User login
In Case You Missed It: COVID
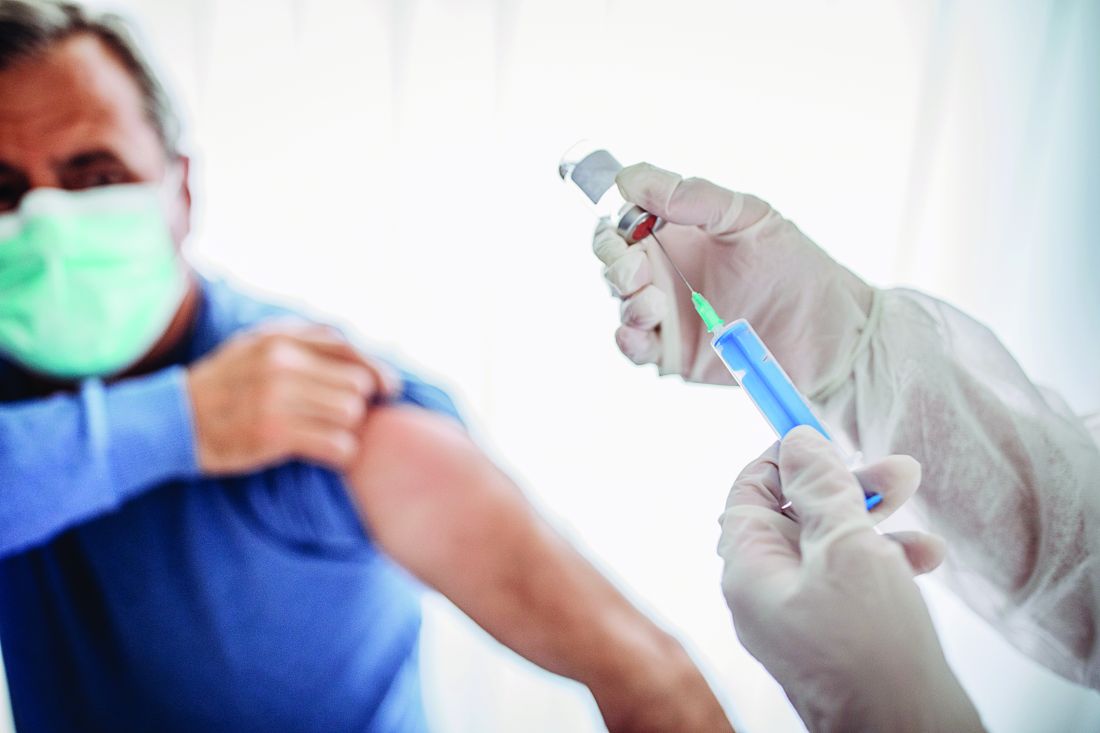
Feds authorize $3 billion to boost vaccine rollout
The CDC will send $3 billion to the states to boost a lagging national COVID-19 vaccination program.
The Department of Health and Human Services announced the new funding as only 30% of the more than 22 million doses of vaccine distributed in the U.S. has been injected into Americans’ arms.
Along with the $3 billion, HHS said another $19 billion is headed to states and jurisdictions to boost COVID-19 testing programs. The amount each state will receive will be determined by population.
The news comes days after President-elect Joe Biden said he planned to release all available doses of vaccine after he takes office on Jan. 20. The Trump administration has been holding back millions of doses to ensure supply of vaccine to provide the necessary second dose for those who received the first shot.
“This funding is another timely investment that will strengthen our nation’s efforts to stop the COVID-19 pandemic in America,” CDC Director Robert Redfield, MD, said in a statement. “Particularly now, it is crucial that states and communities have the resources they need to conduct testing, and to distribute and administer safe, high-quality COVID-19 vaccines safely and equitably.”
Federal officials and public health experts, however, expressed concerns this weekend about Biden’s plan.
Outgoing Trump administration officials and others said they worry that doing so will leave providers without enough second doses for people getting the two-shot vaccines.
If Biden releases all available doses and the vaccine-making process has an issue, they said, that could pose a supply risk.
“We have product that is going through QC right now – quality control – for sterility, identity check that we have tens and tens of millions of product. We always will. But batches fail. Sterility fails ... and then you don’t have a product for that second dose,” Alex Azar, secretary of health and human services, told the American Hospital Association on Jan. 8, according to CNN.
“And frankly, talking about that or encouraging that can really undermine a critical public health need, which is that people come back for their second vaccine,” he said.
One of the main roadblocks in the vaccine rollout has been administering the doses that have already been distributed. The U.S. has shipped 22.1 million doses, and 6.6 million first shots have been given, according to the latest CDC data updated Jan. 8. Mr. Azar and other federal health officials have encouraged states to use their current supply and expand vaccine access to more priority groups.
“We would be delighted to learn that jurisdictions have actually administered many more doses than they are presently reporting,” a spokesman for the U.S. Department of Health and Human Services told CNN. “We are encouraging jurisdictions to expand their priority groups as needed to ensure no vaccine is sitting on the shelf after having been delivered to the jurisdiction-directed locations.”
Releasing more vaccines for first doses could create ethical concerns as well, since people getting vaccines expect to get a second dose in the proper amount of time, according to The Week. Biden’s transition team said on Jan. 8 that he won’t delay the second dose but, instead, plans to ramp up production to stay on track.
To do this well, the federal government should create a coordinated vaccine strategy that sets expectations for an around-the-clock operation and help state and local vaccination programs meet their goals, Leana Wen, MD, a professor at George Washington University, wrote in an editorial for The Washington Post.
“The Biden team’s urgency around vaccinations is commendable,” she added in a Twitter post on Jan. 11. “I’d like to see a guarantee that every 1st dose given will be followed with a timely 2nd dose. Otherwise, there are ethical concerns that could add to vaccine hesitancy.”
Biden has pledged that 100 million doses will be administered in his first 100 days in office. He has grown frustrated as concerns grow that his administration could fall short of the promise, according to Politico. His coronavirus response team has noted several challenges, including what they say is a lack of long-term planning by the Trump administration and an initial refusal to share key information.
“We’re uncovering new information each day, and we’re unearthing – of course – more work to be done,” Vivek Murthy, MD, Biden’s nominee for surgeon general, told Politico.
The team has uncovered staffing shortages, technology problems, and issues with health care insurance coverage. The incoming Biden team has developed several initiatives, such as mobile vaccination units and new federal sites to give shots. It could take weeks to get the vaccine rollout on track, the news outlet reported.
“Will this be challenging? Absolutely,” Anthony Fauci, MD, director of the National Institute of Allergy and Infectious Diseases and Biden’s incoming chief medical adviser on the coronavirus, told Politico. “This is an unprecedented effort to vaccinate the entire country over a period of time that’s fighting against people dying at record numbers. To say it’s not a challenge would be unrealistic. Do I think it can be done? Yes.”
A version of this article first appeared on WebMD.com.
The CDC will send $3 billion to the states to boost a lagging national COVID-19 vaccination program.
The Department of Health and Human Services announced the new funding as only 30% of the more than 22 million doses of vaccine distributed in the U.S. has been injected into Americans’ arms.
Along with the $3 billion, HHS said another $19 billion is headed to states and jurisdictions to boost COVID-19 testing programs. The amount each state will receive will be determined by population.
The news comes days after President-elect Joe Biden said he planned to release all available doses of vaccine after he takes office on Jan. 20. The Trump administration has been holding back millions of doses to ensure supply of vaccine to provide the necessary second dose for those who received the first shot.
“This funding is another timely investment that will strengthen our nation’s efforts to stop the COVID-19 pandemic in America,” CDC Director Robert Redfield, MD, said in a statement. “Particularly now, it is crucial that states and communities have the resources they need to conduct testing, and to distribute and administer safe, high-quality COVID-19 vaccines safely and equitably.”
Federal officials and public health experts, however, expressed concerns this weekend about Biden’s plan.
Outgoing Trump administration officials and others said they worry that doing so will leave providers without enough second doses for people getting the two-shot vaccines.
If Biden releases all available doses and the vaccine-making process has an issue, they said, that could pose a supply risk.
“We have product that is going through QC right now – quality control – for sterility, identity check that we have tens and tens of millions of product. We always will. But batches fail. Sterility fails ... and then you don’t have a product for that second dose,” Alex Azar, secretary of health and human services, told the American Hospital Association on Jan. 8, according to CNN.
“And frankly, talking about that or encouraging that can really undermine a critical public health need, which is that people come back for their second vaccine,” he said.
One of the main roadblocks in the vaccine rollout has been administering the doses that have already been distributed. The U.S. has shipped 22.1 million doses, and 6.6 million first shots have been given, according to the latest CDC data updated Jan. 8. Mr. Azar and other federal health officials have encouraged states to use their current supply and expand vaccine access to more priority groups.
“We would be delighted to learn that jurisdictions have actually administered many more doses than they are presently reporting,” a spokesman for the U.S. Department of Health and Human Services told CNN. “We are encouraging jurisdictions to expand their priority groups as needed to ensure no vaccine is sitting on the shelf after having been delivered to the jurisdiction-directed locations.”
Releasing more vaccines for first doses could create ethical concerns as well, since people getting vaccines expect to get a second dose in the proper amount of time, according to The Week. Biden’s transition team said on Jan. 8 that he won’t delay the second dose but, instead, plans to ramp up production to stay on track.
To do this well, the federal government should create a coordinated vaccine strategy that sets expectations for an around-the-clock operation and help state and local vaccination programs meet their goals, Leana Wen, MD, a professor at George Washington University, wrote in an editorial for The Washington Post.
“The Biden team’s urgency around vaccinations is commendable,” she added in a Twitter post on Jan. 11. “I’d like to see a guarantee that every 1st dose given will be followed with a timely 2nd dose. Otherwise, there are ethical concerns that could add to vaccine hesitancy.”
Biden has pledged that 100 million doses will be administered in his first 100 days in office. He has grown frustrated as concerns grow that his administration could fall short of the promise, according to Politico. His coronavirus response team has noted several challenges, including what they say is a lack of long-term planning by the Trump administration and an initial refusal to share key information.
“We’re uncovering new information each day, and we’re unearthing – of course – more work to be done,” Vivek Murthy, MD, Biden’s nominee for surgeon general, told Politico.
The team has uncovered staffing shortages, technology problems, and issues with health care insurance coverage. The incoming Biden team has developed several initiatives, such as mobile vaccination units and new federal sites to give shots. It could take weeks to get the vaccine rollout on track, the news outlet reported.
“Will this be challenging? Absolutely,” Anthony Fauci, MD, director of the National Institute of Allergy and Infectious Diseases and Biden’s incoming chief medical adviser on the coronavirus, told Politico. “This is an unprecedented effort to vaccinate the entire country over a period of time that’s fighting against people dying at record numbers. To say it’s not a challenge would be unrealistic. Do I think it can be done? Yes.”
A version of this article first appeared on WebMD.com.
The CDC will send $3 billion to the states to boost a lagging national COVID-19 vaccination program.
The Department of Health and Human Services announced the new funding as only 30% of the more than 22 million doses of vaccine distributed in the U.S. has been injected into Americans’ arms.
Along with the $3 billion, HHS said another $19 billion is headed to states and jurisdictions to boost COVID-19 testing programs. The amount each state will receive will be determined by population.
The news comes days after President-elect Joe Biden said he planned to release all available doses of vaccine after he takes office on Jan. 20. The Trump administration has been holding back millions of doses to ensure supply of vaccine to provide the necessary second dose for those who received the first shot.
“This funding is another timely investment that will strengthen our nation’s efforts to stop the COVID-19 pandemic in America,” CDC Director Robert Redfield, MD, said in a statement. “Particularly now, it is crucial that states and communities have the resources they need to conduct testing, and to distribute and administer safe, high-quality COVID-19 vaccines safely and equitably.”
Federal officials and public health experts, however, expressed concerns this weekend about Biden’s plan.
Outgoing Trump administration officials and others said they worry that doing so will leave providers without enough second doses for people getting the two-shot vaccines.
If Biden releases all available doses and the vaccine-making process has an issue, they said, that could pose a supply risk.
“We have product that is going through QC right now – quality control – for sterility, identity check that we have tens and tens of millions of product. We always will. But batches fail. Sterility fails ... and then you don’t have a product for that second dose,” Alex Azar, secretary of health and human services, told the American Hospital Association on Jan. 8, according to CNN.
“And frankly, talking about that or encouraging that can really undermine a critical public health need, which is that people come back for their second vaccine,” he said.
One of the main roadblocks in the vaccine rollout has been administering the doses that have already been distributed. The U.S. has shipped 22.1 million doses, and 6.6 million first shots have been given, according to the latest CDC data updated Jan. 8. Mr. Azar and other federal health officials have encouraged states to use their current supply and expand vaccine access to more priority groups.
“We would be delighted to learn that jurisdictions have actually administered many more doses than they are presently reporting,” a spokesman for the U.S. Department of Health and Human Services told CNN. “We are encouraging jurisdictions to expand their priority groups as needed to ensure no vaccine is sitting on the shelf after having been delivered to the jurisdiction-directed locations.”
Releasing more vaccines for first doses could create ethical concerns as well, since people getting vaccines expect to get a second dose in the proper amount of time, according to The Week. Biden’s transition team said on Jan. 8 that he won’t delay the second dose but, instead, plans to ramp up production to stay on track.
To do this well, the federal government should create a coordinated vaccine strategy that sets expectations for an around-the-clock operation and help state and local vaccination programs meet their goals, Leana Wen, MD, a professor at George Washington University, wrote in an editorial for The Washington Post.
“The Biden team’s urgency around vaccinations is commendable,” she added in a Twitter post on Jan. 11. “I’d like to see a guarantee that every 1st dose given will be followed with a timely 2nd dose. Otherwise, there are ethical concerns that could add to vaccine hesitancy.”
Biden has pledged that 100 million doses will be administered in his first 100 days in office. He has grown frustrated as concerns grow that his administration could fall short of the promise, according to Politico. His coronavirus response team has noted several challenges, including what they say is a lack of long-term planning by the Trump administration and an initial refusal to share key information.
“We’re uncovering new information each day, and we’re unearthing – of course – more work to be done,” Vivek Murthy, MD, Biden’s nominee for surgeon general, told Politico.
The team has uncovered staffing shortages, technology problems, and issues with health care insurance coverage. The incoming Biden team has developed several initiatives, such as mobile vaccination units and new federal sites to give shots. It could take weeks to get the vaccine rollout on track, the news outlet reported.
“Will this be challenging? Absolutely,” Anthony Fauci, MD, director of the National Institute of Allergy and Infectious Diseases and Biden’s incoming chief medical adviser on the coronavirus, told Politico. “This is an unprecedented effort to vaccinate the entire country over a period of time that’s fighting against people dying at record numbers. To say it’s not a challenge would be unrealistic. Do I think it can be done? Yes.”
A version of this article first appeared on WebMD.com.
The psychiatrist and the vaccine
When the long-awaited news of a Food and Drug Administration–approved vaccine came on Dec. 11, 2020, my first thought was that I would wait. I can manage a few more months of Zooming for work, my household is down to two people, I’m not at high risk of dying from COVID, and my husband is not going to be vaccinated any time soon, so a change in my status wouldn’t “free” me. I would rather have “my” vaccine go to a 70-year-old ICU janitor or a bus driver.
The weeks have gone by. I expected there would be kinks, but it has now been a month – one in which COVID rates have soared, and hospitalizations and deaths have risen to unmanageable numbers in some places. Still, vaccines remain in freezers – people are dying while vials of prevention sit unused. I began to think that, when my “turn” came, the better thing was to be vaccinated. We need to have a large segment of the population vaccinated to squelch this virus, and it’s become much less clear to me that, if I yield my turn, it will go into the arm of a bus driver. The process has not been fair, and there are moments of media outrage when one group gets vaccinated before another, so perhaps we have reached point where the goal should not be to get the vaccine into the exact right person in the exact right order, but to get the vaccine into arms according to the protocol that has already been set. Anyone who does not end up in a hospital bed is doing the system a favor.
Mahmood Jahromi, MD, a psychiatrist in private practice in Towson, Md., described the process of vaccination as being similar to a bottleneck traffic jam. “Yes, one must be courteous to the car trying to but in, but no, don’t jam the glue because you are excessively kind. Let the traffic police do their job. When your name is called, go ahead and take it. The system needs to know people are accepting the vaccine, not by begging the authorities to be called ahead of others, but with respect for what is already designed.”
On Friday, Jan. 8, I received information on how to get vaccinated – it seems my “turn” has arrived. An email from the board of physicians informed me that I am in the “1A” category and included a link to sign up for a vaccine in Baltimore – vaccinations would be given until Jan. 29, Mondays to Thursdays from 10 a.m. to 4 p.m. and Fridays from 10 a.m. to 1 p.m. There are no weekend or evening hours, and one might think there would be enough urgency to call for this. The Maryland Psychiatric Society sent out a notice that Sheppard Pratt would be offering vaccines to all behavioral health providers in the state of Maryland during a 2-day clinic. I heard from others that health care workers can now get vaccinated at the Cow Palace (how great is that?) at the Maryland State Fairgrounds and another link was sent for those in Howard County, between Baltimore and Washington.
As I discussed this with colleagues, a couple of issues came up – the most common was one of not wanting to get the vaccine yet because there are others who need it more. Others voiced concern about a vaccine where the long-term effects remain unknown: Is this vaccine safe, might it spur autoimmune problems in the months or years to come? Is it safe for women who plan to become pregnant? Some have insisted it is safe. They say “follow the science” and have dismissed the skepticism. To my read, it makes perfect sense to be wary, but COVID spreads silently and it kills.
With a vaccine where so many are reluctant to get it, including many health care workers, Sue Kim, MD, a psychiatrist in private practice in Lutherville, Md., noted that she has concerns about the safety of the vaccine. “Getting it now is both altruistic and selfish, but letting others go first is also altruistic and selfish. In the meantime, if I get sick, I was too smart for my own good. How do you weigh this ethically?”
My personal feelings have been influenced by a few things. An article in the New York Times highlighted how New York City vaccinated 5 million people for smallpox in just 2 weeks in 1947. I am frustrated knowing that, a month after approval of the first vaccine, only 7 million people have received it in the entire United States. In that time period, millions have contracted COVID and thousands have died. Closer to home, a 45-year-old psychiatrist in Maryland died of COVID, and I have heard more stories about younger people with long-haul neurologic and vascular symptoms. The risk of COVID is feeling higher than it did, and the fact that the first vaccine was authorized after the election somehow makes me feel that it might be safer. Had it been approved right before, I would have worried – perhaps wrongly – that the authorization was a political maneuver, not one based on science.
As we think about what is best for ourselves, our families, our patients, and society as a whole, I believe that those who want the vaccine but don’t feel they should take their place in line before others who are higher risk must ask if it makes sense to wait. Each state is different. While Houston Methodist Hospital is reportedly giving its health care workers a $500 bonus to get the vaccine, Gov. Andrew Cuomo of New York announced that hospitals would be fined $100,000 if they don’t use all of their vaccines within 7 days of receipt and $1 million if they vaccinate anyone out of order. Gov. Cuomo later broadened who could be vaccinated to prevent wasting the vaccine, but there remains an element of being damned if you do and damned if you don’t.
Paul Nestadt, MD, a psychiatrist at Johns Hopkins University, Baltimore, noted that one distribution site initially had to waste unused vaccine when people did not come for their appointments. A waiting list was created for people who could come right away if called to prevent this waste. “To me, this only highlighted that the tier system, while a good idea, does not need to be written in stone. The goal needs to be getting shots in arms, building herd immunity. If there are two arms in front of you, shoot the health care worker or those who are vulnerable. But if there is a healthy arm in reach, it should get any shot made available.”
I registered to be vaccinated. – senior citizens and essential workers are not yet eligible. In Baltimore, vaccinations are available Mondays to Thursdays from 10 a.m. to 4 p.m. and on Fridays from 10 a.m. to 1 p.m. There are no options for early morning or weekend times, but there are slots still available for the coming week. As of this writing, there are 6,100 Marylanders dead, and more than 1,800 COVID patients in hospital beds, and our governor, Larry Hogan, has commercials to “Mask On Maryland” and “Wear the Damn Mask.” I’ll offer some changes: “Wake Up, World” and “Offer the Damn Shot.”
Dr. Miller is coauthor of “Committed: The Battle Over Involuntary Psychiatric Care” (Baltimore: Johns Hopkins University, 2016). She is assistant professor of psychiatry and behavioral sciences at Johns Hopkins University, both in Baltimore. Dr. Miller has no disclosures.
When the long-awaited news of a Food and Drug Administration–approved vaccine came on Dec. 11, 2020, my first thought was that I would wait. I can manage a few more months of Zooming for work, my household is down to two people, I’m not at high risk of dying from COVID, and my husband is not going to be vaccinated any time soon, so a change in my status wouldn’t “free” me. I would rather have “my” vaccine go to a 70-year-old ICU janitor or a bus driver.
The weeks have gone by. I expected there would be kinks, but it has now been a month – one in which COVID rates have soared, and hospitalizations and deaths have risen to unmanageable numbers in some places. Still, vaccines remain in freezers – people are dying while vials of prevention sit unused. I began to think that, when my “turn” came, the better thing was to be vaccinated. We need to have a large segment of the population vaccinated to squelch this virus, and it’s become much less clear to me that, if I yield my turn, it will go into the arm of a bus driver. The process has not been fair, and there are moments of media outrage when one group gets vaccinated before another, so perhaps we have reached point where the goal should not be to get the vaccine into the exact right person in the exact right order, but to get the vaccine into arms according to the protocol that has already been set. Anyone who does not end up in a hospital bed is doing the system a favor.
Mahmood Jahromi, MD, a psychiatrist in private practice in Towson, Md., described the process of vaccination as being similar to a bottleneck traffic jam. “Yes, one must be courteous to the car trying to but in, but no, don’t jam the glue because you are excessively kind. Let the traffic police do their job. When your name is called, go ahead and take it. The system needs to know people are accepting the vaccine, not by begging the authorities to be called ahead of others, but with respect for what is already designed.”
On Friday, Jan. 8, I received information on how to get vaccinated – it seems my “turn” has arrived. An email from the board of physicians informed me that I am in the “1A” category and included a link to sign up for a vaccine in Baltimore – vaccinations would be given until Jan. 29, Mondays to Thursdays from 10 a.m. to 4 p.m. and Fridays from 10 a.m. to 1 p.m. There are no weekend or evening hours, and one might think there would be enough urgency to call for this. The Maryland Psychiatric Society sent out a notice that Sheppard Pratt would be offering vaccines to all behavioral health providers in the state of Maryland during a 2-day clinic. I heard from others that health care workers can now get vaccinated at the Cow Palace (how great is that?) at the Maryland State Fairgrounds and another link was sent for those in Howard County, between Baltimore and Washington.
As I discussed this with colleagues, a couple of issues came up – the most common was one of not wanting to get the vaccine yet because there are others who need it more. Others voiced concern about a vaccine where the long-term effects remain unknown: Is this vaccine safe, might it spur autoimmune problems in the months or years to come? Is it safe for women who plan to become pregnant? Some have insisted it is safe. They say “follow the science” and have dismissed the skepticism. To my read, it makes perfect sense to be wary, but COVID spreads silently and it kills.
With a vaccine where so many are reluctant to get it, including many health care workers, Sue Kim, MD, a psychiatrist in private practice in Lutherville, Md., noted that she has concerns about the safety of the vaccine. “Getting it now is both altruistic and selfish, but letting others go first is also altruistic and selfish. In the meantime, if I get sick, I was too smart for my own good. How do you weigh this ethically?”
My personal feelings have been influenced by a few things. An article in the New York Times highlighted how New York City vaccinated 5 million people for smallpox in just 2 weeks in 1947. I am frustrated knowing that, a month after approval of the first vaccine, only 7 million people have received it in the entire United States. In that time period, millions have contracted COVID and thousands have died. Closer to home, a 45-year-old psychiatrist in Maryland died of COVID, and I have heard more stories about younger people with long-haul neurologic and vascular symptoms. The risk of COVID is feeling higher than it did, and the fact that the first vaccine was authorized after the election somehow makes me feel that it might be safer. Had it been approved right before, I would have worried – perhaps wrongly – that the authorization was a political maneuver, not one based on science.
As we think about what is best for ourselves, our families, our patients, and society as a whole, I believe that those who want the vaccine but don’t feel they should take their place in line before others who are higher risk must ask if it makes sense to wait. Each state is different. While Houston Methodist Hospital is reportedly giving its health care workers a $500 bonus to get the vaccine, Gov. Andrew Cuomo of New York announced that hospitals would be fined $100,000 if they don’t use all of their vaccines within 7 days of receipt and $1 million if they vaccinate anyone out of order. Gov. Cuomo later broadened who could be vaccinated to prevent wasting the vaccine, but there remains an element of being damned if you do and damned if you don’t.
Paul Nestadt, MD, a psychiatrist at Johns Hopkins University, Baltimore, noted that one distribution site initially had to waste unused vaccine when people did not come for their appointments. A waiting list was created for people who could come right away if called to prevent this waste. “To me, this only highlighted that the tier system, while a good idea, does not need to be written in stone. The goal needs to be getting shots in arms, building herd immunity. If there are two arms in front of you, shoot the health care worker or those who are vulnerable. But if there is a healthy arm in reach, it should get any shot made available.”
I registered to be vaccinated. – senior citizens and essential workers are not yet eligible. In Baltimore, vaccinations are available Mondays to Thursdays from 10 a.m. to 4 p.m. and on Fridays from 10 a.m. to 1 p.m. There are no options for early morning or weekend times, but there are slots still available for the coming week. As of this writing, there are 6,100 Marylanders dead, and more than 1,800 COVID patients in hospital beds, and our governor, Larry Hogan, has commercials to “Mask On Maryland” and “Wear the Damn Mask.” I’ll offer some changes: “Wake Up, World” and “Offer the Damn Shot.”
Dr. Miller is coauthor of “Committed: The Battle Over Involuntary Psychiatric Care” (Baltimore: Johns Hopkins University, 2016). She is assistant professor of psychiatry and behavioral sciences at Johns Hopkins University, both in Baltimore. Dr. Miller has no disclosures.
When the long-awaited news of a Food and Drug Administration–approved vaccine came on Dec. 11, 2020, my first thought was that I would wait. I can manage a few more months of Zooming for work, my household is down to two people, I’m not at high risk of dying from COVID, and my husband is not going to be vaccinated any time soon, so a change in my status wouldn’t “free” me. I would rather have “my” vaccine go to a 70-year-old ICU janitor or a bus driver.
The weeks have gone by. I expected there would be kinks, but it has now been a month – one in which COVID rates have soared, and hospitalizations and deaths have risen to unmanageable numbers in some places. Still, vaccines remain in freezers – people are dying while vials of prevention sit unused. I began to think that, when my “turn” came, the better thing was to be vaccinated. We need to have a large segment of the population vaccinated to squelch this virus, and it’s become much less clear to me that, if I yield my turn, it will go into the arm of a bus driver. The process has not been fair, and there are moments of media outrage when one group gets vaccinated before another, so perhaps we have reached point where the goal should not be to get the vaccine into the exact right person in the exact right order, but to get the vaccine into arms according to the protocol that has already been set. Anyone who does not end up in a hospital bed is doing the system a favor.
Mahmood Jahromi, MD, a psychiatrist in private practice in Towson, Md., described the process of vaccination as being similar to a bottleneck traffic jam. “Yes, one must be courteous to the car trying to but in, but no, don’t jam the glue because you are excessively kind. Let the traffic police do their job. When your name is called, go ahead and take it. The system needs to know people are accepting the vaccine, not by begging the authorities to be called ahead of others, but with respect for what is already designed.”
On Friday, Jan. 8, I received information on how to get vaccinated – it seems my “turn” has arrived. An email from the board of physicians informed me that I am in the “1A” category and included a link to sign up for a vaccine in Baltimore – vaccinations would be given until Jan. 29, Mondays to Thursdays from 10 a.m. to 4 p.m. and Fridays from 10 a.m. to 1 p.m. There are no weekend or evening hours, and one might think there would be enough urgency to call for this. The Maryland Psychiatric Society sent out a notice that Sheppard Pratt would be offering vaccines to all behavioral health providers in the state of Maryland during a 2-day clinic. I heard from others that health care workers can now get vaccinated at the Cow Palace (how great is that?) at the Maryland State Fairgrounds and another link was sent for those in Howard County, between Baltimore and Washington.
As I discussed this with colleagues, a couple of issues came up – the most common was one of not wanting to get the vaccine yet because there are others who need it more. Others voiced concern about a vaccine where the long-term effects remain unknown: Is this vaccine safe, might it spur autoimmune problems in the months or years to come? Is it safe for women who plan to become pregnant? Some have insisted it is safe. They say “follow the science” and have dismissed the skepticism. To my read, it makes perfect sense to be wary, but COVID spreads silently and it kills.
With a vaccine where so many are reluctant to get it, including many health care workers, Sue Kim, MD, a psychiatrist in private practice in Lutherville, Md., noted that she has concerns about the safety of the vaccine. “Getting it now is both altruistic and selfish, but letting others go first is also altruistic and selfish. In the meantime, if I get sick, I was too smart for my own good. How do you weigh this ethically?”
My personal feelings have been influenced by a few things. An article in the New York Times highlighted how New York City vaccinated 5 million people for smallpox in just 2 weeks in 1947. I am frustrated knowing that, a month after approval of the first vaccine, only 7 million people have received it in the entire United States. In that time period, millions have contracted COVID and thousands have died. Closer to home, a 45-year-old psychiatrist in Maryland died of COVID, and I have heard more stories about younger people with long-haul neurologic and vascular symptoms. The risk of COVID is feeling higher than it did, and the fact that the first vaccine was authorized after the election somehow makes me feel that it might be safer. Had it been approved right before, I would have worried – perhaps wrongly – that the authorization was a political maneuver, not one based on science.
As we think about what is best for ourselves, our families, our patients, and society as a whole, I believe that those who want the vaccine but don’t feel they should take their place in line before others who are higher risk must ask if it makes sense to wait. Each state is different. While Houston Methodist Hospital is reportedly giving its health care workers a $500 bonus to get the vaccine, Gov. Andrew Cuomo of New York announced that hospitals would be fined $100,000 if they don’t use all of their vaccines within 7 days of receipt and $1 million if they vaccinate anyone out of order. Gov. Cuomo later broadened who could be vaccinated to prevent wasting the vaccine, but there remains an element of being damned if you do and damned if you don’t.
Paul Nestadt, MD, a psychiatrist at Johns Hopkins University, Baltimore, noted that one distribution site initially had to waste unused vaccine when people did not come for their appointments. A waiting list was created for people who could come right away if called to prevent this waste. “To me, this only highlighted that the tier system, while a good idea, does not need to be written in stone. The goal needs to be getting shots in arms, building herd immunity. If there are two arms in front of you, shoot the health care worker or those who are vulnerable. But if there is a healthy arm in reach, it should get any shot made available.”
I registered to be vaccinated. – senior citizens and essential workers are not yet eligible. In Baltimore, vaccinations are available Mondays to Thursdays from 10 a.m. to 4 p.m. and on Fridays from 10 a.m. to 1 p.m. There are no options for early morning or weekend times, but there are slots still available for the coming week. As of this writing, there are 6,100 Marylanders dead, and more than 1,800 COVID patients in hospital beds, and our governor, Larry Hogan, has commercials to “Mask On Maryland” and “Wear the Damn Mask.” I’ll offer some changes: “Wake Up, World” and “Offer the Damn Shot.”
Dr. Miller is coauthor of “Committed: The Battle Over Involuntary Psychiatric Care” (Baltimore: Johns Hopkins University, 2016). She is assistant professor of psychiatry and behavioral sciences at Johns Hopkins University, both in Baltimore. Dr. Miller has no disclosures.
VA Ramps up Vaccinations as COVID-19 Cases Continue to Rise
Updated January 12, 2020
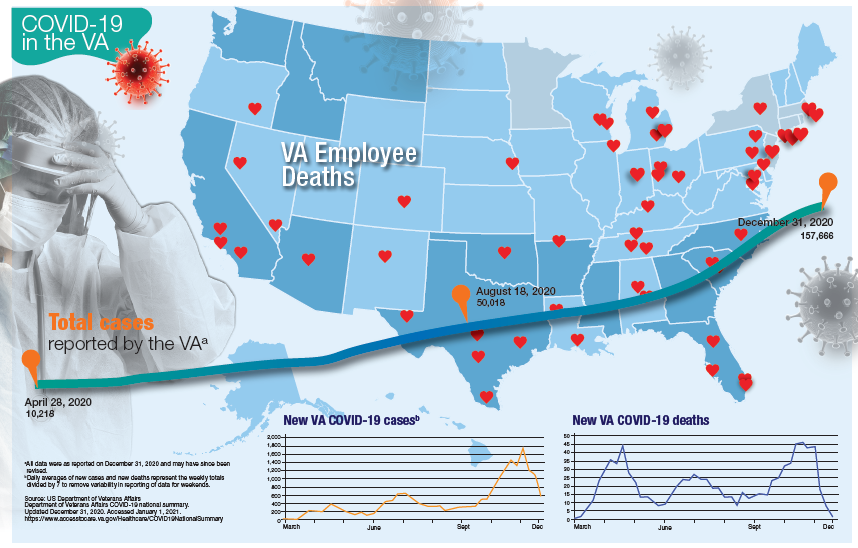
More than 181,000 veterans have contracted the COVID-19 virus and 7,385 have died, according to data released by the US Department of Veterans Affairs (VA) on January 12, 2020. The number of cases and deaths have increased sharply since November 2020. The VA also reports that it has administered at least 1 dose of the 2 approved vaccines to 33,875 veterans and 174,724 employees as of January 6.
Currently, the VA reports nearly 19,000 active cases of COVID-19, including 1,270 among VA employees. One hundred five VA employees have died from COVID-19.
Although facilities across the country are facing increased pressure as the number of cases rise, those in Southern California and Texas are reporting significant infection rates. Thirteen facilities have at least 300 active cases, including facilities in Loma Linda (418), Long Beach (381), Greater Los Angeles (361), and San Diego (274), all in California. In Texas, San Antonio (394), Dallas (370), Temple (338), and Houston (328) have all seen large numbers of active cases. Facilities in Columbia, South Carolina (420); Phoenix (407); Atlanta, Georgia (359); Cleveland, Ohio (352); and Orlando, (341) and Gainesville, Florida (340) also have reported significant numbers of cases.
While early on in the pandemic facilities in New York and New Jersey had reported the largest number of deaths, now nearly every facility has reported at least 1 death. Fourteen facilities have reported at least 100 deaths and 53 have reported between 50 and 99 deaths. The 7,385 VA COVID-19 deaths represent 2.0% of the 375,300 deaths reported in the US by Johns Hopkins University. VA has reported 0.8% of the total number of COVID-19 cases.
The VA also reports the demographic breakdown of its COVID-19 cases. Among the active cases, 56.9% are White, 18.3% Black, 9.4% Hispanic, and 1.4% Native American, Alaska Native, or Pacific Islander.

Updated January 12, 2020
More than 181,000 veterans have contracted the COVID-19 virus and 7,385 have died, according to data released by the US Department of Veterans Affairs (VA) on January 12, 2020. The number of cases and deaths have increased sharply since November 2020. The VA also reports that it has administered at least 1 dose of the 2 approved vaccines to 33,875 veterans and 174,724 employees as of January 6.
Currently, the VA reports nearly 19,000 active cases of COVID-19, including 1,270 among VA employees. One hundred five VA employees have died from COVID-19.
Although facilities across the country are facing increased pressure as the number of cases rise, those in Southern California and Texas are reporting significant infection rates. Thirteen facilities have at least 300 active cases, including facilities in Loma Linda (418), Long Beach (381), Greater Los Angeles (361), and San Diego (274), all in California. In Texas, San Antonio (394), Dallas (370), Temple (338), and Houston (328) have all seen large numbers of active cases. Facilities in Columbia, South Carolina (420); Phoenix (407); Atlanta, Georgia (359); Cleveland, Ohio (352); and Orlando, (341) and Gainesville, Florida (340) also have reported significant numbers of cases.
While early on in the pandemic facilities in New York and New Jersey had reported the largest number of deaths, now nearly every facility has reported at least 1 death. Fourteen facilities have reported at least 100 deaths and 53 have reported between 50 and 99 deaths. The 7,385 VA COVID-19 deaths represent 2.0% of the 375,300 deaths reported in the US by Johns Hopkins University. VA has reported 0.8% of the total number of COVID-19 cases.
The VA also reports the demographic breakdown of its COVID-19 cases. Among the active cases, 56.9% are White, 18.3% Black, 9.4% Hispanic, and 1.4% Native American, Alaska Native, or Pacific Islander.

Updated January 12, 2020
More than 181,000 veterans have contracted the COVID-19 virus and 7,385 have died, according to data released by the US Department of Veterans Affairs (VA) on January 12, 2020. The number of cases and deaths have increased sharply since November 2020. The VA also reports that it has administered at least 1 dose of the 2 approved vaccines to 33,875 veterans and 174,724 employees as of January 6.
Currently, the VA reports nearly 19,000 active cases of COVID-19, including 1,270 among VA employees. One hundred five VA employees have died from COVID-19.
Although facilities across the country are facing increased pressure as the number of cases rise, those in Southern California and Texas are reporting significant infection rates. Thirteen facilities have at least 300 active cases, including facilities in Loma Linda (418), Long Beach (381), Greater Los Angeles (361), and San Diego (274), all in California. In Texas, San Antonio (394), Dallas (370), Temple (338), and Houston (328) have all seen large numbers of active cases. Facilities in Columbia, South Carolina (420); Phoenix (407); Atlanta, Georgia (359); Cleveland, Ohio (352); and Orlando, (341) and Gainesville, Florida (340) also have reported significant numbers of cases.
While early on in the pandemic facilities in New York and New Jersey had reported the largest number of deaths, now nearly every facility has reported at least 1 death. Fourteen facilities have reported at least 100 deaths and 53 have reported between 50 and 99 deaths. The 7,385 VA COVID-19 deaths represent 2.0% of the 375,300 deaths reported in the US by Johns Hopkins University. VA has reported 0.8% of the total number of COVID-19 cases.
The VA also reports the demographic breakdown of its COVID-19 cases. Among the active cases, 56.9% are White, 18.3% Black, 9.4% Hispanic, and 1.4% Native American, Alaska Native, or Pacific Islander.
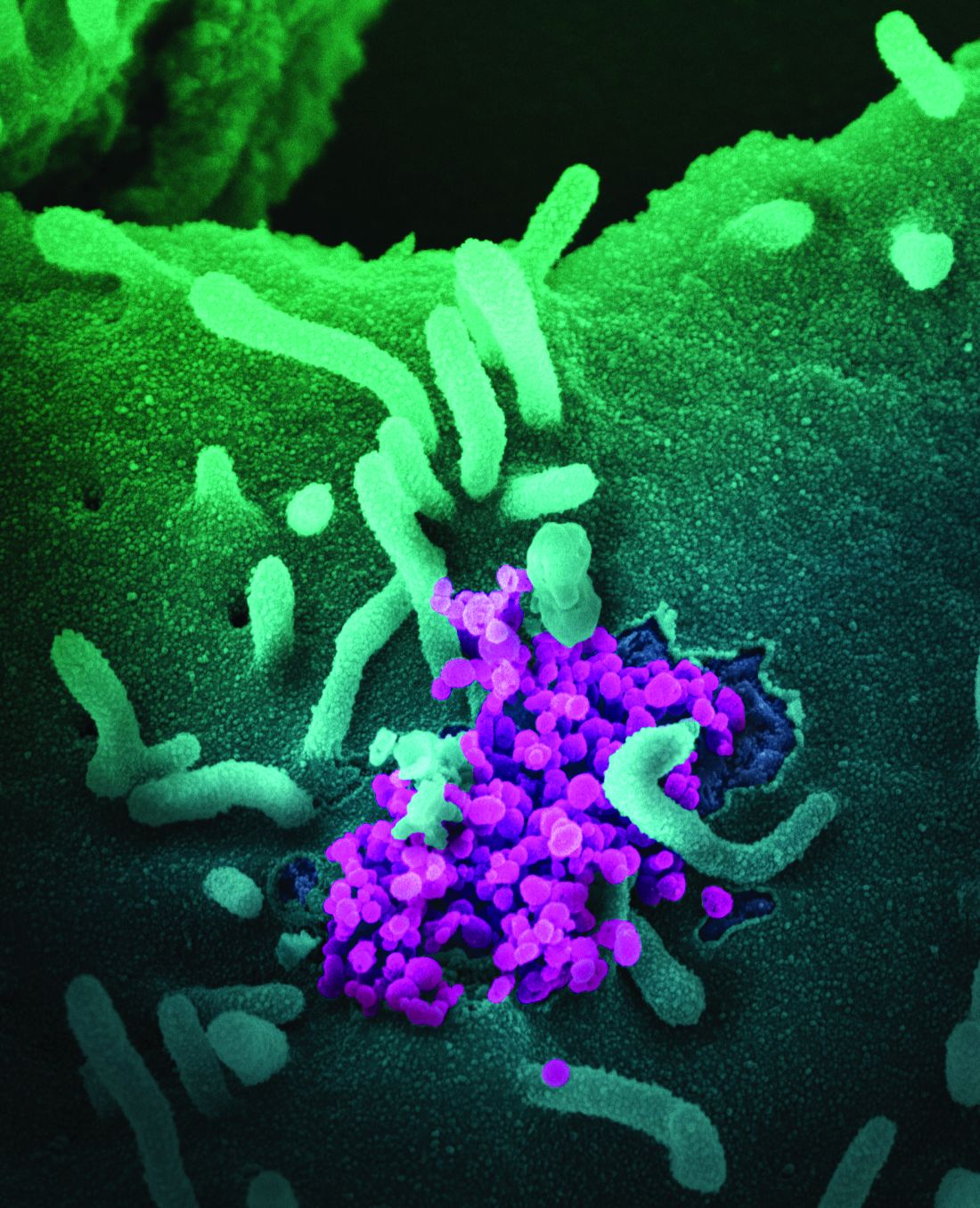
Over half of COVID-19 transmission may occur via asymptomatic people
As COVID-19 cases surge and vaccinations lag, health authorities continue to seek additional ways to mitigate the spread of the novel coronavirus.
Now, a modeling study estimates that more than half of transmissions come from pre-, never-, and asymptomatic individuals, indicating that symptom-based screening will have little effect on spread.
The Centers for Disease Control and Prevention study, published online Jan. 7 in JAMA Network Open, concludes that for optimal control, protective measures such as masking and social distancing should be supplemented with strategic testing of potentially exposed but asymptomatic individuals .
“In the absence of effective and widespread use of therapeutics or vaccines that can shorten or eliminate infectivity, successful control of SARS-CoV-2 cannot rely solely on identifying and isolating symptomatic cases; even if implemented effectively, this strategy would be insufficient,” CDC biologist Michael J. Johansson, PhD, and colleagues warn. “Multiple measures that effectively address transmission risk in the absence of symptoms are imperative to control SARS-CoV-2.”
According to the authors, the effectiveness of some current transmission prevention efforts has been disputed and subject to mixed messaging. Therefore, they decided to model the proportion of COVID-19 infections that are likely the result of individuals who show no symptoms and may be unknowingly infecting others.
“Unfortunately, there continues to be some skepticism about the value of community-wide mitigation efforts for preventing transmission such as masking, distancing, and hand hygiene, particularly for people without symptoms,” corresponding author Jay C. Butler, MD, said in an interview. “So we wanted to have a base assumption about how much transmission occurs from asymptomatic people to underscore the importance of mitigation measures and of creating immunity through vaccine delivery.”
Such a yardstick is especially germane in the context of the new, more transmissible variant. “It really puts [things] in a bigger box and underscores, boldfaces, and italicizes the need to change people’s behaviors and the importance of mitigation,” Dr. Butler said. It also highlights the advisability of targeted strategic testing in congregate settings, schools, and universities, which is already underway.
The analysis
Based on data from several COVID-19 studies from last year, the CDC’s analytical model assumes at baseline that infectiousness peaks at the median point of symptom onset, and that 30% of infected individuals never develop symptoms but are nevertheless 75% as infectious as those who develop overt symptoms.
The investigators then model multiple scenarios of transmission based pre- and never-symptomatic individuals, assuming different incubation and infectious periods, and varying numbers of days from point of infection to symptom onset.
When combined, the models predicts that 59% of all transmission would come from asymptomatic transmission – 35% from presymptomatic individuals and 24% from never-symptomatic individuals.
The findings complement those of an earlier CDC analysis, according to the authors.
The overall proportion of transmission from presymptomatic and never-symptomatic individuals is key to identifying mitigation measures that may be able to control SARS-CoV-2, the authors stated.
For example, they explain, if the infection reproduction number (R) in a particular setting is 2.0, a reduction in transmission of at least 50% is needed in order to reduce R to below 1.0. “Given that in some settings R is likely much greater than 2 and more than half of transmissions may come from individuals who are asymptomatic at the time of transmission, effective control must mitigate transmission risk from people without symptoms,” they wrote.
The authors acknowledge that the study applies a simplistic model to a complex and evolving phenomenon, and that the exact proportions of presymptomatic and never-symptomatic transmission and the incubation periods are not known. They also note symptoms and transmissions appear to vary across different population groups, with older individuals more likely than younger persons to experience symptoms, according to previous studies.
“Assume that everyone is potentially infected”
Other experts agree that expanded testing of asymptomatic individuals is important. “Screening for fever and isolation of symptomatic individuals is a common-sense approach to help prevent spread, but these measures are by no means adequate since it’s been clearly documented that individuals who are either asymptomatic or presymptomatic can still spread the virus,” said Brett Williams, MD, an infectious disease specialist and assistant professor of medicine at Rush University in Chicago.
“As we saw with the White House Rose Garden superspreader outbreak, testing does not reliably exclude infection either because the tested individual has not yet become positive or the test is falsely negative,” Dr. Williams, who was not involved in the CDC study, said in an interview. He further noted that when prevalence is as high as it currently is in the United States, the rate of false negatives will be high because a large proportion of those screened will be unknowingly infected.
At his center, all visitors and staff are screened with a temperature probe on entry, and since the earliest days of the pandemic, universal masking has been required. “Nationally there have been many instances of hospital break room outbreaks because of staff eating lunch together, and these outbreaks also demonstrate the incompleteness of symptomatic isolation,” Dr. Williams said.
For his part, virologist Frank Esper, MD, a pediatric infectious disease specialist at the Cleveland Clinic, said that while it’s been understood for some time that many infected people will not exhibit symptoms, “the question that remains is just how infectious are they?”
Dr. Esper’s takeaway from the modeling study is not so much that we need more screening of possibly exposed but asymptomatic people, but rather testing symptomatic people and tracing their contacts is not enough.
“We need to continue to assume that everyone is potentially infected whether they know it or not. And even though we have ramped up our testing to a much greater capacity than in the first wave, we need to continue to wear masks and socially distance because just identifying people who are sick and isolating or quarantining them is not going to be enough to contain the pandemic.”
And although assumption-based modeling is helpful, it cannot tell us “how many asymptomatic people are actually infected,” said Dr. Esper, who was not involved in the CDC study.
Dr. Esper also pointed out that the study estimates are based on data from early Chinese studies, but the virus has since changed. The new, more transmissible strain in the United States and elsewhere may involve not only more infections but also a longer presymptomatic stage. “So the CDC study may actually undershoot asymptomatic infections,” he said.
He also agreed with the authors that when it comes to infection, not all humans are equal. “Older people tend to be more symptomatic and become symptomatic more quickly so the asymptomatic rate is not the same across board from young people age 20 to older people.”
The bottom line, said David. A. Hirschwerk, MD, an infectious disease specialist at Northwell Health in Manhasset, N.Y., is that these data support the maintenance of protective measures we’ve been taking over the past months. “They support the concept that asymptomatic people are a significant source of transmission and that we need to adhere to mask wearing and social distancing, particularly indoors,” Dr. Hirschwerk, who was not involved in the analysis, said in an interview. “More testing would be better but it has to be fast and it has to be efficient, and there are a lot of challenges to overcome.”
The study was done as part of the CDC’s coronavirus disease 2019 response and was supported solely by federal base and response funding. The authors and commentators have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
As COVID-19 cases surge and vaccinations lag, health authorities continue to seek additional ways to mitigate the spread of the novel coronavirus.
Now, a modeling study estimates that more than half of transmissions come from pre-, never-, and asymptomatic individuals, indicating that symptom-based screening will have little effect on spread.
The Centers for Disease Control and Prevention study, published online Jan. 7 in JAMA Network Open, concludes that for optimal control, protective measures such as masking and social distancing should be supplemented with strategic testing of potentially exposed but asymptomatic individuals .
“In the absence of effective and widespread use of therapeutics or vaccines that can shorten or eliminate infectivity, successful control of SARS-CoV-2 cannot rely solely on identifying and isolating symptomatic cases; even if implemented effectively, this strategy would be insufficient,” CDC biologist Michael J. Johansson, PhD, and colleagues warn. “Multiple measures that effectively address transmission risk in the absence of symptoms are imperative to control SARS-CoV-2.”
According to the authors, the effectiveness of some current transmission prevention efforts has been disputed and subject to mixed messaging. Therefore, they decided to model the proportion of COVID-19 infections that are likely the result of individuals who show no symptoms and may be unknowingly infecting others.
“Unfortunately, there continues to be some skepticism about the value of community-wide mitigation efforts for preventing transmission such as masking, distancing, and hand hygiene, particularly for people without symptoms,” corresponding author Jay C. Butler, MD, said in an interview. “So we wanted to have a base assumption about how much transmission occurs from asymptomatic people to underscore the importance of mitigation measures and of creating immunity through vaccine delivery.”
Such a yardstick is especially germane in the context of the new, more transmissible variant. “It really puts [things] in a bigger box and underscores, boldfaces, and italicizes the need to change people’s behaviors and the importance of mitigation,” Dr. Butler said. It also highlights the advisability of targeted strategic testing in congregate settings, schools, and universities, which is already underway.
The analysis
Based on data from several COVID-19 studies from last year, the CDC’s analytical model assumes at baseline that infectiousness peaks at the median point of symptom onset, and that 30% of infected individuals never develop symptoms but are nevertheless 75% as infectious as those who develop overt symptoms.
The investigators then model multiple scenarios of transmission based pre- and never-symptomatic individuals, assuming different incubation and infectious periods, and varying numbers of days from point of infection to symptom onset.
When combined, the models predicts that 59% of all transmission would come from asymptomatic transmission – 35% from presymptomatic individuals and 24% from never-symptomatic individuals.
The findings complement those of an earlier CDC analysis, according to the authors.
The overall proportion of transmission from presymptomatic and never-symptomatic individuals is key to identifying mitigation measures that may be able to control SARS-CoV-2, the authors stated.
For example, they explain, if the infection reproduction number (R) in a particular setting is 2.0, a reduction in transmission of at least 50% is needed in order to reduce R to below 1.0. “Given that in some settings R is likely much greater than 2 and more than half of transmissions may come from individuals who are asymptomatic at the time of transmission, effective control must mitigate transmission risk from people without symptoms,” they wrote.
The authors acknowledge that the study applies a simplistic model to a complex and evolving phenomenon, and that the exact proportions of presymptomatic and never-symptomatic transmission and the incubation periods are not known. They also note symptoms and transmissions appear to vary across different population groups, with older individuals more likely than younger persons to experience symptoms, according to previous studies.
“Assume that everyone is potentially infected”
Other experts agree that expanded testing of asymptomatic individuals is important. “Screening for fever and isolation of symptomatic individuals is a common-sense approach to help prevent spread, but these measures are by no means adequate since it’s been clearly documented that individuals who are either asymptomatic or presymptomatic can still spread the virus,” said Brett Williams, MD, an infectious disease specialist and assistant professor of medicine at Rush University in Chicago.
“As we saw with the White House Rose Garden superspreader outbreak, testing does not reliably exclude infection either because the tested individual has not yet become positive or the test is falsely negative,” Dr. Williams, who was not involved in the CDC study, said in an interview. He further noted that when prevalence is as high as it currently is in the United States, the rate of false negatives will be high because a large proportion of those screened will be unknowingly infected.
At his center, all visitors and staff are screened with a temperature probe on entry, and since the earliest days of the pandemic, universal masking has been required. “Nationally there have been many instances of hospital break room outbreaks because of staff eating lunch together, and these outbreaks also demonstrate the incompleteness of symptomatic isolation,” Dr. Williams said.
For his part, virologist Frank Esper, MD, a pediatric infectious disease specialist at the Cleveland Clinic, said that while it’s been understood for some time that many infected people will not exhibit symptoms, “the question that remains is just how infectious are they?”
Dr. Esper’s takeaway from the modeling study is not so much that we need more screening of possibly exposed but asymptomatic people, but rather testing symptomatic people and tracing their contacts is not enough.
“We need to continue to assume that everyone is potentially infected whether they know it or not. And even though we have ramped up our testing to a much greater capacity than in the first wave, we need to continue to wear masks and socially distance because just identifying people who are sick and isolating or quarantining them is not going to be enough to contain the pandemic.”
And although assumption-based modeling is helpful, it cannot tell us “how many asymptomatic people are actually infected,” said Dr. Esper, who was not involved in the CDC study.
Dr. Esper also pointed out that the study estimates are based on data from early Chinese studies, but the virus has since changed. The new, more transmissible strain in the United States and elsewhere may involve not only more infections but also a longer presymptomatic stage. “So the CDC study may actually undershoot asymptomatic infections,” he said.
He also agreed with the authors that when it comes to infection, not all humans are equal. “Older people tend to be more symptomatic and become symptomatic more quickly so the asymptomatic rate is not the same across board from young people age 20 to older people.”
The bottom line, said David. A. Hirschwerk, MD, an infectious disease specialist at Northwell Health in Manhasset, N.Y., is that these data support the maintenance of protective measures we’ve been taking over the past months. “They support the concept that asymptomatic people are a significant source of transmission and that we need to adhere to mask wearing and social distancing, particularly indoors,” Dr. Hirschwerk, who was not involved in the analysis, said in an interview. “More testing would be better but it has to be fast and it has to be efficient, and there are a lot of challenges to overcome.”
The study was done as part of the CDC’s coronavirus disease 2019 response and was supported solely by federal base and response funding. The authors and commentators have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
As COVID-19 cases surge and vaccinations lag, health authorities continue to seek additional ways to mitigate the spread of the novel coronavirus.
Now, a modeling study estimates that more than half of transmissions come from pre-, never-, and asymptomatic individuals, indicating that symptom-based screening will have little effect on spread.
The Centers for Disease Control and Prevention study, published online Jan. 7 in JAMA Network Open, concludes that for optimal control, protective measures such as masking and social distancing should be supplemented with strategic testing of potentially exposed but asymptomatic individuals .
“In the absence of effective and widespread use of therapeutics or vaccines that can shorten or eliminate infectivity, successful control of SARS-CoV-2 cannot rely solely on identifying and isolating symptomatic cases; even if implemented effectively, this strategy would be insufficient,” CDC biologist Michael J. Johansson, PhD, and colleagues warn. “Multiple measures that effectively address transmission risk in the absence of symptoms are imperative to control SARS-CoV-2.”
According to the authors, the effectiveness of some current transmission prevention efforts has been disputed and subject to mixed messaging. Therefore, they decided to model the proportion of COVID-19 infections that are likely the result of individuals who show no symptoms and may be unknowingly infecting others.
“Unfortunately, there continues to be some skepticism about the value of community-wide mitigation efforts for preventing transmission such as masking, distancing, and hand hygiene, particularly for people without symptoms,” corresponding author Jay C. Butler, MD, said in an interview. “So we wanted to have a base assumption about how much transmission occurs from asymptomatic people to underscore the importance of mitigation measures and of creating immunity through vaccine delivery.”
Such a yardstick is especially germane in the context of the new, more transmissible variant. “It really puts [things] in a bigger box and underscores, boldfaces, and italicizes the need to change people’s behaviors and the importance of mitigation,” Dr. Butler said. It also highlights the advisability of targeted strategic testing in congregate settings, schools, and universities, which is already underway.
The analysis
Based on data from several COVID-19 studies from last year, the CDC’s analytical model assumes at baseline that infectiousness peaks at the median point of symptom onset, and that 30% of infected individuals never develop symptoms but are nevertheless 75% as infectious as those who develop overt symptoms.
The investigators then model multiple scenarios of transmission based pre- and never-symptomatic individuals, assuming different incubation and infectious periods, and varying numbers of days from point of infection to symptom onset.
When combined, the models predicts that 59% of all transmission would come from asymptomatic transmission – 35% from presymptomatic individuals and 24% from never-symptomatic individuals.
The findings complement those of an earlier CDC analysis, according to the authors.
The overall proportion of transmission from presymptomatic and never-symptomatic individuals is key to identifying mitigation measures that may be able to control SARS-CoV-2, the authors stated.
For example, they explain, if the infection reproduction number (R) in a particular setting is 2.0, a reduction in transmission of at least 50% is needed in order to reduce R to below 1.0. “Given that in some settings R is likely much greater than 2 and more than half of transmissions may come from individuals who are asymptomatic at the time of transmission, effective control must mitigate transmission risk from people without symptoms,” they wrote.
The authors acknowledge that the study applies a simplistic model to a complex and evolving phenomenon, and that the exact proportions of presymptomatic and never-symptomatic transmission and the incubation periods are not known. They also note symptoms and transmissions appear to vary across different population groups, with older individuals more likely than younger persons to experience symptoms, according to previous studies.
“Assume that everyone is potentially infected”
Other experts agree that expanded testing of asymptomatic individuals is important. “Screening for fever and isolation of symptomatic individuals is a common-sense approach to help prevent spread, but these measures are by no means adequate since it’s been clearly documented that individuals who are either asymptomatic or presymptomatic can still spread the virus,” said Brett Williams, MD, an infectious disease specialist and assistant professor of medicine at Rush University in Chicago.
“As we saw with the White House Rose Garden superspreader outbreak, testing does not reliably exclude infection either because the tested individual has not yet become positive or the test is falsely negative,” Dr. Williams, who was not involved in the CDC study, said in an interview. He further noted that when prevalence is as high as it currently is in the United States, the rate of false negatives will be high because a large proportion of those screened will be unknowingly infected.
At his center, all visitors and staff are screened with a temperature probe on entry, and since the earliest days of the pandemic, universal masking has been required. “Nationally there have been many instances of hospital break room outbreaks because of staff eating lunch together, and these outbreaks also demonstrate the incompleteness of symptomatic isolation,” Dr. Williams said.
For his part, virologist Frank Esper, MD, a pediatric infectious disease specialist at the Cleveland Clinic, said that while it’s been understood for some time that many infected people will not exhibit symptoms, “the question that remains is just how infectious are they?”
Dr. Esper’s takeaway from the modeling study is not so much that we need more screening of possibly exposed but asymptomatic people, but rather testing symptomatic people and tracing their contacts is not enough.
“We need to continue to assume that everyone is potentially infected whether they know it or not. And even though we have ramped up our testing to a much greater capacity than in the first wave, we need to continue to wear masks and socially distance because just identifying people who are sick and isolating or quarantining them is not going to be enough to contain the pandemic.”
And although assumption-based modeling is helpful, it cannot tell us “how many asymptomatic people are actually infected,” said Dr. Esper, who was not involved in the CDC study.
Dr. Esper also pointed out that the study estimates are based on data from early Chinese studies, but the virus has since changed. The new, more transmissible strain in the United States and elsewhere may involve not only more infections but also a longer presymptomatic stage. “So the CDC study may actually undershoot asymptomatic infections,” he said.
He also agreed with the authors that when it comes to infection, not all humans are equal. “Older people tend to be more symptomatic and become symptomatic more quickly so the asymptomatic rate is not the same across board from young people age 20 to older people.”
The bottom line, said David. A. Hirschwerk, MD, an infectious disease specialist at Northwell Health in Manhasset, N.Y., is that these data support the maintenance of protective measures we’ve been taking over the past months. “They support the concept that asymptomatic people are a significant source of transmission and that we need to adhere to mask wearing and social distancing, particularly indoors,” Dr. Hirschwerk, who was not involved in the analysis, said in an interview. “More testing would be better but it has to be fast and it has to be efficient, and there are a lot of challenges to overcome.”
The study was done as part of the CDC’s coronavirus disease 2019 response and was supported solely by federal base and response funding. The authors and commentators have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Trust in a Vial
On December 11, 2020, the US Food and Drug Administration (FDA) delivered the holiday gift America was waiting for—approval of the first COVID-19 vaccine. Following the recommendation of its expert advisory panel, the FDA issued its opening emergency use authorization (EUA) for the Pfizer and BioNTech product to be distributed and administered across the country.1 A week after that historic announcement, the FDA issued an EUA to Moderna for a second COVID-19 vaccine.2
An EUA is a misunderstood concept that, like the development of the vaccine itself, appears almost like a magical federal deliverance to a nation at a time when almost every other public health effort has floundered. An EUA is a regulatory process to enable a public health emergency response with medical countermeasures including not only vaccines, but also medications. Earlier in 2020, hydroxychloroquine and remdesivir each received EUAs for treating patients with COVID-19.3 The EUA for hydroxychloroquine was later revoked when more data raised concerns for its efficacy.4 EUAs do not mean the drugs are experimental or that everyone receiving them is participating in a research trial; however, for the sake of safety and science, data continue to be collected and analyzed. Issuance of an EUA indicates that after rigorous examination and an independent advisory board review of data submitted by the manufacturer, the FDA has determined the product and situation meet key criteria: (1) There is a public health emergency that threatens health and life and requires expedited procedures; (2) there are no extant approved products able to treat or prevent the disease; and (3) the known and potential benefits of the product outweigh the known and potential risks.5
The public and even the professional press have celebrated the arrival of this technologic triumph over a virus that had vanquished staggering numbers of lives and livelihoods. Much of the media coverage aptly chose the word “hope” to capture the significance of this unprecedented accomplishment for which so many millions yearned. A Google search for “hope” on the morning of December 20, yielded 339,000,000 results. For example, a headline especially salient for Federal Practitioner readers from the New York Times read, “‘A Shot of Hope’ What the Vaccine is like for Frontline Doctors and Nurses.”6
I want to briefly argue why even though I believe hope in and for the vaccine is desperately needed if we are to survive this long, dark winter, trust in the vaccine can actually usher in the warmth of economic recovery and the light of saved lives. Trust is crucial in 3 main areas if the awe-inspiring hope of the vaccine the EUAs codify is to be fulfilled. The venerable moral and civic virtue of trust has been trivialized and commercialized mostly mentioned in advertising for insurance or real estate companies. Medical virtue-ethicists Edmund Pellegrino and David Thomasma describe trust as the binding force that keeps civilization intact. “Trust is ineradicable in human relationships. Without we could not live in society or attain even the rudiments of a fulfilling life, they explain. “Without trust we could not anticipate the future, and we would therefore be paralyzed into inaction. Yet to trust and entrust is to become vulnerable and dependent on the good will and motivations of those we trust. Trust, ineradicable as it is, is also always problematic.”7
The first area where that trust is the hardest to secure is in the federal government, the actions and messages of which have seemed so inconstant, unjust, and deceptive to many. For enough citizens to roll up their sleeves, they must believe the outgoing and the incoming administrations and legislators can make rational plans translated into sound public health policy that place the good of humanity above other interests and then mobilize the resources of the country to deliver that good with consistency, fairness, and transparency.
The second area is trust in medical science. Long before COVID-19, American attitudes toward vaccines reflected reasonable fears and ridiculous conspiracy theories—both of which are serious obstacles to the breadth of immunization required to achieve herd immunity. Ordinary people must believe that the health care professionals and scientists at the Centers for Disease Control and Prevention and the FDA will never compromise safety for political expediency. Recent polls have shown an increase in the percentage of the population willing to consider vaccination. A December Gallop poll found that 63% of Americans were willing to be vaccinated for COVID-19.8 To raise those numbers high enough to approach herd immunity will require Americans to believe that the scientists who discover the vaccines and the companies that develop them have placed people above profit and ranked the safety of society above individual scientific renown.
Groups that have been the historic objects of exploitation in research and contemporary disparities in health care understandably have more distrust of science and medicine. While public health officials insist that they have developed a system of vaccine distribution that is equitable and prioritizes the sick and old and those who care for them before the rich and powerful, we should not be surprised that our communication of this assurance is viewed with skepticism. As a recent Medscape article advised,
Third we must trust in our fellow citizens to maintain the public health measures of social distancing and mask wearing even after there is widespread vaccination. If we are to reap the benefits of a safe and effective vaccine, we must be a community of immunity, not just isolated inoculated individuals. We as health care practitioners must do all we can to educate the public that the adverse reactions to the vaccine so prominently featured in the media are expected with any new and complex biological product and do not signal risk that outweighs the deadliness of the virus.10
Fourth, and finally, we must trust in ourselves as health care professionals and administrators. We in the DoD, VA, and PHS have the knowledge and skills to endure the onslaught of pain and suffering we will all experience in one way or another in these next long months. We must believe that our courage and compassion can turn a vaccine into vaccinations sufficient to relieve the COVID-19 siege of our hospitals and intensive care units. When that day comes, hope will have been a plan we could trust.
1. US Food and Drug Administration. FDA take key action in fight against COVID-19 by issuing emergency use authorization for first COVID-19 vaccine [press release]. Published December 11, 2020. Accessed December 22, 2020. https://www.fda.gov/news-events/press-announcements/fda-takes-key-action-fight-against-covid-19-issuing-emergency-use-authorization-first-covid-19
2. US Food and Drug Administration. FDA takes additional action in fight against COVID-19 by Issuing emergency use authorization for second COVID-19 vaccine [press release]. Published December 18, 2020. Accessed December 22, 2020. https://www.fda.gov/news-events/press-announcements/fda-takes-additional-action-fight-against-covid-19-issuing-emergency-use-authorization-second-covid
3. US Food and Drug Administration. FDA approves first treatment for COVID-19 [press release]. Published October 22, 2020. Accessed December 20, 2020. https://www.fda.gov/news-events/press-announcements/fda-approves-first-treatment-covid-19
4. US Food and Drug Administration. Coronavirus (COVID-19) update: FDA revokes emergency use authorization for chloroquine and hydroxychloroquine [press release]. Published June 15, 2020. Accessed December 22, 2020. https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-revokes-emergency-use-authorization-chloroquine-and
5. US Food and Drug Administration. Emergency use authorization for vaccines explained. Updated November 20, 2020. Accessed December 22, 2020. https://www.fda.gov/vaccines-blood-biologics/vaccines/emergency-use-authorization-vaccines-explained
6. Healy J, Tompkins L, Burch ADS. ‘A shot of hope’: what the vaccine is like for frontline doctors and nurses. New York Times. Updated December 17, 2020. Accessed December 22, 2020. https://www.nytimes.com/2020/12/14/us/coronavirus-vaccine-doctors-nurses.html
7. Pellegrino E, Thomasma DC. The Virtues in Medical Practice . New York: Oxford University Press; 1993:65.
8. Brenan M. Willingness to get Covid-19 vaccine ticks up to 63% in the U.S. Published December 8, 2020. Accessed December 22, 2020. https://news.gallup.com/poll/327425/willingness-covid-vaccine-ticks.aspx
9. Eldred SM. Trusted messengers may help disenfranchised communities overcome vaccine hesitancy. Published December 17, 2020. Accessed December 22, 2020. https://www.medscape.com/viewarticle/942847
10. Chiu A. ‘Absolutely normal’: Covid vaccine side effects are not reason to avoid the shots, doctors say. Washington Post. Published December 3, 2020. Accessed December 22, 2020. https://www.washingtonpost.com/lifestyle/wellness/vaccine-side-effects-covid/2020/12/02/55bebac0-342c-11eb-8d38-6aea1adb3839_story.html
On December 11, 2020, the US Food and Drug Administration (FDA) delivered the holiday gift America was waiting for—approval of the first COVID-19 vaccine. Following the recommendation of its expert advisory panel, the FDA issued its opening emergency use authorization (EUA) for the Pfizer and BioNTech product to be distributed and administered across the country.1 A week after that historic announcement, the FDA issued an EUA to Moderna for a second COVID-19 vaccine.2
An EUA is a misunderstood concept that, like the development of the vaccine itself, appears almost like a magical federal deliverance to a nation at a time when almost every other public health effort has floundered. An EUA is a regulatory process to enable a public health emergency response with medical countermeasures including not only vaccines, but also medications. Earlier in 2020, hydroxychloroquine and remdesivir each received EUAs for treating patients with COVID-19.3 The EUA for hydroxychloroquine was later revoked when more data raised concerns for its efficacy.4 EUAs do not mean the drugs are experimental or that everyone receiving them is participating in a research trial; however, for the sake of safety and science, data continue to be collected and analyzed. Issuance of an EUA indicates that after rigorous examination and an independent advisory board review of data submitted by the manufacturer, the FDA has determined the product and situation meet key criteria: (1) There is a public health emergency that threatens health and life and requires expedited procedures; (2) there are no extant approved products able to treat or prevent the disease; and (3) the known and potential benefits of the product outweigh the known and potential risks.5
The public and even the professional press have celebrated the arrival of this technologic triumph over a virus that had vanquished staggering numbers of lives and livelihoods. Much of the media coverage aptly chose the word “hope” to capture the significance of this unprecedented accomplishment for which so many millions yearned. A Google search for “hope” on the morning of December 20, yielded 339,000,000 results. For example, a headline especially salient for Federal Practitioner readers from the New York Times read, “‘A Shot of Hope’ What the Vaccine is like for Frontline Doctors and Nurses.”6
I want to briefly argue why even though I believe hope in and for the vaccine is desperately needed if we are to survive this long, dark winter, trust in the vaccine can actually usher in the warmth of economic recovery and the light of saved lives. Trust is crucial in 3 main areas if the awe-inspiring hope of the vaccine the EUAs codify is to be fulfilled. The venerable moral and civic virtue of trust has been trivialized and commercialized mostly mentioned in advertising for insurance or real estate companies. Medical virtue-ethicists Edmund Pellegrino and David Thomasma describe trust as the binding force that keeps civilization intact. “Trust is ineradicable in human relationships. Without we could not live in society or attain even the rudiments of a fulfilling life, they explain. “Without trust we could not anticipate the future, and we would therefore be paralyzed into inaction. Yet to trust and entrust is to become vulnerable and dependent on the good will and motivations of those we trust. Trust, ineradicable as it is, is also always problematic.”7
The first area where that trust is the hardest to secure is in the federal government, the actions and messages of which have seemed so inconstant, unjust, and deceptive to many. For enough citizens to roll up their sleeves, they must believe the outgoing and the incoming administrations and legislators can make rational plans translated into sound public health policy that place the good of humanity above other interests and then mobilize the resources of the country to deliver that good with consistency, fairness, and transparency.
The second area is trust in medical science. Long before COVID-19, American attitudes toward vaccines reflected reasonable fears and ridiculous conspiracy theories—both of which are serious obstacles to the breadth of immunization required to achieve herd immunity. Ordinary people must believe that the health care professionals and scientists at the Centers for Disease Control and Prevention and the FDA will never compromise safety for political expediency. Recent polls have shown an increase in the percentage of the population willing to consider vaccination. A December Gallop poll found that 63% of Americans were willing to be vaccinated for COVID-19.8 To raise those numbers high enough to approach herd immunity will require Americans to believe that the scientists who discover the vaccines and the companies that develop them have placed people above profit and ranked the safety of society above individual scientific renown.
Groups that have been the historic objects of exploitation in research and contemporary disparities in health care understandably have more distrust of science and medicine. While public health officials insist that they have developed a system of vaccine distribution that is equitable and prioritizes the sick and old and those who care for them before the rich and powerful, we should not be surprised that our communication of this assurance is viewed with skepticism. As a recent Medscape article advised,
Third we must trust in our fellow citizens to maintain the public health measures of social distancing and mask wearing even after there is widespread vaccination. If we are to reap the benefits of a safe and effective vaccine, we must be a community of immunity, not just isolated inoculated individuals. We as health care practitioners must do all we can to educate the public that the adverse reactions to the vaccine so prominently featured in the media are expected with any new and complex biological product and do not signal risk that outweighs the deadliness of the virus.10
Fourth, and finally, we must trust in ourselves as health care professionals and administrators. We in the DoD, VA, and PHS have the knowledge and skills to endure the onslaught of pain and suffering we will all experience in one way or another in these next long months. We must believe that our courage and compassion can turn a vaccine into vaccinations sufficient to relieve the COVID-19 siege of our hospitals and intensive care units. When that day comes, hope will have been a plan we could trust.
On December 11, 2020, the US Food and Drug Administration (FDA) delivered the holiday gift America was waiting for—approval of the first COVID-19 vaccine. Following the recommendation of its expert advisory panel, the FDA issued its opening emergency use authorization (EUA) for the Pfizer and BioNTech product to be distributed and administered across the country.1 A week after that historic announcement, the FDA issued an EUA to Moderna for a second COVID-19 vaccine.2
An EUA is a misunderstood concept that, like the development of the vaccine itself, appears almost like a magical federal deliverance to a nation at a time when almost every other public health effort has floundered. An EUA is a regulatory process to enable a public health emergency response with medical countermeasures including not only vaccines, but also medications. Earlier in 2020, hydroxychloroquine and remdesivir each received EUAs for treating patients with COVID-19.3 The EUA for hydroxychloroquine was later revoked when more data raised concerns for its efficacy.4 EUAs do not mean the drugs are experimental or that everyone receiving them is participating in a research trial; however, for the sake of safety and science, data continue to be collected and analyzed. Issuance of an EUA indicates that after rigorous examination and an independent advisory board review of data submitted by the manufacturer, the FDA has determined the product and situation meet key criteria: (1) There is a public health emergency that threatens health and life and requires expedited procedures; (2) there are no extant approved products able to treat or prevent the disease; and (3) the known and potential benefits of the product outweigh the known and potential risks.5
The public and even the professional press have celebrated the arrival of this technologic triumph over a virus that had vanquished staggering numbers of lives and livelihoods. Much of the media coverage aptly chose the word “hope” to capture the significance of this unprecedented accomplishment for which so many millions yearned. A Google search for “hope” on the morning of December 20, yielded 339,000,000 results. For example, a headline especially salient for Federal Practitioner readers from the New York Times read, “‘A Shot of Hope’ What the Vaccine is like for Frontline Doctors and Nurses.”6
I want to briefly argue why even though I believe hope in and for the vaccine is desperately needed if we are to survive this long, dark winter, trust in the vaccine can actually usher in the warmth of economic recovery and the light of saved lives. Trust is crucial in 3 main areas if the awe-inspiring hope of the vaccine the EUAs codify is to be fulfilled. The venerable moral and civic virtue of trust has been trivialized and commercialized mostly mentioned in advertising for insurance or real estate companies. Medical virtue-ethicists Edmund Pellegrino and David Thomasma describe trust as the binding force that keeps civilization intact. “Trust is ineradicable in human relationships. Without we could not live in society or attain even the rudiments of a fulfilling life, they explain. “Without trust we could not anticipate the future, and we would therefore be paralyzed into inaction. Yet to trust and entrust is to become vulnerable and dependent on the good will and motivations of those we trust. Trust, ineradicable as it is, is also always problematic.”7
The first area where that trust is the hardest to secure is in the federal government, the actions and messages of which have seemed so inconstant, unjust, and deceptive to many. For enough citizens to roll up their sleeves, they must believe the outgoing and the incoming administrations and legislators can make rational plans translated into sound public health policy that place the good of humanity above other interests and then mobilize the resources of the country to deliver that good with consistency, fairness, and transparency.
The second area is trust in medical science. Long before COVID-19, American attitudes toward vaccines reflected reasonable fears and ridiculous conspiracy theories—both of which are serious obstacles to the breadth of immunization required to achieve herd immunity. Ordinary people must believe that the health care professionals and scientists at the Centers for Disease Control and Prevention and the FDA will never compromise safety for political expediency. Recent polls have shown an increase in the percentage of the population willing to consider vaccination. A December Gallop poll found that 63% of Americans were willing to be vaccinated for COVID-19.8 To raise those numbers high enough to approach herd immunity will require Americans to believe that the scientists who discover the vaccines and the companies that develop them have placed people above profit and ranked the safety of society above individual scientific renown.
Groups that have been the historic objects of exploitation in research and contemporary disparities in health care understandably have more distrust of science and medicine. While public health officials insist that they have developed a system of vaccine distribution that is equitable and prioritizes the sick and old and those who care for them before the rich and powerful, we should not be surprised that our communication of this assurance is viewed with skepticism. As a recent Medscape article advised,
Third we must trust in our fellow citizens to maintain the public health measures of social distancing and mask wearing even after there is widespread vaccination. If we are to reap the benefits of a safe and effective vaccine, we must be a community of immunity, not just isolated inoculated individuals. We as health care practitioners must do all we can to educate the public that the adverse reactions to the vaccine so prominently featured in the media are expected with any new and complex biological product and do not signal risk that outweighs the deadliness of the virus.10
Fourth, and finally, we must trust in ourselves as health care professionals and administrators. We in the DoD, VA, and PHS have the knowledge and skills to endure the onslaught of pain and suffering we will all experience in one way or another in these next long months. We must believe that our courage and compassion can turn a vaccine into vaccinations sufficient to relieve the COVID-19 siege of our hospitals and intensive care units. When that day comes, hope will have been a plan we could trust.
1. US Food and Drug Administration. FDA take key action in fight against COVID-19 by issuing emergency use authorization for first COVID-19 vaccine [press release]. Published December 11, 2020. Accessed December 22, 2020. https://www.fda.gov/news-events/press-announcements/fda-takes-key-action-fight-against-covid-19-issuing-emergency-use-authorization-first-covid-19
2. US Food and Drug Administration. FDA takes additional action in fight against COVID-19 by Issuing emergency use authorization for second COVID-19 vaccine [press release]. Published December 18, 2020. Accessed December 22, 2020. https://www.fda.gov/news-events/press-announcements/fda-takes-additional-action-fight-against-covid-19-issuing-emergency-use-authorization-second-covid
3. US Food and Drug Administration. FDA approves first treatment for COVID-19 [press release]. Published October 22, 2020. Accessed December 20, 2020. https://www.fda.gov/news-events/press-announcements/fda-approves-first-treatment-covid-19
4. US Food and Drug Administration. Coronavirus (COVID-19) update: FDA revokes emergency use authorization for chloroquine and hydroxychloroquine [press release]. Published June 15, 2020. Accessed December 22, 2020. https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-revokes-emergency-use-authorization-chloroquine-and
5. US Food and Drug Administration. Emergency use authorization for vaccines explained. Updated November 20, 2020. Accessed December 22, 2020. https://www.fda.gov/vaccines-blood-biologics/vaccines/emergency-use-authorization-vaccines-explained
6. Healy J, Tompkins L, Burch ADS. ‘A shot of hope’: what the vaccine is like for frontline doctors and nurses. New York Times. Updated December 17, 2020. Accessed December 22, 2020. https://www.nytimes.com/2020/12/14/us/coronavirus-vaccine-doctors-nurses.html
7. Pellegrino E, Thomasma DC. The Virtues in Medical Practice . New York: Oxford University Press; 1993:65.
8. Brenan M. Willingness to get Covid-19 vaccine ticks up to 63% in the U.S. Published December 8, 2020. Accessed December 22, 2020. https://news.gallup.com/poll/327425/willingness-covid-vaccine-ticks.aspx
9. Eldred SM. Trusted messengers may help disenfranchised communities overcome vaccine hesitancy. Published December 17, 2020. Accessed December 22, 2020. https://www.medscape.com/viewarticle/942847
10. Chiu A. ‘Absolutely normal’: Covid vaccine side effects are not reason to avoid the shots, doctors say. Washington Post. Published December 3, 2020. Accessed December 22, 2020. https://www.washingtonpost.com/lifestyle/wellness/vaccine-side-effects-covid/2020/12/02/55bebac0-342c-11eb-8d38-6aea1adb3839_story.html
1. US Food and Drug Administration. FDA take key action in fight against COVID-19 by issuing emergency use authorization for first COVID-19 vaccine [press release]. Published December 11, 2020. Accessed December 22, 2020. https://www.fda.gov/news-events/press-announcements/fda-takes-key-action-fight-against-covid-19-issuing-emergency-use-authorization-first-covid-19
2. US Food and Drug Administration. FDA takes additional action in fight against COVID-19 by Issuing emergency use authorization for second COVID-19 vaccine [press release]. Published December 18, 2020. Accessed December 22, 2020. https://www.fda.gov/news-events/press-announcements/fda-takes-additional-action-fight-against-covid-19-issuing-emergency-use-authorization-second-covid
3. US Food and Drug Administration. FDA approves first treatment for COVID-19 [press release]. Published October 22, 2020. Accessed December 20, 2020. https://www.fda.gov/news-events/press-announcements/fda-approves-first-treatment-covid-19
4. US Food and Drug Administration. Coronavirus (COVID-19) update: FDA revokes emergency use authorization for chloroquine and hydroxychloroquine [press release]. Published June 15, 2020. Accessed December 22, 2020. https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-revokes-emergency-use-authorization-chloroquine-and
5. US Food and Drug Administration. Emergency use authorization for vaccines explained. Updated November 20, 2020. Accessed December 22, 2020. https://www.fda.gov/vaccines-blood-biologics/vaccines/emergency-use-authorization-vaccines-explained
6. Healy J, Tompkins L, Burch ADS. ‘A shot of hope’: what the vaccine is like for frontline doctors and nurses. New York Times. Updated December 17, 2020. Accessed December 22, 2020. https://www.nytimes.com/2020/12/14/us/coronavirus-vaccine-doctors-nurses.html
7. Pellegrino E, Thomasma DC. The Virtues in Medical Practice . New York: Oxford University Press; 1993:65.
8. Brenan M. Willingness to get Covid-19 vaccine ticks up to 63% in the U.S. Published December 8, 2020. Accessed December 22, 2020. https://news.gallup.com/poll/327425/willingness-covid-vaccine-ticks.aspx
9. Eldred SM. Trusted messengers may help disenfranchised communities overcome vaccine hesitancy. Published December 17, 2020. Accessed December 22, 2020. https://www.medscape.com/viewarticle/942847
10. Chiu A. ‘Absolutely normal’: Covid vaccine side effects are not reason to avoid the shots, doctors say. Washington Post. Published December 3, 2020. Accessed December 22, 2020. https://www.washingtonpost.com/lifestyle/wellness/vaccine-side-effects-covid/2020/12/02/55bebac0-342c-11eb-8d38-6aea1adb3839_story.html
Anaphylaxis cases after COVID-19 vaccine rising but still rare: CDC
Health care providers should be ready to treat rare cases of anaphylaxis following administration of COVID-19 vaccines, federal medical officials have urged. The officials also stressed the importance of continuing vaccinations, despite reports of the rare side effect.
There have been 29 cases of anaphylaxis to date following administration of a COVID-19 vaccine, officials from the Centers for Disease Control and Prevention said in a call with reporters on Jan. 6.
The severe allergic reaction, which appears to be rare, can happen with either the Pfizer-BioNTech vaccine or the rival Moderna product. The Food and Drug Administration granted emergency use authorizations for these two vaccines in December.
Even with the cases seen to date, the COVID-19 vaccines remain a “good value proposition,” Nancy Messonnier, MD, director of the CDC’s National Center for Immunization, said in the call.
There have been about 11.1 cases of anaphylaxis per million doses with the Pfizer-BioNTech COVID-19 vaccine, which is higher than the estimated 1.3 cases per million doses with influenza vaccines, she said. But the low risk of anaphylaxis must be balanced against the threat of COVID-19, which currently claims about 2,000 lives a day in the United States, she said. In addition, many people are reporting long-term complications with COVID-19 even if they recover.
Kept in context, the data on anaphylaxis should not scare people away from getting a COVID-19 vaccine, she added.
“Their risk from COVID and poor outcomes is still more than the risk of a severe outcome from the vaccine,” Dr. Messonnier said. “And fortunately, we know how to treat anaphylaxis.”
Dr. Messonnier urged health care workers administering COVID-19 vaccines to be prepared.
“Anybody administering vaccines needs not just to have the EpiPen available, but frankly, to know how to use it,” Dr. Messonnier said.
MMWR details
The CDC on Jan. 6 also provided an update on anaphylaxis in Morbidity and Mortality Weekly Report (MMWR).
The information included in the report was based on cases reported with the Pfizer-BioNTech vaccine – the first to get emergency use authorization from the FDA. On the call with reporters, CDC officials confirmed there have been additional reports since then and anaphylaxis has been reported with both the Pfizer-BioNTech and Moderna vaccines. CDC officials said they could not give a breakdown of how many cases were linked to each of these products at this time.
Between Dec. 14 and 23, 2020, monitoring by the Vaccine Adverse Event Reporting System detected 21 cases of anaphylaxis after administration of a reported 1,893,360 first doses of the Pfizer-BioNTech COVID-19 vaccine. Most reactions – 71% – occurred within 15 minutes of vaccination.
A version of this article first appeared on Medscape.com.
Health care providers should be ready to treat rare cases of anaphylaxis following administration of COVID-19 vaccines, federal medical officials have urged. The officials also stressed the importance of continuing vaccinations, despite reports of the rare side effect.
There have been 29 cases of anaphylaxis to date following administration of a COVID-19 vaccine, officials from the Centers for Disease Control and Prevention said in a call with reporters on Jan. 6.
The severe allergic reaction, which appears to be rare, can happen with either the Pfizer-BioNTech vaccine or the rival Moderna product. The Food and Drug Administration granted emergency use authorizations for these two vaccines in December.
Even with the cases seen to date, the COVID-19 vaccines remain a “good value proposition,” Nancy Messonnier, MD, director of the CDC’s National Center for Immunization, said in the call.
There have been about 11.1 cases of anaphylaxis per million doses with the Pfizer-BioNTech COVID-19 vaccine, which is higher than the estimated 1.3 cases per million doses with influenza vaccines, she said. But the low risk of anaphylaxis must be balanced against the threat of COVID-19, which currently claims about 2,000 lives a day in the United States, she said. In addition, many people are reporting long-term complications with COVID-19 even if they recover.
Kept in context, the data on anaphylaxis should not scare people away from getting a COVID-19 vaccine, she added.
“Their risk from COVID and poor outcomes is still more than the risk of a severe outcome from the vaccine,” Dr. Messonnier said. “And fortunately, we know how to treat anaphylaxis.”
Dr. Messonnier urged health care workers administering COVID-19 vaccines to be prepared.
“Anybody administering vaccines needs not just to have the EpiPen available, but frankly, to know how to use it,” Dr. Messonnier said.
MMWR details
The CDC on Jan. 6 also provided an update on anaphylaxis in Morbidity and Mortality Weekly Report (MMWR).
The information included in the report was based on cases reported with the Pfizer-BioNTech vaccine – the first to get emergency use authorization from the FDA. On the call with reporters, CDC officials confirmed there have been additional reports since then and anaphylaxis has been reported with both the Pfizer-BioNTech and Moderna vaccines. CDC officials said they could not give a breakdown of how many cases were linked to each of these products at this time.
Between Dec. 14 and 23, 2020, monitoring by the Vaccine Adverse Event Reporting System detected 21 cases of anaphylaxis after administration of a reported 1,893,360 first doses of the Pfizer-BioNTech COVID-19 vaccine. Most reactions – 71% – occurred within 15 minutes of vaccination.
A version of this article first appeared on Medscape.com.
Health care providers should be ready to treat rare cases of anaphylaxis following administration of COVID-19 vaccines, federal medical officials have urged. The officials also stressed the importance of continuing vaccinations, despite reports of the rare side effect.
There have been 29 cases of anaphylaxis to date following administration of a COVID-19 vaccine, officials from the Centers for Disease Control and Prevention said in a call with reporters on Jan. 6.
The severe allergic reaction, which appears to be rare, can happen with either the Pfizer-BioNTech vaccine or the rival Moderna product. The Food and Drug Administration granted emergency use authorizations for these two vaccines in December.
Even with the cases seen to date, the COVID-19 vaccines remain a “good value proposition,” Nancy Messonnier, MD, director of the CDC’s National Center for Immunization, said in the call.
There have been about 11.1 cases of anaphylaxis per million doses with the Pfizer-BioNTech COVID-19 vaccine, which is higher than the estimated 1.3 cases per million doses with influenza vaccines, she said. But the low risk of anaphylaxis must be balanced against the threat of COVID-19, which currently claims about 2,000 lives a day in the United States, she said. In addition, many people are reporting long-term complications with COVID-19 even if they recover.
Kept in context, the data on anaphylaxis should not scare people away from getting a COVID-19 vaccine, she added.
“Their risk from COVID and poor outcomes is still more than the risk of a severe outcome from the vaccine,” Dr. Messonnier said. “And fortunately, we know how to treat anaphylaxis.”
Dr. Messonnier urged health care workers administering COVID-19 vaccines to be prepared.
“Anybody administering vaccines needs not just to have the EpiPen available, but frankly, to know how to use it,” Dr. Messonnier said.
MMWR details
The CDC on Jan. 6 also provided an update on anaphylaxis in Morbidity and Mortality Weekly Report (MMWR).
The information included in the report was based on cases reported with the Pfizer-BioNTech vaccine – the first to get emergency use authorization from the FDA. On the call with reporters, CDC officials confirmed there have been additional reports since then and anaphylaxis has been reported with both the Pfizer-BioNTech and Moderna vaccines. CDC officials said they could not give a breakdown of how many cases were linked to each of these products at this time.
Between Dec. 14 and 23, 2020, monitoring by the Vaccine Adverse Event Reporting System detected 21 cases of anaphylaxis after administration of a reported 1,893,360 first doses of the Pfizer-BioNTech COVID-19 vaccine. Most reactions – 71% – occurred within 15 minutes of vaccination.
A version of this article first appeared on Medscape.com.
Study confirms key COVID-19 risk factors in children
Children and adolescents who receive positive COVID-19 test results are not only more likely to have been in close contact with someone with a confirmed case of the virus but also are less likely to have reported consistent mask use among students and staff inside the school they attended, reported Charlotte V. Hobbs, MD, and colleagues at the University of Mississippi, Jackson.
In partnership with the Centers for Disease Control and Prevention’s COVID-19 Response Team, Dr. Hobbs and colleagues conducted a case-control study of 397 children and adolescents under 18 years of age to assess school, community, and close contact exposures associated with pediatric COVID-19. Patients tested for COVID-19 at outpatient health centers or emergency departments affiliated with the University of Mississippi Medical Center between Sept. 1 and Nov. 5, 2020, were included in the study.
Nearly two-thirds reported that exposure came from family members
Of the total study participants observed, 82 (21%) were under 4 years of age; 214 (54%) were female; 217 (55%) were non-Hispanic black, and 145 (37%) were non-Hispanic white. More than half (53%) sought testing because of COVID-19 symptoms. Of those who tested positive, 66% reported having come into close contact with a COVID-19 case, and 64% reported that those contacts were family members, compared with 15% of contacts who were schoolmates and 27% who were child care classmates.
All participants completed in-person school or child care attendance less than 14 days before testing positive for the virus, including 62% of patients testing positive and 68% of those testing negative. The authors noted that school attendance itself was not found to be associated with any positive test results. In fact, parents in 64% of positive cases and 76% of negative cases reported mask wearing among children and staff inside places of learning.
Of those study participants testing positive who did come into close contact with someone with COVID-19, the contacts were more likely to be family members than school or child care classmates. Specifically, they were more likely, in the 2-week period preceding testing, to have attended gatherings with individuals outside their immediate households, including social events and activities with other children. Parents of students testing positive were also less likely to report consistent indoor mask use among their children older than 2 years and school staff members.
School attendance was not found to increase likelihood of testing positive
Attending in-person school or child care during the 2 weeks before the SARS-CoV-2 test was not associated with greater likelihood of testing positive, the study authors noted, adding that the majority of study respondents reported universal mask use inside school and child care facilities, consistent with Mississippi State Department of Health recommended guidelines.
Dr. Hobbs and colleagues reported at least four limitations of the study. They noted that the study participants may not be representative of youth in other geographic regions of the country. They considered the possibility of unmeasured confounding of participant behaviors that may not have been factored into the study. No attempt was made to verify parent claims of mask use at schools and child care programs. Lastly, they acknowledged that “case or control status might be subject to misclassification because of imperfect sensitivity or specificity of PCR-based testing.
As of Dec. 14, 2020, the CDC reported that 10.2% of all COVID-19 cases in the United States were in children and adolescents under the age of 18.
“Continued efforts to prevent transmission at schools and child care programs are important, as are assessments of various types of activities and exposures to identify risk factors for COVID-19 as children engage in classroom and social interactions.” Promoting behaviors to reduce exposures to the virus among youth in the household, the community, schools, and child care programs is important to preventing outbreaks of the virus at schools, the authors cautioned.
In a separate interview with this news organization, Karalyn Kinsella, MD, general pediatrician in a small group private practice in Cheshire, Conn., said, “What this report tells me is that COVID cases are more common when mask use is inconsistent in schools and at home and in schools that don’t properly adhere to CDC guidelines. Overall, so long as social distancing guidelines are followed, schools are pretty safe places for kids during this pandemic.”
This finding is important, since many families are keeping their children out of school over fears of contracting the virus, she added. Some of the consequences these children are suffering include a lack of social connection and structure, which in some cases is leading to worsening anxiety and depression, and for those with disabilities, such as those who receive physical therapy, occupational therapy, speech or have IEPs, they’re not getting the full benefit of the services that they would otherwise receive in person, she observed.
“I don’t think families really understand the risks of getting together with family or friends “in their bubble” or the risk of continuing sports participation. This is where the majority of COVID cases are coming from,” she said, adding that it is important to discuss this risk with them at appointments. So, when families ask us what we think of in-person learning, I think we should feel fairly confident that the benefit may outweigh the risk.”
Dr. Hobbs and colleagues, and Dr. Kinsella, had no conflicts of interest to report.
SOURCE: MMWR Morb Mortal Wkly Rep. 2020;69:1925-9. doi: 10.15585/mmwr.mm6950e3.
Children and adolescents who receive positive COVID-19 test results are not only more likely to have been in close contact with someone with a confirmed case of the virus but also are less likely to have reported consistent mask use among students and staff inside the school they attended, reported Charlotte V. Hobbs, MD, and colleagues at the University of Mississippi, Jackson.
In partnership with the Centers for Disease Control and Prevention’s COVID-19 Response Team, Dr. Hobbs and colleagues conducted a case-control study of 397 children and adolescents under 18 years of age to assess school, community, and close contact exposures associated with pediatric COVID-19. Patients tested for COVID-19 at outpatient health centers or emergency departments affiliated with the University of Mississippi Medical Center between Sept. 1 and Nov. 5, 2020, were included in the study.
Nearly two-thirds reported that exposure came from family members
Of the total study participants observed, 82 (21%) were under 4 years of age; 214 (54%) were female; 217 (55%) were non-Hispanic black, and 145 (37%) were non-Hispanic white. More than half (53%) sought testing because of COVID-19 symptoms. Of those who tested positive, 66% reported having come into close contact with a COVID-19 case, and 64% reported that those contacts were family members, compared with 15% of contacts who were schoolmates and 27% who were child care classmates.
All participants completed in-person school or child care attendance less than 14 days before testing positive for the virus, including 62% of patients testing positive and 68% of those testing negative. The authors noted that school attendance itself was not found to be associated with any positive test results. In fact, parents in 64% of positive cases and 76% of negative cases reported mask wearing among children and staff inside places of learning.
Of those study participants testing positive who did come into close contact with someone with COVID-19, the contacts were more likely to be family members than school or child care classmates. Specifically, they were more likely, in the 2-week period preceding testing, to have attended gatherings with individuals outside their immediate households, including social events and activities with other children. Parents of students testing positive were also less likely to report consistent indoor mask use among their children older than 2 years and school staff members.
School attendance was not found to increase likelihood of testing positive
Attending in-person school or child care during the 2 weeks before the SARS-CoV-2 test was not associated with greater likelihood of testing positive, the study authors noted, adding that the majority of study respondents reported universal mask use inside school and child care facilities, consistent with Mississippi State Department of Health recommended guidelines.
Dr. Hobbs and colleagues reported at least four limitations of the study. They noted that the study participants may not be representative of youth in other geographic regions of the country. They considered the possibility of unmeasured confounding of participant behaviors that may not have been factored into the study. No attempt was made to verify parent claims of mask use at schools and child care programs. Lastly, they acknowledged that “case or control status might be subject to misclassification because of imperfect sensitivity or specificity of PCR-based testing.
As of Dec. 14, 2020, the CDC reported that 10.2% of all COVID-19 cases in the United States were in children and adolescents under the age of 18.
“Continued efforts to prevent transmission at schools and child care programs are important, as are assessments of various types of activities and exposures to identify risk factors for COVID-19 as children engage in classroom and social interactions.” Promoting behaviors to reduce exposures to the virus among youth in the household, the community, schools, and child care programs is important to preventing outbreaks of the virus at schools, the authors cautioned.
In a separate interview with this news organization, Karalyn Kinsella, MD, general pediatrician in a small group private practice in Cheshire, Conn., said, “What this report tells me is that COVID cases are more common when mask use is inconsistent in schools and at home and in schools that don’t properly adhere to CDC guidelines. Overall, so long as social distancing guidelines are followed, schools are pretty safe places for kids during this pandemic.”
This finding is important, since many families are keeping their children out of school over fears of contracting the virus, she added. Some of the consequences these children are suffering include a lack of social connection and structure, which in some cases is leading to worsening anxiety and depression, and for those with disabilities, such as those who receive physical therapy, occupational therapy, speech or have IEPs, they’re not getting the full benefit of the services that they would otherwise receive in person, she observed.
“I don’t think families really understand the risks of getting together with family or friends “in their bubble” or the risk of continuing sports participation. This is where the majority of COVID cases are coming from,” she said, adding that it is important to discuss this risk with them at appointments. So, when families ask us what we think of in-person learning, I think we should feel fairly confident that the benefit may outweigh the risk.”
Dr. Hobbs and colleagues, and Dr. Kinsella, had no conflicts of interest to report.
SOURCE: MMWR Morb Mortal Wkly Rep. 2020;69:1925-9. doi: 10.15585/mmwr.mm6950e3.
Children and adolescents who receive positive COVID-19 test results are not only more likely to have been in close contact with someone with a confirmed case of the virus but also are less likely to have reported consistent mask use among students and staff inside the school they attended, reported Charlotte V. Hobbs, MD, and colleagues at the University of Mississippi, Jackson.
In partnership with the Centers for Disease Control and Prevention’s COVID-19 Response Team, Dr. Hobbs and colleagues conducted a case-control study of 397 children and adolescents under 18 years of age to assess school, community, and close contact exposures associated with pediatric COVID-19. Patients tested for COVID-19 at outpatient health centers or emergency departments affiliated with the University of Mississippi Medical Center between Sept. 1 and Nov. 5, 2020, were included in the study.
Nearly two-thirds reported that exposure came from family members
Of the total study participants observed, 82 (21%) were under 4 years of age; 214 (54%) were female; 217 (55%) were non-Hispanic black, and 145 (37%) were non-Hispanic white. More than half (53%) sought testing because of COVID-19 symptoms. Of those who tested positive, 66% reported having come into close contact with a COVID-19 case, and 64% reported that those contacts were family members, compared with 15% of contacts who were schoolmates and 27% who were child care classmates.
All participants completed in-person school or child care attendance less than 14 days before testing positive for the virus, including 62% of patients testing positive and 68% of those testing negative. The authors noted that school attendance itself was not found to be associated with any positive test results. In fact, parents in 64% of positive cases and 76% of negative cases reported mask wearing among children and staff inside places of learning.
Of those study participants testing positive who did come into close contact with someone with COVID-19, the contacts were more likely to be family members than school or child care classmates. Specifically, they were more likely, in the 2-week period preceding testing, to have attended gatherings with individuals outside their immediate households, including social events and activities with other children. Parents of students testing positive were also less likely to report consistent indoor mask use among their children older than 2 years and school staff members.
School attendance was not found to increase likelihood of testing positive
Attending in-person school or child care during the 2 weeks before the SARS-CoV-2 test was not associated with greater likelihood of testing positive, the study authors noted, adding that the majority of study respondents reported universal mask use inside school and child care facilities, consistent with Mississippi State Department of Health recommended guidelines.
Dr. Hobbs and colleagues reported at least four limitations of the study. They noted that the study participants may not be representative of youth in other geographic regions of the country. They considered the possibility of unmeasured confounding of participant behaviors that may not have been factored into the study. No attempt was made to verify parent claims of mask use at schools and child care programs. Lastly, they acknowledged that “case or control status might be subject to misclassification because of imperfect sensitivity or specificity of PCR-based testing.
As of Dec. 14, 2020, the CDC reported that 10.2% of all COVID-19 cases in the United States were in children and adolescents under the age of 18.
“Continued efforts to prevent transmission at schools and child care programs are important, as are assessments of various types of activities and exposures to identify risk factors for COVID-19 as children engage in classroom and social interactions.” Promoting behaviors to reduce exposures to the virus among youth in the household, the community, schools, and child care programs is important to preventing outbreaks of the virus at schools, the authors cautioned.
In a separate interview with this news organization, Karalyn Kinsella, MD, general pediatrician in a small group private practice in Cheshire, Conn., said, “What this report tells me is that COVID cases are more common when mask use is inconsistent in schools and at home and in schools that don’t properly adhere to CDC guidelines. Overall, so long as social distancing guidelines are followed, schools are pretty safe places for kids during this pandemic.”
This finding is important, since many families are keeping their children out of school over fears of contracting the virus, she added. Some of the consequences these children are suffering include a lack of social connection and structure, which in some cases is leading to worsening anxiety and depression, and for those with disabilities, such as those who receive physical therapy, occupational therapy, speech or have IEPs, they’re not getting the full benefit of the services that they would otherwise receive in person, she observed.
“I don’t think families really understand the risks of getting together with family or friends “in their bubble” or the risk of continuing sports participation. This is where the majority of COVID cases are coming from,” she said, adding that it is important to discuss this risk with them at appointments. So, when families ask us what we think of in-person learning, I think we should feel fairly confident that the benefit may outweigh the risk.”
Dr. Hobbs and colleagues, and Dr. Kinsella, had no conflicts of interest to report.
SOURCE: MMWR Morb Mortal Wkly Rep. 2020;69:1925-9. doi: 10.15585/mmwr.mm6950e3.
FROM MORBIDITY AND MORTALITY WEEKLY REPORT
IDSA panel updates guidelines on COVID molecular diagnostic tests
Saliva spit tests stack up well against the gold standard for molecular COVID-19 tests – the back-of-the-nose deep swab – without the discomfort and induced coughing or sneezing of the test taker, updated guidelines indicate.
In a press briefing on Jan. 6, the Infectious Diseases Society of America explained the findings of an expert panel that reviewed the literature since the IDSA released its first guidelines in May.
The panel found that saliva tests were especially effective if the test included instructions to cough or clear the throat before spitting into the tube, said panel chair Kimberly E. Hanson, MD, MHS, of University of Utah Health, Salt Lake City.
Throat swab alone less effective
Using a throat swab alone was less effective and missed more cases than the other methods, she said.
The IDSA has updated its recommendation: A saliva test or swabs from either the middle or front of the nose front are preferred to a throat swab alone.
A combination of saliva and swabs from the front and middle of the nose and throat together “looked pretty much equivalent” to the gold-standard deep swab, the panel found.
She acknowledged, however, that multiple swabs exacerbate already challenging supply issues.
Saliva samples do come with challenges, Dr. Hanson noted. A laboratory must validate that its systems can handle the stickier material. And asking a patient to cough necessitates more personal protective equipment for the health care professional.
Each center will have to tailor the specimen type it chooses, based on what resources it has available and the setting – whether in a hospital or a drive-through operation, for instance, she said.
Rapid testing vs. standard
Panel member Angela M. Caliendo, MD, PhD, of Brown University, Providence, R.I., said the panel preferred rapid polymerase chain reaction tests and standard, laboratory-based PCR tests over a rapid isothermal test.
The panel defined rapid tests as those for which results are available within an hour after a test provider has the specimen in hand. They excluded home tests for this category.
The only rapid isothermal test that had enough data on which to issue a recommendation was the ID NOW test (Abbott Labs), she noted.
Rapid PCR tests performed just as well as the standard laboratory-based tests, she said, with a high sensitivity of “97% on average and a very high specificity.”
But the rapid isothermal test had an average sensitivity of only about 80%, compared with the lab-based PCR test, Dr. Caliendo said, yielding a substantial number of false-negative results.
Testing centers will have to weigh the considerable advantages of having results in 15 minutes with a rapid isothermal test and being able to educate positive patients about immediate isolation against the potential for false negatives, which could send positive patients home thinking they don’t have the virus – and thus potentially spreading the disease.
And if a clinician gets a negative result with the rapid isothermal test, but has a strong suspicion the person has COVID or lives in an area with high prevalence, a backup test with a rapid PCR or laboratory-based test should be administered.
“You will miss a certain percentage of people using this rapid isothermal test,” she said.
However, Dr. Caliendo said, if the only available option is the isothermal test, “you should definitely use it because it’s certainly better than not testing at all.”
On a positive note, she said, all the varieties of tests have high specificity, so “you’re not going to see a lot of false-positive results.”
The guidelines back in May didn’t make recommendations on rapid tests, she said, because there weren’t enough data in the literature.
Dr. Caliendo noted that most of the available data were for symptomatic patients, but there are some data that show the amount of virus in the respiratory tract is similar for people with and without symptoms. The panel, therefore, expects that the performance of the various assays would be similar whether or not a person had symptoms.
Testing the immunocompromised
Dr. Hanson said the original recommendation in May was to do molecular testing for asymptomatic people who were awaiting a transplant or were waiting to start immunosuppressive therapy for cancer or an autoimmune disease. Now the current guidelines “make no recommendation for or against screening” in those cases.
Dr. Hanson added that the panel feels that patients awaiting bone marrow and solid organ transplants should have the testing because of the high risks that will result if patients have contracted the virus.
But for those with cancer or an autoimmune disease, the panel decided to leave it up to each physician to assess individual risk and determine whether the patient should be tested.
Home testing
The IDSA guidelines didn’t weigh in on home testing because the products are so new and studies so far have included fewer than 200 patients. But Dr. Caliendo said they clearly perform better earlier in the disease phase – the first 5-7 days – when the amount of the virus is higher.
Dr. Hanson and Dr. Caliendo also fielded a question about what the new virus variant, first discovered in the United Kingdom and now spreading to other countries (including the United States) means for diagnostic testing.
“So far we think with the majority of tests that are [emergency use] authorized, it doesn’t look like this new variant should really affect test performance,” Dr. Hanson said.
The variant has differences in the spike gene, and many of the current tests detect and identify SARS-CoV-2 without the spike gene so they wouldn’t be affected, she added.
Dr. Caliendo agreed: “I think the vast majority of our tests should be in good shape.”
Dr. Hanson and Dr. Caliendo disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Saliva spit tests stack up well against the gold standard for molecular COVID-19 tests – the back-of-the-nose deep swab – without the discomfort and induced coughing or sneezing of the test taker, updated guidelines indicate.
In a press briefing on Jan. 6, the Infectious Diseases Society of America explained the findings of an expert panel that reviewed the literature since the IDSA released its first guidelines in May.
The panel found that saliva tests were especially effective if the test included instructions to cough or clear the throat before spitting into the tube, said panel chair Kimberly E. Hanson, MD, MHS, of University of Utah Health, Salt Lake City.
Throat swab alone less effective
Using a throat swab alone was less effective and missed more cases than the other methods, she said.
The IDSA has updated its recommendation: A saliva test or swabs from either the middle or front of the nose front are preferred to a throat swab alone.
A combination of saliva and swabs from the front and middle of the nose and throat together “looked pretty much equivalent” to the gold-standard deep swab, the panel found.
She acknowledged, however, that multiple swabs exacerbate already challenging supply issues.
Saliva samples do come with challenges, Dr. Hanson noted. A laboratory must validate that its systems can handle the stickier material. And asking a patient to cough necessitates more personal protective equipment for the health care professional.
Each center will have to tailor the specimen type it chooses, based on what resources it has available and the setting – whether in a hospital or a drive-through operation, for instance, she said.
Rapid testing vs. standard
Panel member Angela M. Caliendo, MD, PhD, of Brown University, Providence, R.I., said the panel preferred rapid polymerase chain reaction tests and standard, laboratory-based PCR tests over a rapid isothermal test.
The panel defined rapid tests as those for which results are available within an hour after a test provider has the specimen in hand. They excluded home tests for this category.
The only rapid isothermal test that had enough data on which to issue a recommendation was the ID NOW test (Abbott Labs), she noted.
Rapid PCR tests performed just as well as the standard laboratory-based tests, she said, with a high sensitivity of “97% on average and a very high specificity.”
But the rapid isothermal test had an average sensitivity of only about 80%, compared with the lab-based PCR test, Dr. Caliendo said, yielding a substantial number of false-negative results.
Testing centers will have to weigh the considerable advantages of having results in 15 minutes with a rapid isothermal test and being able to educate positive patients about immediate isolation against the potential for false negatives, which could send positive patients home thinking they don’t have the virus – and thus potentially spreading the disease.
And if a clinician gets a negative result with the rapid isothermal test, but has a strong suspicion the person has COVID or lives in an area with high prevalence, a backup test with a rapid PCR or laboratory-based test should be administered.
“You will miss a certain percentage of people using this rapid isothermal test,” she said.
However, Dr. Caliendo said, if the only available option is the isothermal test, “you should definitely use it because it’s certainly better than not testing at all.”
On a positive note, she said, all the varieties of tests have high specificity, so “you’re not going to see a lot of false-positive results.”
The guidelines back in May didn’t make recommendations on rapid tests, she said, because there weren’t enough data in the literature.
Dr. Caliendo noted that most of the available data were for symptomatic patients, but there are some data that show the amount of virus in the respiratory tract is similar for people with and without symptoms. The panel, therefore, expects that the performance of the various assays would be similar whether or not a person had symptoms.
Testing the immunocompromised
Dr. Hanson said the original recommendation in May was to do molecular testing for asymptomatic people who were awaiting a transplant or were waiting to start immunosuppressive therapy for cancer or an autoimmune disease. Now the current guidelines “make no recommendation for or against screening” in those cases.
Dr. Hanson added that the panel feels that patients awaiting bone marrow and solid organ transplants should have the testing because of the high risks that will result if patients have contracted the virus.
But for those with cancer or an autoimmune disease, the panel decided to leave it up to each physician to assess individual risk and determine whether the patient should be tested.
Home testing
The IDSA guidelines didn’t weigh in on home testing because the products are so new and studies so far have included fewer than 200 patients. But Dr. Caliendo said they clearly perform better earlier in the disease phase – the first 5-7 days – when the amount of the virus is higher.
Dr. Hanson and Dr. Caliendo also fielded a question about what the new virus variant, first discovered in the United Kingdom and now spreading to other countries (including the United States) means for diagnostic testing.
“So far we think with the majority of tests that are [emergency use] authorized, it doesn’t look like this new variant should really affect test performance,” Dr. Hanson said.
The variant has differences in the spike gene, and many of the current tests detect and identify SARS-CoV-2 without the spike gene so they wouldn’t be affected, she added.
Dr. Caliendo agreed: “I think the vast majority of our tests should be in good shape.”
Dr. Hanson and Dr. Caliendo disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Saliva spit tests stack up well against the gold standard for molecular COVID-19 tests – the back-of-the-nose deep swab – without the discomfort and induced coughing or sneezing of the test taker, updated guidelines indicate.
In a press briefing on Jan. 6, the Infectious Diseases Society of America explained the findings of an expert panel that reviewed the literature since the IDSA released its first guidelines in May.
The panel found that saliva tests were especially effective if the test included instructions to cough or clear the throat before spitting into the tube, said panel chair Kimberly E. Hanson, MD, MHS, of University of Utah Health, Salt Lake City.
Throat swab alone less effective
Using a throat swab alone was less effective and missed more cases than the other methods, she said.
The IDSA has updated its recommendation: A saliva test or swabs from either the middle or front of the nose front are preferred to a throat swab alone.
A combination of saliva and swabs from the front and middle of the nose and throat together “looked pretty much equivalent” to the gold-standard deep swab, the panel found.
She acknowledged, however, that multiple swabs exacerbate already challenging supply issues.
Saliva samples do come with challenges, Dr. Hanson noted. A laboratory must validate that its systems can handle the stickier material. And asking a patient to cough necessitates more personal protective equipment for the health care professional.
Each center will have to tailor the specimen type it chooses, based on what resources it has available and the setting – whether in a hospital or a drive-through operation, for instance, she said.
Rapid testing vs. standard
Panel member Angela M. Caliendo, MD, PhD, of Brown University, Providence, R.I., said the panel preferred rapid polymerase chain reaction tests and standard, laboratory-based PCR tests over a rapid isothermal test.
The panel defined rapid tests as those for which results are available within an hour after a test provider has the specimen in hand. They excluded home tests for this category.
The only rapid isothermal test that had enough data on which to issue a recommendation was the ID NOW test (Abbott Labs), she noted.
Rapid PCR tests performed just as well as the standard laboratory-based tests, she said, with a high sensitivity of “97% on average and a very high specificity.”
But the rapid isothermal test had an average sensitivity of only about 80%, compared with the lab-based PCR test, Dr. Caliendo said, yielding a substantial number of false-negative results.
Testing centers will have to weigh the considerable advantages of having results in 15 minutes with a rapid isothermal test and being able to educate positive patients about immediate isolation against the potential for false negatives, which could send positive patients home thinking they don’t have the virus – and thus potentially spreading the disease.
And if a clinician gets a negative result with the rapid isothermal test, but has a strong suspicion the person has COVID or lives in an area with high prevalence, a backup test with a rapid PCR or laboratory-based test should be administered.
“You will miss a certain percentage of people using this rapid isothermal test,” she said.
However, Dr. Caliendo said, if the only available option is the isothermal test, “you should definitely use it because it’s certainly better than not testing at all.”
On a positive note, she said, all the varieties of tests have high specificity, so “you’re not going to see a lot of false-positive results.”
The guidelines back in May didn’t make recommendations on rapid tests, she said, because there weren’t enough data in the literature.
Dr. Caliendo noted that most of the available data were for symptomatic patients, but there are some data that show the amount of virus in the respiratory tract is similar for people with and without symptoms. The panel, therefore, expects that the performance of the various assays would be similar whether or not a person had symptoms.
Testing the immunocompromised
Dr. Hanson said the original recommendation in May was to do molecular testing for asymptomatic people who were awaiting a transplant or were waiting to start immunosuppressive therapy for cancer or an autoimmune disease. Now the current guidelines “make no recommendation for or against screening” in those cases.
Dr. Hanson added that the panel feels that patients awaiting bone marrow and solid organ transplants should have the testing because of the high risks that will result if patients have contracted the virus.
But for those with cancer or an autoimmune disease, the panel decided to leave it up to each physician to assess individual risk and determine whether the patient should be tested.
Home testing
The IDSA guidelines didn’t weigh in on home testing because the products are so new and studies so far have included fewer than 200 patients. But Dr. Caliendo said they clearly perform better earlier in the disease phase – the first 5-7 days – when the amount of the virus is higher.
Dr. Hanson and Dr. Caliendo also fielded a question about what the new virus variant, first discovered in the United Kingdom and now spreading to other countries (including the United States) means for diagnostic testing.
“So far we think with the majority of tests that are [emergency use] authorized, it doesn’t look like this new variant should really affect test performance,” Dr. Hanson said.
The variant has differences in the spike gene, and many of the current tests detect and identify SARS-CoV-2 without the spike gene so they wouldn’t be affected, she added.
Dr. Caliendo agreed: “I think the vast majority of our tests should be in good shape.”
Dr. Hanson and Dr. Caliendo disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Early use of high-titer plasma may prevent severe COVID-19
Administering convalescent plasma that has high levels of antibodies against SARS-CoV-2 within the first 3 days of symptoms was associated with significantly lower chances of progression to severe COVID-19, new evidence demonstrates.
In a trial of 160 older adults with COVID-19, half of whom were randomly assigned to receive plasma and half to receive placebo infusion, treatment with high-titer plasma lowered the relative risk for severe disease by 48% in an intent-to-treat analysis.
“We now have evidence, in the context of a small but well-designed study, that convalescent plasma with high titers of antibody against SARS-CoV-2 administered in the first 3 days of mild symptoms to infected elderly reduces progression of illness and the rate of severe presentations,” senior author Fernando Polack, MD, said in an interview.
“Not any plasma, not any time,” added Dr. Polack, an infectious disease specialist and scientific director at Fundacion INFANT and professor of pediatrics at the University of Buenos Aires. The key, he said, is to select plasma in the upper 28th percentile of IgG antibody concentrations and to administer therapy prior to disease progression.
The study was published online Jan. 6 in The New England Journal of Medicine.
“It’s a very good study and approaches a different population from the PlasmAr study,” Ventura Simonovich, MD, chief of the clinical pharmacology section, Medical Clinic Service, Hospital Italiano de Buenos Aires, said in an interview. “This is the first published randomized controlled trial that shows real benefit in this [older adult] population, the most vulnerable in this disease,” he said.
Dr. Simonovich, who was not affiliated with the current study, was lead author of the PlasmAr trial, which was published in The New England Journal of Medicine Nov. 24, 2020. In that trial, the researchers evaluated adults aged 18 years and older and found no significant benefit with convalescent plasma treatment over placebo for patients with COVID-19 and severe pneumonia.
“We know antibodies work best when given early and in high dose. This is one of the rare reports that validates it in the outpatient setting,” David Sullivan, MD, professor of molecular biology and immunology at Johns Hopkins Bloomberg School of Public Health, Baltimore, said in an interview when asked to comment.
Dr. Sullivan pointed out that most previous studies on convalescent plasma focused on patients with COVID-19 who had severe cases late in the disease course.
Regarding the current study, he said, “The striking thing is treating people within 3 days of illness.”
A more cautious interpretation may be warranted, one expert said. “The study demonstrates the benefit of early intervention. There was a dose-dependent effect, with higher titers providing a greater benefit,” Manoj Menon, MD, MPH, a hematologist and oncologist at the University of Washington, Seattle, said in an interview.
“Taken together, the findings have biologic plausibility and produce more data on the role of convalescent plasma to a relevant age cohort,” he added.
However, Dr. Menon said: “Given the limited sample size, I do not think this study, although well conducted, definitively addresses the role of convalescent plasma for COVID-19. But it does merit additional study.”
A search for clear answers
Treatments that target the early stages of COVID-19 “remain elusive. Few strategies provide benefit, several have failed, and others are being evaluated,” the researchers noted. “In hospitalized patients with COVID-19, the infusion of convalescent plasma against SARS-CoV-2 late in the course of illness has not shown clear benefits and, consequently, the most appropriate antibody concentrations for effective treatment are unclear.”
To learn more, Dr. Polack and colleagues included patients with PCR-confirmed COVID-19 who were aged 75 years or older, regardless of comorbidities. They also included patients aged 65-74 years who had at least one underlying condition. Participants were enrolled at clinical sites or geriatric units in Argentina. The mean age was 77 years, and 62% were women.
In an intent-to-treat analysis, the primary outcome – severe respiratory disease – occurred in 16% of the plasma recipients, vs. 31% of the group that received placebo. The relative risk was 0.52 (95% confidence interval, 0.29-0.94; P = .03).
The number needed to treat to avoid a severe respiratory disease episode was 7 (95% CI, 4-50).
Life-threatening respiratory disease, a secondary outcome, occurred in four people in the plasma group, compared with 10 in the placebo group. Two patients in the treatment group and four patients in the placebo group died.
The researchers also ran a modified intent-to-treat analysis that excluded six participants who experienced severe respiratory disease prior to receiving plasma or placebo. In this analysis, efficacy of plasma therapy increased to 60%.
“Again, this finding suggests that early intervention is critical for efficacy,” the investigators noted.
The investigators, who are based in Argentina, defined their primary endpoint as a respiratory rate of 30 or more breaths per minute and/or an oxygen saturation of less than 93% while breathing ambient air.
Dr. Sullivan pointed out that this is equivalent to the threshold commonly used for hospitalizing people with COVID-19 in the United States. “So it’s equivalent to avoiding hospitalizations. The take-home is high-titer plasma prevents respiratory distress, which equals hospitalization for us.”
Dr. Sullivan is conducting similar research in the United States regarding the use of plasma for treatment or prevention. He and colleagues are evaluating adults aged 18-90 years, “not just the ones at highest risk for going to the hospital,” he said. Enrollment is ongoing.
An inexpensive therapy with global potential?
“Although our trial lacked the statistical power to discern long-term outcomes, the convalescent plasma group appeared to have better outcomes than the placebo group with respect to all secondary endpoints,” the researchers wrote. “Our findings underscore the need to return to the classic approach of treating acute viral infections early, and they define IgG targets that facilitate donor selection.”
Dr. Polack said, “This is an inexpensive solution to mitigate the burden of severe illness in the population most vulnerable to the virus: the elderly. And it has the attraction of being applicable not only in industrialized countries but in many areas of the developing world.”
Convalescent plasma “is a potentially inexpensive alternative to monoclonal antibodies,” the researchers added. Furthermore, “early infusions of convalescent plasma can provide a bridge to recovery for at-risk patients until vaccines become widely available.”
Dr. Polack said the study findings did not surprise him. “We always thought that, as it has been the case in the past with many therapeutic strategies against respiratory and other viral infections, the earlier you treat, the better.
“We just hoped that within 72 hours of symptoms we would be treating early enough – remember that there is a 4- to 5-day incubation period that the virus leverages before the first symptom – and with enough antibody,” he added.
“We are glad it worked,” he said.
The study was supported by the Bill and Melinda Gates Foundation and by the Fundación INFANT Pandemic Fund. Dr. Polack, Dr. Simonovich, and Dr. Sullivan have disclosed various financial relationships industry.
A version of this article first appeared on Medscape.com.
Administering convalescent plasma that has high levels of antibodies against SARS-CoV-2 within the first 3 days of symptoms was associated with significantly lower chances of progression to severe COVID-19, new evidence demonstrates.
In a trial of 160 older adults with COVID-19, half of whom were randomly assigned to receive plasma and half to receive placebo infusion, treatment with high-titer plasma lowered the relative risk for severe disease by 48% in an intent-to-treat analysis.
“We now have evidence, in the context of a small but well-designed study, that convalescent plasma with high titers of antibody against SARS-CoV-2 administered in the first 3 days of mild symptoms to infected elderly reduces progression of illness and the rate of severe presentations,” senior author Fernando Polack, MD, said in an interview.
“Not any plasma, not any time,” added Dr. Polack, an infectious disease specialist and scientific director at Fundacion INFANT and professor of pediatrics at the University of Buenos Aires. The key, he said, is to select plasma in the upper 28th percentile of IgG antibody concentrations and to administer therapy prior to disease progression.
The study was published online Jan. 6 in The New England Journal of Medicine.
“It’s a very good study and approaches a different population from the PlasmAr study,” Ventura Simonovich, MD, chief of the clinical pharmacology section, Medical Clinic Service, Hospital Italiano de Buenos Aires, said in an interview. “This is the first published randomized controlled trial that shows real benefit in this [older adult] population, the most vulnerable in this disease,” he said.
Dr. Simonovich, who was not affiliated with the current study, was lead author of the PlasmAr trial, which was published in The New England Journal of Medicine Nov. 24, 2020. In that trial, the researchers evaluated adults aged 18 years and older and found no significant benefit with convalescent plasma treatment over placebo for patients with COVID-19 and severe pneumonia.
“We know antibodies work best when given early and in high dose. This is one of the rare reports that validates it in the outpatient setting,” David Sullivan, MD, professor of molecular biology and immunology at Johns Hopkins Bloomberg School of Public Health, Baltimore, said in an interview when asked to comment.
Dr. Sullivan pointed out that most previous studies on convalescent plasma focused on patients with COVID-19 who had severe cases late in the disease course.
Regarding the current study, he said, “The striking thing is treating people within 3 days of illness.”
A more cautious interpretation may be warranted, one expert said. “The study demonstrates the benefit of early intervention. There was a dose-dependent effect, with higher titers providing a greater benefit,” Manoj Menon, MD, MPH, a hematologist and oncologist at the University of Washington, Seattle, said in an interview.
“Taken together, the findings have biologic plausibility and produce more data on the role of convalescent plasma to a relevant age cohort,” he added.
However, Dr. Menon said: “Given the limited sample size, I do not think this study, although well conducted, definitively addresses the role of convalescent plasma for COVID-19. But it does merit additional study.”
A search for clear answers
Treatments that target the early stages of COVID-19 “remain elusive. Few strategies provide benefit, several have failed, and others are being evaluated,” the researchers noted. “In hospitalized patients with COVID-19, the infusion of convalescent plasma against SARS-CoV-2 late in the course of illness has not shown clear benefits and, consequently, the most appropriate antibody concentrations for effective treatment are unclear.”
To learn more, Dr. Polack and colleagues included patients with PCR-confirmed COVID-19 who were aged 75 years or older, regardless of comorbidities. They also included patients aged 65-74 years who had at least one underlying condition. Participants were enrolled at clinical sites or geriatric units in Argentina. The mean age was 77 years, and 62% were women.
In an intent-to-treat analysis, the primary outcome – severe respiratory disease – occurred in 16% of the plasma recipients, vs. 31% of the group that received placebo. The relative risk was 0.52 (95% confidence interval, 0.29-0.94; P = .03).
The number needed to treat to avoid a severe respiratory disease episode was 7 (95% CI, 4-50).
Life-threatening respiratory disease, a secondary outcome, occurred in four people in the plasma group, compared with 10 in the placebo group. Two patients in the treatment group and four patients in the placebo group died.
The researchers also ran a modified intent-to-treat analysis that excluded six participants who experienced severe respiratory disease prior to receiving plasma or placebo. In this analysis, efficacy of plasma therapy increased to 60%.
“Again, this finding suggests that early intervention is critical for efficacy,” the investigators noted.
The investigators, who are based in Argentina, defined their primary endpoint as a respiratory rate of 30 or more breaths per minute and/or an oxygen saturation of less than 93% while breathing ambient air.
Dr. Sullivan pointed out that this is equivalent to the threshold commonly used for hospitalizing people with COVID-19 in the United States. “So it’s equivalent to avoiding hospitalizations. The take-home is high-titer plasma prevents respiratory distress, which equals hospitalization for us.”
Dr. Sullivan is conducting similar research in the United States regarding the use of plasma for treatment or prevention. He and colleagues are evaluating adults aged 18-90 years, “not just the ones at highest risk for going to the hospital,” he said. Enrollment is ongoing.
An inexpensive therapy with global potential?
“Although our trial lacked the statistical power to discern long-term outcomes, the convalescent plasma group appeared to have better outcomes than the placebo group with respect to all secondary endpoints,” the researchers wrote. “Our findings underscore the need to return to the classic approach of treating acute viral infections early, and they define IgG targets that facilitate donor selection.”
Dr. Polack said, “This is an inexpensive solution to mitigate the burden of severe illness in the population most vulnerable to the virus: the elderly. And it has the attraction of being applicable not only in industrialized countries but in many areas of the developing world.”
Convalescent plasma “is a potentially inexpensive alternative to monoclonal antibodies,” the researchers added. Furthermore, “early infusions of convalescent plasma can provide a bridge to recovery for at-risk patients until vaccines become widely available.”
Dr. Polack said the study findings did not surprise him. “We always thought that, as it has been the case in the past with many therapeutic strategies against respiratory and other viral infections, the earlier you treat, the better.
“We just hoped that within 72 hours of symptoms we would be treating early enough – remember that there is a 4- to 5-day incubation period that the virus leverages before the first symptom – and with enough antibody,” he added.
“We are glad it worked,” he said.
The study was supported by the Bill and Melinda Gates Foundation and by the Fundación INFANT Pandemic Fund. Dr. Polack, Dr. Simonovich, and Dr. Sullivan have disclosed various financial relationships industry.
A version of this article first appeared on Medscape.com.
Administering convalescent plasma that has high levels of antibodies against SARS-CoV-2 within the first 3 days of symptoms was associated with significantly lower chances of progression to severe COVID-19, new evidence demonstrates.
In a trial of 160 older adults with COVID-19, half of whom were randomly assigned to receive plasma and half to receive placebo infusion, treatment with high-titer plasma lowered the relative risk for severe disease by 48% in an intent-to-treat analysis.
“We now have evidence, in the context of a small but well-designed study, that convalescent plasma with high titers of antibody against SARS-CoV-2 administered in the first 3 days of mild symptoms to infected elderly reduces progression of illness and the rate of severe presentations,” senior author Fernando Polack, MD, said in an interview.
“Not any plasma, not any time,” added Dr. Polack, an infectious disease specialist and scientific director at Fundacion INFANT and professor of pediatrics at the University of Buenos Aires. The key, he said, is to select plasma in the upper 28th percentile of IgG antibody concentrations and to administer therapy prior to disease progression.
The study was published online Jan. 6 in The New England Journal of Medicine.
“It’s a very good study and approaches a different population from the PlasmAr study,” Ventura Simonovich, MD, chief of the clinical pharmacology section, Medical Clinic Service, Hospital Italiano de Buenos Aires, said in an interview. “This is the first published randomized controlled trial that shows real benefit in this [older adult] population, the most vulnerable in this disease,” he said.
Dr. Simonovich, who was not affiliated with the current study, was lead author of the PlasmAr trial, which was published in The New England Journal of Medicine Nov. 24, 2020. In that trial, the researchers evaluated adults aged 18 years and older and found no significant benefit with convalescent plasma treatment over placebo for patients with COVID-19 and severe pneumonia.
“We know antibodies work best when given early and in high dose. This is one of the rare reports that validates it in the outpatient setting,” David Sullivan, MD, professor of molecular biology and immunology at Johns Hopkins Bloomberg School of Public Health, Baltimore, said in an interview when asked to comment.
Dr. Sullivan pointed out that most previous studies on convalescent plasma focused on patients with COVID-19 who had severe cases late in the disease course.
Regarding the current study, he said, “The striking thing is treating people within 3 days of illness.”
A more cautious interpretation may be warranted, one expert said. “The study demonstrates the benefit of early intervention. There was a dose-dependent effect, with higher titers providing a greater benefit,” Manoj Menon, MD, MPH, a hematologist and oncologist at the University of Washington, Seattle, said in an interview.
“Taken together, the findings have biologic plausibility and produce more data on the role of convalescent plasma to a relevant age cohort,” he added.
However, Dr. Menon said: “Given the limited sample size, I do not think this study, although well conducted, definitively addresses the role of convalescent plasma for COVID-19. But it does merit additional study.”
A search for clear answers
Treatments that target the early stages of COVID-19 “remain elusive. Few strategies provide benefit, several have failed, and others are being evaluated,” the researchers noted. “In hospitalized patients with COVID-19, the infusion of convalescent plasma against SARS-CoV-2 late in the course of illness has not shown clear benefits and, consequently, the most appropriate antibody concentrations for effective treatment are unclear.”
To learn more, Dr. Polack and colleagues included patients with PCR-confirmed COVID-19 who were aged 75 years or older, regardless of comorbidities. They also included patients aged 65-74 years who had at least one underlying condition. Participants were enrolled at clinical sites or geriatric units in Argentina. The mean age was 77 years, and 62% were women.
In an intent-to-treat analysis, the primary outcome – severe respiratory disease – occurred in 16% of the plasma recipients, vs. 31% of the group that received placebo. The relative risk was 0.52 (95% confidence interval, 0.29-0.94; P = .03).
The number needed to treat to avoid a severe respiratory disease episode was 7 (95% CI, 4-50).
Life-threatening respiratory disease, a secondary outcome, occurred in four people in the plasma group, compared with 10 in the placebo group. Two patients in the treatment group and four patients in the placebo group died.
The researchers also ran a modified intent-to-treat analysis that excluded six participants who experienced severe respiratory disease prior to receiving plasma or placebo. In this analysis, efficacy of plasma therapy increased to 60%.
“Again, this finding suggests that early intervention is critical for efficacy,” the investigators noted.
The investigators, who are based in Argentina, defined their primary endpoint as a respiratory rate of 30 or more breaths per minute and/or an oxygen saturation of less than 93% while breathing ambient air.
Dr. Sullivan pointed out that this is equivalent to the threshold commonly used for hospitalizing people with COVID-19 in the United States. “So it’s equivalent to avoiding hospitalizations. The take-home is high-titer plasma prevents respiratory distress, which equals hospitalization for us.”
Dr. Sullivan is conducting similar research in the United States regarding the use of plasma for treatment or prevention. He and colleagues are evaluating adults aged 18-90 years, “not just the ones at highest risk for going to the hospital,” he said. Enrollment is ongoing.
An inexpensive therapy with global potential?
“Although our trial lacked the statistical power to discern long-term outcomes, the convalescent plasma group appeared to have better outcomes than the placebo group with respect to all secondary endpoints,” the researchers wrote. “Our findings underscore the need to return to the classic approach of treating acute viral infections early, and they define IgG targets that facilitate donor selection.”
Dr. Polack said, “This is an inexpensive solution to mitigate the burden of severe illness in the population most vulnerable to the virus: the elderly. And it has the attraction of being applicable not only in industrialized countries but in many areas of the developing world.”
Convalescent plasma “is a potentially inexpensive alternative to monoclonal antibodies,” the researchers added. Furthermore, “early infusions of convalescent plasma can provide a bridge to recovery for at-risk patients until vaccines become widely available.”
Dr. Polack said the study findings did not surprise him. “We always thought that, as it has been the case in the past with many therapeutic strategies against respiratory and other viral infections, the earlier you treat, the better.
“We just hoped that within 72 hours of symptoms we would be treating early enough – remember that there is a 4- to 5-day incubation period that the virus leverages before the first symptom – and with enough antibody,” he added.
“We are glad it worked,” he said.
The study was supported by the Bill and Melinda Gates Foundation and by the Fundación INFANT Pandemic Fund. Dr. Polack, Dr. Simonovich, and Dr. Sullivan have disclosed various financial relationships industry.
A version of this article first appeared on Medscape.com.
Guidance issued on COVID vaccine use in patients with dermal fillers
outlining the potential risk and clinical relevance.
The association is not surprising, since other vaccines, including the influenza vaccine, have also been associated with inflammatory reactions in patients with dermal fillers. A warning about inflammatory events from these and other immunologic triggers should be part of routine informed consent, according to Sue Ellen Cox, MD, a coauthor of the guidance and the ASDS president-elect.
“Patients who have had dermal filler should not be discouraged from receiving the vaccine, and those who have received the vaccine should not be discouraged from receiving dermal filler,” Dr. Cox, who practices in Chapel Hill, N.C., said in an interview.
The only available data to assess the risk came from the trial of the Moderna vaccine. Of a total of 15,184 participants who received at least one dose of mRNA-1273, three developed facial or lip swelling that was presumably related to dermal filler. In the placebo group, there were no comparable inflammatory events.
“This is a very small number, but there is no reliable information about the number of patients in either group who had dermal filler, so we do not know the denominator,” Dr. Cox said.
In all three cases, the swelling at the site of dermal filler was observed within 2 days of the vaccination. None were considered a serious adverse event and all resolved. The filler had been administered 2 weeks prior to vaccination in one case, 6 months prior in a second, and time of administration was unknown in the third.
The resolution of the inflammatory reactions associated with the SARS-CoV-2 vaccine is similar to those related to dermal fillers following other immunologic triggers, which not only include other vaccines, but viral or bacterial illnesses and dental procedures. Typically, they are readily controlled with oral corticosteroids, but also typically resolve even in the absence of treatment, according to Dr. Cox.
“The good news is that these will go away,” Dr. Cox said.
The ASDS guidance is meant to alert clinicians and patients to the potential association between inflammatory events and SARS-CoV-2 vaccination in patients with dermal filler, but Dr. Cox said that it will ultimately have very little effect on her own practice. She already employs an informed consent that includes language warning about the potential risk of local reactions to immunological triggers that include vaccines. SARS-CoV-2 vaccination can now be added to examples of potential triggers, but it does not change the importance of informing patients of such triggers, Dr. Cox explained.
Asked if patients should be informed specifically about the association between dermal filler inflammatory reactions and SARS-CoV-2 vaccine, the current ASDS president and first author of the guidance, Mathew Avram, MD, JD, suggested that they should. Although he emphasized that the side effect is clearly rare, he believes it deserves attention.
“We wanted dermatologists and other physicians to be aware of the potential. We focused on the available data but specifically decided not to provide any treatment recommendations at this time,” he said in an interview.
As new data become available, the Soft-Tissue Fillers Guideline Task Force of the ASDS, which provided the guidance, will continue to monitor the relationship between SARS-CoV-2 vaccinations and dermal filler reactions, including other SARS-CoV-2 vaccines and the relative risks for hyaluronic acid and non–hyaluronic acid types of fillers.
“Our guidance was based only on the trial data, but there will soon be tens of millions of patients exposed to several different SARS-CoV-2 vaccines. We may learn things we do not know now, and we plan to communicate to our membership and others any new information as events unfold,” said Dr. Avram, who is director of dermatologic surgery, Massachusetts General Hospital, Boston,
Based on her own expertise in the field, Dr. Cox suggested that administration of SARS-CoV-2 vaccine and administration of dermal filler should be separated by at least 2 weeks regardless of which comes first. Her recommendation is not based on controlled data, but she considers this a prudent interval even if it has not been tested in a controlled study.
The full ASDS guidance is scheduled to appear in an upcoming issue of Dermatologic Surgery.
As new data become available, the Soft-tissue Fillers Guideline Task Force of the ASDS, which provided the guidance, will continue to monitor the relationship between SARS-CoV-2 vaccinations and dermal filler reactions, including other types of vaccines and the relative risks for hyaluronic acid and non–hyaluronic acid types of fillers.
This article was updated 1/7/21.
outlining the potential risk and clinical relevance.
The association is not surprising, since other vaccines, including the influenza vaccine, have also been associated with inflammatory reactions in patients with dermal fillers. A warning about inflammatory events from these and other immunologic triggers should be part of routine informed consent, according to Sue Ellen Cox, MD, a coauthor of the guidance and the ASDS president-elect.
“Patients who have had dermal filler should not be discouraged from receiving the vaccine, and those who have received the vaccine should not be discouraged from receiving dermal filler,” Dr. Cox, who practices in Chapel Hill, N.C., said in an interview.
The only available data to assess the risk came from the trial of the Moderna vaccine. Of a total of 15,184 participants who received at least one dose of mRNA-1273, three developed facial or lip swelling that was presumably related to dermal filler. In the placebo group, there were no comparable inflammatory events.
“This is a very small number, but there is no reliable information about the number of patients in either group who had dermal filler, so we do not know the denominator,” Dr. Cox said.
In all three cases, the swelling at the site of dermal filler was observed within 2 days of the vaccination. None were considered a serious adverse event and all resolved. The filler had been administered 2 weeks prior to vaccination in one case, 6 months prior in a second, and time of administration was unknown in the third.
The resolution of the inflammatory reactions associated with the SARS-CoV-2 vaccine is similar to those related to dermal fillers following other immunologic triggers, which not only include other vaccines, but viral or bacterial illnesses and dental procedures. Typically, they are readily controlled with oral corticosteroids, but also typically resolve even in the absence of treatment, according to Dr. Cox.
“The good news is that these will go away,” Dr. Cox said.
The ASDS guidance is meant to alert clinicians and patients to the potential association between inflammatory events and SARS-CoV-2 vaccination in patients with dermal filler, but Dr. Cox said that it will ultimately have very little effect on her own practice. She already employs an informed consent that includes language warning about the potential risk of local reactions to immunological triggers that include vaccines. SARS-CoV-2 vaccination can now be added to examples of potential triggers, but it does not change the importance of informing patients of such triggers, Dr. Cox explained.
Asked if patients should be informed specifically about the association between dermal filler inflammatory reactions and SARS-CoV-2 vaccine, the current ASDS president and first author of the guidance, Mathew Avram, MD, JD, suggested that they should. Although he emphasized that the side effect is clearly rare, he believes it deserves attention.
“We wanted dermatologists and other physicians to be aware of the potential. We focused on the available data but specifically decided not to provide any treatment recommendations at this time,” he said in an interview.
As new data become available, the Soft-Tissue Fillers Guideline Task Force of the ASDS, which provided the guidance, will continue to monitor the relationship between SARS-CoV-2 vaccinations and dermal filler reactions, including other SARS-CoV-2 vaccines and the relative risks for hyaluronic acid and non–hyaluronic acid types of fillers.
“Our guidance was based only on the trial data, but there will soon be tens of millions of patients exposed to several different SARS-CoV-2 vaccines. We may learn things we do not know now, and we plan to communicate to our membership and others any new information as events unfold,” said Dr. Avram, who is director of dermatologic surgery, Massachusetts General Hospital, Boston,
Based on her own expertise in the field, Dr. Cox suggested that administration of SARS-CoV-2 vaccine and administration of dermal filler should be separated by at least 2 weeks regardless of which comes first. Her recommendation is not based on controlled data, but she considers this a prudent interval even if it has not been tested in a controlled study.
The full ASDS guidance is scheduled to appear in an upcoming issue of Dermatologic Surgery.
As new data become available, the Soft-tissue Fillers Guideline Task Force of the ASDS, which provided the guidance, will continue to monitor the relationship between SARS-CoV-2 vaccinations and dermal filler reactions, including other types of vaccines and the relative risks for hyaluronic acid and non–hyaluronic acid types of fillers.
This article was updated 1/7/21.
outlining the potential risk and clinical relevance.
The association is not surprising, since other vaccines, including the influenza vaccine, have also been associated with inflammatory reactions in patients with dermal fillers. A warning about inflammatory events from these and other immunologic triggers should be part of routine informed consent, according to Sue Ellen Cox, MD, a coauthor of the guidance and the ASDS president-elect.
“Patients who have had dermal filler should not be discouraged from receiving the vaccine, and those who have received the vaccine should not be discouraged from receiving dermal filler,” Dr. Cox, who practices in Chapel Hill, N.C., said in an interview.
The only available data to assess the risk came from the trial of the Moderna vaccine. Of a total of 15,184 participants who received at least one dose of mRNA-1273, three developed facial or lip swelling that was presumably related to dermal filler. In the placebo group, there were no comparable inflammatory events.
“This is a very small number, but there is no reliable information about the number of patients in either group who had dermal filler, so we do not know the denominator,” Dr. Cox said.
In all three cases, the swelling at the site of dermal filler was observed within 2 days of the vaccination. None were considered a serious adverse event and all resolved. The filler had been administered 2 weeks prior to vaccination in one case, 6 months prior in a second, and time of administration was unknown in the third.
The resolution of the inflammatory reactions associated with the SARS-CoV-2 vaccine is similar to those related to dermal fillers following other immunologic triggers, which not only include other vaccines, but viral or bacterial illnesses and dental procedures. Typically, they are readily controlled with oral corticosteroids, but also typically resolve even in the absence of treatment, according to Dr. Cox.
“The good news is that these will go away,” Dr. Cox said.
The ASDS guidance is meant to alert clinicians and patients to the potential association between inflammatory events and SARS-CoV-2 vaccination in patients with dermal filler, but Dr. Cox said that it will ultimately have very little effect on her own practice. She already employs an informed consent that includes language warning about the potential risk of local reactions to immunological triggers that include vaccines. SARS-CoV-2 vaccination can now be added to examples of potential triggers, but it does not change the importance of informing patients of such triggers, Dr. Cox explained.
Asked if patients should be informed specifically about the association between dermal filler inflammatory reactions and SARS-CoV-2 vaccine, the current ASDS president and first author of the guidance, Mathew Avram, MD, JD, suggested that they should. Although he emphasized that the side effect is clearly rare, he believes it deserves attention.
“We wanted dermatologists and other physicians to be aware of the potential. We focused on the available data but specifically decided not to provide any treatment recommendations at this time,” he said in an interview.
As new data become available, the Soft-Tissue Fillers Guideline Task Force of the ASDS, which provided the guidance, will continue to monitor the relationship between SARS-CoV-2 vaccinations and dermal filler reactions, including other SARS-CoV-2 vaccines and the relative risks for hyaluronic acid and non–hyaluronic acid types of fillers.
“Our guidance was based only on the trial data, but there will soon be tens of millions of patients exposed to several different SARS-CoV-2 vaccines. We may learn things we do not know now, and we plan to communicate to our membership and others any new information as events unfold,” said Dr. Avram, who is director of dermatologic surgery, Massachusetts General Hospital, Boston,
Based on her own expertise in the field, Dr. Cox suggested that administration of SARS-CoV-2 vaccine and administration of dermal filler should be separated by at least 2 weeks regardless of which comes first. Her recommendation is not based on controlled data, but she considers this a prudent interval even if it has not been tested in a controlled study.
The full ASDS guidance is scheduled to appear in an upcoming issue of Dermatologic Surgery.
As new data become available, the Soft-tissue Fillers Guideline Task Force of the ASDS, which provided the guidance, will continue to monitor the relationship between SARS-CoV-2 vaccinations and dermal filler reactions, including other types of vaccines and the relative risks for hyaluronic acid and non–hyaluronic acid types of fillers.
This article was updated 1/7/21.