User login
Cardiovascular risk score multipliers suggested for rheumatic diseases
A re-evaluation of cardiovascular risk management guidelines intended for use by rheumatologists may be warranted based on findings from a recently published population-based study of the risks for 12 different cardiovascular disease outcomes in patients with autoimmune diseases.
“The notion that patients with rheumatic diseases are at increased risk of developing cardiovascular diseases has been ongoing for many years,” Nathalie Conrad, PhD, and coauthors wrote in a viewpoint article in Annals of the Rheumatic Diseases.
This has “sparked much debate concerning whether and when to initiate cardiovascular prevention therapies,” they said.
Dr. Conrad was first author on the population-based study published in The Lancet in August 2022 that used linked primary and secondary care records from datasets in the U.K. Clinical Practice Research Datalink involving individuals who were recently diagnosed with any of 19 different autoimmune diseases during an 18-year period stretching from 2000 to 2017 but free of cardiovascular disease until at least 12 months after incident autoimmune disease. “Every single autoimmune disorder we looked at was associated with increased cardiovascular risk,” Dr. Conrad, of the department of public health and primary care at Catholic University Leuven (Belgium), said in an interview.
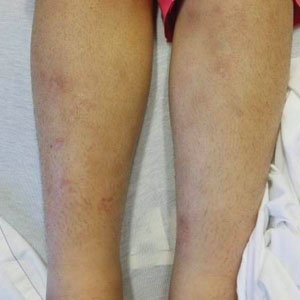
Not only was the risk for cardiovascular disease increased for people with rheumatic diseases by an average of 68%, compared with people without rheumatic diseases, but also the whole spectrum of cardiovascular disorders was seen.
“We saw increases in thromboembolic diseases, degenerative heart diseases, and heart inflammation,” Dr. Conrad said.
Large datasets examined
The idea for the epidemiologic study came from mounting evidence for cardiovascular disease risk among people with autoimmune diseases but not enough to support the design of specific prevention measures.
Dr. Conrad’s Lancet study examined electronic health records of 446,449 individuals with autoimmune diseases and matched them to 2,102,830 individuals without autoimmune disease. This included 160,217 individuals with seven rheumatic diseases: rheumatoid arthritis, polymyalgia rheumatica, vasculitis, systemic lupus erythematosus, Sjögren’s syndrome, ankylosing spondylitis, and systemic sclerosis.
In addition to looking for any evidence of cardiovascular disease, Dr. Conrad and coauthors looked at 12 specific outcomes: atherosclerotic diseases, peripheral arterial disease, stroke or transient ischemic attack, heart failure, valve disorders, thromboembolic disease, atrial fibrillation or flutter, conduction system disease, supraventricular arrhythmias, aortic aneurysm, myocarditis and pericarditis, and infective endocarditis.
CV risk in rheumatic diseases
As might be expected, “greater magnitudes of risk” were seen for individuals with systemic lupus erythematosus and systemic sclerosis than for people in the general population, with the chances of cardiovascular disease being two to four times higher. But what perhaps wasn’t expected was that all rheumatic diseases carried an increased risk for heart or vascular-related problems.
Furthermore, the increased risk could not solely be accounted for by the presence of traditional risk factors, such as blood pressure, smoking, or obesity.
“The background here is that any context of systemic inflammation would be predicted to lead to an increased vascular risk,” Iain McInnes, MD, PhD, professor of medicine and rheumatology at the University of Glasgow, said in an interview. Dr. McInnes was a coauthor of the viewpoint article in Annals of the Rheumatic Diseases.
“The implication is that there may well be increased vascular risk across the whole range of immune-mediated inflammatory diseases,” he added. “We should not, however, infer the magnitude of risk will be the same for each disease.”
What is more intriguing, Dr. McInnes said, is that “we don’t know yet whether there’s one final common pathway that leads to the blood vessel being damaged or whether different diseases might contribute different pathways.”
He added: “A question for the future is to see what are those mechanisms that drive risk across different diseases? And the reason that matters, of course, is that we might want to think about the effectiveness of different therapeutic interventions.”
Determining cardiovascular risk
Dr. Conrad and associates in their viewpoint article suggested that an update to the European Alliance of Associations for Rheumatology guidelines for cardiovascular risk management of rheumatic and musculoskeletal diseases (RMDs) could tailor cardiovascular risk scores to certain diseases.
They suggested that the guidelines could consider a risk multiplier of 2.5 for systemic sclerosis, 2.0 for lupus, and 1.5 for any other rheumatic disease.
“We argue that [EULAR] recommendations should consider this new evidence of poorer cardiovascular health in numerous RMDs and envisage cardiovascular screening and associated prevention measures,” Dr. Conrad said.
While they recognize that risk multipliers aren’t perfect, “they are the best available option until personalized risk prediction tools are developed specifically for patients with RMDs.”
Addressing cardiovascular risk
As a former president of EULAR, Dr. McInnes was keen to point out that “EULAR’s recommendations are evidence based and are rigorously built on [standard operating procedures] that work and have stood the test of time. I’m quite sure that the members of relevant EULAR task forces will be looking at these data, but they’ll be looking at the whole range of literature to see whether change is necessary.”
Good-quality inflammatory disease control will certainly contribute to reducing vascular risk, “but we should not make the assumption that it will be sufficient,” he cautioned. “We still have to be very careful in addressing so called conventional risk factors, but in particular thinking about obesity and cardiometabolic syndrome to be sure that when those are present, that we detect them and we treat them appropriately.”
As to who is best placed to manage a patient’s cardiovascular risk profile, Dr. McInnes said: “I think the rheumatologist has a responsibility to make sure that as much of the patient’s disease spectrum is being treated as possible.”
“As a rheumatologist, I would like to know that those elements of a patient’s disease presentation are being addressed,” whether that is by a primary care physician, cardiologist, diabetologist, or other specialist involved in the optimal management of the patient.
Dr. Conrad acknowledged receiving support from the European Union’s Horizon 2020 Program, the European Society for Cardiology, and grant funding paid to her institution from the Belgian-based Research Foundation Flounders. She also acknowledged receipt of royalties in regard to the intellectual property of a home-monitoring system for heart failure paid to Oxford University Innovation. Dr. McInnes acknowledged financial relationships with many pharmaceutical companies.
*This article was updated 12/30/2022.
A re-evaluation of cardiovascular risk management guidelines intended for use by rheumatologists may be warranted based on findings from a recently published population-based study of the risks for 12 different cardiovascular disease outcomes in patients with autoimmune diseases.
“The notion that patients with rheumatic diseases are at increased risk of developing cardiovascular diseases has been ongoing for many years,” Nathalie Conrad, PhD, and coauthors wrote in a viewpoint article in Annals of the Rheumatic Diseases.
This has “sparked much debate concerning whether and when to initiate cardiovascular prevention therapies,” they said.
Dr. Conrad was first author on the population-based study published in The Lancet in August 2022 that used linked primary and secondary care records from datasets in the U.K. Clinical Practice Research Datalink involving individuals who were recently diagnosed with any of 19 different autoimmune diseases during an 18-year period stretching from 2000 to 2017 but free of cardiovascular disease until at least 12 months after incident autoimmune disease. “Every single autoimmune disorder we looked at was associated with increased cardiovascular risk,” Dr. Conrad, of the department of public health and primary care at Catholic University Leuven (Belgium), said in an interview.
Not only was the risk for cardiovascular disease increased for people with rheumatic diseases by an average of 68%, compared with people without rheumatic diseases, but also the whole spectrum of cardiovascular disorders was seen.
“We saw increases in thromboembolic diseases, degenerative heart diseases, and heart inflammation,” Dr. Conrad said.
Large datasets examined
The idea for the epidemiologic study came from mounting evidence for cardiovascular disease risk among people with autoimmune diseases but not enough to support the design of specific prevention measures.
Dr. Conrad’s Lancet study examined electronic health records of 446,449 individuals with autoimmune diseases and matched them to 2,102,830 individuals without autoimmune disease. This included 160,217 individuals with seven rheumatic diseases: rheumatoid arthritis, polymyalgia rheumatica, vasculitis, systemic lupus erythematosus, Sjögren’s syndrome, ankylosing spondylitis, and systemic sclerosis.
In addition to looking for any evidence of cardiovascular disease, Dr. Conrad and coauthors looked at 12 specific outcomes: atherosclerotic diseases, peripheral arterial disease, stroke or transient ischemic attack, heart failure, valve disorders, thromboembolic disease, atrial fibrillation or flutter, conduction system disease, supraventricular arrhythmias, aortic aneurysm, myocarditis and pericarditis, and infective endocarditis.
CV risk in rheumatic diseases
As might be expected, “greater magnitudes of risk” were seen for individuals with systemic lupus erythematosus and systemic sclerosis than for people in the general population, with the chances of cardiovascular disease being two to four times higher. But what perhaps wasn’t expected was that all rheumatic diseases carried an increased risk for heart or vascular-related problems.
Furthermore, the increased risk could not solely be accounted for by the presence of traditional risk factors, such as blood pressure, smoking, or obesity.
“The background here is that any context of systemic inflammation would be predicted to lead to an increased vascular risk,” Iain McInnes, MD, PhD, professor of medicine and rheumatology at the University of Glasgow, said in an interview. Dr. McInnes was a coauthor of the viewpoint article in Annals of the Rheumatic Diseases.
“The implication is that there may well be increased vascular risk across the whole range of immune-mediated inflammatory diseases,” he added. “We should not, however, infer the magnitude of risk will be the same for each disease.”
What is more intriguing, Dr. McInnes said, is that “we don’t know yet whether there’s one final common pathway that leads to the blood vessel being damaged or whether different diseases might contribute different pathways.”
He added: “A question for the future is to see what are those mechanisms that drive risk across different diseases? And the reason that matters, of course, is that we might want to think about the effectiveness of different therapeutic interventions.”
Determining cardiovascular risk
Dr. Conrad and associates in their viewpoint article suggested that an update to the European Alliance of Associations for Rheumatology guidelines for cardiovascular risk management of rheumatic and musculoskeletal diseases (RMDs) could tailor cardiovascular risk scores to certain diseases.
They suggested that the guidelines could consider a risk multiplier of 2.5 for systemic sclerosis, 2.0 for lupus, and 1.5 for any other rheumatic disease.
“We argue that [EULAR] recommendations should consider this new evidence of poorer cardiovascular health in numerous RMDs and envisage cardiovascular screening and associated prevention measures,” Dr. Conrad said.
While they recognize that risk multipliers aren’t perfect, “they are the best available option until personalized risk prediction tools are developed specifically for patients with RMDs.”
Addressing cardiovascular risk
As a former president of EULAR, Dr. McInnes was keen to point out that “EULAR’s recommendations are evidence based and are rigorously built on [standard operating procedures] that work and have stood the test of time. I’m quite sure that the members of relevant EULAR task forces will be looking at these data, but they’ll be looking at the whole range of literature to see whether change is necessary.”
Good-quality inflammatory disease control will certainly contribute to reducing vascular risk, “but we should not make the assumption that it will be sufficient,” he cautioned. “We still have to be very careful in addressing so called conventional risk factors, but in particular thinking about obesity and cardiometabolic syndrome to be sure that when those are present, that we detect them and we treat them appropriately.”
As to who is best placed to manage a patient’s cardiovascular risk profile, Dr. McInnes said: “I think the rheumatologist has a responsibility to make sure that as much of the patient’s disease spectrum is being treated as possible.”
“As a rheumatologist, I would like to know that those elements of a patient’s disease presentation are being addressed,” whether that is by a primary care physician, cardiologist, diabetologist, or other specialist involved in the optimal management of the patient.
Dr. Conrad acknowledged receiving support from the European Union’s Horizon 2020 Program, the European Society for Cardiology, and grant funding paid to her institution from the Belgian-based Research Foundation Flounders. She also acknowledged receipt of royalties in regard to the intellectual property of a home-monitoring system for heart failure paid to Oxford University Innovation. Dr. McInnes acknowledged financial relationships with many pharmaceutical companies.
*This article was updated 12/30/2022.
A re-evaluation of cardiovascular risk management guidelines intended for use by rheumatologists may be warranted based on findings from a recently published population-based study of the risks for 12 different cardiovascular disease outcomes in patients with autoimmune diseases.
“The notion that patients with rheumatic diseases are at increased risk of developing cardiovascular diseases has been ongoing for many years,” Nathalie Conrad, PhD, and coauthors wrote in a viewpoint article in Annals of the Rheumatic Diseases.
This has “sparked much debate concerning whether and when to initiate cardiovascular prevention therapies,” they said.
Dr. Conrad was first author on the population-based study published in The Lancet in August 2022 that used linked primary and secondary care records from datasets in the U.K. Clinical Practice Research Datalink involving individuals who were recently diagnosed with any of 19 different autoimmune diseases during an 18-year period stretching from 2000 to 2017 but free of cardiovascular disease until at least 12 months after incident autoimmune disease. “Every single autoimmune disorder we looked at was associated with increased cardiovascular risk,” Dr. Conrad, of the department of public health and primary care at Catholic University Leuven (Belgium), said in an interview.
Not only was the risk for cardiovascular disease increased for people with rheumatic diseases by an average of 68%, compared with people without rheumatic diseases, but also the whole spectrum of cardiovascular disorders was seen.
“We saw increases in thromboembolic diseases, degenerative heart diseases, and heart inflammation,” Dr. Conrad said.
Large datasets examined
The idea for the epidemiologic study came from mounting evidence for cardiovascular disease risk among people with autoimmune diseases but not enough to support the design of specific prevention measures.
Dr. Conrad’s Lancet study examined electronic health records of 446,449 individuals with autoimmune diseases and matched them to 2,102,830 individuals without autoimmune disease. This included 160,217 individuals with seven rheumatic diseases: rheumatoid arthritis, polymyalgia rheumatica, vasculitis, systemic lupus erythematosus, Sjögren’s syndrome, ankylosing spondylitis, and systemic sclerosis.
In addition to looking for any evidence of cardiovascular disease, Dr. Conrad and coauthors looked at 12 specific outcomes: atherosclerotic diseases, peripheral arterial disease, stroke or transient ischemic attack, heart failure, valve disorders, thromboembolic disease, atrial fibrillation or flutter, conduction system disease, supraventricular arrhythmias, aortic aneurysm, myocarditis and pericarditis, and infective endocarditis.
CV risk in rheumatic diseases
As might be expected, “greater magnitudes of risk” were seen for individuals with systemic lupus erythematosus and systemic sclerosis than for people in the general population, with the chances of cardiovascular disease being two to four times higher. But what perhaps wasn’t expected was that all rheumatic diseases carried an increased risk for heart or vascular-related problems.
Furthermore, the increased risk could not solely be accounted for by the presence of traditional risk factors, such as blood pressure, smoking, or obesity.
“The background here is that any context of systemic inflammation would be predicted to lead to an increased vascular risk,” Iain McInnes, MD, PhD, professor of medicine and rheumatology at the University of Glasgow, said in an interview. Dr. McInnes was a coauthor of the viewpoint article in Annals of the Rheumatic Diseases.
“The implication is that there may well be increased vascular risk across the whole range of immune-mediated inflammatory diseases,” he added. “We should not, however, infer the magnitude of risk will be the same for each disease.”
What is more intriguing, Dr. McInnes said, is that “we don’t know yet whether there’s one final common pathway that leads to the blood vessel being damaged or whether different diseases might contribute different pathways.”
He added: “A question for the future is to see what are those mechanisms that drive risk across different diseases? And the reason that matters, of course, is that we might want to think about the effectiveness of different therapeutic interventions.”
Determining cardiovascular risk
Dr. Conrad and associates in their viewpoint article suggested that an update to the European Alliance of Associations for Rheumatology guidelines for cardiovascular risk management of rheumatic and musculoskeletal diseases (RMDs) could tailor cardiovascular risk scores to certain diseases.
They suggested that the guidelines could consider a risk multiplier of 2.5 for systemic sclerosis, 2.0 for lupus, and 1.5 for any other rheumatic disease.
“We argue that [EULAR] recommendations should consider this new evidence of poorer cardiovascular health in numerous RMDs and envisage cardiovascular screening and associated prevention measures,” Dr. Conrad said.
While they recognize that risk multipliers aren’t perfect, “they are the best available option until personalized risk prediction tools are developed specifically for patients with RMDs.”
Addressing cardiovascular risk
As a former president of EULAR, Dr. McInnes was keen to point out that “EULAR’s recommendations are evidence based and are rigorously built on [standard operating procedures] that work and have stood the test of time. I’m quite sure that the members of relevant EULAR task forces will be looking at these data, but they’ll be looking at the whole range of literature to see whether change is necessary.”
Good-quality inflammatory disease control will certainly contribute to reducing vascular risk, “but we should not make the assumption that it will be sufficient,” he cautioned. “We still have to be very careful in addressing so called conventional risk factors, but in particular thinking about obesity and cardiometabolic syndrome to be sure that when those are present, that we detect them and we treat them appropriately.”
As to who is best placed to manage a patient’s cardiovascular risk profile, Dr. McInnes said: “I think the rheumatologist has a responsibility to make sure that as much of the patient’s disease spectrum is being treated as possible.”
“As a rheumatologist, I would like to know that those elements of a patient’s disease presentation are being addressed,” whether that is by a primary care physician, cardiologist, diabetologist, or other specialist involved in the optimal management of the patient.
Dr. Conrad acknowledged receiving support from the European Union’s Horizon 2020 Program, the European Society for Cardiology, and grant funding paid to her institution from the Belgian-based Research Foundation Flounders. She also acknowledged receipt of royalties in regard to the intellectual property of a home-monitoring system for heart failure paid to Oxford University Innovation. Dr. McInnes acknowledged financial relationships with many pharmaceutical companies.
*This article was updated 12/30/2022.
FROM ANNALS OF THE RHEUMATIC DISEASES
Immunotherapy drug boosts survival in newly diagnosed ALL
NEW ORLEANS – The immunotherapy drug blinatumomab improves survival as a first-line treatment in certain younger adult patients with B-lineage acute lymphoblastic leukemia, investigators have found. The extremely expensive drug is currently Food and Drug Administration approved for B-lineage ALL in relapsed/refractory cases.
“We feel that this represents a new standard of care for these patients and should be incorporated into their standard therapy,” said lead author and hematologist Mark R. Litzow, MD, of Mayo Clinic in Rochester, Minn., in a news briefing at the annual meeting of the American Society of Hematology.
B-lineage ALL, also known as B-cell ALL, represents 75% of cases of the blood cancer in adults according to the Leukemia & Lymphoma Society. It occurs when there’s an overgrowth of immature white blood cells known as B-cell lymphoblasts. “These are the blast cells that don’t function well and cause these patients to develop infections and bleeding,” Dr. Litzow said.
Treatments include chemotherapy and stem-cell transplants. Blinatumomab, a bispecific T-cell engager molecule, is FDA approved for patients with relapsed/refractory B-lineage ALL and those with morphologic complete remission who still have measurable residual disease (MRD).
As the new study notes, some patients who undergo chemotherapy and reach remission have poor survival outcomes even when there’s no sign of MRD. “Even though we can’t find leukemia in the patients’ bone marrow, it’s still hiding there,” Dr. Litzow said.
The new phase 3, randomized trial aims to determine if adding blinatumomab (Blincyto) to first-line chemotherapy improves outcomes. The drug “brings a normal T cell, part of the immune system, in proximity to a leukemia plasma cell and kills it.”
For the study, researchers from 2013 to 2019 recruited 488 patients aged 30-70 years with newly diagnosed BCR::ABL1 negative B-lineage ALL (median age = 51). The subjects underwent chemotherapy, and then were “randomized to receive an additional four cycles of consolidation chemo or two cycles of blin [blinatumomab] for 28 days each cycle followed by three cycles of consolidation chemo, another 4-week cycle of blinatumomab (third cycle of blinatumomab) followed by an additional cycle of chemo and then a fourth cycle of blinatumomab (step 3),” the researchers reported. “Following completion of consolidation chemo +/– blin, patients were given 2.5 years of POMP [prednisone, vincristine, 6-mercaptopurine, and methotrexate] maintenance therapy timed from the start of the intensification cycle (step 4).”
There were 112 patients in each group. Among MRD-negative patients, 56 patients died – 17 in the blinatumomab arm and 39 in the control arm at the third interim efficacy analysis. At a mean follow-up of 43 months, median overall survival for patients in the blinatumomab arm was not reached vs. 71.4 months in the control group (hazard ratio, 0.42, 95% confidence interval, 0.24-0.75; P = .003).
“The patients that got blinatumomab plus chemotherapy had an improved survival over those that got the standard chemotherapy,” Dr. Litzow said.
Dr. Litzow didn’t discuss the drug’s expense in his presentation. According to a 2019 report, when a daily vial of blinatumomab cost $3,464-$3,815, a treatment course of five month-long cycles could run to $535,000. According to drugs.com, the cost now is $4,740 per vial – more than $660,000 for five cycles.
In an interview, Cleveland Clinic hematologist/oncologist Anjali Advani, MD, said the study is “groundbreaking and one of the most exciting studies to come along in the acute lymphoblastic leukemia field.”
The trial “is one of the first studies to show improvement in outcome in a randomized manner with the addition of a novel agent,” she added. “This will change our standard of care for these patients.”
The National Cancer Institute funded the trial and drug manufacturer Amgen provided the medication and support through a cooperative research and development agreement.
Dr. Litzow discloses relationships with Actinium, Jazz, Syndax, Novartis, Astellas, Amgen, Abbvie, Pluristem and Biosight. Other authors have various disclosures with multiple drugmakers. Dr. Advani discloses relationships with Amgen, Jazz, Nkarta, Taiho, Beam, GMI, Kura, Pfizer, OBI, Incyte, Kite, ImmunoGen, GlycoMimetics, SGN, MacroGenics, and Servier.
NEW ORLEANS – The immunotherapy drug blinatumomab improves survival as a first-line treatment in certain younger adult patients with B-lineage acute lymphoblastic leukemia, investigators have found. The extremely expensive drug is currently Food and Drug Administration approved for B-lineage ALL in relapsed/refractory cases.
“We feel that this represents a new standard of care for these patients and should be incorporated into their standard therapy,” said lead author and hematologist Mark R. Litzow, MD, of Mayo Clinic in Rochester, Minn., in a news briefing at the annual meeting of the American Society of Hematology.
B-lineage ALL, also known as B-cell ALL, represents 75% of cases of the blood cancer in adults according to the Leukemia & Lymphoma Society. It occurs when there’s an overgrowth of immature white blood cells known as B-cell lymphoblasts. “These are the blast cells that don’t function well and cause these patients to develop infections and bleeding,” Dr. Litzow said.
Treatments include chemotherapy and stem-cell transplants. Blinatumomab, a bispecific T-cell engager molecule, is FDA approved for patients with relapsed/refractory B-lineage ALL and those with morphologic complete remission who still have measurable residual disease (MRD).
As the new study notes, some patients who undergo chemotherapy and reach remission have poor survival outcomes even when there’s no sign of MRD. “Even though we can’t find leukemia in the patients’ bone marrow, it’s still hiding there,” Dr. Litzow said.
The new phase 3, randomized trial aims to determine if adding blinatumomab (Blincyto) to first-line chemotherapy improves outcomes. The drug “brings a normal T cell, part of the immune system, in proximity to a leukemia plasma cell and kills it.”
For the study, researchers from 2013 to 2019 recruited 488 patients aged 30-70 years with newly diagnosed BCR::ABL1 negative B-lineage ALL (median age = 51). The subjects underwent chemotherapy, and then were “randomized to receive an additional four cycles of consolidation chemo or two cycles of blin [blinatumomab] for 28 days each cycle followed by three cycles of consolidation chemo, another 4-week cycle of blinatumomab (third cycle of blinatumomab) followed by an additional cycle of chemo and then a fourth cycle of blinatumomab (step 3),” the researchers reported. “Following completion of consolidation chemo +/– blin, patients were given 2.5 years of POMP [prednisone, vincristine, 6-mercaptopurine, and methotrexate] maintenance therapy timed from the start of the intensification cycle (step 4).”
There were 112 patients in each group. Among MRD-negative patients, 56 patients died – 17 in the blinatumomab arm and 39 in the control arm at the third interim efficacy analysis. At a mean follow-up of 43 months, median overall survival for patients in the blinatumomab arm was not reached vs. 71.4 months in the control group (hazard ratio, 0.42, 95% confidence interval, 0.24-0.75; P = .003).
“The patients that got blinatumomab plus chemotherapy had an improved survival over those that got the standard chemotherapy,” Dr. Litzow said.
Dr. Litzow didn’t discuss the drug’s expense in his presentation. According to a 2019 report, when a daily vial of blinatumomab cost $3,464-$3,815, a treatment course of five month-long cycles could run to $535,000. According to drugs.com, the cost now is $4,740 per vial – more than $660,000 for five cycles.
In an interview, Cleveland Clinic hematologist/oncologist Anjali Advani, MD, said the study is “groundbreaking and one of the most exciting studies to come along in the acute lymphoblastic leukemia field.”
The trial “is one of the first studies to show improvement in outcome in a randomized manner with the addition of a novel agent,” she added. “This will change our standard of care for these patients.”
The National Cancer Institute funded the trial and drug manufacturer Amgen provided the medication and support through a cooperative research and development agreement.
Dr. Litzow discloses relationships with Actinium, Jazz, Syndax, Novartis, Astellas, Amgen, Abbvie, Pluristem and Biosight. Other authors have various disclosures with multiple drugmakers. Dr. Advani discloses relationships with Amgen, Jazz, Nkarta, Taiho, Beam, GMI, Kura, Pfizer, OBI, Incyte, Kite, ImmunoGen, GlycoMimetics, SGN, MacroGenics, and Servier.
NEW ORLEANS – The immunotherapy drug blinatumomab improves survival as a first-line treatment in certain younger adult patients with B-lineage acute lymphoblastic leukemia, investigators have found. The extremely expensive drug is currently Food and Drug Administration approved for B-lineage ALL in relapsed/refractory cases.
“We feel that this represents a new standard of care for these patients and should be incorporated into their standard therapy,” said lead author and hematologist Mark R. Litzow, MD, of Mayo Clinic in Rochester, Minn., in a news briefing at the annual meeting of the American Society of Hematology.
B-lineage ALL, also known as B-cell ALL, represents 75% of cases of the blood cancer in adults according to the Leukemia & Lymphoma Society. It occurs when there’s an overgrowth of immature white blood cells known as B-cell lymphoblasts. “These are the blast cells that don’t function well and cause these patients to develop infections and bleeding,” Dr. Litzow said.
Treatments include chemotherapy and stem-cell transplants. Blinatumomab, a bispecific T-cell engager molecule, is FDA approved for patients with relapsed/refractory B-lineage ALL and those with morphologic complete remission who still have measurable residual disease (MRD).
As the new study notes, some patients who undergo chemotherapy and reach remission have poor survival outcomes even when there’s no sign of MRD. “Even though we can’t find leukemia in the patients’ bone marrow, it’s still hiding there,” Dr. Litzow said.
The new phase 3, randomized trial aims to determine if adding blinatumomab (Blincyto) to first-line chemotherapy improves outcomes. The drug “brings a normal T cell, part of the immune system, in proximity to a leukemia plasma cell and kills it.”
For the study, researchers from 2013 to 2019 recruited 488 patients aged 30-70 years with newly diagnosed BCR::ABL1 negative B-lineage ALL (median age = 51). The subjects underwent chemotherapy, and then were “randomized to receive an additional four cycles of consolidation chemo or two cycles of blin [blinatumomab] for 28 days each cycle followed by three cycles of consolidation chemo, another 4-week cycle of blinatumomab (third cycle of blinatumomab) followed by an additional cycle of chemo and then a fourth cycle of blinatumomab (step 3),” the researchers reported. “Following completion of consolidation chemo +/– blin, patients were given 2.5 years of POMP [prednisone, vincristine, 6-mercaptopurine, and methotrexate] maintenance therapy timed from the start of the intensification cycle (step 4).”
There were 112 patients in each group. Among MRD-negative patients, 56 patients died – 17 in the blinatumomab arm and 39 in the control arm at the third interim efficacy analysis. At a mean follow-up of 43 months, median overall survival for patients in the blinatumomab arm was not reached vs. 71.4 months in the control group (hazard ratio, 0.42, 95% confidence interval, 0.24-0.75; P = .003).
“The patients that got blinatumomab plus chemotherapy had an improved survival over those that got the standard chemotherapy,” Dr. Litzow said.
Dr. Litzow didn’t discuss the drug’s expense in his presentation. According to a 2019 report, when a daily vial of blinatumomab cost $3,464-$3,815, a treatment course of five month-long cycles could run to $535,000. According to drugs.com, the cost now is $4,740 per vial – more than $660,000 for five cycles.
In an interview, Cleveland Clinic hematologist/oncologist Anjali Advani, MD, said the study is “groundbreaking and one of the most exciting studies to come along in the acute lymphoblastic leukemia field.”
The trial “is one of the first studies to show improvement in outcome in a randomized manner with the addition of a novel agent,” she added. “This will change our standard of care for these patients.”
The National Cancer Institute funded the trial and drug manufacturer Amgen provided the medication and support through a cooperative research and development agreement.
Dr. Litzow discloses relationships with Actinium, Jazz, Syndax, Novartis, Astellas, Amgen, Abbvie, Pluristem and Biosight. Other authors have various disclosures with multiple drugmakers. Dr. Advani discloses relationships with Amgen, Jazz, Nkarta, Taiho, Beam, GMI, Kura, Pfizer, OBI, Incyte, Kite, ImmunoGen, GlycoMimetics, SGN, MacroGenics, and Servier.
AT ASH 2022
Debating the clinical trial upending colonoscopy practices
This transcript has been edited for clarity.
F. Perry Wilson, MD, MSCE: Hello, and thank you for joining us today for what promises to be a lively discussion about screening for colon cancer.
My name is Perry Wilson. I’m an associate professor of medicine and director of the Clinical and Translational Research Accelerator at the Yale School of Medicine. My new book, “How Medicine Works and When It Doesn’t: Learning Who to Trust to Get and Stay Healthy,” is available for pre-order now anywhere that books are sold.
I’m joined by two wonderful experts. Dr. David Johnson is a professor of medicine and the chief of gastroenterology at the Eastern Virginia School of Medicine. He is the past president of the American College of Gastroenterology. And I’m very encouraged to see that he’s won a Distinguished Educator Award for his efforts in gastroenterology.
I’m also joined by Dr Kenny Lin. He’s a frequent contributor to Medscape and WebMD. He’s a family physician and public health consultant from Lancaster, Pa., and deputy editor of the American Family Physician journal. He’s also a teacher of residents and students at Lancaster General Health and the Penn Medicine Family Medicine Residency program.
So, we have two great educators with us today to hopefully help teach us something about colon cancer and colon cancer screening. Thank you for joining me today.
David A. Johnson, MD: Thanks for having us.
Kenneth W. Lin, MD, MPH: Good to be here.
Dr. Wilson: Colon cancer is the second leading cause of cancer mortality in the United States. A little over 50,000 people die every year in the United States due to colon cancer.
A month ago, I would have said that there was a pretty broad consensus, at least from my perspective, that people should be getting colonoscopies. That’s certainly what we tell our patients.
Then a paper came out in the New England Journal of Medicine, a very prestigious journal, that has caused a lot of consternation online and led to my receiving a lot questions from patients and their family members. Today,
Dr Johnson, can you give us a brief overview of what this trial was about?
Dr. Johnson: This was a randomized trial looking at screening colonoscopy versus no screening test whatsoever. They looked at the outcomes of prevention of cancer and the prevention of colon cancer–related death.
The short answer was that it was disappointing as it relates to colonoscopy. The study looked at patients from four European countries, with data from three of them (Norway, Poland, and Sweden) ultimately analyzed in this report in NEJM. It got a lot of attention because it surprised a lot of people by saying maybe colonoscopy wasn’t quite as good as we thought it was.
They tried to correct that by only looking at the numbers of patients who got their colonoscopy screening, which still showed value, but it was less than that we’ve seen before. There’s lots of reasons for that, which we’ll discuss shortly.
An invitation to a screening
Dr. Wilson: This was a bit of an interesting trial design. I think I’m correct, Dr Lin, that this was the first randomized trial of screening colonoscopy. But they didn’t really randomize people to get a colonoscopy versus not get a colonoscopy. Can you tell us why this differed from that study design, which I’d have thought would be simpler way of assessing this?
Dr. Lin: It’s definitely an important point to highlight about the study. What investigators did was randomize patients to receive an invitation to get a screening colonoscopy. When the trial was set up, they randomized people before they were asked whether they wanted to participate in the study. If you did it the other way around, by first asking them whether they wanted to be in the study and then randomizing them, you would have been assured that more of them probably would have gotten the colonoscopy.
But in this case, they were more interested in figuring out the real-life results of having a national program that invited patients to receive screening colonoscopy. Because we know that everyone that you recommend to get a colonoscopy doesn’t necessarily want to do that, forgets to do it, or something happens that prevents their actually getting it.
When it comes to measuring the effectiveness of the colonoscopy, it perhaps wasn’t the greatest type of study to do that. But I think it did provide some information about what would happen if you invited people to get colonoscopy, in terms of how many would do it and the results overall for that population.
Lower participation numbers than expected
Dr. Wilson: Dr. Johnson, the data show that 42% of people who were in that invitation arm followed through and got their colonoscopy. You’re a gastroenterologist. Does that seem low or about right? Do about half of people who should get a colonoscopy end up getting one?
Dr. Johnson: No, it’s low. In the United States, those numbers are probably in the 70% range. Certainly, the test doesn’t work for people who don’t get the test performed. So, if 42% of those randomized to receive an invitation to get the colonoscopy got one, that really means the majority of patients never got the test.
Dr. Wilson: Certainly, we wouldn’t expect impressive results if they don’t get the test. But on the other hand, I imagine that people who choose to get the test when they’re invited are sort of a different breed. Perhaps they’re more health conscious or living in other healthy ways. Is that something we should worry about when we look at these results?
Dr. Johnson: I don’t think you can stratify based on this study. Factors like ethnicities and diet weren’t really explained. The key element that will hopefully have the major take-home impact is quality. It’s not just the test. It’s how the test is done.
The key results
Dr. Wilson: Let’s start with the big picture. This was a study looking at everyone invited; not the subgroup of people who got the colonoscopy, but the real randomized study population.
Dr. Lin, the study did show that the invited group had a lower risk of colon cancer over the next 10 years. That’s a good thing, I imagine.
Dr. Lin: I think that’s a significant benefit. Initially in the first few years, they had more colon cancers diagnosed. But that’s probably because those were cancers that were already existing and couldn’t be prevented by the test.
But then over the years the curves crossed, and by the end of the average follow-up of 10 years, there was a significantly lower rate of colon cancers being detected. That’s as you would expect, because you’re finding polyps and removing them before they became colon cancer.
Dr. Wilson: Dr. Johnson, is that the natural history of colon cancer? It starts out as a polyp that maybe can be easily removed and doesn’t require more therapy. Is that why screening colonoscopy is helpful?
Dr. Johnson: The ultimate goal of screening is prevention of cancer, rather than detection of cancer. That occurs by identification and complete removal of the polyps that we find that are precancerous. The key is, first, detection, and second, resection. Adequate resection comes down to some very significant issues of quality, which are questions that I’d raised about this study, and we can talk about momentarily.
Dr. Wilson: Absolutely. Let me first go through the two other big findings in this study.
The fact that there were fewer cases of colon cancer over 10 years seems good. But colon cancer mortality was not significantly different in the two groups. Now, of course, we know that not everyone got a colonoscopy. I would have expected though, if you had less colon cancer, you’d have less death from colon cancer.
Dr. Lin, what might explain this disconnect?
Dr. Lin: I think there are a couple of possible explanations.
One explanation is that they just didn’t follow the people long enough. Colon cancer takes a long time to go from an adenoma to cancer, and from cancer to something that would cause the patient’s death. You may need to follow them for longer than the 10 years that most of these patients were followed to see that benefit. I think there probably will be benefit after a while, because if you are removing colon cancers that otherwise would have progressed and metastasized, you often see a benefit.
We also have to consider the other possibility that not all the polyps removed necessarily were going to progress to advanced cancer. Therefore, you weren’t seeing the death benefit because not every polyp that was removed was necessarily going to cause health consequences.
In colonoscopy, quality is key to success
Dr. Wilson: You’re removing things and have no way of knowing in advance which are the bad ones and which aren’t.
Dr. Johnson, you’ve mentioned several times now that the quality of colonoscopy matters here. So, I’m intuiting that it’s not one-size-fits-all, that it’s not all the same. What do you mean by quality of colonoscopy, and what was it in the NEJM study?
Dr. Johnson: Quality colonoscopy is the quality of the whole process. It starts with the warm-up, if you will, and the clean out for the procedure. That allows the colonoscopist to be able to identify precancerous polyps, which we call adenomas (there are other precancerous polyps called sessile serrated lesions).
The identification of adenomas is extremely important. Even a small increase in the detection of those precancerous polyps has benefits. Well-performed studies looking at large databases show that a small, 1% increase in the adenoma detection leads to a 3% decrease in colon cancer and a 5% decrease in colon cancer–related death. There’s a huge array of effect when we talk about small increases in the adenoma detection rate.
Now, let’s go back to this study in NEJM.
If we base quality on the physician performing the colonoscopy, and say that the colonoscopy is achieving the act of getting all the way around the colon, but not all physicians in the study were able to do that, it starts to raise the question about quality, because adenoma detection is so important. Earlier reports from this group [Nordic-European Initiative on Colorectal Cancer Study Group] have shown that the adenoma detection rates have been way below the national thresholds. So, this raises the question of whether they found the polyp, and then whether they resected the polyp. They also don’t tell us where these cancers were. It is about the colonoscopy quality. It’s not the instrument. It’s the process.
An overview of other screening tools
Dr. Wilson: Dr. Lin, colonoscopy, which requires prep and anesthesia, is not the only colon cancer screening method we have. In fact, there are a bunch. I think we’re on board saying it’s probably better to detect colon cancer early than not detect it. But what are our other options aside from colonoscopy that can allow for early detection of colon cancer?
Dr. Lin: For most of my career, there were three options that I presented patients with. The first was the fecal test, which used to be in the form of initial hemoccult tests. These have been mostly replaced by fecal immunochemical testing. But they’re both just basically looking for the presence of blood in the stool. Anyone who has a positive test would be referred for a diagnostic colonoscopy.
The other test besides colonoscopy, which has been largely phased out in the United States, although it is still very much used in Canada and much of Europe, is flexible sigmoidoscopy. Until this study, the tests supported by randomized controlled trials were the fecal tests and flexible sigmoidoscopy.
Interestingly, there was a recent systematic review of flexible sigmoidoscopy looking at four trials and their effects over 15 years. They showed not only a reduction in colon cancer, but also a reduction in colon cancer mortality, and even a small reduction in all-cause mortality.
I believe three out of the four trials were done where the patients were consented and then randomized, so they had a higher uptake of the procedure.
But when you compare this with the colonoscopy trial, it really isn’t that impressive. You would expect a much larger benefit, because obviously you’re looking at the entire colon. But you really didn’t see that. It was, at best, maybe equivalent to sigmoidoscopy, but not a whole lot better.
Dr. Johnson: Perry, you mentioned sedation. It’s important to understand that this particular cohort of patients are from Norway, Sweden, and Poland, where it’s very much the norm to not get sedation for your colonoscopy. Any of the [audience] who have had colonoscopy will tell you that they are not ones to say, “Don’t give me sedation.” The rate of sedation is around 11% in Norway, maybe 23% in Sweden, and around 45% in Poland. So, the examiner and the patient were never really super comfortable.
I’ve done 50,000 colonoscopies in my career, and many nonsedated. We know that taking time increases the finding of polyps and the adequate identification and resection. So, that ability to perform at a high quality is very much impacted when the patients aren’t comfortable.
Dr. Wilson: Dr. Johnson, we brought up flexible sigmoidoscopy. For the patients watching whose doctors are talking to them about screening colonoscopy, what’s the difference?
Dr. Johnson: Flexible sigmoidoscopy is just a short scope examination, in which you see about one-third of the colon. I’ve been in the field for 45 years, and during that time we’ve seen that there’s a progressive increase in the development of cancers above that bottom third of the colon to the higher end, the two-thirds of the colon that you would miss without doing a full colonoscopy. Also, flexible sigmoidoscopy typically does not get covered for sedation.
Again, if you do the exam and find something, then you’re going to have to come back and do an adequate resection with a colonoscopy. So, one-stop-shopping colon cancer screening is not about detection of cancer, it’s about prevention of cancer, and that’s what colonoscopy does.
Patients want convenience, but at what cost?
Dr. Wilson: Dr. Lin, how are your patients in your family practice handling this study? Have conversations changed around colon cancer screening? What are people asking about these days?
Dr. Lin: I don’t think the conversations have changed in my practice that much. When patients ask about this study, we do discuss the limitations, that it wasn’t designed to assess the maximum benefit of getting a colonoscopy because the majority of people assigned to that group didn’t get colonoscopy.
But I think it is an opportunity in primary care to consider the way we present the options to patients. Because I would guess that a majority of primary care physicians, when they present the options, would say colonoscopy is the gold standard and recommend their patients get it. And they only offer fecal testing to patients who don’t want the colonoscopy or really refuse.
That hasn’t been my practice. I’m usually more agnostic, because there are both harms and benefits. If you get a fecal test, the chance of you having a complication from colonoscopy is automatically lower because most of those people will not get colonoscopy. Now obviously, the complications with colonoscopy are pretty rare and usually self-limited, but they do exist. If you’re doing lots and lots of these, eventually you’ll see them. Probably all primary care physicians have patients who’ve had a complication from colonoscopy and may or may not have regretted it depending on how information was presented.
But I feel like this study reinforces my feeling that we ought to be presenting these, and not saying one is superior or inferior to the other. Instead, I’d base it on what the patient’s priorities are. But I feel like this study reinforces my feeling that we ought to be presenting these, and not saying one is superior or inferior to the other. Instead, I’d base it on what the patient’s priorities are. Is your priority finding every single cancer? Do you want to know exactly what the benefit is? I think with colonoscopy, we’re still trying to figure out exactly what the benefit is. Whereas we can say it pretty confidently for fecal tests because we have those randomized trials.
Dr. Wilson: Dr. Johnson, I think patients who are watching need to know, first of all, that if they do the fecal test route, a positive fecal test does lead to colonoscopy. In some sense, all roads lead to colonoscopy once you have a positive screening test. So, I can certainly see the value of just sort of skipping to that point. But what about this risk-versus-benefit relationship? Colonoscopy, albeit a relatively safe procedure, is still a procedure. There is some risk associated with it. If we can get the same benefit from yearly fecal immunochemical testing, is that a better choice potentially, at least for patients at average risk?
Dr. Johnson: The stool-based testing is really more effective for detection of cancer. That’s not screening, where the entire goal is the prevention of cancer. The fecal-based testing, including the stool-based DNA testing, misses the majority of precancerous polyps. And the fecal immunochemical tests, which Dr. Lin just mentioned, misses virtually all of them. We really want to get to the prevention of cancer, meaning identification and removal of polyps, not just screening for cancer.
Dr. Wilson: Do you see anything on the horizon that could unseat colonoscopy as, to quote Dr. Lin, the potential gold standard for screening for colon cancer?
Dr. Johnson: I think not on the horizon for identification and removal of polyps. That’s really the gold standard. Technology continues to advance. We’ll see what happens. But on the short and intermediate horizon, colonoscopy is going to be needed.
We are finding that some patients are starting to acquiesce to stool-based testing because they can do it at home. Maybe they don’t have to do a prep. We’re talking about screening only here, not about the follow-up of patients who have a family history, patients who have colitis, patients who have had colon polyps, or other reasons. Stool-based testing is not an option for the follow-up of those patients.
Convenience testing, in the face of COVID, also has thrown a wrench into things. Patients may have wanted to stay home and do these tests. Again, we need to be proactive, not reactive. We want to prevent cancer, not detect it.
Changing advice in the face of younger screening thresholds
Dr. Wilson: Dr. Lin, I’m 42 years old. I don’t believe I’m at any increased risk of colon cancer based on my family history or other risk factors. I’m 3 years away from when the U.S. Preventive Services Task Force tells me I should potentially consider starting to screen for colon cancer. That recommendation has recently been moved down from 50 years old to 45 years old. So, it’s on my mind as I approach that age. What do you advise younger patients approaching 45 right now in terms of screening for colon cancer?
Dr. Lin: For patients with the risk factors that Dr. Johnson mentioned, I would recommend screening colonoscopy as the initial test.
Assuming you don’t have those risk factors, I present it as we have a couple of different fecal tests. There’s the traditional one that just looks for blood. Then there’s the newer one that also adds DNA, which is more sensitive for colorectal cancer, but a little less specific, which is a problem just because there are more false positives.
But you need to compare that with colonoscopy, which you only need to get done ideally every 10 years if there are no findings. That is more complete. And theoretically, as we’ve been talking about, it would also prevent as well as detect early cancers.
So, I think it’s really down to your preference in terms of how the various factors that come into play, such as convenience of the test and your level of concern about cancer. I do tell patients that family history of cancer is not terribly predictive of whether you get it or not. A lot of people unfortunately who develop colorectal cancer have no previous family history. Diet will come into play to some extent. There are some things that point to increased risk for colorectal cancer if you have a diet high in red meat and things like that. But ultimately, it really is up to the patient. I lay out the options, and whatever they choose, I’m happy to pursue.
But the most important thing is that they do some test, because doing no test is not going to help anyone. I do agree with the notion that the best test is the test that gets done.
Dr. Wilson: Absolutely. I think the NEJM study supports that, even when we’re talking about colonoscopy.
Dr. Johnson, you’ve had some criticisms about the NEJM study, and I think they make sense. At the same time, as this is the first randomized trial of colonoscopy, it’s kind of the only data we have. Are we going to get better data? Are there other studies going on out there that might help shed some light on what’s turning out to be a complicated issue?
Dr. Johnson: Yes, there are ongoing studies. They’re not taking place within the United States, because you couldn’t get through a no-screening option trial. There are comparative studies that are probably still 5 years away looking at stool-based testing.
But again, we have to recognize that if you do these alternative tests that were eloquently discussed by Dr. Lin, and not the colonoscopy, which would be every 10 years with high-quality performance, that you have to annualize or do them in sequence. It’s important that you follow up on those with regularity. It’s not just a one-time test every 10 years for these individual tests.
And any of the time that those tests are ordered, the patient should be instructed that if it’s positive you need a colonoscopy. We’re seeing a lot of slippage on that front for the stool-based testing. Convenience is not the answer. It’s getting the job done.
Dr. Wilson: Would you agree, Dr. Johnson, that for patients that really don’t want to do the colonoscopy for one reason or another, and you’ve done your best in explaining what you think the risks and benefits are, that you’d rather have them get something than nothing?
Dr. Johnson: Absolutely. It comes down to what I recommend and then what you decide. But I still make the point explicit: If we’ve gone through those checkpoints and it’s positive, we agree that you understand that colonoscopy is the next step.
Final take-home messages
Dr. Wilson: Dr. Lin, I’ll turn the last word over to you, as the person who is probably discussing the choice of screening modalities more than any of us, before someone would get referred to someone like Dr. Johnson. What’s your final take-home message about the NEJM study and the state of colon cancer screening in the United States?
Dr. Lin: My take-home points about the study are that there were some limitations, but it is good to finally have a randomized trial of colonoscopy screening 2 decades after we really started doing that in the United States. It won’t immediately change – nor do I think it should – the way we practice and discuss different options. I think that some of Dr. Johnson’s points about making sure that whoever’s doing the colonoscopies for your practices is doing it in a high-quality way are really important. Just as it’s important, if you’re doing the fecal tests, to make sure that all patients who have positives get expeditiously referred for colonoscopy.
Dr. Johnson: Perry, I’d like to make one concluding comment as the gastroenterology expert in this discussion. I’ve had countless questions about this study from my patients and my peers. I tell them the following: Don’t let the headlines mislead you.
When you look at this study, the instrument is not so much the question. We know that getting the test is the first step in colon cancer screening. But we also know that getting the test done, with the highest-quality providers and the best-quality performance, is really the key to optimizing the true value of colonoscopy for colon cancer prevention.
So please don’t lose sight of this when reading the headlines in the media around this study. We really need to analyze the true characteristics of what we call a quality performance, because that’s what drives success and that’s what prevents colon cancer.
Dr. Wilson: Dr. Johnson and Dr. Lin, thank you very much. I appreciate you spending time with me here today and wish you all the best.
I guess I’ll sum up by saying that if you’re getting a colonoscopy, make sure it’s a good one. But do get screened.
This video originally appeared on WebMD. A transcript appeared on Medscape.com.
This transcript has been edited for clarity.
F. Perry Wilson, MD, MSCE: Hello, and thank you for joining us today for what promises to be a lively discussion about screening for colon cancer.
My name is Perry Wilson. I’m an associate professor of medicine and director of the Clinical and Translational Research Accelerator at the Yale School of Medicine. My new book, “How Medicine Works and When It Doesn’t: Learning Who to Trust to Get and Stay Healthy,” is available for pre-order now anywhere that books are sold.
I’m joined by two wonderful experts. Dr. David Johnson is a professor of medicine and the chief of gastroenterology at the Eastern Virginia School of Medicine. He is the past president of the American College of Gastroenterology. And I’m very encouraged to see that he’s won a Distinguished Educator Award for his efforts in gastroenterology.
I’m also joined by Dr Kenny Lin. He’s a frequent contributor to Medscape and WebMD. He’s a family physician and public health consultant from Lancaster, Pa., and deputy editor of the American Family Physician journal. He’s also a teacher of residents and students at Lancaster General Health and the Penn Medicine Family Medicine Residency program.
So, we have two great educators with us today to hopefully help teach us something about colon cancer and colon cancer screening. Thank you for joining me today.
David A. Johnson, MD: Thanks for having us.
Kenneth W. Lin, MD, MPH: Good to be here.
Dr. Wilson: Colon cancer is the second leading cause of cancer mortality in the United States. A little over 50,000 people die every year in the United States due to colon cancer.
A month ago, I would have said that there was a pretty broad consensus, at least from my perspective, that people should be getting colonoscopies. That’s certainly what we tell our patients.
Then a paper came out in the New England Journal of Medicine, a very prestigious journal, that has caused a lot of consternation online and led to my receiving a lot questions from patients and their family members. Today,
Dr Johnson, can you give us a brief overview of what this trial was about?
Dr. Johnson: This was a randomized trial looking at screening colonoscopy versus no screening test whatsoever. They looked at the outcomes of prevention of cancer and the prevention of colon cancer–related death.
The short answer was that it was disappointing as it relates to colonoscopy. The study looked at patients from four European countries, with data from three of them (Norway, Poland, and Sweden) ultimately analyzed in this report in NEJM. It got a lot of attention because it surprised a lot of people by saying maybe colonoscopy wasn’t quite as good as we thought it was.
They tried to correct that by only looking at the numbers of patients who got their colonoscopy screening, which still showed value, but it was less than that we’ve seen before. There’s lots of reasons for that, which we’ll discuss shortly.
An invitation to a screening
Dr. Wilson: This was a bit of an interesting trial design. I think I’m correct, Dr Lin, that this was the first randomized trial of screening colonoscopy. But they didn’t really randomize people to get a colonoscopy versus not get a colonoscopy. Can you tell us why this differed from that study design, which I’d have thought would be simpler way of assessing this?
Dr. Lin: It’s definitely an important point to highlight about the study. What investigators did was randomize patients to receive an invitation to get a screening colonoscopy. When the trial was set up, they randomized people before they were asked whether they wanted to participate in the study. If you did it the other way around, by first asking them whether they wanted to be in the study and then randomizing them, you would have been assured that more of them probably would have gotten the colonoscopy.
But in this case, they were more interested in figuring out the real-life results of having a national program that invited patients to receive screening colonoscopy. Because we know that everyone that you recommend to get a colonoscopy doesn’t necessarily want to do that, forgets to do it, or something happens that prevents their actually getting it.
When it comes to measuring the effectiveness of the colonoscopy, it perhaps wasn’t the greatest type of study to do that. But I think it did provide some information about what would happen if you invited people to get colonoscopy, in terms of how many would do it and the results overall for that population.
Lower participation numbers than expected
Dr. Wilson: Dr. Johnson, the data show that 42% of people who were in that invitation arm followed through and got their colonoscopy. You’re a gastroenterologist. Does that seem low or about right? Do about half of people who should get a colonoscopy end up getting one?
Dr. Johnson: No, it’s low. In the United States, those numbers are probably in the 70% range. Certainly, the test doesn’t work for people who don’t get the test performed. So, if 42% of those randomized to receive an invitation to get the colonoscopy got one, that really means the majority of patients never got the test.
Dr. Wilson: Certainly, we wouldn’t expect impressive results if they don’t get the test. But on the other hand, I imagine that people who choose to get the test when they’re invited are sort of a different breed. Perhaps they’re more health conscious or living in other healthy ways. Is that something we should worry about when we look at these results?
Dr. Johnson: I don’t think you can stratify based on this study. Factors like ethnicities and diet weren’t really explained. The key element that will hopefully have the major take-home impact is quality. It’s not just the test. It’s how the test is done.
The key results
Dr. Wilson: Let’s start with the big picture. This was a study looking at everyone invited; not the subgroup of people who got the colonoscopy, but the real randomized study population.
Dr. Lin, the study did show that the invited group had a lower risk of colon cancer over the next 10 years. That’s a good thing, I imagine.
Dr. Lin: I think that’s a significant benefit. Initially in the first few years, they had more colon cancers diagnosed. But that’s probably because those were cancers that were already existing and couldn’t be prevented by the test.
But then over the years the curves crossed, and by the end of the average follow-up of 10 years, there was a significantly lower rate of colon cancers being detected. That’s as you would expect, because you’re finding polyps and removing them before they became colon cancer.
Dr. Wilson: Dr. Johnson, is that the natural history of colon cancer? It starts out as a polyp that maybe can be easily removed and doesn’t require more therapy. Is that why screening colonoscopy is helpful?
Dr. Johnson: The ultimate goal of screening is prevention of cancer, rather than detection of cancer. That occurs by identification and complete removal of the polyps that we find that are precancerous. The key is, first, detection, and second, resection. Adequate resection comes down to some very significant issues of quality, which are questions that I’d raised about this study, and we can talk about momentarily.
Dr. Wilson: Absolutely. Let me first go through the two other big findings in this study.
The fact that there were fewer cases of colon cancer over 10 years seems good. But colon cancer mortality was not significantly different in the two groups. Now, of course, we know that not everyone got a colonoscopy. I would have expected though, if you had less colon cancer, you’d have less death from colon cancer.
Dr. Lin, what might explain this disconnect?
Dr. Lin: I think there are a couple of possible explanations.
One explanation is that they just didn’t follow the people long enough. Colon cancer takes a long time to go from an adenoma to cancer, and from cancer to something that would cause the patient’s death. You may need to follow them for longer than the 10 years that most of these patients were followed to see that benefit. I think there probably will be benefit after a while, because if you are removing colon cancers that otherwise would have progressed and metastasized, you often see a benefit.
We also have to consider the other possibility that not all the polyps removed necessarily were going to progress to advanced cancer. Therefore, you weren’t seeing the death benefit because not every polyp that was removed was necessarily going to cause health consequences.
In colonoscopy, quality is key to success
Dr. Wilson: You’re removing things and have no way of knowing in advance which are the bad ones and which aren’t.
Dr. Johnson, you’ve mentioned several times now that the quality of colonoscopy matters here. So, I’m intuiting that it’s not one-size-fits-all, that it’s not all the same. What do you mean by quality of colonoscopy, and what was it in the NEJM study?
Dr. Johnson: Quality colonoscopy is the quality of the whole process. It starts with the warm-up, if you will, and the clean out for the procedure. That allows the colonoscopist to be able to identify precancerous polyps, which we call adenomas (there are other precancerous polyps called sessile serrated lesions).
The identification of adenomas is extremely important. Even a small increase in the detection of those precancerous polyps has benefits. Well-performed studies looking at large databases show that a small, 1% increase in the adenoma detection leads to a 3% decrease in colon cancer and a 5% decrease in colon cancer–related death. There’s a huge array of effect when we talk about small increases in the adenoma detection rate.
Now, let’s go back to this study in NEJM.
If we base quality on the physician performing the colonoscopy, and say that the colonoscopy is achieving the act of getting all the way around the colon, but not all physicians in the study were able to do that, it starts to raise the question about quality, because adenoma detection is so important. Earlier reports from this group [Nordic-European Initiative on Colorectal Cancer Study Group] have shown that the adenoma detection rates have been way below the national thresholds. So, this raises the question of whether they found the polyp, and then whether they resected the polyp. They also don’t tell us where these cancers were. It is about the colonoscopy quality. It’s not the instrument. It’s the process.
An overview of other screening tools
Dr. Wilson: Dr. Lin, colonoscopy, which requires prep and anesthesia, is not the only colon cancer screening method we have. In fact, there are a bunch. I think we’re on board saying it’s probably better to detect colon cancer early than not detect it. But what are our other options aside from colonoscopy that can allow for early detection of colon cancer?
Dr. Lin: For most of my career, there were three options that I presented patients with. The first was the fecal test, which used to be in the form of initial hemoccult tests. These have been mostly replaced by fecal immunochemical testing. But they’re both just basically looking for the presence of blood in the stool. Anyone who has a positive test would be referred for a diagnostic colonoscopy.
The other test besides colonoscopy, which has been largely phased out in the United States, although it is still very much used in Canada and much of Europe, is flexible sigmoidoscopy. Until this study, the tests supported by randomized controlled trials were the fecal tests and flexible sigmoidoscopy.
Interestingly, there was a recent systematic review of flexible sigmoidoscopy looking at four trials and their effects over 15 years. They showed not only a reduction in colon cancer, but also a reduction in colon cancer mortality, and even a small reduction in all-cause mortality.
I believe three out of the four trials were done where the patients were consented and then randomized, so they had a higher uptake of the procedure.
But when you compare this with the colonoscopy trial, it really isn’t that impressive. You would expect a much larger benefit, because obviously you’re looking at the entire colon. But you really didn’t see that. It was, at best, maybe equivalent to sigmoidoscopy, but not a whole lot better.
Dr. Johnson: Perry, you mentioned sedation. It’s important to understand that this particular cohort of patients are from Norway, Sweden, and Poland, where it’s very much the norm to not get sedation for your colonoscopy. Any of the [audience] who have had colonoscopy will tell you that they are not ones to say, “Don’t give me sedation.” The rate of sedation is around 11% in Norway, maybe 23% in Sweden, and around 45% in Poland. So, the examiner and the patient were never really super comfortable.
I’ve done 50,000 colonoscopies in my career, and many nonsedated. We know that taking time increases the finding of polyps and the adequate identification and resection. So, that ability to perform at a high quality is very much impacted when the patients aren’t comfortable.
Dr. Wilson: Dr. Johnson, we brought up flexible sigmoidoscopy. For the patients watching whose doctors are talking to them about screening colonoscopy, what’s the difference?
Dr. Johnson: Flexible sigmoidoscopy is just a short scope examination, in which you see about one-third of the colon. I’ve been in the field for 45 years, and during that time we’ve seen that there’s a progressive increase in the development of cancers above that bottom third of the colon to the higher end, the two-thirds of the colon that you would miss without doing a full colonoscopy. Also, flexible sigmoidoscopy typically does not get covered for sedation.
Again, if you do the exam and find something, then you’re going to have to come back and do an adequate resection with a colonoscopy. So, one-stop-shopping colon cancer screening is not about detection of cancer, it’s about prevention of cancer, and that’s what colonoscopy does.
Patients want convenience, but at what cost?
Dr. Wilson: Dr. Lin, how are your patients in your family practice handling this study? Have conversations changed around colon cancer screening? What are people asking about these days?
Dr. Lin: I don’t think the conversations have changed in my practice that much. When patients ask about this study, we do discuss the limitations, that it wasn’t designed to assess the maximum benefit of getting a colonoscopy because the majority of people assigned to that group didn’t get colonoscopy.
But I think it is an opportunity in primary care to consider the way we present the options to patients. Because I would guess that a majority of primary care physicians, when they present the options, would say colonoscopy is the gold standard and recommend their patients get it. And they only offer fecal testing to patients who don’t want the colonoscopy or really refuse.
That hasn’t been my practice. I’m usually more agnostic, because there are both harms and benefits. If you get a fecal test, the chance of you having a complication from colonoscopy is automatically lower because most of those people will not get colonoscopy. Now obviously, the complications with colonoscopy are pretty rare and usually self-limited, but they do exist. If you’re doing lots and lots of these, eventually you’ll see them. Probably all primary care physicians have patients who’ve had a complication from colonoscopy and may or may not have regretted it depending on how information was presented.
But I feel like this study reinforces my feeling that we ought to be presenting these, and not saying one is superior or inferior to the other. Instead, I’d base it on what the patient’s priorities are. But I feel like this study reinforces my feeling that we ought to be presenting these, and not saying one is superior or inferior to the other. Instead, I’d base it on what the patient’s priorities are. Is your priority finding every single cancer? Do you want to know exactly what the benefit is? I think with colonoscopy, we’re still trying to figure out exactly what the benefit is. Whereas we can say it pretty confidently for fecal tests because we have those randomized trials.
Dr. Wilson: Dr. Johnson, I think patients who are watching need to know, first of all, that if they do the fecal test route, a positive fecal test does lead to colonoscopy. In some sense, all roads lead to colonoscopy once you have a positive screening test. So, I can certainly see the value of just sort of skipping to that point. But what about this risk-versus-benefit relationship? Colonoscopy, albeit a relatively safe procedure, is still a procedure. There is some risk associated with it. If we can get the same benefit from yearly fecal immunochemical testing, is that a better choice potentially, at least for patients at average risk?
Dr. Johnson: The stool-based testing is really more effective for detection of cancer. That’s not screening, where the entire goal is the prevention of cancer. The fecal-based testing, including the stool-based DNA testing, misses the majority of precancerous polyps. And the fecal immunochemical tests, which Dr. Lin just mentioned, misses virtually all of them. We really want to get to the prevention of cancer, meaning identification and removal of polyps, not just screening for cancer.
Dr. Wilson: Do you see anything on the horizon that could unseat colonoscopy as, to quote Dr. Lin, the potential gold standard for screening for colon cancer?
Dr. Johnson: I think not on the horizon for identification and removal of polyps. That’s really the gold standard. Technology continues to advance. We’ll see what happens. But on the short and intermediate horizon, colonoscopy is going to be needed.
We are finding that some patients are starting to acquiesce to stool-based testing because they can do it at home. Maybe they don’t have to do a prep. We’re talking about screening only here, not about the follow-up of patients who have a family history, patients who have colitis, patients who have had colon polyps, or other reasons. Stool-based testing is not an option for the follow-up of those patients.
Convenience testing, in the face of COVID, also has thrown a wrench into things. Patients may have wanted to stay home and do these tests. Again, we need to be proactive, not reactive. We want to prevent cancer, not detect it.
Changing advice in the face of younger screening thresholds
Dr. Wilson: Dr. Lin, I’m 42 years old. I don’t believe I’m at any increased risk of colon cancer based on my family history or other risk factors. I’m 3 years away from when the U.S. Preventive Services Task Force tells me I should potentially consider starting to screen for colon cancer. That recommendation has recently been moved down from 50 years old to 45 years old. So, it’s on my mind as I approach that age. What do you advise younger patients approaching 45 right now in terms of screening for colon cancer?
Dr. Lin: For patients with the risk factors that Dr. Johnson mentioned, I would recommend screening colonoscopy as the initial test.
Assuming you don’t have those risk factors, I present it as we have a couple of different fecal tests. There’s the traditional one that just looks for blood. Then there’s the newer one that also adds DNA, which is more sensitive for colorectal cancer, but a little less specific, which is a problem just because there are more false positives.
But you need to compare that with colonoscopy, which you only need to get done ideally every 10 years if there are no findings. That is more complete. And theoretically, as we’ve been talking about, it would also prevent as well as detect early cancers.
So, I think it’s really down to your preference in terms of how the various factors that come into play, such as convenience of the test and your level of concern about cancer. I do tell patients that family history of cancer is not terribly predictive of whether you get it or not. A lot of people unfortunately who develop colorectal cancer have no previous family history. Diet will come into play to some extent. There are some things that point to increased risk for colorectal cancer if you have a diet high in red meat and things like that. But ultimately, it really is up to the patient. I lay out the options, and whatever they choose, I’m happy to pursue.
But the most important thing is that they do some test, because doing no test is not going to help anyone. I do agree with the notion that the best test is the test that gets done.
Dr. Wilson: Absolutely. I think the NEJM study supports that, even when we’re talking about colonoscopy.
Dr. Johnson, you’ve had some criticisms about the NEJM study, and I think they make sense. At the same time, as this is the first randomized trial of colonoscopy, it’s kind of the only data we have. Are we going to get better data? Are there other studies going on out there that might help shed some light on what’s turning out to be a complicated issue?
Dr. Johnson: Yes, there are ongoing studies. They’re not taking place within the United States, because you couldn’t get through a no-screening option trial. There are comparative studies that are probably still 5 years away looking at stool-based testing.
But again, we have to recognize that if you do these alternative tests that were eloquently discussed by Dr. Lin, and not the colonoscopy, which would be every 10 years with high-quality performance, that you have to annualize or do them in sequence. It’s important that you follow up on those with regularity. It’s not just a one-time test every 10 years for these individual tests.
And any of the time that those tests are ordered, the patient should be instructed that if it’s positive you need a colonoscopy. We’re seeing a lot of slippage on that front for the stool-based testing. Convenience is not the answer. It’s getting the job done.
Dr. Wilson: Would you agree, Dr. Johnson, that for patients that really don’t want to do the colonoscopy for one reason or another, and you’ve done your best in explaining what you think the risks and benefits are, that you’d rather have them get something than nothing?
Dr. Johnson: Absolutely. It comes down to what I recommend and then what you decide. But I still make the point explicit: If we’ve gone through those checkpoints and it’s positive, we agree that you understand that colonoscopy is the next step.
Final take-home messages
Dr. Wilson: Dr. Lin, I’ll turn the last word over to you, as the person who is probably discussing the choice of screening modalities more than any of us, before someone would get referred to someone like Dr. Johnson. What’s your final take-home message about the NEJM study and the state of colon cancer screening in the United States?
Dr. Lin: My take-home points about the study are that there were some limitations, but it is good to finally have a randomized trial of colonoscopy screening 2 decades after we really started doing that in the United States. It won’t immediately change – nor do I think it should – the way we practice and discuss different options. I think that some of Dr. Johnson’s points about making sure that whoever’s doing the colonoscopies for your practices is doing it in a high-quality way are really important. Just as it’s important, if you’re doing the fecal tests, to make sure that all patients who have positives get expeditiously referred for colonoscopy.
Dr. Johnson: Perry, I’d like to make one concluding comment as the gastroenterology expert in this discussion. I’ve had countless questions about this study from my patients and my peers. I tell them the following: Don’t let the headlines mislead you.
When you look at this study, the instrument is not so much the question. We know that getting the test is the first step in colon cancer screening. But we also know that getting the test done, with the highest-quality providers and the best-quality performance, is really the key to optimizing the true value of colonoscopy for colon cancer prevention.
So please don’t lose sight of this when reading the headlines in the media around this study. We really need to analyze the true characteristics of what we call a quality performance, because that’s what drives success and that’s what prevents colon cancer.
Dr. Wilson: Dr. Johnson and Dr. Lin, thank you very much. I appreciate you spending time with me here today and wish you all the best.
I guess I’ll sum up by saying that if you’re getting a colonoscopy, make sure it’s a good one. But do get screened.
This video originally appeared on WebMD. A transcript appeared on Medscape.com.
This transcript has been edited for clarity.
F. Perry Wilson, MD, MSCE: Hello, and thank you for joining us today for what promises to be a lively discussion about screening for colon cancer.
My name is Perry Wilson. I’m an associate professor of medicine and director of the Clinical and Translational Research Accelerator at the Yale School of Medicine. My new book, “How Medicine Works and When It Doesn’t: Learning Who to Trust to Get and Stay Healthy,” is available for pre-order now anywhere that books are sold.
I’m joined by two wonderful experts. Dr. David Johnson is a professor of medicine and the chief of gastroenterology at the Eastern Virginia School of Medicine. He is the past president of the American College of Gastroenterology. And I’m very encouraged to see that he’s won a Distinguished Educator Award for his efforts in gastroenterology.
I’m also joined by Dr Kenny Lin. He’s a frequent contributor to Medscape and WebMD. He’s a family physician and public health consultant from Lancaster, Pa., and deputy editor of the American Family Physician journal. He’s also a teacher of residents and students at Lancaster General Health and the Penn Medicine Family Medicine Residency program.
So, we have two great educators with us today to hopefully help teach us something about colon cancer and colon cancer screening. Thank you for joining me today.
David A. Johnson, MD: Thanks for having us.
Kenneth W. Lin, MD, MPH: Good to be here.
Dr. Wilson: Colon cancer is the second leading cause of cancer mortality in the United States. A little over 50,000 people die every year in the United States due to colon cancer.
A month ago, I would have said that there was a pretty broad consensus, at least from my perspective, that people should be getting colonoscopies. That’s certainly what we tell our patients.
Then a paper came out in the New England Journal of Medicine, a very prestigious journal, that has caused a lot of consternation online and led to my receiving a lot questions from patients and their family members. Today,
Dr Johnson, can you give us a brief overview of what this trial was about?
Dr. Johnson: This was a randomized trial looking at screening colonoscopy versus no screening test whatsoever. They looked at the outcomes of prevention of cancer and the prevention of colon cancer–related death.
The short answer was that it was disappointing as it relates to colonoscopy. The study looked at patients from four European countries, with data from three of them (Norway, Poland, and Sweden) ultimately analyzed in this report in NEJM. It got a lot of attention because it surprised a lot of people by saying maybe colonoscopy wasn’t quite as good as we thought it was.
They tried to correct that by only looking at the numbers of patients who got their colonoscopy screening, which still showed value, but it was less than that we’ve seen before. There’s lots of reasons for that, which we’ll discuss shortly.
An invitation to a screening
Dr. Wilson: This was a bit of an interesting trial design. I think I’m correct, Dr Lin, that this was the first randomized trial of screening colonoscopy. But they didn’t really randomize people to get a colonoscopy versus not get a colonoscopy. Can you tell us why this differed from that study design, which I’d have thought would be simpler way of assessing this?
Dr. Lin: It’s definitely an important point to highlight about the study. What investigators did was randomize patients to receive an invitation to get a screening colonoscopy. When the trial was set up, they randomized people before they were asked whether they wanted to participate in the study. If you did it the other way around, by first asking them whether they wanted to be in the study and then randomizing them, you would have been assured that more of them probably would have gotten the colonoscopy.
But in this case, they were more interested in figuring out the real-life results of having a national program that invited patients to receive screening colonoscopy. Because we know that everyone that you recommend to get a colonoscopy doesn’t necessarily want to do that, forgets to do it, or something happens that prevents their actually getting it.
When it comes to measuring the effectiveness of the colonoscopy, it perhaps wasn’t the greatest type of study to do that. But I think it did provide some information about what would happen if you invited people to get colonoscopy, in terms of how many would do it and the results overall for that population.
Lower participation numbers than expected
Dr. Wilson: Dr. Johnson, the data show that 42% of people who were in that invitation arm followed through and got their colonoscopy. You’re a gastroenterologist. Does that seem low or about right? Do about half of people who should get a colonoscopy end up getting one?
Dr. Johnson: No, it’s low. In the United States, those numbers are probably in the 70% range. Certainly, the test doesn’t work for people who don’t get the test performed. So, if 42% of those randomized to receive an invitation to get the colonoscopy got one, that really means the majority of patients never got the test.
Dr. Wilson: Certainly, we wouldn’t expect impressive results if they don’t get the test. But on the other hand, I imagine that people who choose to get the test when they’re invited are sort of a different breed. Perhaps they’re more health conscious or living in other healthy ways. Is that something we should worry about when we look at these results?
Dr. Johnson: I don’t think you can stratify based on this study. Factors like ethnicities and diet weren’t really explained. The key element that will hopefully have the major take-home impact is quality. It’s not just the test. It’s how the test is done.
The key results
Dr. Wilson: Let’s start with the big picture. This was a study looking at everyone invited; not the subgroup of people who got the colonoscopy, but the real randomized study population.
Dr. Lin, the study did show that the invited group had a lower risk of colon cancer over the next 10 years. That’s a good thing, I imagine.
Dr. Lin: I think that’s a significant benefit. Initially in the first few years, they had more colon cancers diagnosed. But that’s probably because those were cancers that were already existing and couldn’t be prevented by the test.
But then over the years the curves crossed, and by the end of the average follow-up of 10 years, there was a significantly lower rate of colon cancers being detected. That’s as you would expect, because you’re finding polyps and removing them before they became colon cancer.
Dr. Wilson: Dr. Johnson, is that the natural history of colon cancer? It starts out as a polyp that maybe can be easily removed and doesn’t require more therapy. Is that why screening colonoscopy is helpful?
Dr. Johnson: The ultimate goal of screening is prevention of cancer, rather than detection of cancer. That occurs by identification and complete removal of the polyps that we find that are precancerous. The key is, first, detection, and second, resection. Adequate resection comes down to some very significant issues of quality, which are questions that I’d raised about this study, and we can talk about momentarily.
Dr. Wilson: Absolutely. Let me first go through the two other big findings in this study.
The fact that there were fewer cases of colon cancer over 10 years seems good. But colon cancer mortality was not significantly different in the two groups. Now, of course, we know that not everyone got a colonoscopy. I would have expected though, if you had less colon cancer, you’d have less death from colon cancer.
Dr. Lin, what might explain this disconnect?
Dr. Lin: I think there are a couple of possible explanations.
One explanation is that they just didn’t follow the people long enough. Colon cancer takes a long time to go from an adenoma to cancer, and from cancer to something that would cause the patient’s death. You may need to follow them for longer than the 10 years that most of these patients were followed to see that benefit. I think there probably will be benefit after a while, because if you are removing colon cancers that otherwise would have progressed and metastasized, you often see a benefit.
We also have to consider the other possibility that not all the polyps removed necessarily were going to progress to advanced cancer. Therefore, you weren’t seeing the death benefit because not every polyp that was removed was necessarily going to cause health consequences.
In colonoscopy, quality is key to success
Dr. Wilson: You’re removing things and have no way of knowing in advance which are the bad ones and which aren’t.
Dr. Johnson, you’ve mentioned several times now that the quality of colonoscopy matters here. So, I’m intuiting that it’s not one-size-fits-all, that it’s not all the same. What do you mean by quality of colonoscopy, and what was it in the NEJM study?
Dr. Johnson: Quality colonoscopy is the quality of the whole process. It starts with the warm-up, if you will, and the clean out for the procedure. That allows the colonoscopist to be able to identify precancerous polyps, which we call adenomas (there are other precancerous polyps called sessile serrated lesions).
The identification of adenomas is extremely important. Even a small increase in the detection of those precancerous polyps has benefits. Well-performed studies looking at large databases show that a small, 1% increase in the adenoma detection leads to a 3% decrease in colon cancer and a 5% decrease in colon cancer–related death. There’s a huge array of effect when we talk about small increases in the adenoma detection rate.
Now, let’s go back to this study in NEJM.
If we base quality on the physician performing the colonoscopy, and say that the colonoscopy is achieving the act of getting all the way around the colon, but not all physicians in the study were able to do that, it starts to raise the question about quality, because adenoma detection is so important. Earlier reports from this group [Nordic-European Initiative on Colorectal Cancer Study Group] have shown that the adenoma detection rates have been way below the national thresholds. So, this raises the question of whether they found the polyp, and then whether they resected the polyp. They also don’t tell us where these cancers were. It is about the colonoscopy quality. It’s not the instrument. It’s the process.
An overview of other screening tools
Dr. Wilson: Dr. Lin, colonoscopy, which requires prep and anesthesia, is not the only colon cancer screening method we have. In fact, there are a bunch. I think we’re on board saying it’s probably better to detect colon cancer early than not detect it. But what are our other options aside from colonoscopy that can allow for early detection of colon cancer?
Dr. Lin: For most of my career, there were three options that I presented patients with. The first was the fecal test, which used to be in the form of initial hemoccult tests. These have been mostly replaced by fecal immunochemical testing. But they’re both just basically looking for the presence of blood in the stool. Anyone who has a positive test would be referred for a diagnostic colonoscopy.
The other test besides colonoscopy, which has been largely phased out in the United States, although it is still very much used in Canada and much of Europe, is flexible sigmoidoscopy. Until this study, the tests supported by randomized controlled trials were the fecal tests and flexible sigmoidoscopy.
Interestingly, there was a recent systematic review of flexible sigmoidoscopy looking at four trials and their effects over 15 years. They showed not only a reduction in colon cancer, but also a reduction in colon cancer mortality, and even a small reduction in all-cause mortality.
I believe three out of the four trials were done where the patients were consented and then randomized, so they had a higher uptake of the procedure.
But when you compare this with the colonoscopy trial, it really isn’t that impressive. You would expect a much larger benefit, because obviously you’re looking at the entire colon. But you really didn’t see that. It was, at best, maybe equivalent to sigmoidoscopy, but not a whole lot better.
Dr. Johnson: Perry, you mentioned sedation. It’s important to understand that this particular cohort of patients are from Norway, Sweden, and Poland, where it’s very much the norm to not get sedation for your colonoscopy. Any of the [audience] who have had colonoscopy will tell you that they are not ones to say, “Don’t give me sedation.” The rate of sedation is around 11% in Norway, maybe 23% in Sweden, and around 45% in Poland. So, the examiner and the patient were never really super comfortable.
I’ve done 50,000 colonoscopies in my career, and many nonsedated. We know that taking time increases the finding of polyps and the adequate identification and resection. So, that ability to perform at a high quality is very much impacted when the patients aren’t comfortable.
Dr. Wilson: Dr. Johnson, we brought up flexible sigmoidoscopy. For the patients watching whose doctors are talking to them about screening colonoscopy, what’s the difference?
Dr. Johnson: Flexible sigmoidoscopy is just a short scope examination, in which you see about one-third of the colon. I’ve been in the field for 45 years, and during that time we’ve seen that there’s a progressive increase in the development of cancers above that bottom third of the colon to the higher end, the two-thirds of the colon that you would miss without doing a full colonoscopy. Also, flexible sigmoidoscopy typically does not get covered for sedation.
Again, if you do the exam and find something, then you’re going to have to come back and do an adequate resection with a colonoscopy. So, one-stop-shopping colon cancer screening is not about detection of cancer, it’s about prevention of cancer, and that’s what colonoscopy does.
Patients want convenience, but at what cost?
Dr. Wilson: Dr. Lin, how are your patients in your family practice handling this study? Have conversations changed around colon cancer screening? What are people asking about these days?
Dr. Lin: I don’t think the conversations have changed in my practice that much. When patients ask about this study, we do discuss the limitations, that it wasn’t designed to assess the maximum benefit of getting a colonoscopy because the majority of people assigned to that group didn’t get colonoscopy.
But I think it is an opportunity in primary care to consider the way we present the options to patients. Because I would guess that a majority of primary care physicians, when they present the options, would say colonoscopy is the gold standard and recommend their patients get it. And they only offer fecal testing to patients who don’t want the colonoscopy or really refuse.
That hasn’t been my practice. I’m usually more agnostic, because there are both harms and benefits. If you get a fecal test, the chance of you having a complication from colonoscopy is automatically lower because most of those people will not get colonoscopy. Now obviously, the complications with colonoscopy are pretty rare and usually self-limited, but they do exist. If you’re doing lots and lots of these, eventually you’ll see them. Probably all primary care physicians have patients who’ve had a complication from colonoscopy and may or may not have regretted it depending on how information was presented.
But I feel like this study reinforces my feeling that we ought to be presenting these, and not saying one is superior or inferior to the other. Instead, I’d base it on what the patient’s priorities are. But I feel like this study reinforces my feeling that we ought to be presenting these, and not saying one is superior or inferior to the other. Instead, I’d base it on what the patient’s priorities are. Is your priority finding every single cancer? Do you want to know exactly what the benefit is? I think with colonoscopy, we’re still trying to figure out exactly what the benefit is. Whereas we can say it pretty confidently for fecal tests because we have those randomized trials.
Dr. Wilson: Dr. Johnson, I think patients who are watching need to know, first of all, that if they do the fecal test route, a positive fecal test does lead to colonoscopy. In some sense, all roads lead to colonoscopy once you have a positive screening test. So, I can certainly see the value of just sort of skipping to that point. But what about this risk-versus-benefit relationship? Colonoscopy, albeit a relatively safe procedure, is still a procedure. There is some risk associated with it. If we can get the same benefit from yearly fecal immunochemical testing, is that a better choice potentially, at least for patients at average risk?
Dr. Johnson: The stool-based testing is really more effective for detection of cancer. That’s not screening, where the entire goal is the prevention of cancer. The fecal-based testing, including the stool-based DNA testing, misses the majority of precancerous polyps. And the fecal immunochemical tests, which Dr. Lin just mentioned, misses virtually all of them. We really want to get to the prevention of cancer, meaning identification and removal of polyps, not just screening for cancer.
Dr. Wilson: Do you see anything on the horizon that could unseat colonoscopy as, to quote Dr. Lin, the potential gold standard for screening for colon cancer?
Dr. Johnson: I think not on the horizon for identification and removal of polyps. That’s really the gold standard. Technology continues to advance. We’ll see what happens. But on the short and intermediate horizon, colonoscopy is going to be needed.
We are finding that some patients are starting to acquiesce to stool-based testing because they can do it at home. Maybe they don’t have to do a prep. We’re talking about screening only here, not about the follow-up of patients who have a family history, patients who have colitis, patients who have had colon polyps, or other reasons. Stool-based testing is not an option for the follow-up of those patients.
Convenience testing, in the face of COVID, also has thrown a wrench into things. Patients may have wanted to stay home and do these tests. Again, we need to be proactive, not reactive. We want to prevent cancer, not detect it.
Changing advice in the face of younger screening thresholds
Dr. Wilson: Dr. Lin, I’m 42 years old. I don’t believe I’m at any increased risk of colon cancer based on my family history or other risk factors. I’m 3 years away from when the U.S. Preventive Services Task Force tells me I should potentially consider starting to screen for colon cancer. That recommendation has recently been moved down from 50 years old to 45 years old. So, it’s on my mind as I approach that age. What do you advise younger patients approaching 45 right now in terms of screening for colon cancer?
Dr. Lin: For patients with the risk factors that Dr. Johnson mentioned, I would recommend screening colonoscopy as the initial test.
Assuming you don’t have those risk factors, I present it as we have a couple of different fecal tests. There’s the traditional one that just looks for blood. Then there’s the newer one that also adds DNA, which is more sensitive for colorectal cancer, but a little less specific, which is a problem just because there are more false positives.
But you need to compare that with colonoscopy, which you only need to get done ideally every 10 years if there are no findings. That is more complete. And theoretically, as we’ve been talking about, it would also prevent as well as detect early cancers.
So, I think it’s really down to your preference in terms of how the various factors that come into play, such as convenience of the test and your level of concern about cancer. I do tell patients that family history of cancer is not terribly predictive of whether you get it or not. A lot of people unfortunately who develop colorectal cancer have no previous family history. Diet will come into play to some extent. There are some things that point to increased risk for colorectal cancer if you have a diet high in red meat and things like that. But ultimately, it really is up to the patient. I lay out the options, and whatever they choose, I’m happy to pursue.
But the most important thing is that they do some test, because doing no test is not going to help anyone. I do agree with the notion that the best test is the test that gets done.
Dr. Wilson: Absolutely. I think the NEJM study supports that, even when we’re talking about colonoscopy.
Dr. Johnson, you’ve had some criticisms about the NEJM study, and I think they make sense. At the same time, as this is the first randomized trial of colonoscopy, it’s kind of the only data we have. Are we going to get better data? Are there other studies going on out there that might help shed some light on what’s turning out to be a complicated issue?
Dr. Johnson: Yes, there are ongoing studies. They’re not taking place within the United States, because you couldn’t get through a no-screening option trial. There are comparative studies that are probably still 5 years away looking at stool-based testing.
But again, we have to recognize that if you do these alternative tests that were eloquently discussed by Dr. Lin, and not the colonoscopy, which would be every 10 years with high-quality performance, that you have to annualize or do them in sequence. It’s important that you follow up on those with regularity. It’s not just a one-time test every 10 years for these individual tests.
And any of the time that those tests are ordered, the patient should be instructed that if it’s positive you need a colonoscopy. We’re seeing a lot of slippage on that front for the stool-based testing. Convenience is not the answer. It’s getting the job done.
Dr. Wilson: Would you agree, Dr. Johnson, that for patients that really don’t want to do the colonoscopy for one reason or another, and you’ve done your best in explaining what you think the risks and benefits are, that you’d rather have them get something than nothing?
Dr. Johnson: Absolutely. It comes down to what I recommend and then what you decide. But I still make the point explicit: If we’ve gone through those checkpoints and it’s positive, we agree that you understand that colonoscopy is the next step.
Final take-home messages
Dr. Wilson: Dr. Lin, I’ll turn the last word over to you, as the person who is probably discussing the choice of screening modalities more than any of us, before someone would get referred to someone like Dr. Johnson. What’s your final take-home message about the NEJM study and the state of colon cancer screening in the United States?
Dr. Lin: My take-home points about the study are that there were some limitations, but it is good to finally have a randomized trial of colonoscopy screening 2 decades after we really started doing that in the United States. It won’t immediately change – nor do I think it should – the way we practice and discuss different options. I think that some of Dr. Johnson’s points about making sure that whoever’s doing the colonoscopies for your practices is doing it in a high-quality way are really important. Just as it’s important, if you’re doing the fecal tests, to make sure that all patients who have positives get expeditiously referred for colonoscopy.
Dr. Johnson: Perry, I’d like to make one concluding comment as the gastroenterology expert in this discussion. I’ve had countless questions about this study from my patients and my peers. I tell them the following: Don’t let the headlines mislead you.
When you look at this study, the instrument is not so much the question. We know that getting the test is the first step in colon cancer screening. But we also know that getting the test done, with the highest-quality providers and the best-quality performance, is really the key to optimizing the true value of colonoscopy for colon cancer prevention.
So please don’t lose sight of this when reading the headlines in the media around this study. We really need to analyze the true characteristics of what we call a quality performance, because that’s what drives success and that’s what prevents colon cancer.
Dr. Wilson: Dr. Johnson and Dr. Lin, thank you very much. I appreciate you spending time with me here today and wish you all the best.
I guess I’ll sum up by saying that if you’re getting a colonoscopy, make sure it’s a good one. But do get screened.
This video originally appeared on WebMD. A transcript appeared on Medscape.com.
Juvenile Dermatomyositis–Associated Panniculitis
To the Editor:
Juvenile dermatomyositis (JDM) is an autoimmune disorder with childhood onset that predominantly affects the muscles and skin, among other organs. Since the recognition of dermatomyositis (DM) more than 100 years ago, a variety of clinical diagnostic criteria have been utilized. Classically, DM presents with muscle weakness and a pathognomonic cutaneous macular, violaceous, erythematous eruption. The juvenile variant is defined by onset prior to 16 years of age. Histologically, these entities are indistinguishable and demonstrate an interface dermatitis with epidermal atrophy. Clinically, JDM has a higher incidence of calcinosis cutis and is not associated with an increased risk for malignancy in contrast to the adult-onset variant.1 Panniculitis is a rare but serious complication in a subset of patients with DM and may represent a precursor to calcinosis cutis.2 We describe a case of JDM-associated panniculitis that was difficult to control with prednisone and rituximab.
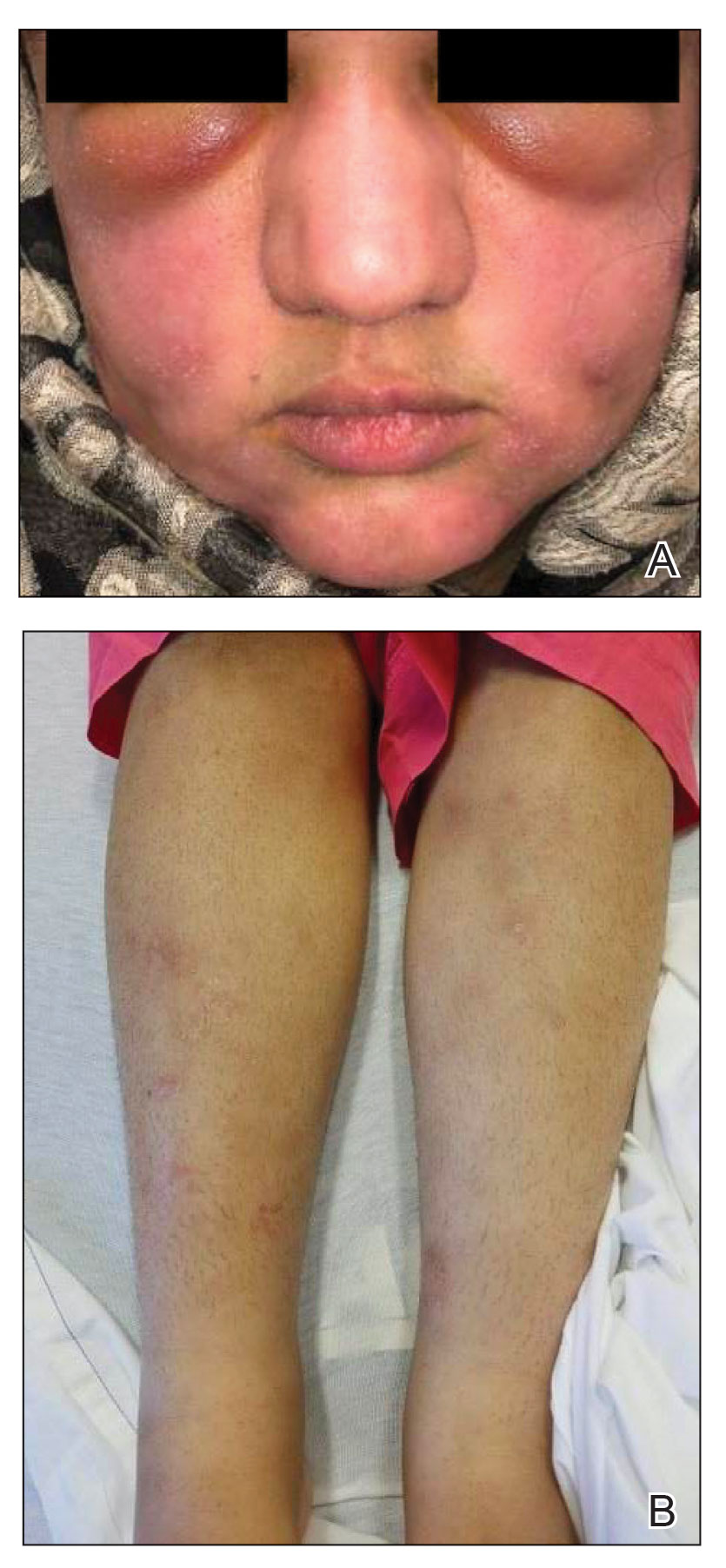
A 21-year-old woman with fever, fatigue, muscle pain, and new-onset swelling of 2 weeks’ duration was admitted to the hospital. She had a 5-year history of intermittent muscle weakness and concomitant rash. Prior to presentation, she had been hospitalized twice for fever of unknown origin, and the source remained undetermined. Physical examination revealed prominent facial and periorbital edema. There was tender nonpitting edema present on all 4 extremities and hyperpigmented indurated nodules on the shins (Figure 1). A full laboratory and imaging workup was performed for autoantibodies and infectious etiologies. The complete blood cell count was notable for pancytopenia, and a thorough infectious workup was negative. Creatine kinase level was within reference range. A biopsy of the right shin was performed, and histopathology revealed a lobular panniculitis with fat necrosis and mixed inflammation with neutrophils with perieccrine involvement as well as an interface dermatitis (Figure 2). Periodic acid–Schiff, Grocott methenamine-silver, and Gram stains were negative. Myositis-specific antibody testing revealed anti-p155/140 autoantibodies, and magnetic resonance imaging did not reveal active myositis within the visualized muscles, consistent with stable nonprogressing DM. A diagnosis of JDM with panniculitis was made. The patient was started on oral prednisone. Subsequently, a trial of rituximab was initiated. Although the patient’s symptoms initially improved, the response was not sustained on rituximab, and the patient was continued on systemic steroids with initiation of cyclosporine.
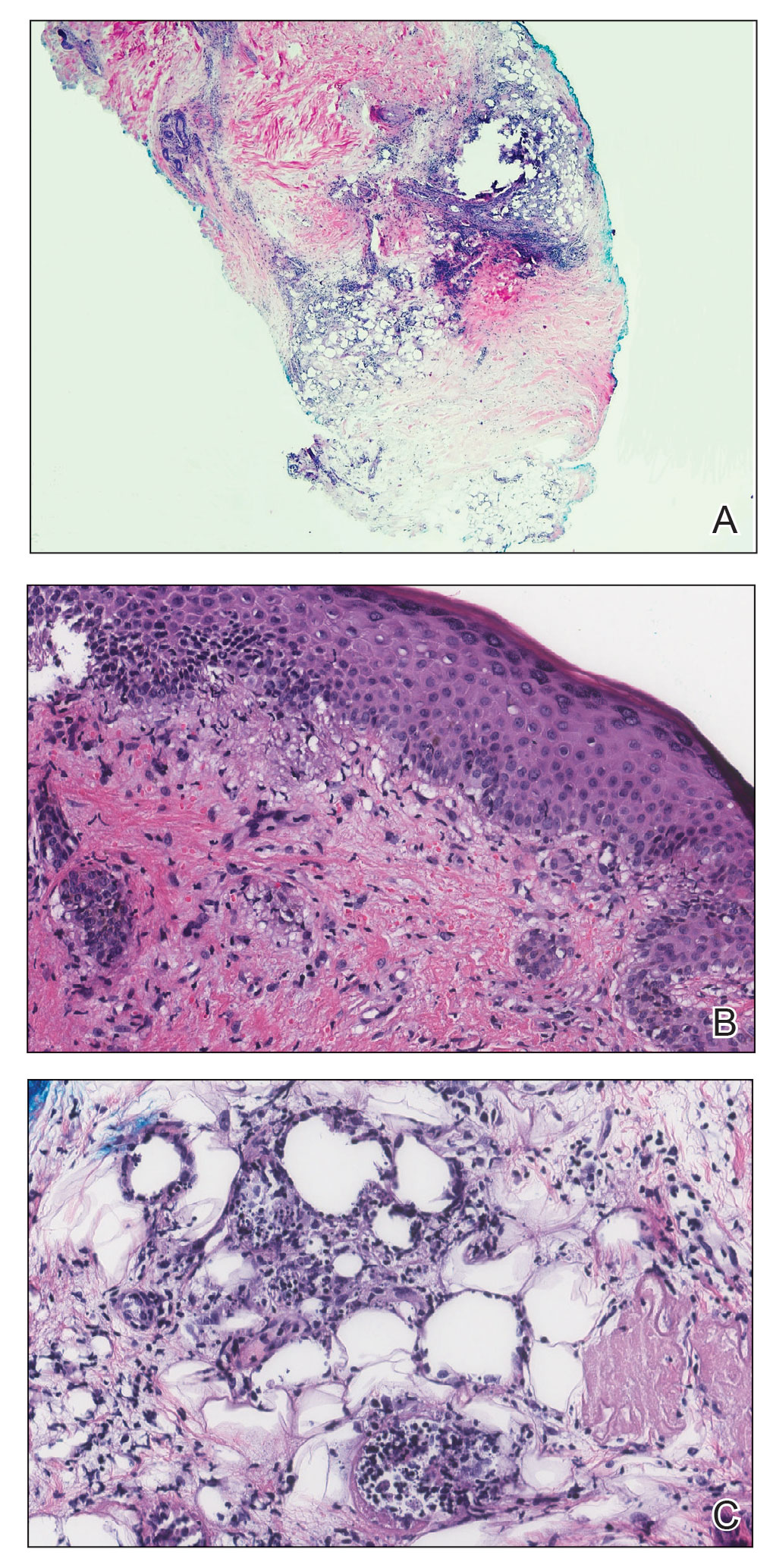
Juvenile dermatomyositis is an autoimmune disorder with childhood onset that involves systemic inflammation of the muscles, skin, and internal organs. It often can present diagnostic and therapeutic challenges.2,3 Bohan and Peter4,5 clinical criteria may help identify potential patients with JDM, but magnetic resonance imaging, electromyography, and muscle biopsy often are required to confirm the diagnosis.6 Skin manifestations include heliotrope rash; V sign; shawl sign; Gottron papules; periorbital edema; and infrequently panniculitis, the subcutaneous inflammation of adipose tissue.3,7
Although panniculitis is found in approximately 10% of skin biopsies in patients with DM, our patient presented with anti-p155/140 antibodies.8-10 Fat involvement in these patients traditionally manifests as lipodystrophy. Panniculitis also may precede calcinosis cutis, a debilitating skin change that may occur in approximately 46% of patients with JDM and can cause severe morbidity.2,6,9
Subcutaneous edema rarely is described in DM-panniculitis, present in only 6% of 86 DM patients in one study.7 The pathophysiology of DM may be due to antibodies that target endothelial cells and activate complement, resulting in the membranolytic attack complex. This leads to microischemia, and microinfarction of the muscle fibers has been suggested to result in edema of the subcutaneous tissue in severe cases.7,11 Microinfarction has been found to be present 2.3 times more often in edematous DM compared with nonedematous DM.7 Subcutaneous edema may be an isolated presentation of DM that arises more quickly with severe disease activity. As such, recommendations have been made to consider edema in future classification schemes.7
Because of the severity of edematous and/or subcutaneous DM, aggressive therapy may be required. First-line therapy consists of corticosteroids with additional immunosuppressants and immunomodulatory agents if adequate response is not achieved.3,12 The effectiveness of rituximab in DM has been suggested.2,12,13 The Rituximab in Myositis (RIM) trial (N=200) was the first double-blind, placebo-controlled, phase 3 clinical trial to assess rituximab’s efficacy in refractory compared with early-onset inflammatory myopathies. Although outcomes were similar in both groups, 83% of patients overall, including the JDM subset, met the definition of improvement.12 In re-examining the RIM trial data and other cases using rituximab to treat inflammatory myopathies, an overall response rate of 78.3% was observed, with 52.1% of patients with DM reporting improvement in skin lesions (N=458, pooled from 48 studies).13 Further analysis of the RIM data revealed that panniculitis affected 10.4% of patients with JDM at baseline, which decreased to 6.8% at 36 weeks of rituximab therapy (N=48).12
As exhibited in our patient, subcutaneous tissue involvement, including calcinosis cutis and panniculitis, is seen more often in JDM than adult DM.2,6 However, panniculitis in anti-p155/140 patients is rare. Our patient also had antibody positivity, which likely predisposed her to a more severe course. Despite not having sustained improvement on rituximab, initiating aggressive therapy earlier in the disease course may be beneficial, and our patient continues with alternative therapies.
- Jorizzo JL, Vleugels RA. Dermatomyositis. In: Bolognia J, Schaffer J, Cerroni L. Dermatology. 4th ed. Elsevier; 2019:681-687.
- Aggarwal R, Loganathan P, Koontz D, et al. Cutaneous improvement in refractory adult and juvenile dermatomyositis after treatment with rituximab. Rheumatology. 2016;56:247-254.
- Santos-Briz A, Calle A, Linos K, et al. Dermatomyositis panniculitis: a clinicopathological and immunohistochemical study of 18 cases. J Eur Acad Dermatol Venereol. 2018;32:1352-1359.
- Bohan A, Peter JB. Polymyositis and dermatomyositis (first of two parts). N Engl J Med. 1975;292:344-347.
- Bohan A, Peter JB. Polymyositis and dermatomyositis (second of two parts). N Engl J Med. 1975;292:403-407.
- Sakurai N, Hino-Shishikura A, Nozawa T, et al. Clinical significance of subcutaneous fat and fascial involvement in juvenile dermatomyositis. Mod Rheumatol. 2019;29:808-813.
- Milisenda JC, Doti PI, Prieto-Gonzalez S, et al. Dermatomyositis presenting with severe subcutaneous edema: five additional cases and review of the literature. Semin Arthritis Rheum. 2014;44:228-233.
- Janis JF, Winkelmann RK. Histopathology of the skin in dermatomyositis: a histopathologic study of 55 cases. Arch Dermatol. 1968;97:640-650.
- van Dongen HM, van Vugt RM, Stoof TJ. Extensive persistent panniculitis in the context of dermatomyositis. J Clin Rheumatol. 2020;26:e187-e188.
- Gunawardena H, Wedderburn LR, North J, et al. Clinical associations of autoantibodies to a p155/140 kDa doublet protein in juvenile dermatomyositis. Rheumatology. 2008;47:324-328.
- Dalakas MC, Hohlfeld R. Polymyositis and dermatomyositis. Lancet. 2003;362:971-982.
- Oddis CV, Reed AM, Aggarwal R, et al. Rituximab in the treatment of refractory adult and juvenile dermatomyositis and adult polymyositis: a randomized, placebo-phase trial. Arthritis Rheum. 2013;65:314-324.
- Fasano S, Gordon P, Hajji R, et al. Rituximab in the treatment of inflammatory myopathies: a review. Rheumatology. 2016;56:26-36.
To the Editor:
Juvenile dermatomyositis (JDM) is an autoimmune disorder with childhood onset that predominantly affects the muscles and skin, among other organs. Since the recognition of dermatomyositis (DM) more than 100 years ago, a variety of clinical diagnostic criteria have been utilized. Classically, DM presents with muscle weakness and a pathognomonic cutaneous macular, violaceous, erythematous eruption. The juvenile variant is defined by onset prior to 16 years of age. Histologically, these entities are indistinguishable and demonstrate an interface dermatitis with epidermal atrophy. Clinically, JDM has a higher incidence of calcinosis cutis and is not associated with an increased risk for malignancy in contrast to the adult-onset variant.1 Panniculitis is a rare but serious complication in a subset of patients with DM and may represent a precursor to calcinosis cutis.2 We describe a case of JDM-associated panniculitis that was difficult to control with prednisone and rituximab.

A 21-year-old woman with fever, fatigue, muscle pain, and new-onset swelling of 2 weeks’ duration was admitted to the hospital. She had a 5-year history of intermittent muscle weakness and concomitant rash. Prior to presentation, she had been hospitalized twice for fever of unknown origin, and the source remained undetermined. Physical examination revealed prominent facial and periorbital edema. There was tender nonpitting edema present on all 4 extremities and hyperpigmented indurated nodules on the shins (Figure 1). A full laboratory and imaging workup was performed for autoantibodies and infectious etiologies. The complete blood cell count was notable for pancytopenia, and a thorough infectious workup was negative. Creatine kinase level was within reference range. A biopsy of the right shin was performed, and histopathology revealed a lobular panniculitis with fat necrosis and mixed inflammation with neutrophils with perieccrine involvement as well as an interface dermatitis (Figure 2). Periodic acid–Schiff, Grocott methenamine-silver, and Gram stains were negative. Myositis-specific antibody testing revealed anti-p155/140 autoantibodies, and magnetic resonance imaging did not reveal active myositis within the visualized muscles, consistent with stable nonprogressing DM. A diagnosis of JDM with panniculitis was made. The patient was started on oral prednisone. Subsequently, a trial of rituximab was initiated. Although the patient’s symptoms initially improved, the response was not sustained on rituximab, and the patient was continued on systemic steroids with initiation of cyclosporine.

Juvenile dermatomyositis is an autoimmune disorder with childhood onset that involves systemic inflammation of the muscles, skin, and internal organs. It often can present diagnostic and therapeutic challenges.2,3 Bohan and Peter4,5 clinical criteria may help identify potential patients with JDM, but magnetic resonance imaging, electromyography, and muscle biopsy often are required to confirm the diagnosis.6 Skin manifestations include heliotrope rash; V sign; shawl sign; Gottron papules; periorbital edema; and infrequently panniculitis, the subcutaneous inflammation of adipose tissue.3,7
Although panniculitis is found in approximately 10% of skin biopsies in patients with DM, our patient presented with anti-p155/140 antibodies.8-10 Fat involvement in these patients traditionally manifests as lipodystrophy. Panniculitis also may precede calcinosis cutis, a debilitating skin change that may occur in approximately 46% of patients with JDM and can cause severe morbidity.2,6,9
Subcutaneous edema rarely is described in DM-panniculitis, present in only 6% of 86 DM patients in one study.7 The pathophysiology of DM may be due to antibodies that target endothelial cells and activate complement, resulting in the membranolytic attack complex. This leads to microischemia, and microinfarction of the muscle fibers has been suggested to result in edema of the subcutaneous tissue in severe cases.7,11 Microinfarction has been found to be present 2.3 times more often in edematous DM compared with nonedematous DM.7 Subcutaneous edema may be an isolated presentation of DM that arises more quickly with severe disease activity. As such, recommendations have been made to consider edema in future classification schemes.7
Because of the severity of edematous and/or subcutaneous DM, aggressive therapy may be required. First-line therapy consists of corticosteroids with additional immunosuppressants and immunomodulatory agents if adequate response is not achieved.3,12 The effectiveness of rituximab in DM has been suggested.2,12,13 The Rituximab in Myositis (RIM) trial (N=200) was the first double-blind, placebo-controlled, phase 3 clinical trial to assess rituximab’s efficacy in refractory compared with early-onset inflammatory myopathies. Although outcomes were similar in both groups, 83% of patients overall, including the JDM subset, met the definition of improvement.12 In re-examining the RIM trial data and other cases using rituximab to treat inflammatory myopathies, an overall response rate of 78.3% was observed, with 52.1% of patients with DM reporting improvement in skin lesions (N=458, pooled from 48 studies).13 Further analysis of the RIM data revealed that panniculitis affected 10.4% of patients with JDM at baseline, which decreased to 6.8% at 36 weeks of rituximab therapy (N=48).12
As exhibited in our patient, subcutaneous tissue involvement, including calcinosis cutis and panniculitis, is seen more often in JDM than adult DM.2,6 However, panniculitis in anti-p155/140 patients is rare. Our patient also had antibody positivity, which likely predisposed her to a more severe course. Despite not having sustained improvement on rituximab, initiating aggressive therapy earlier in the disease course may be beneficial, and our patient continues with alternative therapies.
To the Editor:
Juvenile dermatomyositis (JDM) is an autoimmune disorder with childhood onset that predominantly affects the muscles and skin, among other organs. Since the recognition of dermatomyositis (DM) more than 100 years ago, a variety of clinical diagnostic criteria have been utilized. Classically, DM presents with muscle weakness and a pathognomonic cutaneous macular, violaceous, erythematous eruption. The juvenile variant is defined by onset prior to 16 years of age. Histologically, these entities are indistinguishable and demonstrate an interface dermatitis with epidermal atrophy. Clinically, JDM has a higher incidence of calcinosis cutis and is not associated with an increased risk for malignancy in contrast to the adult-onset variant.1 Panniculitis is a rare but serious complication in a subset of patients with DM and may represent a precursor to calcinosis cutis.2 We describe a case of JDM-associated panniculitis that was difficult to control with prednisone and rituximab.

A 21-year-old woman with fever, fatigue, muscle pain, and new-onset swelling of 2 weeks’ duration was admitted to the hospital. She had a 5-year history of intermittent muscle weakness and concomitant rash. Prior to presentation, she had been hospitalized twice for fever of unknown origin, and the source remained undetermined. Physical examination revealed prominent facial and periorbital edema. There was tender nonpitting edema present on all 4 extremities and hyperpigmented indurated nodules on the shins (Figure 1). A full laboratory and imaging workup was performed for autoantibodies and infectious etiologies. The complete blood cell count was notable for pancytopenia, and a thorough infectious workup was negative. Creatine kinase level was within reference range. A biopsy of the right shin was performed, and histopathology revealed a lobular panniculitis with fat necrosis and mixed inflammation with neutrophils with perieccrine involvement as well as an interface dermatitis (Figure 2). Periodic acid–Schiff, Grocott methenamine-silver, and Gram stains were negative. Myositis-specific antibody testing revealed anti-p155/140 autoantibodies, and magnetic resonance imaging did not reveal active myositis within the visualized muscles, consistent with stable nonprogressing DM. A diagnosis of JDM with panniculitis was made. The patient was started on oral prednisone. Subsequently, a trial of rituximab was initiated. Although the patient’s symptoms initially improved, the response was not sustained on rituximab, and the patient was continued on systemic steroids with initiation of cyclosporine.

Juvenile dermatomyositis is an autoimmune disorder with childhood onset that involves systemic inflammation of the muscles, skin, and internal organs. It often can present diagnostic and therapeutic challenges.2,3 Bohan and Peter4,5 clinical criteria may help identify potential patients with JDM, but magnetic resonance imaging, electromyography, and muscle biopsy often are required to confirm the diagnosis.6 Skin manifestations include heliotrope rash; V sign; shawl sign; Gottron papules; periorbital edema; and infrequently panniculitis, the subcutaneous inflammation of adipose tissue.3,7
Although panniculitis is found in approximately 10% of skin biopsies in patients with DM, our patient presented with anti-p155/140 antibodies.8-10 Fat involvement in these patients traditionally manifests as lipodystrophy. Panniculitis also may precede calcinosis cutis, a debilitating skin change that may occur in approximately 46% of patients with JDM and can cause severe morbidity.2,6,9
Subcutaneous edema rarely is described in DM-panniculitis, present in only 6% of 86 DM patients in one study.7 The pathophysiology of DM may be due to antibodies that target endothelial cells and activate complement, resulting in the membranolytic attack complex. This leads to microischemia, and microinfarction of the muscle fibers has been suggested to result in edema of the subcutaneous tissue in severe cases.7,11 Microinfarction has been found to be present 2.3 times more often in edematous DM compared with nonedematous DM.7 Subcutaneous edema may be an isolated presentation of DM that arises more quickly with severe disease activity. As such, recommendations have been made to consider edema in future classification schemes.7
Because of the severity of edematous and/or subcutaneous DM, aggressive therapy may be required. First-line therapy consists of corticosteroids with additional immunosuppressants and immunomodulatory agents if adequate response is not achieved.3,12 The effectiveness of rituximab in DM has been suggested.2,12,13 The Rituximab in Myositis (RIM) trial (N=200) was the first double-blind, placebo-controlled, phase 3 clinical trial to assess rituximab’s efficacy in refractory compared with early-onset inflammatory myopathies. Although outcomes were similar in both groups, 83% of patients overall, including the JDM subset, met the definition of improvement.12 In re-examining the RIM trial data and other cases using rituximab to treat inflammatory myopathies, an overall response rate of 78.3% was observed, with 52.1% of patients with DM reporting improvement in skin lesions (N=458, pooled from 48 studies).13 Further analysis of the RIM data revealed that panniculitis affected 10.4% of patients with JDM at baseline, which decreased to 6.8% at 36 weeks of rituximab therapy (N=48).12
As exhibited in our patient, subcutaneous tissue involvement, including calcinosis cutis and panniculitis, is seen more often in JDM than adult DM.2,6 However, panniculitis in anti-p155/140 patients is rare. Our patient also had antibody positivity, which likely predisposed her to a more severe course. Despite not having sustained improvement on rituximab, initiating aggressive therapy earlier in the disease course may be beneficial, and our patient continues with alternative therapies.
- Jorizzo JL, Vleugels RA. Dermatomyositis. In: Bolognia J, Schaffer J, Cerroni L. Dermatology. 4th ed. Elsevier; 2019:681-687.
- Aggarwal R, Loganathan P, Koontz D, et al. Cutaneous improvement in refractory adult and juvenile dermatomyositis after treatment with rituximab. Rheumatology. 2016;56:247-254.
- Santos-Briz A, Calle A, Linos K, et al. Dermatomyositis panniculitis: a clinicopathological and immunohistochemical study of 18 cases. J Eur Acad Dermatol Venereol. 2018;32:1352-1359.
- Bohan A, Peter JB. Polymyositis and dermatomyositis (first of two parts). N Engl J Med. 1975;292:344-347.
- Bohan A, Peter JB. Polymyositis and dermatomyositis (second of two parts). N Engl J Med. 1975;292:403-407.
- Sakurai N, Hino-Shishikura A, Nozawa T, et al. Clinical significance of subcutaneous fat and fascial involvement in juvenile dermatomyositis. Mod Rheumatol. 2019;29:808-813.
- Milisenda JC, Doti PI, Prieto-Gonzalez S, et al. Dermatomyositis presenting with severe subcutaneous edema: five additional cases and review of the literature. Semin Arthritis Rheum. 2014;44:228-233.
- Janis JF, Winkelmann RK. Histopathology of the skin in dermatomyositis: a histopathologic study of 55 cases. Arch Dermatol. 1968;97:640-650.
- van Dongen HM, van Vugt RM, Stoof TJ. Extensive persistent panniculitis in the context of dermatomyositis. J Clin Rheumatol. 2020;26:e187-e188.
- Gunawardena H, Wedderburn LR, North J, et al. Clinical associations of autoantibodies to a p155/140 kDa doublet protein in juvenile dermatomyositis. Rheumatology. 2008;47:324-328.
- Dalakas MC, Hohlfeld R. Polymyositis and dermatomyositis. Lancet. 2003;362:971-982.
- Oddis CV, Reed AM, Aggarwal R, et al. Rituximab in the treatment of refractory adult and juvenile dermatomyositis and adult polymyositis: a randomized, placebo-phase trial. Arthritis Rheum. 2013;65:314-324.
- Fasano S, Gordon P, Hajji R, et al. Rituximab in the treatment of inflammatory myopathies: a review. Rheumatology. 2016;56:26-36.
- Jorizzo JL, Vleugels RA. Dermatomyositis. In: Bolognia J, Schaffer J, Cerroni L. Dermatology. 4th ed. Elsevier; 2019:681-687.
- Aggarwal R, Loganathan P, Koontz D, et al. Cutaneous improvement in refractory adult and juvenile dermatomyositis after treatment with rituximab. Rheumatology. 2016;56:247-254.
- Santos-Briz A, Calle A, Linos K, et al. Dermatomyositis panniculitis: a clinicopathological and immunohistochemical study of 18 cases. J Eur Acad Dermatol Venereol. 2018;32:1352-1359.
- Bohan A, Peter JB. Polymyositis and dermatomyositis (first of two parts). N Engl J Med. 1975;292:344-347.
- Bohan A, Peter JB. Polymyositis and dermatomyositis (second of two parts). N Engl J Med. 1975;292:403-407.
- Sakurai N, Hino-Shishikura A, Nozawa T, et al. Clinical significance of subcutaneous fat and fascial involvement in juvenile dermatomyositis. Mod Rheumatol. 2019;29:808-813.
- Milisenda JC, Doti PI, Prieto-Gonzalez S, et al. Dermatomyositis presenting with severe subcutaneous edema: five additional cases and review of the literature. Semin Arthritis Rheum. 2014;44:228-233.
- Janis JF, Winkelmann RK. Histopathology of the skin in dermatomyositis: a histopathologic study of 55 cases. Arch Dermatol. 1968;97:640-650.
- van Dongen HM, van Vugt RM, Stoof TJ. Extensive persistent panniculitis in the context of dermatomyositis. J Clin Rheumatol. 2020;26:e187-e188.
- Gunawardena H, Wedderburn LR, North J, et al. Clinical associations of autoantibodies to a p155/140 kDa doublet protein in juvenile dermatomyositis. Rheumatology. 2008;47:324-328.
- Dalakas MC, Hohlfeld R. Polymyositis and dermatomyositis. Lancet. 2003;362:971-982.
- Oddis CV, Reed AM, Aggarwal R, et al. Rituximab in the treatment of refractory adult and juvenile dermatomyositis and adult polymyositis: a randomized, placebo-phase trial. Arthritis Rheum. 2013;65:314-324.
- Fasano S, Gordon P, Hajji R, et al. Rituximab in the treatment of inflammatory myopathies: a review. Rheumatology. 2016;56:26-36.
Practice Points
- Juvenile dermatomyositis is an autoimmune disorder with childhood onset that predominantly affects the muscles and skin.
- Juvenile dermatomyositis has a higher incidence of calcinosis cutis and is not associated with an increased risk for malignancy in contrast to the adult-onset variant, dermatomyositis (DM).
- Panniculitis is a rare but severe complication of DM, and this subset of DM may be challenging to treat, requiring aggressive therapy.
Skin Manifestations of Complex Regional Pain Syndrome
To the Editor:
Complex regional pain syndrome (CRPS) is a neurologic condition characterized by chronic pain and sensory changes, including allodynia and hyperalgesia, that usually affect the extremities.1,2 The syndrome is defined by the International Association for the Study of Pain (IASP) as a condition that appears regionally after an injury, with a variety of symptoms that often exceed the expected clinical course both in magnitude and duration, causing impairment of motor function and variable progression.3
Although CRPS most often is described following minor peripheral trauma, other precipitating causes include surgery and vascular events.4 Additional features of the condition include autonomic dysfunction, edema, and trophic changes.1 Symptoms of CRPS traditionally present in 3 stages, with notable skin changes most often documented in stages II and III.2
Skin changes are a known manifestation of the syndrome, but reports in the dermatologic literature are scarce. Qureshi and Friedman5 identified only 23 articles in the dermatology literature since 1990 in which skin changes in CRPS were described. We present a patient with a diagnosis of CRPS who developed hyperpigmentation and sclerotic changes, including skin thickening, induration, and skin tightening.
A middle-aged Black woman presented to dermatology for evaluation of progressive hyperpigmentation, hyperhidrosis, and sclerotic changes to the skin. Approximately 3 years prior, the patient was given a diagnosis of CRPS of the hands and feet. Pain symptoms started approximately 3 years prior to the onset of symptoms. Symptoms started in the left hand and eventually spread to the right arm, left leg, and subsequently to the right leg. The first dermatologic change the patient noticed was tightening of the skin in the affected area that led to decreased mobility, which improved over time—partly on its own and partly with physical therapy.
A biopsy performed by an outside dermatologist at the initial presentation demonstrated sclerodermalike changes, which were treated with creams but without improvement. Scleroderma was later ruled out by the same dermatologist. Skin tightening improved over time, with complete resolution approximately 1 year after the onset of symptoms.
Upon presentation to our clinic, the patient reported continuing intermittent flares of CRPS; however, she said she was most concerned about diffuse hyperpigmentation, which spread to include the face, arms, abdomen, legs (Figure), and buttocks and persisted after skin tightening resolved.
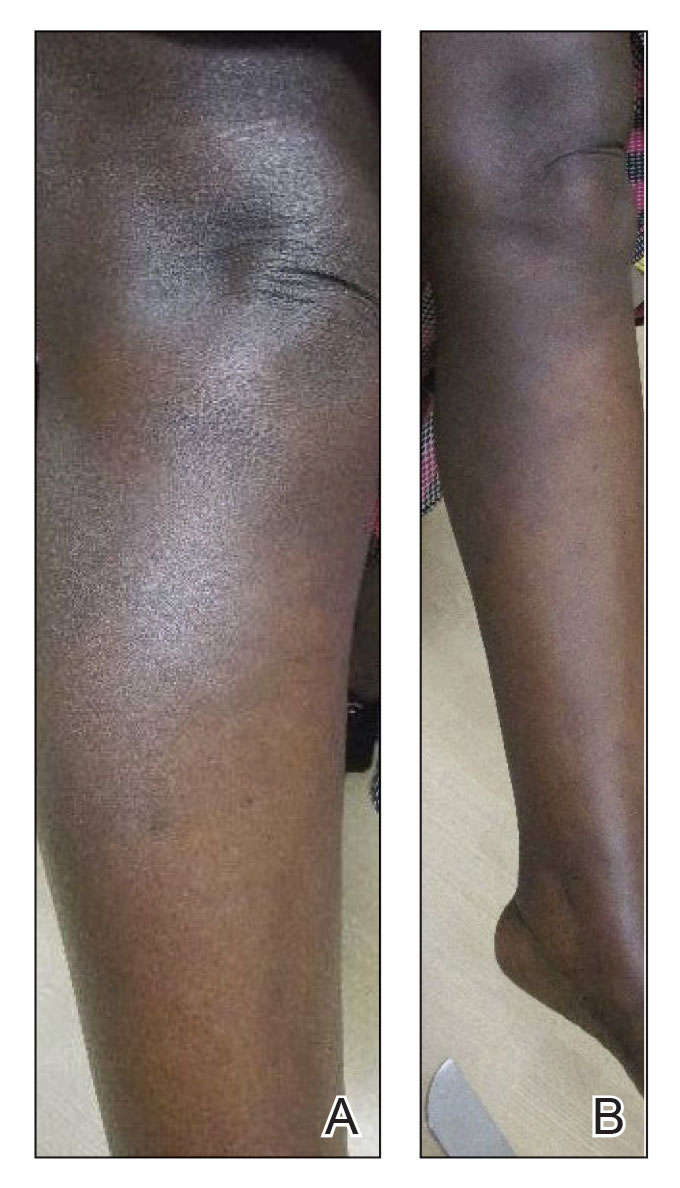
To treat the hyperpigmentation, a decision was made to first focus on a localized area. Facial hyperpigmentation was chosen because it was of greatest concern to the patient. She was instructed to use azelaic acid gel 15% in the morning, tretinoin cream 0.05% at night, and sunscreen daily. The patient had mild improvement in hyperpigmentation after a 4-month period but has been inconsistent in follow-up. She continues to have intermittent flares of CRPS, which may interfere with her response to treatment. In addition to the aforementioned regimen of azelaic acid gel and tretinoin, she has continued to work with a pain specialist to better control the neurologic symptoms and pain associated with her CRPS.
Complex regional pain syndrome, a neurological condition characterized by chronic pain, affects women 3 times more often than men. The syndrome is more common in the fourth and fifth decades of life.1,2
There are 2 subtypes of CRPS. Type I (also known as reflex sympathetic dystrophy) is more common and occurs following minor trauma without peripheral nerve injury. Type II (otherwise known as causalgia) occurs following more notable trauma with injury to a peripheral nerve.1,6 Onset of symptoms most often is secondary to minor peripheral trauma. More common triggers include soft-tissue injury (40%); fractures and subsequent orthopedic surgery (25%); and visceral lesions, such as myocardial infarction and cerebral vascular accident (12%).5 Regardless of the inciting event, prolonged immobilization of a limb has been identified as an important predisposing factor. One study found that 47% of patients who received a diagnosis of CRPS previously underwent immobilization of the same limb.7
The pathogenesis of CRPS has not been fully elucidated. Possible explanations include central nervous system sensitization to thermal, mechanical, and pain stimuli; sympathetic dysfunction leading to vasomotor, pseudomotor, and trophic changes; and inflammatory cytokine release and microcirculatory dysfunction, causing tissue injury.1,2,6
The diagnosis of CRPS is a based on clinical findings. Using the Budapest Criteria established to define CRPS, a clinical diagnosis can be made when all of the following criteria are met: chronic continuing pain disproportionate to any inciting event; 1 or more reported symptoms from 3 or more of the categories of involvement including sensory, vasomotor, pseudomotor, edema, and motor or trophic; 1 or more sign at the time of evaluation in 2 or more of the categories of involvement including sensory, vasomotor, pseudomotor, edema, and motor or trophic.8 Dermatologic findings are a common presenting feature of CRPS and are included in the Budapest Criteria used for diagnosis. In a retrospective chart review (N=26), researchers found that vascular findings were the most common dermatologic manifestation of CRPS—edema in 58% of patients and erythema in 54%.9 Other common manifestations included dermatitis (35%), erythematous papules (23%), and cutaneous atrophy (23%). Hyperpigmentation, which was present in our patient, was seen in 8% of patients in the chart review.9
Complex regional pain syndrome progresses through 3 stages; dermatologic changes are present in each stage and are more severe in later stages. Stage I lasts 2 or 3 months and is characterized by onset of pain, usually burning type, accompanied by allodynia and hyperalgesia. Early vasomotor and pseudomotor changes, such as erythema and edema, may become apparent.1,2 Stage II lasts 3 to 6 months and is characterized by more severe edema and more obvious trophic changes. Functional limitations, such as limited range of motion and muscle weakness, begin to manifest. Stage III—the final and most severe stage—is characterized by obvious hair, skin, and nail changes, as well as functional limitations.1,2 The waxy thickened skin changes and hyperpigmentation observed in our patient are characteristic of stage III. Furthermore, our patient experienced decreased mobility and limited range of motion secondary to tightening of the skin, a characteristic motor change of late-stage CRPS. Although chronic pain and allodynia are the most common characteristics of CRPS, skin changes also can cause notable distress and early dermatologic manifestations can be a chief concern.
Dermatologic management is focused to address the specific skin changes of CRPS. However, traditional treatment of the common dermatologic findings of CRPS is difficult and often unsuccessful; instead, the most successful treatment of skin findings involves controlling the underlying CRPS.9 Current treatment options include removal of any nidus of tissue trauma, sympathetic neural blockade with a local anesthetic, spinal cord stimulation to interrupt dysregulated sympathetic innervation, and physiotherapy or occupational therapy to desensitize skin.1,10
Given the complexity of CRPS and the variability of its presentation, management of the syndrome and its associated dermatologic conditions often requires interdisciplinary care and coordination of multiple specialties. Dermatologists can play an important role in both identification of CRPS and co-management of affected patients. Early diagnosis of CRPS has been universally identified as a key prognostic factor. For that reason, dermatologists should be aware of CRPS and include the syndrome in the differential diagnosis when presented with severe cutaneous findings following trauma either with or without peripheral nerve damage, suggestive of CRPS.
- Sebastin SJ. Complex regional pain syndrome. Indian J Plast Surg. 2011;44:298-307. doi:10.4103/0970-0358.85351
- Kabani R, Brassard A. Dermatological findings in early detection of complex regional pain syndrome. JAMA Dermatol. 2014;150:640-642. doi:10.1001/jamadermatol.2013.7459
- Moseley L. What is complex regional pain syndrome – in plain English. International Association for the Study of Pain website. Published 2009. Accessed December 15, 2022. https://www.iasp-pain.org/publications/relief-news/article/what-is-complex-pain-syndrome-in-plain-english/
- Pak TJ, Martin GM, Magness JL, et al. Reflex sympathetic dystrophy. Review of 140 cases. Minn Med. 1970;53:507-512.
- Qureshi AA, Friedman AJ. Complex regional pain syndrome: what the dermatologist should know. J Drugs Dermatol. 2018;17:532-536.
- Gorodkin R. Complex regional pain syndrome. Rheumatology. 2016;55(suppl 1):i12.
- Araki E, Tanioka M, Miyachi Y, et al. A case of complex regional pain syndrome: an underdiagnosed condition in dermatology. Acta Derm Venereol. 2007;87:440-441. doi:10.2340/00015555-0281
- Pergolizzi JV, LeQuang JA, Nalamachu S, et al. The Budapest criteria for complex regional pain syndrome: the diagnostic challenge. Anaesthesiol Clin Sci Res. 2018;2:1-10. doi:10.35841/anesthesiology.2.1.1-10
- Sundaram S, Webster GF. Vascular diseases are the most common cutaneous manifestations of reflex sympathetic dystrophy. J Am Acad Dermatol. 2001;44:1050-1051. doi:10.1067/mjd.2001.114299
- Taylor RS, Van Buyten J-P, Buchser E. Spinal stimulation for complex regional pain syndrome: a systematic review of the clinical and cost-effectiveness literature and assessment of prognostic factors. Eur J Pain. 2006;10:91-101. doi:10.1016/j.ejpain.2005.02.004
To the Editor:
Complex regional pain syndrome (CRPS) is a neurologic condition characterized by chronic pain and sensory changes, including allodynia and hyperalgesia, that usually affect the extremities.1,2 The syndrome is defined by the International Association for the Study of Pain (IASP) as a condition that appears regionally after an injury, with a variety of symptoms that often exceed the expected clinical course both in magnitude and duration, causing impairment of motor function and variable progression.3
Although CRPS most often is described following minor peripheral trauma, other precipitating causes include surgery and vascular events.4 Additional features of the condition include autonomic dysfunction, edema, and trophic changes.1 Symptoms of CRPS traditionally present in 3 stages, with notable skin changes most often documented in stages II and III.2
Skin changes are a known manifestation of the syndrome, but reports in the dermatologic literature are scarce. Qureshi and Friedman5 identified only 23 articles in the dermatology literature since 1990 in which skin changes in CRPS were described. We present a patient with a diagnosis of CRPS who developed hyperpigmentation and sclerotic changes, including skin thickening, induration, and skin tightening.
A middle-aged Black woman presented to dermatology for evaluation of progressive hyperpigmentation, hyperhidrosis, and sclerotic changes to the skin. Approximately 3 years prior, the patient was given a diagnosis of CRPS of the hands and feet. Pain symptoms started approximately 3 years prior to the onset of symptoms. Symptoms started in the left hand and eventually spread to the right arm, left leg, and subsequently to the right leg. The first dermatologic change the patient noticed was tightening of the skin in the affected area that led to decreased mobility, which improved over time—partly on its own and partly with physical therapy.
A biopsy performed by an outside dermatologist at the initial presentation demonstrated sclerodermalike changes, which were treated with creams but without improvement. Scleroderma was later ruled out by the same dermatologist. Skin tightening improved over time, with complete resolution approximately 1 year after the onset of symptoms.
Upon presentation to our clinic, the patient reported continuing intermittent flares of CRPS; however, she said she was most concerned about diffuse hyperpigmentation, which spread to include the face, arms, abdomen, legs (Figure), and buttocks and persisted after skin tightening resolved.

To treat the hyperpigmentation, a decision was made to first focus on a localized area. Facial hyperpigmentation was chosen because it was of greatest concern to the patient. She was instructed to use azelaic acid gel 15% in the morning, tretinoin cream 0.05% at night, and sunscreen daily. The patient had mild improvement in hyperpigmentation after a 4-month period but has been inconsistent in follow-up. She continues to have intermittent flares of CRPS, which may interfere with her response to treatment. In addition to the aforementioned regimen of azelaic acid gel and tretinoin, she has continued to work with a pain specialist to better control the neurologic symptoms and pain associated with her CRPS.
Complex regional pain syndrome, a neurological condition characterized by chronic pain, affects women 3 times more often than men. The syndrome is more common in the fourth and fifth decades of life.1,2
There are 2 subtypes of CRPS. Type I (also known as reflex sympathetic dystrophy) is more common and occurs following minor trauma without peripheral nerve injury. Type II (otherwise known as causalgia) occurs following more notable trauma with injury to a peripheral nerve.1,6 Onset of symptoms most often is secondary to minor peripheral trauma. More common triggers include soft-tissue injury (40%); fractures and subsequent orthopedic surgery (25%); and visceral lesions, such as myocardial infarction and cerebral vascular accident (12%).5 Regardless of the inciting event, prolonged immobilization of a limb has been identified as an important predisposing factor. One study found that 47% of patients who received a diagnosis of CRPS previously underwent immobilization of the same limb.7
The pathogenesis of CRPS has not been fully elucidated. Possible explanations include central nervous system sensitization to thermal, mechanical, and pain stimuli; sympathetic dysfunction leading to vasomotor, pseudomotor, and trophic changes; and inflammatory cytokine release and microcirculatory dysfunction, causing tissue injury.1,2,6
The diagnosis of CRPS is a based on clinical findings. Using the Budapest Criteria established to define CRPS, a clinical diagnosis can be made when all of the following criteria are met: chronic continuing pain disproportionate to any inciting event; 1 or more reported symptoms from 3 or more of the categories of involvement including sensory, vasomotor, pseudomotor, edema, and motor or trophic; 1 or more sign at the time of evaluation in 2 or more of the categories of involvement including sensory, vasomotor, pseudomotor, edema, and motor or trophic.8 Dermatologic findings are a common presenting feature of CRPS and are included in the Budapest Criteria used for diagnosis. In a retrospective chart review (N=26), researchers found that vascular findings were the most common dermatologic manifestation of CRPS—edema in 58% of patients and erythema in 54%.9 Other common manifestations included dermatitis (35%), erythematous papules (23%), and cutaneous atrophy (23%). Hyperpigmentation, which was present in our patient, was seen in 8% of patients in the chart review.9
Complex regional pain syndrome progresses through 3 stages; dermatologic changes are present in each stage and are more severe in later stages. Stage I lasts 2 or 3 months and is characterized by onset of pain, usually burning type, accompanied by allodynia and hyperalgesia. Early vasomotor and pseudomotor changes, such as erythema and edema, may become apparent.1,2 Stage II lasts 3 to 6 months and is characterized by more severe edema and more obvious trophic changes. Functional limitations, such as limited range of motion and muscle weakness, begin to manifest. Stage III—the final and most severe stage—is characterized by obvious hair, skin, and nail changes, as well as functional limitations.1,2 The waxy thickened skin changes and hyperpigmentation observed in our patient are characteristic of stage III. Furthermore, our patient experienced decreased mobility and limited range of motion secondary to tightening of the skin, a characteristic motor change of late-stage CRPS. Although chronic pain and allodynia are the most common characteristics of CRPS, skin changes also can cause notable distress and early dermatologic manifestations can be a chief concern.
Dermatologic management is focused to address the specific skin changes of CRPS. However, traditional treatment of the common dermatologic findings of CRPS is difficult and often unsuccessful; instead, the most successful treatment of skin findings involves controlling the underlying CRPS.9 Current treatment options include removal of any nidus of tissue trauma, sympathetic neural blockade with a local anesthetic, spinal cord stimulation to interrupt dysregulated sympathetic innervation, and physiotherapy or occupational therapy to desensitize skin.1,10
Given the complexity of CRPS and the variability of its presentation, management of the syndrome and its associated dermatologic conditions often requires interdisciplinary care and coordination of multiple specialties. Dermatologists can play an important role in both identification of CRPS and co-management of affected patients. Early diagnosis of CRPS has been universally identified as a key prognostic factor. For that reason, dermatologists should be aware of CRPS and include the syndrome in the differential diagnosis when presented with severe cutaneous findings following trauma either with or without peripheral nerve damage, suggestive of CRPS.
To the Editor:
Complex regional pain syndrome (CRPS) is a neurologic condition characterized by chronic pain and sensory changes, including allodynia and hyperalgesia, that usually affect the extremities.1,2 The syndrome is defined by the International Association for the Study of Pain (IASP) as a condition that appears regionally after an injury, with a variety of symptoms that often exceed the expected clinical course both in magnitude and duration, causing impairment of motor function and variable progression.3
Although CRPS most often is described following minor peripheral trauma, other precipitating causes include surgery and vascular events.4 Additional features of the condition include autonomic dysfunction, edema, and trophic changes.1 Symptoms of CRPS traditionally present in 3 stages, with notable skin changes most often documented in stages II and III.2
Skin changes are a known manifestation of the syndrome, but reports in the dermatologic literature are scarce. Qureshi and Friedman5 identified only 23 articles in the dermatology literature since 1990 in which skin changes in CRPS were described. We present a patient with a diagnosis of CRPS who developed hyperpigmentation and sclerotic changes, including skin thickening, induration, and skin tightening.
A middle-aged Black woman presented to dermatology for evaluation of progressive hyperpigmentation, hyperhidrosis, and sclerotic changes to the skin. Approximately 3 years prior, the patient was given a diagnosis of CRPS of the hands and feet. Pain symptoms started approximately 3 years prior to the onset of symptoms. Symptoms started in the left hand and eventually spread to the right arm, left leg, and subsequently to the right leg. The first dermatologic change the patient noticed was tightening of the skin in the affected area that led to decreased mobility, which improved over time—partly on its own and partly with physical therapy.
A biopsy performed by an outside dermatologist at the initial presentation demonstrated sclerodermalike changes, which were treated with creams but without improvement. Scleroderma was later ruled out by the same dermatologist. Skin tightening improved over time, with complete resolution approximately 1 year after the onset of symptoms.
Upon presentation to our clinic, the patient reported continuing intermittent flares of CRPS; however, she said she was most concerned about diffuse hyperpigmentation, which spread to include the face, arms, abdomen, legs (Figure), and buttocks and persisted after skin tightening resolved.

To treat the hyperpigmentation, a decision was made to first focus on a localized area. Facial hyperpigmentation was chosen because it was of greatest concern to the patient. She was instructed to use azelaic acid gel 15% in the morning, tretinoin cream 0.05% at night, and sunscreen daily. The patient had mild improvement in hyperpigmentation after a 4-month period but has been inconsistent in follow-up. She continues to have intermittent flares of CRPS, which may interfere with her response to treatment. In addition to the aforementioned regimen of azelaic acid gel and tretinoin, she has continued to work with a pain specialist to better control the neurologic symptoms and pain associated with her CRPS.
Complex regional pain syndrome, a neurological condition characterized by chronic pain, affects women 3 times more often than men. The syndrome is more common in the fourth and fifth decades of life.1,2
There are 2 subtypes of CRPS. Type I (also known as reflex sympathetic dystrophy) is more common and occurs following minor trauma without peripheral nerve injury. Type II (otherwise known as causalgia) occurs following more notable trauma with injury to a peripheral nerve.1,6 Onset of symptoms most often is secondary to minor peripheral trauma. More common triggers include soft-tissue injury (40%); fractures and subsequent orthopedic surgery (25%); and visceral lesions, such as myocardial infarction and cerebral vascular accident (12%).5 Regardless of the inciting event, prolonged immobilization of a limb has been identified as an important predisposing factor. One study found that 47% of patients who received a diagnosis of CRPS previously underwent immobilization of the same limb.7
The pathogenesis of CRPS has not been fully elucidated. Possible explanations include central nervous system sensitization to thermal, mechanical, and pain stimuli; sympathetic dysfunction leading to vasomotor, pseudomotor, and trophic changes; and inflammatory cytokine release and microcirculatory dysfunction, causing tissue injury.1,2,6
The diagnosis of CRPS is a based on clinical findings. Using the Budapest Criteria established to define CRPS, a clinical diagnosis can be made when all of the following criteria are met: chronic continuing pain disproportionate to any inciting event; 1 or more reported symptoms from 3 or more of the categories of involvement including sensory, vasomotor, pseudomotor, edema, and motor or trophic; 1 or more sign at the time of evaluation in 2 or more of the categories of involvement including sensory, vasomotor, pseudomotor, edema, and motor or trophic.8 Dermatologic findings are a common presenting feature of CRPS and are included in the Budapest Criteria used for diagnosis. In a retrospective chart review (N=26), researchers found that vascular findings were the most common dermatologic manifestation of CRPS—edema in 58% of patients and erythema in 54%.9 Other common manifestations included dermatitis (35%), erythematous papules (23%), and cutaneous atrophy (23%). Hyperpigmentation, which was present in our patient, was seen in 8% of patients in the chart review.9
Complex regional pain syndrome progresses through 3 stages; dermatologic changes are present in each stage and are more severe in later stages. Stage I lasts 2 or 3 months and is characterized by onset of pain, usually burning type, accompanied by allodynia and hyperalgesia. Early vasomotor and pseudomotor changes, such as erythema and edema, may become apparent.1,2 Stage II lasts 3 to 6 months and is characterized by more severe edema and more obvious trophic changes. Functional limitations, such as limited range of motion and muscle weakness, begin to manifest. Stage III—the final and most severe stage—is characterized by obvious hair, skin, and nail changes, as well as functional limitations.1,2 The waxy thickened skin changes and hyperpigmentation observed in our patient are characteristic of stage III. Furthermore, our patient experienced decreased mobility and limited range of motion secondary to tightening of the skin, a characteristic motor change of late-stage CRPS. Although chronic pain and allodynia are the most common characteristics of CRPS, skin changes also can cause notable distress and early dermatologic manifestations can be a chief concern.
Dermatologic management is focused to address the specific skin changes of CRPS. However, traditional treatment of the common dermatologic findings of CRPS is difficult and often unsuccessful; instead, the most successful treatment of skin findings involves controlling the underlying CRPS.9 Current treatment options include removal of any nidus of tissue trauma, sympathetic neural blockade with a local anesthetic, spinal cord stimulation to interrupt dysregulated sympathetic innervation, and physiotherapy or occupational therapy to desensitize skin.1,10
Given the complexity of CRPS and the variability of its presentation, management of the syndrome and its associated dermatologic conditions often requires interdisciplinary care and coordination of multiple specialties. Dermatologists can play an important role in both identification of CRPS and co-management of affected patients. Early diagnosis of CRPS has been universally identified as a key prognostic factor. For that reason, dermatologists should be aware of CRPS and include the syndrome in the differential diagnosis when presented with severe cutaneous findings following trauma either with or without peripheral nerve damage, suggestive of CRPS.
- Sebastin SJ. Complex regional pain syndrome. Indian J Plast Surg. 2011;44:298-307. doi:10.4103/0970-0358.85351
- Kabani R, Brassard A. Dermatological findings in early detection of complex regional pain syndrome. JAMA Dermatol. 2014;150:640-642. doi:10.1001/jamadermatol.2013.7459
- Moseley L. What is complex regional pain syndrome – in plain English. International Association for the Study of Pain website. Published 2009. Accessed December 15, 2022. https://www.iasp-pain.org/publications/relief-news/article/what-is-complex-pain-syndrome-in-plain-english/
- Pak TJ, Martin GM, Magness JL, et al. Reflex sympathetic dystrophy. Review of 140 cases. Minn Med. 1970;53:507-512.
- Qureshi AA, Friedman AJ. Complex regional pain syndrome: what the dermatologist should know. J Drugs Dermatol. 2018;17:532-536.
- Gorodkin R. Complex regional pain syndrome. Rheumatology. 2016;55(suppl 1):i12.
- Araki E, Tanioka M, Miyachi Y, et al. A case of complex regional pain syndrome: an underdiagnosed condition in dermatology. Acta Derm Venereol. 2007;87:440-441. doi:10.2340/00015555-0281
- Pergolizzi JV, LeQuang JA, Nalamachu S, et al. The Budapest criteria for complex regional pain syndrome: the diagnostic challenge. Anaesthesiol Clin Sci Res. 2018;2:1-10. doi:10.35841/anesthesiology.2.1.1-10
- Sundaram S, Webster GF. Vascular diseases are the most common cutaneous manifestations of reflex sympathetic dystrophy. J Am Acad Dermatol. 2001;44:1050-1051. doi:10.1067/mjd.2001.114299
- Taylor RS, Van Buyten J-P, Buchser E. Spinal stimulation for complex regional pain syndrome: a systematic review of the clinical and cost-effectiveness literature and assessment of prognostic factors. Eur J Pain. 2006;10:91-101. doi:10.1016/j.ejpain.2005.02.004
- Sebastin SJ. Complex regional pain syndrome. Indian J Plast Surg. 2011;44:298-307. doi:10.4103/0970-0358.85351
- Kabani R, Brassard A. Dermatological findings in early detection of complex regional pain syndrome. JAMA Dermatol. 2014;150:640-642. doi:10.1001/jamadermatol.2013.7459
- Moseley L. What is complex regional pain syndrome – in plain English. International Association for the Study of Pain website. Published 2009. Accessed December 15, 2022. https://www.iasp-pain.org/publications/relief-news/article/what-is-complex-pain-syndrome-in-plain-english/
- Pak TJ, Martin GM, Magness JL, et al. Reflex sympathetic dystrophy. Review of 140 cases. Minn Med. 1970;53:507-512.
- Qureshi AA, Friedman AJ. Complex regional pain syndrome: what the dermatologist should know. J Drugs Dermatol. 2018;17:532-536.
- Gorodkin R. Complex regional pain syndrome. Rheumatology. 2016;55(suppl 1):i12.
- Araki E, Tanioka M, Miyachi Y, et al. A case of complex regional pain syndrome: an underdiagnosed condition in dermatology. Acta Derm Venereol. 2007;87:440-441. doi:10.2340/00015555-0281
- Pergolizzi JV, LeQuang JA, Nalamachu S, et al. The Budapest criteria for complex regional pain syndrome: the diagnostic challenge. Anaesthesiol Clin Sci Res. 2018;2:1-10. doi:10.35841/anesthesiology.2.1.1-10
- Sundaram S, Webster GF. Vascular diseases are the most common cutaneous manifestations of reflex sympathetic dystrophy. J Am Acad Dermatol. 2001;44:1050-1051. doi:10.1067/mjd.2001.114299
- Taylor RS, Van Buyten J-P, Buchser E. Spinal stimulation for complex regional pain syndrome: a systematic review of the clinical and cost-effectiveness literature and assessment of prognostic factors. Eur J Pain. 2006;10:91-101. doi:10.1016/j.ejpain.2005.02.004
PRACTICE POINTS
- Common dermatologic manifestations of complex regional pain syndrome (CRPS), which often are nonspecific and often the presenting symptoms of the syndrome, include allodynia, edema, erythema, hypopigmentation or hyperpigmentation, and petechiae.
- Diagnosis and management of CRPS are the most important steps in treating dermatologic manifestations of the syndrome.
Factors Influencing Patient Preferences for Phototherapy: A Survey Study
Phototherapy—particularly UVB phototherapy, which utilizes UVB rays of specific wavelengths within the UV spectrum—is indicated for a wide variety of dermatoses. In-office and at-home UVB treatments commonly are used, as are salon tanning and sunbathing. When selecting a form of phototherapy, patients are likely to consider safety, cost, effectiveness, insurance issues, and convenience. Research on patient preferences; the reasons for these preferences; and which options patients perceive to be the safest, most cost-effective, efficacious, and convenient is lacking. We aimed to assess the forms of phototherapy that patients would most consider using; the factors influencing patient preferences; and the forms patients perceived as the safest and most cost-effective, efficacious, and convenient.
Methods
Study Participants—We recruited 500 Amazon Mechanical Turk users who were 18 years or older to complete our REDCap-generated survey. The study was approved by the Wake Forest University institutional review board (Winston-Salem, North Carolina).
Evaluation—Participants were asked, “If you were diagnosed with a skin disease that benefited from UV therapy, which of the following forms of UV therapy would you consider choosing?” Participants were instructed to choose all of the forms they would consider using. Available options included in-office UV, at-home UV, home tanning, salon tanning, sunbathing, and other. Participants were asked to select which factors—from safety, cost, effectiveness, issues with insurance, convenience, and other—influenced their decision-making; which form of phototherapy they would most consider along with the factors that influenced their preference for this specific form of phototherapy; and which options they considered to be safest and most cost-effective, efficacious, and convenient. Participants were asked to provide basic sociodemographic information, level of education, income, insurance status (private, Medicare, Medicaid, Veterans Affairs, and uninsured), and distance from the nearest dermatologist.
Statistical Analysis—Descriptive and inferential statistics (χ2 test) were used to analyze the data with a significance set at P<.05.
Results
Five hundred participants completed the survey (Table 1).

Factors Influencing Patient Preferences—When asked to select all forms of phototherapy they would consider, 186 (37.2%) participants selected in-office UVB, 263 (52.6%) selected at-home UV, 141 (28.2%) selected home tanning, 117 (23.4%) selected salon tanning, 191 (38.2%) selected sunbathing, and 3 (0.6%) selected other. Participants who selected in-office UVB as an option were more likely to also select salon tanning (P<.012). No other relationship was found between the UVB options and the tanning options. When asked which factors influenced their phototherapy preferences, 295 (59%) selected convenience, 266 (53.2%) selected effectiveness, 220 (44%) selected safety, 218 (43.6%) selected cost, 72 (14.4%) selected issues with insurance, and 4 (0.8%) selected other. Forms of Phototherapy Patients Consider Using—When asked which form of phototherapy they would most consider using, 179 (35.8%) participants selected at-home UVB, 108 (21.6%) selected sunbathing, 92 (18.4%) selected in-office UVB, 62 (12.4%) selected home-tanning, 57 (11.4%) selected salon tanning, 1 (0.2%) selected other, and 1 participant provided no response (P<.001).
Reasons for Using Phototherapy—Of the 179 who selected at-home UVB, 125 (70%) cited convenience as a reason. Of the 108 who selected salon tanning as their top choice, 62 (57%) cited cost as a reason. Convenience (P<.001), cost (P<.001), and safety (P=.023) were related to top preference. Issues with insurance did not have a statistically significant relationship with the top preference. However, participant insurance type was related to top phototherapy preference (P=.021), with privately insured patients more likely to select in-office UVB, whereas those with Medicaid and Medicare were more likely to select home or salon tanning. Efficacy was not related to top preference. Furthermore, age, gender, education, income, and distance from nearest dermatologist were not related to top preference.
In-office UVB was perceived to be safest (P<.001) and most efficacious (P<.001). Meanwhile, at-home UVB was selected as most convenient (P<.001). Lastly, sunbathing was determined to be most cost-effective (P<.001)(Table 2). Cost-effectiveness had a relationship (P<.001) with the participant’s insurance, as those with private insurance were more likely to select at-home UVB, whereas those with Medicare or Medicaid were more likely to select the tanning options. Additionally, of the54 uninsured participants in the survey, 29 selected sunbathing as the most cost-effective option.

Comment
Phototherapy Treatment—UVB phototherapy at a wavelength of 290 to 320 nm (311–313 nm for narrowband UVB) is used to treat various dermatoses, including psoriasis and atopic dermatitis. UVB alters skin cytokines, induces apoptosis, promotes immunosuppression, causes DNA damage, and decreases the proliferation of dendritic cells and other cells of the innate immune system.1 In-office and at-home UV therapies make use of UVB wavelengths for treatment, while tanning and sunbathing contain not only UVB but also potentially harmful UVA rays. The wavelengths for indoor tanning devices include UVB at 280 to 315 nm and UVA at 315 to 400 nm, which are similar to those of the sun but with a different ratio of UVB to UVA and more intense total UV.2 When in-office and at-home UVB options are not available, various forms of tanning such as salon tanning and sunbathing may be alternatives that are widely used.3 One of the main reasons patients consider alternative phototherapy options is cost, as 1 in-office UVB treatment may cost $140, but a month of unlimited tanning may cost $30 or perhaps nothing if a patient has a gym membership with access to a tanning bed. Lack of insurance benefits covering phototherapy can exacerbate cost burden.4 However, tanning beds are associated with an increased risk for melanoma and nonmelanoma cancers.5,6 Additionally, all forms of phototherapy are associated with photoaging, but it is more intense with tanning and heliotherapy because of the presence of UVA, which penetrates deeper into the dermis.7 Meanwhile, for those who choose UVB therapy, deciding between an in-office and at-home UVB treatment could be a matter of convenience, as patients must consider long trips to the physician’s office; insurance status, as some insurances may not cover at-home UVB; or efficacy, which might be influenced by the presence of a physician or other medical staff. In many cases, patients may not be informed that at-home UVB is an option.
Patient Preferences—At-home UVB therapy was the most popular option in our study population, with most participants (52.6%) considering using it, and 35.9% choosing it as their top choice over all other phototherapy options. Safety, cost, and convenience were all found to be related to the option participants would most consider using. Prior analysis between at-home UVB and in-office UVB for the treatment of psoriasis determined that at-home UVB is as safe and cost-effective as in-office UVB without the inconvenience of the patient having to take time out of the week to visit the physician’s office,8,9 making at-home UVB an option dermatologists may strongly consider for patients who value safety, cost, and convenience. Oddly, efficacy was not related to the top preference, despite being the second highest–cited factor (53.2%) for which forms of phototherapy participants would consider using. For insurance coverage, those with Medicaid and Medicare selected the cheaper tanning options with higher-than-expected frequencies. Although problems with insurance were not related to the top preference, insurance status was related, suggesting that preferences are tied to cost. Of note, while the number of dermatologists that accept Medicare has increased in the last few years, there still remains an uneven distribution of phototherapy clinics. As of 2015, there were 19 million individuals who qualified for Medicare without a clinic within driving distance.10 This problem likely also exists for many Medicaid patients who may not qualify for at-home UVB. In this scenario, tanning or heliotherapy may be effective alternatives.
In-Office vs At-Home Options—Although in-office UVB was the option considered safest (26.2%) and most efficacious (26.8%), it was followed closely by at-home UVB in both categories (safest, 23.8%; most efficacious, 24.2%). Meanwhile, at-home UVB (40.2%) was chosen as the most convenient. Some patients consider tanning options over in-office UVB because of the inconvenience of traveling to an appointment.11 Therefore, at-home tanning may be a convenient alternative for these patients.
Considerations—Although our study was limited to an adult population, issues with convenience exist for the pediatric population as well, as children may need to miss multiple days of school each week to be treated in the office. For these pediatric patients, an at-home unit is preferable; however; issues with insurance coverage remain a challenge.12 Increasing insurance coverage of at-home units for the pediatric population therefore would be most prudent. However, when other options have been exhausted, including in-office UVB, tanning and sunbathing may be viable alternatives because of cost and convenience. In our study, sunbathing (33.2%) was considered the most cost-effective, likely because it does not require expensive equipment or a visit to a salon or physician’s office. Sunbathing has been effective in treating some dermatologic conditions, such as atopic dermatitis.13 However, it may only be effective during certain months and at different latitudes—conditions that make UVB sun rays more accessible—particularly when treating psoriasis.14 Furthermore, sunbathing may not be as cost-effective in patients with average-severity psoriasis compared with conventional psoriasis therapy because of the costs of travel to areas with sufficient UVB rays for treatment.15 Additionally, insurance status was related to which option was selected as the most cost-effective, as 29 (53.7%) of 54 uninsured participants chose sunbathing as the most cost-effective option, while only 92 (34.2%) of 269 privately insured patients selected sunbathing. Therefore, insurance status may be a factor for dermatologists to consider if a patient prefers a treatment that is cost-effective. Overall, dermatologists could perhaps consider guiding patients and optimizing their treatment plans based on the factors most important to the patients while understanding that costs and insurance status may ultimately determine the treatment option.
Limitations—Survey participants were recruited on Amazon Mechanical Turk, which could create sampling bias. Furthermore, these participants were representative of the general public and not exclusively patients on phototherapy, therefore representing the opinions of the general public and not those who may require phototherapy. Furthermore, given the nature of the survey, the study was limited to the adult population.
- Totonchy MB, Chiu MW. UV-based therapy. Dermatol Clin. 2014;32:399-413, ix-x.
- Nilsen LT, Hannevik M, Veierød MB. Ultraviolet exposure from indoor tanning devices: a systematic review. Br J Dermatol. 2016;174:730-740.
- Su J, Pearce DJ, Feldman SR. The role of commercial tanning beds and ultraviolet A light in the treatment of psoriasis. J Dermatolog Treat. 2005;16:324-326.
- Anderson KL, Huang KE, Huang WW, et al. Dermatology residents are prescribing tanning bed treatment. Dermatol Online J. 2016;22:13030/qt19h4k7sx.
- Wehner MR, Shive ML, Chren MM, et al. Indoor tanning and non-melanoma skin cancer: systematic review and meta-analysis. BMJ. 2012;345:e5909.
- Boniol M, Autier P, Boyle P, et al. Cutaneous melanomaattributable to sunbed use: systematic review and meta-analysis. BMJ. 2012;345:E4757.
- Barros NM, Sbroglio LL, Buffara MO, et al. Phototherapy. An Bras Dermatol. 2021;96:397-407.
- Koek MB, Buskens E, van Weelden H, et al. Home versus outpatient ultraviolet B phototherapy for mild to severe psoriasis: pragmatic multicentre randomized controlled non-inferiority trial (PLUTO study). BMJ. 2009;338:b1542.
- Koek MB, Sigurdsson V, van Weelden H, et al. Cost effectiveness of home ultraviolet B phototherapy for psoriasis: economic evaluation of a randomized controlled trial (PLUTO study). BMJ. 2010;340:c1490.
- Tan SY, Buzney E, Mostaghimi A. Trends in phototherapy utilization among Medicare beneficiaries in the United States, 2000 to 2015. J Am Acad Dermatol. 2018;79:672-679.
- Felton S, Adinoff B, Jeon-Slaughter H, et al. The significant health threat from tanning bed use as a self-treatment for psoriasis. J Am Acad Dermatol. 2016;74:1015-1017.
- Juarez MC, Grossberg AL. Phototherapy in the pediatric population. Dermatol Clin. 2020;38:91-108.
- Autio P, Komulainen P, Larni HM. Heliotherapy in atopic dermatitis: a prospective study on climatotherapy using the SCORAD index. Acta Derm Venereol. 2002;82:436-440.
- Krzys´cin JW, Jarosławski J, Rajewska-Wie˛ch B, et al. Effectiveness of heliotherapy for psoriasis clearance in low and mid-latitudinal regions: a theoretical approach. J Photochem Photobiol B. 2012;115:35-41.
- Snellman E, Maljanen T, Aromaa A, et al. Effect of heliotherapy on the cost of psoriasis. Br J Dermatol. 1998;138:288-292.
Phototherapy—particularly UVB phototherapy, which utilizes UVB rays of specific wavelengths within the UV spectrum—is indicated for a wide variety of dermatoses. In-office and at-home UVB treatments commonly are used, as are salon tanning and sunbathing. When selecting a form of phototherapy, patients are likely to consider safety, cost, effectiveness, insurance issues, and convenience. Research on patient preferences; the reasons for these preferences; and which options patients perceive to be the safest, most cost-effective, efficacious, and convenient is lacking. We aimed to assess the forms of phototherapy that patients would most consider using; the factors influencing patient preferences; and the forms patients perceived as the safest and most cost-effective, efficacious, and convenient.
Methods
Study Participants—We recruited 500 Amazon Mechanical Turk users who were 18 years or older to complete our REDCap-generated survey. The study was approved by the Wake Forest University institutional review board (Winston-Salem, North Carolina).
Evaluation—Participants were asked, “If you were diagnosed with a skin disease that benefited from UV therapy, which of the following forms of UV therapy would you consider choosing?” Participants were instructed to choose all of the forms they would consider using. Available options included in-office UV, at-home UV, home tanning, salon tanning, sunbathing, and other. Participants were asked to select which factors—from safety, cost, effectiveness, issues with insurance, convenience, and other—influenced their decision-making; which form of phototherapy they would most consider along with the factors that influenced their preference for this specific form of phototherapy; and which options they considered to be safest and most cost-effective, efficacious, and convenient. Participants were asked to provide basic sociodemographic information, level of education, income, insurance status (private, Medicare, Medicaid, Veterans Affairs, and uninsured), and distance from the nearest dermatologist.
Statistical Analysis—Descriptive and inferential statistics (χ2 test) were used to analyze the data with a significance set at P<.05.
Results
Five hundred participants completed the survey (Table 1).

Factors Influencing Patient Preferences—When asked to select all forms of phototherapy they would consider, 186 (37.2%) participants selected in-office UVB, 263 (52.6%) selected at-home UV, 141 (28.2%) selected home tanning, 117 (23.4%) selected salon tanning, 191 (38.2%) selected sunbathing, and 3 (0.6%) selected other. Participants who selected in-office UVB as an option were more likely to also select salon tanning (P<.012). No other relationship was found between the UVB options and the tanning options. When asked which factors influenced their phototherapy preferences, 295 (59%) selected convenience, 266 (53.2%) selected effectiveness, 220 (44%) selected safety, 218 (43.6%) selected cost, 72 (14.4%) selected issues with insurance, and 4 (0.8%) selected other. Forms of Phototherapy Patients Consider Using—When asked which form of phototherapy they would most consider using, 179 (35.8%) participants selected at-home UVB, 108 (21.6%) selected sunbathing, 92 (18.4%) selected in-office UVB, 62 (12.4%) selected home-tanning, 57 (11.4%) selected salon tanning, 1 (0.2%) selected other, and 1 participant provided no response (P<.001).
Reasons for Using Phototherapy—Of the 179 who selected at-home UVB, 125 (70%) cited convenience as a reason. Of the 108 who selected salon tanning as their top choice, 62 (57%) cited cost as a reason. Convenience (P<.001), cost (P<.001), and safety (P=.023) were related to top preference. Issues with insurance did not have a statistically significant relationship with the top preference. However, participant insurance type was related to top phototherapy preference (P=.021), with privately insured patients more likely to select in-office UVB, whereas those with Medicaid and Medicare were more likely to select home or salon tanning. Efficacy was not related to top preference. Furthermore, age, gender, education, income, and distance from nearest dermatologist were not related to top preference.
In-office UVB was perceived to be safest (P<.001) and most efficacious (P<.001). Meanwhile, at-home UVB was selected as most convenient (P<.001). Lastly, sunbathing was determined to be most cost-effective (P<.001)(Table 2). Cost-effectiveness had a relationship (P<.001) with the participant’s insurance, as those with private insurance were more likely to select at-home UVB, whereas those with Medicare or Medicaid were more likely to select the tanning options. Additionally, of the54 uninsured participants in the survey, 29 selected sunbathing as the most cost-effective option.

Comment
Phototherapy Treatment—UVB phototherapy at a wavelength of 290 to 320 nm (311–313 nm for narrowband UVB) is used to treat various dermatoses, including psoriasis and atopic dermatitis. UVB alters skin cytokines, induces apoptosis, promotes immunosuppression, causes DNA damage, and decreases the proliferation of dendritic cells and other cells of the innate immune system.1 In-office and at-home UV therapies make use of UVB wavelengths for treatment, while tanning and sunbathing contain not only UVB but also potentially harmful UVA rays. The wavelengths for indoor tanning devices include UVB at 280 to 315 nm and UVA at 315 to 400 nm, which are similar to those of the sun but with a different ratio of UVB to UVA and more intense total UV.2 When in-office and at-home UVB options are not available, various forms of tanning such as salon tanning and sunbathing may be alternatives that are widely used.3 One of the main reasons patients consider alternative phototherapy options is cost, as 1 in-office UVB treatment may cost $140, but a month of unlimited tanning may cost $30 or perhaps nothing if a patient has a gym membership with access to a tanning bed. Lack of insurance benefits covering phototherapy can exacerbate cost burden.4 However, tanning beds are associated with an increased risk for melanoma and nonmelanoma cancers.5,6 Additionally, all forms of phototherapy are associated with photoaging, but it is more intense with tanning and heliotherapy because of the presence of UVA, which penetrates deeper into the dermis.7 Meanwhile, for those who choose UVB therapy, deciding between an in-office and at-home UVB treatment could be a matter of convenience, as patients must consider long trips to the physician’s office; insurance status, as some insurances may not cover at-home UVB; or efficacy, which might be influenced by the presence of a physician or other medical staff. In many cases, patients may not be informed that at-home UVB is an option.
Patient Preferences—At-home UVB therapy was the most popular option in our study population, with most participants (52.6%) considering using it, and 35.9% choosing it as their top choice over all other phototherapy options. Safety, cost, and convenience were all found to be related to the option participants would most consider using. Prior analysis between at-home UVB and in-office UVB for the treatment of psoriasis determined that at-home UVB is as safe and cost-effective as in-office UVB without the inconvenience of the patient having to take time out of the week to visit the physician’s office,8,9 making at-home UVB an option dermatologists may strongly consider for patients who value safety, cost, and convenience. Oddly, efficacy was not related to the top preference, despite being the second highest–cited factor (53.2%) for which forms of phototherapy participants would consider using. For insurance coverage, those with Medicaid and Medicare selected the cheaper tanning options with higher-than-expected frequencies. Although problems with insurance were not related to the top preference, insurance status was related, suggesting that preferences are tied to cost. Of note, while the number of dermatologists that accept Medicare has increased in the last few years, there still remains an uneven distribution of phototherapy clinics. As of 2015, there were 19 million individuals who qualified for Medicare without a clinic within driving distance.10 This problem likely also exists for many Medicaid patients who may not qualify for at-home UVB. In this scenario, tanning or heliotherapy may be effective alternatives.
In-Office vs At-Home Options—Although in-office UVB was the option considered safest (26.2%) and most efficacious (26.8%), it was followed closely by at-home UVB in both categories (safest, 23.8%; most efficacious, 24.2%). Meanwhile, at-home UVB (40.2%) was chosen as the most convenient. Some patients consider tanning options over in-office UVB because of the inconvenience of traveling to an appointment.11 Therefore, at-home tanning may be a convenient alternative for these patients.
Considerations—Although our study was limited to an adult population, issues with convenience exist for the pediatric population as well, as children may need to miss multiple days of school each week to be treated in the office. For these pediatric patients, an at-home unit is preferable; however; issues with insurance coverage remain a challenge.12 Increasing insurance coverage of at-home units for the pediatric population therefore would be most prudent. However, when other options have been exhausted, including in-office UVB, tanning and sunbathing may be viable alternatives because of cost and convenience. In our study, sunbathing (33.2%) was considered the most cost-effective, likely because it does not require expensive equipment or a visit to a salon or physician’s office. Sunbathing has been effective in treating some dermatologic conditions, such as atopic dermatitis.13 However, it may only be effective during certain months and at different latitudes—conditions that make UVB sun rays more accessible—particularly when treating psoriasis.14 Furthermore, sunbathing may not be as cost-effective in patients with average-severity psoriasis compared with conventional psoriasis therapy because of the costs of travel to areas with sufficient UVB rays for treatment.15 Additionally, insurance status was related to which option was selected as the most cost-effective, as 29 (53.7%) of 54 uninsured participants chose sunbathing as the most cost-effective option, while only 92 (34.2%) of 269 privately insured patients selected sunbathing. Therefore, insurance status may be a factor for dermatologists to consider if a patient prefers a treatment that is cost-effective. Overall, dermatologists could perhaps consider guiding patients and optimizing their treatment plans based on the factors most important to the patients while understanding that costs and insurance status may ultimately determine the treatment option.
Limitations—Survey participants were recruited on Amazon Mechanical Turk, which could create sampling bias. Furthermore, these participants were representative of the general public and not exclusively patients on phototherapy, therefore representing the opinions of the general public and not those who may require phototherapy. Furthermore, given the nature of the survey, the study was limited to the adult population.
Phototherapy—particularly UVB phototherapy, which utilizes UVB rays of specific wavelengths within the UV spectrum—is indicated for a wide variety of dermatoses. In-office and at-home UVB treatments commonly are used, as are salon tanning and sunbathing. When selecting a form of phototherapy, patients are likely to consider safety, cost, effectiveness, insurance issues, and convenience. Research on patient preferences; the reasons for these preferences; and which options patients perceive to be the safest, most cost-effective, efficacious, and convenient is lacking. We aimed to assess the forms of phototherapy that patients would most consider using; the factors influencing patient preferences; and the forms patients perceived as the safest and most cost-effective, efficacious, and convenient.
Methods
Study Participants—We recruited 500 Amazon Mechanical Turk users who were 18 years or older to complete our REDCap-generated survey. The study was approved by the Wake Forest University institutional review board (Winston-Salem, North Carolina).
Evaluation—Participants were asked, “If you were diagnosed with a skin disease that benefited from UV therapy, which of the following forms of UV therapy would you consider choosing?” Participants were instructed to choose all of the forms they would consider using. Available options included in-office UV, at-home UV, home tanning, salon tanning, sunbathing, and other. Participants were asked to select which factors—from safety, cost, effectiveness, issues with insurance, convenience, and other—influenced their decision-making; which form of phototherapy they would most consider along with the factors that influenced their preference for this specific form of phototherapy; and which options they considered to be safest and most cost-effective, efficacious, and convenient. Participants were asked to provide basic sociodemographic information, level of education, income, insurance status (private, Medicare, Medicaid, Veterans Affairs, and uninsured), and distance from the nearest dermatologist.
Statistical Analysis—Descriptive and inferential statistics (χ2 test) were used to analyze the data with a significance set at P<.05.
Results
Five hundred participants completed the survey (Table 1).

Factors Influencing Patient Preferences—When asked to select all forms of phototherapy they would consider, 186 (37.2%) participants selected in-office UVB, 263 (52.6%) selected at-home UV, 141 (28.2%) selected home tanning, 117 (23.4%) selected salon tanning, 191 (38.2%) selected sunbathing, and 3 (0.6%) selected other. Participants who selected in-office UVB as an option were more likely to also select salon tanning (P<.012). No other relationship was found between the UVB options and the tanning options. When asked which factors influenced their phototherapy preferences, 295 (59%) selected convenience, 266 (53.2%) selected effectiveness, 220 (44%) selected safety, 218 (43.6%) selected cost, 72 (14.4%) selected issues with insurance, and 4 (0.8%) selected other. Forms of Phototherapy Patients Consider Using—When asked which form of phototherapy they would most consider using, 179 (35.8%) participants selected at-home UVB, 108 (21.6%) selected sunbathing, 92 (18.4%) selected in-office UVB, 62 (12.4%) selected home-tanning, 57 (11.4%) selected salon tanning, 1 (0.2%) selected other, and 1 participant provided no response (P<.001).
Reasons for Using Phototherapy—Of the 179 who selected at-home UVB, 125 (70%) cited convenience as a reason. Of the 108 who selected salon tanning as their top choice, 62 (57%) cited cost as a reason. Convenience (P<.001), cost (P<.001), and safety (P=.023) were related to top preference. Issues with insurance did not have a statistically significant relationship with the top preference. However, participant insurance type was related to top phototherapy preference (P=.021), with privately insured patients more likely to select in-office UVB, whereas those with Medicaid and Medicare were more likely to select home or salon tanning. Efficacy was not related to top preference. Furthermore, age, gender, education, income, and distance from nearest dermatologist were not related to top preference.
In-office UVB was perceived to be safest (P<.001) and most efficacious (P<.001). Meanwhile, at-home UVB was selected as most convenient (P<.001). Lastly, sunbathing was determined to be most cost-effective (P<.001)(Table 2). Cost-effectiveness had a relationship (P<.001) with the participant’s insurance, as those with private insurance were more likely to select at-home UVB, whereas those with Medicare or Medicaid were more likely to select the tanning options. Additionally, of the54 uninsured participants in the survey, 29 selected sunbathing as the most cost-effective option.

Comment
Phototherapy Treatment—UVB phototherapy at a wavelength of 290 to 320 nm (311–313 nm for narrowband UVB) is used to treat various dermatoses, including psoriasis and atopic dermatitis. UVB alters skin cytokines, induces apoptosis, promotes immunosuppression, causes DNA damage, and decreases the proliferation of dendritic cells and other cells of the innate immune system.1 In-office and at-home UV therapies make use of UVB wavelengths for treatment, while tanning and sunbathing contain not only UVB but also potentially harmful UVA rays. The wavelengths for indoor tanning devices include UVB at 280 to 315 nm and UVA at 315 to 400 nm, which are similar to those of the sun but with a different ratio of UVB to UVA and more intense total UV.2 When in-office and at-home UVB options are not available, various forms of tanning such as salon tanning and sunbathing may be alternatives that are widely used.3 One of the main reasons patients consider alternative phototherapy options is cost, as 1 in-office UVB treatment may cost $140, but a month of unlimited tanning may cost $30 or perhaps nothing if a patient has a gym membership with access to a tanning bed. Lack of insurance benefits covering phototherapy can exacerbate cost burden.4 However, tanning beds are associated with an increased risk for melanoma and nonmelanoma cancers.5,6 Additionally, all forms of phototherapy are associated with photoaging, but it is more intense with tanning and heliotherapy because of the presence of UVA, which penetrates deeper into the dermis.7 Meanwhile, for those who choose UVB therapy, deciding between an in-office and at-home UVB treatment could be a matter of convenience, as patients must consider long trips to the physician’s office; insurance status, as some insurances may not cover at-home UVB; or efficacy, which might be influenced by the presence of a physician or other medical staff. In many cases, patients may not be informed that at-home UVB is an option.
Patient Preferences—At-home UVB therapy was the most popular option in our study population, with most participants (52.6%) considering using it, and 35.9% choosing it as their top choice over all other phototherapy options. Safety, cost, and convenience were all found to be related to the option participants would most consider using. Prior analysis between at-home UVB and in-office UVB for the treatment of psoriasis determined that at-home UVB is as safe and cost-effective as in-office UVB without the inconvenience of the patient having to take time out of the week to visit the physician’s office,8,9 making at-home UVB an option dermatologists may strongly consider for patients who value safety, cost, and convenience. Oddly, efficacy was not related to the top preference, despite being the second highest–cited factor (53.2%) for which forms of phototherapy participants would consider using. For insurance coverage, those with Medicaid and Medicare selected the cheaper tanning options with higher-than-expected frequencies. Although problems with insurance were not related to the top preference, insurance status was related, suggesting that preferences are tied to cost. Of note, while the number of dermatologists that accept Medicare has increased in the last few years, there still remains an uneven distribution of phototherapy clinics. As of 2015, there were 19 million individuals who qualified for Medicare without a clinic within driving distance.10 This problem likely also exists for many Medicaid patients who may not qualify for at-home UVB. In this scenario, tanning or heliotherapy may be effective alternatives.
In-Office vs At-Home Options—Although in-office UVB was the option considered safest (26.2%) and most efficacious (26.8%), it was followed closely by at-home UVB in both categories (safest, 23.8%; most efficacious, 24.2%). Meanwhile, at-home UVB (40.2%) was chosen as the most convenient. Some patients consider tanning options over in-office UVB because of the inconvenience of traveling to an appointment.11 Therefore, at-home tanning may be a convenient alternative for these patients.
Considerations—Although our study was limited to an adult population, issues with convenience exist for the pediatric population as well, as children may need to miss multiple days of school each week to be treated in the office. For these pediatric patients, an at-home unit is preferable; however; issues with insurance coverage remain a challenge.12 Increasing insurance coverage of at-home units for the pediatric population therefore would be most prudent. However, when other options have been exhausted, including in-office UVB, tanning and sunbathing may be viable alternatives because of cost and convenience. In our study, sunbathing (33.2%) was considered the most cost-effective, likely because it does not require expensive equipment or a visit to a salon or physician’s office. Sunbathing has been effective in treating some dermatologic conditions, such as atopic dermatitis.13 However, it may only be effective during certain months and at different latitudes—conditions that make UVB sun rays more accessible—particularly when treating psoriasis.14 Furthermore, sunbathing may not be as cost-effective in patients with average-severity psoriasis compared with conventional psoriasis therapy because of the costs of travel to areas with sufficient UVB rays for treatment.15 Additionally, insurance status was related to which option was selected as the most cost-effective, as 29 (53.7%) of 54 uninsured participants chose sunbathing as the most cost-effective option, while only 92 (34.2%) of 269 privately insured patients selected sunbathing. Therefore, insurance status may be a factor for dermatologists to consider if a patient prefers a treatment that is cost-effective. Overall, dermatologists could perhaps consider guiding patients and optimizing their treatment plans based on the factors most important to the patients while understanding that costs and insurance status may ultimately determine the treatment option.
Limitations—Survey participants were recruited on Amazon Mechanical Turk, which could create sampling bias. Furthermore, these participants were representative of the general public and not exclusively patients on phototherapy, therefore representing the opinions of the general public and not those who may require phototherapy. Furthermore, given the nature of the survey, the study was limited to the adult population.
- Totonchy MB, Chiu MW. UV-based therapy. Dermatol Clin. 2014;32:399-413, ix-x.
- Nilsen LT, Hannevik M, Veierød MB. Ultraviolet exposure from indoor tanning devices: a systematic review. Br J Dermatol. 2016;174:730-740.
- Su J, Pearce DJ, Feldman SR. The role of commercial tanning beds and ultraviolet A light in the treatment of psoriasis. J Dermatolog Treat. 2005;16:324-326.
- Anderson KL, Huang KE, Huang WW, et al. Dermatology residents are prescribing tanning bed treatment. Dermatol Online J. 2016;22:13030/qt19h4k7sx.
- Wehner MR, Shive ML, Chren MM, et al. Indoor tanning and non-melanoma skin cancer: systematic review and meta-analysis. BMJ. 2012;345:e5909.
- Boniol M, Autier P, Boyle P, et al. Cutaneous melanomaattributable to sunbed use: systematic review and meta-analysis. BMJ. 2012;345:E4757.
- Barros NM, Sbroglio LL, Buffara MO, et al. Phototherapy. An Bras Dermatol. 2021;96:397-407.
- Koek MB, Buskens E, van Weelden H, et al. Home versus outpatient ultraviolet B phototherapy for mild to severe psoriasis: pragmatic multicentre randomized controlled non-inferiority trial (PLUTO study). BMJ. 2009;338:b1542.
- Koek MB, Sigurdsson V, van Weelden H, et al. Cost effectiveness of home ultraviolet B phototherapy for psoriasis: economic evaluation of a randomized controlled trial (PLUTO study). BMJ. 2010;340:c1490.
- Tan SY, Buzney E, Mostaghimi A. Trends in phototherapy utilization among Medicare beneficiaries in the United States, 2000 to 2015. J Am Acad Dermatol. 2018;79:672-679.
- Felton S, Adinoff B, Jeon-Slaughter H, et al. The significant health threat from tanning bed use as a self-treatment for psoriasis. J Am Acad Dermatol. 2016;74:1015-1017.
- Juarez MC, Grossberg AL. Phototherapy in the pediatric population. Dermatol Clin. 2020;38:91-108.
- Autio P, Komulainen P, Larni HM. Heliotherapy in atopic dermatitis: a prospective study on climatotherapy using the SCORAD index. Acta Derm Venereol. 2002;82:436-440.
- Krzys´cin JW, Jarosławski J, Rajewska-Wie˛ch B, et al. Effectiveness of heliotherapy for psoriasis clearance in low and mid-latitudinal regions: a theoretical approach. J Photochem Photobiol B. 2012;115:35-41.
- Snellman E, Maljanen T, Aromaa A, et al. Effect of heliotherapy on the cost of psoriasis. Br J Dermatol. 1998;138:288-292.
- Totonchy MB, Chiu MW. UV-based therapy. Dermatol Clin. 2014;32:399-413, ix-x.
- Nilsen LT, Hannevik M, Veierød MB. Ultraviolet exposure from indoor tanning devices: a systematic review. Br J Dermatol. 2016;174:730-740.
- Su J, Pearce DJ, Feldman SR. The role of commercial tanning beds and ultraviolet A light in the treatment of psoriasis. J Dermatolog Treat. 2005;16:324-326.
- Anderson KL, Huang KE, Huang WW, et al. Dermatology residents are prescribing tanning bed treatment. Dermatol Online J. 2016;22:13030/qt19h4k7sx.
- Wehner MR, Shive ML, Chren MM, et al. Indoor tanning and non-melanoma skin cancer: systematic review and meta-analysis. BMJ. 2012;345:e5909.
- Boniol M, Autier P, Boyle P, et al. Cutaneous melanomaattributable to sunbed use: systematic review and meta-analysis. BMJ. 2012;345:E4757.
- Barros NM, Sbroglio LL, Buffara MO, et al. Phototherapy. An Bras Dermatol. 2021;96:397-407.
- Koek MB, Buskens E, van Weelden H, et al. Home versus outpatient ultraviolet B phototherapy for mild to severe psoriasis: pragmatic multicentre randomized controlled non-inferiority trial (PLUTO study). BMJ. 2009;338:b1542.
- Koek MB, Sigurdsson V, van Weelden H, et al. Cost effectiveness of home ultraviolet B phototherapy for psoriasis: economic evaluation of a randomized controlled trial (PLUTO study). BMJ. 2010;340:c1490.
- Tan SY, Buzney E, Mostaghimi A. Trends in phototherapy utilization among Medicare beneficiaries in the United States, 2000 to 2015. J Am Acad Dermatol. 2018;79:672-679.
- Felton S, Adinoff B, Jeon-Slaughter H, et al. The significant health threat from tanning bed use as a self-treatment for psoriasis. J Am Acad Dermatol. 2016;74:1015-1017.
- Juarez MC, Grossberg AL. Phototherapy in the pediatric population. Dermatol Clin. 2020;38:91-108.
- Autio P, Komulainen P, Larni HM. Heliotherapy in atopic dermatitis: a prospective study on climatotherapy using the SCORAD index. Acta Derm Venereol. 2002;82:436-440.
- Krzys´cin JW, Jarosławski J, Rajewska-Wie˛ch B, et al. Effectiveness of heliotherapy for psoriasis clearance in low and mid-latitudinal regions: a theoretical approach. J Photochem Photobiol B. 2012;115:35-41.
- Snellman E, Maljanen T, Aromaa A, et al. Effect of heliotherapy on the cost of psoriasis. Br J Dermatol. 1998;138:288-292.
Practice Points
- Patients have different priorities when selecting phototherapy, including safety, costs, effectiveness, insurance issues, and convenience.
- By offering and educating patients on all forms of phototherapy, dermatologists may help guide patients to their optimal treatment plan according to patient priorities.
Pyostomatitis Vegetans With Orofacial and Vulvar Granulomatosis in a Pediatric Patient
Case Report
A 7-year-old girl who was otherwise healthy was referred by pediatric gastroenterology for evaluation of cutaneous Crohn disease (CD). The patient had a 4-year history of persistent lip swelling and a 3-year history of asymmetric erythematous labial swelling and perianal erythema with skin tags. She had been applying the calcineurin inhibitor tacrolimus ointment 0.03% 1 or 2 times daily to her lesions with minimal improvement. She did not have a medical history of recurrent or unusual infectious diseases. There was no family history of autoimmune disease.
The patient and her guardian reported intermittent perianal pain but denied constipation, diarrhea, abdominal pain, and blood in the stool. She denied throat and tongue swelling, dysphagia, dyspnea, drooling, facial paralysis, and eyelid edema. She was a well-nourished child whose height and weight percentiles tracked at 30% and 25%, respectively. Physical examination revealed confluent symmetric lip swelling with mild angular cheilitis. Multiple 1- to 2-mm white pustules with pinpoint erosions covered the upper and lower labial mucosa and extended onto the buccal mucosa (Figure 1). She had symmetric erythema and swelling of the left labia majora extending to and involving the left perianal mucosa. Three perianal erythematous skin tags and a perianal fissure were identified.
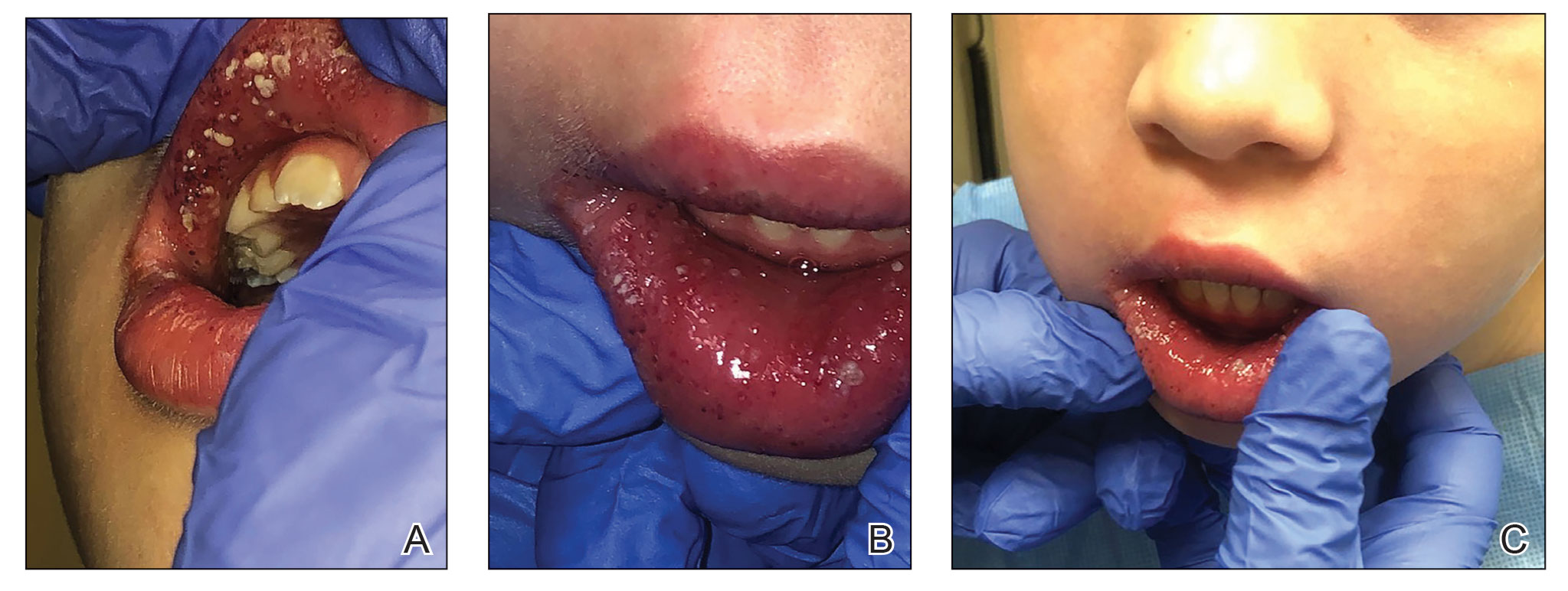
The patient had been assessed 2 years earlier by pediatric dermatology and gastroenterology with an extensive evaluation that favored a diagnosis of cutaneous CD because the combination of orofacial granulomatosis (OFG), vulvar edema, and perianal skin tags is strongly associated.1-3 Contact dermatitis affecting the mouth was considered; however, allergen testing did not demonstrate a trigger.
A trial of a benzoate- and cinnamon-free diet, which has been reported to improve OFG,4 did not provide symptomatic improvement. Topical corticosteroids and tacrolimus reduced the perioral erythema, but the swelling persisted. An infectious cause was considered; however, topical mupirocin had no effect, and amoxicillin resulted in oral candidiasis.
A perianal biopsy revealed a granulomatous dermatitis. Fungal and bacterial cultures were negative. Upper and lower gastrointestinal (GI) endoscopy and a fecal calprotectin assay were not suggestive of inflammatory bowel disease (IBD). A complete blood cell count and QuantiFERON-TB Gold test measuring the immune response to tuberculosis antigens were normal. Chronic granulomatous disease, RAG1/RAG2 deficiency, common variable immunodeficiency, and NOD2 defects were ruled out with normal tests of dihydrorhodamine, quantitative immunoglobulins, and toll-like receptors.
Because of the discomfort associated with the patient’s lesions, she was offered treatment with tumor necrosis factor α inhibitors, including infliximab and adalimumab. These agents had been offered since the onset of symptoms; however, her parents declined systemic medication unless she developed GI involvement. Instead, the tacrolimus concentration was increased to 0.1% applied to the lips, labia, and perianal area, and fluocinonide gel 0.05% applied nightly to the oral pustules was added.
Two months later the patient had notably fewer oral pustules and diminished erythema but only slightly reduced oral, vulvar, and perianal swelling. A trial of oral metronidazole, which has been reported to clear a patient with cutaneous CD,5 was discontinued by her parents after 6 weeks because of a lack of interval improvement.
One year later, a pre-existing perianal skin tag doubled in size and became exquisitely tender. The calprotectin level—previously within reference range at less than 16 μg/g—was now elevated at 149 μg/g (reference range, 1–120 μg/g) and increased to 336 μg/g 3 weeks later. Testing for C-reactive protein, zinc, and stool occult blood; a comprehensive metabolic panel; and a complete blood cell count were unremarkable.
Repeat upper and lower GI endoscopy did not suggest CD. A biopsy using direct immunofluorescence (DIF) was obtained to evaluate for pyostomatitis vegetans (PSV) and rule out
The captured biopsy did not demonstrate the intended pustule; instead, it included less-affected mucosa and was obtained during topical treatment when few pustules and erosions persisted. Pathologic analysis revealed noncaseating granulomas without an increase in microabscesses, neutrophils, or eosinophils (Figure 2). Direct immunofluorescence staining for IgG, IgA, and C3 and indirect immunofluorescence staining for desmoglein-1 and desmoglein-3 antibodies were negative. Although the biopsy did not capture the intended pustule, diagnosis of PV was made based on clinical features and the constellation of cutaneous findings associated with IBD.
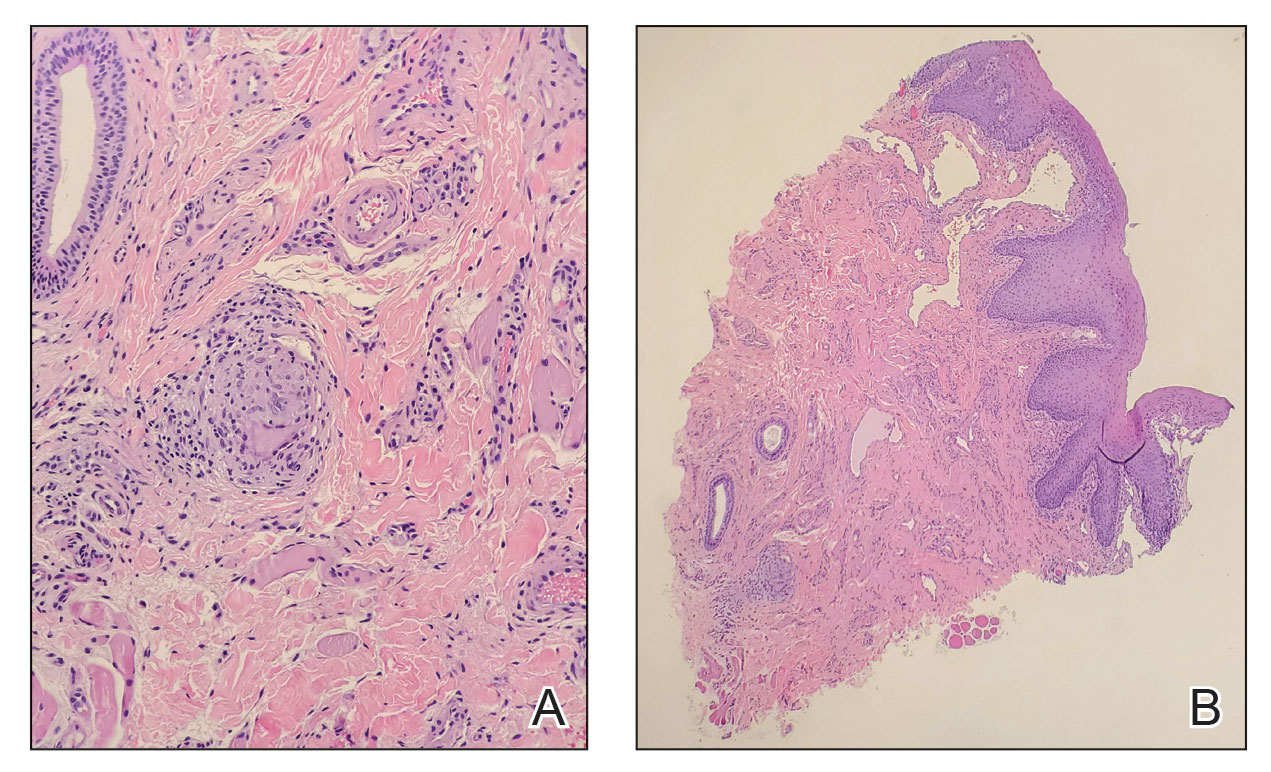
Intralesional triamcinolone, which has been of benefit for pediatric patients with orofacial granulomatosis,1,6,7 was instituted and normalized the vulva and perianal mucosa; however, lip swelling improved only minimally.
Comment
Pyostomatitis vegetans is characterized by multiple white or yellow, friable, miliary pustules that rupture, leaving behind ulcerations and erosions that cause a varying degree of oral pain.8 The disorder can involve any area of the oral mucosa—most often the labia-attached gingiva, soft and hard palates, buccal mucosa, vestibule, and tonsillar areas—but often spares the floor of the mouth and tongue.8-11 The term pyostomatitis vegetans was proposed in 1949 by McCarthy12 when he noted in a patient who presented with the characteristic appearance of the oral mucosa, though cases of vaginal, nasal, and periocular involvement have been reported.8,13,14
Histopathology—Pyostomatitis vegetans displays pseudoepithelial hyperplasia with acanthosis, hyperkeratosis, and intraepithelial or subepithelial microabscesses (or both) with neutrophils and eosinophils.8,9,15 There are a few possible explanations for this patient’s lack of tissue eosinophilia. It has been theorized that the presence of granulomas could mask concurrent PSV16 or that tissue in PSV contains fewer eosinophils as the disorder progresses.11 The oral biopsy obtained from our patient did not capture a pustule, and the condition had noticeably improved with topical tacrolimus at the time of biopsy; therefore, neither neutrophils nor eosinophils were identified. Peripheral eosinophilia, which is present in 42% to 90% of cases of PSV,9,17 can be a diagnostic clue.18 However, PE is associated with IBD,24 which usually occurs with PSV, so the absence of peripheral eosinophilia in our patient may be explained by her lack of bowel disease.
Pathogenesis—The pathogenesis of PSV is unknown. A proposed etiology includes cross-reacting antigens in the bowel and skin secondary to IBD as well as an aberrant immune response to an unidentified factor.8 Pyostomatitis vegetans is considered by many to be the mucosal variant of pyodermatitis vegetans,9,15,19 a neutrophilic dermatosis characterized by asymmetric, crusted, erythematous papulopustules that extend peripherally and coalesce to form large vegetating plaques. These lesions commonly manifest in the axillary folds, groin, and scalp and can involve the face, trunk, and distal extremities.9,18 Infection has been suggested as a cause of PSV, though cultures for pathogenic bacteria, viruses, and fungi consistently show only normal flora.20 Zinc deficiency attributed to malabsorption from CD was reported in an adult with PSV.21 The PSV resolved after 6 weeks of zinc supplementation.
Differential Diagnosis—The main entity in the clinical differential diagnosis for PSV is PVH, which is considered a variant of pemphigus vulgaris. Pemphigus vegetans of Hallopeau presents with pustules and progresses to hyperpigmented vegetative plaques with peripheral hypertrophic granulation tissue.22 The clinical and histological presentation of PVH can be similar to PSV; in PVH, however, DIF demonstrates intercellular IgG and C3 due to circulating IgG autoantibodies specific for desmoglein 3, a cell adhesion molecule.22-24 In PSV, DIF typically is negative for IgG, IgA, and C3.8 Immunohistochemical findings of PSV may overlap with IgA pemphigus, IgG/IgA pemphigus, and IgG pemphigus, which has sparked debate if PSV is an autoimmune blistering disorder or a secondary finding of epithelial injury.9,18,24
Pyostomatitis vegetans is most prevalent in patients aged 20 to 59 years25 but can occur at any age.8,19 Overall, extraintestinal symptoms, including mucocutaneous findings, are common in pediatric patients—in 30% to 71% of children with CD and 21% to 22% of children with ulcerative colitis26—and can predate onset of GI symptoms in 6% of pediatric patients.27
Oral disease is common in CD; manifestations are listed in the Table.28,29 In a prospective study of 48 children with CD, 42% (20/48) had oral manifestations identified at diagnosis28; in a similar study of 25 children, researchers noted that 48% (12/25) had disease-specific oral lesions.29 None of these children recognized the oral findings prior to the onset of systemic symptoms.28 Pyostomatitis vegetans was the least common oral manifestation, reported in 1 of 73 patients in the 2 studies combined.28,29
Two recent articles that looked at PSV in pediatric and adolescent populations identified only 9 patients with PSV.24,30 Only 2 patients (siblings) had documented onset of PSV before 12 years of age,31 which suggests an underlying genetic predisposition in young children.
It has been reported that active or subclinical (ie, asymptomatic with positive endoscopic findings) IBD in adults precedes onset of PSV, which may be considered a sign of relapse.9,30 However, PSV is incredibly rare in children and adolescents and can be an early finding of IBD in children.16,31,32
Our patient has not developed GI involvement since her initial presentation 5 years prior, though another pediatric patient developed symptomatic CD 9 years after onset of OFG.5 A retrospective review of pediatric OFG without CD met criteria for CD at a median of 3.1 years (range, 0.4–6.9 years).33 Regrettably, the early presence of PSV has been associated with future progression to CD and a complicated disease course.12,34
Management—Pyoderma stomatitis vegetans is treated with management of underlying IBD,8 with scarce literature available regarding pediatric patients. Oral lesions have been treated with antiseptics and topical corticosteroids, though these have limited benefit.8 In an adult with IBD, topical tacrolimus initially cleared PSV; however, lesions recurred until mesalamine was initiated.35 Systemic steroids were effective in a 16-year-old patient with CD and PSV,12 but recurrence is common after corticosteroids are stopped.34
Some patients benefit from steroid-sparing medications, such as dapsone, azathioprine, sulfamethoxypyridazine, methotrexate, mycophenolate mofetil, and tumor necrosis factor α inhibitors such as infliximab and adalimumab.8,9,15,23,34,36 A 12-year-old patient with pyodermatitis–PSV without intestinal disease was treated with prednisone, dapsone, and azathioprine with improvement but not complete resolution of oral erosions after 18 weeks of treatment.32 A 15-year-old patient with CD and pyodermatitis–PSV did not show improvement on prednisone, dapsone, and azathioprine but rapidly responded to infliximab.23 Infliximab led to complete clearance of oral lesions in an adult with severe fistulizing CD who developed PSV.11 However, 2 adolescent patients with CD developed PSV while on adalimumab,6,34 though 1 did improve after increasing adalimumab from once to twice weekly.6
Conclusion
The case described here—PSV in a prepubertal 7-year-old with multiple cutaneous findings suggestive of CD, including OFG, perianal and vulvar edema with biopsy-proven noncaseating granulomas, anal skin tags, and an elevated calprotectin level, noted during a cutaneous flare without clinical or endoscopically identified underlying bowel involvement—is an extremely rare presentation. Literature regarding management of PSV primarily is found in the form of case reports and focuses on treating underlying IBD. In patients with intestinal disease, treatment with biologic therapy appears most effective.6,23
ADDENDUM
Interestingly, 3 years after the patient’s original presentation to our clinic, chromosomal sequencing analysis to assess for copy number variants and whole exome gene sequencing identified a variant of unknown significance in the heat shock protein family A member 1-like gene, HSPA1L, which has an unknown mode of inheritance, but the literature suggests that both truncating and missense variants could be associated with individuals with ulcerative colitis, CD, and IBD.37,38 Although we cannot use this information to render a molecular diagnosis, it is highly suspicious that this is the cause of her clinical findings. Additionally, the patient currently is aged 10 years with unchanged cutaneous findings and has not developed gastrointestinal findings of IBD.
- Tuxen AJ, Orchard D. Childhood and adolescent orofacial granulomatosis is strongly associated with Crohn’s disease and responds to intralesional corticosteroids. Australas J Dermatol. 2010;51:124-127. doi:10.1111/j.1440-0960.2010.00627.x
- Vaid RM, Cohen BA. Cutaneous Crohn’s disease in the pediatric population. Pediatr Dermatol. 2010;27:279-281. doi:10.1111/j.1525-1470.2010.01138.x
- van de Scheur MR, van der Waal RIF, van der Waal I, et al. Ano-genital granulomatosis: the counterpart of oro-facial granulomatosis. J Eur Acad Dermatol Venereol. 2003;17:184-189. doi:10.1046/j.1468-3083.2003.00573.x
- Campbell HE, Escudier MP, Patel P, et al. Review article: cinnamon- and benzoate-free diet as a primary treatment for orofacial granulomatosis. Aliment Pharmacol Ther. 2011;34:687-701. doi:10.1111/j.1365-2036.2011.04792.x
- Duhra P, Paul CJ. Metastatic Crohn’s disease responding to metronidazole. Br J Dermatol. 1988;119:87-91. doi:10.1111/j.1365-2133.1988.tb07107.x
- Katsanos KH, Torres J, Roda G, et al. Review article: non-malignant oral manifestations in inflammatory bowel diseases. Aliment Pharmacol Ther. 2015;42:40-60. doi:10.1111/apt.13217
- Schmitz BA, Unkel JH. Symptomatic oral Crohn’s disease in an adolescent. J Dent Child (Chic). 2018;85:66-69.
- Femiano F, Lanza A, Buonaiuto C, et al. Pyostomatitis vegetans: a review of the literature. Med Oral Patol Oral Cir Bucal. 2009;14:E114-E117.
- Clark LG, Tolkachjov SN, Bridges AG, et al. Pyostomatitis vegetans (PSV)–pyodermatitis vegetans (PDV): a clinicopathologic study of 7 cases at a tertiary referral center. J Am Acad Dermatol. 2016;75:578-584. doi:10.1016/j.jaad.2016.03.047
- Hansen LS, Silverman S Jr, Daniels TE. The differential diagnosis of pyostomatitis vegetans and its relation to bowel disease. Oral Surg Oral Med Oral Pathol. 1983;55:363-373. doi:10.1016/0030-4220(83)90191-3
- Cataldo E, Covino MC, Tesone PE. Pyostomatitis vegetans. Oral Surg Oral Med Oral Pathol. 1981;52:172-177. doi:10.1016/0030-4220(81)90316-9
- McCarthy FP. Pyostomatitis vegetans; report of three cases. Arch Derm Syphilol. 1949;60:750-764.
- Bens G, Laharie D, Beylot-Barry M, et al. Successful treatment with infliximab and methotrexate of pyostomatitis vegetans associated with Crohn’s disease. Br J Dermatol. 2003;149:181-184. doi:10.1046/j.1365-2133.2003.05385.x
- Leibovitch I, Ooi C, Huilgol SC, et al. Pyodermatitis–pyostomatitis vegetans of the eyelids: case report and review of the literature. Ophthalmology. 2005;112:1809-1813. doi:10.1016/j.ophtha.2005.04.027
- Ruiz-Roca JA, Berini-Aytés L, Gay-Escoda C. Pyostomatitis vegetans. report of two cases and review of the literature. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2005;99:447-454. doi:10.1016/j.tripleo.2003.08.022
- Molnár T, Farkas K, Nagy F, et al. Third case: another pediatric patient with pyostomatitis vegetans and oral granuloma as one of the initial symptoms of Crohn’s disease. Inflamm Bowel Dis. 2011;17:E122-E123. doi:10.1002/ibd.21791
- Leydhecker W, Lund OE. Eye involvement in pyostomatitis vegetans. Klin Monbl Augenheilkd Augenarztl Fortbild. 1962;141:595-602.
- Thornhill MH, Zakrzewska JM, Gilkes JJ. Pyostomatitis vegetans: report of three cases and review of the literature. J Oral Pathol Med. 1992;21:128-133. doi:10.1111/j.1600-0714.1992.tb00996.x
- Chaudhry SI, Philpot NS, Odell EW, et al. Pyostomatitis vegetans associated with asymptomatic ulcerative colitis: a case report. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1999;87:327-330. doi:10.1016/s1079-2104(99)70217-9
- Konstantopoulou M, O’Dwyer EM, Steele JC, et al. Pyodermatitis–pyostomatitis vegetans complicated by methicillin-resistant Staphylococcus aureus infection. Clin Exp Dermatol. 2005;30:666-668. doi:10.1111/j.1365-2230.2005.01906.x
- Ficarra G, Cicchi P, Amorosi A, et al. Oral Crohn’s disease and pyostomatitis vegetans. an unusual association. Oral Surg Oral Med Oral Pathol. 1993;75:220-224. doi:10.1016/0030-4220(93)90097-n
- Markopoulos AK, Antoniades DZ, Zaraboukas T. Pemphigus vegetans of the oral cavity. Int J Dermatol. 2006;45:425-428. doi:10.1111/j.1365-4632.2004.02480.x
- Nico MMS, Hussein TP, Aoki V, et al. Pyostomatitis vegetans and its relation to inflammatory bowel disease, pyoderma gangrenosum, pyodermatitis vegetans, and pemphigus. J Oral Pathol Med. 2012;41:584-588. doi:10.1111/j.1600-0714.2012.01152.x
- Berzin D, Lahad A, Weiss B, et al. Inflammatory bowel disease presenting with pyodermatitis–pyostomatitis vegetans in a pediatric patient: a case report and review of the literature. Pediatr Dermatol. 2021;38:868-871. doi:10.1111/pde.14625
- Ballo FS, Camisa C, Allen CM. Pyostomatitis vegetans. report of a case and review of the literature. J Am Acad Dermatol. 1989;21:381-387.
- Greuter T, Bertoldo F, Rechner R, et al; Swiss IBD Cohort Study Group. Extraintestinal manifestations of pediatric inflammatory bowel disease: prevalence, presentation, and anti-TNF treatment. J Pediatr Gastroenterol Nutr. 2017;65:200-206. doi:10.1097/MPG.0000000000001455
- Jose FA, Garnett EA, Vittinghoff E, et al. Development of extraintestinal manifestations in pediatric patients with inflammatory bowel disease. Inflamm Bowel Dis. 2009;15:63-68. doi:10.1002/ibd.20604
- Harty S, Fleming P, Rowland M, et al. A prospective study of the oral manifestations of Crohn’s disease. Clin Gastroenterol Hepatol. 2005;3:886-891. doi:10.1016/s1542-3565(05)00424-6
- Pittock S, Drumm B, Fleming P, et al. The oral cavity in Crohn’s disease. J Pediatr. 2001;138:767-771. doi:10.1067/mpd.2001.113008
- Bardasi G, Romagnoli A, Foschini MP, et al. Pyostomatitis vegetans in a pediatric patient with ulcerative colitis: case report of a rare pediatric inflammatory bowel disease extraintestinal manifestation and review of the literature. Eur J Gastroenterol Hepatol. 2020;32:889-892. doi:10.1097/MEG.0000000000001723
- Mesquita Kde C, Costa IM. Case for diagnosis. An Bras Dermatol. 2012;87:929-931. doi:10.1590/s0365-05962012000600022
- Al-Rimawi HS, Hammad MM, Raweily EA, et al. Pyostomatitis vegetans in childhood. Eur J Pediatr. 1998;157:402-405. doi:10.1007/s004310050838
- Chen KL, Diiorio DA, Chiu YE, et al. Pediatric patients with orofacial granulomatosis likely to subsequently develop intestinal Crohn’s disease: brief report. Pediatr Dermatol. 2020;37:1162-1164. doi:10.1111/pde.14390
- Pazheri F, Alkhouri N, Radhakrishnan K. Pyostomatitis vegetans as an oral manifestation of Crohn’s disease in a pediatric patient. Inflamm Bowel Dis. 2010;16:2007. doi:10.1002/ibd.21245.
- Werchniak AE, Storm CA, Plunkett RW, et al. Treatment of pyostomatitis vegetans with topical tacrolimus. J Am Acad Dermatol. 2005;52:722-723. doi:10.1016/j.jaad.2004.11.041
- Stingeni L, Tramontana M, Bassotti G, et al. Pyodermatitis–pyostomatitis vegetans and antibullous pemphigoid antigen 180 autoantibodies: a casual association? Br J Dermatol. 2015;172:811-813. doi:10.1111/bjd.13297
- Takahashi S, Andreoletti G, Chen R, et al. De novo and rare mutations in the HSPA1L heat shock gene associated with inflammatory bowel disease. Genome Med. 2017;9:8. doi:10.1186/s13073-016-0394-9
- Crowley E, Warner N, Pan J, et al. Prevalence and clinical features of inflammatory bowel diseases associated with monogenic variants, identified by whole-exome sequencing in 1000 children at a single center. Gastroenterology. 2020;158:2208-2220. doi:10.1053/j .gastro.2020.02.023
Case Report
A 7-year-old girl who was otherwise healthy was referred by pediatric gastroenterology for evaluation of cutaneous Crohn disease (CD). The patient had a 4-year history of persistent lip swelling and a 3-year history of asymmetric erythematous labial swelling and perianal erythema with skin tags. She had been applying the calcineurin inhibitor tacrolimus ointment 0.03% 1 or 2 times daily to her lesions with minimal improvement. She did not have a medical history of recurrent or unusual infectious diseases. There was no family history of autoimmune disease.
The patient and her guardian reported intermittent perianal pain but denied constipation, diarrhea, abdominal pain, and blood in the stool. She denied throat and tongue swelling, dysphagia, dyspnea, drooling, facial paralysis, and eyelid edema. She was a well-nourished child whose height and weight percentiles tracked at 30% and 25%, respectively. Physical examination revealed confluent symmetric lip swelling with mild angular cheilitis. Multiple 1- to 2-mm white pustules with pinpoint erosions covered the upper and lower labial mucosa and extended onto the buccal mucosa (Figure 1). She had symmetric erythema and swelling of the left labia majora extending to and involving the left perianal mucosa. Three perianal erythematous skin tags and a perianal fissure were identified.

The patient had been assessed 2 years earlier by pediatric dermatology and gastroenterology with an extensive evaluation that favored a diagnosis of cutaneous CD because the combination of orofacial granulomatosis (OFG), vulvar edema, and perianal skin tags is strongly associated.1-3 Contact dermatitis affecting the mouth was considered; however, allergen testing did not demonstrate a trigger.
A trial of a benzoate- and cinnamon-free diet, which has been reported to improve OFG,4 did not provide symptomatic improvement. Topical corticosteroids and tacrolimus reduced the perioral erythema, but the swelling persisted. An infectious cause was considered; however, topical mupirocin had no effect, and amoxicillin resulted in oral candidiasis.
A perianal biopsy revealed a granulomatous dermatitis. Fungal and bacterial cultures were negative. Upper and lower gastrointestinal (GI) endoscopy and a fecal calprotectin assay were not suggestive of inflammatory bowel disease (IBD). A complete blood cell count and QuantiFERON-TB Gold test measuring the immune response to tuberculosis antigens were normal. Chronic granulomatous disease, RAG1/RAG2 deficiency, common variable immunodeficiency, and NOD2 defects were ruled out with normal tests of dihydrorhodamine, quantitative immunoglobulins, and toll-like receptors.
Because of the discomfort associated with the patient’s lesions, she was offered treatment with tumor necrosis factor α inhibitors, including infliximab and adalimumab. These agents had been offered since the onset of symptoms; however, her parents declined systemic medication unless she developed GI involvement. Instead, the tacrolimus concentration was increased to 0.1% applied to the lips, labia, and perianal area, and fluocinonide gel 0.05% applied nightly to the oral pustules was added.
Two months later the patient had notably fewer oral pustules and diminished erythema but only slightly reduced oral, vulvar, and perianal swelling. A trial of oral metronidazole, which has been reported to clear a patient with cutaneous CD,5 was discontinued by her parents after 6 weeks because of a lack of interval improvement.
One year later, a pre-existing perianal skin tag doubled in size and became exquisitely tender. The calprotectin level—previously within reference range at less than 16 μg/g—was now elevated at 149 μg/g (reference range, 1–120 μg/g) and increased to 336 μg/g 3 weeks later. Testing for C-reactive protein, zinc, and stool occult blood; a comprehensive metabolic panel; and a complete blood cell count were unremarkable.
Repeat upper and lower GI endoscopy did not suggest CD. A biopsy using direct immunofluorescence (DIF) was obtained to evaluate for pyostomatitis vegetans (PSV) and rule out
The captured biopsy did not demonstrate the intended pustule; instead, it included less-affected mucosa and was obtained during topical treatment when few pustules and erosions persisted. Pathologic analysis revealed noncaseating granulomas without an increase in microabscesses, neutrophils, or eosinophils (Figure 2). Direct immunofluorescence staining for IgG, IgA, and C3 and indirect immunofluorescence staining for desmoglein-1 and desmoglein-3 antibodies were negative. Although the biopsy did not capture the intended pustule, diagnosis of PV was made based on clinical features and the constellation of cutaneous findings associated with IBD.

Intralesional triamcinolone, which has been of benefit for pediatric patients with orofacial granulomatosis,1,6,7 was instituted and normalized the vulva and perianal mucosa; however, lip swelling improved only minimally.
Comment
Pyostomatitis vegetans is characterized by multiple white or yellow, friable, miliary pustules that rupture, leaving behind ulcerations and erosions that cause a varying degree of oral pain.8 The disorder can involve any area of the oral mucosa—most often the labia-attached gingiva, soft and hard palates, buccal mucosa, vestibule, and tonsillar areas—but often spares the floor of the mouth and tongue.8-11 The term pyostomatitis vegetans was proposed in 1949 by McCarthy12 when he noted in a patient who presented with the characteristic appearance of the oral mucosa, though cases of vaginal, nasal, and periocular involvement have been reported.8,13,14
Histopathology—Pyostomatitis vegetans displays pseudoepithelial hyperplasia with acanthosis, hyperkeratosis, and intraepithelial or subepithelial microabscesses (or both) with neutrophils and eosinophils.8,9,15 There are a few possible explanations for this patient’s lack of tissue eosinophilia. It has been theorized that the presence of granulomas could mask concurrent PSV16 or that tissue in PSV contains fewer eosinophils as the disorder progresses.11 The oral biopsy obtained from our patient did not capture a pustule, and the condition had noticeably improved with topical tacrolimus at the time of biopsy; therefore, neither neutrophils nor eosinophils were identified. Peripheral eosinophilia, which is present in 42% to 90% of cases of PSV,9,17 can be a diagnostic clue.18 However, PE is associated with IBD,24 which usually occurs with PSV, so the absence of peripheral eosinophilia in our patient may be explained by her lack of bowel disease.
Pathogenesis—The pathogenesis of PSV is unknown. A proposed etiology includes cross-reacting antigens in the bowel and skin secondary to IBD as well as an aberrant immune response to an unidentified factor.8 Pyostomatitis vegetans is considered by many to be the mucosal variant of pyodermatitis vegetans,9,15,19 a neutrophilic dermatosis characterized by asymmetric, crusted, erythematous papulopustules that extend peripherally and coalesce to form large vegetating plaques. These lesions commonly manifest in the axillary folds, groin, and scalp and can involve the face, trunk, and distal extremities.9,18 Infection has been suggested as a cause of PSV, though cultures for pathogenic bacteria, viruses, and fungi consistently show only normal flora.20 Zinc deficiency attributed to malabsorption from CD was reported in an adult with PSV.21 The PSV resolved after 6 weeks of zinc supplementation.
Differential Diagnosis—The main entity in the clinical differential diagnosis for PSV is PVH, which is considered a variant of pemphigus vulgaris. Pemphigus vegetans of Hallopeau presents with pustules and progresses to hyperpigmented vegetative plaques with peripheral hypertrophic granulation tissue.22 The clinical and histological presentation of PVH can be similar to PSV; in PVH, however, DIF demonstrates intercellular IgG and C3 due to circulating IgG autoantibodies specific for desmoglein 3, a cell adhesion molecule.22-24 In PSV, DIF typically is negative for IgG, IgA, and C3.8 Immunohistochemical findings of PSV may overlap with IgA pemphigus, IgG/IgA pemphigus, and IgG pemphigus, which has sparked debate if PSV is an autoimmune blistering disorder or a secondary finding of epithelial injury.9,18,24
Pyostomatitis vegetans is most prevalent in patients aged 20 to 59 years25 but can occur at any age.8,19 Overall, extraintestinal symptoms, including mucocutaneous findings, are common in pediatric patients—in 30% to 71% of children with CD and 21% to 22% of children with ulcerative colitis26—and can predate onset of GI symptoms in 6% of pediatric patients.27
Oral disease is common in CD; manifestations are listed in the Table.28,29 In a prospective study of 48 children with CD, 42% (20/48) had oral manifestations identified at diagnosis28; in a similar study of 25 children, researchers noted that 48% (12/25) had disease-specific oral lesions.29 None of these children recognized the oral findings prior to the onset of systemic symptoms.28 Pyostomatitis vegetans was the least common oral manifestation, reported in 1 of 73 patients in the 2 studies combined.28,29

Two recent articles that looked at PSV in pediatric and adolescent populations identified only 9 patients with PSV.24,30 Only 2 patients (siblings) had documented onset of PSV before 12 years of age,31 which suggests an underlying genetic predisposition in young children.
It has been reported that active or subclinical (ie, asymptomatic with positive endoscopic findings) IBD in adults precedes onset of PSV, which may be considered a sign of relapse.9,30 However, PSV is incredibly rare in children and adolescents and can be an early finding of IBD in children.16,31,32
Our patient has not developed GI involvement since her initial presentation 5 years prior, though another pediatric patient developed symptomatic CD 9 years after onset of OFG.5 A retrospective review of pediatric OFG without CD met criteria for CD at a median of 3.1 years (range, 0.4–6.9 years).33 Regrettably, the early presence of PSV has been associated with future progression to CD and a complicated disease course.12,34
Management—Pyoderma stomatitis vegetans is treated with management of underlying IBD,8 with scarce literature available regarding pediatric patients. Oral lesions have been treated with antiseptics and topical corticosteroids, though these have limited benefit.8 In an adult with IBD, topical tacrolimus initially cleared PSV; however, lesions recurred until mesalamine was initiated.35 Systemic steroids were effective in a 16-year-old patient with CD and PSV,12 but recurrence is common after corticosteroids are stopped.34
Some patients benefit from steroid-sparing medications, such as dapsone, azathioprine, sulfamethoxypyridazine, methotrexate, mycophenolate mofetil, and tumor necrosis factor α inhibitors such as infliximab and adalimumab.8,9,15,23,34,36 A 12-year-old patient with pyodermatitis–PSV without intestinal disease was treated with prednisone, dapsone, and azathioprine with improvement but not complete resolution of oral erosions after 18 weeks of treatment.32 A 15-year-old patient with CD and pyodermatitis–PSV did not show improvement on prednisone, dapsone, and azathioprine but rapidly responded to infliximab.23 Infliximab led to complete clearance of oral lesions in an adult with severe fistulizing CD who developed PSV.11 However, 2 adolescent patients with CD developed PSV while on adalimumab,6,34 though 1 did improve after increasing adalimumab from once to twice weekly.6
Conclusion
The case described here—PSV in a prepubertal 7-year-old with multiple cutaneous findings suggestive of CD, including OFG, perianal and vulvar edema with biopsy-proven noncaseating granulomas, anal skin tags, and an elevated calprotectin level, noted during a cutaneous flare without clinical or endoscopically identified underlying bowel involvement—is an extremely rare presentation. Literature regarding management of PSV primarily is found in the form of case reports and focuses on treating underlying IBD. In patients with intestinal disease, treatment with biologic therapy appears most effective.6,23
ADDENDUM
Interestingly, 3 years after the patient’s original presentation to our clinic, chromosomal sequencing analysis to assess for copy number variants and whole exome gene sequencing identified a variant of unknown significance in the heat shock protein family A member 1-like gene, HSPA1L, which has an unknown mode of inheritance, but the literature suggests that both truncating and missense variants could be associated with individuals with ulcerative colitis, CD, and IBD.37,38 Although we cannot use this information to render a molecular diagnosis, it is highly suspicious that this is the cause of her clinical findings. Additionally, the patient currently is aged 10 years with unchanged cutaneous findings and has not developed gastrointestinal findings of IBD.
Case Report
A 7-year-old girl who was otherwise healthy was referred by pediatric gastroenterology for evaluation of cutaneous Crohn disease (CD). The patient had a 4-year history of persistent lip swelling and a 3-year history of asymmetric erythematous labial swelling and perianal erythema with skin tags. She had been applying the calcineurin inhibitor tacrolimus ointment 0.03% 1 or 2 times daily to her lesions with minimal improvement. She did not have a medical history of recurrent or unusual infectious diseases. There was no family history of autoimmune disease.
The patient and her guardian reported intermittent perianal pain but denied constipation, diarrhea, abdominal pain, and blood in the stool. She denied throat and tongue swelling, dysphagia, dyspnea, drooling, facial paralysis, and eyelid edema. She was a well-nourished child whose height and weight percentiles tracked at 30% and 25%, respectively. Physical examination revealed confluent symmetric lip swelling with mild angular cheilitis. Multiple 1- to 2-mm white pustules with pinpoint erosions covered the upper and lower labial mucosa and extended onto the buccal mucosa (Figure 1). She had symmetric erythema and swelling of the left labia majora extending to and involving the left perianal mucosa. Three perianal erythematous skin tags and a perianal fissure were identified.

The patient had been assessed 2 years earlier by pediatric dermatology and gastroenterology with an extensive evaluation that favored a diagnosis of cutaneous CD because the combination of orofacial granulomatosis (OFG), vulvar edema, and perianal skin tags is strongly associated.1-3 Contact dermatitis affecting the mouth was considered; however, allergen testing did not demonstrate a trigger.
A trial of a benzoate- and cinnamon-free diet, which has been reported to improve OFG,4 did not provide symptomatic improvement. Topical corticosteroids and tacrolimus reduced the perioral erythema, but the swelling persisted. An infectious cause was considered; however, topical mupirocin had no effect, and amoxicillin resulted in oral candidiasis.
A perianal biopsy revealed a granulomatous dermatitis. Fungal and bacterial cultures were negative. Upper and lower gastrointestinal (GI) endoscopy and a fecal calprotectin assay were not suggestive of inflammatory bowel disease (IBD). A complete blood cell count and QuantiFERON-TB Gold test measuring the immune response to tuberculosis antigens were normal. Chronic granulomatous disease, RAG1/RAG2 deficiency, common variable immunodeficiency, and NOD2 defects were ruled out with normal tests of dihydrorhodamine, quantitative immunoglobulins, and toll-like receptors.
Because of the discomfort associated with the patient’s lesions, she was offered treatment with tumor necrosis factor α inhibitors, including infliximab and adalimumab. These agents had been offered since the onset of symptoms; however, her parents declined systemic medication unless she developed GI involvement. Instead, the tacrolimus concentration was increased to 0.1% applied to the lips, labia, and perianal area, and fluocinonide gel 0.05% applied nightly to the oral pustules was added.
Two months later the patient had notably fewer oral pustules and diminished erythema but only slightly reduced oral, vulvar, and perianal swelling. A trial of oral metronidazole, which has been reported to clear a patient with cutaneous CD,5 was discontinued by her parents after 6 weeks because of a lack of interval improvement.
One year later, a pre-existing perianal skin tag doubled in size and became exquisitely tender. The calprotectin level—previously within reference range at less than 16 μg/g—was now elevated at 149 μg/g (reference range, 1–120 μg/g) and increased to 336 μg/g 3 weeks later. Testing for C-reactive protein, zinc, and stool occult blood; a comprehensive metabolic panel; and a complete blood cell count were unremarkable.
Repeat upper and lower GI endoscopy did not suggest CD. A biopsy using direct immunofluorescence (DIF) was obtained to evaluate for pyostomatitis vegetans (PSV) and rule out
The captured biopsy did not demonstrate the intended pustule; instead, it included less-affected mucosa and was obtained during topical treatment when few pustules and erosions persisted. Pathologic analysis revealed noncaseating granulomas without an increase in microabscesses, neutrophils, or eosinophils (Figure 2). Direct immunofluorescence staining for IgG, IgA, and C3 and indirect immunofluorescence staining for desmoglein-1 and desmoglein-3 antibodies were negative. Although the biopsy did not capture the intended pustule, diagnosis of PV was made based on clinical features and the constellation of cutaneous findings associated with IBD.

Intralesional triamcinolone, which has been of benefit for pediatric patients with orofacial granulomatosis,1,6,7 was instituted and normalized the vulva and perianal mucosa; however, lip swelling improved only minimally.
Comment
Pyostomatitis vegetans is characterized by multiple white or yellow, friable, miliary pustules that rupture, leaving behind ulcerations and erosions that cause a varying degree of oral pain.8 The disorder can involve any area of the oral mucosa—most often the labia-attached gingiva, soft and hard palates, buccal mucosa, vestibule, and tonsillar areas—but often spares the floor of the mouth and tongue.8-11 The term pyostomatitis vegetans was proposed in 1949 by McCarthy12 when he noted in a patient who presented with the characteristic appearance of the oral mucosa, though cases of vaginal, nasal, and periocular involvement have been reported.8,13,14
Histopathology—Pyostomatitis vegetans displays pseudoepithelial hyperplasia with acanthosis, hyperkeratosis, and intraepithelial or subepithelial microabscesses (or both) with neutrophils and eosinophils.8,9,15 There are a few possible explanations for this patient’s lack of tissue eosinophilia. It has been theorized that the presence of granulomas could mask concurrent PSV16 or that tissue in PSV contains fewer eosinophils as the disorder progresses.11 The oral biopsy obtained from our patient did not capture a pustule, and the condition had noticeably improved with topical tacrolimus at the time of biopsy; therefore, neither neutrophils nor eosinophils were identified. Peripheral eosinophilia, which is present in 42% to 90% of cases of PSV,9,17 can be a diagnostic clue.18 However, PE is associated with IBD,24 which usually occurs with PSV, so the absence of peripheral eosinophilia in our patient may be explained by her lack of bowel disease.
Pathogenesis—The pathogenesis of PSV is unknown. A proposed etiology includes cross-reacting antigens in the bowel and skin secondary to IBD as well as an aberrant immune response to an unidentified factor.8 Pyostomatitis vegetans is considered by many to be the mucosal variant of pyodermatitis vegetans,9,15,19 a neutrophilic dermatosis characterized by asymmetric, crusted, erythematous papulopustules that extend peripherally and coalesce to form large vegetating plaques. These lesions commonly manifest in the axillary folds, groin, and scalp and can involve the face, trunk, and distal extremities.9,18 Infection has been suggested as a cause of PSV, though cultures for pathogenic bacteria, viruses, and fungi consistently show only normal flora.20 Zinc deficiency attributed to malabsorption from CD was reported in an adult with PSV.21 The PSV resolved after 6 weeks of zinc supplementation.
Differential Diagnosis—The main entity in the clinical differential diagnosis for PSV is PVH, which is considered a variant of pemphigus vulgaris. Pemphigus vegetans of Hallopeau presents with pustules and progresses to hyperpigmented vegetative plaques with peripheral hypertrophic granulation tissue.22 The clinical and histological presentation of PVH can be similar to PSV; in PVH, however, DIF demonstrates intercellular IgG and C3 due to circulating IgG autoantibodies specific for desmoglein 3, a cell adhesion molecule.22-24 In PSV, DIF typically is negative for IgG, IgA, and C3.8 Immunohistochemical findings of PSV may overlap with IgA pemphigus, IgG/IgA pemphigus, and IgG pemphigus, which has sparked debate if PSV is an autoimmune blistering disorder or a secondary finding of epithelial injury.9,18,24
Pyostomatitis vegetans is most prevalent in patients aged 20 to 59 years25 but can occur at any age.8,19 Overall, extraintestinal symptoms, including mucocutaneous findings, are common in pediatric patients—in 30% to 71% of children with CD and 21% to 22% of children with ulcerative colitis26—and can predate onset of GI symptoms in 6% of pediatric patients.27
Oral disease is common in CD; manifestations are listed in the Table.28,29 In a prospective study of 48 children with CD, 42% (20/48) had oral manifestations identified at diagnosis28; in a similar study of 25 children, researchers noted that 48% (12/25) had disease-specific oral lesions.29 None of these children recognized the oral findings prior to the onset of systemic symptoms.28 Pyostomatitis vegetans was the least common oral manifestation, reported in 1 of 73 patients in the 2 studies combined.28,29

Two recent articles that looked at PSV in pediatric and adolescent populations identified only 9 patients with PSV.24,30 Only 2 patients (siblings) had documented onset of PSV before 12 years of age,31 which suggests an underlying genetic predisposition in young children.
It has been reported that active or subclinical (ie, asymptomatic with positive endoscopic findings) IBD in adults precedes onset of PSV, which may be considered a sign of relapse.9,30 However, PSV is incredibly rare in children and adolescents and can be an early finding of IBD in children.16,31,32
Our patient has not developed GI involvement since her initial presentation 5 years prior, though another pediatric patient developed symptomatic CD 9 years after onset of OFG.5 A retrospective review of pediatric OFG without CD met criteria for CD at a median of 3.1 years (range, 0.4–6.9 years).33 Regrettably, the early presence of PSV has been associated with future progression to CD and a complicated disease course.12,34
Management—Pyoderma stomatitis vegetans is treated with management of underlying IBD,8 with scarce literature available regarding pediatric patients. Oral lesions have been treated with antiseptics and topical corticosteroids, though these have limited benefit.8 In an adult with IBD, topical tacrolimus initially cleared PSV; however, lesions recurred until mesalamine was initiated.35 Systemic steroids were effective in a 16-year-old patient with CD and PSV,12 but recurrence is common after corticosteroids are stopped.34
Some patients benefit from steroid-sparing medications, such as dapsone, azathioprine, sulfamethoxypyridazine, methotrexate, mycophenolate mofetil, and tumor necrosis factor α inhibitors such as infliximab and adalimumab.8,9,15,23,34,36 A 12-year-old patient with pyodermatitis–PSV without intestinal disease was treated with prednisone, dapsone, and azathioprine with improvement but not complete resolution of oral erosions after 18 weeks of treatment.32 A 15-year-old patient with CD and pyodermatitis–PSV did not show improvement on prednisone, dapsone, and azathioprine but rapidly responded to infliximab.23 Infliximab led to complete clearance of oral lesions in an adult with severe fistulizing CD who developed PSV.11 However, 2 adolescent patients with CD developed PSV while on adalimumab,6,34 though 1 did improve after increasing adalimumab from once to twice weekly.6
Conclusion
The case described here—PSV in a prepubertal 7-year-old with multiple cutaneous findings suggestive of CD, including OFG, perianal and vulvar edema with biopsy-proven noncaseating granulomas, anal skin tags, and an elevated calprotectin level, noted during a cutaneous flare without clinical or endoscopically identified underlying bowel involvement—is an extremely rare presentation. Literature regarding management of PSV primarily is found in the form of case reports and focuses on treating underlying IBD. In patients with intestinal disease, treatment with biologic therapy appears most effective.6,23
ADDENDUM
Interestingly, 3 years after the patient’s original presentation to our clinic, chromosomal sequencing analysis to assess for copy number variants and whole exome gene sequencing identified a variant of unknown significance in the heat shock protein family A member 1-like gene, HSPA1L, which has an unknown mode of inheritance, but the literature suggests that both truncating and missense variants could be associated with individuals with ulcerative colitis, CD, and IBD.37,38 Although we cannot use this information to render a molecular diagnosis, it is highly suspicious that this is the cause of her clinical findings. Additionally, the patient currently is aged 10 years with unchanged cutaneous findings and has not developed gastrointestinal findings of IBD.
- Tuxen AJ, Orchard D. Childhood and adolescent orofacial granulomatosis is strongly associated with Crohn’s disease and responds to intralesional corticosteroids. Australas J Dermatol. 2010;51:124-127. doi:10.1111/j.1440-0960.2010.00627.x
- Vaid RM, Cohen BA. Cutaneous Crohn’s disease in the pediatric population. Pediatr Dermatol. 2010;27:279-281. doi:10.1111/j.1525-1470.2010.01138.x
- van de Scheur MR, van der Waal RIF, van der Waal I, et al. Ano-genital granulomatosis: the counterpart of oro-facial granulomatosis. J Eur Acad Dermatol Venereol. 2003;17:184-189. doi:10.1046/j.1468-3083.2003.00573.x
- Campbell HE, Escudier MP, Patel P, et al. Review article: cinnamon- and benzoate-free diet as a primary treatment for orofacial granulomatosis. Aliment Pharmacol Ther. 2011;34:687-701. doi:10.1111/j.1365-2036.2011.04792.x
- Duhra P, Paul CJ. Metastatic Crohn’s disease responding to metronidazole. Br J Dermatol. 1988;119:87-91. doi:10.1111/j.1365-2133.1988.tb07107.x
- Katsanos KH, Torres J, Roda G, et al. Review article: non-malignant oral manifestations in inflammatory bowel diseases. Aliment Pharmacol Ther. 2015;42:40-60. doi:10.1111/apt.13217
- Schmitz BA, Unkel JH. Symptomatic oral Crohn’s disease in an adolescent. J Dent Child (Chic). 2018;85:66-69.
- Femiano F, Lanza A, Buonaiuto C, et al. Pyostomatitis vegetans: a review of the literature. Med Oral Patol Oral Cir Bucal. 2009;14:E114-E117.
- Clark LG, Tolkachjov SN, Bridges AG, et al. Pyostomatitis vegetans (PSV)–pyodermatitis vegetans (PDV): a clinicopathologic study of 7 cases at a tertiary referral center. J Am Acad Dermatol. 2016;75:578-584. doi:10.1016/j.jaad.2016.03.047
- Hansen LS, Silverman S Jr, Daniels TE. The differential diagnosis of pyostomatitis vegetans and its relation to bowel disease. Oral Surg Oral Med Oral Pathol. 1983;55:363-373. doi:10.1016/0030-4220(83)90191-3
- Cataldo E, Covino MC, Tesone PE. Pyostomatitis vegetans. Oral Surg Oral Med Oral Pathol. 1981;52:172-177. doi:10.1016/0030-4220(81)90316-9
- McCarthy FP. Pyostomatitis vegetans; report of three cases. Arch Derm Syphilol. 1949;60:750-764.
- Bens G, Laharie D, Beylot-Barry M, et al. Successful treatment with infliximab and methotrexate of pyostomatitis vegetans associated with Crohn’s disease. Br J Dermatol. 2003;149:181-184. doi:10.1046/j.1365-2133.2003.05385.x
- Leibovitch I, Ooi C, Huilgol SC, et al. Pyodermatitis–pyostomatitis vegetans of the eyelids: case report and review of the literature. Ophthalmology. 2005;112:1809-1813. doi:10.1016/j.ophtha.2005.04.027
- Ruiz-Roca JA, Berini-Aytés L, Gay-Escoda C. Pyostomatitis vegetans. report of two cases and review of the literature. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2005;99:447-454. doi:10.1016/j.tripleo.2003.08.022
- Molnár T, Farkas K, Nagy F, et al. Third case: another pediatric patient with pyostomatitis vegetans and oral granuloma as one of the initial symptoms of Crohn’s disease. Inflamm Bowel Dis. 2011;17:E122-E123. doi:10.1002/ibd.21791
- Leydhecker W, Lund OE. Eye involvement in pyostomatitis vegetans. Klin Monbl Augenheilkd Augenarztl Fortbild. 1962;141:595-602.
- Thornhill MH, Zakrzewska JM, Gilkes JJ. Pyostomatitis vegetans: report of three cases and review of the literature. J Oral Pathol Med. 1992;21:128-133. doi:10.1111/j.1600-0714.1992.tb00996.x
- Chaudhry SI, Philpot NS, Odell EW, et al. Pyostomatitis vegetans associated with asymptomatic ulcerative colitis: a case report. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1999;87:327-330. doi:10.1016/s1079-2104(99)70217-9
- Konstantopoulou M, O’Dwyer EM, Steele JC, et al. Pyodermatitis–pyostomatitis vegetans complicated by methicillin-resistant Staphylococcus aureus infection. Clin Exp Dermatol. 2005;30:666-668. doi:10.1111/j.1365-2230.2005.01906.x
- Ficarra G, Cicchi P, Amorosi A, et al. Oral Crohn’s disease and pyostomatitis vegetans. an unusual association. Oral Surg Oral Med Oral Pathol. 1993;75:220-224. doi:10.1016/0030-4220(93)90097-n
- Markopoulos AK, Antoniades DZ, Zaraboukas T. Pemphigus vegetans of the oral cavity. Int J Dermatol. 2006;45:425-428. doi:10.1111/j.1365-4632.2004.02480.x
- Nico MMS, Hussein TP, Aoki V, et al. Pyostomatitis vegetans and its relation to inflammatory bowel disease, pyoderma gangrenosum, pyodermatitis vegetans, and pemphigus. J Oral Pathol Med. 2012;41:584-588. doi:10.1111/j.1600-0714.2012.01152.x
- Berzin D, Lahad A, Weiss B, et al. Inflammatory bowel disease presenting with pyodermatitis–pyostomatitis vegetans in a pediatric patient: a case report and review of the literature. Pediatr Dermatol. 2021;38:868-871. doi:10.1111/pde.14625
- Ballo FS, Camisa C, Allen CM. Pyostomatitis vegetans. report of a case and review of the literature. J Am Acad Dermatol. 1989;21:381-387.
- Greuter T, Bertoldo F, Rechner R, et al; Swiss IBD Cohort Study Group. Extraintestinal manifestations of pediatric inflammatory bowel disease: prevalence, presentation, and anti-TNF treatment. J Pediatr Gastroenterol Nutr. 2017;65:200-206. doi:10.1097/MPG.0000000000001455
- Jose FA, Garnett EA, Vittinghoff E, et al. Development of extraintestinal manifestations in pediatric patients with inflammatory bowel disease. Inflamm Bowel Dis. 2009;15:63-68. doi:10.1002/ibd.20604
- Harty S, Fleming P, Rowland M, et al. A prospective study of the oral manifestations of Crohn’s disease. Clin Gastroenterol Hepatol. 2005;3:886-891. doi:10.1016/s1542-3565(05)00424-6
- Pittock S, Drumm B, Fleming P, et al. The oral cavity in Crohn’s disease. J Pediatr. 2001;138:767-771. doi:10.1067/mpd.2001.113008
- Bardasi G, Romagnoli A, Foschini MP, et al. Pyostomatitis vegetans in a pediatric patient with ulcerative colitis: case report of a rare pediatric inflammatory bowel disease extraintestinal manifestation and review of the literature. Eur J Gastroenterol Hepatol. 2020;32:889-892. doi:10.1097/MEG.0000000000001723
- Mesquita Kde C, Costa IM. Case for diagnosis. An Bras Dermatol. 2012;87:929-931. doi:10.1590/s0365-05962012000600022
- Al-Rimawi HS, Hammad MM, Raweily EA, et al. Pyostomatitis vegetans in childhood. Eur J Pediatr. 1998;157:402-405. doi:10.1007/s004310050838
- Chen KL, Diiorio DA, Chiu YE, et al. Pediatric patients with orofacial granulomatosis likely to subsequently develop intestinal Crohn’s disease: brief report. Pediatr Dermatol. 2020;37:1162-1164. doi:10.1111/pde.14390
- Pazheri F, Alkhouri N, Radhakrishnan K. Pyostomatitis vegetans as an oral manifestation of Crohn’s disease in a pediatric patient. Inflamm Bowel Dis. 2010;16:2007. doi:10.1002/ibd.21245.
- Werchniak AE, Storm CA, Plunkett RW, et al. Treatment of pyostomatitis vegetans with topical tacrolimus. J Am Acad Dermatol. 2005;52:722-723. doi:10.1016/j.jaad.2004.11.041
- Stingeni L, Tramontana M, Bassotti G, et al. Pyodermatitis–pyostomatitis vegetans and antibullous pemphigoid antigen 180 autoantibodies: a casual association? Br J Dermatol. 2015;172:811-813. doi:10.1111/bjd.13297
- Takahashi S, Andreoletti G, Chen R, et al. De novo and rare mutations in the HSPA1L heat shock gene associated with inflammatory bowel disease. Genome Med. 2017;9:8. doi:10.1186/s13073-016-0394-9
- Crowley E, Warner N, Pan J, et al. Prevalence and clinical features of inflammatory bowel diseases associated with monogenic variants, identified by whole-exome sequencing in 1000 children at a single center. Gastroenterology. 2020;158:2208-2220. doi:10.1053/j .gastro.2020.02.023
- Tuxen AJ, Orchard D. Childhood and adolescent orofacial granulomatosis is strongly associated with Crohn’s disease and responds to intralesional corticosteroids. Australas J Dermatol. 2010;51:124-127. doi:10.1111/j.1440-0960.2010.00627.x
- Vaid RM, Cohen BA. Cutaneous Crohn’s disease in the pediatric population. Pediatr Dermatol. 2010;27:279-281. doi:10.1111/j.1525-1470.2010.01138.x
- van de Scheur MR, van der Waal RIF, van der Waal I, et al. Ano-genital granulomatosis: the counterpart of oro-facial granulomatosis. J Eur Acad Dermatol Venereol. 2003;17:184-189. doi:10.1046/j.1468-3083.2003.00573.x
- Campbell HE, Escudier MP, Patel P, et al. Review article: cinnamon- and benzoate-free diet as a primary treatment for orofacial granulomatosis. Aliment Pharmacol Ther. 2011;34:687-701. doi:10.1111/j.1365-2036.2011.04792.x
- Duhra P, Paul CJ. Metastatic Crohn’s disease responding to metronidazole. Br J Dermatol. 1988;119:87-91. doi:10.1111/j.1365-2133.1988.tb07107.x
- Katsanos KH, Torres J, Roda G, et al. Review article: non-malignant oral manifestations in inflammatory bowel diseases. Aliment Pharmacol Ther. 2015;42:40-60. doi:10.1111/apt.13217
- Schmitz BA, Unkel JH. Symptomatic oral Crohn’s disease in an adolescent. J Dent Child (Chic). 2018;85:66-69.
- Femiano F, Lanza A, Buonaiuto C, et al. Pyostomatitis vegetans: a review of the literature. Med Oral Patol Oral Cir Bucal. 2009;14:E114-E117.
- Clark LG, Tolkachjov SN, Bridges AG, et al. Pyostomatitis vegetans (PSV)–pyodermatitis vegetans (PDV): a clinicopathologic study of 7 cases at a tertiary referral center. J Am Acad Dermatol. 2016;75:578-584. doi:10.1016/j.jaad.2016.03.047
- Hansen LS, Silverman S Jr, Daniels TE. The differential diagnosis of pyostomatitis vegetans and its relation to bowel disease. Oral Surg Oral Med Oral Pathol. 1983;55:363-373. doi:10.1016/0030-4220(83)90191-3
- Cataldo E, Covino MC, Tesone PE. Pyostomatitis vegetans. Oral Surg Oral Med Oral Pathol. 1981;52:172-177. doi:10.1016/0030-4220(81)90316-9
- McCarthy FP. Pyostomatitis vegetans; report of three cases. Arch Derm Syphilol. 1949;60:750-764.
- Bens G, Laharie D, Beylot-Barry M, et al. Successful treatment with infliximab and methotrexate of pyostomatitis vegetans associated with Crohn’s disease. Br J Dermatol. 2003;149:181-184. doi:10.1046/j.1365-2133.2003.05385.x
- Leibovitch I, Ooi C, Huilgol SC, et al. Pyodermatitis–pyostomatitis vegetans of the eyelids: case report and review of the literature. Ophthalmology. 2005;112:1809-1813. doi:10.1016/j.ophtha.2005.04.027
- Ruiz-Roca JA, Berini-Aytés L, Gay-Escoda C. Pyostomatitis vegetans. report of two cases and review of the literature. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2005;99:447-454. doi:10.1016/j.tripleo.2003.08.022
- Molnár T, Farkas K, Nagy F, et al. Third case: another pediatric patient with pyostomatitis vegetans and oral granuloma as one of the initial symptoms of Crohn’s disease. Inflamm Bowel Dis. 2011;17:E122-E123. doi:10.1002/ibd.21791
- Leydhecker W, Lund OE. Eye involvement in pyostomatitis vegetans. Klin Monbl Augenheilkd Augenarztl Fortbild. 1962;141:595-602.
- Thornhill MH, Zakrzewska JM, Gilkes JJ. Pyostomatitis vegetans: report of three cases and review of the literature. J Oral Pathol Med. 1992;21:128-133. doi:10.1111/j.1600-0714.1992.tb00996.x
- Chaudhry SI, Philpot NS, Odell EW, et al. Pyostomatitis vegetans associated with asymptomatic ulcerative colitis: a case report. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1999;87:327-330. doi:10.1016/s1079-2104(99)70217-9
- Konstantopoulou M, O’Dwyer EM, Steele JC, et al. Pyodermatitis–pyostomatitis vegetans complicated by methicillin-resistant Staphylococcus aureus infection. Clin Exp Dermatol. 2005;30:666-668. doi:10.1111/j.1365-2230.2005.01906.x
- Ficarra G, Cicchi P, Amorosi A, et al. Oral Crohn’s disease and pyostomatitis vegetans. an unusual association. Oral Surg Oral Med Oral Pathol. 1993;75:220-224. doi:10.1016/0030-4220(93)90097-n
- Markopoulos AK, Antoniades DZ, Zaraboukas T. Pemphigus vegetans of the oral cavity. Int J Dermatol. 2006;45:425-428. doi:10.1111/j.1365-4632.2004.02480.x
- Nico MMS, Hussein TP, Aoki V, et al. Pyostomatitis vegetans and its relation to inflammatory bowel disease, pyoderma gangrenosum, pyodermatitis vegetans, and pemphigus. J Oral Pathol Med. 2012;41:584-588. doi:10.1111/j.1600-0714.2012.01152.x
- Berzin D, Lahad A, Weiss B, et al. Inflammatory bowel disease presenting with pyodermatitis–pyostomatitis vegetans in a pediatric patient: a case report and review of the literature. Pediatr Dermatol. 2021;38:868-871. doi:10.1111/pde.14625
- Ballo FS, Camisa C, Allen CM. Pyostomatitis vegetans. report of a case and review of the literature. J Am Acad Dermatol. 1989;21:381-387.
- Greuter T, Bertoldo F, Rechner R, et al; Swiss IBD Cohort Study Group. Extraintestinal manifestations of pediatric inflammatory bowel disease: prevalence, presentation, and anti-TNF treatment. J Pediatr Gastroenterol Nutr. 2017;65:200-206. doi:10.1097/MPG.0000000000001455
- Jose FA, Garnett EA, Vittinghoff E, et al. Development of extraintestinal manifestations in pediatric patients with inflammatory bowel disease. Inflamm Bowel Dis. 2009;15:63-68. doi:10.1002/ibd.20604
- Harty S, Fleming P, Rowland M, et al. A prospective study of the oral manifestations of Crohn’s disease. Clin Gastroenterol Hepatol. 2005;3:886-891. doi:10.1016/s1542-3565(05)00424-6
- Pittock S, Drumm B, Fleming P, et al. The oral cavity in Crohn’s disease. J Pediatr. 2001;138:767-771. doi:10.1067/mpd.2001.113008
- Bardasi G, Romagnoli A, Foschini MP, et al. Pyostomatitis vegetans in a pediatric patient with ulcerative colitis: case report of a rare pediatric inflammatory bowel disease extraintestinal manifestation and review of the literature. Eur J Gastroenterol Hepatol. 2020;32:889-892. doi:10.1097/MEG.0000000000001723
- Mesquita Kde C, Costa IM. Case for diagnosis. An Bras Dermatol. 2012;87:929-931. doi:10.1590/s0365-05962012000600022
- Al-Rimawi HS, Hammad MM, Raweily EA, et al. Pyostomatitis vegetans in childhood. Eur J Pediatr. 1998;157:402-405. doi:10.1007/s004310050838
- Chen KL, Diiorio DA, Chiu YE, et al. Pediatric patients with orofacial granulomatosis likely to subsequently develop intestinal Crohn’s disease: brief report. Pediatr Dermatol. 2020;37:1162-1164. doi:10.1111/pde.14390
- Pazheri F, Alkhouri N, Radhakrishnan K. Pyostomatitis vegetans as an oral manifestation of Crohn’s disease in a pediatric patient. Inflamm Bowel Dis. 2010;16:2007. doi:10.1002/ibd.21245.
- Werchniak AE, Storm CA, Plunkett RW, et al. Treatment of pyostomatitis vegetans with topical tacrolimus. J Am Acad Dermatol. 2005;52:722-723. doi:10.1016/j.jaad.2004.11.041
- Stingeni L, Tramontana M, Bassotti G, et al. Pyodermatitis–pyostomatitis vegetans and antibullous pemphigoid antigen 180 autoantibodies: a casual association? Br J Dermatol. 2015;172:811-813. doi:10.1111/bjd.13297
- Takahashi S, Andreoletti G, Chen R, et al. De novo and rare mutations in the HSPA1L heat shock gene associated with inflammatory bowel disease. Genome Med. 2017;9:8. doi:10.1186/s13073-016-0394-9
- Crowley E, Warner N, Pan J, et al. Prevalence and clinical features of inflammatory bowel diseases associated with monogenic variants, identified by whole-exome sequencing in 1000 children at a single center. Gastroenterology. 2020;158:2208-2220. doi:10.1053/j .gastro.2020.02.023
Practice Points
- Pyostomatitis vegetans (PSV) is a rare manifestation of cutaneous Crohn disease in children and can precede the onset of bowel pathology.
- Although topical and intralesional corticosteroids were beneficial in our patient, systemic corticosteroids and tumor necrosis factor α inhibitors, including infliximab and adalimumab, used to treat underlying inflammatory bowel disease appear to be the most efficacious option for treating PSV.
Children and COVID: New-case counts offer dueling narratives
New COVID-19 cases in children jumped by 66% during the first 2 weeks of December after an 8-week steady period lasting through October and November, according to the American Academy of Pediatrics and the Children’s Hospital Association.
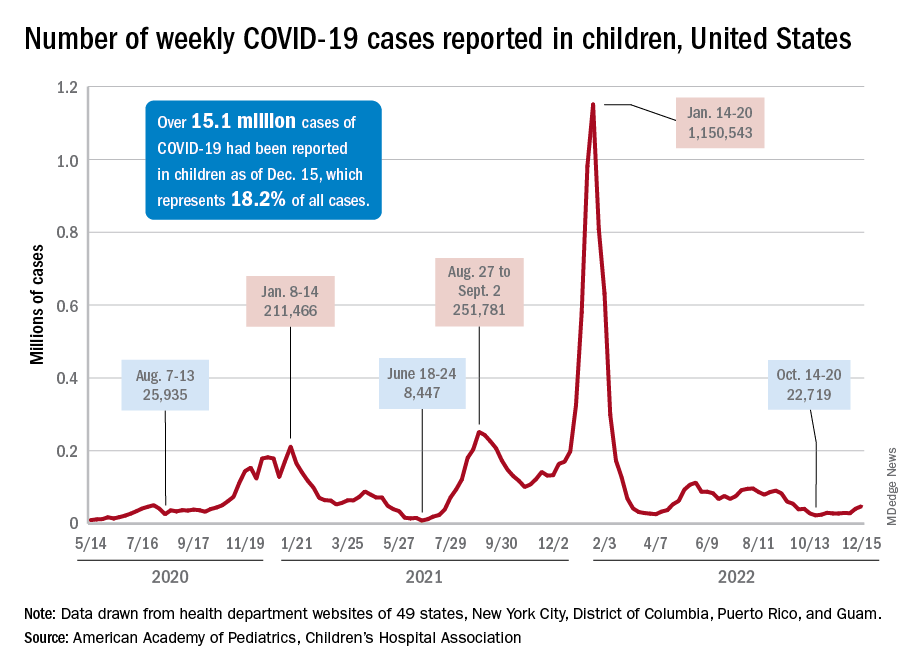
and totaling less than 29,000 for the week of Nov. 25 to Dec. 1. That increase of almost 19,000 cases is the largest over a 2-week period since late July, the AAP and CHA said in their weekly COVID report based on data collected from state and territorial health department websites.
[This publication has been following the AAP/CHA report since the summer of 2020 and continues to share the data for the sake of consistency, but it must be noted that a number of states are no longer updating their public COVID dashboards. As a result, there is now a considerable discrepancy between the AAP/CHA weekly figures and those reported by the Centers for Disease Control and Prevention, which has no such limitations on state data.]
The situation involving new cases over the last 2 weeks is quite different from the CDC’s perspective. The agency does not publish a weekly count, instead offering cumulative cases, which stood at almost 16.1 million as of Dec. 14. Calculating a 2-week total puts the new-case count for Dec. 1-14 at 113,572 among children aged 0-17 years. That is higher than the AAP/CHA count (88,629) for roughly the same period, but it is actually lower than the CDC’s figure (161,832) for the last 2 weeks of November.
The CDC data, in other words, suggest that new cases have gone down in the last 2 weeks, while the AAP and CHA, with their somewhat limited perspective, announced that new cases have gone up.
One COVID-related measure from the CDC that is not contradicted by other sources is hospitalization rates, which had climbed from 0.16 new admissions in children aged 0-17 years with confirmed COVID per 100,000 population on Oct. 22 to 0.29 per 100,000 on Dec. 9. Visits to the emergency department with diagnosed COVID, meanwhile, have been fairly steady so far through December in children, according to the CDC.
New COVID-19 cases in children jumped by 66% during the first 2 weeks of December after an 8-week steady period lasting through October and November, according to the American Academy of Pediatrics and the Children’s Hospital Association.

and totaling less than 29,000 for the week of Nov. 25 to Dec. 1. That increase of almost 19,000 cases is the largest over a 2-week period since late July, the AAP and CHA said in their weekly COVID report based on data collected from state and territorial health department websites.
[This publication has been following the AAP/CHA report since the summer of 2020 and continues to share the data for the sake of consistency, but it must be noted that a number of states are no longer updating their public COVID dashboards. As a result, there is now a considerable discrepancy between the AAP/CHA weekly figures and those reported by the Centers for Disease Control and Prevention, which has no such limitations on state data.]
The situation involving new cases over the last 2 weeks is quite different from the CDC’s perspective. The agency does not publish a weekly count, instead offering cumulative cases, which stood at almost 16.1 million as of Dec. 14. Calculating a 2-week total puts the new-case count for Dec. 1-14 at 113,572 among children aged 0-17 years. That is higher than the AAP/CHA count (88,629) for roughly the same period, but it is actually lower than the CDC’s figure (161,832) for the last 2 weeks of November.
The CDC data, in other words, suggest that new cases have gone down in the last 2 weeks, while the AAP and CHA, with their somewhat limited perspective, announced that new cases have gone up.
One COVID-related measure from the CDC that is not contradicted by other sources is hospitalization rates, which had climbed from 0.16 new admissions in children aged 0-17 years with confirmed COVID per 100,000 population on Oct. 22 to 0.29 per 100,000 on Dec. 9. Visits to the emergency department with diagnosed COVID, meanwhile, have been fairly steady so far through December in children, according to the CDC.
New COVID-19 cases in children jumped by 66% during the first 2 weeks of December after an 8-week steady period lasting through October and November, according to the American Academy of Pediatrics and the Children’s Hospital Association.

and totaling less than 29,000 for the week of Nov. 25 to Dec. 1. That increase of almost 19,000 cases is the largest over a 2-week period since late July, the AAP and CHA said in their weekly COVID report based on data collected from state and territorial health department websites.
[This publication has been following the AAP/CHA report since the summer of 2020 and continues to share the data for the sake of consistency, but it must be noted that a number of states are no longer updating their public COVID dashboards. As a result, there is now a considerable discrepancy between the AAP/CHA weekly figures and those reported by the Centers for Disease Control and Prevention, which has no such limitations on state data.]
The situation involving new cases over the last 2 weeks is quite different from the CDC’s perspective. The agency does not publish a weekly count, instead offering cumulative cases, which stood at almost 16.1 million as of Dec. 14. Calculating a 2-week total puts the new-case count for Dec. 1-14 at 113,572 among children aged 0-17 years. That is higher than the AAP/CHA count (88,629) for roughly the same period, but it is actually lower than the CDC’s figure (161,832) for the last 2 weeks of November.
The CDC data, in other words, suggest that new cases have gone down in the last 2 weeks, while the AAP and CHA, with their somewhat limited perspective, announced that new cases have gone up.
One COVID-related measure from the CDC that is not contradicted by other sources is hospitalization rates, which had climbed from 0.16 new admissions in children aged 0-17 years with confirmed COVID per 100,000 population on Oct. 22 to 0.29 per 100,000 on Dec. 9. Visits to the emergency department with diagnosed COVID, meanwhile, have been fairly steady so far through December in children, according to the CDC.
Vaccinating pregnant women protects infants against severe RSV infection
An investigational vaccine against respiratory syncytial virus (RSV) in pregnant women has been shown to help protect infants against severe disease, according to the vaccine’s manufacturer.
Pfizer recently announced that in the course of a randomized, double-blind, placebo-controlled phase 3 study, the vaccine RSVpreF had an almost 82% efficacy against severe RSV infection in infants from birth through the first 90 days of life, according to a company press release.
The vaccine also had a 69% efficacy against severe disease through the first 6 months of life. A total of 7,400 women had received a single dose of 120 mcg RSVpreF in the late second or third trimester of their pregnancy. There were no signs of safety issues for the mothers or infants.
Due to the good results, the enrollment in the study was halted on the recommendation of the study’s Data Monitoring Committee after achieving a primary endpoint. The company plans to apply for marketing authorization to the U.S. Food and Drug Administration by the end of 2022 and to other regulatory agencies in 2023.
“The directness of the strategy, to vaccinate expectant mothers during pregnancy so that their newborn is then later protected, is new and a very interesting approach,” commented Prof. Ortwin Adams, MD, head of virologic diagnostics at the Institute for Virology of the University Hospital of Düsseldorf (Germany) to the Science Media Centre (SMC).
In terms of the RSV vaccination strategy presented, “the unborn child has taken center stage from the outset.” Because the vaccination route is the placental transfer of antibodies from mother to child (“passive immunity”), “... the medical points of contact for this vaccination will be the gynecologists, not the pediatricians,” Dr. Adams said.
“This concept imitates the natural process, since the mother normally passes immune defenses she acquired through infections to the child via the umbilical cord and her breast milk before and after birth. This procedure is long-proven and practiced worldwide, especially in nonindustrialized countries, for a variety of diseases, including tetanus, whooping cough (pertussis), and viral flu (influenza),” explained Markus Rose, MD, PhD, head of Pediatric Pulmonology at the Olgahospital, Stuttgart, Germany.
The development of an RSV vaccine had ground to a halt for many decades: A tragedy in the 1960s set the whole field of research back. Using the model of the first polio vaccine, scientists had manufactured an experimental vaccine with inactivated viruses. However, tests showed that the vaccine did not protect the children vaccinated, but it actually infected them with RSV, they then fell ill, and two children died. Today, potential RSV vaccines are first tested on adults and not on children.
Few treatment options
RSV causes seasonal epidemics, can lead to bronchiolitis and pneumonia in infants, and is one of the main causes of hospital stays in young children. Monoclonal antibodies are currently the only preventive option, since there is still no vaccine. Usually, 60%-70% of infants and nearly all children younger than 2 years are infected with RSV, but the virus can also trigger pneumonia in adults.
“RSV infections constitute a major public health challenge: It is the most dangerous respiratory virus for young infants, it is also a threat to the chronically ill and immunocompromised of all ages, and [it] is the second most common cause of death worldwide (after malaria) in young children,” stated Dr. Rose.
Recently, pandemic-related measures (face masks, more intense disinfection) meant that the “normal” RSV infections in healthy adults, which usually progress like a mild cold, were prevented, and mothers were unable to pass on as much RSV immune defense to their children. “This was presumably responsible in part for the massive wave of RSV infections in fall and winter of 2021/22,” explained Dr. Rose.
Thomas Mertens, MD, PhD, chair of the Standing Committee on Vaccination at the Robert Koch Institute (STIKO) and former director of the Institute for Virology at Ulm University Hospital, Germany, also noted: “It would be an important and potentially achievable goal to significantly reduce the incidence rate of hospitalizations. In this respect, RSV poses a significant problem for young children, their parents, and the burden on pediatric clinics.”
Final evaluation pending
“I am definitely finding the data interesting, but the original data are needed,” Dr. Mertens said. Once the data are published at a conference or published in a peer-reviewed journal, physicians will be able to better judge the data for themselves, he said.
Dr. Rose characterized the new vaccine as “novel,” including in terms of its composition. Earlier RSV vaccines used the so-called postfusion F protein as their starting point. But it has become known in the meantime that the key to immunogenicity is the continued prefusion state of the apical epitope: Prefusion F-specific memory B cells in adults naturally infected with RSV produce potent neutralizing antibodies.
The new vaccine is bivalent and protects against both RSV A and RSV B.
To date, RSV vaccination directly in young infants have had only had a weak efficacy and were sometimes poorly tolerated. The vaccine presented here is expected to be tested in young adults first, then in school children, then young children.
Through successful vaccination of the entire population, the transfer of RS viruses to young children could be prevented. “To what extent this, or any other RSV vaccine still to be developed on the same basis, will also be effective and well tolerated in young infants is still difficult to assess,” said Dr. Rose.
Dr. Mertens emphasized that all of the study data now needs to be seen as quickly as possible: “This is also a general requirement for transparency from the pharmaceutical companies, which is also rightly criticized.”
This article was originally published in Medscape’s German edition and a version appeared on Medscape.com.
An investigational vaccine against respiratory syncytial virus (RSV) in pregnant women has been shown to help protect infants against severe disease, according to the vaccine’s manufacturer.
Pfizer recently announced that in the course of a randomized, double-blind, placebo-controlled phase 3 study, the vaccine RSVpreF had an almost 82% efficacy against severe RSV infection in infants from birth through the first 90 days of life, according to a company press release.
The vaccine also had a 69% efficacy against severe disease through the first 6 months of life. A total of 7,400 women had received a single dose of 120 mcg RSVpreF in the late second or third trimester of their pregnancy. There were no signs of safety issues for the mothers or infants.
Due to the good results, the enrollment in the study was halted on the recommendation of the study’s Data Monitoring Committee after achieving a primary endpoint. The company plans to apply for marketing authorization to the U.S. Food and Drug Administration by the end of 2022 and to other regulatory agencies in 2023.
“The directness of the strategy, to vaccinate expectant mothers during pregnancy so that their newborn is then later protected, is new and a very interesting approach,” commented Prof. Ortwin Adams, MD, head of virologic diagnostics at the Institute for Virology of the University Hospital of Düsseldorf (Germany) to the Science Media Centre (SMC).
In terms of the RSV vaccination strategy presented, “the unborn child has taken center stage from the outset.” Because the vaccination route is the placental transfer of antibodies from mother to child (“passive immunity”), “... the medical points of contact for this vaccination will be the gynecologists, not the pediatricians,” Dr. Adams said.
“This concept imitates the natural process, since the mother normally passes immune defenses she acquired through infections to the child via the umbilical cord and her breast milk before and after birth. This procedure is long-proven and practiced worldwide, especially in nonindustrialized countries, for a variety of diseases, including tetanus, whooping cough (pertussis), and viral flu (influenza),” explained Markus Rose, MD, PhD, head of Pediatric Pulmonology at the Olgahospital, Stuttgart, Germany.
The development of an RSV vaccine had ground to a halt for many decades: A tragedy in the 1960s set the whole field of research back. Using the model of the first polio vaccine, scientists had manufactured an experimental vaccine with inactivated viruses. However, tests showed that the vaccine did not protect the children vaccinated, but it actually infected them with RSV, they then fell ill, and two children died. Today, potential RSV vaccines are first tested on adults and not on children.
Few treatment options
RSV causes seasonal epidemics, can lead to bronchiolitis and pneumonia in infants, and is one of the main causes of hospital stays in young children. Monoclonal antibodies are currently the only preventive option, since there is still no vaccine. Usually, 60%-70% of infants and nearly all children younger than 2 years are infected with RSV, but the virus can also trigger pneumonia in adults.
“RSV infections constitute a major public health challenge: It is the most dangerous respiratory virus for young infants, it is also a threat to the chronically ill and immunocompromised of all ages, and [it] is the second most common cause of death worldwide (after malaria) in young children,” stated Dr. Rose.
Recently, pandemic-related measures (face masks, more intense disinfection) meant that the “normal” RSV infections in healthy adults, which usually progress like a mild cold, were prevented, and mothers were unable to pass on as much RSV immune defense to their children. “This was presumably responsible in part for the massive wave of RSV infections in fall and winter of 2021/22,” explained Dr. Rose.
Thomas Mertens, MD, PhD, chair of the Standing Committee on Vaccination at the Robert Koch Institute (STIKO) and former director of the Institute for Virology at Ulm University Hospital, Germany, also noted: “It would be an important and potentially achievable goal to significantly reduce the incidence rate of hospitalizations. In this respect, RSV poses a significant problem for young children, their parents, and the burden on pediatric clinics.”
Final evaluation pending
“I am definitely finding the data interesting, but the original data are needed,” Dr. Mertens said. Once the data are published at a conference or published in a peer-reviewed journal, physicians will be able to better judge the data for themselves, he said.
Dr. Rose characterized the new vaccine as “novel,” including in terms of its composition. Earlier RSV vaccines used the so-called postfusion F protein as their starting point. But it has become known in the meantime that the key to immunogenicity is the continued prefusion state of the apical epitope: Prefusion F-specific memory B cells in adults naturally infected with RSV produce potent neutralizing antibodies.
The new vaccine is bivalent and protects against both RSV A and RSV B.
To date, RSV vaccination directly in young infants have had only had a weak efficacy and were sometimes poorly tolerated. The vaccine presented here is expected to be tested in young adults first, then in school children, then young children.
Through successful vaccination of the entire population, the transfer of RS viruses to young children could be prevented. “To what extent this, or any other RSV vaccine still to be developed on the same basis, will also be effective and well tolerated in young infants is still difficult to assess,” said Dr. Rose.
Dr. Mertens emphasized that all of the study data now needs to be seen as quickly as possible: “This is also a general requirement for transparency from the pharmaceutical companies, which is also rightly criticized.”
This article was originally published in Medscape’s German edition and a version appeared on Medscape.com.
An investigational vaccine against respiratory syncytial virus (RSV) in pregnant women has been shown to help protect infants against severe disease, according to the vaccine’s manufacturer.
Pfizer recently announced that in the course of a randomized, double-blind, placebo-controlled phase 3 study, the vaccine RSVpreF had an almost 82% efficacy against severe RSV infection in infants from birth through the first 90 days of life, according to a company press release.
The vaccine also had a 69% efficacy against severe disease through the first 6 months of life. A total of 7,400 women had received a single dose of 120 mcg RSVpreF in the late second or third trimester of their pregnancy. There were no signs of safety issues for the mothers or infants.
Due to the good results, the enrollment in the study was halted on the recommendation of the study’s Data Monitoring Committee after achieving a primary endpoint. The company plans to apply for marketing authorization to the U.S. Food and Drug Administration by the end of 2022 and to other regulatory agencies in 2023.
“The directness of the strategy, to vaccinate expectant mothers during pregnancy so that their newborn is then later protected, is new and a very interesting approach,” commented Prof. Ortwin Adams, MD, head of virologic diagnostics at the Institute for Virology of the University Hospital of Düsseldorf (Germany) to the Science Media Centre (SMC).
In terms of the RSV vaccination strategy presented, “the unborn child has taken center stage from the outset.” Because the vaccination route is the placental transfer of antibodies from mother to child (“passive immunity”), “... the medical points of contact for this vaccination will be the gynecologists, not the pediatricians,” Dr. Adams said.
“This concept imitates the natural process, since the mother normally passes immune defenses she acquired through infections to the child via the umbilical cord and her breast milk before and after birth. This procedure is long-proven and practiced worldwide, especially in nonindustrialized countries, for a variety of diseases, including tetanus, whooping cough (pertussis), and viral flu (influenza),” explained Markus Rose, MD, PhD, head of Pediatric Pulmonology at the Olgahospital, Stuttgart, Germany.
The development of an RSV vaccine had ground to a halt for many decades: A tragedy in the 1960s set the whole field of research back. Using the model of the first polio vaccine, scientists had manufactured an experimental vaccine with inactivated viruses. However, tests showed that the vaccine did not protect the children vaccinated, but it actually infected them with RSV, they then fell ill, and two children died. Today, potential RSV vaccines are first tested on adults and not on children.
Few treatment options
RSV causes seasonal epidemics, can lead to bronchiolitis and pneumonia in infants, and is one of the main causes of hospital stays in young children. Monoclonal antibodies are currently the only preventive option, since there is still no vaccine. Usually, 60%-70% of infants and nearly all children younger than 2 years are infected with RSV, but the virus can also trigger pneumonia in adults.
“RSV infections constitute a major public health challenge: It is the most dangerous respiratory virus for young infants, it is also a threat to the chronically ill and immunocompromised of all ages, and [it] is the second most common cause of death worldwide (after malaria) in young children,” stated Dr. Rose.
Recently, pandemic-related measures (face masks, more intense disinfection) meant that the “normal” RSV infections in healthy adults, which usually progress like a mild cold, were prevented, and mothers were unable to pass on as much RSV immune defense to their children. “This was presumably responsible in part for the massive wave of RSV infections in fall and winter of 2021/22,” explained Dr. Rose.
Thomas Mertens, MD, PhD, chair of the Standing Committee on Vaccination at the Robert Koch Institute (STIKO) and former director of the Institute for Virology at Ulm University Hospital, Germany, also noted: “It would be an important and potentially achievable goal to significantly reduce the incidence rate of hospitalizations. In this respect, RSV poses a significant problem for young children, their parents, and the burden on pediatric clinics.”
Final evaluation pending
“I am definitely finding the data interesting, but the original data are needed,” Dr. Mertens said. Once the data are published at a conference or published in a peer-reviewed journal, physicians will be able to better judge the data for themselves, he said.
Dr. Rose characterized the new vaccine as “novel,” including in terms of its composition. Earlier RSV vaccines used the so-called postfusion F protein as their starting point. But it has become known in the meantime that the key to immunogenicity is the continued prefusion state of the apical epitope: Prefusion F-specific memory B cells in adults naturally infected with RSV produce potent neutralizing antibodies.
The new vaccine is bivalent and protects against both RSV A and RSV B.
To date, RSV vaccination directly in young infants have had only had a weak efficacy and were sometimes poorly tolerated. The vaccine presented here is expected to be tested in young adults first, then in school children, then young children.
Through successful vaccination of the entire population, the transfer of RS viruses to young children could be prevented. “To what extent this, or any other RSV vaccine still to be developed on the same basis, will also be effective and well tolerated in young infants is still difficult to assess,” said Dr. Rose.
Dr. Mertens emphasized that all of the study data now needs to be seen as quickly as possible: “This is also a general requirement for transparency from the pharmaceutical companies, which is also rightly criticized.”
This article was originally published in Medscape’s German edition and a version appeared on Medscape.com.
High lipoprotein(a) levels plus hypertension add to CVD risk
High levels of lipoprotein(a) increase the risk for incident cardiovascular disease (CVD) for hypertensive individuals but not for those without hypertension, a new MESA analysis suggests.
There are ways to test for statistical interaction, “in this case, multiplicative interaction between Lp(a) and hypertension, which suggests that Lp(a) is actually modifying the effect between blood pressure and cardiovascular disease. It’s not simply additive,” senior author Michael D. Shapiro, DO, Wake Forest University, Winston-Salem, N.C., told this news organization.
“So that’s new and I don’t think anybody’s looked at that before.”
Although Lp(a) is recognized as an independent cause of atherosclerotic CVD (ASCVD), the significance of Lp(a) in hypertension has been “virtually untapped,” he noted. A recent prospective study reported that elevated CVD risk was present only in individuals with Lp(a) ≥ 30 mg/dL and hypertension but it included only Chinese participants with stable coronary artery disease.
The current analysis, published online in the journal Hypertension, included 6,674 participants in the ongoing Multi-Ethnic Study of Atherosclerosis (MESA), all free of baseline ASCVD, who were recruited from six communities in the United States and had measured baseline Lp(a), blood pressure, and CVD events data over follow-up from 2000 to 2018.
Participants were stratified into four groups based on the presence or absence of hypertension (defined as 140/90 mm Hg or higher or the use of antihypertensive drugs) and an Lp(a) threshold of 50 mg/dL, as recommended by the American College of Cardiology/American Heart Association cholesterol guideline for consideration as an ASCVD risk-enhancing factor.
Slightly more than half of participants were female (52.8%), 38.6% were White, 27.5% were African American, 22.1% were Hispanic, and 11.9% were Chinese American.
According to the researchers, 809 participants had a CVD event over an average follow-up of 13.9 years, including 7.7% of group 1 with Lp(a) < 50 mg/dL and no hypertension, 8.0% of group 2 with Lp(a) ≥ 50 mg/dL and no hypertension, 16.2% of group 3 with Lp(a) < 50 mg/dL and hypertension, and 18.8% of group 4 with Lp(a) ≥ 50 mg/dL and hypertension.
When compared with group 1 in a fully adjusted Cox proportional model, participants with elevated Lp(a) and no hypertension (group 2) did not have an increased risk of CVD events (hazard ratio [HR], 1.09; 95% confidence interval [CI], 0.79-1.50).
CVD risk, however, was significantly higher in group 3 with normal Lp(a) and hypertension (HR, 1.66; 95% CI, 1.39-1.98) and group 4 with elevated Lp(a) and hypertension (HR, 2.07, 95% CI, 1.63-2.62).
Among all participants with hypertension (groups 3 and 4), Lp(a) was associated with a significant increase in CVD risk (HR, 1.24, 95% CI, 1.01-1.53).
“What I think is interesting here is that in the absence of hypertension, we didn’t really see an increased risk despite having an elevated Lp(a),” said Dr. Shapiro. “What it may indicate is that really for Lp(a) to be associated with risk, there may already need to be some kind of arterial damage that allows the Lp(a) to have its atherogenic impact.
“In other words, in individuals who have totally normal arterial walls, potentially, maybe that is protective enough against Lp(a) that in the absence of any other injurious factor, maybe it’s not an issue,” he said. “That’s a big hypothesis-generating [statement], but hypertension is certainly one of those risk factors that’s known to cause endothelial injury and endothelial dysfunction.”
Dr. Shapiro pointed out that when first measured in MESA, Lp(a) was measured in 4,600 participants who were not on statins, which is important because statins can increase Lp(a) levels.
“When you look just at those participants, those 4,600, you actually do see a relationship between Lp(a) and cardiovascular disease,” he said. “When you look at the whole population, including the 17% who are baseline populations, even when you adjust for statin therapy, we fail to see that, at least in the long-term follow up.”
Nevertheless, he cautioned that hypertension is just one of many traditional cardiovascular risk factors that could affect the relationship between Lp(a) and CVD risk. “I don’t want to suggest that we believe there’s something specifically magical about hypertension and Lp(a). If we chose, say, diabetes or smoking or another traditional risk factor, we may or may not have seen kind of similar results.”
When the investigators stratified the analyses by sex and race/ethnicity, they found that Lp(a) was not associated with CVD risk, regardless of hypertension status. In Black participants, however, greater CVD risk was seen when both elevated Lp(a) and hypertension were present (HR, 2.07, 95% CI, 1.34-3.21; P = .001).
Asked whether the results support one-time universal screening for Lp(a), which is almost exclusively genetically determined, Dr. Shapiro said he supports screening but that this was a secondary analysis and its numbers were modest. He added that median Lp(a) level is higher in African Americans than any other racial/ethnic group but the “most recent data has clarified that, per any absolute level of Lp(a), it appears to confer the same absolute risk in any racial or ethnic group.”
The authors acknowledge that differential loss to follow-up could have resulted in selection bias in the study and that there were relatively few CVD events in group 2, which may have limited the ability to detect differences in groups without hypertension, particularly in the subgroup analyses. Other limitations are the potential for residual confounding and participants may have developed hypertension during follow-up, resulting in misclassification bias.
Further research is needed to better understand the mechanistic link between Lp(a), hypertension, and CVD, Dr. Shapiro said. Further insights also should be provided by the ongoing phase 3 Lp(a) HORIZON trial evaluating the effect of Lp(a) lowering with the investigational antisense drug, pelacarsen, on cardiovascular events in 8,324 patients with established CVD and elevated Lp(a). The study is expected to be completed in May 2025.
The study was supported by contracts from the National Heart, Lung, and Blood Institute and by grants from the National Center for Advanced Translational Sciences. Dr. Shapiro reports participating in scientific advisory boards with Amgen, Novartis, and Novo Nordisk, and consulting for Regeneron.
A version of this article first appeared on Medscape.com.
High levels of lipoprotein(a) increase the risk for incident cardiovascular disease (CVD) for hypertensive individuals but not for those without hypertension, a new MESA analysis suggests.
There are ways to test for statistical interaction, “in this case, multiplicative interaction between Lp(a) and hypertension, which suggests that Lp(a) is actually modifying the effect between blood pressure and cardiovascular disease. It’s not simply additive,” senior author Michael D. Shapiro, DO, Wake Forest University, Winston-Salem, N.C., told this news organization.
“So that’s new and I don’t think anybody’s looked at that before.”
Although Lp(a) is recognized as an independent cause of atherosclerotic CVD (ASCVD), the significance of Lp(a) in hypertension has been “virtually untapped,” he noted. A recent prospective study reported that elevated CVD risk was present only in individuals with Lp(a) ≥ 30 mg/dL and hypertension but it included only Chinese participants with stable coronary artery disease.
The current analysis, published online in the journal Hypertension, included 6,674 participants in the ongoing Multi-Ethnic Study of Atherosclerosis (MESA), all free of baseline ASCVD, who were recruited from six communities in the United States and had measured baseline Lp(a), blood pressure, and CVD events data over follow-up from 2000 to 2018.
Participants were stratified into four groups based on the presence or absence of hypertension (defined as 140/90 mm Hg or higher or the use of antihypertensive drugs) and an Lp(a) threshold of 50 mg/dL, as recommended by the American College of Cardiology/American Heart Association cholesterol guideline for consideration as an ASCVD risk-enhancing factor.
Slightly more than half of participants were female (52.8%), 38.6% were White, 27.5% were African American, 22.1% were Hispanic, and 11.9% were Chinese American.
According to the researchers, 809 participants had a CVD event over an average follow-up of 13.9 years, including 7.7% of group 1 with Lp(a) < 50 mg/dL and no hypertension, 8.0% of group 2 with Lp(a) ≥ 50 mg/dL and no hypertension, 16.2% of group 3 with Lp(a) < 50 mg/dL and hypertension, and 18.8% of group 4 with Lp(a) ≥ 50 mg/dL and hypertension.
When compared with group 1 in a fully adjusted Cox proportional model, participants with elevated Lp(a) and no hypertension (group 2) did not have an increased risk of CVD events (hazard ratio [HR], 1.09; 95% confidence interval [CI], 0.79-1.50).
CVD risk, however, was significantly higher in group 3 with normal Lp(a) and hypertension (HR, 1.66; 95% CI, 1.39-1.98) and group 4 with elevated Lp(a) and hypertension (HR, 2.07, 95% CI, 1.63-2.62).
Among all participants with hypertension (groups 3 and 4), Lp(a) was associated with a significant increase in CVD risk (HR, 1.24, 95% CI, 1.01-1.53).
“What I think is interesting here is that in the absence of hypertension, we didn’t really see an increased risk despite having an elevated Lp(a),” said Dr. Shapiro. “What it may indicate is that really for Lp(a) to be associated with risk, there may already need to be some kind of arterial damage that allows the Lp(a) to have its atherogenic impact.
“In other words, in individuals who have totally normal arterial walls, potentially, maybe that is protective enough against Lp(a) that in the absence of any other injurious factor, maybe it’s not an issue,” he said. “That’s a big hypothesis-generating [statement], but hypertension is certainly one of those risk factors that’s known to cause endothelial injury and endothelial dysfunction.”
Dr. Shapiro pointed out that when first measured in MESA, Lp(a) was measured in 4,600 participants who were not on statins, which is important because statins can increase Lp(a) levels.
“When you look just at those participants, those 4,600, you actually do see a relationship between Lp(a) and cardiovascular disease,” he said. “When you look at the whole population, including the 17% who are baseline populations, even when you adjust for statin therapy, we fail to see that, at least in the long-term follow up.”
Nevertheless, he cautioned that hypertension is just one of many traditional cardiovascular risk factors that could affect the relationship between Lp(a) and CVD risk. “I don’t want to suggest that we believe there’s something specifically magical about hypertension and Lp(a). If we chose, say, diabetes or smoking or another traditional risk factor, we may or may not have seen kind of similar results.”
When the investigators stratified the analyses by sex and race/ethnicity, they found that Lp(a) was not associated with CVD risk, regardless of hypertension status. In Black participants, however, greater CVD risk was seen when both elevated Lp(a) and hypertension were present (HR, 2.07, 95% CI, 1.34-3.21; P = .001).
Asked whether the results support one-time universal screening for Lp(a), which is almost exclusively genetically determined, Dr. Shapiro said he supports screening but that this was a secondary analysis and its numbers were modest. He added that median Lp(a) level is higher in African Americans than any other racial/ethnic group but the “most recent data has clarified that, per any absolute level of Lp(a), it appears to confer the same absolute risk in any racial or ethnic group.”
The authors acknowledge that differential loss to follow-up could have resulted in selection bias in the study and that there were relatively few CVD events in group 2, which may have limited the ability to detect differences in groups without hypertension, particularly in the subgroup analyses. Other limitations are the potential for residual confounding and participants may have developed hypertension during follow-up, resulting in misclassification bias.
Further research is needed to better understand the mechanistic link between Lp(a), hypertension, and CVD, Dr. Shapiro said. Further insights also should be provided by the ongoing phase 3 Lp(a) HORIZON trial evaluating the effect of Lp(a) lowering with the investigational antisense drug, pelacarsen, on cardiovascular events in 8,324 patients with established CVD and elevated Lp(a). The study is expected to be completed in May 2025.
The study was supported by contracts from the National Heart, Lung, and Blood Institute and by grants from the National Center for Advanced Translational Sciences. Dr. Shapiro reports participating in scientific advisory boards with Amgen, Novartis, and Novo Nordisk, and consulting for Regeneron.
A version of this article first appeared on Medscape.com.
High levels of lipoprotein(a) increase the risk for incident cardiovascular disease (CVD) for hypertensive individuals but not for those without hypertension, a new MESA analysis suggests.
There are ways to test for statistical interaction, “in this case, multiplicative interaction between Lp(a) and hypertension, which suggests that Lp(a) is actually modifying the effect between blood pressure and cardiovascular disease. It’s not simply additive,” senior author Michael D. Shapiro, DO, Wake Forest University, Winston-Salem, N.C., told this news organization.
“So that’s new and I don’t think anybody’s looked at that before.”
Although Lp(a) is recognized as an independent cause of atherosclerotic CVD (ASCVD), the significance of Lp(a) in hypertension has been “virtually untapped,” he noted. A recent prospective study reported that elevated CVD risk was present only in individuals with Lp(a) ≥ 30 mg/dL and hypertension but it included only Chinese participants with stable coronary artery disease.
The current analysis, published online in the journal Hypertension, included 6,674 participants in the ongoing Multi-Ethnic Study of Atherosclerosis (MESA), all free of baseline ASCVD, who were recruited from six communities in the United States and had measured baseline Lp(a), blood pressure, and CVD events data over follow-up from 2000 to 2018.
Participants were stratified into four groups based on the presence or absence of hypertension (defined as 140/90 mm Hg or higher or the use of antihypertensive drugs) and an Lp(a) threshold of 50 mg/dL, as recommended by the American College of Cardiology/American Heart Association cholesterol guideline for consideration as an ASCVD risk-enhancing factor.
Slightly more than half of participants were female (52.8%), 38.6% were White, 27.5% were African American, 22.1% were Hispanic, and 11.9% were Chinese American.
According to the researchers, 809 participants had a CVD event over an average follow-up of 13.9 years, including 7.7% of group 1 with Lp(a) < 50 mg/dL and no hypertension, 8.0% of group 2 with Lp(a) ≥ 50 mg/dL and no hypertension, 16.2% of group 3 with Lp(a) < 50 mg/dL and hypertension, and 18.8% of group 4 with Lp(a) ≥ 50 mg/dL and hypertension.
When compared with group 1 in a fully adjusted Cox proportional model, participants with elevated Lp(a) and no hypertension (group 2) did not have an increased risk of CVD events (hazard ratio [HR], 1.09; 95% confidence interval [CI], 0.79-1.50).
CVD risk, however, was significantly higher in group 3 with normal Lp(a) and hypertension (HR, 1.66; 95% CI, 1.39-1.98) and group 4 with elevated Lp(a) and hypertension (HR, 2.07, 95% CI, 1.63-2.62).
Among all participants with hypertension (groups 3 and 4), Lp(a) was associated with a significant increase in CVD risk (HR, 1.24, 95% CI, 1.01-1.53).
“What I think is interesting here is that in the absence of hypertension, we didn’t really see an increased risk despite having an elevated Lp(a),” said Dr. Shapiro. “What it may indicate is that really for Lp(a) to be associated with risk, there may already need to be some kind of arterial damage that allows the Lp(a) to have its atherogenic impact.
“In other words, in individuals who have totally normal arterial walls, potentially, maybe that is protective enough against Lp(a) that in the absence of any other injurious factor, maybe it’s not an issue,” he said. “That’s a big hypothesis-generating [statement], but hypertension is certainly one of those risk factors that’s known to cause endothelial injury and endothelial dysfunction.”
Dr. Shapiro pointed out that when first measured in MESA, Lp(a) was measured in 4,600 participants who were not on statins, which is important because statins can increase Lp(a) levels.
“When you look just at those participants, those 4,600, you actually do see a relationship between Lp(a) and cardiovascular disease,” he said. “When you look at the whole population, including the 17% who are baseline populations, even when you adjust for statin therapy, we fail to see that, at least in the long-term follow up.”
Nevertheless, he cautioned that hypertension is just one of many traditional cardiovascular risk factors that could affect the relationship between Lp(a) and CVD risk. “I don’t want to suggest that we believe there’s something specifically magical about hypertension and Lp(a). If we chose, say, diabetes or smoking or another traditional risk factor, we may or may not have seen kind of similar results.”
When the investigators stratified the analyses by sex and race/ethnicity, they found that Lp(a) was not associated with CVD risk, regardless of hypertension status. In Black participants, however, greater CVD risk was seen when both elevated Lp(a) and hypertension were present (HR, 2.07, 95% CI, 1.34-3.21; P = .001).
Asked whether the results support one-time universal screening for Lp(a), which is almost exclusively genetically determined, Dr. Shapiro said he supports screening but that this was a secondary analysis and its numbers were modest. He added that median Lp(a) level is higher in African Americans than any other racial/ethnic group but the “most recent data has clarified that, per any absolute level of Lp(a), it appears to confer the same absolute risk in any racial or ethnic group.”
The authors acknowledge that differential loss to follow-up could have resulted in selection bias in the study and that there were relatively few CVD events in group 2, which may have limited the ability to detect differences in groups without hypertension, particularly in the subgroup analyses. Other limitations are the potential for residual confounding and participants may have developed hypertension during follow-up, resulting in misclassification bias.
Further research is needed to better understand the mechanistic link between Lp(a), hypertension, and CVD, Dr. Shapiro said. Further insights also should be provided by the ongoing phase 3 Lp(a) HORIZON trial evaluating the effect of Lp(a) lowering with the investigational antisense drug, pelacarsen, on cardiovascular events in 8,324 patients with established CVD and elevated Lp(a). The study is expected to be completed in May 2025.
The study was supported by contracts from the National Heart, Lung, and Blood Institute and by grants from the National Center for Advanced Translational Sciences. Dr. Shapiro reports participating in scientific advisory boards with Amgen, Novartis, and Novo Nordisk, and consulting for Regeneron.
A version of this article first appeared on Medscape.com.
FROM HYPERTENSION