User login
ID Practitioner is an independent news source that provides infectious disease specialists with timely and relevant news and commentary about clinical developments and the impact of health care policy on the infectious disease specialist’s practice. Specialty focus topics include antimicrobial resistance, emerging infections, global ID, hepatitis, HIV, hospital-acquired infections, immunizations and vaccines, influenza, mycoses, pediatric infections, and STIs. Infectious Diseases News is owned by Frontline Medical Communications.
sofosbuvir
ritonavir with dasabuvir
discount
support path
program
ritonavir
greedy
ledipasvir
assistance
viekira pak
vpak
advocacy
needy
protest
abbvie
paritaprevir
ombitasvir
direct-acting antivirals
dasabuvir
gilead
fake-ovir
support
v pak
oasis
harvoni
section[contains(@class, 'footer-nav-section-wrapper')]
div[contains(@class, 'pane-pub-article-idp')]
div[contains(@class, 'pane-medstat-latest-articles-articles-section')]
div[contains(@class, 'pane-pub-home-idp')]
div[contains(@class, 'pane-pub-topic-idp')]
How some COVID-19 vaccines could cause rare blood clots
on April 14, 2021, after the CDC and Food and Drug Administration recommended that states hold off on using it pending a detailed review of six cases of the same kind of rare but serious event – a blood clot in the vessels that drain blood from the brain combined with a large drop in platelets, which increases the risk for bleeding.
This combination can lead to severe strokes that can lead to brain damage or death. Among the six cases reported, which came to light over the past 3 weeks, one person died, according to the CDC. All six were women and ranged in age from 18 to 48 years.
According to a report from the Vaccine Adverse Event Reporting System (VAERS), which is maintained by the Department of Health & Human Services, the woman who died was 45. She developed a gradually worsening headache about a week after receiving the Johnson & Johnson vaccine.
On March 17, the day she came to the hospital, she was dry heaving. Her headache had suddenly gotten much worse, and the left side of her body was weak, which are signs of a stroke. A CT scan revealed both bleeding in her brain and a clot in her cortical vein. She died the following day.
In addition to VAERS, which accepts reports from anyone, the CDC and FDA are monitoring at least eight other safety systems maintained by hospitals, research centers, long-term care facilities, and insurance companies for signs of trouble with the vaccines. VAERS data is searchable and open to the public. Most of these systems are not publicly available to protect patient privacy. It’s unclear which systems detected the six cases cited by federal regulators.
“These are very serious and potentially fatal problems occurring in a healthy young adult. It’s serious and we need to get to the bottom of it,” said Ed Belongia, MD, director of the Center for Clinical Epidemiology and Population Health at the Marshfield (Wis.) Clinic Research Institute. Dr. Belongia leads a research team that helps the CDC monitor vaccine safety and effectiveness.
“Safety is always the highest priority, and I think what we’ve seen here in the past 24 hours is our vaccine safety monitoring system is working,” he said.
Others agree. “I think what CDC and FDA have detected is a rare, but likely real adverse event associated with this vaccine,” said Paul Offit, MD, director of vaccine education at Children’s Hospital of Philadelphia.
Although much is still unknown about these events, they follow a similar pattern of blood clots reported with the AstraZeneca vaccine in Europe. That vaccine is now sold under the brand name Vaxzevria.
This has experts questioning whether all vaccines of this type may cause these rare clots.
“I think it’s likely a class effect,” said Dr. Offit, who was a member of the FDA advisory committee that reviewed clinical trial data on the J&J vaccine before it was authorized for use.
Adenovirus vaccines scrutinized
Both the Johnson & Johnson and Vaxzevria vaccines use an adenovirus to ferry genetic instructions for making the coronaviruses spike protein into our cells.
Adenoviruses are common, relatively simple viruses that normally cause mild cold or flu symptoms. The ones used in the vaccine are disabled so they can’t make us sick. They’re more like Trojan horses.
Once inside our cells, they release the DNA instructions they carry to make the spike protein of the new coronavirus. Those cells then crank out copies of the spike protein, which then get displayed on the outer surface of the cell membrane where they are recognized by the immune system.
The immune system then makes antibodies and other defenses against the spike so that, when the real coronavirus comes along, our bodies are ready to fight the infection.
There’s no question the vaccine works. In clinical trials, the Johnson & Johnson vaccine was 66% percent effective at preventing against moderate to severe COVID-19 infection, and none of the patients who got COVID-19 after vaccination had to be admitted to the hospital or died.
The idea behind using adenoviruses in vaccines isn’t a new one. In a kind of fight-fire-with-fire approach, the idea is to use a virus, which is good at infecting us, to fight a different kind of virus.
Researchers have been working on the concept for about 10 years, but the COVID-19 vaccines that use this technology are some of the first adenovirus-vector vaccines deployed in humans.
Only one other adenovirus vaccine, for Ebola, has been approved for use in humans. It was approved in Europe last year. Before the Johnson & Johnson vaccine, no other adenovirus vector has been available for use in humans in the United States.
There are six adenovirus-vector vaccines for COVID-19. In addition to AstraZeneca and Johnson & Johnson, there’s the Russian-developed vaccine Sputnik V, along with CanSino from China, and the Covishield vaccine in India.
Adenovirus vaccines are more stable than the mRNA vaccines. That makes them easier to store and transport.
But they have a significant downside, too. Because adenoviruses infect humans out in the world, we already make antibodies against them. So there’s always a danger that our immune systems might recognize and react to the vaccine, rendering it ineffective. For that reason, scientists try to carefully select the adenovirus vectors, or carriers, they use.
The two vaccines under investigation for blood clots are slightly different. The Johnson & Johnson vaccine uses the vector AD26, because most of the population lacks preexisting immunity to it. Vaxzevria uses an adenovirus that infects chimpanzees, called ChAdOx1.
Vaxzevria has been widely used in Europe but has not yet been authorized in the United States.
On April 7, the European Medicines Agency, Europe’s counterpart to the FDA, ruled that unusual blood clots with low blood platelets should be listed as rare side effects on the Vaxzevria vaccine.
The decision came after reviewing 62 cases of cerebral venous sinus thrombosis (CVST) linked to the vaccine and 25 cases of another rare type of clot, called a splanchnic vein thrombosis. Splanchnic veins drain blood from the major organs in the digestive system, including the stomach, liver, and intestines; 18 of those events were fatal.
The reports were culled from reporting in Europe and the United Kingdom, where around 25 million people have received the Vaxzevria vaccine, making these clots exceptionally rare, but serious.
So far, six cases of CVST have been reported in the United States, after more than 7 million doses of the Johnson & Johnson vaccines have been administered.
A key question for U.S. regulators will be the background rate for these types of rare combinations of clots and deplenished platelets. The background rate is the number of events that would be expected to occur naturally in a population of unvaccinated people. On a press call on April 13, Peter Marks, MD, PhD, director of the FDA’s Center for Biologics Evaluation and Research, was asked about the frequency of this dangerous combination. He said the combination of low platelets and clots was so rare that it was hard to pinpoint, but might be somewhere between 2 and 14 cases per million people over the course of a year.
The first Johnson & Johnson doses were given in early March. That means the six cases came to light within the first few weeks of use of the vaccine in the United States, a very short amount of time.
“These were six cases per million people for 2 weeks, which is the same thing as 25 million per year, so it’s clearly above the background rate,” Dr. Offit said.
Studies suggest possible mechanism
On April 9, the New England Journal of Medicine published a detailed evaluation of the 11 patients in Germany and Austria who developed the rare clots after their Vaxzevria vaccines.
The study detected rare antibodies to a signaling protein called platelet factor 4, which helps to coordinate clot formation.
These same type of antibodies form in some people given the blood thinning drug heparin. In those reactions, which are also exceptionally rare, the same type of syndrome develops, leading to large, devastating clots that consume circulating platelets.
It’s not yet clear whether people who develop reactions to the vaccines already have some platelet factor 4 antibodies before they are vaccinated, or whether the vaccines somehow spur the body to make these antibodies, which then launch a kind of autoimmune attack.
The researchers on the paper gave the syndrome a name, vaccine-induced thrombotic thrombocytopenia (VITT).
It’s also not clear why more cases seem to be in women than in men. Andrew Eisenberger, MD, an associate professor of hematology and oncology at Columbia University, New York, said the most common causes of cerebral venous sinus thrombosis have to do with conditions that raise estrogen levels, like pregnancy and hormonal contraception.
“Estrogen naturally leads to changes in several clotting proteins in the blood that may predispose to abnormal blood clotting in a few different sites in the body,” he said. “The clotting changes we are encountering with some of COVID-19 vaccines are likely to be synergistic with the effects of estrogen on the blood.”
No matter the cause, the CDC on April 13 alerted doctors to keep a high index of suspicion for VITT in patients who have received the Johnson & Johnson vaccination within the last 2 weeks. In those patients, the usual course of treatment with blood thinning drugs like heparin may be harmful.
Symptoms to watch for include severe headache or backache, new neurologic symptoms, severe abdominal pain, shortness of breath, leg swelling, tiny red spots on the skin, or easy bruising.
Grappling with evidence
The CDC’s Advisory Committee on Immunization Practices will meet today in an emergency session to review the cases and see if any changes are needed to use of the J&J vaccine in the United States.
Last week, for example, the United Kingdom restricted the use of the AstraZeneca vaccine in people aged younger than 30 years, saying the risks and benefits of vaccination are “more finely balanced” for this age group.
With cases of COVID-19 rising again in the United States, and the Johnson & Johnson vaccine currently the most convenient form of protection against the virus, the committee will have to weigh the risks of that infection against the risk of rare clots caused by vaccination.
They will also likely have to rule out whether any of the cases had COVID. At least one study has reported CVST clots in three patients with confirmed COVID infections. In Europe, COVID infection did not seem to play a role in the formation of the clots with low platelets.
Hilda Bastian, PhD, a clinical trials expert who cofounded the Cochrane Collaboration, said it won’t be an easy task. Much will depend on how certain the committee members feel they know about all the events linked to the vaccine.
“That’s the really, really hard issue from my point of view for them right this moment. Have we missed any? Or how many are we likely to have missed?” asked Dr. Bastian, who lives in Australia.
“In a country that size with that fragmented [of] a health care system, how sure can you be that you know them all? That’s going to be a really difficult situation for them to grapple with, the quality of information that they’ve got,” she said.
A version of this article first appeared on Medscape.com.
on April 14, 2021, after the CDC and Food and Drug Administration recommended that states hold off on using it pending a detailed review of six cases of the same kind of rare but serious event – a blood clot in the vessels that drain blood from the brain combined with a large drop in platelets, which increases the risk for bleeding.
This combination can lead to severe strokes that can lead to brain damage or death. Among the six cases reported, which came to light over the past 3 weeks, one person died, according to the CDC. All six were women and ranged in age from 18 to 48 years.
According to a report from the Vaccine Adverse Event Reporting System (VAERS), which is maintained by the Department of Health & Human Services, the woman who died was 45. She developed a gradually worsening headache about a week after receiving the Johnson & Johnson vaccine.
On March 17, the day she came to the hospital, she was dry heaving. Her headache had suddenly gotten much worse, and the left side of her body was weak, which are signs of a stroke. A CT scan revealed both bleeding in her brain and a clot in her cortical vein. She died the following day.
In addition to VAERS, which accepts reports from anyone, the CDC and FDA are monitoring at least eight other safety systems maintained by hospitals, research centers, long-term care facilities, and insurance companies for signs of trouble with the vaccines. VAERS data is searchable and open to the public. Most of these systems are not publicly available to protect patient privacy. It’s unclear which systems detected the six cases cited by federal regulators.
“These are very serious and potentially fatal problems occurring in a healthy young adult. It’s serious and we need to get to the bottom of it,” said Ed Belongia, MD, director of the Center for Clinical Epidemiology and Population Health at the Marshfield (Wis.) Clinic Research Institute. Dr. Belongia leads a research team that helps the CDC monitor vaccine safety and effectiveness.
“Safety is always the highest priority, and I think what we’ve seen here in the past 24 hours is our vaccine safety monitoring system is working,” he said.
Others agree. “I think what CDC and FDA have detected is a rare, but likely real adverse event associated with this vaccine,” said Paul Offit, MD, director of vaccine education at Children’s Hospital of Philadelphia.
Although much is still unknown about these events, they follow a similar pattern of blood clots reported with the AstraZeneca vaccine in Europe. That vaccine is now sold under the brand name Vaxzevria.
This has experts questioning whether all vaccines of this type may cause these rare clots.
“I think it’s likely a class effect,” said Dr. Offit, who was a member of the FDA advisory committee that reviewed clinical trial data on the J&J vaccine before it was authorized for use.
Adenovirus vaccines scrutinized
Both the Johnson & Johnson and Vaxzevria vaccines use an adenovirus to ferry genetic instructions for making the coronaviruses spike protein into our cells.
Adenoviruses are common, relatively simple viruses that normally cause mild cold or flu symptoms. The ones used in the vaccine are disabled so they can’t make us sick. They’re more like Trojan horses.
Once inside our cells, they release the DNA instructions they carry to make the spike protein of the new coronavirus. Those cells then crank out copies of the spike protein, which then get displayed on the outer surface of the cell membrane where they are recognized by the immune system.
The immune system then makes antibodies and other defenses against the spike so that, when the real coronavirus comes along, our bodies are ready to fight the infection.
There’s no question the vaccine works. In clinical trials, the Johnson & Johnson vaccine was 66% percent effective at preventing against moderate to severe COVID-19 infection, and none of the patients who got COVID-19 after vaccination had to be admitted to the hospital or died.
The idea behind using adenoviruses in vaccines isn’t a new one. In a kind of fight-fire-with-fire approach, the idea is to use a virus, which is good at infecting us, to fight a different kind of virus.
Researchers have been working on the concept for about 10 years, but the COVID-19 vaccines that use this technology are some of the first adenovirus-vector vaccines deployed in humans.
Only one other adenovirus vaccine, for Ebola, has been approved for use in humans. It was approved in Europe last year. Before the Johnson & Johnson vaccine, no other adenovirus vector has been available for use in humans in the United States.
There are six adenovirus-vector vaccines for COVID-19. In addition to AstraZeneca and Johnson & Johnson, there’s the Russian-developed vaccine Sputnik V, along with CanSino from China, and the Covishield vaccine in India.
Adenovirus vaccines are more stable than the mRNA vaccines. That makes them easier to store and transport.
But they have a significant downside, too. Because adenoviruses infect humans out in the world, we already make antibodies against them. So there’s always a danger that our immune systems might recognize and react to the vaccine, rendering it ineffective. For that reason, scientists try to carefully select the adenovirus vectors, or carriers, they use.
The two vaccines under investigation for blood clots are slightly different. The Johnson & Johnson vaccine uses the vector AD26, because most of the population lacks preexisting immunity to it. Vaxzevria uses an adenovirus that infects chimpanzees, called ChAdOx1.
Vaxzevria has been widely used in Europe but has not yet been authorized in the United States.
On April 7, the European Medicines Agency, Europe’s counterpart to the FDA, ruled that unusual blood clots with low blood platelets should be listed as rare side effects on the Vaxzevria vaccine.
The decision came after reviewing 62 cases of cerebral venous sinus thrombosis (CVST) linked to the vaccine and 25 cases of another rare type of clot, called a splanchnic vein thrombosis. Splanchnic veins drain blood from the major organs in the digestive system, including the stomach, liver, and intestines; 18 of those events were fatal.
The reports were culled from reporting in Europe and the United Kingdom, where around 25 million people have received the Vaxzevria vaccine, making these clots exceptionally rare, but serious.
So far, six cases of CVST have been reported in the United States, after more than 7 million doses of the Johnson & Johnson vaccines have been administered.
A key question for U.S. regulators will be the background rate for these types of rare combinations of clots and deplenished platelets. The background rate is the number of events that would be expected to occur naturally in a population of unvaccinated people. On a press call on April 13, Peter Marks, MD, PhD, director of the FDA’s Center for Biologics Evaluation and Research, was asked about the frequency of this dangerous combination. He said the combination of low platelets and clots was so rare that it was hard to pinpoint, but might be somewhere between 2 and 14 cases per million people over the course of a year.
The first Johnson & Johnson doses were given in early March. That means the six cases came to light within the first few weeks of use of the vaccine in the United States, a very short amount of time.
“These were six cases per million people for 2 weeks, which is the same thing as 25 million per year, so it’s clearly above the background rate,” Dr. Offit said.
Studies suggest possible mechanism
On April 9, the New England Journal of Medicine published a detailed evaluation of the 11 patients in Germany and Austria who developed the rare clots after their Vaxzevria vaccines.
The study detected rare antibodies to a signaling protein called platelet factor 4, which helps to coordinate clot formation.
These same type of antibodies form in some people given the blood thinning drug heparin. In those reactions, which are also exceptionally rare, the same type of syndrome develops, leading to large, devastating clots that consume circulating platelets.
It’s not yet clear whether people who develop reactions to the vaccines already have some platelet factor 4 antibodies before they are vaccinated, or whether the vaccines somehow spur the body to make these antibodies, which then launch a kind of autoimmune attack.
The researchers on the paper gave the syndrome a name, vaccine-induced thrombotic thrombocytopenia (VITT).
It’s also not clear why more cases seem to be in women than in men. Andrew Eisenberger, MD, an associate professor of hematology and oncology at Columbia University, New York, said the most common causes of cerebral venous sinus thrombosis have to do with conditions that raise estrogen levels, like pregnancy and hormonal contraception.
“Estrogen naturally leads to changes in several clotting proteins in the blood that may predispose to abnormal blood clotting in a few different sites in the body,” he said. “The clotting changes we are encountering with some of COVID-19 vaccines are likely to be synergistic with the effects of estrogen on the blood.”
No matter the cause, the CDC on April 13 alerted doctors to keep a high index of suspicion for VITT in patients who have received the Johnson & Johnson vaccination within the last 2 weeks. In those patients, the usual course of treatment with blood thinning drugs like heparin may be harmful.
Symptoms to watch for include severe headache or backache, new neurologic symptoms, severe abdominal pain, shortness of breath, leg swelling, tiny red spots on the skin, or easy bruising.
Grappling with evidence
The CDC’s Advisory Committee on Immunization Practices will meet today in an emergency session to review the cases and see if any changes are needed to use of the J&J vaccine in the United States.
Last week, for example, the United Kingdom restricted the use of the AstraZeneca vaccine in people aged younger than 30 years, saying the risks and benefits of vaccination are “more finely balanced” for this age group.
With cases of COVID-19 rising again in the United States, and the Johnson & Johnson vaccine currently the most convenient form of protection against the virus, the committee will have to weigh the risks of that infection against the risk of rare clots caused by vaccination.
They will also likely have to rule out whether any of the cases had COVID. At least one study has reported CVST clots in three patients with confirmed COVID infections. In Europe, COVID infection did not seem to play a role in the formation of the clots with low platelets.
Hilda Bastian, PhD, a clinical trials expert who cofounded the Cochrane Collaboration, said it won’t be an easy task. Much will depend on how certain the committee members feel they know about all the events linked to the vaccine.
“That’s the really, really hard issue from my point of view for them right this moment. Have we missed any? Or how many are we likely to have missed?” asked Dr. Bastian, who lives in Australia.
“In a country that size with that fragmented [of] a health care system, how sure can you be that you know them all? That’s going to be a really difficult situation for them to grapple with, the quality of information that they’ve got,” she said.
A version of this article first appeared on Medscape.com.
on April 14, 2021, after the CDC and Food and Drug Administration recommended that states hold off on using it pending a detailed review of six cases of the same kind of rare but serious event – a blood clot in the vessels that drain blood from the brain combined with a large drop in platelets, which increases the risk for bleeding.
This combination can lead to severe strokes that can lead to brain damage or death. Among the six cases reported, which came to light over the past 3 weeks, one person died, according to the CDC. All six were women and ranged in age from 18 to 48 years.
According to a report from the Vaccine Adverse Event Reporting System (VAERS), which is maintained by the Department of Health & Human Services, the woman who died was 45. She developed a gradually worsening headache about a week after receiving the Johnson & Johnson vaccine.
On March 17, the day she came to the hospital, she was dry heaving. Her headache had suddenly gotten much worse, and the left side of her body was weak, which are signs of a stroke. A CT scan revealed both bleeding in her brain and a clot in her cortical vein. She died the following day.
In addition to VAERS, which accepts reports from anyone, the CDC and FDA are monitoring at least eight other safety systems maintained by hospitals, research centers, long-term care facilities, and insurance companies for signs of trouble with the vaccines. VAERS data is searchable and open to the public. Most of these systems are not publicly available to protect patient privacy. It’s unclear which systems detected the six cases cited by federal regulators.
“These are very serious and potentially fatal problems occurring in a healthy young adult. It’s serious and we need to get to the bottom of it,” said Ed Belongia, MD, director of the Center for Clinical Epidemiology and Population Health at the Marshfield (Wis.) Clinic Research Institute. Dr. Belongia leads a research team that helps the CDC monitor vaccine safety and effectiveness.
“Safety is always the highest priority, and I think what we’ve seen here in the past 24 hours is our vaccine safety monitoring system is working,” he said.
Others agree. “I think what CDC and FDA have detected is a rare, but likely real adverse event associated with this vaccine,” said Paul Offit, MD, director of vaccine education at Children’s Hospital of Philadelphia.
Although much is still unknown about these events, they follow a similar pattern of blood clots reported with the AstraZeneca vaccine in Europe. That vaccine is now sold under the brand name Vaxzevria.
This has experts questioning whether all vaccines of this type may cause these rare clots.
“I think it’s likely a class effect,” said Dr. Offit, who was a member of the FDA advisory committee that reviewed clinical trial data on the J&J vaccine before it was authorized for use.
Adenovirus vaccines scrutinized
Both the Johnson & Johnson and Vaxzevria vaccines use an adenovirus to ferry genetic instructions for making the coronaviruses spike protein into our cells.
Adenoviruses are common, relatively simple viruses that normally cause mild cold or flu symptoms. The ones used in the vaccine are disabled so they can’t make us sick. They’re more like Trojan horses.
Once inside our cells, they release the DNA instructions they carry to make the spike protein of the new coronavirus. Those cells then crank out copies of the spike protein, which then get displayed on the outer surface of the cell membrane where they are recognized by the immune system.
The immune system then makes antibodies and other defenses against the spike so that, when the real coronavirus comes along, our bodies are ready to fight the infection.
There’s no question the vaccine works. In clinical trials, the Johnson & Johnson vaccine was 66% percent effective at preventing against moderate to severe COVID-19 infection, and none of the patients who got COVID-19 after vaccination had to be admitted to the hospital or died.
The idea behind using adenoviruses in vaccines isn’t a new one. In a kind of fight-fire-with-fire approach, the idea is to use a virus, which is good at infecting us, to fight a different kind of virus.
Researchers have been working on the concept for about 10 years, but the COVID-19 vaccines that use this technology are some of the first adenovirus-vector vaccines deployed in humans.
Only one other adenovirus vaccine, for Ebola, has been approved for use in humans. It was approved in Europe last year. Before the Johnson & Johnson vaccine, no other adenovirus vector has been available for use in humans in the United States.
There are six adenovirus-vector vaccines for COVID-19. In addition to AstraZeneca and Johnson & Johnson, there’s the Russian-developed vaccine Sputnik V, along with CanSino from China, and the Covishield vaccine in India.
Adenovirus vaccines are more stable than the mRNA vaccines. That makes them easier to store and transport.
But they have a significant downside, too. Because adenoviruses infect humans out in the world, we already make antibodies against them. So there’s always a danger that our immune systems might recognize and react to the vaccine, rendering it ineffective. For that reason, scientists try to carefully select the adenovirus vectors, or carriers, they use.
The two vaccines under investigation for blood clots are slightly different. The Johnson & Johnson vaccine uses the vector AD26, because most of the population lacks preexisting immunity to it. Vaxzevria uses an adenovirus that infects chimpanzees, called ChAdOx1.
Vaxzevria has been widely used in Europe but has not yet been authorized in the United States.
On April 7, the European Medicines Agency, Europe’s counterpart to the FDA, ruled that unusual blood clots with low blood platelets should be listed as rare side effects on the Vaxzevria vaccine.
The decision came after reviewing 62 cases of cerebral venous sinus thrombosis (CVST) linked to the vaccine and 25 cases of another rare type of clot, called a splanchnic vein thrombosis. Splanchnic veins drain blood from the major organs in the digestive system, including the stomach, liver, and intestines; 18 of those events were fatal.
The reports were culled from reporting in Europe and the United Kingdom, where around 25 million people have received the Vaxzevria vaccine, making these clots exceptionally rare, but serious.
So far, six cases of CVST have been reported in the United States, after more than 7 million doses of the Johnson & Johnson vaccines have been administered.
A key question for U.S. regulators will be the background rate for these types of rare combinations of clots and deplenished platelets. The background rate is the number of events that would be expected to occur naturally in a population of unvaccinated people. On a press call on April 13, Peter Marks, MD, PhD, director of the FDA’s Center for Biologics Evaluation and Research, was asked about the frequency of this dangerous combination. He said the combination of low platelets and clots was so rare that it was hard to pinpoint, but might be somewhere between 2 and 14 cases per million people over the course of a year.
The first Johnson & Johnson doses were given in early March. That means the six cases came to light within the first few weeks of use of the vaccine in the United States, a very short amount of time.
“These were six cases per million people for 2 weeks, which is the same thing as 25 million per year, so it’s clearly above the background rate,” Dr. Offit said.
Studies suggest possible mechanism
On April 9, the New England Journal of Medicine published a detailed evaluation of the 11 patients in Germany and Austria who developed the rare clots after their Vaxzevria vaccines.
The study detected rare antibodies to a signaling protein called platelet factor 4, which helps to coordinate clot formation.
These same type of antibodies form in some people given the blood thinning drug heparin. In those reactions, which are also exceptionally rare, the same type of syndrome develops, leading to large, devastating clots that consume circulating platelets.
It’s not yet clear whether people who develop reactions to the vaccines already have some platelet factor 4 antibodies before they are vaccinated, or whether the vaccines somehow spur the body to make these antibodies, which then launch a kind of autoimmune attack.
The researchers on the paper gave the syndrome a name, vaccine-induced thrombotic thrombocytopenia (VITT).
It’s also not clear why more cases seem to be in women than in men. Andrew Eisenberger, MD, an associate professor of hematology and oncology at Columbia University, New York, said the most common causes of cerebral venous sinus thrombosis have to do with conditions that raise estrogen levels, like pregnancy and hormonal contraception.
“Estrogen naturally leads to changes in several clotting proteins in the blood that may predispose to abnormal blood clotting in a few different sites in the body,” he said. “The clotting changes we are encountering with some of COVID-19 vaccines are likely to be synergistic with the effects of estrogen on the blood.”
No matter the cause, the CDC on April 13 alerted doctors to keep a high index of suspicion for VITT in patients who have received the Johnson & Johnson vaccination within the last 2 weeks. In those patients, the usual course of treatment with blood thinning drugs like heparin may be harmful.
Symptoms to watch for include severe headache or backache, new neurologic symptoms, severe abdominal pain, shortness of breath, leg swelling, tiny red spots on the skin, or easy bruising.
Grappling with evidence
The CDC’s Advisory Committee on Immunization Practices will meet today in an emergency session to review the cases and see if any changes are needed to use of the J&J vaccine in the United States.
Last week, for example, the United Kingdom restricted the use of the AstraZeneca vaccine in people aged younger than 30 years, saying the risks and benefits of vaccination are “more finely balanced” for this age group.
With cases of COVID-19 rising again in the United States, and the Johnson & Johnson vaccine currently the most convenient form of protection against the virus, the committee will have to weigh the risks of that infection against the risk of rare clots caused by vaccination.
They will also likely have to rule out whether any of the cases had COVID. At least one study has reported CVST clots in three patients with confirmed COVID infections. In Europe, COVID infection did not seem to play a role in the formation of the clots with low platelets.
Hilda Bastian, PhD, a clinical trials expert who cofounded the Cochrane Collaboration, said it won’t be an easy task. Much will depend on how certain the committee members feel they know about all the events linked to the vaccine.
“That’s the really, really hard issue from my point of view for them right this moment. Have we missed any? Or how many are we likely to have missed?” asked Dr. Bastian, who lives in Australia.
“In a country that size with that fragmented [of] a health care system, how sure can you be that you know them all? That’s going to be a really difficult situation for them to grapple with, the quality of information that they’ve got,” she said.
A version of this article first appeared on Medscape.com.
Hospitalization not rare for children with COVID, study says
Nearly a third of those had severe disease that required mechanical ventilation or admission to an intensive care unit, according to a new study published in JAMA Network Open on April 9.*
That means about 1 in 9 kids with COVID-19 in this cohort needed hospitalization, and about 1 in 28 had severe COVID-19.
“Although most children with COVID-19 experience mild illness, some children develop serious illness that leads to hospitalization, use of invasive mechanical ventilation, and death,” the researchers wrote.
The research team analyzed discharge data from 869 medical facilities in the Premier Healthcare Database Special COVID-19 Release. They looked for COVID-19 patients ages 18 and under who had an in-patient or emergency department visit between March and October 2020.
More than 20,700 children with COVID-19 had an in-patient or an emergency department visit, and 2,430 were hospitalized with COVID-19. Among those, 756 children had severe COVID-19 and were admitted to an intensive care unit or needed mechanical ventilation.
About 53% of the COVID-19 patients were girls, and about 54% were between ages 12-18. In addition, about 29% had at least one chronic condition.
Similar to COVID-19 studies in adults, Hispanic, Latino and Black patients were overrepresented. About 39% of the children were Hispanic or Latino, and 24% were Black. However, the researchers didn’t find an association between severe COVID-19 and race or ethnicity.
The likelihood of severe COVID-19 increased if the patient had at least one chronic condition, was male, or was between ages 2-11.
“Understanding factors associated with severe COVID-19 disease among children could help inform prevention and control strategies,” they added. “Reducing infection risk through community mitigation strategies is critical for protecting children from COVID-19 and preventing poor outcomes.”
As of April 8, more than 3.54 million U.S. children have tested positive for COVID-19, according to the latest report from the American Academy of Pediatrics and Children’s Hospital Association. Cases among children are increasing slightly, with about 73,000 new cases reported during the first week of April.
Children represent about 13.5% of the COVID-19 cases in the country, according to the report. Among the 24 states that provide data, children represented 1% to 3% of all COVID-19 hospitalizations, and less than 2% of all child COVID-19 cases resulted in hospitalization.
“At this time, it appears that severe illness due to COVID-19 is rare among children,” the two groups wrote.
“However, there is an urgent need to collect more data on longer-term impacts of the pandemic on children, including ways the virus may harm the long-term physical health of infected children, as well as its emotional and mental health effects,” they added.
A version of this article first appeared on WebMD.com.
*CORRECTION, 6/7/21 – This story has been corrected to clarify that the patient sample study reflects only those children who presented to an emergency department or received inpatient care for COVID-19 in a hospital network and were included in the Premier Healthcare Database Special COVID-19 Release. A previous version of the story incorrectly implied that 12% of all U.S. children with COVID-19 had required inpatient care.
Nearly a third of those had severe disease that required mechanical ventilation or admission to an intensive care unit, according to a new study published in JAMA Network Open on April 9.*
That means about 1 in 9 kids with COVID-19 in this cohort needed hospitalization, and about 1 in 28 had severe COVID-19.
“Although most children with COVID-19 experience mild illness, some children develop serious illness that leads to hospitalization, use of invasive mechanical ventilation, and death,” the researchers wrote.
The research team analyzed discharge data from 869 medical facilities in the Premier Healthcare Database Special COVID-19 Release. They looked for COVID-19 patients ages 18 and under who had an in-patient or emergency department visit between March and October 2020.
More than 20,700 children with COVID-19 had an in-patient or an emergency department visit, and 2,430 were hospitalized with COVID-19. Among those, 756 children had severe COVID-19 and were admitted to an intensive care unit or needed mechanical ventilation.
About 53% of the COVID-19 patients were girls, and about 54% were between ages 12-18. In addition, about 29% had at least one chronic condition.
Similar to COVID-19 studies in adults, Hispanic, Latino and Black patients were overrepresented. About 39% of the children were Hispanic or Latino, and 24% were Black. However, the researchers didn’t find an association between severe COVID-19 and race or ethnicity.
The likelihood of severe COVID-19 increased if the patient had at least one chronic condition, was male, or was between ages 2-11.
“Understanding factors associated with severe COVID-19 disease among children could help inform prevention and control strategies,” they added. “Reducing infection risk through community mitigation strategies is critical for protecting children from COVID-19 and preventing poor outcomes.”
As of April 8, more than 3.54 million U.S. children have tested positive for COVID-19, according to the latest report from the American Academy of Pediatrics and Children’s Hospital Association. Cases among children are increasing slightly, with about 73,000 new cases reported during the first week of April.
Children represent about 13.5% of the COVID-19 cases in the country, according to the report. Among the 24 states that provide data, children represented 1% to 3% of all COVID-19 hospitalizations, and less than 2% of all child COVID-19 cases resulted in hospitalization.
“At this time, it appears that severe illness due to COVID-19 is rare among children,” the two groups wrote.
“However, there is an urgent need to collect more data on longer-term impacts of the pandemic on children, including ways the virus may harm the long-term physical health of infected children, as well as its emotional and mental health effects,” they added.
A version of this article first appeared on WebMD.com.
*CORRECTION, 6/7/21 – This story has been corrected to clarify that the patient sample study reflects only those children who presented to an emergency department or received inpatient care for COVID-19 in a hospital network and were included in the Premier Healthcare Database Special COVID-19 Release. A previous version of the story incorrectly implied that 12% of all U.S. children with COVID-19 had required inpatient care.
Nearly a third of those had severe disease that required mechanical ventilation or admission to an intensive care unit, according to a new study published in JAMA Network Open on April 9.*
That means about 1 in 9 kids with COVID-19 in this cohort needed hospitalization, and about 1 in 28 had severe COVID-19.
“Although most children with COVID-19 experience mild illness, some children develop serious illness that leads to hospitalization, use of invasive mechanical ventilation, and death,” the researchers wrote.
The research team analyzed discharge data from 869 medical facilities in the Premier Healthcare Database Special COVID-19 Release. They looked for COVID-19 patients ages 18 and under who had an in-patient or emergency department visit between March and October 2020.
More than 20,700 children with COVID-19 had an in-patient or an emergency department visit, and 2,430 were hospitalized with COVID-19. Among those, 756 children had severe COVID-19 and were admitted to an intensive care unit or needed mechanical ventilation.
About 53% of the COVID-19 patients were girls, and about 54% were between ages 12-18. In addition, about 29% had at least one chronic condition.
Similar to COVID-19 studies in adults, Hispanic, Latino and Black patients were overrepresented. About 39% of the children were Hispanic or Latino, and 24% were Black. However, the researchers didn’t find an association between severe COVID-19 and race or ethnicity.
The likelihood of severe COVID-19 increased if the patient had at least one chronic condition, was male, or was between ages 2-11.
“Understanding factors associated with severe COVID-19 disease among children could help inform prevention and control strategies,” they added. “Reducing infection risk through community mitigation strategies is critical for protecting children from COVID-19 and preventing poor outcomes.”
As of April 8, more than 3.54 million U.S. children have tested positive for COVID-19, according to the latest report from the American Academy of Pediatrics and Children’s Hospital Association. Cases among children are increasing slightly, with about 73,000 new cases reported during the first week of April.
Children represent about 13.5% of the COVID-19 cases in the country, according to the report. Among the 24 states that provide data, children represented 1% to 3% of all COVID-19 hospitalizations, and less than 2% of all child COVID-19 cases resulted in hospitalization.
“At this time, it appears that severe illness due to COVID-19 is rare among children,” the two groups wrote.
“However, there is an urgent need to collect more data on longer-term impacts of the pandemic on children, including ways the virus may harm the long-term physical health of infected children, as well as its emotional and mental health effects,” they added.
A version of this article first appeared on WebMD.com.
*CORRECTION, 6/7/21 – This story has been corrected to clarify that the patient sample study reflects only those children who presented to an emergency department or received inpatient care for COVID-19 in a hospital network and were included in the Premier Healthcare Database Special COVID-19 Release. A previous version of the story incorrectly implied that 12% of all U.S. children with COVID-19 had required inpatient care.
How to counsel worried patients about the J&J vaccine news
On April 13, the Centers for Disease Control and Prevention and the Food and Drug Administration issued a joint statement recommending a pause in Johnson & Johnson vaccine administration, pending review of six reported U.S. cases of a rare and severe type of blood clot occurring after receiving the Johnson & Johnson vaccine. To date, more than 6.8 million doses of that vaccine have been given in the United States, so at this point the rate of detected cases of this problem is less than one in a million.
The six cases occurred in women aged 18-48 years, and symptoms occurred 6-13 days after vaccination. In these cases, cerebral venous sinus thrombosis was seen in addition to thrombocytopenia.
Physicians may receive calls from concerned patients who have received a COVID vaccine. However, more than 95% of the vaccine administrations in the United States to date have been the Pfizer and Moderna messenger RNA vaccines. No association between these vaccines and blood clots has been detected. Also, these six cases occurred within 2 weeks of Johnson & Johnson vaccination, so even among those receiving the Johnson & Johnson vaccine, those who are more than 3 weeks out from their vaccination have no need for concern regarding this rare complication.
Physicians should counsel those who have received the Johnson & Johnson vaccine less than 3 weeks ago to watch for easy bruising, gum bleeding, nose bleeds, leg or arm pain or swelling, severe headache or abdominal pain, shortness of breath, or chest pain. If they notice one or more of those symptoms, they should seek medical attention.
The Centers for Disease Control and Prevention will convene a meeting of the Advisory Committee on Immunization Practices on April 14 to review the six U.S. cases of the Johnson & Johnson vaccine and determine their significance.
Several cases of unusual thromboses and thrombocytopenia have been detected after the Oxford AstraZeneca vaccine, which uses the same adenovirus vector technology as the Johnson & Johnson vaccine, but which is not authorized for use in the United States. The Oxford AstraZeneca vaccine uses a recombinant deficient chimpanzee adenovirus to deliver the message to cells to produce antibody against the SARS-CoV-2 spike protein. The Johnson & Johnson vaccine uses a recombinant deficient human adenovirus to deliver this same message.
Two recent reports in the New England Journal of Medicine have reported on thrombosis and thrombocytopenia after the Oxford AstraZeneca vaccine in Europe. Both of these reports identified high levels of IgG antibodies to platelet factor 4–polyanion complexes, similar to the mechanism of heparin-induced thrombocytopenia. The term vaccine-induced immune thrombocytopenia was proposed for this phenomenon. Treatment of this condition involves administration of intravenous immunoglobulin and nonheparin anticoagulants. Recent updates from the World Health Organization report that 169 cases of cerebral venous sinus thrombosis and 53 of splanchnic venous thrombosis occurred after 34 million doses of the Oxford AstraZeneca vaccine was administered in the European Union and United Kingdom.
While this pause in Johnson & Johnson vaccination is disappointing news amid increased cases in parts of the country, the Johnson & Johnson vaccines make up less than 5% of the U.S. vaccine doses administered to date. According to the CDC, more than 122 million Americans have received at least one dose and more than 75 million are fully vaccinated.
Dr. Patterson has received an honorarium from Pfizer for an antifungal symposium and is a subinvestigator for the Novavax vaccine. Her spouse served as a consultant for SCYNEXIS, as a speaker for Gilead Sciences and Basilea, and has received a research grant from the National Institutes of Health for the ACTT remdesivir trial.
A version of this article first appeared on Medscape.com.
On April 13, the Centers for Disease Control and Prevention and the Food and Drug Administration issued a joint statement recommending a pause in Johnson & Johnson vaccine administration, pending review of six reported U.S. cases of a rare and severe type of blood clot occurring after receiving the Johnson & Johnson vaccine. To date, more than 6.8 million doses of that vaccine have been given in the United States, so at this point the rate of detected cases of this problem is less than one in a million.
The six cases occurred in women aged 18-48 years, and symptoms occurred 6-13 days after vaccination. In these cases, cerebral venous sinus thrombosis was seen in addition to thrombocytopenia.
Physicians may receive calls from concerned patients who have received a COVID vaccine. However, more than 95% of the vaccine administrations in the United States to date have been the Pfizer and Moderna messenger RNA vaccines. No association between these vaccines and blood clots has been detected. Also, these six cases occurred within 2 weeks of Johnson & Johnson vaccination, so even among those receiving the Johnson & Johnson vaccine, those who are more than 3 weeks out from their vaccination have no need for concern regarding this rare complication.
Physicians should counsel those who have received the Johnson & Johnson vaccine less than 3 weeks ago to watch for easy bruising, gum bleeding, nose bleeds, leg or arm pain or swelling, severe headache or abdominal pain, shortness of breath, or chest pain. If they notice one or more of those symptoms, they should seek medical attention.
The Centers for Disease Control and Prevention will convene a meeting of the Advisory Committee on Immunization Practices on April 14 to review the six U.S. cases of the Johnson & Johnson vaccine and determine their significance.
Several cases of unusual thromboses and thrombocytopenia have been detected after the Oxford AstraZeneca vaccine, which uses the same adenovirus vector technology as the Johnson & Johnson vaccine, but which is not authorized for use in the United States. The Oxford AstraZeneca vaccine uses a recombinant deficient chimpanzee adenovirus to deliver the message to cells to produce antibody against the SARS-CoV-2 spike protein. The Johnson & Johnson vaccine uses a recombinant deficient human adenovirus to deliver this same message.
Two recent reports in the New England Journal of Medicine have reported on thrombosis and thrombocytopenia after the Oxford AstraZeneca vaccine in Europe. Both of these reports identified high levels of IgG antibodies to platelet factor 4–polyanion complexes, similar to the mechanism of heparin-induced thrombocytopenia. The term vaccine-induced immune thrombocytopenia was proposed for this phenomenon. Treatment of this condition involves administration of intravenous immunoglobulin and nonheparin anticoagulants. Recent updates from the World Health Organization report that 169 cases of cerebral venous sinus thrombosis and 53 of splanchnic venous thrombosis occurred after 34 million doses of the Oxford AstraZeneca vaccine was administered in the European Union and United Kingdom.
While this pause in Johnson & Johnson vaccination is disappointing news amid increased cases in parts of the country, the Johnson & Johnson vaccines make up less than 5% of the U.S. vaccine doses administered to date. According to the CDC, more than 122 million Americans have received at least one dose and more than 75 million are fully vaccinated.
Dr. Patterson has received an honorarium from Pfizer for an antifungal symposium and is a subinvestigator for the Novavax vaccine. Her spouse served as a consultant for SCYNEXIS, as a speaker for Gilead Sciences and Basilea, and has received a research grant from the National Institutes of Health for the ACTT remdesivir trial.
A version of this article first appeared on Medscape.com.
On April 13, the Centers for Disease Control and Prevention and the Food and Drug Administration issued a joint statement recommending a pause in Johnson & Johnson vaccine administration, pending review of six reported U.S. cases of a rare and severe type of blood clot occurring after receiving the Johnson & Johnson vaccine. To date, more than 6.8 million doses of that vaccine have been given in the United States, so at this point the rate of detected cases of this problem is less than one in a million.
The six cases occurred in women aged 18-48 years, and symptoms occurred 6-13 days after vaccination. In these cases, cerebral venous sinus thrombosis was seen in addition to thrombocytopenia.
Physicians may receive calls from concerned patients who have received a COVID vaccine. However, more than 95% of the vaccine administrations in the United States to date have been the Pfizer and Moderna messenger RNA vaccines. No association between these vaccines and blood clots has been detected. Also, these six cases occurred within 2 weeks of Johnson & Johnson vaccination, so even among those receiving the Johnson & Johnson vaccine, those who are more than 3 weeks out from their vaccination have no need for concern regarding this rare complication.
Physicians should counsel those who have received the Johnson & Johnson vaccine less than 3 weeks ago to watch for easy bruising, gum bleeding, nose bleeds, leg or arm pain or swelling, severe headache or abdominal pain, shortness of breath, or chest pain. If they notice one or more of those symptoms, they should seek medical attention.
The Centers for Disease Control and Prevention will convene a meeting of the Advisory Committee on Immunization Practices on April 14 to review the six U.S. cases of the Johnson & Johnson vaccine and determine their significance.
Several cases of unusual thromboses and thrombocytopenia have been detected after the Oxford AstraZeneca vaccine, which uses the same adenovirus vector technology as the Johnson & Johnson vaccine, but which is not authorized for use in the United States. The Oxford AstraZeneca vaccine uses a recombinant deficient chimpanzee adenovirus to deliver the message to cells to produce antibody against the SARS-CoV-2 spike protein. The Johnson & Johnson vaccine uses a recombinant deficient human adenovirus to deliver this same message.
Two recent reports in the New England Journal of Medicine have reported on thrombosis and thrombocytopenia after the Oxford AstraZeneca vaccine in Europe. Both of these reports identified high levels of IgG antibodies to platelet factor 4–polyanion complexes, similar to the mechanism of heparin-induced thrombocytopenia. The term vaccine-induced immune thrombocytopenia was proposed for this phenomenon. Treatment of this condition involves administration of intravenous immunoglobulin and nonheparin anticoagulants. Recent updates from the World Health Organization report that 169 cases of cerebral venous sinus thrombosis and 53 of splanchnic venous thrombosis occurred after 34 million doses of the Oxford AstraZeneca vaccine was administered in the European Union and United Kingdom.
While this pause in Johnson & Johnson vaccination is disappointing news amid increased cases in parts of the country, the Johnson & Johnson vaccines make up less than 5% of the U.S. vaccine doses administered to date. According to the CDC, more than 122 million Americans have received at least one dose and more than 75 million are fully vaccinated.
Dr. Patterson has received an honorarium from Pfizer for an antifungal symposium and is a subinvestigator for the Novavax vaccine. Her spouse served as a consultant for SCYNEXIS, as a speaker for Gilead Sciences and Basilea, and has received a research grant from the National Institutes of Health for the ACTT remdesivir trial.
A version of this article first appeared on Medscape.com.
COVID-19 vaccine failure in patients with blood cancers
COVID vaccines do not work well for patients with hematologic malignancies, new data suggest.
A small study involving 67 such patients shows that nearly half did not produce antibodies and were therefore still at risk of contracting COVID-19, even though they had all received both doses of one of the new mRNA COVID vaccines (Moderna or Pfizer).
“[This] is in stark contrast with the results of phase 1 mRNA vaccine immunogenicity trials, in which robust antibody responses were seen in essentially 100% of participants,” said the authors, led by Mounzer Agha, MD, director of the Mario Lemieux Center for Blood Cancers at the University of Pittsburgh Medical Center’s Hillman Cancer Center.
“Clinicians caring for patients with hematological malignancies and other immunocompromising conditions should be aware of the possibility of COVID-19 vaccine failure,” they emphasized.
“It’s critically important for these patients to be aware of their continued risk [for SARS-CoV-2 infection] and to seek prompt medical attention if they have COVID-19 symptoms, even after vaccination,” Dr. Agha said in a statement.
The study was published online on April 9 as preprint in medRxiv and has not yet undergone peer review.
Antibody responses
The authors analyzed responses in a group of 67 patients who had a hematologic malignancy, including chronic lymphocytic leukemia (CLL), lymphoma, and multiple myeloma. Approximately 45% of the patients were receiving therapy for their cancer at the time of vaccination; the rest were under observation.
All patients received two doses of an mRNA COVID vaccine and so were considered to be fully vaccinated.
Antibody responses for these fully vaccinated patients were then analyzed. The median duration between receipt of the second dose of the vaccine and the antibody test was 23 days.
“In total ... 46.3% ... had a negative antibody result after vaccination and were therefore considered to be vaccine nonresponders,” the authors reported.
The worst responses occurred in patients with CLL, of whom only 23% produced measurable antibodies to either vaccine, although approximately 70% of these patients were not receiving any form of cancer therapy at the time of vaccination.
Older patients were more likely not to have a response to either vaccine compared with younger patients, the investigators added.
In contrast, gender, immunoglobulin G levels, the number of days between the second dose and the measurement of antibodies, and status of cancer therapy did not differ among patients who had a response to the vaccines and those who did not.
“Our findings underscore the importance of adherence to nonpharmaceutical interventions to prevent COVID-19 in hematological malignancy patients,” the authors wrote. This is particularly important, given the fact that among patients with hematologic malignancies who become infected with SARS-CoV-2, the mortality rate is in excess of 30%.
Moreover, among such patients, viral shedding may be prolonged, often lasting several months. As such, “these patients should be advised to wear masks and observe social distancing regardless of vaccination status,” the investigators advised.
As of March 2021, guidance from the Centers for Disease Control and Prevention has allowed gatherings of unmasked people who have been vaccinated and of those at low risk for COVID-19 who have not yet been vaccinated. “As we see more national guidance allowing for unmasked gatherings among vaccinated people, clinicians should counsel their immunocompromised patients about the possibility that COVID-19 vaccines may not fully protect them against SARS-CoV-2,” coauthor Ghady Haidar, MD, assistant professor of medicine at the University of Pittsburgh, said in a statement.
“Our results show that the odds of the vaccine producing an antibody response in people with hematologic malignancies are the equivalent of a coin flip,” he said.
The authors have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
COVID vaccines do not work well for patients with hematologic malignancies, new data suggest.
A small study involving 67 such patients shows that nearly half did not produce antibodies and were therefore still at risk of contracting COVID-19, even though they had all received both doses of one of the new mRNA COVID vaccines (Moderna or Pfizer).
“[This] is in stark contrast with the results of phase 1 mRNA vaccine immunogenicity trials, in which robust antibody responses were seen in essentially 100% of participants,” said the authors, led by Mounzer Agha, MD, director of the Mario Lemieux Center for Blood Cancers at the University of Pittsburgh Medical Center’s Hillman Cancer Center.
“Clinicians caring for patients with hematological malignancies and other immunocompromising conditions should be aware of the possibility of COVID-19 vaccine failure,” they emphasized.
“It’s critically important for these patients to be aware of their continued risk [for SARS-CoV-2 infection] and to seek prompt medical attention if they have COVID-19 symptoms, even after vaccination,” Dr. Agha said in a statement.
The study was published online on April 9 as preprint in medRxiv and has not yet undergone peer review.
Antibody responses
The authors analyzed responses in a group of 67 patients who had a hematologic malignancy, including chronic lymphocytic leukemia (CLL), lymphoma, and multiple myeloma. Approximately 45% of the patients were receiving therapy for their cancer at the time of vaccination; the rest were under observation.
All patients received two doses of an mRNA COVID vaccine and so were considered to be fully vaccinated.
Antibody responses for these fully vaccinated patients were then analyzed. The median duration between receipt of the second dose of the vaccine and the antibody test was 23 days.
“In total ... 46.3% ... had a negative antibody result after vaccination and were therefore considered to be vaccine nonresponders,” the authors reported.
The worst responses occurred in patients with CLL, of whom only 23% produced measurable antibodies to either vaccine, although approximately 70% of these patients were not receiving any form of cancer therapy at the time of vaccination.
Older patients were more likely not to have a response to either vaccine compared with younger patients, the investigators added.
In contrast, gender, immunoglobulin G levels, the number of days between the second dose and the measurement of antibodies, and status of cancer therapy did not differ among patients who had a response to the vaccines and those who did not.
“Our findings underscore the importance of adherence to nonpharmaceutical interventions to prevent COVID-19 in hematological malignancy patients,” the authors wrote. This is particularly important, given the fact that among patients with hematologic malignancies who become infected with SARS-CoV-2, the mortality rate is in excess of 30%.
Moreover, among such patients, viral shedding may be prolonged, often lasting several months. As such, “these patients should be advised to wear masks and observe social distancing regardless of vaccination status,” the investigators advised.
As of March 2021, guidance from the Centers for Disease Control and Prevention has allowed gatherings of unmasked people who have been vaccinated and of those at low risk for COVID-19 who have not yet been vaccinated. “As we see more national guidance allowing for unmasked gatherings among vaccinated people, clinicians should counsel their immunocompromised patients about the possibility that COVID-19 vaccines may not fully protect them against SARS-CoV-2,” coauthor Ghady Haidar, MD, assistant professor of medicine at the University of Pittsburgh, said in a statement.
“Our results show that the odds of the vaccine producing an antibody response in people with hematologic malignancies are the equivalent of a coin flip,” he said.
The authors have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
COVID vaccines do not work well for patients with hematologic malignancies, new data suggest.
A small study involving 67 such patients shows that nearly half did not produce antibodies and were therefore still at risk of contracting COVID-19, even though they had all received both doses of one of the new mRNA COVID vaccines (Moderna or Pfizer).
“[This] is in stark contrast with the results of phase 1 mRNA vaccine immunogenicity trials, in which robust antibody responses were seen in essentially 100% of participants,” said the authors, led by Mounzer Agha, MD, director of the Mario Lemieux Center for Blood Cancers at the University of Pittsburgh Medical Center’s Hillman Cancer Center.
“Clinicians caring for patients with hematological malignancies and other immunocompromising conditions should be aware of the possibility of COVID-19 vaccine failure,” they emphasized.
“It’s critically important for these patients to be aware of their continued risk [for SARS-CoV-2 infection] and to seek prompt medical attention if they have COVID-19 symptoms, even after vaccination,” Dr. Agha said in a statement.
The study was published online on April 9 as preprint in medRxiv and has not yet undergone peer review.
Antibody responses
The authors analyzed responses in a group of 67 patients who had a hematologic malignancy, including chronic lymphocytic leukemia (CLL), lymphoma, and multiple myeloma. Approximately 45% of the patients were receiving therapy for their cancer at the time of vaccination; the rest were under observation.
All patients received two doses of an mRNA COVID vaccine and so were considered to be fully vaccinated.
Antibody responses for these fully vaccinated patients were then analyzed. The median duration between receipt of the second dose of the vaccine and the antibody test was 23 days.
“In total ... 46.3% ... had a negative antibody result after vaccination and were therefore considered to be vaccine nonresponders,” the authors reported.
The worst responses occurred in patients with CLL, of whom only 23% produced measurable antibodies to either vaccine, although approximately 70% of these patients were not receiving any form of cancer therapy at the time of vaccination.
Older patients were more likely not to have a response to either vaccine compared with younger patients, the investigators added.
In contrast, gender, immunoglobulin G levels, the number of days between the second dose and the measurement of antibodies, and status of cancer therapy did not differ among patients who had a response to the vaccines and those who did not.
“Our findings underscore the importance of adherence to nonpharmaceutical interventions to prevent COVID-19 in hematological malignancy patients,” the authors wrote. This is particularly important, given the fact that among patients with hematologic malignancies who become infected with SARS-CoV-2, the mortality rate is in excess of 30%.
Moreover, among such patients, viral shedding may be prolonged, often lasting several months. As such, “these patients should be advised to wear masks and observe social distancing regardless of vaccination status,” the investigators advised.
As of March 2021, guidance from the Centers for Disease Control and Prevention has allowed gatherings of unmasked people who have been vaccinated and of those at low risk for COVID-19 who have not yet been vaccinated. “As we see more national guidance allowing for unmasked gatherings among vaccinated people, clinicians should counsel their immunocompromised patients about the possibility that COVID-19 vaccines may not fully protect them against SARS-CoV-2,” coauthor Ghady Haidar, MD, assistant professor of medicine at the University of Pittsburgh, said in a statement.
“Our results show that the odds of the vaccine producing an antibody response in people with hematologic malignancies are the equivalent of a coin flip,” he said.
The authors have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Data about COVID-19-related skin manifestations in children continue to emerge
Two and stratifying children at risk for serious, systemic illness due to the virus.
In a single-center descriptive study carried out over a 9-month period, researchers in Madrid found that of 50 hospitalized children infected with COVID-19, 21 (42%) had mucocutaneous symptoms, most commonly exanthem, followed by conjunctival hyperemia without secretion and red cracked lips or strawberry tongue. In addition, 18 (36%) fulfilled criteria for Multisystem Inflammatory Syndrome in Children (MIS-C).
“Based on findings in adult patients, the skin manifestations of COVID-19 have been classified under five categories: acral pseudo-chilblain, vesicular eruptions, urticarial lesions, maculopapular eruptions, and livedo or necrosis,” David Andina-Martinez, MD, of Hospital Infantil Universitario Niño Jesús, Madrid, and colleagues wrote in the study, which was published online on April 2 in the Journal of the American Academy of Dermatology.
“Chilblain lesions in healthy children and adolescents have received much attention; these lesions resolve without complications after a few weeks,” they added. “Besides, other cutaneous manifestations of COVID-19 in children have been the matter of case reports or small case series. Nevertheless, the mucocutaneous manifestations in hospitalized children infected with SARS-CoV-2 and their implications on the clinical course have not yet been extensively described.”
In an effort to describe the mucocutaneous manifestations in children hospitalized for COVID-19, the researchers evaluated 50 children up to 18 years of age who were admitted between March 1 and Nov. 30, 2020, to Hospital Infantil Universitario Niño Jesús, which was designated as a pediatric reference center during the peak of the pandemic. The main reasons for admission were respiratory illness (40%) and MIS-C (40%).
Of the 50 patients, 44 (88%) had a positive RT-PCR for SARS-CoV-2 and 6 (12%) met clinical suspicion criteria and had a negative RT-PCR with a positive IgG serology. In 34 patients (68%), a close contact with a suspected or confirmed case of COVID-19 was referred, while the source of the infection remained unknown in the remaining 16 patients (32%).
The researchers reported that 21 patients (42%) had mucocutaneous symptoms, most commonly maculopapular exanthem (86%), conjunctival hyperemia (81%), and red cracked lips or strawberry tongue (43%). In addition, 18 of the 21 patients (86%) fulfilled criteria for MIS-C.
“A tricky thing about MIS-C is that it often manifests 4-5 weeks after a child had COVID-19,” said Christine Ko, MD, professor of dermatology and pathology at Yale University, New Haven, Conn., who was asked to comment on the study. “MIS-C is associated with characteristic bright red lips and a red tongue that might resemble a strawberry. Such oral findings should prompt rapid evaluation for other signs and symptoms. There can be redness of the eyes or other more nonspecific skin findings (large or small areas of redness on the trunk or limbs, sometimes with surface change), but more importantly, fever, a rapid heartbeat, diarrhea, or breathing issues. The risk with MIS-C is a rapid decline in a child’s health, with admission to an intensive care unit.”
Dr. Andina-Martinez and his colleagues also contrast the skin findings of MIS-C, which are not generally on the hands or feet, with the so-called “COVID toe” or finger phenomenon, which has also been associated with SARS-CoV-2, particularly in children. “Only one of the patients in this series had skin involvement of a finger, and it only appeared after recovery from MIS-C,” Dr. Ko noted. “Distinguishing COVID toes from MIS-C is important, as COVID toes has a very good outcome, while MIS-C can have severe consequences, including protracted heart disease.”
In other findings, patients who presented with mucocutaneous signs tended to be older than those without skin signs and they presented at the emergency department with poor general status and extreme tachycardia. They also had higher C-reactive protein and D-dimer levels and lower lymphocyte counts and faced a more than a 10-fold increased risk of being admitted to the PICU, compared with patients who did not have skin signs (OR, 10.24; P = .003).
In a separate study published online on April 7 in JAMA Dermatology, Zachary E. Holcomb, MD, of the combined dermatology residency program at Massachusetts General Hospital, Boston, and colleagues presented what is believed to be the first case report of reactive infectious mucocutaneous eruption (RIME) triggered by SARS-CoV-2. RIME is the preferred term for pediatric patients who present with mucositis and rash (often a scant or even absent skin eruption) triggered by various infectious agents.
The patient, a 17-year-old male, presented to the emergency department with 3 days of mouth pain and nonpainful penile erosions. “One week prior, he experienced transient anosmia and ageusia that had since spontaneously resolved,” the researchers wrote. “At that time, he was tested for SARS-CoV-2 infection via nasopharyngeal polymerase chain reaction (PCR), the results of which were positive.”
At presentation, the patient had no fever, his vital signs were normal, and the physical exam revealed shallow erosions of the vermilion lips and hard palate, circumferential erythematous erosions of the periurethral glans penis, and five small vesicles on the trunk and upper extremities. Serum analysis revealed a normal white blood cell count with mild absolute lymphopenia, slightly elevated creatinine level, normal liver function, slightly elevated C-reactive protein level, and normal ferritin level.
Dr. Holcomb and colleagues made a diagnosis of SARS-CoV-2–associated RIME based on microbiological results, which revealed positive repeated SARS-CoV-2 nasopharyngeal PCR and negative nasopharyngeal PCR testing for Mycoplasma pneumoniae, adenovirus, Chlamydophila pneumoniae, human metapneumovirus, influenza A/B, parainfluenza 1 to 4, rhinovirus, and respiratory syncytial virus. In addition, titers of Mycoplasma pneumoniae IgM levels were negative, but Mycoplasma pneumoniae IgG levels were elevated.
The lesions resolved with 60 mg of oral prednisone taken daily for 4 days. A recurrence of oral mucositis 3 months later responded to 80 mg oral prednisone taken daily for 6 days.
“It’s not surprising that SARS-CoV-2 is yet another trigger for RIME,” said Anna Yasmine Kirkorian, MD, chief of the division of dermatology at Children’s National Hospital, Washington, who was asked to comment about the case report.
“The take-home message is for clinicians to be aware of this association and distinguish these patients from those with MIS-C, because patients with MIS-C require monitoring and urgent systemic treatment. RIME and MIS-C may potentially be distinguished clinically based on the nature of the mucositis (hemorrhagic and erosive in RIME, dry, cracked lips with ‘strawberry tongue’ in MIS-C) but more importantly patients with RIME lack laboratory evidence of severe systemic inflammation,” such as ESR, CRP, or ferritin, she said.
“A final interesting point in this article was the recurrence of mucositis in this patient, which could mean that recurrent mucositis/recurrent RIME might be yet another manifestation of ‘long-COVID’ (now called post-Acute Sequelae of SARS-CoV-2 infection) in some patients,” Dr. Kirkorian added. She noted that the American Academy of Dermatology–International League of Dermatologic Societies COVID-19 Dermatology Registry and articles like these “provide invaluable ‘hot off the presses’ information for clinicians who are facing the protean manifestations of a novel viral epidemic.”
The researchers reported having no financial disclosures.
Two and stratifying children at risk for serious, systemic illness due to the virus.
In a single-center descriptive study carried out over a 9-month period, researchers in Madrid found that of 50 hospitalized children infected with COVID-19, 21 (42%) had mucocutaneous symptoms, most commonly exanthem, followed by conjunctival hyperemia without secretion and red cracked lips or strawberry tongue. In addition, 18 (36%) fulfilled criteria for Multisystem Inflammatory Syndrome in Children (MIS-C).
“Based on findings in adult patients, the skin manifestations of COVID-19 have been classified under five categories: acral pseudo-chilblain, vesicular eruptions, urticarial lesions, maculopapular eruptions, and livedo or necrosis,” David Andina-Martinez, MD, of Hospital Infantil Universitario Niño Jesús, Madrid, and colleagues wrote in the study, which was published online on April 2 in the Journal of the American Academy of Dermatology.
“Chilblain lesions in healthy children and adolescents have received much attention; these lesions resolve without complications after a few weeks,” they added. “Besides, other cutaneous manifestations of COVID-19 in children have been the matter of case reports or small case series. Nevertheless, the mucocutaneous manifestations in hospitalized children infected with SARS-CoV-2 and their implications on the clinical course have not yet been extensively described.”
In an effort to describe the mucocutaneous manifestations in children hospitalized for COVID-19, the researchers evaluated 50 children up to 18 years of age who were admitted between March 1 and Nov. 30, 2020, to Hospital Infantil Universitario Niño Jesús, which was designated as a pediatric reference center during the peak of the pandemic. The main reasons for admission were respiratory illness (40%) and MIS-C (40%).
Of the 50 patients, 44 (88%) had a positive RT-PCR for SARS-CoV-2 and 6 (12%) met clinical suspicion criteria and had a negative RT-PCR with a positive IgG serology. In 34 patients (68%), a close contact with a suspected or confirmed case of COVID-19 was referred, while the source of the infection remained unknown in the remaining 16 patients (32%).
The researchers reported that 21 patients (42%) had mucocutaneous symptoms, most commonly maculopapular exanthem (86%), conjunctival hyperemia (81%), and red cracked lips or strawberry tongue (43%). In addition, 18 of the 21 patients (86%) fulfilled criteria for MIS-C.
“A tricky thing about MIS-C is that it often manifests 4-5 weeks after a child had COVID-19,” said Christine Ko, MD, professor of dermatology and pathology at Yale University, New Haven, Conn., who was asked to comment on the study. “MIS-C is associated with characteristic bright red lips and a red tongue that might resemble a strawberry. Such oral findings should prompt rapid evaluation for other signs and symptoms. There can be redness of the eyes or other more nonspecific skin findings (large or small areas of redness on the trunk or limbs, sometimes with surface change), but more importantly, fever, a rapid heartbeat, diarrhea, or breathing issues. The risk with MIS-C is a rapid decline in a child’s health, with admission to an intensive care unit.”
Dr. Andina-Martinez and his colleagues also contrast the skin findings of MIS-C, which are not generally on the hands or feet, with the so-called “COVID toe” or finger phenomenon, which has also been associated with SARS-CoV-2, particularly in children. “Only one of the patients in this series had skin involvement of a finger, and it only appeared after recovery from MIS-C,” Dr. Ko noted. “Distinguishing COVID toes from MIS-C is important, as COVID toes has a very good outcome, while MIS-C can have severe consequences, including protracted heart disease.”
In other findings, patients who presented with mucocutaneous signs tended to be older than those without skin signs and they presented at the emergency department with poor general status and extreme tachycardia. They also had higher C-reactive protein and D-dimer levels and lower lymphocyte counts and faced a more than a 10-fold increased risk of being admitted to the PICU, compared with patients who did not have skin signs (OR, 10.24; P = .003).
In a separate study published online on April 7 in JAMA Dermatology, Zachary E. Holcomb, MD, of the combined dermatology residency program at Massachusetts General Hospital, Boston, and colleagues presented what is believed to be the first case report of reactive infectious mucocutaneous eruption (RIME) triggered by SARS-CoV-2. RIME is the preferred term for pediatric patients who present with mucositis and rash (often a scant or even absent skin eruption) triggered by various infectious agents.
The patient, a 17-year-old male, presented to the emergency department with 3 days of mouth pain and nonpainful penile erosions. “One week prior, he experienced transient anosmia and ageusia that had since spontaneously resolved,” the researchers wrote. “At that time, he was tested for SARS-CoV-2 infection via nasopharyngeal polymerase chain reaction (PCR), the results of which were positive.”
At presentation, the patient had no fever, his vital signs were normal, and the physical exam revealed shallow erosions of the vermilion lips and hard palate, circumferential erythematous erosions of the periurethral glans penis, and five small vesicles on the trunk and upper extremities. Serum analysis revealed a normal white blood cell count with mild absolute lymphopenia, slightly elevated creatinine level, normal liver function, slightly elevated C-reactive protein level, and normal ferritin level.
Dr. Holcomb and colleagues made a diagnosis of SARS-CoV-2–associated RIME based on microbiological results, which revealed positive repeated SARS-CoV-2 nasopharyngeal PCR and negative nasopharyngeal PCR testing for Mycoplasma pneumoniae, adenovirus, Chlamydophila pneumoniae, human metapneumovirus, influenza A/B, parainfluenza 1 to 4, rhinovirus, and respiratory syncytial virus. In addition, titers of Mycoplasma pneumoniae IgM levels were negative, but Mycoplasma pneumoniae IgG levels were elevated.
The lesions resolved with 60 mg of oral prednisone taken daily for 4 days. A recurrence of oral mucositis 3 months later responded to 80 mg oral prednisone taken daily for 6 days.
“It’s not surprising that SARS-CoV-2 is yet another trigger for RIME,” said Anna Yasmine Kirkorian, MD, chief of the division of dermatology at Children’s National Hospital, Washington, who was asked to comment about the case report.
“The take-home message is for clinicians to be aware of this association and distinguish these patients from those with MIS-C, because patients with MIS-C require monitoring and urgent systemic treatment. RIME and MIS-C may potentially be distinguished clinically based on the nature of the mucositis (hemorrhagic and erosive in RIME, dry, cracked lips with ‘strawberry tongue’ in MIS-C) but more importantly patients with RIME lack laboratory evidence of severe systemic inflammation,” such as ESR, CRP, or ferritin, she said.
“A final interesting point in this article was the recurrence of mucositis in this patient, which could mean that recurrent mucositis/recurrent RIME might be yet another manifestation of ‘long-COVID’ (now called post-Acute Sequelae of SARS-CoV-2 infection) in some patients,” Dr. Kirkorian added. She noted that the American Academy of Dermatology–International League of Dermatologic Societies COVID-19 Dermatology Registry and articles like these “provide invaluable ‘hot off the presses’ information for clinicians who are facing the protean manifestations of a novel viral epidemic.”
The researchers reported having no financial disclosures.
Two and stratifying children at risk for serious, systemic illness due to the virus.
In a single-center descriptive study carried out over a 9-month period, researchers in Madrid found that of 50 hospitalized children infected with COVID-19, 21 (42%) had mucocutaneous symptoms, most commonly exanthem, followed by conjunctival hyperemia without secretion and red cracked lips or strawberry tongue. In addition, 18 (36%) fulfilled criteria for Multisystem Inflammatory Syndrome in Children (MIS-C).
“Based on findings in adult patients, the skin manifestations of COVID-19 have been classified under five categories: acral pseudo-chilblain, vesicular eruptions, urticarial lesions, maculopapular eruptions, and livedo or necrosis,” David Andina-Martinez, MD, of Hospital Infantil Universitario Niño Jesús, Madrid, and colleagues wrote in the study, which was published online on April 2 in the Journal of the American Academy of Dermatology.
“Chilblain lesions in healthy children and adolescents have received much attention; these lesions resolve without complications after a few weeks,” they added. “Besides, other cutaneous manifestations of COVID-19 in children have been the matter of case reports or small case series. Nevertheless, the mucocutaneous manifestations in hospitalized children infected with SARS-CoV-2 and their implications on the clinical course have not yet been extensively described.”
In an effort to describe the mucocutaneous manifestations in children hospitalized for COVID-19, the researchers evaluated 50 children up to 18 years of age who were admitted between March 1 and Nov. 30, 2020, to Hospital Infantil Universitario Niño Jesús, which was designated as a pediatric reference center during the peak of the pandemic. The main reasons for admission were respiratory illness (40%) and MIS-C (40%).
Of the 50 patients, 44 (88%) had a positive RT-PCR for SARS-CoV-2 and 6 (12%) met clinical suspicion criteria and had a negative RT-PCR with a positive IgG serology. In 34 patients (68%), a close contact with a suspected or confirmed case of COVID-19 was referred, while the source of the infection remained unknown in the remaining 16 patients (32%).
The researchers reported that 21 patients (42%) had mucocutaneous symptoms, most commonly maculopapular exanthem (86%), conjunctival hyperemia (81%), and red cracked lips or strawberry tongue (43%). In addition, 18 of the 21 patients (86%) fulfilled criteria for MIS-C.
“A tricky thing about MIS-C is that it often manifests 4-5 weeks after a child had COVID-19,” said Christine Ko, MD, professor of dermatology and pathology at Yale University, New Haven, Conn., who was asked to comment on the study. “MIS-C is associated with characteristic bright red lips and a red tongue that might resemble a strawberry. Such oral findings should prompt rapid evaluation for other signs and symptoms. There can be redness of the eyes or other more nonspecific skin findings (large or small areas of redness on the trunk or limbs, sometimes with surface change), but more importantly, fever, a rapid heartbeat, diarrhea, or breathing issues. The risk with MIS-C is a rapid decline in a child’s health, with admission to an intensive care unit.”
Dr. Andina-Martinez and his colleagues also contrast the skin findings of MIS-C, which are not generally on the hands or feet, with the so-called “COVID toe” or finger phenomenon, which has also been associated with SARS-CoV-2, particularly in children. “Only one of the patients in this series had skin involvement of a finger, and it only appeared after recovery from MIS-C,” Dr. Ko noted. “Distinguishing COVID toes from MIS-C is important, as COVID toes has a very good outcome, while MIS-C can have severe consequences, including protracted heart disease.”
In other findings, patients who presented with mucocutaneous signs tended to be older than those without skin signs and they presented at the emergency department with poor general status and extreme tachycardia. They also had higher C-reactive protein and D-dimer levels and lower lymphocyte counts and faced a more than a 10-fold increased risk of being admitted to the PICU, compared with patients who did not have skin signs (OR, 10.24; P = .003).
In a separate study published online on April 7 in JAMA Dermatology, Zachary E. Holcomb, MD, of the combined dermatology residency program at Massachusetts General Hospital, Boston, and colleagues presented what is believed to be the first case report of reactive infectious mucocutaneous eruption (RIME) triggered by SARS-CoV-2. RIME is the preferred term for pediatric patients who present with mucositis and rash (often a scant or even absent skin eruption) triggered by various infectious agents.
The patient, a 17-year-old male, presented to the emergency department with 3 days of mouth pain and nonpainful penile erosions. “One week prior, he experienced transient anosmia and ageusia that had since spontaneously resolved,” the researchers wrote. “At that time, he was tested for SARS-CoV-2 infection via nasopharyngeal polymerase chain reaction (PCR), the results of which were positive.”
At presentation, the patient had no fever, his vital signs were normal, and the physical exam revealed shallow erosions of the vermilion lips and hard palate, circumferential erythematous erosions of the periurethral glans penis, and five small vesicles on the trunk and upper extremities. Serum analysis revealed a normal white blood cell count with mild absolute lymphopenia, slightly elevated creatinine level, normal liver function, slightly elevated C-reactive protein level, and normal ferritin level.
Dr. Holcomb and colleagues made a diagnosis of SARS-CoV-2–associated RIME based on microbiological results, which revealed positive repeated SARS-CoV-2 nasopharyngeal PCR and negative nasopharyngeal PCR testing for Mycoplasma pneumoniae, adenovirus, Chlamydophila pneumoniae, human metapneumovirus, influenza A/B, parainfluenza 1 to 4, rhinovirus, and respiratory syncytial virus. In addition, titers of Mycoplasma pneumoniae IgM levels were negative, but Mycoplasma pneumoniae IgG levels were elevated.
The lesions resolved with 60 mg of oral prednisone taken daily for 4 days. A recurrence of oral mucositis 3 months later responded to 80 mg oral prednisone taken daily for 6 days.
“It’s not surprising that SARS-CoV-2 is yet another trigger for RIME,” said Anna Yasmine Kirkorian, MD, chief of the division of dermatology at Children’s National Hospital, Washington, who was asked to comment about the case report.
“The take-home message is for clinicians to be aware of this association and distinguish these patients from those with MIS-C, because patients with MIS-C require monitoring and urgent systemic treatment. RIME and MIS-C may potentially be distinguished clinically based on the nature of the mucositis (hemorrhagic and erosive in RIME, dry, cracked lips with ‘strawberry tongue’ in MIS-C) but more importantly patients with RIME lack laboratory evidence of severe systemic inflammation,” such as ESR, CRP, or ferritin, she said.
“A final interesting point in this article was the recurrence of mucositis in this patient, which could mean that recurrent mucositis/recurrent RIME might be yet another manifestation of ‘long-COVID’ (now called post-Acute Sequelae of SARS-CoV-2 infection) in some patients,” Dr. Kirkorian added. She noted that the American Academy of Dermatology–International League of Dermatologic Societies COVID-19 Dermatology Registry and articles like these “provide invaluable ‘hot off the presses’ information for clinicians who are facing the protean manifestations of a novel viral epidemic.”
The researchers reported having no financial disclosures.
COVID-19 in children: New cases on the rise again
The number of new COVID-19 cases in children rose for the third time in the last 4 weeks, reaching the highest point since mid-February, according to a report from the American Academy of Pediatrics and the Children’s Hospital Association.
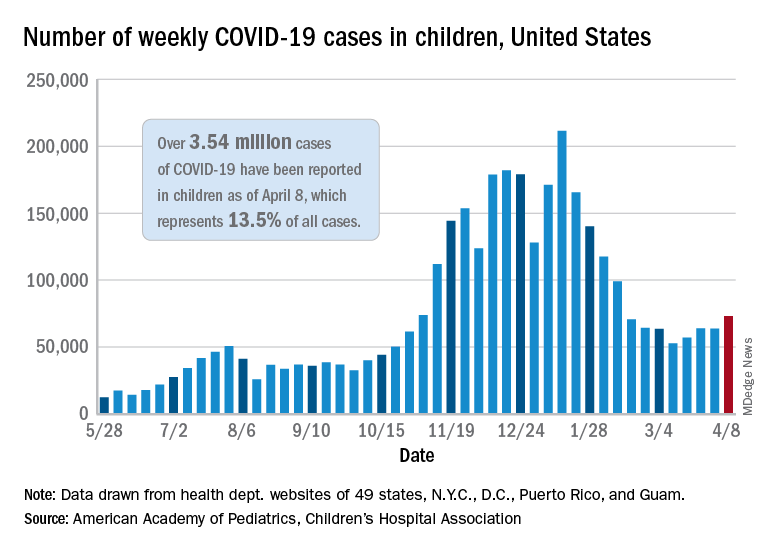
Just over 73,000 cases were reported during the week of April 2-8, up by 14.6% over the previous week. For the latest week, children represented 18.8% of all COVID-19 cases in the United States – also up from the week before and the second-highest proportion seen during the entire pandemic, based on data in the weekly AAP/CHA report.
The 3.54 million children who have been infected with SARS-CoV-2 make up 13.5% of all cases reported in the United States during the pandemic, a figure that climbed again after 2 weeks at 13.4%. The overall rate of infection was just over 4,700 cases per 100,000 children as of April 8, the AAP and CHA said.
State-level data show that Vermont, Michigan, and Maine have been the COVID-19 hotspots over the past 2 weeks. The total number of cases has jumped by almost 19% in Vermont since the week of March 19-25, by 18% in Michigan, and by 12% in Maine, according to the report.
Cumulative data also indicate that the children of Vermont are bearing a greater share of the COVID-19 burden – 21.5% of all cases – than in any other state. North Dakota, meanwhile, has the highest cumulative rate of infection at 9,057 cases per 100,000 children, based on data from 49 states (excluding New York), the District of Columbia, New York City, Puerto Rico, and Guam.
The number of COVID-19–related deaths in children increased by 8 during the week of April 2-8 and now stands at 292, just 0.06% of all deaths reported in the 43 states (along with New York City, Puerto Rico, and Guam) that provide age distributions for mortality data, the AAP and CHA said.
The number of new COVID-19 cases in children rose for the third time in the last 4 weeks, reaching the highest point since mid-February, according to a report from the American Academy of Pediatrics and the Children’s Hospital Association.

Just over 73,000 cases were reported during the week of April 2-8, up by 14.6% over the previous week. For the latest week, children represented 18.8% of all COVID-19 cases in the United States – also up from the week before and the second-highest proportion seen during the entire pandemic, based on data in the weekly AAP/CHA report.
The 3.54 million children who have been infected with SARS-CoV-2 make up 13.5% of all cases reported in the United States during the pandemic, a figure that climbed again after 2 weeks at 13.4%. The overall rate of infection was just over 4,700 cases per 100,000 children as of April 8, the AAP and CHA said.
State-level data show that Vermont, Michigan, and Maine have been the COVID-19 hotspots over the past 2 weeks. The total number of cases has jumped by almost 19% in Vermont since the week of March 19-25, by 18% in Michigan, and by 12% in Maine, according to the report.
Cumulative data also indicate that the children of Vermont are bearing a greater share of the COVID-19 burden – 21.5% of all cases – than in any other state. North Dakota, meanwhile, has the highest cumulative rate of infection at 9,057 cases per 100,000 children, based on data from 49 states (excluding New York), the District of Columbia, New York City, Puerto Rico, and Guam.
The number of COVID-19–related deaths in children increased by 8 during the week of April 2-8 and now stands at 292, just 0.06% of all deaths reported in the 43 states (along with New York City, Puerto Rico, and Guam) that provide age distributions for mortality data, the AAP and CHA said.
The number of new COVID-19 cases in children rose for the third time in the last 4 weeks, reaching the highest point since mid-February, according to a report from the American Academy of Pediatrics and the Children’s Hospital Association.

Just over 73,000 cases were reported during the week of April 2-8, up by 14.6% over the previous week. For the latest week, children represented 18.8% of all COVID-19 cases in the United States – also up from the week before and the second-highest proportion seen during the entire pandemic, based on data in the weekly AAP/CHA report.
The 3.54 million children who have been infected with SARS-CoV-2 make up 13.5% of all cases reported in the United States during the pandemic, a figure that climbed again after 2 weeks at 13.4%. The overall rate of infection was just over 4,700 cases per 100,000 children as of April 8, the AAP and CHA said.
State-level data show that Vermont, Michigan, and Maine have been the COVID-19 hotspots over the past 2 weeks. The total number of cases has jumped by almost 19% in Vermont since the week of March 19-25, by 18% in Michigan, and by 12% in Maine, according to the report.
Cumulative data also indicate that the children of Vermont are bearing a greater share of the COVID-19 burden – 21.5% of all cases – than in any other state. North Dakota, meanwhile, has the highest cumulative rate of infection at 9,057 cases per 100,000 children, based on data from 49 states (excluding New York), the District of Columbia, New York City, Puerto Rico, and Guam.
The number of COVID-19–related deaths in children increased by 8 during the week of April 2-8 and now stands at 292, just 0.06% of all deaths reported in the 43 states (along with New York City, Puerto Rico, and Guam) that provide age distributions for mortality data, the AAP and CHA said.
Next winter may be rough: Models predict ‘considerable surge’ of COVID
It’s likely the United States will see another surge of COVID-19 this winter, warned Christopher Murray, MD, director of the Institute for Health Metrics and Evaluation (IHME) at the University of Washington in Seattle.
Speaking at the national conference of State of Reform on April 8, Dr. Murray cited the seasonality of the SARS-CoV-2 virus, which wanes in the summer and waxes in the winter. The “optimistic forecast” of IHME, which has modeled the course of the pandemic for the past 13 months, is that daily deaths will rise a bit in the next month, then decline from May through August, he said.
“Summer should be fairly quiet in terms of COVID, if vaccinations rise and people don’t stop wearing masks,” Dr. Murray said.
But he added that “a considerable surge will occur over next winter,” because the new variants are more transmissible, and people will likely relax social distancing and mask wearing. The IHME predicts that the percentage of Americans who usually don masks will decline from 73% today to 21% by Aug. 1.
With a rapid decline in mask use and a rise in mobility, there will still be more than 1,000 deaths each day by July 1, Dr. Murray said. In a forecast released the day after Dr. Murray spoke, the IHME predicted that by Aug. 1, there will be a total of 618,523 U.S. deaths from COVID-19. Deaths could be as high as 696,651 if mobility among the vaccinated returns to prepandemic levels, the institute forecasts.
Based on cell phone data, Dr. Murray said, the amount of mobility in the United States has already risen to the level of March 2020, when the pandemic was just getting underway.
Decreased infections
If there’s one piece of good news in the latest IHME report, it’s that the estimated number of people infected (including those not tested) will drop from 111,581 today to a projected 17,502 on Aug. 1. But in a worst-case scenario, with sharply higher mobility among vaccinated people, the case count on that date would only fall to 73,842.
The SARS-CoV-2 variants are another factor of concern. Dr. Murray distinguished between variants like the one first identified in the U.K. (B.1.1.7) and other “escape variants.”
B.1.1.7, which is now the dominant strain in the United States, increases transmission but doesn’t necessarily escape the immune system or vaccines, he explained.
In contrast, if someone is infected with a variant such as the South African or the Brazilian mutations, he said, a previous COVID-19 infection might not protect the person, and vaccines are less effective against those variants.
Cross-variant immunity may range from 0% to 60% for escape variants, based on the slim amount of data now available, Dr. Murray said. In his view, these variants will be the long-term driver of the pandemic in the United States, while the United Kingdom variant is the short-term driver.
The latest data, he said, show that the Pfizer/BioNTech and Moderna vaccines are 75% effective against the escape variants, with lower efficacy for other vaccines. But booster shots may still be required to protect people against some variants.
Human factors
Human behavior will also help determine the course of the pandemic, he noted. Vaccine hesitancy, for example, is still high in the United States.
By the end of May, he predicted, about 180 million people will have received about two doses of vaccine. After that, he said, “vaccination will flatline due to lack of demand.” The two unknowns are how much campaigns to promote vaccination will increase vaccine confidence, and when children will be vaccinated.
In the United States, he said, 69% of adults have been vaccinated or want to get a shot. But that percentage has dropped 5 points since February, and vaccine confidence varies by state.
Dr. Murray emphasized that the winter surge he predicts can be blocked if people change their behaviors. These include a rise in vaccine confidence to 80% and continued mask wearing by most people.
However, if vaccine confidence and mask wearing decline, state governments continue to drop social distancing rules, and the uptake of boosters is low, the winter surge could be more serious, he said.
Double surge
Murray also raised the possibility of a double surge of COVID-19 and influenza this winter. Widely expected last winter, this double surge never materialized here or elsewhere, partly because of mask wearing. But Dr. Murray said it could happen this year: History shows that the flu tends to be stronger in years after weak outbreaks.
He advised hospitals to prepare now for whatever might come later this year. Public health authorities, he said, should speed up vaccination, monitor variants closely with additional sequencing, and try to modify behavior in high-risk groups.
Asked to explain the recent surge of COVID-19 cases in Michigan, Dr. Murray attributed it partly to the spread of the B.1.1.7 (U.K.) variant. But he noted that the U.K. variant has expanded even more widely in some other states that haven’t had an explosive surge like Michigan’s.
Moreover, he noted, Michigan doesn’t have low mask use or high mobility. So the upward spiral of COVID-19 infections there is very concerning, he said.
In regard to the role of children as reservoirs of the virus, Dr. Murray pointed out that views on this have changed around the world. For a while, people thought kids didn’t spread COVID-19 very much. That view shifted when U.K. data showed that child transmission of the B.1.1.7 variant increased by half to 9% of contacts in comparison with the original virus strain.
Dutch data, similarly, showed schools contributing to the latest outbreaks, and some European nations have closed schools. In the United States, the trend is to open them.
A version of this article first appeared on Medscape.com.
It’s likely the United States will see another surge of COVID-19 this winter, warned Christopher Murray, MD, director of the Institute for Health Metrics and Evaluation (IHME) at the University of Washington in Seattle.
Speaking at the national conference of State of Reform on April 8, Dr. Murray cited the seasonality of the SARS-CoV-2 virus, which wanes in the summer and waxes in the winter. The “optimistic forecast” of IHME, which has modeled the course of the pandemic for the past 13 months, is that daily deaths will rise a bit in the next month, then decline from May through August, he said.
“Summer should be fairly quiet in terms of COVID, if vaccinations rise and people don’t stop wearing masks,” Dr. Murray said.
But he added that “a considerable surge will occur over next winter,” because the new variants are more transmissible, and people will likely relax social distancing and mask wearing. The IHME predicts that the percentage of Americans who usually don masks will decline from 73% today to 21% by Aug. 1.
With a rapid decline in mask use and a rise in mobility, there will still be more than 1,000 deaths each day by July 1, Dr. Murray said. In a forecast released the day after Dr. Murray spoke, the IHME predicted that by Aug. 1, there will be a total of 618,523 U.S. deaths from COVID-19. Deaths could be as high as 696,651 if mobility among the vaccinated returns to prepandemic levels, the institute forecasts.
Based on cell phone data, Dr. Murray said, the amount of mobility in the United States has already risen to the level of March 2020, when the pandemic was just getting underway.
Decreased infections
If there’s one piece of good news in the latest IHME report, it’s that the estimated number of people infected (including those not tested) will drop from 111,581 today to a projected 17,502 on Aug. 1. But in a worst-case scenario, with sharply higher mobility among vaccinated people, the case count on that date would only fall to 73,842.
The SARS-CoV-2 variants are another factor of concern. Dr. Murray distinguished between variants like the one first identified in the U.K. (B.1.1.7) and other “escape variants.”
B.1.1.7, which is now the dominant strain in the United States, increases transmission but doesn’t necessarily escape the immune system or vaccines, he explained.
In contrast, if someone is infected with a variant such as the South African or the Brazilian mutations, he said, a previous COVID-19 infection might not protect the person, and vaccines are less effective against those variants.
Cross-variant immunity may range from 0% to 60% for escape variants, based on the slim amount of data now available, Dr. Murray said. In his view, these variants will be the long-term driver of the pandemic in the United States, while the United Kingdom variant is the short-term driver.
The latest data, he said, show that the Pfizer/BioNTech and Moderna vaccines are 75% effective against the escape variants, with lower efficacy for other vaccines. But booster shots may still be required to protect people against some variants.
Human factors
Human behavior will also help determine the course of the pandemic, he noted. Vaccine hesitancy, for example, is still high in the United States.
By the end of May, he predicted, about 180 million people will have received about two doses of vaccine. After that, he said, “vaccination will flatline due to lack of demand.” The two unknowns are how much campaigns to promote vaccination will increase vaccine confidence, and when children will be vaccinated.
In the United States, he said, 69% of adults have been vaccinated or want to get a shot. But that percentage has dropped 5 points since February, and vaccine confidence varies by state.
Dr. Murray emphasized that the winter surge he predicts can be blocked if people change their behaviors. These include a rise in vaccine confidence to 80% and continued mask wearing by most people.
However, if vaccine confidence and mask wearing decline, state governments continue to drop social distancing rules, and the uptake of boosters is low, the winter surge could be more serious, he said.
Double surge
Murray also raised the possibility of a double surge of COVID-19 and influenza this winter. Widely expected last winter, this double surge never materialized here or elsewhere, partly because of mask wearing. But Dr. Murray said it could happen this year: History shows that the flu tends to be stronger in years after weak outbreaks.
He advised hospitals to prepare now for whatever might come later this year. Public health authorities, he said, should speed up vaccination, monitor variants closely with additional sequencing, and try to modify behavior in high-risk groups.
Asked to explain the recent surge of COVID-19 cases in Michigan, Dr. Murray attributed it partly to the spread of the B.1.1.7 (U.K.) variant. But he noted that the U.K. variant has expanded even more widely in some other states that haven’t had an explosive surge like Michigan’s.
Moreover, he noted, Michigan doesn’t have low mask use or high mobility. So the upward spiral of COVID-19 infections there is very concerning, he said.
In regard to the role of children as reservoirs of the virus, Dr. Murray pointed out that views on this have changed around the world. For a while, people thought kids didn’t spread COVID-19 very much. That view shifted when U.K. data showed that child transmission of the B.1.1.7 variant increased by half to 9% of contacts in comparison with the original virus strain.
Dutch data, similarly, showed schools contributing to the latest outbreaks, and some European nations have closed schools. In the United States, the trend is to open them.
A version of this article first appeared on Medscape.com.
It’s likely the United States will see another surge of COVID-19 this winter, warned Christopher Murray, MD, director of the Institute for Health Metrics and Evaluation (IHME) at the University of Washington in Seattle.
Speaking at the national conference of State of Reform on April 8, Dr. Murray cited the seasonality of the SARS-CoV-2 virus, which wanes in the summer and waxes in the winter. The “optimistic forecast” of IHME, which has modeled the course of the pandemic for the past 13 months, is that daily deaths will rise a bit in the next month, then decline from May through August, he said.
“Summer should be fairly quiet in terms of COVID, if vaccinations rise and people don’t stop wearing masks,” Dr. Murray said.
But he added that “a considerable surge will occur over next winter,” because the new variants are more transmissible, and people will likely relax social distancing and mask wearing. The IHME predicts that the percentage of Americans who usually don masks will decline from 73% today to 21% by Aug. 1.
With a rapid decline in mask use and a rise in mobility, there will still be more than 1,000 deaths each day by July 1, Dr. Murray said. In a forecast released the day after Dr. Murray spoke, the IHME predicted that by Aug. 1, there will be a total of 618,523 U.S. deaths from COVID-19. Deaths could be as high as 696,651 if mobility among the vaccinated returns to prepandemic levels, the institute forecasts.
Based on cell phone data, Dr. Murray said, the amount of mobility in the United States has already risen to the level of March 2020, when the pandemic was just getting underway.
Decreased infections
If there’s one piece of good news in the latest IHME report, it’s that the estimated number of people infected (including those not tested) will drop from 111,581 today to a projected 17,502 on Aug. 1. But in a worst-case scenario, with sharply higher mobility among vaccinated people, the case count on that date would only fall to 73,842.
The SARS-CoV-2 variants are another factor of concern. Dr. Murray distinguished between variants like the one first identified in the U.K. (B.1.1.7) and other “escape variants.”
B.1.1.7, which is now the dominant strain in the United States, increases transmission but doesn’t necessarily escape the immune system or vaccines, he explained.
In contrast, if someone is infected with a variant such as the South African or the Brazilian mutations, he said, a previous COVID-19 infection might not protect the person, and vaccines are less effective against those variants.
Cross-variant immunity may range from 0% to 60% for escape variants, based on the slim amount of data now available, Dr. Murray said. In his view, these variants will be the long-term driver of the pandemic in the United States, while the United Kingdom variant is the short-term driver.
The latest data, he said, show that the Pfizer/BioNTech and Moderna vaccines are 75% effective against the escape variants, with lower efficacy for other vaccines. But booster shots may still be required to protect people against some variants.
Human factors
Human behavior will also help determine the course of the pandemic, he noted. Vaccine hesitancy, for example, is still high in the United States.
By the end of May, he predicted, about 180 million people will have received about two doses of vaccine. After that, he said, “vaccination will flatline due to lack of demand.” The two unknowns are how much campaigns to promote vaccination will increase vaccine confidence, and when children will be vaccinated.
In the United States, he said, 69% of adults have been vaccinated or want to get a shot. But that percentage has dropped 5 points since February, and vaccine confidence varies by state.
Dr. Murray emphasized that the winter surge he predicts can be blocked if people change their behaviors. These include a rise in vaccine confidence to 80% and continued mask wearing by most people.
However, if vaccine confidence and mask wearing decline, state governments continue to drop social distancing rules, and the uptake of boosters is low, the winter surge could be more serious, he said.
Double surge
Murray also raised the possibility of a double surge of COVID-19 and influenza this winter. Widely expected last winter, this double surge never materialized here or elsewhere, partly because of mask wearing. But Dr. Murray said it could happen this year: History shows that the flu tends to be stronger in years after weak outbreaks.
He advised hospitals to prepare now for whatever might come later this year. Public health authorities, he said, should speed up vaccination, monitor variants closely with additional sequencing, and try to modify behavior in high-risk groups.
Asked to explain the recent surge of COVID-19 cases in Michigan, Dr. Murray attributed it partly to the spread of the B.1.1.7 (U.K.) variant. But he noted that the U.K. variant has expanded even more widely in some other states that haven’t had an explosive surge like Michigan’s.
Moreover, he noted, Michigan doesn’t have low mask use or high mobility. So the upward spiral of COVID-19 infections there is very concerning, he said.
In regard to the role of children as reservoirs of the virus, Dr. Murray pointed out that views on this have changed around the world. For a while, people thought kids didn’t spread COVID-19 very much. That view shifted when U.K. data showed that child transmission of the B.1.1.7 variant increased by half to 9% of contacts in comparison with the original virus strain.
Dutch data, similarly, showed schools contributing to the latest outbreaks, and some European nations have closed schools. In the United States, the trend is to open them.
A version of this article first appeared on Medscape.com.
FDA, CDC urge pause of J&J COVID vaccine
The Food and Drug Administration and Centers for Disease Control and Prevention on April 13 recommended that use of the Johnson & Johnson COVID-19 vaccine be paused after reports of blood clots in patients receiving the shot, the agencies have announced.
In a statement, FDA said 6.8 million doses of the J&J vaccine have been administered and the agency is investigating six reported cases of a rare and severe blood clot occurring in patients who received the vaccine.
The pause is intended to give time to alert the public to this "very rare" condition, experts said during a joint CDC-FDA media briefing April 13.
"It was clear to us that we needed to alert the public," Janet Woodcock, MD, acting FDA commissioner, said. The move also will allow "time for the healthcare community to learn what they need to know about how to diagnose, treat and report" any additional cases.
The CDC will convene a meeting of the Advisory Committee on Immunization Practices on April 14 to review the cases.
"I know the information today will be very concerning to Americans who have already received the Johnson & Johnson vaccine," said Anne Schuchat, MD, principal deputy director at the CDC.
"For people who got the vaccine more than one month ago, the risk is very low at this time," she added. "For people who recently got the vaccine, in the last couple of weeks, look for symptoms."
Headache, leg pain, abdominal pain, and shortness of breath were among the reported symptoms. All six cases arose within 6 to 13 days of receipt of the Johnson & Johnson vaccine.
Traditional treatment dangerous
Importantly, treatment for traditional blood clots, such as the drug heparin, should not be used for these clots. "The issue here with these types of blood clots is that if one administers the standard treatment we give for blood clots, one can cause tremendous harm or it can be fatal," said Peter Marks, MD, director of the FDA Center for Biologics Evaluation and Research.
If health care providers see people with these symptoms along with a low platelet count or blood clots, they should ask about any recent vaccinations, Dr. Marks added.
Headache is a common side effect of COVID-19 vaccination, Dr. Marks said, but it typically happens within a day or two. In contrast, the headaches associated with these blood clots come 1 to 2 weeks later and were very severe.
Not all of the six women involved in the events had a pre-existing condition or risk factor, Dr. Schuchat said.
Severe but 'extremely rare'
To put the numbers in context, the six reported events occurred among millions of people who received the Johnson & Johnson vaccine to date.
"There have been six reports of a severe stroke-like illness due to low platelet count and more than six million doses of the Johnson & Johnson vaccine have been administered so far," Dr. Schuchat said.
"I would like to stress these events are extremely rare," Dr. Woodcock said, "but we take all reports of adverse events after vaccination very seriously."
The company response
Johnson & Johnson in a statement said, "We are aware of an extremely rare disorder involving people with blood clots in combination with low platelets in a small number of individuals who have received our COVID-19 vaccine. The United States Centers for Disease Control (CDC) and Food and Drug Administration (FDA) are reviewing data involving six reported U.S. cases out of more than 6.8 million doses administered. Out of an abundance of caution, the CDC and FDA have recommended a pause in the use of our vaccine."
The company said they are also reviewing these cases with European regulators and "we have made the decision to proactively delay the rollout of our vaccine in Europe."
Overall vaccinations continuing apace
"This announcement will not have a significant impact on our vaccination plan. Johnson & Johnson vaccine makes up less than 5% of the recorded shots in arms in the United States to date," Jeff Zients, White House COVID-19 Response Coordinator, said in a statement.
"Based on actions taken by the president earlier this year, the United States has secured enough Pfizer and Moderna doses for 300 million Americans. We are working now with our state and federal partners to get anyone scheduled for a J&J vaccine quickly rescheduled for a Pfizer or Moderna vaccine," he added.
The likely duration of the pause remains unclear.
"I know this has been a long and difficult pandemic, and people are tired of the steps they have to take," Dr. Schuchat said. "Steps taken today make sure the health care system is ready to diagnose, treat and report [any additional cases] and the public has the information necessary to stay safe."
A version of this article first appeared on WebMD.com.
This article was updated 4/13/21.
The Food and Drug Administration and Centers for Disease Control and Prevention on April 13 recommended that use of the Johnson & Johnson COVID-19 vaccine be paused after reports of blood clots in patients receiving the shot, the agencies have announced.
In a statement, FDA said 6.8 million doses of the J&J vaccine have been administered and the agency is investigating six reported cases of a rare and severe blood clot occurring in patients who received the vaccine.
The pause is intended to give time to alert the public to this "very rare" condition, experts said during a joint CDC-FDA media briefing April 13.
"It was clear to us that we needed to alert the public," Janet Woodcock, MD, acting FDA commissioner, said. The move also will allow "time for the healthcare community to learn what they need to know about how to diagnose, treat and report" any additional cases.
The CDC will convene a meeting of the Advisory Committee on Immunization Practices on April 14 to review the cases.
"I know the information today will be very concerning to Americans who have already received the Johnson & Johnson vaccine," said Anne Schuchat, MD, principal deputy director at the CDC.
"For people who got the vaccine more than one month ago, the risk is very low at this time," she added. "For people who recently got the vaccine, in the last couple of weeks, look for symptoms."
Headache, leg pain, abdominal pain, and shortness of breath were among the reported symptoms. All six cases arose within 6 to 13 days of receipt of the Johnson & Johnson vaccine.
Traditional treatment dangerous
Importantly, treatment for traditional blood clots, such as the drug heparin, should not be used for these clots. "The issue here with these types of blood clots is that if one administers the standard treatment we give for blood clots, one can cause tremendous harm or it can be fatal," said Peter Marks, MD, director of the FDA Center for Biologics Evaluation and Research.
If health care providers see people with these symptoms along with a low platelet count or blood clots, they should ask about any recent vaccinations, Dr. Marks added.
Headache is a common side effect of COVID-19 vaccination, Dr. Marks said, but it typically happens within a day or two. In contrast, the headaches associated with these blood clots come 1 to 2 weeks later and were very severe.
Not all of the six women involved in the events had a pre-existing condition or risk factor, Dr. Schuchat said.
Severe but 'extremely rare'
To put the numbers in context, the six reported events occurred among millions of people who received the Johnson & Johnson vaccine to date.
"There have been six reports of a severe stroke-like illness due to low platelet count and more than six million doses of the Johnson & Johnson vaccine have been administered so far," Dr. Schuchat said.
"I would like to stress these events are extremely rare," Dr. Woodcock said, "but we take all reports of adverse events after vaccination very seriously."
The company response
Johnson & Johnson in a statement said, "We are aware of an extremely rare disorder involving people with blood clots in combination with low platelets in a small number of individuals who have received our COVID-19 vaccine. The United States Centers for Disease Control (CDC) and Food and Drug Administration (FDA) are reviewing data involving six reported U.S. cases out of more than 6.8 million doses administered. Out of an abundance of caution, the CDC and FDA have recommended a pause in the use of our vaccine."
The company said they are also reviewing these cases with European regulators and "we have made the decision to proactively delay the rollout of our vaccine in Europe."
Overall vaccinations continuing apace
"This announcement will not have a significant impact on our vaccination plan. Johnson & Johnson vaccine makes up less than 5% of the recorded shots in arms in the United States to date," Jeff Zients, White House COVID-19 Response Coordinator, said in a statement.
"Based on actions taken by the president earlier this year, the United States has secured enough Pfizer and Moderna doses for 300 million Americans. We are working now with our state and federal partners to get anyone scheduled for a J&J vaccine quickly rescheduled for a Pfizer or Moderna vaccine," he added.
The likely duration of the pause remains unclear.
"I know this has been a long and difficult pandemic, and people are tired of the steps they have to take," Dr. Schuchat said. "Steps taken today make sure the health care system is ready to diagnose, treat and report [any additional cases] and the public has the information necessary to stay safe."
A version of this article first appeared on WebMD.com.
This article was updated 4/13/21.
The Food and Drug Administration and Centers for Disease Control and Prevention on April 13 recommended that use of the Johnson & Johnson COVID-19 vaccine be paused after reports of blood clots in patients receiving the shot, the agencies have announced.
In a statement, FDA said 6.8 million doses of the J&J vaccine have been administered and the agency is investigating six reported cases of a rare and severe blood clot occurring in patients who received the vaccine.
The pause is intended to give time to alert the public to this "very rare" condition, experts said during a joint CDC-FDA media briefing April 13.
"It was clear to us that we needed to alert the public," Janet Woodcock, MD, acting FDA commissioner, said. The move also will allow "time for the healthcare community to learn what they need to know about how to diagnose, treat and report" any additional cases.
The CDC will convene a meeting of the Advisory Committee on Immunization Practices on April 14 to review the cases.
"I know the information today will be very concerning to Americans who have already received the Johnson & Johnson vaccine," said Anne Schuchat, MD, principal deputy director at the CDC.
"For people who got the vaccine more than one month ago, the risk is very low at this time," she added. "For people who recently got the vaccine, in the last couple of weeks, look for symptoms."
Headache, leg pain, abdominal pain, and shortness of breath were among the reported symptoms. All six cases arose within 6 to 13 days of receipt of the Johnson & Johnson vaccine.
Traditional treatment dangerous
Importantly, treatment for traditional blood clots, such as the drug heparin, should not be used for these clots. "The issue here with these types of blood clots is that if one administers the standard treatment we give for blood clots, one can cause tremendous harm or it can be fatal," said Peter Marks, MD, director of the FDA Center for Biologics Evaluation and Research.
If health care providers see people with these symptoms along with a low platelet count or blood clots, they should ask about any recent vaccinations, Dr. Marks added.
Headache is a common side effect of COVID-19 vaccination, Dr. Marks said, but it typically happens within a day or two. In contrast, the headaches associated with these blood clots come 1 to 2 weeks later and were very severe.
Not all of the six women involved in the events had a pre-existing condition or risk factor, Dr. Schuchat said.
Severe but 'extremely rare'
To put the numbers in context, the six reported events occurred among millions of people who received the Johnson & Johnson vaccine to date.
"There have been six reports of a severe stroke-like illness due to low platelet count and more than six million doses of the Johnson & Johnson vaccine have been administered so far," Dr. Schuchat said.
"I would like to stress these events are extremely rare," Dr. Woodcock said, "but we take all reports of adverse events after vaccination very seriously."
The company response
Johnson & Johnson in a statement said, "We are aware of an extremely rare disorder involving people with blood clots in combination with low platelets in a small number of individuals who have received our COVID-19 vaccine. The United States Centers for Disease Control (CDC) and Food and Drug Administration (FDA) are reviewing data involving six reported U.S. cases out of more than 6.8 million doses administered. Out of an abundance of caution, the CDC and FDA have recommended a pause in the use of our vaccine."
The company said they are also reviewing these cases with European regulators and "we have made the decision to proactively delay the rollout of our vaccine in Europe."
Overall vaccinations continuing apace
"This announcement will not have a significant impact on our vaccination plan. Johnson & Johnson vaccine makes up less than 5% of the recorded shots in arms in the United States to date," Jeff Zients, White House COVID-19 Response Coordinator, said in a statement.
"Based on actions taken by the president earlier this year, the United States has secured enough Pfizer and Moderna doses for 300 million Americans. We are working now with our state and federal partners to get anyone scheduled for a J&J vaccine quickly rescheduled for a Pfizer or Moderna vaccine," he added.
The likely duration of the pause remains unclear.
"I know this has been a long and difficult pandemic, and people are tired of the steps they have to take," Dr. Schuchat said. "Steps taken today make sure the health care system is ready to diagnose, treat and report [any additional cases] and the public has the information necessary to stay safe."
A version of this article first appeared on WebMD.com.
This article was updated 4/13/21.
Study IDs most common lingering symptoms 8 months after mild COVID
Loss of smell, loss of taste, dyspnea, and fatigue are the four most common symptoms that health care professionals in Sweden report 8 months after mild COVID-19 illness, new evidence reveals.
according to the study.
“We see that a substantial portion of health care workers suffer from long-term symptoms after mild COVID-19,” senior author Charlotte Thålin, MD, PhD, said in an interview. She added that loss of smell and taste “may seem trivial, but have a negative impact on work, social, and home life in the long run.”
The study is noteworthy not only for tracking the COVID-19-related experiences of health care workers over time, but also for what it did not find. There was no increased prevalence of cognitive issues – including memory or concentration – that others have linked to what’s often called long-haul COVID-19.
The research letter was published online April 7, 2021, in JAMA.
“Even if you are young and previously healthy, a mild COVID-19 infection may result in long-term consequences,” said Dr. Thålin, from the department of clinical sciences at Danderyd Hospital, Karolinska Institute, Stockholm.
The researchers did not observe an increased risk for long-term symptoms after asymptomatic COVID-19.
Adding to existing evidence
This research letter “adds to the growing body of literature showing that people recovering from COVID have reported a diverse array of symptoms lasting for months after initial infection,” Lekshmi Santhosh, MD, said in an interview. She is physician faculty lead at the University of California, San Francisco Post-COVID OPTIMAL Clinic.
Previous research revealed severe long-term symptoms, including heart palpitations and neurologic impairments, among people hospitalized with COVID-19. However, “there is limited data on the long-term effects after mild COVID-19, and these studies are often hampered by selection bias and without proper control groups,” Dr. Thålin said.
The absence of these more severe symptoms after mild COVID-19 is “reassuring,” she added.
The current findings are part of the ongoing COMMUNITY (COVID-19 Biomarker and Immunity) study looking at long-term immunity. Health care professionals enrolled in the research between April 15 and May 8, 2020, and have initial blood tests repeated every 4 months.
Dr. Thålin, lead author Sebastian Havervall, MD, and their colleagues compared symptom reporting between 323 hospital employees who had mild COVID-19 at least 8 months earlier with 1,072 employees who did not have COVID-19 throughout the study.
The results show that 26% of those who had COVID-19 previously had at least one moderate to severe symptom that lasted more than 2 months, compared with 9% in the control group.
The group with a history of mild COVID-19 was a median 43 years old and 83% were women. The controls were a median 47 years old and 86% were women.
“These data mirror what we have seen across long-term cohorts of patients with COVID-19 infection. Notably, mild illness among previously healthy individuals may be associated with long-term persistent symptoms,” Sarah Jolley, MD, a pulmonologist specializing in critical care at the University of Colorado Hospital in Aurora and director of the Post-COVID Clinic, said in an interview.
“In this cohort, similar to others, this seems to be more pronounced in women,” Dr. Jolley added.
Key findings on functioning
At 8 months, using a smartphone app, participants reported presence, duration, and severity of 23 predefined symptoms. Researchers used the Sheehan Disability Scale to gauge functional impairment.
A total of 11% participants reported at least one symptom that negatively affected work or social or home life at 8 months versus only 2% of the control group.
Seropositive participants were almost two times more likely to report that their long-term symptoms moderately to markedly disrupted their work life, 8% versus 4% of seronegative healthcare workers (relative risk, 1.8; 95%; confidence interval, 1.2-2.9).
Disruptions to a social life from long-term symptoms were 2.5 times more likely in the seropositive group. A total 15% of this cohort reported moderate to marked effects, compared with 6% of the seronegative group (RR, 2.5; 95% CI, 1.8-3.6).
The researchers also inquired about home life disruptions, which were reported by 12% of the seropositive health care workers and 5% of the seronegative participants (RR, 2.3; 95% CI, 1.6-3.4).
The study’s findings “tracks with a lot of the other work we’re seeing,” David Putrino, PT, PhD, director of rehabilitation innovation at Mount Sinai Health System in New York, said in an interview. He and his colleagues are responsible for managing the rehabilitation of patients with long COVID.
Interestingly, the proportion of people with persistent symptoms might be underestimated in this research, Dr. Putrino said. “Antibodies are not an entirely reliable biomarker. So what the researchers are using here is the most conservative measure of who may have had the virus.”
Potential recall bias and the subjective rating of symptoms were possible limitations of the study.
When asked to speculate why researchers did not find higher levels of cognitive dysfunction, Dr. Putrino said that self-reports are generally less reliable than measures like the Montreal Cognitive Assessment for detecting cognitive impairment.
Furthermore, unlike many of the people with long-haul COVID-19 whom he treats clinically – ones who are “really struggling” – the health care workers studied in Sweden are functioning well enough to perform their duties at the hospital, so the study population may not represent the population at large.
More research required
“More research needs to be conducted to investigate the mechanisms underlying these persistent symptoms, and several centers, including UCSF, are conducting research into why this might be,” Dr. Santhosh said.
Dr. Thålin and colleagues plan to continue following participants. “The primary aim of the COMMUNITY study is to investigate long-term immunity after COVID-19, but we will also look into possible underlying pathophysiological mechanisms behind COVID-19–related long-term symptoms,” she said.
“I hope to see that taste and smell will return,” Dr. Thålin added.
“We’re really just starting to understand the long-term effects of COVID-19,” Putrino said. “This is something we’re going to see a lot of moving forward.”
Dr. Thålin, Dr. Santhosh, Dr. Jolley, and Dr. Putrino disclosed no relevant financial relationships. The research was funded by grants from the Knut and Alice Wallenberg Foundation, Jonas and Christina af Jochnick Foundation, Leif Lundblad Family Foundation, Region Stockholm, and Erling-Persson Family Foundation.
A version of this article first appeared on Medscape.com.
Loss of smell, loss of taste, dyspnea, and fatigue are the four most common symptoms that health care professionals in Sweden report 8 months after mild COVID-19 illness, new evidence reveals.
according to the study.
“We see that a substantial portion of health care workers suffer from long-term symptoms after mild COVID-19,” senior author Charlotte Thålin, MD, PhD, said in an interview. She added that loss of smell and taste “may seem trivial, but have a negative impact on work, social, and home life in the long run.”
The study is noteworthy not only for tracking the COVID-19-related experiences of health care workers over time, but also for what it did not find. There was no increased prevalence of cognitive issues – including memory or concentration – that others have linked to what’s often called long-haul COVID-19.
The research letter was published online April 7, 2021, in JAMA.
“Even if you are young and previously healthy, a mild COVID-19 infection may result in long-term consequences,” said Dr. Thålin, from the department of clinical sciences at Danderyd Hospital, Karolinska Institute, Stockholm.
The researchers did not observe an increased risk for long-term symptoms after asymptomatic COVID-19.
Adding to existing evidence
This research letter “adds to the growing body of literature showing that people recovering from COVID have reported a diverse array of symptoms lasting for months after initial infection,” Lekshmi Santhosh, MD, said in an interview. She is physician faculty lead at the University of California, San Francisco Post-COVID OPTIMAL Clinic.
Previous research revealed severe long-term symptoms, including heart palpitations and neurologic impairments, among people hospitalized with COVID-19. However, “there is limited data on the long-term effects after mild COVID-19, and these studies are often hampered by selection bias and without proper control groups,” Dr. Thålin said.
The absence of these more severe symptoms after mild COVID-19 is “reassuring,” she added.
The current findings are part of the ongoing COMMUNITY (COVID-19 Biomarker and Immunity) study looking at long-term immunity. Health care professionals enrolled in the research between April 15 and May 8, 2020, and have initial blood tests repeated every 4 months.
Dr. Thålin, lead author Sebastian Havervall, MD, and their colleagues compared symptom reporting between 323 hospital employees who had mild COVID-19 at least 8 months earlier with 1,072 employees who did not have COVID-19 throughout the study.
The results show that 26% of those who had COVID-19 previously had at least one moderate to severe symptom that lasted more than 2 months, compared with 9% in the control group.
The group with a history of mild COVID-19 was a median 43 years old and 83% were women. The controls were a median 47 years old and 86% were women.
“These data mirror what we have seen across long-term cohorts of patients with COVID-19 infection. Notably, mild illness among previously healthy individuals may be associated with long-term persistent symptoms,” Sarah Jolley, MD, a pulmonologist specializing in critical care at the University of Colorado Hospital in Aurora and director of the Post-COVID Clinic, said in an interview.
“In this cohort, similar to others, this seems to be more pronounced in women,” Dr. Jolley added.
Key findings on functioning
At 8 months, using a smartphone app, participants reported presence, duration, and severity of 23 predefined symptoms. Researchers used the Sheehan Disability Scale to gauge functional impairment.
A total of 11% participants reported at least one symptom that negatively affected work or social or home life at 8 months versus only 2% of the control group.
Seropositive participants were almost two times more likely to report that their long-term symptoms moderately to markedly disrupted their work life, 8% versus 4% of seronegative healthcare workers (relative risk, 1.8; 95%; confidence interval, 1.2-2.9).
Disruptions to a social life from long-term symptoms were 2.5 times more likely in the seropositive group. A total 15% of this cohort reported moderate to marked effects, compared with 6% of the seronegative group (RR, 2.5; 95% CI, 1.8-3.6).
The researchers also inquired about home life disruptions, which were reported by 12% of the seropositive health care workers and 5% of the seronegative participants (RR, 2.3; 95% CI, 1.6-3.4).
The study’s findings “tracks with a lot of the other work we’re seeing,” David Putrino, PT, PhD, director of rehabilitation innovation at Mount Sinai Health System in New York, said in an interview. He and his colleagues are responsible for managing the rehabilitation of patients with long COVID.
Interestingly, the proportion of people with persistent symptoms might be underestimated in this research, Dr. Putrino said. “Antibodies are not an entirely reliable biomarker. So what the researchers are using here is the most conservative measure of who may have had the virus.”
Potential recall bias and the subjective rating of symptoms were possible limitations of the study.
When asked to speculate why researchers did not find higher levels of cognitive dysfunction, Dr. Putrino said that self-reports are generally less reliable than measures like the Montreal Cognitive Assessment for detecting cognitive impairment.
Furthermore, unlike many of the people with long-haul COVID-19 whom he treats clinically – ones who are “really struggling” – the health care workers studied in Sweden are functioning well enough to perform their duties at the hospital, so the study population may not represent the population at large.
More research required
“More research needs to be conducted to investigate the mechanisms underlying these persistent symptoms, and several centers, including UCSF, are conducting research into why this might be,” Dr. Santhosh said.
Dr. Thålin and colleagues plan to continue following participants. “The primary aim of the COMMUNITY study is to investigate long-term immunity after COVID-19, but we will also look into possible underlying pathophysiological mechanisms behind COVID-19–related long-term symptoms,” she said.
“I hope to see that taste and smell will return,” Dr. Thålin added.
“We’re really just starting to understand the long-term effects of COVID-19,” Putrino said. “This is something we’re going to see a lot of moving forward.”
Dr. Thålin, Dr. Santhosh, Dr. Jolley, and Dr. Putrino disclosed no relevant financial relationships. The research was funded by grants from the Knut and Alice Wallenberg Foundation, Jonas and Christina af Jochnick Foundation, Leif Lundblad Family Foundation, Region Stockholm, and Erling-Persson Family Foundation.
A version of this article first appeared on Medscape.com.
Loss of smell, loss of taste, dyspnea, and fatigue are the four most common symptoms that health care professionals in Sweden report 8 months after mild COVID-19 illness, new evidence reveals.
according to the study.
“We see that a substantial portion of health care workers suffer from long-term symptoms after mild COVID-19,” senior author Charlotte Thålin, MD, PhD, said in an interview. She added that loss of smell and taste “may seem trivial, but have a negative impact on work, social, and home life in the long run.”
The study is noteworthy not only for tracking the COVID-19-related experiences of health care workers over time, but also for what it did not find. There was no increased prevalence of cognitive issues – including memory or concentration – that others have linked to what’s often called long-haul COVID-19.
The research letter was published online April 7, 2021, in JAMA.
“Even if you are young and previously healthy, a mild COVID-19 infection may result in long-term consequences,” said Dr. Thålin, from the department of clinical sciences at Danderyd Hospital, Karolinska Institute, Stockholm.
The researchers did not observe an increased risk for long-term symptoms after asymptomatic COVID-19.
Adding to existing evidence
This research letter “adds to the growing body of literature showing that people recovering from COVID have reported a diverse array of symptoms lasting for months after initial infection,” Lekshmi Santhosh, MD, said in an interview. She is physician faculty lead at the University of California, San Francisco Post-COVID OPTIMAL Clinic.
Previous research revealed severe long-term symptoms, including heart palpitations and neurologic impairments, among people hospitalized with COVID-19. However, “there is limited data on the long-term effects after mild COVID-19, and these studies are often hampered by selection bias and without proper control groups,” Dr. Thålin said.
The absence of these more severe symptoms after mild COVID-19 is “reassuring,” she added.
The current findings are part of the ongoing COMMUNITY (COVID-19 Biomarker and Immunity) study looking at long-term immunity. Health care professionals enrolled in the research between April 15 and May 8, 2020, and have initial blood tests repeated every 4 months.
Dr. Thålin, lead author Sebastian Havervall, MD, and their colleagues compared symptom reporting between 323 hospital employees who had mild COVID-19 at least 8 months earlier with 1,072 employees who did not have COVID-19 throughout the study.
The results show that 26% of those who had COVID-19 previously had at least one moderate to severe symptom that lasted more than 2 months, compared with 9% in the control group.
The group with a history of mild COVID-19 was a median 43 years old and 83% were women. The controls were a median 47 years old and 86% were women.
“These data mirror what we have seen across long-term cohorts of patients with COVID-19 infection. Notably, mild illness among previously healthy individuals may be associated with long-term persistent symptoms,” Sarah Jolley, MD, a pulmonologist specializing in critical care at the University of Colorado Hospital in Aurora and director of the Post-COVID Clinic, said in an interview.
“In this cohort, similar to others, this seems to be more pronounced in women,” Dr. Jolley added.
Key findings on functioning
At 8 months, using a smartphone app, participants reported presence, duration, and severity of 23 predefined symptoms. Researchers used the Sheehan Disability Scale to gauge functional impairment.
A total of 11% participants reported at least one symptom that negatively affected work or social or home life at 8 months versus only 2% of the control group.
Seropositive participants were almost two times more likely to report that their long-term symptoms moderately to markedly disrupted their work life, 8% versus 4% of seronegative healthcare workers (relative risk, 1.8; 95%; confidence interval, 1.2-2.9).
Disruptions to a social life from long-term symptoms were 2.5 times more likely in the seropositive group. A total 15% of this cohort reported moderate to marked effects, compared with 6% of the seronegative group (RR, 2.5; 95% CI, 1.8-3.6).
The researchers also inquired about home life disruptions, which were reported by 12% of the seropositive health care workers and 5% of the seronegative participants (RR, 2.3; 95% CI, 1.6-3.4).
The study’s findings “tracks with a lot of the other work we’re seeing,” David Putrino, PT, PhD, director of rehabilitation innovation at Mount Sinai Health System in New York, said in an interview. He and his colleagues are responsible for managing the rehabilitation of patients with long COVID.
Interestingly, the proportion of people with persistent symptoms might be underestimated in this research, Dr. Putrino said. “Antibodies are not an entirely reliable biomarker. So what the researchers are using here is the most conservative measure of who may have had the virus.”
Potential recall bias and the subjective rating of symptoms were possible limitations of the study.
When asked to speculate why researchers did not find higher levels of cognitive dysfunction, Dr. Putrino said that self-reports are generally less reliable than measures like the Montreal Cognitive Assessment for detecting cognitive impairment.
Furthermore, unlike many of the people with long-haul COVID-19 whom he treats clinically – ones who are “really struggling” – the health care workers studied in Sweden are functioning well enough to perform their duties at the hospital, so the study population may not represent the population at large.
More research required
“More research needs to be conducted to investigate the mechanisms underlying these persistent symptoms, and several centers, including UCSF, are conducting research into why this might be,” Dr. Santhosh said.
Dr. Thålin and colleagues plan to continue following participants. “The primary aim of the COMMUNITY study is to investigate long-term immunity after COVID-19, but we will also look into possible underlying pathophysiological mechanisms behind COVID-19–related long-term symptoms,” she said.
“I hope to see that taste and smell will return,” Dr. Thålin added.
“We’re really just starting to understand the long-term effects of COVID-19,” Putrino said. “This is something we’re going to see a lot of moving forward.”
Dr. Thålin, Dr. Santhosh, Dr. Jolley, and Dr. Putrino disclosed no relevant financial relationships. The research was funded by grants from the Knut and Alice Wallenberg Foundation, Jonas and Christina af Jochnick Foundation, Leif Lundblad Family Foundation, Region Stockholm, and Erling-Persson Family Foundation.
A version of this article first appeared on Medscape.com.
U.S. finally hits its stride with COVID-19 vaccination rollouts
Each afternoon, Cyrus Shahpar, MD, the data guru for the White House COVID-19 Response Team, sends an email to staffers with the daily count of COVID-19 vaccinations delivered in the United States.
The numbers, collected from states ahead of the final figures being posted on the Centers for Disease Control and Prevention website, act as a report card of sorts on the team’s efforts.
On Saturday, April 3, it was a new record: 4.1 million vaccinations delivered in a single day, more than the total population of some states.
While the United States has a long way to go before it is done with COVID-19, there’s finally some good news in the nation’s long and blundering slog through the pandemic.
After a rocky start in December 2020 and January 2021, vaccination is happening faster than nearly anyone thought possible. As more people see their friends and family roll up their sleeves, hesitancy is dropping, too.
In settings where large numbers of people are vaccinated, such as nursing homes, COVID-19 cases and deaths have plunged.
Those gains, however, haven’t been shared equally. According to CDC data, 69% of people who are fully vaccinated are White, while just 8% are Black and about 9% are Hispanic, a group that now represents most new COVID-19 cases.
Officials say that’s partly because the vaccines were rolled out to the elderly first. The average life expectancy for Black people in the United States is now age 72, which means there were fewer people of color represented in the first groups to become eligible. Experts are hopeful that underrepresented groups will start to catch up as more states open up vaccinations to younger people.
Based on overall numbers of daily vaccine doses, the United States ranks third, behind China and India. America ranks fourth – behind Israel, the United Kingdom, and Chile – in the total share of the population that’s been vaccinated, according to the website Our World in Data.
A positive development
It’s a stunning turnaround for a country that failed for months to develop effective tests, and still struggles in some quarters to investigate new cases and quarantine their contacts.
The 7-day rolling average of vaccines administered in the United States is currently more than 3 million a day.
“We knew that we needed to get to 3 million a day at some point, if we were going to get most people vaccinated this year, but I don’t think that most people expected it to happen this early,” said Eric Toner, MD, a senior scholar with the Johns Hopkins Center for Health Security in Baltimore.
Before taking office, President Joe Biden pledged to get 100 million shots in arms within his first 100 days in office. After hitting that goal in late March, he doubled it, to 200 million vaccinations by April 30. After first saying all adults should be eligible to get in line for the vaccine by May 1, on April 6, he bumped up that date to April 19.
Some media reports have seen this repeated moving of the goalposts as calculated – an unstated strategy of underpromising and overdelivering with the aim of rebuilding public trust.
But others pointed out that, even if that’s true, the goals being set aren’t easy, and hitting them has never been a given.
“I think the Biden administration really gets a lot of credit for pushing the companies to get more vaccine out faster than they had planned to,” Dr. Toner said. “And the states have really responded as well as the federal government in terms of getting vaccination sites going. So we’re not only getting the vaccines, we’re getting it into people’s arms faster than expected.”
Others agree.
“We’re doing an amazing job, and I think the U.S. is really beginning to bend the curve,” said Carlos del Rio, MD, an infectious disease specialist and distinguished professor of medicine at Emory University, Atlanta.
“I think overall it’s just that everybody’s putting in a ton of work to get it done,” he said.
On April 3, the day the United States hit its vaccination record, he was volunteering to give vaccinations.
“I mean, of all the bad things we do to people as clinicians, this is one thing that people are very happy about, right?” Dr. del Rio said.
He said he vaccinated a young woman who asked if she could video chat with her mom, who was feeling nervous about getting the shot. He answered her mom’s questions, and later that day, she came down to be vaccinated herself.
‘We view it as a war’
The White House COVID-19 Response Team has worked hard to better coordinate the work of so many people at both the federal and state levels, Andy Slavitt, senior adviser for the team, said in an interview.
“We view it as a war, and in a war, you do everything: You bring experienced personnel; you bring all the resources to bear; you create multiple routes,” Mr. Slavitt said. “You don’t leave anything to chance.”
Among the levers the administration has pulled, using the Defense Production Act has helped vaccine manufacturers get needed supplies, Mr. Slavitt said.
The administration has set up an array of Federal Emergency Management Agency–run community vaccination centers and mobile vaccination sites to complement state-led efforts, and it’s activated a federal health law called the Public Readiness and Emergency Preparedness Act, which provides immunity from liability for retired doctors and nurses, among others, who sign up to help give vaccinations. That’s helped get more people into the field giving shots.
The administration also canceled a plan to allocate vaccines to states based on their pace of administration, which would have punished underperforming states. Instead, doses are allocated based on population.
In a media call on April 7, when asked whether the administration would send additional vaccines to Michigan, a state that’s seeing a surge of COVID-19 cases with more transmissible variants, Mr. Slavitt said they weren’t managing vaccine supply “according to some formula.”
He said they were distributing based on population “because that’s fundamental,” but were also locating vaccines “surgically in places that have had the greatest disease and where people have the greatest exposure.”
He said sites like community health centers and retail pharmacies have the power to order vaccines directly from the federal government, which helps get more supply to harder-hit areas.
Mr. Slavitt said hitting 4.1 million daily vaccinations on April 3 was gratifying.
“I’ve seen photographs ... of people breaking down in tears when they get their vaccine, people who are giving standing ovations to active military for taking care of them,” he said, “and I think about people who have gone for a long time without hope, or who have been very scared.
“It’s incredibly encouraging to think about maybe a few million people taking a step back to normal life again,” he said.
A version of this article first appeared on Medscape.com.
Each afternoon, Cyrus Shahpar, MD, the data guru for the White House COVID-19 Response Team, sends an email to staffers with the daily count of COVID-19 vaccinations delivered in the United States.
The numbers, collected from states ahead of the final figures being posted on the Centers for Disease Control and Prevention website, act as a report card of sorts on the team’s efforts.
On Saturday, April 3, it was a new record: 4.1 million vaccinations delivered in a single day, more than the total population of some states.
While the United States has a long way to go before it is done with COVID-19, there’s finally some good news in the nation’s long and blundering slog through the pandemic.
After a rocky start in December 2020 and January 2021, vaccination is happening faster than nearly anyone thought possible. As more people see their friends and family roll up their sleeves, hesitancy is dropping, too.
In settings where large numbers of people are vaccinated, such as nursing homes, COVID-19 cases and deaths have plunged.
Those gains, however, haven’t been shared equally. According to CDC data, 69% of people who are fully vaccinated are White, while just 8% are Black and about 9% are Hispanic, a group that now represents most new COVID-19 cases.
Officials say that’s partly because the vaccines were rolled out to the elderly first. The average life expectancy for Black people in the United States is now age 72, which means there were fewer people of color represented in the first groups to become eligible. Experts are hopeful that underrepresented groups will start to catch up as more states open up vaccinations to younger people.
Based on overall numbers of daily vaccine doses, the United States ranks third, behind China and India. America ranks fourth – behind Israel, the United Kingdom, and Chile – in the total share of the population that’s been vaccinated, according to the website Our World in Data.
A positive development
It’s a stunning turnaround for a country that failed for months to develop effective tests, and still struggles in some quarters to investigate new cases and quarantine their contacts.
The 7-day rolling average of vaccines administered in the United States is currently more than 3 million a day.
“We knew that we needed to get to 3 million a day at some point, if we were going to get most people vaccinated this year, but I don’t think that most people expected it to happen this early,” said Eric Toner, MD, a senior scholar with the Johns Hopkins Center for Health Security in Baltimore.
Before taking office, President Joe Biden pledged to get 100 million shots in arms within his first 100 days in office. After hitting that goal in late March, he doubled it, to 200 million vaccinations by April 30. After first saying all adults should be eligible to get in line for the vaccine by May 1, on April 6, he bumped up that date to April 19.
Some media reports have seen this repeated moving of the goalposts as calculated – an unstated strategy of underpromising and overdelivering with the aim of rebuilding public trust.
But others pointed out that, even if that’s true, the goals being set aren’t easy, and hitting them has never been a given.
“I think the Biden administration really gets a lot of credit for pushing the companies to get more vaccine out faster than they had planned to,” Dr. Toner said. “And the states have really responded as well as the federal government in terms of getting vaccination sites going. So we’re not only getting the vaccines, we’re getting it into people’s arms faster than expected.”
Others agree.
“We’re doing an amazing job, and I think the U.S. is really beginning to bend the curve,” said Carlos del Rio, MD, an infectious disease specialist and distinguished professor of medicine at Emory University, Atlanta.
“I think overall it’s just that everybody’s putting in a ton of work to get it done,” he said.
On April 3, the day the United States hit its vaccination record, he was volunteering to give vaccinations.
“I mean, of all the bad things we do to people as clinicians, this is one thing that people are very happy about, right?” Dr. del Rio said.
He said he vaccinated a young woman who asked if she could video chat with her mom, who was feeling nervous about getting the shot. He answered her mom’s questions, and later that day, she came down to be vaccinated herself.
‘We view it as a war’
The White House COVID-19 Response Team has worked hard to better coordinate the work of so many people at both the federal and state levels, Andy Slavitt, senior adviser for the team, said in an interview.
“We view it as a war, and in a war, you do everything: You bring experienced personnel; you bring all the resources to bear; you create multiple routes,” Mr. Slavitt said. “You don’t leave anything to chance.”
Among the levers the administration has pulled, using the Defense Production Act has helped vaccine manufacturers get needed supplies, Mr. Slavitt said.
The administration has set up an array of Federal Emergency Management Agency–run community vaccination centers and mobile vaccination sites to complement state-led efforts, and it’s activated a federal health law called the Public Readiness and Emergency Preparedness Act, which provides immunity from liability for retired doctors and nurses, among others, who sign up to help give vaccinations. That’s helped get more people into the field giving shots.
The administration also canceled a plan to allocate vaccines to states based on their pace of administration, which would have punished underperforming states. Instead, doses are allocated based on population.
In a media call on April 7, when asked whether the administration would send additional vaccines to Michigan, a state that’s seeing a surge of COVID-19 cases with more transmissible variants, Mr. Slavitt said they weren’t managing vaccine supply “according to some formula.”
He said they were distributing based on population “because that’s fundamental,” but were also locating vaccines “surgically in places that have had the greatest disease and where people have the greatest exposure.”
He said sites like community health centers and retail pharmacies have the power to order vaccines directly from the federal government, which helps get more supply to harder-hit areas.
Mr. Slavitt said hitting 4.1 million daily vaccinations on April 3 was gratifying.
“I’ve seen photographs ... of people breaking down in tears when they get their vaccine, people who are giving standing ovations to active military for taking care of them,” he said, “and I think about people who have gone for a long time without hope, or who have been very scared.
“It’s incredibly encouraging to think about maybe a few million people taking a step back to normal life again,” he said.
A version of this article first appeared on Medscape.com.
Each afternoon, Cyrus Shahpar, MD, the data guru for the White House COVID-19 Response Team, sends an email to staffers with the daily count of COVID-19 vaccinations delivered in the United States.
The numbers, collected from states ahead of the final figures being posted on the Centers for Disease Control and Prevention website, act as a report card of sorts on the team’s efforts.
On Saturday, April 3, it was a new record: 4.1 million vaccinations delivered in a single day, more than the total population of some states.
While the United States has a long way to go before it is done with COVID-19, there’s finally some good news in the nation’s long and blundering slog through the pandemic.
After a rocky start in December 2020 and January 2021, vaccination is happening faster than nearly anyone thought possible. As more people see their friends and family roll up their sleeves, hesitancy is dropping, too.
In settings where large numbers of people are vaccinated, such as nursing homes, COVID-19 cases and deaths have plunged.
Those gains, however, haven’t been shared equally. According to CDC data, 69% of people who are fully vaccinated are White, while just 8% are Black and about 9% are Hispanic, a group that now represents most new COVID-19 cases.
Officials say that’s partly because the vaccines were rolled out to the elderly first. The average life expectancy for Black people in the United States is now age 72, which means there were fewer people of color represented in the first groups to become eligible. Experts are hopeful that underrepresented groups will start to catch up as more states open up vaccinations to younger people.
Based on overall numbers of daily vaccine doses, the United States ranks third, behind China and India. America ranks fourth – behind Israel, the United Kingdom, and Chile – in the total share of the population that’s been vaccinated, according to the website Our World in Data.
A positive development
It’s a stunning turnaround for a country that failed for months to develop effective tests, and still struggles in some quarters to investigate new cases and quarantine their contacts.
The 7-day rolling average of vaccines administered in the United States is currently more than 3 million a day.
“We knew that we needed to get to 3 million a day at some point, if we were going to get most people vaccinated this year, but I don’t think that most people expected it to happen this early,” said Eric Toner, MD, a senior scholar with the Johns Hopkins Center for Health Security in Baltimore.
Before taking office, President Joe Biden pledged to get 100 million shots in arms within his first 100 days in office. After hitting that goal in late March, he doubled it, to 200 million vaccinations by April 30. After first saying all adults should be eligible to get in line for the vaccine by May 1, on April 6, he bumped up that date to April 19.
Some media reports have seen this repeated moving of the goalposts as calculated – an unstated strategy of underpromising and overdelivering with the aim of rebuilding public trust.
But others pointed out that, even if that’s true, the goals being set aren’t easy, and hitting them has never been a given.
“I think the Biden administration really gets a lot of credit for pushing the companies to get more vaccine out faster than they had planned to,” Dr. Toner said. “And the states have really responded as well as the federal government in terms of getting vaccination sites going. So we’re not only getting the vaccines, we’re getting it into people’s arms faster than expected.”
Others agree.
“We’re doing an amazing job, and I think the U.S. is really beginning to bend the curve,” said Carlos del Rio, MD, an infectious disease specialist and distinguished professor of medicine at Emory University, Atlanta.
“I think overall it’s just that everybody’s putting in a ton of work to get it done,” he said.
On April 3, the day the United States hit its vaccination record, he was volunteering to give vaccinations.
“I mean, of all the bad things we do to people as clinicians, this is one thing that people are very happy about, right?” Dr. del Rio said.
He said he vaccinated a young woman who asked if she could video chat with her mom, who was feeling nervous about getting the shot. He answered her mom’s questions, and later that day, she came down to be vaccinated herself.
‘We view it as a war’
The White House COVID-19 Response Team has worked hard to better coordinate the work of so many people at both the federal and state levels, Andy Slavitt, senior adviser for the team, said in an interview.
“We view it as a war, and in a war, you do everything: You bring experienced personnel; you bring all the resources to bear; you create multiple routes,” Mr. Slavitt said. “You don’t leave anything to chance.”
Among the levers the administration has pulled, using the Defense Production Act has helped vaccine manufacturers get needed supplies, Mr. Slavitt said.
The administration has set up an array of Federal Emergency Management Agency–run community vaccination centers and mobile vaccination sites to complement state-led efforts, and it’s activated a federal health law called the Public Readiness and Emergency Preparedness Act, which provides immunity from liability for retired doctors and nurses, among others, who sign up to help give vaccinations. That’s helped get more people into the field giving shots.
The administration also canceled a plan to allocate vaccines to states based on their pace of administration, which would have punished underperforming states. Instead, doses are allocated based on population.
In a media call on April 7, when asked whether the administration would send additional vaccines to Michigan, a state that’s seeing a surge of COVID-19 cases with more transmissible variants, Mr. Slavitt said they weren’t managing vaccine supply “according to some formula.”
He said they were distributing based on population “because that’s fundamental,” but were also locating vaccines “surgically in places that have had the greatest disease and where people have the greatest exposure.”
He said sites like community health centers and retail pharmacies have the power to order vaccines directly from the federal government, which helps get more supply to harder-hit areas.
Mr. Slavitt said hitting 4.1 million daily vaccinations on April 3 was gratifying.
“I’ve seen photographs ... of people breaking down in tears when they get their vaccine, people who are giving standing ovations to active military for taking care of them,” he said, “and I think about people who have gone for a long time without hope, or who have been very scared.
“It’s incredibly encouraging to think about maybe a few million people taking a step back to normal life again,” he said.
A version of this article first appeared on Medscape.com.




