User login
The Official Newspaper of the American Association for Thoracic Surgery
New appropriate use criteria reframe severe aortic stenosis
New appropriate use criteria (AUC) for severe aortic stenosis (AS) run the full gamut of clinical scenarios and treatment options.
“Cardiology is seeing a radical change in the management of aortic stenosis. This new document incorporates all therapies currently available in the world,” said Vinod H. Thourani, MD, who served on the AUC’s writing group on behalf of the American College of Cardiology. “The AUC highlights state-of-the-art therapy for aortic stenosis and, even more importantly, helps clarify the right indications for surgical and transcatheter valve replacement.”
Surgical risk is assessed based on the Society of Thoracic Surgeons Predicted Risk of Mortality score plus additional anatomic and functional considerations that should be assessed by a multidisciplinary heart team. The AUC repeatedly emphasizes this team’s importance. “Multiple comorbidities can change the pathway of treating AS, and this determination is best made by a heart team that at least includes a noninvasive cardiologist, an interventional cardiologist, and a cardiac surgeon,” Dr. Thourani said. “That’s how patients get the best care.”
Historically, aortic stenosis typically was managed medically or with balloon aortic valvuloplasty (BAV) or open aortic valve replacement, Dr. Thourani said. However, BAV is less common now, and indications for surgical or transcatheter aortic valve replacement (SAVR or TAVR) are expanding. Balloon aortic valvuloplasty sometimes does provide palliative treatment or serve as a bridge to a decision, the AUC states. For example, for a high-risk patient with severe aortic stenosis and severe secondary mitral regurgitation, BAV can help the heart team decide whether TAVR alone will improve mitral regurgitation or whether a double valve procedure is preferable.
Regardless of risk score, the AUC considers a wait-and-see approach as potentially appropriate for patients with asymptomatic high-grade AS whose left ventricular ejection fraction is at least 50%, peak aortic valve velocity is 4.0-4.9 m/sec, and exercise stress test is normal and with no predictors of symptom onset or rapid progression. Asymptomatic patients who are likely to become symptomatic but who have a low risk of sudden death are candidates for intervention (rated “appropriate”) or medical management (“may be appropriate”). In contrast, a positive stress test in an otherwise asymptomatic patient merits consideration of SAVR or TAVR regardless of surgical risk. The recommendations for asymptomatic patients reflect a lack of head-to-head trials in this population, Dr. Thourani said. “We don’t have good randomized data to show one therapy is better than another.”
Symptomatic, high-gradient, severe AS with associated coronary artery disease merits consideration of SAVR with coronary artery bypass graft or, in some cases, TAVR with percutaneous coronary intervention, according to the AUC. Less evidence supports SAVR with PCI. “Optimal management of coronary artery disease in patients with AS is a complex decision process requiring clinical, anatomical, and technical considerations that is best achieved with close collaboration between heart team members,” the authors stress.
The document covers other valvular and structural heart conditions that commonly accompany severe AS, such as symptomatic AS with bicuspid aortic valve and ascending aortic dilation. “Although there remains an increasing prevalence of transcatheter valve usage in bicuspid aortic valve, the standard of care remains surgical therapy, especially in patients who have a dilated aorta,” Dr. Thourani said.
For the first time, the AUC also addresses failing aortic valve prostheses, presenting six relevant clinical scenarios. The AUC consistently recommends SAVR, although the use of TAVR has “dramatically increased” in these patients, Dr. Thourani said. “Long-term data are still pending, but TAVR appears to be a less morbid procedure, when done appropriately.”
The societies involved in creating the AUC statement were the American Association for Thoracic Surgery, American Heart Association, American Society of Echocardiography, European Association for Cardio-Thoracic Surgery, Heart Valve Society, Society of Cardiovascular Anesthesiologists, Society for Cardiovascular Angiography and Interventions, Society of Cardiovascular Computed Tomography, Society for Cardiovascular Magnetic Resonance, and Society of Thoracic Surgeons.
Dr. Thourani disclosed ties to Edwards Lifesciences, St. Jude Medical, Abbott, Boston Scientific, and Medtronic.
New appropriate use criteria (AUC) for severe aortic stenosis (AS) run the full gamut of clinical scenarios and treatment options.
“Cardiology is seeing a radical change in the management of aortic stenosis. This new document incorporates all therapies currently available in the world,” said Vinod H. Thourani, MD, who served on the AUC’s writing group on behalf of the American College of Cardiology. “The AUC highlights state-of-the-art therapy for aortic stenosis and, even more importantly, helps clarify the right indications for surgical and transcatheter valve replacement.”
Surgical risk is assessed based on the Society of Thoracic Surgeons Predicted Risk of Mortality score plus additional anatomic and functional considerations that should be assessed by a multidisciplinary heart team. The AUC repeatedly emphasizes this team’s importance. “Multiple comorbidities can change the pathway of treating AS, and this determination is best made by a heart team that at least includes a noninvasive cardiologist, an interventional cardiologist, and a cardiac surgeon,” Dr. Thourani said. “That’s how patients get the best care.”
Historically, aortic stenosis typically was managed medically or with balloon aortic valvuloplasty (BAV) or open aortic valve replacement, Dr. Thourani said. However, BAV is less common now, and indications for surgical or transcatheter aortic valve replacement (SAVR or TAVR) are expanding. Balloon aortic valvuloplasty sometimes does provide palliative treatment or serve as a bridge to a decision, the AUC states. For example, for a high-risk patient with severe aortic stenosis and severe secondary mitral regurgitation, BAV can help the heart team decide whether TAVR alone will improve mitral regurgitation or whether a double valve procedure is preferable.
Regardless of risk score, the AUC considers a wait-and-see approach as potentially appropriate for patients with asymptomatic high-grade AS whose left ventricular ejection fraction is at least 50%, peak aortic valve velocity is 4.0-4.9 m/sec, and exercise stress test is normal and with no predictors of symptom onset or rapid progression. Asymptomatic patients who are likely to become symptomatic but who have a low risk of sudden death are candidates for intervention (rated “appropriate”) or medical management (“may be appropriate”). In contrast, a positive stress test in an otherwise asymptomatic patient merits consideration of SAVR or TAVR regardless of surgical risk. The recommendations for asymptomatic patients reflect a lack of head-to-head trials in this population, Dr. Thourani said. “We don’t have good randomized data to show one therapy is better than another.”
Symptomatic, high-gradient, severe AS with associated coronary artery disease merits consideration of SAVR with coronary artery bypass graft or, in some cases, TAVR with percutaneous coronary intervention, according to the AUC. Less evidence supports SAVR with PCI. “Optimal management of coronary artery disease in patients with AS is a complex decision process requiring clinical, anatomical, and technical considerations that is best achieved with close collaboration between heart team members,” the authors stress.
The document covers other valvular and structural heart conditions that commonly accompany severe AS, such as symptomatic AS with bicuspid aortic valve and ascending aortic dilation. “Although there remains an increasing prevalence of transcatheter valve usage in bicuspid aortic valve, the standard of care remains surgical therapy, especially in patients who have a dilated aorta,” Dr. Thourani said.
For the first time, the AUC also addresses failing aortic valve prostheses, presenting six relevant clinical scenarios. The AUC consistently recommends SAVR, although the use of TAVR has “dramatically increased” in these patients, Dr. Thourani said. “Long-term data are still pending, but TAVR appears to be a less morbid procedure, when done appropriately.”
The societies involved in creating the AUC statement were the American Association for Thoracic Surgery, American Heart Association, American Society of Echocardiography, European Association for Cardio-Thoracic Surgery, Heart Valve Society, Society of Cardiovascular Anesthesiologists, Society for Cardiovascular Angiography and Interventions, Society of Cardiovascular Computed Tomography, Society for Cardiovascular Magnetic Resonance, and Society of Thoracic Surgeons.
Dr. Thourani disclosed ties to Edwards Lifesciences, St. Jude Medical, Abbott, Boston Scientific, and Medtronic.
New appropriate use criteria (AUC) for severe aortic stenosis (AS) run the full gamut of clinical scenarios and treatment options.
“Cardiology is seeing a radical change in the management of aortic stenosis. This new document incorporates all therapies currently available in the world,” said Vinod H. Thourani, MD, who served on the AUC’s writing group on behalf of the American College of Cardiology. “The AUC highlights state-of-the-art therapy for aortic stenosis and, even more importantly, helps clarify the right indications for surgical and transcatheter valve replacement.”
Surgical risk is assessed based on the Society of Thoracic Surgeons Predicted Risk of Mortality score plus additional anatomic and functional considerations that should be assessed by a multidisciplinary heart team. The AUC repeatedly emphasizes this team’s importance. “Multiple comorbidities can change the pathway of treating AS, and this determination is best made by a heart team that at least includes a noninvasive cardiologist, an interventional cardiologist, and a cardiac surgeon,” Dr. Thourani said. “That’s how patients get the best care.”
Historically, aortic stenosis typically was managed medically or with balloon aortic valvuloplasty (BAV) or open aortic valve replacement, Dr. Thourani said. However, BAV is less common now, and indications for surgical or transcatheter aortic valve replacement (SAVR or TAVR) are expanding. Balloon aortic valvuloplasty sometimes does provide palliative treatment or serve as a bridge to a decision, the AUC states. For example, for a high-risk patient with severe aortic stenosis and severe secondary mitral regurgitation, BAV can help the heart team decide whether TAVR alone will improve mitral regurgitation or whether a double valve procedure is preferable.
Regardless of risk score, the AUC considers a wait-and-see approach as potentially appropriate for patients with asymptomatic high-grade AS whose left ventricular ejection fraction is at least 50%, peak aortic valve velocity is 4.0-4.9 m/sec, and exercise stress test is normal and with no predictors of symptom onset or rapid progression. Asymptomatic patients who are likely to become symptomatic but who have a low risk of sudden death are candidates for intervention (rated “appropriate”) or medical management (“may be appropriate”). In contrast, a positive stress test in an otherwise asymptomatic patient merits consideration of SAVR or TAVR regardless of surgical risk. The recommendations for asymptomatic patients reflect a lack of head-to-head trials in this population, Dr. Thourani said. “We don’t have good randomized data to show one therapy is better than another.”
Symptomatic, high-gradient, severe AS with associated coronary artery disease merits consideration of SAVR with coronary artery bypass graft or, in some cases, TAVR with percutaneous coronary intervention, according to the AUC. Less evidence supports SAVR with PCI. “Optimal management of coronary artery disease in patients with AS is a complex decision process requiring clinical, anatomical, and technical considerations that is best achieved with close collaboration between heart team members,” the authors stress.
The document covers other valvular and structural heart conditions that commonly accompany severe AS, such as symptomatic AS with bicuspid aortic valve and ascending aortic dilation. “Although there remains an increasing prevalence of transcatheter valve usage in bicuspid aortic valve, the standard of care remains surgical therapy, especially in patients who have a dilated aorta,” Dr. Thourani said.
For the first time, the AUC also addresses failing aortic valve prostheses, presenting six relevant clinical scenarios. The AUC consistently recommends SAVR, although the use of TAVR has “dramatically increased” in these patients, Dr. Thourani said. “Long-term data are still pending, but TAVR appears to be a less morbid procedure, when done appropriately.”
The societies involved in creating the AUC statement were the American Association for Thoracic Surgery, American Heart Association, American Society of Echocardiography, European Association for Cardio-Thoracic Surgery, Heart Valve Society, Society of Cardiovascular Anesthesiologists, Society for Cardiovascular Angiography and Interventions, Society of Cardiovascular Computed Tomography, Society for Cardiovascular Magnetic Resonance, and Society of Thoracic Surgeons.
Dr. Thourani disclosed ties to Edwards Lifesciences, St. Jude Medical, Abbott, Boston Scientific, and Medtronic.
FROM THE JOURNAL OF THE AMERICAN COLLEGE OF CARDIOLOGY
Role grows for heart failure patient-reported outcomes
DALLAS – The Food and Drug Administration is keenly seeking patient-reported outcomes as endpoints in cardiovascular drug or device trials, particularly for heart failure patients, but the bar remains high for getting such an outcome into labeling, said agency officials who regulate cardiovascular disease therapies.
The FDA issued guidance nearly 8 years ago on how to integrate patient-reported outcome (PRO) measures into medical product development, but so far no heart failure drug nor device has met the agency’s standards for documented success in improving a PRO, despite the clear need for these patients to receive patient-centered care, clinicians said.

“We don’t yet have a patient-reported outcome in a label for heart failure,” Paul A. Heidenreich, MD, said during a session on PROs at the annual scientific meeting of the Heart Failure Society of America. He voiced hope that a PRO might end up on the label of a heart failure drug or device sometime in 2018. “Almost half of FDA submissions now include a PRO” as part of the data package, added Dr. Heidenreich, a cardiologist and professor of medicine at Stanford (Calif.) University.
For years, PROs for heart failure weren’t often used in trials, and they remain largely absent from routine practice – an absence Dr. Heidenreich lamented. “Just focusing on mortality in heart failure is really not patient centered,” he said.

Heart failure physicians “are very good at disease-centered care” that focuses on survival and reducing hospitalizations, but “survival is often not as important to patients,” noted Mary Norine Walsh, MD, medical director of the heart failure and cardiac transplantation program at St. Vincent Medical Group in Indianapolis. She suggested “tailoring treatment to improve patient symptoms, physical function, and quality of life” without necessarily reducing hospital readmissions or increasing survival rates. “Self-reported measures have more meaning for patients,” she said, and called for using PROs to better target interventions to the patients who can most benefit from them.
Two FDA representatives who spoke during the session agreed on the importance of PROs and attested to the agency’s interest in greater reliance on them.

“PROs are a critical complement to the other measures made in device trials,” said Bram Zuckerman, MD, director of the FDA’s division of cardiovascular devices. “We need PRO information because it reflects important aspects of patients’ health-related quality of life.”
The most commonly used PRO measures in device trials today are the Kansas City Cardiomyopathy Questionnaire (J Am Coll Cardiol. 2000 Apr;35[5]:1245-55) and the Minnesota Living With Heart Failure questionnaire, he noted.
“Neither is perfect, but there is a track record in heart failure device development that these two PROs can be helpful.” The FDA’s cardiovascular device division “wants to use PRO information,” Dr. Zuckerman said.
“All-cause mortality is the most unbiased endpoint, but there is interest in PROs,” agreed Ebony Dashiell-Aje, PhD, from the FDA’s office of new drugs in the Center for Drug Evaluation and Research. She highlighted the encouragement that the FDA gave to drug and device developers to include PROs in trials, both in its 2009 guidance document as well as in a “roadmap” from the agency on how to measure PROs in clinical trials. “Unfortunately, in heart failure we struggle to find tools that can adequately measure the patient’s perspective and be sensitive enough to detect a treatment benefit,” she said.
Norman Stockbridge, MD, director of the division of cardiovascular and renal products in the agency’s Office of Drug Evaluation, cited even bigger barriers to FDA approval of PROs as labeled effects from drugs or devices.
Getting a PRO endpoint supported by clinical-trial results that qualify it for an FDA label faces two big challenges. One challenge, he said, is “how much of an effect we need to see in a complex scoring algorithm to know that patients actually received some benefit in a disease that often varies from day to day and from week to week.” The second challenge is that, “in a disease with a high background rate of bad outcomes, you need some evidence that the benefit [from the treatment] is worth any risk,” which is something that can be hard to prove in heart failure when many patients don’t live more than 2 years with the disease, Dr. Stockbridge said in an interview.
“You need to be able to make the argument that the [PRO] benefit is likely perceptible to patients, but that is only half the problem. The other half is whether the developer can rule out that survival is not less than it would have been with no treatment. If patients take this, will they feel better but have a greater risk of being hurt?”
So far, no drug or device developer has succeeded in proving this to the FDA, despite the agency’s 2009 guidance on how it could be done.
That guidance “is one of the two worst and most destructive guidance documents we ever published,” Dr. Stockbridge declared.
Dr. Walsh, Dr. Heidenreich, Dr. Zuckerman, Dr. Dashiell-Aje, and Dr. Stockbridge had no relevant disclosures.
[email protected]
On Twitter @mitchelzoler
DALLAS – The Food and Drug Administration is keenly seeking patient-reported outcomes as endpoints in cardiovascular drug or device trials, particularly for heart failure patients, but the bar remains high for getting such an outcome into labeling, said agency officials who regulate cardiovascular disease therapies.
The FDA issued guidance nearly 8 years ago on how to integrate patient-reported outcome (PRO) measures into medical product development, but so far no heart failure drug nor device has met the agency’s standards for documented success in improving a PRO, despite the clear need for these patients to receive patient-centered care, clinicians said.

“We don’t yet have a patient-reported outcome in a label for heart failure,” Paul A. Heidenreich, MD, said during a session on PROs at the annual scientific meeting of the Heart Failure Society of America. He voiced hope that a PRO might end up on the label of a heart failure drug or device sometime in 2018. “Almost half of FDA submissions now include a PRO” as part of the data package, added Dr. Heidenreich, a cardiologist and professor of medicine at Stanford (Calif.) University.
For years, PROs for heart failure weren’t often used in trials, and they remain largely absent from routine practice – an absence Dr. Heidenreich lamented. “Just focusing on mortality in heart failure is really not patient centered,” he said.

Heart failure physicians “are very good at disease-centered care” that focuses on survival and reducing hospitalizations, but “survival is often not as important to patients,” noted Mary Norine Walsh, MD, medical director of the heart failure and cardiac transplantation program at St. Vincent Medical Group in Indianapolis. She suggested “tailoring treatment to improve patient symptoms, physical function, and quality of life” without necessarily reducing hospital readmissions or increasing survival rates. “Self-reported measures have more meaning for patients,” she said, and called for using PROs to better target interventions to the patients who can most benefit from them.
Two FDA representatives who spoke during the session agreed on the importance of PROs and attested to the agency’s interest in greater reliance on them.

“PROs are a critical complement to the other measures made in device trials,” said Bram Zuckerman, MD, director of the FDA’s division of cardiovascular devices. “We need PRO information because it reflects important aspects of patients’ health-related quality of life.”
The most commonly used PRO measures in device trials today are the Kansas City Cardiomyopathy Questionnaire (J Am Coll Cardiol. 2000 Apr;35[5]:1245-55) and the Minnesota Living With Heart Failure questionnaire, he noted.
“Neither is perfect, but there is a track record in heart failure device development that these two PROs can be helpful.” The FDA’s cardiovascular device division “wants to use PRO information,” Dr. Zuckerman said.
“All-cause mortality is the most unbiased endpoint, but there is interest in PROs,” agreed Ebony Dashiell-Aje, PhD, from the FDA’s office of new drugs in the Center for Drug Evaluation and Research. She highlighted the encouragement that the FDA gave to drug and device developers to include PROs in trials, both in its 2009 guidance document as well as in a “roadmap” from the agency on how to measure PROs in clinical trials. “Unfortunately, in heart failure we struggle to find tools that can adequately measure the patient’s perspective and be sensitive enough to detect a treatment benefit,” she said.
Norman Stockbridge, MD, director of the division of cardiovascular and renal products in the agency’s Office of Drug Evaluation, cited even bigger barriers to FDA approval of PROs as labeled effects from drugs or devices.
Getting a PRO endpoint supported by clinical-trial results that qualify it for an FDA label faces two big challenges. One challenge, he said, is “how much of an effect we need to see in a complex scoring algorithm to know that patients actually received some benefit in a disease that often varies from day to day and from week to week.” The second challenge is that, “in a disease with a high background rate of bad outcomes, you need some evidence that the benefit [from the treatment] is worth any risk,” which is something that can be hard to prove in heart failure when many patients don’t live more than 2 years with the disease, Dr. Stockbridge said in an interview.
“You need to be able to make the argument that the [PRO] benefit is likely perceptible to patients, but that is only half the problem. The other half is whether the developer can rule out that survival is not less than it would have been with no treatment. If patients take this, will they feel better but have a greater risk of being hurt?”
So far, no drug or device developer has succeeded in proving this to the FDA, despite the agency’s 2009 guidance on how it could be done.
That guidance “is one of the two worst and most destructive guidance documents we ever published,” Dr. Stockbridge declared.
Dr. Walsh, Dr. Heidenreich, Dr. Zuckerman, Dr. Dashiell-Aje, and Dr. Stockbridge had no relevant disclosures.
[email protected]
On Twitter @mitchelzoler
DALLAS – The Food and Drug Administration is keenly seeking patient-reported outcomes as endpoints in cardiovascular drug or device trials, particularly for heart failure patients, but the bar remains high for getting such an outcome into labeling, said agency officials who regulate cardiovascular disease therapies.
The FDA issued guidance nearly 8 years ago on how to integrate patient-reported outcome (PRO) measures into medical product development, but so far no heart failure drug nor device has met the agency’s standards for documented success in improving a PRO, despite the clear need for these patients to receive patient-centered care, clinicians said.

“We don’t yet have a patient-reported outcome in a label for heart failure,” Paul A. Heidenreich, MD, said during a session on PROs at the annual scientific meeting of the Heart Failure Society of America. He voiced hope that a PRO might end up on the label of a heart failure drug or device sometime in 2018. “Almost half of FDA submissions now include a PRO” as part of the data package, added Dr. Heidenreich, a cardiologist and professor of medicine at Stanford (Calif.) University.
For years, PROs for heart failure weren’t often used in trials, and they remain largely absent from routine practice – an absence Dr. Heidenreich lamented. “Just focusing on mortality in heart failure is really not patient centered,” he said.

Heart failure physicians “are very good at disease-centered care” that focuses on survival and reducing hospitalizations, but “survival is often not as important to patients,” noted Mary Norine Walsh, MD, medical director of the heart failure and cardiac transplantation program at St. Vincent Medical Group in Indianapolis. She suggested “tailoring treatment to improve patient symptoms, physical function, and quality of life” without necessarily reducing hospital readmissions or increasing survival rates. “Self-reported measures have more meaning for patients,” she said, and called for using PROs to better target interventions to the patients who can most benefit from them.
Two FDA representatives who spoke during the session agreed on the importance of PROs and attested to the agency’s interest in greater reliance on them.

“PROs are a critical complement to the other measures made in device trials,” said Bram Zuckerman, MD, director of the FDA’s division of cardiovascular devices. “We need PRO information because it reflects important aspects of patients’ health-related quality of life.”
The most commonly used PRO measures in device trials today are the Kansas City Cardiomyopathy Questionnaire (J Am Coll Cardiol. 2000 Apr;35[5]:1245-55) and the Minnesota Living With Heart Failure questionnaire, he noted.
“Neither is perfect, but there is a track record in heart failure device development that these two PROs can be helpful.” The FDA’s cardiovascular device division “wants to use PRO information,” Dr. Zuckerman said.
“All-cause mortality is the most unbiased endpoint, but there is interest in PROs,” agreed Ebony Dashiell-Aje, PhD, from the FDA’s office of new drugs in the Center for Drug Evaluation and Research. She highlighted the encouragement that the FDA gave to drug and device developers to include PROs in trials, both in its 2009 guidance document as well as in a “roadmap” from the agency on how to measure PROs in clinical trials. “Unfortunately, in heart failure we struggle to find tools that can adequately measure the patient’s perspective and be sensitive enough to detect a treatment benefit,” she said.
Norman Stockbridge, MD, director of the division of cardiovascular and renal products in the agency’s Office of Drug Evaluation, cited even bigger barriers to FDA approval of PROs as labeled effects from drugs or devices.
Getting a PRO endpoint supported by clinical-trial results that qualify it for an FDA label faces two big challenges. One challenge, he said, is “how much of an effect we need to see in a complex scoring algorithm to know that patients actually received some benefit in a disease that often varies from day to day and from week to week.” The second challenge is that, “in a disease with a high background rate of bad outcomes, you need some evidence that the benefit [from the treatment] is worth any risk,” which is something that can be hard to prove in heart failure when many patients don’t live more than 2 years with the disease, Dr. Stockbridge said in an interview.
“You need to be able to make the argument that the [PRO] benefit is likely perceptible to patients, but that is only half the problem. The other half is whether the developer can rule out that survival is not less than it would have been with no treatment. If patients take this, will they feel better but have a greater risk of being hurt?”
So far, no drug or device developer has succeeded in proving this to the FDA, despite the agency’s 2009 guidance on how it could be done.
That guidance “is one of the two worst and most destructive guidance documents we ever published,” Dr. Stockbridge declared.
Dr. Walsh, Dr. Heidenreich, Dr. Zuckerman, Dr. Dashiell-Aje, and Dr. Stockbridge had no relevant disclosures.
[email protected]
On Twitter @mitchelzoler
EXPERT ANALYSIS FROM THE HFSA ANNUAL SCIENTIFIC MEETING
Fentanyl in the cath lab questioned
BARCELONA – The current routine use of intravenous fentanyl in the cardiac catheterization lab for patient comfort during coronary angiography has been called into question by the results of a double-blind randomized trial presented at the annual congress of the European Society of Cardiology.
The trial, known as PACIFY, showed that IV fentanyl delayed absorption of the oral P2Y12 inhibitor ticagrelor (Brilinta) by up to 4 hours. That’s a disturbing finding that could account for the relatively high risk of stent thrombosis in the first hours after percutaneous coronary intervention, according to lead investigator John W. McEvoy, MD, a cardiologist at Johns Hopkins University in Baltimore.
“These data challenge the routine and nonselective use of fentanyl for cardiac catheterization and PCI, particularly when rapid platelet inhibition is desirable,” he said, adding, “This would represent a significant change in U.S. cath lab practice.”
PACIFY (Platelet Aggregation After Ticagrelor Inhibition and Fentanyl) was a single-center trial in which 212 patients undergoing PCI were randomized in double-blind fashion to fentanyl or no fentanyl on top of a local anesthetic and IV midazolam (Versed). In addition, the 70 subjects undergoing PCI with stent placement received a 180-mg loading dose of ticagrelor intraprocedurally.
The primary endpoint was ticagrelor plasma concentration during the first 24 hours after the drug’s administration. Secondary endpoints were patients’ self-reported maximum pain during the procedure and platelet inhibition at 2 hours.
The plasma concentration time area under the curve over the course of 24 hours was superior in the no-fentanyl group by a margin of 3,441 ng/mL–1 per hour to 2,016 ng/mL–1 per hour. Moreover, 37% of fentanyl recipients displayed high platelet reactivity at 2 hours as measured by light transmission platelet aggregometry, compared with none of the no-fentanyl controls.
Pain was similarly well controlled in both treatment arms, casting doubt on the widespread belief among U.S. interventionalists that routine administration of fentanyl in the cath lab is necessary for patient comfort. Patients in the control arm could receive bailout fentanyl upon request; only two did so.
Dr. McEvoy reported having no financial conflicts regarding this study, which was conducted free of commercial support.
BARCELONA – The current routine use of intravenous fentanyl in the cardiac catheterization lab for patient comfort during coronary angiography has been called into question by the results of a double-blind randomized trial presented at the annual congress of the European Society of Cardiology.
The trial, known as PACIFY, showed that IV fentanyl delayed absorption of the oral P2Y12 inhibitor ticagrelor (Brilinta) by up to 4 hours. That’s a disturbing finding that could account for the relatively high risk of stent thrombosis in the first hours after percutaneous coronary intervention, according to lead investigator John W. McEvoy, MD, a cardiologist at Johns Hopkins University in Baltimore.
“These data challenge the routine and nonselective use of fentanyl for cardiac catheterization and PCI, particularly when rapid platelet inhibition is desirable,” he said, adding, “This would represent a significant change in U.S. cath lab practice.”
PACIFY (Platelet Aggregation After Ticagrelor Inhibition and Fentanyl) was a single-center trial in which 212 patients undergoing PCI were randomized in double-blind fashion to fentanyl or no fentanyl on top of a local anesthetic and IV midazolam (Versed). In addition, the 70 subjects undergoing PCI with stent placement received a 180-mg loading dose of ticagrelor intraprocedurally.
The primary endpoint was ticagrelor plasma concentration during the first 24 hours after the drug’s administration. Secondary endpoints were patients’ self-reported maximum pain during the procedure and platelet inhibition at 2 hours.
The plasma concentration time area under the curve over the course of 24 hours was superior in the no-fentanyl group by a margin of 3,441 ng/mL–1 per hour to 2,016 ng/mL–1 per hour. Moreover, 37% of fentanyl recipients displayed high platelet reactivity at 2 hours as measured by light transmission platelet aggregometry, compared with none of the no-fentanyl controls.
Pain was similarly well controlled in both treatment arms, casting doubt on the widespread belief among U.S. interventionalists that routine administration of fentanyl in the cath lab is necessary for patient comfort. Patients in the control arm could receive bailout fentanyl upon request; only two did so.
Dr. McEvoy reported having no financial conflicts regarding this study, which was conducted free of commercial support.
BARCELONA – The current routine use of intravenous fentanyl in the cardiac catheterization lab for patient comfort during coronary angiography has been called into question by the results of a double-blind randomized trial presented at the annual congress of the European Society of Cardiology.
The trial, known as PACIFY, showed that IV fentanyl delayed absorption of the oral P2Y12 inhibitor ticagrelor (Brilinta) by up to 4 hours. That’s a disturbing finding that could account for the relatively high risk of stent thrombosis in the first hours after percutaneous coronary intervention, according to lead investigator John W. McEvoy, MD, a cardiologist at Johns Hopkins University in Baltimore.
“These data challenge the routine and nonselective use of fentanyl for cardiac catheterization and PCI, particularly when rapid platelet inhibition is desirable,” he said, adding, “This would represent a significant change in U.S. cath lab practice.”
PACIFY (Platelet Aggregation After Ticagrelor Inhibition and Fentanyl) was a single-center trial in which 212 patients undergoing PCI were randomized in double-blind fashion to fentanyl or no fentanyl on top of a local anesthetic and IV midazolam (Versed). In addition, the 70 subjects undergoing PCI with stent placement received a 180-mg loading dose of ticagrelor intraprocedurally.
The primary endpoint was ticagrelor plasma concentration during the first 24 hours after the drug’s administration. Secondary endpoints were patients’ self-reported maximum pain during the procedure and platelet inhibition at 2 hours.
The plasma concentration time area under the curve over the course of 24 hours was superior in the no-fentanyl group by a margin of 3,441 ng/mL–1 per hour to 2,016 ng/mL–1 per hour. Moreover, 37% of fentanyl recipients displayed high platelet reactivity at 2 hours as measured by light transmission platelet aggregometry, compared with none of the no-fentanyl controls.
Pain was similarly well controlled in both treatment arms, casting doubt on the widespread belief among U.S. interventionalists that routine administration of fentanyl in the cath lab is necessary for patient comfort. Patients in the control arm could receive bailout fentanyl upon request; only two did so.
Dr. McEvoy reported having no financial conflicts regarding this study, which was conducted free of commercial support.
AT THE ESC CONGRESS 2017
Key clinical point:
Major finding: High platelet reactivity at 2 hours was present in 37% of patients who underwent coronary angiography with IV fentanyl and in none randomized to going without the opiate.
Data source: PACIFY, a single-center, double-blind, randomized trial included 212 patients undergoing coronary angiography.
Disclosures: The presenter reported having no financial conflicts regarding this study, which was conducted free of commercial support.
Over 40% of Americans have experience with medical errors
More than 40% of adults have either experienced a medical error or been involved in the care of someone who did, according to a recent national survey.
Specifically, 21% of American adults said that they have personally experienced a medical error and 31% said that they have been involved in the care of another person who experienced an error. The combined total, which includes some overlap, was 41% in the survey conducted by the Institute for Healthcare Improvement (IHI)/National Patient Safety Foundation (NPSF) and National Opinion Research Center (NORC), a nonpartisan research institution at the University of Chicago, .
Medical errors were defined for respondents as “mistakes [that] sometimes result in no harm, while other times they may result in additional or prolonged treatment, emotional distress, disability, or death,” investigators at IHI/NPSF and NORC said in their report.
A misdiagnosed medical problem was the most common type of medical error, reported by 59% of those with error experience. The next most common type of error was a mistake during a test, surgery, or treatment, which was mentioned by 46% of those with error experience, followed by a diagnosis that didn’t make sense (42%), lack of respect (39%), and incorrect instructions about follow-up care (29%), the IHI/HPSF and NORC report indicated.
Feelings of disrespect were more common among younger respondents: 46% of those aged 18-44 years said they were not treated with respect by a health care provider, compared with 34% of those aged 45 years and older. No differences in disrespect were seen with regard to socioeconomic status, health literacy, or English language proficiency. Those who spoke a language other than English at home, however, were more than twice as likely to get the wrong medication from a physician (34%) than were those who did not (15%), the report showed.
The survey, which had a sampling error of plus or minus 3.2 percentage points, was conducted between May 12, 2017, and June 26, 2017, and involved 2,536 respondents. It was conducted with support from Medtronic.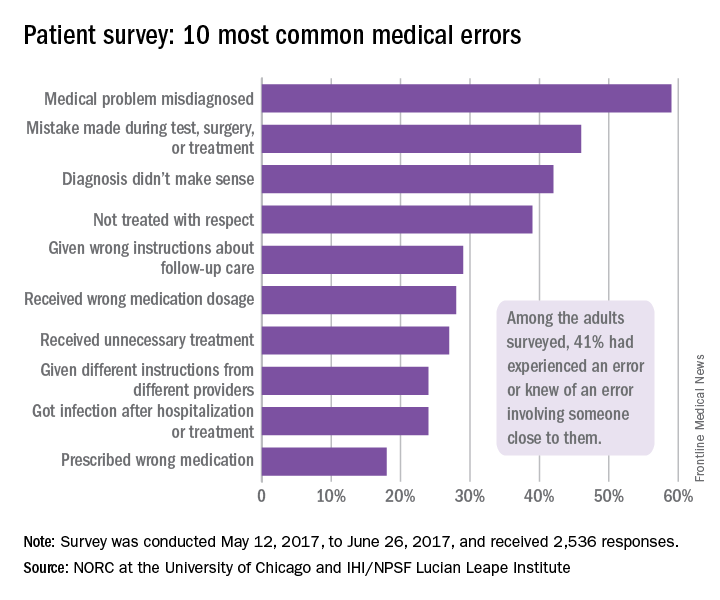
More than 40% of adults have either experienced a medical error or been involved in the care of someone who did, according to a recent national survey.
Specifically, 21% of American adults said that they have personally experienced a medical error and 31% said that they have been involved in the care of another person who experienced an error. The combined total, which includes some overlap, was 41% in the survey conducted by the Institute for Healthcare Improvement (IHI)/National Patient Safety Foundation (NPSF) and National Opinion Research Center (NORC), a nonpartisan research institution at the University of Chicago, .
Medical errors were defined for respondents as “mistakes [that] sometimes result in no harm, while other times they may result in additional or prolonged treatment, emotional distress, disability, or death,” investigators at IHI/NPSF and NORC said in their report.
A misdiagnosed medical problem was the most common type of medical error, reported by 59% of those with error experience. The next most common type of error was a mistake during a test, surgery, or treatment, which was mentioned by 46% of those with error experience, followed by a diagnosis that didn’t make sense (42%), lack of respect (39%), and incorrect instructions about follow-up care (29%), the IHI/HPSF and NORC report indicated.
Feelings of disrespect were more common among younger respondents: 46% of those aged 18-44 years said they were not treated with respect by a health care provider, compared with 34% of those aged 45 years and older. No differences in disrespect were seen with regard to socioeconomic status, health literacy, or English language proficiency. Those who spoke a language other than English at home, however, were more than twice as likely to get the wrong medication from a physician (34%) than were those who did not (15%), the report showed.
The survey, which had a sampling error of plus or minus 3.2 percentage points, was conducted between May 12, 2017, and June 26, 2017, and involved 2,536 respondents. It was conducted with support from Medtronic.
More than 40% of adults have either experienced a medical error or been involved in the care of someone who did, according to a recent national survey.
Specifically, 21% of American adults said that they have personally experienced a medical error and 31% said that they have been involved in the care of another person who experienced an error. The combined total, which includes some overlap, was 41% in the survey conducted by the Institute for Healthcare Improvement (IHI)/National Patient Safety Foundation (NPSF) and National Opinion Research Center (NORC), a nonpartisan research institution at the University of Chicago, .
Medical errors were defined for respondents as “mistakes [that] sometimes result in no harm, while other times they may result in additional or prolonged treatment, emotional distress, disability, or death,” investigators at IHI/NPSF and NORC said in their report.
A misdiagnosed medical problem was the most common type of medical error, reported by 59% of those with error experience. The next most common type of error was a mistake during a test, surgery, or treatment, which was mentioned by 46% of those with error experience, followed by a diagnosis that didn’t make sense (42%), lack of respect (39%), and incorrect instructions about follow-up care (29%), the IHI/HPSF and NORC report indicated.
Feelings of disrespect were more common among younger respondents: 46% of those aged 18-44 years said they were not treated with respect by a health care provider, compared with 34% of those aged 45 years and older. No differences in disrespect were seen with regard to socioeconomic status, health literacy, or English language proficiency. Those who spoke a language other than English at home, however, were more than twice as likely to get the wrong medication from a physician (34%) than were those who did not (15%), the report showed.
The survey, which had a sampling error of plus or minus 3.2 percentage points, was conducted between May 12, 2017, and June 26, 2017, and involved 2,536 respondents. It was conducted with support from Medtronic.
Two Senators reach deal on a health law fix
After nearly 2 months of negotiations, key senators said on Oct. 17 that they have reached a bipartisan deal on a proposal intended to stabilize the Affordable Care Act’s insurance market, which has been rocked by recent actions by President Donald Trump.
Sens. Lamar Alexander (R-Tenn.) and Patty Murray (D-Wash.), respectively the chairman and the top Democrat of the Senate Health, Education, Labor, and Pensions Committee, negotiated the emerging deal. The milestone agreement, they said, would guarantee payment of cost-sharing reduction subsidies that help some policyholders with low incomes afford their deductibles and other out-of-pocket costs for 2 years, 2018 and 2019.
President Trump announced on Oct. 12 that he would stop funding the subsidies, which also have been the subject of a long-running lawsuit.
Even if it fails to become law, the deal marks a singular achievement that has been almost completely missing in Congress for the past 8 years – a bipartisan compromise on how to make the nation’s health insurance system work.
“This is an agreement I am proud to support,” Sen. Murray said on the Senate floor, “because of the message it sends about how to get things done.”
The proposal – which will require 60 votes to pass the Senate and agreement from a still-dubious House of Representatives – also would restore $110 million in ACA outreach funding cut by the Trump administration. That funding would help guide eligible individuals to sign up for coverage on the health insurance exchanges during the open enrollment period that runs from Nov. 1 to Dec. 15.
In exchange for those provisions, urged by Democrats and state officials, Republicans would win some changes to make it easier for states to apply for waivers that would let them experiment with alternative ways to provide and subsidize health insurance. The deal also would allow the sale of less comprehensive catastrophic plans in the health exchanges. Currently, such plans can be sold only to those under age 30 years.
On the Senate floor, Sen. Alexander said, “This agreement avoids chaos. I don’t know a Democrat or a Republican who benefits from chaos.”
Senate Majority Leader Mitch McConnell (R-Ky.) reserved judgment about the deal.
Both parties still have some major disagreements when it comes to health care, Senate Minority Leader Chuck Schumer (D-N.Y.) told reporters on Oct. 17, but “I think there’s a growing consensus that in the short term we need stability in the markets. So we’ve achieved stability if this agreement becomes law.”
More than 60 senators have already participated in the meetings that led to the deal, Sen. Alexander said on the Senate floor. But the path to passage in the House is uncertain – with many conservatives vehemently opposed to anything that could be construed as helping the ACA succeed.
Rep. Mark Walker (R-N.C.), chairman of the conservative Republican Study Committee, tweeted on Oct. 17: “The GOP should focus on repealing & replacing Obamacare, not trying to save it. This bailout is unacceptable.”
Both Sen. Murray and Sen. Alexander said that they were still struggling over language to make sure that if the cost-sharing payments are resumed, insurers would not receive a windfall by keeping both those payments and the higher premiums that many states are allowing in anticipation of the payments being ended.
“We want to make sure that the cost-sharing payments go to the benefit of consumers, not the insurance companies,” Sen. Alexander said.
President Trump, who as recently as Oct. 16 called the cost-sharing subsidies “a payoff” to insurance companies, took credit for the negotiations. “If I didn’t cut the CSRs, they wouldn’t be meeting,” he said. That was not, in fact, the case. The negotiations had picked up some weeks ago after being called off earlier in September while the Senate tried for one last-ditch repeal vote.
On Oct. 13, White House Budget Director Mick Mulvaney told Politico that the president would not allow a short-term fix, calling a restoration of the cost-sharing reduction funds “corporate welfare and bailouts for the insurance companies.”
But on Oct. 17 the president hailed the deal. “We think it’s going to not only save money, but give people much better health care with a very, very much smaller premium spike,” he told reporters.
Kaiser Health News is a national health policy news service that is part of the nonpartisan Henry J. Kaiser Family Foundation.
After nearly 2 months of negotiations, key senators said on Oct. 17 that they have reached a bipartisan deal on a proposal intended to stabilize the Affordable Care Act’s insurance market, which has been rocked by recent actions by President Donald Trump.
Sens. Lamar Alexander (R-Tenn.) and Patty Murray (D-Wash.), respectively the chairman and the top Democrat of the Senate Health, Education, Labor, and Pensions Committee, negotiated the emerging deal. The milestone agreement, they said, would guarantee payment of cost-sharing reduction subsidies that help some policyholders with low incomes afford their deductibles and other out-of-pocket costs for 2 years, 2018 and 2019.
President Trump announced on Oct. 12 that he would stop funding the subsidies, which also have been the subject of a long-running lawsuit.
Even if it fails to become law, the deal marks a singular achievement that has been almost completely missing in Congress for the past 8 years – a bipartisan compromise on how to make the nation’s health insurance system work.
“This is an agreement I am proud to support,” Sen. Murray said on the Senate floor, “because of the message it sends about how to get things done.”
The proposal – which will require 60 votes to pass the Senate and agreement from a still-dubious House of Representatives – also would restore $110 million in ACA outreach funding cut by the Trump administration. That funding would help guide eligible individuals to sign up for coverage on the health insurance exchanges during the open enrollment period that runs from Nov. 1 to Dec. 15.
In exchange for those provisions, urged by Democrats and state officials, Republicans would win some changes to make it easier for states to apply for waivers that would let them experiment with alternative ways to provide and subsidize health insurance. The deal also would allow the sale of less comprehensive catastrophic plans in the health exchanges. Currently, such plans can be sold only to those under age 30 years.
On the Senate floor, Sen. Alexander said, “This agreement avoids chaos. I don’t know a Democrat or a Republican who benefits from chaos.”
Senate Majority Leader Mitch McConnell (R-Ky.) reserved judgment about the deal.
Both parties still have some major disagreements when it comes to health care, Senate Minority Leader Chuck Schumer (D-N.Y.) told reporters on Oct. 17, but “I think there’s a growing consensus that in the short term we need stability in the markets. So we’ve achieved stability if this agreement becomes law.”
More than 60 senators have already participated in the meetings that led to the deal, Sen. Alexander said on the Senate floor. But the path to passage in the House is uncertain – with many conservatives vehemently opposed to anything that could be construed as helping the ACA succeed.
Rep. Mark Walker (R-N.C.), chairman of the conservative Republican Study Committee, tweeted on Oct. 17: “The GOP should focus on repealing & replacing Obamacare, not trying to save it. This bailout is unacceptable.”
Both Sen. Murray and Sen. Alexander said that they were still struggling over language to make sure that if the cost-sharing payments are resumed, insurers would not receive a windfall by keeping both those payments and the higher premiums that many states are allowing in anticipation of the payments being ended.
“We want to make sure that the cost-sharing payments go to the benefit of consumers, not the insurance companies,” Sen. Alexander said.
President Trump, who as recently as Oct. 16 called the cost-sharing subsidies “a payoff” to insurance companies, took credit for the negotiations. “If I didn’t cut the CSRs, they wouldn’t be meeting,” he said. That was not, in fact, the case. The negotiations had picked up some weeks ago after being called off earlier in September while the Senate tried for one last-ditch repeal vote.
On Oct. 13, White House Budget Director Mick Mulvaney told Politico that the president would not allow a short-term fix, calling a restoration of the cost-sharing reduction funds “corporate welfare and bailouts for the insurance companies.”
But on Oct. 17 the president hailed the deal. “We think it’s going to not only save money, but give people much better health care with a very, very much smaller premium spike,” he told reporters.
Kaiser Health News is a national health policy news service that is part of the nonpartisan Henry J. Kaiser Family Foundation.
After nearly 2 months of negotiations, key senators said on Oct. 17 that they have reached a bipartisan deal on a proposal intended to stabilize the Affordable Care Act’s insurance market, which has been rocked by recent actions by President Donald Trump.
Sens. Lamar Alexander (R-Tenn.) and Patty Murray (D-Wash.), respectively the chairman and the top Democrat of the Senate Health, Education, Labor, and Pensions Committee, negotiated the emerging deal. The milestone agreement, they said, would guarantee payment of cost-sharing reduction subsidies that help some policyholders with low incomes afford their deductibles and other out-of-pocket costs for 2 years, 2018 and 2019.
President Trump announced on Oct. 12 that he would stop funding the subsidies, which also have been the subject of a long-running lawsuit.
Even if it fails to become law, the deal marks a singular achievement that has been almost completely missing in Congress for the past 8 years – a bipartisan compromise on how to make the nation’s health insurance system work.
“This is an agreement I am proud to support,” Sen. Murray said on the Senate floor, “because of the message it sends about how to get things done.”
The proposal – which will require 60 votes to pass the Senate and agreement from a still-dubious House of Representatives – also would restore $110 million in ACA outreach funding cut by the Trump administration. That funding would help guide eligible individuals to sign up for coverage on the health insurance exchanges during the open enrollment period that runs from Nov. 1 to Dec. 15.
In exchange for those provisions, urged by Democrats and state officials, Republicans would win some changes to make it easier for states to apply for waivers that would let them experiment with alternative ways to provide and subsidize health insurance. The deal also would allow the sale of less comprehensive catastrophic plans in the health exchanges. Currently, such plans can be sold only to those under age 30 years.
On the Senate floor, Sen. Alexander said, “This agreement avoids chaos. I don’t know a Democrat or a Republican who benefits from chaos.”
Senate Majority Leader Mitch McConnell (R-Ky.) reserved judgment about the deal.
Both parties still have some major disagreements when it comes to health care, Senate Minority Leader Chuck Schumer (D-N.Y.) told reporters on Oct. 17, but “I think there’s a growing consensus that in the short term we need stability in the markets. So we’ve achieved stability if this agreement becomes law.”
More than 60 senators have already participated in the meetings that led to the deal, Sen. Alexander said on the Senate floor. But the path to passage in the House is uncertain – with many conservatives vehemently opposed to anything that could be construed as helping the ACA succeed.
Rep. Mark Walker (R-N.C.), chairman of the conservative Republican Study Committee, tweeted on Oct. 17: “The GOP should focus on repealing & replacing Obamacare, not trying to save it. This bailout is unacceptable.”
Both Sen. Murray and Sen. Alexander said that they were still struggling over language to make sure that if the cost-sharing payments are resumed, insurers would not receive a windfall by keeping both those payments and the higher premiums that many states are allowing in anticipation of the payments being ended.
“We want to make sure that the cost-sharing payments go to the benefit of consumers, not the insurance companies,” Sen. Alexander said.
President Trump, who as recently as Oct. 16 called the cost-sharing subsidies “a payoff” to insurance companies, took credit for the negotiations. “If I didn’t cut the CSRs, they wouldn’t be meeting,” he said. That was not, in fact, the case. The negotiations had picked up some weeks ago after being called off earlier in September while the Senate tried for one last-ditch repeal vote.
On Oct. 13, White House Budget Director Mick Mulvaney told Politico that the president would not allow a short-term fix, calling a restoration of the cost-sharing reduction funds “corporate welfare and bailouts for the insurance companies.”
But on Oct. 17 the president hailed the deal. “We think it’s going to not only save money, but give people much better health care with a very, very much smaller premium spike,” he told reporters.
Kaiser Health News is a national health policy news service that is part of the nonpartisan Henry J. Kaiser Family Foundation.
CardioMEMS shows real-world success as use expands
DALLAS – Management of outpatients with advanced heart failure using an implanted pulmonary artery pressure monitor continues to show real-world efficacy and safety at least as impressive as in the pivotal trial for the device.
Data from the first waves of patients to receive the CardioMEMS implanted pulmonary artery pressure (PAP) monitor since it got Food and Drug Administration marketing approval in May 2014 also showed steady uptake of this fluid volume management strategy for patients with advanced heart failure, despite Medicare reimbursement issues in some U.S. regions, J. Thomas Heywood, MD, said at the at the annual scientific meeting of the Heart Failure Society of America. He estimated that more than 6,000 U.S. heart failure patients have now had a CardioMEMS PAP monitor implanted.

“The clinicians using CardioMEMS now have a lot more experience” than they had during the trial, he said in an interview. “They have more experience using the device, they know what treatments to use to lower PAP more effectively, and they are now convinced that patients will benefit from reducing diastolic PAP.”
Dr. Heywood estimated that tens of thousands more U.S. heart failure patients with New York Heart Association class III disease and a recent history of at least one heart failure hospitalization are eligible to receive an implanted PAP monitor, dwarfing the more than 6,000 patients who received a device so far.
The postapproval study
The newest efficacy data come from the first 300 patients enrolled in the CardioMEMS HF System Post Approval Study, a registry of patients receiving an implanted PAP monitor funded by the device’s manufacturer and scheduled to include a total of 1,200 patients. Dr. Heywood said full enrollment was on track for completion by the end of October 2017.
The first 300 patients enrolled in the postapproval study were older than the CHAMPION cohort; they averaged about 69 years of age, compared with about 62 years in CHAMPION, were more often women (38% vs. 28% in CHAMPION), and were more likely to have heart failure with preserved ejection fraction (41% vs. about 22%).

A similar pattern existed for the 6-month cumulative tally of PAP area under the curve, which showed an average rise of 42 mm Hg/day in the CHAMPION control patients, an average drop of 160 mm Hg/day in the CHAMPION patients managed using their CardioMEMS data, and a drop of 281 mm Hg/day in the 300 postapproval study patients.
“We’re now using the implanted sensor in a broader population of patients, and one wonders whether the effect will be diluted. What we see is at least as good as in the CHAMPION trial. This is just an early snapshot, but it is exciting that we see no erosion of the benefit. It’s a great indication that the correct patients are receiving it,” Dr. Raval said while presenting a poster at the meeting.
Further scrutiny of the same 300 patients showed another feature of the impact of PAP monitoring on patient outcomes: The first 90 days with the PAP monitor in place led to a greater number of tweaks in patient treatment and a steady fall in PAP. During days 91-180, PAP tended to level off, the number of medication adjustments dropped, and heart failure hospitalizations fell even more than in the first 90 days, Joanna M. Joly, MD, reported in a separate poster at the meeting.

The data showed “effective reduction” of PAP during the second half of the study despite fewer medication adjustments. How was that possible? Patients who transmit data on their PAPs undergo “modeling of their behavior” based on the feedback they receive from the device, Dr. Joly suggested. Regular measurement of their PAP and seeing how the number relates to their clinical status helps patients “understand the impact of their nonadherence to diet and their medications.” Another factor could be the growing familiarity clinicians develop over time with PAP fluctuations that individual patients display repeatedly that are usually self-correcting. Also, patients may undergo “hemodynamic remodeling” that results in improved self-correction of minor shifts in fluid volume and vascular tone, she said.
This pattern of a reduced need for interventions after the first 90 days with a PAP implant suggests that many patients managed this way may be able to transition to care largely delivered by local providers, or even play a greater role in their own self-care once their PAP and clinical state stabilizes, Dr. Joly said.
The findings imply that by the end of the first 90 days, “patients accept the device and manage themselves better. It becomes basically a behavioral device” that helps patients better optimize their diet and behavior, Dr. Raval observed.
Safety holds steady
Continued real-world use of PAP monitoring has also resulted in new safety insights. During the first 3 years when the CardioMEMS device was on the U.S. market, May 2014–May 2017, the FDA’s adverse event reporting system for devices, the Manufacturer and User Facility Device Experience (MAUDE) received reports on 177 unique adverse events in 155 patients implanted with a PAP monitor, Muthiah Vaduganathan, MD, reported at the meeting. During the same 3-year period, he estimated that at least 5,500 U.S. patients had received a CardioMEMS device, based on data Dr. Vaduganathan obtained from the manufacturer, Abbott. This works out to an adverse event rate of about 2.8%, virtually identical to the rate reported from CHAMPION, noted Dr. Vaduganathan, a cardiologist also at Brigham and Women’s.

Analysis of both the 22 deaths as well as the episodes of pulmonary artery injury or hemoptysis showed that the preponderance occurred relatively early after introduction for U.S. use, suggesting that “a learning curve may exist for the most serious complications,” he said. “Improved safety and device durability may result from careful patient selection, increased operator training, and refined technologies.”
Dr. Vaduganathan cautioned that the MAUDE database is limited by its bias toward serious adverse events, selective reporting, and lack of adjudication for the reported events. Concurrently with his report at the meeting, a written version appeared online (JAMA Cardiol. 2017 Sep 18. doi:10.1001/jamacardio.2017.3791).
“The adverse event rate was reassuringly low, well below the accepted threshold for device safety. It bodes favorably for the device,” he said in an interview.
“But with a passive surveillance system like MAUDE, adverse events are likely underreported; we see in MAUDE the most severe adverse events. There is certainly a larger spectrum of more minor events that we are not seeing, but I think these numbers accurately reflect serious events.” A full registry of every U.S. patient who receives the device, similar to what’s in place for U.S. patients who undergo transcatheter aortic valve replacement, would provide a more complete picture of the risks, Dr. Vaduganathan suggested.
He also voiced some surprise about the frequency of pulmonary artery injury, which was not as apparent in the 550 total patients enrolled in CHAMPION. Clinicians who place the PAP monitor are required to first take a training program, but the manufacturer has no mandated minimum number of placements an operator must assist on before launching a new CardioMEMS practice, Dr. Vaduganathan said. Many of the pulmonary artery injuries reported to MAUDE resulted from wire perforations that resulted from loss of wire control, he noted.
Clarifying the optimal CardioMEMS recipients
PAP monitoring for patients with advanced heart failure “is a major advance for certain patients who have historically been very challenging to manage,” especially patients with heart failure with preserved ejection fraction, which has few other treatment options. But “it’s often difficult to know when to pull the trigger” and proceed with placing a PAP monitor in an eligible patient, he said. “Greater experience will help us better understand that,” Dr. Vaduganathan predicted.
Dr. Heywood said that, in addition to the standard criteria of NYHA class III symptoms and a recent history of a heart failure hospitalization, the other clinical feature he looks for in a patient who is a possible CardioMEMS recipient is a persistently elevated systolic PAP as measured using echocardiography.
“These are patients with evidence of an ongoing hemodynamic problem despite treatment, and I need more data to do a better job of getting their PAP down.” Although the PAP that patients self-measure once they have the device in place is their diastolic PAP, measuring systolic PAP by echo is usually a good surrogate for finding patients who also have a persistently elevated diastolic PAP, he explained.
Another important selection criterion is to look for the patients who are dying from heart failure rather than with heart failure, Dr. Heywood added.
“If heart failure is the major thing wrong, then we can improve their quality of life” by guiding fluid management with regular PAP measurement, especially patients with preserved left ventricular ejection fraction who have few other treatment options right now, he said.
The CardioMEMS HF System Post Approval Study is sponsored by Abbott, which markets CardioMEMS. Dr Heywood has been a consultant to and/or has received research funding from Abbott as well as Impedimed, Medtronic, Novartis, and Otsuka. Dr. Raval has been a consultant to Abbott. Dr. Joly and Dr. Vaduganathan had no disclosures.
[email protected]
On Twitter @mitchelzoler
DALLAS – Management of outpatients with advanced heart failure using an implanted pulmonary artery pressure monitor continues to show real-world efficacy and safety at least as impressive as in the pivotal trial for the device.
Data from the first waves of patients to receive the CardioMEMS implanted pulmonary artery pressure (PAP) monitor since it got Food and Drug Administration marketing approval in May 2014 also showed steady uptake of this fluid volume management strategy for patients with advanced heart failure, despite Medicare reimbursement issues in some U.S. regions, J. Thomas Heywood, MD, said at the at the annual scientific meeting of the Heart Failure Society of America. He estimated that more than 6,000 U.S. heart failure patients have now had a CardioMEMS PAP monitor implanted.

“The clinicians using CardioMEMS now have a lot more experience” than they had during the trial, he said in an interview. “They have more experience using the device, they know what treatments to use to lower PAP more effectively, and they are now convinced that patients will benefit from reducing diastolic PAP.”
Dr. Heywood estimated that tens of thousands more U.S. heart failure patients with New York Heart Association class III disease and a recent history of at least one heart failure hospitalization are eligible to receive an implanted PAP monitor, dwarfing the more than 6,000 patients who received a device so far.
The postapproval study
The newest efficacy data come from the first 300 patients enrolled in the CardioMEMS HF System Post Approval Study, a registry of patients receiving an implanted PAP monitor funded by the device’s manufacturer and scheduled to include a total of 1,200 patients. Dr. Heywood said full enrollment was on track for completion by the end of October 2017.
The first 300 patients enrolled in the postapproval study were older than the CHAMPION cohort; they averaged about 69 years of age, compared with about 62 years in CHAMPION, were more often women (38% vs. 28% in CHAMPION), and were more likely to have heart failure with preserved ejection fraction (41% vs. about 22%).

A similar pattern existed for the 6-month cumulative tally of PAP area under the curve, which showed an average rise of 42 mm Hg/day in the CHAMPION control patients, an average drop of 160 mm Hg/day in the CHAMPION patients managed using their CardioMEMS data, and a drop of 281 mm Hg/day in the 300 postapproval study patients.
“We’re now using the implanted sensor in a broader population of patients, and one wonders whether the effect will be diluted. What we see is at least as good as in the CHAMPION trial. This is just an early snapshot, but it is exciting that we see no erosion of the benefit. It’s a great indication that the correct patients are receiving it,” Dr. Raval said while presenting a poster at the meeting.
Further scrutiny of the same 300 patients showed another feature of the impact of PAP monitoring on patient outcomes: The first 90 days with the PAP monitor in place led to a greater number of tweaks in patient treatment and a steady fall in PAP. During days 91-180, PAP tended to level off, the number of medication adjustments dropped, and heart failure hospitalizations fell even more than in the first 90 days, Joanna M. Joly, MD, reported in a separate poster at the meeting.

The data showed “effective reduction” of PAP during the second half of the study despite fewer medication adjustments. How was that possible? Patients who transmit data on their PAPs undergo “modeling of their behavior” based on the feedback they receive from the device, Dr. Joly suggested. Regular measurement of their PAP and seeing how the number relates to their clinical status helps patients “understand the impact of their nonadherence to diet and their medications.” Another factor could be the growing familiarity clinicians develop over time with PAP fluctuations that individual patients display repeatedly that are usually self-correcting. Also, patients may undergo “hemodynamic remodeling” that results in improved self-correction of minor shifts in fluid volume and vascular tone, she said.
This pattern of a reduced need for interventions after the first 90 days with a PAP implant suggests that many patients managed this way may be able to transition to care largely delivered by local providers, or even play a greater role in their own self-care once their PAP and clinical state stabilizes, Dr. Joly said.
The findings imply that by the end of the first 90 days, “patients accept the device and manage themselves better. It becomes basically a behavioral device” that helps patients better optimize their diet and behavior, Dr. Raval observed.
Safety holds steady
Continued real-world use of PAP monitoring has also resulted in new safety insights. During the first 3 years when the CardioMEMS device was on the U.S. market, May 2014–May 2017, the FDA’s adverse event reporting system for devices, the Manufacturer and User Facility Device Experience (MAUDE) received reports on 177 unique adverse events in 155 patients implanted with a PAP monitor, Muthiah Vaduganathan, MD, reported at the meeting. During the same 3-year period, he estimated that at least 5,500 U.S. patients had received a CardioMEMS device, based on data Dr. Vaduganathan obtained from the manufacturer, Abbott. This works out to an adverse event rate of about 2.8%, virtually identical to the rate reported from CHAMPION, noted Dr. Vaduganathan, a cardiologist also at Brigham and Women’s.

Analysis of both the 22 deaths as well as the episodes of pulmonary artery injury or hemoptysis showed that the preponderance occurred relatively early after introduction for U.S. use, suggesting that “a learning curve may exist for the most serious complications,” he said. “Improved safety and device durability may result from careful patient selection, increased operator training, and refined technologies.”
Dr. Vaduganathan cautioned that the MAUDE database is limited by its bias toward serious adverse events, selective reporting, and lack of adjudication for the reported events. Concurrently with his report at the meeting, a written version appeared online (JAMA Cardiol. 2017 Sep 18. doi:10.1001/jamacardio.2017.3791).
“The adverse event rate was reassuringly low, well below the accepted threshold for device safety. It bodes favorably for the device,” he said in an interview.
“But with a passive surveillance system like MAUDE, adverse events are likely underreported; we see in MAUDE the most severe adverse events. There is certainly a larger spectrum of more minor events that we are not seeing, but I think these numbers accurately reflect serious events.” A full registry of every U.S. patient who receives the device, similar to what’s in place for U.S. patients who undergo transcatheter aortic valve replacement, would provide a more complete picture of the risks, Dr. Vaduganathan suggested.
He also voiced some surprise about the frequency of pulmonary artery injury, which was not as apparent in the 550 total patients enrolled in CHAMPION. Clinicians who place the PAP monitor are required to first take a training program, but the manufacturer has no mandated minimum number of placements an operator must assist on before launching a new CardioMEMS practice, Dr. Vaduganathan said. Many of the pulmonary artery injuries reported to MAUDE resulted from wire perforations that resulted from loss of wire control, he noted.
Clarifying the optimal CardioMEMS recipients
PAP monitoring for patients with advanced heart failure “is a major advance for certain patients who have historically been very challenging to manage,” especially patients with heart failure with preserved ejection fraction, which has few other treatment options. But “it’s often difficult to know when to pull the trigger” and proceed with placing a PAP monitor in an eligible patient, he said. “Greater experience will help us better understand that,” Dr. Vaduganathan predicted.
Dr. Heywood said that, in addition to the standard criteria of NYHA class III symptoms and a recent history of a heart failure hospitalization, the other clinical feature he looks for in a patient who is a possible CardioMEMS recipient is a persistently elevated systolic PAP as measured using echocardiography.
“These are patients with evidence of an ongoing hemodynamic problem despite treatment, and I need more data to do a better job of getting their PAP down.” Although the PAP that patients self-measure once they have the device in place is their diastolic PAP, measuring systolic PAP by echo is usually a good surrogate for finding patients who also have a persistently elevated diastolic PAP, he explained.
Another important selection criterion is to look for the patients who are dying from heart failure rather than with heart failure, Dr. Heywood added.
“If heart failure is the major thing wrong, then we can improve their quality of life” by guiding fluid management with regular PAP measurement, especially patients with preserved left ventricular ejection fraction who have few other treatment options right now, he said.
The CardioMEMS HF System Post Approval Study is sponsored by Abbott, which markets CardioMEMS. Dr Heywood has been a consultant to and/or has received research funding from Abbott as well as Impedimed, Medtronic, Novartis, and Otsuka. Dr. Raval has been a consultant to Abbott. Dr. Joly and Dr. Vaduganathan had no disclosures.
[email protected]
On Twitter @mitchelzoler
DALLAS – Management of outpatients with advanced heart failure using an implanted pulmonary artery pressure monitor continues to show real-world efficacy and safety at least as impressive as in the pivotal trial for the device.
Data from the first waves of patients to receive the CardioMEMS implanted pulmonary artery pressure (PAP) monitor since it got Food and Drug Administration marketing approval in May 2014 also showed steady uptake of this fluid volume management strategy for patients with advanced heart failure, despite Medicare reimbursement issues in some U.S. regions, J. Thomas Heywood, MD, said at the at the annual scientific meeting of the Heart Failure Society of America. He estimated that more than 6,000 U.S. heart failure patients have now had a CardioMEMS PAP monitor implanted.

“The clinicians using CardioMEMS now have a lot more experience” than they had during the trial, he said in an interview. “They have more experience using the device, they know what treatments to use to lower PAP more effectively, and they are now convinced that patients will benefit from reducing diastolic PAP.”
Dr. Heywood estimated that tens of thousands more U.S. heart failure patients with New York Heart Association class III disease and a recent history of at least one heart failure hospitalization are eligible to receive an implanted PAP monitor, dwarfing the more than 6,000 patients who received a device so far.
The postapproval study
The newest efficacy data come from the first 300 patients enrolled in the CardioMEMS HF System Post Approval Study, a registry of patients receiving an implanted PAP monitor funded by the device’s manufacturer and scheduled to include a total of 1,200 patients. Dr. Heywood said full enrollment was on track for completion by the end of October 2017.
The first 300 patients enrolled in the postapproval study were older than the CHAMPION cohort; they averaged about 69 years of age, compared with about 62 years in CHAMPION, were more often women (38% vs. 28% in CHAMPION), and were more likely to have heart failure with preserved ejection fraction (41% vs. about 22%).

A similar pattern existed for the 6-month cumulative tally of PAP area under the curve, which showed an average rise of 42 mm Hg/day in the CHAMPION control patients, an average drop of 160 mm Hg/day in the CHAMPION patients managed using their CardioMEMS data, and a drop of 281 mm Hg/day in the 300 postapproval study patients.
“We’re now using the implanted sensor in a broader population of patients, and one wonders whether the effect will be diluted. What we see is at least as good as in the CHAMPION trial. This is just an early snapshot, but it is exciting that we see no erosion of the benefit. It’s a great indication that the correct patients are receiving it,” Dr. Raval said while presenting a poster at the meeting.
Further scrutiny of the same 300 patients showed another feature of the impact of PAP monitoring on patient outcomes: The first 90 days with the PAP monitor in place led to a greater number of tweaks in patient treatment and a steady fall in PAP. During days 91-180, PAP tended to level off, the number of medication adjustments dropped, and heart failure hospitalizations fell even more than in the first 90 days, Joanna M. Joly, MD, reported in a separate poster at the meeting.

The data showed “effective reduction” of PAP during the second half of the study despite fewer medication adjustments. How was that possible? Patients who transmit data on their PAPs undergo “modeling of their behavior” based on the feedback they receive from the device, Dr. Joly suggested. Regular measurement of their PAP and seeing how the number relates to their clinical status helps patients “understand the impact of their nonadherence to diet and their medications.” Another factor could be the growing familiarity clinicians develop over time with PAP fluctuations that individual patients display repeatedly that are usually self-correcting. Also, patients may undergo “hemodynamic remodeling” that results in improved self-correction of minor shifts in fluid volume and vascular tone, she said.
This pattern of a reduced need for interventions after the first 90 days with a PAP implant suggests that many patients managed this way may be able to transition to care largely delivered by local providers, or even play a greater role in their own self-care once their PAP and clinical state stabilizes, Dr. Joly said.
The findings imply that by the end of the first 90 days, “patients accept the device and manage themselves better. It becomes basically a behavioral device” that helps patients better optimize their diet and behavior, Dr. Raval observed.
Safety holds steady
Continued real-world use of PAP monitoring has also resulted in new safety insights. During the first 3 years when the CardioMEMS device was on the U.S. market, May 2014–May 2017, the FDA’s adverse event reporting system for devices, the Manufacturer and User Facility Device Experience (MAUDE) received reports on 177 unique adverse events in 155 patients implanted with a PAP monitor, Muthiah Vaduganathan, MD, reported at the meeting. During the same 3-year period, he estimated that at least 5,500 U.S. patients had received a CardioMEMS device, based on data Dr. Vaduganathan obtained from the manufacturer, Abbott. This works out to an adverse event rate of about 2.8%, virtually identical to the rate reported from CHAMPION, noted Dr. Vaduganathan, a cardiologist also at Brigham and Women’s.

Analysis of both the 22 deaths as well as the episodes of pulmonary artery injury or hemoptysis showed that the preponderance occurred relatively early after introduction for U.S. use, suggesting that “a learning curve may exist for the most serious complications,” he said. “Improved safety and device durability may result from careful patient selection, increased operator training, and refined technologies.”
Dr. Vaduganathan cautioned that the MAUDE database is limited by its bias toward serious adverse events, selective reporting, and lack of adjudication for the reported events. Concurrently with his report at the meeting, a written version appeared online (JAMA Cardiol. 2017 Sep 18. doi:10.1001/jamacardio.2017.3791).
“The adverse event rate was reassuringly low, well below the accepted threshold for device safety. It bodes favorably for the device,” he said in an interview.
“But with a passive surveillance system like MAUDE, adverse events are likely underreported; we see in MAUDE the most severe adverse events. There is certainly a larger spectrum of more minor events that we are not seeing, but I think these numbers accurately reflect serious events.” A full registry of every U.S. patient who receives the device, similar to what’s in place for U.S. patients who undergo transcatheter aortic valve replacement, would provide a more complete picture of the risks, Dr. Vaduganathan suggested.
He also voiced some surprise about the frequency of pulmonary artery injury, which was not as apparent in the 550 total patients enrolled in CHAMPION. Clinicians who place the PAP monitor are required to first take a training program, but the manufacturer has no mandated minimum number of placements an operator must assist on before launching a new CardioMEMS practice, Dr. Vaduganathan said. Many of the pulmonary artery injuries reported to MAUDE resulted from wire perforations that resulted from loss of wire control, he noted.
Clarifying the optimal CardioMEMS recipients
PAP monitoring for patients with advanced heart failure “is a major advance for certain patients who have historically been very challenging to manage,” especially patients with heart failure with preserved ejection fraction, which has few other treatment options. But “it’s often difficult to know when to pull the trigger” and proceed with placing a PAP monitor in an eligible patient, he said. “Greater experience will help us better understand that,” Dr. Vaduganathan predicted.
Dr. Heywood said that, in addition to the standard criteria of NYHA class III symptoms and a recent history of a heart failure hospitalization, the other clinical feature he looks for in a patient who is a possible CardioMEMS recipient is a persistently elevated systolic PAP as measured using echocardiography.
“These are patients with evidence of an ongoing hemodynamic problem despite treatment, and I need more data to do a better job of getting their PAP down.” Although the PAP that patients self-measure once they have the device in place is their diastolic PAP, measuring systolic PAP by echo is usually a good surrogate for finding patients who also have a persistently elevated diastolic PAP, he explained.
Another important selection criterion is to look for the patients who are dying from heart failure rather than with heart failure, Dr. Heywood added.
“If heart failure is the major thing wrong, then we can improve their quality of life” by guiding fluid management with regular PAP measurement, especially patients with preserved left ventricular ejection fraction who have few other treatment options right now, he said.
The CardioMEMS HF System Post Approval Study is sponsored by Abbott, which markets CardioMEMS. Dr Heywood has been a consultant to and/or has received research funding from Abbott as well as Impedimed, Medtronic, Novartis, and Otsuka. Dr. Raval has been a consultant to Abbott. Dr. Joly and Dr. Vaduganathan had no disclosures.
[email protected]
On Twitter @mitchelzoler
AT THE HFSA ANNUAL SCIENTIFIC MEETING
MI, stroke risk from HFrEF surpasses HFpEF
DALLAS – Patients newly diagnosed with heart failure with reduced ejection fraction had about an 8% incidence of MIs during the subsequent 9 months, and a 5% incidence of ischemic strokes in a retrospective review of more than 1,600 community-dwelling U.S. patients.
The MI and ischemic stroke incidence rates in heart failure patients with reduced ejection fraction (HFrEF) were both significantly higher than in more than 4,000 patients with heart failure with preserved ejection fraction (HFpEF), Gregg C. Fonarow, MD, said while presenting a poster at the annual scientific meeting of the Heart Failure Society of America.
The findings suggest that greater attention is needed to reduce the risks for MI and stroke in HFrEF patients, suggested Dr. Fonarow, professor and cochief of cardiology at the University of California, Los Angeles, and his associates in their poster.
The study used claims data collected during July 2009-September 2016 from more than 10 million people enrolled in the United Health Group, who received care at more than 650 hospitals and about 6,600 clinics. The study included all patients diagnosed with heart failure during a hospital or emergency room visit and who had no history of a heart failure diagnosis or episode during the preceding 18 months, a left ventricular ejection fraction measurement made close to the time of the index encounter, and no stroke or MI apparent at the time of the index event. The study included 1,622 patients with HFrEF, defined as a left ventricular ejection fraction of less than 40%, 4,288 with HFpEF, defined as an ejection fraction of 50% or more, and 1,095 with heart failure with a borderline ejection fraction of 40%-49%.
The HFrEF patients had an average ejection fraction of 28%, they averaged 72 years old, 36% were women, and 8% had a prior stroke. The HFpEF patients averaged 74 years old, their average ejection fraction was 61%, 55% were women, and 11% had a prior stroke. Follow-up data on all patients were available for an average of nearly 9 months following their index heart failure event, with some patients followed as long as 1 year.
During follow-up, the incidence of ischemic stroke was 5.4% in the HFrEF patients and 3.9% in those with HFpEF, a difference that worked out to a statistically significant 40% higher ischemic stroke rate in HFrEF patients after adjustment for baseline differences between the two patient groups, Dr. Fonarow reported. The patients with a borderline ejection fraction had a 3.7% stroke incidence that fell short of a significant difference, compared with the HFrEF patient.The rate of new MIs during follow-up was 7.5% in the HFrEF patients and 3.2% in the HFpEF patients, a statistically significant 2.5-fold relatively higher MI rate with HFrEF, a statistically significant difference after adjustments. The MI incidence in patients with a borderline ejection fraction was 5.9%
[email protected]
On Twitter @mitchelzoler
DALLAS – Patients newly diagnosed with heart failure with reduced ejection fraction had about an 8% incidence of MIs during the subsequent 9 months, and a 5% incidence of ischemic strokes in a retrospective review of more than 1,600 community-dwelling U.S. patients.
The MI and ischemic stroke incidence rates in heart failure patients with reduced ejection fraction (HFrEF) were both significantly higher than in more than 4,000 patients with heart failure with preserved ejection fraction (HFpEF), Gregg C. Fonarow, MD, said while presenting a poster at the annual scientific meeting of the Heart Failure Society of America.
The findings suggest that greater attention is needed to reduce the risks for MI and stroke in HFrEF patients, suggested Dr. Fonarow, professor and cochief of cardiology at the University of California, Los Angeles, and his associates in their poster.
The study used claims data collected during July 2009-September 2016 from more than 10 million people enrolled in the United Health Group, who received care at more than 650 hospitals and about 6,600 clinics. The study included all patients diagnosed with heart failure during a hospital or emergency room visit and who had no history of a heart failure diagnosis or episode during the preceding 18 months, a left ventricular ejection fraction measurement made close to the time of the index encounter, and no stroke or MI apparent at the time of the index event. The study included 1,622 patients with HFrEF, defined as a left ventricular ejection fraction of less than 40%, 4,288 with HFpEF, defined as an ejection fraction of 50% or more, and 1,095 with heart failure with a borderline ejection fraction of 40%-49%.
The HFrEF patients had an average ejection fraction of 28%, they averaged 72 years old, 36% were women, and 8% had a prior stroke. The HFpEF patients averaged 74 years old, their average ejection fraction was 61%, 55% were women, and 11% had a prior stroke. Follow-up data on all patients were available for an average of nearly 9 months following their index heart failure event, with some patients followed as long as 1 year.
During follow-up, the incidence of ischemic stroke was 5.4% in the HFrEF patients and 3.9% in those with HFpEF, a difference that worked out to a statistically significant 40% higher ischemic stroke rate in HFrEF patients after adjustment for baseline differences between the two patient groups, Dr. Fonarow reported. The patients with a borderline ejection fraction had a 3.7% stroke incidence that fell short of a significant difference, compared with the HFrEF patient.The rate of new MIs during follow-up was 7.5% in the HFrEF patients and 3.2% in the HFpEF patients, a statistically significant 2.5-fold relatively higher MI rate with HFrEF, a statistically significant difference after adjustments. The MI incidence in patients with a borderline ejection fraction was 5.9%
[email protected]
On Twitter @mitchelzoler
DALLAS – Patients newly diagnosed with heart failure with reduced ejection fraction had about an 8% incidence of MIs during the subsequent 9 months, and a 5% incidence of ischemic strokes in a retrospective review of more than 1,600 community-dwelling U.S. patients.
The MI and ischemic stroke incidence rates in heart failure patients with reduced ejection fraction (HFrEF) were both significantly higher than in more than 4,000 patients with heart failure with preserved ejection fraction (HFpEF), Gregg C. Fonarow, MD, said while presenting a poster at the annual scientific meeting of the Heart Failure Society of America.
The findings suggest that greater attention is needed to reduce the risks for MI and stroke in HFrEF patients, suggested Dr. Fonarow, professor and cochief of cardiology at the University of California, Los Angeles, and his associates in their poster.
The study used claims data collected during July 2009-September 2016 from more than 10 million people enrolled in the United Health Group, who received care at more than 650 hospitals and about 6,600 clinics. The study included all patients diagnosed with heart failure during a hospital or emergency room visit and who had no history of a heart failure diagnosis or episode during the preceding 18 months, a left ventricular ejection fraction measurement made close to the time of the index encounter, and no stroke or MI apparent at the time of the index event. The study included 1,622 patients with HFrEF, defined as a left ventricular ejection fraction of less than 40%, 4,288 with HFpEF, defined as an ejection fraction of 50% or more, and 1,095 with heart failure with a borderline ejection fraction of 40%-49%.
The HFrEF patients had an average ejection fraction of 28%, they averaged 72 years old, 36% were women, and 8% had a prior stroke. The HFpEF patients averaged 74 years old, their average ejection fraction was 61%, 55% were women, and 11% had a prior stroke. Follow-up data on all patients were available for an average of nearly 9 months following their index heart failure event, with some patients followed as long as 1 year.
During follow-up, the incidence of ischemic stroke was 5.4% in the HFrEF patients and 3.9% in those with HFpEF, a difference that worked out to a statistically significant 40% higher ischemic stroke rate in HFrEF patients after adjustment for baseline differences between the two patient groups, Dr. Fonarow reported. The patients with a borderline ejection fraction had a 3.7% stroke incidence that fell short of a significant difference, compared with the HFrEF patient.The rate of new MIs during follow-up was 7.5% in the HFrEF patients and 3.2% in the HFpEF patients, a statistically significant 2.5-fold relatively higher MI rate with HFrEF, a statistically significant difference after adjustments. The MI incidence in patients with a borderline ejection fraction was 5.9%
[email protected]
On Twitter @mitchelzoler
AT THE HFSA ANNUAL SCIENTIFIC MEETING
Key clinical point:
Major finding: HFrEF patients had a 40% higher incidence of stroke and a 2.5-fold higher incidence of MI, compared with HFpEF patients.
Data source: Retrospective review of 7,005 U.S. patients newly diagnosed with heart failure.
Disclosures: The study was funded by Janssen. Dr. Fonarow had no relevant disclosures.
Ideal intubation position still unknown
In critically ill adults undergoing endotracheal intubation, the ramped position does not significantly improve oxygenation compared with the sniffing position, according to results of a multicenter, randomized trial of 260 patients treated in an intensive care unit.
Moreover, “[ramped] position appeared to worsen glottic view and increase the number of attempts required for successful intubation,” wrote Matthew W. Semler, MD, of Vanderbilt University Medical Center, Nashville, Tenn., and his coauthors (Chest. 2017 Oct. doi: 10.1016/j.chest.2017.03.061).
The ramped and sniffing positions are the two most common patient positions used during emergent intubation, according to investigators. The sniffing position is characterized by supine torso, neck flexed forward, and head extended, while ramped position involves elevating the torso and head.
Some believe the ramped position may offer superior anatomic alignment of the upper airway; however, only a few observational studies suggest it is associated with fewer complications than the sniffing position, the authors wrote.
Accordingly, they conducted a multicenter randomized trial with a primary endpoint of lowest arterial oxygen saturation, hypothesizing that the endpoint would be higher for the ramped position: “Our primary outcome of lowest arterial oxygen saturation is an established endpoint in ICU intubation trials, and is linked to periprocedural cardiac arrest and death,” they wrote.
The investigators instead found that median lowest arterial oxygen saturation was not statistically different between groups, at 93% for the ramped position, and 92% for the sniffing position (P = 0.27), published data show.
Further results showed that the ramped position appeared to be associated with poor glottic view and more difficult intubation. The incidence of grade III (only epiglottis) or grade IV (no visible glottis structures) views were 25.4% for ramped vs. 11.5% for sniffing (P = .01), while the rate of first-attempt intubation was 76.2% for ramped vs 85.4% for sniffing (P = .02).
While the findings are compelling, the authors were forthcoming about the potential limitations of the study and differences compared with earlier investigations. Notably, they said, all prior controlled trials of patient positioning during endotracheal intubation were conducted in the operating room, rather than in the ICU.
Also, the operators’ skill levels may further explain differences in this study’s outcomes from those of similar studies, the researchers noted. Earlier studies included patients intubated by one or two senior anesthesiologists from one center, while this trial involved 30 operators across multiple centers, with the average operator having performed 60 previous intubations. “Thus, our findings may generalize to settings in which airway management is performed by trainees, but whether results would be similar among expert operators remains unknown,” the investigators noted.
The authors reported no potential conflicts of interest. One coauthor reported serving on an advisory board for Avisa Pharma.
Editorialists praised the multicenter, randomized design of this study, and its total recruitment of 260 patients. They also noted several limitations of the study that “could shed some light” on the group’s conclusions (Chest. 2017 Oct. doi: 10.1016/j.chest.2017.06.002).
“The results diverge from [operating room] literature of the past 15 years that suggest that the ramped position is the preferred intubation position for obese patients or those with an anticipated difficult airway.” This may have been caused by shortcomings of this study’s design and differences between it and other research exploring the topic of patient positioning during endotracheal intubation, they wrote.
The study lacked a prespecified algorithm for preoxygenation and the operators had relatively low amounts of experience with intubations. Finally, the beds used in this study could contribute to the divergences between this intensive care unit experience and the operating room literature. The operating room table is narrower, firmer, and more stable, while by contrast, the ICU bed is wider and softer, they noted. This “may make initial positioning, maintenance of positioning, and accessing the patient’s head more difficult.”
Nevertheless, “[this] important study provides ideas for further study of optimal positioning in the ICU and adds valuable data to the sparse literature on the subject in the ICU setting,” they concluded.
James Aaron Scott, DO, Jens Matthias Walz, MD, FCCP, and Stephen O. Heard, MD, FCCP, are in the department of anesthesiology and perioperative medicine, UMass Memorial Medical Center, Worcester, Mass. The authors reported no conflicts of interest. These comments are based on their editorial.
Editorialists praised the multicenter, randomized design of this study, and its total recruitment of 260 patients. They also noted several limitations of the study that “could shed some light” on the group’s conclusions (Chest. 2017 Oct. doi: 10.1016/j.chest.2017.06.002).
“The results diverge from [operating room] literature of the past 15 years that suggest that the ramped position is the preferred intubation position for obese patients or those with an anticipated difficult airway.” This may have been caused by shortcomings of this study’s design and differences between it and other research exploring the topic of patient positioning during endotracheal intubation, they wrote.
The study lacked a prespecified algorithm for preoxygenation and the operators had relatively low amounts of experience with intubations. Finally, the beds used in this study could contribute to the divergences between this intensive care unit experience and the operating room literature. The operating room table is narrower, firmer, and more stable, while by contrast, the ICU bed is wider and softer, they noted. This “may make initial positioning, maintenance of positioning, and accessing the patient’s head more difficult.”
Nevertheless, “[this] important study provides ideas for further study of optimal positioning in the ICU and adds valuable data to the sparse literature on the subject in the ICU setting,” they concluded.
James Aaron Scott, DO, Jens Matthias Walz, MD, FCCP, and Stephen O. Heard, MD, FCCP, are in the department of anesthesiology and perioperative medicine, UMass Memorial Medical Center, Worcester, Mass. The authors reported no conflicts of interest. These comments are based on their editorial.
Editorialists praised the multicenter, randomized design of this study, and its total recruitment of 260 patients. They also noted several limitations of the study that “could shed some light” on the group’s conclusions (Chest. 2017 Oct. doi: 10.1016/j.chest.2017.06.002).
“The results diverge from [operating room] literature of the past 15 years that suggest that the ramped position is the preferred intubation position for obese patients or those with an anticipated difficult airway.” This may have been caused by shortcomings of this study’s design and differences between it and other research exploring the topic of patient positioning during endotracheal intubation, they wrote.
The study lacked a prespecified algorithm for preoxygenation and the operators had relatively low amounts of experience with intubations. Finally, the beds used in this study could contribute to the divergences between this intensive care unit experience and the operating room literature. The operating room table is narrower, firmer, and more stable, while by contrast, the ICU bed is wider and softer, they noted. This “may make initial positioning, maintenance of positioning, and accessing the patient’s head more difficult.”
Nevertheless, “[this] important study provides ideas for further study of optimal positioning in the ICU and adds valuable data to the sparse literature on the subject in the ICU setting,” they concluded.
James Aaron Scott, DO, Jens Matthias Walz, MD, FCCP, and Stephen O. Heard, MD, FCCP, are in the department of anesthesiology and perioperative medicine, UMass Memorial Medical Center, Worcester, Mass. The authors reported no conflicts of interest. These comments are based on their editorial.
In critically ill adults undergoing endotracheal intubation, the ramped position does not significantly improve oxygenation compared with the sniffing position, according to results of a multicenter, randomized trial of 260 patients treated in an intensive care unit.
Moreover, “[ramped] position appeared to worsen glottic view and increase the number of attempts required for successful intubation,” wrote Matthew W. Semler, MD, of Vanderbilt University Medical Center, Nashville, Tenn., and his coauthors (Chest. 2017 Oct. doi: 10.1016/j.chest.2017.03.061).
The ramped and sniffing positions are the two most common patient positions used during emergent intubation, according to investigators. The sniffing position is characterized by supine torso, neck flexed forward, and head extended, while ramped position involves elevating the torso and head.
Some believe the ramped position may offer superior anatomic alignment of the upper airway; however, only a few observational studies suggest it is associated with fewer complications than the sniffing position, the authors wrote.
Accordingly, they conducted a multicenter randomized trial with a primary endpoint of lowest arterial oxygen saturation, hypothesizing that the endpoint would be higher for the ramped position: “Our primary outcome of lowest arterial oxygen saturation is an established endpoint in ICU intubation trials, and is linked to periprocedural cardiac arrest and death,” they wrote.
The investigators instead found that median lowest arterial oxygen saturation was not statistically different between groups, at 93% for the ramped position, and 92% for the sniffing position (P = 0.27), published data show.
Further results showed that the ramped position appeared to be associated with poor glottic view and more difficult intubation. The incidence of grade III (only epiglottis) or grade IV (no visible glottis structures) views were 25.4% for ramped vs. 11.5% for sniffing (P = .01), while the rate of first-attempt intubation was 76.2% for ramped vs 85.4% for sniffing (P = .02).
While the findings are compelling, the authors were forthcoming about the potential limitations of the study and differences compared with earlier investigations. Notably, they said, all prior controlled trials of patient positioning during endotracheal intubation were conducted in the operating room, rather than in the ICU.
Also, the operators’ skill levels may further explain differences in this study’s outcomes from those of similar studies, the researchers noted. Earlier studies included patients intubated by one or two senior anesthesiologists from one center, while this trial involved 30 operators across multiple centers, with the average operator having performed 60 previous intubations. “Thus, our findings may generalize to settings in which airway management is performed by trainees, but whether results would be similar among expert operators remains unknown,” the investigators noted.
The authors reported no potential conflicts of interest. One coauthor reported serving on an advisory board for Avisa Pharma.
In critically ill adults undergoing endotracheal intubation, the ramped position does not significantly improve oxygenation compared with the sniffing position, according to results of a multicenter, randomized trial of 260 patients treated in an intensive care unit.
Moreover, “[ramped] position appeared to worsen glottic view and increase the number of attempts required for successful intubation,” wrote Matthew W. Semler, MD, of Vanderbilt University Medical Center, Nashville, Tenn., and his coauthors (Chest. 2017 Oct. doi: 10.1016/j.chest.2017.03.061).
The ramped and sniffing positions are the two most common patient positions used during emergent intubation, according to investigators. The sniffing position is characterized by supine torso, neck flexed forward, and head extended, while ramped position involves elevating the torso and head.
Some believe the ramped position may offer superior anatomic alignment of the upper airway; however, only a few observational studies suggest it is associated with fewer complications than the sniffing position, the authors wrote.
Accordingly, they conducted a multicenter randomized trial with a primary endpoint of lowest arterial oxygen saturation, hypothesizing that the endpoint would be higher for the ramped position: “Our primary outcome of lowest arterial oxygen saturation is an established endpoint in ICU intubation trials, and is linked to periprocedural cardiac arrest and death,” they wrote.
The investigators instead found that median lowest arterial oxygen saturation was not statistically different between groups, at 93% for the ramped position, and 92% for the sniffing position (P = 0.27), published data show.
Further results showed that the ramped position appeared to be associated with poor glottic view and more difficult intubation. The incidence of grade III (only epiglottis) or grade IV (no visible glottis structures) views were 25.4% for ramped vs. 11.5% for sniffing (P = .01), while the rate of first-attempt intubation was 76.2% for ramped vs 85.4% for sniffing (P = .02).
While the findings are compelling, the authors were forthcoming about the potential limitations of the study and differences compared with earlier investigations. Notably, they said, all prior controlled trials of patient positioning during endotracheal intubation were conducted in the operating room, rather than in the ICU.
Also, the operators’ skill levels may further explain differences in this study’s outcomes from those of similar studies, the researchers noted. Earlier studies included patients intubated by one or two senior anesthesiologists from one center, while this trial involved 30 operators across multiple centers, with the average operator having performed 60 previous intubations. “Thus, our findings may generalize to settings in which airway management is performed by trainees, but whether results would be similar among expert operators remains unknown,” the investigators noted.
The authors reported no potential conflicts of interest. One coauthor reported serving on an advisory board for Avisa Pharma.
FROM CHEST
Key clinical point: During endotracheal intubation of critically ill adults, use of the ramped position did not significantly improve oxygenation compared with the sniffing position, and it increased the number of attempts needed to achieve successful intubation.
Major finding: The median lowest arterial oxygen saturation was 93% for the ramped position and 92% for the sniffing position (P = .27).
Data source: Multicenter, randomized trial of 260 critically ill adults undergoing endotracheal intubation.
Disclosures: The authors reported no potential conflicts of interest. One coauthor reported serving on an advisory board for Avisa Pharma.
Higher stroke risk found for TAVR versus SAVR
SAN DIEGO – There was an 86% greater risk of ischemic stroke after transcatheter aortic valve replacement, compared with surgical aortic valve replacement, and a more than sixfold increased risk of hemorrhagic stroke, in a review of more than 44,000 patients in the Nationwide Readmissions Database who were followed out to a year after having one procedure or the other.
“Our data suggest an elevated risk of both ischemic and hemorrhagic stroke after TAVR [transcatheter aortic valve replacement]. I see a lot of people that have strokes after” TAVR, so “I wasn’t all that surprised that there is an increased risk, but could I have guessed it would have been so high? No.” Perhaps “we are offering it to people we shouldn’t be offering it to,” said lead investigator Laura Stein, MD, a vascular neurology fellow at Mount Sinai Hospital, New York.
The 2013 Nationwide Readmissions Database used in the study captured more than 14 million readmissions in the United States across all payers and the uninsured. “We used this database because its captures all comers” and reflects “more real-world practice,” Dr. Stein said.
There were 6,015 TAVR and 38,624 SAVR cases in 2013, and the team found consistently elevated cumulative risks of ischemic and hemorrhagic stroke after TAVR, compared with SAVR, according to a presentation at the annual meeting of the American Neurological Association.
Compared with SAVR, the hazard ratio for ischemic stroke with TAVR was 1.86 (95% confidence interval, 1.12-3.08; P = .016) and, for hemorrhagic stroke, 6.17 (95% CI, 1.97-19.33; P = .0018). Dr. Stein declined to release absolute numbers of strokes in the two groups, pending publication.
“A lot of attention is being paid to this topic because there has been a push, a lot of it by the device makers, to prove that [TAVR] outcomes are just as good as with traditional surgery, and that we should be offering [TAVR] to more people with higher risk factor profiles who might not have been offered repair otherwise. Our job is to help patients make the most informed decisions. Having another source of data like [ours]” adds to the conversation about risks and benefits, she said.
The investigators adjusted for a large number of potential confounders to make sure the comparison was as fair as possible given the limits of database reviews. Among other variables, they controlled for baseline cardiovascular risk factors, carotid artery disease, heart failure, obesity, smoking, surgical complications, mortality, and illness severity scores, as well as hospital size, teaching hospital status, and urban versus rural location.
“What we can’t know is what medications these patients were on that might have increased their bleeding or ischemia risk. Also, we were relying on coding done by other people,” Dr. Stein said.
The next step is to look at the impact of stenting and other concomitant procedures. “We were surprised by the number of people that had multiple procedures at the same time.” The ultimate goal is to develop a risk score to help patients and doctors decide between the two procedures, she said.
Meanwhile, the team found no difference in stroke risk between coronary artery bypass grafting and percutaneous coronary interventions in the 2013 database.
Three was no industry funding for the work, and Dr. Stein did not have any relevant disclosures.
SAN DIEGO – There was an 86% greater risk of ischemic stroke after transcatheter aortic valve replacement, compared with surgical aortic valve replacement, and a more than sixfold increased risk of hemorrhagic stroke, in a review of more than 44,000 patients in the Nationwide Readmissions Database who were followed out to a year after having one procedure or the other.
“Our data suggest an elevated risk of both ischemic and hemorrhagic stroke after TAVR [transcatheter aortic valve replacement]. I see a lot of people that have strokes after” TAVR, so “I wasn’t all that surprised that there is an increased risk, but could I have guessed it would have been so high? No.” Perhaps “we are offering it to people we shouldn’t be offering it to,” said lead investigator Laura Stein, MD, a vascular neurology fellow at Mount Sinai Hospital, New York.
The 2013 Nationwide Readmissions Database used in the study captured more than 14 million readmissions in the United States across all payers and the uninsured. “We used this database because its captures all comers” and reflects “more real-world practice,” Dr. Stein said.
There were 6,015 TAVR and 38,624 SAVR cases in 2013, and the team found consistently elevated cumulative risks of ischemic and hemorrhagic stroke after TAVR, compared with SAVR, according to a presentation at the annual meeting of the American Neurological Association.
Compared with SAVR, the hazard ratio for ischemic stroke with TAVR was 1.86 (95% confidence interval, 1.12-3.08; P = .016) and, for hemorrhagic stroke, 6.17 (95% CI, 1.97-19.33; P = .0018). Dr. Stein declined to release absolute numbers of strokes in the two groups, pending publication.
“A lot of attention is being paid to this topic because there has been a push, a lot of it by the device makers, to prove that [TAVR] outcomes are just as good as with traditional surgery, and that we should be offering [TAVR] to more people with higher risk factor profiles who might not have been offered repair otherwise. Our job is to help patients make the most informed decisions. Having another source of data like [ours]” adds to the conversation about risks and benefits, she said.
The investigators adjusted for a large number of potential confounders to make sure the comparison was as fair as possible given the limits of database reviews. Among other variables, they controlled for baseline cardiovascular risk factors, carotid artery disease, heart failure, obesity, smoking, surgical complications, mortality, and illness severity scores, as well as hospital size, teaching hospital status, and urban versus rural location.
“What we can’t know is what medications these patients were on that might have increased their bleeding or ischemia risk. Also, we were relying on coding done by other people,” Dr. Stein said.
The next step is to look at the impact of stenting and other concomitant procedures. “We were surprised by the number of people that had multiple procedures at the same time.” The ultimate goal is to develop a risk score to help patients and doctors decide between the two procedures, she said.
Meanwhile, the team found no difference in stroke risk between coronary artery bypass grafting and percutaneous coronary interventions in the 2013 database.
Three was no industry funding for the work, and Dr. Stein did not have any relevant disclosures.
SAN DIEGO – There was an 86% greater risk of ischemic stroke after transcatheter aortic valve replacement, compared with surgical aortic valve replacement, and a more than sixfold increased risk of hemorrhagic stroke, in a review of more than 44,000 patients in the Nationwide Readmissions Database who were followed out to a year after having one procedure or the other.
“Our data suggest an elevated risk of both ischemic and hemorrhagic stroke after TAVR [transcatheter aortic valve replacement]. I see a lot of people that have strokes after” TAVR, so “I wasn’t all that surprised that there is an increased risk, but could I have guessed it would have been so high? No.” Perhaps “we are offering it to people we shouldn’t be offering it to,” said lead investigator Laura Stein, MD, a vascular neurology fellow at Mount Sinai Hospital, New York.
The 2013 Nationwide Readmissions Database used in the study captured more than 14 million readmissions in the United States across all payers and the uninsured. “We used this database because its captures all comers” and reflects “more real-world practice,” Dr. Stein said.
There were 6,015 TAVR and 38,624 SAVR cases in 2013, and the team found consistently elevated cumulative risks of ischemic and hemorrhagic stroke after TAVR, compared with SAVR, according to a presentation at the annual meeting of the American Neurological Association.
Compared with SAVR, the hazard ratio for ischemic stroke with TAVR was 1.86 (95% confidence interval, 1.12-3.08; P = .016) and, for hemorrhagic stroke, 6.17 (95% CI, 1.97-19.33; P = .0018). Dr. Stein declined to release absolute numbers of strokes in the two groups, pending publication.
“A lot of attention is being paid to this topic because there has been a push, a lot of it by the device makers, to prove that [TAVR] outcomes are just as good as with traditional surgery, and that we should be offering [TAVR] to more people with higher risk factor profiles who might not have been offered repair otherwise. Our job is to help patients make the most informed decisions. Having another source of data like [ours]” adds to the conversation about risks and benefits, she said.
The investigators adjusted for a large number of potential confounders to make sure the comparison was as fair as possible given the limits of database reviews. Among other variables, they controlled for baseline cardiovascular risk factors, carotid artery disease, heart failure, obesity, smoking, surgical complications, mortality, and illness severity scores, as well as hospital size, teaching hospital status, and urban versus rural location.
“What we can’t know is what medications these patients were on that might have increased their bleeding or ischemia risk. Also, we were relying on coding done by other people,” Dr. Stein said.
The next step is to look at the impact of stenting and other concomitant procedures. “We were surprised by the number of people that had multiple procedures at the same time.” The ultimate goal is to develop a risk score to help patients and doctors decide between the two procedures, she said.
Meanwhile, the team found no difference in stroke risk between coronary artery bypass grafting and percutaneous coronary interventions in the 2013 database.
Three was no industry funding for the work, and Dr. Stein did not have any relevant disclosures.
AT ANA 2017
Key clinical point:
Major finding: There was an 86% greater risk of ischemic stroke after transcatheter aortic valve replacement, compared with surgical aortic valve replacement, and a more than sixfold increased risk of hemorrhagic stroke.
Data source: Database review of more than 44,000 patients
Disclosures: Three was no industry funding for the work, and the lead investigator did not have any relevant disclosures.
Trump halts ACA cost-sharing reduction subsidy payments
“The Democrats ObamaCare [sic] is imploding. Massive subsidy payments to their pet insurance companies has stopped. Dems should call me to fix!” President Trump tweeted Oct. 13.
The action prompted criticism from medical organizations and health insurers alike.
In a statement, American Medical Association President David Barbe said he was “deeply discouraged” by the cuts. “This most recent action by the administration creates still more uncertainty in the ACA marketplace just as the abbreviated open enrollment period is about to begin, further undermining the law and threatening access to meaningful health insurance coverage for millions of Americans,” Dr. Barbe said.
“We need constructive solutions that increase consumer choice, lower consumer costs, and stabilize local markets,” America’s Health Insurance Plans and the Blue Cross Blue Shield Association said in a joint statement. “Terminating this critical program will do just the opposite. This action will make it harder for patients to access the care they need. Costs will go up and choice will be restricted.”
The U.S. Senate Committee on Health, Education, Labor & Pensions was working on a bipartisan, narrowly focused bill that would have, among other things, codified cost-sharing reduction payments in legislation for at least a year, after witnesses from across the political spectrum testified during four hearings that maintenance of the payments would bring stability to the individual market. That effort was derailed when Senate Majority Leader Mitch McConnell (R-Ky.) instead pushed a broad repeal-and-replace effort, which never reached the Senate floor for consideration when it was clear the action did not have enough votes to pass.
The president’s move follows an executive order that could further destabilize the individual market through broader use of association health plans and an expansion of short-term health insurance plans, neither of which would have to meet the coverage and benefits requirements of the Affordable Care Act.
“The Democrats ObamaCare [sic] is imploding. Massive subsidy payments to their pet insurance companies has stopped. Dems should call me to fix!” President Trump tweeted Oct. 13.
The action prompted criticism from medical organizations and health insurers alike.
In a statement, American Medical Association President David Barbe said he was “deeply discouraged” by the cuts. “This most recent action by the administration creates still more uncertainty in the ACA marketplace just as the abbreviated open enrollment period is about to begin, further undermining the law and threatening access to meaningful health insurance coverage for millions of Americans,” Dr. Barbe said.
“We need constructive solutions that increase consumer choice, lower consumer costs, and stabilize local markets,” America’s Health Insurance Plans and the Blue Cross Blue Shield Association said in a joint statement. “Terminating this critical program will do just the opposite. This action will make it harder for patients to access the care they need. Costs will go up and choice will be restricted.”
The U.S. Senate Committee on Health, Education, Labor & Pensions was working on a bipartisan, narrowly focused bill that would have, among other things, codified cost-sharing reduction payments in legislation for at least a year, after witnesses from across the political spectrum testified during four hearings that maintenance of the payments would bring stability to the individual market. That effort was derailed when Senate Majority Leader Mitch McConnell (R-Ky.) instead pushed a broad repeal-and-replace effort, which never reached the Senate floor for consideration when it was clear the action did not have enough votes to pass.
The president’s move follows an executive order that could further destabilize the individual market through broader use of association health plans and an expansion of short-term health insurance plans, neither of which would have to meet the coverage and benefits requirements of the Affordable Care Act.
“The Democrats ObamaCare [sic] is imploding. Massive subsidy payments to their pet insurance companies has stopped. Dems should call me to fix!” President Trump tweeted Oct. 13.
The action prompted criticism from medical organizations and health insurers alike.
In a statement, American Medical Association President David Barbe said he was “deeply discouraged” by the cuts. “This most recent action by the administration creates still more uncertainty in the ACA marketplace just as the abbreviated open enrollment period is about to begin, further undermining the law and threatening access to meaningful health insurance coverage for millions of Americans,” Dr. Barbe said.
“We need constructive solutions that increase consumer choice, lower consumer costs, and stabilize local markets,” America’s Health Insurance Plans and the Blue Cross Blue Shield Association said in a joint statement. “Terminating this critical program will do just the opposite. This action will make it harder for patients to access the care they need. Costs will go up and choice will be restricted.”
The U.S. Senate Committee on Health, Education, Labor & Pensions was working on a bipartisan, narrowly focused bill that would have, among other things, codified cost-sharing reduction payments in legislation for at least a year, after witnesses from across the political spectrum testified during four hearings that maintenance of the payments would bring stability to the individual market. That effort was derailed when Senate Majority Leader Mitch McConnell (R-Ky.) instead pushed a broad repeal-and-replace effort, which never reached the Senate floor for consideration when it was clear the action did not have enough votes to pass.
The president’s move follows an executive order that could further destabilize the individual market through broader use of association health plans and an expansion of short-term health insurance plans, neither of which would have to meet the coverage and benefits requirements of the Affordable Care Act.









