User login
Minidose edoxaban may safely cut AFib stroke risk in the frail, very elderly
suggests a randomized trial conducted in Japan.
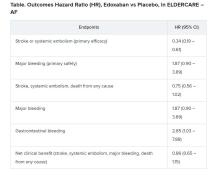
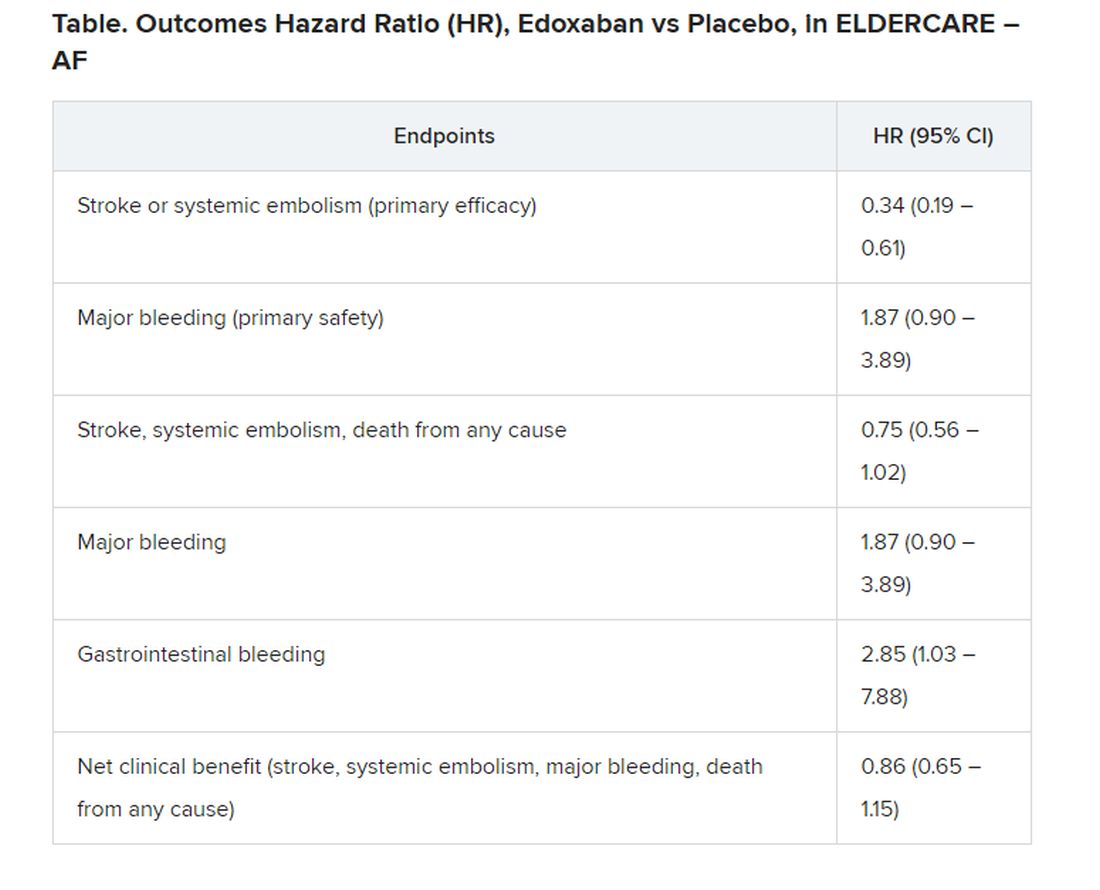
Many of the study’s 984 mostly octogenarian patients were objectively frail with poor renal function, low body weight, a history of serious bleeding, or other conditions that made them poor candidates for regular-dose oral anticoagulation. Yet those who took the factor Xa inhibitor edoxaban (Savaysa) at the off-label dosage of 15 mg once daily showed a two-thirds drop in risk for stroke or systemic embolism (P < .001), compared with patients who received placebo. There were no fatal bleeds and virtually no intracranial hemorrhages.
For such high-risk patients with nonvalvular AFib who otherwise would not be given an OAC, edoxaban 15 mg “can be an acceptable treatment option in decreasing the risk of devastating stroke”; however, “it may increase the risk of gastrointestinal bleeding, so care should be given in every patient,” said Ken Okumura, MD, PhD. Indeed, the rate of gastrointestinal bleeding tripled among the patients who received edoxaban, compared with those given placebo, at about 2.3% per year versus 0.8% per year.
Although their 87% increased risk for major bleeding did not reach significance, it hit close, with a P value of .09 in the trial, called Edoxaban Low-Dose for Elder Care Atrial Fibrillation Patients (ELDERCARE-AF).
Dr. Okumura, of Saiseikai Kumamoto (Japan) Hospital, presented the study August 30 during the virtual annual congress of the European Society of Cardiology. He is lead author of an article describing the study, which was simultaneously published in the New England Journal of Medicine.
Many patients with AFib suffer strokes if they are not given oral anticoagulation because of “fear of major bleeding caused by standard OAC therapy,” Dr. Okumura noted. Others are inappropriately administered antiplatelets or anticoagulants at conventional dosages. “There is no standard of practice in Japan for patients like those in the present trial,” Dr. Okumura said. “However, I believe the present study opens a new possible path of thromboprophylaxis in such high-risk patients.”
Even with its relatively few bleeding events, ELDERCARE-AF “does suggest that the risk of the worst types of bleeds is not that high,” said Daniel E. Singer, MD, of Massachusetts General Hospital, Boston. “Gastrointestinal bleeding is annoying, and it will probably stop people from taking their edoxaban, but for the most part it doesn’t kill people.”
Moreover, he added, the trial suggests that low-dose edoxaban, in exchange for a steep reduction in thromboembolic risk, “doesn’t add to your risk of intracranial hemorrhage!”
ELDERCARE-AF may give practitioners “yet another reason to rethink” whether a low-dose DOAC such as edoxaban 15 mg/day may well be a good approach for such patients with AFib who are not receiving standard-dose OAC because of a perceived high risk for serious bleeding, said Dr. Singer, who was not involved in the study.
The trial randomly and evenly assigned 984 patients with AF in Japan to take either edoxaban 15 mg/day or placebo. The patients, who were at least 80 years old and had a CHADS2 score of 2 or higher, were judged inappropriate candidates for OAC at dosages approved for stroke prevention.
The mean age of the patients was 86.6, more than a decade older than patients “in the previous landmark clinical trials of direct oral anticoagulants,” and were 5-10 years older than the general AFib population, reported Dr. Okumura and colleagues.
Their mean weight was 52 kg, and mean creatinine clearance was 36.3 mL/min; 41% were classified as frail according to validated assessment tools.
Of the 303 patients who did not complete the trial, 158 voluntarily withdrew for various reasons. The withdrawal rate was similar in the two treatment arms. Outcomes were analyzed by intention to treat, the report noted.
The annualized rate of stroke or systemic embolism, the primary efficacy endpoint, was 2.3% for those who received edoxaban and 6.7% for the control group. Corresponding rates for the primary safety endpoint, major bleeding as determined by International Society on Thrombosis and Hemostasis criteria, were 3.3% and 1.8%, respectively.
“The question is, can the Food and Drug Administration act on this information? I doubt it can. What will be needed is to reproduce the study in a U.S. population to see if it holds,” Dr. Singer proposed.
“Edoxaban isn’t used much in the U.S. This could heighten interest. And who knows, there may be a gold rush,” he said, if the strategy were to pan out for the other DOACs, rivaroxaban (Xarelto), apixaban (Eliquis), and dabigatran (Pradaxa).
ELDERCARE-AF was funded by Daiichi Sankyo, from which Dr. Okumura reported receiving grants and personal fees; he also disclosed personal fees from Daiichi Sankyo, Boehringer Ingelheim, Bristol-Myers Squibb, Medtronic, Johnson & Johnson, and Bayer.
A version of this article originally appeared on Medscape.com.
suggests a randomized trial conducted in Japan.
Many of the study’s 984 mostly octogenarian patients were objectively frail with poor renal function, low body weight, a history of serious bleeding, or other conditions that made them poor candidates for regular-dose oral anticoagulation. Yet those who took the factor Xa inhibitor edoxaban (Savaysa) at the off-label dosage of 15 mg once daily showed a two-thirds drop in risk for stroke or systemic embolism (P < .001), compared with patients who received placebo. There were no fatal bleeds and virtually no intracranial hemorrhages.
For such high-risk patients with nonvalvular AFib who otherwise would not be given an OAC, edoxaban 15 mg “can be an acceptable treatment option in decreasing the risk of devastating stroke”; however, “it may increase the risk of gastrointestinal bleeding, so care should be given in every patient,” said Ken Okumura, MD, PhD. Indeed, the rate of gastrointestinal bleeding tripled among the patients who received edoxaban, compared with those given placebo, at about 2.3% per year versus 0.8% per year.
Although their 87% increased risk for major bleeding did not reach significance, it hit close, with a P value of .09 in the trial, called Edoxaban Low-Dose for Elder Care Atrial Fibrillation Patients (ELDERCARE-AF).
Dr. Okumura, of Saiseikai Kumamoto (Japan) Hospital, presented the study August 30 during the virtual annual congress of the European Society of Cardiology. He is lead author of an article describing the study, which was simultaneously published in the New England Journal of Medicine.
Many patients with AFib suffer strokes if they are not given oral anticoagulation because of “fear of major bleeding caused by standard OAC therapy,” Dr. Okumura noted. Others are inappropriately administered antiplatelets or anticoagulants at conventional dosages. “There is no standard of practice in Japan for patients like those in the present trial,” Dr. Okumura said. “However, I believe the present study opens a new possible path of thromboprophylaxis in such high-risk patients.”
Even with its relatively few bleeding events, ELDERCARE-AF “does suggest that the risk of the worst types of bleeds is not that high,” said Daniel E. Singer, MD, of Massachusetts General Hospital, Boston. “Gastrointestinal bleeding is annoying, and it will probably stop people from taking their edoxaban, but for the most part it doesn’t kill people.”
Moreover, he added, the trial suggests that low-dose edoxaban, in exchange for a steep reduction in thromboembolic risk, “doesn’t add to your risk of intracranial hemorrhage!”
ELDERCARE-AF may give practitioners “yet another reason to rethink” whether a low-dose DOAC such as edoxaban 15 mg/day may well be a good approach for such patients with AFib who are not receiving standard-dose OAC because of a perceived high risk for serious bleeding, said Dr. Singer, who was not involved in the study.
The trial randomly and evenly assigned 984 patients with AF in Japan to take either edoxaban 15 mg/day or placebo. The patients, who were at least 80 years old and had a CHADS2 score of 2 or higher, were judged inappropriate candidates for OAC at dosages approved for stroke prevention.
The mean age of the patients was 86.6, more than a decade older than patients “in the previous landmark clinical trials of direct oral anticoagulants,” and were 5-10 years older than the general AFib population, reported Dr. Okumura and colleagues.
Their mean weight was 52 kg, and mean creatinine clearance was 36.3 mL/min; 41% were classified as frail according to validated assessment tools.
Of the 303 patients who did not complete the trial, 158 voluntarily withdrew for various reasons. The withdrawal rate was similar in the two treatment arms. Outcomes were analyzed by intention to treat, the report noted.
The annualized rate of stroke or systemic embolism, the primary efficacy endpoint, was 2.3% for those who received edoxaban and 6.7% for the control group. Corresponding rates for the primary safety endpoint, major bleeding as determined by International Society on Thrombosis and Hemostasis criteria, were 3.3% and 1.8%, respectively.
“The question is, can the Food and Drug Administration act on this information? I doubt it can. What will be needed is to reproduce the study in a U.S. population to see if it holds,” Dr. Singer proposed.
“Edoxaban isn’t used much in the U.S. This could heighten interest. And who knows, there may be a gold rush,” he said, if the strategy were to pan out for the other DOACs, rivaroxaban (Xarelto), apixaban (Eliquis), and dabigatran (Pradaxa).
ELDERCARE-AF was funded by Daiichi Sankyo, from which Dr. Okumura reported receiving grants and personal fees; he also disclosed personal fees from Daiichi Sankyo, Boehringer Ingelheim, Bristol-Myers Squibb, Medtronic, Johnson & Johnson, and Bayer.
A version of this article originally appeared on Medscape.com.
suggests a randomized trial conducted in Japan.
Many of the study’s 984 mostly octogenarian patients were objectively frail with poor renal function, low body weight, a history of serious bleeding, or other conditions that made them poor candidates for regular-dose oral anticoagulation. Yet those who took the factor Xa inhibitor edoxaban (Savaysa) at the off-label dosage of 15 mg once daily showed a two-thirds drop in risk for stroke or systemic embolism (P < .001), compared with patients who received placebo. There were no fatal bleeds and virtually no intracranial hemorrhages.
For such high-risk patients with nonvalvular AFib who otherwise would not be given an OAC, edoxaban 15 mg “can be an acceptable treatment option in decreasing the risk of devastating stroke”; however, “it may increase the risk of gastrointestinal bleeding, so care should be given in every patient,” said Ken Okumura, MD, PhD. Indeed, the rate of gastrointestinal bleeding tripled among the patients who received edoxaban, compared with those given placebo, at about 2.3% per year versus 0.8% per year.
Although their 87% increased risk for major bleeding did not reach significance, it hit close, with a P value of .09 in the trial, called Edoxaban Low-Dose for Elder Care Atrial Fibrillation Patients (ELDERCARE-AF).
Dr. Okumura, of Saiseikai Kumamoto (Japan) Hospital, presented the study August 30 during the virtual annual congress of the European Society of Cardiology. He is lead author of an article describing the study, which was simultaneously published in the New England Journal of Medicine.
Many patients with AFib suffer strokes if they are not given oral anticoagulation because of “fear of major bleeding caused by standard OAC therapy,” Dr. Okumura noted. Others are inappropriately administered antiplatelets or anticoagulants at conventional dosages. “There is no standard of practice in Japan for patients like those in the present trial,” Dr. Okumura said. “However, I believe the present study opens a new possible path of thromboprophylaxis in such high-risk patients.”
Even with its relatively few bleeding events, ELDERCARE-AF “does suggest that the risk of the worst types of bleeds is not that high,” said Daniel E. Singer, MD, of Massachusetts General Hospital, Boston. “Gastrointestinal bleeding is annoying, and it will probably stop people from taking their edoxaban, but for the most part it doesn’t kill people.”
Moreover, he added, the trial suggests that low-dose edoxaban, in exchange for a steep reduction in thromboembolic risk, “doesn’t add to your risk of intracranial hemorrhage!”
ELDERCARE-AF may give practitioners “yet another reason to rethink” whether a low-dose DOAC such as edoxaban 15 mg/day may well be a good approach for such patients with AFib who are not receiving standard-dose OAC because of a perceived high risk for serious bleeding, said Dr. Singer, who was not involved in the study.
The trial randomly and evenly assigned 984 patients with AF in Japan to take either edoxaban 15 mg/day or placebo. The patients, who were at least 80 years old and had a CHADS2 score of 2 or higher, were judged inappropriate candidates for OAC at dosages approved for stroke prevention.
The mean age of the patients was 86.6, more than a decade older than patients “in the previous landmark clinical trials of direct oral anticoagulants,” and were 5-10 years older than the general AFib population, reported Dr. Okumura and colleagues.
Their mean weight was 52 kg, and mean creatinine clearance was 36.3 mL/min; 41% were classified as frail according to validated assessment tools.
Of the 303 patients who did not complete the trial, 158 voluntarily withdrew for various reasons. The withdrawal rate was similar in the two treatment arms. Outcomes were analyzed by intention to treat, the report noted.
The annualized rate of stroke or systemic embolism, the primary efficacy endpoint, was 2.3% for those who received edoxaban and 6.7% for the control group. Corresponding rates for the primary safety endpoint, major bleeding as determined by International Society on Thrombosis and Hemostasis criteria, were 3.3% and 1.8%, respectively.
“The question is, can the Food and Drug Administration act on this information? I doubt it can. What will be needed is to reproduce the study in a U.S. population to see if it holds,” Dr. Singer proposed.
“Edoxaban isn’t used much in the U.S. This could heighten interest. And who knows, there may be a gold rush,” he said, if the strategy were to pan out for the other DOACs, rivaroxaban (Xarelto), apixaban (Eliquis), and dabigatran (Pradaxa).
ELDERCARE-AF was funded by Daiichi Sankyo, from which Dr. Okumura reported receiving grants and personal fees; he also disclosed personal fees from Daiichi Sankyo, Boehringer Ingelheim, Bristol-Myers Squibb, Medtronic, Johnson & Johnson, and Bayer.
A version of this article originally appeared on Medscape.com.
FROM ESC CONGRESS 2020
IMPACT-AFib: Single mailing fails to budge oral anticoagulant uptake for AFib
A single educational mailing sent by several U.S. health plans to their patients with atrial fibrillation who were candidates for oral anticoagulation, but had not yet started a regimen, failed to boost them over their prescription hurdle and facilitate starting an antithrombotic regimen.
By 1 year following the intervention, a mere 10% of patients in both the intervention and a control arm of the randomized trial had begun treatment, with no signal of incremental uptake because of the mailing, Sean D. Pokorney, MD, said at the virtual annual congress of the European Society of Cardiology. Included in the mailing was an educational letter citing the patient’s atrial fibrillation (AFib) diagnosis, a statement regarding their suitability for oral anticoagulation, some information about the treatment, and a suggestion that recipients discuss this with their personal physician.
Dr. Pokorney acknowledged that the single mailing to patients may not have been adequate to capture patients’ attention and trigger an action, and that repeated messaging via multiple platforms and in coordination with interventions aimed at their health care providers may be what’s needed.
“It will take repeated interventions and engagements. We will need different methods to move the needle,” said Dr. Pokorney, a cardiac electrophysiologist at Duke University in Durham, N.C. The goal is to “empower patients to talk with their health care providers, and to become agents of change” in their care, he explained, but the single, mailed prod wasn’t enough.
An earlier study run by Dr. Pokorney and several of his colleagues used a broader panel of interventions aimed at both patients and clinicians to encourage increased prescribing of oral anticoagulants in five middle income countries, and documented successfully increasing the uptake rate by threefold compared with control patients (Lancet. 2017 Oct 14;390[10104]:1737-46). The current study tested the efficacy of a “much lower-impact intervention,” he admitted.
“The data are “sobering and eye-opening,” said Kalyanam Shivkumar, MD, a cardiac electrophysiologist and professor of medicine at the University of California, Los Angeles. “We’re stuck with this big challenge,” the gap between “what medicine can do and what it actually does” when evidence-based interventions fail to gain traction in everyday practice, he said in an interview.
The numbers collected during the new study highlighted the treatment gap. The IMPACT-AFib study randomized 23,546 patients with AFib and a CHA2DS2-VASc score of at least 2, denoting a stroke risk that warrants oral anticoagulation, to the intervention group, and 23,787 patients to the control arm. The patient selection process began with nearly 200,000 patients who met these criteria, but the researchers excluded 67% because they were already on an oral anticoagulant regimen, an uptake level that roughly matched the 50%-60% level usually seen among U.S. patients, Dr. Pokorney noted. That number coupled with the incremental uptake rate of only 10% of the enrolled patients during the trial, despite their uniform suitability for treatment, underscored how low uptake rates tend to remain stuck over time.
Enrolled patients averaged 78 years of age, with nearly two-thirds at least 75 years old, and with an average CHA2DS2-VASc score of 4.5.
The trial featured a novel design as the first clinical trial to take advantage of the Sentinel program for phase 4 data collection and study devised by the Food and Drug Administration, said Dr. Pokorney. The Sentinel program relies on data partners to provide information; for the IMPACT-AFib study, data came from five large U.S. health systems: Aetna, HealthCore, Humana, Harvard Pilgrim Healthcare, and Optum. Each of these systems sent the mailing to their targeted member patients.
In addition to sending just a single, mailed intervention, the study may have also been limited by the mailing’s content. The educational text, presented by Dr. Pokorney during his talk, focused largely on the potential risks of oral anticoagulation, the limited availability of antidote agents, potential drug and food interactions, and a brief entry about the risk for stroke associated with AFib along with a chart that a patient could use to hand calculate their CHA2DS2-VASc score. What the mailing lacked was discussion of the benefits of oral anticoagulation, noted study discussant Christophe LeClercq, MD, a cardiac electrophysiologist and professor of cardiology at the University of Rennes, France.
IMPACT-AFib received no commercial funding, and Dr. Pokorney and Dr. Shivkumar had no disclosures. Dr. Leclercq has received honoraria from Abbott, Biotronik, Boston Scientific, Livanova, and Medtronic.
A single educational mailing sent by several U.S. health plans to their patients with atrial fibrillation who were candidates for oral anticoagulation, but had not yet started a regimen, failed to boost them over their prescription hurdle and facilitate starting an antithrombotic regimen.
By 1 year following the intervention, a mere 10% of patients in both the intervention and a control arm of the randomized trial had begun treatment, with no signal of incremental uptake because of the mailing, Sean D. Pokorney, MD, said at the virtual annual congress of the European Society of Cardiology. Included in the mailing was an educational letter citing the patient’s atrial fibrillation (AFib) diagnosis, a statement regarding their suitability for oral anticoagulation, some information about the treatment, and a suggestion that recipients discuss this with their personal physician.
Dr. Pokorney acknowledged that the single mailing to patients may not have been adequate to capture patients’ attention and trigger an action, and that repeated messaging via multiple platforms and in coordination with interventions aimed at their health care providers may be what’s needed.
“It will take repeated interventions and engagements. We will need different methods to move the needle,” said Dr. Pokorney, a cardiac electrophysiologist at Duke University in Durham, N.C. The goal is to “empower patients to talk with their health care providers, and to become agents of change” in their care, he explained, but the single, mailed prod wasn’t enough.
An earlier study run by Dr. Pokorney and several of his colleagues used a broader panel of interventions aimed at both patients and clinicians to encourage increased prescribing of oral anticoagulants in five middle income countries, and documented successfully increasing the uptake rate by threefold compared with control patients (Lancet. 2017 Oct 14;390[10104]:1737-46). The current study tested the efficacy of a “much lower-impact intervention,” he admitted.
“The data are “sobering and eye-opening,” said Kalyanam Shivkumar, MD, a cardiac electrophysiologist and professor of medicine at the University of California, Los Angeles. “We’re stuck with this big challenge,” the gap between “what medicine can do and what it actually does” when evidence-based interventions fail to gain traction in everyday practice, he said in an interview.
The numbers collected during the new study highlighted the treatment gap. The IMPACT-AFib study randomized 23,546 patients with AFib and a CHA2DS2-VASc score of at least 2, denoting a stroke risk that warrants oral anticoagulation, to the intervention group, and 23,787 patients to the control arm. The patient selection process began with nearly 200,000 patients who met these criteria, but the researchers excluded 67% because they were already on an oral anticoagulant regimen, an uptake level that roughly matched the 50%-60% level usually seen among U.S. patients, Dr. Pokorney noted. That number coupled with the incremental uptake rate of only 10% of the enrolled patients during the trial, despite their uniform suitability for treatment, underscored how low uptake rates tend to remain stuck over time.
Enrolled patients averaged 78 years of age, with nearly two-thirds at least 75 years old, and with an average CHA2DS2-VASc score of 4.5.
The trial featured a novel design as the first clinical trial to take advantage of the Sentinel program for phase 4 data collection and study devised by the Food and Drug Administration, said Dr. Pokorney. The Sentinel program relies on data partners to provide information; for the IMPACT-AFib study, data came from five large U.S. health systems: Aetna, HealthCore, Humana, Harvard Pilgrim Healthcare, and Optum. Each of these systems sent the mailing to their targeted member patients.
In addition to sending just a single, mailed intervention, the study may have also been limited by the mailing’s content. The educational text, presented by Dr. Pokorney during his talk, focused largely on the potential risks of oral anticoagulation, the limited availability of antidote agents, potential drug and food interactions, and a brief entry about the risk for stroke associated with AFib along with a chart that a patient could use to hand calculate their CHA2DS2-VASc score. What the mailing lacked was discussion of the benefits of oral anticoagulation, noted study discussant Christophe LeClercq, MD, a cardiac electrophysiologist and professor of cardiology at the University of Rennes, France.
IMPACT-AFib received no commercial funding, and Dr. Pokorney and Dr. Shivkumar had no disclosures. Dr. Leclercq has received honoraria from Abbott, Biotronik, Boston Scientific, Livanova, and Medtronic.
A single educational mailing sent by several U.S. health plans to their patients with atrial fibrillation who were candidates for oral anticoagulation, but had not yet started a regimen, failed to boost them over their prescription hurdle and facilitate starting an antithrombotic regimen.
By 1 year following the intervention, a mere 10% of patients in both the intervention and a control arm of the randomized trial had begun treatment, with no signal of incremental uptake because of the mailing, Sean D. Pokorney, MD, said at the virtual annual congress of the European Society of Cardiology. Included in the mailing was an educational letter citing the patient’s atrial fibrillation (AFib) diagnosis, a statement regarding their suitability for oral anticoagulation, some information about the treatment, and a suggestion that recipients discuss this with their personal physician.
Dr. Pokorney acknowledged that the single mailing to patients may not have been adequate to capture patients’ attention and trigger an action, and that repeated messaging via multiple platforms and in coordination with interventions aimed at their health care providers may be what’s needed.
“It will take repeated interventions and engagements. We will need different methods to move the needle,” said Dr. Pokorney, a cardiac electrophysiologist at Duke University in Durham, N.C. The goal is to “empower patients to talk with their health care providers, and to become agents of change” in their care, he explained, but the single, mailed prod wasn’t enough.
An earlier study run by Dr. Pokorney and several of his colleagues used a broader panel of interventions aimed at both patients and clinicians to encourage increased prescribing of oral anticoagulants in five middle income countries, and documented successfully increasing the uptake rate by threefold compared with control patients (Lancet. 2017 Oct 14;390[10104]:1737-46). The current study tested the efficacy of a “much lower-impact intervention,” he admitted.
“The data are “sobering and eye-opening,” said Kalyanam Shivkumar, MD, a cardiac electrophysiologist and professor of medicine at the University of California, Los Angeles. “We’re stuck with this big challenge,” the gap between “what medicine can do and what it actually does” when evidence-based interventions fail to gain traction in everyday practice, he said in an interview.
The numbers collected during the new study highlighted the treatment gap. The IMPACT-AFib study randomized 23,546 patients with AFib and a CHA2DS2-VASc score of at least 2, denoting a stroke risk that warrants oral anticoagulation, to the intervention group, and 23,787 patients to the control arm. The patient selection process began with nearly 200,000 patients who met these criteria, but the researchers excluded 67% because they were already on an oral anticoagulant regimen, an uptake level that roughly matched the 50%-60% level usually seen among U.S. patients, Dr. Pokorney noted. That number coupled with the incremental uptake rate of only 10% of the enrolled patients during the trial, despite their uniform suitability for treatment, underscored how low uptake rates tend to remain stuck over time.
Enrolled patients averaged 78 years of age, with nearly two-thirds at least 75 years old, and with an average CHA2DS2-VASc score of 4.5.
The trial featured a novel design as the first clinical trial to take advantage of the Sentinel program for phase 4 data collection and study devised by the Food and Drug Administration, said Dr. Pokorney. The Sentinel program relies on data partners to provide information; for the IMPACT-AFib study, data came from five large U.S. health systems: Aetna, HealthCore, Humana, Harvard Pilgrim Healthcare, and Optum. Each of these systems sent the mailing to their targeted member patients.
In addition to sending just a single, mailed intervention, the study may have also been limited by the mailing’s content. The educational text, presented by Dr. Pokorney during his talk, focused largely on the potential risks of oral anticoagulation, the limited availability of antidote agents, potential drug and food interactions, and a brief entry about the risk for stroke associated with AFib along with a chart that a patient could use to hand calculate their CHA2DS2-VASc score. What the mailing lacked was discussion of the benefits of oral anticoagulation, noted study discussant Christophe LeClercq, MD, a cardiac electrophysiologist and professor of cardiology at the University of Rennes, France.
IMPACT-AFib received no commercial funding, and Dr. Pokorney and Dr. Shivkumar had no disclosures. Dr. Leclercq has received honoraria from Abbott, Biotronik, Boston Scientific, Livanova, and Medtronic.
FROM ESC CONGRESS 2020
REALITY trial supports restrictive transfusion in anemic MI
in the landmark REALITY trial.
Randomized trial data already support a restrictive transfusion strategy in patients undergoing cardiac and noncardiac surgery, as well as in other settings. Those trials deliberately excluded patients with acute myocardial ischemia.
Cardiologists have been loath to adopt a restrictive strategy in the absence of persuasive supporting evidence because of a theoretic concern that low hemoglobin might be particularly harmful to ischemic myocardium. Anemia occurs in 5%-10% patients with MI, and clinicians have been eager for evidence-based guidance on how to best manage it.
“Blood is a precious resource and transfusion is costly, logistically cumbersome, and has side effects,” Philippe Gabriel Steg, MD, chair of the REALITY trial, noted in presenting the study results at the virtual annual congress of the European Society of Cardiology.
REALITY was the first-ever large randomized trial of a restrictive versus liberal transfusion strategy in acute MI. The study, which featured a noninferiority design, included 668 stable patients with acute MI and anemia with a hemoglobin of 7-10 g/dL at 35 hospitals in France and Spain. Participants were randomized to a restrictive strategy in which transfusion was withheld unless the hemoglobin dropped to 8 g/dL or less, or to a conventional liberal strategy triggered by a hemoglobin of 10 g/dL or lower. The transfusion target was a hemoglobin level of 8-10 g/dL in the restrictive strategy group and greater than 11 g/dL in the liberal transfusion group. In the restrictive transfusion group, 36% received at least one RBC transfusion, as did 87% in the liberal transfusion study arm. The restrictive strategy group used 414 fewer units of blood.
The two coprimary endpoints were 30-day major adverse cardiovascular events and cost-effectiveness. The 30-day composite of all-cause mortality, reinfarction, stroke, and emergency percutaneous coronary intervention for myocardial ischemia occurred in 11% of the restrictive transfusion group and 14% of the liberal transfusion group. The resultant 21% relative risk reduction established that the restrictive strategy was noninferior. Of note, all of the individual components of the composite endpoint numerically favored the restrictive approach.
In terms of safety, patients in the restrictive transfusion group were significantly less likely to develop an infection, by a margin of 0% versus 1.5%. The rate of acute lung injury was also significantly lower in the restrictive group: 0.3%, compared with 2.2%. The median hospital length of stay was identical at 7 days in both groups.
The cost-effectiveness analysis concluded that the restrictive transfusion strategy had an 84% probability of being both less expensive and more effective.
Patients were enrolled in REALITY regardless of whether they had active bleeding, as long as the bleeding wasn’t deemed massive and life-threatening. Notably, there was no difference in the results of restrictive versus liberal transfusion regardless of whether active bleeding was present, nor did baseline hemoglobin or the presence or absence of preexisting anemia affect the results.
Dr. Steg noted that a much larger randomized trial of restrictive versus liberal transfusion in the setting of acute MI with anemia is underway in the United States and Canada. The 3,000-patient MINT trial, sponsored by the National Institutes of Health, is testing the superiority of restrictive transfusion, rather than its noninferiority, as in REALITY. Results are a couple of years away.
“I think that will be an important piece of additional evidence,” he said.
Discussant Marco Roffi, MD, didn’t mince words.
“I really love the REALITY trial,” declared Dr. Roffi, professor and vice chairman of the cardiology department and director of the interventional cardiology unit at University Hospital of Geneva.
He ticked off a series of reasons: The trial addressed a common clinical dilemma about which there has been essentially no prior high-quality evidence, it provided convincing results, and it carried important implications for responsible stewardship of the blood supply.
“REALITY allows clinicians to comfortably refrain from transfusing anemic patients presenting with myocardial infarction, and this should lead to a reduction in the consumption of blood products,” Dr. Roffi said.
He applauded the investigators for their success in obtaining public funding for a study lacking a commercial hook. And as a clinical investigator, he was particularly impressed by one of the technical details about the REALITY trial: “I was amazed by the fact that the observed event rates virtually corresponded to the estimated ones used for the power calculations. This is rarely the case in such a trial.”
Dr. Roffi said the REALITY findings should have an immediate impact on clinical practice, as well as on the brand new 2020 ESC guidelines on the management of non–ST-elevation ACS issued during the ESC virtual congress.
The freshly inked guidelines state: “Based on inconsistent study results and the lack of adequately powered randomized, controlled trials, a restrictive policy of transfusion in anemic patients with MI may be considered.” As of today, Dr. Roffi argued, the phrase “may be considered” ought to be replaced by the stronger phrase “should be considered.”
During the discussion period, he was asked if it’s appropriate to extrapolate the REALITY results to patients undergoing transcatheter aortic valve replacement, among whom anemia is highly prevalent.
“I think this is a different patient population. Nevertheless, the concept of being restrictive is one that in my opinion now remains until proven otherwise. So we are being very restrictive in these patients,” he replied.
Asked about possible mechanisms by which liberal transfusion might have detrimental effects in acute MI patients, Dr. Steg cited several, including evidence that transfusion may not improve oxygen delivery to as great an extent as traditionally thought. There is also the risk of volume overload, increased blood viscosity, and enhanced platelet aggregation and activation, which could promote myocardial ischemia.
The REALITY trial was funded by the French Ministry of Health and the Spanish Ministry of Economy and Competitiveness with no commercial support. Outside the scope of the trial, Dr. Steg reported receiving research grants from Bayer, Merck, Servier, and Sanofi as well as serving as a consultant to numerous pharmaceutical companies.
in the landmark REALITY trial.
Randomized trial data already support a restrictive transfusion strategy in patients undergoing cardiac and noncardiac surgery, as well as in other settings. Those trials deliberately excluded patients with acute myocardial ischemia.
Cardiologists have been loath to adopt a restrictive strategy in the absence of persuasive supporting evidence because of a theoretic concern that low hemoglobin might be particularly harmful to ischemic myocardium. Anemia occurs in 5%-10% patients with MI, and clinicians have been eager for evidence-based guidance on how to best manage it.
“Blood is a precious resource and transfusion is costly, logistically cumbersome, and has side effects,” Philippe Gabriel Steg, MD, chair of the REALITY trial, noted in presenting the study results at the virtual annual congress of the European Society of Cardiology.
REALITY was the first-ever large randomized trial of a restrictive versus liberal transfusion strategy in acute MI. The study, which featured a noninferiority design, included 668 stable patients with acute MI and anemia with a hemoglobin of 7-10 g/dL at 35 hospitals in France and Spain. Participants were randomized to a restrictive strategy in which transfusion was withheld unless the hemoglobin dropped to 8 g/dL or less, or to a conventional liberal strategy triggered by a hemoglobin of 10 g/dL or lower. The transfusion target was a hemoglobin level of 8-10 g/dL in the restrictive strategy group and greater than 11 g/dL in the liberal transfusion group. In the restrictive transfusion group, 36% received at least one RBC transfusion, as did 87% in the liberal transfusion study arm. The restrictive strategy group used 414 fewer units of blood.
The two coprimary endpoints were 30-day major adverse cardiovascular events and cost-effectiveness. The 30-day composite of all-cause mortality, reinfarction, stroke, and emergency percutaneous coronary intervention for myocardial ischemia occurred in 11% of the restrictive transfusion group and 14% of the liberal transfusion group. The resultant 21% relative risk reduction established that the restrictive strategy was noninferior. Of note, all of the individual components of the composite endpoint numerically favored the restrictive approach.
In terms of safety, patients in the restrictive transfusion group were significantly less likely to develop an infection, by a margin of 0% versus 1.5%. The rate of acute lung injury was also significantly lower in the restrictive group: 0.3%, compared with 2.2%. The median hospital length of stay was identical at 7 days in both groups.
The cost-effectiveness analysis concluded that the restrictive transfusion strategy had an 84% probability of being both less expensive and more effective.
Patients were enrolled in REALITY regardless of whether they had active bleeding, as long as the bleeding wasn’t deemed massive and life-threatening. Notably, there was no difference in the results of restrictive versus liberal transfusion regardless of whether active bleeding was present, nor did baseline hemoglobin or the presence or absence of preexisting anemia affect the results.
Dr. Steg noted that a much larger randomized trial of restrictive versus liberal transfusion in the setting of acute MI with anemia is underway in the United States and Canada. The 3,000-patient MINT trial, sponsored by the National Institutes of Health, is testing the superiority of restrictive transfusion, rather than its noninferiority, as in REALITY. Results are a couple of years away.
“I think that will be an important piece of additional evidence,” he said.
Discussant Marco Roffi, MD, didn’t mince words.
“I really love the REALITY trial,” declared Dr. Roffi, professor and vice chairman of the cardiology department and director of the interventional cardiology unit at University Hospital of Geneva.
He ticked off a series of reasons: The trial addressed a common clinical dilemma about which there has been essentially no prior high-quality evidence, it provided convincing results, and it carried important implications for responsible stewardship of the blood supply.
“REALITY allows clinicians to comfortably refrain from transfusing anemic patients presenting with myocardial infarction, and this should lead to a reduction in the consumption of blood products,” Dr. Roffi said.
He applauded the investigators for their success in obtaining public funding for a study lacking a commercial hook. And as a clinical investigator, he was particularly impressed by one of the technical details about the REALITY trial: “I was amazed by the fact that the observed event rates virtually corresponded to the estimated ones used for the power calculations. This is rarely the case in such a trial.”
Dr. Roffi said the REALITY findings should have an immediate impact on clinical practice, as well as on the brand new 2020 ESC guidelines on the management of non–ST-elevation ACS issued during the ESC virtual congress.
The freshly inked guidelines state: “Based on inconsistent study results and the lack of adequately powered randomized, controlled trials, a restrictive policy of transfusion in anemic patients with MI may be considered.” As of today, Dr. Roffi argued, the phrase “may be considered” ought to be replaced by the stronger phrase “should be considered.”
During the discussion period, he was asked if it’s appropriate to extrapolate the REALITY results to patients undergoing transcatheter aortic valve replacement, among whom anemia is highly prevalent.
“I think this is a different patient population. Nevertheless, the concept of being restrictive is one that in my opinion now remains until proven otherwise. So we are being very restrictive in these patients,” he replied.
Asked about possible mechanisms by which liberal transfusion might have detrimental effects in acute MI patients, Dr. Steg cited several, including evidence that transfusion may not improve oxygen delivery to as great an extent as traditionally thought. There is also the risk of volume overload, increased blood viscosity, and enhanced platelet aggregation and activation, which could promote myocardial ischemia.
The REALITY trial was funded by the French Ministry of Health and the Spanish Ministry of Economy and Competitiveness with no commercial support. Outside the scope of the trial, Dr. Steg reported receiving research grants from Bayer, Merck, Servier, and Sanofi as well as serving as a consultant to numerous pharmaceutical companies.
in the landmark REALITY trial.
Randomized trial data already support a restrictive transfusion strategy in patients undergoing cardiac and noncardiac surgery, as well as in other settings. Those trials deliberately excluded patients with acute myocardial ischemia.
Cardiologists have been loath to adopt a restrictive strategy in the absence of persuasive supporting evidence because of a theoretic concern that low hemoglobin might be particularly harmful to ischemic myocardium. Anemia occurs in 5%-10% patients with MI, and clinicians have been eager for evidence-based guidance on how to best manage it.
“Blood is a precious resource and transfusion is costly, logistically cumbersome, and has side effects,” Philippe Gabriel Steg, MD, chair of the REALITY trial, noted in presenting the study results at the virtual annual congress of the European Society of Cardiology.
REALITY was the first-ever large randomized trial of a restrictive versus liberal transfusion strategy in acute MI. The study, which featured a noninferiority design, included 668 stable patients with acute MI and anemia with a hemoglobin of 7-10 g/dL at 35 hospitals in France and Spain. Participants were randomized to a restrictive strategy in which transfusion was withheld unless the hemoglobin dropped to 8 g/dL or less, or to a conventional liberal strategy triggered by a hemoglobin of 10 g/dL or lower. The transfusion target was a hemoglobin level of 8-10 g/dL in the restrictive strategy group and greater than 11 g/dL in the liberal transfusion group. In the restrictive transfusion group, 36% received at least one RBC transfusion, as did 87% in the liberal transfusion study arm. The restrictive strategy group used 414 fewer units of blood.
The two coprimary endpoints were 30-day major adverse cardiovascular events and cost-effectiveness. The 30-day composite of all-cause mortality, reinfarction, stroke, and emergency percutaneous coronary intervention for myocardial ischemia occurred in 11% of the restrictive transfusion group and 14% of the liberal transfusion group. The resultant 21% relative risk reduction established that the restrictive strategy was noninferior. Of note, all of the individual components of the composite endpoint numerically favored the restrictive approach.
In terms of safety, patients in the restrictive transfusion group were significantly less likely to develop an infection, by a margin of 0% versus 1.5%. The rate of acute lung injury was also significantly lower in the restrictive group: 0.3%, compared with 2.2%. The median hospital length of stay was identical at 7 days in both groups.
The cost-effectiveness analysis concluded that the restrictive transfusion strategy had an 84% probability of being both less expensive and more effective.
Patients were enrolled in REALITY regardless of whether they had active bleeding, as long as the bleeding wasn’t deemed massive and life-threatening. Notably, there was no difference in the results of restrictive versus liberal transfusion regardless of whether active bleeding was present, nor did baseline hemoglobin or the presence or absence of preexisting anemia affect the results.
Dr. Steg noted that a much larger randomized trial of restrictive versus liberal transfusion in the setting of acute MI with anemia is underway in the United States and Canada. The 3,000-patient MINT trial, sponsored by the National Institutes of Health, is testing the superiority of restrictive transfusion, rather than its noninferiority, as in REALITY. Results are a couple of years away.
“I think that will be an important piece of additional evidence,” he said.
Discussant Marco Roffi, MD, didn’t mince words.
“I really love the REALITY trial,” declared Dr. Roffi, professor and vice chairman of the cardiology department and director of the interventional cardiology unit at University Hospital of Geneva.
He ticked off a series of reasons: The trial addressed a common clinical dilemma about which there has been essentially no prior high-quality evidence, it provided convincing results, and it carried important implications for responsible stewardship of the blood supply.
“REALITY allows clinicians to comfortably refrain from transfusing anemic patients presenting with myocardial infarction, and this should lead to a reduction in the consumption of blood products,” Dr. Roffi said.
He applauded the investigators for their success in obtaining public funding for a study lacking a commercial hook. And as a clinical investigator, he was particularly impressed by one of the technical details about the REALITY trial: “I was amazed by the fact that the observed event rates virtually corresponded to the estimated ones used for the power calculations. This is rarely the case in such a trial.”
Dr. Roffi said the REALITY findings should have an immediate impact on clinical practice, as well as on the brand new 2020 ESC guidelines on the management of non–ST-elevation ACS issued during the ESC virtual congress.
The freshly inked guidelines state: “Based on inconsistent study results and the lack of adequately powered randomized, controlled trials, a restrictive policy of transfusion in anemic patients with MI may be considered.” As of today, Dr. Roffi argued, the phrase “may be considered” ought to be replaced by the stronger phrase “should be considered.”
During the discussion period, he was asked if it’s appropriate to extrapolate the REALITY results to patients undergoing transcatheter aortic valve replacement, among whom anemia is highly prevalent.
“I think this is a different patient population. Nevertheless, the concept of being restrictive is one that in my opinion now remains until proven otherwise. So we are being very restrictive in these patients,” he replied.
Asked about possible mechanisms by which liberal transfusion might have detrimental effects in acute MI patients, Dr. Steg cited several, including evidence that transfusion may not improve oxygen delivery to as great an extent as traditionally thought. There is also the risk of volume overload, increased blood viscosity, and enhanced platelet aggregation and activation, which could promote myocardial ischemia.
The REALITY trial was funded by the French Ministry of Health and the Spanish Ministry of Economy and Competitiveness with no commercial support. Outside the scope of the trial, Dr. Steg reported receiving research grants from Bayer, Merck, Servier, and Sanofi as well as serving as a consultant to numerous pharmaceutical companies.
REPORTING FROM ESC CONGRESS 2020
First randomized trial reassures on ACEIs, ARBs in COVID-19
The first randomized study to compare continuing versus stopping ACE inhibitors or angiotensin receptor blockers (ARBs) for patients with COVID-19 has shown no difference in key outcomes between the two approaches.
The BRACE CORONA trial – conducted in patients had been taking an ACE inhibitor or an ARB on a long-term basis and who were subsequently hospitalized with COVID-19 – showed no difference in the primary endpoint of number of days alive and out of hospital among those whose medication was suspended for 30 days and those who continued undergoing treatment with these agents.
“Because these data indicate that there is no clinical benefit from routinely interrupting these medications in hospitalized patients with mild to moderate COVID-19, they should generally be continued for those with an indication,” principal investigator Renato Lopes, MD, of Duke Clinical Research Institute, Durham, N.C., concluded.
The BRACE CORONA trial was presented at the European Society of Cardiology Congress 2020 on Sept. 1.
Dr. Lopes explained that there are two conflicting hypotheses about the role of ACE inhibitors and ARBs in COVID-19.
One hypothesis suggests that use of these drugs could be harmful by increasing the expression of ACE2 receptors (which the SARS-CoV-2 virus uses to gain entry into cells), thus potentially enhancing viral binding and viral entry. The other suggests that ACE inhibitors and ARBs could be protective by reducing production of angiotensin II and enhancing the generation of angiotensin 1-7, which attenuates inflammation and fibrosis and therefore could attenuate lung injury.
The BRACE CORONA trial was an academic-led randomized study that tested two strategies: temporarily stopping the ACE inhibitor/ARB for 30 days or continuing these drugs for patients who had been taking these medications on a long-term basis and were hospitalized with a confirmed diagnosis of COVID-19.
The primary outcome was the number of days alive and out of hospital at 30 days. Patients who were using more than three antihypertensive drugs or sacubitril/valsartan or who were hemodynamically unstable at presentation were excluded from the study.
The trial enrolled 659 patients from 29 sites in Brazil. The mean age of patients was 56 years, 40% were women, and 52% were obese. ACE inhibitors were being taken by 15% of the trial participants; ARBs were being taken by 85%. The median duration of ACE inhibitor/ARB treatment was 5 years.
Patients were a median of 6 days from COVID-19 symptom onset. For 30% of the patients, oxygen saturation was below 94% at entry. In terms of COVID-19 symptoms, 57% were classified as mild, and 43% as moderate.
Those with severe COVID-19 symptoms who needed intubation or vasoactive drugs were excluded. Antihypertensive therapy would generally be discontinued in these patients anyway, Dr. Lopes said.
Results showed that the average number of days alive and out of hospital was 21.9 days for patients who stopped taking ACE inhibitors/ARBs and 22.9 days for patients who continued taking these medications. The average difference between groups was –1.1 days.
The average ratio of days alive and out of hospital between the suspending and continuing groups was 0.95 (95% CI, 0.90-1.01; P = .09).
The proportion of patients alive and out of hospital by the end of 30 days in the suspending ACE inhibitor/ARB group was 91.8% versus 95% in the continuing group.
A similar 30-day mortality rate was seen for patients who continued and those who suspended ACE inhibitor/ARB therapy, at 2.8% and 2.7%, respectively (hazard ratio, 0.97). The median number of days that patients were alive and out of hospital was 25 in both groups.
Dr. Lopes said that there was no difference between the two groups with regard to many other secondary outcomes. These included COVID-19 disease progression (need for intubation, ventilation, need for vasoactive drugs, or imaging results) and cardiovascular endpoints (MI, stroke, thromboembolic events, worsening heart failure, myocarditis, or hypertensive crisis).
“Our results endorse with reliable and more definitive data what most medical and cardiovascular societies are recommending – that patients do not stop ACE inhibitor or ARB medication. This has been based on observational data so far, but BRACE CORONA now provides randomized data to support this recommendation,” Dr. Lopes concluded.
Dr. Lopes noted that several subgroups had been prespecified for analysis. Factors included age, obesity, difference between ACE inhibitors/ARBs, difference in oxygen saturation at presentation, time since COVID-19 symptom onset, degree of lung involvement on CT, and symptom severity on presentation.
“We saw very consistent effects of our main findings across all these subgroups, and we plan to report more details of these in the near future,” he said.
Protective for older patients?
The discussant of the study at the ESC Hotline session, Gianfranco Parati, MD, University of Milan-Bicocca and San Luca Hospital, Milan, congratulated Lopes and his team for conducting this important trial at such a difficult time.
He pointed out that patients in the BRACE CORONA trial were quite young (average age, 56 years) and that observational data so far suggest that ACE inhibitors and ARBs have a stronger protective effect in older COVID-19 patients.
He also noted that the percentage of patients alive and out of hospital at 30 days was higher for the patients who continued on treatment in this study (95% vs. 91.8%), which suggested an advantage in maintaining the medication.
Dr. Lopes replied that one-quarter of the population in the BRACE CORONA trial was older than 65 years, which he said was a “reasonable number.”
“Subgroup analysis by age did not show a significant interaction, but the effect of continuing treatment does seem to be more favorable in older patients and also in those who were sicker and had more comorbidities,” he added.
Dr. Parati also suggested that it would have been difficult to discern differences between ACE inhibitors and ARBs in the BRACE CORONA trial, because so few patents were taking ACE inhibitors; the follow-up period of 30 days was relatively short, inasmuch as these drugs may have long-term effects; and it would have been difficult to show differences in the main outcomes used in the study – mortality and time out of hospital – in these patients with mild to moderate disease.
Franz H. Messerli, MD, and Christoph Gräni, MD, University of Bern (Switzerland), said in a joint statement: “The BRACE CORONA trial provides answers to what we know from retrospective studies: if you have already COVID, don’t stop renin-angiotensin system blocker medication.”
But they added that the study does not answer the question about the risk/benefit of ACE inhibitors or ARBs with regard to possible enhanced viral entry through the ACE2 receptor. “What about all those on these drugs who are not infected with COVID? Do they need to stop them? We simply don’t know yet,” they said.
Dr. Messerli and Dr. Gräni added that they would like to see a study that compared patients before SARS-CoV-2 infection who were without hypertension, patients with hypertension who were taking ACE inhibitors or ARBs, and patients with hypertension taking other antihypertensive drugs.
The BRACE CORONA trial was sponsored by D’Or Institute for Research and Education and the Brazilian Clinical Research Institute. Dr. Lopes has disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
The first randomized study to compare continuing versus stopping ACE inhibitors or angiotensin receptor blockers (ARBs) for patients with COVID-19 has shown no difference in key outcomes between the two approaches.
The BRACE CORONA trial – conducted in patients had been taking an ACE inhibitor or an ARB on a long-term basis and who were subsequently hospitalized with COVID-19 – showed no difference in the primary endpoint of number of days alive and out of hospital among those whose medication was suspended for 30 days and those who continued undergoing treatment with these agents.
“Because these data indicate that there is no clinical benefit from routinely interrupting these medications in hospitalized patients with mild to moderate COVID-19, they should generally be continued for those with an indication,” principal investigator Renato Lopes, MD, of Duke Clinical Research Institute, Durham, N.C., concluded.
The BRACE CORONA trial was presented at the European Society of Cardiology Congress 2020 on Sept. 1.
Dr. Lopes explained that there are two conflicting hypotheses about the role of ACE inhibitors and ARBs in COVID-19.
One hypothesis suggests that use of these drugs could be harmful by increasing the expression of ACE2 receptors (which the SARS-CoV-2 virus uses to gain entry into cells), thus potentially enhancing viral binding and viral entry. The other suggests that ACE inhibitors and ARBs could be protective by reducing production of angiotensin II and enhancing the generation of angiotensin 1-7, which attenuates inflammation and fibrosis and therefore could attenuate lung injury.
The BRACE CORONA trial was an academic-led randomized study that tested two strategies: temporarily stopping the ACE inhibitor/ARB for 30 days or continuing these drugs for patients who had been taking these medications on a long-term basis and were hospitalized with a confirmed diagnosis of COVID-19.
The primary outcome was the number of days alive and out of hospital at 30 days. Patients who were using more than three antihypertensive drugs or sacubitril/valsartan or who were hemodynamically unstable at presentation were excluded from the study.
The trial enrolled 659 patients from 29 sites in Brazil. The mean age of patients was 56 years, 40% were women, and 52% were obese. ACE inhibitors were being taken by 15% of the trial participants; ARBs were being taken by 85%. The median duration of ACE inhibitor/ARB treatment was 5 years.
Patients were a median of 6 days from COVID-19 symptom onset. For 30% of the patients, oxygen saturation was below 94% at entry. In terms of COVID-19 symptoms, 57% were classified as mild, and 43% as moderate.
Those with severe COVID-19 symptoms who needed intubation or vasoactive drugs were excluded. Antihypertensive therapy would generally be discontinued in these patients anyway, Dr. Lopes said.
Results showed that the average number of days alive and out of hospital was 21.9 days for patients who stopped taking ACE inhibitors/ARBs and 22.9 days for patients who continued taking these medications. The average difference between groups was –1.1 days.
The average ratio of days alive and out of hospital between the suspending and continuing groups was 0.95 (95% CI, 0.90-1.01; P = .09).
The proportion of patients alive and out of hospital by the end of 30 days in the suspending ACE inhibitor/ARB group was 91.8% versus 95% in the continuing group.
A similar 30-day mortality rate was seen for patients who continued and those who suspended ACE inhibitor/ARB therapy, at 2.8% and 2.7%, respectively (hazard ratio, 0.97). The median number of days that patients were alive and out of hospital was 25 in both groups.
Dr. Lopes said that there was no difference between the two groups with regard to many other secondary outcomes. These included COVID-19 disease progression (need for intubation, ventilation, need for vasoactive drugs, or imaging results) and cardiovascular endpoints (MI, stroke, thromboembolic events, worsening heart failure, myocarditis, or hypertensive crisis).
“Our results endorse with reliable and more definitive data what most medical and cardiovascular societies are recommending – that patients do not stop ACE inhibitor or ARB medication. This has been based on observational data so far, but BRACE CORONA now provides randomized data to support this recommendation,” Dr. Lopes concluded.
Dr. Lopes noted that several subgroups had been prespecified for analysis. Factors included age, obesity, difference between ACE inhibitors/ARBs, difference in oxygen saturation at presentation, time since COVID-19 symptom onset, degree of lung involvement on CT, and symptom severity on presentation.
“We saw very consistent effects of our main findings across all these subgroups, and we plan to report more details of these in the near future,” he said.
Protective for older patients?
The discussant of the study at the ESC Hotline session, Gianfranco Parati, MD, University of Milan-Bicocca and San Luca Hospital, Milan, congratulated Lopes and his team for conducting this important trial at such a difficult time.
He pointed out that patients in the BRACE CORONA trial were quite young (average age, 56 years) and that observational data so far suggest that ACE inhibitors and ARBs have a stronger protective effect in older COVID-19 patients.
He also noted that the percentage of patients alive and out of hospital at 30 days was higher for the patients who continued on treatment in this study (95% vs. 91.8%), which suggested an advantage in maintaining the medication.
Dr. Lopes replied that one-quarter of the population in the BRACE CORONA trial was older than 65 years, which he said was a “reasonable number.”
“Subgroup analysis by age did not show a significant interaction, but the effect of continuing treatment does seem to be more favorable in older patients and also in those who were sicker and had more comorbidities,” he added.
Dr. Parati also suggested that it would have been difficult to discern differences between ACE inhibitors and ARBs in the BRACE CORONA trial, because so few patents were taking ACE inhibitors; the follow-up period of 30 days was relatively short, inasmuch as these drugs may have long-term effects; and it would have been difficult to show differences in the main outcomes used in the study – mortality and time out of hospital – in these patients with mild to moderate disease.
Franz H. Messerli, MD, and Christoph Gräni, MD, University of Bern (Switzerland), said in a joint statement: “The BRACE CORONA trial provides answers to what we know from retrospective studies: if you have already COVID, don’t stop renin-angiotensin system blocker medication.”
But they added that the study does not answer the question about the risk/benefit of ACE inhibitors or ARBs with regard to possible enhanced viral entry through the ACE2 receptor. “What about all those on these drugs who are not infected with COVID? Do they need to stop them? We simply don’t know yet,” they said.
Dr. Messerli and Dr. Gräni added that they would like to see a study that compared patients before SARS-CoV-2 infection who were without hypertension, patients with hypertension who were taking ACE inhibitors or ARBs, and patients with hypertension taking other antihypertensive drugs.
The BRACE CORONA trial was sponsored by D’Or Institute for Research and Education and the Brazilian Clinical Research Institute. Dr. Lopes has disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
The first randomized study to compare continuing versus stopping ACE inhibitors or angiotensin receptor blockers (ARBs) for patients with COVID-19 has shown no difference in key outcomes between the two approaches.
The BRACE CORONA trial – conducted in patients had been taking an ACE inhibitor or an ARB on a long-term basis and who were subsequently hospitalized with COVID-19 – showed no difference in the primary endpoint of number of days alive and out of hospital among those whose medication was suspended for 30 days and those who continued undergoing treatment with these agents.
“Because these data indicate that there is no clinical benefit from routinely interrupting these medications in hospitalized patients with mild to moderate COVID-19, they should generally be continued for those with an indication,” principal investigator Renato Lopes, MD, of Duke Clinical Research Institute, Durham, N.C., concluded.
The BRACE CORONA trial was presented at the European Society of Cardiology Congress 2020 on Sept. 1.
Dr. Lopes explained that there are two conflicting hypotheses about the role of ACE inhibitors and ARBs in COVID-19.
One hypothesis suggests that use of these drugs could be harmful by increasing the expression of ACE2 receptors (which the SARS-CoV-2 virus uses to gain entry into cells), thus potentially enhancing viral binding and viral entry. The other suggests that ACE inhibitors and ARBs could be protective by reducing production of angiotensin II and enhancing the generation of angiotensin 1-7, which attenuates inflammation and fibrosis and therefore could attenuate lung injury.
The BRACE CORONA trial was an academic-led randomized study that tested two strategies: temporarily stopping the ACE inhibitor/ARB for 30 days or continuing these drugs for patients who had been taking these medications on a long-term basis and were hospitalized with a confirmed diagnosis of COVID-19.
The primary outcome was the number of days alive and out of hospital at 30 days. Patients who were using more than three antihypertensive drugs or sacubitril/valsartan or who were hemodynamically unstable at presentation were excluded from the study.
The trial enrolled 659 patients from 29 sites in Brazil. The mean age of patients was 56 years, 40% were women, and 52% were obese. ACE inhibitors were being taken by 15% of the trial participants; ARBs were being taken by 85%. The median duration of ACE inhibitor/ARB treatment was 5 years.
Patients were a median of 6 days from COVID-19 symptom onset. For 30% of the patients, oxygen saturation was below 94% at entry. In terms of COVID-19 symptoms, 57% were classified as mild, and 43% as moderate.
Those with severe COVID-19 symptoms who needed intubation or vasoactive drugs were excluded. Antihypertensive therapy would generally be discontinued in these patients anyway, Dr. Lopes said.
Results showed that the average number of days alive and out of hospital was 21.9 days for patients who stopped taking ACE inhibitors/ARBs and 22.9 days for patients who continued taking these medications. The average difference between groups was –1.1 days.
The average ratio of days alive and out of hospital between the suspending and continuing groups was 0.95 (95% CI, 0.90-1.01; P = .09).
The proportion of patients alive and out of hospital by the end of 30 days in the suspending ACE inhibitor/ARB group was 91.8% versus 95% in the continuing group.
A similar 30-day mortality rate was seen for patients who continued and those who suspended ACE inhibitor/ARB therapy, at 2.8% and 2.7%, respectively (hazard ratio, 0.97). The median number of days that patients were alive and out of hospital was 25 in both groups.
Dr. Lopes said that there was no difference between the two groups with regard to many other secondary outcomes. These included COVID-19 disease progression (need for intubation, ventilation, need for vasoactive drugs, or imaging results) and cardiovascular endpoints (MI, stroke, thromboembolic events, worsening heart failure, myocarditis, or hypertensive crisis).
“Our results endorse with reliable and more definitive data what most medical and cardiovascular societies are recommending – that patients do not stop ACE inhibitor or ARB medication. This has been based on observational data so far, but BRACE CORONA now provides randomized data to support this recommendation,” Dr. Lopes concluded.
Dr. Lopes noted that several subgroups had been prespecified for analysis. Factors included age, obesity, difference between ACE inhibitors/ARBs, difference in oxygen saturation at presentation, time since COVID-19 symptom onset, degree of lung involvement on CT, and symptom severity on presentation.
“We saw very consistent effects of our main findings across all these subgroups, and we plan to report more details of these in the near future,” he said.
Protective for older patients?
The discussant of the study at the ESC Hotline session, Gianfranco Parati, MD, University of Milan-Bicocca and San Luca Hospital, Milan, congratulated Lopes and his team for conducting this important trial at such a difficult time.
He pointed out that patients in the BRACE CORONA trial were quite young (average age, 56 years) and that observational data so far suggest that ACE inhibitors and ARBs have a stronger protective effect in older COVID-19 patients.
He also noted that the percentage of patients alive and out of hospital at 30 days was higher for the patients who continued on treatment in this study (95% vs. 91.8%), which suggested an advantage in maintaining the medication.
Dr. Lopes replied that one-quarter of the population in the BRACE CORONA trial was older than 65 years, which he said was a “reasonable number.”
“Subgroup analysis by age did not show a significant interaction, but the effect of continuing treatment does seem to be more favorable in older patients and also in those who were sicker and had more comorbidities,” he added.
Dr. Parati also suggested that it would have been difficult to discern differences between ACE inhibitors and ARBs in the BRACE CORONA trial, because so few patents were taking ACE inhibitors; the follow-up period of 30 days was relatively short, inasmuch as these drugs may have long-term effects; and it would have been difficult to show differences in the main outcomes used in the study – mortality and time out of hospital – in these patients with mild to moderate disease.
Franz H. Messerli, MD, and Christoph Gräni, MD, University of Bern (Switzerland), said in a joint statement: “The BRACE CORONA trial provides answers to what we know from retrospective studies: if you have already COVID, don’t stop renin-angiotensin system blocker medication.”
But they added that the study does not answer the question about the risk/benefit of ACE inhibitors or ARBs with regard to possible enhanced viral entry through the ACE2 receptor. “What about all those on these drugs who are not infected with COVID? Do they need to stop them? We simply don’t know yet,” they said.
Dr. Messerli and Dr. Gräni added that they would like to see a study that compared patients before SARS-CoV-2 infection who were without hypertension, patients with hypertension who were taking ACE inhibitors or ARBs, and patients with hypertension taking other antihypertensive drugs.
The BRACE CORONA trial was sponsored by D’Or Institute for Research and Education and the Brazilian Clinical Research Institute. Dr. Lopes has disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
HOME-PE trial clarifies which pulmonary embolism patients to treat at home
The pragmatic Hestia criteria proved as safe as the more structured, points-based simplified Pulmonary Embolism Severity Index (sPESI) score for selection of patients with acute pulmonary embolism for outpatient care in the large, randomized HOME-PE trial presented at the virtual annual congress of the European Society of Cardiology.
“These results support outpatient management of acute pulmonary embolism patients using either the Hestia method or the sPESI score with the option for the physician-in-charge to override the decision. In hospitals organized for outpatient management, both triaging strategies enable more than a third of pulmonary embolism patients to be managed at home with a low rate of complications,” Pierre-Marie Roy, MD, said in presenting the HOME-PE findings.
The study clarifies a transatlantic controversy regarding how best to triage patients with acute pulmonary embolism (PE) for outpatient care. The answer? It’s basically a tie between the points-based sPESI score recommended in the current ESC guidelines (Eur Respir J. 2019 Oct 9;54[3]:1901647) and the Hestia method endorsed in the American College of Chest Physician guidelines (Chest. 2016 Feb;149[2]:315-52).
The sPESI is a validated tool that grants 1 point each for age over 80 years, background cardiopulmonary disease, a systolic blood pressure below 100 mm Hg, cancer, a heart rate of 110 bpm or more, and an oxygen saturation level below 90%. A patient needs a score of zero to be eligible for outpatient management. In contrast, the Hestia method relies upon 11 simple bedside criteria rather than a points system, explained Dr. Roy of University Hospital of Angers, France (J Thromb Haemost. 2011 Aug;9[8]:1500-7).
HOME-PE was a randomized, open-label, noninferiority trial conducted at 26 hospitals in France, Belgium, Switzerland, and the Netherlands. The study included 1,974 patients presenting to the emergency department with non–high-risk acute PE as defined by hemodynamic stability. About 39% of patients in the Hestia group were eligible for outpatient care on the basis of ‘no’ answers regarding all 11 criteria, while 48% of patients had an sPESI score of 0 and were thus initially considered appropriate for outpatient management.
However, the investigators recognized that no scoring system for acute PE is perfect, and that the judgment of a physician with extensive experience in managing this life-threatening condition counts for a lot. So they stipulated that a patient’s physician-in-charge could overrule a decision for early discharge. This happened 29% of the time in patients with a sPESI score of 0, as compared with a 3% overrule rate with the Hestia rule. The physician-in-charge also moved small numbers of patients who were Hestia or sPESI positive into the outpatient care group. As a result, a similar proportion of patients in both groups were discharged home within 24 hours for outpatient treatment: 38% of the total Hestia group and 37% in the sPESI arm.
Major adverse event rates were reassuringly low in both groups managed on an outpatient basis. The composite of recurrent venous thromboembolism, bleeding, or death within 30 days occurred in 1.3% of Hestia outpatients and 1.1% of sPESI outpatients. Among patients managed in the hospital, these rates were 5.6% in the Hestia group and 4.7% in the sPESI group.
Discussant Stavros V. Konstantinides, MD, who chaired the ESC guideline committee, asked rhetorically, “who’s happy with the HOME-PE trial? I think everybody.”
“The Hestia criteria integrate the feasibility of family support of the individual patient. This is a good thing. And eligibility based on the Hestia criteria, unlike sPESI, does not require age younger than 80 years or no cancer, and it appears from the HOME-PE study that this is okay,” observed Dr. Konstantinides of the Center for Thrombosis and Hemostasis at the University of Mainz (Germany).
In an interview, Hadley Wilson, MD, called the HOME-PE trial “transformative” and predicted it will change clinical practice. He was particularly impressed with the high quality of the trial, noting that 87% of participants managed as outpatients received a direct oral anticoagulant.
The Hestia rule is simpler and more user-friendly. And greater use of this triaging strategy might have advantages in terms of economics and health care utilization by potentially encouraging movement of decision-making regarding outpatient management of acute PE out of the hospital wards and into emergency departments, said Dr. Wilson, executive vice chair of the Sanger Heart and Vascular Institute and a cardiologist at the University of North Carolina at Chapel Hill.
Dr. Roy reported receiving research grants to conduct HOME-PE from the French Ministry of Health, the study sponsor. In addition, he is on scientific advisory boards and/or speakers’ panels for Bayer, Boehringer Ingelheim, Bristol-Myers Squibb, Pfizer, Aspen, Daiichi Sankyo, and Sanofi Aventis.
The pragmatic Hestia criteria proved as safe as the more structured, points-based simplified Pulmonary Embolism Severity Index (sPESI) score for selection of patients with acute pulmonary embolism for outpatient care in the large, randomized HOME-PE trial presented at the virtual annual congress of the European Society of Cardiology.
“These results support outpatient management of acute pulmonary embolism patients using either the Hestia method or the sPESI score with the option for the physician-in-charge to override the decision. In hospitals organized for outpatient management, both triaging strategies enable more than a third of pulmonary embolism patients to be managed at home with a low rate of complications,” Pierre-Marie Roy, MD, said in presenting the HOME-PE findings.
The study clarifies a transatlantic controversy regarding how best to triage patients with acute pulmonary embolism (PE) for outpatient care. The answer? It’s basically a tie between the points-based sPESI score recommended in the current ESC guidelines (Eur Respir J. 2019 Oct 9;54[3]:1901647) and the Hestia method endorsed in the American College of Chest Physician guidelines (Chest. 2016 Feb;149[2]:315-52).
The sPESI is a validated tool that grants 1 point each for age over 80 years, background cardiopulmonary disease, a systolic blood pressure below 100 mm Hg, cancer, a heart rate of 110 bpm or more, and an oxygen saturation level below 90%. A patient needs a score of zero to be eligible for outpatient management. In contrast, the Hestia method relies upon 11 simple bedside criteria rather than a points system, explained Dr. Roy of University Hospital of Angers, France (J Thromb Haemost. 2011 Aug;9[8]:1500-7).
HOME-PE was a randomized, open-label, noninferiority trial conducted at 26 hospitals in France, Belgium, Switzerland, and the Netherlands. The study included 1,974 patients presenting to the emergency department with non–high-risk acute PE as defined by hemodynamic stability. About 39% of patients in the Hestia group were eligible for outpatient care on the basis of ‘no’ answers regarding all 11 criteria, while 48% of patients had an sPESI score of 0 and were thus initially considered appropriate for outpatient management.
However, the investigators recognized that no scoring system for acute PE is perfect, and that the judgment of a physician with extensive experience in managing this life-threatening condition counts for a lot. So they stipulated that a patient’s physician-in-charge could overrule a decision for early discharge. This happened 29% of the time in patients with a sPESI score of 0, as compared with a 3% overrule rate with the Hestia rule. The physician-in-charge also moved small numbers of patients who were Hestia or sPESI positive into the outpatient care group. As a result, a similar proportion of patients in both groups were discharged home within 24 hours for outpatient treatment: 38% of the total Hestia group and 37% in the sPESI arm.
Major adverse event rates were reassuringly low in both groups managed on an outpatient basis. The composite of recurrent venous thromboembolism, bleeding, or death within 30 days occurred in 1.3% of Hestia outpatients and 1.1% of sPESI outpatients. Among patients managed in the hospital, these rates were 5.6% in the Hestia group and 4.7% in the sPESI group.
Discussant Stavros V. Konstantinides, MD, who chaired the ESC guideline committee, asked rhetorically, “who’s happy with the HOME-PE trial? I think everybody.”
“The Hestia criteria integrate the feasibility of family support of the individual patient. This is a good thing. And eligibility based on the Hestia criteria, unlike sPESI, does not require age younger than 80 years or no cancer, and it appears from the HOME-PE study that this is okay,” observed Dr. Konstantinides of the Center for Thrombosis and Hemostasis at the University of Mainz (Germany).
In an interview, Hadley Wilson, MD, called the HOME-PE trial “transformative” and predicted it will change clinical practice. He was particularly impressed with the high quality of the trial, noting that 87% of participants managed as outpatients received a direct oral anticoagulant.
The Hestia rule is simpler and more user-friendly. And greater use of this triaging strategy might have advantages in terms of economics and health care utilization by potentially encouraging movement of decision-making regarding outpatient management of acute PE out of the hospital wards and into emergency departments, said Dr. Wilson, executive vice chair of the Sanger Heart and Vascular Institute and a cardiologist at the University of North Carolina at Chapel Hill.
Dr. Roy reported receiving research grants to conduct HOME-PE from the French Ministry of Health, the study sponsor. In addition, he is on scientific advisory boards and/or speakers’ panels for Bayer, Boehringer Ingelheim, Bristol-Myers Squibb, Pfizer, Aspen, Daiichi Sankyo, and Sanofi Aventis.
The pragmatic Hestia criteria proved as safe as the more structured, points-based simplified Pulmonary Embolism Severity Index (sPESI) score for selection of patients with acute pulmonary embolism for outpatient care in the large, randomized HOME-PE trial presented at the virtual annual congress of the European Society of Cardiology.
“These results support outpatient management of acute pulmonary embolism patients using either the Hestia method or the sPESI score with the option for the physician-in-charge to override the decision. In hospitals organized for outpatient management, both triaging strategies enable more than a third of pulmonary embolism patients to be managed at home with a low rate of complications,” Pierre-Marie Roy, MD, said in presenting the HOME-PE findings.
The study clarifies a transatlantic controversy regarding how best to triage patients with acute pulmonary embolism (PE) for outpatient care. The answer? It’s basically a tie between the points-based sPESI score recommended in the current ESC guidelines (Eur Respir J. 2019 Oct 9;54[3]:1901647) and the Hestia method endorsed in the American College of Chest Physician guidelines (Chest. 2016 Feb;149[2]:315-52).
The sPESI is a validated tool that grants 1 point each for age over 80 years, background cardiopulmonary disease, a systolic blood pressure below 100 mm Hg, cancer, a heart rate of 110 bpm or more, and an oxygen saturation level below 90%. A patient needs a score of zero to be eligible for outpatient management. In contrast, the Hestia method relies upon 11 simple bedside criteria rather than a points system, explained Dr. Roy of University Hospital of Angers, France (J Thromb Haemost. 2011 Aug;9[8]:1500-7).
HOME-PE was a randomized, open-label, noninferiority trial conducted at 26 hospitals in France, Belgium, Switzerland, and the Netherlands. The study included 1,974 patients presenting to the emergency department with non–high-risk acute PE as defined by hemodynamic stability. About 39% of patients in the Hestia group were eligible for outpatient care on the basis of ‘no’ answers regarding all 11 criteria, while 48% of patients had an sPESI score of 0 and were thus initially considered appropriate for outpatient management.
However, the investigators recognized that no scoring system for acute PE is perfect, and that the judgment of a physician with extensive experience in managing this life-threatening condition counts for a lot. So they stipulated that a patient’s physician-in-charge could overrule a decision for early discharge. This happened 29% of the time in patients with a sPESI score of 0, as compared with a 3% overrule rate with the Hestia rule. The physician-in-charge also moved small numbers of patients who were Hestia or sPESI positive into the outpatient care group. As a result, a similar proportion of patients in both groups were discharged home within 24 hours for outpatient treatment: 38% of the total Hestia group and 37% in the sPESI arm.
Major adverse event rates were reassuringly low in both groups managed on an outpatient basis. The composite of recurrent venous thromboembolism, bleeding, or death within 30 days occurred in 1.3% of Hestia outpatients and 1.1% of sPESI outpatients. Among patients managed in the hospital, these rates were 5.6% in the Hestia group and 4.7% in the sPESI group.
Discussant Stavros V. Konstantinides, MD, who chaired the ESC guideline committee, asked rhetorically, “who’s happy with the HOME-PE trial? I think everybody.”
“The Hestia criteria integrate the feasibility of family support of the individual patient. This is a good thing. And eligibility based on the Hestia criteria, unlike sPESI, does not require age younger than 80 years or no cancer, and it appears from the HOME-PE study that this is okay,” observed Dr. Konstantinides of the Center for Thrombosis and Hemostasis at the University of Mainz (Germany).
In an interview, Hadley Wilson, MD, called the HOME-PE trial “transformative” and predicted it will change clinical practice. He was particularly impressed with the high quality of the trial, noting that 87% of participants managed as outpatients received a direct oral anticoagulant.
The Hestia rule is simpler and more user-friendly. And greater use of this triaging strategy might have advantages in terms of economics and health care utilization by potentially encouraging movement of decision-making regarding outpatient management of acute PE out of the hospital wards and into emergency departments, said Dr. Wilson, executive vice chair of the Sanger Heart and Vascular Institute and a cardiologist at the University of North Carolina at Chapel Hill.
Dr. Roy reported receiving research grants to conduct HOME-PE from the French Ministry of Health, the study sponsor. In addition, he is on scientific advisory boards and/or speakers’ panels for Bayer, Boehringer Ingelheim, Bristol-Myers Squibb, Pfizer, Aspen, Daiichi Sankyo, and Sanofi Aventis.
REPORTING FROM ESC CONGRESS 2020
ESC’s revised NSTE-ACS guidelines embrace hsT, personalized anti-ischemia treatments
The first revision since 2015 to the European Society of Cardiology’s guidelines for diagnosing and managing non ST-elevation acute coronary syndrome placed increased reliance on high-sensitivity cardiac troponin testing for diagnosis, and embraced coronary CT to rule out lower-risk patients.
It also highlighted the need for personalized antiplatelet regimens, systems of care, and quality improvement.
The society released the new guidelines on August 29 (Eur Heart J. 2020 Aug 29;doi: 10.1093/eurheartj/ehaa575), and then devoted a session to them the next day at the virtual annual congress of the European Society of Cardiology to highlight some of the key updates, starting with the further emphasis placed on high-sensitivity cardiac troponin (hs-cTn), a reliance that contrasts with what remains inconsistent use of this metric in U.S. practice.
An hs-cTn test is the “preferred” diagnostic test and a “key” testing element, said Marco Roffi, MD, professor and director of interventional cardiology at University Hospital, Geneva, and a member of the guideline committee. He also stressed an update to the time frame of initial hs-cTn testing, which now involves a baseline reading and then a second measure after 2 hours to discern how the marker level is evolving with time. The guidelines advise against measuring any other biomarker of myocardial injury.
U.S. lags in measuring high-sensitivity cardiac troponin
U.S. medical systems and centers “are not uniform in adopting hs-cTn,” noted Richard J. Kovacs, MD, professor of cardiology at the Indiana University School of Medicine in Indianapolis. “The new European guidelines should spur U.S. institutions to at least take a close look at the advantages of hs-cTn. There is a strong case that it leads to more efficient emergency care and allows for quicker decisions and triage,” added Dr. Kovacs in an interview.
The new guideline’s emphasis on hs-cTn should hasten broader uptake in U.S. practice, agreed Deepak L. Bhatt, MD, professor of medicine at Harvard Medical School in Boston and a member of the guideline-writing panel
Another plus of the guidelines is its endorsement of an “organized approach to risk assessment” early on in these patients, said Dr. Kovacs, who is also the immediate past-president of the American College of Cardiology (ACC). An ACC committee is developing a new set of recommendations for managing patients with cardiac chest pain and is on track for release in 2021. It would represent the first update to U.S. guidelines for non ST-elevation ACS patients since 2014.
The new ESC guidelines give coronary CT angiography a class Ia rating as an alternative to invasive coronary angiography for assessing patients with a low or intermediate risk of having coronary disease, a “tremendous upgrade,” commented Ashish Pershad, MD, an interventional cardiologist at Banner-University Medical Heart Institute in Phoenix. While he welcomes this support for using coronary CT angiography in this setting, he acknowledged that the method remains limited in availability as it requires highly trained technicians to obtain good images and experienced clinicians to interpret the results.
Personalizing antiplatelet and antithrombotic treatments
Notable revisions to medical treatments to minimize ischemia included an admonition not to use routine pretreatment with a P2Y12 receptor inhibitor (such as clopidogrel) before testing determines coronary anatomy.
Not using one of these antiplatelet drugs upfront on all patients “is a tremendous change,” Dr. Pershad said in an interview. Many patients currently get these drugs while awaiting an angiogram, but a more selected and deferred antiplatelet approach would be better when angiography shows that some patients need coronary bypass surgery, he noted. Recent study results have shown no added benefit from pretreatment, and its use can be especially problematic for patients who are slated for a planned invasive strategy, said Dr. Bhatt.
Dr. Pershad, Dr. Bhatt, and Dr. Kovacs all praised the overall emphasis on a personalized approach to treating patients with antiplatelet and antithrombotic drugs, with endorsement of a flexible approach to treatment intensity and duration. The guideline acknowledges the need to take into account a patient’s bleeding risk and comorbidities, and specifically endorsed the Academic Research Consortium’s formula for identifying and stratifying high bleeding risk (Eur Heart J. 2019 Aug 14;40[31]:2632-53).
The new guidelines also provide guidance on how to apply recent study results that addressed balancing efficacy and safety when pairing an antiplatelet drug with a direct-acting oral anticoagulant (DOAC) for patients who potentially need both drug classes, such as patients with atrial fibrillation and a recent ACS event. “It’s tremendous to get clarity on this issue; there’s been a lot of uncertainty,” said Dr. Pershad. The guidelines call for a week of triple therapy with a DOAC, aspirin, and a second antiplatelet drug, followed by 12 months on a DOAC plus a single antiplatelet drug, and then the DOAC alone as the “default” strategy for most patients, but also presents alternative options for patients with high risk for either bleeding or ischemia.
The new guidelines also give much-needed direction on how to apply an invasive strategy, with an emphasis on immediate intervention for within 2 hours for very-high-risk patients, and early intervention within 24 hours for high-risk patients. Adhering to this timetable can mean increasing catheter laboratory availability on an urgent basis over weekends, Dr. Bhatt noted.
Improving quality of care
A novel section in the new guidelines was devoted to nine quality measures that can help health systems and medical centers monitor their adherence to the guideline recommendations, track their performance relative to peer institutions, and follow changes in performance that result from quality improvement steps. It’s something of a “futuristic” step for a guideline to take, with a goal of persuading administrators to implement tracking of these measures and improve patient outcomes, noted Dr. Bhatt.
“It’s very important to see that this is not just a set of guidelines but also a tool to improve quality of care,” commented Dr. Kovacs. The key to success in this effort will be to follow registered patients, set benchmarks that systems can aspire to achieve, and use this to improve the quality of care.
Until now, optimizing care for patients with NSTE-ACS has been “challenging,” he said. “The focus must be on moving toward systems of care” that can provide consistent patient evaluation and care, and do it quickly, said Dr. Kovacs.
Dr. Roffi has received research funding from Biotronik, Boston Scientific, GE Healthcare, and Medtronic. Dr. Kovacs was formerly an employee of Eli Lilly. Dr. Bhatt has been a consultant to and has received research funding from several companies. Dr. Pershad had no disclosures.
The first revision since 2015 to the European Society of Cardiology’s guidelines for diagnosing and managing non ST-elevation acute coronary syndrome placed increased reliance on high-sensitivity cardiac troponin testing for diagnosis, and embraced coronary CT to rule out lower-risk patients.
It also highlighted the need for personalized antiplatelet regimens, systems of care, and quality improvement.
The society released the new guidelines on August 29 (Eur Heart J. 2020 Aug 29;doi: 10.1093/eurheartj/ehaa575), and then devoted a session to them the next day at the virtual annual congress of the European Society of Cardiology to highlight some of the key updates, starting with the further emphasis placed on high-sensitivity cardiac troponin (hs-cTn), a reliance that contrasts with what remains inconsistent use of this metric in U.S. practice.
An hs-cTn test is the “preferred” diagnostic test and a “key” testing element, said Marco Roffi, MD, professor and director of interventional cardiology at University Hospital, Geneva, and a member of the guideline committee. He also stressed an update to the time frame of initial hs-cTn testing, which now involves a baseline reading and then a second measure after 2 hours to discern how the marker level is evolving with time. The guidelines advise against measuring any other biomarker of myocardial injury.
U.S. lags in measuring high-sensitivity cardiac troponin
U.S. medical systems and centers “are not uniform in adopting hs-cTn,” noted Richard J. Kovacs, MD, professor of cardiology at the Indiana University School of Medicine in Indianapolis. “The new European guidelines should spur U.S. institutions to at least take a close look at the advantages of hs-cTn. There is a strong case that it leads to more efficient emergency care and allows for quicker decisions and triage,” added Dr. Kovacs in an interview.
The new guideline’s emphasis on hs-cTn should hasten broader uptake in U.S. practice, agreed Deepak L. Bhatt, MD, professor of medicine at Harvard Medical School in Boston and a member of the guideline-writing panel
Another plus of the guidelines is its endorsement of an “organized approach to risk assessment” early on in these patients, said Dr. Kovacs, who is also the immediate past-president of the American College of Cardiology (ACC). An ACC committee is developing a new set of recommendations for managing patients with cardiac chest pain and is on track for release in 2021. It would represent the first update to U.S. guidelines for non ST-elevation ACS patients since 2014.
The new ESC guidelines give coronary CT angiography a class Ia rating as an alternative to invasive coronary angiography for assessing patients with a low or intermediate risk of having coronary disease, a “tremendous upgrade,” commented Ashish Pershad, MD, an interventional cardiologist at Banner-University Medical Heart Institute in Phoenix. While he welcomes this support for using coronary CT angiography in this setting, he acknowledged that the method remains limited in availability as it requires highly trained technicians to obtain good images and experienced clinicians to interpret the results.
Personalizing antiplatelet and antithrombotic treatments
Notable revisions to medical treatments to minimize ischemia included an admonition not to use routine pretreatment with a P2Y12 receptor inhibitor (such as clopidogrel) before testing determines coronary anatomy.
Not using one of these antiplatelet drugs upfront on all patients “is a tremendous change,” Dr. Pershad said in an interview. Many patients currently get these drugs while awaiting an angiogram, but a more selected and deferred antiplatelet approach would be better when angiography shows that some patients need coronary bypass surgery, he noted. Recent study results have shown no added benefit from pretreatment, and its use can be especially problematic for patients who are slated for a planned invasive strategy, said Dr. Bhatt.
Dr. Pershad, Dr. Bhatt, and Dr. Kovacs all praised the overall emphasis on a personalized approach to treating patients with antiplatelet and antithrombotic drugs, with endorsement of a flexible approach to treatment intensity and duration. The guideline acknowledges the need to take into account a patient’s bleeding risk and comorbidities, and specifically endorsed the Academic Research Consortium’s formula for identifying and stratifying high bleeding risk (Eur Heart J. 2019 Aug 14;40[31]:2632-53).
The new guidelines also provide guidance on how to apply recent study results that addressed balancing efficacy and safety when pairing an antiplatelet drug with a direct-acting oral anticoagulant (DOAC) for patients who potentially need both drug classes, such as patients with atrial fibrillation and a recent ACS event. “It’s tremendous to get clarity on this issue; there’s been a lot of uncertainty,” said Dr. Pershad. The guidelines call for a week of triple therapy with a DOAC, aspirin, and a second antiplatelet drug, followed by 12 months on a DOAC plus a single antiplatelet drug, and then the DOAC alone as the “default” strategy for most patients, but also presents alternative options for patients with high risk for either bleeding or ischemia.
The new guidelines also give much-needed direction on how to apply an invasive strategy, with an emphasis on immediate intervention for within 2 hours for very-high-risk patients, and early intervention within 24 hours for high-risk patients. Adhering to this timetable can mean increasing catheter laboratory availability on an urgent basis over weekends, Dr. Bhatt noted.
Improving quality of care
A novel section in the new guidelines was devoted to nine quality measures that can help health systems and medical centers monitor their adherence to the guideline recommendations, track their performance relative to peer institutions, and follow changes in performance that result from quality improvement steps. It’s something of a “futuristic” step for a guideline to take, with a goal of persuading administrators to implement tracking of these measures and improve patient outcomes, noted Dr. Bhatt.
“It’s very important to see that this is not just a set of guidelines but also a tool to improve quality of care,” commented Dr. Kovacs. The key to success in this effort will be to follow registered patients, set benchmarks that systems can aspire to achieve, and use this to improve the quality of care.
Until now, optimizing care for patients with NSTE-ACS has been “challenging,” he said. “The focus must be on moving toward systems of care” that can provide consistent patient evaluation and care, and do it quickly, said Dr. Kovacs.
Dr. Roffi has received research funding from Biotronik, Boston Scientific, GE Healthcare, and Medtronic. Dr. Kovacs was formerly an employee of Eli Lilly. Dr. Bhatt has been a consultant to and has received research funding from several companies. Dr. Pershad had no disclosures.
The first revision since 2015 to the European Society of Cardiology’s guidelines for diagnosing and managing non ST-elevation acute coronary syndrome placed increased reliance on high-sensitivity cardiac troponin testing for diagnosis, and embraced coronary CT to rule out lower-risk patients.
It also highlighted the need for personalized antiplatelet regimens, systems of care, and quality improvement.
The society released the new guidelines on August 29 (Eur Heart J. 2020 Aug 29;doi: 10.1093/eurheartj/ehaa575), and then devoted a session to them the next day at the virtual annual congress of the European Society of Cardiology to highlight some of the key updates, starting with the further emphasis placed on high-sensitivity cardiac troponin (hs-cTn), a reliance that contrasts with what remains inconsistent use of this metric in U.S. practice.
An hs-cTn test is the “preferred” diagnostic test and a “key” testing element, said Marco Roffi, MD, professor and director of interventional cardiology at University Hospital, Geneva, and a member of the guideline committee. He also stressed an update to the time frame of initial hs-cTn testing, which now involves a baseline reading and then a second measure after 2 hours to discern how the marker level is evolving with time. The guidelines advise against measuring any other biomarker of myocardial injury.
U.S. lags in measuring high-sensitivity cardiac troponin
U.S. medical systems and centers “are not uniform in adopting hs-cTn,” noted Richard J. Kovacs, MD, professor of cardiology at the Indiana University School of Medicine in Indianapolis. “The new European guidelines should spur U.S. institutions to at least take a close look at the advantages of hs-cTn. There is a strong case that it leads to more efficient emergency care and allows for quicker decisions and triage,” added Dr. Kovacs in an interview.
The new guideline’s emphasis on hs-cTn should hasten broader uptake in U.S. practice, agreed Deepak L. Bhatt, MD, professor of medicine at Harvard Medical School in Boston and a member of the guideline-writing panel
Another plus of the guidelines is its endorsement of an “organized approach to risk assessment” early on in these patients, said Dr. Kovacs, who is also the immediate past-president of the American College of Cardiology (ACC). An ACC committee is developing a new set of recommendations for managing patients with cardiac chest pain and is on track for release in 2021. It would represent the first update to U.S. guidelines for non ST-elevation ACS patients since 2014.
The new ESC guidelines give coronary CT angiography a class Ia rating as an alternative to invasive coronary angiography for assessing patients with a low or intermediate risk of having coronary disease, a “tremendous upgrade,” commented Ashish Pershad, MD, an interventional cardiologist at Banner-University Medical Heart Institute in Phoenix. While he welcomes this support for using coronary CT angiography in this setting, he acknowledged that the method remains limited in availability as it requires highly trained technicians to obtain good images and experienced clinicians to interpret the results.
Personalizing antiplatelet and antithrombotic treatments
Notable revisions to medical treatments to minimize ischemia included an admonition not to use routine pretreatment with a P2Y12 receptor inhibitor (such as clopidogrel) before testing determines coronary anatomy.
Not using one of these antiplatelet drugs upfront on all patients “is a tremendous change,” Dr. Pershad said in an interview. Many patients currently get these drugs while awaiting an angiogram, but a more selected and deferred antiplatelet approach would be better when angiography shows that some patients need coronary bypass surgery, he noted. Recent study results have shown no added benefit from pretreatment, and its use can be especially problematic for patients who are slated for a planned invasive strategy, said Dr. Bhatt.
Dr. Pershad, Dr. Bhatt, and Dr. Kovacs all praised the overall emphasis on a personalized approach to treating patients with antiplatelet and antithrombotic drugs, with endorsement of a flexible approach to treatment intensity and duration. The guideline acknowledges the need to take into account a patient’s bleeding risk and comorbidities, and specifically endorsed the Academic Research Consortium’s formula for identifying and stratifying high bleeding risk (Eur Heart J. 2019 Aug 14;40[31]:2632-53).
The new guidelines also provide guidance on how to apply recent study results that addressed balancing efficacy and safety when pairing an antiplatelet drug with a direct-acting oral anticoagulant (DOAC) for patients who potentially need both drug classes, such as patients with atrial fibrillation and a recent ACS event. “It’s tremendous to get clarity on this issue; there’s been a lot of uncertainty,” said Dr. Pershad. The guidelines call for a week of triple therapy with a DOAC, aspirin, and a second antiplatelet drug, followed by 12 months on a DOAC plus a single antiplatelet drug, and then the DOAC alone as the “default” strategy for most patients, but also presents alternative options for patients with high risk for either bleeding or ischemia.
The new guidelines also give much-needed direction on how to apply an invasive strategy, with an emphasis on immediate intervention for within 2 hours for very-high-risk patients, and early intervention within 24 hours for high-risk patients. Adhering to this timetable can mean increasing catheter laboratory availability on an urgent basis over weekends, Dr. Bhatt noted.
Improving quality of care
A novel section in the new guidelines was devoted to nine quality measures that can help health systems and medical centers monitor their adherence to the guideline recommendations, track their performance relative to peer institutions, and follow changes in performance that result from quality improvement steps. It’s something of a “futuristic” step for a guideline to take, with a goal of persuading administrators to implement tracking of these measures and improve patient outcomes, noted Dr. Bhatt.
“It’s very important to see that this is not just a set of guidelines but also a tool to improve quality of care,” commented Dr. Kovacs. The key to success in this effort will be to follow registered patients, set benchmarks that systems can aspire to achieve, and use this to improve the quality of care.
Until now, optimizing care for patients with NSTE-ACS has been “challenging,” he said. “The focus must be on moving toward systems of care” that can provide consistent patient evaluation and care, and do it quickly, said Dr. Kovacs.
Dr. Roffi has received research funding from Biotronik, Boston Scientific, GE Healthcare, and Medtronic. Dr. Kovacs was formerly an employee of Eli Lilly. Dr. Bhatt has been a consultant to and has received research funding from several companies. Dr. Pershad had no disclosures.
FROM ESC CONGRESS 2020
Evolocumab safe and effective in pediatric FH
The PCSK9 monoclonal antibody evolocumab (Repatha) was well tolerated and effectively lowered LDL cholesterol by 38% compared with placebo in a randomized controlled trial in pediatric patients with heterozygous familial hypercholesterolemia (FH) already taking statins with or without ezetimibe.
“HAUSER-RCT is the largest study and the first placebo-controlled randomized trial of a PCSK9 inhibitor in pediatric FH,” senior author Daniel Gaudet, MD, PhD, Universite de Montreal, said in an interview.
“The study showed good safety and efficacy of the drug in this population, with an excellent 44% reduction in LDL cholesterol compared with 6% in the placebo group.”
The trial also found evolocumab to be well tolerated in this group, with adverse effects similar in the active and placebo groups.
“Some people have wondered about using a drug with a monthly injection in a pediatric population, but this was not an issue in our study,” Dr. Gaudet said. “The idea of a monthly injection was well received, and no patient withdrew because of this.”
The HAUSER-RCT trial was presented on Aug. 29 at the virtual annual congress of the European Society of Cardiology (ESC Congress 2020) and simultaneously published online in the New England Journal of Medicine.
“With patients recruited from 23 countries in five continents, the study provides an accurate picture of the safety and efficacy of evolocumab in pediatric FH patients worldwide,” Dr. Gaudet said.
The 24-week, randomized, double-blind, placebo-controlled trial involved 157 patients aged 10-17 years with heterozygous FH already taking statins with or without ezetimibe and who had an LDL cholesterol level of 130 mg/dL or more and a triglyceride level of 400 mg/dL or less.
They were randomly assigned in a 2:1 ratio to receive monthly subcutaneous injections of evolocumab (420 mg) or placebo.
Results showed that at week 24, the mean percentage change from baseline in LDL cholesterol level was −44.5% in the evolocumab group and −6.2% in the placebo group, giving a difference of −38.3 percentage points (P < .001).
The absolute change in the LDL cholesterol level was −77.5 mg/dL in the evolocumab group and −9.0 mg/dL in the placebo group, giving a difference of −68.6 mg/dL (P < .001).
Results for all secondary lipid variables were significantly better with evolocumab than with placebo. The incidence of adverse events that occurred during the treatment period was similar in the evolocumab and placebo groups. Laboratory abnormalities did not differ between groups.
Dr. Gaudet noted that FH is the most common genetic disease worldwide, affecting 1 in 250 people. “It is very treatable, so it is important to identify these patients, but it is massively underdiagnosed, with only around 15%-20% of patients with the condition having been identified,” he said.
“The vast majority of pediatric FH patients can reach target LDL levels with statins and ezetimibe, but there are 5%-10% of patients who may need additional therapy. We have now shown that evolocumab is safe and effective for these patients and can be used to fill this gap,” Dr. Gaudet said. “We can now say that we can cover all situations in treating FH whatever the severity of the disease.”
However, the challenge remains to improve the diagnosis of FH. “If there is one person with FH in a family, then it is essential that the whole extended family is tested. Our toolbox for treating this condition is now sufficiently effective, so there is no reason not to diagnose this disease,” Dr. Gaudet stressed.
The HAUSER-RCT study was supported by Amgen. Gaudet reports grants and personal fees from Amgen during the conduct of the study.
A version of this article originally appeared on Medscape.com.
The PCSK9 monoclonal antibody evolocumab (Repatha) was well tolerated and effectively lowered LDL cholesterol by 38% compared with placebo in a randomized controlled trial in pediatric patients with heterozygous familial hypercholesterolemia (FH) already taking statins with or without ezetimibe.
“HAUSER-RCT is the largest study and the first placebo-controlled randomized trial of a PCSK9 inhibitor in pediatric FH,” senior author Daniel Gaudet, MD, PhD, Universite de Montreal, said in an interview.
“The study showed good safety and efficacy of the drug in this population, with an excellent 44% reduction in LDL cholesterol compared with 6% in the placebo group.”
The trial also found evolocumab to be well tolerated in this group, with adverse effects similar in the active and placebo groups.
“Some people have wondered about using a drug with a monthly injection in a pediatric population, but this was not an issue in our study,” Dr. Gaudet said. “The idea of a monthly injection was well received, and no patient withdrew because of this.”
The HAUSER-RCT trial was presented on Aug. 29 at the virtual annual congress of the European Society of Cardiology (ESC Congress 2020) and simultaneously published online in the New England Journal of Medicine.
“With patients recruited from 23 countries in five continents, the study provides an accurate picture of the safety and efficacy of evolocumab in pediatric FH patients worldwide,” Dr. Gaudet said.
The 24-week, randomized, double-blind, placebo-controlled trial involved 157 patients aged 10-17 years with heterozygous FH already taking statins with or without ezetimibe and who had an LDL cholesterol level of 130 mg/dL or more and a triglyceride level of 400 mg/dL or less.
They were randomly assigned in a 2:1 ratio to receive monthly subcutaneous injections of evolocumab (420 mg) or placebo.
Results showed that at week 24, the mean percentage change from baseline in LDL cholesterol level was −44.5% in the evolocumab group and −6.2% in the placebo group, giving a difference of −38.3 percentage points (P < .001).
The absolute change in the LDL cholesterol level was −77.5 mg/dL in the evolocumab group and −9.0 mg/dL in the placebo group, giving a difference of −68.6 mg/dL (P < .001).
Results for all secondary lipid variables were significantly better with evolocumab than with placebo. The incidence of adverse events that occurred during the treatment period was similar in the evolocumab and placebo groups. Laboratory abnormalities did not differ between groups.
Dr. Gaudet noted that FH is the most common genetic disease worldwide, affecting 1 in 250 people. “It is very treatable, so it is important to identify these patients, but it is massively underdiagnosed, with only around 15%-20% of patients with the condition having been identified,” he said.
“The vast majority of pediatric FH patients can reach target LDL levels with statins and ezetimibe, but there are 5%-10% of patients who may need additional therapy. We have now shown that evolocumab is safe and effective for these patients and can be used to fill this gap,” Dr. Gaudet said. “We can now say that we can cover all situations in treating FH whatever the severity of the disease.”
However, the challenge remains to improve the diagnosis of FH. “If there is one person with FH in a family, then it is essential that the whole extended family is tested. Our toolbox for treating this condition is now sufficiently effective, so there is no reason not to diagnose this disease,” Dr. Gaudet stressed.
The HAUSER-RCT study was supported by Amgen. Gaudet reports grants and personal fees from Amgen during the conduct of the study.
A version of this article originally appeared on Medscape.com.
The PCSK9 monoclonal antibody evolocumab (Repatha) was well tolerated and effectively lowered LDL cholesterol by 38% compared with placebo in a randomized controlled trial in pediatric patients with heterozygous familial hypercholesterolemia (FH) already taking statins with or without ezetimibe.
“HAUSER-RCT is the largest study and the first placebo-controlled randomized trial of a PCSK9 inhibitor in pediatric FH,” senior author Daniel Gaudet, MD, PhD, Universite de Montreal, said in an interview.
“The study showed good safety and efficacy of the drug in this population, with an excellent 44% reduction in LDL cholesterol compared with 6% in the placebo group.”
The trial also found evolocumab to be well tolerated in this group, with adverse effects similar in the active and placebo groups.
“Some people have wondered about using a drug with a monthly injection in a pediatric population, but this was not an issue in our study,” Dr. Gaudet said. “The idea of a monthly injection was well received, and no patient withdrew because of this.”
The HAUSER-RCT trial was presented on Aug. 29 at the virtual annual congress of the European Society of Cardiology (ESC Congress 2020) and simultaneously published online in the New England Journal of Medicine.
“With patients recruited from 23 countries in five continents, the study provides an accurate picture of the safety and efficacy of evolocumab in pediatric FH patients worldwide,” Dr. Gaudet said.
The 24-week, randomized, double-blind, placebo-controlled trial involved 157 patients aged 10-17 years with heterozygous FH already taking statins with or without ezetimibe and who had an LDL cholesterol level of 130 mg/dL or more and a triglyceride level of 400 mg/dL or less.
They were randomly assigned in a 2:1 ratio to receive monthly subcutaneous injections of evolocumab (420 mg) or placebo.
Results showed that at week 24, the mean percentage change from baseline in LDL cholesterol level was −44.5% in the evolocumab group and −6.2% in the placebo group, giving a difference of −38.3 percentage points (P < .001).
The absolute change in the LDL cholesterol level was −77.5 mg/dL in the evolocumab group and −9.0 mg/dL in the placebo group, giving a difference of −68.6 mg/dL (P < .001).
Results for all secondary lipid variables were significantly better with evolocumab than with placebo. The incidence of adverse events that occurred during the treatment period was similar in the evolocumab and placebo groups. Laboratory abnormalities did not differ between groups.
Dr. Gaudet noted that FH is the most common genetic disease worldwide, affecting 1 in 250 people. “It is very treatable, so it is important to identify these patients, but it is massively underdiagnosed, with only around 15%-20% of patients with the condition having been identified,” he said.
“The vast majority of pediatric FH patients can reach target LDL levels with statins and ezetimibe, but there are 5%-10% of patients who may need additional therapy. We have now shown that evolocumab is safe and effective for these patients and can be used to fill this gap,” Dr. Gaudet said. “We can now say that we can cover all situations in treating FH whatever the severity of the disease.”
However, the challenge remains to improve the diagnosis of FH. “If there is one person with FH in a family, then it is essential that the whole extended family is tested. Our toolbox for treating this condition is now sufficiently effective, so there is no reason not to diagnose this disease,” Dr. Gaudet stressed.
The HAUSER-RCT study was supported by Amgen. Gaudet reports grants and personal fees from Amgen during the conduct of the study.
A version of this article originally appeared on Medscape.com.
Gut bacteria linked to cardiovascular, other health conditions
Microorganisms in the human digestive tract are linked to 29 specific health conditions, including chronic obstructive pulmonary disease, high blood pressure, and type 2 diabetes, according to a genome analysis in more than 400,000 individuals.
Although previous studies have suggested a link between gut microbiota and diseases in humans, “the extent to which the human gut microbiome can be considered a determinant of disease and healthy aging remains unknown,” Hilde E. Groot, MD, of the University of Groningen (The Netherlands), said in a presentation at the virtual annual congress of the European Society of Cardiology.
To identify the spectrum of diseases linked to the gut microbiome, the researchers identified 422,417 unrelated adults of White British ancestry with genotype and matching genetic data. The average age of the participants was 57 years and 46% were male.
The researchers conducted a phenomewide association study including 35 distinct single-nucleotide polymorphisms (SNPs) that are known to influence the microbiome of the human gut.
Overall, seven SNPs were significantly associated with 29 disease outcomes including hypertension, type 2 diabetes, hypercholesterolemia, heart failure, renal failure, and osteoarthritis.
In addition, after a further sensitivity analysis using a Mendelian randomization (MR) approach, associations between Ruminococcus flavefaciens and hypertension and between Clostridium and platelet count might point to a causal link, the researchers said.
“Over the past few years, the amount of research concerning the human gut microbiome and the associations with health and disease has tremendously increased. However, most studies investigated one or a few traits. The strength of our study is the possibility to cover a wide range of traits simultaneously within one population,” Dr. Groot said in an interview.
“Our data support the hypothesis that the human gut microbiome is a complex system, involved in many pathophysiological mechanisms in the human body. So, our results are additional to earlier research and strengthen this hypothesis,” Dr. Groot added.
“Microbiota and their metabolites might be of importance in the interplay between overlapping pathophysiological processes, and could serve as potential therapeutic targets for the maintenance of health and prevention and treatment of cardiovascular diseases. However, before it is possible to give advice for the public and medical practice, further research is needed to study causality,” she emphasized.
“Currently, it is too soon to advise patients concerning their microbiome,” Dr. Groot noted. “However, genetic studies like ours might help other researchers to study causality between the gut microbiome and particular traits, which might potentially lead to new therapeutic targets. Next to genetic variants as a proxy, we’re currently studying the gut microbiome composition in myocardial infarction patients and healthy controls in a longitudinal setting.”
“Previous studies have suggested a potential link between the gut microbiome and the development of cardiovascular disease, type 2 diabetes mellitus, and other chronic disorders,” Carol Ann Remme, MD, of the Amsterdam University Medical Center, said in an interview. “However, it is challenging to study the effect of gut microbiome composition in large patient cohorts. As an alternative approach, the study authors showed in a very large population that genetic variants previously shown to influence gut microbiome composition were significantly associated with conditions such as hypertension, type 2 diabetes, hypercholesterolemia, and heart failure.”
The study is unique in that it employed a very large cohort of more than 400,000 individuals, which is typically required to be able to draw clear conclusions, Dr. Remme continued. “The authors were able to further refine their findings by linking genetic variants known to influence specific gut bacteria to some particular disorders,” she noted.
“It is becoming increasingly clear that an individual’s gut microbiome composition, which is defined by both genetic and environmental factors such as diet, may affect his/her susceptibility to certain diseases – including cardiovascular – in addition to disease progression and outcome,” said Dr. Remme. “This may ultimately lead to development of novel, personalized strategies for risk stratification in addition to potential preventive measures targeting the gut microbiome. I expect this area of research will become increasingly important in the coming years.”
The study received no outside funding. Dr. Groot and colleagues had no financial conflicts to disclose. Dr. Remme had no financial conflicts to disclose.
Microorganisms in the human digestive tract are linked to 29 specific health conditions, including chronic obstructive pulmonary disease, high blood pressure, and type 2 diabetes, according to a genome analysis in more than 400,000 individuals.
Although previous studies have suggested a link between gut microbiota and diseases in humans, “the extent to which the human gut microbiome can be considered a determinant of disease and healthy aging remains unknown,” Hilde E. Groot, MD, of the University of Groningen (The Netherlands), said in a presentation at the virtual annual congress of the European Society of Cardiology.
To identify the spectrum of diseases linked to the gut microbiome, the researchers identified 422,417 unrelated adults of White British ancestry with genotype and matching genetic data. The average age of the participants was 57 years and 46% were male.
The researchers conducted a phenomewide association study including 35 distinct single-nucleotide polymorphisms (SNPs) that are known to influence the microbiome of the human gut.
Overall, seven SNPs were significantly associated with 29 disease outcomes including hypertension, type 2 diabetes, hypercholesterolemia, heart failure, renal failure, and osteoarthritis.
In addition, after a further sensitivity analysis using a Mendelian randomization (MR) approach, associations between Ruminococcus flavefaciens and hypertension and between Clostridium and platelet count might point to a causal link, the researchers said.
“Over the past few years, the amount of research concerning the human gut microbiome and the associations with health and disease has tremendously increased. However, most studies investigated one or a few traits. The strength of our study is the possibility to cover a wide range of traits simultaneously within one population,” Dr. Groot said in an interview.
“Our data support the hypothesis that the human gut microbiome is a complex system, involved in many pathophysiological mechanisms in the human body. So, our results are additional to earlier research and strengthen this hypothesis,” Dr. Groot added.
“Microbiota and their metabolites might be of importance in the interplay between overlapping pathophysiological processes, and could serve as potential therapeutic targets for the maintenance of health and prevention and treatment of cardiovascular diseases. However, before it is possible to give advice for the public and medical practice, further research is needed to study causality,” she emphasized.
“Currently, it is too soon to advise patients concerning their microbiome,” Dr. Groot noted. “However, genetic studies like ours might help other researchers to study causality between the gut microbiome and particular traits, which might potentially lead to new therapeutic targets. Next to genetic variants as a proxy, we’re currently studying the gut microbiome composition in myocardial infarction patients and healthy controls in a longitudinal setting.”
“Previous studies have suggested a potential link between the gut microbiome and the development of cardiovascular disease, type 2 diabetes mellitus, and other chronic disorders,” Carol Ann Remme, MD, of the Amsterdam University Medical Center, said in an interview. “However, it is challenging to study the effect of gut microbiome composition in large patient cohorts. As an alternative approach, the study authors showed in a very large population that genetic variants previously shown to influence gut microbiome composition were significantly associated with conditions such as hypertension, type 2 diabetes, hypercholesterolemia, and heart failure.”
The study is unique in that it employed a very large cohort of more than 400,000 individuals, which is typically required to be able to draw clear conclusions, Dr. Remme continued. “The authors were able to further refine their findings by linking genetic variants known to influence specific gut bacteria to some particular disorders,” she noted.
“It is becoming increasingly clear that an individual’s gut microbiome composition, which is defined by both genetic and environmental factors such as diet, may affect his/her susceptibility to certain diseases – including cardiovascular – in addition to disease progression and outcome,” said Dr. Remme. “This may ultimately lead to development of novel, personalized strategies for risk stratification in addition to potential preventive measures targeting the gut microbiome. I expect this area of research will become increasingly important in the coming years.”
The study received no outside funding. Dr. Groot and colleagues had no financial conflicts to disclose. Dr. Remme had no financial conflicts to disclose.
Microorganisms in the human digestive tract are linked to 29 specific health conditions, including chronic obstructive pulmonary disease, high blood pressure, and type 2 diabetes, according to a genome analysis in more than 400,000 individuals.
Although previous studies have suggested a link between gut microbiota and diseases in humans, “the extent to which the human gut microbiome can be considered a determinant of disease and healthy aging remains unknown,” Hilde E. Groot, MD, of the University of Groningen (The Netherlands), said in a presentation at the virtual annual congress of the European Society of Cardiology.
To identify the spectrum of diseases linked to the gut microbiome, the researchers identified 422,417 unrelated adults of White British ancestry with genotype and matching genetic data. The average age of the participants was 57 years and 46% were male.
The researchers conducted a phenomewide association study including 35 distinct single-nucleotide polymorphisms (SNPs) that are known to influence the microbiome of the human gut.
Overall, seven SNPs were significantly associated with 29 disease outcomes including hypertension, type 2 diabetes, hypercholesterolemia, heart failure, renal failure, and osteoarthritis.
In addition, after a further sensitivity analysis using a Mendelian randomization (MR) approach, associations between Ruminococcus flavefaciens and hypertension and between Clostridium and platelet count might point to a causal link, the researchers said.
“Over the past few years, the amount of research concerning the human gut microbiome and the associations with health and disease has tremendously increased. However, most studies investigated one or a few traits. The strength of our study is the possibility to cover a wide range of traits simultaneously within one population,” Dr. Groot said in an interview.
“Our data support the hypothesis that the human gut microbiome is a complex system, involved in many pathophysiological mechanisms in the human body. So, our results are additional to earlier research and strengthen this hypothesis,” Dr. Groot added.
“Microbiota and their metabolites might be of importance in the interplay between overlapping pathophysiological processes, and could serve as potential therapeutic targets for the maintenance of health and prevention and treatment of cardiovascular diseases. However, before it is possible to give advice for the public and medical practice, further research is needed to study causality,” she emphasized.
“Currently, it is too soon to advise patients concerning their microbiome,” Dr. Groot noted. “However, genetic studies like ours might help other researchers to study causality between the gut microbiome and particular traits, which might potentially lead to new therapeutic targets. Next to genetic variants as a proxy, we’re currently studying the gut microbiome composition in myocardial infarction patients and healthy controls in a longitudinal setting.”
“Previous studies have suggested a potential link between the gut microbiome and the development of cardiovascular disease, type 2 diabetes mellitus, and other chronic disorders,” Carol Ann Remme, MD, of the Amsterdam University Medical Center, said in an interview. “However, it is challenging to study the effect of gut microbiome composition in large patient cohorts. As an alternative approach, the study authors showed in a very large population that genetic variants previously shown to influence gut microbiome composition were significantly associated with conditions such as hypertension, type 2 diabetes, hypercholesterolemia, and heart failure.”
The study is unique in that it employed a very large cohort of more than 400,000 individuals, which is typically required to be able to draw clear conclusions, Dr. Remme continued. “The authors were able to further refine their findings by linking genetic variants known to influence specific gut bacteria to some particular disorders,” she noted.
“It is becoming increasingly clear that an individual’s gut microbiome composition, which is defined by both genetic and environmental factors such as diet, may affect his/her susceptibility to certain diseases – including cardiovascular – in addition to disease progression and outcome,” said Dr. Remme. “This may ultimately lead to development of novel, personalized strategies for risk stratification in addition to potential preventive measures targeting the gut microbiome. I expect this area of research will become increasingly important in the coming years.”
The study received no outside funding. Dr. Groot and colleagues had no financial conflicts to disclose. Dr. Remme had no financial conflicts to disclose.
FROM ESC CONGRESS 2020
ATPCI: Trimetazidine fizzles for post-PCI angina
Adding trimetazidine to optimal medical therapy does not improve outcomes following successful percutaneous coronary intervention (PCI) for stable angina or a non–ST-elevated myocardial infarction, results of the ATPCI trial show.
There was no benefit for the composite primary endpoint of cardiac death, hospitalization for cardiac events, or recurrent/persistent angina requiring an addition, switch, or increased dose of antianginal therapies, or requiring coronary angiography (hazard ratio, 0.98; 95% confidence interval, 0.88-1.09).
Further, there were no between-group differences in any of the individual components of the endpoint or any prespecified subgroups, Roberto Ferrari, MD, professor of cardiology at the University of Ferrara (Italy), reported in a Hot Line session at the digital European Society of Cardiology Congress 2020.
“I think one of the reasons why we couldn’t see any results was really due to this population was extremely well treated,” he said. “Almost all of them were receiving either a beta-blocker or calcium blocker and, on top of this, they had a successful angioplasty and that is what we should do, at least according to ESC guidelines.”
Research has shown that about 85% to 90% of patients have a change in New York Heart Association angina class within 30 days of PCI, leaving very little angina leftover to treat, observed Magnus Ohman, MD, director of the advanced coronary disease program at Duke University, Durham, N.C., who was not involved in the study.
“The fundamental question is whether this was the right study. Is this agent ineffective, or is it just that it was studied in the wrong population? That to me is really the crux of the matter,” he said in an interview.
There is potential benefit in chronic angina, which reflects the level II recommendation by the ESC, said Dr. Ohman. “Those patients typically require more therapy and, in the ideal world of treating angina, you need both physiological and metabolic agents to treat angina and trimetazidine is one metabolic agent.”
Trimetazidine is not available in the United States, but the anti-ischemic metabolic agent is recommended as second-line therapy for angina after beta-blockers and calcium-channel blockers in the 2019 ESC guidelines on chronic coronary syndrome.
Unlike other commonly used first- and second-line antianginal drugs, trimetazidine is devoid of hemodynamic effects, Dr. Ferrari said. It improves myocardial utilization by favoring glucose to fatty acids, thus allowing anaerobic adenosine triphosphate formation and preventing acidosis.
In the absence of contemporary data on the prognostic benefits of antianginal drugs in post-PCI patients, ATPCI investigators at 365 centers in 27 countries randomly assigned 6007 patients with stable angina or non–ST-segment MI after successful elective or urgent PCI to optimal medical therapy alone or with trimetazidine, 35 mg modified-release twice daily.
Patients with severe heart failure, valvular disease, arrhythmia, renal failure or acute ST-elevation MI were excluded.
Most patients (77% male) had Canadian Class Cardiovascular Society class III/IV angina (58%) and were receiving aspirin plus a P2Y12 inhibitor (97%), lipid-lowering agent (96.6%), renin-angiotensin inhibitors (82.2%), and beta-blockers (83.9%). A quarter were receiving calcium-channel blockers (27.6%). In all, 2517 patients had an urgent PCI and 3490 had an elective PCI.
After a median follow-up of 47.5 months, the composite primary endpoint occurred in 23.3% of the trimetazidine group and 23.7% of the control group, according to the study, which was published simultaneously in The Lancet.
The incidence of the individual components was similar:
- Cardiac death: 2.1% vs. 2.6% (HR, 0.81)
- Hospital admission for cardiac events: 13.4% vs. 13.4% (HR, 1.01)
- Angina leading to coronary angiography: 16.9% vs. 16.6% (HR, 1.02)
- Angina leading to increase/switch in antianginal drugs (HR, 1.01)
There was no between-group difference in the composite major secondary endpoint, which included the primary endpoint components plus ischemia leading to coronary angiography and an increase or switch in antianginal therapies. This outcome occurred in 23.5% and 24.0% of patients in the trimetazidine and control groups, respectively (HR, 0.98; 95% CI, 0.88-1.08).
Results were also similar when the primary endpoint was analyzed based on whether patients underwent elective PCI (HR, 0.94; 95% CI, 0.82-1.08) or urgent PCI (HR, 1.04; 95% CI, 0.88-1.22), Dr. Ferrari reported.
Given the lack of observed efficacy, trimetazidine has no use or place in the population studied, said Stephan Windecker, MD, the formal discussant for the study and chair of cardiology at Bern (Switzerland) University Hospital. “Notwithstanding, I think we have to recognize that the optimal medical therapy is so potent and has been well implemented in this trial that any additional medication beyond this is just unable to exploit additional benefit.”
The study was supported by Servier. Dr. Ferrari received fees, honoraria, and travel expenses from Servier. Dr. Ohman reports no relevant financial conflicts of interest. Dr. Windecker is an unpaid member of the steering/executive group for trials funded by Abbott, Abiomed, Amgen, BMS, Boston Scientific, Biotronik, Cardiovalve, Edwards Lifesciences, MedAlliancé, Medtronic, Polares, Sinomed, V-Wave, and Xeltis but has not received personal payments from any pharmaceutical or device company.
This article first appeared on Medscape.com.
Adding trimetazidine to optimal medical therapy does not improve outcomes following successful percutaneous coronary intervention (PCI) for stable angina or a non–ST-elevated myocardial infarction, results of the ATPCI trial show.
There was no benefit for the composite primary endpoint of cardiac death, hospitalization for cardiac events, or recurrent/persistent angina requiring an addition, switch, or increased dose of antianginal therapies, or requiring coronary angiography (hazard ratio, 0.98; 95% confidence interval, 0.88-1.09).
Further, there were no between-group differences in any of the individual components of the endpoint or any prespecified subgroups, Roberto Ferrari, MD, professor of cardiology at the University of Ferrara (Italy), reported in a Hot Line session at the digital European Society of Cardiology Congress 2020.
“I think one of the reasons why we couldn’t see any results was really due to this population was extremely well treated,” he said. “Almost all of them were receiving either a beta-blocker or calcium blocker and, on top of this, they had a successful angioplasty and that is what we should do, at least according to ESC guidelines.”
Research has shown that about 85% to 90% of patients have a change in New York Heart Association angina class within 30 days of PCI, leaving very little angina leftover to treat, observed Magnus Ohman, MD, director of the advanced coronary disease program at Duke University, Durham, N.C., who was not involved in the study.
“The fundamental question is whether this was the right study. Is this agent ineffective, or is it just that it was studied in the wrong population? That to me is really the crux of the matter,” he said in an interview.
There is potential benefit in chronic angina, which reflects the level II recommendation by the ESC, said Dr. Ohman. “Those patients typically require more therapy and, in the ideal world of treating angina, you need both physiological and metabolic agents to treat angina and trimetazidine is one metabolic agent.”
Trimetazidine is not available in the United States, but the anti-ischemic metabolic agent is recommended as second-line therapy for angina after beta-blockers and calcium-channel blockers in the 2019 ESC guidelines on chronic coronary syndrome.
Unlike other commonly used first- and second-line antianginal drugs, trimetazidine is devoid of hemodynamic effects, Dr. Ferrari said. It improves myocardial utilization by favoring glucose to fatty acids, thus allowing anaerobic adenosine triphosphate formation and preventing acidosis.
In the absence of contemporary data on the prognostic benefits of antianginal drugs in post-PCI patients, ATPCI investigators at 365 centers in 27 countries randomly assigned 6007 patients with stable angina or non–ST-segment MI after successful elective or urgent PCI to optimal medical therapy alone or with trimetazidine, 35 mg modified-release twice daily.
Patients with severe heart failure, valvular disease, arrhythmia, renal failure or acute ST-elevation MI were excluded.
Most patients (77% male) had Canadian Class Cardiovascular Society class III/IV angina (58%) and were receiving aspirin plus a P2Y12 inhibitor (97%), lipid-lowering agent (96.6%), renin-angiotensin inhibitors (82.2%), and beta-blockers (83.9%). A quarter were receiving calcium-channel blockers (27.6%). In all, 2517 patients had an urgent PCI and 3490 had an elective PCI.
After a median follow-up of 47.5 months, the composite primary endpoint occurred in 23.3% of the trimetazidine group and 23.7% of the control group, according to the study, which was published simultaneously in The Lancet.
The incidence of the individual components was similar:
- Cardiac death: 2.1% vs. 2.6% (HR, 0.81)
- Hospital admission for cardiac events: 13.4% vs. 13.4% (HR, 1.01)
- Angina leading to coronary angiography: 16.9% vs. 16.6% (HR, 1.02)
- Angina leading to increase/switch in antianginal drugs (HR, 1.01)
There was no between-group difference in the composite major secondary endpoint, which included the primary endpoint components plus ischemia leading to coronary angiography and an increase or switch in antianginal therapies. This outcome occurred in 23.5% and 24.0% of patients in the trimetazidine and control groups, respectively (HR, 0.98; 95% CI, 0.88-1.08).
Results were also similar when the primary endpoint was analyzed based on whether patients underwent elective PCI (HR, 0.94; 95% CI, 0.82-1.08) or urgent PCI (HR, 1.04; 95% CI, 0.88-1.22), Dr. Ferrari reported.
Given the lack of observed efficacy, trimetazidine has no use or place in the population studied, said Stephan Windecker, MD, the formal discussant for the study and chair of cardiology at Bern (Switzerland) University Hospital. “Notwithstanding, I think we have to recognize that the optimal medical therapy is so potent and has been well implemented in this trial that any additional medication beyond this is just unable to exploit additional benefit.”
The study was supported by Servier. Dr. Ferrari received fees, honoraria, and travel expenses from Servier. Dr. Ohman reports no relevant financial conflicts of interest. Dr. Windecker is an unpaid member of the steering/executive group for trials funded by Abbott, Abiomed, Amgen, BMS, Boston Scientific, Biotronik, Cardiovalve, Edwards Lifesciences, MedAlliancé, Medtronic, Polares, Sinomed, V-Wave, and Xeltis but has not received personal payments from any pharmaceutical or device company.
This article first appeared on Medscape.com.
Adding trimetazidine to optimal medical therapy does not improve outcomes following successful percutaneous coronary intervention (PCI) for stable angina or a non–ST-elevated myocardial infarction, results of the ATPCI trial show.
There was no benefit for the composite primary endpoint of cardiac death, hospitalization for cardiac events, or recurrent/persistent angina requiring an addition, switch, or increased dose of antianginal therapies, or requiring coronary angiography (hazard ratio, 0.98; 95% confidence interval, 0.88-1.09).
Further, there were no between-group differences in any of the individual components of the endpoint or any prespecified subgroups, Roberto Ferrari, MD, professor of cardiology at the University of Ferrara (Italy), reported in a Hot Line session at the digital European Society of Cardiology Congress 2020.
“I think one of the reasons why we couldn’t see any results was really due to this population was extremely well treated,” he said. “Almost all of them were receiving either a beta-blocker or calcium blocker and, on top of this, they had a successful angioplasty and that is what we should do, at least according to ESC guidelines.”
Research has shown that about 85% to 90% of patients have a change in New York Heart Association angina class within 30 days of PCI, leaving very little angina leftover to treat, observed Magnus Ohman, MD, director of the advanced coronary disease program at Duke University, Durham, N.C., who was not involved in the study.
“The fundamental question is whether this was the right study. Is this agent ineffective, or is it just that it was studied in the wrong population? That to me is really the crux of the matter,” he said in an interview.
There is potential benefit in chronic angina, which reflects the level II recommendation by the ESC, said Dr. Ohman. “Those patients typically require more therapy and, in the ideal world of treating angina, you need both physiological and metabolic agents to treat angina and trimetazidine is one metabolic agent.”
Trimetazidine is not available in the United States, but the anti-ischemic metabolic agent is recommended as second-line therapy for angina after beta-blockers and calcium-channel blockers in the 2019 ESC guidelines on chronic coronary syndrome.
Unlike other commonly used first- and second-line antianginal drugs, trimetazidine is devoid of hemodynamic effects, Dr. Ferrari said. It improves myocardial utilization by favoring glucose to fatty acids, thus allowing anaerobic adenosine triphosphate formation and preventing acidosis.
In the absence of contemporary data on the prognostic benefits of antianginal drugs in post-PCI patients, ATPCI investigators at 365 centers in 27 countries randomly assigned 6007 patients with stable angina or non–ST-segment MI after successful elective or urgent PCI to optimal medical therapy alone or with trimetazidine, 35 mg modified-release twice daily.
Patients with severe heart failure, valvular disease, arrhythmia, renal failure or acute ST-elevation MI were excluded.
Most patients (77% male) had Canadian Class Cardiovascular Society class III/IV angina (58%) and were receiving aspirin plus a P2Y12 inhibitor (97%), lipid-lowering agent (96.6%), renin-angiotensin inhibitors (82.2%), and beta-blockers (83.9%). A quarter were receiving calcium-channel blockers (27.6%). In all, 2517 patients had an urgent PCI and 3490 had an elective PCI.
After a median follow-up of 47.5 months, the composite primary endpoint occurred in 23.3% of the trimetazidine group and 23.7% of the control group, according to the study, which was published simultaneously in The Lancet.
The incidence of the individual components was similar:
- Cardiac death: 2.1% vs. 2.6% (HR, 0.81)
- Hospital admission for cardiac events: 13.4% vs. 13.4% (HR, 1.01)
- Angina leading to coronary angiography: 16.9% vs. 16.6% (HR, 1.02)
- Angina leading to increase/switch in antianginal drugs (HR, 1.01)
There was no between-group difference in the composite major secondary endpoint, which included the primary endpoint components plus ischemia leading to coronary angiography and an increase or switch in antianginal therapies. This outcome occurred in 23.5% and 24.0% of patients in the trimetazidine and control groups, respectively (HR, 0.98; 95% CI, 0.88-1.08).
Results were also similar when the primary endpoint was analyzed based on whether patients underwent elective PCI (HR, 0.94; 95% CI, 0.82-1.08) or urgent PCI (HR, 1.04; 95% CI, 0.88-1.22), Dr. Ferrari reported.
Given the lack of observed efficacy, trimetazidine has no use or place in the population studied, said Stephan Windecker, MD, the formal discussant for the study and chair of cardiology at Bern (Switzerland) University Hospital. “Notwithstanding, I think we have to recognize that the optimal medical therapy is so potent and has been well implemented in this trial that any additional medication beyond this is just unable to exploit additional benefit.”
The study was supported by Servier. Dr. Ferrari received fees, honoraria, and travel expenses from Servier. Dr. Ohman reports no relevant financial conflicts of interest. Dr. Windecker is an unpaid member of the steering/executive group for trials funded by Abbott, Abiomed, Amgen, BMS, Boston Scientific, Biotronik, Cardiovalve, Edwards Lifesciences, MedAlliancé, Medtronic, Polares, Sinomed, V-Wave, and Xeltis but has not received personal payments from any pharmaceutical or device company.
This article first appeared on Medscape.com.
DAPA-CKD: SGLT2 inhibitor benefit extends to chronic kidney disease without diabetes
In the DAPA-CKD trial, treatment with the SGLT2 inhibitor dapagliflozin (Farxiga) cut the incidence of substantially worsened chronic kidney disease by an average of 39% compared with placebo when added to standard treatment, with a number needed to treat of 19 to prevent one primary outcome event after a median of 2.4 years.
The level of benefit was similar in both the one-third of enrolled patients without diabetes and in the two-thirds with diabetes, showing a statistically significant 50% cut in the primary endpoint among patients without diabetes, Hiddo J.L. Heerspink, MD, reported at the virtual annual congress of the European Society of Cardiology.
“We found that dapagliflozin delayed the initiation of dialysis, and reduced the number of deaths,” regardless of diabetes status, Dr. Heerspink, of University Medical Centre Groningen, the Netherlands, said during a press conference. “DAPA-CKD trial has shown dapagliflozin’s potential as a long-awaited new treatment for patients with chronic kidney disease.”
This finding ushers in a “completely new era in chronic kidney disease management,” said Janani Rangaswami, MD, a nephrologist and cardiorenal syndrome specialist at Einstein Medical Center in Philadelphia. “It’s good news” for these patients.
The results showed that dapagliflozin is the first “game changing” drug for chronic kidney disease in 2 decades, following the introduction of angiotensin converting enzyme inhibitors and angiotensin receptor blockers, she said in an interview. And given the consistency of the findings with the results from several other studies that documented meaningful renal protection by several different SGLT2 inhibitors, the results from this single trial also convincingly establish dapagliflozin as a standard-of-care agent to use on the types of patients the study enrolled, she said in an interview.
Representing many real-world patients
The DAPA-CKD trial enrolled 4,304 patients with albuminuria based on having a urinary albumin-to-creatinine ratio of at least 200 mg/g, and an estimated glomerular filtration rate (eGFR) of 25-75 mL/min per 1.73 m2 (with 90% of patients having an eGFR of less than 60 mL/min per 1.73 m2), and 97% were on treatment with a renin-angiotensin system–blocking drug. The primary endpoint was the combined rate of a drop in eGFR of at least 50% from baseline, progression to end stage renal disease, or renal or cardiovascular death; the between-group difference in this composite was driven primarily by both preserved eGFR and by prevention of end stage renal disease.
This represents both an appropriate target population, and meaningful endpoints, Dr. Rangaswami said. The study was “very representative of who we see in real-world practice,” a group that likely includes “hundreds of thousands” of U.S. patients with nondiabetic chronic kidney disease, she estimated.
Another notable finding was that 14% of the enrolled patients had eGFR values at baseline of 25-29 mL/min per 1.73 m2, pegging them as having stage 4 chronic kidney disease, and the median baseline eGFR was 43 mL/min per 1.73 m2, but dapagliflozin treatment was as safe and effective in these patients as it was in enrolled patients with a higher level of retained renal activity. This experience should give clinicians greater confidence about using dapagliflozin and other drugs in the sodium-glucose cotransporter (SGLT) 2 inhibitor class in patients with substantially depressed renal function, Dr. Rangaswami said.
“We now need to be more proactive about treating patients with more advanced kidney disease who can still benefit” from dapagliflozin treatment. “The sooner you intervene the better,” to slow further progression, but the new findings show “benefit even when treating patients with lower eGFRs. There is still hope to prevent or delay dialysis.”
A heart-kidney connection
Dapagliflozin treatment also cut all-cause mortality by a statistically significant, relative 31%, and another secondary-endpoint analysis showed a statistically significant 29% relative reduction in the rate of cardiovascular death or heart failure hospitalization, a benefit seen consistently in several prior studies of SGLT2 inhibitors, but possibly unexpected here because enrolled patients underwent no selection for a history of heart failure or any other cardiovascular disease. But the finding shouldn’t surprise, because “chronic kidney disease is an independent risk factor for cardiovascular disease across the board, and especially for heart failure,” noted Dr. Rangaswami.
“Heart and kidney disease is one big spectrum,” and the collected experience of several trials that have now proven the efficacy of SGLT2 inhibitors among patients with heart failure with reduced ejection fraction or with chronic kidney disease, regardless of their glycemic control, shows how broadly this drug class can benefit patients across the breadth of this spectrum, she said.
DAPA-CKD was funded by AstraZeneca, the company that markets dapagliflozin (Farxiga). Dr. Heerspink has been a consultant to and received research funding from AstraZeneca and from several other companies. Dr. Rangaswami had no disclosures.
In the DAPA-CKD trial, treatment with the SGLT2 inhibitor dapagliflozin (Farxiga) cut the incidence of substantially worsened chronic kidney disease by an average of 39% compared with placebo when added to standard treatment, with a number needed to treat of 19 to prevent one primary outcome event after a median of 2.4 years.
The level of benefit was similar in both the one-third of enrolled patients without diabetes and in the two-thirds with diabetes, showing a statistically significant 50% cut in the primary endpoint among patients without diabetes, Hiddo J.L. Heerspink, MD, reported at the virtual annual congress of the European Society of Cardiology.
“We found that dapagliflozin delayed the initiation of dialysis, and reduced the number of deaths,” regardless of diabetes status, Dr. Heerspink, of University Medical Centre Groningen, the Netherlands, said during a press conference. “DAPA-CKD trial has shown dapagliflozin’s potential as a long-awaited new treatment for patients with chronic kidney disease.”
This finding ushers in a “completely new era in chronic kidney disease management,” said Janani Rangaswami, MD, a nephrologist and cardiorenal syndrome specialist at Einstein Medical Center in Philadelphia. “It’s good news” for these patients.
The results showed that dapagliflozin is the first “game changing” drug for chronic kidney disease in 2 decades, following the introduction of angiotensin converting enzyme inhibitors and angiotensin receptor blockers, she said in an interview. And given the consistency of the findings with the results from several other studies that documented meaningful renal protection by several different SGLT2 inhibitors, the results from this single trial also convincingly establish dapagliflozin as a standard-of-care agent to use on the types of patients the study enrolled, she said in an interview.
Representing many real-world patients
The DAPA-CKD trial enrolled 4,304 patients with albuminuria based on having a urinary albumin-to-creatinine ratio of at least 200 mg/g, and an estimated glomerular filtration rate (eGFR) of 25-75 mL/min per 1.73 m2 (with 90% of patients having an eGFR of less than 60 mL/min per 1.73 m2), and 97% were on treatment with a renin-angiotensin system–blocking drug. The primary endpoint was the combined rate of a drop in eGFR of at least 50% from baseline, progression to end stage renal disease, or renal or cardiovascular death; the between-group difference in this composite was driven primarily by both preserved eGFR and by prevention of end stage renal disease.
This represents both an appropriate target population, and meaningful endpoints, Dr. Rangaswami said. The study was “very representative of who we see in real-world practice,” a group that likely includes “hundreds of thousands” of U.S. patients with nondiabetic chronic kidney disease, she estimated.
Another notable finding was that 14% of the enrolled patients had eGFR values at baseline of 25-29 mL/min per 1.73 m2, pegging them as having stage 4 chronic kidney disease, and the median baseline eGFR was 43 mL/min per 1.73 m2, but dapagliflozin treatment was as safe and effective in these patients as it was in enrolled patients with a higher level of retained renal activity. This experience should give clinicians greater confidence about using dapagliflozin and other drugs in the sodium-glucose cotransporter (SGLT) 2 inhibitor class in patients with substantially depressed renal function, Dr. Rangaswami said.
“We now need to be more proactive about treating patients with more advanced kidney disease who can still benefit” from dapagliflozin treatment. “The sooner you intervene the better,” to slow further progression, but the new findings show “benefit even when treating patients with lower eGFRs. There is still hope to prevent or delay dialysis.”
A heart-kidney connection
Dapagliflozin treatment also cut all-cause mortality by a statistically significant, relative 31%, and another secondary-endpoint analysis showed a statistically significant 29% relative reduction in the rate of cardiovascular death or heart failure hospitalization, a benefit seen consistently in several prior studies of SGLT2 inhibitors, but possibly unexpected here because enrolled patients underwent no selection for a history of heart failure or any other cardiovascular disease. But the finding shouldn’t surprise, because “chronic kidney disease is an independent risk factor for cardiovascular disease across the board, and especially for heart failure,” noted Dr. Rangaswami.
“Heart and kidney disease is one big spectrum,” and the collected experience of several trials that have now proven the efficacy of SGLT2 inhibitors among patients with heart failure with reduced ejection fraction or with chronic kidney disease, regardless of their glycemic control, shows how broadly this drug class can benefit patients across the breadth of this spectrum, she said.
DAPA-CKD was funded by AstraZeneca, the company that markets dapagliflozin (Farxiga). Dr. Heerspink has been a consultant to and received research funding from AstraZeneca and from several other companies. Dr. Rangaswami had no disclosures.
In the DAPA-CKD trial, treatment with the SGLT2 inhibitor dapagliflozin (Farxiga) cut the incidence of substantially worsened chronic kidney disease by an average of 39% compared with placebo when added to standard treatment, with a number needed to treat of 19 to prevent one primary outcome event after a median of 2.4 years.
The level of benefit was similar in both the one-third of enrolled patients without diabetes and in the two-thirds with diabetes, showing a statistically significant 50% cut in the primary endpoint among patients without diabetes, Hiddo J.L. Heerspink, MD, reported at the virtual annual congress of the European Society of Cardiology.
“We found that dapagliflozin delayed the initiation of dialysis, and reduced the number of deaths,” regardless of diabetes status, Dr. Heerspink, of University Medical Centre Groningen, the Netherlands, said during a press conference. “DAPA-CKD trial has shown dapagliflozin’s potential as a long-awaited new treatment for patients with chronic kidney disease.”
This finding ushers in a “completely new era in chronic kidney disease management,” said Janani Rangaswami, MD, a nephrologist and cardiorenal syndrome specialist at Einstein Medical Center in Philadelphia. “It’s good news” for these patients.
The results showed that dapagliflozin is the first “game changing” drug for chronic kidney disease in 2 decades, following the introduction of angiotensin converting enzyme inhibitors and angiotensin receptor blockers, she said in an interview. And given the consistency of the findings with the results from several other studies that documented meaningful renal protection by several different SGLT2 inhibitors, the results from this single trial also convincingly establish dapagliflozin as a standard-of-care agent to use on the types of patients the study enrolled, she said in an interview.
Representing many real-world patients
The DAPA-CKD trial enrolled 4,304 patients with albuminuria based on having a urinary albumin-to-creatinine ratio of at least 200 mg/g, and an estimated glomerular filtration rate (eGFR) of 25-75 mL/min per 1.73 m2 (with 90% of patients having an eGFR of less than 60 mL/min per 1.73 m2), and 97% were on treatment with a renin-angiotensin system–blocking drug. The primary endpoint was the combined rate of a drop in eGFR of at least 50% from baseline, progression to end stage renal disease, or renal or cardiovascular death; the between-group difference in this composite was driven primarily by both preserved eGFR and by prevention of end stage renal disease.
This represents both an appropriate target population, and meaningful endpoints, Dr. Rangaswami said. The study was “very representative of who we see in real-world practice,” a group that likely includes “hundreds of thousands” of U.S. patients with nondiabetic chronic kidney disease, she estimated.
Another notable finding was that 14% of the enrolled patients had eGFR values at baseline of 25-29 mL/min per 1.73 m2, pegging them as having stage 4 chronic kidney disease, and the median baseline eGFR was 43 mL/min per 1.73 m2, but dapagliflozin treatment was as safe and effective in these patients as it was in enrolled patients with a higher level of retained renal activity. This experience should give clinicians greater confidence about using dapagliflozin and other drugs in the sodium-glucose cotransporter (SGLT) 2 inhibitor class in patients with substantially depressed renal function, Dr. Rangaswami said.
“We now need to be more proactive about treating patients with more advanced kidney disease who can still benefit” from dapagliflozin treatment. “The sooner you intervene the better,” to slow further progression, but the new findings show “benefit even when treating patients with lower eGFRs. There is still hope to prevent or delay dialysis.”
A heart-kidney connection
Dapagliflozin treatment also cut all-cause mortality by a statistically significant, relative 31%, and another secondary-endpoint analysis showed a statistically significant 29% relative reduction in the rate of cardiovascular death or heart failure hospitalization, a benefit seen consistently in several prior studies of SGLT2 inhibitors, but possibly unexpected here because enrolled patients underwent no selection for a history of heart failure or any other cardiovascular disease. But the finding shouldn’t surprise, because “chronic kidney disease is an independent risk factor for cardiovascular disease across the board, and especially for heart failure,” noted Dr. Rangaswami.
“Heart and kidney disease is one big spectrum,” and the collected experience of several trials that have now proven the efficacy of SGLT2 inhibitors among patients with heart failure with reduced ejection fraction or with chronic kidney disease, regardless of their glycemic control, shows how broadly this drug class can benefit patients across the breadth of this spectrum, she said.
DAPA-CKD was funded by AstraZeneca, the company that markets dapagliflozin (Farxiga). Dr. Heerspink has been a consultant to and received research funding from AstraZeneca and from several other companies. Dr. Rangaswami had no disclosures.
FROM ESC CONGRESS 2020