User login
In Case You Missed It: COVID
First COVID-19 human challenge study provides insights
A small droplet that contains the coronavirus can infect someone with COVID-19, according to recent results from the first COVID-19 human challenge study, which were published in Nature Medicine.
Human challenge trials deliberately infect healthy volunteers to understand how an infection occurs and develops. In the first human challenge study for COVID-19, people were infected with the SARS-CoV-2 virus to better understand what has happened during the pandemic.
“Really, there’s no other type of study where you can do that, because normally, patients only come to your attention if they have developed symptoms, and so you miss all of those preceding days when the infection is brewing,” Christopher Chiu, MD, PhD, the lead study author and an infectious disease doctor and immunologist at Imperial College London, told CNN.
Starting in March 2021, Dr. Chiu and colleagues carefully selected 36 volunteers aged 18-30 years who didn’t have any risk factors for severe COVID-19, such as being overweight or having kidney, liver, heart, lung or blood problems. Participants also signed an extensive informed consent form, CNN reported.
The researchers conducted the trial in phases for safety. The first 10 participants who were infected received remdesivir, the antiviral drug, to reduce their chances of progressing to severe COVID-19. The research team also had monoclonal antibodies on hand in case any volunteers developed more severe symptoms. Ultimately, the researchers said, remdesivir was unnecessary, and they didn’t need to use the antibodies.
As part of the study, the participants had a small droplet of fluid that contained the original coronavirus strain inserted into their nose through a long tube. They stayed at London’s Royal Free Hospital for 2 weeks and were monitored by doctors 24 hours a day in rooms that had special air flow to keep the virus from spreading.
Of the 36 participants, 18 became infected, including two who never developed symptoms. The others had mild cases with symptoms such as congestion, sneezing, stuffy nose, and sore throat. Some also had headaches, muscle and joint pain, fatigue, and fever.
About 83% of participants who contracted COVID-19 lost their sense of smell to some degree, and nine people couldn’t smell at all. The symptom improved for most participants within 90 days, though one person still hadn’t fully regained their sense of smell about six months after the study ended.
The research team reported several other findings:
- Small amounts of the virus can make someone sick. About 10 mcm, or the amount in a single droplet that someone sneezes or coughs, can lead to infection.
- About 40 hours after the virus was inserted into a participant’s nose, the virus could be detected in the back of the throat.
- It took about 58 hours for the virus to appear on swabs from the nose, where the viral load eventually increased even more.
- COVID-19 has a short incubation period. It takes about 2 days after infection for someone to begin shedding the virus to others.
- People become contagious and shed high amounts of the virus before they show symptoms.
- In addition, infected people can shed high levels of the virus even if they don’t develop any symptoms.
- The study volunteers shed the virus for about 6 days on average, though some shed the virus for up to 12 days, even if they didn’t have symptoms.
- Lateral flow tests, which are used for rapid at-home tests, work well when an infected person is contagious. These tests could diagnose infection before 70%-80% of the viable virus had been generated.
The findings emphasized the importance of contagious people covering their mouth and nose when sick to protect others, Dr. Chiu told CNN.
None of the study volunteers developed lung issues as part of their infection, CNN reported. Dr. Chiu said that’s likely because they were young, healthy and received tiny amounts of the virus. All of the participants will be followed for a year to monitor for potential long-term effects.
Throughout the study, the research team also conducted cognitive tests to check the participants’ short-term memory and reaction time. The researchers are still analyzing the data, but the results “will really be informative,” Dr. Chiu told CNN.
Now the research team will conduct another human challenge trial, which will include vaccinated people who will be infected with the Delta variant. The researchers intend to study participants’ immune responses, which could provide valuable insights about new variants and vaccines.
“While there are differences in transmissibility due to the emergence of variants, such as Delta and Omicron, fundamentally, this is the same disease and the same factors will be responsible for protecting it,” Dr. Chiu said in a statement.
The research team will also study the 18 participants who didn’t get sick in the first human challenge trial. They didn’t develop antibodies, Dr. Chiu told CNN, despite receiving the same dose of the virus as those who got sick.
Before the study, all of the participants were screened for antibodies to other viruses, such as the original SARS virus. That means the volunteers weren’t cross-protected, and other factors may play into why some people don’t contract COVID-19. Future studies could help researchers provide better advice about protection if new variants emerge or a future pandemic occurs.
“There are lots of other things that help protect us,” Dr. Chiu said. “There are barriers in the nose. There are different kinds of proteins and things which are very ancient, primordial, protective systems ... and we’re really interested in trying to understand what those are.”
A version of this article first appeared on WebMD.com.
A small droplet that contains the coronavirus can infect someone with COVID-19, according to recent results from the first COVID-19 human challenge study, which were published in Nature Medicine.
Human challenge trials deliberately infect healthy volunteers to understand how an infection occurs and develops. In the first human challenge study for COVID-19, people were infected with the SARS-CoV-2 virus to better understand what has happened during the pandemic.
“Really, there’s no other type of study where you can do that, because normally, patients only come to your attention if they have developed symptoms, and so you miss all of those preceding days when the infection is brewing,” Christopher Chiu, MD, PhD, the lead study author and an infectious disease doctor and immunologist at Imperial College London, told CNN.
Starting in March 2021, Dr. Chiu and colleagues carefully selected 36 volunteers aged 18-30 years who didn’t have any risk factors for severe COVID-19, such as being overweight or having kidney, liver, heart, lung or blood problems. Participants also signed an extensive informed consent form, CNN reported.
The researchers conducted the trial in phases for safety. The first 10 participants who were infected received remdesivir, the antiviral drug, to reduce their chances of progressing to severe COVID-19. The research team also had monoclonal antibodies on hand in case any volunteers developed more severe symptoms. Ultimately, the researchers said, remdesivir was unnecessary, and they didn’t need to use the antibodies.
As part of the study, the participants had a small droplet of fluid that contained the original coronavirus strain inserted into their nose through a long tube. They stayed at London’s Royal Free Hospital for 2 weeks and were monitored by doctors 24 hours a day in rooms that had special air flow to keep the virus from spreading.
Of the 36 participants, 18 became infected, including two who never developed symptoms. The others had mild cases with symptoms such as congestion, sneezing, stuffy nose, and sore throat. Some also had headaches, muscle and joint pain, fatigue, and fever.
About 83% of participants who contracted COVID-19 lost their sense of smell to some degree, and nine people couldn’t smell at all. The symptom improved for most participants within 90 days, though one person still hadn’t fully regained their sense of smell about six months after the study ended.
The research team reported several other findings:
- Small amounts of the virus can make someone sick. About 10 mcm, or the amount in a single droplet that someone sneezes or coughs, can lead to infection.
- About 40 hours after the virus was inserted into a participant’s nose, the virus could be detected in the back of the throat.
- It took about 58 hours for the virus to appear on swabs from the nose, where the viral load eventually increased even more.
- COVID-19 has a short incubation period. It takes about 2 days after infection for someone to begin shedding the virus to others.
- People become contagious and shed high amounts of the virus before they show symptoms.
- In addition, infected people can shed high levels of the virus even if they don’t develop any symptoms.
- The study volunteers shed the virus for about 6 days on average, though some shed the virus for up to 12 days, even if they didn’t have symptoms.
- Lateral flow tests, which are used for rapid at-home tests, work well when an infected person is contagious. These tests could diagnose infection before 70%-80% of the viable virus had been generated.
The findings emphasized the importance of contagious people covering their mouth and nose when sick to protect others, Dr. Chiu told CNN.
None of the study volunteers developed lung issues as part of their infection, CNN reported. Dr. Chiu said that’s likely because they were young, healthy and received tiny amounts of the virus. All of the participants will be followed for a year to monitor for potential long-term effects.
Throughout the study, the research team also conducted cognitive tests to check the participants’ short-term memory and reaction time. The researchers are still analyzing the data, but the results “will really be informative,” Dr. Chiu told CNN.
Now the research team will conduct another human challenge trial, which will include vaccinated people who will be infected with the Delta variant. The researchers intend to study participants’ immune responses, which could provide valuable insights about new variants and vaccines.
“While there are differences in transmissibility due to the emergence of variants, such as Delta and Omicron, fundamentally, this is the same disease and the same factors will be responsible for protecting it,” Dr. Chiu said in a statement.
The research team will also study the 18 participants who didn’t get sick in the first human challenge trial. They didn’t develop antibodies, Dr. Chiu told CNN, despite receiving the same dose of the virus as those who got sick.
Before the study, all of the participants were screened for antibodies to other viruses, such as the original SARS virus. That means the volunteers weren’t cross-protected, and other factors may play into why some people don’t contract COVID-19. Future studies could help researchers provide better advice about protection if new variants emerge or a future pandemic occurs.
“There are lots of other things that help protect us,” Dr. Chiu said. “There are barriers in the nose. There are different kinds of proteins and things which are very ancient, primordial, protective systems ... and we’re really interested in trying to understand what those are.”
A version of this article first appeared on WebMD.com.
A small droplet that contains the coronavirus can infect someone with COVID-19, according to recent results from the first COVID-19 human challenge study, which were published in Nature Medicine.
Human challenge trials deliberately infect healthy volunteers to understand how an infection occurs and develops. In the first human challenge study for COVID-19, people were infected with the SARS-CoV-2 virus to better understand what has happened during the pandemic.
“Really, there’s no other type of study where you can do that, because normally, patients only come to your attention if they have developed symptoms, and so you miss all of those preceding days when the infection is brewing,” Christopher Chiu, MD, PhD, the lead study author and an infectious disease doctor and immunologist at Imperial College London, told CNN.
Starting in March 2021, Dr. Chiu and colleagues carefully selected 36 volunteers aged 18-30 years who didn’t have any risk factors for severe COVID-19, such as being overweight or having kidney, liver, heart, lung or blood problems. Participants also signed an extensive informed consent form, CNN reported.
The researchers conducted the trial in phases for safety. The first 10 participants who were infected received remdesivir, the antiviral drug, to reduce their chances of progressing to severe COVID-19. The research team also had monoclonal antibodies on hand in case any volunteers developed more severe symptoms. Ultimately, the researchers said, remdesivir was unnecessary, and they didn’t need to use the antibodies.
As part of the study, the participants had a small droplet of fluid that contained the original coronavirus strain inserted into their nose through a long tube. They stayed at London’s Royal Free Hospital for 2 weeks and were monitored by doctors 24 hours a day in rooms that had special air flow to keep the virus from spreading.
Of the 36 participants, 18 became infected, including two who never developed symptoms. The others had mild cases with symptoms such as congestion, sneezing, stuffy nose, and sore throat. Some also had headaches, muscle and joint pain, fatigue, and fever.
About 83% of participants who contracted COVID-19 lost their sense of smell to some degree, and nine people couldn’t smell at all. The symptom improved for most participants within 90 days, though one person still hadn’t fully regained their sense of smell about six months after the study ended.
The research team reported several other findings:
- Small amounts of the virus can make someone sick. About 10 mcm, or the amount in a single droplet that someone sneezes or coughs, can lead to infection.
- About 40 hours after the virus was inserted into a participant’s nose, the virus could be detected in the back of the throat.
- It took about 58 hours for the virus to appear on swabs from the nose, where the viral load eventually increased even more.
- COVID-19 has a short incubation period. It takes about 2 days after infection for someone to begin shedding the virus to others.
- People become contagious and shed high amounts of the virus before they show symptoms.
- In addition, infected people can shed high levels of the virus even if they don’t develop any symptoms.
- The study volunteers shed the virus for about 6 days on average, though some shed the virus for up to 12 days, even if they didn’t have symptoms.
- Lateral flow tests, which are used for rapid at-home tests, work well when an infected person is contagious. These tests could diagnose infection before 70%-80% of the viable virus had been generated.
The findings emphasized the importance of contagious people covering their mouth and nose when sick to protect others, Dr. Chiu told CNN.
None of the study volunteers developed lung issues as part of their infection, CNN reported. Dr. Chiu said that’s likely because they were young, healthy and received tiny amounts of the virus. All of the participants will be followed for a year to monitor for potential long-term effects.
Throughout the study, the research team also conducted cognitive tests to check the participants’ short-term memory and reaction time. The researchers are still analyzing the data, but the results “will really be informative,” Dr. Chiu told CNN.
Now the research team will conduct another human challenge trial, which will include vaccinated people who will be infected with the Delta variant. The researchers intend to study participants’ immune responses, which could provide valuable insights about new variants and vaccines.
“While there are differences in transmissibility due to the emergence of variants, such as Delta and Omicron, fundamentally, this is the same disease and the same factors will be responsible for protecting it,” Dr. Chiu said in a statement.
The research team will also study the 18 participants who didn’t get sick in the first human challenge trial. They didn’t develop antibodies, Dr. Chiu told CNN, despite receiving the same dose of the virus as those who got sick.
Before the study, all of the participants were screened for antibodies to other viruses, such as the original SARS virus. That means the volunteers weren’t cross-protected, and other factors may play into why some people don’t contract COVID-19. Future studies could help researchers provide better advice about protection if new variants emerge or a future pandemic occurs.
“There are lots of other things that help protect us,” Dr. Chiu said. “There are barriers in the nose. There are different kinds of proteins and things which are very ancient, primordial, protective systems ... and we’re really interested in trying to understand what those are.”
A version of this article first appeared on WebMD.com.
FROM NATURE MEDICINE
Ivermectin doesn’t help treat COVID-19, large study finds
according to results from a large clinical trial published in the New England Journal of Medicine.
The findings pretty much rule out the drug as a treatment for COVID-19, the study authors wrote.
“There’s really no sign of any benefit,” David Boulware, MD, one of the coauthors and an infectious disease specialist at the University of Minnesota, Minneapolis, told the New York Times.
The researchers shared a summary of the results in August 2021 during an online presentation hosted by the National Institutes of Health. The full data hadn’t been published until now.
“Now that people can dive into the details and the data, hopefully that will steer the majority of doctors away from ivermectin toward other therapies,” Dr. Boulware said.
In the trial, the research team compared more than 1,350 people infected with the coronavirus in Brazil who received either ivermectin or a placebo as treatment.
Between March and August 2021, 679 patients received a daily dose of ivermectin over the course of 3 days. The researchers found that ivermectin didn’t reduce the risk that people with COVID-19 would be hospitalized or go to an ED within 28 days after treatment.
In addition, the researchers looked at particular groups to understand if some patients benefited for some reason, such as taking ivermectin sooner after testing positive for COVID-19. But those who took the drug during the first 3 days after a positive coronavirus test ended up doing worse than those in the placebo group. The drug also didn’t help patients recover sooner.
The researchers found “no important effects” of treatment with ivermectin on the number of days people spent in the hospital, the number of days hospitalized people needed mechanical ventilation, or the risk of death.
Ivermectin has become a controversial focal point during the pandemic.
For decades, the drug has been widely used to treat parasitic infections. At the beginning of the pandemic, researchers checked thousands of existing drugs against the coronavirus to determine if a potential treatment already existed. Laboratory experiments on cells suggested that ivermectin might work, the New York Times reported.
But some researchers noted that the experiments worked because a high concentration of ivermectin was used, a much higher dose than would be safe for people. Despite the concerns, some doctors began prescribing ivermectin to patients. After receiving reports of people who needed medical attention, particularly after using formulations intended for livestock, the Food and Drug Administration issued a warning that the drug wasn’t approved to be used for COVID-19.
Researchers around the world have done small clinical trials to understand whether ivermectin treats COVID-19, the newspaper reported. At the end of 2020, Andrew Hill, MD, a virologist at the University of Liverpool in England, reviewed the results from 23 trials and concluded that the drug could lower the risk of death from COVID-19. He published the results in July 2021, but later reports found that many of the studies were flawed, and at least one was fraudulent.
Dr. Hill retracted his original study and began another analysis, which was published in January 2022. In this review, he and his colleagues focused on studies that were least likely to be biased. They found that ivermectin was not helpful.
Recently, Dr. Hill and associates ran another analysis using the new data from the Brazil trial, and once again they saw no benefit.
Several clinical trials are still testing ivermectin as a treatment, the New York Times reported, with results expected in upcoming months. After reviewing the data from the Brazil trial, which tested ivermectin and a variety of other drugs against COVID-19, some infectious disease experts say they’ll likely see more of the same – that ivermectin doesn’t help people with COVID-19.
“I welcome the results of the other clinical trials and will view them with an open mind,” Paul Sax, MD, an infectious disease expert at Brigham and Women’s Hospital, Boston, who has been watching the data on the drug throughout the pandemic, told the New York Times.
“But at some point, it will become a waste of resources to continue studying an unpromising approach,” he said.
A version of this article first appeared on WebMD.com.
according to results from a large clinical trial published in the New England Journal of Medicine.
The findings pretty much rule out the drug as a treatment for COVID-19, the study authors wrote.
“There’s really no sign of any benefit,” David Boulware, MD, one of the coauthors and an infectious disease specialist at the University of Minnesota, Minneapolis, told the New York Times.
The researchers shared a summary of the results in August 2021 during an online presentation hosted by the National Institutes of Health. The full data hadn’t been published until now.
“Now that people can dive into the details and the data, hopefully that will steer the majority of doctors away from ivermectin toward other therapies,” Dr. Boulware said.
In the trial, the research team compared more than 1,350 people infected with the coronavirus in Brazil who received either ivermectin or a placebo as treatment.
Between March and August 2021, 679 patients received a daily dose of ivermectin over the course of 3 days. The researchers found that ivermectin didn’t reduce the risk that people with COVID-19 would be hospitalized or go to an ED within 28 days after treatment.
In addition, the researchers looked at particular groups to understand if some patients benefited for some reason, such as taking ivermectin sooner after testing positive for COVID-19. But those who took the drug during the first 3 days after a positive coronavirus test ended up doing worse than those in the placebo group. The drug also didn’t help patients recover sooner.
The researchers found “no important effects” of treatment with ivermectin on the number of days people spent in the hospital, the number of days hospitalized people needed mechanical ventilation, or the risk of death.
Ivermectin has become a controversial focal point during the pandemic.
For decades, the drug has been widely used to treat parasitic infections. At the beginning of the pandemic, researchers checked thousands of existing drugs against the coronavirus to determine if a potential treatment already existed. Laboratory experiments on cells suggested that ivermectin might work, the New York Times reported.
But some researchers noted that the experiments worked because a high concentration of ivermectin was used, a much higher dose than would be safe for people. Despite the concerns, some doctors began prescribing ivermectin to patients. After receiving reports of people who needed medical attention, particularly after using formulations intended for livestock, the Food and Drug Administration issued a warning that the drug wasn’t approved to be used for COVID-19.
Researchers around the world have done small clinical trials to understand whether ivermectin treats COVID-19, the newspaper reported. At the end of 2020, Andrew Hill, MD, a virologist at the University of Liverpool in England, reviewed the results from 23 trials and concluded that the drug could lower the risk of death from COVID-19. He published the results in July 2021, but later reports found that many of the studies were flawed, and at least one was fraudulent.
Dr. Hill retracted his original study and began another analysis, which was published in January 2022. In this review, he and his colleagues focused on studies that were least likely to be biased. They found that ivermectin was not helpful.
Recently, Dr. Hill and associates ran another analysis using the new data from the Brazil trial, and once again they saw no benefit.
Several clinical trials are still testing ivermectin as a treatment, the New York Times reported, with results expected in upcoming months. After reviewing the data from the Brazil trial, which tested ivermectin and a variety of other drugs against COVID-19, some infectious disease experts say they’ll likely see more of the same – that ivermectin doesn’t help people with COVID-19.
“I welcome the results of the other clinical trials and will view them with an open mind,” Paul Sax, MD, an infectious disease expert at Brigham and Women’s Hospital, Boston, who has been watching the data on the drug throughout the pandemic, told the New York Times.
“But at some point, it will become a waste of resources to continue studying an unpromising approach,” he said.
A version of this article first appeared on WebMD.com.
according to results from a large clinical trial published in the New England Journal of Medicine.
The findings pretty much rule out the drug as a treatment for COVID-19, the study authors wrote.
“There’s really no sign of any benefit,” David Boulware, MD, one of the coauthors and an infectious disease specialist at the University of Minnesota, Minneapolis, told the New York Times.
The researchers shared a summary of the results in August 2021 during an online presentation hosted by the National Institutes of Health. The full data hadn’t been published until now.
“Now that people can dive into the details and the data, hopefully that will steer the majority of doctors away from ivermectin toward other therapies,” Dr. Boulware said.
In the trial, the research team compared more than 1,350 people infected with the coronavirus in Brazil who received either ivermectin or a placebo as treatment.
Between March and August 2021, 679 patients received a daily dose of ivermectin over the course of 3 days. The researchers found that ivermectin didn’t reduce the risk that people with COVID-19 would be hospitalized or go to an ED within 28 days after treatment.
In addition, the researchers looked at particular groups to understand if some patients benefited for some reason, such as taking ivermectin sooner after testing positive for COVID-19. But those who took the drug during the first 3 days after a positive coronavirus test ended up doing worse than those in the placebo group. The drug also didn’t help patients recover sooner.
The researchers found “no important effects” of treatment with ivermectin on the number of days people spent in the hospital, the number of days hospitalized people needed mechanical ventilation, or the risk of death.
Ivermectin has become a controversial focal point during the pandemic.
For decades, the drug has been widely used to treat parasitic infections. At the beginning of the pandemic, researchers checked thousands of existing drugs against the coronavirus to determine if a potential treatment already existed. Laboratory experiments on cells suggested that ivermectin might work, the New York Times reported.
But some researchers noted that the experiments worked because a high concentration of ivermectin was used, a much higher dose than would be safe for people. Despite the concerns, some doctors began prescribing ivermectin to patients. After receiving reports of people who needed medical attention, particularly after using formulations intended for livestock, the Food and Drug Administration issued a warning that the drug wasn’t approved to be used for COVID-19.
Researchers around the world have done small clinical trials to understand whether ivermectin treats COVID-19, the newspaper reported. At the end of 2020, Andrew Hill, MD, a virologist at the University of Liverpool in England, reviewed the results from 23 trials and concluded that the drug could lower the risk of death from COVID-19. He published the results in July 2021, but later reports found that many of the studies were flawed, and at least one was fraudulent.
Dr. Hill retracted his original study and began another analysis, which was published in January 2022. In this review, he and his colleagues focused on studies that were least likely to be biased. They found that ivermectin was not helpful.
Recently, Dr. Hill and associates ran another analysis using the new data from the Brazil trial, and once again they saw no benefit.
Several clinical trials are still testing ivermectin as a treatment, the New York Times reported, with results expected in upcoming months. After reviewing the data from the Brazil trial, which tested ivermectin and a variety of other drugs against COVID-19, some infectious disease experts say they’ll likely see more of the same – that ivermectin doesn’t help people with COVID-19.
“I welcome the results of the other clinical trials and will view them with an open mind,” Paul Sax, MD, an infectious disease expert at Brigham and Women’s Hospital, Boston, who has been watching the data on the drug throughout the pandemic, told the New York Times.
“But at some point, it will become a waste of resources to continue studying an unpromising approach,” he said.
A version of this article first appeared on WebMD.com.
FROM THE NEW ENGLAND JOURNAL OF MEDICINE
Psychotropic med use tied to ‘striking’ post-COVID dementia risk
, new research suggests.
Results from a large study of more than 1,700 patients who had been hospitalized with COVID showed a greater than twofold increased risk for post-COVID dementia in those taking antipsychotics and mood stabilizers/anticonvulsants – medications often used to treat schizophrenia, psychosis, bipolar disorder, and seizures.
“We know that pre-existing psychiatric illness is associated with poor COVID-19 outcomes, but our study is the first to show an association with certain psychiatric medications and dementia,” co-investigator Liron Sinvani, MD, the Feinstein Institutes for Medical Research, Manhasset, New York, said in an interview.
“Our study highlights the potential interaction between baseline neuropsychiatric disease, psychotropic medications, COVID-19, and dementia,” Dr. Sinvani added.
The findings were published online March 18 in Frontiers in Medicine.
‘Striking’ dementia rate
Using electronic health records, the researchers evaluated pre-COVID psychotropic medication use and post-COVID dementia onset in 1,755 adults aged 65 and older. All were hospitalized with COVID-19 at Northwell Health between March 1 and April 20, 2020.
A “striking” 13% of the participants (n = 223) developed dementia within 1-year of follow-up, the investigators report.
Among the 438 patients (25%) exposed to at least one psychotropic medication before COVID-19, 105 (24%) developed dementia in the year following COVID versus 118 of 1,317 (9%) patients with no pre-COVID exposure to psychotropic medication (odds ratio, 3.2; 95% confidence interval, 2.37-4.32).
Both pre-COVID psychotropic medication use (OR, 2.7; 95% CI, 1.8-4.0, P < .001) and delirium (OR, 3.0; 95% CI, 1.9-4.6, P < .001) were significantly associated with post-COVID dementia at 1 year.
In a sensitivity analysis in the subset of 423 patients with at least one documented neurologic or psychiatric diagnosis at the time of COVID admission, and after adjusting for confounding factors, pre-COVID psychotropic medication use remained significantly linked to post-COVID dementia onset (OR, 3.09; 95% CI, 1.5-6.6, P = .002).
Drug classes most strongly associated with 1-year post-COVID dementia onset were antipsychotics (OR, 2.8, 95% CI, 1.7-4.4, P < .001) and mood stabilizers/anticonvulsants (OR, 2.4, 95% CI, 1.39-4.02, P = .001).
In a further exploratory analysis, the psychotropics valproic acid (multiple brands) and haloperidol (Haldol) had the largest association with post-COVID dementia.
Antidepressants as a class were not associated with post-COVID dementia, but the potential effects of two commonly prescribed antidepressants in older adults, mirtazapine (Remeron) and escitalopram (Lexapro), “warrant further investigation,” the researchers note.
Predictive risk marker?
“This research shows that psychotropic medications can be considered a predictive risk marker for post-COVID dementia. In patients taking psychotropic medications, COVID-19 could have accelerated progression of dementia after hospitalization,” lead author Yun Freudenberg-Hua, MD, the Feinstein Institutes, said in a news release.
It is unclear why psychotropic medications may raise the risk for dementia onset after COVID, the investigators note.
“It is intuitive that psychotropic medications indicate pre-existing neuropsychiatric conditions in which COVID-19 occurs. It is possible that psychotropic medications may potentiate the neurostructural changes that have been found in the brain of those who have recovered from COVID-19,” they write.
The sensitivity analysis in patients with documented neurologic and psychiatric diagnoses supports this interpretation.
COVID-19 may also accelerate the underlying brain disorders for which psychotropic medications were prescribed, leading to the greater incidence of post-COVID dementia, the researchers write.
“It is important to note that this study is in no way recommending people should stop taking antipsychotics but simply that clinicians need to factor in a patient’s medication history while considering post-COVID aftereffects,” Dr. Freudenberg-Hua said.
“Given that the number of patients with dementia is projected to triple in the next 30 years, these findings have significant public health implications,” Dr. Sinvani added.
She noted that “care partners and health care professionals” should look for early signs of dementia, such as forgetfulness and depressive symptoms, in their patients.
“Future studies must continue to evaluate these associations, which are key for potential future interventions to prevent dementia,” Dr. Sinvani said.
The study was funded by the National Institutes of Health. Dr. Freudenberg-Hua co-owns stock and stock options from Regeneron Pharmaceuticals. Dr. Sinvani has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, new research suggests.
Results from a large study of more than 1,700 patients who had been hospitalized with COVID showed a greater than twofold increased risk for post-COVID dementia in those taking antipsychotics and mood stabilizers/anticonvulsants – medications often used to treat schizophrenia, psychosis, bipolar disorder, and seizures.
“We know that pre-existing psychiatric illness is associated with poor COVID-19 outcomes, but our study is the first to show an association with certain psychiatric medications and dementia,” co-investigator Liron Sinvani, MD, the Feinstein Institutes for Medical Research, Manhasset, New York, said in an interview.
“Our study highlights the potential interaction between baseline neuropsychiatric disease, psychotropic medications, COVID-19, and dementia,” Dr. Sinvani added.
The findings were published online March 18 in Frontiers in Medicine.
‘Striking’ dementia rate
Using electronic health records, the researchers evaluated pre-COVID psychotropic medication use and post-COVID dementia onset in 1,755 adults aged 65 and older. All were hospitalized with COVID-19 at Northwell Health between March 1 and April 20, 2020.
A “striking” 13% of the participants (n = 223) developed dementia within 1-year of follow-up, the investigators report.
Among the 438 patients (25%) exposed to at least one psychotropic medication before COVID-19, 105 (24%) developed dementia in the year following COVID versus 118 of 1,317 (9%) patients with no pre-COVID exposure to psychotropic medication (odds ratio, 3.2; 95% confidence interval, 2.37-4.32).
Both pre-COVID psychotropic medication use (OR, 2.7; 95% CI, 1.8-4.0, P < .001) and delirium (OR, 3.0; 95% CI, 1.9-4.6, P < .001) were significantly associated with post-COVID dementia at 1 year.
In a sensitivity analysis in the subset of 423 patients with at least one documented neurologic or psychiatric diagnosis at the time of COVID admission, and after adjusting for confounding factors, pre-COVID psychotropic medication use remained significantly linked to post-COVID dementia onset (OR, 3.09; 95% CI, 1.5-6.6, P = .002).
Drug classes most strongly associated with 1-year post-COVID dementia onset were antipsychotics (OR, 2.8, 95% CI, 1.7-4.4, P < .001) and mood stabilizers/anticonvulsants (OR, 2.4, 95% CI, 1.39-4.02, P = .001).
In a further exploratory analysis, the psychotropics valproic acid (multiple brands) and haloperidol (Haldol) had the largest association with post-COVID dementia.
Antidepressants as a class were not associated with post-COVID dementia, but the potential effects of two commonly prescribed antidepressants in older adults, mirtazapine (Remeron) and escitalopram (Lexapro), “warrant further investigation,” the researchers note.
Predictive risk marker?
“This research shows that psychotropic medications can be considered a predictive risk marker for post-COVID dementia. In patients taking psychotropic medications, COVID-19 could have accelerated progression of dementia after hospitalization,” lead author Yun Freudenberg-Hua, MD, the Feinstein Institutes, said in a news release.
It is unclear why psychotropic medications may raise the risk for dementia onset after COVID, the investigators note.
“It is intuitive that psychotropic medications indicate pre-existing neuropsychiatric conditions in which COVID-19 occurs. It is possible that psychotropic medications may potentiate the neurostructural changes that have been found in the brain of those who have recovered from COVID-19,” they write.
The sensitivity analysis in patients with documented neurologic and psychiatric diagnoses supports this interpretation.
COVID-19 may also accelerate the underlying brain disorders for which psychotropic medications were prescribed, leading to the greater incidence of post-COVID dementia, the researchers write.
“It is important to note that this study is in no way recommending people should stop taking antipsychotics but simply that clinicians need to factor in a patient’s medication history while considering post-COVID aftereffects,” Dr. Freudenberg-Hua said.
“Given that the number of patients with dementia is projected to triple in the next 30 years, these findings have significant public health implications,” Dr. Sinvani added.
She noted that “care partners and health care professionals” should look for early signs of dementia, such as forgetfulness and depressive symptoms, in their patients.
“Future studies must continue to evaluate these associations, which are key for potential future interventions to prevent dementia,” Dr. Sinvani said.
The study was funded by the National Institutes of Health. Dr. Freudenberg-Hua co-owns stock and stock options from Regeneron Pharmaceuticals. Dr. Sinvani has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, new research suggests.
Results from a large study of more than 1,700 patients who had been hospitalized with COVID showed a greater than twofold increased risk for post-COVID dementia in those taking antipsychotics and mood stabilizers/anticonvulsants – medications often used to treat schizophrenia, psychosis, bipolar disorder, and seizures.
“We know that pre-existing psychiatric illness is associated with poor COVID-19 outcomes, but our study is the first to show an association with certain psychiatric medications and dementia,” co-investigator Liron Sinvani, MD, the Feinstein Institutes for Medical Research, Manhasset, New York, said in an interview.
“Our study highlights the potential interaction between baseline neuropsychiatric disease, psychotropic medications, COVID-19, and dementia,” Dr. Sinvani added.
The findings were published online March 18 in Frontiers in Medicine.
‘Striking’ dementia rate
Using electronic health records, the researchers evaluated pre-COVID psychotropic medication use and post-COVID dementia onset in 1,755 adults aged 65 and older. All were hospitalized with COVID-19 at Northwell Health between March 1 and April 20, 2020.
A “striking” 13% of the participants (n = 223) developed dementia within 1-year of follow-up, the investigators report.
Among the 438 patients (25%) exposed to at least one psychotropic medication before COVID-19, 105 (24%) developed dementia in the year following COVID versus 118 of 1,317 (9%) patients with no pre-COVID exposure to psychotropic medication (odds ratio, 3.2; 95% confidence interval, 2.37-4.32).
Both pre-COVID psychotropic medication use (OR, 2.7; 95% CI, 1.8-4.0, P < .001) and delirium (OR, 3.0; 95% CI, 1.9-4.6, P < .001) were significantly associated with post-COVID dementia at 1 year.
In a sensitivity analysis in the subset of 423 patients with at least one documented neurologic or psychiatric diagnosis at the time of COVID admission, and after adjusting for confounding factors, pre-COVID psychotropic medication use remained significantly linked to post-COVID dementia onset (OR, 3.09; 95% CI, 1.5-6.6, P = .002).
Drug classes most strongly associated with 1-year post-COVID dementia onset were antipsychotics (OR, 2.8, 95% CI, 1.7-4.4, P < .001) and mood stabilizers/anticonvulsants (OR, 2.4, 95% CI, 1.39-4.02, P = .001).
In a further exploratory analysis, the psychotropics valproic acid (multiple brands) and haloperidol (Haldol) had the largest association with post-COVID dementia.
Antidepressants as a class were not associated with post-COVID dementia, but the potential effects of two commonly prescribed antidepressants in older adults, mirtazapine (Remeron) and escitalopram (Lexapro), “warrant further investigation,” the researchers note.
Predictive risk marker?
“This research shows that psychotropic medications can be considered a predictive risk marker for post-COVID dementia. In patients taking psychotropic medications, COVID-19 could have accelerated progression of dementia after hospitalization,” lead author Yun Freudenberg-Hua, MD, the Feinstein Institutes, said in a news release.
It is unclear why psychotropic medications may raise the risk for dementia onset after COVID, the investigators note.
“It is intuitive that psychotropic medications indicate pre-existing neuropsychiatric conditions in which COVID-19 occurs. It is possible that psychotropic medications may potentiate the neurostructural changes that have been found in the brain of those who have recovered from COVID-19,” they write.
The sensitivity analysis in patients with documented neurologic and psychiatric diagnoses supports this interpretation.
COVID-19 may also accelerate the underlying brain disorders for which psychotropic medications were prescribed, leading to the greater incidence of post-COVID dementia, the researchers write.
“It is important to note that this study is in no way recommending people should stop taking antipsychotics but simply that clinicians need to factor in a patient’s medication history while considering post-COVID aftereffects,” Dr. Freudenberg-Hua said.
“Given that the number of patients with dementia is projected to triple in the next 30 years, these findings have significant public health implications,” Dr. Sinvani added.
She noted that “care partners and health care professionals” should look for early signs of dementia, such as forgetfulness and depressive symptoms, in their patients.
“Future studies must continue to evaluate these associations, which are key for potential future interventions to prevent dementia,” Dr. Sinvani said.
The study was funded by the National Institutes of Health. Dr. Freudenberg-Hua co-owns stock and stock options from Regeneron Pharmaceuticals. Dr. Sinvani has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM FRONTIERS IN MEDICINE
Children and COVID: The long goodbye continues
COVID-19 continues to be a diminishing issue for U.S. children, as the number of new cases declined for the ninth consecutive week, based on data from the American Academy of Pediatrics and the Children’s Hospital Association.
the AAP and CHA said in their weekly COVID report. The most recently infected children brought the total number of COVID-19 cases to just over 12.8 million since the pandemic began.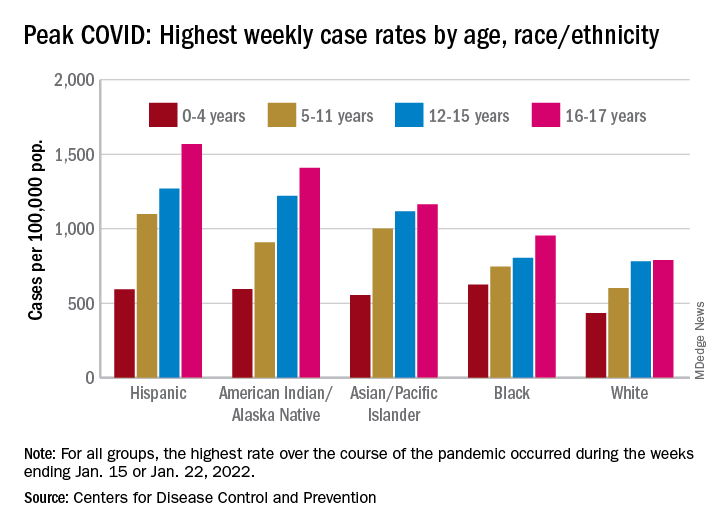
Other measures of COVID occurrence in children, such as hospital admissions and emergency department visits, also followed recent downward trends, although the sizes of the declines are beginning to decrease. Admissions dropped by 13.3% during the week ending March 26, but that followed declines of 25%, 20%, 26.5% and 24.4% for the 4 previous weeks, data from the Centers for Disease Control and Prevention show.
The slowdown in ED visits started a couple of weeks earlier, but the decline is still ongoing. As of March 25, ED visits with a confirmed COVID diagnosis represented just 0.4% of all visits for children aged 0-11 years, down from 1.1% on Feb. 25 and a peak of 14.3% on Jan. 15. For children aged 12-15, the latest figure is just 0.2%, compared with 0.5% on Feb. 25 and a peak of 14.3% on Jan. 9, the CDC reported on its COVID Data Tracker.
Although he was speaking of the nation as a whole and not specifically of children, Anthony Fauci, MD, the director of the National Institute of Allergy and Infectious Diseases, recently told the Washington Post that, “unless something changes dramatically,” another major surge isn’t on the horizon.
That sentiment, however, was not entirely shared by Moderna’s chief medical officer, Paul Burton, MD, PhD. In an interview with WebMD, he said that another COVID wave is inevitable and that it’s too soon to dismantle the vaccine infrastructure: “We’ve come so far. We’ve put so much into this to now take our foot off the gas. I think it would be a mistake for public health worldwide.”
Disparities during the Omicron surge
As the country puts Omicron in its rear view mirror, a quick look back at the CDC data shows some differences in how children were affected. At the surge’s peak in early to mid-January, Hispanic children were the most likely to get COVID-19, with incidence highest in the older groups. (See graph.)
At their peak week of Jan. 2-8, Hispanic children aged 16-17 years had a COVID rate of 1,568 cases per 100,000 population, versus 790 per 100,000 for White children, whose peak occurred a week later, from Jan. 9 to 15. Hispanic children aged 5-11 (1,098 per 100,000) and 12-15 (1,269 per 100,000) also had the highest recorded rates of the largest racial/ethnic groups, while Black children had the highest one-week rate, 625 per 100,000, among the 0- to 4-year-olds, according to the CDC.
COVID-19 continues to be a diminishing issue for U.S. children, as the number of new cases declined for the ninth consecutive week, based on data from the American Academy of Pediatrics and the Children’s Hospital Association.
the AAP and CHA said in their weekly COVID report. The most recently infected children brought the total number of COVID-19 cases to just over 12.8 million since the pandemic began.
Other measures of COVID occurrence in children, such as hospital admissions and emergency department visits, also followed recent downward trends, although the sizes of the declines are beginning to decrease. Admissions dropped by 13.3% during the week ending March 26, but that followed declines of 25%, 20%, 26.5% and 24.4% for the 4 previous weeks, data from the Centers for Disease Control and Prevention show.
The slowdown in ED visits started a couple of weeks earlier, but the decline is still ongoing. As of March 25, ED visits with a confirmed COVID diagnosis represented just 0.4% of all visits for children aged 0-11 years, down from 1.1% on Feb. 25 and a peak of 14.3% on Jan. 15. For children aged 12-15, the latest figure is just 0.2%, compared with 0.5% on Feb. 25 and a peak of 14.3% on Jan. 9, the CDC reported on its COVID Data Tracker.
Although he was speaking of the nation as a whole and not specifically of children, Anthony Fauci, MD, the director of the National Institute of Allergy and Infectious Diseases, recently told the Washington Post that, “unless something changes dramatically,” another major surge isn’t on the horizon.
That sentiment, however, was not entirely shared by Moderna’s chief medical officer, Paul Burton, MD, PhD. In an interview with WebMD, he said that another COVID wave is inevitable and that it’s too soon to dismantle the vaccine infrastructure: “We’ve come so far. We’ve put so much into this to now take our foot off the gas. I think it would be a mistake for public health worldwide.”
Disparities during the Omicron surge
As the country puts Omicron in its rear view mirror, a quick look back at the CDC data shows some differences in how children were affected. At the surge’s peak in early to mid-January, Hispanic children were the most likely to get COVID-19, with incidence highest in the older groups. (See graph.)
At their peak week of Jan. 2-8, Hispanic children aged 16-17 years had a COVID rate of 1,568 cases per 100,000 population, versus 790 per 100,000 for White children, whose peak occurred a week later, from Jan. 9 to 15. Hispanic children aged 5-11 (1,098 per 100,000) and 12-15 (1,269 per 100,000) also had the highest recorded rates of the largest racial/ethnic groups, while Black children had the highest one-week rate, 625 per 100,000, among the 0- to 4-year-olds, according to the CDC.
COVID-19 continues to be a diminishing issue for U.S. children, as the number of new cases declined for the ninth consecutive week, based on data from the American Academy of Pediatrics and the Children’s Hospital Association.
the AAP and CHA said in their weekly COVID report. The most recently infected children brought the total number of COVID-19 cases to just over 12.8 million since the pandemic began.
Other measures of COVID occurrence in children, such as hospital admissions and emergency department visits, also followed recent downward trends, although the sizes of the declines are beginning to decrease. Admissions dropped by 13.3% during the week ending March 26, but that followed declines of 25%, 20%, 26.5% and 24.4% for the 4 previous weeks, data from the Centers for Disease Control and Prevention show.
The slowdown in ED visits started a couple of weeks earlier, but the decline is still ongoing. As of March 25, ED visits with a confirmed COVID diagnosis represented just 0.4% of all visits for children aged 0-11 years, down from 1.1% on Feb. 25 and a peak of 14.3% on Jan. 15. For children aged 12-15, the latest figure is just 0.2%, compared with 0.5% on Feb. 25 and a peak of 14.3% on Jan. 9, the CDC reported on its COVID Data Tracker.
Although he was speaking of the nation as a whole and not specifically of children, Anthony Fauci, MD, the director of the National Institute of Allergy and Infectious Diseases, recently told the Washington Post that, “unless something changes dramatically,” another major surge isn’t on the horizon.
That sentiment, however, was not entirely shared by Moderna’s chief medical officer, Paul Burton, MD, PhD. In an interview with WebMD, he said that another COVID wave is inevitable and that it’s too soon to dismantle the vaccine infrastructure: “We’ve come so far. We’ve put so much into this to now take our foot off the gas. I think it would be a mistake for public health worldwide.”
Disparities during the Omicron surge
As the country puts Omicron in its rear view mirror, a quick look back at the CDC data shows some differences in how children were affected. At the surge’s peak in early to mid-January, Hispanic children were the most likely to get COVID-19, with incidence highest in the older groups. (See graph.)
At their peak week of Jan. 2-8, Hispanic children aged 16-17 years had a COVID rate of 1,568 cases per 100,000 population, versus 790 per 100,000 for White children, whose peak occurred a week later, from Jan. 9 to 15. Hispanic children aged 5-11 (1,098 per 100,000) and 12-15 (1,269 per 100,000) also had the highest recorded rates of the largest racial/ethnic groups, while Black children had the highest one-week rate, 625 per 100,000, among the 0- to 4-year-olds, according to the CDC.
As FDA OKs another COVID booster, some experts question need
, even though many top infectious disease experts questioned the need before the agency’s decision.
The FDA granted emergency use authorization for both Pfizer and Moderna to offer the second booster – and fourth shot overall – for adults over 50 as well as those over 18 with compromised immune systems.
The Centers for Control and Prevention must still sign off before those doses start reaching American arms. That approval could come at any time.
“The general consensus, certainly the CDC’s consensus, is that the current vaccines are still really quite effective against Omicron and this new BA.2 variant in keeping people out of the hospital, and preventing the development of severe disease,” William Schaffner, MD, an infectious disease specialist at Vanderbilt University in Nashville said prior to the FDA’s announcement March 29.
Of the 217.4 million Americans who are “fully vaccinated,” i.e., received two doses of either Pfizer or Moderna’s vaccines or one dose of the Johnson & Johnson vaccine, only 45% have also received a booster shot, according to the CDC.
“Given that, there’s no need at the moment for the general population to get a fourth inoculation,” Dr. Schaffner says. “Our current focus ought to be on making sure that as many people as possible get that [first] booster who are eligible.”
Monica Gandhi, MD, an infectious disease specialist at the University of California, San Francisco, agreed that another booster for everyone was unnecessary. The only people who would need a fourth shot (or third, if they had the Johnson & Johnson vaccine initially) are those over age 65 or 70 years, Dr. Gandhi says.
“Older people need those antibodies up high because they’re more susceptible to severe breakthroughs,” she said, also before the latest development.
To boost or not to boost
Daniel Kuritzkes, MD, chief of infectious diseases at Brigham & Women’s Hospital in Boston, said the timing of a booster and who should be eligible depends on what the nation is trying to achieve with its vaccination strategy.
“Is the goal to prevent any symptomatic infection with COVID-19, is the goal to prevent the spread of COVID-19, or is the goal to prevent severe disease that requires hospitalization?” asked Dr. Kuritzkes.
The current vaccine — with a booster — has prevented severe disease, he said.
An Israeli study showed, for instance, that a third Pfizer dose was 93% effective against hospitalization, 92% effective against severe illness, and 81% effective against death.
A just-published study in the New England Journal of Medicine found that a booster of the Pfizer vaccine was 95% effective against COVID-19 infection and that it did not raise any new safety issues.
A small Israeli study, also published in NEJM, of a fourth Pfizer dose given to health care workers found that it prevented symptomatic infection and illness, but that it was much less effective than previous doses — maybe 65% effective against symptomatic illness, the authors write.
Giving Americans another booster now — which has been shown to lose some effectiveness after about 4 months — means it might not offer protection this fall and winter, when there could be a seasonal surge of the virus, Dr. Kuritzkes says.
And, even if people receive boosters every few months, they are still likely to get a mild respiratory virus infection, he said.
“I’m pretty convinced that we cannot boost ourselves out of this pandemic,” said Dr. Kuritzkes. “We need to first of all ensure there’s global immunization so that all the people who have not been vaccinated at all get vaccinated. That’s far more important than boosting people a fourth time.”
Booster confusion
The April 6 FDA meeting of the agency’s Vaccines and Related Biological Products Advisory Committee comes as the two major COVID vaccine makers — Pfizer and Moderna — have applied for emergency use authorization for an additional booster.
Pfizer had asked for authorization for a fourth shot in patients over age 65 years, while Moderna wanted a booster to be available to all Americans over 18. The FDA instead granted authorization to both companies for those over 50 and anyone 18 or older who is immunocompromised.
What this means for the committee’s April 6 meeting is not clear. The original agenda says the committee will consider the evidence on safety and effectiveness of the additional vaccine doses and discuss how to set up a process — similar to that used for the influenza vaccine — to be able to determine the makeup of COVID vaccines as new variants emerge. That could lay the groundwork for an annual COVID shot, if needed.
The FDA advisers will not make recommendations nor vote on whether — and which — Americans should get a COVID booster. That is the job of the CDC’s Advisory Committee on Immunization Practices (ACIP).
The last time a booster was considered, CDC Director Rochelle Walensky, MD, overrode the committee and recommended that all Americans — not just older individuals — get an additional COVID shot, which became the first booster.
That past action worries Dr. Gandhi, who calls it confusing, and says it may have contributed to the fact that less than half of Americans have since chosen to get a booster.
Dr. Schaffner says he expects the FDA to authorize emergency use for fourth doses of the Pfizer and Moderna vaccines, but he doesn’t think the CDC committee will recommend routine use. As was seen before, however, the CDC director does not have to follow the committee’s advice.
The members of ACIP “might be more conservative or narrower in scope in terms of recommending who needs to be boosted and when boosting is appropriate,” Dr. Kuritzkes says.
Dr. Gandhi says she’s concerned the FDA’s deliberations could be swayed by Moderna and Pfizer’s influence and that “pharmaceutical companies are going to have more of a say than they should in the scientific process.”
There are similar worries for Dr. Schaffner. He says he’s “a bit grumpy” that the vaccine makers have been using press releases to argue for boosters.
“Press releases are no way to make vaccine recommendations,” Dr. Schaffner said, adding that he “would advise [vaccine makers] to sit down and be quiet and let the FDA and CDC advisory committee do their thing.”
Moderna Chief Medical Officer Paul Burton, MD, however, told WebMD last week that the signs point to why a fourth shot may be needed.
“We see waning of effectiveness, antibody levels come down, and certainly effectiveness against Omicron comes down in 3 to 6 months,” Burton said. “The natural history, from what we’re seeing around the world, is that BA.2 is definitely here, it’s highly transmissible, and I think we are going to get an additional wave of BA.2 here in the United States.”
Another wave is coming, he said, and “I think there will be waning of effectiveness. We need to be prepared for that, so that’s why we need the fourth dose.”
Supply issues?
Meanwhile, the United Kingdom has begun offering boosters to anyone over 75, and Sweden’s health authority has recommended a fourth shot to people over age 80.
That puts pressure on the United States — at least on its politicians and policymakers — to, in a sense, keep up, said the infectious disease specialists.
Indeed, the White House has been keeping fourth shots in the news, warning that it is running out of money to ensure that all Americans would have access to one, if recommended.
On March 23, outgoing White House COVID-19 Response Coordinator Jeff Zients said the federal government had enough vaccine for the immunocompromised to get a fourth dose “and, if authorized in the coming weeks, enough supply for fourth doses for our most vulnerable, including seniors.”
But he warned that without congressional approval of a COVID-19 funding package, “We can’t procure the necessary vaccine supply to support fourth shots for all Americans.”
Mr. Zients also noted that other countries, including Japan, Vietnam, and the Philippines had already secured future booster doses and added, “We should be securing additional supply right now.”
Dr. Schaffner says that while it would be nice to “have a booster on the shelf,” the United States needs to put more effort into creating a globally-coordinated process for ensuring that vaccines match circulating strains and that they are manufactured on a timely basis.
He says he and others “have been reminding the public that the COVID pandemic may indeed be diminishing and moving into the endemic, but that doesn’t mean COVID is over or finished or disappeared.”
Dr. Schaffner says that it may be that “perhaps we’d need a periodic reminder to our immune system to remain protected. In other words, we might have to get boosted perhaps annually like we do with influenza.”
A version of this article first appeared on WebMD.com.
, even though many top infectious disease experts questioned the need before the agency’s decision.
The FDA granted emergency use authorization for both Pfizer and Moderna to offer the second booster – and fourth shot overall – for adults over 50 as well as those over 18 with compromised immune systems.
The Centers for Control and Prevention must still sign off before those doses start reaching American arms. That approval could come at any time.
“The general consensus, certainly the CDC’s consensus, is that the current vaccines are still really quite effective against Omicron and this new BA.2 variant in keeping people out of the hospital, and preventing the development of severe disease,” William Schaffner, MD, an infectious disease specialist at Vanderbilt University in Nashville said prior to the FDA’s announcement March 29.
Of the 217.4 million Americans who are “fully vaccinated,” i.e., received two doses of either Pfizer or Moderna’s vaccines or one dose of the Johnson & Johnson vaccine, only 45% have also received a booster shot, according to the CDC.
“Given that, there’s no need at the moment for the general population to get a fourth inoculation,” Dr. Schaffner says. “Our current focus ought to be on making sure that as many people as possible get that [first] booster who are eligible.”
Monica Gandhi, MD, an infectious disease specialist at the University of California, San Francisco, agreed that another booster for everyone was unnecessary. The only people who would need a fourth shot (or third, if they had the Johnson & Johnson vaccine initially) are those over age 65 or 70 years, Dr. Gandhi says.
“Older people need those antibodies up high because they’re more susceptible to severe breakthroughs,” she said, also before the latest development.
To boost or not to boost
Daniel Kuritzkes, MD, chief of infectious diseases at Brigham & Women’s Hospital in Boston, said the timing of a booster and who should be eligible depends on what the nation is trying to achieve with its vaccination strategy.
“Is the goal to prevent any symptomatic infection with COVID-19, is the goal to prevent the spread of COVID-19, or is the goal to prevent severe disease that requires hospitalization?” asked Dr. Kuritzkes.
The current vaccine — with a booster — has prevented severe disease, he said.
An Israeli study showed, for instance, that a third Pfizer dose was 93% effective against hospitalization, 92% effective against severe illness, and 81% effective against death.
A just-published study in the New England Journal of Medicine found that a booster of the Pfizer vaccine was 95% effective against COVID-19 infection and that it did not raise any new safety issues.
A small Israeli study, also published in NEJM, of a fourth Pfizer dose given to health care workers found that it prevented symptomatic infection and illness, but that it was much less effective than previous doses — maybe 65% effective against symptomatic illness, the authors write.
Giving Americans another booster now — which has been shown to lose some effectiveness after about 4 months — means it might not offer protection this fall and winter, when there could be a seasonal surge of the virus, Dr. Kuritzkes says.
And, even if people receive boosters every few months, they are still likely to get a mild respiratory virus infection, he said.
“I’m pretty convinced that we cannot boost ourselves out of this pandemic,” said Dr. Kuritzkes. “We need to first of all ensure there’s global immunization so that all the people who have not been vaccinated at all get vaccinated. That’s far more important than boosting people a fourth time.”
Booster confusion
The April 6 FDA meeting of the agency’s Vaccines and Related Biological Products Advisory Committee comes as the two major COVID vaccine makers — Pfizer and Moderna — have applied for emergency use authorization for an additional booster.
Pfizer had asked for authorization for a fourth shot in patients over age 65 years, while Moderna wanted a booster to be available to all Americans over 18. The FDA instead granted authorization to both companies for those over 50 and anyone 18 or older who is immunocompromised.
What this means for the committee’s April 6 meeting is not clear. The original agenda says the committee will consider the evidence on safety and effectiveness of the additional vaccine doses and discuss how to set up a process — similar to that used for the influenza vaccine — to be able to determine the makeup of COVID vaccines as new variants emerge. That could lay the groundwork for an annual COVID shot, if needed.
The FDA advisers will not make recommendations nor vote on whether — and which — Americans should get a COVID booster. That is the job of the CDC’s Advisory Committee on Immunization Practices (ACIP).
The last time a booster was considered, CDC Director Rochelle Walensky, MD, overrode the committee and recommended that all Americans — not just older individuals — get an additional COVID shot, which became the first booster.
That past action worries Dr. Gandhi, who calls it confusing, and says it may have contributed to the fact that less than half of Americans have since chosen to get a booster.
Dr. Schaffner says he expects the FDA to authorize emergency use for fourth doses of the Pfizer and Moderna vaccines, but he doesn’t think the CDC committee will recommend routine use. As was seen before, however, the CDC director does not have to follow the committee’s advice.
The members of ACIP “might be more conservative or narrower in scope in terms of recommending who needs to be boosted and when boosting is appropriate,” Dr. Kuritzkes says.
Dr. Gandhi says she’s concerned the FDA’s deliberations could be swayed by Moderna and Pfizer’s influence and that “pharmaceutical companies are going to have more of a say than they should in the scientific process.”
There are similar worries for Dr. Schaffner. He says he’s “a bit grumpy” that the vaccine makers have been using press releases to argue for boosters.
“Press releases are no way to make vaccine recommendations,” Dr. Schaffner said, adding that he “would advise [vaccine makers] to sit down and be quiet and let the FDA and CDC advisory committee do their thing.”
Moderna Chief Medical Officer Paul Burton, MD, however, told WebMD last week that the signs point to why a fourth shot may be needed.
“We see waning of effectiveness, antibody levels come down, and certainly effectiveness against Omicron comes down in 3 to 6 months,” Burton said. “The natural history, from what we’re seeing around the world, is that BA.2 is definitely here, it’s highly transmissible, and I think we are going to get an additional wave of BA.2 here in the United States.”
Another wave is coming, he said, and “I think there will be waning of effectiveness. We need to be prepared for that, so that’s why we need the fourth dose.”
Supply issues?
Meanwhile, the United Kingdom has begun offering boosters to anyone over 75, and Sweden’s health authority has recommended a fourth shot to people over age 80.
That puts pressure on the United States — at least on its politicians and policymakers — to, in a sense, keep up, said the infectious disease specialists.
Indeed, the White House has been keeping fourth shots in the news, warning that it is running out of money to ensure that all Americans would have access to one, if recommended.
On March 23, outgoing White House COVID-19 Response Coordinator Jeff Zients said the federal government had enough vaccine for the immunocompromised to get a fourth dose “and, if authorized in the coming weeks, enough supply for fourth doses for our most vulnerable, including seniors.”
But he warned that without congressional approval of a COVID-19 funding package, “We can’t procure the necessary vaccine supply to support fourth shots for all Americans.”
Mr. Zients also noted that other countries, including Japan, Vietnam, and the Philippines had already secured future booster doses and added, “We should be securing additional supply right now.”
Dr. Schaffner says that while it would be nice to “have a booster on the shelf,” the United States needs to put more effort into creating a globally-coordinated process for ensuring that vaccines match circulating strains and that they are manufactured on a timely basis.
He says he and others “have been reminding the public that the COVID pandemic may indeed be diminishing and moving into the endemic, but that doesn’t mean COVID is over or finished or disappeared.”
Dr. Schaffner says that it may be that “perhaps we’d need a periodic reminder to our immune system to remain protected. In other words, we might have to get boosted perhaps annually like we do with influenza.”
A version of this article first appeared on WebMD.com.
, even though many top infectious disease experts questioned the need before the agency’s decision.
The FDA granted emergency use authorization for both Pfizer and Moderna to offer the second booster – and fourth shot overall – for adults over 50 as well as those over 18 with compromised immune systems.
The Centers for Control and Prevention must still sign off before those doses start reaching American arms. That approval could come at any time.
“The general consensus, certainly the CDC’s consensus, is that the current vaccines are still really quite effective against Omicron and this new BA.2 variant in keeping people out of the hospital, and preventing the development of severe disease,” William Schaffner, MD, an infectious disease specialist at Vanderbilt University in Nashville said prior to the FDA’s announcement March 29.
Of the 217.4 million Americans who are “fully vaccinated,” i.e., received two doses of either Pfizer or Moderna’s vaccines or one dose of the Johnson & Johnson vaccine, only 45% have also received a booster shot, according to the CDC.
“Given that, there’s no need at the moment for the general population to get a fourth inoculation,” Dr. Schaffner says. “Our current focus ought to be on making sure that as many people as possible get that [first] booster who are eligible.”
Monica Gandhi, MD, an infectious disease specialist at the University of California, San Francisco, agreed that another booster for everyone was unnecessary. The only people who would need a fourth shot (or third, if they had the Johnson & Johnson vaccine initially) are those over age 65 or 70 years, Dr. Gandhi says.
“Older people need those antibodies up high because they’re more susceptible to severe breakthroughs,” she said, also before the latest development.
To boost or not to boost
Daniel Kuritzkes, MD, chief of infectious diseases at Brigham & Women’s Hospital in Boston, said the timing of a booster and who should be eligible depends on what the nation is trying to achieve with its vaccination strategy.
“Is the goal to prevent any symptomatic infection with COVID-19, is the goal to prevent the spread of COVID-19, or is the goal to prevent severe disease that requires hospitalization?” asked Dr. Kuritzkes.
The current vaccine — with a booster — has prevented severe disease, he said.
An Israeli study showed, for instance, that a third Pfizer dose was 93% effective against hospitalization, 92% effective against severe illness, and 81% effective against death.
A just-published study in the New England Journal of Medicine found that a booster of the Pfizer vaccine was 95% effective against COVID-19 infection and that it did not raise any new safety issues.
A small Israeli study, also published in NEJM, of a fourth Pfizer dose given to health care workers found that it prevented symptomatic infection and illness, but that it was much less effective than previous doses — maybe 65% effective against symptomatic illness, the authors write.
Giving Americans another booster now — which has been shown to lose some effectiveness after about 4 months — means it might not offer protection this fall and winter, when there could be a seasonal surge of the virus, Dr. Kuritzkes says.
And, even if people receive boosters every few months, they are still likely to get a mild respiratory virus infection, he said.
“I’m pretty convinced that we cannot boost ourselves out of this pandemic,” said Dr. Kuritzkes. “We need to first of all ensure there’s global immunization so that all the people who have not been vaccinated at all get vaccinated. That’s far more important than boosting people a fourth time.”
Booster confusion
The April 6 FDA meeting of the agency’s Vaccines and Related Biological Products Advisory Committee comes as the two major COVID vaccine makers — Pfizer and Moderna — have applied for emergency use authorization for an additional booster.
Pfizer had asked for authorization for a fourth shot in patients over age 65 years, while Moderna wanted a booster to be available to all Americans over 18. The FDA instead granted authorization to both companies for those over 50 and anyone 18 or older who is immunocompromised.
What this means for the committee’s April 6 meeting is not clear. The original agenda says the committee will consider the evidence on safety and effectiveness of the additional vaccine doses and discuss how to set up a process — similar to that used for the influenza vaccine — to be able to determine the makeup of COVID vaccines as new variants emerge. That could lay the groundwork for an annual COVID shot, if needed.
The FDA advisers will not make recommendations nor vote on whether — and which — Americans should get a COVID booster. That is the job of the CDC’s Advisory Committee on Immunization Practices (ACIP).
The last time a booster was considered, CDC Director Rochelle Walensky, MD, overrode the committee and recommended that all Americans — not just older individuals — get an additional COVID shot, which became the first booster.
That past action worries Dr. Gandhi, who calls it confusing, and says it may have contributed to the fact that less than half of Americans have since chosen to get a booster.
Dr. Schaffner says he expects the FDA to authorize emergency use for fourth doses of the Pfizer and Moderna vaccines, but he doesn’t think the CDC committee will recommend routine use. As was seen before, however, the CDC director does not have to follow the committee’s advice.
The members of ACIP “might be more conservative or narrower in scope in terms of recommending who needs to be boosted and when boosting is appropriate,” Dr. Kuritzkes says.
Dr. Gandhi says she’s concerned the FDA’s deliberations could be swayed by Moderna and Pfizer’s influence and that “pharmaceutical companies are going to have more of a say than they should in the scientific process.”
There are similar worries for Dr. Schaffner. He says he’s “a bit grumpy” that the vaccine makers have been using press releases to argue for boosters.
“Press releases are no way to make vaccine recommendations,” Dr. Schaffner said, adding that he “would advise [vaccine makers] to sit down and be quiet and let the FDA and CDC advisory committee do their thing.”
Moderna Chief Medical Officer Paul Burton, MD, however, told WebMD last week that the signs point to why a fourth shot may be needed.
“We see waning of effectiveness, antibody levels come down, and certainly effectiveness against Omicron comes down in 3 to 6 months,” Burton said. “The natural history, from what we’re seeing around the world, is that BA.2 is definitely here, it’s highly transmissible, and I think we are going to get an additional wave of BA.2 here in the United States.”
Another wave is coming, he said, and “I think there will be waning of effectiveness. We need to be prepared for that, so that’s why we need the fourth dose.”
Supply issues?
Meanwhile, the United Kingdom has begun offering boosters to anyone over 75, and Sweden’s health authority has recommended a fourth shot to people over age 80.
That puts pressure on the United States — at least on its politicians and policymakers — to, in a sense, keep up, said the infectious disease specialists.
Indeed, the White House has been keeping fourth shots in the news, warning that it is running out of money to ensure that all Americans would have access to one, if recommended.
On March 23, outgoing White House COVID-19 Response Coordinator Jeff Zients said the federal government had enough vaccine for the immunocompromised to get a fourth dose “and, if authorized in the coming weeks, enough supply for fourth doses for our most vulnerable, including seniors.”
But he warned that without congressional approval of a COVID-19 funding package, “We can’t procure the necessary vaccine supply to support fourth shots for all Americans.”
Mr. Zients also noted that other countries, including Japan, Vietnam, and the Philippines had already secured future booster doses and added, “We should be securing additional supply right now.”
Dr. Schaffner says that while it would be nice to “have a booster on the shelf,” the United States needs to put more effort into creating a globally-coordinated process for ensuring that vaccines match circulating strains and that they are manufactured on a timely basis.
He says he and others “have been reminding the public that the COVID pandemic may indeed be diminishing and moving into the endemic, but that doesn’t mean COVID is over or finished or disappeared.”
Dr. Schaffner says that it may be that “perhaps we’d need a periodic reminder to our immune system to remain protected. In other words, we might have to get boosted perhaps annually like we do with influenza.”
A version of this article first appeared on WebMD.com.
‘Staggeringly high’ rates of psychiatric symptoms after COVID-19
DENVER – Neurocognitive and psychiatric symptoms of mental illness, including posttraumatic stress disorder, are alarmingly high among patients who have previously had COVID-19 – even among those who were not hospitalized with the virus, new research shows.
The findings are from an online survey of more than 800 respondents.
“Regardless of how long ago they had been infected with COVID-19, all respondents had persistent symptoms,” co-investigator Beth Patterson, MSc, adjunct clinical professor at McMaster University, MacAnxiety Research Centre, Hamilton, Ont., told this news organization.
“The take-home message for clinicians is to be aware that if you have patients who had COVID-19, it’s quite likely that they may also experience a psychiatric issue and that they may have reduced resilience and lower quality-of-life [issues],” Ms. Patterson said.
The survey results were presented here at the Anxiety and Depression Association of America (ADAA) Conference 2022.
100% report symptoms
The study included 827 respondents (81% women) to an online survey who had contracted COVID.
Using validated symptom severity scores, respondents were assessed for mental health and neurocognitive issues, as well as some physical and quality-of-life factors.
Remarkably, all participants (100%) reported having current, persistent symptoms of COVID. In addition, 88% (n = 729) reported persistent neurocognitive symptoms, even though only 15.5% reported they had been hospitalized for COVID.
Of those hospitalized, 28.9% were treated in the intensive care unit; 42.2% stayed in hospital for less than 1 week; and 13.3% remained hospitalized for at least 3 weeks.
Data were not available on how long it had been since the patients were diagnosed or hospitalized, but most participants (68%) said they had not returned to normal functioning since contracting COVID.
The most common persistent symptoms were fatigue (75.9%), brain fog (67.9%), difficulty concentrating (61%), and weakness (51.2%).
More than half of respondents reported neurocognitive symptoms, including poor memory (57.4%) and word-finding problems in processing information (46.9%). Only 11% reported no persistent neurocognitive symptoms.
A total of 41.7% of respondents reported anxiety using the Generalized Anxiety Disorder-7 (GAD-7) scale, and rates of depression were 61.4% as assessed with the Patient Health Questionnaire (PHQ-9).
Rates of probable posttraumatic stress disorder were 40.5% as assessed via the PTSD checklist (PCL-5).
Although it wasn’t possible to use diagnostic screens, the assessment scores suggest strikingly high rates of mental health disorders among the respondents, Ms. Patterson said.
“When we look at the mean scores on the validated scales, we see percentages of probable diagnoses that are staggeringly higher than you would find in the population,” she added.
Of note, about 44% of respondents reported having had mental health treatment in the past, and 33.7% were receiving current mental health treatment.
Although the study had no control group, the findings are consistent with larger studies that have had comparator groups, including research recently published in the BMJ.
Poor understanding of COVID’s fallout
In an editorial accompanying the BMJ study, Scott Weich, MD, Mental Health Research Unit, School of Health and Related Research, University of Sheffield, United Kingdom, emphasized the need to better understand the lingering mental health aspects of COVID-19 infection.
“Our attachment to syndromal phenotypes means that we have learned remarkably little about the causes of mental ill health – in this case psychopathology associated with a viral pandemic,” Dr. Weich writes.
Dr. Weich called for improved efforts to understanding long COVID, as well as the establishment of more effective responses to the mental health fallout from the pandemic.
Commenting on the current study, Dr. Weich elaborated on the challenges in disentangling the causes of mental health effects in illness.
“In terms of other viruses, etc., there is a long history of debate and pitched battles between those that attribute mental health effects to predominantly biological processes, [involving] immunological and other responses, and those who understand these responses are mediated by psychological and social processes,” he noted.
“The story of myalgic encephalomyelitis/chronic fatigue syndrome speaks volumes about these different positions, and how difficult it can be to find a middle ground,” he said.
“This has been going on for centuries and may never be fully resolved, at least until we have clearer and more definitive evidence of pathophysiology, though this seems incredibly elusive,” Dr. Weich said.
The authors and Dr. Weich have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
DENVER – Neurocognitive and psychiatric symptoms of mental illness, including posttraumatic stress disorder, are alarmingly high among patients who have previously had COVID-19 – even among those who were not hospitalized with the virus, new research shows.
The findings are from an online survey of more than 800 respondents.
“Regardless of how long ago they had been infected with COVID-19, all respondents had persistent symptoms,” co-investigator Beth Patterson, MSc, adjunct clinical professor at McMaster University, MacAnxiety Research Centre, Hamilton, Ont., told this news organization.
“The take-home message for clinicians is to be aware that if you have patients who had COVID-19, it’s quite likely that they may also experience a psychiatric issue and that they may have reduced resilience and lower quality-of-life [issues],” Ms. Patterson said.
The survey results were presented here at the Anxiety and Depression Association of America (ADAA) Conference 2022.
100% report symptoms
The study included 827 respondents (81% women) to an online survey who had contracted COVID.
Using validated symptom severity scores, respondents were assessed for mental health and neurocognitive issues, as well as some physical and quality-of-life factors.
Remarkably, all participants (100%) reported having current, persistent symptoms of COVID. In addition, 88% (n = 729) reported persistent neurocognitive symptoms, even though only 15.5% reported they had been hospitalized for COVID.
Of those hospitalized, 28.9% were treated in the intensive care unit; 42.2% stayed in hospital for less than 1 week; and 13.3% remained hospitalized for at least 3 weeks.
Data were not available on how long it had been since the patients were diagnosed or hospitalized, but most participants (68%) said they had not returned to normal functioning since contracting COVID.
The most common persistent symptoms were fatigue (75.9%), brain fog (67.9%), difficulty concentrating (61%), and weakness (51.2%).
More than half of respondents reported neurocognitive symptoms, including poor memory (57.4%) and word-finding problems in processing information (46.9%). Only 11% reported no persistent neurocognitive symptoms.
A total of 41.7% of respondents reported anxiety using the Generalized Anxiety Disorder-7 (GAD-7) scale, and rates of depression were 61.4% as assessed with the Patient Health Questionnaire (PHQ-9).
Rates of probable posttraumatic stress disorder were 40.5% as assessed via the PTSD checklist (PCL-5).
Although it wasn’t possible to use diagnostic screens, the assessment scores suggest strikingly high rates of mental health disorders among the respondents, Ms. Patterson said.
“When we look at the mean scores on the validated scales, we see percentages of probable diagnoses that are staggeringly higher than you would find in the population,” she added.
Of note, about 44% of respondents reported having had mental health treatment in the past, and 33.7% were receiving current mental health treatment.
Although the study had no control group, the findings are consistent with larger studies that have had comparator groups, including research recently published in the BMJ.
Poor understanding of COVID’s fallout
In an editorial accompanying the BMJ study, Scott Weich, MD, Mental Health Research Unit, School of Health and Related Research, University of Sheffield, United Kingdom, emphasized the need to better understand the lingering mental health aspects of COVID-19 infection.
“Our attachment to syndromal phenotypes means that we have learned remarkably little about the causes of mental ill health – in this case psychopathology associated with a viral pandemic,” Dr. Weich writes.
Dr. Weich called for improved efforts to understanding long COVID, as well as the establishment of more effective responses to the mental health fallout from the pandemic.
Commenting on the current study, Dr. Weich elaborated on the challenges in disentangling the causes of mental health effects in illness.
“In terms of other viruses, etc., there is a long history of debate and pitched battles between those that attribute mental health effects to predominantly biological processes, [involving] immunological and other responses, and those who understand these responses are mediated by psychological and social processes,” he noted.
“The story of myalgic encephalomyelitis/chronic fatigue syndrome speaks volumes about these different positions, and how difficult it can be to find a middle ground,” he said.
“This has been going on for centuries and may never be fully resolved, at least until we have clearer and more definitive evidence of pathophysiology, though this seems incredibly elusive,” Dr. Weich said.
The authors and Dr. Weich have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
DENVER – Neurocognitive and psychiatric symptoms of mental illness, including posttraumatic stress disorder, are alarmingly high among patients who have previously had COVID-19 – even among those who were not hospitalized with the virus, new research shows.
The findings are from an online survey of more than 800 respondents.
“Regardless of how long ago they had been infected with COVID-19, all respondents had persistent symptoms,” co-investigator Beth Patterson, MSc, adjunct clinical professor at McMaster University, MacAnxiety Research Centre, Hamilton, Ont., told this news organization.
“The take-home message for clinicians is to be aware that if you have patients who had COVID-19, it’s quite likely that they may also experience a psychiatric issue and that they may have reduced resilience and lower quality-of-life [issues],” Ms. Patterson said.
The survey results were presented here at the Anxiety and Depression Association of America (ADAA) Conference 2022.
100% report symptoms
The study included 827 respondents (81% women) to an online survey who had contracted COVID.
Using validated symptom severity scores, respondents were assessed for mental health and neurocognitive issues, as well as some physical and quality-of-life factors.
Remarkably, all participants (100%) reported having current, persistent symptoms of COVID. In addition, 88% (n = 729) reported persistent neurocognitive symptoms, even though only 15.5% reported they had been hospitalized for COVID.
Of those hospitalized, 28.9% were treated in the intensive care unit; 42.2% stayed in hospital for less than 1 week; and 13.3% remained hospitalized for at least 3 weeks.
Data were not available on how long it had been since the patients were diagnosed or hospitalized, but most participants (68%) said they had not returned to normal functioning since contracting COVID.
The most common persistent symptoms were fatigue (75.9%), brain fog (67.9%), difficulty concentrating (61%), and weakness (51.2%).
More than half of respondents reported neurocognitive symptoms, including poor memory (57.4%) and word-finding problems in processing information (46.9%). Only 11% reported no persistent neurocognitive symptoms.
A total of 41.7% of respondents reported anxiety using the Generalized Anxiety Disorder-7 (GAD-7) scale, and rates of depression were 61.4% as assessed with the Patient Health Questionnaire (PHQ-9).
Rates of probable posttraumatic stress disorder were 40.5% as assessed via the PTSD checklist (PCL-5).
Although it wasn’t possible to use diagnostic screens, the assessment scores suggest strikingly high rates of mental health disorders among the respondents, Ms. Patterson said.
“When we look at the mean scores on the validated scales, we see percentages of probable diagnoses that are staggeringly higher than you would find in the population,” she added.
Of note, about 44% of respondents reported having had mental health treatment in the past, and 33.7% were receiving current mental health treatment.
Although the study had no control group, the findings are consistent with larger studies that have had comparator groups, including research recently published in the BMJ.
Poor understanding of COVID’s fallout
In an editorial accompanying the BMJ study, Scott Weich, MD, Mental Health Research Unit, School of Health and Related Research, University of Sheffield, United Kingdom, emphasized the need to better understand the lingering mental health aspects of COVID-19 infection.
“Our attachment to syndromal phenotypes means that we have learned remarkably little about the causes of mental ill health – in this case psychopathology associated with a viral pandemic,” Dr. Weich writes.
Dr. Weich called for improved efforts to understanding long COVID, as well as the establishment of more effective responses to the mental health fallout from the pandemic.
Commenting on the current study, Dr. Weich elaborated on the challenges in disentangling the causes of mental health effects in illness.
“In terms of other viruses, etc., there is a long history of debate and pitched battles between those that attribute mental health effects to predominantly biological processes, [involving] immunological and other responses, and those who understand these responses are mediated by psychological and social processes,” he noted.
“The story of myalgic encephalomyelitis/chronic fatigue syndrome speaks volumes about these different positions, and how difficult it can be to find a middle ground,” he said.
“This has been going on for centuries and may never be fully resolved, at least until we have clearer and more definitive evidence of pathophysiology, though this seems incredibly elusive,” Dr. Weich said.
The authors and Dr. Weich have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
AT ADAA 2022
Different variants may cause different long COVID symptoms: Study
Long COVID symptoms may differ depending on which SARS-CoV-2 variant is behind a person’s infection, a new study shows.
The data from Italy compared long COVID symptoms reported by patients infected with SARS-CoV-2 from March to December 2020 (when the original, or “Wuhan,” variant was dominant) with those reported by patients infected from January to April 2021 (B.1.1.7-, or Alpha variant-dominant). It showed a substantial change in the pattern of neurological and cognitive/emotional problems – the latter mostly seen with the Alpha variant.
Infectious disease specialist Michele Spinicci, MD, from the University of Florence and Careggi University Hospital, Italy, led the work. “Many of the symptoms reported in this study have been measured [before], but this is the first time they have been linked to different COVID-19 variants,” he told this news organization. “Findings in patients with long COVID were focused on neurological and psychological difficulties.”
However, he pointed out that much remains to be understood about long COVID in terms of symptoms, diagnosis, and treatment.
“Long COVID is a huge area that involves many different fields of medicine, so there is not one single piece of advice to give on management. There’s lots to consider when evaluating a long COVID patient,” he said.
Results showed that when the Alpha variant was the dominant variant, the prevalence of myalgia (10%), dyspnea (42%), brain fog/mental confusion (17%), and anxiety/depression (13%) significantly increased relative to the wild-type (original, Wuhan) variant, while anosmia (2%), dysgeusia (4%), and impaired hearing (1%) were less common.
When the wild-type (original, Wuhan) variant was dominant, fatigue (37%), insomnia (16%), dysgeusia (11%), and impaired hearing (5%) were all more common than with the Alpha variant. Dyspnea (33%), brain fog (10%), myalgia (4%), and anxiety/depression (6%) were less common.
Overall, 76% of the patients in the trial reported at least one persistent symptom, while the most common reported symptoms were dyspnea (37%) and chronic fatigue (36%), followed by insomnia (16%), visual disorders (13%), and brain fog (13%).
The findings come from an early-release abstract that will be presented at the European Congress of Clinical Microbiology & Infectious Diseases (ECCMID) 2022, in Lisbon, Portugal, in a few weeks’ time.
‘The take-home point’
Michael A. Horberg, MD, associate medical director, Kaiser Permanente – Mid-Atlantic Permanente Medical Group, Rockville, Maryland, has recently presented data on symptoms seen with long COVID in over 28,000 people, as reported by this news organization, at the Conference on Retroviruses and Opportunistic Infections 2022. These people were infected with the wild-type virus.
Commenting on the study by Dr. Spinicci, he said: “The issue is that as we go along the COVID lifespan from acute to long COVID, what prompts patients to seek medical attention may change. If symptoms are not severe or were not well publicized previously, patients may not see the need to seek care or evaluation. As such, it doesn’t surprise me to find these changes over time, independent of any potential biological activity of the virus or its consequences.”
Dr. Horberg noted that their own study results are consistent with those of Dr. Spinicci et al. from March to December 2020 (original, Wuhan variant). “To me, the take-home point is long COVID is real, and physicians need to be on the lookout for it. However, not all symptoms are due to long COVID, and we need to keep the time course of symptoms during evaluation of such patients.”
Also providing comment on the findings was Debby Bogaert, MD, chair of Pediatric Medicine, University of Edinburgh. Reflecting on whether the symptoms were due to long COVID or another underlying disease, she said: “The number of patients with ongoing symptoms is very high, therefore [it is] unlikely that all of this is re-emergence of underlying or previous health problems. The type of symptoms reported are also as reported by other cohorts, so not unexpected. And irrespective of the root cause, they require care.”
Dr. Bogaert also noted that the data reiterate that COVID-19 is a new disease, and that “new variants might show shifting clinical pictures, not only regarding severity and symptoms of acute disease, but possibly also regarding sequela,” and that this, “underlines the importance of ongoing surveillance of variants, and ongoing evaluation of the acute and long-term clinical picture accompanying these, to ensure we adapt our public health approaches, clinical treatment plans, and long-term follow-up when and where needed.”
Dr. Bogaert stressed that only by keeping track of the changes in symptoms both acute and long-term – by patients and doctors – would the best patient care be provided.
“Patients need to know so they can report these back to their doctors, and doctors need to know over time that the picture of sequela might shift, so sequela are recognized early, and these patients receive the appropriate follow-up treatment,” she said. These shifting patterns might also apply to community patients as well as those hospitalized with COVID-19.
Study details
The retrospective, observational study included 428 patients, 59% men, with a mean age of 64 years, who had been treated at the Careggi University Hospital’s post-COVID outpatient service between June 2020 and June 2021, when the original form of SARS-CoV-2, and later the Alpha variant, were circulating, with some overlap.
All patients had been hospitalized with COVID-19 and discharged 4-12 weeks prior to attending the outpatient post-COVID service. They were asked to complete a questionnaire on persistent symptoms at the median of 53 days after being discharged from the hospital. In addition, data on medical history, microbiological and clinical COVID-19 course, self-reported symptoms (at the point of the follow-up visit), and patient demographics were obtained from electronic medical records.
Newer variants being studied
Upon analysis of long COVID symptoms according to treatment given during the acute phase using multivariate analysis, increasing oxygen support (odds ratio, 1.4; 95% confidence interval, 1.1-1.8), use of immunosuppressant drugs (OR, 6.4; 95% CI, 1.5-28), and female sex (OR, 1.8; 95% CI, 1.1-2.9) were associated with a higher risk for long COVID symptoms, while patients with type 2 diabetes (OR, 0.4; 95% CI, 0.2-0.7) had a lower risk of developing long COVID symptoms.
When asked whether the increased anxiety and depression seen with the Alpha variant might be also linked to the fact that people are living through hard times, with lockdowns, economic difficulties, possible illness, and even fatalities among family and friends due to COVID, Dr. Spinicci pointed out that “it’s a preliminary study, and there are lots of factors that we didn’t explore. It’s difficult to arrive at definite conclusions about long COVID because so much remains unknown. There are lots of external and environmental factors in the general population that might contribute to these findings.”
Dr. Spinicci has continued to enroll patients from later periods of the pandemic, including patients who were infected with the Delta and Omicron variants of SARS-CoV-2.
“We’re interested in finding out if these other variants are also associated with different phenotypes of long COVID. This study is part of our follow-up program here in the hospital where lots of different specialties are following patients for 20 months,” he said.
Dr. Horberg noted that one criticism of this study is that it was unclear whether the researchers accounted for pre-existing conditions. “They note the co-morbidities in the table 1, but don’t say how they accounted for that in their analyses. We found a lot of what patients were calling ‘long COVID’ were exacerbations of co-morbidities but not a new condition.”
Dr. Spinicci and his coauthors acknowledged that the study was observational. And, as such, it does not prove cause and effect, and they could not confirm which variant of the virus caused the infection in different patients, which may limit the conclusions that can be drawn.
“Future research should focus on the potential impacts of variants of concern and vaccination status on ongoing symptoms,” Spinicci said.
Early release of an abstract will be presented at the European Congress of Clinical Microbiology & Infectious Diseases (ECCMID) 2022, in Lisbon, Portugal, April 23-26, 2022. Abstract 02768.
Dr. Spinicci and Dr. Horberg have disclosed no relevant financial relationships. Dr. Bogaert declared that she is on the program committee of ECCMID; she has been a member of SIGN/NICE COVID-19 rapid guideline: managing the long-term effects of COVID-19; and she is involved in multiple ongoing COVID-related studies, both acute and long-term sequela (funding MRC, CSO, ZonMw).
A version of this article first appeared on Medscape.com.
Long COVID symptoms may differ depending on which SARS-CoV-2 variant is behind a person’s infection, a new study shows.
The data from Italy compared long COVID symptoms reported by patients infected with SARS-CoV-2 from March to December 2020 (when the original, or “Wuhan,” variant was dominant) with those reported by patients infected from January to April 2021 (B.1.1.7-, or Alpha variant-dominant). It showed a substantial change in the pattern of neurological and cognitive/emotional problems – the latter mostly seen with the Alpha variant.
Infectious disease specialist Michele Spinicci, MD, from the University of Florence and Careggi University Hospital, Italy, led the work. “Many of the symptoms reported in this study have been measured [before], but this is the first time they have been linked to different COVID-19 variants,” he told this news organization. “Findings in patients with long COVID were focused on neurological and psychological difficulties.”
However, he pointed out that much remains to be understood about long COVID in terms of symptoms, diagnosis, and treatment.
“Long COVID is a huge area that involves many different fields of medicine, so there is not one single piece of advice to give on management. There’s lots to consider when evaluating a long COVID patient,” he said.
Results showed that when the Alpha variant was the dominant variant, the prevalence of myalgia (10%), dyspnea (42%), brain fog/mental confusion (17%), and anxiety/depression (13%) significantly increased relative to the wild-type (original, Wuhan) variant, while anosmia (2%), dysgeusia (4%), and impaired hearing (1%) were less common.
When the wild-type (original, Wuhan) variant was dominant, fatigue (37%), insomnia (16%), dysgeusia (11%), and impaired hearing (5%) were all more common than with the Alpha variant. Dyspnea (33%), brain fog (10%), myalgia (4%), and anxiety/depression (6%) were less common.
Overall, 76% of the patients in the trial reported at least one persistent symptom, while the most common reported symptoms were dyspnea (37%) and chronic fatigue (36%), followed by insomnia (16%), visual disorders (13%), and brain fog (13%).
The findings come from an early-release abstract that will be presented at the European Congress of Clinical Microbiology & Infectious Diseases (ECCMID) 2022, in Lisbon, Portugal, in a few weeks’ time.
‘The take-home point’
Michael A. Horberg, MD, associate medical director, Kaiser Permanente – Mid-Atlantic Permanente Medical Group, Rockville, Maryland, has recently presented data on symptoms seen with long COVID in over 28,000 people, as reported by this news organization, at the Conference on Retroviruses and Opportunistic Infections 2022. These people were infected with the wild-type virus.
Commenting on the study by Dr. Spinicci, he said: “The issue is that as we go along the COVID lifespan from acute to long COVID, what prompts patients to seek medical attention may change. If symptoms are not severe or were not well publicized previously, patients may not see the need to seek care or evaluation. As such, it doesn’t surprise me to find these changes over time, independent of any potential biological activity of the virus or its consequences.”
Dr. Horberg noted that their own study results are consistent with those of Dr. Spinicci et al. from March to December 2020 (original, Wuhan variant). “To me, the take-home point is long COVID is real, and physicians need to be on the lookout for it. However, not all symptoms are due to long COVID, and we need to keep the time course of symptoms during evaluation of such patients.”
Also providing comment on the findings was Debby Bogaert, MD, chair of Pediatric Medicine, University of Edinburgh. Reflecting on whether the symptoms were due to long COVID or another underlying disease, she said: “The number of patients with ongoing symptoms is very high, therefore [it is] unlikely that all of this is re-emergence of underlying or previous health problems. The type of symptoms reported are also as reported by other cohorts, so not unexpected. And irrespective of the root cause, they require care.”
Dr. Bogaert also noted that the data reiterate that COVID-19 is a new disease, and that “new variants might show shifting clinical pictures, not only regarding severity and symptoms of acute disease, but possibly also regarding sequela,” and that this, “underlines the importance of ongoing surveillance of variants, and ongoing evaluation of the acute and long-term clinical picture accompanying these, to ensure we adapt our public health approaches, clinical treatment plans, and long-term follow-up when and where needed.”
Dr. Bogaert stressed that only by keeping track of the changes in symptoms both acute and long-term – by patients and doctors – would the best patient care be provided.
“Patients need to know so they can report these back to their doctors, and doctors need to know over time that the picture of sequela might shift, so sequela are recognized early, and these patients receive the appropriate follow-up treatment,” she said. These shifting patterns might also apply to community patients as well as those hospitalized with COVID-19.
Study details
The retrospective, observational study included 428 patients, 59% men, with a mean age of 64 years, who had been treated at the Careggi University Hospital’s post-COVID outpatient service between June 2020 and June 2021, when the original form of SARS-CoV-2, and later the Alpha variant, were circulating, with some overlap.
All patients had been hospitalized with COVID-19 and discharged 4-12 weeks prior to attending the outpatient post-COVID service. They were asked to complete a questionnaire on persistent symptoms at the median of 53 days after being discharged from the hospital. In addition, data on medical history, microbiological and clinical COVID-19 course, self-reported symptoms (at the point of the follow-up visit), and patient demographics were obtained from electronic medical records.
Newer variants being studied
Upon analysis of long COVID symptoms according to treatment given during the acute phase using multivariate analysis, increasing oxygen support (odds ratio, 1.4; 95% confidence interval, 1.1-1.8), use of immunosuppressant drugs (OR, 6.4; 95% CI, 1.5-28), and female sex (OR, 1.8; 95% CI, 1.1-2.9) were associated with a higher risk for long COVID symptoms, while patients with type 2 diabetes (OR, 0.4; 95% CI, 0.2-0.7) had a lower risk of developing long COVID symptoms.
When asked whether the increased anxiety and depression seen with the Alpha variant might be also linked to the fact that people are living through hard times, with lockdowns, economic difficulties, possible illness, and even fatalities among family and friends due to COVID, Dr. Spinicci pointed out that “it’s a preliminary study, and there are lots of factors that we didn’t explore. It’s difficult to arrive at definite conclusions about long COVID because so much remains unknown. There are lots of external and environmental factors in the general population that might contribute to these findings.”
Dr. Spinicci has continued to enroll patients from later periods of the pandemic, including patients who were infected with the Delta and Omicron variants of SARS-CoV-2.
“We’re interested in finding out if these other variants are also associated with different phenotypes of long COVID. This study is part of our follow-up program here in the hospital where lots of different specialties are following patients for 20 months,” he said.
Dr. Horberg noted that one criticism of this study is that it was unclear whether the researchers accounted for pre-existing conditions. “They note the co-morbidities in the table 1, but don’t say how they accounted for that in their analyses. We found a lot of what patients were calling ‘long COVID’ were exacerbations of co-morbidities but not a new condition.”
Dr. Spinicci and his coauthors acknowledged that the study was observational. And, as such, it does not prove cause and effect, and they could not confirm which variant of the virus caused the infection in different patients, which may limit the conclusions that can be drawn.
“Future research should focus on the potential impacts of variants of concern and vaccination status on ongoing symptoms,” Spinicci said.
Early release of an abstract will be presented at the European Congress of Clinical Microbiology & Infectious Diseases (ECCMID) 2022, in Lisbon, Portugal, April 23-26, 2022. Abstract 02768.
Dr. Spinicci and Dr. Horberg have disclosed no relevant financial relationships. Dr. Bogaert declared that she is on the program committee of ECCMID; she has been a member of SIGN/NICE COVID-19 rapid guideline: managing the long-term effects of COVID-19; and she is involved in multiple ongoing COVID-related studies, both acute and long-term sequela (funding MRC, CSO, ZonMw).
A version of this article first appeared on Medscape.com.
Long COVID symptoms may differ depending on which SARS-CoV-2 variant is behind a person’s infection, a new study shows.
The data from Italy compared long COVID symptoms reported by patients infected with SARS-CoV-2 from March to December 2020 (when the original, or “Wuhan,” variant was dominant) with those reported by patients infected from January to April 2021 (B.1.1.7-, or Alpha variant-dominant). It showed a substantial change in the pattern of neurological and cognitive/emotional problems – the latter mostly seen with the Alpha variant.
Infectious disease specialist Michele Spinicci, MD, from the University of Florence and Careggi University Hospital, Italy, led the work. “Many of the symptoms reported in this study have been measured [before], but this is the first time they have been linked to different COVID-19 variants,” he told this news organization. “Findings in patients with long COVID were focused on neurological and psychological difficulties.”
However, he pointed out that much remains to be understood about long COVID in terms of symptoms, diagnosis, and treatment.
“Long COVID is a huge area that involves many different fields of medicine, so there is not one single piece of advice to give on management. There’s lots to consider when evaluating a long COVID patient,” he said.
Results showed that when the Alpha variant was the dominant variant, the prevalence of myalgia (10%), dyspnea (42%), brain fog/mental confusion (17%), and anxiety/depression (13%) significantly increased relative to the wild-type (original, Wuhan) variant, while anosmia (2%), dysgeusia (4%), and impaired hearing (1%) were less common.
When the wild-type (original, Wuhan) variant was dominant, fatigue (37%), insomnia (16%), dysgeusia (11%), and impaired hearing (5%) were all more common than with the Alpha variant. Dyspnea (33%), brain fog (10%), myalgia (4%), and anxiety/depression (6%) were less common.
Overall, 76% of the patients in the trial reported at least one persistent symptom, while the most common reported symptoms were dyspnea (37%) and chronic fatigue (36%), followed by insomnia (16%), visual disorders (13%), and brain fog (13%).
The findings come from an early-release abstract that will be presented at the European Congress of Clinical Microbiology & Infectious Diseases (ECCMID) 2022, in Lisbon, Portugal, in a few weeks’ time.
‘The take-home point’
Michael A. Horberg, MD, associate medical director, Kaiser Permanente – Mid-Atlantic Permanente Medical Group, Rockville, Maryland, has recently presented data on symptoms seen with long COVID in over 28,000 people, as reported by this news organization, at the Conference on Retroviruses and Opportunistic Infections 2022. These people were infected with the wild-type virus.
Commenting on the study by Dr. Spinicci, he said: “The issue is that as we go along the COVID lifespan from acute to long COVID, what prompts patients to seek medical attention may change. If symptoms are not severe or were not well publicized previously, patients may not see the need to seek care or evaluation. As such, it doesn’t surprise me to find these changes over time, independent of any potential biological activity of the virus or its consequences.”
Dr. Horberg noted that their own study results are consistent with those of Dr. Spinicci et al. from March to December 2020 (original, Wuhan variant). “To me, the take-home point is long COVID is real, and physicians need to be on the lookout for it. However, not all symptoms are due to long COVID, and we need to keep the time course of symptoms during evaluation of such patients.”
Also providing comment on the findings was Debby Bogaert, MD, chair of Pediatric Medicine, University of Edinburgh. Reflecting on whether the symptoms were due to long COVID or another underlying disease, she said: “The number of patients with ongoing symptoms is very high, therefore [it is] unlikely that all of this is re-emergence of underlying or previous health problems. The type of symptoms reported are also as reported by other cohorts, so not unexpected. And irrespective of the root cause, they require care.”
Dr. Bogaert also noted that the data reiterate that COVID-19 is a new disease, and that “new variants might show shifting clinical pictures, not only regarding severity and symptoms of acute disease, but possibly also regarding sequela,” and that this, “underlines the importance of ongoing surveillance of variants, and ongoing evaluation of the acute and long-term clinical picture accompanying these, to ensure we adapt our public health approaches, clinical treatment plans, and long-term follow-up when and where needed.”
Dr. Bogaert stressed that only by keeping track of the changes in symptoms both acute and long-term – by patients and doctors – would the best patient care be provided.
“Patients need to know so they can report these back to their doctors, and doctors need to know over time that the picture of sequela might shift, so sequela are recognized early, and these patients receive the appropriate follow-up treatment,” she said. These shifting patterns might also apply to community patients as well as those hospitalized with COVID-19.
Study details
The retrospective, observational study included 428 patients, 59% men, with a mean age of 64 years, who had been treated at the Careggi University Hospital’s post-COVID outpatient service between June 2020 and June 2021, when the original form of SARS-CoV-2, and later the Alpha variant, were circulating, with some overlap.
All patients had been hospitalized with COVID-19 and discharged 4-12 weeks prior to attending the outpatient post-COVID service. They were asked to complete a questionnaire on persistent symptoms at the median of 53 days after being discharged from the hospital. In addition, data on medical history, microbiological and clinical COVID-19 course, self-reported symptoms (at the point of the follow-up visit), and patient demographics were obtained from electronic medical records.
Newer variants being studied
Upon analysis of long COVID symptoms according to treatment given during the acute phase using multivariate analysis, increasing oxygen support (odds ratio, 1.4; 95% confidence interval, 1.1-1.8), use of immunosuppressant drugs (OR, 6.4; 95% CI, 1.5-28), and female sex (OR, 1.8; 95% CI, 1.1-2.9) were associated with a higher risk for long COVID symptoms, while patients with type 2 diabetes (OR, 0.4; 95% CI, 0.2-0.7) had a lower risk of developing long COVID symptoms.
When asked whether the increased anxiety and depression seen with the Alpha variant might be also linked to the fact that people are living through hard times, with lockdowns, economic difficulties, possible illness, and even fatalities among family and friends due to COVID, Dr. Spinicci pointed out that “it’s a preliminary study, and there are lots of factors that we didn’t explore. It’s difficult to arrive at definite conclusions about long COVID because so much remains unknown. There are lots of external and environmental factors in the general population that might contribute to these findings.”
Dr. Spinicci has continued to enroll patients from later periods of the pandemic, including patients who were infected with the Delta and Omicron variants of SARS-CoV-2.
“We’re interested in finding out if these other variants are also associated with different phenotypes of long COVID. This study is part of our follow-up program here in the hospital where lots of different specialties are following patients for 20 months,” he said.
Dr. Horberg noted that one criticism of this study is that it was unclear whether the researchers accounted for pre-existing conditions. “They note the co-morbidities in the table 1, but don’t say how they accounted for that in their analyses. We found a lot of what patients were calling ‘long COVID’ were exacerbations of co-morbidities but not a new condition.”
Dr. Spinicci and his coauthors acknowledged that the study was observational. And, as such, it does not prove cause and effect, and they could not confirm which variant of the virus caused the infection in different patients, which may limit the conclusions that can be drawn.
“Future research should focus on the potential impacts of variants of concern and vaccination status on ongoing symptoms,” Spinicci said.
Early release of an abstract will be presented at the European Congress of Clinical Microbiology & Infectious Diseases (ECCMID) 2022, in Lisbon, Portugal, April 23-26, 2022. Abstract 02768.
Dr. Spinicci and Dr. Horberg have disclosed no relevant financial relationships. Dr. Bogaert declared that she is on the program committee of ECCMID; she has been a member of SIGN/NICE COVID-19 rapid guideline: managing the long-term effects of COVID-19; and she is involved in multiple ongoing COVID-related studies, both acute and long-term sequela (funding MRC, CSO, ZonMw).
A version of this article first appeared on Medscape.com.
CROI 2022
Even moderate exercise offers strong shield from COVID-19
in its participants.
Researchers identified 65,361 members of a South African private health plan who had a COVID-19 diagnosis from March 2020 to June 2021 and matched them with physical activity data during the 2 years prior to the country’s March 2020 lockdown captured by smart devices, and clocked gym attendance and mass event participation in a voluntary healthy lifestyle behavior program linked to the insurer.
In all, 20.4% of participants had engaged in low levels of at least moderate-intensity physical activity per week (0-59 minutes), 34.5% in moderate levels (60-149 minutes), and 45.1% in high levels (150 minutes or more).
Overall, 11.1% were hospitalized as a result of COVID-19, 2.4% were admitted to the ICU, 1.3% required a ventilator, and 1.6% died.
As reported in the British Journal of Sports Medicine, analyses adjusted for demographic and other risk factors showed that, with COVID-19 infection, people with high versus low physical activity had a 34% lower risk for hospitalization (risk ratio, 0.66; 95% confidence interval, 0.63-0.70), a 41% lower risk for ICU admission (RR, 0.59; 95% CI, 0.52-0.66), a 45% lower risk of requiring ventilation (RR, 0.55; 95% CI, 0.47-0.64), and a 42% lower risk for death (RR, 0.58; 95% CI, 0.50-0.68).
Even moderate physical exercise, below the recommended guidelines of at least 150 minutes per week, was associated with several benefits, such as a 13% lower risk for hospitalization (RR, 0.87; 95% CI, 0.82-0.91), a 20% lower risk for ICU admission (RR, 0.80; 95% CI, 0.71-0.89), a 27% lower risk of requiring ventilation (RR, 0.73; 95% CI, 0.62-0.84), and a21% lower risk for death (RR, 0.79; 95% CI, 0.69-0.91).
“Should we come across further waves of this pandemic, our advice from a medical point of view should be to promote and facilitate exercise,” senior author Jon Patricios, MD, Wits Sport and Health, University of the Witwatersrand, Johannesburg, South Africa, said in an interview. “The likelihood is that exercise and vaccination are going to be the two most significant interventions in terms of helping to offload the health care system rather than face the catastrophic events endured a year or so ago.”
The study showed that males are at greater risk than females for severe COVID-19 outcomes, as were patients with essential hypertension, diabetes, and chronic renal disease.
It also suggests that the protective benefit of exercise extends to HIV-positive patients and those with rheumatoid arthritis, two groups previously not evaluated, the authors noted.
The results are comparable with previous reports of self-reported exercise and COVID-19 from the United States and South Korea, although the effect of even moderate exercise was more significant, possibly due to the use of direct measures of exercise rather than self-report, Dr. Patricios suggested.
Previous data suggest that regular physical activity may protect against many viral infections including influenza, rhinovirus, and the reactivation of latent herpes viruses, he noted. However, emerging evidence also points to significant decreases in physical activity during the pandemic.
“Regular physical activity should be a message that is strongly, strongly advocated for, particularly in less well-developed countries where we don’t have access or the resources to afford pharmacological interventions in many of these scenarios,” Dr. Patricios said. “It’s frustrating that the message is not driven strongly enough. It should be part of every government’s agenda.”
The cohort all being members of a medical insurance plan could imply some selection bias based on affordability and limit generalizability of the results, the authors noted. Other limitations include a lack of data on sociodemographic criteria such as education, income, and race, as well as behavioral risk factors such as smoking and diet.
Dr. Patricios and one coauthor are editors of the British Journal of Sports Medicine. Several coauthors are employees of Discovery Health, Johannesburg.
A version of this article first appeared on Medscape.com.
in its participants.
Researchers identified 65,361 members of a South African private health plan who had a COVID-19 diagnosis from March 2020 to June 2021 and matched them with physical activity data during the 2 years prior to the country’s March 2020 lockdown captured by smart devices, and clocked gym attendance and mass event participation in a voluntary healthy lifestyle behavior program linked to the insurer.
In all, 20.4% of participants had engaged in low levels of at least moderate-intensity physical activity per week (0-59 minutes), 34.5% in moderate levels (60-149 minutes), and 45.1% in high levels (150 minutes or more).
Overall, 11.1% were hospitalized as a result of COVID-19, 2.4% were admitted to the ICU, 1.3% required a ventilator, and 1.6% died.
As reported in the British Journal of Sports Medicine, analyses adjusted for demographic and other risk factors showed that, with COVID-19 infection, people with high versus low physical activity had a 34% lower risk for hospitalization (risk ratio, 0.66; 95% confidence interval, 0.63-0.70), a 41% lower risk for ICU admission (RR, 0.59; 95% CI, 0.52-0.66), a 45% lower risk of requiring ventilation (RR, 0.55; 95% CI, 0.47-0.64), and a 42% lower risk for death (RR, 0.58; 95% CI, 0.50-0.68).
Even moderate physical exercise, below the recommended guidelines of at least 150 minutes per week, was associated with several benefits, such as a 13% lower risk for hospitalization (RR, 0.87; 95% CI, 0.82-0.91), a 20% lower risk for ICU admission (RR, 0.80; 95% CI, 0.71-0.89), a 27% lower risk of requiring ventilation (RR, 0.73; 95% CI, 0.62-0.84), and a21% lower risk for death (RR, 0.79; 95% CI, 0.69-0.91).
“Should we come across further waves of this pandemic, our advice from a medical point of view should be to promote and facilitate exercise,” senior author Jon Patricios, MD, Wits Sport and Health, University of the Witwatersrand, Johannesburg, South Africa, said in an interview. “The likelihood is that exercise and vaccination are going to be the two most significant interventions in terms of helping to offload the health care system rather than face the catastrophic events endured a year or so ago.”
The study showed that males are at greater risk than females for severe COVID-19 outcomes, as were patients with essential hypertension, diabetes, and chronic renal disease.
It also suggests that the protective benefit of exercise extends to HIV-positive patients and those with rheumatoid arthritis, two groups previously not evaluated, the authors noted.
The results are comparable with previous reports of self-reported exercise and COVID-19 from the United States and South Korea, although the effect of even moderate exercise was more significant, possibly due to the use of direct measures of exercise rather than self-report, Dr. Patricios suggested.
Previous data suggest that regular physical activity may protect against many viral infections including influenza, rhinovirus, and the reactivation of latent herpes viruses, he noted. However, emerging evidence also points to significant decreases in physical activity during the pandemic.
“Regular physical activity should be a message that is strongly, strongly advocated for, particularly in less well-developed countries where we don’t have access or the resources to afford pharmacological interventions in many of these scenarios,” Dr. Patricios said. “It’s frustrating that the message is not driven strongly enough. It should be part of every government’s agenda.”
The cohort all being members of a medical insurance plan could imply some selection bias based on affordability and limit generalizability of the results, the authors noted. Other limitations include a lack of data on sociodemographic criteria such as education, income, and race, as well as behavioral risk factors such as smoking and diet.
Dr. Patricios and one coauthor are editors of the British Journal of Sports Medicine. Several coauthors are employees of Discovery Health, Johannesburg.
A version of this article first appeared on Medscape.com.
in its participants.
Researchers identified 65,361 members of a South African private health plan who had a COVID-19 diagnosis from March 2020 to June 2021 and matched them with physical activity data during the 2 years prior to the country’s March 2020 lockdown captured by smart devices, and clocked gym attendance and mass event participation in a voluntary healthy lifestyle behavior program linked to the insurer.
In all, 20.4% of participants had engaged in low levels of at least moderate-intensity physical activity per week (0-59 minutes), 34.5% in moderate levels (60-149 minutes), and 45.1% in high levels (150 minutes or more).
Overall, 11.1% were hospitalized as a result of COVID-19, 2.4% were admitted to the ICU, 1.3% required a ventilator, and 1.6% died.
As reported in the British Journal of Sports Medicine, analyses adjusted for demographic and other risk factors showed that, with COVID-19 infection, people with high versus low physical activity had a 34% lower risk for hospitalization (risk ratio, 0.66; 95% confidence interval, 0.63-0.70), a 41% lower risk for ICU admission (RR, 0.59; 95% CI, 0.52-0.66), a 45% lower risk of requiring ventilation (RR, 0.55; 95% CI, 0.47-0.64), and a 42% lower risk for death (RR, 0.58; 95% CI, 0.50-0.68).
Even moderate physical exercise, below the recommended guidelines of at least 150 minutes per week, was associated with several benefits, such as a 13% lower risk for hospitalization (RR, 0.87; 95% CI, 0.82-0.91), a 20% lower risk for ICU admission (RR, 0.80; 95% CI, 0.71-0.89), a 27% lower risk of requiring ventilation (RR, 0.73; 95% CI, 0.62-0.84), and a21% lower risk for death (RR, 0.79; 95% CI, 0.69-0.91).
“Should we come across further waves of this pandemic, our advice from a medical point of view should be to promote and facilitate exercise,” senior author Jon Patricios, MD, Wits Sport and Health, University of the Witwatersrand, Johannesburg, South Africa, said in an interview. “The likelihood is that exercise and vaccination are going to be the two most significant interventions in terms of helping to offload the health care system rather than face the catastrophic events endured a year or so ago.”
The study showed that males are at greater risk than females for severe COVID-19 outcomes, as were patients with essential hypertension, diabetes, and chronic renal disease.
It also suggests that the protective benefit of exercise extends to HIV-positive patients and those with rheumatoid arthritis, two groups previously not evaluated, the authors noted.
The results are comparable with previous reports of self-reported exercise and COVID-19 from the United States and South Korea, although the effect of even moderate exercise was more significant, possibly due to the use of direct measures of exercise rather than self-report, Dr. Patricios suggested.
Previous data suggest that regular physical activity may protect against many viral infections including influenza, rhinovirus, and the reactivation of latent herpes viruses, he noted. However, emerging evidence also points to significant decreases in physical activity during the pandemic.
“Regular physical activity should be a message that is strongly, strongly advocated for, particularly in less well-developed countries where we don’t have access or the resources to afford pharmacological interventions in many of these scenarios,” Dr. Patricios said. “It’s frustrating that the message is not driven strongly enough. It should be part of every government’s agenda.”
The cohort all being members of a medical insurance plan could imply some selection bias based on affordability and limit generalizability of the results, the authors noted. Other limitations include a lack of data on sociodemographic criteria such as education, income, and race, as well as behavioral risk factors such as smoking and diet.
Dr. Patricios and one coauthor are editors of the British Journal of Sports Medicine. Several coauthors are employees of Discovery Health, Johannesburg.
A version of this article first appeared on Medscape.com.
FROM THE BRITISH JOURNAL OF SPORTS MEDICINE
COVID-19 infection linked to risk of cutaneous autoimmune and vascular diseases
BOSTON – . This predominately favored systemic disease states with cutaneous involvement, rather than skin-limited processes.
The findings come from a large multicenter analysis that Zachary Holcomb, MD, presented during a late-breaking abstract session at the annual meeting of the American Academy of Dermatology.
“Viral triggers have been implicated in the pathogenesis of rheumatologic disease, but information regarding development of autoimmune disease following SARS-CoV-2 infection is limited,” said Dr. Holcomb, chief resident in the Harvard Combined Internal Medicine–Dermatology Residency, Boston. “Given its proposed thromboinflammatory pathobiology, we hypothesized that SARS-CoV-2 infection increases the risk of development of autoimmune disease with cutaneous manifestations and sought to define incidence rates of newly-diagnosed autoimmune diseases following SARS-CoV-2 infection.”
The researchers drew from the TriNetX Dataworks platform, an online cloud-based system that contains aggregated and deidentified patient information from about 75 million patients across 48 health care organizations. The infected cohort was defined as having a positive lab test for severe SARS-CoV-2 within the study window using Logical Observation Identifiers Names and Codes (LOINCs). Healthy controls consisted of a documented health care contact (inpatient or outpatient visit) during the study window without a positive SARS-CoV-2 lab test. Each cohort included patients aged 18-65 at the time of the study, and patients with previously diagnosed cutaneous autoimmune or vascular diseases were excluded from the analysis.
After propensity matching, the COVID-19 infected cohort and the healthy cohort included 1,904,864 patients each, with no baseline differences in age at index event, ethnicity, race, or sex. The study window was between April 1, 2020, and Oct. 1, 2020. The index event was a COVID-19 infection for the infected group and first documented health care contact in the healthy control group. The researchers looked at a window of 60 days following this index event for new incidence of cutaneous or vascular disease.
In the realm of connective tissue and related diseases, they found the incidence was increased among the COVID-19 infected group compared with controls for dermatomyositis (risk ratio, 2.273; P = .0196), scleroderma (RR, 1.959; P = .0001), and systemic lupus erythematosus (RR, 1.401; P < .0001). They also noted a significant decrease in the new incidence of alopecia areata in the COVID-19 infected group compared with controls (RR, 0.527; P < .0001).
No significant differences in the incidence of bullous and papulosquamous diseases were observed between the two groups. However, sarcoidosis was significantly more common in the COVID-19–infected group compared with controls (RR, 2.086; P < .001). “When taking all of these autoinflammatory diseases as a whole, there was an increased incidence in the COVID-19 infected group overall with a RR of 1.168 (P < .0001),” Dr. Holcomb said.
In the realm of vascular skin diseases, there was an increased incidence in the COVID-19 infected group in acrocyanosis (RR, 2.825; P < .001), Raynaud’s phenomenon (RR, 1.462; P < .0001), cutaneous small vessel vasculitis (RR, 1.714; P < .0001), granulomatosis with polyangiitis (RR, 2.667; P = .0002), and temporal arteritis (RR, 1.900; P = .0038).
“Interestingly, despite the academic and lay press reports of COVID toes, we did not see that in our data related to the COVID-infected group,” he said.
Dr. Holcomb acknowledged certain limitations of the study, including a narrow study window with a relatively short follow-up. “We were able to propensity match based on baseline demographics but not necessarily so based on health status and prior autoimmune disease,” he said. In addition, since the study was limited to those aged 18-65, the results may not be generalizable to pediatric and elderly patients, he said.
He described the study findings as “somewhat hypothesis-generating.” For instance, “why would we have more of a systemic process [at play?]. Our theory is that the severe inflammatory nature of COVID-19 leads to a lot of internal organ damage and exposure of autoantigens in that process, with relative skin sparing.”
One of the session moderators, Robert Paul Dellavalle, MD, PhD, professor of dermatology at the University of Colorado, Aurora, characterized the findings as “intriguing” but preliminary. “It would be interesting to look at more recent cohorts and see how vaccination for COVID-19 would impact the incidence rates of some of these diseases,” he said.
When asked for comment, Jeffrey A. Sparks, MD, MMSc, a rheumatologist at Brigham and Women's Hospital and assistant professor of medicine at Harvard Medical School, both in Boston, said, "This is an interesting study that should be followed up. Viral triggers have been known to precede autoimmune diseases so it will be very important to understand whether COVID-19 also impacts systemic autoimmune rheumatic diseases. I would be interested in differences in surveillance between the infection and control groups early in the pandemic. Many patients were avoiding interaction with the health care system at that point."
Dr. Holcomb reported having no financial disclosures. Dr. Dellavalle disclosed that he is a consultant for Altus Labs and ParaPRO LLC. He has received grants and research funding from Pfizer.
* This story was updated on 3/29/22.
BOSTON – . This predominately favored systemic disease states with cutaneous involvement, rather than skin-limited processes.
The findings come from a large multicenter analysis that Zachary Holcomb, MD, presented during a late-breaking abstract session at the annual meeting of the American Academy of Dermatology.
“Viral triggers have been implicated in the pathogenesis of rheumatologic disease, but information regarding development of autoimmune disease following SARS-CoV-2 infection is limited,” said Dr. Holcomb, chief resident in the Harvard Combined Internal Medicine–Dermatology Residency, Boston. “Given its proposed thromboinflammatory pathobiology, we hypothesized that SARS-CoV-2 infection increases the risk of development of autoimmune disease with cutaneous manifestations and sought to define incidence rates of newly-diagnosed autoimmune diseases following SARS-CoV-2 infection.”
The researchers drew from the TriNetX Dataworks platform, an online cloud-based system that contains aggregated and deidentified patient information from about 75 million patients across 48 health care organizations. The infected cohort was defined as having a positive lab test for severe SARS-CoV-2 within the study window using Logical Observation Identifiers Names and Codes (LOINCs). Healthy controls consisted of a documented health care contact (inpatient or outpatient visit) during the study window without a positive SARS-CoV-2 lab test. Each cohort included patients aged 18-65 at the time of the study, and patients with previously diagnosed cutaneous autoimmune or vascular diseases were excluded from the analysis.
After propensity matching, the COVID-19 infected cohort and the healthy cohort included 1,904,864 patients each, with no baseline differences in age at index event, ethnicity, race, or sex. The study window was between April 1, 2020, and Oct. 1, 2020. The index event was a COVID-19 infection for the infected group and first documented health care contact in the healthy control group. The researchers looked at a window of 60 days following this index event for new incidence of cutaneous or vascular disease.
In the realm of connective tissue and related diseases, they found the incidence was increased among the COVID-19 infected group compared with controls for dermatomyositis (risk ratio, 2.273; P = .0196), scleroderma (RR, 1.959; P = .0001), and systemic lupus erythematosus (RR, 1.401; P < .0001). They also noted a significant decrease in the new incidence of alopecia areata in the COVID-19 infected group compared with controls (RR, 0.527; P < .0001).
No significant differences in the incidence of bullous and papulosquamous diseases were observed between the two groups. However, sarcoidosis was significantly more common in the COVID-19–infected group compared with controls (RR, 2.086; P < .001). “When taking all of these autoinflammatory diseases as a whole, there was an increased incidence in the COVID-19 infected group overall with a RR of 1.168 (P < .0001),” Dr. Holcomb said.
In the realm of vascular skin diseases, there was an increased incidence in the COVID-19 infected group in acrocyanosis (RR, 2.825; P < .001), Raynaud’s phenomenon (RR, 1.462; P < .0001), cutaneous small vessel vasculitis (RR, 1.714; P < .0001), granulomatosis with polyangiitis (RR, 2.667; P = .0002), and temporal arteritis (RR, 1.900; P = .0038).
“Interestingly, despite the academic and lay press reports of COVID toes, we did not see that in our data related to the COVID-infected group,” he said.
Dr. Holcomb acknowledged certain limitations of the study, including a narrow study window with a relatively short follow-up. “We were able to propensity match based on baseline demographics but not necessarily so based on health status and prior autoimmune disease,” he said. In addition, since the study was limited to those aged 18-65, the results may not be generalizable to pediatric and elderly patients, he said.
He described the study findings as “somewhat hypothesis-generating.” For instance, “why would we have more of a systemic process [at play?]. Our theory is that the severe inflammatory nature of COVID-19 leads to a lot of internal organ damage and exposure of autoantigens in that process, with relative skin sparing.”
One of the session moderators, Robert Paul Dellavalle, MD, PhD, professor of dermatology at the University of Colorado, Aurora, characterized the findings as “intriguing” but preliminary. “It would be interesting to look at more recent cohorts and see how vaccination for COVID-19 would impact the incidence rates of some of these diseases,” he said.
When asked for comment, Jeffrey A. Sparks, MD, MMSc, a rheumatologist at Brigham and Women's Hospital and assistant professor of medicine at Harvard Medical School, both in Boston, said, "This is an interesting study that should be followed up. Viral triggers have been known to precede autoimmune diseases so it will be very important to understand whether COVID-19 also impacts systemic autoimmune rheumatic diseases. I would be interested in differences in surveillance between the infection and control groups early in the pandemic. Many patients were avoiding interaction with the health care system at that point."
Dr. Holcomb reported having no financial disclosures. Dr. Dellavalle disclosed that he is a consultant for Altus Labs and ParaPRO LLC. He has received grants and research funding from Pfizer.
* This story was updated on 3/29/22.
BOSTON – . This predominately favored systemic disease states with cutaneous involvement, rather than skin-limited processes.
The findings come from a large multicenter analysis that Zachary Holcomb, MD, presented during a late-breaking abstract session at the annual meeting of the American Academy of Dermatology.
“Viral triggers have been implicated in the pathogenesis of rheumatologic disease, but information regarding development of autoimmune disease following SARS-CoV-2 infection is limited,” said Dr. Holcomb, chief resident in the Harvard Combined Internal Medicine–Dermatology Residency, Boston. “Given its proposed thromboinflammatory pathobiology, we hypothesized that SARS-CoV-2 infection increases the risk of development of autoimmune disease with cutaneous manifestations and sought to define incidence rates of newly-diagnosed autoimmune diseases following SARS-CoV-2 infection.”
The researchers drew from the TriNetX Dataworks platform, an online cloud-based system that contains aggregated and deidentified patient information from about 75 million patients across 48 health care organizations. The infected cohort was defined as having a positive lab test for severe SARS-CoV-2 within the study window using Logical Observation Identifiers Names and Codes (LOINCs). Healthy controls consisted of a documented health care contact (inpatient or outpatient visit) during the study window without a positive SARS-CoV-2 lab test. Each cohort included patients aged 18-65 at the time of the study, and patients with previously diagnosed cutaneous autoimmune or vascular diseases were excluded from the analysis.
After propensity matching, the COVID-19 infected cohort and the healthy cohort included 1,904,864 patients each, with no baseline differences in age at index event, ethnicity, race, or sex. The study window was between April 1, 2020, and Oct. 1, 2020. The index event was a COVID-19 infection for the infected group and first documented health care contact in the healthy control group. The researchers looked at a window of 60 days following this index event for new incidence of cutaneous or vascular disease.
In the realm of connective tissue and related diseases, they found the incidence was increased among the COVID-19 infected group compared with controls for dermatomyositis (risk ratio, 2.273; P = .0196), scleroderma (RR, 1.959; P = .0001), and systemic lupus erythematosus (RR, 1.401; P < .0001). They also noted a significant decrease in the new incidence of alopecia areata in the COVID-19 infected group compared with controls (RR, 0.527; P < .0001).
No significant differences in the incidence of bullous and papulosquamous diseases were observed between the two groups. However, sarcoidosis was significantly more common in the COVID-19–infected group compared with controls (RR, 2.086; P < .001). “When taking all of these autoinflammatory diseases as a whole, there was an increased incidence in the COVID-19 infected group overall with a RR of 1.168 (P < .0001),” Dr. Holcomb said.
In the realm of vascular skin diseases, there was an increased incidence in the COVID-19 infected group in acrocyanosis (RR, 2.825; P < .001), Raynaud’s phenomenon (RR, 1.462; P < .0001), cutaneous small vessel vasculitis (RR, 1.714; P < .0001), granulomatosis with polyangiitis (RR, 2.667; P = .0002), and temporal arteritis (RR, 1.900; P = .0038).
“Interestingly, despite the academic and lay press reports of COVID toes, we did not see that in our data related to the COVID-infected group,” he said.
Dr. Holcomb acknowledged certain limitations of the study, including a narrow study window with a relatively short follow-up. “We were able to propensity match based on baseline demographics but not necessarily so based on health status and prior autoimmune disease,” he said. In addition, since the study was limited to those aged 18-65, the results may not be generalizable to pediatric and elderly patients, he said.
He described the study findings as “somewhat hypothesis-generating.” For instance, “why would we have more of a systemic process [at play?]. Our theory is that the severe inflammatory nature of COVID-19 leads to a lot of internal organ damage and exposure of autoantigens in that process, with relative skin sparing.”
One of the session moderators, Robert Paul Dellavalle, MD, PhD, professor of dermatology at the University of Colorado, Aurora, characterized the findings as “intriguing” but preliminary. “It would be interesting to look at more recent cohorts and see how vaccination for COVID-19 would impact the incidence rates of some of these diseases,” he said.
When asked for comment, Jeffrey A. Sparks, MD, MMSc, a rheumatologist at Brigham and Women's Hospital and assistant professor of medicine at Harvard Medical School, both in Boston, said, "This is an interesting study that should be followed up. Viral triggers have been known to precede autoimmune diseases so it will be very important to understand whether COVID-19 also impacts systemic autoimmune rheumatic diseases. I would be interested in differences in surveillance between the infection and control groups early in the pandemic. Many patients were avoiding interaction with the health care system at that point."
Dr. Holcomb reported having no financial disclosures. Dr. Dellavalle disclosed that he is a consultant for Altus Labs and ParaPRO LLC. He has received grants and research funding from Pfizer.
* This story was updated on 3/29/22.
AT AAD 2022
Neuropsychiatric outcomes similar for hospitalized COVID-19 patients and non–COVID-19 patients
Hospitalized COVID-19 survivors showed greater cognitive impairment 6 months later, compared with patients hospitalized for other causes, but the overall disease burden was similar, based on data from 85 adults with COVID-19.
Previous studies have shown that cognitive and neuropsychiatric symptoms can occur from 2-6 months after COVID-19 recovery, and such symptoms are known to be associated with hospitalization for other severe medical conditions, Vardan Nersesjan, MD, of Copenhagen University Hospital, and colleagues wrote.
However, it remains unknown if COVID-19 is associated with a unique pattern of cognitive and mental impairment compared with other similarly severe medical conditions, they said.
In a study published in JAMA Psychiatry (2022 Mar 23. doi: 10.1001/jamapsychiatry.2022.0284), the researchers identified 85 adult COVID-19 survivors and 61 controls with non-COVID medical conditions who were treated and released between July 2020 and July 2021. The COVID-19 patients and controls were matched for age, sex, and ICU status. Cognitive impairment was assessed using the Mini-International Neuropsychiatric Interview, the Montreal Cognitive Assessment (MoCA), neurologic examination, and a semistructured interview to determine subjective symptoms.
The primary outcomes were the total scores on the MoCA and any new-onset psychiatric diagnoses. Secondary outcomes included specific psychiatric diagnoses such as depression, neurologic examination findings, and self-reported neuropsychiatric and cognitive symptoms. The mean age of the COVID-19 patients was 56.8 years, and 42% were women.
At 6 months’ follow-up, cognitive status was significantly lower in COVID-19 survivors, compared with controls, based on total geometric mean MoCA scores (26.7 vs. 27.5, P = .01). However, cognitive status improved significantly from 19.2 at hospital discharge to 26.1 at 6 months in 15 of the COVID-19 patients (P = .004), the researchers noted.
New-onset psychiatric diagnoses occurred in 16 COVID-19 patients and 12 of the controls (19% vs. 20%); this difference was not significant.
Secondary outcomes were not significantly different at 6 months between the groups, with the exception of anosmia, which was significantly more common in the COVID-19 patients; however, the significance disappeared in adjusted analysis, the researchers said.
The study findings were limited by several factors including the inability to prove causality because of the case-control feature and by the inability to detect small differences in neuropsychiatric outcomes, the researchers noted.
However, the results were strengthened by the use of a prospectively matched control group with similar disease severity admitted to the same hospital in the same time frame. Although the overall burden of neuropsychiatric and neurologic symptoms and diagnoses appeared similar in COVID-19 patients and those with other medical conditions, more research in larger populations is needed to determine smaller differences in neuropsychiatric profiles, the researchers noted.
Study fills research gap
The study is important at this time because, although prolonged neuropsychiatric and cognitive symptoms have been reported after COVID-19, the field lacked prospective case-control studies with well-matched controls to investigate whether these outcomes differed from those seen in other critical illnesses that had also required hospitalization, corresponding author Michael E. Benros, MD, of the Mental Health Center, Copenhagen, said in an interview.
“I was surprised that there was a significant worse cognitive functioning among COVID-19 patients 6 months after symptom onset also when compared to this well-matched control group that had been hospitalized for non–COVID-19 illness, although the absolute difference between the groups in cognition score were small,” said Dr. Benros. “Another notable finding is the large improvement in cognitive functioning from discharge to follow-up,” he added on behalf of himself and fellow corresponding author Daniel Kondziella, MD.
The study results show that cognitive function affected by COVID-19 and critical illness as observed at discharge showed a substantial improvement at 6 months after symptom onset, said Dr. Benros. “However, the cognitive function was significantly worse among severely ill COVID-19 patients 6 months after symptom onset when compared to a matched control group of individuals hospitalized for non–COVID-19 illness, although this difference in cognitive function was rather small in absolute numbers, and smaller than what had been suggested by other studies that lacked control groups. Strikingly, neuropsychiatric disorders were similar across the two groups, which was also the case when looking at neuropsychiatric symptoms.
“Larger prospective case-control studies of neuropsychiatric and cognitive functioning after COVID-19, compared with matched controls are still needed to detect smaller differences, and more detailed cognitive domains, and with longer follow-up time, which we are currently conducting,” Dr. Benros said.
Controlled studies will help planning
“Lingering neuropsychiatric complications are common after COVID-19, but only controlled studies can tell us whether these complications are specific to COVID-19, rather than a general effect of having been medically ill,” Alasdair G. Rooney, MRCPsych MD PhD, of the University of Edinburgh, said in an interview. “The answer matters ultimately because COVID-19 is a new disease; societies and health care services need to be able to plan for its specific consequences.”
The health status of the control group is important as well. “Most previous studies had compared COVID-19 survivors against healthy controls or patients from a historical database. This new study compared COVID-19 survivors against those hospitalized for other medical causes over the same period,” Dr. Rooney said. “This is a more stringent test of whether COVID-19 has specific neurocognitive and neuropsychiatric consequences.
“The study found that new-onset neuropsychiatric diagnoses and symptoms were no more likely to occur after COVID-19 than after similarly severe medical illnesses,” Dr. Rooney said. “This negative finding runs counter to some earlier studies and may surprise some.” The findings need to be replicated in larger samples, but the current study shows the importance of prospectively recruiting active controls.
“In a subgroup analysis, some patients showed good improvement in cognitive scores between discharge and follow-up. While unsurprising, this is encouraging and suggests that the early postdischarge months are an important time for neurocognitive recovery,” Dr. Rooney noted.
“The findings suggest that COVID-19 may impair attention more selectively than other medical causes of hospitalization. COVID-19 survivors may also be at higher risk of significant overall cognitive impairment than survivors of similarly severe medical illnesses, after a similar duration,” said Dr. Rooney. “If the results are replicated by other prospective studies, they would suggest that there is something about COVID-19 that causes clinically significant neurocognitive difficulties in a minority of survivors.
“Larger well-controlled studies are required, with longer follow-up and more detailed neurocognitive testing,” as the duration of impairment and scope for further recovery are not known, Dr. Rooney added. Also unknown is whether COVID-19 affects attention permanently, or whether recovery is simply slower after COVID-19 compared to other medical illnesses.
“Knowing who is at the greatest risk of severe cognitive impairment after COVID-19 is important and likely to allow tailoring of more effective shielding strategies,” said Dr. Rooney. “This study was conducted before the widespread availability of vaccines for COVID-19. Long-term neuropsychiatric outcomes in vaccinated patients remain largely unknown. Arguably, these are now more important to understand, as future COVID-19 waves will occur mainly among vaccinated individuals.”
The study was supported by the Lundbeck Foundation and the Novo Nordisk Foundation. Lead author Dr. Nersesjan had no financial conflicts to disclose. Dr. Benros reported grants from Lundbeck Foundation and Novo Nordisk Foundation during the conduct of the study. Dr. Rooney had no financial conflicts to disclose.
This article was updated 3/25/22.
Hospitalized COVID-19 survivors showed greater cognitive impairment 6 months later, compared with patients hospitalized for other causes, but the overall disease burden was similar, based on data from 85 adults with COVID-19.
Previous studies have shown that cognitive and neuropsychiatric symptoms can occur from 2-6 months after COVID-19 recovery, and such symptoms are known to be associated with hospitalization for other severe medical conditions, Vardan Nersesjan, MD, of Copenhagen University Hospital, and colleagues wrote.
However, it remains unknown if COVID-19 is associated with a unique pattern of cognitive and mental impairment compared with other similarly severe medical conditions, they said.
In a study published in JAMA Psychiatry (2022 Mar 23. doi: 10.1001/jamapsychiatry.2022.0284), the researchers identified 85 adult COVID-19 survivors and 61 controls with non-COVID medical conditions who were treated and released between July 2020 and July 2021. The COVID-19 patients and controls were matched for age, sex, and ICU status. Cognitive impairment was assessed using the Mini-International Neuropsychiatric Interview, the Montreal Cognitive Assessment (MoCA), neurologic examination, and a semistructured interview to determine subjective symptoms.
The primary outcomes were the total scores on the MoCA and any new-onset psychiatric diagnoses. Secondary outcomes included specific psychiatric diagnoses such as depression, neurologic examination findings, and self-reported neuropsychiatric and cognitive symptoms. The mean age of the COVID-19 patients was 56.8 years, and 42% were women.
At 6 months’ follow-up, cognitive status was significantly lower in COVID-19 survivors, compared with controls, based on total geometric mean MoCA scores (26.7 vs. 27.5, P = .01). However, cognitive status improved significantly from 19.2 at hospital discharge to 26.1 at 6 months in 15 of the COVID-19 patients (P = .004), the researchers noted.
New-onset psychiatric diagnoses occurred in 16 COVID-19 patients and 12 of the controls (19% vs. 20%); this difference was not significant.
Secondary outcomes were not significantly different at 6 months between the groups, with the exception of anosmia, which was significantly more common in the COVID-19 patients; however, the significance disappeared in adjusted analysis, the researchers said.
The study findings were limited by several factors including the inability to prove causality because of the case-control feature and by the inability to detect small differences in neuropsychiatric outcomes, the researchers noted.
However, the results were strengthened by the use of a prospectively matched control group with similar disease severity admitted to the same hospital in the same time frame. Although the overall burden of neuropsychiatric and neurologic symptoms and diagnoses appeared similar in COVID-19 patients and those with other medical conditions, more research in larger populations is needed to determine smaller differences in neuropsychiatric profiles, the researchers noted.
Study fills research gap
The study is important at this time because, although prolonged neuropsychiatric and cognitive symptoms have been reported after COVID-19, the field lacked prospective case-control studies with well-matched controls to investigate whether these outcomes differed from those seen in other critical illnesses that had also required hospitalization, corresponding author Michael E. Benros, MD, of the Mental Health Center, Copenhagen, said in an interview.
“I was surprised that there was a significant worse cognitive functioning among COVID-19 patients 6 months after symptom onset also when compared to this well-matched control group that had been hospitalized for non–COVID-19 illness, although the absolute difference between the groups in cognition score were small,” said Dr. Benros. “Another notable finding is the large improvement in cognitive functioning from discharge to follow-up,” he added on behalf of himself and fellow corresponding author Daniel Kondziella, MD.
The study results show that cognitive function affected by COVID-19 and critical illness as observed at discharge showed a substantial improvement at 6 months after symptom onset, said Dr. Benros. “However, the cognitive function was significantly worse among severely ill COVID-19 patients 6 months after symptom onset when compared to a matched control group of individuals hospitalized for non–COVID-19 illness, although this difference in cognitive function was rather small in absolute numbers, and smaller than what had been suggested by other studies that lacked control groups. Strikingly, neuropsychiatric disorders were similar across the two groups, which was also the case when looking at neuropsychiatric symptoms.
“Larger prospective case-control studies of neuropsychiatric and cognitive functioning after COVID-19, compared with matched controls are still needed to detect smaller differences, and more detailed cognitive domains, and with longer follow-up time, which we are currently conducting,” Dr. Benros said.
Controlled studies will help planning
“Lingering neuropsychiatric complications are common after COVID-19, but only controlled studies can tell us whether these complications are specific to COVID-19, rather than a general effect of having been medically ill,” Alasdair G. Rooney, MRCPsych MD PhD, of the University of Edinburgh, said in an interview. “The answer matters ultimately because COVID-19 is a new disease; societies and health care services need to be able to plan for its specific consequences.”
The health status of the control group is important as well. “Most previous studies had compared COVID-19 survivors against healthy controls or patients from a historical database. This new study compared COVID-19 survivors against those hospitalized for other medical causes over the same period,” Dr. Rooney said. “This is a more stringent test of whether COVID-19 has specific neurocognitive and neuropsychiatric consequences.
“The study found that new-onset neuropsychiatric diagnoses and symptoms were no more likely to occur after COVID-19 than after similarly severe medical illnesses,” Dr. Rooney said. “This negative finding runs counter to some earlier studies and may surprise some.” The findings need to be replicated in larger samples, but the current study shows the importance of prospectively recruiting active controls.
“In a subgroup analysis, some patients showed good improvement in cognitive scores between discharge and follow-up. While unsurprising, this is encouraging and suggests that the early postdischarge months are an important time for neurocognitive recovery,” Dr. Rooney noted.
“The findings suggest that COVID-19 may impair attention more selectively than other medical causes of hospitalization. COVID-19 survivors may also be at higher risk of significant overall cognitive impairment than survivors of similarly severe medical illnesses, after a similar duration,” said Dr. Rooney. “If the results are replicated by other prospective studies, they would suggest that there is something about COVID-19 that causes clinically significant neurocognitive difficulties in a minority of survivors.
“Larger well-controlled studies are required, with longer follow-up and more detailed neurocognitive testing,” as the duration of impairment and scope for further recovery are not known, Dr. Rooney added. Also unknown is whether COVID-19 affects attention permanently, or whether recovery is simply slower after COVID-19 compared to other medical illnesses.
“Knowing who is at the greatest risk of severe cognitive impairment after COVID-19 is important and likely to allow tailoring of more effective shielding strategies,” said Dr. Rooney. “This study was conducted before the widespread availability of vaccines for COVID-19. Long-term neuropsychiatric outcomes in vaccinated patients remain largely unknown. Arguably, these are now more important to understand, as future COVID-19 waves will occur mainly among vaccinated individuals.”
The study was supported by the Lundbeck Foundation and the Novo Nordisk Foundation. Lead author Dr. Nersesjan had no financial conflicts to disclose. Dr. Benros reported grants from Lundbeck Foundation and Novo Nordisk Foundation during the conduct of the study. Dr. Rooney had no financial conflicts to disclose.
This article was updated 3/25/22.
Hospitalized COVID-19 survivors showed greater cognitive impairment 6 months later, compared with patients hospitalized for other causes, but the overall disease burden was similar, based on data from 85 adults with COVID-19.
Previous studies have shown that cognitive and neuropsychiatric symptoms can occur from 2-6 months after COVID-19 recovery, and such symptoms are known to be associated with hospitalization for other severe medical conditions, Vardan Nersesjan, MD, of Copenhagen University Hospital, and colleagues wrote.
However, it remains unknown if COVID-19 is associated with a unique pattern of cognitive and mental impairment compared with other similarly severe medical conditions, they said.
In a study published in JAMA Psychiatry (2022 Mar 23. doi: 10.1001/jamapsychiatry.2022.0284), the researchers identified 85 adult COVID-19 survivors and 61 controls with non-COVID medical conditions who were treated and released between July 2020 and July 2021. The COVID-19 patients and controls were matched for age, sex, and ICU status. Cognitive impairment was assessed using the Mini-International Neuropsychiatric Interview, the Montreal Cognitive Assessment (MoCA), neurologic examination, and a semistructured interview to determine subjective symptoms.
The primary outcomes were the total scores on the MoCA and any new-onset psychiatric diagnoses. Secondary outcomes included specific psychiatric diagnoses such as depression, neurologic examination findings, and self-reported neuropsychiatric and cognitive symptoms. The mean age of the COVID-19 patients was 56.8 years, and 42% were women.
At 6 months’ follow-up, cognitive status was significantly lower in COVID-19 survivors, compared with controls, based on total geometric mean MoCA scores (26.7 vs. 27.5, P = .01). However, cognitive status improved significantly from 19.2 at hospital discharge to 26.1 at 6 months in 15 of the COVID-19 patients (P = .004), the researchers noted.
New-onset psychiatric diagnoses occurred in 16 COVID-19 patients and 12 of the controls (19% vs. 20%); this difference was not significant.
Secondary outcomes were not significantly different at 6 months between the groups, with the exception of anosmia, which was significantly more common in the COVID-19 patients; however, the significance disappeared in adjusted analysis, the researchers said.
The study findings were limited by several factors including the inability to prove causality because of the case-control feature and by the inability to detect small differences in neuropsychiatric outcomes, the researchers noted.
However, the results were strengthened by the use of a prospectively matched control group with similar disease severity admitted to the same hospital in the same time frame. Although the overall burden of neuropsychiatric and neurologic symptoms and diagnoses appeared similar in COVID-19 patients and those with other medical conditions, more research in larger populations is needed to determine smaller differences in neuropsychiatric profiles, the researchers noted.
Study fills research gap
The study is important at this time because, although prolonged neuropsychiatric and cognitive symptoms have been reported after COVID-19, the field lacked prospective case-control studies with well-matched controls to investigate whether these outcomes differed from those seen in other critical illnesses that had also required hospitalization, corresponding author Michael E. Benros, MD, of the Mental Health Center, Copenhagen, said in an interview.
“I was surprised that there was a significant worse cognitive functioning among COVID-19 patients 6 months after symptom onset also when compared to this well-matched control group that had been hospitalized for non–COVID-19 illness, although the absolute difference between the groups in cognition score were small,” said Dr. Benros. “Another notable finding is the large improvement in cognitive functioning from discharge to follow-up,” he added on behalf of himself and fellow corresponding author Daniel Kondziella, MD.
The study results show that cognitive function affected by COVID-19 and critical illness as observed at discharge showed a substantial improvement at 6 months after symptom onset, said Dr. Benros. “However, the cognitive function was significantly worse among severely ill COVID-19 patients 6 months after symptom onset when compared to a matched control group of individuals hospitalized for non–COVID-19 illness, although this difference in cognitive function was rather small in absolute numbers, and smaller than what had been suggested by other studies that lacked control groups. Strikingly, neuropsychiatric disorders were similar across the two groups, which was also the case when looking at neuropsychiatric symptoms.
“Larger prospective case-control studies of neuropsychiatric and cognitive functioning after COVID-19, compared with matched controls are still needed to detect smaller differences, and more detailed cognitive domains, and with longer follow-up time, which we are currently conducting,” Dr. Benros said.
Controlled studies will help planning
“Lingering neuropsychiatric complications are common after COVID-19, but only controlled studies can tell us whether these complications are specific to COVID-19, rather than a general effect of having been medically ill,” Alasdair G. Rooney, MRCPsych MD PhD, of the University of Edinburgh, said in an interview. “The answer matters ultimately because COVID-19 is a new disease; societies and health care services need to be able to plan for its specific consequences.”
The health status of the control group is important as well. “Most previous studies had compared COVID-19 survivors against healthy controls or patients from a historical database. This new study compared COVID-19 survivors against those hospitalized for other medical causes over the same period,” Dr. Rooney said. “This is a more stringent test of whether COVID-19 has specific neurocognitive and neuropsychiatric consequences.
“The study found that new-onset neuropsychiatric diagnoses and symptoms were no more likely to occur after COVID-19 than after similarly severe medical illnesses,” Dr. Rooney said. “This negative finding runs counter to some earlier studies and may surprise some.” The findings need to be replicated in larger samples, but the current study shows the importance of prospectively recruiting active controls.
“In a subgroup analysis, some patients showed good improvement in cognitive scores between discharge and follow-up. While unsurprising, this is encouraging and suggests that the early postdischarge months are an important time for neurocognitive recovery,” Dr. Rooney noted.
“The findings suggest that COVID-19 may impair attention more selectively than other medical causes of hospitalization. COVID-19 survivors may also be at higher risk of significant overall cognitive impairment than survivors of similarly severe medical illnesses, after a similar duration,” said Dr. Rooney. “If the results are replicated by other prospective studies, they would suggest that there is something about COVID-19 that causes clinically significant neurocognitive difficulties in a minority of survivors.
“Larger well-controlled studies are required, with longer follow-up and more detailed neurocognitive testing,” as the duration of impairment and scope for further recovery are not known, Dr. Rooney added. Also unknown is whether COVID-19 affects attention permanently, or whether recovery is simply slower after COVID-19 compared to other medical illnesses.
“Knowing who is at the greatest risk of severe cognitive impairment after COVID-19 is important and likely to allow tailoring of more effective shielding strategies,” said Dr. Rooney. “This study was conducted before the widespread availability of vaccines for COVID-19. Long-term neuropsychiatric outcomes in vaccinated patients remain largely unknown. Arguably, these are now more important to understand, as future COVID-19 waves will occur mainly among vaccinated individuals.”
The study was supported by the Lundbeck Foundation and the Novo Nordisk Foundation. Lead author Dr. Nersesjan had no financial conflicts to disclose. Dr. Benros reported grants from Lundbeck Foundation and Novo Nordisk Foundation during the conduct of the study. Dr. Rooney had no financial conflicts to disclose.
This article was updated 3/25/22.
FROM JAMA PSYCHIATRY








