User login
Burnout Is Rampant, But Oncologists Can Turn the Tide
SAN DIEGO—Before the pandemic, an estimated one-third of oncologists worldwide suffered a high level of burnout. Cancer physicians face many of the same risk factors as their colleagues—high workloads, lack of autonomy, and no support—along with the added pressure of working in a medical field where patients often die. Then COVID-19 hit, and the burnout crisis got even worse.
This tide can be reversed with a focus on best practices and resilience, a mental health researcher told cancer professionals at the September 2022 annual meeting of the Association of VA Hematology/Oncology. Assessments, long-term interventions, and communication are all key, said Fay J. Hlubocky, PhD, MA, a clinical health psychologist and ethicist at the University of Chicago.
Even simple actions like taking time for “mindful moments” and checking in with a colleague can make a difference, she said. But institutions must act, she said. “Long-term tailored strategies are incredibly important to promote well-being.”
Hlubocky, who led an American Society of Clinical Oncology committee on burnout prior to the pandemic, noted that statistics about burnout in American medicine and oncology specifically, are grim. In 2017, a systematic review and meta-analysis found that significant numbers of oncologists suffered from high burnout (32%), high psychiatric morbidity (27%), depression (at least 12%), and alcohol misuse (as many as 30%).
The pandemic piled on more stressors. In the second half of 2020, researchers interviewed 25 American oncologists in focus groups and found that their “underlying oncologist burnout exacerbated stressors associated with disruptions in care, education, research, financial practice health, and telemedicine. Many feared delays in cancer screening, diagnosis, and treatment [and] strongly considered working part-time or taking early retirement.”
As one participant put it, “everyone is seeing a lot of death and heartache and social isolation and anger that they’re not used to encountering and in very new and different ways.”
Major contributors to oncologist burnout, Hlubocky said, include moral distress, moral injury, and compassion fatigue. “Moral distress occurs when that individual believes he or she knows the right thing to do, but institutional constraints make it really difficult to do what is right,” Hlubocky said. “The individual is aware of the moral problem, acknowledges and takes moral responsibility, makes some moral judgments, but yet—as a result of these constraints — participates in perceived moral wrongdoing.”
Moral injury refers to the damage that can be caused by moral distress or by witnessing acts that violate morals, such as during military service. Compassion fatigue, meanwhile, is defined by the American Stress Institute as “a low level, chronic clouding of caring and concern for others in your life.”
What can be done? Hlubocky highlighted multiple interventions, such as adjustment of work patterns, cognitive behavioral therapy, and training in mindfulness, relaxation, and communication. One strategy is to adopt multiple in-person interventions simultaneously.
But first it’s crucial for administrators to understand the problem in a specific workplace: “You have to know what’s going on in your organization to intervene on it,” she said. “There are multiple tools that have been validated in other health care fields and can be used on a regular basis over time to measure burnout, satisfaction, and engagement.”
For individuals, other strategies include daily check-ins with colleagues to catch signs of stress, she said, as Toronto oncologists started doing amid the pandemic. The check-ins can include simple questions like: How are you doing? How are you feeling? Are you sleeping, eating and exercising? Do you need help?
As for resilience, Hlubocky said it must grow at the individual level. “We can't rely so much on the organization. We need to develop our personal resilience in order for professional resilience to flourish again, and we have to do a lot to protect ourselves. It’s about focusing on the strength of the individual—that empowerment to rise above adversity, that vitality, that engagement, that self-efficacy. It supports health and enhances coping, and it is the key element of physician and clinician well-being.”
Research into resilience offers guidance about how to achieve it, she said. A 2013 German study of 200 physicians found that the most resilient physicians change their attitudes and behaviors, take time off, set boundaries, spend time with family and friends, and ask colleagues for help. And they gained resilience, the study found, by getting older and becoming more experienced.
Hlubocky pointed to several useful resources for burned-out medical professionals, including mindfulness, cognitive behavioral therapy and breathing apps: She highlighted Breathe2Relax, Headspace, MoodGYM, Stress Gym, and guided audio files from the University of California at San Diego. And she said ASCO has resources on combatting burnout and promoting well-being.
Hlubocky has no relevant disclosures.
SAN DIEGO—Before the pandemic, an estimated one-third of oncologists worldwide suffered a high level of burnout. Cancer physicians face many of the same risk factors as their colleagues—high workloads, lack of autonomy, and no support—along with the added pressure of working in a medical field where patients often die. Then COVID-19 hit, and the burnout crisis got even worse.
This tide can be reversed with a focus on best practices and resilience, a mental health researcher told cancer professionals at the September 2022 annual meeting of the Association of VA Hematology/Oncology. Assessments, long-term interventions, and communication are all key, said Fay J. Hlubocky, PhD, MA, a clinical health psychologist and ethicist at the University of Chicago.
Even simple actions like taking time for “mindful moments” and checking in with a colleague can make a difference, she said. But institutions must act, she said. “Long-term tailored strategies are incredibly important to promote well-being.”
Hlubocky, who led an American Society of Clinical Oncology committee on burnout prior to the pandemic, noted that statistics about burnout in American medicine and oncology specifically, are grim. In 2017, a systematic review and meta-analysis found that significant numbers of oncologists suffered from high burnout (32%), high psychiatric morbidity (27%), depression (at least 12%), and alcohol misuse (as many as 30%).
The pandemic piled on more stressors. In the second half of 2020, researchers interviewed 25 American oncologists in focus groups and found that their “underlying oncologist burnout exacerbated stressors associated with disruptions in care, education, research, financial practice health, and telemedicine. Many feared delays in cancer screening, diagnosis, and treatment [and] strongly considered working part-time or taking early retirement.”
As one participant put it, “everyone is seeing a lot of death and heartache and social isolation and anger that they’re not used to encountering and in very new and different ways.”
Major contributors to oncologist burnout, Hlubocky said, include moral distress, moral injury, and compassion fatigue. “Moral distress occurs when that individual believes he or she knows the right thing to do, but institutional constraints make it really difficult to do what is right,” Hlubocky said. “The individual is aware of the moral problem, acknowledges and takes moral responsibility, makes some moral judgments, but yet—as a result of these constraints — participates in perceived moral wrongdoing.”
Moral injury refers to the damage that can be caused by moral distress or by witnessing acts that violate morals, such as during military service. Compassion fatigue, meanwhile, is defined by the American Stress Institute as “a low level, chronic clouding of caring and concern for others in your life.”
What can be done? Hlubocky highlighted multiple interventions, such as adjustment of work patterns, cognitive behavioral therapy, and training in mindfulness, relaxation, and communication. One strategy is to adopt multiple in-person interventions simultaneously.
But first it’s crucial for administrators to understand the problem in a specific workplace: “You have to know what’s going on in your organization to intervene on it,” she said. “There are multiple tools that have been validated in other health care fields and can be used on a regular basis over time to measure burnout, satisfaction, and engagement.”
For individuals, other strategies include daily check-ins with colleagues to catch signs of stress, she said, as Toronto oncologists started doing amid the pandemic. The check-ins can include simple questions like: How are you doing? How are you feeling? Are you sleeping, eating and exercising? Do you need help?
As for resilience, Hlubocky said it must grow at the individual level. “We can't rely so much on the organization. We need to develop our personal resilience in order for professional resilience to flourish again, and we have to do a lot to protect ourselves. It’s about focusing on the strength of the individual—that empowerment to rise above adversity, that vitality, that engagement, that self-efficacy. It supports health and enhances coping, and it is the key element of physician and clinician well-being.”
Research into resilience offers guidance about how to achieve it, she said. A 2013 German study of 200 physicians found that the most resilient physicians change their attitudes and behaviors, take time off, set boundaries, spend time with family and friends, and ask colleagues for help. And they gained resilience, the study found, by getting older and becoming more experienced.
Hlubocky pointed to several useful resources for burned-out medical professionals, including mindfulness, cognitive behavioral therapy and breathing apps: She highlighted Breathe2Relax, Headspace, MoodGYM, Stress Gym, and guided audio files from the University of California at San Diego. And she said ASCO has resources on combatting burnout and promoting well-being.
Hlubocky has no relevant disclosures.
SAN DIEGO—Before the pandemic, an estimated one-third of oncologists worldwide suffered a high level of burnout. Cancer physicians face many of the same risk factors as their colleagues—high workloads, lack of autonomy, and no support—along with the added pressure of working in a medical field where patients often die. Then COVID-19 hit, and the burnout crisis got even worse.
This tide can be reversed with a focus on best practices and resilience, a mental health researcher told cancer professionals at the September 2022 annual meeting of the Association of VA Hematology/Oncology. Assessments, long-term interventions, and communication are all key, said Fay J. Hlubocky, PhD, MA, a clinical health psychologist and ethicist at the University of Chicago.
Even simple actions like taking time for “mindful moments” and checking in with a colleague can make a difference, she said. But institutions must act, she said. “Long-term tailored strategies are incredibly important to promote well-being.”
Hlubocky, who led an American Society of Clinical Oncology committee on burnout prior to the pandemic, noted that statistics about burnout in American medicine and oncology specifically, are grim. In 2017, a systematic review and meta-analysis found that significant numbers of oncologists suffered from high burnout (32%), high psychiatric morbidity (27%), depression (at least 12%), and alcohol misuse (as many as 30%).
The pandemic piled on more stressors. In the second half of 2020, researchers interviewed 25 American oncologists in focus groups and found that their “underlying oncologist burnout exacerbated stressors associated with disruptions in care, education, research, financial practice health, and telemedicine. Many feared delays in cancer screening, diagnosis, and treatment [and] strongly considered working part-time or taking early retirement.”
As one participant put it, “everyone is seeing a lot of death and heartache and social isolation and anger that they’re not used to encountering and in very new and different ways.”
Major contributors to oncologist burnout, Hlubocky said, include moral distress, moral injury, and compassion fatigue. “Moral distress occurs when that individual believes he or she knows the right thing to do, but institutional constraints make it really difficult to do what is right,” Hlubocky said. “The individual is aware of the moral problem, acknowledges and takes moral responsibility, makes some moral judgments, but yet—as a result of these constraints — participates in perceived moral wrongdoing.”
Moral injury refers to the damage that can be caused by moral distress or by witnessing acts that violate morals, such as during military service. Compassion fatigue, meanwhile, is defined by the American Stress Institute as “a low level, chronic clouding of caring and concern for others in your life.”
What can be done? Hlubocky highlighted multiple interventions, such as adjustment of work patterns, cognitive behavioral therapy, and training in mindfulness, relaxation, and communication. One strategy is to adopt multiple in-person interventions simultaneously.
But first it’s crucial for administrators to understand the problem in a specific workplace: “You have to know what’s going on in your organization to intervene on it,” she said. “There are multiple tools that have been validated in other health care fields and can be used on a regular basis over time to measure burnout, satisfaction, and engagement.”
For individuals, other strategies include daily check-ins with colleagues to catch signs of stress, she said, as Toronto oncologists started doing amid the pandemic. The check-ins can include simple questions like: How are you doing? How are you feeling? Are you sleeping, eating and exercising? Do you need help?
As for resilience, Hlubocky said it must grow at the individual level. “We can't rely so much on the organization. We need to develop our personal resilience in order for professional resilience to flourish again, and we have to do a lot to protect ourselves. It’s about focusing on the strength of the individual—that empowerment to rise above adversity, that vitality, that engagement, that self-efficacy. It supports health and enhances coping, and it is the key element of physician and clinician well-being.”
Research into resilience offers guidance about how to achieve it, she said. A 2013 German study of 200 physicians found that the most resilient physicians change their attitudes and behaviors, take time off, set boundaries, spend time with family and friends, and ask colleagues for help. And they gained resilience, the study found, by getting older and becoming more experienced.
Hlubocky pointed to several useful resources for burned-out medical professionals, including mindfulness, cognitive behavioral therapy and breathing apps: She highlighted Breathe2Relax, Headspace, MoodGYM, Stress Gym, and guided audio files from the University of California at San Diego. And she said ASCO has resources on combatting burnout and promoting well-being.
Hlubocky has no relevant disclosures.
Roselyn Tso confirmed to head Indian Health Service
It took 609 days, but the US Senate has finally (unanimously) confirmed President Biden’s choice to head the Indian Health Service (IHS: Roselyn Tso.)
President Biden nominated Tso in March 2022, and she was formally sworn in on September 27, 2022. The long-awaited confirmation filled a space that hadn’t had a permanent director since Michael Weahkee, a Pueblo of Zuni citizen, stepped down in 2021. In the interim, Elizabeth Fowler, of the Comanche Nation, served as acting director.
Tso’s resume includes almost 40 years of professional experience working at all levels of the IHS. Before taking over as IHS director, she led the IHS Navajo area, the largest IHS regional area, managing more than 4000 employees and a budget of nearly $1 billion.
She also brings “decades of lived experience as a member of the Navajo Nation,” she said in a 40-minute Senate hearing with the US Senate Committee on Indian Affairs in May.
The first Navajo Nation citizen to head the IHS (and only the second woman to do so), Tso introduced herself in Navajo: Deeschii’nii (Start of the Red Streak People) and born for Hashk’aa hadzohi (Yucca Fruit Strung Out). “This is a historic achievement for all of our Navajo people and tribal nations across the country,” Navajo Nation President Jonathan Nez said. “To have one of our own Navajo members in the highest position with IHS is remarkable.”
Tso spoke of having to “navigate the services provided by the Agency for myself, family, and friends.” Her personal and professional backgrounds, she said, help her understand how patients experience the system and how that can be improved. “The health care provided at IHS is critical for those we serve. I understand this not just because I work there,” she said. “My family relies on IHS. My friends rely on IHS. I rely on the IHS.”
The long lacuna in confirming a permanent IHS director left the Native peoples particularly vulnerable—when the COVID-19 pandemic essentially worsened the existing problems they faced, such as diabetes mellitus and cancer. Life expectancy for Native people fell by more than 6 years between 2019 and 2021, to 65 years, compared with the US average of 76 years.
Without a full-time IHS leader, the National Council of Urban Indian Health said in a statement, tribal nations and other Native health care providers struggled to raise and address the issues they were facing amid the pandemic. “Since the resignation of Rear Admiral Weahkee, there have been countless requests from Indian Country calling on Congress and the Administration to nominate a new IHS director to address the growing health disparities experienced by AI/ANs.”
Tso laid out her priorities in her May testimony: creating a more unified health care system using the latest technology to develop centralized systems; improving accountability, transparency, and patient safety; addressing workforce needs and challenges, improving recruitment and retention.
Meeting her goals, she noted, would take “strong partnerships and communication with our Tribal partners…. Each tribe has unique needs, and those needs cannot be met if you do not understand them.”
Last year, President Joseph R. Biden asked Congress to significantly increase IHS funding, but his proposal was cut to $400 million. “For years, IHS has been funded at a rate that is far below its level of need, and the results of this historical neglect can be seen in the disparities in health outcomes for AI/AN people,” William Smith, Valdez Native Tribe, Chairman of the National Indian Health Board (NIHB), wrote to the Senate Committee on Indian Affairs, on the topic of the next IHS director. “Perhaps one of the greatest challenges facing the [Indian, tribal and urban] system is the chronic and severe underfunding and budgetary instability for health care and public health services infrastructure and delivery. Since its creation in 1955, IHS has been chronically underfunded, with annual appropriations never exceeding 50% of demonstrated need. This underfunding has contributed to substandard investment in health delivery systems, some of the worst health disparities among any US population and a severe lack of public health infrastructure and services for our people. At the start of the COVID-19 pandemic these vulnerabilities were starkly exposed and while Congress moved decisively to invest into Tribal health and public health, the new Director must work to maintain these one-time investments.”
Stacy Bohlen, NIHB chief executive, told The Oklahoman that tribal leaders will look to Tso to press Congress for more money and to secure mandatory full funding for IHS—in contrast with the current annual appropriations, where Congress includes IHS in much larger budget bills. “When those bills stall, so does the money tribal clinics need to pay employees and suppliers,” making it hard to recruit and retain employees. “In the Indian Health System,” Bohlen says, “we simply can’t afford that kind of vulnerability.”
Securing advance appropriations and, ultimately, full mandatory funding for IHS, Smith wrote in his letter to the Senate committee, “fulfills the commitment made to our people generations ago and breaks down the systemic healthcare funding inequities the federal government tolerates for Tribes.”
Tso emphasized her intent to “improve the physical, mental, social, and spiritual health and well-being of all American Indians and Alaskan Natives served by the Agency.” Tso “understands the healthcare needs that many first people of this country deal with,” President Nez said. “Her work ethic, value system and approach to problem solving demonstrates the resilience of Indigenous peoples and the commitment to combat the systemic inequities that impact tribal nations.”
It took 609 days, but the US Senate has finally (unanimously) confirmed President Biden’s choice to head the Indian Health Service (IHS: Roselyn Tso.)
President Biden nominated Tso in March 2022, and she was formally sworn in on September 27, 2022. The long-awaited confirmation filled a space that hadn’t had a permanent director since Michael Weahkee, a Pueblo of Zuni citizen, stepped down in 2021. In the interim, Elizabeth Fowler, of the Comanche Nation, served as acting director.
Tso’s resume includes almost 40 years of professional experience working at all levels of the IHS. Before taking over as IHS director, she led the IHS Navajo area, the largest IHS regional area, managing more than 4000 employees and a budget of nearly $1 billion.
She also brings “decades of lived experience as a member of the Navajo Nation,” she said in a 40-minute Senate hearing with the US Senate Committee on Indian Affairs in May.
The first Navajo Nation citizen to head the IHS (and only the second woman to do so), Tso introduced herself in Navajo: Deeschii’nii (Start of the Red Streak People) and born for Hashk’aa hadzohi (Yucca Fruit Strung Out). “This is a historic achievement for all of our Navajo people and tribal nations across the country,” Navajo Nation President Jonathan Nez said. “To have one of our own Navajo members in the highest position with IHS is remarkable.”
Tso spoke of having to “navigate the services provided by the Agency for myself, family, and friends.” Her personal and professional backgrounds, she said, help her understand how patients experience the system and how that can be improved. “The health care provided at IHS is critical for those we serve. I understand this not just because I work there,” she said. “My family relies on IHS. My friends rely on IHS. I rely on the IHS.”
The long lacuna in confirming a permanent IHS director left the Native peoples particularly vulnerable—when the COVID-19 pandemic essentially worsened the existing problems they faced, such as diabetes mellitus and cancer. Life expectancy for Native people fell by more than 6 years between 2019 and 2021, to 65 years, compared with the US average of 76 years.
Without a full-time IHS leader, the National Council of Urban Indian Health said in a statement, tribal nations and other Native health care providers struggled to raise and address the issues they were facing amid the pandemic. “Since the resignation of Rear Admiral Weahkee, there have been countless requests from Indian Country calling on Congress and the Administration to nominate a new IHS director to address the growing health disparities experienced by AI/ANs.”
Tso laid out her priorities in her May testimony: creating a more unified health care system using the latest technology to develop centralized systems; improving accountability, transparency, and patient safety; addressing workforce needs and challenges, improving recruitment and retention.
Meeting her goals, she noted, would take “strong partnerships and communication with our Tribal partners…. Each tribe has unique needs, and those needs cannot be met if you do not understand them.”
Last year, President Joseph R. Biden asked Congress to significantly increase IHS funding, but his proposal was cut to $400 million. “For years, IHS has been funded at a rate that is far below its level of need, and the results of this historical neglect can be seen in the disparities in health outcomes for AI/AN people,” William Smith, Valdez Native Tribe, Chairman of the National Indian Health Board (NIHB), wrote to the Senate Committee on Indian Affairs, on the topic of the next IHS director. “Perhaps one of the greatest challenges facing the [Indian, tribal and urban] system is the chronic and severe underfunding and budgetary instability for health care and public health services infrastructure and delivery. Since its creation in 1955, IHS has been chronically underfunded, with annual appropriations never exceeding 50% of demonstrated need. This underfunding has contributed to substandard investment in health delivery systems, some of the worst health disparities among any US population and a severe lack of public health infrastructure and services for our people. At the start of the COVID-19 pandemic these vulnerabilities were starkly exposed and while Congress moved decisively to invest into Tribal health and public health, the new Director must work to maintain these one-time investments.”
Stacy Bohlen, NIHB chief executive, told The Oklahoman that tribal leaders will look to Tso to press Congress for more money and to secure mandatory full funding for IHS—in contrast with the current annual appropriations, where Congress includes IHS in much larger budget bills. “When those bills stall, so does the money tribal clinics need to pay employees and suppliers,” making it hard to recruit and retain employees. “In the Indian Health System,” Bohlen says, “we simply can’t afford that kind of vulnerability.”
Securing advance appropriations and, ultimately, full mandatory funding for IHS, Smith wrote in his letter to the Senate committee, “fulfills the commitment made to our people generations ago and breaks down the systemic healthcare funding inequities the federal government tolerates for Tribes.”
Tso emphasized her intent to “improve the physical, mental, social, and spiritual health and well-being of all American Indians and Alaskan Natives served by the Agency.” Tso “understands the healthcare needs that many first people of this country deal with,” President Nez said. “Her work ethic, value system and approach to problem solving demonstrates the resilience of Indigenous peoples and the commitment to combat the systemic inequities that impact tribal nations.”
It took 609 days, but the US Senate has finally (unanimously) confirmed President Biden’s choice to head the Indian Health Service (IHS: Roselyn Tso.)
President Biden nominated Tso in March 2022, and she was formally sworn in on September 27, 2022. The long-awaited confirmation filled a space that hadn’t had a permanent director since Michael Weahkee, a Pueblo of Zuni citizen, stepped down in 2021. In the interim, Elizabeth Fowler, of the Comanche Nation, served as acting director.
Tso’s resume includes almost 40 years of professional experience working at all levels of the IHS. Before taking over as IHS director, she led the IHS Navajo area, the largest IHS regional area, managing more than 4000 employees and a budget of nearly $1 billion.
She also brings “decades of lived experience as a member of the Navajo Nation,” she said in a 40-minute Senate hearing with the US Senate Committee on Indian Affairs in May.
The first Navajo Nation citizen to head the IHS (and only the second woman to do so), Tso introduced herself in Navajo: Deeschii’nii (Start of the Red Streak People) and born for Hashk’aa hadzohi (Yucca Fruit Strung Out). “This is a historic achievement for all of our Navajo people and tribal nations across the country,” Navajo Nation President Jonathan Nez said. “To have one of our own Navajo members in the highest position with IHS is remarkable.”
Tso spoke of having to “navigate the services provided by the Agency for myself, family, and friends.” Her personal and professional backgrounds, she said, help her understand how patients experience the system and how that can be improved. “The health care provided at IHS is critical for those we serve. I understand this not just because I work there,” she said. “My family relies on IHS. My friends rely on IHS. I rely on the IHS.”
The long lacuna in confirming a permanent IHS director left the Native peoples particularly vulnerable—when the COVID-19 pandemic essentially worsened the existing problems they faced, such as diabetes mellitus and cancer. Life expectancy for Native people fell by more than 6 years between 2019 and 2021, to 65 years, compared with the US average of 76 years.
Without a full-time IHS leader, the National Council of Urban Indian Health said in a statement, tribal nations and other Native health care providers struggled to raise and address the issues they were facing amid the pandemic. “Since the resignation of Rear Admiral Weahkee, there have been countless requests from Indian Country calling on Congress and the Administration to nominate a new IHS director to address the growing health disparities experienced by AI/ANs.”
Tso laid out her priorities in her May testimony: creating a more unified health care system using the latest technology to develop centralized systems; improving accountability, transparency, and patient safety; addressing workforce needs and challenges, improving recruitment and retention.
Meeting her goals, she noted, would take “strong partnerships and communication with our Tribal partners…. Each tribe has unique needs, and those needs cannot be met if you do not understand them.”
Last year, President Joseph R. Biden asked Congress to significantly increase IHS funding, but his proposal was cut to $400 million. “For years, IHS has been funded at a rate that is far below its level of need, and the results of this historical neglect can be seen in the disparities in health outcomes for AI/AN people,” William Smith, Valdez Native Tribe, Chairman of the National Indian Health Board (NIHB), wrote to the Senate Committee on Indian Affairs, on the topic of the next IHS director. “Perhaps one of the greatest challenges facing the [Indian, tribal and urban] system is the chronic and severe underfunding and budgetary instability for health care and public health services infrastructure and delivery. Since its creation in 1955, IHS has been chronically underfunded, with annual appropriations never exceeding 50% of demonstrated need. This underfunding has contributed to substandard investment in health delivery systems, some of the worst health disparities among any US population and a severe lack of public health infrastructure and services for our people. At the start of the COVID-19 pandemic these vulnerabilities were starkly exposed and while Congress moved decisively to invest into Tribal health and public health, the new Director must work to maintain these one-time investments.”
Stacy Bohlen, NIHB chief executive, told The Oklahoman that tribal leaders will look to Tso to press Congress for more money and to secure mandatory full funding for IHS—in contrast with the current annual appropriations, where Congress includes IHS in much larger budget bills. “When those bills stall, so does the money tribal clinics need to pay employees and suppliers,” making it hard to recruit and retain employees. “In the Indian Health System,” Bohlen says, “we simply can’t afford that kind of vulnerability.”
Securing advance appropriations and, ultimately, full mandatory funding for IHS, Smith wrote in his letter to the Senate committee, “fulfills the commitment made to our people generations ago and breaks down the systemic healthcare funding inequities the federal government tolerates for Tribes.”
Tso emphasized her intent to “improve the physical, mental, social, and spiritual health and well-being of all American Indians and Alaskan Natives served by the Agency.” Tso “understands the healthcare needs that many first people of this country deal with,” President Nez said. “Her work ethic, value system and approach to problem solving demonstrates the resilience of Indigenous peoples and the commitment to combat the systemic inequities that impact tribal nations.”
Air pollution tied to stroke risk, subsequent CV events, and death
Results of a UK biobank study show high levels of air pollution were associated with an increased risk of transition from health to a first stroke and subsequent progression to cardiovascular (CV) events and death.
“These results indicate that understanding and reducing the effects of air pollutants on different transition stages in stroke will be beneficial in managing people’s health and preventing the occurrence and progression of stroke,” study investigator Hualiang Lin, PhD, of Sun Yat-sen University School of Public Health, Guangzhou, China, said in a news release.
The study was published online in the journal Neurology.
A way to stop stroke progression?
The researchers assessed air pollution exposure in 318,752 people (mean age, 56) from the UK biobank database. None had a history of stroke or heart disease at the start of the study. Annual concentrations of air pollution near where people lived were estimated through land-use regressions.
During an average follow-up of 12 years, 5,967 people had a stroke, 2,985 developed post-stroke CVD, and 1,020 died.
After adjusting for confounding factors, every 5 µg/m3 increase in exposure to fine particulate matter (PM2.5) was associated with a 24% increase in transition from healthy to first stroke (hazard ratio, 1.24; 95% confidence interval, 1.10-1.40) and a 30% increase in transition from being healthy to dying (HR, 1.30; 95% CI, 1.21-1.40).
PM2.5 is less than 2.5 microns in diameter and includes fly ash from coal combustion. The World Health Organization recommends that annual PM2.5 exposure should not exceed 5 µg/m3.
Those who had a stroke during the study had an average exposure of 10.03 µg/m3 of PM2.5, compared with 9.97 µg/m3 for those who did not have a stroke.
The air pollutants nitrogen oxide and nitrogen dioxide were also associated with an increased risk of stroke and death, but the associations were weaker.
“More research is needed, but it’s possible that decreasing exposure to heavy levels of air pollution could play a role in reducing the progression of stroke,” Dr. Lin said.
“People can reduce their exposure by staying indoors on heavy pollution days, reducing their outdoor exercise, wearing masks to filter out particulate matter, and using air purifiers,” Dr. Lin added.
Public policy implications
Reached for comment, Steffen E. Petersen, MD, MPH, professor of cardiovascular medicine, Barts Health NHS Trust, London, said the study “elegantly confirms the increased risk of stroke due to air pollution in the UK Biobank population study but interestingly suggests that the impact of air pollution may continue to adversely impact cardiovascular health even after the stroke occurred.”
“This is further evidence to inform policymakers to tackle air pollution and get levels below the recommended levels,” Dr. Petersen said.
“On a personal level, everyone, including stroke patients, may wish to consider personal measures to reduce exposure to air pollution, such as avoiding walking along polluted streets and rather take a less polluted route away from the main roads,” Dr. Petersen added.
The study had no targeted funding. Dr. Lin and Dr. Petersen report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Results of a UK biobank study show high levels of air pollution were associated with an increased risk of transition from health to a first stroke and subsequent progression to cardiovascular (CV) events and death.
“These results indicate that understanding and reducing the effects of air pollutants on different transition stages in stroke will be beneficial in managing people’s health and preventing the occurrence and progression of stroke,” study investigator Hualiang Lin, PhD, of Sun Yat-sen University School of Public Health, Guangzhou, China, said in a news release.
The study was published online in the journal Neurology.
A way to stop stroke progression?
The researchers assessed air pollution exposure in 318,752 people (mean age, 56) from the UK biobank database. None had a history of stroke or heart disease at the start of the study. Annual concentrations of air pollution near where people lived were estimated through land-use regressions.
During an average follow-up of 12 years, 5,967 people had a stroke, 2,985 developed post-stroke CVD, and 1,020 died.
After adjusting for confounding factors, every 5 µg/m3 increase in exposure to fine particulate matter (PM2.5) was associated with a 24% increase in transition from healthy to first stroke (hazard ratio, 1.24; 95% confidence interval, 1.10-1.40) and a 30% increase in transition from being healthy to dying (HR, 1.30; 95% CI, 1.21-1.40).
PM2.5 is less than 2.5 microns in diameter and includes fly ash from coal combustion. The World Health Organization recommends that annual PM2.5 exposure should not exceed 5 µg/m3.
Those who had a stroke during the study had an average exposure of 10.03 µg/m3 of PM2.5, compared with 9.97 µg/m3 for those who did not have a stroke.
The air pollutants nitrogen oxide and nitrogen dioxide were also associated with an increased risk of stroke and death, but the associations were weaker.
“More research is needed, but it’s possible that decreasing exposure to heavy levels of air pollution could play a role in reducing the progression of stroke,” Dr. Lin said.
“People can reduce their exposure by staying indoors on heavy pollution days, reducing their outdoor exercise, wearing masks to filter out particulate matter, and using air purifiers,” Dr. Lin added.
Public policy implications
Reached for comment, Steffen E. Petersen, MD, MPH, professor of cardiovascular medicine, Barts Health NHS Trust, London, said the study “elegantly confirms the increased risk of stroke due to air pollution in the UK Biobank population study but interestingly suggests that the impact of air pollution may continue to adversely impact cardiovascular health even after the stroke occurred.”
“This is further evidence to inform policymakers to tackle air pollution and get levels below the recommended levels,” Dr. Petersen said.
“On a personal level, everyone, including stroke patients, may wish to consider personal measures to reduce exposure to air pollution, such as avoiding walking along polluted streets and rather take a less polluted route away from the main roads,” Dr. Petersen added.
The study had no targeted funding. Dr. Lin and Dr. Petersen report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Results of a UK biobank study show high levels of air pollution were associated with an increased risk of transition from health to a first stroke and subsequent progression to cardiovascular (CV) events and death.
“These results indicate that understanding and reducing the effects of air pollutants on different transition stages in stroke will be beneficial in managing people’s health and preventing the occurrence and progression of stroke,” study investigator Hualiang Lin, PhD, of Sun Yat-sen University School of Public Health, Guangzhou, China, said in a news release.
The study was published online in the journal Neurology.
A way to stop stroke progression?
The researchers assessed air pollution exposure in 318,752 people (mean age, 56) from the UK biobank database. None had a history of stroke or heart disease at the start of the study. Annual concentrations of air pollution near where people lived were estimated through land-use regressions.
During an average follow-up of 12 years, 5,967 people had a stroke, 2,985 developed post-stroke CVD, and 1,020 died.
After adjusting for confounding factors, every 5 µg/m3 increase in exposure to fine particulate matter (PM2.5) was associated with a 24% increase in transition from healthy to first stroke (hazard ratio, 1.24; 95% confidence interval, 1.10-1.40) and a 30% increase in transition from being healthy to dying (HR, 1.30; 95% CI, 1.21-1.40).
PM2.5 is less than 2.5 microns in diameter and includes fly ash from coal combustion. The World Health Organization recommends that annual PM2.5 exposure should not exceed 5 µg/m3.
Those who had a stroke during the study had an average exposure of 10.03 µg/m3 of PM2.5, compared with 9.97 µg/m3 for those who did not have a stroke.
The air pollutants nitrogen oxide and nitrogen dioxide were also associated with an increased risk of stroke and death, but the associations were weaker.
“More research is needed, but it’s possible that decreasing exposure to heavy levels of air pollution could play a role in reducing the progression of stroke,” Dr. Lin said.
“People can reduce their exposure by staying indoors on heavy pollution days, reducing their outdoor exercise, wearing masks to filter out particulate matter, and using air purifiers,” Dr. Lin added.
Public policy implications
Reached for comment, Steffen E. Petersen, MD, MPH, professor of cardiovascular medicine, Barts Health NHS Trust, London, said the study “elegantly confirms the increased risk of stroke due to air pollution in the UK Biobank population study but interestingly suggests that the impact of air pollution may continue to adversely impact cardiovascular health even after the stroke occurred.”
“This is further evidence to inform policymakers to tackle air pollution and get levels below the recommended levels,” Dr. Petersen said.
“On a personal level, everyone, including stroke patients, may wish to consider personal measures to reduce exposure to air pollution, such as avoiding walking along polluted streets and rather take a less polluted route away from the main roads,” Dr. Petersen added.
The study had no targeted funding. Dr. Lin and Dr. Petersen report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Weighted blankets promote melatonin release, may improve sleep
, compared with a lighter blanket of only about 2.4% of body weight.
This suggests that weighted blankets may help promote sleep in patients suffering from insomnia, according to the results from the small, in-laboratory crossover study.
“Melatonin is produced by the pineal gland and plays an essential role in sleep timing,” lead author Elisa Meth, PhD student, Uppsala University, Sweden, and colleagues observe.
“Using a weighted blanket increased melatonin concentration in saliva by about 30%,” Ms. Meth added in a statement.
“Future studies should investigate whether the stimulatory effect on melatonin secretion remains when using a weighted blanket over more extended periods,” the researchers observe, and caution that “it is also unclear whether the observed increase in melatonin is therapeutically relevant.”
The study was published online in the Journal of Sleep Research.
Weighted blankets are commercially available at least in some countries in Scandinavia and Germany, as examples, and in general, they are sold for therapeutic purposes. And at least one study found that weighted blankets were an effective and safe intervention for insomnia in patients with major depressive disorder, bipolar disorder, generalized anxiety disorder, and attention deficit hyperactivity disorder and led to improvements in daytime symptoms and levels of activity.
Study done in healthy volunteers
The study involved a total of 26 healthy volunteers, 15 men and 11 women, none of whom had any sleep issues. “The day before the first testing session, the participants visited the laboratory for an adaptation night,” the authors observe. There were two experimental test nights, one in which the weighted blanket was used and the second during which the lighter blanket was used.
On the test nights, lights were dimmed between 9 PM and 11 PM and participants used a weighted blanket covering the extremities, abdomen, and chest 1 hour before and during 8 hours of sleep. As the authors explain, the filling of the weighted blanket consisted of honed glass pearls, combined with polyester wadding, which corresponded to 12.2% of participants’ body weight.
“Saliva was collected every 20 minutes between 22:00 and 23:00,” Ms. Meth and colleagues note. Participants’ subjective sleepiness was also assessed every 20 minutes using the Karolinska Sleepiness Scale both before the hour that lights were turned off and the next morning.
“Sleep duration in each experimental night was recorded with the OURA ring,” investigators explain.
The OURA ring is a commercial multisensor wearable device that measures physiological variables indicative of sleep. Investigators focused on total sleep duration as the primary outcome measure.
On average, salivary melatonin concentrations rose by about 5.8 pg/mL between 10 PM and 11 PM (P < .001), but the average increase in salivary melatonin concentrations was greater under weighted blanket conditions at 6.6 pg/mL, compared with 5.0 pg/mL during the lighter blanket session (P = .011).
Oxytocin in turn rose by about 315 pg/mL initially, but this rise was only transient, and over time, no significant difference in oxytocin levels was observed between the two blanket conditions. There were also no differences in cortisol levels or the activity of the sympathetic nervous system between the weighted and light blanket sessions.
Importantly, as well, no significant differences were seen in the level of sleepiness between participants when either blanket was used nor was there a significant difference in total sleep duration.
“Our study cannot identify the underlying mechanism for the observed stimulatory effects of the weighted blanket on melatonin,” the investigators caution.
However, one explanation could be that the pressure exerted by the weighted blanket activates cutaneous sensory afferent nerves, carrying information to the brain. The region where the sensory information is delivered stimulates oxytocinergic neurons that can promote calm and well-being and decrease fear, stress, and pain. In addition, these neurons also connect to the pineal gland to influence the release of melatonin, the authors explain.
Melatonin often viewed in the wrong context
Senior author Christian Benedict, PhD, associate professor of pharmacology, Uppsala University, Sweden, explained that some people think of melatonin in the wrong context.
In point of fact, “it’s not a sleep-promoting hormone. It prepares your body and brain for the biological night ... [and] sleep coincides with the biological night, but it’s not like you take melatonin and you have a very nice uninterrupted slumber – this is not true,” he told this news organization.
He also noted that certain groups respond to melatonin better than others. For example, children with attention deficit hyperactivity disorder may have some benefit from melatonin supplements, as may the elderly who can no longer produce sufficient amounts of melatonin and for whom supplements may help promote the timing of sleep.
However, the bottom line is that, even in those who do respond to melatonin supplements, they likely do so through a placebo effect that meta-analyses have shown plays a powerful role in promoting sleep.
Dr. Benedict also stressed that just because the body makes melatonin, itself, does not mean that melatonin supplements are necessarily “safe.”
“We know melatonin has some impact on puberty – it may delay the onset of puberty – and we know that it can also impair blood glucose, so when people are eating and have a lot of melatonin on board, the melatonin will tell the pancreas to turn off insulin production, which can give rise to hyperglycemia,” he said.
However, Dr. Benedict cautioned that weighted blankets don’t come cheap. A quick Google search brings up examples that cost upwards of $350. “MDs can say try one if you can afford these blankets, but perhaps people can use several less costly blankets,” he said. “But I definitely think if there are cheap options, why not?” he concluded.
Dr. Benedict has reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, compared with a lighter blanket of only about 2.4% of body weight.
This suggests that weighted blankets may help promote sleep in patients suffering from insomnia, according to the results from the small, in-laboratory crossover study.
“Melatonin is produced by the pineal gland and plays an essential role in sleep timing,” lead author Elisa Meth, PhD student, Uppsala University, Sweden, and colleagues observe.
“Using a weighted blanket increased melatonin concentration in saliva by about 30%,” Ms. Meth added in a statement.
“Future studies should investigate whether the stimulatory effect on melatonin secretion remains when using a weighted blanket over more extended periods,” the researchers observe, and caution that “it is also unclear whether the observed increase in melatonin is therapeutically relevant.”
The study was published online in the Journal of Sleep Research.
Weighted blankets are commercially available at least in some countries in Scandinavia and Germany, as examples, and in general, they are sold for therapeutic purposes. And at least one study found that weighted blankets were an effective and safe intervention for insomnia in patients with major depressive disorder, bipolar disorder, generalized anxiety disorder, and attention deficit hyperactivity disorder and led to improvements in daytime symptoms and levels of activity.
Study done in healthy volunteers
The study involved a total of 26 healthy volunteers, 15 men and 11 women, none of whom had any sleep issues. “The day before the first testing session, the participants visited the laboratory for an adaptation night,” the authors observe. There were two experimental test nights, one in which the weighted blanket was used and the second during which the lighter blanket was used.
On the test nights, lights were dimmed between 9 PM and 11 PM and participants used a weighted blanket covering the extremities, abdomen, and chest 1 hour before and during 8 hours of sleep. As the authors explain, the filling of the weighted blanket consisted of honed glass pearls, combined with polyester wadding, which corresponded to 12.2% of participants’ body weight.
“Saliva was collected every 20 minutes between 22:00 and 23:00,” Ms. Meth and colleagues note. Participants’ subjective sleepiness was also assessed every 20 minutes using the Karolinska Sleepiness Scale both before the hour that lights were turned off and the next morning.
“Sleep duration in each experimental night was recorded with the OURA ring,” investigators explain.
The OURA ring is a commercial multisensor wearable device that measures physiological variables indicative of sleep. Investigators focused on total sleep duration as the primary outcome measure.
On average, salivary melatonin concentrations rose by about 5.8 pg/mL between 10 PM and 11 PM (P < .001), but the average increase in salivary melatonin concentrations was greater under weighted blanket conditions at 6.6 pg/mL, compared with 5.0 pg/mL during the lighter blanket session (P = .011).
Oxytocin in turn rose by about 315 pg/mL initially, but this rise was only transient, and over time, no significant difference in oxytocin levels was observed between the two blanket conditions. There were also no differences in cortisol levels or the activity of the sympathetic nervous system between the weighted and light blanket sessions.
Importantly, as well, no significant differences were seen in the level of sleepiness between participants when either blanket was used nor was there a significant difference in total sleep duration.
“Our study cannot identify the underlying mechanism for the observed stimulatory effects of the weighted blanket on melatonin,” the investigators caution.
However, one explanation could be that the pressure exerted by the weighted blanket activates cutaneous sensory afferent nerves, carrying information to the brain. The region where the sensory information is delivered stimulates oxytocinergic neurons that can promote calm and well-being and decrease fear, stress, and pain. In addition, these neurons also connect to the pineal gland to influence the release of melatonin, the authors explain.
Melatonin often viewed in the wrong context
Senior author Christian Benedict, PhD, associate professor of pharmacology, Uppsala University, Sweden, explained that some people think of melatonin in the wrong context.
In point of fact, “it’s not a sleep-promoting hormone. It prepares your body and brain for the biological night ... [and] sleep coincides with the biological night, but it’s not like you take melatonin and you have a very nice uninterrupted slumber – this is not true,” he told this news organization.
He also noted that certain groups respond to melatonin better than others. For example, children with attention deficit hyperactivity disorder may have some benefit from melatonin supplements, as may the elderly who can no longer produce sufficient amounts of melatonin and for whom supplements may help promote the timing of sleep.
However, the bottom line is that, even in those who do respond to melatonin supplements, they likely do so through a placebo effect that meta-analyses have shown plays a powerful role in promoting sleep.
Dr. Benedict also stressed that just because the body makes melatonin, itself, does not mean that melatonin supplements are necessarily “safe.”
“We know melatonin has some impact on puberty – it may delay the onset of puberty – and we know that it can also impair blood glucose, so when people are eating and have a lot of melatonin on board, the melatonin will tell the pancreas to turn off insulin production, which can give rise to hyperglycemia,” he said.
However, Dr. Benedict cautioned that weighted blankets don’t come cheap. A quick Google search brings up examples that cost upwards of $350. “MDs can say try one if you can afford these blankets, but perhaps people can use several less costly blankets,” he said. “But I definitely think if there are cheap options, why not?” he concluded.
Dr. Benedict has reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, compared with a lighter blanket of only about 2.4% of body weight.
This suggests that weighted blankets may help promote sleep in patients suffering from insomnia, according to the results from the small, in-laboratory crossover study.
“Melatonin is produced by the pineal gland and plays an essential role in sleep timing,” lead author Elisa Meth, PhD student, Uppsala University, Sweden, and colleagues observe.
“Using a weighted blanket increased melatonin concentration in saliva by about 30%,” Ms. Meth added in a statement.
“Future studies should investigate whether the stimulatory effect on melatonin secretion remains when using a weighted blanket over more extended periods,” the researchers observe, and caution that “it is also unclear whether the observed increase in melatonin is therapeutically relevant.”
The study was published online in the Journal of Sleep Research.
Weighted blankets are commercially available at least in some countries in Scandinavia and Germany, as examples, and in general, they are sold for therapeutic purposes. And at least one study found that weighted blankets were an effective and safe intervention for insomnia in patients with major depressive disorder, bipolar disorder, generalized anxiety disorder, and attention deficit hyperactivity disorder and led to improvements in daytime symptoms and levels of activity.
Study done in healthy volunteers
The study involved a total of 26 healthy volunteers, 15 men and 11 women, none of whom had any sleep issues. “The day before the first testing session, the participants visited the laboratory for an adaptation night,” the authors observe. There were two experimental test nights, one in which the weighted blanket was used and the second during which the lighter blanket was used.
On the test nights, lights were dimmed between 9 PM and 11 PM and participants used a weighted blanket covering the extremities, abdomen, and chest 1 hour before and during 8 hours of sleep. As the authors explain, the filling of the weighted blanket consisted of honed glass pearls, combined with polyester wadding, which corresponded to 12.2% of participants’ body weight.
“Saliva was collected every 20 minutes between 22:00 and 23:00,” Ms. Meth and colleagues note. Participants’ subjective sleepiness was also assessed every 20 minutes using the Karolinska Sleepiness Scale both before the hour that lights were turned off and the next morning.
“Sleep duration in each experimental night was recorded with the OURA ring,” investigators explain.
The OURA ring is a commercial multisensor wearable device that measures physiological variables indicative of sleep. Investigators focused on total sleep duration as the primary outcome measure.
On average, salivary melatonin concentrations rose by about 5.8 pg/mL between 10 PM and 11 PM (P < .001), but the average increase in salivary melatonin concentrations was greater under weighted blanket conditions at 6.6 pg/mL, compared with 5.0 pg/mL during the lighter blanket session (P = .011).
Oxytocin in turn rose by about 315 pg/mL initially, but this rise was only transient, and over time, no significant difference in oxytocin levels was observed between the two blanket conditions. There were also no differences in cortisol levels or the activity of the sympathetic nervous system between the weighted and light blanket sessions.
Importantly, as well, no significant differences were seen in the level of sleepiness between participants when either blanket was used nor was there a significant difference in total sleep duration.
“Our study cannot identify the underlying mechanism for the observed stimulatory effects of the weighted blanket on melatonin,” the investigators caution.
However, one explanation could be that the pressure exerted by the weighted blanket activates cutaneous sensory afferent nerves, carrying information to the brain. The region where the sensory information is delivered stimulates oxytocinergic neurons that can promote calm and well-being and decrease fear, stress, and pain. In addition, these neurons also connect to the pineal gland to influence the release of melatonin, the authors explain.
Melatonin often viewed in the wrong context
Senior author Christian Benedict, PhD, associate professor of pharmacology, Uppsala University, Sweden, explained that some people think of melatonin in the wrong context.
In point of fact, “it’s not a sleep-promoting hormone. It prepares your body and brain for the biological night ... [and] sleep coincides with the biological night, but it’s not like you take melatonin and you have a very nice uninterrupted slumber – this is not true,” he told this news organization.
He also noted that certain groups respond to melatonin better than others. For example, children with attention deficit hyperactivity disorder may have some benefit from melatonin supplements, as may the elderly who can no longer produce sufficient amounts of melatonin and for whom supplements may help promote the timing of sleep.
However, the bottom line is that, even in those who do respond to melatonin supplements, they likely do so through a placebo effect that meta-analyses have shown plays a powerful role in promoting sleep.
Dr. Benedict also stressed that just because the body makes melatonin, itself, does not mean that melatonin supplements are necessarily “safe.”
“We know melatonin has some impact on puberty – it may delay the onset of puberty – and we know that it can also impair blood glucose, so when people are eating and have a lot of melatonin on board, the melatonin will tell the pancreas to turn off insulin production, which can give rise to hyperglycemia,” he said.
However, Dr. Benedict cautioned that weighted blankets don’t come cheap. A quick Google search brings up examples that cost upwards of $350. “MDs can say try one if you can afford these blankets, but perhaps people can use several less costly blankets,” he said. “But I definitely think if there are cheap options, why not?” he concluded.
Dr. Benedict has reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM THE JOURNAL OF SLEEP RESEARCH
Youth killed by guns in U.S. equals classroom a day
according to the American Academy of Pediatrics.
Preventing firearm-related injuries and deaths in children and youth “demands a public safety approach like regulation of motor vehicles,” the group said.
The organization on Oct. 8 released an updated policy statement and technical report about gun violence and children at its 2022 annual meeting in Anaheim, Calif. The reports were published in the journal Pediatrics, and the authors plan to discuss them during the conference.
“Each day, 28 U.S. children and teens – the equivalent of a high school classroom – die from gun violence, making it the No. 1 killer of youth through age 24,” the AAP said in a statement about the reports. “The national death rate is significantly higher than all other high-income countries combined, largely due to an alarming increase in suicides and homicides that do not make national headlines.”
Firearms have become the leading cause of death among children in the United States.
In 2020, guns caused 10,197 deaths of Americans younger than 24, according to the Society for Adolescent Health and Medicine.
In 2015, more than 7,200 American youth were killed by firearms. That same year in 28 other high-income countries – which combined would have had a population twice that of the United States – just 685 youth were killed by firearms, according to the AAP.
Separately at the AAP conference, physicians are presenting new research about gun violence and children. And on Oct. 10, a pediatrician who was at Uvalde Memorial Hospital in Texas after the deadly school shooting in May is scheduled to address attendees. The doctor, Roy Guerrero, MD, testified on Capitol Hill to advocate for gun control after the shooting at Robb Elementary School, which killed 19 children and two adults.
“This is not a simple problem, and it cannot be fixed with a simple solution,” Lois K. Lee, MD, MPH, said in the AAP news release. Dr. Lee chairs the AAP Council on Injury, Violence, and Poison Prevention that wrote the new reports. “Pediatricians as a start can offer families guidance and education on more safely storing guns. AAP also calls for supporting legislation that, much like the common-sense requirements for obtaining a driver’s license, would improve gun ownership safety.”
Many deaths occur at home
The rate of homicide from firearms in U.S. youth, especially those aged 15-24 years, increased by 14% during the past decade, and the rate of suicide from firearms increased by 39%, according to the AAP.
Homicides account for 58% of youth firearm deaths, whereas suicides account for 37%. Another 2% of youth firearm deaths are unintentional, and 1% result from law enforcement actions, the group said.
Among children 12 years old and younger, about 85% of firearm deaths occur at home. Teen firearm deaths are about as likely to occur at home (39%) as on the street or sidewalk (38%), according to research based on 2014 data.
“School shootings represent a relatively new phenomenon over the last half-century, and the United States has the highest rate of school shootings in the world,” the AAP technical report noted. Between 1966 and 2008, according to the group, 44 such shootings occurred in the United States, or an average of about one per year. Fast forward a few years and the violence became dramatically worse: Between 2013 and 2015, officials counted 154 school shootings – or about one per week.
Still, school shootings are responsible for less than 1% of all firearm deaths among children 17 years or younger in the United States. While school shootings “receive a tremendous amount of attention,” the report stated, other child firearm deaths may be less likely to make national headlines.
“Many firearm tragedies escape public attention because they occur in a home, sometimes in a child’s own home or at a friend’s house, or their neighbor’s or grandparent’s residence,” Eric W. Fleegler, MD, MPH, Boston Children’s Hospital, a co-author of the new reports, said in a statement from AAP. “Research tells us that families tend to underestimate how children will behave when they encounter a gun and miscalculate the risks. Suicide risks are also a huge concern, especially in families where teens are struggling with their mental health.”
AAP-recommended actions include:
- Mental health screenings and safe gun storage education provided by clinicians as part of routine patient visits
- Increased funding for violence intervention programs in hospital and community settings
- Regulation of firearms like other consumer products, with national requirements that address training, licensing, insurance coverage, registration of individuals purchasing firearms, and safe storage
- The use of technology that allows only authorized users to pull the trigger
- Universal background checks that use federal databases and information from local police before all gun purchases
- Extreme risk protection order laws, or “red flag laws,” that prohibit individuals at risk for harming themselves or others from purchasing or owning a firearm
- More funding for firearm injury and prevention research.
A noticeable increase in the ED
Irma Ugalde, MD, associate professor and director of pediatric emergency medicine research at McGovern Medical School at UTHealth Houston, noticed that firearm-related injuries in children at her hospital were more common during the COVID-19 pandemic, even as pediatric emergency department visits decreased overall.
She and her colleagues studied the trends and reported their findings at the AAP meeting.
“We saw a drop in pediatric admissions overall,” Dr. Ugalde said in a statement about the study. “But what was really noticeable was that trauma was still very prevalent – in fact probably more so – and we were seeing more firearm injuries.”
The researchers found that firearm injuries in children rose from 88 cases in 2019 to 118 in 2020. The number of incidents remained elevated in 2021, with 115 cases.
In addition, the researchers found an initial increase in injuries occurring at home where the shooter was a known family member or friend, and in cases involving firearms that were not properly stored.
By comparison, pediatric ED visits overall decreased by 34.2% from 2019 to 2020, and by 11.8% from 2019 to 2021.
The increase in firearm injuries coincided with an increase in gun sales in the United States, the researchers noted.
“National and statewide initiatives to mitigate the risk of firearm-related injury and death are necessary,” Dr. Ugalde’s group said. “We recommend that health care workers remain vigilant about screening for potential risk factors and safe storage of firearms.”
Accidental injuries
Daniel D. Guzman, MD, with Cook Children’s Health Care Center, Fort Worth, Tex., conducted a study focused on unintentional firearm injuries in children. Dr. Guzman’s group analyzed data from 204 patients younger than age 19 seen at Cook Children’s from January 2015 to June 2021.
Dr. Guzman and his colleagues examined outcomes for injuries caused by powder guns – shotguns, rifles, and handguns – and air-power guns that shoot BBs and pellets.
The researchers found that 29% of the unintentional firearm injuries occurred with powder guns and 71% with air-power weapons, often BB guns.
“It is important that all firearms, powdered and air-powered, be stored safely in a lock box or safe,” Dr. Guzman said in a statement. To that end, Cook Children’s has developed a program called Aim for Safety to teach children and parents about the dangers of unsupervised play with BB guns and pellet guns, as well as the importance of storing all firearms unloaded and in a locked safe.
A version of this article first appeared on Medscape.com.
according to the American Academy of Pediatrics.
Preventing firearm-related injuries and deaths in children and youth “demands a public safety approach like regulation of motor vehicles,” the group said.
The organization on Oct. 8 released an updated policy statement and technical report about gun violence and children at its 2022 annual meeting in Anaheim, Calif. The reports were published in the journal Pediatrics, and the authors plan to discuss them during the conference.
“Each day, 28 U.S. children and teens – the equivalent of a high school classroom – die from gun violence, making it the No. 1 killer of youth through age 24,” the AAP said in a statement about the reports. “The national death rate is significantly higher than all other high-income countries combined, largely due to an alarming increase in suicides and homicides that do not make national headlines.”
Firearms have become the leading cause of death among children in the United States.
In 2020, guns caused 10,197 deaths of Americans younger than 24, according to the Society for Adolescent Health and Medicine.
In 2015, more than 7,200 American youth were killed by firearms. That same year in 28 other high-income countries – which combined would have had a population twice that of the United States – just 685 youth were killed by firearms, according to the AAP.
Separately at the AAP conference, physicians are presenting new research about gun violence and children. And on Oct. 10, a pediatrician who was at Uvalde Memorial Hospital in Texas after the deadly school shooting in May is scheduled to address attendees. The doctor, Roy Guerrero, MD, testified on Capitol Hill to advocate for gun control after the shooting at Robb Elementary School, which killed 19 children and two adults.
“This is not a simple problem, and it cannot be fixed with a simple solution,” Lois K. Lee, MD, MPH, said in the AAP news release. Dr. Lee chairs the AAP Council on Injury, Violence, and Poison Prevention that wrote the new reports. “Pediatricians as a start can offer families guidance and education on more safely storing guns. AAP also calls for supporting legislation that, much like the common-sense requirements for obtaining a driver’s license, would improve gun ownership safety.”
Many deaths occur at home
The rate of homicide from firearms in U.S. youth, especially those aged 15-24 years, increased by 14% during the past decade, and the rate of suicide from firearms increased by 39%, according to the AAP.
Homicides account for 58% of youth firearm deaths, whereas suicides account for 37%. Another 2% of youth firearm deaths are unintentional, and 1% result from law enforcement actions, the group said.
Among children 12 years old and younger, about 85% of firearm deaths occur at home. Teen firearm deaths are about as likely to occur at home (39%) as on the street or sidewalk (38%), according to research based on 2014 data.
“School shootings represent a relatively new phenomenon over the last half-century, and the United States has the highest rate of school shootings in the world,” the AAP technical report noted. Between 1966 and 2008, according to the group, 44 such shootings occurred in the United States, or an average of about one per year. Fast forward a few years and the violence became dramatically worse: Between 2013 and 2015, officials counted 154 school shootings – or about one per week.
Still, school shootings are responsible for less than 1% of all firearm deaths among children 17 years or younger in the United States. While school shootings “receive a tremendous amount of attention,” the report stated, other child firearm deaths may be less likely to make national headlines.
“Many firearm tragedies escape public attention because they occur in a home, sometimes in a child’s own home or at a friend’s house, or their neighbor’s or grandparent’s residence,” Eric W. Fleegler, MD, MPH, Boston Children’s Hospital, a co-author of the new reports, said in a statement from AAP. “Research tells us that families tend to underestimate how children will behave when they encounter a gun and miscalculate the risks. Suicide risks are also a huge concern, especially in families where teens are struggling with their mental health.”
AAP-recommended actions include:
- Mental health screenings and safe gun storage education provided by clinicians as part of routine patient visits
- Increased funding for violence intervention programs in hospital and community settings
- Regulation of firearms like other consumer products, with national requirements that address training, licensing, insurance coverage, registration of individuals purchasing firearms, and safe storage
- The use of technology that allows only authorized users to pull the trigger
- Universal background checks that use federal databases and information from local police before all gun purchases
- Extreme risk protection order laws, or “red flag laws,” that prohibit individuals at risk for harming themselves or others from purchasing or owning a firearm
- More funding for firearm injury and prevention research.
A noticeable increase in the ED
Irma Ugalde, MD, associate professor and director of pediatric emergency medicine research at McGovern Medical School at UTHealth Houston, noticed that firearm-related injuries in children at her hospital were more common during the COVID-19 pandemic, even as pediatric emergency department visits decreased overall.
She and her colleagues studied the trends and reported their findings at the AAP meeting.
“We saw a drop in pediatric admissions overall,” Dr. Ugalde said in a statement about the study. “But what was really noticeable was that trauma was still very prevalent – in fact probably more so – and we were seeing more firearm injuries.”
The researchers found that firearm injuries in children rose from 88 cases in 2019 to 118 in 2020. The number of incidents remained elevated in 2021, with 115 cases.
In addition, the researchers found an initial increase in injuries occurring at home where the shooter was a known family member or friend, and in cases involving firearms that were not properly stored.
By comparison, pediatric ED visits overall decreased by 34.2% from 2019 to 2020, and by 11.8% from 2019 to 2021.
The increase in firearm injuries coincided with an increase in gun sales in the United States, the researchers noted.
“National and statewide initiatives to mitigate the risk of firearm-related injury and death are necessary,” Dr. Ugalde’s group said. “We recommend that health care workers remain vigilant about screening for potential risk factors and safe storage of firearms.”
Accidental injuries
Daniel D. Guzman, MD, with Cook Children’s Health Care Center, Fort Worth, Tex., conducted a study focused on unintentional firearm injuries in children. Dr. Guzman’s group analyzed data from 204 patients younger than age 19 seen at Cook Children’s from January 2015 to June 2021.
Dr. Guzman and his colleagues examined outcomes for injuries caused by powder guns – shotguns, rifles, and handguns – and air-power guns that shoot BBs and pellets.
The researchers found that 29% of the unintentional firearm injuries occurred with powder guns and 71% with air-power weapons, often BB guns.
“It is important that all firearms, powdered and air-powered, be stored safely in a lock box or safe,” Dr. Guzman said in a statement. To that end, Cook Children’s has developed a program called Aim for Safety to teach children and parents about the dangers of unsupervised play with BB guns and pellet guns, as well as the importance of storing all firearms unloaded and in a locked safe.
A version of this article first appeared on Medscape.com.
according to the American Academy of Pediatrics.
Preventing firearm-related injuries and deaths in children and youth “demands a public safety approach like regulation of motor vehicles,” the group said.
The organization on Oct. 8 released an updated policy statement and technical report about gun violence and children at its 2022 annual meeting in Anaheim, Calif. The reports were published in the journal Pediatrics, and the authors plan to discuss them during the conference.
“Each day, 28 U.S. children and teens – the equivalent of a high school classroom – die from gun violence, making it the No. 1 killer of youth through age 24,” the AAP said in a statement about the reports. “The national death rate is significantly higher than all other high-income countries combined, largely due to an alarming increase in suicides and homicides that do not make national headlines.”
Firearms have become the leading cause of death among children in the United States.
In 2020, guns caused 10,197 deaths of Americans younger than 24, according to the Society for Adolescent Health and Medicine.
In 2015, more than 7,200 American youth were killed by firearms. That same year in 28 other high-income countries – which combined would have had a population twice that of the United States – just 685 youth were killed by firearms, according to the AAP.
Separately at the AAP conference, physicians are presenting new research about gun violence and children. And on Oct. 10, a pediatrician who was at Uvalde Memorial Hospital in Texas after the deadly school shooting in May is scheduled to address attendees. The doctor, Roy Guerrero, MD, testified on Capitol Hill to advocate for gun control after the shooting at Robb Elementary School, which killed 19 children and two adults.
“This is not a simple problem, and it cannot be fixed with a simple solution,” Lois K. Lee, MD, MPH, said in the AAP news release. Dr. Lee chairs the AAP Council on Injury, Violence, and Poison Prevention that wrote the new reports. “Pediatricians as a start can offer families guidance and education on more safely storing guns. AAP also calls for supporting legislation that, much like the common-sense requirements for obtaining a driver’s license, would improve gun ownership safety.”
Many deaths occur at home
The rate of homicide from firearms in U.S. youth, especially those aged 15-24 years, increased by 14% during the past decade, and the rate of suicide from firearms increased by 39%, according to the AAP.
Homicides account for 58% of youth firearm deaths, whereas suicides account for 37%. Another 2% of youth firearm deaths are unintentional, and 1% result from law enforcement actions, the group said.
Among children 12 years old and younger, about 85% of firearm deaths occur at home. Teen firearm deaths are about as likely to occur at home (39%) as on the street or sidewalk (38%), according to research based on 2014 data.
“School shootings represent a relatively new phenomenon over the last half-century, and the United States has the highest rate of school shootings in the world,” the AAP technical report noted. Between 1966 and 2008, according to the group, 44 such shootings occurred in the United States, or an average of about one per year. Fast forward a few years and the violence became dramatically worse: Between 2013 and 2015, officials counted 154 school shootings – or about one per week.
Still, school shootings are responsible for less than 1% of all firearm deaths among children 17 years or younger in the United States. While school shootings “receive a tremendous amount of attention,” the report stated, other child firearm deaths may be less likely to make national headlines.
“Many firearm tragedies escape public attention because they occur in a home, sometimes in a child’s own home or at a friend’s house, or their neighbor’s or grandparent’s residence,” Eric W. Fleegler, MD, MPH, Boston Children’s Hospital, a co-author of the new reports, said in a statement from AAP. “Research tells us that families tend to underestimate how children will behave when they encounter a gun and miscalculate the risks. Suicide risks are also a huge concern, especially in families where teens are struggling with their mental health.”
AAP-recommended actions include:
- Mental health screenings and safe gun storage education provided by clinicians as part of routine patient visits
- Increased funding for violence intervention programs in hospital and community settings
- Regulation of firearms like other consumer products, with national requirements that address training, licensing, insurance coverage, registration of individuals purchasing firearms, and safe storage
- The use of technology that allows only authorized users to pull the trigger
- Universal background checks that use federal databases and information from local police before all gun purchases
- Extreme risk protection order laws, or “red flag laws,” that prohibit individuals at risk for harming themselves or others from purchasing or owning a firearm
- More funding for firearm injury and prevention research.
A noticeable increase in the ED
Irma Ugalde, MD, associate professor and director of pediatric emergency medicine research at McGovern Medical School at UTHealth Houston, noticed that firearm-related injuries in children at her hospital were more common during the COVID-19 pandemic, even as pediatric emergency department visits decreased overall.
She and her colleagues studied the trends and reported their findings at the AAP meeting.
“We saw a drop in pediatric admissions overall,” Dr. Ugalde said in a statement about the study. “But what was really noticeable was that trauma was still very prevalent – in fact probably more so – and we were seeing more firearm injuries.”
The researchers found that firearm injuries in children rose from 88 cases in 2019 to 118 in 2020. The number of incidents remained elevated in 2021, with 115 cases.
In addition, the researchers found an initial increase in injuries occurring at home where the shooter was a known family member or friend, and in cases involving firearms that were not properly stored.
By comparison, pediatric ED visits overall decreased by 34.2% from 2019 to 2020, and by 11.8% from 2019 to 2021.
The increase in firearm injuries coincided with an increase in gun sales in the United States, the researchers noted.
“National and statewide initiatives to mitigate the risk of firearm-related injury and death are necessary,” Dr. Ugalde’s group said. “We recommend that health care workers remain vigilant about screening for potential risk factors and safe storage of firearms.”
Accidental injuries
Daniel D. Guzman, MD, with Cook Children’s Health Care Center, Fort Worth, Tex., conducted a study focused on unintentional firearm injuries in children. Dr. Guzman’s group analyzed data from 204 patients younger than age 19 seen at Cook Children’s from January 2015 to June 2021.
Dr. Guzman and his colleagues examined outcomes for injuries caused by powder guns – shotguns, rifles, and handguns – and air-power guns that shoot BBs and pellets.
The researchers found that 29% of the unintentional firearm injuries occurred with powder guns and 71% with air-power weapons, often BB guns.
“It is important that all firearms, powdered and air-powered, be stored safely in a lock box or safe,” Dr. Guzman said in a statement. To that end, Cook Children’s has developed a program called Aim for Safety to teach children and parents about the dangers of unsupervised play with BB guns and pellet guns, as well as the importance of storing all firearms unloaded and in a locked safe.
A version of this article first appeared on Medscape.com.
FROM PEDIATRICS
Firm Mobile Nodule on the Scalp
The Diagnosis: Metastatic Carcinoid Tumor
Carcinoid tumors are derived from neuroendocrine cell compartments and generally arise in the gastrointestinal tract, with a quarter of carcinoids arising in the small bowel.1 Carcinoid tumors have an incidence of approximately 2 to 5 per 100,000 patients.2 Metastasis of carcinoids is approximately 31.2% to 46.7%.1 Metastasis to the skin is uncommon; we present a rare case of a carcinoid tumor of the terminal ileum with metastasis to the scalp.
Unlike our patient, most patients with carcinoid tumors have an indolent clinical course. The most common cutaneous symptom is flushing, which occurs in 75% of patients.3 Secreted vasoactive peptides such as serotonin may cause other symptoms such as tachycardia, diarrhea, and bronchospasm; together, these symptoms comprise carcinoid syndrome. Carcinoid syndrome requires metastasis of the tumor to the liver or a site outside of the gastrointestinal tract because the liver will metabolize the secreted serotonin. However, even in patients with liver metastasis, carcinoid syndrome only occurs in approximately 10% of patients.4 Common skin findings of carcinoid syndrome include pellagralike dermatitis, flushing, and sclerodermalike changes.5 Our patient experienced several episodes of presyncope with symptoms of dyspnea, lightheadedness, and flushing but did not have bronchospasm or recurrent diarrhea. Intramuscular octreotide improved some symptoms.
The scalp accounts for approximately 15% of cutaneous metastases, the most common being from the lung, renal, and breast cancers.6 Cutaneous metastases of carcinoid tumors are rare. A PubMed search of articles indexed for MEDLINE using the terms metastatic AND [carcinoid OR neuroendocrine] tumors AND [skin OR cutaneous] revealed 47 cases.7-11 Similar to other skin metastases, cutaneous metastases of carcinoid tumors commonly present as firm erythematous nodules of varying sizes that may be asymptomatic, tender, or pruritic (Figure 1). Cases of carcinoid tumors with cutaneous metastasis as the initial and only presenting sign are exceedingly rare.12
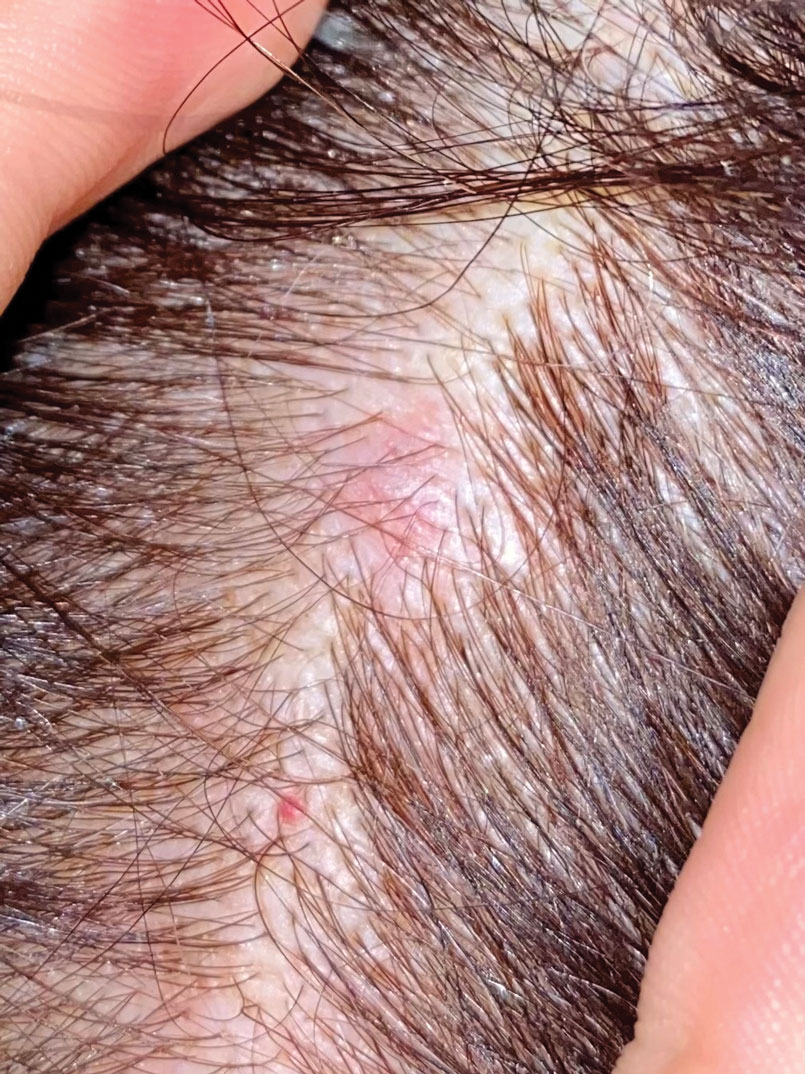
Histology of carcinoid tumors reveals a dermal neoplasm composed of loosely cohesive, mildly atypical, polygonal cells with salt-and-pepper chromatin and eosinophilic cytoplasm, which are similar findings to the primary tumor. The cells may grow in the typical trabecular or organoid neuroendocrine pattern or exhibit a pseudoglandular growth pattern with prominent vessels (quiz image, top).12 Positive chromogranin and synaptophysin immunostaining are the most common and reliable markers used for the diagnosis of carcinoid tumors.
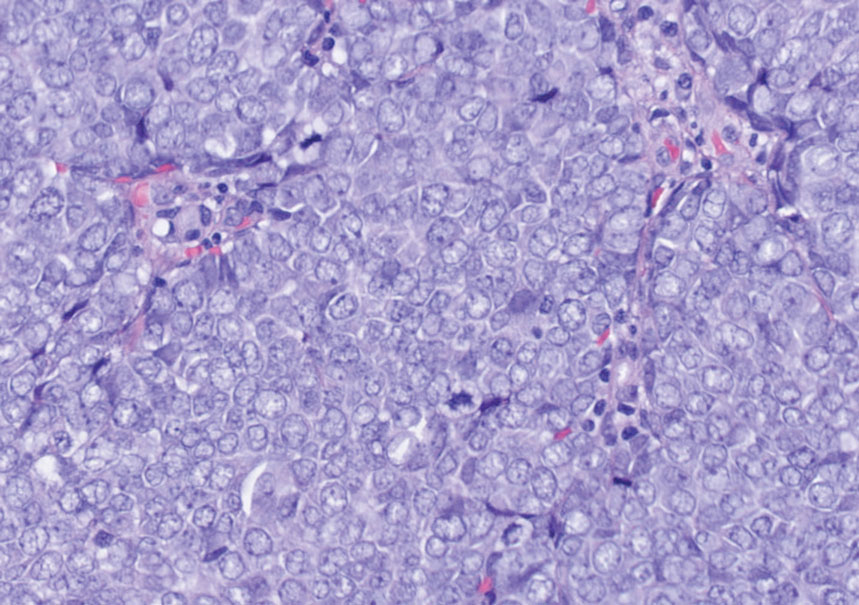
An important histopathologic differential diagnosis is the aggressive Merkel cell carcinoma, which also demonstrates homogenous salt-and-pepper chromatin but exhibits a higher mitotic rate and positive cytokeratin 20 staining (Figure 2).13 Basal cell carcinoma (BCC) also may display similar features, including a blue tumor at scanning magnification and nodular or infiltrative growth patterns. The cell morphology of BCC is characterized by islands of basaloid cells with minimal cytoplasm and frequent apoptosis, connecting to the epidermis with peripheral palisading, retraction artifact, and a myxoid stroma; BCC lacks the salt-and-pepper chromatin commonly seen in carcinoid tumors (Figure 3). Basal cell carcinoma is characterized by positive BerEP4 (epithelial cell adhesion molecule immunostain), cytokeratin 5/6, and cytokeratin 14 uptake. Cytokeratin 20, often used to diagnose Merkel cell carcinoma, is negative in BCC. Chromogranin and synaptophysin occasionally may be positive in BCC.14
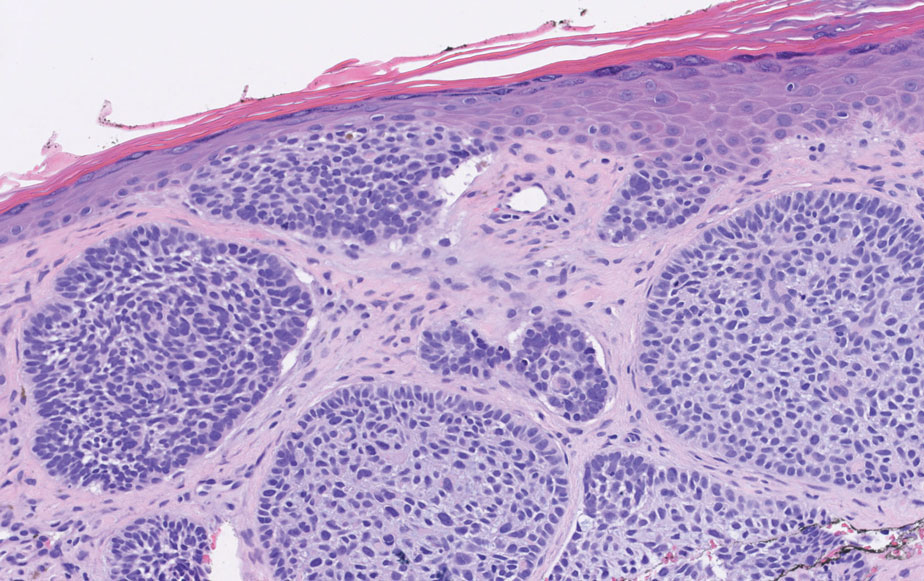
The superficial Ewing sarcoma family of tumors also may be included in the differential diagnosis of small round cell tumors of the skin, but they are very rare. These tumors possess strong positive membranous staining of cytokeratin 99 and also can stain positively for synaptophysin and chromogranin.15 Epithelial membrane antigen, which is negative in Ewing sarcomas, is positive in carcinoid tumors.16 Neuroendocrine tumors of all sites share similar basic morphologic patterns, and multiple primary tumors should be considered, including small cell lung carcinoma (Figure 4).17,18 Red granulations and true glandular lumina typically are not seen in the lungs but are common in gastrointestinal carcinoids.18 Regarding immunohistochemistry, TTF-1 is negative and CDX2 is positive in gastroenteropancreatic carcinoids, suggesting that these 2 markers can help distinguish carcinoids of unknown primary origin.19
Metastases in carcinoid tumors are common, with one study noting that the highest frequency of small intestinal metastases was from the ileal subset.20 At the time of diagnosis, 58% to 64% of patients with small intestine carcinoid tumors already had nonlocalized disease, with frequent sites being the lymph nodes (89.8%), liver (44.1%), lungs (13.6%), and peritoneum (13.6%). Regional and distant metastases are associated with substantially worse prognoses, with survival rates of 71.7% and 38.5%, respectively.1 Treatment of symptomatic unresectable disease focuses on symptomatic management with somatostatin analogs that also control tumor growth.21
We present a rare case of scalp metastasis of a carcinoid tumor of the terminal ileum. Distant metastasis is associated with poorer prognosis and should be considered in patients with a known history of a carcinoid tumor.
Acknowledgment—We would like to acknowledge the Research Histology and Tissue Imaging Core at University of Illinois Chicago Research Resources Center for the immunohistochemistry studies.
- Modlin IM, Lye KD, Kidd M. A 5-decade analysis of 13,715 carcinoid tumors. Cancer. 2003;97:934-959.
- Lawrence B, Gustafsson BI, Chan A, et al. The epidemiology of gastroenteropancreatic neuroendocrine tumors. Endocrinol Metab Clin North Am. 2011;40:1-18, vii.
- Sabir S, James WD, Schuchter LM. Cutaneous manifestations of cancer. Curr Opin Oncol. 1999;11:139-144.
- Tomassetti P. Clinical aspects of carcinoid tumours. Italian J Gastroenterol Hepatol. 1999;31(suppl 2):S143-S146.
- Bell HK, Poston GJ, Vora J, et al. Cutaneous manifestations of the malignant carcinoid syndrome. Br J Dermatol. 2005;152:71-75.
- Lookingbill DP, Spangler N, Helm KF. Cutaneous metastases in patients with metastatic carcinoma: a retrospective study of 4020 patients. J Am Acad Dermatol. 1993;29(2 pt 1):228-236.
- Garcia A, Mays S, Silapunt S. Metastatic neuroendocrine carcinoma in the skin. Dermatol Online J. 2017;23:13030/qt9052w9x1.
- Ciliberti MP, Carbonara R, Grillo A, et al. Unexpected response to palliative radiotherapy for subcutaneous metastases of an advanced small cell pancreatic neuroendocrine carcinoma: a case report of two different radiation schedules. BMC Cancer. 2020;20:311.
- Devnani B, Kumar R, Pathy S, et al. Cutaneous metastases from neuroendocrine carcinoma of the cervix: an unusual metastatic lesion from an uncommon malignancy. Curr Probl Cancer. 2018; 42:527-533.
- Falto-Aizpurua L, Seyfer S, Krishnan B, et al. Cutaneous metastasis of a pulmonary carcinoid tumor. Cutis. 2017;99:E13-E15.
- Dhingra R, Tse JY, Saif MW. Cutaneous metastasis of gastroenteropancreatic neuroendocrine tumors (GEP-Nets)[published online September 8, 2018]. JOP. 2018;19.
- Jedrych J, Busam K, Klimstra DS, et al. Cutaneous metastases as an initial manifestation of visceral well-differentiated neuroendocrine tumor: a report of four cases and a review of literature. J Cutan Pathol. 2014;41:113-122.
- Lloyd RV. Practical markers used in the diagnosis of neuroendocrine tumors. Endocr Pathol. 2003;14:293-301.
- Stanoszek LM, Wang GY, Harms PW. Histologic mimics of basal cell carcinoma. Arch Pathol Lab Med. 2017;141:1490-1502.
- Machado I, Llombart B, Calabuig-Fariñas S, et al. Superficial Ewing’s sarcoma family of tumors: a clinicopathological study with differential diagnoses. J Cutan Pathol. 2011;38:636-643.
- D’Cruze L, Dutta R, Rao S, et al. The role of immunohistochemistry in the analysis of the spectrum of small round cell tumours at a tertiary care centre. J Clin Diagn Res. 2013;7:1377-1382.
- Chirila DN, Turdeanu NA, Constantea NA, et al. Multiple malignant tumors. Chirurgia (Bucur). 2013;108:498-502.
- Rekhtman N. Neuroendocrine tumors of the lung: an update. Arch Pathol Lab Med. 2010;134:1628-1638.
- Lin X, Saad RS, Luckasevic TM, et al. Diagnostic value of CDX-2 and TTF-1 expressions in separating metastatic neuroendocrine neoplasms of unknown origin. Appl Immunohistochem Mol Morphol. 2007;15:407-414.
- Olney JR, Urdaneta LF, Al-Jurf AS, et al. Carcinoid tumors of the gastrointestinal tract. Am Surg. 1985;51:37-41.
- Strosberg JR, Halfdanarson TR, Bellizzi AM, et al. The North American Neuroendocrine Tumor Society consensus guidelines for surveillance and medical management of midgut neuroendocrine tumors. Pancreas. 2017;46:707-714.
The Diagnosis: Metastatic Carcinoid Tumor
Carcinoid tumors are derived from neuroendocrine cell compartments and generally arise in the gastrointestinal tract, with a quarter of carcinoids arising in the small bowel.1 Carcinoid tumors have an incidence of approximately 2 to 5 per 100,000 patients.2 Metastasis of carcinoids is approximately 31.2% to 46.7%.1 Metastasis to the skin is uncommon; we present a rare case of a carcinoid tumor of the terminal ileum with metastasis to the scalp.
Unlike our patient, most patients with carcinoid tumors have an indolent clinical course. The most common cutaneous symptom is flushing, which occurs in 75% of patients.3 Secreted vasoactive peptides such as serotonin may cause other symptoms such as tachycardia, diarrhea, and bronchospasm; together, these symptoms comprise carcinoid syndrome. Carcinoid syndrome requires metastasis of the tumor to the liver or a site outside of the gastrointestinal tract because the liver will metabolize the secreted serotonin. However, even in patients with liver metastasis, carcinoid syndrome only occurs in approximately 10% of patients.4 Common skin findings of carcinoid syndrome include pellagralike dermatitis, flushing, and sclerodermalike changes.5 Our patient experienced several episodes of presyncope with symptoms of dyspnea, lightheadedness, and flushing but did not have bronchospasm or recurrent diarrhea. Intramuscular octreotide improved some symptoms.
The scalp accounts for approximately 15% of cutaneous metastases, the most common being from the lung, renal, and breast cancers.6 Cutaneous metastases of carcinoid tumors are rare. A PubMed search of articles indexed for MEDLINE using the terms metastatic AND [carcinoid OR neuroendocrine] tumors AND [skin OR cutaneous] revealed 47 cases.7-11 Similar to other skin metastases, cutaneous metastases of carcinoid tumors commonly present as firm erythematous nodules of varying sizes that may be asymptomatic, tender, or pruritic (Figure 1). Cases of carcinoid tumors with cutaneous metastasis as the initial and only presenting sign are exceedingly rare.12

Histology of carcinoid tumors reveals a dermal neoplasm composed of loosely cohesive, mildly atypical, polygonal cells with salt-and-pepper chromatin and eosinophilic cytoplasm, which are similar findings to the primary tumor. The cells may grow in the typical trabecular or organoid neuroendocrine pattern or exhibit a pseudoglandular growth pattern with prominent vessels (quiz image, top).12 Positive chromogranin and synaptophysin immunostaining are the most common and reliable markers used for the diagnosis of carcinoid tumors.

An important histopathologic differential diagnosis is the aggressive Merkel cell carcinoma, which also demonstrates homogenous salt-and-pepper chromatin but exhibits a higher mitotic rate and positive cytokeratin 20 staining (Figure 2).13 Basal cell carcinoma (BCC) also may display similar features, including a blue tumor at scanning magnification and nodular or infiltrative growth patterns. The cell morphology of BCC is characterized by islands of basaloid cells with minimal cytoplasm and frequent apoptosis, connecting to the epidermis with peripheral palisading, retraction artifact, and a myxoid stroma; BCC lacks the salt-and-pepper chromatin commonly seen in carcinoid tumors (Figure 3). Basal cell carcinoma is characterized by positive BerEP4 (epithelial cell adhesion molecule immunostain), cytokeratin 5/6, and cytokeratin 14 uptake. Cytokeratin 20, often used to diagnose Merkel cell carcinoma, is negative in BCC. Chromogranin and synaptophysin occasionally may be positive in BCC.14

The superficial Ewing sarcoma family of tumors also may be included in the differential diagnosis of small round cell tumors of the skin, but they are very rare. These tumors possess strong positive membranous staining of cytokeratin 99 and also can stain positively for synaptophysin and chromogranin.15 Epithelial membrane antigen, which is negative in Ewing sarcomas, is positive in carcinoid tumors.16 Neuroendocrine tumors of all sites share similar basic morphologic patterns, and multiple primary tumors should be considered, including small cell lung carcinoma (Figure 4).17,18 Red granulations and true glandular lumina typically are not seen in the lungs but are common in gastrointestinal carcinoids.18 Regarding immunohistochemistry, TTF-1 is negative and CDX2 is positive in gastroenteropancreatic carcinoids, suggesting that these 2 markers can help distinguish carcinoids of unknown primary origin.19

Metastases in carcinoid tumors are common, with one study noting that the highest frequency of small intestinal metastases was from the ileal subset.20 At the time of diagnosis, 58% to 64% of patients with small intestine carcinoid tumors already had nonlocalized disease, with frequent sites being the lymph nodes (89.8%), liver (44.1%), lungs (13.6%), and peritoneum (13.6%). Regional and distant metastases are associated with substantially worse prognoses, with survival rates of 71.7% and 38.5%, respectively.1 Treatment of symptomatic unresectable disease focuses on symptomatic management with somatostatin analogs that also control tumor growth.21
We present a rare case of scalp metastasis of a carcinoid tumor of the terminal ileum. Distant metastasis is associated with poorer prognosis and should be considered in patients with a known history of a carcinoid tumor.
Acknowledgment—We would like to acknowledge the Research Histology and Tissue Imaging Core at University of Illinois Chicago Research Resources Center for the immunohistochemistry studies.
The Diagnosis: Metastatic Carcinoid Tumor
Carcinoid tumors are derived from neuroendocrine cell compartments and generally arise in the gastrointestinal tract, with a quarter of carcinoids arising in the small bowel.1 Carcinoid tumors have an incidence of approximately 2 to 5 per 100,000 patients.2 Metastasis of carcinoids is approximately 31.2% to 46.7%.1 Metastasis to the skin is uncommon; we present a rare case of a carcinoid tumor of the terminal ileum with metastasis to the scalp.
Unlike our patient, most patients with carcinoid tumors have an indolent clinical course. The most common cutaneous symptom is flushing, which occurs in 75% of patients.3 Secreted vasoactive peptides such as serotonin may cause other symptoms such as tachycardia, diarrhea, and bronchospasm; together, these symptoms comprise carcinoid syndrome. Carcinoid syndrome requires metastasis of the tumor to the liver or a site outside of the gastrointestinal tract because the liver will metabolize the secreted serotonin. However, even in patients with liver metastasis, carcinoid syndrome only occurs in approximately 10% of patients.4 Common skin findings of carcinoid syndrome include pellagralike dermatitis, flushing, and sclerodermalike changes.5 Our patient experienced several episodes of presyncope with symptoms of dyspnea, lightheadedness, and flushing but did not have bronchospasm or recurrent diarrhea. Intramuscular octreotide improved some symptoms.
The scalp accounts for approximately 15% of cutaneous metastases, the most common being from the lung, renal, and breast cancers.6 Cutaneous metastases of carcinoid tumors are rare. A PubMed search of articles indexed for MEDLINE using the terms metastatic AND [carcinoid OR neuroendocrine] tumors AND [skin OR cutaneous] revealed 47 cases.7-11 Similar to other skin metastases, cutaneous metastases of carcinoid tumors commonly present as firm erythematous nodules of varying sizes that may be asymptomatic, tender, or pruritic (Figure 1). Cases of carcinoid tumors with cutaneous metastasis as the initial and only presenting sign are exceedingly rare.12

Histology of carcinoid tumors reveals a dermal neoplasm composed of loosely cohesive, mildly atypical, polygonal cells with salt-and-pepper chromatin and eosinophilic cytoplasm, which are similar findings to the primary tumor. The cells may grow in the typical trabecular or organoid neuroendocrine pattern or exhibit a pseudoglandular growth pattern with prominent vessels (quiz image, top).12 Positive chromogranin and synaptophysin immunostaining are the most common and reliable markers used for the diagnosis of carcinoid tumors.

An important histopathologic differential diagnosis is the aggressive Merkel cell carcinoma, which also demonstrates homogenous salt-and-pepper chromatin but exhibits a higher mitotic rate and positive cytokeratin 20 staining (Figure 2).13 Basal cell carcinoma (BCC) also may display similar features, including a blue tumor at scanning magnification and nodular or infiltrative growth patterns. The cell morphology of BCC is characterized by islands of basaloid cells with minimal cytoplasm and frequent apoptosis, connecting to the epidermis with peripheral palisading, retraction artifact, and a myxoid stroma; BCC lacks the salt-and-pepper chromatin commonly seen in carcinoid tumors (Figure 3). Basal cell carcinoma is characterized by positive BerEP4 (epithelial cell adhesion molecule immunostain), cytokeratin 5/6, and cytokeratin 14 uptake. Cytokeratin 20, often used to diagnose Merkel cell carcinoma, is negative in BCC. Chromogranin and synaptophysin occasionally may be positive in BCC.14

The superficial Ewing sarcoma family of tumors also may be included in the differential diagnosis of small round cell tumors of the skin, but they are very rare. These tumors possess strong positive membranous staining of cytokeratin 99 and also can stain positively for synaptophysin and chromogranin.15 Epithelial membrane antigen, which is negative in Ewing sarcomas, is positive in carcinoid tumors.16 Neuroendocrine tumors of all sites share similar basic morphologic patterns, and multiple primary tumors should be considered, including small cell lung carcinoma (Figure 4).17,18 Red granulations and true glandular lumina typically are not seen in the lungs but are common in gastrointestinal carcinoids.18 Regarding immunohistochemistry, TTF-1 is negative and CDX2 is positive in gastroenteropancreatic carcinoids, suggesting that these 2 markers can help distinguish carcinoids of unknown primary origin.19

Metastases in carcinoid tumors are common, with one study noting that the highest frequency of small intestinal metastases was from the ileal subset.20 At the time of diagnosis, 58% to 64% of patients with small intestine carcinoid tumors already had nonlocalized disease, with frequent sites being the lymph nodes (89.8%), liver (44.1%), lungs (13.6%), and peritoneum (13.6%). Regional and distant metastases are associated with substantially worse prognoses, with survival rates of 71.7% and 38.5%, respectively.1 Treatment of symptomatic unresectable disease focuses on symptomatic management with somatostatin analogs that also control tumor growth.21
We present a rare case of scalp metastasis of a carcinoid tumor of the terminal ileum. Distant metastasis is associated with poorer prognosis and should be considered in patients with a known history of a carcinoid tumor.
Acknowledgment—We would like to acknowledge the Research Histology and Tissue Imaging Core at University of Illinois Chicago Research Resources Center for the immunohistochemistry studies.
- Modlin IM, Lye KD, Kidd M. A 5-decade analysis of 13,715 carcinoid tumors. Cancer. 2003;97:934-959.
- Lawrence B, Gustafsson BI, Chan A, et al. The epidemiology of gastroenteropancreatic neuroendocrine tumors. Endocrinol Metab Clin North Am. 2011;40:1-18, vii.
- Sabir S, James WD, Schuchter LM. Cutaneous manifestations of cancer. Curr Opin Oncol. 1999;11:139-144.
- Tomassetti P. Clinical aspects of carcinoid tumours. Italian J Gastroenterol Hepatol. 1999;31(suppl 2):S143-S146.
- Bell HK, Poston GJ, Vora J, et al. Cutaneous manifestations of the malignant carcinoid syndrome. Br J Dermatol. 2005;152:71-75.
- Lookingbill DP, Spangler N, Helm KF. Cutaneous metastases in patients with metastatic carcinoma: a retrospective study of 4020 patients. J Am Acad Dermatol. 1993;29(2 pt 1):228-236.
- Garcia A, Mays S, Silapunt S. Metastatic neuroendocrine carcinoma in the skin. Dermatol Online J. 2017;23:13030/qt9052w9x1.
- Ciliberti MP, Carbonara R, Grillo A, et al. Unexpected response to palliative radiotherapy for subcutaneous metastases of an advanced small cell pancreatic neuroendocrine carcinoma: a case report of two different radiation schedules. BMC Cancer. 2020;20:311.
- Devnani B, Kumar R, Pathy S, et al. Cutaneous metastases from neuroendocrine carcinoma of the cervix: an unusual metastatic lesion from an uncommon malignancy. Curr Probl Cancer. 2018; 42:527-533.
- Falto-Aizpurua L, Seyfer S, Krishnan B, et al. Cutaneous metastasis of a pulmonary carcinoid tumor. Cutis. 2017;99:E13-E15.
- Dhingra R, Tse JY, Saif MW. Cutaneous metastasis of gastroenteropancreatic neuroendocrine tumors (GEP-Nets)[published online September 8, 2018]. JOP. 2018;19.
- Jedrych J, Busam K, Klimstra DS, et al. Cutaneous metastases as an initial manifestation of visceral well-differentiated neuroendocrine tumor: a report of four cases and a review of literature. J Cutan Pathol. 2014;41:113-122.
- Lloyd RV. Practical markers used in the diagnosis of neuroendocrine tumors. Endocr Pathol. 2003;14:293-301.
- Stanoszek LM, Wang GY, Harms PW. Histologic mimics of basal cell carcinoma. Arch Pathol Lab Med. 2017;141:1490-1502.
- Machado I, Llombart B, Calabuig-Fariñas S, et al. Superficial Ewing’s sarcoma family of tumors: a clinicopathological study with differential diagnoses. J Cutan Pathol. 2011;38:636-643.
- D’Cruze L, Dutta R, Rao S, et al. The role of immunohistochemistry in the analysis of the spectrum of small round cell tumours at a tertiary care centre. J Clin Diagn Res. 2013;7:1377-1382.
- Chirila DN, Turdeanu NA, Constantea NA, et al. Multiple malignant tumors. Chirurgia (Bucur). 2013;108:498-502.
- Rekhtman N. Neuroendocrine tumors of the lung: an update. Arch Pathol Lab Med. 2010;134:1628-1638.
- Lin X, Saad RS, Luckasevic TM, et al. Diagnostic value of CDX-2 and TTF-1 expressions in separating metastatic neuroendocrine neoplasms of unknown origin. Appl Immunohistochem Mol Morphol. 2007;15:407-414.
- Olney JR, Urdaneta LF, Al-Jurf AS, et al. Carcinoid tumors of the gastrointestinal tract. Am Surg. 1985;51:37-41.
- Strosberg JR, Halfdanarson TR, Bellizzi AM, et al. The North American Neuroendocrine Tumor Society consensus guidelines for surveillance and medical management of midgut neuroendocrine tumors. Pancreas. 2017;46:707-714.
- Modlin IM, Lye KD, Kidd M. A 5-decade analysis of 13,715 carcinoid tumors. Cancer. 2003;97:934-959.
- Lawrence B, Gustafsson BI, Chan A, et al. The epidemiology of gastroenteropancreatic neuroendocrine tumors. Endocrinol Metab Clin North Am. 2011;40:1-18, vii.
- Sabir S, James WD, Schuchter LM. Cutaneous manifestations of cancer. Curr Opin Oncol. 1999;11:139-144.
- Tomassetti P. Clinical aspects of carcinoid tumours. Italian J Gastroenterol Hepatol. 1999;31(suppl 2):S143-S146.
- Bell HK, Poston GJ, Vora J, et al. Cutaneous manifestations of the malignant carcinoid syndrome. Br J Dermatol. 2005;152:71-75.
- Lookingbill DP, Spangler N, Helm KF. Cutaneous metastases in patients with metastatic carcinoma: a retrospective study of 4020 patients. J Am Acad Dermatol. 1993;29(2 pt 1):228-236.
- Garcia A, Mays S, Silapunt S. Metastatic neuroendocrine carcinoma in the skin. Dermatol Online J. 2017;23:13030/qt9052w9x1.
- Ciliberti MP, Carbonara R, Grillo A, et al. Unexpected response to palliative radiotherapy for subcutaneous metastases of an advanced small cell pancreatic neuroendocrine carcinoma: a case report of two different radiation schedules. BMC Cancer. 2020;20:311.
- Devnani B, Kumar R, Pathy S, et al. Cutaneous metastases from neuroendocrine carcinoma of the cervix: an unusual metastatic lesion from an uncommon malignancy. Curr Probl Cancer. 2018; 42:527-533.
- Falto-Aizpurua L, Seyfer S, Krishnan B, et al. Cutaneous metastasis of a pulmonary carcinoid tumor. Cutis. 2017;99:E13-E15.
- Dhingra R, Tse JY, Saif MW. Cutaneous metastasis of gastroenteropancreatic neuroendocrine tumors (GEP-Nets)[published online September 8, 2018]. JOP. 2018;19.
- Jedrych J, Busam K, Klimstra DS, et al. Cutaneous metastases as an initial manifestation of visceral well-differentiated neuroendocrine tumor: a report of four cases and a review of literature. J Cutan Pathol. 2014;41:113-122.
- Lloyd RV. Practical markers used in the diagnosis of neuroendocrine tumors. Endocr Pathol. 2003;14:293-301.
- Stanoszek LM, Wang GY, Harms PW. Histologic mimics of basal cell carcinoma. Arch Pathol Lab Med. 2017;141:1490-1502.
- Machado I, Llombart B, Calabuig-Fariñas S, et al. Superficial Ewing’s sarcoma family of tumors: a clinicopathological study with differential diagnoses. J Cutan Pathol. 2011;38:636-643.
- D’Cruze L, Dutta R, Rao S, et al. The role of immunohistochemistry in the analysis of the spectrum of small round cell tumours at a tertiary care centre. J Clin Diagn Res. 2013;7:1377-1382.
- Chirila DN, Turdeanu NA, Constantea NA, et al. Multiple malignant tumors. Chirurgia (Bucur). 2013;108:498-502.
- Rekhtman N. Neuroendocrine tumors of the lung: an update. Arch Pathol Lab Med. 2010;134:1628-1638.
- Lin X, Saad RS, Luckasevic TM, et al. Diagnostic value of CDX-2 and TTF-1 expressions in separating metastatic neuroendocrine neoplasms of unknown origin. Appl Immunohistochem Mol Morphol. 2007;15:407-414.
- Olney JR, Urdaneta LF, Al-Jurf AS, et al. Carcinoid tumors of the gastrointestinal tract. Am Surg. 1985;51:37-41.
- Strosberg JR, Halfdanarson TR, Bellizzi AM, et al. The North American Neuroendocrine Tumor Society consensus guidelines for surveillance and medical management of midgut neuroendocrine tumors. Pancreas. 2017;46:707-714.
A 47-year-old woman was admitted to the hospital with abdominal pain and flushing. She had a history of a midgut carcinoid that originated in the ileum with metastasis to the colon, liver, and pancreas. Dermatologic examination revealed a firm, nontender, mobile, 7-mm scalp nodule with a pink-purple overlying epidermis. The lesion was associated with a slight decrease in hair density. A 4-mm punch biopsy was performed.

Pumping iron improves longevity in older adults
with the strongest effects observed when the two types of exercise are combined, new research shows.
“The novel finding from our study is that weight lifting is independently associated with lower all-cause and CVD-specific mortality, regardless of aerobic activity,” first author Jessica Gorzelitz, PhD, said in an interview.
“What’s less surprising – but consistent and nonetheless noteworthy – is that weight lifting in combination with aerobic exercise provides the lowest...risk for mortality in older adults,” added Dr. Gorzelitz, an assistant professor of health promotion in the department of health and human physiology at the University of Iowa, Iowa City.
Those who undertook weight lifting and aerobic exercise in combination had around a 40% lower risk of death than those who reported no moderate to vigorous aerobic activity or weight lifting. The findings were recently published online in the British Journal of Sports Medicine.
Physical activity guidelines generally recommend regular moderate to vigorous aerobic physical activity, in addition to at least 2 days per week of muscle-strengthening exercise for all major muscle groups for adults to improve health and boost longevity.
However, few observational studies have examined the association between muscle strengthening and mortality, and even fewer have looked specifically at the benefits of weight lifting, Dr. Gorzelitz said.
Benefit of weight lifting stronger in women than men
To investigate, Dr. Gorzelitz and coauthors evaluated data on participants in the Prostate, Lung, Colorectal and Ovarian Cancer Screening Trial, which, initiated in 1993, and involved adults aged 55-74 at 10 U.S. cancer centers.
Thirteen years into the trial, in 2006, participants completed follow-up questionnaires that included an assessment of weight lifting (not included in a baseline survey).
Among 99,713 participants involved in the current analysis, the mean age at the time of the follow-up questionnaire was 71.3 years. Participants had a mean body mass index of 27.8 kg/m2 and 52.6% were women.
Only about a quarter of adults (23%) reported any weight lifting activity within the previous 12 months, with fewer, at 16%, reporting regular weight lifting of between one and six times per week.
Participants’ physical aerobic activity was also assessed. Physical activity guidelines (2018) recommend at least 150-300 minutes per week of moderate-intensity aerobic physical activity or 75-150 minutes per week of vigorous intensity aerobic activity or an equal combination of the two. Overall, 23.6% of participants reported activity that met the guideline for moderate to vigorous physical activity, and 8% exceeded it.
Over a median follow-up of about 9 years, 28,477 deaths occurred.
Those reporting weight lifting had a 9% lower risk of combined all-cause mortality and CVD mortality, after adjustment for any moderate to vigorous physical activity (each hazard ratio, 0.91).
Adults who met aerobic activity recommendations but did not weight lift had a 32% lower risk of all-cause mortality (HR, 0.68), while those who also reported weight lifting 1-2 times per week in addition to the aerobic activity had as much as a 41% lower risk of death (HR, 0.59), compared with adults reporting no moderate to vigorous aerobic activity or weight lifting.
The benefit of weight lifting in terms of cancer mortality was only observed without adjustment for moderate to vigorous physical activity, and was therefore considered null, which Dr. Gorzelitz said was somewhat surprising. “We will examine this association further because there could still be a signal there,” she said, noting other studies have shown that muscle strengthening activity is associated with lower cancer-specific mortality.
Of note, the benefit of weight lifting appeared stronger in women versus men, Dr. Gorzelitz said.
What are the mechanisms?
Underscoring that the results show only associations and not causation, Dr. Gorzelitz speculated that mechanisms behind a mortality benefit could include known favorable physiological changes of weight lifting.
“If people are weight lifting [to a degree] to reap strength benefits, we generally see improvement in body composition, including reductions in fat and improvements in lean tissue, and we know that those changes are associated with mortality, so it could be that the weight lifting is driving the strength or body composition,” she said.
The full body response involved in weight lifting could also play a key role, she noted.
With weight lifting, “the muscles have to redirect more blood flow, the heart is pumping harder, the lungs breathe more and when the muscles are worked in that fashion, there could be other system-wide adaptations,” she said.
Furthermore, social aspects could play a role, Dr. Gorzelitz observed.
“Unlike muscle strengthening [activities] that can be done in the home setting, weight lifting typically has to be done in recreational facilities or other community centers, and considering that this is an older adult population, that social interaction could be very key for preventing isolation.”
Important limitations include that the study did not determine the nature of the weight lifting, including the duration of the weight lifting sessions or type of weight, which could feasibly range from small hand-held weights to heavier weight lifting.
The study also couldn’t show how long participants had engaged in weight lifting in terms of months or years, hence, the duration needed to see a mortality benefit was not established.
Nevertheless, the study’s finding that the group with the lowest benefits was the one reporting no aerobic or weight lifting exercise underscores the benefits of even small amounts of exercise.
“I think it’s really important to promote the importance of adding muscle strengthening, but also of any physical activity,” Dr. Gorzelitz said. “Start small, but something is better than nothing.”
The authors reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
with the strongest effects observed when the two types of exercise are combined, new research shows.
“The novel finding from our study is that weight lifting is independently associated with lower all-cause and CVD-specific mortality, regardless of aerobic activity,” first author Jessica Gorzelitz, PhD, said in an interview.
“What’s less surprising – but consistent and nonetheless noteworthy – is that weight lifting in combination with aerobic exercise provides the lowest...risk for mortality in older adults,” added Dr. Gorzelitz, an assistant professor of health promotion in the department of health and human physiology at the University of Iowa, Iowa City.
Those who undertook weight lifting and aerobic exercise in combination had around a 40% lower risk of death than those who reported no moderate to vigorous aerobic activity or weight lifting. The findings were recently published online in the British Journal of Sports Medicine.
Physical activity guidelines generally recommend regular moderate to vigorous aerobic physical activity, in addition to at least 2 days per week of muscle-strengthening exercise for all major muscle groups for adults to improve health and boost longevity.
However, few observational studies have examined the association between muscle strengthening and mortality, and even fewer have looked specifically at the benefits of weight lifting, Dr. Gorzelitz said.
Benefit of weight lifting stronger in women than men
To investigate, Dr. Gorzelitz and coauthors evaluated data on participants in the Prostate, Lung, Colorectal and Ovarian Cancer Screening Trial, which, initiated in 1993, and involved adults aged 55-74 at 10 U.S. cancer centers.
Thirteen years into the trial, in 2006, participants completed follow-up questionnaires that included an assessment of weight lifting (not included in a baseline survey).
Among 99,713 participants involved in the current analysis, the mean age at the time of the follow-up questionnaire was 71.3 years. Participants had a mean body mass index of 27.8 kg/m2 and 52.6% were women.
Only about a quarter of adults (23%) reported any weight lifting activity within the previous 12 months, with fewer, at 16%, reporting regular weight lifting of between one and six times per week.
Participants’ physical aerobic activity was also assessed. Physical activity guidelines (2018) recommend at least 150-300 minutes per week of moderate-intensity aerobic physical activity or 75-150 minutes per week of vigorous intensity aerobic activity or an equal combination of the two. Overall, 23.6% of participants reported activity that met the guideline for moderate to vigorous physical activity, and 8% exceeded it.
Over a median follow-up of about 9 years, 28,477 deaths occurred.
Those reporting weight lifting had a 9% lower risk of combined all-cause mortality and CVD mortality, after adjustment for any moderate to vigorous physical activity (each hazard ratio, 0.91).
Adults who met aerobic activity recommendations but did not weight lift had a 32% lower risk of all-cause mortality (HR, 0.68), while those who also reported weight lifting 1-2 times per week in addition to the aerobic activity had as much as a 41% lower risk of death (HR, 0.59), compared with adults reporting no moderate to vigorous aerobic activity or weight lifting.
The benefit of weight lifting in terms of cancer mortality was only observed without adjustment for moderate to vigorous physical activity, and was therefore considered null, which Dr. Gorzelitz said was somewhat surprising. “We will examine this association further because there could still be a signal there,” she said, noting other studies have shown that muscle strengthening activity is associated with lower cancer-specific mortality.
Of note, the benefit of weight lifting appeared stronger in women versus men, Dr. Gorzelitz said.
What are the mechanisms?
Underscoring that the results show only associations and not causation, Dr. Gorzelitz speculated that mechanisms behind a mortality benefit could include known favorable physiological changes of weight lifting.
“If people are weight lifting [to a degree] to reap strength benefits, we generally see improvement in body composition, including reductions in fat and improvements in lean tissue, and we know that those changes are associated with mortality, so it could be that the weight lifting is driving the strength or body composition,” she said.
The full body response involved in weight lifting could also play a key role, she noted.
With weight lifting, “the muscles have to redirect more blood flow, the heart is pumping harder, the lungs breathe more and when the muscles are worked in that fashion, there could be other system-wide adaptations,” she said.
Furthermore, social aspects could play a role, Dr. Gorzelitz observed.
“Unlike muscle strengthening [activities] that can be done in the home setting, weight lifting typically has to be done in recreational facilities or other community centers, and considering that this is an older adult population, that social interaction could be very key for preventing isolation.”
Important limitations include that the study did not determine the nature of the weight lifting, including the duration of the weight lifting sessions or type of weight, which could feasibly range from small hand-held weights to heavier weight lifting.
The study also couldn’t show how long participants had engaged in weight lifting in terms of months or years, hence, the duration needed to see a mortality benefit was not established.
Nevertheless, the study’s finding that the group with the lowest benefits was the one reporting no aerobic or weight lifting exercise underscores the benefits of even small amounts of exercise.
“I think it’s really important to promote the importance of adding muscle strengthening, but also of any physical activity,” Dr. Gorzelitz said. “Start small, but something is better than nothing.”
The authors reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
with the strongest effects observed when the two types of exercise are combined, new research shows.
“The novel finding from our study is that weight lifting is independently associated with lower all-cause and CVD-specific mortality, regardless of aerobic activity,” first author Jessica Gorzelitz, PhD, said in an interview.
“What’s less surprising – but consistent and nonetheless noteworthy – is that weight lifting in combination with aerobic exercise provides the lowest...risk for mortality in older adults,” added Dr. Gorzelitz, an assistant professor of health promotion in the department of health and human physiology at the University of Iowa, Iowa City.
Those who undertook weight lifting and aerobic exercise in combination had around a 40% lower risk of death than those who reported no moderate to vigorous aerobic activity or weight lifting. The findings were recently published online in the British Journal of Sports Medicine.
Physical activity guidelines generally recommend regular moderate to vigorous aerobic physical activity, in addition to at least 2 days per week of muscle-strengthening exercise for all major muscle groups for adults to improve health and boost longevity.
However, few observational studies have examined the association between muscle strengthening and mortality, and even fewer have looked specifically at the benefits of weight lifting, Dr. Gorzelitz said.
Benefit of weight lifting stronger in women than men
To investigate, Dr. Gorzelitz and coauthors evaluated data on participants in the Prostate, Lung, Colorectal and Ovarian Cancer Screening Trial, which, initiated in 1993, and involved adults aged 55-74 at 10 U.S. cancer centers.
Thirteen years into the trial, in 2006, participants completed follow-up questionnaires that included an assessment of weight lifting (not included in a baseline survey).
Among 99,713 participants involved in the current analysis, the mean age at the time of the follow-up questionnaire was 71.3 years. Participants had a mean body mass index of 27.8 kg/m2 and 52.6% were women.
Only about a quarter of adults (23%) reported any weight lifting activity within the previous 12 months, with fewer, at 16%, reporting regular weight lifting of between one and six times per week.
Participants’ physical aerobic activity was also assessed. Physical activity guidelines (2018) recommend at least 150-300 minutes per week of moderate-intensity aerobic physical activity or 75-150 minutes per week of vigorous intensity aerobic activity or an equal combination of the two. Overall, 23.6% of participants reported activity that met the guideline for moderate to vigorous physical activity, and 8% exceeded it.
Over a median follow-up of about 9 years, 28,477 deaths occurred.
Those reporting weight lifting had a 9% lower risk of combined all-cause mortality and CVD mortality, after adjustment for any moderate to vigorous physical activity (each hazard ratio, 0.91).
Adults who met aerobic activity recommendations but did not weight lift had a 32% lower risk of all-cause mortality (HR, 0.68), while those who also reported weight lifting 1-2 times per week in addition to the aerobic activity had as much as a 41% lower risk of death (HR, 0.59), compared with adults reporting no moderate to vigorous aerobic activity or weight lifting.
The benefit of weight lifting in terms of cancer mortality was only observed without adjustment for moderate to vigorous physical activity, and was therefore considered null, which Dr. Gorzelitz said was somewhat surprising. “We will examine this association further because there could still be a signal there,” she said, noting other studies have shown that muscle strengthening activity is associated with lower cancer-specific mortality.
Of note, the benefit of weight lifting appeared stronger in women versus men, Dr. Gorzelitz said.
What are the mechanisms?
Underscoring that the results show only associations and not causation, Dr. Gorzelitz speculated that mechanisms behind a mortality benefit could include known favorable physiological changes of weight lifting.
“If people are weight lifting [to a degree] to reap strength benefits, we generally see improvement in body composition, including reductions in fat and improvements in lean tissue, and we know that those changes are associated with mortality, so it could be that the weight lifting is driving the strength or body composition,” she said.
The full body response involved in weight lifting could also play a key role, she noted.
With weight lifting, “the muscles have to redirect more blood flow, the heart is pumping harder, the lungs breathe more and when the muscles are worked in that fashion, there could be other system-wide adaptations,” she said.
Furthermore, social aspects could play a role, Dr. Gorzelitz observed.
“Unlike muscle strengthening [activities] that can be done in the home setting, weight lifting typically has to be done in recreational facilities or other community centers, and considering that this is an older adult population, that social interaction could be very key for preventing isolation.”
Important limitations include that the study did not determine the nature of the weight lifting, including the duration of the weight lifting sessions or type of weight, which could feasibly range from small hand-held weights to heavier weight lifting.
The study also couldn’t show how long participants had engaged in weight lifting in terms of months or years, hence, the duration needed to see a mortality benefit was not established.
Nevertheless, the study’s finding that the group with the lowest benefits was the one reporting no aerobic or weight lifting exercise underscores the benefits of even small amounts of exercise.
“I think it’s really important to promote the importance of adding muscle strengthening, but also of any physical activity,” Dr. Gorzelitz said. “Start small, but something is better than nothing.”
The authors reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM THE BRITISH JOURNAL OF SPORTS MEDICINE
Colonoscopy lowers CRC risk and death, but not by much: NordICC
VIENNA – the 10-year follow-up of the large, multicenter, randomized Northern-European Initiative on Colorectal Cancer (NordICC) trial shows.
In effect, this means the number needed to invite to undergo screening to prevent one case of colorectal cancer is 455 (95% confidence interval, 270-1,429), the researchers determined.
The results were presented at the United European Gastroenterology Week 2022 meeting and were published simultaneously in The New England Journal of Medicine.
The results of the study, which was designed to be truly population based and to mimic national colorectal cancer screening programs, provide an estimate of the effect of screening colonoscopy in the general population.
The primary outcome was determined on an intention-to-screen basis. All persons who were invited to undergo colonoscopy screening were compared with people who received usual care (that is, received no invitation or screening). At UEG 2022, the researchers presented the interim 10-year colorectal cancer risk, which was found to be 0.98%, compared to 1.20%. This represents a risk reduction of 18% among colonoscopy invitees (risk ratio, 0.82; 95% CI, 0.70-0.93). During the study period, 259 cases of colorectal cancer were diagnosed in the invited group versus622 in the usual-care group.
The risk of death from colorectal cancer was 0.28% in the invited group and 0.31% in the usual-care group (RR, 0.90; 95% CI, 0.64-1.16). The risk of death from any cause was similar in both the invited group and the usual-care group, at 11.03% and 11.04%, respectively (RR, 0.99; 95% CI, 0.96-1.04).
The authors noted that the benefit would have been greater had more people undergone screening; only 42% of those who were invited actually underwent colonoscopy. In an adjusted analysis, had all those who had been invited to undergo screening undergone colonoscopy, the 10-year risk of colorectal cancer would have decreased from 1.22% to 0.84%, and the risk of colorectal cancer–related death would have fallen from 0.30% to 0.15%.
The researchers, led by gastroenterologist Michael Bretthauer, MD, from the department of medicine, gastrointestinal endoscopy, University of Oslo, who presented the data at UEG 2022 on behalf of the NordICC study group, acknowledged that, despite the “observed appreciable reductions in relative risks, the absolute risks of the risk of colorectal cancer and even more so of colorectal cancer–related death were lower than those in previous screening trials and lower than what we anticipated when the trial was planned.”
However, they add that “optimism related to the effects of screening on colorectal cancer–related death may be warranted in light of the 50% decrease observed in adjusted per-protocol analyses.”
With his coauthors, Dr. Bretthauer wrote that even their adjusted findings “probably underestimated the benefit because, as in most other large-scale trials of colorectal cancer screening, we could not adjust for all important confounders in all countries.”
Dr. Bretthauer also noted that results were similar to those achieved through sigmoidoscopy screening. By close comparison, sigmoidoscopy studies show the risk of colorectal cancer is reduced between 33% and 40%, according to per protocol analyses. “These results suggest that colonoscopy screening might not be substantially better in reducing the risk of colorectal cancer than sigmoidoscopy.”
Real-world, population-based study
NordICC is an ongoing, pragmatic study and is the first randomized trial to quantify the possible benefit of colonoscopy screening on risk of colorectal cancer and related death.
Researchers recruited healthy men and women from registries in Poland, Norway, Sweden, and the Netherlands between 2009 and 2014. Most participants came from Poland (54,258), followed by Norway (26,411) and Sweden (3,646). Data from the Netherlands could not be included owing to data protection law.
At baseline, 84,585 participants aged 55-64 years were randomly assigned in a 1:2 ratio either to receive an invitation to undergo a single screening colonoscopy (28,220; invited) or to undergo usual care in each participant country (56,365; no invitation or screening).
Any colorectal cancer lesions detected were removed, whenever possible. The primary endpoints were the risks of colorectal cancer and colorectal cancer–related death. The secondary endpoint was death from any cause.
‘Modest effectiveness,’ but longer follow-up to give fuller picture
In an editorial that accompanied publication of the study, Jason A. Dominitz, MD, from the division of gastroenterology, University of Washington, Seattle, and Douglas J. Robertson, MD, from White River Junction (Vt.) Veterans Affairs Medical Center, commented on the possible reasons for the low reduction in incident cancer and deaths seen in NordICC.
They pointed out that cohort studies suggest a 40%-69% decrease in the incidence of colorectal cancer and a 29%-88% decrease in the risk of death with colonoscopy. However, they noted that “cohort studies probably overestimate the real-world effectiveness of colonoscopy because of the inability to adjust for important factors such as incomplete adherence to testing and the tendency of healthier persons to seek preventive care.”
Referring to Dr. Bretthauer’s point about attendance to screening, Dr. Dominitz and Dr. Robertson added that, in the United States, colonoscopy is the predominant form of screening for colorectal cancer and that in countries where colonoscopy is less established, participation may be very different.
“The actual effectiveness of colonoscopy in populations that are more accepting of colonoscopy could more closely resemble the effectiveness shown in the per-protocol analysis in this trial,” they wrote.
The editorialists also pointed out that the benefits of screening colonoscopy take time to be realized “because the incidence of colorectal cancer is initially increased when presymptomatic cancers are identified.” A repeat and final analysis of the NordICC data is due at 15 years’ follow-up.
In addition, they noted that “colonoscopy is highly operator dependent” and that the adenoma detection rate is variable and affects cancer risk and related mortality.
Given the “modest effectiveness” of screening colonoscopy in the trial, they asserted that, “if the trial truly represents the real-world performance of population-based screening colonoscopy, it might be hard to justify the risk and expense of this form of screening when simpler, less-invasive strategies (e.g., sigmoidoscopy and FIT [fecal immunochemical test]) are available.”
However, they also noted that “additional analyses, including longer follow up and results from other ongoing comparative effectiveness trials, will help us to fully understand the benefits of this test.”
Also commenting on the study was Michiel Maas, MD, from the department of gastroenterology and hepatology, Radboud UMC, Nijmegen, the Netherlands, told this news organization that he agreed that the absolute effect on colorectal cancer risk or colorectal cancer–related death was not as high as expected and may be disappointing.
But Dr. Maas said that “around half of the patients in the study did not undergo colonoscopy, which may have negatively impacted the results.
“An additional factor, which can be influential in colonoscopy studies, is the potential variability in detection rates between operators/endoscopists,” he said.
Looking to the future, Dr. Maas noted that “AI [artificial intelligence] or computer-aided detection can level this playing field in detection rates.
“Nevertheless, this is a very interesting study, which sheds a new light on the efficacy on screening colonoscopies,” he said.
Dr. Bretthauer has relationships with Paion, Cybernet, and the Norwegian Council of Research. Dr. Dominitz is cochair of VA Cooperative Studies Program #577: “Colonoscopy vs. Fecal Immunochemical Test (FIT) in Reducing Mortality from Colorectal Cancer” (the CONFIRM Study), which is funded by the Department of Veterans Affairs. Dr. Robertson is national cochair (with Dr. Dominitz) of the CONFIRM trial and has received personal fees from Freenome outside of the submitted work. Dr. Maas reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
VIENNA – the 10-year follow-up of the large, multicenter, randomized Northern-European Initiative on Colorectal Cancer (NordICC) trial shows.
In effect, this means the number needed to invite to undergo screening to prevent one case of colorectal cancer is 455 (95% confidence interval, 270-1,429), the researchers determined.
The results were presented at the United European Gastroenterology Week 2022 meeting and were published simultaneously in The New England Journal of Medicine.
The results of the study, which was designed to be truly population based and to mimic national colorectal cancer screening programs, provide an estimate of the effect of screening colonoscopy in the general population.
The primary outcome was determined on an intention-to-screen basis. All persons who were invited to undergo colonoscopy screening were compared with people who received usual care (that is, received no invitation or screening). At UEG 2022, the researchers presented the interim 10-year colorectal cancer risk, which was found to be 0.98%, compared to 1.20%. This represents a risk reduction of 18% among colonoscopy invitees (risk ratio, 0.82; 95% CI, 0.70-0.93). During the study period, 259 cases of colorectal cancer were diagnosed in the invited group versus622 in the usual-care group.
The risk of death from colorectal cancer was 0.28% in the invited group and 0.31% in the usual-care group (RR, 0.90; 95% CI, 0.64-1.16). The risk of death from any cause was similar in both the invited group and the usual-care group, at 11.03% and 11.04%, respectively (RR, 0.99; 95% CI, 0.96-1.04).
The authors noted that the benefit would have been greater had more people undergone screening; only 42% of those who were invited actually underwent colonoscopy. In an adjusted analysis, had all those who had been invited to undergo screening undergone colonoscopy, the 10-year risk of colorectal cancer would have decreased from 1.22% to 0.84%, and the risk of colorectal cancer–related death would have fallen from 0.30% to 0.15%.
The researchers, led by gastroenterologist Michael Bretthauer, MD, from the department of medicine, gastrointestinal endoscopy, University of Oslo, who presented the data at UEG 2022 on behalf of the NordICC study group, acknowledged that, despite the “observed appreciable reductions in relative risks, the absolute risks of the risk of colorectal cancer and even more so of colorectal cancer–related death were lower than those in previous screening trials and lower than what we anticipated when the trial was planned.”
However, they add that “optimism related to the effects of screening on colorectal cancer–related death may be warranted in light of the 50% decrease observed in adjusted per-protocol analyses.”
With his coauthors, Dr. Bretthauer wrote that even their adjusted findings “probably underestimated the benefit because, as in most other large-scale trials of colorectal cancer screening, we could not adjust for all important confounders in all countries.”
Dr. Bretthauer also noted that results were similar to those achieved through sigmoidoscopy screening. By close comparison, sigmoidoscopy studies show the risk of colorectal cancer is reduced between 33% and 40%, according to per protocol analyses. “These results suggest that colonoscopy screening might not be substantially better in reducing the risk of colorectal cancer than sigmoidoscopy.”
Real-world, population-based study
NordICC is an ongoing, pragmatic study and is the first randomized trial to quantify the possible benefit of colonoscopy screening on risk of colorectal cancer and related death.
Researchers recruited healthy men and women from registries in Poland, Norway, Sweden, and the Netherlands between 2009 and 2014. Most participants came from Poland (54,258), followed by Norway (26,411) and Sweden (3,646). Data from the Netherlands could not be included owing to data protection law.
At baseline, 84,585 participants aged 55-64 years were randomly assigned in a 1:2 ratio either to receive an invitation to undergo a single screening colonoscopy (28,220; invited) or to undergo usual care in each participant country (56,365; no invitation or screening).
Any colorectal cancer lesions detected were removed, whenever possible. The primary endpoints were the risks of colorectal cancer and colorectal cancer–related death. The secondary endpoint was death from any cause.
‘Modest effectiveness,’ but longer follow-up to give fuller picture
In an editorial that accompanied publication of the study, Jason A. Dominitz, MD, from the division of gastroenterology, University of Washington, Seattle, and Douglas J. Robertson, MD, from White River Junction (Vt.) Veterans Affairs Medical Center, commented on the possible reasons for the low reduction in incident cancer and deaths seen in NordICC.
They pointed out that cohort studies suggest a 40%-69% decrease in the incidence of colorectal cancer and a 29%-88% decrease in the risk of death with colonoscopy. However, they noted that “cohort studies probably overestimate the real-world effectiveness of colonoscopy because of the inability to adjust for important factors such as incomplete adherence to testing and the tendency of healthier persons to seek preventive care.”
Referring to Dr. Bretthauer’s point about attendance to screening, Dr. Dominitz and Dr. Robertson added that, in the United States, colonoscopy is the predominant form of screening for colorectal cancer and that in countries where colonoscopy is less established, participation may be very different.
“The actual effectiveness of colonoscopy in populations that are more accepting of colonoscopy could more closely resemble the effectiveness shown in the per-protocol analysis in this trial,” they wrote.
The editorialists also pointed out that the benefits of screening colonoscopy take time to be realized “because the incidence of colorectal cancer is initially increased when presymptomatic cancers are identified.” A repeat and final analysis of the NordICC data is due at 15 years’ follow-up.
In addition, they noted that “colonoscopy is highly operator dependent” and that the adenoma detection rate is variable and affects cancer risk and related mortality.
Given the “modest effectiveness” of screening colonoscopy in the trial, they asserted that, “if the trial truly represents the real-world performance of population-based screening colonoscopy, it might be hard to justify the risk and expense of this form of screening when simpler, less-invasive strategies (e.g., sigmoidoscopy and FIT [fecal immunochemical test]) are available.”
However, they also noted that “additional analyses, including longer follow up and results from other ongoing comparative effectiveness trials, will help us to fully understand the benefits of this test.”
Also commenting on the study was Michiel Maas, MD, from the department of gastroenterology and hepatology, Radboud UMC, Nijmegen, the Netherlands, told this news organization that he agreed that the absolute effect on colorectal cancer risk or colorectal cancer–related death was not as high as expected and may be disappointing.
But Dr. Maas said that “around half of the patients in the study did not undergo colonoscopy, which may have negatively impacted the results.
“An additional factor, which can be influential in colonoscopy studies, is the potential variability in detection rates between operators/endoscopists,” he said.
Looking to the future, Dr. Maas noted that “AI [artificial intelligence] or computer-aided detection can level this playing field in detection rates.
“Nevertheless, this is a very interesting study, which sheds a new light on the efficacy on screening colonoscopies,” he said.
Dr. Bretthauer has relationships with Paion, Cybernet, and the Norwegian Council of Research. Dr. Dominitz is cochair of VA Cooperative Studies Program #577: “Colonoscopy vs. Fecal Immunochemical Test (FIT) in Reducing Mortality from Colorectal Cancer” (the CONFIRM Study), which is funded by the Department of Veterans Affairs. Dr. Robertson is national cochair (with Dr. Dominitz) of the CONFIRM trial and has received personal fees from Freenome outside of the submitted work. Dr. Maas reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
VIENNA – the 10-year follow-up of the large, multicenter, randomized Northern-European Initiative on Colorectal Cancer (NordICC) trial shows.
In effect, this means the number needed to invite to undergo screening to prevent one case of colorectal cancer is 455 (95% confidence interval, 270-1,429), the researchers determined.
The results were presented at the United European Gastroenterology Week 2022 meeting and were published simultaneously in The New England Journal of Medicine.
The results of the study, which was designed to be truly population based and to mimic national colorectal cancer screening programs, provide an estimate of the effect of screening colonoscopy in the general population.
The primary outcome was determined on an intention-to-screen basis. All persons who were invited to undergo colonoscopy screening were compared with people who received usual care (that is, received no invitation or screening). At UEG 2022, the researchers presented the interim 10-year colorectal cancer risk, which was found to be 0.98%, compared to 1.20%. This represents a risk reduction of 18% among colonoscopy invitees (risk ratio, 0.82; 95% CI, 0.70-0.93). During the study period, 259 cases of colorectal cancer were diagnosed in the invited group versus622 in the usual-care group.
The risk of death from colorectal cancer was 0.28% in the invited group and 0.31% in the usual-care group (RR, 0.90; 95% CI, 0.64-1.16). The risk of death from any cause was similar in both the invited group and the usual-care group, at 11.03% and 11.04%, respectively (RR, 0.99; 95% CI, 0.96-1.04).
The authors noted that the benefit would have been greater had more people undergone screening; only 42% of those who were invited actually underwent colonoscopy. In an adjusted analysis, had all those who had been invited to undergo screening undergone colonoscopy, the 10-year risk of colorectal cancer would have decreased from 1.22% to 0.84%, and the risk of colorectal cancer–related death would have fallen from 0.30% to 0.15%.
The researchers, led by gastroenterologist Michael Bretthauer, MD, from the department of medicine, gastrointestinal endoscopy, University of Oslo, who presented the data at UEG 2022 on behalf of the NordICC study group, acknowledged that, despite the “observed appreciable reductions in relative risks, the absolute risks of the risk of colorectal cancer and even more so of colorectal cancer–related death were lower than those in previous screening trials and lower than what we anticipated when the trial was planned.”
However, they add that “optimism related to the effects of screening on colorectal cancer–related death may be warranted in light of the 50% decrease observed in adjusted per-protocol analyses.”
With his coauthors, Dr. Bretthauer wrote that even their adjusted findings “probably underestimated the benefit because, as in most other large-scale trials of colorectal cancer screening, we could not adjust for all important confounders in all countries.”
Dr. Bretthauer also noted that results were similar to those achieved through sigmoidoscopy screening. By close comparison, sigmoidoscopy studies show the risk of colorectal cancer is reduced between 33% and 40%, according to per protocol analyses. “These results suggest that colonoscopy screening might not be substantially better in reducing the risk of colorectal cancer than sigmoidoscopy.”
Real-world, population-based study
NordICC is an ongoing, pragmatic study and is the first randomized trial to quantify the possible benefit of colonoscopy screening on risk of colorectal cancer and related death.
Researchers recruited healthy men and women from registries in Poland, Norway, Sweden, and the Netherlands between 2009 and 2014. Most participants came from Poland (54,258), followed by Norway (26,411) and Sweden (3,646). Data from the Netherlands could not be included owing to data protection law.
At baseline, 84,585 participants aged 55-64 years were randomly assigned in a 1:2 ratio either to receive an invitation to undergo a single screening colonoscopy (28,220; invited) or to undergo usual care in each participant country (56,365; no invitation or screening).
Any colorectal cancer lesions detected were removed, whenever possible. The primary endpoints were the risks of colorectal cancer and colorectal cancer–related death. The secondary endpoint was death from any cause.
‘Modest effectiveness,’ but longer follow-up to give fuller picture
In an editorial that accompanied publication of the study, Jason A. Dominitz, MD, from the division of gastroenterology, University of Washington, Seattle, and Douglas J. Robertson, MD, from White River Junction (Vt.) Veterans Affairs Medical Center, commented on the possible reasons for the low reduction in incident cancer and deaths seen in NordICC.
They pointed out that cohort studies suggest a 40%-69% decrease in the incidence of colorectal cancer and a 29%-88% decrease in the risk of death with colonoscopy. However, they noted that “cohort studies probably overestimate the real-world effectiveness of colonoscopy because of the inability to adjust for important factors such as incomplete adherence to testing and the tendency of healthier persons to seek preventive care.”
Referring to Dr. Bretthauer’s point about attendance to screening, Dr. Dominitz and Dr. Robertson added that, in the United States, colonoscopy is the predominant form of screening for colorectal cancer and that in countries where colonoscopy is less established, participation may be very different.
“The actual effectiveness of colonoscopy in populations that are more accepting of colonoscopy could more closely resemble the effectiveness shown in the per-protocol analysis in this trial,” they wrote.
The editorialists also pointed out that the benefits of screening colonoscopy take time to be realized “because the incidence of colorectal cancer is initially increased when presymptomatic cancers are identified.” A repeat and final analysis of the NordICC data is due at 15 years’ follow-up.
In addition, they noted that “colonoscopy is highly operator dependent” and that the adenoma detection rate is variable and affects cancer risk and related mortality.
Given the “modest effectiveness” of screening colonoscopy in the trial, they asserted that, “if the trial truly represents the real-world performance of population-based screening colonoscopy, it might be hard to justify the risk and expense of this form of screening when simpler, less-invasive strategies (e.g., sigmoidoscopy and FIT [fecal immunochemical test]) are available.”
However, they also noted that “additional analyses, including longer follow up and results from other ongoing comparative effectiveness trials, will help us to fully understand the benefits of this test.”
Also commenting on the study was Michiel Maas, MD, from the department of gastroenterology and hepatology, Radboud UMC, Nijmegen, the Netherlands, told this news organization that he agreed that the absolute effect on colorectal cancer risk or colorectal cancer–related death was not as high as expected and may be disappointing.
But Dr. Maas said that “around half of the patients in the study did not undergo colonoscopy, which may have negatively impacted the results.
“An additional factor, which can be influential in colonoscopy studies, is the potential variability in detection rates between operators/endoscopists,” he said.
Looking to the future, Dr. Maas noted that “AI [artificial intelligence] or computer-aided detection can level this playing field in detection rates.
“Nevertheless, this is a very interesting study, which sheds a new light on the efficacy on screening colonoscopies,” he said.
Dr. Bretthauer has relationships with Paion, Cybernet, and the Norwegian Council of Research. Dr. Dominitz is cochair of VA Cooperative Studies Program #577: “Colonoscopy vs. Fecal Immunochemical Test (FIT) in Reducing Mortality from Colorectal Cancer” (the CONFIRM Study), which is funded by the Department of Veterans Affairs. Dr. Robertson is national cochair (with Dr. Dominitz) of the CONFIRM trial and has received personal fees from Freenome outside of the submitted work. Dr. Maas reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM UEG 2022
Gut microbiota disruption a driver of aggression in schizophrenia?
However, at least one expert expressed concerns over the study’s conclusions.
Results from a study of 50 inpatients with schizophrenia showed significantly higher pro-inflammation, pro-oxidation, and leaky gut biomarkers in those with aggression vs. their peers who did not display aggression.
In addition, those with aggression showed less alpha diversity and evenness of the fecal bacterial community, lower levels of several beneficial gut bacteria, and higher levels of the fecal genera Prevotella.
Six short-chain fatty acids (SCFAs) and six neurotransmitters were also lower in the aggression vs. no-aggression groups.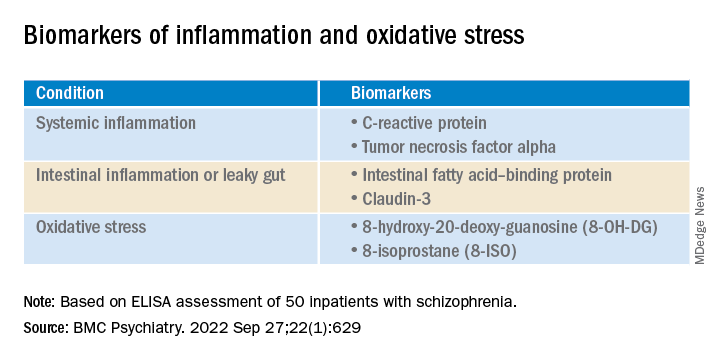
“The present study was the first to compare the state of inflammation, oxidation, intestinal microbiota, and metabolites” in inpatients with schizophrenia and aggression, compared with those who did not show aggression, write the investigators, led by Hongxin Deng, department of psychiatry, Zhumadian (China) Psychiatric Hospital.
“Results indicate pro-inflammation, pro-oxidation and leaky gut phenotypes relating to enteric dysbacteriosis and microbial SCFAs feature the aggression in [individuals with schizophrenia], which provides clues for future microbial-based or anti-inflammatory/oxidative therapies on aggression,” they add.
The findings were published online in BMC Psychiatry.
Unknown pathogenesis
Although emerging evidence suggests that schizophrenia “may augment the propensity for aggression incidence about fourfold to sevenfold,” the pathogenesis of aggression “remains largely unknown,” the investigators note.
The same researchers previously found an association between the systemic pro-inflammation response and the onset or severity of aggression in schizophrenia, “possibly caused by leaky gut-induced bacterial translocation.”
The researchers suggest that peripheral cytokines “could cross the blood-brain barrier, thus precipitating changes in mood and behavior through hypothalamic-pituitary-adrenal axis.”
However, they note that the pro-inflammation phenotype is “often a synergistic effect of multiple causes.” Of these, chronic pro-oxidative stress has been shown to contribute to aggression onset in intermittent explosive disorder, but this association has rarely been confirmed in patients with schizophrenia.
In addition, increasing evidence points to enteric dysbacteriosis and dysbiosis of intestinal flora metabolites, including SCFAs or neurotransmitters, as potentially “integral parts of psychiatric disorders’ pathophysiology” by changing the state of both oxidative stress and inflammation.
The investigators hypothesized that the systemic pro-inflammation phenotype in aggression-affected schizophrenia cases “involves alterations to gut microbiota and its metabolites, leaky gut, and oxidative stress.” However, the profiles of these variables and their interrelationships have been “poorly investigated” in inpatients with schizophrenia and aggression.
To fill this gap, they assessed adult psychiatric inpatients with schizophrenia and aggressive behaviors and inpatients with schizophrenia but no aggressive behavior within 1 week before admission (n = 25 per group; mean age, 33.52 years, and 32.88 years, respectively; 68% and 64% women, respectively).
They collected stool samples from each patient and used enzyme-linked immunoassay (ELISA) to detect fecal calprotectin protein, an indicator of intestinal inflammation. They also collected fasting peripheral blood samples, using ELISA to detect several biomarkers.
The researchers also used the Modified Overt Aggression Scale (MOAS) to characterize aggressive behaviors and the Positive and Negative Syndrome Scale to characterize psychiatric symptoms.
‘Vital role’
Significantly higher biomarkers for systemic pro-inflammation, pro-oxidation and leaky gut were found in the aggression vs the no-aggression group (all P < .05).
After controlling for potential confounders, the researchers also found positive associations between MOAS scores and biomarkers, both serum and fecal.
There were also positive associations between serum 8-hydroxy-20-deoxy-guanosine (8-OH-DG) or 8-isoprostane (8-ISO) and systemic inflammatory biomarkers (all R > 0; P < .05).
In addition, the alpha diversity and evenness of the fecal bacterial community were lower in the aggression vs. no aggression groups.
When the researchers compared the relative abundance of the top 15 genera composition of intestinal microflora in the two groups, Bacteroides, Faecalibacterium, Blautia, Bifidobacterium, Collinsella, and Eubacterium coprostanoligenes were “remarkably reduced” in the group with aggression, whereas the abundance of fecal genera Prevotella was significantly increased (all corrected P < .001).
In the patients who had schizophrenia with aggression, levels of six SCFAs and six neurotransmitters were much lower than in the patients with schizophrenia but no aggression (all P < .05).
Inpatients with schizophrenia and aggression “had dramatically increased serum level of 8-OH-DG (nucleic acid oxidation biomarker) and 8-ISO (lipid oxidation biomarker) than those without, and further correlation analysis also showed positive correlativity between pro-oxidation and systemic pro-inflammation response or aggression severity,” the investigators write.
The findings “collectively suggest the cocontributory role of systemic pro-inflammation and pro-oxidation in the development of aggression” in schizophrenia, they add. “Gut dysbacteriosis with leaky gut seems to play a vital role in the pathophysiology.”
Correlation vs. causality
Commenting for this article, Emeran Mayer, MD, distinguished research professor of medicine at the G. Oppenheimer Center for Neurobiology of Stress and Resilience and UCLA Brain Gut Microbiome Center, Los Angeles, said that “at first glance, it is interesting that the behavioral trait of aggression but not the diagnosis of schizophrenia showed the differences in markers of systemic inflammation, increased gut permeability, and microbiome parameters.”
However, like many such descriptive studies, the research is flawed by comparing two patient groups and concluding causality between the biomarkers and the behavior traits, added Dr. Mayer, who was not involved with the study.
The study’s shortcomings include its small sample size as well as several confounding factors – particularly diet, sleep, exercise, and stress and anxiety levels – that were not considered, he said. The study also lacked a control group with high levels of aggression but without schizophrenia.
“The observed changes in intestinal permeability, unscientifically referred to as ‘leaky gut,’ as well as the gut microbiome differences, could be secondary to chronically increased sympathetic nervous system activation in the high aggression group,” Dr. Mayer said. “This is an interesting hypothesis which should be discussed and should have been addressed in this study.”
The differences in gut microbial composition and SCFA production “could be secondary to differences in plant-based diet components,” Dr. Mayer speculated, wondering how well dietary intake was controlled.
“Overall, it is an interesting descriptive study, which unfortunately does not contribute significantly to a better understanding of the role of the brain-gut microbiome system in schizophrenic patients,” he said.
The study was funded by a grant from China Postdoctoral Science Foundation. The investigators have reported no relevant financial relationships. Dr. Mayer is a scientific advisory board member of Danone, Axial Therapeutics, Viome, Amare, Mahana Therapeutics, Pendulum, Bloom Biosciences, and APC Microbiome Ireland.
A version of this article first appeared on Medscape.com.
However, at least one expert expressed concerns over the study’s conclusions.
Results from a study of 50 inpatients with schizophrenia showed significantly higher pro-inflammation, pro-oxidation, and leaky gut biomarkers in those with aggression vs. their peers who did not display aggression.
In addition, those with aggression showed less alpha diversity and evenness of the fecal bacterial community, lower levels of several beneficial gut bacteria, and higher levels of the fecal genera Prevotella.
Six short-chain fatty acids (SCFAs) and six neurotransmitters were also lower in the aggression vs. no-aggression groups.
“The present study was the first to compare the state of inflammation, oxidation, intestinal microbiota, and metabolites” in inpatients with schizophrenia and aggression, compared with those who did not show aggression, write the investigators, led by Hongxin Deng, department of psychiatry, Zhumadian (China) Psychiatric Hospital.
“Results indicate pro-inflammation, pro-oxidation and leaky gut phenotypes relating to enteric dysbacteriosis and microbial SCFAs feature the aggression in [individuals with schizophrenia], which provides clues for future microbial-based or anti-inflammatory/oxidative therapies on aggression,” they add.
The findings were published online in BMC Psychiatry.
Unknown pathogenesis
Although emerging evidence suggests that schizophrenia “may augment the propensity for aggression incidence about fourfold to sevenfold,” the pathogenesis of aggression “remains largely unknown,” the investigators note.
The same researchers previously found an association between the systemic pro-inflammation response and the onset or severity of aggression in schizophrenia, “possibly caused by leaky gut-induced bacterial translocation.”
The researchers suggest that peripheral cytokines “could cross the blood-brain barrier, thus precipitating changes in mood and behavior through hypothalamic-pituitary-adrenal axis.”
However, they note that the pro-inflammation phenotype is “often a synergistic effect of multiple causes.” Of these, chronic pro-oxidative stress has been shown to contribute to aggression onset in intermittent explosive disorder, but this association has rarely been confirmed in patients with schizophrenia.
In addition, increasing evidence points to enteric dysbacteriosis and dysbiosis of intestinal flora metabolites, including SCFAs or neurotransmitters, as potentially “integral parts of psychiatric disorders’ pathophysiology” by changing the state of both oxidative stress and inflammation.
The investigators hypothesized that the systemic pro-inflammation phenotype in aggression-affected schizophrenia cases “involves alterations to gut microbiota and its metabolites, leaky gut, and oxidative stress.” However, the profiles of these variables and their interrelationships have been “poorly investigated” in inpatients with schizophrenia and aggression.
To fill this gap, they assessed adult psychiatric inpatients with schizophrenia and aggressive behaviors and inpatients with schizophrenia but no aggressive behavior within 1 week before admission (n = 25 per group; mean age, 33.52 years, and 32.88 years, respectively; 68% and 64% women, respectively).
They collected stool samples from each patient and used enzyme-linked immunoassay (ELISA) to detect fecal calprotectin protein, an indicator of intestinal inflammation. They also collected fasting peripheral blood samples, using ELISA to detect several biomarkers.
The researchers also used the Modified Overt Aggression Scale (MOAS) to characterize aggressive behaviors and the Positive and Negative Syndrome Scale to characterize psychiatric symptoms.
‘Vital role’
Significantly higher biomarkers for systemic pro-inflammation, pro-oxidation and leaky gut were found in the aggression vs the no-aggression group (all P < .05).
After controlling for potential confounders, the researchers also found positive associations between MOAS scores and biomarkers, both serum and fecal.
There were also positive associations between serum 8-hydroxy-20-deoxy-guanosine (8-OH-DG) or 8-isoprostane (8-ISO) and systemic inflammatory biomarkers (all R > 0; P < .05).
In addition, the alpha diversity and evenness of the fecal bacterial community were lower in the aggression vs. no aggression groups.
When the researchers compared the relative abundance of the top 15 genera composition of intestinal microflora in the two groups, Bacteroides, Faecalibacterium, Blautia, Bifidobacterium, Collinsella, and Eubacterium coprostanoligenes were “remarkably reduced” in the group with aggression, whereas the abundance of fecal genera Prevotella was significantly increased (all corrected P < .001).
In the patients who had schizophrenia with aggression, levels of six SCFAs and six neurotransmitters were much lower than in the patients with schizophrenia but no aggression (all P < .05).
Inpatients with schizophrenia and aggression “had dramatically increased serum level of 8-OH-DG (nucleic acid oxidation biomarker) and 8-ISO (lipid oxidation biomarker) than those without, and further correlation analysis also showed positive correlativity between pro-oxidation and systemic pro-inflammation response or aggression severity,” the investigators write.
The findings “collectively suggest the cocontributory role of systemic pro-inflammation and pro-oxidation in the development of aggression” in schizophrenia, they add. “Gut dysbacteriosis with leaky gut seems to play a vital role in the pathophysiology.”
Correlation vs. causality
Commenting for this article, Emeran Mayer, MD, distinguished research professor of medicine at the G. Oppenheimer Center for Neurobiology of Stress and Resilience and UCLA Brain Gut Microbiome Center, Los Angeles, said that “at first glance, it is interesting that the behavioral trait of aggression but not the diagnosis of schizophrenia showed the differences in markers of systemic inflammation, increased gut permeability, and microbiome parameters.”
However, like many such descriptive studies, the research is flawed by comparing two patient groups and concluding causality between the biomarkers and the behavior traits, added Dr. Mayer, who was not involved with the study.
The study’s shortcomings include its small sample size as well as several confounding factors – particularly diet, sleep, exercise, and stress and anxiety levels – that were not considered, he said. The study also lacked a control group with high levels of aggression but without schizophrenia.
“The observed changes in intestinal permeability, unscientifically referred to as ‘leaky gut,’ as well as the gut microbiome differences, could be secondary to chronically increased sympathetic nervous system activation in the high aggression group,” Dr. Mayer said. “This is an interesting hypothesis which should be discussed and should have been addressed in this study.”
The differences in gut microbial composition and SCFA production “could be secondary to differences in plant-based diet components,” Dr. Mayer speculated, wondering how well dietary intake was controlled.
“Overall, it is an interesting descriptive study, which unfortunately does not contribute significantly to a better understanding of the role of the brain-gut microbiome system in schizophrenic patients,” he said.
The study was funded by a grant from China Postdoctoral Science Foundation. The investigators have reported no relevant financial relationships. Dr. Mayer is a scientific advisory board member of Danone, Axial Therapeutics, Viome, Amare, Mahana Therapeutics, Pendulum, Bloom Biosciences, and APC Microbiome Ireland.
A version of this article first appeared on Medscape.com.
However, at least one expert expressed concerns over the study’s conclusions.
Results from a study of 50 inpatients with schizophrenia showed significantly higher pro-inflammation, pro-oxidation, and leaky gut biomarkers in those with aggression vs. their peers who did not display aggression.
In addition, those with aggression showed less alpha diversity and evenness of the fecal bacterial community, lower levels of several beneficial gut bacteria, and higher levels of the fecal genera Prevotella.
Six short-chain fatty acids (SCFAs) and six neurotransmitters were also lower in the aggression vs. no-aggression groups.
“The present study was the first to compare the state of inflammation, oxidation, intestinal microbiota, and metabolites” in inpatients with schizophrenia and aggression, compared with those who did not show aggression, write the investigators, led by Hongxin Deng, department of psychiatry, Zhumadian (China) Psychiatric Hospital.
“Results indicate pro-inflammation, pro-oxidation and leaky gut phenotypes relating to enteric dysbacteriosis and microbial SCFAs feature the aggression in [individuals with schizophrenia], which provides clues for future microbial-based or anti-inflammatory/oxidative therapies on aggression,” they add.
The findings were published online in BMC Psychiatry.
Unknown pathogenesis
Although emerging evidence suggests that schizophrenia “may augment the propensity for aggression incidence about fourfold to sevenfold,” the pathogenesis of aggression “remains largely unknown,” the investigators note.
The same researchers previously found an association between the systemic pro-inflammation response and the onset or severity of aggression in schizophrenia, “possibly caused by leaky gut-induced bacterial translocation.”
The researchers suggest that peripheral cytokines “could cross the blood-brain barrier, thus precipitating changes in mood and behavior through hypothalamic-pituitary-adrenal axis.”
However, they note that the pro-inflammation phenotype is “often a synergistic effect of multiple causes.” Of these, chronic pro-oxidative stress has been shown to contribute to aggression onset in intermittent explosive disorder, but this association has rarely been confirmed in patients with schizophrenia.
In addition, increasing evidence points to enteric dysbacteriosis and dysbiosis of intestinal flora metabolites, including SCFAs or neurotransmitters, as potentially “integral parts of psychiatric disorders’ pathophysiology” by changing the state of both oxidative stress and inflammation.
The investigators hypothesized that the systemic pro-inflammation phenotype in aggression-affected schizophrenia cases “involves alterations to gut microbiota and its metabolites, leaky gut, and oxidative stress.” However, the profiles of these variables and their interrelationships have been “poorly investigated” in inpatients with schizophrenia and aggression.
To fill this gap, they assessed adult psychiatric inpatients with schizophrenia and aggressive behaviors and inpatients with schizophrenia but no aggressive behavior within 1 week before admission (n = 25 per group; mean age, 33.52 years, and 32.88 years, respectively; 68% and 64% women, respectively).
They collected stool samples from each patient and used enzyme-linked immunoassay (ELISA) to detect fecal calprotectin protein, an indicator of intestinal inflammation. They also collected fasting peripheral blood samples, using ELISA to detect several biomarkers.
The researchers also used the Modified Overt Aggression Scale (MOAS) to characterize aggressive behaviors and the Positive and Negative Syndrome Scale to characterize psychiatric symptoms.
‘Vital role’
Significantly higher biomarkers for systemic pro-inflammation, pro-oxidation and leaky gut were found in the aggression vs the no-aggression group (all P < .05).
After controlling for potential confounders, the researchers also found positive associations between MOAS scores and biomarkers, both serum and fecal.
There were also positive associations between serum 8-hydroxy-20-deoxy-guanosine (8-OH-DG) or 8-isoprostane (8-ISO) and systemic inflammatory biomarkers (all R > 0; P < .05).
In addition, the alpha diversity and evenness of the fecal bacterial community were lower in the aggression vs. no aggression groups.
When the researchers compared the relative abundance of the top 15 genera composition of intestinal microflora in the two groups, Bacteroides, Faecalibacterium, Blautia, Bifidobacterium, Collinsella, and Eubacterium coprostanoligenes were “remarkably reduced” in the group with aggression, whereas the abundance of fecal genera Prevotella was significantly increased (all corrected P < .001).
In the patients who had schizophrenia with aggression, levels of six SCFAs and six neurotransmitters were much lower than in the patients with schizophrenia but no aggression (all P < .05).
Inpatients with schizophrenia and aggression “had dramatically increased serum level of 8-OH-DG (nucleic acid oxidation biomarker) and 8-ISO (lipid oxidation biomarker) than those without, and further correlation analysis also showed positive correlativity between pro-oxidation and systemic pro-inflammation response or aggression severity,” the investigators write.
The findings “collectively suggest the cocontributory role of systemic pro-inflammation and pro-oxidation in the development of aggression” in schizophrenia, they add. “Gut dysbacteriosis with leaky gut seems to play a vital role in the pathophysiology.”
Correlation vs. causality
Commenting for this article, Emeran Mayer, MD, distinguished research professor of medicine at the G. Oppenheimer Center for Neurobiology of Stress and Resilience and UCLA Brain Gut Microbiome Center, Los Angeles, said that “at first glance, it is interesting that the behavioral trait of aggression but not the diagnosis of schizophrenia showed the differences in markers of systemic inflammation, increased gut permeability, and microbiome parameters.”
However, like many such descriptive studies, the research is flawed by comparing two patient groups and concluding causality between the biomarkers and the behavior traits, added Dr. Mayer, who was not involved with the study.
The study’s shortcomings include its small sample size as well as several confounding factors – particularly diet, sleep, exercise, and stress and anxiety levels – that were not considered, he said. The study also lacked a control group with high levels of aggression but without schizophrenia.
“The observed changes in intestinal permeability, unscientifically referred to as ‘leaky gut,’ as well as the gut microbiome differences, could be secondary to chronically increased sympathetic nervous system activation in the high aggression group,” Dr. Mayer said. “This is an interesting hypothesis which should be discussed and should have been addressed in this study.”
The differences in gut microbial composition and SCFA production “could be secondary to differences in plant-based diet components,” Dr. Mayer speculated, wondering how well dietary intake was controlled.
“Overall, it is an interesting descriptive study, which unfortunately does not contribute significantly to a better understanding of the role of the brain-gut microbiome system in schizophrenic patients,” he said.
The study was funded by a grant from China Postdoctoral Science Foundation. The investigators have reported no relevant financial relationships. Dr. Mayer is a scientific advisory board member of Danone, Axial Therapeutics, Viome, Amare, Mahana Therapeutics, Pendulum, Bloom Biosciences, and APC Microbiome Ireland.
A version of this article first appeared on Medscape.com.
FROM BMC PSYCHIATRY
Antioxidant-rich diet may reduce Helicobacter pylori risk
People who eat a balanced diet with sufficient antioxidants from fruits and vegetables may face reduced risks for Heliobacter pylori infections, according to a new report.
In particular, patients with an H. pylori infection were more likely to score lower on the Dietary Antioxidant Index (DAI), which was created to consider a diet’s entire antioxidant profile.
“Available evidence indicates that diet has an important role in developing H. pylori infection. Therefore, protective dietary factors are important from a public health point of view,” Farzad Shidfar, a professor of nutrition at the Iran University of Medical Sciences, Tehran, and member of the university’s colorectal research center, and colleagues write.
“While some nutritional research has widely focused on single nutrients or foods in diet-disease relations, the overall diet could be more informative because humans typically consume a combination of nutrients and foods,” they write. “Dietary indices such as DAI are one of the approaches for this purpose.”
The study was published online in BMC Gastroenterology.
Measuring antioxidant intake
Previous research has indicated an inverse association between the DAI and inflammatory diseases, the study authors write, including gastric cancer, colorectal cancer, nonalcoholic fatty liver disease, and obesity. Studies have also indicated that H. pylori infection is related to deficiencies in vitamins A, C, and E, which have antioxidant properties.
In a case-control study, the research team compared the dietary intake of 148 patients with H. pylori to 302 healthy controls without infection. The patients in the H. pylori–positive group were recruited between June 2021 and November 2021 from the gastroenterology clinic at Rasoul-e-Akram Hospital in Tehran, where they were newly diagnosed with active infection and not yet under treatment.
The researchers calculated the DAI based on dietary intake information from a validated, 168-item food frequency questionnaire used in Iran. The participants were asked about their dietary intake based on the average day, week, month, and year. They also discussed serving sizes of food items, and to increase the accuracy of estimates, interviewers showed household measurements or serving sizes to confirm the measurements with participants.
The average age of the study participants was 39 years, and about 60% were women. Compared with the healthy controls, those with H. pylori were significantly older, had higher body mass index, and smoked more.
Overall, patients with H. pylori had a significantly lower intake of vitamin A, vitamin E, manganese, and selenium. Other differences in dietary intake – for vitamin C and zinc – were not significant.
The average total DAI was significantly higher in the healthy controls, at 7.67, as compared with 3.57 in the patients with H. pylori. The risk for infection decreased as continuous DAI increased.
After adjusting for several variables, the researchers found that participants with less than the median DAI values had an increased risk of developing an H. pylori infection.
“A balanced diet, especially high consumption of fruits and vegetables, might protect people against the consequences of H. pylori infection,” the study authors write. “On the contrary, a diet full of carbohydrates and sweets is related to a higher H. pylori infection prevalence.”
Why a good diet may help combat infection
The findings are consistent with other studies that have noted a higher intake of fruits and vegetables among healthy people compared with those who have H. pylori infections, the study authors write. Animal studies have also indicated that taking vitamins A, C, and E and selenium can lead to a reduction in H. pylori growth.
“Several biologically plausible reasons may explain why dietary antioxidants might be, either directly or indirectly, a protective factor against H. pylori infection,” the researchers write. “It is well-known that antioxidants, with their free radical scavenging activities, can inhibit the growth of H. pylori.”
H. pylori is urease-positive and can synthesize a large amount of urease for ammonia production to neutralize gastric acid, which allows it to colonize in the stomach epithelium, the study authors write. Vitamin C inhibits urease activity and improves the stimulation of granulocytes, macrophages, lymphocytes, and immunoglobulin production. Other nutrients, such as zinc, may inhibit the urease enzyme and prevent H. pylori adhesion to gastric tissues, they write.
“Dietary elements have previously been shown to dramatically alter pathogenic responses to H. pylori infections,” Richard Peek Jr., MD, professor of medicine and director of gastroenterology at Vanderbilt University Medical Center, Nashville, Tenn., told this news organization.
Dr. Peek, who wasn’t involved with this study, and colleagues found that iron deficiency is linked with altered bile metabolism, which can promote H. pylori–induced gastric carcinogenesis.
“The current study is important, as it suggests that shifting to a diet rich in antioxidants may be beneficial in terms of H. pylori infection,” he said.
At the same time, Dr. Peek expressed caution about generalizing the results across populations.
“Most of the persons enrolled in this study were likely infected with H. pylori as children,” he noted. “Therefore, the inverse role of antioxidant-rich diets and H. pylori infection must be interpreted with caution.”
Future studies should confirm the findings in other groups and determine whether antioxidant-rich diets limit the diseases caused by H. pylori infection, Dr. Peek added.
The study was not funded by any research center, and the authors declared no conflicts of interest. Dr. Peek reported no relevant disclosures.
A version of this article first appeared on Medscape.com.
People who eat a balanced diet with sufficient antioxidants from fruits and vegetables may face reduced risks for Heliobacter pylori infections, according to a new report.
In particular, patients with an H. pylori infection were more likely to score lower on the Dietary Antioxidant Index (DAI), which was created to consider a diet’s entire antioxidant profile.
“Available evidence indicates that diet has an important role in developing H. pylori infection. Therefore, protective dietary factors are important from a public health point of view,” Farzad Shidfar, a professor of nutrition at the Iran University of Medical Sciences, Tehran, and member of the university’s colorectal research center, and colleagues write.
“While some nutritional research has widely focused on single nutrients or foods in diet-disease relations, the overall diet could be more informative because humans typically consume a combination of nutrients and foods,” they write. “Dietary indices such as DAI are one of the approaches for this purpose.”
The study was published online in BMC Gastroenterology.
Measuring antioxidant intake
Previous research has indicated an inverse association between the DAI and inflammatory diseases, the study authors write, including gastric cancer, colorectal cancer, nonalcoholic fatty liver disease, and obesity. Studies have also indicated that H. pylori infection is related to deficiencies in vitamins A, C, and E, which have antioxidant properties.
In a case-control study, the research team compared the dietary intake of 148 patients with H. pylori to 302 healthy controls without infection. The patients in the H. pylori–positive group were recruited between June 2021 and November 2021 from the gastroenterology clinic at Rasoul-e-Akram Hospital in Tehran, where they were newly diagnosed with active infection and not yet under treatment.
The researchers calculated the DAI based on dietary intake information from a validated, 168-item food frequency questionnaire used in Iran. The participants were asked about their dietary intake based on the average day, week, month, and year. They also discussed serving sizes of food items, and to increase the accuracy of estimates, interviewers showed household measurements or serving sizes to confirm the measurements with participants.
The average age of the study participants was 39 years, and about 60% were women. Compared with the healthy controls, those with H. pylori were significantly older, had higher body mass index, and smoked more.
Overall, patients with H. pylori had a significantly lower intake of vitamin A, vitamin E, manganese, and selenium. Other differences in dietary intake – for vitamin C and zinc – were not significant.
The average total DAI was significantly higher in the healthy controls, at 7.67, as compared with 3.57 in the patients with H. pylori. The risk for infection decreased as continuous DAI increased.
After adjusting for several variables, the researchers found that participants with less than the median DAI values had an increased risk of developing an H. pylori infection.
“A balanced diet, especially high consumption of fruits and vegetables, might protect people against the consequences of H. pylori infection,” the study authors write. “On the contrary, a diet full of carbohydrates and sweets is related to a higher H. pylori infection prevalence.”
Why a good diet may help combat infection
The findings are consistent with other studies that have noted a higher intake of fruits and vegetables among healthy people compared with those who have H. pylori infections, the study authors write. Animal studies have also indicated that taking vitamins A, C, and E and selenium can lead to a reduction in H. pylori growth.
“Several biologically plausible reasons may explain why dietary antioxidants might be, either directly or indirectly, a protective factor against H. pylori infection,” the researchers write. “It is well-known that antioxidants, with their free radical scavenging activities, can inhibit the growth of H. pylori.”
H. pylori is urease-positive and can synthesize a large amount of urease for ammonia production to neutralize gastric acid, which allows it to colonize in the stomach epithelium, the study authors write. Vitamin C inhibits urease activity and improves the stimulation of granulocytes, macrophages, lymphocytes, and immunoglobulin production. Other nutrients, such as zinc, may inhibit the urease enzyme and prevent H. pylori adhesion to gastric tissues, they write.
“Dietary elements have previously been shown to dramatically alter pathogenic responses to H. pylori infections,” Richard Peek Jr., MD, professor of medicine and director of gastroenterology at Vanderbilt University Medical Center, Nashville, Tenn., told this news organization.
Dr. Peek, who wasn’t involved with this study, and colleagues found that iron deficiency is linked with altered bile metabolism, which can promote H. pylori–induced gastric carcinogenesis.
“The current study is important, as it suggests that shifting to a diet rich in antioxidants may be beneficial in terms of H. pylori infection,” he said.
At the same time, Dr. Peek expressed caution about generalizing the results across populations.
“Most of the persons enrolled in this study were likely infected with H. pylori as children,” he noted. “Therefore, the inverse role of antioxidant-rich diets and H. pylori infection must be interpreted with caution.”
Future studies should confirm the findings in other groups and determine whether antioxidant-rich diets limit the diseases caused by H. pylori infection, Dr. Peek added.
The study was not funded by any research center, and the authors declared no conflicts of interest. Dr. Peek reported no relevant disclosures.
A version of this article first appeared on Medscape.com.
People who eat a balanced diet with sufficient antioxidants from fruits and vegetables may face reduced risks for Heliobacter pylori infections, according to a new report.
In particular, patients with an H. pylori infection were more likely to score lower on the Dietary Antioxidant Index (DAI), which was created to consider a diet’s entire antioxidant profile.
“Available evidence indicates that diet has an important role in developing H. pylori infection. Therefore, protective dietary factors are important from a public health point of view,” Farzad Shidfar, a professor of nutrition at the Iran University of Medical Sciences, Tehran, and member of the university’s colorectal research center, and colleagues write.
“While some nutritional research has widely focused on single nutrients or foods in diet-disease relations, the overall diet could be more informative because humans typically consume a combination of nutrients and foods,” they write. “Dietary indices such as DAI are one of the approaches for this purpose.”
The study was published online in BMC Gastroenterology.
Measuring antioxidant intake
Previous research has indicated an inverse association between the DAI and inflammatory diseases, the study authors write, including gastric cancer, colorectal cancer, nonalcoholic fatty liver disease, and obesity. Studies have also indicated that H. pylori infection is related to deficiencies in vitamins A, C, and E, which have antioxidant properties.
In a case-control study, the research team compared the dietary intake of 148 patients with H. pylori to 302 healthy controls without infection. The patients in the H. pylori–positive group were recruited between June 2021 and November 2021 from the gastroenterology clinic at Rasoul-e-Akram Hospital in Tehran, where they were newly diagnosed with active infection and not yet under treatment.
The researchers calculated the DAI based on dietary intake information from a validated, 168-item food frequency questionnaire used in Iran. The participants were asked about their dietary intake based on the average day, week, month, and year. They also discussed serving sizes of food items, and to increase the accuracy of estimates, interviewers showed household measurements or serving sizes to confirm the measurements with participants.
The average age of the study participants was 39 years, and about 60% were women. Compared with the healthy controls, those with H. pylori were significantly older, had higher body mass index, and smoked more.
Overall, patients with H. pylori had a significantly lower intake of vitamin A, vitamin E, manganese, and selenium. Other differences in dietary intake – for vitamin C and zinc – were not significant.
The average total DAI was significantly higher in the healthy controls, at 7.67, as compared with 3.57 in the patients with H. pylori. The risk for infection decreased as continuous DAI increased.
After adjusting for several variables, the researchers found that participants with less than the median DAI values had an increased risk of developing an H. pylori infection.
“A balanced diet, especially high consumption of fruits and vegetables, might protect people against the consequences of H. pylori infection,” the study authors write. “On the contrary, a diet full of carbohydrates and sweets is related to a higher H. pylori infection prevalence.”
Why a good diet may help combat infection
The findings are consistent with other studies that have noted a higher intake of fruits and vegetables among healthy people compared with those who have H. pylori infections, the study authors write. Animal studies have also indicated that taking vitamins A, C, and E and selenium can lead to a reduction in H. pylori growth.
“Several biologically plausible reasons may explain why dietary antioxidants might be, either directly or indirectly, a protective factor against H. pylori infection,” the researchers write. “It is well-known that antioxidants, with their free radical scavenging activities, can inhibit the growth of H. pylori.”
H. pylori is urease-positive and can synthesize a large amount of urease for ammonia production to neutralize gastric acid, which allows it to colonize in the stomach epithelium, the study authors write. Vitamin C inhibits urease activity and improves the stimulation of granulocytes, macrophages, lymphocytes, and immunoglobulin production. Other nutrients, such as zinc, may inhibit the urease enzyme and prevent H. pylori adhesion to gastric tissues, they write.
“Dietary elements have previously been shown to dramatically alter pathogenic responses to H. pylori infections,” Richard Peek Jr., MD, professor of medicine and director of gastroenterology at Vanderbilt University Medical Center, Nashville, Tenn., told this news organization.
Dr. Peek, who wasn’t involved with this study, and colleagues found that iron deficiency is linked with altered bile metabolism, which can promote H. pylori–induced gastric carcinogenesis.
“The current study is important, as it suggests that shifting to a diet rich in antioxidants may be beneficial in terms of H. pylori infection,” he said.
At the same time, Dr. Peek expressed caution about generalizing the results across populations.
“Most of the persons enrolled in this study were likely infected with H. pylori as children,” he noted. “Therefore, the inverse role of antioxidant-rich diets and H. pylori infection must be interpreted with caution.”
Future studies should confirm the findings in other groups and determine whether antioxidant-rich diets limit the diseases caused by H. pylori infection, Dr. Peek added.
The study was not funded by any research center, and the authors declared no conflicts of interest. Dr. Peek reported no relevant disclosures.
A version of this article first appeared on Medscape.com.
FROM BMC GASTROENTEROLOGY