User login
Bivalirudin edges unfractionated heparin for PCI in acute coronary syndrome patients
SAN DIEGO– The antithrombin drug bivalirudin received a boost, compared with unfractionated heparin, as the safer drug for preventing ischemic events in patients with acute coronary syndrome undergoing percutaneous coronary intervention in results from a multicenter, randomized trial with more than 7,000 patients.
Although the two primary endpoints from this head-to-head comparison showed no statistically significant differences between the two agents, prespecified secondary endpoints showed that treatment with bivalirudin (Angiomax) during percutaneous coronary intervention (PCI) resulted in significantly fewer deaths after 30 days and significantly fewer major bleeding events, compared with unfractionated heparin (UFH), Dr. Marco Valgimigli said at the annual meeting of the American College of Cardiology. Participating physicians administered the UFH with an antiplatelet glycoprotein IIb/IIIa inhibitor (at the operator’s discretion) 26% of the time.
In the antithrombin-randomization analysis of the MATRIX (Minimizing Adverse Hemorrhagic Events by Transradial Access Site and Systemic Implementation of Angiox) trial, which included 7,213 of the 8,404 acute coronary syndrome patients enrolled in MATRIX, treatment with bivalirudin or UFH led to similar combined rates of all-cause death, myocardial infarction, or stroke, as well as similar rates of death, MI, stroke, and major bleeds, said Dr. Valgimigli, an interventional cardiologist at Erasmus University Medical Center in Rotterdam, the Netherlands. A separate analysis of MATRIX focused on PCI outcomes with transradial vs. transfemoral access (Lancet 2015 [doi:10.1016/S0140-6736(15)60292-6]).
But treatment with bivalirudin cut the 30-day rate of all-cause death by an absolute 0.6%, driven by a concurrent cut in cardiovascular death by 0.7%, which meant that treatment with bivalirudin reduced 30-day cardiovascular deaths by one event for about every 150 patients treated, compared with patients treated with UFH, he reported. Another secondary-endpoint analysis showed that bivalirudin treatment cut the 30-day rate of major bleeding events by an absolute 1.1%, but also increased the rate of definite stent thrombosis by an absolute 0.4%, both statistically significant differences. These important differences got diluted in the primary, combined endpoints by a relatively large number of periprocedural MIs that occurred at an equal rate in both arms of the study, Dr. Valgimigli said.
The antithrombin results from MATRIX followed mixed results in several prior comparisons of bivalirudin and UFH for percutaneous coronary intervention acute coronary syndrome patients (JAMA 2015 [doi:10.1001/jama.2015.2345]). A year ago, results from the HEAT-PCI (Unfractionated Heparin Versus Bivalirudin in Primary Percutaneous Coronary Intervention) trial (Lancet 2014;384:1849-58), which enrolled 1,829 ST-elevation MI patients at one U.K. center, showed a significant reduction in ischemic events and no increased bleeding in patients treated with UFH, compared with those who received bivalirudin, a finding that experts now say seemed to lead to increased use of UFH in both U.S. and global practice relative to the more expensive bivalirudin. But the MATRIX results, as well as results published on the same day as the MATRIX report from the Chinese BRIGHT (Bivalirudin vs. Heparin With or Without Tirofiban During Primary Percutaneous Coronary Intervention in Acute Myocardial Infarction) study (JAMA 2015 [doi:10.1001/jama.2015.232]) that also compared bivalirudin and UFH, seemed to throw the balance of evidence back in bivalirudin’s favor.
MATRIX “was a win for bivalirudin,” commented Dr. Sanjit S. Jolly, an interventional cardiologist at McMaster University in Hamilton, Ont. “Clearly mortality is the most important endpoint, and the totality of data from all the trials suggest that bivalirudin reduced mortality, compared with UFH. I think that physicians who stopped using bivalirudin after HEAT-PCI may go back to bivalirudin,” he said during a press conference at the meeting.
“Following HEAT-PCI there was a shift to greater use of UFH and more selective use of bivalirudin, even in the United States, and especially for patients with ST-elevation MI,” commented Dr. David E. Kandzari , director of interventional cardiology at the Piedmont Heart Institute in Atlanta. The new MATRIX findings “will probably prompt clinicians to revisit this given that MATRIX was the largest study to compare bivalirudin and UFH. That is not to discount the HEAT-PCI findings, but that was a much smaller trial and at a single center.”
Bivalirudin treatment results in additional expense, compared with UFH, and physicians are “under pressure to cut costs, so following the HEAT-PCI results, there was a big reduction in bivalirudin use. But with the subsequent BRIGHT results and now the MATRIX results, I think there will be an uptick again in bivalirudin use,” commented Dr. Cindy L. Grines, an interventional cardiologist at the Detroit Medical Center. The MATRIX results make her “more confident about the benefit from bivalirudin,” she said in an interview.
MATRIX was an investigator-initiated study that received grant support from Terumo and the Medicines Co. Dr. Valgimigli had no disclosures. Dr. Jolly has been a consultant to AstraZeneca, a speaker on behalf of St. Jude, and received research grants from Medtronic. Dr. Kandzari has been a consultant to Medtronic and Boston Scientific and has received research support from Abbott Vascular, Biotronik, Boston Scientific, and Medtronic. Dr. Grines has been a consultant to and received honoraria from Abbott Vascular, the Medicines Co., Merck, and the Volcano Group.
On Twitter @mitchelzoler
SAN DIEGO– The antithrombin drug bivalirudin received a boost, compared with unfractionated heparin, as the safer drug for preventing ischemic events in patients with acute coronary syndrome undergoing percutaneous coronary intervention in results from a multicenter, randomized trial with more than 7,000 patients.
Although the two primary endpoints from this head-to-head comparison showed no statistically significant differences between the two agents, prespecified secondary endpoints showed that treatment with bivalirudin (Angiomax) during percutaneous coronary intervention (PCI) resulted in significantly fewer deaths after 30 days and significantly fewer major bleeding events, compared with unfractionated heparin (UFH), Dr. Marco Valgimigli said at the annual meeting of the American College of Cardiology. Participating physicians administered the UFH with an antiplatelet glycoprotein IIb/IIIa inhibitor (at the operator’s discretion) 26% of the time.
In the antithrombin-randomization analysis of the MATRIX (Minimizing Adverse Hemorrhagic Events by Transradial Access Site and Systemic Implementation of Angiox) trial, which included 7,213 of the 8,404 acute coronary syndrome patients enrolled in MATRIX, treatment with bivalirudin or UFH led to similar combined rates of all-cause death, myocardial infarction, or stroke, as well as similar rates of death, MI, stroke, and major bleeds, said Dr. Valgimigli, an interventional cardiologist at Erasmus University Medical Center in Rotterdam, the Netherlands. A separate analysis of MATRIX focused on PCI outcomes with transradial vs. transfemoral access (Lancet 2015 [doi:10.1016/S0140-6736(15)60292-6]).
But treatment with bivalirudin cut the 30-day rate of all-cause death by an absolute 0.6%, driven by a concurrent cut in cardiovascular death by 0.7%, which meant that treatment with bivalirudin reduced 30-day cardiovascular deaths by one event for about every 150 patients treated, compared with patients treated with UFH, he reported. Another secondary-endpoint analysis showed that bivalirudin treatment cut the 30-day rate of major bleeding events by an absolute 1.1%, but also increased the rate of definite stent thrombosis by an absolute 0.4%, both statistically significant differences. These important differences got diluted in the primary, combined endpoints by a relatively large number of periprocedural MIs that occurred at an equal rate in both arms of the study, Dr. Valgimigli said.
The antithrombin results from MATRIX followed mixed results in several prior comparisons of bivalirudin and UFH for percutaneous coronary intervention acute coronary syndrome patients (JAMA 2015 [doi:10.1001/jama.2015.2345]). A year ago, results from the HEAT-PCI (Unfractionated Heparin Versus Bivalirudin in Primary Percutaneous Coronary Intervention) trial (Lancet 2014;384:1849-58), which enrolled 1,829 ST-elevation MI patients at one U.K. center, showed a significant reduction in ischemic events and no increased bleeding in patients treated with UFH, compared with those who received bivalirudin, a finding that experts now say seemed to lead to increased use of UFH in both U.S. and global practice relative to the more expensive bivalirudin. But the MATRIX results, as well as results published on the same day as the MATRIX report from the Chinese BRIGHT (Bivalirudin vs. Heparin With or Without Tirofiban During Primary Percutaneous Coronary Intervention in Acute Myocardial Infarction) study (JAMA 2015 [doi:10.1001/jama.2015.232]) that also compared bivalirudin and UFH, seemed to throw the balance of evidence back in bivalirudin’s favor.
MATRIX “was a win for bivalirudin,” commented Dr. Sanjit S. Jolly, an interventional cardiologist at McMaster University in Hamilton, Ont. “Clearly mortality is the most important endpoint, and the totality of data from all the trials suggest that bivalirudin reduced mortality, compared with UFH. I think that physicians who stopped using bivalirudin after HEAT-PCI may go back to bivalirudin,” he said during a press conference at the meeting.
“Following HEAT-PCI there was a shift to greater use of UFH and more selective use of bivalirudin, even in the United States, and especially for patients with ST-elevation MI,” commented Dr. David E. Kandzari , director of interventional cardiology at the Piedmont Heart Institute in Atlanta. The new MATRIX findings “will probably prompt clinicians to revisit this given that MATRIX was the largest study to compare bivalirudin and UFH. That is not to discount the HEAT-PCI findings, but that was a much smaller trial and at a single center.”
Bivalirudin treatment results in additional expense, compared with UFH, and physicians are “under pressure to cut costs, so following the HEAT-PCI results, there was a big reduction in bivalirudin use. But with the subsequent BRIGHT results and now the MATRIX results, I think there will be an uptick again in bivalirudin use,” commented Dr. Cindy L. Grines, an interventional cardiologist at the Detroit Medical Center. The MATRIX results make her “more confident about the benefit from bivalirudin,” she said in an interview.
MATRIX was an investigator-initiated study that received grant support from Terumo and the Medicines Co. Dr. Valgimigli had no disclosures. Dr. Jolly has been a consultant to AstraZeneca, a speaker on behalf of St. Jude, and received research grants from Medtronic. Dr. Kandzari has been a consultant to Medtronic and Boston Scientific and has received research support from Abbott Vascular, Biotronik, Boston Scientific, and Medtronic. Dr. Grines has been a consultant to and received honoraria from Abbott Vascular, the Medicines Co., Merck, and the Volcano Group.
On Twitter @mitchelzoler
SAN DIEGO– The antithrombin drug bivalirudin received a boost, compared with unfractionated heparin, as the safer drug for preventing ischemic events in patients with acute coronary syndrome undergoing percutaneous coronary intervention in results from a multicenter, randomized trial with more than 7,000 patients.
Although the two primary endpoints from this head-to-head comparison showed no statistically significant differences between the two agents, prespecified secondary endpoints showed that treatment with bivalirudin (Angiomax) during percutaneous coronary intervention (PCI) resulted in significantly fewer deaths after 30 days and significantly fewer major bleeding events, compared with unfractionated heparin (UFH), Dr. Marco Valgimigli said at the annual meeting of the American College of Cardiology. Participating physicians administered the UFH with an antiplatelet glycoprotein IIb/IIIa inhibitor (at the operator’s discretion) 26% of the time.
In the antithrombin-randomization analysis of the MATRIX (Minimizing Adverse Hemorrhagic Events by Transradial Access Site and Systemic Implementation of Angiox) trial, which included 7,213 of the 8,404 acute coronary syndrome patients enrolled in MATRIX, treatment with bivalirudin or UFH led to similar combined rates of all-cause death, myocardial infarction, or stroke, as well as similar rates of death, MI, stroke, and major bleeds, said Dr. Valgimigli, an interventional cardiologist at Erasmus University Medical Center in Rotterdam, the Netherlands. A separate analysis of MATRIX focused on PCI outcomes with transradial vs. transfemoral access (Lancet 2015 [doi:10.1016/S0140-6736(15)60292-6]).
But treatment with bivalirudin cut the 30-day rate of all-cause death by an absolute 0.6%, driven by a concurrent cut in cardiovascular death by 0.7%, which meant that treatment with bivalirudin reduced 30-day cardiovascular deaths by one event for about every 150 patients treated, compared with patients treated with UFH, he reported. Another secondary-endpoint analysis showed that bivalirudin treatment cut the 30-day rate of major bleeding events by an absolute 1.1%, but also increased the rate of definite stent thrombosis by an absolute 0.4%, both statistically significant differences. These important differences got diluted in the primary, combined endpoints by a relatively large number of periprocedural MIs that occurred at an equal rate in both arms of the study, Dr. Valgimigli said.
The antithrombin results from MATRIX followed mixed results in several prior comparisons of bivalirudin and UFH for percutaneous coronary intervention acute coronary syndrome patients (JAMA 2015 [doi:10.1001/jama.2015.2345]). A year ago, results from the HEAT-PCI (Unfractionated Heparin Versus Bivalirudin in Primary Percutaneous Coronary Intervention) trial (Lancet 2014;384:1849-58), which enrolled 1,829 ST-elevation MI patients at one U.K. center, showed a significant reduction in ischemic events and no increased bleeding in patients treated with UFH, compared with those who received bivalirudin, a finding that experts now say seemed to lead to increased use of UFH in both U.S. and global practice relative to the more expensive bivalirudin. But the MATRIX results, as well as results published on the same day as the MATRIX report from the Chinese BRIGHT (Bivalirudin vs. Heparin With or Without Tirofiban During Primary Percutaneous Coronary Intervention in Acute Myocardial Infarction) study (JAMA 2015 [doi:10.1001/jama.2015.232]) that also compared bivalirudin and UFH, seemed to throw the balance of evidence back in bivalirudin’s favor.
MATRIX “was a win for bivalirudin,” commented Dr. Sanjit S. Jolly, an interventional cardiologist at McMaster University in Hamilton, Ont. “Clearly mortality is the most important endpoint, and the totality of data from all the trials suggest that bivalirudin reduced mortality, compared with UFH. I think that physicians who stopped using bivalirudin after HEAT-PCI may go back to bivalirudin,” he said during a press conference at the meeting.
“Following HEAT-PCI there was a shift to greater use of UFH and more selective use of bivalirudin, even in the United States, and especially for patients with ST-elevation MI,” commented Dr. David E. Kandzari , director of interventional cardiology at the Piedmont Heart Institute in Atlanta. The new MATRIX findings “will probably prompt clinicians to revisit this given that MATRIX was the largest study to compare bivalirudin and UFH. That is not to discount the HEAT-PCI findings, but that was a much smaller trial and at a single center.”
Bivalirudin treatment results in additional expense, compared with UFH, and physicians are “under pressure to cut costs, so following the HEAT-PCI results, there was a big reduction in bivalirudin use. But with the subsequent BRIGHT results and now the MATRIX results, I think there will be an uptick again in bivalirudin use,” commented Dr. Cindy L. Grines, an interventional cardiologist at the Detroit Medical Center. The MATRIX results make her “more confident about the benefit from bivalirudin,” she said in an interview.
MATRIX was an investigator-initiated study that received grant support from Terumo and the Medicines Co. Dr. Valgimigli had no disclosures. Dr. Jolly has been a consultant to AstraZeneca, a speaker on behalf of St. Jude, and received research grants from Medtronic. Dr. Kandzari has been a consultant to Medtronic and Boston Scientific and has received research support from Abbott Vascular, Biotronik, Boston Scientific, and Medtronic. Dr. Grines has been a consultant to and received honoraria from Abbott Vascular, the Medicines Co., Merck, and the Volcano Group.
On Twitter @mitchelzoler
AT ACC 15
Key clinical point: In acute coronary syndrome patients undergoing percutaneous coronary intervention, bivalirudin led to significantly fewer deaths and major bleeds than did unfractionated heparin.
Major finding: Treatment with bivalirudin cut all-cause, 30-day mortality by an absolute 0.6%, compared with unfractionated heparin.
Data source: MATRIX, a multicenter, randomized trial that enrolled 7,213 acute coronary syndrome patients undergoing percutaneous coronary intervention.
Disclosures: MATRIX was an investigator-initiated study that received grant support from Terumo and the Medicines Co. Dr. Valgimigli had no disclosures. Dr. Jolly has been a consultant to AstraZeneca, a speaker on behalf of St. Jude, and received research grants from Medtronic. Dr. Kandzari has been a consultant to Medtronic and Boston Scientific and has received research support from Abbott Vascular, Biotronik, Boston Scientific, and Medtronic. Dr. Grines has been a consultant to and received honoraria from Abbott Vascular, the Medicines Co., Merck, and the Volcano Group.
LEGACY: Weight loss markedly improves atrial fibrillation
SAN DIEGO – Maintenance of long-term weight loss in obese or overweight individuals with atrial fibrillation was associated with nearly a sixfold greater likelihood of freedom from recurrent AF during nearly 5 years of active follow-up in the LEGACY study.
“The effect was dose dependent. The most important finding is that 46% of patients with at least a 10% weight loss were free from AF without the use of drugs or ablation procedures through nearly 5 years, versus 22% of those with 3%-9% weight loss, and just 13% with less than a 3% weight loss,” Dr. Rajeev K. Pathak said at the annual meeting of the American College of Cardiology.
LEGACY (Long-Term Effect of Goal-Directed Weight Management in an Atrial Fibrillation Cohort: A Long-Term Follow-Up Study) included 355 overweight or obese participants with paroxsymal or persistent AF who were offered the chance to participate in a dedicated weight-loss clinic. Regular participation in this clinic proved to be a key factor in losing weight, keeping it off, and reducing AF burden; the more frequently patients attended the quarterly sessions the better the outcomes, noted Dr. Pathak, a cardiologist and electrophysiology fellow at the University of Adelaide (Australia).
Of the 355 participants, 135 lost at least 10% of their body weight, 103 had a 3%-9% drop in body weight, and 117 had less than a 3% loss or a weight gain.
Year-by-year weight trends had a significant impact on outcome. The 141 patients with linear weight loss had a 76% AF-free rate with or without the use of drugs or ablation, compared with a 59% in the 179 patients with weight fluctuations and the 38% rate in those with no loss or a weight gain. Atrial fibrillation status was assessed by 7-day Holter monitoring at least annually.
Weight fluctuation – defined as a 1% or greater change in weight between two consecutive annual follow-ups – dampened the benefits conferred by weight loss. Patients who experienced more than a 5% weight fluctuation were 2.2-fold more likely to experience AF recurrence than were those without weight fluctuation.
Patients with a sustained 10% weight loss were 5.6-fold more likely to achieve long-term freedom from AF than were patients with lesser or no weight loss. The goal was 10% weight loss rather than a body mass index of 25 kg/m2 or less because once AF patients get down to a BMI below 27 kg/m2 the incremental benefit of each additional 1 BMI point in terms of freedom from AF becomes much smaller, said Dr. Pathak.
Weight loss also showed a dose-dependent effect on various cardiovascular risk factors. For example, mean systolic blood pressure fell by 18 mm Hg in subjects with at least a 10% weight loss, by 10 mm Hg with a 3%-9% loss, and by 7 mm Hg with a lesser weight loss. Triglycerides, LDL cholesterol, and glycemic control improved in similar fashion. In addition, weight loss showed beneficial effects on cardiac structure, with dose-dependent reductions in left atrial volume indexed for body surface area as well as interventricular septal thickness, Dr. Pathak continued.
He described the weight loss clinic as a “very simple” structured motivational and goal-directed program with face-to-face counseling.
“We have one patient, one physician, no props. We sit with the patient, discuss areas we can improve, then we devise a low-carb, low-fat, high-protein diet in consultation with the patient. Patients maintain a lifestyle journal where they log their meals and exercise. We prescribe at least 200 minutes of moderate-intensity activity per week. Eating is a behavioral pattern, and we have found this approach to be a very effective behavioral tool. Because the plan is developed in consultation with the patient, we’ve found patients tend to adhere to what they have planned,” he explained.
Discussant Dr. Bernard J. Gersh praised LEGACY as “a really wonderful study – very important.
“There are years of epidemiologic evidence suggesting that obesity contributes in a major way to the epidemic of atrial fibrillation, and you’ve taken it a step further,” added Dr. Gersh, professor of medicine at the Mayo Clinic in Rochester, Minn.
Playing devil’s advocate, he asked whether the observed reduction in AF might have nothing to do with weight loss, but could be explained simply by LEGACY perhaps having enrolled a highly compliant group of patients who agreed to attend a clinic and were more willing to take their medications.
Dr. Pathak rejected the compliance factor as an explanation for the results. He noted that while cardiovascular risk factors improved with greater weight loss, the need for antihypertensive, lipid-lowering, and antiarrhythmic medications decreased.
“The effect can’t possibly be due to increased compliance with medications, but rather it’s a true effect of the weight loss itself. So I think this is a true clinical effect and not an epiphenomenon,” he replied.
Dr. Pathak added that he and his coinvestigators are organizing a randomized controlled confirmatory study.
Dr. Prediman K. Shah, who chaired a press conference where the LEGACY study was highlighted, said the study provided him with one of the major take-home lessons from ACC 15.
“We can argue about the mechanism of atrial fibrillation till kingdom come, but the fact is that the association is very strong that weight loss is associated with a reduced burden of atrial fibrillation, and with a very robust magnitude of benefit. That’s one of the messages that I’ll take home with me from this meeting: The next time I see my fat patient with atrial fibrillation, I’m putting him on a weight-reducing diet as the first approach,” declared Dr. Shah, professor of medicine at UCLA and director of the Oppenheimer Atherosclerosis Research Center at Cedars-Sinai Medical Center in Los Angeles.
Dr. Pathak reported having no financial conflicts regarding this study, which was supported by university funds.
Simultaneously with Dr. Pathak’s presentation at ACC 15, the LEGACY study was published online (J. Am. Coll. Cardiol. 2015 [doi: 10.1016/j.jacc.2015.03.002])
SAN DIEGO – Maintenance of long-term weight loss in obese or overweight individuals with atrial fibrillation was associated with nearly a sixfold greater likelihood of freedom from recurrent AF during nearly 5 years of active follow-up in the LEGACY study.
“The effect was dose dependent. The most important finding is that 46% of patients with at least a 10% weight loss were free from AF without the use of drugs or ablation procedures through nearly 5 years, versus 22% of those with 3%-9% weight loss, and just 13% with less than a 3% weight loss,” Dr. Rajeev K. Pathak said at the annual meeting of the American College of Cardiology.
LEGACY (Long-Term Effect of Goal-Directed Weight Management in an Atrial Fibrillation Cohort: A Long-Term Follow-Up Study) included 355 overweight or obese participants with paroxsymal or persistent AF who were offered the chance to participate in a dedicated weight-loss clinic. Regular participation in this clinic proved to be a key factor in losing weight, keeping it off, and reducing AF burden; the more frequently patients attended the quarterly sessions the better the outcomes, noted Dr. Pathak, a cardiologist and electrophysiology fellow at the University of Adelaide (Australia).
Of the 355 participants, 135 lost at least 10% of their body weight, 103 had a 3%-9% drop in body weight, and 117 had less than a 3% loss or a weight gain.
Year-by-year weight trends had a significant impact on outcome. The 141 patients with linear weight loss had a 76% AF-free rate with or without the use of drugs or ablation, compared with a 59% in the 179 patients with weight fluctuations and the 38% rate in those with no loss or a weight gain. Atrial fibrillation status was assessed by 7-day Holter monitoring at least annually.
Weight fluctuation – defined as a 1% or greater change in weight between two consecutive annual follow-ups – dampened the benefits conferred by weight loss. Patients who experienced more than a 5% weight fluctuation were 2.2-fold more likely to experience AF recurrence than were those without weight fluctuation.
Patients with a sustained 10% weight loss were 5.6-fold more likely to achieve long-term freedom from AF than were patients with lesser or no weight loss. The goal was 10% weight loss rather than a body mass index of 25 kg/m2 or less because once AF patients get down to a BMI below 27 kg/m2 the incremental benefit of each additional 1 BMI point in terms of freedom from AF becomes much smaller, said Dr. Pathak.
Weight loss also showed a dose-dependent effect on various cardiovascular risk factors. For example, mean systolic blood pressure fell by 18 mm Hg in subjects with at least a 10% weight loss, by 10 mm Hg with a 3%-9% loss, and by 7 mm Hg with a lesser weight loss. Triglycerides, LDL cholesterol, and glycemic control improved in similar fashion. In addition, weight loss showed beneficial effects on cardiac structure, with dose-dependent reductions in left atrial volume indexed for body surface area as well as interventricular septal thickness, Dr. Pathak continued.
He described the weight loss clinic as a “very simple” structured motivational and goal-directed program with face-to-face counseling.
“We have one patient, one physician, no props. We sit with the patient, discuss areas we can improve, then we devise a low-carb, low-fat, high-protein diet in consultation with the patient. Patients maintain a lifestyle journal where they log their meals and exercise. We prescribe at least 200 minutes of moderate-intensity activity per week. Eating is a behavioral pattern, and we have found this approach to be a very effective behavioral tool. Because the plan is developed in consultation with the patient, we’ve found patients tend to adhere to what they have planned,” he explained.
Discussant Dr. Bernard J. Gersh praised LEGACY as “a really wonderful study – very important.
“There are years of epidemiologic evidence suggesting that obesity contributes in a major way to the epidemic of atrial fibrillation, and you’ve taken it a step further,” added Dr. Gersh, professor of medicine at the Mayo Clinic in Rochester, Minn.
Playing devil’s advocate, he asked whether the observed reduction in AF might have nothing to do with weight loss, but could be explained simply by LEGACY perhaps having enrolled a highly compliant group of patients who agreed to attend a clinic and were more willing to take their medications.
Dr. Pathak rejected the compliance factor as an explanation for the results. He noted that while cardiovascular risk factors improved with greater weight loss, the need for antihypertensive, lipid-lowering, and antiarrhythmic medications decreased.
“The effect can’t possibly be due to increased compliance with medications, but rather it’s a true effect of the weight loss itself. So I think this is a true clinical effect and not an epiphenomenon,” he replied.
Dr. Pathak added that he and his coinvestigators are organizing a randomized controlled confirmatory study.
Dr. Prediman K. Shah, who chaired a press conference where the LEGACY study was highlighted, said the study provided him with one of the major take-home lessons from ACC 15.
“We can argue about the mechanism of atrial fibrillation till kingdom come, but the fact is that the association is very strong that weight loss is associated with a reduced burden of atrial fibrillation, and with a very robust magnitude of benefit. That’s one of the messages that I’ll take home with me from this meeting: The next time I see my fat patient with atrial fibrillation, I’m putting him on a weight-reducing diet as the first approach,” declared Dr. Shah, professor of medicine at UCLA and director of the Oppenheimer Atherosclerosis Research Center at Cedars-Sinai Medical Center in Los Angeles.
Dr. Pathak reported having no financial conflicts regarding this study, which was supported by university funds.
Simultaneously with Dr. Pathak’s presentation at ACC 15, the LEGACY study was published online (J. Am. Coll. Cardiol. 2015 [doi: 10.1016/j.jacc.2015.03.002])
SAN DIEGO – Maintenance of long-term weight loss in obese or overweight individuals with atrial fibrillation was associated with nearly a sixfold greater likelihood of freedom from recurrent AF during nearly 5 years of active follow-up in the LEGACY study.
“The effect was dose dependent. The most important finding is that 46% of patients with at least a 10% weight loss were free from AF without the use of drugs or ablation procedures through nearly 5 years, versus 22% of those with 3%-9% weight loss, and just 13% with less than a 3% weight loss,” Dr. Rajeev K. Pathak said at the annual meeting of the American College of Cardiology.
LEGACY (Long-Term Effect of Goal-Directed Weight Management in an Atrial Fibrillation Cohort: A Long-Term Follow-Up Study) included 355 overweight or obese participants with paroxsymal or persistent AF who were offered the chance to participate in a dedicated weight-loss clinic. Regular participation in this clinic proved to be a key factor in losing weight, keeping it off, and reducing AF burden; the more frequently patients attended the quarterly sessions the better the outcomes, noted Dr. Pathak, a cardiologist and electrophysiology fellow at the University of Adelaide (Australia).
Of the 355 participants, 135 lost at least 10% of their body weight, 103 had a 3%-9% drop in body weight, and 117 had less than a 3% loss or a weight gain.
Year-by-year weight trends had a significant impact on outcome. The 141 patients with linear weight loss had a 76% AF-free rate with or without the use of drugs or ablation, compared with a 59% in the 179 patients with weight fluctuations and the 38% rate in those with no loss or a weight gain. Atrial fibrillation status was assessed by 7-day Holter monitoring at least annually.
Weight fluctuation – defined as a 1% or greater change in weight between two consecutive annual follow-ups – dampened the benefits conferred by weight loss. Patients who experienced more than a 5% weight fluctuation were 2.2-fold more likely to experience AF recurrence than were those without weight fluctuation.
Patients with a sustained 10% weight loss were 5.6-fold more likely to achieve long-term freedom from AF than were patients with lesser or no weight loss. The goal was 10% weight loss rather than a body mass index of 25 kg/m2 or less because once AF patients get down to a BMI below 27 kg/m2 the incremental benefit of each additional 1 BMI point in terms of freedom from AF becomes much smaller, said Dr. Pathak.
Weight loss also showed a dose-dependent effect on various cardiovascular risk factors. For example, mean systolic blood pressure fell by 18 mm Hg in subjects with at least a 10% weight loss, by 10 mm Hg with a 3%-9% loss, and by 7 mm Hg with a lesser weight loss. Triglycerides, LDL cholesterol, and glycemic control improved in similar fashion. In addition, weight loss showed beneficial effects on cardiac structure, with dose-dependent reductions in left atrial volume indexed for body surface area as well as interventricular septal thickness, Dr. Pathak continued.
He described the weight loss clinic as a “very simple” structured motivational and goal-directed program with face-to-face counseling.
“We have one patient, one physician, no props. We sit with the patient, discuss areas we can improve, then we devise a low-carb, low-fat, high-protein diet in consultation with the patient. Patients maintain a lifestyle journal where they log their meals and exercise. We prescribe at least 200 minutes of moderate-intensity activity per week. Eating is a behavioral pattern, and we have found this approach to be a very effective behavioral tool. Because the plan is developed in consultation with the patient, we’ve found patients tend to adhere to what they have planned,” he explained.
Discussant Dr. Bernard J. Gersh praised LEGACY as “a really wonderful study – very important.
“There are years of epidemiologic evidence suggesting that obesity contributes in a major way to the epidemic of atrial fibrillation, and you’ve taken it a step further,” added Dr. Gersh, professor of medicine at the Mayo Clinic in Rochester, Minn.
Playing devil’s advocate, he asked whether the observed reduction in AF might have nothing to do with weight loss, but could be explained simply by LEGACY perhaps having enrolled a highly compliant group of patients who agreed to attend a clinic and were more willing to take their medications.
Dr. Pathak rejected the compliance factor as an explanation for the results. He noted that while cardiovascular risk factors improved with greater weight loss, the need for antihypertensive, lipid-lowering, and antiarrhythmic medications decreased.
“The effect can’t possibly be due to increased compliance with medications, but rather it’s a true effect of the weight loss itself. So I think this is a true clinical effect and not an epiphenomenon,” he replied.
Dr. Pathak added that he and his coinvestigators are organizing a randomized controlled confirmatory study.
Dr. Prediman K. Shah, who chaired a press conference where the LEGACY study was highlighted, said the study provided him with one of the major take-home lessons from ACC 15.
“We can argue about the mechanism of atrial fibrillation till kingdom come, but the fact is that the association is very strong that weight loss is associated with a reduced burden of atrial fibrillation, and with a very robust magnitude of benefit. That’s one of the messages that I’ll take home with me from this meeting: The next time I see my fat patient with atrial fibrillation, I’m putting him on a weight-reducing diet as the first approach,” declared Dr. Shah, professor of medicine at UCLA and director of the Oppenheimer Atherosclerosis Research Center at Cedars-Sinai Medical Center in Los Angeles.
Dr. Pathak reported having no financial conflicts regarding this study, which was supported by university funds.
Simultaneously with Dr. Pathak’s presentation at ACC 15, the LEGACY study was published online (J. Am. Coll. Cardiol. 2015 [doi: 10.1016/j.jacc.2015.03.002])
AT ACC 15
Key clinical point: Nearly half of overweight or obese patients with atrial fibrillation who achieved at least a 10% sustained weight loss remained arrhythmia free without resort to antiarrhythmic drugs or ablation procedures during nearly 5 years of follow-up.
Major finding: The weight-loss-associated freedom from recurrent AF was accompanied by improvements in cardiac structure as well as improved cardiovascular risk factors despite lesser use of risk factor-modifying drugs.
Data source: An observational study of 355 overweight or obese patients with atrial fibrillation who agreed to participate in a weight loss clinic.
Disclosures: The LEGACY study was supported by university funds. The presenter reported no financial conflicts.
Updated IMPROVE-IT results show bigger benefits
SAN DIEGO– The sensational initial presentation of the landmark IMPROVE-IT trial at last November’s AHA Scientific Sessions told only half the story of the actual clinical impact of lipid-lowering with the combination of ezetimibe and simvastatin as opposed to simvastatin alone.
That’s because the main analysis included only the first cardiovascular event patients experienced. Many participants with a nonfatal first event went on to have a second, third, or even a fourth event. And while additional events haven’t traditionally been counted in acute coronary syndrome clinical trials, they should be, Sabina A. Murphy said at the annual meeting of the American College of Cardiology.
“All events, not just first events, are important to patients and clinicians. Total events have implications for patient morbidity, clinical management, need for recurrent hospitalization, and total costs,” said Ms. Murphy, head of biostatistics at the TIMI Study Group at Brigham and Women’s Hospital, Boston.
Analyses that include recurrent events are common in some other medical fields, including oncology and rheumatology, she added.
In last November’s initial findings from IMPROVE-IT (Improved Reduction of Outcomes: Vytorin Efficacy International Trial), the combination of 10 mg ezetimibe/40 mg simvastatin (Vytorin) daily resulted in a modest yet statistically significant 6.4% relative risk reduction in the primary composite endpoint, compared with 40 mg of simvastatin alone, reported Dr. Christopher P. Cannon, professor of medicine at Harvard Medical School, Boston.
Most observers hailed this result as clinically important, since IMPROVE-IT was the first study to show that lowering cholesterol with a nonstatin reduces cardiovascular events, and it showed that the lower a patient’s LDL cholesterol, the better the outcomes: The mean LDL with combination therapy was 54 mg/dL, compared with 70 mg/dL in controls.
The double-blind study included 18,144 patients randomized within 10 days of an acute coronary syndrome. The primary composite outcome comprised cardiovascular death, nonfatal MI, nonfatal stroke, rehospitalization for unstable angina, and coronary revascularization. The 6.4% relative risk reduction was based solely on first events, of which there were 5,314 during a median 6 years of follow-up. But there were also 4,231 subsequent primary endpoint events, including 2,297 second events, 972 third events, and 456 fourth ones.
While 170 fewer first events occurred with combination therapy than with simvastatin alone, there were also 251 fewer additional or recurrent cardiovascular events in the ezetimibe/simvastatin group which were not incorporated in the initial analysis.
Here’s the key point: While the relative risk reduction for first primary endpoint events was 6.4% with combination therapy, the risk of additional events was reduced by 12%, resulting in a more substantial 9% relative risk reduction for total events.
For the prespecified secondary composite endpoint consisting of death due to coronary heart disease, nonfatal MI, or urgent coronary revascularization, the initial analysis based solely upon first events showed an 8.8% relative risk reduction with ezetimibe/simvastatin. But the fuller view includes a 21% reduction in the risk of additional events, resulting in a 15% relative risk reduction for total events during a median 6 years of follow-up.
In an exploratory analysis examining the harder endpoints of cardiovascular death, nonfatal MI, or cerebrovascular accident, the relative risk reduction for total events was 12% with combination therapy. Ezetimibe/simvastatin was associated with a 13% reduction in all nonfatal MIs occurring during follow-up and a 23% reduction in nonfatal strokes, according to Ms. Murphy. Among every 1,000 IMPROVE-IT participants on ezetimibe/simvastatin rather than simvastatin alone for 1 year there were 11 fewer total primary outcome events, including 5 fewer nonfatal MIs, 2 less nonfatal strokes, and 4 fewer revascularization procedures.
She reported serving as a consultant to Merck and receiving research grants from AstraZeneca, Daiichi Sanko, GlaxoSmithKline, and Merck.
SAN DIEGO– The sensational initial presentation of the landmark IMPROVE-IT trial at last November’s AHA Scientific Sessions told only half the story of the actual clinical impact of lipid-lowering with the combination of ezetimibe and simvastatin as opposed to simvastatin alone.
That’s because the main analysis included only the first cardiovascular event patients experienced. Many participants with a nonfatal first event went on to have a second, third, or even a fourth event. And while additional events haven’t traditionally been counted in acute coronary syndrome clinical trials, they should be, Sabina A. Murphy said at the annual meeting of the American College of Cardiology.
“All events, not just first events, are important to patients and clinicians. Total events have implications for patient morbidity, clinical management, need for recurrent hospitalization, and total costs,” said Ms. Murphy, head of biostatistics at the TIMI Study Group at Brigham and Women’s Hospital, Boston.
Analyses that include recurrent events are common in some other medical fields, including oncology and rheumatology, she added.
In last November’s initial findings from IMPROVE-IT (Improved Reduction of Outcomes: Vytorin Efficacy International Trial), the combination of 10 mg ezetimibe/40 mg simvastatin (Vytorin) daily resulted in a modest yet statistically significant 6.4% relative risk reduction in the primary composite endpoint, compared with 40 mg of simvastatin alone, reported Dr. Christopher P. Cannon, professor of medicine at Harvard Medical School, Boston.
Most observers hailed this result as clinically important, since IMPROVE-IT was the first study to show that lowering cholesterol with a nonstatin reduces cardiovascular events, and it showed that the lower a patient’s LDL cholesterol, the better the outcomes: The mean LDL with combination therapy was 54 mg/dL, compared with 70 mg/dL in controls.
The double-blind study included 18,144 patients randomized within 10 days of an acute coronary syndrome. The primary composite outcome comprised cardiovascular death, nonfatal MI, nonfatal stroke, rehospitalization for unstable angina, and coronary revascularization. The 6.4% relative risk reduction was based solely on first events, of which there were 5,314 during a median 6 years of follow-up. But there were also 4,231 subsequent primary endpoint events, including 2,297 second events, 972 third events, and 456 fourth ones.
While 170 fewer first events occurred with combination therapy than with simvastatin alone, there were also 251 fewer additional or recurrent cardiovascular events in the ezetimibe/simvastatin group which were not incorporated in the initial analysis.
Here’s the key point: While the relative risk reduction for first primary endpoint events was 6.4% with combination therapy, the risk of additional events was reduced by 12%, resulting in a more substantial 9% relative risk reduction for total events.
For the prespecified secondary composite endpoint consisting of death due to coronary heart disease, nonfatal MI, or urgent coronary revascularization, the initial analysis based solely upon first events showed an 8.8% relative risk reduction with ezetimibe/simvastatin. But the fuller view includes a 21% reduction in the risk of additional events, resulting in a 15% relative risk reduction for total events during a median 6 years of follow-up.
In an exploratory analysis examining the harder endpoints of cardiovascular death, nonfatal MI, or cerebrovascular accident, the relative risk reduction for total events was 12% with combination therapy. Ezetimibe/simvastatin was associated with a 13% reduction in all nonfatal MIs occurring during follow-up and a 23% reduction in nonfatal strokes, according to Ms. Murphy. Among every 1,000 IMPROVE-IT participants on ezetimibe/simvastatin rather than simvastatin alone for 1 year there were 11 fewer total primary outcome events, including 5 fewer nonfatal MIs, 2 less nonfatal strokes, and 4 fewer revascularization procedures.
She reported serving as a consultant to Merck and receiving research grants from AstraZeneca, Daiichi Sanko, GlaxoSmithKline, and Merck.
SAN DIEGO– The sensational initial presentation of the landmark IMPROVE-IT trial at last November’s AHA Scientific Sessions told only half the story of the actual clinical impact of lipid-lowering with the combination of ezetimibe and simvastatin as opposed to simvastatin alone.
That’s because the main analysis included only the first cardiovascular event patients experienced. Many participants with a nonfatal first event went on to have a second, third, or even a fourth event. And while additional events haven’t traditionally been counted in acute coronary syndrome clinical trials, they should be, Sabina A. Murphy said at the annual meeting of the American College of Cardiology.
“All events, not just first events, are important to patients and clinicians. Total events have implications for patient morbidity, clinical management, need for recurrent hospitalization, and total costs,” said Ms. Murphy, head of biostatistics at the TIMI Study Group at Brigham and Women’s Hospital, Boston.
Analyses that include recurrent events are common in some other medical fields, including oncology and rheumatology, she added.
In last November’s initial findings from IMPROVE-IT (Improved Reduction of Outcomes: Vytorin Efficacy International Trial), the combination of 10 mg ezetimibe/40 mg simvastatin (Vytorin) daily resulted in a modest yet statistically significant 6.4% relative risk reduction in the primary composite endpoint, compared with 40 mg of simvastatin alone, reported Dr. Christopher P. Cannon, professor of medicine at Harvard Medical School, Boston.
Most observers hailed this result as clinically important, since IMPROVE-IT was the first study to show that lowering cholesterol with a nonstatin reduces cardiovascular events, and it showed that the lower a patient’s LDL cholesterol, the better the outcomes: The mean LDL with combination therapy was 54 mg/dL, compared with 70 mg/dL in controls.
The double-blind study included 18,144 patients randomized within 10 days of an acute coronary syndrome. The primary composite outcome comprised cardiovascular death, nonfatal MI, nonfatal stroke, rehospitalization for unstable angina, and coronary revascularization. The 6.4% relative risk reduction was based solely on first events, of which there were 5,314 during a median 6 years of follow-up. But there were also 4,231 subsequent primary endpoint events, including 2,297 second events, 972 third events, and 456 fourth ones.
While 170 fewer first events occurred with combination therapy than with simvastatin alone, there were also 251 fewer additional or recurrent cardiovascular events in the ezetimibe/simvastatin group which were not incorporated in the initial analysis.
Here’s the key point: While the relative risk reduction for first primary endpoint events was 6.4% with combination therapy, the risk of additional events was reduced by 12%, resulting in a more substantial 9% relative risk reduction for total events.
For the prespecified secondary composite endpoint consisting of death due to coronary heart disease, nonfatal MI, or urgent coronary revascularization, the initial analysis based solely upon first events showed an 8.8% relative risk reduction with ezetimibe/simvastatin. But the fuller view includes a 21% reduction in the risk of additional events, resulting in a 15% relative risk reduction for total events during a median 6 years of follow-up.
In an exploratory analysis examining the harder endpoints of cardiovascular death, nonfatal MI, or cerebrovascular accident, the relative risk reduction for total events was 12% with combination therapy. Ezetimibe/simvastatin was associated with a 13% reduction in all nonfatal MIs occurring during follow-up and a 23% reduction in nonfatal strokes, according to Ms. Murphy. Among every 1,000 IMPROVE-IT participants on ezetimibe/simvastatin rather than simvastatin alone for 1 year there were 11 fewer total primary outcome events, including 5 fewer nonfatal MIs, 2 less nonfatal strokes, and 4 fewer revascularization procedures.
She reported serving as a consultant to Merck and receiving research grants from AstraZeneca, Daiichi Sanko, GlaxoSmithKline, and Merck.
AT ACC 15
Key clinical point: Adding up the total number of cardiovascular events avoided by a medication in IMPROVE-IT rather than just the first one – as has been customary – showed further benefit of combination therapy.
Major finding: While the combination of ezetimibe and simvastatin reduced the risk of a first cardiovascular event by 6.4% compared to simvastatin alone, as previously reported, a new analysis shows the combo reduced total events – including the additional ones that followed a patient’s first – by a more robust 9%.
Data source: IMPROVE-IT, a randomized, double-blind, prospective study of more than 18,000 patients enrolled within 10 days of an acute coronary syndrome.
Disclosures: The presenter disclosed ties to Merck, which sponsored the study.
Cardiologists honor a fallen colleague
It’s extremely unusual for a cardiologist or cardiac surgeon to die in the line of duty, but that tragedy occurred last January in Boston when the enraged son of a patient mortally shot Dr. Michael J. Davidson while he was on the job as director of endovascular cardiac surgery at Brigham and Women’s Hospital in Boston.
Dr. Davidson had been an active coinvestigator in the PARTNER study since it began in 2007 to make the first direct comparison of a transcatheter aortic valve replacement (TAVR) system against aortic-valve replacement with conventional heart surgery.
Because of Dr. Davidson’s long and active involvement with the PARTNER trial, his colleagues decided to dedicate the study’s 5-year follow-up findings to him, making the announcement during the first public release of the 5-year results in mid-March at the annual meeting of the American College of Cardiology.
“On behalf of the PARTNER team, we would like to dedicate this study – the 5-year outcomes – to Mike Davidson,” said Dr. Michael J. Mack as he finished his podium presentation of the report.
Preceding Dr. Mack’s talk, the session began with brief remarks about Dr. Davidson from Dr. Martin B. Leon, coleader of the PARTNER trial, and then the airing of a 6-minute video featuring several of Dr. Davidson’s colleagues recalling his unique career and accomplishments.
Notable in their comments was the outline they provided of the unusual training and career path Dr. Davidson forged for himself, based on his remarkably prescient realization a decade or more ago that the future of cardiology and cardiac surgery lay in fusing the two into a hybrid discipline.
Dr. Davidson’s colleagues cited the training he undertook to become both a fully qualified cardiac surgeon and a skilled interventional cardiologist, turning himself into an embodiment of the “heart team.” Several in the video called him “visionary” for recognizing this fusion as an important step toward the future of treating heart disease.
The poignancy of the moment did not stop there.
After the video ended and before Dr. Mack delivered the session’s first talk, ACC president Dr. Patrick T. O’Gara presented a posthumous distinguished-service award from the ACC to Dr. Davidson – with Dr. O’Gara handing the award to the fallen surgeon’s parents, including his father, Dr. Robert M. Davidson, a long-time ACC fellow and former clinical chief of cardiology at Cedars-Sinai Medical Center in Los Angeles.
Following the award, Dr. Athena Poppas, chair of the meeting’s program committee and cochair for the latebreaker session on heart valve replacement, stressed that the entire session was dedicated to honor Dr. Michael Davidson.
Perhaps most moving of all were the small white buttons that Dr. O’Gara, Dr. Davidson’s parents, and others wore on their lapels during the session, featuring a blue heart and the initials MJD. It combined for an affecting tribute to someone who had played a central role in transforming heart-valve replacement and then was murdered for doing this work.
On Twitter @mitchelzoler
It’s extremely unusual for a cardiologist or cardiac surgeon to die in the line of duty, but that tragedy occurred last January in Boston when the enraged son of a patient mortally shot Dr. Michael J. Davidson while he was on the job as director of endovascular cardiac surgery at Brigham and Women’s Hospital in Boston.
Dr. Davidson had been an active coinvestigator in the PARTNER study since it began in 2007 to make the first direct comparison of a transcatheter aortic valve replacement (TAVR) system against aortic-valve replacement with conventional heart surgery.
Because of Dr. Davidson’s long and active involvement with the PARTNER trial, his colleagues decided to dedicate the study’s 5-year follow-up findings to him, making the announcement during the first public release of the 5-year results in mid-March at the annual meeting of the American College of Cardiology.
“On behalf of the PARTNER team, we would like to dedicate this study – the 5-year outcomes – to Mike Davidson,” said Dr. Michael J. Mack as he finished his podium presentation of the report.
Preceding Dr. Mack’s talk, the session began with brief remarks about Dr. Davidson from Dr. Martin B. Leon, coleader of the PARTNER trial, and then the airing of a 6-minute video featuring several of Dr. Davidson’s colleagues recalling his unique career and accomplishments.
Notable in their comments was the outline they provided of the unusual training and career path Dr. Davidson forged for himself, based on his remarkably prescient realization a decade or more ago that the future of cardiology and cardiac surgery lay in fusing the two into a hybrid discipline.
Dr. Davidson’s colleagues cited the training he undertook to become both a fully qualified cardiac surgeon and a skilled interventional cardiologist, turning himself into an embodiment of the “heart team.” Several in the video called him “visionary” for recognizing this fusion as an important step toward the future of treating heart disease.
The poignancy of the moment did not stop there.
After the video ended and before Dr. Mack delivered the session’s first talk, ACC president Dr. Patrick T. O’Gara presented a posthumous distinguished-service award from the ACC to Dr. Davidson – with Dr. O’Gara handing the award to the fallen surgeon’s parents, including his father, Dr. Robert M. Davidson, a long-time ACC fellow and former clinical chief of cardiology at Cedars-Sinai Medical Center in Los Angeles.
Following the award, Dr. Athena Poppas, chair of the meeting’s program committee and cochair for the latebreaker session on heart valve replacement, stressed that the entire session was dedicated to honor Dr. Michael Davidson.
Perhaps most moving of all were the small white buttons that Dr. O’Gara, Dr. Davidson’s parents, and others wore on their lapels during the session, featuring a blue heart and the initials MJD. It combined for an affecting tribute to someone who had played a central role in transforming heart-valve replacement and then was murdered for doing this work.
On Twitter @mitchelzoler
It’s extremely unusual for a cardiologist or cardiac surgeon to die in the line of duty, but that tragedy occurred last January in Boston when the enraged son of a patient mortally shot Dr. Michael J. Davidson while he was on the job as director of endovascular cardiac surgery at Brigham and Women’s Hospital in Boston.
Dr. Davidson had been an active coinvestigator in the PARTNER study since it began in 2007 to make the first direct comparison of a transcatheter aortic valve replacement (TAVR) system against aortic-valve replacement with conventional heart surgery.
Because of Dr. Davidson’s long and active involvement with the PARTNER trial, his colleagues decided to dedicate the study’s 5-year follow-up findings to him, making the announcement during the first public release of the 5-year results in mid-March at the annual meeting of the American College of Cardiology.
“On behalf of the PARTNER team, we would like to dedicate this study – the 5-year outcomes – to Mike Davidson,” said Dr. Michael J. Mack as he finished his podium presentation of the report.
Preceding Dr. Mack’s talk, the session began with brief remarks about Dr. Davidson from Dr. Martin B. Leon, coleader of the PARTNER trial, and then the airing of a 6-minute video featuring several of Dr. Davidson’s colleagues recalling his unique career and accomplishments.
Notable in their comments was the outline they provided of the unusual training and career path Dr. Davidson forged for himself, based on his remarkably prescient realization a decade or more ago that the future of cardiology and cardiac surgery lay in fusing the two into a hybrid discipline.
Dr. Davidson’s colleagues cited the training he undertook to become both a fully qualified cardiac surgeon and a skilled interventional cardiologist, turning himself into an embodiment of the “heart team.” Several in the video called him “visionary” for recognizing this fusion as an important step toward the future of treating heart disease.
The poignancy of the moment did not stop there.
After the video ended and before Dr. Mack delivered the session’s first talk, ACC president Dr. Patrick T. O’Gara presented a posthumous distinguished-service award from the ACC to Dr. Davidson – with Dr. O’Gara handing the award to the fallen surgeon’s parents, including his father, Dr. Robert M. Davidson, a long-time ACC fellow and former clinical chief of cardiology at Cedars-Sinai Medical Center in Los Angeles.
Following the award, Dr. Athena Poppas, chair of the meeting’s program committee and cochair for the latebreaker session on heart valve replacement, stressed that the entire session was dedicated to honor Dr. Michael Davidson.
Perhaps most moving of all were the small white buttons that Dr. O’Gara, Dr. Davidson’s parents, and others wore on their lapels during the session, featuring a blue heart and the initials MJD. It combined for an affecting tribute to someone who had played a central role in transforming heart-valve replacement and then was murdered for doing this work.
On Twitter @mitchelzoler
Mindfulness training improved angina in women with nonobstructive CAD
SAN DIEGO– Mindfulness-based stress reduction was an effective adjunct to medical therapy in women with angina and no obstructive coronary artery disease, a small pilot study indicated.
A standard 8-week Kabat-Zinn mindfulness-based stress reduction training course resulted in significant reductions in angina frequency and severity, as well as in self-reported depression, anxiety, and perceived stress, compared with medical therapy alone in the crossover study, Dr. Vedant Pargaonkar reported at the annual meeting of the American College of Cardiology.
The mindfulness training did not, however, produce any significant effect on vascular function or levels of the inflammatory biomarkers interleukin-6 and C-reactive protein, added Dr. Pargaonkar of Stanford (Calif.) University.
Mindfulness training has been successfully employed in a variety of patient populations to improve quality of life and emotional resilience. Dutch investigators recently reported promising results in patients who underwent percutaneous coronary intervention (J. Behav. Med. 2014;37:135-44).
Dr. Pargaonkar presented a prospective study involving 19 women, mean age 53 years, with angina triggered by emotional stress. All participants had undergone coronary angiography, which uniformly showed no obstructive coronary artery disease. Participants were randomized to receive either the 8-week mindfulness-based stress reduction course plus standard anti-anginal medications or medications alone while being wait-listed for the mindfulness training.
Patients underwent a battery of tests at baseline, again after 8 weeks, and in the control group a third time once they completed the 8-week mindfulness course. The testing included the Seattle Angina Questionnaire, the Beck Depression Inventory-II, the State-Trait Anxiety Scale, the Perceived Stress Scale, Rosenberg’s Self-Esteem Scale, the Kentucky Inventory of Mindfulness Skills, the SF-36, peripheral vascular function assessment via flow-mediated dilation testing, and measurement of systemic inflammatory biomarkers.
Eight weeks of wait-list control didn’t result in any significant changes from baseline on any measures. However, after women completed the structured mindfulness-based stress reduction program, they experienced a significant reduction in angina frequency, from three or more times per week to one to two. Their physical limitations due to angina improved from moderate to slight, or from slight at baseline to none.
They also demonstrated significant reductions in their scores on the validated measures of depression, anxiety, and perceived stress, as well as significant improvement on the Kentucky Mindfulness Scale. Most of the improvements were in the absolute 10%-15% range.
Dr. Pargaonkar observed that the major limitations of his pilot study are the small sample size and the lack of follow-up to assess the durability of the benefits.
The study was conducted free of commercial support. Dr. Pargaonkar reported having no financial conflicts.
SAN DIEGO– Mindfulness-based stress reduction was an effective adjunct to medical therapy in women with angina and no obstructive coronary artery disease, a small pilot study indicated.
A standard 8-week Kabat-Zinn mindfulness-based stress reduction training course resulted in significant reductions in angina frequency and severity, as well as in self-reported depression, anxiety, and perceived stress, compared with medical therapy alone in the crossover study, Dr. Vedant Pargaonkar reported at the annual meeting of the American College of Cardiology.
The mindfulness training did not, however, produce any significant effect on vascular function or levels of the inflammatory biomarkers interleukin-6 and C-reactive protein, added Dr. Pargaonkar of Stanford (Calif.) University.
Mindfulness training has been successfully employed in a variety of patient populations to improve quality of life and emotional resilience. Dutch investigators recently reported promising results in patients who underwent percutaneous coronary intervention (J. Behav. Med. 2014;37:135-44).
Dr. Pargaonkar presented a prospective study involving 19 women, mean age 53 years, with angina triggered by emotional stress. All participants had undergone coronary angiography, which uniformly showed no obstructive coronary artery disease. Participants were randomized to receive either the 8-week mindfulness-based stress reduction course plus standard anti-anginal medications or medications alone while being wait-listed for the mindfulness training.
Patients underwent a battery of tests at baseline, again after 8 weeks, and in the control group a third time once they completed the 8-week mindfulness course. The testing included the Seattle Angina Questionnaire, the Beck Depression Inventory-II, the State-Trait Anxiety Scale, the Perceived Stress Scale, Rosenberg’s Self-Esteem Scale, the Kentucky Inventory of Mindfulness Skills, the SF-36, peripheral vascular function assessment via flow-mediated dilation testing, and measurement of systemic inflammatory biomarkers.
Eight weeks of wait-list control didn’t result in any significant changes from baseline on any measures. However, after women completed the structured mindfulness-based stress reduction program, they experienced a significant reduction in angina frequency, from three or more times per week to one to two. Their physical limitations due to angina improved from moderate to slight, or from slight at baseline to none.
They also demonstrated significant reductions in their scores on the validated measures of depression, anxiety, and perceived stress, as well as significant improvement on the Kentucky Mindfulness Scale. Most of the improvements were in the absolute 10%-15% range.
Dr. Pargaonkar observed that the major limitations of his pilot study are the small sample size and the lack of follow-up to assess the durability of the benefits.
The study was conducted free of commercial support. Dr. Pargaonkar reported having no financial conflicts.
SAN DIEGO– Mindfulness-based stress reduction was an effective adjunct to medical therapy in women with angina and no obstructive coronary artery disease, a small pilot study indicated.
A standard 8-week Kabat-Zinn mindfulness-based stress reduction training course resulted in significant reductions in angina frequency and severity, as well as in self-reported depression, anxiety, and perceived stress, compared with medical therapy alone in the crossover study, Dr. Vedant Pargaonkar reported at the annual meeting of the American College of Cardiology.
The mindfulness training did not, however, produce any significant effect on vascular function or levels of the inflammatory biomarkers interleukin-6 and C-reactive protein, added Dr. Pargaonkar of Stanford (Calif.) University.
Mindfulness training has been successfully employed in a variety of patient populations to improve quality of life and emotional resilience. Dutch investigators recently reported promising results in patients who underwent percutaneous coronary intervention (J. Behav. Med. 2014;37:135-44).
Dr. Pargaonkar presented a prospective study involving 19 women, mean age 53 years, with angina triggered by emotional stress. All participants had undergone coronary angiography, which uniformly showed no obstructive coronary artery disease. Participants were randomized to receive either the 8-week mindfulness-based stress reduction course plus standard anti-anginal medications or medications alone while being wait-listed for the mindfulness training.
Patients underwent a battery of tests at baseline, again after 8 weeks, and in the control group a third time once they completed the 8-week mindfulness course. The testing included the Seattle Angina Questionnaire, the Beck Depression Inventory-II, the State-Trait Anxiety Scale, the Perceived Stress Scale, Rosenberg’s Self-Esteem Scale, the Kentucky Inventory of Mindfulness Skills, the SF-36, peripheral vascular function assessment via flow-mediated dilation testing, and measurement of systemic inflammatory biomarkers.
Eight weeks of wait-list control didn’t result in any significant changes from baseline on any measures. However, after women completed the structured mindfulness-based stress reduction program, they experienced a significant reduction in angina frequency, from three or more times per week to one to two. Their physical limitations due to angina improved from moderate to slight, or from slight at baseline to none.
They also demonstrated significant reductions in their scores on the validated measures of depression, anxiety, and perceived stress, as well as significant improvement on the Kentucky Mindfulness Scale. Most of the improvements were in the absolute 10%-15% range.
Dr. Pargaonkar observed that the major limitations of his pilot study are the small sample size and the lack of follow-up to assess the durability of the benefits.
The study was conducted free of commercial support. Dr. Pargaonkar reported having no financial conflicts.
AT ACC 15
Key clinical point: Women without obstructive coronary artery disease who experience angina in response to emotional stress benefit from mindfulness-based stress reduction training.
Major finding: Angina frequency decreased from three or more attacks per week to one to two per week.
Data source: A randomized, prospective, crossover pilot study involving 19 women.
Disclosures: The study was conducted free of commercial support. Dr. Pargaonkar reported having no financial conflicts.
Anticipation runs high for coming megatrials in general cardiology
SAN DIEGO – Preventive cardiovascular medicine will get “a big boost” from ongoing major randomized controlled trials due to report results during the next several years, Dr. Eric D. Peterson predicted at the annual meeting of the American College of Cardiology.
He presented an overview of eagerly anticipated clinical trials approaching completion in three of the hottest areas of research in general cardiology: new cardiovascular prevention agents and strategies; innovative health policy initiatives; and optimal antithrombotic regimens across the range of chronic coronary artery disease, peripheral vascular disease, patients with atrial fibrillation who’ve undergone percutaneous coronary intervention, and cerebrovascular disease.
Dr. Peterson is executive director of the Duke Clinical Research Institute (DCRI), a major player in some of these studies. To gain additional perspective regarding what’s in store, he consulted a who’s who list of eminent American cardiology clinical trialists as to what’s going to be big in 2016-2017 and shortly beyond.
One thing’s clear looking out at the research horizon: Megatrials enrolling tens of thousands of patients to answer key clinical questions are not a thing of the past.
Far from it.
Cardiovascular prevention
Exciting pivotal phase III cardiovascular prevention trials are well underway in the areas of lipid modification, diabetes, and hypertension. In addition, the possible preventive effect of vitamin D and omega-3 free fatty acid supplementation seen in hypothesis-generating epidemiologic studies is finally being put to a definitive test in a 26,000-subject randomized trial known as VITAL (the Vitamin D and omega-3 trial).
Lipids: All eyes are on ongoing pivotal phase III randomized clinical outcome trials of three monoclonal antibodies that inhibit proprotein convertase subtilisin kexin type 9 (PCSK9): the 22,500-patient FOURIER trial of evolocumab, which includes 9,000 patients older than 65 years; the 18,000-patient ODYSSEY Outcomes trial of alirocumab; and the SPIRE-1 and SPIRE-2 trials of bococizumab totaling 18,300 patients.
In phase II studies, these agents have generated enormous excitement, because they safely achieve unprecedented LDL lowering, with early hints of improved clinical outcomes beyond what’s achievable with today’s drugs, noted Dr. Peterson, professor of medicine at Duke University in Durham, N.C.
CETP inhibitors: The cholesteryl ester transfer protein inhibitors torcetrapib and dalcetrapib flamed out in clinical trials because of safety concerns and lack of clinical benefit. But pivotal phase III trials of two other CETP inhibitors are well underway: anacetrapib, which is the focus of the 30,000-patient REVEAL trial; and evacetrapib, featured in the 11,000-patient ACCELERATE trial. The CETP inhibitors simultaneously boost HDL while reducing LDL.
Diabetes: The search continues for new drugs that not only enhance diabetes control but also improve cardiovascular outcomes, or at the very least are safe in diabetes patients with coronary disease.
Next up is the nearly 15,000-patient Trial Evaluating Cardiovascular Outcomes with Sitagliptin (TECOS), coordinated by the DCRI and University of Oxford. It’s due to be presented in June at the annual meeting of the American Diabetes Association in Boston. Trials of other dipeptidyl peptidase-4 inhibitors have shown evidence of an increased risk of heart failure, specifically with saxagliptin in the SAVOR-TIMI 53 trial and alogliptin in EXAMINE, so a lot is riding on TECOS.
The glucagon-like peptide-1 (GLP-1) agonists are under study in cardiovascular event-driven randomized clinical trials collectively totaling more than 33,000 patients with type 2 diabetes. The Exenatide Study of Cardiovascular Event Lowering (EXSCEL), also coordinated by the DCRI and University of Oxford, includes 14,000 randomized patients. Other major trials of GLP-1 agonists are ELIXA (lixisenatide), LEADER (liraglutide), and REWIND (dulaglutide).
In addition, ongoing phase III trials of sodium glucose cotransporter 2 (SGLT-2) inhibitors with cardiovascular endpoints include the Canagliflozin Cardiovascular Assessment Study (CANVAS) and a 7,000-patient study of empagliflozin known as EMPA-REG OUTCOME.
Hypertension: The Systolic Blood Pressure Intervention Trial (SPRINT) is an NIH-funded, randomized trial designed to finally answer a question bothering physicians for years: What’s the right amount of blood pressure lowering?
Nearly 9,400 high-risk patients with clinical or subclinical cardiovascular disease have been randomized to a target systolic blood pressure of less than 120 mm Hg, compared with less than 140 mm Hg. The primary composite endpoint is cardiovascular death, nonfatal MI or stroke, or hospitalization for acute coronary syndrome. Secondary endpoints focus on cognitive impairment, dementia, and rates of progression of chronic kidney disease. Results are expected in 2018.
Vitamin D: The VITAL trial, also sponsored by the NIH, has randomized 25,875 initially healthy men and women to 2,000 IU of vitamin D3 per day or placebo, and further randomized them to 1 g/day of omega-3 fatty acids or placebo. Key endpoints are total cancers, MI, stroke, and cardiovascular death. Results are coming in 2017.
Optimal antithrombotic therapy
Chronic coronary artery disease: It’s remarkable that physicians still don’t know the right dose of aspirin to use, even though the drug has been around since 1897. The answer is finally on the way. It will come from the ADAPTABLE eTrial, the first comparative effectiveness research project funded by the Patient-Centered Outcomes Research Institute (PCORI).
Twenty thousand U.S. patients with known atherosclerotic cardiovascular disease and at least one extra risk factor will be randomized to daily aspirin at 81 mg or 325 mg and followed for a maximum of 30 months. The primary efficacy endpoint is all-cause mortality, nonfatal MI, or nonfatal stroke. The primary safety endpoint is major bleeding.
This is a groundbreaking innovative study: It’s a 20,000-patient trial budgeted at a mere $10 million – peanuts in the world of megatrials. By comparison, the NIH-funded SPRINT hypertension trial includes less than half as many patients, yet the price tag is $114 million.
The bargain-basement cost of the aspirin trial is possible because follow-up will be via electronic medical records and claims data provided every 3 months by 29 health data networks participating in PCORI’s National Patient-Centered Clinical Research Network (PCORnet). The aspirin study is sort of a shakedown cruise for an exciting new cost-effective approach to conducting comparative effectiveness research.
“This study could be a game changer,” Dr. Peterson declared. “Whether you think the research question is important or not, the ability to utilize electronic health records in long-term patient follow-up in randomized trials will be revolutionary in terms of answering key clinical questions cost effectively.”
Peripheral vascular disease: In search of the antithrombotic regimen that optimizes limb salvage while minimizing vascular event rate, the EUCLID trial has randomized 13,500 patients with symptomatic peripheral artery disease to ticagrelor at 90 mg BID or clopidogrel at 75 mg/day. Follow-up will be for 18 months, with the primary endpoint a composite of cardiovascular death, nonfatal MI, or ischemic stroke.
Atrial fibrillation patients who undergo coronary stent placement: The 2,100-patient PIONEER AF-PCI study, now recruiting, is evaluating different combinations of rivaroxaban in various dosing regimens coupled with clopidogrel, warfarin, and/or aspirin.
Stroke: The SOCRATES trial is recruiting 9,600 patients with acute ischemic stroke or high-risk transient ischemic attack to be randomized within 24 hours to ticagrelor at 90 mg BID or aspirin at 100 mg/day. As with PIONEER, results from SOCRATES are anticipated next year.
Health policy and implementation
The ARTEMIS study poses the question of whether providing a guideline-directed medication gratis will improve patient adherence and/or clinical outcomes.
Some 9,000 high-cardiovascular-risk patients at 300 sites are being randomized to free ticagrelor or clopidogrel – no copay – for 1 year or to usual care. Outcomes include choice of medication, adherence, and major adverse cardiovascular events.
“If this works, there’s the possibility that insurers can be persuaded to pay for medications and basically give them away as a means of ultimately resulting in better outcomes for patients and lower costs,” Dr. Peterson said.
IMPACT AF is an international quality improvement project aimed at improving the use of oral anticoagulation therapy in high-risk patients with atrial fibrillation in Argentina, Brazil, China, India, and Romania. Participating centers will be randomized to receive a provider education and feedback program or not. The primary outcome is a change from baseline through year 1 in the proportion of patients with atrial fibrillation taking an oral anticoagulant.
In summing up the state of research in general cardiology, Dr. Peterson observed, “The way we do trials is changing. The size of the studies is changing. But ultimately, we will find better ways to treat patients, and perhaps we can find better ways to encourage patients to take their medications long term.”
Dr. Peterson reported serving as a consultant to AstraZeneca Boehringer Ingelheim, Genentech, Janssen, and Sanofi, and receiving research funding from Eli Lilly and Janssen.
SAN DIEGO – Preventive cardiovascular medicine will get “a big boost” from ongoing major randomized controlled trials due to report results during the next several years, Dr. Eric D. Peterson predicted at the annual meeting of the American College of Cardiology.
He presented an overview of eagerly anticipated clinical trials approaching completion in three of the hottest areas of research in general cardiology: new cardiovascular prevention agents and strategies; innovative health policy initiatives; and optimal antithrombotic regimens across the range of chronic coronary artery disease, peripheral vascular disease, patients with atrial fibrillation who’ve undergone percutaneous coronary intervention, and cerebrovascular disease.
Dr. Peterson is executive director of the Duke Clinical Research Institute (DCRI), a major player in some of these studies. To gain additional perspective regarding what’s in store, he consulted a who’s who list of eminent American cardiology clinical trialists as to what’s going to be big in 2016-2017 and shortly beyond.
One thing’s clear looking out at the research horizon: Megatrials enrolling tens of thousands of patients to answer key clinical questions are not a thing of the past.
Far from it.
Cardiovascular prevention
Exciting pivotal phase III cardiovascular prevention trials are well underway in the areas of lipid modification, diabetes, and hypertension. In addition, the possible preventive effect of vitamin D and omega-3 free fatty acid supplementation seen in hypothesis-generating epidemiologic studies is finally being put to a definitive test in a 26,000-subject randomized trial known as VITAL (the Vitamin D and omega-3 trial).
Lipids: All eyes are on ongoing pivotal phase III randomized clinical outcome trials of three monoclonal antibodies that inhibit proprotein convertase subtilisin kexin type 9 (PCSK9): the 22,500-patient FOURIER trial of evolocumab, which includes 9,000 patients older than 65 years; the 18,000-patient ODYSSEY Outcomes trial of alirocumab; and the SPIRE-1 and SPIRE-2 trials of bococizumab totaling 18,300 patients.
In phase II studies, these agents have generated enormous excitement, because they safely achieve unprecedented LDL lowering, with early hints of improved clinical outcomes beyond what’s achievable with today’s drugs, noted Dr. Peterson, professor of medicine at Duke University in Durham, N.C.
CETP inhibitors: The cholesteryl ester transfer protein inhibitors torcetrapib and dalcetrapib flamed out in clinical trials because of safety concerns and lack of clinical benefit. But pivotal phase III trials of two other CETP inhibitors are well underway: anacetrapib, which is the focus of the 30,000-patient REVEAL trial; and evacetrapib, featured in the 11,000-patient ACCELERATE trial. The CETP inhibitors simultaneously boost HDL while reducing LDL.
Diabetes: The search continues for new drugs that not only enhance diabetes control but also improve cardiovascular outcomes, or at the very least are safe in diabetes patients with coronary disease.
Next up is the nearly 15,000-patient Trial Evaluating Cardiovascular Outcomes with Sitagliptin (TECOS), coordinated by the DCRI and University of Oxford. It’s due to be presented in June at the annual meeting of the American Diabetes Association in Boston. Trials of other dipeptidyl peptidase-4 inhibitors have shown evidence of an increased risk of heart failure, specifically with saxagliptin in the SAVOR-TIMI 53 trial and alogliptin in EXAMINE, so a lot is riding on TECOS.
The glucagon-like peptide-1 (GLP-1) agonists are under study in cardiovascular event-driven randomized clinical trials collectively totaling more than 33,000 patients with type 2 diabetes. The Exenatide Study of Cardiovascular Event Lowering (EXSCEL), also coordinated by the DCRI and University of Oxford, includes 14,000 randomized patients. Other major trials of GLP-1 agonists are ELIXA (lixisenatide), LEADER (liraglutide), and REWIND (dulaglutide).
In addition, ongoing phase III trials of sodium glucose cotransporter 2 (SGLT-2) inhibitors with cardiovascular endpoints include the Canagliflozin Cardiovascular Assessment Study (CANVAS) and a 7,000-patient study of empagliflozin known as EMPA-REG OUTCOME.
Hypertension: The Systolic Blood Pressure Intervention Trial (SPRINT) is an NIH-funded, randomized trial designed to finally answer a question bothering physicians for years: What’s the right amount of blood pressure lowering?
Nearly 9,400 high-risk patients with clinical or subclinical cardiovascular disease have been randomized to a target systolic blood pressure of less than 120 mm Hg, compared with less than 140 mm Hg. The primary composite endpoint is cardiovascular death, nonfatal MI or stroke, or hospitalization for acute coronary syndrome. Secondary endpoints focus on cognitive impairment, dementia, and rates of progression of chronic kidney disease. Results are expected in 2018.
Vitamin D: The VITAL trial, also sponsored by the NIH, has randomized 25,875 initially healthy men and women to 2,000 IU of vitamin D3 per day or placebo, and further randomized them to 1 g/day of omega-3 fatty acids or placebo. Key endpoints are total cancers, MI, stroke, and cardiovascular death. Results are coming in 2017.
Optimal antithrombotic therapy
Chronic coronary artery disease: It’s remarkable that physicians still don’t know the right dose of aspirin to use, even though the drug has been around since 1897. The answer is finally on the way. It will come from the ADAPTABLE eTrial, the first comparative effectiveness research project funded by the Patient-Centered Outcomes Research Institute (PCORI).
Twenty thousand U.S. patients with known atherosclerotic cardiovascular disease and at least one extra risk factor will be randomized to daily aspirin at 81 mg or 325 mg and followed for a maximum of 30 months. The primary efficacy endpoint is all-cause mortality, nonfatal MI, or nonfatal stroke. The primary safety endpoint is major bleeding.
This is a groundbreaking innovative study: It’s a 20,000-patient trial budgeted at a mere $10 million – peanuts in the world of megatrials. By comparison, the NIH-funded SPRINT hypertension trial includes less than half as many patients, yet the price tag is $114 million.
The bargain-basement cost of the aspirin trial is possible because follow-up will be via electronic medical records and claims data provided every 3 months by 29 health data networks participating in PCORI’s National Patient-Centered Clinical Research Network (PCORnet). The aspirin study is sort of a shakedown cruise for an exciting new cost-effective approach to conducting comparative effectiveness research.
“This study could be a game changer,” Dr. Peterson declared. “Whether you think the research question is important or not, the ability to utilize electronic health records in long-term patient follow-up in randomized trials will be revolutionary in terms of answering key clinical questions cost effectively.”
Peripheral vascular disease: In search of the antithrombotic regimen that optimizes limb salvage while minimizing vascular event rate, the EUCLID trial has randomized 13,500 patients with symptomatic peripheral artery disease to ticagrelor at 90 mg BID or clopidogrel at 75 mg/day. Follow-up will be for 18 months, with the primary endpoint a composite of cardiovascular death, nonfatal MI, or ischemic stroke.
Atrial fibrillation patients who undergo coronary stent placement: The 2,100-patient PIONEER AF-PCI study, now recruiting, is evaluating different combinations of rivaroxaban in various dosing regimens coupled with clopidogrel, warfarin, and/or aspirin.
Stroke: The SOCRATES trial is recruiting 9,600 patients with acute ischemic stroke or high-risk transient ischemic attack to be randomized within 24 hours to ticagrelor at 90 mg BID or aspirin at 100 mg/day. As with PIONEER, results from SOCRATES are anticipated next year.
Health policy and implementation
The ARTEMIS study poses the question of whether providing a guideline-directed medication gratis will improve patient adherence and/or clinical outcomes.
Some 9,000 high-cardiovascular-risk patients at 300 sites are being randomized to free ticagrelor or clopidogrel – no copay – for 1 year or to usual care. Outcomes include choice of medication, adherence, and major adverse cardiovascular events.
“If this works, there’s the possibility that insurers can be persuaded to pay for medications and basically give them away as a means of ultimately resulting in better outcomes for patients and lower costs,” Dr. Peterson said.
IMPACT AF is an international quality improvement project aimed at improving the use of oral anticoagulation therapy in high-risk patients with atrial fibrillation in Argentina, Brazil, China, India, and Romania. Participating centers will be randomized to receive a provider education and feedback program or not. The primary outcome is a change from baseline through year 1 in the proportion of patients with atrial fibrillation taking an oral anticoagulant.
In summing up the state of research in general cardiology, Dr. Peterson observed, “The way we do trials is changing. The size of the studies is changing. But ultimately, we will find better ways to treat patients, and perhaps we can find better ways to encourage patients to take their medications long term.”
Dr. Peterson reported serving as a consultant to AstraZeneca Boehringer Ingelheim, Genentech, Janssen, and Sanofi, and receiving research funding from Eli Lilly and Janssen.
SAN DIEGO – Preventive cardiovascular medicine will get “a big boost” from ongoing major randomized controlled trials due to report results during the next several years, Dr. Eric D. Peterson predicted at the annual meeting of the American College of Cardiology.
He presented an overview of eagerly anticipated clinical trials approaching completion in three of the hottest areas of research in general cardiology: new cardiovascular prevention agents and strategies; innovative health policy initiatives; and optimal antithrombotic regimens across the range of chronic coronary artery disease, peripheral vascular disease, patients with atrial fibrillation who’ve undergone percutaneous coronary intervention, and cerebrovascular disease.
Dr. Peterson is executive director of the Duke Clinical Research Institute (DCRI), a major player in some of these studies. To gain additional perspective regarding what’s in store, he consulted a who’s who list of eminent American cardiology clinical trialists as to what’s going to be big in 2016-2017 and shortly beyond.
One thing’s clear looking out at the research horizon: Megatrials enrolling tens of thousands of patients to answer key clinical questions are not a thing of the past.
Far from it.
Cardiovascular prevention
Exciting pivotal phase III cardiovascular prevention trials are well underway in the areas of lipid modification, diabetes, and hypertension. In addition, the possible preventive effect of vitamin D and omega-3 free fatty acid supplementation seen in hypothesis-generating epidemiologic studies is finally being put to a definitive test in a 26,000-subject randomized trial known as VITAL (the Vitamin D and omega-3 trial).
Lipids: All eyes are on ongoing pivotal phase III randomized clinical outcome trials of three monoclonal antibodies that inhibit proprotein convertase subtilisin kexin type 9 (PCSK9): the 22,500-patient FOURIER trial of evolocumab, which includes 9,000 patients older than 65 years; the 18,000-patient ODYSSEY Outcomes trial of alirocumab; and the SPIRE-1 and SPIRE-2 trials of bococizumab totaling 18,300 patients.
In phase II studies, these agents have generated enormous excitement, because they safely achieve unprecedented LDL lowering, with early hints of improved clinical outcomes beyond what’s achievable with today’s drugs, noted Dr. Peterson, professor of medicine at Duke University in Durham, N.C.
CETP inhibitors: The cholesteryl ester transfer protein inhibitors torcetrapib and dalcetrapib flamed out in clinical trials because of safety concerns and lack of clinical benefit. But pivotal phase III trials of two other CETP inhibitors are well underway: anacetrapib, which is the focus of the 30,000-patient REVEAL trial; and evacetrapib, featured in the 11,000-patient ACCELERATE trial. The CETP inhibitors simultaneously boost HDL while reducing LDL.
Diabetes: The search continues for new drugs that not only enhance diabetes control but also improve cardiovascular outcomes, or at the very least are safe in diabetes patients with coronary disease.
Next up is the nearly 15,000-patient Trial Evaluating Cardiovascular Outcomes with Sitagliptin (TECOS), coordinated by the DCRI and University of Oxford. It’s due to be presented in June at the annual meeting of the American Diabetes Association in Boston. Trials of other dipeptidyl peptidase-4 inhibitors have shown evidence of an increased risk of heart failure, specifically with saxagliptin in the SAVOR-TIMI 53 trial and alogliptin in EXAMINE, so a lot is riding on TECOS.
The glucagon-like peptide-1 (GLP-1) agonists are under study in cardiovascular event-driven randomized clinical trials collectively totaling more than 33,000 patients with type 2 diabetes. The Exenatide Study of Cardiovascular Event Lowering (EXSCEL), also coordinated by the DCRI and University of Oxford, includes 14,000 randomized patients. Other major trials of GLP-1 agonists are ELIXA (lixisenatide), LEADER (liraglutide), and REWIND (dulaglutide).
In addition, ongoing phase III trials of sodium glucose cotransporter 2 (SGLT-2) inhibitors with cardiovascular endpoints include the Canagliflozin Cardiovascular Assessment Study (CANVAS) and a 7,000-patient study of empagliflozin known as EMPA-REG OUTCOME.
Hypertension: The Systolic Blood Pressure Intervention Trial (SPRINT) is an NIH-funded, randomized trial designed to finally answer a question bothering physicians for years: What’s the right amount of blood pressure lowering?
Nearly 9,400 high-risk patients with clinical or subclinical cardiovascular disease have been randomized to a target systolic blood pressure of less than 120 mm Hg, compared with less than 140 mm Hg. The primary composite endpoint is cardiovascular death, nonfatal MI or stroke, or hospitalization for acute coronary syndrome. Secondary endpoints focus on cognitive impairment, dementia, and rates of progression of chronic kidney disease. Results are expected in 2018.
Vitamin D: The VITAL trial, also sponsored by the NIH, has randomized 25,875 initially healthy men and women to 2,000 IU of vitamin D3 per day or placebo, and further randomized them to 1 g/day of omega-3 fatty acids or placebo. Key endpoints are total cancers, MI, stroke, and cardiovascular death. Results are coming in 2017.
Optimal antithrombotic therapy
Chronic coronary artery disease: It’s remarkable that physicians still don’t know the right dose of aspirin to use, even though the drug has been around since 1897. The answer is finally on the way. It will come from the ADAPTABLE eTrial, the first comparative effectiveness research project funded by the Patient-Centered Outcomes Research Institute (PCORI).
Twenty thousand U.S. patients with known atherosclerotic cardiovascular disease and at least one extra risk factor will be randomized to daily aspirin at 81 mg or 325 mg and followed for a maximum of 30 months. The primary efficacy endpoint is all-cause mortality, nonfatal MI, or nonfatal stroke. The primary safety endpoint is major bleeding.
This is a groundbreaking innovative study: It’s a 20,000-patient trial budgeted at a mere $10 million – peanuts in the world of megatrials. By comparison, the NIH-funded SPRINT hypertension trial includes less than half as many patients, yet the price tag is $114 million.
The bargain-basement cost of the aspirin trial is possible because follow-up will be via electronic medical records and claims data provided every 3 months by 29 health data networks participating in PCORI’s National Patient-Centered Clinical Research Network (PCORnet). The aspirin study is sort of a shakedown cruise for an exciting new cost-effective approach to conducting comparative effectiveness research.
“This study could be a game changer,” Dr. Peterson declared. “Whether you think the research question is important or not, the ability to utilize electronic health records in long-term patient follow-up in randomized trials will be revolutionary in terms of answering key clinical questions cost effectively.”
Peripheral vascular disease: In search of the antithrombotic regimen that optimizes limb salvage while minimizing vascular event rate, the EUCLID trial has randomized 13,500 patients with symptomatic peripheral artery disease to ticagrelor at 90 mg BID or clopidogrel at 75 mg/day. Follow-up will be for 18 months, with the primary endpoint a composite of cardiovascular death, nonfatal MI, or ischemic stroke.
Atrial fibrillation patients who undergo coronary stent placement: The 2,100-patient PIONEER AF-PCI study, now recruiting, is evaluating different combinations of rivaroxaban in various dosing regimens coupled with clopidogrel, warfarin, and/or aspirin.
Stroke: The SOCRATES trial is recruiting 9,600 patients with acute ischemic stroke or high-risk transient ischemic attack to be randomized within 24 hours to ticagrelor at 90 mg BID or aspirin at 100 mg/day. As with PIONEER, results from SOCRATES are anticipated next year.
Health policy and implementation
The ARTEMIS study poses the question of whether providing a guideline-directed medication gratis will improve patient adherence and/or clinical outcomes.
Some 9,000 high-cardiovascular-risk patients at 300 sites are being randomized to free ticagrelor or clopidogrel – no copay – for 1 year or to usual care. Outcomes include choice of medication, adherence, and major adverse cardiovascular events.
“If this works, there’s the possibility that insurers can be persuaded to pay for medications and basically give them away as a means of ultimately resulting in better outcomes for patients and lower costs,” Dr. Peterson said.
IMPACT AF is an international quality improvement project aimed at improving the use of oral anticoagulation therapy in high-risk patients with atrial fibrillation in Argentina, Brazil, China, India, and Romania. Participating centers will be randomized to receive a provider education and feedback program or not. The primary outcome is a change from baseline through year 1 in the proportion of patients with atrial fibrillation taking an oral anticoagulant.
In summing up the state of research in general cardiology, Dr. Peterson observed, “The way we do trials is changing. The size of the studies is changing. But ultimately, we will find better ways to treat patients, and perhaps we can find better ways to encourage patients to take their medications long term.”
Dr. Peterson reported serving as a consultant to AstraZeneca Boehringer Ingelheim, Genentech, Janssen, and Sanofi, and receiving research funding from Eli Lilly and Janssen.
EXPERT ANALYSIS FROM ACC 15
Advanced age no barrier to aggressive heart attack treatment
SAN DIEGO – Patients aged 80 years and older benefit from more invasive early treatment after non-ST-elevation myocardial infarction or unstable angina, the After Eighty Trial showed.
After a median follow-up of 1.5 years, an invasive strategy that included coronary angiography significantly reduced the primary endpoint of myocardial infarction (MI), need for urgent revascularization, stroke, and death from 61% with optimal medical treatment to 41% (risk ratio, 0.48; P value < .00001).
That drop was driven primarily by significantly fewer MIs (17% vs. 30%; RR, 0.50; P = .0003) and urgent revascularizations (2% vs. 11%; RR, 0.19; P = .0001), lead author Dr. Nicolai Tegn reported at the American College of Cardiology/Cardiovascular Research Foundation Innovation in Intervention Summit.
There were no significant differences between the invasive and conservative strategy groups in rates of stroke (3% vs. 6%; RR, 0.61; P = .26) or all-cause death (25% vs. 27%; RR, 0.87; P = .53).
The composite of death and MI, however, significantly favored the invasive group (35% vs. 48%; RR, 0.54; P < .0001), he said during a latebreaking clinical trial session.
After Eighty randomly assigned 457 patients, aged 80 years or older, to either optimal medical therapy with no invasive treatments or coronary angiography at a percutaneous coronary intervention (PCI) center the day after inclusion, plus optimal medical therapy after about 4-5 hours if PCI was not performed or about 6-18 hours if it was. Of the 225 patients receiving angiography, 48% went on to balloon angioplasty and/or coronary stenting, and 3% had bypass surgery.
Patients 80 years or older account for roughly one-third of all patients with non-STEMI and unstable angina, but they are underrepresented in clinical trials. As a result, the role of an early invasive strategy, and even an invasive strategy at all, in those elderly patients is still a subject of debate, observed Dr. Tegn, a cardiologist from Rikshospitalet, Oslo University Hospital, Oslo.
The study demonstrated that an invasive strategy is superior to a conservative strategy in patients at least 80 years with NSTEMI or unstable angina, he concluded.
After Eighty is a welcome study because of the under-representation of the elderly in clinical trials, Dr. David Kandzari, director of interventional cardiology at the Piedmont Heart Center in Atlanta, said during a press briefing at the meeting. But it raises the challenge of identifying patients in clinical practice with the same qualifying characteristics, he added, given that the study population represents only 10% of the entire screened population,
There was also a fairly high prevalence of patients who angiographically did not have significant coronary artery disease, yet MI and stroke rates were quite considerable.
That said, “the study in other ways reminds us that the coronary anatomy does not know the age of the patient, meaning that the findings of a benefit of an early invasive strategy seem consistent with previous studies we know across the management of patients with acute coronary syndromes,” Dr. Kandzari said.
After Eighty investigators screened 4,187 elderly patients presenting at 17 community hospitals in Norway with non-STEMI or unstable angina, with or without ST-segment depression in ECG, and normal or elevated troponin T or I levels. Patients had to have no chest pain or other ischemic symptoms after medical treatment and mobilization.
In all, 3,730 patients were excluded for life expectancy less than 12 months because of a serious comorbidity; ongoing or recent bleeding; inability to comply with protocol; clinically unstable including ongoing ischemia; refusal to participate; logistic reasons; or other reasons.
The average age was 84.7 years in the invasive group (range 80-93 years) and 84.9 years in the conservative group (range 80-94 years).
Medical treatment during the index admission included 75 mg aspirin in 97% of both the invasive and conservative groups, clopidogrel (85% vs. 82%), ticagrelor (both 5%), angiotensin-converting enzyme (ACE) inhibitor/angiotensin receptor blockers (ARB) (43% vs. 50%), beta blocker (83% vs. 85%), statins (90% vs. 85%), loop or thiazide diuretics (41% vs. 33%), calcium channel blocker (20% vs. 21%), nitrates (45% vs. 55%), warfarin (17% vs. 9%), low molecular-weight heparin (both 76% ), and dabigatran in one patient in each group.
Medical therapy at discharge in the invasive and conservative groups was aspirin (both 93%), clopidogrel (both 72%), ticagrelor (both 4%), ACE inhibitor/ARB (52% vs. 54%), beta blockers (both 84%), statins (90% vs. 86%), diuretics (45% vs. 38%), calcium channel blocker (24% vs. 23%), nitrates (34% vs. 48%), warfarin (21% vs. 14%), rivaroxaban (three patients in both groups), and dabigatran (one patient vs. six patients).
There were no differences in bleeding rates between the two groups, Dr. Tegn said. Four major bleeding events were reported in both groups, while 23 patients in the invasive group and 16 patients in the conservative group had minor bleeds.
The Norwegian Health Association sponsored the study. Dr. Tegn reported nothing to disclose. Dr. Kandazari reported research and grant support from Abbott Vascular, Biotronic, Boston Scientific, and Medtronic, and minor consulting honoraria from Boston Scientific and Medtronic.
SAN DIEGO – Patients aged 80 years and older benefit from more invasive early treatment after non-ST-elevation myocardial infarction or unstable angina, the After Eighty Trial showed.
After a median follow-up of 1.5 years, an invasive strategy that included coronary angiography significantly reduced the primary endpoint of myocardial infarction (MI), need for urgent revascularization, stroke, and death from 61% with optimal medical treatment to 41% (risk ratio, 0.48; P value < .00001).
That drop was driven primarily by significantly fewer MIs (17% vs. 30%; RR, 0.50; P = .0003) and urgent revascularizations (2% vs. 11%; RR, 0.19; P = .0001), lead author Dr. Nicolai Tegn reported at the American College of Cardiology/Cardiovascular Research Foundation Innovation in Intervention Summit.
There were no significant differences between the invasive and conservative strategy groups in rates of stroke (3% vs. 6%; RR, 0.61; P = .26) or all-cause death (25% vs. 27%; RR, 0.87; P = .53).
The composite of death and MI, however, significantly favored the invasive group (35% vs. 48%; RR, 0.54; P < .0001), he said during a latebreaking clinical trial session.
After Eighty randomly assigned 457 patients, aged 80 years or older, to either optimal medical therapy with no invasive treatments or coronary angiography at a percutaneous coronary intervention (PCI) center the day after inclusion, plus optimal medical therapy after about 4-5 hours if PCI was not performed or about 6-18 hours if it was. Of the 225 patients receiving angiography, 48% went on to balloon angioplasty and/or coronary stenting, and 3% had bypass surgery.
Patients 80 years or older account for roughly one-third of all patients with non-STEMI and unstable angina, but they are underrepresented in clinical trials. As a result, the role of an early invasive strategy, and even an invasive strategy at all, in those elderly patients is still a subject of debate, observed Dr. Tegn, a cardiologist from Rikshospitalet, Oslo University Hospital, Oslo.
The study demonstrated that an invasive strategy is superior to a conservative strategy in patients at least 80 years with NSTEMI or unstable angina, he concluded.
After Eighty is a welcome study because of the under-representation of the elderly in clinical trials, Dr. David Kandzari, director of interventional cardiology at the Piedmont Heart Center in Atlanta, said during a press briefing at the meeting. But it raises the challenge of identifying patients in clinical practice with the same qualifying characteristics, he added, given that the study population represents only 10% of the entire screened population,
There was also a fairly high prevalence of patients who angiographically did not have significant coronary artery disease, yet MI and stroke rates were quite considerable.
That said, “the study in other ways reminds us that the coronary anatomy does not know the age of the patient, meaning that the findings of a benefit of an early invasive strategy seem consistent with previous studies we know across the management of patients with acute coronary syndromes,” Dr. Kandzari said.
After Eighty investigators screened 4,187 elderly patients presenting at 17 community hospitals in Norway with non-STEMI or unstable angina, with or without ST-segment depression in ECG, and normal or elevated troponin T or I levels. Patients had to have no chest pain or other ischemic symptoms after medical treatment and mobilization.
In all, 3,730 patients were excluded for life expectancy less than 12 months because of a serious comorbidity; ongoing or recent bleeding; inability to comply with protocol; clinically unstable including ongoing ischemia; refusal to participate; logistic reasons; or other reasons.
The average age was 84.7 years in the invasive group (range 80-93 years) and 84.9 years in the conservative group (range 80-94 years).
Medical treatment during the index admission included 75 mg aspirin in 97% of both the invasive and conservative groups, clopidogrel (85% vs. 82%), ticagrelor (both 5%), angiotensin-converting enzyme (ACE) inhibitor/angiotensin receptor blockers (ARB) (43% vs. 50%), beta blocker (83% vs. 85%), statins (90% vs. 85%), loop or thiazide diuretics (41% vs. 33%), calcium channel blocker (20% vs. 21%), nitrates (45% vs. 55%), warfarin (17% vs. 9%), low molecular-weight heparin (both 76% ), and dabigatran in one patient in each group.
Medical therapy at discharge in the invasive and conservative groups was aspirin (both 93%), clopidogrel (both 72%), ticagrelor (both 4%), ACE inhibitor/ARB (52% vs. 54%), beta blockers (both 84%), statins (90% vs. 86%), diuretics (45% vs. 38%), calcium channel blocker (24% vs. 23%), nitrates (34% vs. 48%), warfarin (21% vs. 14%), rivaroxaban (three patients in both groups), and dabigatran (one patient vs. six patients).
There were no differences in bleeding rates between the two groups, Dr. Tegn said. Four major bleeding events were reported in both groups, while 23 patients in the invasive group and 16 patients in the conservative group had minor bleeds.
The Norwegian Health Association sponsored the study. Dr. Tegn reported nothing to disclose. Dr. Kandazari reported research and grant support from Abbott Vascular, Biotronic, Boston Scientific, and Medtronic, and minor consulting honoraria from Boston Scientific and Medtronic.
SAN DIEGO – Patients aged 80 years and older benefit from more invasive early treatment after non-ST-elevation myocardial infarction or unstable angina, the After Eighty Trial showed.
After a median follow-up of 1.5 years, an invasive strategy that included coronary angiography significantly reduced the primary endpoint of myocardial infarction (MI), need for urgent revascularization, stroke, and death from 61% with optimal medical treatment to 41% (risk ratio, 0.48; P value < .00001).
That drop was driven primarily by significantly fewer MIs (17% vs. 30%; RR, 0.50; P = .0003) and urgent revascularizations (2% vs. 11%; RR, 0.19; P = .0001), lead author Dr. Nicolai Tegn reported at the American College of Cardiology/Cardiovascular Research Foundation Innovation in Intervention Summit.
There were no significant differences between the invasive and conservative strategy groups in rates of stroke (3% vs. 6%; RR, 0.61; P = .26) or all-cause death (25% vs. 27%; RR, 0.87; P = .53).
The composite of death and MI, however, significantly favored the invasive group (35% vs. 48%; RR, 0.54; P < .0001), he said during a latebreaking clinical trial session.
After Eighty randomly assigned 457 patients, aged 80 years or older, to either optimal medical therapy with no invasive treatments or coronary angiography at a percutaneous coronary intervention (PCI) center the day after inclusion, plus optimal medical therapy after about 4-5 hours if PCI was not performed or about 6-18 hours if it was. Of the 225 patients receiving angiography, 48% went on to balloon angioplasty and/or coronary stenting, and 3% had bypass surgery.
Patients 80 years or older account for roughly one-third of all patients with non-STEMI and unstable angina, but they are underrepresented in clinical trials. As a result, the role of an early invasive strategy, and even an invasive strategy at all, in those elderly patients is still a subject of debate, observed Dr. Tegn, a cardiologist from Rikshospitalet, Oslo University Hospital, Oslo.
The study demonstrated that an invasive strategy is superior to a conservative strategy in patients at least 80 years with NSTEMI or unstable angina, he concluded.
After Eighty is a welcome study because of the under-representation of the elderly in clinical trials, Dr. David Kandzari, director of interventional cardiology at the Piedmont Heart Center in Atlanta, said during a press briefing at the meeting. But it raises the challenge of identifying patients in clinical practice with the same qualifying characteristics, he added, given that the study population represents only 10% of the entire screened population,
There was also a fairly high prevalence of patients who angiographically did not have significant coronary artery disease, yet MI and stroke rates were quite considerable.
That said, “the study in other ways reminds us that the coronary anatomy does not know the age of the patient, meaning that the findings of a benefit of an early invasive strategy seem consistent with previous studies we know across the management of patients with acute coronary syndromes,” Dr. Kandzari said.
After Eighty investigators screened 4,187 elderly patients presenting at 17 community hospitals in Norway with non-STEMI or unstable angina, with or without ST-segment depression in ECG, and normal or elevated troponin T or I levels. Patients had to have no chest pain or other ischemic symptoms after medical treatment and mobilization.
In all, 3,730 patients were excluded for life expectancy less than 12 months because of a serious comorbidity; ongoing or recent bleeding; inability to comply with protocol; clinically unstable including ongoing ischemia; refusal to participate; logistic reasons; or other reasons.
The average age was 84.7 years in the invasive group (range 80-93 years) and 84.9 years in the conservative group (range 80-94 years).
Medical treatment during the index admission included 75 mg aspirin in 97% of both the invasive and conservative groups, clopidogrel (85% vs. 82%), ticagrelor (both 5%), angiotensin-converting enzyme (ACE) inhibitor/angiotensin receptor blockers (ARB) (43% vs. 50%), beta blocker (83% vs. 85%), statins (90% vs. 85%), loop or thiazide diuretics (41% vs. 33%), calcium channel blocker (20% vs. 21%), nitrates (45% vs. 55%), warfarin (17% vs. 9%), low molecular-weight heparin (both 76% ), and dabigatran in one patient in each group.
Medical therapy at discharge in the invasive and conservative groups was aspirin (both 93%), clopidogrel (both 72%), ticagrelor (both 4%), ACE inhibitor/ARB (52% vs. 54%), beta blockers (both 84%), statins (90% vs. 86%), diuretics (45% vs. 38%), calcium channel blocker (24% vs. 23%), nitrates (34% vs. 48%), warfarin (21% vs. 14%), rivaroxaban (three patients in both groups), and dabigatran (one patient vs. six patients).
There were no differences in bleeding rates between the two groups, Dr. Tegn said. Four major bleeding events were reported in both groups, while 23 patients in the invasive group and 16 patients in the conservative group had minor bleeds.
The Norwegian Health Association sponsored the study. Dr. Tegn reported nothing to disclose. Dr. Kandazari reported research and grant support from Abbott Vascular, Biotronic, Boston Scientific, and Medtronic, and minor consulting honoraria from Boston Scientific and Medtronic.
AT ACC/CRF i2 SUMMIT
Key clinical point: An early invasive treatment strategy improved most outcomes in patients aged 80 years and older with acute coronary syndromes.
Major finding: Myocardial infarction, need for urgent revascularization, stroke, and death were significantly lower with invasive vs. conservative care (41% vs. 61%; risk ratio, 0.48; P < .00001).
Data source: Randomized study in 457 patients aged 80 years or older with non-STEMI or unstable angina.
Disclosures: The Norwegian Health Association sponsored the study. Dr. Tegn reported nothing to disclose. Dr. Kandzari reported research and grant support from Abbott Vascular, Biotronic, Boston Scientific, and Medtronic, and minor consulting honoraria from Boston Scientific and Medtronic.
MOOD-HF: SSRI ineffective in heart failure with depression
SAN DIEGO – Patients with major depressive disorder and heart failure with reduced ejection fraction fared no better with a selective serotonin reuptake inhibitor than with placebo in terms of heart failure outcomes or improved psychological well being in MOOD-HF, the first large randomized trial to examine the issue.
“MOOD-HF does not provide a rationale for the use of escitalopram in patients with systolic heart failure and comorbid depression. MOOD-HF suggests, however, that optimal heart failure management resulting in improved signs and symptoms might possibly be a means to ameliorate comorbid depression,” Dr. Christiane E. Angermann said at the annual meeting of the American College of Cardiology.
Moreover, exploratory secondary analysis suggested “a very subtle long-term effect of the antidepressant that may not be so favorable” in patients with more severe heart failure, she added.
“It seems that some of our patients take antidepressants in vain, and we should care more about the heart failure medications,” said Dr. Angermann, chairman of the MOOD-HF trial and director of clinical research at the Comprehensive Heart Failure Center at the University of Wurzburg (Germany).
MOOD-HF was an ambitious clinical trial conducted at 16 German academic medical centers. The study began with the screening – via the Patient Health Questionnaire–9 – of more than 11,000 patients who had heart failure with reduced ejection fraction. A psychiatrist using the Structured Clinical Interview for Depression eventually diagnosed a subgroup as having major depressive disorder. Ultimately, 372 patients with an average left ventricular ejection fraction of 35% were randomized in double-blind fashion to escitalopram (Lexapro) or placebo for a planned 24 months.
The study rationale stemmed from the well-established finding that depression is three to five times more common in heart failure patients than in the general population, and that the severity of depression in these patients shows a dose-response relationship with their risks of all-cause mortality and heart failure hospitalization. The primary study hypothesis in MOOD-HF was that treating the comorbid depression would result in a reduced risk of all-cause mortality and unplanned hospitalization for any cause.
“Never before had an ethics committee been persuaded to allow patients with major depressive disorder to be on a placebo for 2 years,” said Dr. Angermann. “It took a lot of discussion with the psychiatrists, addressing whether these were depressed patients with comorbid heart failure or patients with heart failure and comorbid depression. We ended up agreeing that since these patients mostly die from heart failure, therefore this is the main disease. And since there was no evidence from other cardiovascular studies that antidepressants were of use, we were allowed to do the study,” she explained.
The trial was halted early for futility at the request of the data safety monitoring board after a median of 18 months. At that point, the primary composite endpoint of death or unplanned hospitalization had occurred in 116 patients in the escitalopram group and 119 on placebo.
A key secondary endpoint – improvement in the Montgomery-Asberg Depression Rating Scale (MADRS) at 12 weeks – was also a virtual dead heat. From a baseline score of 21, both the escitalopram and placebo groups showed a clinically meaningful improvement of roughly 9 points. The improvement in MADRS scores in the control group piqued investigators’ interest because no psychotherapy or counseling was involved in the trial.
The target dose of escitalopram was 20 mg/day, with uptitration to that goal taking place over the first 12 weeks. The mean dose of the antidepressant at week 12 was 13.7 mg/day. During the course of the study, however, the target dose dropped to 10 mg/day in patients over age 65 in accordance with updated regulatory guidance regarding possible proarrhythmic QTc prolongation in older patients. As it turned out, QTc prolongation was not an issue in MOOD-HF. Indeed, there was no difference between the two groups in terms of any serious adverse events. Adherence to the study medication was good, Dr. Angermann continued.
While the dose of escitalopram was being uptitrated during the first 12 weeks, so was guideline-directed medical therapy for heart failure in both study arms. The patients improved from being at 56% of the recommended dose of ACE inhibitor/angiotensin receptor blocker therapy at baseline to 64%, and from 58% of the target dose of beta-blocker therapy to 63%. That, rather than the antidepressant, appeared to be responsible for the improvement in heart failure, she said.
Both study arms showed similar improvements over time in New York Heart Association functional class and left ventricular ejection fraction. But levels of N-terminal pro-B-type natriuretic peptide (NT-proBNP) – an important biomarker of heart failure severity – improved only in the placebo-treated controls. The same held true for reduction in left ventricular end diastolic diameter.
“This suggests a somewhat attenuated improvement in heart failure in the escitalopram group,” the cardiologist observed.
The investigators conducted a post hoc analysis to generate a hypothesis-generating risk score for the primary outcome. One point each was given for age greater than 62.6 years, a baseline NT-proBNP level in excess of 807 ng/L, a heart rate greater than 68 bpm, being New York Heart Association class III/IV, and having a left ventricular end diastolic diameter in excess of 59 mm. These were thresholds that identified patients in the top half of the study population for each of these variables.
In patients with a risk score of 0-2, escitalopram was associated with a highly significant 33% reduction in the risk of death or unplanned hospitalization compared with placebo. However, in patients with a score of 3-5, the escitalopram group had a 52% greater risk of the primary endpoint than controls.
“This suggests a heterogeneous pathophysiology and an impact of individual disease profiles on the effects of the study medication,” Dr. Angermann said.
MOOD-HF was funded chiefly by the German Ministry of Education and Research and the University of Wurzburg. Lundbeck provided the escitalopram gratis. Dr. Angermann reported serving as a consultant to ResMed, Novartis, and Vifor and receiving research grants from Lundbeck, Alere, and Boehringer Ingelheim.
SAN DIEGO – Patients with major depressive disorder and heart failure with reduced ejection fraction fared no better with a selective serotonin reuptake inhibitor than with placebo in terms of heart failure outcomes or improved psychological well being in MOOD-HF, the first large randomized trial to examine the issue.
“MOOD-HF does not provide a rationale for the use of escitalopram in patients with systolic heart failure and comorbid depression. MOOD-HF suggests, however, that optimal heart failure management resulting in improved signs and symptoms might possibly be a means to ameliorate comorbid depression,” Dr. Christiane E. Angermann said at the annual meeting of the American College of Cardiology.
Moreover, exploratory secondary analysis suggested “a very subtle long-term effect of the antidepressant that may not be so favorable” in patients with more severe heart failure, she added.
“It seems that some of our patients take antidepressants in vain, and we should care more about the heart failure medications,” said Dr. Angermann, chairman of the MOOD-HF trial and director of clinical research at the Comprehensive Heart Failure Center at the University of Wurzburg (Germany).
MOOD-HF was an ambitious clinical trial conducted at 16 German academic medical centers. The study began with the screening – via the Patient Health Questionnaire–9 – of more than 11,000 patients who had heart failure with reduced ejection fraction. A psychiatrist using the Structured Clinical Interview for Depression eventually diagnosed a subgroup as having major depressive disorder. Ultimately, 372 patients with an average left ventricular ejection fraction of 35% were randomized in double-blind fashion to escitalopram (Lexapro) or placebo for a planned 24 months.
The study rationale stemmed from the well-established finding that depression is three to five times more common in heart failure patients than in the general population, and that the severity of depression in these patients shows a dose-response relationship with their risks of all-cause mortality and heart failure hospitalization. The primary study hypothesis in MOOD-HF was that treating the comorbid depression would result in a reduced risk of all-cause mortality and unplanned hospitalization for any cause.
“Never before had an ethics committee been persuaded to allow patients with major depressive disorder to be on a placebo for 2 years,” said Dr. Angermann. “It took a lot of discussion with the psychiatrists, addressing whether these were depressed patients with comorbid heart failure or patients with heart failure and comorbid depression. We ended up agreeing that since these patients mostly die from heart failure, therefore this is the main disease. And since there was no evidence from other cardiovascular studies that antidepressants were of use, we were allowed to do the study,” she explained.
The trial was halted early for futility at the request of the data safety monitoring board after a median of 18 months. At that point, the primary composite endpoint of death or unplanned hospitalization had occurred in 116 patients in the escitalopram group and 119 on placebo.
A key secondary endpoint – improvement in the Montgomery-Asberg Depression Rating Scale (MADRS) at 12 weeks – was also a virtual dead heat. From a baseline score of 21, both the escitalopram and placebo groups showed a clinically meaningful improvement of roughly 9 points. The improvement in MADRS scores in the control group piqued investigators’ interest because no psychotherapy or counseling was involved in the trial.
The target dose of escitalopram was 20 mg/day, with uptitration to that goal taking place over the first 12 weeks. The mean dose of the antidepressant at week 12 was 13.7 mg/day. During the course of the study, however, the target dose dropped to 10 mg/day in patients over age 65 in accordance with updated regulatory guidance regarding possible proarrhythmic QTc prolongation in older patients. As it turned out, QTc prolongation was not an issue in MOOD-HF. Indeed, there was no difference between the two groups in terms of any serious adverse events. Adherence to the study medication was good, Dr. Angermann continued.
While the dose of escitalopram was being uptitrated during the first 12 weeks, so was guideline-directed medical therapy for heart failure in both study arms. The patients improved from being at 56% of the recommended dose of ACE inhibitor/angiotensin receptor blocker therapy at baseline to 64%, and from 58% of the target dose of beta-blocker therapy to 63%. That, rather than the antidepressant, appeared to be responsible for the improvement in heart failure, she said.
Both study arms showed similar improvements over time in New York Heart Association functional class and left ventricular ejection fraction. But levels of N-terminal pro-B-type natriuretic peptide (NT-proBNP) – an important biomarker of heart failure severity – improved only in the placebo-treated controls. The same held true for reduction in left ventricular end diastolic diameter.
“This suggests a somewhat attenuated improvement in heart failure in the escitalopram group,” the cardiologist observed.
The investigators conducted a post hoc analysis to generate a hypothesis-generating risk score for the primary outcome. One point each was given for age greater than 62.6 years, a baseline NT-proBNP level in excess of 807 ng/L, a heart rate greater than 68 bpm, being New York Heart Association class III/IV, and having a left ventricular end diastolic diameter in excess of 59 mm. These were thresholds that identified patients in the top half of the study population for each of these variables.
In patients with a risk score of 0-2, escitalopram was associated with a highly significant 33% reduction in the risk of death or unplanned hospitalization compared with placebo. However, in patients with a score of 3-5, the escitalopram group had a 52% greater risk of the primary endpoint than controls.
“This suggests a heterogeneous pathophysiology and an impact of individual disease profiles on the effects of the study medication,” Dr. Angermann said.
MOOD-HF was funded chiefly by the German Ministry of Education and Research and the University of Wurzburg. Lundbeck provided the escitalopram gratis. Dr. Angermann reported serving as a consultant to ResMed, Novartis, and Vifor and receiving research grants from Lundbeck, Alere, and Boehringer Ingelheim.
SAN DIEGO – Patients with major depressive disorder and heart failure with reduced ejection fraction fared no better with a selective serotonin reuptake inhibitor than with placebo in terms of heart failure outcomes or improved psychological well being in MOOD-HF, the first large randomized trial to examine the issue.
“MOOD-HF does not provide a rationale for the use of escitalopram in patients with systolic heart failure and comorbid depression. MOOD-HF suggests, however, that optimal heart failure management resulting in improved signs and symptoms might possibly be a means to ameliorate comorbid depression,” Dr. Christiane E. Angermann said at the annual meeting of the American College of Cardiology.
Moreover, exploratory secondary analysis suggested “a very subtle long-term effect of the antidepressant that may not be so favorable” in patients with more severe heart failure, she added.
“It seems that some of our patients take antidepressants in vain, and we should care more about the heart failure medications,” said Dr. Angermann, chairman of the MOOD-HF trial and director of clinical research at the Comprehensive Heart Failure Center at the University of Wurzburg (Germany).
MOOD-HF was an ambitious clinical trial conducted at 16 German academic medical centers. The study began with the screening – via the Patient Health Questionnaire–9 – of more than 11,000 patients who had heart failure with reduced ejection fraction. A psychiatrist using the Structured Clinical Interview for Depression eventually diagnosed a subgroup as having major depressive disorder. Ultimately, 372 patients with an average left ventricular ejection fraction of 35% were randomized in double-blind fashion to escitalopram (Lexapro) or placebo for a planned 24 months.
The study rationale stemmed from the well-established finding that depression is three to five times more common in heart failure patients than in the general population, and that the severity of depression in these patients shows a dose-response relationship with their risks of all-cause mortality and heart failure hospitalization. The primary study hypothesis in MOOD-HF was that treating the comorbid depression would result in a reduced risk of all-cause mortality and unplanned hospitalization for any cause.
“Never before had an ethics committee been persuaded to allow patients with major depressive disorder to be on a placebo for 2 years,” said Dr. Angermann. “It took a lot of discussion with the psychiatrists, addressing whether these were depressed patients with comorbid heart failure or patients with heart failure and comorbid depression. We ended up agreeing that since these patients mostly die from heart failure, therefore this is the main disease. And since there was no evidence from other cardiovascular studies that antidepressants were of use, we were allowed to do the study,” she explained.
The trial was halted early for futility at the request of the data safety monitoring board after a median of 18 months. At that point, the primary composite endpoint of death or unplanned hospitalization had occurred in 116 patients in the escitalopram group and 119 on placebo.
A key secondary endpoint – improvement in the Montgomery-Asberg Depression Rating Scale (MADRS) at 12 weeks – was also a virtual dead heat. From a baseline score of 21, both the escitalopram and placebo groups showed a clinically meaningful improvement of roughly 9 points. The improvement in MADRS scores in the control group piqued investigators’ interest because no psychotherapy or counseling was involved in the trial.
The target dose of escitalopram was 20 mg/day, with uptitration to that goal taking place over the first 12 weeks. The mean dose of the antidepressant at week 12 was 13.7 mg/day. During the course of the study, however, the target dose dropped to 10 mg/day in patients over age 65 in accordance with updated regulatory guidance regarding possible proarrhythmic QTc prolongation in older patients. As it turned out, QTc prolongation was not an issue in MOOD-HF. Indeed, there was no difference between the two groups in terms of any serious adverse events. Adherence to the study medication was good, Dr. Angermann continued.
While the dose of escitalopram was being uptitrated during the first 12 weeks, so was guideline-directed medical therapy for heart failure in both study arms. The patients improved from being at 56% of the recommended dose of ACE inhibitor/angiotensin receptor blocker therapy at baseline to 64%, and from 58% of the target dose of beta-blocker therapy to 63%. That, rather than the antidepressant, appeared to be responsible for the improvement in heart failure, she said.
Both study arms showed similar improvements over time in New York Heart Association functional class and left ventricular ejection fraction. But levels of N-terminal pro-B-type natriuretic peptide (NT-proBNP) – an important biomarker of heart failure severity – improved only in the placebo-treated controls. The same held true for reduction in left ventricular end diastolic diameter.
“This suggests a somewhat attenuated improvement in heart failure in the escitalopram group,” the cardiologist observed.
The investigators conducted a post hoc analysis to generate a hypothesis-generating risk score for the primary outcome. One point each was given for age greater than 62.6 years, a baseline NT-proBNP level in excess of 807 ng/L, a heart rate greater than 68 bpm, being New York Heart Association class III/IV, and having a left ventricular end diastolic diameter in excess of 59 mm. These were thresholds that identified patients in the top half of the study population for each of these variables.
In patients with a risk score of 0-2, escitalopram was associated with a highly significant 33% reduction in the risk of death or unplanned hospitalization compared with placebo. However, in patients with a score of 3-5, the escitalopram group had a 52% greater risk of the primary endpoint than controls.
“This suggests a heterogeneous pathophysiology and an impact of individual disease profiles on the effects of the study medication,” Dr. Angermann said.
MOOD-HF was funded chiefly by the German Ministry of Education and Research and the University of Wurzburg. Lundbeck provided the escitalopram gratis. Dr. Angermann reported serving as a consultant to ResMed, Novartis, and Vifor and receiving research grants from Lundbeck, Alere, and Boehringer Ingelheim.
AT ACC 15
Key clinical point: Prescribing escitalopram in patients with major depressive disorder and heart failure with reduced ejection fraction did not improve either disease any more than did placebo.
Major finding: The study was stopped for reasons of futility at a median of 18.6 months into a planned 24-month course of treatment.
Data source: MOOD-HF, a randomized, double-blind, placebo-controlled, prospective, 16-center study involving 372 patients with major depressive disorder and heart failure.
Disclosures: The trial was funded chiefly by the German Ministry of Education and Research and the University of Wurzburg. Lundbeck provided the escitalopram gratis. The presenter disclosed ties with Lundbeck, Alere, Boehringer Ingelheim, ResMed, Novartis, and Vifor.
Catheter treatment for PAH outperforms medication
SAN DIEGO – Percutaneous pulmonary artery denervation for the treatment of pulmonary arterial hypertension safely resulted in significantly greater improvement in functional capacity and hemodynamics compared with medication, in a controlled before-and-after study.
A particularly noteworthy secondary finding in the study was that rehospitalizations during the first 6 months after pulmonary artery denervation (PADN) occurred just one-third as frequently as in the 6-month preprocedural period on standard medications, Dr. Shao-Liang Chen said at the annual meeting of the American College of Cardiology.
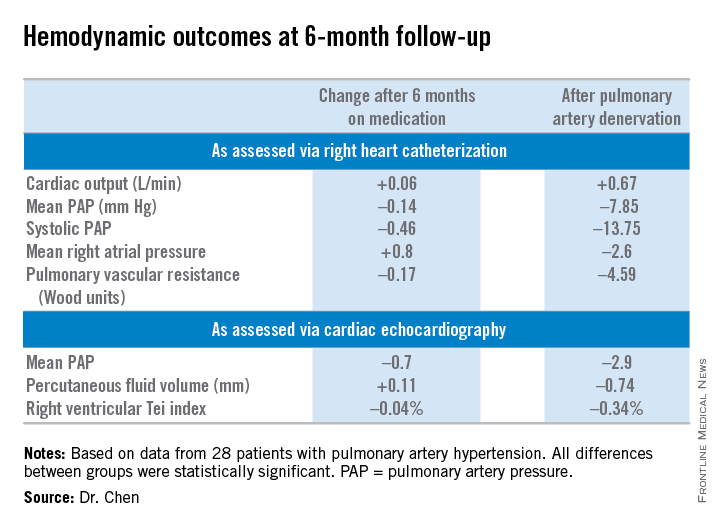
He and his coinvestigators, including Dr. Gregg W. Stone of Columbia University in New York, developed a percutaneous catheter-based method of destroying the pulmonary baroreceptor structure located at the bifurcation area of the middle pulmonary artery. Along the way, they redefined the understanding of the pathogenesis of pulmonary artery hypertension (PAH) by demonstrating that local sympathetic nerve activity plays a pivotal role in modulating the elevations of mean pulmonary artery pressure (mPAP) and pulmonary vascular resistance (PVR), which are the disease hallmarks.
Dr. Chen and coinvestigators previously reported the first-in-man study of PADN, which demonstrated safety and short-term efficacy (J. Am. Coll. Cardiol. 2013;62:1092-100). At ACC 15, Dr. Chen presented the findings of the new PADN-2 study, which expands upon the first study by including more patients and longer and more comprehensive follow-up.
The study comprised 28 patients with PAH, including 11 with idiopathic PAH and 8 with pulmonary hypertension caused by left ventricular disease. All of them underwent medication washout followed by right heart catheterization and echocardiography for baseline off-drug hemodynamic measurements as well as a 6-minute walk distance test of their functional capacity. Then they went back on medications for 6 months, after which they underwent repeat testing. Then their medications were discontinued and they underwent PADN. Six months after the procedure, still off medications, they were retested once again.
The primary study endpoint was change in 6-minute walk distance. After 6 months of medication it improved from 361 to 373 meters, a modest 3.9% gain over off-drug baseline. In contrast, 6-minute walk distance grew from 358 to 423 meters 6 months after PADN, a clinically important 23.9% improvement, reported Dr. Chen, a cardiologist at First Hospital of Nanjing (China) Medical University.
Multiple secondary hemodynamic endpoints also showed significantly greater improvement with PADN than medical therapy.
Twelve predefined clinical events – mostly involving worsening PAH – occurred during medical management, compared with three in the 6 months following PADN.
In addition, there were 12 hospitalizations during the 6 months on medical management compared with only 4 after the same patients underwent PADN. Health care costs averaged $35,000 per patient during the 6-month study period on medication compared with $6,000 per patient in the first 6 months after PADN.
There were no deaths, aneurysms, access site hematomas, or thrombotic events during either study period.
Further randomized, controlled trials are planned to explore the possibility that the benefits seen in the PADN-2 trial will result in reduced mortality in patients with PAH, according to Dr. Chen.
The PADN-2 trial was sponsored by Nanjing Medical University. Dr. Chen reported serving as a consultant to MicroPort.
SAN DIEGO – Percutaneous pulmonary artery denervation for the treatment of pulmonary arterial hypertension safely resulted in significantly greater improvement in functional capacity and hemodynamics compared with medication, in a controlled before-and-after study.
A particularly noteworthy secondary finding in the study was that rehospitalizations during the first 6 months after pulmonary artery denervation (PADN) occurred just one-third as frequently as in the 6-month preprocedural period on standard medications, Dr. Shao-Liang Chen said at the annual meeting of the American College of Cardiology.

He and his coinvestigators, including Dr. Gregg W. Stone of Columbia University in New York, developed a percutaneous catheter-based method of destroying the pulmonary baroreceptor structure located at the bifurcation area of the middle pulmonary artery. Along the way, they redefined the understanding of the pathogenesis of pulmonary artery hypertension (PAH) by demonstrating that local sympathetic nerve activity plays a pivotal role in modulating the elevations of mean pulmonary artery pressure (mPAP) and pulmonary vascular resistance (PVR), which are the disease hallmarks.
Dr. Chen and coinvestigators previously reported the first-in-man study of PADN, which demonstrated safety and short-term efficacy (J. Am. Coll. Cardiol. 2013;62:1092-100). At ACC 15, Dr. Chen presented the findings of the new PADN-2 study, which expands upon the first study by including more patients and longer and more comprehensive follow-up.
The study comprised 28 patients with PAH, including 11 with idiopathic PAH and 8 with pulmonary hypertension caused by left ventricular disease. All of them underwent medication washout followed by right heart catheterization and echocardiography for baseline off-drug hemodynamic measurements as well as a 6-minute walk distance test of their functional capacity. Then they went back on medications for 6 months, after which they underwent repeat testing. Then their medications were discontinued and they underwent PADN. Six months after the procedure, still off medications, they were retested once again.
The primary study endpoint was change in 6-minute walk distance. After 6 months of medication it improved from 361 to 373 meters, a modest 3.9% gain over off-drug baseline. In contrast, 6-minute walk distance grew from 358 to 423 meters 6 months after PADN, a clinically important 23.9% improvement, reported Dr. Chen, a cardiologist at First Hospital of Nanjing (China) Medical University.
Multiple secondary hemodynamic endpoints also showed significantly greater improvement with PADN than medical therapy.
Twelve predefined clinical events – mostly involving worsening PAH – occurred during medical management, compared with three in the 6 months following PADN.
In addition, there were 12 hospitalizations during the 6 months on medical management compared with only 4 after the same patients underwent PADN. Health care costs averaged $35,000 per patient during the 6-month study period on medication compared with $6,000 per patient in the first 6 months after PADN.
There were no deaths, aneurysms, access site hematomas, or thrombotic events during either study period.
Further randomized, controlled trials are planned to explore the possibility that the benefits seen in the PADN-2 trial will result in reduced mortality in patients with PAH, according to Dr. Chen.
The PADN-2 trial was sponsored by Nanjing Medical University. Dr. Chen reported serving as a consultant to MicroPort.
SAN DIEGO – Percutaneous pulmonary artery denervation for the treatment of pulmonary arterial hypertension safely resulted in significantly greater improvement in functional capacity and hemodynamics compared with medication, in a controlled before-and-after study.
A particularly noteworthy secondary finding in the study was that rehospitalizations during the first 6 months after pulmonary artery denervation (PADN) occurred just one-third as frequently as in the 6-month preprocedural period on standard medications, Dr. Shao-Liang Chen said at the annual meeting of the American College of Cardiology.

He and his coinvestigators, including Dr. Gregg W. Stone of Columbia University in New York, developed a percutaneous catheter-based method of destroying the pulmonary baroreceptor structure located at the bifurcation area of the middle pulmonary artery. Along the way, they redefined the understanding of the pathogenesis of pulmonary artery hypertension (PAH) by demonstrating that local sympathetic nerve activity plays a pivotal role in modulating the elevations of mean pulmonary artery pressure (mPAP) and pulmonary vascular resistance (PVR), which are the disease hallmarks.
Dr. Chen and coinvestigators previously reported the first-in-man study of PADN, which demonstrated safety and short-term efficacy (J. Am. Coll. Cardiol. 2013;62:1092-100). At ACC 15, Dr. Chen presented the findings of the new PADN-2 study, which expands upon the first study by including more patients and longer and more comprehensive follow-up.
The study comprised 28 patients with PAH, including 11 with idiopathic PAH and 8 with pulmonary hypertension caused by left ventricular disease. All of them underwent medication washout followed by right heart catheterization and echocardiography for baseline off-drug hemodynamic measurements as well as a 6-minute walk distance test of their functional capacity. Then they went back on medications for 6 months, after which they underwent repeat testing. Then their medications were discontinued and they underwent PADN. Six months after the procedure, still off medications, they were retested once again.
The primary study endpoint was change in 6-minute walk distance. After 6 months of medication it improved from 361 to 373 meters, a modest 3.9% gain over off-drug baseline. In contrast, 6-minute walk distance grew from 358 to 423 meters 6 months after PADN, a clinically important 23.9% improvement, reported Dr. Chen, a cardiologist at First Hospital of Nanjing (China) Medical University.
Multiple secondary hemodynamic endpoints also showed significantly greater improvement with PADN than medical therapy.
Twelve predefined clinical events – mostly involving worsening PAH – occurred during medical management, compared with three in the 6 months following PADN.
In addition, there were 12 hospitalizations during the 6 months on medical management compared with only 4 after the same patients underwent PADN. Health care costs averaged $35,000 per patient during the 6-month study period on medication compared with $6,000 per patient in the first 6 months after PADN.
There were no deaths, aneurysms, access site hematomas, or thrombotic events during either study period.
Further randomized, controlled trials are planned to explore the possibility that the benefits seen in the PADN-2 trial will result in reduced mortality in patients with PAH, according to Dr. Chen.
The PADN-2 trial was sponsored by Nanjing Medical University. Dr. Chen reported serving as a consultant to MicroPort.
AT ACC 15
Key clinical point: Pulmonary artery denervation for PAH showed promise in a small study.
Major finding: Six-minute walk distance improved by 3.9% after 6 months of medication, compared with 23.9% in testing done 6 months after percutaneous pulmonary artery denervation.
Data source: The PADN-2 trial, a prospective before-and-after study of 28 patients with pulmonary arterial hypertension.
Disclosures: Nanjing Medical University funded the study. The presenter reported serving as a consultant to MicroPort.
Cardiovascular safety evidence for alogliptin reassuring
SAN DIEGO – No adverse interaction occurred between the dipeptidyl peptidase–4 inhibitor alogliptin and ACE inhibitors in patients with high cardiovascular risk and type 2 diabetes enrolled in the EXAMINE trial.
This is reassuring news that puts to rest concerns that patients on these two classes of medications might experience an increase in cardiovascular events, Dr. Christopher P. Cannon said at the annual meeting of the American College of Cardiology.
These concerns arose from evidence suggesting the possibility that DPP-4 inhibition in the presence of higher-dose ACE inhibitor therapy might activate the sympathetic nervous system through an increase in substance P, with a resultant elevated risk of serious cardiovascular events.
This hypothesis was tested in a secondary analysis of the EXAMINE (Examination of Cardiovascular Outcomes With Alogliptin Versus Standard of Care) trial. In EXAMINE, 5,380 patients with type 2 diabetes and a history of an acute coronary syndrome within the previous 90 days were randomized in a double-blind fashion to oral alogliptin (Nesina) or placebo on top of standard guideline-directed medical therapy for type 2 diabetes and cardiovascular risk factors. They were prospectively followed for a median of 18 months and a maximum of 40 months.
The primary results of EXAMINE have been reported previously: Among patients with type 2 diabetes and a recent acute coronary syndrome, major adverse cardiovascular events weren’t increased with agoliptin, compared with placebo (N. Engl. J. Med. 2013;369:1327-35). But because of subsequent theoretical safety questions raised about dual therapy with agoliptin and an ACE inhibitor – and in light of the fact that 3,323 participants in EXAMINE were on background ACE inhibitor therapy – the investigators decided to perform this new secondary analysis, according to Dr. Cannon, professor of medicine at Harvard Medical School, Boston.
The composite primary endpoint comprised of cardiovascular death, nonfatal MI, and nonfatal stroke occurred in 11.4% of subjects on alogliptin plus an ACE inhibitor, compared with 11.8% of those on placebo plus an ACE inhibitor, 11.2% of patients on alogliptin without an ACE inhibitor, and 11.9% of those not on alogliptin or an ACE inhibitor.
The secondary composite endpoint of cardiovascular death or hospitalization for heart failure occurred in 6.8% of patients on alogliptin and an ACE inhibitor, 7.2% of those on placebo plus an ACE inhibitor, 8.5% of subjects on alogliptin but no ACE inhibitor, and 8.0% of those on neither – again, with no significant differences.
The EXAMINE trial was funded by Takeda Pharmaceuticals. Dr. Cannon reported serving as a consultant to that company and numerous other pharmaceutical companies.
SAN DIEGO – No adverse interaction occurred between the dipeptidyl peptidase–4 inhibitor alogliptin and ACE inhibitors in patients with high cardiovascular risk and type 2 diabetes enrolled in the EXAMINE trial.
This is reassuring news that puts to rest concerns that patients on these two classes of medications might experience an increase in cardiovascular events, Dr. Christopher P. Cannon said at the annual meeting of the American College of Cardiology.
These concerns arose from evidence suggesting the possibility that DPP-4 inhibition in the presence of higher-dose ACE inhibitor therapy might activate the sympathetic nervous system through an increase in substance P, with a resultant elevated risk of serious cardiovascular events.
This hypothesis was tested in a secondary analysis of the EXAMINE (Examination of Cardiovascular Outcomes With Alogliptin Versus Standard of Care) trial. In EXAMINE, 5,380 patients with type 2 diabetes and a history of an acute coronary syndrome within the previous 90 days were randomized in a double-blind fashion to oral alogliptin (Nesina) or placebo on top of standard guideline-directed medical therapy for type 2 diabetes and cardiovascular risk factors. They were prospectively followed for a median of 18 months and a maximum of 40 months.
The primary results of EXAMINE have been reported previously: Among patients with type 2 diabetes and a recent acute coronary syndrome, major adverse cardiovascular events weren’t increased with agoliptin, compared with placebo (N. Engl. J. Med. 2013;369:1327-35). But because of subsequent theoretical safety questions raised about dual therapy with agoliptin and an ACE inhibitor – and in light of the fact that 3,323 participants in EXAMINE were on background ACE inhibitor therapy – the investigators decided to perform this new secondary analysis, according to Dr. Cannon, professor of medicine at Harvard Medical School, Boston.
The composite primary endpoint comprised of cardiovascular death, nonfatal MI, and nonfatal stroke occurred in 11.4% of subjects on alogliptin plus an ACE inhibitor, compared with 11.8% of those on placebo plus an ACE inhibitor, 11.2% of patients on alogliptin without an ACE inhibitor, and 11.9% of those not on alogliptin or an ACE inhibitor.
The secondary composite endpoint of cardiovascular death or hospitalization for heart failure occurred in 6.8% of patients on alogliptin and an ACE inhibitor, 7.2% of those on placebo plus an ACE inhibitor, 8.5% of subjects on alogliptin but no ACE inhibitor, and 8.0% of those on neither – again, with no significant differences.
The EXAMINE trial was funded by Takeda Pharmaceuticals. Dr. Cannon reported serving as a consultant to that company and numerous other pharmaceutical companies.
SAN DIEGO – No adverse interaction occurred between the dipeptidyl peptidase–4 inhibitor alogliptin and ACE inhibitors in patients with high cardiovascular risk and type 2 diabetes enrolled in the EXAMINE trial.
This is reassuring news that puts to rest concerns that patients on these two classes of medications might experience an increase in cardiovascular events, Dr. Christopher P. Cannon said at the annual meeting of the American College of Cardiology.
These concerns arose from evidence suggesting the possibility that DPP-4 inhibition in the presence of higher-dose ACE inhibitor therapy might activate the sympathetic nervous system through an increase in substance P, with a resultant elevated risk of serious cardiovascular events.
This hypothesis was tested in a secondary analysis of the EXAMINE (Examination of Cardiovascular Outcomes With Alogliptin Versus Standard of Care) trial. In EXAMINE, 5,380 patients with type 2 diabetes and a history of an acute coronary syndrome within the previous 90 days were randomized in a double-blind fashion to oral alogliptin (Nesina) or placebo on top of standard guideline-directed medical therapy for type 2 diabetes and cardiovascular risk factors. They were prospectively followed for a median of 18 months and a maximum of 40 months.
The primary results of EXAMINE have been reported previously: Among patients with type 2 diabetes and a recent acute coronary syndrome, major adverse cardiovascular events weren’t increased with agoliptin, compared with placebo (N. Engl. J. Med. 2013;369:1327-35). But because of subsequent theoretical safety questions raised about dual therapy with agoliptin and an ACE inhibitor – and in light of the fact that 3,323 participants in EXAMINE were on background ACE inhibitor therapy – the investigators decided to perform this new secondary analysis, according to Dr. Cannon, professor of medicine at Harvard Medical School, Boston.
The composite primary endpoint comprised of cardiovascular death, nonfatal MI, and nonfatal stroke occurred in 11.4% of subjects on alogliptin plus an ACE inhibitor, compared with 11.8% of those on placebo plus an ACE inhibitor, 11.2% of patients on alogliptin without an ACE inhibitor, and 11.9% of those not on alogliptin or an ACE inhibitor.
The secondary composite endpoint of cardiovascular death or hospitalization for heart failure occurred in 6.8% of patients on alogliptin and an ACE inhibitor, 7.2% of those on placebo plus an ACE inhibitor, 8.5% of subjects on alogliptin but no ACE inhibitor, and 8.0% of those on neither – again, with no significant differences.
The EXAMINE trial was funded by Takeda Pharmaceuticals. Dr. Cannon reported serving as a consultant to that company and numerous other pharmaceutical companies.
AT ACC 15
Key clinical point: Concomitant use of an ACE inhibitor and alogliptin by patients with type 2 diabetes and high cardiovascular risk isn’t associated with an increase in serious cardiovascular events.
Major finding: Patients on alogliptin and an ACE inhibitor had an 11.4% incidence of cardiovascular death or nonfatal MI or stroke during a median 18 months’ follow-up, while those on an ACE inhibitor plus placebo rather than alogliptin had a statistically similar 11.8% rate.
Data source: A secondary analysis of the 5,380-patient, randomized double-blind EXAMINE trial.
Disclosures: The EXAMINE trial was sponsored by Takeda Pharmaceuticals. The presenter serves as a consultant to Takeda and numerous other pharmaceutical companies.