User login
ID Practitioner is an independent news source that provides infectious disease specialists with timely and relevant news and commentary about clinical developments and the impact of health care policy on the infectious disease specialist’s practice. Specialty focus topics include antimicrobial resistance, emerging infections, global ID, hepatitis, HIV, hospital-acquired infections, immunizations and vaccines, influenza, mycoses, pediatric infections, and STIs. Infectious Diseases News is owned by Frontline Medical Communications.
sofosbuvir
ritonavir with dasabuvir
discount
support path
program
ritonavir
greedy
ledipasvir
assistance
viekira pak
vpak
advocacy
needy
protest
abbvie
paritaprevir
ombitasvir
direct-acting antivirals
dasabuvir
gilead
fake-ovir
support
v pak
oasis
harvoni
section[contains(@class, 'footer-nav-section-wrapper')]
div[contains(@class, 'pane-pub-article-idp')]
div[contains(@class, 'pane-medstat-latest-articles-articles-section')]
div[contains(@class, 'pane-pub-home-idp')]
div[contains(@class, 'pane-pub-topic-idp')]
University taking aim at racial disparities in COVID vaccine trials
Although recent months have seen the arrival of several promising vaccines to combat COVID-19, many researchers have been concerned about the shortage of Black and Latinx volunteers in their pivotal trials.
Minority groups have long been underrepresented in clinical research. The pandemic’s inequitable fallout has heightened the need for more inclusive COVID-19 trials. By one estimate, Black Americans are three times more likely to become infected with SARS-Cov-2 and twice as likely to die from it, compared with their White counterparts.
It was therefore welcome news this past November when the Maryland-based biotech company Novavax unveiled their plans to boost participation among specific minority groups during the phase 3 trial of their COVID-19 vaccine candidate NVX-CoV2373. To help them in their efforts, the company tapped Howard University, in Washington, D.C., to be a clinical test site. The goal was to enroll 300 Black and Latinx volunteers through a recruitment registry at the Coronavirus Prevention Network.
“We have seen quite a good number of participants in the registry, and many are African American, who are the ones we are trying to reach in the trial,” explained Siham Mahgoub, MD, medical director of the Center of Infectious Diseases Management and Research and principal investigator for the Novavax trial at Howard University, Washington. “It’s very important for people of color to participate in the trial because we want to make sure these vaccines work in people of color,” Dr. Mahgoub said.
Over the years, Howard University has hosted several important clinical trials and studies, and its participation in the multi-institutional Georgetown–Howard Universities Center for Clinical and Translational Science consortium brings crucial infrastructural value. By bringing this vaccine trial to one of the most esteemed historically Black colleges or universities (HBCUs), researchers hoped to address a sense of hesitancy among possible participants that is prompted in part by the tragic history of medical testing in the Black community.
“The community trusts Howard,” said Dr. Mahgoub. “I think it’s great having Howard and an HBCU host this trial, because these are people who look like them.”
Lisa M. Dunkle, MD, vice president and global medical lead for coronavirus vaccine at Novavax, explained that, in addition to Howard being located close to the company’s headquarters, the university seemed like a great fit for the overall mission.
“As part of our goal to achieve a representative trial population that includes communities who are disproportionately impacted by the pandemic, we sought out some of the HBCUs to include in our trial sites. We hoped that this might encourage people of color to enroll and to increase their comfort level with vaccines in general,” Dr. Dunkle said.
Building more representative clinical trials
For decades, research on some of the most groundbreaking vaccines and treatments have been based on the results of studies conducted with predominately White participants, despite the fact that a much more demographically varied general population would ultimately receive them. This has led to calls to include people of different races and ethnic backgrounds in trials.
Homogeneity in clinical trials is discouraged, but trials are not heavily regulated in this regard. In 1993, Congress passed the Revitalization Act, which requires that trials that are conducted by the National Institutes of Health include women and members of minority groups among their cohorts. However, the number or proportion of such participants is not specified.
Underrepresentation in clinical trials also reflects a general unwillingness by members of ethnic minorities to volunteer because of the deeply unsettling history of such trials in minority communities. Among some Black persons, it is not uncommon for names like Tuskegee, Henrietta Lacks, and J. Marion Simms to be mentioned when giving reasons for not participating.
“There is certainly some dark history in how minorities have been treated by our health care system, and it’s not surprising that there is some fear and distrust,” said Dr. Dunkle. “By recruiting people of color into clinical trials that are governed with strict standards, we can begin to change perceptions and attitudes.”
Vaccine hesitancy is not only rooted in the past. The current state of medical care also has some potential trial participants worried. Misinformation, inequity in health care access, and low health literacy contribute to the current fears of scientific development.
A trial designed to engender trust
Having information about the vaccine come from trusted voices in the community is a key means of overcoming hesitancy. Howard University President Wayne Frederick, MD, reached out to a pastor of a local Black church to have more participants enroll in the trial. One who answered the call to action was Stephanie Williams, an elementary school teacher in Montgomery County, Maryland. When she saw that her pastor was participating in the Novavax trial and when she considered the devastation she had seen from COVID-19, she was on board.
“We had about three sessions where he shared his experiences. He also shared some links to read about it more,” Ms. Williams said. “When I saw that he took it, that gave me a lot of confidence. Since I’m going be going into the classroom, I wanted to be sure that I was well protected.”
Transparency is key to gaining more participation, explained Dr. Maghoub. Webinar-based information sessions have proven particularly important in achieving this.
“We do a lot of explaining in very simple language to make sure everyone understands about the vaccine. The participants have time to ask questions during the webinar, and at any time [during the trial], if a participant feels that it is not right for them, they can stop. They have time to learn about the trial and give consent. People often think they are like guinea pigs in trials, but they are not. They must give consent.”
There are signs that the approach has been successful. Over a period of 4-5 weeks, the Howard site enrolled 150 participants, of whom 30% were Black and 20% were Latinx.
Novavax has been in business for more than 3 decades but hasn’t seen the booming success that their competitors have. The company has noted progress in developing vaccines against Middle East respiratory syndrome and severe acute respiratory syndrome. However, they missed the mark in clinical trials, failing twice in 3 years to develop a respiratory syncytial virus vaccine administered through maternal immunizations.
From being on the verge of closing, Novavax has since made a dramatic turnaround after former President Trump awarded the company $1.6 billion dollars in July 2020 as part of Operation Warp Speed. If trial results are promising, the Novavax vaccine could enter the market in a few months, representing not only a new therapeutic option but perhaps a new model for building inclusivity in clinical trials.
A version of this article first appeared on Medscape.com.
Although recent months have seen the arrival of several promising vaccines to combat COVID-19, many researchers have been concerned about the shortage of Black and Latinx volunteers in their pivotal trials.
Minority groups have long been underrepresented in clinical research. The pandemic’s inequitable fallout has heightened the need for more inclusive COVID-19 trials. By one estimate, Black Americans are three times more likely to become infected with SARS-Cov-2 and twice as likely to die from it, compared with their White counterparts.
It was therefore welcome news this past November when the Maryland-based biotech company Novavax unveiled their plans to boost participation among specific minority groups during the phase 3 trial of their COVID-19 vaccine candidate NVX-CoV2373. To help them in their efforts, the company tapped Howard University, in Washington, D.C., to be a clinical test site. The goal was to enroll 300 Black and Latinx volunteers through a recruitment registry at the Coronavirus Prevention Network.
“We have seen quite a good number of participants in the registry, and many are African American, who are the ones we are trying to reach in the trial,” explained Siham Mahgoub, MD, medical director of the Center of Infectious Diseases Management and Research and principal investigator for the Novavax trial at Howard University, Washington. “It’s very important for people of color to participate in the trial because we want to make sure these vaccines work in people of color,” Dr. Mahgoub said.
Over the years, Howard University has hosted several important clinical trials and studies, and its participation in the multi-institutional Georgetown–Howard Universities Center for Clinical and Translational Science consortium brings crucial infrastructural value. By bringing this vaccine trial to one of the most esteemed historically Black colleges or universities (HBCUs), researchers hoped to address a sense of hesitancy among possible participants that is prompted in part by the tragic history of medical testing in the Black community.
“The community trusts Howard,” said Dr. Mahgoub. “I think it’s great having Howard and an HBCU host this trial, because these are people who look like them.”
Lisa M. Dunkle, MD, vice president and global medical lead for coronavirus vaccine at Novavax, explained that, in addition to Howard being located close to the company’s headquarters, the university seemed like a great fit for the overall mission.
“As part of our goal to achieve a representative trial population that includes communities who are disproportionately impacted by the pandemic, we sought out some of the HBCUs to include in our trial sites. We hoped that this might encourage people of color to enroll and to increase their comfort level with vaccines in general,” Dr. Dunkle said.
Building more representative clinical trials
For decades, research on some of the most groundbreaking vaccines and treatments have been based on the results of studies conducted with predominately White participants, despite the fact that a much more demographically varied general population would ultimately receive them. This has led to calls to include people of different races and ethnic backgrounds in trials.
Homogeneity in clinical trials is discouraged, but trials are not heavily regulated in this regard. In 1993, Congress passed the Revitalization Act, which requires that trials that are conducted by the National Institutes of Health include women and members of minority groups among their cohorts. However, the number or proportion of such participants is not specified.
Underrepresentation in clinical trials also reflects a general unwillingness by members of ethnic minorities to volunteer because of the deeply unsettling history of such trials in minority communities. Among some Black persons, it is not uncommon for names like Tuskegee, Henrietta Lacks, and J. Marion Simms to be mentioned when giving reasons for not participating.
“There is certainly some dark history in how minorities have been treated by our health care system, and it’s not surprising that there is some fear and distrust,” said Dr. Dunkle. “By recruiting people of color into clinical trials that are governed with strict standards, we can begin to change perceptions and attitudes.”
Vaccine hesitancy is not only rooted in the past. The current state of medical care also has some potential trial participants worried. Misinformation, inequity in health care access, and low health literacy contribute to the current fears of scientific development.
A trial designed to engender trust
Having information about the vaccine come from trusted voices in the community is a key means of overcoming hesitancy. Howard University President Wayne Frederick, MD, reached out to a pastor of a local Black church to have more participants enroll in the trial. One who answered the call to action was Stephanie Williams, an elementary school teacher in Montgomery County, Maryland. When she saw that her pastor was participating in the Novavax trial and when she considered the devastation she had seen from COVID-19, she was on board.
“We had about three sessions where he shared his experiences. He also shared some links to read about it more,” Ms. Williams said. “When I saw that he took it, that gave me a lot of confidence. Since I’m going be going into the classroom, I wanted to be sure that I was well protected.”
Transparency is key to gaining more participation, explained Dr. Maghoub. Webinar-based information sessions have proven particularly important in achieving this.
“We do a lot of explaining in very simple language to make sure everyone understands about the vaccine. The participants have time to ask questions during the webinar, and at any time [during the trial], if a participant feels that it is not right for them, they can stop. They have time to learn about the trial and give consent. People often think they are like guinea pigs in trials, but they are not. They must give consent.”
There are signs that the approach has been successful. Over a period of 4-5 weeks, the Howard site enrolled 150 participants, of whom 30% were Black and 20% were Latinx.
Novavax has been in business for more than 3 decades but hasn’t seen the booming success that their competitors have. The company has noted progress in developing vaccines against Middle East respiratory syndrome and severe acute respiratory syndrome. However, they missed the mark in clinical trials, failing twice in 3 years to develop a respiratory syncytial virus vaccine administered through maternal immunizations.
From being on the verge of closing, Novavax has since made a dramatic turnaround after former President Trump awarded the company $1.6 billion dollars in July 2020 as part of Operation Warp Speed. If trial results are promising, the Novavax vaccine could enter the market in a few months, representing not only a new therapeutic option but perhaps a new model for building inclusivity in clinical trials.
A version of this article first appeared on Medscape.com.
Although recent months have seen the arrival of several promising vaccines to combat COVID-19, many researchers have been concerned about the shortage of Black and Latinx volunteers in their pivotal trials.
Minority groups have long been underrepresented in clinical research. The pandemic’s inequitable fallout has heightened the need for more inclusive COVID-19 trials. By one estimate, Black Americans are three times more likely to become infected with SARS-Cov-2 and twice as likely to die from it, compared with their White counterparts.
It was therefore welcome news this past November when the Maryland-based biotech company Novavax unveiled their plans to boost participation among specific minority groups during the phase 3 trial of their COVID-19 vaccine candidate NVX-CoV2373. To help them in their efforts, the company tapped Howard University, in Washington, D.C., to be a clinical test site. The goal was to enroll 300 Black and Latinx volunteers through a recruitment registry at the Coronavirus Prevention Network.
“We have seen quite a good number of participants in the registry, and many are African American, who are the ones we are trying to reach in the trial,” explained Siham Mahgoub, MD, medical director of the Center of Infectious Diseases Management and Research and principal investigator for the Novavax trial at Howard University, Washington. “It’s very important for people of color to participate in the trial because we want to make sure these vaccines work in people of color,” Dr. Mahgoub said.
Over the years, Howard University has hosted several important clinical trials and studies, and its participation in the multi-institutional Georgetown–Howard Universities Center for Clinical and Translational Science consortium brings crucial infrastructural value. By bringing this vaccine trial to one of the most esteemed historically Black colleges or universities (HBCUs), researchers hoped to address a sense of hesitancy among possible participants that is prompted in part by the tragic history of medical testing in the Black community.
“The community trusts Howard,” said Dr. Mahgoub. “I think it’s great having Howard and an HBCU host this trial, because these are people who look like them.”
Lisa M. Dunkle, MD, vice president and global medical lead for coronavirus vaccine at Novavax, explained that, in addition to Howard being located close to the company’s headquarters, the university seemed like a great fit for the overall mission.
“As part of our goal to achieve a representative trial population that includes communities who are disproportionately impacted by the pandemic, we sought out some of the HBCUs to include in our trial sites. We hoped that this might encourage people of color to enroll and to increase their comfort level with vaccines in general,” Dr. Dunkle said.
Building more representative clinical trials
For decades, research on some of the most groundbreaking vaccines and treatments have been based on the results of studies conducted with predominately White participants, despite the fact that a much more demographically varied general population would ultimately receive them. This has led to calls to include people of different races and ethnic backgrounds in trials.
Homogeneity in clinical trials is discouraged, but trials are not heavily regulated in this regard. In 1993, Congress passed the Revitalization Act, which requires that trials that are conducted by the National Institutes of Health include women and members of minority groups among their cohorts. However, the number or proportion of such participants is not specified.
Underrepresentation in clinical trials also reflects a general unwillingness by members of ethnic minorities to volunteer because of the deeply unsettling history of such trials in minority communities. Among some Black persons, it is not uncommon for names like Tuskegee, Henrietta Lacks, and J. Marion Simms to be mentioned when giving reasons for not participating.
“There is certainly some dark history in how minorities have been treated by our health care system, and it’s not surprising that there is some fear and distrust,” said Dr. Dunkle. “By recruiting people of color into clinical trials that are governed with strict standards, we can begin to change perceptions and attitudes.”
Vaccine hesitancy is not only rooted in the past. The current state of medical care also has some potential trial participants worried. Misinformation, inequity in health care access, and low health literacy contribute to the current fears of scientific development.
A trial designed to engender trust
Having information about the vaccine come from trusted voices in the community is a key means of overcoming hesitancy. Howard University President Wayne Frederick, MD, reached out to a pastor of a local Black church to have more participants enroll in the trial. One who answered the call to action was Stephanie Williams, an elementary school teacher in Montgomery County, Maryland. When she saw that her pastor was participating in the Novavax trial and when she considered the devastation she had seen from COVID-19, she was on board.
“We had about three sessions where he shared his experiences. He also shared some links to read about it more,” Ms. Williams said. “When I saw that he took it, that gave me a lot of confidence. Since I’m going be going into the classroom, I wanted to be sure that I was well protected.”
Transparency is key to gaining more participation, explained Dr. Maghoub. Webinar-based information sessions have proven particularly important in achieving this.
“We do a lot of explaining in very simple language to make sure everyone understands about the vaccine. The participants have time to ask questions during the webinar, and at any time [during the trial], if a participant feels that it is not right for them, they can stop. They have time to learn about the trial and give consent. People often think they are like guinea pigs in trials, but they are not. They must give consent.”
There are signs that the approach has been successful. Over a period of 4-5 weeks, the Howard site enrolled 150 participants, of whom 30% were Black and 20% were Latinx.
Novavax has been in business for more than 3 decades but hasn’t seen the booming success that their competitors have. The company has noted progress in developing vaccines against Middle East respiratory syndrome and severe acute respiratory syndrome. However, they missed the mark in clinical trials, failing twice in 3 years to develop a respiratory syncytial virus vaccine administered through maternal immunizations.
From being on the verge of closing, Novavax has since made a dramatic turnaround after former President Trump awarded the company $1.6 billion dollars in July 2020 as part of Operation Warp Speed. If trial results are promising, the Novavax vaccine could enter the market in a few months, representing not only a new therapeutic option but perhaps a new model for building inclusivity in clinical trials.
A version of this article first appeared on Medscape.com.
Antimicrobial, pH-modulating gel shows promise in preventing common STIs
An investigational vaginal gel significantly reduced urogenital chlamydia and gonorrhea in women at high risk for infection, compared with placebo, opening up new possibilities for an on-demand prevention option. Investigators of a randomized trial reported these findings in the American Journal of Obstetrics and Gynecology.
Rates of Chlamydia trachomatis (CT) and Neisseria gonorrhoeae (GC) are on the rise in the United States, despite wide availability of male and female condoms to prevent sexually transmitted infections. This suggests that women need a more discrete method that they can better control. Other vaginal microbicides developed over the last few decades haven’t performed well in protecting against STIs or HIV in clinical trials.
The slightly alkaline nature of human semen has the potential to neutralize vaginal pH after intercourse, creating a more vulnerable environment for STIs. EVO100 is an investigational antimicrobial, bioadhesive vaginal gel that contains L-lactic acid, citric acid, and potassium bitartrate. In preclinical studies, it was highly effective at buffering the alkaline properties of human semen and maintaining vaginal pH levels. Patients generally tolerated it well, aside from some reports of vaginal itching and burning.
In the AMPREVENCE study, a double-blinded, placebo-controlled, randomized, phase 2b/3 trial, Todd Chappell, MD, of Adams Patterson Gynecology & Obstetrics, Memphis, and colleagues tested the efficacy and safety of EVO100 to prevent chlamydia and gonorrhea.
Investigators randomized 1:1,860 healthy, sexually active women to receive either EVO100 (n = 426) or placebo (n = 434). Participants had either been diagnosed or treated for these STIs up to 16 weeks prior to enrollment. Among those enrolled, 335 women in the EVO100 arm and 335 women in the placebo arm completed the study.
From this cohort, 764 women (EVO100: n = 376; placebo: n = 388) reported any use of either product. These women represented the “safety analysis population,” a predefined population for statistical analysis.
Participants averaged nearly 28 years of age, had a median body mass index of 28.9 kg/m2, and represented several racial/ethnic groups: White (54.3% [467/860]), African American (41.6% [358/860]), and non-Hispanic/Latinx ethnicity (67.1% [577/860]).
The women were instructed to apply the drug within 1 hour of initiating sexual intercourse. Investigators scheduled follow-up visits every 4 weeks during the 16-week study period, to obtain repeat CT/GC assessments, review diary entries, and to collect information about adverse effects and use of concomitant medications. During enrollment, participants consented to return to the clinic at each study visit. If a woman missed a visit, the study site would follow-up by telephone after the missed assessment visit.
Participants reported a mean number of 16 coital events (EVO100, 15.7 [13.5]; placebo, 16.3 [15.8]). EVO100 significantly reduced STI incidence for both types of STIs. CT infection rates among EVO100 users was 4.8% (14/289), half of what it was in placebo users (9.7% [28/290]) (P = .0256). The investigational method was even more successful in GC-analysis–eligible women: infection rates averaged 0.7% (2/280), compared with 3.2% (9/277) in the placebo group, a relative risk reduction of 78% (P = .0316).
Examining electronic diary entries of the participants, investigators reported similar adherence rates among the two treatment arms. However, additional sensitivity analyses in CT-eligible and GC-eligible populations on adherence yielded notably different results.
EVO100 users in the CT population who used the product as directed 100% of the time were significantly less likely to become infected, compared with the placebo group (2.3% vs. 16.9%, P = .0012). However, investigators found no significant differences in infection rates among women with poorer adherence rates in the two groups. Comparatively, they found no major differences in GC infection rates between the control and EVO100 groups, regardless of adherence rates, likely because of the small number of GC infections reported. Observed adverse events correlated with the drug’s known safety profile.
Most of the participants said they would likely recommend EVO100 to other women and continue using this preventive treatment.
A small GC subgroup caused by fewer infection cases and reliance on participant self-reporting of coital incidents may have limited the study’s results. “While use of the electronic diaries is helpful for collection of study data, it may encourage compliance and efficacy that may be higher in the ‘real-world’ population outside of the setting of a clinical trial,” noted Dr. Chappell and colleagues.
According to the investigators, this is the first prospective, randomized trial to study the use of an antimicrobial bioadhesive vaginal gel for preventing CT and GC infection. “EVO100 has the potential of fulfilling an unmet need in women’s sexual health as a new on-demand, woman-controlled option that reduces the risk of urogenital CT and GC infections,” the authors concluded.
The Food and Drug Administration has already approved EVO100 as a contraceptive option (Phexxi), Dr. Chappell said in an interview. Next steps are to conduct a phase 3 trial, which is currently underway. “If the findings are positive, we will submit to the FDA for review and approval of EVO100” for preventing these STIs.
These are promising results, Catherine Cansino, MD, MPH, an associate clinical professor with the department of obstetrics and gynecology at the University of California, Davis, said in an interview. It’s always helpful to look at effective treatments, “especially those that aren’t traditional antibiotics in order to decrease the risk of antibiotic resistance,” said Dr. Cansino, who was not part of the study. This is why EVO100 is such an attractive option.
Future studies should look at a broader population, she continued. “The population this study looked at is not the general population – these women had an infection at some point, previously,” which means they are potentially at higher risk for reinfection. “Looking at what their likelihood is of getting infected again, it’s hard to know if this would be the same or different from the general population.” If the drug appears to cause a decrease in new infections, the relative risk reduction is actually greater than what’s reported. If the reinfection rate for this population is lower because people who’ve had infections are practicing safer sex, the relative risk reduction would be lower, explained Dr. Cansino.
Dr. Chappell and several coauthors received research funding from Evofem Biosciences.
An investigational vaginal gel significantly reduced urogenital chlamydia and gonorrhea in women at high risk for infection, compared with placebo, opening up new possibilities for an on-demand prevention option. Investigators of a randomized trial reported these findings in the American Journal of Obstetrics and Gynecology.
Rates of Chlamydia trachomatis (CT) and Neisseria gonorrhoeae (GC) are on the rise in the United States, despite wide availability of male and female condoms to prevent sexually transmitted infections. This suggests that women need a more discrete method that they can better control. Other vaginal microbicides developed over the last few decades haven’t performed well in protecting against STIs or HIV in clinical trials.
The slightly alkaline nature of human semen has the potential to neutralize vaginal pH after intercourse, creating a more vulnerable environment for STIs. EVO100 is an investigational antimicrobial, bioadhesive vaginal gel that contains L-lactic acid, citric acid, and potassium bitartrate. In preclinical studies, it was highly effective at buffering the alkaline properties of human semen and maintaining vaginal pH levels. Patients generally tolerated it well, aside from some reports of vaginal itching and burning.
In the AMPREVENCE study, a double-blinded, placebo-controlled, randomized, phase 2b/3 trial, Todd Chappell, MD, of Adams Patterson Gynecology & Obstetrics, Memphis, and colleagues tested the efficacy and safety of EVO100 to prevent chlamydia and gonorrhea.
Investigators randomized 1:1,860 healthy, sexually active women to receive either EVO100 (n = 426) or placebo (n = 434). Participants had either been diagnosed or treated for these STIs up to 16 weeks prior to enrollment. Among those enrolled, 335 women in the EVO100 arm and 335 women in the placebo arm completed the study.
From this cohort, 764 women (EVO100: n = 376; placebo: n = 388) reported any use of either product. These women represented the “safety analysis population,” a predefined population for statistical analysis.
Participants averaged nearly 28 years of age, had a median body mass index of 28.9 kg/m2, and represented several racial/ethnic groups: White (54.3% [467/860]), African American (41.6% [358/860]), and non-Hispanic/Latinx ethnicity (67.1% [577/860]).
The women were instructed to apply the drug within 1 hour of initiating sexual intercourse. Investigators scheduled follow-up visits every 4 weeks during the 16-week study period, to obtain repeat CT/GC assessments, review diary entries, and to collect information about adverse effects and use of concomitant medications. During enrollment, participants consented to return to the clinic at each study visit. If a woman missed a visit, the study site would follow-up by telephone after the missed assessment visit.
Participants reported a mean number of 16 coital events (EVO100, 15.7 [13.5]; placebo, 16.3 [15.8]). EVO100 significantly reduced STI incidence for both types of STIs. CT infection rates among EVO100 users was 4.8% (14/289), half of what it was in placebo users (9.7% [28/290]) (P = .0256). The investigational method was even more successful in GC-analysis–eligible women: infection rates averaged 0.7% (2/280), compared with 3.2% (9/277) in the placebo group, a relative risk reduction of 78% (P = .0316).
Examining electronic diary entries of the participants, investigators reported similar adherence rates among the two treatment arms. However, additional sensitivity analyses in CT-eligible and GC-eligible populations on adherence yielded notably different results.
EVO100 users in the CT population who used the product as directed 100% of the time were significantly less likely to become infected, compared with the placebo group (2.3% vs. 16.9%, P = .0012). However, investigators found no significant differences in infection rates among women with poorer adherence rates in the two groups. Comparatively, they found no major differences in GC infection rates between the control and EVO100 groups, regardless of adherence rates, likely because of the small number of GC infections reported. Observed adverse events correlated with the drug’s known safety profile.
Most of the participants said they would likely recommend EVO100 to other women and continue using this preventive treatment.
A small GC subgroup caused by fewer infection cases and reliance on participant self-reporting of coital incidents may have limited the study’s results. “While use of the electronic diaries is helpful for collection of study data, it may encourage compliance and efficacy that may be higher in the ‘real-world’ population outside of the setting of a clinical trial,” noted Dr. Chappell and colleagues.
According to the investigators, this is the first prospective, randomized trial to study the use of an antimicrobial bioadhesive vaginal gel for preventing CT and GC infection. “EVO100 has the potential of fulfilling an unmet need in women’s sexual health as a new on-demand, woman-controlled option that reduces the risk of urogenital CT and GC infections,” the authors concluded.
The Food and Drug Administration has already approved EVO100 as a contraceptive option (Phexxi), Dr. Chappell said in an interview. Next steps are to conduct a phase 3 trial, which is currently underway. “If the findings are positive, we will submit to the FDA for review and approval of EVO100” for preventing these STIs.
These are promising results, Catherine Cansino, MD, MPH, an associate clinical professor with the department of obstetrics and gynecology at the University of California, Davis, said in an interview. It’s always helpful to look at effective treatments, “especially those that aren’t traditional antibiotics in order to decrease the risk of antibiotic resistance,” said Dr. Cansino, who was not part of the study. This is why EVO100 is such an attractive option.
Future studies should look at a broader population, she continued. “The population this study looked at is not the general population – these women had an infection at some point, previously,” which means they are potentially at higher risk for reinfection. “Looking at what their likelihood is of getting infected again, it’s hard to know if this would be the same or different from the general population.” If the drug appears to cause a decrease in new infections, the relative risk reduction is actually greater than what’s reported. If the reinfection rate for this population is lower because people who’ve had infections are practicing safer sex, the relative risk reduction would be lower, explained Dr. Cansino.
Dr. Chappell and several coauthors received research funding from Evofem Biosciences.
An investigational vaginal gel significantly reduced urogenital chlamydia and gonorrhea in women at high risk for infection, compared with placebo, opening up new possibilities for an on-demand prevention option. Investigators of a randomized trial reported these findings in the American Journal of Obstetrics and Gynecology.
Rates of Chlamydia trachomatis (CT) and Neisseria gonorrhoeae (GC) are on the rise in the United States, despite wide availability of male and female condoms to prevent sexually transmitted infections. This suggests that women need a more discrete method that they can better control. Other vaginal microbicides developed over the last few decades haven’t performed well in protecting against STIs or HIV in clinical trials.
The slightly alkaline nature of human semen has the potential to neutralize vaginal pH after intercourse, creating a more vulnerable environment for STIs. EVO100 is an investigational antimicrobial, bioadhesive vaginal gel that contains L-lactic acid, citric acid, and potassium bitartrate. In preclinical studies, it was highly effective at buffering the alkaline properties of human semen and maintaining vaginal pH levels. Patients generally tolerated it well, aside from some reports of vaginal itching and burning.
In the AMPREVENCE study, a double-blinded, placebo-controlled, randomized, phase 2b/3 trial, Todd Chappell, MD, of Adams Patterson Gynecology & Obstetrics, Memphis, and colleagues tested the efficacy and safety of EVO100 to prevent chlamydia and gonorrhea.
Investigators randomized 1:1,860 healthy, sexually active women to receive either EVO100 (n = 426) or placebo (n = 434). Participants had either been diagnosed or treated for these STIs up to 16 weeks prior to enrollment. Among those enrolled, 335 women in the EVO100 arm and 335 women in the placebo arm completed the study.
From this cohort, 764 women (EVO100: n = 376; placebo: n = 388) reported any use of either product. These women represented the “safety analysis population,” a predefined population for statistical analysis.
Participants averaged nearly 28 years of age, had a median body mass index of 28.9 kg/m2, and represented several racial/ethnic groups: White (54.3% [467/860]), African American (41.6% [358/860]), and non-Hispanic/Latinx ethnicity (67.1% [577/860]).
The women were instructed to apply the drug within 1 hour of initiating sexual intercourse. Investigators scheduled follow-up visits every 4 weeks during the 16-week study period, to obtain repeat CT/GC assessments, review diary entries, and to collect information about adverse effects and use of concomitant medications. During enrollment, participants consented to return to the clinic at each study visit. If a woman missed a visit, the study site would follow-up by telephone after the missed assessment visit.
Participants reported a mean number of 16 coital events (EVO100, 15.7 [13.5]; placebo, 16.3 [15.8]). EVO100 significantly reduced STI incidence for both types of STIs. CT infection rates among EVO100 users was 4.8% (14/289), half of what it was in placebo users (9.7% [28/290]) (P = .0256). The investigational method was even more successful in GC-analysis–eligible women: infection rates averaged 0.7% (2/280), compared with 3.2% (9/277) in the placebo group, a relative risk reduction of 78% (P = .0316).
Examining electronic diary entries of the participants, investigators reported similar adherence rates among the two treatment arms. However, additional sensitivity analyses in CT-eligible and GC-eligible populations on adherence yielded notably different results.
EVO100 users in the CT population who used the product as directed 100% of the time were significantly less likely to become infected, compared with the placebo group (2.3% vs. 16.9%, P = .0012). However, investigators found no significant differences in infection rates among women with poorer adherence rates in the two groups. Comparatively, they found no major differences in GC infection rates between the control and EVO100 groups, regardless of adherence rates, likely because of the small number of GC infections reported. Observed adverse events correlated with the drug’s known safety profile.
Most of the participants said they would likely recommend EVO100 to other women and continue using this preventive treatment.
A small GC subgroup caused by fewer infection cases and reliance on participant self-reporting of coital incidents may have limited the study’s results. “While use of the electronic diaries is helpful for collection of study data, it may encourage compliance and efficacy that may be higher in the ‘real-world’ population outside of the setting of a clinical trial,” noted Dr. Chappell and colleagues.
According to the investigators, this is the first prospective, randomized trial to study the use of an antimicrobial bioadhesive vaginal gel for preventing CT and GC infection. “EVO100 has the potential of fulfilling an unmet need in women’s sexual health as a new on-demand, woman-controlled option that reduces the risk of urogenital CT and GC infections,” the authors concluded.
The Food and Drug Administration has already approved EVO100 as a contraceptive option (Phexxi), Dr. Chappell said in an interview. Next steps are to conduct a phase 3 trial, which is currently underway. “If the findings are positive, we will submit to the FDA for review and approval of EVO100” for preventing these STIs.
These are promising results, Catherine Cansino, MD, MPH, an associate clinical professor with the department of obstetrics and gynecology at the University of California, Davis, said in an interview. It’s always helpful to look at effective treatments, “especially those that aren’t traditional antibiotics in order to decrease the risk of antibiotic resistance,” said Dr. Cansino, who was not part of the study. This is why EVO100 is such an attractive option.
Future studies should look at a broader population, she continued. “The population this study looked at is not the general population – these women had an infection at some point, previously,” which means they are potentially at higher risk for reinfection. “Looking at what their likelihood is of getting infected again, it’s hard to know if this would be the same or different from the general population.” If the drug appears to cause a decrease in new infections, the relative risk reduction is actually greater than what’s reported. If the reinfection rate for this population is lower because people who’ve had infections are practicing safer sex, the relative risk reduction would be lower, explained Dr. Cansino.
Dr. Chappell and several coauthors received research funding from Evofem Biosciences.
FROM THE AMERICAN JOURNAL OF OBSTETRICS AND GYNECOLOGY
‘Beyond a reasonable doubt’: COVID-19 brain health fallout is real, severe
COVID-19 survivors face a sharply elevated risk of developing psychiatric or neurologic disorders in the 6 months after they contract the virus – a danger that mounts with symptom severity, new research shows.
In what is purported to be the largest study of its kind to date, results showed that among 236,379 COVID-19 patients, one-third were diagnosed with at least 1 of 14 psychiatric or neurologic disorders within a 6-month span.
The rate of illnesses, which ranged from depression to stroke, rose sharply among those with COVID-19 symptoms acute enough to require hospitalization.
“If we look at patients who were hospitalized, that rate increased to 39%, and then increased to about just under 1 in 2 patients who needed ICU admission at the time of the COVID-19 diagnosis,” Maxime Taquet, PhD, University of Oxford (England) department of psychiatry, said at a media briefing.
Incidence jumps to almost two-thirds in patients with encephalopathy at the time of COVID-19 diagnosis, he added.
The study, which examined the brain health of 236,379 survivors of COVID-19 via a U.S. database of 81 million electronic health records, was published online April 6 in The Lancet Psychiatry.
High rate of neurologic, psychiatric disorders
The research team looked at the first-time diagnosis or recurrence of 14 neurologic and psychiatric outcomes in patients with confirmed SARS-CoV-2 infections. They also compared the brain health of this cohort with a control group of those with influenza or with non–COVID-19 respiratory infections over the same period.
All study participants were older than 10 years, diagnosed with COVID-19 on or after Jan. 20, 2020, and still alive as of Dec. 13, 2020.
The psychiatric and neurologic conditions examined included intracranial hemorrhage; ischemic stroke; parkinsonism; Guillain-Barré syndrome; nerve, nerve root and plexus disorders; myoneural junction and muscle disease; encephalitis; dementia; psychotic, mood, and anxiety disorders; substance use disorder; and insomnia.
The investigators used hospitalization, intensive care admissions, and encephalopathy as an indication of the severity of COVID-19 symptoms.
The study benchmarked the primary cohort with four populations of patients diagnosed in the same period with nonrespiratory illnesses, including skin infection, urolithiasis, bone fractures, and pulmonary embolisms.
Results showed that substantially more COVID-19 patients were diagnosed with a neurologic or psychiatric disorder compared with those with other respiratory illnesses.
“On average, in terms of the relative numbers, there was a 44% increased risk of having a neurological or psychiatric diagnosis after COVID-19 than after the flu and a 16% increased risk compared to other respiratory tract infections,” Dr. Taquet told reporters.
Health services should be prepared for an increase in psychiatric and neurologic issues in the months to come, he said, adding that further investigations are needed into why, and how, the coronavirus affects brain health.
Largest study to date
Although previous research suggests a link between the two, this is the largest study of its kind, examines a wider range of neurologic outcomes, and spans the longest time frame to date, said study coinvestigator Paul Harrison, BM BCh, associate head of the University of Oxford department of psychiatry.
There was a lower incidence of mood and anxiety disorders vs. neurologic disorders in patients with severe COVID-19 symptoms, a finding that Dr. Harrison said may indicate pandemic-related psychological stress is driving these disorders vs. biological factors.
“This paper follows up on an earlier study we did where we found much the same association, and our view is that a lot of the mental health consequences of COVID are … to do with the stress of knowing that one has had COVID and all the implications that go with that, rather than its being a direct effect, for example, of the virus on the brain, or of the immune response to the virus on the brain,” he added.
In contrast, neurologic diagnoses were more likely to be “mediated by some direct consequence of the COVID infection,” he added.
Psychosis and dementia, for instance, were less frequent in the overall COVID-19 population but became much more frequent among those with severe symptoms. The research team said these findings, along with those related to the incidence of ischemic stroke, were “concerning.”
“We found that 1 in 50 patients with COVID-19 go on to have an ischemic stroke in the 6 months after the COVID-19 illness,” Dr. Taquet told reporters. “And that rate increased to 1 in 11 patients if we look at patients with encephalopathy at the time of the COVID-19 diagnosis.”
Rates of brain hemorrhages also rose sharply among those with acute symptoms. Just over 1 in 200 total COVID-19 patients were diagnosed with this neurological condition, but that jumped to 1 in 25 of those who experienced encephalopathy at the time of their COVID-19 diagnosis.
Need for replication
Study coauthor Masud Husain, PhD, of University of Oxford’s cognitive neurology department, told reporters that while there is evidence from other neurologic studies that the virus can access the brain, there has been little sign the neurons themselves are affected.
“There isn’t much evidence that the virus itself attacks neurons in the brain, but it can cause inflammation, and it can activate inflammatory cells in the brain,” he said.
“And those effects are probably very important in some of the biological effects on the brain. In addition, of course, we know that the virus can change clotting and the likelihood of thrombosis in the blood, and those effects can also impact upon the brain,” he added.
Dr. Harrison said it would be helpful to replicate the results garnered from the U.S. database in other populations.
“It goes without saying that replication of these results with other electronic health records and in other countries is a priority,” he said, adding that investigations are essential into how and why the virus affects brain health.
Dr. Harrison cited a U.K. Research and Innovation–funded study called COVID CNS that will follow patients with neurologic and/or psychiatric issues during acute COVID-19 in hopes of exploring possible causes.
Beyond a reasonable doubt
Commenting on the findings, Sir Simon Wessely, MD, Regius chair of psychiatry, King’s College London, said in a release: “This is a very important paper. It confirms beyond any reasonable doubt that COVID-19 affects both brain and mind in equal measure.”
Some of these effects, including stroke and anxiety disorders, were already known, but others such as dementia and psychosis were less well known, he added.
“What is very new is the comparisons with all respiratory viruses or influenza, which suggests that these increases are specifically related to COVID-19, and not a general impact of viral infection,” Dr. Wessely said. “In general, the worse the illness, the greater the neurological or psychiatric outcomes, which is perhaps not surprising.
“The worst outcomes were in those with encephalopathy – inflammation of the brain – again, not surprising. The association with dementia was, however, small and might reflect diagnostic issues, whilst so far there doesn’t seem early evidence of a link with parkinsonism, which was a major factor after the great Spanish Flu pandemic, although the authors caution that it is too early to rule this out.”
A version of this article first appeared on Medscape.com.
COVID-19 survivors face a sharply elevated risk of developing psychiatric or neurologic disorders in the 6 months after they contract the virus – a danger that mounts with symptom severity, new research shows.
In what is purported to be the largest study of its kind to date, results showed that among 236,379 COVID-19 patients, one-third were diagnosed with at least 1 of 14 psychiatric or neurologic disorders within a 6-month span.
The rate of illnesses, which ranged from depression to stroke, rose sharply among those with COVID-19 symptoms acute enough to require hospitalization.
“If we look at patients who were hospitalized, that rate increased to 39%, and then increased to about just under 1 in 2 patients who needed ICU admission at the time of the COVID-19 diagnosis,” Maxime Taquet, PhD, University of Oxford (England) department of psychiatry, said at a media briefing.
Incidence jumps to almost two-thirds in patients with encephalopathy at the time of COVID-19 diagnosis, he added.
The study, which examined the brain health of 236,379 survivors of COVID-19 via a U.S. database of 81 million electronic health records, was published online April 6 in The Lancet Psychiatry.
High rate of neurologic, psychiatric disorders
The research team looked at the first-time diagnosis or recurrence of 14 neurologic and psychiatric outcomes in patients with confirmed SARS-CoV-2 infections. They also compared the brain health of this cohort with a control group of those with influenza or with non–COVID-19 respiratory infections over the same period.
All study participants were older than 10 years, diagnosed with COVID-19 on or after Jan. 20, 2020, and still alive as of Dec. 13, 2020.
The psychiatric and neurologic conditions examined included intracranial hemorrhage; ischemic stroke; parkinsonism; Guillain-Barré syndrome; nerve, nerve root and plexus disorders; myoneural junction and muscle disease; encephalitis; dementia; psychotic, mood, and anxiety disorders; substance use disorder; and insomnia.
The investigators used hospitalization, intensive care admissions, and encephalopathy as an indication of the severity of COVID-19 symptoms.
The study benchmarked the primary cohort with four populations of patients diagnosed in the same period with nonrespiratory illnesses, including skin infection, urolithiasis, bone fractures, and pulmonary embolisms.
Results showed that substantially more COVID-19 patients were diagnosed with a neurologic or psychiatric disorder compared with those with other respiratory illnesses.
“On average, in terms of the relative numbers, there was a 44% increased risk of having a neurological or psychiatric diagnosis after COVID-19 than after the flu and a 16% increased risk compared to other respiratory tract infections,” Dr. Taquet told reporters.
Health services should be prepared for an increase in psychiatric and neurologic issues in the months to come, he said, adding that further investigations are needed into why, and how, the coronavirus affects brain health.
Largest study to date
Although previous research suggests a link between the two, this is the largest study of its kind, examines a wider range of neurologic outcomes, and spans the longest time frame to date, said study coinvestigator Paul Harrison, BM BCh, associate head of the University of Oxford department of psychiatry.
There was a lower incidence of mood and anxiety disorders vs. neurologic disorders in patients with severe COVID-19 symptoms, a finding that Dr. Harrison said may indicate pandemic-related psychological stress is driving these disorders vs. biological factors.
“This paper follows up on an earlier study we did where we found much the same association, and our view is that a lot of the mental health consequences of COVID are … to do with the stress of knowing that one has had COVID and all the implications that go with that, rather than its being a direct effect, for example, of the virus on the brain, or of the immune response to the virus on the brain,” he added.
In contrast, neurologic diagnoses were more likely to be “mediated by some direct consequence of the COVID infection,” he added.
Psychosis and dementia, for instance, were less frequent in the overall COVID-19 population but became much more frequent among those with severe symptoms. The research team said these findings, along with those related to the incidence of ischemic stroke, were “concerning.”
“We found that 1 in 50 patients with COVID-19 go on to have an ischemic stroke in the 6 months after the COVID-19 illness,” Dr. Taquet told reporters. “And that rate increased to 1 in 11 patients if we look at patients with encephalopathy at the time of the COVID-19 diagnosis.”
Rates of brain hemorrhages also rose sharply among those with acute symptoms. Just over 1 in 200 total COVID-19 patients were diagnosed with this neurological condition, but that jumped to 1 in 25 of those who experienced encephalopathy at the time of their COVID-19 diagnosis.
Need for replication
Study coauthor Masud Husain, PhD, of University of Oxford’s cognitive neurology department, told reporters that while there is evidence from other neurologic studies that the virus can access the brain, there has been little sign the neurons themselves are affected.
“There isn’t much evidence that the virus itself attacks neurons in the brain, but it can cause inflammation, and it can activate inflammatory cells in the brain,” he said.
“And those effects are probably very important in some of the biological effects on the brain. In addition, of course, we know that the virus can change clotting and the likelihood of thrombosis in the blood, and those effects can also impact upon the brain,” he added.
Dr. Harrison said it would be helpful to replicate the results garnered from the U.S. database in other populations.
“It goes without saying that replication of these results with other electronic health records and in other countries is a priority,” he said, adding that investigations are essential into how and why the virus affects brain health.
Dr. Harrison cited a U.K. Research and Innovation–funded study called COVID CNS that will follow patients with neurologic and/or psychiatric issues during acute COVID-19 in hopes of exploring possible causes.
Beyond a reasonable doubt
Commenting on the findings, Sir Simon Wessely, MD, Regius chair of psychiatry, King’s College London, said in a release: “This is a very important paper. It confirms beyond any reasonable doubt that COVID-19 affects both brain and mind in equal measure.”
Some of these effects, including stroke and anxiety disorders, were already known, but others such as dementia and psychosis were less well known, he added.
“What is very new is the comparisons with all respiratory viruses or influenza, which suggests that these increases are specifically related to COVID-19, and not a general impact of viral infection,” Dr. Wessely said. “In general, the worse the illness, the greater the neurological or psychiatric outcomes, which is perhaps not surprising.
“The worst outcomes were in those with encephalopathy – inflammation of the brain – again, not surprising. The association with dementia was, however, small and might reflect diagnostic issues, whilst so far there doesn’t seem early evidence of a link with parkinsonism, which was a major factor after the great Spanish Flu pandemic, although the authors caution that it is too early to rule this out.”
A version of this article first appeared on Medscape.com.
COVID-19 survivors face a sharply elevated risk of developing psychiatric or neurologic disorders in the 6 months after they contract the virus – a danger that mounts with symptom severity, new research shows.
In what is purported to be the largest study of its kind to date, results showed that among 236,379 COVID-19 patients, one-third were diagnosed with at least 1 of 14 psychiatric or neurologic disorders within a 6-month span.
The rate of illnesses, which ranged from depression to stroke, rose sharply among those with COVID-19 symptoms acute enough to require hospitalization.
“If we look at patients who were hospitalized, that rate increased to 39%, and then increased to about just under 1 in 2 patients who needed ICU admission at the time of the COVID-19 diagnosis,” Maxime Taquet, PhD, University of Oxford (England) department of psychiatry, said at a media briefing.
Incidence jumps to almost two-thirds in patients with encephalopathy at the time of COVID-19 diagnosis, he added.
The study, which examined the brain health of 236,379 survivors of COVID-19 via a U.S. database of 81 million electronic health records, was published online April 6 in The Lancet Psychiatry.
High rate of neurologic, psychiatric disorders
The research team looked at the first-time diagnosis or recurrence of 14 neurologic and psychiatric outcomes in patients with confirmed SARS-CoV-2 infections. They also compared the brain health of this cohort with a control group of those with influenza or with non–COVID-19 respiratory infections over the same period.
All study participants were older than 10 years, diagnosed with COVID-19 on or after Jan. 20, 2020, and still alive as of Dec. 13, 2020.
The psychiatric and neurologic conditions examined included intracranial hemorrhage; ischemic stroke; parkinsonism; Guillain-Barré syndrome; nerve, nerve root and plexus disorders; myoneural junction and muscle disease; encephalitis; dementia; psychotic, mood, and anxiety disorders; substance use disorder; and insomnia.
The investigators used hospitalization, intensive care admissions, and encephalopathy as an indication of the severity of COVID-19 symptoms.
The study benchmarked the primary cohort with four populations of patients diagnosed in the same period with nonrespiratory illnesses, including skin infection, urolithiasis, bone fractures, and pulmonary embolisms.
Results showed that substantially more COVID-19 patients were diagnosed with a neurologic or psychiatric disorder compared with those with other respiratory illnesses.
“On average, in terms of the relative numbers, there was a 44% increased risk of having a neurological or psychiatric diagnosis after COVID-19 than after the flu and a 16% increased risk compared to other respiratory tract infections,” Dr. Taquet told reporters.
Health services should be prepared for an increase in psychiatric and neurologic issues in the months to come, he said, adding that further investigations are needed into why, and how, the coronavirus affects brain health.
Largest study to date
Although previous research suggests a link between the two, this is the largest study of its kind, examines a wider range of neurologic outcomes, and spans the longest time frame to date, said study coinvestigator Paul Harrison, BM BCh, associate head of the University of Oxford department of psychiatry.
There was a lower incidence of mood and anxiety disorders vs. neurologic disorders in patients with severe COVID-19 symptoms, a finding that Dr. Harrison said may indicate pandemic-related psychological stress is driving these disorders vs. biological factors.
“This paper follows up on an earlier study we did where we found much the same association, and our view is that a lot of the mental health consequences of COVID are … to do with the stress of knowing that one has had COVID and all the implications that go with that, rather than its being a direct effect, for example, of the virus on the brain, or of the immune response to the virus on the brain,” he added.
In contrast, neurologic diagnoses were more likely to be “mediated by some direct consequence of the COVID infection,” he added.
Psychosis and dementia, for instance, were less frequent in the overall COVID-19 population but became much more frequent among those with severe symptoms. The research team said these findings, along with those related to the incidence of ischemic stroke, were “concerning.”
“We found that 1 in 50 patients with COVID-19 go on to have an ischemic stroke in the 6 months after the COVID-19 illness,” Dr. Taquet told reporters. “And that rate increased to 1 in 11 patients if we look at patients with encephalopathy at the time of the COVID-19 diagnosis.”
Rates of brain hemorrhages also rose sharply among those with acute symptoms. Just over 1 in 200 total COVID-19 patients were diagnosed with this neurological condition, but that jumped to 1 in 25 of those who experienced encephalopathy at the time of their COVID-19 diagnosis.
Need for replication
Study coauthor Masud Husain, PhD, of University of Oxford’s cognitive neurology department, told reporters that while there is evidence from other neurologic studies that the virus can access the brain, there has been little sign the neurons themselves are affected.
“There isn’t much evidence that the virus itself attacks neurons in the brain, but it can cause inflammation, and it can activate inflammatory cells in the brain,” he said.
“And those effects are probably very important in some of the biological effects on the brain. In addition, of course, we know that the virus can change clotting and the likelihood of thrombosis in the blood, and those effects can also impact upon the brain,” he added.
Dr. Harrison said it would be helpful to replicate the results garnered from the U.S. database in other populations.
“It goes without saying that replication of these results with other electronic health records and in other countries is a priority,” he said, adding that investigations are essential into how and why the virus affects brain health.
Dr. Harrison cited a U.K. Research and Innovation–funded study called COVID CNS that will follow patients with neurologic and/or psychiatric issues during acute COVID-19 in hopes of exploring possible causes.
Beyond a reasonable doubt
Commenting on the findings, Sir Simon Wessely, MD, Regius chair of psychiatry, King’s College London, said in a release: “This is a very important paper. It confirms beyond any reasonable doubt that COVID-19 affects both brain and mind in equal measure.”
Some of these effects, including stroke and anxiety disorders, were already known, but others such as dementia and psychosis were less well known, he added.
“What is very new is the comparisons with all respiratory viruses or influenza, which suggests that these increases are specifically related to COVID-19, and not a general impact of viral infection,” Dr. Wessely said. “In general, the worse the illness, the greater the neurological or psychiatric outcomes, which is perhaps not surprising.
“The worst outcomes were in those with encephalopathy – inflammation of the brain – again, not surprising. The association with dementia was, however, small and might reflect diagnostic issues, whilst so far there doesn’t seem early evidence of a link with parkinsonism, which was a major factor after the great Spanish Flu pandemic, although the authors caution that it is too early to rule this out.”
A version of this article first appeared on Medscape.com.
About one in five clinicians considers quitting because of pandemic
a new survey of more than 5,000 clinicians at an academic medical center illustrates.
About one in five people reported considering leaving the workforce because of the challenges of working during the COVID-19 pandemic. In addition, 30% reported they are considering cutting back work hours.
“There are a substantial number of employees and trainees who are experiencing major stress and work disruptions because of the pandemic,” lead author Rebecca K. Delaney, PhD, said in an interview. “It is particularly alarming that people who have spent 5 or more years in training for their specialty are struggling with their work, so much so that they have even considered leaving the workforce or reducing their hours.”
“Being a caregiver adds another layer of difficulty for faculty, staff, and trainees who are trying to manage work and child care,” added Dr. Delaney, a researcher in the department of population health sciences, University of Utah, Salt Lake City.
The study was published online April 2 in JAMA Network Open.
“This looks like an excellent survey,” Carol A Bernstein, MD, said in an interview when asked to comment. “I do not think it provides particularly new information as these challenges in the workplace, especially for women during COVID, have been well documented in the media and the medical literature to date.”
“That said, to the extent that data helps drive solutions, I would hope that information such as this would be considered as strong further evidence that health care systems must pay close attention to the wellbeing of the workforce,” added Dr. Bernstein, professor and vice chair of faculty development and well-being, departments of psychiatry and behavioral sciences and obstetrics and gynecology and women’s health, Montefiore Medical Center/Albert Einstein College of Medicine, New York.
When the pandemic hits home
A total of 42% of the American workforce rapidly transitioned to working from home at the onset of the COVID-19 pandemic. At the same time, many employees had to provide child care and assistance with schoolwork. This placed a burden on many individuals at academic medical centers, and women in particular.
“Women comprise 74.9% of hospital employees, many of whom are essential clinical workers,” the researchers noted. “The extent of the needs and difficulties for these workers during the pandemic remain largely unknown.”
To learn more, Dr. Delaney, senior author Angie Fagerlin, PhD, and their colleagues emailed a Qualtrics survey to 27,700 faculty, staff, and trainees at University of Utah Health. The survey was conducted Aug. 5-20, 2020 as part of a quality improvement initiative. All responses were anonymous.
Survey questions included if, because of the pandemic, people had considered leaving the workforce, considered reducing their hours, or experienced reduced productivity. The researchers also asked about career impacts and potential solutions in terms of “work culture adaptations.”
Respondents with children aged under 18 years also were asked about child care options. Dr. Delaney and colleagues also inquired about race and ethnicity because they hypothesized that employees from underrepresented groups would likely experience the pandemic differently.
The mean age of the 5,951 (21%) faculty, staff, and trainees who completed the survey was 40 years. A majority of respondents were women, reflecting the higher proportion of women within the health system.
A majority (86%) identified as White or European American. About two-thirds of respondents (66%) were staff, 16% were faculty, and 13% were trainees.
COVID-19 career concerns
Overall, 1,061 respondents (21%) “moderately or very seriously” considered leaving the workforce and 1,505 (30%) considered reducing hours. Respondents who were younger, married, a member of an underrepresented racial/ethnic group, and worked in a clinical setting were more likely to consider leaving the workforce.
The survey showed 27% felt their productivity increased whereas 39% believed their productivity decreased.
Of the 2,412 survey participants with children aged 18 years or younger, 66% reported that they did not have child care fully available.
“Failure to address and provide for child care has long been one of the many significant deficits in U.S. health care systems,” said Dr. Bernstein, lead author of a March 2021 report evaluating staff emotional support at Montefiore Medical Center during the pandemic in The Joint Commission Journal on Quality and Patient Safety.
Furthermore, 47% were “moderately or very seriously worried” about COVID-19 impacting their career development.
Women trainees were significantly more likely than male counterparts to consider leaving the workforce and reducing their work hours. Women in a faculty or trainee role were also more likely to worry about COVID-19’s impact on their career, compared with men, and compared with women in staff positions.
“It was disheartening to have our data support the gender and racial/ethnic disparity that has been highlighted in the media during the pandemic,” Dr. Delaney said. “Women and in some cases racial/ethnic groups that are underrepresented in medicine were most likely to consider leaving the workforce, reducing hours, and were worried about their career development.
“It is critical that we strategically address these important disparities,” she said.
Women also are disproportionately affected by burnout, particularly during the pandemic, according to an analysis of Medscape’s Physician Burnout and Suicide Report.
Furthermore, the COVID-19 pandemic has shifted the medical specialties now considered highest risk for burnout: critical care physicians ranked first in the report, followed by rheumatologists and infectious disease specialists.
Potential solutions
“Given the disproportionate impact COVID-19 has on employees of health systems, institutions must find ways to support their employees, both in terms of workplace cultural adaptations and assistance with familial responsibilities,” the researchers noted.
Telecommuting policies, scheduling flexibility, and expanding employee support programs are potential solutions. Institutional policies also could address the educational and direct care needs of employee children.
Limitations of the study include its generalizability beyond employees of University of Utah Health. Also, respondents included a lower proportion of racial and ethnic groups, compared with national figures, “although this is mostly accounted for by the overall low population of such groups in the state of Utah,” the researchers added.
“Our results suggest that respondents were struggling during the COVID-19 pandemic,” the researchers noted. “As a result, even after investing substantial amounts of time in years of training, many were considering leaving the workforce because of stress and caregiving responsibilities related to the pandemic.”
The Jon M. Huntsman Presidential Endowed Chair supported the work with a financial award to Dr. Fagerlin. Dr. Delaney and Dr. Bernstein disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
a new survey of more than 5,000 clinicians at an academic medical center illustrates.
About one in five people reported considering leaving the workforce because of the challenges of working during the COVID-19 pandemic. In addition, 30% reported they are considering cutting back work hours.
“There are a substantial number of employees and trainees who are experiencing major stress and work disruptions because of the pandemic,” lead author Rebecca K. Delaney, PhD, said in an interview. “It is particularly alarming that people who have spent 5 or more years in training for their specialty are struggling with their work, so much so that they have even considered leaving the workforce or reducing their hours.”
“Being a caregiver adds another layer of difficulty for faculty, staff, and trainees who are trying to manage work and child care,” added Dr. Delaney, a researcher in the department of population health sciences, University of Utah, Salt Lake City.
The study was published online April 2 in JAMA Network Open.
“This looks like an excellent survey,” Carol A Bernstein, MD, said in an interview when asked to comment. “I do not think it provides particularly new information as these challenges in the workplace, especially for women during COVID, have been well documented in the media and the medical literature to date.”
“That said, to the extent that data helps drive solutions, I would hope that information such as this would be considered as strong further evidence that health care systems must pay close attention to the wellbeing of the workforce,” added Dr. Bernstein, professor and vice chair of faculty development and well-being, departments of psychiatry and behavioral sciences and obstetrics and gynecology and women’s health, Montefiore Medical Center/Albert Einstein College of Medicine, New York.
When the pandemic hits home
A total of 42% of the American workforce rapidly transitioned to working from home at the onset of the COVID-19 pandemic. At the same time, many employees had to provide child care and assistance with schoolwork. This placed a burden on many individuals at academic medical centers, and women in particular.
“Women comprise 74.9% of hospital employees, many of whom are essential clinical workers,” the researchers noted. “The extent of the needs and difficulties for these workers during the pandemic remain largely unknown.”
To learn more, Dr. Delaney, senior author Angie Fagerlin, PhD, and their colleagues emailed a Qualtrics survey to 27,700 faculty, staff, and trainees at University of Utah Health. The survey was conducted Aug. 5-20, 2020 as part of a quality improvement initiative. All responses were anonymous.
Survey questions included if, because of the pandemic, people had considered leaving the workforce, considered reducing their hours, or experienced reduced productivity. The researchers also asked about career impacts and potential solutions in terms of “work culture adaptations.”
Respondents with children aged under 18 years also were asked about child care options. Dr. Delaney and colleagues also inquired about race and ethnicity because they hypothesized that employees from underrepresented groups would likely experience the pandemic differently.
The mean age of the 5,951 (21%) faculty, staff, and trainees who completed the survey was 40 years. A majority of respondents were women, reflecting the higher proportion of women within the health system.
A majority (86%) identified as White or European American. About two-thirds of respondents (66%) were staff, 16% were faculty, and 13% were trainees.
COVID-19 career concerns
Overall, 1,061 respondents (21%) “moderately or very seriously” considered leaving the workforce and 1,505 (30%) considered reducing hours. Respondents who were younger, married, a member of an underrepresented racial/ethnic group, and worked in a clinical setting were more likely to consider leaving the workforce.
The survey showed 27% felt their productivity increased whereas 39% believed their productivity decreased.
Of the 2,412 survey participants with children aged 18 years or younger, 66% reported that they did not have child care fully available.
“Failure to address and provide for child care has long been one of the many significant deficits in U.S. health care systems,” said Dr. Bernstein, lead author of a March 2021 report evaluating staff emotional support at Montefiore Medical Center during the pandemic in The Joint Commission Journal on Quality and Patient Safety.
Furthermore, 47% were “moderately or very seriously worried” about COVID-19 impacting their career development.
Women trainees were significantly more likely than male counterparts to consider leaving the workforce and reducing their work hours. Women in a faculty or trainee role were also more likely to worry about COVID-19’s impact on their career, compared with men, and compared with women in staff positions.
“It was disheartening to have our data support the gender and racial/ethnic disparity that has been highlighted in the media during the pandemic,” Dr. Delaney said. “Women and in some cases racial/ethnic groups that are underrepresented in medicine were most likely to consider leaving the workforce, reducing hours, and were worried about their career development.
“It is critical that we strategically address these important disparities,” she said.
Women also are disproportionately affected by burnout, particularly during the pandemic, according to an analysis of Medscape’s Physician Burnout and Suicide Report.
Furthermore, the COVID-19 pandemic has shifted the medical specialties now considered highest risk for burnout: critical care physicians ranked first in the report, followed by rheumatologists and infectious disease specialists.
Potential solutions
“Given the disproportionate impact COVID-19 has on employees of health systems, institutions must find ways to support their employees, both in terms of workplace cultural adaptations and assistance with familial responsibilities,” the researchers noted.
Telecommuting policies, scheduling flexibility, and expanding employee support programs are potential solutions. Institutional policies also could address the educational and direct care needs of employee children.
Limitations of the study include its generalizability beyond employees of University of Utah Health. Also, respondents included a lower proportion of racial and ethnic groups, compared with national figures, “although this is mostly accounted for by the overall low population of such groups in the state of Utah,” the researchers added.
“Our results suggest that respondents were struggling during the COVID-19 pandemic,” the researchers noted. “As a result, even after investing substantial amounts of time in years of training, many were considering leaving the workforce because of stress and caregiving responsibilities related to the pandemic.”
The Jon M. Huntsman Presidential Endowed Chair supported the work with a financial award to Dr. Fagerlin. Dr. Delaney and Dr. Bernstein disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
a new survey of more than 5,000 clinicians at an academic medical center illustrates.
About one in five people reported considering leaving the workforce because of the challenges of working during the COVID-19 pandemic. In addition, 30% reported they are considering cutting back work hours.
“There are a substantial number of employees and trainees who are experiencing major stress and work disruptions because of the pandemic,” lead author Rebecca K. Delaney, PhD, said in an interview. “It is particularly alarming that people who have spent 5 or more years in training for their specialty are struggling with their work, so much so that they have even considered leaving the workforce or reducing their hours.”
“Being a caregiver adds another layer of difficulty for faculty, staff, and trainees who are trying to manage work and child care,” added Dr. Delaney, a researcher in the department of population health sciences, University of Utah, Salt Lake City.
The study was published online April 2 in JAMA Network Open.
“This looks like an excellent survey,” Carol A Bernstein, MD, said in an interview when asked to comment. “I do not think it provides particularly new information as these challenges in the workplace, especially for women during COVID, have been well documented in the media and the medical literature to date.”
“That said, to the extent that data helps drive solutions, I would hope that information such as this would be considered as strong further evidence that health care systems must pay close attention to the wellbeing of the workforce,” added Dr. Bernstein, professor and vice chair of faculty development and well-being, departments of psychiatry and behavioral sciences and obstetrics and gynecology and women’s health, Montefiore Medical Center/Albert Einstein College of Medicine, New York.
When the pandemic hits home
A total of 42% of the American workforce rapidly transitioned to working from home at the onset of the COVID-19 pandemic. At the same time, many employees had to provide child care and assistance with schoolwork. This placed a burden on many individuals at academic medical centers, and women in particular.
“Women comprise 74.9% of hospital employees, many of whom are essential clinical workers,” the researchers noted. “The extent of the needs and difficulties for these workers during the pandemic remain largely unknown.”
To learn more, Dr. Delaney, senior author Angie Fagerlin, PhD, and their colleagues emailed a Qualtrics survey to 27,700 faculty, staff, and trainees at University of Utah Health. The survey was conducted Aug. 5-20, 2020 as part of a quality improvement initiative. All responses were anonymous.
Survey questions included if, because of the pandemic, people had considered leaving the workforce, considered reducing their hours, or experienced reduced productivity. The researchers also asked about career impacts and potential solutions in terms of “work culture adaptations.”
Respondents with children aged under 18 years also were asked about child care options. Dr. Delaney and colleagues also inquired about race and ethnicity because they hypothesized that employees from underrepresented groups would likely experience the pandemic differently.
The mean age of the 5,951 (21%) faculty, staff, and trainees who completed the survey was 40 years. A majority of respondents were women, reflecting the higher proportion of women within the health system.
A majority (86%) identified as White or European American. About two-thirds of respondents (66%) were staff, 16% were faculty, and 13% were trainees.
COVID-19 career concerns
Overall, 1,061 respondents (21%) “moderately or very seriously” considered leaving the workforce and 1,505 (30%) considered reducing hours. Respondents who were younger, married, a member of an underrepresented racial/ethnic group, and worked in a clinical setting were more likely to consider leaving the workforce.
The survey showed 27% felt their productivity increased whereas 39% believed their productivity decreased.
Of the 2,412 survey participants with children aged 18 years or younger, 66% reported that they did not have child care fully available.
“Failure to address and provide for child care has long been one of the many significant deficits in U.S. health care systems,” said Dr. Bernstein, lead author of a March 2021 report evaluating staff emotional support at Montefiore Medical Center during the pandemic in The Joint Commission Journal on Quality and Patient Safety.
Furthermore, 47% were “moderately or very seriously worried” about COVID-19 impacting their career development.
Women trainees were significantly more likely than male counterparts to consider leaving the workforce and reducing their work hours. Women in a faculty or trainee role were also more likely to worry about COVID-19’s impact on their career, compared with men, and compared with women in staff positions.
“It was disheartening to have our data support the gender and racial/ethnic disparity that has been highlighted in the media during the pandemic,” Dr. Delaney said. “Women and in some cases racial/ethnic groups that are underrepresented in medicine were most likely to consider leaving the workforce, reducing hours, and were worried about their career development.
“It is critical that we strategically address these important disparities,” she said.
Women also are disproportionately affected by burnout, particularly during the pandemic, according to an analysis of Medscape’s Physician Burnout and Suicide Report.
Furthermore, the COVID-19 pandemic has shifted the medical specialties now considered highest risk for burnout: critical care physicians ranked first in the report, followed by rheumatologists and infectious disease specialists.
Potential solutions
“Given the disproportionate impact COVID-19 has on employees of health systems, institutions must find ways to support their employees, both in terms of workplace cultural adaptations and assistance with familial responsibilities,” the researchers noted.
Telecommuting policies, scheduling flexibility, and expanding employee support programs are potential solutions. Institutional policies also could address the educational and direct care needs of employee children.
Limitations of the study include its generalizability beyond employees of University of Utah Health. Also, respondents included a lower proportion of racial and ethnic groups, compared with national figures, “although this is mostly accounted for by the overall low population of such groups in the state of Utah,” the researchers added.
“Our results suggest that respondents were struggling during the COVID-19 pandemic,” the researchers noted. “As a result, even after investing substantial amounts of time in years of training, many were considering leaving the workforce because of stress and caregiving responsibilities related to the pandemic.”
The Jon M. Huntsman Presidential Endowed Chair supported the work with a financial award to Dr. Fagerlin. Dr. Delaney and Dr. Bernstein disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Deaths tied to reprocessed urologic endoscopes, FDA warns
The U.S. Food and Drug Administration is warning health care providers about the risk for potentially life-threatening infections associated with reprocessed endoscopes used for viewing the urinary tract, including cystoscopes, cystouerthroscopes, and ureteroscopes.
The federal agency is investigating more than 450 medical device reports, including three reports of deaths, received between Jan. 1, 2017, and Feb. 20, 2021, that describe post-procedure infections and other possible contamination problems associated with the reprocessing or cleaning and sterilization of the devices.
Although it’s early in the investigation, on the basis of available data, the FDA believes the risk for infection is low.
“We are very concerned about the three reported deaths – outside of the United States – associated with these infections, and we’re acting fast to communicate with health care providers and the public about what we know and what is still an emerging issue,” Jeff Shuren, MD, JD, director of the FDA’s Center for Devices and Radiological Health, said in a statement released on April 1.
Manufacturer Olympus Corporation submitted three reports of deaths attributed to a bacterial infection. In two of those reports, the infection was linked to a forceps/irrigation plug, an accessory component used to control water flow and enable access to the working channel of the endoscope. Lab tests confirmed that the bacteria that caused the infection was present in the forceps/irrigation plug.
The FDA said the third victim’s death involved a cystoscope that did not pass a leak test. It is possible that the damaged device was a factor in the patient’s becoming infected.
It’s not known to what degree the reported infections or patient comorbidities played a part in the patient deaths. The FDA also hasn’t concluded that any specific manufacturer or brand of these devices is associated with higher risks than others.
The FDA released recommendations for processing and using these devices and emphasized the importance of following manufacturers’ labeling and reprocessing instructions to minimize the risk for infection.
In addition to following reprocessing instructions, the recommendations include not using a device that has failed a leak test, developing schedules for routine device inspection and maintenance, and discussing the potential benefits and risks associated with procedures involving reprocessed urologic endoscopes with patients.
The newly reported concerns with urologic endoscopes are similar to problems associated with reprocessed duodenoscopes. In 2018, the FDA warned about higher-than-expected contamination rates for reprocessed duodenoscopes. The FDA has taken action on infections related to the reprocessing of duodenoscopes. In 2015, it required postmarket safety studies and the updating of sampling and culturing protocols. In 2019, the FDA approved single-use duodenoscopes in an effort to curb infections.
A version of this article first appeared on Medscape.com.
The U.S. Food and Drug Administration is warning health care providers about the risk for potentially life-threatening infections associated with reprocessed endoscopes used for viewing the urinary tract, including cystoscopes, cystouerthroscopes, and ureteroscopes.
The federal agency is investigating more than 450 medical device reports, including three reports of deaths, received between Jan. 1, 2017, and Feb. 20, 2021, that describe post-procedure infections and other possible contamination problems associated with the reprocessing or cleaning and sterilization of the devices.
Although it’s early in the investigation, on the basis of available data, the FDA believes the risk for infection is low.
“We are very concerned about the three reported deaths – outside of the United States – associated with these infections, and we’re acting fast to communicate with health care providers and the public about what we know and what is still an emerging issue,” Jeff Shuren, MD, JD, director of the FDA’s Center for Devices and Radiological Health, said in a statement released on April 1.
Manufacturer Olympus Corporation submitted three reports of deaths attributed to a bacterial infection. In two of those reports, the infection was linked to a forceps/irrigation plug, an accessory component used to control water flow and enable access to the working channel of the endoscope. Lab tests confirmed that the bacteria that caused the infection was present in the forceps/irrigation plug.
The FDA said the third victim’s death involved a cystoscope that did not pass a leak test. It is possible that the damaged device was a factor in the patient’s becoming infected.
It’s not known to what degree the reported infections or patient comorbidities played a part in the patient deaths. The FDA also hasn’t concluded that any specific manufacturer or brand of these devices is associated with higher risks than others.
The FDA released recommendations for processing and using these devices and emphasized the importance of following manufacturers’ labeling and reprocessing instructions to minimize the risk for infection.
In addition to following reprocessing instructions, the recommendations include not using a device that has failed a leak test, developing schedules for routine device inspection and maintenance, and discussing the potential benefits and risks associated with procedures involving reprocessed urologic endoscopes with patients.
The newly reported concerns with urologic endoscopes are similar to problems associated with reprocessed duodenoscopes. In 2018, the FDA warned about higher-than-expected contamination rates for reprocessed duodenoscopes. The FDA has taken action on infections related to the reprocessing of duodenoscopes. In 2015, it required postmarket safety studies and the updating of sampling and culturing protocols. In 2019, the FDA approved single-use duodenoscopes in an effort to curb infections.
A version of this article first appeared on Medscape.com.
The U.S. Food and Drug Administration is warning health care providers about the risk for potentially life-threatening infections associated with reprocessed endoscopes used for viewing the urinary tract, including cystoscopes, cystouerthroscopes, and ureteroscopes.
The federal agency is investigating more than 450 medical device reports, including three reports of deaths, received between Jan. 1, 2017, and Feb. 20, 2021, that describe post-procedure infections and other possible contamination problems associated with the reprocessing or cleaning and sterilization of the devices.
Although it’s early in the investigation, on the basis of available data, the FDA believes the risk for infection is low.
“We are very concerned about the three reported deaths – outside of the United States – associated with these infections, and we’re acting fast to communicate with health care providers and the public about what we know and what is still an emerging issue,” Jeff Shuren, MD, JD, director of the FDA’s Center for Devices and Radiological Health, said in a statement released on April 1.
Manufacturer Olympus Corporation submitted three reports of deaths attributed to a bacterial infection. In two of those reports, the infection was linked to a forceps/irrigation plug, an accessory component used to control water flow and enable access to the working channel of the endoscope. Lab tests confirmed that the bacteria that caused the infection was present in the forceps/irrigation plug.
The FDA said the third victim’s death involved a cystoscope that did not pass a leak test. It is possible that the damaged device was a factor in the patient’s becoming infected.
It’s not known to what degree the reported infections or patient comorbidities played a part in the patient deaths. The FDA also hasn’t concluded that any specific manufacturer or brand of these devices is associated with higher risks than others.
The FDA released recommendations for processing and using these devices and emphasized the importance of following manufacturers’ labeling and reprocessing instructions to minimize the risk for infection.
In addition to following reprocessing instructions, the recommendations include not using a device that has failed a leak test, developing schedules for routine device inspection and maintenance, and discussing the potential benefits and risks associated with procedures involving reprocessed urologic endoscopes with patients.
The newly reported concerns with urologic endoscopes are similar to problems associated with reprocessed duodenoscopes. In 2018, the FDA warned about higher-than-expected contamination rates for reprocessed duodenoscopes. The FDA has taken action on infections related to the reprocessing of duodenoscopes. In 2015, it required postmarket safety studies and the updating of sampling and culturing protocols. In 2019, the FDA approved single-use duodenoscopes in an effort to curb infections.
A version of this article first appeared on Medscape.com.
List of COVID-19 high-risk comorbidities expanded
The list of medical according to the Centers for Disease Control and Prevention.
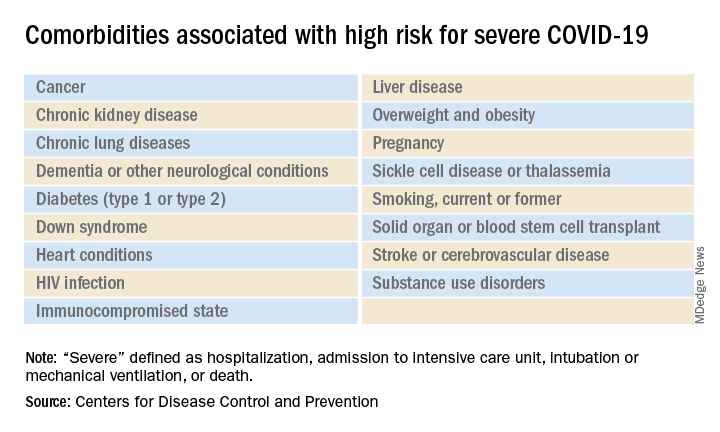
The CDC’s latest list consists of 17 conditions or groups of related conditions that may increase patients’ risk of developing severe outcomes of COVID-19, the CDC said on a web page intended for the general public.
On a separate page, the CDC defines severe outcomes “as hospitalization, admission to the intensive care unit, intubation or mechanical ventilation, or death.”
Asthma is included in the newly expanded list with other chronic lung diseases such as chronic obstructive pulmonary disease and cystic fibrosis; the list’s heart disease entry covers coronary artery disease, heart failure, cardiomyopathies, and hypertension, the CDC said.
The list of medical according to the Centers for Disease Control and Prevention.

The CDC’s latest list consists of 17 conditions or groups of related conditions that may increase patients’ risk of developing severe outcomes of COVID-19, the CDC said on a web page intended for the general public.
On a separate page, the CDC defines severe outcomes “as hospitalization, admission to the intensive care unit, intubation or mechanical ventilation, or death.”
Asthma is included in the newly expanded list with other chronic lung diseases such as chronic obstructive pulmonary disease and cystic fibrosis; the list’s heart disease entry covers coronary artery disease, heart failure, cardiomyopathies, and hypertension, the CDC said.
The list of medical according to the Centers for Disease Control and Prevention.

The CDC’s latest list consists of 17 conditions or groups of related conditions that may increase patients’ risk of developing severe outcomes of COVID-19, the CDC said on a web page intended for the general public.
On a separate page, the CDC defines severe outcomes “as hospitalization, admission to the intensive care unit, intubation or mechanical ventilation, or death.”
Asthma is included in the newly expanded list with other chronic lung diseases such as chronic obstructive pulmonary disease and cystic fibrosis; the list’s heart disease entry covers coronary artery disease, heart failure, cardiomyopathies, and hypertension, the CDC said.
Risk for erectile dysfunction sixfold higher in men with COVID-19
COVID-19 increases the risk of developing erectile dysfunction (ED) by nearly sixfold, according to data from the first study to investigate the association between ED and COVID-19 in young men in a real-life setting.
Men with ED are more than five times more likely to have COVID-19 (odds ratio, 5.27).
For men with a history of COVID-19, the odds ratio of developing ED was 5.66. The strength of the association remained after adjusting for factors considered to affect ED.
The study, which was led by Emmanuele A. Jannini, MD, professor of endocrinology and medical sexology, University of Rome Tor Vergata, was published on March 20 in Andrology.
‘Mask up to keep it up’
ED can be both a short-term and a long-term complication of COVID-19, Dr. Jannini suggests.
“When offered, men should have the COVID vaccination. It also gives a whole new meaning to wearing the mask – mask up to keep it up,” he said. “It could possibly have the added benefit of preventing sexual dysfunction.”
He points out that older age, diabetes, high body mass index, and smoking increase the risk of contracting COVID-19.
“These are the same as risk factors for ED. Results of our study agree with the pathophysiological mechanisms linking ED, endothelial dysfunction, and COVID-19. Basically, endothelial dysfunction is common in both conditions [COVID-10 and ED].
“We would like to find some sort of biomarker of endothelial dysfunction post COVID, because it seems that there are many sequelae that coexist for a long time after infection,” added Dr. Jannini. “Asking a patient if they have ED after COVID might provide a measure of systemic wellness.”
Allan Pacey, MD, professor of andrology at the University of Sheffield (England), welcomed the research, noting, “This seems to be a well-conducted study. However, at the moment, the relationship is just a correlation, and it might be that some of the comorbidities that increased the men’s chances of getting a significant COVID-19 infection may have also independently increased their chances of erectile dysfunction.
“But the authors offer a plausible mechanism by which COVID-19 may impact directly on erectile function,” agrees Dr. Pacey. However, “There’s more work to be done,” he said. “I’d also argue it’s a good reason for men to wear a mask, practice social distancing, and take the vaccine when it’s offered to them.”
Urologist John Mulhall, MD, from Memorial Sloan Kettering Cancer Center, New York, remarked, “It was a highly preliminary study, but the data are suggestive of a potential link between COVID-19 infection and ED.
“However, it raises enough questions such that further large, more long-term analyses are required to define causation. Future studies assessing testosterone levels and erectile hemodynamics will be needed to provide definite evidence of a causative link,» he stressed.
Erectile problems a ‘hallmark’ of systemic endothelial dysfunction
Prior research has suggested that asymptomatic COVID-19 could be associated with subclinical microvascular involvement with long-term cardiovascular effects.
“Indeed, COVID-19 is by all means an endothelial disease, in which systemic manifestations ... can potentially be due to alterations in the endothelial thrombotic/fibrinolytic balance,” emphasized Dr. Jannini. “In addition, endothelial cells express many of the cofactors used by SARS-CoV-2 to invade host cells.
“Erectile dysfunction has often been considered a hallmark of endothelial dysfunction, and as such, a potential association between ED and COVID-19 has also been postulated and underpinned the investigation in this study,” he explained.
The study was predicated on the fact that ED is often considered a clinical marker of impaired overall health status, which often features cardiovascular events at an early age. It aimed to investigate the bidirectional relationship between COVID-19 and ED. It asked whether ED could be a risk factor for contracting COVID-19 and whether having COVID-19 predisposes to developing ED.
“This would possibly suggest that men with ED, due to the underlying conditions which impair erectile response, could also be more susceptible to contracting COVID-19,” said Dr. Jannini.
Data were drawn from the Sex@COVID online survey, which was conducted from April 7 to May 4, 2020, in Italy. The survey included 6,821 participants aged 18 years or older (4,177 women; 2,644 men; mean age, 32.83 ± 11.24 years). Participants were stratified on the basis of marital status and sexual activity during lockdown. From these participants, 985 sexually active men were identified, among whom 25 (2.54%) reported having tested positive for COVID-19. These persons were matched with 75 COVID-19–negative men using propensity score matching in a 1:3 ratio.
The researchers used standardized psychometric tools to measure the effects of lockdown and social distancing on the intrapsychic, relational, and sexual health of the participants.
Erectile function was measured with the International Index of Erectile Function or the Sexual Health Inventory for Men, which are often used in clinical settings. In light of the two-way interaction between sexual activity and psychological well-being, results were adjusted for any influence of anxiety and depression, which were measured with recognized scales for use in patients with a history of COVID-19.
Results showed that the prevalence of ED was significantly higher among men who self-reported a history of COVID-19, compared with a matching COVID-negative population (28% vs. 9.33%; P = .027).
After adjusting for variables that are considered to have a bearing on the development of ED, such as psychological status, age, and BMI, the odds ratio for developing ED after having had COVID-19 was 5.66 (95% confidence interval, 1.50-24.01).
Similarly, after adjusting for age and BMI, men with ED were more likely to have COVID‐19 (OR, 5.27; 95% CI, 1.49-20.09).
The authors note that persons who experience “a sudden onset or worsening of ED might also consider precautionary quarantine or nasopharyngeal swab, as COVID‐19 might act as a potential initiating trigger for the onset of erectile impairment, or an aggravating factor for its progression to more severe forms.”
Similarly, patients who have ED “should consider their erectile impairment as a sign of possible underlying conditions that could increase the likelihood of suffering from COVID‐19,” they write.
Dr. Mulhall highlighted several limitations of the study, including its retrospective nature, recall bias associated with the use of online questionnaires, and the inclusion of COVID‐19 diagnoses that were based on the response to the survey rather than on testing with nasopharyngeal swabs. In addition, comorbidity data were incomplete, and there was no indication of duration after COVID-19 infection, the severity of COVID-19, or the severity of ED.
The authors have disclosed no relevant financial relationships. Dr. Pacey is chairman of the advisory committee of the U.K. National External Quality Assurance Schemes in Andrology, editor-in-chief of Human Fertility, trustee of the Progress Educational Trust, and trustee of the British Fertility Society (all unpaid). Dr. Mulhall has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
COVID-19 increases the risk of developing erectile dysfunction (ED) by nearly sixfold, according to data from the first study to investigate the association between ED and COVID-19 in young men in a real-life setting.
Men with ED are more than five times more likely to have COVID-19 (odds ratio, 5.27).
For men with a history of COVID-19, the odds ratio of developing ED was 5.66. The strength of the association remained after adjusting for factors considered to affect ED.
The study, which was led by Emmanuele A. Jannini, MD, professor of endocrinology and medical sexology, University of Rome Tor Vergata, was published on March 20 in Andrology.
‘Mask up to keep it up’
ED can be both a short-term and a long-term complication of COVID-19, Dr. Jannini suggests.
“When offered, men should have the COVID vaccination. It also gives a whole new meaning to wearing the mask – mask up to keep it up,” he said. “It could possibly have the added benefit of preventing sexual dysfunction.”
He points out that older age, diabetes, high body mass index, and smoking increase the risk of contracting COVID-19.
“These are the same as risk factors for ED. Results of our study agree with the pathophysiological mechanisms linking ED, endothelial dysfunction, and COVID-19. Basically, endothelial dysfunction is common in both conditions [COVID-10 and ED].
“We would like to find some sort of biomarker of endothelial dysfunction post COVID, because it seems that there are many sequelae that coexist for a long time after infection,” added Dr. Jannini. “Asking a patient if they have ED after COVID might provide a measure of systemic wellness.”
Allan Pacey, MD, professor of andrology at the University of Sheffield (England), welcomed the research, noting, “This seems to be a well-conducted study. However, at the moment, the relationship is just a correlation, and it might be that some of the comorbidities that increased the men’s chances of getting a significant COVID-19 infection may have also independently increased their chances of erectile dysfunction.
“But the authors offer a plausible mechanism by which COVID-19 may impact directly on erectile function,” agrees Dr. Pacey. However, “There’s more work to be done,” he said. “I’d also argue it’s a good reason for men to wear a mask, practice social distancing, and take the vaccine when it’s offered to them.”
Urologist John Mulhall, MD, from Memorial Sloan Kettering Cancer Center, New York, remarked, “It was a highly preliminary study, but the data are suggestive of a potential link between COVID-19 infection and ED.
“However, it raises enough questions such that further large, more long-term analyses are required to define causation. Future studies assessing testosterone levels and erectile hemodynamics will be needed to provide definite evidence of a causative link,» he stressed.
Erectile problems a ‘hallmark’ of systemic endothelial dysfunction
Prior research has suggested that asymptomatic COVID-19 could be associated with subclinical microvascular involvement with long-term cardiovascular effects.
“Indeed, COVID-19 is by all means an endothelial disease, in which systemic manifestations ... can potentially be due to alterations in the endothelial thrombotic/fibrinolytic balance,” emphasized Dr. Jannini. “In addition, endothelial cells express many of the cofactors used by SARS-CoV-2 to invade host cells.
“Erectile dysfunction has often been considered a hallmark of endothelial dysfunction, and as such, a potential association between ED and COVID-19 has also been postulated and underpinned the investigation in this study,” he explained.
The study was predicated on the fact that ED is often considered a clinical marker of impaired overall health status, which often features cardiovascular events at an early age. It aimed to investigate the bidirectional relationship between COVID-19 and ED. It asked whether ED could be a risk factor for contracting COVID-19 and whether having COVID-19 predisposes to developing ED.
“This would possibly suggest that men with ED, due to the underlying conditions which impair erectile response, could also be more susceptible to contracting COVID-19,” said Dr. Jannini.
Data were drawn from the Sex@COVID online survey, which was conducted from April 7 to May 4, 2020, in Italy. The survey included 6,821 participants aged 18 years or older (4,177 women; 2,644 men; mean age, 32.83 ± 11.24 years). Participants were stratified on the basis of marital status and sexual activity during lockdown. From these participants, 985 sexually active men were identified, among whom 25 (2.54%) reported having tested positive for COVID-19. These persons were matched with 75 COVID-19–negative men using propensity score matching in a 1:3 ratio.
The researchers used standardized psychometric tools to measure the effects of lockdown and social distancing on the intrapsychic, relational, and sexual health of the participants.
Erectile function was measured with the International Index of Erectile Function or the Sexual Health Inventory for Men, which are often used in clinical settings. In light of the two-way interaction between sexual activity and psychological well-being, results were adjusted for any influence of anxiety and depression, which were measured with recognized scales for use in patients with a history of COVID-19.
Results showed that the prevalence of ED was significantly higher among men who self-reported a history of COVID-19, compared with a matching COVID-negative population (28% vs. 9.33%; P = .027).
After adjusting for variables that are considered to have a bearing on the development of ED, such as psychological status, age, and BMI, the odds ratio for developing ED after having had COVID-19 was 5.66 (95% confidence interval, 1.50-24.01).
Similarly, after adjusting for age and BMI, men with ED were more likely to have COVID‐19 (OR, 5.27; 95% CI, 1.49-20.09).
The authors note that persons who experience “a sudden onset or worsening of ED might also consider precautionary quarantine or nasopharyngeal swab, as COVID‐19 might act as a potential initiating trigger for the onset of erectile impairment, or an aggravating factor for its progression to more severe forms.”
Similarly, patients who have ED “should consider their erectile impairment as a sign of possible underlying conditions that could increase the likelihood of suffering from COVID‐19,” they write.
Dr. Mulhall highlighted several limitations of the study, including its retrospective nature, recall bias associated with the use of online questionnaires, and the inclusion of COVID‐19 diagnoses that were based on the response to the survey rather than on testing with nasopharyngeal swabs. In addition, comorbidity data were incomplete, and there was no indication of duration after COVID-19 infection, the severity of COVID-19, or the severity of ED.
The authors have disclosed no relevant financial relationships. Dr. Pacey is chairman of the advisory committee of the U.K. National External Quality Assurance Schemes in Andrology, editor-in-chief of Human Fertility, trustee of the Progress Educational Trust, and trustee of the British Fertility Society (all unpaid). Dr. Mulhall has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
COVID-19 increases the risk of developing erectile dysfunction (ED) by nearly sixfold, according to data from the first study to investigate the association between ED and COVID-19 in young men in a real-life setting.
Men with ED are more than five times more likely to have COVID-19 (odds ratio, 5.27).
For men with a history of COVID-19, the odds ratio of developing ED was 5.66. The strength of the association remained after adjusting for factors considered to affect ED.
The study, which was led by Emmanuele A. Jannini, MD, professor of endocrinology and medical sexology, University of Rome Tor Vergata, was published on March 20 in Andrology.
‘Mask up to keep it up’
ED can be both a short-term and a long-term complication of COVID-19, Dr. Jannini suggests.
“When offered, men should have the COVID vaccination. It also gives a whole new meaning to wearing the mask – mask up to keep it up,” he said. “It could possibly have the added benefit of preventing sexual dysfunction.”
He points out that older age, diabetes, high body mass index, and smoking increase the risk of contracting COVID-19.
“These are the same as risk factors for ED. Results of our study agree with the pathophysiological mechanisms linking ED, endothelial dysfunction, and COVID-19. Basically, endothelial dysfunction is common in both conditions [COVID-10 and ED].
“We would like to find some sort of biomarker of endothelial dysfunction post COVID, because it seems that there are many sequelae that coexist for a long time after infection,” added Dr. Jannini. “Asking a patient if they have ED after COVID might provide a measure of systemic wellness.”
Allan Pacey, MD, professor of andrology at the University of Sheffield (England), welcomed the research, noting, “This seems to be a well-conducted study. However, at the moment, the relationship is just a correlation, and it might be that some of the comorbidities that increased the men’s chances of getting a significant COVID-19 infection may have also independently increased their chances of erectile dysfunction.
“But the authors offer a plausible mechanism by which COVID-19 may impact directly on erectile function,” agrees Dr. Pacey. However, “There’s more work to be done,” he said. “I’d also argue it’s a good reason for men to wear a mask, practice social distancing, and take the vaccine when it’s offered to them.”
Urologist John Mulhall, MD, from Memorial Sloan Kettering Cancer Center, New York, remarked, “It was a highly preliminary study, but the data are suggestive of a potential link between COVID-19 infection and ED.
“However, it raises enough questions such that further large, more long-term analyses are required to define causation. Future studies assessing testosterone levels and erectile hemodynamics will be needed to provide definite evidence of a causative link,» he stressed.
Erectile problems a ‘hallmark’ of systemic endothelial dysfunction
Prior research has suggested that asymptomatic COVID-19 could be associated with subclinical microvascular involvement with long-term cardiovascular effects.
“Indeed, COVID-19 is by all means an endothelial disease, in which systemic manifestations ... can potentially be due to alterations in the endothelial thrombotic/fibrinolytic balance,” emphasized Dr. Jannini. “In addition, endothelial cells express many of the cofactors used by SARS-CoV-2 to invade host cells.
“Erectile dysfunction has often been considered a hallmark of endothelial dysfunction, and as such, a potential association between ED and COVID-19 has also been postulated and underpinned the investigation in this study,” he explained.
The study was predicated on the fact that ED is often considered a clinical marker of impaired overall health status, which often features cardiovascular events at an early age. It aimed to investigate the bidirectional relationship between COVID-19 and ED. It asked whether ED could be a risk factor for contracting COVID-19 and whether having COVID-19 predisposes to developing ED.
“This would possibly suggest that men with ED, due to the underlying conditions which impair erectile response, could also be more susceptible to contracting COVID-19,” said Dr. Jannini.
Data were drawn from the Sex@COVID online survey, which was conducted from April 7 to May 4, 2020, in Italy. The survey included 6,821 participants aged 18 years or older (4,177 women; 2,644 men; mean age, 32.83 ± 11.24 years). Participants were stratified on the basis of marital status and sexual activity during lockdown. From these participants, 985 sexually active men were identified, among whom 25 (2.54%) reported having tested positive for COVID-19. These persons were matched with 75 COVID-19–negative men using propensity score matching in a 1:3 ratio.
The researchers used standardized psychometric tools to measure the effects of lockdown and social distancing on the intrapsychic, relational, and sexual health of the participants.
Erectile function was measured with the International Index of Erectile Function or the Sexual Health Inventory for Men, which are often used in clinical settings. In light of the two-way interaction between sexual activity and psychological well-being, results were adjusted for any influence of anxiety and depression, which were measured with recognized scales for use in patients with a history of COVID-19.
Results showed that the prevalence of ED was significantly higher among men who self-reported a history of COVID-19, compared with a matching COVID-negative population (28% vs. 9.33%; P = .027).
After adjusting for variables that are considered to have a bearing on the development of ED, such as psychological status, age, and BMI, the odds ratio for developing ED after having had COVID-19 was 5.66 (95% confidence interval, 1.50-24.01).
Similarly, after adjusting for age and BMI, men with ED were more likely to have COVID‐19 (OR, 5.27; 95% CI, 1.49-20.09).
The authors note that persons who experience “a sudden onset or worsening of ED might also consider precautionary quarantine or nasopharyngeal swab, as COVID‐19 might act as a potential initiating trigger for the onset of erectile impairment, or an aggravating factor for its progression to more severe forms.”
Similarly, patients who have ED “should consider their erectile impairment as a sign of possible underlying conditions that could increase the likelihood of suffering from COVID‐19,” they write.
Dr. Mulhall highlighted several limitations of the study, including its retrospective nature, recall bias associated with the use of online questionnaires, and the inclusion of COVID‐19 diagnoses that were based on the response to the survey rather than on testing with nasopharyngeal swabs. In addition, comorbidity data were incomplete, and there was no indication of duration after COVID-19 infection, the severity of COVID-19, or the severity of ED.
The authors have disclosed no relevant financial relationships. Dr. Pacey is chairman of the advisory committee of the U.K. National External Quality Assurance Schemes in Andrology, editor-in-chief of Human Fertility, trustee of the Progress Educational Trust, and trustee of the British Fertility Society (all unpaid). Dr. Mulhall has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
New guidelines on antibiotic prescribing focus on shorter courses
An antibiotic course of 5 days is usually just as effective as longer courses but with fewer side effects and decreased overall antibiotic exposure for a number of common bacterial conditions, according to new clinical guidelines published by the American College of Physicians.
The guidelines focus on treatment of uncomplicated cases involving pneumonia, urinary tract infections (UTIs), cellulitis, chronic obstructive pulmonary disease (COPD) exacerbations, and acute bronchitis. The goal of the guidelines is to continue improving antibiotic stewardship given the increasing threat of antibiotic resistance and the adverse effects of antibiotics.
“Any use of antibiotics (including necessary use) has downstream effects outside of treating infection,” Dawn Nolt, MD, MPH, a professor of pediatric infection disease at Oregon Health & Science University, Portland, said in an interview. Dr. Nolt was not involved in developing these guidelines. “Undesirable outcomes include allergic reactions, diarrhea, and antibiotic-resistant bacteria. When we reduce unnecessary antibiotic, we reduce undesirable outcomes,” she said.
According to background information in the paper, 1 in 10 patients receives an antibiotic prescription during visits, yet nearly a third of these (30%) are unnecessary and last too long, especially for sinusitis and bronchitis. Meanwhile, overuse of antibiotics, particularly broad-spectrum ones, leads to resistance and adverse effects in up to 20% of patients.
“Prescribing practices can vary based on the type of provider, the setting where the antibiotic is being prescribed, what geographic area you are looking at, the medical reason for which the antibiotic is being prescribed, the actual germ being targeted, and the type of patient,” Dr. Nolt said. “But this variability can be reduced when prescribing providers are aware and follow best practice standards as through this article.”
The new ACP guidelines are a distillation of recommendations from preexisting infectious disease organizations, Dr. Nolt said, but aimed specifically at those practicing internal medicine.
“We define appropriate antibiotic use as prescribing the right antibiotic at the right dose for the right duration for a specific condition,” Rachael A. Lee, MD, MSPH, of the University of Alabama at Birmingham, and colleagues wrote in the article detailing the new guidelines. “Despite evidence and guidelines supporting shorter durations of antibiotic use, many physicians do not prescribe short-course therapy, frequently defaulting to 10-day courses regardless of the condition.”
The reasons for this default response vary. Though some clinicians prescribe longer courses specifically to prevent antibiotic resistance, no evidence shows that continuing to take antibiotics after symptoms have resolved actually reduces likelihood of resistance, the authors noted.
“In fact, resistance is a documented side effect of prolonged antibiotic use due to natural selection pressure,” they wrote.
Another common reason is habit.
“This was the ‘conventional wisdom’ for so long, just trying to make sure all bacteria causing the infection were completely eradicated, with no stragglers that had been exposed to the antibiotic but were not gone and now could evolve into resistant organisms,” Jacqueline W. Fincher, MD, a primary care physician and president of the ACP, said in an interview. “While antibiotic stewardship has been very important for over a decade, we now have more recent head-to-head studies/data showing that, in these four conditions, shorter courses of treatment are just as efficacious with less side effects and adverse events.”
The researchers reviewed all existing clinical guidelines related to bronchitis with COPD exacerbations, community-acquired pneumonia, UTIs, and cellulitis, as well as any other relevant studies in the literature. Although they did not conduct a formal systematic review, they compiled the guidelines specifically for all internists, family physicians and other clinicians caring for patients with these conditions.
“Although most patients with these infections will be seen in the outpatient setting, these best-practice advice statements also apply to patients who present in the inpatient setting,” the authors wrote. They also note the importance of ensuring the patient has the correct diagnosis and appropriate corresponding antibiotic prescription. “If a patient is not improving with appropriate antibiotics, it is important for the clinician to reassess for other causes of symptoms rather than defaulting to a longer duration of antibiotic therapy,” they wrote, calling a longer course “the exception and not the rule.”
Acute bronchitis with COPD exacerbations
Antibiotic treatment for COPD exacerbations and acute uncomplicated bronchitis with signs of a bacterial infection should last no longer than 5 days. The authors define this condition as an acute respiratory infection with a normal chest x-ray, most often caused by a virus. Although patients with bronchitis do not automatically need antibiotics if there’s no evidence of pneumonia, the authors did advise antibiotics in cases involving COPD and a high likelihood of bacterial infection. Clinicians should base their choice of antibiotics on the most common bacterial etiology: Haemophilus influenzae, Streptococcus pneumoniae, and Moraxella catarrhalis. Ideal candidates for therapy may include aminopenicillin with clavulanic acid, a macrolide, or a tetracycline.
Community-acquired pneumonia
The initial course of antibiotics should be at least 5 days for pneumonia and only extended after considering validated evidence of the patient’s clinical stability, such as resuming normal vital signs, mental activity, and the ability to eat. Multiple randomized, controlled trials have shown no improved benefit from longer courses, though longer courses are linked to increased adverse events and mortality.
Again, antibiotics used should “cover common pathogens, such as S. pneumoniae, H. influenzae, Mycoplasma pneumoniae, and Staphylococcus aureus, and atypical pathogens, such as Legionella species,” the authors wrote. Options include “amoxicillin, doxycycline, or a macrolide for healthy adults or a beta-lactam with a macrolide or a respiratory fluoroquinolone in patients with comorbidities.”
UTIs: Uncomplicated cystitis and pyelonephritis
For women’s bacterial cystitis – 75% of which is caused by Escherichia coli – the guidelines recommend nitrofurantoin for 5 days, trimethoprim-sulfamethoxazole for 3 days, or fosfomycin as a single dose. For uncomplicated pyelonephritis in both men and women, clinicians can consider fluoroquinolones for 5-7 days or trimethoprim-sulfamethoxazole for 14 days, depending on antibiotic susceptibility.
This recommendation does not include UTIs in women who are pregnant or UTIs with other functional abnormalities present, such as obstruction. The authors also intentionally left out acute bacterial prostatitis because of its complexity and how long it can take to treat.
Cellulitis
MRSA, which has been increasing in prevalence, is a leading cause of skin and soft-tissue infections, such as necrotizing infections, cellulitis, and erysipelas. Unless the patient has penetrating trauma, evidence of MRSA infection elsewhere, injection drug use, nasal colonization of MRSA, or systemic inflammatory response syndrome, the guidelines recommend a 5- to 6-day course of cephalosporin, penicillin, or clindamycin, extended only if the infection has not improved in 5 days. Further research can narrow down the most appropriate treatment course.
This guidance does not apply to purulent cellulitis, such as conditions with abscesses, furuncles, or carbuncles that typically require incision and drainage.
Continuing to get the message out
Dr. Fincher emphasized the importance of continuing to disseminate messaging for clinicians about reducing unnecessary antibiotic use.
“In medicine we are constantly bombarded with new information. It is those patients and disease states that we see and treat every day that are especially important for us as physicians and other clinicians to keep our skills and knowledge base up to date when it comes to use of antibiotics,” Dr. Fincher said in an interview. “We just need to continue to educate and push out the data, guidelines, and recommendations.”
Dr. Nolt added that it’s important to emphasize how to translate these national recommendations into local practices since local guidance can also raise awareness and encourage local compliance.
Other strategies for reducing overuse of antibiotics “include restriction on antibiotics available at health care systems (formulary restriction), not allowing use of antibiotics unless there is discussion about the patient’s case (preauthorization), and reviewing cases of patients on antibiotics and advising on next steps (prospective audit and feedback),” she said.
The research was funded by the ACP. Dr. Lee has received personal fees from this news organization and Prime Education. Dr. Fincher owns stock in Johnson & Johnson and Procter and Gamble. Dr. Nolt and the article’s coauthors disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
An antibiotic course of 5 days is usually just as effective as longer courses but with fewer side effects and decreased overall antibiotic exposure for a number of common bacterial conditions, according to new clinical guidelines published by the American College of Physicians.
The guidelines focus on treatment of uncomplicated cases involving pneumonia, urinary tract infections (UTIs), cellulitis, chronic obstructive pulmonary disease (COPD) exacerbations, and acute bronchitis. The goal of the guidelines is to continue improving antibiotic stewardship given the increasing threat of antibiotic resistance and the adverse effects of antibiotics.
“Any use of antibiotics (including necessary use) has downstream effects outside of treating infection,” Dawn Nolt, MD, MPH, a professor of pediatric infection disease at Oregon Health & Science University, Portland, said in an interview. Dr. Nolt was not involved in developing these guidelines. “Undesirable outcomes include allergic reactions, diarrhea, and antibiotic-resistant bacteria. When we reduce unnecessary antibiotic, we reduce undesirable outcomes,” she said.
According to background information in the paper, 1 in 10 patients receives an antibiotic prescription during visits, yet nearly a third of these (30%) are unnecessary and last too long, especially for sinusitis and bronchitis. Meanwhile, overuse of antibiotics, particularly broad-spectrum ones, leads to resistance and adverse effects in up to 20% of patients.
“Prescribing practices can vary based on the type of provider, the setting where the antibiotic is being prescribed, what geographic area you are looking at, the medical reason for which the antibiotic is being prescribed, the actual germ being targeted, and the type of patient,” Dr. Nolt said. “But this variability can be reduced when prescribing providers are aware and follow best practice standards as through this article.”
The new ACP guidelines are a distillation of recommendations from preexisting infectious disease organizations, Dr. Nolt said, but aimed specifically at those practicing internal medicine.
“We define appropriate antibiotic use as prescribing the right antibiotic at the right dose for the right duration for a specific condition,” Rachael A. Lee, MD, MSPH, of the University of Alabama at Birmingham, and colleagues wrote in the article detailing the new guidelines. “Despite evidence and guidelines supporting shorter durations of antibiotic use, many physicians do not prescribe short-course therapy, frequently defaulting to 10-day courses regardless of the condition.”
The reasons for this default response vary. Though some clinicians prescribe longer courses specifically to prevent antibiotic resistance, no evidence shows that continuing to take antibiotics after symptoms have resolved actually reduces likelihood of resistance, the authors noted.
“In fact, resistance is a documented side effect of prolonged antibiotic use due to natural selection pressure,” they wrote.
Another common reason is habit.
“This was the ‘conventional wisdom’ for so long, just trying to make sure all bacteria causing the infection were completely eradicated, with no stragglers that had been exposed to the antibiotic but were not gone and now could evolve into resistant organisms,” Jacqueline W. Fincher, MD, a primary care physician and president of the ACP, said in an interview. “While antibiotic stewardship has been very important for over a decade, we now have more recent head-to-head studies/data showing that, in these four conditions, shorter courses of treatment are just as efficacious with less side effects and adverse events.”
The researchers reviewed all existing clinical guidelines related to bronchitis with COPD exacerbations, community-acquired pneumonia, UTIs, and cellulitis, as well as any other relevant studies in the literature. Although they did not conduct a formal systematic review, they compiled the guidelines specifically for all internists, family physicians and other clinicians caring for patients with these conditions.
“Although most patients with these infections will be seen in the outpatient setting, these best-practice advice statements also apply to patients who present in the inpatient setting,” the authors wrote. They also note the importance of ensuring the patient has the correct diagnosis and appropriate corresponding antibiotic prescription. “If a patient is not improving with appropriate antibiotics, it is important for the clinician to reassess for other causes of symptoms rather than defaulting to a longer duration of antibiotic therapy,” they wrote, calling a longer course “the exception and not the rule.”
Acute bronchitis with COPD exacerbations
Antibiotic treatment for COPD exacerbations and acute uncomplicated bronchitis with signs of a bacterial infection should last no longer than 5 days. The authors define this condition as an acute respiratory infection with a normal chest x-ray, most often caused by a virus. Although patients with bronchitis do not automatically need antibiotics if there’s no evidence of pneumonia, the authors did advise antibiotics in cases involving COPD and a high likelihood of bacterial infection. Clinicians should base their choice of antibiotics on the most common bacterial etiology: Haemophilus influenzae, Streptococcus pneumoniae, and Moraxella catarrhalis. Ideal candidates for therapy may include aminopenicillin with clavulanic acid, a macrolide, or a tetracycline.
Community-acquired pneumonia
The initial course of antibiotics should be at least 5 days for pneumonia and only extended after considering validated evidence of the patient’s clinical stability, such as resuming normal vital signs, mental activity, and the ability to eat. Multiple randomized, controlled trials have shown no improved benefit from longer courses, though longer courses are linked to increased adverse events and mortality.
Again, antibiotics used should “cover common pathogens, such as S. pneumoniae, H. influenzae, Mycoplasma pneumoniae, and Staphylococcus aureus, and atypical pathogens, such as Legionella species,” the authors wrote. Options include “amoxicillin, doxycycline, or a macrolide for healthy adults or a beta-lactam with a macrolide or a respiratory fluoroquinolone in patients with comorbidities.”
UTIs: Uncomplicated cystitis and pyelonephritis
For women’s bacterial cystitis – 75% of which is caused by Escherichia coli – the guidelines recommend nitrofurantoin for 5 days, trimethoprim-sulfamethoxazole for 3 days, or fosfomycin as a single dose. For uncomplicated pyelonephritis in both men and women, clinicians can consider fluoroquinolones for 5-7 days or trimethoprim-sulfamethoxazole for 14 days, depending on antibiotic susceptibility.
This recommendation does not include UTIs in women who are pregnant or UTIs with other functional abnormalities present, such as obstruction. The authors also intentionally left out acute bacterial prostatitis because of its complexity and how long it can take to treat.
Cellulitis
MRSA, which has been increasing in prevalence, is a leading cause of skin and soft-tissue infections, such as necrotizing infections, cellulitis, and erysipelas. Unless the patient has penetrating trauma, evidence of MRSA infection elsewhere, injection drug use, nasal colonization of MRSA, or systemic inflammatory response syndrome, the guidelines recommend a 5- to 6-day course of cephalosporin, penicillin, or clindamycin, extended only if the infection has not improved in 5 days. Further research can narrow down the most appropriate treatment course.
This guidance does not apply to purulent cellulitis, such as conditions with abscesses, furuncles, or carbuncles that typically require incision and drainage.
Continuing to get the message out
Dr. Fincher emphasized the importance of continuing to disseminate messaging for clinicians about reducing unnecessary antibiotic use.
“In medicine we are constantly bombarded with new information. It is those patients and disease states that we see and treat every day that are especially important for us as physicians and other clinicians to keep our skills and knowledge base up to date when it comes to use of antibiotics,” Dr. Fincher said in an interview. “We just need to continue to educate and push out the data, guidelines, and recommendations.”
Dr. Nolt added that it’s important to emphasize how to translate these national recommendations into local practices since local guidance can also raise awareness and encourage local compliance.
Other strategies for reducing overuse of antibiotics “include restriction on antibiotics available at health care systems (formulary restriction), not allowing use of antibiotics unless there is discussion about the patient’s case (preauthorization), and reviewing cases of patients on antibiotics and advising on next steps (prospective audit and feedback),” she said.
The research was funded by the ACP. Dr. Lee has received personal fees from this news organization and Prime Education. Dr. Fincher owns stock in Johnson & Johnson and Procter and Gamble. Dr. Nolt and the article’s coauthors disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
An antibiotic course of 5 days is usually just as effective as longer courses but with fewer side effects and decreased overall antibiotic exposure for a number of common bacterial conditions, according to new clinical guidelines published by the American College of Physicians.
The guidelines focus on treatment of uncomplicated cases involving pneumonia, urinary tract infections (UTIs), cellulitis, chronic obstructive pulmonary disease (COPD) exacerbations, and acute bronchitis. The goal of the guidelines is to continue improving antibiotic stewardship given the increasing threat of antibiotic resistance and the adverse effects of antibiotics.
“Any use of antibiotics (including necessary use) has downstream effects outside of treating infection,” Dawn Nolt, MD, MPH, a professor of pediatric infection disease at Oregon Health & Science University, Portland, said in an interview. Dr. Nolt was not involved in developing these guidelines. “Undesirable outcomes include allergic reactions, diarrhea, and antibiotic-resistant bacteria. When we reduce unnecessary antibiotic, we reduce undesirable outcomes,” she said.
According to background information in the paper, 1 in 10 patients receives an antibiotic prescription during visits, yet nearly a third of these (30%) are unnecessary and last too long, especially for sinusitis and bronchitis. Meanwhile, overuse of antibiotics, particularly broad-spectrum ones, leads to resistance and adverse effects in up to 20% of patients.
“Prescribing practices can vary based on the type of provider, the setting where the antibiotic is being prescribed, what geographic area you are looking at, the medical reason for which the antibiotic is being prescribed, the actual germ being targeted, and the type of patient,” Dr. Nolt said. “But this variability can be reduced when prescribing providers are aware and follow best practice standards as through this article.”
The new ACP guidelines are a distillation of recommendations from preexisting infectious disease organizations, Dr. Nolt said, but aimed specifically at those practicing internal medicine.
“We define appropriate antibiotic use as prescribing the right antibiotic at the right dose for the right duration for a specific condition,” Rachael A. Lee, MD, MSPH, of the University of Alabama at Birmingham, and colleagues wrote in the article detailing the new guidelines. “Despite evidence and guidelines supporting shorter durations of antibiotic use, many physicians do not prescribe short-course therapy, frequently defaulting to 10-day courses regardless of the condition.”
The reasons for this default response vary. Though some clinicians prescribe longer courses specifically to prevent antibiotic resistance, no evidence shows that continuing to take antibiotics after symptoms have resolved actually reduces likelihood of resistance, the authors noted.
“In fact, resistance is a documented side effect of prolonged antibiotic use due to natural selection pressure,” they wrote.
Another common reason is habit.
“This was the ‘conventional wisdom’ for so long, just trying to make sure all bacteria causing the infection were completely eradicated, with no stragglers that had been exposed to the antibiotic but were not gone and now could evolve into resistant organisms,” Jacqueline W. Fincher, MD, a primary care physician and president of the ACP, said in an interview. “While antibiotic stewardship has been very important for over a decade, we now have more recent head-to-head studies/data showing that, in these four conditions, shorter courses of treatment are just as efficacious with less side effects and adverse events.”
The researchers reviewed all existing clinical guidelines related to bronchitis with COPD exacerbations, community-acquired pneumonia, UTIs, and cellulitis, as well as any other relevant studies in the literature. Although they did not conduct a formal systematic review, they compiled the guidelines specifically for all internists, family physicians and other clinicians caring for patients with these conditions.
“Although most patients with these infections will be seen in the outpatient setting, these best-practice advice statements also apply to patients who present in the inpatient setting,” the authors wrote. They also note the importance of ensuring the patient has the correct diagnosis and appropriate corresponding antibiotic prescription. “If a patient is not improving with appropriate antibiotics, it is important for the clinician to reassess for other causes of symptoms rather than defaulting to a longer duration of antibiotic therapy,” they wrote, calling a longer course “the exception and not the rule.”
Acute bronchitis with COPD exacerbations
Antibiotic treatment for COPD exacerbations and acute uncomplicated bronchitis with signs of a bacterial infection should last no longer than 5 days. The authors define this condition as an acute respiratory infection with a normal chest x-ray, most often caused by a virus. Although patients with bronchitis do not automatically need antibiotics if there’s no evidence of pneumonia, the authors did advise antibiotics in cases involving COPD and a high likelihood of bacterial infection. Clinicians should base their choice of antibiotics on the most common bacterial etiology: Haemophilus influenzae, Streptococcus pneumoniae, and Moraxella catarrhalis. Ideal candidates for therapy may include aminopenicillin with clavulanic acid, a macrolide, or a tetracycline.
Community-acquired pneumonia
The initial course of antibiotics should be at least 5 days for pneumonia and only extended after considering validated evidence of the patient’s clinical stability, such as resuming normal vital signs, mental activity, and the ability to eat. Multiple randomized, controlled trials have shown no improved benefit from longer courses, though longer courses are linked to increased adverse events and mortality.
Again, antibiotics used should “cover common pathogens, such as S. pneumoniae, H. influenzae, Mycoplasma pneumoniae, and Staphylococcus aureus, and atypical pathogens, such as Legionella species,” the authors wrote. Options include “amoxicillin, doxycycline, or a macrolide for healthy adults or a beta-lactam with a macrolide or a respiratory fluoroquinolone in patients with comorbidities.”
UTIs: Uncomplicated cystitis and pyelonephritis
For women’s bacterial cystitis – 75% of which is caused by Escherichia coli – the guidelines recommend nitrofurantoin for 5 days, trimethoprim-sulfamethoxazole for 3 days, or fosfomycin as a single dose. For uncomplicated pyelonephritis in both men and women, clinicians can consider fluoroquinolones for 5-7 days or trimethoprim-sulfamethoxazole for 14 days, depending on antibiotic susceptibility.
This recommendation does not include UTIs in women who are pregnant or UTIs with other functional abnormalities present, such as obstruction. The authors also intentionally left out acute bacterial prostatitis because of its complexity and how long it can take to treat.
Cellulitis
MRSA, which has been increasing in prevalence, is a leading cause of skin and soft-tissue infections, such as necrotizing infections, cellulitis, and erysipelas. Unless the patient has penetrating trauma, evidence of MRSA infection elsewhere, injection drug use, nasal colonization of MRSA, or systemic inflammatory response syndrome, the guidelines recommend a 5- to 6-day course of cephalosporin, penicillin, or clindamycin, extended only if the infection has not improved in 5 days. Further research can narrow down the most appropriate treatment course.
This guidance does not apply to purulent cellulitis, such as conditions with abscesses, furuncles, or carbuncles that typically require incision and drainage.
Continuing to get the message out
Dr. Fincher emphasized the importance of continuing to disseminate messaging for clinicians about reducing unnecessary antibiotic use.
“In medicine we are constantly bombarded with new information. It is those patients and disease states that we see and treat every day that are especially important for us as physicians and other clinicians to keep our skills and knowledge base up to date when it comes to use of antibiotics,” Dr. Fincher said in an interview. “We just need to continue to educate and push out the data, guidelines, and recommendations.”
Dr. Nolt added that it’s important to emphasize how to translate these national recommendations into local practices since local guidance can also raise awareness and encourage local compliance.
Other strategies for reducing overuse of antibiotics “include restriction on antibiotics available at health care systems (formulary restriction), not allowing use of antibiotics unless there is discussion about the patient’s case (preauthorization), and reviewing cases of patients on antibiotics and advising on next steps (prospective audit and feedback),” she said.
The research was funded by the ACP. Dr. Lee has received personal fees from this news organization and Prime Education. Dr. Fincher owns stock in Johnson & Johnson and Procter and Gamble. Dr. Nolt and the article’s coauthors disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Researchers stress importance of second COVID-19 vaccine dose for infliximab users
Patients being treated with infliximab had weakened immune responses to the first dose of the ChAdOx1 nCoV-19 (Oxford/AstraZeneca) and BNT162b2 (Pfizer/BioNTech) vaccines, compared with patients on vedolizumab (Entyvio), although a very significant number of patients from both groups seroconverted after their second dose, according to a new U.K. study of patients with inflammatory bowel disease (IBD).
“Antibody testing and adapted vaccine schedules should be considered to protect these at-risk patients,” Nicholas A. Kennedy, PhD, MBBS, of the University of Exeter (England) and colleagues wrote in a preprint published March 29 on MedRxiv.
Infliximab is an anti–tumor necrosis factor (anti-TNF) monoclonal antibody that’s approved to treat adult and pediatric Crohn’s disease and ulcerative colitis, as well as rheumatoid arthritis, ankylosing spondylitis, psoriatic arthritis, and plaque psoriasis, whereas vedolizumab, a gut selective anti-integrin alpha4beta7 monoclonal antibody that is not associated with impaired systemic immune responses, is approved to treat Crohn’s disease and ulcerative colitis in adults.
A previous study from Kennedy and colleagues revealed that IBD patients on infliximab showed a weakened COVID-19 antibody response compared with patients on vedolizumab. To determine if treatment with anti-TNF drugs impacted the efficacy of the first shot of these two-dose COVID-19 vaccines, the researchers used data from the CLARITY IBD study to assess 865 infliximab- and 428 vedolizumab-treated participants without evidence of prior SARS-CoV-2 infection who had received uninterrupted biologic therapy since being recruited between Sept. 22 and Dec. 23, 2020.
In the 3-10 weeks after initial vaccination, geometric mean concentrations for SARS-CoV-2 anti-spike protein receptor-binding protein antibodies were lower in patients on infliximab, compared with patients on vedolizumab for both the Pfizer (6.0 U/mL [5.9] versus 28.8 U/mL [5.4], P < .0001) and AstraZeneca (4.7 U/mL [4.9] versus 13.8 U/mL [5.9]; P < .0001) vaccines. The researchers’ multivariable models reinforced those findings, with antibody concentrations lower in infliximab-treated patients for both the Pfizer (fold change, 0.29; 95% confidence interval, 0.21-0.40; P < .0001) and AstraZeneca (FC, 0.39; 95% CI, 0.30-0.51; P < .0001) vaccines.
After second doses of the two-dose Pfizer vaccine, 85% of patients on infliximab and 86% of patients on vedolizumab seroconverted (P = .68); similarly high seroconversion rates were seen in patients who had been infected with SARS-CoV-2 prior to receiving either vaccine. Several patient characteristics were associated with lower antibody concentrations regardless of vaccine type: being 60 years or older, use of immunomodulators, having Crohn’s disease, and being a smoker. Alternatively, non-White ethnicity was associated with higher antibody concentrations.
Evidence has ‘unclear clinical significance’
“These data, which require peer review, do not change my opinion on the safety and efficacy of COVID-19 vaccines in patients taking TNF inhibitors such as infliximab as monotherapy for the treatment of psoriatic disease,” Joel M. Gelfand MD, director of the psoriasis and phototherapy treatment center at the University of Pennsylvania, Philadelphia, said in an interview.
“First, two peer-reviewed studies found good antibody response in patients on TNF inhibitors receiving COVID-19 vaccines (doi: 10.1136/annrheumdis-2021-220289; 10.1136/annrheumdis-2021-220272). Second, antibody responses were robust in the small cohort that received the second dose of a COVID-19 vaccine. We already know that, for the two messenger RNA-based vaccines available under emergency use authorization in the U.S., a second dose is required for optimal efficacy. Thus, evidence of a reduced antibody response after just one dose is of unclear clinical significance. Third, antibody responses are only a surrogate marker, and a low antibody response doesn’t necessarily mean the patient will not be protected by the vaccine.”
Focus on the second dose of a two-dose regimen
“Tell me about the response in people who got both doses of a vaccine that you’re supposed to get both doses of,” Jeffrey Curtis, MD, professor of medicine in the division of clinical immunology and rheumatology at the University of Alabama at Birmingham, said in an interview. “The number of patients in that subset was small [n = 27] but in my opinion that’s the most clinically relevant analysis and the one that patients and clinicians want answered.”
He also emphasized the uncertainty around what ‘protection’ means in these early days of studying COVID-19 vaccine responses. “You can define seroprotection or seroconversion as some absolute level of an antibody response, but if you want to say ‘Mrs. Smith, your antibody level was X,’ on whatever arbitrary scale with whoever’s arbitrary lab test, nobody actually knows that Mrs. Smith is now protected from SARS-CoV-2, or how protected,” he said.
“What is not terribly controversial is: If you can’t detect antibodies, the vaccine didn’t ‘take,’ if you will. But if I tell you that the mean antibody level was X with one drug and then 2X with another drug, does that mean that you’re twice as protected? We don’t know that. I’m fearful that people are looking at these studies and thinking that more is better. It might be, but we don’t know that to be true.”
Debating the cause of weakened immune responses
“The biological plausibility of being on an anti-TNF affecting your immune reaction to a messenger RNA or even a replication-deficient viral vector vaccine doesn’t make sense,” David T. Rubin, MD, professor of medicine at the University of Chicago and chair of the National Scientific Advisory Committee of the Crohn’s and Colitis Foundation, said in an interview.
“I’m sure immunologists may differ with me on this, but given what we have come to appreciate about these vaccine mechanisms, this finding doesn’t make intuitive sense. So we need to make sure that, when this happens, we look to the next studies and try to understand, was there any other confounder that may have resulted in these findings that was not adequately adjusted for or addressed in some other way?
“When you have a study of this size, you argue, ‘Because it’s so large, any effect that was seen must be real,’ ” he added. “Alternatively, to have a study of this size, by its very nature you are limited in being able to control for certain other factors or differences between the groups.”
That said, he commended the authors for their study and acknowledged the potential questions it raises about the single-shot Johnson & Johnson vaccine. “If you only get one and you’re on infliximab, this study implies that maybe that’s not enough,” he said. “Despite the fact that Johnson & Johnson was approved as a single dose, it may be necessary to think about it as the first of two, or maybe it’s not the preferred vaccine in this group of patients.”
The study was supported by the Royal Devon and Exeter and Hull University Hospital Foundation NHS Trusts and unrestricted educational grants from Biogen (Switzerland), Celltrion Healthcare (South Korea), Galapagos NV (Belgium), and F. Hoffmann-La Roche (Switzerland). The authors acknowledged numerous potential conflicts of interest, including receiving grants, personal fees, and nonfinancial support from various pharmaceutical companies.
Patients being treated with infliximab had weakened immune responses to the first dose of the ChAdOx1 nCoV-19 (Oxford/AstraZeneca) and BNT162b2 (Pfizer/BioNTech) vaccines, compared with patients on vedolizumab (Entyvio), although a very significant number of patients from both groups seroconverted after their second dose, according to a new U.K. study of patients with inflammatory bowel disease (IBD).
“Antibody testing and adapted vaccine schedules should be considered to protect these at-risk patients,” Nicholas A. Kennedy, PhD, MBBS, of the University of Exeter (England) and colleagues wrote in a preprint published March 29 on MedRxiv.
Infliximab is an anti–tumor necrosis factor (anti-TNF) monoclonal antibody that’s approved to treat adult and pediatric Crohn’s disease and ulcerative colitis, as well as rheumatoid arthritis, ankylosing spondylitis, psoriatic arthritis, and plaque psoriasis, whereas vedolizumab, a gut selective anti-integrin alpha4beta7 monoclonal antibody that is not associated with impaired systemic immune responses, is approved to treat Crohn’s disease and ulcerative colitis in adults.
A previous study from Kennedy and colleagues revealed that IBD patients on infliximab showed a weakened COVID-19 antibody response compared with patients on vedolizumab. To determine if treatment with anti-TNF drugs impacted the efficacy of the first shot of these two-dose COVID-19 vaccines, the researchers used data from the CLARITY IBD study to assess 865 infliximab- and 428 vedolizumab-treated participants without evidence of prior SARS-CoV-2 infection who had received uninterrupted biologic therapy since being recruited between Sept. 22 and Dec. 23, 2020.
In the 3-10 weeks after initial vaccination, geometric mean concentrations for SARS-CoV-2 anti-spike protein receptor-binding protein antibodies were lower in patients on infliximab, compared with patients on vedolizumab for both the Pfizer (6.0 U/mL [5.9] versus 28.8 U/mL [5.4], P < .0001) and AstraZeneca (4.7 U/mL [4.9] versus 13.8 U/mL [5.9]; P < .0001) vaccines. The researchers’ multivariable models reinforced those findings, with antibody concentrations lower in infliximab-treated patients for both the Pfizer (fold change, 0.29; 95% confidence interval, 0.21-0.40; P < .0001) and AstraZeneca (FC, 0.39; 95% CI, 0.30-0.51; P < .0001) vaccines.
After second doses of the two-dose Pfizer vaccine, 85% of patients on infliximab and 86% of patients on vedolizumab seroconverted (P = .68); similarly high seroconversion rates were seen in patients who had been infected with SARS-CoV-2 prior to receiving either vaccine. Several patient characteristics were associated with lower antibody concentrations regardless of vaccine type: being 60 years or older, use of immunomodulators, having Crohn’s disease, and being a smoker. Alternatively, non-White ethnicity was associated with higher antibody concentrations.
Evidence has ‘unclear clinical significance’
“These data, which require peer review, do not change my opinion on the safety and efficacy of COVID-19 vaccines in patients taking TNF inhibitors such as infliximab as monotherapy for the treatment of psoriatic disease,” Joel M. Gelfand MD, director of the psoriasis and phototherapy treatment center at the University of Pennsylvania, Philadelphia, said in an interview.
“First, two peer-reviewed studies found good antibody response in patients on TNF inhibitors receiving COVID-19 vaccines (doi: 10.1136/annrheumdis-2021-220289; 10.1136/annrheumdis-2021-220272). Second, antibody responses were robust in the small cohort that received the second dose of a COVID-19 vaccine. We already know that, for the two messenger RNA-based vaccines available under emergency use authorization in the U.S., a second dose is required for optimal efficacy. Thus, evidence of a reduced antibody response after just one dose is of unclear clinical significance. Third, antibody responses are only a surrogate marker, and a low antibody response doesn’t necessarily mean the patient will not be protected by the vaccine.”
Focus on the second dose of a two-dose regimen
“Tell me about the response in people who got both doses of a vaccine that you’re supposed to get both doses of,” Jeffrey Curtis, MD, professor of medicine in the division of clinical immunology and rheumatology at the University of Alabama at Birmingham, said in an interview. “The number of patients in that subset was small [n = 27] but in my opinion that’s the most clinically relevant analysis and the one that patients and clinicians want answered.”
He also emphasized the uncertainty around what ‘protection’ means in these early days of studying COVID-19 vaccine responses. “You can define seroprotection or seroconversion as some absolute level of an antibody response, but if you want to say ‘Mrs. Smith, your antibody level was X,’ on whatever arbitrary scale with whoever’s arbitrary lab test, nobody actually knows that Mrs. Smith is now protected from SARS-CoV-2, or how protected,” he said.
“What is not terribly controversial is: If you can’t detect antibodies, the vaccine didn’t ‘take,’ if you will. But if I tell you that the mean antibody level was X with one drug and then 2X with another drug, does that mean that you’re twice as protected? We don’t know that. I’m fearful that people are looking at these studies and thinking that more is better. It might be, but we don’t know that to be true.”
Debating the cause of weakened immune responses
“The biological plausibility of being on an anti-TNF affecting your immune reaction to a messenger RNA or even a replication-deficient viral vector vaccine doesn’t make sense,” David T. Rubin, MD, professor of medicine at the University of Chicago and chair of the National Scientific Advisory Committee of the Crohn’s and Colitis Foundation, said in an interview.
“I’m sure immunologists may differ with me on this, but given what we have come to appreciate about these vaccine mechanisms, this finding doesn’t make intuitive sense. So we need to make sure that, when this happens, we look to the next studies and try to understand, was there any other confounder that may have resulted in these findings that was not adequately adjusted for or addressed in some other way?
“When you have a study of this size, you argue, ‘Because it’s so large, any effect that was seen must be real,’ ” he added. “Alternatively, to have a study of this size, by its very nature you are limited in being able to control for certain other factors or differences between the groups.”
That said, he commended the authors for their study and acknowledged the potential questions it raises about the single-shot Johnson & Johnson vaccine. “If you only get one and you’re on infliximab, this study implies that maybe that’s not enough,” he said. “Despite the fact that Johnson & Johnson was approved as a single dose, it may be necessary to think about it as the first of two, or maybe it’s not the preferred vaccine in this group of patients.”
The study was supported by the Royal Devon and Exeter and Hull University Hospital Foundation NHS Trusts and unrestricted educational grants from Biogen (Switzerland), Celltrion Healthcare (South Korea), Galapagos NV (Belgium), and F. Hoffmann-La Roche (Switzerland). The authors acknowledged numerous potential conflicts of interest, including receiving grants, personal fees, and nonfinancial support from various pharmaceutical companies.
Patients being treated with infliximab had weakened immune responses to the first dose of the ChAdOx1 nCoV-19 (Oxford/AstraZeneca) and BNT162b2 (Pfizer/BioNTech) vaccines, compared with patients on vedolizumab (Entyvio), although a very significant number of patients from both groups seroconverted after their second dose, according to a new U.K. study of patients with inflammatory bowel disease (IBD).
“Antibody testing and adapted vaccine schedules should be considered to protect these at-risk patients,” Nicholas A. Kennedy, PhD, MBBS, of the University of Exeter (England) and colleagues wrote in a preprint published March 29 on MedRxiv.
Infliximab is an anti–tumor necrosis factor (anti-TNF) monoclonal antibody that’s approved to treat adult and pediatric Crohn’s disease and ulcerative colitis, as well as rheumatoid arthritis, ankylosing spondylitis, psoriatic arthritis, and plaque psoriasis, whereas vedolizumab, a gut selective anti-integrin alpha4beta7 monoclonal antibody that is not associated with impaired systemic immune responses, is approved to treat Crohn’s disease and ulcerative colitis in adults.
A previous study from Kennedy and colleagues revealed that IBD patients on infliximab showed a weakened COVID-19 antibody response compared with patients on vedolizumab. To determine if treatment with anti-TNF drugs impacted the efficacy of the first shot of these two-dose COVID-19 vaccines, the researchers used data from the CLARITY IBD study to assess 865 infliximab- and 428 vedolizumab-treated participants without evidence of prior SARS-CoV-2 infection who had received uninterrupted biologic therapy since being recruited between Sept. 22 and Dec. 23, 2020.
In the 3-10 weeks after initial vaccination, geometric mean concentrations for SARS-CoV-2 anti-spike protein receptor-binding protein antibodies were lower in patients on infliximab, compared with patients on vedolizumab for both the Pfizer (6.0 U/mL [5.9] versus 28.8 U/mL [5.4], P < .0001) and AstraZeneca (4.7 U/mL [4.9] versus 13.8 U/mL [5.9]; P < .0001) vaccines. The researchers’ multivariable models reinforced those findings, with antibody concentrations lower in infliximab-treated patients for both the Pfizer (fold change, 0.29; 95% confidence interval, 0.21-0.40; P < .0001) and AstraZeneca (FC, 0.39; 95% CI, 0.30-0.51; P < .0001) vaccines.
After second doses of the two-dose Pfizer vaccine, 85% of patients on infliximab and 86% of patients on vedolizumab seroconverted (P = .68); similarly high seroconversion rates were seen in patients who had been infected with SARS-CoV-2 prior to receiving either vaccine. Several patient characteristics were associated with lower antibody concentrations regardless of vaccine type: being 60 years or older, use of immunomodulators, having Crohn’s disease, and being a smoker. Alternatively, non-White ethnicity was associated with higher antibody concentrations.
Evidence has ‘unclear clinical significance’
“These data, which require peer review, do not change my opinion on the safety and efficacy of COVID-19 vaccines in patients taking TNF inhibitors such as infliximab as monotherapy for the treatment of psoriatic disease,” Joel M. Gelfand MD, director of the psoriasis and phototherapy treatment center at the University of Pennsylvania, Philadelphia, said in an interview.
“First, two peer-reviewed studies found good antibody response in patients on TNF inhibitors receiving COVID-19 vaccines (doi: 10.1136/annrheumdis-2021-220289; 10.1136/annrheumdis-2021-220272). Second, antibody responses were robust in the small cohort that received the second dose of a COVID-19 vaccine. We already know that, for the two messenger RNA-based vaccines available under emergency use authorization in the U.S., a second dose is required for optimal efficacy. Thus, evidence of a reduced antibody response after just one dose is of unclear clinical significance. Third, antibody responses are only a surrogate marker, and a low antibody response doesn’t necessarily mean the patient will not be protected by the vaccine.”
Focus on the second dose of a two-dose regimen
“Tell me about the response in people who got both doses of a vaccine that you’re supposed to get both doses of,” Jeffrey Curtis, MD, professor of medicine in the division of clinical immunology and rheumatology at the University of Alabama at Birmingham, said in an interview. “The number of patients in that subset was small [n = 27] but in my opinion that’s the most clinically relevant analysis and the one that patients and clinicians want answered.”
He also emphasized the uncertainty around what ‘protection’ means in these early days of studying COVID-19 vaccine responses. “You can define seroprotection or seroconversion as some absolute level of an antibody response, but if you want to say ‘Mrs. Smith, your antibody level was X,’ on whatever arbitrary scale with whoever’s arbitrary lab test, nobody actually knows that Mrs. Smith is now protected from SARS-CoV-2, or how protected,” he said.
“What is not terribly controversial is: If you can’t detect antibodies, the vaccine didn’t ‘take,’ if you will. But if I tell you that the mean antibody level was X with one drug and then 2X with another drug, does that mean that you’re twice as protected? We don’t know that. I’m fearful that people are looking at these studies and thinking that more is better. It might be, but we don’t know that to be true.”
Debating the cause of weakened immune responses
“The biological plausibility of being on an anti-TNF affecting your immune reaction to a messenger RNA or even a replication-deficient viral vector vaccine doesn’t make sense,” David T. Rubin, MD, professor of medicine at the University of Chicago and chair of the National Scientific Advisory Committee of the Crohn’s and Colitis Foundation, said in an interview.
“I’m sure immunologists may differ with me on this, but given what we have come to appreciate about these vaccine mechanisms, this finding doesn’t make intuitive sense. So we need to make sure that, when this happens, we look to the next studies and try to understand, was there any other confounder that may have resulted in these findings that was not adequately adjusted for or addressed in some other way?
“When you have a study of this size, you argue, ‘Because it’s so large, any effect that was seen must be real,’ ” he added. “Alternatively, to have a study of this size, by its very nature you are limited in being able to control for certain other factors or differences between the groups.”
That said, he commended the authors for their study and acknowledged the potential questions it raises about the single-shot Johnson & Johnson vaccine. “If you only get one and you’re on infliximab, this study implies that maybe that’s not enough,” he said. “Despite the fact that Johnson & Johnson was approved as a single dose, it may be necessary to think about it as the first of two, or maybe it’s not the preferred vaccine in this group of patients.”
The study was supported by the Royal Devon and Exeter and Hull University Hospital Foundation NHS Trusts and unrestricted educational grants from Biogen (Switzerland), Celltrion Healthcare (South Korea), Galapagos NV (Belgium), and F. Hoffmann-La Roche (Switzerland). The authors acknowledged numerous potential conflicts of interest, including receiving grants, personal fees, and nonfinancial support from various pharmaceutical companies.
FROM MEDRXIV
Study suggests no added risk of blood clots in COVID-19 outpatients
The incidence of venous thromboembolism (VTE) in nonhospitalized patients with COVID-19 was not significantly different from patients without the infectious disease, according to a new study published in JAMA Internal Medicine.
National Institutes of Health guidelines recommend blood thinners to prevent blood clots in patients hospitalized with COVID-19. However, the new study provides more insight on the best treatment approach for COVID-19 outpatients.
“[COVID-19’s] rapid global progression and impact has caused us to make and modify treatment decisions at a pace that we never have in modern medicine,” study author Nareg Roubinian, MD, an investigator at Kaiser Permanente, Oakland, Calif., said in an interview.
“As with other potential therapies for COVID-19, blood thinners need to be prospectively studied in a clinical trial to determine if they improve patient outcomes,” Dr. Roubinian added.
The increased risk of blood clots in patients hospitalized with COVID-19 has been a major issue throughout the pandemic. In fact, one study published in November 2020 found that more than half of patients hospitalized with the illness have prothrombotic antiphospholipid (aPL) autoantibodies in their blood, which could contribute to venous and arterial thromboembolism.
Although it was clear many hospitalized patients diagnosed with COVID-19 were developing more clots, researchers of the current study were not sure if this trend would also be seen in outpatients.
“Most people with COVID-19 do not need to be hospitalized, and we needed to know how often patients outside the hospital were having blood clots,” said Dr. Roubinian.
For the study, Dr. Roubinian and colleagues examined data on 220,588 patients who were members of Kaiser Permanente Northern California health plan and were tested for COVID-19 between Feb. 25 and Aug. 31, 2020. They then reported on the 30-day incidence of outpatient and hospital-associated blood clots following the COVID-19 diagnosis. Patients who were asymptomatic at the time of testing or had received anticoagulants within the last year were excluded.
“We knew from other studies that patients with COVID-19 often get sicker in the first few weeks after infection. What we didn’t know was whether COVID-19 patients were developing blood clots but not pneumonia or were developing blood clots at the same time as they developed pneumonia,” said Dr. Roubinian, an intensive care doctor with the Permanente Medical Group in Oakland, Calif. “Following the patients for 30 days allowed us to focus on the time period from infection to when blood clots were most likely to develop.”
Researchers found that of the cohort who took the COVID-19 test, 11.8% had a positive result. Within 30 days of the COVID-19 test, 0.8% of patients with a positive result were diagnosed with VTE compared to 0.5% of those who received a negative test result. They also found that viral testing took place in an outpatient setting for 59.1% of the patients with a positive viral test who later developed VTE. Of those patients, 76.1% had to be hospitalized.
Dr. Roubinian said he was surprised to see that the blood clotting in outpatients with COVID-19 was similar in frequency to what he saw in patients without the infection.
“Our findings suggest that blood clots do occur in COVID-19 patients but not on a scale where we need to put all or many COVID outpatients on blood thinners,” he said. “As with other potential therapies for COVID-19, blood thinners need to be prospectively studied in a clinical trial to determine if they improve patient outcomes.”
In December 2020, three trials investigating the risk and benefits of increased levels of anticoagulation in hospitalized COVID-19 patients were paused because of safety issues. The trials would have enrolled critically ill COVID-19 patients for whom therapeutic doses of anticoagulation drugs showed no benefit.
Anticoagulants are associated with bleeding risks, including prolonged nosebleeds and vomiting or coughing up blood.
Instead of prescribing the routine use of thromboprophylactic drugs to COVID-19 outpatients, Dr. Roubinian believes it would be helpful to learn how to determine whether a patient at risk of becoming sick or being hospitalized would benefit from being treated with such drugs.
Dr. Roubinian reported receiving grants from the National Institutes of Health and the National Heart, Lung, and Blood Institute during the conduct of the study.
The incidence of venous thromboembolism (VTE) in nonhospitalized patients with COVID-19 was not significantly different from patients without the infectious disease, according to a new study published in JAMA Internal Medicine.
National Institutes of Health guidelines recommend blood thinners to prevent blood clots in patients hospitalized with COVID-19. However, the new study provides more insight on the best treatment approach for COVID-19 outpatients.
“[COVID-19’s] rapid global progression and impact has caused us to make and modify treatment decisions at a pace that we never have in modern medicine,” study author Nareg Roubinian, MD, an investigator at Kaiser Permanente, Oakland, Calif., said in an interview.
“As with other potential therapies for COVID-19, blood thinners need to be prospectively studied in a clinical trial to determine if they improve patient outcomes,” Dr. Roubinian added.
The increased risk of blood clots in patients hospitalized with COVID-19 has been a major issue throughout the pandemic. In fact, one study published in November 2020 found that more than half of patients hospitalized with the illness have prothrombotic antiphospholipid (aPL) autoantibodies in their blood, which could contribute to venous and arterial thromboembolism.
Although it was clear many hospitalized patients diagnosed with COVID-19 were developing more clots, researchers of the current study were not sure if this trend would also be seen in outpatients.
“Most people with COVID-19 do not need to be hospitalized, and we needed to know how often patients outside the hospital were having blood clots,” said Dr. Roubinian.
For the study, Dr. Roubinian and colleagues examined data on 220,588 patients who were members of Kaiser Permanente Northern California health plan and were tested for COVID-19 between Feb. 25 and Aug. 31, 2020. They then reported on the 30-day incidence of outpatient and hospital-associated blood clots following the COVID-19 diagnosis. Patients who were asymptomatic at the time of testing or had received anticoagulants within the last year were excluded.
“We knew from other studies that patients with COVID-19 often get sicker in the first few weeks after infection. What we didn’t know was whether COVID-19 patients were developing blood clots but not pneumonia or were developing blood clots at the same time as they developed pneumonia,” said Dr. Roubinian, an intensive care doctor with the Permanente Medical Group in Oakland, Calif. “Following the patients for 30 days allowed us to focus on the time period from infection to when blood clots were most likely to develop.”
Researchers found that of the cohort who took the COVID-19 test, 11.8% had a positive result. Within 30 days of the COVID-19 test, 0.8% of patients with a positive result were diagnosed with VTE compared to 0.5% of those who received a negative test result. They also found that viral testing took place in an outpatient setting for 59.1% of the patients with a positive viral test who later developed VTE. Of those patients, 76.1% had to be hospitalized.
Dr. Roubinian said he was surprised to see that the blood clotting in outpatients with COVID-19 was similar in frequency to what he saw in patients without the infection.
“Our findings suggest that blood clots do occur in COVID-19 patients but not on a scale where we need to put all or many COVID outpatients on blood thinners,” he said. “As with other potential therapies for COVID-19, blood thinners need to be prospectively studied in a clinical trial to determine if they improve patient outcomes.”
In December 2020, three trials investigating the risk and benefits of increased levels of anticoagulation in hospitalized COVID-19 patients were paused because of safety issues. The trials would have enrolled critically ill COVID-19 patients for whom therapeutic doses of anticoagulation drugs showed no benefit.
Anticoagulants are associated with bleeding risks, including prolonged nosebleeds and vomiting or coughing up blood.
Instead of prescribing the routine use of thromboprophylactic drugs to COVID-19 outpatients, Dr. Roubinian believes it would be helpful to learn how to determine whether a patient at risk of becoming sick or being hospitalized would benefit from being treated with such drugs.
Dr. Roubinian reported receiving grants from the National Institutes of Health and the National Heart, Lung, and Blood Institute during the conduct of the study.
The incidence of venous thromboembolism (VTE) in nonhospitalized patients with COVID-19 was not significantly different from patients without the infectious disease, according to a new study published in JAMA Internal Medicine.
National Institutes of Health guidelines recommend blood thinners to prevent blood clots in patients hospitalized with COVID-19. However, the new study provides more insight on the best treatment approach for COVID-19 outpatients.
“[COVID-19’s] rapid global progression and impact has caused us to make and modify treatment decisions at a pace that we never have in modern medicine,” study author Nareg Roubinian, MD, an investigator at Kaiser Permanente, Oakland, Calif., said in an interview.
“As with other potential therapies for COVID-19, blood thinners need to be prospectively studied in a clinical trial to determine if they improve patient outcomes,” Dr. Roubinian added.
The increased risk of blood clots in patients hospitalized with COVID-19 has been a major issue throughout the pandemic. In fact, one study published in November 2020 found that more than half of patients hospitalized with the illness have prothrombotic antiphospholipid (aPL) autoantibodies in their blood, which could contribute to venous and arterial thromboembolism.
Although it was clear many hospitalized patients diagnosed with COVID-19 were developing more clots, researchers of the current study were not sure if this trend would also be seen in outpatients.
“Most people with COVID-19 do not need to be hospitalized, and we needed to know how often patients outside the hospital were having blood clots,” said Dr. Roubinian.
For the study, Dr. Roubinian and colleagues examined data on 220,588 patients who were members of Kaiser Permanente Northern California health plan and were tested for COVID-19 between Feb. 25 and Aug. 31, 2020. They then reported on the 30-day incidence of outpatient and hospital-associated blood clots following the COVID-19 diagnosis. Patients who were asymptomatic at the time of testing or had received anticoagulants within the last year were excluded.
“We knew from other studies that patients with COVID-19 often get sicker in the first few weeks after infection. What we didn’t know was whether COVID-19 patients were developing blood clots but not pneumonia or were developing blood clots at the same time as they developed pneumonia,” said Dr. Roubinian, an intensive care doctor with the Permanente Medical Group in Oakland, Calif. “Following the patients for 30 days allowed us to focus on the time period from infection to when blood clots were most likely to develop.”
Researchers found that of the cohort who took the COVID-19 test, 11.8% had a positive result. Within 30 days of the COVID-19 test, 0.8% of patients with a positive result were diagnosed with VTE compared to 0.5% of those who received a negative test result. They also found that viral testing took place in an outpatient setting for 59.1% of the patients with a positive viral test who later developed VTE. Of those patients, 76.1% had to be hospitalized.
Dr. Roubinian said he was surprised to see that the blood clotting in outpatients with COVID-19 was similar in frequency to what he saw in patients without the infection.
“Our findings suggest that blood clots do occur in COVID-19 patients but not on a scale where we need to put all or many COVID outpatients on blood thinners,” he said. “As with other potential therapies for COVID-19, blood thinners need to be prospectively studied in a clinical trial to determine if they improve patient outcomes.”
In December 2020, three trials investigating the risk and benefits of increased levels of anticoagulation in hospitalized COVID-19 patients were paused because of safety issues. The trials would have enrolled critically ill COVID-19 patients for whom therapeutic doses of anticoagulation drugs showed no benefit.
Anticoagulants are associated with bleeding risks, including prolonged nosebleeds and vomiting or coughing up blood.
Instead of prescribing the routine use of thromboprophylactic drugs to COVID-19 outpatients, Dr. Roubinian believes it would be helpful to learn how to determine whether a patient at risk of becoming sick or being hospitalized would benefit from being treated with such drugs.
Dr. Roubinian reported receiving grants from the National Institutes of Health and the National Heart, Lung, and Blood Institute during the conduct of the study.