User login
Held every two years International Headache Society (IHS): International Headache Congress (IHC 2015)
IHC: IV dihydroergotamine linked to increased thrombosis risk
VALENCIA, SPAIN – Inpatient high-dose intravenous dihydroergotamine delivered through a peripherally inserted central catheter or midline catheter for treatment of refractory headache appears to be associated with an increased risk of venous thrombosis, according to a retrospective case-control study.
“In patients with PICC or midline catheter placement, a low threshold for consideration of ultrasound investigation and consideration of prophylactic anticoagulation are appropriate. But patients with peripheral IVs had a very low thrombosis rate, so it seems reasonable to continue not to anticoagulate these patients,” Dr. Amy Tso said at the International Headache Congress.
Most headache centers rely upon a 5-day inpatient course of IV dihydroergotamine (DHE) as their workhorse therapy for the most refractory patients with chronic migraine or chronic cluster headache. But at the Headache Center of the University of California, San Francisco, where this internationally popular regimen was pioneered (Neurology 2011;77:1827-32), investigators have concluded that the risk of venous thrombosis when the drug is delivered by PICC or midline catheter is suspiciously high in what is a generally healthy ambulatory patient population aside from their severe headaches.
Dr. Tso, a neurologist at the university, presented a new retrospective analysis of 604 consecutive admissions for this therapy in 450 adult and pediatric patients. ‘Venous thrombosis occurred in 6.8% of 264 severe headache patients whose dihydroergotamine was administered through a PICC line or midline catheter. The venous thrombosis rate was 6.4% in 157 patients treated through a PICC line and 7.5% in 107 whose DHE was given through a midline catheter. Eleven of the 18 affected patients had deep vein thrombosis warranting 3 months of oral anticoagulation, and 2 others developed pulmonary embolism.
In contrast, venous thrombosis occurred in 1 of 272 patients treated via a peripheral IV line.
In this study, venous thrombosis risk was unrelated to total DHE dose, age, gender, or the presence or absence of aura.
Since most studies of VTE risk in patients with a PICC or midline catheter have been conducted in cancer patients getting chemotherapy, patients on antibiotics for serious infections, or individuals on total parenteral nutrition – populations very different from headache patients – Dr. Tso opted to use as a control group for her study of 56 headache patients who received a 10-day inpatient course of IV lidocaine at the UCSF Headache Center; not one of them developed venous thrombosis, regardless of whether the lidocaine was given through a PICC, peripheral IV line, or midline catheter, she reported at the meeting, which was sponsored by the International Headache Society and the American Headache Society.
Physicians routinely use a peripheral IV line at the start of the 5-day course of inpatient DHE, during which patients receive 1 mg of the drug every 8 hours. However, DHE is a potent vasoconstrictor, and when the veins clamp down, as occurs often, it’s common practice to switch to a PICC or midline catheter to maintain vascular access and continue treatment.
“I certainly don’t think this new information about venous thrombosis risk would lead us to not give the therapy. The data supporting the use of this protocol in treating chronic migraine and chronic cluster headache have established that it’s so useful,” she said in an interview. “But I’ll have more reticence to put in a central line, and when I do I’ll have a lower threshold for getting an ultrasound when a patient complains of discomfort.”
Dr. Tso reported having no financial conflicts regarding this study.
VALENCIA, SPAIN – Inpatient high-dose intravenous dihydroergotamine delivered through a peripherally inserted central catheter or midline catheter for treatment of refractory headache appears to be associated with an increased risk of venous thrombosis, according to a retrospective case-control study.
“In patients with PICC or midline catheter placement, a low threshold for consideration of ultrasound investigation and consideration of prophylactic anticoagulation are appropriate. But patients with peripheral IVs had a very low thrombosis rate, so it seems reasonable to continue not to anticoagulate these patients,” Dr. Amy Tso said at the International Headache Congress.
Most headache centers rely upon a 5-day inpatient course of IV dihydroergotamine (DHE) as their workhorse therapy for the most refractory patients with chronic migraine or chronic cluster headache. But at the Headache Center of the University of California, San Francisco, where this internationally popular regimen was pioneered (Neurology 2011;77:1827-32), investigators have concluded that the risk of venous thrombosis when the drug is delivered by PICC or midline catheter is suspiciously high in what is a generally healthy ambulatory patient population aside from their severe headaches.
Dr. Tso, a neurologist at the university, presented a new retrospective analysis of 604 consecutive admissions for this therapy in 450 adult and pediatric patients. ‘Venous thrombosis occurred in 6.8% of 264 severe headache patients whose dihydroergotamine was administered through a PICC line or midline catheter. The venous thrombosis rate was 6.4% in 157 patients treated through a PICC line and 7.5% in 107 whose DHE was given through a midline catheter. Eleven of the 18 affected patients had deep vein thrombosis warranting 3 months of oral anticoagulation, and 2 others developed pulmonary embolism.
In contrast, venous thrombosis occurred in 1 of 272 patients treated via a peripheral IV line.
In this study, venous thrombosis risk was unrelated to total DHE dose, age, gender, or the presence or absence of aura.
Since most studies of VTE risk in patients with a PICC or midline catheter have been conducted in cancer patients getting chemotherapy, patients on antibiotics for serious infections, or individuals on total parenteral nutrition – populations very different from headache patients – Dr. Tso opted to use as a control group for her study of 56 headache patients who received a 10-day inpatient course of IV lidocaine at the UCSF Headache Center; not one of them developed venous thrombosis, regardless of whether the lidocaine was given through a PICC, peripheral IV line, or midline catheter, she reported at the meeting, which was sponsored by the International Headache Society and the American Headache Society.
Physicians routinely use a peripheral IV line at the start of the 5-day course of inpatient DHE, during which patients receive 1 mg of the drug every 8 hours. However, DHE is a potent vasoconstrictor, and when the veins clamp down, as occurs often, it’s common practice to switch to a PICC or midline catheter to maintain vascular access and continue treatment.
“I certainly don’t think this new information about venous thrombosis risk would lead us to not give the therapy. The data supporting the use of this protocol in treating chronic migraine and chronic cluster headache have established that it’s so useful,” she said in an interview. “But I’ll have more reticence to put in a central line, and when I do I’ll have a lower threshold for getting an ultrasound when a patient complains of discomfort.”
Dr. Tso reported having no financial conflicts regarding this study.
VALENCIA, SPAIN – Inpatient high-dose intravenous dihydroergotamine delivered through a peripherally inserted central catheter or midline catheter for treatment of refractory headache appears to be associated with an increased risk of venous thrombosis, according to a retrospective case-control study.
“In patients with PICC or midline catheter placement, a low threshold for consideration of ultrasound investigation and consideration of prophylactic anticoagulation are appropriate. But patients with peripheral IVs had a very low thrombosis rate, so it seems reasonable to continue not to anticoagulate these patients,” Dr. Amy Tso said at the International Headache Congress.
Most headache centers rely upon a 5-day inpatient course of IV dihydroergotamine (DHE) as their workhorse therapy for the most refractory patients with chronic migraine or chronic cluster headache. But at the Headache Center of the University of California, San Francisco, where this internationally popular regimen was pioneered (Neurology 2011;77:1827-32), investigators have concluded that the risk of venous thrombosis when the drug is delivered by PICC or midline catheter is suspiciously high in what is a generally healthy ambulatory patient population aside from their severe headaches.
Dr. Tso, a neurologist at the university, presented a new retrospective analysis of 604 consecutive admissions for this therapy in 450 adult and pediatric patients. ‘Venous thrombosis occurred in 6.8% of 264 severe headache patients whose dihydroergotamine was administered through a PICC line or midline catheter. The venous thrombosis rate was 6.4% in 157 patients treated through a PICC line and 7.5% in 107 whose DHE was given through a midline catheter. Eleven of the 18 affected patients had deep vein thrombosis warranting 3 months of oral anticoagulation, and 2 others developed pulmonary embolism.
In contrast, venous thrombosis occurred in 1 of 272 patients treated via a peripheral IV line.
In this study, venous thrombosis risk was unrelated to total DHE dose, age, gender, or the presence or absence of aura.
Since most studies of VTE risk in patients with a PICC or midline catheter have been conducted in cancer patients getting chemotherapy, patients on antibiotics for serious infections, or individuals on total parenteral nutrition – populations very different from headache patients – Dr. Tso opted to use as a control group for her study of 56 headache patients who received a 10-day inpatient course of IV lidocaine at the UCSF Headache Center; not one of them developed venous thrombosis, regardless of whether the lidocaine was given through a PICC, peripheral IV line, or midline catheter, she reported at the meeting, which was sponsored by the International Headache Society and the American Headache Society.
Physicians routinely use a peripheral IV line at the start of the 5-day course of inpatient DHE, during which patients receive 1 mg of the drug every 8 hours. However, DHE is a potent vasoconstrictor, and when the veins clamp down, as occurs often, it’s common practice to switch to a PICC or midline catheter to maintain vascular access and continue treatment.
“I certainly don’t think this new information about venous thrombosis risk would lead us to not give the therapy. The data supporting the use of this protocol in treating chronic migraine and chronic cluster headache have established that it’s so useful,” she said in an interview. “But I’ll have more reticence to put in a central line, and when I do I’ll have a lower threshold for getting an ultrasound when a patient complains of discomfort.”
Dr. Tso reported having no financial conflicts regarding this study.
AT IHC 2015
Key clinical point: Multiday inpatient IV dihydroergotamine delivered by PICC or midline catheter carries an increased risk of venous thrombosis.
Major finding: Venous thrombosis occurred in 6.8% of 264 severe headache patients whose dihydroergotamine was administered through a PICC line or midline catheter.
Data source: A retrospective, single-center, case-control study of 604 admissions involving 450 patients with chronic refractory headache who underwent a 5-day inpatient course of IV dihydroergotamine.
Disclosures: Dr. Tso reported no financial conflicts regarding this study.
Patient unhappiness with migraine prophylaxis options abounds
VALENCIA, SPAIN – How dissatisfied are migraine patients with their prophylactic medication options?
Here’s a clue: Roughly two-thirds of migraine patients who initiate topiramate, a beta-blocker, or a tricyclic antidepressant for prophylaxis have given up on prophylactic therapy altogether within 9 months, Dr. Robert Lenz reported at the International Headache Congress.
Moreover, treatment gaps longer than 90 days in duration are the rule rather than the exception within the first year after starting prophylactic therapy. Seventy-eight percent of patients who start on topiramate for migraine prophylaxis have such a gap in therapy within the first year, as do 80% who start on a beta-blocker, and 85% on a tricyclic antidepressant. These prolonged treatment gaps occur early: a mean of 95 days after commencing treatment. And only 9%-12% of patients restart prophylaxis after a gap, added Dr. Lenz of Amgen in Thousand Oaks, Calif.
He presented an analysis of patterns of prophylaxis use in 107,122 migraine patients who initiated therapy during 2008-2011. Forty-nine percent went on topiramate for this purpose, 30% on a tricyclic antidepressant, and 21% on a beta-blocker. The researchers used Medicare claims data as well as information from the Truven Health Analytics MarketScan Commercial Claims and Encounters database.
Despite the generally poor persistence with prophylactic therapy documented in this study, few patients were interested in switching to a different preventive medication or adding a second one. Indeed, only 12%-14% of patients did so within the first year after starting prophylaxis, Dr. Lenz noted at the meeting, which was sponsored by the International Headache Society and the American Headache Society.
Eighty-one percent of patients who started on migraine prophylaxis also used acute migraine medications during the first year. Forty-eight percent of the study population filled an average of 4.4 prescriptions for a triptan during that period, whereas 38% used a triptan in the year prior to going on prophylaxis.
More disturbingly, Dr. Lenz said, 53% of patients who initiated prophylactic migraine therapy also received opioids during that first year; indeed, they filled an average of 6.1 prescriptions for opioids during the year. However, upon exclusion of patients who carried a diagnosis of another chronic pain condition, such as low back pain, arthritis, or neuropathic pain, 40% of the remaining patients who started migraine prophylaxis received opioids during the study year, filling an average of 3.8 prescriptions.
The study was funded by Dr. Lenz’ employer, Amgen, which is among the pharmaceutical companies developing a new class of monoclonal antibodies to calcitonin gene-related peptide (CGRP) for the prevention of migraine.
VALENCIA, SPAIN – How dissatisfied are migraine patients with their prophylactic medication options?
Here’s a clue: Roughly two-thirds of migraine patients who initiate topiramate, a beta-blocker, or a tricyclic antidepressant for prophylaxis have given up on prophylactic therapy altogether within 9 months, Dr. Robert Lenz reported at the International Headache Congress.
Moreover, treatment gaps longer than 90 days in duration are the rule rather than the exception within the first year after starting prophylactic therapy. Seventy-eight percent of patients who start on topiramate for migraine prophylaxis have such a gap in therapy within the first year, as do 80% who start on a beta-blocker, and 85% on a tricyclic antidepressant. These prolonged treatment gaps occur early: a mean of 95 days after commencing treatment. And only 9%-12% of patients restart prophylaxis after a gap, added Dr. Lenz of Amgen in Thousand Oaks, Calif.
He presented an analysis of patterns of prophylaxis use in 107,122 migraine patients who initiated therapy during 2008-2011. Forty-nine percent went on topiramate for this purpose, 30% on a tricyclic antidepressant, and 21% on a beta-blocker. The researchers used Medicare claims data as well as information from the Truven Health Analytics MarketScan Commercial Claims and Encounters database.
Despite the generally poor persistence with prophylactic therapy documented in this study, few patients were interested in switching to a different preventive medication or adding a second one. Indeed, only 12%-14% of patients did so within the first year after starting prophylaxis, Dr. Lenz noted at the meeting, which was sponsored by the International Headache Society and the American Headache Society.
Eighty-one percent of patients who started on migraine prophylaxis also used acute migraine medications during the first year. Forty-eight percent of the study population filled an average of 4.4 prescriptions for a triptan during that period, whereas 38% used a triptan in the year prior to going on prophylaxis.
More disturbingly, Dr. Lenz said, 53% of patients who initiated prophylactic migraine therapy also received opioids during that first year; indeed, they filled an average of 6.1 prescriptions for opioids during the year. However, upon exclusion of patients who carried a diagnosis of another chronic pain condition, such as low back pain, arthritis, or neuropathic pain, 40% of the remaining patients who started migraine prophylaxis received opioids during the study year, filling an average of 3.8 prescriptions.
The study was funded by Dr. Lenz’ employer, Amgen, which is among the pharmaceutical companies developing a new class of monoclonal antibodies to calcitonin gene-related peptide (CGRP) for the prevention of migraine.
VALENCIA, SPAIN – How dissatisfied are migraine patients with their prophylactic medication options?
Here’s a clue: Roughly two-thirds of migraine patients who initiate topiramate, a beta-blocker, or a tricyclic antidepressant for prophylaxis have given up on prophylactic therapy altogether within 9 months, Dr. Robert Lenz reported at the International Headache Congress.
Moreover, treatment gaps longer than 90 days in duration are the rule rather than the exception within the first year after starting prophylactic therapy. Seventy-eight percent of patients who start on topiramate for migraine prophylaxis have such a gap in therapy within the first year, as do 80% who start on a beta-blocker, and 85% on a tricyclic antidepressant. These prolonged treatment gaps occur early: a mean of 95 days after commencing treatment. And only 9%-12% of patients restart prophylaxis after a gap, added Dr. Lenz of Amgen in Thousand Oaks, Calif.
He presented an analysis of patterns of prophylaxis use in 107,122 migraine patients who initiated therapy during 2008-2011. Forty-nine percent went on topiramate for this purpose, 30% on a tricyclic antidepressant, and 21% on a beta-blocker. The researchers used Medicare claims data as well as information from the Truven Health Analytics MarketScan Commercial Claims and Encounters database.
Despite the generally poor persistence with prophylactic therapy documented in this study, few patients were interested in switching to a different preventive medication or adding a second one. Indeed, only 12%-14% of patients did so within the first year after starting prophylaxis, Dr. Lenz noted at the meeting, which was sponsored by the International Headache Society and the American Headache Society.
Eighty-one percent of patients who started on migraine prophylaxis also used acute migraine medications during the first year. Forty-eight percent of the study population filled an average of 4.4 prescriptions for a triptan during that period, whereas 38% used a triptan in the year prior to going on prophylaxis.
More disturbingly, Dr. Lenz said, 53% of patients who initiated prophylactic migraine therapy also received opioids during that first year; indeed, they filled an average of 6.1 prescriptions for opioids during the year. However, upon exclusion of patients who carried a diagnosis of another chronic pain condition, such as low back pain, arthritis, or neuropathic pain, 40% of the remaining patients who started migraine prophylaxis received opioids during the study year, filling an average of 3.8 prescriptions.
The study was funded by Dr. Lenz’ employer, Amgen, which is among the pharmaceutical companies developing a new class of monoclonal antibodies to calcitonin gene-related peptide (CGRP) for the prevention of migraine.
AT IHC 2015
Key clinical point: Migraine patients are deeply dissatisfied with their current options for prophylactic therapy.
Major finding: About one-third of patients who initiate prophylactic drug therapy for migraine are still on preventive therapy 9 months later.
Data source: An analysis of claims data for more than 107,000 U.S. patients who initiated prophylactic migraine therapy.
Disclosures: The study was sponsored by Amgen and presented by a company employee.
Botox effective in pediatric chronic migraine
VALENCIA, SPAIN – OnabotulinumtoxinA injections are safe, well tolerated, and effective for chronic migraine in adolescents refractory to medications, Dr. Ilya Bragin reported at the International Headache Congress.
“Overall, botulinum toxin type A proved to be an excellent treatment option for this group,” said Dr. Bragin, a neurologist at the State University of New York Upstate Medical Center at Syracuse.
He reported on 19 adolescents, mean age 15.7 years, seen for chronic migraine at a pediatric headache clinic. All had failed to respond to multiple standard medications, including amitriptyline and topiramate. The teens were treated with onabotulinumtoxinA (Botox) injections using the standard Food and Drug Administration–specified adult protocol.
This was off-label therapy, since Botox is FDA approved for the treatment of headaches only in adults. However, as was noted by numerous speakers at the meeting, which was sponsored by the International Headache Society and the American Headache Society, the great majority of drug prescribing for pediatric headaches is off label.
Indeed, the only FDA-approved medications for pediatric migraine are topiramate (Topamax) and almotriptan (Axert) for use in patients 12 years of age and older, rizatriptan (Maxalt) starting as early as age 6 years, and – as of May 2015 – Treximet, a proprietary sumatriptan/naproxen sodium combination approved for use in patients as young as 12 years of age.
Dr. Bragin reported that after an average of two injection cycles, the adolescents’ migraine severity decreased by a mean of nearly 3 points on a 1-10 visual analog scale, from 7.7 at baseline to 4.8. Mean headache frequency fell from 24.8 days per month to 15.
Drilling down further into the results, 12 patients had severe migraine at baseline as defined by a pain score of 7-10. After treatment, 5 of the 12 were still categorized as severe, 1 moderate, 4 mild – meaning they had a pain score of 1-3 – and 2 patients became headache free.
Among the seven patients with moderate chronic migraine at baseline, five remained in the moderate category post treatment, one became mild, and one patient experienced headache resolution.
Thus, although the study sample size was limited, it appears from these results that adolescents with the most severe chronic migraine at baseline see the most benefit, the neurologist observed.
Dr. Bragin reported having no financial conflicts regarding this study.
VALENCIA, SPAIN – OnabotulinumtoxinA injections are safe, well tolerated, and effective for chronic migraine in adolescents refractory to medications, Dr. Ilya Bragin reported at the International Headache Congress.
“Overall, botulinum toxin type A proved to be an excellent treatment option for this group,” said Dr. Bragin, a neurologist at the State University of New York Upstate Medical Center at Syracuse.
He reported on 19 adolescents, mean age 15.7 years, seen for chronic migraine at a pediatric headache clinic. All had failed to respond to multiple standard medications, including amitriptyline and topiramate. The teens were treated with onabotulinumtoxinA (Botox) injections using the standard Food and Drug Administration–specified adult protocol.
This was off-label therapy, since Botox is FDA approved for the treatment of headaches only in adults. However, as was noted by numerous speakers at the meeting, which was sponsored by the International Headache Society and the American Headache Society, the great majority of drug prescribing for pediatric headaches is off label.
Indeed, the only FDA-approved medications for pediatric migraine are topiramate (Topamax) and almotriptan (Axert) for use in patients 12 years of age and older, rizatriptan (Maxalt) starting as early as age 6 years, and – as of May 2015 – Treximet, a proprietary sumatriptan/naproxen sodium combination approved for use in patients as young as 12 years of age.
Dr. Bragin reported that after an average of two injection cycles, the adolescents’ migraine severity decreased by a mean of nearly 3 points on a 1-10 visual analog scale, from 7.7 at baseline to 4.8. Mean headache frequency fell from 24.8 days per month to 15.
Drilling down further into the results, 12 patients had severe migraine at baseline as defined by a pain score of 7-10. After treatment, 5 of the 12 were still categorized as severe, 1 moderate, 4 mild – meaning they had a pain score of 1-3 – and 2 patients became headache free.
Among the seven patients with moderate chronic migraine at baseline, five remained in the moderate category post treatment, one became mild, and one patient experienced headache resolution.
Thus, although the study sample size was limited, it appears from these results that adolescents with the most severe chronic migraine at baseline see the most benefit, the neurologist observed.
Dr. Bragin reported having no financial conflicts regarding this study.
VALENCIA, SPAIN – OnabotulinumtoxinA injections are safe, well tolerated, and effective for chronic migraine in adolescents refractory to medications, Dr. Ilya Bragin reported at the International Headache Congress.
“Overall, botulinum toxin type A proved to be an excellent treatment option for this group,” said Dr. Bragin, a neurologist at the State University of New York Upstate Medical Center at Syracuse.
He reported on 19 adolescents, mean age 15.7 years, seen for chronic migraine at a pediatric headache clinic. All had failed to respond to multiple standard medications, including amitriptyline and topiramate. The teens were treated with onabotulinumtoxinA (Botox) injections using the standard Food and Drug Administration–specified adult protocol.
This was off-label therapy, since Botox is FDA approved for the treatment of headaches only in adults. However, as was noted by numerous speakers at the meeting, which was sponsored by the International Headache Society and the American Headache Society, the great majority of drug prescribing for pediatric headaches is off label.
Indeed, the only FDA-approved medications for pediatric migraine are topiramate (Topamax) and almotriptan (Axert) for use in patients 12 years of age and older, rizatriptan (Maxalt) starting as early as age 6 years, and – as of May 2015 – Treximet, a proprietary sumatriptan/naproxen sodium combination approved for use in patients as young as 12 years of age.
Dr. Bragin reported that after an average of two injection cycles, the adolescents’ migraine severity decreased by a mean of nearly 3 points on a 1-10 visual analog scale, from 7.7 at baseline to 4.8. Mean headache frequency fell from 24.8 days per month to 15.
Drilling down further into the results, 12 patients had severe migraine at baseline as defined by a pain score of 7-10. After treatment, 5 of the 12 were still categorized as severe, 1 moderate, 4 mild – meaning they had a pain score of 1-3 – and 2 patients became headache free.
Among the seven patients with moderate chronic migraine at baseline, five remained in the moderate category post treatment, one became mild, and one patient experienced headache resolution.
Thus, although the study sample size was limited, it appears from these results that adolescents with the most severe chronic migraine at baseline see the most benefit, the neurologist observed.
Dr. Bragin reported having no financial conflicts regarding this study.
AT IHC 2015
Key clinical point: OnabotulinumtoxinA injections appear to be well tolerated and effective for refractory chronic migraine in adolescents.
Major finding: Mean pain scores on a 1-10 scale improved from 7.7 at baseline to 4.8 after 2 standard injection cycles.
Data source: A retrospective case series of 19 adolescents treated for refractory chronic migraine at a pediatric headache clinic.
Disclosures: The presenter reported having no financial conflicts regarding this unsponsored study.
Worsening migraine in pregnancy linked to adverse outcomes
VALENCIA, SPAIN – Women who present with acute severe migraine during pregnancy are at increased risk for adverse pregnancy outcomes and should be seen in a high-risk pregnancy clinic, Dr. Tracy B. Grossman asserted at the International Headache Congress.
“We should not be seeing these patients in a regular ob.gyn./generalist’s office because oftentimes we need input from neurology, and we need extra surveillance for both the fetus and the mother,” she said at the meeting sponsored by the International Headache Society and the American Headache Society.
Dr. Grossman presented a retrospective study of 90 consecutive pregnant patients who presented with acute severe migraine and obtained a neurology consult at Montefiore Medical Center, New York, where she is an ob.gyn. resident.
“These patients are different from most migraine patients because most migraine patients actually see improvement of symptoms during pregnancy. So this is a special group of patients with worsening and refractory migraine,” she noted in an interview.
Most were in their third trimester and had migraine without aura. Twenty-four presented with status migrainosus, a migraine for 15 or more days a month for more than 3 months.
Forty-nine of the 90 patients (54%) experienced one or more adverse pregnancy outcomes. Of note, the 28% preterm delivery rate was nearly three times the national average of 11% as reported by the March of Dimes. The preeclampsia rate was 20.5%, compared with a national rate of just 3%-4%. The 19.2% low birth weight rate was more than double the 8% national average. The cesarean section rate was 30.8%.
The study hypothesis was that the migraine-with-aura group would have higher preeclampsia, preterm delivery, and low birth weight rates, as has been reported by some other investigators in what is a sparse literature. Not so, Dr. Grossman said, because most of these patients didn’t have aura.
“So it can’t be purely an aura/vascular phenomenon that’s resulting in these adverse outcomes. These high rates of adverse pregnancy outcomes aren’t easily explainable. There’s something going on here that we haven’t teased out yet as to why these migraine patients are special,” she continued.
Their risk of adverse pregnancy outcomes wasn’t related to the headache medications they took. Sixty-two patients received a combination of oral and intravenous therapy with acetaminophen, metoclopramide, and dihydroergotamine. But 30% of patients were briefly on barbiturates, and 30% were on oxycodone or codeine; these are drugs of concern during pregnancy, yet there was no associated increase in adverse pregnancy outcomes, compared with the women who weren’t on those drugs or who indeed weren’t on any headache medications at all.
Dr. Grossman’s own therapeutic preference in patients with severe migraine in pregnancy is a peripheral nerve block with bupivacaine and lidocaine.
“It works for the majority of people – we don’t quite know why – and it’s a local therapy that avoids fetal exposure to systemic medications,” she observed.
Dr. Grossman reported no financial conflicts with regard to her study, which was carried out without industry support.
VALENCIA, SPAIN – Women who present with acute severe migraine during pregnancy are at increased risk for adverse pregnancy outcomes and should be seen in a high-risk pregnancy clinic, Dr. Tracy B. Grossman asserted at the International Headache Congress.
“We should not be seeing these patients in a regular ob.gyn./generalist’s office because oftentimes we need input from neurology, and we need extra surveillance for both the fetus and the mother,” she said at the meeting sponsored by the International Headache Society and the American Headache Society.
Dr. Grossman presented a retrospective study of 90 consecutive pregnant patients who presented with acute severe migraine and obtained a neurology consult at Montefiore Medical Center, New York, where she is an ob.gyn. resident.
“These patients are different from most migraine patients because most migraine patients actually see improvement of symptoms during pregnancy. So this is a special group of patients with worsening and refractory migraine,” she noted in an interview.
Most were in their third trimester and had migraine without aura. Twenty-four presented with status migrainosus, a migraine for 15 or more days a month for more than 3 months.
Forty-nine of the 90 patients (54%) experienced one or more adverse pregnancy outcomes. Of note, the 28% preterm delivery rate was nearly three times the national average of 11% as reported by the March of Dimes. The preeclampsia rate was 20.5%, compared with a national rate of just 3%-4%. The 19.2% low birth weight rate was more than double the 8% national average. The cesarean section rate was 30.8%.
The study hypothesis was that the migraine-with-aura group would have higher preeclampsia, preterm delivery, and low birth weight rates, as has been reported by some other investigators in what is a sparse literature. Not so, Dr. Grossman said, because most of these patients didn’t have aura.
“So it can’t be purely an aura/vascular phenomenon that’s resulting in these adverse outcomes. These high rates of adverse pregnancy outcomes aren’t easily explainable. There’s something going on here that we haven’t teased out yet as to why these migraine patients are special,” she continued.
Their risk of adverse pregnancy outcomes wasn’t related to the headache medications they took. Sixty-two patients received a combination of oral and intravenous therapy with acetaminophen, metoclopramide, and dihydroergotamine. But 30% of patients were briefly on barbiturates, and 30% were on oxycodone or codeine; these are drugs of concern during pregnancy, yet there was no associated increase in adverse pregnancy outcomes, compared with the women who weren’t on those drugs or who indeed weren’t on any headache medications at all.
Dr. Grossman’s own therapeutic preference in patients with severe migraine in pregnancy is a peripheral nerve block with bupivacaine and lidocaine.
“It works for the majority of people – we don’t quite know why – and it’s a local therapy that avoids fetal exposure to systemic medications,” she observed.
Dr. Grossman reported no financial conflicts with regard to her study, which was carried out without industry support.
VALENCIA, SPAIN – Women who present with acute severe migraine during pregnancy are at increased risk for adverse pregnancy outcomes and should be seen in a high-risk pregnancy clinic, Dr. Tracy B. Grossman asserted at the International Headache Congress.
“We should not be seeing these patients in a regular ob.gyn./generalist’s office because oftentimes we need input from neurology, and we need extra surveillance for both the fetus and the mother,” she said at the meeting sponsored by the International Headache Society and the American Headache Society.
Dr. Grossman presented a retrospective study of 90 consecutive pregnant patients who presented with acute severe migraine and obtained a neurology consult at Montefiore Medical Center, New York, where she is an ob.gyn. resident.
“These patients are different from most migraine patients because most migraine patients actually see improvement of symptoms during pregnancy. So this is a special group of patients with worsening and refractory migraine,” she noted in an interview.
Most were in their third trimester and had migraine without aura. Twenty-four presented with status migrainosus, a migraine for 15 or more days a month for more than 3 months.
Forty-nine of the 90 patients (54%) experienced one or more adverse pregnancy outcomes. Of note, the 28% preterm delivery rate was nearly three times the national average of 11% as reported by the March of Dimes. The preeclampsia rate was 20.5%, compared with a national rate of just 3%-4%. The 19.2% low birth weight rate was more than double the 8% national average. The cesarean section rate was 30.8%.
The study hypothesis was that the migraine-with-aura group would have higher preeclampsia, preterm delivery, and low birth weight rates, as has been reported by some other investigators in what is a sparse literature. Not so, Dr. Grossman said, because most of these patients didn’t have aura.
“So it can’t be purely an aura/vascular phenomenon that’s resulting in these adverse outcomes. These high rates of adverse pregnancy outcomes aren’t easily explainable. There’s something going on here that we haven’t teased out yet as to why these migraine patients are special,” she continued.
Their risk of adverse pregnancy outcomes wasn’t related to the headache medications they took. Sixty-two patients received a combination of oral and intravenous therapy with acetaminophen, metoclopramide, and dihydroergotamine. But 30% of patients were briefly on barbiturates, and 30% were on oxycodone or codeine; these are drugs of concern during pregnancy, yet there was no associated increase in adverse pregnancy outcomes, compared with the women who weren’t on those drugs or who indeed weren’t on any headache medications at all.
Dr. Grossman’s own therapeutic preference in patients with severe migraine in pregnancy is a peripheral nerve block with bupivacaine and lidocaine.
“It works for the majority of people – we don’t quite know why – and it’s a local therapy that avoids fetal exposure to systemic medications,” she observed.
Dr. Grossman reported no financial conflicts with regard to her study, which was carried out without industry support.
AT IHC 2015
Key clinical point: Patients who present with acute severe migraine during pregnancy should be seen in a high-risk pregnancy clinic.
Major finding: Forty-nine of 90 (54%) consecutive pregnant women who presented with acute severe migraine subsequently had one or more adverse pregnancy outcomes.
Data source: This was a retrospective single-center study.
Disclosures: Dr. Grossman reported having no financial conflicts regarding this study, carried out free of commercial support.
IHC: In medication overuse headache, think ‘stress reduction’
VALENCIA, SPAIN – Medication overuse headache is strongly associated with sky-high stress levels and several unhealthy – yet modifiable – lifestyle behaviors, according to a large Danish population-based study.
“High stress plus smoking, low physical activity, or obesity has synergistic effects in medication overuse headache. So stress reduction is highly relevant in medication overuse headache [MOH] management,” Dr. Rigmor H. Jensen reported at the International Headache Congress.
Stopping the offending medications remains central to the successful treatment of MOH. And while bearing in mind that association doesn’t prove causality, the findings of the Danish study suggest that stress reduction and lifestyle modification, in addition to their many recognized mental and physical health benefits, might also have some MOH-specific effects, according to Dr. Jensen, professor of neurology and director of the Danish Headache Center at the University of Copenhagen.
She presented the results of a questionnaire survey sent to a representative sample comprising 129,150 Danish adults. The survey focused on headache symptoms, lifestyle, and stress as measured by the validated 10-question perceived stress scale. The survey response rate was 53%, a figure so high as to likely elicit envy among U.S. researchers.
A total of 3.4% of the 68,518 respondents were classified as having chronic headache based upon self-report of headache on at least 15 days per month for 3 months. This group was then further categorized as having MOH by the standard International Classification of Headache Disorders definition – as did 1.8% of the total study population – or chronic headache without medication overuse.
Of adults with MOH, 58% scored in the top fifth of the total study population in terms of stress level. Those with chronic headache without medication overuse were less well represented at the high end of the stress spectrum: 46% of them were in the top stress quintile, as were 18% of people without chronic headache, she reported at the meeting sponsored by the International Headache Society and the American Headache Society.
Dr. Jensen and coinvestigators examined the relationship between MOH, stress level, and five unhealthy lifestyle behaviors: daily smoking, excessive alcohol intake, physical inactivity, obesity, and illicit drug use. In multivariate logistic regression analysis, smoking, physical inactivity, and obesity were strongly associated with MOH, while excessive drinking and illicit drug use were not.
Women in the top quintile for stress were 3.8-fold more likely to have MOH if they were a smoker than if they smoked but were in any of the four lower-stress quintiles, 3.5-fold more likely to have MOH if sedentary and high rather than lower stress, and 2.9-fold more likely if obese. For men in the top quintile for stress, the respective odds ratios for MOH were all in the 5-5.6 range for the three lifestyles.
After controlling for stress level, the odds of having MOH were greatest among individuals with all three unhealthy behaviors, compared with those with none: a 5.1-fold increase among men and 2.8-fold increase in women.
In terms of a theoretic goal for stress reduction that might be a useful target as part of the long-term management of MOH, it appears from the Danish data that patients wouldn’t need to attain super-relaxed, below-average stress levels. The independent association between stress and MOH was statistically significant only for individuals in the top two stress quintiles. Men in the fifth or top quintile for stress were 10.3-fold more likely to have MOH than those in the first quintile, while those in the fourth quintile were 4.3-fold more likely to have MOH than those in the first. In women the associations were less dramatic: a 3.9-fold increased risk of MOH if they were in the fifth stress quintile and a 2-fold increase in the fourth.
This study was funded by Danish governmental research agencies. Dr. Jensen reported having no financial conflicts.
VALENCIA, SPAIN – Medication overuse headache is strongly associated with sky-high stress levels and several unhealthy – yet modifiable – lifestyle behaviors, according to a large Danish population-based study.
“High stress plus smoking, low physical activity, or obesity has synergistic effects in medication overuse headache. So stress reduction is highly relevant in medication overuse headache [MOH] management,” Dr. Rigmor H. Jensen reported at the International Headache Congress.
Stopping the offending medications remains central to the successful treatment of MOH. And while bearing in mind that association doesn’t prove causality, the findings of the Danish study suggest that stress reduction and lifestyle modification, in addition to their many recognized mental and physical health benefits, might also have some MOH-specific effects, according to Dr. Jensen, professor of neurology and director of the Danish Headache Center at the University of Copenhagen.
She presented the results of a questionnaire survey sent to a representative sample comprising 129,150 Danish adults. The survey focused on headache symptoms, lifestyle, and stress as measured by the validated 10-question perceived stress scale. The survey response rate was 53%, a figure so high as to likely elicit envy among U.S. researchers.
A total of 3.4% of the 68,518 respondents were classified as having chronic headache based upon self-report of headache on at least 15 days per month for 3 months. This group was then further categorized as having MOH by the standard International Classification of Headache Disorders definition – as did 1.8% of the total study population – or chronic headache without medication overuse.
Of adults with MOH, 58% scored in the top fifth of the total study population in terms of stress level. Those with chronic headache without medication overuse were less well represented at the high end of the stress spectrum: 46% of them were in the top stress quintile, as were 18% of people without chronic headache, she reported at the meeting sponsored by the International Headache Society and the American Headache Society.
Dr. Jensen and coinvestigators examined the relationship between MOH, stress level, and five unhealthy lifestyle behaviors: daily smoking, excessive alcohol intake, physical inactivity, obesity, and illicit drug use. In multivariate logistic regression analysis, smoking, physical inactivity, and obesity were strongly associated with MOH, while excessive drinking and illicit drug use were not.
Women in the top quintile for stress were 3.8-fold more likely to have MOH if they were a smoker than if they smoked but were in any of the four lower-stress quintiles, 3.5-fold more likely to have MOH if sedentary and high rather than lower stress, and 2.9-fold more likely if obese. For men in the top quintile for stress, the respective odds ratios for MOH were all in the 5-5.6 range for the three lifestyles.
After controlling for stress level, the odds of having MOH were greatest among individuals with all three unhealthy behaviors, compared with those with none: a 5.1-fold increase among men and 2.8-fold increase in women.
In terms of a theoretic goal for stress reduction that might be a useful target as part of the long-term management of MOH, it appears from the Danish data that patients wouldn’t need to attain super-relaxed, below-average stress levels. The independent association between stress and MOH was statistically significant only for individuals in the top two stress quintiles. Men in the fifth or top quintile for stress were 10.3-fold more likely to have MOH than those in the first quintile, while those in the fourth quintile were 4.3-fold more likely to have MOH than those in the first. In women the associations were less dramatic: a 3.9-fold increased risk of MOH if they were in the fifth stress quintile and a 2-fold increase in the fourth.
This study was funded by Danish governmental research agencies. Dr. Jensen reported having no financial conflicts.
VALENCIA, SPAIN – Medication overuse headache is strongly associated with sky-high stress levels and several unhealthy – yet modifiable – lifestyle behaviors, according to a large Danish population-based study.
“High stress plus smoking, low physical activity, or obesity has synergistic effects in medication overuse headache. So stress reduction is highly relevant in medication overuse headache [MOH] management,” Dr. Rigmor H. Jensen reported at the International Headache Congress.
Stopping the offending medications remains central to the successful treatment of MOH. And while bearing in mind that association doesn’t prove causality, the findings of the Danish study suggest that stress reduction and lifestyle modification, in addition to their many recognized mental and physical health benefits, might also have some MOH-specific effects, according to Dr. Jensen, professor of neurology and director of the Danish Headache Center at the University of Copenhagen.
She presented the results of a questionnaire survey sent to a representative sample comprising 129,150 Danish adults. The survey focused on headache symptoms, lifestyle, and stress as measured by the validated 10-question perceived stress scale. The survey response rate was 53%, a figure so high as to likely elicit envy among U.S. researchers.
A total of 3.4% of the 68,518 respondents were classified as having chronic headache based upon self-report of headache on at least 15 days per month for 3 months. This group was then further categorized as having MOH by the standard International Classification of Headache Disorders definition – as did 1.8% of the total study population – or chronic headache without medication overuse.
Of adults with MOH, 58% scored in the top fifth of the total study population in terms of stress level. Those with chronic headache without medication overuse were less well represented at the high end of the stress spectrum: 46% of them were in the top stress quintile, as were 18% of people without chronic headache, she reported at the meeting sponsored by the International Headache Society and the American Headache Society.
Dr. Jensen and coinvestigators examined the relationship between MOH, stress level, and five unhealthy lifestyle behaviors: daily smoking, excessive alcohol intake, physical inactivity, obesity, and illicit drug use. In multivariate logistic regression analysis, smoking, physical inactivity, and obesity were strongly associated with MOH, while excessive drinking and illicit drug use were not.
Women in the top quintile for stress were 3.8-fold more likely to have MOH if they were a smoker than if they smoked but were in any of the four lower-stress quintiles, 3.5-fold more likely to have MOH if sedentary and high rather than lower stress, and 2.9-fold more likely if obese. For men in the top quintile for stress, the respective odds ratios for MOH were all in the 5-5.6 range for the three lifestyles.
After controlling for stress level, the odds of having MOH were greatest among individuals with all three unhealthy behaviors, compared with those with none: a 5.1-fold increase among men and 2.8-fold increase in women.
In terms of a theoretic goal for stress reduction that might be a useful target as part of the long-term management of MOH, it appears from the Danish data that patients wouldn’t need to attain super-relaxed, below-average stress levels. The independent association between stress and MOH was statistically significant only for individuals in the top two stress quintiles. Men in the fifth or top quintile for stress were 10.3-fold more likely to have MOH than those in the first quintile, while those in the fourth quintile were 4.3-fold more likely to have MOH than those in the first. In women the associations were less dramatic: a 3.9-fold increased risk of MOH if they were in the fifth stress quintile and a 2-fold increase in the fourth.
This study was funded by Danish governmental research agencies. Dr. Jensen reported having no financial conflicts.
AT IHC 2015
Key clinical point: High stress and unhealthy behaviors figure prominently in people with medication overuse headache – and addressing those issues can only help.
Major finding: Fifty-eight percent of Danes with medication overuse headache are in the top 20% of the population in terms of perceived stress level.
Data source: This was a population-based, cross-sectional study of 68,518 Danish adults, 1.8% of whom had medication overuse headache.
Disclosures: This study was funded by Danish governmental research agencies. The presenter reported having no financial conflicts.
IHC: Transcranial magnetic stimulation effective for medication overuse headache
VALENCIA, SPAIN – Single-pulse transcranial magnetic stimulation shows promise for treatment of migraine with medication overuse headache, according to a pilot study.
Twenty-eight severely affected patients participated in this prospective, open-label study of single-pulse transcranial magnetic stimulation (sTMS). They were instructed to use the device for at least 3 months, with an option to continue for another 3 months.
The proprietary SpringTMS system was approved by the Food and Drug Administration and the U.K.’s National Institute for Clinical Excellence for treatment of acute migraine on the basis of a positive randomized, double-blind, sham-controlled clinical trial (Lancet Neurol. 2010;9:373-80), but it hasn’t previously been evaluated in the common and clinically challenging setting of migraine complicated by medication overuse, Dr. Farooq Maniyar noted at the International Headache Congress.
The reasons participants gave for enrolling in the study varied. Most felt their current medications had intolerable side effects and/or weren’t sufficiently beneficial. Some preferred the possibility of a nondrug method of managing their headaches, explained Dr. Maniyar, a consulting neurologist at Basildon and Thurrock University Hospitals NHS Foundation Trust, Basildon, England, and the Royal London Hospital of Barts Health NHS Trust.
The key results include:
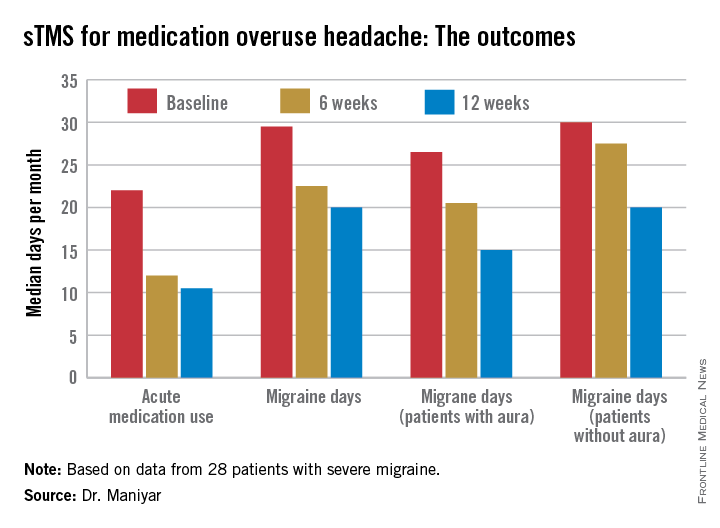
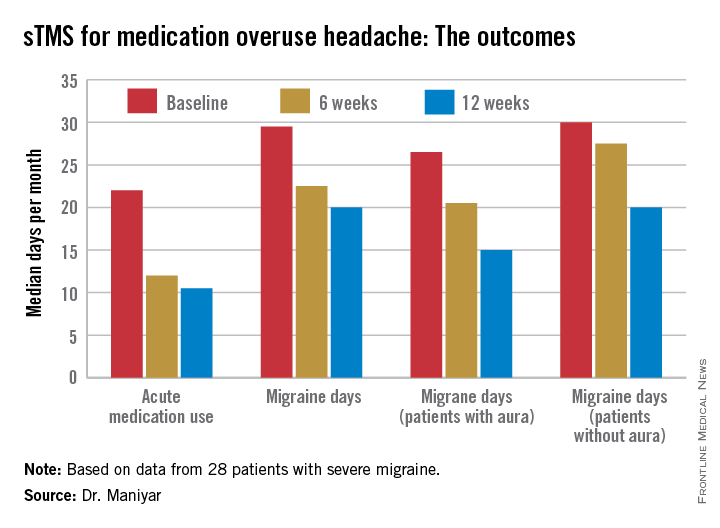
• Twenty-four of 28 patients (86%) reported a reduction in their days of acute medication use per month, while 2 patients reported an increase in acute medications.
• Nineteen patients (68%) experienced fewer migraine days per month, and 7 of the 19 had a 50% or greater reduction.
• The number of patients who rated their migraine pain as excruciating or severe plunged from 19 at baseline to 3 at 3 months, an 84% drop.
• Self-reported headache attack duration, which averaged 1 hour at baseline, decreased in 15 patients, remained unchanged in 9, and increased in 4.
• The Headache Impact Test (HIT-6) score, which reflects patient perception of the impact the migraine headaches have on daily life, was rated severe at baseline – meaning a score of at least 60 – by 26 of 28 participants. At 3 months, this number had decreased from 26 to 18.
“I think it’s fair to say that sTMS may be a useful option in treatment of medication overuse headache,” Dr. Maniyar declared at the meeting sponsored by the International Headache Society and the American Headache Society.
The sTMS regimen was beneficial in migraine patients with or without aura. Although the 12 patients who had migraine with aura showed a consistent trend for better outcomes, Dr. Maniyar said the patient numbers in the study were too small to draw definite conclusions on this score.
After 3 months, five patients discontinued sTMS because of inconvenience or lack of benefit. Four others were lost to follow-up. Of the remaining 19, 16 reported reduced days of acute medication usage at 6 months, compared with baseline. HIT-6 scores in the 19 patients with 6-month treatment data were comparable to their scores at 3 months.
No adverse events occurred in connection with the study.
Dr. Maniyar said the study’s major limitations are its relatively small sample size – the results need a confirmatory study – and the open-label, uncontrolled study design.
“We can’t rule out the possibility of a placebo effect for sTMS,” he observed.
The portable, noninvasive SpringTMS device delivers a brief pulse of magnetic energy to the back of the head in order to disrupt the migraine headache. The daily treatment regimen for the first month involved giving two pulses, followed 15 minutes later by another two, then repeating the sequence roughly 12 hours later. In addition, patients were instructed to use the device at the onset of headaches. Patients were allowed to use a maximum of 16 pulses per day in month 1, 24 per day in month 2, and up to 32 daily during month 3.
The study was sponsored by eNeura, which markets the SpringTMS device. Dr. Maniyar reported serving as a consultant to the company.
VALENCIA, SPAIN – Single-pulse transcranial magnetic stimulation shows promise for treatment of migraine with medication overuse headache, according to a pilot study.
Twenty-eight severely affected patients participated in this prospective, open-label study of single-pulse transcranial magnetic stimulation (sTMS). They were instructed to use the device for at least 3 months, with an option to continue for another 3 months.
The proprietary SpringTMS system was approved by the Food and Drug Administration and the U.K.’s National Institute for Clinical Excellence for treatment of acute migraine on the basis of a positive randomized, double-blind, sham-controlled clinical trial (Lancet Neurol. 2010;9:373-80), but it hasn’t previously been evaluated in the common and clinically challenging setting of migraine complicated by medication overuse, Dr. Farooq Maniyar noted at the International Headache Congress.

The reasons participants gave for enrolling in the study varied. Most felt their current medications had intolerable side effects and/or weren’t sufficiently beneficial. Some preferred the possibility of a nondrug method of managing their headaches, explained Dr. Maniyar, a consulting neurologist at Basildon and Thurrock University Hospitals NHS Foundation Trust, Basildon, England, and the Royal London Hospital of Barts Health NHS Trust.
The key results include:
• Twenty-four of 28 patients (86%) reported a reduction in their days of acute medication use per month, while 2 patients reported an increase in acute medications.
• Nineteen patients (68%) experienced fewer migraine days per month, and 7 of the 19 had a 50% or greater reduction.
• The number of patients who rated their migraine pain as excruciating or severe plunged from 19 at baseline to 3 at 3 months, an 84% drop.
• Self-reported headache attack duration, which averaged 1 hour at baseline, decreased in 15 patients, remained unchanged in 9, and increased in 4.
• The Headache Impact Test (HIT-6) score, which reflects patient perception of the impact the migraine headaches have on daily life, was rated severe at baseline – meaning a score of at least 60 – by 26 of 28 participants. At 3 months, this number had decreased from 26 to 18.
“I think it’s fair to say that sTMS may be a useful option in treatment of medication overuse headache,” Dr. Maniyar declared at the meeting sponsored by the International Headache Society and the American Headache Society.
The sTMS regimen was beneficial in migraine patients with or without aura. Although the 12 patients who had migraine with aura showed a consistent trend for better outcomes, Dr. Maniyar said the patient numbers in the study were too small to draw definite conclusions on this score.
After 3 months, five patients discontinued sTMS because of inconvenience or lack of benefit. Four others were lost to follow-up. Of the remaining 19, 16 reported reduced days of acute medication usage at 6 months, compared with baseline. HIT-6 scores in the 19 patients with 6-month treatment data were comparable to their scores at 3 months.
No adverse events occurred in connection with the study.
Dr. Maniyar said the study’s major limitations are its relatively small sample size – the results need a confirmatory study – and the open-label, uncontrolled study design.
“We can’t rule out the possibility of a placebo effect for sTMS,” he observed.
The portable, noninvasive SpringTMS device delivers a brief pulse of magnetic energy to the back of the head in order to disrupt the migraine headache. The daily treatment regimen for the first month involved giving two pulses, followed 15 minutes later by another two, then repeating the sequence roughly 12 hours later. In addition, patients were instructed to use the device at the onset of headaches. Patients were allowed to use a maximum of 16 pulses per day in month 1, 24 per day in month 2, and up to 32 daily during month 3.
The study was sponsored by eNeura, which markets the SpringTMS device. Dr. Maniyar reported serving as a consultant to the company.
VALENCIA, SPAIN – Single-pulse transcranial magnetic stimulation shows promise for treatment of migraine with medication overuse headache, according to a pilot study.
Twenty-eight severely affected patients participated in this prospective, open-label study of single-pulse transcranial magnetic stimulation (sTMS). They were instructed to use the device for at least 3 months, with an option to continue for another 3 months.
The proprietary SpringTMS system was approved by the Food and Drug Administration and the U.K.’s National Institute for Clinical Excellence for treatment of acute migraine on the basis of a positive randomized, double-blind, sham-controlled clinical trial (Lancet Neurol. 2010;9:373-80), but it hasn’t previously been evaluated in the common and clinically challenging setting of migraine complicated by medication overuse, Dr. Farooq Maniyar noted at the International Headache Congress.

The reasons participants gave for enrolling in the study varied. Most felt their current medications had intolerable side effects and/or weren’t sufficiently beneficial. Some preferred the possibility of a nondrug method of managing their headaches, explained Dr. Maniyar, a consulting neurologist at Basildon and Thurrock University Hospitals NHS Foundation Trust, Basildon, England, and the Royal London Hospital of Barts Health NHS Trust.
The key results include:
• Twenty-four of 28 patients (86%) reported a reduction in their days of acute medication use per month, while 2 patients reported an increase in acute medications.
• Nineteen patients (68%) experienced fewer migraine days per month, and 7 of the 19 had a 50% or greater reduction.
• The number of patients who rated their migraine pain as excruciating or severe plunged from 19 at baseline to 3 at 3 months, an 84% drop.
• Self-reported headache attack duration, which averaged 1 hour at baseline, decreased in 15 patients, remained unchanged in 9, and increased in 4.
• The Headache Impact Test (HIT-6) score, which reflects patient perception of the impact the migraine headaches have on daily life, was rated severe at baseline – meaning a score of at least 60 – by 26 of 28 participants. At 3 months, this number had decreased from 26 to 18.
“I think it’s fair to say that sTMS may be a useful option in treatment of medication overuse headache,” Dr. Maniyar declared at the meeting sponsored by the International Headache Society and the American Headache Society.
The sTMS regimen was beneficial in migraine patients with or without aura. Although the 12 patients who had migraine with aura showed a consistent trend for better outcomes, Dr. Maniyar said the patient numbers in the study were too small to draw definite conclusions on this score.
After 3 months, five patients discontinued sTMS because of inconvenience or lack of benefit. Four others were lost to follow-up. Of the remaining 19, 16 reported reduced days of acute medication usage at 6 months, compared with baseline. HIT-6 scores in the 19 patients with 6-month treatment data were comparable to their scores at 3 months.
No adverse events occurred in connection with the study.
Dr. Maniyar said the study’s major limitations are its relatively small sample size – the results need a confirmatory study – and the open-label, uncontrolled study design.
“We can’t rule out the possibility of a placebo effect for sTMS,” he observed.
The portable, noninvasive SpringTMS device delivers a brief pulse of magnetic energy to the back of the head in order to disrupt the migraine headache. The daily treatment regimen for the first month involved giving two pulses, followed 15 minutes later by another two, then repeating the sequence roughly 12 hours later. In addition, patients were instructed to use the device at the onset of headaches. Patients were allowed to use a maximum of 16 pulses per day in month 1, 24 per day in month 2, and up to 32 daily during month 3.
The study was sponsored by eNeura, which markets the SpringTMS device. Dr. Maniyar reported serving as a consultant to the company.
AT IHC 2015
Key clinical point:The SpringTMS device shows promising results as a safe and effective nondrug therapeutic option in migraine with medication overuse headache.
Major finding: The median number of days of acute medication use per month went from 22 at baseline to 12 after 6 weeks of self-treatment with single-pulse transcranial magnetic stimulation to 10.5 days after 3 months.
Data source: This was a prospective, open-label, uncontrolled pilot study involving 28 patients with severe migraine complicated by medication overuse headache.
Disclosures: The study was sponsored by eNeura, which markets the SpringTMS device. The presenter reported serving as a consultant to the company.
IHC: Real-world data support Botox in chronic migraine
VALENCIA, SPAIN – OnabotulinumtoxinA injections for the treatment of refractory chronic migraine halved the number of days of work per month missed because of migraine while quadrupling the number of crystal-clear, headache-free days in a large, prospective, real-world study.
“OnabotulinumtoxinA [Botox] is a valuable addition to current treatment options in patients with chronic migraine,” Dr. Modar Khalil concluded in summarizing his study findings at the International Headache Congress.
He presented a prospective study of 465 patients with chronic migraine treated with onabotulinumtoxinA in Hull, England.
The results paralleled the findings of the earlier PREEMPT (Phase-3 Research Evaluating Migraine Prophylaxis Therapy) study program which led to marketing approval of onabotulinumtoxinA for chronic migraine in the United Kingdom as well as the United States. However, the Hull patients were more representative of the sort of chronic migraine patients typically seen in tertiary headache clinics. Their chronic migraine was more severe than in the PREEMPT population: 97% of the Hull group had failed three or more preventive therapies at baseline, compared with only one-third of PREEMPT participants, noted Dr. Khalil of the Hull Royal Infirmary.
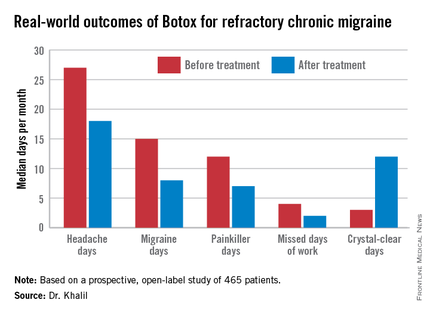
The Hull patients had a median 4-year history of chronic migraine. Prior to undergoing their first cycle of onabotulinumtoxinA therapy they averaged 27 total headache days and 15 migraine days per month.
The three coprimary study endpoints were at least a 50% reduction in headache days in the first month after onabotulinumtoxinA therapy, compared with the month before, at least a 50% reduction in migraine days, or a doubling of the number of crystal-clear days.
Fifty-nine percent of patients achieved at least one of those targets, 39% reached two, and 21% attained all three. The goal most frequently met was the 50% or greater reduction in monthly migraine days, achieved by 47% of patients. Forty-six percent met the target for an increase in crystal-clear days, while 27% of patients experienced at least a 50% reduction in headache days, Dr. Khalil said at the meeting sponsored by the International Headache Society and the American Headache Society.
The highest response rate was seen in patients with what he termed moderate-frequency chronic migraine as defined by 21-25 days of headache per month. Seventy-four percent of those patients met at least one of the three coprimary endpoints. In contrast, patients with a baseline headache frequency of 26-30 days per month had a 50% response rate, while those with 16-20 headache days had a 59% response rate.
A tougher standard – a 75% or greater reduction in either migraine days or headache days or at least a threefold increase in crystal-clear days – was achieved in one-third of patients. Fourteen percent met any two of the goals, and 6% met all three goals at the higher bar.
The median number of work days missed per month due to headache fell from 4 to 2 in the study population.
The most common adverse event was neck stiffness, reported by 16% of treated patients. In addition, 15% reported injection site pain lasting at least 24 hours, 9% experienced drooping of the upper eyelid, and 1.5% had difficulty swallowing.
Dr. Khalil noted that while a real-world, prospective, observational study such as this offers advantages over a controlled clinical trial, which often enrolls a select patient population, it has the disadvantage of not having an active comparator arm. That’s important because it’s well known that migraine studies tend to have a high placebo response rate.
In other Botox-related news at the IHC Congress, Rami Burstein, Ph.D., received the 2015 International Headache Society Cephalalgia Award given by the editorial board of the journal Cephalalgia for the most important paper in the field of headache medicine published in that journal in the past year. Dr. Burstein, professor of anesthesiology at Harvard Medical School, Boston, earned the honor, which comes with a 10,000 euro award, for his work in elucidating the mechanism of benefit for Botox in migraine (Cephalalgia 2014;34:853-69).
Dr. Khalil’s study was supported by Allergan. He reported having no financial conflicts.
VALENCIA, SPAIN – OnabotulinumtoxinA injections for the treatment of refractory chronic migraine halved the number of days of work per month missed because of migraine while quadrupling the number of crystal-clear, headache-free days in a large, prospective, real-world study.
“OnabotulinumtoxinA [Botox] is a valuable addition to current treatment options in patients with chronic migraine,” Dr. Modar Khalil concluded in summarizing his study findings at the International Headache Congress.
He presented a prospective study of 465 patients with chronic migraine treated with onabotulinumtoxinA in Hull, England.
The results paralleled the findings of the earlier PREEMPT (Phase-3 Research Evaluating Migraine Prophylaxis Therapy) study program which led to marketing approval of onabotulinumtoxinA for chronic migraine in the United Kingdom as well as the United States. However, the Hull patients were more representative of the sort of chronic migraine patients typically seen in tertiary headache clinics. Their chronic migraine was more severe than in the PREEMPT population: 97% of the Hull group had failed three or more preventive therapies at baseline, compared with only one-third of PREEMPT participants, noted Dr. Khalil of the Hull Royal Infirmary.
The Hull patients had a median 4-year history of chronic migraine. Prior to undergoing their first cycle of onabotulinumtoxinA therapy they averaged 27 total headache days and 15 migraine days per month.
The three coprimary study endpoints were at least a 50% reduction in headache days in the first month after onabotulinumtoxinA therapy, compared with the month before, at least a 50% reduction in migraine days, or a doubling of the number of crystal-clear days.

Fifty-nine percent of patients achieved at least one of those targets, 39% reached two, and 21% attained all three. The goal most frequently met was the 50% or greater reduction in monthly migraine days, achieved by 47% of patients. Forty-six percent met the target for an increase in crystal-clear days, while 27% of patients experienced at least a 50% reduction in headache days, Dr. Khalil said at the meeting sponsored by the International Headache Society and the American Headache Society.
The highest response rate was seen in patients with what he termed moderate-frequency chronic migraine as defined by 21-25 days of headache per month. Seventy-four percent of those patients met at least one of the three coprimary endpoints. In contrast, patients with a baseline headache frequency of 26-30 days per month had a 50% response rate, while those with 16-20 headache days had a 59% response rate.
A tougher standard – a 75% or greater reduction in either migraine days or headache days or at least a threefold increase in crystal-clear days – was achieved in one-third of patients. Fourteen percent met any two of the goals, and 6% met all three goals at the higher bar.
The median number of work days missed per month due to headache fell from 4 to 2 in the study population.
The most common adverse event was neck stiffness, reported by 16% of treated patients. In addition, 15% reported injection site pain lasting at least 24 hours, 9% experienced drooping of the upper eyelid, and 1.5% had difficulty swallowing.
Dr. Khalil noted that while a real-world, prospective, observational study such as this offers advantages over a controlled clinical trial, which often enrolls a select patient population, it has the disadvantage of not having an active comparator arm. That’s important because it’s well known that migraine studies tend to have a high placebo response rate.
In other Botox-related news at the IHC Congress, Rami Burstein, Ph.D., received the 2015 International Headache Society Cephalalgia Award given by the editorial board of the journal Cephalalgia for the most important paper in the field of headache medicine published in that journal in the past year. Dr. Burstein, professor of anesthesiology at Harvard Medical School, Boston, earned the honor, which comes with a 10,000 euro award, for his work in elucidating the mechanism of benefit for Botox in migraine (Cephalalgia 2014;34:853-69).
Dr. Khalil’s study was supported by Allergan. He reported having no financial conflicts.
VALENCIA, SPAIN – OnabotulinumtoxinA injections for the treatment of refractory chronic migraine halved the number of days of work per month missed because of migraine while quadrupling the number of crystal-clear, headache-free days in a large, prospective, real-world study.
“OnabotulinumtoxinA [Botox] is a valuable addition to current treatment options in patients with chronic migraine,” Dr. Modar Khalil concluded in summarizing his study findings at the International Headache Congress.
He presented a prospective study of 465 patients with chronic migraine treated with onabotulinumtoxinA in Hull, England.
The results paralleled the findings of the earlier PREEMPT (Phase-3 Research Evaluating Migraine Prophylaxis Therapy) study program which led to marketing approval of onabotulinumtoxinA for chronic migraine in the United Kingdom as well as the United States. However, the Hull patients were more representative of the sort of chronic migraine patients typically seen in tertiary headache clinics. Their chronic migraine was more severe than in the PREEMPT population: 97% of the Hull group had failed three or more preventive therapies at baseline, compared with only one-third of PREEMPT participants, noted Dr. Khalil of the Hull Royal Infirmary.
The Hull patients had a median 4-year history of chronic migraine. Prior to undergoing their first cycle of onabotulinumtoxinA therapy they averaged 27 total headache days and 15 migraine days per month.
The three coprimary study endpoints were at least a 50% reduction in headache days in the first month after onabotulinumtoxinA therapy, compared with the month before, at least a 50% reduction in migraine days, or a doubling of the number of crystal-clear days.

Fifty-nine percent of patients achieved at least one of those targets, 39% reached two, and 21% attained all three. The goal most frequently met was the 50% or greater reduction in monthly migraine days, achieved by 47% of patients. Forty-six percent met the target for an increase in crystal-clear days, while 27% of patients experienced at least a 50% reduction in headache days, Dr. Khalil said at the meeting sponsored by the International Headache Society and the American Headache Society.
The highest response rate was seen in patients with what he termed moderate-frequency chronic migraine as defined by 21-25 days of headache per month. Seventy-four percent of those patients met at least one of the three coprimary endpoints. In contrast, patients with a baseline headache frequency of 26-30 days per month had a 50% response rate, while those with 16-20 headache days had a 59% response rate.
A tougher standard – a 75% or greater reduction in either migraine days or headache days or at least a threefold increase in crystal-clear days – was achieved in one-third of patients. Fourteen percent met any two of the goals, and 6% met all three goals at the higher bar.
The median number of work days missed per month due to headache fell from 4 to 2 in the study population.
The most common adverse event was neck stiffness, reported by 16% of treated patients. In addition, 15% reported injection site pain lasting at least 24 hours, 9% experienced drooping of the upper eyelid, and 1.5% had difficulty swallowing.
Dr. Khalil noted that while a real-world, prospective, observational study such as this offers advantages over a controlled clinical trial, which often enrolls a select patient population, it has the disadvantage of not having an active comparator arm. That’s important because it’s well known that migraine studies tend to have a high placebo response rate.
In other Botox-related news at the IHC Congress, Rami Burstein, Ph.D., received the 2015 International Headache Society Cephalalgia Award given by the editorial board of the journal Cephalalgia for the most important paper in the field of headache medicine published in that journal in the past year. Dr. Burstein, professor of anesthesiology at Harvard Medical School, Boston, earned the honor, which comes with a 10,000 euro award, for his work in elucidating the mechanism of benefit for Botox in migraine (Cephalalgia 2014;34:853-69).
Dr. Khalil’s study was supported by Allergan. He reported having no financial conflicts.
AT IHC 2015
Key clinical point: OnabotulinumtoxinA (Botox) injections have a high treatment success rate in real-world chronic migraine patients who’ve failed to respond to at least three preventive therapies.
Major finding: Fifty-nine percent of refractory chronic migraine patients showed a meaningful response to onabotulinumtoxinA therapy based upon a significant reduction in either monthly total headache or migraine days or an increase in completely headache-free days.
Data source: This was a prospective open-label study involving 465 unselected, real-world, patients with refractory chronic migraine treated with Botox at a tertiary headache center.
Disclosures: The study was supported by Allergan. The presenter reported having no financial conflicts.