User login
American Heart Association (AHA): Scientific Sessions 2015
AHA: New emphasis on percent LDL reduction on-treatment
ORLANDO – The individual variability in percent reduction in LDL cholesterol levels in response to high-intensity statin therapy is far greater than generally appreciated, and this has important implications for clinical practice, Dr. Paul M. Ridker said at the American Heart Association scientific sessions.
A new secondary analysis from the landmark JUPITER trial highlighted this substantial variability in percent reduction in LDL cholesterol on 20 mg/day of rosuvastatin (Crestor). Moreover, it showed that the size of this reduction was directly related to the magnitude of reduction in cardiovascular events.
“These data provide general support for the concept of introducing percent reduction in LDL cholesterol into broader clinical practice,” said Dr. Ridker, director of the Center for Cardiovascular Disease Prevention at Brigham and Women’s Hospital and professor of medicine at Harvard Medical School, Boston.
The concept of percent LDL reduction as a treatment target is already widely embedded in the ACC/AHA, European Society of Cardiology, and Canadian Cardiovascular Society cholesterol management guidelines, he noted.
For example, the 2013 ACC/AHA guidelines state that lower-risk individuals who qualify for statin therapy should receive a moderate-intensity statin regimen capable of reducing LDL by 30%-50% from baseline, while higher-risk patients should be placed on a high-intensity statin, described as an agent that gives a 50% or greater reduction in LDL. The new JUPITER analysis makes the case for featuring percent LDL reduction more prominently as an explicit personalized treatment target in the guidelines, Dr. Ridker continued.
In JUPITER, 17,802 apparently healthy subjects with an LDL cholesterol level below 130 mg/dL were randomized to rosuvastatin or placebo. The trial was halted early, after a median of 1.9 years, because the rosuvastatin group showed a compelling 44% reduction in the composite endpoint of MI, stroke, unstable angina treated by revascularization, or cardiovascular death (N Engl J Med. 2008 Nov 20;359[21]:2195-320).
In JUPITER, rosuvastatin reduced LDL cholesterol by an average of 50% in the 7,856 treated patients. But as the new analysis demonstrates, individual variability in response was huge, ranging from no LDL reduction at all to a greater than 85% reduction. And cardiovascular event rates varied accordingly: from 11.2 events per 1,000 person-years with placebo to 9.2 in rosuvastatin-treated patients with no LDL reduction, 6.7 in those with less than a 50% drop in LDL, and 4.8 events per 1,000 person-years in subjects with a greater than 50% reduction in LDL on rosuvastatin. Thus, the one-half of rosuvastatin-treated patients who had more than a 50% decrease in LDL had an adjusted 59% reduction in major cardiovascular events, compared with placebo, while those with a drop of less than 50% in LDL had a 39% risk reduction.
The same exceptionally wide individual variability was seen in on-treatment reductions in apolipoprotein B cholesterol and non–HDL cholesterol levels, and once again, the magnitude of the percent reduction in these lipids tracked with the size of the reduction in cardiovascular events.
This new analysis from JUPITER essentially confirms the findings of an earlier meta-analysis of eight randomized controlled trials with more than 38,000 patients assigned to statin therapy. The meta-analysis showed very large interindividual variations in reductions in LDL, non–HDL cholesterol, and apolipoprotein B in response to high-dose statin therapy. Moreover, patients who achieved very low LDL levels on-treatment had a lower risk of cardiovascular events than those who achieved more moderate LDL reductions (J Am Coll Cardiol. 2014 Aug 5;64[5]:485-94).
Dr. Ridker said the new findings from JUPITER and the meta-analysis, in addition to their implications for clinical practice, could also be relevant in the future with regard to treatment decisions regarding when to prescribe proprotein convertase subtilisin–kexin type 9 (PCSK9) inhibitors, assuming ongoing clinical trials ultimately show that this novel and expensive class of superpotent LDL-lowering agents reduces the risk of cardiovascular events.
He noted that 20% of rosuvastatin-treated JUPITER participants had a greater than 60% reduction in LDL. In theory, he explained, this might be the group where the PCSK9 inhibitors would have the least benefit because those patients already have a 70%-80% reduction in LDL on a high-intensity statin.
On the other hand, the 35% of JUPITER participants with an on-treatment LDL reduction ranging from zero to less than 40% would have the greatest theoretic benefit from a PCSK9 inhibitor, while patients who obtained a 40%-60% LDL reduction on rosuvastatin would be expected to derive an intermediate benefit from the new drugs.
Discussant Michael J. Pencina, Ph.D., said the current U.S. cholesterol management guidelines focus heavily on cardiovascular risk as determined by the risk calculator equation. This needs to be balanced by a more explicit focus on assessment of the anticipated benefit of therapy, he added. For this reason, he agreed with Dr. Ridker’s call to incorporate measurement of percent reduction in lipid levels into individualized assessment of therapeutic benefit.
It will be important for the ongoing randomized trials of PCSK9 inhibitors to report results stratified by the percent reduction in LDL cholesterol achieved by background statin therapy. This will be useful, as Dr. Ridker said, in figuring out how best to allocate this new class of lipid-lowering medications, added Dr. Pencina, professor of biostatistics and bioinformatics at Duke University, Durham, N.C.
Dr. Ridker reported receiving research grants from AstraZeneca, Pfizer, Amgen, and the National Institutes of Health.
ORLANDO – The individual variability in percent reduction in LDL cholesterol levels in response to high-intensity statin therapy is far greater than generally appreciated, and this has important implications for clinical practice, Dr. Paul M. Ridker said at the American Heart Association scientific sessions.
A new secondary analysis from the landmark JUPITER trial highlighted this substantial variability in percent reduction in LDL cholesterol on 20 mg/day of rosuvastatin (Crestor). Moreover, it showed that the size of this reduction was directly related to the magnitude of reduction in cardiovascular events.
“These data provide general support for the concept of introducing percent reduction in LDL cholesterol into broader clinical practice,” said Dr. Ridker, director of the Center for Cardiovascular Disease Prevention at Brigham and Women’s Hospital and professor of medicine at Harvard Medical School, Boston.
The concept of percent LDL reduction as a treatment target is already widely embedded in the ACC/AHA, European Society of Cardiology, and Canadian Cardiovascular Society cholesterol management guidelines, he noted.
For example, the 2013 ACC/AHA guidelines state that lower-risk individuals who qualify for statin therapy should receive a moderate-intensity statin regimen capable of reducing LDL by 30%-50% from baseline, while higher-risk patients should be placed on a high-intensity statin, described as an agent that gives a 50% or greater reduction in LDL. The new JUPITER analysis makes the case for featuring percent LDL reduction more prominently as an explicit personalized treatment target in the guidelines, Dr. Ridker continued.
In JUPITER, 17,802 apparently healthy subjects with an LDL cholesterol level below 130 mg/dL were randomized to rosuvastatin or placebo. The trial was halted early, after a median of 1.9 years, because the rosuvastatin group showed a compelling 44% reduction in the composite endpoint of MI, stroke, unstable angina treated by revascularization, or cardiovascular death (N Engl J Med. 2008 Nov 20;359[21]:2195-320).
In JUPITER, rosuvastatin reduced LDL cholesterol by an average of 50% in the 7,856 treated patients. But as the new analysis demonstrates, individual variability in response was huge, ranging from no LDL reduction at all to a greater than 85% reduction. And cardiovascular event rates varied accordingly: from 11.2 events per 1,000 person-years with placebo to 9.2 in rosuvastatin-treated patients with no LDL reduction, 6.7 in those with less than a 50% drop in LDL, and 4.8 events per 1,000 person-years in subjects with a greater than 50% reduction in LDL on rosuvastatin. Thus, the one-half of rosuvastatin-treated patients who had more than a 50% decrease in LDL had an adjusted 59% reduction in major cardiovascular events, compared with placebo, while those with a drop of less than 50% in LDL had a 39% risk reduction.
The same exceptionally wide individual variability was seen in on-treatment reductions in apolipoprotein B cholesterol and non–HDL cholesterol levels, and once again, the magnitude of the percent reduction in these lipids tracked with the size of the reduction in cardiovascular events.
This new analysis from JUPITER essentially confirms the findings of an earlier meta-analysis of eight randomized controlled trials with more than 38,000 patients assigned to statin therapy. The meta-analysis showed very large interindividual variations in reductions in LDL, non–HDL cholesterol, and apolipoprotein B in response to high-dose statin therapy. Moreover, patients who achieved very low LDL levels on-treatment had a lower risk of cardiovascular events than those who achieved more moderate LDL reductions (J Am Coll Cardiol. 2014 Aug 5;64[5]:485-94).
Dr. Ridker said the new findings from JUPITER and the meta-analysis, in addition to their implications for clinical practice, could also be relevant in the future with regard to treatment decisions regarding when to prescribe proprotein convertase subtilisin–kexin type 9 (PCSK9) inhibitors, assuming ongoing clinical trials ultimately show that this novel and expensive class of superpotent LDL-lowering agents reduces the risk of cardiovascular events.
He noted that 20% of rosuvastatin-treated JUPITER participants had a greater than 60% reduction in LDL. In theory, he explained, this might be the group where the PCSK9 inhibitors would have the least benefit because those patients already have a 70%-80% reduction in LDL on a high-intensity statin.
On the other hand, the 35% of JUPITER participants with an on-treatment LDL reduction ranging from zero to less than 40% would have the greatest theoretic benefit from a PCSK9 inhibitor, while patients who obtained a 40%-60% LDL reduction on rosuvastatin would be expected to derive an intermediate benefit from the new drugs.
Discussant Michael J. Pencina, Ph.D., said the current U.S. cholesterol management guidelines focus heavily on cardiovascular risk as determined by the risk calculator equation. This needs to be balanced by a more explicit focus on assessment of the anticipated benefit of therapy, he added. For this reason, he agreed with Dr. Ridker’s call to incorporate measurement of percent reduction in lipid levels into individualized assessment of therapeutic benefit.
It will be important for the ongoing randomized trials of PCSK9 inhibitors to report results stratified by the percent reduction in LDL cholesterol achieved by background statin therapy. This will be useful, as Dr. Ridker said, in figuring out how best to allocate this new class of lipid-lowering medications, added Dr. Pencina, professor of biostatistics and bioinformatics at Duke University, Durham, N.C.
Dr. Ridker reported receiving research grants from AstraZeneca, Pfizer, Amgen, and the National Institutes of Health.
ORLANDO – The individual variability in percent reduction in LDL cholesterol levels in response to high-intensity statin therapy is far greater than generally appreciated, and this has important implications for clinical practice, Dr. Paul M. Ridker said at the American Heart Association scientific sessions.
A new secondary analysis from the landmark JUPITER trial highlighted this substantial variability in percent reduction in LDL cholesterol on 20 mg/day of rosuvastatin (Crestor). Moreover, it showed that the size of this reduction was directly related to the magnitude of reduction in cardiovascular events.
“These data provide general support for the concept of introducing percent reduction in LDL cholesterol into broader clinical practice,” said Dr. Ridker, director of the Center for Cardiovascular Disease Prevention at Brigham and Women’s Hospital and professor of medicine at Harvard Medical School, Boston.
The concept of percent LDL reduction as a treatment target is already widely embedded in the ACC/AHA, European Society of Cardiology, and Canadian Cardiovascular Society cholesterol management guidelines, he noted.
For example, the 2013 ACC/AHA guidelines state that lower-risk individuals who qualify for statin therapy should receive a moderate-intensity statin regimen capable of reducing LDL by 30%-50% from baseline, while higher-risk patients should be placed on a high-intensity statin, described as an agent that gives a 50% or greater reduction in LDL. The new JUPITER analysis makes the case for featuring percent LDL reduction more prominently as an explicit personalized treatment target in the guidelines, Dr. Ridker continued.
In JUPITER, 17,802 apparently healthy subjects with an LDL cholesterol level below 130 mg/dL were randomized to rosuvastatin or placebo. The trial was halted early, after a median of 1.9 years, because the rosuvastatin group showed a compelling 44% reduction in the composite endpoint of MI, stroke, unstable angina treated by revascularization, or cardiovascular death (N Engl J Med. 2008 Nov 20;359[21]:2195-320).
In JUPITER, rosuvastatin reduced LDL cholesterol by an average of 50% in the 7,856 treated patients. But as the new analysis demonstrates, individual variability in response was huge, ranging from no LDL reduction at all to a greater than 85% reduction. And cardiovascular event rates varied accordingly: from 11.2 events per 1,000 person-years with placebo to 9.2 in rosuvastatin-treated patients with no LDL reduction, 6.7 in those with less than a 50% drop in LDL, and 4.8 events per 1,000 person-years in subjects with a greater than 50% reduction in LDL on rosuvastatin. Thus, the one-half of rosuvastatin-treated patients who had more than a 50% decrease in LDL had an adjusted 59% reduction in major cardiovascular events, compared with placebo, while those with a drop of less than 50% in LDL had a 39% risk reduction.
The same exceptionally wide individual variability was seen in on-treatment reductions in apolipoprotein B cholesterol and non–HDL cholesterol levels, and once again, the magnitude of the percent reduction in these lipids tracked with the size of the reduction in cardiovascular events.
This new analysis from JUPITER essentially confirms the findings of an earlier meta-analysis of eight randomized controlled trials with more than 38,000 patients assigned to statin therapy. The meta-analysis showed very large interindividual variations in reductions in LDL, non–HDL cholesterol, and apolipoprotein B in response to high-dose statin therapy. Moreover, patients who achieved very low LDL levels on-treatment had a lower risk of cardiovascular events than those who achieved more moderate LDL reductions (J Am Coll Cardiol. 2014 Aug 5;64[5]:485-94).
Dr. Ridker said the new findings from JUPITER and the meta-analysis, in addition to their implications for clinical practice, could also be relevant in the future with regard to treatment decisions regarding when to prescribe proprotein convertase subtilisin–kexin type 9 (PCSK9) inhibitors, assuming ongoing clinical trials ultimately show that this novel and expensive class of superpotent LDL-lowering agents reduces the risk of cardiovascular events.
He noted that 20% of rosuvastatin-treated JUPITER participants had a greater than 60% reduction in LDL. In theory, he explained, this might be the group where the PCSK9 inhibitors would have the least benefit because those patients already have a 70%-80% reduction in LDL on a high-intensity statin.
On the other hand, the 35% of JUPITER participants with an on-treatment LDL reduction ranging from zero to less than 40% would have the greatest theoretic benefit from a PCSK9 inhibitor, while patients who obtained a 40%-60% LDL reduction on rosuvastatin would be expected to derive an intermediate benefit from the new drugs.
Discussant Michael J. Pencina, Ph.D., said the current U.S. cholesterol management guidelines focus heavily on cardiovascular risk as determined by the risk calculator equation. This needs to be balanced by a more explicit focus on assessment of the anticipated benefit of therapy, he added. For this reason, he agreed with Dr. Ridker’s call to incorporate measurement of percent reduction in lipid levels into individualized assessment of therapeutic benefit.
It will be important for the ongoing randomized trials of PCSK9 inhibitors to report results stratified by the percent reduction in LDL cholesterol achieved by background statin therapy. This will be useful, as Dr. Ridker said, in figuring out how best to allocate this new class of lipid-lowering medications, added Dr. Pencina, professor of biostatistics and bioinformatics at Duke University, Durham, N.C.
Dr. Ridker reported receiving research grants from AstraZeneca, Pfizer, Amgen, and the National Institutes of Health.
AT THE AHA SCIENTIFIC SESSIONS
Key clinical point: An individual’s percent reduction in LDL cholesterol achieved on statin therapy is a clinically important measurement.
Major finding: The percent LDL cholesterol lowering achieved with high-intensity statin therapy varies widely between individuals and tracks closely with the magnitude of cardiovascular risk reduction.
Data source: A secondary analysis of the JUPITER trial, in which more than 17,000 apparently healthy subjects were randomized to 20 mg/day of rosuvastatin or placebo.
Disclosures: The presenter reported receiving research grants from AstraZeneca, Pfizer, Amgen, and the National Institutes of Health.
AHA: DAPT prevents migraines after atrial-septal defect closure
ORLANDO – Dual antiplatelet therapy with clopidogrel and aspirin was more effective and about as safe as aspirin alone for preventing and mitigating migraine headaches in patients who underwent transcatheter atrial-septal defect closure.
The finding both solidified dual-antiplatelet therapy (DAPT) as default treatment following transcatheter atrial-septal defect (ASD) closure, and advanced thrombotic etiology as a plausible explanation for at least some types of migraine headaches, especially those that follow this type of procedure, Dr. Josep Rodés-Cabau said at the American Heart Association scientific sessions.
Prior results indicated a roughly 15% incidence of new-onset migraines following transcatheter ASD closure, and in the current trial patients in the control arm, who received 80 mg/day of aspirin for 3 months, had a 22% rate of new-onset migraines, compared with a 10% rate among patients randomized to postprocedure treatment with 80 mg aspirin plus 75 mg clopidogrel daily, reported Dr. Rodés-Cabau, a cardiologist at the Quebec Heart and Lung Institute of Laval University in Quebec City.
For the study’ primary endpoint, the average number of days with migraine per month during the first 3 months following transcatheter ASD closure, treatment with aspirin alone produced a 1.4-day rate in 87 patients, compared with a 0.4 day per month average rate in patients who received clopidogrel plus aspirin, a 62% relative risk reduction that was statistically significant, Dr Rodés-Cabau said.
He and his associates ran the CANOA (Clopidogrel for the Prevention of New-Onset Migraine Headache Following Transcatheter Closure of Atrial Septal Defects) trial at six Canadian centers. They enrolled patients who underwent transcatheter ASD repair with the AMPLATZER device and had no history of migraine. The patients’ average age was 49 years, and the average device size was 22 mm. The researchers defined migraines based on 2004 criteria of the International Headache Society (Cephalagia 2004;24 suppl 1:9-160).
Dr. Rodés-Cabau noted that the modest-appearing effect of DAPT on the average number of migraine days per month can be explained by the relatively small percentage of enrolled patients who actually developed migraines. In addition to substantially cutting the number of patients with a migraine, DAPT also produced an important benefit specifically for patients who developed migraines by cutting the number with moderately or severely disabling headaches from eight patients in the aspirin monotherapy arm to zero patients in the DAPT arm.
The adverse event profile in both arms was mild and statistically similar. Five DAPT patients had minor bleeding events, compared with one patient in the aspirin monotherapy arm, a difference that was not statistically significant. No patient in either arm experienced a major bleeding episode during 3 months on study treatment.
Concurrent with Dr. Rodés-Cabau’s report at the meeting the results appeared in an online article (JAMA 2015 Nov 9. doi: 10.1001/jama.2015.13919).
On Twitter @mitchelzoler
Most American centers now routinely treat patients who undergo transcatheter atrial-septal defect closure with dual antiplatelet therapy for 3-6 months, and the results of the CANOA study will only accelerate wider adoption of this approach. The findings of this well-run study support the idea that, in at least a significant percentage of patients with new-onset migraine following closure, the apparent cause is microemboli production, possibly along with serotonin release. Other possible links between closure and migraine could explain other cases. Unfortunately, the study did not try to measure thrombi formation in patients.

|
| Mitchel L. Zoler/Frontline Medical News Dr. J. Dawn Abbott |
The CANOA trial fulfilled its primary endpoint, a reduction in average migraine days per month. The overall rate of new-onset migraine in the control arm, 22%, is a bit higher than we might have expected, but when patients are asked to maintain headache diaries, their awareness of headache would be high. The results are not necessarily generalizable to patients who undergo atrial-septal defect closure using devices other than the AMPLATZER device used in this study.
The study did not address the optimal duration of treatment, although 3 months is reasonable, and it would be useful to more closely examine nonresponders to try to gain better insight into the mechanism of effect from dual-antiplatelet therapy in these patients.
Dual antiplatelet therapy poses an increased bleeding risk, compared with aspirin monotherapy. When applying these results to individual patients, it will be important to take into account each patient’s potential risk and benefit from treatment to decide whether treatment seems warranted.
Dr. J. Dawn Abbott is an interventional cardiologist at Brown University in Providence, R.I. She had no disclosures. She made these comments as designated discussant for the report and in an interview.
Most American centers now routinely treat patients who undergo transcatheter atrial-septal defect closure with dual antiplatelet therapy for 3-6 months, and the results of the CANOA study will only accelerate wider adoption of this approach. The findings of this well-run study support the idea that, in at least a significant percentage of patients with new-onset migraine following closure, the apparent cause is microemboli production, possibly along with serotonin release. Other possible links between closure and migraine could explain other cases. Unfortunately, the study did not try to measure thrombi formation in patients.

|
| Mitchel L. Zoler/Frontline Medical News Dr. J. Dawn Abbott |
The CANOA trial fulfilled its primary endpoint, a reduction in average migraine days per month. The overall rate of new-onset migraine in the control arm, 22%, is a bit higher than we might have expected, but when patients are asked to maintain headache diaries, their awareness of headache would be high. The results are not necessarily generalizable to patients who undergo atrial-septal defect closure using devices other than the AMPLATZER device used in this study.
The study did not address the optimal duration of treatment, although 3 months is reasonable, and it would be useful to more closely examine nonresponders to try to gain better insight into the mechanism of effect from dual-antiplatelet therapy in these patients.
Dual antiplatelet therapy poses an increased bleeding risk, compared with aspirin monotherapy. When applying these results to individual patients, it will be important to take into account each patient’s potential risk and benefit from treatment to decide whether treatment seems warranted.
Dr. J. Dawn Abbott is an interventional cardiologist at Brown University in Providence, R.I. She had no disclosures. She made these comments as designated discussant for the report and in an interview.
Most American centers now routinely treat patients who undergo transcatheter atrial-septal defect closure with dual antiplatelet therapy for 3-6 months, and the results of the CANOA study will only accelerate wider adoption of this approach. The findings of this well-run study support the idea that, in at least a significant percentage of patients with new-onset migraine following closure, the apparent cause is microemboli production, possibly along with serotonin release. Other possible links between closure and migraine could explain other cases. Unfortunately, the study did not try to measure thrombi formation in patients.

|
| Mitchel L. Zoler/Frontline Medical News Dr. J. Dawn Abbott |
The CANOA trial fulfilled its primary endpoint, a reduction in average migraine days per month. The overall rate of new-onset migraine in the control arm, 22%, is a bit higher than we might have expected, but when patients are asked to maintain headache diaries, their awareness of headache would be high. The results are not necessarily generalizable to patients who undergo atrial-septal defect closure using devices other than the AMPLATZER device used in this study.
The study did not address the optimal duration of treatment, although 3 months is reasonable, and it would be useful to more closely examine nonresponders to try to gain better insight into the mechanism of effect from dual-antiplatelet therapy in these patients.
Dual antiplatelet therapy poses an increased bleeding risk, compared with aspirin monotherapy. When applying these results to individual patients, it will be important to take into account each patient’s potential risk and benefit from treatment to decide whether treatment seems warranted.
Dr. J. Dawn Abbott is an interventional cardiologist at Brown University in Providence, R.I. She had no disclosures. She made these comments as designated discussant for the report and in an interview.
ORLANDO – Dual antiplatelet therapy with clopidogrel and aspirin was more effective and about as safe as aspirin alone for preventing and mitigating migraine headaches in patients who underwent transcatheter atrial-septal defect closure.
The finding both solidified dual-antiplatelet therapy (DAPT) as default treatment following transcatheter atrial-septal defect (ASD) closure, and advanced thrombotic etiology as a plausible explanation for at least some types of migraine headaches, especially those that follow this type of procedure, Dr. Josep Rodés-Cabau said at the American Heart Association scientific sessions.
Prior results indicated a roughly 15% incidence of new-onset migraines following transcatheter ASD closure, and in the current trial patients in the control arm, who received 80 mg/day of aspirin for 3 months, had a 22% rate of new-onset migraines, compared with a 10% rate among patients randomized to postprocedure treatment with 80 mg aspirin plus 75 mg clopidogrel daily, reported Dr. Rodés-Cabau, a cardiologist at the Quebec Heart and Lung Institute of Laval University in Quebec City.
For the study’ primary endpoint, the average number of days with migraine per month during the first 3 months following transcatheter ASD closure, treatment with aspirin alone produced a 1.4-day rate in 87 patients, compared with a 0.4 day per month average rate in patients who received clopidogrel plus aspirin, a 62% relative risk reduction that was statistically significant, Dr Rodés-Cabau said.
He and his associates ran the CANOA (Clopidogrel for the Prevention of New-Onset Migraine Headache Following Transcatheter Closure of Atrial Septal Defects) trial at six Canadian centers. They enrolled patients who underwent transcatheter ASD repair with the AMPLATZER device and had no history of migraine. The patients’ average age was 49 years, and the average device size was 22 mm. The researchers defined migraines based on 2004 criteria of the International Headache Society (Cephalagia 2004;24 suppl 1:9-160).
Dr. Rodés-Cabau noted that the modest-appearing effect of DAPT on the average number of migraine days per month can be explained by the relatively small percentage of enrolled patients who actually developed migraines. In addition to substantially cutting the number of patients with a migraine, DAPT also produced an important benefit specifically for patients who developed migraines by cutting the number with moderately or severely disabling headaches from eight patients in the aspirin monotherapy arm to zero patients in the DAPT arm.
The adverse event profile in both arms was mild and statistically similar. Five DAPT patients had minor bleeding events, compared with one patient in the aspirin monotherapy arm, a difference that was not statistically significant. No patient in either arm experienced a major bleeding episode during 3 months on study treatment.
Concurrent with Dr. Rodés-Cabau’s report at the meeting the results appeared in an online article (JAMA 2015 Nov 9. doi: 10.1001/jama.2015.13919).
On Twitter @mitchelzoler
ORLANDO – Dual antiplatelet therapy with clopidogrel and aspirin was more effective and about as safe as aspirin alone for preventing and mitigating migraine headaches in patients who underwent transcatheter atrial-septal defect closure.
The finding both solidified dual-antiplatelet therapy (DAPT) as default treatment following transcatheter atrial-septal defect (ASD) closure, and advanced thrombotic etiology as a plausible explanation for at least some types of migraine headaches, especially those that follow this type of procedure, Dr. Josep Rodés-Cabau said at the American Heart Association scientific sessions.
Prior results indicated a roughly 15% incidence of new-onset migraines following transcatheter ASD closure, and in the current trial patients in the control arm, who received 80 mg/day of aspirin for 3 months, had a 22% rate of new-onset migraines, compared with a 10% rate among patients randomized to postprocedure treatment with 80 mg aspirin plus 75 mg clopidogrel daily, reported Dr. Rodés-Cabau, a cardiologist at the Quebec Heart and Lung Institute of Laval University in Quebec City.
For the study’ primary endpoint, the average number of days with migraine per month during the first 3 months following transcatheter ASD closure, treatment with aspirin alone produced a 1.4-day rate in 87 patients, compared with a 0.4 day per month average rate in patients who received clopidogrel plus aspirin, a 62% relative risk reduction that was statistically significant, Dr Rodés-Cabau said.
He and his associates ran the CANOA (Clopidogrel for the Prevention of New-Onset Migraine Headache Following Transcatheter Closure of Atrial Septal Defects) trial at six Canadian centers. They enrolled patients who underwent transcatheter ASD repair with the AMPLATZER device and had no history of migraine. The patients’ average age was 49 years, and the average device size was 22 mm. The researchers defined migraines based on 2004 criteria of the International Headache Society (Cephalagia 2004;24 suppl 1:9-160).
Dr. Rodés-Cabau noted that the modest-appearing effect of DAPT on the average number of migraine days per month can be explained by the relatively small percentage of enrolled patients who actually developed migraines. In addition to substantially cutting the number of patients with a migraine, DAPT also produced an important benefit specifically for patients who developed migraines by cutting the number with moderately or severely disabling headaches from eight patients in the aspirin monotherapy arm to zero patients in the DAPT arm.
The adverse event profile in both arms was mild and statistically similar. Five DAPT patients had minor bleeding events, compared with one patient in the aspirin monotherapy arm, a difference that was not statistically significant. No patient in either arm experienced a major bleeding episode during 3 months on study treatment.
Concurrent with Dr. Rodés-Cabau’s report at the meeting the results appeared in an online article (JAMA 2015 Nov 9. doi: 10.1001/jama.2015.13919).
On Twitter @mitchelzoler
AT THE AHA SCIENTIFIC SESSIONS
Key clinical point: Three months of dual antiplatelet therapy surpassed aspirin monotherapy for preventing new-onset migraine headaches following transcatheter atrial-septal defect closure.
Major finding: Patients on DAPT had 0.4 migraine days per month, compared with a 1.4-day per month rate with aspirin monotherapy.
Data source: CANOA, a randomized trial with 171 patients run at six Canadian centers.
Disclosures: CANOA was investigator initiated. It received partial funding with unrestricted grants from Sanofi and St. Jude Medical. St. Jude markets the AMPLATZER device. Dr. Josep Rodés-Cabau had no disclosures. Dr. Abbott had no disclosures.
AHA: HFpEF, HFrEF cause similar acute hospitalization rates
ORLANDO – The number of Americans hospitalized for acute decompensated heart failure (ADHF) with preserved ejection fraction during 2003-2012 nearly equaled the number hospitalized with ADHF with reduced ejection fraction, in an analysis of more than 5 million hospitalized heart failure patients tracked in a national-sample database.
But the profile of patients hospitalized with ADHF with preserved ejection fraction (HFpEF) differed from patients hospitalized with acute heart failure and reduced ejection fraction (HFrEF), with a substantially higher percentage of women and patients aged 75 years or older, Dr. Parag Goyal said at the American Heart Association scientific sessions.
The analysis also showed the strongest correlate for in-hospital mortality among HFpEF patients hospitalized with acute decompensation was a pulmonary circulation disorder, such as pulmonary hypertension, which nearly doubled the rate of in-hospital death among HFpEF patients. Other strong correlates of mortality during hospitalization were liver disease, which was linked with about a 50% boost in hospitalized mortality; and chronic renal failure, which was tied to a roughly one-third higher mortality, said Dr. Goyal, a cardiologist at New York–Presbyterian Hospital.
His study used data collected by the Nationwide Inpatient Sample, which included data on more than 388 million hospitalized U.S. patients during 2003-2012, including 5,046,879 hospitalized with acute heart failure. This total included 2,329,391 patients (46%) diagnosed with HFpEF and 2,717,488 patients (54%) diagnosed with HFrEF.
The HFpEF patients’ average age was 76 years, with 60% at least 75 years old, while the HFrEF patients’ average age was 72 years, with 49% age 75 years or older. Nearly two-thirds of the HFpEF patients were women, compared with 42% in the HFrEF group. The HFrEF patients also had a substantially higher prevalence of coronary artery disease, 59%, compared with 41% in the HFpEF group. The prevalence of several comorbidities – including diabetes, hypertension, and chronic renal failure – were each roughly similar in both subgroups, but the obesity rate of 19% in the HFpEF patients substantially exceeded the 12% rate in HFrEF patients.
In-hospital mortality ran 4.3% in the HFpEF patients and 5.1% in the HFrEF patients, a 13% relative-risk reduction that was statistically significant. But average length of stay was similar between the two groups, about 7 days with either type of heart failure.
Dr. Goyal and his associates also examined time trends during 2003-2012. During this period, the percentage of patients with HFpEF aged 75 years or older rose from 57% to 60%. Even more notably, the percentage of men with HFpEF rose from 31% in 2003 to 37% in 2012. Furthermore, the reduced in-hospital mortality during the period was largely driven by mortality reductions among HFpEF patients aged 65 years or older. A multivariate analysis for significant correlates of in-hospital mortality identified age 75 years or older, male sex, and white race in both the HFpEF subgroup and in those with HFrEF. Older age had the highest impact, linked with about a 60% relatively higher mortality rate in patients with either type of heart failure.
The multivariate analysis also identified three comorbidities linked with in-hospital mortality. A pulmonary circulation disorder was associated with a 90% higher mortality rate among HFpEF patients and a 79% higher rate among those with HFrEF. Liver disease and chronic renal disease linked with smaller mortality increases for both heart failure types. The presence of treatable comorbidities, including hypertension, diabetes, and coronary artery disease, linked with significantly lower in-hospital mortality rates. Dr. Goyal speculated that the reduced mortality resulted from successful treatment of these conditions.
On Twitter @mitchelzoler
ORLANDO – The number of Americans hospitalized for acute decompensated heart failure (ADHF) with preserved ejection fraction during 2003-2012 nearly equaled the number hospitalized with ADHF with reduced ejection fraction, in an analysis of more than 5 million hospitalized heart failure patients tracked in a national-sample database.
But the profile of patients hospitalized with ADHF with preserved ejection fraction (HFpEF) differed from patients hospitalized with acute heart failure and reduced ejection fraction (HFrEF), with a substantially higher percentage of women and patients aged 75 years or older, Dr. Parag Goyal said at the American Heart Association scientific sessions.
The analysis also showed the strongest correlate for in-hospital mortality among HFpEF patients hospitalized with acute decompensation was a pulmonary circulation disorder, such as pulmonary hypertension, which nearly doubled the rate of in-hospital death among HFpEF patients. Other strong correlates of mortality during hospitalization were liver disease, which was linked with about a 50% boost in hospitalized mortality; and chronic renal failure, which was tied to a roughly one-third higher mortality, said Dr. Goyal, a cardiologist at New York–Presbyterian Hospital.
His study used data collected by the Nationwide Inpatient Sample, which included data on more than 388 million hospitalized U.S. patients during 2003-2012, including 5,046,879 hospitalized with acute heart failure. This total included 2,329,391 patients (46%) diagnosed with HFpEF and 2,717,488 patients (54%) diagnosed with HFrEF.
The HFpEF patients’ average age was 76 years, with 60% at least 75 years old, while the HFrEF patients’ average age was 72 years, with 49% age 75 years or older. Nearly two-thirds of the HFpEF patients were women, compared with 42% in the HFrEF group. The HFrEF patients also had a substantially higher prevalence of coronary artery disease, 59%, compared with 41% in the HFpEF group. The prevalence of several comorbidities – including diabetes, hypertension, and chronic renal failure – were each roughly similar in both subgroups, but the obesity rate of 19% in the HFpEF patients substantially exceeded the 12% rate in HFrEF patients.
In-hospital mortality ran 4.3% in the HFpEF patients and 5.1% in the HFrEF patients, a 13% relative-risk reduction that was statistically significant. But average length of stay was similar between the two groups, about 7 days with either type of heart failure.
Dr. Goyal and his associates also examined time trends during 2003-2012. During this period, the percentage of patients with HFpEF aged 75 years or older rose from 57% to 60%. Even more notably, the percentage of men with HFpEF rose from 31% in 2003 to 37% in 2012. Furthermore, the reduced in-hospital mortality during the period was largely driven by mortality reductions among HFpEF patients aged 65 years or older. A multivariate analysis for significant correlates of in-hospital mortality identified age 75 years or older, male sex, and white race in both the HFpEF subgroup and in those with HFrEF. Older age had the highest impact, linked with about a 60% relatively higher mortality rate in patients with either type of heart failure.
The multivariate analysis also identified three comorbidities linked with in-hospital mortality. A pulmonary circulation disorder was associated with a 90% higher mortality rate among HFpEF patients and a 79% higher rate among those with HFrEF. Liver disease and chronic renal disease linked with smaller mortality increases for both heart failure types. The presence of treatable comorbidities, including hypertension, diabetes, and coronary artery disease, linked with significantly lower in-hospital mortality rates. Dr. Goyal speculated that the reduced mortality resulted from successful treatment of these conditions.
On Twitter @mitchelzoler
ORLANDO – The number of Americans hospitalized for acute decompensated heart failure (ADHF) with preserved ejection fraction during 2003-2012 nearly equaled the number hospitalized with ADHF with reduced ejection fraction, in an analysis of more than 5 million hospitalized heart failure patients tracked in a national-sample database.
But the profile of patients hospitalized with ADHF with preserved ejection fraction (HFpEF) differed from patients hospitalized with acute heart failure and reduced ejection fraction (HFrEF), with a substantially higher percentage of women and patients aged 75 years or older, Dr. Parag Goyal said at the American Heart Association scientific sessions.
The analysis also showed the strongest correlate for in-hospital mortality among HFpEF patients hospitalized with acute decompensation was a pulmonary circulation disorder, such as pulmonary hypertension, which nearly doubled the rate of in-hospital death among HFpEF patients. Other strong correlates of mortality during hospitalization were liver disease, which was linked with about a 50% boost in hospitalized mortality; and chronic renal failure, which was tied to a roughly one-third higher mortality, said Dr. Goyal, a cardiologist at New York–Presbyterian Hospital.
His study used data collected by the Nationwide Inpatient Sample, which included data on more than 388 million hospitalized U.S. patients during 2003-2012, including 5,046,879 hospitalized with acute heart failure. This total included 2,329,391 patients (46%) diagnosed with HFpEF and 2,717,488 patients (54%) diagnosed with HFrEF.
The HFpEF patients’ average age was 76 years, with 60% at least 75 years old, while the HFrEF patients’ average age was 72 years, with 49% age 75 years or older. Nearly two-thirds of the HFpEF patients were women, compared with 42% in the HFrEF group. The HFrEF patients also had a substantially higher prevalence of coronary artery disease, 59%, compared with 41% in the HFpEF group. The prevalence of several comorbidities – including diabetes, hypertension, and chronic renal failure – were each roughly similar in both subgroups, but the obesity rate of 19% in the HFpEF patients substantially exceeded the 12% rate in HFrEF patients.
In-hospital mortality ran 4.3% in the HFpEF patients and 5.1% in the HFrEF patients, a 13% relative-risk reduction that was statistically significant. But average length of stay was similar between the two groups, about 7 days with either type of heart failure.
Dr. Goyal and his associates also examined time trends during 2003-2012. During this period, the percentage of patients with HFpEF aged 75 years or older rose from 57% to 60%. Even more notably, the percentage of men with HFpEF rose from 31% in 2003 to 37% in 2012. Furthermore, the reduced in-hospital mortality during the period was largely driven by mortality reductions among HFpEF patients aged 65 years or older. A multivariate analysis for significant correlates of in-hospital mortality identified age 75 years or older, male sex, and white race in both the HFpEF subgroup and in those with HFrEF. Older age had the highest impact, linked with about a 60% relatively higher mortality rate in patients with either type of heart failure.
The multivariate analysis also identified three comorbidities linked with in-hospital mortality. A pulmonary circulation disorder was associated with a 90% higher mortality rate among HFpEF patients and a 79% higher rate among those with HFrEF. Liver disease and chronic renal disease linked with smaller mortality increases for both heart failure types. The presence of treatable comorbidities, including hypertension, diabetes, and coronary artery disease, linked with significantly lower in-hospital mortality rates. Dr. Goyal speculated that the reduced mortality resulted from successful treatment of these conditions.
On Twitter @mitchelzoler
AT THE AHA SCIENTIFIC SESSIONS
Key clinical point: Heart failure with preserved ejection fraction closely tracked to heart failure with reduced ejection fraction for causing U.S. heart failure hospitalizations.
Major finding: Among U.S. heart failure patients hospitalized during 2003-2012, 46% had preserved ejection fraction and 54% had reduced ejection fraction.
Data source: Retrospective analysis of 5 million U.S. patients hospitalized for heart failure during 2003-2012 and included in the Nationwide Inpatient Sample.
Disclosures: Dr. Goyal had no disclosures.
AHA: Older Breast Cancer Patients More Likely to Die of Heart Disease Than Malignancy
ORLANDO – Women diagnosed with localized breast cancer while in their 70s have a higher mortality from cardiovascular disease than from their breast cancer, according to new data from the Women’s Health Initiative.
“Identification and treatment of cardiovascular disease risk factors among older women with breast cancer will likely improve survivorship and should be a high priority, especially for older women with incident localized breast cancer,” Na-Jin Park, Ph.D., said at the American Heart Association scientific sessions.
She presented an analysis that included 101,916 women who were free of cardiovascular disease and breast cancer upon enrollment in the Women’s Health Initiative (WHI) at age 50-79 years during 1993-1998. During follow-up in this prospective cohort study, 4,340 of them developed invasive breast cancer. The diagnosis occurred an average of 5 years into the study, and patients were followed for 10 years afterwards. “Forty-one percent of women with breast cancer already had cardiovascular risk factors at baseline, way before their breast cancer diagnosis,” noted Dr. Park of the University of Pittsburgh.
Among women diagnosed with breast cancer in their 50s, 40% of all deaths were from breast cancer and 15% were caused by cardiovascular disease. In contrast, the cumulative impact of atherosclerosis was far more prominent in women diagnosed with breast cancer at a more advanced age. Indeed, among women diagnosed with breast cancer in their 70s, about 15% of deaths were caused by breast cancer, while 25% resulted from cardiovascular disease.
The number of baseline cardiovascular risk factors present at enrollment in the WHI turned out to be a powerful determinant of the likelihood of acute MI or death as a result of coronary heart disease in participants who developed breast cancer.
Of the 4,340 women who later developed invasive breast cancer, 2,562 were free of hypertension, diabetes, and hypercholesterolemia and were nonsmokers upon enrollment. In an age-adjusted analysis in which this risk factor–free group served as the reference population, the risk of MI after breast cancer diagnosis was increased 1.65-fold in those with a single baseline risk factor, 3.2-fold in those with two, and 5.8-fold in women with three cardiovascular risk factors. Similarly, the breast cancer patients’ risk of CHD death climbed stepwise by 1.78-, 2.28-, and 3.6-fold as the number of baseline cardiovascular risk factors increased from one to three.
The greatest risk was seen in breast cancer patients who at WHI enrollment were current smokers with an additional cardiovascular risk factor. They had a 9.6-fold greater risk of an acute MI after developing breast cancer, compared with breast cancer patients with none of the baseline cardiovascular risk factors. They also had a 7.7-fold increased risk of CHD death.
The WHI was funded by the National Heart, Lung, and Blood Institute. Dr. Park reported having no financial conflicts of interest.
ORLANDO – Women diagnosed with localized breast cancer while in their 70s have a higher mortality from cardiovascular disease than from their breast cancer, according to new data from the Women’s Health Initiative.
“Identification and treatment of cardiovascular disease risk factors among older women with breast cancer will likely improve survivorship and should be a high priority, especially for older women with incident localized breast cancer,” Na-Jin Park, Ph.D., said at the American Heart Association scientific sessions.
She presented an analysis that included 101,916 women who were free of cardiovascular disease and breast cancer upon enrollment in the Women’s Health Initiative (WHI) at age 50-79 years during 1993-1998. During follow-up in this prospective cohort study, 4,340 of them developed invasive breast cancer. The diagnosis occurred an average of 5 years into the study, and patients were followed for 10 years afterwards. “Forty-one percent of women with breast cancer already had cardiovascular risk factors at baseline, way before their breast cancer diagnosis,” noted Dr. Park of the University of Pittsburgh.
Among women diagnosed with breast cancer in their 50s, 40% of all deaths were from breast cancer and 15% were caused by cardiovascular disease. In contrast, the cumulative impact of atherosclerosis was far more prominent in women diagnosed with breast cancer at a more advanced age. Indeed, among women diagnosed with breast cancer in their 70s, about 15% of deaths were caused by breast cancer, while 25% resulted from cardiovascular disease.
The number of baseline cardiovascular risk factors present at enrollment in the WHI turned out to be a powerful determinant of the likelihood of acute MI or death as a result of coronary heart disease in participants who developed breast cancer.
Of the 4,340 women who later developed invasive breast cancer, 2,562 were free of hypertension, diabetes, and hypercholesterolemia and were nonsmokers upon enrollment. In an age-adjusted analysis in which this risk factor–free group served as the reference population, the risk of MI after breast cancer diagnosis was increased 1.65-fold in those with a single baseline risk factor, 3.2-fold in those with two, and 5.8-fold in women with three cardiovascular risk factors. Similarly, the breast cancer patients’ risk of CHD death climbed stepwise by 1.78-, 2.28-, and 3.6-fold as the number of baseline cardiovascular risk factors increased from one to three.
The greatest risk was seen in breast cancer patients who at WHI enrollment were current smokers with an additional cardiovascular risk factor. They had a 9.6-fold greater risk of an acute MI after developing breast cancer, compared with breast cancer patients with none of the baseline cardiovascular risk factors. They also had a 7.7-fold increased risk of CHD death.
The WHI was funded by the National Heart, Lung, and Blood Institute. Dr. Park reported having no financial conflicts of interest.
ORLANDO – Women diagnosed with localized breast cancer while in their 70s have a higher mortality from cardiovascular disease than from their breast cancer, according to new data from the Women’s Health Initiative.
“Identification and treatment of cardiovascular disease risk factors among older women with breast cancer will likely improve survivorship and should be a high priority, especially for older women with incident localized breast cancer,” Na-Jin Park, Ph.D., said at the American Heart Association scientific sessions.
She presented an analysis that included 101,916 women who were free of cardiovascular disease and breast cancer upon enrollment in the Women’s Health Initiative (WHI) at age 50-79 years during 1993-1998. During follow-up in this prospective cohort study, 4,340 of them developed invasive breast cancer. The diagnosis occurred an average of 5 years into the study, and patients were followed for 10 years afterwards. “Forty-one percent of women with breast cancer already had cardiovascular risk factors at baseline, way before their breast cancer diagnosis,” noted Dr. Park of the University of Pittsburgh.
Among women diagnosed with breast cancer in their 50s, 40% of all deaths were from breast cancer and 15% were caused by cardiovascular disease. In contrast, the cumulative impact of atherosclerosis was far more prominent in women diagnosed with breast cancer at a more advanced age. Indeed, among women diagnosed with breast cancer in their 70s, about 15% of deaths were caused by breast cancer, while 25% resulted from cardiovascular disease.
The number of baseline cardiovascular risk factors present at enrollment in the WHI turned out to be a powerful determinant of the likelihood of acute MI or death as a result of coronary heart disease in participants who developed breast cancer.
Of the 4,340 women who later developed invasive breast cancer, 2,562 were free of hypertension, diabetes, and hypercholesterolemia and were nonsmokers upon enrollment. In an age-adjusted analysis in which this risk factor–free group served as the reference population, the risk of MI after breast cancer diagnosis was increased 1.65-fold in those with a single baseline risk factor, 3.2-fold in those with two, and 5.8-fold in women with three cardiovascular risk factors. Similarly, the breast cancer patients’ risk of CHD death climbed stepwise by 1.78-, 2.28-, and 3.6-fold as the number of baseline cardiovascular risk factors increased from one to three.
The greatest risk was seen in breast cancer patients who at WHI enrollment were current smokers with an additional cardiovascular risk factor. They had a 9.6-fold greater risk of an acute MI after developing breast cancer, compared with breast cancer patients with none of the baseline cardiovascular risk factors. They also had a 7.7-fold increased risk of CHD death.
The WHI was funded by the National Heart, Lung, and Blood Institute. Dr. Park reported having no financial conflicts of interest.
AT THE AHA SCIENTIFIC SESSIONS
AHA: Older breast cancer patients more likely to die of heart disease than malignancy
ORLANDO – Women diagnosed with localized breast cancer while in their 70s have a higher mortality from cardiovascular disease than from their breast cancer, according to new data from the Women’s Health Initiative.
“Identification and treatment of cardiovascular disease risk factors among older women with breast cancer will likely improve survivorship and should be a high priority, especially for older women with incident localized breast cancer,” Na-Jin Park, Ph.D., said at the American Heart Association scientific sessions.
She presented an analysis that included 101,916 women who were free of cardiovascular disease and breast cancer upon enrollment in the Women’s Health Initiative (WHI) at age 50-79 years during 1993-1998. During follow-up in this prospective cohort study, 4,340 of them developed invasive breast cancer. The diagnosis occurred an average of 5 years into the study, and patients were followed for 10 years afterwards. “Forty-one percent of women with breast cancer already had cardiovascular risk factors at baseline, way before their breast cancer diagnosis,” noted Dr. Park of the University of Pittsburgh.
Among women diagnosed with breast cancer in their 50s, 40% of all deaths were from breast cancer and 15% were caused by cardiovascular disease. In contrast, the cumulative impact of atherosclerosis was far more prominent in women diagnosed with breast cancer at a more advanced age. Indeed, among women diagnosed with breast cancer in their 70s, about 15% of deaths were caused by breast cancer, while 25% resulted from cardiovascular disease.
The number of baseline cardiovascular risk factors present at enrollment in the WHI turned out to be a powerful determinant of the likelihood of acute MI or death as a result of coronary heart disease in participants who developed breast cancer.
Of the 4,340 women who later developed invasive breast cancer, 2,562 were free of hypertension, diabetes, and hypercholesterolemia and were nonsmokers upon enrollment. In an age-adjusted analysis in which this risk factor–free group served as the reference population, the risk of MI after breast cancer diagnosis was increased 1.65-fold in those with a single baseline risk factor, 3.2-fold in those with two, and 5.8-fold in women with three cardiovascular risk factors. Similarly, the breast cancer patients’ risk of CHD death climbed stepwise by 1.78-, 2.28-, and 3.6-fold as the number of baseline cardiovascular risk factors increased from one to three.
The greatest risk was seen in breast cancer patients who at WHI enrollment were current smokers with an additional cardiovascular risk factor. They had a 9.6-fold greater risk of an acute MI after developing breast cancer, compared with breast cancer patients with none of the baseline cardiovascular risk factors. They also had a 7.7-fold increased risk of CHD death.
The WHI was funded by the National Heart, Lung, and Blood Institute. Dr. Park reported having no financial conflicts of interest.
ORLANDO – Women diagnosed with localized breast cancer while in their 70s have a higher mortality from cardiovascular disease than from their breast cancer, according to new data from the Women’s Health Initiative.
“Identification and treatment of cardiovascular disease risk factors among older women with breast cancer will likely improve survivorship and should be a high priority, especially for older women with incident localized breast cancer,” Na-Jin Park, Ph.D., said at the American Heart Association scientific sessions.
She presented an analysis that included 101,916 women who were free of cardiovascular disease and breast cancer upon enrollment in the Women’s Health Initiative (WHI) at age 50-79 years during 1993-1998. During follow-up in this prospective cohort study, 4,340 of them developed invasive breast cancer. The diagnosis occurred an average of 5 years into the study, and patients were followed for 10 years afterwards. “Forty-one percent of women with breast cancer already had cardiovascular risk factors at baseline, way before their breast cancer diagnosis,” noted Dr. Park of the University of Pittsburgh.
Among women diagnosed with breast cancer in their 50s, 40% of all deaths were from breast cancer and 15% were caused by cardiovascular disease. In contrast, the cumulative impact of atherosclerosis was far more prominent in women diagnosed with breast cancer at a more advanced age. Indeed, among women diagnosed with breast cancer in their 70s, about 15% of deaths were caused by breast cancer, while 25% resulted from cardiovascular disease.
The number of baseline cardiovascular risk factors present at enrollment in the WHI turned out to be a powerful determinant of the likelihood of acute MI or death as a result of coronary heart disease in participants who developed breast cancer.
Of the 4,340 women who later developed invasive breast cancer, 2,562 were free of hypertension, diabetes, and hypercholesterolemia and were nonsmokers upon enrollment. In an age-adjusted analysis in which this risk factor–free group served as the reference population, the risk of MI after breast cancer diagnosis was increased 1.65-fold in those with a single baseline risk factor, 3.2-fold in those with two, and 5.8-fold in women with three cardiovascular risk factors. Similarly, the breast cancer patients’ risk of CHD death climbed stepwise by 1.78-, 2.28-, and 3.6-fold as the number of baseline cardiovascular risk factors increased from one to three.
The greatest risk was seen in breast cancer patients who at WHI enrollment were current smokers with an additional cardiovascular risk factor. They had a 9.6-fold greater risk of an acute MI after developing breast cancer, compared with breast cancer patients with none of the baseline cardiovascular risk factors. They also had a 7.7-fold increased risk of CHD death.
The WHI was funded by the National Heart, Lung, and Blood Institute. Dr. Park reported having no financial conflicts of interest.
ORLANDO – Women diagnosed with localized breast cancer while in their 70s have a higher mortality from cardiovascular disease than from their breast cancer, according to new data from the Women’s Health Initiative.
“Identification and treatment of cardiovascular disease risk factors among older women with breast cancer will likely improve survivorship and should be a high priority, especially for older women with incident localized breast cancer,” Na-Jin Park, Ph.D., said at the American Heart Association scientific sessions.
She presented an analysis that included 101,916 women who were free of cardiovascular disease and breast cancer upon enrollment in the Women’s Health Initiative (WHI) at age 50-79 years during 1993-1998. During follow-up in this prospective cohort study, 4,340 of them developed invasive breast cancer. The diagnosis occurred an average of 5 years into the study, and patients were followed for 10 years afterwards. “Forty-one percent of women with breast cancer already had cardiovascular risk factors at baseline, way before their breast cancer diagnosis,” noted Dr. Park of the University of Pittsburgh.
Among women diagnosed with breast cancer in their 50s, 40% of all deaths were from breast cancer and 15% were caused by cardiovascular disease. In contrast, the cumulative impact of atherosclerosis was far more prominent in women diagnosed with breast cancer at a more advanced age. Indeed, among women diagnosed with breast cancer in their 70s, about 15% of deaths were caused by breast cancer, while 25% resulted from cardiovascular disease.
The number of baseline cardiovascular risk factors present at enrollment in the WHI turned out to be a powerful determinant of the likelihood of acute MI or death as a result of coronary heart disease in participants who developed breast cancer.
Of the 4,340 women who later developed invasive breast cancer, 2,562 were free of hypertension, diabetes, and hypercholesterolemia and were nonsmokers upon enrollment. In an age-adjusted analysis in which this risk factor–free group served as the reference population, the risk of MI after breast cancer diagnosis was increased 1.65-fold in those with a single baseline risk factor, 3.2-fold in those with two, and 5.8-fold in women with three cardiovascular risk factors. Similarly, the breast cancer patients’ risk of CHD death climbed stepwise by 1.78-, 2.28-, and 3.6-fold as the number of baseline cardiovascular risk factors increased from one to three.
The greatest risk was seen in breast cancer patients who at WHI enrollment were current smokers with an additional cardiovascular risk factor. They had a 9.6-fold greater risk of an acute MI after developing breast cancer, compared with breast cancer patients with none of the baseline cardiovascular risk factors. They also had a 7.7-fold increased risk of CHD death.
The WHI was funded by the National Heart, Lung, and Blood Institute. Dr. Park reported having no financial conflicts of interest.
AT THE AHA SCIENTIFIC SESSIONS
Key clinical point: Among women diagnosed with breast cancer in their 70s, more will die from cardiovascular disease than from their malignancy.
Major finding: Among women diagnosed with breast cancer in their 70s, only about 15% of deaths were caused by breast cancer, while 25% resulted from cardiovascular disease.
Data source: This prospective cohort analysis from the Women’s Health Initiative included 101,916 women free of cardiovascular disease and breast cancer upon enrollment, of whom 4,340 later developed invasive breast cancer.
Disclosures: The Women’s Health Initiative was funded by the National Heart, Lung, and Blood Institute. The presenter reported having no financial conflicts of interest.
AHA: Low-dose beta-blockers may provide equivalent cardioprotection
ORLANDO – Low-dose beta-blockade appears to be at least as effective as standard full-dose beta-blocker therapy for cardioprotection in patients who’ve had an acute coronary syndrome, Dr. Jason E. Allen reported at the American Heart Association scientific sessions.
“This is an important observation, because in clinical practice patients often don’t tolerate high-dose beta-blockers. They prefer not to be on them. They experience hemodynamic issues like orthostatic hypotension and bradycardia. Plus, at times it’s important to have blood pressure room for other things – comorbid conditions like diabetes, where ACE inhibitors might also have an important role,” said Dr. Allen of Intermountain Medical Center in Salt Lake City.
While beta-blockade is a cornerstone of optimal medical management for reducing cardiovascular events in patients with symptomatic coronary artery disease, the high doses recommended today are based on decades-old randomized trials, which may not be entirely applicable in the current era of advanced medical and interventional therapies. That’s why he and his coinvestigators conducted a retrospective observational study using the Intermountain Healthcare database for the years 1993-2013.
They identified 7,158 patients with angiographically documented CAD who were discharged on beta-blocker therapy following an ACS. Eighty-five percent were discharged on a low-dose beta-blocker, defined by investigators as 25% or less of standard-dose metoprolol tartrate at 200 mg/day or its equivalent. Only 15% were discharged on high-dose therapy, defined as at least 100 mg/day of metoprolol or its equivalent.
The study endpoint was the incidence of the composite endpoint of all-cause mortality, MI, or stroke during follow-up. Because the group on high-dose beta-blockers typically had higher baseline rates of hypertension and hyperlipidemia, and a higher body mass index, propensity scores were utilized to adjust for these differences in a Cox proportional hazards analysis.
The incidence of the composite major adverse cardiovascular event (MACE) outcome from discharge through 6 months of follow-up was 4.3% among patients on a low-dose beta-blocker, which was equivalent to but not statistically superior to the 4.6% rate in those on high-dose therapy.
Among patients who didn’t have a MACE event in the first 6 months of follow-up, the incidence during months 7-24 was 4.8% in the low-dose group and 5.7% in the high-dose group, which once again was not a statistically significant difference. Of note, rates of all three components of the composite endpoint were numerically lower in the low-dose group.
A third analysis was restricted to the 6,844 patients who never switched between low- and high-dose therapy during the entire 24-month follow-up period. The composite MACE rate was 8.9% in the low-dose group, which was statistically equivalent to the 9.7% rate in patients on high-dose beta-blocker therapy throughout.
Dr. Allen was quick to point out that an observational study such as this can’t provide definitive proof. That would have to come from a randomized, controlled trial of high- versus low-dose beta-blocker therapy for secondary prevention. Such a trial also would be helpful in determining the mechanism of the putative equivalence seen in the Intermountain Healthcare study.
Dr. Allen’s study was conducted with institutional funds. He reported having no financial conflicts.
ORLANDO – Low-dose beta-blockade appears to be at least as effective as standard full-dose beta-blocker therapy for cardioprotection in patients who’ve had an acute coronary syndrome, Dr. Jason E. Allen reported at the American Heart Association scientific sessions.
“This is an important observation, because in clinical practice patients often don’t tolerate high-dose beta-blockers. They prefer not to be on them. They experience hemodynamic issues like orthostatic hypotension and bradycardia. Plus, at times it’s important to have blood pressure room for other things – comorbid conditions like diabetes, where ACE inhibitors might also have an important role,” said Dr. Allen of Intermountain Medical Center in Salt Lake City.
While beta-blockade is a cornerstone of optimal medical management for reducing cardiovascular events in patients with symptomatic coronary artery disease, the high doses recommended today are based on decades-old randomized trials, which may not be entirely applicable in the current era of advanced medical and interventional therapies. That’s why he and his coinvestigators conducted a retrospective observational study using the Intermountain Healthcare database for the years 1993-2013.
They identified 7,158 patients with angiographically documented CAD who were discharged on beta-blocker therapy following an ACS. Eighty-five percent were discharged on a low-dose beta-blocker, defined by investigators as 25% or less of standard-dose metoprolol tartrate at 200 mg/day or its equivalent. Only 15% were discharged on high-dose therapy, defined as at least 100 mg/day of metoprolol or its equivalent.
The study endpoint was the incidence of the composite endpoint of all-cause mortality, MI, or stroke during follow-up. Because the group on high-dose beta-blockers typically had higher baseline rates of hypertension and hyperlipidemia, and a higher body mass index, propensity scores were utilized to adjust for these differences in a Cox proportional hazards analysis.
The incidence of the composite major adverse cardiovascular event (MACE) outcome from discharge through 6 months of follow-up was 4.3% among patients on a low-dose beta-blocker, which was equivalent to but not statistically superior to the 4.6% rate in those on high-dose therapy.
Among patients who didn’t have a MACE event in the first 6 months of follow-up, the incidence during months 7-24 was 4.8% in the low-dose group and 5.7% in the high-dose group, which once again was not a statistically significant difference. Of note, rates of all three components of the composite endpoint were numerically lower in the low-dose group.
A third analysis was restricted to the 6,844 patients who never switched between low- and high-dose therapy during the entire 24-month follow-up period. The composite MACE rate was 8.9% in the low-dose group, which was statistically equivalent to the 9.7% rate in patients on high-dose beta-blocker therapy throughout.
Dr. Allen was quick to point out that an observational study such as this can’t provide definitive proof. That would have to come from a randomized, controlled trial of high- versus low-dose beta-blocker therapy for secondary prevention. Such a trial also would be helpful in determining the mechanism of the putative equivalence seen in the Intermountain Healthcare study.
Dr. Allen’s study was conducted with institutional funds. He reported having no financial conflicts.
ORLANDO – Low-dose beta-blockade appears to be at least as effective as standard full-dose beta-blocker therapy for cardioprotection in patients who’ve had an acute coronary syndrome, Dr. Jason E. Allen reported at the American Heart Association scientific sessions.
“This is an important observation, because in clinical practice patients often don’t tolerate high-dose beta-blockers. They prefer not to be on them. They experience hemodynamic issues like orthostatic hypotension and bradycardia. Plus, at times it’s important to have blood pressure room for other things – comorbid conditions like diabetes, where ACE inhibitors might also have an important role,” said Dr. Allen of Intermountain Medical Center in Salt Lake City.
While beta-blockade is a cornerstone of optimal medical management for reducing cardiovascular events in patients with symptomatic coronary artery disease, the high doses recommended today are based on decades-old randomized trials, which may not be entirely applicable in the current era of advanced medical and interventional therapies. That’s why he and his coinvestigators conducted a retrospective observational study using the Intermountain Healthcare database for the years 1993-2013.
They identified 7,158 patients with angiographically documented CAD who were discharged on beta-blocker therapy following an ACS. Eighty-five percent were discharged on a low-dose beta-blocker, defined by investigators as 25% or less of standard-dose metoprolol tartrate at 200 mg/day or its equivalent. Only 15% were discharged on high-dose therapy, defined as at least 100 mg/day of metoprolol or its equivalent.
The study endpoint was the incidence of the composite endpoint of all-cause mortality, MI, or stroke during follow-up. Because the group on high-dose beta-blockers typically had higher baseline rates of hypertension and hyperlipidemia, and a higher body mass index, propensity scores were utilized to adjust for these differences in a Cox proportional hazards analysis.
The incidence of the composite major adverse cardiovascular event (MACE) outcome from discharge through 6 months of follow-up was 4.3% among patients on a low-dose beta-blocker, which was equivalent to but not statistically superior to the 4.6% rate in those on high-dose therapy.
Among patients who didn’t have a MACE event in the first 6 months of follow-up, the incidence during months 7-24 was 4.8% in the low-dose group and 5.7% in the high-dose group, which once again was not a statistically significant difference. Of note, rates of all three components of the composite endpoint were numerically lower in the low-dose group.
A third analysis was restricted to the 6,844 patients who never switched between low- and high-dose therapy during the entire 24-month follow-up period. The composite MACE rate was 8.9% in the low-dose group, which was statistically equivalent to the 9.7% rate in patients on high-dose beta-blocker therapy throughout.
Dr. Allen was quick to point out that an observational study such as this can’t provide definitive proof. That would have to come from a randomized, controlled trial of high- versus low-dose beta-blocker therapy for secondary prevention. Such a trial also would be helpful in determining the mechanism of the putative equivalence seen in the Intermountain Healthcare study.
Dr. Allen’s study was conducted with institutional funds. He reported having no financial conflicts.
AT THE AHA SCIENTIFIC SESSIONS
Key clinical point: When it comes to secondary cardiovascular prevention following an acute coronary syndrome, low-dose beta-blockade may be as effective as high-dose.
Major finding: The rate of all-cause mortality, MI, or stroke during 24 months after discharge following an acute coronary syndrome was 8.9% in patients on a low-dose beta-blocker throughout, equivalent to the 9.7% rate in those on high-dose therapy throughout.
Data source: This retrospective observational study included 7,158 patients discharged on beta-blocker therapy after an acute coronary syndrome and followed for 24 months.
Disclosures: This study was conducted with institutional funds. The presenter reported having no financial conflicts.
AHA: Asthma History Boosts Heart Disease Risk in Postmenopausal Women
ORLANDO – A history of asthma was independently associated with a 24% increase in the risk of new-onset coronary heart disease among postmenopausal women in an analysis from the Women’s Health Initiative.
The study cohort included 90,168 women aged 50-79 years who were free of cardiovascular disease at enrollment in the Women’s Health Initiative (WHI), of whom 6,921 reported a history of physician-diagnosed asthma at baseline. During follow-up in the prospective study, the incidence of a CHD event was 8.6% in subjects with a history of asthma and 6.97% in the no-asthma group, Dr. Fady Y. Marmoush reported at the American Heart Association scientific sessions.
Moreover, the incidence of a first cardiovascular event was 11.6% in the asthma group, compared with 9.7% in the no-asthma controls, added Dr. Marmoush of Memorial Hospital of Rhode Island, Pawtucket.
The asthma group had an absolute 1%-2% greater baseline prevalence of hypertension, diabetes, and family history of CHD. Those with asthma also were more likely to be obese. On the other hand, they were less likely to have ever smoked.
In a multivariate analysis adjusted for these and other potential confounders, including age, dyslipidemia, and waist-hip ratio, the women with a history of asthma had a 24% greater risk of CHD during prospective follow-up in the WHI, as well as a 21% increased rate of cardiovascular events, including stroke, compared with the no-asthma group.
Thus, a history of asthma could be a useful consideration – a tie breaker of sorts – in older women whose calculated 10-year atherosclerotic cardiovascular disease risk based on the standard risk factors places them on the borderline as candidates for statin therapy. The most likely mechanism for the observed association between asthma history and increased risk of cardiovascular disease is the chronic inflammatory state that’s a hallmark of asthma accelerating the atherosclerotic process, which also is inflammatory, she said.
The WHI is funded by the National Heart, Lung, and Blood Institute. Dr. Marmoush reported having no financial conflicts.
ORLANDO – A history of asthma was independently associated with a 24% increase in the risk of new-onset coronary heart disease among postmenopausal women in an analysis from the Women’s Health Initiative.
The study cohort included 90,168 women aged 50-79 years who were free of cardiovascular disease at enrollment in the Women’s Health Initiative (WHI), of whom 6,921 reported a history of physician-diagnosed asthma at baseline. During follow-up in the prospective study, the incidence of a CHD event was 8.6% in subjects with a history of asthma and 6.97% in the no-asthma group, Dr. Fady Y. Marmoush reported at the American Heart Association scientific sessions.
Moreover, the incidence of a first cardiovascular event was 11.6% in the asthma group, compared with 9.7% in the no-asthma controls, added Dr. Marmoush of Memorial Hospital of Rhode Island, Pawtucket.
The asthma group had an absolute 1%-2% greater baseline prevalence of hypertension, diabetes, and family history of CHD. Those with asthma also were more likely to be obese. On the other hand, they were less likely to have ever smoked.
In a multivariate analysis adjusted for these and other potential confounders, including age, dyslipidemia, and waist-hip ratio, the women with a history of asthma had a 24% greater risk of CHD during prospective follow-up in the WHI, as well as a 21% increased rate of cardiovascular events, including stroke, compared with the no-asthma group.
Thus, a history of asthma could be a useful consideration – a tie breaker of sorts – in older women whose calculated 10-year atherosclerotic cardiovascular disease risk based on the standard risk factors places them on the borderline as candidates for statin therapy. The most likely mechanism for the observed association between asthma history and increased risk of cardiovascular disease is the chronic inflammatory state that’s a hallmark of asthma accelerating the atherosclerotic process, which also is inflammatory, she said.
The WHI is funded by the National Heart, Lung, and Blood Institute. Dr. Marmoush reported having no financial conflicts.
ORLANDO – A history of asthma was independently associated with a 24% increase in the risk of new-onset coronary heart disease among postmenopausal women in an analysis from the Women’s Health Initiative.
The study cohort included 90,168 women aged 50-79 years who were free of cardiovascular disease at enrollment in the Women’s Health Initiative (WHI), of whom 6,921 reported a history of physician-diagnosed asthma at baseline. During follow-up in the prospective study, the incidence of a CHD event was 8.6% in subjects with a history of asthma and 6.97% in the no-asthma group, Dr. Fady Y. Marmoush reported at the American Heart Association scientific sessions.
Moreover, the incidence of a first cardiovascular event was 11.6% in the asthma group, compared with 9.7% in the no-asthma controls, added Dr. Marmoush of Memorial Hospital of Rhode Island, Pawtucket.
The asthma group had an absolute 1%-2% greater baseline prevalence of hypertension, diabetes, and family history of CHD. Those with asthma also were more likely to be obese. On the other hand, they were less likely to have ever smoked.
In a multivariate analysis adjusted for these and other potential confounders, including age, dyslipidemia, and waist-hip ratio, the women with a history of asthma had a 24% greater risk of CHD during prospective follow-up in the WHI, as well as a 21% increased rate of cardiovascular events, including stroke, compared with the no-asthma group.
Thus, a history of asthma could be a useful consideration – a tie breaker of sorts – in older women whose calculated 10-year atherosclerotic cardiovascular disease risk based on the standard risk factors places them on the borderline as candidates for statin therapy. The most likely mechanism for the observed association between asthma history and increased risk of cardiovascular disease is the chronic inflammatory state that’s a hallmark of asthma accelerating the atherosclerotic process, which also is inflammatory, she said.
The WHI is funded by the National Heart, Lung, and Blood Institute. Dr. Marmoush reported having no financial conflicts.
AT THE AHA SCIENTIFIC SESSIONS
AHA: Asthma history boosts heart disease risk in postmenopausal women
ORLANDO – A history of asthma was independently associated with a 24% increase in the risk of new-onset coronary heart disease among postmenopausal women in an analysis from the Women’s Health Initiative.
The study cohort included 90,168 women aged 50-79 years who were free of cardiovascular disease at enrollment in the Women’s Health Initiative (WHI), of whom 6,921 reported a history of physician-diagnosed asthma at baseline. During follow-up in the prospective study, the incidence of a CHD event was 8.6% in subjects with a history of asthma and 6.97% in the no-asthma group, Dr. Fady Y. Marmoush reported at the American Heart Association scientific sessions.
Moreover, the incidence of a first cardiovascular event was 11.6% in the asthma group, compared with 9.7% in the no-asthma controls, added Dr. Marmoush of Memorial Hospital of Rhode Island, Pawtucket.
The asthma group had an absolute 1%-2% greater baseline prevalence of hypertension, diabetes, and family history of CHD. Those with asthma also were more likely to be obese. On the other hand, they were less likely to have ever smoked.
In a multivariate analysis adjusted for these and other potential confounders, including age, dyslipidemia, and waist-hip ratio, the women with a history of asthma had a 24% greater risk of CHD during prospective follow-up in the WHI, as well as a 21% increased rate of cardiovascular events, including stroke, compared with the no-asthma group.
Thus, a history of asthma could be a useful consideration – a tie breaker of sorts – in older women whose calculated 10-year atherosclerotic cardiovascular disease risk based on the standard risk factors places them on the borderline as candidates for statin therapy. The most likely mechanism for the observed association between asthma history and increased risk of cardiovascular disease is the chronic inflammatory state that’s a hallmark of asthma accelerating the atherosclerotic process, which also is inflammatory, she said.
The WHI is funded by the National Heart, Lung, and Blood Institute. Dr. Marmoush reported having no financial conflicts.
ORLANDO – A history of asthma was independently associated with a 24% increase in the risk of new-onset coronary heart disease among postmenopausal women in an analysis from the Women’s Health Initiative.
The study cohort included 90,168 women aged 50-79 years who were free of cardiovascular disease at enrollment in the Women’s Health Initiative (WHI), of whom 6,921 reported a history of physician-diagnosed asthma at baseline. During follow-up in the prospective study, the incidence of a CHD event was 8.6% in subjects with a history of asthma and 6.97% in the no-asthma group, Dr. Fady Y. Marmoush reported at the American Heart Association scientific sessions.
Moreover, the incidence of a first cardiovascular event was 11.6% in the asthma group, compared with 9.7% in the no-asthma controls, added Dr. Marmoush of Memorial Hospital of Rhode Island, Pawtucket.
The asthma group had an absolute 1%-2% greater baseline prevalence of hypertension, diabetes, and family history of CHD. Those with asthma also were more likely to be obese. On the other hand, they were less likely to have ever smoked.
In a multivariate analysis adjusted for these and other potential confounders, including age, dyslipidemia, and waist-hip ratio, the women with a history of asthma had a 24% greater risk of CHD during prospective follow-up in the WHI, as well as a 21% increased rate of cardiovascular events, including stroke, compared with the no-asthma group.
Thus, a history of asthma could be a useful consideration – a tie breaker of sorts – in older women whose calculated 10-year atherosclerotic cardiovascular disease risk based on the standard risk factors places them on the borderline as candidates for statin therapy. The most likely mechanism for the observed association between asthma history and increased risk of cardiovascular disease is the chronic inflammatory state that’s a hallmark of asthma accelerating the atherosclerotic process, which also is inflammatory, she said.
The WHI is funded by the National Heart, Lung, and Blood Institute. Dr. Marmoush reported having no financial conflicts.
ORLANDO – A history of asthma was independently associated with a 24% increase in the risk of new-onset coronary heart disease among postmenopausal women in an analysis from the Women’s Health Initiative.
The study cohort included 90,168 women aged 50-79 years who were free of cardiovascular disease at enrollment in the Women’s Health Initiative (WHI), of whom 6,921 reported a history of physician-diagnosed asthma at baseline. During follow-up in the prospective study, the incidence of a CHD event was 8.6% in subjects with a history of asthma and 6.97% in the no-asthma group, Dr. Fady Y. Marmoush reported at the American Heart Association scientific sessions.
Moreover, the incidence of a first cardiovascular event was 11.6% in the asthma group, compared with 9.7% in the no-asthma controls, added Dr. Marmoush of Memorial Hospital of Rhode Island, Pawtucket.
The asthma group had an absolute 1%-2% greater baseline prevalence of hypertension, diabetes, and family history of CHD. Those with asthma also were more likely to be obese. On the other hand, they were less likely to have ever smoked.
In a multivariate analysis adjusted for these and other potential confounders, including age, dyslipidemia, and waist-hip ratio, the women with a history of asthma had a 24% greater risk of CHD during prospective follow-up in the WHI, as well as a 21% increased rate of cardiovascular events, including stroke, compared with the no-asthma group.
Thus, a history of asthma could be a useful consideration – a tie breaker of sorts – in older women whose calculated 10-year atherosclerotic cardiovascular disease risk based on the standard risk factors places them on the borderline as candidates for statin therapy. The most likely mechanism for the observed association between asthma history and increased risk of cardiovascular disease is the chronic inflammatory state that’s a hallmark of asthma accelerating the atherosclerotic process, which also is inflammatory, she said.
The WHI is funded by the National Heart, Lung, and Blood Institute. Dr. Marmoush reported having no financial conflicts.
AT THE AHA SCIENTIFIC SESSIONS
Key clinical point: A history of asthma appears to substantially increase the risk of a first cardiovascular event in postmenopausal women.
Major finding: In a multivariate analysis adjusted for conventional cardiovascular risk factors, older women with a history of asthma had a 24% higher risk for a first coronary heart disease event and 21% greater risk of a cardiovascular event than those without such a history.
Data source: The Women’s Health Initiative is a prospective cohort study. This analysis included 90,168 women aged 50-79 at enrollment, of whom 6,921 reported a history of physician-diagnosed asthma.
Disclosures: The study is supported by the National Heart, Lung, and Blood Institute. The presenter reported having no financial conflicts.
VIDEO: Monitoring helps only adherent heart failure patients
ORLANDO – A multipronged approach to following and managing heart failure patients closely after they are hospitalized for acute decompensation led to significant reductions in subsequent rehospitalization or death in a randomized trial, but only in the subgroup of patients who actually adhered to the program.
The main message from the study was “this type of telemonitoring should not get used on everyone,” said Dr. Michael K. Ong in an interview at the American Heart Association scientific sessions. “A key issue is who are the people who would benefit” from an intensified at-home monitoring program following hospitalization for an acute heart failure episode.
Another issue is that new monitoring technologies introduced after launch of the BEAT-HF (Better Effectiveness After Transition–Heart Failure) trial more than 4 years ago have produced unobtrusive and implantable monitoring devices that could help boost monitoring compliance, said Dr. Ong, an internist at the University of California, Los Angeles.
“We all monitor our patients remotely on a variety of ways,” commented Dr. Mariell Jessup, professor of medicine and heart failure specialist and at the University of Pennsylvania in Philadelphia. “Depending on our resources and technology, we might use implantable monitors or have nurses call patients, and have patients send us emails. There is a wide range of telemonitoring available. But we need to find out what works. An enormous effort has been made to enhance patients’ ability to monitor themselves, so they can take charge of their disease,” Dr. Jessup said.
BEAT-HF randomized 1,437 patients with confirmed heart failure and an index hospitalization to an intensive monitoring and education program or usual care during 2011-2013 at six academic health centers in California. Patients averaged 73 years old, and most patients had class III New York Heart Association heart failure, with three quarters having either class III or IV.
The intensive program included three elements:
• An in-hospital education program.
• A schedule of nine follow-up telephone calls by a registered nurse starting 2-3 days post discharge and continuing out to 6 months. Patients in the intervention arm completed a median of six of these calls.
• Telemonitoring of daily measurement of weight, blood pressure, and heart rate using electronically linked monitoring devices supplied to each patient. The monitoring equipment actually was used by 83% of the 715 patients randomized to this arm, and at 180 days, 52% of the patients in this arm had transmitted more than half of their daily measurement updates.
The study showed no significant benefit from the intensive monitoring arm compared with usual care for the primary endpoint of all-cause hospitalizations after 180 days, Dr. Ong reported. However, in a post hoc analysis that divided the intervention arm patients into those with more than 50% days with monitoring information sent and those with 50% or less, the rehospitalization rate was 61% among the patients who complied 50% or less of the time with daily home monitoring, and 41% in patients with greater than 50% compliance, a one-third relative drop. The more-compliant patients also substantially and significantly reduced their mortality rates at both 30 and 180 days, compared with the less-adherent patients in the intervention arm.
Additional studies must now examine how to optimize adherence and better match patients with various monitoring techniques. “If patients won’t use a treatment, they won’t benefit,” said Dr. Ong. Finding out what makes people adherent and encourage them to participate is the next research issue, he added.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
On Twitter @mitchelzoler
We are now in the second decade of research aimed at finding the most effective ways to monitor patients following a hospitalization for acute heart failure decompensation. Interest runs high to find the best ways to monitor these patients and treat them with interventions intended to cut the rate of future decompensation events and hospitalizations and mortality.
Despite all this, research and interest studies have not yet clearly identified monitoring and intervention strategies that are consistently effective. In fact, sometimes so many monitoring strategies are begun by both health systems and by payers that it can become can become confusing.

|
Dr. Mary Norine Walsh |
A key issue is, who receives the monitoring data and what do they do with it? The way that physicians and nurses act on monitoring data really matters, and ideally, patients should also know their monitoring data and be an active part of maintaining their stability.
At the center where I work, we routinely educate patients during their hospitalization on the importance of maintaining a low-sodium diet and daily weight monitoring. Daily weights as a way to track the fluid-balance status of patients has been unfairly criticized, as new technology has made implantable monitors routinely available. Although they are routinely available, implanted technologies are not yet for the masses. I am a firm believer in the value of daily weights.
At my center, we put a paper weight chart in each patient’s room, recorded in pounds, so that patients can track their weight fluctuations themselves. We try to educate and indoctrinate our heart failure patients to the importance of tracking their weight, and tell them to bring the charts they maintain at home to their clinic visits. We even instruct selected patients who have taken good, personal control of their heart failure to adjust their daily furosemide dosage themselves – within specified limits and while keeping us informed – when they see their weight tracking up or down.
The better patients with heart failure understand the tight relationship between their lifestyle choices and their status, the better it is for their long-term success.
Dr. Mary Norine Walsh is medical director of the Heart Failure and Cardiac Transplantation program at the St. Vincent Heart Center in Indianapolis. She had no disclosures. She made these comments in an interview.
We are now in the second decade of research aimed at finding the most effective ways to monitor patients following a hospitalization for acute heart failure decompensation. Interest runs high to find the best ways to monitor these patients and treat them with interventions intended to cut the rate of future decompensation events and hospitalizations and mortality.
Despite all this, research and interest studies have not yet clearly identified monitoring and intervention strategies that are consistently effective. In fact, sometimes so many monitoring strategies are begun by both health systems and by payers that it can become can become confusing.

|
Dr. Mary Norine Walsh |
A key issue is, who receives the monitoring data and what do they do with it? The way that physicians and nurses act on monitoring data really matters, and ideally, patients should also know their monitoring data and be an active part of maintaining their stability.
At the center where I work, we routinely educate patients during their hospitalization on the importance of maintaining a low-sodium diet and daily weight monitoring. Daily weights as a way to track the fluid-balance status of patients has been unfairly criticized, as new technology has made implantable monitors routinely available. Although they are routinely available, implanted technologies are not yet for the masses. I am a firm believer in the value of daily weights.
At my center, we put a paper weight chart in each patient’s room, recorded in pounds, so that patients can track their weight fluctuations themselves. We try to educate and indoctrinate our heart failure patients to the importance of tracking their weight, and tell them to bring the charts they maintain at home to their clinic visits. We even instruct selected patients who have taken good, personal control of their heart failure to adjust their daily furosemide dosage themselves – within specified limits and while keeping us informed – when they see their weight tracking up or down.
The better patients with heart failure understand the tight relationship between their lifestyle choices and their status, the better it is for their long-term success.
Dr. Mary Norine Walsh is medical director of the Heart Failure and Cardiac Transplantation program at the St. Vincent Heart Center in Indianapolis. She had no disclosures. She made these comments in an interview.
We are now in the second decade of research aimed at finding the most effective ways to monitor patients following a hospitalization for acute heart failure decompensation. Interest runs high to find the best ways to monitor these patients and treat them with interventions intended to cut the rate of future decompensation events and hospitalizations and mortality.
Despite all this, research and interest studies have not yet clearly identified monitoring and intervention strategies that are consistently effective. In fact, sometimes so many monitoring strategies are begun by both health systems and by payers that it can become can become confusing.

|
Dr. Mary Norine Walsh |
A key issue is, who receives the monitoring data and what do they do with it? The way that physicians and nurses act on monitoring data really matters, and ideally, patients should also know their monitoring data and be an active part of maintaining their stability.
At the center where I work, we routinely educate patients during their hospitalization on the importance of maintaining a low-sodium diet and daily weight monitoring. Daily weights as a way to track the fluid-balance status of patients has been unfairly criticized, as new technology has made implantable monitors routinely available. Although they are routinely available, implanted technologies are not yet for the masses. I am a firm believer in the value of daily weights.
At my center, we put a paper weight chart in each patient’s room, recorded in pounds, so that patients can track their weight fluctuations themselves. We try to educate and indoctrinate our heart failure patients to the importance of tracking their weight, and tell them to bring the charts they maintain at home to their clinic visits. We even instruct selected patients who have taken good, personal control of their heart failure to adjust their daily furosemide dosage themselves – within specified limits and while keeping us informed – when they see their weight tracking up or down.
The better patients with heart failure understand the tight relationship between their lifestyle choices and their status, the better it is for their long-term success.
Dr. Mary Norine Walsh is medical director of the Heart Failure and Cardiac Transplantation program at the St. Vincent Heart Center in Indianapolis. She had no disclosures. She made these comments in an interview.
ORLANDO – A multipronged approach to following and managing heart failure patients closely after they are hospitalized for acute decompensation led to significant reductions in subsequent rehospitalization or death in a randomized trial, but only in the subgroup of patients who actually adhered to the program.
The main message from the study was “this type of telemonitoring should not get used on everyone,” said Dr. Michael K. Ong in an interview at the American Heart Association scientific sessions. “A key issue is who are the people who would benefit” from an intensified at-home monitoring program following hospitalization for an acute heart failure episode.
Another issue is that new monitoring technologies introduced after launch of the BEAT-HF (Better Effectiveness After Transition–Heart Failure) trial more than 4 years ago have produced unobtrusive and implantable monitoring devices that could help boost monitoring compliance, said Dr. Ong, an internist at the University of California, Los Angeles.
“We all monitor our patients remotely on a variety of ways,” commented Dr. Mariell Jessup, professor of medicine and heart failure specialist and at the University of Pennsylvania in Philadelphia. “Depending on our resources and technology, we might use implantable monitors or have nurses call patients, and have patients send us emails. There is a wide range of telemonitoring available. But we need to find out what works. An enormous effort has been made to enhance patients’ ability to monitor themselves, so they can take charge of their disease,” Dr. Jessup said.
BEAT-HF randomized 1,437 patients with confirmed heart failure and an index hospitalization to an intensive monitoring and education program or usual care during 2011-2013 at six academic health centers in California. Patients averaged 73 years old, and most patients had class III New York Heart Association heart failure, with three quarters having either class III or IV.
The intensive program included three elements:
• An in-hospital education program.
• A schedule of nine follow-up telephone calls by a registered nurse starting 2-3 days post discharge and continuing out to 6 months. Patients in the intervention arm completed a median of six of these calls.
• Telemonitoring of daily measurement of weight, blood pressure, and heart rate using electronically linked monitoring devices supplied to each patient. The monitoring equipment actually was used by 83% of the 715 patients randomized to this arm, and at 180 days, 52% of the patients in this arm had transmitted more than half of their daily measurement updates.
The study showed no significant benefit from the intensive monitoring arm compared with usual care for the primary endpoint of all-cause hospitalizations after 180 days, Dr. Ong reported. However, in a post hoc analysis that divided the intervention arm patients into those with more than 50% days with monitoring information sent and those with 50% or less, the rehospitalization rate was 61% among the patients who complied 50% or less of the time with daily home monitoring, and 41% in patients with greater than 50% compliance, a one-third relative drop. The more-compliant patients also substantially and significantly reduced their mortality rates at both 30 and 180 days, compared with the less-adherent patients in the intervention arm.
Additional studies must now examine how to optimize adherence and better match patients with various monitoring techniques. “If patients won’t use a treatment, they won’t benefit,” said Dr. Ong. Finding out what makes people adherent and encourage them to participate is the next research issue, he added.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
On Twitter @mitchelzoler
ORLANDO – A multipronged approach to following and managing heart failure patients closely after they are hospitalized for acute decompensation led to significant reductions in subsequent rehospitalization or death in a randomized trial, but only in the subgroup of patients who actually adhered to the program.
The main message from the study was “this type of telemonitoring should not get used on everyone,” said Dr. Michael K. Ong in an interview at the American Heart Association scientific sessions. “A key issue is who are the people who would benefit” from an intensified at-home monitoring program following hospitalization for an acute heart failure episode.
Another issue is that new monitoring technologies introduced after launch of the BEAT-HF (Better Effectiveness After Transition–Heart Failure) trial more than 4 years ago have produced unobtrusive and implantable monitoring devices that could help boost monitoring compliance, said Dr. Ong, an internist at the University of California, Los Angeles.
“We all monitor our patients remotely on a variety of ways,” commented Dr. Mariell Jessup, professor of medicine and heart failure specialist and at the University of Pennsylvania in Philadelphia. “Depending on our resources and technology, we might use implantable monitors or have nurses call patients, and have patients send us emails. There is a wide range of telemonitoring available. But we need to find out what works. An enormous effort has been made to enhance patients’ ability to monitor themselves, so they can take charge of their disease,” Dr. Jessup said.
BEAT-HF randomized 1,437 patients with confirmed heart failure and an index hospitalization to an intensive monitoring and education program or usual care during 2011-2013 at six academic health centers in California. Patients averaged 73 years old, and most patients had class III New York Heart Association heart failure, with three quarters having either class III or IV.
The intensive program included three elements:
• An in-hospital education program.
• A schedule of nine follow-up telephone calls by a registered nurse starting 2-3 days post discharge and continuing out to 6 months. Patients in the intervention arm completed a median of six of these calls.
• Telemonitoring of daily measurement of weight, blood pressure, and heart rate using electronically linked monitoring devices supplied to each patient. The monitoring equipment actually was used by 83% of the 715 patients randomized to this arm, and at 180 days, 52% of the patients in this arm had transmitted more than half of their daily measurement updates.
The study showed no significant benefit from the intensive monitoring arm compared with usual care for the primary endpoint of all-cause hospitalizations after 180 days, Dr. Ong reported. However, in a post hoc analysis that divided the intervention arm patients into those with more than 50% days with monitoring information sent and those with 50% or less, the rehospitalization rate was 61% among the patients who complied 50% or less of the time with daily home monitoring, and 41% in patients with greater than 50% compliance, a one-third relative drop. The more-compliant patients also substantially and significantly reduced their mortality rates at both 30 and 180 days, compared with the less-adherent patients in the intervention arm.
Additional studies must now examine how to optimize adherence and better match patients with various monitoring techniques. “If patients won’t use a treatment, they won’t benefit,” said Dr. Ong. Finding out what makes people adherent and encourage them to participate is the next research issue, he added.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
On Twitter @mitchelzoler
AT THE AHA SCIENTIFIC SESSIONS
Key clinical point: Remote monitoring failed to reduce mortality or hospitalizations for all patients during the 180 days following heart failure hospitalization, but was effective for patients who adhered to the program.
Major finding: Telemonitoring more than half the time cut 180-day readmissions by a third relative to usual care.
Data source: The BEAT-HF study, which enrolled 1,437 patients hospitalized for acute heart failure at six California centers.
Disclosures: BEAT-HF had no commercial sponsors. Dr. Ong had no disclosures. Dr. Jessup had no disclosures.
AHA: Coronary calcium personalizes ACC/AHA risk calculator
ORLANDO – Combining the coronary artery calcium score with the ACC/AHA cardiovascular risk calculator tool enables physicians to refine their decision making about who to recommend for statin therapy, Dr. Salman Waheed reported at the American Heart Association scientific sessions.
He presented an analysis of 1,225 asymptomatic adults followed for a median of 3.9 years in the observational arm of the St. Francis Health Study, an early landmark prospective study of the relationship between electron beam CT coronary artery calcium (CAC) score and cardiovascular event risk.
The 217 subjects who today would not be recommended for statin therapy on the basis of a 10-year atherosclerotic cardiovascular disease risk of 5%-7.4% as determined by the risk calculator included in the 2013 ACC/AHA cholesterol management guidelines (Circulation. 2014 Jun 24;129[25 Suppl 2]:S1-45) would be reclassified as warranting statin therapy if they had a CAC greater than 0, as was the case for 169 of the 217 (78%). Indeed, the presence of a CAC of 1 or more boosted their estimated 10-year risk to 10.8%.
On the other hand, there were 510 patients who would be classified as high risk by the ACC/AHA clinical risk calculator, with a 10-year risk of 7.5%-20%. Taking their CAC score into account would result in 73 being reclassified as low risk and becoming no longer statin candidates because their CAC of 0 was associated with a 10-year event risk of less than 1%. In contrast, for the 447 remaining subjects with a CAC greater than 0, the 10-year risk climbed to 21.9%, according to Dr. Waheed of the University of Kansas, Kansas City.
The composite outcome utilized in this analysis from the St. Francis Heart Study was comprised of nonfatal MI, coronary death, stroke, peripheral arterial revascularization, or coronary revascularization. Of note, heart failure wasn’t included.
Among the 545 subjects deemed at low cardiovascular risk because they weren’t eligible for statin therapy according to the 2013 ACC/AHA guidelines, 209 would be recategorized as high risk on the basis of a CAC score at or above the 80th percentile adjusted for age and gender. The adjusted risk of a cardiovascular event in the high CAC/low clinical risk group was 24.9-fold greater than in the low CAC/low clinical risk group.
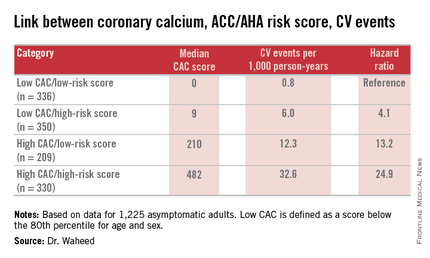
“Among those eligible for statin therapy based upon current guidelines, high CAC portends a sixfold higher outcome risk than low CAC,” Dr. Waheed added.
The magnitude of CAC progression over the course of 4 years was similar across the baseline risk categories; however, the absolute CAC progression was greater among those with a high CAC at baseline.
Audience member Dr. Daniel S. Berman observed that the results of Dr. Waheed’s study are highly concordant with an earlier report from the Multi-Ethnic Study of Atherosclerosis in which a CAC of 0 was quite common in patients for whom statins would be recommended under the current ACC/AHA guidelines.
“Your data support the idea that stratification based upon CAC could more personalize the statin recommendations,” said Dr. Berman, chief of cardiac imaging and nuclear cardiology at Cedars-Sinai Medical Center and professor of medicine at the University of California, Los Angeles.
Another audience member, Dr. Donald M. Lloyd-Jones, one of the architects of the current guidelines, commented that the analyses from the St. Francis Heart Study and the Multi-Ethnic Study of Atherosclerosis suggest CAC screening coupled with the ACC/AHA risk score provides added value in a select group of patients.
“Your analysis continues to reinforce the point that we probably shouldn’t be doing universal CAC screening because in the people with a 10-year risk of less than 5% the yield is low and the CAC didn’t change anything, while in the people with a 10-year risk of 20% or higher the yield is incredibly high and the CAC didn’t change anything,” observed Dr. Lloyd-Jones, professor and chair of the department of preventive medicine at Northwestern University, Chicago.
“So CAC really is for those intermediate-risk folks where we’re on the bubble, where we might consider withholding therapy. And I’d love to see a trial to show that’s safe, by the way, but maybe that day will come. But we certainly would be comfortable up-classifying somebody if more CAC is present than there should be,” he added.
Dr. Waheed reported having no financial conflicts regarding his study.
ORLANDO – Combining the coronary artery calcium score with the ACC/AHA cardiovascular risk calculator tool enables physicians to refine their decision making about who to recommend for statin therapy, Dr. Salman Waheed reported at the American Heart Association scientific sessions.
He presented an analysis of 1,225 asymptomatic adults followed for a median of 3.9 years in the observational arm of the St. Francis Health Study, an early landmark prospective study of the relationship between electron beam CT coronary artery calcium (CAC) score and cardiovascular event risk.
The 217 subjects who today would not be recommended for statin therapy on the basis of a 10-year atherosclerotic cardiovascular disease risk of 5%-7.4% as determined by the risk calculator included in the 2013 ACC/AHA cholesterol management guidelines (Circulation. 2014 Jun 24;129[25 Suppl 2]:S1-45) would be reclassified as warranting statin therapy if they had a CAC greater than 0, as was the case for 169 of the 217 (78%). Indeed, the presence of a CAC of 1 or more boosted their estimated 10-year risk to 10.8%.
On the other hand, there were 510 patients who would be classified as high risk by the ACC/AHA clinical risk calculator, with a 10-year risk of 7.5%-20%. Taking their CAC score into account would result in 73 being reclassified as low risk and becoming no longer statin candidates because their CAC of 0 was associated with a 10-year event risk of less than 1%. In contrast, for the 447 remaining subjects with a CAC greater than 0, the 10-year risk climbed to 21.9%, according to Dr. Waheed of the University of Kansas, Kansas City.
The composite outcome utilized in this analysis from the St. Francis Heart Study was comprised of nonfatal MI, coronary death, stroke, peripheral arterial revascularization, or coronary revascularization. Of note, heart failure wasn’t included.
Among the 545 subjects deemed at low cardiovascular risk because they weren’t eligible for statin therapy according to the 2013 ACC/AHA guidelines, 209 would be recategorized as high risk on the basis of a CAC score at or above the 80th percentile adjusted for age and gender. The adjusted risk of a cardiovascular event in the high CAC/low clinical risk group was 24.9-fold greater than in the low CAC/low clinical risk group.

“Among those eligible for statin therapy based upon current guidelines, high CAC portends a sixfold higher outcome risk than low CAC,” Dr. Waheed added.
The magnitude of CAC progression over the course of 4 years was similar across the baseline risk categories; however, the absolute CAC progression was greater among those with a high CAC at baseline.
Audience member Dr. Daniel S. Berman observed that the results of Dr. Waheed’s study are highly concordant with an earlier report from the Multi-Ethnic Study of Atherosclerosis in which a CAC of 0 was quite common in patients for whom statins would be recommended under the current ACC/AHA guidelines.
“Your data support the idea that stratification based upon CAC could more personalize the statin recommendations,” said Dr. Berman, chief of cardiac imaging and nuclear cardiology at Cedars-Sinai Medical Center and professor of medicine at the University of California, Los Angeles.
Another audience member, Dr. Donald M. Lloyd-Jones, one of the architects of the current guidelines, commented that the analyses from the St. Francis Heart Study and the Multi-Ethnic Study of Atherosclerosis suggest CAC screening coupled with the ACC/AHA risk score provides added value in a select group of patients.
“Your analysis continues to reinforce the point that we probably shouldn’t be doing universal CAC screening because in the people with a 10-year risk of less than 5% the yield is low and the CAC didn’t change anything, while in the people with a 10-year risk of 20% or higher the yield is incredibly high and the CAC didn’t change anything,” observed Dr. Lloyd-Jones, professor and chair of the department of preventive medicine at Northwestern University, Chicago.
“So CAC really is for those intermediate-risk folks where we’re on the bubble, where we might consider withholding therapy. And I’d love to see a trial to show that’s safe, by the way, but maybe that day will come. But we certainly would be comfortable up-classifying somebody if more CAC is present than there should be,” he added.
Dr. Waheed reported having no financial conflicts regarding his study.
ORLANDO – Combining the coronary artery calcium score with the ACC/AHA cardiovascular risk calculator tool enables physicians to refine their decision making about who to recommend for statin therapy, Dr. Salman Waheed reported at the American Heart Association scientific sessions.
He presented an analysis of 1,225 asymptomatic adults followed for a median of 3.9 years in the observational arm of the St. Francis Health Study, an early landmark prospective study of the relationship between electron beam CT coronary artery calcium (CAC) score and cardiovascular event risk.
The 217 subjects who today would not be recommended for statin therapy on the basis of a 10-year atherosclerotic cardiovascular disease risk of 5%-7.4% as determined by the risk calculator included in the 2013 ACC/AHA cholesterol management guidelines (Circulation. 2014 Jun 24;129[25 Suppl 2]:S1-45) would be reclassified as warranting statin therapy if they had a CAC greater than 0, as was the case for 169 of the 217 (78%). Indeed, the presence of a CAC of 1 or more boosted their estimated 10-year risk to 10.8%.
On the other hand, there were 510 patients who would be classified as high risk by the ACC/AHA clinical risk calculator, with a 10-year risk of 7.5%-20%. Taking their CAC score into account would result in 73 being reclassified as low risk and becoming no longer statin candidates because their CAC of 0 was associated with a 10-year event risk of less than 1%. In contrast, for the 447 remaining subjects with a CAC greater than 0, the 10-year risk climbed to 21.9%, according to Dr. Waheed of the University of Kansas, Kansas City.
The composite outcome utilized in this analysis from the St. Francis Heart Study was comprised of nonfatal MI, coronary death, stroke, peripheral arterial revascularization, or coronary revascularization. Of note, heart failure wasn’t included.
Among the 545 subjects deemed at low cardiovascular risk because they weren’t eligible for statin therapy according to the 2013 ACC/AHA guidelines, 209 would be recategorized as high risk on the basis of a CAC score at or above the 80th percentile adjusted for age and gender. The adjusted risk of a cardiovascular event in the high CAC/low clinical risk group was 24.9-fold greater than in the low CAC/low clinical risk group.

“Among those eligible for statin therapy based upon current guidelines, high CAC portends a sixfold higher outcome risk than low CAC,” Dr. Waheed added.
The magnitude of CAC progression over the course of 4 years was similar across the baseline risk categories; however, the absolute CAC progression was greater among those with a high CAC at baseline.
Audience member Dr. Daniel S. Berman observed that the results of Dr. Waheed’s study are highly concordant with an earlier report from the Multi-Ethnic Study of Atherosclerosis in which a CAC of 0 was quite common in patients for whom statins would be recommended under the current ACC/AHA guidelines.
“Your data support the idea that stratification based upon CAC could more personalize the statin recommendations,” said Dr. Berman, chief of cardiac imaging and nuclear cardiology at Cedars-Sinai Medical Center and professor of medicine at the University of California, Los Angeles.
Another audience member, Dr. Donald M. Lloyd-Jones, one of the architects of the current guidelines, commented that the analyses from the St. Francis Heart Study and the Multi-Ethnic Study of Atherosclerosis suggest CAC screening coupled with the ACC/AHA risk score provides added value in a select group of patients.
“Your analysis continues to reinforce the point that we probably shouldn’t be doing universal CAC screening because in the people with a 10-year risk of less than 5% the yield is low and the CAC didn’t change anything, while in the people with a 10-year risk of 20% or higher the yield is incredibly high and the CAC didn’t change anything,” observed Dr. Lloyd-Jones, professor and chair of the department of preventive medicine at Northwestern University, Chicago.
“So CAC really is for those intermediate-risk folks where we’re on the bubble, where we might consider withholding therapy. And I’d love to see a trial to show that’s safe, by the way, but maybe that day will come. But we certainly would be comfortable up-classifying somebody if more CAC is present than there should be,” he added.
Dr. Waheed reported having no financial conflicts regarding his study.
AT THE AHA SCIENTIFIC SESSIONS
Key clinical point: Measuring coronary artery calcium provides added value in refining the 10-year atherosclerotic cardiovascular disease risk, especially in patients with an intermediate-risk score on the ACC/AHA risk calculator.
Major finding: Seventy-eight percent of a group of patients for whom statin therapy wouldn’t be recommended under the current ACC/AHA guidelines because their estimated 10-year event risk was 5%-7.5% would be reclassified as warranting statin therapy because their coronary artery calcium score was greater than 0, pushing their estimated risk to 10.8%.
Data source: A retrospective analysis of data on 1,225 asymptomatic adults followed prospectively with coronary artery calcium measurements in the St. Francis Health Study.
Disclosures: The study presenter reported having no financial conflicts of interest.