Nurses Association, have called for mandatory vaccination of all health care workers.
Meanwhile, many businesses are requiring workers to be vaccinated before returning to offices and other workplaces. Colleges across the country are mandating the shots for students and staff. And some states and cities are also returning to mask mandates, including Hawaii; Louisiana; Washington, D.C.; San Francisco; and Los Angeles.
Experts say the 90 million unvaccinated Americans are most at risk from COVID and have helped the new Delta variant gain a foothold and spread, posing a risk of “breakthrough” cases even in vaccinated people.
Delta is more contagious and causes more severe disease than other known variants of the virus, according to the CDC. It is also more contagious than the viruses that cause Middle East respiratory syndrome, severe acute respiratory syndrome, Ebola, the common cold, flu, and smallpox
Calls for mandates grow
With Delta helping to drive new spikes in COVID cases, some vaccinated Americans argue that the federal government should be taking a harder line with holdouts. Others have even advocated withholding government stimulus checks or tax credits from vaccine refusers and cutting federal funding to states that don’t meet vaccine targets.
Eric Jaffe, a creative writer and producer from Florida who is vaccinated, said he would like to see government agencies and private businesses do more to put pressure on unvaccinated Americans to get the shot.
“In the interest of public safety, I believe the government and private businesses need to [make] life difficult for the unvaccinated,” said Mr. Jaffe, 29, whose parents both contracted the virus but recovered. “They should not be allowed to dine at restaurants, ride public transportation, attend concerts, or broadly be in spaces with large concentrations of people without passing a COVID test at the door.
“They’ll stand in long lines and be inconvenienced at every turn, while vaccinated people get to fly through security, TSA PreCheck-style. The holdouts at [this] point are beyond convincing. The vaccinated should be able to return to a level of normalcy, and the unvaccinated should face restrictions. Any other dynamic puts the stress on citizens who did the right thing.”
Elif Akcali, 49, who teaches engineering at the University of Florida, Gainesville, worries that the rights of people who refuse the vaccine are being put ahead of those of vaccinated people. She’s also concerned for people who face greater COVID risks, including health care workers and children too young to be inoculated.
“Each infection is an opportunity for the virus to evolve into a stronger version in itself,” said Ms. Akcali, who felt such a sense of relief when she received her vaccination that she teared up. “Each hospitalization is an unnecessary burden to health care workers and the system. Each death brings heartbreak to someone in their circle.”
Ed Berliner, an Emmy Award–winning broadcast journalist and Florida-based media specialist, blames social media for spreading misinformation that has taken root with unvaccinated Americans.
“When America rallied together to combat polio, there were two things we didn’t have. One was a lack of the sewer-dwelling, troll-infested social media, which has become the main source of news for the less intelligent and arrogant,” said Mr. Berliner, CEO of Entourage Media and host of The Man in the Arena, a talk show. “Second, children were dying across the country, and that made people sit up and take notice.”
Mr. Berliner, who knows two people who’ve died from COVID and who received the vaccine early, also believes too many political leaders are still fueling falsehoods that are giving unvaccinated Americans a license to refuse the shot.
“We are also here because governments and officials spend too little time being brutally honest, choosing instead to dance around issues with soft words,” he said. “The first words out of their mouths should have been: ‘What we are doing is trying to save lives. Help us save your life and that of everyone else.’ Would it have made a difference? We will never know.”
Shon Neyland, senior pastor at the Highland Christian Center church in Portland, Ore., said vaccine tensions have divided his congregation, with about half refusing the shot by his estimation. But he said it’s important to understand why some are making that choice, rather than rage at them and hammer home the benefits of the shot.
Many vaccine holdouts don’t trust the government or medical establishment or have bought into political arguments against the shot, he says. Some conservative evangelicals are also swayed by spiritual beliefs that COVID-19 is a sign of “biblical end-times prophesies” and the vaccine is “the mark of the beast.”
But he has tried to counter those beliefs and biases, arguing they are false and unfounded, urging members of his church to get the vaccine, and partnering with local health officials to run clinics to deliver it.
“I gently try to show them that the vaccine is for our own good and, in fact, is a blessing from God, and it’s up to us to accept the blessing [so] we can get back to somewhat of normalcy,” said Mr. Neyland, author of “The Courage to Stand: A New America.”
“I also believe that to get a vaccine this quick, this was nothing short of a miracle to turn the tide so quickly. Now, for us to resist, it would cause us to continue to suffer and lose lives. And you can’t turn away from the lives that have already been lost.”
Mr. Hyman fears we may not have seen the worst of the pandemic and that the Delta variant won’t be the last or most virulent mutation to emerge.
“The number of unvaccinated people is allowing this virus to continue circulating in the community,” he noted. “And while I have a tremendous amount of confidence that the vaccine protects me now from Delta, I have less confidence that it’s going to protect me from whatever [variant] comes next.
“So, I have a tremendous amount of concern for my own health and safety and welfare, and that of the people that I love. But I’m also concerned about what’s it going to do to businesses [and] the economy. Are we going to have more shutdowns if cases continue trending up? I’m very concerned as to what this could do [to] the country.”
A version of this article first appeared on WebMD.com.
Outraged at vaccine-hesitant people, some are even calling for mandates requiring all Americans to get inoculated, arguing the holdouts are allowing the Delta coronavirus variant to gain traction and reverse the progress the United States was making against the virus.
“I am angry, I am resentful, and I think it’s a fair and appropriate response,” said Jonathan Hyman, a Berea, Ohio, attorney who blames the unvaccinated for the backslide in pandemic progress.
Mr. Hyman has been following the difficult guidelines health experts have been urging from the beginning. He has been masking up, avoiding large gatherings, postponing travel, and he signed up to receive the vaccine as soon as it was available.
“We have been responsible, I did everything I was supposed to do,” said Mr. Hyman, 48, who didn’t visit his parents for 18 months to keep them safe. “Yet here we are, 16, 17 months later, and it feels like we’re in the exact same place we were last summer, and it’s all because some people refuse to do the responsible things they were told to do.”
James Simmons, a retired South Florida high school finance teacher, is also angered by the vaccine holdouts, citing new spikes in COVID-19 infections, hospitalization rates, and deaths across the country – nearly all of which are among unvaccinated people.
“I can’t fathom the fact that people have seen over 600,000 Americans die from COVID, yet are resistant to a vaccine that provides direct protection for themselves and others,” said Mr. Simmons, 63, who received the shot early. “Their irresponsible decision is an affront to those of us who are vaccinated and still wear masks for the benefit of our society.”
Melissa Martin, an Atlanta resident who contracted a serious case of COVID-19 in September 2020, says it is “perplexing and frustrating” that so many Americans are refusing the vaccine. She believes the anger so many vaccinated people feel is tied to fear.
“I believe at the core of this anger is a fear of losing the ones we love,” said Ms. Martin, 55, who has been vaccinated, as has her fiancé, Shane McGeehin. “I was very angry last year after contracting COVID. The experience of having COVID was negative physically, emotionally, and socially.”
She recalled arguing with friends and relatives who downplayed how severe the virus was and who still refuse vaccination, despite seeing how COVID affected her.
“I am trying to understand why they feel the way they do,” she said, “but I would describe the emotions I have now towards those who do not get the vaccine as frustration, confusion, and disbelief.”
Leana Wen, MD, an emergency medicine doctor and public health policy professor at George Washington University, said such sentiments are common and justified.
“I understand that feeling of frustration and anger, because it is the unvaccinated who are setting back the progress that we’ve made [because of] the many sacrifices that many people have undergone,” said Dr. Wen, author of the newly published book “Lifelines: A Doctor’s Journey in the Fight for Public Health.”
“I think it is appropriate for the vaccinated to feel like they’re being punished right now,” she said. “We as a country had the opportunity to beat this virus – to return to prepandemic normal [life] and have our kids go back to school without worrying about coronavirus and our economy fully recovering. We came so close to achieving this, but we didn’t, and now COVID-19 is surging again. The vaccinated are having to pay the price for the choices that some have made to not end this pandemic.”
COVID rising, driving anger
The rising anger among vaccinated Americans comes as health officials are reporting huge spikes in new cases, hospitalizations, and deaths. Meanwhile, only about half of all Americans fully vaccinated, according to the Centers for Disease Control and Prevention.
Per Aug. 6 estimates from the CDC, the nation is averaging more than 100,000 new cases every day – the highest levels seen since February.
Southern states, with the lowest vaccination rates in the country, have been particularly hard-hit. Florida and Louisiana recently set 7-day records for new cases and hospitalizations, beating previous peaks last summer. Those two states, along with Mississippi, North Carolina, South Carolina, Tennessee, Kentucky, and Georgia, account for 41% of all new COVID-19 hospitalizations in the country, according to the CDC.
“It’s time to start blaming the unvaccinated folks, not the regular folks,” an angry Gov. Kay Ivey (R) of Alabama, told reporters. “It’s the unvaccinated folks that are letting us down.”
In response to the resurgence in cases, President Joe Biden has ordered new vaccine mandates for millions of federal workers.
California started requiring health care professionals to be vaccinated in August 2021, removing the option for unvaccinated employees to submit to regular testing.
New York City became the first in the country to require proof of vaccination for all workers and customers to enter restaurants, gyms, concert halls, movie theaters, and Broadway venues.
Nearly 60 major medical organizations, including the American Medical Association and the American Nurses Association, have called for mandatory vaccination of all health care workers.
Meanwhile, many businesses are requiring workers to be vaccinated before returning to offices and other workplaces. Colleges across the country are mandating the shots for students and staff. And some states and cities are also returning to mask mandates, including Hawaii; Louisiana; Washington, D.C.; San Francisco; and Los Angeles.
Experts say the 90 million unvaccinated Americans are most at risk from COVID and have helped the new Delta variant gain a foothold and spread, posing a risk of “breakthrough” cases even in vaccinated people.
Delta is more contagious and causes more severe disease than other known variants of the virus, according to the CDC. It is also more contagious than the viruses that cause Middle East respiratory syndrome, severe acute respiratory syndrome, Ebola, the common cold, flu, and smallpox
Calls for mandates grow
With Delta helping to drive new spikes in COVID cases, some vaccinated Americans argue that the federal government should be taking a harder line with holdouts. Others have even advocated withholding government stimulus checks or tax credits from vaccine refusers and cutting federal funding to states that don’t meet vaccine targets.
Eric Jaffe, a creative writer and producer from Florida who is vaccinated, said he would like to see government agencies and private businesses do more to put pressure on unvaccinated Americans to get the shot.
“In the interest of public safety, I believe the government and private businesses need to [make] life difficult for the unvaccinated,” said Mr. Jaffe, 29, whose parents both contracted the virus but recovered. “They should not be allowed to dine at restaurants, ride public transportation, attend concerts, or broadly be in spaces with large concentrations of people without passing a COVID test at the door.
“They’ll stand in long lines and be inconvenienced at every turn, while vaccinated people get to fly through security, TSA PreCheck-style. The holdouts at [this] point are beyond convincing. The vaccinated should be able to return to a level of normalcy, and the unvaccinated should face restrictions. Any other dynamic puts the stress on citizens who did the right thing.”
Elif Akcali, 49, who teaches engineering at the University of Florida, Gainesville, worries that the rights of people who refuse the vaccine are being put ahead of those of vaccinated people. She’s also concerned for people who face greater COVID risks, including health care workers and children too young to be inoculated.
“Each infection is an opportunity for the virus to evolve into a stronger version in itself,” said Ms. Akcali, who felt such a sense of relief when she received her vaccination that she teared up. “Each hospitalization is an unnecessary burden to health care workers and the system. Each death brings heartbreak to someone in their circle.”
Ed Berliner, an Emmy Award–winning broadcast journalist and Florida-based media specialist, blames social media for spreading misinformation that has taken root with unvaccinated Americans.
“When America rallied together to combat polio, there were two things we didn’t have. One was a lack of the sewer-dwelling, troll-infested social media, which has become the main source of news for the less intelligent and arrogant,” said Mr. Berliner, CEO of Entourage Media and host of The Man in the Arena, a talk show. “Second, children were dying across the country, and that made people sit up and take notice.”
Mr. Berliner, who knows two people who’ve died from COVID and who received the vaccine early, also believes too many political leaders are still fueling falsehoods that are giving unvaccinated Americans a license to refuse the shot.
“We are also here because governments and officials spend too little time being brutally honest, choosing instead to dance around issues with soft words,” he said. “The first words out of their mouths should have been: ‘What we are doing is trying to save lives. Help us save your life and that of everyone else.’ Would it have made a difference? We will never know.”
Shon Neyland, senior pastor at the Highland Christian Center church in Portland, Ore., said vaccine tensions have divided his congregation, with about half refusing the shot by his estimation. But he said it’s important to understand why some are making that choice, rather than rage at them and hammer home the benefits of the shot.
Many vaccine holdouts don’t trust the government or medical establishment or have bought into political arguments against the shot, he says. Some conservative evangelicals are also swayed by spiritual beliefs that COVID-19 is a sign of “biblical end-times prophesies” and the vaccine is “the mark of the beast.”
But he has tried to counter those beliefs and biases, arguing they are false and unfounded, urging members of his church to get the vaccine, and partnering with local health officials to run clinics to deliver it.
“I gently try to show them that the vaccine is for our own good and, in fact, is a blessing from God, and it’s up to us to accept the blessing [so] we can get back to somewhat of normalcy,” said Mr. Neyland, author of “The Courage to Stand: A New America.”
“I also believe that to get a vaccine this quick, this was nothing short of a miracle to turn the tide so quickly. Now, for us to resist, it would cause us to continue to suffer and lose lives. And you can’t turn away from the lives that have already been lost.”
Mr. Hyman fears we may not have seen the worst of the pandemic and that the Delta variant won’t be the last or most virulent mutation to emerge.
“The number of unvaccinated people is allowing this virus to continue circulating in the community,” he noted. “And while I have a tremendous amount of confidence that the vaccine protects me now from Delta, I have less confidence that it’s going to protect me from whatever [variant] comes next.
“So, I have a tremendous amount of concern for my own health and safety and welfare, and that of the people that I love. But I’m also concerned about what’s it going to do to businesses [and] the economy. Are we going to have more shutdowns if cases continue trending up? I’m very concerned as to what this could do [to] the country.”
A version of this article first appeared on WebMD.com.
Surge of new child COVID cases continues for 6th consecutive week
The current COVID-19 surge has brought new cases in children to their highest level since February, according to a new report.
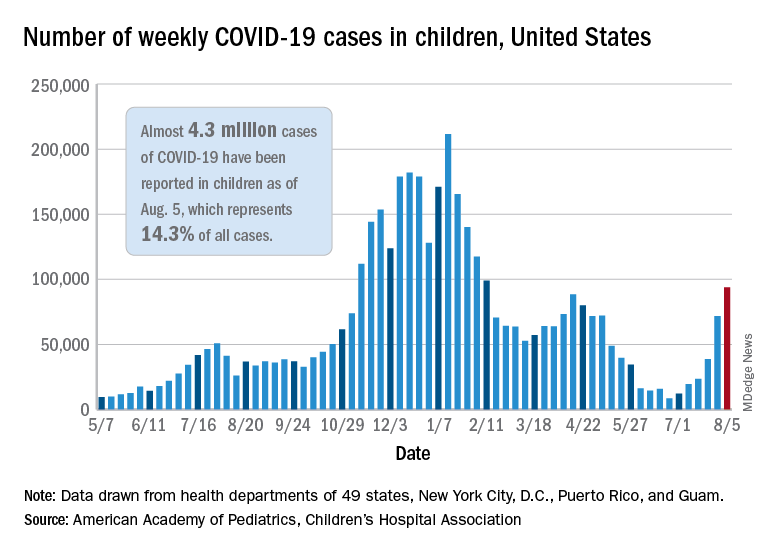
New pediatric cases rose for the 6th straight week, with almost 94,000 reported for the week ending Aug. 5.
That weekly total was up by 31% over the previous week and by over 1,000% since late June, when the new-case figure was at its lowest point (8,447) since early in the pandemic, the American Academy of Pediatrics and the Children’s Hospital Association said. COVID-related deaths – 13 for the week – were also higher than at any time since March 2021.
Almost 4.3 million children have been infected with SARS-CoV-2, which is 14.3% of all cases reported in 49 states (excluding New York), the District of Columbia, New York City, Puerto Rico, and Guam. Children represented 15.0% of the new cases reported in those jurisdictions during the week ending Aug. 5, the AAP and CHA said in their weekly report.
Another measure that has been trending upward recently is vaccine initiation among 12- to 15-year-olds, although the latest weekly total is still well below the high of 1.4 million seen in May. First-time vaccinations reached almost 411,000 for the week of Aug. 3-9, marking the fourth consecutive increase in that age group, the Centers for Disease Control and Prevention said on its COVID Data Tracker. Vaccinations also increased, although more modestly, for 16- and 17-year-olds in the most recent week.
Cumulative figures for children aged 12-17 show that almost 10.4 million have received at least one dose and that 7.7 million are fully vaccinated as of Aug. 9. By age group, 42.2% of those aged 12-15 have received at least one dose, and 30.4% have completed the vaccine regimen. Among those aged 16-17 years, 52.2% have gotten their first dose, and 41.4% are fully vaccinated, according to the COVID Data Tracker.
Looking at vaccination rates on the state level shows that only 20% of children aged 12-17 in Wyoming and 21% in Mississippi have gotten at least one dose as of Aug. 4, while Massachusetts is up to 68% and Vermont reports 70%. Rates for full vaccination range from 11% in Mississippi and Alabama to 61% in Vermont, based on an AAP analysis of CDC data, which is not available for Idaho.
The current COVID-19 surge has brought new cases in children to their highest level since February, according to a new report.

New pediatric cases rose for the 6th straight week, with almost 94,000 reported for the week ending Aug. 5.
That weekly total was up by 31% over the previous week and by over 1,000% since late June, when the new-case figure was at its lowest point (8,447) since early in the pandemic, the American Academy of Pediatrics and the Children’s Hospital Association said. COVID-related deaths – 13 for the week – were also higher than at any time since March 2021.
Almost 4.3 million children have been infected with SARS-CoV-2, which is 14.3% of all cases reported in 49 states (excluding New York), the District of Columbia, New York City, Puerto Rico, and Guam. Children represented 15.0% of the new cases reported in those jurisdictions during the week ending Aug. 5, the AAP and CHA said in their weekly report.
Another measure that has been trending upward recently is vaccine initiation among 12- to 15-year-olds, although the latest weekly total is still well below the high of 1.4 million seen in May. First-time vaccinations reached almost 411,000 for the week of Aug. 3-9, marking the fourth consecutive increase in that age group, the Centers for Disease Control and Prevention said on its COVID Data Tracker. Vaccinations also increased, although more modestly, for 16- and 17-year-olds in the most recent week.
Cumulative figures for children aged 12-17 show that almost 10.4 million have received at least one dose and that 7.7 million are fully vaccinated as of Aug. 9. By age group, 42.2% of those aged 12-15 have received at least one dose, and 30.4% have completed the vaccine regimen. Among those aged 16-17 years, 52.2% have gotten their first dose, and 41.4% are fully vaccinated, according to the COVID Data Tracker.
Looking at vaccination rates on the state level shows that only 20% of children aged 12-17 in Wyoming and 21% in Mississippi have gotten at least one dose as of Aug. 4, while Massachusetts is up to 68% and Vermont reports 70%. Rates for full vaccination range from 11% in Mississippi and Alabama to 61% in Vermont, based on an AAP analysis of CDC data, which is not available for Idaho.
The current COVID-19 surge has brought new cases in children to their highest level since February, according to a new report.

New pediatric cases rose for the 6th straight week, with almost 94,000 reported for the week ending Aug. 5.
That weekly total was up by 31% over the previous week and by over 1,000% since late June, when the new-case figure was at its lowest point (8,447) since early in the pandemic, the American Academy of Pediatrics and the Children’s Hospital Association said. COVID-related deaths – 13 for the week – were also higher than at any time since March 2021.
Almost 4.3 million children have been infected with SARS-CoV-2, which is 14.3% of all cases reported in 49 states (excluding New York), the District of Columbia, New York City, Puerto Rico, and Guam. Children represented 15.0% of the new cases reported in those jurisdictions during the week ending Aug. 5, the AAP and CHA said in their weekly report.
Another measure that has been trending upward recently is vaccine initiation among 12- to 15-year-olds, although the latest weekly total is still well below the high of 1.4 million seen in May. First-time vaccinations reached almost 411,000 for the week of Aug. 3-9, marking the fourth consecutive increase in that age group, the Centers for Disease Control and Prevention said on its COVID Data Tracker. Vaccinations also increased, although more modestly, for 16- and 17-year-olds in the most recent week.
Cumulative figures for children aged 12-17 show that almost 10.4 million have received at least one dose and that 7.7 million are fully vaccinated as of Aug. 9. By age group, 42.2% of those aged 12-15 have received at least one dose, and 30.4% have completed the vaccine regimen. Among those aged 16-17 years, 52.2% have gotten their first dose, and 41.4% are fully vaccinated, according to the COVID Data Tracker.
Looking at vaccination rates on the state level shows that only 20% of children aged 12-17 in Wyoming and 21% in Mississippi have gotten at least one dose as of Aug. 4, while Massachusetts is up to 68% and Vermont reports 70%. Rates for full vaccination range from 11% in Mississippi and Alabama to 61% in Vermont, based on an AAP analysis of CDC data, which is not available for Idaho.
Global youth depression and anxiety doubled during pandemic
The COVID-19 pandemic doubled international rates of child and adolescent psychological disorders, according to results of a meta-analysis.
In the first year of the pandemic, an estimated one in four youth across various regions of the globe experienced clinically elevated depression symptoms, while one in five experienced clinically elevated anxiety symptoms. These pooled estimates, which increased over time, are double prepandemic estimates, according to Nicole Racine, PhD, RPsych, a clinical psychologist at the University of Calgary (Alta.) and colleagues.
Their meta-analysis of 29 studies, comprising 80,879 young people worldwide aged 18 years or less, found pooled prevalence estimates of clinically elevated youth depression and anxiety of 25.2% (95% confidence interval, 21.2%-29.7%) and 20.5% (95% CI, 17.2%-24.4%), respectively.
“The prevalence of depression and anxiety symptoms during COVID-19 [has] doubled, compared with prepandemic estimates, and moderator analyses revealed that prevalence rates were higher when collected later in the pandemic, in older adolescents, and in girls,” the researchers write online in JAMA Pediatrics.
Prepandemic estimates of clinically significant generalized anxiety and depressive symptoms in large youth cohorts were approximately 11.6% and 12.9%, respectively, the authors say.
The increases revealed in these international findings have implications for targeted mental health resource planning.
“One difficulty in the literature is that there are large discrepancies on the prevalence of child depression and anxiety during the COVID-19 pandemic, with published rates between 2% and 68%,” corresponding author Sheri Madigan, PhD, RPsych, of the University of Calgary department of psychology, said in an interview. “By conducting a synthesis of the 29 studies on over 80,000 children, we were able to determine that, on average across these studies, 25% of youth are experiencing depression and 20% are experiencing anxiety during the COVID-19 pandemic.”
The cohort
The mean age in the combined global cohort was 13 years (range 4.1-17.6 ), and the mean proportion of females was 52.7% (standard deviation) 12.3%). The findings were based on international data published from Jan. 1, 2020, to Feb. 16, 2021, in studies conducted in the Middle East (n = 1), Europe (n = 4), South America (n = 2), North America (n = 6), and East Asia (n = 16). Notably absent were data from most of Latin America and the Middle East, Africa, South East Asia, and the Pacific Islands.
As the year progressed, the prevalence of depressive symptoms rose (b = .26; 95% CI, .06-46) with the number of months elapsed. Prevalence rates also rose as both age (b = 0.08, 95% CI, 0.01-0.15), and the percentage of females in samples increased (b = .03; 95% CI, 0.01-0.05).
The authors surmise that this cumulative worsening might be because of prolonged social isolation, family financial difficulties, missed milestones, and school disruptions, which are compounded over time. A second possibility is that studies conducted in the earlier months of the pandemic were more likely to be conducted in East Asia, where the self-reported prevalence of mental health symptoms tends to be lower.
The findings highlight an urgent need for intervention and recovery efforts and also indicate the need to consider individual differences when determining targets for intervention, including age, sex, and exposure to COVID-19 stressors), they add.
Even more concerning, recent data from the Centers for Disease Control and Prevention suggest that the pandemic spurred an increase in suspected suicide attempts by teenage girls. In the United Kingdom, acute mental health presentations to emergency care tripled over 2019 at one pediatric facility during the pandemic.
The authors attribute the toll on the psychological well-being of the world’s young people to pandemic-mandated restrictions. Those entailed loss of peer interactions, social isolation, and reduced contact with support figures such as teachers, and, “In addition, schools are often a primary location for receiving psychological services, with 80% of children relying on school-based services to address their mental health needs.” For many children, these services were rendered unavailable owing to school closures, Dr. Madigan and associates write.
In the context of clinical practice, doctors play a critical role. “With school closures, the physician’s office may be the only mental health checkpoint for youth,” Dr. Madigan said “So I recommend that family physicians screen for, and/or ask children and youth, about their mental health.”
On the home front, emerging research suggests that a predictable home environment can protect children’s mental well-being, with less depression and fewer behavioral problems observed in families adhering to regular routines during COVID-19. “Thus, a tangible solution to help mitigate the adverse effects of COVID-19 on youth is working with children and families to implement consistent and predictable routines around schoolwork, sleep, screen use, and physical activity,” the authors write.
They also point to the need for research on the long-term effects of the pandemic on mental health, including studies in order to “augment understanding of the implications of this crisis on the mental health trajectories of today’s children and youth.”
In an accompanying editorial, Tami D. Benton, MD, psychiatrist-in-chief at Children’s Hospital of Philadelphia, and colleagues, who were not involved in the meta-analysis, note certain limitations to the study. First, the included studies are based on self- or parent-reported symptoms. Second, the studies, more than half of which (55.2%) were done in China, may not be generalizable to all regions of the world, where 90% of children live in low- or middle-income countries.
Still, they write,“The increased mental health needs identified in the meta-analysis call for immediate action for every country. Our responses must consider the range of child mental health infrastructures available, which vary across countries, with some having well-developed and coordinated mental health services, while others have informal, limited, underfunded, or fragmented systems of care.”
Empirically supported and culturally appropriate intervention strategies for children and families according to countries and communities will be crucial, they stress.
“This meta-analysis provides the most complete evidence to date on the toll the COVID-19 pandemic has taken on child and adolescent mental health,” said Katie A. McLaughlin, PhD, a professor of psychology at Harvard University in Boston, who was not involved in the study. “The results confirm the substantial increases in symptoms of youth depression and anxiety that many clinicians and researchers have observed during the pandemic and highlight the critical need for greater investments in mental health services for children and adolescents.”
This study received no specific funding other than research support to the investigators from nonprivate entities. The authors disclosed no relevant conflicts of interest. Dr. Benton and associates and Dr. McLaughlin declared no competing interests.
The COVID-19 pandemic doubled international rates of child and adolescent psychological disorders, according to results of a meta-analysis.
In the first year of the pandemic, an estimated one in four youth across various regions of the globe experienced clinically elevated depression symptoms, while one in five experienced clinically elevated anxiety symptoms. These pooled estimates, which increased over time, are double prepandemic estimates, according to Nicole Racine, PhD, RPsych, a clinical psychologist at the University of Calgary (Alta.) and colleagues.
Their meta-analysis of 29 studies, comprising 80,879 young people worldwide aged 18 years or less, found pooled prevalence estimates of clinically elevated youth depression and anxiety of 25.2% (95% confidence interval, 21.2%-29.7%) and 20.5% (95% CI, 17.2%-24.4%), respectively.
“The prevalence of depression and anxiety symptoms during COVID-19 [has] doubled, compared with prepandemic estimates, and moderator analyses revealed that prevalence rates were higher when collected later in the pandemic, in older adolescents, and in girls,” the researchers write online in JAMA Pediatrics.
Prepandemic estimates of clinically significant generalized anxiety and depressive symptoms in large youth cohorts were approximately 11.6% and 12.9%, respectively, the authors say.
The increases revealed in these international findings have implications for targeted mental health resource planning.
“One difficulty in the literature is that there are large discrepancies on the prevalence of child depression and anxiety during the COVID-19 pandemic, with published rates between 2% and 68%,” corresponding author Sheri Madigan, PhD, RPsych, of the University of Calgary department of psychology, said in an interview. “By conducting a synthesis of the 29 studies on over 80,000 children, we were able to determine that, on average across these studies, 25% of youth are experiencing depression and 20% are experiencing anxiety during the COVID-19 pandemic.”
The cohort
The mean age in the combined global cohort was 13 years (range 4.1-17.6 ), and the mean proportion of females was 52.7% (standard deviation) 12.3%). The findings were based on international data published from Jan. 1, 2020, to Feb. 16, 2021, in studies conducted in the Middle East (n = 1), Europe (n = 4), South America (n = 2), North America (n = 6), and East Asia (n = 16). Notably absent were data from most of Latin America and the Middle East, Africa, South East Asia, and the Pacific Islands.
As the year progressed, the prevalence of depressive symptoms rose (b = .26; 95% CI, .06-46) with the number of months elapsed. Prevalence rates also rose as both age (b = 0.08, 95% CI, 0.01-0.15), and the percentage of females in samples increased (b = .03; 95% CI, 0.01-0.05).
The authors surmise that this cumulative worsening might be because of prolonged social isolation, family financial difficulties, missed milestones, and school disruptions, which are compounded over time. A second possibility is that studies conducted in the earlier months of the pandemic were more likely to be conducted in East Asia, where the self-reported prevalence of mental health symptoms tends to be lower.
The findings highlight an urgent need for intervention and recovery efforts and also indicate the need to consider individual differences when determining targets for intervention, including age, sex, and exposure to COVID-19 stressors), they add.
Even more concerning, recent data from the Centers for Disease Control and Prevention suggest that the pandemic spurred an increase in suspected suicide attempts by teenage girls. In the United Kingdom, acute mental health presentations to emergency care tripled over 2019 at one pediatric facility during the pandemic.
The authors attribute the toll on the psychological well-being of the world’s young people to pandemic-mandated restrictions. Those entailed loss of peer interactions, social isolation, and reduced contact with support figures such as teachers, and, “In addition, schools are often a primary location for receiving psychological services, with 80% of children relying on school-based services to address their mental health needs.” For many children, these services were rendered unavailable owing to school closures, Dr. Madigan and associates write.
In the context of clinical practice, doctors play a critical role. “With school closures, the physician’s office may be the only mental health checkpoint for youth,” Dr. Madigan said “So I recommend that family physicians screen for, and/or ask children and youth, about their mental health.”
On the home front, emerging research suggests that a predictable home environment can protect children’s mental well-being, with less depression and fewer behavioral problems observed in families adhering to regular routines during COVID-19. “Thus, a tangible solution to help mitigate the adverse effects of COVID-19 on youth is working with children and families to implement consistent and predictable routines around schoolwork, sleep, screen use, and physical activity,” the authors write.
They also point to the need for research on the long-term effects of the pandemic on mental health, including studies in order to “augment understanding of the implications of this crisis on the mental health trajectories of today’s children and youth.”
In an accompanying editorial, Tami D. Benton, MD, psychiatrist-in-chief at Children’s Hospital of Philadelphia, and colleagues, who were not involved in the meta-analysis, note certain limitations to the study. First, the included studies are based on self- or parent-reported symptoms. Second, the studies, more than half of which (55.2%) were done in China, may not be generalizable to all regions of the world, where 90% of children live in low- or middle-income countries.
Still, they write,“The increased mental health needs identified in the meta-analysis call for immediate action for every country. Our responses must consider the range of child mental health infrastructures available, which vary across countries, with some having well-developed and coordinated mental health services, while others have informal, limited, underfunded, or fragmented systems of care.”
Empirically supported and culturally appropriate intervention strategies for children and families according to countries and communities will be crucial, they stress.
“This meta-analysis provides the most complete evidence to date on the toll the COVID-19 pandemic has taken on child and adolescent mental health,” said Katie A. McLaughlin, PhD, a professor of psychology at Harvard University in Boston, who was not involved in the study. “The results confirm the substantial increases in symptoms of youth depression and anxiety that many clinicians and researchers have observed during the pandemic and highlight the critical need for greater investments in mental health services for children and adolescents.”
This study received no specific funding other than research support to the investigators from nonprivate entities. The authors disclosed no relevant conflicts of interest. Dr. Benton and associates and Dr. McLaughlin declared no competing interests.
The COVID-19 pandemic doubled international rates of child and adolescent psychological disorders, according to results of a meta-analysis.
In the first year of the pandemic, an estimated one in four youth across various regions of the globe experienced clinically elevated depression symptoms, while one in five experienced clinically elevated anxiety symptoms. These pooled estimates, which increased over time, are double prepandemic estimates, according to Nicole Racine, PhD, RPsych, a clinical psychologist at the University of Calgary (Alta.) and colleagues.
Their meta-analysis of 29 studies, comprising 80,879 young people worldwide aged 18 years or less, found pooled prevalence estimates of clinically elevated youth depression and anxiety of 25.2% (95% confidence interval, 21.2%-29.7%) and 20.5% (95% CI, 17.2%-24.4%), respectively.
“The prevalence of depression and anxiety symptoms during COVID-19 [has] doubled, compared with prepandemic estimates, and moderator analyses revealed that prevalence rates were higher when collected later in the pandemic, in older adolescents, and in girls,” the researchers write online in JAMA Pediatrics.
Prepandemic estimates of clinically significant generalized anxiety and depressive symptoms in large youth cohorts were approximately 11.6% and 12.9%, respectively, the authors say.
The increases revealed in these international findings have implications for targeted mental health resource planning.
“One difficulty in the literature is that there are large discrepancies on the prevalence of child depression and anxiety during the COVID-19 pandemic, with published rates between 2% and 68%,” corresponding author Sheri Madigan, PhD, RPsych, of the University of Calgary department of psychology, said in an interview. “By conducting a synthesis of the 29 studies on over 80,000 children, we were able to determine that, on average across these studies, 25% of youth are experiencing depression and 20% are experiencing anxiety during the COVID-19 pandemic.”
The cohort
The mean age in the combined global cohort was 13 years (range 4.1-17.6 ), and the mean proportion of females was 52.7% (standard deviation) 12.3%). The findings were based on international data published from Jan. 1, 2020, to Feb. 16, 2021, in studies conducted in the Middle East (n = 1), Europe (n = 4), South America (n = 2), North America (n = 6), and East Asia (n = 16). Notably absent were data from most of Latin America and the Middle East, Africa, South East Asia, and the Pacific Islands.
As the year progressed, the prevalence of depressive symptoms rose (b = .26; 95% CI, .06-46) with the number of months elapsed. Prevalence rates also rose as both age (b = 0.08, 95% CI, 0.01-0.15), and the percentage of females in samples increased (b = .03; 95% CI, 0.01-0.05).
The authors surmise that this cumulative worsening might be because of prolonged social isolation, family financial difficulties, missed milestones, and school disruptions, which are compounded over time. A second possibility is that studies conducted in the earlier months of the pandemic were more likely to be conducted in East Asia, where the self-reported prevalence of mental health symptoms tends to be lower.
The findings highlight an urgent need for intervention and recovery efforts and also indicate the need to consider individual differences when determining targets for intervention, including age, sex, and exposure to COVID-19 stressors), they add.
Even more concerning, recent data from the Centers for Disease Control and Prevention suggest that the pandemic spurred an increase in suspected suicide attempts by teenage girls. In the United Kingdom, acute mental health presentations to emergency care tripled over 2019 at one pediatric facility during the pandemic.
The authors attribute the toll on the psychological well-being of the world’s young people to pandemic-mandated restrictions. Those entailed loss of peer interactions, social isolation, and reduced contact with support figures such as teachers, and, “In addition, schools are often a primary location for receiving psychological services, with 80% of children relying on school-based services to address their mental health needs.” For many children, these services were rendered unavailable owing to school closures, Dr. Madigan and associates write.
In the context of clinical practice, doctors play a critical role. “With school closures, the physician’s office may be the only mental health checkpoint for youth,” Dr. Madigan said “So I recommend that family physicians screen for, and/or ask children and youth, about their mental health.”
On the home front, emerging research suggests that a predictable home environment can protect children’s mental well-being, with less depression and fewer behavioral problems observed in families adhering to regular routines during COVID-19. “Thus, a tangible solution to help mitigate the adverse effects of COVID-19 on youth is working with children and families to implement consistent and predictable routines around schoolwork, sleep, screen use, and physical activity,” the authors write.
They also point to the need for research on the long-term effects of the pandemic on mental health, including studies in order to “augment understanding of the implications of this crisis on the mental health trajectories of today’s children and youth.”
In an accompanying editorial, Tami D. Benton, MD, psychiatrist-in-chief at Children’s Hospital of Philadelphia, and colleagues, who were not involved in the meta-analysis, note certain limitations to the study. First, the included studies are based on self- or parent-reported symptoms. Second, the studies, more than half of which (55.2%) were done in China, may not be generalizable to all regions of the world, where 90% of children live in low- or middle-income countries.
Still, they write,“The increased mental health needs identified in the meta-analysis call for immediate action for every country. Our responses must consider the range of child mental health infrastructures available, which vary across countries, with some having well-developed and coordinated mental health services, while others have informal, limited, underfunded, or fragmented systems of care.”
Empirically supported and culturally appropriate intervention strategies for children and families according to countries and communities will be crucial, they stress.
“This meta-analysis provides the most complete evidence to date on the toll the COVID-19 pandemic has taken on child and adolescent mental health,” said Katie A. McLaughlin, PhD, a professor of psychology at Harvard University in Boston, who was not involved in the study. “The results confirm the substantial increases in symptoms of youth depression and anxiety that many clinicians and researchers have observed during the pandemic and highlight the critical need for greater investments in mental health services for children and adolescents.”
This study received no specific funding other than research support to the investigators from nonprivate entities. The authors disclosed no relevant conflicts of interest. Dr. Benton and associates and Dr. McLaughlin declared no competing interests.
Microlearning during the pandemic
How to become a hospitalist
The vast amounts of information generated this past year related to the COVID-19 pandemic was a feat of wonder – recommendations and guidelines on the hospital level and on the national level came in a flurry, more often overwhelming and confusing than clarifying for the frontline provider. In addition, “routine” hospital care for non-infected patients and improvement processes had to continue as we all dealt with the whirlwind of increasing COVID cases, torrents of new guidelines, and educating our trainees.
Thus, the individual-level question: how does a clinician stay engaged and distill the relentless stream of new information?
In Spring 2020, when the first patients with COVID were admitted, our hospital medicine section was tasked to create a surge plan. This included organizing, orienting, and educating off-service providers on how to become hospitalists. Undoubtedly, the call to arms for our center was heard, and many responded. However, backgrounds were diverse in specialty – clinicians and trainees from psychiatry, general surgery, and various fellowships all answered. It was an exhausting and inefficient effort to produce the material, hold webinars, and schedule training, especially for those who were more removed from a hospital medicine experience. We knew we had to come up with an alternative plan moving forward.
Thus, the systems-level question: how does a health care system educate its clinicians, or any other health care providers, when reallocation of their talents and skills is both necessary, time-sensitive, and occuring during a period where new information is constantly being produced and changing?
To reach the most clinicians as possible, with the most succinct and distilled information, we had to come up with a method to do so. Ultimately, in considering the situation at hand, we had to understand who we were as the provider of the information, and who the recipient would be. We would like to share the initiatives and processes by which we constructed our solution to the two questions – microlearning through hospital podcasting.
Learning from our health care colleagues
With the initial webinars and training sessions for our staff, we assessed our learners’ motivations and background in managing in a hospital medicine capacity. Overall, we discovered that our trainees and clinicians have an innate drive to learn; all of them recognized the importance of keeping up with evidence-based information. However, the difficulty highlighted was the individual time available to dedicate to acquiring new information and awareness of new information being available to the health care sector during the chaotic times of the pandemic.
From our section’s perspective, we had a difficulty with coordinating among multiple professional development groups within our hospital, cost, and resources to execute training. These difficulties between providing knowledge and receiving knowledge have already been expertly analyzed.1
Parallel to this, the pedagogic paradigm shifts as we progress through our careers – the methods and skills we used in school contrast in many ways with those we use on a daily basis when it comes to learning. Instead of dedicating hours at a time to new challenges in our workflow or our interests, we watch videos, search retailers for product solutions, check our email correspondence, and peruse social media accounts several times a day. Information comes at us very quickly, but in small pieces.
One such innovation in pedagogy is the practice of microlearning. This refers to the use of small lesson modules and short-term activities intended to teach and reinforce concepts.2 It is the opposite of “macrolearning,” which is the principle of dedicating reading material, structured coursework, and traditional knowledge evaluation in the form of exams to reinforce learning. Certainly, microlearning has other names as well – “just-in-time,” “just-enough,” and “micro-courses” are a few synonyms seen in the current literature. Though a highly relevant concept for our situation, translating it to an endproduct for our trainees and clinicians required more thought.
From theory to application
Microlearning allows for faster delivery of information – fewer things to write means shorter course distribution times, allowing the learner to respond faster to changing educational goals and training demands. Microlearning is flexible – “micro-courses” can give a broad overview of a subject or cover complex topics broken down into simple parts. In addition, micro-learning promotes retention of key concepts – given the length of each lesson, repetition of the topic by the learner is possible at any point in time. The whole experience is similar to checking your favorite social media application on your smartphone.
Certainly, many examples of the application of microlearning are available in the health care sector – pharmaceutical and nursing training both have utilized the theory extensively.3-4 However, in many instances, individuals were still required to sit at a workstation to complete modules and lessons. We envisioned our application of microlearning to be “on-the-go,” without necessarily requiring a computer workstation or laptop to complete.
In thinking about how social media attracts and influences clinicians, many content creators on social media come to mind. In addition, most, if not all, have branched into various social media platforms – podcasting, blogging, YouTube, for example. In thinking about our colleagues and trainees, we wanted a platform that they could take on the go, without the need to focus their visual attention (such as while driving or running). Ultimately, we believe the podcast would be the best platform to disseminate our information.
Podcasting is not foreign to medicine. A variety of medical podcasts exist, whether produced by major medical journals or by various independent health care practitioners. Both, however, have their drawbacks – the podcasts created by major medical journals are typically a summary of the publication’s content and are less engaging. Alternatively, podcasts produced by independent creators are certainly engaging and entertaining, and have a wealth of information, but the line is often blurred between just that: education and entertainment. In both instances, there is no follow-up or feedback offered to the learner in the form of surveys, or other types of feedback, which is arguably an important piece in any form of pedagogy. Thus, we sought to strike a balance between the two forms for our purposes.
Process of two podcasts
Our section was aware of the two aims during the pandemic – (1) disseminate new information regarding COVID-19 to the rest of our staff members and trainees as quickly as possible, and (2) maintain and improve the current quality of care of our patients. Thus, we sought to apply the reach and efficiency of the podcasting medium to provide ongoing education and feedback with respect to these two aims.
“The Cure” podcast. We recognized the constant flow of new COVID-19 information and updates and we wanted to find a readily accessible platform to reach staff with timely updates. Our marketing & communications team later helped us realize that the content we wanted to share was relevant to our patients and the community, so we formatted the material to be practical and easily digestible- something that may help an individual make decisions at the bedside as well as have conversations at the dinner table. Most recently, we engaged with our human resources department to use our platform in orienting new hires with the goal of helping staff familiarize with the institutions policies, procedures, and job aids that keep staff and patients safe.
“Antibiotry” podcast. Prior to the COVID-19 pandemic, our antibiotic stewardship group noticed an increase in antibiotic use on our medical floors. This is monitored not only through internal metrics by our pharmacy department, but also via the SAAR (standardized antibiotic administration ratio). Both sources demonstrated an increase in antibiotic use, greater than expected. An initiative was formed between our hospital medicine and infectious disease sections, and our pharmacy department to raise awareness of this increase in use, provide education to our trainees, and to create systems solutions for clinicians.
Initially, we sought to hold in-person sessions once a month for our trainees. This was led by a senior resident at the time. Topics of discussion were geared towards clinical decision making regarding empiric antibiotic use on the hospital medicine service. At the same time, our team published empiric antibiotic use guidelines, accessible through our electronic medical record. In addition, the resident leader gave a voluntary survey at the end of the session to assess not only confidence of antibiotic use, but also baseline knowledge regarding antibiotics in various clinical scenarios. This survey was repeated at the end of the resident group’s month-long rotation. Altogether, each in-person session was no longer than 10 minutes.
Unfortunately, the initiative was just gaining momentum when the COVID-19 pandemic was declared. However, we sought to take this challenge and translate it into an opportunity.
We directed our focus towards stewardship during pandemic times. Initially, our resident leader sent out email primers, approximately 3-5 minute reads, as a substitute for the in-person sessions. Our primers’ uniqueness was in its incorporation of prescription pattern data that was developed by our resident leader and our initiative’s data analyst. In doing so, we provided professional feedback regarding our antibiotic use based on the clinical indication. This was a powerful tool to not only engage our learners and staff clinicians, but also as a benchmarking tool for continued quality improvement.
But email primers are not engaging, and despite the ubiquity of teleconferencing, it was difficult to ask our housestaff to break from their morning rounds for a 10 minute tele-meeting. Thus, we devised a podcast method of education – 5-10 minute audio clips with conversation regarding a topic of discussion. This way, our trainees and learners can access episodes of education on their own time throughout the pandemic without disrupting their workflow. Given the brevity of, but high-yield content in, each episode, it would not only be convenient for listeners to access and repeat, but also for the podcaster (our resident leader) to create, as recording of the audio portion takes anywhere between 10-20 minutes for each episode, with postprocessing similarly fast.
The interdisciplinary nature of continued medical education cannot be stressed enough. With the help of our professional development team and their educators, we were able to centralize our podcast and attach surveys and additional graphics for each episode, if appropriate. This additional detail allowed for feedback, engagement with our learners, and the chance to provide additional educational points, if the learner was interested. Given the integrated nature of this platform, quality metrics could easily be recorded in the form of “click” data and various other more conventional metrics, such as listener counts and the duration of each podcast played.
Future applications and initiatives
Thus far, we have had great success in the reception and use of both podcasts within our institution as an application of microlearning. “The Cure” has been widely listened to by all hospital staff from various services; it has caught the attention of state-wide radio programs, and plans to expand it into the community are being discussed.
As for “Antibiotry” podcast, the concept has been lauded by our medical educators. Given its centralization within our institution, we are able to publish institution-based data as a form of professional and educational feedback to our trainees and staff physicians. This is currently coupled with the development of a provider dashboard, visualizing antibiotic prescriptions and narrowing patterns of practice within our medicine department. We plan to expand “Antibiotry” to other services at the hospital.
For both podcasts, the steps it took to achieve the final product from the microlearning concept were possible through a combination of institutional need and a motivated team. We are fortunate to have highly energetic individuals, making the coordination and planning with our hospitalists, various sub-specialists, and professional development teams straightforward. As the team grows with more individuals interested in the initiatives, keen insight into interests, individual clinical expertise, presentation skills, and technical skills ought to be carefully weighed to sustain our podcasts most efficiently, and perhaps expand them through different social media platforms.
Our objective for sustainability is through the continued outreach to and recruitment of residents and medical students, who can play key roles in the development of future projects related to these educational innovations. Both microlearning podcasts were developed through the initial planning, trial and error, and execution by two resident leaders. Their initiative and motivation to educate our institution through these platforms were highly unique; their pathfinding set the foundation for sustainability and expansion to other services.
Of course, one of the key measures we would like to investigate is whether our microlearning platform translates to improved patient outcomes. Regarding “Antibiotry,” we hope to see a decrease in unnecessary broad-spectrum antibiotic use by drawing attention to clinician practice patterns. Quality and outcome metrics will continue to be developed and measured. In addition to patient care metrics, further investigation of pedagogical metrics will be conducted, especially in the evolving realm of graduate and continuing medical education.
Measuring educational quality is neither a new ethical nor philosophical debate – neither does it carry a definitive answer. Further help from education experts may be needed to assess the quality of the information provided and its impact on our learners.
Conclusion
Medicine is ever-changing – the guidelines and criteria for patient care and pathology that we learned in medical school have likely changed. There is no single “best” method of learning new information in medicine, simply due to the breadth and volume of such information generated on a daily basis. This poses both a challenge for present-day clinicians and trainees, and a stimulus for change in the methods of acquiring, absorbing, and applying new information to clinical decision making and practice.
We have found that podcasting is a well-received medium of information transfer that is convenient for both the learner and the content creator. Through the podcast format, we were able to distill non-engaging pieces of education and information and transform them into short-duration lessons that the learner can listen to at their own convenience. This proved to be especially handy during the chaos of the pandemic, not only for dissemination of information regarding the management of COVID-19, but also for sustaining quality improvement goals within our institution.
Further investigation on patient outcomes and information quality are the planned next steps. In addition, expansion of other microlearning media, such as group SMS texting, YouTube videos, and Twitter, ought to be considered. Though many publications discuss the theory, potential benefits, and predicted pitfalls of microlearning, few assess the real-world application of microlearning to the clinical setting for medical education.
So what did we learn? We should think of microlearning as moments when you turn to your smartphone or tablet in order to discover something, answer a question, or complete a task. These are moments when decisions are made and knowledge is reinforced. The goal is to capture these moments and fill them with essential pieces of information.
We offer these suggestions as a place to start. The microlearning platform allows for the collection of data on the interaction between user and course content. The data collected can be used for continuous quality improvement of the curriculum. Microlearning is a dynamic platform where creative ideas are encouraged and a multi-disciplinary approach is valuable to keeping an audience engaged. In the future, we hope to be able to correlate microlearning courses to provider performance and measurable patient outcomes.
Dr. Mercado is medical director at Alice Peck Day Memorial Hospital, and associate hospital epidemiologist, Dartmouth-Hitchcock Medical Center, both in Lebanon, N.H., and assistant professor at the Geisel School of Medicine at Dartmouth, Hanover, N.H. Dr. Feng is a Fellow in the Leadership/Preventive Medicine Program in the Department of Internal Medicine at Dartmouth-Hitchcock Medical Center.
References
1. Duggan F and Banwell L. Constructing a model of effective information dissemination in a crisis. Information Research. 2004;9(3). Paper 178 [Available at http://InformationR.net/ir/9-3/paper178.html].
2. Filipe HP, et al. Microlearning to improve CPD learning objectives. Clin Teach. 2020 Dec;17(6):695-699. doi: 10.1111/tct.13208.
3. Hegerius A, et al. E-Learning in Pharmacovigilance: An Evaluation of Microlearning-Based Modules Developed by Uppsala Monitoring Centre. Drug Saf. 2020 Nov;43(11):1171-1180. doi: 10.1007/s40264-020-00981-w.
4. Orwoll B, et al. Gamification and Microlearning for Engagement With Quality Improvement (GAMEQI): A Bundled Digital Intervention for the Prevention of Central Line-Associated Bloodstream Infection. Am J Med Qual. Jan/Feb 2018;33(1):21-29. doi: 10.1177/1062860617706542.
How to become a hospitalist
How to become a hospitalist
The vast amounts of information generated this past year related to the COVID-19 pandemic was a feat of wonder – recommendations and guidelines on the hospital level and on the national level came in a flurry, more often overwhelming and confusing than clarifying for the frontline provider. In addition, “routine” hospital care for non-infected patients and improvement processes had to continue as we all dealt with the whirlwind of increasing COVID cases, torrents of new guidelines, and educating our trainees.
Thus, the individual-level question: how does a clinician stay engaged and distill the relentless stream of new information?
In Spring 2020, when the first patients with COVID were admitted, our hospital medicine section was tasked to create a surge plan. This included organizing, orienting, and educating off-service providers on how to become hospitalists. Undoubtedly, the call to arms for our center was heard, and many responded. However, backgrounds were diverse in specialty – clinicians and trainees from psychiatry, general surgery, and various fellowships all answered. It was an exhausting and inefficient effort to produce the material, hold webinars, and schedule training, especially for those who were more removed from a hospital medicine experience. We knew we had to come up with an alternative plan moving forward.
Thus, the systems-level question: how does a health care system educate its clinicians, or any other health care providers, when reallocation of their talents and skills is both necessary, time-sensitive, and occuring during a period where new information is constantly being produced and changing?
To reach the most clinicians as possible, with the most succinct and distilled information, we had to come up with a method to do so. Ultimately, in considering the situation at hand, we had to understand who we were as the provider of the information, and who the recipient would be. We would like to share the initiatives and processes by which we constructed our solution to the two questions – microlearning through hospital podcasting.
Learning from our health care colleagues
With the initial webinars and training sessions for our staff, we assessed our learners’ motivations and background in managing in a hospital medicine capacity. Overall, we discovered that our trainees and clinicians have an innate drive to learn; all of them recognized the importance of keeping up with evidence-based information. However, the difficulty highlighted was the individual time available to dedicate to acquiring new information and awareness of new information being available to the health care sector during the chaotic times of the pandemic.
From our section’s perspective, we had a difficulty with coordinating among multiple professional development groups within our hospital, cost, and resources to execute training. These difficulties between providing knowledge and receiving knowledge have already been expertly analyzed.1
Parallel to this, the pedagogic paradigm shifts as we progress through our careers – the methods and skills we used in school contrast in many ways with those we use on a daily basis when it comes to learning. Instead of dedicating hours at a time to new challenges in our workflow or our interests, we watch videos, search retailers for product solutions, check our email correspondence, and peruse social media accounts several times a day. Information comes at us very quickly, but in small pieces.
One such innovation in pedagogy is the practice of microlearning. This refers to the use of small lesson modules and short-term activities intended to teach and reinforce concepts.2 It is the opposite of “macrolearning,” which is the principle of dedicating reading material, structured coursework, and traditional knowledge evaluation in the form of exams to reinforce learning. Certainly, microlearning has other names as well – “just-in-time,” “just-enough,” and “micro-courses” are a few synonyms seen in the current literature. Though a highly relevant concept for our situation, translating it to an endproduct for our trainees and clinicians required more thought.
From theory to application
Microlearning allows for faster delivery of information – fewer things to write means shorter course distribution times, allowing the learner to respond faster to changing educational goals and training demands. Microlearning is flexible – “micro-courses” can give a broad overview of a subject or cover complex topics broken down into simple parts. In addition, micro-learning promotes retention of key concepts – given the length of each lesson, repetition of the topic by the learner is possible at any point in time. The whole experience is similar to checking your favorite social media application on your smartphone.
Certainly, many examples of the application of microlearning are available in the health care sector – pharmaceutical and nursing training both have utilized the theory extensively.3-4 However, in many instances, individuals were still required to sit at a workstation to complete modules and lessons. We envisioned our application of microlearning to be “on-the-go,” without necessarily requiring a computer workstation or laptop to complete.
In thinking about how social media attracts and influences clinicians, many content creators on social media come to mind. In addition, most, if not all, have branched into various social media platforms – podcasting, blogging, YouTube, for example. In thinking about our colleagues and trainees, we wanted a platform that they could take on the go, without the need to focus their visual attention (such as while driving or running). Ultimately, we believe the podcast would be the best platform to disseminate our information.
Podcasting is not foreign to medicine. A variety of medical podcasts exist, whether produced by major medical journals or by various independent health care practitioners. Both, however, have their drawbacks – the podcasts created by major medical journals are typically a summary of the publication’s content and are less engaging. Alternatively, podcasts produced by independent creators are certainly engaging and entertaining, and have a wealth of information, but the line is often blurred between just that: education and entertainment. In both instances, there is no follow-up or feedback offered to the learner in the form of surveys, or other types of feedback, which is arguably an important piece in any form of pedagogy. Thus, we sought to strike a balance between the two forms for our purposes.
Process of two podcasts
Our section was aware of the two aims during the pandemic – (1) disseminate new information regarding COVID-19 to the rest of our staff members and trainees as quickly as possible, and (2) maintain and improve the current quality of care of our patients. Thus, we sought to apply the reach and efficiency of the podcasting medium to provide ongoing education and feedback with respect to these two aims.
“The Cure” podcast. We recognized the constant flow of new COVID-19 information and updates and we wanted to find a readily accessible platform to reach staff with timely updates. Our marketing & communications team later helped us realize that the content we wanted to share was relevant to our patients and the community, so we formatted the material to be practical and easily digestible- something that may help an individual make decisions at the bedside as well as have conversations at the dinner table. Most recently, we engaged with our human resources department to use our platform in orienting new hires with the goal of helping staff familiarize with the institutions policies, procedures, and job aids that keep staff and patients safe.
“Antibiotry” podcast. Prior to the COVID-19 pandemic, our antibiotic stewardship group noticed an increase in antibiotic use on our medical floors. This is monitored not only through internal metrics by our pharmacy department, but also via the SAAR (standardized antibiotic administration ratio). Both sources demonstrated an increase in antibiotic use, greater than expected. An initiative was formed between our hospital medicine and infectious disease sections, and our pharmacy department to raise awareness of this increase in use, provide education to our trainees, and to create systems solutions for clinicians.
Initially, we sought to hold in-person sessions once a month for our trainees. This was led by a senior resident at the time. Topics of discussion were geared towards clinical decision making regarding empiric antibiotic use on the hospital medicine service. At the same time, our team published empiric antibiotic use guidelines, accessible through our electronic medical record. In addition, the resident leader gave a voluntary survey at the end of the session to assess not only confidence of antibiotic use, but also baseline knowledge regarding antibiotics in various clinical scenarios. This survey was repeated at the end of the resident group’s month-long rotation. Altogether, each in-person session was no longer than 10 minutes.
Unfortunately, the initiative was just gaining momentum when the COVID-19 pandemic was declared. However, we sought to take this challenge and translate it into an opportunity.
We directed our focus towards stewardship during pandemic times. Initially, our resident leader sent out email primers, approximately 3-5 minute reads, as a substitute for the in-person sessions. Our primers’ uniqueness was in its incorporation of prescription pattern data that was developed by our resident leader and our initiative’s data analyst. In doing so, we provided professional feedback regarding our antibiotic use based on the clinical indication. This was a powerful tool to not only engage our learners and staff clinicians, but also as a benchmarking tool for continued quality improvement.
But email primers are not engaging, and despite the ubiquity of teleconferencing, it was difficult to ask our housestaff to break from their morning rounds for a 10 minute tele-meeting. Thus, we devised a podcast method of education – 5-10 minute audio clips with conversation regarding a topic of discussion. This way, our trainees and learners can access episodes of education on their own time throughout the pandemic without disrupting their workflow. Given the brevity of, but high-yield content in, each episode, it would not only be convenient for listeners to access and repeat, but also for the podcaster (our resident leader) to create, as recording of the audio portion takes anywhere between 10-20 minutes for each episode, with postprocessing similarly fast.
The interdisciplinary nature of continued medical education cannot be stressed enough. With the help of our professional development team and their educators, we were able to centralize our podcast and attach surveys and additional graphics for each episode, if appropriate. This additional detail allowed for feedback, engagement with our learners, and the chance to provide additional educational points, if the learner was interested. Given the integrated nature of this platform, quality metrics could easily be recorded in the form of “click” data and various other more conventional metrics, such as listener counts and the duration of each podcast played.
Future applications and initiatives
Thus far, we have had great success in the reception and use of both podcasts within our institution as an application of microlearning. “The Cure” has been widely listened to by all hospital staff from various services; it has caught the attention of state-wide radio programs, and plans to expand it into the community are being discussed.
As for “Antibiotry” podcast, the concept has been lauded by our medical educators. Given its centralization within our institution, we are able to publish institution-based data as a form of professional and educational feedback to our trainees and staff physicians. This is currently coupled with the development of a provider dashboard, visualizing antibiotic prescriptions and narrowing patterns of practice within our medicine department. We plan to expand “Antibiotry” to other services at the hospital.
For both podcasts, the steps it took to achieve the final product from the microlearning concept were possible through a combination of institutional need and a motivated team. We are fortunate to have highly energetic individuals, making the coordination and planning with our hospitalists, various sub-specialists, and professional development teams straightforward. As the team grows with more individuals interested in the initiatives, keen insight into interests, individual clinical expertise, presentation skills, and technical skills ought to be carefully weighed to sustain our podcasts most efficiently, and perhaps expand them through different social media platforms.
Our objective for sustainability is through the continued outreach to and recruitment of residents and medical students, who can play key roles in the development of future projects related to these educational innovations. Both microlearning podcasts were developed through the initial planning, trial and error, and execution by two resident leaders. Their initiative and motivation to educate our institution through these platforms were highly unique; their pathfinding set the foundation for sustainability and expansion to other services.
Of course, one of the key measures we would like to investigate is whether our microlearning platform translates to improved patient outcomes. Regarding “Antibiotry,” we hope to see a decrease in unnecessary broad-spectrum antibiotic use by drawing attention to clinician practice patterns. Quality and outcome metrics will continue to be developed and measured. In addition to patient care metrics, further investigation of pedagogical metrics will be conducted, especially in the evolving realm of graduate and continuing medical education.
Measuring educational quality is neither a new ethical nor philosophical debate – neither does it carry a definitive answer. Further help from education experts may be needed to assess the quality of the information provided and its impact on our learners.
Conclusion
Medicine is ever-changing – the guidelines and criteria for patient care and pathology that we learned in medical school have likely changed. There is no single “best” method of learning new information in medicine, simply due to the breadth and volume of such information generated on a daily basis. This poses both a challenge for present-day clinicians and trainees, and a stimulus for change in the methods of acquiring, absorbing, and applying new information to clinical decision making and practice.
We have found that podcasting is a well-received medium of information transfer that is convenient for both the learner and the content creator. Through the podcast format, we were able to distill non-engaging pieces of education and information and transform them into short-duration lessons that the learner can listen to at their own convenience. This proved to be especially handy during the chaos of the pandemic, not only for dissemination of information regarding the management of COVID-19, but also for sustaining quality improvement goals within our institution.
Further investigation on patient outcomes and information quality are the planned next steps. In addition, expansion of other microlearning media, such as group SMS texting, YouTube videos, and Twitter, ought to be considered. Though many publications discuss the theory, potential benefits, and predicted pitfalls of microlearning, few assess the real-world application of microlearning to the clinical setting for medical education.
So what did we learn? We should think of microlearning as moments when you turn to your smartphone or tablet in order to discover something, answer a question, or complete a task. These are moments when decisions are made and knowledge is reinforced. The goal is to capture these moments and fill them with essential pieces of information.
We offer these suggestions as a place to start. The microlearning platform allows for the collection of data on the interaction between user and course content. The data collected can be used for continuous quality improvement of the curriculum. Microlearning is a dynamic platform where creative ideas are encouraged and a multi-disciplinary approach is valuable to keeping an audience engaged. In the future, we hope to be able to correlate microlearning courses to provider performance and measurable patient outcomes.
Dr. Mercado is medical director at Alice Peck Day Memorial Hospital, and associate hospital epidemiologist, Dartmouth-Hitchcock Medical Center, both in Lebanon, N.H., and assistant professor at the Geisel School of Medicine at Dartmouth, Hanover, N.H. Dr. Feng is a Fellow in the Leadership/Preventive Medicine Program in the Department of Internal Medicine at Dartmouth-Hitchcock Medical Center.
References
1. Duggan F and Banwell L. Constructing a model of effective information dissemination in a crisis. Information Research. 2004;9(3). Paper 178 [Available at http://InformationR.net/ir/9-3/paper178.html].
2. Filipe HP, et al. Microlearning to improve CPD learning objectives. Clin Teach. 2020 Dec;17(6):695-699. doi: 10.1111/tct.13208.
3. Hegerius A, et al. E-Learning in Pharmacovigilance: An Evaluation of Microlearning-Based Modules Developed by Uppsala Monitoring Centre. Drug Saf. 2020 Nov;43(11):1171-1180. doi: 10.1007/s40264-020-00981-w.
4. Orwoll B, et al. Gamification and Microlearning for Engagement With Quality Improvement (GAMEQI): A Bundled Digital Intervention for the Prevention of Central Line-Associated Bloodstream Infection. Am J Med Qual. Jan/Feb 2018;33(1):21-29. doi: 10.1177/1062860617706542.
The vast amounts of information generated this past year related to the COVID-19 pandemic was a feat of wonder – recommendations and guidelines on the hospital level and on the national level came in a flurry, more often overwhelming and confusing than clarifying for the frontline provider. In addition, “routine” hospital care for non-infected patients and improvement processes had to continue as we all dealt with the whirlwind of increasing COVID cases, torrents of new guidelines, and educating our trainees.
Thus, the individual-level question: how does a clinician stay engaged and distill the relentless stream of new information?
In Spring 2020, when the first patients with COVID were admitted, our hospital medicine section was tasked to create a surge plan. This included organizing, orienting, and educating off-service providers on how to become hospitalists. Undoubtedly, the call to arms for our center was heard, and many responded. However, backgrounds were diverse in specialty – clinicians and trainees from psychiatry, general surgery, and various fellowships all answered. It was an exhausting and inefficient effort to produce the material, hold webinars, and schedule training, especially for those who were more removed from a hospital medicine experience. We knew we had to come up with an alternative plan moving forward.
Thus, the systems-level question: how does a health care system educate its clinicians, or any other health care providers, when reallocation of their talents and skills is both necessary, time-sensitive, and occuring during a period where new information is constantly being produced and changing?
To reach the most clinicians as possible, with the most succinct and distilled information, we had to come up with a method to do so. Ultimately, in considering the situation at hand, we had to understand who we were as the provider of the information, and who the recipient would be. We would like to share the initiatives and processes by which we constructed our solution to the two questions – microlearning through hospital podcasting.
Learning from our health care colleagues
With the initial webinars and training sessions for our staff, we assessed our learners’ motivations and background in managing in a hospital medicine capacity. Overall, we discovered that our trainees and clinicians have an innate drive to learn; all of them recognized the importance of keeping up with evidence-based information. However, the difficulty highlighted was the individual time available to dedicate to acquiring new information and awareness of new information being available to the health care sector during the chaotic times of the pandemic.
From our section’s perspective, we had a difficulty with coordinating among multiple professional development groups within our hospital, cost, and resources to execute training. These difficulties between providing knowledge and receiving knowledge have already been expertly analyzed.1
Parallel to this, the pedagogic paradigm shifts as we progress through our careers – the methods and skills we used in school contrast in many ways with those we use on a daily basis when it comes to learning. Instead of dedicating hours at a time to new challenges in our workflow or our interests, we watch videos, search retailers for product solutions, check our email correspondence, and peruse social media accounts several times a day. Information comes at us very quickly, but in small pieces.
One such innovation in pedagogy is the practice of microlearning. This refers to the use of small lesson modules and short-term activities intended to teach and reinforce concepts.2 It is the opposite of “macrolearning,” which is the principle of dedicating reading material, structured coursework, and traditional knowledge evaluation in the form of exams to reinforce learning. Certainly, microlearning has other names as well – “just-in-time,” “just-enough,” and “micro-courses” are a few synonyms seen in the current literature. Though a highly relevant concept for our situation, translating it to an endproduct for our trainees and clinicians required more thought.
From theory to application
Microlearning allows for faster delivery of information – fewer things to write means shorter course distribution times, allowing the learner to respond faster to changing educational goals and training demands. Microlearning is flexible – “micro-courses” can give a broad overview of a subject or cover complex topics broken down into simple parts. In addition, micro-learning promotes retention of key concepts – given the length of each lesson, repetition of the topic by the learner is possible at any point in time. The whole experience is similar to checking your favorite social media application on your smartphone.
Certainly, many examples of the application of microlearning are available in the health care sector – pharmaceutical and nursing training both have utilized the theory extensively.3-4 However, in many instances, individuals were still required to sit at a workstation to complete modules and lessons. We envisioned our application of microlearning to be “on-the-go,” without necessarily requiring a computer workstation or laptop to complete.
In thinking about how social media attracts and influences clinicians, many content creators on social media come to mind. In addition, most, if not all, have branched into various social media platforms – podcasting, blogging, YouTube, for example. In thinking about our colleagues and trainees, we wanted a platform that they could take on the go, without the need to focus their visual attention (such as while driving or running). Ultimately, we believe the podcast would be the best platform to disseminate our information.
Podcasting is not foreign to medicine. A variety of medical podcasts exist, whether produced by major medical journals or by various independent health care practitioners. Both, however, have their drawbacks – the podcasts created by major medical journals are typically a summary of the publication’s content and are less engaging. Alternatively, podcasts produced by independent creators are certainly engaging and entertaining, and have a wealth of information, but the line is often blurred between just that: education and entertainment. In both instances, there is no follow-up or feedback offered to the learner in the form of surveys, or other types of feedback, which is arguably an important piece in any form of pedagogy. Thus, we sought to strike a balance between the two forms for our purposes.
Process of two podcasts
Our section was aware of the two aims during the pandemic – (1) disseminate new information regarding COVID-19 to the rest of our staff members and trainees as quickly as possible, and (2) maintain and improve the current quality of care of our patients. Thus, we sought to apply the reach and efficiency of the podcasting medium to provide ongoing education and feedback with respect to these two aims.
“The Cure” podcast. We recognized the constant flow of new COVID-19 information and updates and we wanted to find a readily accessible platform to reach staff with timely updates. Our marketing & communications team later helped us realize that the content we wanted to share was relevant to our patients and the community, so we formatted the material to be practical and easily digestible- something that may help an individual make decisions at the bedside as well as have conversations at the dinner table. Most recently, we engaged with our human resources department to use our platform in orienting new hires with the goal of helping staff familiarize with the institutions policies, procedures, and job aids that keep staff and patients safe.
“Antibiotry” podcast. Prior to the COVID-19 pandemic, our antibiotic stewardship group noticed an increase in antibiotic use on our medical floors. This is monitored not only through internal metrics by our pharmacy department, but also via the SAAR (standardized antibiotic administration ratio). Both sources demonstrated an increase in antibiotic use, greater than expected. An initiative was formed between our hospital medicine and infectious disease sections, and our pharmacy department to raise awareness of this increase in use, provide education to our trainees, and to create systems solutions for clinicians.
Initially, we sought to hold in-person sessions once a month for our trainees. This was led by a senior resident at the time. Topics of discussion were geared towards clinical decision making regarding empiric antibiotic use on the hospital medicine service. At the same time, our team published empiric antibiotic use guidelines, accessible through our electronic medical record. In addition, the resident leader gave a voluntary survey at the end of the session to assess not only confidence of antibiotic use, but also baseline knowledge regarding antibiotics in various clinical scenarios. This survey was repeated at the end of the resident group’s month-long rotation. Altogether, each in-person session was no longer than 10 minutes.
Unfortunately, the initiative was just gaining momentum when the COVID-19 pandemic was declared. However, we sought to take this challenge and translate it into an opportunity.
We directed our focus towards stewardship during pandemic times. Initially, our resident leader sent out email primers, approximately 3-5 minute reads, as a substitute for the in-person sessions. Our primers’ uniqueness was in its incorporation of prescription pattern data that was developed by our resident leader and our initiative’s data analyst. In doing so, we provided professional feedback regarding our antibiotic use based on the clinical indication. This was a powerful tool to not only engage our learners and staff clinicians, but also as a benchmarking tool for continued quality improvement.
But email primers are not engaging, and despite the ubiquity of teleconferencing, it was difficult to ask our housestaff to break from their morning rounds for a 10 minute tele-meeting. Thus, we devised a podcast method of education – 5-10 minute audio clips with conversation regarding a topic of discussion. This way, our trainees and learners can access episodes of education on their own time throughout the pandemic without disrupting their workflow. Given the brevity of, but high-yield content in, each episode, it would not only be convenient for listeners to access and repeat, but also for the podcaster (our resident leader) to create, as recording of the audio portion takes anywhere between 10-20 minutes for each episode, with postprocessing similarly fast.
The interdisciplinary nature of continued medical education cannot be stressed enough. With the help of our professional development team and their educators, we were able to centralize our podcast and attach surveys and additional graphics for each episode, if appropriate. This additional detail allowed for feedback, engagement with our learners, and the chance to provide additional educational points, if the learner was interested. Given the integrated nature of this platform, quality metrics could easily be recorded in the form of “click” data and various other more conventional metrics, such as listener counts and the duration of each podcast played.
Future applications and initiatives
Thus far, we have had great success in the reception and use of both podcasts within our institution as an application of microlearning. “The Cure” has been widely listened to by all hospital staff from various services; it has caught the attention of state-wide radio programs, and plans to expand it into the community are being discussed.
As for “Antibiotry” podcast, the concept has been lauded by our medical educators. Given its centralization within our institution, we are able to publish institution-based data as a form of professional and educational feedback to our trainees and staff physicians. This is currently coupled with the development of a provider dashboard, visualizing antibiotic prescriptions and narrowing patterns of practice within our medicine department. We plan to expand “Antibiotry” to other services at the hospital.
For both podcasts, the steps it took to achieve the final product from the microlearning concept were possible through a combination of institutional need and a motivated team. We are fortunate to have highly energetic individuals, making the coordination and planning with our hospitalists, various sub-specialists, and professional development teams straightforward. As the team grows with more individuals interested in the initiatives, keen insight into interests, individual clinical expertise, presentation skills, and technical skills ought to be carefully weighed to sustain our podcasts most efficiently, and perhaps expand them through different social media platforms.
Our objective for sustainability is through the continued outreach to and recruitment of residents and medical students, who can play key roles in the development of future projects related to these educational innovations. Both microlearning podcasts were developed through the initial planning, trial and error, and execution by two resident leaders. Their initiative and motivation to educate our institution through these platforms were highly unique; their pathfinding set the foundation for sustainability and expansion to other services.
Of course, one of the key measures we would like to investigate is whether our microlearning platform translates to improved patient outcomes. Regarding “Antibiotry,” we hope to see a decrease in unnecessary broad-spectrum antibiotic use by drawing attention to clinician practice patterns. Quality and outcome metrics will continue to be developed and measured. In addition to patient care metrics, further investigation of pedagogical metrics will be conducted, especially in the evolving realm of graduate and continuing medical education.
Measuring educational quality is neither a new ethical nor philosophical debate – neither does it carry a definitive answer. Further help from education experts may be needed to assess the quality of the information provided and its impact on our learners.
Conclusion
Medicine is ever-changing – the guidelines and criteria for patient care and pathology that we learned in medical school have likely changed. There is no single “best” method of learning new information in medicine, simply due to the breadth and volume of such information generated on a daily basis. This poses both a challenge for present-day clinicians and trainees, and a stimulus for change in the methods of acquiring, absorbing, and applying new information to clinical decision making and practice.
We have found that podcasting is a well-received medium of information transfer that is convenient for both the learner and the content creator. Through the podcast format, we were able to distill non-engaging pieces of education and information and transform them into short-duration lessons that the learner can listen to at their own convenience. This proved to be especially handy during the chaos of the pandemic, not only for dissemination of information regarding the management of COVID-19, but also for sustaining quality improvement goals within our institution.
Further investigation on patient outcomes and information quality are the planned next steps. In addition, expansion of other microlearning media, such as group SMS texting, YouTube videos, and Twitter, ought to be considered. Though many publications discuss the theory, potential benefits, and predicted pitfalls of microlearning, few assess the real-world application of microlearning to the clinical setting for medical education.
So what did we learn? We should think of microlearning as moments when you turn to your smartphone or tablet in order to discover something, answer a question, or complete a task. These are moments when decisions are made and knowledge is reinforced. The goal is to capture these moments and fill them with essential pieces of information.
We offer these suggestions as a place to start. The microlearning platform allows for the collection of data on the interaction between user and course content. The data collected can be used for continuous quality improvement of the curriculum. Microlearning is a dynamic platform where creative ideas are encouraged and a multi-disciplinary approach is valuable to keeping an audience engaged. In the future, we hope to be able to correlate microlearning courses to provider performance and measurable patient outcomes.
Dr. Mercado is medical director at Alice Peck Day Memorial Hospital, and associate hospital epidemiologist, Dartmouth-Hitchcock Medical Center, both in Lebanon, N.H., and assistant professor at the Geisel School of Medicine at Dartmouth, Hanover, N.H. Dr. Feng is a Fellow in the Leadership/Preventive Medicine Program in the Department of Internal Medicine at Dartmouth-Hitchcock Medical Center.
References
1. Duggan F and Banwell L. Constructing a model of effective information dissemination in a crisis. Information Research. 2004;9(3). Paper 178 [Available at http://InformationR.net/ir/9-3/paper178.html].
2. Filipe HP, et al. Microlearning to improve CPD learning objectives. Clin Teach. 2020 Dec;17(6):695-699. doi: 10.1111/tct.13208.
3. Hegerius A, et al. E-Learning in Pharmacovigilance: An Evaluation of Microlearning-Based Modules Developed by Uppsala Monitoring Centre. Drug Saf. 2020 Nov;43(11):1171-1180. doi: 10.1007/s40264-020-00981-w.
4. Orwoll B, et al. Gamification and Microlearning for Engagement With Quality Improvement (GAMEQI): A Bundled Digital Intervention for the Prevention of Central Line-Associated Bloodstream Infection. Am J Med Qual. Jan/Feb 2018;33(1):21-29. doi: 10.1177/1062860617706542.
CDC: Vaccination may cut risk of COVID reinfection in half
The Centers for Disease Control and Prevention has recommended that everyone get a COVID-19 vaccine, even if they’ve had the virus before. Yet many skeptics have held off getting the shots, believing that immunity generated by their previous infection will protect them if they should encounter the virus again.
A new study published in the CDC’s Morbidity and Mortality Weekly Report pokes holes in this notion. It shows people who have recovered from COVID-19 but haven’t been vaccinated have more than double the risk of testing positive for the virus again, compared with someone who was vaccinated after an initial infection.
The study looked at 738 Kentucky residents who had an initial bout of COVID-19 in 2020. About 250 of them tested positive for COVID-19 a second time between May and July of 2021, when the Delta variant became dominant in the United States.
The study matched each person who’d been reinfected with two people of the same sex and roughly the same age who had caught their initial COVID infection within the same week. The researchers then cross-matched those cases with data from Kentucky’s Immunization Registry.
They found that those who were unvaccinated had more than double the risk of being reinfected during the Delta wave. Partial vaccination appeared to have no significant impact on the risk of reinfection.
Among those who were reinfected, 20% were fully vaccinated, while 34% of those who did not get reinfected were fully vaccinated.
The study is observational, meaning it can’t show cause and effect; and the researchers had no information on the severity of the infections. Alyson Cavanaugh, PhD, a member of the CDC’s Epidemic Intelligence Service who led the study, said it is possible that some of the people who tested positive a second time had asymptomatic infections that were picked up through routine screening.
Still, the study backs up previous research and suggests that vaccination offers important additional protection.
“Our laboratory studies have shown that there’s an added benefit of vaccine for people who’ve had previous COVID-19. This is a real-world, epidemiologic study that found that among people who’d previously already had COVID-19, those who were vaccinated had lower odds of being reinfected,” Dr. Cavanaugh said.
“If you have had COVID-19 before, please still get vaccinated,” said CDC Director Rochelle Walensky, MD, in a written media statement. “This study shows you are twice as likely to get infected again if you are unvaccinated. Getting the vaccine is the best way to protect yourself and others around you, especially as the more contagious Delta variant spreads around the country.”
In a White House COVID-19 Response Team briefing in May, Anthony S. Fauci, MD, director of the National Institute of Allergy and Infectious Disease, explained why vaccines create stronger immunity than infection. He highlighted new research showing that two doses of an mRNA vaccine produce levels of neutralizing antibodies that are up to 10 times higher than the levels found in the blood of people who’ve recovered from COVID-19. Vaccines also enhance B cells and T cells in people who’ve recovered from COVID-19, which broadens the spectrum of protection and helps to fend off variants.
The study has some important limitations, which the authors acknowledged. The first is that second infections weren’t confirmed with genetic sequencing, so the researchers couldn’t definitively tell if a person tested positive a second time because they caught a new virus, or if they were somehow still shedding virus from their first infection. Given that the tests were at least 5 months apart, though, the researchers think reinfection is the most likely explanation.
Another bias in the study could have something to do with vaccination. Vaccinated people may have been less likely to be tested for COVID-19 after their vaccines, so the association or reinfection with a lack of vaccination may be overestimated.
Also, people who were vaccinated at federal sites or in another state were not logged in the state’s immunization registry, which may have skewed the data.
A version of this article first appeared on Medscape.com.
The Centers for Disease Control and Prevention has recommended that everyone get a COVID-19 vaccine, even if they’ve had the virus before. Yet many skeptics have held off getting the shots, believing that immunity generated by their previous infection will protect them if they should encounter the virus again.
A new study published in the CDC’s Morbidity and Mortality Weekly Report pokes holes in this notion. It shows people who have recovered from COVID-19 but haven’t been vaccinated have more than double the risk of testing positive for the virus again, compared with someone who was vaccinated after an initial infection.
The study looked at 738 Kentucky residents who had an initial bout of COVID-19 in 2020. About 250 of them tested positive for COVID-19 a second time between May and July of 2021, when the Delta variant became dominant in the United States.
The study matched each person who’d been reinfected with two people of the same sex and roughly the same age who had caught their initial COVID infection within the same week. The researchers then cross-matched those cases with data from Kentucky’s Immunization Registry.
They found that those who were unvaccinated had more than double the risk of being reinfected during the Delta wave. Partial vaccination appeared to have no significant impact on the risk of reinfection.
Among those who were reinfected, 20% were fully vaccinated, while 34% of those who did not get reinfected were fully vaccinated.
The study is observational, meaning it can’t show cause and effect; and the researchers had no information on the severity of the infections. Alyson Cavanaugh, PhD, a member of the CDC’s Epidemic Intelligence Service who led the study, said it is possible that some of the people who tested positive a second time had asymptomatic infections that were picked up through routine screening.
Still, the study backs up previous research and suggests that vaccination offers important additional protection.
“Our laboratory studies have shown that there’s an added benefit of vaccine for people who’ve had previous COVID-19. This is a real-world, epidemiologic study that found that among people who’d previously already had COVID-19, those who were vaccinated had lower odds of being reinfected,” Dr. Cavanaugh said.
“If you have had COVID-19 before, please still get vaccinated,” said CDC Director Rochelle Walensky, MD, in a written media statement. “This study shows you are twice as likely to get infected again if you are unvaccinated. Getting the vaccine is the best way to protect yourself and others around you, especially as the more contagious Delta variant spreads around the country.”
In a White House COVID-19 Response Team briefing in May, Anthony S. Fauci, MD, director of the National Institute of Allergy and Infectious Disease, explained why vaccines create stronger immunity than infection. He highlighted new research showing that two doses of an mRNA vaccine produce levels of neutralizing antibodies that are up to 10 times higher than the levels found in the blood of people who’ve recovered from COVID-19. Vaccines also enhance B cells and T cells in people who’ve recovered from COVID-19, which broadens the spectrum of protection and helps to fend off variants.
The study has some important limitations, which the authors acknowledged. The first is that second infections weren’t confirmed with genetic sequencing, so the researchers couldn’t definitively tell if a person tested positive a second time because they caught a new virus, or if they were somehow still shedding virus from their first infection. Given that the tests were at least 5 months apart, though, the researchers think reinfection is the most likely explanation.
Another bias in the study could have something to do with vaccination. Vaccinated people may have been less likely to be tested for COVID-19 after their vaccines, so the association or reinfection with a lack of vaccination may be overestimated.
Also, people who were vaccinated at federal sites or in another state were not logged in the state’s immunization registry, which may have skewed the data.
A version of this article first appeared on Medscape.com.
The Centers for Disease Control and Prevention has recommended that everyone get a COVID-19 vaccine, even if they’ve had the virus before. Yet many skeptics have held off getting the shots, believing that immunity generated by their previous infection will protect them if they should encounter the virus again.
A new study published in the CDC’s Morbidity and Mortality Weekly Report pokes holes in this notion. It shows people who have recovered from COVID-19 but haven’t been vaccinated have more than double the risk of testing positive for the virus again, compared with someone who was vaccinated after an initial infection.
The study looked at 738 Kentucky residents who had an initial bout of COVID-19 in 2020. About 250 of them tested positive for COVID-19 a second time between May and July of 2021, when the Delta variant became dominant in the United States.
The study matched each person who’d been reinfected with two people of the same sex and roughly the same age who had caught their initial COVID infection within the same week. The researchers then cross-matched those cases with data from Kentucky’s Immunization Registry.
They found that those who were unvaccinated had more than double the risk of being reinfected during the Delta wave. Partial vaccination appeared to have no significant impact on the risk of reinfection.
Among those who were reinfected, 20% were fully vaccinated, while 34% of those who did not get reinfected were fully vaccinated.
The study is observational, meaning it can’t show cause and effect; and the researchers had no information on the severity of the infections. Alyson Cavanaugh, PhD, a member of the CDC’s Epidemic Intelligence Service who led the study, said it is possible that some of the people who tested positive a second time had asymptomatic infections that were picked up through routine screening.
Still, the study backs up previous research and suggests that vaccination offers important additional protection.
“Our laboratory studies have shown that there’s an added benefit of vaccine for people who’ve had previous COVID-19. This is a real-world, epidemiologic study that found that among people who’d previously already had COVID-19, those who were vaccinated had lower odds of being reinfected,” Dr. Cavanaugh said.
“If you have had COVID-19 before, please still get vaccinated,” said CDC Director Rochelle Walensky, MD, in a written media statement. “This study shows you are twice as likely to get infected again if you are unvaccinated. Getting the vaccine is the best way to protect yourself and others around you, especially as the more contagious Delta variant spreads around the country.”
In a White House COVID-19 Response Team briefing in May, Anthony S. Fauci, MD, director of the National Institute of Allergy and Infectious Disease, explained why vaccines create stronger immunity than infection. He highlighted new research showing that two doses of an mRNA vaccine produce levels of neutralizing antibodies that are up to 10 times higher than the levels found in the blood of people who’ve recovered from COVID-19. Vaccines also enhance B cells and T cells in people who’ve recovered from COVID-19, which broadens the spectrum of protection and helps to fend off variants.
The study has some important limitations, which the authors acknowledged. The first is that second infections weren’t confirmed with genetic sequencing, so the researchers couldn’t definitively tell if a person tested positive a second time because they caught a new virus, or if they were somehow still shedding virus from their first infection. Given that the tests were at least 5 months apart, though, the researchers think reinfection is the most likely explanation.
Another bias in the study could have something to do with vaccination. Vaccinated people may have been less likely to be tested for COVID-19 after their vaccines, so the association or reinfection with a lack of vaccination may be overestimated.
Also, people who were vaccinated at federal sites or in another state were not logged in the state’s immunization registry, which may have skewed the data.
A version of this article first appeared on Medscape.com.
Myasthenic Crisis After Recurrent COVID-19 Infection
A patient with myasthenia gravis who survived 2 COVID-19 infections required plasmapheresis to recover from an acute crisis.
COVID-19 is still in the early stages of understanding, although it is known to be complicated by individual patient comorbidities. The management and treatment of COVID-19 continues to quickly evolve as more is discovered regarding the virus. Multiple treatments have been preliminarily tested and used under a Food and Drug Administration emergency use authorization (EUA) determination. The long-term success of these therapies, however, is yet to be determined. Additionally, if a patient has a second clinical presentation for COVID-19, it is not known whether this represents latency with subsequent reactivation from the previous infection or a second de novo infection. The uncertainty calls into question the duration of immunity, if any, following a primary infection.
COVID-19 management becomes more complicated when patients have complex medical conditions, such as myasthenia gravis (MG). This autoimmune neuromuscular disorder can present with varying weakness, and many patients are on immunomodulator medications. The weakness can worsen into a myasthenic crisis (MC), resulting in profound weakness of the respiratory muscles. Therefore, patients with MG are at increased risk for COVID-19 and may have a more complicated course when infected.
Our patient with MG presented for severe COVID-19 symptoms twice and later developed MC. He received 2 treatment modalities available under an EUA (remdesivir and convalescent plasma) for COVID-19, resulting in symptom resolution and a negative polymerize chain reaction (PCR) test result for the virus. However, after receiving his typical maintenance therapy of IV immunoglobulin (IVIG) for his MG, he again developed symptoms consistent with COVID-19 and tested positive. After recovering from the second episode of COVID-19, the patient went into MC requiring plasmapheresis.
Case Presentation
A 56-year-old male, US Army veteran presented to Carl R. Darnall Army Medical Center emergency department (ED) 6 days after testing positive for COVID-19, with worsening sputum, cough, congestion, dyspnea, and fever. Due to his MG, the patient had a home oxygen monitor and reported that his oxygenation saturation dropped below 90% with minimal exertion. His medical history was significant for MG, status postthymectomy and radiation treatment, left hemidiaphragm paralysis secondary to phrenic nerve injury, and corticosteroid-induced insulin-dependent diabetes mellitus. His current home medications included pyridostigmine 60 mg 3 times a day, mycophenolate (MMF) 1500 mg twice daily, IV immunoglobulin (IVIG) every 3 weeks, insulin aspart up to 16 U per meal, insulin glargine 30 U twice a day, dulaglutide 0.75 mg every week, and metformin 1000 mg twice daily.
On initial examination, the patient’s heart rate (HR) was 111 beats/min, respiratory rate (RR), 22 breaths/min, blood pressure (BP), 138/88 mm Hg, temperature, 100.9 oF, and his initial pulse oximetry, 91% on room air. On physical examination, the patient was tachypneic, though without other signs of respiratory distress. Lung auscultation revealed no adventitial lung sounds. His cardiac examination was notable only for tachycardia. His neurologic examination demonstrated intact cranial nerves, with 5 out of 5 (scale 1 to 5) strength throughout the upper and lower extremities, sensation was intact to light touch, and he had normal cerebellar function. The rest of the examination was normal.
Initial laboratory investigation was notable for a white blood cell count of 14.15x103 cells/mcL with 84% neutrophils, and 6% lymphocytes. Additional tests revealed a C-reactive protein (CRP) level, 17.97 mg/dL (reference range, 0-0.5 mg/dL), ferritin level, 647 ng/mL (reference range, 22-274 ng/mL), d-dimer, 0.64 mcg/mL (reference range, 0-0.47mcg/mL), and a repeated positive COVID-19 PCR test. A portable chest X-ray showed bibasilar opacities (Figure 1).
The patient was diagnosed with COVID-19 and admitted to the intensive care unit (ICU). In the ICU, the patient received 1 U of convalescent plasma (CP) and started on a course of IV remdesivir 100 mg/d consistent with the EUA. He also received a 5-day course of ceftriaxone and azithromycin for possible community acquired pneumonia (CAP). As part of the patient’s MG maintenance medications, he received IVIG 4 g while in the ICU. Throughout his ICU stay, he required supplemental nasal cannula oxygenation to maintain his oxygen saturation > 93%. After 8 days in the ICU, his oxygen requirements decreased, and the patient was transferred out of the ICU and remdesivir was discontinued. On hospital day 10, a repeat COVID-19 PCR test was negative, inflammatory markers returned to within normal limits, and a repeat chest X-ray showed improvement from admission (Figure 2). Having recovered significantly, he was discharged home.
Three weeks later, the patient again presented to the MTF with 3 days of dyspnea, cough, fever, nausea, and vomiting. One day before symptom onset, he had received his maintenance IVIG infusion. The patient reported that his home oxygen saturation was 82% with minimal exertion. On ED presentation his HR was 107 beats/min, RR, 28 breaths/min, temperature, 98.1 oF, BP 118/71 mm Hg, and oxygen saturation, 92% on 2L nasal cannula. His examination was most notable for tachypnea with accessory muscle use. At this time, his neurologic examination was unchanged from prior admission with grossly intact cranial nerves and symmetric 5 of 5 motor strength in all extremities.
At this second ED visit, laboratory results demonstrated a CRP of 3.44 mg/dL, ferritin 2019 ng/mL, d-dimer, 3.39 mcg/mL, and a positive COVID-19 PCR result. His chest X-ray demonstrated new peripheral opacities compared with the X-ray at discharge (Figure 3). He required ICU admission again for his COVID-19 symptoms.
During his ICU course he continued to require supplemental oxygen by nasal cannula, though never required intubation. This second admission, he was again treated empirically for CAP with levofloxacin 750 mg daily for 5 days. He was discharged after 14 days with symptom resolution and down trending of inflammatory markers, though he was not retested for COVID-19.
Four days after his second discharge, he presented to the ED for a third time with diffuse weakness, dysphagia, and dysarthria of 1 day. His HR was 87/beats/min; RR, 17 breaths/min; temperature, 98.7 oF; BP, 144/81 mm Hg; and oxygen saturation, 98% on room air. His examination was significant for slurred speech, bilateral ptosis, 3 of 5 strength in bilateral finger flexion/abduction, wrist extension, knee and ankle flexion/extension; 4 of 5 strength in bilateral proximal muscle testing of deltoid, and hip; normal sensation, cerebellar function and reflexes. His negative inspiratory force (NIF) maximal effort was −30 cmH2O. He was determined to be in MC without evidence of COIVD-19 symptoms, and laboratory results were within normal limits, including a negative COVID-19 PCR. As he received IVIG as maintenance therapy, plasmapheresis was recommended to treat his MC, which required transfer to an outside civilian facility.
At the outside hospital, the patient underwent 5 rounds of plasmapheresis over 10 days. By the third treatment his strength had returned with resolution of the bulbar symptoms and no supplemental oxygen requirements. The patient was discharged and continued his original dosages of MMF and pyridostigmine. At 3 months, he remained asymptomatic from a COVID-19 standpoint and stable from a MG standpoint.
Discussion
Reinfection with the COVID-19 has been continuously debated with alternative explanations suggested for a positive test after a previous negative PCR test in the setting of symptom resolution.1,2 Proposed causes include dynamic PCR results due to prolonged viral shedding and inaccurate or poorly sensitive tests. The repeat positive cases in these scenarios, however, occurred in asymptomatic patients.1,2 COVID-19 shedding averages 20 to 22 days after symptom onset but has been seen up to 36 days after symptom resolution.2,3 This would suggest that fluctuating results during the immediate postsymptom period may be due to variations in viral shedding load and or sampling error—especially in asymptomatic patients. On the other hand, patients who experience return of symptoms days to weeks after previous convalescence leave clinicians wondering whether this represents clinical latency with reactivation or COVID-19 reinfection. A separate case of initial COVID-19 in a patient that had subsequent clinical recovery with a negative PCR developed recurrent respiratory symptoms and had a positive PCR test only 10 days later, further highlighting the reinfection vs reactivation issue of COVID-19.2 Further understanding of this issue may have implications on the extent of natural immunity following primary infection; potential vaccine dosage schedules; and global public health policies.
Although reactivation may be plausible given his immunomodulatory therapy, our patient’s second COVID-19 symptoms started 40 days after the initial symptoms, and 26 days after the initial course resolution; previous cases of return of severe symptoms occurred between 3 and 6 days.1 Given our patient’s time course between resolution and return of symptoms, if latency is the mechanism at play, this case demonstrates an exceptionally longer latency period than the ones that have been reported. Additionally, if latency is an issue in COVID-19, using remdesivir as a treatment further complicates the understanding of this disease.
Remdesivir, a nucleoside analogue antiviral, was shown to benefit recovery in patients with severe symptoms in the Adaptive COVID-19 Treatment Trial-1 study.4 Our patient had originally been placed on a 10-day course; however, on treatment day 8, his symptoms resolved and the remdesivir was discontinued. This is a similar finding to half the patients in the 10-day arm of the study by McCreary and colleagues.5 Although our patient was asymptomatic 4 weeks after the start of remdesivir, consistent with the majority of patients in the McCreary 10-day study arm, further comparison of the presented patient is limited due to study length and follow-up considerations.5 No previous data exist on reactivation, reinfection, or long-term mortality after being treated with remdesivir for COVID-19 infection.
IVIG is being studied in the treatment of COVID-19 and bears consideration as it relates to our patient. There is no evidence that IVIG used in the treatment of autoimmune diseases increases the risk of infection compared with that of other medications used in the treatment of such diseases. Furthermore, the current guidance from the MG expert panel does not suggest that IVIG increases the risk of contracting COVID-19 aside from the risks of exposure to hospital infrastructure.6 Yet the guidance does not discuss the use of IVIG for MG in patients who are already symptomatic from COVID-19 or for patients recovering from the clinical disease or does it discuss a possible compounding risk of thromboembolic events associated with IVIG and COVID-19.6,7 Our patient received his maintenance IVIG during his first admission without any worsening of symptoms or increased oxygen requirements. The day following our patient’s next scheduled IVIG infusion—while asymptomatic—he again developed respiratory symptoms; this could suggest that IVIG did not contribute to his second clinical course nor protect against.
CP is a treatment modality that has been used and studied in previous infectious outbreaks such as the first severe acute respiratory syndrome, and the H1N1 influenza virus.8 Current data on CP for COVID-19 are limited, but early descriptive studies have shown a benefit in improvement of symptoms 5 days sooner in those requiring supplemental oxygen, but no benefit for those requiring mechanical ventilation.9 Like patients that benefitted in these studies, our patient received CP early, 6 days after first testing positive and onset of symptoms. This patient’s reinfection or return of symptoms draws into question the hindrance or even prevention of long-term immunity from administration of CP.
COVID-19 presents many challenges when managing this patient’s coexisting MG, especially as the patient was already being treated with immunosuppressing therapies. The guidance does recommend continuation of standard MG therapies during hospitalizations, including immunosuppression medications such as MMF.6 Immunosuppression is associated with worsened severity of COVID-19 symptoms, although no relation exists to degree of immunosuppression and severity.7,10 To the best of our knowledge there has been no case report of reinfection or reactivation of COVID-19 associated with immunosuppressive agents used in the treatment of MG.
Our patient also was taking pyridostigmine for the treatment of his MG. There is no evidence this medication increases the risk of infection; but the cholinergic activity can increase bronchial secretions, which could theoretically worsen the COVID-19 respiratory symptoms.6,11 During both ICU admissions, our patient continued pyridostigmine use, observing complete return to baseline after discharge. Given the possible association with worsened respiratory outcomes after the second ICU admission, the balance between managing MG symptoms and COVID-19 symptoms needs further examination.
The patient was in MC during his third presentation to the ED. Although respiratory symptoms may be difficult to differentiate from COVID-19, the additional neurologic symptoms seen in this patient allowed for quick determination of the need for MC treatment. There are many potential etiologies contributing to the development of the MC presented here, and it was likely due to multifactorial precipitants. A common cause of MC is viral upper respiratory infections, further challenging the care of these patients during this pandemic.12 Many medications have been cited as causing a MC, 2 of which our patient received during admission for COVID-19: azithromycin and levoquin.12 Although the patient did not receive hydroxychloroquine, which was still being considered as an appropriate COVID-19 treatment at the time, it also is a drug known for precipitating MC and its use scrutinized in patients with MG.12
A key aspect to diagnosing and guiding therapies in myasthenic crisis in addition to the clinical symptoms of acute weakness is respiratory assessment through the nonaerosolizing NIF test.12 Our patient’s NIF measured < 30 cmH2O when in MC, while the reference range is < 75 cmH2O, and for mechanical ventilation is recommended at 20 cmH2O. Although the patient was maintaining O2 saturation > 95%, his NIF value was concerning, and preparations were made in case of precipitous decline. Compounding the NIF assessment in this patient is his history of left phrenic nerve palsy. Without a documented baseline NIF, results were limited in determining his diaphragm strength.13 Treatment for MC includes IVIG or plasmapheresis, since this patient had failed his maintenance therapy IVIG, plasmapheresis was coordinated for definitive therapy.
Conclusions
Federal facilities have seen an increase in the amount of respiratory complaints over the past months. Although COVID-19 is a concerning diagnosis, it is crucial to consider comorbidities in the diagnostic workup of each, even with a previous recent diagnosis of COVID-19. As treatment recommendations for COVID-19 continue to fluctuate coupled with the limitations and difficulties associated with MG patients, so too treatment and evaluation must be carefully considered at each presentation.
1. Gousseff M, Penot P, Gallay L, et al. Clinical recurrences of COVID-19 symptoms after recovery: viral relapse, reinfection or inflammatory rebound? J Infect. 2020;81(5):816-846. doi:10.1016/j.jinf.2020.06.073
2. Duggan NM, Ludy SM, Shannon BC, Reisner AT, Wilcox SR. Is novel coronavirus 2019 reinfection possible? Interpreting dynamic SARS-CoV-2 test results. Am J Emerg Med. 2021;39:256.e1-256.e3. doi:10.1016/j.ajem.2020.06.079
3. Li J, Zhang L, Liu B, Song D. Case report: viral shedding for 60 days in a woman with COVID-19. Am J Trop Med Hyg. 2020;102(6):1210-1213. doi:10.4269/ajtmh.20-0275
4. Beigel JH, Tomashek KM, Dodd LE. Remdesivir for the treatment of Covid-19 - preliminary report. Reply. N Engl J Med. 2020;383(10):994. doi:10.1056/NEJMc2022236
5. McCreary EK, Angus DC. Efficacy of remdesivir in COVID-19. JAMA. 2020;324(11):1041-1042. doi:10.1001/jama.2020.16337
6. International MG/COVID-19 Working Group; Jacob S, Muppidi S, Gordon A, et al. Guidance for the management of myasthenia gravis (MG) and Lambert-Eaton myasthenic syndrome (LEMS) during the COVID-19 pandemic. J Neurol Sci. 2020;412:116803. doi:10.1016/j.jns.2020.116803
7. Anand P, Slama MCC, Kaku M, et al. COVID-19 in patients with myasthenia gravis. Muscle Nerve. 2020;62(2):254-258. doi:10.1002/mus.26918
8. Wooding DJ, Bach H. Treatment of COVID-19 with convalescent plasma: lessons from past coronavirus outbreaks. Clin Microbiol Infect. 2020;26(10):1436-1446. doi:10.1016/j.cmi.2020.08.005
9. Salazar E, Perez KK, Ashraf M, et al. Treatment of coronavirus disease 2019 (covid-19) patients with convalescent plasma. Am J Pathol. 2020;190(8):1680-1690. doi:10.1016/j.ajpath.2020.05.014
10. Ryan C, Minc A, Caceres J, et al. Predicting severe outcomes in Covid-19 related illness using only patient demographics, comorbidities and symptoms [published online ahead of print, 2020 Sep 9]. Am J Emerg Med. 2020;S0735-6757(20)30809-3. doi:10.1016/j.ajem.2020.09.017
11. Singh S, Govindarajan R. COVID-19 and generalized myasthenia gravis exacerbation: a case report. Clin Neurol Neurosurg. 2020;196:106045. doi:10.1016/j.clineuro.2020.106045
12. Wendell LC, Levine JM. Myasthenic crisis. Neurohospitalist. 2011;1(1):16-22. doi:10.1177/1941875210382918
13. Dubé BP, Dres M. Diaphragm dysfunction: diagnostic approaches and management strategies. J Clin Med. 2016;5(12):113. Published 2016 Dec 5. doi:10.3390/jcm5120113
A patient with myasthenia gravis who survived 2 COVID-19 infections required plasmapheresis to recover from an acute crisis.
A patient with myasthenia gravis who survived 2 COVID-19 infections required plasmapheresis to recover from an acute crisis.
COVID-19 is still in the early stages of understanding, although it is known to be complicated by individual patient comorbidities. The management and treatment of COVID-19 continues to quickly evolve as more is discovered regarding the virus. Multiple treatments have been preliminarily tested and used under a Food and Drug Administration emergency use authorization (EUA) determination. The long-term success of these therapies, however, is yet to be determined. Additionally, if a patient has a second clinical presentation for COVID-19, it is not known whether this represents latency with subsequent reactivation from the previous infection or a second de novo infection. The uncertainty calls into question the duration of immunity, if any, following a primary infection.
COVID-19 management becomes more complicated when patients have complex medical conditions, such as myasthenia gravis (MG). This autoimmune neuromuscular disorder can present with varying weakness, and many patients are on immunomodulator medications. The weakness can worsen into a myasthenic crisis (MC), resulting in profound weakness of the respiratory muscles. Therefore, patients with MG are at increased risk for COVID-19 and may have a more complicated course when infected.
Our patient with MG presented for severe COVID-19 symptoms twice and later developed MC. He received 2 treatment modalities available under an EUA (remdesivir and convalescent plasma) for COVID-19, resulting in symptom resolution and a negative polymerize chain reaction (PCR) test result for the virus. However, after receiving his typical maintenance therapy of IV immunoglobulin (IVIG) for his MG, he again developed symptoms consistent with COVID-19 and tested positive. After recovering from the second episode of COVID-19, the patient went into MC requiring plasmapheresis.
Case Presentation
A 56-year-old male, US Army veteran presented to Carl R. Darnall Army Medical Center emergency department (ED) 6 days after testing positive for COVID-19, with worsening sputum, cough, congestion, dyspnea, and fever. Due to his MG, the patient had a home oxygen monitor and reported that his oxygenation saturation dropped below 90% with minimal exertion. His medical history was significant for MG, status postthymectomy and radiation treatment, left hemidiaphragm paralysis secondary to phrenic nerve injury, and corticosteroid-induced insulin-dependent diabetes mellitus. His current home medications included pyridostigmine 60 mg 3 times a day, mycophenolate (MMF) 1500 mg twice daily, IV immunoglobulin (IVIG) every 3 weeks, insulin aspart up to 16 U per meal, insulin glargine 30 U twice a day, dulaglutide 0.75 mg every week, and metformin 1000 mg twice daily.
On initial examination, the patient’s heart rate (HR) was 111 beats/min, respiratory rate (RR), 22 breaths/min, blood pressure (BP), 138/88 mm Hg, temperature, 100.9 oF, and his initial pulse oximetry, 91% on room air. On physical examination, the patient was tachypneic, though without other signs of respiratory distress. Lung auscultation revealed no adventitial lung sounds. His cardiac examination was notable only for tachycardia. His neurologic examination demonstrated intact cranial nerves, with 5 out of 5 (scale 1 to 5) strength throughout the upper and lower extremities, sensation was intact to light touch, and he had normal cerebellar function. The rest of the examination was normal.
Initial laboratory investigation was notable for a white blood cell count of 14.15x103 cells/mcL with 84% neutrophils, and 6% lymphocytes. Additional tests revealed a C-reactive protein (CRP) level, 17.97 mg/dL (reference range, 0-0.5 mg/dL), ferritin level, 647 ng/mL (reference range, 22-274 ng/mL), d-dimer, 0.64 mcg/mL (reference range, 0-0.47mcg/mL), and a repeated positive COVID-19 PCR test. A portable chest X-ray showed bibasilar opacities (Figure 1).
The patient was diagnosed with COVID-19 and admitted to the intensive care unit (ICU). In the ICU, the patient received 1 U of convalescent plasma (CP) and started on a course of IV remdesivir 100 mg/d consistent with the EUA. He also received a 5-day course of ceftriaxone and azithromycin for possible community acquired pneumonia (CAP). As part of the patient’s MG maintenance medications, he received IVIG 4 g while in the ICU. Throughout his ICU stay, he required supplemental nasal cannula oxygenation to maintain his oxygen saturation > 93%. After 8 days in the ICU, his oxygen requirements decreased, and the patient was transferred out of the ICU and remdesivir was discontinued. On hospital day 10, a repeat COVID-19 PCR test was negative, inflammatory markers returned to within normal limits, and a repeat chest X-ray showed improvement from admission (Figure 2). Having recovered significantly, he was discharged home.
Three weeks later, the patient again presented to the MTF with 3 days of dyspnea, cough, fever, nausea, and vomiting. One day before symptom onset, he had received his maintenance IVIG infusion. The patient reported that his home oxygen saturation was 82% with minimal exertion. On ED presentation his HR was 107 beats/min, RR, 28 breaths/min, temperature, 98.1 oF, BP 118/71 mm Hg, and oxygen saturation, 92% on 2L nasal cannula. His examination was most notable for tachypnea with accessory muscle use. At this time, his neurologic examination was unchanged from prior admission with grossly intact cranial nerves and symmetric 5 of 5 motor strength in all extremities.
At this second ED visit, laboratory results demonstrated a CRP of 3.44 mg/dL, ferritin 2019 ng/mL, d-dimer, 3.39 mcg/mL, and a positive COVID-19 PCR result. His chest X-ray demonstrated new peripheral opacities compared with the X-ray at discharge (Figure 3). He required ICU admission again for his COVID-19 symptoms.
During his ICU course he continued to require supplemental oxygen by nasal cannula, though never required intubation. This second admission, he was again treated empirically for CAP with levofloxacin 750 mg daily for 5 days. He was discharged after 14 days with symptom resolution and down trending of inflammatory markers, though he was not retested for COVID-19.
Four days after his second discharge, he presented to the ED for a third time with diffuse weakness, dysphagia, and dysarthria of 1 day. His HR was 87/beats/min; RR, 17 breaths/min; temperature, 98.7 oF; BP, 144/81 mm Hg; and oxygen saturation, 98% on room air. His examination was significant for slurred speech, bilateral ptosis, 3 of 5 strength in bilateral finger flexion/abduction, wrist extension, knee and ankle flexion/extension; 4 of 5 strength in bilateral proximal muscle testing of deltoid, and hip; normal sensation, cerebellar function and reflexes. His negative inspiratory force (NIF) maximal effort was −30 cmH2O. He was determined to be in MC without evidence of COIVD-19 symptoms, and laboratory results were within normal limits, including a negative COVID-19 PCR. As he received IVIG as maintenance therapy, plasmapheresis was recommended to treat his MC, which required transfer to an outside civilian facility.
At the outside hospital, the patient underwent 5 rounds of plasmapheresis over 10 days. By the third treatment his strength had returned with resolution of the bulbar symptoms and no supplemental oxygen requirements. The patient was discharged and continued his original dosages of MMF and pyridostigmine. At 3 months, he remained asymptomatic from a COVID-19 standpoint and stable from a MG standpoint.
Discussion
Reinfection with the COVID-19 has been continuously debated with alternative explanations suggested for a positive test after a previous negative PCR test in the setting of symptom resolution.1,2 Proposed causes include dynamic PCR results due to prolonged viral shedding and inaccurate or poorly sensitive tests. The repeat positive cases in these scenarios, however, occurred in asymptomatic patients.1,2 COVID-19 shedding averages 20 to 22 days after symptom onset but has been seen up to 36 days after symptom resolution.2,3 This would suggest that fluctuating results during the immediate postsymptom period may be due to variations in viral shedding load and or sampling error—especially in asymptomatic patients. On the other hand, patients who experience return of symptoms days to weeks after previous convalescence leave clinicians wondering whether this represents clinical latency with reactivation or COVID-19 reinfection. A separate case of initial COVID-19 in a patient that had subsequent clinical recovery with a negative PCR developed recurrent respiratory symptoms and had a positive PCR test only 10 days later, further highlighting the reinfection vs reactivation issue of COVID-19.2 Further understanding of this issue may have implications on the extent of natural immunity following primary infection; potential vaccine dosage schedules; and global public health policies.
Although reactivation may be plausible given his immunomodulatory therapy, our patient’s second COVID-19 symptoms started 40 days after the initial symptoms, and 26 days after the initial course resolution; previous cases of return of severe symptoms occurred between 3 and 6 days.1 Given our patient’s time course between resolution and return of symptoms, if latency is the mechanism at play, this case demonstrates an exceptionally longer latency period than the ones that have been reported. Additionally, if latency is an issue in COVID-19, using remdesivir as a treatment further complicates the understanding of this disease.
Remdesivir, a nucleoside analogue antiviral, was shown to benefit recovery in patients with severe symptoms in the Adaptive COVID-19 Treatment Trial-1 study.4 Our patient had originally been placed on a 10-day course; however, on treatment day 8, his symptoms resolved and the remdesivir was discontinued. This is a similar finding to half the patients in the 10-day arm of the study by McCreary and colleagues.5 Although our patient was asymptomatic 4 weeks after the start of remdesivir, consistent with the majority of patients in the McCreary 10-day study arm, further comparison of the presented patient is limited due to study length and follow-up considerations.5 No previous data exist on reactivation, reinfection, or long-term mortality after being treated with remdesivir for COVID-19 infection.
IVIG is being studied in the treatment of COVID-19 and bears consideration as it relates to our patient. There is no evidence that IVIG used in the treatment of autoimmune diseases increases the risk of infection compared with that of other medications used in the treatment of such diseases. Furthermore, the current guidance from the MG expert panel does not suggest that IVIG increases the risk of contracting COVID-19 aside from the risks of exposure to hospital infrastructure.6 Yet the guidance does not discuss the use of IVIG for MG in patients who are already symptomatic from COVID-19 or for patients recovering from the clinical disease or does it discuss a possible compounding risk of thromboembolic events associated with IVIG and COVID-19.6,7 Our patient received his maintenance IVIG during his first admission without any worsening of symptoms or increased oxygen requirements. The day following our patient’s next scheduled IVIG infusion—while asymptomatic—he again developed respiratory symptoms; this could suggest that IVIG did not contribute to his second clinical course nor protect against.
CP is a treatment modality that has been used and studied in previous infectious outbreaks such as the first severe acute respiratory syndrome, and the H1N1 influenza virus.8 Current data on CP for COVID-19 are limited, but early descriptive studies have shown a benefit in improvement of symptoms 5 days sooner in those requiring supplemental oxygen, but no benefit for those requiring mechanical ventilation.9 Like patients that benefitted in these studies, our patient received CP early, 6 days after first testing positive and onset of symptoms. This patient’s reinfection or return of symptoms draws into question the hindrance or even prevention of long-term immunity from administration of CP.
COVID-19 presents many challenges when managing this patient’s coexisting MG, especially as the patient was already being treated with immunosuppressing therapies. The guidance does recommend continuation of standard MG therapies during hospitalizations, including immunosuppression medications such as MMF.6 Immunosuppression is associated with worsened severity of COVID-19 symptoms, although no relation exists to degree of immunosuppression and severity.7,10 To the best of our knowledge there has been no case report of reinfection or reactivation of COVID-19 associated with immunosuppressive agents used in the treatment of MG.
Our patient also was taking pyridostigmine for the treatment of his MG. There is no evidence this medication increases the risk of infection; but the cholinergic activity can increase bronchial secretions, which could theoretically worsen the COVID-19 respiratory symptoms.6,11 During both ICU admissions, our patient continued pyridostigmine use, observing complete return to baseline after discharge. Given the possible association with worsened respiratory outcomes after the second ICU admission, the balance between managing MG symptoms and COVID-19 symptoms needs further examination.
The patient was in MC during his third presentation to the ED. Although respiratory symptoms may be difficult to differentiate from COVID-19, the additional neurologic symptoms seen in this patient allowed for quick determination of the need for MC treatment. There are many potential etiologies contributing to the development of the MC presented here, and it was likely due to multifactorial precipitants. A common cause of MC is viral upper respiratory infections, further challenging the care of these patients during this pandemic.12 Many medications have been cited as causing a MC, 2 of which our patient received during admission for COVID-19: azithromycin and levoquin.12 Although the patient did not receive hydroxychloroquine, which was still being considered as an appropriate COVID-19 treatment at the time, it also is a drug known for precipitating MC and its use scrutinized in patients with MG.12
A key aspect to diagnosing and guiding therapies in myasthenic crisis in addition to the clinical symptoms of acute weakness is respiratory assessment through the nonaerosolizing NIF test.12 Our patient’s NIF measured < 30 cmH2O when in MC, while the reference range is < 75 cmH2O, and for mechanical ventilation is recommended at 20 cmH2O. Although the patient was maintaining O2 saturation > 95%, his NIF value was concerning, and preparations were made in case of precipitous decline. Compounding the NIF assessment in this patient is his history of left phrenic nerve palsy. Without a documented baseline NIF, results were limited in determining his diaphragm strength.13 Treatment for MC includes IVIG or plasmapheresis, since this patient had failed his maintenance therapy IVIG, plasmapheresis was coordinated for definitive therapy.
Conclusions
Federal facilities have seen an increase in the amount of respiratory complaints over the past months. Although COVID-19 is a concerning diagnosis, it is crucial to consider comorbidities in the diagnostic workup of each, even with a previous recent diagnosis of COVID-19. As treatment recommendations for COVID-19 continue to fluctuate coupled with the limitations and difficulties associated with MG patients, so too treatment and evaluation must be carefully considered at each presentation.
COVID-19 is still in the early stages of understanding, although it is known to be complicated by individual patient comorbidities. The management and treatment of COVID-19 continues to quickly evolve as more is discovered regarding the virus. Multiple treatments have been preliminarily tested and used under a Food and Drug Administration emergency use authorization (EUA) determination. The long-term success of these therapies, however, is yet to be determined. Additionally, if a patient has a second clinical presentation for COVID-19, it is not known whether this represents latency with subsequent reactivation from the previous infection or a second de novo infection. The uncertainty calls into question the duration of immunity, if any, following a primary infection.
COVID-19 management becomes more complicated when patients have complex medical conditions, such as myasthenia gravis (MG). This autoimmune neuromuscular disorder can present with varying weakness, and many patients are on immunomodulator medications. The weakness can worsen into a myasthenic crisis (MC), resulting in profound weakness of the respiratory muscles. Therefore, patients with MG are at increased risk for COVID-19 and may have a more complicated course when infected.
Our patient with MG presented for severe COVID-19 symptoms twice and later developed MC. He received 2 treatment modalities available under an EUA (remdesivir and convalescent plasma) for COVID-19, resulting in symptom resolution and a negative polymerize chain reaction (PCR) test result for the virus. However, after receiving his typical maintenance therapy of IV immunoglobulin (IVIG) for his MG, he again developed symptoms consistent with COVID-19 and tested positive. After recovering from the second episode of COVID-19, the patient went into MC requiring plasmapheresis.
Case Presentation
A 56-year-old male, US Army veteran presented to Carl R. Darnall Army Medical Center emergency department (ED) 6 days after testing positive for COVID-19, with worsening sputum, cough, congestion, dyspnea, and fever. Due to his MG, the patient had a home oxygen monitor and reported that his oxygenation saturation dropped below 90% with minimal exertion. His medical history was significant for MG, status postthymectomy and radiation treatment, left hemidiaphragm paralysis secondary to phrenic nerve injury, and corticosteroid-induced insulin-dependent diabetes mellitus. His current home medications included pyridostigmine 60 mg 3 times a day, mycophenolate (MMF) 1500 mg twice daily, IV immunoglobulin (IVIG) every 3 weeks, insulin aspart up to 16 U per meal, insulin glargine 30 U twice a day, dulaglutide 0.75 mg every week, and metformin 1000 mg twice daily.
On initial examination, the patient’s heart rate (HR) was 111 beats/min, respiratory rate (RR), 22 breaths/min, blood pressure (BP), 138/88 mm Hg, temperature, 100.9 oF, and his initial pulse oximetry, 91% on room air. On physical examination, the patient was tachypneic, though without other signs of respiratory distress. Lung auscultation revealed no adventitial lung sounds. His cardiac examination was notable only for tachycardia. His neurologic examination demonstrated intact cranial nerves, with 5 out of 5 (scale 1 to 5) strength throughout the upper and lower extremities, sensation was intact to light touch, and he had normal cerebellar function. The rest of the examination was normal.
Initial laboratory investigation was notable for a white blood cell count of 14.15x103 cells/mcL with 84% neutrophils, and 6% lymphocytes. Additional tests revealed a C-reactive protein (CRP) level, 17.97 mg/dL (reference range, 0-0.5 mg/dL), ferritin level, 647 ng/mL (reference range, 22-274 ng/mL), d-dimer, 0.64 mcg/mL (reference range, 0-0.47mcg/mL), and a repeated positive COVID-19 PCR test. A portable chest X-ray showed bibasilar opacities (Figure 1).
The patient was diagnosed with COVID-19 and admitted to the intensive care unit (ICU). In the ICU, the patient received 1 U of convalescent plasma (CP) and started on a course of IV remdesivir 100 mg/d consistent with the EUA. He also received a 5-day course of ceftriaxone and azithromycin for possible community acquired pneumonia (CAP). As part of the patient’s MG maintenance medications, he received IVIG 4 g while in the ICU. Throughout his ICU stay, he required supplemental nasal cannula oxygenation to maintain his oxygen saturation > 93%. After 8 days in the ICU, his oxygen requirements decreased, and the patient was transferred out of the ICU and remdesivir was discontinued. On hospital day 10, a repeat COVID-19 PCR test was negative, inflammatory markers returned to within normal limits, and a repeat chest X-ray showed improvement from admission (Figure 2). Having recovered significantly, he was discharged home.
Three weeks later, the patient again presented to the MTF with 3 days of dyspnea, cough, fever, nausea, and vomiting. One day before symptom onset, he had received his maintenance IVIG infusion. The patient reported that his home oxygen saturation was 82% with minimal exertion. On ED presentation his HR was 107 beats/min, RR, 28 breaths/min, temperature, 98.1 oF, BP 118/71 mm Hg, and oxygen saturation, 92% on 2L nasal cannula. His examination was most notable for tachypnea with accessory muscle use. At this time, his neurologic examination was unchanged from prior admission with grossly intact cranial nerves and symmetric 5 of 5 motor strength in all extremities.
At this second ED visit, laboratory results demonstrated a CRP of 3.44 mg/dL, ferritin 2019 ng/mL, d-dimer, 3.39 mcg/mL, and a positive COVID-19 PCR result. His chest X-ray demonstrated new peripheral opacities compared with the X-ray at discharge (Figure 3). He required ICU admission again for his COVID-19 symptoms.
During his ICU course he continued to require supplemental oxygen by nasal cannula, though never required intubation. This second admission, he was again treated empirically for CAP with levofloxacin 750 mg daily for 5 days. He was discharged after 14 days with symptom resolution and down trending of inflammatory markers, though he was not retested for COVID-19.
Four days after his second discharge, he presented to the ED for a third time with diffuse weakness, dysphagia, and dysarthria of 1 day. His HR was 87/beats/min; RR, 17 breaths/min; temperature, 98.7 oF; BP, 144/81 mm Hg; and oxygen saturation, 98% on room air. His examination was significant for slurred speech, bilateral ptosis, 3 of 5 strength in bilateral finger flexion/abduction, wrist extension, knee and ankle flexion/extension; 4 of 5 strength in bilateral proximal muscle testing of deltoid, and hip; normal sensation, cerebellar function and reflexes. His negative inspiratory force (NIF) maximal effort was −30 cmH2O. He was determined to be in MC without evidence of COIVD-19 symptoms, and laboratory results were within normal limits, including a negative COVID-19 PCR. As he received IVIG as maintenance therapy, plasmapheresis was recommended to treat his MC, which required transfer to an outside civilian facility.
At the outside hospital, the patient underwent 5 rounds of plasmapheresis over 10 days. By the third treatment his strength had returned with resolution of the bulbar symptoms and no supplemental oxygen requirements. The patient was discharged and continued his original dosages of MMF and pyridostigmine. At 3 months, he remained asymptomatic from a COVID-19 standpoint and stable from a MG standpoint.
Discussion
Reinfection with the COVID-19 has been continuously debated with alternative explanations suggested for a positive test after a previous negative PCR test in the setting of symptom resolution.1,2 Proposed causes include dynamic PCR results due to prolonged viral shedding and inaccurate or poorly sensitive tests. The repeat positive cases in these scenarios, however, occurred in asymptomatic patients.1,2 COVID-19 shedding averages 20 to 22 days after symptom onset but has been seen up to 36 days after symptom resolution.2,3 This would suggest that fluctuating results during the immediate postsymptom period may be due to variations in viral shedding load and or sampling error—especially in asymptomatic patients. On the other hand, patients who experience return of symptoms days to weeks after previous convalescence leave clinicians wondering whether this represents clinical latency with reactivation or COVID-19 reinfection. A separate case of initial COVID-19 in a patient that had subsequent clinical recovery with a negative PCR developed recurrent respiratory symptoms and had a positive PCR test only 10 days later, further highlighting the reinfection vs reactivation issue of COVID-19.2 Further understanding of this issue may have implications on the extent of natural immunity following primary infection; potential vaccine dosage schedules; and global public health policies.
Although reactivation may be plausible given his immunomodulatory therapy, our patient’s second COVID-19 symptoms started 40 days after the initial symptoms, and 26 days after the initial course resolution; previous cases of return of severe symptoms occurred between 3 and 6 days.1 Given our patient’s time course between resolution and return of symptoms, if latency is the mechanism at play, this case demonstrates an exceptionally longer latency period than the ones that have been reported. Additionally, if latency is an issue in COVID-19, using remdesivir as a treatment further complicates the understanding of this disease.
Remdesivir, a nucleoside analogue antiviral, was shown to benefit recovery in patients with severe symptoms in the Adaptive COVID-19 Treatment Trial-1 study.4 Our patient had originally been placed on a 10-day course; however, on treatment day 8, his symptoms resolved and the remdesivir was discontinued. This is a similar finding to half the patients in the 10-day arm of the study by McCreary and colleagues.5 Although our patient was asymptomatic 4 weeks after the start of remdesivir, consistent with the majority of patients in the McCreary 10-day study arm, further comparison of the presented patient is limited due to study length and follow-up considerations.5 No previous data exist on reactivation, reinfection, or long-term mortality after being treated with remdesivir for COVID-19 infection.
IVIG is being studied in the treatment of COVID-19 and bears consideration as it relates to our patient. There is no evidence that IVIG used in the treatment of autoimmune diseases increases the risk of infection compared with that of other medications used in the treatment of such diseases. Furthermore, the current guidance from the MG expert panel does not suggest that IVIG increases the risk of contracting COVID-19 aside from the risks of exposure to hospital infrastructure.6 Yet the guidance does not discuss the use of IVIG for MG in patients who are already symptomatic from COVID-19 or for patients recovering from the clinical disease or does it discuss a possible compounding risk of thromboembolic events associated with IVIG and COVID-19.6,7 Our patient received his maintenance IVIG during his first admission without any worsening of symptoms or increased oxygen requirements. The day following our patient’s next scheduled IVIG infusion—while asymptomatic—he again developed respiratory symptoms; this could suggest that IVIG did not contribute to his second clinical course nor protect against.
CP is a treatment modality that has been used and studied in previous infectious outbreaks such as the first severe acute respiratory syndrome, and the H1N1 influenza virus.8 Current data on CP for COVID-19 are limited, but early descriptive studies have shown a benefit in improvement of symptoms 5 days sooner in those requiring supplemental oxygen, but no benefit for those requiring mechanical ventilation.9 Like patients that benefitted in these studies, our patient received CP early, 6 days after first testing positive and onset of symptoms. This patient’s reinfection or return of symptoms draws into question the hindrance or even prevention of long-term immunity from administration of CP.
COVID-19 presents many challenges when managing this patient’s coexisting MG, especially as the patient was already being treated with immunosuppressing therapies. The guidance does recommend continuation of standard MG therapies during hospitalizations, including immunosuppression medications such as MMF.6 Immunosuppression is associated with worsened severity of COVID-19 symptoms, although no relation exists to degree of immunosuppression and severity.7,10 To the best of our knowledge there has been no case report of reinfection or reactivation of COVID-19 associated with immunosuppressive agents used in the treatment of MG.
Our patient also was taking pyridostigmine for the treatment of his MG. There is no evidence this medication increases the risk of infection; but the cholinergic activity can increase bronchial secretions, which could theoretically worsen the COVID-19 respiratory symptoms.6,11 During both ICU admissions, our patient continued pyridostigmine use, observing complete return to baseline after discharge. Given the possible association with worsened respiratory outcomes after the second ICU admission, the balance between managing MG symptoms and COVID-19 symptoms needs further examination.
The patient was in MC during his third presentation to the ED. Although respiratory symptoms may be difficult to differentiate from COVID-19, the additional neurologic symptoms seen in this patient allowed for quick determination of the need for MC treatment. There are many potential etiologies contributing to the development of the MC presented here, and it was likely due to multifactorial precipitants. A common cause of MC is viral upper respiratory infections, further challenging the care of these patients during this pandemic.12 Many medications have been cited as causing a MC, 2 of which our patient received during admission for COVID-19: azithromycin and levoquin.12 Although the patient did not receive hydroxychloroquine, which was still being considered as an appropriate COVID-19 treatment at the time, it also is a drug known for precipitating MC and its use scrutinized in patients with MG.12
A key aspect to diagnosing and guiding therapies in myasthenic crisis in addition to the clinical symptoms of acute weakness is respiratory assessment through the nonaerosolizing NIF test.12 Our patient’s NIF measured < 30 cmH2O when in MC, while the reference range is < 75 cmH2O, and for mechanical ventilation is recommended at 20 cmH2O. Although the patient was maintaining O2 saturation > 95%, his NIF value was concerning, and preparations were made in case of precipitous decline. Compounding the NIF assessment in this patient is his history of left phrenic nerve palsy. Without a documented baseline NIF, results were limited in determining his diaphragm strength.13 Treatment for MC includes IVIG or plasmapheresis, since this patient had failed his maintenance therapy IVIG, plasmapheresis was coordinated for definitive therapy.
Conclusions
Federal facilities have seen an increase in the amount of respiratory complaints over the past months. Although COVID-19 is a concerning diagnosis, it is crucial to consider comorbidities in the diagnostic workup of each, even with a previous recent diagnosis of COVID-19. As treatment recommendations for COVID-19 continue to fluctuate coupled with the limitations and difficulties associated with MG patients, so too treatment and evaluation must be carefully considered at each presentation.
1. Gousseff M, Penot P, Gallay L, et al. Clinical recurrences of COVID-19 symptoms after recovery: viral relapse, reinfection or inflammatory rebound? J Infect. 2020;81(5):816-846. doi:10.1016/j.jinf.2020.06.073
2. Duggan NM, Ludy SM, Shannon BC, Reisner AT, Wilcox SR. Is novel coronavirus 2019 reinfection possible? Interpreting dynamic SARS-CoV-2 test results. Am J Emerg Med. 2021;39:256.e1-256.e3. doi:10.1016/j.ajem.2020.06.079
3. Li J, Zhang L, Liu B, Song D. Case report: viral shedding for 60 days in a woman with COVID-19. Am J Trop Med Hyg. 2020;102(6):1210-1213. doi:10.4269/ajtmh.20-0275
4. Beigel JH, Tomashek KM, Dodd LE. Remdesivir for the treatment of Covid-19 - preliminary report. Reply. N Engl J Med. 2020;383(10):994. doi:10.1056/NEJMc2022236
5. McCreary EK, Angus DC. Efficacy of remdesivir in COVID-19. JAMA. 2020;324(11):1041-1042. doi:10.1001/jama.2020.16337
6. International MG/COVID-19 Working Group; Jacob S, Muppidi S, Gordon A, et al. Guidance for the management of myasthenia gravis (MG) and Lambert-Eaton myasthenic syndrome (LEMS) during the COVID-19 pandemic. J Neurol Sci. 2020;412:116803. doi:10.1016/j.jns.2020.116803
7. Anand P, Slama MCC, Kaku M, et al. COVID-19 in patients with myasthenia gravis. Muscle Nerve. 2020;62(2):254-258. doi:10.1002/mus.26918
8. Wooding DJ, Bach H. Treatment of COVID-19 with convalescent plasma: lessons from past coronavirus outbreaks. Clin Microbiol Infect. 2020;26(10):1436-1446. doi:10.1016/j.cmi.2020.08.005
9. Salazar E, Perez KK, Ashraf M, et al. Treatment of coronavirus disease 2019 (covid-19) patients with convalescent plasma. Am J Pathol. 2020;190(8):1680-1690. doi:10.1016/j.ajpath.2020.05.014
10. Ryan C, Minc A, Caceres J, et al. Predicting severe outcomes in Covid-19 related illness using only patient demographics, comorbidities and symptoms [published online ahead of print, 2020 Sep 9]. Am J Emerg Med. 2020;S0735-6757(20)30809-3. doi:10.1016/j.ajem.2020.09.017
11. Singh S, Govindarajan R. COVID-19 and generalized myasthenia gravis exacerbation: a case report. Clin Neurol Neurosurg. 2020;196:106045. doi:10.1016/j.clineuro.2020.106045
12. Wendell LC, Levine JM. Myasthenic crisis. Neurohospitalist. 2011;1(1):16-22. doi:10.1177/1941875210382918
13. Dubé BP, Dres M. Diaphragm dysfunction: diagnostic approaches and management strategies. J Clin Med. 2016;5(12):113. Published 2016 Dec 5. doi:10.3390/jcm5120113
1. Gousseff M, Penot P, Gallay L, et al. Clinical recurrences of COVID-19 symptoms after recovery: viral relapse, reinfection or inflammatory rebound? J Infect. 2020;81(5):816-846. doi:10.1016/j.jinf.2020.06.073
2. Duggan NM, Ludy SM, Shannon BC, Reisner AT, Wilcox SR. Is novel coronavirus 2019 reinfection possible? Interpreting dynamic SARS-CoV-2 test results. Am J Emerg Med. 2021;39:256.e1-256.e3. doi:10.1016/j.ajem.2020.06.079
3. Li J, Zhang L, Liu B, Song D. Case report: viral shedding for 60 days in a woman with COVID-19. Am J Trop Med Hyg. 2020;102(6):1210-1213. doi:10.4269/ajtmh.20-0275
4. Beigel JH, Tomashek KM, Dodd LE. Remdesivir for the treatment of Covid-19 - preliminary report. Reply. N Engl J Med. 2020;383(10):994. doi:10.1056/NEJMc2022236
5. McCreary EK, Angus DC. Efficacy of remdesivir in COVID-19. JAMA. 2020;324(11):1041-1042. doi:10.1001/jama.2020.16337
6. International MG/COVID-19 Working Group; Jacob S, Muppidi S, Gordon A, et al. Guidance for the management of myasthenia gravis (MG) and Lambert-Eaton myasthenic syndrome (LEMS) during the COVID-19 pandemic. J Neurol Sci. 2020;412:116803. doi:10.1016/j.jns.2020.116803
7. Anand P, Slama MCC, Kaku M, et al. COVID-19 in patients with myasthenia gravis. Muscle Nerve. 2020;62(2):254-258. doi:10.1002/mus.26918
8. Wooding DJ, Bach H. Treatment of COVID-19 with convalescent plasma: lessons from past coronavirus outbreaks. Clin Microbiol Infect. 2020;26(10):1436-1446. doi:10.1016/j.cmi.2020.08.005
9. Salazar E, Perez KK, Ashraf M, et al. Treatment of coronavirus disease 2019 (covid-19) patients with convalescent plasma. Am J Pathol. 2020;190(8):1680-1690. doi:10.1016/j.ajpath.2020.05.014
10. Ryan C, Minc A, Caceres J, et al. Predicting severe outcomes in Covid-19 related illness using only patient demographics, comorbidities and symptoms [published online ahead of print, 2020 Sep 9]. Am J Emerg Med. 2020;S0735-6757(20)30809-3. doi:10.1016/j.ajem.2020.09.017
11. Singh S, Govindarajan R. COVID-19 and generalized myasthenia gravis exacerbation: a case report. Clin Neurol Neurosurg. 2020;196:106045. doi:10.1016/j.clineuro.2020.106045
12. Wendell LC, Levine JM. Myasthenic crisis. Neurohospitalist. 2011;1(1):16-22. doi:10.1177/1941875210382918
13. Dubé BP, Dres M. Diaphragm dysfunction: diagnostic approaches and management strategies. J Clin Med. 2016;5(12):113. Published 2016 Dec 5. doi:10.3390/jcm5120113
Fauci says ‘unprecedented’ conditions could influence COVID vaccine approval for kids
“From a public health standpoint, I think we have an evolving situation,” said Anthony S. Fauci, MD, director of the National Institute of Allergy and Infectious Diseases, in a moderated session with Lee Beers, MD, president of the American Academy of Pediatrics, at the virtual Pediatric Hospital Medicine annual conference.
The reasons for this shift remain unclear, he said.
Dr. Beers emphasized the ability of pediatric hospitalists to be flexible in the face of uncertainty and the evolving virus, and asked Dr. Fauci to elaborate on the unique traits of the delta variant that make it especially challenging.
“There is no doubt that delta transmits much more efficiently than the alpha variant or any other variant,” Dr. Fauci said. The transmissibility is evident in comparisons of the level of virus in the nasopharynx of the delta variant, compared with the original alpha COVID-19 virus – delta is as much as 1,000 times higher, he explained.
In addition, the level of virus in the nasopharynx of vaccinated individuals who develop breakthrough infections with the delta variant is similar to the levels in unvaccinated individuals who are infected with the delta variant.
The delta variant is “the tough guy on the block” at the moment, Dr. Fauci said.
Dr. Fauci also responded to a question on the lack of winter viruses, such as RSV and the flu, last winter, but the surge in these viruses over the summer.
This winter’s activity remains uncertain, Dr. Fauci said. However, he speculated “with a strong dose of humility and modesty” that viruses tend to have niches, some are seasonal, and the winter viruses that were displaced by COVID-19 hit harder in the summer instead. “If I were a [non-COVID] virus looking for a niche, I would be really confused,” he said. “I don’t know what will happen this winter, but if we get good control over COVID-19 by winter, we could have a very vengeful influenza season,” he said. “This is speculation, I don’t have any data for this,” he cautioned.
Dr. Beers raised the issue of back-to-school safety, and the updated AAP guidance for universal masking for K-12 students. “Our guidance about return to school gets updated as the situation changes and we gain a better understanding of how kids can get to school safely,” she said. A combination of factors affect back-to-school guidance, including the ineligibility of children younger than 12 years to be vaccinated, the number of adolescents who are eligible but have not been vaccinated, and the challenge for educators to navigate which children should wear masks, Dr. Beers said.
“We want to get vaccines for our youngest kids as soon as safely possible,” Dr. Beers emphasized. She noted that the same urgency is needed to provide vaccines for children as for adults, although “we have to do it safely, and be sure and feel confident in the data.”
When asked to comment about the status of FDA authorization of COVID-19 vaccines for younger children, Dr. Fauci described the current situation as one that “might require some unprecedented and unique action” on the part of the FDA, which tends to move cautiously because of safety considerations. However, concerns about adverse events might get in the way of protecting children against what “you are really worried about,” in this case COVID-19 and its variants, he said. Despite the breakthrough infections, “vaccination continues to very adequately protect people from getting severe disease,” he emphasized.
Dr. Fauci also said that he believes the current data support boosters for the immune compromised; however “it is a different story about the general vaccinated population and the vaccinated elderly,” he said. Sooner or later most people will likely need boosters; “the question is who, when, and how soon,” he noted.
Dr. Fauci wrapped up the session with kudos and support for the pediatric health care community. “As a nonpediatrician, I have a great deal of respect for the job you are doing,” he said. “Keep up the great work.”
Dr. Beers echoed this sentiment, saying that she was “continually awed, impressed, and inspired” by how the pediatric hospitalists are navigating the ever-changing pandemic environment.
“From a public health standpoint, I think we have an evolving situation,” said Anthony S. Fauci, MD, director of the National Institute of Allergy and Infectious Diseases, in a moderated session with Lee Beers, MD, president of the American Academy of Pediatrics, at the virtual Pediatric Hospital Medicine annual conference.
The reasons for this shift remain unclear, he said.
Dr. Beers emphasized the ability of pediatric hospitalists to be flexible in the face of uncertainty and the evolving virus, and asked Dr. Fauci to elaborate on the unique traits of the delta variant that make it especially challenging.
“There is no doubt that delta transmits much more efficiently than the alpha variant or any other variant,” Dr. Fauci said. The transmissibility is evident in comparisons of the level of virus in the nasopharynx of the delta variant, compared with the original alpha COVID-19 virus – delta is as much as 1,000 times higher, he explained.
In addition, the level of virus in the nasopharynx of vaccinated individuals who develop breakthrough infections with the delta variant is similar to the levels in unvaccinated individuals who are infected with the delta variant.
The delta variant is “the tough guy on the block” at the moment, Dr. Fauci said.
Dr. Fauci also responded to a question on the lack of winter viruses, such as RSV and the flu, last winter, but the surge in these viruses over the summer.
This winter’s activity remains uncertain, Dr. Fauci said. However, he speculated “with a strong dose of humility and modesty” that viruses tend to have niches, some are seasonal, and the winter viruses that were displaced by COVID-19 hit harder in the summer instead. “If I were a [non-COVID] virus looking for a niche, I would be really confused,” he said. “I don’t know what will happen this winter, but if we get good control over COVID-19 by winter, we could have a very vengeful influenza season,” he said. “This is speculation, I don’t have any data for this,” he cautioned.
Dr. Beers raised the issue of back-to-school safety, and the updated AAP guidance for universal masking for K-12 students. “Our guidance about return to school gets updated as the situation changes and we gain a better understanding of how kids can get to school safely,” she said. A combination of factors affect back-to-school guidance, including the ineligibility of children younger than 12 years to be vaccinated, the number of adolescents who are eligible but have not been vaccinated, and the challenge for educators to navigate which children should wear masks, Dr. Beers said.
“We want to get vaccines for our youngest kids as soon as safely possible,” Dr. Beers emphasized. She noted that the same urgency is needed to provide vaccines for children as for adults, although “we have to do it safely, and be sure and feel confident in the data.”
When asked to comment about the status of FDA authorization of COVID-19 vaccines for younger children, Dr. Fauci described the current situation as one that “might require some unprecedented and unique action” on the part of the FDA, which tends to move cautiously because of safety considerations. However, concerns about adverse events might get in the way of protecting children against what “you are really worried about,” in this case COVID-19 and its variants, he said. Despite the breakthrough infections, “vaccination continues to very adequately protect people from getting severe disease,” he emphasized.
Dr. Fauci also said that he believes the current data support boosters for the immune compromised; however “it is a different story about the general vaccinated population and the vaccinated elderly,” he said. Sooner or later most people will likely need boosters; “the question is who, when, and how soon,” he noted.
Dr. Fauci wrapped up the session with kudos and support for the pediatric health care community. “As a nonpediatrician, I have a great deal of respect for the job you are doing,” he said. “Keep up the great work.”
Dr. Beers echoed this sentiment, saying that she was “continually awed, impressed, and inspired” by how the pediatric hospitalists are navigating the ever-changing pandemic environment.
“From a public health standpoint, I think we have an evolving situation,” said Anthony S. Fauci, MD, director of the National Institute of Allergy and Infectious Diseases, in a moderated session with Lee Beers, MD, president of the American Academy of Pediatrics, at the virtual Pediatric Hospital Medicine annual conference.
The reasons for this shift remain unclear, he said.
Dr. Beers emphasized the ability of pediatric hospitalists to be flexible in the face of uncertainty and the evolving virus, and asked Dr. Fauci to elaborate on the unique traits of the delta variant that make it especially challenging.
“There is no doubt that delta transmits much more efficiently than the alpha variant or any other variant,” Dr. Fauci said. The transmissibility is evident in comparisons of the level of virus in the nasopharynx of the delta variant, compared with the original alpha COVID-19 virus – delta is as much as 1,000 times higher, he explained.
In addition, the level of virus in the nasopharynx of vaccinated individuals who develop breakthrough infections with the delta variant is similar to the levels in unvaccinated individuals who are infected with the delta variant.
The delta variant is “the tough guy on the block” at the moment, Dr. Fauci said.
Dr. Fauci also responded to a question on the lack of winter viruses, such as RSV and the flu, last winter, but the surge in these viruses over the summer.
This winter’s activity remains uncertain, Dr. Fauci said. However, he speculated “with a strong dose of humility and modesty” that viruses tend to have niches, some are seasonal, and the winter viruses that were displaced by COVID-19 hit harder in the summer instead. “If I were a [non-COVID] virus looking for a niche, I would be really confused,” he said. “I don’t know what will happen this winter, but if we get good control over COVID-19 by winter, we could have a very vengeful influenza season,” he said. “This is speculation, I don’t have any data for this,” he cautioned.
Dr. Beers raised the issue of back-to-school safety, and the updated AAP guidance for universal masking for K-12 students. “Our guidance about return to school gets updated as the situation changes and we gain a better understanding of how kids can get to school safely,” she said. A combination of factors affect back-to-school guidance, including the ineligibility of children younger than 12 years to be vaccinated, the number of adolescents who are eligible but have not been vaccinated, and the challenge for educators to navigate which children should wear masks, Dr. Beers said.
“We want to get vaccines for our youngest kids as soon as safely possible,” Dr. Beers emphasized. She noted that the same urgency is needed to provide vaccines for children as for adults, although “we have to do it safely, and be sure and feel confident in the data.”
When asked to comment about the status of FDA authorization of COVID-19 vaccines for younger children, Dr. Fauci described the current situation as one that “might require some unprecedented and unique action” on the part of the FDA, which tends to move cautiously because of safety considerations. However, concerns about adverse events might get in the way of protecting children against what “you are really worried about,” in this case COVID-19 and its variants, he said. Despite the breakthrough infections, “vaccination continues to very adequately protect people from getting severe disease,” he emphasized.
Dr. Fauci also said that he believes the current data support boosters for the immune compromised; however “it is a different story about the general vaccinated population and the vaccinated elderly,” he said. Sooner or later most people will likely need boosters; “the question is who, when, and how soon,” he noted.
Dr. Fauci wrapped up the session with kudos and support for the pediatric health care community. “As a nonpediatrician, I have a great deal of respect for the job you are doing,” he said. “Keep up the great work.”
Dr. Beers echoed this sentiment, saying that she was “continually awed, impressed, and inspired” by how the pediatric hospitalists are navigating the ever-changing pandemic environment.
FROM PHM 2021
Injectable monoclonal antibodies prevent COVID-19 in trial
according to results of a randomized, double-blind, placebo-controlled clinical trial published online August 4, 2021, in the New England Journal of Medicine.
The cocktail of the monoclonal antibodies casirivimab and imdevimab (REGEN-COV, Regeneron Pharmaceuticals) reduced participants’ relative risk of infection by 72%, compared with placebo within the first week. After the first week, risk reduction increased to 93%.
“Long after you would be exposed by your household, there is an enduring effect that prevents you from community spread,” said David Wohl, MD, professor of medicine in the division of infectious diseases at the University of North Carolina at Chapel Hill, who was a site investigator for the trial but not a study author.
Participants were enrolled within 96 hours after someone in their household tested positive for SARS-CoV-2. Participants were randomly assigned to receive 1,200 mg of REGEN-COV subcutaneously or a placebo. Based on serologic testing, study participants showed no evidence of current or previous SARS-CoV-2 infection. The median age of participants was 42.9, but 45% were male teenagers (ages 12-17).
In the group that received REGEN-COV, 11 out of 753 participants developed symptomatic COVID-19, compared with 59 out of 752 participants who received placebo. The relative risk reduction for the study’s 4-week period was 81.4% (P < .001). Of the participants that did develop a SARS-CoV-2 infection, those that received REGEN-COV were less likely to be symptomatic. Asymptomatic infections developed in 25 participants who received REGEN-COV versus 48 in the placebo group. The relative risk of developing any SARS-CoV-2 infection, symptomatic or asymptomatic, was reduced by 66.4% with REGEN-COV (P < .001).
Among the patients who were symptomatic, symptoms subsided within a median of 1.2 weeks for the group that received REGEN-COV, 2 weeks earlier than the placebo group. These patients also had a shorter duration of a high viral load (>104 copies/mL). Few adverse events were reported in the treatment or placebo groups. Monoclonal antibodies “seem to be incredibly safe,” Dr. Wohl said.
“These monoclonal antibodies have proven they can reduce the viral replication in the nose,” said study author Myron Cohen, MD, an infectious disease specialist and professor of epidemiology at the University of North Carolina.
The Food and Drug Administration first granted REGEN-COV emergency use authorization (EUA) in November 2020 for use in patients with mild or moderate COVID-19 who were also at high risk for progressing to severe COVID-19. At that time, the cocktail of monoclonal antibodies was delivered by a single intravenous infusion.
In January, Regeneron first announced the success of this trial of the subcutaneous injection for exposed household contacts based on early results, and in June of 2021, the FDA expanded the EUA to include a subcutaneous delivery when IV is not feasible. On July 30, the EUA was expanded again to include prophylactic use in exposed patients based on these trial results.
The U.S. government has purchased approximately 1.5 million doses of REGEN-COV from Regeneron and has agreed to make the treatments free of charge to patients.
But despite being free, available, and backed by promising data, monoclonal antibodies as a therapeutic answer to COVID-19 still hasn’t really taken off. “The problem is, it first requires knowledge and awareness,” Dr. Wohl said. “A lot [of people] don’t know this exists. To be honest, vaccination has taken up all the oxygen in the room.”
Dr. Cohen agreed. One reason for the slow uptake may be because the drug supply is owned by the government and not a pharmaceutical company. There hasn’t been a typical marketing push to make physicians and consumers aware. Additionally, “the logistics are daunting,” Dr. Cohen said. The office spaces where many physicians care for patients “often aren’t appropriate for patients who think they have SARS-CoV-2.”
“Right now, there’s not a mechanism” to administer the drug to people who could benefit from it, Dr. Wohl said. Eligible patients are either immunocompromised and unlikely to mount a sufficient immune response with vaccination, or not fully vaccinated. They should have been exposed to an infected individual or have a high likelihood of exposure due to where they live, such as in a prison or nursing home. Local doctors are unlikely to be the primary administrators of the drug, Dr. Wohl added. “How do we operationalize this for people who fit the criteria?”
There’s also an issue of timing. REGEN-COV is most effective when given early, Dr. Cohen said. “[Monoclonal antibodies] really only work well in the replication phase.” Many patients who would be eligible delay care until they’ve had symptoms for several days, when REGEN-COV would no longer have the desired effect.
Eventually, Dr. Wohl suspects demand will increase when people realize REGEN-COV can help those with COVID-19 and those who have been exposed. But before then, “we do have to think about how to integrate this into a workflow people can access without being confused.”
The trial was done before there was widespread vaccination, so it’s unclear what the results mean for people who have been vaccinated. Dr. Cohen and Dr. Wohl said there are ongoing conversations about whether monoclonal antibodies could be complementary to vaccination and if there’s potential for continued monthly use of these therapies.
Cohen and Wohl reported no relevant financial relationships. The trial was supported by Regeneron Pharmaceuticals, F. Hoffmann–La Roche, the National Institute of Allergy and Infectious Diseases, NIH, and the COVID-19 Prevention Network.
A version of this article first appeared on Medscape.com.
according to results of a randomized, double-blind, placebo-controlled clinical trial published online August 4, 2021, in the New England Journal of Medicine.
The cocktail of the monoclonal antibodies casirivimab and imdevimab (REGEN-COV, Regeneron Pharmaceuticals) reduced participants’ relative risk of infection by 72%, compared with placebo within the first week. After the first week, risk reduction increased to 93%.
“Long after you would be exposed by your household, there is an enduring effect that prevents you from community spread,” said David Wohl, MD, professor of medicine in the division of infectious diseases at the University of North Carolina at Chapel Hill, who was a site investigator for the trial but not a study author.
Participants were enrolled within 96 hours after someone in their household tested positive for SARS-CoV-2. Participants were randomly assigned to receive 1,200 mg of REGEN-COV subcutaneously or a placebo. Based on serologic testing, study participants showed no evidence of current or previous SARS-CoV-2 infection. The median age of participants was 42.9, but 45% were male teenagers (ages 12-17).
In the group that received REGEN-COV, 11 out of 753 participants developed symptomatic COVID-19, compared with 59 out of 752 participants who received placebo. The relative risk reduction for the study’s 4-week period was 81.4% (P < .001). Of the participants that did develop a SARS-CoV-2 infection, those that received REGEN-COV were less likely to be symptomatic. Asymptomatic infections developed in 25 participants who received REGEN-COV versus 48 in the placebo group. The relative risk of developing any SARS-CoV-2 infection, symptomatic or asymptomatic, was reduced by 66.4% with REGEN-COV (P < .001).
Among the patients who were symptomatic, symptoms subsided within a median of 1.2 weeks for the group that received REGEN-COV, 2 weeks earlier than the placebo group. These patients also had a shorter duration of a high viral load (>104 copies/mL). Few adverse events were reported in the treatment or placebo groups. Monoclonal antibodies “seem to be incredibly safe,” Dr. Wohl said.
“These monoclonal antibodies have proven they can reduce the viral replication in the nose,” said study author Myron Cohen, MD, an infectious disease specialist and professor of epidemiology at the University of North Carolina.
The Food and Drug Administration first granted REGEN-COV emergency use authorization (EUA) in November 2020 for use in patients with mild or moderate COVID-19 who were also at high risk for progressing to severe COVID-19. At that time, the cocktail of monoclonal antibodies was delivered by a single intravenous infusion.
In January, Regeneron first announced the success of this trial of the subcutaneous injection for exposed household contacts based on early results, and in June of 2021, the FDA expanded the EUA to include a subcutaneous delivery when IV is not feasible. On July 30, the EUA was expanded again to include prophylactic use in exposed patients based on these trial results.
The U.S. government has purchased approximately 1.5 million doses of REGEN-COV from Regeneron and has agreed to make the treatments free of charge to patients.
But despite being free, available, and backed by promising data, monoclonal antibodies as a therapeutic answer to COVID-19 still hasn’t really taken off. “The problem is, it first requires knowledge and awareness,” Dr. Wohl said. “A lot [of people] don’t know this exists. To be honest, vaccination has taken up all the oxygen in the room.”
Dr. Cohen agreed. One reason for the slow uptake may be because the drug supply is owned by the government and not a pharmaceutical company. There hasn’t been a typical marketing push to make physicians and consumers aware. Additionally, “the logistics are daunting,” Dr. Cohen said. The office spaces where many physicians care for patients “often aren’t appropriate for patients who think they have SARS-CoV-2.”
“Right now, there’s not a mechanism” to administer the drug to people who could benefit from it, Dr. Wohl said. Eligible patients are either immunocompromised and unlikely to mount a sufficient immune response with vaccination, or not fully vaccinated. They should have been exposed to an infected individual or have a high likelihood of exposure due to where they live, such as in a prison or nursing home. Local doctors are unlikely to be the primary administrators of the drug, Dr. Wohl added. “How do we operationalize this for people who fit the criteria?”
There’s also an issue of timing. REGEN-COV is most effective when given early, Dr. Cohen said. “[Monoclonal antibodies] really only work well in the replication phase.” Many patients who would be eligible delay care until they’ve had symptoms for several days, when REGEN-COV would no longer have the desired effect.
Eventually, Dr. Wohl suspects demand will increase when people realize REGEN-COV can help those with COVID-19 and those who have been exposed. But before then, “we do have to think about how to integrate this into a workflow people can access without being confused.”
The trial was done before there was widespread vaccination, so it’s unclear what the results mean for people who have been vaccinated. Dr. Cohen and Dr. Wohl said there are ongoing conversations about whether monoclonal antibodies could be complementary to vaccination and if there’s potential for continued monthly use of these therapies.
Cohen and Wohl reported no relevant financial relationships. The trial was supported by Regeneron Pharmaceuticals, F. Hoffmann–La Roche, the National Institute of Allergy and Infectious Diseases, NIH, and the COVID-19 Prevention Network.
A version of this article first appeared on Medscape.com.
according to results of a randomized, double-blind, placebo-controlled clinical trial published online August 4, 2021, in the New England Journal of Medicine.
The cocktail of the monoclonal antibodies casirivimab and imdevimab (REGEN-COV, Regeneron Pharmaceuticals) reduced participants’ relative risk of infection by 72%, compared with placebo within the first week. After the first week, risk reduction increased to 93%.
“Long after you would be exposed by your household, there is an enduring effect that prevents you from community spread,” said David Wohl, MD, professor of medicine in the division of infectious diseases at the University of North Carolina at Chapel Hill, who was a site investigator for the trial but not a study author.
Participants were enrolled within 96 hours after someone in their household tested positive for SARS-CoV-2. Participants were randomly assigned to receive 1,200 mg of REGEN-COV subcutaneously or a placebo. Based on serologic testing, study participants showed no evidence of current or previous SARS-CoV-2 infection. The median age of participants was 42.9, but 45% were male teenagers (ages 12-17).
In the group that received REGEN-COV, 11 out of 753 participants developed symptomatic COVID-19, compared with 59 out of 752 participants who received placebo. The relative risk reduction for the study’s 4-week period was 81.4% (P < .001). Of the participants that did develop a SARS-CoV-2 infection, those that received REGEN-COV were less likely to be symptomatic. Asymptomatic infections developed in 25 participants who received REGEN-COV versus 48 in the placebo group. The relative risk of developing any SARS-CoV-2 infection, symptomatic or asymptomatic, was reduced by 66.4% with REGEN-COV (P < .001).
Among the patients who were symptomatic, symptoms subsided within a median of 1.2 weeks for the group that received REGEN-COV, 2 weeks earlier than the placebo group. These patients also had a shorter duration of a high viral load (>104 copies/mL). Few adverse events were reported in the treatment or placebo groups. Monoclonal antibodies “seem to be incredibly safe,” Dr. Wohl said.
“These monoclonal antibodies have proven they can reduce the viral replication in the nose,” said study author Myron Cohen, MD, an infectious disease specialist and professor of epidemiology at the University of North Carolina.
The Food and Drug Administration first granted REGEN-COV emergency use authorization (EUA) in November 2020 for use in patients with mild or moderate COVID-19 who were also at high risk for progressing to severe COVID-19. At that time, the cocktail of monoclonal antibodies was delivered by a single intravenous infusion.
In January, Regeneron first announced the success of this trial of the subcutaneous injection for exposed household contacts based on early results, and in June of 2021, the FDA expanded the EUA to include a subcutaneous delivery when IV is not feasible. On July 30, the EUA was expanded again to include prophylactic use in exposed patients based on these trial results.
The U.S. government has purchased approximately 1.5 million doses of REGEN-COV from Regeneron and has agreed to make the treatments free of charge to patients.
But despite being free, available, and backed by promising data, monoclonal antibodies as a therapeutic answer to COVID-19 still hasn’t really taken off. “The problem is, it first requires knowledge and awareness,” Dr. Wohl said. “A lot [of people] don’t know this exists. To be honest, vaccination has taken up all the oxygen in the room.”
Dr. Cohen agreed. One reason for the slow uptake may be because the drug supply is owned by the government and not a pharmaceutical company. There hasn’t been a typical marketing push to make physicians and consumers aware. Additionally, “the logistics are daunting,” Dr. Cohen said. The office spaces where many physicians care for patients “often aren’t appropriate for patients who think they have SARS-CoV-2.”
“Right now, there’s not a mechanism” to administer the drug to people who could benefit from it, Dr. Wohl said. Eligible patients are either immunocompromised and unlikely to mount a sufficient immune response with vaccination, or not fully vaccinated. They should have been exposed to an infected individual or have a high likelihood of exposure due to where they live, such as in a prison or nursing home. Local doctors are unlikely to be the primary administrators of the drug, Dr. Wohl added. “How do we operationalize this for people who fit the criteria?”
There’s also an issue of timing. REGEN-COV is most effective when given early, Dr. Cohen said. “[Monoclonal antibodies] really only work well in the replication phase.” Many patients who would be eligible delay care until they’ve had symptoms for several days, when REGEN-COV would no longer have the desired effect.
Eventually, Dr. Wohl suspects demand will increase when people realize REGEN-COV can help those with COVID-19 and those who have been exposed. But before then, “we do have to think about how to integrate this into a workflow people can access without being confused.”
The trial was done before there was widespread vaccination, so it’s unclear what the results mean for people who have been vaccinated. Dr. Cohen and Dr. Wohl said there are ongoing conversations about whether monoclonal antibodies could be complementary to vaccination and if there’s potential for continued monthly use of these therapies.
Cohen and Wohl reported no relevant financial relationships. The trial was supported by Regeneron Pharmaceuticals, F. Hoffmann–La Roche, the National Institute of Allergy and Infectious Diseases, NIH, and the COVID-19 Prevention Network.
A version of this article first appeared on Medscape.com.
Moderna says boosters may be needed after 6 months
Moderna says neutralizing antibodies generated by its COVID-19 vaccine against three variants of the virus that causes the disease waned substantially 6 months after the second dose.
Because of this, the company expects an increase in breakthrough infections with a need for boosters before winter.
In an experiment, a 50-mg dose of the vaccine, given as a third shot, boosted levels of antibodies in 20 previously vaccinated people by 32 times against the Beta variant, by 44 times against the Gamma variant, and by 42 times against Delta.
The new data was presented in an earnings call to investors and is based on a small study that hasn’t yet been published in medical literature.
The company also said its vaccine remained highly effective at preventing severe COVID outcomes through 6 months.
Last week, Pfizer released early data suggesting a similar drop in protection from its vaccine. The company also showed a third dose substantially boosted protection, including against the Delta variant.
The new results come just 1 day after the World Health Organization implored wealthy nations to hold off on third doses until more of the world’s population could get a first dose.
More than 80% of the 4 billion vaccine doses given around the world have been distributed to high-income countries.
A version of this article first appeared on WebMD.com.
Moderna says neutralizing antibodies generated by its COVID-19 vaccine against three variants of the virus that causes the disease waned substantially 6 months after the second dose.
Because of this, the company expects an increase in breakthrough infections with a need for boosters before winter.
In an experiment, a 50-mg dose of the vaccine, given as a third shot, boosted levels of antibodies in 20 previously vaccinated people by 32 times against the Beta variant, by 44 times against the Gamma variant, and by 42 times against Delta.
The new data was presented in an earnings call to investors and is based on a small study that hasn’t yet been published in medical literature.
The company also said its vaccine remained highly effective at preventing severe COVID outcomes through 6 months.
Last week, Pfizer released early data suggesting a similar drop in protection from its vaccine. The company also showed a third dose substantially boosted protection, including against the Delta variant.
The new results come just 1 day after the World Health Organization implored wealthy nations to hold off on third doses until more of the world’s population could get a first dose.
More than 80% of the 4 billion vaccine doses given around the world have been distributed to high-income countries.
A version of this article first appeared on WebMD.com.
Moderna says neutralizing antibodies generated by its COVID-19 vaccine against three variants of the virus that causes the disease waned substantially 6 months after the second dose.
Because of this, the company expects an increase in breakthrough infections with a need for boosters before winter.
In an experiment, a 50-mg dose of the vaccine, given as a third shot, boosted levels of antibodies in 20 previously vaccinated people by 32 times against the Beta variant, by 44 times against the Gamma variant, and by 42 times against Delta.
The new data was presented in an earnings call to investors and is based on a small study that hasn’t yet been published in medical literature.
The company also said its vaccine remained highly effective at preventing severe COVID outcomes through 6 months.
Last week, Pfizer released early data suggesting a similar drop in protection from its vaccine. The company also showed a third dose substantially boosted protection, including against the Delta variant.
The new results come just 1 day after the World Health Organization implored wealthy nations to hold off on third doses until more of the world’s population could get a first dose.
More than 80% of the 4 billion vaccine doses given around the world have been distributed to high-income countries.
A version of this article first appeared on WebMD.com.
Despite retraction, study using fraudulent Surgisphere data still cited
A retracted study on the safety of blood pressure medications in patients with COVID-19 continues to be cited nearly a year later, new research shows.
The study in question, published on May 1, 2020, in the New England Journal of Medicine, showed no increased risk for in-hospital death with the use of ACE inhibitors or angiotensin-receptor blockers (ARBs) in hospitalized patients with COVID-19.
Concerns about the veracity of the Surgisphere database used for the study, however, led to a June 4 retraction and to the June 13 retraction of a second study, published in the Lancet, that focused on hydroxychloroquine as a COVID-19 treatment.
Although the Surgisphere scandal caused a global reckoning of COVID-19 scientific studies, the new analysis identified 652 citations of the NEJM article as of May 31.
More than a third of the citations occurred in the first 2 months after the retraction, 54% were at least 3 months later, and 2.8% at least 6 months later. In May, 11 months after the article was retracted, it was cited 21 times, senior author Emily G. McDonald, MD, MSc, McGill University, Montreal, and colleagues reported in a research letter in JAMA Internal Medicine.
“In early May and June there were already more than 200 citations in one of the world’s leading scientific journals, so I do believe it was a highly influential article early on and had an impact on different types of studies or research taking place,” she said in an interview.
Dr. McDonald said she’s also “certain that it impacted patient care,” observing that when there are no guidelines available on how to manage patients, physicians will turn to the most recent evidence in the most reputable journals.
“In the case of ACE [inhibitors] and ARBs, although the study was based on fraudulent data, we were lucky that the overall message was in the end probably correct, but that might not have been the case for another study or dataset,” she said.
Early in the pandemic, concerns existed that ACE inhibitors and ARBs could be harmful, increasing the expression of ACE2 receptors, which the SARS-CoV-2 virus uses to gain entry into cells. The first randomized trial to examine the issue, BRACE CORONA, showed no clinical benefit to interrupting use of the agents in hospitalized patients. An observational study suggested ACE inhibitors may even be protective.
Of two high-profile retractions, McDonald said they chose to bypass the hydroxychloroquine study, which had an eye-popping Altmetric attention score of 23,084, compared with 3,727 for the NEJM paper, because it may have been cited for “other” reasons. “We wanted to focus less on the politics and more on the problem of retracted work.”
The team found that researchers across the globe were citing the retracted ACE/ARB paper (18.7% in the United States, 8.1% in Italy, and 44% other countries). Most citations were used to support a statement in the main text of a study, but in nearly 3% of cases, the data were incorporated into new analyses.
Just 17.6% of the studies cited or noted the retraction. “For sure, that was surprising to us. We suspected it, but our study confirmed it,” Dr. McDonald said.
Although retracted articles can be identified by a watermark or line of text, in some cases that can be easily missed, she noted. What’s more, not all citation software points out when a study has been retracted, a fate shared by the copyediting process.
“There are a lot of mechanisms in place and, in general, what’s happening is rare but there isn’t a perfect automated system solution to absolutely prevent this from happening,” she said. “It’s still subject to human error.”
The findings also have to be taken in the context of a rapidly emerging pandemic and the unprecedented torrent of scientific papers released over the past year.
“That might have contributed to why this happened, but the takeaway message is that this can happen despite our best efforts, and we need to challenge ourselves to come up with a system solution to prevent this from happening in the future,” Dr. McDonald said. “Current mechanisms are probably capturing 95% of it, but we need to do better.”
Limitations of the present analysis are that it was limited to the single retracted study; used only a single search engine, Google Scholar, to identify the citing works; and that additional citations may have been missed, the authors noted.
McDonald and coauthor Todd C. Lee, MD, report being signatories on a public letter calling for the retraction of the Surgisphere papers. Dr. Lee also reported receiving research support from Fonds De Recherche du Quebec-Sante during the conduct of the study.
A version of this article first appeared on Medscape.com.
A retracted study on the safety of blood pressure medications in patients with COVID-19 continues to be cited nearly a year later, new research shows.
The study in question, published on May 1, 2020, in the New England Journal of Medicine, showed no increased risk for in-hospital death with the use of ACE inhibitors or angiotensin-receptor blockers (ARBs) in hospitalized patients with COVID-19.
Concerns about the veracity of the Surgisphere database used for the study, however, led to a June 4 retraction and to the June 13 retraction of a second study, published in the Lancet, that focused on hydroxychloroquine as a COVID-19 treatment.
Although the Surgisphere scandal caused a global reckoning of COVID-19 scientific studies, the new analysis identified 652 citations of the NEJM article as of May 31.
More than a third of the citations occurred in the first 2 months after the retraction, 54% were at least 3 months later, and 2.8% at least 6 months later. In May, 11 months after the article was retracted, it was cited 21 times, senior author Emily G. McDonald, MD, MSc, McGill University, Montreal, and colleagues reported in a research letter in JAMA Internal Medicine.
“In early May and June there were already more than 200 citations in one of the world’s leading scientific journals, so I do believe it was a highly influential article early on and had an impact on different types of studies or research taking place,” she said in an interview.
Dr. McDonald said she’s also “certain that it impacted patient care,” observing that when there are no guidelines available on how to manage patients, physicians will turn to the most recent evidence in the most reputable journals.
“In the case of ACE [inhibitors] and ARBs, although the study was based on fraudulent data, we were lucky that the overall message was in the end probably correct, but that might not have been the case for another study or dataset,” she said.
Early in the pandemic, concerns existed that ACE inhibitors and ARBs could be harmful, increasing the expression of ACE2 receptors, which the SARS-CoV-2 virus uses to gain entry into cells. The first randomized trial to examine the issue, BRACE CORONA, showed no clinical benefit to interrupting use of the agents in hospitalized patients. An observational study suggested ACE inhibitors may even be protective.
Of two high-profile retractions, McDonald said they chose to bypass the hydroxychloroquine study, which had an eye-popping Altmetric attention score of 23,084, compared with 3,727 for the NEJM paper, because it may have been cited for “other” reasons. “We wanted to focus less on the politics and more on the problem of retracted work.”
The team found that researchers across the globe were citing the retracted ACE/ARB paper (18.7% in the United States, 8.1% in Italy, and 44% other countries). Most citations were used to support a statement in the main text of a study, but in nearly 3% of cases, the data were incorporated into new analyses.
Just 17.6% of the studies cited or noted the retraction. “For sure, that was surprising to us. We suspected it, but our study confirmed it,” Dr. McDonald said.
Although retracted articles can be identified by a watermark or line of text, in some cases that can be easily missed, she noted. What’s more, not all citation software points out when a study has been retracted, a fate shared by the copyediting process.
“There are a lot of mechanisms in place and, in general, what’s happening is rare but there isn’t a perfect automated system solution to absolutely prevent this from happening,” she said. “It’s still subject to human error.”
The findings also have to be taken in the context of a rapidly emerging pandemic and the unprecedented torrent of scientific papers released over the past year.
“That might have contributed to why this happened, but the takeaway message is that this can happen despite our best efforts, and we need to challenge ourselves to come up with a system solution to prevent this from happening in the future,” Dr. McDonald said. “Current mechanisms are probably capturing 95% of it, but we need to do better.”
Limitations of the present analysis are that it was limited to the single retracted study; used only a single search engine, Google Scholar, to identify the citing works; and that additional citations may have been missed, the authors noted.
McDonald and coauthor Todd C. Lee, MD, report being signatories on a public letter calling for the retraction of the Surgisphere papers. Dr. Lee also reported receiving research support from Fonds De Recherche du Quebec-Sante during the conduct of the study.
A version of this article first appeared on Medscape.com.
A retracted study on the safety of blood pressure medications in patients with COVID-19 continues to be cited nearly a year later, new research shows.
The study in question, published on May 1, 2020, in the New England Journal of Medicine, showed no increased risk for in-hospital death with the use of ACE inhibitors or angiotensin-receptor blockers (ARBs) in hospitalized patients with COVID-19.
Concerns about the veracity of the Surgisphere database used for the study, however, led to a June 4 retraction and to the June 13 retraction of a second study, published in the Lancet, that focused on hydroxychloroquine as a COVID-19 treatment.
Although the Surgisphere scandal caused a global reckoning of COVID-19 scientific studies, the new analysis identified 652 citations of the NEJM article as of May 31.
More than a third of the citations occurred in the first 2 months after the retraction, 54% were at least 3 months later, and 2.8% at least 6 months later. In May, 11 months after the article was retracted, it was cited 21 times, senior author Emily G. McDonald, MD, MSc, McGill University, Montreal, and colleagues reported in a research letter in JAMA Internal Medicine.
“In early May and June there were already more than 200 citations in one of the world’s leading scientific journals, so I do believe it was a highly influential article early on and had an impact on different types of studies or research taking place,” she said in an interview.
Dr. McDonald said she’s also “certain that it impacted patient care,” observing that when there are no guidelines available on how to manage patients, physicians will turn to the most recent evidence in the most reputable journals.
“In the case of ACE [inhibitors] and ARBs, although the study was based on fraudulent data, we were lucky that the overall message was in the end probably correct, but that might not have been the case for another study or dataset,” she said.
Early in the pandemic, concerns existed that ACE inhibitors and ARBs could be harmful, increasing the expression of ACE2 receptors, which the SARS-CoV-2 virus uses to gain entry into cells. The first randomized trial to examine the issue, BRACE CORONA, showed no clinical benefit to interrupting use of the agents in hospitalized patients. An observational study suggested ACE inhibitors may even be protective.
Of two high-profile retractions, McDonald said they chose to bypass the hydroxychloroquine study, which had an eye-popping Altmetric attention score of 23,084, compared with 3,727 for the NEJM paper, because it may have been cited for “other” reasons. “We wanted to focus less on the politics and more on the problem of retracted work.”
The team found that researchers across the globe were citing the retracted ACE/ARB paper (18.7% in the United States, 8.1% in Italy, and 44% other countries). Most citations were used to support a statement in the main text of a study, but in nearly 3% of cases, the data were incorporated into new analyses.
Just 17.6% of the studies cited or noted the retraction. “For sure, that was surprising to us. We suspected it, but our study confirmed it,” Dr. McDonald said.
Although retracted articles can be identified by a watermark or line of text, in some cases that can be easily missed, she noted. What’s more, not all citation software points out when a study has been retracted, a fate shared by the copyediting process.
“There are a lot of mechanisms in place and, in general, what’s happening is rare but there isn’t a perfect automated system solution to absolutely prevent this from happening,” she said. “It’s still subject to human error.”
The findings also have to be taken in the context of a rapidly emerging pandemic and the unprecedented torrent of scientific papers released over the past year.
“That might have contributed to why this happened, but the takeaway message is that this can happen despite our best efforts, and we need to challenge ourselves to come up with a system solution to prevent this from happening in the future,” Dr. McDonald said. “Current mechanisms are probably capturing 95% of it, but we need to do better.”
Limitations of the present analysis are that it was limited to the single retracted study; used only a single search engine, Google Scholar, to identify the citing works; and that additional citations may have been missed, the authors noted.
McDonald and coauthor Todd C. Lee, MD, report being signatories on a public letter calling for the retraction of the Surgisphere papers. Dr. Lee also reported receiving research support from Fonds De Recherche du Quebec-Sante during the conduct of the study.
A version of this article first appeared on Medscape.com.