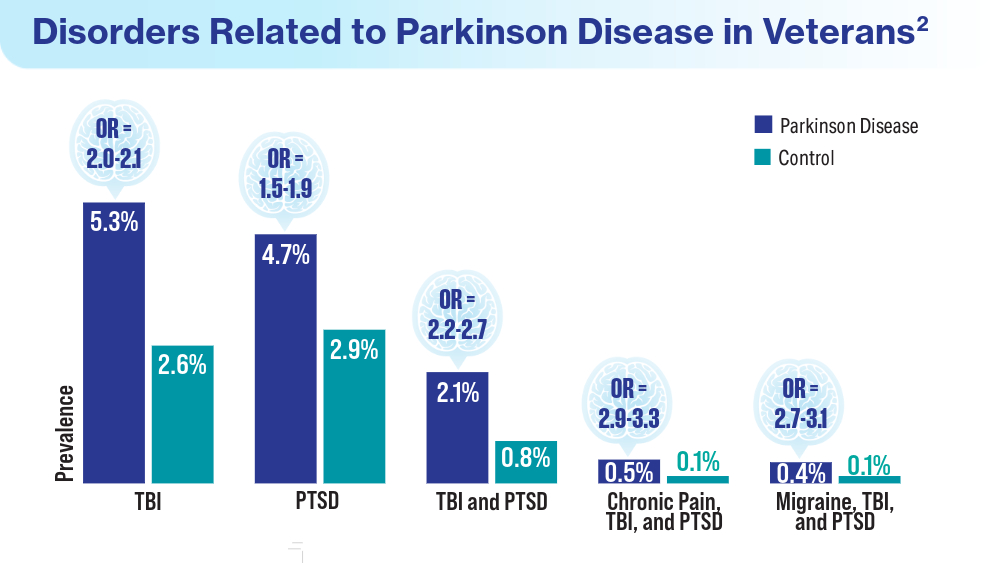
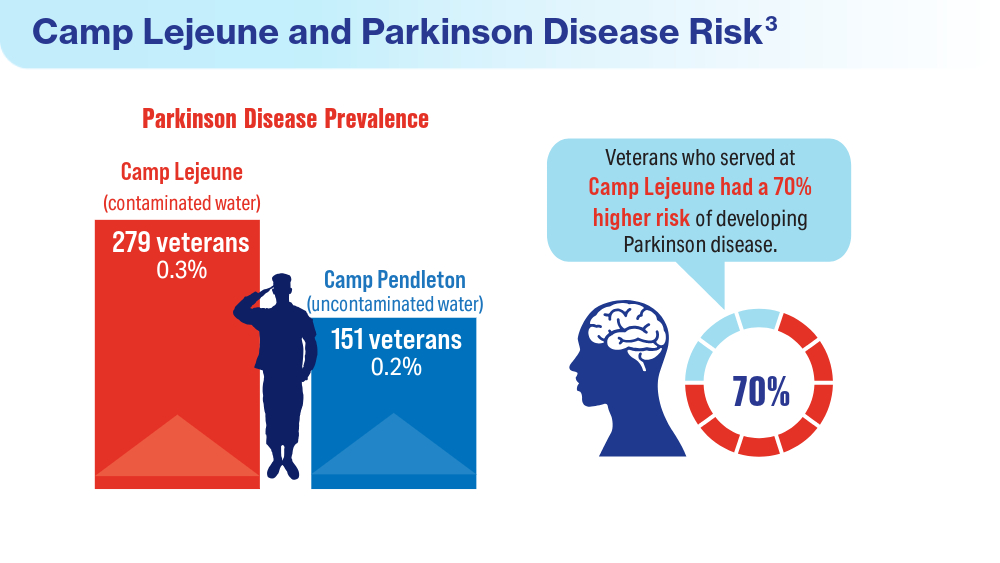
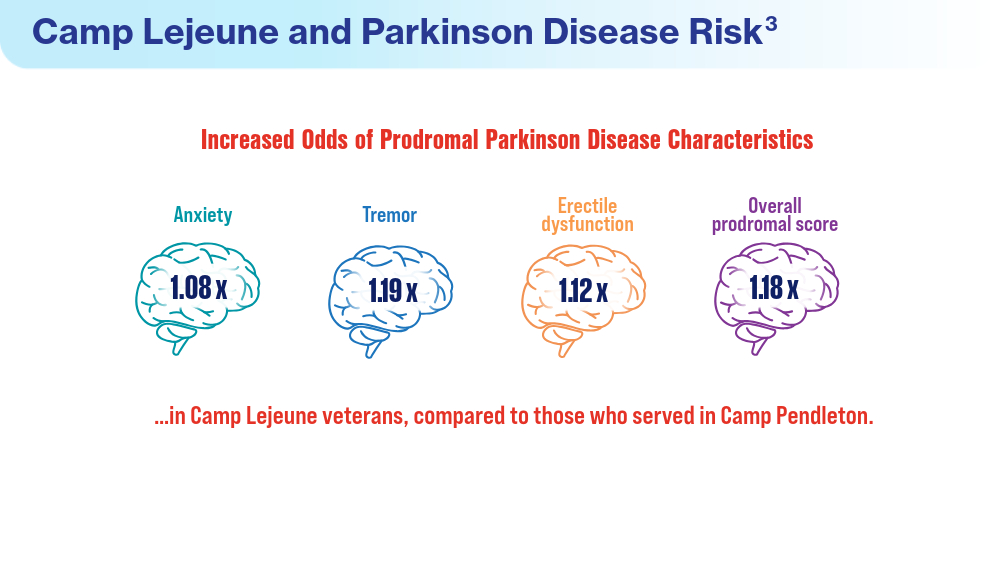
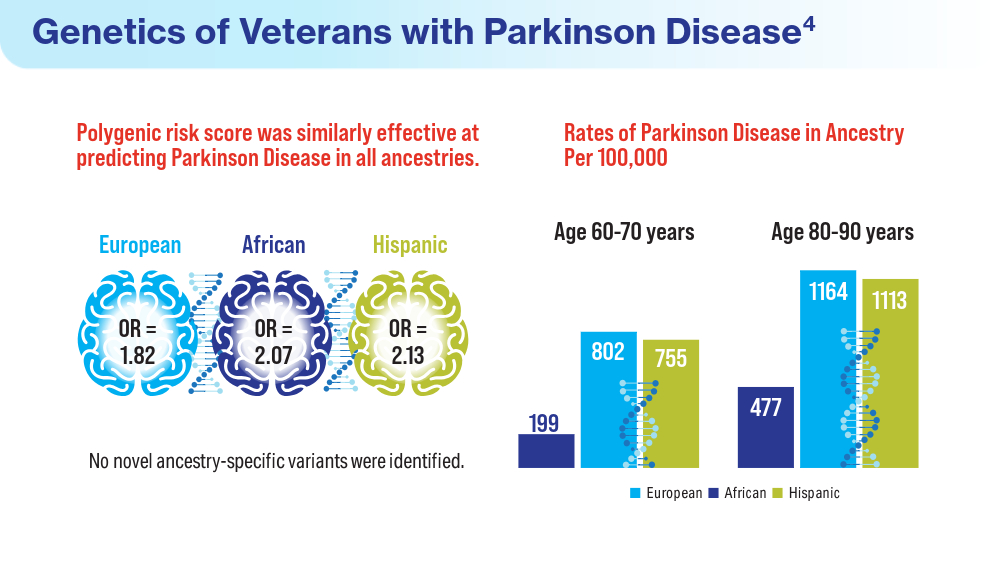
User login
Could This COPD Treatment’s Cost Put It Out of Reach for Many?
Ensifentrine (Ohtuvayre), a novel medication for the treatment of chronic obstructive pulmonary disease (COPD) recently approved by the US Food and Drug Administration, has been shown to reduce COPD exacerbations and may improve the quality of life for patients, but these potential benefits come at a high annual cost, authors of a cost-effectiveness analysis say.
Ensifentrine is a first-in-class selective dual inhibitor of both phosphodiesterase 3 (PDE-3) and PDE-4, combining both bronchodilator and nonsteroidal anti-inflammatory effects in a single molecule. The drug is delivered through a standard jet nebulizer.
In the phase 3 ENHANCE 1 and 2 trials, ensifentrine significantly improved lung function based on the primary outcome of average forced expiratory volume in 1 second (FEV1) within 0-12 hours of administration compared with placebo. In addition, patients were found to tolerate the inhaled treatment well, with similar proportions of ensifentrine- and placebo-assigned patients reporting treatment-emergent adverse events. The most common treatment-emergent adverse events were nasopharyngitis, hypertension, and back pain, reported in < 3% of the ensifentrine group.
High Cost Barrier
But as authors of the analysis from the Boston-based Institute for Clinical and Economic Review (ICER) found, ICER is an independent, nonprofit research institute that conducts evidence-based reviews of healthcare interventions, including prescription drugs, other treatments, and diagnostic tests.
“Current evidence shows that ensifentrine decreases COPD exacerbations when used in combination with some current inhaled therapies, but there are uncertainties about how much benefit it may add to unstudied combinations of inhaled treatments,” said David Rind, MD, chief medical officer of ICER, in a statement.
In an interview, Dr. Rind noted that the high price of ensifentrine may lead payers to restrict access to an otherwise promising new therapy. “Obviously many drugs in the US are overpriced, and this one, too, looks like it is overpriced. That causes ongoing financial toxicity for individual patients and it causes problems for the entire US health system, because when we pay too much for drugs we don’t have money for other things. So I’m worried about the fact that this price is too high compared to the benefit it provides,” he said.
As previously reported, as many as 1 in 6 persons with COPD in the United States miss or delay COPD medication doses owing to high drug costs. “I think that the pricing they chose is going to cause lots of barriers to people getting access and that insurance companies will throw up barriers. Primary care physicians like me won’t even try to get approval for a drug like this given the hoops we will be made to jump through, and so fewer people will get this drug,” Dr. Rind said. He pointed out that a lower wholesale acquisition cost could encourage higher volume sales, affording the drug maker a comparable profit to the higher cost but lower volume option.
Good Drug, High Price
An independent appraisal committee for ICER determined that “current evidence is adequate to demonstrate a net health benefit for ensifentrine added to maintenance therapy when compared to maintenance therapy alone.”
But ICER also issued an access and affordability alert “to signal to stakeholders and policymakers that the amount of added health care costs associated with a new service may be difficult for the health system to absorb over the short term without displacing other needed services.” ICER recommends that payers should include coverage for smoking cessation therapies, and that drug manufacturers “set prices that will foster affordability and good access for all patients by aligning prices with the patient-centered therapeutic value of their treatments.”
“This looks like a pretty good drug,” Dr. Rind said. “It looks quite safe, and I think there will be a lot of patients, particularly those who are having frequent exacerbations, who this would be appropriate for, particularly once they’ve maxed out existing therapies, but maybe even earlier than that. And if the price comes down to the point that patients can really access this and providers can access it, people really should look at this as a potential therapy.”
Drug Not Yet Available?
However, providers have not yet had experience to gauge the new medication. “We haven’t been able to prescribe it yet,” said Corinne Young, MSN, FNP-C, FCCP, director of advance practice provider and clinical services for Colorado Springs Pulmonary Consultants and president and founder of the Association of Pulmonary Advanced Practice Providers. She learned that “they were going to release it to select specialty pharmacies in the third quarter of 2024. But all the ones we call do not have it, and no one knows who does. They haven’t sent any reps into the field in my area, so we don’t have any points of contact either,” she said.
Verona Pharma stated it anticipates ensifentrine to be available in the third quarter of 2024 “through an exclusive network of accredited specialty pharmacies.”
Funding for the ICER report came from nonprofit foundations. No funding came from health insurers, pharmacy benefit managers, or life science companies. Dr. Rind had no disclosures relevant to ensifentrine or Verona Pharma. Ms. Young is a member of the CHEST Physician Editorial Board.
A version of this article first appeared on Medscape.com.
Ensifentrine (Ohtuvayre), a novel medication for the treatment of chronic obstructive pulmonary disease (COPD) recently approved by the US Food and Drug Administration, has been shown to reduce COPD exacerbations and may improve the quality of life for patients, but these potential benefits come at a high annual cost, authors of a cost-effectiveness analysis say.
Ensifentrine is a first-in-class selective dual inhibitor of both phosphodiesterase 3 (PDE-3) and PDE-4, combining both bronchodilator and nonsteroidal anti-inflammatory effects in a single molecule. The drug is delivered through a standard jet nebulizer.
In the phase 3 ENHANCE 1 and 2 trials, ensifentrine significantly improved lung function based on the primary outcome of average forced expiratory volume in 1 second (FEV1) within 0-12 hours of administration compared with placebo. In addition, patients were found to tolerate the inhaled treatment well, with similar proportions of ensifentrine- and placebo-assigned patients reporting treatment-emergent adverse events. The most common treatment-emergent adverse events were nasopharyngitis, hypertension, and back pain, reported in < 3% of the ensifentrine group.
High Cost Barrier
But as authors of the analysis from the Boston-based Institute for Clinical and Economic Review (ICER) found, ICER is an independent, nonprofit research institute that conducts evidence-based reviews of healthcare interventions, including prescription drugs, other treatments, and diagnostic tests.
“Current evidence shows that ensifentrine decreases COPD exacerbations when used in combination with some current inhaled therapies, but there are uncertainties about how much benefit it may add to unstudied combinations of inhaled treatments,” said David Rind, MD, chief medical officer of ICER, in a statement.
In an interview, Dr. Rind noted that the high price of ensifentrine may lead payers to restrict access to an otherwise promising new therapy. “Obviously many drugs in the US are overpriced, and this one, too, looks like it is overpriced. That causes ongoing financial toxicity for individual patients and it causes problems for the entire US health system, because when we pay too much for drugs we don’t have money for other things. So I’m worried about the fact that this price is too high compared to the benefit it provides,” he said.
As previously reported, as many as 1 in 6 persons with COPD in the United States miss or delay COPD medication doses owing to high drug costs. “I think that the pricing they chose is going to cause lots of barriers to people getting access and that insurance companies will throw up barriers. Primary care physicians like me won’t even try to get approval for a drug like this given the hoops we will be made to jump through, and so fewer people will get this drug,” Dr. Rind said. He pointed out that a lower wholesale acquisition cost could encourage higher volume sales, affording the drug maker a comparable profit to the higher cost but lower volume option.
Good Drug, High Price
An independent appraisal committee for ICER determined that “current evidence is adequate to demonstrate a net health benefit for ensifentrine added to maintenance therapy when compared to maintenance therapy alone.”
But ICER also issued an access and affordability alert “to signal to stakeholders and policymakers that the amount of added health care costs associated with a new service may be difficult for the health system to absorb over the short term without displacing other needed services.” ICER recommends that payers should include coverage for smoking cessation therapies, and that drug manufacturers “set prices that will foster affordability and good access for all patients by aligning prices with the patient-centered therapeutic value of their treatments.”
“This looks like a pretty good drug,” Dr. Rind said. “It looks quite safe, and I think there will be a lot of patients, particularly those who are having frequent exacerbations, who this would be appropriate for, particularly once they’ve maxed out existing therapies, but maybe even earlier than that. And if the price comes down to the point that patients can really access this and providers can access it, people really should look at this as a potential therapy.”
Drug Not Yet Available?
However, providers have not yet had experience to gauge the new medication. “We haven’t been able to prescribe it yet,” said Corinne Young, MSN, FNP-C, FCCP, director of advance practice provider and clinical services for Colorado Springs Pulmonary Consultants and president and founder of the Association of Pulmonary Advanced Practice Providers. She learned that “they were going to release it to select specialty pharmacies in the third quarter of 2024. But all the ones we call do not have it, and no one knows who does. They haven’t sent any reps into the field in my area, so we don’t have any points of contact either,” she said.
Verona Pharma stated it anticipates ensifentrine to be available in the third quarter of 2024 “through an exclusive network of accredited specialty pharmacies.”
Funding for the ICER report came from nonprofit foundations. No funding came from health insurers, pharmacy benefit managers, or life science companies. Dr. Rind had no disclosures relevant to ensifentrine or Verona Pharma. Ms. Young is a member of the CHEST Physician Editorial Board.
A version of this article first appeared on Medscape.com.
Ensifentrine (Ohtuvayre), a novel medication for the treatment of chronic obstructive pulmonary disease (COPD) recently approved by the US Food and Drug Administration, has been shown to reduce COPD exacerbations and may improve the quality of life for patients, but these potential benefits come at a high annual cost, authors of a cost-effectiveness analysis say.
Ensifentrine is a first-in-class selective dual inhibitor of both phosphodiesterase 3 (PDE-3) and PDE-4, combining both bronchodilator and nonsteroidal anti-inflammatory effects in a single molecule. The drug is delivered through a standard jet nebulizer.
In the phase 3 ENHANCE 1 and 2 trials, ensifentrine significantly improved lung function based on the primary outcome of average forced expiratory volume in 1 second (FEV1) within 0-12 hours of administration compared with placebo. In addition, patients were found to tolerate the inhaled treatment well, with similar proportions of ensifentrine- and placebo-assigned patients reporting treatment-emergent adverse events. The most common treatment-emergent adverse events were nasopharyngitis, hypertension, and back pain, reported in < 3% of the ensifentrine group.
High Cost Barrier
But as authors of the analysis from the Boston-based Institute for Clinical and Economic Review (ICER) found, ICER is an independent, nonprofit research institute that conducts evidence-based reviews of healthcare interventions, including prescription drugs, other treatments, and diagnostic tests.
“Current evidence shows that ensifentrine decreases COPD exacerbations when used in combination with some current inhaled therapies, but there are uncertainties about how much benefit it may add to unstudied combinations of inhaled treatments,” said David Rind, MD, chief medical officer of ICER, in a statement.
In an interview, Dr. Rind noted that the high price of ensifentrine may lead payers to restrict access to an otherwise promising new therapy. “Obviously many drugs in the US are overpriced, and this one, too, looks like it is overpriced. That causes ongoing financial toxicity for individual patients and it causes problems for the entire US health system, because when we pay too much for drugs we don’t have money for other things. So I’m worried about the fact that this price is too high compared to the benefit it provides,” he said.
As previously reported, as many as 1 in 6 persons with COPD in the United States miss or delay COPD medication doses owing to high drug costs. “I think that the pricing they chose is going to cause lots of barriers to people getting access and that insurance companies will throw up barriers. Primary care physicians like me won’t even try to get approval for a drug like this given the hoops we will be made to jump through, and so fewer people will get this drug,” Dr. Rind said. He pointed out that a lower wholesale acquisition cost could encourage higher volume sales, affording the drug maker a comparable profit to the higher cost but lower volume option.
Good Drug, High Price
An independent appraisal committee for ICER determined that “current evidence is adequate to demonstrate a net health benefit for ensifentrine added to maintenance therapy when compared to maintenance therapy alone.”
But ICER also issued an access and affordability alert “to signal to stakeholders and policymakers that the amount of added health care costs associated with a new service may be difficult for the health system to absorb over the short term without displacing other needed services.” ICER recommends that payers should include coverage for smoking cessation therapies, and that drug manufacturers “set prices that will foster affordability and good access for all patients by aligning prices with the patient-centered therapeutic value of their treatments.”
“This looks like a pretty good drug,” Dr. Rind said. “It looks quite safe, and I think there will be a lot of patients, particularly those who are having frequent exacerbations, who this would be appropriate for, particularly once they’ve maxed out existing therapies, but maybe even earlier than that. And if the price comes down to the point that patients can really access this and providers can access it, people really should look at this as a potential therapy.”
Drug Not Yet Available?
However, providers have not yet had experience to gauge the new medication. “We haven’t been able to prescribe it yet,” said Corinne Young, MSN, FNP-C, FCCP, director of advance practice provider and clinical services for Colorado Springs Pulmonary Consultants and president and founder of the Association of Pulmonary Advanced Practice Providers. She learned that “they were going to release it to select specialty pharmacies in the third quarter of 2024. But all the ones we call do not have it, and no one knows who does. They haven’t sent any reps into the field in my area, so we don’t have any points of contact either,” she said.
Verona Pharma stated it anticipates ensifentrine to be available in the third quarter of 2024 “through an exclusive network of accredited specialty pharmacies.”
Funding for the ICER report came from nonprofit foundations. No funding came from health insurers, pharmacy benefit managers, or life science companies. Dr. Rind had no disclosures relevant to ensifentrine or Verona Pharma. Ms. Young is a member of the CHEST Physician Editorial Board.
A version of this article first appeared on Medscape.com.
ABIM Revokes Two Physicians’ Certifications Over Accusations of COVID Misinformation
The American Board of Internal Medicine (ABIM) has revoked certification for two physicians known for leading an organization that promotes ivermectin as a treatment for COVID-19.
Pierre Kory, MD, is no longer certified in critical care medicine, pulmonary disease, and internal medicine, according to the ABIM website. Paul Ellis Marik, MD, is no longer certified in critical care medicine or internal medicine.
Dr. Marik is the chief scientific officer and Dr. Kory is president emeritus of the Front Line COVID-19 Critical Care Alliance, a group they founded in March 2020. and also offers treatments for Lyme disease.
Ivermectin was proven to not be of use in treating COVID. Studies purporting to show a benefit were later linked to errors, and some were found to have been based on potentially fraudulent research.
The ABIM declined to comment when asked by this news organization about its action. Its website indicates that “revoked” indicates “loss of certification due to disciplinary action for which ABIM has determined that the conduct underlying the sanction does not warrant a defined pathway for restoration of certification at the time of disciplinary sanction.”
In a statement emailed to this news organization, Dr. Kory and Dr. Marik said, “we believe this decision represents a dangerous shift away from the foundation principles of medical discourse and scientific debate that have historically been the bedrock of medical education associations.”
The FLCCC said in the statement that it, along with Dr. Kory and Dr. Marik, are “evaluating options to challenge these decisions.”
Dr. Kory and Dr. Marik said they were notified in May 2022 that they were facing a potential ABIM disciplinary action. An ABIM committee recommended the revocation in July 2023, saying the two men were spreading “false or inaccurate medical information,” according to FLCCC. Dr. Kory and Dr. Marik lost an appeal.
In a 2023 statement, Dr. Kory and Dr. Marik called the ABIM action an “attack on freedom of speech.”
“This isn’t a free speech question,” said Arthur L. Caplan, PhD, the Drs. William F. and Virginia Connolly Mitty Professor of Bioethics at NYU Grossman School of Medicine’s Department of Population Health, New York City. “You do have the right to free speech, but you don’t have the right to practice outside of the standard of care boundaries,” he told this news organization.
The ABIM action “is the field standing up and saying, ‘These are the limits of what you can do,’” said Dr. Caplan. It means the profession is rejecting those “who are involved in things that harm patients or delay them getting accepted treatments,” he said. Caplan noted that a disciplinary action had been a long time in coming — 3 years since the first battles over ivermectin.
Wendy Parmet, JD, Matthews Distinguished University Professor of Law at Northeastern University School of Public Policy and Urban Affairs, Boston, said that misinformation spread by physicians is especially harmful because it comes with an air of credibility.
“We certainly want people to be able to dissent,” Ms. Parmet told this news organization. To engender trust, any sanctions by a professional board should be done in a deliberative process with a strong evidentiary base, she said.
“You want to leave sufficient room for discourse and discussion within the profession, and you don’t want the board to enforce a narrow, rigid orthodoxy,” she said. But in cases where people are “peddling information that is way outside the consensus” or are “profiting off of it, for the profession to take no action, that is, I think, detrimental also to the trust in the profession,” she said.
She was not surprised that Dr. Kory and Dr. Marik would fight to retain certification. “Board certification is an important, very worthwhile thing to have,” she said. “Losing it is not trivial.”
Dr. Kory, who is licensed in California, New York, and Wisconsin, “does not require this certification for his independent practice but is evaluating next steps with attorneys,” according to the statement from FLCCC.
Dr. Marik, whose Virginia medical license expired in 2022, “is no longer treating patients and has dedicated his time and efforts to the FLCCC Alliance,” the statement said.
Dr. Caplan served as a director, officer, partner, employee, advisor, consultant, or trustee for Johnson & Johnson’s Panel for Compassionate Drug Use (unpaid position) and is a contributing author and advisor for this news organization. Ms. Parmet reports no relevant financial relationships.
A version of this article appeared on Medscape.com.
The American Board of Internal Medicine (ABIM) has revoked certification for two physicians known for leading an organization that promotes ivermectin as a treatment for COVID-19.
Pierre Kory, MD, is no longer certified in critical care medicine, pulmonary disease, and internal medicine, according to the ABIM website. Paul Ellis Marik, MD, is no longer certified in critical care medicine or internal medicine.
Dr. Marik is the chief scientific officer and Dr. Kory is president emeritus of the Front Line COVID-19 Critical Care Alliance, a group they founded in March 2020. and also offers treatments for Lyme disease.
Ivermectin was proven to not be of use in treating COVID. Studies purporting to show a benefit were later linked to errors, and some were found to have been based on potentially fraudulent research.
The ABIM declined to comment when asked by this news organization about its action. Its website indicates that “revoked” indicates “loss of certification due to disciplinary action for which ABIM has determined that the conduct underlying the sanction does not warrant a defined pathway for restoration of certification at the time of disciplinary sanction.”
In a statement emailed to this news organization, Dr. Kory and Dr. Marik said, “we believe this decision represents a dangerous shift away from the foundation principles of medical discourse and scientific debate that have historically been the bedrock of medical education associations.”
The FLCCC said in the statement that it, along with Dr. Kory and Dr. Marik, are “evaluating options to challenge these decisions.”
Dr. Kory and Dr. Marik said they were notified in May 2022 that they were facing a potential ABIM disciplinary action. An ABIM committee recommended the revocation in July 2023, saying the two men were spreading “false or inaccurate medical information,” according to FLCCC. Dr. Kory and Dr. Marik lost an appeal.
In a 2023 statement, Dr. Kory and Dr. Marik called the ABIM action an “attack on freedom of speech.”
“This isn’t a free speech question,” said Arthur L. Caplan, PhD, the Drs. William F. and Virginia Connolly Mitty Professor of Bioethics at NYU Grossman School of Medicine’s Department of Population Health, New York City. “You do have the right to free speech, but you don’t have the right to practice outside of the standard of care boundaries,” he told this news organization.
The ABIM action “is the field standing up and saying, ‘These are the limits of what you can do,’” said Dr. Caplan. It means the profession is rejecting those “who are involved in things that harm patients or delay them getting accepted treatments,” he said. Caplan noted that a disciplinary action had been a long time in coming — 3 years since the first battles over ivermectin.
Wendy Parmet, JD, Matthews Distinguished University Professor of Law at Northeastern University School of Public Policy and Urban Affairs, Boston, said that misinformation spread by physicians is especially harmful because it comes with an air of credibility.
“We certainly want people to be able to dissent,” Ms. Parmet told this news organization. To engender trust, any sanctions by a professional board should be done in a deliberative process with a strong evidentiary base, she said.
“You want to leave sufficient room for discourse and discussion within the profession, and you don’t want the board to enforce a narrow, rigid orthodoxy,” she said. But in cases where people are “peddling information that is way outside the consensus” or are “profiting off of it, for the profession to take no action, that is, I think, detrimental also to the trust in the profession,” she said.
She was not surprised that Dr. Kory and Dr. Marik would fight to retain certification. “Board certification is an important, very worthwhile thing to have,” she said. “Losing it is not trivial.”
Dr. Kory, who is licensed in California, New York, and Wisconsin, “does not require this certification for his independent practice but is evaluating next steps with attorneys,” according to the statement from FLCCC.
Dr. Marik, whose Virginia medical license expired in 2022, “is no longer treating patients and has dedicated his time and efforts to the FLCCC Alliance,” the statement said.
Dr. Caplan served as a director, officer, partner, employee, advisor, consultant, or trustee for Johnson & Johnson’s Panel for Compassionate Drug Use (unpaid position) and is a contributing author and advisor for this news organization. Ms. Parmet reports no relevant financial relationships.
A version of this article appeared on Medscape.com.
The American Board of Internal Medicine (ABIM) has revoked certification for two physicians known for leading an organization that promotes ivermectin as a treatment for COVID-19.
Pierre Kory, MD, is no longer certified in critical care medicine, pulmonary disease, and internal medicine, according to the ABIM website. Paul Ellis Marik, MD, is no longer certified in critical care medicine or internal medicine.
Dr. Marik is the chief scientific officer and Dr. Kory is president emeritus of the Front Line COVID-19 Critical Care Alliance, a group they founded in March 2020. and also offers treatments for Lyme disease.
Ivermectin was proven to not be of use in treating COVID. Studies purporting to show a benefit were later linked to errors, and some were found to have been based on potentially fraudulent research.
The ABIM declined to comment when asked by this news organization about its action. Its website indicates that “revoked” indicates “loss of certification due to disciplinary action for which ABIM has determined that the conduct underlying the sanction does not warrant a defined pathway for restoration of certification at the time of disciplinary sanction.”
In a statement emailed to this news organization, Dr. Kory and Dr. Marik said, “we believe this decision represents a dangerous shift away from the foundation principles of medical discourse and scientific debate that have historically been the bedrock of medical education associations.”
The FLCCC said in the statement that it, along with Dr. Kory and Dr. Marik, are “evaluating options to challenge these decisions.”
Dr. Kory and Dr. Marik said they were notified in May 2022 that they were facing a potential ABIM disciplinary action. An ABIM committee recommended the revocation in July 2023, saying the two men were spreading “false or inaccurate medical information,” according to FLCCC. Dr. Kory and Dr. Marik lost an appeal.
In a 2023 statement, Dr. Kory and Dr. Marik called the ABIM action an “attack on freedom of speech.”
“This isn’t a free speech question,” said Arthur L. Caplan, PhD, the Drs. William F. and Virginia Connolly Mitty Professor of Bioethics at NYU Grossman School of Medicine’s Department of Population Health, New York City. “You do have the right to free speech, but you don’t have the right to practice outside of the standard of care boundaries,” he told this news organization.
The ABIM action “is the field standing up and saying, ‘These are the limits of what you can do,’” said Dr. Caplan. It means the profession is rejecting those “who are involved in things that harm patients or delay them getting accepted treatments,” he said. Caplan noted that a disciplinary action had been a long time in coming — 3 years since the first battles over ivermectin.
Wendy Parmet, JD, Matthews Distinguished University Professor of Law at Northeastern University School of Public Policy and Urban Affairs, Boston, said that misinformation spread by physicians is especially harmful because it comes with an air of credibility.
“We certainly want people to be able to dissent,” Ms. Parmet told this news organization. To engender trust, any sanctions by a professional board should be done in a deliberative process with a strong evidentiary base, she said.
“You want to leave sufficient room for discourse and discussion within the profession, and you don’t want the board to enforce a narrow, rigid orthodoxy,” she said. But in cases where people are “peddling information that is way outside the consensus” or are “profiting off of it, for the profession to take no action, that is, I think, detrimental also to the trust in the profession,” she said.
She was not surprised that Dr. Kory and Dr. Marik would fight to retain certification. “Board certification is an important, very worthwhile thing to have,” she said. “Losing it is not trivial.”
Dr. Kory, who is licensed in California, New York, and Wisconsin, “does not require this certification for his independent practice but is evaluating next steps with attorneys,” according to the statement from FLCCC.
Dr. Marik, whose Virginia medical license expired in 2022, “is no longer treating patients and has dedicated his time and efforts to the FLCCC Alliance,” the statement said.
Dr. Caplan served as a director, officer, partner, employee, advisor, consultant, or trustee for Johnson & Johnson’s Panel for Compassionate Drug Use (unpaid position) and is a contributing author and advisor for this news organization. Ms. Parmet reports no relevant financial relationships.
A version of this article appeared on Medscape.com.
Data Trends 2024: Transgender and Gender-Affirming Care
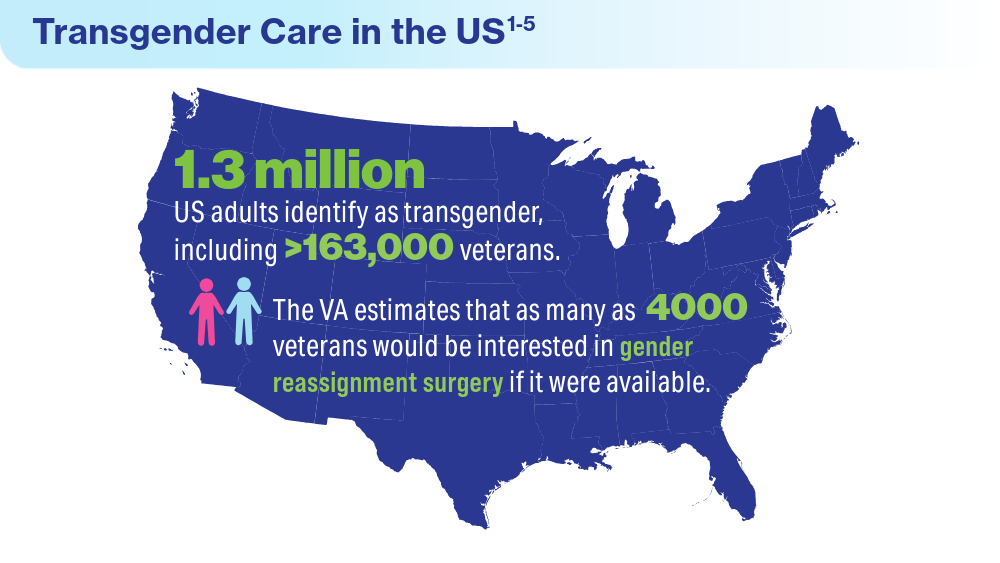
- Herman JL, Flores AR, O’Neill KK. How many adults and youth identify as transgender in the United States? UCLA School of Law Williams Institute. June 2022. Accessed April 15, 2024. https://williamsinstitute.law.ucla.edu/publications/trans-adults-united-states/
Boyer TL, Youk AO, Haas AP, et al. Suicide, homicide, and all-cause mortality among transgender and cisgender patients in the Veterans Health Administration. LGBT Health. 2021;8(3):173-180. doi:10.1089/lgbt.2020.0235
James SE, Herman JL, Rankin S, Keisling M, Mottet L, Anafi M. The report of the 2015 U.S. transgender survey. National Center for Transgender Equality. 2016. https://transequality.org/sites/default/files/docs/usts/USTS-Full-Report-Dec17.pdf
Jasuja GK, Reisman JI, Rao SR, et al. Social stressors and health among older transgender and gender diverse veterans. LGBT Health. 2023;10(2):148-157. doi:10.1089/lgbt.2022.0012
Shane L. VA again delays decision on transgender surgery options. Military Times. February 26, 2024. Accessed April 30, 2024. https://www.militarytimes.com/veterans/2024/02/26/va-again-delays-decision-on-transgender-surgery-options/
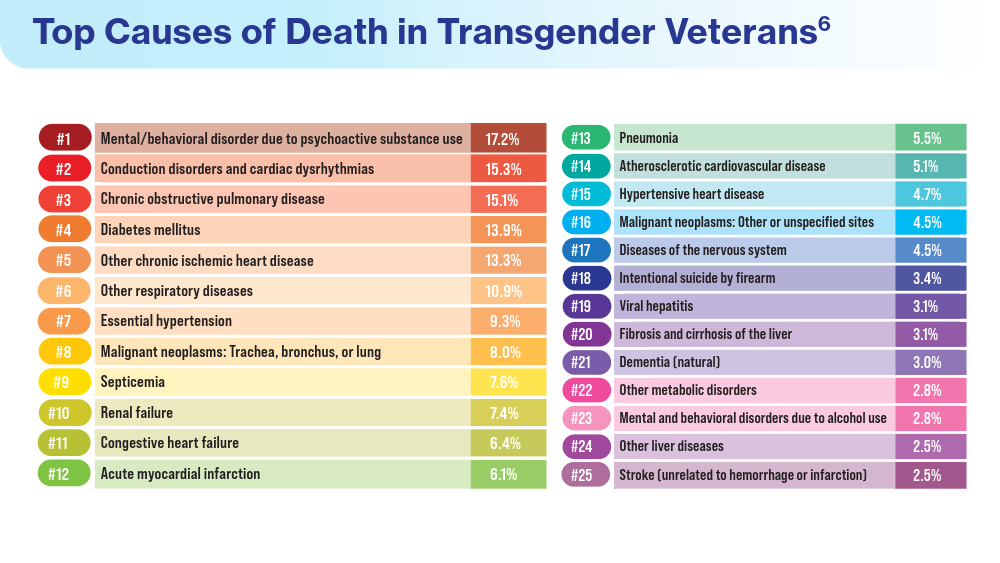
Henderson ER, Boyer TL, Wolfe HL, Blosnich JR. Causes of death of transgender and gender diverse veterans. Am J Prev Med. 2024;66(4):664-671. doi:10.1016/j.amepre.2023.11.014
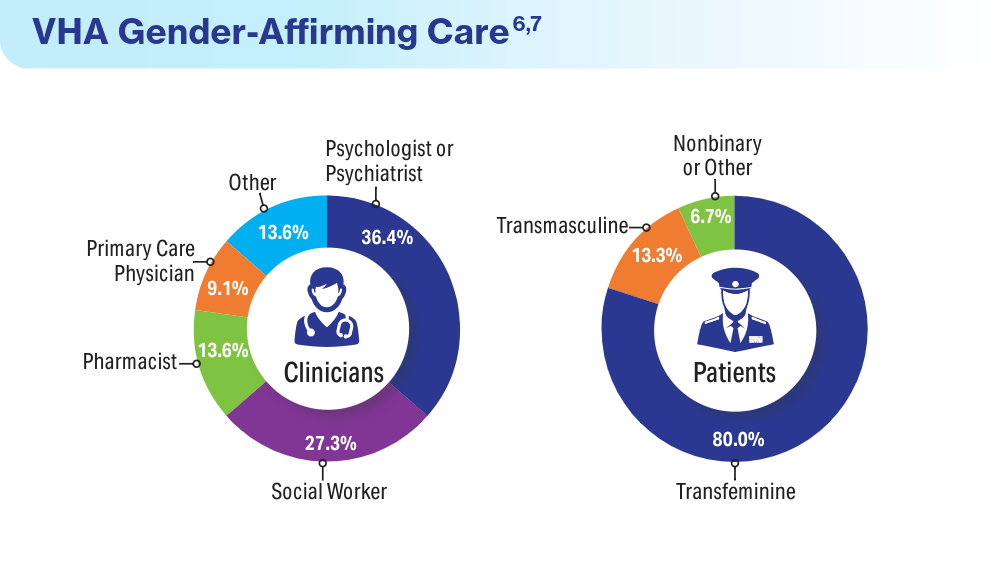
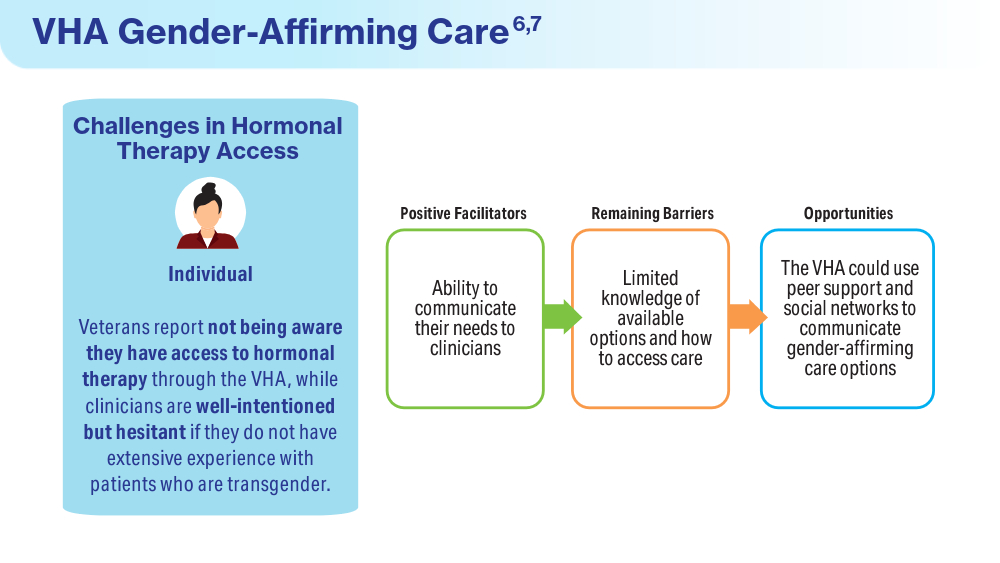
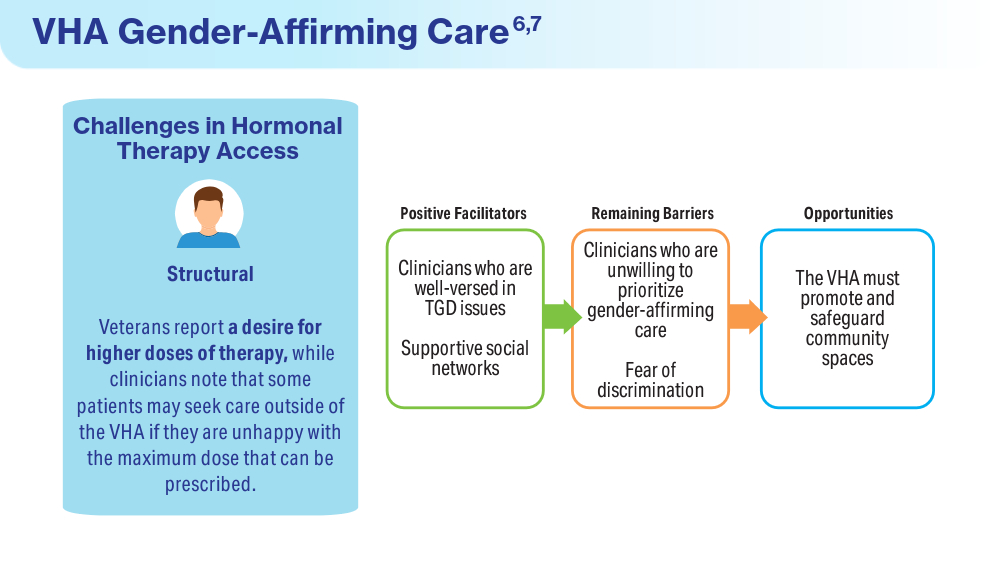
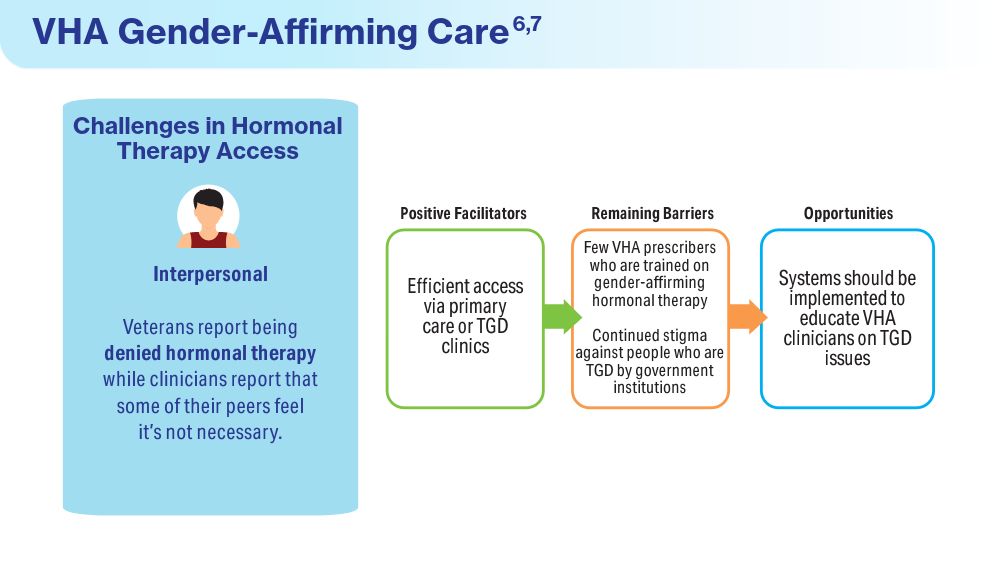
Wolfe HL, Boyer TL, Shipherd JC, Kauth MR, Jasuja GK, Blosnich JR. Barriers and facilitators to gender-affirming hormone therapy in the Veterans Health Administration. Ann Behav Med. 202316;57(12):1014-1023. doi:10.1093/abm/kaad035
- Herman JL, Flores AR, O’Neill KK. How many adults and youth identify as transgender in the United States? UCLA School of Law Williams Institute. June 2022. Accessed April 15, 2024. https://williamsinstitute.law.ucla.edu/publications/trans-adults-united-states/
Boyer TL, Youk AO, Haas AP, et al. Suicide, homicide, and all-cause mortality among transgender and cisgender patients in the Veterans Health Administration. LGBT Health. 2021;8(3):173-180. doi:10.1089/lgbt.2020.0235
James SE, Herman JL, Rankin S, Keisling M, Mottet L, Anafi M. The report of the 2015 U.S. transgender survey. National Center for Transgender Equality. 2016. https://transequality.org/sites/default/files/docs/usts/USTS-Full-Report-Dec17.pdf
Jasuja GK, Reisman JI, Rao SR, et al. Social stressors and health among older transgender and gender diverse veterans. LGBT Health. 2023;10(2):148-157. doi:10.1089/lgbt.2022.0012
Shane L. VA again delays decision on transgender surgery options. Military Times. February 26, 2024. Accessed April 30, 2024. https://www.militarytimes.com/veterans/2024/02/26/va-again-delays-decision-on-transgender-surgery-options/
Henderson ER, Boyer TL, Wolfe HL, Blosnich JR. Causes of death of transgender and gender diverse veterans. Am J Prev Med. 2024;66(4):664-671. doi:10.1016/j.amepre.2023.11.014
Wolfe HL, Boyer TL, Shipherd JC, Kauth MR, Jasuja GK, Blosnich JR. Barriers and facilitators to gender-affirming hormone therapy in the Veterans Health Administration. Ann Behav Med. 202316;57(12):1014-1023. doi:10.1093/abm/kaad035
- Herman JL, Flores AR, O’Neill KK. How many adults and youth identify as transgender in the United States? UCLA School of Law Williams Institute. June 2022. Accessed April 15, 2024. https://williamsinstitute.law.ucla.edu/publications/trans-adults-united-states/
Boyer TL, Youk AO, Haas AP, et al. Suicide, homicide, and all-cause mortality among transgender and cisgender patients in the Veterans Health Administration. LGBT Health. 2021;8(3):173-180. doi:10.1089/lgbt.2020.0235
James SE, Herman JL, Rankin S, Keisling M, Mottet L, Anafi M. The report of the 2015 U.S. transgender survey. National Center for Transgender Equality. 2016. https://transequality.org/sites/default/files/docs/usts/USTS-Full-Report-Dec17.pdf
Jasuja GK, Reisman JI, Rao SR, et al. Social stressors and health among older transgender and gender diverse veterans. LGBT Health. 2023;10(2):148-157. doi:10.1089/lgbt.2022.0012
Shane L. VA again delays decision on transgender surgery options. Military Times. February 26, 2024. Accessed April 30, 2024. https://www.militarytimes.com/veterans/2024/02/26/va-again-delays-decision-on-transgender-surgery-options/
Henderson ER, Boyer TL, Wolfe HL, Blosnich JR. Causes of death of transgender and gender diverse veterans. Am J Prev Med. 2024;66(4):664-671. doi:10.1016/j.amepre.2023.11.014
Wolfe HL, Boyer TL, Shipherd JC, Kauth MR, Jasuja GK, Blosnich JR. Barriers and facilitators to gender-affirming hormone therapy in the Veterans Health Administration. Ann Behav Med. 202316;57(12):1014-1023. doi:10.1093/abm/kaad035
FDA Grants Livdelzi Accelerated Approval for Primary Biliary Cholangitis
, or as monotherapy in those who can’t tolerate UDCA.
Livdelzi, a selective agonist of peroxisome proliferator–activated receptor delta, is not recommended in adults who have or develop decompensated cirrhosis.
PBC is a rare, chronic, autoimmune disease of the bile ducts that affects roughly 130,000 Americans, primarily women, and can cause liver damage and possible liver failure if untreated. The disease currently has no cure.
The FDA approved Livdelzi based largely on results of the phase 3 RESPONSE study, in which the drug significantly improved liver biomarkers of disease activity and bothersome symptoms of pruritus in adults with PBC.
The primary endpoint of the trial was a biochemical response, defined as an alkaline phosphatase (ALP) level < 1.67 times the upper limit of the normal range, with a decrease of 15% or more from baseline, and a normal total bilirubin level, at 12 months.
After 12 months, 62% of patients taking Livdelzi met the primary endpoint vs 20% of patients taking placebo.
In addition, significantly more patients taking Livdelzi than placebo had normalization of the ALP level (25% vs 0%). The average decrease in ALP from baseline was 42.4% in the Livdelzi group vs 4.3% in the placebo group.
At 12 months, alanine aminotransferase and gamma-glutamyl transferase levels were reduced by 23.5% and 39.1%, respectively, in the Livdelzi group compared with 6.5% and 11.4%, respectively, in the placebo group.
A key secondary endpoint was change in patient-reported pruritus.
At baseline, 38.3% of patients in the Livdelzi group and 35.4% of those in the placebo group had moderate to severe pruritus, with a daily numerical rating scale (NRS) score ≥ 4 out of 10.
Among these patients, the reduction from baseline in the pruritus NRS score at month 6 was significantly greater with Livdelzi than with placebo (change from baseline, -3.2 vs -1.7 points). These improvements were sustained through 12 months.
Improvements on the 5-D Itch Scale in both the moderate- to severe-pruritis population and the overall population also favored Livdelzi over placebo for itch relief, which had a positive impact on sleep.
“The availability of a new treatment option that can help reduce [the] intense itching while also improving biomarkers of active liver disease is a milestone for our community,” Carol Roberts, president, The PBCers Organization, said in a news release announcing the approval.
The most common adverse reactions with Livdelzi were headache, abdominal pain, nausea, abdominal distension, and dizziness.
The company noted that the FDA granted accelerated approval for Livdelzi based on a reduction of ALP. Improvement in survival or prevention of liver decompensation events have not been demonstrated. Continued approval of Livdelzi for PBC may be contingent on verification and description of clinical benefit in confirmatory trial(s).
A version of this article appeared on Medscape.com.
, or as monotherapy in those who can’t tolerate UDCA.
Livdelzi, a selective agonist of peroxisome proliferator–activated receptor delta, is not recommended in adults who have or develop decompensated cirrhosis.
PBC is a rare, chronic, autoimmune disease of the bile ducts that affects roughly 130,000 Americans, primarily women, and can cause liver damage and possible liver failure if untreated. The disease currently has no cure.
The FDA approved Livdelzi based largely on results of the phase 3 RESPONSE study, in which the drug significantly improved liver biomarkers of disease activity and bothersome symptoms of pruritus in adults with PBC.
The primary endpoint of the trial was a biochemical response, defined as an alkaline phosphatase (ALP) level < 1.67 times the upper limit of the normal range, with a decrease of 15% or more from baseline, and a normal total bilirubin level, at 12 months.
After 12 months, 62% of patients taking Livdelzi met the primary endpoint vs 20% of patients taking placebo.
In addition, significantly more patients taking Livdelzi than placebo had normalization of the ALP level (25% vs 0%). The average decrease in ALP from baseline was 42.4% in the Livdelzi group vs 4.3% in the placebo group.
At 12 months, alanine aminotransferase and gamma-glutamyl transferase levels were reduced by 23.5% and 39.1%, respectively, in the Livdelzi group compared with 6.5% and 11.4%, respectively, in the placebo group.
A key secondary endpoint was change in patient-reported pruritus.
At baseline, 38.3% of patients in the Livdelzi group and 35.4% of those in the placebo group had moderate to severe pruritus, with a daily numerical rating scale (NRS) score ≥ 4 out of 10.
Among these patients, the reduction from baseline in the pruritus NRS score at month 6 was significantly greater with Livdelzi than with placebo (change from baseline, -3.2 vs -1.7 points). These improvements were sustained through 12 months.
Improvements on the 5-D Itch Scale in both the moderate- to severe-pruritis population and the overall population also favored Livdelzi over placebo for itch relief, which had a positive impact on sleep.
“The availability of a new treatment option that can help reduce [the] intense itching while also improving biomarkers of active liver disease is a milestone for our community,” Carol Roberts, president, The PBCers Organization, said in a news release announcing the approval.
The most common adverse reactions with Livdelzi were headache, abdominal pain, nausea, abdominal distension, and dizziness.
The company noted that the FDA granted accelerated approval for Livdelzi based on a reduction of ALP. Improvement in survival or prevention of liver decompensation events have not been demonstrated. Continued approval of Livdelzi for PBC may be contingent on verification and description of clinical benefit in confirmatory trial(s).
A version of this article appeared on Medscape.com.
, or as monotherapy in those who can’t tolerate UDCA.
Livdelzi, a selective agonist of peroxisome proliferator–activated receptor delta, is not recommended in adults who have or develop decompensated cirrhosis.
PBC is a rare, chronic, autoimmune disease of the bile ducts that affects roughly 130,000 Americans, primarily women, and can cause liver damage and possible liver failure if untreated. The disease currently has no cure.
The FDA approved Livdelzi based largely on results of the phase 3 RESPONSE study, in which the drug significantly improved liver biomarkers of disease activity and bothersome symptoms of pruritus in adults with PBC.
The primary endpoint of the trial was a biochemical response, defined as an alkaline phosphatase (ALP) level < 1.67 times the upper limit of the normal range, with a decrease of 15% or more from baseline, and a normal total bilirubin level, at 12 months.
After 12 months, 62% of patients taking Livdelzi met the primary endpoint vs 20% of patients taking placebo.
In addition, significantly more patients taking Livdelzi than placebo had normalization of the ALP level (25% vs 0%). The average decrease in ALP from baseline was 42.4% in the Livdelzi group vs 4.3% in the placebo group.
At 12 months, alanine aminotransferase and gamma-glutamyl transferase levels were reduced by 23.5% and 39.1%, respectively, in the Livdelzi group compared with 6.5% and 11.4%, respectively, in the placebo group.
A key secondary endpoint was change in patient-reported pruritus.
At baseline, 38.3% of patients in the Livdelzi group and 35.4% of those in the placebo group had moderate to severe pruritus, with a daily numerical rating scale (NRS) score ≥ 4 out of 10.
Among these patients, the reduction from baseline in the pruritus NRS score at month 6 was significantly greater with Livdelzi than with placebo (change from baseline, -3.2 vs -1.7 points). These improvements were sustained through 12 months.
Improvements on the 5-D Itch Scale in both the moderate- to severe-pruritis population and the overall population also favored Livdelzi over placebo for itch relief, which had a positive impact on sleep.
“The availability of a new treatment option that can help reduce [the] intense itching while also improving biomarkers of active liver disease is a milestone for our community,” Carol Roberts, president, The PBCers Organization, said in a news release announcing the approval.
The most common adverse reactions with Livdelzi were headache, abdominal pain, nausea, abdominal distension, and dizziness.
The company noted that the FDA granted accelerated approval for Livdelzi based on a reduction of ALP. Improvement in survival or prevention of liver decompensation events have not been demonstrated. Continued approval of Livdelzi for PBC may be contingent on verification and description of clinical benefit in confirmatory trial(s).
A version of this article appeared on Medscape.com.
CBD Use in Pregnant People Double That of Nonpregnant Counterparts
Pregnant women in a large North American sample reported nearly double the rate of cannabidiol (CBD) use compared with nonpregnant women, new data published in a research letter in Obstetrics & Gynecology indicates.
Healthcare providers should be aware of the high rate of CBD use in pregnancy, especially as legal use of cannabis is increasing faster than evidence on outcomes for exposed offspring, note the researchers, led by Devika Bhatia, MD, from the Department of Psychiatry, Colorado School of Medicine, University of Colorado Anschutz Medical Campus in Aurora.
In an accompanying editorial, Torri D. Metz, MD, MS, deputy editor for obstetrics for Obstetrics & Gynecology, writes that the study “is critically important.” She points out that pregnant individuals may perceive that CBD is a safe drug to use in pregnancy, despite there being essentially no data examining whether or not this is the case.
Large Dataset From United States and Canada
Researchers used data from the International Cannabis Policy Study (2019-2021), a repeated cross-sectional survey of people aged 16-65 years in the United States and Canada. There were 66,457 women in the sample, including 1096 pregnant women.
Particularly concerning, the authors write, is the prenatal use of CBD-only products. Those products are advertised to contain only CBD, rather than tetrahydrocannabinol (THC). They point out CBD-only products are often legal in North America and often marketed as supplements.
The prevalence of CBD-only use in pregnant women in the study was 20.4% compared with 11.3% among nonpregnant women, P < .001. The top reason for use by pregnant women was anxiety (58.4%). Other top reasons included depression (40.3%), posttraumatic stress disorder (32.1%), pain (52.3%), headache (35.6%), and nausea or vomiting (31.9%).
“Nonpregnant women were significantly more likely to report using CBD for pain, sleep, general well-being, and ‘other’ physical or mental health reasons, or to not use CBD for mental health,” the authors write, adding that the reasons for CBD use highlight drivers that may be important to address in treating pregnant patients.
Provider Endorsement in Some Cases
Dr. Metz, associate professor of obstetrics and gynecology with the University of Utah Health in Salt Lake City, says in some cases women may be getting endorsement of CBD use from their provider or at least implied support when CBD is prescribed. In the study, pregnant women had 2.33 times greater adjusted odds of having a CBD prescription than nonpregnant women (95% confidence interval, 1.27-2.88).
She points to another cross-sectional study of more than 10,000 participants using PRAMS (Pregnancy Risk Assessment Monitoring System) data that found that “from 2017 to 2019, 63% of pregnant women reported that they were not told to avoid cannabis use in pregnancy, and 8% noted that they were advised to use cannabis by their prenatal care practitioner.”
The American College of Obstetricians and Gynecologists recommends against prescribing cannabis products for pregnant or lactating women.
Studies that have explored THC and its metabolites have shown “a consistent association between cannabis use and decreased fetal growth,” Dr. Metz noted. “There also remain persistent concerns about the long-term neurodevelopmental effects of maternal cannabis use on the fetus and, subsequently, the newborn.”
Limitations of the study include the self-reported responses and participants’ ability to accurately distinguish between CBD-only and THC-containing products.
Because self-reports of CBD use in pregnancy may be drastically underestimated and nonreliable, Dr. Metz writes, development of blood and urine screens to help detect CBD product use “will be helpful in moving the field forward.”
Study senior author David Hammond, PhD, has been a paid expert witness on behalf of public health authorities in response to legal challenges from the cannabis, tobacco, vaping, and food industries. Other authors did not report any potential conflicts. Dr. Metz reports personal fees from Pfizer, and grants from Pfizer for her role as a site principal investigator for SARS-CoV-2 vaccination and for her role as a site PI for RSV vaccination in pregnancy study.
Pregnant women in a large North American sample reported nearly double the rate of cannabidiol (CBD) use compared with nonpregnant women, new data published in a research letter in Obstetrics & Gynecology indicates.
Healthcare providers should be aware of the high rate of CBD use in pregnancy, especially as legal use of cannabis is increasing faster than evidence on outcomes for exposed offspring, note the researchers, led by Devika Bhatia, MD, from the Department of Psychiatry, Colorado School of Medicine, University of Colorado Anschutz Medical Campus in Aurora.
In an accompanying editorial, Torri D. Metz, MD, MS, deputy editor for obstetrics for Obstetrics & Gynecology, writes that the study “is critically important.” She points out that pregnant individuals may perceive that CBD is a safe drug to use in pregnancy, despite there being essentially no data examining whether or not this is the case.
Large Dataset From United States and Canada
Researchers used data from the International Cannabis Policy Study (2019-2021), a repeated cross-sectional survey of people aged 16-65 years in the United States and Canada. There were 66,457 women in the sample, including 1096 pregnant women.
Particularly concerning, the authors write, is the prenatal use of CBD-only products. Those products are advertised to contain only CBD, rather than tetrahydrocannabinol (THC). They point out CBD-only products are often legal in North America and often marketed as supplements.
The prevalence of CBD-only use in pregnant women in the study was 20.4% compared with 11.3% among nonpregnant women, P < .001. The top reason for use by pregnant women was anxiety (58.4%). Other top reasons included depression (40.3%), posttraumatic stress disorder (32.1%), pain (52.3%), headache (35.6%), and nausea or vomiting (31.9%).
“Nonpregnant women were significantly more likely to report using CBD for pain, sleep, general well-being, and ‘other’ physical or mental health reasons, or to not use CBD for mental health,” the authors write, adding that the reasons for CBD use highlight drivers that may be important to address in treating pregnant patients.
Provider Endorsement in Some Cases
Dr. Metz, associate professor of obstetrics and gynecology with the University of Utah Health in Salt Lake City, says in some cases women may be getting endorsement of CBD use from their provider or at least implied support when CBD is prescribed. In the study, pregnant women had 2.33 times greater adjusted odds of having a CBD prescription than nonpregnant women (95% confidence interval, 1.27-2.88).
She points to another cross-sectional study of more than 10,000 participants using PRAMS (Pregnancy Risk Assessment Monitoring System) data that found that “from 2017 to 2019, 63% of pregnant women reported that they were not told to avoid cannabis use in pregnancy, and 8% noted that they were advised to use cannabis by their prenatal care practitioner.”
The American College of Obstetricians and Gynecologists recommends against prescribing cannabis products for pregnant or lactating women.
Studies that have explored THC and its metabolites have shown “a consistent association between cannabis use and decreased fetal growth,” Dr. Metz noted. “There also remain persistent concerns about the long-term neurodevelopmental effects of maternal cannabis use on the fetus and, subsequently, the newborn.”
Limitations of the study include the self-reported responses and participants’ ability to accurately distinguish between CBD-only and THC-containing products.
Because self-reports of CBD use in pregnancy may be drastically underestimated and nonreliable, Dr. Metz writes, development of blood and urine screens to help detect CBD product use “will be helpful in moving the field forward.”
Study senior author David Hammond, PhD, has been a paid expert witness on behalf of public health authorities in response to legal challenges from the cannabis, tobacco, vaping, and food industries. Other authors did not report any potential conflicts. Dr. Metz reports personal fees from Pfizer, and grants from Pfizer for her role as a site principal investigator for SARS-CoV-2 vaccination and for her role as a site PI for RSV vaccination in pregnancy study.
Pregnant women in a large North American sample reported nearly double the rate of cannabidiol (CBD) use compared with nonpregnant women, new data published in a research letter in Obstetrics & Gynecology indicates.
Healthcare providers should be aware of the high rate of CBD use in pregnancy, especially as legal use of cannabis is increasing faster than evidence on outcomes for exposed offspring, note the researchers, led by Devika Bhatia, MD, from the Department of Psychiatry, Colorado School of Medicine, University of Colorado Anschutz Medical Campus in Aurora.
In an accompanying editorial, Torri D. Metz, MD, MS, deputy editor for obstetrics for Obstetrics & Gynecology, writes that the study “is critically important.” She points out that pregnant individuals may perceive that CBD is a safe drug to use in pregnancy, despite there being essentially no data examining whether or not this is the case.
Large Dataset From United States and Canada
Researchers used data from the International Cannabis Policy Study (2019-2021), a repeated cross-sectional survey of people aged 16-65 years in the United States and Canada. There were 66,457 women in the sample, including 1096 pregnant women.
Particularly concerning, the authors write, is the prenatal use of CBD-only products. Those products are advertised to contain only CBD, rather than tetrahydrocannabinol (THC). They point out CBD-only products are often legal in North America and often marketed as supplements.
The prevalence of CBD-only use in pregnant women in the study was 20.4% compared with 11.3% among nonpregnant women, P < .001. The top reason for use by pregnant women was anxiety (58.4%). Other top reasons included depression (40.3%), posttraumatic stress disorder (32.1%), pain (52.3%), headache (35.6%), and nausea or vomiting (31.9%).
“Nonpregnant women were significantly more likely to report using CBD for pain, sleep, general well-being, and ‘other’ physical or mental health reasons, or to not use CBD for mental health,” the authors write, adding that the reasons for CBD use highlight drivers that may be important to address in treating pregnant patients.
Provider Endorsement in Some Cases
Dr. Metz, associate professor of obstetrics and gynecology with the University of Utah Health in Salt Lake City, says in some cases women may be getting endorsement of CBD use from their provider or at least implied support when CBD is prescribed. In the study, pregnant women had 2.33 times greater adjusted odds of having a CBD prescription than nonpregnant women (95% confidence interval, 1.27-2.88).
She points to another cross-sectional study of more than 10,000 participants using PRAMS (Pregnancy Risk Assessment Monitoring System) data that found that “from 2017 to 2019, 63% of pregnant women reported that they were not told to avoid cannabis use in pregnancy, and 8% noted that they were advised to use cannabis by their prenatal care practitioner.”
The American College of Obstetricians and Gynecologists recommends against prescribing cannabis products for pregnant or lactating women.
Studies that have explored THC and its metabolites have shown “a consistent association between cannabis use and decreased fetal growth,” Dr. Metz noted. “There also remain persistent concerns about the long-term neurodevelopmental effects of maternal cannabis use on the fetus and, subsequently, the newborn.”
Limitations of the study include the self-reported responses and participants’ ability to accurately distinguish between CBD-only and THC-containing products.
Because self-reports of CBD use in pregnancy may be drastically underestimated and nonreliable, Dr. Metz writes, development of blood and urine screens to help detect CBD product use “will be helpful in moving the field forward.”
Study senior author David Hammond, PhD, has been a paid expert witness on behalf of public health authorities in response to legal challenges from the cannabis, tobacco, vaping, and food industries. Other authors did not report any potential conflicts. Dr. Metz reports personal fees from Pfizer, and grants from Pfizer for her role as a site principal investigator for SARS-CoV-2 vaccination and for her role as a site PI for RSV vaccination in pregnancy study.
FROM OBSTETRICS & GYNECOLOGY
A Checklist for Compounded Semaglutide or Tirzepatide
Consider this: If you’re taking your children to the beach, how do you protect them from drowning? You don’t tell them, “Don’t go into the ocean.” You teach them how to swim; you give them floaties; and you accompany them in the water and go in only when a lifeguard is present. In other words, you give them all the tools to protect themselves because you know they will go into the ocean anyway.
Patients are diving into the ocean. Patients with obesity, who know that a treatment for their disease exists but is inaccessible, are diving into the ocean. Unfortunately, they are diving in without floaties or a lifeguard, and well-meaning bystanders are simply telling them to not go.
Compounded peptides are an ocean of alternative therapies. Even though compounding pharmacists need specialized training, facilities and equipment need to be properly certified, and final dosage forms need extensive testing, pharmacies are not equal when it comes to sterile compounding. Regulatory agencies such as the US Food and Drug Administration (FDA) have expressed caution around compounded semaglutide. Professional societies such as the Obesity Medicine Association (OMA) advise against compounded peptides because they lack clinical trials that prove their safety and efficacy. Ask any individual doctor and you are likely to receive a range of opinions.
As an endocrinologist specializing in obesity, I practice evidence-based medicine as much as possible. However, I also recognize how today’s dysfunctional medical system compels patients to dive into that ocean in search of an alternative solution.
With the help of pharmacists, compounding pharmacists, researchers, and clinicians, here is a checklist for patients who seek compounded semaglutide or tirzepatide:
- Check the state licensing board website to see if there have been any complaints or disciplinary actions made against the pharmacy facility. These government-maintained websites vary in searchability and user-friendliness, but you are specifically looking for whether the FDA ever issued a 483 form.
- Ask for the pharmacy’s state board inspection report. There should be at least one of these reports, issued at the pharmacy’s founding, and there may be more depending on individual state regulations on frequencies of inspections.
- Ask if the compounding pharmacy is accredited by the Pharmacy Compounding Accreditation Board (PCAB). Accreditation is an extra optional step that some compounding pharmacies take to be legitimized by a third party.
- Ask if the pharmacy follows Current Good Manufacturing Practice (CGMP). CGMP is not required of 503a pharmacies, which are pharmacies that provide semaglutide or tirzepatide directly to patients, but following CGMP means an extra level of quality assurance. The bare minimum for anyone doing sterile compounding in the United States is to meet the standards found in the US Pharmacopeia, chapter <797>, Sterile Compounding.
- Ask your compounding pharmacy where they source the medication’s active pharmaceutical ingredient (API).
- Find out if this supplier is registered with the FDA by searching here or here.
- Confirm that semaglutide base, not semaglutide salt, is used in the compounding process.
- Request a certificate of analysis (COA) of the active pharmaceutical ingredient, which should be semaglutide base. This shows you whether the medication has impurities or byproducts due to its manufacturing process.
- Ask if they have third-party confirmation of potency, stability, and sterility testing of the final product.
In generating this guidance, I’m not endorsing compounded peptides, and in fact, I recognize that there is nothing keeping small-time compounding pharmacies from skirting some of these quality measures, falsifying records, and flying under the radar. However, I hope this checklist serves as a starting point for education and risk mitigation. If a compounder is unwilling or unable to answer these questions, consider it a red flag and look elsewhere.
In an ideal world, the state regulators or the FDA would proactively supervise instead of reactively monitor; trusted compounding pharmacies would be systematically activated to ease medication shortages; and patients with obesity would have access to safe and efficacious treatments for their disease. Until then, we as providers can acknowledge the disappointments of our healthcare system while still developing realistic and individualized solutions that prioritize patient care and safety.
Dr. Tchang is assistant professor, Clinical Medicine, Division of Endocrinology, Diabetes, and Metabolism, Weill Cornell Medicine, and a physician, Department of Medicine, Iris Cantor Women’s Health Center, Comprehensive Weight Control Center, New York. She is an adviser for Novo Nordisk, which manufactures Wegovy, and an adviser for Ro, a telehealth company that offers compounded semaglutide, and serves or has served as a director, officer, partner, employee, advisor, consultant, or trustee for Gelesis and Novo Nordisk.
A version of this article first appeared on Medscape.com.
Consider this: If you’re taking your children to the beach, how do you protect them from drowning? You don’t tell them, “Don’t go into the ocean.” You teach them how to swim; you give them floaties; and you accompany them in the water and go in only when a lifeguard is present. In other words, you give them all the tools to protect themselves because you know they will go into the ocean anyway.
Patients are diving into the ocean. Patients with obesity, who know that a treatment for their disease exists but is inaccessible, are diving into the ocean. Unfortunately, they are diving in without floaties or a lifeguard, and well-meaning bystanders are simply telling them to not go.
Compounded peptides are an ocean of alternative therapies. Even though compounding pharmacists need specialized training, facilities and equipment need to be properly certified, and final dosage forms need extensive testing, pharmacies are not equal when it comes to sterile compounding. Regulatory agencies such as the US Food and Drug Administration (FDA) have expressed caution around compounded semaglutide. Professional societies such as the Obesity Medicine Association (OMA) advise against compounded peptides because they lack clinical trials that prove their safety and efficacy. Ask any individual doctor and you are likely to receive a range of opinions.
As an endocrinologist specializing in obesity, I practice evidence-based medicine as much as possible. However, I also recognize how today’s dysfunctional medical system compels patients to dive into that ocean in search of an alternative solution.
With the help of pharmacists, compounding pharmacists, researchers, and clinicians, here is a checklist for patients who seek compounded semaglutide or tirzepatide:
- Check the state licensing board website to see if there have been any complaints or disciplinary actions made against the pharmacy facility. These government-maintained websites vary in searchability and user-friendliness, but you are specifically looking for whether the FDA ever issued a 483 form.
- Ask for the pharmacy’s state board inspection report. There should be at least one of these reports, issued at the pharmacy’s founding, and there may be more depending on individual state regulations on frequencies of inspections.
- Ask if the compounding pharmacy is accredited by the Pharmacy Compounding Accreditation Board (PCAB). Accreditation is an extra optional step that some compounding pharmacies take to be legitimized by a third party.
- Ask if the pharmacy follows Current Good Manufacturing Practice (CGMP). CGMP is not required of 503a pharmacies, which are pharmacies that provide semaglutide or tirzepatide directly to patients, but following CGMP means an extra level of quality assurance. The bare minimum for anyone doing sterile compounding in the United States is to meet the standards found in the US Pharmacopeia, chapter <797>, Sterile Compounding.
- Ask your compounding pharmacy where they source the medication’s active pharmaceutical ingredient (API).
- Find out if this supplier is registered with the FDA by searching here or here.
- Confirm that semaglutide base, not semaglutide salt, is used in the compounding process.
- Request a certificate of analysis (COA) of the active pharmaceutical ingredient, which should be semaglutide base. This shows you whether the medication has impurities or byproducts due to its manufacturing process.
- Ask if they have third-party confirmation of potency, stability, and sterility testing of the final product.
In generating this guidance, I’m not endorsing compounded peptides, and in fact, I recognize that there is nothing keeping small-time compounding pharmacies from skirting some of these quality measures, falsifying records, and flying under the radar. However, I hope this checklist serves as a starting point for education and risk mitigation. If a compounder is unwilling or unable to answer these questions, consider it a red flag and look elsewhere.
In an ideal world, the state regulators or the FDA would proactively supervise instead of reactively monitor; trusted compounding pharmacies would be systematically activated to ease medication shortages; and patients with obesity would have access to safe and efficacious treatments for their disease. Until then, we as providers can acknowledge the disappointments of our healthcare system while still developing realistic and individualized solutions that prioritize patient care and safety.
Dr. Tchang is assistant professor, Clinical Medicine, Division of Endocrinology, Diabetes, and Metabolism, Weill Cornell Medicine, and a physician, Department of Medicine, Iris Cantor Women’s Health Center, Comprehensive Weight Control Center, New York. She is an adviser for Novo Nordisk, which manufactures Wegovy, and an adviser for Ro, a telehealth company that offers compounded semaglutide, and serves or has served as a director, officer, partner, employee, advisor, consultant, or trustee for Gelesis and Novo Nordisk.
A version of this article first appeared on Medscape.com.
Consider this: If you’re taking your children to the beach, how do you protect them from drowning? You don’t tell them, “Don’t go into the ocean.” You teach them how to swim; you give them floaties; and you accompany them in the water and go in only when a lifeguard is present. In other words, you give them all the tools to protect themselves because you know they will go into the ocean anyway.
Patients are diving into the ocean. Patients with obesity, who know that a treatment for their disease exists but is inaccessible, are diving into the ocean. Unfortunately, they are diving in without floaties or a lifeguard, and well-meaning bystanders are simply telling them to not go.
Compounded peptides are an ocean of alternative therapies. Even though compounding pharmacists need specialized training, facilities and equipment need to be properly certified, and final dosage forms need extensive testing, pharmacies are not equal when it comes to sterile compounding. Regulatory agencies such as the US Food and Drug Administration (FDA) have expressed caution around compounded semaglutide. Professional societies such as the Obesity Medicine Association (OMA) advise against compounded peptides because they lack clinical trials that prove their safety and efficacy. Ask any individual doctor and you are likely to receive a range of opinions.
As an endocrinologist specializing in obesity, I practice evidence-based medicine as much as possible. However, I also recognize how today’s dysfunctional medical system compels patients to dive into that ocean in search of an alternative solution.
With the help of pharmacists, compounding pharmacists, researchers, and clinicians, here is a checklist for patients who seek compounded semaglutide or tirzepatide:
- Check the state licensing board website to see if there have been any complaints or disciplinary actions made against the pharmacy facility. These government-maintained websites vary in searchability and user-friendliness, but you are specifically looking for whether the FDA ever issued a 483 form.
- Ask for the pharmacy’s state board inspection report. There should be at least one of these reports, issued at the pharmacy’s founding, and there may be more depending on individual state regulations on frequencies of inspections.
- Ask if the compounding pharmacy is accredited by the Pharmacy Compounding Accreditation Board (PCAB). Accreditation is an extra optional step that some compounding pharmacies take to be legitimized by a third party.
- Ask if the pharmacy follows Current Good Manufacturing Practice (CGMP). CGMP is not required of 503a pharmacies, which are pharmacies that provide semaglutide or tirzepatide directly to patients, but following CGMP means an extra level of quality assurance. The bare minimum for anyone doing sterile compounding in the United States is to meet the standards found in the US Pharmacopeia, chapter <797>, Sterile Compounding.
- Ask your compounding pharmacy where they source the medication’s active pharmaceutical ingredient (API).
- Find out if this supplier is registered with the FDA by searching here or here.
- Confirm that semaglutide base, not semaglutide salt, is used in the compounding process.
- Request a certificate of analysis (COA) of the active pharmaceutical ingredient, which should be semaglutide base. This shows you whether the medication has impurities or byproducts due to its manufacturing process.
- Ask if they have third-party confirmation of potency, stability, and sterility testing of the final product.
In generating this guidance, I’m not endorsing compounded peptides, and in fact, I recognize that there is nothing keeping small-time compounding pharmacies from skirting some of these quality measures, falsifying records, and flying under the radar. However, I hope this checklist serves as a starting point for education and risk mitigation. If a compounder is unwilling or unable to answer these questions, consider it a red flag and look elsewhere.
In an ideal world, the state regulators or the FDA would proactively supervise instead of reactively monitor; trusted compounding pharmacies would be systematically activated to ease medication shortages; and patients with obesity would have access to safe and efficacious treatments for their disease. Until then, we as providers can acknowledge the disappointments of our healthcare system while still developing realistic and individualized solutions that prioritize patient care and safety.
Dr. Tchang is assistant professor, Clinical Medicine, Division of Endocrinology, Diabetes, and Metabolism, Weill Cornell Medicine, and a physician, Department of Medicine, Iris Cantor Women’s Health Center, Comprehensive Weight Control Center, New York. She is an adviser for Novo Nordisk, which manufactures Wegovy, and an adviser for Ro, a telehealth company that offers compounded semaglutide, and serves or has served as a director, officer, partner, employee, advisor, consultant, or trustee for Gelesis and Novo Nordisk.
A version of this article first appeared on Medscape.com.
PTSD Needs a New Name, Experts Say — Here’s Why
In a bid to reduce stigma and improve treatment rates, for inclusion in the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition, Text Revision (DSM-5-TR). The APA’s policy is that a rolling name change is available if the current term is determined to be harmful.
Currently led by anesthesiologist Eugene Lipov, MD, clinical assistant professor, University of Illinois Chicago, and chief medical officer of Stella Center, also in Chicago, the formal request for the proposed name change to the APA’s DSM-5-TR Steering Committee in August 2023.
The APA Steering Committee rejected the proposed name change in November 2023, citing a “lack of convincing evidence.” However, Dr. Lipov and colleagues remain undeterred and continue to advocate for the change.
“The word ‘disorder’ is both imprecise and stigmatizing,” Dr. Lipov said. “Because of stigma, many people with PTSD — especially those in the military — don’t get help, which my research has demonstrated.”
Patients are more likely to seek help if their symptoms are framed as manifestations of an injury that is diagnosable and treatable, like a broken leg, Dr. Lipov said. “Stigma can kill in very real ways, since delayed care or lack of care can directly lead to suicides, thus satisfying the reduce harm requirement for the name change.”
Neurobiology of Trauma
Dr. Lipov grew up with a veteran father affected by PTSD and a mother with debilitating depression who eventually took her life. “I understand the impact of trauma very well,” he said.
Although not a psychiatrist, Dr. Lipov pioneered a highly successful treatment for PTSD by adapting an anesthetic technique — the stellate ganglion block (SGB) — to reverse many trauma symptoms through the process of “rebooting.”
This involves reversing the activity of the sympathetic nervous system — the fight-or-flight response — to the pretrauma state by anesthetizing the sympathetic ganglion in the neck. Investigating how SGB can help ameliorate the symptoms of PTSD led him to investigate and describe the neurobiology of PTSD and the mechanism of action of SGB.
The impact of SGD on PTSD was supported by a small neuroimaging study demonstrating that the right amygdala — the area of the brain associated with the fear response — was overactivated in patients with PTSD but that this region was deactivated after the administration of SGB, Dr. Lipov said.
“I believe that psychiatric conditions are actually physiologic brain changes that can be measured by advanced neuroimaging technologies and then physiologically treated,” he stated.
He noted that a growing body of literature suggests that use of the SGB for PTSD can be effective “because PTSD has a neurobiological basis and is essentially caused by an actual injury to the brain.”
A Natural Response, Not a Disorder
Dr. Lipov’s clinical work treating PTSD as a brain injury led him to connect with Frank Ochberg, MD, a founding board member of the International Society for Traumatic Stress Studies, former associate director of the National Institute of Mental Health, and former director of the Michigan Department of Mental Health.
In 2012, Dr. Ochberg teamed up with retired Army General Peter Chiarelli and Jonathan Shay, MD, PhD, author of Achilles in Vietnam: Combat Trauma and the Undoing of Character, to petition the DSM-5 Steering Committee to change the name of PTSD to PTSI in the upcoming DSM-5.
Dr. Ochberg explained that Gen. Chiarelli believed the term “disorder” suggests a preexisting issue prior to enlistment, potentially making an individual appear “weak.” He noted that this stigma is particularly troubling for military personnel, who often avoid seeking so they are not perceived as vulnerable, which can lead to potentially dire consequences, including suicide.
“We received endorsements from many quarters, not only advocates for service members or veterans,” Dr. Ochberg said.
This included feminists like Gloria Steinem, who championed the rights of women who had survived rape, incest, and domestic violence. As one advocate put it: “The natural human reaction to a life-threatening event should not be labeled a disorder.”
The DSM-5 Steering Committee declined to change the name. “Their feeling was that if we change the word ‘disorder’ to something else, we’d have to change every condition in the DSM that’s called a ‘disorder’. And they felt there really was nothing wrong with the word,” said Dr. Ochberg.
However, Dr. Lipov noted that other diagnoses have undergone name changes in the DSM for the sake of accuracy or stigma reduction. For example, the term mental retardation (DSM-IV) was changed to intellectual disability in DSM-5, and gender identity disorder was changed to gender dysphoria.
A decade later, Dr. Lipov decided to try again. To bolster his contention, he conducted a telephone survey of 1025 individuals. Of these, about 50% had a PTSD diagnosis.
Approximately two thirds of respondents agreed that a name change to PTSI would reduce the stigma associated with the term “PTSD.” Over half said it would increase the likelihood they would seek medical help. Those diagnosed with PTSD were most likely to endorse the name change.
Dr. Lipov conducts an ongoing survey of psychiatrists to ascertain their views on the potential name change and hopes to include findings in future research and communication with the DSM-5 Steering Committee. In addition, he has developed a new survey that expands upon his original survey, which specifically looked at individuals with PTSD.
“The new survey includes a wide range of people, many of whom have never been diagnosed. One of the questions we ask is whether they’ve ever heard of PTSD, and then we ask them about their reaction to the term,” he said.
A Barrier to Care
Psychiatrist Marcel Green, MD, director of Hudson Mind in New York City, refers to himself as an “interventional psychiatrist,” as he employs a comprehensive approach that includes not only medication and psychotherapy but also specialized techniques like SBG for severe anxiety-related physical symptoms and certain pain conditions.
Dr. Green, who is not involved in the name change initiative, agrees that the term “disorder” carries more stigma than “injury” for many groups, including those who have experienced childhood trauma, those struggling with substance abuse, or who are from backgrounds or peer groups where seeking mental health care is stigmatized.
Patients like these “are looking to me to give them a language to frame what they’re going through, and I tell them their symptoms are consistent with PTSD,” he said. “But they tell me don’t see themselves as having a disorder, which hinders their pursuit of care.”
Framing the condition as an “injury” also aligns with the approach of using biologic interventions to address the injury. Dr. Green has found SGB helpful in treating substance abuse disorder too, “which is a form of escape from the hyperactivation that accompanies PTSD.” And after the procedure, “they’re more receptive to therapy.”
Unfortunately, said Dr. Lipov, the DSM Steering Committee rejected his proposed name change, stating that the “concept of disorder as a dividing line from, eg, normal reactions to stress, is a core concept in the DSM, and the term has only rarely been removed.”
Moreover, the committee “did not see sufficient evidence ... that the name PTSD is stigmatizing and actually deters people with the disorder from seeking treatment who would not be deterred from doing so by PTSI.”
‘An Avenue for Dignity’
Ken Duckworth, MD, chief medical officer of the National Alliance on Mental Illness (NAMI), noted that the organization does not have an official position on this issue. However, he shared his own personal perspective.
There may be merit in the proposed name change, said Dr. Duckworth, but more evidence is needed. “If it’s clear, after rigorous studies have been performed and there’s compelling data, that calling it a ‘disorder’ rather than an ‘injury’ is actually preventing people from getting the care they need, then it merits serious attention.”
If so, Dr. Duckworth would be “interested in having a conversation with the policy team at NAMI to start to see if we could activate the DSM Committee.”
Roger McIntyre, MD, professor of psychiatry and pharmacology at the University of Toronto in Ontario, Canada, and head of the Mood Disorders Psychopharmacology Unit, said the name change initiative is a “really interesting proposal.”
Dr. McIntyre, chairman and executive director of the Brain and Cognition Discovery Foundation, also in Toronto, who is not involved in the initiative, has also heard “many people say that the term ‘disorder’ is stigmatizing and might even come across as pejorative in some ways.”
By contrast, “the word ‘injury’ parallels physical injury, and what we currently call ‘PTSD’ is a psychological or emotional injury no less devastating than torn tissue or broken bones,” added Dr. McIntyre, who is also the chairman of the board of the Depression and Bipolar Support Alliance.
Dr. Ochberg agreed. “In the military, ‘injury’ opens up an avenue for dignity, for a medal. Being injured and learning how to deal with an injury is part of having yet another honorable task that comes from being an honorable person who did an honorable thing.”
While disappointed, Dr. Lipov does not plan to give up on his vision. “I will continue to amass evidence that the word ‘PTSD’ is stigmatizing and indeed does prevent people from seeking care and will resubmit the proposal to the DSM Steering Committee when I have gathered a larger body of compelling evidence.”
Currently, Dr. Lipov is in active discussions with the special operations force of the US Army to obtain more evidence. “This will be the follow-up to bolster the opinion of Peter Chiarelli,” he said. “It is known that suicide and PTSD are highly related. This is especially urgent and relevant because recent data suggest suicide rate of military personnel in the VA may be as high as 44 per day,” Dr. Lipov said.
Dr. Lipov is the chief medical officer and an investor in the Stella Center. Dr. Green performs SGBs as part of his psychiatric practice. Drs. Ochberg, McIntyre, and Duckworth reported no relevant financial relationships.
A version of this article appeared on Medscape.com.
In a bid to reduce stigma and improve treatment rates, for inclusion in the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition, Text Revision (DSM-5-TR). The APA’s policy is that a rolling name change is available if the current term is determined to be harmful.
Currently led by anesthesiologist Eugene Lipov, MD, clinical assistant professor, University of Illinois Chicago, and chief medical officer of Stella Center, also in Chicago, the formal request for the proposed name change to the APA’s DSM-5-TR Steering Committee in August 2023.
The APA Steering Committee rejected the proposed name change in November 2023, citing a “lack of convincing evidence.” However, Dr. Lipov and colleagues remain undeterred and continue to advocate for the change.
“The word ‘disorder’ is both imprecise and stigmatizing,” Dr. Lipov said. “Because of stigma, many people with PTSD — especially those in the military — don’t get help, which my research has demonstrated.”
Patients are more likely to seek help if their symptoms are framed as manifestations of an injury that is diagnosable and treatable, like a broken leg, Dr. Lipov said. “Stigma can kill in very real ways, since delayed care or lack of care can directly lead to suicides, thus satisfying the reduce harm requirement for the name change.”
Neurobiology of Trauma
Dr. Lipov grew up with a veteran father affected by PTSD and a mother with debilitating depression who eventually took her life. “I understand the impact of trauma very well,” he said.
Although not a psychiatrist, Dr. Lipov pioneered a highly successful treatment for PTSD by adapting an anesthetic technique — the stellate ganglion block (SGB) — to reverse many trauma symptoms through the process of “rebooting.”
This involves reversing the activity of the sympathetic nervous system — the fight-or-flight response — to the pretrauma state by anesthetizing the sympathetic ganglion in the neck. Investigating how SGB can help ameliorate the symptoms of PTSD led him to investigate and describe the neurobiology of PTSD and the mechanism of action of SGB.
The impact of SGD on PTSD was supported by a small neuroimaging study demonstrating that the right amygdala — the area of the brain associated with the fear response — was overactivated in patients with PTSD but that this region was deactivated after the administration of SGB, Dr. Lipov said.
“I believe that psychiatric conditions are actually physiologic brain changes that can be measured by advanced neuroimaging technologies and then physiologically treated,” he stated.
He noted that a growing body of literature suggests that use of the SGB for PTSD can be effective “because PTSD has a neurobiological basis and is essentially caused by an actual injury to the brain.”
A Natural Response, Not a Disorder
Dr. Lipov’s clinical work treating PTSD as a brain injury led him to connect with Frank Ochberg, MD, a founding board member of the International Society for Traumatic Stress Studies, former associate director of the National Institute of Mental Health, and former director of the Michigan Department of Mental Health.
In 2012, Dr. Ochberg teamed up with retired Army General Peter Chiarelli and Jonathan Shay, MD, PhD, author of Achilles in Vietnam: Combat Trauma and the Undoing of Character, to petition the DSM-5 Steering Committee to change the name of PTSD to PTSI in the upcoming DSM-5.
Dr. Ochberg explained that Gen. Chiarelli believed the term “disorder” suggests a preexisting issue prior to enlistment, potentially making an individual appear “weak.” He noted that this stigma is particularly troubling for military personnel, who often avoid seeking so they are not perceived as vulnerable, which can lead to potentially dire consequences, including suicide.
“We received endorsements from many quarters, not only advocates for service members or veterans,” Dr. Ochberg said.
This included feminists like Gloria Steinem, who championed the rights of women who had survived rape, incest, and domestic violence. As one advocate put it: “The natural human reaction to a life-threatening event should not be labeled a disorder.”
The DSM-5 Steering Committee declined to change the name. “Their feeling was that if we change the word ‘disorder’ to something else, we’d have to change every condition in the DSM that’s called a ‘disorder’. And they felt there really was nothing wrong with the word,” said Dr. Ochberg.
However, Dr. Lipov noted that other diagnoses have undergone name changes in the DSM for the sake of accuracy or stigma reduction. For example, the term mental retardation (DSM-IV) was changed to intellectual disability in DSM-5, and gender identity disorder was changed to gender dysphoria.
A decade later, Dr. Lipov decided to try again. To bolster his contention, he conducted a telephone survey of 1025 individuals. Of these, about 50% had a PTSD diagnosis.
Approximately two thirds of respondents agreed that a name change to PTSI would reduce the stigma associated with the term “PTSD.” Over half said it would increase the likelihood they would seek medical help. Those diagnosed with PTSD were most likely to endorse the name change.
Dr. Lipov conducts an ongoing survey of psychiatrists to ascertain their views on the potential name change and hopes to include findings in future research and communication with the DSM-5 Steering Committee. In addition, he has developed a new survey that expands upon his original survey, which specifically looked at individuals with PTSD.
“The new survey includes a wide range of people, many of whom have never been diagnosed. One of the questions we ask is whether they’ve ever heard of PTSD, and then we ask them about their reaction to the term,” he said.
A Barrier to Care
Psychiatrist Marcel Green, MD, director of Hudson Mind in New York City, refers to himself as an “interventional psychiatrist,” as he employs a comprehensive approach that includes not only medication and psychotherapy but also specialized techniques like SBG for severe anxiety-related physical symptoms and certain pain conditions.
Dr. Green, who is not involved in the name change initiative, agrees that the term “disorder” carries more stigma than “injury” for many groups, including those who have experienced childhood trauma, those struggling with substance abuse, or who are from backgrounds or peer groups where seeking mental health care is stigmatized.
Patients like these “are looking to me to give them a language to frame what they’re going through, and I tell them their symptoms are consistent with PTSD,” he said. “But they tell me don’t see themselves as having a disorder, which hinders their pursuit of care.”
Framing the condition as an “injury” also aligns with the approach of using biologic interventions to address the injury. Dr. Green has found SGB helpful in treating substance abuse disorder too, “which is a form of escape from the hyperactivation that accompanies PTSD.” And after the procedure, “they’re more receptive to therapy.”
Unfortunately, said Dr. Lipov, the DSM Steering Committee rejected his proposed name change, stating that the “concept of disorder as a dividing line from, eg, normal reactions to stress, is a core concept in the DSM, and the term has only rarely been removed.”
Moreover, the committee “did not see sufficient evidence ... that the name PTSD is stigmatizing and actually deters people with the disorder from seeking treatment who would not be deterred from doing so by PTSI.”
‘An Avenue for Dignity’
Ken Duckworth, MD, chief medical officer of the National Alliance on Mental Illness (NAMI), noted that the organization does not have an official position on this issue. However, he shared his own personal perspective.
There may be merit in the proposed name change, said Dr. Duckworth, but more evidence is needed. “If it’s clear, after rigorous studies have been performed and there’s compelling data, that calling it a ‘disorder’ rather than an ‘injury’ is actually preventing people from getting the care they need, then it merits serious attention.”
If so, Dr. Duckworth would be “interested in having a conversation with the policy team at NAMI to start to see if we could activate the DSM Committee.”
Roger McIntyre, MD, professor of psychiatry and pharmacology at the University of Toronto in Ontario, Canada, and head of the Mood Disorders Psychopharmacology Unit, said the name change initiative is a “really interesting proposal.”
Dr. McIntyre, chairman and executive director of the Brain and Cognition Discovery Foundation, also in Toronto, who is not involved in the initiative, has also heard “many people say that the term ‘disorder’ is stigmatizing and might even come across as pejorative in some ways.”
By contrast, “the word ‘injury’ parallels physical injury, and what we currently call ‘PTSD’ is a psychological or emotional injury no less devastating than torn tissue or broken bones,” added Dr. McIntyre, who is also the chairman of the board of the Depression and Bipolar Support Alliance.
Dr. Ochberg agreed. “In the military, ‘injury’ opens up an avenue for dignity, for a medal. Being injured and learning how to deal with an injury is part of having yet another honorable task that comes from being an honorable person who did an honorable thing.”
While disappointed, Dr. Lipov does not plan to give up on his vision. “I will continue to amass evidence that the word ‘PTSD’ is stigmatizing and indeed does prevent people from seeking care and will resubmit the proposal to the DSM Steering Committee when I have gathered a larger body of compelling evidence.”
Currently, Dr. Lipov is in active discussions with the special operations force of the US Army to obtain more evidence. “This will be the follow-up to bolster the opinion of Peter Chiarelli,” he said. “It is known that suicide and PTSD are highly related. This is especially urgent and relevant because recent data suggest suicide rate of military personnel in the VA may be as high as 44 per day,” Dr. Lipov said.
Dr. Lipov is the chief medical officer and an investor in the Stella Center. Dr. Green performs SGBs as part of his psychiatric practice. Drs. Ochberg, McIntyre, and Duckworth reported no relevant financial relationships.
A version of this article appeared on Medscape.com.
In a bid to reduce stigma and improve treatment rates, for inclusion in the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition, Text Revision (DSM-5-TR). The APA’s policy is that a rolling name change is available if the current term is determined to be harmful.
Currently led by anesthesiologist Eugene Lipov, MD, clinical assistant professor, University of Illinois Chicago, and chief medical officer of Stella Center, also in Chicago, the formal request for the proposed name change to the APA’s DSM-5-TR Steering Committee in August 2023.
The APA Steering Committee rejected the proposed name change in November 2023, citing a “lack of convincing evidence.” However, Dr. Lipov and colleagues remain undeterred and continue to advocate for the change.
“The word ‘disorder’ is both imprecise and stigmatizing,” Dr. Lipov said. “Because of stigma, many people with PTSD — especially those in the military — don’t get help, which my research has demonstrated.”
Patients are more likely to seek help if their symptoms are framed as manifestations of an injury that is diagnosable and treatable, like a broken leg, Dr. Lipov said. “Stigma can kill in very real ways, since delayed care or lack of care can directly lead to suicides, thus satisfying the reduce harm requirement for the name change.”
Neurobiology of Trauma
Dr. Lipov grew up with a veteran father affected by PTSD and a mother with debilitating depression who eventually took her life. “I understand the impact of trauma very well,” he said.
Although not a psychiatrist, Dr. Lipov pioneered a highly successful treatment for PTSD by adapting an anesthetic technique — the stellate ganglion block (SGB) — to reverse many trauma symptoms through the process of “rebooting.”
This involves reversing the activity of the sympathetic nervous system — the fight-or-flight response — to the pretrauma state by anesthetizing the sympathetic ganglion in the neck. Investigating how SGB can help ameliorate the symptoms of PTSD led him to investigate and describe the neurobiology of PTSD and the mechanism of action of SGB.
The impact of SGD on PTSD was supported by a small neuroimaging study demonstrating that the right amygdala — the area of the brain associated with the fear response — was overactivated in patients with PTSD but that this region was deactivated after the administration of SGB, Dr. Lipov said.
“I believe that psychiatric conditions are actually physiologic brain changes that can be measured by advanced neuroimaging technologies and then physiologically treated,” he stated.
He noted that a growing body of literature suggests that use of the SGB for PTSD can be effective “because PTSD has a neurobiological basis and is essentially caused by an actual injury to the brain.”
A Natural Response, Not a Disorder
Dr. Lipov’s clinical work treating PTSD as a brain injury led him to connect with Frank Ochberg, MD, a founding board member of the International Society for Traumatic Stress Studies, former associate director of the National Institute of Mental Health, and former director of the Michigan Department of Mental Health.
In 2012, Dr. Ochberg teamed up with retired Army General Peter Chiarelli and Jonathan Shay, MD, PhD, author of Achilles in Vietnam: Combat Trauma and the Undoing of Character, to petition the DSM-5 Steering Committee to change the name of PTSD to PTSI in the upcoming DSM-5.
Dr. Ochberg explained that Gen. Chiarelli believed the term “disorder” suggests a preexisting issue prior to enlistment, potentially making an individual appear “weak.” He noted that this stigma is particularly troubling for military personnel, who often avoid seeking so they are not perceived as vulnerable, which can lead to potentially dire consequences, including suicide.
“We received endorsements from many quarters, not only advocates for service members or veterans,” Dr. Ochberg said.
This included feminists like Gloria Steinem, who championed the rights of women who had survived rape, incest, and domestic violence. As one advocate put it: “The natural human reaction to a life-threatening event should not be labeled a disorder.”
The DSM-5 Steering Committee declined to change the name. “Their feeling was that if we change the word ‘disorder’ to something else, we’d have to change every condition in the DSM that’s called a ‘disorder’. And they felt there really was nothing wrong with the word,” said Dr. Ochberg.
However, Dr. Lipov noted that other diagnoses have undergone name changes in the DSM for the sake of accuracy or stigma reduction. For example, the term mental retardation (DSM-IV) was changed to intellectual disability in DSM-5, and gender identity disorder was changed to gender dysphoria.
A decade later, Dr. Lipov decided to try again. To bolster his contention, he conducted a telephone survey of 1025 individuals. Of these, about 50% had a PTSD diagnosis.
Approximately two thirds of respondents agreed that a name change to PTSI would reduce the stigma associated with the term “PTSD.” Over half said it would increase the likelihood they would seek medical help. Those diagnosed with PTSD were most likely to endorse the name change.
Dr. Lipov conducts an ongoing survey of psychiatrists to ascertain their views on the potential name change and hopes to include findings in future research and communication with the DSM-5 Steering Committee. In addition, he has developed a new survey that expands upon his original survey, which specifically looked at individuals with PTSD.
“The new survey includes a wide range of people, many of whom have never been diagnosed. One of the questions we ask is whether they’ve ever heard of PTSD, and then we ask them about their reaction to the term,” he said.
A Barrier to Care
Psychiatrist Marcel Green, MD, director of Hudson Mind in New York City, refers to himself as an “interventional psychiatrist,” as he employs a comprehensive approach that includes not only medication and psychotherapy but also specialized techniques like SBG for severe anxiety-related physical symptoms and certain pain conditions.
Dr. Green, who is not involved in the name change initiative, agrees that the term “disorder” carries more stigma than “injury” for many groups, including those who have experienced childhood trauma, those struggling with substance abuse, or who are from backgrounds or peer groups where seeking mental health care is stigmatized.
Patients like these “are looking to me to give them a language to frame what they’re going through, and I tell them their symptoms are consistent with PTSD,” he said. “But they tell me don’t see themselves as having a disorder, which hinders their pursuit of care.”
Framing the condition as an “injury” also aligns with the approach of using biologic interventions to address the injury. Dr. Green has found SGB helpful in treating substance abuse disorder too, “which is a form of escape from the hyperactivation that accompanies PTSD.” And after the procedure, “they’re more receptive to therapy.”
Unfortunately, said Dr. Lipov, the DSM Steering Committee rejected his proposed name change, stating that the “concept of disorder as a dividing line from, eg, normal reactions to stress, is a core concept in the DSM, and the term has only rarely been removed.”
Moreover, the committee “did not see sufficient evidence ... that the name PTSD is stigmatizing and actually deters people with the disorder from seeking treatment who would not be deterred from doing so by PTSI.”
‘An Avenue for Dignity’
Ken Duckworth, MD, chief medical officer of the National Alliance on Mental Illness (NAMI), noted that the organization does not have an official position on this issue. However, he shared his own personal perspective.
There may be merit in the proposed name change, said Dr. Duckworth, but more evidence is needed. “If it’s clear, after rigorous studies have been performed and there’s compelling data, that calling it a ‘disorder’ rather than an ‘injury’ is actually preventing people from getting the care they need, then it merits serious attention.”
If so, Dr. Duckworth would be “interested in having a conversation with the policy team at NAMI to start to see if we could activate the DSM Committee.”
Roger McIntyre, MD, professor of psychiatry and pharmacology at the University of Toronto in Ontario, Canada, and head of the Mood Disorders Psychopharmacology Unit, said the name change initiative is a “really interesting proposal.”
Dr. McIntyre, chairman and executive director of the Brain and Cognition Discovery Foundation, also in Toronto, who is not involved in the initiative, has also heard “many people say that the term ‘disorder’ is stigmatizing and might even come across as pejorative in some ways.”
By contrast, “the word ‘injury’ parallels physical injury, and what we currently call ‘PTSD’ is a psychological or emotional injury no less devastating than torn tissue or broken bones,” added Dr. McIntyre, who is also the chairman of the board of the Depression and Bipolar Support Alliance.
Dr. Ochberg agreed. “In the military, ‘injury’ opens up an avenue for dignity, for a medal. Being injured and learning how to deal with an injury is part of having yet another honorable task that comes from being an honorable person who did an honorable thing.”
While disappointed, Dr. Lipov does not plan to give up on his vision. “I will continue to amass evidence that the word ‘PTSD’ is stigmatizing and indeed does prevent people from seeking care and will resubmit the proposal to the DSM Steering Committee when I have gathered a larger body of compelling evidence.”
Currently, Dr. Lipov is in active discussions with the special operations force of the US Army to obtain more evidence. “This will be the follow-up to bolster the opinion of Peter Chiarelli,” he said. “It is known that suicide and PTSD are highly related. This is especially urgent and relevant because recent data suggest suicide rate of military personnel in the VA may be as high as 44 per day,” Dr. Lipov said.
Dr. Lipov is the chief medical officer and an investor in the Stella Center. Dr. Green performs SGBs as part of his psychiatric practice. Drs. Ochberg, McIntyre, and Duckworth reported no relevant financial relationships.
A version of this article appeared on Medscape.com.
Data Trends 2024: Women's Health
Mahorter S, Vinekar K, Shaw JG, et al. Variations in provision of long-acting reversible contraception across Veterans Health Administration facilities. J Gen Intern Med. 2023;38(suppl 3):865-867. doi:10.1007/s11606-023-08123-5
Quinn DA, Sileanu FE, Zhao X, Mor MK, Judge-Golden C, Callegari LS, Borrero S. History of unintended pregnancy and patterns of contraceptive use among racial and ethnic minority women veterans. Am J Obstet Gynecol. 2020;223(4):564.e1-564.e13. doi:10.1016/j.ajog.2020.02.042
Wolgemuth TE, Cuddeback M, Callegari LS, Rodriguez KL, Zhao X, Borrero S. Perceived Barriers and Facilitators to Contraceptive Use Among Women Veterans Accessing the Veterans Affairs Healthcare System. Womens Health Issues. 2020;30(1):57-63. doi:10.1016/j.whi.2019.08.005
Gawron LM, He T, Lewis L, Fudin H, Callegari LS, Turok DK, Stevens V. Oral emergency contraception provision in the Veterans Health Administration: a retrospective cohort study. J Gen Intern Med. 2022;37(suppl 3):685-689. doi:10.1007/s11606-022-07596-0
Gardella CM, Borgerding J, Maier MM, Beste LA. Chlamydial and gonococcal infections and adverse reproductive health conditions among patients assigned female at birth in the Veterans Health Administration. Sex Transm Dis. 2024;51(5):p 320-324. doi:10.1097/OLQ.0000000000001932
Katon JG, Bossick AS, Tartaglione EV, et al. Assessing racial disparities in access, use, and outcomes for pregnant and postpartum veterans and their infants in Veterans Health Administration. J Womens Health (Larchmt). 2023;32(7):757-766. doi:10.1089/jwh.2022.0507
Katon J, Bossick A, Tartaglione E, et al. Survey of Veterans Receiving VA Maternity Care Benefits: A Report Sponsored by the VHA Office of Women's Health Department of Veterans Affairs. VA Office of Women's Health: Washington, DC; 2021.
Frayne SM, Phibbs SC, Saechao F, et al. Sourcebook: Women Veterans in the Veterans Health Administration. Vol 4. Longitudinal Trends in Sociodemographics, Utilization, Health Profile, and Geographic Distribution. Veterans Health Administration, Department of Veterans Affairs: Washington, DC; 2018.
March of Dimes Peristats: Birth. 2022. Updated January 2024. Accessed May 15, 2024. https://www.marchofdimes.org/peristats/
Katon JG, Hoggatt KJ, Balasubramanian V, et al. Reproductive health diagnoses of women veterans using Department of Veterans Affairs health care. Med Care. 2015;53(4 Suppl 1):S63–S67. doi:10.1097/MLR.0000000000000295
Kroll-Desrosiers A, Wallace KF, Higgins DM, Martino S, Mattocks KM. Musculoskeletal pain during pregnancy among veterans: associations with health and health care utilization. Womens Health Issues. 2024;34(1):90-97. doi:10.1016/j.whi.2023.07.004
Mahorter S, Vinekar K, Shaw JG, et al. Variations in provision of long-acting reversible contraception across Veterans Health Administration facilities. J Gen Intern Med. 2023;38(suppl 3):865-867. doi:10.1007/s11606-023-08123-5
Quinn DA, Sileanu FE, Zhao X, Mor MK, Judge-Golden C, Callegari LS, Borrero S. History of unintended pregnancy and patterns of contraceptive use among racial and ethnic minority women veterans. Am J Obstet Gynecol. 2020;223(4):564.e1-564.e13. doi:10.1016/j.ajog.2020.02.042
Wolgemuth TE, Cuddeback M, Callegari LS, Rodriguez KL, Zhao X, Borrero S. Perceived Barriers and Facilitators to Contraceptive Use Among Women Veterans Accessing the Veterans Affairs Healthcare System. Womens Health Issues. 2020;30(1):57-63. doi:10.1016/j.whi.2019.08.005
Gawron LM, He T, Lewis L, Fudin H, Callegari LS, Turok DK, Stevens V. Oral emergency contraception provision in the Veterans Health Administration: a retrospective cohort study. J Gen Intern Med. 2022;37(suppl 3):685-689. doi:10.1007/s11606-022-07596-0
Gardella CM, Borgerding J, Maier MM, Beste LA. Chlamydial and gonococcal infections and adverse reproductive health conditions among patients assigned female at birth in the Veterans Health Administration. Sex Transm Dis. 2024;51(5):p 320-324. doi:10.1097/OLQ.0000000000001932
Katon JG, Bossick AS, Tartaglione EV, et al. Assessing racial disparities in access, use, and outcomes for pregnant and postpartum veterans and their infants in Veterans Health Administration. J Womens Health (Larchmt). 2023;32(7):757-766. doi:10.1089/jwh.2022.0507
Katon J, Bossick A, Tartaglione E, et al. Survey of Veterans Receiving VA Maternity Care Benefits: A Report Sponsored by the VHA Office of Women's Health Department of Veterans Affairs. VA Office of Women's Health: Washington, DC; 2021.
Frayne SM, Phibbs SC, Saechao F, et al. Sourcebook: Women Veterans in the Veterans Health Administration. Vol 4. Longitudinal Trends in Sociodemographics, Utilization, Health Profile, and Geographic Distribution. Veterans Health Administration, Department of Veterans Affairs: Washington, DC; 2018.
March of Dimes Peristats: Birth. 2022. Updated January 2024. Accessed May 15, 2024. https://www.marchofdimes.org/peristats/
Katon JG, Hoggatt KJ, Balasubramanian V, et al. Reproductive health diagnoses of women veterans using Department of Veterans Affairs health care. Med Care. 2015;53(4 Suppl 1):S63–S67. doi:10.1097/MLR.0000000000000295
Kroll-Desrosiers A, Wallace KF, Higgins DM, Martino S, Mattocks KM. Musculoskeletal pain during pregnancy among veterans: associations with health and health care utilization. Womens Health Issues. 2024;34(1):90-97. doi:10.1016/j.whi.2023.07.004
Mahorter S, Vinekar K, Shaw JG, et al. Variations in provision of long-acting reversible contraception across Veterans Health Administration facilities. J Gen Intern Med. 2023;38(suppl 3):865-867. doi:10.1007/s11606-023-08123-5
Quinn DA, Sileanu FE, Zhao X, Mor MK, Judge-Golden C, Callegari LS, Borrero S. History of unintended pregnancy and patterns of contraceptive use among racial and ethnic minority women veterans. Am J Obstet Gynecol. 2020;223(4):564.e1-564.e13. doi:10.1016/j.ajog.2020.02.042
Wolgemuth TE, Cuddeback M, Callegari LS, Rodriguez KL, Zhao X, Borrero S. Perceived Barriers and Facilitators to Contraceptive Use Among Women Veterans Accessing the Veterans Affairs Healthcare System. Womens Health Issues. 2020;30(1):57-63. doi:10.1016/j.whi.2019.08.005
Gawron LM, He T, Lewis L, Fudin H, Callegari LS, Turok DK, Stevens V. Oral emergency contraception provision in the Veterans Health Administration: a retrospective cohort study. J Gen Intern Med. 2022;37(suppl 3):685-689. doi:10.1007/s11606-022-07596-0
Gardella CM, Borgerding J, Maier MM, Beste LA. Chlamydial and gonococcal infections and adverse reproductive health conditions among patients assigned female at birth in the Veterans Health Administration. Sex Transm Dis. 2024;51(5):p 320-324. doi:10.1097/OLQ.0000000000001932
Katon JG, Bossick AS, Tartaglione EV, et al. Assessing racial disparities in access, use, and outcomes for pregnant and postpartum veterans and their infants in Veterans Health Administration. J Womens Health (Larchmt). 2023;32(7):757-766. doi:10.1089/jwh.2022.0507
Katon J, Bossick A, Tartaglione E, et al. Survey of Veterans Receiving VA Maternity Care Benefits: A Report Sponsored by the VHA Office of Women's Health Department of Veterans Affairs. VA Office of Women's Health: Washington, DC; 2021.
Frayne SM, Phibbs SC, Saechao F, et al. Sourcebook: Women Veterans in the Veterans Health Administration. Vol 4. Longitudinal Trends in Sociodemographics, Utilization, Health Profile, and Geographic Distribution. Veterans Health Administration, Department of Veterans Affairs: Washington, DC; 2018.
March of Dimes Peristats: Birth. 2022. Updated January 2024. Accessed May 15, 2024. https://www.marchofdimes.org/peristats/
Katon JG, Hoggatt KJ, Balasubramanian V, et al. Reproductive health diagnoses of women veterans using Department of Veterans Affairs health care. Med Care. 2015;53(4 Suppl 1):S63–S67. doi:10.1097/MLR.0000000000000295
Kroll-Desrosiers A, Wallace KF, Higgins DM, Martino S, Mattocks KM. Musculoskeletal pain during pregnancy among veterans: associations with health and health care utilization. Womens Health Issues. 2024;34(1):90-97. doi:10.1016/j.whi.2023.07.004
Data Trends 2024: Parkinson Disease
- Power MC, Parthasarathy V, Gianattasio KZ, et al. Investigation of the association of military employment and Parkinson’s disease with a validated Parkinson’s disease case-finding strategy. Brain Inj. 2023;37(5):383-387. doi:10.1080/02699052.2022.2158234
- Scott GD, Neilson LE, Woltjer R, Quinn JF, Lim MM. Lifelong association of disorders related to military trauma with subsequent Parkinson’s disease. Mov Disord. 2023;38(8):1483-1492. doi:10.1002/mds.29457
- Goldman SM, Weaver FM, Stroupe KT, et al. Risk of Parkinson disease among service members at Marine Corps Base Camp Lejeune. JAMA Neurol. 2023;80(7):673-681. doi:10.1001/jamaneurol.2023.1168
- Pankratz N, Cole BR, Beutel KM, Liao KP, Ashe J. Parkinson disease genetics extended to African and Hispanic ancestries in the VA Million Veteran Program. Neurol Genet. 2023;10(1):e200110. doi:10.1212/NXG.0000000000200110
- Power MC, Parthasarathy V, Gianattasio KZ, et al. Investigation of the association of military employment and Parkinson’s disease with a validated Parkinson’s disease case-finding strategy. Brain Inj. 2023;37(5):383-387. doi:10.1080/02699052.2022.2158234
- Scott GD, Neilson LE, Woltjer R, Quinn JF, Lim MM. Lifelong association of disorders related to military trauma with subsequent Parkinson’s disease. Mov Disord. 2023;38(8):1483-1492. doi:10.1002/mds.29457
- Goldman SM, Weaver FM, Stroupe KT, et al. Risk of Parkinson disease among service members at Marine Corps Base Camp Lejeune. JAMA Neurol. 2023;80(7):673-681. doi:10.1001/jamaneurol.2023.1168
- Pankratz N, Cole BR, Beutel KM, Liao KP, Ashe J. Parkinson disease genetics extended to African and Hispanic ancestries in the VA Million Veteran Program. Neurol Genet. 2023;10(1):e200110. doi:10.1212/NXG.0000000000200110
- Power MC, Parthasarathy V, Gianattasio KZ, et al. Investigation of the association of military employment and Parkinson’s disease with a validated Parkinson’s disease case-finding strategy. Brain Inj. 2023;37(5):383-387. doi:10.1080/02699052.2022.2158234
- Scott GD, Neilson LE, Woltjer R, Quinn JF, Lim MM. Lifelong association of disorders related to military trauma with subsequent Parkinson’s disease. Mov Disord. 2023;38(8):1483-1492. doi:10.1002/mds.29457
- Goldman SM, Weaver FM, Stroupe KT, et al. Risk of Parkinson disease among service members at Marine Corps Base Camp Lejeune. JAMA Neurol. 2023;80(7):673-681. doi:10.1001/jamaneurol.2023.1168
- Pankratz N, Cole BR, Beutel KM, Liao KP, Ashe J. Parkinson disease genetics extended to African and Hispanic ancestries in the VA Million Veteran Program. Neurol Genet. 2023;10(1):e200110. doi:10.1212/NXG.0000000000200110
Type 2 Diabetes Fracture Risk Likely Due to Impaired Physical Function
, according to a Swedish prospective observational study in JAMA Network Open.
The study was conducted in more than 3000 Swedish women by Mattias Lorentzon, MD, a professor of geriatric medicine at Gothenburg University, and chief physician at the Osteoporosis Clinic at Sahlgrenska University Hospital in Mölndal, and colleagues.
Older women with T2D had higher BMD, better bone microarchitecture, and a similar bone material strength index (BMSi) but poorer physical performance and higher fracture risk than women without diabetes.
Women with T2D had 9.1% higher body weight, a 9.5% higher body mass index (BMI), and 6.3% higher appendicular lean mass index (lean mass divided by height squared) than controls.
The T2D group also had a lower prevalence of reported osteoporosis medication use vs controls: 3.4% vs 7.5%, respectively.
Prolonged diabetes treatment and insulin use were associated with higher fracture risk and poorer physical performance despite better bone characteristics.
“Our results demonstrate that checking and monitoring physical function is important to identify diabetes patients with a high risk of fractures and suggest that improving physical function may be important to reduce the risk of fractures in these patients,” Dr. Lorentzon told this news organization.
He speculated that the better bone microarchitecture in women with T2D could be due to both higher body weight and adiposity as well as to hormonal differences such as higher estradiol levels.
Study Details
A fractures study was performed in the Gothenburg area from March 2013 to May 2016 with follow-up of incident fracture data completed in March 2023. Data were collected from questionnaires and through examination of anthropometrics, physical function, and bone measurements using dual-energy x-ray absorptiometry and high-resolution peripheral computed tomography. A subsample underwent bone microindentation to assess BMSi.
Among the cohort’s 3008 women, ages 75-80 (mean, 77.8), 294 patients with T2D were compared with 2714 same-age unaffected women.
During a median follow-up of 7.3 years, 1071 incident fractures, 853 major osteoporotic fractures, and 232 hip fractures occurred. In models adjusted for age, BMI, clinical risk factors, and femoral neck BMD, T2D was associated with an increased risk of any fracture: hazard ratio (HR), 1.26; (95% CI, 1.04-1.54), and major osteoporotic fracture (HR, 1.25; 95% CI, 1.00-1.56).
Most fractures were due to falls, with the most common affected sites being the forearm, upper arm, spine, and hip, Dr. Lorentzon said.
Among the findings:
- In bone microarchitecture, women with T2D had higher BMD at all sites: total hip, 4.4% higher; femoral neck, 4.9% higher; and lumbar spine, 5.2% higher.
- At the tibia, the T2D group had 7.4% greater cortical area and 1.3% greater density, as well as 8.7% higher trabecular bone volume fraction.
“Our findings regarding BMD are consistent with previous publications showing higher BMD in individuals with T2D compared with those without diabetes,” Dr. Lorentzon said. A 2012 meta-analysis, for example, showed higher BMD levels in T2D patients. “Some smaller studies, however, have found worse bone microstructure and lower bone material strength in contrast to the results from our study,” Dr. Lorentzon said.
- There was no difference in BMSi, with a mean of 78 in both groups.
- The T2D group had lower performance on all physical function tests: a 9.7% lower grip strength, 9.9% slower gait speed, and 13.9% slower timed up-and-go time than women without diabetes.
“We found all parameters regarding physical function, such as muscle strength, balance, and performance, were much worse in women with diabetes than in those without,” Dr. Lorentzon said. “Dizziness could also be a contributor to the increased risk of falls, but this factor was not investigated in our study.”
Commenting on the study but not involved in it, Anthony J. Pick, MD, an endocrinologist at Northwestern Medicine Lake Forest Hospital in Lake Forest, Illinois, said sarcopenia is a common and often under-recognized problem in older adults and is especially prevalent in T2D, obesity, and heart failure. “I believe that ‘exercise is medicine’ is a key concept for metabolic and osteoporosis patients — and wellness and longevity in general — and I certainly hope studies like this drive awareness of the importance of engaging in strengthening exercises.”
Dr. Pick noted some nuances in this study suggesting there may be some impairments in bone quality beyond the strength and fall risk issue, “so this is likely a complex area.”
This study was supported by the Swedish Research Council, the Inga-Britt and Arne Lundberg Foundation, and Sahlgrenska University Hospital. Dr. Lorentzon reported personal fees from UCB Pharma, Amgen, Parexel International, Astellas, and Gedeon Richter outside the submitted work. Coauthor Dr. Johansson reported lecture fees from Union Chimique Belge (UCB) Pharma outside the submitted work. Dr. Axelsson reported personal fees from Amgen, Meda/Mylan, and Lilly outside the submitted work. Dr. Pick had no relevant conflicts of interest.
, according to a Swedish prospective observational study in JAMA Network Open.
The study was conducted in more than 3000 Swedish women by Mattias Lorentzon, MD, a professor of geriatric medicine at Gothenburg University, and chief physician at the Osteoporosis Clinic at Sahlgrenska University Hospital in Mölndal, and colleagues.
Older women with T2D had higher BMD, better bone microarchitecture, and a similar bone material strength index (BMSi) but poorer physical performance and higher fracture risk than women without diabetes.
Women with T2D had 9.1% higher body weight, a 9.5% higher body mass index (BMI), and 6.3% higher appendicular lean mass index (lean mass divided by height squared) than controls.
The T2D group also had a lower prevalence of reported osteoporosis medication use vs controls: 3.4% vs 7.5%, respectively.
Prolonged diabetes treatment and insulin use were associated with higher fracture risk and poorer physical performance despite better bone characteristics.
“Our results demonstrate that checking and monitoring physical function is important to identify diabetes patients with a high risk of fractures and suggest that improving physical function may be important to reduce the risk of fractures in these patients,” Dr. Lorentzon told this news organization.
He speculated that the better bone microarchitecture in women with T2D could be due to both higher body weight and adiposity as well as to hormonal differences such as higher estradiol levels.
Study Details
A fractures study was performed in the Gothenburg area from March 2013 to May 2016 with follow-up of incident fracture data completed in March 2023. Data were collected from questionnaires and through examination of anthropometrics, physical function, and bone measurements using dual-energy x-ray absorptiometry and high-resolution peripheral computed tomography. A subsample underwent bone microindentation to assess BMSi.
Among the cohort’s 3008 women, ages 75-80 (mean, 77.8), 294 patients with T2D were compared with 2714 same-age unaffected women.
During a median follow-up of 7.3 years, 1071 incident fractures, 853 major osteoporotic fractures, and 232 hip fractures occurred. In models adjusted for age, BMI, clinical risk factors, and femoral neck BMD, T2D was associated with an increased risk of any fracture: hazard ratio (HR), 1.26; (95% CI, 1.04-1.54), and major osteoporotic fracture (HR, 1.25; 95% CI, 1.00-1.56).
Most fractures were due to falls, with the most common affected sites being the forearm, upper arm, spine, and hip, Dr. Lorentzon said.
Among the findings:
- In bone microarchitecture, women with T2D had higher BMD at all sites: total hip, 4.4% higher; femoral neck, 4.9% higher; and lumbar spine, 5.2% higher.
- At the tibia, the T2D group had 7.4% greater cortical area and 1.3% greater density, as well as 8.7% higher trabecular bone volume fraction.
“Our findings regarding BMD are consistent with previous publications showing higher BMD in individuals with T2D compared with those without diabetes,” Dr. Lorentzon said. A 2012 meta-analysis, for example, showed higher BMD levels in T2D patients. “Some smaller studies, however, have found worse bone microstructure and lower bone material strength in contrast to the results from our study,” Dr. Lorentzon said.
- There was no difference in BMSi, with a mean of 78 in both groups.
- The T2D group had lower performance on all physical function tests: a 9.7% lower grip strength, 9.9% slower gait speed, and 13.9% slower timed up-and-go time than women without diabetes.
“We found all parameters regarding physical function, such as muscle strength, balance, and performance, were much worse in women with diabetes than in those without,” Dr. Lorentzon said. “Dizziness could also be a contributor to the increased risk of falls, but this factor was not investigated in our study.”
Commenting on the study but not involved in it, Anthony J. Pick, MD, an endocrinologist at Northwestern Medicine Lake Forest Hospital in Lake Forest, Illinois, said sarcopenia is a common and often under-recognized problem in older adults and is especially prevalent in T2D, obesity, and heart failure. “I believe that ‘exercise is medicine’ is a key concept for metabolic and osteoporosis patients — and wellness and longevity in general — and I certainly hope studies like this drive awareness of the importance of engaging in strengthening exercises.”
Dr. Pick noted some nuances in this study suggesting there may be some impairments in bone quality beyond the strength and fall risk issue, “so this is likely a complex area.”
This study was supported by the Swedish Research Council, the Inga-Britt and Arne Lundberg Foundation, and Sahlgrenska University Hospital. Dr. Lorentzon reported personal fees from UCB Pharma, Amgen, Parexel International, Astellas, and Gedeon Richter outside the submitted work. Coauthor Dr. Johansson reported lecture fees from Union Chimique Belge (UCB) Pharma outside the submitted work. Dr. Axelsson reported personal fees from Amgen, Meda/Mylan, and Lilly outside the submitted work. Dr. Pick had no relevant conflicts of interest.
, according to a Swedish prospective observational study in JAMA Network Open.
The study was conducted in more than 3000 Swedish women by Mattias Lorentzon, MD, a professor of geriatric medicine at Gothenburg University, and chief physician at the Osteoporosis Clinic at Sahlgrenska University Hospital in Mölndal, and colleagues.
Older women with T2D had higher BMD, better bone microarchitecture, and a similar bone material strength index (BMSi) but poorer physical performance and higher fracture risk than women without diabetes.
Women with T2D had 9.1% higher body weight, a 9.5% higher body mass index (BMI), and 6.3% higher appendicular lean mass index (lean mass divided by height squared) than controls.
The T2D group also had a lower prevalence of reported osteoporosis medication use vs controls: 3.4% vs 7.5%, respectively.
Prolonged diabetes treatment and insulin use were associated with higher fracture risk and poorer physical performance despite better bone characteristics.
“Our results demonstrate that checking and monitoring physical function is important to identify diabetes patients with a high risk of fractures and suggest that improving physical function may be important to reduce the risk of fractures in these patients,” Dr. Lorentzon told this news organization.
He speculated that the better bone microarchitecture in women with T2D could be due to both higher body weight and adiposity as well as to hormonal differences such as higher estradiol levels.
Study Details
A fractures study was performed in the Gothenburg area from March 2013 to May 2016 with follow-up of incident fracture data completed in March 2023. Data were collected from questionnaires and through examination of anthropometrics, physical function, and bone measurements using dual-energy x-ray absorptiometry and high-resolution peripheral computed tomography. A subsample underwent bone microindentation to assess BMSi.
Among the cohort’s 3008 women, ages 75-80 (mean, 77.8), 294 patients with T2D were compared with 2714 same-age unaffected women.
During a median follow-up of 7.3 years, 1071 incident fractures, 853 major osteoporotic fractures, and 232 hip fractures occurred. In models adjusted for age, BMI, clinical risk factors, and femoral neck BMD, T2D was associated with an increased risk of any fracture: hazard ratio (HR), 1.26; (95% CI, 1.04-1.54), and major osteoporotic fracture (HR, 1.25; 95% CI, 1.00-1.56).
Most fractures were due to falls, with the most common affected sites being the forearm, upper arm, spine, and hip, Dr. Lorentzon said.
Among the findings:
- In bone microarchitecture, women with T2D had higher BMD at all sites: total hip, 4.4% higher; femoral neck, 4.9% higher; and lumbar spine, 5.2% higher.
- At the tibia, the T2D group had 7.4% greater cortical area and 1.3% greater density, as well as 8.7% higher trabecular bone volume fraction.
“Our findings regarding BMD are consistent with previous publications showing higher BMD in individuals with T2D compared with those without diabetes,” Dr. Lorentzon said. A 2012 meta-analysis, for example, showed higher BMD levels in T2D patients. “Some smaller studies, however, have found worse bone microstructure and lower bone material strength in contrast to the results from our study,” Dr. Lorentzon said.
- There was no difference in BMSi, with a mean of 78 in both groups.
- The T2D group had lower performance on all physical function tests: a 9.7% lower grip strength, 9.9% slower gait speed, and 13.9% slower timed up-and-go time than women without diabetes.
“We found all parameters regarding physical function, such as muscle strength, balance, and performance, were much worse in women with diabetes than in those without,” Dr. Lorentzon said. “Dizziness could also be a contributor to the increased risk of falls, but this factor was not investigated in our study.”
Commenting on the study but not involved in it, Anthony J. Pick, MD, an endocrinologist at Northwestern Medicine Lake Forest Hospital in Lake Forest, Illinois, said sarcopenia is a common and often under-recognized problem in older adults and is especially prevalent in T2D, obesity, and heart failure. “I believe that ‘exercise is medicine’ is a key concept for metabolic and osteoporosis patients — and wellness and longevity in general — and I certainly hope studies like this drive awareness of the importance of engaging in strengthening exercises.”
Dr. Pick noted some nuances in this study suggesting there may be some impairments in bone quality beyond the strength and fall risk issue, “so this is likely a complex area.”
This study was supported by the Swedish Research Council, the Inga-Britt and Arne Lundberg Foundation, and Sahlgrenska University Hospital. Dr. Lorentzon reported personal fees from UCB Pharma, Amgen, Parexel International, Astellas, and Gedeon Richter outside the submitted work. Coauthor Dr. Johansson reported lecture fees from Union Chimique Belge (UCB) Pharma outside the submitted work. Dr. Axelsson reported personal fees from Amgen, Meda/Mylan, and Lilly outside the submitted work. Dr. Pick had no relevant conflicts of interest.
FROM JAMA NETWORK OPEN