User login
Lifestyle guidance app may be effective in NASH
After 48 weeks, nonalcoholic fatty liver disease activity scores (NAS) improved in 13 out of 19 patients who used the NASH app developed by CureApp, according to Masaya Sato of the University of Tokyo and colleagues.
If confirmed by a controlled trial, these preliminary results could show promise for digital therapeutics, the researchers stated in an article published in The American Journal of Gastroenterology.
“The widespread use of smartphones, which can process and communicate data in real time, makes them an ideal platform for therapeutic interventions,” they said.
Although lifestyle changes can reduce NASH activity, many patients have difficulty keeping up these changes. Not enough counselors are available to guide patients in healthy practices, and hiring the counselors is expensive, the researchers wrote.
Smartphone applications aimed at instilling healthy behavior have been tried in diabetes, smoking, hypertension, alcoholism, and even cancer, they noted. They wanted to see whether something similar could be done with NASH.
The researchers recruited 19 patients with biopsy-confirmed NASH who consumed no more than moderate amounts of alcohol and had a body mass index (BMI) of at least 25 kg/m2. Their mean age was 52 years, mean BMI was 32, and mean NAS was 5.0.
The patients downloaded the NASH app onto their phones and entered their baseline profile information, including age, gender, diet and exercise practices, and social characteristics. On the basis of this information and daily weight measurements, the system proposed lifestyle improvement programs tailored to each individual. Its chatbot presented them in the form of behavioral goals and lectures from virtual nurses.
While patients used the app for 48 weeks, they also received standard outpatient care for NASH from live physicians, who also promoted the use of the app and provided additional education related to NASH.
The patients underwent liver biopsies within 90 days prior to beginning the study and at the end of 48 weeks. The researchers compared the changes in these patients versus those in a hypothetical control group, which they based on the placebo group in a previous study.
In the patients who used the app, the mean NAS change from baseline to week 48, the main endpoint, was –2.05 (95% confidence interval, –3.00 to –1.11). This result was statistically significant compared with the hypothetical control group, in which the mean change in NAS was –0.7 (P < .001).
In 11 of the patients, NAS decreased by at least 2 points without worsening of liver fibrosis. In eight patients, the researchers observed resolution of steatohepatitis, which they defined as disappearance of hepatocyte ballooning.
In 12 patients with stage F2 or F3 fibrosis, the average stage went from 2.5 to 2.0 (P = .02). No patient with stage F1 fibrosis showed a reduction in fibrosis stage. The scores for steatosis decreased in 11 patients, for lobular inflammation in 9 patients, and for ballooning in 10 patients.
The patients lost an average of 8.3% of their body weight, which was significant, compared with their baseline (P < .001). The patients also notched significant reductions in average serum levels of AST, ALT, gamma-glutamyltransferase, alkaline phosphatase, and triglycerides.
The researchers noted that the lack of a real control group and the small size of the study population limited the importance of their findings. A larger randomized, controlled trial is needed to confirm their results.
During the study, physicians browsed the patients’ data and provided them with feedback about it, the researchers wrote. But the study did not measure the amount of time the physicians spent on this activity.
CureApp founded the study, and one of the authors is a consultant for the company.
A version of this article first appeared on Medscape.com.
After 48 weeks, nonalcoholic fatty liver disease activity scores (NAS) improved in 13 out of 19 patients who used the NASH app developed by CureApp, according to Masaya Sato of the University of Tokyo and colleagues.
If confirmed by a controlled trial, these preliminary results could show promise for digital therapeutics, the researchers stated in an article published in The American Journal of Gastroenterology.
“The widespread use of smartphones, which can process and communicate data in real time, makes them an ideal platform for therapeutic interventions,” they said.
Although lifestyle changes can reduce NASH activity, many patients have difficulty keeping up these changes. Not enough counselors are available to guide patients in healthy practices, and hiring the counselors is expensive, the researchers wrote.
Smartphone applications aimed at instilling healthy behavior have been tried in diabetes, smoking, hypertension, alcoholism, and even cancer, they noted. They wanted to see whether something similar could be done with NASH.
The researchers recruited 19 patients with biopsy-confirmed NASH who consumed no more than moderate amounts of alcohol and had a body mass index (BMI) of at least 25 kg/m2. Their mean age was 52 years, mean BMI was 32, and mean NAS was 5.0.
The patients downloaded the NASH app onto their phones and entered their baseline profile information, including age, gender, diet and exercise practices, and social characteristics. On the basis of this information and daily weight measurements, the system proposed lifestyle improvement programs tailored to each individual. Its chatbot presented them in the form of behavioral goals and lectures from virtual nurses.
While patients used the app for 48 weeks, they also received standard outpatient care for NASH from live physicians, who also promoted the use of the app and provided additional education related to NASH.
The patients underwent liver biopsies within 90 days prior to beginning the study and at the end of 48 weeks. The researchers compared the changes in these patients versus those in a hypothetical control group, which they based on the placebo group in a previous study.
In the patients who used the app, the mean NAS change from baseline to week 48, the main endpoint, was –2.05 (95% confidence interval, –3.00 to –1.11). This result was statistically significant compared with the hypothetical control group, in which the mean change in NAS was –0.7 (P < .001).
In 11 of the patients, NAS decreased by at least 2 points without worsening of liver fibrosis. In eight patients, the researchers observed resolution of steatohepatitis, which they defined as disappearance of hepatocyte ballooning.
In 12 patients with stage F2 or F3 fibrosis, the average stage went from 2.5 to 2.0 (P = .02). No patient with stage F1 fibrosis showed a reduction in fibrosis stage. The scores for steatosis decreased in 11 patients, for lobular inflammation in 9 patients, and for ballooning in 10 patients.
The patients lost an average of 8.3% of their body weight, which was significant, compared with their baseline (P < .001). The patients also notched significant reductions in average serum levels of AST, ALT, gamma-glutamyltransferase, alkaline phosphatase, and triglycerides.
The researchers noted that the lack of a real control group and the small size of the study population limited the importance of their findings. A larger randomized, controlled trial is needed to confirm their results.
During the study, physicians browsed the patients’ data and provided them with feedback about it, the researchers wrote. But the study did not measure the amount of time the physicians spent on this activity.
CureApp founded the study, and one of the authors is a consultant for the company.
A version of this article first appeared on Medscape.com.
After 48 weeks, nonalcoholic fatty liver disease activity scores (NAS) improved in 13 out of 19 patients who used the NASH app developed by CureApp, according to Masaya Sato of the University of Tokyo and colleagues.
If confirmed by a controlled trial, these preliminary results could show promise for digital therapeutics, the researchers stated in an article published in The American Journal of Gastroenterology.
“The widespread use of smartphones, which can process and communicate data in real time, makes them an ideal platform for therapeutic interventions,” they said.
Although lifestyle changes can reduce NASH activity, many patients have difficulty keeping up these changes. Not enough counselors are available to guide patients in healthy practices, and hiring the counselors is expensive, the researchers wrote.
Smartphone applications aimed at instilling healthy behavior have been tried in diabetes, smoking, hypertension, alcoholism, and even cancer, they noted. They wanted to see whether something similar could be done with NASH.
The researchers recruited 19 patients with biopsy-confirmed NASH who consumed no more than moderate amounts of alcohol and had a body mass index (BMI) of at least 25 kg/m2. Their mean age was 52 years, mean BMI was 32, and mean NAS was 5.0.
The patients downloaded the NASH app onto their phones and entered their baseline profile information, including age, gender, diet and exercise practices, and social characteristics. On the basis of this information and daily weight measurements, the system proposed lifestyle improvement programs tailored to each individual. Its chatbot presented them in the form of behavioral goals and lectures from virtual nurses.
While patients used the app for 48 weeks, they also received standard outpatient care for NASH from live physicians, who also promoted the use of the app and provided additional education related to NASH.
The patients underwent liver biopsies within 90 days prior to beginning the study and at the end of 48 weeks. The researchers compared the changes in these patients versus those in a hypothetical control group, which they based on the placebo group in a previous study.
In the patients who used the app, the mean NAS change from baseline to week 48, the main endpoint, was –2.05 (95% confidence interval, –3.00 to –1.11). This result was statistically significant compared with the hypothetical control group, in which the mean change in NAS was –0.7 (P < .001).
In 11 of the patients, NAS decreased by at least 2 points without worsening of liver fibrosis. In eight patients, the researchers observed resolution of steatohepatitis, which they defined as disappearance of hepatocyte ballooning.
In 12 patients with stage F2 or F3 fibrosis, the average stage went from 2.5 to 2.0 (P = .02). No patient with stage F1 fibrosis showed a reduction in fibrosis stage. The scores for steatosis decreased in 11 patients, for lobular inflammation in 9 patients, and for ballooning in 10 patients.
The patients lost an average of 8.3% of their body weight, which was significant, compared with their baseline (P < .001). The patients also notched significant reductions in average serum levels of AST, ALT, gamma-glutamyltransferase, alkaline phosphatase, and triglycerides.
The researchers noted that the lack of a real control group and the small size of the study population limited the importance of their findings. A larger randomized, controlled trial is needed to confirm their results.
During the study, physicians browsed the patients’ data and provided them with feedback about it, the researchers wrote. But the study did not measure the amount of time the physicians spent on this activity.
CureApp founded the study, and one of the authors is a consultant for the company.
A version of this article first appeared on Medscape.com.
FROM THE AMERICAN JOURNAL OF GASTROENTEROLOGY
Endocarditis tied to drug use on the rise, spiked during COVID
A new study provides more evidence that endocarditis associated with drug use is a significant and growing health concern, and further demonstrates that this risk has been exacerbated by the COVID-19 pandemic.
The rate of infective endocarditis among individuals in the United States with opioid or cocaine use disorder increased in the 11-year period 2011 to 2022, with the steepest increase logged during the COVID-19 pandemic (2021-2022), according to the study.
A diagnosis of COVID-19 more than doubled the risk for a new diagnosis of endocarditis in patients with either cocaine (hazard ratio, 2.24) or opioid use disorder (HR, 2.23).
“Our data suggests that, in addition to the major social disruption from the pandemic, including disrupted access to health care, COVID-19 infection itself is a significant risk factor for new diagnosis of endocarditis in drug using populations,” authors Nora Volkow, MD, director of the National Institute on Drug Abuse, and colleagues wrote.
“Drug-using populations, particularly those who use cocaine or opioids, have some of the highest risk for endocarditis, and here we show that having a COVID-19 diagnoses further increases this risk,” they added.
The study was published online in Molecular Psychiatry.
The researchers analyzed electronic health record data collected from January 2011 to August 2022 for more than 109 million people across the United States, including more than 736,000 with an opioid use disorder and more than 379,000 with a cocaine use disorder.
In 2011, there were 4 cases of endocarditis per day for every 1 million people with opioid use disorder. By 2022, the rate had increased to 30 cases per day per 1 million people with opioid use disorder.
For people with cocaine use disorder, cases of endocarditis increased from 5 per 1 million in 2011 to 23 per 1 million in 2022.
Among individuals with cocaine or opioid use disorder, the risk of being hospitalized within 180 days following a diagnosis of endocarditis was higher in those with than without COVID-19 (67.5% vs. 58.7%; HR, 1.21).
The risk of dying within 180 days following new diagnosis of endocarditis was also higher in those with than without COVID-19 (9.2% vs. 8%; HR, 1.16).
The study also showed that Black and Hispanic individuals had a lower risk for COVID-19-associated endocarditis than non-Hispanic White individuals, which is consistent with a higher prevalence of injection drug use in non-Hispanic White populations, compared with Black or Hispanic populations, the researchers pointed out.
Dr. Volkow and colleagues said their findings highlight the need to screen drug users for endocarditis and link them to infectious disease and addiction treatment if they contract COVID-19.
“People with substance use disorder already face major impediments to proper health care due to lack of access and stigma,” Dr. Volkow said in a news release.
“Proven techniques like syringe service programs, which help people avoid infection from reused or shared injection equipment, can help prevent this often fatal and costly condition,” Dr. Volkow added.
The authors said it will also be important to determine exactly how SARS-CoV-2 viral infection exacerbates the risk for endocarditis in drug users.
Support for the study was provided by the National Institute on Aging, National Institute on Alcohol Abuse and Alcoholism, the Clinical and Translational Science Collaborative of Cleveland, and the National Cancer Institute Case Comprehensive Cancer Center. The authors reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
A new study provides more evidence that endocarditis associated with drug use is a significant and growing health concern, and further demonstrates that this risk has been exacerbated by the COVID-19 pandemic.
The rate of infective endocarditis among individuals in the United States with opioid or cocaine use disorder increased in the 11-year period 2011 to 2022, with the steepest increase logged during the COVID-19 pandemic (2021-2022), according to the study.
A diagnosis of COVID-19 more than doubled the risk for a new diagnosis of endocarditis in patients with either cocaine (hazard ratio, 2.24) or opioid use disorder (HR, 2.23).
“Our data suggests that, in addition to the major social disruption from the pandemic, including disrupted access to health care, COVID-19 infection itself is a significant risk factor for new diagnosis of endocarditis in drug using populations,” authors Nora Volkow, MD, director of the National Institute on Drug Abuse, and colleagues wrote.
“Drug-using populations, particularly those who use cocaine or opioids, have some of the highest risk for endocarditis, and here we show that having a COVID-19 diagnoses further increases this risk,” they added.
The study was published online in Molecular Psychiatry.
The researchers analyzed electronic health record data collected from January 2011 to August 2022 for more than 109 million people across the United States, including more than 736,000 with an opioid use disorder and more than 379,000 with a cocaine use disorder.
In 2011, there were 4 cases of endocarditis per day for every 1 million people with opioid use disorder. By 2022, the rate had increased to 30 cases per day per 1 million people with opioid use disorder.
For people with cocaine use disorder, cases of endocarditis increased from 5 per 1 million in 2011 to 23 per 1 million in 2022.
Among individuals with cocaine or opioid use disorder, the risk of being hospitalized within 180 days following a diagnosis of endocarditis was higher in those with than without COVID-19 (67.5% vs. 58.7%; HR, 1.21).
The risk of dying within 180 days following new diagnosis of endocarditis was also higher in those with than without COVID-19 (9.2% vs. 8%; HR, 1.16).
The study also showed that Black and Hispanic individuals had a lower risk for COVID-19-associated endocarditis than non-Hispanic White individuals, which is consistent with a higher prevalence of injection drug use in non-Hispanic White populations, compared with Black or Hispanic populations, the researchers pointed out.
Dr. Volkow and colleagues said their findings highlight the need to screen drug users for endocarditis and link them to infectious disease and addiction treatment if they contract COVID-19.
“People with substance use disorder already face major impediments to proper health care due to lack of access and stigma,” Dr. Volkow said in a news release.
“Proven techniques like syringe service programs, which help people avoid infection from reused or shared injection equipment, can help prevent this often fatal and costly condition,” Dr. Volkow added.
The authors said it will also be important to determine exactly how SARS-CoV-2 viral infection exacerbates the risk for endocarditis in drug users.
Support for the study was provided by the National Institute on Aging, National Institute on Alcohol Abuse and Alcoholism, the Clinical and Translational Science Collaborative of Cleveland, and the National Cancer Institute Case Comprehensive Cancer Center. The authors reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
A new study provides more evidence that endocarditis associated with drug use is a significant and growing health concern, and further demonstrates that this risk has been exacerbated by the COVID-19 pandemic.
The rate of infective endocarditis among individuals in the United States with opioid or cocaine use disorder increased in the 11-year period 2011 to 2022, with the steepest increase logged during the COVID-19 pandemic (2021-2022), according to the study.
A diagnosis of COVID-19 more than doubled the risk for a new diagnosis of endocarditis in patients with either cocaine (hazard ratio, 2.24) or opioid use disorder (HR, 2.23).
“Our data suggests that, in addition to the major social disruption from the pandemic, including disrupted access to health care, COVID-19 infection itself is a significant risk factor for new diagnosis of endocarditis in drug using populations,” authors Nora Volkow, MD, director of the National Institute on Drug Abuse, and colleagues wrote.
“Drug-using populations, particularly those who use cocaine or opioids, have some of the highest risk for endocarditis, and here we show that having a COVID-19 diagnoses further increases this risk,” they added.
The study was published online in Molecular Psychiatry.
The researchers analyzed electronic health record data collected from January 2011 to August 2022 for more than 109 million people across the United States, including more than 736,000 with an opioid use disorder and more than 379,000 with a cocaine use disorder.
In 2011, there were 4 cases of endocarditis per day for every 1 million people with opioid use disorder. By 2022, the rate had increased to 30 cases per day per 1 million people with opioid use disorder.
For people with cocaine use disorder, cases of endocarditis increased from 5 per 1 million in 2011 to 23 per 1 million in 2022.
Among individuals with cocaine or opioid use disorder, the risk of being hospitalized within 180 days following a diagnosis of endocarditis was higher in those with than without COVID-19 (67.5% vs. 58.7%; HR, 1.21).
The risk of dying within 180 days following new diagnosis of endocarditis was also higher in those with than without COVID-19 (9.2% vs. 8%; HR, 1.16).
The study also showed that Black and Hispanic individuals had a lower risk for COVID-19-associated endocarditis than non-Hispanic White individuals, which is consistent with a higher prevalence of injection drug use in non-Hispanic White populations, compared with Black or Hispanic populations, the researchers pointed out.
Dr. Volkow and colleagues said their findings highlight the need to screen drug users for endocarditis and link them to infectious disease and addiction treatment if they contract COVID-19.
“People with substance use disorder already face major impediments to proper health care due to lack of access and stigma,” Dr. Volkow said in a news release.
“Proven techniques like syringe service programs, which help people avoid infection from reused or shared injection equipment, can help prevent this often fatal and costly condition,” Dr. Volkow added.
The authors said it will also be important to determine exactly how SARS-CoV-2 viral infection exacerbates the risk for endocarditis in drug users.
Support for the study was provided by the National Institute on Aging, National Institute on Alcohol Abuse and Alcoholism, the Clinical and Translational Science Collaborative of Cleveland, and the National Cancer Institute Case Comprehensive Cancer Center. The authors reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM MOLECULAR PSYCHIATRY
A starting point for precision medicine in type 1 diabetes
MADRID – With type 1 diabetes, there can be great differences in terms of epidemiology, genetics, and possible constituent causes, as well as in the course of the disease before and after diagnosis. This point was made evident in the Can We Perform Precision Medicine in T1D? conference.
At the 63rd Congress of the Spanish Society of Endocrinology (SEEN), María José Redondo, MD, PhD, director of research in the division of diabetes and endocrinology at Texas Children’s Hospital Baylor College of Medicine in Houston, noted that delving into this evidence is the “clue” to implementing precision medicine strategies.
“Physiopathologically, there are different forms of type 1 diabetes that must be considered in the therapeutic approach. The objective is to describe this heterogeneity to discover the etiopathogenesis underlying it, so that endotypes can be defined and thus apply precision medicine. This is the paradigm followed by the European Association for the Study of Diabetes (EASD), the American Diabetes Association (ADA), and other organizations,” said Dr. Redondo.
She added that there have been significant advances in knowledge of factors that account for these epidemiologic and genetic variations. “For example, immunological processes appear to be different in children who develop type 1 diabetes at a young age, compared with those who present with the disease later in life.”
Metabolic factors are also involved in the development of type 1 diabetes in adolescents and adults, “and this metabolic heterogeneity is a very important aspect, since we currently use only glucose to diagnose diabetes and especially to classify it as type 1 when other factors should really be measured, such as C-peptide, since it has been seen that people with high levels of this peptide present a process that is closer to type 2 diabetes and have atypical characteristics for type 1 diabetes that are more like type 2 diabetes (obesity, older age, lack of typically genetic factors associated with type 1 diabetes),” noted Dr. Redondo.
Eluding classification
The specialist added that this evidence suggests a need to review the classification of the different types of diabetes. “The current general classification distinguishes type 1 diabetes, type 2 diabetes, gestational diabetes, monogenic (neonatal) diabetes, monogenic diabetes associated with cystic fibrosis, pancreatogenic, steroid-induced, and posttransplantation diabetes. However, in clinical practice, cases that are very difficult to diagnose and classify emerge, such as autoimmune diabetes, type 1 diabetes in people with insulin resistance, positive antibodies for type 2 diabetes, for example, in children with obesity (in which it is not known whether it is type 1 or type 2 diabetes), drug-induced diabetes in cases of insulin resistance, autoimmune type 1 diabetes with persistent C-peptide, or monogenic diabetes in people with obesity.
“Therefore, the current classification does not help to guide prevention or treatment, and the heterogeneity of the pathology is not as clear as we would like. Since, for example, insulin resistance affects both types of diabetes, inflammation exists in both cases, and the genes that give beta cell secretion defects exist in monogenic diabetes and probably in type 2 diabetes as well. It can be argued that type 2 diabetes is like a backdrop to a lot of diabetes that we know of so far and that it interacts with other factors that have happened to the particular person,” said Dr. Redondo.
“Furthermore, it has been shown that metformin can improve insulin resistance and cardiovascular events in patients with type 1 diabetes with obesity. On the other hand, most patients with type 2 diabetes do not need insulin after diagnosis, except for pediatric patients and those with positive antibodies who require insulin quickly. Added to this is the inability to differentiate between responders and nonresponders to immunomodulators in the prevention of type 1 diabetes, all of which highlights that there are pathogenic processes that can appear in different types of diabetes, which is why the current classification leaves out cases that do not clearly fit into a single disease type, while many people with the same diagnosis actually have very different diseases,” she pointed out.
Toward precision diagnostics
“Encapsulating” all these factors is the first step to applying precision medicine in type 1 diabetes, an area, Dr. Redondo explained, in which concrete actions are being carried out. “One of these actions is to determine BMI [body mass index], which has been incorporated into the diabetes prediction strategy that we use in clinical trials, since we know that people with a high BMI, along with other factors, clearly have a different risk. Likewise, we’ve seen that teplizumab could work better in the prevention of type 1 diabetes in individuals with anti-islet antibodies and that people who have the DR4 gene respond better than those who don’t have it and that those with the DR3 gene respond worse.”
Other recent advances along these lines involve the identification of treatments that can delay or even prevent the development of type 1 diabetes in people with positive antibodies, as well as the development of algorithms and models to predict who will develop the disease, thus placing preventive treatments within reach.
“The objective is to use all available information from each individual to understand the etiology and pathogenesis of the disease at a given moment, knowing that changes occur throughout life, and this also applies to other types of diabetes. The next step is to discover and test pathogenesis-focused therapeutic strategies with the most clinical impact in each patient at any given time,” said Dr. Redondo.
Technological tools
The specialist referred to recent advances in diabetes technology, especially semiclosed systems (such as a sensor/pump) that, in her opinion, have radically changed the control of the disease. “However, the main objective is to make type 1 diabetes preventable or reversible in people who have developed it,” she said.
Fernando Gómez-Peralta, MD, PhD, elected coordinator of the Diabetes Department at SEEN and head of the endocrinology and nutrition unit of General Hospital of Segovia, Spain, spoke about these technological advances in his presentation, “Technology and Diabetes: Clinical Experiences,” which was organized in collaboration with the Spanish Diabetes Society.
According to this expert, technological and digital tools are changing the daily lives of people with this disease. “Continuous glucose monitoring and new connected insulin pen and cap systems have increased the benefits for users of treatment with new insulins, for example,” said Dr. Gómez-Peralta.
He explained that most systems make it possible to access complete data regarding glycemic control and the treatment received and to share them with caregivers, professionals, and family members. “Some integrated insulin pump and sensor systems have self-adjusting insulin therapy algorithms that have been shown to greatly increase time-to-target glucose and reduce hypoglycemic events,” he said.
“Regarding glucose monitoring, there are devices with a longer duration (up to 2 weeks) and precision that are characterized by easier use for the patient, avoiding the need for calibration, with annoying capillary blood glucose levels.”
In the case of insulin administration, it is anticipated that in the future, some models will have very interesting features, Dr. Gómez-Peralta said. “Integrated closed-loop glucose sensor and insulin pump systems that have self-adjusting algorithms, regardless of the user, are highly effective and safe, and clearly improve glycemic control.
“For users of insulin injections, connected pens allow the integration of dynamic glucose information with doses, as well as the integration of user support tools for insulin adjustment,” Dr. Gómez-Peralta added.
The specialist stressed that a challenge for the future is to reduce the digital divide so as to increase the capacity and motivation to access these options. “In the coming years, health systems will have to face significant cost so that these systems are made available to all patients, and it is necessary to provide the systems with more material and human resources so that they can be integrated with our endocrinology and diabetes services and units.”
Dr. Redondo and Dr. Gómez-Peralta have disclosed no relevant financial relationships.
This article was translated from the Medscape Spanish edition and a version appeared on Medscape.com.
MADRID – With type 1 diabetes, there can be great differences in terms of epidemiology, genetics, and possible constituent causes, as well as in the course of the disease before and after diagnosis. This point was made evident in the Can We Perform Precision Medicine in T1D? conference.
At the 63rd Congress of the Spanish Society of Endocrinology (SEEN), María José Redondo, MD, PhD, director of research in the division of diabetes and endocrinology at Texas Children’s Hospital Baylor College of Medicine in Houston, noted that delving into this evidence is the “clue” to implementing precision medicine strategies.
“Physiopathologically, there are different forms of type 1 diabetes that must be considered in the therapeutic approach. The objective is to describe this heterogeneity to discover the etiopathogenesis underlying it, so that endotypes can be defined and thus apply precision medicine. This is the paradigm followed by the European Association for the Study of Diabetes (EASD), the American Diabetes Association (ADA), and other organizations,” said Dr. Redondo.
She added that there have been significant advances in knowledge of factors that account for these epidemiologic and genetic variations. “For example, immunological processes appear to be different in children who develop type 1 diabetes at a young age, compared with those who present with the disease later in life.”
Metabolic factors are also involved in the development of type 1 diabetes in adolescents and adults, “and this metabolic heterogeneity is a very important aspect, since we currently use only glucose to diagnose diabetes and especially to classify it as type 1 when other factors should really be measured, such as C-peptide, since it has been seen that people with high levels of this peptide present a process that is closer to type 2 diabetes and have atypical characteristics for type 1 diabetes that are more like type 2 diabetes (obesity, older age, lack of typically genetic factors associated with type 1 diabetes),” noted Dr. Redondo.
Eluding classification
The specialist added that this evidence suggests a need to review the classification of the different types of diabetes. “The current general classification distinguishes type 1 diabetes, type 2 diabetes, gestational diabetes, monogenic (neonatal) diabetes, monogenic diabetes associated with cystic fibrosis, pancreatogenic, steroid-induced, and posttransplantation diabetes. However, in clinical practice, cases that are very difficult to diagnose and classify emerge, such as autoimmune diabetes, type 1 diabetes in people with insulin resistance, positive antibodies for type 2 diabetes, for example, in children with obesity (in which it is not known whether it is type 1 or type 2 diabetes), drug-induced diabetes in cases of insulin resistance, autoimmune type 1 diabetes with persistent C-peptide, or monogenic diabetes in people with obesity.
“Therefore, the current classification does not help to guide prevention or treatment, and the heterogeneity of the pathology is not as clear as we would like. Since, for example, insulin resistance affects both types of diabetes, inflammation exists in both cases, and the genes that give beta cell secretion defects exist in monogenic diabetes and probably in type 2 diabetes as well. It can be argued that type 2 diabetes is like a backdrop to a lot of diabetes that we know of so far and that it interacts with other factors that have happened to the particular person,” said Dr. Redondo.
“Furthermore, it has been shown that metformin can improve insulin resistance and cardiovascular events in patients with type 1 diabetes with obesity. On the other hand, most patients with type 2 diabetes do not need insulin after diagnosis, except for pediatric patients and those with positive antibodies who require insulin quickly. Added to this is the inability to differentiate between responders and nonresponders to immunomodulators in the prevention of type 1 diabetes, all of which highlights that there are pathogenic processes that can appear in different types of diabetes, which is why the current classification leaves out cases that do not clearly fit into a single disease type, while many people with the same diagnosis actually have very different diseases,” she pointed out.
Toward precision diagnostics
“Encapsulating” all these factors is the first step to applying precision medicine in type 1 diabetes, an area, Dr. Redondo explained, in which concrete actions are being carried out. “One of these actions is to determine BMI [body mass index], which has been incorporated into the diabetes prediction strategy that we use in clinical trials, since we know that people with a high BMI, along with other factors, clearly have a different risk. Likewise, we’ve seen that teplizumab could work better in the prevention of type 1 diabetes in individuals with anti-islet antibodies and that people who have the DR4 gene respond better than those who don’t have it and that those with the DR3 gene respond worse.”
Other recent advances along these lines involve the identification of treatments that can delay or even prevent the development of type 1 diabetes in people with positive antibodies, as well as the development of algorithms and models to predict who will develop the disease, thus placing preventive treatments within reach.
“The objective is to use all available information from each individual to understand the etiology and pathogenesis of the disease at a given moment, knowing that changes occur throughout life, and this also applies to other types of diabetes. The next step is to discover and test pathogenesis-focused therapeutic strategies with the most clinical impact in each patient at any given time,” said Dr. Redondo.
Technological tools
The specialist referred to recent advances in diabetes technology, especially semiclosed systems (such as a sensor/pump) that, in her opinion, have radically changed the control of the disease. “However, the main objective is to make type 1 diabetes preventable or reversible in people who have developed it,” she said.
Fernando Gómez-Peralta, MD, PhD, elected coordinator of the Diabetes Department at SEEN and head of the endocrinology and nutrition unit of General Hospital of Segovia, Spain, spoke about these technological advances in his presentation, “Technology and Diabetes: Clinical Experiences,” which was organized in collaboration with the Spanish Diabetes Society.
According to this expert, technological and digital tools are changing the daily lives of people with this disease. “Continuous glucose monitoring and new connected insulin pen and cap systems have increased the benefits for users of treatment with new insulins, for example,” said Dr. Gómez-Peralta.
He explained that most systems make it possible to access complete data regarding glycemic control and the treatment received and to share them with caregivers, professionals, and family members. “Some integrated insulin pump and sensor systems have self-adjusting insulin therapy algorithms that have been shown to greatly increase time-to-target glucose and reduce hypoglycemic events,” he said.
“Regarding glucose monitoring, there are devices with a longer duration (up to 2 weeks) and precision that are characterized by easier use for the patient, avoiding the need for calibration, with annoying capillary blood glucose levels.”
In the case of insulin administration, it is anticipated that in the future, some models will have very interesting features, Dr. Gómez-Peralta said. “Integrated closed-loop glucose sensor and insulin pump systems that have self-adjusting algorithms, regardless of the user, are highly effective and safe, and clearly improve glycemic control.
“For users of insulin injections, connected pens allow the integration of dynamic glucose information with doses, as well as the integration of user support tools for insulin adjustment,” Dr. Gómez-Peralta added.
The specialist stressed that a challenge for the future is to reduce the digital divide so as to increase the capacity and motivation to access these options. “In the coming years, health systems will have to face significant cost so that these systems are made available to all patients, and it is necessary to provide the systems with more material and human resources so that they can be integrated with our endocrinology and diabetes services and units.”
Dr. Redondo and Dr. Gómez-Peralta have disclosed no relevant financial relationships.
This article was translated from the Medscape Spanish edition and a version appeared on Medscape.com.
MADRID – With type 1 diabetes, there can be great differences in terms of epidemiology, genetics, and possible constituent causes, as well as in the course of the disease before and after diagnosis. This point was made evident in the Can We Perform Precision Medicine in T1D? conference.
At the 63rd Congress of the Spanish Society of Endocrinology (SEEN), María José Redondo, MD, PhD, director of research in the division of diabetes and endocrinology at Texas Children’s Hospital Baylor College of Medicine in Houston, noted that delving into this evidence is the “clue” to implementing precision medicine strategies.
“Physiopathologically, there are different forms of type 1 diabetes that must be considered in the therapeutic approach. The objective is to describe this heterogeneity to discover the etiopathogenesis underlying it, so that endotypes can be defined and thus apply precision medicine. This is the paradigm followed by the European Association for the Study of Diabetes (EASD), the American Diabetes Association (ADA), and other organizations,” said Dr. Redondo.
She added that there have been significant advances in knowledge of factors that account for these epidemiologic and genetic variations. “For example, immunological processes appear to be different in children who develop type 1 diabetes at a young age, compared with those who present with the disease later in life.”
Metabolic factors are also involved in the development of type 1 diabetes in adolescents and adults, “and this metabolic heterogeneity is a very important aspect, since we currently use only glucose to diagnose diabetes and especially to classify it as type 1 when other factors should really be measured, such as C-peptide, since it has been seen that people with high levels of this peptide present a process that is closer to type 2 diabetes and have atypical characteristics for type 1 diabetes that are more like type 2 diabetes (obesity, older age, lack of typically genetic factors associated with type 1 diabetes),” noted Dr. Redondo.
Eluding classification
The specialist added that this evidence suggests a need to review the classification of the different types of diabetes. “The current general classification distinguishes type 1 diabetes, type 2 diabetes, gestational diabetes, monogenic (neonatal) diabetes, monogenic diabetes associated with cystic fibrosis, pancreatogenic, steroid-induced, and posttransplantation diabetes. However, in clinical practice, cases that are very difficult to diagnose and classify emerge, such as autoimmune diabetes, type 1 diabetes in people with insulin resistance, positive antibodies for type 2 diabetes, for example, in children with obesity (in which it is not known whether it is type 1 or type 2 diabetes), drug-induced diabetes in cases of insulin resistance, autoimmune type 1 diabetes with persistent C-peptide, or monogenic diabetes in people with obesity.
“Therefore, the current classification does not help to guide prevention or treatment, and the heterogeneity of the pathology is not as clear as we would like. Since, for example, insulin resistance affects both types of diabetes, inflammation exists in both cases, and the genes that give beta cell secretion defects exist in monogenic diabetes and probably in type 2 diabetes as well. It can be argued that type 2 diabetes is like a backdrop to a lot of diabetes that we know of so far and that it interacts with other factors that have happened to the particular person,” said Dr. Redondo.
“Furthermore, it has been shown that metformin can improve insulin resistance and cardiovascular events in patients with type 1 diabetes with obesity. On the other hand, most patients with type 2 diabetes do not need insulin after diagnosis, except for pediatric patients and those with positive antibodies who require insulin quickly. Added to this is the inability to differentiate between responders and nonresponders to immunomodulators in the prevention of type 1 diabetes, all of which highlights that there are pathogenic processes that can appear in different types of diabetes, which is why the current classification leaves out cases that do not clearly fit into a single disease type, while many people with the same diagnosis actually have very different diseases,” she pointed out.
Toward precision diagnostics
“Encapsulating” all these factors is the first step to applying precision medicine in type 1 diabetes, an area, Dr. Redondo explained, in which concrete actions are being carried out. “One of these actions is to determine BMI [body mass index], which has been incorporated into the diabetes prediction strategy that we use in clinical trials, since we know that people with a high BMI, along with other factors, clearly have a different risk. Likewise, we’ve seen that teplizumab could work better in the prevention of type 1 diabetes in individuals with anti-islet antibodies and that people who have the DR4 gene respond better than those who don’t have it and that those with the DR3 gene respond worse.”
Other recent advances along these lines involve the identification of treatments that can delay or even prevent the development of type 1 diabetes in people with positive antibodies, as well as the development of algorithms and models to predict who will develop the disease, thus placing preventive treatments within reach.
“The objective is to use all available information from each individual to understand the etiology and pathogenesis of the disease at a given moment, knowing that changes occur throughout life, and this also applies to other types of diabetes. The next step is to discover and test pathogenesis-focused therapeutic strategies with the most clinical impact in each patient at any given time,” said Dr. Redondo.
Technological tools
The specialist referred to recent advances in diabetes technology, especially semiclosed systems (such as a sensor/pump) that, in her opinion, have radically changed the control of the disease. “However, the main objective is to make type 1 diabetes preventable or reversible in people who have developed it,” she said.
Fernando Gómez-Peralta, MD, PhD, elected coordinator of the Diabetes Department at SEEN and head of the endocrinology and nutrition unit of General Hospital of Segovia, Spain, spoke about these technological advances in his presentation, “Technology and Diabetes: Clinical Experiences,” which was organized in collaboration with the Spanish Diabetes Society.
According to this expert, technological and digital tools are changing the daily lives of people with this disease. “Continuous glucose monitoring and new connected insulin pen and cap systems have increased the benefits for users of treatment with new insulins, for example,” said Dr. Gómez-Peralta.
He explained that most systems make it possible to access complete data regarding glycemic control and the treatment received and to share them with caregivers, professionals, and family members. “Some integrated insulin pump and sensor systems have self-adjusting insulin therapy algorithms that have been shown to greatly increase time-to-target glucose and reduce hypoglycemic events,” he said.
“Regarding glucose monitoring, there are devices with a longer duration (up to 2 weeks) and precision that are characterized by easier use for the patient, avoiding the need for calibration, with annoying capillary blood glucose levels.”
In the case of insulin administration, it is anticipated that in the future, some models will have very interesting features, Dr. Gómez-Peralta said. “Integrated closed-loop glucose sensor and insulin pump systems that have self-adjusting algorithms, regardless of the user, are highly effective and safe, and clearly improve glycemic control.
“For users of insulin injections, connected pens allow the integration of dynamic glucose information with doses, as well as the integration of user support tools for insulin adjustment,” Dr. Gómez-Peralta added.
The specialist stressed that a challenge for the future is to reduce the digital divide so as to increase the capacity and motivation to access these options. “In the coming years, health systems will have to face significant cost so that these systems are made available to all patients, and it is necessary to provide the systems with more material and human resources so that they can be integrated with our endocrinology and diabetes services and units.”
Dr. Redondo and Dr. Gómez-Peralta have disclosed no relevant financial relationships.
This article was translated from the Medscape Spanish edition and a version appeared on Medscape.com.
Lesions on upper arms
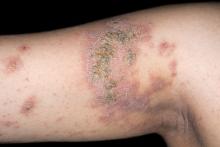
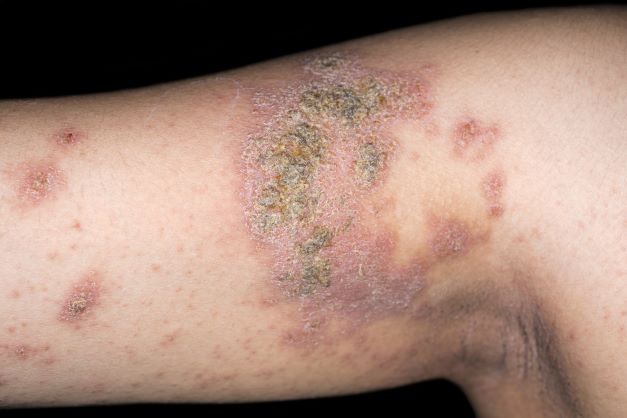
The patient is diagnosed with atopic dermatitis (AD) complicated by skin infection.
AD is the most common chronic pruritic inflammatory skin disorder that affects both children and adults. In the United States, up to 18% of children and 7% of adults are affected. Atopic dermatitis is associated with diminished quality of life, including disruption in activities of daily living, sleep disturbance, depression, and anxiety. Moreover, patients with AD have an increased risk for infections. A significantly higher prevalence of cutaneous and systemic infections is seen in patients with AD compared with individuals without AD.
Bacterial infections are common in AD and are usually caused by Staphylococcus aureus. Examples include impetigo, which typically presents with oozing serum that dries, resulting in a honey-crusted appearance surrounded by an erythematous base. Fluid-filled blisters (bullous impetigo) may also be present, which can be mistaken for eczema herpeticum (EH).
Nonpurulent skin and soft tissue infections (SSTIs) include erysipelas and cellulitis. In most cases, these infections begin in a focal skin area but spread rapidly across the affected sites such as the arms, legs, trunk, or face. Signs typically include focal erythema, swelling, warmth, and tenderness; fever and bacteremia may also be present.
Purulent SSTIs present as skin abscesses, involving fluctuant or nonfluctuant nodules or pustules surrounded by an erythematous swelling; the lesions may also be tender and warm. Methicillin-resistant S aureus (MRSA) is a common cause of purulent SSTIs.
Systemic complications of SSTI in AD may include bacteremia, osteomyelitis, septic arthritis, or bursitis; less often, endocarditis and staphylococcal scalded skin syndrome may occur. Clinicians should maintain a high index of suspicion for these complications in patients who present with an ill-looking appearance, lethargy, focal point tenderness of the bone, joint swelling, heart murmur, and widespread desquamation. Persistent elevated inflammatory markers (eg, C-reactive protein or erythrocyte sedimentation rate) should increase the level of suspicion.
Nonbacterial infections can occur concurrently with bacterial skin infections and the two can be difficult to distinguish. For example, EH results from the local spread of herpes simplex virus, which has a predilection for AD lesions. Early during EH, skin lesions appear as superficial clusters of dome‐shaped vesicles and/or small, round, punched‐out erosions. With progression, the lesions may become superficially infected with S aureus and may develop the characteristic honey-colored scale of impetigo.
Factors that contribute to the increased prevalence of infections in AD include skin barrier defects, suppression of cutaneous innate immunity by type 2 inflammation, S aureus colonization, allergen sensitivity, filaggrin loss-of-function mutation, and cutaneous dysbiosis.
Daily skin hydration and moisturization is a fundamental component of treatment for any patient with AD, both to treat the AD and prevent infection. Patients with AD should bathe daily, followed by gentle drying and application of a moisturizer or a prescribed topical medication. Standard topical anti-inflammatory medications, including topical corticosteroids and topical calcineurin inhibitors, improve skin barrier functions and have been reported to decrease S aureus colonization in AD lesions. Similarly, the monoclonal antibody dupilumab has been shown to decrease S aureus colonization and increase microbial diversity.
In the presence of an uncomplicated, nonpurulent skin infection, a beta-lactam antibiotic that covers both S aureus and beta-hemolytic streptococci (eg, cefazolin or cephalexin) may be sufficient, depending on clinical response or culture and in consideration of local epidemiology and resistance patterns. For patients with AD who present with a skin abscess, history of MRSA colonization, close contacts with a history of skin infections, or recent hospitalization, coverage for MRSA should be considered. Acceptable oral options for MRSA skin infections include clindamycin, doxycycline, trimethoprim-sulfamethoxazole, and linezolid, assuming that the isolate is susceptible in vitro. Topical mupirocin ointment can be used for patients with minor, localized skin infections (eg, impetigo).
William D. James, MD, Professor, Department of Dermatology, University of Pennsylvania, Philadelphia.
Disclosure: William D. James, MD, has disclosed the following relevant financial relationships:
Received income in an amount equal to or greater than $250 from: Elsevier.
Image Quizzes are fictional or fictionalized clinical scenarios intended to provide evidence-based educational takeaways.
The patient is diagnosed with atopic dermatitis (AD) complicated by skin infection.
AD is the most common chronic pruritic inflammatory skin disorder that affects both children and adults. In the United States, up to 18% of children and 7% of adults are affected. Atopic dermatitis is associated with diminished quality of life, including disruption in activities of daily living, sleep disturbance, depression, and anxiety. Moreover, patients with AD have an increased risk for infections. A significantly higher prevalence of cutaneous and systemic infections is seen in patients with AD compared with individuals without AD.
Bacterial infections are common in AD and are usually caused by Staphylococcus aureus. Examples include impetigo, which typically presents with oozing serum that dries, resulting in a honey-crusted appearance surrounded by an erythematous base. Fluid-filled blisters (bullous impetigo) may also be present, which can be mistaken for eczema herpeticum (EH).
Nonpurulent skin and soft tissue infections (SSTIs) include erysipelas and cellulitis. In most cases, these infections begin in a focal skin area but spread rapidly across the affected sites such as the arms, legs, trunk, or face. Signs typically include focal erythema, swelling, warmth, and tenderness; fever and bacteremia may also be present.
Purulent SSTIs present as skin abscesses, involving fluctuant or nonfluctuant nodules or pustules surrounded by an erythematous swelling; the lesions may also be tender and warm. Methicillin-resistant S aureus (MRSA) is a common cause of purulent SSTIs.
Systemic complications of SSTI in AD may include bacteremia, osteomyelitis, septic arthritis, or bursitis; less often, endocarditis and staphylococcal scalded skin syndrome may occur. Clinicians should maintain a high index of suspicion for these complications in patients who present with an ill-looking appearance, lethargy, focal point tenderness of the bone, joint swelling, heart murmur, and widespread desquamation. Persistent elevated inflammatory markers (eg, C-reactive protein or erythrocyte sedimentation rate) should increase the level of suspicion.
Nonbacterial infections can occur concurrently with bacterial skin infections and the two can be difficult to distinguish. For example, EH results from the local spread of herpes simplex virus, which has a predilection for AD lesions. Early during EH, skin lesions appear as superficial clusters of dome‐shaped vesicles and/or small, round, punched‐out erosions. With progression, the lesions may become superficially infected with S aureus and may develop the characteristic honey-colored scale of impetigo.
Factors that contribute to the increased prevalence of infections in AD include skin barrier defects, suppression of cutaneous innate immunity by type 2 inflammation, S aureus colonization, allergen sensitivity, filaggrin loss-of-function mutation, and cutaneous dysbiosis.
Daily skin hydration and moisturization is a fundamental component of treatment for any patient with AD, both to treat the AD and prevent infection. Patients with AD should bathe daily, followed by gentle drying and application of a moisturizer or a prescribed topical medication. Standard topical anti-inflammatory medications, including topical corticosteroids and topical calcineurin inhibitors, improve skin barrier functions and have been reported to decrease S aureus colonization in AD lesions. Similarly, the monoclonal antibody dupilumab has been shown to decrease S aureus colonization and increase microbial diversity.
In the presence of an uncomplicated, nonpurulent skin infection, a beta-lactam antibiotic that covers both S aureus and beta-hemolytic streptococci (eg, cefazolin or cephalexin) may be sufficient, depending on clinical response or culture and in consideration of local epidemiology and resistance patterns. For patients with AD who present with a skin abscess, history of MRSA colonization, close contacts with a history of skin infections, or recent hospitalization, coverage for MRSA should be considered. Acceptable oral options for MRSA skin infections include clindamycin, doxycycline, trimethoprim-sulfamethoxazole, and linezolid, assuming that the isolate is susceptible in vitro. Topical mupirocin ointment can be used for patients with minor, localized skin infections (eg, impetigo).
William D. James, MD, Professor, Department of Dermatology, University of Pennsylvania, Philadelphia.
Disclosure: William D. James, MD, has disclosed the following relevant financial relationships:
Received income in an amount equal to or greater than $250 from: Elsevier.
Image Quizzes are fictional or fictionalized clinical scenarios intended to provide evidence-based educational takeaways.
The patient is diagnosed with atopic dermatitis (AD) complicated by skin infection.
AD is the most common chronic pruritic inflammatory skin disorder that affects both children and adults. In the United States, up to 18% of children and 7% of adults are affected. Atopic dermatitis is associated with diminished quality of life, including disruption in activities of daily living, sleep disturbance, depression, and anxiety. Moreover, patients with AD have an increased risk for infections. A significantly higher prevalence of cutaneous and systemic infections is seen in patients with AD compared with individuals without AD.
Bacterial infections are common in AD and are usually caused by Staphylococcus aureus. Examples include impetigo, which typically presents with oozing serum that dries, resulting in a honey-crusted appearance surrounded by an erythematous base. Fluid-filled blisters (bullous impetigo) may also be present, which can be mistaken for eczema herpeticum (EH).
Nonpurulent skin and soft tissue infections (SSTIs) include erysipelas and cellulitis. In most cases, these infections begin in a focal skin area but spread rapidly across the affected sites such as the arms, legs, trunk, or face. Signs typically include focal erythema, swelling, warmth, and tenderness; fever and bacteremia may also be present.
Purulent SSTIs present as skin abscesses, involving fluctuant or nonfluctuant nodules or pustules surrounded by an erythematous swelling; the lesions may also be tender and warm. Methicillin-resistant S aureus (MRSA) is a common cause of purulent SSTIs.
Systemic complications of SSTI in AD may include bacteremia, osteomyelitis, septic arthritis, or bursitis; less often, endocarditis and staphylococcal scalded skin syndrome may occur. Clinicians should maintain a high index of suspicion for these complications in patients who present with an ill-looking appearance, lethargy, focal point tenderness of the bone, joint swelling, heart murmur, and widespread desquamation. Persistent elevated inflammatory markers (eg, C-reactive protein or erythrocyte sedimentation rate) should increase the level of suspicion.
Nonbacterial infections can occur concurrently with bacterial skin infections and the two can be difficult to distinguish. For example, EH results from the local spread of herpes simplex virus, which has a predilection for AD lesions. Early during EH, skin lesions appear as superficial clusters of dome‐shaped vesicles and/or small, round, punched‐out erosions. With progression, the lesions may become superficially infected with S aureus and may develop the characteristic honey-colored scale of impetigo.
Factors that contribute to the increased prevalence of infections in AD include skin barrier defects, suppression of cutaneous innate immunity by type 2 inflammation, S aureus colonization, allergen sensitivity, filaggrin loss-of-function mutation, and cutaneous dysbiosis.
Daily skin hydration and moisturization is a fundamental component of treatment for any patient with AD, both to treat the AD and prevent infection. Patients with AD should bathe daily, followed by gentle drying and application of a moisturizer or a prescribed topical medication. Standard topical anti-inflammatory medications, including topical corticosteroids and topical calcineurin inhibitors, improve skin barrier functions and have been reported to decrease S aureus colonization in AD lesions. Similarly, the monoclonal antibody dupilumab has been shown to decrease S aureus colonization and increase microbial diversity.
In the presence of an uncomplicated, nonpurulent skin infection, a beta-lactam antibiotic that covers both S aureus and beta-hemolytic streptococci (eg, cefazolin or cephalexin) may be sufficient, depending on clinical response or culture and in consideration of local epidemiology and resistance patterns. For patients with AD who present with a skin abscess, history of MRSA colonization, close contacts with a history of skin infections, or recent hospitalization, coverage for MRSA should be considered. Acceptable oral options for MRSA skin infections include clindamycin, doxycycline, trimethoprim-sulfamethoxazole, and linezolid, assuming that the isolate is susceptible in vitro. Topical mupirocin ointment can be used for patients with minor, localized skin infections (eg, impetigo).
William D. James, MD, Professor, Department of Dermatology, University of Pennsylvania, Philadelphia.
Disclosure: William D. James, MD, has disclosed the following relevant financial relationships:
Received income in an amount equal to or greater than $250 from: Elsevier.
Image Quizzes are fictional or fictionalized clinical scenarios intended to provide evidence-based educational takeaways.
An 8-year-old girl presents with pruritic lesions on her upper arms. As an infant, the patient was treated for widespread dermatitis with topical steroids and emollients; recently, after a long symptom-free period, she has had multiple bouts of dermatitis on her face, knees, ankles, and elbows. According to the patient's mother, the patient bathes every 2-3 days to not dry out her skin. At the current visit, physical examination reveals scaly patches and plaques with a honey-colored crust surrounded by an erythematous base. No other family members are experiencing symptoms. There is a positive family history for atopy and asthma.
New PDT therapy for CTCL to be reviewed by FDA
based on phase 3 findings published in JAMA Dermatology.
The treatment employs an ointment formulation of synthetic hypericin (HyBryte), a photosensitizer, that is preferentially absorbed into malignant cells and activated with visible light – rather than ultraviolet light – approximately 24 hours later. Investigators saw significant clinical responses in both patch and plaque type lesions and across races during the 24-week placebo-controlled, double-blinded, phase 3, randomized clinical trial.
“Traditional phototherapy, ultraviolet B phototherapy, has a limited depth of penetration, so patients with thicker plaque lesions don’t respond as well ... and UVB phototherapy typically is less effective in penetrating pigmented skin,” Ellen J. Kim, MD, lead author of the FLASH phase 3 trial, said in an interview.
Visible light in the yellow-red spectrum (500-650 nm) “penetrates deeper into the skin” and is nonmutagenic in vitro, so “theoretically it should have a much more favorable long-term safety profile,” said Dr. Kim, a dermatologist at the University of Pennsylvania, Philadelphia.
Currently, she said, the risk of secondary malignancies inherent with UV PDT, including melanoma, is a deterrent for some patients, especially “patients with really fair skin and a history of skin cancer.”
Hypericin PDT also seems well suited for use with an at-home light unit. “In our field, it’s not about which therapy is [universally] better or best, but a matter of what works best for each patient at that moment in time, depending on the side-effect profile and other issues such as access,” Dr. Kim said. “It will be great to have another option for an incurable disease that requires chronic management.”
Mycosis fungoides (MF)/CTCL is considered an orphan disease, and the treatment has received orphan drug and fast track designations from the FDA, and orphan designation from the European Medicines Agency, according to a press release from its developer, Soligenix. The company is anticipating potential approval in the second half of 2023 and is targeting early 2024 for a U.S. launch, the statement said.
Phase 3 results
The pivotal trial involved 169 patients at 39 academic and community-based U.S. medical centers and consisted of several 6-week cycles of twice-weekly treatment punctuated by 2-week breaks. In cycle 1, patients were randomized 2:1 to receive hypericin or placebo treatment of three index lesions. Cycle 2 involved the crossover of placebo patients to active treatment of index lesions, and cycle 3 (optional) involved open-label treatment of all desired lesions (index and nonindex).
The trial defined the primary endpoint in phase 1 as 50% or greater improvement in the modified Composite Assessment of Index Lesion Severity score – a tool that’s endorsed by U.S. and international MF/CTCL specialty group consensus guidelines. For cycles 2 and 3, open-label response rates were secondary endpoints. Responses were assessed after 2-week rest periods to allow for treatment-induced skin reactions to subside.
After one cycle of treatment, topical hypericin PDT was more effective than placebo (an index lesion response rate of 16% vs. 4%; P =.04). The index lesion response rate with treatment increased to 40% after two cycles and 49% after three cycles. All were statistically significant changes.
Response rates were similar in patch and plaque-type lesions and regardless of age, sex, race, stage IA versus IB, time since diagnosis, and number of prior therapies. Adverse events were primarily mild application-site skin reactions. No serious drug-related adverse events occurred, Dr. Kim said, and “we had a low drop-out rate overall.”
Into the real world
The 24-week phase 3 trial duration is short, considering that “typically, phototherapy takes between 4 to 24 months [to achieve] full responses in CTCL,” Dr. Kim said in the interview.
So with real-world application, she said, “we’ll want to see where the overall response peaks with longer treatment, what the effects are of continuous treatment without any built-in breaks, and whether we will indeed see less skin cancer development in patients who are at higher risk of developing skin cancers from light treatment.”
Such questions will be explored as part of a new 4-year, 50-patient, open-label, multicenter study with the primary aim of investigating home-based hypericin PDT therapy in a supervised setting, said Dr. Kim, principal investigator of this study. Patients who are doing well after 6 weeks of twice-weekly therapy will be given at-home light units to continue therapy and achieve 1 year of treatment with no breaks. They will be monitored with video-based telemedicine.
“Long term, having a home unit should really improve patient access and compliance and hopefully effectiveness,” Dr. Kim said. Based on the phase 3 experience, “we think that continuous treatment will be well tolerated and that we may see greater responses.”
On Dec. 19, Soligenix announced that enrollment had begun in a phase 2a study of synthetic hypericin for treating patients with mild to moderate psoriasis.
Dr. Kim reported to JAMA Dermatology grants from Innate Pharma and Galderma; consulting/advisory fees from Almirall, Galderma, and Helsinn; and honoraria from Ology and UptoDate.
based on phase 3 findings published in JAMA Dermatology.
The treatment employs an ointment formulation of synthetic hypericin (HyBryte), a photosensitizer, that is preferentially absorbed into malignant cells and activated with visible light – rather than ultraviolet light – approximately 24 hours later. Investigators saw significant clinical responses in both patch and plaque type lesions and across races during the 24-week placebo-controlled, double-blinded, phase 3, randomized clinical trial.
“Traditional phototherapy, ultraviolet B phototherapy, has a limited depth of penetration, so patients with thicker plaque lesions don’t respond as well ... and UVB phototherapy typically is less effective in penetrating pigmented skin,” Ellen J. Kim, MD, lead author of the FLASH phase 3 trial, said in an interview.
Visible light in the yellow-red spectrum (500-650 nm) “penetrates deeper into the skin” and is nonmutagenic in vitro, so “theoretically it should have a much more favorable long-term safety profile,” said Dr. Kim, a dermatologist at the University of Pennsylvania, Philadelphia.
Currently, she said, the risk of secondary malignancies inherent with UV PDT, including melanoma, is a deterrent for some patients, especially “patients with really fair skin and a history of skin cancer.”
Hypericin PDT also seems well suited for use with an at-home light unit. “In our field, it’s not about which therapy is [universally] better or best, but a matter of what works best for each patient at that moment in time, depending on the side-effect profile and other issues such as access,” Dr. Kim said. “It will be great to have another option for an incurable disease that requires chronic management.”
Mycosis fungoides (MF)/CTCL is considered an orphan disease, and the treatment has received orphan drug and fast track designations from the FDA, and orphan designation from the European Medicines Agency, according to a press release from its developer, Soligenix. The company is anticipating potential approval in the second half of 2023 and is targeting early 2024 for a U.S. launch, the statement said.
Phase 3 results
The pivotal trial involved 169 patients at 39 academic and community-based U.S. medical centers and consisted of several 6-week cycles of twice-weekly treatment punctuated by 2-week breaks. In cycle 1, patients were randomized 2:1 to receive hypericin or placebo treatment of three index lesions. Cycle 2 involved the crossover of placebo patients to active treatment of index lesions, and cycle 3 (optional) involved open-label treatment of all desired lesions (index and nonindex).
The trial defined the primary endpoint in phase 1 as 50% or greater improvement in the modified Composite Assessment of Index Lesion Severity score – a tool that’s endorsed by U.S. and international MF/CTCL specialty group consensus guidelines. For cycles 2 and 3, open-label response rates were secondary endpoints. Responses were assessed after 2-week rest periods to allow for treatment-induced skin reactions to subside.
After one cycle of treatment, topical hypericin PDT was more effective than placebo (an index lesion response rate of 16% vs. 4%; P =.04). The index lesion response rate with treatment increased to 40% after two cycles and 49% after three cycles. All were statistically significant changes.
Response rates were similar in patch and plaque-type lesions and regardless of age, sex, race, stage IA versus IB, time since diagnosis, and number of prior therapies. Adverse events were primarily mild application-site skin reactions. No serious drug-related adverse events occurred, Dr. Kim said, and “we had a low drop-out rate overall.”
Into the real world
The 24-week phase 3 trial duration is short, considering that “typically, phototherapy takes between 4 to 24 months [to achieve] full responses in CTCL,” Dr. Kim said in the interview.
So with real-world application, she said, “we’ll want to see where the overall response peaks with longer treatment, what the effects are of continuous treatment without any built-in breaks, and whether we will indeed see less skin cancer development in patients who are at higher risk of developing skin cancers from light treatment.”
Such questions will be explored as part of a new 4-year, 50-patient, open-label, multicenter study with the primary aim of investigating home-based hypericin PDT therapy in a supervised setting, said Dr. Kim, principal investigator of this study. Patients who are doing well after 6 weeks of twice-weekly therapy will be given at-home light units to continue therapy and achieve 1 year of treatment with no breaks. They will be monitored with video-based telemedicine.
“Long term, having a home unit should really improve patient access and compliance and hopefully effectiveness,” Dr. Kim said. Based on the phase 3 experience, “we think that continuous treatment will be well tolerated and that we may see greater responses.”
On Dec. 19, Soligenix announced that enrollment had begun in a phase 2a study of synthetic hypericin for treating patients with mild to moderate psoriasis.
Dr. Kim reported to JAMA Dermatology grants from Innate Pharma and Galderma; consulting/advisory fees from Almirall, Galderma, and Helsinn; and honoraria from Ology and UptoDate.
based on phase 3 findings published in JAMA Dermatology.
The treatment employs an ointment formulation of synthetic hypericin (HyBryte), a photosensitizer, that is preferentially absorbed into malignant cells and activated with visible light – rather than ultraviolet light – approximately 24 hours later. Investigators saw significant clinical responses in both patch and plaque type lesions and across races during the 24-week placebo-controlled, double-blinded, phase 3, randomized clinical trial.
“Traditional phototherapy, ultraviolet B phototherapy, has a limited depth of penetration, so patients with thicker plaque lesions don’t respond as well ... and UVB phototherapy typically is less effective in penetrating pigmented skin,” Ellen J. Kim, MD, lead author of the FLASH phase 3 trial, said in an interview.
Visible light in the yellow-red spectrum (500-650 nm) “penetrates deeper into the skin” and is nonmutagenic in vitro, so “theoretically it should have a much more favorable long-term safety profile,” said Dr. Kim, a dermatologist at the University of Pennsylvania, Philadelphia.
Currently, she said, the risk of secondary malignancies inherent with UV PDT, including melanoma, is a deterrent for some patients, especially “patients with really fair skin and a history of skin cancer.”
Hypericin PDT also seems well suited for use with an at-home light unit. “In our field, it’s not about which therapy is [universally] better or best, but a matter of what works best for each patient at that moment in time, depending on the side-effect profile and other issues such as access,” Dr. Kim said. “It will be great to have another option for an incurable disease that requires chronic management.”
Mycosis fungoides (MF)/CTCL is considered an orphan disease, and the treatment has received orphan drug and fast track designations from the FDA, and orphan designation from the European Medicines Agency, according to a press release from its developer, Soligenix. The company is anticipating potential approval in the second half of 2023 and is targeting early 2024 for a U.S. launch, the statement said.
Phase 3 results
The pivotal trial involved 169 patients at 39 academic and community-based U.S. medical centers and consisted of several 6-week cycles of twice-weekly treatment punctuated by 2-week breaks. In cycle 1, patients were randomized 2:1 to receive hypericin or placebo treatment of three index lesions. Cycle 2 involved the crossover of placebo patients to active treatment of index lesions, and cycle 3 (optional) involved open-label treatment of all desired lesions (index and nonindex).
The trial defined the primary endpoint in phase 1 as 50% or greater improvement in the modified Composite Assessment of Index Lesion Severity score – a tool that’s endorsed by U.S. and international MF/CTCL specialty group consensus guidelines. For cycles 2 and 3, open-label response rates were secondary endpoints. Responses were assessed after 2-week rest periods to allow for treatment-induced skin reactions to subside.
After one cycle of treatment, topical hypericin PDT was more effective than placebo (an index lesion response rate of 16% vs. 4%; P =.04). The index lesion response rate with treatment increased to 40% after two cycles and 49% after three cycles. All were statistically significant changes.
Response rates were similar in patch and plaque-type lesions and regardless of age, sex, race, stage IA versus IB, time since diagnosis, and number of prior therapies. Adverse events were primarily mild application-site skin reactions. No serious drug-related adverse events occurred, Dr. Kim said, and “we had a low drop-out rate overall.”
Into the real world
The 24-week phase 3 trial duration is short, considering that “typically, phototherapy takes between 4 to 24 months [to achieve] full responses in CTCL,” Dr. Kim said in the interview.
So with real-world application, she said, “we’ll want to see where the overall response peaks with longer treatment, what the effects are of continuous treatment without any built-in breaks, and whether we will indeed see less skin cancer development in patients who are at higher risk of developing skin cancers from light treatment.”
Such questions will be explored as part of a new 4-year, 50-patient, open-label, multicenter study with the primary aim of investigating home-based hypericin PDT therapy in a supervised setting, said Dr. Kim, principal investigator of this study. Patients who are doing well after 6 weeks of twice-weekly therapy will be given at-home light units to continue therapy and achieve 1 year of treatment with no breaks. They will be monitored with video-based telemedicine.
“Long term, having a home unit should really improve patient access and compliance and hopefully effectiveness,” Dr. Kim said. Based on the phase 3 experience, “we think that continuous treatment will be well tolerated and that we may see greater responses.”
On Dec. 19, Soligenix announced that enrollment had begun in a phase 2a study of synthetic hypericin for treating patients with mild to moderate psoriasis.
Dr. Kim reported to JAMA Dermatology grants from Innate Pharma and Galderma; consulting/advisory fees from Almirall, Galderma, and Helsinn; and honoraria from Ology and UptoDate.
Dietary interventions can support IBD treatment
For Crohn’s disease, a diet low in refined carbohydrates and a symptoms-guided diet appeared to help with remission, yet reduction of refined carbohydrates or red meat didn’t reduce the risk of relapse. For ulcerative colitis, solid food diets were similar to control measures.
“The Internet has a dizzying array of diet variants touted to benefit inflammation and IBD, which has led to much confusion among patients, and even clinicians, over what is truly effective or not,” Berkeley Limketkai, MD, PhD, director of clinical research at the Center for Inflammatory Bowel Disease at the University of California, Los Angeles, said in an interview.
“Even experiences shared by well-meaning individuals might not be generalizable to others,” he said. “The lack of clarity on what is or is not effective motivated us to perform this systematic review and meta-analysis.”
The study was published online in Clinical Gastroenterology and Hepatology.
Analyzing diets
Some nutritional therapies, such as exclusive enteral nutrition, have good evidence to support their use in the treatment of IBD, Dr. Limketkai said. However, patients often find maintaining a liquid diet difficult, particularly over a long period of time, so clinicians and patients have been interested in solid food diets as a treatment for IBD.
In 2019, Dr. Limketkai and colleagues conducted a systematic review and meta-analysis of randomized controlled trials focused on solid food diets for IBD that was published with the Cochrane Collaboration. At that time, the data were considered sparse, and the certainty of evidence was very low or low. Since then, several high-quality trials have been published.
For this study, Dr. Limketkai and colleagues conducted an updated review of 36 studies and a meta-analysis of 27 studies that compared a solid food diet with a control diet in patients with Crohn’s disease or ulcerative colitis. The intervention arm had to involve a well-defined diet, not merely a “usual” diet.
Among the studies, 12 evaluated dietary interventions for inducing clinical remission in patients with active Crohn’s disease, and 639 patients were involved. Overall, a low–refined carbohydrate diet was superior to a high-carbohydrate diet or a low-fiber diet. In addition, a symptoms-guided diet, which sequentially eliminated foods that aggravated a patient’s symptoms, was superior to conventional nutrition advice. However, the studies had serious imprecisions and very low certainty of evidence.
Compared with respective controls, a highly restrictive organic diet, a low-microparticle diet, and a low-calcium diet were ineffective at inducing remission of Crohn’s disease. Studies focused on immunoglobulin G-based measures were also inconsistent.
When comparing diets touted to benefit patients with Crohn’s disease, the Specific Carbohydrate Diet was similar to the Mediterranean diet and the whole-food diet, though the certainty of evidence was low. Partial enteral nutrition was similar to exclusive enteral nutrition, though there was substantial statistical heterogeneity between studies and very low certainty of evidence.
For maintenance of Crohn’s disease remission, researchers evaluated 14 studies that included 1,211 patients with inactive disease. Partial enteral nutrition appeared to reduce the risk of relapse, although evidence certainty was very low. In contrast, reducing red meat or refined carbohydrates did not lower the risk of relapse.
“These findings seemingly contradict our belief that red meat and refined carbohydrates have proinflammatory effects, although there are other studies that appear to show inconsistent, weak, or no association between consumption of unprocessed red meat and disease,” Dr. Limketkai said. “The caveat is that our findings are based on weak evidence, which may change as more studies are performed over time.”
For induction of remission in ulcerative colitis, researchers evaluated three studies that included 124 participants with active disease. When compared with participants’ usual diet, there was no benefit from a diet that excluded symptom-provoking foods, fried foods, refined carbohydrates, additives, preservatives, most condiments, spices, and beverages other than boiled water. Other studies found no benefit from eliminating cow milk protein or gluten.
For maintenance of ulcerative colitis remission, they looked at four studies that included 101 patients with inactive disease. Overall, there was no benefit from a carrageenan-free diet, anti-inflammatory diet, or cow milk protein elimination diet.
Helping patients
Although the certainty of evidence remains very low or low for most dietary trials in IBD, the emerging data suggest that nutrition plays an important role in IBD management and should be considered in the overall treatment plan for patients, the study authors wrote.
“Patients continue to look for ways to control their IBD, particularly with diet. Providers continue to struggle with making evidence-based recommendations about dietary interventions for IBD. This systematic review is a useful tool for providers to advise their patients,” James D. Lewis, MD, associate director of the inflammatory bowel diseases program at the University of Pennsylvania, Philadelphia, said in an interview.
Dr. Lewis, who wasn’t involved with this study, has researched dietary interventions for IBD. He and his colleagues have found that reducing red meat does not lower the rate of Crohn’s disease flares and that the Mediterranean diet and Specific Carbohydrate Diet appear to be similar for inducing clinical remission.
Based on this review, partial enteral nutrition could be an option for patients with Crohn’s disease, Dr. Lewis said.
“Partial enteral nutrition is much easier than exclusive enteral nutrition for patients,” he said. “However, there remains uncertainty as to whether the solid food component of a partial enteral nutrition approach impacts outcomes.”
As more dietary studies become available, the certainty of evidence could improve and lead to better recommendations for patients, Dr. Limketkai and colleagues wrote. They are conducting several studies focused on the concept of precision nutrition.
“While certain diets may be helpful and effective for IBD, different diets work differently in different people. This concept is no different than the fact that different IBD medications work differently in different individuals,” Dr. Limketkai said. “However, given the current state of evidence for dietary interventions in IBD, we still have a long path of research ahead of us.”
The study received no funding. The study authors reported no conflicts of interest. Dr. Lewis reported no relevant disclosures.
A version of this article first appeared on Medscape.com.
For Crohn’s disease, a diet low in refined carbohydrates and a symptoms-guided diet appeared to help with remission, yet reduction of refined carbohydrates or red meat didn’t reduce the risk of relapse. For ulcerative colitis, solid food diets were similar to control measures.
“The Internet has a dizzying array of diet variants touted to benefit inflammation and IBD, which has led to much confusion among patients, and even clinicians, over what is truly effective or not,” Berkeley Limketkai, MD, PhD, director of clinical research at the Center for Inflammatory Bowel Disease at the University of California, Los Angeles, said in an interview.
“Even experiences shared by well-meaning individuals might not be generalizable to others,” he said. “The lack of clarity on what is or is not effective motivated us to perform this systematic review and meta-analysis.”
The study was published online in Clinical Gastroenterology and Hepatology.
Analyzing diets
Some nutritional therapies, such as exclusive enteral nutrition, have good evidence to support their use in the treatment of IBD, Dr. Limketkai said. However, patients often find maintaining a liquid diet difficult, particularly over a long period of time, so clinicians and patients have been interested in solid food diets as a treatment for IBD.
In 2019, Dr. Limketkai and colleagues conducted a systematic review and meta-analysis of randomized controlled trials focused on solid food diets for IBD that was published with the Cochrane Collaboration. At that time, the data were considered sparse, and the certainty of evidence was very low or low. Since then, several high-quality trials have been published.
For this study, Dr. Limketkai and colleagues conducted an updated review of 36 studies and a meta-analysis of 27 studies that compared a solid food diet with a control diet in patients with Crohn’s disease or ulcerative colitis. The intervention arm had to involve a well-defined diet, not merely a “usual” diet.
Among the studies, 12 evaluated dietary interventions for inducing clinical remission in patients with active Crohn’s disease, and 639 patients were involved. Overall, a low–refined carbohydrate diet was superior to a high-carbohydrate diet or a low-fiber diet. In addition, a symptoms-guided diet, which sequentially eliminated foods that aggravated a patient’s symptoms, was superior to conventional nutrition advice. However, the studies had serious imprecisions and very low certainty of evidence.
Compared with respective controls, a highly restrictive organic diet, a low-microparticle diet, and a low-calcium diet were ineffective at inducing remission of Crohn’s disease. Studies focused on immunoglobulin G-based measures were also inconsistent.
When comparing diets touted to benefit patients with Crohn’s disease, the Specific Carbohydrate Diet was similar to the Mediterranean diet and the whole-food diet, though the certainty of evidence was low. Partial enteral nutrition was similar to exclusive enteral nutrition, though there was substantial statistical heterogeneity between studies and very low certainty of evidence.
For maintenance of Crohn’s disease remission, researchers evaluated 14 studies that included 1,211 patients with inactive disease. Partial enteral nutrition appeared to reduce the risk of relapse, although evidence certainty was very low. In contrast, reducing red meat or refined carbohydrates did not lower the risk of relapse.
“These findings seemingly contradict our belief that red meat and refined carbohydrates have proinflammatory effects, although there are other studies that appear to show inconsistent, weak, or no association between consumption of unprocessed red meat and disease,” Dr. Limketkai said. “The caveat is that our findings are based on weak evidence, which may change as more studies are performed over time.”
For induction of remission in ulcerative colitis, researchers evaluated three studies that included 124 participants with active disease. When compared with participants’ usual diet, there was no benefit from a diet that excluded symptom-provoking foods, fried foods, refined carbohydrates, additives, preservatives, most condiments, spices, and beverages other than boiled water. Other studies found no benefit from eliminating cow milk protein or gluten.
For maintenance of ulcerative colitis remission, they looked at four studies that included 101 patients with inactive disease. Overall, there was no benefit from a carrageenan-free diet, anti-inflammatory diet, or cow milk protein elimination diet.
Helping patients
Although the certainty of evidence remains very low or low for most dietary trials in IBD, the emerging data suggest that nutrition plays an important role in IBD management and should be considered in the overall treatment plan for patients, the study authors wrote.
“Patients continue to look for ways to control their IBD, particularly with diet. Providers continue to struggle with making evidence-based recommendations about dietary interventions for IBD. This systematic review is a useful tool for providers to advise their patients,” James D. Lewis, MD, associate director of the inflammatory bowel diseases program at the University of Pennsylvania, Philadelphia, said in an interview.
Dr. Lewis, who wasn’t involved with this study, has researched dietary interventions for IBD. He and his colleagues have found that reducing red meat does not lower the rate of Crohn’s disease flares and that the Mediterranean diet and Specific Carbohydrate Diet appear to be similar for inducing clinical remission.
Based on this review, partial enteral nutrition could be an option for patients with Crohn’s disease, Dr. Lewis said.
“Partial enteral nutrition is much easier than exclusive enteral nutrition for patients,” he said. “However, there remains uncertainty as to whether the solid food component of a partial enteral nutrition approach impacts outcomes.”
As more dietary studies become available, the certainty of evidence could improve and lead to better recommendations for patients, Dr. Limketkai and colleagues wrote. They are conducting several studies focused on the concept of precision nutrition.
“While certain diets may be helpful and effective for IBD, different diets work differently in different people. This concept is no different than the fact that different IBD medications work differently in different individuals,” Dr. Limketkai said. “However, given the current state of evidence for dietary interventions in IBD, we still have a long path of research ahead of us.”
The study received no funding. The study authors reported no conflicts of interest. Dr. Lewis reported no relevant disclosures.
A version of this article first appeared on Medscape.com.
For Crohn’s disease, a diet low in refined carbohydrates and a symptoms-guided diet appeared to help with remission, yet reduction of refined carbohydrates or red meat didn’t reduce the risk of relapse. For ulcerative colitis, solid food diets were similar to control measures.
“The Internet has a dizzying array of diet variants touted to benefit inflammation and IBD, which has led to much confusion among patients, and even clinicians, over what is truly effective or not,” Berkeley Limketkai, MD, PhD, director of clinical research at the Center for Inflammatory Bowel Disease at the University of California, Los Angeles, said in an interview.
“Even experiences shared by well-meaning individuals might not be generalizable to others,” he said. “The lack of clarity on what is or is not effective motivated us to perform this systematic review and meta-analysis.”
The study was published online in Clinical Gastroenterology and Hepatology.
Analyzing diets
Some nutritional therapies, such as exclusive enteral nutrition, have good evidence to support their use in the treatment of IBD, Dr. Limketkai said. However, patients often find maintaining a liquid diet difficult, particularly over a long period of time, so clinicians and patients have been interested in solid food diets as a treatment for IBD.
In 2019, Dr. Limketkai and colleagues conducted a systematic review and meta-analysis of randomized controlled trials focused on solid food diets for IBD that was published with the Cochrane Collaboration. At that time, the data were considered sparse, and the certainty of evidence was very low or low. Since then, several high-quality trials have been published.
For this study, Dr. Limketkai and colleagues conducted an updated review of 36 studies and a meta-analysis of 27 studies that compared a solid food diet with a control diet in patients with Crohn’s disease or ulcerative colitis. The intervention arm had to involve a well-defined diet, not merely a “usual” diet.
Among the studies, 12 evaluated dietary interventions for inducing clinical remission in patients with active Crohn’s disease, and 639 patients were involved. Overall, a low–refined carbohydrate diet was superior to a high-carbohydrate diet or a low-fiber diet. In addition, a symptoms-guided diet, which sequentially eliminated foods that aggravated a patient’s symptoms, was superior to conventional nutrition advice. However, the studies had serious imprecisions and very low certainty of evidence.
Compared with respective controls, a highly restrictive organic diet, a low-microparticle diet, and a low-calcium diet were ineffective at inducing remission of Crohn’s disease. Studies focused on immunoglobulin G-based measures were also inconsistent.
When comparing diets touted to benefit patients with Crohn’s disease, the Specific Carbohydrate Diet was similar to the Mediterranean diet and the whole-food diet, though the certainty of evidence was low. Partial enteral nutrition was similar to exclusive enteral nutrition, though there was substantial statistical heterogeneity between studies and very low certainty of evidence.
For maintenance of Crohn’s disease remission, researchers evaluated 14 studies that included 1,211 patients with inactive disease. Partial enteral nutrition appeared to reduce the risk of relapse, although evidence certainty was very low. In contrast, reducing red meat or refined carbohydrates did not lower the risk of relapse.
“These findings seemingly contradict our belief that red meat and refined carbohydrates have proinflammatory effects, although there are other studies that appear to show inconsistent, weak, or no association between consumption of unprocessed red meat and disease,” Dr. Limketkai said. “The caveat is that our findings are based on weak evidence, which may change as more studies are performed over time.”
For induction of remission in ulcerative colitis, researchers evaluated three studies that included 124 participants with active disease. When compared with participants’ usual diet, there was no benefit from a diet that excluded symptom-provoking foods, fried foods, refined carbohydrates, additives, preservatives, most condiments, spices, and beverages other than boiled water. Other studies found no benefit from eliminating cow milk protein or gluten.
For maintenance of ulcerative colitis remission, they looked at four studies that included 101 patients with inactive disease. Overall, there was no benefit from a carrageenan-free diet, anti-inflammatory diet, or cow milk protein elimination diet.
Helping patients
Although the certainty of evidence remains very low or low for most dietary trials in IBD, the emerging data suggest that nutrition plays an important role in IBD management and should be considered in the overall treatment plan for patients, the study authors wrote.
“Patients continue to look for ways to control their IBD, particularly with diet. Providers continue to struggle with making evidence-based recommendations about dietary interventions for IBD. This systematic review is a useful tool for providers to advise their patients,” James D. Lewis, MD, associate director of the inflammatory bowel diseases program at the University of Pennsylvania, Philadelphia, said in an interview.
Dr. Lewis, who wasn’t involved with this study, has researched dietary interventions for IBD. He and his colleagues have found that reducing red meat does not lower the rate of Crohn’s disease flares and that the Mediterranean diet and Specific Carbohydrate Diet appear to be similar for inducing clinical remission.
Based on this review, partial enteral nutrition could be an option for patients with Crohn’s disease, Dr. Lewis said.
“Partial enteral nutrition is much easier than exclusive enteral nutrition for patients,” he said. “However, there remains uncertainty as to whether the solid food component of a partial enteral nutrition approach impacts outcomes.”
As more dietary studies become available, the certainty of evidence could improve and lead to better recommendations for patients, Dr. Limketkai and colleagues wrote. They are conducting several studies focused on the concept of precision nutrition.
“While certain diets may be helpful and effective for IBD, different diets work differently in different people. This concept is no different than the fact that different IBD medications work differently in different individuals,” Dr. Limketkai said. “However, given the current state of evidence for dietary interventions in IBD, we still have a long path of research ahead of us.”
The study received no funding. The study authors reported no conflicts of interest. Dr. Lewis reported no relevant disclosures.
A version of this article first appeared on Medscape.com.
FROM CLINICAL GASTROENTEROLOGY AND HEPATOLOGY
All the National Health Service wants for Christmas is tea and biscuits
Three cups of tea, two biscuit packs, and a Christmas study from the BMJ
Warning: The following content may contain excessive Britishness. Continue at your own risk.
It’s no secret that the world economy is in an … interesting spot right now. Belt tightening is occurring around the world despite the holiday season, and hospitals across the pond in Great Britain are no exception.
It was a simple sign that prompted the study, published in the Christmas edition of the BMJ: “Please do not take excessive quantities of these refreshments.” And if we all know one thing, you do not get between Brits and their tea and biscuits. So the researchers behind the study drafted a survey and sent it around to nearly 2,000 British health care workers and asked what they considered to be excessive consumption of work-provided hot drinks and biscuits.
In the hot drinks department (tea and coffee, though we appreciate the two people who voiced a preference for free hot whiskey, if it was available) the survey participants decreed that 3.32 drinks was the maximum before consumption became excessive. That’s pretty close to the actual number of hot drinks respondents drank daily (3.04), so it’s pretty fair to say that British health care workers do a good job of self-limiting.
It’s much the same story with biscuits: Health care workers reported that consuming 2.25 packets of free biscuits would be excessive. Notably, doctors would take more than nondoctors (2.35 vs. 2.14 – typical doctor behavior), and those who had been in their role for less than 2 years would consume nearly 3 packets a day before calling it quits.
The study did not include an official cost analysis, but calculations conducted on a biscuit wrapper (that’s not a joke, by the way) estimated that the combined cost for providing every National Health Service employee with three free drinks and two free biscuit packages a day would be about 160 million pounds a year. Now, that’s a lot of money for tea and biscuits, but, they added, it’s a meager 0.1% of the NHS annual budget. They also noted that most employees consider free hot drinks a more valuable workplace perk than free support for mental health.
In conclusion, the authors wrote, “As a target for cost-saving initiatives, limiting free refreshment consumption is really scraping the biscuit barrel (although some limits on hot whiskey availability may be necessary), and implementing, or continuing, perks that improve staff morale seems justifiable. … Healthcare employers should allow biscuits and hot drinks to be freely available to staff, and they should leave these grateful recipients to judge for themselves what constitutes reasonable consumption.”
Now there’s a Christmas sentiment we can all get behind.
We come not to bury sugar, but to improve it
When we think about sugar, healthy isn’t the first thing that comes to mind. Research also shows that artificial sweeteners, as well as processed foods in general, are bad for your body and brain. People, however, love the stuff. That’s why one of the leading brands in processed foods, Kraft Heinz, partnered with the Wyss Institute for Biologically Inspired Engineering at Harvard to find a way to reduce consumers’ sugar consumption.
The question that Kraft Heinz presented to Wyss was this: How could it reduce the fructose in its products without losing the functionality of regular sugar.
The Wyss team’s approach seems pretty simple: Use a naturally occurring enzyme to convert sugar to fiber. The trick was to add the enzymes into the food so they could convert the sugar to fiber after being consumed. The enzymes also needed to be able to be added to existing food products without changing their existing recipes, Kraft Heinz insisted.
How does it work? The crafted enzyme is encapsulated to remain dormant in the food until exposed to an increased pH level, as is found in the GI tract between the stomach and the intestine. It reduces the amount of sugar absorbed in the bloodstream and creates a healthy prebiotic fiber, the institute explained.
This opens a whole new window for consumers. People with diabetes can enjoy their favorite cookies from time to time, while parents can feel less guilty about their children bathing their chicken nuggets in unholy amounts of ketchup.
New genes, or not new genes? That is the question
… and the police report that no capybaras were harmed in the incident. What a relief. Now Action News 8 brings you Carol Espinosa’s exclusive interview with legendary scientist and zombie, Charles Darwin.
Carol: Thanks, Daryl. Tell us, Prof. Darwin, what have you been up to lately?
Prof. Darwin: Please, Carol, call me Chuck. As always, I’ve got my hands full with the whole evolution thing. The big news right now is a study published in Cell Reports that offers evidence of the continuing evolution of humans. Can I eat your brain now?
Carol: No, Chuck, you may not. So people are still evolving? It sure seems like we’ve reverted to survival of the dumbest.
Chuck Darwin: Good one, Carol, but evolution hasn’t stopped. The investigators used a previously published dataset of functionally relevant new genes to create an ancestral tree comparing humans with other vertebrate species. By tracking the genes across evolution, they found 155 from regions of unique DNA that arose from scratch and not from duplication events in the existing genome. That’s a big deal.
Carol: Anything made from scratch is always better. Everyone knows that. What else can you tell us, Chuck?
Chuck Darwin: So these 155 genes didn’t exist when humans separated from chimpanzees nearly 7 million years ago. Turns out that 44 of them are associated with growth defects in cell cultures and three “have disease-associated DNA markers that point to connections with ailments such as muscular dystrophy, retinitis pigmentosa, and Alazami syndrome.” At least that’s what the investigators said in a written statement. I must say, Carol, that your brain is looking particularly delicious tonight.
Carol: Ironic. For years I’ve been hoping a man would appreciate me for my brain, and now I get this. Back to you, Daryl.
Three cups of tea, two biscuit packs, and a Christmas study from the BMJ
Warning: The following content may contain excessive Britishness. Continue at your own risk.
It’s no secret that the world economy is in an … interesting spot right now. Belt tightening is occurring around the world despite the holiday season, and hospitals across the pond in Great Britain are no exception.
It was a simple sign that prompted the study, published in the Christmas edition of the BMJ: “Please do not take excessive quantities of these refreshments.” And if we all know one thing, you do not get between Brits and their tea and biscuits. So the researchers behind the study drafted a survey and sent it around to nearly 2,000 British health care workers and asked what they considered to be excessive consumption of work-provided hot drinks and biscuits.
In the hot drinks department (tea and coffee, though we appreciate the two people who voiced a preference for free hot whiskey, if it was available) the survey participants decreed that 3.32 drinks was the maximum before consumption became excessive. That’s pretty close to the actual number of hot drinks respondents drank daily (3.04), so it’s pretty fair to say that British health care workers do a good job of self-limiting.
It’s much the same story with biscuits: Health care workers reported that consuming 2.25 packets of free biscuits would be excessive. Notably, doctors would take more than nondoctors (2.35 vs. 2.14 – typical doctor behavior), and those who had been in their role for less than 2 years would consume nearly 3 packets a day before calling it quits.
The study did not include an official cost analysis, but calculations conducted on a biscuit wrapper (that’s not a joke, by the way) estimated that the combined cost for providing every National Health Service employee with three free drinks and two free biscuit packages a day would be about 160 million pounds a year. Now, that’s a lot of money for tea and biscuits, but, they added, it’s a meager 0.1% of the NHS annual budget. They also noted that most employees consider free hot drinks a more valuable workplace perk than free support for mental health.
In conclusion, the authors wrote, “As a target for cost-saving initiatives, limiting free refreshment consumption is really scraping the biscuit barrel (although some limits on hot whiskey availability may be necessary), and implementing, or continuing, perks that improve staff morale seems justifiable. … Healthcare employers should allow biscuits and hot drinks to be freely available to staff, and they should leave these grateful recipients to judge for themselves what constitutes reasonable consumption.”
Now there’s a Christmas sentiment we can all get behind.
We come not to bury sugar, but to improve it
When we think about sugar, healthy isn’t the first thing that comes to mind. Research also shows that artificial sweeteners, as well as processed foods in general, are bad for your body and brain. People, however, love the stuff. That’s why one of the leading brands in processed foods, Kraft Heinz, partnered with the Wyss Institute for Biologically Inspired Engineering at Harvard to find a way to reduce consumers’ sugar consumption.
The question that Kraft Heinz presented to Wyss was this: How could it reduce the fructose in its products without losing the functionality of regular sugar.
The Wyss team’s approach seems pretty simple: Use a naturally occurring enzyme to convert sugar to fiber. The trick was to add the enzymes into the food so they could convert the sugar to fiber after being consumed. The enzymes also needed to be able to be added to existing food products without changing their existing recipes, Kraft Heinz insisted.
How does it work? The crafted enzyme is encapsulated to remain dormant in the food until exposed to an increased pH level, as is found in the GI tract between the stomach and the intestine. It reduces the amount of sugar absorbed in the bloodstream and creates a healthy prebiotic fiber, the institute explained.
This opens a whole new window for consumers. People with diabetes can enjoy their favorite cookies from time to time, while parents can feel less guilty about their children bathing their chicken nuggets in unholy amounts of ketchup.
New genes, or not new genes? That is the question
… and the police report that no capybaras were harmed in the incident. What a relief. Now Action News 8 brings you Carol Espinosa’s exclusive interview with legendary scientist and zombie, Charles Darwin.
Carol: Thanks, Daryl. Tell us, Prof. Darwin, what have you been up to lately?
Prof. Darwin: Please, Carol, call me Chuck. As always, I’ve got my hands full with the whole evolution thing. The big news right now is a study published in Cell Reports that offers evidence of the continuing evolution of humans. Can I eat your brain now?
Carol: No, Chuck, you may not. So people are still evolving? It sure seems like we’ve reverted to survival of the dumbest.
Chuck Darwin: Good one, Carol, but evolution hasn’t stopped. The investigators used a previously published dataset of functionally relevant new genes to create an ancestral tree comparing humans with other vertebrate species. By tracking the genes across evolution, they found 155 from regions of unique DNA that arose from scratch and not from duplication events in the existing genome. That’s a big deal.
Carol: Anything made from scratch is always better. Everyone knows that. What else can you tell us, Chuck?
Chuck Darwin: So these 155 genes didn’t exist when humans separated from chimpanzees nearly 7 million years ago. Turns out that 44 of them are associated with growth defects in cell cultures and three “have disease-associated DNA markers that point to connections with ailments such as muscular dystrophy, retinitis pigmentosa, and Alazami syndrome.” At least that’s what the investigators said in a written statement. I must say, Carol, that your brain is looking particularly delicious tonight.
Carol: Ironic. For years I’ve been hoping a man would appreciate me for my brain, and now I get this. Back to you, Daryl.
Three cups of tea, two biscuit packs, and a Christmas study from the BMJ
Warning: The following content may contain excessive Britishness. Continue at your own risk.
It’s no secret that the world economy is in an … interesting spot right now. Belt tightening is occurring around the world despite the holiday season, and hospitals across the pond in Great Britain are no exception.
It was a simple sign that prompted the study, published in the Christmas edition of the BMJ: “Please do not take excessive quantities of these refreshments.” And if we all know one thing, you do not get between Brits and their tea and biscuits. So the researchers behind the study drafted a survey and sent it around to nearly 2,000 British health care workers and asked what they considered to be excessive consumption of work-provided hot drinks and biscuits.
In the hot drinks department (tea and coffee, though we appreciate the two people who voiced a preference for free hot whiskey, if it was available) the survey participants decreed that 3.32 drinks was the maximum before consumption became excessive. That’s pretty close to the actual number of hot drinks respondents drank daily (3.04), so it’s pretty fair to say that British health care workers do a good job of self-limiting.
It’s much the same story with biscuits: Health care workers reported that consuming 2.25 packets of free biscuits would be excessive. Notably, doctors would take more than nondoctors (2.35 vs. 2.14 – typical doctor behavior), and those who had been in their role for less than 2 years would consume nearly 3 packets a day before calling it quits.
The study did not include an official cost analysis, but calculations conducted on a biscuit wrapper (that’s not a joke, by the way) estimated that the combined cost for providing every National Health Service employee with three free drinks and two free biscuit packages a day would be about 160 million pounds a year. Now, that’s a lot of money for tea and biscuits, but, they added, it’s a meager 0.1% of the NHS annual budget. They also noted that most employees consider free hot drinks a more valuable workplace perk than free support for mental health.
In conclusion, the authors wrote, “As a target for cost-saving initiatives, limiting free refreshment consumption is really scraping the biscuit barrel (although some limits on hot whiskey availability may be necessary), and implementing, or continuing, perks that improve staff morale seems justifiable. … Healthcare employers should allow biscuits and hot drinks to be freely available to staff, and they should leave these grateful recipients to judge for themselves what constitutes reasonable consumption.”
Now there’s a Christmas sentiment we can all get behind.
We come not to bury sugar, but to improve it
When we think about sugar, healthy isn’t the first thing that comes to mind. Research also shows that artificial sweeteners, as well as processed foods in general, are bad for your body and brain. People, however, love the stuff. That’s why one of the leading brands in processed foods, Kraft Heinz, partnered with the Wyss Institute for Biologically Inspired Engineering at Harvard to find a way to reduce consumers’ sugar consumption.
The question that Kraft Heinz presented to Wyss was this: How could it reduce the fructose in its products without losing the functionality of regular sugar.
The Wyss team’s approach seems pretty simple: Use a naturally occurring enzyme to convert sugar to fiber. The trick was to add the enzymes into the food so they could convert the sugar to fiber after being consumed. The enzymes also needed to be able to be added to existing food products without changing their existing recipes, Kraft Heinz insisted.
How does it work? The crafted enzyme is encapsulated to remain dormant in the food until exposed to an increased pH level, as is found in the GI tract between the stomach and the intestine. It reduces the amount of sugar absorbed in the bloodstream and creates a healthy prebiotic fiber, the institute explained.
This opens a whole new window for consumers. People with diabetes can enjoy their favorite cookies from time to time, while parents can feel less guilty about their children bathing their chicken nuggets in unholy amounts of ketchup.
New genes, or not new genes? That is the question
… and the police report that no capybaras were harmed in the incident. What a relief. Now Action News 8 brings you Carol Espinosa’s exclusive interview with legendary scientist and zombie, Charles Darwin.
Carol: Thanks, Daryl. Tell us, Prof. Darwin, what have you been up to lately?
Prof. Darwin: Please, Carol, call me Chuck. As always, I’ve got my hands full with the whole evolution thing. The big news right now is a study published in Cell Reports that offers evidence of the continuing evolution of humans. Can I eat your brain now?
Carol: No, Chuck, you may not. So people are still evolving? It sure seems like we’ve reverted to survival of the dumbest.
Chuck Darwin: Good one, Carol, but evolution hasn’t stopped. The investigators used a previously published dataset of functionally relevant new genes to create an ancestral tree comparing humans with other vertebrate species. By tracking the genes across evolution, they found 155 from regions of unique DNA that arose from scratch and not from duplication events in the existing genome. That’s a big deal.
Carol: Anything made from scratch is always better. Everyone knows that. What else can you tell us, Chuck?
Chuck Darwin: So these 155 genes didn’t exist when humans separated from chimpanzees nearly 7 million years ago. Turns out that 44 of them are associated with growth defects in cell cultures and three “have disease-associated DNA markers that point to connections with ailments such as muscular dystrophy, retinitis pigmentosa, and Alazami syndrome.” At least that’s what the investigators said in a written statement. I must say, Carol, that your brain is looking particularly delicious tonight.
Carol: Ironic. For years I’ve been hoping a man would appreciate me for my brain, and now I get this. Back to you, Daryl.
GLP-1 agonists for weight loss: What you need to know
Obesity and overweight, with or without metabolic dysregulation, pose vexing problems for many patients with mood, anxiety, or psychotic disorders. More than one-half of individuals with severe mental illnesses are obese or overweight,1 resulting from multiple factors that may include psychiatric symptoms (eg, anergia and hyperphagia), poor dietary choices, sedentary lifestyle, underlying inflammatory processes, medical comorbidities, and iatrogenic consequences of certain medications. Unfortunately, numerous psychotropic medications can increase weight and appetite due to a variety of mechanisms, including antihistaminergic effects, direct appetite-stimulating effects, and proclivities to cause insulin resistance. While individual agents can vary, a recent review identified an overall 2-fold increased risk for rapid, significant weight gain during treatment with antipsychotics as a class.2 In addition to lifestyle modifications (diet and exercise), many pharmacologic strategies have been proposed to counter iatrogenic weight gain, including appetite suppressants (eg, pro-dopaminergic agents such as phentermine, stimulants, and amantadine), pro-anorectant anticonvulsants (eg, topiramate or zonisamide), opioid receptor antagonists (eg, olanzapine/samidorphan or naltrexone) and oral hypoglycemics such as metformin. However, the magnitude of impact for most of these agents to reverse iatrogenic weight gain tends to be modest, particularly once significant weight gain (ie, ≥7% of initial body weight) has already occurred.
Pharmacologic strategies to modulate or enhance the effects of insulin hold particular importance for combatting psychotropic-associated weight gain. Insulin transports glucose from the intravascular space to end organs for fuel consumption; to varying degrees, second-generation antipsychotics (SGAs) and some other psychotropic medications can cause insulin resistance. This in turn leads to excessive storage of underutilized glucose in the liver (glycogenesis), the potential for developing fatty liver (ie, nonalcoholic steatohepatitis), and conversion of excess carbohydrates to fatty acids and triglycerides, with subsequent storage in adipose tissue. Medications that can enhance the activity of insulin (so-called incretin mimetics) can help to overcome insulin resistance caused by SGAs (and potentially by other psychotropic medications) and essentially lead to weight loss through enhanced “fuel efficiency.”
Metformin, typically dosed up to 1,000 mg twice daily with meals, has increasingly become recognized as a first-line strategy to attenuate weight gain and glycemic dysregulation from SGAs via its ability to reduce insulin resistance. Yet meta-analyses have shown that although results are significantly better than placebo, overall long-term weight loss from metformin alone tends to be rather modest (<4 kg) and associated with a reduction in body mass index (BMI) of only approximately 1 point.3 Psychiatrists (and other clinicians who prescribe psychotropic medications that can cause weight gain or metabolic dysregulation) therefore need to become familiar with alternative or adjunctive weight loss options. The use of a relatively new class of incretin mimetics called glucagon-like peptide 1 (GLP-1) agonists (Table) has been associated with profound and often dramatic weight loss and improvement of glycemic parameters in patients with obesity and glycemic dysregulation.
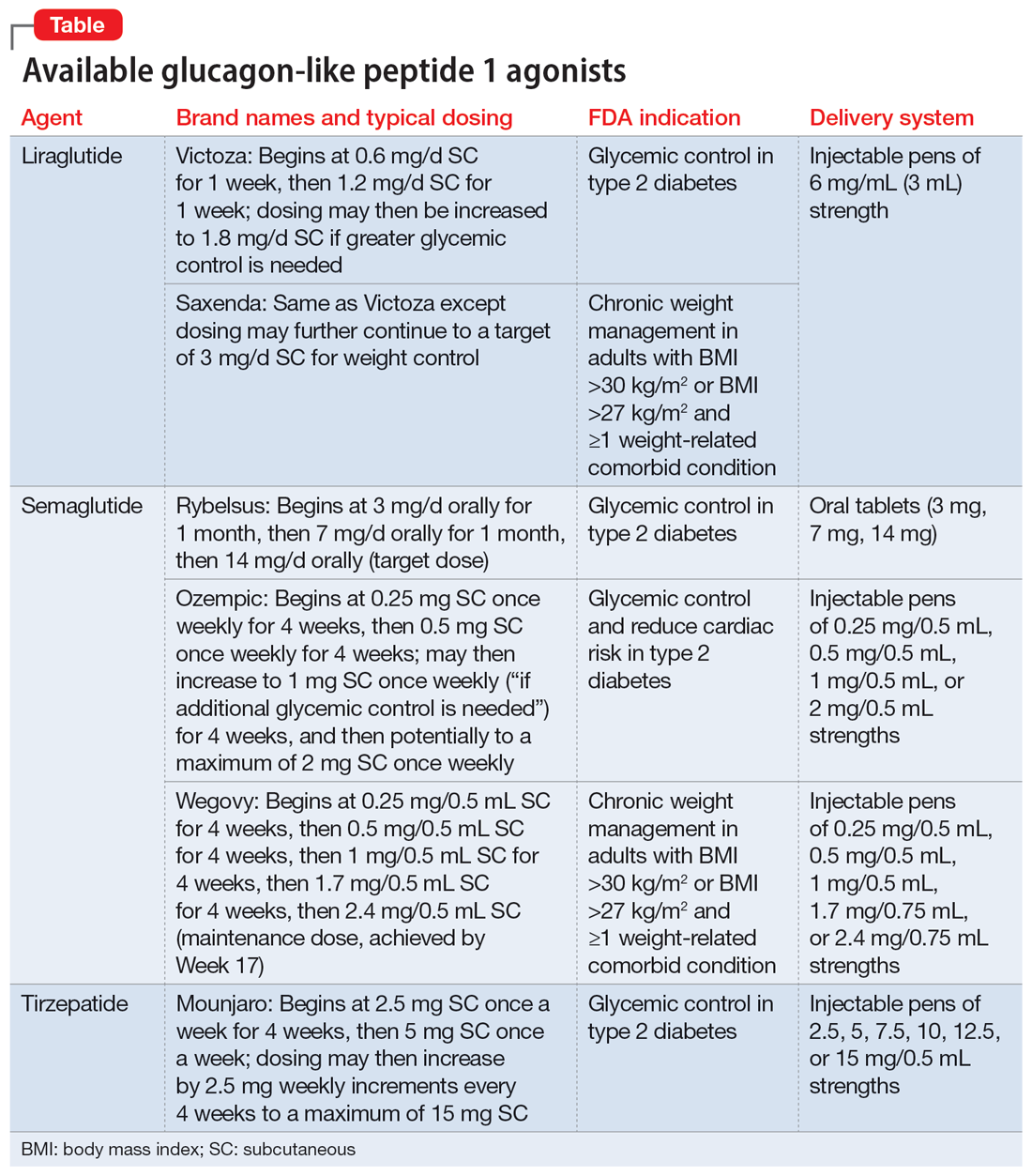
What are GLP-1 agonists?
GLP-1 is a hormone secreted by L cells in the intestinal mucosa in response to food. GLP-1 agonists reduce blood sugar by increasing insulin secretion, decreasing glucagon release (thus downregulating further increases in blood sugar), and reducing insulin resistance. GLP-1 agonists also reduce appetite by directly stimulating the satiety center and slowing gastric emptying and GI motility. In addition to GLP-1 agonism, some medications in this family (notably tirzepatide) also agonize a second hormone, glucose-dependent insulinotropic polypeptide, which can further induce insulin secretion as well as decrease stomach acid secretion, potentially delivering an even more substantial reduction in appetite and weight.
Routes of administration and FDA indications
Due to limited bioavailability, most GLP-1 agonists require subcutaneous (SC) injections (the sole exception is the Rybelsus brand of semaglutide, which comes in a daily pill form). Most are FDA-approved not specifically for weight loss but for patients with type 2 diabetes (defined as a hemoglobin A1C ≥6.5% or a fasting blood glucose level ≥126 mg/dL). Weight loss represents a secondary outcome for GLP-1 agonists FDA-approved for glycemic control in patients with type 2 diabetes. The 2 current exceptions to this classification are the Wegovy brand of semaglutide (ie, dosing of 2.4 mg) and the Saxenda brand of liraglutide, both of which carry FDA indications for chronic weight management alone (when paired with dietary and lifestyle modification) in individuals who are obese (BMI >30 kg/m2) regardless of the presence or absence of diabetes, or for persons who are overweight (BMI >27 kg/m2) and have ≥1 weight-related comorbid condition (eg, hypertension, type 2 diabetes, or dyslipidemia). Although patients at risk for diabetes (ie, prediabetes, defined as a hemoglobin A1C 5.7% to 6.4% or a fasting blood glucose level 100 to 125 mg/dL) were included in FDA registration trials of Saxenda or Wegovy, prediabetes is not an FDA indication for any GLP-1 agonist.
Data in weight loss
Most of the existing empirical data on weight loss with GLP-1 agonists come from studies of individuals who are overweight or obese, with or without type 2 diabetes, rather than from studies using these agents to counteract iatrogenic weight gain. In a retrospective cohort study of patients with type 2 diabetes, coadministration with serotonergic antidepressants (eg, citalopram/escitalopram) was associated with attenuation of the weight loss effects of GLP-1 agonists.4
Liraglutide currently is the sole GLP-1 agonist studied for treating SGA-associated weight gain. A 16-week randomized trial compared once-daily SC injected liraglutide vs placebo in patients with schizophrenia who incurred weight gain and prediabetes after taking olanzapine or clozapine.5 Significantly more patients taking liraglutide than placebo developed normal glucose tolerance (64% vs 16%), and body weight decreased by a mean of 5.3 kg.
Continue to: In studies of semaglutide...
In studies of semaglutide for overweight/obese patients with type 2 diabetes or prediabetes, clinical trials of oral semaglutide (Rybelsus) found a mean weight loss over 26 weeks of -1.0 kg with dosing at 7 mg/d and -2.6 kg with dosing at 14 mg/d.6 A 68-week placebo-controlled trial of semaglutide (dosed at 2.4 mg SC weekly) for overweight/obese adults who did not have diabetes yielded a -15.3 kg weight loss (vs -2.6 kg with placebo); one-half of those who received semaglutide lost 15% of their initial body weight (Figure 1A and Figure 1B).7 Similar findings with semaglutide 2.4 mg SC weekly (Wegovy) were observed in overweight/obese adolescents, with 73% of participants losing ≥5% of their baseline weight.8 A comparative randomized trial in patients with type 2 diabetes also found modestly but significantly greater weight loss with oral semaglutide than with SC liraglutide.9
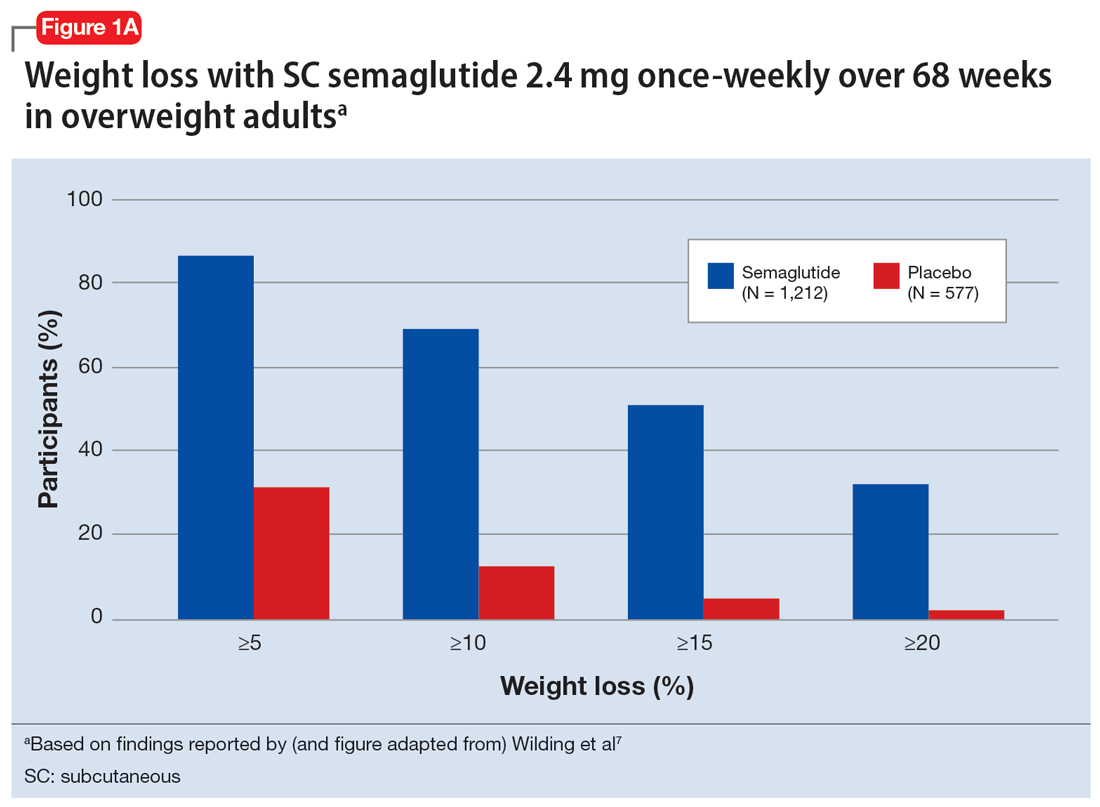
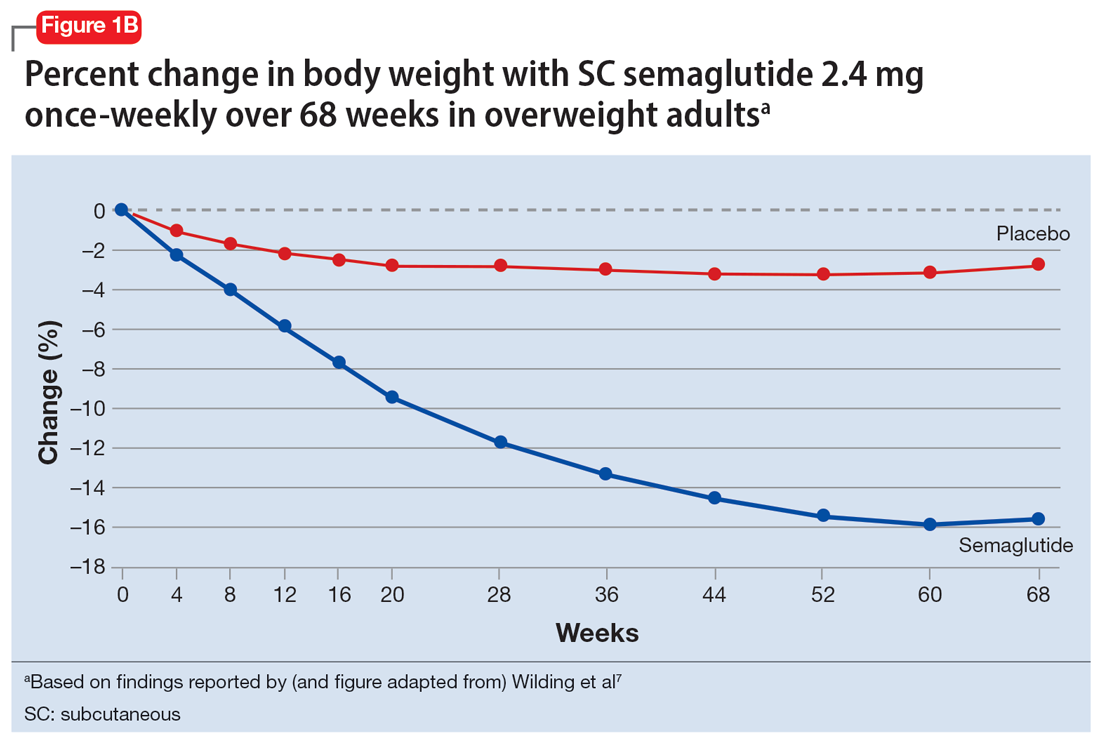
In a 72-week study of tirzepatide specifically for weight loss in nondiabetic patients who were overweight or obese, findings were especially dramatic (Figure 2A and Figure 2B).10 An overall 15% decrease in body weight was observed with 5 mg/week dosing alongside a 19.5% decrease in body weight with 10 mg/week dosing and a 20.9% weight reduction with 15 mg/week dosing.10 As noted in Figure 2B, the observed pattern of weight loss occurred along an exponential decay curve. Notably, a comparative study of tirzepatide vs once-weekly semaglutide (1 mg) in patients with type 2 diabetes11 found significantly greater dose-dependent weight loss with tirzepatide than semaglutide (-1.9 kg at 5 mg, -3.6 kg at 10 mg, and -5.5 kg at 15 mg)—although the somewhat low dosing of semaglutide may have limited its optimal possible weight loss benefit.
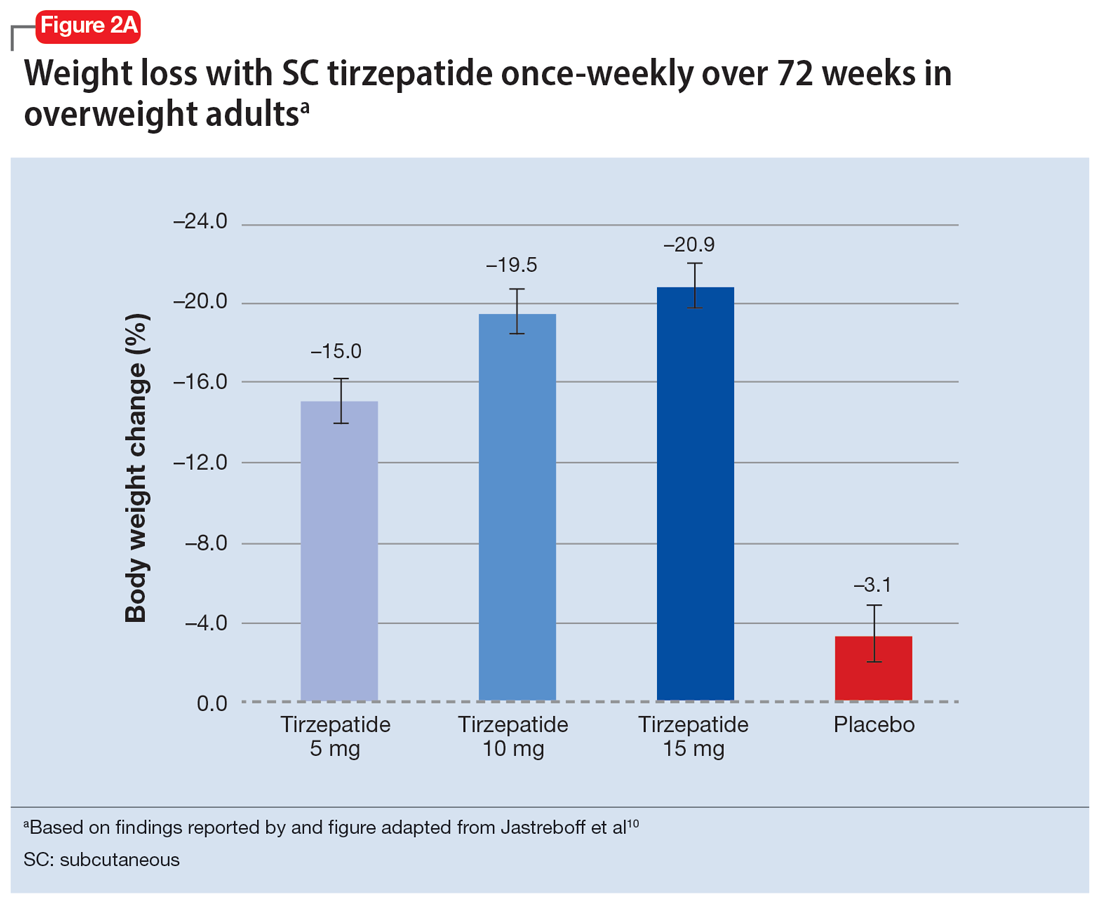
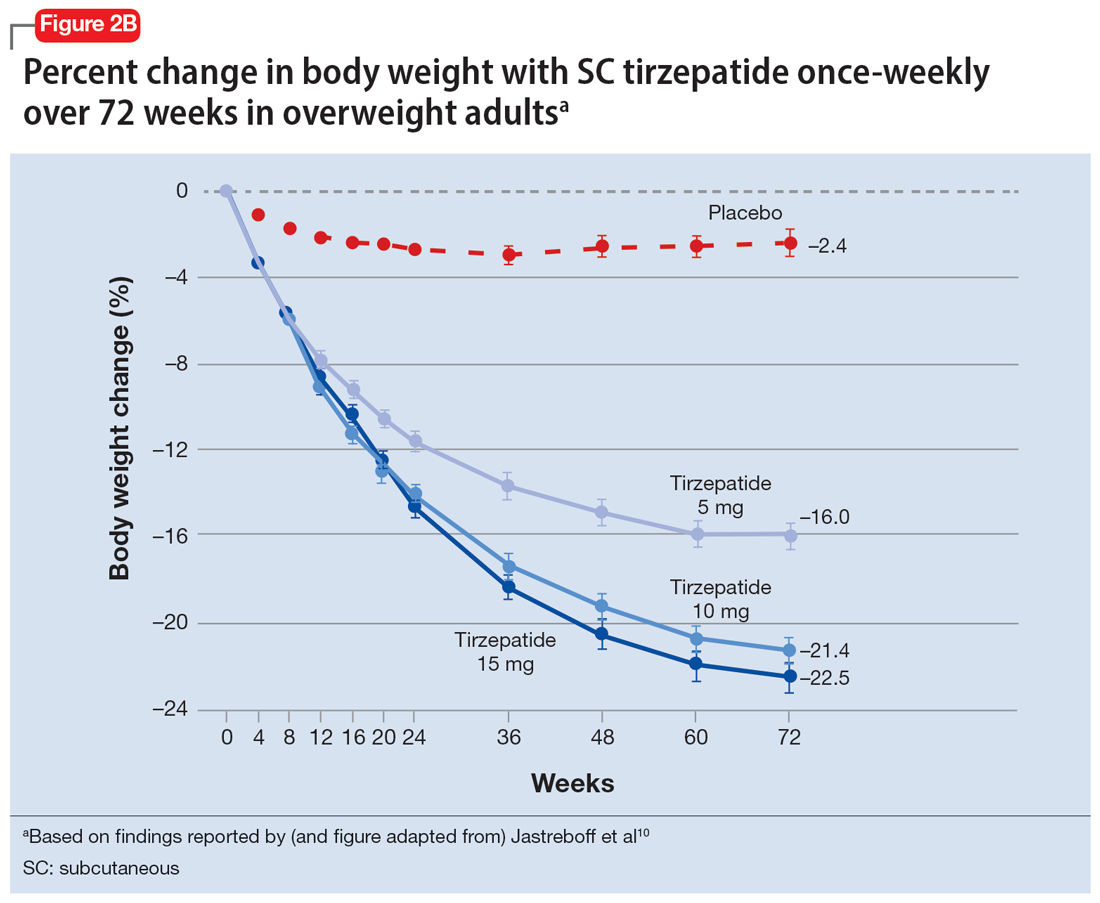
Tolerability
Adverse effects with GLP-1 agonists are mainly gastrointestinal (eg, nausea, vomiting, abdominal pain, diarrhea, or constipation)5-11 and generally transient. SC administration is performed in fatty tissue of the abdomen, thigh, or upper arm; site rotation is recommended to minimize injection site pain. All GLP-1 agonists carry manufacturers’ warning and precaution statements identifying the rare potential for acute pancreatitis, acute gall bladder disease, acute kidney injury, and hypoglycemia. Animal studies also have suggested an increased, dose-dependent risk for thyroid C-cell tumors with GLP-1 agonists; this has not been observed in human trials, although postmarketing pharmacovigilance reports have identified cases of medullary thyroid carcinoma in patients who took liraglutide. A manufacturer’s boxed warning indicates that a personal or family history of medullary carcinoma of the thyroid poses a contraindication for taking semaglutide, liraglutide, or tirzepatide.
Initial evidence prompts additional questions
GLP-1 agonists represent an emerging class of novel agents that can modulate glycemic dysregulation and overweight/obesity, often with dramatic results whose magnitude rivals the efficacy of bariatric surgery. Once-weekly formulations of semaglutide (Wegovy) and daily liraglutide (Saxenda) are FDA-approved for weight loss in patients who are overweight or obese while other existing formulations are approved solely for patients with type 2 diabetes, although it is likely that broader indications for weight loss (regardless of glycemic status) are forthcoming. Targeted use of GLP-1 agonists to counteract SGA-associated weight gain is supported by a handful of preliminary reports, with additional studies likely to come. Unanswered questions include:
- When should GLP-1 agonists be considered within a treatment algorithm for iatrogenic weight gain relative to other antidote strategies such as metformin or appetite-suppressing anticonvulsants?
- How effective might GLP-1 agonists be for iatrogenic weight gain from non-SGA psychotropic medications, such as serotonergic antidepressants?
- When and how can GLP-1 agonists be safely coprescribed with other nonincretin mimetic weight loss medications?
- When should psychiatrists prescribe GLP-1 agonists, or do so collaboratively with primary care physicians or endocrinologists, particularly in patients with metabolic syndrome?
Followers of the rapidly emerging literature in this area will likely find themselves best positioned to address these and other questions about optimal management of psychotropic-induced weight gain for the patients they treat.
Bottom Line
The use of glucagon-like peptide 1 (GLP-1) agonists, a relatively new class of incretin mimetics, has been associated with profound and often dramatic weight loss and improvement of glycemic parameters in patients with obesity and glycemic dysregulation. Preliminary reports support the potential targeted use of GLP-1 agonists to counteract weight gain associated with second-generation antipsychotics.
Related Resources
- Singh F, Allen A, Ianni A. Managing metabolic syndrome in patients with schizophrenia. Current Psychiatry. 2020;19(12):20-24,26. doi:10.12788/cp.0064
- Ard J, Fitch A, Fruh S, et al. Weight loss and maintenance related to the mechanism of action of glucagon-like peptide 1 receptor agonists. Adv Ther. 2021;38(6):2821- 2839. doi:10.1007/s12325-021-01710-0
Drug Brand Names
Amantadine • Gocovri
Citalopram • Celexa
Clozapine • Clozaril
Escitalopram • Lexapro
Liraglutide • Victoza, Saxenda
Metformin • Glucophage
Naltrexone • ReVia
Olanzapine • Zyprexa
Olanzapine/samidorphan • Lybalvi
Phentermine • Ionamin
Semaglutide • Rybelsus, Ozempic, Wegovy
Tirzepatide • Mounjaro
Topiramate • Topamax
Zonisamide • Zonegran
1. Afzal M, Siddiqi N, Ahmad B, et al. Prevalence of overweight and obesity in people with severe mental illness: systematic review and meta-analysis. Front Endocrinol (Lausanne). 2021;25;12:769309.
2. Barton BB, Segger F, Fischer K, et al. Update on weight-gain caused by antipsychotics: a systematic review and meta-analysis. Expert Opin Drug Safety. 2020;19(3):295-314.
3. de Silva AV, Suraweera C, Ratnatunga SS, et al. Metformin in prevention and treatment of antipsychotic induced weight gain: a systematic review and meta-analysis. BMC Psychiatry. 2016;16(1):341.
4. Durell N, Franks R, Coon S, et al. Effects of antidepressants on glucagon-like peptide-1 receptor agonist-related weight loss. J Pharm Technol. 2022;38(5):283-288.
5. Larsen JR, Vedtofte L, Jakobsen MSL, et al. Effect of liraglutide treatment on prediabetes and overweight or obesity in clozapine- or olanzapine-treated patients with schizophrenia spectrum disorder: a randomized clinical trial. JAMA Psychiatry. 2017;74(7):719-728.
6. Aroda VR, Rosenstock J, Terauchi Y, et al. PIONEER 1: randomized clinical trial of the efficacy and safety of oral semaglutide monotherapy in comparison with placebo in patients with type 2 diabetes. Diabetes Care. 2019;42(9):1724-1732.
7. Wilding JPH, Batterham RL, Calanna S, et al. Once-weekly semaglutide in adults with overweight or obesity. N Engl J Med. 2021;384(11):989-1002.
8. Weghuber D, Barrett T, Barrientos-Pérez M, et al. Once-weekly semaglutide in adolescents with obesity. N Engl J Med. Published online November 2, 2022. doi:10.1056/NEJMoa2208601.
9. Pratley R, Amod A, Hoff ST, et al. Oral semaglutide versus subcutaneous liraglutide and placebo in type 2 diabetes (PIONEER 4): a randomized, double-blind, phase 3a trial. Lancet. 2019;394(10192):39-50.
10. Jastreboff AM, Aronne LJ, Ahmad NN, et al. Tirzepatide once weekly for the treatment of obesity. N Engl J Med. 2022;387(3):205-216.
11. Frías JP, Davies MJ, Rosenstock J, et al. Tirzepatide versus semaglutide once weekly in patients with type 2 diabetes. N Engl J Med. 2021;385(6):503-515.
Obesity and overweight, with or without metabolic dysregulation, pose vexing problems for many patients with mood, anxiety, or psychotic disorders. More than one-half of individuals with severe mental illnesses are obese or overweight,1 resulting from multiple factors that may include psychiatric symptoms (eg, anergia and hyperphagia), poor dietary choices, sedentary lifestyle, underlying inflammatory processes, medical comorbidities, and iatrogenic consequences of certain medications. Unfortunately, numerous psychotropic medications can increase weight and appetite due to a variety of mechanisms, including antihistaminergic effects, direct appetite-stimulating effects, and proclivities to cause insulin resistance. While individual agents can vary, a recent review identified an overall 2-fold increased risk for rapid, significant weight gain during treatment with antipsychotics as a class.2 In addition to lifestyle modifications (diet and exercise), many pharmacologic strategies have been proposed to counter iatrogenic weight gain, including appetite suppressants (eg, pro-dopaminergic agents such as phentermine, stimulants, and amantadine), pro-anorectant anticonvulsants (eg, topiramate or zonisamide), opioid receptor antagonists (eg, olanzapine/samidorphan or naltrexone) and oral hypoglycemics such as metformin. However, the magnitude of impact for most of these agents to reverse iatrogenic weight gain tends to be modest, particularly once significant weight gain (ie, ≥7% of initial body weight) has already occurred.
Pharmacologic strategies to modulate or enhance the effects of insulin hold particular importance for combatting psychotropic-associated weight gain. Insulin transports glucose from the intravascular space to end organs for fuel consumption; to varying degrees, second-generation antipsychotics (SGAs) and some other psychotropic medications can cause insulin resistance. This in turn leads to excessive storage of underutilized glucose in the liver (glycogenesis), the potential for developing fatty liver (ie, nonalcoholic steatohepatitis), and conversion of excess carbohydrates to fatty acids and triglycerides, with subsequent storage in adipose tissue. Medications that can enhance the activity of insulin (so-called incretin mimetics) can help to overcome insulin resistance caused by SGAs (and potentially by other psychotropic medications) and essentially lead to weight loss through enhanced “fuel efficiency.”
Metformin, typically dosed up to 1,000 mg twice daily with meals, has increasingly become recognized as a first-line strategy to attenuate weight gain and glycemic dysregulation from SGAs via its ability to reduce insulin resistance. Yet meta-analyses have shown that although results are significantly better than placebo, overall long-term weight loss from metformin alone tends to be rather modest (<4 kg) and associated with a reduction in body mass index (BMI) of only approximately 1 point.3 Psychiatrists (and other clinicians who prescribe psychotropic medications that can cause weight gain or metabolic dysregulation) therefore need to become familiar with alternative or adjunctive weight loss options. The use of a relatively new class of incretin mimetics called glucagon-like peptide 1 (GLP-1) agonists (Table) has been associated with profound and often dramatic weight loss and improvement of glycemic parameters in patients with obesity and glycemic dysregulation.

What are GLP-1 agonists?
GLP-1 is a hormone secreted by L cells in the intestinal mucosa in response to food. GLP-1 agonists reduce blood sugar by increasing insulin secretion, decreasing glucagon release (thus downregulating further increases in blood sugar), and reducing insulin resistance. GLP-1 agonists also reduce appetite by directly stimulating the satiety center and slowing gastric emptying and GI motility. In addition to GLP-1 agonism, some medications in this family (notably tirzepatide) also agonize a second hormone, glucose-dependent insulinotropic polypeptide, which can further induce insulin secretion as well as decrease stomach acid secretion, potentially delivering an even more substantial reduction in appetite and weight.
Routes of administration and FDA indications
Due to limited bioavailability, most GLP-1 agonists require subcutaneous (SC) injections (the sole exception is the Rybelsus brand of semaglutide, which comes in a daily pill form). Most are FDA-approved not specifically for weight loss but for patients with type 2 diabetes (defined as a hemoglobin A1C ≥6.5% or a fasting blood glucose level ≥126 mg/dL). Weight loss represents a secondary outcome for GLP-1 agonists FDA-approved for glycemic control in patients with type 2 diabetes. The 2 current exceptions to this classification are the Wegovy brand of semaglutide (ie, dosing of 2.4 mg) and the Saxenda brand of liraglutide, both of which carry FDA indications for chronic weight management alone (when paired with dietary and lifestyle modification) in individuals who are obese (BMI >30 kg/m2) regardless of the presence or absence of diabetes, or for persons who are overweight (BMI >27 kg/m2) and have ≥1 weight-related comorbid condition (eg, hypertension, type 2 diabetes, or dyslipidemia). Although patients at risk for diabetes (ie, prediabetes, defined as a hemoglobin A1C 5.7% to 6.4% or a fasting blood glucose level 100 to 125 mg/dL) were included in FDA registration trials of Saxenda or Wegovy, prediabetes is not an FDA indication for any GLP-1 agonist.
Data in weight loss
Most of the existing empirical data on weight loss with GLP-1 agonists come from studies of individuals who are overweight or obese, with or without type 2 diabetes, rather than from studies using these agents to counteract iatrogenic weight gain. In a retrospective cohort study of patients with type 2 diabetes, coadministration with serotonergic antidepressants (eg, citalopram/escitalopram) was associated with attenuation of the weight loss effects of GLP-1 agonists.4
Liraglutide currently is the sole GLP-1 agonist studied for treating SGA-associated weight gain. A 16-week randomized trial compared once-daily SC injected liraglutide vs placebo in patients with schizophrenia who incurred weight gain and prediabetes after taking olanzapine or clozapine.5 Significantly more patients taking liraglutide than placebo developed normal glucose tolerance (64% vs 16%), and body weight decreased by a mean of 5.3 kg.
Continue to: In studies of semaglutide...
In studies of semaglutide for overweight/obese patients with type 2 diabetes or prediabetes, clinical trials of oral semaglutide (Rybelsus) found a mean weight loss over 26 weeks of -1.0 kg with dosing at 7 mg/d and -2.6 kg with dosing at 14 mg/d.6 A 68-week placebo-controlled trial of semaglutide (dosed at 2.4 mg SC weekly) for overweight/obese adults who did not have diabetes yielded a -15.3 kg weight loss (vs -2.6 kg with placebo); one-half of those who received semaglutide lost 15% of their initial body weight (Figure 1A and Figure 1B).7 Similar findings with semaglutide 2.4 mg SC weekly (Wegovy) were observed in overweight/obese adolescents, with 73% of participants losing ≥5% of their baseline weight.8 A comparative randomized trial in patients with type 2 diabetes also found modestly but significantly greater weight loss with oral semaglutide than with SC liraglutide.9


In a 72-week study of tirzepatide specifically for weight loss in nondiabetic patients who were overweight or obese, findings were especially dramatic (Figure 2A and Figure 2B).10 An overall 15% decrease in body weight was observed with 5 mg/week dosing alongside a 19.5% decrease in body weight with 10 mg/week dosing and a 20.9% weight reduction with 15 mg/week dosing.10 As noted in Figure 2B, the observed pattern of weight loss occurred along an exponential decay curve. Notably, a comparative study of tirzepatide vs once-weekly semaglutide (1 mg) in patients with type 2 diabetes11 found significantly greater dose-dependent weight loss with tirzepatide than semaglutide (-1.9 kg at 5 mg, -3.6 kg at 10 mg, and -5.5 kg at 15 mg)—although the somewhat low dosing of semaglutide may have limited its optimal possible weight loss benefit.


Tolerability
Adverse effects with GLP-1 agonists are mainly gastrointestinal (eg, nausea, vomiting, abdominal pain, diarrhea, or constipation)5-11 and generally transient. SC administration is performed in fatty tissue of the abdomen, thigh, or upper arm; site rotation is recommended to minimize injection site pain. All GLP-1 agonists carry manufacturers’ warning and precaution statements identifying the rare potential for acute pancreatitis, acute gall bladder disease, acute kidney injury, and hypoglycemia. Animal studies also have suggested an increased, dose-dependent risk for thyroid C-cell tumors with GLP-1 agonists; this has not been observed in human trials, although postmarketing pharmacovigilance reports have identified cases of medullary thyroid carcinoma in patients who took liraglutide. A manufacturer’s boxed warning indicates that a personal or family history of medullary carcinoma of the thyroid poses a contraindication for taking semaglutide, liraglutide, or tirzepatide.
Initial evidence prompts additional questions
GLP-1 agonists represent an emerging class of novel agents that can modulate glycemic dysregulation and overweight/obesity, often with dramatic results whose magnitude rivals the efficacy of bariatric surgery. Once-weekly formulations of semaglutide (Wegovy) and daily liraglutide (Saxenda) are FDA-approved for weight loss in patients who are overweight or obese while other existing formulations are approved solely for patients with type 2 diabetes, although it is likely that broader indications for weight loss (regardless of glycemic status) are forthcoming. Targeted use of GLP-1 agonists to counteract SGA-associated weight gain is supported by a handful of preliminary reports, with additional studies likely to come. Unanswered questions include:
- When should GLP-1 agonists be considered within a treatment algorithm for iatrogenic weight gain relative to other antidote strategies such as metformin or appetite-suppressing anticonvulsants?
- How effective might GLP-1 agonists be for iatrogenic weight gain from non-SGA psychotropic medications, such as serotonergic antidepressants?
- When and how can GLP-1 agonists be safely coprescribed with other nonincretin mimetic weight loss medications?
- When should psychiatrists prescribe GLP-1 agonists, or do so collaboratively with primary care physicians or endocrinologists, particularly in patients with metabolic syndrome?
Followers of the rapidly emerging literature in this area will likely find themselves best positioned to address these and other questions about optimal management of psychotropic-induced weight gain for the patients they treat.
Bottom Line
The use of glucagon-like peptide 1 (GLP-1) agonists, a relatively new class of incretin mimetics, has been associated with profound and often dramatic weight loss and improvement of glycemic parameters in patients with obesity and glycemic dysregulation. Preliminary reports support the potential targeted use of GLP-1 agonists to counteract weight gain associated with second-generation antipsychotics.
Related Resources
- Singh F, Allen A, Ianni A. Managing metabolic syndrome in patients with schizophrenia. Current Psychiatry. 2020;19(12):20-24,26. doi:10.12788/cp.0064
- Ard J, Fitch A, Fruh S, et al. Weight loss and maintenance related to the mechanism of action of glucagon-like peptide 1 receptor agonists. Adv Ther. 2021;38(6):2821- 2839. doi:10.1007/s12325-021-01710-0
Drug Brand Names
Amantadine • Gocovri
Citalopram • Celexa
Clozapine • Clozaril
Escitalopram • Lexapro
Liraglutide • Victoza, Saxenda
Metformin • Glucophage
Naltrexone • ReVia
Olanzapine • Zyprexa
Olanzapine/samidorphan • Lybalvi
Phentermine • Ionamin
Semaglutide • Rybelsus, Ozempic, Wegovy
Tirzepatide • Mounjaro
Topiramate • Topamax
Zonisamide • Zonegran
Obesity and overweight, with or without metabolic dysregulation, pose vexing problems for many patients with mood, anxiety, or psychotic disorders. More than one-half of individuals with severe mental illnesses are obese or overweight,1 resulting from multiple factors that may include psychiatric symptoms (eg, anergia and hyperphagia), poor dietary choices, sedentary lifestyle, underlying inflammatory processes, medical comorbidities, and iatrogenic consequences of certain medications. Unfortunately, numerous psychotropic medications can increase weight and appetite due to a variety of mechanisms, including antihistaminergic effects, direct appetite-stimulating effects, and proclivities to cause insulin resistance. While individual agents can vary, a recent review identified an overall 2-fold increased risk for rapid, significant weight gain during treatment with antipsychotics as a class.2 In addition to lifestyle modifications (diet and exercise), many pharmacologic strategies have been proposed to counter iatrogenic weight gain, including appetite suppressants (eg, pro-dopaminergic agents such as phentermine, stimulants, and amantadine), pro-anorectant anticonvulsants (eg, topiramate or zonisamide), opioid receptor antagonists (eg, olanzapine/samidorphan or naltrexone) and oral hypoglycemics such as metformin. However, the magnitude of impact for most of these agents to reverse iatrogenic weight gain tends to be modest, particularly once significant weight gain (ie, ≥7% of initial body weight) has already occurred.
Pharmacologic strategies to modulate or enhance the effects of insulin hold particular importance for combatting psychotropic-associated weight gain. Insulin transports glucose from the intravascular space to end organs for fuel consumption; to varying degrees, second-generation antipsychotics (SGAs) and some other psychotropic medications can cause insulin resistance. This in turn leads to excessive storage of underutilized glucose in the liver (glycogenesis), the potential for developing fatty liver (ie, nonalcoholic steatohepatitis), and conversion of excess carbohydrates to fatty acids and triglycerides, with subsequent storage in adipose tissue. Medications that can enhance the activity of insulin (so-called incretin mimetics) can help to overcome insulin resistance caused by SGAs (and potentially by other psychotropic medications) and essentially lead to weight loss through enhanced “fuel efficiency.”
Metformin, typically dosed up to 1,000 mg twice daily with meals, has increasingly become recognized as a first-line strategy to attenuate weight gain and glycemic dysregulation from SGAs via its ability to reduce insulin resistance. Yet meta-analyses have shown that although results are significantly better than placebo, overall long-term weight loss from metformin alone tends to be rather modest (<4 kg) and associated with a reduction in body mass index (BMI) of only approximately 1 point.3 Psychiatrists (and other clinicians who prescribe psychotropic medications that can cause weight gain or metabolic dysregulation) therefore need to become familiar with alternative or adjunctive weight loss options. The use of a relatively new class of incretin mimetics called glucagon-like peptide 1 (GLP-1) agonists (Table) has been associated with profound and often dramatic weight loss and improvement of glycemic parameters in patients with obesity and glycemic dysregulation.

What are GLP-1 agonists?
GLP-1 is a hormone secreted by L cells in the intestinal mucosa in response to food. GLP-1 agonists reduce blood sugar by increasing insulin secretion, decreasing glucagon release (thus downregulating further increases in blood sugar), and reducing insulin resistance. GLP-1 agonists also reduce appetite by directly stimulating the satiety center and slowing gastric emptying and GI motility. In addition to GLP-1 agonism, some medications in this family (notably tirzepatide) also agonize a second hormone, glucose-dependent insulinotropic polypeptide, which can further induce insulin secretion as well as decrease stomach acid secretion, potentially delivering an even more substantial reduction in appetite and weight.
Routes of administration and FDA indications
Due to limited bioavailability, most GLP-1 agonists require subcutaneous (SC) injections (the sole exception is the Rybelsus brand of semaglutide, which comes in a daily pill form). Most are FDA-approved not specifically for weight loss but for patients with type 2 diabetes (defined as a hemoglobin A1C ≥6.5% or a fasting blood glucose level ≥126 mg/dL). Weight loss represents a secondary outcome for GLP-1 agonists FDA-approved for glycemic control in patients with type 2 diabetes. The 2 current exceptions to this classification are the Wegovy brand of semaglutide (ie, dosing of 2.4 mg) and the Saxenda brand of liraglutide, both of which carry FDA indications for chronic weight management alone (when paired with dietary and lifestyle modification) in individuals who are obese (BMI >30 kg/m2) regardless of the presence or absence of diabetes, or for persons who are overweight (BMI >27 kg/m2) and have ≥1 weight-related comorbid condition (eg, hypertension, type 2 diabetes, or dyslipidemia). Although patients at risk for diabetes (ie, prediabetes, defined as a hemoglobin A1C 5.7% to 6.4% or a fasting blood glucose level 100 to 125 mg/dL) were included in FDA registration trials of Saxenda or Wegovy, prediabetes is not an FDA indication for any GLP-1 agonist.
Data in weight loss
Most of the existing empirical data on weight loss with GLP-1 agonists come from studies of individuals who are overweight or obese, with or without type 2 diabetes, rather than from studies using these agents to counteract iatrogenic weight gain. In a retrospective cohort study of patients with type 2 diabetes, coadministration with serotonergic antidepressants (eg, citalopram/escitalopram) was associated with attenuation of the weight loss effects of GLP-1 agonists.4
Liraglutide currently is the sole GLP-1 agonist studied for treating SGA-associated weight gain. A 16-week randomized trial compared once-daily SC injected liraglutide vs placebo in patients with schizophrenia who incurred weight gain and prediabetes after taking olanzapine or clozapine.5 Significantly more patients taking liraglutide than placebo developed normal glucose tolerance (64% vs 16%), and body weight decreased by a mean of 5.3 kg.
Continue to: In studies of semaglutide...
In studies of semaglutide for overweight/obese patients with type 2 diabetes or prediabetes, clinical trials of oral semaglutide (Rybelsus) found a mean weight loss over 26 weeks of -1.0 kg with dosing at 7 mg/d and -2.6 kg with dosing at 14 mg/d.6 A 68-week placebo-controlled trial of semaglutide (dosed at 2.4 mg SC weekly) for overweight/obese adults who did not have diabetes yielded a -15.3 kg weight loss (vs -2.6 kg with placebo); one-half of those who received semaglutide lost 15% of their initial body weight (Figure 1A and Figure 1B).7 Similar findings with semaglutide 2.4 mg SC weekly (Wegovy) were observed in overweight/obese adolescents, with 73% of participants losing ≥5% of their baseline weight.8 A comparative randomized trial in patients with type 2 diabetes also found modestly but significantly greater weight loss with oral semaglutide than with SC liraglutide.9


In a 72-week study of tirzepatide specifically for weight loss in nondiabetic patients who were overweight or obese, findings were especially dramatic (Figure 2A and Figure 2B).10 An overall 15% decrease in body weight was observed with 5 mg/week dosing alongside a 19.5% decrease in body weight with 10 mg/week dosing and a 20.9% weight reduction with 15 mg/week dosing.10 As noted in Figure 2B, the observed pattern of weight loss occurred along an exponential decay curve. Notably, a comparative study of tirzepatide vs once-weekly semaglutide (1 mg) in patients with type 2 diabetes11 found significantly greater dose-dependent weight loss with tirzepatide than semaglutide (-1.9 kg at 5 mg, -3.6 kg at 10 mg, and -5.5 kg at 15 mg)—although the somewhat low dosing of semaglutide may have limited its optimal possible weight loss benefit.


Tolerability
Adverse effects with GLP-1 agonists are mainly gastrointestinal (eg, nausea, vomiting, abdominal pain, diarrhea, or constipation)5-11 and generally transient. SC administration is performed in fatty tissue of the abdomen, thigh, or upper arm; site rotation is recommended to minimize injection site pain. All GLP-1 agonists carry manufacturers’ warning and precaution statements identifying the rare potential for acute pancreatitis, acute gall bladder disease, acute kidney injury, and hypoglycemia. Animal studies also have suggested an increased, dose-dependent risk for thyroid C-cell tumors with GLP-1 agonists; this has not been observed in human trials, although postmarketing pharmacovigilance reports have identified cases of medullary thyroid carcinoma in patients who took liraglutide. A manufacturer’s boxed warning indicates that a personal or family history of medullary carcinoma of the thyroid poses a contraindication for taking semaglutide, liraglutide, or tirzepatide.
Initial evidence prompts additional questions
GLP-1 agonists represent an emerging class of novel agents that can modulate glycemic dysregulation and overweight/obesity, often with dramatic results whose magnitude rivals the efficacy of bariatric surgery. Once-weekly formulations of semaglutide (Wegovy) and daily liraglutide (Saxenda) are FDA-approved for weight loss in patients who are overweight or obese while other existing formulations are approved solely for patients with type 2 diabetes, although it is likely that broader indications for weight loss (regardless of glycemic status) are forthcoming. Targeted use of GLP-1 agonists to counteract SGA-associated weight gain is supported by a handful of preliminary reports, with additional studies likely to come. Unanswered questions include:
- When should GLP-1 agonists be considered within a treatment algorithm for iatrogenic weight gain relative to other antidote strategies such as metformin or appetite-suppressing anticonvulsants?
- How effective might GLP-1 agonists be for iatrogenic weight gain from non-SGA psychotropic medications, such as serotonergic antidepressants?
- When and how can GLP-1 agonists be safely coprescribed with other nonincretin mimetic weight loss medications?
- When should psychiatrists prescribe GLP-1 agonists, or do so collaboratively with primary care physicians or endocrinologists, particularly in patients with metabolic syndrome?
Followers of the rapidly emerging literature in this area will likely find themselves best positioned to address these and other questions about optimal management of psychotropic-induced weight gain for the patients they treat.
Bottom Line
The use of glucagon-like peptide 1 (GLP-1) agonists, a relatively new class of incretin mimetics, has been associated with profound and often dramatic weight loss and improvement of glycemic parameters in patients with obesity and glycemic dysregulation. Preliminary reports support the potential targeted use of GLP-1 agonists to counteract weight gain associated with second-generation antipsychotics.
Related Resources
- Singh F, Allen A, Ianni A. Managing metabolic syndrome in patients with schizophrenia. Current Psychiatry. 2020;19(12):20-24,26. doi:10.12788/cp.0064
- Ard J, Fitch A, Fruh S, et al. Weight loss and maintenance related to the mechanism of action of glucagon-like peptide 1 receptor agonists. Adv Ther. 2021;38(6):2821- 2839. doi:10.1007/s12325-021-01710-0
Drug Brand Names
Amantadine • Gocovri
Citalopram • Celexa
Clozapine • Clozaril
Escitalopram • Lexapro
Liraglutide • Victoza, Saxenda
Metformin • Glucophage
Naltrexone • ReVia
Olanzapine • Zyprexa
Olanzapine/samidorphan • Lybalvi
Phentermine • Ionamin
Semaglutide • Rybelsus, Ozempic, Wegovy
Tirzepatide • Mounjaro
Topiramate • Topamax
Zonisamide • Zonegran
1. Afzal M, Siddiqi N, Ahmad B, et al. Prevalence of overweight and obesity in people with severe mental illness: systematic review and meta-analysis. Front Endocrinol (Lausanne). 2021;25;12:769309.
2. Barton BB, Segger F, Fischer K, et al. Update on weight-gain caused by antipsychotics: a systematic review and meta-analysis. Expert Opin Drug Safety. 2020;19(3):295-314.
3. de Silva AV, Suraweera C, Ratnatunga SS, et al. Metformin in prevention and treatment of antipsychotic induced weight gain: a systematic review and meta-analysis. BMC Psychiatry. 2016;16(1):341.
4. Durell N, Franks R, Coon S, et al. Effects of antidepressants on glucagon-like peptide-1 receptor agonist-related weight loss. J Pharm Technol. 2022;38(5):283-288.
5. Larsen JR, Vedtofte L, Jakobsen MSL, et al. Effect of liraglutide treatment on prediabetes and overweight or obesity in clozapine- or olanzapine-treated patients with schizophrenia spectrum disorder: a randomized clinical trial. JAMA Psychiatry. 2017;74(7):719-728.
6. Aroda VR, Rosenstock J, Terauchi Y, et al. PIONEER 1: randomized clinical trial of the efficacy and safety of oral semaglutide monotherapy in comparison with placebo in patients with type 2 diabetes. Diabetes Care. 2019;42(9):1724-1732.
7. Wilding JPH, Batterham RL, Calanna S, et al. Once-weekly semaglutide in adults with overweight or obesity. N Engl J Med. 2021;384(11):989-1002.
8. Weghuber D, Barrett T, Barrientos-Pérez M, et al. Once-weekly semaglutide in adolescents with obesity. N Engl J Med. Published online November 2, 2022. doi:10.1056/NEJMoa2208601.
9. Pratley R, Amod A, Hoff ST, et al. Oral semaglutide versus subcutaneous liraglutide and placebo in type 2 diabetes (PIONEER 4): a randomized, double-blind, phase 3a trial. Lancet. 2019;394(10192):39-50.
10. Jastreboff AM, Aronne LJ, Ahmad NN, et al. Tirzepatide once weekly for the treatment of obesity. N Engl J Med. 2022;387(3):205-216.
11. Frías JP, Davies MJ, Rosenstock J, et al. Tirzepatide versus semaglutide once weekly in patients with type 2 diabetes. N Engl J Med. 2021;385(6):503-515.
1. Afzal M, Siddiqi N, Ahmad B, et al. Prevalence of overweight and obesity in people with severe mental illness: systematic review and meta-analysis. Front Endocrinol (Lausanne). 2021;25;12:769309.
2. Barton BB, Segger F, Fischer K, et al. Update on weight-gain caused by antipsychotics: a systematic review and meta-analysis. Expert Opin Drug Safety. 2020;19(3):295-314.
3. de Silva AV, Suraweera C, Ratnatunga SS, et al. Metformin in prevention and treatment of antipsychotic induced weight gain: a systematic review and meta-analysis. BMC Psychiatry. 2016;16(1):341.
4. Durell N, Franks R, Coon S, et al. Effects of antidepressants on glucagon-like peptide-1 receptor agonist-related weight loss. J Pharm Technol. 2022;38(5):283-288.
5. Larsen JR, Vedtofte L, Jakobsen MSL, et al. Effect of liraglutide treatment on prediabetes and overweight or obesity in clozapine- or olanzapine-treated patients with schizophrenia spectrum disorder: a randomized clinical trial. JAMA Psychiatry. 2017;74(7):719-728.
6. Aroda VR, Rosenstock J, Terauchi Y, et al. PIONEER 1: randomized clinical trial of the efficacy and safety of oral semaglutide monotherapy in comparison with placebo in patients with type 2 diabetes. Diabetes Care. 2019;42(9):1724-1732.
7. Wilding JPH, Batterham RL, Calanna S, et al. Once-weekly semaglutide in adults with overweight or obesity. N Engl J Med. 2021;384(11):989-1002.
8. Weghuber D, Barrett T, Barrientos-Pérez M, et al. Once-weekly semaglutide in adolescents with obesity. N Engl J Med. Published online November 2, 2022. doi:10.1056/NEJMoa2208601.
9. Pratley R, Amod A, Hoff ST, et al. Oral semaglutide versus subcutaneous liraglutide and placebo in type 2 diabetes (PIONEER 4): a randomized, double-blind, phase 3a trial. Lancet. 2019;394(10192):39-50.
10. Jastreboff AM, Aronne LJ, Ahmad NN, et al. Tirzepatide once weekly for the treatment of obesity. N Engl J Med. 2022;387(3):205-216.
11. Frías JP, Davies MJ, Rosenstock J, et al. Tirzepatide versus semaglutide once weekly in patients with type 2 diabetes. N Engl J Med. 2021;385(6):503-515.
Managing excited catatonia: A suggested approach
Catatonia is often difficult to identify and treat. The excited catatonia subtype can be particularly challenging to diagnose because it can present with symptoms similar to those seen in mania or psychosis. In this article, we present 3 cases of excited catatonia that illustrate how to identify it, how to treat the catatonia as well as the underlying pathology, and factors to consider during this process to mitigate the risk of adverse outcomes. We also outline a treatment algorithm we used for the 3 cases. Although we describe using this approach for patients with excited catatonia, it is generalizable to other types of catatonia.
Many causes, varying presentations
Catatonia is a psychomotor syndrome characterized by mutism, negativism, stereotypy, waxy flexibility, and other symptoms.1 It is defined by the presence of ≥3 of the 12 symptoms listed in the Table.2 Causes of catatonia include metabolic abnormalities, endocrine disorders, drug intoxication, neurodevelopmental disorders, medication adverse effects, psychosis, and mood disorders.1,3
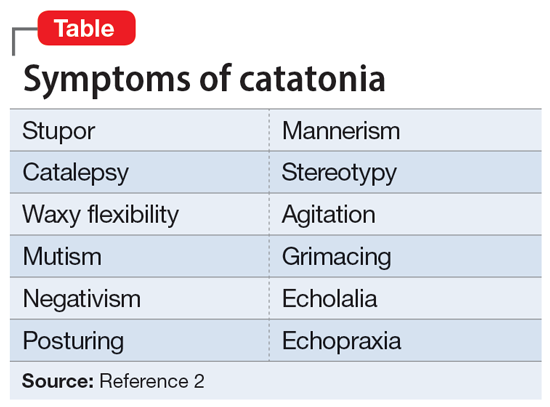
A subtype of this syndrome, excited catatonia, can present with restlessness, agitation, emotional lability, poor sleep, and altered mental status in addition to the more typical symptoms.1,4 Because excited catatonia can resemble mania or psychosis, it is particularly challenging to identify the underlying disorder causing it and appropriate treatment. Fink et al4 discussed how clinicians have interpreted the different presentations of excited catatonia to gain insight into the underlying diagnosis. If the patient’s thought process appears disorganized, psychosis may be suspected.4 If the patient is delusional and grandiose, they may be manic, and when altered mental status dominates the presentation, delirium may be the culprit.4
Regardless of the underlying cause, the first step is to treat the catatonia. Benzodiazepines and electroconvulsive therapy (ECT) are the most well validated treatments for catatonia and have been used to treat excited catatonia.1 Excited catatonia is often misdiagnosed and subsequently mistreated. In the following 3 cases, excited catatonia was successfully identified and treated using the same approach (Figure).
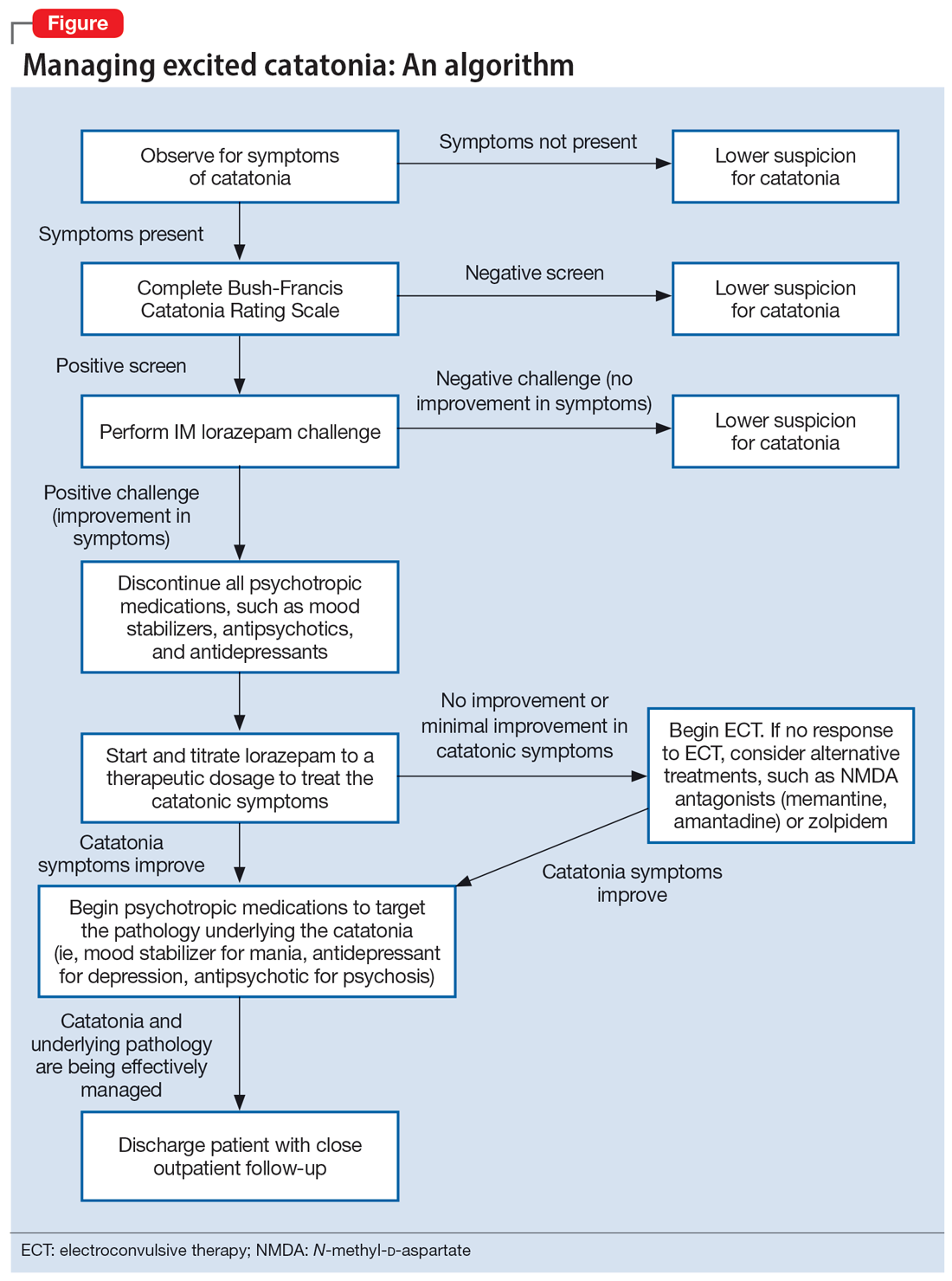
Case 1
Mr. A, age 27, has a history of bipolar I disorder. He was brought to the hospital by ambulance after being found to be yelling and acting belligerently, and he was admitted to the inpatient psychiatry unit for manic decompensation due to medication nonadherence. He was started on divalproex sodium 500 mg twice a day for mood stabilization, risperidone 1 mg twice a day for adjunct mood stabilization and psychosis, and lorazepam 1 mg 3 times a day for agitation. Mr. A exhibited odd behavior; he would take off his clothes in the hallway, run around the unit, and randomly yell at staff or to himself. At other times, he would stay silent, repeat the same statements, or oddly posture in the hallway for minutes at a time. These behaviors were seen primarily in the hour or 2 preceding lorazepam administration and improved after he received lorazepam.
Mr. A’s treating team completed the Bush-Francis Catatonia Rating Scale (BFCRS), which yielded a positive catatonia screen of 7/14. As a result, divalproex sodium and risperidone were held, and lorazepam was increased to 2 mg twice a day.
After several days, Mr. A was no longer acting oddly and was able to speak more spontaneously; however, he began to exhibit overt signs of mania. He would speak rapidly and make grandiose claims about managing millions of dollars as the CEO of a famous company. Divalproex sodium was restarted at 500 mg twice a day and increased to 500 mg 3 times a day for mood stabilization. Mr. A continued to receive lorazepam 2 mg 3 times a day for catatonia, and risperidone was restarted at 1 mg twice a day to more effectively target his manic symptoms. Risperidone was increased to 2 mg twice a day. After this change, Mr. A’s grandiosity dissipated, his speech normalized, and his thought process became organized. He was discharged on lorazepam 2 mg 3 times a day, divalproex sodium 500 mg 3 times a day, and risperidone 2 mg twice a day. Mr. A’s length of stay (LOS) for this admission was 11 days.
Continue to: Case 2
Case 2
Mr. B, age 49, presented with irritability and odd posturing. He has a history of schizoaffective disorder, bipolar type for which he was receiving a maintenance regimen of lithium 600 mg/d at bedtime and risperidone 2 mg/d at bedtime. He had multiple previous psychiatric admissions for catatonia. On this admission, Mr. B was irritable and difficult to redirect. He yelled at staff members and had a stiff gait. The BFCRS yielded a positive screening score of 3/14 and a severity score of 8/23. As a result, the treatment team conducted a lorazepam challenge.
After Mr. B received lorazepam 1 mg IM, his thought organization and irritability improved, which allowed him to have a coherent conversation with the interviewer. His gait stiffness also improved. His risperidone and lithium were held, and oral lorazepam 1 mg 3 times a day was started for catatonia. Lorazepam was gradually increased to 4 mg 3 times a day. Mr. B became euthymic and redirectable, and had an improved gait. However, he was also tangential and hyperverbal; these symptoms were indicative of the underlying mania that precipitated his catatonia.
Divalproex sodium extended release (ER) was started and increased to 1,500 mg/d at bedtime for mood stabilization. Lithium was restarted and increased to 300 mg twice a day for adjunct mood stabilization. Risperidone was not restarted. Toward the end of his admission, Mr. B was noted to be overly sedated, so the lorazepam dosage was decreased. He was discharged on lorazepam 2 mg 3 times a day, divalproex sodium ER 1,500 mg/d at bedtime, and lithium 300 mg twice a day. At discharge, Mr. B was calm and euthymic, with a linear thought process. His LOS was 25 days.
Case 3
Mr. C, age 62, presented to the emergency department (ED) because he had exhibited erratic behavior and had not slept for the past week. He has a history of bipolar I disorder, hypothyroidism, diabetes, and hypertension. For many years, he had been stable on divalproex sodium ER 2,500 mg/d at bedtime for mood stabilization and clozapine 100 mg/d at bedtime for adjunct mood stabilization and psychosis. In the ED, Mr. C was irritable, distractible, and tangential. On admission, he was speaking slowly with increased speech latency in response to questions, exhibiting stereotypy, repeating statements over and over, and walking very slowly.
The BFCRS yielded a positive screening score of 5/14 and a severity score of 10/23. Lorazepam 1 mg IM was administered. After 15 minutes, Mr. C’s speech, gait, and distractibility improved. As a result, clozapine and divalproex sodium were held, and he was started on oral lorazepam 1 mg 3 times a day. After several days, Mr. C was speaking fluently and no longer exhibiting stereotypy or having outbursts where he would make repetitive statements. However, he was tangential and irritable at times, which were signs of his underlying mania. Divalproex sodium ER was restarted at 250 mg/d at bedtime for mood stabilization and gradually increased to 2,500 mg/d at bedtime. Clozapine was also restarted at 25 mg/d at bedtime and gradually increased to 200 mg/d at bedtime. The lorazepam was gradually tapered and discontinued over the course of 3 weeks due to oversedation.
Continue to: At discharge...
At discharge, Mr. C was euthymic, calm, linear, and goal-directed. He was discharged on divalproex sodium ER 2,500 mg/d at bedtime and clozapine 200 mg/d at bedtime. His LOS for this admission was 22 days.
A stepwise approach can improve outcomes
The Figure outlines the method we used to manage excited catatonia in these 3 cases. Each of these patients exhibited signs of excited catatonia, but because those symptoms were nearly identical to those of mania, it was initially difficult to identify catatonia. Excited catatonia was suspected after more typical catatonic symptoms—such as a stiff gait, slowed speech, and stereotypy—were observed. The BFCRS was completed to get an objective measure of the likelihood that the patient was catatonic. In all 3 cases, the BFCRS resulted in a positive screen for catatonia. Following this, the patients described in Case 2 and Case 3 received a lorazepam challenge, which confirmed their catatonia. No lorazepam challenge was performed in Case 1 because the patient was already receiving lorazepam when the BFCRS was completed. Although most catatonic patients will respond to a lorazepam challenge, not all will. Therefore, clinicians should maintain some degree of suspicion for catatonia if a patient has a positive screen on the BFCRS but a negative lorazepam challenge.
In all 3 cases, after catatonia was confirmed, the patient’s psychotropic medications were discontinued. In all 3 cases, the antipsychotic was held to prevent progression to neuroleptic malignant syndrome (NMS) or malignant catatonia. Rasmussen et al3 found that 3.6% of the catatonic patients in their sample who were treated with antipsychotics developed NMS. A review of prospective studies looking at patients treated with antipsychotics found the incidence of NMS was .07% to 1.8%.5 Because NMS is often clinically indistinguishable from malignant catatonia,4,6 this incidence of NMS may have represented an increased incidence in malignant catatonia.
In all 3 cases, the mood stabilizer was held to prevent it from complicating the clinical picture. Discontinuing the mood stabilizer and focusing on treating the catatonia before targeting the underlying mania increased the likelihood of differentiating the patient’s catatonic symptoms from manic symptoms. This resulted in more precise medication selection and titration by allowing us to identify the specific symptoms that were being targeted by each medication.
Oral lorazepam was prescribed to target catatonia in all 3 cases, and the dosage was gradually increased until symptoms began to resolve. As the catatonia resolved, the manic symptoms became more easily identifiable, and at this point a mood stabilizer was started and titrated to a therapeutic dose to target the mania. In Case 1 and Case 3, the antipsychotic was restarted to treat the mania more effectively. It was not restarted in Case 2 because the patient’s mania was effectively being managed by 2 mood stabilizers. The risks and benefits of starting an antipsychotic in a catatonic or recently catatonic patient should be carefully considered. In the 2 cases where the antipsychotic was restarted, the patients were closely monitored, and there were no signs of NMS or malignant catatonia.
Continue to: As discharge approached...
As discharge approached, the dosages of oral lorazepam were reevaluated. Catatonic patients can typically tolerate high doses of benzodiazepines without becoming overly sedated, but each patient has a different threshold at which the dosage causes oversedation. In all 3 patients, lorazepam was initially titrated to a dose that treated their catatonic symptoms without causing intolerable sedation. In Case 2 and Case 3, as the catatonia began to resolve, the patients became increasingly sedated on their existing lorazepam dosage, so it was decreased. Because the patient in Case 1 did not become overly sedated, his lorazepam dosage did not need to be reduced.
For 2 of these patients, our approach resulted in a shorter LOS compared to their previous hospitalizations. The LOS in Case 2 was 25 days; 5 years earlier, he had a 49-day LOS for mania and catatonia. During the past admission, the identification and treatment of the catatonia was delayed, which resulted in the patient requiring multiple transfers to the medical unit for unstable vital signs. The LOS in Case 3 was 22 days; 6 months prior to this admission, the patient had 2 psychiatric admissions that totaled 37 days. Although the patient’s presentation in the 2 previous admissions was similar to his presentation as described in Case 3, catatonia had not been identified or treated in either admission. Since his catatonia and mania were treated in Case 3, he has not required a readmission. The patient in Case 1 was previously hospitalized, but information about the LOS of these admissions was not available. These results suggest that early identification and treatment of catatonia via the approach we used can improve patient outcomes.
Bottom Line
Excited catatonia can be challenging to diagnose and treat because it can present with symptoms similar to those seen in mania or psychosis. We describe 3 cases in which we used a stepwise approach to optimize treatment and improve outcomes for patients with excited catatonia. This approach may work equally well for other catatonia subtypes.
Related Resources
- Dubovsky SL, Dubovsky AN. Catatonia: how to identify and treat it. Current Psychiatry. 2018;17(8):16-26.
- Crouse EL, Joel B. Moran JB. Catatonia: recognition, management, and prevention of complications. Current Psychiatry. 2018;17(12):45-49.
Drug Brand Names
Clozapine • Clozaril
Lithium • Eskalith, Lithobid
Lorazepam • Ativan
Risperidone • Risperdal
Divalproex sodium • Depakote
1. Fink M, Taylor MA. The many varieties of catatonia. Eur Arch Psychiatry Clin Neurosci. 2001;251(Suppl 1):8-13.
2. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. American Psychiatric Association; 2013:119-121.
3. Rasmussen SA, Mazurek MF, Rosebush PI. Catatonia: our current understanding of its diagnosis, treatment and pathophysiology. World J Psychiatry. 2016;6(4):391-398.
4. Fink M, Taylor MA. Catatonia: A Clinician’s Guide to Diagnosis and Treatment. Cambridge University Press; 2003.
5. Adityanjee, Aderibigbe YA, Matthews T. Epidemiology of neuroleptic malignant syndrome. Clin Neuropharmacol. 1999;22(3):151-158.
6. Strawn JR, Keck PE Jr, Caroff SN. Neuroleptic malignant syndrome. Am J Psychiatry. 2007;164(6):870-876.
Catatonia is often difficult to identify and treat. The excited catatonia subtype can be particularly challenging to diagnose because it can present with symptoms similar to those seen in mania or psychosis. In this article, we present 3 cases of excited catatonia that illustrate how to identify it, how to treat the catatonia as well as the underlying pathology, and factors to consider during this process to mitigate the risk of adverse outcomes. We also outline a treatment algorithm we used for the 3 cases. Although we describe using this approach for patients with excited catatonia, it is generalizable to other types of catatonia.
Many causes, varying presentations
Catatonia is a psychomotor syndrome characterized by mutism, negativism, stereotypy, waxy flexibility, and other symptoms.1 It is defined by the presence of ≥3 of the 12 symptoms listed in the Table.2 Causes of catatonia include metabolic abnormalities, endocrine disorders, drug intoxication, neurodevelopmental disorders, medication adverse effects, psychosis, and mood disorders.1,3

A subtype of this syndrome, excited catatonia, can present with restlessness, agitation, emotional lability, poor sleep, and altered mental status in addition to the more typical symptoms.1,4 Because excited catatonia can resemble mania or psychosis, it is particularly challenging to identify the underlying disorder causing it and appropriate treatment. Fink et al4 discussed how clinicians have interpreted the different presentations of excited catatonia to gain insight into the underlying diagnosis. If the patient’s thought process appears disorganized, psychosis may be suspected.4 If the patient is delusional and grandiose, they may be manic, and when altered mental status dominates the presentation, delirium may be the culprit.4
Regardless of the underlying cause, the first step is to treat the catatonia. Benzodiazepines and electroconvulsive therapy (ECT) are the most well validated treatments for catatonia and have been used to treat excited catatonia.1 Excited catatonia is often misdiagnosed and subsequently mistreated. In the following 3 cases, excited catatonia was successfully identified and treated using the same approach (Figure).

Case 1
Mr. A, age 27, has a history of bipolar I disorder. He was brought to the hospital by ambulance after being found to be yelling and acting belligerently, and he was admitted to the inpatient psychiatry unit for manic decompensation due to medication nonadherence. He was started on divalproex sodium 500 mg twice a day for mood stabilization, risperidone 1 mg twice a day for adjunct mood stabilization and psychosis, and lorazepam 1 mg 3 times a day for agitation. Mr. A exhibited odd behavior; he would take off his clothes in the hallway, run around the unit, and randomly yell at staff or to himself. At other times, he would stay silent, repeat the same statements, or oddly posture in the hallway for minutes at a time. These behaviors were seen primarily in the hour or 2 preceding lorazepam administration and improved after he received lorazepam.
Mr. A’s treating team completed the Bush-Francis Catatonia Rating Scale (BFCRS), which yielded a positive catatonia screen of 7/14. As a result, divalproex sodium and risperidone were held, and lorazepam was increased to 2 mg twice a day.
After several days, Mr. A was no longer acting oddly and was able to speak more spontaneously; however, he began to exhibit overt signs of mania. He would speak rapidly and make grandiose claims about managing millions of dollars as the CEO of a famous company. Divalproex sodium was restarted at 500 mg twice a day and increased to 500 mg 3 times a day for mood stabilization. Mr. A continued to receive lorazepam 2 mg 3 times a day for catatonia, and risperidone was restarted at 1 mg twice a day to more effectively target his manic symptoms. Risperidone was increased to 2 mg twice a day. After this change, Mr. A’s grandiosity dissipated, his speech normalized, and his thought process became organized. He was discharged on lorazepam 2 mg 3 times a day, divalproex sodium 500 mg 3 times a day, and risperidone 2 mg twice a day. Mr. A’s length of stay (LOS) for this admission was 11 days.
Continue to: Case 2
Case 2
Mr. B, age 49, presented with irritability and odd posturing. He has a history of schizoaffective disorder, bipolar type for which he was receiving a maintenance regimen of lithium 600 mg/d at bedtime and risperidone 2 mg/d at bedtime. He had multiple previous psychiatric admissions for catatonia. On this admission, Mr. B was irritable and difficult to redirect. He yelled at staff members and had a stiff gait. The BFCRS yielded a positive screening score of 3/14 and a severity score of 8/23. As a result, the treatment team conducted a lorazepam challenge.
After Mr. B received lorazepam 1 mg IM, his thought organization and irritability improved, which allowed him to have a coherent conversation with the interviewer. His gait stiffness also improved. His risperidone and lithium were held, and oral lorazepam 1 mg 3 times a day was started for catatonia. Lorazepam was gradually increased to 4 mg 3 times a day. Mr. B became euthymic and redirectable, and had an improved gait. However, he was also tangential and hyperverbal; these symptoms were indicative of the underlying mania that precipitated his catatonia.
Divalproex sodium extended release (ER) was started and increased to 1,500 mg/d at bedtime for mood stabilization. Lithium was restarted and increased to 300 mg twice a day for adjunct mood stabilization. Risperidone was not restarted. Toward the end of his admission, Mr. B was noted to be overly sedated, so the lorazepam dosage was decreased. He was discharged on lorazepam 2 mg 3 times a day, divalproex sodium ER 1,500 mg/d at bedtime, and lithium 300 mg twice a day. At discharge, Mr. B was calm and euthymic, with a linear thought process. His LOS was 25 days.
Case 3
Mr. C, age 62, presented to the emergency department (ED) because he had exhibited erratic behavior and had not slept for the past week. He has a history of bipolar I disorder, hypothyroidism, diabetes, and hypertension. For many years, he had been stable on divalproex sodium ER 2,500 mg/d at bedtime for mood stabilization and clozapine 100 mg/d at bedtime for adjunct mood stabilization and psychosis. In the ED, Mr. C was irritable, distractible, and tangential. On admission, he was speaking slowly with increased speech latency in response to questions, exhibiting stereotypy, repeating statements over and over, and walking very slowly.
The BFCRS yielded a positive screening score of 5/14 and a severity score of 10/23. Lorazepam 1 mg IM was administered. After 15 minutes, Mr. C’s speech, gait, and distractibility improved. As a result, clozapine and divalproex sodium were held, and he was started on oral lorazepam 1 mg 3 times a day. After several days, Mr. C was speaking fluently and no longer exhibiting stereotypy or having outbursts where he would make repetitive statements. However, he was tangential and irritable at times, which were signs of his underlying mania. Divalproex sodium ER was restarted at 250 mg/d at bedtime for mood stabilization and gradually increased to 2,500 mg/d at bedtime. Clozapine was also restarted at 25 mg/d at bedtime and gradually increased to 200 mg/d at bedtime. The lorazepam was gradually tapered and discontinued over the course of 3 weeks due to oversedation.
Continue to: At discharge...
At discharge, Mr. C was euthymic, calm, linear, and goal-directed. He was discharged on divalproex sodium ER 2,500 mg/d at bedtime and clozapine 200 mg/d at bedtime. His LOS for this admission was 22 days.
A stepwise approach can improve outcomes
The Figure outlines the method we used to manage excited catatonia in these 3 cases. Each of these patients exhibited signs of excited catatonia, but because those symptoms were nearly identical to those of mania, it was initially difficult to identify catatonia. Excited catatonia was suspected after more typical catatonic symptoms—such as a stiff gait, slowed speech, and stereotypy—were observed. The BFCRS was completed to get an objective measure of the likelihood that the patient was catatonic. In all 3 cases, the BFCRS resulted in a positive screen for catatonia. Following this, the patients described in Case 2 and Case 3 received a lorazepam challenge, which confirmed their catatonia. No lorazepam challenge was performed in Case 1 because the patient was already receiving lorazepam when the BFCRS was completed. Although most catatonic patients will respond to a lorazepam challenge, not all will. Therefore, clinicians should maintain some degree of suspicion for catatonia if a patient has a positive screen on the BFCRS but a negative lorazepam challenge.
In all 3 cases, after catatonia was confirmed, the patient’s psychotropic medications were discontinued. In all 3 cases, the antipsychotic was held to prevent progression to neuroleptic malignant syndrome (NMS) or malignant catatonia. Rasmussen et al3 found that 3.6% of the catatonic patients in their sample who were treated with antipsychotics developed NMS. A review of prospective studies looking at patients treated with antipsychotics found the incidence of NMS was .07% to 1.8%.5 Because NMS is often clinically indistinguishable from malignant catatonia,4,6 this incidence of NMS may have represented an increased incidence in malignant catatonia.
In all 3 cases, the mood stabilizer was held to prevent it from complicating the clinical picture. Discontinuing the mood stabilizer and focusing on treating the catatonia before targeting the underlying mania increased the likelihood of differentiating the patient’s catatonic symptoms from manic symptoms. This resulted in more precise medication selection and titration by allowing us to identify the specific symptoms that were being targeted by each medication.
Oral lorazepam was prescribed to target catatonia in all 3 cases, and the dosage was gradually increased until symptoms began to resolve. As the catatonia resolved, the manic symptoms became more easily identifiable, and at this point a mood stabilizer was started and titrated to a therapeutic dose to target the mania. In Case 1 and Case 3, the antipsychotic was restarted to treat the mania more effectively. It was not restarted in Case 2 because the patient’s mania was effectively being managed by 2 mood stabilizers. The risks and benefits of starting an antipsychotic in a catatonic or recently catatonic patient should be carefully considered. In the 2 cases where the antipsychotic was restarted, the patients were closely monitored, and there were no signs of NMS or malignant catatonia.
Continue to: As discharge approached...
As discharge approached, the dosages of oral lorazepam were reevaluated. Catatonic patients can typically tolerate high doses of benzodiazepines without becoming overly sedated, but each patient has a different threshold at which the dosage causes oversedation. In all 3 patients, lorazepam was initially titrated to a dose that treated their catatonic symptoms without causing intolerable sedation. In Case 2 and Case 3, as the catatonia began to resolve, the patients became increasingly sedated on their existing lorazepam dosage, so it was decreased. Because the patient in Case 1 did not become overly sedated, his lorazepam dosage did not need to be reduced.
For 2 of these patients, our approach resulted in a shorter LOS compared to their previous hospitalizations. The LOS in Case 2 was 25 days; 5 years earlier, he had a 49-day LOS for mania and catatonia. During the past admission, the identification and treatment of the catatonia was delayed, which resulted in the patient requiring multiple transfers to the medical unit for unstable vital signs. The LOS in Case 3 was 22 days; 6 months prior to this admission, the patient had 2 psychiatric admissions that totaled 37 days. Although the patient’s presentation in the 2 previous admissions was similar to his presentation as described in Case 3, catatonia had not been identified or treated in either admission. Since his catatonia and mania were treated in Case 3, he has not required a readmission. The patient in Case 1 was previously hospitalized, but information about the LOS of these admissions was not available. These results suggest that early identification and treatment of catatonia via the approach we used can improve patient outcomes.
Bottom Line
Excited catatonia can be challenging to diagnose and treat because it can present with symptoms similar to those seen in mania or psychosis. We describe 3 cases in which we used a stepwise approach to optimize treatment and improve outcomes for patients with excited catatonia. This approach may work equally well for other catatonia subtypes.
Related Resources
- Dubovsky SL, Dubovsky AN. Catatonia: how to identify and treat it. Current Psychiatry. 2018;17(8):16-26.
- Crouse EL, Joel B. Moran JB. Catatonia: recognition, management, and prevention of complications. Current Psychiatry. 2018;17(12):45-49.
Drug Brand Names
Clozapine • Clozaril
Lithium • Eskalith, Lithobid
Lorazepam • Ativan
Risperidone • Risperdal
Divalproex sodium • Depakote
Catatonia is often difficult to identify and treat. The excited catatonia subtype can be particularly challenging to diagnose because it can present with symptoms similar to those seen in mania or psychosis. In this article, we present 3 cases of excited catatonia that illustrate how to identify it, how to treat the catatonia as well as the underlying pathology, and factors to consider during this process to mitigate the risk of adverse outcomes. We also outline a treatment algorithm we used for the 3 cases. Although we describe using this approach for patients with excited catatonia, it is generalizable to other types of catatonia.
Many causes, varying presentations
Catatonia is a psychomotor syndrome characterized by mutism, negativism, stereotypy, waxy flexibility, and other symptoms.1 It is defined by the presence of ≥3 of the 12 symptoms listed in the Table.2 Causes of catatonia include metabolic abnormalities, endocrine disorders, drug intoxication, neurodevelopmental disorders, medication adverse effects, psychosis, and mood disorders.1,3

A subtype of this syndrome, excited catatonia, can present with restlessness, agitation, emotional lability, poor sleep, and altered mental status in addition to the more typical symptoms.1,4 Because excited catatonia can resemble mania or psychosis, it is particularly challenging to identify the underlying disorder causing it and appropriate treatment. Fink et al4 discussed how clinicians have interpreted the different presentations of excited catatonia to gain insight into the underlying diagnosis. If the patient’s thought process appears disorganized, psychosis may be suspected.4 If the patient is delusional and grandiose, they may be manic, and when altered mental status dominates the presentation, delirium may be the culprit.4
Regardless of the underlying cause, the first step is to treat the catatonia. Benzodiazepines and electroconvulsive therapy (ECT) are the most well validated treatments for catatonia and have been used to treat excited catatonia.1 Excited catatonia is often misdiagnosed and subsequently mistreated. In the following 3 cases, excited catatonia was successfully identified and treated using the same approach (Figure).

Case 1
Mr. A, age 27, has a history of bipolar I disorder. He was brought to the hospital by ambulance after being found to be yelling and acting belligerently, and he was admitted to the inpatient psychiatry unit for manic decompensation due to medication nonadherence. He was started on divalproex sodium 500 mg twice a day for mood stabilization, risperidone 1 mg twice a day for adjunct mood stabilization and psychosis, and lorazepam 1 mg 3 times a day for agitation. Mr. A exhibited odd behavior; he would take off his clothes in the hallway, run around the unit, and randomly yell at staff or to himself. At other times, he would stay silent, repeat the same statements, or oddly posture in the hallway for minutes at a time. These behaviors were seen primarily in the hour or 2 preceding lorazepam administration and improved after he received lorazepam.
Mr. A’s treating team completed the Bush-Francis Catatonia Rating Scale (BFCRS), which yielded a positive catatonia screen of 7/14. As a result, divalproex sodium and risperidone were held, and lorazepam was increased to 2 mg twice a day.
After several days, Mr. A was no longer acting oddly and was able to speak more spontaneously; however, he began to exhibit overt signs of mania. He would speak rapidly and make grandiose claims about managing millions of dollars as the CEO of a famous company. Divalproex sodium was restarted at 500 mg twice a day and increased to 500 mg 3 times a day for mood stabilization. Mr. A continued to receive lorazepam 2 mg 3 times a day for catatonia, and risperidone was restarted at 1 mg twice a day to more effectively target his manic symptoms. Risperidone was increased to 2 mg twice a day. After this change, Mr. A’s grandiosity dissipated, his speech normalized, and his thought process became organized. He was discharged on lorazepam 2 mg 3 times a day, divalproex sodium 500 mg 3 times a day, and risperidone 2 mg twice a day. Mr. A’s length of stay (LOS) for this admission was 11 days.
Continue to: Case 2
Case 2
Mr. B, age 49, presented with irritability and odd posturing. He has a history of schizoaffective disorder, bipolar type for which he was receiving a maintenance regimen of lithium 600 mg/d at bedtime and risperidone 2 mg/d at bedtime. He had multiple previous psychiatric admissions for catatonia. On this admission, Mr. B was irritable and difficult to redirect. He yelled at staff members and had a stiff gait. The BFCRS yielded a positive screening score of 3/14 and a severity score of 8/23. As a result, the treatment team conducted a lorazepam challenge.
After Mr. B received lorazepam 1 mg IM, his thought organization and irritability improved, which allowed him to have a coherent conversation with the interviewer. His gait stiffness also improved. His risperidone and lithium were held, and oral lorazepam 1 mg 3 times a day was started for catatonia. Lorazepam was gradually increased to 4 mg 3 times a day. Mr. B became euthymic and redirectable, and had an improved gait. However, he was also tangential and hyperverbal; these symptoms were indicative of the underlying mania that precipitated his catatonia.
Divalproex sodium extended release (ER) was started and increased to 1,500 mg/d at bedtime for mood stabilization. Lithium was restarted and increased to 300 mg twice a day for adjunct mood stabilization. Risperidone was not restarted. Toward the end of his admission, Mr. B was noted to be overly sedated, so the lorazepam dosage was decreased. He was discharged on lorazepam 2 mg 3 times a day, divalproex sodium ER 1,500 mg/d at bedtime, and lithium 300 mg twice a day. At discharge, Mr. B was calm and euthymic, with a linear thought process. His LOS was 25 days.
Case 3
Mr. C, age 62, presented to the emergency department (ED) because he had exhibited erratic behavior and had not slept for the past week. He has a history of bipolar I disorder, hypothyroidism, diabetes, and hypertension. For many years, he had been stable on divalproex sodium ER 2,500 mg/d at bedtime for mood stabilization and clozapine 100 mg/d at bedtime for adjunct mood stabilization and psychosis. In the ED, Mr. C was irritable, distractible, and tangential. On admission, he was speaking slowly with increased speech latency in response to questions, exhibiting stereotypy, repeating statements over and over, and walking very slowly.
The BFCRS yielded a positive screening score of 5/14 and a severity score of 10/23. Lorazepam 1 mg IM was administered. After 15 minutes, Mr. C’s speech, gait, and distractibility improved. As a result, clozapine and divalproex sodium were held, and he was started on oral lorazepam 1 mg 3 times a day. After several days, Mr. C was speaking fluently and no longer exhibiting stereotypy or having outbursts where he would make repetitive statements. However, he was tangential and irritable at times, which were signs of his underlying mania. Divalproex sodium ER was restarted at 250 mg/d at bedtime for mood stabilization and gradually increased to 2,500 mg/d at bedtime. Clozapine was also restarted at 25 mg/d at bedtime and gradually increased to 200 mg/d at bedtime. The lorazepam was gradually tapered and discontinued over the course of 3 weeks due to oversedation.
Continue to: At discharge...
At discharge, Mr. C was euthymic, calm, linear, and goal-directed. He was discharged on divalproex sodium ER 2,500 mg/d at bedtime and clozapine 200 mg/d at bedtime. His LOS for this admission was 22 days.
A stepwise approach can improve outcomes
The Figure outlines the method we used to manage excited catatonia in these 3 cases. Each of these patients exhibited signs of excited catatonia, but because those symptoms were nearly identical to those of mania, it was initially difficult to identify catatonia. Excited catatonia was suspected after more typical catatonic symptoms—such as a stiff gait, slowed speech, and stereotypy—were observed. The BFCRS was completed to get an objective measure of the likelihood that the patient was catatonic. In all 3 cases, the BFCRS resulted in a positive screen for catatonia. Following this, the patients described in Case 2 and Case 3 received a lorazepam challenge, which confirmed their catatonia. No lorazepam challenge was performed in Case 1 because the patient was already receiving lorazepam when the BFCRS was completed. Although most catatonic patients will respond to a lorazepam challenge, not all will. Therefore, clinicians should maintain some degree of suspicion for catatonia if a patient has a positive screen on the BFCRS but a negative lorazepam challenge.
In all 3 cases, after catatonia was confirmed, the patient’s psychotropic medications were discontinued. In all 3 cases, the antipsychotic was held to prevent progression to neuroleptic malignant syndrome (NMS) or malignant catatonia. Rasmussen et al3 found that 3.6% of the catatonic patients in their sample who were treated with antipsychotics developed NMS. A review of prospective studies looking at patients treated with antipsychotics found the incidence of NMS was .07% to 1.8%.5 Because NMS is often clinically indistinguishable from malignant catatonia,4,6 this incidence of NMS may have represented an increased incidence in malignant catatonia.
In all 3 cases, the mood stabilizer was held to prevent it from complicating the clinical picture. Discontinuing the mood stabilizer and focusing on treating the catatonia before targeting the underlying mania increased the likelihood of differentiating the patient’s catatonic symptoms from manic symptoms. This resulted in more precise medication selection and titration by allowing us to identify the specific symptoms that were being targeted by each medication.
Oral lorazepam was prescribed to target catatonia in all 3 cases, and the dosage was gradually increased until symptoms began to resolve. As the catatonia resolved, the manic symptoms became more easily identifiable, and at this point a mood stabilizer was started and titrated to a therapeutic dose to target the mania. In Case 1 and Case 3, the antipsychotic was restarted to treat the mania more effectively. It was not restarted in Case 2 because the patient’s mania was effectively being managed by 2 mood stabilizers. The risks and benefits of starting an antipsychotic in a catatonic or recently catatonic patient should be carefully considered. In the 2 cases where the antipsychotic was restarted, the patients were closely monitored, and there were no signs of NMS or malignant catatonia.
Continue to: As discharge approached...
As discharge approached, the dosages of oral lorazepam were reevaluated. Catatonic patients can typically tolerate high doses of benzodiazepines without becoming overly sedated, but each patient has a different threshold at which the dosage causes oversedation. In all 3 patients, lorazepam was initially titrated to a dose that treated their catatonic symptoms without causing intolerable sedation. In Case 2 and Case 3, as the catatonia began to resolve, the patients became increasingly sedated on their existing lorazepam dosage, so it was decreased. Because the patient in Case 1 did not become overly sedated, his lorazepam dosage did not need to be reduced.
For 2 of these patients, our approach resulted in a shorter LOS compared to their previous hospitalizations. The LOS in Case 2 was 25 days; 5 years earlier, he had a 49-day LOS for mania and catatonia. During the past admission, the identification and treatment of the catatonia was delayed, which resulted in the patient requiring multiple transfers to the medical unit for unstable vital signs. The LOS in Case 3 was 22 days; 6 months prior to this admission, the patient had 2 psychiatric admissions that totaled 37 days. Although the patient’s presentation in the 2 previous admissions was similar to his presentation as described in Case 3, catatonia had not been identified or treated in either admission. Since his catatonia and mania were treated in Case 3, he has not required a readmission. The patient in Case 1 was previously hospitalized, but information about the LOS of these admissions was not available. These results suggest that early identification and treatment of catatonia via the approach we used can improve patient outcomes.
Bottom Line
Excited catatonia can be challenging to diagnose and treat because it can present with symptoms similar to those seen in mania or psychosis. We describe 3 cases in which we used a stepwise approach to optimize treatment and improve outcomes for patients with excited catatonia. This approach may work equally well for other catatonia subtypes.
Related Resources
- Dubovsky SL, Dubovsky AN. Catatonia: how to identify and treat it. Current Psychiatry. 2018;17(8):16-26.
- Crouse EL, Joel B. Moran JB. Catatonia: recognition, management, and prevention of complications. Current Psychiatry. 2018;17(12):45-49.
Drug Brand Names
Clozapine • Clozaril
Lithium • Eskalith, Lithobid
Lorazepam • Ativan
Risperidone • Risperdal
Divalproex sodium • Depakote
1. Fink M, Taylor MA. The many varieties of catatonia. Eur Arch Psychiatry Clin Neurosci. 2001;251(Suppl 1):8-13.
2. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. American Psychiatric Association; 2013:119-121.
3. Rasmussen SA, Mazurek MF, Rosebush PI. Catatonia: our current understanding of its diagnosis, treatment and pathophysiology. World J Psychiatry. 2016;6(4):391-398.
4. Fink M, Taylor MA. Catatonia: A Clinician’s Guide to Diagnosis and Treatment. Cambridge University Press; 2003.
5. Adityanjee, Aderibigbe YA, Matthews T. Epidemiology of neuroleptic malignant syndrome. Clin Neuropharmacol. 1999;22(3):151-158.
6. Strawn JR, Keck PE Jr, Caroff SN. Neuroleptic malignant syndrome. Am J Psychiatry. 2007;164(6):870-876.
1. Fink M, Taylor MA. The many varieties of catatonia. Eur Arch Psychiatry Clin Neurosci. 2001;251(Suppl 1):8-13.
2. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. American Psychiatric Association; 2013:119-121.
3. Rasmussen SA, Mazurek MF, Rosebush PI. Catatonia: our current understanding of its diagnosis, treatment and pathophysiology. World J Psychiatry. 2016;6(4):391-398.
4. Fink M, Taylor MA. Catatonia: A Clinician’s Guide to Diagnosis and Treatment. Cambridge University Press; 2003.
5. Adityanjee, Aderibigbe YA, Matthews T. Epidemiology of neuroleptic malignant syndrome. Clin Neuropharmacol. 1999;22(3):151-158.
6. Strawn JR, Keck PE Jr, Caroff SN. Neuroleptic malignant syndrome. Am J Psychiatry. 2007;164(6):870-876.
Intentional deaths continue to rise among U.S. children
The homicide rate among children in the United States rose by more than 4% per year since 2013 but jumped nearly 28% from 2019 to 2020, new data show.
Although long-term trends varied by region and demographics, with some groups and areas seeing declines in killings, the increases were the highest among Black children and boys aged 11-17, according to the researchers, who attribute the surge in violent deaths to a recent rise in firearm-related killings in children. Gun violence is now the leading cause of death for children in the United States, claiming what the American Academy of Pediatrics has equated to a classroomful of lives each day.
“There are troubling recent rate increases among several groups, warranting immediate attention, with some racial and ethnic disparities persisting for more than 20 years,” said Rebecca F. Wilson, PhD, of the U.S. Centers for Disease Control and Prevention, who helped conduct the study.
Dr. Wilson and her colleagues, whose findings appear in JAMA Pediatrics, examined data on 38,362 homicide victims in the United States aged 0-17 years who were killed between 1999 and 2020.
The nation’s overall homicide rate for youth fell by 5.6% per year from 2007 to 2013 before reversing course. Between 2013 and 2020, the overall rate rose 4.3% annually.
The figures show that not all children are affected equally. The rate of child homicide has fallen significantly for girls, infants, and children ages 5 years and under – whose deaths often result from caregiver neglect or violence – as well as Asian or Pacific Islanders, Whites, and those living in the Northeast.
But the child homicide rate in the South increased 6.4% per year between 2013 and 2020, while that of children in both rural America and in cities is also rising after years of decline, according to the researchers.
The suspected perpetrator was known in about 64% of child killings. Nearly 80% of those perpetrators were male.
Dr. Wilson and her colleagues also note that the COVID-19 pandemic appears to have precipitated a wave of gun-related violence among children – a link borne out by another recent paper in JAMA Pediatrics. (Recent data suggest that intentional firearm injuries are often misclassified as accidental.)
The study found that gun-related injuries in youth remained elevated through 2021, with non-Hispanic Black children and those with public insurance making up greater proportions of victims during the pandemic. The researchers identified 1,815 firearm injuries per month before the pandemic and 2,759 per month during the outbreak, a 52% increase.
Although the two studies look at different data, both show that Black children are most affected by gun violence, experts said.
“This demonstrates a critical issue for the medical, public health, and legal communities: While homicide is often presented as a criminal justice problem, it is increasingly a racial justice problem,” said Katherine E. Hoops, MD, of the Center for Gun Violence Solutions at Johns Hopkins Bloomberg School of Public Health, Baltimore.
In an editorial about the homicide study, researchers at the University of Pennsylvania, Philadelphia, called the violent deaths “preventable and unacceptable.” Eliminating such deaths “must be among our first priorities,” they wrote.
The editorial authors also noted that researchers know relatively little about nonfatal violent injuries such as those involving firearms. “These injuries are important not only because they may have life-altering consequences for children and families but also because understanding only the most severe form of any health condition (death) will hamper our ability to design and evaluate prevention strategies,” they wrote.
Dr. Wilson’s group identified different causes of youth homicide for different age groups – and the potential interventions for each differ. Although the youngest children are more likely to die from abuse or neglect, those aged 6-10 years were most likely to die by firearm, often associated with abuse that ends in suicide. Meanwhile, adolescents aged 11-17 were more subject to peer violence.
For Dr. Hoops, “each of these differences has important policy implications, including the need for policies that address structural racism, poverty, and systematic disadvantage – but also firearm safe storage to prevent youth violence and suicide [and] reduction of access to lethal means, such as through extreme risk protective orders when someone is at risk of harming themselves or others.”
Dr. Wilson agreed. “We know child homicides are preventable,” she said. “The rate decrease for some groups is encouraging, yet more can be done to protect all children.”
A version of this article first appeared on Medscape.com.
The homicide rate among children in the United States rose by more than 4% per year since 2013 but jumped nearly 28% from 2019 to 2020, new data show.
Although long-term trends varied by region and demographics, with some groups and areas seeing declines in killings, the increases were the highest among Black children and boys aged 11-17, according to the researchers, who attribute the surge in violent deaths to a recent rise in firearm-related killings in children. Gun violence is now the leading cause of death for children in the United States, claiming what the American Academy of Pediatrics has equated to a classroomful of lives each day.
“There are troubling recent rate increases among several groups, warranting immediate attention, with some racial and ethnic disparities persisting for more than 20 years,” said Rebecca F. Wilson, PhD, of the U.S. Centers for Disease Control and Prevention, who helped conduct the study.
Dr. Wilson and her colleagues, whose findings appear in JAMA Pediatrics, examined data on 38,362 homicide victims in the United States aged 0-17 years who were killed between 1999 and 2020.
The nation’s overall homicide rate for youth fell by 5.6% per year from 2007 to 2013 before reversing course. Between 2013 and 2020, the overall rate rose 4.3% annually.
The figures show that not all children are affected equally. The rate of child homicide has fallen significantly for girls, infants, and children ages 5 years and under – whose deaths often result from caregiver neglect or violence – as well as Asian or Pacific Islanders, Whites, and those living in the Northeast.
But the child homicide rate in the South increased 6.4% per year between 2013 and 2020, while that of children in both rural America and in cities is also rising after years of decline, according to the researchers.
The suspected perpetrator was known in about 64% of child killings. Nearly 80% of those perpetrators were male.
Dr. Wilson and her colleagues also note that the COVID-19 pandemic appears to have precipitated a wave of gun-related violence among children – a link borne out by another recent paper in JAMA Pediatrics. (Recent data suggest that intentional firearm injuries are often misclassified as accidental.)
The study found that gun-related injuries in youth remained elevated through 2021, with non-Hispanic Black children and those with public insurance making up greater proportions of victims during the pandemic. The researchers identified 1,815 firearm injuries per month before the pandemic and 2,759 per month during the outbreak, a 52% increase.
Although the two studies look at different data, both show that Black children are most affected by gun violence, experts said.
“This demonstrates a critical issue for the medical, public health, and legal communities: While homicide is often presented as a criminal justice problem, it is increasingly a racial justice problem,” said Katherine E. Hoops, MD, of the Center for Gun Violence Solutions at Johns Hopkins Bloomberg School of Public Health, Baltimore.
In an editorial about the homicide study, researchers at the University of Pennsylvania, Philadelphia, called the violent deaths “preventable and unacceptable.” Eliminating such deaths “must be among our first priorities,” they wrote.
The editorial authors also noted that researchers know relatively little about nonfatal violent injuries such as those involving firearms. “These injuries are important not only because they may have life-altering consequences for children and families but also because understanding only the most severe form of any health condition (death) will hamper our ability to design and evaluate prevention strategies,” they wrote.
Dr. Wilson’s group identified different causes of youth homicide for different age groups – and the potential interventions for each differ. Although the youngest children are more likely to die from abuse or neglect, those aged 6-10 years were most likely to die by firearm, often associated with abuse that ends in suicide. Meanwhile, adolescents aged 11-17 were more subject to peer violence.
For Dr. Hoops, “each of these differences has important policy implications, including the need for policies that address structural racism, poverty, and systematic disadvantage – but also firearm safe storage to prevent youth violence and suicide [and] reduction of access to lethal means, such as through extreme risk protective orders when someone is at risk of harming themselves or others.”
Dr. Wilson agreed. “We know child homicides are preventable,” she said. “The rate decrease for some groups is encouraging, yet more can be done to protect all children.”
A version of this article first appeared on Medscape.com.
The homicide rate among children in the United States rose by more than 4% per year since 2013 but jumped nearly 28% from 2019 to 2020, new data show.
Although long-term trends varied by region and demographics, with some groups and areas seeing declines in killings, the increases were the highest among Black children and boys aged 11-17, according to the researchers, who attribute the surge in violent deaths to a recent rise in firearm-related killings in children. Gun violence is now the leading cause of death for children in the United States, claiming what the American Academy of Pediatrics has equated to a classroomful of lives each day.
“There are troubling recent rate increases among several groups, warranting immediate attention, with some racial and ethnic disparities persisting for more than 20 years,” said Rebecca F. Wilson, PhD, of the U.S. Centers for Disease Control and Prevention, who helped conduct the study.
Dr. Wilson and her colleagues, whose findings appear in JAMA Pediatrics, examined data on 38,362 homicide victims in the United States aged 0-17 years who were killed between 1999 and 2020.
The nation’s overall homicide rate for youth fell by 5.6% per year from 2007 to 2013 before reversing course. Between 2013 and 2020, the overall rate rose 4.3% annually.
The figures show that not all children are affected equally. The rate of child homicide has fallen significantly for girls, infants, and children ages 5 years and under – whose deaths often result from caregiver neglect or violence – as well as Asian or Pacific Islanders, Whites, and those living in the Northeast.
But the child homicide rate in the South increased 6.4% per year between 2013 and 2020, while that of children in both rural America and in cities is also rising after years of decline, according to the researchers.
The suspected perpetrator was known in about 64% of child killings. Nearly 80% of those perpetrators were male.
Dr. Wilson and her colleagues also note that the COVID-19 pandemic appears to have precipitated a wave of gun-related violence among children – a link borne out by another recent paper in JAMA Pediatrics. (Recent data suggest that intentional firearm injuries are often misclassified as accidental.)
The study found that gun-related injuries in youth remained elevated through 2021, with non-Hispanic Black children and those with public insurance making up greater proportions of victims during the pandemic. The researchers identified 1,815 firearm injuries per month before the pandemic and 2,759 per month during the outbreak, a 52% increase.
Although the two studies look at different data, both show that Black children are most affected by gun violence, experts said.
“This demonstrates a critical issue for the medical, public health, and legal communities: While homicide is often presented as a criminal justice problem, it is increasingly a racial justice problem,” said Katherine E. Hoops, MD, of the Center for Gun Violence Solutions at Johns Hopkins Bloomberg School of Public Health, Baltimore.
In an editorial about the homicide study, researchers at the University of Pennsylvania, Philadelphia, called the violent deaths “preventable and unacceptable.” Eliminating such deaths “must be among our first priorities,” they wrote.
The editorial authors also noted that researchers know relatively little about nonfatal violent injuries such as those involving firearms. “These injuries are important not only because they may have life-altering consequences for children and families but also because understanding only the most severe form of any health condition (death) will hamper our ability to design and evaluate prevention strategies,” they wrote.
Dr. Wilson’s group identified different causes of youth homicide for different age groups – and the potential interventions for each differ. Although the youngest children are more likely to die from abuse or neglect, those aged 6-10 years were most likely to die by firearm, often associated with abuse that ends in suicide. Meanwhile, adolescents aged 11-17 were more subject to peer violence.
For Dr. Hoops, “each of these differences has important policy implications, including the need for policies that address structural racism, poverty, and systematic disadvantage – but also firearm safe storage to prevent youth violence and suicide [and] reduction of access to lethal means, such as through extreme risk protective orders when someone is at risk of harming themselves or others.”
Dr. Wilson agreed. “We know child homicides are preventable,” she said. “The rate decrease for some groups is encouraging, yet more can be done to protect all children.”
A version of this article first appeared on Medscape.com.