User login
Pacific Dermatologic Association (PDA): Annual Meeting
Management of dysplastic nevi varies widely
PARK CITY, UTAH – When you ask clinicians why they elect to reexcise dysplastic nevi, you’re likely to get a variety of answers, according to Dr. Douglas Grossman.
“Sometimes they have no reason all,” Dr. Grossman, professor of dermatology at the University of Utah, Salt Lake City, said at the annual meeting of the Pacific Dermatologic Association. “Sometimes the answer is ‘to prevent recurrence.’ Sometimes it’s to ensure complete removal of the lesion, to confirm the diagnosis, to rule out melanoma, or for ‘therapeutic’ purposes.”
Dysplastic nevus margins are often positive, “because the melanocytes in these lesions tend to extend up to 2 mm beyond clinical margins,” Dr. Grossman said. “I always try to remove the entire clinical lesion as well as 1-2 mm around the lesion to avoid positive margins.”
A survey of 101 Chicago Dermatological Society members found wide variation in clinical practice concerning how clinicians manage dysplastic nevi if the margins are clear vs. positive, and based on the degree of atypia (Arch Dermatol. 2012;148[2]:259-60). The greatest quantitative shift in decision making (from observe to reexcise) was seen for dysplastic nevi with moderate dysplasia. Specifically, the decision to reexcise dysplastic nevi with moderate dysplasia ranged from 9% (for clear margins) to 81% (for positive margins) of respondents. “The margin status is driving the management,” said Dr. Grossman, who was not involved with the study.
In his opinion, two “unspoken fears” are also impacting the way clinicians treat dysplastic nevi. The first is that the nevus will recur in the scar years later and be indistinguishable from melanoma – the so-called pseudomelanoma phenomenon. The second fear is that residual nevus cells left behind will transform to melanoma. Evidence for these two possibilities, however, is lacking, Dr. Grossman said. According to a large study of pseudomelanoma phenomena, recurrence was rare and easily distinguishable from cases of melanoma with regression (Modern Pathol. 2009;22:611-7). “Almost all of them had recurred within a year, so if a nevus is going to recur, it’s usually going to recur within that first year,” he said.
As for the issue of recurrence, Dr. Grossman led a research team that evaluated 271 nevus biopsy sites in 115 patients (J Am Acad Dermatol. 2010;62[4]:591-6). At greater than 2 years of follow-up they observed a recurrence rate of 3.6% for dysplastic nevi, compared with 3.3% for nondysplastic nevi, “which is similar,” he said. In terms of melanoma development, one study of 28 incompletely removed dysplastic nevi found that no melanomas developed during 5 years of follow-up (Am J Dermatopathol. 1985;7 Suppl:93-7).
Dr. Grossman referenced four cases in the medical literature in which the diagnosis was changed upon reexcision from dysplastic nevus to melanoma. “The question here is whether the initial biopsy or the reexcision was the correct diagnosis,” he said. “Development of melanoma at the site of a previously biopsied dysplastic nevus is exceedingly rare, and has not been described beyond a few case reports. The most likely explanation for how this could occur would be sampling error. If you have a melanoma arising at the site, maybe it was melanoma to start with, and the diagnosis was missed on the original biopsy. It could also be the case of pseudomelanoma phenomenon where it’s a nevus to start with but it comes back looking more atypical, so it’s incorrectly diagnosed as melanoma. A final possibility, which is the least likely, is that the few nevus cells left behind transformed into melanoma.”
According to a recent consensus statement from the Pigmented Lesion Subcommittee of the Melanoma Prevention Working Group, mild/moderate dysplastic nevi with clear margins do not need reexcision (JAMA Dermatol. 2015;151[2]:212-8). The working group also recommends that mild dysplastic nevi with positive margins may be safely observed and that observation for moderately dysplastic nevi with positive margins “may be a reasonable option.”
Dr. Grossman reported having no financial disclosures.
PARK CITY, UTAH – When you ask clinicians why they elect to reexcise dysplastic nevi, you’re likely to get a variety of answers, according to Dr. Douglas Grossman.
“Sometimes they have no reason all,” Dr. Grossman, professor of dermatology at the University of Utah, Salt Lake City, said at the annual meeting of the Pacific Dermatologic Association. “Sometimes the answer is ‘to prevent recurrence.’ Sometimes it’s to ensure complete removal of the lesion, to confirm the diagnosis, to rule out melanoma, or for ‘therapeutic’ purposes.”
Dysplastic nevus margins are often positive, “because the melanocytes in these lesions tend to extend up to 2 mm beyond clinical margins,” Dr. Grossman said. “I always try to remove the entire clinical lesion as well as 1-2 mm around the lesion to avoid positive margins.”
A survey of 101 Chicago Dermatological Society members found wide variation in clinical practice concerning how clinicians manage dysplastic nevi if the margins are clear vs. positive, and based on the degree of atypia (Arch Dermatol. 2012;148[2]:259-60). The greatest quantitative shift in decision making (from observe to reexcise) was seen for dysplastic nevi with moderate dysplasia. Specifically, the decision to reexcise dysplastic nevi with moderate dysplasia ranged from 9% (for clear margins) to 81% (for positive margins) of respondents. “The margin status is driving the management,” said Dr. Grossman, who was not involved with the study.
In his opinion, two “unspoken fears” are also impacting the way clinicians treat dysplastic nevi. The first is that the nevus will recur in the scar years later and be indistinguishable from melanoma – the so-called pseudomelanoma phenomenon. The second fear is that residual nevus cells left behind will transform to melanoma. Evidence for these two possibilities, however, is lacking, Dr. Grossman said. According to a large study of pseudomelanoma phenomena, recurrence was rare and easily distinguishable from cases of melanoma with regression (Modern Pathol. 2009;22:611-7). “Almost all of them had recurred within a year, so if a nevus is going to recur, it’s usually going to recur within that first year,” he said.
As for the issue of recurrence, Dr. Grossman led a research team that evaluated 271 nevus biopsy sites in 115 patients (J Am Acad Dermatol. 2010;62[4]:591-6). At greater than 2 years of follow-up they observed a recurrence rate of 3.6% for dysplastic nevi, compared with 3.3% for nondysplastic nevi, “which is similar,” he said. In terms of melanoma development, one study of 28 incompletely removed dysplastic nevi found that no melanomas developed during 5 years of follow-up (Am J Dermatopathol. 1985;7 Suppl:93-7).
Dr. Grossman referenced four cases in the medical literature in which the diagnosis was changed upon reexcision from dysplastic nevus to melanoma. “The question here is whether the initial biopsy or the reexcision was the correct diagnosis,” he said. “Development of melanoma at the site of a previously biopsied dysplastic nevus is exceedingly rare, and has not been described beyond a few case reports. The most likely explanation for how this could occur would be sampling error. If you have a melanoma arising at the site, maybe it was melanoma to start with, and the diagnosis was missed on the original biopsy. It could also be the case of pseudomelanoma phenomenon where it’s a nevus to start with but it comes back looking more atypical, so it’s incorrectly diagnosed as melanoma. A final possibility, which is the least likely, is that the few nevus cells left behind transformed into melanoma.”
According to a recent consensus statement from the Pigmented Lesion Subcommittee of the Melanoma Prevention Working Group, mild/moderate dysplastic nevi with clear margins do not need reexcision (JAMA Dermatol. 2015;151[2]:212-8). The working group also recommends that mild dysplastic nevi with positive margins may be safely observed and that observation for moderately dysplastic nevi with positive margins “may be a reasonable option.”
Dr. Grossman reported having no financial disclosures.
PARK CITY, UTAH – When you ask clinicians why they elect to reexcise dysplastic nevi, you’re likely to get a variety of answers, according to Dr. Douglas Grossman.
“Sometimes they have no reason all,” Dr. Grossman, professor of dermatology at the University of Utah, Salt Lake City, said at the annual meeting of the Pacific Dermatologic Association. “Sometimes the answer is ‘to prevent recurrence.’ Sometimes it’s to ensure complete removal of the lesion, to confirm the diagnosis, to rule out melanoma, or for ‘therapeutic’ purposes.”
Dysplastic nevus margins are often positive, “because the melanocytes in these lesions tend to extend up to 2 mm beyond clinical margins,” Dr. Grossman said. “I always try to remove the entire clinical lesion as well as 1-2 mm around the lesion to avoid positive margins.”
A survey of 101 Chicago Dermatological Society members found wide variation in clinical practice concerning how clinicians manage dysplastic nevi if the margins are clear vs. positive, and based on the degree of atypia (Arch Dermatol. 2012;148[2]:259-60). The greatest quantitative shift in decision making (from observe to reexcise) was seen for dysplastic nevi with moderate dysplasia. Specifically, the decision to reexcise dysplastic nevi with moderate dysplasia ranged from 9% (for clear margins) to 81% (for positive margins) of respondents. “The margin status is driving the management,” said Dr. Grossman, who was not involved with the study.
In his opinion, two “unspoken fears” are also impacting the way clinicians treat dysplastic nevi. The first is that the nevus will recur in the scar years later and be indistinguishable from melanoma – the so-called pseudomelanoma phenomenon. The second fear is that residual nevus cells left behind will transform to melanoma. Evidence for these two possibilities, however, is lacking, Dr. Grossman said. According to a large study of pseudomelanoma phenomena, recurrence was rare and easily distinguishable from cases of melanoma with regression (Modern Pathol. 2009;22:611-7). “Almost all of them had recurred within a year, so if a nevus is going to recur, it’s usually going to recur within that first year,” he said.
As for the issue of recurrence, Dr. Grossman led a research team that evaluated 271 nevus biopsy sites in 115 patients (J Am Acad Dermatol. 2010;62[4]:591-6). At greater than 2 years of follow-up they observed a recurrence rate of 3.6% for dysplastic nevi, compared with 3.3% for nondysplastic nevi, “which is similar,” he said. In terms of melanoma development, one study of 28 incompletely removed dysplastic nevi found that no melanomas developed during 5 years of follow-up (Am J Dermatopathol. 1985;7 Suppl:93-7).
Dr. Grossman referenced four cases in the medical literature in which the diagnosis was changed upon reexcision from dysplastic nevus to melanoma. “The question here is whether the initial biopsy or the reexcision was the correct diagnosis,” he said. “Development of melanoma at the site of a previously biopsied dysplastic nevus is exceedingly rare, and has not been described beyond a few case reports. The most likely explanation for how this could occur would be sampling error. If you have a melanoma arising at the site, maybe it was melanoma to start with, and the diagnosis was missed on the original biopsy. It could also be the case of pseudomelanoma phenomenon where it’s a nevus to start with but it comes back looking more atypical, so it’s incorrectly diagnosed as melanoma. A final possibility, which is the least likely, is that the few nevus cells left behind transformed into melanoma.”
According to a recent consensus statement from the Pigmented Lesion Subcommittee of the Melanoma Prevention Working Group, mild/moderate dysplastic nevi with clear margins do not need reexcision (JAMA Dermatol. 2015;151[2]:212-8). The working group also recommends that mild dysplastic nevi with positive margins may be safely observed and that observation for moderately dysplastic nevi with positive margins “may be a reasonable option.”
Dr. Grossman reported having no financial disclosures.
EXPERT ANALYSIS FROM PDA 2015
Celiac disease ‘as common as psoriasis’
PARK CITY, UTAH – Dr. John J. Zone first began to study gluten sensitivity in 1977, an interest that left some of his clinician colleagues wondering why.
“Everybody told me I was crazy – that this was extremely rare. So I always say I was gluten when gluten wasn’t cool,” Dr. Zone, professor and chairman of dermatology at the University of Utah, Salt Lake City, told attendees at the annual meeting of the Pacific Dermatologic Association.
These days, it’s hard to shop in a food market without noticing all the gluten-free foods available, from pizza dough to beer. Many restaurants also serve gluten-free dishes. But is it hype, or is gluten sensitivity that common? Five percent of people in the United States “will say they are gluten sensitive,” he said. “In fact, 1% of Caucasians actually have celiac disease and 1% of Caucasians have gluten sensitivity that can be documented by challenge but don’t have celiac disease, while 3% have nothing.”
Gluten is a group of proteins contained in wheat, barley, and rye that is insoluble in water. Dr. Zone described celiac disease as a “spectrum of disease” characterized by inflammation of the small intestinal mucosa that occurs with the ingestion of gluten. The condition improves when gluten is removed from the diet. From a genetic standpoint, having a predisposition to express human leukocyte antigen-DQ2 or HLA-DQ8 is required for a diagnosis of celiac disease (CD). An estimated 20%-25% of whites “have that HLA background, but it is rare in Asians,” he said. “The receptors coded by HLA genes are essential for the processing of the gliadin antigen in CD.”
The hallmark for CD is a blood test for immunoglobulin A (IgA) anti-tissue transglutaminase antibodies, which are detectable in patients with untreated disease. “You should be able to get that test for $50 or $60 in any laboratory in the country,” Dr. Zone said. “It’s about 98% reliable. You also want to do a total serum IgA to rule out IgA-deficiency.”
CD clusters with other autoimmune disorders such as Addison’s disease, autoimmune thyroiditis, atrophic gastritis, systemic lupus erythematosus, rheumatoid arthritis, myasthenia gravis, and vitiligo. It’s also common in Down syndrome. “Many patients with histological inflammation have atypical intestinal symptoms or none at all,” he said. “Clinical studies have shown that only 15%-20% of CD patients identified by serology and confirmed by biopsy have classical symptoms of diarrhea and malabsorption.” The presenting symptom in patients with celiac disease may be limited to only aphthous stomatitis, eczema, alopecia areata, psoriasis, or diabetes, along with fatigue or anemia.
Researchers who analyzed the prevalence of CD in the United States estimated the risk to be 1:133 among individuals deemed not to be at risk, 1:56 in symptomatic patients, 1:39 in second-degree relatives, and 1:22 in first-degree relatives (Arch Intern Med. 2003;163[3]:286-92.). “We tested 2,100 people in Utah and found the prevalence among first-degree relatives was 1:12,” Dr. Zone said. “The point is that CD is common, not rare. It’s as common as psoriasis. It profoundly affects the immune system, which is the modulator of inflammatory skin disease.”
Dr. Zone reported having no financial disclosures.
PARK CITY, UTAH – Dr. John J. Zone first began to study gluten sensitivity in 1977, an interest that left some of his clinician colleagues wondering why.
“Everybody told me I was crazy – that this was extremely rare. So I always say I was gluten when gluten wasn’t cool,” Dr. Zone, professor and chairman of dermatology at the University of Utah, Salt Lake City, told attendees at the annual meeting of the Pacific Dermatologic Association.
These days, it’s hard to shop in a food market without noticing all the gluten-free foods available, from pizza dough to beer. Many restaurants also serve gluten-free dishes. But is it hype, or is gluten sensitivity that common? Five percent of people in the United States “will say they are gluten sensitive,” he said. “In fact, 1% of Caucasians actually have celiac disease and 1% of Caucasians have gluten sensitivity that can be documented by challenge but don’t have celiac disease, while 3% have nothing.”
Gluten is a group of proteins contained in wheat, barley, and rye that is insoluble in water. Dr. Zone described celiac disease as a “spectrum of disease” characterized by inflammation of the small intestinal mucosa that occurs with the ingestion of gluten. The condition improves when gluten is removed from the diet. From a genetic standpoint, having a predisposition to express human leukocyte antigen-DQ2 or HLA-DQ8 is required for a diagnosis of celiac disease (CD). An estimated 20%-25% of whites “have that HLA background, but it is rare in Asians,” he said. “The receptors coded by HLA genes are essential for the processing of the gliadin antigen in CD.”
The hallmark for CD is a blood test for immunoglobulin A (IgA) anti-tissue transglutaminase antibodies, which are detectable in patients with untreated disease. “You should be able to get that test for $50 or $60 in any laboratory in the country,” Dr. Zone said. “It’s about 98% reliable. You also want to do a total serum IgA to rule out IgA-deficiency.”
CD clusters with other autoimmune disorders such as Addison’s disease, autoimmune thyroiditis, atrophic gastritis, systemic lupus erythematosus, rheumatoid arthritis, myasthenia gravis, and vitiligo. It’s also common in Down syndrome. “Many patients with histological inflammation have atypical intestinal symptoms or none at all,” he said. “Clinical studies have shown that only 15%-20% of CD patients identified by serology and confirmed by biopsy have classical symptoms of diarrhea and malabsorption.” The presenting symptom in patients with celiac disease may be limited to only aphthous stomatitis, eczema, alopecia areata, psoriasis, or diabetes, along with fatigue or anemia.
Researchers who analyzed the prevalence of CD in the United States estimated the risk to be 1:133 among individuals deemed not to be at risk, 1:56 in symptomatic patients, 1:39 in second-degree relatives, and 1:22 in first-degree relatives (Arch Intern Med. 2003;163[3]:286-92.). “We tested 2,100 people in Utah and found the prevalence among first-degree relatives was 1:12,” Dr. Zone said. “The point is that CD is common, not rare. It’s as common as psoriasis. It profoundly affects the immune system, which is the modulator of inflammatory skin disease.”
Dr. Zone reported having no financial disclosures.
PARK CITY, UTAH – Dr. John J. Zone first began to study gluten sensitivity in 1977, an interest that left some of his clinician colleagues wondering why.
“Everybody told me I was crazy – that this was extremely rare. So I always say I was gluten when gluten wasn’t cool,” Dr. Zone, professor and chairman of dermatology at the University of Utah, Salt Lake City, told attendees at the annual meeting of the Pacific Dermatologic Association.
These days, it’s hard to shop in a food market without noticing all the gluten-free foods available, from pizza dough to beer. Many restaurants also serve gluten-free dishes. But is it hype, or is gluten sensitivity that common? Five percent of people in the United States “will say they are gluten sensitive,” he said. “In fact, 1% of Caucasians actually have celiac disease and 1% of Caucasians have gluten sensitivity that can be documented by challenge but don’t have celiac disease, while 3% have nothing.”
Gluten is a group of proteins contained in wheat, barley, and rye that is insoluble in water. Dr. Zone described celiac disease as a “spectrum of disease” characterized by inflammation of the small intestinal mucosa that occurs with the ingestion of gluten. The condition improves when gluten is removed from the diet. From a genetic standpoint, having a predisposition to express human leukocyte antigen-DQ2 or HLA-DQ8 is required for a diagnosis of celiac disease (CD). An estimated 20%-25% of whites “have that HLA background, but it is rare in Asians,” he said. “The receptors coded by HLA genes are essential for the processing of the gliadin antigen in CD.”
The hallmark for CD is a blood test for immunoglobulin A (IgA) anti-tissue transglutaminase antibodies, which are detectable in patients with untreated disease. “You should be able to get that test for $50 or $60 in any laboratory in the country,” Dr. Zone said. “It’s about 98% reliable. You also want to do a total serum IgA to rule out IgA-deficiency.”
CD clusters with other autoimmune disorders such as Addison’s disease, autoimmune thyroiditis, atrophic gastritis, systemic lupus erythematosus, rheumatoid arthritis, myasthenia gravis, and vitiligo. It’s also common in Down syndrome. “Many patients with histological inflammation have atypical intestinal symptoms or none at all,” he said. “Clinical studies have shown that only 15%-20% of CD patients identified by serology and confirmed by biopsy have classical symptoms of diarrhea and malabsorption.” The presenting symptom in patients with celiac disease may be limited to only aphthous stomatitis, eczema, alopecia areata, psoriasis, or diabetes, along with fatigue or anemia.
Researchers who analyzed the prevalence of CD in the United States estimated the risk to be 1:133 among individuals deemed not to be at risk, 1:56 in symptomatic patients, 1:39 in second-degree relatives, and 1:22 in first-degree relatives (Arch Intern Med. 2003;163[3]:286-92.). “We tested 2,100 people in Utah and found the prevalence among first-degree relatives was 1:12,” Dr. Zone said. “The point is that CD is common, not rare. It’s as common as psoriasis. It profoundly affects the immune system, which is the modulator of inflammatory skin disease.”
Dr. Zone reported having no financial disclosures.
EXPERT ANALYSIS FROM PDA 2015
Simple screening tool can help detect psoriatic arthritis
PARK CITY, UTAH – Since most people with psoriatic arthritis (PsA) develop psoriasis before joint symptoms, it’s helpful to have a simple screening test for the condition.
One of Dr. Philip Mease’s favorite PsA screening tools is the Psoriasis Epidemiology Screening Test (PEST), which was first described at the 2009 annual meeting of the Group for Research and Assessment of Psoriasis and Psoriatic Arthritis (GRAPPA) and consists of five simple questions. They are: Have you ever had a swollen joint (or joints)? Has a doctor ever told you that you have arthritis? Do your fingernails or toes have holes or pits? Have you had pain in your heel? Have you had a finger or toe that was completely swollen and painful for no apparent reason? (Clin Exp Rheumatol. 2009;27:469-74).
“Just these five simple questions, or trying to remember a few of them, can help you in your review of systems,” Dr. Mease, director of arthritis research at Swedish Medical Center, Seattle, said at the annual meeting of the Pacific Dermatologic Association. “I think patients appreciate it when you look beyond the skin in your questioning. These can pick up [PsA] with a high sensitivity and specificity” of 0.92 and 0.78, respectively.
He went on to discuss current PsA treatment approaches. According to an evidence review that he and his associates in GRAPPA published in 2009, biologics (anti–tumor necrosis factor inhibitors) as a group were found to be effective in all five domains of the disease: peripheral arthritis, skin and nail disease, axial disease, dactylitis, and enthesitis, while the oral disease-modifying antirheumatic drugs (DMARDs) were effective for peripheral arthritis and skin and nail disease.
Other treatments that were found effective are: psoralen and UVA/UVB for skin and nail disease, physiotherapy for axial disease, intra-articular steroids for peripheral arthritis, and NSAIDs for peripheral arthritis and axial disease (J Rheum. 2009;33:1417-21). “Patients with mild disease can be tried on NSAIDs, especially in a patient with monoarticular disease, but for the most part we need to move on to using systemic medication,” he said. Updated recommendations from GRAPPA include new data regarding ustekinumab, apremilast, and secukinumab, as well as data on comorbidities (J Rheumatol. 2015;42[6]:1052-5).
According to Dr. Mease, controlled trials of DMARDs in PsA patients have yielded treatment effects that range from marginal in the joints to marginal or none at all in the skin. Data from the Methotrexate in Psoriatic Arthritis trial conducted in the United Kingdom and published in 2012 showed no evidence that methotrexate improves inflammatory synovitis in active PsA (Rheumatol. 2012;51:1368-77).
“There were issues with this trial, including the fact that it took 5 years to enroll patients, and many dropped out, so I don’t think it’s a very reliable study,” said Dr. Mease, who is also a professor of medicine in the division of rheumatology at the University of Washington, Seattle. “Currently, Amgen is in the process of starting a trial in which the goal is to enroll 840 subjects with early PsA who are being randomized to methotrexate alone, Enbrel alone, or Enbrel plus methotrexate. This, I think, is going to give us a better answer about the effectiveness of methotrexate. It will also teach us about whether there’s a value in combining an anti-TNF inhibitor with methotrexate. We still don’t know the answer to that question.”
The most recent data on methotrexate come from an open-label trial known as TICOPA, which used a tight control treatment paradigm through 48 weeks of treatment. A subanalysis of 188 patients treated with methotrexate through 12 weeks was presented at the 2015 meeting of the European League Against Rheumatism. It revealed that 41% of patients achieved an ACR 20, 22% achieved minimal disease activity, 62% experienced an improvement in dactylitis, and 25% experienced an improvement in enthesitis.
“So we have a few data suggesting that methotrexate may be modestly effective in treating PsA,” Dr. Mease said. “We often will start with methotrexate unless the patient has really aggressive disease activity. If they get some effect from the drug but not enough, we’ll often add a biologic agent but often keep some methotrexate in the background, even at 10 mg per week, in order to reduce immunogenicity from a biologic.”
Dr. Mease disclosed that he has received research grants, consultation fees, and/or speaker honoraria from AbbVie, Amgen, Bristol-Myers Squibb, Celgene, Crescendo Bioscience, Genentech, GlaxoSmithKline, Janssen, Eli Lilly, Merck, Novartis, Pfizer, and UCB.
PARK CITY, UTAH – Since most people with psoriatic arthritis (PsA) develop psoriasis before joint symptoms, it’s helpful to have a simple screening test for the condition.
One of Dr. Philip Mease’s favorite PsA screening tools is the Psoriasis Epidemiology Screening Test (PEST), which was first described at the 2009 annual meeting of the Group for Research and Assessment of Psoriasis and Psoriatic Arthritis (GRAPPA) and consists of five simple questions. They are: Have you ever had a swollen joint (or joints)? Has a doctor ever told you that you have arthritis? Do your fingernails or toes have holes or pits? Have you had pain in your heel? Have you had a finger or toe that was completely swollen and painful for no apparent reason? (Clin Exp Rheumatol. 2009;27:469-74).
“Just these five simple questions, or trying to remember a few of them, can help you in your review of systems,” Dr. Mease, director of arthritis research at Swedish Medical Center, Seattle, said at the annual meeting of the Pacific Dermatologic Association. “I think patients appreciate it when you look beyond the skin in your questioning. These can pick up [PsA] with a high sensitivity and specificity” of 0.92 and 0.78, respectively.
He went on to discuss current PsA treatment approaches. According to an evidence review that he and his associates in GRAPPA published in 2009, biologics (anti–tumor necrosis factor inhibitors) as a group were found to be effective in all five domains of the disease: peripheral arthritis, skin and nail disease, axial disease, dactylitis, and enthesitis, while the oral disease-modifying antirheumatic drugs (DMARDs) were effective for peripheral arthritis and skin and nail disease.
Other treatments that were found effective are: psoralen and UVA/UVB for skin and nail disease, physiotherapy for axial disease, intra-articular steroids for peripheral arthritis, and NSAIDs for peripheral arthritis and axial disease (J Rheum. 2009;33:1417-21). “Patients with mild disease can be tried on NSAIDs, especially in a patient with monoarticular disease, but for the most part we need to move on to using systemic medication,” he said. Updated recommendations from GRAPPA include new data regarding ustekinumab, apremilast, and secukinumab, as well as data on comorbidities (J Rheumatol. 2015;42[6]:1052-5).
According to Dr. Mease, controlled trials of DMARDs in PsA patients have yielded treatment effects that range from marginal in the joints to marginal or none at all in the skin. Data from the Methotrexate in Psoriatic Arthritis trial conducted in the United Kingdom and published in 2012 showed no evidence that methotrexate improves inflammatory synovitis in active PsA (Rheumatol. 2012;51:1368-77).
“There were issues with this trial, including the fact that it took 5 years to enroll patients, and many dropped out, so I don’t think it’s a very reliable study,” said Dr. Mease, who is also a professor of medicine in the division of rheumatology at the University of Washington, Seattle. “Currently, Amgen is in the process of starting a trial in which the goal is to enroll 840 subjects with early PsA who are being randomized to methotrexate alone, Enbrel alone, or Enbrel plus methotrexate. This, I think, is going to give us a better answer about the effectiveness of methotrexate. It will also teach us about whether there’s a value in combining an anti-TNF inhibitor with methotrexate. We still don’t know the answer to that question.”
The most recent data on methotrexate come from an open-label trial known as TICOPA, which used a tight control treatment paradigm through 48 weeks of treatment. A subanalysis of 188 patients treated with methotrexate through 12 weeks was presented at the 2015 meeting of the European League Against Rheumatism. It revealed that 41% of patients achieved an ACR 20, 22% achieved minimal disease activity, 62% experienced an improvement in dactylitis, and 25% experienced an improvement in enthesitis.
“So we have a few data suggesting that methotrexate may be modestly effective in treating PsA,” Dr. Mease said. “We often will start with methotrexate unless the patient has really aggressive disease activity. If they get some effect from the drug but not enough, we’ll often add a biologic agent but often keep some methotrexate in the background, even at 10 mg per week, in order to reduce immunogenicity from a biologic.”
Dr. Mease disclosed that he has received research grants, consultation fees, and/or speaker honoraria from AbbVie, Amgen, Bristol-Myers Squibb, Celgene, Crescendo Bioscience, Genentech, GlaxoSmithKline, Janssen, Eli Lilly, Merck, Novartis, Pfizer, and UCB.
PARK CITY, UTAH – Since most people with psoriatic arthritis (PsA) develop psoriasis before joint symptoms, it’s helpful to have a simple screening test for the condition.
One of Dr. Philip Mease’s favorite PsA screening tools is the Psoriasis Epidemiology Screening Test (PEST), which was first described at the 2009 annual meeting of the Group for Research and Assessment of Psoriasis and Psoriatic Arthritis (GRAPPA) and consists of five simple questions. They are: Have you ever had a swollen joint (or joints)? Has a doctor ever told you that you have arthritis? Do your fingernails or toes have holes or pits? Have you had pain in your heel? Have you had a finger or toe that was completely swollen and painful for no apparent reason? (Clin Exp Rheumatol. 2009;27:469-74).
“Just these five simple questions, or trying to remember a few of them, can help you in your review of systems,” Dr. Mease, director of arthritis research at Swedish Medical Center, Seattle, said at the annual meeting of the Pacific Dermatologic Association. “I think patients appreciate it when you look beyond the skin in your questioning. These can pick up [PsA] with a high sensitivity and specificity” of 0.92 and 0.78, respectively.
He went on to discuss current PsA treatment approaches. According to an evidence review that he and his associates in GRAPPA published in 2009, biologics (anti–tumor necrosis factor inhibitors) as a group were found to be effective in all five domains of the disease: peripheral arthritis, skin and nail disease, axial disease, dactylitis, and enthesitis, while the oral disease-modifying antirheumatic drugs (DMARDs) were effective for peripheral arthritis and skin and nail disease.
Other treatments that were found effective are: psoralen and UVA/UVB for skin and nail disease, physiotherapy for axial disease, intra-articular steroids for peripheral arthritis, and NSAIDs for peripheral arthritis and axial disease (J Rheum. 2009;33:1417-21). “Patients with mild disease can be tried on NSAIDs, especially in a patient with monoarticular disease, but for the most part we need to move on to using systemic medication,” he said. Updated recommendations from GRAPPA include new data regarding ustekinumab, apremilast, and secukinumab, as well as data on comorbidities (J Rheumatol. 2015;42[6]:1052-5).
According to Dr. Mease, controlled trials of DMARDs in PsA patients have yielded treatment effects that range from marginal in the joints to marginal or none at all in the skin. Data from the Methotrexate in Psoriatic Arthritis trial conducted in the United Kingdom and published in 2012 showed no evidence that methotrexate improves inflammatory synovitis in active PsA (Rheumatol. 2012;51:1368-77).
“There were issues with this trial, including the fact that it took 5 years to enroll patients, and many dropped out, so I don’t think it’s a very reliable study,” said Dr. Mease, who is also a professor of medicine in the division of rheumatology at the University of Washington, Seattle. “Currently, Amgen is in the process of starting a trial in which the goal is to enroll 840 subjects with early PsA who are being randomized to methotrexate alone, Enbrel alone, or Enbrel plus methotrexate. This, I think, is going to give us a better answer about the effectiveness of methotrexate. It will also teach us about whether there’s a value in combining an anti-TNF inhibitor with methotrexate. We still don’t know the answer to that question.”
The most recent data on methotrexate come from an open-label trial known as TICOPA, which used a tight control treatment paradigm through 48 weeks of treatment. A subanalysis of 188 patients treated with methotrexate through 12 weeks was presented at the 2015 meeting of the European League Against Rheumatism. It revealed that 41% of patients achieved an ACR 20, 22% achieved minimal disease activity, 62% experienced an improvement in dactylitis, and 25% experienced an improvement in enthesitis.
“So we have a few data suggesting that methotrexate may be modestly effective in treating PsA,” Dr. Mease said. “We often will start with methotrexate unless the patient has really aggressive disease activity. If they get some effect from the drug but not enough, we’ll often add a biologic agent but often keep some methotrexate in the background, even at 10 mg per week, in order to reduce immunogenicity from a biologic.”
Dr. Mease disclosed that he has received research grants, consultation fees, and/or speaker honoraria from AbbVie, Amgen, Bristol-Myers Squibb, Celgene, Crescendo Bioscience, Genentech, GlaxoSmithKline, Janssen, Eli Lilly, Merck, Novartis, Pfizer, and UCB.
EXPERT ANALYSIS AT PDA 2015
Understanding of Mycoplasma-induced mucositis improving
PARK CITY, UTAH – According to emerging evidence in the medical literature, Mycoplasma pneumoniae–induced rash and mucositis is a distinct clinical entity with a distinct etiology and a good prognosis.
At the annual meeting of the Pacific Dermatologic Association, Dr. Erin Mathes shared tips on how to distinguish potentially life-threatening causes of rash and mucositis in children. “You want to think about their age, demographics, and history of medication exposures,” said Dr. Mathes, a pediatric dermatologist at the University of California, San Francisco. “Kids are exposed to viruses and bacteria all the time when they’re in day care. Also think about fever, whether or not they’re sick or well appearing, and the morphology.”
According to a retrospective study of 55 children with Stevens-Johnson Syndrome/toxic epidermal necrolysis (SJS/TEN) at the Hospital for Sick Children, Toronto, and Boston Children’s Hospital between 2000 and 2007, the top three causes of these rare conditions were antiepileptic drugs (29%), followed by M. pneumoniae infection (25%) and antibiotics (20%) (Pediatrics 2011;128[4]:723-8).
Dr. Mathes said that she learned from her mentor, Dr. Ilona Frieden, director of pediatric dermatology at UCSF Benioff Children’s Hospital, that in cases of M. pneumoniae-induced rash and mucositis (MIRM), 90% of the disease burden is the mucositis, and the remaining 10% is cutaneous findings. “So when you see mucositis far out of proportion to rash, think Mycoplasma,” Dr. Mathes said.
She and her associates at UCSF conducted a systematic review of all published cases on Mycoplasma-induced rash and mucositis and included 202 cases from 95 reports in the medical literature (J Am Acad Dermatol. 2015;72:239-45). The mean age of patients was 12 years, 66% were male, and most had a mucosal morphology (oral in 94% of cases, ocular in 82%, and genitourinary findings in 63%). “About one-third of patients had mucosal involvement alone, which is important to remember,” she said.
The patients’ skin involvement was generally mild; 46% was considered to be sparse or scattered, and 19% had moderate involvement. Extensive skin involvement “is rare, but it can happen,” Dr. Mathes said. Outcomes for the 202 patients were “generally good,” with 81% having no sequelae. The rates of recurrence and mucosal complications such as scarring around the mouth were both 8%, the rate of pigmentary alterations was 6%, and the rate of mortality was 3%, “but those cases were prior to the introduction of antibiotics in the 1940s,” she emphasized. “Children, in general, are not dying of Mycoplasma-associated rash and mucositis.”
A study published on July 25, 2015 in Pediatrics (doi:10.1542/peds.2015-0278) described an outbreak of eight Mycoplasma-associated SJS cases at Children’s Hospital Colorado, Aurora, that occurred over a 2-month period. In a case-control analysis comparing hospitalized SJS cases with and without evidence of M. pneumoniae infection, the researchers found that cases of Mycoplasma-associated SJS were significantly more likely to have pneumonia (odds ratio, 10), preceding respiratory symptoms (OR, 30), an erythrocyte sedimentation rate of greater than 35 mg/dL (OR, 22.8), and fewer than three affected skin sites (OR, 4.5).
Dr. Mathes was part of a research team that published diagnostic criteria for MIRM. These include less than 10% body surface area affected, involvement of two or more mucosal sites, the presence of few vesiculobullous lesions/scattered atypical targets with or without targetoid lesions, and clinical and laboratory evidence of atypical pneumonia (J Am Acad Dermatol. 2015;72:239-45).
“Rare cases can have more body surface area detachment, or no rash at all,” she said.
Existing tests for Mycoplasma infection are “not that great,” she continued. “It depends on the manufacturer and what is used as the gold standard.” IgM and IgG have specificity ranges from 25% to 100% and sensitivity ranges from 52% to 100%, she said, while polymerase chain reaction is rapidly becoming the gold standard. “That is usually from a nasal wash or bronchoalveolar lavage. It is very sensitive, but is almost too sensitive, because you can actually shed bacteria in your nasal pharynx for 4 months after an infection. That can lead to false positives.”
At UCSF, Dr. Mathes and her colleagues tend to treat MIRM cases with azithromycin followed by supportive care. “We occasionally use steroids but rarely use IVIG [intravenous immunoglobulin],” she said.
Dr. Mathes reported having no financial disclosures.
PARK CITY, UTAH – According to emerging evidence in the medical literature, Mycoplasma pneumoniae–induced rash and mucositis is a distinct clinical entity with a distinct etiology and a good prognosis.
At the annual meeting of the Pacific Dermatologic Association, Dr. Erin Mathes shared tips on how to distinguish potentially life-threatening causes of rash and mucositis in children. “You want to think about their age, demographics, and history of medication exposures,” said Dr. Mathes, a pediatric dermatologist at the University of California, San Francisco. “Kids are exposed to viruses and bacteria all the time when they’re in day care. Also think about fever, whether or not they’re sick or well appearing, and the morphology.”
According to a retrospective study of 55 children with Stevens-Johnson Syndrome/toxic epidermal necrolysis (SJS/TEN) at the Hospital for Sick Children, Toronto, and Boston Children’s Hospital between 2000 and 2007, the top three causes of these rare conditions were antiepileptic drugs (29%), followed by M. pneumoniae infection (25%) and antibiotics (20%) (Pediatrics 2011;128[4]:723-8).
Dr. Mathes said that she learned from her mentor, Dr. Ilona Frieden, director of pediatric dermatology at UCSF Benioff Children’s Hospital, that in cases of M. pneumoniae-induced rash and mucositis (MIRM), 90% of the disease burden is the mucositis, and the remaining 10% is cutaneous findings. “So when you see mucositis far out of proportion to rash, think Mycoplasma,” Dr. Mathes said.
She and her associates at UCSF conducted a systematic review of all published cases on Mycoplasma-induced rash and mucositis and included 202 cases from 95 reports in the medical literature (J Am Acad Dermatol. 2015;72:239-45). The mean age of patients was 12 years, 66% were male, and most had a mucosal morphology (oral in 94% of cases, ocular in 82%, and genitourinary findings in 63%). “About one-third of patients had mucosal involvement alone, which is important to remember,” she said.
The patients’ skin involvement was generally mild; 46% was considered to be sparse or scattered, and 19% had moderate involvement. Extensive skin involvement “is rare, but it can happen,” Dr. Mathes said. Outcomes for the 202 patients were “generally good,” with 81% having no sequelae. The rates of recurrence and mucosal complications such as scarring around the mouth were both 8%, the rate of pigmentary alterations was 6%, and the rate of mortality was 3%, “but those cases were prior to the introduction of antibiotics in the 1940s,” she emphasized. “Children, in general, are not dying of Mycoplasma-associated rash and mucositis.”
A study published on July 25, 2015 in Pediatrics (doi:10.1542/peds.2015-0278) described an outbreak of eight Mycoplasma-associated SJS cases at Children’s Hospital Colorado, Aurora, that occurred over a 2-month period. In a case-control analysis comparing hospitalized SJS cases with and without evidence of M. pneumoniae infection, the researchers found that cases of Mycoplasma-associated SJS were significantly more likely to have pneumonia (odds ratio, 10), preceding respiratory symptoms (OR, 30), an erythrocyte sedimentation rate of greater than 35 mg/dL (OR, 22.8), and fewer than three affected skin sites (OR, 4.5).
Dr. Mathes was part of a research team that published diagnostic criteria for MIRM. These include less than 10% body surface area affected, involvement of two or more mucosal sites, the presence of few vesiculobullous lesions/scattered atypical targets with or without targetoid lesions, and clinical and laboratory evidence of atypical pneumonia (J Am Acad Dermatol. 2015;72:239-45).
“Rare cases can have more body surface area detachment, or no rash at all,” she said.
Existing tests for Mycoplasma infection are “not that great,” she continued. “It depends on the manufacturer and what is used as the gold standard.” IgM and IgG have specificity ranges from 25% to 100% and sensitivity ranges from 52% to 100%, she said, while polymerase chain reaction is rapidly becoming the gold standard. “That is usually from a nasal wash or bronchoalveolar lavage. It is very sensitive, but is almost too sensitive, because you can actually shed bacteria in your nasal pharynx for 4 months after an infection. That can lead to false positives.”
At UCSF, Dr. Mathes and her colleagues tend to treat MIRM cases with azithromycin followed by supportive care. “We occasionally use steroids but rarely use IVIG [intravenous immunoglobulin],” she said.
Dr. Mathes reported having no financial disclosures.
PARK CITY, UTAH – According to emerging evidence in the medical literature, Mycoplasma pneumoniae–induced rash and mucositis is a distinct clinical entity with a distinct etiology and a good prognosis.
At the annual meeting of the Pacific Dermatologic Association, Dr. Erin Mathes shared tips on how to distinguish potentially life-threatening causes of rash and mucositis in children. “You want to think about their age, demographics, and history of medication exposures,” said Dr. Mathes, a pediatric dermatologist at the University of California, San Francisco. “Kids are exposed to viruses and bacteria all the time when they’re in day care. Also think about fever, whether or not they’re sick or well appearing, and the morphology.”
According to a retrospective study of 55 children with Stevens-Johnson Syndrome/toxic epidermal necrolysis (SJS/TEN) at the Hospital for Sick Children, Toronto, and Boston Children’s Hospital between 2000 and 2007, the top three causes of these rare conditions were antiepileptic drugs (29%), followed by M. pneumoniae infection (25%) and antibiotics (20%) (Pediatrics 2011;128[4]:723-8).
Dr. Mathes said that she learned from her mentor, Dr. Ilona Frieden, director of pediatric dermatology at UCSF Benioff Children’s Hospital, that in cases of M. pneumoniae-induced rash and mucositis (MIRM), 90% of the disease burden is the mucositis, and the remaining 10% is cutaneous findings. “So when you see mucositis far out of proportion to rash, think Mycoplasma,” Dr. Mathes said.
She and her associates at UCSF conducted a systematic review of all published cases on Mycoplasma-induced rash and mucositis and included 202 cases from 95 reports in the medical literature (J Am Acad Dermatol. 2015;72:239-45). The mean age of patients was 12 years, 66% were male, and most had a mucosal morphology (oral in 94% of cases, ocular in 82%, and genitourinary findings in 63%). “About one-third of patients had mucosal involvement alone, which is important to remember,” she said.
The patients’ skin involvement was generally mild; 46% was considered to be sparse or scattered, and 19% had moderate involvement. Extensive skin involvement “is rare, but it can happen,” Dr. Mathes said. Outcomes for the 202 patients were “generally good,” with 81% having no sequelae. The rates of recurrence and mucosal complications such as scarring around the mouth were both 8%, the rate of pigmentary alterations was 6%, and the rate of mortality was 3%, “but those cases were prior to the introduction of antibiotics in the 1940s,” she emphasized. “Children, in general, are not dying of Mycoplasma-associated rash and mucositis.”
A study published on July 25, 2015 in Pediatrics (doi:10.1542/peds.2015-0278) described an outbreak of eight Mycoplasma-associated SJS cases at Children’s Hospital Colorado, Aurora, that occurred over a 2-month period. In a case-control analysis comparing hospitalized SJS cases with and without evidence of M. pneumoniae infection, the researchers found that cases of Mycoplasma-associated SJS were significantly more likely to have pneumonia (odds ratio, 10), preceding respiratory symptoms (OR, 30), an erythrocyte sedimentation rate of greater than 35 mg/dL (OR, 22.8), and fewer than three affected skin sites (OR, 4.5).
Dr. Mathes was part of a research team that published diagnostic criteria for MIRM. These include less than 10% body surface area affected, involvement of two or more mucosal sites, the presence of few vesiculobullous lesions/scattered atypical targets with or without targetoid lesions, and clinical and laboratory evidence of atypical pneumonia (J Am Acad Dermatol. 2015;72:239-45).
“Rare cases can have more body surface area detachment, or no rash at all,” she said.
Existing tests for Mycoplasma infection are “not that great,” she continued. “It depends on the manufacturer and what is used as the gold standard.” IgM and IgG have specificity ranges from 25% to 100% and sensitivity ranges from 52% to 100%, she said, while polymerase chain reaction is rapidly becoming the gold standard. “That is usually from a nasal wash or bronchoalveolar lavage. It is very sensitive, but is almost too sensitive, because you can actually shed bacteria in your nasal pharynx for 4 months after an infection. That can lead to false positives.”
At UCSF, Dr. Mathes and her colleagues tend to treat MIRM cases with azithromycin followed by supportive care. “We occasionally use steroids but rarely use IVIG [intravenous immunoglobulin],” she said.
Dr. Mathes reported having no financial disclosures.
EXPERT ANALYSIS AT PDA 2015
Pruritus in the elderly often linked to atopic-like dermatosis
PARK CITY, UTAH – When an otherwise healthy 75-year-old patient presents with persistent pruritus as the chief complaint, the first thing to do is rule out specific dermatologic disorders, according to Dr. Kevin C. Wang.
“Thankfully, most of the patients complaining of pruritus have visible dermatoses,” Dr. Wang said at the annual meeting of the Pacific Dermatologic Association. “According to a review of more than 150 elderly patients in the outpatient setting who presented with a chief complaint of persistent pruritus, the five most common diagnoses were atopic-like dermatosis, lichen simplex chronicus/prurigo nodularis, subacute prurigo, transient acantholytic dermatosis, and neuropathic disease.”
Treatment directed at the primary element triggering the pruritus is most effective. “The best treatment for these patients will likely involve multiple modalities/combination therapy, as there is no one major pathway pathophysiologically,” said Dr. Wang of the department of dermatology at Stanford (Calif.) University and the Palo Alto VA Hospital.
“Pruritus can be quite debilitating,” said Dr. Wang, who also is the principal investigator of a research lab at Stanford. “I have not met an itchy patient who has said that it has not ruined their lives somehow: whether it’s their work, social life, things that they like to do. Also, many elderly veterans are already quite debilitated functionally in the first place, so it is a huge problem.”
An estimated 2% of all dermatology visits are for pruritus, “but it’s probably more than that, because a lot of those complaints don’t come up until you ask the patient in person,” he said. “This issue is also important because as physicians we really don’t have any specific ‘itch blockers.’ We just use drugs developed for other conditions that happen to work, but not often enough.”
Dr. Wang then went on to share some of the concepts raised in a 2011 article by Dr. Timothy G. Berger and Dr. Martin Steinhoff of the University of California, San Francisco. They suggested that pruritic conditions afflicting the elderly are the results of a variety of age-related changes they termed “eruptions of senescence” (Semin Cutan Med Surg. 2011;30[2]:113-7).
As people age, Dr. Wang said, the immune system “becomes much more proinflammatory, including significant aberration of T- and B-cell populations. More importantly, the immune system develops an allergic Th2 phenotype, where you have loss of naive T cells as the immune repertoire becomes populated with ‘committed’ T and B cells, and a preponderance of Th2 cells. This means you have an impaired ability to respond to new antigens, with a greater propensity for autoimmune responses, and lingering, low-grade inflammation.”
Aging also brings structural changes to the epidermal barrier, he continued. Specifically, the surface pH becomes less acidic. This is problematic because enzymes that are required to process lipids function best at acidic pH. “You also have a reduction in the rate of barrier repair, and decreased production of filaggrin and aquaporin-3,” he said. “In combination, this impaired barrier has two direct consequences: Barrier failure may lead to increased development of contact dermatitis, because the impaired barrier may not prevent penetration of potential antigens into the epidermis, and when the barrier fails, the cytokines released to induce barrier repair are proinflammatory, resulting in dermatitis.”
He reported having no financial disclosures.
PARK CITY, UTAH – When an otherwise healthy 75-year-old patient presents with persistent pruritus as the chief complaint, the first thing to do is rule out specific dermatologic disorders, according to Dr. Kevin C. Wang.
“Thankfully, most of the patients complaining of pruritus have visible dermatoses,” Dr. Wang said at the annual meeting of the Pacific Dermatologic Association. “According to a review of more than 150 elderly patients in the outpatient setting who presented with a chief complaint of persistent pruritus, the five most common diagnoses were atopic-like dermatosis, lichen simplex chronicus/prurigo nodularis, subacute prurigo, transient acantholytic dermatosis, and neuropathic disease.”
Treatment directed at the primary element triggering the pruritus is most effective. “The best treatment for these patients will likely involve multiple modalities/combination therapy, as there is no one major pathway pathophysiologically,” said Dr. Wang of the department of dermatology at Stanford (Calif.) University and the Palo Alto VA Hospital.
“Pruritus can be quite debilitating,” said Dr. Wang, who also is the principal investigator of a research lab at Stanford. “I have not met an itchy patient who has said that it has not ruined their lives somehow: whether it’s their work, social life, things that they like to do. Also, many elderly veterans are already quite debilitated functionally in the first place, so it is a huge problem.”
An estimated 2% of all dermatology visits are for pruritus, “but it’s probably more than that, because a lot of those complaints don’t come up until you ask the patient in person,” he said. “This issue is also important because as physicians we really don’t have any specific ‘itch blockers.’ We just use drugs developed for other conditions that happen to work, but not often enough.”
Dr. Wang then went on to share some of the concepts raised in a 2011 article by Dr. Timothy G. Berger and Dr. Martin Steinhoff of the University of California, San Francisco. They suggested that pruritic conditions afflicting the elderly are the results of a variety of age-related changes they termed “eruptions of senescence” (Semin Cutan Med Surg. 2011;30[2]:113-7).
As people age, Dr. Wang said, the immune system “becomes much more proinflammatory, including significant aberration of T- and B-cell populations. More importantly, the immune system develops an allergic Th2 phenotype, where you have loss of naive T cells as the immune repertoire becomes populated with ‘committed’ T and B cells, and a preponderance of Th2 cells. This means you have an impaired ability to respond to new antigens, with a greater propensity for autoimmune responses, and lingering, low-grade inflammation.”
Aging also brings structural changes to the epidermal barrier, he continued. Specifically, the surface pH becomes less acidic. This is problematic because enzymes that are required to process lipids function best at acidic pH. “You also have a reduction in the rate of barrier repair, and decreased production of filaggrin and aquaporin-3,” he said. “In combination, this impaired barrier has two direct consequences: Barrier failure may lead to increased development of contact dermatitis, because the impaired barrier may not prevent penetration of potential antigens into the epidermis, and when the barrier fails, the cytokines released to induce barrier repair are proinflammatory, resulting in dermatitis.”
He reported having no financial disclosures.
PARK CITY, UTAH – When an otherwise healthy 75-year-old patient presents with persistent pruritus as the chief complaint, the first thing to do is rule out specific dermatologic disorders, according to Dr. Kevin C. Wang.
“Thankfully, most of the patients complaining of pruritus have visible dermatoses,” Dr. Wang said at the annual meeting of the Pacific Dermatologic Association. “According to a review of more than 150 elderly patients in the outpatient setting who presented with a chief complaint of persistent pruritus, the five most common diagnoses were atopic-like dermatosis, lichen simplex chronicus/prurigo nodularis, subacute prurigo, transient acantholytic dermatosis, and neuropathic disease.”
Treatment directed at the primary element triggering the pruritus is most effective. “The best treatment for these patients will likely involve multiple modalities/combination therapy, as there is no one major pathway pathophysiologically,” said Dr. Wang of the department of dermatology at Stanford (Calif.) University and the Palo Alto VA Hospital.
“Pruritus can be quite debilitating,” said Dr. Wang, who also is the principal investigator of a research lab at Stanford. “I have not met an itchy patient who has said that it has not ruined their lives somehow: whether it’s their work, social life, things that they like to do. Also, many elderly veterans are already quite debilitated functionally in the first place, so it is a huge problem.”
An estimated 2% of all dermatology visits are for pruritus, “but it’s probably more than that, because a lot of those complaints don’t come up until you ask the patient in person,” he said. “This issue is also important because as physicians we really don’t have any specific ‘itch blockers.’ We just use drugs developed for other conditions that happen to work, but not often enough.”
Dr. Wang then went on to share some of the concepts raised in a 2011 article by Dr. Timothy G. Berger and Dr. Martin Steinhoff of the University of California, San Francisco. They suggested that pruritic conditions afflicting the elderly are the results of a variety of age-related changes they termed “eruptions of senescence” (Semin Cutan Med Surg. 2011;30[2]:113-7).
As people age, Dr. Wang said, the immune system “becomes much more proinflammatory, including significant aberration of T- and B-cell populations. More importantly, the immune system develops an allergic Th2 phenotype, where you have loss of naive T cells as the immune repertoire becomes populated with ‘committed’ T and B cells, and a preponderance of Th2 cells. This means you have an impaired ability to respond to new antigens, with a greater propensity for autoimmune responses, and lingering, low-grade inflammation.”
Aging also brings structural changes to the epidermal barrier, he continued. Specifically, the surface pH becomes less acidic. This is problematic because enzymes that are required to process lipids function best at acidic pH. “You also have a reduction in the rate of barrier repair, and decreased production of filaggrin and aquaporin-3,” he said. “In combination, this impaired barrier has two direct consequences: Barrier failure may lead to increased development of contact dermatitis, because the impaired barrier may not prevent penetration of potential antigens into the epidermis, and when the barrier fails, the cytokines released to induce barrier repair are proinflammatory, resulting in dermatitis.”
He reported having no financial disclosures.
EXPERT ANALYSIS FROM PDA 2015
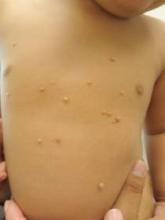
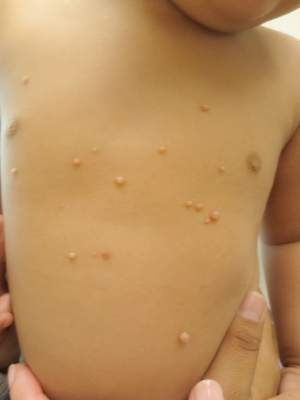
Expert Shares Treatment Tips for Molluscum Contagiosum and Warts
PARK CITY, UTAH – In her practice as a pediatric dermatologist, Dr. Sheryll L. Vanderhooft sees her share of children who present with molluscum contagiosum and warts. At the annual meeting of the Pacific Dermatologic Association, she shared her approach to caring for patients who present with these two common conditions.
The root cause of molluscum contagiosum is a poxvirus infection that spreads by skin-to-skin contact. It takes an average of 6 months to 2 years for the host immune response to occur. “During that time parents panic and some need a lot of hand-holding,” said Dr. Vanderhooft, professor of pediatric dermatology at the University of Utah School of Medicine, Salt Lake City. “They seek treatment primarily due to associated dermatitis triggered or exacerbated by the virus. There’s also the risk of spreading the condition to other children and the stigma of visible lesions.”
No FDA-approved therapies for molluscum contagiosum currently exist. “There are many options, but none of them are guaranteed to hasten resolution,” she said. “This is a very important point to deliver to parents before you start trying to do a lot of therapy.” Common treatment options are cryotherapy and cantharadin destruction, curettage, treatment with topical imiquimod or retinoids, oral cimetidine, and injections of Candida antigen.
According to two retrospective studies of Candida antigen injection, complete clearance occurred in 56% of patients after an average of 3 treatments, while partial clearance occurred in 28-38% of patients after an average of 4 treatments (Pediatr. Dermatol. 2008; 25:189-92 and Pediatr. Dermatol 2011; 28:254-8). The main side effect was local pain from the injections.
Antiviral and antitumor effects are seen with imiquimod cream through activation of the innate immunity and upregulation of cytokines, such as interferon-alpha, Dr. Vanderhooft said. It’s FDA approved for genital and perianal warts in patients older than 12 years of age, as well as for actinic keratosis, superficial basal cell carcinoma, and antiviral-resistant HSV in patients older than 12 years of age. In a prospective study, researchers compared imiquimod cream 5 times per week up to 16 weeks, with cryotherapy for the treatment of molluscum contagiosum in children (Pediatr. Dermatol. 2010; 27[4]:388-94). More than half of patients (60%) achieved clearance by week 6 and 92% achieved clearance by week 12. There were no relapses observed at six months. “Liquid nitrogen cleared the lesions faster, but caused more post-inflammatory dyspigmentation, and there were a few relapses in this group,” Dr. Vanderhooft said.
The downside to imiquimod treatment is that it requires prolonged use, it’s expensive, “and a lot of insurance companies will not pay for it because it’s not FDA approved for young children,” she said. “It can also cause irritant dermatitis, which is a difficult problem when you already have a kid who may be atopic and you’re trying to treat molluscum.” Also, according to the package insert for Aldara cream, imiquimod administered three times per week for 16 weeks was not shown to be superior to vehicle alone in two randomized, controlled trials that were completed in 2006 but never published. There were 323 children in one study, with complete clearance at 18 weeks in 24% of children treated with imiquimod, compared with 26% of children treated with vehicle alone. There were 379 in the second study with complete clearance in 24% and 28% of children, respectively.
Children who were treated with imiquimod in these two studies were more likely to experience application site reactions, otitis media, and conjunctivitis. In addition, a pharmacokinetic study of 22 children with molluscum involving at least 10% of body surface area showed systemically detectable drug levels after single and multiple doses 3 times a week for 4 weeks. The findings from these three studies led to changes in the FDA approved package insert in 2008. Section 1.4 of the “indications and usage” section of the package insert now reads: “Aldara cream has been evaluated in children ages 2012 years with molluscum contagiosum and these studies failed to demonstrate efficacy.” With that in mind, Dr. Vanderhooft’s recommendation “is to either stop prescribing it in children or prescribe it once daily or twice daily as tolerated, not every other day.”
While molluscum contagiosum is caused by a poxvirus, warts are growths on the skin caused by infection with human papillomavirus (HPV). On average, 75% of children will develop immunity to HPV within 3 years, “whether you do nothing, or whether you try to treat it,” she said. “Parents seek treatment when they are large, spreading, or causing social stigma. There are many treatment options, but none are guaranteed to hasten resolution. This needs to be driven home to patients when you’re counseling them.” Numerous treatment options exist, ranging from destruction with cryotherapy and pulsed-dye laser to salicylic acid and various topical or injectable agents. The only FDA-approved option is imiquimod cream, which is approved for genital and perianal warts only in patients 12 years of age and older.
In Dr. Vanderhooft’s experience, Candida antigen injections benefit some patients. After injection of 0.3 mL of Candida antigen into 1 or 2 warts at monthly intervals, researchers in one study observed complete clearance in 87% of patients after an average of 3.5 treatments, while 7% demonstrated no improvement after an average of 3.75 treatments (Pediatr. Dermatol. 2008; 25: 189-92).
Zinc supplementation is another option, she said. In a randomized trial, researchers who evaluated oral zinc supplementation versus placebo for two months found complete clearance in 20 out of 23 patients in the treatment group (87%), compared with none of the 20 patients in the placebo group (Br. J. Dermatol; 2002; 146[3]:423-31). All patients in the zinc group reported nausea. “That’s what has limited zinc therapy in my patient population,” Dr. Vanderhooft said.
In a more recent study, researchers conducted a placebo-controlled study of zinc sulfate 10 mg/kg per day up to 600 mg per day for up to two months (J. Am. Acad. Dermatol. 2009; 60[4]:706-8). Complete clearance of all warts was achieved in 78% of patients in the treatment group, compared with 13% in the placebo group. No recurrence of warts was observed at the six-month follow-up.
Topical 5-FU for warts has also been evaluated. One study of once or twice daily application of 5-FU under occlusion for 6 weeks demonstrated improvement in 88% of patients, including 13% with complete clearance (Br. J. Dermatol; 2011; 165[2]:233-46). No blood levels of the drug were detected. “It’s thought to be safe and well-tolerated, but over-the-counter salicylic acid has better efficacy and is quite a bit cheaper,” Dr. Vanderhooft said.
Another strategy for recalcitrant warts involves administration of squaric acid dibutyl ester. In a recent retrospective chart review conducted over a 10.5-year period, researchers evaluated 72 children with recalcitrant warts who had failed therapy with multiple agents and were followed for a period of 6 months to 11 years (Pediatr. Dermatol. 2015; 32:85-90). The protocol involved sensitizing the children to 2% squaric acid dibutyl ester (SADBE). The treatment then started two weeks later, with 0.4% SADBE applied 3 times per week initially, with an extra day added per week as tolerated to reach daily use if possible. The researchers found that 40 of the 48 (83%) patients in whom treatment outcomes could be obtained reported complete resolution of their warts. Seventy percent of patients used a maximum concentration of 0.4% SADBE and 60% of patients reported no adverse effects. The average time to reduction in size of warts was 2.6 months after sensitization, and treatment continued for a mean of 8 months.
Dr. Vanderhooft reported having no financial disclosures.
PARK CITY, UTAH – In her practice as a pediatric dermatologist, Dr. Sheryll L. Vanderhooft sees her share of children who present with molluscum contagiosum and warts. At the annual meeting of the Pacific Dermatologic Association, she shared her approach to caring for patients who present with these two common conditions.
The root cause of molluscum contagiosum is a poxvirus infection that spreads by skin-to-skin contact. It takes an average of 6 months to 2 years for the host immune response to occur. “During that time parents panic and some need a lot of hand-holding,” said Dr. Vanderhooft, professor of pediatric dermatology at the University of Utah School of Medicine, Salt Lake City. “They seek treatment primarily due to associated dermatitis triggered or exacerbated by the virus. There’s also the risk of spreading the condition to other children and the stigma of visible lesions.”
No FDA-approved therapies for molluscum contagiosum currently exist. “There are many options, but none of them are guaranteed to hasten resolution,” she said. “This is a very important point to deliver to parents before you start trying to do a lot of therapy.” Common treatment options are cryotherapy and cantharadin destruction, curettage, treatment with topical imiquimod or retinoids, oral cimetidine, and injections of Candida antigen.
According to two retrospective studies of Candida antigen injection, complete clearance occurred in 56% of patients after an average of 3 treatments, while partial clearance occurred in 28-38% of patients after an average of 4 treatments (Pediatr. Dermatol. 2008; 25:189-92 and Pediatr. Dermatol 2011; 28:254-8). The main side effect was local pain from the injections.
Antiviral and antitumor effects are seen with imiquimod cream through activation of the innate immunity and upregulation of cytokines, such as interferon-alpha, Dr. Vanderhooft said. It’s FDA approved for genital and perianal warts in patients older than 12 years of age, as well as for actinic keratosis, superficial basal cell carcinoma, and antiviral-resistant HSV in patients older than 12 years of age. In a prospective study, researchers compared imiquimod cream 5 times per week up to 16 weeks, with cryotherapy for the treatment of molluscum contagiosum in children (Pediatr. Dermatol. 2010; 27[4]:388-94). More than half of patients (60%) achieved clearance by week 6 and 92% achieved clearance by week 12. There were no relapses observed at six months. “Liquid nitrogen cleared the lesions faster, but caused more post-inflammatory dyspigmentation, and there were a few relapses in this group,” Dr. Vanderhooft said.
The downside to imiquimod treatment is that it requires prolonged use, it’s expensive, “and a lot of insurance companies will not pay for it because it’s not FDA approved for young children,” she said. “It can also cause irritant dermatitis, which is a difficult problem when you already have a kid who may be atopic and you’re trying to treat molluscum.” Also, according to the package insert for Aldara cream, imiquimod administered three times per week for 16 weeks was not shown to be superior to vehicle alone in two randomized, controlled trials that were completed in 2006 but never published. There were 323 children in one study, with complete clearance at 18 weeks in 24% of children treated with imiquimod, compared with 26% of children treated with vehicle alone. There were 379 in the second study with complete clearance in 24% and 28% of children, respectively.
Children who were treated with imiquimod in these two studies were more likely to experience application site reactions, otitis media, and conjunctivitis. In addition, a pharmacokinetic study of 22 children with molluscum involving at least 10% of body surface area showed systemically detectable drug levels after single and multiple doses 3 times a week for 4 weeks. The findings from these three studies led to changes in the FDA approved package insert in 2008. Section 1.4 of the “indications and usage” section of the package insert now reads: “Aldara cream has been evaluated in children ages 2012 years with molluscum contagiosum and these studies failed to demonstrate efficacy.” With that in mind, Dr. Vanderhooft’s recommendation “is to either stop prescribing it in children or prescribe it once daily or twice daily as tolerated, not every other day.”
While molluscum contagiosum is caused by a poxvirus, warts are growths on the skin caused by infection with human papillomavirus (HPV). On average, 75% of children will develop immunity to HPV within 3 years, “whether you do nothing, or whether you try to treat it,” she said. “Parents seek treatment when they are large, spreading, or causing social stigma. There are many treatment options, but none are guaranteed to hasten resolution. This needs to be driven home to patients when you’re counseling them.” Numerous treatment options exist, ranging from destruction with cryotherapy and pulsed-dye laser to salicylic acid and various topical or injectable agents. The only FDA-approved option is imiquimod cream, which is approved for genital and perianal warts only in patients 12 years of age and older.
In Dr. Vanderhooft’s experience, Candida antigen injections benefit some patients. After injection of 0.3 mL of Candida antigen into 1 or 2 warts at monthly intervals, researchers in one study observed complete clearance in 87% of patients after an average of 3.5 treatments, while 7% demonstrated no improvement after an average of 3.75 treatments (Pediatr. Dermatol. 2008; 25: 189-92).
Zinc supplementation is another option, she said. In a randomized trial, researchers who evaluated oral zinc supplementation versus placebo for two months found complete clearance in 20 out of 23 patients in the treatment group (87%), compared with none of the 20 patients in the placebo group (Br. J. Dermatol; 2002; 146[3]:423-31). All patients in the zinc group reported nausea. “That’s what has limited zinc therapy in my patient population,” Dr. Vanderhooft said.
In a more recent study, researchers conducted a placebo-controlled study of zinc sulfate 10 mg/kg per day up to 600 mg per day for up to two months (J. Am. Acad. Dermatol. 2009; 60[4]:706-8). Complete clearance of all warts was achieved in 78% of patients in the treatment group, compared with 13% in the placebo group. No recurrence of warts was observed at the six-month follow-up.
Topical 5-FU for warts has also been evaluated. One study of once or twice daily application of 5-FU under occlusion for 6 weeks demonstrated improvement in 88% of patients, including 13% with complete clearance (Br. J. Dermatol; 2011; 165[2]:233-46). No blood levels of the drug were detected. “It’s thought to be safe and well-tolerated, but over-the-counter salicylic acid has better efficacy and is quite a bit cheaper,” Dr. Vanderhooft said.
Another strategy for recalcitrant warts involves administration of squaric acid dibutyl ester. In a recent retrospective chart review conducted over a 10.5-year period, researchers evaluated 72 children with recalcitrant warts who had failed therapy with multiple agents and were followed for a period of 6 months to 11 years (Pediatr. Dermatol. 2015; 32:85-90). The protocol involved sensitizing the children to 2% squaric acid dibutyl ester (SADBE). The treatment then started two weeks later, with 0.4% SADBE applied 3 times per week initially, with an extra day added per week as tolerated to reach daily use if possible. The researchers found that 40 of the 48 (83%) patients in whom treatment outcomes could be obtained reported complete resolution of their warts. Seventy percent of patients used a maximum concentration of 0.4% SADBE and 60% of patients reported no adverse effects. The average time to reduction in size of warts was 2.6 months after sensitization, and treatment continued for a mean of 8 months.
Dr. Vanderhooft reported having no financial disclosures.
PARK CITY, UTAH – In her practice as a pediatric dermatologist, Dr. Sheryll L. Vanderhooft sees her share of children who present with molluscum contagiosum and warts. At the annual meeting of the Pacific Dermatologic Association, she shared her approach to caring for patients who present with these two common conditions.
The root cause of molluscum contagiosum is a poxvirus infection that spreads by skin-to-skin contact. It takes an average of 6 months to 2 years for the host immune response to occur. “During that time parents panic and some need a lot of hand-holding,” said Dr. Vanderhooft, professor of pediatric dermatology at the University of Utah School of Medicine, Salt Lake City. “They seek treatment primarily due to associated dermatitis triggered or exacerbated by the virus. There’s also the risk of spreading the condition to other children and the stigma of visible lesions.”
No FDA-approved therapies for molluscum contagiosum currently exist. “There are many options, but none of them are guaranteed to hasten resolution,” she said. “This is a very important point to deliver to parents before you start trying to do a lot of therapy.” Common treatment options are cryotherapy and cantharadin destruction, curettage, treatment with topical imiquimod or retinoids, oral cimetidine, and injections of Candida antigen.
According to two retrospective studies of Candida antigen injection, complete clearance occurred in 56% of patients after an average of 3 treatments, while partial clearance occurred in 28-38% of patients after an average of 4 treatments (Pediatr. Dermatol. 2008; 25:189-92 and Pediatr. Dermatol 2011; 28:254-8). The main side effect was local pain from the injections.
Antiviral and antitumor effects are seen with imiquimod cream through activation of the innate immunity and upregulation of cytokines, such as interferon-alpha, Dr. Vanderhooft said. It’s FDA approved for genital and perianal warts in patients older than 12 years of age, as well as for actinic keratosis, superficial basal cell carcinoma, and antiviral-resistant HSV in patients older than 12 years of age. In a prospective study, researchers compared imiquimod cream 5 times per week up to 16 weeks, with cryotherapy for the treatment of molluscum contagiosum in children (Pediatr. Dermatol. 2010; 27[4]:388-94). More than half of patients (60%) achieved clearance by week 6 and 92% achieved clearance by week 12. There were no relapses observed at six months. “Liquid nitrogen cleared the lesions faster, but caused more post-inflammatory dyspigmentation, and there were a few relapses in this group,” Dr. Vanderhooft said.
The downside to imiquimod treatment is that it requires prolonged use, it’s expensive, “and a lot of insurance companies will not pay for it because it’s not FDA approved for young children,” she said. “It can also cause irritant dermatitis, which is a difficult problem when you already have a kid who may be atopic and you’re trying to treat molluscum.” Also, according to the package insert for Aldara cream, imiquimod administered three times per week for 16 weeks was not shown to be superior to vehicle alone in two randomized, controlled trials that were completed in 2006 but never published. There were 323 children in one study, with complete clearance at 18 weeks in 24% of children treated with imiquimod, compared with 26% of children treated with vehicle alone. There were 379 in the second study with complete clearance in 24% and 28% of children, respectively.
Children who were treated with imiquimod in these two studies were more likely to experience application site reactions, otitis media, and conjunctivitis. In addition, a pharmacokinetic study of 22 children with molluscum involving at least 10% of body surface area showed systemically detectable drug levels after single and multiple doses 3 times a week for 4 weeks. The findings from these three studies led to changes in the FDA approved package insert in 2008. Section 1.4 of the “indications and usage” section of the package insert now reads: “Aldara cream has been evaluated in children ages 2012 years with molluscum contagiosum and these studies failed to demonstrate efficacy.” With that in mind, Dr. Vanderhooft’s recommendation “is to either stop prescribing it in children or prescribe it once daily or twice daily as tolerated, not every other day.”
While molluscum contagiosum is caused by a poxvirus, warts are growths on the skin caused by infection with human papillomavirus (HPV). On average, 75% of children will develop immunity to HPV within 3 years, “whether you do nothing, or whether you try to treat it,” she said. “Parents seek treatment when they are large, spreading, or causing social stigma. There are many treatment options, but none are guaranteed to hasten resolution. This needs to be driven home to patients when you’re counseling them.” Numerous treatment options exist, ranging from destruction with cryotherapy and pulsed-dye laser to salicylic acid and various topical or injectable agents. The only FDA-approved option is imiquimod cream, which is approved for genital and perianal warts only in patients 12 years of age and older.
In Dr. Vanderhooft’s experience, Candida antigen injections benefit some patients. After injection of 0.3 mL of Candida antigen into 1 or 2 warts at monthly intervals, researchers in one study observed complete clearance in 87% of patients after an average of 3.5 treatments, while 7% demonstrated no improvement after an average of 3.75 treatments (Pediatr. Dermatol. 2008; 25: 189-92).
Zinc supplementation is another option, she said. In a randomized trial, researchers who evaluated oral zinc supplementation versus placebo for two months found complete clearance in 20 out of 23 patients in the treatment group (87%), compared with none of the 20 patients in the placebo group (Br. J. Dermatol; 2002; 146[3]:423-31). All patients in the zinc group reported nausea. “That’s what has limited zinc therapy in my patient population,” Dr. Vanderhooft said.
In a more recent study, researchers conducted a placebo-controlled study of zinc sulfate 10 mg/kg per day up to 600 mg per day for up to two months (J. Am. Acad. Dermatol. 2009; 60[4]:706-8). Complete clearance of all warts was achieved in 78% of patients in the treatment group, compared with 13% in the placebo group. No recurrence of warts was observed at the six-month follow-up.
Topical 5-FU for warts has also been evaluated. One study of once or twice daily application of 5-FU under occlusion for 6 weeks demonstrated improvement in 88% of patients, including 13% with complete clearance (Br. J. Dermatol; 2011; 165[2]:233-46). No blood levels of the drug were detected. “It’s thought to be safe and well-tolerated, but over-the-counter salicylic acid has better efficacy and is quite a bit cheaper,” Dr. Vanderhooft said.
Another strategy for recalcitrant warts involves administration of squaric acid dibutyl ester. In a recent retrospective chart review conducted over a 10.5-year period, researchers evaluated 72 children with recalcitrant warts who had failed therapy with multiple agents and were followed for a period of 6 months to 11 years (Pediatr. Dermatol. 2015; 32:85-90). The protocol involved sensitizing the children to 2% squaric acid dibutyl ester (SADBE). The treatment then started two weeks later, with 0.4% SADBE applied 3 times per week initially, with an extra day added per week as tolerated to reach daily use if possible. The researchers found that 40 of the 48 (83%) patients in whom treatment outcomes could be obtained reported complete resolution of their warts. Seventy percent of patients used a maximum concentration of 0.4% SADBE and 60% of patients reported no adverse effects. The average time to reduction in size of warts was 2.6 months after sensitization, and treatment continued for a mean of 8 months.
Dr. Vanderhooft reported having no financial disclosures.
EXPERT ANALYSIS AT PDA 2015
Expert shares treatment tips for molluscum contagiosum and warts
PARK CITY, UTAH – In her practice as a pediatric dermatologist, Dr. Sheryll L. Vanderhooft sees her share of children who present with molluscum contagiosum and warts. At the annual meeting of the Pacific Dermatologic Association, she shared her approach to caring for patients who present with these two common conditions.
The root cause of molluscum contagiosum is a poxvirus infection that spreads by skin-to-skin contact. It takes an average of 6 months to 2 years for the host immune response to occur. “During that time parents panic and some need a lot of hand-holding,” said Dr. Vanderhooft, professor of pediatric dermatology at the University of Utah School of Medicine, Salt Lake City. “They seek treatment primarily due to associated dermatitis triggered or exacerbated by the virus. There’s also the risk of spreading the condition to other children and the stigma of visible lesions.”
No FDA-approved therapies for molluscum contagiosum currently exist. “There are many options, but none of them are guaranteed to hasten resolution,” she said. “This is a very important point to deliver to parents before you start trying to do a lot of therapy.” Common treatment options are cryotherapy and cantharadin destruction, curettage, treatment with topical imiquimod or retinoids, oral cimetidine, and injections of Candida antigen.
According to two retrospective studies of Candida antigen injection, complete clearance occurred in 56% of patients after an average of 3 treatments, while partial clearance occurred in 28-38% of patients after an average of 4 treatments (Pediatr. Dermatol. 2008; 25:189-92 and Pediatr. Dermatol 2011; 28:254-8). The main side effect was local pain from the injections.
Antiviral and antitumor effects are seen with imiquimod cream through activation of the innate immunity and upregulation of cytokines, such as interferon-alpha, Dr. Vanderhooft said. It’s FDA approved for genital and perianal warts in patients older than 12 years of age, as well as for actinic keratosis, superficial basal cell carcinoma, and antiviral-resistant HSV in patients older than 12 years of age. In a prospective study, researchers compared imiquimod cream 5 times per week up to 16 weeks, with cryotherapy for the treatment of molluscum contagiosum in children (Pediatr. Dermatol. 2010; 27[4]:388-94). More than half of patients (60%) achieved clearance by week 6 and 92% achieved clearance by week 12. There were no relapses observed at six months. “Liquid nitrogen cleared the lesions faster, but caused more post-inflammatory dyspigmentation, and there were a few relapses in this group,” Dr. Vanderhooft said.
The downside to imiquimod treatment is that it requires prolonged use, it’s expensive, “and a lot of insurance companies will not pay for it because it’s not FDA approved for young children,” she said. “It can also cause irritant dermatitis, which is a difficult problem when you already have a kid who may be atopic and you’re trying to treat molluscum.” Also, according to the package insert for Aldara cream, imiquimod administered three times per week for 16 weeks was not shown to be superior to vehicle alone in two randomized, controlled trials that were completed in 2006 but never published. There were 323 children in one study, with complete clearance at 18 weeks in 24% of children treated with imiquimod, compared with 26% of children treated with vehicle alone. There were 379 in the second study with complete clearance in 24% and 28% of children, respectively.
Children who were treated with imiquimod in these two studies were more likely to experience application site reactions, otitis media, and conjunctivitis. In addition, a pharmacokinetic study of 22 children with molluscum involving at least 10% of body surface area showed systemically detectable drug levels after single and multiple doses 3 times a week for 4 weeks. The findings from these three studies led to changes in the FDA approved package insert in 2008. Section 1.4 of the “indications and usage” section of the package insert now reads: “Aldara cream has been evaluated in children ages 2012 years with molluscum contagiosum and these studies failed to demonstrate efficacy.” With that in mind, Dr. Vanderhooft’s recommendation “is to either stop prescribing it in children or prescribe it once daily or twice daily as tolerated, not every other day.”
While molluscum contagiosum is caused by a poxvirus, warts are growths on the skin caused by infection with human papillomavirus (HPV). On average, 75% of children will develop immunity to HPV within 3 years, “whether you do nothing, or whether you try to treat it,” she said. “Parents seek treatment when they are large, spreading, or causing social stigma. There are many treatment options, but none are guaranteed to hasten resolution. This needs to be driven home to patients when you’re counseling them.” Numerous treatment options exist, ranging from destruction with cryotherapy and pulsed-dye laser to salicylic acid and various topical or injectable agents. The only FDA-approved option is imiquimod cream, which is approved for genital and perianal warts only in patients 12 years of age and older.
In Dr. Vanderhooft’s experience, Candida antigen injections benefit some patients. After injection of 0.3 mL of Candida antigen into 1 or 2 warts at monthly intervals, researchers in one study observed complete clearance in 87% of patients after an average of 3.5 treatments, while 7% demonstrated no improvement after an average of 3.75 treatments (Pediatr. Dermatol. 2008; 25: 189-92).
Zinc supplementation is another option, she said. In a randomized trial, researchers who evaluated oral zinc supplementation versus placebo for two months found complete clearance in 20 out of 23 patients in the treatment group (87%), compared with none of the 20 patients in the placebo group (Br. J. Dermatol; 2002; 146[3]:423-31). All patients in the zinc group reported nausea. “That’s what has limited zinc therapy in my patient population,” Dr. Vanderhooft said.
In a more recent study, researchers conducted a placebo-controlled study of zinc sulfate 10 mg/kg per day up to 600 mg per day for up to two months (J. Am. Acad. Dermatol. 2009; 60[4]:706-8). Complete clearance of all warts was achieved in 78% of patients in the treatment group, compared with 13% in the placebo group. No recurrence of warts was observed at the six-month follow-up.
Topical 5-FU for warts has also been evaluated. One study of once or twice daily application of 5-FU under occlusion for 6 weeks demonstrated improvement in 88% of patients, including 13% with complete clearance (Br. J. Dermatol; 2011; 165[2]:233-46). No blood levels of the drug were detected. “It’s thought to be safe and well-tolerated, but over-the-counter salicylic acid has better efficacy and is quite a bit cheaper,” Dr. Vanderhooft said.
Another strategy for recalcitrant warts involves administration of squaric acid dibutyl ester. In a recent retrospective chart review conducted over a 10.5-year period, researchers evaluated 72 children with recalcitrant warts who had failed therapy with multiple agents and were followed for a period of 6 months to 11 years (Pediatr. Dermatol. 2015; 32:85-90). The protocol involved sensitizing the children to 2% squaric acid dibutyl ester (SADBE). The treatment then started two weeks later, with 0.4% SADBE applied 3 times per week initially, with an extra day added per week as tolerated to reach daily use if possible. The researchers found that 40 of the 48 (83%) patients in whom treatment outcomes could be obtained reported complete resolution of their warts. Seventy percent of patients used a maximum concentration of 0.4% SADBE and 60% of patients reported no adverse effects. The average time to reduction in size of warts was 2.6 months after sensitization, and treatment continued for a mean of 8 months.
Dr. Vanderhooft reported having no financial disclosures.
PARK CITY, UTAH – In her practice as a pediatric dermatologist, Dr. Sheryll L. Vanderhooft sees her share of children who present with molluscum contagiosum and warts. At the annual meeting of the Pacific Dermatologic Association, she shared her approach to caring for patients who present with these two common conditions.
The root cause of molluscum contagiosum is a poxvirus infection that spreads by skin-to-skin contact. It takes an average of 6 months to 2 years for the host immune response to occur. “During that time parents panic and some need a lot of hand-holding,” said Dr. Vanderhooft, professor of pediatric dermatology at the University of Utah School of Medicine, Salt Lake City. “They seek treatment primarily due to associated dermatitis triggered or exacerbated by the virus. There’s also the risk of spreading the condition to other children and the stigma of visible lesions.”
No FDA-approved therapies for molluscum contagiosum currently exist. “There are many options, but none of them are guaranteed to hasten resolution,” she said. “This is a very important point to deliver to parents before you start trying to do a lot of therapy.” Common treatment options are cryotherapy and cantharadin destruction, curettage, treatment with topical imiquimod or retinoids, oral cimetidine, and injections of Candida antigen.
According to two retrospective studies of Candida antigen injection, complete clearance occurred in 56% of patients after an average of 3 treatments, while partial clearance occurred in 28-38% of patients after an average of 4 treatments (Pediatr. Dermatol. 2008; 25:189-92 and Pediatr. Dermatol 2011; 28:254-8). The main side effect was local pain from the injections.
Antiviral and antitumor effects are seen with imiquimod cream through activation of the innate immunity and upregulation of cytokines, such as interferon-alpha, Dr. Vanderhooft said. It’s FDA approved for genital and perianal warts in patients older than 12 years of age, as well as for actinic keratosis, superficial basal cell carcinoma, and antiviral-resistant HSV in patients older than 12 years of age. In a prospective study, researchers compared imiquimod cream 5 times per week up to 16 weeks, with cryotherapy for the treatment of molluscum contagiosum in children (Pediatr. Dermatol. 2010; 27[4]:388-94). More than half of patients (60%) achieved clearance by week 6 and 92% achieved clearance by week 12. There were no relapses observed at six months. “Liquid nitrogen cleared the lesions faster, but caused more post-inflammatory dyspigmentation, and there were a few relapses in this group,” Dr. Vanderhooft said.
The downside to imiquimod treatment is that it requires prolonged use, it’s expensive, “and a lot of insurance companies will not pay for it because it’s not FDA approved for young children,” she said. “It can also cause irritant dermatitis, which is a difficult problem when you already have a kid who may be atopic and you’re trying to treat molluscum.” Also, according to the package insert for Aldara cream, imiquimod administered three times per week for 16 weeks was not shown to be superior to vehicle alone in two randomized, controlled trials that were completed in 2006 but never published. There were 323 children in one study, with complete clearance at 18 weeks in 24% of children treated with imiquimod, compared with 26% of children treated with vehicle alone. There were 379 in the second study with complete clearance in 24% and 28% of children, respectively.
Children who were treated with imiquimod in these two studies were more likely to experience application site reactions, otitis media, and conjunctivitis. In addition, a pharmacokinetic study of 22 children with molluscum involving at least 10% of body surface area showed systemically detectable drug levels after single and multiple doses 3 times a week for 4 weeks. The findings from these three studies led to changes in the FDA approved package insert in 2008. Section 1.4 of the “indications and usage” section of the package insert now reads: “Aldara cream has been evaluated in children ages 2012 years with molluscum contagiosum and these studies failed to demonstrate efficacy.” With that in mind, Dr. Vanderhooft’s recommendation “is to either stop prescribing it in children or prescribe it once daily or twice daily as tolerated, not every other day.”
While molluscum contagiosum is caused by a poxvirus, warts are growths on the skin caused by infection with human papillomavirus (HPV). On average, 75% of children will develop immunity to HPV within 3 years, “whether you do nothing, or whether you try to treat it,” she said. “Parents seek treatment when they are large, spreading, or causing social stigma. There are many treatment options, but none are guaranteed to hasten resolution. This needs to be driven home to patients when you’re counseling them.” Numerous treatment options exist, ranging from destruction with cryotherapy and pulsed-dye laser to salicylic acid and various topical or injectable agents. The only FDA-approved option is imiquimod cream, which is approved for genital and perianal warts only in patients 12 years of age and older.
In Dr. Vanderhooft’s experience, Candida antigen injections benefit some patients. After injection of 0.3 mL of Candida antigen into 1 or 2 warts at monthly intervals, researchers in one study observed complete clearance in 87% of patients after an average of 3.5 treatments, while 7% demonstrated no improvement after an average of 3.75 treatments (Pediatr. Dermatol. 2008; 25: 189-92).
Zinc supplementation is another option, she said. In a randomized trial, researchers who evaluated oral zinc supplementation versus placebo for two months found complete clearance in 20 out of 23 patients in the treatment group (87%), compared with none of the 20 patients in the placebo group (Br. J. Dermatol; 2002; 146[3]:423-31). All patients in the zinc group reported nausea. “That’s what has limited zinc therapy in my patient population,” Dr. Vanderhooft said.
In a more recent study, researchers conducted a placebo-controlled study of zinc sulfate 10 mg/kg per day up to 600 mg per day for up to two months (J. Am. Acad. Dermatol. 2009; 60[4]:706-8). Complete clearance of all warts was achieved in 78% of patients in the treatment group, compared with 13% in the placebo group. No recurrence of warts was observed at the six-month follow-up.
Topical 5-FU for warts has also been evaluated. One study of once or twice daily application of 5-FU under occlusion for 6 weeks demonstrated improvement in 88% of patients, including 13% with complete clearance (Br. J. Dermatol; 2011; 165[2]:233-46). No blood levels of the drug were detected. “It’s thought to be safe and well-tolerated, but over-the-counter salicylic acid has better efficacy and is quite a bit cheaper,” Dr. Vanderhooft said.
Another strategy for recalcitrant warts involves administration of squaric acid dibutyl ester. In a recent retrospective chart review conducted over a 10.5-year period, researchers evaluated 72 children with recalcitrant warts who had failed therapy with multiple agents and were followed for a period of 6 months to 11 years (Pediatr. Dermatol. 2015; 32:85-90). The protocol involved sensitizing the children to 2% squaric acid dibutyl ester (SADBE). The treatment then started two weeks later, with 0.4% SADBE applied 3 times per week initially, with an extra day added per week as tolerated to reach daily use if possible. The researchers found that 40 of the 48 (83%) patients in whom treatment outcomes could be obtained reported complete resolution of their warts. Seventy percent of patients used a maximum concentration of 0.4% SADBE and 60% of patients reported no adverse effects. The average time to reduction in size of warts was 2.6 months after sensitization, and treatment continued for a mean of 8 months.
Dr. Vanderhooft reported having no financial disclosures.
PARK CITY, UTAH – In her practice as a pediatric dermatologist, Dr. Sheryll L. Vanderhooft sees her share of children who present with molluscum contagiosum and warts. At the annual meeting of the Pacific Dermatologic Association, she shared her approach to caring for patients who present with these two common conditions.
The root cause of molluscum contagiosum is a poxvirus infection that spreads by skin-to-skin contact. It takes an average of 6 months to 2 years for the host immune response to occur. “During that time parents panic and some need a lot of hand-holding,” said Dr. Vanderhooft, professor of pediatric dermatology at the University of Utah School of Medicine, Salt Lake City. “They seek treatment primarily due to associated dermatitis triggered or exacerbated by the virus. There’s also the risk of spreading the condition to other children and the stigma of visible lesions.”
No FDA-approved therapies for molluscum contagiosum currently exist. “There are many options, but none of them are guaranteed to hasten resolution,” she said. “This is a very important point to deliver to parents before you start trying to do a lot of therapy.” Common treatment options are cryotherapy and cantharadin destruction, curettage, treatment with topical imiquimod or retinoids, oral cimetidine, and injections of Candida antigen.
According to two retrospective studies of Candida antigen injection, complete clearance occurred in 56% of patients after an average of 3 treatments, while partial clearance occurred in 28-38% of patients after an average of 4 treatments (Pediatr. Dermatol. 2008; 25:189-92 and Pediatr. Dermatol 2011; 28:254-8). The main side effect was local pain from the injections.
Antiviral and antitumor effects are seen with imiquimod cream through activation of the innate immunity and upregulation of cytokines, such as interferon-alpha, Dr. Vanderhooft said. It’s FDA approved for genital and perianal warts in patients older than 12 years of age, as well as for actinic keratosis, superficial basal cell carcinoma, and antiviral-resistant HSV in patients older than 12 years of age. In a prospective study, researchers compared imiquimod cream 5 times per week up to 16 weeks, with cryotherapy for the treatment of molluscum contagiosum in children (Pediatr. Dermatol. 2010; 27[4]:388-94). More than half of patients (60%) achieved clearance by week 6 and 92% achieved clearance by week 12. There were no relapses observed at six months. “Liquid nitrogen cleared the lesions faster, but caused more post-inflammatory dyspigmentation, and there were a few relapses in this group,” Dr. Vanderhooft said.
The downside to imiquimod treatment is that it requires prolonged use, it’s expensive, “and a lot of insurance companies will not pay for it because it’s not FDA approved for young children,” she said. “It can also cause irritant dermatitis, which is a difficult problem when you already have a kid who may be atopic and you’re trying to treat molluscum.” Also, according to the package insert for Aldara cream, imiquimod administered three times per week for 16 weeks was not shown to be superior to vehicle alone in two randomized, controlled trials that were completed in 2006 but never published. There were 323 children in one study, with complete clearance at 18 weeks in 24% of children treated with imiquimod, compared with 26% of children treated with vehicle alone. There were 379 in the second study with complete clearance in 24% and 28% of children, respectively.
Children who were treated with imiquimod in these two studies were more likely to experience application site reactions, otitis media, and conjunctivitis. In addition, a pharmacokinetic study of 22 children with molluscum involving at least 10% of body surface area showed systemically detectable drug levels after single and multiple doses 3 times a week for 4 weeks. The findings from these three studies led to changes in the FDA approved package insert in 2008. Section 1.4 of the “indications and usage” section of the package insert now reads: “Aldara cream has been evaluated in children ages 2012 years with molluscum contagiosum and these studies failed to demonstrate efficacy.” With that in mind, Dr. Vanderhooft’s recommendation “is to either stop prescribing it in children or prescribe it once daily or twice daily as tolerated, not every other day.”
While molluscum contagiosum is caused by a poxvirus, warts are growths on the skin caused by infection with human papillomavirus (HPV). On average, 75% of children will develop immunity to HPV within 3 years, “whether you do nothing, or whether you try to treat it,” she said. “Parents seek treatment when they are large, spreading, or causing social stigma. There are many treatment options, but none are guaranteed to hasten resolution. This needs to be driven home to patients when you’re counseling them.” Numerous treatment options exist, ranging from destruction with cryotherapy and pulsed-dye laser to salicylic acid and various topical or injectable agents. The only FDA-approved option is imiquimod cream, which is approved for genital and perianal warts only in patients 12 years of age and older.
In Dr. Vanderhooft’s experience, Candida antigen injections benefit some patients. After injection of 0.3 mL of Candida antigen into 1 or 2 warts at monthly intervals, researchers in one study observed complete clearance in 87% of patients after an average of 3.5 treatments, while 7% demonstrated no improvement after an average of 3.75 treatments (Pediatr. Dermatol. 2008; 25: 189-92).
Zinc supplementation is another option, she said. In a randomized trial, researchers who evaluated oral zinc supplementation versus placebo for two months found complete clearance in 20 out of 23 patients in the treatment group (87%), compared with none of the 20 patients in the placebo group (Br. J. Dermatol; 2002; 146[3]:423-31). All patients in the zinc group reported nausea. “That’s what has limited zinc therapy in my patient population,” Dr. Vanderhooft said.
In a more recent study, researchers conducted a placebo-controlled study of zinc sulfate 10 mg/kg per day up to 600 mg per day for up to two months (J. Am. Acad. Dermatol. 2009; 60[4]:706-8). Complete clearance of all warts was achieved in 78% of patients in the treatment group, compared with 13% in the placebo group. No recurrence of warts was observed at the six-month follow-up.
Topical 5-FU for warts has also been evaluated. One study of once or twice daily application of 5-FU under occlusion for 6 weeks demonstrated improvement in 88% of patients, including 13% with complete clearance (Br. J. Dermatol; 2011; 165[2]:233-46). No blood levels of the drug were detected. “It’s thought to be safe and well-tolerated, but over-the-counter salicylic acid has better efficacy and is quite a bit cheaper,” Dr. Vanderhooft said.
Another strategy for recalcitrant warts involves administration of squaric acid dibutyl ester. In a recent retrospective chart review conducted over a 10.5-year period, researchers evaluated 72 children with recalcitrant warts who had failed therapy with multiple agents and were followed for a period of 6 months to 11 years (Pediatr. Dermatol. 2015; 32:85-90). The protocol involved sensitizing the children to 2% squaric acid dibutyl ester (SADBE). The treatment then started two weeks later, with 0.4% SADBE applied 3 times per week initially, with an extra day added per week as tolerated to reach daily use if possible. The researchers found that 40 of the 48 (83%) patients in whom treatment outcomes could be obtained reported complete resolution of their warts. Seventy percent of patients used a maximum concentration of 0.4% SADBE and 60% of patients reported no adverse effects. The average time to reduction in size of warts was 2.6 months after sensitization, and treatment continued for a mean of 8 months.
Dr. Vanderhooft reported having no financial disclosures.
EXPERT ANALYSIS AT PDA 2015
Treatment options for lentigo maligna far from perfect
PARK CITY, UTAH – Dr. Glen M. Bowen has been working to improve the surgical treatment of lentigo maligna ever since he joined the Huntsman Cancer Institute at the University of Utah 16 years ago. A retrospective review from Memorial Sloan-Kettering Cancer Center found that on average, 7.1-mm margins are required to remove lentigo maligna (LM) (J. Am. Acad. Dermatol. 2008;58[1]:142-8).
“If you have an LM with a 10-mm diameter to begin with, 7.1-mm margins give you a final surgical diameter of 24.2 mm,” Dr. Bowen said at the annual meeting of the Pacific Dermatologic Association. “These are very morbid surgeries in cosmetically sensitive areas for a relatively low-risk tumor.”
The risk of LM progressing to an invasive melanoma is not known, but is estimated to range between 5% and 33%. Of 2,016 patients treated for LM at the Huntsman Cancer Institute, 522 have been treated with neo-adjuvant topical imiquimod 5% cream followed by a conservative staged excision with 2-mm margins with a recurrence rate of 2.3% during median follow-up of 5 years. Of their recurrences, about 20% recurred with invasion. All recurrences to date have been less than 1 mm in depth (stage IA), which has an estimated mortality risk of 5% at 5 years. “A 5% mortality rate of the 20% that recur with invasion of the 2.3% that recur after surgery yields a mortality risk of 0.023%,” Dr. Bowen said. “Due to the very low risk of actually dying from a recurrent LM, very large and morbid surgical defects strike me as a punishment that doesn’t fit the crime in terms of the cost-benefit ratio.”
He and his associates at the Huntsman Cancer Institute have observed some deaths in patients who presented with LM melanoma (invasive melanoma) but have not observed a single death in patients who presented with LM in situ that subsequently recurred. For these reasons, Dr. Bowen favors pretreating LM with imiquimod 5% cream followed by a conservative staged excision, a process that substantially decreases the size of the surgical defects.
His current treatment protocol involves a five-step process that begins with removing all of the visible lentigo maligna to rule out invasion, since 16% of LMs referred to him have harbored invasion when removed and are upgraded from stage 0 to IA. “I am not going to use a topical cream on an invasive melanoma,” he said. “After an excisional biopsy with minimal margins, I usually close the defect with a purse-string suture because it avoids removing standing cones and consequently enlarging the treatment area.”
Second, he traces a template of the LM border on transparent plastic and places a tiny tattoo in the center of the biopsy site to enable pinpoint placement of the template at the time of surgery.
Third, he treats the site with imiquimod 5% cream Monday through Friday for 2-3 months and sees the patient monthly for dosage adjustments when needed. The fourth step involves enabling the site to recover for 2-6 months to allow for resolution of the inflammatory infiltrate. The final step involves re-excising around the original template with 2-mm margins for confirmation with the use of a negative control taken from an equally sun-exposed site taken some distance away from the LM. “Caucasians will have atypical junctional melanocytic hyperplasia (AJMH), which must be subtracted out as background,” he said. “Otherwise, if you hold a non–sun-exposed site as your standard for a negative margin, you will never stop cutting.”
Dr. Bowen likes to use frozen radial sections with routine staining with H and E and immunostaining with MART-1 (Melan-A) and SOX-10. Processing takes 2 hours, he continued, “so I put in relaxing sutures, which will stretch out nicely over 2 hours so I can usually close the defects primarily.”
In Dr. Bowen’s opinion, topical imiquimod as monotherapy for LM is not safe, since about 30% of patients treated with imiquimod will still harbor residual LM. “In our dataset, about 70% have no residual LM, 20% have residual LM in the center but negative perimeter margins, and 10% have LM touching a perimeter margin and require a second stage,” he said. “Taken together, 90% of patients pretreated with imiquimod will be cleared in one stage of surgery with 2 mm margins.”
Making the distinction between LM and AJMH common to chronically sun-damaged skin is no easy task. Dr. Bowen cited a concordance study between dermatopathologists interpreting staged excisional margins on permanent sections for LM where the concordance was only moderate at best. In this study, the use of a negative control improved the concordance rate on “difficult” cases from 46% to 76%; P = .001 (Arch Dermatol. 2003 May;139(5):595-604). “What we really need is a molecular marker that will tell us if a melanocyte is malignant or not,” he said. “All we have now are immunostains that tell you if it’s melanocyte but nothing more.” He went on to say that in multivariate analysis in two studies of the histologic features of LM, the only feature that consistently predicted the difference between LM and AJMH was the melanocyte density and its ratio to the negative control (Dermatol Surg. 2011;37(5):657-63 and J. Plast. Reconstr. Aesthet. Surg. 2014;67(10):1322-32). “The MART-1 immunostain is extremely sensitive, but it makes the slide somewhat muddy, so it’s hard to do an accurate cell count,” he said. “For that reason, we also use a SOX-10 immunostain which is very specific but not as sensitive. I believe that the truth lies somewhere in between those two immunostains in light of a positive control from our lab and a negative control from the patient.”
He concluded that the neoadjuvant use of imiquimod followed by a conservative staged excision “allows me to clear 90% of LM with a 2 mm margin with a recurrence rate of 2.3% in patients with a mean follow-up of 5-years or greater.”
Dr. Bowen reported having no financial disclosures.
PARK CITY, UTAH – Dr. Glen M. Bowen has been working to improve the surgical treatment of lentigo maligna ever since he joined the Huntsman Cancer Institute at the University of Utah 16 years ago. A retrospective review from Memorial Sloan-Kettering Cancer Center found that on average, 7.1-mm margins are required to remove lentigo maligna (LM) (J. Am. Acad. Dermatol. 2008;58[1]:142-8).
“If you have an LM with a 10-mm diameter to begin with, 7.1-mm margins give you a final surgical diameter of 24.2 mm,” Dr. Bowen said at the annual meeting of the Pacific Dermatologic Association. “These are very morbid surgeries in cosmetically sensitive areas for a relatively low-risk tumor.”
The risk of LM progressing to an invasive melanoma is not known, but is estimated to range between 5% and 33%. Of 2,016 patients treated for LM at the Huntsman Cancer Institute, 522 have been treated with neo-adjuvant topical imiquimod 5% cream followed by a conservative staged excision with 2-mm margins with a recurrence rate of 2.3% during median follow-up of 5 years. Of their recurrences, about 20% recurred with invasion. All recurrences to date have been less than 1 mm in depth (stage IA), which has an estimated mortality risk of 5% at 5 years. “A 5% mortality rate of the 20% that recur with invasion of the 2.3% that recur after surgery yields a mortality risk of 0.023%,” Dr. Bowen said. “Due to the very low risk of actually dying from a recurrent LM, very large and morbid surgical defects strike me as a punishment that doesn’t fit the crime in terms of the cost-benefit ratio.”
He and his associates at the Huntsman Cancer Institute have observed some deaths in patients who presented with LM melanoma (invasive melanoma) but have not observed a single death in patients who presented with LM in situ that subsequently recurred. For these reasons, Dr. Bowen favors pretreating LM with imiquimod 5% cream followed by a conservative staged excision, a process that substantially decreases the size of the surgical defects.
His current treatment protocol involves a five-step process that begins with removing all of the visible lentigo maligna to rule out invasion, since 16% of LMs referred to him have harbored invasion when removed and are upgraded from stage 0 to IA. “I am not going to use a topical cream on an invasive melanoma,” he said. “After an excisional biopsy with minimal margins, I usually close the defect with a purse-string suture because it avoids removing standing cones and consequently enlarging the treatment area.”
Second, he traces a template of the LM border on transparent plastic and places a tiny tattoo in the center of the biopsy site to enable pinpoint placement of the template at the time of surgery.
Third, he treats the site with imiquimod 5% cream Monday through Friday for 2-3 months and sees the patient monthly for dosage adjustments when needed. The fourth step involves enabling the site to recover for 2-6 months to allow for resolution of the inflammatory infiltrate. The final step involves re-excising around the original template with 2-mm margins for confirmation with the use of a negative control taken from an equally sun-exposed site taken some distance away from the LM. “Caucasians will have atypical junctional melanocytic hyperplasia (AJMH), which must be subtracted out as background,” he said. “Otherwise, if you hold a non–sun-exposed site as your standard for a negative margin, you will never stop cutting.”
Dr. Bowen likes to use frozen radial sections with routine staining with H and E and immunostaining with MART-1 (Melan-A) and SOX-10. Processing takes 2 hours, he continued, “so I put in relaxing sutures, which will stretch out nicely over 2 hours so I can usually close the defects primarily.”
In Dr. Bowen’s opinion, topical imiquimod as monotherapy for LM is not safe, since about 30% of patients treated with imiquimod will still harbor residual LM. “In our dataset, about 70% have no residual LM, 20% have residual LM in the center but negative perimeter margins, and 10% have LM touching a perimeter margin and require a second stage,” he said. “Taken together, 90% of patients pretreated with imiquimod will be cleared in one stage of surgery with 2 mm margins.”
Making the distinction between LM and AJMH common to chronically sun-damaged skin is no easy task. Dr. Bowen cited a concordance study between dermatopathologists interpreting staged excisional margins on permanent sections for LM where the concordance was only moderate at best. In this study, the use of a negative control improved the concordance rate on “difficult” cases from 46% to 76%; P = .001 (Arch Dermatol. 2003 May;139(5):595-604). “What we really need is a molecular marker that will tell us if a melanocyte is malignant or not,” he said. “All we have now are immunostains that tell you if it’s melanocyte but nothing more.” He went on to say that in multivariate analysis in two studies of the histologic features of LM, the only feature that consistently predicted the difference between LM and AJMH was the melanocyte density and its ratio to the negative control (Dermatol Surg. 2011;37(5):657-63 and J. Plast. Reconstr. Aesthet. Surg. 2014;67(10):1322-32). “The MART-1 immunostain is extremely sensitive, but it makes the slide somewhat muddy, so it’s hard to do an accurate cell count,” he said. “For that reason, we also use a SOX-10 immunostain which is very specific but not as sensitive. I believe that the truth lies somewhere in between those two immunostains in light of a positive control from our lab and a negative control from the patient.”
He concluded that the neoadjuvant use of imiquimod followed by a conservative staged excision “allows me to clear 90% of LM with a 2 mm margin with a recurrence rate of 2.3% in patients with a mean follow-up of 5-years or greater.”
Dr. Bowen reported having no financial disclosures.
PARK CITY, UTAH – Dr. Glen M. Bowen has been working to improve the surgical treatment of lentigo maligna ever since he joined the Huntsman Cancer Institute at the University of Utah 16 years ago. A retrospective review from Memorial Sloan-Kettering Cancer Center found that on average, 7.1-mm margins are required to remove lentigo maligna (LM) (J. Am. Acad. Dermatol. 2008;58[1]:142-8).
“If you have an LM with a 10-mm diameter to begin with, 7.1-mm margins give you a final surgical diameter of 24.2 mm,” Dr. Bowen said at the annual meeting of the Pacific Dermatologic Association. “These are very morbid surgeries in cosmetically sensitive areas for a relatively low-risk tumor.”
The risk of LM progressing to an invasive melanoma is not known, but is estimated to range between 5% and 33%. Of 2,016 patients treated for LM at the Huntsman Cancer Institute, 522 have been treated with neo-adjuvant topical imiquimod 5% cream followed by a conservative staged excision with 2-mm margins with a recurrence rate of 2.3% during median follow-up of 5 years. Of their recurrences, about 20% recurred with invasion. All recurrences to date have been less than 1 mm in depth (stage IA), which has an estimated mortality risk of 5% at 5 years. “A 5% mortality rate of the 20% that recur with invasion of the 2.3% that recur after surgery yields a mortality risk of 0.023%,” Dr. Bowen said. “Due to the very low risk of actually dying from a recurrent LM, very large and morbid surgical defects strike me as a punishment that doesn’t fit the crime in terms of the cost-benefit ratio.”
He and his associates at the Huntsman Cancer Institute have observed some deaths in patients who presented with LM melanoma (invasive melanoma) but have not observed a single death in patients who presented with LM in situ that subsequently recurred. For these reasons, Dr. Bowen favors pretreating LM with imiquimod 5% cream followed by a conservative staged excision, a process that substantially decreases the size of the surgical defects.
His current treatment protocol involves a five-step process that begins with removing all of the visible lentigo maligna to rule out invasion, since 16% of LMs referred to him have harbored invasion when removed and are upgraded from stage 0 to IA. “I am not going to use a topical cream on an invasive melanoma,” he said. “After an excisional biopsy with minimal margins, I usually close the defect with a purse-string suture because it avoids removing standing cones and consequently enlarging the treatment area.”
Second, he traces a template of the LM border on transparent plastic and places a tiny tattoo in the center of the biopsy site to enable pinpoint placement of the template at the time of surgery.
Third, he treats the site with imiquimod 5% cream Monday through Friday for 2-3 months and sees the patient monthly for dosage adjustments when needed. The fourth step involves enabling the site to recover for 2-6 months to allow for resolution of the inflammatory infiltrate. The final step involves re-excising around the original template with 2-mm margins for confirmation with the use of a negative control taken from an equally sun-exposed site taken some distance away from the LM. “Caucasians will have atypical junctional melanocytic hyperplasia (AJMH), which must be subtracted out as background,” he said. “Otherwise, if you hold a non–sun-exposed site as your standard for a negative margin, you will never stop cutting.”
Dr. Bowen likes to use frozen radial sections with routine staining with H and E and immunostaining with MART-1 (Melan-A) and SOX-10. Processing takes 2 hours, he continued, “so I put in relaxing sutures, which will stretch out nicely over 2 hours so I can usually close the defects primarily.”
In Dr. Bowen’s opinion, topical imiquimod as monotherapy for LM is not safe, since about 30% of patients treated with imiquimod will still harbor residual LM. “In our dataset, about 70% have no residual LM, 20% have residual LM in the center but negative perimeter margins, and 10% have LM touching a perimeter margin and require a second stage,” he said. “Taken together, 90% of patients pretreated with imiquimod will be cleared in one stage of surgery with 2 mm margins.”
Making the distinction between LM and AJMH common to chronically sun-damaged skin is no easy task. Dr. Bowen cited a concordance study between dermatopathologists interpreting staged excisional margins on permanent sections for LM where the concordance was only moderate at best. In this study, the use of a negative control improved the concordance rate on “difficult” cases from 46% to 76%; P = .001 (Arch Dermatol. 2003 May;139(5):595-604). “What we really need is a molecular marker that will tell us if a melanocyte is malignant or not,” he said. “All we have now are immunostains that tell you if it’s melanocyte but nothing more.” He went on to say that in multivariate analysis in two studies of the histologic features of LM, the only feature that consistently predicted the difference between LM and AJMH was the melanocyte density and its ratio to the negative control (Dermatol Surg. 2011;37(5):657-63 and J. Plast. Reconstr. Aesthet. Surg. 2014;67(10):1322-32). “The MART-1 immunostain is extremely sensitive, but it makes the slide somewhat muddy, so it’s hard to do an accurate cell count,” he said. “For that reason, we also use a SOX-10 immunostain which is very specific but not as sensitive. I believe that the truth lies somewhere in between those two immunostains in light of a positive control from our lab and a negative control from the patient.”
He concluded that the neoadjuvant use of imiquimod followed by a conservative staged excision “allows me to clear 90% of LM with a 2 mm margin with a recurrence rate of 2.3% in patients with a mean follow-up of 5-years or greater.”
Dr. Bowen reported having no financial disclosures.
EXPERT ANALYSIS from PDA 2015
Biologic Treatment for Psoriasis Found to Have CVD Benefits
PARK CITY, UTAH – Evidence is mounting that the use of biologic therapies is associated with certain cardiovascular benefits in patients with psoriasis.
However, until the strength of the evidence becomes more robust, Dr. Jashin J. Wu does not recommend that patients are prescribed tumor necrosis factor (TNF) inhibitors specifically to reduce the risk of cardiovascular disease. “If systemic therapy is a consideration in the setting of CVD [cardiovascular disease] risk, it would appear that TNF inhibitors and methotrexate offer the best evidence of benefit,” Dr. Wu, director of the psoriasis clinic and director of dermatology research in the department of dermatology at Kaiser Permanente Los Angeles Medical Center, said at the annual meeting of the Pacific Dermatologic Association.
The National Psoriasis Foundation recently published a consensus statement about the potential impact of current therapies on cardiovascular disease, including the risk of diabetes (J Am Acad Dermatol. 2014;70[1]:168-77). In a retrospective study of 121,280 patients with rheumatoid arthritis (RA) or psoriasis, drug regimens were categorized into four mutually exclusive groups: TNF inhibitors with or without other disease-modifying antirheumatic drugs (DMARDs), methotrexate without TNF inhibitors or hydroxychloroquine, hydroxychloroquine without TNF inhibitors or methotrexate; and other nonbiologic DMARDs without TNF inhibitors, methotrexate or hydroxychloroquine (referent group). The researchers found that adjusted Cox proportional hazards for the risk of diabetes were lower among those on TNF inhibitors (hazard ratio, 0.62; 95% confidence interval, 0.42-0.91) and hydroxychloroquine (HR, 0.54; 95% CI, 0.36-0.80), compared with those on methotrexate (HR, 0.77, 95% CI, 0.53-1.13) (JAMA. 2011 Jun 22;305[24]:2525-31).
Increased carotid intimal media thickness (IMT) is also an independent predictor of CVD. In a prospective, observational study, 20 psoriasis patients received a 12-week treatment of TNF inhibitors (Ann Rheum Dis. 2011 Apr;70[4]:705-6). After 12 weeks, 9 patients continued on their TNF-inhibitor regimen (group 1), while 11 discontinued treatment because of financial constraints (group 2). Another 20 psoriasis patients who did not receive biologic therapy served as controls (group 3). The researchers measured IMT by carotid ultrasound at baseline, week 12 and at 2 years. They found that between baseline and 2 years, IMT decreased from 0.70 mm to 0.63 mm in group 1, from 0.71 to 0.67 in group 2, and increased from 0.79 to 0.82 in group 3. “This seems to suggest that the use of TNF inhibitors may improve atherosclerosis,” said Dr. Wu, who is currently running seven clinical trials of psoriasis patients. Similar findings were seen in a larger study that examined 224 patients with psoriatic arthritis and followed them for a mean of 55 months: 124 on TNF inhibitors, 104 on DMARDs, and 305 matched controls (Arterioscler Thromb Vasc Biol. 2011 Mar;31[3]:705-120).
In a separate study of 16 psoriasis patients treated with TNF inhibitors, researchers used ultrasound to measure IMT of carotid and brachial arteries after 6 months of therapy (J Am Acad Dermatol. 2013 Oct;69[4]:523-9). At baseline, all 16 patients had an IMT greater than normal. In those without initial calcified atherosclerotic plaques, 13 of 16 had a significant decrease of IMT (P = .0002). In those with initial calcified atherosclerotic plaques, 3 of 16 patients had a nonsignificant increase in IMT.
Increased arterial stiffness is another independent predictor of CVD. In a controlled study of 55 patients with psoriatic arthritis, RA, or ankylosing spondylitis, researchers used aortic pulse-wave velocity to measure aortic stiffness. Almost 35% of the patients (36) received TNF inhibitors while the remaining 19 did not receive treatment (Am J Hypertens. 2012 Jun;25[6]:644-50). After 1 year of follow-up, use of TNF inhibitors was associated with a significant improvement in aortic pulse-wave velocity (P = .02) and reduced carotid IMT progression (P = .04).
Other evidence suggests that systemic therapy with TNF inhibitors is associated with the reduction of cardiovascular events, including myocardial infarction. In a study led by Dr. Wu, 8,845 Kaiser Permanente patients with psoriasis were evaluated between Jan. 1, 2004, and Nov. 30, 2010, and placed into one of three groups: treatment with a TNF inhibitor, treatment with oral therapy or phototherapy, or treatment with a topical agent (referent group) (Arch Dermatol. 2012 Nov;148[11]:1244-50). On multivariate analysis, the investigators found that the use of TNF inhibitors was associated with a significant reduction in the rate of incident MI (HR, 0.50; P = .003), compared with the use of topical agents.
Dr. Wu went on to note that C-reactive protein (CRP) is a predictor of CVD such as MI, peripheral arterial disease, and sudden cardiac death. In a retrospective cohort study, Dr. Wu and his associates evaluated Kaiser Permanente patients with psoriasis, psoriatic arthritis, or RA who were treated with a combination of TNF plus methotrexate, or with methotrexate alone from Jan. 1, 2002, to July 31, 2011 (J. Am. Acad. Dermatol. 2015;72[5]:917-9). Only patients in the combination therapy group had a clinically and statistically significant decrease in CRP, compared with baseline (a mean decrease of 5.18 mg/dL). No significant changes between the two groups were observed in terms of BMI, blood pressure, fasting glucose, hemoglobin A1C, total cholesterol, LDL cholesterol, HDL cholesterol, triglycerides, or alanine aminotransferase.
HDL cholesterol composition and function have also been studied in psoriasis patients. In one trial, HDL cholesterol was isolated from 15 psoriasis patients at baseline and after effective topical and/or systemic psoriasis therapy, and from 15 healthy controls matched for age and sex (J. Invest. Dermatol. 2014;134[3]:635-42). The researchers found that HDL cholesterol from psoriasis patients showed a significantly impaired capability to mobilize cholesterol from macrophages. However, psoriasis therapy recovered HDL cholesterol composition and function, but had no effect on serum HDL cholesterol levels.
A better understanding of the cardiovascular effects of biologic therapy should be gleaned from the Vascular Inflammation in Psoriasis Trial, which includes $3.8 million in funding from the National Institutes of Health, to prospectively study the effect of therapy on biomarkers and on positron emission tomography with 18fluorodeoxyglucose (FDG-PET)/CT scan. The 1-year study just completed enrolling patients into one of three treatment groups: adalimumab, UVB phototherapy, or placebo. Biomarkers such as CRP, lipids, and imaging will be assessed at baseline, weeks 4, 8, 12, and then every 12 weeks. “The theory is that adalimumab, but not UVB phototherapy, will improve biomarkers and reduce vascular inflammation as measured by FDG-PET/CT scan,” Dr. Wu said. “Aggressive systemic therapy may alter the natural history of cardiovascular disease.”
Current recommendations from the National Psoriasis Foundation call for an assessment of blood pressure, pulse, and body mass index every 2 years, and an assessment of fasting blood glucose and lipid levels every 5 years, or every 2 years if the patient has additional risk factors.
Dr. Wu reported that he has received research and consulting fees from numerous pharmaceutical companies, including AbbVie and Amgen. These go to his employer.
PARK CITY, UTAH – Evidence is mounting that the use of biologic therapies is associated with certain cardiovascular benefits in patients with psoriasis.
However, until the strength of the evidence becomes more robust, Dr. Jashin J. Wu does not recommend that patients are prescribed tumor necrosis factor (TNF) inhibitors specifically to reduce the risk of cardiovascular disease. “If systemic therapy is a consideration in the setting of CVD [cardiovascular disease] risk, it would appear that TNF inhibitors and methotrexate offer the best evidence of benefit,” Dr. Wu, director of the psoriasis clinic and director of dermatology research in the department of dermatology at Kaiser Permanente Los Angeles Medical Center, said at the annual meeting of the Pacific Dermatologic Association.
The National Psoriasis Foundation recently published a consensus statement about the potential impact of current therapies on cardiovascular disease, including the risk of diabetes (J Am Acad Dermatol. 2014;70[1]:168-77). In a retrospective study of 121,280 patients with rheumatoid arthritis (RA) or psoriasis, drug regimens were categorized into four mutually exclusive groups: TNF inhibitors with or without other disease-modifying antirheumatic drugs (DMARDs), methotrexate without TNF inhibitors or hydroxychloroquine, hydroxychloroquine without TNF inhibitors or methotrexate; and other nonbiologic DMARDs without TNF inhibitors, methotrexate or hydroxychloroquine (referent group). The researchers found that adjusted Cox proportional hazards for the risk of diabetes were lower among those on TNF inhibitors (hazard ratio, 0.62; 95% confidence interval, 0.42-0.91) and hydroxychloroquine (HR, 0.54; 95% CI, 0.36-0.80), compared with those on methotrexate (HR, 0.77, 95% CI, 0.53-1.13) (JAMA. 2011 Jun 22;305[24]:2525-31).
Increased carotid intimal media thickness (IMT) is also an independent predictor of CVD. In a prospective, observational study, 20 psoriasis patients received a 12-week treatment of TNF inhibitors (Ann Rheum Dis. 2011 Apr;70[4]:705-6). After 12 weeks, 9 patients continued on their TNF-inhibitor regimen (group 1), while 11 discontinued treatment because of financial constraints (group 2). Another 20 psoriasis patients who did not receive biologic therapy served as controls (group 3). The researchers measured IMT by carotid ultrasound at baseline, week 12 and at 2 years. They found that between baseline and 2 years, IMT decreased from 0.70 mm to 0.63 mm in group 1, from 0.71 to 0.67 in group 2, and increased from 0.79 to 0.82 in group 3. “This seems to suggest that the use of TNF inhibitors may improve atherosclerosis,” said Dr. Wu, who is currently running seven clinical trials of psoriasis patients. Similar findings were seen in a larger study that examined 224 patients with psoriatic arthritis and followed them for a mean of 55 months: 124 on TNF inhibitors, 104 on DMARDs, and 305 matched controls (Arterioscler Thromb Vasc Biol. 2011 Mar;31[3]:705-120).
In a separate study of 16 psoriasis patients treated with TNF inhibitors, researchers used ultrasound to measure IMT of carotid and brachial arteries after 6 months of therapy (J Am Acad Dermatol. 2013 Oct;69[4]:523-9). At baseline, all 16 patients had an IMT greater than normal. In those without initial calcified atherosclerotic plaques, 13 of 16 had a significant decrease of IMT (P = .0002). In those with initial calcified atherosclerotic plaques, 3 of 16 patients had a nonsignificant increase in IMT.
Increased arterial stiffness is another independent predictor of CVD. In a controlled study of 55 patients with psoriatic arthritis, RA, or ankylosing spondylitis, researchers used aortic pulse-wave velocity to measure aortic stiffness. Almost 35% of the patients (36) received TNF inhibitors while the remaining 19 did not receive treatment (Am J Hypertens. 2012 Jun;25[6]:644-50). After 1 year of follow-up, use of TNF inhibitors was associated with a significant improvement in aortic pulse-wave velocity (P = .02) and reduced carotid IMT progression (P = .04).
Other evidence suggests that systemic therapy with TNF inhibitors is associated with the reduction of cardiovascular events, including myocardial infarction. In a study led by Dr. Wu, 8,845 Kaiser Permanente patients with psoriasis were evaluated between Jan. 1, 2004, and Nov. 30, 2010, and placed into one of three groups: treatment with a TNF inhibitor, treatment with oral therapy or phototherapy, or treatment with a topical agent (referent group) (Arch Dermatol. 2012 Nov;148[11]:1244-50). On multivariate analysis, the investigators found that the use of TNF inhibitors was associated with a significant reduction in the rate of incident MI (HR, 0.50; P = .003), compared with the use of topical agents.
Dr. Wu went on to note that C-reactive protein (CRP) is a predictor of CVD such as MI, peripheral arterial disease, and sudden cardiac death. In a retrospective cohort study, Dr. Wu and his associates evaluated Kaiser Permanente patients with psoriasis, psoriatic arthritis, or RA who were treated with a combination of TNF plus methotrexate, or with methotrexate alone from Jan. 1, 2002, to July 31, 2011 (J. Am. Acad. Dermatol. 2015;72[5]:917-9). Only patients in the combination therapy group had a clinically and statistically significant decrease in CRP, compared with baseline (a mean decrease of 5.18 mg/dL). No significant changes between the two groups were observed in terms of BMI, blood pressure, fasting glucose, hemoglobin A1C, total cholesterol, LDL cholesterol, HDL cholesterol, triglycerides, or alanine aminotransferase.
HDL cholesterol composition and function have also been studied in psoriasis patients. In one trial, HDL cholesterol was isolated from 15 psoriasis patients at baseline and after effective topical and/or systemic psoriasis therapy, and from 15 healthy controls matched for age and sex (J. Invest. Dermatol. 2014;134[3]:635-42). The researchers found that HDL cholesterol from psoriasis patients showed a significantly impaired capability to mobilize cholesterol from macrophages. However, psoriasis therapy recovered HDL cholesterol composition and function, but had no effect on serum HDL cholesterol levels.
A better understanding of the cardiovascular effects of biologic therapy should be gleaned from the Vascular Inflammation in Psoriasis Trial, which includes $3.8 million in funding from the National Institutes of Health, to prospectively study the effect of therapy on biomarkers and on positron emission tomography with 18fluorodeoxyglucose (FDG-PET)/CT scan. The 1-year study just completed enrolling patients into one of three treatment groups: adalimumab, UVB phototherapy, or placebo. Biomarkers such as CRP, lipids, and imaging will be assessed at baseline, weeks 4, 8, 12, and then every 12 weeks. “The theory is that adalimumab, but not UVB phototherapy, will improve biomarkers and reduce vascular inflammation as measured by FDG-PET/CT scan,” Dr. Wu said. “Aggressive systemic therapy may alter the natural history of cardiovascular disease.”
Current recommendations from the National Psoriasis Foundation call for an assessment of blood pressure, pulse, and body mass index every 2 years, and an assessment of fasting blood glucose and lipid levels every 5 years, or every 2 years if the patient has additional risk factors.
Dr. Wu reported that he has received research and consulting fees from numerous pharmaceutical companies, including AbbVie and Amgen. These go to his employer.
PARK CITY, UTAH – Evidence is mounting that the use of biologic therapies is associated with certain cardiovascular benefits in patients with psoriasis.
However, until the strength of the evidence becomes more robust, Dr. Jashin J. Wu does not recommend that patients are prescribed tumor necrosis factor (TNF) inhibitors specifically to reduce the risk of cardiovascular disease. “If systemic therapy is a consideration in the setting of CVD [cardiovascular disease] risk, it would appear that TNF inhibitors and methotrexate offer the best evidence of benefit,” Dr. Wu, director of the psoriasis clinic and director of dermatology research in the department of dermatology at Kaiser Permanente Los Angeles Medical Center, said at the annual meeting of the Pacific Dermatologic Association.
The National Psoriasis Foundation recently published a consensus statement about the potential impact of current therapies on cardiovascular disease, including the risk of diabetes (J Am Acad Dermatol. 2014;70[1]:168-77). In a retrospective study of 121,280 patients with rheumatoid arthritis (RA) or psoriasis, drug regimens were categorized into four mutually exclusive groups: TNF inhibitors with or without other disease-modifying antirheumatic drugs (DMARDs), methotrexate without TNF inhibitors or hydroxychloroquine, hydroxychloroquine without TNF inhibitors or methotrexate; and other nonbiologic DMARDs without TNF inhibitors, methotrexate or hydroxychloroquine (referent group). The researchers found that adjusted Cox proportional hazards for the risk of diabetes were lower among those on TNF inhibitors (hazard ratio, 0.62; 95% confidence interval, 0.42-0.91) and hydroxychloroquine (HR, 0.54; 95% CI, 0.36-0.80), compared with those on methotrexate (HR, 0.77, 95% CI, 0.53-1.13) (JAMA. 2011 Jun 22;305[24]:2525-31).
Increased carotid intimal media thickness (IMT) is also an independent predictor of CVD. In a prospective, observational study, 20 psoriasis patients received a 12-week treatment of TNF inhibitors (Ann Rheum Dis. 2011 Apr;70[4]:705-6). After 12 weeks, 9 patients continued on their TNF-inhibitor regimen (group 1), while 11 discontinued treatment because of financial constraints (group 2). Another 20 psoriasis patients who did not receive biologic therapy served as controls (group 3). The researchers measured IMT by carotid ultrasound at baseline, week 12 and at 2 years. They found that between baseline and 2 years, IMT decreased from 0.70 mm to 0.63 mm in group 1, from 0.71 to 0.67 in group 2, and increased from 0.79 to 0.82 in group 3. “This seems to suggest that the use of TNF inhibitors may improve atherosclerosis,” said Dr. Wu, who is currently running seven clinical trials of psoriasis patients. Similar findings were seen in a larger study that examined 224 patients with psoriatic arthritis and followed them for a mean of 55 months: 124 on TNF inhibitors, 104 on DMARDs, and 305 matched controls (Arterioscler Thromb Vasc Biol. 2011 Mar;31[3]:705-120).
In a separate study of 16 psoriasis patients treated with TNF inhibitors, researchers used ultrasound to measure IMT of carotid and brachial arteries after 6 months of therapy (J Am Acad Dermatol. 2013 Oct;69[4]:523-9). At baseline, all 16 patients had an IMT greater than normal. In those without initial calcified atherosclerotic plaques, 13 of 16 had a significant decrease of IMT (P = .0002). In those with initial calcified atherosclerotic plaques, 3 of 16 patients had a nonsignificant increase in IMT.
Increased arterial stiffness is another independent predictor of CVD. In a controlled study of 55 patients with psoriatic arthritis, RA, or ankylosing spondylitis, researchers used aortic pulse-wave velocity to measure aortic stiffness. Almost 35% of the patients (36) received TNF inhibitors while the remaining 19 did not receive treatment (Am J Hypertens. 2012 Jun;25[6]:644-50). After 1 year of follow-up, use of TNF inhibitors was associated with a significant improvement in aortic pulse-wave velocity (P = .02) and reduced carotid IMT progression (P = .04).
Other evidence suggests that systemic therapy with TNF inhibitors is associated with the reduction of cardiovascular events, including myocardial infarction. In a study led by Dr. Wu, 8,845 Kaiser Permanente patients with psoriasis were evaluated between Jan. 1, 2004, and Nov. 30, 2010, and placed into one of three groups: treatment with a TNF inhibitor, treatment with oral therapy or phototherapy, or treatment with a topical agent (referent group) (Arch Dermatol. 2012 Nov;148[11]:1244-50). On multivariate analysis, the investigators found that the use of TNF inhibitors was associated with a significant reduction in the rate of incident MI (HR, 0.50; P = .003), compared with the use of topical agents.
Dr. Wu went on to note that C-reactive protein (CRP) is a predictor of CVD such as MI, peripheral arterial disease, and sudden cardiac death. In a retrospective cohort study, Dr. Wu and his associates evaluated Kaiser Permanente patients with psoriasis, psoriatic arthritis, or RA who were treated with a combination of TNF plus methotrexate, or with methotrexate alone from Jan. 1, 2002, to July 31, 2011 (J. Am. Acad. Dermatol. 2015;72[5]:917-9). Only patients in the combination therapy group had a clinically and statistically significant decrease in CRP, compared with baseline (a mean decrease of 5.18 mg/dL). No significant changes between the two groups were observed in terms of BMI, blood pressure, fasting glucose, hemoglobin A1C, total cholesterol, LDL cholesterol, HDL cholesterol, triglycerides, or alanine aminotransferase.
HDL cholesterol composition and function have also been studied in psoriasis patients. In one trial, HDL cholesterol was isolated from 15 psoriasis patients at baseline and after effective topical and/or systemic psoriasis therapy, and from 15 healthy controls matched for age and sex (J. Invest. Dermatol. 2014;134[3]:635-42). The researchers found that HDL cholesterol from psoriasis patients showed a significantly impaired capability to mobilize cholesterol from macrophages. However, psoriasis therapy recovered HDL cholesterol composition and function, but had no effect on serum HDL cholesterol levels.
A better understanding of the cardiovascular effects of biologic therapy should be gleaned from the Vascular Inflammation in Psoriasis Trial, which includes $3.8 million in funding from the National Institutes of Health, to prospectively study the effect of therapy on biomarkers and on positron emission tomography with 18fluorodeoxyglucose (FDG-PET)/CT scan. The 1-year study just completed enrolling patients into one of three treatment groups: adalimumab, UVB phototherapy, or placebo. Biomarkers such as CRP, lipids, and imaging will be assessed at baseline, weeks 4, 8, 12, and then every 12 weeks. “The theory is that adalimumab, but not UVB phototherapy, will improve biomarkers and reduce vascular inflammation as measured by FDG-PET/CT scan,” Dr. Wu said. “Aggressive systemic therapy may alter the natural history of cardiovascular disease.”
Current recommendations from the National Psoriasis Foundation call for an assessment of blood pressure, pulse, and body mass index every 2 years, and an assessment of fasting blood glucose and lipid levels every 5 years, or every 2 years if the patient has additional risk factors.
Dr. Wu reported that he has received research and consulting fees from numerous pharmaceutical companies, including AbbVie and Amgen. These go to his employer.
EXPERT ANALYSIS AT PDA 2015
Biologic treatment for psoriasis found to have CVD benefits
PARK CITY, UTAH – Evidence is mounting that the use of biologic therapies is associated with certain cardiovascular benefits in patients with psoriasis.
However, until the strength of the evidence becomes more robust, Dr. Jashin J. Wu does not recommend that patients are prescribed tumor necrosis factor (TNF) inhibitors specifically to reduce the risk of cardiovascular disease. “If systemic therapy is a consideration in the setting of CVD [cardiovascular disease] risk, it would appear that TNF inhibitors and methotrexate offer the best evidence of benefit,” Dr. Wu, director of the psoriasis clinic and director of dermatology research in the department of dermatology at Kaiser Permanente Los Angeles Medical Center, said at the annual meeting of the Pacific Dermatologic Association.
The National Psoriasis Foundation recently published a consensus statement about the potential impact of current therapies on cardiovascular disease, including the risk of diabetes (J Am Acad Dermatol. 2014;70[1]:168-77). In a retrospective study of 121,280 patients with rheumatoid arthritis (RA) or psoriasis, drug regimens were categorized into four mutually exclusive groups: TNF inhibitors with or without other disease-modifying antirheumatic drugs (DMARDs), methotrexate without TNF inhibitors or hydroxychloroquine, hydroxychloroquine without TNF inhibitors or methotrexate; and other nonbiologic DMARDs without TNF inhibitors, methotrexate or hydroxychloroquine (referent group). The researchers found that adjusted Cox proportional hazards for the risk of diabetes were lower among those on TNF inhibitors (hazard ratio, 0.62; 95% confidence interval, 0.42-0.91) and hydroxychloroquine (HR, 0.54; 95% CI, 0.36-0.80), compared with those on methotrexate (HR, 0.77, 95% CI, 0.53-1.13) (JAMA. 2011 Jun 22;305[24]:2525-31).
Increased carotid intimal media thickness (IMT) is also an independent predictor of CVD. In a prospective, observational study, 20 psoriasis patients received a 12-week treatment of TNF inhibitors (Ann Rheum Dis. 2011 Apr;70[4]:705-6). After 12 weeks, 9 patients continued on their TNF-inhibitor regimen (group 1), while 11 discontinued treatment because of financial constraints (group 2). Another 20 psoriasis patients who did not receive biologic therapy served as controls (group 3). The researchers measured IMT by carotid ultrasound at baseline, week 12 and at 2 years. They found that between baseline and 2 years, IMT decreased from 0.70 mm to 0.63 mm in group 1, from 0.71 to 0.67 in group 2, and increased from 0.79 to 0.82 in group 3. “This seems to suggest that the use of TNF inhibitors may improve atherosclerosis,” said Dr. Wu, who is currently running seven clinical trials of psoriasis patients. Similar findings were seen in a larger study that examined 224 patients with psoriatic arthritis and followed them for a mean of 55 months: 124 on TNF inhibitors, 104 on DMARDs, and 305 matched controls (Arterioscler Thromb Vasc Biol. 2011 Mar;31[3]:705-120).
In a separate study of 16 psoriasis patients treated with TNF inhibitors, researchers used ultrasound to measure IMT of carotid and brachial arteries after 6 months of therapy (J Am Acad Dermatol. 2013 Oct;69[4]:523-9). At baseline, all 16 patients had an IMT greater than normal. In those without initial calcified atherosclerotic plaques, 13 of 16 had a significant decrease of IMT (P = .0002). In those with initial calcified atherosclerotic plaques, 3 of 16 patients had a nonsignificant increase in IMT.
Increased arterial stiffness is another independent predictor of CVD. In a controlled study of 55 patients with psoriatic arthritis, RA, or ankylosing spondylitis, researchers used aortic pulse-wave velocity to measure aortic stiffness. Almost 35% of the patients (36) received TNF inhibitors while the remaining 19 did not receive treatment (Am J Hypertens. 2012 Jun;25[6]:644-50). After 1 year of follow-up, use of TNF inhibitors was associated with a significant improvement in aortic pulse-wave velocity (P = .02) and reduced carotid IMT progression (P = .04).
Other evidence suggests that systemic therapy with TNF inhibitors is associated with the reduction of cardiovascular events, including myocardial infarction. In a study led by Dr. Wu, 8,845 Kaiser Permanente patients with psoriasis were evaluated between Jan. 1, 2004, and Nov. 30, 2010, and placed into one of three groups: treatment with a TNF inhibitor, treatment with oral therapy or phototherapy, or treatment with a topical agent (referent group) (Arch Dermatol. 2012 Nov;148[11]:1244-50). On multivariate analysis, the investigators found that the use of TNF inhibitors was associated with a significant reduction in the rate of incident MI (HR, 0.50; P = .003), compared with the use of topical agents.
Dr. Wu went on to note that C-reactive protein (CRP) is a predictor of CVD such as MI, peripheral arterial disease, and sudden cardiac death. In a retrospective cohort study, Dr. Wu and his associates evaluated Kaiser Permanente patients with psoriasis, psoriatic arthritis, or RA who were treated with a combination of TNF plus methotrexate, or with methotrexate alone from Jan. 1, 2002, to July 31, 2011 (J. Am. Acad. Dermatol. 2015;72[5]:917-9). Only patients in the combination therapy group had a clinically and statistically significant decrease in CRP, compared with baseline (a mean decrease of 5.18 mg/dL). No significant changes between the two groups were observed in terms of BMI, blood pressure, fasting glucose, hemoglobin A1C, total cholesterol, LDL cholesterol, HDL cholesterol, triglycerides, or alanine aminotransferase.
HDL cholesterol composition and function have also been studied in psoriasis patients. In one trial, HDL cholesterol was isolated from 15 psoriasis patients at baseline and after effective topical and/or systemic psoriasis therapy, and from 15 healthy controls matched for age and sex (J. Invest. Dermatol. 2014;134[3]:635-42). The researchers found that HDL cholesterol from psoriasis patients showed a significantly impaired capability to mobilize cholesterol from macrophages. However, psoriasis therapy recovered HDL cholesterol composition and function, but had no effect on serum HDL cholesterol levels.
A better understanding of the cardiovascular effects of biologic therapy should be gleaned from the Vascular Inflammation in Psoriasis Trial, which includes $3.8 million in funding from the National Institutes of Health, to prospectively study the effect of therapy on biomarkers and on positron emission tomography with 18fluorodeoxyglucose (FDG-PET)/CT scan. The 1-year study just completed enrolling patients into one of three treatment groups: adalimumab, UVB phototherapy, or placebo. Biomarkers such as CRP, lipids, and imaging will be assessed at baseline, weeks 4, 8, 12, and then every 12 weeks. “The theory is that adalimumab, but not UVB phototherapy, will improve biomarkers and reduce vascular inflammation as measured by FDG-PET/CT scan,” Dr. Wu said. “Aggressive systemic therapy may alter the natural history of cardiovascular disease.”
Current recommendations from the National Psoriasis Foundation call for an assessment of blood pressure, pulse, and body mass index every 2 years, and an assessment of fasting blood glucose and lipid levels every 5 years, or every 2 years if the patient has additional risk factors.
Dr. Wu reported that he has received research and consulting fees from numerous pharmaceutical companies, including AbbVie and Amgen. These go to his employer.
PARK CITY, UTAH – Evidence is mounting that the use of biologic therapies is associated with certain cardiovascular benefits in patients with psoriasis.
However, until the strength of the evidence becomes more robust, Dr. Jashin J. Wu does not recommend that patients are prescribed tumor necrosis factor (TNF) inhibitors specifically to reduce the risk of cardiovascular disease. “If systemic therapy is a consideration in the setting of CVD [cardiovascular disease] risk, it would appear that TNF inhibitors and methotrexate offer the best evidence of benefit,” Dr. Wu, director of the psoriasis clinic and director of dermatology research in the department of dermatology at Kaiser Permanente Los Angeles Medical Center, said at the annual meeting of the Pacific Dermatologic Association.
The National Psoriasis Foundation recently published a consensus statement about the potential impact of current therapies on cardiovascular disease, including the risk of diabetes (J Am Acad Dermatol. 2014;70[1]:168-77). In a retrospective study of 121,280 patients with rheumatoid arthritis (RA) or psoriasis, drug regimens were categorized into four mutually exclusive groups: TNF inhibitors with or without other disease-modifying antirheumatic drugs (DMARDs), methotrexate without TNF inhibitors or hydroxychloroquine, hydroxychloroquine without TNF inhibitors or methotrexate; and other nonbiologic DMARDs without TNF inhibitors, methotrexate or hydroxychloroquine (referent group). The researchers found that adjusted Cox proportional hazards for the risk of diabetes were lower among those on TNF inhibitors (hazard ratio, 0.62; 95% confidence interval, 0.42-0.91) and hydroxychloroquine (HR, 0.54; 95% CI, 0.36-0.80), compared with those on methotrexate (HR, 0.77, 95% CI, 0.53-1.13) (JAMA. 2011 Jun 22;305[24]:2525-31).
Increased carotid intimal media thickness (IMT) is also an independent predictor of CVD. In a prospective, observational study, 20 psoriasis patients received a 12-week treatment of TNF inhibitors (Ann Rheum Dis. 2011 Apr;70[4]:705-6). After 12 weeks, 9 patients continued on their TNF-inhibitor regimen (group 1), while 11 discontinued treatment because of financial constraints (group 2). Another 20 psoriasis patients who did not receive biologic therapy served as controls (group 3). The researchers measured IMT by carotid ultrasound at baseline, week 12 and at 2 years. They found that between baseline and 2 years, IMT decreased from 0.70 mm to 0.63 mm in group 1, from 0.71 to 0.67 in group 2, and increased from 0.79 to 0.82 in group 3. “This seems to suggest that the use of TNF inhibitors may improve atherosclerosis,” said Dr. Wu, who is currently running seven clinical trials of psoriasis patients. Similar findings were seen in a larger study that examined 224 patients with psoriatic arthritis and followed them for a mean of 55 months: 124 on TNF inhibitors, 104 on DMARDs, and 305 matched controls (Arterioscler Thromb Vasc Biol. 2011 Mar;31[3]:705-120).
In a separate study of 16 psoriasis patients treated with TNF inhibitors, researchers used ultrasound to measure IMT of carotid and brachial arteries after 6 months of therapy (J Am Acad Dermatol. 2013 Oct;69[4]:523-9). At baseline, all 16 patients had an IMT greater than normal. In those without initial calcified atherosclerotic plaques, 13 of 16 had a significant decrease of IMT (P = .0002). In those with initial calcified atherosclerotic plaques, 3 of 16 patients had a nonsignificant increase in IMT.
Increased arterial stiffness is another independent predictor of CVD. In a controlled study of 55 patients with psoriatic arthritis, RA, or ankylosing spondylitis, researchers used aortic pulse-wave velocity to measure aortic stiffness. Almost 35% of the patients (36) received TNF inhibitors while the remaining 19 did not receive treatment (Am J Hypertens. 2012 Jun;25[6]:644-50). After 1 year of follow-up, use of TNF inhibitors was associated with a significant improvement in aortic pulse-wave velocity (P = .02) and reduced carotid IMT progression (P = .04).
Other evidence suggests that systemic therapy with TNF inhibitors is associated with the reduction of cardiovascular events, including myocardial infarction. In a study led by Dr. Wu, 8,845 Kaiser Permanente patients with psoriasis were evaluated between Jan. 1, 2004, and Nov. 30, 2010, and placed into one of three groups: treatment with a TNF inhibitor, treatment with oral therapy or phototherapy, or treatment with a topical agent (referent group) (Arch Dermatol. 2012 Nov;148[11]:1244-50). On multivariate analysis, the investigators found that the use of TNF inhibitors was associated with a significant reduction in the rate of incident MI (HR, 0.50; P = .003), compared with the use of topical agents.
Dr. Wu went on to note that C-reactive protein (CRP) is a predictor of CVD such as MI, peripheral arterial disease, and sudden cardiac death. In a retrospective cohort study, Dr. Wu and his associates evaluated Kaiser Permanente patients with psoriasis, psoriatic arthritis, or RA who were treated with a combination of TNF plus methotrexate, or with methotrexate alone from Jan. 1, 2002, to July 31, 2011 (J. Am. Acad. Dermatol. 2015;72[5]:917-9). Only patients in the combination therapy group had a clinically and statistically significant decrease in CRP, compared with baseline (a mean decrease of 5.18 mg/dL). No significant changes between the two groups were observed in terms of BMI, blood pressure, fasting glucose, hemoglobin A1C, total cholesterol, LDL cholesterol, HDL cholesterol, triglycerides, or alanine aminotransferase.
HDL cholesterol composition and function have also been studied in psoriasis patients. In one trial, HDL cholesterol was isolated from 15 psoriasis patients at baseline and after effective topical and/or systemic psoriasis therapy, and from 15 healthy controls matched for age and sex (J. Invest. Dermatol. 2014;134[3]:635-42). The researchers found that HDL cholesterol from psoriasis patients showed a significantly impaired capability to mobilize cholesterol from macrophages. However, psoriasis therapy recovered HDL cholesterol composition and function, but had no effect on serum HDL cholesterol levels.
A better understanding of the cardiovascular effects of biologic therapy should be gleaned from the Vascular Inflammation in Psoriasis Trial, which includes $3.8 million in funding from the National Institutes of Health, to prospectively study the effect of therapy on biomarkers and on positron emission tomography with 18fluorodeoxyglucose (FDG-PET)/CT scan. The 1-year study just completed enrolling patients into one of three treatment groups: adalimumab, UVB phototherapy, or placebo. Biomarkers such as CRP, lipids, and imaging will be assessed at baseline, weeks 4, 8, 12, and then every 12 weeks. “The theory is that adalimumab, but not UVB phototherapy, will improve biomarkers and reduce vascular inflammation as measured by FDG-PET/CT scan,” Dr. Wu said. “Aggressive systemic therapy may alter the natural history of cardiovascular disease.”
Current recommendations from the National Psoriasis Foundation call for an assessment of blood pressure, pulse, and body mass index every 2 years, and an assessment of fasting blood glucose and lipid levels every 5 years, or every 2 years if the patient has additional risk factors.
Dr. Wu reported that he has received research and consulting fees from numerous pharmaceutical companies, including AbbVie and Amgen. These go to his employer.
PARK CITY, UTAH – Evidence is mounting that the use of biologic therapies is associated with certain cardiovascular benefits in patients with psoriasis.
However, until the strength of the evidence becomes more robust, Dr. Jashin J. Wu does not recommend that patients are prescribed tumor necrosis factor (TNF) inhibitors specifically to reduce the risk of cardiovascular disease. “If systemic therapy is a consideration in the setting of CVD [cardiovascular disease] risk, it would appear that TNF inhibitors and methotrexate offer the best evidence of benefit,” Dr. Wu, director of the psoriasis clinic and director of dermatology research in the department of dermatology at Kaiser Permanente Los Angeles Medical Center, said at the annual meeting of the Pacific Dermatologic Association.
The National Psoriasis Foundation recently published a consensus statement about the potential impact of current therapies on cardiovascular disease, including the risk of diabetes (J Am Acad Dermatol. 2014;70[1]:168-77). In a retrospective study of 121,280 patients with rheumatoid arthritis (RA) or psoriasis, drug regimens were categorized into four mutually exclusive groups: TNF inhibitors with or without other disease-modifying antirheumatic drugs (DMARDs), methotrexate without TNF inhibitors or hydroxychloroquine, hydroxychloroquine without TNF inhibitors or methotrexate; and other nonbiologic DMARDs without TNF inhibitors, methotrexate or hydroxychloroquine (referent group). The researchers found that adjusted Cox proportional hazards for the risk of diabetes were lower among those on TNF inhibitors (hazard ratio, 0.62; 95% confidence interval, 0.42-0.91) and hydroxychloroquine (HR, 0.54; 95% CI, 0.36-0.80), compared with those on methotrexate (HR, 0.77, 95% CI, 0.53-1.13) (JAMA. 2011 Jun 22;305[24]:2525-31).
Increased carotid intimal media thickness (IMT) is also an independent predictor of CVD. In a prospective, observational study, 20 psoriasis patients received a 12-week treatment of TNF inhibitors (Ann Rheum Dis. 2011 Apr;70[4]:705-6). After 12 weeks, 9 patients continued on their TNF-inhibitor regimen (group 1), while 11 discontinued treatment because of financial constraints (group 2). Another 20 psoriasis patients who did not receive biologic therapy served as controls (group 3). The researchers measured IMT by carotid ultrasound at baseline, week 12 and at 2 years. They found that between baseline and 2 years, IMT decreased from 0.70 mm to 0.63 mm in group 1, from 0.71 to 0.67 in group 2, and increased from 0.79 to 0.82 in group 3. “This seems to suggest that the use of TNF inhibitors may improve atherosclerosis,” said Dr. Wu, who is currently running seven clinical trials of psoriasis patients. Similar findings were seen in a larger study that examined 224 patients with psoriatic arthritis and followed them for a mean of 55 months: 124 on TNF inhibitors, 104 on DMARDs, and 305 matched controls (Arterioscler Thromb Vasc Biol. 2011 Mar;31[3]:705-120).
In a separate study of 16 psoriasis patients treated with TNF inhibitors, researchers used ultrasound to measure IMT of carotid and brachial arteries after 6 months of therapy (J Am Acad Dermatol. 2013 Oct;69[4]:523-9). At baseline, all 16 patients had an IMT greater than normal. In those without initial calcified atherosclerotic plaques, 13 of 16 had a significant decrease of IMT (P = .0002). In those with initial calcified atherosclerotic plaques, 3 of 16 patients had a nonsignificant increase in IMT.
Increased arterial stiffness is another independent predictor of CVD. In a controlled study of 55 patients with psoriatic arthritis, RA, or ankylosing spondylitis, researchers used aortic pulse-wave velocity to measure aortic stiffness. Almost 35% of the patients (36) received TNF inhibitors while the remaining 19 did not receive treatment (Am J Hypertens. 2012 Jun;25[6]:644-50). After 1 year of follow-up, use of TNF inhibitors was associated with a significant improvement in aortic pulse-wave velocity (P = .02) and reduced carotid IMT progression (P = .04).
Other evidence suggests that systemic therapy with TNF inhibitors is associated with the reduction of cardiovascular events, including myocardial infarction. In a study led by Dr. Wu, 8,845 Kaiser Permanente patients with psoriasis were evaluated between Jan. 1, 2004, and Nov. 30, 2010, and placed into one of three groups: treatment with a TNF inhibitor, treatment with oral therapy or phototherapy, or treatment with a topical agent (referent group) (Arch Dermatol. 2012 Nov;148[11]:1244-50). On multivariate analysis, the investigators found that the use of TNF inhibitors was associated with a significant reduction in the rate of incident MI (HR, 0.50; P = .003), compared with the use of topical agents.
Dr. Wu went on to note that C-reactive protein (CRP) is a predictor of CVD such as MI, peripheral arterial disease, and sudden cardiac death. In a retrospective cohort study, Dr. Wu and his associates evaluated Kaiser Permanente patients with psoriasis, psoriatic arthritis, or RA who were treated with a combination of TNF plus methotrexate, or with methotrexate alone from Jan. 1, 2002, to July 31, 2011 (J. Am. Acad. Dermatol. 2015;72[5]:917-9). Only patients in the combination therapy group had a clinically and statistically significant decrease in CRP, compared with baseline (a mean decrease of 5.18 mg/dL). No significant changes between the two groups were observed in terms of BMI, blood pressure, fasting glucose, hemoglobin A1C, total cholesterol, LDL cholesterol, HDL cholesterol, triglycerides, or alanine aminotransferase.
HDL cholesterol composition and function have also been studied in psoriasis patients. In one trial, HDL cholesterol was isolated from 15 psoriasis patients at baseline and after effective topical and/or systemic psoriasis therapy, and from 15 healthy controls matched for age and sex (J. Invest. Dermatol. 2014;134[3]:635-42). The researchers found that HDL cholesterol from psoriasis patients showed a significantly impaired capability to mobilize cholesterol from macrophages. However, psoriasis therapy recovered HDL cholesterol composition and function, but had no effect on serum HDL cholesterol levels.
A better understanding of the cardiovascular effects of biologic therapy should be gleaned from the Vascular Inflammation in Psoriasis Trial, which includes $3.8 million in funding from the National Institutes of Health, to prospectively study the effect of therapy on biomarkers and on positron emission tomography with 18fluorodeoxyglucose (FDG-PET)/CT scan. The 1-year study just completed enrolling patients into one of three treatment groups: adalimumab, UVB phototherapy, or placebo. Biomarkers such as CRP, lipids, and imaging will be assessed at baseline, weeks 4, 8, 12, and then every 12 weeks. “The theory is that adalimumab, but not UVB phototherapy, will improve biomarkers and reduce vascular inflammation as measured by FDG-PET/CT scan,” Dr. Wu said. “Aggressive systemic therapy may alter the natural history of cardiovascular disease.”
Current recommendations from the National Psoriasis Foundation call for an assessment of blood pressure, pulse, and body mass index every 2 years, and an assessment of fasting blood glucose and lipid levels every 5 years, or every 2 years if the patient has additional risk factors.
Dr. Wu reported that he has received research and consulting fees from numerous pharmaceutical companies, including AbbVie and Amgen. These go to his employer.
EXPERT ANALYSIS AT PDA 2015