User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
nav[contains(@class, 'nav-ce-stack nav-ce-stack__large-screen')]
header[@id='header']
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'main-prefix')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
div[contains(@class, 'view-medstat-quiz-listing-panes')]
div[contains(@class, 'pane-article-sidebar-latest-news')]
Large vessel stroke linked to AstraZeneca COVID vaccine
The three cases (one of which was fatal) occurred in two women and one man in their 30s or 40s and involved blockages of the carotid and middle cerebral artery. Two of the three patients also had venous thrombosis involving the portal and cerebral venous system. All three also had extremely low platelet counts, confirmed antibodies to platelet factor 4, and raised D-dimer levels, all characteristic of the vaccine-induced immune thrombotic thrombocytopenia (VITT) reaction associated with the AstraZeneca vaccine.
They are described in detail in a letter published online on May 25 in the Journal of Neurology, Neurosurgery & Psychiatry
“These are [the] first detailed reports of arterial stroke believed to be caused by VITT after the AstraZeneca COVID vaccine, although stroke has been mentioned previously in the VITT data,” said senior author David Werring, PhD, FRCP.
“VITT has more commonly presented as CVST [Cerebral venous sinus thrombosis] which is stroke caused by a venous thrombosis; these cases are showing that it can also cause stroke caused by an arterial thrombosis,” explained Dr. Werring, professor of clinical neurology at the Stroke Research Centre, University College London.
“In patients who present with ischemic stroke, especially younger patients, and who have had the AstraZeneca vaccine within the past month, clinicians need to consider VITT as a possible cause, as there is a specific treatment needed for this syndrome,” he said.
Young patients presenting with ischemic stroke after receiving the AstraZeneca vaccine should urgently be evaluated for VITT with laboratory tests, including platelet count, D-dimers, fibrinogen, and anti-PF4 antibodies, the authors wrote, and then managed by a multidisciplinary team, including hematology, neurology, stroke, neurosurgery, and neuroradiology, for rapid access to treatments including intravenous immune globulin, methylprednisolone, plasmapheresis, and nonheparin anticoagulants such as fondaparinux, argatroban, or direct oral anticoagulants.
Dr. Werring noted that these reports do not add anything to the overall risk/benefit of the vaccine, as they are only describing three cases. “While VITT is very serious, the benefit of the vaccine still outweighs its risks,” he said. “Around 40% of patients hospitalized with COVID-19 experience some sort of thrombosis and about 1.5% have an ischemic stroke. Whereas latest figures from the U.K. estimate the incidence of VITT with the AstraZeneca vaccine of 1 in 50,000 to 1 in 100,000.
“Our report doesn’t suggest that VITT is more common than these latest figures estimate, but we are just drawing attention to an alternative presentation,” he added.
Three cases
The first patient in the current case series, a woman in her 30s, experienced an intermittent headache on the right side and around her eyes 6 days after the vaccine. Five days later, she awoke feeling drowsy and with weakness to her left face, arm, and leg.
Imaging revealed a blocked right middle cerebral artery with brain infarction and clots in the right portal vein. She underwent brain surgery to reduce the pressure in her skull, plasma removal and replacement, and received the anticoagulant fondaparinux, but she still unfortunately died.
The second patient, a woman in her late 30s, presented with headache, confusion, weakness in her left arm, and loss of vision on the left side 12 days after having received the vaccine. Imaging showed occlusion of both carotid arteries, as well as pulmonary embolism and a left cerebral venous sinus thrombosis.
Her platelet count increased following plasma removal and replacement and intravenous corticosteroids, and her condition improved after fondaparinux treatment.
The third patient, a man in his early 40s, presented 3 weeks after receiving his vaccination with problems speaking. Imaging showed a clot in the left middle cerebral artery, but there was no evidence of clots in the cerebral venous sinuses. He received a platelet and plasma transfusion, and fondaparinux, and remains stable.
High index of suspicion required
In a linked commentary, Hugh Markus, PhD, FRCP, professor of stroke medicine at the University of Cambridge, United Kingdom, wrote: “This report emphasizes that the immune mediated coagulopathy can also cause arterial thrombosis, including ischemic stroke, although venous thrombosis and especially cerebral venous sinus thrombosis appear more frequent.
“During the current period of COVID vaccination, a high index of suspicion is required to identify thrombotic episodes following vaccination,” he added. “However, it is important to remember that these side effects are rare and much less common than both cerebral venous thrombosis and ischemic stroke associated with COVID-19 infection itself.”
Risk/benefit unaltered
Several experts who commented on these reports for the Science Media Centre all agreed with Dr. Werring and Dr. Markus that these reports do not alter the current risk/benefit estimates with the vaccine.
Ian Douglas, PhD, professor of pharmacoepidemiology, London School of Hygiene & Tropical Medicine, who sits on the U.K.’s Medicines and Healthcare Products Regulatory Agency’s Pharmacovigilance Expert Advisory Group, said: “The picture regarding the rare syndrome of blood clots combined with low platelet counts associated with the AstraZeneca vaccine is becoming clearer. Until now, the cases described have tended to involve clots in veins such as cerebral vein thrombosis. In this series of three case reports, we now have some evidence that the types of blood vessels affected include arteries as well as veins.”
“It’s important to stress that such cases remain very rare, and it’s certainly much rarer in people who have had the AstraZeneca vaccine than it is in people affected by COVID-19 itself,” Dr. Douglas emphasized.
“The description of the cases suggests the patients involved presented with the same kind of symptoms as already described in cases involving cerebral vein thrombosis, and they don’t suggest patients need to be on the alert for anything different,” he added.
“However, the emergence of details like this will help guide health professionals who may be faced with similar cases in future; the sooner such cases are recognized, the more chance they will quickly receive the right kind of treatment, hopefully leading to better outcomes.”
Will Lester, MBChB, PhD, consultant hematologist, University Hospitals Birmingham NHS Foundation Trust, said: “VITT remains a rare complication, and patients with a history of thrombosis, including stroke, should not consider themselves to be at any higher risk of this type of rare thrombosis after vaccination, and COVID infection itself is a significant risk for stroke and other types of thrombosis.”
Many countries have paused use of the AstraZeneca vaccine because of its link to the VITT syndrome or restricted its use to older people as the VITT reaction appears to be slightly more common in younger people. In the United Kingdom, the current recommendation is that individuals under 40 years of age should be offered an alternative to the AstraZeneca vaccine where possible.
A version of this article first appeared on Medscape.com.
The three cases (one of which was fatal) occurred in two women and one man in their 30s or 40s and involved blockages of the carotid and middle cerebral artery. Two of the three patients also had venous thrombosis involving the portal and cerebral venous system. All three also had extremely low platelet counts, confirmed antibodies to platelet factor 4, and raised D-dimer levels, all characteristic of the vaccine-induced immune thrombotic thrombocytopenia (VITT) reaction associated with the AstraZeneca vaccine.
They are described in detail in a letter published online on May 25 in the Journal of Neurology, Neurosurgery & Psychiatry
“These are [the] first detailed reports of arterial stroke believed to be caused by VITT after the AstraZeneca COVID vaccine, although stroke has been mentioned previously in the VITT data,” said senior author David Werring, PhD, FRCP.
“VITT has more commonly presented as CVST [Cerebral venous sinus thrombosis] which is stroke caused by a venous thrombosis; these cases are showing that it can also cause stroke caused by an arterial thrombosis,” explained Dr. Werring, professor of clinical neurology at the Stroke Research Centre, University College London.
“In patients who present with ischemic stroke, especially younger patients, and who have had the AstraZeneca vaccine within the past month, clinicians need to consider VITT as a possible cause, as there is a specific treatment needed for this syndrome,” he said.
Young patients presenting with ischemic stroke after receiving the AstraZeneca vaccine should urgently be evaluated for VITT with laboratory tests, including platelet count, D-dimers, fibrinogen, and anti-PF4 antibodies, the authors wrote, and then managed by a multidisciplinary team, including hematology, neurology, stroke, neurosurgery, and neuroradiology, for rapid access to treatments including intravenous immune globulin, methylprednisolone, plasmapheresis, and nonheparin anticoagulants such as fondaparinux, argatroban, or direct oral anticoagulants.
Dr. Werring noted that these reports do not add anything to the overall risk/benefit of the vaccine, as they are only describing three cases. “While VITT is very serious, the benefit of the vaccine still outweighs its risks,” he said. “Around 40% of patients hospitalized with COVID-19 experience some sort of thrombosis and about 1.5% have an ischemic stroke. Whereas latest figures from the U.K. estimate the incidence of VITT with the AstraZeneca vaccine of 1 in 50,000 to 1 in 100,000.
“Our report doesn’t suggest that VITT is more common than these latest figures estimate, but we are just drawing attention to an alternative presentation,” he added.
Three cases
The first patient in the current case series, a woman in her 30s, experienced an intermittent headache on the right side and around her eyes 6 days after the vaccine. Five days later, she awoke feeling drowsy and with weakness to her left face, arm, and leg.
Imaging revealed a blocked right middle cerebral artery with brain infarction and clots in the right portal vein. She underwent brain surgery to reduce the pressure in her skull, plasma removal and replacement, and received the anticoagulant fondaparinux, but she still unfortunately died.
The second patient, a woman in her late 30s, presented with headache, confusion, weakness in her left arm, and loss of vision on the left side 12 days after having received the vaccine. Imaging showed occlusion of both carotid arteries, as well as pulmonary embolism and a left cerebral venous sinus thrombosis.
Her platelet count increased following plasma removal and replacement and intravenous corticosteroids, and her condition improved after fondaparinux treatment.
The third patient, a man in his early 40s, presented 3 weeks after receiving his vaccination with problems speaking. Imaging showed a clot in the left middle cerebral artery, but there was no evidence of clots in the cerebral venous sinuses. He received a platelet and plasma transfusion, and fondaparinux, and remains stable.
High index of suspicion required
In a linked commentary, Hugh Markus, PhD, FRCP, professor of stroke medicine at the University of Cambridge, United Kingdom, wrote: “This report emphasizes that the immune mediated coagulopathy can also cause arterial thrombosis, including ischemic stroke, although venous thrombosis and especially cerebral venous sinus thrombosis appear more frequent.
“During the current period of COVID vaccination, a high index of suspicion is required to identify thrombotic episodes following vaccination,” he added. “However, it is important to remember that these side effects are rare and much less common than both cerebral venous thrombosis and ischemic stroke associated with COVID-19 infection itself.”
Risk/benefit unaltered
Several experts who commented on these reports for the Science Media Centre all agreed with Dr. Werring and Dr. Markus that these reports do not alter the current risk/benefit estimates with the vaccine.
Ian Douglas, PhD, professor of pharmacoepidemiology, London School of Hygiene & Tropical Medicine, who sits on the U.K.’s Medicines and Healthcare Products Regulatory Agency’s Pharmacovigilance Expert Advisory Group, said: “The picture regarding the rare syndrome of blood clots combined with low platelet counts associated with the AstraZeneca vaccine is becoming clearer. Until now, the cases described have tended to involve clots in veins such as cerebral vein thrombosis. In this series of three case reports, we now have some evidence that the types of blood vessels affected include arteries as well as veins.”
“It’s important to stress that such cases remain very rare, and it’s certainly much rarer in people who have had the AstraZeneca vaccine than it is in people affected by COVID-19 itself,” Dr. Douglas emphasized.
“The description of the cases suggests the patients involved presented with the same kind of symptoms as already described in cases involving cerebral vein thrombosis, and they don’t suggest patients need to be on the alert for anything different,” he added.
“However, the emergence of details like this will help guide health professionals who may be faced with similar cases in future; the sooner such cases are recognized, the more chance they will quickly receive the right kind of treatment, hopefully leading to better outcomes.”
Will Lester, MBChB, PhD, consultant hematologist, University Hospitals Birmingham NHS Foundation Trust, said: “VITT remains a rare complication, and patients with a history of thrombosis, including stroke, should not consider themselves to be at any higher risk of this type of rare thrombosis after vaccination, and COVID infection itself is a significant risk for stroke and other types of thrombosis.”
Many countries have paused use of the AstraZeneca vaccine because of its link to the VITT syndrome or restricted its use to older people as the VITT reaction appears to be slightly more common in younger people. In the United Kingdom, the current recommendation is that individuals under 40 years of age should be offered an alternative to the AstraZeneca vaccine where possible.
A version of this article first appeared on Medscape.com.
The three cases (one of which was fatal) occurred in two women and one man in their 30s or 40s and involved blockages of the carotid and middle cerebral artery. Two of the three patients also had venous thrombosis involving the portal and cerebral venous system. All three also had extremely low platelet counts, confirmed antibodies to platelet factor 4, and raised D-dimer levels, all characteristic of the vaccine-induced immune thrombotic thrombocytopenia (VITT) reaction associated with the AstraZeneca vaccine.
They are described in detail in a letter published online on May 25 in the Journal of Neurology, Neurosurgery & Psychiatry
“These are [the] first detailed reports of arterial stroke believed to be caused by VITT after the AstraZeneca COVID vaccine, although stroke has been mentioned previously in the VITT data,” said senior author David Werring, PhD, FRCP.
“VITT has more commonly presented as CVST [Cerebral venous sinus thrombosis] which is stroke caused by a venous thrombosis; these cases are showing that it can also cause stroke caused by an arterial thrombosis,” explained Dr. Werring, professor of clinical neurology at the Stroke Research Centre, University College London.
“In patients who present with ischemic stroke, especially younger patients, and who have had the AstraZeneca vaccine within the past month, clinicians need to consider VITT as a possible cause, as there is a specific treatment needed for this syndrome,” he said.
Young patients presenting with ischemic stroke after receiving the AstraZeneca vaccine should urgently be evaluated for VITT with laboratory tests, including platelet count, D-dimers, fibrinogen, and anti-PF4 antibodies, the authors wrote, and then managed by a multidisciplinary team, including hematology, neurology, stroke, neurosurgery, and neuroradiology, for rapid access to treatments including intravenous immune globulin, methylprednisolone, plasmapheresis, and nonheparin anticoagulants such as fondaparinux, argatroban, or direct oral anticoagulants.
Dr. Werring noted that these reports do not add anything to the overall risk/benefit of the vaccine, as they are only describing three cases. “While VITT is very serious, the benefit of the vaccine still outweighs its risks,” he said. “Around 40% of patients hospitalized with COVID-19 experience some sort of thrombosis and about 1.5% have an ischemic stroke. Whereas latest figures from the U.K. estimate the incidence of VITT with the AstraZeneca vaccine of 1 in 50,000 to 1 in 100,000.
“Our report doesn’t suggest that VITT is more common than these latest figures estimate, but we are just drawing attention to an alternative presentation,” he added.
Three cases
The first patient in the current case series, a woman in her 30s, experienced an intermittent headache on the right side and around her eyes 6 days after the vaccine. Five days later, she awoke feeling drowsy and with weakness to her left face, arm, and leg.
Imaging revealed a blocked right middle cerebral artery with brain infarction and clots in the right portal vein. She underwent brain surgery to reduce the pressure in her skull, plasma removal and replacement, and received the anticoagulant fondaparinux, but she still unfortunately died.
The second patient, a woman in her late 30s, presented with headache, confusion, weakness in her left arm, and loss of vision on the left side 12 days after having received the vaccine. Imaging showed occlusion of both carotid arteries, as well as pulmonary embolism and a left cerebral venous sinus thrombosis.
Her platelet count increased following plasma removal and replacement and intravenous corticosteroids, and her condition improved after fondaparinux treatment.
The third patient, a man in his early 40s, presented 3 weeks after receiving his vaccination with problems speaking. Imaging showed a clot in the left middle cerebral artery, but there was no evidence of clots in the cerebral venous sinuses. He received a platelet and plasma transfusion, and fondaparinux, and remains stable.
High index of suspicion required
In a linked commentary, Hugh Markus, PhD, FRCP, professor of stroke medicine at the University of Cambridge, United Kingdom, wrote: “This report emphasizes that the immune mediated coagulopathy can also cause arterial thrombosis, including ischemic stroke, although venous thrombosis and especially cerebral venous sinus thrombosis appear more frequent.
“During the current period of COVID vaccination, a high index of suspicion is required to identify thrombotic episodes following vaccination,” he added. “However, it is important to remember that these side effects are rare and much less common than both cerebral venous thrombosis and ischemic stroke associated with COVID-19 infection itself.”
Risk/benefit unaltered
Several experts who commented on these reports for the Science Media Centre all agreed with Dr. Werring and Dr. Markus that these reports do not alter the current risk/benefit estimates with the vaccine.
Ian Douglas, PhD, professor of pharmacoepidemiology, London School of Hygiene & Tropical Medicine, who sits on the U.K.’s Medicines and Healthcare Products Regulatory Agency’s Pharmacovigilance Expert Advisory Group, said: “The picture regarding the rare syndrome of blood clots combined with low platelet counts associated with the AstraZeneca vaccine is becoming clearer. Until now, the cases described have tended to involve clots in veins such as cerebral vein thrombosis. In this series of three case reports, we now have some evidence that the types of blood vessels affected include arteries as well as veins.”
“It’s important to stress that such cases remain very rare, and it’s certainly much rarer in people who have had the AstraZeneca vaccine than it is in people affected by COVID-19 itself,” Dr. Douglas emphasized.
“The description of the cases suggests the patients involved presented with the same kind of symptoms as already described in cases involving cerebral vein thrombosis, and they don’t suggest patients need to be on the alert for anything different,” he added.
“However, the emergence of details like this will help guide health professionals who may be faced with similar cases in future; the sooner such cases are recognized, the more chance they will quickly receive the right kind of treatment, hopefully leading to better outcomes.”
Will Lester, MBChB, PhD, consultant hematologist, University Hospitals Birmingham NHS Foundation Trust, said: “VITT remains a rare complication, and patients with a history of thrombosis, including stroke, should not consider themselves to be at any higher risk of this type of rare thrombosis after vaccination, and COVID infection itself is a significant risk for stroke and other types of thrombosis.”
Many countries have paused use of the AstraZeneca vaccine because of its link to the VITT syndrome or restricted its use to older people as the VITT reaction appears to be slightly more common in younger people. In the United Kingdom, the current recommendation is that individuals under 40 years of age should be offered an alternative to the AstraZeneca vaccine where possible.
A version of this article first appeared on Medscape.com.
Benefit from cooling temps for cardiac arrest does not differ in randomized trial
The first randomized controlled trial to compare specific temperatures for therapeutic hypothermia in comatose survivors of out-of-hospital cardiac arrest showed no differences in major outcomes, according to a single-center, double-blind study.
In the CAPITAL-CHILL trial, cooling temperatures of 31° C and 34° C were compared to explore the hypothesis that a lower temperature would improve major outcomes, explained Michel Le May, MD.
No differences for the primary composite outcome of all-cause mortality or poor neurologic outcome at 180 days were observed, he reported at the annual scientific sessions of the American College of Cardiology.
The study was completed over a period of almost 7 years in patients presumed to have had an out-of-hospital cardiac arrest and who were unconscious when they reached a center affiliated with the Ottawa Heart Institute, where Dr. Le May directs the regional STEMI (ST-elevation myocardial infarction) program. The initial rhythm at the time of the cardiac arrest was not an entry criterion.
Of 389 patients enrolled, the intention-to-treat analysis included 184 randomized to a cooling temperature of 31° C group and 183 to a temperature of 34° C. The assigned target temperature, reached with an endovascular device, was known only by the managing nurses.
31° C and 34° C are equivalent
There was a small numerical disadvantage for the lower temperature assignment, but none reached statistical significance. This was true of the primary outcome (48.4% vs. 45.4% for the higher temperature) and its components of mortality (43.5% vs. 41.0%) and poor neurologic outcome (4.9% vs. 4.4%). Poor neurologic outcome was defined as a Disability Rating Scale score of greater than 5.
Deaths were most common in the early part of the 180-day follow-up in both arms. On a Kaplan-Meier survival graph, Dr. Le May showed curves that he characterized as “almost superimposable.”
There were no significant differences for any subgroup stratifications, such as age 75 years or older versus younger, males versus females, presence versus absence or an initial shockable rhythm, percutaneous coronary intervention (PCI) within 24 hours versus later, and STEMI versus non-STEMI. In these analyses, the higher temperature was associated with a potential trend for benefit among females and those with a shockable rhythm.
There was no signal for a difference in neurologic outcomes on the Disability Rating Scale or the Modified Rankin Scale. On the latter, for example, 46% of those in the 31° C group and 44% of these in the 34° C group had a score of four or greater at the end of follow-up.
The baseline characteristics of the two groups were similar. About 80% were male; the average age was roughly 62 years. More than 80% of the cardiac arrests were witnessed with CPR being administered by bystanders in nearly 70%. Nearly 40% had a STEMI.
Interventions were similar. Almost all patients underwent coronary angiography, of which nearly 60% received a percutaneous coronary intervention. More than 50% received a stent. The time from arrest to randomization was slightly longer in the 31° C group (228 vs. 204 minutes). The time to balloon inflation from arrival at the cardiac center was also slightly longer (73 vs. 60 minutes).
There was a trend for an increased rate of seizures in the 31° C group (12.5% vs. 7.1%; P = .08), but other secondary outcomes, including pneumonia (67.8% vs. 63.4%), renal replacement therapy (9.2% vs. 9.3%), and stroke (4.4% vs. 1.6%), were similar in the 31° C and 34° C groups, respectively.
Bleeding, whether measured by transfusion (19.6% vs. 22.4%) or TIMI major bleed (23.4% vs. 19.7%) were similar in the 31° C and 34° C groups, respectively. Thrombosis, whether measured by stent thrombosis (1.2% vs. 2.2%) or deep venous thrombosis (11.4% vs. 10.9%) were similar in these two groups, respectively.
The length of stay in the cardiac intensive care unit was significantly greater in the 31° C group (10 vs. 7 days; P = .004). Some of this increased length of stay can be attributed to the longer rewarming process required for the greater cooling, according to Dr. Le May, but he acknowledged that it is not clear this provides a full explanation.
More trials like CAPITAL-CHILL needed
The validity of these findings is supported by several strengths of the methodology, according to Jeanne E. Poole, MD, director of the arrhythmia service and electrophysiology laboratory, University of Washington, Seattle. This includes the reliance of an endovascular device, which can accelerate the time to the target temperature and assure the precision with which it is reached and maintained.
Dr. Poole did note that many of the primary and secondary measures, including the rates of stroke, seizures, and major bleeds, even though not significantly different, favored the higher temperature. The slightly longer door-to-balloon times might have been a factor. For the higher rate of pneumonia in the 31° C group, she questioned whether the longer period of ventilation linked to a longer period of rewarming might have been a factor.
However, Dr. Poole praised the CAPITAL-CHILL trial for drawing attention to a group of patients for whom survival rates remain “dismally low.” She indicated that these types of high-level trials are needed to look for strategies to improve outcomes.
Dr. Le May and Dr. Poole report no potential conflicts of interest.
The first randomized controlled trial to compare specific temperatures for therapeutic hypothermia in comatose survivors of out-of-hospital cardiac arrest showed no differences in major outcomes, according to a single-center, double-blind study.
In the CAPITAL-CHILL trial, cooling temperatures of 31° C and 34° C were compared to explore the hypothesis that a lower temperature would improve major outcomes, explained Michel Le May, MD.
No differences for the primary composite outcome of all-cause mortality or poor neurologic outcome at 180 days were observed, he reported at the annual scientific sessions of the American College of Cardiology.
The study was completed over a period of almost 7 years in patients presumed to have had an out-of-hospital cardiac arrest and who were unconscious when they reached a center affiliated with the Ottawa Heart Institute, where Dr. Le May directs the regional STEMI (ST-elevation myocardial infarction) program. The initial rhythm at the time of the cardiac arrest was not an entry criterion.
Of 389 patients enrolled, the intention-to-treat analysis included 184 randomized to a cooling temperature of 31° C group and 183 to a temperature of 34° C. The assigned target temperature, reached with an endovascular device, was known only by the managing nurses.
31° C and 34° C are equivalent
There was a small numerical disadvantage for the lower temperature assignment, but none reached statistical significance. This was true of the primary outcome (48.4% vs. 45.4% for the higher temperature) and its components of mortality (43.5% vs. 41.0%) and poor neurologic outcome (4.9% vs. 4.4%). Poor neurologic outcome was defined as a Disability Rating Scale score of greater than 5.
Deaths were most common in the early part of the 180-day follow-up in both arms. On a Kaplan-Meier survival graph, Dr. Le May showed curves that he characterized as “almost superimposable.”
There were no significant differences for any subgroup stratifications, such as age 75 years or older versus younger, males versus females, presence versus absence or an initial shockable rhythm, percutaneous coronary intervention (PCI) within 24 hours versus later, and STEMI versus non-STEMI. In these analyses, the higher temperature was associated with a potential trend for benefit among females and those with a shockable rhythm.
There was no signal for a difference in neurologic outcomes on the Disability Rating Scale or the Modified Rankin Scale. On the latter, for example, 46% of those in the 31° C group and 44% of these in the 34° C group had a score of four or greater at the end of follow-up.
The baseline characteristics of the two groups were similar. About 80% were male; the average age was roughly 62 years. More than 80% of the cardiac arrests were witnessed with CPR being administered by bystanders in nearly 70%. Nearly 40% had a STEMI.
Interventions were similar. Almost all patients underwent coronary angiography, of which nearly 60% received a percutaneous coronary intervention. More than 50% received a stent. The time from arrest to randomization was slightly longer in the 31° C group (228 vs. 204 minutes). The time to balloon inflation from arrival at the cardiac center was also slightly longer (73 vs. 60 minutes).
There was a trend for an increased rate of seizures in the 31° C group (12.5% vs. 7.1%; P = .08), but other secondary outcomes, including pneumonia (67.8% vs. 63.4%), renal replacement therapy (9.2% vs. 9.3%), and stroke (4.4% vs. 1.6%), were similar in the 31° C and 34° C groups, respectively.
Bleeding, whether measured by transfusion (19.6% vs. 22.4%) or TIMI major bleed (23.4% vs. 19.7%) were similar in the 31° C and 34° C groups, respectively. Thrombosis, whether measured by stent thrombosis (1.2% vs. 2.2%) or deep venous thrombosis (11.4% vs. 10.9%) were similar in these two groups, respectively.
The length of stay in the cardiac intensive care unit was significantly greater in the 31° C group (10 vs. 7 days; P = .004). Some of this increased length of stay can be attributed to the longer rewarming process required for the greater cooling, according to Dr. Le May, but he acknowledged that it is not clear this provides a full explanation.
More trials like CAPITAL-CHILL needed
The validity of these findings is supported by several strengths of the methodology, according to Jeanne E. Poole, MD, director of the arrhythmia service and electrophysiology laboratory, University of Washington, Seattle. This includes the reliance of an endovascular device, which can accelerate the time to the target temperature and assure the precision with which it is reached and maintained.
Dr. Poole did note that many of the primary and secondary measures, including the rates of stroke, seizures, and major bleeds, even though not significantly different, favored the higher temperature. The slightly longer door-to-balloon times might have been a factor. For the higher rate of pneumonia in the 31° C group, she questioned whether the longer period of ventilation linked to a longer period of rewarming might have been a factor.
However, Dr. Poole praised the CAPITAL-CHILL trial for drawing attention to a group of patients for whom survival rates remain “dismally low.” She indicated that these types of high-level trials are needed to look for strategies to improve outcomes.
Dr. Le May and Dr. Poole report no potential conflicts of interest.
The first randomized controlled trial to compare specific temperatures for therapeutic hypothermia in comatose survivors of out-of-hospital cardiac arrest showed no differences in major outcomes, according to a single-center, double-blind study.
In the CAPITAL-CHILL trial, cooling temperatures of 31° C and 34° C were compared to explore the hypothesis that a lower temperature would improve major outcomes, explained Michel Le May, MD.
No differences for the primary composite outcome of all-cause mortality or poor neurologic outcome at 180 days were observed, he reported at the annual scientific sessions of the American College of Cardiology.
The study was completed over a period of almost 7 years in patients presumed to have had an out-of-hospital cardiac arrest and who were unconscious when they reached a center affiliated with the Ottawa Heart Institute, where Dr. Le May directs the regional STEMI (ST-elevation myocardial infarction) program. The initial rhythm at the time of the cardiac arrest was not an entry criterion.
Of 389 patients enrolled, the intention-to-treat analysis included 184 randomized to a cooling temperature of 31° C group and 183 to a temperature of 34° C. The assigned target temperature, reached with an endovascular device, was known only by the managing nurses.
31° C and 34° C are equivalent
There was a small numerical disadvantage for the lower temperature assignment, but none reached statistical significance. This was true of the primary outcome (48.4% vs. 45.4% for the higher temperature) and its components of mortality (43.5% vs. 41.0%) and poor neurologic outcome (4.9% vs. 4.4%). Poor neurologic outcome was defined as a Disability Rating Scale score of greater than 5.
Deaths were most common in the early part of the 180-day follow-up in both arms. On a Kaplan-Meier survival graph, Dr. Le May showed curves that he characterized as “almost superimposable.”
There were no significant differences for any subgroup stratifications, such as age 75 years or older versus younger, males versus females, presence versus absence or an initial shockable rhythm, percutaneous coronary intervention (PCI) within 24 hours versus later, and STEMI versus non-STEMI. In these analyses, the higher temperature was associated with a potential trend for benefit among females and those with a shockable rhythm.
There was no signal for a difference in neurologic outcomes on the Disability Rating Scale or the Modified Rankin Scale. On the latter, for example, 46% of those in the 31° C group and 44% of these in the 34° C group had a score of four or greater at the end of follow-up.
The baseline characteristics of the two groups were similar. About 80% were male; the average age was roughly 62 years. More than 80% of the cardiac arrests were witnessed with CPR being administered by bystanders in nearly 70%. Nearly 40% had a STEMI.
Interventions were similar. Almost all patients underwent coronary angiography, of which nearly 60% received a percutaneous coronary intervention. More than 50% received a stent. The time from arrest to randomization was slightly longer in the 31° C group (228 vs. 204 minutes). The time to balloon inflation from arrival at the cardiac center was also slightly longer (73 vs. 60 minutes).
There was a trend for an increased rate of seizures in the 31° C group (12.5% vs. 7.1%; P = .08), but other secondary outcomes, including pneumonia (67.8% vs. 63.4%), renal replacement therapy (9.2% vs. 9.3%), and stroke (4.4% vs. 1.6%), were similar in the 31° C and 34° C groups, respectively.
Bleeding, whether measured by transfusion (19.6% vs. 22.4%) or TIMI major bleed (23.4% vs. 19.7%) were similar in the 31° C and 34° C groups, respectively. Thrombosis, whether measured by stent thrombosis (1.2% vs. 2.2%) or deep venous thrombosis (11.4% vs. 10.9%) were similar in these two groups, respectively.
The length of stay in the cardiac intensive care unit was significantly greater in the 31° C group (10 vs. 7 days; P = .004). Some of this increased length of stay can be attributed to the longer rewarming process required for the greater cooling, according to Dr. Le May, but he acknowledged that it is not clear this provides a full explanation.
More trials like CAPITAL-CHILL needed
The validity of these findings is supported by several strengths of the methodology, according to Jeanne E. Poole, MD, director of the arrhythmia service and electrophysiology laboratory, University of Washington, Seattle. This includes the reliance of an endovascular device, which can accelerate the time to the target temperature and assure the precision with which it is reached and maintained.
Dr. Poole did note that many of the primary and secondary measures, including the rates of stroke, seizures, and major bleeds, even though not significantly different, favored the higher temperature. The slightly longer door-to-balloon times might have been a factor. For the higher rate of pneumonia in the 31° C group, she questioned whether the longer period of ventilation linked to a longer period of rewarming might have been a factor.
However, Dr. Poole praised the CAPITAL-CHILL trial for drawing attention to a group of patients for whom survival rates remain “dismally low.” She indicated that these types of high-level trials are needed to look for strategies to improve outcomes.
Dr. Le May and Dr. Poole report no potential conflicts of interest.
FROM ACC 2021
The end of happy hour? No safe level of alcohol for the brain
There is no safe amount of alcohol consumption for the brain; even moderate drinking adversely affects brain structure and function, according a British study of more 25,000 adults.
“This is one of the largest studies of alcohol and brain health to date,” Anya Topiwala, DPhil, University of Oxford (England), told this news organization.
“There have been previous claims the relationship between alcohol and brain health are J-shaped (ie., small amounts are protective), but we formally tested this and did not find it to be the case. In fact, we found that any level of alcohol was associated with poorer brain health, compared to no alcohol,” Dr. Topiwala added.
The study, which has not yet been peer reviewed, was published online May 12 in MedRxiv.
Global impact on the brain
Participants provided detailed information on their alcohol intake. The cohort included 691 never-drinkers, 617 former drinkers, and 24,069 current drinkers.
Median alcohol intake was 13.5 units (102 g) weekly. Almost half of the sample (48.2%) were drinking above current UK low-risk guidelines (14 units, 112 g weekly), but few were heavy drinkers (>50 units, 400 g weekly).
After adjusting for all known potential confounders and multiple comparisons, a higher volume of alcohol consumed per week was associated with lower gray matter in “almost all areas of the brain,” Dr. Topiwala said in an interview.
Alcohol consumption accounted for up to 0.8% of gray matter volume variance. “The size of the effect is small, albeit greater than any other modifiable risk factor. These brain changes have been previously linked to aging, poorer performance on memory changes, and dementia,” Dr. Topiwala said.
Widespread negative associations were also found between drinking alcohol and all the measures of white matter integrity that were assessed. There was a significant positive association between alcohol consumption and resting-state functional connectivity.
Higher blood pressure and body mass index “steepened” the negative associations between alcohol and brain health, and binge drinking had additive negative effects on brain structure beyond the absolute volume consumed.
There was no evidence that the risk for alcohol-related brain harm differs according to the type of alcohol consumed (wine, beer, or spirits).
A key limitation of the study is that the study population from the UK Biobank represents a sample that is healthier, better educated, and less deprived and is characterized by less ethnic diversity than the general population. “As with any observational study, we cannot infer causality from association,” the authors note.
What remains unclear, they say, is the duration of drinking needed to cause an effect on the brain. It may be that vulnerability is increased during periods of life in which dynamic brain changes occur, such as adolescence and older age.
They also note that some studies of alcohol-dependent individuals have suggested that at least some brain damage is reversible upon abstinence. Whether that is true for moderate drinkers is unknown.
On the basis of their findings, there is “no safe dose of alcohol for the brain,” Dr. Topiwala and colleagues conclude. They suggest that current low-risk drinking guidelines be revisited to take account of brain effects.
Experts weigh in
Several experts weighed in on the study in a statement from the nonprofit UK Science Media Center.
Paul Matthews, MD, head of the department of brain sciences, Imperial College London, noted that this “carefully performed preliminary report extends our earlier UK Dementia Research Institute study of a smaller group from same UK Biobank population also showing that even moderate drinking is associated with greater atrophy of the brain, as well as injury to the heart and liver.”
Dr. Matthews said the investigators’ conclusion that there is no safe threshold below which alcohol consumption has no toxic effects “echoes our own. We join with them in suggesting that current public health guidelines concerning alcohol consumption may need to be revisited.”
Rebecca Dewey, PhD, research fellow in neuroimaging, University of Nottingham (England), cautioned that “the degree to which very small changes in brain volume are harmful” is unknown.
“While there was no threshold under which alcohol consumption did not cause changes in the brain, there may a degree of brain volume difference that is irrelevant to brain health. We don’t know what these people’s brains looked like before they drank alcohol, so the brain may have learned to cope/compensate,” Dewey said.
Sadie Boniface, PhD, head of research at the Institute of Alcohol Studies and visiting researcher at King’s College London, said, “While we can’t yet say for sure whether there is ‘no safe level’ of alcohol regarding brain health at the moment, it has been known for decades that heavy drinking is bad for brain health.
“We also shouldn’t forget alcohol affects all parts of the body and there are multiple health risks. For example, it is already known there is ‘no safe level’ of alcohol consumption for the seven types of cancer caused by alcohol, as identified by the UK chief medical officers,” Dr. Boniface said.
The study was supported in part by the Wellcome Trust, Li Ka Shing Center for Health Information and Discovery, the National Institutes of Health, and the UK Medical Research Council. Dr. Topiwala, Dr. Boniface, Dr. Dewey, and Dr. Matthews have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
There is no safe amount of alcohol consumption for the brain; even moderate drinking adversely affects brain structure and function, according a British study of more 25,000 adults.
“This is one of the largest studies of alcohol and brain health to date,” Anya Topiwala, DPhil, University of Oxford (England), told this news organization.
“There have been previous claims the relationship between alcohol and brain health are J-shaped (ie., small amounts are protective), but we formally tested this and did not find it to be the case. In fact, we found that any level of alcohol was associated with poorer brain health, compared to no alcohol,” Dr. Topiwala added.
The study, which has not yet been peer reviewed, was published online May 12 in MedRxiv.
Global impact on the brain
Participants provided detailed information on their alcohol intake. The cohort included 691 never-drinkers, 617 former drinkers, and 24,069 current drinkers.
Median alcohol intake was 13.5 units (102 g) weekly. Almost half of the sample (48.2%) were drinking above current UK low-risk guidelines (14 units, 112 g weekly), but few were heavy drinkers (>50 units, 400 g weekly).
After adjusting for all known potential confounders and multiple comparisons, a higher volume of alcohol consumed per week was associated with lower gray matter in “almost all areas of the brain,” Dr. Topiwala said in an interview.
Alcohol consumption accounted for up to 0.8% of gray matter volume variance. “The size of the effect is small, albeit greater than any other modifiable risk factor. These brain changes have been previously linked to aging, poorer performance on memory changes, and dementia,” Dr. Topiwala said.
Widespread negative associations were also found between drinking alcohol and all the measures of white matter integrity that were assessed. There was a significant positive association between alcohol consumption and resting-state functional connectivity.
Higher blood pressure and body mass index “steepened” the negative associations between alcohol and brain health, and binge drinking had additive negative effects on brain structure beyond the absolute volume consumed.
There was no evidence that the risk for alcohol-related brain harm differs according to the type of alcohol consumed (wine, beer, or spirits).
A key limitation of the study is that the study population from the UK Biobank represents a sample that is healthier, better educated, and less deprived and is characterized by less ethnic diversity than the general population. “As with any observational study, we cannot infer causality from association,” the authors note.
What remains unclear, they say, is the duration of drinking needed to cause an effect on the brain. It may be that vulnerability is increased during periods of life in which dynamic brain changes occur, such as adolescence and older age.
They also note that some studies of alcohol-dependent individuals have suggested that at least some brain damage is reversible upon abstinence. Whether that is true for moderate drinkers is unknown.
On the basis of their findings, there is “no safe dose of alcohol for the brain,” Dr. Topiwala and colleagues conclude. They suggest that current low-risk drinking guidelines be revisited to take account of brain effects.
Experts weigh in
Several experts weighed in on the study in a statement from the nonprofit UK Science Media Center.
Paul Matthews, MD, head of the department of brain sciences, Imperial College London, noted that this “carefully performed preliminary report extends our earlier UK Dementia Research Institute study of a smaller group from same UK Biobank population also showing that even moderate drinking is associated with greater atrophy of the brain, as well as injury to the heart and liver.”
Dr. Matthews said the investigators’ conclusion that there is no safe threshold below which alcohol consumption has no toxic effects “echoes our own. We join with them in suggesting that current public health guidelines concerning alcohol consumption may need to be revisited.”
Rebecca Dewey, PhD, research fellow in neuroimaging, University of Nottingham (England), cautioned that “the degree to which very small changes in brain volume are harmful” is unknown.
“While there was no threshold under which alcohol consumption did not cause changes in the brain, there may a degree of brain volume difference that is irrelevant to brain health. We don’t know what these people’s brains looked like before they drank alcohol, so the brain may have learned to cope/compensate,” Dewey said.
Sadie Boniface, PhD, head of research at the Institute of Alcohol Studies and visiting researcher at King’s College London, said, “While we can’t yet say for sure whether there is ‘no safe level’ of alcohol regarding brain health at the moment, it has been known for decades that heavy drinking is bad for brain health.
“We also shouldn’t forget alcohol affects all parts of the body and there are multiple health risks. For example, it is already known there is ‘no safe level’ of alcohol consumption for the seven types of cancer caused by alcohol, as identified by the UK chief medical officers,” Dr. Boniface said.
The study was supported in part by the Wellcome Trust, Li Ka Shing Center for Health Information and Discovery, the National Institutes of Health, and the UK Medical Research Council. Dr. Topiwala, Dr. Boniface, Dr. Dewey, and Dr. Matthews have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
There is no safe amount of alcohol consumption for the brain; even moderate drinking adversely affects brain structure and function, according a British study of more 25,000 adults.
“This is one of the largest studies of alcohol and brain health to date,” Anya Topiwala, DPhil, University of Oxford (England), told this news organization.
“There have been previous claims the relationship between alcohol and brain health are J-shaped (ie., small amounts are protective), but we formally tested this and did not find it to be the case. In fact, we found that any level of alcohol was associated with poorer brain health, compared to no alcohol,” Dr. Topiwala added.
The study, which has not yet been peer reviewed, was published online May 12 in MedRxiv.
Global impact on the brain
Participants provided detailed information on their alcohol intake. The cohort included 691 never-drinkers, 617 former drinkers, and 24,069 current drinkers.
Median alcohol intake was 13.5 units (102 g) weekly. Almost half of the sample (48.2%) were drinking above current UK low-risk guidelines (14 units, 112 g weekly), but few were heavy drinkers (>50 units, 400 g weekly).
After adjusting for all known potential confounders and multiple comparisons, a higher volume of alcohol consumed per week was associated with lower gray matter in “almost all areas of the brain,” Dr. Topiwala said in an interview.
Alcohol consumption accounted for up to 0.8% of gray matter volume variance. “The size of the effect is small, albeit greater than any other modifiable risk factor. These brain changes have been previously linked to aging, poorer performance on memory changes, and dementia,” Dr. Topiwala said.
Widespread negative associations were also found between drinking alcohol and all the measures of white matter integrity that were assessed. There was a significant positive association between alcohol consumption and resting-state functional connectivity.
Higher blood pressure and body mass index “steepened” the negative associations between alcohol and brain health, and binge drinking had additive negative effects on brain structure beyond the absolute volume consumed.
There was no evidence that the risk for alcohol-related brain harm differs according to the type of alcohol consumed (wine, beer, or spirits).
A key limitation of the study is that the study population from the UK Biobank represents a sample that is healthier, better educated, and less deprived and is characterized by less ethnic diversity than the general population. “As with any observational study, we cannot infer causality from association,” the authors note.
What remains unclear, they say, is the duration of drinking needed to cause an effect on the brain. It may be that vulnerability is increased during periods of life in which dynamic brain changes occur, such as adolescence and older age.
They also note that some studies of alcohol-dependent individuals have suggested that at least some brain damage is reversible upon abstinence. Whether that is true for moderate drinkers is unknown.
On the basis of their findings, there is “no safe dose of alcohol for the brain,” Dr. Topiwala and colleagues conclude. They suggest that current low-risk drinking guidelines be revisited to take account of brain effects.
Experts weigh in
Several experts weighed in on the study in a statement from the nonprofit UK Science Media Center.
Paul Matthews, MD, head of the department of brain sciences, Imperial College London, noted that this “carefully performed preliminary report extends our earlier UK Dementia Research Institute study of a smaller group from same UK Biobank population also showing that even moderate drinking is associated with greater atrophy of the brain, as well as injury to the heart and liver.”
Dr. Matthews said the investigators’ conclusion that there is no safe threshold below which alcohol consumption has no toxic effects “echoes our own. We join with them in suggesting that current public health guidelines concerning alcohol consumption may need to be revisited.”
Rebecca Dewey, PhD, research fellow in neuroimaging, University of Nottingham (England), cautioned that “the degree to which very small changes in brain volume are harmful” is unknown.
“While there was no threshold under which alcohol consumption did not cause changes in the brain, there may a degree of brain volume difference that is irrelevant to brain health. We don’t know what these people’s brains looked like before they drank alcohol, so the brain may have learned to cope/compensate,” Dewey said.
Sadie Boniface, PhD, head of research at the Institute of Alcohol Studies and visiting researcher at King’s College London, said, “While we can’t yet say for sure whether there is ‘no safe level’ of alcohol regarding brain health at the moment, it has been known for decades that heavy drinking is bad for brain health.
“We also shouldn’t forget alcohol affects all parts of the body and there are multiple health risks. For example, it is already known there is ‘no safe level’ of alcohol consumption for the seven types of cancer caused by alcohol, as identified by the UK chief medical officers,” Dr. Boniface said.
The study was supported in part by the Wellcome Trust, Li Ka Shing Center for Health Information and Discovery, the National Institutes of Health, and the UK Medical Research Council. Dr. Topiwala, Dr. Boniface, Dr. Dewey, and Dr. Matthews have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
AHA reassures myocarditis rare after COVID vaccination, benefits overwhelm risks
The benefits of COVID-19 vaccination “enormously outweigh” the rare possible risk for heart-related complications, including myocarditis, the American Heart Association/American Stroke Association (ASA) says in new statement.
The message follows a Centers for Disease Control and Prevention report that the agency is monitoring the Vaccine Adverse Events Reporting System (VAERS) and the Vaccine Safety Datalink (VSD) for cases of myocarditis that have been associated with the mRNA vaccines against SARS-CoV-2 from Pfizer and Moderna.
The “relatively few” reported cases myocarditis in adolescents or young adults have involved males more often than females, more often followed the second dose rather than the first, and were usually seen in the 4 days after vaccination, the CDC’s COVID-19 Vaccine Safety Technical Work Group (VaST) found.
“Most cases appear to be mild, and follow-up of cases is ongoing,” the CDC says. “Within CDC safety monitoring systems, rates of myocarditis reports in the window following COVID-19 vaccination have not differed from expected baseline rates.”
In their statement, the AHA/ASA “strongly urge” all adults and children 12 years and older to receive a COVID-19 vaccine as soon as possible.
“The evidence continues to indicate that the COVID-19 vaccines are nearly 100% effective at preventing death and hospitalization due to COVID-19 infection,” the groups say.
Although the investigation of cases of myocarditis related to COVID-19 vaccination is ongoing, the AHA/ASA notes that myocarditis is typically the result of an actual viral infection, “and it is yet to be determined if these cases have any correlation to receiving a COVID-19 vaccine.”
“We’ve lost hundreds of children, and there have been thousands who have been hospitalized, thousands who developed an inflammatory syndrome, and one of the pieces of that can be myocarditis,” Richard Besser, MD, president and CEO of the Robert Wood Johnson Foundation (RWJF), said today on ABC’s Good Morning America.
Still, “from my perspective, the risk of COVID is so much greater than any theoretical risk from the vaccine,” said Dr. Besser, former acting director of the CDC.
The symptoms that can occur after COVID-19 vaccination include tiredness, headache, muscle pain, chills, fever, and nausea, reminds the AHA/ASA statement. Such symptoms would “typically appear within 24-48 hours and usually pass within 36-48 hours after receiving the vaccine.”
All health care providers should be aware of the “very rare” adverse events that could be related to a COVID-19 vaccine, including myocarditis, blood clots, low platelets, and symptoms of severe inflammation, it says.
“Health care professionals should strongly consider inquiring about the timing of any recent COVID vaccination among patients presenting with these conditions, as needed, in order to provide appropriate treatment quickly,” the statement advises.
A version of this article first appeared on Medscape.com.
The benefits of COVID-19 vaccination “enormously outweigh” the rare possible risk for heart-related complications, including myocarditis, the American Heart Association/American Stroke Association (ASA) says in new statement.
The message follows a Centers for Disease Control and Prevention report that the agency is monitoring the Vaccine Adverse Events Reporting System (VAERS) and the Vaccine Safety Datalink (VSD) for cases of myocarditis that have been associated with the mRNA vaccines against SARS-CoV-2 from Pfizer and Moderna.
The “relatively few” reported cases myocarditis in adolescents or young adults have involved males more often than females, more often followed the second dose rather than the first, and were usually seen in the 4 days after vaccination, the CDC’s COVID-19 Vaccine Safety Technical Work Group (VaST) found.
“Most cases appear to be mild, and follow-up of cases is ongoing,” the CDC says. “Within CDC safety monitoring systems, rates of myocarditis reports in the window following COVID-19 vaccination have not differed from expected baseline rates.”
In their statement, the AHA/ASA “strongly urge” all adults and children 12 years and older to receive a COVID-19 vaccine as soon as possible.
“The evidence continues to indicate that the COVID-19 vaccines are nearly 100% effective at preventing death and hospitalization due to COVID-19 infection,” the groups say.
Although the investigation of cases of myocarditis related to COVID-19 vaccination is ongoing, the AHA/ASA notes that myocarditis is typically the result of an actual viral infection, “and it is yet to be determined if these cases have any correlation to receiving a COVID-19 vaccine.”
“We’ve lost hundreds of children, and there have been thousands who have been hospitalized, thousands who developed an inflammatory syndrome, and one of the pieces of that can be myocarditis,” Richard Besser, MD, president and CEO of the Robert Wood Johnson Foundation (RWJF), said today on ABC’s Good Morning America.
Still, “from my perspective, the risk of COVID is so much greater than any theoretical risk from the vaccine,” said Dr. Besser, former acting director of the CDC.
The symptoms that can occur after COVID-19 vaccination include tiredness, headache, muscle pain, chills, fever, and nausea, reminds the AHA/ASA statement. Such symptoms would “typically appear within 24-48 hours and usually pass within 36-48 hours after receiving the vaccine.”
All health care providers should be aware of the “very rare” adverse events that could be related to a COVID-19 vaccine, including myocarditis, blood clots, low platelets, and symptoms of severe inflammation, it says.
“Health care professionals should strongly consider inquiring about the timing of any recent COVID vaccination among patients presenting with these conditions, as needed, in order to provide appropriate treatment quickly,” the statement advises.
A version of this article first appeared on Medscape.com.
The benefits of COVID-19 vaccination “enormously outweigh” the rare possible risk for heart-related complications, including myocarditis, the American Heart Association/American Stroke Association (ASA) says in new statement.
The message follows a Centers for Disease Control and Prevention report that the agency is monitoring the Vaccine Adverse Events Reporting System (VAERS) and the Vaccine Safety Datalink (VSD) for cases of myocarditis that have been associated with the mRNA vaccines against SARS-CoV-2 from Pfizer and Moderna.
The “relatively few” reported cases myocarditis in adolescents or young adults have involved males more often than females, more often followed the second dose rather than the first, and were usually seen in the 4 days after vaccination, the CDC’s COVID-19 Vaccine Safety Technical Work Group (VaST) found.
“Most cases appear to be mild, and follow-up of cases is ongoing,” the CDC says. “Within CDC safety monitoring systems, rates of myocarditis reports in the window following COVID-19 vaccination have not differed from expected baseline rates.”
In their statement, the AHA/ASA “strongly urge” all adults and children 12 years and older to receive a COVID-19 vaccine as soon as possible.
“The evidence continues to indicate that the COVID-19 vaccines are nearly 100% effective at preventing death and hospitalization due to COVID-19 infection,” the groups say.
Although the investigation of cases of myocarditis related to COVID-19 vaccination is ongoing, the AHA/ASA notes that myocarditis is typically the result of an actual viral infection, “and it is yet to be determined if these cases have any correlation to receiving a COVID-19 vaccine.”
“We’ve lost hundreds of children, and there have been thousands who have been hospitalized, thousands who developed an inflammatory syndrome, and one of the pieces of that can be myocarditis,” Richard Besser, MD, president and CEO of the Robert Wood Johnson Foundation (RWJF), said today on ABC’s Good Morning America.
Still, “from my perspective, the risk of COVID is so much greater than any theoretical risk from the vaccine,” said Dr. Besser, former acting director of the CDC.
The symptoms that can occur after COVID-19 vaccination include tiredness, headache, muscle pain, chills, fever, and nausea, reminds the AHA/ASA statement. Such symptoms would “typically appear within 24-48 hours and usually pass within 36-48 hours after receiving the vaccine.”
All health care providers should be aware of the “very rare” adverse events that could be related to a COVID-19 vaccine, including myocarditis, blood clots, low platelets, and symptoms of severe inflammation, it says.
“Health care professionals should strongly consider inquiring about the timing of any recent COVID vaccination among patients presenting with these conditions, as needed, in order to provide appropriate treatment quickly,” the statement advises.
A version of this article first appeared on Medscape.com.
Physicians’ trust in health care leadership drops in pandemic
according to a survey conducted by NORC at the University of Chicago on behalf of the American Board of Internal Medicine Foundation.
Survey results, released May 21, indicate that 30% of physicians say their trust in the U.S. health care system and health care leadership has decreased during the pandemic. Only 18% reported an increase in trust.
Physicians, however, have great trust in their fellow clinicians.
In the survey of 600 physicians, 94% said they trust doctors within their practice; 85% trusted doctors outside of their practice; and 89% trusted nurses. That trust increased during the pandemic, with 41% saying their trust in fellow physicians rose and 37% saying their trust in nurses did.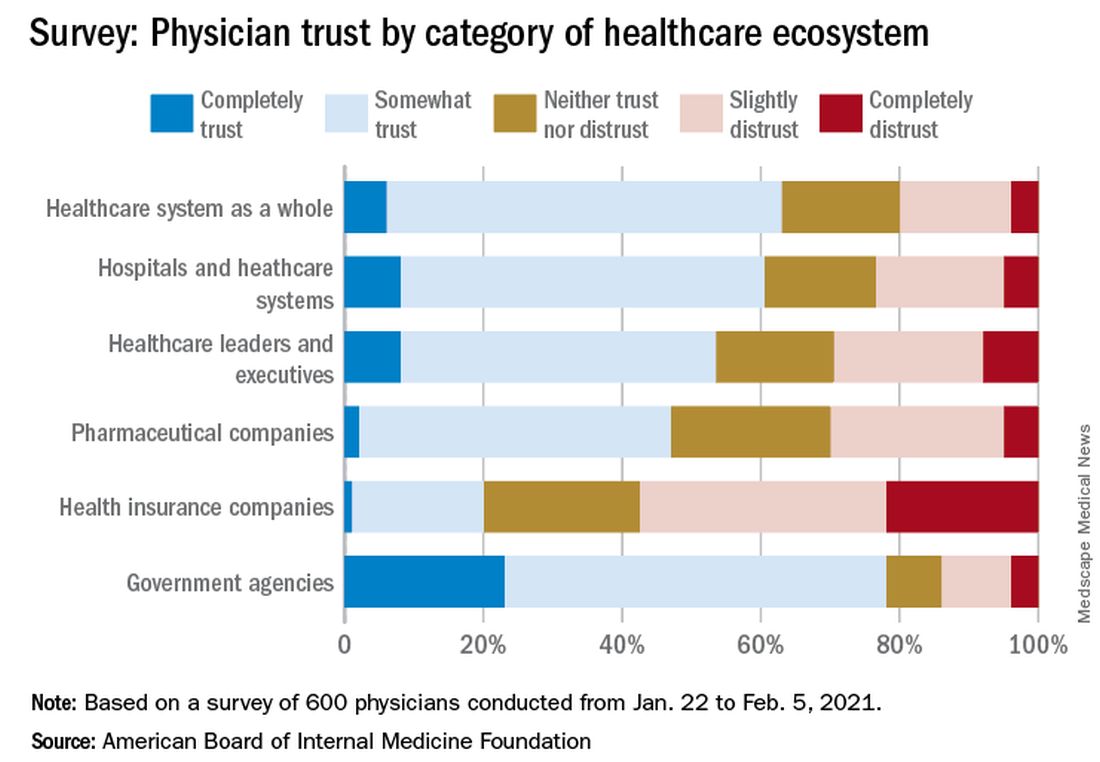
In a separate survey, NORC asked patients about their trust in various aspects of health care. Among 2,069 respondents, a wide majority reported that they trust doctors (84%) and nurses (85%), but only 64% trusted the health care system as a whole. One in three consumers (32%) said their trust in the health care system decreased during the pandemic, compared with 11% who said their trust increased.
The ABIM Foundation released the research findings on May 21 as part of Building Trust, a national campaign that aims to boost trust among patients, clinicians, system leaders, researchers, and others.
Richard J. Baron, MD, president and chief executive officer of the ABIM Foundation, said in an interview, “Clearly there’s lower trust in health care organization leaders and executives, and that’s troubling.
“Science by itself is not enough,” he said. “Becoming trustworthy has to be a core project of everybody in health care.”
Deterioration in physicians’ trust during the pandemic comes in part from failed promises of adequate personal protective equipment and some physicians’ loss of income as a result of the crisis, Dr. Baron said.
He added that the vaccine rollout was very uneven and that policies as to which elective procedures could be performed were handled differently in different parts of the country.
He also noted that, early on, transparency was lacking as to how many COVID patients hospitals were treating, which may have contributed to the decrease in trust in the system.
Fear of being known as ‘the COVID hospital’
Hospitals were afraid of being known as “the COVID hospital” and losing patients who were afraid to come there, Dr. Baron said.
He said the COVID-19 epidemic exacerbated problems regarding trust, but that trust has been declining for some time. The Building Trust campaign will focus on solutions in breaches of trust as physicians move increasingly toward being employees of huge systems, according to Dr. Baron.
However, trust works both ways, Dr. Baron notes. Physicians can be champions for their health care system or “throw the system under the bus,” he said.
For example, if a patient complains about the appointment system, clinicians who trust their institutions may say the system usually works and that they will try to make sure the patient has a better experience next time. Clinicians without trust may say they agree that the health care system doesn’t know what it is doing, and patients may further lose confidence when physicians validate their complaint, and patients may then go elsewhere.
78% of patients trust primary care doctor
When asked whether they trust their primary care physician, 78% of patients said yes. However, trust in doctors was higher among people who were older (90%), White (82%), or had high income (89%). Among people reporting lower trust, 25% said their physician spends too little time with them, and 14% said their doctor does not know or listen to them.
The survey shows that government agencies have work to do to earn trust. Responses indicate that 43% of physicians said they have “complete trust” in government health care agencies, such as the U.S. Food and Drug Administration and the Centers for Disease Control and Prevention, which is substantially higher than other parts of the health care system. However, trust in agencies declined for 43% of physician respondents and increased for 21%.
Dhruv Khullar, MD, MPP, of the department of health policy and economics at Weill Cornell Medical College in New York, told this news organization the survey results match what he sees anecdotally in medicine – that physicians have been losing trust in the system but not in their colleagues.
He said the sample size of 600 is enough to be influential, though he said he would like to know the response rate, which was not calculated for this survey.
He added that, in large part, physicians’ lack of trust in their systems may come from generally being asked to see more patients and to meet more metrics during the same or shorter periods.
Physicians’ lack of trust in the system can have significant consequences, he said. It can lead to burnout, which has been linked with poorer quality of care and physician turnover, he noted.
COVID-19 led some physicians to wonder whether their system had their best interests at heart, insofar as access to adequate medicines and supplies as well as emotional support were inconsistent, Dr. Khullar said.
He said that to regain trust health care systems need to ask themselves questions in three areas. The first is whether their goals are focused on the best interest of the organization or the best interest of the patient.
“Next is competency,” Dr. Khullar said. “Maybe your motives are right, but are you able to deliver? Are you delivering a good product, whether clinical services or something else?”
The third area is transparency, he said. “Are you going to be honest and forthright in what we’re doing and where we’re going?”
Caroline Pearson, senior vice president of health care strategy for NORC, said the emailed survey was conducted between Dec. 29, 2020, and Feb. 5, 2021, with a health care survey partner that maintains a nationwide panel of physicians across specialties.
She said this report is fairly novel insofar as surveys are more typically conducted regarding patients’ trust of their doctors or of the health care system.
Ms. Pearson said because health care is delivered in teams, understanding the level of trust among the entities helps ensure that care will be delivered effectively and seamlessly with high quality.
“We want our patients to trust our doctors, but we really want doctors to trust each other and trust the hospitals and systems in which they’re working,” she said.
Dr. Baron, Ms. Pearson, and Dr. Khullar report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
according to a survey conducted by NORC at the University of Chicago on behalf of the American Board of Internal Medicine Foundation.
Survey results, released May 21, indicate that 30% of physicians say their trust in the U.S. health care system and health care leadership has decreased during the pandemic. Only 18% reported an increase in trust.
Physicians, however, have great trust in their fellow clinicians.
In the survey of 600 physicians, 94% said they trust doctors within their practice; 85% trusted doctors outside of their practice; and 89% trusted nurses. That trust increased during the pandemic, with 41% saying their trust in fellow physicians rose and 37% saying their trust in nurses did.
In a separate survey, NORC asked patients about their trust in various aspects of health care. Among 2,069 respondents, a wide majority reported that they trust doctors (84%) and nurses (85%), but only 64% trusted the health care system as a whole. One in three consumers (32%) said their trust in the health care system decreased during the pandemic, compared with 11% who said their trust increased.
The ABIM Foundation released the research findings on May 21 as part of Building Trust, a national campaign that aims to boost trust among patients, clinicians, system leaders, researchers, and others.
Richard J. Baron, MD, president and chief executive officer of the ABIM Foundation, said in an interview, “Clearly there’s lower trust in health care organization leaders and executives, and that’s troubling.
“Science by itself is not enough,” he said. “Becoming trustworthy has to be a core project of everybody in health care.”
Deterioration in physicians’ trust during the pandemic comes in part from failed promises of adequate personal protective equipment and some physicians’ loss of income as a result of the crisis, Dr. Baron said.
He added that the vaccine rollout was very uneven and that policies as to which elective procedures could be performed were handled differently in different parts of the country.
He also noted that, early on, transparency was lacking as to how many COVID patients hospitals were treating, which may have contributed to the decrease in trust in the system.
Fear of being known as ‘the COVID hospital’
Hospitals were afraid of being known as “the COVID hospital” and losing patients who were afraid to come there, Dr. Baron said.
He said the COVID-19 epidemic exacerbated problems regarding trust, but that trust has been declining for some time. The Building Trust campaign will focus on solutions in breaches of trust as physicians move increasingly toward being employees of huge systems, according to Dr. Baron.
However, trust works both ways, Dr. Baron notes. Physicians can be champions for their health care system or “throw the system under the bus,” he said.
For example, if a patient complains about the appointment system, clinicians who trust their institutions may say the system usually works and that they will try to make sure the patient has a better experience next time. Clinicians without trust may say they agree that the health care system doesn’t know what it is doing, and patients may further lose confidence when physicians validate their complaint, and patients may then go elsewhere.
78% of patients trust primary care doctor
When asked whether they trust their primary care physician, 78% of patients said yes. However, trust in doctors was higher among people who were older (90%), White (82%), or had high income (89%). Among people reporting lower trust, 25% said their physician spends too little time with them, and 14% said their doctor does not know or listen to them.
The survey shows that government agencies have work to do to earn trust. Responses indicate that 43% of physicians said they have “complete trust” in government health care agencies, such as the U.S. Food and Drug Administration and the Centers for Disease Control and Prevention, which is substantially higher than other parts of the health care system. However, trust in agencies declined for 43% of physician respondents and increased for 21%.
Dhruv Khullar, MD, MPP, of the department of health policy and economics at Weill Cornell Medical College in New York, told this news organization the survey results match what he sees anecdotally in medicine – that physicians have been losing trust in the system but not in their colleagues.
He said the sample size of 600 is enough to be influential, though he said he would like to know the response rate, which was not calculated for this survey.
He added that, in large part, physicians’ lack of trust in their systems may come from generally being asked to see more patients and to meet more metrics during the same or shorter periods.
Physicians’ lack of trust in the system can have significant consequences, he said. It can lead to burnout, which has been linked with poorer quality of care and physician turnover, he noted.
COVID-19 led some physicians to wonder whether their system had their best interests at heart, insofar as access to adequate medicines and supplies as well as emotional support were inconsistent, Dr. Khullar said.
He said that to regain trust health care systems need to ask themselves questions in three areas. The first is whether their goals are focused on the best interest of the organization or the best interest of the patient.
“Next is competency,” Dr. Khullar said. “Maybe your motives are right, but are you able to deliver? Are you delivering a good product, whether clinical services or something else?”
The third area is transparency, he said. “Are you going to be honest and forthright in what we’re doing and where we’re going?”
Caroline Pearson, senior vice president of health care strategy for NORC, said the emailed survey was conducted between Dec. 29, 2020, and Feb. 5, 2021, with a health care survey partner that maintains a nationwide panel of physicians across specialties.
She said this report is fairly novel insofar as surveys are more typically conducted regarding patients’ trust of their doctors or of the health care system.
Ms. Pearson said because health care is delivered in teams, understanding the level of trust among the entities helps ensure that care will be delivered effectively and seamlessly with high quality.
“We want our patients to trust our doctors, but we really want doctors to trust each other and trust the hospitals and systems in which they’re working,” she said.
Dr. Baron, Ms. Pearson, and Dr. Khullar report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
according to a survey conducted by NORC at the University of Chicago on behalf of the American Board of Internal Medicine Foundation.
Survey results, released May 21, indicate that 30% of physicians say their trust in the U.S. health care system and health care leadership has decreased during the pandemic. Only 18% reported an increase in trust.
Physicians, however, have great trust in their fellow clinicians.
In the survey of 600 physicians, 94% said they trust doctors within their practice; 85% trusted doctors outside of their practice; and 89% trusted nurses. That trust increased during the pandemic, with 41% saying their trust in fellow physicians rose and 37% saying their trust in nurses did.
In a separate survey, NORC asked patients about their trust in various aspects of health care. Among 2,069 respondents, a wide majority reported that they trust doctors (84%) and nurses (85%), but only 64% trusted the health care system as a whole. One in three consumers (32%) said their trust in the health care system decreased during the pandemic, compared with 11% who said their trust increased.
The ABIM Foundation released the research findings on May 21 as part of Building Trust, a national campaign that aims to boost trust among patients, clinicians, system leaders, researchers, and others.
Richard J. Baron, MD, president and chief executive officer of the ABIM Foundation, said in an interview, “Clearly there’s lower trust in health care organization leaders and executives, and that’s troubling.
“Science by itself is not enough,” he said. “Becoming trustworthy has to be a core project of everybody in health care.”
Deterioration in physicians’ trust during the pandemic comes in part from failed promises of adequate personal protective equipment and some physicians’ loss of income as a result of the crisis, Dr. Baron said.
He added that the vaccine rollout was very uneven and that policies as to which elective procedures could be performed were handled differently in different parts of the country.
He also noted that, early on, transparency was lacking as to how many COVID patients hospitals were treating, which may have contributed to the decrease in trust in the system.
Fear of being known as ‘the COVID hospital’
Hospitals were afraid of being known as “the COVID hospital” and losing patients who were afraid to come there, Dr. Baron said.
He said the COVID-19 epidemic exacerbated problems regarding trust, but that trust has been declining for some time. The Building Trust campaign will focus on solutions in breaches of trust as physicians move increasingly toward being employees of huge systems, according to Dr. Baron.
However, trust works both ways, Dr. Baron notes. Physicians can be champions for their health care system or “throw the system under the bus,” he said.
For example, if a patient complains about the appointment system, clinicians who trust their institutions may say the system usually works and that they will try to make sure the patient has a better experience next time. Clinicians without trust may say they agree that the health care system doesn’t know what it is doing, and patients may further lose confidence when physicians validate their complaint, and patients may then go elsewhere.
78% of patients trust primary care doctor
When asked whether they trust their primary care physician, 78% of patients said yes. However, trust in doctors was higher among people who were older (90%), White (82%), or had high income (89%). Among people reporting lower trust, 25% said their physician spends too little time with them, and 14% said their doctor does not know or listen to them.
The survey shows that government agencies have work to do to earn trust. Responses indicate that 43% of physicians said they have “complete trust” in government health care agencies, such as the U.S. Food and Drug Administration and the Centers for Disease Control and Prevention, which is substantially higher than other parts of the health care system. However, trust in agencies declined for 43% of physician respondents and increased for 21%.
Dhruv Khullar, MD, MPP, of the department of health policy and economics at Weill Cornell Medical College in New York, told this news organization the survey results match what he sees anecdotally in medicine – that physicians have been losing trust in the system but not in their colleagues.
He said the sample size of 600 is enough to be influential, though he said he would like to know the response rate, which was not calculated for this survey.
He added that, in large part, physicians’ lack of trust in their systems may come from generally being asked to see more patients and to meet more metrics during the same or shorter periods.
Physicians’ lack of trust in the system can have significant consequences, he said. It can lead to burnout, which has been linked with poorer quality of care and physician turnover, he noted.
COVID-19 led some physicians to wonder whether their system had their best interests at heart, insofar as access to adequate medicines and supplies as well as emotional support were inconsistent, Dr. Khullar said.
He said that to regain trust health care systems need to ask themselves questions in three areas. The first is whether their goals are focused on the best interest of the organization or the best interest of the patient.
“Next is competency,” Dr. Khullar said. “Maybe your motives are right, but are you able to deliver? Are you delivering a good product, whether clinical services or something else?”
The third area is transparency, he said. “Are you going to be honest and forthright in what we’re doing and where we’re going?”
Caroline Pearson, senior vice president of health care strategy for NORC, said the emailed survey was conducted between Dec. 29, 2020, and Feb. 5, 2021, with a health care survey partner that maintains a nationwide panel of physicians across specialties.
She said this report is fairly novel insofar as surveys are more typically conducted regarding patients’ trust of their doctors or of the health care system.
Ms. Pearson said because health care is delivered in teams, understanding the level of trust among the entities helps ensure that care will be delivered effectively and seamlessly with high quality.
“We want our patients to trust our doctors, but we really want doctors to trust each other and trust the hospitals and systems in which they’re working,” she said.
Dr. Baron, Ms. Pearson, and Dr. Khullar report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Young adults with epilepsy face higher mental illness risks
Young adults with epilepsy experience higher rates of anxiety, depression, and suicidality, compared with their counterparts in the general population, a new study shows.
The findings, based on a study of 144 young adults with epilepsy (YAWE), was published recently in Epilepsy & Behavior.
“People with epilepsy (PWE) are at a significantly higher risk of experiencing mental health difficulties, compared with healthy controls and individuals with other [long-term conditions] such as asthma and diabetes,” according to Rachel Batchelor, MSc, and Michelle D. Taylor, PhD, of the University of London (England) in Surrey.
Young adulthood, which encompasses people aged 18-25 years, has been identified as “a peak age of onset for anxiety and depression,” but mental health in young adults with epilepsy in particular has not been well studied, they wrote.
The survey measured current mental health symptoms, including anxiety, depression, and suicidality, as well as sociodemographic and epilepsy-related factors, coping strategies, and social support (Epilepsy Behav. 2021 May;118:107911. doi: 10.1016/j.yebeh.2021.107911).
The average age of the respondents was 21.6 years, 61% were female, and 88% were of White British ethnicity. A total of 88 participants were single, 48 were in a relationship, and 8 were married or engaged. About one-third (38%) worked full-time, and 28.5% were full-time university students, 18.8% worked part-time, and 8.3% were unemployed and not students. The average age of seizure onset was 12.4 years.
Overall, 116 (80.6%) of the survey respondents met the criteria for anxiety, 110 (76.4%) for depression, and 51 (35.4%) for suicidality.
Ratings of all three of these conditions were significantly higher in females, compared with males, the researchers noted. Anxiety, depression, and suicidality also were rated higher for individuals who waited more than 1 year vs. less than 1 year for an epilepsy diagnosis from the time of seizure onset, for those suffering from anti-seizure medication side effects vs. no side effects, and for those with comorbid conditions vs. no comorbid conditions.
Avoidant-focused coping strategies were positively correlated with anxiety, depression, and suicidality, while problem-focused coping and meaning-focused coping were negatively correlated, the researchers said. In addition, those who reported greater levels of support from friends had lower rates of anxiety and depression, and those who reported greater levels of support from family had lower rates of suicidality.
The study findings were limited by several factors, including the relatively homogenous population, and the absence of data on current anxiety and depression medications and additional professional support, the researchers noted.
However, the results extend the research on mental health in people with epilepsy, and the study is the first known to focus on the young adult population with epilepsy, they said.
“The high rates of anxiety, depression, and suicidality underscore the need for better integration of mental health provision into epilepsy care,” the researchers wrote. “While it would be premature to base recommendations for treating anxiety, depression, and suicidality in YAWE on the current study, investigating the efficacy of psychological interventions (for example, [acceptance and commitment therapy], [compassion-focused therapy], peer support, and family-based [therapy]) designed to address the psychosocial variables shown to independently predict mental health outcomes in YAWE would be worthy future research avenues,” they concluded.
The study received no outside funding, and the researchers disclosed no financial conflicts.
Young adults with epilepsy experience higher rates of anxiety, depression, and suicidality, compared with their counterparts in the general population, a new study shows.
The findings, based on a study of 144 young adults with epilepsy (YAWE), was published recently in Epilepsy & Behavior.
“People with epilepsy (PWE) are at a significantly higher risk of experiencing mental health difficulties, compared with healthy controls and individuals with other [long-term conditions] such as asthma and diabetes,” according to Rachel Batchelor, MSc, and Michelle D. Taylor, PhD, of the University of London (England) in Surrey.
Young adulthood, which encompasses people aged 18-25 years, has been identified as “a peak age of onset for anxiety and depression,” but mental health in young adults with epilepsy in particular has not been well studied, they wrote.
The survey measured current mental health symptoms, including anxiety, depression, and suicidality, as well as sociodemographic and epilepsy-related factors, coping strategies, and social support (Epilepsy Behav. 2021 May;118:107911. doi: 10.1016/j.yebeh.2021.107911).
The average age of the respondents was 21.6 years, 61% were female, and 88% were of White British ethnicity. A total of 88 participants were single, 48 were in a relationship, and 8 were married or engaged. About one-third (38%) worked full-time, and 28.5% were full-time university students, 18.8% worked part-time, and 8.3% were unemployed and not students. The average age of seizure onset was 12.4 years.
Overall, 116 (80.6%) of the survey respondents met the criteria for anxiety, 110 (76.4%) for depression, and 51 (35.4%) for suicidality.
Ratings of all three of these conditions were significantly higher in females, compared with males, the researchers noted. Anxiety, depression, and suicidality also were rated higher for individuals who waited more than 1 year vs. less than 1 year for an epilepsy diagnosis from the time of seizure onset, for those suffering from anti-seizure medication side effects vs. no side effects, and for those with comorbid conditions vs. no comorbid conditions.
Avoidant-focused coping strategies were positively correlated with anxiety, depression, and suicidality, while problem-focused coping and meaning-focused coping were negatively correlated, the researchers said. In addition, those who reported greater levels of support from friends had lower rates of anxiety and depression, and those who reported greater levels of support from family had lower rates of suicidality.
The study findings were limited by several factors, including the relatively homogenous population, and the absence of data on current anxiety and depression medications and additional professional support, the researchers noted.
However, the results extend the research on mental health in people with epilepsy, and the study is the first known to focus on the young adult population with epilepsy, they said.
“The high rates of anxiety, depression, and suicidality underscore the need for better integration of mental health provision into epilepsy care,” the researchers wrote. “While it would be premature to base recommendations for treating anxiety, depression, and suicidality in YAWE on the current study, investigating the efficacy of psychological interventions (for example, [acceptance and commitment therapy], [compassion-focused therapy], peer support, and family-based [therapy]) designed to address the psychosocial variables shown to independently predict mental health outcomes in YAWE would be worthy future research avenues,” they concluded.
The study received no outside funding, and the researchers disclosed no financial conflicts.
Young adults with epilepsy experience higher rates of anxiety, depression, and suicidality, compared with their counterparts in the general population, a new study shows.
The findings, based on a study of 144 young adults with epilepsy (YAWE), was published recently in Epilepsy & Behavior.
“People with epilepsy (PWE) are at a significantly higher risk of experiencing mental health difficulties, compared with healthy controls and individuals with other [long-term conditions] such as asthma and diabetes,” according to Rachel Batchelor, MSc, and Michelle D. Taylor, PhD, of the University of London (England) in Surrey.
Young adulthood, which encompasses people aged 18-25 years, has been identified as “a peak age of onset for anxiety and depression,” but mental health in young adults with epilepsy in particular has not been well studied, they wrote.
The survey measured current mental health symptoms, including anxiety, depression, and suicidality, as well as sociodemographic and epilepsy-related factors, coping strategies, and social support (Epilepsy Behav. 2021 May;118:107911. doi: 10.1016/j.yebeh.2021.107911).
The average age of the respondents was 21.6 years, 61% were female, and 88% were of White British ethnicity. A total of 88 participants were single, 48 were in a relationship, and 8 were married or engaged. About one-third (38%) worked full-time, and 28.5% were full-time university students, 18.8% worked part-time, and 8.3% were unemployed and not students. The average age of seizure onset was 12.4 years.
Overall, 116 (80.6%) of the survey respondents met the criteria for anxiety, 110 (76.4%) for depression, and 51 (35.4%) for suicidality.
Ratings of all three of these conditions were significantly higher in females, compared with males, the researchers noted. Anxiety, depression, and suicidality also were rated higher for individuals who waited more than 1 year vs. less than 1 year for an epilepsy diagnosis from the time of seizure onset, for those suffering from anti-seizure medication side effects vs. no side effects, and for those with comorbid conditions vs. no comorbid conditions.
Avoidant-focused coping strategies were positively correlated with anxiety, depression, and suicidality, while problem-focused coping and meaning-focused coping were negatively correlated, the researchers said. In addition, those who reported greater levels of support from friends had lower rates of anxiety and depression, and those who reported greater levels of support from family had lower rates of suicidality.
The study findings were limited by several factors, including the relatively homogenous population, and the absence of data on current anxiety and depression medications and additional professional support, the researchers noted.
However, the results extend the research on mental health in people with epilepsy, and the study is the first known to focus on the young adult population with epilepsy, they said.
“The high rates of anxiety, depression, and suicidality underscore the need for better integration of mental health provision into epilepsy care,” the researchers wrote. “While it would be premature to base recommendations for treating anxiety, depression, and suicidality in YAWE on the current study, investigating the efficacy of psychological interventions (for example, [acceptance and commitment therapy], [compassion-focused therapy], peer support, and family-based [therapy]) designed to address the psychosocial variables shown to independently predict mental health outcomes in YAWE would be worthy future research avenues,” they concluded.
The study received no outside funding, and the researchers disclosed no financial conflicts.
FROM EPILEPSY & BEHAVIOR
Psychosis, depression tied to neurodegeneration in Parkinson’s
Depression and psychosis are significantly associated with neuronal loss and gliosis – but not with Lewy body scores – in Parkinson’s disease, data from analyses of the brains of 175 patients suggest.
Previous research has suggested a link between neuronal loss and depression in Parkinson’s disease (PD) but the impact of Lewy bodies has not been well studied, Nicole Mercado Fischer, MPH, of Johns Hopkins University, Baltimore, and colleagues wrote.
Evaluating Lewy body scores and neuronal loss/gliosis in the substantia nigra pars compacta (SN) and locus coeruleus (LC) could increase understanding of pathophysiology in PD, they said.
In a study published in the American Journal of Geriatric Psychiatry, the researchers analyzed the brains of 175 individuals with a primary diagnosis of PD.
A total of 98 participants had diagnoses of psychosis, 88 had depression, and 55 had anxiety. The average age of onset for PD was 62.4 years; 67.4% of the subjects were male, and 97.8% were White. The mean duration of illness was 16 years, and the average age at death was 78 years.
Psychosis was significantly associated with severe neuronal loss and gliosis in both the LC and SN (P = .048 and P = .042, respectively). Depression was significantly associated with severe neuronal loss in the SN (P = .042) but not in the LC. Anxiety was not associated with severe neuronal loss in either brain region. These results remained significant after a multivariate analysis, the researchers noted. However, Lewy body scores were not associated with any neuropsychiatric symptom, and severity of neuronal loss and gliosis was not correlated with Lewy body scores.
The study findings were limited by several factors, including the retrospective design and inability to collect pathology data for all patients, the researchers noted. Also, in some cases, the collection of clinical data and observation of brain tissue pathology took place years apart, and the researchers did not assess medication records.
However, the results were strengthened by the large sample size and “further support the notion that in vivo clinical symptoms of PD are either not caused by Lewy body pathology or that the relationship is confounded by the time of autopsy,” they said. and eventually by using new functional imaging techniques in vivo.”
The researchers had no financial conflicts to disclose. Two coauthors were supported in part by the National Institutes of Health.
Depression and psychosis are significantly associated with neuronal loss and gliosis – but not with Lewy body scores – in Parkinson’s disease, data from analyses of the brains of 175 patients suggest.
Previous research has suggested a link between neuronal loss and depression in Parkinson’s disease (PD) but the impact of Lewy bodies has not been well studied, Nicole Mercado Fischer, MPH, of Johns Hopkins University, Baltimore, and colleagues wrote.
Evaluating Lewy body scores and neuronal loss/gliosis in the substantia nigra pars compacta (SN) and locus coeruleus (LC) could increase understanding of pathophysiology in PD, they said.
In a study published in the American Journal of Geriatric Psychiatry, the researchers analyzed the brains of 175 individuals with a primary diagnosis of PD.
A total of 98 participants had diagnoses of psychosis, 88 had depression, and 55 had anxiety. The average age of onset for PD was 62.4 years; 67.4% of the subjects were male, and 97.8% were White. The mean duration of illness was 16 years, and the average age at death was 78 years.
Psychosis was significantly associated with severe neuronal loss and gliosis in both the LC and SN (P = .048 and P = .042, respectively). Depression was significantly associated with severe neuronal loss in the SN (P = .042) but not in the LC. Anxiety was not associated with severe neuronal loss in either brain region. These results remained significant after a multivariate analysis, the researchers noted. However, Lewy body scores were not associated with any neuropsychiatric symptom, and severity of neuronal loss and gliosis was not correlated with Lewy body scores.
The study findings were limited by several factors, including the retrospective design and inability to collect pathology data for all patients, the researchers noted. Also, in some cases, the collection of clinical data and observation of brain tissue pathology took place years apart, and the researchers did not assess medication records.
However, the results were strengthened by the large sample size and “further support the notion that in vivo clinical symptoms of PD are either not caused by Lewy body pathology or that the relationship is confounded by the time of autopsy,” they said. and eventually by using new functional imaging techniques in vivo.”
The researchers had no financial conflicts to disclose. Two coauthors were supported in part by the National Institutes of Health.
Depression and psychosis are significantly associated with neuronal loss and gliosis – but not with Lewy body scores – in Parkinson’s disease, data from analyses of the brains of 175 patients suggest.
Previous research has suggested a link between neuronal loss and depression in Parkinson’s disease (PD) but the impact of Lewy bodies has not been well studied, Nicole Mercado Fischer, MPH, of Johns Hopkins University, Baltimore, and colleagues wrote.
Evaluating Lewy body scores and neuronal loss/gliosis in the substantia nigra pars compacta (SN) and locus coeruleus (LC) could increase understanding of pathophysiology in PD, they said.
In a study published in the American Journal of Geriatric Psychiatry, the researchers analyzed the brains of 175 individuals with a primary diagnosis of PD.
A total of 98 participants had diagnoses of psychosis, 88 had depression, and 55 had anxiety. The average age of onset for PD was 62.4 years; 67.4% of the subjects were male, and 97.8% were White. The mean duration of illness was 16 years, and the average age at death was 78 years.
Psychosis was significantly associated with severe neuronal loss and gliosis in both the LC and SN (P = .048 and P = .042, respectively). Depression was significantly associated with severe neuronal loss in the SN (P = .042) but not in the LC. Anxiety was not associated with severe neuronal loss in either brain region. These results remained significant after a multivariate analysis, the researchers noted. However, Lewy body scores were not associated with any neuropsychiatric symptom, and severity of neuronal loss and gliosis was not correlated with Lewy body scores.
The study findings were limited by several factors, including the retrospective design and inability to collect pathology data for all patients, the researchers noted. Also, in some cases, the collection of clinical data and observation of brain tissue pathology took place years apart, and the researchers did not assess medication records.
However, the results were strengthened by the large sample size and “further support the notion that in vivo clinical symptoms of PD are either not caused by Lewy body pathology or that the relationship is confounded by the time of autopsy,” they said. and eventually by using new functional imaging techniques in vivo.”
The researchers had no financial conflicts to disclose. Two coauthors were supported in part by the National Institutes of Health.
FROM THE AMERICAN JOURNAL OF GERIATRIC PSYCHIATRY
Final SPRINT data confirm lower BP is better
The results include data on some outcome events from the trial that had yet to be adjudicated when the primary analysis was released in 2015, as well as posttrial observational follow-up data collected through July 2016.
The data confirm and enhance the earlier findings and show that “lower is better” when it comes to blood pressure, primary investigator Cora E. Lewis, MD, professor and chair, department of epidemiology, University of Alabama at Birmingham, said in an interview.
Final results of the Systolic Blood Pressure Intervention Trial (SPRINT) were published in the May 20 issue of the New England Journal of Medicine.
For the trial, researchers enrolled 9,361 adults 50 years and older with a SBP between 130 and 180 mm Hg who were at increased risk for cardiovascular disease (CVD) but did not have a history of diabetes or stroke. Patients were randomly assigned to an intensive treatment target (SBP < 120 mm Hg) or a standard treatment target (SBP < 140 mm Hg).
In the final analysis, the rate of the primary outcome was 1.77% per year in the intensive-treatment group and 2.40% per year in the standard-treatment group (hazard ratio [HR], 0.73; 95% confidence interval [CR], 0.63-0.86; P < .001), similar to the earlier SPRINT findings.
All-cause mortality was 1.06% per year in the intensive-treatment group and 1.41% per year in the standard-treatment group (HR, 0.75; 95% CI, 0.61-0.92; P = .006), again similar to the previous findings.
“These results were highly statistically significant. It is remarkable in a trial powered for a composite CVD outcome to obtain a significant benefit for total mortality,” Dr. Lewis said.
She noted that one criticism of the initial SPRINT results was that, for the components of the primary outcome, only heart failure and death due to CVD were significantly lower in the intensively treated group.
“Heart failure can be difficult to diagnose from records in a clinical trial, and the critiques were that this was shaky evidence, given that more participants treated to less than 120 were on diuretics, which could decrease swelling, a key symptom of heart failure,” she explained.
“In these final results, SPRINT found that risk of myocardial infarction, heart failure, and death from CVD were significantly lower in the group treated to less than 120, and risk of the primary outcome, excluding heart failure, was still significantly lower in the more intensively treated group,” she noted.
After the trial phase ended, blood pressure treatment was returned to the participants’ usual source of medical care and the trial treatment goals were no longer pursued. SPRINT continued to collect data on the outcomes through July 2016. During this time, SBP rose 6.9 mm Hg in the intensive-treatment group and 2.6 mm Hg in the standard-treatment group.
“Putting all the data together from the trial phase and the phase after randomized interventions had been stopped, there was still a significant benefit for the more intensive treatment on the primary outcome and on death from all causes,” Dr. Lewis said.
In addition, a separate new analysis based on all the data showed significantly fewer first and recurrent primary outcome events with intensive treatment than with standard treatment (435 vs. 552; HR, 0.78; 95% CI, 0.69-0.89; P < .001).
Manageable risk
The pattern of safety events in the final analysis was similar to the 2015 report. In the intervention period, rates of serious adverse events overall did not differ significantly between the groups. However, rates of hypotension, electrolyte abnormalities, syncope (none leading to injurious falls), and acute kidney injury were higher in the intensive-treatment group.
As in other SPRINT reports, “acute kidney injury safety events were generally mild and there was nearly complete recovery of kidney function within 1 year,” Dr. Lewis said. “This and other analyses we have published indicate this is probably a hemodynamic effect.”
“Intensive treatment can be well tolerated and is generally safe with proper patient selection and monitoring. There are advantages to intensive therapy, and some risks, but I don’t think the risks are such that we should just throw the idea of more intensive treatment out the window,” Dr. Lewis said.
Reached for comment, Carlos G. Santos-Gallego, MD, from the Icahn School of Medicine at Mount Sinai in New York, said there has been “controversy” over whether intensive blood pressure control targeting systolic to below 120 mm Hg is beneficial.
“The original SPRINT trial is incredibly important, in that it conclusively demonstrated that among patients with hypertension and increased cardiovascular risk, targeting systolic blood pressure to below 120 mm Hg resulted in lower rates of adverse cardiovascular events and, importantly, all-cause mortality," compared with the conventional target of 140 mm Hg, he said in an interview.
“This final report of the SPRINT trial basically consolidates, confirms, and corroborates the original SPRINT data,” he noted. However, the final data are “more robust, with additional primary outcome events and all events having been adjudicated by a central committee, and there is an additional observation period of 1 extra year in which the treatment has been discontinued,” he said.
“Over time, we are becoming more and more certain that lower is better with blood pressure. We still have a long way to go, but the cardiology community is slowly becoming more intense in our treatment of blood pressure for our patients,” Dr. Santos-Gallego said.
The potential adverse effects of intensive blood pressure control are “very manageable,” he added.
Support for SPRINT was provided by the National Institutes of Health. Full disclosures for authors are available in the original article. Dr. Santos-Gallego has no relevant disclosures.
A version of this article first appeared on Medscape.com.
The results include data on some outcome events from the trial that had yet to be adjudicated when the primary analysis was released in 2015, as well as posttrial observational follow-up data collected through July 2016.
The data confirm and enhance the earlier findings and show that “lower is better” when it comes to blood pressure, primary investigator Cora E. Lewis, MD, professor and chair, department of epidemiology, University of Alabama at Birmingham, said in an interview.
Final results of the Systolic Blood Pressure Intervention Trial (SPRINT) were published in the May 20 issue of the New England Journal of Medicine.
For the trial, researchers enrolled 9,361 adults 50 years and older with a SBP between 130 and 180 mm Hg who were at increased risk for cardiovascular disease (CVD) but did not have a history of diabetes or stroke. Patients were randomly assigned to an intensive treatment target (SBP < 120 mm Hg) or a standard treatment target (SBP < 140 mm Hg).
In the final analysis, the rate of the primary outcome was 1.77% per year in the intensive-treatment group and 2.40% per year in the standard-treatment group (hazard ratio [HR], 0.73; 95% confidence interval [CR], 0.63-0.86; P < .001), similar to the earlier SPRINT findings.
All-cause mortality was 1.06% per year in the intensive-treatment group and 1.41% per year in the standard-treatment group (HR, 0.75; 95% CI, 0.61-0.92; P = .006), again similar to the previous findings.
“These results were highly statistically significant. It is remarkable in a trial powered for a composite CVD outcome to obtain a significant benefit for total mortality,” Dr. Lewis said.
She noted that one criticism of the initial SPRINT results was that, for the components of the primary outcome, only heart failure and death due to CVD were significantly lower in the intensively treated group.
“Heart failure can be difficult to diagnose from records in a clinical trial, and the critiques were that this was shaky evidence, given that more participants treated to less than 120 were on diuretics, which could decrease swelling, a key symptom of heart failure,” she explained.
“In these final results, SPRINT found that risk of myocardial infarction, heart failure, and death from CVD were significantly lower in the group treated to less than 120, and risk of the primary outcome, excluding heart failure, was still significantly lower in the more intensively treated group,” she noted.
After the trial phase ended, blood pressure treatment was returned to the participants’ usual source of medical care and the trial treatment goals were no longer pursued. SPRINT continued to collect data on the outcomes through July 2016. During this time, SBP rose 6.9 mm Hg in the intensive-treatment group and 2.6 mm Hg in the standard-treatment group.
“Putting all the data together from the trial phase and the phase after randomized interventions had been stopped, there was still a significant benefit for the more intensive treatment on the primary outcome and on death from all causes,” Dr. Lewis said.
In addition, a separate new analysis based on all the data showed significantly fewer first and recurrent primary outcome events with intensive treatment than with standard treatment (435 vs. 552; HR, 0.78; 95% CI, 0.69-0.89; P < .001).
Manageable risk
The pattern of safety events in the final analysis was similar to the 2015 report. In the intervention period, rates of serious adverse events overall did not differ significantly between the groups. However, rates of hypotension, electrolyte abnormalities, syncope (none leading to injurious falls), and acute kidney injury were higher in the intensive-treatment group.
As in other SPRINT reports, “acute kidney injury safety events were generally mild and there was nearly complete recovery of kidney function within 1 year,” Dr. Lewis said. “This and other analyses we have published indicate this is probably a hemodynamic effect.”
“Intensive treatment can be well tolerated and is generally safe with proper patient selection and monitoring. There are advantages to intensive therapy, and some risks, but I don’t think the risks are such that we should just throw the idea of more intensive treatment out the window,” Dr. Lewis said.
Reached for comment, Carlos G. Santos-Gallego, MD, from the Icahn School of Medicine at Mount Sinai in New York, said there has been “controversy” over whether intensive blood pressure control targeting systolic to below 120 mm Hg is beneficial.
“The original SPRINT trial is incredibly important, in that it conclusively demonstrated that among patients with hypertension and increased cardiovascular risk, targeting systolic blood pressure to below 120 mm Hg resulted in lower rates of adverse cardiovascular events and, importantly, all-cause mortality," compared with the conventional target of 140 mm Hg, he said in an interview.
“This final report of the SPRINT trial basically consolidates, confirms, and corroborates the original SPRINT data,” he noted. However, the final data are “more robust, with additional primary outcome events and all events having been adjudicated by a central committee, and there is an additional observation period of 1 extra year in which the treatment has been discontinued,” he said.
“Over time, we are becoming more and more certain that lower is better with blood pressure. We still have a long way to go, but the cardiology community is slowly becoming more intense in our treatment of blood pressure for our patients,” Dr. Santos-Gallego said.
The potential adverse effects of intensive blood pressure control are “very manageable,” he added.
Support for SPRINT was provided by the National Institutes of Health. Full disclosures for authors are available in the original article. Dr. Santos-Gallego has no relevant disclosures.
A version of this article first appeared on Medscape.com.
The results include data on some outcome events from the trial that had yet to be adjudicated when the primary analysis was released in 2015, as well as posttrial observational follow-up data collected through July 2016.
The data confirm and enhance the earlier findings and show that “lower is better” when it comes to blood pressure, primary investigator Cora E. Lewis, MD, professor and chair, department of epidemiology, University of Alabama at Birmingham, said in an interview.
Final results of the Systolic Blood Pressure Intervention Trial (SPRINT) were published in the May 20 issue of the New England Journal of Medicine.
For the trial, researchers enrolled 9,361 adults 50 years and older with a SBP between 130 and 180 mm Hg who were at increased risk for cardiovascular disease (CVD) but did not have a history of diabetes or stroke. Patients were randomly assigned to an intensive treatment target (SBP < 120 mm Hg) or a standard treatment target (SBP < 140 mm Hg).
In the final analysis, the rate of the primary outcome was 1.77% per year in the intensive-treatment group and 2.40% per year in the standard-treatment group (hazard ratio [HR], 0.73; 95% confidence interval [CR], 0.63-0.86; P < .001), similar to the earlier SPRINT findings.
All-cause mortality was 1.06% per year in the intensive-treatment group and 1.41% per year in the standard-treatment group (HR, 0.75; 95% CI, 0.61-0.92; P = .006), again similar to the previous findings.
“These results were highly statistically significant. It is remarkable in a trial powered for a composite CVD outcome to obtain a significant benefit for total mortality,” Dr. Lewis said.
She noted that one criticism of the initial SPRINT results was that, for the components of the primary outcome, only heart failure and death due to CVD were significantly lower in the intensively treated group.
“Heart failure can be difficult to diagnose from records in a clinical trial, and the critiques were that this was shaky evidence, given that more participants treated to less than 120 were on diuretics, which could decrease swelling, a key symptom of heart failure,” she explained.
“In these final results, SPRINT found that risk of myocardial infarction, heart failure, and death from CVD were significantly lower in the group treated to less than 120, and risk of the primary outcome, excluding heart failure, was still significantly lower in the more intensively treated group,” she noted.
After the trial phase ended, blood pressure treatment was returned to the participants’ usual source of medical care and the trial treatment goals were no longer pursued. SPRINT continued to collect data on the outcomes through July 2016. During this time, SBP rose 6.9 mm Hg in the intensive-treatment group and 2.6 mm Hg in the standard-treatment group.
“Putting all the data together from the trial phase and the phase after randomized interventions had been stopped, there was still a significant benefit for the more intensive treatment on the primary outcome and on death from all causes,” Dr. Lewis said.
In addition, a separate new analysis based on all the data showed significantly fewer first and recurrent primary outcome events with intensive treatment than with standard treatment (435 vs. 552; HR, 0.78; 95% CI, 0.69-0.89; P < .001).
Manageable risk
The pattern of safety events in the final analysis was similar to the 2015 report. In the intervention period, rates of serious adverse events overall did not differ significantly between the groups. However, rates of hypotension, electrolyte abnormalities, syncope (none leading to injurious falls), and acute kidney injury were higher in the intensive-treatment group.
As in other SPRINT reports, “acute kidney injury safety events were generally mild and there was nearly complete recovery of kidney function within 1 year,” Dr. Lewis said. “This and other analyses we have published indicate this is probably a hemodynamic effect.”
“Intensive treatment can be well tolerated and is generally safe with proper patient selection and monitoring. There are advantages to intensive therapy, and some risks, but I don’t think the risks are such that we should just throw the idea of more intensive treatment out the window,” Dr. Lewis said.
Reached for comment, Carlos G. Santos-Gallego, MD, from the Icahn School of Medicine at Mount Sinai in New York, said there has been “controversy” over whether intensive blood pressure control targeting systolic to below 120 mm Hg is beneficial.
“The original SPRINT trial is incredibly important, in that it conclusively demonstrated that among patients with hypertension and increased cardiovascular risk, targeting systolic blood pressure to below 120 mm Hg resulted in lower rates of adverse cardiovascular events and, importantly, all-cause mortality," compared with the conventional target of 140 mm Hg, he said in an interview.
“This final report of the SPRINT trial basically consolidates, confirms, and corroborates the original SPRINT data,” he noted. However, the final data are “more robust, with additional primary outcome events and all events having been adjudicated by a central committee, and there is an additional observation period of 1 extra year in which the treatment has been discontinued,” he said.
“Over time, we are becoming more and more certain that lower is better with blood pressure. We still have a long way to go, but the cardiology community is slowly becoming more intense in our treatment of blood pressure for our patients,” Dr. Santos-Gallego said.
The potential adverse effects of intensive blood pressure control are “very manageable,” he added.
Support for SPRINT was provided by the National Institutes of Health. Full disclosures for authors are available in the original article. Dr. Santos-Gallego has no relevant disclosures.
A version of this article first appeared on Medscape.com.
Risk factors identified for late seizure relapse after epilepsy surgery
according to a new study on the factors most associated with seizure recurrence in drug-resistant epilepsy.
“As our study analyzed late seizure relapse, our results are not applicable for short‐term seizure control. Vice versa, results for short‐term outcomes should not be transferred to long‐term outcomes,” Stephan Petrik of the Epilepsy Center at the University of Freiburg (Germany) and colleagues wrote. The study was published in the May 2021 issue of Epilepsia.
To assess the variables that increase risk of late seizure recurrence following surgery, the researchers retrospectively studied the medical records of patients who underwent resective epilepsy surgery at the University Hospital Freiburg (Germany) between 1999 and 2015. Of the 1,278 initial patients, a group of 99 participants (7.7%) with seizure relapses after at least 2 years of complete seizure freedom were matched with controls experiencing long-term seizure freedom. The two groups had similar mean durations of epilepsy from onset to surgery: 13.9 years in the relapse group and 13.0 years in the control group.
The mean follow-up was 9.7 years (standard deviation, 4.0; range, 2.9-18.5) in the relapse group and 8.2 years (SD, 3.5; range, 2.2-18.3) in the control group. The mean time to late seizure recurrence was 56.6 months, and two-thirds of patients relapsed in the 5 years after surgery. Twenty of the relapse patients only experienced a single seizure, and 41% of the patients who reported more than one seizure had a frequency of less than one per month.
The type of resection had no discernible impact on outcomes, although anterior temporal lobe resection did trend toward being associated with recurrence (odds ratio, 2.7; 95% confidence interval, 0.93-8.89; P = .06). Incomplete resection was significantly associated with late relapse but did not seem to affect timing: the mean duration of seizure freedom was 56.5 months with complete resection and 58.5 months with incomplete resection (P = .62). Additional preoperative PET scans were performed on 45% of patients in the relapse group, compared with 29% in the control group.
After multivariate analysis, predictors for late relapse included incomplete resection (OR, 3.81; 95% CI, 1.79-8.53; P < .001); the existence of additional, potentially epileptogenic lesions in the contralateral hemisphere on presurgical MRI (OR, 3.36; 95% CI, 1.18-10.62; P = .03); epilepsy onset during the first year of life (OR, 4.24; 95% CI, 1.4-15.89; P = .02); and preoperative PET scans being performed (OR, 2.47; 95% CI, 1.25-4.97; P = .01). Though use of preoperative and postoperative antiepileptic drugs (AEDs) was higher in the relapse group, along with complete withdrawal being more common in the control group (68%, compared with 51%), neither was deemed significant in multivariate analysis.
What to do about seizure relapse risk factors
“This is one of the best analyses of the factors that contribute to late seizure relapse,” Gregory K. Bergey, MD, director of the Johns Hopkins Epilepsy Center in Baltimore, said in an interview. “Am I surprised by their results? Not necessarily.”
What did jump out, he said, was AED use not being a predictor of recurrence, as well as all the patients with late relapse having lesional epilepsy. “As they point out, you can have relapse with nonlesional epilepsy, but very often it happens in the first year or 2. If someone is 2 years out and doesn’t have a lesion, they’re probably more likely to remain seizure free.”
Despite the researchers’ comprehensive review of risk factors, the question remains: What to do with this information?
“They’ve done a very good job of identifying that 7.7% of 1,200 who are at risk of a late relapse,” he said. “Now, take those patients with high-risk factors and launch a trial where you keep medicines the same or do something that would alter that outcome.”
“The problem is,” he added, “that’s a 10-year study. It’s easy for me to sit here and call for one of those. But still, as valuable as this was, it’s a retrospective study. Now you have to say, what are the implications of this? What can we do in the prospective fashion?”
The authors acknowledged their study’s other limitations, including a lack of information on the reasons for an incomplete resection, a notable decrease in follow-up visits more than 5 years after surgery, and potential selection bias. They added, however, that “matching by age at surgery, gender, and time to relapse/last follow‐up” should have helped reduce any significant bias.
No potential conflicts of interest were disclosed.
according to a new study on the factors most associated with seizure recurrence in drug-resistant epilepsy.
“As our study analyzed late seizure relapse, our results are not applicable for short‐term seizure control. Vice versa, results for short‐term outcomes should not be transferred to long‐term outcomes,” Stephan Petrik of the Epilepsy Center at the University of Freiburg (Germany) and colleagues wrote. The study was published in the May 2021 issue of Epilepsia.
To assess the variables that increase risk of late seizure recurrence following surgery, the researchers retrospectively studied the medical records of patients who underwent resective epilepsy surgery at the University Hospital Freiburg (Germany) between 1999 and 2015. Of the 1,278 initial patients, a group of 99 participants (7.7%) with seizure relapses after at least 2 years of complete seizure freedom were matched with controls experiencing long-term seizure freedom. The two groups had similar mean durations of epilepsy from onset to surgery: 13.9 years in the relapse group and 13.0 years in the control group.
The mean follow-up was 9.7 years (standard deviation, 4.0; range, 2.9-18.5) in the relapse group and 8.2 years (SD, 3.5; range, 2.2-18.3) in the control group. The mean time to late seizure recurrence was 56.6 months, and two-thirds of patients relapsed in the 5 years after surgery. Twenty of the relapse patients only experienced a single seizure, and 41% of the patients who reported more than one seizure had a frequency of less than one per month.
The type of resection had no discernible impact on outcomes, although anterior temporal lobe resection did trend toward being associated with recurrence (odds ratio, 2.7; 95% confidence interval, 0.93-8.89; P = .06). Incomplete resection was significantly associated with late relapse but did not seem to affect timing: the mean duration of seizure freedom was 56.5 months with complete resection and 58.5 months with incomplete resection (P = .62). Additional preoperative PET scans were performed on 45% of patients in the relapse group, compared with 29% in the control group.
After multivariate analysis, predictors for late relapse included incomplete resection (OR, 3.81; 95% CI, 1.79-8.53; P < .001); the existence of additional, potentially epileptogenic lesions in the contralateral hemisphere on presurgical MRI (OR, 3.36; 95% CI, 1.18-10.62; P = .03); epilepsy onset during the first year of life (OR, 4.24; 95% CI, 1.4-15.89; P = .02); and preoperative PET scans being performed (OR, 2.47; 95% CI, 1.25-4.97; P = .01). Though use of preoperative and postoperative antiepileptic drugs (AEDs) was higher in the relapse group, along with complete withdrawal being more common in the control group (68%, compared with 51%), neither was deemed significant in multivariate analysis.
What to do about seizure relapse risk factors
“This is one of the best analyses of the factors that contribute to late seizure relapse,” Gregory K. Bergey, MD, director of the Johns Hopkins Epilepsy Center in Baltimore, said in an interview. “Am I surprised by their results? Not necessarily.”
What did jump out, he said, was AED use not being a predictor of recurrence, as well as all the patients with late relapse having lesional epilepsy. “As they point out, you can have relapse with nonlesional epilepsy, but very often it happens in the first year or 2. If someone is 2 years out and doesn’t have a lesion, they’re probably more likely to remain seizure free.”
Despite the researchers’ comprehensive review of risk factors, the question remains: What to do with this information?
“They’ve done a very good job of identifying that 7.7% of 1,200 who are at risk of a late relapse,” he said. “Now, take those patients with high-risk factors and launch a trial where you keep medicines the same or do something that would alter that outcome.”
“The problem is,” he added, “that’s a 10-year study. It’s easy for me to sit here and call for one of those. But still, as valuable as this was, it’s a retrospective study. Now you have to say, what are the implications of this? What can we do in the prospective fashion?”
The authors acknowledged their study’s other limitations, including a lack of information on the reasons for an incomplete resection, a notable decrease in follow-up visits more than 5 years after surgery, and potential selection bias. They added, however, that “matching by age at surgery, gender, and time to relapse/last follow‐up” should have helped reduce any significant bias.
No potential conflicts of interest were disclosed.
according to a new study on the factors most associated with seizure recurrence in drug-resistant epilepsy.
“As our study analyzed late seizure relapse, our results are not applicable for short‐term seizure control. Vice versa, results for short‐term outcomes should not be transferred to long‐term outcomes,” Stephan Petrik of the Epilepsy Center at the University of Freiburg (Germany) and colleagues wrote. The study was published in the May 2021 issue of Epilepsia.
To assess the variables that increase risk of late seizure recurrence following surgery, the researchers retrospectively studied the medical records of patients who underwent resective epilepsy surgery at the University Hospital Freiburg (Germany) between 1999 and 2015. Of the 1,278 initial patients, a group of 99 participants (7.7%) with seizure relapses after at least 2 years of complete seizure freedom were matched with controls experiencing long-term seizure freedom. The two groups had similar mean durations of epilepsy from onset to surgery: 13.9 years in the relapse group and 13.0 years in the control group.
The mean follow-up was 9.7 years (standard deviation, 4.0; range, 2.9-18.5) in the relapse group and 8.2 years (SD, 3.5; range, 2.2-18.3) in the control group. The mean time to late seizure recurrence was 56.6 months, and two-thirds of patients relapsed in the 5 years after surgery. Twenty of the relapse patients only experienced a single seizure, and 41% of the patients who reported more than one seizure had a frequency of less than one per month.
The type of resection had no discernible impact on outcomes, although anterior temporal lobe resection did trend toward being associated with recurrence (odds ratio, 2.7; 95% confidence interval, 0.93-8.89; P = .06). Incomplete resection was significantly associated with late relapse but did not seem to affect timing: the mean duration of seizure freedom was 56.5 months with complete resection and 58.5 months with incomplete resection (P = .62). Additional preoperative PET scans were performed on 45% of patients in the relapse group, compared with 29% in the control group.
After multivariate analysis, predictors for late relapse included incomplete resection (OR, 3.81; 95% CI, 1.79-8.53; P < .001); the existence of additional, potentially epileptogenic lesions in the contralateral hemisphere on presurgical MRI (OR, 3.36; 95% CI, 1.18-10.62; P = .03); epilepsy onset during the first year of life (OR, 4.24; 95% CI, 1.4-15.89; P = .02); and preoperative PET scans being performed (OR, 2.47; 95% CI, 1.25-4.97; P = .01). Though use of preoperative and postoperative antiepileptic drugs (AEDs) was higher in the relapse group, along with complete withdrawal being more common in the control group (68%, compared with 51%), neither was deemed significant in multivariate analysis.
What to do about seizure relapse risk factors
“This is one of the best analyses of the factors that contribute to late seizure relapse,” Gregory K. Bergey, MD, director of the Johns Hopkins Epilepsy Center in Baltimore, said in an interview. “Am I surprised by their results? Not necessarily.”
What did jump out, he said, was AED use not being a predictor of recurrence, as well as all the patients with late relapse having lesional epilepsy. “As they point out, you can have relapse with nonlesional epilepsy, but very often it happens in the first year or 2. If someone is 2 years out and doesn’t have a lesion, they’re probably more likely to remain seizure free.”
Despite the researchers’ comprehensive review of risk factors, the question remains: What to do with this information?
“They’ve done a very good job of identifying that 7.7% of 1,200 who are at risk of a late relapse,” he said. “Now, take those patients with high-risk factors and launch a trial where you keep medicines the same or do something that would alter that outcome.”
“The problem is,” he added, “that’s a 10-year study. It’s easy for me to sit here and call for one of those. But still, as valuable as this was, it’s a retrospective study. Now you have to say, what are the implications of this? What can we do in the prospective fashion?”
The authors acknowledged their study’s other limitations, including a lack of information on the reasons for an incomplete resection, a notable decrease in follow-up visits more than 5 years after surgery, and potential selection bias. They added, however, that “matching by age at surgery, gender, and time to relapse/last follow‐up” should have helped reduce any significant bias.
No potential conflicts of interest were disclosed.
FROM EPILEPSIA
A new take on breathing and a performance-enhancing placebo
No ifs, ands, or butt ventilators
Breathing, on most days, is a pretty simple task. You inhale, the oxygen goes in, fills your lungs, becomes carbon dioxide, and is exhaled. But as certain recent events have made very clear, some diseases make this task difficult, which is where ventilators come in. The issue is, some patients can’t really use ventilators.
Enter a new study from Japan, which tested the ability of mice and pigs to absorb oxygen through the rectum. Yes, breathing through the butt. It’s not actually such a far-fetched idea; several aquatic animals such as sea cucumbers and catfish absorb oxygen through their intestines, and as any drunken frat boy can tell you after a good butt chug, other chemicals can absolutely be absorbed by human intestines.
After an initial successful experiment where a group of mice had their intestines scrubbed, had pure oxygen inserted enterally, and were exposed to a hypoxic environment, the researchers decided to step up their game and avoid the exhaustive act of digestive scrubbing by enlisting the aid of something out of science fiction: perfluorocarbon. If you haven’t seen “The Abyss,” this liquid can absorb massive amounts of oxygen, so you can actually breathe it in the same way you do with air.
In part two of the experiment, a group of hypoxic mice and pigs had perfluorocarbon inserted into their anuses, while another group got saline solution. The saline group did not fare well, but the animals that got perfluorocarbon had their hypoxic symptoms relieved within minutes.
The effectiveness of this procedure in humans clearly has yet to be tested, and while it may not be useful in all, or even most, situations, it is always beneficial to have more ways to combat a problem. Just don’t tell the frat boys: They’ll be hooking oxygen tanks up to their butts and chanting: “Breathe! Breathe! Breathe!”
Better, stronger, faster … pinker
Many people, most of whom aren’t even athletes, commit huge amounts of time, effort, and expense to improve their athletic performance. But what if there’s an easier way?
Research conducted at the University of Westminster (England) showed that participants could, with one fairly simple intervention, get on a treadmill and run 212 meters further in 30 minutes, increasing their speed by an average of 4.4%. Not only that, but “feelings of pleasure were also enhanced, meaning participants found running more enjoyable,” according to a statement from the university.
Is this amazing intervention a new wonder drug? No. Is it a super special nutritional supplement? Negatory. An energy drink that “gives you wiiings”? Nope. The latest designer steroid? Nyet.
Like we said, it’s simple, and it’s pink. Literally, the color pink. We will explain.
Each of the 10 study subjects completed two 30-minute trials on the treadmill. For one, they were given a clear, artificially sweetened drink while they were running. For the other, they received the exact same drink colored pink with food dye. Pink did better. So to recap the last month in our column, faster looks pink, and skinny smells like lemons.
Once again, science demonstrates that you can’t go wrong by fooling a brain. Next week, LOTME tries to find out if purple makes you funnier.
Hey … I’m singing here!
Noise pollution has been linked to plenty of negative outcomes, but the latest target is the poor baby zebra finch.
Researchers at the Max Planck Institute of Ornithology in Germany say traffic noise disrupts the timing of vocal development and impairs learning in the flying finches. The noise was also shown to suppress their immune systems, because of lingering stress.
The good news is that the birds with noise-induced stress sang as much as their peers in a control group, so the delay in development “was not due to a lack of vocal practice,” according to researchers. However, one long-term effect could be that zebra finch birdsongs could change over time due to noise-induced copying errors. Imagine a really long game of birdsong telephone – the song at the beginning is unlikely to be the song years from now.
While not mentioned in the study, one could also imagine that due to all that exposure to traffic, young zebra finches could be developing a salty dialect and impatience with fellow finches taking up too much space on the same tree branch. Hopefully, they don’t give others “the bird.”
Slimy soap
Remember at the beginning of the pandemic when it was almost impossible to find sufficient hand-washing supplies? Just when you thought you’d tried everything, there is soap made from snail slime.
Snail slime, surprisingly, has many beneficial properties for humans. The slime has antiaging and skin healing properties and is actually used in some Korean beauty supplies. The snails even use the slime to help fix their shells if they become damaged.
Happily, no snails are harmed in the slime extraction and making of the soap. Snail farmer Damien Desrochers says, “I only touch it with my finger, you see it’s not violent, it’s simple.”
As you can probably imagine, a lot of slime is needed to have a steady supply of this soap, so Mr. Desrochers has systems in place to get enough slime. Approximately 40 snails are needed to make 15 bars of soap, and he hopes to produce about 3,000 bars in the first year.
Nothing really surprises us anymore in the beauty world: People put eggs in their hair and bee venom on their skin, so what’s wrong with a little snail slime?
No ifs, ands, or butt ventilators
Breathing, on most days, is a pretty simple task. You inhale, the oxygen goes in, fills your lungs, becomes carbon dioxide, and is exhaled. But as certain recent events have made very clear, some diseases make this task difficult, which is where ventilators come in. The issue is, some patients can’t really use ventilators.
Enter a new study from Japan, which tested the ability of mice and pigs to absorb oxygen through the rectum. Yes, breathing through the butt. It’s not actually such a far-fetched idea; several aquatic animals such as sea cucumbers and catfish absorb oxygen through their intestines, and as any drunken frat boy can tell you after a good butt chug, other chemicals can absolutely be absorbed by human intestines.
After an initial successful experiment where a group of mice had their intestines scrubbed, had pure oxygen inserted enterally, and were exposed to a hypoxic environment, the researchers decided to step up their game and avoid the exhaustive act of digestive scrubbing by enlisting the aid of something out of science fiction: perfluorocarbon. If you haven’t seen “The Abyss,” this liquid can absorb massive amounts of oxygen, so you can actually breathe it in the same way you do with air.
In part two of the experiment, a group of hypoxic mice and pigs had perfluorocarbon inserted into their anuses, while another group got saline solution. The saline group did not fare well, but the animals that got perfluorocarbon had their hypoxic symptoms relieved within minutes.
The effectiveness of this procedure in humans clearly has yet to be tested, and while it may not be useful in all, or even most, situations, it is always beneficial to have more ways to combat a problem. Just don’t tell the frat boys: They’ll be hooking oxygen tanks up to their butts and chanting: “Breathe! Breathe! Breathe!”
Better, stronger, faster … pinker
Many people, most of whom aren’t even athletes, commit huge amounts of time, effort, and expense to improve their athletic performance. But what if there’s an easier way?
Research conducted at the University of Westminster (England) showed that participants could, with one fairly simple intervention, get on a treadmill and run 212 meters further in 30 minutes, increasing their speed by an average of 4.4%. Not only that, but “feelings of pleasure were also enhanced, meaning participants found running more enjoyable,” according to a statement from the university.
Is this amazing intervention a new wonder drug? No. Is it a super special nutritional supplement? Negatory. An energy drink that “gives you wiiings”? Nope. The latest designer steroid? Nyet.
Like we said, it’s simple, and it’s pink. Literally, the color pink. We will explain.
Each of the 10 study subjects completed two 30-minute trials on the treadmill. For one, they were given a clear, artificially sweetened drink while they were running. For the other, they received the exact same drink colored pink with food dye. Pink did better. So to recap the last month in our column, faster looks pink, and skinny smells like lemons.
Once again, science demonstrates that you can’t go wrong by fooling a brain. Next week, LOTME tries to find out if purple makes you funnier.
Hey … I’m singing here!
Noise pollution has been linked to plenty of negative outcomes, but the latest target is the poor baby zebra finch.
Researchers at the Max Planck Institute of Ornithology in Germany say traffic noise disrupts the timing of vocal development and impairs learning in the flying finches. The noise was also shown to suppress their immune systems, because of lingering stress.
The good news is that the birds with noise-induced stress sang as much as their peers in a control group, so the delay in development “was not due to a lack of vocal practice,” according to researchers. However, one long-term effect could be that zebra finch birdsongs could change over time due to noise-induced copying errors. Imagine a really long game of birdsong telephone – the song at the beginning is unlikely to be the song years from now.
While not mentioned in the study, one could also imagine that due to all that exposure to traffic, young zebra finches could be developing a salty dialect and impatience with fellow finches taking up too much space on the same tree branch. Hopefully, they don’t give others “the bird.”
Slimy soap
Remember at the beginning of the pandemic when it was almost impossible to find sufficient hand-washing supplies? Just when you thought you’d tried everything, there is soap made from snail slime.
Snail slime, surprisingly, has many beneficial properties for humans. The slime has antiaging and skin healing properties and is actually used in some Korean beauty supplies. The snails even use the slime to help fix their shells if they become damaged.
Happily, no snails are harmed in the slime extraction and making of the soap. Snail farmer Damien Desrochers says, “I only touch it with my finger, you see it’s not violent, it’s simple.”
As you can probably imagine, a lot of slime is needed to have a steady supply of this soap, so Mr. Desrochers has systems in place to get enough slime. Approximately 40 snails are needed to make 15 bars of soap, and he hopes to produce about 3,000 bars in the first year.
Nothing really surprises us anymore in the beauty world: People put eggs in their hair and bee venom on their skin, so what’s wrong with a little snail slime?
No ifs, ands, or butt ventilators
Breathing, on most days, is a pretty simple task. You inhale, the oxygen goes in, fills your lungs, becomes carbon dioxide, and is exhaled. But as certain recent events have made very clear, some diseases make this task difficult, which is where ventilators come in. The issue is, some patients can’t really use ventilators.
Enter a new study from Japan, which tested the ability of mice and pigs to absorb oxygen through the rectum. Yes, breathing through the butt. It’s not actually such a far-fetched idea; several aquatic animals such as sea cucumbers and catfish absorb oxygen through their intestines, and as any drunken frat boy can tell you after a good butt chug, other chemicals can absolutely be absorbed by human intestines.
After an initial successful experiment where a group of mice had their intestines scrubbed, had pure oxygen inserted enterally, and were exposed to a hypoxic environment, the researchers decided to step up their game and avoid the exhaustive act of digestive scrubbing by enlisting the aid of something out of science fiction: perfluorocarbon. If you haven’t seen “The Abyss,” this liquid can absorb massive amounts of oxygen, so you can actually breathe it in the same way you do with air.
In part two of the experiment, a group of hypoxic mice and pigs had perfluorocarbon inserted into their anuses, while another group got saline solution. The saline group did not fare well, but the animals that got perfluorocarbon had their hypoxic symptoms relieved within minutes.
The effectiveness of this procedure in humans clearly has yet to be tested, and while it may not be useful in all, or even most, situations, it is always beneficial to have more ways to combat a problem. Just don’t tell the frat boys: They’ll be hooking oxygen tanks up to their butts and chanting: “Breathe! Breathe! Breathe!”
Better, stronger, faster … pinker
Many people, most of whom aren’t even athletes, commit huge amounts of time, effort, and expense to improve their athletic performance. But what if there’s an easier way?
Research conducted at the University of Westminster (England) showed that participants could, with one fairly simple intervention, get on a treadmill and run 212 meters further in 30 minutes, increasing their speed by an average of 4.4%. Not only that, but “feelings of pleasure were also enhanced, meaning participants found running more enjoyable,” according to a statement from the university.
Is this amazing intervention a new wonder drug? No. Is it a super special nutritional supplement? Negatory. An energy drink that “gives you wiiings”? Nope. The latest designer steroid? Nyet.
Like we said, it’s simple, and it’s pink. Literally, the color pink. We will explain.
Each of the 10 study subjects completed two 30-minute trials on the treadmill. For one, they were given a clear, artificially sweetened drink while they were running. For the other, they received the exact same drink colored pink with food dye. Pink did better. So to recap the last month in our column, faster looks pink, and skinny smells like lemons.
Once again, science demonstrates that you can’t go wrong by fooling a brain. Next week, LOTME tries to find out if purple makes you funnier.
Hey … I’m singing here!
Noise pollution has been linked to plenty of negative outcomes, but the latest target is the poor baby zebra finch.
Researchers at the Max Planck Institute of Ornithology in Germany say traffic noise disrupts the timing of vocal development and impairs learning in the flying finches. The noise was also shown to suppress their immune systems, because of lingering stress.
The good news is that the birds with noise-induced stress sang as much as their peers in a control group, so the delay in development “was not due to a lack of vocal practice,” according to researchers. However, one long-term effect could be that zebra finch birdsongs could change over time due to noise-induced copying errors. Imagine a really long game of birdsong telephone – the song at the beginning is unlikely to be the song years from now.
While not mentioned in the study, one could also imagine that due to all that exposure to traffic, young zebra finches could be developing a salty dialect and impatience with fellow finches taking up too much space on the same tree branch. Hopefully, they don’t give others “the bird.”
Slimy soap
Remember at the beginning of the pandemic when it was almost impossible to find sufficient hand-washing supplies? Just when you thought you’d tried everything, there is soap made from snail slime.
Snail slime, surprisingly, has many beneficial properties for humans. The slime has antiaging and skin healing properties and is actually used in some Korean beauty supplies. The snails even use the slime to help fix their shells if they become damaged.
Happily, no snails are harmed in the slime extraction and making of the soap. Snail farmer Damien Desrochers says, “I only touch it with my finger, you see it’s not violent, it’s simple.”
As you can probably imagine, a lot of slime is needed to have a steady supply of this soap, so Mr. Desrochers has systems in place to get enough slime. Approximately 40 snails are needed to make 15 bars of soap, and he hopes to produce about 3,000 bars in the first year.
Nothing really surprises us anymore in the beauty world: People put eggs in their hair and bee venom on their skin, so what’s wrong with a little snail slime?










