User login
Bimekizumab Shows Promising Outcomes in PsA, With or Without Methotrexate
Key clinical point: Bimekizumab demonstrated sustained efficacy and was well tolerated for 52 weeks, regardless of concomitant methotrexate use, in patients with psoriatic arthritis (PsA) who were biologic-naive or intolerant to tumor necrosis factor inhibitors (TNFi-IR).
Major finding: Through week 52, nearly half of the patients receiving bimekizumab with or without methotrexate achieved a ≥50% improvement in American College of Rheumatology response (biologic-naive ~55%; TNFi-IR ~48-56%) and minimal disease activity (biologic-naive ~55%; TNFi-IR ~47%). The rates of experiencing at least one treatment emergent adverse event were similar across the subgroups.
Study details: This post hoc analysis of phase 3 trials (BE OPTIMAL, BE COMPLETE, and BE VITAL) included biologic-naive (n = 852) or TNFi-IR (n = 400) patients with PsA who received bimekizumab, placebo with crossover to bimekizumab at week 16, or adalimumab, with or without methotrexate.
Disclosures: This study was funded by UCB Pharma and supported by the NIHR Manchester Biomedical Research Centre, UK. Two authors declared being employees of or holding stocks in UCB. Several authors declared having other ties with UCB and other sources.
Source: McInnes IB, Mease PJ, Tanaka Y, et al. Efficacy and safety of bimekizumab in patients with psoriatic arthritis with or without methotrexate: 52-week results from two phase 3 studies. ACR Open Rheumatol. 2024 (Jul 30). Doi: 10.1002/acr2.11727 Source
Key clinical point: Bimekizumab demonstrated sustained efficacy and was well tolerated for 52 weeks, regardless of concomitant methotrexate use, in patients with psoriatic arthritis (PsA) who were biologic-naive or intolerant to tumor necrosis factor inhibitors (TNFi-IR).
Major finding: Through week 52, nearly half of the patients receiving bimekizumab with or without methotrexate achieved a ≥50% improvement in American College of Rheumatology response (biologic-naive ~55%; TNFi-IR ~48-56%) and minimal disease activity (biologic-naive ~55%; TNFi-IR ~47%). The rates of experiencing at least one treatment emergent adverse event were similar across the subgroups.
Study details: This post hoc analysis of phase 3 trials (BE OPTIMAL, BE COMPLETE, and BE VITAL) included biologic-naive (n = 852) or TNFi-IR (n = 400) patients with PsA who received bimekizumab, placebo with crossover to bimekizumab at week 16, or adalimumab, with or without methotrexate.
Disclosures: This study was funded by UCB Pharma and supported by the NIHR Manchester Biomedical Research Centre, UK. Two authors declared being employees of or holding stocks in UCB. Several authors declared having other ties with UCB and other sources.
Source: McInnes IB, Mease PJ, Tanaka Y, et al. Efficacy and safety of bimekizumab in patients with psoriatic arthritis with or without methotrexate: 52-week results from two phase 3 studies. ACR Open Rheumatol. 2024 (Jul 30). Doi: 10.1002/acr2.11727 Source
Key clinical point: Bimekizumab demonstrated sustained efficacy and was well tolerated for 52 weeks, regardless of concomitant methotrexate use, in patients with psoriatic arthritis (PsA) who were biologic-naive or intolerant to tumor necrosis factor inhibitors (TNFi-IR).
Major finding: Through week 52, nearly half of the patients receiving bimekizumab with or without methotrexate achieved a ≥50% improvement in American College of Rheumatology response (biologic-naive ~55%; TNFi-IR ~48-56%) and minimal disease activity (biologic-naive ~55%; TNFi-IR ~47%). The rates of experiencing at least one treatment emergent adverse event were similar across the subgroups.
Study details: This post hoc analysis of phase 3 trials (BE OPTIMAL, BE COMPLETE, and BE VITAL) included biologic-naive (n = 852) or TNFi-IR (n = 400) patients with PsA who received bimekizumab, placebo with crossover to bimekizumab at week 16, or adalimumab, with or without methotrexate.
Disclosures: This study was funded by UCB Pharma and supported by the NIHR Manchester Biomedical Research Centre, UK. Two authors declared being employees of or holding stocks in UCB. Several authors declared having other ties with UCB and other sources.
Source: McInnes IB, Mease PJ, Tanaka Y, et al. Efficacy and safety of bimekizumab in patients with psoriatic arthritis with or without methotrexate: 52-week results from two phase 3 studies. ACR Open Rheumatol. 2024 (Jul 30). Doi: 10.1002/acr2.11727 Source
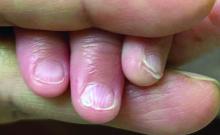
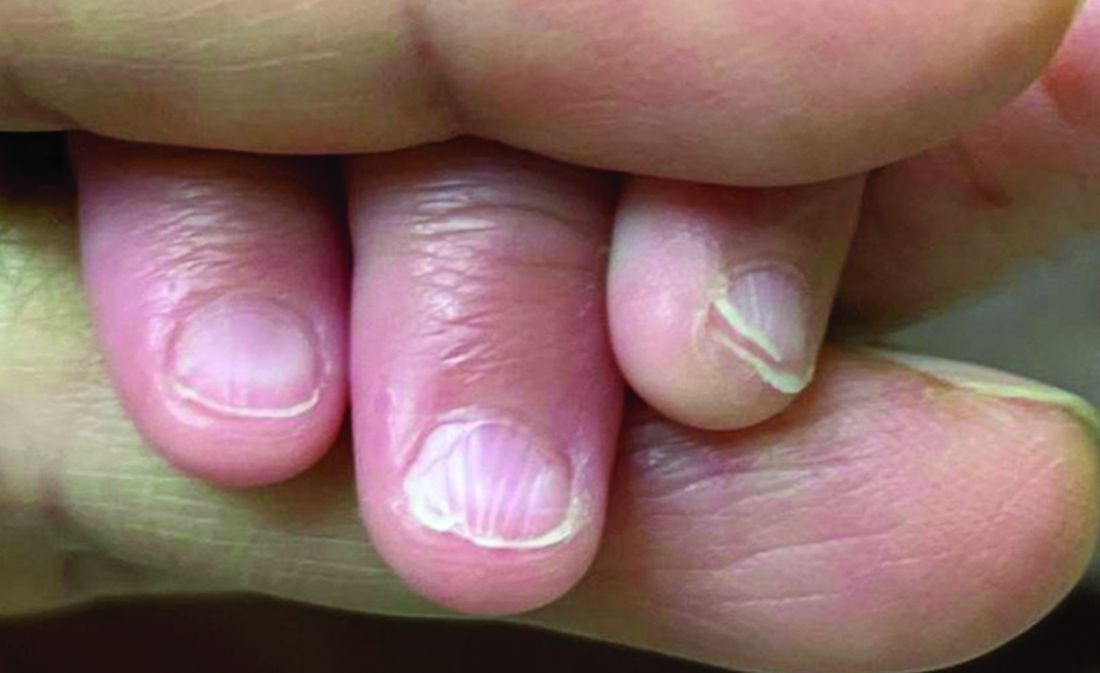
A 7-Month-Old Female Presented With Nail Changes
Given the clinical presentation and the absence of other systemic or dermatological findings, the diagnosis of chevron nails was made.
Discussion
The condition is characterized by transverse ridges on the nails that converge towards the center, forming a V or chevron shape. This condition was first described by Perry et al. and later by Shuster et al., who explained that the condition might result from axial growth of the nail with synchronous growth occurring from a chevron-shaped growing edge of the nail root. Alternatively, Shuster suggested that sequential growth, with localized variation in the nail production rate, could propagate a wave from the center of the nail to the edge.
The etiology of chevron nails is not well understood, but it is believed to result from temporary disruptions in the nail matrix, possibly related to minor illness or physiological stress during infancy.
In the case of our 7-month-old patient, the history of mild upper respiratory infections might have contributed to the development of chevron nails. However, the lack of other significant illness, skin involvement, or systemic findings supports the benign and self-limiting nature of this condition. Parents were reassured that chevron nails typically resolve on their own as the child grows and that no specific treatment is necessary.
Differential Diagnosis
The differential diagnosis of transverse nail changes in children includes other conditions such as trachyonychia, lichen planus, Darier disease, and pachyonychia congenita.
Trachyonychia, also known as “sandpaper nails,” trachyonychia is characterized by the roughening of the nail surface, giving it a dull and ridged appearance. The condition may affect all 20 nails and is often associated with underlying dermatological conditions such as lichen planus or alopecia areata. Unlike chevron nails, trachyonychia presents with more diffuse nail changes and does not typically feature the distinct V-shaped ridging seen in this patient.
Lichen planus is an inflammatory condition that can affect the skin, mucous membranes, and nails. Nail involvement in lichen planus can lead to longitudinal ridging, thinning, and sometimes even complete nail loss. The absence of other characteristic features of lichen planus, such as violaceous papules on the skin or white lacy patterns on mucous membranes (Wickham striae), makes this diagnosis less likely in our patient.
Darier disease, also known as keratosis follicularis, is a genetic disorder characterized by greasy, warty papules primarily on seborrheic areas of the skin, nail abnormalities, and sometimes mucosal involvement. Nail changes in Darier disease include longitudinal red and white streaks, V-shaped notching at the free edge of the nails, and subungual hyperkeratosis. These nail changes are more severe and distinct than the simple transverse ridging seen in chevron nails. The absence of other clinical signs of Darier disease, such as skin papules or characteristic nail notching, makes this diagnosis unlikely in our patient.
Pachyonychia congenita is a rare genetic disorder characterized by thickened nails (pachyonychia), painful plantar keratoderma, and sometimes oral leukokeratosis. The condition typically presents with significant nail thickening and other systemic findings, which were absent in our patient. The distinct pattern of V-shaped ridging observed in chevron nails does not align with the typical presentation of pachyonychia congenita.
Next Steps
No specific treatment is required for chevron nails. The condition is typically self-resolving, and the nails usually return to a normal appearance as the child continues to grow. Parents were advised to monitor the nails for any changes or new symptoms and were reassured about the benign nature of the findings. Follow-up was scheduled to ensure the resolution of the condition as the child develops.
Conclusion
Chevron nails are an important consideration in the differential diagnosis of transverse nail ridging in infants and young children. While the condition is benign and self-limiting, it is crucial to differentiate it from other nail dystrophies, such as trachyonychia, lichen planus, Darier disease, and pachyonychia congenita, which may require further investigation or intervention. Awareness of chevron nails can help prevent unnecessary worry and provide reassurance to parents and caregivers.
Dr. Matiz is a pediatric dermatologist at Southern California Permanente Medical Group, San Diego.
Suggested Reading
Delano S, Belazarian L. Chevron nails: A normal variant in the pediatric population. Pediatr Dermatol. 2014 Jan-Feb;31(1):e24-5. doi: 10.1111/pde.12193.
John JM et al. Chevron nail — An under-recognised normal variant of nail development. Arch Dis Child. 2024 Jul 18;109(8):648. doi: 10.1136/archdischild-2024-326975.
Shuster S. The significance of chevron nails. Br J Dermatol. 1996;135:151–152. doi: 10.1046/j.1365-2133.1996.d01-961.x.
Starace M et al. Nail disorders in children. Skin Appendage Disord. 2018 Oct;4(4):217-229. doi: 10.1159/000486020.
Given the clinical presentation and the absence of other systemic or dermatological findings, the diagnosis of chevron nails was made.
Discussion
The condition is characterized by transverse ridges on the nails that converge towards the center, forming a V or chevron shape. This condition was first described by Perry et al. and later by Shuster et al., who explained that the condition might result from axial growth of the nail with synchronous growth occurring from a chevron-shaped growing edge of the nail root. Alternatively, Shuster suggested that sequential growth, with localized variation in the nail production rate, could propagate a wave from the center of the nail to the edge.
The etiology of chevron nails is not well understood, but it is believed to result from temporary disruptions in the nail matrix, possibly related to minor illness or physiological stress during infancy.
In the case of our 7-month-old patient, the history of mild upper respiratory infections might have contributed to the development of chevron nails. However, the lack of other significant illness, skin involvement, or systemic findings supports the benign and self-limiting nature of this condition. Parents were reassured that chevron nails typically resolve on their own as the child grows and that no specific treatment is necessary.
Differential Diagnosis
The differential diagnosis of transverse nail changes in children includes other conditions such as trachyonychia, lichen planus, Darier disease, and pachyonychia congenita.
Trachyonychia, also known as “sandpaper nails,” trachyonychia is characterized by the roughening of the nail surface, giving it a dull and ridged appearance. The condition may affect all 20 nails and is often associated with underlying dermatological conditions such as lichen planus or alopecia areata. Unlike chevron nails, trachyonychia presents with more diffuse nail changes and does not typically feature the distinct V-shaped ridging seen in this patient.
Lichen planus is an inflammatory condition that can affect the skin, mucous membranes, and nails. Nail involvement in lichen planus can lead to longitudinal ridging, thinning, and sometimes even complete nail loss. The absence of other characteristic features of lichen planus, such as violaceous papules on the skin or white lacy patterns on mucous membranes (Wickham striae), makes this diagnosis less likely in our patient.
Darier disease, also known as keratosis follicularis, is a genetic disorder characterized by greasy, warty papules primarily on seborrheic areas of the skin, nail abnormalities, and sometimes mucosal involvement. Nail changes in Darier disease include longitudinal red and white streaks, V-shaped notching at the free edge of the nails, and subungual hyperkeratosis. These nail changes are more severe and distinct than the simple transverse ridging seen in chevron nails. The absence of other clinical signs of Darier disease, such as skin papules or characteristic nail notching, makes this diagnosis unlikely in our patient.
Pachyonychia congenita is a rare genetic disorder characterized by thickened nails (pachyonychia), painful plantar keratoderma, and sometimes oral leukokeratosis. The condition typically presents with significant nail thickening and other systemic findings, which were absent in our patient. The distinct pattern of V-shaped ridging observed in chevron nails does not align with the typical presentation of pachyonychia congenita.
Next Steps
No specific treatment is required for chevron nails. The condition is typically self-resolving, and the nails usually return to a normal appearance as the child continues to grow. Parents were advised to monitor the nails for any changes or new symptoms and were reassured about the benign nature of the findings. Follow-up was scheduled to ensure the resolution of the condition as the child develops.
Conclusion
Chevron nails are an important consideration in the differential diagnosis of transverse nail ridging in infants and young children. While the condition is benign and self-limiting, it is crucial to differentiate it from other nail dystrophies, such as trachyonychia, lichen planus, Darier disease, and pachyonychia congenita, which may require further investigation or intervention. Awareness of chevron nails can help prevent unnecessary worry and provide reassurance to parents and caregivers.
Dr. Matiz is a pediatric dermatologist at Southern California Permanente Medical Group, San Diego.
Suggested Reading
Delano S, Belazarian L. Chevron nails: A normal variant in the pediatric population. Pediatr Dermatol. 2014 Jan-Feb;31(1):e24-5. doi: 10.1111/pde.12193.
John JM et al. Chevron nail — An under-recognised normal variant of nail development. Arch Dis Child. 2024 Jul 18;109(8):648. doi: 10.1136/archdischild-2024-326975.
Shuster S. The significance of chevron nails. Br J Dermatol. 1996;135:151–152. doi: 10.1046/j.1365-2133.1996.d01-961.x.
Starace M et al. Nail disorders in children. Skin Appendage Disord. 2018 Oct;4(4):217-229. doi: 10.1159/000486020.
Given the clinical presentation and the absence of other systemic or dermatological findings, the diagnosis of chevron nails was made.
Discussion
The condition is characterized by transverse ridges on the nails that converge towards the center, forming a V or chevron shape. This condition was first described by Perry et al. and later by Shuster et al., who explained that the condition might result from axial growth of the nail with synchronous growth occurring from a chevron-shaped growing edge of the nail root. Alternatively, Shuster suggested that sequential growth, with localized variation in the nail production rate, could propagate a wave from the center of the nail to the edge.
The etiology of chevron nails is not well understood, but it is believed to result from temporary disruptions in the nail matrix, possibly related to minor illness or physiological stress during infancy.
In the case of our 7-month-old patient, the history of mild upper respiratory infections might have contributed to the development of chevron nails. However, the lack of other significant illness, skin involvement, or systemic findings supports the benign and self-limiting nature of this condition. Parents were reassured that chevron nails typically resolve on their own as the child grows and that no specific treatment is necessary.
Differential Diagnosis
The differential diagnosis of transverse nail changes in children includes other conditions such as trachyonychia, lichen planus, Darier disease, and pachyonychia congenita.
Trachyonychia, also known as “sandpaper nails,” trachyonychia is characterized by the roughening of the nail surface, giving it a dull and ridged appearance. The condition may affect all 20 nails and is often associated with underlying dermatological conditions such as lichen planus or alopecia areata. Unlike chevron nails, trachyonychia presents with more diffuse nail changes and does not typically feature the distinct V-shaped ridging seen in this patient.
Lichen planus is an inflammatory condition that can affect the skin, mucous membranes, and nails. Nail involvement in lichen planus can lead to longitudinal ridging, thinning, and sometimes even complete nail loss. The absence of other characteristic features of lichen planus, such as violaceous papules on the skin or white lacy patterns on mucous membranes (Wickham striae), makes this diagnosis less likely in our patient.
Darier disease, also known as keratosis follicularis, is a genetic disorder characterized by greasy, warty papules primarily on seborrheic areas of the skin, nail abnormalities, and sometimes mucosal involvement. Nail changes in Darier disease include longitudinal red and white streaks, V-shaped notching at the free edge of the nails, and subungual hyperkeratosis. These nail changes are more severe and distinct than the simple transverse ridging seen in chevron nails. The absence of other clinical signs of Darier disease, such as skin papules or characteristic nail notching, makes this diagnosis unlikely in our patient.
Pachyonychia congenita is a rare genetic disorder characterized by thickened nails (pachyonychia), painful plantar keratoderma, and sometimes oral leukokeratosis. The condition typically presents with significant nail thickening and other systemic findings, which were absent in our patient. The distinct pattern of V-shaped ridging observed in chevron nails does not align with the typical presentation of pachyonychia congenita.
Next Steps
No specific treatment is required for chevron nails. The condition is typically self-resolving, and the nails usually return to a normal appearance as the child continues to grow. Parents were advised to monitor the nails for any changes or new symptoms and were reassured about the benign nature of the findings. Follow-up was scheduled to ensure the resolution of the condition as the child develops.
Conclusion
Chevron nails are an important consideration in the differential diagnosis of transverse nail ridging in infants and young children. While the condition is benign and self-limiting, it is crucial to differentiate it from other nail dystrophies, such as trachyonychia, lichen planus, Darier disease, and pachyonychia congenita, which may require further investigation or intervention. Awareness of chevron nails can help prevent unnecessary worry and provide reassurance to parents and caregivers.
Dr. Matiz is a pediatric dermatologist at Southern California Permanente Medical Group, San Diego.
Suggested Reading
Delano S, Belazarian L. Chevron nails: A normal variant in the pediatric population. Pediatr Dermatol. 2014 Jan-Feb;31(1):e24-5. doi: 10.1111/pde.12193.
John JM et al. Chevron nail — An under-recognised normal variant of nail development. Arch Dis Child. 2024 Jul 18;109(8):648. doi: 10.1136/archdischild-2024-326975.
Shuster S. The significance of chevron nails. Br J Dermatol. 1996;135:151–152. doi: 10.1046/j.1365-2133.1996.d01-961.x.
Starace M et al. Nail disorders in children. Skin Appendage Disord. 2018 Oct;4(4):217-229. doi: 10.1159/000486020.
There was no family history of similar nail findings and no relatives had a history of chronic skin conditions or congenital nail disorders.
On physical examination, several of the child’s fingernails exhibited distinct longitudinal ridges, with a characteristic pattern where the ridges converged at the center of the nail, forming a V-shape. There were no other concerning dermatologic findings, such as rashes, plaques, or erosions, and the skin and hair appeared otherwise normal. The rest of the physical exam was unremarkable.
After Rapid Weight Loss, Monitor Antiobesity Drug Dosing
A patient who developed atrial fibrillation resulting from the failure to adjust the levothyroxine dose after rapid, significant weight loss while on the antiobesity drug tirzepatide (Zepbound) serves as a key reminder in managing patients experiencing rapid weight loss, either from antiobesity medications or any other means: Patients taking medications with weight-based dosing need to have their doses closely monitored.
“Failing to monitor and adjust dosing of these [and other] medications during a period of rapid weight loss may lead to supratherapeutic — even toxic — levels, as was seen in this [case],” underscore the authors of an editorial regarding the Teachable Moment case, published in JAMA Internal Medicine.
Toxicities from excessive doses can have a range of detrimental effects. In terms of thyroid medicine, the failure to adjust levothyroxine treatment for hypothyroidism in cases of rapid weight loss can lead to thyrotoxicosis, and in older patients in particular, a resulting thyrotropin level < 0.1 mIU/L is associated with as much as a threefold increased risk for atrial fibrillation, as observed in the report.
Case Demonstrates Risks
The case involved a 62-year-old man with obesity, hypothyroidism, and type 1 diabetes who presented to the emergency department with palpitations, excessive sweating, confusion, fever, and hand tremors. Upon being diagnosed with atrial fibrillation, the patient was immediately treated.
His medical history revealed the underlying culprit: Six months earlier, the patient had started treatment with the gastric inhibitory polypeptide (GIP)/glucagon-like peptide (GLP) 1 dual agonist tirzepatide. As is typical with the drug, the patient’s weight quickly plummeted, dropping from a starting body mass index of 44.4 down to 31.2 after 6 months and a decrease in body weight from 132 kg to 93 kg (a loss of 39 kg [approximately 86 lb]).
When he was prescribed tirzepatide, 2.5 mg weekly, for obesity, the patient had been recommended to increase the dose every 4 weeks as tolerated and, importantly, to have a follow-up visit in a month. But because he lived in different states seasonally, the follow-up never occurred.
Upon his emergency department visit, the patient’s thyrotropin level had dropped from 1.9 mIU/L at the first visit 6 months earlier to 0.001 mIU/L (well within the atrial fibrillation risk range), and his free thyroxine level (fT4) was 7.26 ng/ dL — substantially outside of the normal range of about 0.9-1.7 ng/dL for adults.
“The patient had 4-times higher fT4 levels of the upper limit,” first author Kagan E. Karakus, MD, of the Barbara Davis Center for Diabetes, University of Colorado Anschutz Medical Campus, Aurora, told this news organization. “That is why he had experienced the adverse event of atrial fibrillation.”
Thyrotoxicosis Symptoms Can Be ‘Insidious,’ Levothyroxine Should Be Monitored
Although tirzepatide has not been approved by the US Food and Drug Administration for the treatment of type 1 diabetes, obesity is on the rise among patients with this disorder and recent research has shown a more than 10% reduction in body weight in 6 months and significant reductions in A1c with various doses.
Of note, in the current case, although the patient’s levothyroxine dose was not adjusted, his insulin dose was gradually self-decreased during his tirzepatide treatment to prevent hypoglycemia.
“If insulin treatment is excessive in diabetes, it causes hypoglycemia, [and] people with type 1 diabetes will recognize the signs of hypoglycemia related to excessive insulin earlier,” Dr. Karakus said.
If symptoms appear, patients can reduce their insulin doses on their own; however, the symptoms of thyrotoxicosis caused by excessive levothyroxine can be more insidious compared with hypoglycemia, he explained.
“Although patients can change their insulin doses, they cannot change the levothyroxine doses since it requires a blood test [thyroid-stimulating hormone; TSH] and a new prescription of the new dose.”
The key lesson is that “following levothyroxine treatment initiation or dose adjustment, 4-6 weeks is the optimal duration to recheck [the] thyrotropin level and adjust the dose as needed,” Dr. Karakus said.
Key Medications to Monitor
Other common outpatient medications that should be closely monitored in patients experiencing rapid weight loss, by any method, range from anticoagulants, anticonvulsants, and antituberculosis drugs to antibiotics and antifungals, the authors note.
Of note, medications with a narrow therapeutic index include phenytoin, warfarin, lithium carbonate, digoxin theophylline, tacrolimus, valproic acid, carbamazepine, and cyclosporine.
The failure to make necessary dose adjustments “is seen more often since the newer antiobesity drugs reduce a great amount of weight within months, almost as rapidly as bariatric surgery,” Dr. Karakus said.
“It is very important for physicians to be aware of the weight-based medications and narrow therapeutic index medications since their doses should be adjusted carefully, especially during weight loss,” he added.
Furthermore, “the patient should also know that weight reduction medication may cause adverse effects like nausea, vomiting and also may affect metabolism of other medications such that some medication doses should be adjusted regularly.”
In the editorial published with the study, Tyrone A. Johnson, MD, of the Department of Medicine, University of California, San Francisco, and colleagues note that the need for close monitoring is particularly important with older patients, who, in addition to having a higher likelihood of comorbidities, commonly have polypharmacy that could increase the potential for adverse effects.
Another key area concern is the emergence of direct-to-consumer avenues for GLP-1/GIP agonists for the many who either cannot afford or do not have access to the drugs, providing further opportunities for treatment without appropriate clinical oversight, they add.
Overall, the case “highlights the potential dangers underlying under-supervised prescribing of GLP-1/GIP receptor agonists and affirms the need for strong partnerships between patients and their clinicians during their use,” they wrote.
“These medications are best used in collaboration with continuity care teams, in context of a patient’s entire health, and in comprehensive risk-benefit assessment throughout the entire duration of treatment.”
A Caveat: Subclinical Levothyroxine Dosing
Commenting on the study, Matthew Ettleson, MD, a clinical instructor of medicine in the Section of Endocrinology, Diabetes, & Metabolism, University of Chicago, noted the important caveat that patients with hypothyroidism are commonly on subclinical doses, with varying dose adjustment needs.
“The patient in the case was clearly on a replacement level dose. However, many patients are on low doses of levothyroxine (75 µg or lower) for subclinical hypothyroidism, and, in general, I think the risks are lower with patients with subclinical hypothyroidism on lower doses of levothyroxine,” he told this news organization.
Because of that, “frequent TSH monitoring may be excessive in this population,” he said. “I would hesitate to empirically lower the dose with weight loss, unless it was clear that the patient was unlikely to follow up.
“Checking TSH at a more frequent interval and adjusting the dose accordingly should be adequate to prevent situations like this case.”
Dr. Karakus, Dr. Ettleson, and the editorial authors had no relevant disclosures to report.
A version of this article appeared on Medscape.com.
A patient who developed atrial fibrillation resulting from the failure to adjust the levothyroxine dose after rapid, significant weight loss while on the antiobesity drug tirzepatide (Zepbound) serves as a key reminder in managing patients experiencing rapid weight loss, either from antiobesity medications or any other means: Patients taking medications with weight-based dosing need to have their doses closely monitored.
“Failing to monitor and adjust dosing of these [and other] medications during a period of rapid weight loss may lead to supratherapeutic — even toxic — levels, as was seen in this [case],” underscore the authors of an editorial regarding the Teachable Moment case, published in JAMA Internal Medicine.
Toxicities from excessive doses can have a range of detrimental effects. In terms of thyroid medicine, the failure to adjust levothyroxine treatment for hypothyroidism in cases of rapid weight loss can lead to thyrotoxicosis, and in older patients in particular, a resulting thyrotropin level < 0.1 mIU/L is associated with as much as a threefold increased risk for atrial fibrillation, as observed in the report.
Case Demonstrates Risks
The case involved a 62-year-old man with obesity, hypothyroidism, and type 1 diabetes who presented to the emergency department with palpitations, excessive sweating, confusion, fever, and hand tremors. Upon being diagnosed with atrial fibrillation, the patient was immediately treated.
His medical history revealed the underlying culprit: Six months earlier, the patient had started treatment with the gastric inhibitory polypeptide (GIP)/glucagon-like peptide (GLP) 1 dual agonist tirzepatide. As is typical with the drug, the patient’s weight quickly plummeted, dropping from a starting body mass index of 44.4 down to 31.2 after 6 months and a decrease in body weight from 132 kg to 93 kg (a loss of 39 kg [approximately 86 lb]).
When he was prescribed tirzepatide, 2.5 mg weekly, for obesity, the patient had been recommended to increase the dose every 4 weeks as tolerated and, importantly, to have a follow-up visit in a month. But because he lived in different states seasonally, the follow-up never occurred.
Upon his emergency department visit, the patient’s thyrotropin level had dropped from 1.9 mIU/L at the first visit 6 months earlier to 0.001 mIU/L (well within the atrial fibrillation risk range), and his free thyroxine level (fT4) was 7.26 ng/ dL — substantially outside of the normal range of about 0.9-1.7 ng/dL for adults.
“The patient had 4-times higher fT4 levels of the upper limit,” first author Kagan E. Karakus, MD, of the Barbara Davis Center for Diabetes, University of Colorado Anschutz Medical Campus, Aurora, told this news organization. “That is why he had experienced the adverse event of atrial fibrillation.”
Thyrotoxicosis Symptoms Can Be ‘Insidious,’ Levothyroxine Should Be Monitored
Although tirzepatide has not been approved by the US Food and Drug Administration for the treatment of type 1 diabetes, obesity is on the rise among patients with this disorder and recent research has shown a more than 10% reduction in body weight in 6 months and significant reductions in A1c with various doses.
Of note, in the current case, although the patient’s levothyroxine dose was not adjusted, his insulin dose was gradually self-decreased during his tirzepatide treatment to prevent hypoglycemia.
“If insulin treatment is excessive in diabetes, it causes hypoglycemia, [and] people with type 1 diabetes will recognize the signs of hypoglycemia related to excessive insulin earlier,” Dr. Karakus said.
If symptoms appear, patients can reduce their insulin doses on their own; however, the symptoms of thyrotoxicosis caused by excessive levothyroxine can be more insidious compared with hypoglycemia, he explained.
“Although patients can change their insulin doses, they cannot change the levothyroxine doses since it requires a blood test [thyroid-stimulating hormone; TSH] and a new prescription of the new dose.”
The key lesson is that “following levothyroxine treatment initiation or dose adjustment, 4-6 weeks is the optimal duration to recheck [the] thyrotropin level and adjust the dose as needed,” Dr. Karakus said.
Key Medications to Monitor
Other common outpatient medications that should be closely monitored in patients experiencing rapid weight loss, by any method, range from anticoagulants, anticonvulsants, and antituberculosis drugs to antibiotics and antifungals, the authors note.
Of note, medications with a narrow therapeutic index include phenytoin, warfarin, lithium carbonate, digoxin theophylline, tacrolimus, valproic acid, carbamazepine, and cyclosporine.
The failure to make necessary dose adjustments “is seen more often since the newer antiobesity drugs reduce a great amount of weight within months, almost as rapidly as bariatric surgery,” Dr. Karakus said.
“It is very important for physicians to be aware of the weight-based medications and narrow therapeutic index medications since their doses should be adjusted carefully, especially during weight loss,” he added.
Furthermore, “the patient should also know that weight reduction medication may cause adverse effects like nausea, vomiting and also may affect metabolism of other medications such that some medication doses should be adjusted regularly.”
In the editorial published with the study, Tyrone A. Johnson, MD, of the Department of Medicine, University of California, San Francisco, and colleagues note that the need for close monitoring is particularly important with older patients, who, in addition to having a higher likelihood of comorbidities, commonly have polypharmacy that could increase the potential for adverse effects.
Another key area concern is the emergence of direct-to-consumer avenues for GLP-1/GIP agonists for the many who either cannot afford or do not have access to the drugs, providing further opportunities for treatment without appropriate clinical oversight, they add.
Overall, the case “highlights the potential dangers underlying under-supervised prescribing of GLP-1/GIP receptor agonists and affirms the need for strong partnerships between patients and their clinicians during their use,” they wrote.
“These medications are best used in collaboration with continuity care teams, in context of a patient’s entire health, and in comprehensive risk-benefit assessment throughout the entire duration of treatment.”
A Caveat: Subclinical Levothyroxine Dosing
Commenting on the study, Matthew Ettleson, MD, a clinical instructor of medicine in the Section of Endocrinology, Diabetes, & Metabolism, University of Chicago, noted the important caveat that patients with hypothyroidism are commonly on subclinical doses, with varying dose adjustment needs.
“The patient in the case was clearly on a replacement level dose. However, many patients are on low doses of levothyroxine (75 µg or lower) for subclinical hypothyroidism, and, in general, I think the risks are lower with patients with subclinical hypothyroidism on lower doses of levothyroxine,” he told this news organization.
Because of that, “frequent TSH monitoring may be excessive in this population,” he said. “I would hesitate to empirically lower the dose with weight loss, unless it was clear that the patient was unlikely to follow up.
“Checking TSH at a more frequent interval and adjusting the dose accordingly should be adequate to prevent situations like this case.”
Dr. Karakus, Dr. Ettleson, and the editorial authors had no relevant disclosures to report.
A version of this article appeared on Medscape.com.
A patient who developed atrial fibrillation resulting from the failure to adjust the levothyroxine dose after rapid, significant weight loss while on the antiobesity drug tirzepatide (Zepbound) serves as a key reminder in managing patients experiencing rapid weight loss, either from antiobesity medications or any other means: Patients taking medications with weight-based dosing need to have their doses closely monitored.
“Failing to monitor and adjust dosing of these [and other] medications during a period of rapid weight loss may lead to supratherapeutic — even toxic — levels, as was seen in this [case],” underscore the authors of an editorial regarding the Teachable Moment case, published in JAMA Internal Medicine.
Toxicities from excessive doses can have a range of detrimental effects. In terms of thyroid medicine, the failure to adjust levothyroxine treatment for hypothyroidism in cases of rapid weight loss can lead to thyrotoxicosis, and in older patients in particular, a resulting thyrotropin level < 0.1 mIU/L is associated with as much as a threefold increased risk for atrial fibrillation, as observed in the report.
Case Demonstrates Risks
The case involved a 62-year-old man with obesity, hypothyroidism, and type 1 diabetes who presented to the emergency department with palpitations, excessive sweating, confusion, fever, and hand tremors. Upon being diagnosed with atrial fibrillation, the patient was immediately treated.
His medical history revealed the underlying culprit: Six months earlier, the patient had started treatment with the gastric inhibitory polypeptide (GIP)/glucagon-like peptide (GLP) 1 dual agonist tirzepatide. As is typical with the drug, the patient’s weight quickly plummeted, dropping from a starting body mass index of 44.4 down to 31.2 after 6 months and a decrease in body weight from 132 kg to 93 kg (a loss of 39 kg [approximately 86 lb]).
When he was prescribed tirzepatide, 2.5 mg weekly, for obesity, the patient had been recommended to increase the dose every 4 weeks as tolerated and, importantly, to have a follow-up visit in a month. But because he lived in different states seasonally, the follow-up never occurred.
Upon his emergency department visit, the patient’s thyrotropin level had dropped from 1.9 mIU/L at the first visit 6 months earlier to 0.001 mIU/L (well within the atrial fibrillation risk range), and his free thyroxine level (fT4) was 7.26 ng/ dL — substantially outside of the normal range of about 0.9-1.7 ng/dL for adults.
“The patient had 4-times higher fT4 levels of the upper limit,” first author Kagan E. Karakus, MD, of the Barbara Davis Center for Diabetes, University of Colorado Anschutz Medical Campus, Aurora, told this news organization. “That is why he had experienced the adverse event of atrial fibrillation.”
Thyrotoxicosis Symptoms Can Be ‘Insidious,’ Levothyroxine Should Be Monitored
Although tirzepatide has not been approved by the US Food and Drug Administration for the treatment of type 1 diabetes, obesity is on the rise among patients with this disorder and recent research has shown a more than 10% reduction in body weight in 6 months and significant reductions in A1c with various doses.
Of note, in the current case, although the patient’s levothyroxine dose was not adjusted, his insulin dose was gradually self-decreased during his tirzepatide treatment to prevent hypoglycemia.
“If insulin treatment is excessive in diabetes, it causes hypoglycemia, [and] people with type 1 diabetes will recognize the signs of hypoglycemia related to excessive insulin earlier,” Dr. Karakus said.
If symptoms appear, patients can reduce their insulin doses on their own; however, the symptoms of thyrotoxicosis caused by excessive levothyroxine can be more insidious compared with hypoglycemia, he explained.
“Although patients can change their insulin doses, they cannot change the levothyroxine doses since it requires a blood test [thyroid-stimulating hormone; TSH] and a new prescription of the new dose.”
The key lesson is that “following levothyroxine treatment initiation or dose adjustment, 4-6 weeks is the optimal duration to recheck [the] thyrotropin level and adjust the dose as needed,” Dr. Karakus said.
Key Medications to Monitor
Other common outpatient medications that should be closely monitored in patients experiencing rapid weight loss, by any method, range from anticoagulants, anticonvulsants, and antituberculosis drugs to antibiotics and antifungals, the authors note.
Of note, medications with a narrow therapeutic index include phenytoin, warfarin, lithium carbonate, digoxin theophylline, tacrolimus, valproic acid, carbamazepine, and cyclosporine.
The failure to make necessary dose adjustments “is seen more often since the newer antiobesity drugs reduce a great amount of weight within months, almost as rapidly as bariatric surgery,” Dr. Karakus said.
“It is very important for physicians to be aware of the weight-based medications and narrow therapeutic index medications since their doses should be adjusted carefully, especially during weight loss,” he added.
Furthermore, “the patient should also know that weight reduction medication may cause adverse effects like nausea, vomiting and also may affect metabolism of other medications such that some medication doses should be adjusted regularly.”
In the editorial published with the study, Tyrone A. Johnson, MD, of the Department of Medicine, University of California, San Francisco, and colleagues note that the need for close monitoring is particularly important with older patients, who, in addition to having a higher likelihood of comorbidities, commonly have polypharmacy that could increase the potential for adverse effects.
Another key area concern is the emergence of direct-to-consumer avenues for GLP-1/GIP agonists for the many who either cannot afford or do not have access to the drugs, providing further opportunities for treatment without appropriate clinical oversight, they add.
Overall, the case “highlights the potential dangers underlying under-supervised prescribing of GLP-1/GIP receptor agonists and affirms the need for strong partnerships between patients and their clinicians during their use,” they wrote.
“These medications are best used in collaboration with continuity care teams, in context of a patient’s entire health, and in comprehensive risk-benefit assessment throughout the entire duration of treatment.”
A Caveat: Subclinical Levothyroxine Dosing
Commenting on the study, Matthew Ettleson, MD, a clinical instructor of medicine in the Section of Endocrinology, Diabetes, & Metabolism, University of Chicago, noted the important caveat that patients with hypothyroidism are commonly on subclinical doses, with varying dose adjustment needs.
“The patient in the case was clearly on a replacement level dose. However, many patients are on low doses of levothyroxine (75 µg or lower) for subclinical hypothyroidism, and, in general, I think the risks are lower with patients with subclinical hypothyroidism on lower doses of levothyroxine,” he told this news organization.
Because of that, “frequent TSH monitoring may be excessive in this population,” he said. “I would hesitate to empirically lower the dose with weight loss, unless it was clear that the patient was unlikely to follow up.
“Checking TSH at a more frequent interval and adjusting the dose accordingly should be adequate to prevent situations like this case.”
Dr. Karakus, Dr. Ettleson, and the editorial authors had no relevant disclosures to report.
A version of this article appeared on Medscape.com.
Could Dry Fasting Aid in Metabolic Disorders, Diabetes?
Dry fasting, the practice of going without food and water, has enthusiastic advocates on TikTok, X, YouTube, and other social media platforms. Devotees claim a wide range of health effects, but medical professionals advise caution to ensure that the practice does more good than harm, especially for individuals with diabetes.
Purported benefits and risks vary, depending on who is following the regimen and how long they abstain from food and water. Advocates on social media assert that dry fasting makes “intuition skyrocket” and puts autophagy on “overdrive.” Although such statements may rev up followers, there is little evidence to support these and many other dry-fasting claims. In fact, several physicians warned about unintended consequences.
“I had one patient who followed this fasting method often, and over time she developed kidney stones that led to a severe infection,” said Deena Adimoolam, MD, an endocrinologist in private practice in New York City and New Jersey. “Lack of both water and food can fuel hunger and increase the likelihood of overeating or binge eating once the fast is completed, which does not lead to weight loss. Untreated dehydration can lead to loss of consciousness.”
“For individuals with type 2 diabetes, dehydration can exacerbate hyperglycemia and increase the risk of complications such as diabetic ketoacidosis (DKA),” said Abeer Bader, lead clinical nutrition specialist at the Massachusetts General Hospital Weight Center in Boston. “Research also consistently shows that adequate hydration is crucial for maintaining physical and cognitive performance.”
, Ms. Bader noted. “Prolonged dry fasting can result in nutrient deficiencies. For individuals with diabetes, maintaining adequate nutrition is crucial to manage blood sugar levels and overall health. The lack of both food and water can exacerbate deficiencies.”
Joanne Bruno, MD, an endocrinologist at NYU Langone Health, added, “Certain medications used for the management of type 2 diabetes, such as SGLT2 inhibitors, can cause dehydration. It is critical that patients stay well hydrated while on these medications to avoid serious side effects such as euglycemic DKA.”
What Exactly Is Dry Fasting?
Defining dry fasting, like any kind of fasting, has remained a challenge, according to authors of the first international consensus on fasting terminology, published on July 25 in Cell Metabolism. The clinical terminology “has remained heterogeneous and often confusing, with similar terms being used to define different fasting regimens ... reflecting the manifold contexts in which fasting is practiced.”
Indeed, dry fasting was among the most discussed terms by the consensus panel and went through several rounds before the panelists came to agreement. A few experts were critical of the practice, whereas those familiar with religious fasting traditions, such as during Ramadan, were clear about the importance of including this term in the consensus process.
“The dissent was resolved by the clarification that this form of fasting has historical and geographical extensions and that the present consensus process did not aim at evaluating therapeutic effectiveness or safety for any term defined,” the authors wrote.
The panel concluded that dry fasting is not the same as total or complete fasting because the latter can include water (such as water-only fasting). Their final definition of dry fasting is ‘’a fasting regimen during which a voluntary abstinence from all foods and beverages, including water, is practiced for a certain period of time.’’
Different types of fasting regimens, such as intermittent fasting, may include dry fasting, in which case it is referred to as “intermittent dry fasting.” This is defined in the consensus as intermittent fasting regimens that involve abstaining from food and fluid intake during the fasting interval, which typically lasts 9-20 hours.
Most dry fasts, including religious ones, are maintained for a specific interval and are followed by a refeeding period. These fasts are not starvation, defined as no food or water intake for days.
What the Evidence Says
All that said, dry fasting by any other name remains dry fasting. “Abundant” evidence from animal studies suggests the potential of various types of fasting for disease prevention and treatment in humans, noted the authors of the consensus report, Along with the risks described above, small studies have explored short-term effects in people, all of which have yet to be established by larger and longer-term studies.
In a recent small study, researchers at Baylor College of Medicine, Houston, Texas, reported that dawn-to-dusk dry fasting for 30 days reduced levels of inflammatory cytokines in the 13 participants with a high body mass index. Earlier work by the group showed that dawn-to-dusk dry fasting for 30 days induced “anti-atherosclerotic, anti-inflammatory, and anti-tumorigenic proteome” in peripheral blood mononuclear cells of 14 individuals with metabolic syndrome (The researchers declined to comment for this article.)
Importantly, the health effects can vary among individuals for unknown reasons, found a recent cross-sectional study of fasting blood glucose (FBG) changes in 181 patients with type 2 diabetes during Ramadan intermittent fasting (RIF), which involves dry fasting during daylight hours for 1 month. The researchers classified participants into three groups: reduced average FBG levels (44%), no change in FBG levels (24%), and increased FBG levels (32%). The authors wrote that further studies are needed to identify factors associated with the differences and to identify “those who are great candidates for RIF.”
In contrast to some of the concerns expressed by clinicians, an exploratory study of daytime dry fasting among 34 healthy Baha’i volunteers in Germany concluded that the 19-day regimen “is safe, has no negative effects on hydration, can improve fat metabolism and can cause transient phase shifts of circadian rhythms.” The authors acknowledge that a larger number and more diverse participants are needed to validate the findings and assess the impact on long-term health.
What to Advise Patients
For patients who want to fast as part of their weight loss regimen or to help manage diabetes, clinicians can consider suggesting “alternate ways of eating that might achieve similar goals,” Ms. Bader said. One is intermittent fasting without dry fasting: the 16:8 method (16 hours of fasting, 8 hours of eating) or the 5:2 method (normal eating for 5 days, reduced calorie intake for 2 days), which can support improved insulin sensitivity and metabolic health.
Caloric restriction can also work if the patient maintains a balanced diet that includes all essential nutrients, she said. A low-carbohydrate diet that focuses on limiting carbohydrate intake while increasing consumption of lean proteins and healthy fats has been shown to lower blood sugar levels and improve insulin sensitivity.
Other healthy strategies for patients include the Mediterranean diet, which emphasizes whole grains, fruits, vegetables, nuts, seeds, olive oil, and lean proteins such as fish, or a similar plant-based diet with less animal protein. Ms. Bader advises cultivating mindful eating, which involves paying attention to hunger and fullness cues, making thoughtful food choices, and focusing on being present during meals.
“Each of these dietary strategies offers potential benefits for managing type 2 diabetes and improving overall health,” Ms. Bader said. “I have not had any patients who have tried dry fasting specifically. However, I have encountered scenarios where individuals abstained from food and beverages due to religious practices. In those cases, we focused on ensuring that they maintained proper hydration and balanced nutrition during their eating periods to manage their diabetes effectively and prevent complications.”
Overall, Dr. Adimoolam suggests that clinicians help patients find a weight-loss plan that works best for them based on understanding the calories in the foods they like and don’t like. For fasting regimens, patients can be encouraged to choose one with fluids when possible, as well as intervals of time to fast and eat that work best for their lifestyle.
Ms. Bader, Dr. Bruno, and Dr. Adimoolam report no relevant conflicts.
A version of this article appeared on Medscape.com.
Dry fasting, the practice of going without food and water, has enthusiastic advocates on TikTok, X, YouTube, and other social media platforms. Devotees claim a wide range of health effects, but medical professionals advise caution to ensure that the practice does more good than harm, especially for individuals with diabetes.
Purported benefits and risks vary, depending on who is following the regimen and how long they abstain from food and water. Advocates on social media assert that dry fasting makes “intuition skyrocket” and puts autophagy on “overdrive.” Although such statements may rev up followers, there is little evidence to support these and many other dry-fasting claims. In fact, several physicians warned about unintended consequences.
“I had one patient who followed this fasting method often, and over time she developed kidney stones that led to a severe infection,” said Deena Adimoolam, MD, an endocrinologist in private practice in New York City and New Jersey. “Lack of both water and food can fuel hunger and increase the likelihood of overeating or binge eating once the fast is completed, which does not lead to weight loss. Untreated dehydration can lead to loss of consciousness.”
“For individuals with type 2 diabetes, dehydration can exacerbate hyperglycemia and increase the risk of complications such as diabetic ketoacidosis (DKA),” said Abeer Bader, lead clinical nutrition specialist at the Massachusetts General Hospital Weight Center in Boston. “Research also consistently shows that adequate hydration is crucial for maintaining physical and cognitive performance.”
, Ms. Bader noted. “Prolonged dry fasting can result in nutrient deficiencies. For individuals with diabetes, maintaining adequate nutrition is crucial to manage blood sugar levels and overall health. The lack of both food and water can exacerbate deficiencies.”
Joanne Bruno, MD, an endocrinologist at NYU Langone Health, added, “Certain medications used for the management of type 2 diabetes, such as SGLT2 inhibitors, can cause dehydration. It is critical that patients stay well hydrated while on these medications to avoid serious side effects such as euglycemic DKA.”
What Exactly Is Dry Fasting?
Defining dry fasting, like any kind of fasting, has remained a challenge, according to authors of the first international consensus on fasting terminology, published on July 25 in Cell Metabolism. The clinical terminology “has remained heterogeneous and often confusing, with similar terms being used to define different fasting regimens ... reflecting the manifold contexts in which fasting is practiced.”
Indeed, dry fasting was among the most discussed terms by the consensus panel and went through several rounds before the panelists came to agreement. A few experts were critical of the practice, whereas those familiar with religious fasting traditions, such as during Ramadan, were clear about the importance of including this term in the consensus process.
“The dissent was resolved by the clarification that this form of fasting has historical and geographical extensions and that the present consensus process did not aim at evaluating therapeutic effectiveness or safety for any term defined,” the authors wrote.
The panel concluded that dry fasting is not the same as total or complete fasting because the latter can include water (such as water-only fasting). Their final definition of dry fasting is ‘’a fasting regimen during which a voluntary abstinence from all foods and beverages, including water, is practiced for a certain period of time.’’
Different types of fasting regimens, such as intermittent fasting, may include dry fasting, in which case it is referred to as “intermittent dry fasting.” This is defined in the consensus as intermittent fasting regimens that involve abstaining from food and fluid intake during the fasting interval, which typically lasts 9-20 hours.
Most dry fasts, including religious ones, are maintained for a specific interval and are followed by a refeeding period. These fasts are not starvation, defined as no food or water intake for days.
What the Evidence Says
All that said, dry fasting by any other name remains dry fasting. “Abundant” evidence from animal studies suggests the potential of various types of fasting for disease prevention and treatment in humans, noted the authors of the consensus report, Along with the risks described above, small studies have explored short-term effects in people, all of which have yet to be established by larger and longer-term studies.
In a recent small study, researchers at Baylor College of Medicine, Houston, Texas, reported that dawn-to-dusk dry fasting for 30 days reduced levels of inflammatory cytokines in the 13 participants with a high body mass index. Earlier work by the group showed that dawn-to-dusk dry fasting for 30 days induced “anti-atherosclerotic, anti-inflammatory, and anti-tumorigenic proteome” in peripheral blood mononuclear cells of 14 individuals with metabolic syndrome (The researchers declined to comment for this article.)
Importantly, the health effects can vary among individuals for unknown reasons, found a recent cross-sectional study of fasting blood glucose (FBG) changes in 181 patients with type 2 diabetes during Ramadan intermittent fasting (RIF), which involves dry fasting during daylight hours for 1 month. The researchers classified participants into three groups: reduced average FBG levels (44%), no change in FBG levels (24%), and increased FBG levels (32%). The authors wrote that further studies are needed to identify factors associated with the differences and to identify “those who are great candidates for RIF.”
In contrast to some of the concerns expressed by clinicians, an exploratory study of daytime dry fasting among 34 healthy Baha’i volunteers in Germany concluded that the 19-day regimen “is safe, has no negative effects on hydration, can improve fat metabolism and can cause transient phase shifts of circadian rhythms.” The authors acknowledge that a larger number and more diverse participants are needed to validate the findings and assess the impact on long-term health.
What to Advise Patients
For patients who want to fast as part of their weight loss regimen or to help manage diabetes, clinicians can consider suggesting “alternate ways of eating that might achieve similar goals,” Ms. Bader said. One is intermittent fasting without dry fasting: the 16:8 method (16 hours of fasting, 8 hours of eating) or the 5:2 method (normal eating for 5 days, reduced calorie intake for 2 days), which can support improved insulin sensitivity and metabolic health.
Caloric restriction can also work if the patient maintains a balanced diet that includes all essential nutrients, she said. A low-carbohydrate diet that focuses on limiting carbohydrate intake while increasing consumption of lean proteins and healthy fats has been shown to lower blood sugar levels and improve insulin sensitivity.
Other healthy strategies for patients include the Mediterranean diet, which emphasizes whole grains, fruits, vegetables, nuts, seeds, olive oil, and lean proteins such as fish, or a similar plant-based diet with less animal protein. Ms. Bader advises cultivating mindful eating, which involves paying attention to hunger and fullness cues, making thoughtful food choices, and focusing on being present during meals.
“Each of these dietary strategies offers potential benefits for managing type 2 diabetes and improving overall health,” Ms. Bader said. “I have not had any patients who have tried dry fasting specifically. However, I have encountered scenarios where individuals abstained from food and beverages due to religious practices. In those cases, we focused on ensuring that they maintained proper hydration and balanced nutrition during their eating periods to manage their diabetes effectively and prevent complications.”
Overall, Dr. Adimoolam suggests that clinicians help patients find a weight-loss plan that works best for them based on understanding the calories in the foods they like and don’t like. For fasting regimens, patients can be encouraged to choose one with fluids when possible, as well as intervals of time to fast and eat that work best for their lifestyle.
Ms. Bader, Dr. Bruno, and Dr. Adimoolam report no relevant conflicts.
A version of this article appeared on Medscape.com.
Dry fasting, the practice of going without food and water, has enthusiastic advocates on TikTok, X, YouTube, and other social media platforms. Devotees claim a wide range of health effects, but medical professionals advise caution to ensure that the practice does more good than harm, especially for individuals with diabetes.
Purported benefits and risks vary, depending on who is following the regimen and how long they abstain from food and water. Advocates on social media assert that dry fasting makes “intuition skyrocket” and puts autophagy on “overdrive.” Although such statements may rev up followers, there is little evidence to support these and many other dry-fasting claims. In fact, several physicians warned about unintended consequences.
“I had one patient who followed this fasting method often, and over time she developed kidney stones that led to a severe infection,” said Deena Adimoolam, MD, an endocrinologist in private practice in New York City and New Jersey. “Lack of both water and food can fuel hunger and increase the likelihood of overeating or binge eating once the fast is completed, which does not lead to weight loss. Untreated dehydration can lead to loss of consciousness.”
“For individuals with type 2 diabetes, dehydration can exacerbate hyperglycemia and increase the risk of complications such as diabetic ketoacidosis (DKA),” said Abeer Bader, lead clinical nutrition specialist at the Massachusetts General Hospital Weight Center in Boston. “Research also consistently shows that adequate hydration is crucial for maintaining physical and cognitive performance.”
, Ms. Bader noted. “Prolonged dry fasting can result in nutrient deficiencies. For individuals with diabetes, maintaining adequate nutrition is crucial to manage blood sugar levels and overall health. The lack of both food and water can exacerbate deficiencies.”
Joanne Bruno, MD, an endocrinologist at NYU Langone Health, added, “Certain medications used for the management of type 2 diabetes, such as SGLT2 inhibitors, can cause dehydration. It is critical that patients stay well hydrated while on these medications to avoid serious side effects such as euglycemic DKA.”
What Exactly Is Dry Fasting?
Defining dry fasting, like any kind of fasting, has remained a challenge, according to authors of the first international consensus on fasting terminology, published on July 25 in Cell Metabolism. The clinical terminology “has remained heterogeneous and often confusing, with similar terms being used to define different fasting regimens ... reflecting the manifold contexts in which fasting is practiced.”
Indeed, dry fasting was among the most discussed terms by the consensus panel and went through several rounds before the panelists came to agreement. A few experts were critical of the practice, whereas those familiar with religious fasting traditions, such as during Ramadan, were clear about the importance of including this term in the consensus process.
“The dissent was resolved by the clarification that this form of fasting has historical and geographical extensions and that the present consensus process did not aim at evaluating therapeutic effectiveness or safety for any term defined,” the authors wrote.
The panel concluded that dry fasting is not the same as total or complete fasting because the latter can include water (such as water-only fasting). Their final definition of dry fasting is ‘’a fasting regimen during which a voluntary abstinence from all foods and beverages, including water, is practiced for a certain period of time.’’
Different types of fasting regimens, such as intermittent fasting, may include dry fasting, in which case it is referred to as “intermittent dry fasting.” This is defined in the consensus as intermittent fasting regimens that involve abstaining from food and fluid intake during the fasting interval, which typically lasts 9-20 hours.
Most dry fasts, including religious ones, are maintained for a specific interval and are followed by a refeeding period. These fasts are not starvation, defined as no food or water intake for days.
What the Evidence Says
All that said, dry fasting by any other name remains dry fasting. “Abundant” evidence from animal studies suggests the potential of various types of fasting for disease prevention and treatment in humans, noted the authors of the consensus report, Along with the risks described above, small studies have explored short-term effects in people, all of which have yet to be established by larger and longer-term studies.
In a recent small study, researchers at Baylor College of Medicine, Houston, Texas, reported that dawn-to-dusk dry fasting for 30 days reduced levels of inflammatory cytokines in the 13 participants with a high body mass index. Earlier work by the group showed that dawn-to-dusk dry fasting for 30 days induced “anti-atherosclerotic, anti-inflammatory, and anti-tumorigenic proteome” in peripheral blood mononuclear cells of 14 individuals with metabolic syndrome (The researchers declined to comment for this article.)
Importantly, the health effects can vary among individuals for unknown reasons, found a recent cross-sectional study of fasting blood glucose (FBG) changes in 181 patients with type 2 diabetes during Ramadan intermittent fasting (RIF), which involves dry fasting during daylight hours for 1 month. The researchers classified participants into three groups: reduced average FBG levels (44%), no change in FBG levels (24%), and increased FBG levels (32%). The authors wrote that further studies are needed to identify factors associated with the differences and to identify “those who are great candidates for RIF.”
In contrast to some of the concerns expressed by clinicians, an exploratory study of daytime dry fasting among 34 healthy Baha’i volunteers in Germany concluded that the 19-day regimen “is safe, has no negative effects on hydration, can improve fat metabolism and can cause transient phase shifts of circadian rhythms.” The authors acknowledge that a larger number and more diverse participants are needed to validate the findings and assess the impact on long-term health.
What to Advise Patients
For patients who want to fast as part of their weight loss regimen or to help manage diabetes, clinicians can consider suggesting “alternate ways of eating that might achieve similar goals,” Ms. Bader said. One is intermittent fasting without dry fasting: the 16:8 method (16 hours of fasting, 8 hours of eating) or the 5:2 method (normal eating for 5 days, reduced calorie intake for 2 days), which can support improved insulin sensitivity and metabolic health.
Caloric restriction can also work if the patient maintains a balanced diet that includes all essential nutrients, she said. A low-carbohydrate diet that focuses on limiting carbohydrate intake while increasing consumption of lean proteins and healthy fats has been shown to lower blood sugar levels and improve insulin sensitivity.
Other healthy strategies for patients include the Mediterranean diet, which emphasizes whole grains, fruits, vegetables, nuts, seeds, olive oil, and lean proteins such as fish, or a similar plant-based diet with less animal protein. Ms. Bader advises cultivating mindful eating, which involves paying attention to hunger and fullness cues, making thoughtful food choices, and focusing on being present during meals.
“Each of these dietary strategies offers potential benefits for managing type 2 diabetes and improving overall health,” Ms. Bader said. “I have not had any patients who have tried dry fasting specifically. However, I have encountered scenarios where individuals abstained from food and beverages due to religious practices. In those cases, we focused on ensuring that they maintained proper hydration and balanced nutrition during their eating periods to manage their diabetes effectively and prevent complications.”
Overall, Dr. Adimoolam suggests that clinicians help patients find a weight-loss plan that works best for them based on understanding the calories in the foods they like and don’t like. For fasting regimens, patients can be encouraged to choose one with fluids when possible, as well as intervals of time to fast and eat that work best for their lifestyle.
Ms. Bader, Dr. Bruno, and Dr. Adimoolam report no relevant conflicts.
A version of this article appeared on Medscape.com.
1 in 4 Unresponsive Coma Patients May Retain Some Awareness
“We found that at least 1 in 4 patients who are unresponsive to commands might actually be quite present and highly cognitive,” said study investigator Nicholas D. Schiff, MD, Feil Family Brain & Mind Research Institute and Department of Neurology, Weill Cornell Medicine, Rockefeller University Hospital, New York.
“In other words, if you go to the bedside and carefully examine someone with a severe brain injury and find no evidence of responsiveness, no one has been able to give you an a priori number to say how likely you are to be wrong in thinking this person is actually unaware, not processing language, and not capable of high-level cognitive work. And the answer to that now is at least 1 in 4 times.”
The findings were published online in The New England Journal of Medicine.
Clinical Implications?
Cognitive motor dissociation (CMD) is a condition whereby patients with a severe brain injury who are unresponsive to commands at the bedside show brain activity on functional MRI (fMRI) or electroencephalography (EEG) when presented with selective motor imagery commands, such as “imagine playing tennis,” or “ imagine opening and closing your hand.”
Previous research shows that CMD is present in 10%-20% of people with a disorder of consciousness, a rate similar to that in patients with acute or chronic brain injury.
Understanding that a patient who appears unconscious has signs of cognitive processing could change the way clinicians and family interact with such individuals. Unresponsive patients who are aware may eventually be able to harness emerging communication technologies such as brain-computer interfaces.
In addition, knowing an individual’s CMD status could aid in prognosis. “We know from one study that there’s a four times increased likelihood that patients will be independent in a year in their function if they have cognitive motor dissociation,” said Dr. Schiff.
Unlike most previous studies of CMD, which were conducted at single sites and had relatively small cohorts, this new study included 353 adults with a disorder of consciousness (mean age, 37.9 years; 64% male) at six multinational sites.
Participants were recruited using a variety of methods, including consecutive enrollment of critically ill patients in the intensive care unit and enrollment of those with chronic illness or injury who were in the postacute phase of brain injury.
Response to Commands
Study participants were at different stages of recovery from an acute brain injury that had occurred an average of 8 months before the study started.
To determine the presence or absence of an observable response to commands among participants, trained staff used the Coma Recovery Scale–Revised (CRS-R); scores on this instrument range from 0 to 23, and higher scores indicate better neurobehavioral function.
About 40% of individuals were diagnosed with coma or vegetative state, 29% with minimally conscious state–minus, and 22% with minimally conscious state–plus. In all, 10% had emerged from a minimally conscious state.
Researchers assessed response to timed and repeated commands using fMRI or EEG in participants without an observable response to verbal commands, including those with a behavioral diagnosis of coma, vegetative state, or minimally conscious state–minus, and in participants with an observable response to verbal commands.
Of the 353 study participants, 61% underwent at least one fMRI assessment and 74% at least one EEG assessment. Both fMRI and EEG were performed in 35% of participants.
Dr. Schiff explained the two assessment types provide slightly different information, in that they measuring different types of brain signals. He also noted that although “every medical center in the world” has EEG, many do not have fMRI.
The brain imaging assessments captured brain activity within the motor area of the frontal cortex when tasked with motor imagery.
Of the 241 participants deemed to be in a coma or vegetative state or minimally conscious state–minus on the basis of CRS-R score, 60 (25%) had a response to commands on task-based fMRI, task-based EEG, or both.
The percentage of participants with CMD varied across study sites, from 2% to 45%, but Dr. Schiff said the reason for this is unclear.
The proportion of participants with CMD may have been even higher if all individuals had been assessed with both imaging techniques, he said.
Higher Rate of Awareness Than in Previous Research
The investigators noted that the percentage of participants with CMD in their study was up to 10 percentage points higher than in previous studies. This may be due to the multimodal approach that classified participants undergoing assessment with both fMRI and EEG on the basis of responses on either technique, they said.
The median age was lower among participants with CMD than those without CMD (30.5 years vs 45.3 years).
Compared with participants without CMD, a higher percentage of those with such dissociation had brain trauma as an etiologic factor (65% vs 38%) and a diagnosis of minimally conscious state–minus on the CRS-R (53% vs 38%).
Among people with CMD, 18% were assessed with fMRI only, 22% with EEG only, and 60% with both fMRI and EEG.
Dr. Schiff noted that the use of both fMRI and EEG appears to be more sensitive in detecting brain activity during tasks compared with use of one of these techniques alone.
Of the 112 participants with a diagnosis of minimally conscious state–plus or who had emerged from the minimally conscious state, 38% had a response to commands on task-based fMRI, task-based EEG, or both. Among these participants, 23% were assessed with fMRI only, 19% with EEG only, and 58% with both fMRI and EEG.
Research shows “it’s very clear that people with severe brain injury continue to get better over time,” noted Dr. Schiff. “Every month and week matters, and so it probably is the case that a lot of these patients are picking up the level of recovery, and the later we go out to measure them, the more likely we are to find people who are CMD than not.”
These new results should prompt further study to explore whether detection of CMD can lead to improved outcomes, the investigators noted. “In addition, the standardization, validation, and simplification of task-based fMRI and EEG methods that are used to detect cognitive motor dissociation are needed to prompt widespread clinical integration of these techniques and investigation of the bioethical implications of the findings.”
All study participants with chronic brain injury had survived their initial illness or injury and had access to a research facility with advanced fMRI and EEG capabilities. “This survival bias may reflect greater cognitive reserve and resilience over time among the participants. As such, the results of our study may not be generalizable to the overall population of patients with cognitive motor dissociation,” the investigators wrote.
Another study limitation was that participating sites used heterogeneous strategies to acquire, analyze, and interpret data, which led to differences in the number, type, and ordering of the cognitive tasks assessed on fMRI and EEG.
“These differences, along with variations in recruitment strategies and participant characteristics, may have contributed to the unequal percentage of participants with cognitive motor dissociation observed at each site. Our findings may therefore not be generalizable across all centers,” the researchers wrote.
Only a few academic medical centers have the specially trained personnel and techniques needed to assess patients for CMD — which, the researchers noted, limits the feasibility of performing these assessments in general practice.
Challenging Research
Commenting on the research, Aarti Sarwal, MD, professor of neurology and section chief, Neurocritical Care, Virginia Commonwealth University, Richmond, Virginia, noted that this was a “very challenging” study to perform, given that only a few academic centers are equipped to perform both fMRI and quantitative EEG analysis.
“In general, finding patients this far out, who have access to clinical, radiological, and electrophysiological testing and were provided good care enough to receive these, is a mammoth task in itself.”
Dr. Sarwal said the study builds on efforts of the Curing Coma campaign , a clinical, scientific, and public health effort of the Neurocritical Care Society to tackle the concept of coma as a treatable medical entity.
“It continues to highlight the challenges of prognostication in acute brain injured patients by showing a higher presence of cognitive function than previously perceived,” she said.
Dr. Sarwal believes that the study’s largest impact is underscoring the need for more research into understanding the degree and quality of cognitive processing in patients with a disorder of consciousness. But it also underlines the need for a “healthy debate” on the cost/benefit analysis of pursuing such research, given the limited number of patients with access to resources.
“This debate needs to include the caregivers and families outside the traditional realms of stakeholders overseeing the science.”
Although communication with comatose patients is still “a ways away,” this research is “a step in the right direction,” said Dr. Sarwal.
The study was funded by the James S. McDonnell Foundation and others. Dr. Schiff and Dr. Sarwal report no relevant financial disclosures.
A version of this article first appeared on Medscape.com.
“We found that at least 1 in 4 patients who are unresponsive to commands might actually be quite present and highly cognitive,” said study investigator Nicholas D. Schiff, MD, Feil Family Brain & Mind Research Institute and Department of Neurology, Weill Cornell Medicine, Rockefeller University Hospital, New York.
“In other words, if you go to the bedside and carefully examine someone with a severe brain injury and find no evidence of responsiveness, no one has been able to give you an a priori number to say how likely you are to be wrong in thinking this person is actually unaware, not processing language, and not capable of high-level cognitive work. And the answer to that now is at least 1 in 4 times.”
The findings were published online in The New England Journal of Medicine.
Clinical Implications?
Cognitive motor dissociation (CMD) is a condition whereby patients with a severe brain injury who are unresponsive to commands at the bedside show brain activity on functional MRI (fMRI) or electroencephalography (EEG) when presented with selective motor imagery commands, such as “imagine playing tennis,” or “ imagine opening and closing your hand.”
Previous research shows that CMD is present in 10%-20% of people with a disorder of consciousness, a rate similar to that in patients with acute or chronic brain injury.
Understanding that a patient who appears unconscious has signs of cognitive processing could change the way clinicians and family interact with such individuals. Unresponsive patients who are aware may eventually be able to harness emerging communication technologies such as brain-computer interfaces.
In addition, knowing an individual’s CMD status could aid in prognosis. “We know from one study that there’s a four times increased likelihood that patients will be independent in a year in their function if they have cognitive motor dissociation,” said Dr. Schiff.
Unlike most previous studies of CMD, which were conducted at single sites and had relatively small cohorts, this new study included 353 adults with a disorder of consciousness (mean age, 37.9 years; 64% male) at six multinational sites.
Participants were recruited using a variety of methods, including consecutive enrollment of critically ill patients in the intensive care unit and enrollment of those with chronic illness or injury who were in the postacute phase of brain injury.
Response to Commands
Study participants were at different stages of recovery from an acute brain injury that had occurred an average of 8 months before the study started.
To determine the presence or absence of an observable response to commands among participants, trained staff used the Coma Recovery Scale–Revised (CRS-R); scores on this instrument range from 0 to 23, and higher scores indicate better neurobehavioral function.
About 40% of individuals were diagnosed with coma or vegetative state, 29% with minimally conscious state–minus, and 22% with minimally conscious state–plus. In all, 10% had emerged from a minimally conscious state.
Researchers assessed response to timed and repeated commands using fMRI or EEG in participants without an observable response to verbal commands, including those with a behavioral diagnosis of coma, vegetative state, or minimally conscious state–minus, and in participants with an observable response to verbal commands.
Of the 353 study participants, 61% underwent at least one fMRI assessment and 74% at least one EEG assessment. Both fMRI and EEG were performed in 35% of participants.
Dr. Schiff explained the two assessment types provide slightly different information, in that they measuring different types of brain signals. He also noted that although “every medical center in the world” has EEG, many do not have fMRI.
The brain imaging assessments captured brain activity within the motor area of the frontal cortex when tasked with motor imagery.
Of the 241 participants deemed to be in a coma or vegetative state or minimally conscious state–minus on the basis of CRS-R score, 60 (25%) had a response to commands on task-based fMRI, task-based EEG, or both.
The percentage of participants with CMD varied across study sites, from 2% to 45%, but Dr. Schiff said the reason for this is unclear.
The proportion of participants with CMD may have been even higher if all individuals had been assessed with both imaging techniques, he said.
Higher Rate of Awareness Than in Previous Research
The investigators noted that the percentage of participants with CMD in their study was up to 10 percentage points higher than in previous studies. This may be due to the multimodal approach that classified participants undergoing assessment with both fMRI and EEG on the basis of responses on either technique, they said.
The median age was lower among participants with CMD than those without CMD (30.5 years vs 45.3 years).
Compared with participants without CMD, a higher percentage of those with such dissociation had brain trauma as an etiologic factor (65% vs 38%) and a diagnosis of minimally conscious state–minus on the CRS-R (53% vs 38%).
Among people with CMD, 18% were assessed with fMRI only, 22% with EEG only, and 60% with both fMRI and EEG.
Dr. Schiff noted that the use of both fMRI and EEG appears to be more sensitive in detecting brain activity during tasks compared with use of one of these techniques alone.
Of the 112 participants with a diagnosis of minimally conscious state–plus or who had emerged from the minimally conscious state, 38% had a response to commands on task-based fMRI, task-based EEG, or both. Among these participants, 23% were assessed with fMRI only, 19% with EEG only, and 58% with both fMRI and EEG.
Research shows “it’s very clear that people with severe brain injury continue to get better over time,” noted Dr. Schiff. “Every month and week matters, and so it probably is the case that a lot of these patients are picking up the level of recovery, and the later we go out to measure them, the more likely we are to find people who are CMD than not.”
These new results should prompt further study to explore whether detection of CMD can lead to improved outcomes, the investigators noted. “In addition, the standardization, validation, and simplification of task-based fMRI and EEG methods that are used to detect cognitive motor dissociation are needed to prompt widespread clinical integration of these techniques and investigation of the bioethical implications of the findings.”
All study participants with chronic brain injury had survived their initial illness or injury and had access to a research facility with advanced fMRI and EEG capabilities. “This survival bias may reflect greater cognitive reserve and resilience over time among the participants. As such, the results of our study may not be generalizable to the overall population of patients with cognitive motor dissociation,” the investigators wrote.
Another study limitation was that participating sites used heterogeneous strategies to acquire, analyze, and interpret data, which led to differences in the number, type, and ordering of the cognitive tasks assessed on fMRI and EEG.
“These differences, along with variations in recruitment strategies and participant characteristics, may have contributed to the unequal percentage of participants with cognitive motor dissociation observed at each site. Our findings may therefore not be generalizable across all centers,” the researchers wrote.
Only a few academic medical centers have the specially trained personnel and techniques needed to assess patients for CMD — which, the researchers noted, limits the feasibility of performing these assessments in general practice.
Challenging Research
Commenting on the research, Aarti Sarwal, MD, professor of neurology and section chief, Neurocritical Care, Virginia Commonwealth University, Richmond, Virginia, noted that this was a “very challenging” study to perform, given that only a few academic centers are equipped to perform both fMRI and quantitative EEG analysis.
“In general, finding patients this far out, who have access to clinical, radiological, and electrophysiological testing and were provided good care enough to receive these, is a mammoth task in itself.”
Dr. Sarwal said the study builds on efforts of the Curing Coma campaign , a clinical, scientific, and public health effort of the Neurocritical Care Society to tackle the concept of coma as a treatable medical entity.
“It continues to highlight the challenges of prognostication in acute brain injured patients by showing a higher presence of cognitive function than previously perceived,” she said.
Dr. Sarwal believes that the study’s largest impact is underscoring the need for more research into understanding the degree and quality of cognitive processing in patients with a disorder of consciousness. But it also underlines the need for a “healthy debate” on the cost/benefit analysis of pursuing such research, given the limited number of patients with access to resources.
“This debate needs to include the caregivers and families outside the traditional realms of stakeholders overseeing the science.”
Although communication with comatose patients is still “a ways away,” this research is “a step in the right direction,” said Dr. Sarwal.
The study was funded by the James S. McDonnell Foundation and others. Dr. Schiff and Dr. Sarwal report no relevant financial disclosures.
A version of this article first appeared on Medscape.com.
“We found that at least 1 in 4 patients who are unresponsive to commands might actually be quite present and highly cognitive,” said study investigator Nicholas D. Schiff, MD, Feil Family Brain & Mind Research Institute and Department of Neurology, Weill Cornell Medicine, Rockefeller University Hospital, New York.
“In other words, if you go to the bedside and carefully examine someone with a severe brain injury and find no evidence of responsiveness, no one has been able to give you an a priori number to say how likely you are to be wrong in thinking this person is actually unaware, not processing language, and not capable of high-level cognitive work. And the answer to that now is at least 1 in 4 times.”
The findings were published online in The New England Journal of Medicine.
Clinical Implications?
Cognitive motor dissociation (CMD) is a condition whereby patients with a severe brain injury who are unresponsive to commands at the bedside show brain activity on functional MRI (fMRI) or electroencephalography (EEG) when presented with selective motor imagery commands, such as “imagine playing tennis,” or “ imagine opening and closing your hand.”
Previous research shows that CMD is present in 10%-20% of people with a disorder of consciousness, a rate similar to that in patients with acute or chronic brain injury.
Understanding that a patient who appears unconscious has signs of cognitive processing could change the way clinicians and family interact with such individuals. Unresponsive patients who are aware may eventually be able to harness emerging communication technologies such as brain-computer interfaces.
In addition, knowing an individual’s CMD status could aid in prognosis. “We know from one study that there’s a four times increased likelihood that patients will be independent in a year in their function if they have cognitive motor dissociation,” said Dr. Schiff.
Unlike most previous studies of CMD, which were conducted at single sites and had relatively small cohorts, this new study included 353 adults with a disorder of consciousness (mean age, 37.9 years; 64% male) at six multinational sites.
Participants were recruited using a variety of methods, including consecutive enrollment of critically ill patients in the intensive care unit and enrollment of those with chronic illness or injury who were in the postacute phase of brain injury.
Response to Commands
Study participants were at different stages of recovery from an acute brain injury that had occurred an average of 8 months before the study started.
To determine the presence or absence of an observable response to commands among participants, trained staff used the Coma Recovery Scale–Revised (CRS-R); scores on this instrument range from 0 to 23, and higher scores indicate better neurobehavioral function.
About 40% of individuals were diagnosed with coma or vegetative state, 29% with minimally conscious state–minus, and 22% with minimally conscious state–plus. In all, 10% had emerged from a minimally conscious state.
Researchers assessed response to timed and repeated commands using fMRI or EEG in participants without an observable response to verbal commands, including those with a behavioral diagnosis of coma, vegetative state, or minimally conscious state–minus, and in participants with an observable response to verbal commands.
Of the 353 study participants, 61% underwent at least one fMRI assessment and 74% at least one EEG assessment. Both fMRI and EEG were performed in 35% of participants.
Dr. Schiff explained the two assessment types provide slightly different information, in that they measuring different types of brain signals. He also noted that although “every medical center in the world” has EEG, many do not have fMRI.
The brain imaging assessments captured brain activity within the motor area of the frontal cortex when tasked with motor imagery.
Of the 241 participants deemed to be in a coma or vegetative state or minimally conscious state–minus on the basis of CRS-R score, 60 (25%) had a response to commands on task-based fMRI, task-based EEG, or both.
The percentage of participants with CMD varied across study sites, from 2% to 45%, but Dr. Schiff said the reason for this is unclear.
The proportion of participants with CMD may have been even higher if all individuals had been assessed with both imaging techniques, he said.
Higher Rate of Awareness Than in Previous Research
The investigators noted that the percentage of participants with CMD in their study was up to 10 percentage points higher than in previous studies. This may be due to the multimodal approach that classified participants undergoing assessment with both fMRI and EEG on the basis of responses on either technique, they said.
The median age was lower among participants with CMD than those without CMD (30.5 years vs 45.3 years).
Compared with participants without CMD, a higher percentage of those with such dissociation had brain trauma as an etiologic factor (65% vs 38%) and a diagnosis of minimally conscious state–minus on the CRS-R (53% vs 38%).
Among people with CMD, 18% were assessed with fMRI only, 22% with EEG only, and 60% with both fMRI and EEG.
Dr. Schiff noted that the use of both fMRI and EEG appears to be more sensitive in detecting brain activity during tasks compared with use of one of these techniques alone.
Of the 112 participants with a diagnosis of minimally conscious state–plus or who had emerged from the minimally conscious state, 38% had a response to commands on task-based fMRI, task-based EEG, or both. Among these participants, 23% were assessed with fMRI only, 19% with EEG only, and 58% with both fMRI and EEG.
Research shows “it’s very clear that people with severe brain injury continue to get better over time,” noted Dr. Schiff. “Every month and week matters, and so it probably is the case that a lot of these patients are picking up the level of recovery, and the later we go out to measure them, the more likely we are to find people who are CMD than not.”
These new results should prompt further study to explore whether detection of CMD can lead to improved outcomes, the investigators noted. “In addition, the standardization, validation, and simplification of task-based fMRI and EEG methods that are used to detect cognitive motor dissociation are needed to prompt widespread clinical integration of these techniques and investigation of the bioethical implications of the findings.”
All study participants with chronic brain injury had survived their initial illness or injury and had access to a research facility with advanced fMRI and EEG capabilities. “This survival bias may reflect greater cognitive reserve and resilience over time among the participants. As such, the results of our study may not be generalizable to the overall population of patients with cognitive motor dissociation,” the investigators wrote.
Another study limitation was that participating sites used heterogeneous strategies to acquire, analyze, and interpret data, which led to differences in the number, type, and ordering of the cognitive tasks assessed on fMRI and EEG.
“These differences, along with variations in recruitment strategies and participant characteristics, may have contributed to the unequal percentage of participants with cognitive motor dissociation observed at each site. Our findings may therefore not be generalizable across all centers,” the researchers wrote.
Only a few academic medical centers have the specially trained personnel and techniques needed to assess patients for CMD — which, the researchers noted, limits the feasibility of performing these assessments in general practice.
Challenging Research
Commenting on the research, Aarti Sarwal, MD, professor of neurology and section chief, Neurocritical Care, Virginia Commonwealth University, Richmond, Virginia, noted that this was a “very challenging” study to perform, given that only a few academic centers are equipped to perform both fMRI and quantitative EEG analysis.
“In general, finding patients this far out, who have access to clinical, radiological, and electrophysiological testing and were provided good care enough to receive these, is a mammoth task in itself.”
Dr. Sarwal said the study builds on efforts of the Curing Coma campaign , a clinical, scientific, and public health effort of the Neurocritical Care Society to tackle the concept of coma as a treatable medical entity.
“It continues to highlight the challenges of prognostication in acute brain injured patients by showing a higher presence of cognitive function than previously perceived,” she said.
Dr. Sarwal believes that the study’s largest impact is underscoring the need for more research into understanding the degree and quality of cognitive processing in patients with a disorder of consciousness. But it also underlines the need for a “healthy debate” on the cost/benefit analysis of pursuing such research, given the limited number of patients with access to resources.
“This debate needs to include the caregivers and families outside the traditional realms of stakeholders overseeing the science.”
Although communication with comatose patients is still “a ways away,” this research is “a step in the right direction,” said Dr. Sarwal.
The study was funded by the James S. McDonnell Foundation and others. Dr. Schiff and Dr. Sarwal report no relevant financial disclosures.
A version of this article first appeared on Medscape.com.
FROM THE NEW ENGLAND JOURNAL OF MEDICINE
Chronic Back Pain in Patients With Psoriasis, Uveitis, or Colitis: How Often Is It Axial Spondyloarthritis?
TOPLINE:
Patients with psoriasis, uveitis, or colitis who present with undiagnosed chronic back pain should be referred to a rheumatologist for the assessment of axial spondyloarthritis (axSpA), with MRI being a more accurate diagnostic method than clinical features.
METHODOLOGY:
- Researchers assessed the prevalence of axSpA according to the extra-articular presentation and human leukocyte antigen B27 (HLA-B27) status in two Canadian cohorts (SASPIC 1 and 2).
- Overall, 363 adult patients aged ≤ 45 years with psoriasis, uveitis, or colitis who presented with chronic undiagnosed back and/or buttock pain lasting 3 months or more were included.
- Participants were referred to rheumatologists with expertise in axSpA for structured diagnostic evaluations, including history, physical exam, levels of C-reactive protein, HLA-B27 status, and imaging studies.
- An MRI of the sacroiliac joints was conducted in all patients in the SASPIC-2 cohort and in 62.3% of those in the SASPIC-1 cohort.
- The primary outcome was the proportion of patients diagnosed with axSpA after final global evaluation, and the secondary outcome was the impact of MRI on diagnosis and classification.
TAKEAWAY:
- AxSpA diagnoses were made in 46.7% with psoriasis, 61.6% with uveitis, and 46.8% with colitis in the SASPIC-1 cohort and in 23.5%, 57.9%, and 23.3%, respectively, in the SASPIC-2 cohort.
- Being positive for HLA-B27 was linked to the presence of axSpA in 56%-88% of those in both the cohorts.
- Musculoskeletal clinical features were not helpful in differentiating between patients with and without axSpA.
- In both the cohorts, the MRI of the sacroiliac joints was indicative of axSpA in a significantly greater number of patients with psoriasis, uveitis, or colitis who were diagnosed with axSpA than in those not diagnosed with axSpA (P < .05 for all).
IN PRACTICE:
“Our data supports the benefit of recent referral recommendations that advocate referral to a rheumatologist of patients with chronic back pain and extra-articular features related to axSpA,” the authors wrote.
SOURCE:
The study was led by Walter P. Maksymowych, MB ChB, University of Alberta, Edmonton, Alberta, Canada. It was published online in Arthritis & Rheumatology.
LIMITATIONS:
MRI readers had to rely on their own expertise to decide if an MRI was indeed positive and thus indicative of axSpA. This study included only patients with undiagnosed back pain, and a longer follow-up duration could have led to a higher number of patients being diagnosed with axial inflammation. In SASPIC-1, local rheumatologists conducted MRI evaluations of the spinal lesions only when necessary, while in SASPIC-2, MRI of only the sacroiliac joints was required.
DISCLOSURES:
SASPIC-1 was supported by AbbVie Canada and Janssen Canada, and SASPIC-2 was supported by AbbVie Canada. The authors disclosed receiving grants, consulting fees, speaking fees, and/or honoraria and having other ties with AbbVie and several other pharmaceutical companies.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
Patients with psoriasis, uveitis, or colitis who present with undiagnosed chronic back pain should be referred to a rheumatologist for the assessment of axial spondyloarthritis (axSpA), with MRI being a more accurate diagnostic method than clinical features.
METHODOLOGY:
- Researchers assessed the prevalence of axSpA according to the extra-articular presentation and human leukocyte antigen B27 (HLA-B27) status in two Canadian cohorts (SASPIC 1 and 2).
- Overall, 363 adult patients aged ≤ 45 years with psoriasis, uveitis, or colitis who presented with chronic undiagnosed back and/or buttock pain lasting 3 months or more were included.
- Participants were referred to rheumatologists with expertise in axSpA for structured diagnostic evaluations, including history, physical exam, levels of C-reactive protein, HLA-B27 status, and imaging studies.
- An MRI of the sacroiliac joints was conducted in all patients in the SASPIC-2 cohort and in 62.3% of those in the SASPIC-1 cohort.
- The primary outcome was the proportion of patients diagnosed with axSpA after final global evaluation, and the secondary outcome was the impact of MRI on diagnosis and classification.
TAKEAWAY:
- AxSpA diagnoses were made in 46.7% with psoriasis, 61.6% with uveitis, and 46.8% with colitis in the SASPIC-1 cohort and in 23.5%, 57.9%, and 23.3%, respectively, in the SASPIC-2 cohort.
- Being positive for HLA-B27 was linked to the presence of axSpA in 56%-88% of those in both the cohorts.
- Musculoskeletal clinical features were not helpful in differentiating between patients with and without axSpA.
- In both the cohorts, the MRI of the sacroiliac joints was indicative of axSpA in a significantly greater number of patients with psoriasis, uveitis, or colitis who were diagnosed with axSpA than in those not diagnosed with axSpA (P < .05 for all).
IN PRACTICE:
“Our data supports the benefit of recent referral recommendations that advocate referral to a rheumatologist of patients with chronic back pain and extra-articular features related to axSpA,” the authors wrote.
SOURCE:
The study was led by Walter P. Maksymowych, MB ChB, University of Alberta, Edmonton, Alberta, Canada. It was published online in Arthritis & Rheumatology.
LIMITATIONS:
MRI readers had to rely on their own expertise to decide if an MRI was indeed positive and thus indicative of axSpA. This study included only patients with undiagnosed back pain, and a longer follow-up duration could have led to a higher number of patients being diagnosed with axial inflammation. In SASPIC-1, local rheumatologists conducted MRI evaluations of the spinal lesions only when necessary, while in SASPIC-2, MRI of only the sacroiliac joints was required.
DISCLOSURES:
SASPIC-1 was supported by AbbVie Canada and Janssen Canada, and SASPIC-2 was supported by AbbVie Canada. The authors disclosed receiving grants, consulting fees, speaking fees, and/or honoraria and having other ties with AbbVie and several other pharmaceutical companies.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
Patients with psoriasis, uveitis, or colitis who present with undiagnosed chronic back pain should be referred to a rheumatologist for the assessment of axial spondyloarthritis (axSpA), with MRI being a more accurate diagnostic method than clinical features.
METHODOLOGY:
- Researchers assessed the prevalence of axSpA according to the extra-articular presentation and human leukocyte antigen B27 (HLA-B27) status in two Canadian cohorts (SASPIC 1 and 2).
- Overall, 363 adult patients aged ≤ 45 years with psoriasis, uveitis, or colitis who presented with chronic undiagnosed back and/or buttock pain lasting 3 months or more were included.
- Participants were referred to rheumatologists with expertise in axSpA for structured diagnostic evaluations, including history, physical exam, levels of C-reactive protein, HLA-B27 status, and imaging studies.
- An MRI of the sacroiliac joints was conducted in all patients in the SASPIC-2 cohort and in 62.3% of those in the SASPIC-1 cohort.
- The primary outcome was the proportion of patients diagnosed with axSpA after final global evaluation, and the secondary outcome was the impact of MRI on diagnosis and classification.
TAKEAWAY:
- AxSpA diagnoses were made in 46.7% with psoriasis, 61.6% with uveitis, and 46.8% with colitis in the SASPIC-1 cohort and in 23.5%, 57.9%, and 23.3%, respectively, in the SASPIC-2 cohort.
- Being positive for HLA-B27 was linked to the presence of axSpA in 56%-88% of those in both the cohorts.
- Musculoskeletal clinical features were not helpful in differentiating between patients with and without axSpA.
- In both the cohorts, the MRI of the sacroiliac joints was indicative of axSpA in a significantly greater number of patients with psoriasis, uveitis, or colitis who were diagnosed with axSpA than in those not diagnosed with axSpA (P < .05 for all).
IN PRACTICE:
“Our data supports the benefit of recent referral recommendations that advocate referral to a rheumatologist of patients with chronic back pain and extra-articular features related to axSpA,” the authors wrote.
SOURCE:
The study was led by Walter P. Maksymowych, MB ChB, University of Alberta, Edmonton, Alberta, Canada. It was published online in Arthritis & Rheumatology.
LIMITATIONS:
MRI readers had to rely on their own expertise to decide if an MRI was indeed positive and thus indicative of axSpA. This study included only patients with undiagnosed back pain, and a longer follow-up duration could have led to a higher number of patients being diagnosed with axial inflammation. In SASPIC-1, local rheumatologists conducted MRI evaluations of the spinal lesions only when necessary, while in SASPIC-2, MRI of only the sacroiliac joints was required.
DISCLOSURES:
SASPIC-1 was supported by AbbVie Canada and Janssen Canada, and SASPIC-2 was supported by AbbVie Canada. The authors disclosed receiving grants, consulting fees, speaking fees, and/or honoraria and having other ties with AbbVie and several other pharmaceutical companies.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
Dementia Deemed Highly Preventable: Here’s How
A new report on the preventability of dementia is both exciting and paradigm-shifting. The new study, published in The Lancet by the Lancet Commission on Dementia, estimates that .
This is paradigm-shifting because dementia is often perceived as an inevitable consequence of the aging process, with a major genetic component. But this study suggests that modifying these risk factors can benefit everyone, irrespective of genetic risk, and that it’s important to have a life-course approach. It’s never too early or too late to start to modify these factors.
We’ve known for a long time that many chronic diseases are highly preventable and modifiable. Some that come to mind are type 2 diabetes, coronary heart disease, and even certain forms of cancer. Modifiable risk factors include cigarette smoking, diet, physical activity, and maintaining a healthy weight. This study suggests that many of the same risk factors and more are relevant to reducing risk for dementia.
Let’s go through the risk factors, many of which are behavioral. These risk factors include lifestyle factors such as lack of physical activity, cigarette smoking, excessive alcohol consumption, and obesity. The cardiovascular or vascular-specific risk factors include not only those behavioral factors but also hypertension, high LDL cholesterol, and diabetes. Cognitive engagement–specific risk factors include social isolation, which is a major risk factor for dementia, as well as untreated hearing or vision loss, which can exacerbate social isolation and depression, and low educational attainment, which can be related to less cognitive engagement.
They also mention traumatic brain injury from an accident or contact sports without head protection as a risk factor, and the environmental risk factor of air pollution or poor air quality.
Two of these risk factors are new since the previous report in 2020: elevated LDL cholesterol and untreated vision loss, both of which are quite treatable. Overall, these findings suggest that a lot can be done to lower dementia risk, but it requires individual behavior modifications as well as a comprehensive approach with involvement of the healthcare system for improved screening, access, and public policy to reduce air pollution.
Some of these risk factors are more relevant to women, especially the social isolation that is so common later in life in women. In the United States, close to two out of three patients with dementia are women.
So, informing our patients about these risk factors and what can be done in terms of behavior modification, increased screening, and treatment for these conditions can go a long way in helping our patients reduce their risk for dementia.
Dr. Manson is professor of medicine and the Michael and Lee Bell Professor of Women’s Health, Harvard Medical School, chief, Division of Preventive Medicine, Brigham and Women’s Hospital, Boston, and past president, North American Menopause Society, 2011-2012. She disclosed receiving study pill donation and infrastructure support from Mars Symbioscience (for the COSMOS trial).
A version of this article appeared on Medscape.com.
A new report on the preventability of dementia is both exciting and paradigm-shifting. The new study, published in The Lancet by the Lancet Commission on Dementia, estimates that .
This is paradigm-shifting because dementia is often perceived as an inevitable consequence of the aging process, with a major genetic component. But this study suggests that modifying these risk factors can benefit everyone, irrespective of genetic risk, and that it’s important to have a life-course approach. It’s never too early or too late to start to modify these factors.
We’ve known for a long time that many chronic diseases are highly preventable and modifiable. Some that come to mind are type 2 diabetes, coronary heart disease, and even certain forms of cancer. Modifiable risk factors include cigarette smoking, diet, physical activity, and maintaining a healthy weight. This study suggests that many of the same risk factors and more are relevant to reducing risk for dementia.
Let’s go through the risk factors, many of which are behavioral. These risk factors include lifestyle factors such as lack of physical activity, cigarette smoking, excessive alcohol consumption, and obesity. The cardiovascular or vascular-specific risk factors include not only those behavioral factors but also hypertension, high LDL cholesterol, and diabetes. Cognitive engagement–specific risk factors include social isolation, which is a major risk factor for dementia, as well as untreated hearing or vision loss, which can exacerbate social isolation and depression, and low educational attainment, which can be related to less cognitive engagement.
They also mention traumatic brain injury from an accident or contact sports without head protection as a risk factor, and the environmental risk factor of air pollution or poor air quality.
Two of these risk factors are new since the previous report in 2020: elevated LDL cholesterol and untreated vision loss, both of which are quite treatable. Overall, these findings suggest that a lot can be done to lower dementia risk, but it requires individual behavior modifications as well as a comprehensive approach with involvement of the healthcare system for improved screening, access, and public policy to reduce air pollution.
Some of these risk factors are more relevant to women, especially the social isolation that is so common later in life in women. In the United States, close to two out of three patients with dementia are women.
So, informing our patients about these risk factors and what can be done in terms of behavior modification, increased screening, and treatment for these conditions can go a long way in helping our patients reduce their risk for dementia.
Dr. Manson is professor of medicine and the Michael and Lee Bell Professor of Women’s Health, Harvard Medical School, chief, Division of Preventive Medicine, Brigham and Women’s Hospital, Boston, and past president, North American Menopause Society, 2011-2012. She disclosed receiving study pill donation and infrastructure support from Mars Symbioscience (for the COSMOS trial).
A version of this article appeared on Medscape.com.
A new report on the preventability of dementia is both exciting and paradigm-shifting. The new study, published in The Lancet by the Lancet Commission on Dementia, estimates that .
This is paradigm-shifting because dementia is often perceived as an inevitable consequence of the aging process, with a major genetic component. But this study suggests that modifying these risk factors can benefit everyone, irrespective of genetic risk, and that it’s important to have a life-course approach. It’s never too early or too late to start to modify these factors.
We’ve known for a long time that many chronic diseases are highly preventable and modifiable. Some that come to mind are type 2 diabetes, coronary heart disease, and even certain forms of cancer. Modifiable risk factors include cigarette smoking, diet, physical activity, and maintaining a healthy weight. This study suggests that many of the same risk factors and more are relevant to reducing risk for dementia.
Let’s go through the risk factors, many of which are behavioral. These risk factors include lifestyle factors such as lack of physical activity, cigarette smoking, excessive alcohol consumption, and obesity. The cardiovascular or vascular-specific risk factors include not only those behavioral factors but also hypertension, high LDL cholesterol, and diabetes. Cognitive engagement–specific risk factors include social isolation, which is a major risk factor for dementia, as well as untreated hearing or vision loss, which can exacerbate social isolation and depression, and low educational attainment, which can be related to less cognitive engagement.
They also mention traumatic brain injury from an accident or contact sports without head protection as a risk factor, and the environmental risk factor of air pollution or poor air quality.
Two of these risk factors are new since the previous report in 2020: elevated LDL cholesterol and untreated vision loss, both of which are quite treatable. Overall, these findings suggest that a lot can be done to lower dementia risk, but it requires individual behavior modifications as well as a comprehensive approach with involvement of the healthcare system for improved screening, access, and public policy to reduce air pollution.
Some of these risk factors are more relevant to women, especially the social isolation that is so common later in life in women. In the United States, close to two out of three patients with dementia are women.
So, informing our patients about these risk factors and what can be done in terms of behavior modification, increased screening, and treatment for these conditions can go a long way in helping our patients reduce their risk for dementia.
Dr. Manson is professor of medicine and the Michael and Lee Bell Professor of Women’s Health, Harvard Medical School, chief, Division of Preventive Medicine, Brigham and Women’s Hospital, Boston, and past president, North American Menopause Society, 2011-2012. She disclosed receiving study pill donation and infrastructure support from Mars Symbioscience (for the COSMOS trial).
A version of this article appeared on Medscape.com.
FTC Interim Report on Pharmacy Middlemen Is First Step of Many Needed in Addressing Drug Costs, Access
Rising consolidation among pharmacy benefit managers (PBMs) allows the companies to profit at the expense of patients and independent pharmacists. That’s the conclusion of a recent Federal Trade Commission (FTC) report on interim findings from the agency’s ongoing investigation of PBMs.
Lawmakers are increasingly scrutinizing the industry amid growing concern among physicians and consumers about how PBMs exploit their market dominance. The top six PBMs managed 94% of US drug claims in 2023, with the majority handled by the industry’s three giants: CVS Caremark, Cigna’s Express Scripts, and United Healthcare’s OptumRx.
PBMs manage prescription drug benefits for health insurers, Medicare Part D drug plans, and large employers. They act as middlemen between health insurers and pharmacies, developing formularies of covered drugs and promising savings from the discounts and rebates they negotiate with drugmakers.
The FTC’s interim report found that the giant PBMs often exercise significant control over what drugs are available and at what price and which pharmacies patients can use to access their prescribed medications. Consumers suffer as a result, the report concluded.
Madelaine A. Feldman, MD, vice president for advocacy and government affairs for the Coalition of State Rheumatology Organizations, shared her perspective on the FTC report in an email Q&A with this news organization. She is affiliated with The Rheumatology Group, based in Metairie, Louisiana.
Dr. Feldman has long tracked the PBM industry and appeared as a witness before influential government panels, including the House Energy and Commerce Committee. She has highlighted for lawmakers the challenges physicians face in helping patients get needed medicines.
For example, she shared cases of PBMs steering patients toward the more expensive of three widely used rheumatoid arthritis medicines that have a similar mechanism of action, the Janus kinase (JAK) inhibitors, Dr. Feldman said.
One of the drugs cost roughly half of the other two — about $30,000 per year vs $65,000-$70,000. Yet only the two expensive drugs were included in the PBM formulary. As a result, the cheapest drug holds only a sliver of market share; the remainder is dominated by the two expensive products, she told the House Oversight and Accountability Committee in 2021.
This Q&A has been edited for length and clarity.
What would you want federal and state policymakers to do in response to the FTC’s report?
I think Congress needs to clearly delineate the differences between anticompetitive pharmacy issues, drug pricing issues, and their effect on formulary construction issues.
Lawmakers should demand more transparency and consider legislation that would remove perverse incentives that prompt PBMs to choose higher priced drugs for their formularies.
That may require other regulatory or legislative actions to ensure lower prices (not higher kickbacks) are incentivized. Ultimately, in order to gain true competition within the health insurance business, these oligopolies of multiple businesses need to be broken up. Anything less seems to be nibbling around the edges and allows the Big Three to continue their “whack-a mole” in circumventing piecemeal regulatory and legislative policies.
You’ve followed PBM practices closely for many years. Was there anything in this interim FTC report that surprised you?
Though not surprised, I am glad that it was released because it had been a year in investigation and there were many requests for some type of substantive report.
Two things that are missing that I feel are paramount are investigating how the three big PBMs are causing physical harm to patients as a result of the profit component in formulary construction and the profound financial impact of hidden PBM profit centers in self-insured employer health plans.
What we have seen over the years is the result of the perverse incentives for the PBMs to prefer the most profitable medications on their formularies.
They use utilization management tools such as step therapy, nonmedical switching, and exclusions to maintain their formularies’ profitability. These tools have been shown to delay and deny the proper care of patients, resulting in not just monetary but physical harm as well.
I would think the physical harm done to patients in manipulating the formularies should be addressed in this report as well and, in fact, may be the most important aspect of consumer protection of this issue.
In terms of the FTC’s mission to not “unduly burden” legitimate business, I would like to see the sector of self-insured employers addressed.
The report details how PBMs steer prescriptions to their affiliated pharmacies. The FTC says that can push smaller pharmacies out of the market, ultimately leading to higher costs and lower quality services for people. What’s your perspective?
Having more community pharmacies is better than having less. We are seeing more “pharmacy deserts” in rural areas as a result of many community pharmacies having to close.
The FTC voted 4-1 to allow staff to issue the interim report, with Commissioner Melissa Holyoak voting no. And some FTC commissioners seem divided on the usefulness of the report. Why?
Commissioner Holyoak states the “the Report leaves us without a better understanding of the competition concerns surrounding PBMs or how consumers are impacted by PBM practices.”
I do agree with her that the harm to patients’ medical status was not even addressed as far as I could tell in this report. There are multiple news articles and reports on the harms inflicted upon patients by the UM tools that drive the construction of ever changing formularies, all based on contracting with manufacturers that result in the highest profit for the PBM.
Holyoak also states, “Among other critical conclusions, the Report does not address the seemingly contradictory conclusions in the 2005 Report that PBMs, including vertically owned PBMs, generated cost savings for consumers.”
That may be true, but in 2005, the rise of PBMs was just beginning and the huge vertical and horizontal integration had yet to begin. Also, 2005 was still in the beginning of the biologic drug deluge, which did create competition to get on the formulary. Since then, PBMs have done nothing to control the rise in prices but instead, apparently have used the competition to get higher price concessions from manufacturers based on a percentage of the list price to line their pockets.
Commissioner Ferguson agreed with releasing the report but he had many issues with this report including the lack of PBM response.
I do agree with him that the FTC should have used some type of “force” to get the information they needed from the PBMs. The Big Three are known for obfuscation and delaying providing information to legislative and regulatory agencies.
A version of this article appeared on Medscape.com.
Rising consolidation among pharmacy benefit managers (PBMs) allows the companies to profit at the expense of patients and independent pharmacists. That’s the conclusion of a recent Federal Trade Commission (FTC) report on interim findings from the agency’s ongoing investigation of PBMs.
Lawmakers are increasingly scrutinizing the industry amid growing concern among physicians and consumers about how PBMs exploit their market dominance. The top six PBMs managed 94% of US drug claims in 2023, with the majority handled by the industry’s three giants: CVS Caremark, Cigna’s Express Scripts, and United Healthcare’s OptumRx.
PBMs manage prescription drug benefits for health insurers, Medicare Part D drug plans, and large employers. They act as middlemen between health insurers and pharmacies, developing formularies of covered drugs and promising savings from the discounts and rebates they negotiate with drugmakers.
The FTC’s interim report found that the giant PBMs often exercise significant control over what drugs are available and at what price and which pharmacies patients can use to access their prescribed medications. Consumers suffer as a result, the report concluded.
Madelaine A. Feldman, MD, vice president for advocacy and government affairs for the Coalition of State Rheumatology Organizations, shared her perspective on the FTC report in an email Q&A with this news organization. She is affiliated with The Rheumatology Group, based in Metairie, Louisiana.
Dr. Feldman has long tracked the PBM industry and appeared as a witness before influential government panels, including the House Energy and Commerce Committee. She has highlighted for lawmakers the challenges physicians face in helping patients get needed medicines.
For example, she shared cases of PBMs steering patients toward the more expensive of three widely used rheumatoid arthritis medicines that have a similar mechanism of action, the Janus kinase (JAK) inhibitors, Dr. Feldman said.
One of the drugs cost roughly half of the other two — about $30,000 per year vs $65,000-$70,000. Yet only the two expensive drugs were included in the PBM formulary. As a result, the cheapest drug holds only a sliver of market share; the remainder is dominated by the two expensive products, she told the House Oversight and Accountability Committee in 2021.
This Q&A has been edited for length and clarity.
What would you want federal and state policymakers to do in response to the FTC’s report?
I think Congress needs to clearly delineate the differences between anticompetitive pharmacy issues, drug pricing issues, and their effect on formulary construction issues.
Lawmakers should demand more transparency and consider legislation that would remove perverse incentives that prompt PBMs to choose higher priced drugs for their formularies.
That may require other regulatory or legislative actions to ensure lower prices (not higher kickbacks) are incentivized. Ultimately, in order to gain true competition within the health insurance business, these oligopolies of multiple businesses need to be broken up. Anything less seems to be nibbling around the edges and allows the Big Three to continue their “whack-a mole” in circumventing piecemeal regulatory and legislative policies.
You’ve followed PBM practices closely for many years. Was there anything in this interim FTC report that surprised you?
Though not surprised, I am glad that it was released because it had been a year in investigation and there were many requests for some type of substantive report.
Two things that are missing that I feel are paramount are investigating how the three big PBMs are causing physical harm to patients as a result of the profit component in formulary construction and the profound financial impact of hidden PBM profit centers in self-insured employer health plans.
What we have seen over the years is the result of the perverse incentives for the PBMs to prefer the most profitable medications on their formularies.
They use utilization management tools such as step therapy, nonmedical switching, and exclusions to maintain their formularies’ profitability. These tools have been shown to delay and deny the proper care of patients, resulting in not just monetary but physical harm as well.
I would think the physical harm done to patients in manipulating the formularies should be addressed in this report as well and, in fact, may be the most important aspect of consumer protection of this issue.
In terms of the FTC’s mission to not “unduly burden” legitimate business, I would like to see the sector of self-insured employers addressed.
The report details how PBMs steer prescriptions to their affiliated pharmacies. The FTC says that can push smaller pharmacies out of the market, ultimately leading to higher costs and lower quality services for people. What’s your perspective?
Having more community pharmacies is better than having less. We are seeing more “pharmacy deserts” in rural areas as a result of many community pharmacies having to close.
The FTC voted 4-1 to allow staff to issue the interim report, with Commissioner Melissa Holyoak voting no. And some FTC commissioners seem divided on the usefulness of the report. Why?
Commissioner Holyoak states the “the Report leaves us without a better understanding of the competition concerns surrounding PBMs or how consumers are impacted by PBM practices.”
I do agree with her that the harm to patients’ medical status was not even addressed as far as I could tell in this report. There are multiple news articles and reports on the harms inflicted upon patients by the UM tools that drive the construction of ever changing formularies, all based on contracting with manufacturers that result in the highest profit for the PBM.
Holyoak also states, “Among other critical conclusions, the Report does not address the seemingly contradictory conclusions in the 2005 Report that PBMs, including vertically owned PBMs, generated cost savings for consumers.”
That may be true, but in 2005, the rise of PBMs was just beginning and the huge vertical and horizontal integration had yet to begin. Also, 2005 was still in the beginning of the biologic drug deluge, which did create competition to get on the formulary. Since then, PBMs have done nothing to control the rise in prices but instead, apparently have used the competition to get higher price concessions from manufacturers based on a percentage of the list price to line their pockets.
Commissioner Ferguson agreed with releasing the report but he had many issues with this report including the lack of PBM response.
I do agree with him that the FTC should have used some type of “force” to get the information they needed from the PBMs. The Big Three are known for obfuscation and delaying providing information to legislative and regulatory agencies.
A version of this article appeared on Medscape.com.
Rising consolidation among pharmacy benefit managers (PBMs) allows the companies to profit at the expense of patients and independent pharmacists. That’s the conclusion of a recent Federal Trade Commission (FTC) report on interim findings from the agency’s ongoing investigation of PBMs.
Lawmakers are increasingly scrutinizing the industry amid growing concern among physicians and consumers about how PBMs exploit their market dominance. The top six PBMs managed 94% of US drug claims in 2023, with the majority handled by the industry’s three giants: CVS Caremark, Cigna’s Express Scripts, and United Healthcare’s OptumRx.
PBMs manage prescription drug benefits for health insurers, Medicare Part D drug plans, and large employers. They act as middlemen between health insurers and pharmacies, developing formularies of covered drugs and promising savings from the discounts and rebates they negotiate with drugmakers.
The FTC’s interim report found that the giant PBMs often exercise significant control over what drugs are available and at what price and which pharmacies patients can use to access their prescribed medications. Consumers suffer as a result, the report concluded.
Madelaine A. Feldman, MD, vice president for advocacy and government affairs for the Coalition of State Rheumatology Organizations, shared her perspective on the FTC report in an email Q&A with this news organization. She is affiliated with The Rheumatology Group, based in Metairie, Louisiana.
Dr. Feldman has long tracked the PBM industry and appeared as a witness before influential government panels, including the House Energy and Commerce Committee. She has highlighted for lawmakers the challenges physicians face in helping patients get needed medicines.
For example, she shared cases of PBMs steering patients toward the more expensive of three widely used rheumatoid arthritis medicines that have a similar mechanism of action, the Janus kinase (JAK) inhibitors, Dr. Feldman said.
One of the drugs cost roughly half of the other two — about $30,000 per year vs $65,000-$70,000. Yet only the two expensive drugs were included in the PBM formulary. As a result, the cheapest drug holds only a sliver of market share; the remainder is dominated by the two expensive products, she told the House Oversight and Accountability Committee in 2021.
This Q&A has been edited for length and clarity.
What would you want federal and state policymakers to do in response to the FTC’s report?
I think Congress needs to clearly delineate the differences between anticompetitive pharmacy issues, drug pricing issues, and their effect on formulary construction issues.
Lawmakers should demand more transparency and consider legislation that would remove perverse incentives that prompt PBMs to choose higher priced drugs for their formularies.
That may require other regulatory or legislative actions to ensure lower prices (not higher kickbacks) are incentivized. Ultimately, in order to gain true competition within the health insurance business, these oligopolies of multiple businesses need to be broken up. Anything less seems to be nibbling around the edges and allows the Big Three to continue their “whack-a mole” in circumventing piecemeal regulatory and legislative policies.
You’ve followed PBM practices closely for many years. Was there anything in this interim FTC report that surprised you?
Though not surprised, I am glad that it was released because it had been a year in investigation and there were many requests for some type of substantive report.
Two things that are missing that I feel are paramount are investigating how the three big PBMs are causing physical harm to patients as a result of the profit component in formulary construction and the profound financial impact of hidden PBM profit centers in self-insured employer health plans.
What we have seen over the years is the result of the perverse incentives for the PBMs to prefer the most profitable medications on their formularies.
They use utilization management tools such as step therapy, nonmedical switching, and exclusions to maintain their formularies’ profitability. These tools have been shown to delay and deny the proper care of patients, resulting in not just monetary but physical harm as well.
I would think the physical harm done to patients in manipulating the formularies should be addressed in this report as well and, in fact, may be the most important aspect of consumer protection of this issue.
In terms of the FTC’s mission to not “unduly burden” legitimate business, I would like to see the sector of self-insured employers addressed.
The report details how PBMs steer prescriptions to their affiliated pharmacies. The FTC says that can push smaller pharmacies out of the market, ultimately leading to higher costs and lower quality services for people. What’s your perspective?
Having more community pharmacies is better than having less. We are seeing more “pharmacy deserts” in rural areas as a result of many community pharmacies having to close.
The FTC voted 4-1 to allow staff to issue the interim report, with Commissioner Melissa Holyoak voting no. And some FTC commissioners seem divided on the usefulness of the report. Why?
Commissioner Holyoak states the “the Report leaves us without a better understanding of the competition concerns surrounding PBMs or how consumers are impacted by PBM practices.”
I do agree with her that the harm to patients’ medical status was not even addressed as far as I could tell in this report. There are multiple news articles and reports on the harms inflicted upon patients by the UM tools that drive the construction of ever changing formularies, all based on contracting with manufacturers that result in the highest profit for the PBM.
Holyoak also states, “Among other critical conclusions, the Report does not address the seemingly contradictory conclusions in the 2005 Report that PBMs, including vertically owned PBMs, generated cost savings for consumers.”
That may be true, but in 2005, the rise of PBMs was just beginning and the huge vertical and horizontal integration had yet to begin. Also, 2005 was still in the beginning of the biologic drug deluge, which did create competition to get on the formulary. Since then, PBMs have done nothing to control the rise in prices but instead, apparently have used the competition to get higher price concessions from manufacturers based on a percentage of the list price to line their pockets.
Commissioner Ferguson agreed with releasing the report but he had many issues with this report including the lack of PBM response.
I do agree with him that the FTC should have used some type of “force” to get the information they needed from the PBMs. The Big Three are known for obfuscation and delaying providing information to legislative and regulatory agencies.
A version of this article appeared on Medscape.com.
Rimegepant Relieves Pain in Acute Migraine
Key clinical point: Rimegepant was more effective than placebo in reducing pain and most bothersome symptoms in patients with moderate to severe migraine, while also showing favorable safety and tolerability.
Major findings: At 2 hours post dose, rimegepant was more effective than placebo in providing freedom from pain (risk difference 4.9; P = .0298) and the most bothersome symptoms (risk difference 8.9; P = .0016). Similar proportions of participants in the rimegepant vs placebo group experienced at least one adverse event (12.6% vs 10.7%), with nausea (0.9% vs 1.1%) and dizziness (0.7% vs 0.4%) being the most common adverse events.
Study details: This randomized, double-blind, placebo-controlled trial (Safety and Efficacy Study in Adult Subjects With Acute Migraines, NCT03235479) included 1084 patients with migraine with or without aura who were randomly assigned to receive 75 mg rimegepant (n = 543) or placebo (n = 541).
Disclosures: This study was supported by Biohaven Pharmaceuticals (acquired by Pfizer, Inc.). Four authors declared being employees or stockholders of Biohaven Pharmaceuticals, Pfizer, or having other ties with various sources.
Source: Lipton RB, Thiry A, Morris BA, Croop R. Efficacy and safety of rimegepant 75 mg oral tablet, a CGRP receptor antagonist, for the acute treatment of migraine: A randomized, double-blind, placebo-controlled trial. J Pain Res. 2024;17:2431-2441 (Jul 22). Doi: 10.2147/JPR.S453806 Source
Key clinical point: Rimegepant was more effective than placebo in reducing pain and most bothersome symptoms in patients with moderate to severe migraine, while also showing favorable safety and tolerability.
Major findings: At 2 hours post dose, rimegepant was more effective than placebo in providing freedom from pain (risk difference 4.9; P = .0298) and the most bothersome symptoms (risk difference 8.9; P = .0016). Similar proportions of participants in the rimegepant vs placebo group experienced at least one adverse event (12.6% vs 10.7%), with nausea (0.9% vs 1.1%) and dizziness (0.7% vs 0.4%) being the most common adverse events.
Study details: This randomized, double-blind, placebo-controlled trial (Safety and Efficacy Study in Adult Subjects With Acute Migraines, NCT03235479) included 1084 patients with migraine with or without aura who were randomly assigned to receive 75 mg rimegepant (n = 543) or placebo (n = 541).
Disclosures: This study was supported by Biohaven Pharmaceuticals (acquired by Pfizer, Inc.). Four authors declared being employees or stockholders of Biohaven Pharmaceuticals, Pfizer, or having other ties with various sources.
Source: Lipton RB, Thiry A, Morris BA, Croop R. Efficacy and safety of rimegepant 75 mg oral tablet, a CGRP receptor antagonist, for the acute treatment of migraine: A randomized, double-blind, placebo-controlled trial. J Pain Res. 2024;17:2431-2441 (Jul 22). Doi: 10.2147/JPR.S453806 Source
Key clinical point: Rimegepant was more effective than placebo in reducing pain and most bothersome symptoms in patients with moderate to severe migraine, while also showing favorable safety and tolerability.
Major findings: At 2 hours post dose, rimegepant was more effective than placebo in providing freedom from pain (risk difference 4.9; P = .0298) and the most bothersome symptoms (risk difference 8.9; P = .0016). Similar proportions of participants in the rimegepant vs placebo group experienced at least one adverse event (12.6% vs 10.7%), with nausea (0.9% vs 1.1%) and dizziness (0.7% vs 0.4%) being the most common adverse events.
Study details: This randomized, double-blind, placebo-controlled trial (Safety and Efficacy Study in Adult Subjects With Acute Migraines, NCT03235479) included 1084 patients with migraine with or without aura who were randomly assigned to receive 75 mg rimegepant (n = 543) or placebo (n = 541).
Disclosures: This study was supported by Biohaven Pharmaceuticals (acquired by Pfizer, Inc.). Four authors declared being employees or stockholders of Biohaven Pharmaceuticals, Pfizer, or having other ties with various sources.
Source: Lipton RB, Thiry A, Morris BA, Croop R. Efficacy and safety of rimegepant 75 mg oral tablet, a CGRP receptor antagonist, for the acute treatment of migraine: A randomized, double-blind, placebo-controlled trial. J Pain Res. 2024;17:2431-2441 (Jul 22). Doi: 10.2147/JPR.S453806 Source
Hypertension Responsible for Detrimental Effects of Leisure Screen Time on Migraine
Key clinical point: This Mendelian randomization study showed that leisure screen time (LST) worsens migraine, whereas moderate to vigorous physical activity (MVPA) alleviates it, with hypertension and diastolic blood pressure (DBP) being responsible for the effects of MVPA or LST on migraine.
Major findings: Genetically predicted LST was associated with a significantly increased risk for migraine (odds ratio [OR] 1.28; P < .001), whereas MVPA was linked to a significantly reduced risk (OR 0.73; P = .000006). Hypertension mediated 4.86% and 24.81% of the effects of MVPA and LST on migraine risk, respectively, and DBP accounted for 4.66% of the effects of MVPA on migraine risk.
Study details: This study included 18,477 patients with migraine and 287,837 control individuals without migraine from the FinnGen consortium and 26,052 patients with migraine and 487,214 control individuals without migraine from the large-scale genome-wide association studies.
Disclosures: This study was supported by grants from the National Natural Science Foundation of China and others. The authors declared no conflicts of interest.
Source: Gan Q, Song E, Zhang L, et al. The role of hypertension in the relationship between leisure screen time, physical activity and migraine: A 2-sample Mendelian randomization study. J Headache Pain. 2024;25:122 (Jul 24). Doi: 10.1186/s10194-024-01820-4 Source
Key clinical point: This Mendelian randomization study showed that leisure screen time (LST) worsens migraine, whereas moderate to vigorous physical activity (MVPA) alleviates it, with hypertension and diastolic blood pressure (DBP) being responsible for the effects of MVPA or LST on migraine.
Major findings: Genetically predicted LST was associated with a significantly increased risk for migraine (odds ratio [OR] 1.28; P < .001), whereas MVPA was linked to a significantly reduced risk (OR 0.73; P = .000006). Hypertension mediated 4.86% and 24.81% of the effects of MVPA and LST on migraine risk, respectively, and DBP accounted for 4.66% of the effects of MVPA on migraine risk.
Study details: This study included 18,477 patients with migraine and 287,837 control individuals without migraine from the FinnGen consortium and 26,052 patients with migraine and 487,214 control individuals without migraine from the large-scale genome-wide association studies.
Disclosures: This study was supported by grants from the National Natural Science Foundation of China and others. The authors declared no conflicts of interest.
Source: Gan Q, Song E, Zhang L, et al. The role of hypertension in the relationship between leisure screen time, physical activity and migraine: A 2-sample Mendelian randomization study. J Headache Pain. 2024;25:122 (Jul 24). Doi: 10.1186/s10194-024-01820-4 Source
Key clinical point: This Mendelian randomization study showed that leisure screen time (LST) worsens migraine, whereas moderate to vigorous physical activity (MVPA) alleviates it, with hypertension and diastolic blood pressure (DBP) being responsible for the effects of MVPA or LST on migraine.
Major findings: Genetically predicted LST was associated with a significantly increased risk for migraine (odds ratio [OR] 1.28; P < .001), whereas MVPA was linked to a significantly reduced risk (OR 0.73; P = .000006). Hypertension mediated 4.86% and 24.81% of the effects of MVPA and LST on migraine risk, respectively, and DBP accounted for 4.66% of the effects of MVPA on migraine risk.
Study details: This study included 18,477 patients with migraine and 287,837 control individuals without migraine from the FinnGen consortium and 26,052 patients with migraine and 487,214 control individuals without migraine from the large-scale genome-wide association studies.
Disclosures: This study was supported by grants from the National Natural Science Foundation of China and others. The authors declared no conflicts of interest.
Source: Gan Q, Song E, Zhang L, et al. The role of hypertension in the relationship between leisure screen time, physical activity and migraine: A 2-sample Mendelian randomization study. J Headache Pain. 2024;25:122 (Jul 24). Doi: 10.1186/s10194-024-01820-4 Source