User login
Pacific Dermatologic Association (PDA): Annual Meeting
Inpatient dermatologist offers three rules to diagnose by
SAN FRANCISCO – Three rules can guide inpatient dermatology for the most effective practice, according to Dr. Lindy P. Fox, director of the dermatology consultation service at the University of California, San Francisco.
First, more than one infection or etiology may account for the clinical picture, especially in immunocompromised patients. If there’s more than one morphology present, work up each one separately, she said.
Second, most cases require some degree of clinicopathologic correlation.
Third, for inpatient dermatology it helps to think of "zebras," because that’s how you’ll make the rare diagnosis, she said at the annual meeting of the Pacific Dermatologic Association.
She provided tips on some of the more common morphologies encountered in hospital dermatology:
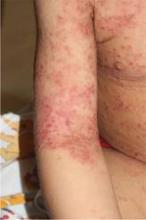
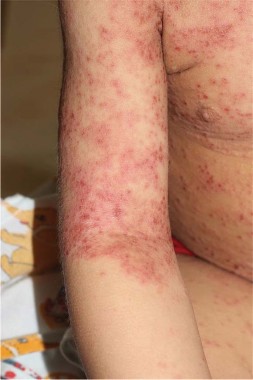
• Morbilliform. Most commonly, morbilliform brings to mind a drug eruption, viral exanthema, graft vs. host disease (GVHD), and other entities that can present in this pattern. Rarely in immunocompromised patients, disseminated histoplasmosis, cryptococcosis, or coccidiomycosis can mimic a drug eruption, so it’s important in those situations to biopsy what you think might be a drug eruption.
Acute graft vs. host disease on average occurs 25 days after a transplant, but hyperacute GVHD occurs 14 days post transplant, so think of this before your oncologist tells you the patient’s graft hasn’t taken, she said. Two features of GVHD morbilliform eruption distinguish it from other differential diagnoses: a perifollicular accentuation, and an acral distribution around the ears, hand, and periungual areas. A patient’s nausea, vomiting, diarrhea, and hyperbilirubinemia support the diagnosis.
• Papules/papulonodules. The differential diagnosis is long – fungal diseases, septic emboli, Sweet’s syndrome, leukemia or lymphoma cutis, Kaposi sarcoma, cutaneous metastasis, and sarcoidosis, among others. But if you see purple plumb-colored papules, the list shrinks to lymphoma, Kaposi sarcoma, bacillary angiomatosis, melanoma, or cutaneous metastasis.
• Cellulitic plaques. These bring to mind cellulitis or its mimic, stasis dermatitis, as well as some deep fungal infections, carcinoma erysipeloides, neutrophilic diseases, and acute inflammatory edema of the ICU. Keep in mind that cryptococcal cellulitis is the most common presentation of Cryptococcus in an organ transplant recipient. Unlike bacterial cellulitis, it tends to be bilateral. It is almost never purely a cutaneous disease. "If you ever see Cryptococcus on the skin, you are obligated to look for the systemic source of infection," she said.
• Palpable purpura. This typically points to small or mixed-vessel vasculitis. But you can also have secondary hemorrhage into a papular process; it can be due to leukemia, lymphoma cutis, or Sweet’s syndrome; or it can be a cutaneous reaction to cytarabine. What Dr. Fox said she commonly sees are purpuric papules on the lower extremities. If looked at closely, there’s a morbilliform eruption that can spread to the trunk in a few days. Biopsy typically demonstrates spongiosis, which is a "very reassuring" sign that this is a benign and self-limiting reaction to cytarabine. The reaction may or may not recur if the patient takes the drug again. "Steroids do help, and patients do very well," she said.
• Retiform purpura. Another very long differential diagnosis can be organized by thinking about potential causes as vascular (in the vessel wall) or intravascular (in the lumen of the wall). Lumen involvement is a sign of embolization or thrombosis. "If you see concomitant thrombosis and vasculitis truly on the same day early on in lesion development, that differential diagnosis shrinks considerably. There are very few things that can do that: cryoglobulinemia, septic vasculitis, and exposure to levamisole," Dr. Fox said.
• Sclerodermoid. The long differential diagnosis includes carcinoma en cuirasse, radiation dermatitis, GVHD, scleroderma, paraffinoma, lipodermatosclerosis, scleredema, and scleromyxedema, among others. Dr. Fox said she saw an unusual presentation in a woman with "antibiotic refractory cellulitis" who had developed a migrating paraffinoma after mineral oil injections to her calves for cosmetic purposes.
• Necrotic papules/plaques. One of the many potential causes – calciphylaxis – can be thought of as analogous to a myocardial infarction that occurs in the skin, she said. Patients more often than not have a long history of vascular stenosis and medial arterial calcification. An acute thrombotic event occurs, and the patient presents with calciphylaxis with tissue ischemia, pain, and stellate purpuric plaques. "This implies that we need to think about our therapeutics differently," she said. Address the calcium, phosphorous, and parathyroid hormone, as usual, but also consider treating the thrombus with tissue plasminogen activator. Don’t use warfarin; many cases of calciphylaxis are triggered by warfarin, and the drug promotes calcification, Dr. Fox said at the annual meeting of the Pacific Dermatologic Association.
• Ulcers. The most common causes in the hospital are venous insufficiency, pyoderma gangrenosum, deep fungal infection, and chronic viral infections. Chronic herpes simplex virus frequently is the culprit in bedridden, immunosuppressed patients. "Any buttock ulcer or any decubitis ulcer that I see gets ruled out for concomitant herpes simplex virus because I find it probably more than 50% of the time," she said.
• Pustules. One cause – disseminated candidiasis – can mimic acne in the immunosuppressed patient. The lesions tend to have a pale center that sometimes is pustular. "You can KOH that center to prove that you’ve got candidiasis," she said. If there’s no pustule, diagnosing disseminated candidiasis can be difficult because the collection of organisms is so small that it’s easily missed on serial sectioning by dermatopathology. Ask for serial sectioning and staining to identify the organism.
• Erythema. Dr. Fox said she divides erythemas into macular, subacute, and erosive. Macular erythema can be a toxin-mediated erythema or due to drug eruption, GVHD, viral exanthema, or Kawasaki disease. Eosinophilia is a common finding in toxin-mediated erythema. "I use it to help rule [erythema] in, rather than to help rule it out," she said.
When a subacute or chronic erythroderma is due to drug hypersensitivity reaction, be aware of two long-term sequellae: thyroid disruption and late-onset cardiac involvement presenting as life-threatening heart failure in people you wouldn’t expect to have heart problems. Dr. Fox said she checks thyroid-stimulating hormone levels monthly for 6 months and warns patients to go to the emergency department if they develop heart symptoms and tell the staff there to call the dermatologist.
One of the lesser-known causes of erosive erythroderma is toxic erythema of chemotherapy. It can take a lot of charts and talking with the primary medical team to make sure this is not toxic erythema necrolysislike GVHD, she said.
Dr. Fox reported having no relevant financial disclosures.
On Twitter @sherryboschert
SAN FRANCISCO – Three rules can guide inpatient dermatology for the most effective practice, according to Dr. Lindy P. Fox, director of the dermatology consultation service at the University of California, San Francisco.
First, more than one infection or etiology may account for the clinical picture, especially in immunocompromised patients. If there’s more than one morphology present, work up each one separately, she said.
Second, most cases require some degree of clinicopathologic correlation.
Third, for inpatient dermatology it helps to think of "zebras," because that’s how you’ll make the rare diagnosis, she said at the annual meeting of the Pacific Dermatologic Association.
She provided tips on some of the more common morphologies encountered in hospital dermatology:
• Morbilliform. Most commonly, morbilliform brings to mind a drug eruption, viral exanthema, graft vs. host disease (GVHD), and other entities that can present in this pattern. Rarely in immunocompromised patients, disseminated histoplasmosis, cryptococcosis, or coccidiomycosis can mimic a drug eruption, so it’s important in those situations to biopsy what you think might be a drug eruption.
Acute graft vs. host disease on average occurs 25 days after a transplant, but hyperacute GVHD occurs 14 days post transplant, so think of this before your oncologist tells you the patient’s graft hasn’t taken, she said. Two features of GVHD morbilliform eruption distinguish it from other differential diagnoses: a perifollicular accentuation, and an acral distribution around the ears, hand, and periungual areas. A patient’s nausea, vomiting, diarrhea, and hyperbilirubinemia support the diagnosis.
• Papules/papulonodules. The differential diagnosis is long – fungal diseases, septic emboli, Sweet’s syndrome, leukemia or lymphoma cutis, Kaposi sarcoma, cutaneous metastasis, and sarcoidosis, among others. But if you see purple plumb-colored papules, the list shrinks to lymphoma, Kaposi sarcoma, bacillary angiomatosis, melanoma, or cutaneous metastasis.
• Cellulitic plaques. These bring to mind cellulitis or its mimic, stasis dermatitis, as well as some deep fungal infections, carcinoma erysipeloides, neutrophilic diseases, and acute inflammatory edema of the ICU. Keep in mind that cryptococcal cellulitis is the most common presentation of Cryptococcus in an organ transplant recipient. Unlike bacterial cellulitis, it tends to be bilateral. It is almost never purely a cutaneous disease. "If you ever see Cryptococcus on the skin, you are obligated to look for the systemic source of infection," she said.
• Palpable purpura. This typically points to small or mixed-vessel vasculitis. But you can also have secondary hemorrhage into a papular process; it can be due to leukemia, lymphoma cutis, or Sweet’s syndrome; or it can be a cutaneous reaction to cytarabine. What Dr. Fox said she commonly sees are purpuric papules on the lower extremities. If looked at closely, there’s a morbilliform eruption that can spread to the trunk in a few days. Biopsy typically demonstrates spongiosis, which is a "very reassuring" sign that this is a benign and self-limiting reaction to cytarabine. The reaction may or may not recur if the patient takes the drug again. "Steroids do help, and patients do very well," she said.
• Retiform purpura. Another very long differential diagnosis can be organized by thinking about potential causes as vascular (in the vessel wall) or intravascular (in the lumen of the wall). Lumen involvement is a sign of embolization or thrombosis. "If you see concomitant thrombosis and vasculitis truly on the same day early on in lesion development, that differential diagnosis shrinks considerably. There are very few things that can do that: cryoglobulinemia, septic vasculitis, and exposure to levamisole," Dr. Fox said.
• Sclerodermoid. The long differential diagnosis includes carcinoma en cuirasse, radiation dermatitis, GVHD, scleroderma, paraffinoma, lipodermatosclerosis, scleredema, and scleromyxedema, among others. Dr. Fox said she saw an unusual presentation in a woman with "antibiotic refractory cellulitis" who had developed a migrating paraffinoma after mineral oil injections to her calves for cosmetic purposes.
• Necrotic papules/plaques. One of the many potential causes – calciphylaxis – can be thought of as analogous to a myocardial infarction that occurs in the skin, she said. Patients more often than not have a long history of vascular stenosis and medial arterial calcification. An acute thrombotic event occurs, and the patient presents with calciphylaxis with tissue ischemia, pain, and stellate purpuric plaques. "This implies that we need to think about our therapeutics differently," she said. Address the calcium, phosphorous, and parathyroid hormone, as usual, but also consider treating the thrombus with tissue plasminogen activator. Don’t use warfarin; many cases of calciphylaxis are triggered by warfarin, and the drug promotes calcification, Dr. Fox said at the annual meeting of the Pacific Dermatologic Association.
• Ulcers. The most common causes in the hospital are venous insufficiency, pyoderma gangrenosum, deep fungal infection, and chronic viral infections. Chronic herpes simplex virus frequently is the culprit in bedridden, immunosuppressed patients. "Any buttock ulcer or any decubitis ulcer that I see gets ruled out for concomitant herpes simplex virus because I find it probably more than 50% of the time," she said.
• Pustules. One cause – disseminated candidiasis – can mimic acne in the immunosuppressed patient. The lesions tend to have a pale center that sometimes is pustular. "You can KOH that center to prove that you’ve got candidiasis," she said. If there’s no pustule, diagnosing disseminated candidiasis can be difficult because the collection of organisms is so small that it’s easily missed on serial sectioning by dermatopathology. Ask for serial sectioning and staining to identify the organism.
• Erythema. Dr. Fox said she divides erythemas into macular, subacute, and erosive. Macular erythema can be a toxin-mediated erythema or due to drug eruption, GVHD, viral exanthema, or Kawasaki disease. Eosinophilia is a common finding in toxin-mediated erythema. "I use it to help rule [erythema] in, rather than to help rule it out," she said.
When a subacute or chronic erythroderma is due to drug hypersensitivity reaction, be aware of two long-term sequellae: thyroid disruption and late-onset cardiac involvement presenting as life-threatening heart failure in people you wouldn’t expect to have heart problems. Dr. Fox said she checks thyroid-stimulating hormone levels monthly for 6 months and warns patients to go to the emergency department if they develop heart symptoms and tell the staff there to call the dermatologist.
One of the lesser-known causes of erosive erythroderma is toxic erythema of chemotherapy. It can take a lot of charts and talking with the primary medical team to make sure this is not toxic erythema necrolysislike GVHD, she said.
Dr. Fox reported having no relevant financial disclosures.
On Twitter @sherryboschert
SAN FRANCISCO – Three rules can guide inpatient dermatology for the most effective practice, according to Dr. Lindy P. Fox, director of the dermatology consultation service at the University of California, San Francisco.
First, more than one infection or etiology may account for the clinical picture, especially in immunocompromised patients. If there’s more than one morphology present, work up each one separately, she said.
Second, most cases require some degree of clinicopathologic correlation.
Third, for inpatient dermatology it helps to think of "zebras," because that’s how you’ll make the rare diagnosis, she said at the annual meeting of the Pacific Dermatologic Association.
She provided tips on some of the more common morphologies encountered in hospital dermatology:
• Morbilliform. Most commonly, morbilliform brings to mind a drug eruption, viral exanthema, graft vs. host disease (GVHD), and other entities that can present in this pattern. Rarely in immunocompromised patients, disseminated histoplasmosis, cryptococcosis, or coccidiomycosis can mimic a drug eruption, so it’s important in those situations to biopsy what you think might be a drug eruption.
Acute graft vs. host disease on average occurs 25 days after a transplant, but hyperacute GVHD occurs 14 days post transplant, so think of this before your oncologist tells you the patient’s graft hasn’t taken, she said. Two features of GVHD morbilliform eruption distinguish it from other differential diagnoses: a perifollicular accentuation, and an acral distribution around the ears, hand, and periungual areas. A patient’s nausea, vomiting, diarrhea, and hyperbilirubinemia support the diagnosis.
• Papules/papulonodules. The differential diagnosis is long – fungal diseases, septic emboli, Sweet’s syndrome, leukemia or lymphoma cutis, Kaposi sarcoma, cutaneous metastasis, and sarcoidosis, among others. But if you see purple plumb-colored papules, the list shrinks to lymphoma, Kaposi sarcoma, bacillary angiomatosis, melanoma, or cutaneous metastasis.
• Cellulitic plaques. These bring to mind cellulitis or its mimic, stasis dermatitis, as well as some deep fungal infections, carcinoma erysipeloides, neutrophilic diseases, and acute inflammatory edema of the ICU. Keep in mind that cryptococcal cellulitis is the most common presentation of Cryptococcus in an organ transplant recipient. Unlike bacterial cellulitis, it tends to be bilateral. It is almost never purely a cutaneous disease. "If you ever see Cryptococcus on the skin, you are obligated to look for the systemic source of infection," she said.
• Palpable purpura. This typically points to small or mixed-vessel vasculitis. But you can also have secondary hemorrhage into a papular process; it can be due to leukemia, lymphoma cutis, or Sweet’s syndrome; or it can be a cutaneous reaction to cytarabine. What Dr. Fox said she commonly sees are purpuric papules on the lower extremities. If looked at closely, there’s a morbilliform eruption that can spread to the trunk in a few days. Biopsy typically demonstrates spongiosis, which is a "very reassuring" sign that this is a benign and self-limiting reaction to cytarabine. The reaction may or may not recur if the patient takes the drug again. "Steroids do help, and patients do very well," she said.
• Retiform purpura. Another very long differential diagnosis can be organized by thinking about potential causes as vascular (in the vessel wall) or intravascular (in the lumen of the wall). Lumen involvement is a sign of embolization or thrombosis. "If you see concomitant thrombosis and vasculitis truly on the same day early on in lesion development, that differential diagnosis shrinks considerably. There are very few things that can do that: cryoglobulinemia, septic vasculitis, and exposure to levamisole," Dr. Fox said.
• Sclerodermoid. The long differential diagnosis includes carcinoma en cuirasse, radiation dermatitis, GVHD, scleroderma, paraffinoma, lipodermatosclerosis, scleredema, and scleromyxedema, among others. Dr. Fox said she saw an unusual presentation in a woman with "antibiotic refractory cellulitis" who had developed a migrating paraffinoma after mineral oil injections to her calves for cosmetic purposes.
• Necrotic papules/plaques. One of the many potential causes – calciphylaxis – can be thought of as analogous to a myocardial infarction that occurs in the skin, she said. Patients more often than not have a long history of vascular stenosis and medial arterial calcification. An acute thrombotic event occurs, and the patient presents with calciphylaxis with tissue ischemia, pain, and stellate purpuric plaques. "This implies that we need to think about our therapeutics differently," she said. Address the calcium, phosphorous, and parathyroid hormone, as usual, but also consider treating the thrombus with tissue plasminogen activator. Don’t use warfarin; many cases of calciphylaxis are triggered by warfarin, and the drug promotes calcification, Dr. Fox said at the annual meeting of the Pacific Dermatologic Association.
• Ulcers. The most common causes in the hospital are venous insufficiency, pyoderma gangrenosum, deep fungal infection, and chronic viral infections. Chronic herpes simplex virus frequently is the culprit in bedridden, immunosuppressed patients. "Any buttock ulcer or any decubitis ulcer that I see gets ruled out for concomitant herpes simplex virus because I find it probably more than 50% of the time," she said.
• Pustules. One cause – disseminated candidiasis – can mimic acne in the immunosuppressed patient. The lesions tend to have a pale center that sometimes is pustular. "You can KOH that center to prove that you’ve got candidiasis," she said. If there’s no pustule, diagnosing disseminated candidiasis can be difficult because the collection of organisms is so small that it’s easily missed on serial sectioning by dermatopathology. Ask for serial sectioning and staining to identify the organism.
• Erythema. Dr. Fox said she divides erythemas into macular, subacute, and erosive. Macular erythema can be a toxin-mediated erythema or due to drug eruption, GVHD, viral exanthema, or Kawasaki disease. Eosinophilia is a common finding in toxin-mediated erythema. "I use it to help rule [erythema] in, rather than to help rule it out," she said.
When a subacute or chronic erythroderma is due to drug hypersensitivity reaction, be aware of two long-term sequellae: thyroid disruption and late-onset cardiac involvement presenting as life-threatening heart failure in people you wouldn’t expect to have heart problems. Dr. Fox said she checks thyroid-stimulating hormone levels monthly for 6 months and warns patients to go to the emergency department if they develop heart symptoms and tell the staff there to call the dermatologist.
One of the lesser-known causes of erosive erythroderma is toxic erythema of chemotherapy. It can take a lot of charts and talking with the primary medical team to make sure this is not toxic erythema necrolysislike GVHD, she said.
Dr. Fox reported having no relevant financial disclosures.
On Twitter @sherryboschert
EXPERT ANALYSIS FROM THE PDA ANNUAL MEETING
Big changes ahead for dermatology
Dr. Howard I. Maibach is a big believer in science-based practice, or as he puts it, a "deeply orthodox member" of evidence-based medicine. But he went beyond these parameters to draw on his decades of experience as a dermatologist in forecasting where the specialty is headed, based on his keen observations and anecdotal experience.
Dr. Maibach’s predictions for dermatology potentially have huge ramifications for training and clinical practice.
For one thing, board examinations will evolve from testing memory to testing how well physicians can look up information and whether they know the right algorithms for when to seek consultations. Current recertification exams are "based upon a system from the 19th century. It’s going to go," he said at the annual meeting of the Pacific Dermatologic Association in San Francisco.
Because of the expansion of knowledge and specialization, medical institutions – especially medical schools – will rely on cooperative teams and silos of excellence to help each other. In some parts of the United States, groups of dermatologists may do consultations only, and no direct care. The specialty will develop algorithms for deciding when to get consultant help early, when to wait, and when a consultant is of no value, said Dr. Maibach, professor of dermatology at the University of California, San Francisco.
Health education of consumers and patients will increase, starting from grades 5 or 6, he suggested. Medical residencies and fellowships will emphasize an introduction to lifelong learning in the subject. And medicine will mimic some other successful industries by instituting months-long sabbaticals every 5 years or so for additional training.
Why does he think this? Here are the trends that Dr. Maibach perceives, and which he believes will underlie the changes ahead. What do you think?
• There is a functional shortage of dermatologists. On Dr. Maibach’s weekly telephone hour, he frequently gets asked for help in arranging for a patient to be seen sooner in places like Kansas, where there’s a 6-month waiting list to see dermatologists, he said. "Clearly, society is going to demand a change."
• Dermatologic knowledge is expanding rapidly, which will lead to greater specialization and changes in training that will include some practitioners who are more specialized than today’s dermatologists and some who are less specialized.
• The development and use of smartphone "apps" will become ubiquitous in 3-5 years, with ever-more-powerful and useful apps. "You’re going to say three words, and all of the stuff that you learned in your residency is going to be updated in moments," he said.
• More and more physician assistants and nurse practitioners will be employed to help deal with the functional shortage of U.S. dermatologists.
• Whole new classes of assistants will be created, some of whom may be certified after 6 weeks of training and some of whom will be uncertified. "Your patients will learn to live with them," Dr. Maibach said. "If you go around the hospitals of California, there are many people acting as nurses who don’t even have an LVN degree and who are doing a wonderful job."
• Aestheticians will increase their role in dermatology practices.
• In all countries of the world, not just the United States, government will play an increasing role in medical care. "Your governments, unless you’re totally cash oriented, are going to be more deeply involved," he said.
• Specialties will increasingly subspecialize. "I predict the major training centers will have 10-15 subspecialties. I could see there will be one porphyria clinic for all of California and Utah in the not-too-distant future," he said.
• There’s enormous room for innovation, especially as evidence-based medicine takes hold, which will continue to change medical practice.
• People will live longer, exacerbating some of these trends and perhaps creating gerontologic dermatologists.
• Rationing will grow. "We have it already. We don’t like to talk about it in public, but you’re going to see more and more pseudo- or actual rationing of care," he said.
• Physician assistants and nurse practitioners who practice 10 or 100 miles from their supervising physicians eventually will become freestanding practitioners themselves.
• Younger dermatologists who already are rejecting long work hours will shorten the work week to 30 or 32 hours.
• The nascent use of teledermatology will increase.
• Some medical schools will offer 3-year degrees instead of the traditional 4 years. "It’s already started," Dr. Maibach said.
• Residencies may evolve into two or three tiers of varying lengths to more rapidly produce the numbers of physicians needed to meet public demand.
• More nondermatologists, such as general physicians or gynecologists, will offer cosmetic services, sometimes after brief training of questionable validity. Dr. Maibach recently was invited to take a 3-day course in laser medicine with the claim that after only 3 days, "you’ll be able to use a laser."
Dermatologists can either fight change or aid it. "That’s a personal decision," Dr. Maibach said, but big changes are ahead either way.
What do you think of his predictions? Are you seeing them happening already? Do you have other predictions of your own? Look into your own crystal ball and let us know.
Dr. Maibach reported having no financial disclosures.
–Sherry Boschert
On Twitter @sherryboschert
Dr. Howard I. Maibach is a big believer in science-based practice, or as he puts it, a "deeply orthodox member" of evidence-based medicine. But he went beyond these parameters to draw on his decades of experience as a dermatologist in forecasting where the specialty is headed, based on his keen observations and anecdotal experience.
Dr. Maibach’s predictions for dermatology potentially have huge ramifications for training and clinical practice.
For one thing, board examinations will evolve from testing memory to testing how well physicians can look up information and whether they know the right algorithms for when to seek consultations. Current recertification exams are "based upon a system from the 19th century. It’s going to go," he said at the annual meeting of the Pacific Dermatologic Association in San Francisco.
Because of the expansion of knowledge and specialization, medical institutions – especially medical schools – will rely on cooperative teams and silos of excellence to help each other. In some parts of the United States, groups of dermatologists may do consultations only, and no direct care. The specialty will develop algorithms for deciding when to get consultant help early, when to wait, and when a consultant is of no value, said Dr. Maibach, professor of dermatology at the University of California, San Francisco.
Health education of consumers and patients will increase, starting from grades 5 or 6, he suggested. Medical residencies and fellowships will emphasize an introduction to lifelong learning in the subject. And medicine will mimic some other successful industries by instituting months-long sabbaticals every 5 years or so for additional training.
Why does he think this? Here are the trends that Dr. Maibach perceives, and which he believes will underlie the changes ahead. What do you think?
• There is a functional shortage of dermatologists. On Dr. Maibach’s weekly telephone hour, he frequently gets asked for help in arranging for a patient to be seen sooner in places like Kansas, where there’s a 6-month waiting list to see dermatologists, he said. "Clearly, society is going to demand a change."
• Dermatologic knowledge is expanding rapidly, which will lead to greater specialization and changes in training that will include some practitioners who are more specialized than today’s dermatologists and some who are less specialized.
• The development and use of smartphone "apps" will become ubiquitous in 3-5 years, with ever-more-powerful and useful apps. "You’re going to say three words, and all of the stuff that you learned in your residency is going to be updated in moments," he said.
• More and more physician assistants and nurse practitioners will be employed to help deal with the functional shortage of U.S. dermatologists.
• Whole new classes of assistants will be created, some of whom may be certified after 6 weeks of training and some of whom will be uncertified. "Your patients will learn to live with them," Dr. Maibach said. "If you go around the hospitals of California, there are many people acting as nurses who don’t even have an LVN degree and who are doing a wonderful job."
• Aestheticians will increase their role in dermatology practices.
• In all countries of the world, not just the United States, government will play an increasing role in medical care. "Your governments, unless you’re totally cash oriented, are going to be more deeply involved," he said.
• Specialties will increasingly subspecialize. "I predict the major training centers will have 10-15 subspecialties. I could see there will be one porphyria clinic for all of California and Utah in the not-too-distant future," he said.
• There’s enormous room for innovation, especially as evidence-based medicine takes hold, which will continue to change medical practice.
• People will live longer, exacerbating some of these trends and perhaps creating gerontologic dermatologists.
• Rationing will grow. "We have it already. We don’t like to talk about it in public, but you’re going to see more and more pseudo- or actual rationing of care," he said.
• Physician assistants and nurse practitioners who practice 10 or 100 miles from their supervising physicians eventually will become freestanding practitioners themselves.
• Younger dermatologists who already are rejecting long work hours will shorten the work week to 30 or 32 hours.
• The nascent use of teledermatology will increase.
• Some medical schools will offer 3-year degrees instead of the traditional 4 years. "It’s already started," Dr. Maibach said.
• Residencies may evolve into two or three tiers of varying lengths to more rapidly produce the numbers of physicians needed to meet public demand.
• More nondermatologists, such as general physicians or gynecologists, will offer cosmetic services, sometimes after brief training of questionable validity. Dr. Maibach recently was invited to take a 3-day course in laser medicine with the claim that after only 3 days, "you’ll be able to use a laser."
Dermatologists can either fight change or aid it. "That’s a personal decision," Dr. Maibach said, but big changes are ahead either way.
What do you think of his predictions? Are you seeing them happening already? Do you have other predictions of your own? Look into your own crystal ball and let us know.
Dr. Maibach reported having no financial disclosures.
–Sherry Boschert
On Twitter @sherryboschert
Dr. Howard I. Maibach is a big believer in science-based practice, or as he puts it, a "deeply orthodox member" of evidence-based medicine. But he went beyond these parameters to draw on his decades of experience as a dermatologist in forecasting where the specialty is headed, based on his keen observations and anecdotal experience.
Dr. Maibach’s predictions for dermatology potentially have huge ramifications for training and clinical practice.
For one thing, board examinations will evolve from testing memory to testing how well physicians can look up information and whether they know the right algorithms for when to seek consultations. Current recertification exams are "based upon a system from the 19th century. It’s going to go," he said at the annual meeting of the Pacific Dermatologic Association in San Francisco.
Because of the expansion of knowledge and specialization, medical institutions – especially medical schools – will rely on cooperative teams and silos of excellence to help each other. In some parts of the United States, groups of dermatologists may do consultations only, and no direct care. The specialty will develop algorithms for deciding when to get consultant help early, when to wait, and when a consultant is of no value, said Dr. Maibach, professor of dermatology at the University of California, San Francisco.
Health education of consumers and patients will increase, starting from grades 5 or 6, he suggested. Medical residencies and fellowships will emphasize an introduction to lifelong learning in the subject. And medicine will mimic some other successful industries by instituting months-long sabbaticals every 5 years or so for additional training.
Why does he think this? Here are the trends that Dr. Maibach perceives, and which he believes will underlie the changes ahead. What do you think?
• There is a functional shortage of dermatologists. On Dr. Maibach’s weekly telephone hour, he frequently gets asked for help in arranging for a patient to be seen sooner in places like Kansas, where there’s a 6-month waiting list to see dermatologists, he said. "Clearly, society is going to demand a change."
• Dermatologic knowledge is expanding rapidly, which will lead to greater specialization and changes in training that will include some practitioners who are more specialized than today’s dermatologists and some who are less specialized.
• The development and use of smartphone "apps" will become ubiquitous in 3-5 years, with ever-more-powerful and useful apps. "You’re going to say three words, and all of the stuff that you learned in your residency is going to be updated in moments," he said.
• More and more physician assistants and nurse practitioners will be employed to help deal with the functional shortage of U.S. dermatologists.
• Whole new classes of assistants will be created, some of whom may be certified after 6 weeks of training and some of whom will be uncertified. "Your patients will learn to live with them," Dr. Maibach said. "If you go around the hospitals of California, there are many people acting as nurses who don’t even have an LVN degree and who are doing a wonderful job."
• Aestheticians will increase their role in dermatology practices.
• In all countries of the world, not just the United States, government will play an increasing role in medical care. "Your governments, unless you’re totally cash oriented, are going to be more deeply involved," he said.
• Specialties will increasingly subspecialize. "I predict the major training centers will have 10-15 subspecialties. I could see there will be one porphyria clinic for all of California and Utah in the not-too-distant future," he said.
• There’s enormous room for innovation, especially as evidence-based medicine takes hold, which will continue to change medical practice.
• People will live longer, exacerbating some of these trends and perhaps creating gerontologic dermatologists.
• Rationing will grow. "We have it already. We don’t like to talk about it in public, but you’re going to see more and more pseudo- or actual rationing of care," he said.
• Physician assistants and nurse practitioners who practice 10 or 100 miles from their supervising physicians eventually will become freestanding practitioners themselves.
• Younger dermatologists who already are rejecting long work hours will shorten the work week to 30 or 32 hours.
• The nascent use of teledermatology will increase.
• Some medical schools will offer 3-year degrees instead of the traditional 4 years. "It’s already started," Dr. Maibach said.
• Residencies may evolve into two or three tiers of varying lengths to more rapidly produce the numbers of physicians needed to meet public demand.
• More nondermatologists, such as general physicians or gynecologists, will offer cosmetic services, sometimes after brief training of questionable validity. Dr. Maibach recently was invited to take a 3-day course in laser medicine with the claim that after only 3 days, "you’ll be able to use a laser."
Dermatologists can either fight change or aid it. "That’s a personal decision," Dr. Maibach said, but big changes are ahead either way.
What do you think of his predictions? Are you seeing them happening already? Do you have other predictions of your own? Look into your own crystal ball and let us know.
Dr. Maibach reported having no financial disclosures.
–Sherry Boschert
On Twitter @sherryboschert
Don’t Rush to Lymph Node Biopsy
SAN FRANCISCO – Don’t recommend sentinel lymph node biopsy in patients with a melanoma depth of 0.75 mm or thinner unless there are true high-risk features, Dr. Susan Swetter advised at the annual meeting of the Pacific Dermatologic Association.
Increasing numbers of patients with thin melanomas being referred for consideration of sentinel lymph node biopsy inspired some revisions in the 2013 National Comprehensive Cancer Network melanoma guidelines regarding clinical stage, patient work-up, and treatment, said Dr. Swetter, professor of dermatology at Stanford (Calif.) University.
"Because of the issue of upstaging T1a and T1b melanomas based on the mitotic rate, we have seen a dramatic rise in cases referred to the academic centers for sentinel lymph node biopsy consideration," Dr. Swetter said.
The experts convened by the National Comprehensive Cancer Network (NCCN) in 2012 to revise the guidelines reviewed the evidence and found that thickness is the only consistent predictor of sentinel lymph node positivity for thin melanomas 1 mm or less in depth. They revised the stratification of stage IA and IB melanomas according to their risk for sentinel node metastasis rather than on the basis of the American Joint Committee on Cancer stage.
Essentially, tumors up to 0.75 mm in thickness have a low risk of metastasis (2%-2.5%) regardless of the mitotic rate. Tumors with a thickness of 0.76-1mm, particularly those with a high mitotic rate, have a higher risk of metastasis (approximately 5%), and are "very eligible" for sentinel lymph node biopsy, said Dr. Swetter, who was a member of the guidelines committee. "The 0.75-mm cutoff does not change the stage of the patient" but is a way of stratifying the risk for a positive sentinel node biopsy (J. Natl. Compr. Canc. Netw. 2013;11:395-407).
Guidelines from the American Academy of Dermatology in 2011 did not recommend sentinel lymph node biopsy for any T1a tumor up to 1 mm in depth because the AAD committee felt that the risk of a positive biopsy result should be at least 10% to justify the procedure, said Dr. Swetter, who also was on the committee that drafted those guidelines.
A key footnote in the NCCN 2013 recommendations: Sentinel node biopsy is not recommended for primary melanomas up to 0.75 mm unless there is significant uncertainty about the adequacy of microstaging, "meaning it’s a transected tumor or a nonrepresentative biopsy," Dr. Swetter explained. Sentinel node biopsy may be considered for melanomas 0.76-1 mm thick in the appropriate clinical context.
There is little consensus on what puts a thin melanoma (up to 1 mm) at high risk for metastasis, other than primary tumor thickness. Risk factors include a high mitotic rate (though the NCCN guidelines don’t specify a number), Dr. Swetter noted. Ulceration and lymphovascular invasion increase the risk but are rare.
"Consider sentinel lymph node biopsy in thin melanomas on an individual basis," Dr. Swetter said.
In general, don’t recommend sentinel lymph node biopsy for primary melanomas up to 0.75 mm thick unless there is a very high mitotic rate, lymphovascular invasion, ulceration, a nonrepresentative biopsy (with a large clinical residual lesion), or a partial biopsy (particularly one with deep transection of the tumor and inadequate microstaging), she said. A Clark level IV or V also might be cause for a sentinel node biopsy for a thin tumor, but "we only use Clark level when the mitotic rate can’t be assessed," she noted. "I can’t think of a case in the last 4 years where I’ve paid attention to the Clark level as a reason to pursue treatment in a patient."
One other "risk factor" for sentinel lymph node biopsy, even in a patient with a very thin melanoma and low mitotic rate, is a strong patient preference for the procedure, Dr. Swetter added. Dermatologists should try to educate patients and explain that their chances of a positive result may be fairly negligible, but if "they are determined to have sentinel lymph node biopsy, they will have it done," she said.
Dr. Swetter reported having no relevant financial disclosures.
On Twitter @sherryboschert
SAN FRANCISCO – Don’t recommend sentinel lymph node biopsy in patients with a melanoma depth of 0.75 mm or thinner unless there are true high-risk features, Dr. Susan Swetter advised at the annual meeting of the Pacific Dermatologic Association.
Increasing numbers of patients with thin melanomas being referred for consideration of sentinel lymph node biopsy inspired some revisions in the 2013 National Comprehensive Cancer Network melanoma guidelines regarding clinical stage, patient work-up, and treatment, said Dr. Swetter, professor of dermatology at Stanford (Calif.) University.
"Because of the issue of upstaging T1a and T1b melanomas based on the mitotic rate, we have seen a dramatic rise in cases referred to the academic centers for sentinel lymph node biopsy consideration," Dr. Swetter said.
The experts convened by the National Comprehensive Cancer Network (NCCN) in 2012 to revise the guidelines reviewed the evidence and found that thickness is the only consistent predictor of sentinel lymph node positivity for thin melanomas 1 mm or less in depth. They revised the stratification of stage IA and IB melanomas according to their risk for sentinel node metastasis rather than on the basis of the American Joint Committee on Cancer stage.
Essentially, tumors up to 0.75 mm in thickness have a low risk of metastasis (2%-2.5%) regardless of the mitotic rate. Tumors with a thickness of 0.76-1mm, particularly those with a high mitotic rate, have a higher risk of metastasis (approximately 5%), and are "very eligible" for sentinel lymph node biopsy, said Dr. Swetter, who was a member of the guidelines committee. "The 0.75-mm cutoff does not change the stage of the patient" but is a way of stratifying the risk for a positive sentinel node biopsy (J. Natl. Compr. Canc. Netw. 2013;11:395-407).
Guidelines from the American Academy of Dermatology in 2011 did not recommend sentinel lymph node biopsy for any T1a tumor up to 1 mm in depth because the AAD committee felt that the risk of a positive biopsy result should be at least 10% to justify the procedure, said Dr. Swetter, who also was on the committee that drafted those guidelines.
A key footnote in the NCCN 2013 recommendations: Sentinel node biopsy is not recommended for primary melanomas up to 0.75 mm unless there is significant uncertainty about the adequacy of microstaging, "meaning it’s a transected tumor or a nonrepresentative biopsy," Dr. Swetter explained. Sentinel node biopsy may be considered for melanomas 0.76-1 mm thick in the appropriate clinical context.
There is little consensus on what puts a thin melanoma (up to 1 mm) at high risk for metastasis, other than primary tumor thickness. Risk factors include a high mitotic rate (though the NCCN guidelines don’t specify a number), Dr. Swetter noted. Ulceration and lymphovascular invasion increase the risk but are rare.
"Consider sentinel lymph node biopsy in thin melanomas on an individual basis," Dr. Swetter said.
In general, don’t recommend sentinel lymph node biopsy for primary melanomas up to 0.75 mm thick unless there is a very high mitotic rate, lymphovascular invasion, ulceration, a nonrepresentative biopsy (with a large clinical residual lesion), or a partial biopsy (particularly one with deep transection of the tumor and inadequate microstaging), she said. A Clark level IV or V also might be cause for a sentinel node biopsy for a thin tumor, but "we only use Clark level when the mitotic rate can’t be assessed," she noted. "I can’t think of a case in the last 4 years where I’ve paid attention to the Clark level as a reason to pursue treatment in a patient."
One other "risk factor" for sentinel lymph node biopsy, even in a patient with a very thin melanoma and low mitotic rate, is a strong patient preference for the procedure, Dr. Swetter added. Dermatologists should try to educate patients and explain that their chances of a positive result may be fairly negligible, but if "they are determined to have sentinel lymph node biopsy, they will have it done," she said.
Dr. Swetter reported having no relevant financial disclosures.
On Twitter @sherryboschert
SAN FRANCISCO – Don’t recommend sentinel lymph node biopsy in patients with a melanoma depth of 0.75 mm or thinner unless there are true high-risk features, Dr. Susan Swetter advised at the annual meeting of the Pacific Dermatologic Association.
Increasing numbers of patients with thin melanomas being referred for consideration of sentinel lymph node biopsy inspired some revisions in the 2013 National Comprehensive Cancer Network melanoma guidelines regarding clinical stage, patient work-up, and treatment, said Dr. Swetter, professor of dermatology at Stanford (Calif.) University.
"Because of the issue of upstaging T1a and T1b melanomas based on the mitotic rate, we have seen a dramatic rise in cases referred to the academic centers for sentinel lymph node biopsy consideration," Dr. Swetter said.
The experts convened by the National Comprehensive Cancer Network (NCCN) in 2012 to revise the guidelines reviewed the evidence and found that thickness is the only consistent predictor of sentinel lymph node positivity for thin melanomas 1 mm or less in depth. They revised the stratification of stage IA and IB melanomas according to their risk for sentinel node metastasis rather than on the basis of the American Joint Committee on Cancer stage.
Essentially, tumors up to 0.75 mm in thickness have a low risk of metastasis (2%-2.5%) regardless of the mitotic rate. Tumors with a thickness of 0.76-1mm, particularly those with a high mitotic rate, have a higher risk of metastasis (approximately 5%), and are "very eligible" for sentinel lymph node biopsy, said Dr. Swetter, who was a member of the guidelines committee. "The 0.75-mm cutoff does not change the stage of the patient" but is a way of stratifying the risk for a positive sentinel node biopsy (J. Natl. Compr. Canc. Netw. 2013;11:395-407).
Guidelines from the American Academy of Dermatology in 2011 did not recommend sentinel lymph node biopsy for any T1a tumor up to 1 mm in depth because the AAD committee felt that the risk of a positive biopsy result should be at least 10% to justify the procedure, said Dr. Swetter, who also was on the committee that drafted those guidelines.
A key footnote in the NCCN 2013 recommendations: Sentinel node biopsy is not recommended for primary melanomas up to 0.75 mm unless there is significant uncertainty about the adequacy of microstaging, "meaning it’s a transected tumor or a nonrepresentative biopsy," Dr. Swetter explained. Sentinel node biopsy may be considered for melanomas 0.76-1 mm thick in the appropriate clinical context.
There is little consensus on what puts a thin melanoma (up to 1 mm) at high risk for metastasis, other than primary tumor thickness. Risk factors include a high mitotic rate (though the NCCN guidelines don’t specify a number), Dr. Swetter noted. Ulceration and lymphovascular invasion increase the risk but are rare.
"Consider sentinel lymph node biopsy in thin melanomas on an individual basis," Dr. Swetter said.
In general, don’t recommend sentinel lymph node biopsy for primary melanomas up to 0.75 mm thick unless there is a very high mitotic rate, lymphovascular invasion, ulceration, a nonrepresentative biopsy (with a large clinical residual lesion), or a partial biopsy (particularly one with deep transection of the tumor and inadequate microstaging), she said. A Clark level IV or V also might be cause for a sentinel node biopsy for a thin tumor, but "we only use Clark level when the mitotic rate can’t be assessed," she noted. "I can’t think of a case in the last 4 years where I’ve paid attention to the Clark level as a reason to pursue treatment in a patient."
One other "risk factor" for sentinel lymph node biopsy, even in a patient with a very thin melanoma and low mitotic rate, is a strong patient preference for the procedure, Dr. Swetter added. Dermatologists should try to educate patients and explain that their chances of a positive result may be fairly negligible, but if "they are determined to have sentinel lymph node biopsy, they will have it done," she said.
Dr. Swetter reported having no relevant financial disclosures.
On Twitter @sherryboschert
EXPERT ANALYSIS FROM THE PDA ANNUAL MEETING
Don’t rush to lymph node biopsy for thin melanomas, expert says
SAN FRANCISCO – Don’t recommend sentinel lymph node biopsy in patients with a melanoma depth of 0.75 mm or thinner unless there are true high-risk features, Dr. Susan Swetter advised at the annual meeting of the Pacific Dermatologic Association.
Increasing numbers of patients with thin melanomas being referred for consideration of sentinel lymph node biopsy inspired some revisions in the 2013 National Comprehensive Cancer Network melanoma guidelines regarding clinical stage, patient work-up, and treatment, said Dr. Swetter, professor of dermatology at Stanford (Calif.) University.
"Because of the issue of upstaging T1a and T1b melanomas based on the mitotic rate, we have seen a dramatic rise in cases referred to the academic centers for sentinel lymph node biopsy consideration," Dr. Swetter said.
The experts convened by the National Comprehensive Cancer Network (NCCN) in 2012 to revise the guidelines reviewed the evidence and found that thickness is the only consistent predictor of sentinel lymph node positivity for thin melanomas 1 mm or less in depth. They revised the stratification of stage IA and IB melanomas according to their risk for sentinel node metastasis rather than on the basis of the American Joint Committee on Cancer stage.
Essentially, tumors up to 0.75 mm in thickness have a low risk of metastasis (2%-2.5%) regardless of the mitotic rate. Tumors with a thickness of 0.76-1mm, particularly those with a high mitotic rate, have a higher risk of metastasis (approximately 5%), and are "very eligible" for sentinel lymph node biopsy, said Dr. Swetter, who was a member of the guidelines committee. "The 0.75-mm cutoff does not change the stage of the patient" but is a way of stratifying the risk for a positive sentinel node biopsy (J. Natl. Compr. Canc. Netw. 2013;11:395-407).
Guidelines from the American Academy of Dermatology in 2011 did not recommend sentinel lymph node biopsy for any T1a tumor up to 1 mm in depth because the AAD committee felt that the risk of a positive biopsy result should be at least 10% to justify the procedure, said Dr. Swetter, who also was on the committee that drafted those guidelines.
A key footnote in the NCCN 2013 recommendations: Sentinel node biopsy is not recommended for primary melanomas up to 0.75 mm unless there is significant uncertainty about the adequacy of microstaging, "meaning it’s a transected tumor or a nonrepresentative biopsy," Dr. Swetter explained. Sentinel node biopsy may be considered for melanomas 0.76-1 mm thick in the appropriate clinical context.
There is little consensus on what puts a thin melanoma (up to 1 mm) at high risk for metastasis, other than primary tumor thickness. Risk factors include a high mitotic rate (though the NCCN guidelines don’t specify a number), Dr. Swetter noted. Ulceration and lymphovascular invasion increase the risk but are rare.
"Consider sentinel lymph node biopsy in thin melanomas on an individual basis," Dr. Swetter said.
In general, don’t recommend sentinel lymph node biopsy for primary melanomas up to 0.75 mm thick unless there is a very high mitotic rate, lymphovascular invasion, ulceration, a nonrepresentative biopsy (with a large clinical residual lesion), or a partial biopsy (particularly one with deep transection of the tumor and inadequate microstaging), she said. A Clark level IV or V also might be cause for a sentinel node biopsy for a thin tumor, but "we only use Clark level when the mitotic rate can’t be assessed," she noted. "I can’t think of a case in the last 4 years where I’ve paid attention to the Clark level as a reason to pursue treatment in a patient."
One other "risk factor" for sentinel lymph node biopsy, even in a patient with a very thin melanoma and low mitotic rate, is a strong patient preference for the procedure, Dr. Swetter added. Dermatologists should try to educate patients and explain that their chances of a positive result may be fairly negligible, but if "they are determined to have sentinel lymph node biopsy, they will have it done," she said.
Dr. Swetter reported having no relevant financial disclosures.
On Twitter @sherryboschert
SAN FRANCISCO – Don’t recommend sentinel lymph node biopsy in patients with a melanoma depth of 0.75 mm or thinner unless there are true high-risk features, Dr. Susan Swetter advised at the annual meeting of the Pacific Dermatologic Association.
Increasing numbers of patients with thin melanomas being referred for consideration of sentinel lymph node biopsy inspired some revisions in the 2013 National Comprehensive Cancer Network melanoma guidelines regarding clinical stage, patient work-up, and treatment, said Dr. Swetter, professor of dermatology at Stanford (Calif.) University.
"Because of the issue of upstaging T1a and T1b melanomas based on the mitotic rate, we have seen a dramatic rise in cases referred to the academic centers for sentinel lymph node biopsy consideration," Dr. Swetter said.
The experts convened by the National Comprehensive Cancer Network (NCCN) in 2012 to revise the guidelines reviewed the evidence and found that thickness is the only consistent predictor of sentinel lymph node positivity for thin melanomas 1 mm or less in depth. They revised the stratification of stage IA and IB melanomas according to their risk for sentinel node metastasis rather than on the basis of the American Joint Committee on Cancer stage.
Essentially, tumors up to 0.75 mm in thickness have a low risk of metastasis (2%-2.5%) regardless of the mitotic rate. Tumors with a thickness of 0.76-1mm, particularly those with a high mitotic rate, have a higher risk of metastasis (approximately 5%), and are "very eligible" for sentinel lymph node biopsy, said Dr. Swetter, who was a member of the guidelines committee. "The 0.75-mm cutoff does not change the stage of the patient" but is a way of stratifying the risk for a positive sentinel node biopsy (J. Natl. Compr. Canc. Netw. 2013;11:395-407).
Guidelines from the American Academy of Dermatology in 2011 did not recommend sentinel lymph node biopsy for any T1a tumor up to 1 mm in depth because the AAD committee felt that the risk of a positive biopsy result should be at least 10% to justify the procedure, said Dr. Swetter, who also was on the committee that drafted those guidelines.
A key footnote in the NCCN 2013 recommendations: Sentinel node biopsy is not recommended for primary melanomas up to 0.75 mm unless there is significant uncertainty about the adequacy of microstaging, "meaning it’s a transected tumor or a nonrepresentative biopsy," Dr. Swetter explained. Sentinel node biopsy may be considered for melanomas 0.76-1 mm thick in the appropriate clinical context.
There is little consensus on what puts a thin melanoma (up to 1 mm) at high risk for metastasis, other than primary tumor thickness. Risk factors include a high mitotic rate (though the NCCN guidelines don’t specify a number), Dr. Swetter noted. Ulceration and lymphovascular invasion increase the risk but are rare.
"Consider sentinel lymph node biopsy in thin melanomas on an individual basis," Dr. Swetter said.
In general, don’t recommend sentinel lymph node biopsy for primary melanomas up to 0.75 mm thick unless there is a very high mitotic rate, lymphovascular invasion, ulceration, a nonrepresentative biopsy (with a large clinical residual lesion), or a partial biopsy (particularly one with deep transection of the tumor and inadequate microstaging), she said. A Clark level IV or V also might be cause for a sentinel node biopsy for a thin tumor, but "we only use Clark level when the mitotic rate can’t be assessed," she noted. "I can’t think of a case in the last 4 years where I’ve paid attention to the Clark level as a reason to pursue treatment in a patient."
One other "risk factor" for sentinel lymph node biopsy, even in a patient with a very thin melanoma and low mitotic rate, is a strong patient preference for the procedure, Dr. Swetter added. Dermatologists should try to educate patients and explain that their chances of a positive result may be fairly negligible, but if "they are determined to have sentinel lymph node biopsy, they will have it done," she said.
Dr. Swetter reported having no relevant financial disclosures.
On Twitter @sherryboschert
SAN FRANCISCO – Don’t recommend sentinel lymph node biopsy in patients with a melanoma depth of 0.75 mm or thinner unless there are true high-risk features, Dr. Susan Swetter advised at the annual meeting of the Pacific Dermatologic Association.
Increasing numbers of patients with thin melanomas being referred for consideration of sentinel lymph node biopsy inspired some revisions in the 2013 National Comprehensive Cancer Network melanoma guidelines regarding clinical stage, patient work-up, and treatment, said Dr. Swetter, professor of dermatology at Stanford (Calif.) University.
"Because of the issue of upstaging T1a and T1b melanomas based on the mitotic rate, we have seen a dramatic rise in cases referred to the academic centers for sentinel lymph node biopsy consideration," Dr. Swetter said.
The experts convened by the National Comprehensive Cancer Network (NCCN) in 2012 to revise the guidelines reviewed the evidence and found that thickness is the only consistent predictor of sentinel lymph node positivity for thin melanomas 1 mm or less in depth. They revised the stratification of stage IA and IB melanomas according to their risk for sentinel node metastasis rather than on the basis of the American Joint Committee on Cancer stage.
Essentially, tumors up to 0.75 mm in thickness have a low risk of metastasis (2%-2.5%) regardless of the mitotic rate. Tumors with a thickness of 0.76-1mm, particularly those with a high mitotic rate, have a higher risk of metastasis (approximately 5%), and are "very eligible" for sentinel lymph node biopsy, said Dr. Swetter, who was a member of the guidelines committee. "The 0.75-mm cutoff does not change the stage of the patient" but is a way of stratifying the risk for a positive sentinel node biopsy (J. Natl. Compr. Canc. Netw. 2013;11:395-407).
Guidelines from the American Academy of Dermatology in 2011 did not recommend sentinel lymph node biopsy for any T1a tumor up to 1 mm in depth because the AAD committee felt that the risk of a positive biopsy result should be at least 10% to justify the procedure, said Dr. Swetter, who also was on the committee that drafted those guidelines.
A key footnote in the NCCN 2013 recommendations: Sentinel node biopsy is not recommended for primary melanomas up to 0.75 mm unless there is significant uncertainty about the adequacy of microstaging, "meaning it’s a transected tumor or a nonrepresentative biopsy," Dr. Swetter explained. Sentinel node biopsy may be considered for melanomas 0.76-1 mm thick in the appropriate clinical context.
There is little consensus on what puts a thin melanoma (up to 1 mm) at high risk for metastasis, other than primary tumor thickness. Risk factors include a high mitotic rate (though the NCCN guidelines don’t specify a number), Dr. Swetter noted. Ulceration and lymphovascular invasion increase the risk but are rare.
"Consider sentinel lymph node biopsy in thin melanomas on an individual basis," Dr. Swetter said.
In general, don’t recommend sentinel lymph node biopsy for primary melanomas up to 0.75 mm thick unless there is a very high mitotic rate, lymphovascular invasion, ulceration, a nonrepresentative biopsy (with a large clinical residual lesion), or a partial biopsy (particularly one with deep transection of the tumor and inadequate microstaging), she said. A Clark level IV or V also might be cause for a sentinel node biopsy for a thin tumor, but "we only use Clark level when the mitotic rate can’t be assessed," she noted. "I can’t think of a case in the last 4 years where I’ve paid attention to the Clark level as a reason to pursue treatment in a patient."
One other "risk factor" for sentinel lymph node biopsy, even in a patient with a very thin melanoma and low mitotic rate, is a strong patient preference for the procedure, Dr. Swetter added. Dermatologists should try to educate patients and explain that their chances of a positive result may be fairly negligible, but if "they are determined to have sentinel lymph node biopsy, they will have it done," she said.
Dr. Swetter reported having no relevant financial disclosures.
On Twitter @sherryboschert
EXPERT ANALYSIS FROM THE PDA ANNUAL MEETING
New GI, topical approaches target rosacea
SAN FRANCISCO – Things are looking rosier for the treatment of rosacea, according to Dr. Kanade Shinkai.
"Two exciting new treatments" are likely to change clinical practices around this difficult-to-treat disease while researchers continue to gather more data on their use, she said at the annual meeting of the Pacific Dermatologic Association.
Dr. Shinkai said she was encouraged by preliminary data on treating rosacea indirectly by using off-label rifaximin for small intestinal bacterial overgrowth and by a new formulation of brimonidine approved in August as the first topical agent for the erythema of rosacea.
The GI approach looks promising enough to incorporate into her practice, said Dr. Shinkai of the University of California, San Francisco.
That strategy grew out of a 2008 study showing that 52 (46%) of 113 patients with rosacea had small intestinal bacterial overgrowth (SIBO) compared with 3 (5%) of 60 healthy matched controls. Treating SIBO with the antibiotic rifaximin (a cousin of rifampin) at 400 mg thrice daily for 10 days cleared rosacea in 35 (78%) of the 45 patients in whom SIBO was eradicated. Cleared rosacea remained clear for up to 9 months in 96% of patients. In two patients, both rosacea and SIBO recurred (Clin. Gastroenterol. Hepatol. 2008;6:759-64).
Investigators hypothesized that SIBO might modulate cytokines by increasing tumor necrosis factor–alpha, suppressing interleukin-17, or increasing the T helper cell type 1 pathway gene to drive skin inflammation. Mechanistically, the gut could be inducing molecular mimicry that results in extraintestinal disease due to these cytokine triggers, said Dr. Shinkai.
A more recent pilot study showed that 32 (51%) of 63 patients with rosacea had SIBO compared with 7 (23%) of 30 control subjects in the general population. Among 28 patients who took rifaximin for their SIBO, rosacea cleared in 46%, moderately improved in 25%, and mildly improved in 11%. No improvement was seen in 18% (J. Am. Acad. Dermatol. 2013;68:875-6).
"Rosacea can be very difficult to treat. This is at least one avenue that we can ask about," she said. Patients with rosacea who have more GI symptoms "will be the ones that I’ll target with rifaximin."
Most cases of rosacea in the study were confirmed by dermatologists. The studies used a positive lactulose/glucose breath test as a surrogate for diagnosing SIBO, a less-invasive strategy than the standard for diagnosis: jejunal aspirate. Larger double-blind studies that include jejunal aspirates probably will be needed "to really prove that this is a worthy treatment," Dr. Shinkai said.
The other addition to the regimen for rosacea is the new 0.33% topical brimonidine gel (Mirvaso), approved by the Food and Drug Administration in August 2013 for the treatment of the facial erythema of rosacea in adults. Each gram of gel contains 5 mg of brimonidine tartrate, equivalent to 3.3 mg of brimonidine free base, according to Galderma, which markets the drug.
"I think that’s a very important breakthrough considering what we tell patients now for erythema, which is to avoid their triggers (sun exposure, hot spicy foods, hot liquid) or to consider laser therapy, which is not an option for many patients because of the insurance issue," she said.
Brimonidine is a highly selective alpha2-adrenergic agonist that is very vasoconstrictive. The drug has a known safety and efficacy profile from its long-term use as a treatment for glaucoma, but the topical gel is a new formulation.
Approval was based on two Galderma-funded pivotal trials of various doses of the gel in a total of 391 patients, which showed dose-dependent reductions in erythema for 12 hours and a two-grade improvement in erythema ratings by clinicians, patients, and Chroma Meter (Br. J. Dermatol. 2012;166:633-41).
The drug was fast acting and well tolerated, Dr. Shinkai said. Stopping the medication did cause a kind of tachyphylaxis seen with other alpha-adrenergics used to treat nasal or conjunctival congestion, where stopping the drug increased erythema or edema. Two patients in the studies developed mild, transient decreases in intraocular pressure, probably a result of inadvertently getting the gel in the eye, she said. Both studies were short, with 4 weeks of follow-up.
"I think the jury is still out" until more data become available on long-term use of brimonidine for erythema in rosacea. "However, I think this is a really exciting new development" because it adds to the few tools available for treatment, she said.
Dr. Shinkai reported having no relevant financial disclosures.
On Twitter @sherryboschert
SAN FRANCISCO – Things are looking rosier for the treatment of rosacea, according to Dr. Kanade Shinkai.
"Two exciting new treatments" are likely to change clinical practices around this difficult-to-treat disease while researchers continue to gather more data on their use, she said at the annual meeting of the Pacific Dermatologic Association.
Dr. Shinkai said she was encouraged by preliminary data on treating rosacea indirectly by using off-label rifaximin for small intestinal bacterial overgrowth and by a new formulation of brimonidine approved in August as the first topical agent for the erythema of rosacea.
The GI approach looks promising enough to incorporate into her practice, said Dr. Shinkai of the University of California, San Francisco.
That strategy grew out of a 2008 study showing that 52 (46%) of 113 patients with rosacea had small intestinal bacterial overgrowth (SIBO) compared with 3 (5%) of 60 healthy matched controls. Treating SIBO with the antibiotic rifaximin (a cousin of rifampin) at 400 mg thrice daily for 10 days cleared rosacea in 35 (78%) of the 45 patients in whom SIBO was eradicated. Cleared rosacea remained clear for up to 9 months in 96% of patients. In two patients, both rosacea and SIBO recurred (Clin. Gastroenterol. Hepatol. 2008;6:759-64).
Investigators hypothesized that SIBO might modulate cytokines by increasing tumor necrosis factor–alpha, suppressing interleukin-17, or increasing the T helper cell type 1 pathway gene to drive skin inflammation. Mechanistically, the gut could be inducing molecular mimicry that results in extraintestinal disease due to these cytokine triggers, said Dr. Shinkai.
A more recent pilot study showed that 32 (51%) of 63 patients with rosacea had SIBO compared with 7 (23%) of 30 control subjects in the general population. Among 28 patients who took rifaximin for their SIBO, rosacea cleared in 46%, moderately improved in 25%, and mildly improved in 11%. No improvement was seen in 18% (J. Am. Acad. Dermatol. 2013;68:875-6).
"Rosacea can be very difficult to treat. This is at least one avenue that we can ask about," she said. Patients with rosacea who have more GI symptoms "will be the ones that I’ll target with rifaximin."
Most cases of rosacea in the study were confirmed by dermatologists. The studies used a positive lactulose/glucose breath test as a surrogate for diagnosing SIBO, a less-invasive strategy than the standard for diagnosis: jejunal aspirate. Larger double-blind studies that include jejunal aspirates probably will be needed "to really prove that this is a worthy treatment," Dr. Shinkai said.
The other addition to the regimen for rosacea is the new 0.33% topical brimonidine gel (Mirvaso), approved by the Food and Drug Administration in August 2013 for the treatment of the facial erythema of rosacea in adults. Each gram of gel contains 5 mg of brimonidine tartrate, equivalent to 3.3 mg of brimonidine free base, according to Galderma, which markets the drug.
"I think that’s a very important breakthrough considering what we tell patients now for erythema, which is to avoid their triggers (sun exposure, hot spicy foods, hot liquid) or to consider laser therapy, which is not an option for many patients because of the insurance issue," she said.
Brimonidine is a highly selective alpha2-adrenergic agonist that is very vasoconstrictive. The drug has a known safety and efficacy profile from its long-term use as a treatment for glaucoma, but the topical gel is a new formulation.
Approval was based on two Galderma-funded pivotal trials of various doses of the gel in a total of 391 patients, which showed dose-dependent reductions in erythema for 12 hours and a two-grade improvement in erythema ratings by clinicians, patients, and Chroma Meter (Br. J. Dermatol. 2012;166:633-41).
The drug was fast acting and well tolerated, Dr. Shinkai said. Stopping the medication did cause a kind of tachyphylaxis seen with other alpha-adrenergics used to treat nasal or conjunctival congestion, where stopping the drug increased erythema or edema. Two patients in the studies developed mild, transient decreases in intraocular pressure, probably a result of inadvertently getting the gel in the eye, she said. Both studies were short, with 4 weeks of follow-up.
"I think the jury is still out" until more data become available on long-term use of brimonidine for erythema in rosacea. "However, I think this is a really exciting new development" because it adds to the few tools available for treatment, she said.
Dr. Shinkai reported having no relevant financial disclosures.
On Twitter @sherryboschert
SAN FRANCISCO – Things are looking rosier for the treatment of rosacea, according to Dr. Kanade Shinkai.
"Two exciting new treatments" are likely to change clinical practices around this difficult-to-treat disease while researchers continue to gather more data on their use, she said at the annual meeting of the Pacific Dermatologic Association.
Dr. Shinkai said she was encouraged by preliminary data on treating rosacea indirectly by using off-label rifaximin for small intestinal bacterial overgrowth and by a new formulation of brimonidine approved in August as the first topical agent for the erythema of rosacea.
The GI approach looks promising enough to incorporate into her practice, said Dr. Shinkai of the University of California, San Francisco.
That strategy grew out of a 2008 study showing that 52 (46%) of 113 patients with rosacea had small intestinal bacterial overgrowth (SIBO) compared with 3 (5%) of 60 healthy matched controls. Treating SIBO with the antibiotic rifaximin (a cousin of rifampin) at 400 mg thrice daily for 10 days cleared rosacea in 35 (78%) of the 45 patients in whom SIBO was eradicated. Cleared rosacea remained clear for up to 9 months in 96% of patients. In two patients, both rosacea and SIBO recurred (Clin. Gastroenterol. Hepatol. 2008;6:759-64).
Investigators hypothesized that SIBO might modulate cytokines by increasing tumor necrosis factor–alpha, suppressing interleukin-17, or increasing the T helper cell type 1 pathway gene to drive skin inflammation. Mechanistically, the gut could be inducing molecular mimicry that results in extraintestinal disease due to these cytokine triggers, said Dr. Shinkai.
A more recent pilot study showed that 32 (51%) of 63 patients with rosacea had SIBO compared with 7 (23%) of 30 control subjects in the general population. Among 28 patients who took rifaximin for their SIBO, rosacea cleared in 46%, moderately improved in 25%, and mildly improved in 11%. No improvement was seen in 18% (J. Am. Acad. Dermatol. 2013;68:875-6).
"Rosacea can be very difficult to treat. This is at least one avenue that we can ask about," she said. Patients with rosacea who have more GI symptoms "will be the ones that I’ll target with rifaximin."
Most cases of rosacea in the study were confirmed by dermatologists. The studies used a positive lactulose/glucose breath test as a surrogate for diagnosing SIBO, a less-invasive strategy than the standard for diagnosis: jejunal aspirate. Larger double-blind studies that include jejunal aspirates probably will be needed "to really prove that this is a worthy treatment," Dr. Shinkai said.
The other addition to the regimen for rosacea is the new 0.33% topical brimonidine gel (Mirvaso), approved by the Food and Drug Administration in August 2013 for the treatment of the facial erythema of rosacea in adults. Each gram of gel contains 5 mg of brimonidine tartrate, equivalent to 3.3 mg of brimonidine free base, according to Galderma, which markets the drug.
"I think that’s a very important breakthrough considering what we tell patients now for erythema, which is to avoid their triggers (sun exposure, hot spicy foods, hot liquid) or to consider laser therapy, which is not an option for many patients because of the insurance issue," she said.
Brimonidine is a highly selective alpha2-adrenergic agonist that is very vasoconstrictive. The drug has a known safety and efficacy profile from its long-term use as a treatment for glaucoma, but the topical gel is a new formulation.
Approval was based on two Galderma-funded pivotal trials of various doses of the gel in a total of 391 patients, which showed dose-dependent reductions in erythema for 12 hours and a two-grade improvement in erythema ratings by clinicians, patients, and Chroma Meter (Br. J. Dermatol. 2012;166:633-41).
The drug was fast acting and well tolerated, Dr. Shinkai said. Stopping the medication did cause a kind of tachyphylaxis seen with other alpha-adrenergics used to treat nasal or conjunctival congestion, where stopping the drug increased erythema or edema. Two patients in the studies developed mild, transient decreases in intraocular pressure, probably a result of inadvertently getting the gel in the eye, she said. Both studies were short, with 4 weeks of follow-up.
"I think the jury is still out" until more data become available on long-term use of brimonidine for erythema in rosacea. "However, I think this is a really exciting new development" because it adds to the few tools available for treatment, she said.
Dr. Shinkai reported having no relevant financial disclosures.
On Twitter @sherryboschert
EXPERT ANALYSIS FROM THE PDA ANNUAL MEETING
Restrict Mohs surgery or risk drop in reimbursement
SAN FRANCISCO – Dermatologists and dermatologic surgeons risk a decreased reimbursement value for Mohs surgery if they don’t review the published criteria on its appropriate use, according to Dr. Sumaira Aasi.
Although it can be difficult, it is worth the effort to convince some patients that electrodessication, a curettage procedure, or some other management strategy is appropriate for their skin lesions, she said at the annual meeting of the Pacific Dermatologic Association.
"The reason is, at the end of the day, we might be killing the goose that laid the golden egg ourselves" if too many Mohs surgeries are done for inappropriate reasons, said Dr. Aasi of Stanford (Calif.) University.
SDr. Aasi urged the audience at the meeting to study criteria published in 2012 for the appropriate use of Mohs surgery, and to pay particular attention to scenarios deemed inappropriate for the procedure (J. Am. Acad. Dermatol. 2012;67:531-50).
There are an estimated 4 million nonmelanoma skin cancers in the United States each year, and the use of Mohs surgery increased by 400% from 1995 to 2009, Dr. Aasi said (Dermatol. Clin. 2012;30:167-75).
Some experts argue that the increase is a response to the increase in skin cancer cases, and others say the increasing number of Mohs surgeons is driving the rising rate of the procedure. "Regardless, it’s a problem," Dr. Aasi said.
Federal reimbursement programs don’t care if there is an epidemic of skin cancer, she said. If a surgery is being "overused," it is considered overvalued, and reimbursement rates are subject to change, she explained.
The top two CPT codes flagged by the federal government as potentially misvalued in the July 19, 2013, Federal Register were codes 17311 for Mohs surgery on the face, head, and neck and 17313 for Mohs surgery on the trunk and extremities, Dr. Aasi noted.
The 2012 appropriate-use criteria were created to help maintain the value of Mohs surgery in Medicare reimbursements, Dr. Aasi said. The American Academy of Dermatology, the American Society of Dermatologic Surgery, the American College of Mohs Surgery, and the American Society of Mohs Surgery convened a consensus panel of eight Mohs surgeons and nine non-Mohs surgeons. The panel considered 270 clinical scenarios and rated 74% appropriate for Mohs surgery, 17% inappropriate, and 9% uncertain, meaning there were conflicting data in the literature or not enough information to make a determination of the appropriateness of Mohs surgery in that scenario.
"I still think that Mohs surgeons in this panel were a little highly represented," Dr. Aasi said. Overall, the indications the panel deemed appropriate for Mohs surgery were "pretty generous," she added.
The consensus panel was asked to implicitly consider cost in deciding on the appropriateness of Mohs surgery for each scenario. "It’s an implicit consideration but a very important consideration," said Dr. Aasi.
Invasive melanoma was not included in the scenarios because if its complexity, but some scenarios did include melanoma in situ or lentigo maligna melanoma. The scenarios included different body areas, aggressive or nonaggressive skin cancers, tumor size, and the patient’s immune status. Agreement by at least 12 panel members was considered a consensus on the use of Mohs surgery.
Examples of scenarios that were considered inappropriate for Mohs surgery included nonaggressive basal cell or squamous cell carcinomas smaller than 2 cm on the trunk or extremities. Other lesions deemed inappropriate for Mohs included primary actinic keratosis (AK) with focal squamous cell cancer in situ, bowenoid AK, or squamous cell carcinoma in situ AK-type, labels that Dr. Aasi found "worrisome" because their definitions are unclear.
"As a Mohs surgeon, I found it a little surprising that they had to consider scenarios where actinic keratoses were being considered a possibility for Mohs surgery," she said.
Physicians can use the appropriate-use criteria to talk with other health care providers, third-party payers, and patients, said Dr. Aasi. Talking with patients can be most difficult, because if they’ve heard of Mohs surgery, they often want "the technique where I [the patient] know right away that I’m going to be clear," she said.
When Mohs surgery is inappropriate, "I try to convince them that, no, you don’t need it. It’s like an atomic bomb being used to kill a flea," she said.
The appropriate-use criteria do not compare different treatment modalities with Mohs surgery, Dr. Aasi emphasized, but they aim to state simply whether or not Mohs surgery would be appropriate in selected scenarios.
"I urge everyone to read" the criteria, she said.
Dr. Aasi reported having no financial disclosures.
On Twitter @sherryboschert
SAN FRANCISCO – Dermatologists and dermatologic surgeons risk a decreased reimbursement value for Mohs surgery if they don’t review the published criteria on its appropriate use, according to Dr. Sumaira Aasi.
Although it can be difficult, it is worth the effort to convince some patients that electrodessication, a curettage procedure, or some other management strategy is appropriate for their skin lesions, she said at the annual meeting of the Pacific Dermatologic Association.
"The reason is, at the end of the day, we might be killing the goose that laid the golden egg ourselves" if too many Mohs surgeries are done for inappropriate reasons, said Dr. Aasi of Stanford (Calif.) University.
SDr. Aasi urged the audience at the meeting to study criteria published in 2012 for the appropriate use of Mohs surgery, and to pay particular attention to scenarios deemed inappropriate for the procedure (J. Am. Acad. Dermatol. 2012;67:531-50).
There are an estimated 4 million nonmelanoma skin cancers in the United States each year, and the use of Mohs surgery increased by 400% from 1995 to 2009, Dr. Aasi said (Dermatol. Clin. 2012;30:167-75).
Some experts argue that the increase is a response to the increase in skin cancer cases, and others say the increasing number of Mohs surgeons is driving the rising rate of the procedure. "Regardless, it’s a problem," Dr. Aasi said.
Federal reimbursement programs don’t care if there is an epidemic of skin cancer, she said. If a surgery is being "overused," it is considered overvalued, and reimbursement rates are subject to change, she explained.
The top two CPT codes flagged by the federal government as potentially misvalued in the July 19, 2013, Federal Register were codes 17311 for Mohs surgery on the face, head, and neck and 17313 for Mohs surgery on the trunk and extremities, Dr. Aasi noted.
The 2012 appropriate-use criteria were created to help maintain the value of Mohs surgery in Medicare reimbursements, Dr. Aasi said. The American Academy of Dermatology, the American Society of Dermatologic Surgery, the American College of Mohs Surgery, and the American Society of Mohs Surgery convened a consensus panel of eight Mohs surgeons and nine non-Mohs surgeons. The panel considered 270 clinical scenarios and rated 74% appropriate for Mohs surgery, 17% inappropriate, and 9% uncertain, meaning there were conflicting data in the literature or not enough information to make a determination of the appropriateness of Mohs surgery in that scenario.
"I still think that Mohs surgeons in this panel were a little highly represented," Dr. Aasi said. Overall, the indications the panel deemed appropriate for Mohs surgery were "pretty generous," she added.
The consensus panel was asked to implicitly consider cost in deciding on the appropriateness of Mohs surgery for each scenario. "It’s an implicit consideration but a very important consideration," said Dr. Aasi.
Invasive melanoma was not included in the scenarios because if its complexity, but some scenarios did include melanoma in situ or lentigo maligna melanoma. The scenarios included different body areas, aggressive or nonaggressive skin cancers, tumor size, and the patient’s immune status. Agreement by at least 12 panel members was considered a consensus on the use of Mohs surgery.
Examples of scenarios that were considered inappropriate for Mohs surgery included nonaggressive basal cell or squamous cell carcinomas smaller than 2 cm on the trunk or extremities. Other lesions deemed inappropriate for Mohs included primary actinic keratosis (AK) with focal squamous cell cancer in situ, bowenoid AK, or squamous cell carcinoma in situ AK-type, labels that Dr. Aasi found "worrisome" because their definitions are unclear.
"As a Mohs surgeon, I found it a little surprising that they had to consider scenarios where actinic keratoses were being considered a possibility for Mohs surgery," she said.
Physicians can use the appropriate-use criteria to talk with other health care providers, third-party payers, and patients, said Dr. Aasi. Talking with patients can be most difficult, because if they’ve heard of Mohs surgery, they often want "the technique where I [the patient] know right away that I’m going to be clear," she said.
When Mohs surgery is inappropriate, "I try to convince them that, no, you don’t need it. It’s like an atomic bomb being used to kill a flea," she said.
The appropriate-use criteria do not compare different treatment modalities with Mohs surgery, Dr. Aasi emphasized, but they aim to state simply whether or not Mohs surgery would be appropriate in selected scenarios.
"I urge everyone to read" the criteria, she said.
Dr. Aasi reported having no financial disclosures.
On Twitter @sherryboschert
SAN FRANCISCO – Dermatologists and dermatologic surgeons risk a decreased reimbursement value for Mohs surgery if they don’t review the published criteria on its appropriate use, according to Dr. Sumaira Aasi.
Although it can be difficult, it is worth the effort to convince some patients that electrodessication, a curettage procedure, or some other management strategy is appropriate for their skin lesions, she said at the annual meeting of the Pacific Dermatologic Association.
"The reason is, at the end of the day, we might be killing the goose that laid the golden egg ourselves" if too many Mohs surgeries are done for inappropriate reasons, said Dr. Aasi of Stanford (Calif.) University.
SDr. Aasi urged the audience at the meeting to study criteria published in 2012 for the appropriate use of Mohs surgery, and to pay particular attention to scenarios deemed inappropriate for the procedure (J. Am. Acad. Dermatol. 2012;67:531-50).
There are an estimated 4 million nonmelanoma skin cancers in the United States each year, and the use of Mohs surgery increased by 400% from 1995 to 2009, Dr. Aasi said (Dermatol. Clin. 2012;30:167-75).
Some experts argue that the increase is a response to the increase in skin cancer cases, and others say the increasing number of Mohs surgeons is driving the rising rate of the procedure. "Regardless, it’s a problem," Dr. Aasi said.
Federal reimbursement programs don’t care if there is an epidemic of skin cancer, she said. If a surgery is being "overused," it is considered overvalued, and reimbursement rates are subject to change, she explained.
The top two CPT codes flagged by the federal government as potentially misvalued in the July 19, 2013, Federal Register were codes 17311 for Mohs surgery on the face, head, and neck and 17313 for Mohs surgery on the trunk and extremities, Dr. Aasi noted.
The 2012 appropriate-use criteria were created to help maintain the value of Mohs surgery in Medicare reimbursements, Dr. Aasi said. The American Academy of Dermatology, the American Society of Dermatologic Surgery, the American College of Mohs Surgery, and the American Society of Mohs Surgery convened a consensus panel of eight Mohs surgeons and nine non-Mohs surgeons. The panel considered 270 clinical scenarios and rated 74% appropriate for Mohs surgery, 17% inappropriate, and 9% uncertain, meaning there were conflicting data in the literature or not enough information to make a determination of the appropriateness of Mohs surgery in that scenario.
"I still think that Mohs surgeons in this panel were a little highly represented," Dr. Aasi said. Overall, the indications the panel deemed appropriate for Mohs surgery were "pretty generous," she added.
The consensus panel was asked to implicitly consider cost in deciding on the appropriateness of Mohs surgery for each scenario. "It’s an implicit consideration but a very important consideration," said Dr. Aasi.
Invasive melanoma was not included in the scenarios because if its complexity, but some scenarios did include melanoma in situ or lentigo maligna melanoma. The scenarios included different body areas, aggressive or nonaggressive skin cancers, tumor size, and the patient’s immune status. Agreement by at least 12 panel members was considered a consensus on the use of Mohs surgery.
Examples of scenarios that were considered inappropriate for Mohs surgery included nonaggressive basal cell or squamous cell carcinomas smaller than 2 cm on the trunk or extremities. Other lesions deemed inappropriate for Mohs included primary actinic keratosis (AK) with focal squamous cell cancer in situ, bowenoid AK, or squamous cell carcinoma in situ AK-type, labels that Dr. Aasi found "worrisome" because their definitions are unclear.
"As a Mohs surgeon, I found it a little surprising that they had to consider scenarios where actinic keratoses were being considered a possibility for Mohs surgery," she said.
Physicians can use the appropriate-use criteria to talk with other health care providers, third-party payers, and patients, said Dr. Aasi. Talking with patients can be most difficult, because if they’ve heard of Mohs surgery, they often want "the technique where I [the patient] know right away that I’m going to be clear," she said.
When Mohs surgery is inappropriate, "I try to convince them that, no, you don’t need it. It’s like an atomic bomb being used to kill a flea," she said.
The appropriate-use criteria do not compare different treatment modalities with Mohs surgery, Dr. Aasi emphasized, but they aim to state simply whether or not Mohs surgery would be appropriate in selected scenarios.
"I urge everyone to read" the criteria, she said.
Dr. Aasi reported having no financial disclosures.
On Twitter @sherryboschert
EXPERT ANALYSIS FROM THE PDA ANNUAL MEETING
Add education, vitamin D to eczema management
SAN FRANCISCO – Treatment for severe eczema should begin with education rather than first-line agents, according to Dr. Timothy G. Berger.
Data reported within the past year from a Japanese study convinced Dr. Berger to modify his therapeutic ladder for severe eczema, he said.
He now starts with greater patient and parental education before prescribing first-line treatments and checks vitamin D levels in patients with severe eczema and frequent infection. He chooses among systemic immunomodulators based on a recent practice update. And as a last resort, for the most difficult cases, he uses omalizumab or photophoresis, he said.
Dr. Berger described his treatment ladder approach at the annual meeting of the Pacific Dermatologic Association:
• First rung: education. He used to start up the therapeutic ladder by prescribing topical steroids, moisturization, and antihistamines. Data from the Japanese study persuaded him to insert more intensive education before sending patients and parents on their way.
The study randomized the mothers of 59 children with moderate to severe atopic dermatitis to attend a 2-day parental education program about managing the disease or to receive a booklet about the disease and conventional care. Six months later, patients in the education group had "dramatically better scores" on the Scoring Atopic Dermatitis (SCORAD) tool and on measures of itching, sleeplessness, and family impact, said Dr. Berger, professor of clinical dermatology at the University of California, San Francisco.
SCORAD scores improved in the education group from 40 at baseline to 15 after treatment, compared with a change from 42 to 27 in the control group (Pediatr. Dermatol. 2013;30:438-43). Parents in the education group also reported less anxiety about steroid use. The patients ranged in age from 6 months to 6 years.
"I think that education is really critical," Dr. Berger said.
• Second rung: first-line treatments. With this foundation of education, Dr. Berger starts patients on topical steroids, moisturizers, and antihistamines.
• Third rung: checking vitamin D levels. A comparison of 95 patients with atopic dermatitis and 58 control patients in another study found that low levels of vitamin D were no more likely in one group than in the other, but frequent skin infections were more likely in the subgroup of patients with atopic dermatitis and low vitamin D levels compared with patients who had the disease and normal vitamin D levels. Vitamin D supplementation in the patients with atopic dermatitis and low vitamin D levels significantly improved SCORAD scores (J. Am. Acad. Dermatol. 2013;69:238-44).
In that study, more than 80% of patients with atopic dermatitis and levels of 25-hydroxyvitamin D below 30 ng/mL developed skin infections compared with less than 20% of patients with atopic dermatitis whose vitamin D levels were above 30 ng/mL, Dr. Berger said. That 30-ng/mL cutoff is the dividing line between normal and abnormal or "insufficient" vitamin D levels, "which I’m not so sure how to interpret," he said. In general, levels below 20 ng/mL are considered vitamin D deficiency.
Now when Dr. Berger sees a patient with atopic dermatitis and frequent infections, he checks vitamin D and looks for levels that are deficient or even close to 10 ng/mL. These patients improve on vitamin D supplementation, he said. Vitamin D is required to deliver antimicrobial peptides to the skin surface, which is the presumed mechanism by which this helps.
He recently used this strategy in a patient with refractory lupus, frequent skin infections, and low vitamin D, he said. Both her lupus and skin infections improved with vitamin D supplementation.
• Fourth, fifth, and sixth rungs. Next he prescribes soak and smear, the conventional therapy of skin hydration and smearing on of topical corticosteroids. Beyond that, phototherapy and then possibly day treatment with ultraviolet light and application of coal tar remain options on his therapeutic ladder. "Day treatment with tar and light works very well for a patient that doesn’t have bullous pemphigoid," he said.
• Seventh rung: systemic immunomodulators. Guidance for choosing among these agents comes from a recent practice update from the American Academy of Allergy, Asthma, and Immunology; the American College of Allergy, Asthma, and Immunology; and the Joint Council of Allergy, Asthma, and Immunology (J. Allergy Clin. Immunol. 2013;131:295-9).
Cyclosporine works fastest, especially if started at higher doses, the recommendations say, but "my experience is that in older patients, it’s hard to use," Dr. Berger added. Oral tacrolimus also works, according to the Dr. Berger, as well as the guidelines.
Beyond that, methotrexate and azathioprine appear to be equally effective, with approximately 80% of patients responding in 3-6 weeks. Ordering an assay to detect deficiency of the thiopurine methyltransferase enzyme helps avoid toxicity from the drug in some patients. Because one study showed that increasing the dose of methotrexate does not help if the patient fails to respond to 15 mg/wk, Dr. Berger said he tries to get to 15 mg/wk for 4-6 weeks and holds that dose if there’s a response.
Another study showed that eczema cleared or almost cleared in around 60% of patients treated with mycophenolate mofetil. "I certainly have used it, but it’s unpredictable and it takes longer to start," he said. "You wait a couple of months to see if it’s going to work and then 20%-30% of the time it’s not going to be good enough."
• Final rung: newer agents. The same practice update recommends a list of newer agents "if you can afford it," Dr. Berger said. Approximately 60% of patients respond, slowly but steadily, to treatment with subcutaneous omalizumab 150-450 mg every 2 weeks. Some patients in studies managed to get off unsustainable levels of systemic steroids with the help of omalizumab. "There really isn’t a rebound" flare when omalizumab is stopped, as happens with cyclosporine, Dr. Berger said. "You kind of get better, then creep along. So if the quality of life is moderately bad, you get to less bad."
Tumor necrosis factor inhibitors work as induction therapy but not for maintenance, so they’re "probably not useful," he said. Anecdotal reports suggest that high-dose intravenous immunoglobulin may help.
For the most refractory patients, extracorporeal photophoresis can improve SCORAD scores by 50%. "The itch and SCORAD drop pretty quickly, but you have to maintain it" with omalizumab or another strategy, Dr. Berger said.
"If everything absolutely fails, that’s a backup," he said. "It does suggest that there will be new ways to manage patients that we don’t have available now."
Dr. Berger reported having no relevant financial disclosures.
On Twitter @sherryboschert
SAN FRANCISCO – Treatment for severe eczema should begin with education rather than first-line agents, according to Dr. Timothy G. Berger.
Data reported within the past year from a Japanese study convinced Dr. Berger to modify his therapeutic ladder for severe eczema, he said.
He now starts with greater patient and parental education before prescribing first-line treatments and checks vitamin D levels in patients with severe eczema and frequent infection. He chooses among systemic immunomodulators based on a recent practice update. And as a last resort, for the most difficult cases, he uses omalizumab or photophoresis, he said.
Dr. Berger described his treatment ladder approach at the annual meeting of the Pacific Dermatologic Association:
• First rung: education. He used to start up the therapeutic ladder by prescribing topical steroids, moisturization, and antihistamines. Data from the Japanese study persuaded him to insert more intensive education before sending patients and parents on their way.
The study randomized the mothers of 59 children with moderate to severe atopic dermatitis to attend a 2-day parental education program about managing the disease or to receive a booklet about the disease and conventional care. Six months later, patients in the education group had "dramatically better scores" on the Scoring Atopic Dermatitis (SCORAD) tool and on measures of itching, sleeplessness, and family impact, said Dr. Berger, professor of clinical dermatology at the University of California, San Francisco.
SCORAD scores improved in the education group from 40 at baseline to 15 after treatment, compared with a change from 42 to 27 in the control group (Pediatr. Dermatol. 2013;30:438-43). Parents in the education group also reported less anxiety about steroid use. The patients ranged in age from 6 months to 6 years.
"I think that education is really critical," Dr. Berger said.
• Second rung: first-line treatments. With this foundation of education, Dr. Berger starts patients on topical steroids, moisturizers, and antihistamines.
• Third rung: checking vitamin D levels. A comparison of 95 patients with atopic dermatitis and 58 control patients in another study found that low levels of vitamin D were no more likely in one group than in the other, but frequent skin infections were more likely in the subgroup of patients with atopic dermatitis and low vitamin D levels compared with patients who had the disease and normal vitamin D levels. Vitamin D supplementation in the patients with atopic dermatitis and low vitamin D levels significantly improved SCORAD scores (J. Am. Acad. Dermatol. 2013;69:238-44).
In that study, more than 80% of patients with atopic dermatitis and levels of 25-hydroxyvitamin D below 30 ng/mL developed skin infections compared with less than 20% of patients with atopic dermatitis whose vitamin D levels were above 30 ng/mL, Dr. Berger said. That 30-ng/mL cutoff is the dividing line between normal and abnormal or "insufficient" vitamin D levels, "which I’m not so sure how to interpret," he said. In general, levels below 20 ng/mL are considered vitamin D deficiency.
Now when Dr. Berger sees a patient with atopic dermatitis and frequent infections, he checks vitamin D and looks for levels that are deficient or even close to 10 ng/mL. These patients improve on vitamin D supplementation, he said. Vitamin D is required to deliver antimicrobial peptides to the skin surface, which is the presumed mechanism by which this helps.
He recently used this strategy in a patient with refractory lupus, frequent skin infections, and low vitamin D, he said. Both her lupus and skin infections improved with vitamin D supplementation.
• Fourth, fifth, and sixth rungs. Next he prescribes soak and smear, the conventional therapy of skin hydration and smearing on of topical corticosteroids. Beyond that, phototherapy and then possibly day treatment with ultraviolet light and application of coal tar remain options on his therapeutic ladder. "Day treatment with tar and light works very well for a patient that doesn’t have bullous pemphigoid," he said.
• Seventh rung: systemic immunomodulators. Guidance for choosing among these agents comes from a recent practice update from the American Academy of Allergy, Asthma, and Immunology; the American College of Allergy, Asthma, and Immunology; and the Joint Council of Allergy, Asthma, and Immunology (J. Allergy Clin. Immunol. 2013;131:295-9).
Cyclosporine works fastest, especially if started at higher doses, the recommendations say, but "my experience is that in older patients, it’s hard to use," Dr. Berger added. Oral tacrolimus also works, according to the Dr. Berger, as well as the guidelines.
Beyond that, methotrexate and azathioprine appear to be equally effective, with approximately 80% of patients responding in 3-6 weeks. Ordering an assay to detect deficiency of the thiopurine methyltransferase enzyme helps avoid toxicity from the drug in some patients. Because one study showed that increasing the dose of methotrexate does not help if the patient fails to respond to 15 mg/wk, Dr. Berger said he tries to get to 15 mg/wk for 4-6 weeks and holds that dose if there’s a response.
Another study showed that eczema cleared or almost cleared in around 60% of patients treated with mycophenolate mofetil. "I certainly have used it, but it’s unpredictable and it takes longer to start," he said. "You wait a couple of months to see if it’s going to work and then 20%-30% of the time it’s not going to be good enough."
• Final rung: newer agents. The same practice update recommends a list of newer agents "if you can afford it," Dr. Berger said. Approximately 60% of patients respond, slowly but steadily, to treatment with subcutaneous omalizumab 150-450 mg every 2 weeks. Some patients in studies managed to get off unsustainable levels of systemic steroids with the help of omalizumab. "There really isn’t a rebound" flare when omalizumab is stopped, as happens with cyclosporine, Dr. Berger said. "You kind of get better, then creep along. So if the quality of life is moderately bad, you get to less bad."
Tumor necrosis factor inhibitors work as induction therapy but not for maintenance, so they’re "probably not useful," he said. Anecdotal reports suggest that high-dose intravenous immunoglobulin may help.
For the most refractory patients, extracorporeal photophoresis can improve SCORAD scores by 50%. "The itch and SCORAD drop pretty quickly, but you have to maintain it" with omalizumab or another strategy, Dr. Berger said.
"If everything absolutely fails, that’s a backup," he said. "It does suggest that there will be new ways to manage patients that we don’t have available now."
Dr. Berger reported having no relevant financial disclosures.
On Twitter @sherryboschert
SAN FRANCISCO – Treatment for severe eczema should begin with education rather than first-line agents, according to Dr. Timothy G. Berger.
Data reported within the past year from a Japanese study convinced Dr. Berger to modify his therapeutic ladder for severe eczema, he said.
He now starts with greater patient and parental education before prescribing first-line treatments and checks vitamin D levels in patients with severe eczema and frequent infection. He chooses among systemic immunomodulators based on a recent practice update. And as a last resort, for the most difficult cases, he uses omalizumab or photophoresis, he said.
Dr. Berger described his treatment ladder approach at the annual meeting of the Pacific Dermatologic Association:
• First rung: education. He used to start up the therapeutic ladder by prescribing topical steroids, moisturization, and antihistamines. Data from the Japanese study persuaded him to insert more intensive education before sending patients and parents on their way.
The study randomized the mothers of 59 children with moderate to severe atopic dermatitis to attend a 2-day parental education program about managing the disease or to receive a booklet about the disease and conventional care. Six months later, patients in the education group had "dramatically better scores" on the Scoring Atopic Dermatitis (SCORAD) tool and on measures of itching, sleeplessness, and family impact, said Dr. Berger, professor of clinical dermatology at the University of California, San Francisco.
SCORAD scores improved in the education group from 40 at baseline to 15 after treatment, compared with a change from 42 to 27 in the control group (Pediatr. Dermatol. 2013;30:438-43). Parents in the education group also reported less anxiety about steroid use. The patients ranged in age from 6 months to 6 years.
"I think that education is really critical," Dr. Berger said.
• Second rung: first-line treatments. With this foundation of education, Dr. Berger starts patients on topical steroids, moisturizers, and antihistamines.
• Third rung: checking vitamin D levels. A comparison of 95 patients with atopic dermatitis and 58 control patients in another study found that low levels of vitamin D were no more likely in one group than in the other, but frequent skin infections were more likely in the subgroup of patients with atopic dermatitis and low vitamin D levels compared with patients who had the disease and normal vitamin D levels. Vitamin D supplementation in the patients with atopic dermatitis and low vitamin D levels significantly improved SCORAD scores (J. Am. Acad. Dermatol. 2013;69:238-44).
In that study, more than 80% of patients with atopic dermatitis and levels of 25-hydroxyvitamin D below 30 ng/mL developed skin infections compared with less than 20% of patients with atopic dermatitis whose vitamin D levels were above 30 ng/mL, Dr. Berger said. That 30-ng/mL cutoff is the dividing line between normal and abnormal or "insufficient" vitamin D levels, "which I’m not so sure how to interpret," he said. In general, levels below 20 ng/mL are considered vitamin D deficiency.
Now when Dr. Berger sees a patient with atopic dermatitis and frequent infections, he checks vitamin D and looks for levels that are deficient or even close to 10 ng/mL. These patients improve on vitamin D supplementation, he said. Vitamin D is required to deliver antimicrobial peptides to the skin surface, which is the presumed mechanism by which this helps.
He recently used this strategy in a patient with refractory lupus, frequent skin infections, and low vitamin D, he said. Both her lupus and skin infections improved with vitamin D supplementation.
• Fourth, fifth, and sixth rungs. Next he prescribes soak and smear, the conventional therapy of skin hydration and smearing on of topical corticosteroids. Beyond that, phototherapy and then possibly day treatment with ultraviolet light and application of coal tar remain options on his therapeutic ladder. "Day treatment with tar and light works very well for a patient that doesn’t have bullous pemphigoid," he said.
• Seventh rung: systemic immunomodulators. Guidance for choosing among these agents comes from a recent practice update from the American Academy of Allergy, Asthma, and Immunology; the American College of Allergy, Asthma, and Immunology; and the Joint Council of Allergy, Asthma, and Immunology (J. Allergy Clin. Immunol. 2013;131:295-9).
Cyclosporine works fastest, especially if started at higher doses, the recommendations say, but "my experience is that in older patients, it’s hard to use," Dr. Berger added. Oral tacrolimus also works, according to the Dr. Berger, as well as the guidelines.
Beyond that, methotrexate and azathioprine appear to be equally effective, with approximately 80% of patients responding in 3-6 weeks. Ordering an assay to detect deficiency of the thiopurine methyltransferase enzyme helps avoid toxicity from the drug in some patients. Because one study showed that increasing the dose of methotrexate does not help if the patient fails to respond to 15 mg/wk, Dr. Berger said he tries to get to 15 mg/wk for 4-6 weeks and holds that dose if there’s a response.
Another study showed that eczema cleared or almost cleared in around 60% of patients treated with mycophenolate mofetil. "I certainly have used it, but it’s unpredictable and it takes longer to start," he said. "You wait a couple of months to see if it’s going to work and then 20%-30% of the time it’s not going to be good enough."
• Final rung: newer agents. The same practice update recommends a list of newer agents "if you can afford it," Dr. Berger said. Approximately 60% of patients respond, slowly but steadily, to treatment with subcutaneous omalizumab 150-450 mg every 2 weeks. Some patients in studies managed to get off unsustainable levels of systemic steroids with the help of omalizumab. "There really isn’t a rebound" flare when omalizumab is stopped, as happens with cyclosporine, Dr. Berger said. "You kind of get better, then creep along. So if the quality of life is moderately bad, you get to less bad."
Tumor necrosis factor inhibitors work as induction therapy but not for maintenance, so they’re "probably not useful," he said. Anecdotal reports suggest that high-dose intravenous immunoglobulin may help.
For the most refractory patients, extracorporeal photophoresis can improve SCORAD scores by 50%. "The itch and SCORAD drop pretty quickly, but you have to maintain it" with omalizumab or another strategy, Dr. Berger said.
"If everything absolutely fails, that’s a backup," he said. "It does suggest that there will be new ways to manage patients that we don’t have available now."
Dr. Berger reported having no relevant financial disclosures.
On Twitter @sherryboschert
EXPERT ANALYSIS FROM THE PDA ANNUAL MEETING
Isotretinoin duration changes game for solid facial edema
SAN FRANCISCO – None of Dr. Timothy G. Berger’s patients with solid facial edema had responded to treatment with oral isotretinoin, so he was puzzled when New York dermatologists reported success in five out of five cases of patients with Morbihan disease, considered by most clinicians as a late-stage complication of rosacea. What was the secret to their success?
The answer: The successful patients took high doses for a prolonged treatment period – at least 6 months – before there was any sign of improvement.
"I was bailing out too soon. I didn’t treat long enough," said Dr. Berger of the University of California, San Francisco. "I’m now changing the way that I approach these patients," he said during a presentation at the annual meeting of the Pacific Dermatologic Association.
The average dose for the five patients in the report was 60 mg/day (ranging from 40 to 80 mg/day), with a mean treatment period of 16 months (and a range of 10 to 24 months). Patients received a mean cumulative dose of isotretinoin of 285 mg/kg (ranging from 170 to 491 mg/kg). The mean disease-free follow-up period was 9 months (ranging from 1 to 24 months), the researchers wrote (Arch. Derm. 2012;148:1395-8). However, the researchers noted that they saw no substantial clinical improvement in the patients until at least 6 months.
Both the daily and cumulative doses used in the study are high, Dr. Berger noted. "If you have patients with solid facial edema and you give them oral retinoids, you have to tell them ahead of time, ‘We have to get you up to this dose and we have to treat you for at least 6 months,’ " he said.
Solid facial edema "is a really difficult disease to treat," Dr. Berger said. Conventional therapy for solid facial edema includes systemic anti-inflammatory drugs, but the clinical response often is unsatisfactory, he noted. The findings were limited by the small number of patients, but the results represent an encouraging option for a challenging condition.
"This paper taught me a lot," Dr. Berger added.
Dr. Berger reported having no financial disclosures.
On Twitter @sherryboschert
SAN FRANCISCO – None of Dr. Timothy G. Berger’s patients with solid facial edema had responded to treatment with oral isotretinoin, so he was puzzled when New York dermatologists reported success in five out of five cases of patients with Morbihan disease, considered by most clinicians as a late-stage complication of rosacea. What was the secret to their success?
The answer: The successful patients took high doses for a prolonged treatment period – at least 6 months – before there was any sign of improvement.
"I was bailing out too soon. I didn’t treat long enough," said Dr. Berger of the University of California, San Francisco. "I’m now changing the way that I approach these patients," he said during a presentation at the annual meeting of the Pacific Dermatologic Association.
The average dose for the five patients in the report was 60 mg/day (ranging from 40 to 80 mg/day), with a mean treatment period of 16 months (and a range of 10 to 24 months). Patients received a mean cumulative dose of isotretinoin of 285 mg/kg (ranging from 170 to 491 mg/kg). The mean disease-free follow-up period was 9 months (ranging from 1 to 24 months), the researchers wrote (Arch. Derm. 2012;148:1395-8). However, the researchers noted that they saw no substantial clinical improvement in the patients until at least 6 months.
Both the daily and cumulative doses used in the study are high, Dr. Berger noted. "If you have patients with solid facial edema and you give them oral retinoids, you have to tell them ahead of time, ‘We have to get you up to this dose and we have to treat you for at least 6 months,’ " he said.
Solid facial edema "is a really difficult disease to treat," Dr. Berger said. Conventional therapy for solid facial edema includes systemic anti-inflammatory drugs, but the clinical response often is unsatisfactory, he noted. The findings were limited by the small number of patients, but the results represent an encouraging option for a challenging condition.
"This paper taught me a lot," Dr. Berger added.
Dr. Berger reported having no financial disclosures.
On Twitter @sherryboschert
SAN FRANCISCO – None of Dr. Timothy G. Berger’s patients with solid facial edema had responded to treatment with oral isotretinoin, so he was puzzled when New York dermatologists reported success in five out of five cases of patients with Morbihan disease, considered by most clinicians as a late-stage complication of rosacea. What was the secret to their success?
The answer: The successful patients took high doses for a prolonged treatment period – at least 6 months – before there was any sign of improvement.
"I was bailing out too soon. I didn’t treat long enough," said Dr. Berger of the University of California, San Francisco. "I’m now changing the way that I approach these patients," he said during a presentation at the annual meeting of the Pacific Dermatologic Association.
The average dose for the five patients in the report was 60 mg/day (ranging from 40 to 80 mg/day), with a mean treatment period of 16 months (and a range of 10 to 24 months). Patients received a mean cumulative dose of isotretinoin of 285 mg/kg (ranging from 170 to 491 mg/kg). The mean disease-free follow-up period was 9 months (ranging from 1 to 24 months), the researchers wrote (Arch. Derm. 2012;148:1395-8). However, the researchers noted that they saw no substantial clinical improvement in the patients until at least 6 months.
Both the daily and cumulative doses used in the study are high, Dr. Berger noted. "If you have patients with solid facial edema and you give them oral retinoids, you have to tell them ahead of time, ‘We have to get you up to this dose and we have to treat you for at least 6 months,’ " he said.
Solid facial edema "is a really difficult disease to treat," Dr. Berger said. Conventional therapy for solid facial edema includes systemic anti-inflammatory drugs, but the clinical response often is unsatisfactory, he noted. The findings were limited by the small number of patients, but the results represent an encouraging option for a challenging condition.
"This paper taught me a lot," Dr. Berger added.
Dr. Berger reported having no financial disclosures.
On Twitter @sherryboschert
EXPERT ANALYSIS AT THE ANNUAL MEETING OF THE PACIFIC DERMATOLOGIC ASSOCIATION
‘Eczema coxsackium’ cutaneous eruptions characterized
SAN FRANCISCO – A study of cases at seven academic pediatric dermatology centers has more precisely described the cutaneous eruptions caused by a severe form of hand, foot, and mouth disease in U.S. children.
Dermatologists don’t see many cases of hand, foot, and mouth disease because these usually are routine viral infections managed by pediatricians. In the spring of 2012, however, Dr. Erin Mathes and her associates started seeing children with the fevers and GI symptoms typical of hand, foot, and mouth disease, but with a very severe rash with severe blisters, vesicles, and erosions instead of the milder papulovesicles of the hands, feet, buttocks, and mouth seen with the classic disease.
"We were left scratching our heads wondering exactly what was going on" until the Centers for Disease Control and Prevention reported an outbreak of severe hand, foot, and mouth disease associated with coxsackievirus A6 (CVA6) that also had been reported in several other countries (MMWR 2012;61:213-4).
"These reports said that the rash was severe, but they didn’t characterize it very carefully," so she and her colleagues across the country set out to describe the cutaneous manifestations in U.S. patients more specifically, said Dr. Mathes of the University of California, San Francisco.
The 80 cases they reviewed were treated between July 2011 and June 2012 at seven pediatric academic centers. Seventeen were caused by CVA6 infection that was confirmed by polymerase chain reaction (PCR) testing and 63 cases met predefined clinical criteria for inclusion as likely CVA6 infection, she reported at the annual meeting of the Pacific Dermatologic Association. Patients ranged in age from 4 months to 16 years.
Nearly all of the patients had vesiculobullous and erosive eruptions (99% of patients). "Some of the smaller vesicles were more widespread than in classic hand, foot, and mouth disease. We saw a striking perioral distribution," she said. "And then some of our younger patients had quite large blisters," such as a 4-cm blister on the foot of a 4-month-old baby (Pediatrics 2013;132:e149-57 [doi: 10.1542/peds.2012-3175]).
The most interesting finding was an eczema herpeticum–like rash in 55% of patients. From a distance, all of the patients looked like they had eczema herpeticum – erosions and vesicles in areas that previously were affected by atopic dermatitis but closer inspection showed that they weren’t as monomorphous or as "punched out" as erosions from eczema herpeticum. "So, there are some morphologic clues that this is not eczema herpeticum," she said. Most patients tested negative for herpes simplex virus (HSV).
CVA6 now can be added to the short list of HSV-1, varicella, and coxsackievirus A16 as viruses that have been reported to cause a Kaposi’s varicelliform eruption, in which preexisting dermatitis becomes superinfected with virus. Dr. Mathes proposed that the CVA6 eruption be called "eczema coxsackium." "We like this [term] because it brings to mind eczema herpeticum and yet indicates that the virus is coxsackie rather than a herpes virus," she said.
Nineteen percent of patients had locus minoris infection without previous atopic dermatitis and developed blisters and erosions in areas of sunburn, laceration, or irritant dermatitis.
Gianotti-Crosti–like eruptions with papulovesicles on only the cheeks, arms, and legs were seen in 36% of patients.
One of the rarer morphologies that "was confusing to both the pediatricians and the dermatologists" was a petechial and purpuric eruption in 17% of patients, more commonly in older children and in acral areas, she said. Biopsies from several patients showed no evidence of vasculitis, and several patients had confirmed CVA6 infection. "It was more consistent with a viral eruption," she said.
Among nail changes in 25% of patients, Beau’s lines and onychomadesis were common. These probably would have been seen more if patients were followed longitudinally, but the study captured data from just one visit, she noted.
Only half of patients had oropharyngeal ulcerations (50%), a lower percentage than with classic hand, foot, and mouth disease. Dr. Mathes’ impression was that these ulcers tended to occur more commonly in anterior locations, compared with classic disease.
Ten patients were hospitalized for an average of 12 days, mainly for diagnostic work-ups and empiric antibiotic treatment. All recovered with supportive care. There were no cases of severe systemic illness, in contrast with published case series from Asia in which several patients developed aseptic meningitis and other neurologic complications, she said.
Dr. Mathes and her colleagues were wondering if they’d see eczema coxsackium in 2013 until she saw her first case of the year recently. "We think we’ll probably see it in lower numbers given that there is some herd immunity now, and some children will have immunity to it," she said.
The diagnosis is a clinical one. Look for features of hand, foot, and mouth disease with severe rash, and do bacterial and viral cultures to rule out other infections. "We did have some patients who had a superinfection with Staph in addition to their viral infection," she said. Coxsackievirus will not grow in culture; if you want to confirm the diagnosis, use PCR. If you see a severe case in a patient hospitalized with neurologic or cardiac complications, send specimens for diagnostic confirmation to your local health department or the CDC, she advised.
The international reports of severe hand, foot, and mouth disease from CVA6 infection began in 2012 in Finland, and then came from Singapore and Japan. The international reports noted a severe rash, onychomadesis and desquamation, rare neurologic complications, and a high prevalence of infection in school-age children or adults, probably because of low herd immunity. The international investigators were able to obtain the virus for analysis from nail clippings from the patients who had onychomadesis, Dr. Mathes noted.
She reported having no financial disclosures.
On Twitter @sherryboschert
SAN FRANCISCO – A study of cases at seven academic pediatric dermatology centers has more precisely described the cutaneous eruptions caused by a severe form of hand, foot, and mouth disease in U.S. children.
Dermatologists don’t see many cases of hand, foot, and mouth disease because these usually are routine viral infections managed by pediatricians. In the spring of 2012, however, Dr. Erin Mathes and her associates started seeing children with the fevers and GI symptoms typical of hand, foot, and mouth disease, but with a very severe rash with severe blisters, vesicles, and erosions instead of the milder papulovesicles of the hands, feet, buttocks, and mouth seen with the classic disease.
"We were left scratching our heads wondering exactly what was going on" until the Centers for Disease Control and Prevention reported an outbreak of severe hand, foot, and mouth disease associated with coxsackievirus A6 (CVA6) that also had been reported in several other countries (MMWR 2012;61:213-4).
"These reports said that the rash was severe, but they didn’t characterize it very carefully," so she and her colleagues across the country set out to describe the cutaneous manifestations in U.S. patients more specifically, said Dr. Mathes of the University of California, San Francisco.
The 80 cases they reviewed were treated between July 2011 and June 2012 at seven pediatric academic centers. Seventeen were caused by CVA6 infection that was confirmed by polymerase chain reaction (PCR) testing and 63 cases met predefined clinical criteria for inclusion as likely CVA6 infection, she reported at the annual meeting of the Pacific Dermatologic Association. Patients ranged in age from 4 months to 16 years.
Nearly all of the patients had vesiculobullous and erosive eruptions (99% of patients). "Some of the smaller vesicles were more widespread than in classic hand, foot, and mouth disease. We saw a striking perioral distribution," she said. "And then some of our younger patients had quite large blisters," such as a 4-cm blister on the foot of a 4-month-old baby (Pediatrics 2013;132:e149-57 [doi: 10.1542/peds.2012-3175]).
The most interesting finding was an eczema herpeticum–like rash in 55% of patients. From a distance, all of the patients looked like they had eczema herpeticum – erosions and vesicles in areas that previously were affected by atopic dermatitis but closer inspection showed that they weren’t as monomorphous or as "punched out" as erosions from eczema herpeticum. "So, there are some morphologic clues that this is not eczema herpeticum," she said. Most patients tested negative for herpes simplex virus (HSV).
CVA6 now can be added to the short list of HSV-1, varicella, and coxsackievirus A16 as viruses that have been reported to cause a Kaposi’s varicelliform eruption, in which preexisting dermatitis becomes superinfected with virus. Dr. Mathes proposed that the CVA6 eruption be called "eczema coxsackium." "We like this [term] because it brings to mind eczema herpeticum and yet indicates that the virus is coxsackie rather than a herpes virus," she said.
Nineteen percent of patients had locus minoris infection without previous atopic dermatitis and developed blisters and erosions in areas of sunburn, laceration, or irritant dermatitis.
Gianotti-Crosti–like eruptions with papulovesicles on only the cheeks, arms, and legs were seen in 36% of patients.
One of the rarer morphologies that "was confusing to both the pediatricians and the dermatologists" was a petechial and purpuric eruption in 17% of patients, more commonly in older children and in acral areas, she said. Biopsies from several patients showed no evidence of vasculitis, and several patients had confirmed CVA6 infection. "It was more consistent with a viral eruption," she said.
Among nail changes in 25% of patients, Beau’s lines and onychomadesis were common. These probably would have been seen more if patients were followed longitudinally, but the study captured data from just one visit, she noted.
Only half of patients had oropharyngeal ulcerations (50%), a lower percentage than with classic hand, foot, and mouth disease. Dr. Mathes’ impression was that these ulcers tended to occur more commonly in anterior locations, compared with classic disease.
Ten patients were hospitalized for an average of 12 days, mainly for diagnostic work-ups and empiric antibiotic treatment. All recovered with supportive care. There were no cases of severe systemic illness, in contrast with published case series from Asia in which several patients developed aseptic meningitis and other neurologic complications, she said.
Dr. Mathes and her colleagues were wondering if they’d see eczema coxsackium in 2013 until she saw her first case of the year recently. "We think we’ll probably see it in lower numbers given that there is some herd immunity now, and some children will have immunity to it," she said.
The diagnosis is a clinical one. Look for features of hand, foot, and mouth disease with severe rash, and do bacterial and viral cultures to rule out other infections. "We did have some patients who had a superinfection with Staph in addition to their viral infection," she said. Coxsackievirus will not grow in culture; if you want to confirm the diagnosis, use PCR. If you see a severe case in a patient hospitalized with neurologic or cardiac complications, send specimens for diagnostic confirmation to your local health department or the CDC, she advised.
The international reports of severe hand, foot, and mouth disease from CVA6 infection began in 2012 in Finland, and then came from Singapore and Japan. The international reports noted a severe rash, onychomadesis and desquamation, rare neurologic complications, and a high prevalence of infection in school-age children or adults, probably because of low herd immunity. The international investigators were able to obtain the virus for analysis from nail clippings from the patients who had onychomadesis, Dr. Mathes noted.
She reported having no financial disclosures.
On Twitter @sherryboschert
SAN FRANCISCO – A study of cases at seven academic pediatric dermatology centers has more precisely described the cutaneous eruptions caused by a severe form of hand, foot, and mouth disease in U.S. children.
Dermatologists don’t see many cases of hand, foot, and mouth disease because these usually are routine viral infections managed by pediatricians. In the spring of 2012, however, Dr. Erin Mathes and her associates started seeing children with the fevers and GI symptoms typical of hand, foot, and mouth disease, but with a very severe rash with severe blisters, vesicles, and erosions instead of the milder papulovesicles of the hands, feet, buttocks, and mouth seen with the classic disease.
"We were left scratching our heads wondering exactly what was going on" until the Centers for Disease Control and Prevention reported an outbreak of severe hand, foot, and mouth disease associated with coxsackievirus A6 (CVA6) that also had been reported in several other countries (MMWR 2012;61:213-4).
"These reports said that the rash was severe, but they didn’t characterize it very carefully," so she and her colleagues across the country set out to describe the cutaneous manifestations in U.S. patients more specifically, said Dr. Mathes of the University of California, San Francisco.
The 80 cases they reviewed were treated between July 2011 and June 2012 at seven pediatric academic centers. Seventeen were caused by CVA6 infection that was confirmed by polymerase chain reaction (PCR) testing and 63 cases met predefined clinical criteria for inclusion as likely CVA6 infection, she reported at the annual meeting of the Pacific Dermatologic Association. Patients ranged in age from 4 months to 16 years.
Nearly all of the patients had vesiculobullous and erosive eruptions (99% of patients). "Some of the smaller vesicles were more widespread than in classic hand, foot, and mouth disease. We saw a striking perioral distribution," she said. "And then some of our younger patients had quite large blisters," such as a 4-cm blister on the foot of a 4-month-old baby (Pediatrics 2013;132:e149-57 [doi: 10.1542/peds.2012-3175]).
The most interesting finding was an eczema herpeticum–like rash in 55% of patients. From a distance, all of the patients looked like they had eczema herpeticum – erosions and vesicles in areas that previously were affected by atopic dermatitis but closer inspection showed that they weren’t as monomorphous or as "punched out" as erosions from eczema herpeticum. "So, there are some morphologic clues that this is not eczema herpeticum," she said. Most patients tested negative for herpes simplex virus (HSV).
CVA6 now can be added to the short list of HSV-1, varicella, and coxsackievirus A16 as viruses that have been reported to cause a Kaposi’s varicelliform eruption, in which preexisting dermatitis becomes superinfected with virus. Dr. Mathes proposed that the CVA6 eruption be called "eczema coxsackium." "We like this [term] because it brings to mind eczema herpeticum and yet indicates that the virus is coxsackie rather than a herpes virus," she said.
Nineteen percent of patients had locus minoris infection without previous atopic dermatitis and developed blisters and erosions in areas of sunburn, laceration, or irritant dermatitis.
Gianotti-Crosti–like eruptions with papulovesicles on only the cheeks, arms, and legs were seen in 36% of patients.
One of the rarer morphologies that "was confusing to both the pediatricians and the dermatologists" was a petechial and purpuric eruption in 17% of patients, more commonly in older children and in acral areas, she said. Biopsies from several patients showed no evidence of vasculitis, and several patients had confirmed CVA6 infection. "It was more consistent with a viral eruption," she said.
Among nail changes in 25% of patients, Beau’s lines and onychomadesis were common. These probably would have been seen more if patients were followed longitudinally, but the study captured data from just one visit, she noted.
Only half of patients had oropharyngeal ulcerations (50%), a lower percentage than with classic hand, foot, and mouth disease. Dr. Mathes’ impression was that these ulcers tended to occur more commonly in anterior locations, compared with classic disease.
Ten patients were hospitalized for an average of 12 days, mainly for diagnostic work-ups and empiric antibiotic treatment. All recovered with supportive care. There were no cases of severe systemic illness, in contrast with published case series from Asia in which several patients developed aseptic meningitis and other neurologic complications, she said.
Dr. Mathes and her colleagues were wondering if they’d see eczema coxsackium in 2013 until she saw her first case of the year recently. "We think we’ll probably see it in lower numbers given that there is some herd immunity now, and some children will have immunity to it," she said.
The diagnosis is a clinical one. Look for features of hand, foot, and mouth disease with severe rash, and do bacterial and viral cultures to rule out other infections. "We did have some patients who had a superinfection with Staph in addition to their viral infection," she said. Coxsackievirus will not grow in culture; if you want to confirm the diagnosis, use PCR. If you see a severe case in a patient hospitalized with neurologic or cardiac complications, send specimens for diagnostic confirmation to your local health department or the CDC, she advised.
The international reports of severe hand, foot, and mouth disease from CVA6 infection began in 2012 in Finland, and then came from Singapore and Japan. The international reports noted a severe rash, onychomadesis and desquamation, rare neurologic complications, and a high prevalence of infection in school-age children or adults, probably because of low herd immunity. The international investigators were able to obtain the virus for analysis from nail clippings from the patients who had onychomadesis, Dr. Mathes noted.
She reported having no financial disclosures.
On Twitter @sherryboschert
AT THE ANNUAL MEETING OF THE PACIFIC DERMATOLOGIC ASSOCIATION
Major finding: The most common cutaneous findings were vesiculobullous and erosive eruptions in 99% of patients, eczema herpeticum-like rash in 55%, Gianotti-Crosti–like eruptions in 36%, nail changes in 25%, and oropharyngeal ulcerations in 50%.
Data source: Retrospective, multicenter review of 80 pediatric patients with atypical cases of hand, foot, and mouth disease caused by a confirmed CVA6 infection or that met predefined criteria for infection.
Disclosures: Dr. Mathes reported having no financial disclosures.
When wounds won’t heal, try these strategies
SAN FRANCISCO – When your first or second attempts to help a skin wound heal haven’t worked, ask yourself three questions, Dr. Theodora Mauro suggested:
• Have you diagnosed the wound correctly?
• What systemic conditions are keeping the wound from healing?
• Have you tailored your treatment to the wound correctly?
Most dermatologists are pretty good at diagnosing ulcers that are caused by single etiologies. What Dr. Mauro sees more commonly in her busy referral practice, however, are wounds that won’t heal because of a combination of causes – often venous insufficiency plus something else, she said at the annual meeting of the Pacific Dermatologic Association.
"That can make the diagnosis a little more confusing," said Dr. Mauro, professor of dermatology at the University of California, San Francisco and chief of the dermatology service at the San Francisco Veterans Affairs Medical Center.
Ulcer types
The most common skin ulcer in the United States is due to venous insufficiency. These patients tend to be spread among dermatologists, vascular surgeons, and podiatrists and "are not well served by being scattered among different disciplines," she said.
Arterial ulcers make up 6%-10% of skin ulcers in the medical literature, and the incidence of diabetic ulcers is increasing, she added. Pressure ulcers are becoming more common as the population ages.
Morphology and the location of ulcers usually can distinguish the different types, but don’t forget to check the patient’s pulses and sensations, Dr. Mauro said. If you can feel pedal pulses, the patient is very likely to have a normal ankle brachial index (greater than 0.8). "It’s an easy thing to do and very helpful," she said. Also, break a Q-tip cotton swab in half and poke the patient with the sharp end. "You’d be surprised at the number of people who have altered sensation" that’s contributing to the ulceration.
A common "combination" that gets missed is a nonmelanoma skin cancer and an ulcer at that site from venous insufficiency. Consider taking biopsies of nonhealing ulcers to look for skin cancer, she said. Long-standing ulcers can develop squamous cell carcinoma within them. A basal cell carcinoma can look like a healing ulcer, but not heal.
Bullous diseases also can be hidden in ulcers, particularly in the elderly. "There seems to be a two-hit thing with venous insufficiency where you have a little bit of blistering disease and you have a lot of hydrostatic pressure, and people will get their bullae on their legs long before they get it anyplace else," Dr. Mauro said. "You put compression on and they come back next week and now they have intact bullae. Think about that as another cause."
Less common ulcerative problems that can be confusing include pyoderma gangrenosum (which may be half as common as people think, studies suggest), an underlying vasculitis, or mycobacterial infections. If you see a nonhealing hyperkeratotic lesion, consider an atypical mycobacterial infection. In Dr. Mauro’s region, these usually are in patients who went mountain biking and inoculated themselves through an ankle scratch or in patients exposed to the organism through infected water during a pedicure.
Systemic conditions
Wound healing can be inhibited by things that doctors do, and by things that patients do.
Taking antimetabolite medications (such as hydroxyurea or methotrexate), prednisone, or nonsteroidal anti-inflammatory drugs can impair wound healing, as does smoking. Radiation to the site also inhibits healing. Think twice before irradiating a basal cell carcinoma on the leg of a patient with venous insufficiency. "It’s much better to either try some topical medication or just bite the bullet, do surgery, and put the Unna boot on after the surgery, because if you irradiate [the basal cell carcinoma] and it ulcerates, then it’s really hard to heal," she said.
Coexisting cancer or arterial disease can inhibit wound healing. If you check pulses and can’t feel them, send the patient for an ankle brachial index to determine if there’s arterial disease, in which case the patient should see a vascular surgeon, she said.
When you’re taking patients’ histories, ask what they’re eating so you can assess whether they’re getting enough protein, zinc, and vitamin C. "You’d be surprised at the number of nutritional deficiencies that we see" in patients with nonhealing wounds. Her clinic picks up a zinc deficiency three to four times per year and a protein deficiency approximately twice a year. "All of these are things that you need to make collagen and granulation tissue," she explained. Supplementation with Ensure can provide these nutrients, but a less-expensive option is Carnation Breakfast Essentials, at about one-fifth the cost, she added.
Tailor therapy appropriately
Dressings can make wounds worse. Most commonly, putting a hydrocolloid dressing (such as DuoDERM) on an ulcer that has a lot of drainage will macerate and enlarge the wound. Putting stiff foams or self-adherent wraps such as Coban on wounds with a lot of edema can create secondary ulcers. And avoid compression on the legs of people with arterial insufficiency, Dr. Mauro said.
Artifactual ulcers from patients scratching or picking at wounds are increasing, she said. These patients usually will not report that they’re messing with the wound, but your level of suspicion should increase if the ulcer is above the knee. Hydrocolloid dressings work pretty well for this problem. Consider sending some of these patients to an expert in the growing field of medication therapy for dysesthesia.
Ask patients about home remedies, because they will put all kinds of things on wounds to try and help them heal. Neomycin can cause contact dermatitis. Patients may apply full-strength hydrogen peroxide, thinking that they need a disinfectant, which tends to damage the epithelium and can impair wound healing. If they insist on using hydrogen peroxide, recommend a half-strength solution.
For patients with venous insufficiency, the hydrostatic pressure from sitting can be nearly as bad as from standing. Ask patients with nonhealing wounds what they do at home and at work. If they’re sitting much of that time, recommend products to alleviate the pressure. Dr. Mauro said she has virtually become a saleswoman for La-Z-Boy furniture during her medical career because La-Z-Boy products allow patients to eat, work at computers, and function in positions with less hydrostatic pressure.
When to refer
Consider referring patients with chronic wounds that have not lost at least 50% of their width and height within 6 weeks. When a wound has been present long enough, the physiology shifts from acute to chronic. "It’s as if the body decides that it’s tired of trying to heal it and its new equilibrium is just going to be, ‘I have a wound,’ " she said.
If you want to try treating chronic wounds, applying Promogran Prisma matrix wound dressing in those without good granulation tissue may stimulate granulation tissue, she said.
Debriding an ulcer is "kind of a poor man’s way of getting platelet-derived growth factor," she added. Dr. Mauro applies a compounded 30% lidocaine ("but you can use any topical lidocaine"), leaves it on for 20-25 minutes, and debrides using a curette, which is much more precise than a scalpel. "What you’re trying to do is ‘reboot the computer’ and trick the ulcer into thinking it’s an acute ulcer rather than a chronic one," she said.
Dr. Mauro said she refers patients with arterial insufficiency to vascular surgeons. She sends patients with diabetes to podiatrists, preferably before they develop ulcers. The podiatrist can regularly trim nails for patients with poor sight and loss of sensation who may cut themselves, and can design footwear to avoid pressure that could cause an ulcer. "That will keep you out of trouble" with diabetic patients, she said.
Dr. Mauro has been a consultant for Unilever, but not in a wound-healing capacity.
On Twitter @sherryboschert
SAN FRANCISCO – When your first or second attempts to help a skin wound heal haven’t worked, ask yourself three questions, Dr. Theodora Mauro suggested:
• Have you diagnosed the wound correctly?
• What systemic conditions are keeping the wound from healing?
• Have you tailored your treatment to the wound correctly?
Most dermatologists are pretty good at diagnosing ulcers that are caused by single etiologies. What Dr. Mauro sees more commonly in her busy referral practice, however, are wounds that won’t heal because of a combination of causes – often venous insufficiency plus something else, she said at the annual meeting of the Pacific Dermatologic Association.
"That can make the diagnosis a little more confusing," said Dr. Mauro, professor of dermatology at the University of California, San Francisco and chief of the dermatology service at the San Francisco Veterans Affairs Medical Center.
Ulcer types
The most common skin ulcer in the United States is due to venous insufficiency. These patients tend to be spread among dermatologists, vascular surgeons, and podiatrists and "are not well served by being scattered among different disciplines," she said.
Arterial ulcers make up 6%-10% of skin ulcers in the medical literature, and the incidence of diabetic ulcers is increasing, she added. Pressure ulcers are becoming more common as the population ages.
Morphology and the location of ulcers usually can distinguish the different types, but don’t forget to check the patient’s pulses and sensations, Dr. Mauro said. If you can feel pedal pulses, the patient is very likely to have a normal ankle brachial index (greater than 0.8). "It’s an easy thing to do and very helpful," she said. Also, break a Q-tip cotton swab in half and poke the patient with the sharp end. "You’d be surprised at the number of people who have altered sensation" that’s contributing to the ulceration.
A common "combination" that gets missed is a nonmelanoma skin cancer and an ulcer at that site from venous insufficiency. Consider taking biopsies of nonhealing ulcers to look for skin cancer, she said. Long-standing ulcers can develop squamous cell carcinoma within them. A basal cell carcinoma can look like a healing ulcer, but not heal.
Bullous diseases also can be hidden in ulcers, particularly in the elderly. "There seems to be a two-hit thing with venous insufficiency where you have a little bit of blistering disease and you have a lot of hydrostatic pressure, and people will get their bullae on their legs long before they get it anyplace else," Dr. Mauro said. "You put compression on and they come back next week and now they have intact bullae. Think about that as another cause."
Less common ulcerative problems that can be confusing include pyoderma gangrenosum (which may be half as common as people think, studies suggest), an underlying vasculitis, or mycobacterial infections. If you see a nonhealing hyperkeratotic lesion, consider an atypical mycobacterial infection. In Dr. Mauro’s region, these usually are in patients who went mountain biking and inoculated themselves through an ankle scratch or in patients exposed to the organism through infected water during a pedicure.
Systemic conditions
Wound healing can be inhibited by things that doctors do, and by things that patients do.
Taking antimetabolite medications (such as hydroxyurea or methotrexate), prednisone, or nonsteroidal anti-inflammatory drugs can impair wound healing, as does smoking. Radiation to the site also inhibits healing. Think twice before irradiating a basal cell carcinoma on the leg of a patient with venous insufficiency. "It’s much better to either try some topical medication or just bite the bullet, do surgery, and put the Unna boot on after the surgery, because if you irradiate [the basal cell carcinoma] and it ulcerates, then it’s really hard to heal," she said.
Coexisting cancer or arterial disease can inhibit wound healing. If you check pulses and can’t feel them, send the patient for an ankle brachial index to determine if there’s arterial disease, in which case the patient should see a vascular surgeon, she said.
When you’re taking patients’ histories, ask what they’re eating so you can assess whether they’re getting enough protein, zinc, and vitamin C. "You’d be surprised at the number of nutritional deficiencies that we see" in patients with nonhealing wounds. Her clinic picks up a zinc deficiency three to four times per year and a protein deficiency approximately twice a year. "All of these are things that you need to make collagen and granulation tissue," she explained. Supplementation with Ensure can provide these nutrients, but a less-expensive option is Carnation Breakfast Essentials, at about one-fifth the cost, she added.
Tailor therapy appropriately
Dressings can make wounds worse. Most commonly, putting a hydrocolloid dressing (such as DuoDERM) on an ulcer that has a lot of drainage will macerate and enlarge the wound. Putting stiff foams or self-adherent wraps such as Coban on wounds with a lot of edema can create secondary ulcers. And avoid compression on the legs of people with arterial insufficiency, Dr. Mauro said.
Artifactual ulcers from patients scratching or picking at wounds are increasing, she said. These patients usually will not report that they’re messing with the wound, but your level of suspicion should increase if the ulcer is above the knee. Hydrocolloid dressings work pretty well for this problem. Consider sending some of these patients to an expert in the growing field of medication therapy for dysesthesia.
Ask patients about home remedies, because they will put all kinds of things on wounds to try and help them heal. Neomycin can cause contact dermatitis. Patients may apply full-strength hydrogen peroxide, thinking that they need a disinfectant, which tends to damage the epithelium and can impair wound healing. If they insist on using hydrogen peroxide, recommend a half-strength solution.
For patients with venous insufficiency, the hydrostatic pressure from sitting can be nearly as bad as from standing. Ask patients with nonhealing wounds what they do at home and at work. If they’re sitting much of that time, recommend products to alleviate the pressure. Dr. Mauro said she has virtually become a saleswoman for La-Z-Boy furniture during her medical career because La-Z-Boy products allow patients to eat, work at computers, and function in positions with less hydrostatic pressure.
When to refer
Consider referring patients with chronic wounds that have not lost at least 50% of their width and height within 6 weeks. When a wound has been present long enough, the physiology shifts from acute to chronic. "It’s as if the body decides that it’s tired of trying to heal it and its new equilibrium is just going to be, ‘I have a wound,’ " she said.
If you want to try treating chronic wounds, applying Promogran Prisma matrix wound dressing in those without good granulation tissue may stimulate granulation tissue, she said.
Debriding an ulcer is "kind of a poor man’s way of getting platelet-derived growth factor," she added. Dr. Mauro applies a compounded 30% lidocaine ("but you can use any topical lidocaine"), leaves it on for 20-25 minutes, and debrides using a curette, which is much more precise than a scalpel. "What you’re trying to do is ‘reboot the computer’ and trick the ulcer into thinking it’s an acute ulcer rather than a chronic one," she said.
Dr. Mauro said she refers patients with arterial insufficiency to vascular surgeons. She sends patients with diabetes to podiatrists, preferably before they develop ulcers. The podiatrist can regularly trim nails for patients with poor sight and loss of sensation who may cut themselves, and can design footwear to avoid pressure that could cause an ulcer. "That will keep you out of trouble" with diabetic patients, she said.
Dr. Mauro has been a consultant for Unilever, but not in a wound-healing capacity.
On Twitter @sherryboschert
SAN FRANCISCO – When your first or second attempts to help a skin wound heal haven’t worked, ask yourself three questions, Dr. Theodora Mauro suggested:
• Have you diagnosed the wound correctly?
• What systemic conditions are keeping the wound from healing?
• Have you tailored your treatment to the wound correctly?
Most dermatologists are pretty good at diagnosing ulcers that are caused by single etiologies. What Dr. Mauro sees more commonly in her busy referral practice, however, are wounds that won’t heal because of a combination of causes – often venous insufficiency plus something else, she said at the annual meeting of the Pacific Dermatologic Association.
"That can make the diagnosis a little more confusing," said Dr. Mauro, professor of dermatology at the University of California, San Francisco and chief of the dermatology service at the San Francisco Veterans Affairs Medical Center.
Ulcer types
The most common skin ulcer in the United States is due to venous insufficiency. These patients tend to be spread among dermatologists, vascular surgeons, and podiatrists and "are not well served by being scattered among different disciplines," she said.
Arterial ulcers make up 6%-10% of skin ulcers in the medical literature, and the incidence of diabetic ulcers is increasing, she added. Pressure ulcers are becoming more common as the population ages.
Morphology and the location of ulcers usually can distinguish the different types, but don’t forget to check the patient’s pulses and sensations, Dr. Mauro said. If you can feel pedal pulses, the patient is very likely to have a normal ankle brachial index (greater than 0.8). "It’s an easy thing to do and very helpful," she said. Also, break a Q-tip cotton swab in half and poke the patient with the sharp end. "You’d be surprised at the number of people who have altered sensation" that’s contributing to the ulceration.
A common "combination" that gets missed is a nonmelanoma skin cancer and an ulcer at that site from venous insufficiency. Consider taking biopsies of nonhealing ulcers to look for skin cancer, she said. Long-standing ulcers can develop squamous cell carcinoma within them. A basal cell carcinoma can look like a healing ulcer, but not heal.
Bullous diseases also can be hidden in ulcers, particularly in the elderly. "There seems to be a two-hit thing with venous insufficiency where you have a little bit of blistering disease and you have a lot of hydrostatic pressure, and people will get their bullae on their legs long before they get it anyplace else," Dr. Mauro said. "You put compression on and they come back next week and now they have intact bullae. Think about that as another cause."
Less common ulcerative problems that can be confusing include pyoderma gangrenosum (which may be half as common as people think, studies suggest), an underlying vasculitis, or mycobacterial infections. If you see a nonhealing hyperkeratotic lesion, consider an atypical mycobacterial infection. In Dr. Mauro’s region, these usually are in patients who went mountain biking and inoculated themselves through an ankle scratch or in patients exposed to the organism through infected water during a pedicure.
Systemic conditions
Wound healing can be inhibited by things that doctors do, and by things that patients do.
Taking antimetabolite medications (such as hydroxyurea or methotrexate), prednisone, or nonsteroidal anti-inflammatory drugs can impair wound healing, as does smoking. Radiation to the site also inhibits healing. Think twice before irradiating a basal cell carcinoma on the leg of a patient with venous insufficiency. "It’s much better to either try some topical medication or just bite the bullet, do surgery, and put the Unna boot on after the surgery, because if you irradiate [the basal cell carcinoma] and it ulcerates, then it’s really hard to heal," she said.
Coexisting cancer or arterial disease can inhibit wound healing. If you check pulses and can’t feel them, send the patient for an ankle brachial index to determine if there’s arterial disease, in which case the patient should see a vascular surgeon, she said.
When you’re taking patients’ histories, ask what they’re eating so you can assess whether they’re getting enough protein, zinc, and vitamin C. "You’d be surprised at the number of nutritional deficiencies that we see" in patients with nonhealing wounds. Her clinic picks up a zinc deficiency three to four times per year and a protein deficiency approximately twice a year. "All of these are things that you need to make collagen and granulation tissue," she explained. Supplementation with Ensure can provide these nutrients, but a less-expensive option is Carnation Breakfast Essentials, at about one-fifth the cost, she added.
Tailor therapy appropriately
Dressings can make wounds worse. Most commonly, putting a hydrocolloid dressing (such as DuoDERM) on an ulcer that has a lot of drainage will macerate and enlarge the wound. Putting stiff foams or self-adherent wraps such as Coban on wounds with a lot of edema can create secondary ulcers. And avoid compression on the legs of people with arterial insufficiency, Dr. Mauro said.
Artifactual ulcers from patients scratching or picking at wounds are increasing, she said. These patients usually will not report that they’re messing with the wound, but your level of suspicion should increase if the ulcer is above the knee. Hydrocolloid dressings work pretty well for this problem. Consider sending some of these patients to an expert in the growing field of medication therapy for dysesthesia.
Ask patients about home remedies, because they will put all kinds of things on wounds to try and help them heal. Neomycin can cause contact dermatitis. Patients may apply full-strength hydrogen peroxide, thinking that they need a disinfectant, which tends to damage the epithelium and can impair wound healing. If they insist on using hydrogen peroxide, recommend a half-strength solution.
For patients with venous insufficiency, the hydrostatic pressure from sitting can be nearly as bad as from standing. Ask patients with nonhealing wounds what they do at home and at work. If they’re sitting much of that time, recommend products to alleviate the pressure. Dr. Mauro said she has virtually become a saleswoman for La-Z-Boy furniture during her medical career because La-Z-Boy products allow patients to eat, work at computers, and function in positions with less hydrostatic pressure.
When to refer
Consider referring patients with chronic wounds that have not lost at least 50% of their width and height within 6 weeks. When a wound has been present long enough, the physiology shifts from acute to chronic. "It’s as if the body decides that it’s tired of trying to heal it and its new equilibrium is just going to be, ‘I have a wound,’ " she said.
If you want to try treating chronic wounds, applying Promogran Prisma matrix wound dressing in those without good granulation tissue may stimulate granulation tissue, she said.
Debriding an ulcer is "kind of a poor man’s way of getting platelet-derived growth factor," she added. Dr. Mauro applies a compounded 30% lidocaine ("but you can use any topical lidocaine"), leaves it on for 20-25 minutes, and debrides using a curette, which is much more precise than a scalpel. "What you’re trying to do is ‘reboot the computer’ and trick the ulcer into thinking it’s an acute ulcer rather than a chronic one," she said.
Dr. Mauro said she refers patients with arterial insufficiency to vascular surgeons. She sends patients with diabetes to podiatrists, preferably before they develop ulcers. The podiatrist can regularly trim nails for patients with poor sight and loss of sensation who may cut themselves, and can design footwear to avoid pressure that could cause an ulcer. "That will keep you out of trouble" with diabetic patients, she said.
Dr. Mauro has been a consultant for Unilever, but not in a wound-healing capacity.
On Twitter @sherryboschert
EXPERT ANALYSIS FROM THE ANNUAL MEETING OF THE PACIFIC DERMATOLOGIC ASSOCIATION