User login
Borderline personality disorder: 6 studies of psychosocial interventions
SECOND OF 2 PARTS
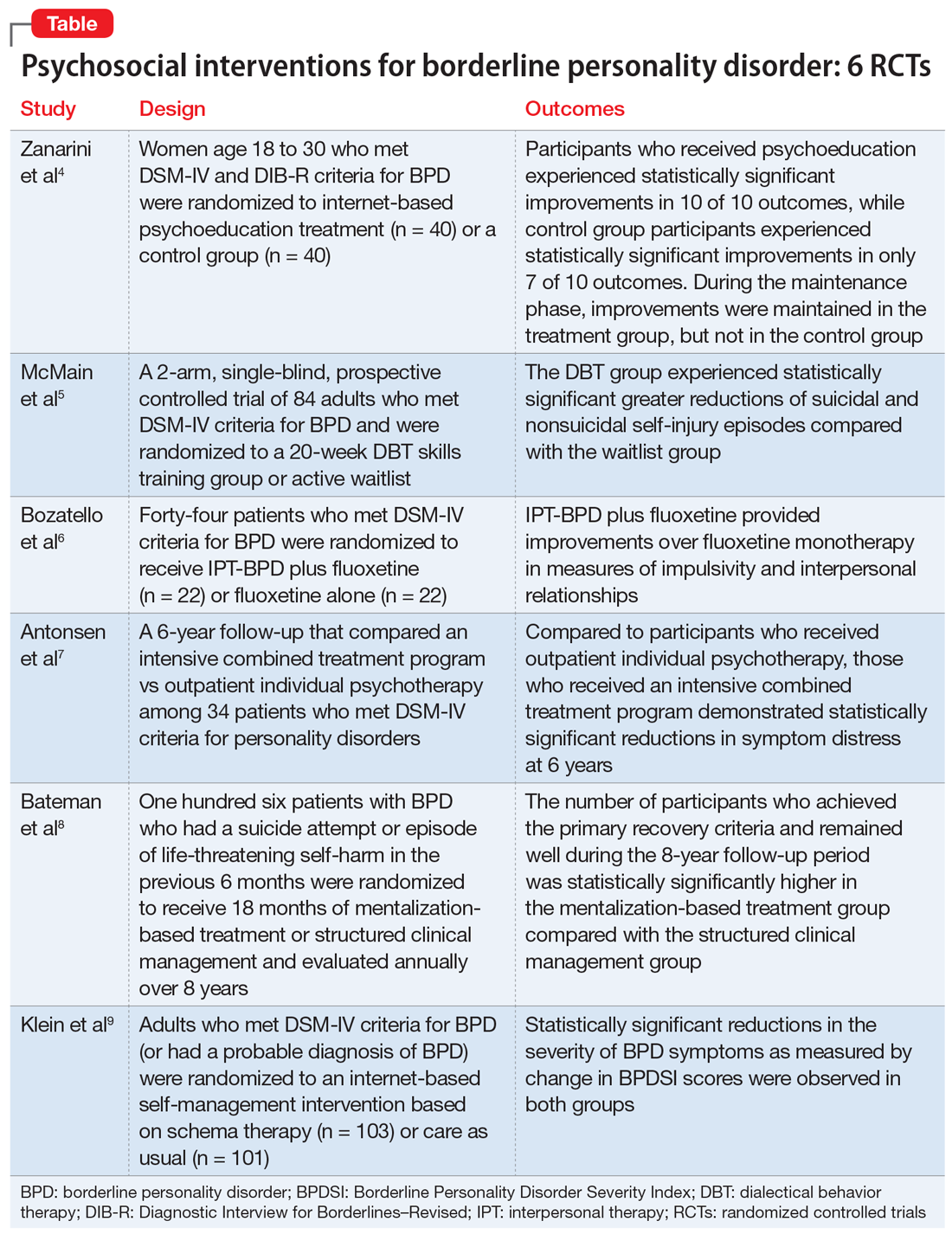
Borderline personality disorder (BPD) is associated with serious impairment in psychosocial functioning.1 It is characterized by an ongoing pattern of mood instability, cognitive distortions, problems with self-image, and impulsive behavior that often results in problems in relationships. As a result, patients with BPD tend to utilize more mental health services than patients with other personality disorders or major depressive disorder.2
Some clinicians believe BPD is difficult to treat. While historically there has been little consensus on the best treatments for this disorder, current options include both pharmacologic and psychological interventions. In Part 1 of this 2-part article, we focused on 6 studies that evaluated biological interventions.3 Here in Part 2, we focus on findings from 6 recent randomized controlled trials (RCTs) of psychosocial interventions for BPD
1. Zanarini MC, Conkey LC, Temes CM, et al. Randomized controlled trial of web-based psychoeducation for women with borderline personality disorder. J Clin Psychiatry. 2018;79(3):16m11153. doi: 10.4088/JCP.16m11153
Research has shown that BPD is a treatable illness with a more favorable prognosis than previously believed. Despite this, patients often experience difficulty accessing the most up-to-date information on BPD, which can impede their treatment. A 2008 study by Zanarini et al10 of younger female patients with BPD demonstrated that immediate, in-person psychoeducation improved impulsivity and relationships. Widespread implementation of this program proved problematic, however, due to cost and personnel constraints. To resolve this issue, researchers developed an internet-based version of the program. In a 2018 follow-up study, Zanarini et al4 examined the effect of this internet-based psychoeducation program on symptoms of BPD.
Continue to: Study design...
Study design
- Women (age 18 to 30) who met DSM-IV and Diagnostic Interview for Borderlines–Revised criteria for BPD were randomized to an internet-based psychoeducation treatment group (n = 40) or a control group (n = 40).
- Ten outcomes concerning symptom severity and psychosocial functioning were assessed during weeks 1 to 12 (acute phase) and at months 6, 9, and 12 (maintenance phase) using the self-report version of the Zanarini Rating Scale for BPD (ZAN-BPD), the Borderline Evaluation of Severity over Time, the Sheehan Disability Scale, the Clinically Useful Depression Outcome Scale, the Clinically Useful Anxiety Outcome Scale, and Weissman’s Social Adjustment Scale (SAS).
Outcomes
- In the acute phase, treatment group participants experienced statistically significant improvements in all 10 outcomes. Control group participants demonstrated similar results, achieving statistically significant improvements in 7 of 10 outcomes.
- Compared to the control group, the treatment group experienced a more significant reduction in impulsivity and improvement in psychosocial functioning as measured by the ZAN-BPD and SAS.
- In the maintenance phase, treatment group participants achieved statistically significant improvements in 9 of 10 outcomes, whereas control group participants demonstrated statistically significant improvements in only 3 of 10 outcomes.
- Compared to the control group, the treatment group also demonstrated a significantly greater improvement in all 4 sector scores and the total score of the ZAN-BP
Conclusions/limitations
- In patients with BPD, internet-based psychoeducation reduced symptom severity and improved psychosocial functioning, with effects lasting 1 year. Treatment group participants experienced clinically significant improvements in all outcomes measured during the acute phase of the study; most improvements were maintained over 1 year.
- While the control group initially saw similar improvements in most measurements, these improvements were not maintained as effectively over 1 year.
- Limitations include a female-only population, the restricted age range of participants, and recruitment exclusively from volunteers.
2. McMain SF, Guimond T, Barnhart R, et al. A randomized trial of brief dialectical behaviour therapy skills training in suicidal patients suffering from borderline disorder. Acta Psychiatr Scand. 2017;135(2):138-148.
Standard dialectical behavior therapy (DBT) is an effective treatment for BPD; however, access is often limited by shortages of clinicians and resources. Therefore, it has become increasingly common for clinical settings to offer patients only the skills training component of DBT, which requires fewer resources. While several clinical trials examining brief DBT skills–only treatment for BPD have shown promising results, it is unclear how effective this intervention is at reducing suicidal or nonsuicidal self-injury (NSSI) episodes. McMain et al5 explored the effectiveness of brief DBT skills–only adjunctive treatment on the rates of suicidal and NSSI episodes in patients with BPD.
Study design
- In this 2-arm, single-blind, prospective controlled trial, 84 adults who met DSM-IV criteria for BPD were randomized to a 20-week DBT skills training group (DBT group) or an active waitlist (WL group). No restrictions on additional psychosocial or pharmacologic treatments were imposed on either group.
- The primary outcome was the frequency of suicidal and NSSI episodes as measured by the Lifetime Suicide Attempt Self-Injury Interview and the Deliberate Self-Harm Inventory (DSHI). Measurements occurred at baseline, 10 weeks, 20 weeks, and 3 months posttreatment (32 weeks).
- Secondary outcomes included changes in health care utilization, BPD symptoms, and coping. These were assessed using the Treatment History Interview-2, Borderline Symptom List-23, State-Trait Anger Expression Inventory, Symptom Checklist-90-revised (SCL-90-R), Barratt Impulsiveness Scale-11, Beck Depression Inventory (BDI)-II, Social Adjustment Scale Self-report, Difficulties in Emotion Regulation Scale, Distress Tolerance Scale, and Kentucky Inventory of Mindfulness Scale.
Outcomes
- At Week 32, compared to the WL group, the DBT group showed statistically significant greater reductions in the frequency of suicidal and NSSI episodes as measured by the LSASI but not by the DSHI. The DBT group experienced statistically significant improvements in distress tolerance and emotion regulation over the WL group at all points, but no difference on mindfulness. The DBT group achieved greater reductions in anger over time as compared to the WL group.
- At Week 20, compared to the WL group, the DBT group showed significant improvements in social adjustment, symptom distress, and borderline symptoms. There were no significant group differences on impulsivity. Between-group differences in the number of hospital admissions favored the DBT group at 10 and 20 weeks, but not at 32 weeks. There was no statistically significant difference between the groups with respect to the number of emergency department visits.
- Analyses of group differences in clinical improvement as measured by the SCL-90-R revealed statistically reliable and clinically significant changes in the DBT group over the WL group at 20 weeks, but not at 32 weeks.
Conclusions/limitations
- Brief DBT skills training reduced suicidal and NSSI episodes in patients with BPD. Participants in the DBT group also demonstrated greater improvement in anger, distress tolerance, and emotion regulation compared to the control group. These results were evident 3 months after treatment. However, any gains in health care utilization, social adjustment, symptom distress, and borderline symptoms diminished or did not differ from waitlist participants at Week 32. At that time, participants in the DBT group demonstrated a similar level of symptomatology as the WL group.
- Limitations include the use of an active waitlist control group, allowance of concurrent treatments, the absence of an active therapeutic comparator group, use of self-report measures, use of an instrument with unknown psychometric properties, and a relatively short 3-month follow-up period.
3. Bozzatello P, Bellino S. Combined therapy with interpersonal psychotherapy adapted for borderline personality disorder: a two-years follow-up. Psychiatry Res. 2016;240:151-156.
Psychotherapeutic options for treating BPD include DBT, mentalization-based treatment, schema-focused therapy, transference-based psychotherapy, and systems training for emotional predictability and problem solving. More recently, interpersonal therapy also has been adapted for BPD (IPT-BPD). However, thus far no trials have investigated the long-term effects of this therapy on BPD. In 2010, Bellino et al11 published a 32-week RCT examining the effect of IPT-BPD on BPD. They concluded that IPT-BPD plus fluoxetine was superior to fluoxetine alone in improving symptoms and quality of life. The present study by Bozzatello et al6 examined whether the benefits of IPT-BPD plus fluoxetine demonstrated in the 2010 study persisted over a 24-month follow-up.
Continue to: Study design...
Study design
- In the 2010 study by Bellino et al,11 55 outpatients who met DSM-IV criteria for BPD were randomized to receive IPT-BPD plus fluoxetine (combined therapy) or fluoxetine alone for 32 weeks. Forty-four participants completed a 24-month follow-up study (n = 22 for IPT-BPD plus fluoxetine, n = 22 for fluoxetine only).
- Clinical assessments were performed at 6, 12, and 24 months, and used the same instruments as the original study, including the Clinical Global Impression Scale–Severity item, Hamilton Depression Rating Scale, Hamilton Anxiety Rating Scale (HARS), Social and Occupational Functioning Assessment, Satisfaction Profile (SAT-P), and the Borderline Personality Disorder Severity Index (BPDSI).
Outcomes
- While the original study demonstrated that combined therapy had a clinically significant effect over fluoxetine alone on both HARS score and the BPDSI item “affective instability” at 32 weeks, this advantage was maintained only at the 6-month assessment.
- The improvements that the combined therapy provided over fluoxetine monotherapy on the BPDSI items of “impulsivity” and “interpersonal relationships” as well as the SAT-P factors of social and psychological functioning at 32 weeks were preserved at 24 months. No additional improvements were seen.
Conclusions/limitations
- The improvements in impulsivity, interpersonal functioning, social functioning, and psychological functioning at 32 weeks seen with IPT-BPD plus fluoxetine compared with fluoxetine alone persisted for 2 years after completing therapy; no further improvements were seen.
- The improvements to anxiety and affective instability that combined therapy demonstrated over fluoxetine monotherapy at 32 weeks were not maintained at 24 months.
- Limitations include a small sample size, exclusion of psychiatric comorbidities, and a lack of assessment of session or medication adherence.
4. Antonsen BT, Kvarstein EH, Urnes Ø, et al. Favourable outcome of long-term combined psychotherapy for patients with borderline personality disorder: six-year follow-up of a randomized study. Psychother Res. 2017;27(1):51-63.
While many studies have demonstrated the benefits of psychotherapy for treating personality disorders, there is limited research of how different levels of psychotherapy may impact treatment outcomes. An RCT called the Ullevål Personality Project (UPP)12 compared an intensive combined treatment program (CP) with outpatient individual psychotherapy (OIP) in patients with personality disorders. The CP program consisted of short-term day-hospital treatment followed by outpatient combined group and individual psychotherapy. The outcomes this RCT evaluated included suicide attempts, suicidal thoughts, self-injury, psychosocial functioning, symptom distress, and interpersonal and personality problems. A 6-year follow-up concluded there were no differences in outcomes between the 2 treatment groups. However, in this RCT, Antonsen et al7 examined whether CP produced statistically significant benefits over OIP in a subset of patients with BPD.
Study design
- In the UPP trial,12 117 patients who met DSM-IV criteria for personality disorders (excluding antisocial and schizotypal personality disorder) were randomized to receive 18 weeks of day hospital psychotherapy followed by CP or OIP. Fifty-two participants in the UPP were diagnosed with BPD, and 34 of these participants completed the 6-year follow-up investigation.
- Symptom distress, psychosocial functioning, interpersonal problems, quality of life, personality functioning, and self-harm/suicidal thoughts/suicide attempts were assessed at baseline, 8 months, 18 months, 3 years, and 6 years using the SCL-90-R Global Severity Index (GSI), BDI, Global Assessment of Functioning (GAF), Work and Social Adjustment Scale (WSAS), Quality of Life 10-point scale (QOL), Circumplex of Interpersonal Problems (CIP), and the 60-item short form of the Severity Indices of Personality Problems (SIPP-118) questionnaire.
Outcomes
- Compared to the OIP group, the CP group demonstrated statistically significant reductions in symptom distress at Year 6 as measured by the SCL-90-R GSI. Between Years 3 and 6, the CP group continued to show improvements in psychosocial functioning as demonstrated by improvements in GAF and WSAS scores. The OIP group’s scores worsened during this time. Compared to the OIP group, participants in the CP group also had significantly better outcomes on the SIPP-118 domains of self-control and identity integration.
- There were no significant differences between groups on the proportion of participants who engaged in self-harm or experienced suicidal thoughts or attempts. There were no significant differences in outcomes between the treatment groups on the CIP, BDI, or QOL.
- Participants in CP group tended to use fewer psychotropic medications than those in the OIP group over time, but this difference was not statistically significant. The 2 groups did not differ in use of health care services over the last year.
- Avoidant personality disorder (AVPD) did not have a significant moderator effect on GAF score. Comorbid AVPD was a negative predictor of GAF score, independent of the group.
Conclusions/limitations
- Both groups experienced a remission rate of 90% at 6-year follow-up. Compared with the OIP group, participants in the CP group experienced significantly greater reductions in symptom distress and improvements in self-control and identity integration at 6 years. Between Years 3 and 6, participants in the CP group experienced significant improvements in psychosocial functioning compared with OIP group participants. The 2 groups did not differ on other outcomes, including the CIP, BDI, QOL, suicidal thoughts, suicidal attempts, self-harm, and health care utilization.
- Despite statistically significant differences in GAF scores favoring the CP group over the OIP group during Years 3 to 6, GAF scores did not differ significantly in the final year, which suggests that symptomatic remission does not equal functional improvement.
- Limitations include a lack of control for intensity or length of treatment in statistical analyses, small sample size, lack of correction for multiple testing, lack of an a priori power analysis, missing data and potential violation of the missing at random assumption, use of therapists’ preferred treatment method/practice, and a lack of control for other treatments.
5. Bateman A, Constantinou MP, Fonagy P, et al. Eight-year prospective follow-up of mentalization-based treatment versus structured clinical management for people with borderline personality disorder. Personal Disord. 2021;12(4):291-299.
The efficacy of various psychotherapies for symptoms of BPD has been well established. However, there is limited evidence that these effects persist over time. In 2009, Bateman et al13 conducted an 18-month RCT comparing the effectiveness of outpatient mentalization-based treatment (MBT) against structured clinical management (SCM) for patients with BPD. Both groups experienced substantial improvements, but patients assigned to MBT demonstrated greater improvement in clinically significant problems, including suicide attempts and hospitalizations. In a 2021 follow-up to this study, Bateman et al8 investigated whether the MBT group’s gains in the primary outcomes (absence of severe self-harm, suicide attempts, and inpatient admissions in the previous 12 months), social functioning, vocational engagement, and mental health service usage were maintained throughout an 8-year follow-up period.
Continue to: Study design...
Study design
- In the 2009 trial, Bateman et al13 randomized adult participants who met DSM-IV criteria for BPD and had a suicide attempt or episode of life-threatening self-harm in the past 6 months to receive 18 months of MBT or SCM. The primary outcome was crisis events, defined as a composite of suicidal and severe self-injurious behaviors and hospitalizations. The 2021 Bateman et al8 study expanded this investigation by collecting additional data on a yearly basis for 8 years.
- Of the 134 original participants, 98 agreed to complete the follow-up. Due to attrition, the follow-up period was limited to 8 years. At each yearly visit, researchers collected information on the primary outcome, the absence of severe self-harm, suicide attempts, and inpatient admissions in the previous 12 months.
- Secondary measures were collected mainly through a modified version of the Client Service Receipt Inventory and included critical incidents, psychiatric and medical hospital and community services, employment and other personally meaningful activity, psychoactive medication, and other mental health treatments.
Outcomes
- The number of participants who met diagnostic criteria for BPD at the 1-year follow-up was significantly lower in the MBT group compared with the SCM group. To improve participant retention, this outcome was not evaluated at later visits.
- The number of participants who achieved the primary recovery criteria of the original trial (absence of severe self-harm, suicide attempts, and inpatient admissions in the previous 12 months) and remained well throughout the entire follow-up period was significantly higher in the MBT group compared with the SCM group. The average number of years during which participants failed to meet recovery criteria was significantly greater in the SCM group compared with the MBT group.
- When controlling for age, treatment group was a significant predictor of recovery during the follow-up period. Overall, significantly fewer participants in the MBT group experienced critical incidents during the follow-up period.
- The SCM group used crisis mental health services for a significantly greater number of follow-up years than the MBT group, although the likelihood of ever using crisis services did not statistically differ between the groups. Both groups had similar use of outpatient mental health services, primary care services, and nonmental health medical services. Compared to the SCM group, the MBT group had significantly fewer professional support service visits and significantly fewer outpatient psychiatrist visits.
- MBT group participants spent more time in education, were less likely to be unemployed, and were less likely to use social care interventions than SCM group participants. Although those in the MBT group spent more months engaged in purposeful activity, there was no significant difference between the groups in the proportion of participants who did not engage in purposeful activity.
- The MBT group spent fewer months receiving psychotherapeutic medication compared with the SCM group. The variables that yielded significant 2-way interactions were eating disorder, substance use disorder, and physical abuse, suggesting greater benefit from MBT with these concurrent diagnoses. Younger age was associated with better outcomes.
Conclusions/limitations
- This study demonstrated that patients with BPD significantly benefited from specialized therapies such as MBT.
- At the 1 follow-up visit, the number of participants who met diagnostic criteria for BPD was significantly lower in the MBT group compared with the SCM group.
- The number of participants who had achieved the primary recovery criteria and remained well during the 8-year follow-up period was significantly higher in the MBT group compared to the SCM group.
- Limitations include increasing attrition over time, possible allegiance effects and unmasking of research assistants, lack of self-report questionnaires, and the potentially erroneous conclusion that increased use of services equates to poorer treatment response and greater need for support.
6. Klein JP, Hauer-von Mauschwitz A, Berger T, et al. Effectiveness and safety of the adjunctive use of an internet-based self-management intervention for borderline personality disorder in addition to care as usual: results from a randomised controlled trial. BMJ Open. 2021;11(9):e047771.
Fewer than 1 in 4 patients with BPD have access to effective psychotherapies. The use of internet-based self-management interventions (SMIs) developed from evidence-based psychotherapies can help close this treatment gap. Although the efficacy of SMIs for several mental disorders has been demonstrated in multiple meta-analyses, results for BPD are mixed. In this study, Klein et al9 examined the effectiveness and safety of the adjunctive use of an SMI based on schema therapy in addition to care as usual (CAU) in patients with BPD.
Study design
- In a 12-month, rater-blind, controlled parallel group trial, adults who had a total BPDSI score ≥15 and either a diagnosis of BPD according to DSM-IV criteria or a probable diagnosis of BPD (if they had also received a BPD diagnosis from their treating physician) were randomized to an internet-based SMI based on schema therapy called priovi (n = 103) or CAU (n = 101). Participants could complete the SMI content in approximately 6 months but were recommended to use the intervention for the entire year.
- Participation in psychotherapy and psychiatric treatment, including pharmacotherapy, was permitted. At baseline, 74% of participants were receiving psychotherapy and 88% were receiving psychiatric treatment.
- The primary outcome was change in BPDSI score at 12 months. The primary safety outcome was the number of serious adverse events at 12 months. Secondary outcomes included BPD severity, depressive symptoms, anxiety symptoms, quality of life, uncontrolled internet use, negative treatment effects, and satisfaction with the intervention. Most assessments were measured at baseline, 3 months, 6 months, 9 months, and 12 months.
Outcomes
- Large reductions in the severity of BPD symptoms as measured by change in BPDSI score was observed in both groups. Although the average reduction in BPDSI score was greater in the SMI group, this difference was not statistically significant from the CAU group.
- There was no statistically significant difference in the number of serious adverse events between groups at any time.
Conclusions/limitations
- Treatment with SMI did not result in improved outcomes over CAU. Although the average reduction in BPDSI score was greater in the SMI group compared to the CAU group, this difference was not statistically significant.
- The authors cautioned that the smaller-than-expected between-groups effect size must be interpreted against the background of an unexpectedly large effect in the CAU group. In fact, the CAU group pre/post effect was comparable to the pre/post effect of intensive specialized DBT treatment groups in previous RCTs.
- The authors also suggested that the high percentage of participants who received psychotherapy did not allow for an additional benefit from SMI.
- Limitations include recruitment method, lack of systematic assessment of accidental unblinding, high exclusion rate due to failure of the participant or treating clinician to provide confirmation of diagnosis, and the use of a serious adverse events assessment that is not psychometrically validated.
Bottom Line
Evidence from randomized controlled trials suggests that internet-based psychoeducation, brief dialectical behavior therapy skills-only treatment, interpersonal therapy, a program that combines day treatment with individual and group psychotherapy, and mentalization-based treatment can improve symptoms and quality of life for patients with borderline personality disorder.
1. Skodol AE, Gunderson JG, McGlashan TM, et al. Functional impairment in patients with schizotypal, borderline, avoidant, or obsessive-compulsive personality disorder. Am J Psychiatry. 2002;159(2):276-83.
2. Bender DS, Dolan RT, Skodol AE, et al. Treatment utilization by patients with personality disorders. Am J Psychiatry. 2001;158(2):295-302.
3. Saeed SA, Kallis AC. Borderline personality disorder: 6 studies of biological interventions. Current Psychiatry. 2021;20(11):26-30,34-36.
4. Zanarini MC, Conkey LC, Temes CM, et al. Randomized controlled trial of web-based psychoeducation for women with borderline personality disorder. J Clin Psychiatry. 2018;79(3):16m11153. doi:10.4088/JCP.16m11153
5. McMain SF, Guimond T, Barnhart R, et al. A randomized trial of brief dialectical behaviour therapy skills training in suicidal patients suffering from borderline disorder. Acta Psychiatr Scand. 2017;135(2):138-148. doi:10.1111/acps.12664
6. Bozzatello P, Bellino S. Combined therapy with interpersonal psychotherapy adapted for borderline personality disorder: a two-years follow-up. Psychiatry Res. 2016;240:151-156. doi:10.1016/j.psychres.2016.04.014
7. Antonsen BT, Kvarstein EH, Urnes Ø, et al. Favourable outcome of long-term combined psychotherapy for patients with borderline personality disorder: six-year follow-up of a randomized study. Psychother Res. 2017;27(1):51-63. doi:10.1080/10503307.2015.1072283
8. Bateman A, Constantinou MP, Fonagy P, et al. Eight-year prospective follow-up of mentalization-based treatment versus structured clinical management for people with borderline personality disorder. Personal Disord. 2021;12(4):291-299. doi:10.1037/per0000422
9. Klein JP, Hauer-von Mauschwitz A, Berger T, et al. Effectiveness and safety of the adjunctive use of an internet-based self-management intervention for borderline personality disorder in addition to care as usual: results from a randomised controlled trial. BMJ Open. 2021;11(9):e047771. doi:10.1136/bmjopen-2020-047771
10. Zanarini MC, Frankenburg FR. A preliminary, randomized trial of psychoeducation for women with borderline personality disorder. J Pers Disord. 2008;22(3):284-290.
11. Bellino S, Rinaldi C, Bogetto F. Adaptation of interpersonal psychotherapy to borderline personality disorder: a comparison of combined therapy and single pharmacotherapy. Can J Psychiatry. 2010;55(2):74-81.
12. Arnevik E, Wilberg T, Urnes Ø, et al. Psychotherapy for personality disorders: short-term day hospital psychotherapy versus outpatient individual therapy – a randomized controlled study. Eur Psychiatry. 2009,24(2):71-78. doi:10.1016/j.eurpsy.2008.09.004
13. Bateman A, Fonagy P. Randomized controlled trial of outpatient mentalization-based treatment versus structured clinical management for borderline personality disorder. Am J Psychiatry. 2009;166(12):1355-1364.
SECOND OF 2 PARTS
Borderline personality disorder (BPD) is associated with serious impairment in psychosocial functioning.1 It is characterized by an ongoing pattern of mood instability, cognitive distortions, problems with self-image, and impulsive behavior that often results in problems in relationships. As a result, patients with BPD tend to utilize more mental health services than patients with other personality disorders or major depressive disorder.2
Some clinicians believe BPD is difficult to treat. While historically there has been little consensus on the best treatments for this disorder, current options include both pharmacologic and psychological interventions. In Part 1 of this 2-part article, we focused on 6 studies that evaluated biological interventions.3 Here in Part 2, we focus on findings from 6 recent randomized controlled trials (RCTs) of psychosocial interventions for BPD

1. Zanarini MC, Conkey LC, Temes CM, et al. Randomized controlled trial of web-based psychoeducation for women with borderline personality disorder. J Clin Psychiatry. 2018;79(3):16m11153. doi: 10.4088/JCP.16m11153
Research has shown that BPD is a treatable illness with a more favorable prognosis than previously believed. Despite this, patients often experience difficulty accessing the most up-to-date information on BPD, which can impede their treatment. A 2008 study by Zanarini et al10 of younger female patients with BPD demonstrated that immediate, in-person psychoeducation improved impulsivity and relationships. Widespread implementation of this program proved problematic, however, due to cost and personnel constraints. To resolve this issue, researchers developed an internet-based version of the program. In a 2018 follow-up study, Zanarini et al4 examined the effect of this internet-based psychoeducation program on symptoms of BPD.
Continue to: Study design...
Study design
- Women (age 18 to 30) who met DSM-IV and Diagnostic Interview for Borderlines–Revised criteria for BPD were randomized to an internet-based psychoeducation treatment group (n = 40) or a control group (n = 40).
- Ten outcomes concerning symptom severity and psychosocial functioning were assessed during weeks 1 to 12 (acute phase) and at months 6, 9, and 12 (maintenance phase) using the self-report version of the Zanarini Rating Scale for BPD (ZAN-BPD), the Borderline Evaluation of Severity over Time, the Sheehan Disability Scale, the Clinically Useful Depression Outcome Scale, the Clinically Useful Anxiety Outcome Scale, and Weissman’s Social Adjustment Scale (SAS).
Outcomes
- In the acute phase, treatment group participants experienced statistically significant improvements in all 10 outcomes. Control group participants demonstrated similar results, achieving statistically significant improvements in 7 of 10 outcomes.
- Compared to the control group, the treatment group experienced a more significant reduction in impulsivity and improvement in psychosocial functioning as measured by the ZAN-BPD and SAS.
- In the maintenance phase, treatment group participants achieved statistically significant improvements in 9 of 10 outcomes, whereas control group participants demonstrated statistically significant improvements in only 3 of 10 outcomes.
- Compared to the control group, the treatment group also demonstrated a significantly greater improvement in all 4 sector scores and the total score of the ZAN-BP
Conclusions/limitations
- In patients with BPD, internet-based psychoeducation reduced symptom severity and improved psychosocial functioning, with effects lasting 1 year. Treatment group participants experienced clinically significant improvements in all outcomes measured during the acute phase of the study; most improvements were maintained over 1 year.
- While the control group initially saw similar improvements in most measurements, these improvements were not maintained as effectively over 1 year.
- Limitations include a female-only population, the restricted age range of participants, and recruitment exclusively from volunteers.
2. McMain SF, Guimond T, Barnhart R, et al. A randomized trial of brief dialectical behaviour therapy skills training in suicidal patients suffering from borderline disorder. Acta Psychiatr Scand. 2017;135(2):138-148.
Standard dialectical behavior therapy (DBT) is an effective treatment for BPD; however, access is often limited by shortages of clinicians and resources. Therefore, it has become increasingly common for clinical settings to offer patients only the skills training component of DBT, which requires fewer resources. While several clinical trials examining brief DBT skills–only treatment for BPD have shown promising results, it is unclear how effective this intervention is at reducing suicidal or nonsuicidal self-injury (NSSI) episodes. McMain et al5 explored the effectiveness of brief DBT skills–only adjunctive treatment on the rates of suicidal and NSSI episodes in patients with BPD.
Study design
- In this 2-arm, single-blind, prospective controlled trial, 84 adults who met DSM-IV criteria for BPD were randomized to a 20-week DBT skills training group (DBT group) or an active waitlist (WL group). No restrictions on additional psychosocial or pharmacologic treatments were imposed on either group.
- The primary outcome was the frequency of suicidal and NSSI episodes as measured by the Lifetime Suicide Attempt Self-Injury Interview and the Deliberate Self-Harm Inventory (DSHI). Measurements occurred at baseline, 10 weeks, 20 weeks, and 3 months posttreatment (32 weeks).
- Secondary outcomes included changes in health care utilization, BPD symptoms, and coping. These were assessed using the Treatment History Interview-2, Borderline Symptom List-23, State-Trait Anger Expression Inventory, Symptom Checklist-90-revised (SCL-90-R), Barratt Impulsiveness Scale-11, Beck Depression Inventory (BDI)-II, Social Adjustment Scale Self-report, Difficulties in Emotion Regulation Scale, Distress Tolerance Scale, and Kentucky Inventory of Mindfulness Scale.
Outcomes
- At Week 32, compared to the WL group, the DBT group showed statistically significant greater reductions in the frequency of suicidal and NSSI episodes as measured by the LSASI but not by the DSHI. The DBT group experienced statistically significant improvements in distress tolerance and emotion regulation over the WL group at all points, but no difference on mindfulness. The DBT group achieved greater reductions in anger over time as compared to the WL group.
- At Week 20, compared to the WL group, the DBT group showed significant improvements in social adjustment, symptom distress, and borderline symptoms. There were no significant group differences on impulsivity. Between-group differences in the number of hospital admissions favored the DBT group at 10 and 20 weeks, but not at 32 weeks. There was no statistically significant difference between the groups with respect to the number of emergency department visits.
- Analyses of group differences in clinical improvement as measured by the SCL-90-R revealed statistically reliable and clinically significant changes in the DBT group over the WL group at 20 weeks, but not at 32 weeks.
Conclusions/limitations
- Brief DBT skills training reduced suicidal and NSSI episodes in patients with BPD. Participants in the DBT group also demonstrated greater improvement in anger, distress tolerance, and emotion regulation compared to the control group. These results were evident 3 months after treatment. However, any gains in health care utilization, social adjustment, symptom distress, and borderline symptoms diminished or did not differ from waitlist participants at Week 32. At that time, participants in the DBT group demonstrated a similar level of symptomatology as the WL group.
- Limitations include the use of an active waitlist control group, allowance of concurrent treatments, the absence of an active therapeutic comparator group, use of self-report measures, use of an instrument with unknown psychometric properties, and a relatively short 3-month follow-up period.
3. Bozzatello P, Bellino S. Combined therapy with interpersonal psychotherapy adapted for borderline personality disorder: a two-years follow-up. Psychiatry Res. 2016;240:151-156.
Psychotherapeutic options for treating BPD include DBT, mentalization-based treatment, schema-focused therapy, transference-based psychotherapy, and systems training for emotional predictability and problem solving. More recently, interpersonal therapy also has been adapted for BPD (IPT-BPD). However, thus far no trials have investigated the long-term effects of this therapy on BPD. In 2010, Bellino et al11 published a 32-week RCT examining the effect of IPT-BPD on BPD. They concluded that IPT-BPD plus fluoxetine was superior to fluoxetine alone in improving symptoms and quality of life. The present study by Bozzatello et al6 examined whether the benefits of IPT-BPD plus fluoxetine demonstrated in the 2010 study persisted over a 24-month follow-up.
Continue to: Study design...
Study design
- In the 2010 study by Bellino et al,11 55 outpatients who met DSM-IV criteria for BPD were randomized to receive IPT-BPD plus fluoxetine (combined therapy) or fluoxetine alone for 32 weeks. Forty-four participants completed a 24-month follow-up study (n = 22 for IPT-BPD plus fluoxetine, n = 22 for fluoxetine only).
- Clinical assessments were performed at 6, 12, and 24 months, and used the same instruments as the original study, including the Clinical Global Impression Scale–Severity item, Hamilton Depression Rating Scale, Hamilton Anxiety Rating Scale (HARS), Social and Occupational Functioning Assessment, Satisfaction Profile (SAT-P), and the Borderline Personality Disorder Severity Index (BPDSI).
Outcomes
- While the original study demonstrated that combined therapy had a clinically significant effect over fluoxetine alone on both HARS score and the BPDSI item “affective instability” at 32 weeks, this advantage was maintained only at the 6-month assessment.
- The improvements that the combined therapy provided over fluoxetine monotherapy on the BPDSI items of “impulsivity” and “interpersonal relationships” as well as the SAT-P factors of social and psychological functioning at 32 weeks were preserved at 24 months. No additional improvements were seen.
Conclusions/limitations
- The improvements in impulsivity, interpersonal functioning, social functioning, and psychological functioning at 32 weeks seen with IPT-BPD plus fluoxetine compared with fluoxetine alone persisted for 2 years after completing therapy; no further improvements were seen.
- The improvements to anxiety and affective instability that combined therapy demonstrated over fluoxetine monotherapy at 32 weeks were not maintained at 24 months.
- Limitations include a small sample size, exclusion of psychiatric comorbidities, and a lack of assessment of session or medication adherence.
4. Antonsen BT, Kvarstein EH, Urnes Ø, et al. Favourable outcome of long-term combined psychotherapy for patients with borderline personality disorder: six-year follow-up of a randomized study. Psychother Res. 2017;27(1):51-63.
While many studies have demonstrated the benefits of psychotherapy for treating personality disorders, there is limited research of how different levels of psychotherapy may impact treatment outcomes. An RCT called the Ullevål Personality Project (UPP)12 compared an intensive combined treatment program (CP) with outpatient individual psychotherapy (OIP) in patients with personality disorders. The CP program consisted of short-term day-hospital treatment followed by outpatient combined group and individual psychotherapy. The outcomes this RCT evaluated included suicide attempts, suicidal thoughts, self-injury, psychosocial functioning, symptom distress, and interpersonal and personality problems. A 6-year follow-up concluded there were no differences in outcomes between the 2 treatment groups. However, in this RCT, Antonsen et al7 examined whether CP produced statistically significant benefits over OIP in a subset of patients with BPD.
Study design
- In the UPP trial,12 117 patients who met DSM-IV criteria for personality disorders (excluding antisocial and schizotypal personality disorder) were randomized to receive 18 weeks of day hospital psychotherapy followed by CP or OIP. Fifty-two participants in the UPP were diagnosed with BPD, and 34 of these participants completed the 6-year follow-up investigation.
- Symptom distress, psychosocial functioning, interpersonal problems, quality of life, personality functioning, and self-harm/suicidal thoughts/suicide attempts were assessed at baseline, 8 months, 18 months, 3 years, and 6 years using the SCL-90-R Global Severity Index (GSI), BDI, Global Assessment of Functioning (GAF), Work and Social Adjustment Scale (WSAS), Quality of Life 10-point scale (QOL), Circumplex of Interpersonal Problems (CIP), and the 60-item short form of the Severity Indices of Personality Problems (SIPP-118) questionnaire.
Outcomes
- Compared to the OIP group, the CP group demonstrated statistically significant reductions in symptom distress at Year 6 as measured by the SCL-90-R GSI. Between Years 3 and 6, the CP group continued to show improvements in psychosocial functioning as demonstrated by improvements in GAF and WSAS scores. The OIP group’s scores worsened during this time. Compared to the OIP group, participants in the CP group also had significantly better outcomes on the SIPP-118 domains of self-control and identity integration.
- There were no significant differences between groups on the proportion of participants who engaged in self-harm or experienced suicidal thoughts or attempts. There were no significant differences in outcomes between the treatment groups on the CIP, BDI, or QOL.
- Participants in CP group tended to use fewer psychotropic medications than those in the OIP group over time, but this difference was not statistically significant. The 2 groups did not differ in use of health care services over the last year.
- Avoidant personality disorder (AVPD) did not have a significant moderator effect on GAF score. Comorbid AVPD was a negative predictor of GAF score, independent of the group.
Conclusions/limitations
- Both groups experienced a remission rate of 90% at 6-year follow-up. Compared with the OIP group, participants in the CP group experienced significantly greater reductions in symptom distress and improvements in self-control and identity integration at 6 years. Between Years 3 and 6, participants in the CP group experienced significant improvements in psychosocial functioning compared with OIP group participants. The 2 groups did not differ on other outcomes, including the CIP, BDI, QOL, suicidal thoughts, suicidal attempts, self-harm, and health care utilization.
- Despite statistically significant differences in GAF scores favoring the CP group over the OIP group during Years 3 to 6, GAF scores did not differ significantly in the final year, which suggests that symptomatic remission does not equal functional improvement.
- Limitations include a lack of control for intensity or length of treatment in statistical analyses, small sample size, lack of correction for multiple testing, lack of an a priori power analysis, missing data and potential violation of the missing at random assumption, use of therapists’ preferred treatment method/practice, and a lack of control for other treatments.
5. Bateman A, Constantinou MP, Fonagy P, et al. Eight-year prospective follow-up of mentalization-based treatment versus structured clinical management for people with borderline personality disorder. Personal Disord. 2021;12(4):291-299.
The efficacy of various psychotherapies for symptoms of BPD has been well established. However, there is limited evidence that these effects persist over time. In 2009, Bateman et al13 conducted an 18-month RCT comparing the effectiveness of outpatient mentalization-based treatment (MBT) against structured clinical management (SCM) for patients with BPD. Both groups experienced substantial improvements, but patients assigned to MBT demonstrated greater improvement in clinically significant problems, including suicide attempts and hospitalizations. In a 2021 follow-up to this study, Bateman et al8 investigated whether the MBT group’s gains in the primary outcomes (absence of severe self-harm, suicide attempts, and inpatient admissions in the previous 12 months), social functioning, vocational engagement, and mental health service usage were maintained throughout an 8-year follow-up period.
Continue to: Study design...
Study design
- In the 2009 trial, Bateman et al13 randomized adult participants who met DSM-IV criteria for BPD and had a suicide attempt or episode of life-threatening self-harm in the past 6 months to receive 18 months of MBT or SCM. The primary outcome was crisis events, defined as a composite of suicidal and severe self-injurious behaviors and hospitalizations. The 2021 Bateman et al8 study expanded this investigation by collecting additional data on a yearly basis for 8 years.
- Of the 134 original participants, 98 agreed to complete the follow-up. Due to attrition, the follow-up period was limited to 8 years. At each yearly visit, researchers collected information on the primary outcome, the absence of severe self-harm, suicide attempts, and inpatient admissions in the previous 12 months.
- Secondary measures were collected mainly through a modified version of the Client Service Receipt Inventory and included critical incidents, psychiatric and medical hospital and community services, employment and other personally meaningful activity, psychoactive medication, and other mental health treatments.
Outcomes
- The number of participants who met diagnostic criteria for BPD at the 1-year follow-up was significantly lower in the MBT group compared with the SCM group. To improve participant retention, this outcome was not evaluated at later visits.
- The number of participants who achieved the primary recovery criteria of the original trial (absence of severe self-harm, suicide attempts, and inpatient admissions in the previous 12 months) and remained well throughout the entire follow-up period was significantly higher in the MBT group compared with the SCM group. The average number of years during which participants failed to meet recovery criteria was significantly greater in the SCM group compared with the MBT group.
- When controlling for age, treatment group was a significant predictor of recovery during the follow-up period. Overall, significantly fewer participants in the MBT group experienced critical incidents during the follow-up period.
- The SCM group used crisis mental health services for a significantly greater number of follow-up years than the MBT group, although the likelihood of ever using crisis services did not statistically differ between the groups. Both groups had similar use of outpatient mental health services, primary care services, and nonmental health medical services. Compared to the SCM group, the MBT group had significantly fewer professional support service visits and significantly fewer outpatient psychiatrist visits.
- MBT group participants spent more time in education, were less likely to be unemployed, and were less likely to use social care interventions than SCM group participants. Although those in the MBT group spent more months engaged in purposeful activity, there was no significant difference between the groups in the proportion of participants who did not engage in purposeful activity.
- The MBT group spent fewer months receiving psychotherapeutic medication compared with the SCM group. The variables that yielded significant 2-way interactions were eating disorder, substance use disorder, and physical abuse, suggesting greater benefit from MBT with these concurrent diagnoses. Younger age was associated with better outcomes.
Conclusions/limitations
- This study demonstrated that patients with BPD significantly benefited from specialized therapies such as MBT.
- At the 1 follow-up visit, the number of participants who met diagnostic criteria for BPD was significantly lower in the MBT group compared with the SCM group.
- The number of participants who had achieved the primary recovery criteria and remained well during the 8-year follow-up period was significantly higher in the MBT group compared to the SCM group.
- Limitations include increasing attrition over time, possible allegiance effects and unmasking of research assistants, lack of self-report questionnaires, and the potentially erroneous conclusion that increased use of services equates to poorer treatment response and greater need for support.
6. Klein JP, Hauer-von Mauschwitz A, Berger T, et al. Effectiveness and safety of the adjunctive use of an internet-based self-management intervention for borderline personality disorder in addition to care as usual: results from a randomised controlled trial. BMJ Open. 2021;11(9):e047771.
Fewer than 1 in 4 patients with BPD have access to effective psychotherapies. The use of internet-based self-management interventions (SMIs) developed from evidence-based psychotherapies can help close this treatment gap. Although the efficacy of SMIs for several mental disorders has been demonstrated in multiple meta-analyses, results for BPD are mixed. In this study, Klein et al9 examined the effectiveness and safety of the adjunctive use of an SMI based on schema therapy in addition to care as usual (CAU) in patients with BPD.
Study design
- In a 12-month, rater-blind, controlled parallel group trial, adults who had a total BPDSI score ≥15 and either a diagnosis of BPD according to DSM-IV criteria or a probable diagnosis of BPD (if they had also received a BPD diagnosis from their treating physician) were randomized to an internet-based SMI based on schema therapy called priovi (n = 103) or CAU (n = 101). Participants could complete the SMI content in approximately 6 months but were recommended to use the intervention for the entire year.
- Participation in psychotherapy and psychiatric treatment, including pharmacotherapy, was permitted. At baseline, 74% of participants were receiving psychotherapy and 88% were receiving psychiatric treatment.
- The primary outcome was change in BPDSI score at 12 months. The primary safety outcome was the number of serious adverse events at 12 months. Secondary outcomes included BPD severity, depressive symptoms, anxiety symptoms, quality of life, uncontrolled internet use, negative treatment effects, and satisfaction with the intervention. Most assessments were measured at baseline, 3 months, 6 months, 9 months, and 12 months.
Outcomes
- Large reductions in the severity of BPD symptoms as measured by change in BPDSI score was observed in both groups. Although the average reduction in BPDSI score was greater in the SMI group, this difference was not statistically significant from the CAU group.
- There was no statistically significant difference in the number of serious adverse events between groups at any time.
Conclusions/limitations
- Treatment with SMI did not result in improved outcomes over CAU. Although the average reduction in BPDSI score was greater in the SMI group compared to the CAU group, this difference was not statistically significant.
- The authors cautioned that the smaller-than-expected between-groups effect size must be interpreted against the background of an unexpectedly large effect in the CAU group. In fact, the CAU group pre/post effect was comparable to the pre/post effect of intensive specialized DBT treatment groups in previous RCTs.
- The authors also suggested that the high percentage of participants who received psychotherapy did not allow for an additional benefit from SMI.
- Limitations include recruitment method, lack of systematic assessment of accidental unblinding, high exclusion rate due to failure of the participant or treating clinician to provide confirmation of diagnosis, and the use of a serious adverse events assessment that is not psychometrically validated.
Bottom Line
Evidence from randomized controlled trials suggests that internet-based psychoeducation, brief dialectical behavior therapy skills-only treatment, interpersonal therapy, a program that combines day treatment with individual and group psychotherapy, and mentalization-based treatment can improve symptoms and quality of life for patients with borderline personality disorder.
SECOND OF 2 PARTS
Borderline personality disorder (BPD) is associated with serious impairment in psychosocial functioning.1 It is characterized by an ongoing pattern of mood instability, cognitive distortions, problems with self-image, and impulsive behavior that often results in problems in relationships. As a result, patients with BPD tend to utilize more mental health services than patients with other personality disorders or major depressive disorder.2
Some clinicians believe BPD is difficult to treat. While historically there has been little consensus on the best treatments for this disorder, current options include both pharmacologic and psychological interventions. In Part 1 of this 2-part article, we focused on 6 studies that evaluated biological interventions.3 Here in Part 2, we focus on findings from 6 recent randomized controlled trials (RCTs) of psychosocial interventions for BPD

1. Zanarini MC, Conkey LC, Temes CM, et al. Randomized controlled trial of web-based psychoeducation for women with borderline personality disorder. J Clin Psychiatry. 2018;79(3):16m11153. doi: 10.4088/JCP.16m11153
Research has shown that BPD is a treatable illness with a more favorable prognosis than previously believed. Despite this, patients often experience difficulty accessing the most up-to-date information on BPD, which can impede their treatment. A 2008 study by Zanarini et al10 of younger female patients with BPD demonstrated that immediate, in-person psychoeducation improved impulsivity and relationships. Widespread implementation of this program proved problematic, however, due to cost and personnel constraints. To resolve this issue, researchers developed an internet-based version of the program. In a 2018 follow-up study, Zanarini et al4 examined the effect of this internet-based psychoeducation program on symptoms of BPD.
Continue to: Study design...
Study design
- Women (age 18 to 30) who met DSM-IV and Diagnostic Interview for Borderlines–Revised criteria for BPD were randomized to an internet-based psychoeducation treatment group (n = 40) or a control group (n = 40).
- Ten outcomes concerning symptom severity and psychosocial functioning were assessed during weeks 1 to 12 (acute phase) and at months 6, 9, and 12 (maintenance phase) using the self-report version of the Zanarini Rating Scale for BPD (ZAN-BPD), the Borderline Evaluation of Severity over Time, the Sheehan Disability Scale, the Clinically Useful Depression Outcome Scale, the Clinically Useful Anxiety Outcome Scale, and Weissman’s Social Adjustment Scale (SAS).
Outcomes
- In the acute phase, treatment group participants experienced statistically significant improvements in all 10 outcomes. Control group participants demonstrated similar results, achieving statistically significant improvements in 7 of 10 outcomes.
- Compared to the control group, the treatment group experienced a more significant reduction in impulsivity and improvement in psychosocial functioning as measured by the ZAN-BPD and SAS.
- In the maintenance phase, treatment group participants achieved statistically significant improvements in 9 of 10 outcomes, whereas control group participants demonstrated statistically significant improvements in only 3 of 10 outcomes.
- Compared to the control group, the treatment group also demonstrated a significantly greater improvement in all 4 sector scores and the total score of the ZAN-BP
Conclusions/limitations
- In patients with BPD, internet-based psychoeducation reduced symptom severity and improved psychosocial functioning, with effects lasting 1 year. Treatment group participants experienced clinically significant improvements in all outcomes measured during the acute phase of the study; most improvements were maintained over 1 year.
- While the control group initially saw similar improvements in most measurements, these improvements were not maintained as effectively over 1 year.
- Limitations include a female-only population, the restricted age range of participants, and recruitment exclusively from volunteers.
2. McMain SF, Guimond T, Barnhart R, et al. A randomized trial of brief dialectical behaviour therapy skills training in suicidal patients suffering from borderline disorder. Acta Psychiatr Scand. 2017;135(2):138-148.
Standard dialectical behavior therapy (DBT) is an effective treatment for BPD; however, access is often limited by shortages of clinicians and resources. Therefore, it has become increasingly common for clinical settings to offer patients only the skills training component of DBT, which requires fewer resources. While several clinical trials examining brief DBT skills–only treatment for BPD have shown promising results, it is unclear how effective this intervention is at reducing suicidal or nonsuicidal self-injury (NSSI) episodes. McMain et al5 explored the effectiveness of brief DBT skills–only adjunctive treatment on the rates of suicidal and NSSI episodes in patients with BPD.
Study design
- In this 2-arm, single-blind, prospective controlled trial, 84 adults who met DSM-IV criteria for BPD were randomized to a 20-week DBT skills training group (DBT group) or an active waitlist (WL group). No restrictions on additional psychosocial or pharmacologic treatments were imposed on either group.
- The primary outcome was the frequency of suicidal and NSSI episodes as measured by the Lifetime Suicide Attempt Self-Injury Interview and the Deliberate Self-Harm Inventory (DSHI). Measurements occurred at baseline, 10 weeks, 20 weeks, and 3 months posttreatment (32 weeks).
- Secondary outcomes included changes in health care utilization, BPD symptoms, and coping. These were assessed using the Treatment History Interview-2, Borderline Symptom List-23, State-Trait Anger Expression Inventory, Symptom Checklist-90-revised (SCL-90-R), Barratt Impulsiveness Scale-11, Beck Depression Inventory (BDI)-II, Social Adjustment Scale Self-report, Difficulties in Emotion Regulation Scale, Distress Tolerance Scale, and Kentucky Inventory of Mindfulness Scale.
Outcomes
- At Week 32, compared to the WL group, the DBT group showed statistically significant greater reductions in the frequency of suicidal and NSSI episodes as measured by the LSASI but not by the DSHI. The DBT group experienced statistically significant improvements in distress tolerance and emotion regulation over the WL group at all points, but no difference on mindfulness. The DBT group achieved greater reductions in anger over time as compared to the WL group.
- At Week 20, compared to the WL group, the DBT group showed significant improvements in social adjustment, symptom distress, and borderline symptoms. There were no significant group differences on impulsivity. Between-group differences in the number of hospital admissions favored the DBT group at 10 and 20 weeks, but not at 32 weeks. There was no statistically significant difference between the groups with respect to the number of emergency department visits.
- Analyses of group differences in clinical improvement as measured by the SCL-90-R revealed statistically reliable and clinically significant changes in the DBT group over the WL group at 20 weeks, but not at 32 weeks.
Conclusions/limitations
- Brief DBT skills training reduced suicidal and NSSI episodes in patients with BPD. Participants in the DBT group also demonstrated greater improvement in anger, distress tolerance, and emotion regulation compared to the control group. These results were evident 3 months after treatment. However, any gains in health care utilization, social adjustment, symptom distress, and borderline symptoms diminished or did not differ from waitlist participants at Week 32. At that time, participants in the DBT group demonstrated a similar level of symptomatology as the WL group.
- Limitations include the use of an active waitlist control group, allowance of concurrent treatments, the absence of an active therapeutic comparator group, use of self-report measures, use of an instrument with unknown psychometric properties, and a relatively short 3-month follow-up period.
3. Bozzatello P, Bellino S. Combined therapy with interpersonal psychotherapy adapted for borderline personality disorder: a two-years follow-up. Psychiatry Res. 2016;240:151-156.
Psychotherapeutic options for treating BPD include DBT, mentalization-based treatment, schema-focused therapy, transference-based psychotherapy, and systems training for emotional predictability and problem solving. More recently, interpersonal therapy also has been adapted for BPD (IPT-BPD). However, thus far no trials have investigated the long-term effects of this therapy on BPD. In 2010, Bellino et al11 published a 32-week RCT examining the effect of IPT-BPD on BPD. They concluded that IPT-BPD plus fluoxetine was superior to fluoxetine alone in improving symptoms and quality of life. The present study by Bozzatello et al6 examined whether the benefits of IPT-BPD plus fluoxetine demonstrated in the 2010 study persisted over a 24-month follow-up.
Continue to: Study design...
Study design
- In the 2010 study by Bellino et al,11 55 outpatients who met DSM-IV criteria for BPD were randomized to receive IPT-BPD plus fluoxetine (combined therapy) or fluoxetine alone for 32 weeks. Forty-four participants completed a 24-month follow-up study (n = 22 for IPT-BPD plus fluoxetine, n = 22 for fluoxetine only).
- Clinical assessments were performed at 6, 12, and 24 months, and used the same instruments as the original study, including the Clinical Global Impression Scale–Severity item, Hamilton Depression Rating Scale, Hamilton Anxiety Rating Scale (HARS), Social and Occupational Functioning Assessment, Satisfaction Profile (SAT-P), and the Borderline Personality Disorder Severity Index (BPDSI).
Outcomes
- While the original study demonstrated that combined therapy had a clinically significant effect over fluoxetine alone on both HARS score and the BPDSI item “affective instability” at 32 weeks, this advantage was maintained only at the 6-month assessment.
- The improvements that the combined therapy provided over fluoxetine monotherapy on the BPDSI items of “impulsivity” and “interpersonal relationships” as well as the SAT-P factors of social and psychological functioning at 32 weeks were preserved at 24 months. No additional improvements were seen.
Conclusions/limitations
- The improvements in impulsivity, interpersonal functioning, social functioning, and psychological functioning at 32 weeks seen with IPT-BPD plus fluoxetine compared with fluoxetine alone persisted for 2 years after completing therapy; no further improvements were seen.
- The improvements to anxiety and affective instability that combined therapy demonstrated over fluoxetine monotherapy at 32 weeks were not maintained at 24 months.
- Limitations include a small sample size, exclusion of psychiatric comorbidities, and a lack of assessment of session or medication adherence.
4. Antonsen BT, Kvarstein EH, Urnes Ø, et al. Favourable outcome of long-term combined psychotherapy for patients with borderline personality disorder: six-year follow-up of a randomized study. Psychother Res. 2017;27(1):51-63.
While many studies have demonstrated the benefits of psychotherapy for treating personality disorders, there is limited research of how different levels of psychotherapy may impact treatment outcomes. An RCT called the Ullevål Personality Project (UPP)12 compared an intensive combined treatment program (CP) with outpatient individual psychotherapy (OIP) in patients with personality disorders. The CP program consisted of short-term day-hospital treatment followed by outpatient combined group and individual psychotherapy. The outcomes this RCT evaluated included suicide attempts, suicidal thoughts, self-injury, psychosocial functioning, symptom distress, and interpersonal and personality problems. A 6-year follow-up concluded there were no differences in outcomes between the 2 treatment groups. However, in this RCT, Antonsen et al7 examined whether CP produced statistically significant benefits over OIP in a subset of patients with BPD.
Study design
- In the UPP trial,12 117 patients who met DSM-IV criteria for personality disorders (excluding antisocial and schizotypal personality disorder) were randomized to receive 18 weeks of day hospital psychotherapy followed by CP or OIP. Fifty-two participants in the UPP were diagnosed with BPD, and 34 of these participants completed the 6-year follow-up investigation.
- Symptom distress, psychosocial functioning, interpersonal problems, quality of life, personality functioning, and self-harm/suicidal thoughts/suicide attempts were assessed at baseline, 8 months, 18 months, 3 years, and 6 years using the SCL-90-R Global Severity Index (GSI), BDI, Global Assessment of Functioning (GAF), Work and Social Adjustment Scale (WSAS), Quality of Life 10-point scale (QOL), Circumplex of Interpersonal Problems (CIP), and the 60-item short form of the Severity Indices of Personality Problems (SIPP-118) questionnaire.
Outcomes
- Compared to the OIP group, the CP group demonstrated statistically significant reductions in symptom distress at Year 6 as measured by the SCL-90-R GSI. Between Years 3 and 6, the CP group continued to show improvements in psychosocial functioning as demonstrated by improvements in GAF and WSAS scores. The OIP group’s scores worsened during this time. Compared to the OIP group, participants in the CP group also had significantly better outcomes on the SIPP-118 domains of self-control and identity integration.
- There were no significant differences between groups on the proportion of participants who engaged in self-harm or experienced suicidal thoughts or attempts. There were no significant differences in outcomes between the treatment groups on the CIP, BDI, or QOL.
- Participants in CP group tended to use fewer psychotropic medications than those in the OIP group over time, but this difference was not statistically significant. The 2 groups did not differ in use of health care services over the last year.
- Avoidant personality disorder (AVPD) did not have a significant moderator effect on GAF score. Comorbid AVPD was a negative predictor of GAF score, independent of the group.
Conclusions/limitations
- Both groups experienced a remission rate of 90% at 6-year follow-up. Compared with the OIP group, participants in the CP group experienced significantly greater reductions in symptom distress and improvements in self-control and identity integration at 6 years. Between Years 3 and 6, participants in the CP group experienced significant improvements in psychosocial functioning compared with OIP group participants. The 2 groups did not differ on other outcomes, including the CIP, BDI, QOL, suicidal thoughts, suicidal attempts, self-harm, and health care utilization.
- Despite statistically significant differences in GAF scores favoring the CP group over the OIP group during Years 3 to 6, GAF scores did not differ significantly in the final year, which suggests that symptomatic remission does not equal functional improvement.
- Limitations include a lack of control for intensity or length of treatment in statistical analyses, small sample size, lack of correction for multiple testing, lack of an a priori power analysis, missing data and potential violation of the missing at random assumption, use of therapists’ preferred treatment method/practice, and a lack of control for other treatments.
5. Bateman A, Constantinou MP, Fonagy P, et al. Eight-year prospective follow-up of mentalization-based treatment versus structured clinical management for people with borderline personality disorder. Personal Disord. 2021;12(4):291-299.
The efficacy of various psychotherapies for symptoms of BPD has been well established. However, there is limited evidence that these effects persist over time. In 2009, Bateman et al13 conducted an 18-month RCT comparing the effectiveness of outpatient mentalization-based treatment (MBT) against structured clinical management (SCM) for patients with BPD. Both groups experienced substantial improvements, but patients assigned to MBT demonstrated greater improvement in clinically significant problems, including suicide attempts and hospitalizations. In a 2021 follow-up to this study, Bateman et al8 investigated whether the MBT group’s gains in the primary outcomes (absence of severe self-harm, suicide attempts, and inpatient admissions in the previous 12 months), social functioning, vocational engagement, and mental health service usage were maintained throughout an 8-year follow-up period.
Continue to: Study design...
Study design
- In the 2009 trial, Bateman et al13 randomized adult participants who met DSM-IV criteria for BPD and had a suicide attempt or episode of life-threatening self-harm in the past 6 months to receive 18 months of MBT or SCM. The primary outcome was crisis events, defined as a composite of suicidal and severe self-injurious behaviors and hospitalizations. The 2021 Bateman et al8 study expanded this investigation by collecting additional data on a yearly basis for 8 years.
- Of the 134 original participants, 98 agreed to complete the follow-up. Due to attrition, the follow-up period was limited to 8 years. At each yearly visit, researchers collected information on the primary outcome, the absence of severe self-harm, suicide attempts, and inpatient admissions in the previous 12 months.
- Secondary measures were collected mainly through a modified version of the Client Service Receipt Inventory and included critical incidents, psychiatric and medical hospital and community services, employment and other personally meaningful activity, psychoactive medication, and other mental health treatments.
Outcomes
- The number of participants who met diagnostic criteria for BPD at the 1-year follow-up was significantly lower in the MBT group compared with the SCM group. To improve participant retention, this outcome was not evaluated at later visits.
- The number of participants who achieved the primary recovery criteria of the original trial (absence of severe self-harm, suicide attempts, and inpatient admissions in the previous 12 months) and remained well throughout the entire follow-up period was significantly higher in the MBT group compared with the SCM group. The average number of years during which participants failed to meet recovery criteria was significantly greater in the SCM group compared with the MBT group.
- When controlling for age, treatment group was a significant predictor of recovery during the follow-up period. Overall, significantly fewer participants in the MBT group experienced critical incidents during the follow-up period.
- The SCM group used crisis mental health services for a significantly greater number of follow-up years than the MBT group, although the likelihood of ever using crisis services did not statistically differ between the groups. Both groups had similar use of outpatient mental health services, primary care services, and nonmental health medical services. Compared to the SCM group, the MBT group had significantly fewer professional support service visits and significantly fewer outpatient psychiatrist visits.
- MBT group participants spent more time in education, were less likely to be unemployed, and were less likely to use social care interventions than SCM group participants. Although those in the MBT group spent more months engaged in purposeful activity, there was no significant difference between the groups in the proportion of participants who did not engage in purposeful activity.
- The MBT group spent fewer months receiving psychotherapeutic medication compared with the SCM group. The variables that yielded significant 2-way interactions were eating disorder, substance use disorder, and physical abuse, suggesting greater benefit from MBT with these concurrent diagnoses. Younger age was associated with better outcomes.
Conclusions/limitations
- This study demonstrated that patients with BPD significantly benefited from specialized therapies such as MBT.
- At the 1 follow-up visit, the number of participants who met diagnostic criteria for BPD was significantly lower in the MBT group compared with the SCM group.
- The number of participants who had achieved the primary recovery criteria and remained well during the 8-year follow-up period was significantly higher in the MBT group compared to the SCM group.
- Limitations include increasing attrition over time, possible allegiance effects and unmasking of research assistants, lack of self-report questionnaires, and the potentially erroneous conclusion that increased use of services equates to poorer treatment response and greater need for support.
6. Klein JP, Hauer-von Mauschwitz A, Berger T, et al. Effectiveness and safety of the adjunctive use of an internet-based self-management intervention for borderline personality disorder in addition to care as usual: results from a randomised controlled trial. BMJ Open. 2021;11(9):e047771.
Fewer than 1 in 4 patients with BPD have access to effective psychotherapies. The use of internet-based self-management interventions (SMIs) developed from evidence-based psychotherapies can help close this treatment gap. Although the efficacy of SMIs for several mental disorders has been demonstrated in multiple meta-analyses, results for BPD are mixed. In this study, Klein et al9 examined the effectiveness and safety of the adjunctive use of an SMI based on schema therapy in addition to care as usual (CAU) in patients with BPD.
Study design
- In a 12-month, rater-blind, controlled parallel group trial, adults who had a total BPDSI score ≥15 and either a diagnosis of BPD according to DSM-IV criteria or a probable diagnosis of BPD (if they had also received a BPD diagnosis from their treating physician) were randomized to an internet-based SMI based on schema therapy called priovi (n = 103) or CAU (n = 101). Participants could complete the SMI content in approximately 6 months but were recommended to use the intervention for the entire year.
- Participation in psychotherapy and psychiatric treatment, including pharmacotherapy, was permitted. At baseline, 74% of participants were receiving psychotherapy and 88% were receiving psychiatric treatment.
- The primary outcome was change in BPDSI score at 12 months. The primary safety outcome was the number of serious adverse events at 12 months. Secondary outcomes included BPD severity, depressive symptoms, anxiety symptoms, quality of life, uncontrolled internet use, negative treatment effects, and satisfaction with the intervention. Most assessments were measured at baseline, 3 months, 6 months, 9 months, and 12 months.
Outcomes
- Large reductions in the severity of BPD symptoms as measured by change in BPDSI score was observed in both groups. Although the average reduction in BPDSI score was greater in the SMI group, this difference was not statistically significant from the CAU group.
- There was no statistically significant difference in the number of serious adverse events between groups at any time.
Conclusions/limitations
- Treatment with SMI did not result in improved outcomes over CAU. Although the average reduction in BPDSI score was greater in the SMI group compared to the CAU group, this difference was not statistically significant.
- The authors cautioned that the smaller-than-expected between-groups effect size must be interpreted against the background of an unexpectedly large effect in the CAU group. In fact, the CAU group pre/post effect was comparable to the pre/post effect of intensive specialized DBT treatment groups in previous RCTs.
- The authors also suggested that the high percentage of participants who received psychotherapy did not allow for an additional benefit from SMI.
- Limitations include recruitment method, lack of systematic assessment of accidental unblinding, high exclusion rate due to failure of the participant or treating clinician to provide confirmation of diagnosis, and the use of a serious adverse events assessment that is not psychometrically validated.
Bottom Line
Evidence from randomized controlled trials suggests that internet-based psychoeducation, brief dialectical behavior therapy skills-only treatment, interpersonal therapy, a program that combines day treatment with individual and group psychotherapy, and mentalization-based treatment can improve symptoms and quality of life for patients with borderline personality disorder.
1. Skodol AE, Gunderson JG, McGlashan TM, et al. Functional impairment in patients with schizotypal, borderline, avoidant, or obsessive-compulsive personality disorder. Am J Psychiatry. 2002;159(2):276-83.
2. Bender DS, Dolan RT, Skodol AE, et al. Treatment utilization by patients with personality disorders. Am J Psychiatry. 2001;158(2):295-302.
3. Saeed SA, Kallis AC. Borderline personality disorder: 6 studies of biological interventions. Current Psychiatry. 2021;20(11):26-30,34-36.
4. Zanarini MC, Conkey LC, Temes CM, et al. Randomized controlled trial of web-based psychoeducation for women with borderline personality disorder. J Clin Psychiatry. 2018;79(3):16m11153. doi:10.4088/JCP.16m11153
5. McMain SF, Guimond T, Barnhart R, et al. A randomized trial of brief dialectical behaviour therapy skills training in suicidal patients suffering from borderline disorder. Acta Psychiatr Scand. 2017;135(2):138-148. doi:10.1111/acps.12664
6. Bozzatello P, Bellino S. Combined therapy with interpersonal psychotherapy adapted for borderline personality disorder: a two-years follow-up. Psychiatry Res. 2016;240:151-156. doi:10.1016/j.psychres.2016.04.014
7. Antonsen BT, Kvarstein EH, Urnes Ø, et al. Favourable outcome of long-term combined psychotherapy for patients with borderline personality disorder: six-year follow-up of a randomized study. Psychother Res. 2017;27(1):51-63. doi:10.1080/10503307.2015.1072283
8. Bateman A, Constantinou MP, Fonagy P, et al. Eight-year prospective follow-up of mentalization-based treatment versus structured clinical management for people with borderline personality disorder. Personal Disord. 2021;12(4):291-299. doi:10.1037/per0000422
9. Klein JP, Hauer-von Mauschwitz A, Berger T, et al. Effectiveness and safety of the adjunctive use of an internet-based self-management intervention for borderline personality disorder in addition to care as usual: results from a randomised controlled trial. BMJ Open. 2021;11(9):e047771. doi:10.1136/bmjopen-2020-047771
10. Zanarini MC, Frankenburg FR. A preliminary, randomized trial of psychoeducation for women with borderline personality disorder. J Pers Disord. 2008;22(3):284-290.
11. Bellino S, Rinaldi C, Bogetto F. Adaptation of interpersonal psychotherapy to borderline personality disorder: a comparison of combined therapy and single pharmacotherapy. Can J Psychiatry. 2010;55(2):74-81.
12. Arnevik E, Wilberg T, Urnes Ø, et al. Psychotherapy for personality disorders: short-term day hospital psychotherapy versus outpatient individual therapy – a randomized controlled study. Eur Psychiatry. 2009,24(2):71-78. doi:10.1016/j.eurpsy.2008.09.004
13. Bateman A, Fonagy P. Randomized controlled trial of outpatient mentalization-based treatment versus structured clinical management for borderline personality disorder. Am J Psychiatry. 2009;166(12):1355-1364.
1. Skodol AE, Gunderson JG, McGlashan TM, et al. Functional impairment in patients with schizotypal, borderline, avoidant, or obsessive-compulsive personality disorder. Am J Psychiatry. 2002;159(2):276-83.
2. Bender DS, Dolan RT, Skodol AE, et al. Treatment utilization by patients with personality disorders. Am J Psychiatry. 2001;158(2):295-302.
3. Saeed SA, Kallis AC. Borderline personality disorder: 6 studies of biological interventions. Current Psychiatry. 2021;20(11):26-30,34-36.
4. Zanarini MC, Conkey LC, Temes CM, et al. Randomized controlled trial of web-based psychoeducation for women with borderline personality disorder. J Clin Psychiatry. 2018;79(3):16m11153. doi:10.4088/JCP.16m11153
5. McMain SF, Guimond T, Barnhart R, et al. A randomized trial of brief dialectical behaviour therapy skills training in suicidal patients suffering from borderline disorder. Acta Psychiatr Scand. 2017;135(2):138-148. doi:10.1111/acps.12664
6. Bozzatello P, Bellino S. Combined therapy with interpersonal psychotherapy adapted for borderline personality disorder: a two-years follow-up. Psychiatry Res. 2016;240:151-156. doi:10.1016/j.psychres.2016.04.014
7. Antonsen BT, Kvarstein EH, Urnes Ø, et al. Favourable outcome of long-term combined psychotherapy for patients with borderline personality disorder: six-year follow-up of a randomized study. Psychother Res. 2017;27(1):51-63. doi:10.1080/10503307.2015.1072283
8. Bateman A, Constantinou MP, Fonagy P, et al. Eight-year prospective follow-up of mentalization-based treatment versus structured clinical management for people with borderline personality disorder. Personal Disord. 2021;12(4):291-299. doi:10.1037/per0000422
9. Klein JP, Hauer-von Mauschwitz A, Berger T, et al. Effectiveness and safety of the adjunctive use of an internet-based self-management intervention for borderline personality disorder in addition to care as usual: results from a randomised controlled trial. BMJ Open. 2021;11(9):e047771. doi:10.1136/bmjopen-2020-047771
10. Zanarini MC, Frankenburg FR. A preliminary, randomized trial of psychoeducation for women with borderline personality disorder. J Pers Disord. 2008;22(3):284-290.
11. Bellino S, Rinaldi C, Bogetto F. Adaptation of interpersonal psychotherapy to borderline personality disorder: a comparison of combined therapy and single pharmacotherapy. Can J Psychiatry. 2010;55(2):74-81.
12. Arnevik E, Wilberg T, Urnes Ø, et al. Psychotherapy for personality disorders: short-term day hospital psychotherapy versus outpatient individual therapy – a randomized controlled study. Eur Psychiatry. 2009,24(2):71-78. doi:10.1016/j.eurpsy.2008.09.004
13. Bateman A, Fonagy P. Randomized controlled trial of outpatient mentalization-based treatment versus structured clinical management for borderline personality disorder. Am J Psychiatry. 2009;166(12):1355-1364.
Psychiatric partial hospitalization programs: What you need to know
Psychiatric partial hospitalization programs (PHPs), previously known as “day hospitals,” serve to bridge the gap between inpatient and outpatient facilities by providing intensive, highly structured outpatient behavioral health services (typically several hours of psychotherapy each weekday for most days of the week). The concept of PHPs has existed since at least the 1950s, but such programs started to become more common in the United States as the result of legislation passed in 1963 (Box1-3). In this article, I provide a brief introductory review of PHPs, while acknowledging that most research on PHPs was conducted years ago and is rather limited.
Box
The concept of partial hospitalization programs (PHPs) was developed before the 1950s.1 However, in the United States, PHPs did not take hold until Congress passed the Community Mental Health Act of 1963, which required that PHPs must be a core component of Community Mental Health Centers (CMHCs). The Omnibus Budget Reconciliation Acts of 1987 and 1990 required Medicare to pay for PHPs affiliated with or based in CMHCs and psychiatric hospitals, which resulted in a proliferation of PHPs across the country. The number of CMHCbased PHPs grew from 296 in 1993 to 769 in 1997.2 By 2016, more than one-third (38.7%) of all metropolitan hospitals and 11.4% of nonmetropolitan hospitals in the United States provided PHP services.3 This growth was also partially the result of private health insurance companies and the managed care industry clamping down on inpatient hospital stays and approving PHP care to reduce costs. In recent years, freestanding PHPs that are not affiliated with a CMHC or hospital have opened to serve high-functioning patients who do not want inpatient hospitalization or the stigma associated with it.
PHPs: What they are, and how they work
The term “partial hospitalization” is fraught with confusion because initially it was used to contrast such services from full hospitalization. Historically, it was used to describe services for patients who had been discharged home from a state hospital and attended a program on the hospital grounds during the day as outpatients. In reality, today’s PHPs are “day treatment” programs, but the terminology has stuck.
PHPs are neither an inpatient service nor a strict outpatient service, but rather a midground along the continuum of treatment intensity between the 2 traditional types of psychiatric services for patients with a range of mental illness of varying severity. The Association for Ambulatory Behavioral Healthcare, which has set standards and guidelines for PHPs, defines a PHP as “an ambulatory treatment program that includes the major diagnostic, medical, psychiatric, psychosocial, and prevocational treatment modalities designed for patients with serious mental disorders who require coordinated intensive, comprehensive, and multidisciplinary treatment not provided in an outpatient clinical setting.”4 PHPs can render acute care as an alternative to inpatient treatment, provide transitional stabilization treatment between an inpatient stay and traditional outpatient treatment (once a week or less frequent), and function as a supplement to traditional outpatient treatment.
Medicare has established criteria that PHPs must meet to qualify for reimbursement5; these criteria are now widely accepted as standards of care by the insurance industry. To meet the Medicare criteria, PHP treatment must be active and structured to provide an individualized treatment plan that incorporates coordination of services to meet the particular needs of the patient.5 It must include a multidisciplinary team approach to patient care under the direction of a physician, and the treatment goals must be measurable, functional, time-framed, medically necessary, and directly related to the reason for admission.5 The physician must certify the medical necessity for admission by documenting that the patient has a diagnosis of an acute Axis I mental disorder, a level of functioning that includes severe impairments in multiple areas of daily life, and a “reasonable expectation” that the disorder and level of functioning will improve as a result of the treatment.5
The Joint Commission (formerly JCAHO) lumps day treatment, intensive outpatient, partial hospitalization, and adult day care services into a single category of an ambulatory health care environment offering an organized day or night program of assessment, treatment, care, services, habilitation, or rehabilitation for individuals who do not require 24-hour care.6 For behavioral health, this may be a structured, ongoing program that typically meets 2 to 5 times a week for 2 to 5 hours per day.6
Most PHPs for adult patients provide services during the day 5 days per week and average 5 to 6 hours of programming per day. Night or evening programming may be a good option for patients who work during the day. Typically, treatment is provided in a group therapy format, with individual therapy at least once a week. Group therapy may include cognitive-behavioral therapy, coping with grief and loss, trauma recovery, conflict resolution, stress management, anger control, and behavioral modification. Family therapy is provided as needed, but usually is mandatory for children and adolescents. Many PHPs offer intensive outpatient programs (IOPs) as a step down to further facilitate a patient’s adjustment to psychosocial and family functioning while returning to work on a part-time basis. IOPs typically provide 3 to 4 hours of service per day 3 days per week. While not evidence-based, the typical duration of PHP treatment is 4 to 6 weeks, followed by an additional 2 to 4 weeks of IOP.
Continue to: Advantages and disadvantages...
Advantages and disadvantages
Based on a qualitative literature review, Neffinger1 outlined potential advantages and disadvantages of PHPs vs inpatient hospitalization (Table1). While these have not been empirically studied, they may be useful to consider when determining if a PHP would be beneficial for a specific patient.
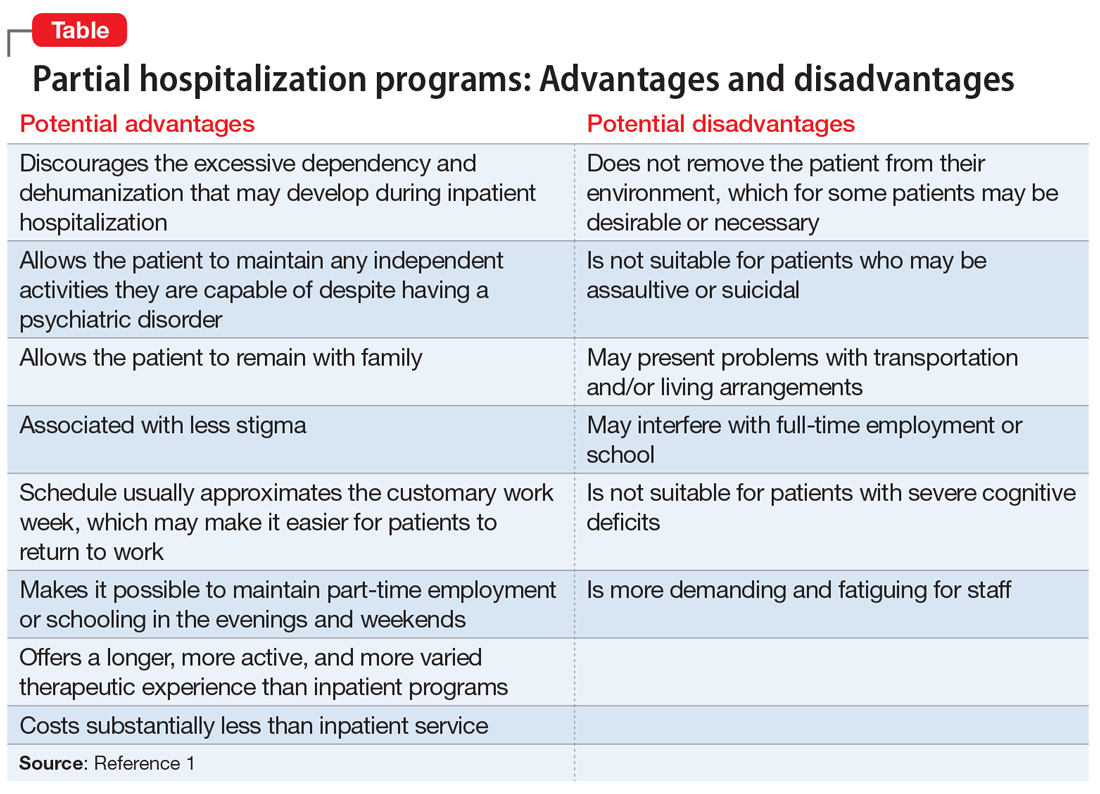
Which factors are most therapeutic?
Research on which factors of PHPs are of therapeutic value is quite limited and primarily consists of surveys of small numbers of participants. Hoge et al7 explored the active therapeutic factors responsible for change in the Connecticut Mental Health Center PHP by comparing responses of 20 patients with those of their clinicians. Ninety-five percent of patients rated structure as the top therapeutic factor, followed by interpersonal contact, medication, and altruism. Other factors that were rated as important by 40% or fewer participants were catharsis, learning, mobilization of family support, connection to community, universality, patient autonomy, successful completion, security, feedback on behavior, and practice at home. In a British study8 that used a similar method, patients reported that counseling was the most helpful aspect of treatment, followed by medical treatment, while staff picked groups followed by a planned approach.
Evidence supports PHPs’ effectiveness
Some research has suggested that PHPs can be effective, both clinically and in terms of cost. Marshall et al9 conducted a systematic review of the effectiveness of an acute day hospital vs inpatient admission vs outpatient care that included 9 trials. They concluded that psychiatric inpatient admissions could be reduced by at least 23% if patients were diverted to an acute day hospital. They also found some evidence that day treatment programs may be superior to outpatient care in improving symptoms in nonpsychotic patients who are refractory to outpatient treatment. In a systematic review of 18 studies, high rates of satisfaction with PHP services suggested PHPs have an advantage over inpatient treatment within 1 year of discharge; patients and families were more satisfied with PHPs.10 In a study of 197 urban, socioeconomically disadvantaged, severely ill patients, Sledge et al11 compared the clinical outcomes of those who participated in day hospital programs with those of patients who received inpatient care. They found that while overall the 2 approaches produced similar outcomes, there were slightly more positive effects for day hospital programs in measures of symptoms, overall functioning, and social functioning. In terms of cost effectiveness, a 1978 study found that even after correcting for differences in treatment days between inpatient and PHP services, there was a significant financial advantage with PHP (costs were one-third less), primarily because of lower costs per day.12 In another study, PHP cost savings were 20%, and potential savings were higher for nonpsychotic patients.13
Are PHPs appropriate for children and adolescents?
Studies of PHPs for adolescents found that patients made gains in peer relationships, behavioral and academic performance, and control of their emotions.14,15 A review of PHPs’ effectiveness for children suggested that 66% to 99% of treated patients demonstrated improvement and successful return to community-based schools, and family functioning was a major factor in improvement.14 In a follow-up study that surveyed patients via telephone >1 year after discharge from a PHP, almost 80% of the children and adolescents were either “doing OK” or were “well-adjusted.”14 Only 22.5% required inpatient or residential treatment; the majority were doing well in school, with only 7% failing.14 In addition, 60% of parents reported satisfaction with treatment, and 85% reported functional improvement in their children.
Factors that predict PHP success or failure
In an analysis of a day treatment program that provided 4 months of intensive psychodynamic, group-oriented milieu treatment for patients with long-standing personality disorders, Rosie et al16 found 3 factors that contributed to the success of the PHP:
- optimal treatment-patient matching
- judicious use of authority in milieu therapy
- maintaining close relationships with referral sources.
In a study that compared 58 patients who completed an Ottawa hospital PHP and 44 who did not complete the program, psychological mindedness and chronicity of psychiatric illness were found to predict completion.17 However, a study of 59 females with anorexia nervosa who were transferred from inpatient care to an eating disorder day hospital program found that a longer duration of illness, amenorrhea, and a lower body mass index were associated with PHP treatment failure and inpatient rehospitalization.18 One study found that for individuals who were referred to a PHP from inpatient care, suicidal ideation and greater psychotic symptoms were associated with acute inpatient rehospitalization.19 Other factors associated with PHP nonattendance and treatment failure include limited personal and economic resources, high rates of substance abuse disorders, multiple admissions, and disability.20 In a study of 103 alcohol-dependent patients who completed IOP treatment, 64% were abstinent at 6 months follow-up; relapse was associated with a longer duration of alcohol dependence and a higher number of prior treatments, while favorable outcomes were associated with a lower degree of depression, anxiety, and craving.21 Patients with cocaine dependence who completed an IOP showed significant improvements in addiction scores and had more favorable outcomes in employment status and psychological problems if they stayed longer in treatment.22
Bottom Line
Psychiatric partial hospitalization programs (PHPs) provide a transition from inpatient hospitalization to outpatient treatment for patients who need further stabilization, or serve as an alternative to inpatient treatment for patients who don’t need or want inpatient hospitalization. PHPs can be as effective as inpatient treatment for all but the most seriously ill patients, and are more cost-effective than inpatient treatment.
1. Neffinger GG. Partial hospitalization: an overview. J Community Psychol. 1981;9(3):262-269.
2. Leung MY, Drozd EM, Healy DA, et al. Impacts associated with the Medicare Psychiatric PPS: a study of partial hospitalization programs. February 2009. Accessed January 8, 2022. https://www.cms.gov/Research-Statistics-Data-and-Systems/Statistics-Trends-and-Reports/Reports/downloads/Leung_PHP_PPS_2010.pdf
3. Williams T, Borders TF, Jasinski L. Partial Psychiatric Hospitalization Program Availability in Non-Metropolitan and Non-Metropolitan Hospitals Nationally. Rural and Underserved Health Research Center; 2019.
4. Rosser J, Michael S, eds. Standards and guidelines for partial hospital programs and intensive outpatient programs. Association for Ambulatory Behavioral Healthcare. 2018. Accessed January 8, 2022. https://aabh.org/wp-content/uploads/2021/05/2021-SandG-Final.pdf
5. CMS Manual System PUB. 100-02 Medicare Benefit Policy: Partial Hospitalization Services, Department of Health and Human Services. Centers for Medicare & Medicaid Services; 2004:6-9.
6. Joint Commission. Guide to Joint Commission Behavioral Healthcare Accreditation. Joint Commission; 2007:36.
7. Hoge MA, Farrell SP, Munchel ME, et al. Therapeutic factors in partial hospitalization. Psychiatry. 1988;51(2):199-210.
8. Ricketts T, Kirshbaum MN. Helpfulness of mental health day care: client and staff views. J Adv Nurs. 1994;20(2):297-306.
9. Marshall M, Crowther R, Almaraz-Serrano A, et al. Systematic reviews of the effectiveness of day care for people with severe mental disorders: (1) acute day hospital versus admission; (2) vocational rehabilitation; (3) day hospital versus outpatient care. Health Technol Assess. 2001;5(21):1-75.
10. Horvitz-Lennon M, Normand SL, Gaccione P, et al. Partial versus full hospitalization for adults in psychiatric distress: a systematic review of the published literature (1957-1997). Am J Psychiatry. 2001;158(5):676-685.
11. Sledge WH, Tebes J, Rakfeldt J, et al. Day hospital/crisis respite care versus inpatient care, Part I: Clinical outcomes. Am J Psychiatry. 1996;153(8):1065-1073.
12. Fink EB, Longabaugh R, Stout R. The paradoxical underutilization of partial hospitalization. Am J Psychiatry. 1978;135(6):713-716.
13. Sledge WH, Tebes J, Wolff N, et al. Day hospital/crisis respite care versus inpatient care, Part II: service utilization and costs. Am J Psychiatry. 1996;153(8):1074-1083.
14. Kiser LJ. Treatment-effectiveness research in child and adolescent partial hospitalization. Psychiatr Hosp. 1991;22(2):51-8.
15. Milin R, Coupland K, Walker S, et al. Outcome and follow-up study of an adolescent psychiatric day treatment school program. J Am Acad Child Adolesc Psychiatry. 2000;39(3):320-328.
16. Rosie JS, Azim HF, Piper WE, et al. Effective psychiatric day treatment: historical lessons. Psychiatr Serv. 1995;46(10):1019-1026.
17. Tasca GA, Balfour L, Bissada H, et al. Treatment completion and outcome in a partial hospitalization program: interactions among patient variables. Psychotherapy Res. 1999;9(2):232-247.
18. Howard WT, Evans KK, Quintero-Howard CV, et al. Predictors of success or failure of transition to day hospital treatment for inpatients with anorexia nervosa. Am J Psychiatry. 1999;156(11):1697-1702.
19. Beard C, Hearon BA, Lee J, et al. When partial hospitalization fails: risk factors for inpatient hospitalization. J Nerv Ment Dis. 2016;204(6):431-436.
20. Lieberman PB, Guggenheim FG. Reasons for patient nonattendance during acute partial hospitalization. Psychiatr Serv. 2016;67(6):684-687.
21. Bottlender M, Soyka M. Efficacy of an intensive outpatient rehabilitation program in alcoholism: predictors of outcome 6 months after treatment. Eur Addict Res. 2005;11(3):132-137.
22. Gottheil E, Weinstein SP, Sterling RC, et al. A randomized controlled study of the effectiveness of intensive outpatient treatment for cocaine dependence. Psychiatr Serv. 1998;49(6):782-787.
Psychiatric partial hospitalization programs (PHPs), previously known as “day hospitals,” serve to bridge the gap between inpatient and outpatient facilities by providing intensive, highly structured outpatient behavioral health services (typically several hours of psychotherapy each weekday for most days of the week). The concept of PHPs has existed since at least the 1950s, but such programs started to become more common in the United States as the result of legislation passed in 1963 (Box1-3). In this article, I provide a brief introductory review of PHPs, while acknowledging that most research on PHPs was conducted years ago and is rather limited.
Box
The concept of partial hospitalization programs (PHPs) was developed before the 1950s.1 However, in the United States, PHPs did not take hold until Congress passed the Community Mental Health Act of 1963, which required that PHPs must be a core component of Community Mental Health Centers (CMHCs). The Omnibus Budget Reconciliation Acts of 1987 and 1990 required Medicare to pay for PHPs affiliated with or based in CMHCs and psychiatric hospitals, which resulted in a proliferation of PHPs across the country. The number of CMHCbased PHPs grew from 296 in 1993 to 769 in 1997.2 By 2016, more than one-third (38.7%) of all metropolitan hospitals and 11.4% of nonmetropolitan hospitals in the United States provided PHP services.3 This growth was also partially the result of private health insurance companies and the managed care industry clamping down on inpatient hospital stays and approving PHP care to reduce costs. In recent years, freestanding PHPs that are not affiliated with a CMHC or hospital have opened to serve high-functioning patients who do not want inpatient hospitalization or the stigma associated with it.
PHPs: What they are, and how they work
The term “partial hospitalization” is fraught with confusion because initially it was used to contrast such services from full hospitalization. Historically, it was used to describe services for patients who had been discharged home from a state hospital and attended a program on the hospital grounds during the day as outpatients. In reality, today’s PHPs are “day treatment” programs, but the terminology has stuck.
PHPs are neither an inpatient service nor a strict outpatient service, but rather a midground along the continuum of treatment intensity between the 2 traditional types of psychiatric services for patients with a range of mental illness of varying severity. The Association for Ambulatory Behavioral Healthcare, which has set standards and guidelines for PHPs, defines a PHP as “an ambulatory treatment program that includes the major diagnostic, medical, psychiatric, psychosocial, and prevocational treatment modalities designed for patients with serious mental disorders who require coordinated intensive, comprehensive, and multidisciplinary treatment not provided in an outpatient clinical setting.”4 PHPs can render acute care as an alternative to inpatient treatment, provide transitional stabilization treatment between an inpatient stay and traditional outpatient treatment (once a week or less frequent), and function as a supplement to traditional outpatient treatment.
Medicare has established criteria that PHPs must meet to qualify for reimbursement5; these criteria are now widely accepted as standards of care by the insurance industry. To meet the Medicare criteria, PHP treatment must be active and structured to provide an individualized treatment plan that incorporates coordination of services to meet the particular needs of the patient.5 It must include a multidisciplinary team approach to patient care under the direction of a physician, and the treatment goals must be measurable, functional, time-framed, medically necessary, and directly related to the reason for admission.5 The physician must certify the medical necessity for admission by documenting that the patient has a diagnosis of an acute Axis I mental disorder, a level of functioning that includes severe impairments in multiple areas of daily life, and a “reasonable expectation” that the disorder and level of functioning will improve as a result of the treatment.5
The Joint Commission (formerly JCAHO) lumps day treatment, intensive outpatient, partial hospitalization, and adult day care services into a single category of an ambulatory health care environment offering an organized day or night program of assessment, treatment, care, services, habilitation, or rehabilitation for individuals who do not require 24-hour care.6 For behavioral health, this may be a structured, ongoing program that typically meets 2 to 5 times a week for 2 to 5 hours per day.6
Most PHPs for adult patients provide services during the day 5 days per week and average 5 to 6 hours of programming per day. Night or evening programming may be a good option for patients who work during the day. Typically, treatment is provided in a group therapy format, with individual therapy at least once a week. Group therapy may include cognitive-behavioral therapy, coping with grief and loss, trauma recovery, conflict resolution, stress management, anger control, and behavioral modification. Family therapy is provided as needed, but usually is mandatory for children and adolescents. Many PHPs offer intensive outpatient programs (IOPs) as a step down to further facilitate a patient’s adjustment to psychosocial and family functioning while returning to work on a part-time basis. IOPs typically provide 3 to 4 hours of service per day 3 days per week. While not evidence-based, the typical duration of PHP treatment is 4 to 6 weeks, followed by an additional 2 to 4 weeks of IOP.
Continue to: Advantages and disadvantages...
Advantages and disadvantages
Based on a qualitative literature review, Neffinger1 outlined potential advantages and disadvantages of PHPs vs inpatient hospitalization (Table1). While these have not been empirically studied, they may be useful to consider when determining if a PHP would be beneficial for a specific patient.

Which factors are most therapeutic?
Research on which factors of PHPs are of therapeutic value is quite limited and primarily consists of surveys of small numbers of participants. Hoge et al7 explored the active therapeutic factors responsible for change in the Connecticut Mental Health Center PHP by comparing responses of 20 patients with those of their clinicians. Ninety-five percent of patients rated structure as the top therapeutic factor, followed by interpersonal contact, medication, and altruism. Other factors that were rated as important by 40% or fewer participants were catharsis, learning, mobilization of family support, connection to community, universality, patient autonomy, successful completion, security, feedback on behavior, and practice at home. In a British study8 that used a similar method, patients reported that counseling was the most helpful aspect of treatment, followed by medical treatment, while staff picked groups followed by a planned approach.
Evidence supports PHPs’ effectiveness
Some research has suggested that PHPs can be effective, both clinically and in terms of cost. Marshall et al9 conducted a systematic review of the effectiveness of an acute day hospital vs inpatient admission vs outpatient care that included 9 trials. They concluded that psychiatric inpatient admissions could be reduced by at least 23% if patients were diverted to an acute day hospital. They also found some evidence that day treatment programs may be superior to outpatient care in improving symptoms in nonpsychotic patients who are refractory to outpatient treatment. In a systematic review of 18 studies, high rates of satisfaction with PHP services suggested PHPs have an advantage over inpatient treatment within 1 year of discharge; patients and families were more satisfied with PHPs.10 In a study of 197 urban, socioeconomically disadvantaged, severely ill patients, Sledge et al11 compared the clinical outcomes of those who participated in day hospital programs with those of patients who received inpatient care. They found that while overall the 2 approaches produced similar outcomes, there were slightly more positive effects for day hospital programs in measures of symptoms, overall functioning, and social functioning. In terms of cost effectiveness, a 1978 study found that even after correcting for differences in treatment days between inpatient and PHP services, there was a significant financial advantage with PHP (costs were one-third less), primarily because of lower costs per day.12 In another study, PHP cost savings were 20%, and potential savings were higher for nonpsychotic patients.13
Are PHPs appropriate for children and adolescents?
Studies of PHPs for adolescents found that patients made gains in peer relationships, behavioral and academic performance, and control of their emotions.14,15 A review of PHPs’ effectiveness for children suggested that 66% to 99% of treated patients demonstrated improvement and successful return to community-based schools, and family functioning was a major factor in improvement.14 In a follow-up study that surveyed patients via telephone >1 year after discharge from a PHP, almost 80% of the children and adolescents were either “doing OK” or were “well-adjusted.”14 Only 22.5% required inpatient or residential treatment; the majority were doing well in school, with only 7% failing.14 In addition, 60% of parents reported satisfaction with treatment, and 85% reported functional improvement in their children.
Factors that predict PHP success or failure
In an analysis of a day treatment program that provided 4 months of intensive psychodynamic, group-oriented milieu treatment for patients with long-standing personality disorders, Rosie et al16 found 3 factors that contributed to the success of the PHP:
- optimal treatment-patient matching
- judicious use of authority in milieu therapy
- maintaining close relationships with referral sources.
In a study that compared 58 patients who completed an Ottawa hospital PHP and 44 who did not complete the program, psychological mindedness and chronicity of psychiatric illness were found to predict completion.17 However, a study of 59 females with anorexia nervosa who were transferred from inpatient care to an eating disorder day hospital program found that a longer duration of illness, amenorrhea, and a lower body mass index were associated with PHP treatment failure and inpatient rehospitalization.18 One study found that for individuals who were referred to a PHP from inpatient care, suicidal ideation and greater psychotic symptoms were associated with acute inpatient rehospitalization.19 Other factors associated with PHP nonattendance and treatment failure include limited personal and economic resources, high rates of substance abuse disorders, multiple admissions, and disability.20 In a study of 103 alcohol-dependent patients who completed IOP treatment, 64% were abstinent at 6 months follow-up; relapse was associated with a longer duration of alcohol dependence and a higher number of prior treatments, while favorable outcomes were associated with a lower degree of depression, anxiety, and craving.21 Patients with cocaine dependence who completed an IOP showed significant improvements in addiction scores and had more favorable outcomes in employment status and psychological problems if they stayed longer in treatment.22
Bottom Line
Psychiatric partial hospitalization programs (PHPs) provide a transition from inpatient hospitalization to outpatient treatment for patients who need further stabilization, or serve as an alternative to inpatient treatment for patients who don’t need or want inpatient hospitalization. PHPs can be as effective as inpatient treatment for all but the most seriously ill patients, and are more cost-effective than inpatient treatment.
Psychiatric partial hospitalization programs (PHPs), previously known as “day hospitals,” serve to bridge the gap between inpatient and outpatient facilities by providing intensive, highly structured outpatient behavioral health services (typically several hours of psychotherapy each weekday for most days of the week). The concept of PHPs has existed since at least the 1950s, but such programs started to become more common in the United States as the result of legislation passed in 1963 (Box1-3). In this article, I provide a brief introductory review of PHPs, while acknowledging that most research on PHPs was conducted years ago and is rather limited.
Box
The concept of partial hospitalization programs (PHPs) was developed before the 1950s.1 However, in the United States, PHPs did not take hold until Congress passed the Community Mental Health Act of 1963, which required that PHPs must be a core component of Community Mental Health Centers (CMHCs). The Omnibus Budget Reconciliation Acts of 1987 and 1990 required Medicare to pay for PHPs affiliated with or based in CMHCs and psychiatric hospitals, which resulted in a proliferation of PHPs across the country. The number of CMHCbased PHPs grew from 296 in 1993 to 769 in 1997.2 By 2016, more than one-third (38.7%) of all metropolitan hospitals and 11.4% of nonmetropolitan hospitals in the United States provided PHP services.3 This growth was also partially the result of private health insurance companies and the managed care industry clamping down on inpatient hospital stays and approving PHP care to reduce costs. In recent years, freestanding PHPs that are not affiliated with a CMHC or hospital have opened to serve high-functioning patients who do not want inpatient hospitalization or the stigma associated with it.
PHPs: What they are, and how they work
The term “partial hospitalization” is fraught with confusion because initially it was used to contrast such services from full hospitalization. Historically, it was used to describe services for patients who had been discharged home from a state hospital and attended a program on the hospital grounds during the day as outpatients. In reality, today’s PHPs are “day treatment” programs, but the terminology has stuck.
PHPs are neither an inpatient service nor a strict outpatient service, but rather a midground along the continuum of treatment intensity between the 2 traditional types of psychiatric services for patients with a range of mental illness of varying severity. The Association for Ambulatory Behavioral Healthcare, which has set standards and guidelines for PHPs, defines a PHP as “an ambulatory treatment program that includes the major diagnostic, medical, psychiatric, psychosocial, and prevocational treatment modalities designed for patients with serious mental disorders who require coordinated intensive, comprehensive, and multidisciplinary treatment not provided in an outpatient clinical setting.”4 PHPs can render acute care as an alternative to inpatient treatment, provide transitional stabilization treatment between an inpatient stay and traditional outpatient treatment (once a week or less frequent), and function as a supplement to traditional outpatient treatment.
Medicare has established criteria that PHPs must meet to qualify for reimbursement5; these criteria are now widely accepted as standards of care by the insurance industry. To meet the Medicare criteria, PHP treatment must be active and structured to provide an individualized treatment plan that incorporates coordination of services to meet the particular needs of the patient.5 It must include a multidisciplinary team approach to patient care under the direction of a physician, and the treatment goals must be measurable, functional, time-framed, medically necessary, and directly related to the reason for admission.5 The physician must certify the medical necessity for admission by documenting that the patient has a diagnosis of an acute Axis I mental disorder, a level of functioning that includes severe impairments in multiple areas of daily life, and a “reasonable expectation” that the disorder and level of functioning will improve as a result of the treatment.5
The Joint Commission (formerly JCAHO) lumps day treatment, intensive outpatient, partial hospitalization, and adult day care services into a single category of an ambulatory health care environment offering an organized day or night program of assessment, treatment, care, services, habilitation, or rehabilitation for individuals who do not require 24-hour care.6 For behavioral health, this may be a structured, ongoing program that typically meets 2 to 5 times a week for 2 to 5 hours per day.6
Most PHPs for adult patients provide services during the day 5 days per week and average 5 to 6 hours of programming per day. Night or evening programming may be a good option for patients who work during the day. Typically, treatment is provided in a group therapy format, with individual therapy at least once a week. Group therapy may include cognitive-behavioral therapy, coping with grief and loss, trauma recovery, conflict resolution, stress management, anger control, and behavioral modification. Family therapy is provided as needed, but usually is mandatory for children and adolescents. Many PHPs offer intensive outpatient programs (IOPs) as a step down to further facilitate a patient’s adjustment to psychosocial and family functioning while returning to work on a part-time basis. IOPs typically provide 3 to 4 hours of service per day 3 days per week. While not evidence-based, the typical duration of PHP treatment is 4 to 6 weeks, followed by an additional 2 to 4 weeks of IOP.
Continue to: Advantages and disadvantages...
Advantages and disadvantages
Based on a qualitative literature review, Neffinger1 outlined potential advantages and disadvantages of PHPs vs inpatient hospitalization (Table1). While these have not been empirically studied, they may be useful to consider when determining if a PHP would be beneficial for a specific patient.

Which factors are most therapeutic?
Research on which factors of PHPs are of therapeutic value is quite limited and primarily consists of surveys of small numbers of participants. Hoge et al7 explored the active therapeutic factors responsible for change in the Connecticut Mental Health Center PHP by comparing responses of 20 patients with those of their clinicians. Ninety-five percent of patients rated structure as the top therapeutic factor, followed by interpersonal contact, medication, and altruism. Other factors that were rated as important by 40% or fewer participants were catharsis, learning, mobilization of family support, connection to community, universality, patient autonomy, successful completion, security, feedback on behavior, and practice at home. In a British study8 that used a similar method, patients reported that counseling was the most helpful aspect of treatment, followed by medical treatment, while staff picked groups followed by a planned approach.
Evidence supports PHPs’ effectiveness
Some research has suggested that PHPs can be effective, both clinically and in terms of cost. Marshall et al9 conducted a systematic review of the effectiveness of an acute day hospital vs inpatient admission vs outpatient care that included 9 trials. They concluded that psychiatric inpatient admissions could be reduced by at least 23% if patients were diverted to an acute day hospital. They also found some evidence that day treatment programs may be superior to outpatient care in improving symptoms in nonpsychotic patients who are refractory to outpatient treatment. In a systematic review of 18 studies, high rates of satisfaction with PHP services suggested PHPs have an advantage over inpatient treatment within 1 year of discharge; patients and families were more satisfied with PHPs.10 In a study of 197 urban, socioeconomically disadvantaged, severely ill patients, Sledge et al11 compared the clinical outcomes of those who participated in day hospital programs with those of patients who received inpatient care. They found that while overall the 2 approaches produced similar outcomes, there were slightly more positive effects for day hospital programs in measures of symptoms, overall functioning, and social functioning. In terms of cost effectiveness, a 1978 study found that even after correcting for differences in treatment days between inpatient and PHP services, there was a significant financial advantage with PHP (costs were one-third less), primarily because of lower costs per day.12 In another study, PHP cost savings were 20%, and potential savings were higher for nonpsychotic patients.13
Are PHPs appropriate for children and adolescents?
Studies of PHPs for adolescents found that patients made gains in peer relationships, behavioral and academic performance, and control of their emotions.14,15 A review of PHPs’ effectiveness for children suggested that 66% to 99% of treated patients demonstrated improvement and successful return to community-based schools, and family functioning was a major factor in improvement.14 In a follow-up study that surveyed patients via telephone >1 year after discharge from a PHP, almost 80% of the children and adolescents were either “doing OK” or were “well-adjusted.”14 Only 22.5% required inpatient or residential treatment; the majority were doing well in school, with only 7% failing.14 In addition, 60% of parents reported satisfaction with treatment, and 85% reported functional improvement in their children.
Factors that predict PHP success or failure
In an analysis of a day treatment program that provided 4 months of intensive psychodynamic, group-oriented milieu treatment for patients with long-standing personality disorders, Rosie et al16 found 3 factors that contributed to the success of the PHP:
- optimal treatment-patient matching
- judicious use of authority in milieu therapy
- maintaining close relationships with referral sources.
In a study that compared 58 patients who completed an Ottawa hospital PHP and 44 who did not complete the program, psychological mindedness and chronicity of psychiatric illness were found to predict completion.17 However, a study of 59 females with anorexia nervosa who were transferred from inpatient care to an eating disorder day hospital program found that a longer duration of illness, amenorrhea, and a lower body mass index were associated with PHP treatment failure and inpatient rehospitalization.18 One study found that for individuals who were referred to a PHP from inpatient care, suicidal ideation and greater psychotic symptoms were associated with acute inpatient rehospitalization.19 Other factors associated with PHP nonattendance and treatment failure include limited personal and economic resources, high rates of substance abuse disorders, multiple admissions, and disability.20 In a study of 103 alcohol-dependent patients who completed IOP treatment, 64% were abstinent at 6 months follow-up; relapse was associated with a longer duration of alcohol dependence and a higher number of prior treatments, while favorable outcomes were associated with a lower degree of depression, anxiety, and craving.21 Patients with cocaine dependence who completed an IOP showed significant improvements in addiction scores and had more favorable outcomes in employment status and psychological problems if they stayed longer in treatment.22
Bottom Line
Psychiatric partial hospitalization programs (PHPs) provide a transition from inpatient hospitalization to outpatient treatment for patients who need further stabilization, or serve as an alternative to inpatient treatment for patients who don’t need or want inpatient hospitalization. PHPs can be as effective as inpatient treatment for all but the most seriously ill patients, and are more cost-effective than inpatient treatment.
1. Neffinger GG. Partial hospitalization: an overview. J Community Psychol. 1981;9(3):262-269.
2. Leung MY, Drozd EM, Healy DA, et al. Impacts associated with the Medicare Psychiatric PPS: a study of partial hospitalization programs. February 2009. Accessed January 8, 2022. https://www.cms.gov/Research-Statistics-Data-and-Systems/Statistics-Trends-and-Reports/Reports/downloads/Leung_PHP_PPS_2010.pdf
3. Williams T, Borders TF, Jasinski L. Partial Psychiatric Hospitalization Program Availability in Non-Metropolitan and Non-Metropolitan Hospitals Nationally. Rural and Underserved Health Research Center; 2019.
4. Rosser J, Michael S, eds. Standards and guidelines for partial hospital programs and intensive outpatient programs. Association for Ambulatory Behavioral Healthcare. 2018. Accessed January 8, 2022. https://aabh.org/wp-content/uploads/2021/05/2021-SandG-Final.pdf
5. CMS Manual System PUB. 100-02 Medicare Benefit Policy: Partial Hospitalization Services, Department of Health and Human Services. Centers for Medicare & Medicaid Services; 2004:6-9.
6. Joint Commission. Guide to Joint Commission Behavioral Healthcare Accreditation. Joint Commission; 2007:36.
7. Hoge MA, Farrell SP, Munchel ME, et al. Therapeutic factors in partial hospitalization. Psychiatry. 1988;51(2):199-210.
8. Ricketts T, Kirshbaum MN. Helpfulness of mental health day care: client and staff views. J Adv Nurs. 1994;20(2):297-306.
9. Marshall M, Crowther R, Almaraz-Serrano A, et al. Systematic reviews of the effectiveness of day care for people with severe mental disorders: (1) acute day hospital versus admission; (2) vocational rehabilitation; (3) day hospital versus outpatient care. Health Technol Assess. 2001;5(21):1-75.
10. Horvitz-Lennon M, Normand SL, Gaccione P, et al. Partial versus full hospitalization for adults in psychiatric distress: a systematic review of the published literature (1957-1997). Am J Psychiatry. 2001;158(5):676-685.
11. Sledge WH, Tebes J, Rakfeldt J, et al. Day hospital/crisis respite care versus inpatient care, Part I: Clinical outcomes. Am J Psychiatry. 1996;153(8):1065-1073.
12. Fink EB, Longabaugh R, Stout R. The paradoxical underutilization of partial hospitalization. Am J Psychiatry. 1978;135(6):713-716.
13. Sledge WH, Tebes J, Wolff N, et al. Day hospital/crisis respite care versus inpatient care, Part II: service utilization and costs. Am J Psychiatry. 1996;153(8):1074-1083.
14. Kiser LJ. Treatment-effectiveness research in child and adolescent partial hospitalization. Psychiatr Hosp. 1991;22(2):51-8.
15. Milin R, Coupland K, Walker S, et al. Outcome and follow-up study of an adolescent psychiatric day treatment school program. J Am Acad Child Adolesc Psychiatry. 2000;39(3):320-328.
16. Rosie JS, Azim HF, Piper WE, et al. Effective psychiatric day treatment: historical lessons. Psychiatr Serv. 1995;46(10):1019-1026.
17. Tasca GA, Balfour L, Bissada H, et al. Treatment completion and outcome in a partial hospitalization program: interactions among patient variables. Psychotherapy Res. 1999;9(2):232-247.
18. Howard WT, Evans KK, Quintero-Howard CV, et al. Predictors of success or failure of transition to day hospital treatment for inpatients with anorexia nervosa. Am J Psychiatry. 1999;156(11):1697-1702.
19. Beard C, Hearon BA, Lee J, et al. When partial hospitalization fails: risk factors for inpatient hospitalization. J Nerv Ment Dis. 2016;204(6):431-436.
20. Lieberman PB, Guggenheim FG. Reasons for patient nonattendance during acute partial hospitalization. Psychiatr Serv. 2016;67(6):684-687.
21. Bottlender M, Soyka M. Efficacy of an intensive outpatient rehabilitation program in alcoholism: predictors of outcome 6 months after treatment. Eur Addict Res. 2005;11(3):132-137.
22. Gottheil E, Weinstein SP, Sterling RC, et al. A randomized controlled study of the effectiveness of intensive outpatient treatment for cocaine dependence. Psychiatr Serv. 1998;49(6):782-787.
1. Neffinger GG. Partial hospitalization: an overview. J Community Psychol. 1981;9(3):262-269.
2. Leung MY, Drozd EM, Healy DA, et al. Impacts associated with the Medicare Psychiatric PPS: a study of partial hospitalization programs. February 2009. Accessed January 8, 2022. https://www.cms.gov/Research-Statistics-Data-and-Systems/Statistics-Trends-and-Reports/Reports/downloads/Leung_PHP_PPS_2010.pdf
3. Williams T, Borders TF, Jasinski L. Partial Psychiatric Hospitalization Program Availability in Non-Metropolitan and Non-Metropolitan Hospitals Nationally. Rural and Underserved Health Research Center; 2019.
4. Rosser J, Michael S, eds. Standards and guidelines for partial hospital programs and intensive outpatient programs. Association for Ambulatory Behavioral Healthcare. 2018. Accessed January 8, 2022. https://aabh.org/wp-content/uploads/2021/05/2021-SandG-Final.pdf
5. CMS Manual System PUB. 100-02 Medicare Benefit Policy: Partial Hospitalization Services, Department of Health and Human Services. Centers for Medicare & Medicaid Services; 2004:6-9.
6. Joint Commission. Guide to Joint Commission Behavioral Healthcare Accreditation. Joint Commission; 2007:36.
7. Hoge MA, Farrell SP, Munchel ME, et al. Therapeutic factors in partial hospitalization. Psychiatry. 1988;51(2):199-210.
8. Ricketts T, Kirshbaum MN. Helpfulness of mental health day care: client and staff views. J Adv Nurs. 1994;20(2):297-306.
9. Marshall M, Crowther R, Almaraz-Serrano A, et al. Systematic reviews of the effectiveness of day care for people with severe mental disorders: (1) acute day hospital versus admission; (2) vocational rehabilitation; (3) day hospital versus outpatient care. Health Technol Assess. 2001;5(21):1-75.
10. Horvitz-Lennon M, Normand SL, Gaccione P, et al. Partial versus full hospitalization for adults in psychiatric distress: a systematic review of the published literature (1957-1997). Am J Psychiatry. 2001;158(5):676-685.
11. Sledge WH, Tebes J, Rakfeldt J, et al. Day hospital/crisis respite care versus inpatient care, Part I: Clinical outcomes. Am J Psychiatry. 1996;153(8):1065-1073.
12. Fink EB, Longabaugh R, Stout R. The paradoxical underutilization of partial hospitalization. Am J Psychiatry. 1978;135(6):713-716.
13. Sledge WH, Tebes J, Wolff N, et al. Day hospital/crisis respite care versus inpatient care, Part II: service utilization and costs. Am J Psychiatry. 1996;153(8):1074-1083.
14. Kiser LJ. Treatment-effectiveness research in child and adolescent partial hospitalization. Psychiatr Hosp. 1991;22(2):51-8.
15. Milin R, Coupland K, Walker S, et al. Outcome and follow-up study of an adolescent psychiatric day treatment school program. J Am Acad Child Adolesc Psychiatry. 2000;39(3):320-328.
16. Rosie JS, Azim HF, Piper WE, et al. Effective psychiatric day treatment: historical lessons. Psychiatr Serv. 1995;46(10):1019-1026.
17. Tasca GA, Balfour L, Bissada H, et al. Treatment completion and outcome in a partial hospitalization program: interactions among patient variables. Psychotherapy Res. 1999;9(2):232-247.
18. Howard WT, Evans KK, Quintero-Howard CV, et al. Predictors of success or failure of transition to day hospital treatment for inpatients with anorexia nervosa. Am J Psychiatry. 1999;156(11):1697-1702.
19. Beard C, Hearon BA, Lee J, et al. When partial hospitalization fails: risk factors for inpatient hospitalization. J Nerv Ment Dis. 2016;204(6):431-436.
20. Lieberman PB, Guggenheim FG. Reasons for patient nonattendance during acute partial hospitalization. Psychiatr Serv. 2016;67(6):684-687.
21. Bottlender M, Soyka M. Efficacy of an intensive outpatient rehabilitation program in alcoholism: predictors of outcome 6 months after treatment. Eur Addict Res. 2005;11(3):132-137.
22. Gottheil E, Weinstein SP, Sterling RC, et al. A randomized controlled study of the effectiveness of intensive outpatient treatment for cocaine dependence. Psychiatr Serv. 1998;49(6):782-787.
It’s time for moonshot thinking in psychiatry
“I believe that this nation should commit itself to achieving the goal, before the decade is out, of landing a man on the Moon and returning him safely to Earth.”
President John F. Kennedy, May 25, 1961
Despite significant progress, there remain many unmet needs in psychiatry. These include a granular understanding of the neurobiology of various psychopathologies, an objective and valid diagnostic schema, and disease-modifying treatments for chronic and disabling psychiatric disorders. Several moonshots are needed to address those festering needs.
A “moonshot” is an extremely ambitious, dramatic, imaginative, and inspiring goal. Landing on the Moon was generally believed to be impossible when President Kennedy boldly set that as a goal for the United States in 1961. Yet, 8 short years later, on July 20, 1969, Neil Armstrong stepped off the lunar module ladder onto the Moon’s surface, a feat that captured the imagination of the nation and the world. I distinctly remember watching it on television with amazement as a young boy. It was a surreal experience. That’s what achieving a moonshot feels like.
Successful organizations should always have 1 or more moonshots (American Psychiatric Association and National Institute of Mental Health [NIMH], are you listening?). Setting lofty goals that require monumental determination and effort to accomplish will have a transformative, long-lasting impact. The construction of the Panama Canal to connect 2 oceans and the Manhattan Project to develop the first nuclear bomb, which ended World War II, are examples of moonshots that continue to reverberate. A more recent moonshot is the driverless car, which in the past was a laughable idea but is now a reality that will change society and the world in many ways. Innovative billionaire moguls now speak loudly about colonizing Mars, which sounds improbable and highly risky, but it’s a moonshot that may be achieved within a few years. Establishing world peace is a moonshot that requires collective Kennedy-esque vision and motivation among world leaders, which currently is sadly lacking.
So, for contemporary psychiatry, what is the equivalent of landing on the Moon? Here is the list that pops in my brain’s mind (let us know which of these would be your top 3 moonshots by taking our survey at https://bit.ly/3qkKqTa):
- A cure for schizophrenia (across positive, negative, and cognitive symptom domains)
- A cure for mood disorders, unipolar and bipolar (including suicide)
- A cure for anxiety disorders
- A cure for obsessive-compulsive disorder
- A cure for posttraumatic stress disorder
- A cure for alcoholism/addiction
- A cure for autism
- A cure for Alzheimer’s disease and other dementias
- A cure for personality disorders, especially antisocial and borderline
- A cure for the visceral hatred across political parties that permeates our society (obviously not a psychiatric category, but perhaps it should be added to DSM because it is so destructive).
Those moonshots may be regarded as absurd, and totally unachievable, but so was landing on the Moon, until it was accomplished. Psychiatry must stop thinking small and being content with tiny advances (which is like changing the chairs to more comfortable sofas on the deck of the Titanic and calling it “progress…”). Psychiatry needs to be unified under the flag of “moonshot thinking” by several visionary and transformative leaders to start believing in a miraculously better future for our patients. But to pave the way for moonshots in psychiatry, the leading organizations must collaborate closely to open the door for unprecedented scientific and medical breakthroughs of a moonshot by:
- Lobbying effectively to secure massive funding for research from federal, state, corporate, and foundation sources (perhaps convincing the Gates Foundation that schizophrenia is as devastating worldwide as malaria may bring a few badly needed billions into psychiatric brain research).
- Reminding members of Congress that in the United States, costs associated with psychiatric brain disorders total an estimated $700 billion annually,1 and that this must be addressed by boosting the meager NIMH budget by at least an order of magnitude. The NIMH should disproportionately invest its resources on severe brain disorders such as schizophrenia because breakthrough advances in its neurobiology will provide unprecedented insights to the pathophysiology of other severe psychiatric brain disorders.
- Partnering intimately with the pharmaceutical industry in a powerful public-private coalition to exploit the extensive research infrastructure of this industry.
- Creating the necessary army of researchers (physician-scientists) by providing huge incentives to medical students and psychiatric residents to pursue careers in neuroscience research. Incentives can include paying for an individual’s entire medical education and research training, and providing generous salaries that match or exceed the income of a very successful clinical practice.
- Convincing all psychiatric clinicians to support research by referring patients to research projects. Clinical psychiatrists are badly needed to care for the population, but they must be reminded that every treatment they are using today was a research project in the past, and that the research of today will evolve into the treatments (or cures) of tomorrow.
Pursuing lofty moonshots via innovative research is very likely to enhance serendipity and lead to unexpected discoveries along the way. As Louis Pasteur said, “chance only favors the prepared mind.”2 Moonshot thinking in psychiatry today is more feasible than ever before because of the many advances in research methods (neuroimaging, pluripotent cells, optogenetics, CRISPR, etc) and complex data management technologies (big data, machine learning, artificial intelligence), each of which qualifies as a preparatory moonshot in its own right.
Given the tragic consequences of psychiatric brain disorders, it is imperative that we “think big.” Humanity expects us to do that. We must envision the future of psychiatry as dramatically different from the present. Moonshot thinking is the indispensable vehicle to take us there.
1. Discovery Mood and Anxiety Program. The rising cost of mental health and substance abuse in the United States. Accessed January 13, 2022. https://discoverymood.com/blog/cost-of-mental-health-increase/
2. Wikiquote. Louis Pasteur. Accessed January 10, 2022. https://en.wikiquote.org/wiki/Louis_Pasteur
“I believe that this nation should commit itself to achieving the goal, before the decade is out, of landing a man on the Moon and returning him safely to Earth.”
President John F. Kennedy, May 25, 1961
Despite significant progress, there remain many unmet needs in psychiatry. These include a granular understanding of the neurobiology of various psychopathologies, an objective and valid diagnostic schema, and disease-modifying treatments for chronic and disabling psychiatric disorders. Several moonshots are needed to address those festering needs.
A “moonshot” is an extremely ambitious, dramatic, imaginative, and inspiring goal. Landing on the Moon was generally believed to be impossible when President Kennedy boldly set that as a goal for the United States in 1961. Yet, 8 short years later, on July 20, 1969, Neil Armstrong stepped off the lunar module ladder onto the Moon’s surface, a feat that captured the imagination of the nation and the world. I distinctly remember watching it on television with amazement as a young boy. It was a surreal experience. That’s what achieving a moonshot feels like.
Successful organizations should always have 1 or more moonshots (American Psychiatric Association and National Institute of Mental Health [NIMH], are you listening?). Setting lofty goals that require monumental determination and effort to accomplish will have a transformative, long-lasting impact. The construction of the Panama Canal to connect 2 oceans and the Manhattan Project to develop the first nuclear bomb, which ended World War II, are examples of moonshots that continue to reverberate. A more recent moonshot is the driverless car, which in the past was a laughable idea but is now a reality that will change society and the world in many ways. Innovative billionaire moguls now speak loudly about colonizing Mars, which sounds improbable and highly risky, but it’s a moonshot that may be achieved within a few years. Establishing world peace is a moonshot that requires collective Kennedy-esque vision and motivation among world leaders, which currently is sadly lacking.
So, for contemporary psychiatry, what is the equivalent of landing on the Moon? Here is the list that pops in my brain’s mind (let us know which of these would be your top 3 moonshots by taking our survey at https://bit.ly/3qkKqTa):
- A cure for schizophrenia (across positive, negative, and cognitive symptom domains)
- A cure for mood disorders, unipolar and bipolar (including suicide)
- A cure for anxiety disorders
- A cure for obsessive-compulsive disorder
- A cure for posttraumatic stress disorder
- A cure for alcoholism/addiction
- A cure for autism
- A cure for Alzheimer’s disease and other dementias
- A cure for personality disorders, especially antisocial and borderline
- A cure for the visceral hatred across political parties that permeates our society (obviously not a psychiatric category, but perhaps it should be added to DSM because it is so destructive).
Those moonshots may be regarded as absurd, and totally unachievable, but so was landing on the Moon, until it was accomplished. Psychiatry must stop thinking small and being content with tiny advances (which is like changing the chairs to more comfortable sofas on the deck of the Titanic and calling it “progress…”). Psychiatry needs to be unified under the flag of “moonshot thinking” by several visionary and transformative leaders to start believing in a miraculously better future for our patients. But to pave the way for moonshots in psychiatry, the leading organizations must collaborate closely to open the door for unprecedented scientific and medical breakthroughs of a moonshot by:
- Lobbying effectively to secure massive funding for research from federal, state, corporate, and foundation sources (perhaps convincing the Gates Foundation that schizophrenia is as devastating worldwide as malaria may bring a few badly needed billions into psychiatric brain research).
- Reminding members of Congress that in the United States, costs associated with psychiatric brain disorders total an estimated $700 billion annually,1 and that this must be addressed by boosting the meager NIMH budget by at least an order of magnitude. The NIMH should disproportionately invest its resources on severe brain disorders such as schizophrenia because breakthrough advances in its neurobiology will provide unprecedented insights to the pathophysiology of other severe psychiatric brain disorders.
- Partnering intimately with the pharmaceutical industry in a powerful public-private coalition to exploit the extensive research infrastructure of this industry.
- Creating the necessary army of researchers (physician-scientists) by providing huge incentives to medical students and psychiatric residents to pursue careers in neuroscience research. Incentives can include paying for an individual’s entire medical education and research training, and providing generous salaries that match or exceed the income of a very successful clinical practice.
- Convincing all psychiatric clinicians to support research by referring patients to research projects. Clinical psychiatrists are badly needed to care for the population, but they must be reminded that every treatment they are using today was a research project in the past, and that the research of today will evolve into the treatments (or cures) of tomorrow.
Pursuing lofty moonshots via innovative research is very likely to enhance serendipity and lead to unexpected discoveries along the way. As Louis Pasteur said, “chance only favors the prepared mind.”2 Moonshot thinking in psychiatry today is more feasible than ever before because of the many advances in research methods (neuroimaging, pluripotent cells, optogenetics, CRISPR, etc) and complex data management technologies (big data, machine learning, artificial intelligence), each of which qualifies as a preparatory moonshot in its own right.
Given the tragic consequences of psychiatric brain disorders, it is imperative that we “think big.” Humanity expects us to do that. We must envision the future of psychiatry as dramatically different from the present. Moonshot thinking is the indispensable vehicle to take us there.
“I believe that this nation should commit itself to achieving the goal, before the decade is out, of landing a man on the Moon and returning him safely to Earth.”
President John F. Kennedy, May 25, 1961
Despite significant progress, there remain many unmet needs in psychiatry. These include a granular understanding of the neurobiology of various psychopathologies, an objective and valid diagnostic schema, and disease-modifying treatments for chronic and disabling psychiatric disorders. Several moonshots are needed to address those festering needs.
A “moonshot” is an extremely ambitious, dramatic, imaginative, and inspiring goal. Landing on the Moon was generally believed to be impossible when President Kennedy boldly set that as a goal for the United States in 1961. Yet, 8 short years later, on July 20, 1969, Neil Armstrong stepped off the lunar module ladder onto the Moon’s surface, a feat that captured the imagination of the nation and the world. I distinctly remember watching it on television with amazement as a young boy. It was a surreal experience. That’s what achieving a moonshot feels like.
Successful organizations should always have 1 or more moonshots (American Psychiatric Association and National Institute of Mental Health [NIMH], are you listening?). Setting lofty goals that require monumental determination and effort to accomplish will have a transformative, long-lasting impact. The construction of the Panama Canal to connect 2 oceans and the Manhattan Project to develop the first nuclear bomb, which ended World War II, are examples of moonshots that continue to reverberate. A more recent moonshot is the driverless car, which in the past was a laughable idea but is now a reality that will change society and the world in many ways. Innovative billionaire moguls now speak loudly about colonizing Mars, which sounds improbable and highly risky, but it’s a moonshot that may be achieved within a few years. Establishing world peace is a moonshot that requires collective Kennedy-esque vision and motivation among world leaders, which currently is sadly lacking.
So, for contemporary psychiatry, what is the equivalent of landing on the Moon? Here is the list that pops in my brain’s mind (let us know which of these would be your top 3 moonshots by taking our survey at https://bit.ly/3qkKqTa):
- A cure for schizophrenia (across positive, negative, and cognitive symptom domains)
- A cure for mood disorders, unipolar and bipolar (including suicide)
- A cure for anxiety disorders
- A cure for obsessive-compulsive disorder
- A cure for posttraumatic stress disorder
- A cure for alcoholism/addiction
- A cure for autism
- A cure for Alzheimer’s disease and other dementias
- A cure for personality disorders, especially antisocial and borderline
- A cure for the visceral hatred across political parties that permeates our society (obviously not a psychiatric category, but perhaps it should be added to DSM because it is so destructive).
Those moonshots may be regarded as absurd, and totally unachievable, but so was landing on the Moon, until it was accomplished. Psychiatry must stop thinking small and being content with tiny advances (which is like changing the chairs to more comfortable sofas on the deck of the Titanic and calling it “progress…”). Psychiatry needs to be unified under the flag of “moonshot thinking” by several visionary and transformative leaders to start believing in a miraculously better future for our patients. But to pave the way for moonshots in psychiatry, the leading organizations must collaborate closely to open the door for unprecedented scientific and medical breakthroughs of a moonshot by:
- Lobbying effectively to secure massive funding for research from federal, state, corporate, and foundation sources (perhaps convincing the Gates Foundation that schizophrenia is as devastating worldwide as malaria may bring a few badly needed billions into psychiatric brain research).
- Reminding members of Congress that in the United States, costs associated with psychiatric brain disorders total an estimated $700 billion annually,1 and that this must be addressed by boosting the meager NIMH budget by at least an order of magnitude. The NIMH should disproportionately invest its resources on severe brain disorders such as schizophrenia because breakthrough advances in its neurobiology will provide unprecedented insights to the pathophysiology of other severe psychiatric brain disorders.
- Partnering intimately with the pharmaceutical industry in a powerful public-private coalition to exploit the extensive research infrastructure of this industry.
- Creating the necessary army of researchers (physician-scientists) by providing huge incentives to medical students and psychiatric residents to pursue careers in neuroscience research. Incentives can include paying for an individual’s entire medical education and research training, and providing generous salaries that match or exceed the income of a very successful clinical practice.
- Convincing all psychiatric clinicians to support research by referring patients to research projects. Clinical psychiatrists are badly needed to care for the population, but they must be reminded that every treatment they are using today was a research project in the past, and that the research of today will evolve into the treatments (or cures) of tomorrow.
Pursuing lofty moonshots via innovative research is very likely to enhance serendipity and lead to unexpected discoveries along the way. As Louis Pasteur said, “chance only favors the prepared mind.”2 Moonshot thinking in psychiatry today is more feasible than ever before because of the many advances in research methods (neuroimaging, pluripotent cells, optogenetics, CRISPR, etc) and complex data management technologies (big data, machine learning, artificial intelligence), each of which qualifies as a preparatory moonshot in its own right.
Given the tragic consequences of psychiatric brain disorders, it is imperative that we “think big.” Humanity expects us to do that. We must envision the future of psychiatry as dramatically different from the present. Moonshot thinking is the indispensable vehicle to take us there.
1. Discovery Mood and Anxiety Program. The rising cost of mental health and substance abuse in the United States. Accessed January 13, 2022. https://discoverymood.com/blog/cost-of-mental-health-increase/
2. Wikiquote. Louis Pasteur. Accessed January 10, 2022. https://en.wikiquote.org/wiki/Louis_Pasteur
1. Discovery Mood and Anxiety Program. The rising cost of mental health and substance abuse in the United States. Accessed January 13, 2022. https://discoverymood.com/blog/cost-of-mental-health-increase/
2. Wikiquote. Louis Pasteur. Accessed January 10, 2022. https://en.wikiquote.org/wiki/Louis_Pasteur
Honor thy parents? Understanding parricide and associated spree killings
Mr. B, age 37, presents to a community mental health center for an appointment following a recent emergency department visit. He is diagnosed with schizophrenia, and has been treated for approximately 1 year. Six months ago, Mr. B stopped taking his antipsychotic due to its adverse effects. Despite compliance with another agent, he has become increasingly disorganized and paranoid.
He now believes that his mother, with whom he has lived all his life and who serves as his guardian, is poisoning his food and trying to kill him. She is an employee at a local grocery store, and Mr. B has expressed concern that her coworkers are assisting her in the plot to kill him.
Following a home visit, Mr. B’s case manager indicates that the patient showed them the collection of weapons he is amassing to “defend” himself. This leads to a concern for the safety of the patient, his mother, and others.
Although parricide—killing one’s parent—is a relatively rare event, its sensationalistic nature has long captured the attention of headline writers and the general public. This article discusses the diagnostic and demographic factors that may be seen among individuals who kill their parents, with an emphasis on those who commit matricide (murder of one’s mother) and associated spree killings, where an individual kills multiple people within a single brief but contiguous time period. Understanding these characteristics can help clinicians identify and more safely manage patients who may be at risk of harming their parents in addition to others.
Characteristics of perpetrators of parricide
Worldwide, approximately 2% to 4% of homicides involve parricide, or killing one’s parent.1,2 Most offenders are men in early adulthood, though a proportion are adolescents and some are women.1,3 They are often single, unemployed, and live with the parent prior to the killing.1 Patricide occurs more frequently than matricide.4 In the United States, approximately 150 fathers and 100 mothers are killed by their child each year.5
In a study of all homicides in England and Wales between 1997 and 2014, two-thirds of parricide offenders had previously been diagnosed with a mental disorder.1 One-third were diagnosed with schizophrenia.1 In a Canadian study focusing on 43 adult perpetrators found not criminally responsible,6 most were experiencing psychotic symptoms at the time of parricide; symptoms of a personality disorder were the second-most prevalent symptoms. Similarly, Bourget et al4 studied Canadian coroner records for 64 parents killed by their children. Of the children involved in those parricides, 15% attempted suicide after the killing. Two-thirds of the male offenders evidenced delusional thinking, and/or excessive violence (overkill) was common. Some cases (16%) followed an argument, and some of those perpetrators were intoxicated or psychotic. From our clinical experience, when there are identifiable nonpsychotic triggers, they often can be small things such as an argument over food, smoking, or video games. Often, the perpetrator was financially dependent on their parents and were “trapped in a difficult/hostile/dependence/love relationship” with that parent.6 Adolescent males who kill their parents may not have psychosis7; however, they may be victims of longstanding serious abuse at the hands of their parents. These perpetrators often express relief rather than remorse after committing murder.
Three categories to classify the perpetrators of parricide have been proposed: severely abused, severely mentally ill, and “dangerously antisocial.”3 While severe mental illness was most common in adult defendants, severe abuse was most common in adolescent offenders. There may be significant commonalities between adolescent and adult perpetrators. A more recent latent class analysis by Bojanic et al1 indicated 3 unique types of parricide offenders (Table 1).
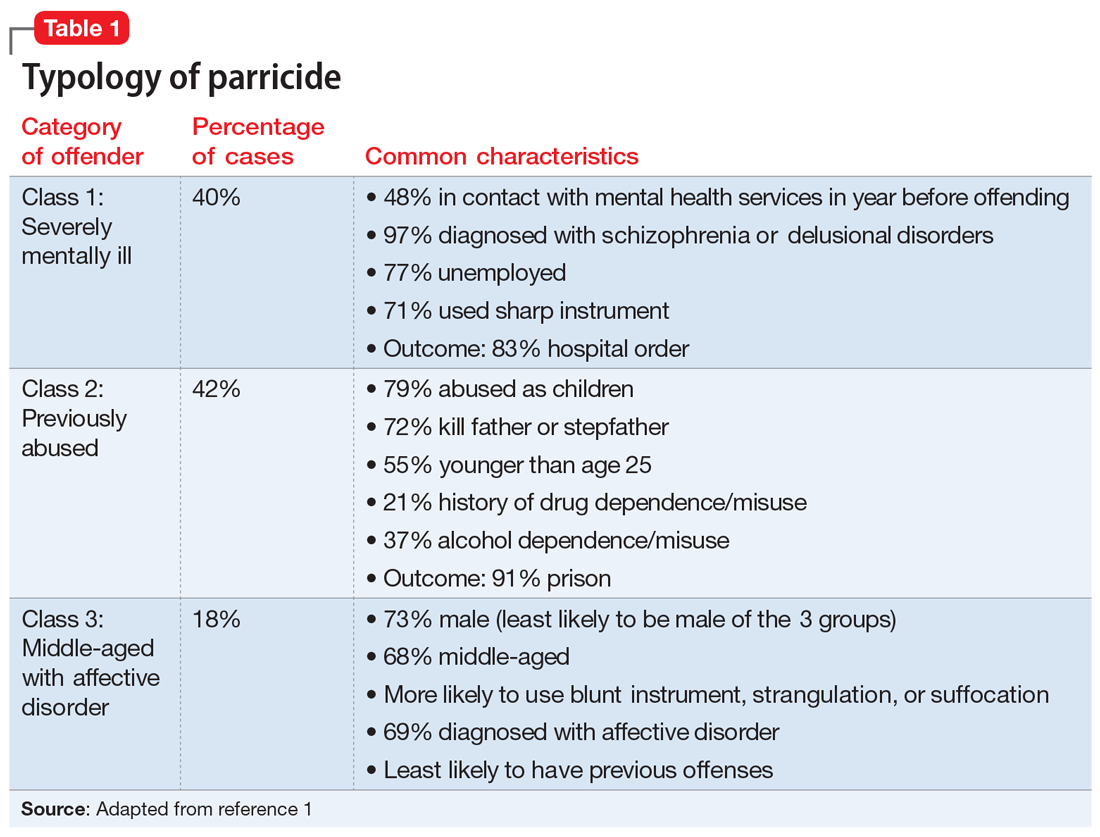
Continue to: Matricide: A closer look...
Matricide: A closer look
Though multiple studies have found a higher rate of psychosis among perpetrators of matricide, it is important to note that most people with psychotic disorders would never kill their mother. These events, however, tend to grab headlines and may be highly focused upon by the general population. In addition, matricide may be part of a larger crime that draws additional attention. For example, the 1966 University of Texas Bell Tower shooter and the 2012 Sandy Hook Elementary school shooter both killed their mothers before engaging in mass homicide. Often in cases of matricide, a longer-term dysfunctional relationship existed between the mother and the child. The mother is frequently described as controlling and intrusive and the (often adult) child as overly dependent, while the father may be absent or ineffectual. Hostility and mutual dependence are usual hallmarks of these relationships.8
However, in some cases where an individual with a psychotic disorder kills their mother, there may have been a traditional nurturing relationship.8 Alternative motivations unrelated to psychosis must be considered, including crimes motivated by money/inheritance or those perpetrated out of nonpsychotic anger. Green8 described motive categories for matricide that include paranoid and persecutory, altruistic, and other. In the “paranoid and persecutory” group, delusional beliefs about the mother occur; for example, the perpetrator may believe their mother was the devil. Sexual elements are found in some cases.8 Alternatively, the “altruistic” group demonstrated rather selfless reasons for killing, such as altruistic infanticide cases, or killing out of love.9 The altruistic matricide perpetrator may believe their mother is unwell, which may be a delusion or actually true. Finally, the “other” category contains cases related to jealousy, rage, and impulsivity.
In a study of 15 matricidal men in New York conducted by Campion et al,10 individuals seen by forensic psychiatric services for the crime of matricide included those with schizophrenia, substance-induced psychosis, and impulse control disorders. The authors noted there was often “a serious chronic derangement in the relationships of most matricidal men and their mothers.” Psychometric testing in these cases indicated feelings of dependency, weakness, and difficulty accepting an adult role separate from their mother. Some had conceptualized their mothers as a threat to their masculinity, while others had become enraged at their mothers.
Prevention requires addressing underlying issues
As described above, several factors are common among individuals who commit parricide, and these can be used to develop prevention strategies that focus on addressing underlying issues (Table 2). It is important to consider the relationship dynamics between the potential victim and perpetrator, as well as the motive, rather than focusing solely on mental illness or substance misuse.2
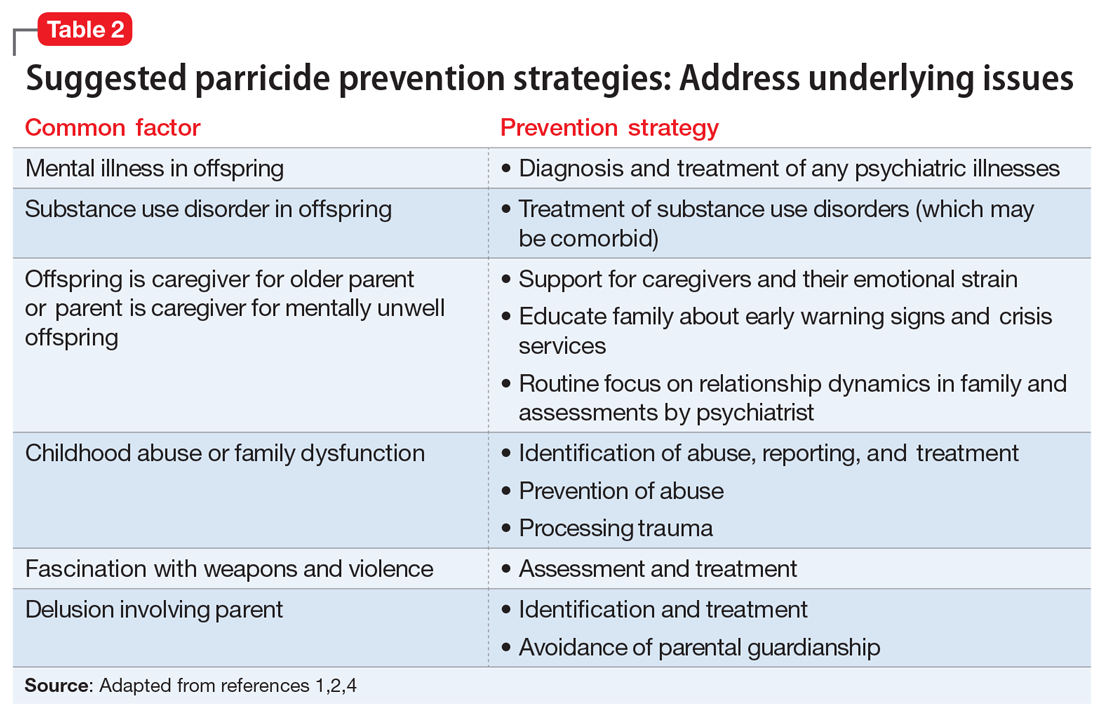
Spree killings that start as parricide
Although spree killing is a relatively rare event, a subset of spree killings involve parricide. One infamous recent event occurred in 2012 at Sandy Hook Elementary School, where the gunman killed his mother before going to the school and killing 26 additional people, many of whom were children.11,12 Because such events are rare, and because in these cases there is a high likelihood that the perpetrator is deceased (eg, died by suicide or killed by the police), much remains unknown about specific motivations and causative factors.
Information is often pieced together from postmortem reviews, which can be hampered by hindsight/recall bias and lack of contemporaneous documentation. Even worse, when these events occur, they may lead to a bias that all parricides or mass murders follow the pattern of the most recent case. This can result in overgeneralization of an individual’s history as being actionable risk violence factors for all potential parricide cases both by the public (eg, “My sister’s son has autism, and the Sandy Hook shooter was reported to have autism—should I be worried for my sister?”) and professionals (eg, “Will I be blamed for the next Sandy Hook by not taking more aggressive action even though I am not sure it is clinically warranted?”).
To identify trends for individuals committing parricide who engage in mass murder events (such as spree killing), we reviewed the 2000-2019 FBI active shooter list.12 Of the 333 events identified, 46 could be classified as domestic violence situations (eg, the perpetrator was in a romantic or familial relationship/former relationship and engaged in an active shooting incident involving at least 1 person from that relationship). We classified 11 of those 46 cases as parricide. Ten of those 11 parricides involved a child killing a parent (Table 3), and the other involved a grandchild killing a grandfather who served as their primary caregiver. Of the 11 incidents, mothers were involved (killed or wounded) in 4, and father figures (including the grandfather serving as a father and a stepfather) were killed in 9, with 2 incidents involving both parents. In 4 of the 11 parricides, other family members were killed in addition to the parent (including siblings, grandparents, or extended family). When considering spree shooters who committed parricide, 4 alleged perpetrators died by suicide, 1 was killed at the scene, and the rest were apprehended. The most common active shooting site endangering the public was an educational location (5), followed by commerce locations (4), with 2 involving open spaces. Eight of the 11 parricides occurred before the event was designated as an active shooting. The mean age for a parricide plus spree shooter was 23, once the oldest (age 61) and youngest (age 14) were removed from the calculation. The majority of the cases fell into the age range of 16 to 25 (n = 6), followed by 3 individuals who were age 26 to 31 (n = 3). All suspected individuals were male.
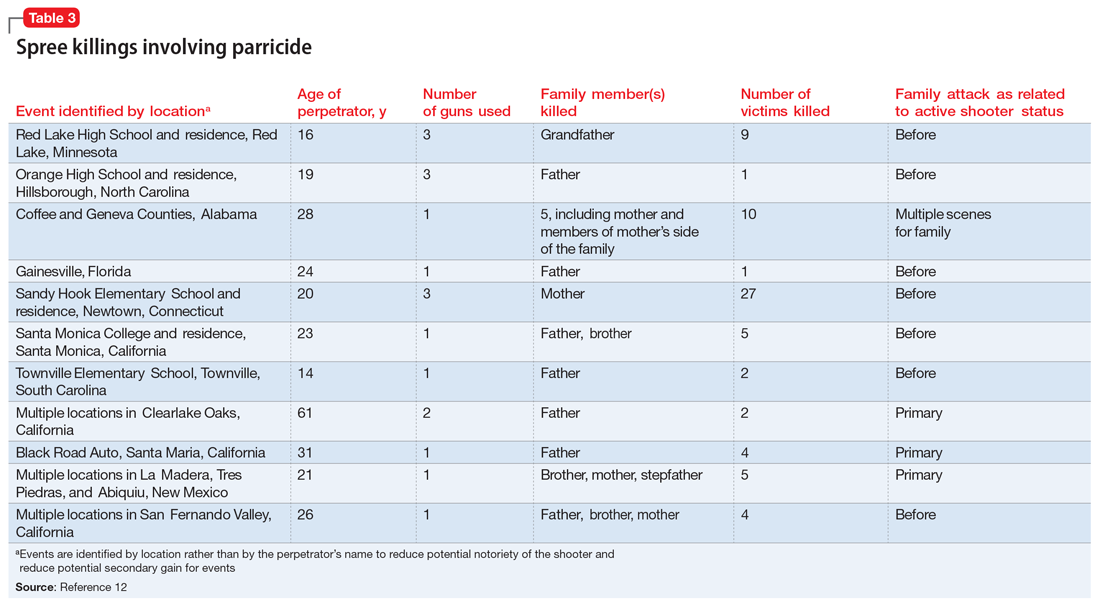
It is difficult to ascertain the existence of prior mental health care because perpetrators’ medical records may be confidential, juvenile court records may be sealed, and there may not even be a trial due to death or suicide (leading to limited forensic psychiatry examination or public testimony). Among those apprehended, many individuals raise some form of mental health defense, but the validity of their diagnosis may be undermined by concerns of possible malingering, especially in cases where the individual did not have a history of psychiatric treatment prior to the event.11 In summary, based on FBI data,12 spree shooters who committed parricide were usually male, in their late adolescence or early 20s, and more frequently targeted father figures. They often committed the parricide first and at a different location from later “active” shootings. Police were usually not aware of the parricide until after the spree is over.
Continue to: Parricide and society...
Parricide and society
For centuries, mothers and fathers have been honored and revered. Therefore, it is not surprising that killing of one’s mother or father attracts a great deal of macabre interest. Examples of parricide are present throughout popular culture, in mythology, comic books, movies, and television. As all psychiatrists know, Oedipus killed his father and married his mother. Other popular culture examples include: Grant Morrison’s Arkham Asylum: A Serious House on Serious Earth, Alfred Hitchcock’s Psycho, Oliver Stone’s Natural Born Killers, Peter Jackson’s Heavenly Creatures, The Affair drama series, and Star Wars: The Force Awakens.13,14
CASE CONTINUED
In Mr. B’s case, it is imperative for the treatment team to inquire about his history of violence, paying particular attention to prior violent acts towards his mother. His clinicians should consider hospitalization with the guardian’s consent if the danger appears imminent, especially considering the presence of weapons at home. They should attempt to stabilize Mr. B on effective, tolerable medications to ameliorate his psychosis, and to refer him for long-term psychotherapy to address difficult dynamic issues in the family relationship and encourage compliance with treatment. These steps may help avert a tragedy.
Bottom Line
Individuals who commit parricide often have a history of psychosis, a mood disorder, childhood abuse, and/or difficult relationship dynamics with the parent they kill. Some go on a spree killing in the community. Through careful consideration of individual risk factors, psychiatrists may help prevent some cases of parent murder by a child and possibly more tragedy in the community.
1. Bojanié L, Flynn S, Gianatsi M, et al. The typology of parricide and the role of mental illness: data-driven approach. Aggress Behav. 2020;46(6):516-522.
2. Pinals DS. Parricide. In Friedman SH, ed. Family Murder: Pathologies of Love and Hate. American Psychiatric Publishing; 2019:113-138.
3. Heide KM. Matricide and stepmatricide victims and offenders: an empirical analysis of US arrest data. Behav Sci Law. 2013;31(2):203-214.
4. Bourget D, Gagné P, Labelle ME. Parricide: a comparative study of matricide versus patricide. J Am Acad Psychiatry Law. 2007;35(3):306-312.
5. Heide KM, Frei A. Matricide: a critique of the literature. Trauma Violence Abuse. 2010;11(1):3-17.
6. Marleau JD, Auclair N, Millaud F. Comparison of factors associated with parricide in adults and adolescents. J Fam Viol. 2006;21:321-325.
7. West SG, Feldsher M. Parricide: characteristics of sons and daughters who kill their parents. Current Psychiatry. 2010;9(11):20-38.
8. Green CM. Matricide by sons. Med Sci Law. 1981;21(3):207-214.
9. Friedman SH. Conclusions. In Friedman SH, ed. Family Murder: Pathologies of Love and Hate. American Psychiatric Publishing; 2019:161-164.
10. Campion J, Cravens JM, Rotholc A, et al. A study of 15 matricidal men. Am J Psychiatry. 1985;142(3):312-317.
11. Hall RCW, Friedman SH, Sorrentino R, et al. The myth of school shooters and psychotropic medications. Behav Sci Law. 2019;37(5):540-558.
12. Department of Justice Federal Bureau of Investigation. Active Shooter Incidents: 20-Year Review, 2000-2019. June 1, 2021. Accessed October 12, 2021. https://www.fbi.gov/file-repository/active-shooter-incidents-20-year-review-2000-2019-060121.pdf/view
13. Friedman SH, Hall RCW. Star Wars: The Force Awakens, forensic teaching about matricide. J Am Acad Psychiatry Law. 2017;45(1):128-130.
14. Friedman SH, Hall RCW. Deadly and dysfunctional family dynamics: when fiction mirrors fact. In: Packer S, Fredrick DR, eds. Welcome to Arkham Asylum: Essays on Psychiatry and the Gotham City Institution. McFarland; 2019:65-75.
Mr. B, age 37, presents to a community mental health center for an appointment following a recent emergency department visit. He is diagnosed with schizophrenia, and has been treated for approximately 1 year. Six months ago, Mr. B stopped taking his antipsychotic due to its adverse effects. Despite compliance with another agent, he has become increasingly disorganized and paranoid.
He now believes that his mother, with whom he has lived all his life and who serves as his guardian, is poisoning his food and trying to kill him. She is an employee at a local grocery store, and Mr. B has expressed concern that her coworkers are assisting her in the plot to kill him.
Following a home visit, Mr. B’s case manager indicates that the patient showed them the collection of weapons he is amassing to “defend” himself. This leads to a concern for the safety of the patient, his mother, and others.
Although parricide—killing one’s parent—is a relatively rare event, its sensationalistic nature has long captured the attention of headline writers and the general public. This article discusses the diagnostic and demographic factors that may be seen among individuals who kill their parents, with an emphasis on those who commit matricide (murder of one’s mother) and associated spree killings, where an individual kills multiple people within a single brief but contiguous time period. Understanding these characteristics can help clinicians identify and more safely manage patients who may be at risk of harming their parents in addition to others.
Characteristics of perpetrators of parricide
Worldwide, approximately 2% to 4% of homicides involve parricide, or killing one’s parent.1,2 Most offenders are men in early adulthood, though a proportion are adolescents and some are women.1,3 They are often single, unemployed, and live with the parent prior to the killing.1 Patricide occurs more frequently than matricide.4 In the United States, approximately 150 fathers and 100 mothers are killed by their child each year.5
In a study of all homicides in England and Wales between 1997 and 2014, two-thirds of parricide offenders had previously been diagnosed with a mental disorder.1 One-third were diagnosed with schizophrenia.1 In a Canadian study focusing on 43 adult perpetrators found not criminally responsible,6 most were experiencing psychotic symptoms at the time of parricide; symptoms of a personality disorder were the second-most prevalent symptoms. Similarly, Bourget et al4 studied Canadian coroner records for 64 parents killed by their children. Of the children involved in those parricides, 15% attempted suicide after the killing. Two-thirds of the male offenders evidenced delusional thinking, and/or excessive violence (overkill) was common. Some cases (16%) followed an argument, and some of those perpetrators were intoxicated or psychotic. From our clinical experience, when there are identifiable nonpsychotic triggers, they often can be small things such as an argument over food, smoking, or video games. Often, the perpetrator was financially dependent on their parents and were “trapped in a difficult/hostile/dependence/love relationship” with that parent.6 Adolescent males who kill their parents may not have psychosis7; however, they may be victims of longstanding serious abuse at the hands of their parents. These perpetrators often express relief rather than remorse after committing murder.
Three categories to classify the perpetrators of parricide have been proposed: severely abused, severely mentally ill, and “dangerously antisocial.”3 While severe mental illness was most common in adult defendants, severe abuse was most common in adolescent offenders. There may be significant commonalities between adolescent and adult perpetrators. A more recent latent class analysis by Bojanic et al1 indicated 3 unique types of parricide offenders (Table 1).

Continue to: Matricide: A closer look...
Matricide: A closer look
Though multiple studies have found a higher rate of psychosis among perpetrators of matricide, it is important to note that most people with psychotic disorders would never kill their mother. These events, however, tend to grab headlines and may be highly focused upon by the general population. In addition, matricide may be part of a larger crime that draws additional attention. For example, the 1966 University of Texas Bell Tower shooter and the 2012 Sandy Hook Elementary school shooter both killed their mothers before engaging in mass homicide. Often in cases of matricide, a longer-term dysfunctional relationship existed between the mother and the child. The mother is frequently described as controlling and intrusive and the (often adult) child as overly dependent, while the father may be absent or ineffectual. Hostility and mutual dependence are usual hallmarks of these relationships.8
However, in some cases where an individual with a psychotic disorder kills their mother, there may have been a traditional nurturing relationship.8 Alternative motivations unrelated to psychosis must be considered, including crimes motivated by money/inheritance or those perpetrated out of nonpsychotic anger. Green8 described motive categories for matricide that include paranoid and persecutory, altruistic, and other. In the “paranoid and persecutory” group, delusional beliefs about the mother occur; for example, the perpetrator may believe their mother was the devil. Sexual elements are found in some cases.8 Alternatively, the “altruistic” group demonstrated rather selfless reasons for killing, such as altruistic infanticide cases, or killing out of love.9 The altruistic matricide perpetrator may believe their mother is unwell, which may be a delusion or actually true. Finally, the “other” category contains cases related to jealousy, rage, and impulsivity.
In a study of 15 matricidal men in New York conducted by Campion et al,10 individuals seen by forensic psychiatric services for the crime of matricide included those with schizophrenia, substance-induced psychosis, and impulse control disorders. The authors noted there was often “a serious chronic derangement in the relationships of most matricidal men and their mothers.” Psychometric testing in these cases indicated feelings of dependency, weakness, and difficulty accepting an adult role separate from their mother. Some had conceptualized their mothers as a threat to their masculinity, while others had become enraged at their mothers.
Prevention requires addressing underlying issues
As described above, several factors are common among individuals who commit parricide, and these can be used to develop prevention strategies that focus on addressing underlying issues (Table 2). It is important to consider the relationship dynamics between the potential victim and perpetrator, as well as the motive, rather than focusing solely on mental illness or substance misuse.2

Spree killings that start as parricide
Although spree killing is a relatively rare event, a subset of spree killings involve parricide. One infamous recent event occurred in 2012 at Sandy Hook Elementary School, where the gunman killed his mother before going to the school and killing 26 additional people, many of whom were children.11,12 Because such events are rare, and because in these cases there is a high likelihood that the perpetrator is deceased (eg, died by suicide or killed by the police), much remains unknown about specific motivations and causative factors.
Information is often pieced together from postmortem reviews, which can be hampered by hindsight/recall bias and lack of contemporaneous documentation. Even worse, when these events occur, they may lead to a bias that all parricides or mass murders follow the pattern of the most recent case. This can result in overgeneralization of an individual’s history as being actionable risk violence factors for all potential parricide cases both by the public (eg, “My sister’s son has autism, and the Sandy Hook shooter was reported to have autism—should I be worried for my sister?”) and professionals (eg, “Will I be blamed for the next Sandy Hook by not taking more aggressive action even though I am not sure it is clinically warranted?”).
To identify trends for individuals committing parricide who engage in mass murder events (such as spree killing), we reviewed the 2000-2019 FBI active shooter list.12 Of the 333 events identified, 46 could be classified as domestic violence situations (eg, the perpetrator was in a romantic or familial relationship/former relationship and engaged in an active shooting incident involving at least 1 person from that relationship). We classified 11 of those 46 cases as parricide. Ten of those 11 parricides involved a child killing a parent (Table 3), and the other involved a grandchild killing a grandfather who served as their primary caregiver. Of the 11 incidents, mothers were involved (killed or wounded) in 4, and father figures (including the grandfather serving as a father and a stepfather) were killed in 9, with 2 incidents involving both parents. In 4 of the 11 parricides, other family members were killed in addition to the parent (including siblings, grandparents, or extended family). When considering spree shooters who committed parricide, 4 alleged perpetrators died by suicide, 1 was killed at the scene, and the rest were apprehended. The most common active shooting site endangering the public was an educational location (5), followed by commerce locations (4), with 2 involving open spaces. Eight of the 11 parricides occurred before the event was designated as an active shooting. The mean age for a parricide plus spree shooter was 23, once the oldest (age 61) and youngest (age 14) were removed from the calculation. The majority of the cases fell into the age range of 16 to 25 (n = 6), followed by 3 individuals who were age 26 to 31 (n = 3). All suspected individuals were male.

It is difficult to ascertain the existence of prior mental health care because perpetrators’ medical records may be confidential, juvenile court records may be sealed, and there may not even be a trial due to death or suicide (leading to limited forensic psychiatry examination or public testimony). Among those apprehended, many individuals raise some form of mental health defense, but the validity of their diagnosis may be undermined by concerns of possible malingering, especially in cases where the individual did not have a history of psychiatric treatment prior to the event.11 In summary, based on FBI data,12 spree shooters who committed parricide were usually male, in their late adolescence or early 20s, and more frequently targeted father figures. They often committed the parricide first and at a different location from later “active” shootings. Police were usually not aware of the parricide until after the spree is over.
Continue to: Parricide and society...
Parricide and society
For centuries, mothers and fathers have been honored and revered. Therefore, it is not surprising that killing of one’s mother or father attracts a great deal of macabre interest. Examples of parricide are present throughout popular culture, in mythology, comic books, movies, and television. As all psychiatrists know, Oedipus killed his father and married his mother. Other popular culture examples include: Grant Morrison’s Arkham Asylum: A Serious House on Serious Earth, Alfred Hitchcock’s Psycho, Oliver Stone’s Natural Born Killers, Peter Jackson’s Heavenly Creatures, The Affair drama series, and Star Wars: The Force Awakens.13,14
CASE CONTINUED
In Mr. B’s case, it is imperative for the treatment team to inquire about his history of violence, paying particular attention to prior violent acts towards his mother. His clinicians should consider hospitalization with the guardian’s consent if the danger appears imminent, especially considering the presence of weapons at home. They should attempt to stabilize Mr. B on effective, tolerable medications to ameliorate his psychosis, and to refer him for long-term psychotherapy to address difficult dynamic issues in the family relationship and encourage compliance with treatment. These steps may help avert a tragedy.
Bottom Line
Individuals who commit parricide often have a history of psychosis, a mood disorder, childhood abuse, and/or difficult relationship dynamics with the parent they kill. Some go on a spree killing in the community. Through careful consideration of individual risk factors, psychiatrists may help prevent some cases of parent murder by a child and possibly more tragedy in the community.
Mr. B, age 37, presents to a community mental health center for an appointment following a recent emergency department visit. He is diagnosed with schizophrenia, and has been treated for approximately 1 year. Six months ago, Mr. B stopped taking his antipsychotic due to its adverse effects. Despite compliance with another agent, he has become increasingly disorganized and paranoid.
He now believes that his mother, with whom he has lived all his life and who serves as his guardian, is poisoning his food and trying to kill him. She is an employee at a local grocery store, and Mr. B has expressed concern that her coworkers are assisting her in the plot to kill him.
Following a home visit, Mr. B’s case manager indicates that the patient showed them the collection of weapons he is amassing to “defend” himself. This leads to a concern for the safety of the patient, his mother, and others.
Although parricide—killing one’s parent—is a relatively rare event, its sensationalistic nature has long captured the attention of headline writers and the general public. This article discusses the diagnostic and demographic factors that may be seen among individuals who kill their parents, with an emphasis on those who commit matricide (murder of one’s mother) and associated spree killings, where an individual kills multiple people within a single brief but contiguous time period. Understanding these characteristics can help clinicians identify and more safely manage patients who may be at risk of harming their parents in addition to others.
Characteristics of perpetrators of parricide
Worldwide, approximately 2% to 4% of homicides involve parricide, or killing one’s parent.1,2 Most offenders are men in early adulthood, though a proportion are adolescents and some are women.1,3 They are often single, unemployed, and live with the parent prior to the killing.1 Patricide occurs more frequently than matricide.4 In the United States, approximately 150 fathers and 100 mothers are killed by their child each year.5
In a study of all homicides in England and Wales between 1997 and 2014, two-thirds of parricide offenders had previously been diagnosed with a mental disorder.1 One-third were diagnosed with schizophrenia.1 In a Canadian study focusing on 43 adult perpetrators found not criminally responsible,6 most were experiencing psychotic symptoms at the time of parricide; symptoms of a personality disorder were the second-most prevalent symptoms. Similarly, Bourget et al4 studied Canadian coroner records for 64 parents killed by their children. Of the children involved in those parricides, 15% attempted suicide after the killing. Two-thirds of the male offenders evidenced delusional thinking, and/or excessive violence (overkill) was common. Some cases (16%) followed an argument, and some of those perpetrators were intoxicated or psychotic. From our clinical experience, when there are identifiable nonpsychotic triggers, they often can be small things such as an argument over food, smoking, or video games. Often, the perpetrator was financially dependent on their parents and were “trapped in a difficult/hostile/dependence/love relationship” with that parent.6 Adolescent males who kill their parents may not have psychosis7; however, they may be victims of longstanding serious abuse at the hands of their parents. These perpetrators often express relief rather than remorse after committing murder.
Three categories to classify the perpetrators of parricide have been proposed: severely abused, severely mentally ill, and “dangerously antisocial.”3 While severe mental illness was most common in adult defendants, severe abuse was most common in adolescent offenders. There may be significant commonalities between adolescent and adult perpetrators. A more recent latent class analysis by Bojanic et al1 indicated 3 unique types of parricide offenders (Table 1).

Continue to: Matricide: A closer look...
Matricide: A closer look
Though multiple studies have found a higher rate of psychosis among perpetrators of matricide, it is important to note that most people with psychotic disorders would never kill their mother. These events, however, tend to grab headlines and may be highly focused upon by the general population. In addition, matricide may be part of a larger crime that draws additional attention. For example, the 1966 University of Texas Bell Tower shooter and the 2012 Sandy Hook Elementary school shooter both killed their mothers before engaging in mass homicide. Often in cases of matricide, a longer-term dysfunctional relationship existed between the mother and the child. The mother is frequently described as controlling and intrusive and the (often adult) child as overly dependent, while the father may be absent or ineffectual. Hostility and mutual dependence are usual hallmarks of these relationships.8
However, in some cases where an individual with a psychotic disorder kills their mother, there may have been a traditional nurturing relationship.8 Alternative motivations unrelated to psychosis must be considered, including crimes motivated by money/inheritance or those perpetrated out of nonpsychotic anger. Green8 described motive categories for matricide that include paranoid and persecutory, altruistic, and other. In the “paranoid and persecutory” group, delusional beliefs about the mother occur; for example, the perpetrator may believe their mother was the devil. Sexual elements are found in some cases.8 Alternatively, the “altruistic” group demonstrated rather selfless reasons for killing, such as altruistic infanticide cases, or killing out of love.9 The altruistic matricide perpetrator may believe their mother is unwell, which may be a delusion or actually true. Finally, the “other” category contains cases related to jealousy, rage, and impulsivity.
In a study of 15 matricidal men in New York conducted by Campion et al,10 individuals seen by forensic psychiatric services for the crime of matricide included those with schizophrenia, substance-induced psychosis, and impulse control disorders. The authors noted there was often “a serious chronic derangement in the relationships of most matricidal men and their mothers.” Psychometric testing in these cases indicated feelings of dependency, weakness, and difficulty accepting an adult role separate from their mother. Some had conceptualized their mothers as a threat to their masculinity, while others had become enraged at their mothers.
Prevention requires addressing underlying issues
As described above, several factors are common among individuals who commit parricide, and these can be used to develop prevention strategies that focus on addressing underlying issues (Table 2). It is important to consider the relationship dynamics between the potential victim and perpetrator, as well as the motive, rather than focusing solely on mental illness or substance misuse.2

Spree killings that start as parricide
Although spree killing is a relatively rare event, a subset of spree killings involve parricide. One infamous recent event occurred in 2012 at Sandy Hook Elementary School, where the gunman killed his mother before going to the school and killing 26 additional people, many of whom were children.11,12 Because such events are rare, and because in these cases there is a high likelihood that the perpetrator is deceased (eg, died by suicide or killed by the police), much remains unknown about specific motivations and causative factors.
Information is often pieced together from postmortem reviews, which can be hampered by hindsight/recall bias and lack of contemporaneous documentation. Even worse, when these events occur, they may lead to a bias that all parricides or mass murders follow the pattern of the most recent case. This can result in overgeneralization of an individual’s history as being actionable risk violence factors for all potential parricide cases both by the public (eg, “My sister’s son has autism, and the Sandy Hook shooter was reported to have autism—should I be worried for my sister?”) and professionals (eg, “Will I be blamed for the next Sandy Hook by not taking more aggressive action even though I am not sure it is clinically warranted?”).
To identify trends for individuals committing parricide who engage in mass murder events (such as spree killing), we reviewed the 2000-2019 FBI active shooter list.12 Of the 333 events identified, 46 could be classified as domestic violence situations (eg, the perpetrator was in a romantic or familial relationship/former relationship and engaged in an active shooting incident involving at least 1 person from that relationship). We classified 11 of those 46 cases as parricide. Ten of those 11 parricides involved a child killing a parent (Table 3), and the other involved a grandchild killing a grandfather who served as their primary caregiver. Of the 11 incidents, mothers were involved (killed or wounded) in 4, and father figures (including the grandfather serving as a father and a stepfather) were killed in 9, with 2 incidents involving both parents. In 4 of the 11 parricides, other family members were killed in addition to the parent (including siblings, grandparents, or extended family). When considering spree shooters who committed parricide, 4 alleged perpetrators died by suicide, 1 was killed at the scene, and the rest were apprehended. The most common active shooting site endangering the public was an educational location (5), followed by commerce locations (4), with 2 involving open spaces. Eight of the 11 parricides occurred before the event was designated as an active shooting. The mean age for a parricide plus spree shooter was 23, once the oldest (age 61) and youngest (age 14) were removed from the calculation. The majority of the cases fell into the age range of 16 to 25 (n = 6), followed by 3 individuals who were age 26 to 31 (n = 3). All suspected individuals were male.

It is difficult to ascertain the existence of prior mental health care because perpetrators’ medical records may be confidential, juvenile court records may be sealed, and there may not even be a trial due to death or suicide (leading to limited forensic psychiatry examination or public testimony). Among those apprehended, many individuals raise some form of mental health defense, but the validity of their diagnosis may be undermined by concerns of possible malingering, especially in cases where the individual did not have a history of psychiatric treatment prior to the event.11 In summary, based on FBI data,12 spree shooters who committed parricide were usually male, in their late adolescence or early 20s, and more frequently targeted father figures. They often committed the parricide first and at a different location from later “active” shootings. Police were usually not aware of the parricide until after the spree is over.
Continue to: Parricide and society...
Parricide and society
For centuries, mothers and fathers have been honored and revered. Therefore, it is not surprising that killing of one’s mother or father attracts a great deal of macabre interest. Examples of parricide are present throughout popular culture, in mythology, comic books, movies, and television. As all psychiatrists know, Oedipus killed his father and married his mother. Other popular culture examples include: Grant Morrison’s Arkham Asylum: A Serious House on Serious Earth, Alfred Hitchcock’s Psycho, Oliver Stone’s Natural Born Killers, Peter Jackson’s Heavenly Creatures, The Affair drama series, and Star Wars: The Force Awakens.13,14
CASE CONTINUED
In Mr. B’s case, it is imperative for the treatment team to inquire about his history of violence, paying particular attention to prior violent acts towards his mother. His clinicians should consider hospitalization with the guardian’s consent if the danger appears imminent, especially considering the presence of weapons at home. They should attempt to stabilize Mr. B on effective, tolerable medications to ameliorate his psychosis, and to refer him for long-term psychotherapy to address difficult dynamic issues in the family relationship and encourage compliance with treatment. These steps may help avert a tragedy.
Bottom Line
Individuals who commit parricide often have a history of psychosis, a mood disorder, childhood abuse, and/or difficult relationship dynamics with the parent they kill. Some go on a spree killing in the community. Through careful consideration of individual risk factors, psychiatrists may help prevent some cases of parent murder by a child and possibly more tragedy in the community.
1. Bojanié L, Flynn S, Gianatsi M, et al. The typology of parricide and the role of mental illness: data-driven approach. Aggress Behav. 2020;46(6):516-522.
2. Pinals DS. Parricide. In Friedman SH, ed. Family Murder: Pathologies of Love and Hate. American Psychiatric Publishing; 2019:113-138.
3. Heide KM. Matricide and stepmatricide victims and offenders: an empirical analysis of US arrest data. Behav Sci Law. 2013;31(2):203-214.
4. Bourget D, Gagné P, Labelle ME. Parricide: a comparative study of matricide versus patricide. J Am Acad Psychiatry Law. 2007;35(3):306-312.
5. Heide KM, Frei A. Matricide: a critique of the literature. Trauma Violence Abuse. 2010;11(1):3-17.
6. Marleau JD, Auclair N, Millaud F. Comparison of factors associated with parricide in adults and adolescents. J Fam Viol. 2006;21:321-325.
7. West SG, Feldsher M. Parricide: characteristics of sons and daughters who kill their parents. Current Psychiatry. 2010;9(11):20-38.
8. Green CM. Matricide by sons. Med Sci Law. 1981;21(3):207-214.
9. Friedman SH. Conclusions. In Friedman SH, ed. Family Murder: Pathologies of Love and Hate. American Psychiatric Publishing; 2019:161-164.
10. Campion J, Cravens JM, Rotholc A, et al. A study of 15 matricidal men. Am J Psychiatry. 1985;142(3):312-317.
11. Hall RCW, Friedman SH, Sorrentino R, et al. The myth of school shooters and psychotropic medications. Behav Sci Law. 2019;37(5):540-558.
12. Department of Justice Federal Bureau of Investigation. Active Shooter Incidents: 20-Year Review, 2000-2019. June 1, 2021. Accessed October 12, 2021. https://www.fbi.gov/file-repository/active-shooter-incidents-20-year-review-2000-2019-060121.pdf/view
13. Friedman SH, Hall RCW. Star Wars: The Force Awakens, forensic teaching about matricide. J Am Acad Psychiatry Law. 2017;45(1):128-130.
14. Friedman SH, Hall RCW. Deadly and dysfunctional family dynamics: when fiction mirrors fact. In: Packer S, Fredrick DR, eds. Welcome to Arkham Asylum: Essays on Psychiatry and the Gotham City Institution. McFarland; 2019:65-75.
1. Bojanié L, Flynn S, Gianatsi M, et al. The typology of parricide and the role of mental illness: data-driven approach. Aggress Behav. 2020;46(6):516-522.
2. Pinals DS. Parricide. In Friedman SH, ed. Family Murder: Pathologies of Love and Hate. American Psychiatric Publishing; 2019:113-138.
3. Heide KM. Matricide and stepmatricide victims and offenders: an empirical analysis of US arrest data. Behav Sci Law. 2013;31(2):203-214.
4. Bourget D, Gagné P, Labelle ME. Parricide: a comparative study of matricide versus patricide. J Am Acad Psychiatry Law. 2007;35(3):306-312.
5. Heide KM, Frei A. Matricide: a critique of the literature. Trauma Violence Abuse. 2010;11(1):3-17.
6. Marleau JD, Auclair N, Millaud F. Comparison of factors associated with parricide in adults and adolescents. J Fam Viol. 2006;21:321-325.
7. West SG, Feldsher M. Parricide: characteristics of sons and daughters who kill their parents. Current Psychiatry. 2010;9(11):20-38.
8. Green CM. Matricide by sons. Med Sci Law. 1981;21(3):207-214.
9. Friedman SH. Conclusions. In Friedman SH, ed. Family Murder: Pathologies of Love and Hate. American Psychiatric Publishing; 2019:161-164.
10. Campion J, Cravens JM, Rotholc A, et al. A study of 15 matricidal men. Am J Psychiatry. 1985;142(3):312-317.
11. Hall RCW, Friedman SH, Sorrentino R, et al. The myth of school shooters and psychotropic medications. Behav Sci Law. 2019;37(5):540-558.
12. Department of Justice Federal Bureau of Investigation. Active Shooter Incidents: 20-Year Review, 2000-2019. June 1, 2021. Accessed October 12, 2021. https://www.fbi.gov/file-repository/active-shooter-incidents-20-year-review-2000-2019-060121.pdf/view
13. Friedman SH, Hall RCW. Star Wars: The Force Awakens, forensic teaching about matricide. J Am Acad Psychiatry Law. 2017;45(1):128-130.
14. Friedman SH, Hall RCW. Deadly and dysfunctional family dynamics: when fiction mirrors fact. In: Packer S, Fredrick DR, eds. Welcome to Arkham Asylum: Essays on Psychiatry and the Gotham City Institution. McFarland; 2019:65-75.
Antipsychotic-induced priapism: Mitigating the risk
Mr. J, age 35, is brought to the hospital from prison due to priapism that does not improve with treatment. He says he has had priapism 5 times previously, with the first incidence occurring “years ago” due to trazodone.
Recently, he has been receiving risperidone, which the treatment team believes is the cause of his current priapism. His medical history includes asthma, schizophrenia, hypertension, seizures, and sickle cell trait. Mr. J is experiencing auditory hallucinations, which he describes as “continuous, neutral voices that are annoying.” He would like relief from his auditory hallucinations and is willing to change his antipsychotic, but does not want additional treatment for his priapism. His present medications include risperidone, 1 mg twice a day, escitalopram, 10 mg/d, benztropine, 1 mg twice a day, and phenytoin, 500 mg/d at bedtime.
Priapism is a prolonged, persistent, and often painful erection that occurs without sexual stimulation. Although relatively rare, it can result in potentially serious long-term complications, including impotence and gangrene, and requires immediate evaluation and management.
There are 2 types of priapism: nonischemic, or “high-flow,” priapism, and ischemic, or “low-flow,” priapism (Table 1). While nonischemic priapism is typically caused by penile or perineal trauma, ischemic priapism can occur as a result of medications, including antipsychotics, antidepressants, anxiolytics, and antihypertensives, or hematological conditions such as sickle cell disease.1 Other risk factors associated with priapism include substance abuse, hyperprolactinemia, diabetes, and liver disease.4
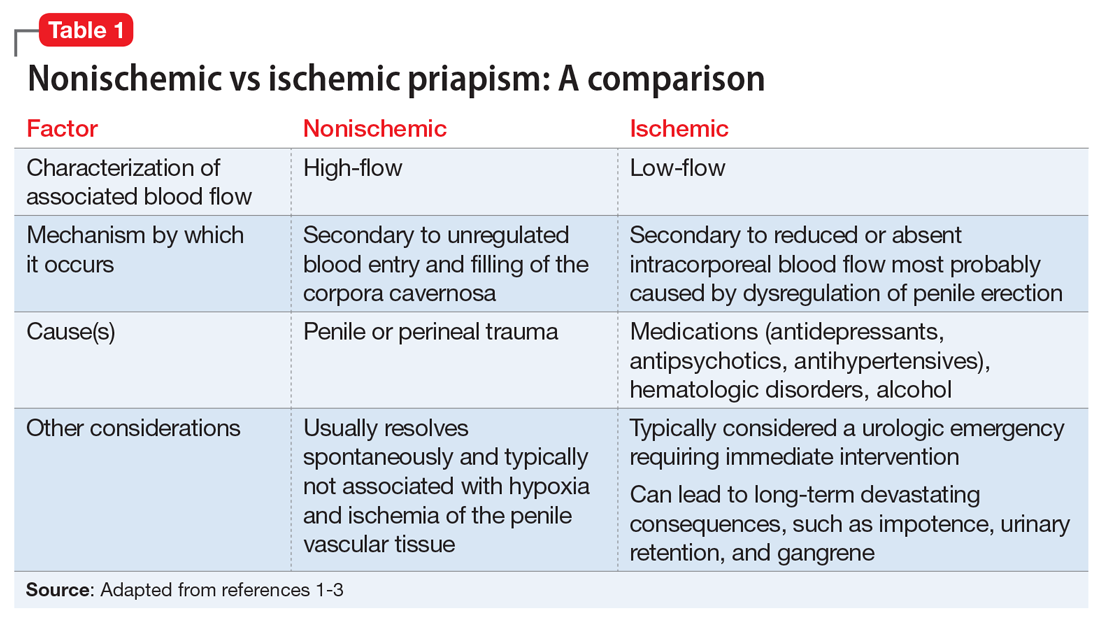
Antipsychotic-induced priapism
Medication-induced priapism is a rare adverse drug reaction (ADR). Of the medication classes associated with priapism, antipsychotics have the highest incidence and account for approximately 20% of all cases.1
The mechanism of priapism associated with antipsychotics is thought to be related to alpha-1 blockade in the corpora cavernosa of the penis. Although antipsychotics within each class share common characteristics, each agent has a unique profile of receptor affinities. As such, antipsychotics have varying affinities for the alpha-adrenergic receptor (Table 2). Agents such as ziprasidone, chlorpromazine, and risperidone—which have the highest affinity for the alpha-1 adrenoceptors—may be more likely to cause priapism compared with agents with lower affinity, such as olanzapine. Priapism may occur at any time during antipsychotic treatment, and does not appear to be dose-related.
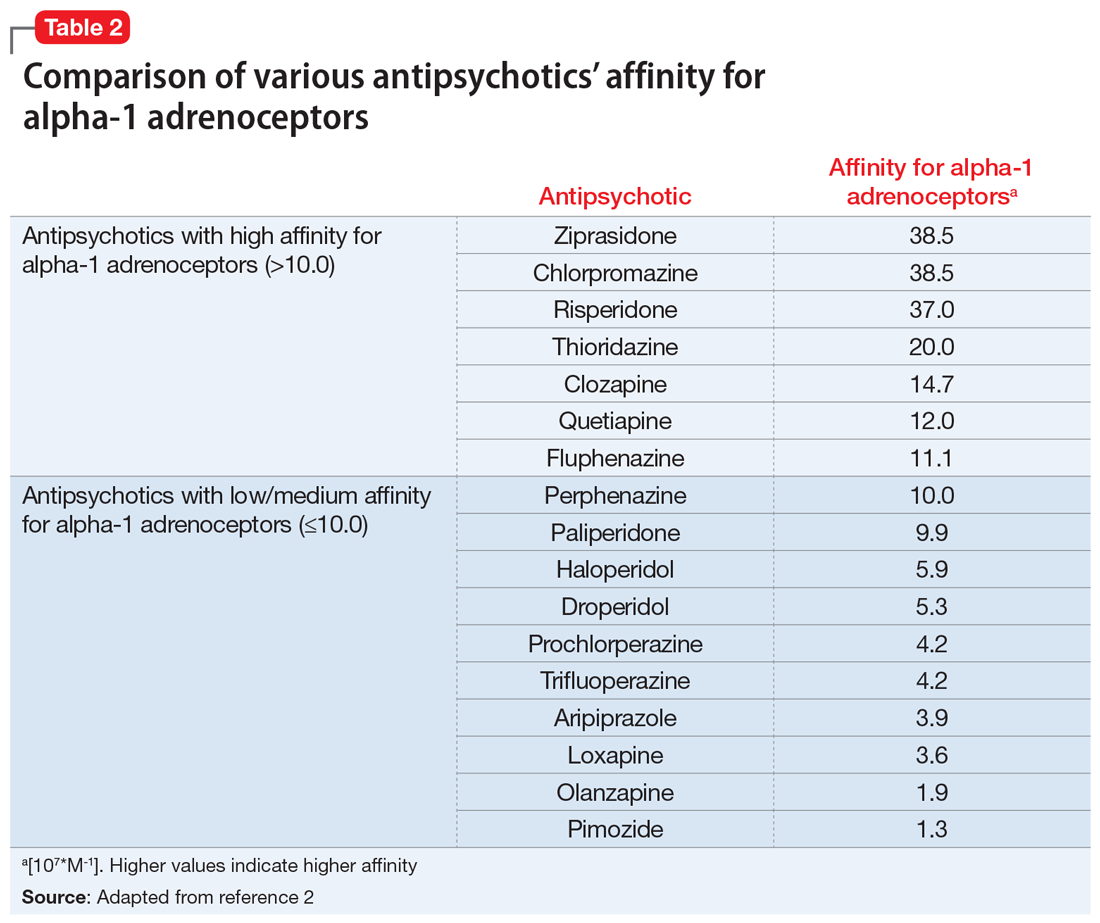
Continue to: Antipsychotic drug interactions and priapism...
Antipsychotic drug interactions and priapism
Patients who are receiving multiple medications as treatment for chronic medical or psychiatric conditions have an increased likelihood of experiencing drug-drug interactions (DDIs) that lead to adverse effects.
Various case reports have described priapism as a result of DDIs related to antipsychotic agents combined with other psychotropic or nonpsychotropic medications.3 Most of these DDIs have been attributed to the cytochrome P450 (CYP) family of enzymes, including CYP2D6, CYP1A2, and CYP3A4/5, which are major enzymes implicated in the metabolism of antipsychotics (Table 3).
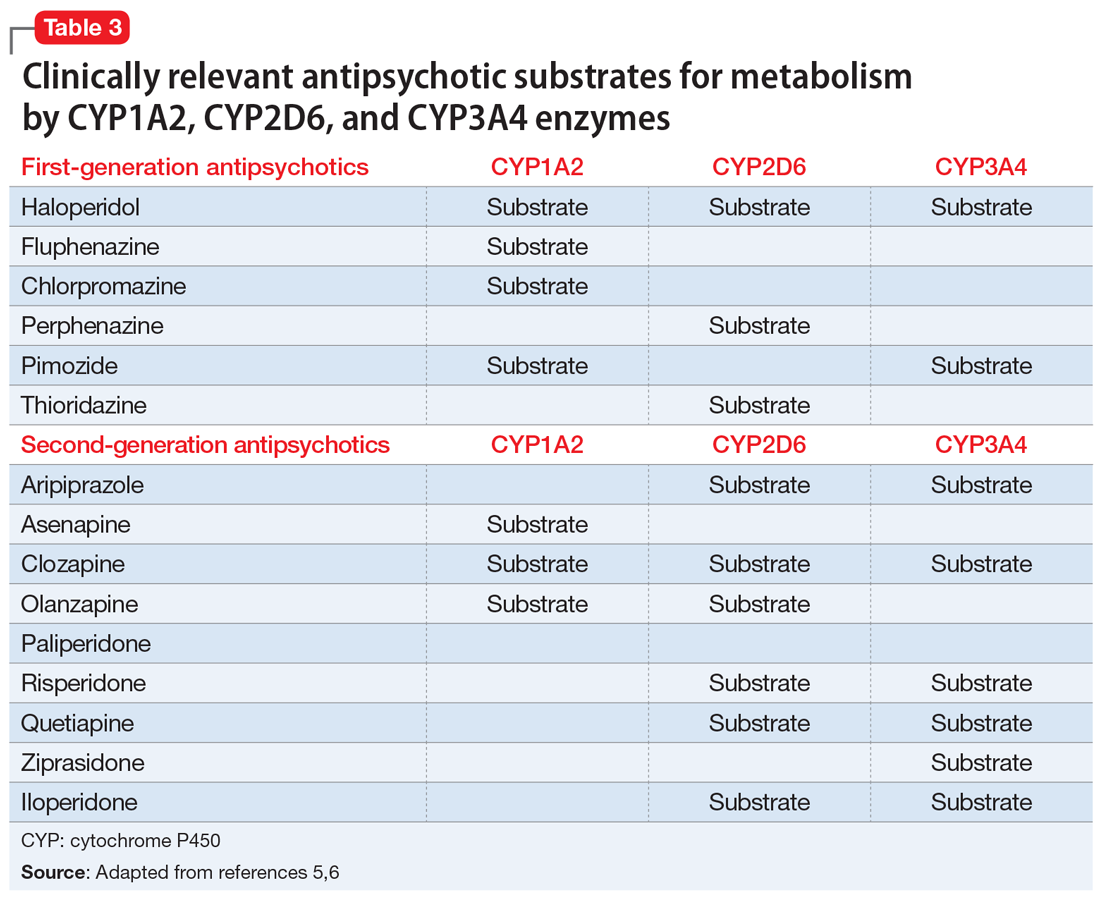
It is imperative to be vigilant during the concomitant administration of antipsychotics with other medications that may be substrates, inducers, or inhibitors of CYP enzymes, as this could alter the metabolism and kinetics of the antipsychotic and result in ADRs such as priapism. For example, drug interactions exist between strong CYP2D6 inhibitors—such as the antidepressants paroxetine, fluoxetine, and bupropion—and antipsychotics that are substrates of CYP2D6, such as risperidone, aripiprazole, haloperidol, and perphenazine. This interaction can lead to higher levels of the antipsychotic, which would increase the patient’s risk of experiencing ADRs. Because psychotic illnesses and depression/anxiety often coexist, it is not uncommon for individuals with these conditions to be receiving both an antipsychotic and an antidepressant.
Because there is a high incidence of comorbidities such as HIV and cardiovascular disease among individuals with mental illnesses, clinicians must also be cognizant of any nonpsychotropic medications the patient may be taking. For instance, clinically relevant DDIs exist between protease inhibitors, such as ritonavir, a strong CYP3A4 inhibitor, and antipsychotics that are substrates of CYP3A4, such as pimozide, aripiprazole, and quetiapine.
Mitigating the risk of priapism
Although there are associated risk factors for priapism, there are no concrete indicators to predict the onset or development of the condition. The best predictor may be a history of prolonged and painless erections.3
As such, when choosing an antipsychotic, it is critical to screen the patient for the previously mentioned risk factors, including the presence of medications with strong alpha-1 receptor affinity and CYP interactions, especially to minimize the risk of recurrence of priapism in those with prior or similar episodes. Management of patients with priapism due to antipsychotics has involved reducing the dose of the offending agent and/or changing the medication to one with a lower alpha-adrenergic affinity (Table 22).
Similar to most situations, management is patient-specific and depends on several factors, including the severity of the patient’s psychiatric disease, history/severity of priapism and treatment, concurrent medication list, etc. For example, although clozapine is considered to have relatively high affinity for the alpha-1 receptor, it is also the agent of choice for treatment-refractory schizophrenia. Risks and benefits must be weighed on a individualized basis. Case reports have described symptom improvement via lowering the dose of clozapine and adding on or switching to an antipsychotic agent with minimal alpha-1 receptor affinity.4
After considering Mr. J’s history, risk factors, and preferences, the treatment team discontinues risperidone and initiates haloperidol, 5 mg twice a day. Soon after, Mr. J no longer experiences priapism.
1. Weiner DM, Lowe FC. Psychotropic drug-induced priapism. Mol Diag Ther 9. 1998;371-379. doi:10.2165/00023210-199809050-00004
2. Andersohn F, Schmedt N, Weinmann S, et al. Priapism associated with antipsychotics: role of alpha1 adrenoceptor affinity. J Clin Psychopharmacol. 2010;30(1):68-71. doi:10.1097/JCP.0b013e3181c8273d
3. Sood S, James W, Bailon MJ. Priapism associated with atypical antipsychotic medications: a review. Int Clin Psychopharmacol. 2008;23(1):9-17.
4. Sinkeviciute I, Kroken RA, Johnsen E. Priapism in antipsychotic drug use: a rare but important side effect. Case Rep Psychiatry. 2012;2012:496364. doi:10.1155/2012/496364
5. Mora F, Martín JDD, Zubillaga E, et al. CYP450 and its implications in the clinical use of antipsychotic drugs. Clin Exp Pharmacol. 2015;5(176):1-10. doi:10.4172/2161-1459.1000176
6. Puangpetch A, Vanwong N, Nuntamool N, et al. CYP2D6 polymorphisms and their influence on risperidone treatment. Pharmgenomics Pers Med. 2016;9:131-147. doi:10.2147/PGPM.S107772
Mr. J, age 35, is brought to the hospital from prison due to priapism that does not improve with treatment. He says he has had priapism 5 times previously, with the first incidence occurring “years ago” due to trazodone.
Recently, he has been receiving risperidone, which the treatment team believes is the cause of his current priapism. His medical history includes asthma, schizophrenia, hypertension, seizures, and sickle cell trait. Mr. J is experiencing auditory hallucinations, which he describes as “continuous, neutral voices that are annoying.” He would like relief from his auditory hallucinations and is willing to change his antipsychotic, but does not want additional treatment for his priapism. His present medications include risperidone, 1 mg twice a day, escitalopram, 10 mg/d, benztropine, 1 mg twice a day, and phenytoin, 500 mg/d at bedtime.
Priapism is a prolonged, persistent, and often painful erection that occurs without sexual stimulation. Although relatively rare, it can result in potentially serious long-term complications, including impotence and gangrene, and requires immediate evaluation and management.
There are 2 types of priapism: nonischemic, or “high-flow,” priapism, and ischemic, or “low-flow,” priapism (Table 1). While nonischemic priapism is typically caused by penile or perineal trauma, ischemic priapism can occur as a result of medications, including antipsychotics, antidepressants, anxiolytics, and antihypertensives, or hematological conditions such as sickle cell disease.1 Other risk factors associated with priapism include substance abuse, hyperprolactinemia, diabetes, and liver disease.4

Antipsychotic-induced priapism
Medication-induced priapism is a rare adverse drug reaction (ADR). Of the medication classes associated with priapism, antipsychotics have the highest incidence and account for approximately 20% of all cases.1
The mechanism of priapism associated with antipsychotics is thought to be related to alpha-1 blockade in the corpora cavernosa of the penis. Although antipsychotics within each class share common characteristics, each agent has a unique profile of receptor affinities. As such, antipsychotics have varying affinities for the alpha-adrenergic receptor (Table 2). Agents such as ziprasidone, chlorpromazine, and risperidone—which have the highest affinity for the alpha-1 adrenoceptors—may be more likely to cause priapism compared with agents with lower affinity, such as olanzapine. Priapism may occur at any time during antipsychotic treatment, and does not appear to be dose-related.

Continue to: Antipsychotic drug interactions and priapism...
Antipsychotic drug interactions and priapism
Patients who are receiving multiple medications as treatment for chronic medical or psychiatric conditions have an increased likelihood of experiencing drug-drug interactions (DDIs) that lead to adverse effects.
Various case reports have described priapism as a result of DDIs related to antipsychotic agents combined with other psychotropic or nonpsychotropic medications.3 Most of these DDIs have been attributed to the cytochrome P450 (CYP) family of enzymes, including CYP2D6, CYP1A2, and CYP3A4/5, which are major enzymes implicated in the metabolism of antipsychotics (Table 3).

It is imperative to be vigilant during the concomitant administration of antipsychotics with other medications that may be substrates, inducers, or inhibitors of CYP enzymes, as this could alter the metabolism and kinetics of the antipsychotic and result in ADRs such as priapism. For example, drug interactions exist between strong CYP2D6 inhibitors—such as the antidepressants paroxetine, fluoxetine, and bupropion—and antipsychotics that are substrates of CYP2D6, such as risperidone, aripiprazole, haloperidol, and perphenazine. This interaction can lead to higher levels of the antipsychotic, which would increase the patient’s risk of experiencing ADRs. Because psychotic illnesses and depression/anxiety often coexist, it is not uncommon for individuals with these conditions to be receiving both an antipsychotic and an antidepressant.
Because there is a high incidence of comorbidities such as HIV and cardiovascular disease among individuals with mental illnesses, clinicians must also be cognizant of any nonpsychotropic medications the patient may be taking. For instance, clinically relevant DDIs exist between protease inhibitors, such as ritonavir, a strong CYP3A4 inhibitor, and antipsychotics that are substrates of CYP3A4, such as pimozide, aripiprazole, and quetiapine.
Mitigating the risk of priapism
Although there are associated risk factors for priapism, there are no concrete indicators to predict the onset or development of the condition. The best predictor may be a history of prolonged and painless erections.3
As such, when choosing an antipsychotic, it is critical to screen the patient for the previously mentioned risk factors, including the presence of medications with strong alpha-1 receptor affinity and CYP interactions, especially to minimize the risk of recurrence of priapism in those with prior or similar episodes. Management of patients with priapism due to antipsychotics has involved reducing the dose of the offending agent and/or changing the medication to one with a lower alpha-adrenergic affinity (Table 22).
Similar to most situations, management is patient-specific and depends on several factors, including the severity of the patient’s psychiatric disease, history/severity of priapism and treatment, concurrent medication list, etc. For example, although clozapine is considered to have relatively high affinity for the alpha-1 receptor, it is also the agent of choice for treatment-refractory schizophrenia. Risks and benefits must be weighed on a individualized basis. Case reports have described symptom improvement via lowering the dose of clozapine and adding on or switching to an antipsychotic agent with minimal alpha-1 receptor affinity.4
After considering Mr. J’s history, risk factors, and preferences, the treatment team discontinues risperidone and initiates haloperidol, 5 mg twice a day. Soon after, Mr. J no longer experiences priapism.
Mr. J, age 35, is brought to the hospital from prison due to priapism that does not improve with treatment. He says he has had priapism 5 times previously, with the first incidence occurring “years ago” due to trazodone.
Recently, he has been receiving risperidone, which the treatment team believes is the cause of his current priapism. His medical history includes asthma, schizophrenia, hypertension, seizures, and sickle cell trait. Mr. J is experiencing auditory hallucinations, which he describes as “continuous, neutral voices that are annoying.” He would like relief from his auditory hallucinations and is willing to change his antipsychotic, but does not want additional treatment for his priapism. His present medications include risperidone, 1 mg twice a day, escitalopram, 10 mg/d, benztropine, 1 mg twice a day, and phenytoin, 500 mg/d at bedtime.
Priapism is a prolonged, persistent, and often painful erection that occurs without sexual stimulation. Although relatively rare, it can result in potentially serious long-term complications, including impotence and gangrene, and requires immediate evaluation and management.
There are 2 types of priapism: nonischemic, or “high-flow,” priapism, and ischemic, or “low-flow,” priapism (Table 1). While nonischemic priapism is typically caused by penile or perineal trauma, ischemic priapism can occur as a result of medications, including antipsychotics, antidepressants, anxiolytics, and antihypertensives, or hematological conditions such as sickle cell disease.1 Other risk factors associated with priapism include substance abuse, hyperprolactinemia, diabetes, and liver disease.4

Antipsychotic-induced priapism
Medication-induced priapism is a rare adverse drug reaction (ADR). Of the medication classes associated with priapism, antipsychotics have the highest incidence and account for approximately 20% of all cases.1
The mechanism of priapism associated with antipsychotics is thought to be related to alpha-1 blockade in the corpora cavernosa of the penis. Although antipsychotics within each class share common characteristics, each agent has a unique profile of receptor affinities. As such, antipsychotics have varying affinities for the alpha-adrenergic receptor (Table 2). Agents such as ziprasidone, chlorpromazine, and risperidone—which have the highest affinity for the alpha-1 adrenoceptors—may be more likely to cause priapism compared with agents with lower affinity, such as olanzapine. Priapism may occur at any time during antipsychotic treatment, and does not appear to be dose-related.

Continue to: Antipsychotic drug interactions and priapism...
Antipsychotic drug interactions and priapism
Patients who are receiving multiple medications as treatment for chronic medical or psychiatric conditions have an increased likelihood of experiencing drug-drug interactions (DDIs) that lead to adverse effects.
Various case reports have described priapism as a result of DDIs related to antipsychotic agents combined with other psychotropic or nonpsychotropic medications.3 Most of these DDIs have been attributed to the cytochrome P450 (CYP) family of enzymes, including CYP2D6, CYP1A2, and CYP3A4/5, which are major enzymes implicated in the metabolism of antipsychotics (Table 3).

It is imperative to be vigilant during the concomitant administration of antipsychotics with other medications that may be substrates, inducers, or inhibitors of CYP enzymes, as this could alter the metabolism and kinetics of the antipsychotic and result in ADRs such as priapism. For example, drug interactions exist between strong CYP2D6 inhibitors—such as the antidepressants paroxetine, fluoxetine, and bupropion—and antipsychotics that are substrates of CYP2D6, such as risperidone, aripiprazole, haloperidol, and perphenazine. This interaction can lead to higher levels of the antipsychotic, which would increase the patient’s risk of experiencing ADRs. Because psychotic illnesses and depression/anxiety often coexist, it is not uncommon for individuals with these conditions to be receiving both an antipsychotic and an antidepressant.
Because there is a high incidence of comorbidities such as HIV and cardiovascular disease among individuals with mental illnesses, clinicians must also be cognizant of any nonpsychotropic medications the patient may be taking. For instance, clinically relevant DDIs exist between protease inhibitors, such as ritonavir, a strong CYP3A4 inhibitor, and antipsychotics that are substrates of CYP3A4, such as pimozide, aripiprazole, and quetiapine.
Mitigating the risk of priapism
Although there are associated risk factors for priapism, there are no concrete indicators to predict the onset or development of the condition. The best predictor may be a history of prolonged and painless erections.3
As such, when choosing an antipsychotic, it is critical to screen the patient for the previously mentioned risk factors, including the presence of medications with strong alpha-1 receptor affinity and CYP interactions, especially to minimize the risk of recurrence of priapism in those with prior or similar episodes. Management of patients with priapism due to antipsychotics has involved reducing the dose of the offending agent and/or changing the medication to one with a lower alpha-adrenergic affinity (Table 22).
Similar to most situations, management is patient-specific and depends on several factors, including the severity of the patient’s psychiatric disease, history/severity of priapism and treatment, concurrent medication list, etc. For example, although clozapine is considered to have relatively high affinity for the alpha-1 receptor, it is also the agent of choice for treatment-refractory schizophrenia. Risks and benefits must be weighed on a individualized basis. Case reports have described symptom improvement via lowering the dose of clozapine and adding on or switching to an antipsychotic agent with minimal alpha-1 receptor affinity.4
After considering Mr. J’s history, risk factors, and preferences, the treatment team discontinues risperidone and initiates haloperidol, 5 mg twice a day. Soon after, Mr. J no longer experiences priapism.
1. Weiner DM, Lowe FC. Psychotropic drug-induced priapism. Mol Diag Ther 9. 1998;371-379. doi:10.2165/00023210-199809050-00004
2. Andersohn F, Schmedt N, Weinmann S, et al. Priapism associated with antipsychotics: role of alpha1 adrenoceptor affinity. J Clin Psychopharmacol. 2010;30(1):68-71. doi:10.1097/JCP.0b013e3181c8273d
3. Sood S, James W, Bailon MJ. Priapism associated with atypical antipsychotic medications: a review. Int Clin Psychopharmacol. 2008;23(1):9-17.
4. Sinkeviciute I, Kroken RA, Johnsen E. Priapism in antipsychotic drug use: a rare but important side effect. Case Rep Psychiatry. 2012;2012:496364. doi:10.1155/2012/496364
5. Mora F, Martín JDD, Zubillaga E, et al. CYP450 and its implications in the clinical use of antipsychotic drugs. Clin Exp Pharmacol. 2015;5(176):1-10. doi:10.4172/2161-1459.1000176
6. Puangpetch A, Vanwong N, Nuntamool N, et al. CYP2D6 polymorphisms and their influence on risperidone treatment. Pharmgenomics Pers Med. 2016;9:131-147. doi:10.2147/PGPM.S107772
1. Weiner DM, Lowe FC. Psychotropic drug-induced priapism. Mol Diag Ther 9. 1998;371-379. doi:10.2165/00023210-199809050-00004
2. Andersohn F, Schmedt N, Weinmann S, et al. Priapism associated with antipsychotics: role of alpha1 adrenoceptor affinity. J Clin Psychopharmacol. 2010;30(1):68-71. doi:10.1097/JCP.0b013e3181c8273d
3. Sood S, James W, Bailon MJ. Priapism associated with atypical antipsychotic medications: a review. Int Clin Psychopharmacol. 2008;23(1):9-17.
4. Sinkeviciute I, Kroken RA, Johnsen E. Priapism in antipsychotic drug use: a rare but important side effect. Case Rep Psychiatry. 2012;2012:496364. doi:10.1155/2012/496364
5. Mora F, Martín JDD, Zubillaga E, et al. CYP450 and its implications in the clinical use of antipsychotic drugs. Clin Exp Pharmacol. 2015;5(176):1-10. doi:10.4172/2161-1459.1000176
6. Puangpetch A, Vanwong N, Nuntamool N, et al. CYP2D6 polymorphisms and their influence on risperidone treatment. Pharmgenomics Pers Med. 2016;9:131-147. doi:10.2147/PGPM.S107772
Depressed and awkward: Is it more than that?
CASE Treatment-resistant MDD
Ms. P, age 21, presents to the outpatient clinic. She has diagnoses of treatment-resistant major depressive disorder (MDD) and schizoid personality disorder (SPD). Ms. P was diagnosed with MDD 3 years ago after reporting symptoms of prevailing sadness for approximately 8 years, described as feelings of worthlessness, anhedonia, social withdrawal, and decreased hygiene and self-care behaviors, as well as suicidal ideation and self-harm. SPD was diagnosed 1 year earlier based on her “odd” behaviors and disheveled appearance following observation and in collateral with her family. Her odd behaviors are described as spending most of her time alone, preferring solitary activities, and having little contact with people other than her parents.
Ms. P reports that she was previously treated with citalopram, 20 mg/d, bupropion, 150 mg/d, aripiprazole, 3.75 mg/d, topiramate, 100 mg twice daily, and melatonin, 9 mg/d at bedtime, but discontinued follow-up appointments and medications after no significant improvement in symptoms.
[polldaddy:11027942]
The authors’ observations
The term “schizoid” first made its debut in the medical community to describe the prodromal social withdrawal and isolation observed in schizophrenia.1 The use of schizoid to describe a personality type first occurred in DSM-III in 1980.2 SPD is a Cluster A personality disorder that groups personalities characterized by common traits that are “odd” or “eccentric” and may resemble the positive and/or negative symptoms of schizophrenia.3,4 Relatively uncommon in clinical settings, SPD includes individuals who do not desire or enjoy close relationships. Those afflicted with SPD will be described as isolated, aloof, and detached from social relationships with others, even immediate family members. Individuals with SPD may appear indifferent to criticism and praise, and may take pleasure in only a few activities. They may exhibit a general absence of affective range, which contributes to their characterization as flat, blunted, or emotionally vacant. SPD is more commonly diagnosed in males and may be present in childhood and adolescence. These children are typified by solitariness, poor peer relationships, and underachievement in school. SPD impacts 3.1% to 4.9% of the United States population and approximately 1% of community populations.5,6
EVALUATION Persistent depressive symptoms
Ms. P is accompanied by her parents for the examination. She reports a chronic, persistent sad mood, hopelessness, anergia, insomnia, anhedonia, and decreased concentration and appetite. She says she experiences episodes of intense worry, along with tension, restlessness, feelings of being on the edge, irritability, and difficulty relaxing. Socially, she is withdrawn, preferring to stay alone in her room most of the day watching YouTube or trying to write stories. She has 2 friends with whom she does not interact with in person, but rather through digital means. Ms. P has never enjoyed attending school and feels “nervous” when she is around people. She has difficulty expressing her thoughts and often looks to her parents for help. Her parents add that getting Ms. P to attend school was a struggle, which resulted in periods of home schooling throughout high school.
The treating team prescribes citalopram, 10 mg/d, and aripiprazole, 2 mg/d. On subsequent follow-up visits, Ms. P’s depression improves with an increase in citalopram to 40 mg/d. Psychotherapy is added to her treatment plan to help address the persistent social deficits, odd behavior, and anxieties.
Continue to: Evaluation Psychological assessment...
EVALUATION Psychological assessment
At her psychotherapy intake appointment with the clinical neuropsychologist, Ms. P is dressed in purple from head to toe and sits clutching her purse and looking at the ground. She is overweight with clean, fitting clothing. Ms. P takes a secondary role during most of the interview, allowing her parents to answer most questions. When asked why she is starting therapy, Ms. P replies, “Well, I’ve been using the bathroom a lot.” She describes a feeling of comfort and calmness while in the restroom. Suddenly, she asks her parents to exit the exam room for a moment. Once they leave, she leans in and whispers, “Have you ever heard of self-sabotage? I think that’s what I’m doing.”
Her mood is euthymic, with a blunted affect. She scores 2 on the Patient Health Questionnaire-9 (PHQ-9) and 10 on the Generalized Anxiety Disorder 7-item scale (GAD-7), which indicates the positive impact of medication on her depressive symptoms but continuing moderate anxious distress. She endorses fear of the night, insomnia, and suicidal ideation. She reports an unusual “constant itching sensation,” resulting in hours of repetitive excoriation. Physical examination reveals several significant scars and scabs covering her bilateral upper and lower extremities. Her vocational history is brief; she had held 2 entry-level customer service positions that lasted <1 year. She was fired due to excessive bathroom use.
As the interview progresses, the intake clinician’s background in neuropsychological assessment facilitates screening for possible developmental disorders. Given the nature of the referral and psychotherapy intake, a full neuropsychological assessment is not conducted. The clinician emphasizes verbal abstraction and theory of mind. Ms. P’s IQ was estimated to be average by Wide Range Achievement Test 4 word reading and interview questions about her academic history. Questions are abstracted from the Autism Diagnostic Observation Schedule, Module 4, to assess for conversation ability, emotional insight, awareness and expression, relationships, and areas of functioning in daily living. Developmental history questions, such as those found on the Adaptive Behavior Assessment System, 3rd edition, help guide developmental information provided by parents in the areas of communication, emotion and eye-gaze, gestures, sensory function, language, social functioning, hygiene behavior, and specific interests.
Ms. P’s mother describes a normal pregnancy and delivery; however, she states that Ms. P was “born with problems,” including difficulty with rooting and sucking, and required gastrointestinal intubation until age 3. Cyclical vomiting followed normal food consumption. Ambulation, language acquisition, toilet training, and hygiene behavior were delayed. Ms. P experienced improvements with early intervention in intensive physical and occupational therapy.
Ms. P’s hygiene is well below average, and she requires cueing from her parents. She attended general education until she reached high school, when she began special education. She was sensitive to sensory stimulation from infancy, with sensory sensitivity to textures. Ms. P continues to report sensory sensitivity and lapses in hygiene.
She has difficulty establishing and maintaining relationships with her peers, and prefers solitary activities. Ms. P has no history of romantic relationships, although she does desire one. When asked about her understanding of various relationships, Ms. P’s responses are stereotyped, such as “I know someone is my friend because they are nice to me” and “People get married because they love each other.” She struggles to offer greater insight into the nuances that form lasting relationships and bonds. Ms. P struggles to imitate and describe the physical and internal cues of several basic emotions (eg, fear, joy, anger).
Her conversational and social skills are assessed by asking her to engage in a conversation with the examiner as if meeting for the first time. Her speech is reciprocal, aprosodic, and delayed. The conversation is one-sided, and the examiner fills in several awkward pauses. Ms. P’s gaze at times is intense and prolonged, especially when responding to questions. She tends to use descriptive statements (eg, “I like your purple pen, I like your shirt”) to engage in conversation, rather than gathering more information through reflective statements, questions, or expressing a shared interest.
Ms. P’s verbal abstraction is screened using questions from the Wechsler Adult Intelligence Scale, 4th edition Similarities subtest, to which she provides several responses within normal limits. Her understanding of colloquial speech is assessed by asking her the meaning of common phrases (eg, “Get knocked down 9 times, get up 10,” “Jack and Jill are 2 peas in a pod”). On many occasions, she is able to limit her response to 1 word, (eg, “resiliency”), demonstrating intact ability to decipher idioms.
[polldaddy:11027971]
The authors’ observations
Upon reflection of Ms. P’s clinical presentation and history of developmental delays, social deficits, sensory sensitivity since infancy, and repetitive behaviors (all which continue to impact her), the clinical team concluded that the diagnosis of autism spectrum disorder (ASD) helps explain the patient’s “odd” behaviors, more so than SPD.
ASD is a heterogenous, complex neuropsychiatric disorder characterized by a persistent deficit in social reciprocity, verbal, and nonverbal communication, and includes a pattern of restricted, repetitive and/or stereotyped behaviors and/or interests.5 The term “autismus” is Greek meaning “self,” and was first used to classify the qualities of “morbid self-admiration” observed in prodromal schizophrenia.7

To properly distinguish these disorders, keep in mind that patients with ASD have repetitive and restricted patterns of behaviors or interests that are not found in SPD, and experience deficits in forming, maintaining, and understanding relationships since they lack those skills, while patients with SPD are more prone to desire solitary activities and limited relationships.5,9
There has been an increased interest in determining why for some patients the diagnosis of ASD is delayed until they reach adulthood. Limited or no access to the patient’s childhood caregiver to obtain a developmental history, as well as generational differences on what constitutes typical childhood behavior, could contribute to a delayed diagnosis of ASD until adulthood. Some patients develop camouflaging strategies that allow them to navigate social expectations to a limited degree, such as learning stock phrases, imitating gestures, and telling anecdotes. Another factor to consider is that co-occurring psychiatric disorders may take center stage when patients present for mental health services.10 Fusar-Poli et al11 investigated the characteristics of patients who received a diagnosis of ASD in adulthood. They found that the median time from the initial clinical evaluation to diagnosis of ASD in adulthood was 11 years. In adults identified with ASD, their cognitive abilities ranged from average to above average, and they required less support. Additionally, they also had higher rates of being previously diagnosed with psychotic disorders and personality disorders.11
It is important to keep in mind that the wide spectrum of autism as currently defined by DSM-5 and its overlap of symptoms with other psychiatric disorders can make the diagnosis challenging for both child and adolescent psychiatrists and adult psychiatrists and might help explain why severe cases of ASD are more readily identified earlier than milder cases of ASD.10
Ms. P’s case is also an example of how women are more likely than men to be overlooked when evaluated for ASD. According to DSM-5, the estimated gender ratio for ASD is believed to be 4:1 (male:female).5 However, upon systematic review and meta-analysis, Loomes et al12 found that the gender ratio may be closer to 3:1 (male:female). These authors suggested that diagnostic bias and a failure of passive case ascertainment to estimate gender ratios as stated by DSM-5 in identifying ASD might explain the lower gender ratio.12 A growing body of evidence suggests that ASD is different in males and females. A 2019 qualitative study by Milner et al13 found that female participants reported using masking and camouflaging strategies to appear neurotypical. Compensatory behaviors were found to be linked to a delay in diagnosis and support for ASD.13
Cognitive ability as measured by IQ has also been found to be a factor in receiving a diagnosis of ASD. In a 2010 secondary analysis of a population-based study of the prevalence of ASD, Giarelli et al14found that girls with cognitive impairments as measured by IQ were less likely to be diagnosed with ASD than boys with cognitive impairment, despite meeting the criteria for ASD. Females tend to exhibit fewer repetitive behaviors than males, and tend to be more likely to show accompanying intellectual disability, which suggests that females with ASD may go unrecognized when they exhibit average intelligence with less impairment of behavior and subtler manifestation of social and communication deficits.15 Consequently, females tend to receive this diagnosis later than males.
Continue to: Treatment...
TREATMENT Adding CBT
At an interdisciplinary session several weeks later that includes Ms. P and her parents, the treatment team discusses the revised diagnoses of ASD and MDD, a treatment recommendation for cognitive-behavioral therapy (CBT), and continued use of medication. At this session, Ms. P discloses that she has not been consistent with her medication regimen since her last appointment, which helps explain the increase in her PHQ-9 score from 2 to 14 and GAD-7 score
[polldaddy:11027990]
The authors’ observations
CBT can be helpful in improving medication adherence, developing coping skills, and modifying maladaptive behaviors.

OUTCOME Improvement with psychotherapy
Ms. P and family agree with the team’s recommendations. The aims of Ms. P’s psychotherapy are to maintain medication compliance; implement behavioral modification, vocational rehabilitation, and community engagement; develop social skills; increase functional independence; and develop coping skills for depression and anxiety.
Bottom Line
The prevalence of schizoid personality disorder (SPD) is low, and its symptoms overlap with those of autism spectrum disorder. Therefore, before diagnosing SPD in an adult patient, it is important to obtain a detailed developmental history and include an interdisciplinary team to assess for autism spectrum disorder.
1. Fariba K, Gupta V. Schizoid personality disorder. StatPearls Publishing. Updated June 9, 2021. Accessed January 6, 2021. https://www.ncbi.nlm.nih.gov/books/NBK559234/
2. Diagnostic and Statistical Manual of Mental Disorders: DSM-III. 3rd ed rev. American Psychiatric Association; 1987.
3. Esterberg ML, Goulding SM, Walker EF. Cluster A personality disorders: schizotypal, schizoid and paranoid personality disorders in childhood and adolescence. J Psychopathol Behav Assess. 2010;32(4):515-528. doi:10.1007/s10862-010-9183-8
4. Kalus O, Bernstein DP, Siever LJ. Schizoid personality disorder: a review of current status and implications for DSM-IV. Journal of Personality Disorders. 1993;7(1), 43-52.
5. Diagnostic and Statistical Manual of Mental Disorders: DSM-5. 5th ed. American Psychiatric Association; 2013.
6. Eaton NR, Greene AL. Personality disorders: community prevalence and socio-demographic correlates. Curr Opin Psychol. 2018;21:28-32. doi:10.1016/j.copsyc.2017.09.001
7. Vatano
8. Ritsner MS. Handbook of Schizophrenia Spectrum Disorders, Volume I: Conceptual Issues and Neurobiological Advances. Springer; 2011.
9. Cook ML, Zhang Y, Constantino JN. On the continuity between autistic and schizoid personality disorder trait burden: a prospective study in adolescence. J Nerv Ment Dis. 2020;208(2):94-100. doi:10.1097/NMD.0000000000001105
10. Lai MC, Baron-Cohen S. Identifying the lost generation of adults with autism spectrum conditions. Lancet Psychiatry. 2015;2(11):1013-1027. doi:10.1016/S2215-0366(15)00277-1
11. Fusar-Poli L, Brondino N, Politi P, et al. Missed diagnoses and misdiagnoses of adults with autism spectrum disorder. Eur Arch Psychiatry Clin Neurosci. 2020;10.1007/s00406-020-01189-2. doi:10.1007/s00406-020-01189-w
12. Loomes R, Hull L, Mandy WPL. What is the male-to-female ratio in autism spectrum disorder? A systematic review and meta-analysis. J Am Acad Child Adolesc Psychiatry. 2017;56(6):466-474. doi:10.1016/j.jaac.2017.03.013
13. Milner V, McIntosh H, Colvert E, et al. A qualitative exploration of the female experience of autism spectrum disorder (ASD). J Autism Dev Disord. 2019;49(6):2389-2402. doi:10.1007/s10803-019-03906-4
14. Giarelli E, Wiggins LD, Rice CE, et al. Sex differences in the evaluation and diagnosis of autism spectrum disorders among children. Disabil Health J. 2010;3(2):107-116. doi:10.1016/j.dhjo.2009.07.001
15. Frazier TW, Georgiades S, Bishop SL, et al. Behavioral and cognitive characteristics of females and males with autism in the Simons Simplex Collection. J Am Acad Child Adolesc Psychiatry. 2014;53(3):329-40.e403. doi:10.1016/j.jaac.2013.12.004
16. Julius RJ, Novitsky MA Jr, et al. Medication adherence: a review of the literature and implications for clinical practice. J Psychiatr Pract. 2009;15(1):34-44. doi:10.1097/01.pra.0000344917.43780.77
17. Spain D, Sin J, Chalder T, et al. Cognitive behaviour therapy for adults with autism spectrum disorders and psychiatric co-morbidity: a review. Research in Autism Spectrum Disorders. 2015;9, 151-162. https://doi.org/10.1016/j.rasd.2014.10.019
18. Bishop-Fitzpatrick L, Minshew NJ, Eack SM. A systematic review of psychosocial interventions for adults with autism spectrum disorders. J Autism Dev Disord. 2013;43(3):687-694. doi:10.1007/s10803-012-1615-8
CASE Treatment-resistant MDD
Ms. P, age 21, presents to the outpatient clinic. She has diagnoses of treatment-resistant major depressive disorder (MDD) and schizoid personality disorder (SPD). Ms. P was diagnosed with MDD 3 years ago after reporting symptoms of prevailing sadness for approximately 8 years, described as feelings of worthlessness, anhedonia, social withdrawal, and decreased hygiene and self-care behaviors, as well as suicidal ideation and self-harm. SPD was diagnosed 1 year earlier based on her “odd” behaviors and disheveled appearance following observation and in collateral with her family. Her odd behaviors are described as spending most of her time alone, preferring solitary activities, and having little contact with people other than her parents.
Ms. P reports that she was previously treated with citalopram, 20 mg/d, bupropion, 150 mg/d, aripiprazole, 3.75 mg/d, topiramate, 100 mg twice daily, and melatonin, 9 mg/d at bedtime, but discontinued follow-up appointments and medications after no significant improvement in symptoms.
[polldaddy:11027942]
The authors’ observations
The term “schizoid” first made its debut in the medical community to describe the prodromal social withdrawal and isolation observed in schizophrenia.1 The use of schizoid to describe a personality type first occurred in DSM-III in 1980.2 SPD is a Cluster A personality disorder that groups personalities characterized by common traits that are “odd” or “eccentric” and may resemble the positive and/or negative symptoms of schizophrenia.3,4 Relatively uncommon in clinical settings, SPD includes individuals who do not desire or enjoy close relationships. Those afflicted with SPD will be described as isolated, aloof, and detached from social relationships with others, even immediate family members. Individuals with SPD may appear indifferent to criticism and praise, and may take pleasure in only a few activities. They may exhibit a general absence of affective range, which contributes to their characterization as flat, blunted, or emotionally vacant. SPD is more commonly diagnosed in males and may be present in childhood and adolescence. These children are typified by solitariness, poor peer relationships, and underachievement in school. SPD impacts 3.1% to 4.9% of the United States population and approximately 1% of community populations.5,6
EVALUATION Persistent depressive symptoms
Ms. P is accompanied by her parents for the examination. She reports a chronic, persistent sad mood, hopelessness, anergia, insomnia, anhedonia, and decreased concentration and appetite. She says she experiences episodes of intense worry, along with tension, restlessness, feelings of being on the edge, irritability, and difficulty relaxing. Socially, she is withdrawn, preferring to stay alone in her room most of the day watching YouTube or trying to write stories. She has 2 friends with whom she does not interact with in person, but rather through digital means. Ms. P has never enjoyed attending school and feels “nervous” when she is around people. She has difficulty expressing her thoughts and often looks to her parents for help. Her parents add that getting Ms. P to attend school was a struggle, which resulted in periods of home schooling throughout high school.
The treating team prescribes citalopram, 10 mg/d, and aripiprazole, 2 mg/d. On subsequent follow-up visits, Ms. P’s depression improves with an increase in citalopram to 40 mg/d. Psychotherapy is added to her treatment plan to help address the persistent social deficits, odd behavior, and anxieties.
Continue to: Evaluation Psychological assessment...
EVALUATION Psychological assessment
At her psychotherapy intake appointment with the clinical neuropsychologist, Ms. P is dressed in purple from head to toe and sits clutching her purse and looking at the ground. She is overweight with clean, fitting clothing. Ms. P takes a secondary role during most of the interview, allowing her parents to answer most questions. When asked why she is starting therapy, Ms. P replies, “Well, I’ve been using the bathroom a lot.” She describes a feeling of comfort and calmness while in the restroom. Suddenly, she asks her parents to exit the exam room for a moment. Once they leave, she leans in and whispers, “Have you ever heard of self-sabotage? I think that’s what I’m doing.”
Her mood is euthymic, with a blunted affect. She scores 2 on the Patient Health Questionnaire-9 (PHQ-9) and 10 on the Generalized Anxiety Disorder 7-item scale (GAD-7), which indicates the positive impact of medication on her depressive symptoms but continuing moderate anxious distress. She endorses fear of the night, insomnia, and suicidal ideation. She reports an unusual “constant itching sensation,” resulting in hours of repetitive excoriation. Physical examination reveals several significant scars and scabs covering her bilateral upper and lower extremities. Her vocational history is brief; she had held 2 entry-level customer service positions that lasted <1 year. She was fired due to excessive bathroom use.
As the interview progresses, the intake clinician’s background in neuropsychological assessment facilitates screening for possible developmental disorders. Given the nature of the referral and psychotherapy intake, a full neuropsychological assessment is not conducted. The clinician emphasizes verbal abstraction and theory of mind. Ms. P’s IQ was estimated to be average by Wide Range Achievement Test 4 word reading and interview questions about her academic history. Questions are abstracted from the Autism Diagnostic Observation Schedule, Module 4, to assess for conversation ability, emotional insight, awareness and expression, relationships, and areas of functioning in daily living. Developmental history questions, such as those found on the Adaptive Behavior Assessment System, 3rd edition, help guide developmental information provided by parents in the areas of communication, emotion and eye-gaze, gestures, sensory function, language, social functioning, hygiene behavior, and specific interests.
Ms. P’s mother describes a normal pregnancy and delivery; however, she states that Ms. P was “born with problems,” including difficulty with rooting and sucking, and required gastrointestinal intubation until age 3. Cyclical vomiting followed normal food consumption. Ambulation, language acquisition, toilet training, and hygiene behavior were delayed. Ms. P experienced improvements with early intervention in intensive physical and occupational therapy.
Ms. P’s hygiene is well below average, and she requires cueing from her parents. She attended general education until she reached high school, when she began special education. She was sensitive to sensory stimulation from infancy, with sensory sensitivity to textures. Ms. P continues to report sensory sensitivity and lapses in hygiene.
She has difficulty establishing and maintaining relationships with her peers, and prefers solitary activities. Ms. P has no history of romantic relationships, although she does desire one. When asked about her understanding of various relationships, Ms. P’s responses are stereotyped, such as “I know someone is my friend because they are nice to me” and “People get married because they love each other.” She struggles to offer greater insight into the nuances that form lasting relationships and bonds. Ms. P struggles to imitate and describe the physical and internal cues of several basic emotions (eg, fear, joy, anger).
Her conversational and social skills are assessed by asking her to engage in a conversation with the examiner as if meeting for the first time. Her speech is reciprocal, aprosodic, and delayed. The conversation is one-sided, and the examiner fills in several awkward pauses. Ms. P’s gaze at times is intense and prolonged, especially when responding to questions. She tends to use descriptive statements (eg, “I like your purple pen, I like your shirt”) to engage in conversation, rather than gathering more information through reflective statements, questions, or expressing a shared interest.
Ms. P’s verbal abstraction is screened using questions from the Wechsler Adult Intelligence Scale, 4th edition Similarities subtest, to which she provides several responses within normal limits. Her understanding of colloquial speech is assessed by asking her the meaning of common phrases (eg, “Get knocked down 9 times, get up 10,” “Jack and Jill are 2 peas in a pod”). On many occasions, she is able to limit her response to 1 word, (eg, “resiliency”), demonstrating intact ability to decipher idioms.
[polldaddy:11027971]
The authors’ observations
Upon reflection of Ms. P’s clinical presentation and history of developmental delays, social deficits, sensory sensitivity since infancy, and repetitive behaviors (all which continue to impact her), the clinical team concluded that the diagnosis of autism spectrum disorder (ASD) helps explain the patient’s “odd” behaviors, more so than SPD.
ASD is a heterogenous, complex neuropsychiatric disorder characterized by a persistent deficit in social reciprocity, verbal, and nonverbal communication, and includes a pattern of restricted, repetitive and/or stereotyped behaviors and/or interests.5 The term “autismus” is Greek meaning “self,” and was first used to classify the qualities of “morbid self-admiration” observed in prodromal schizophrenia.7

To properly distinguish these disorders, keep in mind that patients with ASD have repetitive and restricted patterns of behaviors or interests that are not found in SPD, and experience deficits in forming, maintaining, and understanding relationships since they lack those skills, while patients with SPD are more prone to desire solitary activities and limited relationships.5,9
There has been an increased interest in determining why for some patients the diagnosis of ASD is delayed until they reach adulthood. Limited or no access to the patient’s childhood caregiver to obtain a developmental history, as well as generational differences on what constitutes typical childhood behavior, could contribute to a delayed diagnosis of ASD until adulthood. Some patients develop camouflaging strategies that allow them to navigate social expectations to a limited degree, such as learning stock phrases, imitating gestures, and telling anecdotes. Another factor to consider is that co-occurring psychiatric disorders may take center stage when patients present for mental health services.10 Fusar-Poli et al11 investigated the characteristics of patients who received a diagnosis of ASD in adulthood. They found that the median time from the initial clinical evaluation to diagnosis of ASD in adulthood was 11 years. In adults identified with ASD, their cognitive abilities ranged from average to above average, and they required less support. Additionally, they also had higher rates of being previously diagnosed with psychotic disorders and personality disorders.11
It is important to keep in mind that the wide spectrum of autism as currently defined by DSM-5 and its overlap of symptoms with other psychiatric disorders can make the diagnosis challenging for both child and adolescent psychiatrists and adult psychiatrists and might help explain why severe cases of ASD are more readily identified earlier than milder cases of ASD.10
Ms. P’s case is also an example of how women are more likely than men to be overlooked when evaluated for ASD. According to DSM-5, the estimated gender ratio for ASD is believed to be 4:1 (male:female).5 However, upon systematic review and meta-analysis, Loomes et al12 found that the gender ratio may be closer to 3:1 (male:female). These authors suggested that diagnostic bias and a failure of passive case ascertainment to estimate gender ratios as stated by DSM-5 in identifying ASD might explain the lower gender ratio.12 A growing body of evidence suggests that ASD is different in males and females. A 2019 qualitative study by Milner et al13 found that female participants reported using masking and camouflaging strategies to appear neurotypical. Compensatory behaviors were found to be linked to a delay in diagnosis and support for ASD.13
Cognitive ability as measured by IQ has also been found to be a factor in receiving a diagnosis of ASD. In a 2010 secondary analysis of a population-based study of the prevalence of ASD, Giarelli et al14found that girls with cognitive impairments as measured by IQ were less likely to be diagnosed with ASD than boys with cognitive impairment, despite meeting the criteria for ASD. Females tend to exhibit fewer repetitive behaviors than males, and tend to be more likely to show accompanying intellectual disability, which suggests that females with ASD may go unrecognized when they exhibit average intelligence with less impairment of behavior and subtler manifestation of social and communication deficits.15 Consequently, females tend to receive this diagnosis later than males.
Continue to: Treatment...
TREATMENT Adding CBT
At an interdisciplinary session several weeks later that includes Ms. P and her parents, the treatment team discusses the revised diagnoses of ASD and MDD, a treatment recommendation for cognitive-behavioral therapy (CBT), and continued use of medication. At this session, Ms. P discloses that she has not been consistent with her medication regimen since her last appointment, which helps explain the increase in her PHQ-9 score from 2 to 14 and GAD-7 score
[polldaddy:11027990]
The authors’ observations
CBT can be helpful in improving medication adherence, developing coping skills, and modifying maladaptive behaviors.

OUTCOME Improvement with psychotherapy
Ms. P and family agree with the team’s recommendations. The aims of Ms. P’s psychotherapy are to maintain medication compliance; implement behavioral modification, vocational rehabilitation, and community engagement; develop social skills; increase functional independence; and develop coping skills for depression and anxiety.
Bottom Line
The prevalence of schizoid personality disorder (SPD) is low, and its symptoms overlap with those of autism spectrum disorder. Therefore, before diagnosing SPD in an adult patient, it is important to obtain a detailed developmental history and include an interdisciplinary team to assess for autism spectrum disorder.
CASE Treatment-resistant MDD
Ms. P, age 21, presents to the outpatient clinic. She has diagnoses of treatment-resistant major depressive disorder (MDD) and schizoid personality disorder (SPD). Ms. P was diagnosed with MDD 3 years ago after reporting symptoms of prevailing sadness for approximately 8 years, described as feelings of worthlessness, anhedonia, social withdrawal, and decreased hygiene and self-care behaviors, as well as suicidal ideation and self-harm. SPD was diagnosed 1 year earlier based on her “odd” behaviors and disheveled appearance following observation and in collateral with her family. Her odd behaviors are described as spending most of her time alone, preferring solitary activities, and having little contact with people other than her parents.
Ms. P reports that she was previously treated with citalopram, 20 mg/d, bupropion, 150 mg/d, aripiprazole, 3.75 mg/d, topiramate, 100 mg twice daily, and melatonin, 9 mg/d at bedtime, but discontinued follow-up appointments and medications after no significant improvement in symptoms.
[polldaddy:11027942]
The authors’ observations
The term “schizoid” first made its debut in the medical community to describe the prodromal social withdrawal and isolation observed in schizophrenia.1 The use of schizoid to describe a personality type first occurred in DSM-III in 1980.2 SPD is a Cluster A personality disorder that groups personalities characterized by common traits that are “odd” or “eccentric” and may resemble the positive and/or negative symptoms of schizophrenia.3,4 Relatively uncommon in clinical settings, SPD includes individuals who do not desire or enjoy close relationships. Those afflicted with SPD will be described as isolated, aloof, and detached from social relationships with others, even immediate family members. Individuals with SPD may appear indifferent to criticism and praise, and may take pleasure in only a few activities. They may exhibit a general absence of affective range, which contributes to their characterization as flat, blunted, or emotionally vacant. SPD is more commonly diagnosed in males and may be present in childhood and adolescence. These children are typified by solitariness, poor peer relationships, and underachievement in school. SPD impacts 3.1% to 4.9% of the United States population and approximately 1% of community populations.5,6
EVALUATION Persistent depressive symptoms
Ms. P is accompanied by her parents for the examination. She reports a chronic, persistent sad mood, hopelessness, anergia, insomnia, anhedonia, and decreased concentration and appetite. She says she experiences episodes of intense worry, along with tension, restlessness, feelings of being on the edge, irritability, and difficulty relaxing. Socially, she is withdrawn, preferring to stay alone in her room most of the day watching YouTube or trying to write stories. She has 2 friends with whom she does not interact with in person, but rather through digital means. Ms. P has never enjoyed attending school and feels “nervous” when she is around people. She has difficulty expressing her thoughts and often looks to her parents for help. Her parents add that getting Ms. P to attend school was a struggle, which resulted in periods of home schooling throughout high school.
The treating team prescribes citalopram, 10 mg/d, and aripiprazole, 2 mg/d. On subsequent follow-up visits, Ms. P’s depression improves with an increase in citalopram to 40 mg/d. Psychotherapy is added to her treatment plan to help address the persistent social deficits, odd behavior, and anxieties.
Continue to: Evaluation Psychological assessment...
EVALUATION Psychological assessment
At her psychotherapy intake appointment with the clinical neuropsychologist, Ms. P is dressed in purple from head to toe and sits clutching her purse and looking at the ground. She is overweight with clean, fitting clothing. Ms. P takes a secondary role during most of the interview, allowing her parents to answer most questions. When asked why she is starting therapy, Ms. P replies, “Well, I’ve been using the bathroom a lot.” She describes a feeling of comfort and calmness while in the restroom. Suddenly, she asks her parents to exit the exam room for a moment. Once they leave, she leans in and whispers, “Have you ever heard of self-sabotage? I think that’s what I’m doing.”
Her mood is euthymic, with a blunted affect. She scores 2 on the Patient Health Questionnaire-9 (PHQ-9) and 10 on the Generalized Anxiety Disorder 7-item scale (GAD-7), which indicates the positive impact of medication on her depressive symptoms but continuing moderate anxious distress. She endorses fear of the night, insomnia, and suicidal ideation. She reports an unusual “constant itching sensation,” resulting in hours of repetitive excoriation. Physical examination reveals several significant scars and scabs covering her bilateral upper and lower extremities. Her vocational history is brief; she had held 2 entry-level customer service positions that lasted <1 year. She was fired due to excessive bathroom use.
As the interview progresses, the intake clinician’s background in neuropsychological assessment facilitates screening for possible developmental disorders. Given the nature of the referral and psychotherapy intake, a full neuropsychological assessment is not conducted. The clinician emphasizes verbal abstraction and theory of mind. Ms. P’s IQ was estimated to be average by Wide Range Achievement Test 4 word reading and interview questions about her academic history. Questions are abstracted from the Autism Diagnostic Observation Schedule, Module 4, to assess for conversation ability, emotional insight, awareness and expression, relationships, and areas of functioning in daily living. Developmental history questions, such as those found on the Adaptive Behavior Assessment System, 3rd edition, help guide developmental information provided by parents in the areas of communication, emotion and eye-gaze, gestures, sensory function, language, social functioning, hygiene behavior, and specific interests.
Ms. P’s mother describes a normal pregnancy and delivery; however, she states that Ms. P was “born with problems,” including difficulty with rooting and sucking, and required gastrointestinal intubation until age 3. Cyclical vomiting followed normal food consumption. Ambulation, language acquisition, toilet training, and hygiene behavior were delayed. Ms. P experienced improvements with early intervention in intensive physical and occupational therapy.
Ms. P’s hygiene is well below average, and she requires cueing from her parents. She attended general education until she reached high school, when she began special education. She was sensitive to sensory stimulation from infancy, with sensory sensitivity to textures. Ms. P continues to report sensory sensitivity and lapses in hygiene.
She has difficulty establishing and maintaining relationships with her peers, and prefers solitary activities. Ms. P has no history of romantic relationships, although she does desire one. When asked about her understanding of various relationships, Ms. P’s responses are stereotyped, such as “I know someone is my friend because they are nice to me” and “People get married because they love each other.” She struggles to offer greater insight into the nuances that form lasting relationships and bonds. Ms. P struggles to imitate and describe the physical and internal cues of several basic emotions (eg, fear, joy, anger).
Her conversational and social skills are assessed by asking her to engage in a conversation with the examiner as if meeting for the first time. Her speech is reciprocal, aprosodic, and delayed. The conversation is one-sided, and the examiner fills in several awkward pauses. Ms. P’s gaze at times is intense and prolonged, especially when responding to questions. She tends to use descriptive statements (eg, “I like your purple pen, I like your shirt”) to engage in conversation, rather than gathering more information through reflective statements, questions, or expressing a shared interest.
Ms. P’s verbal abstraction is screened using questions from the Wechsler Adult Intelligence Scale, 4th edition Similarities subtest, to which she provides several responses within normal limits. Her understanding of colloquial speech is assessed by asking her the meaning of common phrases (eg, “Get knocked down 9 times, get up 10,” “Jack and Jill are 2 peas in a pod”). On many occasions, she is able to limit her response to 1 word, (eg, “resiliency”), demonstrating intact ability to decipher idioms.
[polldaddy:11027971]
The authors’ observations
Upon reflection of Ms. P’s clinical presentation and history of developmental delays, social deficits, sensory sensitivity since infancy, and repetitive behaviors (all which continue to impact her), the clinical team concluded that the diagnosis of autism spectrum disorder (ASD) helps explain the patient’s “odd” behaviors, more so than SPD.
ASD is a heterogenous, complex neuropsychiatric disorder characterized by a persistent deficit in social reciprocity, verbal, and nonverbal communication, and includes a pattern of restricted, repetitive and/or stereotyped behaviors and/or interests.5 The term “autismus” is Greek meaning “self,” and was first used to classify the qualities of “morbid self-admiration” observed in prodromal schizophrenia.7

To properly distinguish these disorders, keep in mind that patients with ASD have repetitive and restricted patterns of behaviors or interests that are not found in SPD, and experience deficits in forming, maintaining, and understanding relationships since they lack those skills, while patients with SPD are more prone to desire solitary activities and limited relationships.5,9
There has been an increased interest in determining why for some patients the diagnosis of ASD is delayed until they reach adulthood. Limited or no access to the patient’s childhood caregiver to obtain a developmental history, as well as generational differences on what constitutes typical childhood behavior, could contribute to a delayed diagnosis of ASD until adulthood. Some patients develop camouflaging strategies that allow them to navigate social expectations to a limited degree, such as learning stock phrases, imitating gestures, and telling anecdotes. Another factor to consider is that co-occurring psychiatric disorders may take center stage when patients present for mental health services.10 Fusar-Poli et al11 investigated the characteristics of patients who received a diagnosis of ASD in adulthood. They found that the median time from the initial clinical evaluation to diagnosis of ASD in adulthood was 11 years. In adults identified with ASD, their cognitive abilities ranged from average to above average, and they required less support. Additionally, they also had higher rates of being previously diagnosed with psychotic disorders and personality disorders.11
It is important to keep in mind that the wide spectrum of autism as currently defined by DSM-5 and its overlap of symptoms with other psychiatric disorders can make the diagnosis challenging for both child and adolescent psychiatrists and adult psychiatrists and might help explain why severe cases of ASD are more readily identified earlier than milder cases of ASD.10
Ms. P’s case is also an example of how women are more likely than men to be overlooked when evaluated for ASD. According to DSM-5, the estimated gender ratio for ASD is believed to be 4:1 (male:female).5 However, upon systematic review and meta-analysis, Loomes et al12 found that the gender ratio may be closer to 3:1 (male:female). These authors suggested that diagnostic bias and a failure of passive case ascertainment to estimate gender ratios as stated by DSM-5 in identifying ASD might explain the lower gender ratio.12 A growing body of evidence suggests that ASD is different in males and females. A 2019 qualitative study by Milner et al13 found that female participants reported using masking and camouflaging strategies to appear neurotypical. Compensatory behaviors were found to be linked to a delay in diagnosis and support for ASD.13
Cognitive ability as measured by IQ has also been found to be a factor in receiving a diagnosis of ASD. In a 2010 secondary analysis of a population-based study of the prevalence of ASD, Giarelli et al14found that girls with cognitive impairments as measured by IQ were less likely to be diagnosed with ASD than boys with cognitive impairment, despite meeting the criteria for ASD. Females tend to exhibit fewer repetitive behaviors than males, and tend to be more likely to show accompanying intellectual disability, which suggests that females with ASD may go unrecognized when they exhibit average intelligence with less impairment of behavior and subtler manifestation of social and communication deficits.15 Consequently, females tend to receive this diagnosis later than males.
Continue to: Treatment...
TREATMENT Adding CBT
At an interdisciplinary session several weeks later that includes Ms. P and her parents, the treatment team discusses the revised diagnoses of ASD and MDD, a treatment recommendation for cognitive-behavioral therapy (CBT), and continued use of medication. At this session, Ms. P discloses that she has not been consistent with her medication regimen since her last appointment, which helps explain the increase in her PHQ-9 score from 2 to 14 and GAD-7 score
[polldaddy:11027990]
The authors’ observations
CBT can be helpful in improving medication adherence, developing coping skills, and modifying maladaptive behaviors.

OUTCOME Improvement with psychotherapy
Ms. P and family agree with the team’s recommendations. The aims of Ms. P’s psychotherapy are to maintain medication compliance; implement behavioral modification, vocational rehabilitation, and community engagement; develop social skills; increase functional independence; and develop coping skills for depression and anxiety.
Bottom Line
The prevalence of schizoid personality disorder (SPD) is low, and its symptoms overlap with those of autism spectrum disorder. Therefore, before diagnosing SPD in an adult patient, it is important to obtain a detailed developmental history and include an interdisciplinary team to assess for autism spectrum disorder.
1. Fariba K, Gupta V. Schizoid personality disorder. StatPearls Publishing. Updated June 9, 2021. Accessed January 6, 2021. https://www.ncbi.nlm.nih.gov/books/NBK559234/
2. Diagnostic and Statistical Manual of Mental Disorders: DSM-III. 3rd ed rev. American Psychiatric Association; 1987.
3. Esterberg ML, Goulding SM, Walker EF. Cluster A personality disorders: schizotypal, schizoid and paranoid personality disorders in childhood and adolescence. J Psychopathol Behav Assess. 2010;32(4):515-528. doi:10.1007/s10862-010-9183-8
4. Kalus O, Bernstein DP, Siever LJ. Schizoid personality disorder: a review of current status and implications for DSM-IV. Journal of Personality Disorders. 1993;7(1), 43-52.
5. Diagnostic and Statistical Manual of Mental Disorders: DSM-5. 5th ed. American Psychiatric Association; 2013.
6. Eaton NR, Greene AL. Personality disorders: community prevalence and socio-demographic correlates. Curr Opin Psychol. 2018;21:28-32. doi:10.1016/j.copsyc.2017.09.001
7. Vatano
8. Ritsner MS. Handbook of Schizophrenia Spectrum Disorders, Volume I: Conceptual Issues and Neurobiological Advances. Springer; 2011.
9. Cook ML, Zhang Y, Constantino JN. On the continuity between autistic and schizoid personality disorder trait burden: a prospective study in adolescence. J Nerv Ment Dis. 2020;208(2):94-100. doi:10.1097/NMD.0000000000001105
10. Lai MC, Baron-Cohen S. Identifying the lost generation of adults with autism spectrum conditions. Lancet Psychiatry. 2015;2(11):1013-1027. doi:10.1016/S2215-0366(15)00277-1
11. Fusar-Poli L, Brondino N, Politi P, et al. Missed diagnoses and misdiagnoses of adults with autism spectrum disorder. Eur Arch Psychiatry Clin Neurosci. 2020;10.1007/s00406-020-01189-2. doi:10.1007/s00406-020-01189-w
12. Loomes R, Hull L, Mandy WPL. What is the male-to-female ratio in autism spectrum disorder? A systematic review and meta-analysis. J Am Acad Child Adolesc Psychiatry. 2017;56(6):466-474. doi:10.1016/j.jaac.2017.03.013
13. Milner V, McIntosh H, Colvert E, et al. A qualitative exploration of the female experience of autism spectrum disorder (ASD). J Autism Dev Disord. 2019;49(6):2389-2402. doi:10.1007/s10803-019-03906-4
14. Giarelli E, Wiggins LD, Rice CE, et al. Sex differences in the evaluation and diagnosis of autism spectrum disorders among children. Disabil Health J. 2010;3(2):107-116. doi:10.1016/j.dhjo.2009.07.001
15. Frazier TW, Georgiades S, Bishop SL, et al. Behavioral and cognitive characteristics of females and males with autism in the Simons Simplex Collection. J Am Acad Child Adolesc Psychiatry. 2014;53(3):329-40.e403. doi:10.1016/j.jaac.2013.12.004
16. Julius RJ, Novitsky MA Jr, et al. Medication adherence: a review of the literature and implications for clinical practice. J Psychiatr Pract. 2009;15(1):34-44. doi:10.1097/01.pra.0000344917.43780.77
17. Spain D, Sin J, Chalder T, et al. Cognitive behaviour therapy for adults with autism spectrum disorders and psychiatric co-morbidity: a review. Research in Autism Spectrum Disorders. 2015;9, 151-162. https://doi.org/10.1016/j.rasd.2014.10.019
18. Bishop-Fitzpatrick L, Minshew NJ, Eack SM. A systematic review of psychosocial interventions for adults with autism spectrum disorders. J Autism Dev Disord. 2013;43(3):687-694. doi:10.1007/s10803-012-1615-8
1. Fariba K, Gupta V. Schizoid personality disorder. StatPearls Publishing. Updated June 9, 2021. Accessed January 6, 2021. https://www.ncbi.nlm.nih.gov/books/NBK559234/
2. Diagnostic and Statistical Manual of Mental Disorders: DSM-III. 3rd ed rev. American Psychiatric Association; 1987.
3. Esterberg ML, Goulding SM, Walker EF. Cluster A personality disorders: schizotypal, schizoid and paranoid personality disorders in childhood and adolescence. J Psychopathol Behav Assess. 2010;32(4):515-528. doi:10.1007/s10862-010-9183-8
4. Kalus O, Bernstein DP, Siever LJ. Schizoid personality disorder: a review of current status and implications for DSM-IV. Journal of Personality Disorders. 1993;7(1), 43-52.
5. Diagnostic and Statistical Manual of Mental Disorders: DSM-5. 5th ed. American Psychiatric Association; 2013.
6. Eaton NR, Greene AL. Personality disorders: community prevalence and socio-demographic correlates. Curr Opin Psychol. 2018;21:28-32. doi:10.1016/j.copsyc.2017.09.001
7. Vatano
8. Ritsner MS. Handbook of Schizophrenia Spectrum Disorders, Volume I: Conceptual Issues and Neurobiological Advances. Springer; 2011.
9. Cook ML, Zhang Y, Constantino JN. On the continuity between autistic and schizoid personality disorder trait burden: a prospective study in adolescence. J Nerv Ment Dis. 2020;208(2):94-100. doi:10.1097/NMD.0000000000001105
10. Lai MC, Baron-Cohen S. Identifying the lost generation of adults with autism spectrum conditions. Lancet Psychiatry. 2015;2(11):1013-1027. doi:10.1016/S2215-0366(15)00277-1
11. Fusar-Poli L, Brondino N, Politi P, et al. Missed diagnoses and misdiagnoses of adults with autism spectrum disorder. Eur Arch Psychiatry Clin Neurosci. 2020;10.1007/s00406-020-01189-2. doi:10.1007/s00406-020-01189-w
12. Loomes R, Hull L, Mandy WPL. What is the male-to-female ratio in autism spectrum disorder? A systematic review and meta-analysis. J Am Acad Child Adolesc Psychiatry. 2017;56(6):466-474. doi:10.1016/j.jaac.2017.03.013
13. Milner V, McIntosh H, Colvert E, et al. A qualitative exploration of the female experience of autism spectrum disorder (ASD). J Autism Dev Disord. 2019;49(6):2389-2402. doi:10.1007/s10803-019-03906-4
14. Giarelli E, Wiggins LD, Rice CE, et al. Sex differences in the evaluation and diagnosis of autism spectrum disorders among children. Disabil Health J. 2010;3(2):107-116. doi:10.1016/j.dhjo.2009.07.001
15. Frazier TW, Georgiades S, Bishop SL, et al. Behavioral and cognitive characteristics of females and males with autism in the Simons Simplex Collection. J Am Acad Child Adolesc Psychiatry. 2014;53(3):329-40.e403. doi:10.1016/j.jaac.2013.12.004
16. Julius RJ, Novitsky MA Jr, et al. Medication adherence: a review of the literature and implications for clinical practice. J Psychiatr Pract. 2009;15(1):34-44. doi:10.1097/01.pra.0000344917.43780.77
17. Spain D, Sin J, Chalder T, et al. Cognitive behaviour therapy for adults with autism spectrum disorders and psychiatric co-morbidity: a review. Research in Autism Spectrum Disorders. 2015;9, 151-162. https://doi.org/10.1016/j.rasd.2014.10.019
18. Bishop-Fitzpatrick L, Minshew NJ, Eack SM. A systematic review of psychosocial interventions for adults with autism spectrum disorders. J Autism Dev Disord. 2013;43(3):687-694. doi:10.1007/s10803-012-1615-8
The skill of administering IM medications: 3 questions to consider
The intramuscular (IM) route is commonly used to administer medication in various clinical settings. Even when an IM medication is administered appropriately, patient factors such as high subcutaneous tissue, greater body mass index, and gender can lower the success rate of injections.1 A key but infrequently discussed issue is the skill of the individual administering the IM medication. Incorrectly administering an IM medication can lead to complications, such as abscesses, nerve injury, and skeletal muscle fibrosis.2 Poor IM injection technique can impact patient care and safety.1 For example, a poorly administered antipsychotic medication might lead to the patient receiving a subtherapeutic dose, and could prompt a clinician to ask, “Does this agitated patient need more emergent medication because the medication being given is not effective, or because the medication is not being administered properly?”
This article offers 3 questions to ask when clinicians are evaluating how IM medications are being administered in their clinical setting.
1. Who is administering the medication?
Is the person a registered nurse, licensed psychiatric technician, certified nursing assistant, licensed vocational nurse, or medical assistant? What a specific clinician is permitted to do in one state may not be permitted in another state. For example, in the state of Washington, under certain conditions a medical assistant is allowed to administer an IM medication.3
2. What is the individual’s training in administering IM medications?
Has the person been trained in the proper technique, depending on the body location? Is the injection being properly prepared? Is the correct needle gauge being used?
3. What is the individual’s comfort level with administering IM medications?
Is the person comfortable administering medication only when a patient is calm? Or are they comfortable administering medication when a patient is agitated and being physically held or in 4-point restraints, such as in inpatient psychiatric units or emergency departments?
1. Soliman E, Ranjan S, Xu T, et al. A narrative review of the success of intramuscular gluteal injections and its impact in psychiatry. Biodes Manuf. 2018;1(3):161-170.
2. Nicoll LH, Hesby A. Intramuscular injection: an integrative research review and guideline for evidence-based practice. Appl Nurs Res. 2002;15(3):149-162.
3. Washington State Legislature. WAC 246-827-0240. Medical assistant-certified—Administering medications and injections. Accessed January 10, 2022. https://apps.leg.wa.gov/wac/default.aspx?cite=246-827-0240
The intramuscular (IM) route is commonly used to administer medication in various clinical settings. Even when an IM medication is administered appropriately, patient factors such as high subcutaneous tissue, greater body mass index, and gender can lower the success rate of injections.1 A key but infrequently discussed issue is the skill of the individual administering the IM medication. Incorrectly administering an IM medication can lead to complications, such as abscesses, nerve injury, and skeletal muscle fibrosis.2 Poor IM injection technique can impact patient care and safety.1 For example, a poorly administered antipsychotic medication might lead to the patient receiving a subtherapeutic dose, and could prompt a clinician to ask, “Does this agitated patient need more emergent medication because the medication being given is not effective, or because the medication is not being administered properly?”
This article offers 3 questions to ask when clinicians are evaluating how IM medications are being administered in their clinical setting.
1. Who is administering the medication?
Is the person a registered nurse, licensed psychiatric technician, certified nursing assistant, licensed vocational nurse, or medical assistant? What a specific clinician is permitted to do in one state may not be permitted in another state. For example, in the state of Washington, under certain conditions a medical assistant is allowed to administer an IM medication.3
2. What is the individual’s training in administering IM medications?
Has the person been trained in the proper technique, depending on the body location? Is the injection being properly prepared? Is the correct needle gauge being used?
3. What is the individual’s comfort level with administering IM medications?
Is the person comfortable administering medication only when a patient is calm? Or are they comfortable administering medication when a patient is agitated and being physically held or in 4-point restraints, such as in inpatient psychiatric units or emergency departments?
The intramuscular (IM) route is commonly used to administer medication in various clinical settings. Even when an IM medication is administered appropriately, patient factors such as high subcutaneous tissue, greater body mass index, and gender can lower the success rate of injections.1 A key but infrequently discussed issue is the skill of the individual administering the IM medication. Incorrectly administering an IM medication can lead to complications, such as abscesses, nerve injury, and skeletal muscle fibrosis.2 Poor IM injection technique can impact patient care and safety.1 For example, a poorly administered antipsychotic medication might lead to the patient receiving a subtherapeutic dose, and could prompt a clinician to ask, “Does this agitated patient need more emergent medication because the medication being given is not effective, or because the medication is not being administered properly?”
This article offers 3 questions to ask when clinicians are evaluating how IM medications are being administered in their clinical setting.
1. Who is administering the medication?
Is the person a registered nurse, licensed psychiatric technician, certified nursing assistant, licensed vocational nurse, or medical assistant? What a specific clinician is permitted to do in one state may not be permitted in another state. For example, in the state of Washington, under certain conditions a medical assistant is allowed to administer an IM medication.3
2. What is the individual’s training in administering IM medications?
Has the person been trained in the proper technique, depending on the body location? Is the injection being properly prepared? Is the correct needle gauge being used?
3. What is the individual’s comfort level with administering IM medications?
Is the person comfortable administering medication only when a patient is calm? Or are they comfortable administering medication when a patient is agitated and being physically held or in 4-point restraints, such as in inpatient psychiatric units or emergency departments?
1. Soliman E, Ranjan S, Xu T, et al. A narrative review of the success of intramuscular gluteal injections and its impact in psychiatry. Biodes Manuf. 2018;1(3):161-170.
2. Nicoll LH, Hesby A. Intramuscular injection: an integrative research review and guideline for evidence-based practice. Appl Nurs Res. 2002;15(3):149-162.
3. Washington State Legislature. WAC 246-827-0240. Medical assistant-certified—Administering medications and injections. Accessed January 10, 2022. https://apps.leg.wa.gov/wac/default.aspx?cite=246-827-0240
1. Soliman E, Ranjan S, Xu T, et al. A narrative review of the success of intramuscular gluteal injections and its impact in psychiatry. Biodes Manuf. 2018;1(3):161-170.
2. Nicoll LH, Hesby A. Intramuscular injection: an integrative research review and guideline for evidence-based practice. Appl Nurs Res. 2002;15(3):149-162.
3. Washington State Legislature. WAC 246-827-0240. Medical assistant-certified—Administering medications and injections. Accessed January 10, 2022. https://apps.leg.wa.gov/wac/default.aspx?cite=246-827-0240
Intermittent fasting: What to tell patients
Intermittent fasting is the purposeful, restricted intake of food (and sometimes water), usually for health or religious reasons. Common forms are alternative-day fasting or time-restricted fasting, with variable ratios of days or hours for fasting and eating/drinking.1 For example, fasting during Ramadan, the ninth month of the Islamic calendar, occurs from dawn to sunset, for a variable duration due to latitude and seasonal shifts.2 Clinicians are likely to care for a patient who occasionally fasts. While there are potential benefits of fasting, clinicians need to consider the implications for patients who fast, particularly those receiving psychotropic medications.
Potential benefits for weight loss, mood
Some research suggests fasting is popular and may have benefits for an individual’s physical and mental health. In a 2020 online poll (N = 1,241), 24% of respondents said they had tried intermittent fasting, and 87% said the practice was very effective (50%) or somewhat effective (37%) in helping them lose weight.3 While more randomized control trials are needed to examine the practice’s effectiveness in promoting and maintaining weight loss, fasting has been linked to better glucose control in both humans and animals, and patients may have better adherence with fasting compared to caloric restriction alone.1 Improved mood, alertness, tranquility, and sometimes euphoria have been documented among individuals who fast, but these benefits may not be sustained.4 A prospective study of 462 participants who fasted during Ramadan found the practice reduced depression in patients with diabetes, possibly due to mindfulness, decreased inflammation from improved insulin sensitivity, and/or social cohesion.5
Be aware of the potential risks
Fasting may either improve or destabilize mood in people with bipolar disorder by disrupting circadian rhythm and sleep.2 Fasting might exacerbate underlying eating disorders.2 Increased dehydration escalates the risk for orthostatic hypotension, which might require discontinuing clozapine.6 Hypotension and toxicity might arise during lithium pharmacotherapy. The Table4 summarizes things to consider when caring for a patient who fasts while receiving pharmacotherapy.

Provide patients with guidance
Advise patients not to fast if you believe it might exacerbate their mental illness, and encourage them to discuss with their primary care physicians any potential worsening of physical illnesses.2 When caring for a patient who fasts for religious reasons, consider consulting with the patient’s religious leaders.2 If patients choose to fast, monitor them for mood destabilization and/or medication adverse effects. If possible, avoid altering drug treatment regimens during fasting, and carefully monitor whenever a pharmaceutical change is necessary. When appropriate, the use of long-acting injectable medications may minimize adverse effects while maintaining mood stability. Encourage patients who fast to ensure they remain hydrated and practice sleep hygiene while they fast.7
1. Dong TA, Sandesara PB, Dhindsa DS, et al. Intermittent fasting: a heart healthy dietary pattern? Am J Med. 2020;133(8):901-907.
2. Fond G, Macgregor A, Leboyer M, et al. Fasting in mood disorders: neurobiology and effectiveness. A review of the literature. Psychiatry Res. 2013;209(3):253-258.
3. Ballard J. Americans say this popular diet is effective and inexpensive. YouGov. February 24, 2020. Accessed January 6, 2022. https://today.yougov.com/topics/food/articles-reports/2020/02/24/most-effective-diet-intermittent-fasting-poll
4. Furqan Z, Awaad R, Kurdyak P, et al. Considerations for clinicians treating Muslim patients with psychiatric disorders during Ramadan. Lancet Psychiatry. 2019;6(7):556-557.
5. Al-Ozairi E, AlAwadhi MM, Al-Ozairi A, et al. A prospective study of the effect of fasting during the month of Ramadan on depression and diabetes distress in people with type 2 diabetes. Diabet Res Clin Pract. 2019;153:145-149.
6. Chehovich C, Demler TL, Leppien E. Impact of Ramadan fasting on medical and psychiatric health. Int Clin Psychopharmacol. 2019;34(6):317-322.
7. Farooq S, Nazar Z, Akhtar J, et al. Effect of fasting during Ramadan on serum lithium level and mental state in bipolar affective disorder. Int Clin Psychopharmacol. 2010;25(6):323-327.
Intermittent fasting is the purposeful, restricted intake of food (and sometimes water), usually for health or religious reasons. Common forms are alternative-day fasting or time-restricted fasting, with variable ratios of days or hours for fasting and eating/drinking.1 For example, fasting during Ramadan, the ninth month of the Islamic calendar, occurs from dawn to sunset, for a variable duration due to latitude and seasonal shifts.2 Clinicians are likely to care for a patient who occasionally fasts. While there are potential benefits of fasting, clinicians need to consider the implications for patients who fast, particularly those receiving psychotropic medications.
Potential benefits for weight loss, mood
Some research suggests fasting is popular and may have benefits for an individual’s physical and mental health. In a 2020 online poll (N = 1,241), 24% of respondents said they had tried intermittent fasting, and 87% said the practice was very effective (50%) or somewhat effective (37%) in helping them lose weight.3 While more randomized control trials are needed to examine the practice’s effectiveness in promoting and maintaining weight loss, fasting has been linked to better glucose control in both humans and animals, and patients may have better adherence with fasting compared to caloric restriction alone.1 Improved mood, alertness, tranquility, and sometimes euphoria have been documented among individuals who fast, but these benefits may not be sustained.4 A prospective study of 462 participants who fasted during Ramadan found the practice reduced depression in patients with diabetes, possibly due to mindfulness, decreased inflammation from improved insulin sensitivity, and/or social cohesion.5
Be aware of the potential risks
Fasting may either improve or destabilize mood in people with bipolar disorder by disrupting circadian rhythm and sleep.2 Fasting might exacerbate underlying eating disorders.2 Increased dehydration escalates the risk for orthostatic hypotension, which might require discontinuing clozapine.6 Hypotension and toxicity might arise during lithium pharmacotherapy. The Table4 summarizes things to consider when caring for a patient who fasts while receiving pharmacotherapy.

Provide patients with guidance
Advise patients not to fast if you believe it might exacerbate their mental illness, and encourage them to discuss with their primary care physicians any potential worsening of physical illnesses.2 When caring for a patient who fasts for religious reasons, consider consulting with the patient’s religious leaders.2 If patients choose to fast, monitor them for mood destabilization and/or medication adverse effects. If possible, avoid altering drug treatment regimens during fasting, and carefully monitor whenever a pharmaceutical change is necessary. When appropriate, the use of long-acting injectable medications may minimize adverse effects while maintaining mood stability. Encourage patients who fast to ensure they remain hydrated and practice sleep hygiene while they fast.7
Intermittent fasting is the purposeful, restricted intake of food (and sometimes water), usually for health or religious reasons. Common forms are alternative-day fasting or time-restricted fasting, with variable ratios of days or hours for fasting and eating/drinking.1 For example, fasting during Ramadan, the ninth month of the Islamic calendar, occurs from dawn to sunset, for a variable duration due to latitude and seasonal shifts.2 Clinicians are likely to care for a patient who occasionally fasts. While there are potential benefits of fasting, clinicians need to consider the implications for patients who fast, particularly those receiving psychotropic medications.
Potential benefits for weight loss, mood
Some research suggests fasting is popular and may have benefits for an individual’s physical and mental health. In a 2020 online poll (N = 1,241), 24% of respondents said they had tried intermittent fasting, and 87% said the practice was very effective (50%) or somewhat effective (37%) in helping them lose weight.3 While more randomized control trials are needed to examine the practice’s effectiveness in promoting and maintaining weight loss, fasting has been linked to better glucose control in both humans and animals, and patients may have better adherence with fasting compared to caloric restriction alone.1 Improved mood, alertness, tranquility, and sometimes euphoria have been documented among individuals who fast, but these benefits may not be sustained.4 A prospective study of 462 participants who fasted during Ramadan found the practice reduced depression in patients with diabetes, possibly due to mindfulness, decreased inflammation from improved insulin sensitivity, and/or social cohesion.5
Be aware of the potential risks
Fasting may either improve or destabilize mood in people with bipolar disorder by disrupting circadian rhythm and sleep.2 Fasting might exacerbate underlying eating disorders.2 Increased dehydration escalates the risk for orthostatic hypotension, which might require discontinuing clozapine.6 Hypotension and toxicity might arise during lithium pharmacotherapy. The Table4 summarizes things to consider when caring for a patient who fasts while receiving pharmacotherapy.

Provide patients with guidance
Advise patients not to fast if you believe it might exacerbate their mental illness, and encourage them to discuss with their primary care physicians any potential worsening of physical illnesses.2 When caring for a patient who fasts for religious reasons, consider consulting with the patient’s religious leaders.2 If patients choose to fast, monitor them for mood destabilization and/or medication adverse effects. If possible, avoid altering drug treatment regimens during fasting, and carefully monitor whenever a pharmaceutical change is necessary. When appropriate, the use of long-acting injectable medications may minimize adverse effects while maintaining mood stability. Encourage patients who fast to ensure they remain hydrated and practice sleep hygiene while they fast.7
1. Dong TA, Sandesara PB, Dhindsa DS, et al. Intermittent fasting: a heart healthy dietary pattern? Am J Med. 2020;133(8):901-907.
2. Fond G, Macgregor A, Leboyer M, et al. Fasting in mood disorders: neurobiology and effectiveness. A review of the literature. Psychiatry Res. 2013;209(3):253-258.
3. Ballard J. Americans say this popular diet is effective and inexpensive. YouGov. February 24, 2020. Accessed January 6, 2022. https://today.yougov.com/topics/food/articles-reports/2020/02/24/most-effective-diet-intermittent-fasting-poll
4. Furqan Z, Awaad R, Kurdyak P, et al. Considerations for clinicians treating Muslim patients with psychiatric disorders during Ramadan. Lancet Psychiatry. 2019;6(7):556-557.
5. Al-Ozairi E, AlAwadhi MM, Al-Ozairi A, et al. A prospective study of the effect of fasting during the month of Ramadan on depression and diabetes distress in people with type 2 diabetes. Diabet Res Clin Pract. 2019;153:145-149.
6. Chehovich C, Demler TL, Leppien E. Impact of Ramadan fasting on medical and psychiatric health. Int Clin Psychopharmacol. 2019;34(6):317-322.
7. Farooq S, Nazar Z, Akhtar J, et al. Effect of fasting during Ramadan on serum lithium level and mental state in bipolar affective disorder. Int Clin Psychopharmacol. 2010;25(6):323-327.
1. Dong TA, Sandesara PB, Dhindsa DS, et al. Intermittent fasting: a heart healthy dietary pattern? Am J Med. 2020;133(8):901-907.
2. Fond G, Macgregor A, Leboyer M, et al. Fasting in mood disorders: neurobiology and effectiveness. A review of the literature. Psychiatry Res. 2013;209(3):253-258.
3. Ballard J. Americans say this popular diet is effective and inexpensive. YouGov. February 24, 2020. Accessed January 6, 2022. https://today.yougov.com/topics/food/articles-reports/2020/02/24/most-effective-diet-intermittent-fasting-poll
4. Furqan Z, Awaad R, Kurdyak P, et al. Considerations for clinicians treating Muslim patients with psychiatric disorders during Ramadan. Lancet Psychiatry. 2019;6(7):556-557.
5. Al-Ozairi E, AlAwadhi MM, Al-Ozairi A, et al. A prospective study of the effect of fasting during the month of Ramadan on depression and diabetes distress in people with type 2 diabetes. Diabet Res Clin Pract. 2019;153:145-149.
6. Chehovich C, Demler TL, Leppien E. Impact of Ramadan fasting on medical and psychiatric health. Int Clin Psychopharmacol. 2019;34(6):317-322.
7. Farooq S, Nazar Z, Akhtar J, et al. Effect of fasting during Ramadan on serum lithium level and mental state in bipolar affective disorder. Int Clin Psychopharmacol. 2010;25(6):323-327.
Rheumatology News celebrates 20 years
This time around, we’ll examine the first issue of Rheumatology News, which was published in February 2002. You can read the first-ever issue at the "PDF Download" link above.
In that premiere issue, information about early treatment of rheumatoid arthritis with tumor necrosis factor inhibitors and other disease-modifying antirheumatic drugs (DMARDs) featured prominently. A front-page story described new findings showing that early treatment of RA with DMARDs could reduce disease-related disability by one-third or more. A second article described quantitative improvement in MRI-detected synovitis in patients with early RA who were treated with infliximab for 14 weeks, while another reported on clinically relevant responses seen in 66% of patients treated with adalimumab plus methotrexate in a trial of patients with an inadequate response to methotrexate alone.
In other RA news, a report focused on low rates of preventive health care services and screening for other disorders in women, including Pap smears, mammograms, and influenza vaccinations. Another story suggested the possibility that methotrexate may elevate cancer risk in patients with RA. An analysis of two separate prospective studies indicated that women who regularly drink decaffeinated coffee may be at higher risk for developing RA.
Another page 1 story examined the potential of new drugs bosentan and epoprostenol for treating pulmonary arterial hypertension associated with various forms of connective tissue disease.
Etanercept was the focus of two articles, one announcing its approval for psoriatic arthritis, and another describing a small trial of the biologic in treating moderate to severe ankylosing spondylitis.
In osteoarthritis news, a front-page report described two placebo-controlled studies of oral glucosamine sulfate supplementation that suggested the formulation might slow the progression of joint space narrowing in postmenopausal women, and another article noted how a combined formulation of tramadol and acetaminophen reduced OA pain flares.
Readers were also treated to a pro and con editorial debate between Frederick Wolfe, MD, and Thomas J. Romano, MD, on whether trauma causes fibromyalgia.
Looking ahead
Throughout 2022, look for articles examining the past and future of rheumatology, including:
- The rise of women in the field;
- the rise of biologic and targeted synthetic disease-modifying antirheumatic drugs;
- the history and ongoing influence of OMERACT (Outcome Measures in Rheumatology);
- the growth and future of ACR-EULAR collaborations;
- progress and future directions of pediatric rheumatology; and
- the growth in understanding how sociodemographics and racial/ethnic identity affect access to and acceptance and receipt of rheumatologic care.
Are there any topics you think would be valuable to cover in light of Rheumatology News’ 20th anniversary? The editorial staff welcomes your suggestions. Please share them by emailing us at [email protected].
This time around, we’ll examine the first issue of Rheumatology News, which was published in February 2002. You can read the first-ever issue at the "PDF Download" link above.
In that premiere issue, information about early treatment of rheumatoid arthritis with tumor necrosis factor inhibitors and other disease-modifying antirheumatic drugs (DMARDs) featured prominently. A front-page story described new findings showing that early treatment of RA with DMARDs could reduce disease-related disability by one-third or more. A second article described quantitative improvement in MRI-detected synovitis in patients with early RA who were treated with infliximab for 14 weeks, while another reported on clinically relevant responses seen in 66% of patients treated with adalimumab plus methotrexate in a trial of patients with an inadequate response to methotrexate alone.
In other RA news, a report focused on low rates of preventive health care services and screening for other disorders in women, including Pap smears, mammograms, and influenza vaccinations. Another story suggested the possibility that methotrexate may elevate cancer risk in patients with RA. An analysis of two separate prospective studies indicated that women who regularly drink decaffeinated coffee may be at higher risk for developing RA.
Another page 1 story examined the potential of new drugs bosentan and epoprostenol for treating pulmonary arterial hypertension associated with various forms of connective tissue disease.
Etanercept was the focus of two articles, one announcing its approval for psoriatic arthritis, and another describing a small trial of the biologic in treating moderate to severe ankylosing spondylitis.
In osteoarthritis news, a front-page report described two placebo-controlled studies of oral glucosamine sulfate supplementation that suggested the formulation might slow the progression of joint space narrowing in postmenopausal women, and another article noted how a combined formulation of tramadol and acetaminophen reduced OA pain flares.
Readers were also treated to a pro and con editorial debate between Frederick Wolfe, MD, and Thomas J. Romano, MD, on whether trauma causes fibromyalgia.
Looking ahead
Throughout 2022, look for articles examining the past and future of rheumatology, including:
- The rise of women in the field;
- the rise of biologic and targeted synthetic disease-modifying antirheumatic drugs;
- the history and ongoing influence of OMERACT (Outcome Measures in Rheumatology);
- the growth and future of ACR-EULAR collaborations;
- progress and future directions of pediatric rheumatology; and
- the growth in understanding how sociodemographics and racial/ethnic identity affect access to and acceptance and receipt of rheumatologic care.
Are there any topics you think would be valuable to cover in light of Rheumatology News’ 20th anniversary? The editorial staff welcomes your suggestions. Please share them by emailing us at [email protected].
This time around, we’ll examine the first issue of Rheumatology News, which was published in February 2002. You can read the first-ever issue at the "PDF Download" link above.
In that premiere issue, information about early treatment of rheumatoid arthritis with tumor necrosis factor inhibitors and other disease-modifying antirheumatic drugs (DMARDs) featured prominently. A front-page story described new findings showing that early treatment of RA with DMARDs could reduce disease-related disability by one-third or more. A second article described quantitative improvement in MRI-detected synovitis in patients with early RA who were treated with infliximab for 14 weeks, while another reported on clinically relevant responses seen in 66% of patients treated with adalimumab plus methotrexate in a trial of patients with an inadequate response to methotrexate alone.
In other RA news, a report focused on low rates of preventive health care services and screening for other disorders in women, including Pap smears, mammograms, and influenza vaccinations. Another story suggested the possibility that methotrexate may elevate cancer risk in patients with RA. An analysis of two separate prospective studies indicated that women who regularly drink decaffeinated coffee may be at higher risk for developing RA.
Another page 1 story examined the potential of new drugs bosentan and epoprostenol for treating pulmonary arterial hypertension associated with various forms of connective tissue disease.
Etanercept was the focus of two articles, one announcing its approval for psoriatic arthritis, and another describing a small trial of the biologic in treating moderate to severe ankylosing spondylitis.
In osteoarthritis news, a front-page report described two placebo-controlled studies of oral glucosamine sulfate supplementation that suggested the formulation might slow the progression of joint space narrowing in postmenopausal women, and another article noted how a combined formulation of tramadol and acetaminophen reduced OA pain flares.
Readers were also treated to a pro and con editorial debate between Frederick Wolfe, MD, and Thomas J. Romano, MD, on whether trauma causes fibromyalgia.
Looking ahead
Throughout 2022, look for articles examining the past and future of rheumatology, including:
- The rise of women in the field;
- the rise of biologic and targeted synthetic disease-modifying antirheumatic drugs;
- the history and ongoing influence of OMERACT (Outcome Measures in Rheumatology);
- the growth and future of ACR-EULAR collaborations;
- progress and future directions of pediatric rheumatology; and
- the growth in understanding how sociodemographics and racial/ethnic identity affect access to and acceptance and receipt of rheumatologic care.
Are there any topics you think would be valuable to cover in light of Rheumatology News’ 20th anniversary? The editorial staff welcomes your suggestions. Please share them by emailing us at [email protected].
The management of inflammatory bowel disease in pregnancy
Inflammatory bowel disease (IBD) incidence is rising globally.1-3 In the United States, we have seen a 123% increase in prevalence of IBD among adults and a 133% increase among children from 2007 to 2016, with an annual percentage change of 9.9%.1 The rise of IBD in young people, and the overall higher prevalence in women compared with men, make pregnancy and IBD a topic of increasing importance for gastroenterologists.1 Here, we will discuss management and expectations in women with IBD before conception, during pregnancy, and post partum.
Preconception
Disease activity
Achieving both clinical and endoscopic remission of disease prior to conception is the key to ensuring the best maternal and fetal outcomes. Patients with IBD who conceive while in remission remain in remission 80% of the time.4,5 On the other hand, those who conceive while their disease is active may continue to have active or worsening disease in nearly 70% of cases.4 Active disease has been associated with an increased incidence of preterm birth, low birth weight, and small-for-gestational-age birth.6-8 Active disease can also exacerbate malnutrition and result in poor maternal weight gain, which is associated with intrauterine growth restriction.9,7 Pregnancy outcomes in patients with IBD and quiescent disease are similar to those in the general population.10,11
Health care maintenance
Optimizing maternal health prior to conception is critical. Alcohol, tobacco, recreational drugs, and marijuana should all be avoided. Opioids should be tapered off prior to conception, as continued use may result in neonatal opioid withdrawal syndrome and long-term neurodevelopmental consequences.12,13 In addition, aiming for a healthy body mass index between 18 and 25 months prior to conception allows for better overall pregnancy outcomes.13 Appropriate cancer screening includes colon cancer screening in those with more than 8 years of colitis, regular pap smear for cervical cancer, and annual total body skin cancer examinations for patients on thiopurines and biologic therapies.14
Nutrition
Folic acid supplementation with at least 400 micrograms (mcg) daily is necessary for all women planning pregnancy. Patients with small bowel involvement or history of small bowel resection should have a folate intake of a minimum of 2 grams per day. Adequate vitamin D levels (at least 20 ng/mL) are recommended in all women with IBD. Those with malabsorption should be screened for deficiencies in vitamin B12, folate, and iron.13 These nutritional markers should be evaluated prepregnancy, during the first trimester, and thereafter as needed.15-18
Preconception counseling
Steroid-free remission for at least 3 months prior to conception is recommended and is associated with reduced risk of flare during pregnancy.16,19 IBD medications needed to control disease activity are generally safe preconception and during pregnancy, with some exception (Table).
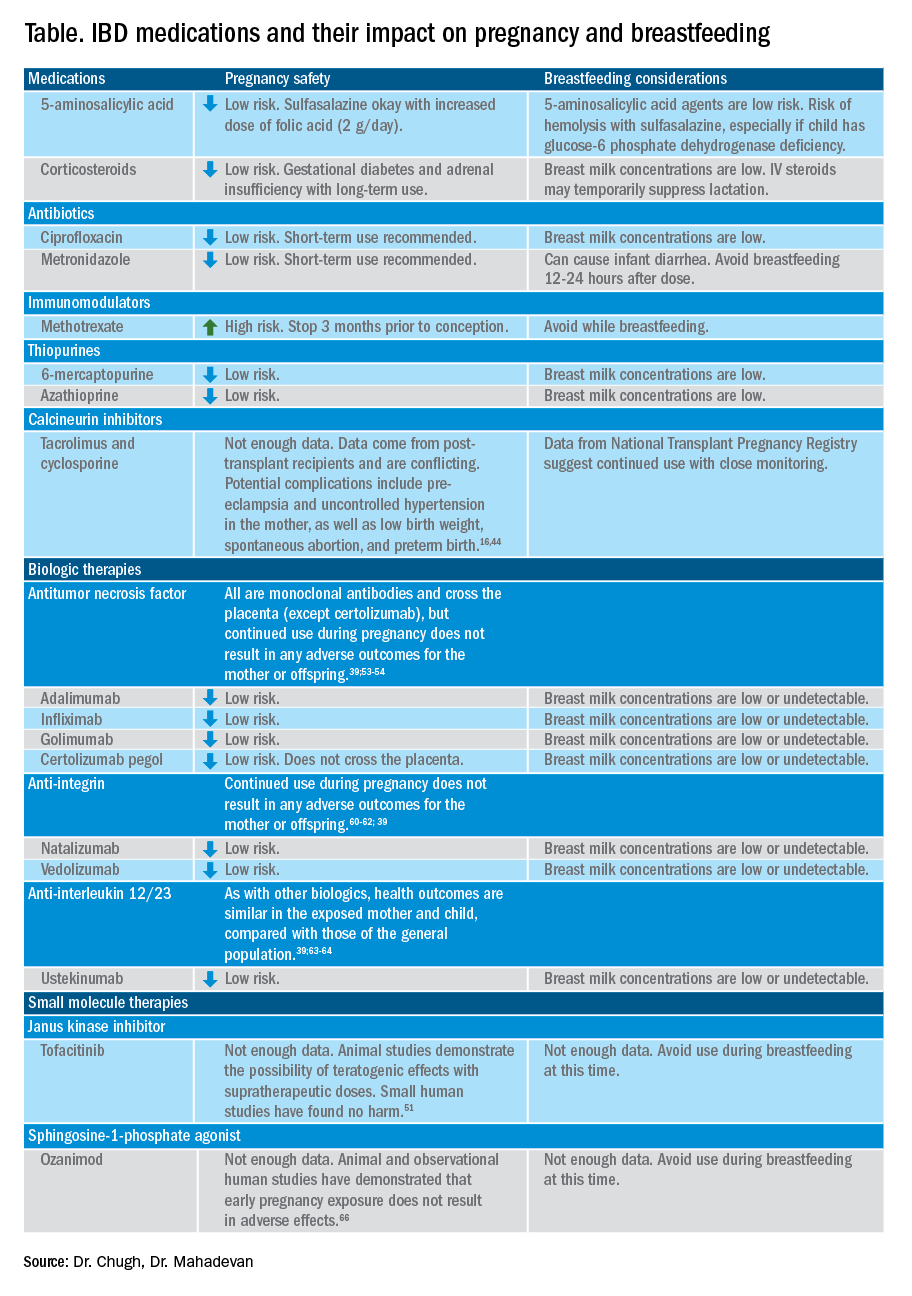
Misconceptions regarding heritability of IBD have sometimes discouraged men and women from having children. While genetics may increase susceptibility, environmental and other factors are involved as well. The concordance rates for monozygotic twins range from 33.3%-58.3% for Crohn’s disease and 13.4%-27.9% for ulcerative colitis (UC).20 The risk of a child developing IBD is higher in those who have multiple relatives with IBD and whose parents had IBD at the time of conception.21 While genetic testing for IBD loci is available, it is not commonly performed at this time as many genes are involved.22
Pregnancy
Coordinated care
A complete team of specialists with coordinated care among all providers is needed for optimal maternal and fetal outcomes.23,24 A gastroenterologist, ideally an IBD specialist, should follow the patient throughout pregnancy, seeing the patient at least once during the first or second trimester and as needed during pregnancy.16 A high-risk obstetrician or maternal-fetal medicine specialist should be involved early in pregnancy, as well. Open communication among all disciplines ensures that a common message is conveyed to the patient.16,24 A nutritionist, mental health provider, and lactation specialist knowledgeable about IBD drugs may be of assistance, as well.16
Disease activity
While women with IBD are at increased risk of spontaneous abortion, preterm birth, and labor complications, this risk is mitigated by controlling disease activity.25 The risk of preterm birth, small-for-gestational-age birth, and delivery via C-section is much higher in women with moderate-to-high disease activity, compared with those with low disease activity.26 The presence of active perianal disease mandates C-section over vaginal delivery. Fourth-degree lacerations following vaginal delivery are most common among those patients with perianal disease.26,27 Stillbirths were shown to be increased only in those with active IBD when compared with non-IBD comparators and inactive IBD.28-31;11
Noninvasive methods for disease monitoring are preferred in pregnancy, but serum markers such as erythrocyte sedimentation rate and C-reactive protein may not be reliable in the pregnant patient (Figure).32 Fecal calprotectin does rise in correlation with disease activity, but exact thresholds have not been validated in pregnancy.33,34
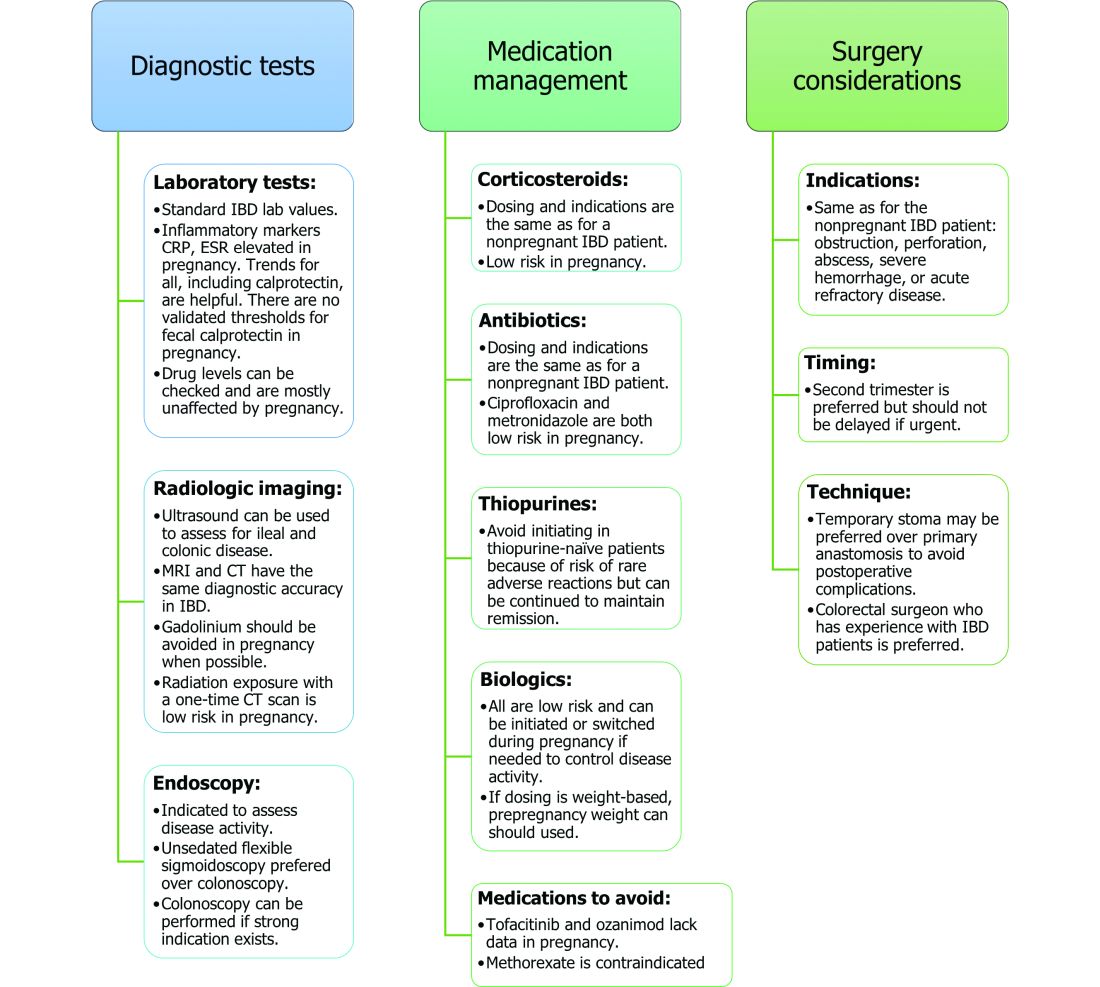
An unsedated, unprepped flexible sigmoidoscopy can be safely performed throughout pregnancy.35 When there is a strong indication, a complete colonoscopy can be performed in the pregnant patient as well.36 Current American Society for Gastrointestinal Endoscopy (ASGE) guidelines suggest placing the patient in the left lateral tilt position to avoid decreased maternal and placental perfusion via compression of the aorta or inferior vena cava and performing endoscopy during the second trimester, although trimester-specific timing is not always feasible by indication.37
Medication use and safety
IBD medications are a priority topic of concern among pregnant patients or those considering conception.38 Comprehensive data from the PIANO (Pregnancy in Inflammatory Bowel Disease and Neonatal Outcomes) registry has shown that most IBD drugs do not result in adverse pregnancy outcomes and should be continued.39 The use of biologics and thiopurines, either in combination or alone, is not related to an increased risk of congenital malformations, spontaneous abortion, preterm birth, low birth weight, or infections during the child’s first year of life.7,39 Developmental milestones also remain unaffected.39 Here, we will discuss safety considerations during pregnancy (see Table).
5-aminosalycylic acid. 5-aminosalicylic acid (5-ASA) agents are generally low risk during pregnancy and should be continued.40-41 Sulfasalazine does interfere with folate metabolism, but by increasing folic acid supplementation to 2 grams per day, sulfasalazine can be continued throughout pregnancy, as well.42
Corticosteroids. Intrapartum corticosteroid use is associated with an increased risk of gestational diabetes and adrenal insufficiency when used long term.43-45 Short-term use may, however, be necessary to control an acute flare. The lowest dose for the shortest duration possible is recommended. Because of its high first-pass metabolism, budesonide is considered low risk in pregnancy.
Methotrexate. Methotrexate needs to be stopped at least 3 months prior to conception and should be avoided throughout pregnancy. Use during pregnancy can result in spontaneous abortions, as well as embryotoxicity.46
Thiopurines (6-mercaptopurine and azathioprine). Patients who are taking thiopurines prior to conception to maintain remission can continue to do so. Data on thiopurines from the PIANO registry has shown no increase in spontaneous abortions, congenital malformations, low birth weight, preterm birth, rates of infection in the child, or developmental delays.47-51
Calcineurin inhibitors (cyclosporine and tacrolimus). Calcineurin inhibitors are reserved for the management of acute severe UC. Safety data on calcineurin inhibitors is conflicting, and there is not enough information at this time to identify risk during pregnancy. Cyclosporine can be used for salvage therapy if absolutely needed, and there are case reports of its successful using during pregnancy.16,52
Biologic therapies. With the exception of certolizumab, all of the currently used biologics are actively transported across the placenta.39,53,54 Intrapartum use of biologic therapies does not worsen pregnancy or neonatal outcomes, including the risk for intensive care unit admission, infections, and developmental milestones.39,47
While drug concentrations may vary slightly during pregnancy, these changes are not substantial enough to warrant more frequent monitoring or dose adjustments, and prepregnancy weight should be used for dosing.55,56
Antitumor necrosis factor agents used in IBD include infliximab, adalimumab, certolizumab, and golimumab.57 All are low risk for pregnant patients and their offspring. Dosage timings can be adjusted, but not stopped, to minimize exposure to the child; however, it cannot be adjusted for certolizumab pegol because of its lack of placental transfer.58-59
Natalizumab and vedolizumab are integrin receptor antagonists and are also low risk in pregnancy.57;60-62;39
Ustekinumab, an interleukin-12/23 antagonist, can be found in infant serum and cord blood, as well. Health outcomes are similar in the exposed mother and child, however, compared with those of the general population.39;63-64
Small molecule drugs. Unlike monoclonal antibodies, which do not cross the placenta in large amounts until early in the second trimester, small molecules can cross in the first trimester during the critical period of organogenesis.
The two small molecule agents currently approved for use in UC are tofacitinib, a janus kinase inhibitor, and ozanimod, a sphingosine-1-phosphate receptor agonist.65-66 Further data are still needed to make recommendations on the use of tofacitinib and ozanimod in pregnancy. At this time, we recommend weighing the risks (unknown risk to human pregnancy) vs. benefits (controlled disease activity with clear risk of harm to mother and baby from flare) in the individual patient before counseling on use in pregnancy.
Delivery
Mode of delivery
The mode of delivery should be determined by the obstetrician. C-section is recommended for patients with active perianal disease or, in some cases, a history of ileal pouch anal anastomosis (IPAA).67-68 Vaginal delivery in the setting of perianal disease has been shown to increase the risk of fourth-degree laceration and anal sphincter dysfunction in the future.26-27 Anorectal motility may be impacted by IPAA construction and vaginal delivery independently of each other. It is therefore suggested that vaginal delivery be avoided in patients with a history of IPAA to avoid compounding the risk. Some studies do not show clear harm from vaginal delivery in the setting of IPAA, however, and informed decision making among all stakeholders should be had.27;69-70
Anticoagulation
The incidence of venous thromboembolism (VTE) is elevated in patients with IBD during pregnancy, and up to 12 weeks postpartum, compared with pregnant patients without IBD.71-72 VTE for prophylaxis is indicated in the pregnant patient while hospitalized and potentially thereafter depending on the patient’s risk factors, which may include obesity, prior personal history of VTE, heart failure, and prolonged immobility. Unfractionated heparin, low molecular weight heparin, and warfarin are safe for breastfeeding women.16,73
Postpartum care of mother
There is a risk of postpartum flare, occurring in about one third of patients in the first 6 months postpartum.74-75 De-escalating therapy during delivery or immediately postpartum is a predictor of a postpartum flare.75 If no infection is present and the timing interval is appropriate, biologic therapies should be continued and can be resumed 24 hours after a vaginal delivery and 48 hours after a C-section.16,76
NSAIDs and opioids can be used for pain relief but should be avoided in the long-term to prevent flares (NSAIDs) and infant sedation (associated with opioids) when used while breastfeeding.77 The LactMed database is an excellent resource for clarification on risk of medication use while breastfeeding.78
In particular, contraception should be addressed postpartum. Exogenous estrogen use increases the risk of VTE, which is already increased in IBD; nonestrogen containing, long-acting reversible contraception is preferred.79-80 Progestin-only implants or intrauterine devices may be used first line. The efficacy of oral contraceptives is theoretically reduced in those with rapid bowel transit, active small bowel inflammation, and prior small bowel resection, so adding another form of contraception is recommended.16,81
Source: American Gastroenterological Association
Postdelivery care of baby
Breastfeeding
Guidelines regarding medication use during breastfeeding are similar to those in pregnancy (see Table). Breastfeeding on biologics and thiopurines can continue without interruption in the child. Thiopurine concentrations in breast milk are low or undetectable.82,78 TNF receptor antagonists, anti-integrin therapies, and ustekinumab are found in low to undetectable levels in breast milk, as well.78
On the other hand, the active metabolite of methotrexate is detectable in breast milk and most sources recommend not breastfeeding on methotrexate. At doses used in IBD (15-25 milligrams per week), some experts have suggested avoiding breastfeeding for 24 hours following a dose.57,78 It is the practice of this author to recommend not breastfeeding at all on methotrexate.
5-ASA therapies are low risk for breastfeeding, but alternatives to sulfasalazine are preferred. The sulfapyridine metabolite transfers to breast milk and may cause hemolysis in infants born with a glucose-6-phosphate dehydrogenase deficiency.78
With regards to calcineurin inhibitors, tacrolimus appears in breast milk in low quantities, while cyclosporine levels are variable. Data from the National Transplantation Pregnancy Registry suggest that these medications can be used at the time of breastfeeding with close monitoring.78
There is not enough data on small molecule therapies at this time to support breastfeeding safety, and it is our practice to not recommend breastfeeding in this scenario.
The transfer of steroids to the child via breast milk does occur but at subtherapeutic levels.16 Budesonide has high first pass metabolism and is low risk during breastfeeding.83-84 As far as is known, IBD maintenance medications do not suppress lactation. The use of intravenous corticosteroids can, however, temporarily decrease milk production.16,85
Vaccines
Vaccination of infants can proceed as indicated by the Center for Disease Control and Prevention guidelines, with one exception. If the child’s mother was exposed to any biologic agents (not including certolizumab) during the third trimester, any live vaccines should be withheld in the first 6 months of life. In the United States, this restriction currently only applies to the rotavirus vaccine, which is administered starting at the age of 2 months.16,86 Notably, inadvertent administration of the rotavirus vaccine in the biologic-exposed child does not appear to result in any adverse effects.87 Immunity is achieved even if the child is exposed to IBD therapies through breast milk.88
Developmental milestones
Infant exposure to biologics and thiopurines has not been shown to result in any developmental delays. The PIANO study measured developmental milestones at 48 months from birth and found no differences when compared with validated population norms.39 A separate study observing childhood development up to 7 years of age in patients born to mothers with IBD found similar cognitive scores and motor development when compared with those born to mothers without IBD.89
Conclusion
Women considering conception should be optimized prior to pregnancy and maintained on appropriate medications throughout pregnancy and lactation to achieve a healthy pregnancy for both mother and baby. To date, biologics and thiopurines are not associated with adverse pregnancy outcomes. More data are needed for small molecules.
Dr. Chugh is an advanced inflammatory bowel disease fellow in the division of gastroenterology at the University of California San Francisco. Dr. Mahadevan is professor of medicine and codirector at the Center for Colitis and Crohn’s Disease in the division of gastroenterology at the University of California San Francisco. Dr. Mahadevan has potential conflicts related to AbbVie, Janssen, BMS, Takeda, Pfizer, Lilly, Gilead, Arena, and Prometheus Biosciences.
References
1. Ye Y et al. Inflamm Bowel Dis. 2020;26:619-25.
2. Sykora J et al. World J Gastroenterol. 2018;24:2741-63.
3. Murakami Y et al. J Gastroenterol 2019;54:1070-7.
4. Hashash JG and Kane S. Gastroenterol Hepatol. (N Y) 2015;11:96-102.
5. Miller JP. J R Soc Med. 1986;79:221-5.
6. Cornish J et al. Gut. 2007;56:830-7.
7. Leung KK et al. Inflamm Bowel Dis. 2021;27:550-62.
8. O’Toole A et al. Dig Dis Sci. 2015;60:2750-61.
9. Nguyen GC et al. Inflamm Bowel Dis. 2008;14:1105-11.
10. Lee HH et al. Aliment Pharmacol Ther. 2020;51:861-9.
11. Kim MA et al. J Crohns Colitis. 2021;15:719-32.
12. Conradt E et al. Pediatrics. 2019;144.
13. ACOG Committee Opinion No. 762: Prepregnancy Counseling. Obstet Gynecol. 2019;133:e78-e89.
14. Farraye FA et al. Am J Gastroenterol. 2017;112:241-58.
15. Lee S et al. J Crohns Colitis. 2018;12:702-9.
16. Mahadevan U et al. Inflamm Bowel Dis. 2019;25:627-41.
17. Ward MG et al. Inflamm. Bowel Dis 2015;21:2839-47.
18. Battat R et al. Inflamm Bowel Dis. 2014;20:1120-8.
19. Pedersen N et al. Aliment Pharmacol Ther. 2013;38:501-12.
20. Annese V. Pharmacol Res. 2020;159:104892.
21. Bennett RA et al. Gastroenterology. 1991;100:1638-43.
22. Turpin W et al. Inflamm Bowel Dis. 2018;24:1133-48.
23. de Lima A et al. Clin Gastroenterol Hepatol. 2016;14:1285-92 e1.
24. Selinger C et al. Frontline Gastroenterol. 2021;12:182-7.
25. Mahadevan U et al. Gastroenterology. 2007;133:1106-12.
26. Hatch Q et al. Dis Colon Rectum. 2014;57:174-8.
27. Foulon A et al. Inflamm Bowel Dis. 2017;23:712-20.
28. Norgard B et al. Am J Gastroenterol. 2007;102:1947-54.
29. Broms G et al. Scand J Gastroenterol 2016;51:1462-9.
30. Meyer A et al. Aliment Pharmacol Ther. 2020;52:1480-90.
31. Kammerlander H et al. Inflamm Bowel Dis. 2017;23:1011-8.
32. Tandon P et al. J Clin Gastroenterol. 2019;53:574-81.
33. Kammerlander H et al. Inflamm Bowel Dis. 2018;24:839-48.
34. Julsgaard M et al. Inflamm Bowel Dis. 2017;23:1240-6.
35. Ko MS et al. Dig Dis Sci. 2020;65:2979-85.
36. Cappell MS et al. J Reprod Med. 2010;55:115-23.
37. Committee ASoP et al. Gastrointest Endosc. 2012;76:18-24.
38. Aboubakr A et al. Dig Dis Sci. 2021;66:1829-35.
39. Mahadevan U et al. Gastroenterology. 2021;160:1131-9.
40. Diav-Citrin O et al. Gastroenterology. 1998;114:23-8.
41. Rahimi R et al. Reprod Toxicol. 2008;25:271-5.
42. Norgard B et al. Aliment Pharmacol Ther. 2001;15:483-6.
43. Leung YP et al. J Crohns Colitis. 2015;9:223-30.
44. Schulze H et al. Aliment Pharmacol Ther. 2014;40:991-1008.
45. Szymanska E et al. J Gynecol Obstet Hum Reprod. 2021;50:101777.
46. Weber-Schoendorfer C et al. Arthritis Rheumatol. 2014;66:1101-10.
47. Nielsen OH et al. Clin Gastroenterol Hepatol. 2022 Jan;20(1):74-87.e3.
48. Coelho J et al. Gut. 2011;60:198-203.
49. Sheikh M et al. J Crohns Colitis. 2015;9:680-4.
50. Kanis SL et al. Clin Gastroenterol Hepatol. 2017;15:1232-41 e1.
51. Mahadevan U et al. Inflamm Bowel Dis. 2018;24:2494-500.
52. Rosen MH et al. Inflamm Bowel Dis. 2020;26:971-3.
53. Porter C et al. J Reprod Immunol. 2016;116:7-12.
54. Mahadevan U et al. Clin Gastroenterol Hepatol. 2013;11:286-92; quiz e24.
55. Picardo S and Seow CH. Best Pract Res Clin Gastroenterol. 2020;44-5:101670.
56. Flanagan E et al. Aliment Pharmacol Ther. 2020;52:1551-62.
57. Singh S et al. Gastroenterology. 2021;160:2512-56 e9.
58. de Lima A et al. Gut. 2016;65:1261-8.
59. Julsgaard M et al. Inflamm Bowel Dis. 2020;26:93-102.
60. Wils P et al. Aliment Pharmacol Ther. 2021;53:460-70.
61. Mahadevan U et al. Aliment Pharmacol Ther. 2017;45:941-50.
62. Bar-Gil Shitrit A et al. Am J Gastroenterol. 2019;114:1172-5.
63. Klenske E et al. J Crohns Colitis. 2019;13:267-9.
64. Matro R et al. Gastroenterology. 2018;155:696-704.
65. Feuerstein JD et al. Gastroenterology. 2020;158:1450-61.
66. Sandborn WJ et al. J Crohns Colitis. 2021 Jul 5;15(7):1120-1129.
67. Lamb CA et al. Gut. 2019;68:s1-s106.
68. Nguyen GC et al. Gastroenterology. 2016;150:734-57 e1.
69. Ravid A et al. Dis Colon Rectum. 2002;45:1283-8.
70. Seligman NS et al. J Matern Fetal Neonatal Med. 2011;24:525-30.
71. Kim YH et al. Medicine (Baltimore). 2019;98:e17309.
72. Hansen AT et al. J Thromb Haemost. 2017;15:702-8.
73. Bates SM et al. J Thromb Thrombolysis. 2016;41:92-128.
74. Bennett A et al. Inflamm Bowel Dis. 2021 May 17;izab104.
75. Yu A et al. Inflamm Bowel Dis. 2020;26:1926-32.
76. Mahadevan U et al. Gastroenterology. 2017;152:451-62 e2.
77. Long MD et al. J Clin Gastroenterol. 2016;50:152-6.
78. Drugs and Lactation Database (LactMed). 2006 ed. Bethesda, MD: National Library of Medicine (US), 2006-2021.
79. Khalili H et al. Gut. 2013;62:1153-9.
80. Long MD and Hutfless S. Gastroenterology. 2016;150:1518-20.
81. Centers for Disease Control and Prevention. U S. Medical Eligibility Criteria for Contraceptive Use, 2010. MMWR Recomm Rep. 2010;59:1-86.
82. Angelberger S et al. J Crohns Colitis. 2011;5:95-100.
83. Vestergaard T et al. Scand J Gastroenterol. 2018;53:1459-62.
84. Beaulieu DB et al. Inflamm Bowel Dis. 2009;15:25-8.
85. Anderson PO. Breastfeed Med. 2017;12:199-201.
86. Wodi AP et al. MMWR Morb Mortal Wkly Rep. 2021;70:189-92.
87. Chiarella-Redfern H et al. Inflamm Bowel Dis. 2022 Jan 5;28(1):79-86.
88. Beaulieu DB et al. Clin Gastroenterol Hepatol. 2018;16:99-105.
89. Friedman S et al. J Crohns Colitis. 2020 Dec 2;14(12):1709-1716.
Inflammatory bowel disease (IBD) incidence is rising globally.1-3 In the United States, we have seen a 123% increase in prevalence of IBD among adults and a 133% increase among children from 2007 to 2016, with an annual percentage change of 9.9%.1 The rise of IBD in young people, and the overall higher prevalence in women compared with men, make pregnancy and IBD a topic of increasing importance for gastroenterologists.1 Here, we will discuss management and expectations in women with IBD before conception, during pregnancy, and post partum.
Preconception
Disease activity
Achieving both clinical and endoscopic remission of disease prior to conception is the key to ensuring the best maternal and fetal outcomes. Patients with IBD who conceive while in remission remain in remission 80% of the time.4,5 On the other hand, those who conceive while their disease is active may continue to have active or worsening disease in nearly 70% of cases.4 Active disease has been associated with an increased incidence of preterm birth, low birth weight, and small-for-gestational-age birth.6-8 Active disease can also exacerbate malnutrition and result in poor maternal weight gain, which is associated with intrauterine growth restriction.9,7 Pregnancy outcomes in patients with IBD and quiescent disease are similar to those in the general population.10,11
Health care maintenance
Optimizing maternal health prior to conception is critical. Alcohol, tobacco, recreational drugs, and marijuana should all be avoided. Opioids should be tapered off prior to conception, as continued use may result in neonatal opioid withdrawal syndrome and long-term neurodevelopmental consequences.12,13 In addition, aiming for a healthy body mass index between 18 and 25 months prior to conception allows for better overall pregnancy outcomes.13 Appropriate cancer screening includes colon cancer screening in those with more than 8 years of colitis, regular pap smear for cervical cancer, and annual total body skin cancer examinations for patients on thiopurines and biologic therapies.14
Nutrition
Folic acid supplementation with at least 400 micrograms (mcg) daily is necessary for all women planning pregnancy. Patients with small bowel involvement or history of small bowel resection should have a folate intake of a minimum of 2 grams per day. Adequate vitamin D levels (at least 20 ng/mL) are recommended in all women with IBD. Those with malabsorption should be screened for deficiencies in vitamin B12, folate, and iron.13 These nutritional markers should be evaluated prepregnancy, during the first trimester, and thereafter as needed.15-18
Preconception counseling
Steroid-free remission for at least 3 months prior to conception is recommended and is associated with reduced risk of flare during pregnancy.16,19 IBD medications needed to control disease activity are generally safe preconception and during pregnancy, with some exception (Table).

Misconceptions regarding heritability of IBD have sometimes discouraged men and women from having children. While genetics may increase susceptibility, environmental and other factors are involved as well. The concordance rates for monozygotic twins range from 33.3%-58.3% for Crohn’s disease and 13.4%-27.9% for ulcerative colitis (UC).20 The risk of a child developing IBD is higher in those who have multiple relatives with IBD and whose parents had IBD at the time of conception.21 While genetic testing for IBD loci is available, it is not commonly performed at this time as many genes are involved.22
Pregnancy
Coordinated care
A complete team of specialists with coordinated care among all providers is needed for optimal maternal and fetal outcomes.23,24 A gastroenterologist, ideally an IBD specialist, should follow the patient throughout pregnancy, seeing the patient at least once during the first or second trimester and as needed during pregnancy.16 A high-risk obstetrician or maternal-fetal medicine specialist should be involved early in pregnancy, as well. Open communication among all disciplines ensures that a common message is conveyed to the patient.16,24 A nutritionist, mental health provider, and lactation specialist knowledgeable about IBD drugs may be of assistance, as well.16
Disease activity
While women with IBD are at increased risk of spontaneous abortion, preterm birth, and labor complications, this risk is mitigated by controlling disease activity.25 The risk of preterm birth, small-for-gestational-age birth, and delivery via C-section is much higher in women with moderate-to-high disease activity, compared with those with low disease activity.26 The presence of active perianal disease mandates C-section over vaginal delivery. Fourth-degree lacerations following vaginal delivery are most common among those patients with perianal disease.26,27 Stillbirths were shown to be increased only in those with active IBD when compared with non-IBD comparators and inactive IBD.28-31;11
Noninvasive methods for disease monitoring are preferred in pregnancy, but serum markers such as erythrocyte sedimentation rate and C-reactive protein may not be reliable in the pregnant patient (Figure).32 Fecal calprotectin does rise in correlation with disease activity, but exact thresholds have not been validated in pregnancy.33,34

An unsedated, unprepped flexible sigmoidoscopy can be safely performed throughout pregnancy.35 When there is a strong indication, a complete colonoscopy can be performed in the pregnant patient as well.36 Current American Society for Gastrointestinal Endoscopy (ASGE) guidelines suggest placing the patient in the left lateral tilt position to avoid decreased maternal and placental perfusion via compression of the aorta or inferior vena cava and performing endoscopy during the second trimester, although trimester-specific timing is not always feasible by indication.37
Medication use and safety
IBD medications are a priority topic of concern among pregnant patients or those considering conception.38 Comprehensive data from the PIANO (Pregnancy in Inflammatory Bowel Disease and Neonatal Outcomes) registry has shown that most IBD drugs do not result in adverse pregnancy outcomes and should be continued.39 The use of biologics and thiopurines, either in combination or alone, is not related to an increased risk of congenital malformations, spontaneous abortion, preterm birth, low birth weight, or infections during the child’s first year of life.7,39 Developmental milestones also remain unaffected.39 Here, we will discuss safety considerations during pregnancy (see Table).
5-aminosalycylic acid. 5-aminosalicylic acid (5-ASA) agents are generally low risk during pregnancy and should be continued.40-41 Sulfasalazine does interfere with folate metabolism, but by increasing folic acid supplementation to 2 grams per day, sulfasalazine can be continued throughout pregnancy, as well.42
Corticosteroids. Intrapartum corticosteroid use is associated with an increased risk of gestational diabetes and adrenal insufficiency when used long term.43-45 Short-term use may, however, be necessary to control an acute flare. The lowest dose for the shortest duration possible is recommended. Because of its high first-pass metabolism, budesonide is considered low risk in pregnancy.
Methotrexate. Methotrexate needs to be stopped at least 3 months prior to conception and should be avoided throughout pregnancy. Use during pregnancy can result in spontaneous abortions, as well as embryotoxicity.46
Thiopurines (6-mercaptopurine and azathioprine). Patients who are taking thiopurines prior to conception to maintain remission can continue to do so. Data on thiopurines from the PIANO registry has shown no increase in spontaneous abortions, congenital malformations, low birth weight, preterm birth, rates of infection in the child, or developmental delays.47-51
Calcineurin inhibitors (cyclosporine and tacrolimus). Calcineurin inhibitors are reserved for the management of acute severe UC. Safety data on calcineurin inhibitors is conflicting, and there is not enough information at this time to identify risk during pregnancy. Cyclosporine can be used for salvage therapy if absolutely needed, and there are case reports of its successful using during pregnancy.16,52
Biologic therapies. With the exception of certolizumab, all of the currently used biologics are actively transported across the placenta.39,53,54 Intrapartum use of biologic therapies does not worsen pregnancy or neonatal outcomes, including the risk for intensive care unit admission, infections, and developmental milestones.39,47
While drug concentrations may vary slightly during pregnancy, these changes are not substantial enough to warrant more frequent monitoring or dose adjustments, and prepregnancy weight should be used for dosing.55,56
Antitumor necrosis factor agents used in IBD include infliximab, adalimumab, certolizumab, and golimumab.57 All are low risk for pregnant patients and their offspring. Dosage timings can be adjusted, but not stopped, to minimize exposure to the child; however, it cannot be adjusted for certolizumab pegol because of its lack of placental transfer.58-59
Natalizumab and vedolizumab are integrin receptor antagonists and are also low risk in pregnancy.57;60-62;39
Ustekinumab, an interleukin-12/23 antagonist, can be found in infant serum and cord blood, as well. Health outcomes are similar in the exposed mother and child, however, compared with those of the general population.39;63-64
Small molecule drugs. Unlike monoclonal antibodies, which do not cross the placenta in large amounts until early in the second trimester, small molecules can cross in the first trimester during the critical period of organogenesis.
The two small molecule agents currently approved for use in UC are tofacitinib, a janus kinase inhibitor, and ozanimod, a sphingosine-1-phosphate receptor agonist.65-66 Further data are still needed to make recommendations on the use of tofacitinib and ozanimod in pregnancy. At this time, we recommend weighing the risks (unknown risk to human pregnancy) vs. benefits (controlled disease activity with clear risk of harm to mother and baby from flare) in the individual patient before counseling on use in pregnancy.
Delivery
Mode of delivery
The mode of delivery should be determined by the obstetrician. C-section is recommended for patients with active perianal disease or, in some cases, a history of ileal pouch anal anastomosis (IPAA).67-68 Vaginal delivery in the setting of perianal disease has been shown to increase the risk of fourth-degree laceration and anal sphincter dysfunction in the future.26-27 Anorectal motility may be impacted by IPAA construction and vaginal delivery independently of each other. It is therefore suggested that vaginal delivery be avoided in patients with a history of IPAA to avoid compounding the risk. Some studies do not show clear harm from vaginal delivery in the setting of IPAA, however, and informed decision making among all stakeholders should be had.27;69-70
Anticoagulation
The incidence of venous thromboembolism (VTE) is elevated in patients with IBD during pregnancy, and up to 12 weeks postpartum, compared with pregnant patients without IBD.71-72 VTE for prophylaxis is indicated in the pregnant patient while hospitalized and potentially thereafter depending on the patient’s risk factors, which may include obesity, prior personal history of VTE, heart failure, and prolonged immobility. Unfractionated heparin, low molecular weight heparin, and warfarin are safe for breastfeeding women.16,73
Postpartum care of mother
There is a risk of postpartum flare, occurring in about one third of patients in the first 6 months postpartum.74-75 De-escalating therapy during delivery or immediately postpartum is a predictor of a postpartum flare.75 If no infection is present and the timing interval is appropriate, biologic therapies should be continued and can be resumed 24 hours after a vaginal delivery and 48 hours after a C-section.16,76
NSAIDs and opioids can be used for pain relief but should be avoided in the long-term to prevent flares (NSAIDs) and infant sedation (associated with opioids) when used while breastfeeding.77 The LactMed database is an excellent resource for clarification on risk of medication use while breastfeeding.78
In particular, contraception should be addressed postpartum. Exogenous estrogen use increases the risk of VTE, which is already increased in IBD; nonestrogen containing, long-acting reversible contraception is preferred.79-80 Progestin-only implants or intrauterine devices may be used first line. The efficacy of oral contraceptives is theoretically reduced in those with rapid bowel transit, active small bowel inflammation, and prior small bowel resection, so adding another form of contraception is recommended.16,81
Source: American Gastroenterological Association
Postdelivery care of baby
Breastfeeding
Guidelines regarding medication use during breastfeeding are similar to those in pregnancy (see Table). Breastfeeding on biologics and thiopurines can continue without interruption in the child. Thiopurine concentrations in breast milk are low or undetectable.82,78 TNF receptor antagonists, anti-integrin therapies, and ustekinumab are found in low to undetectable levels in breast milk, as well.78
On the other hand, the active metabolite of methotrexate is detectable in breast milk and most sources recommend not breastfeeding on methotrexate. At doses used in IBD (15-25 milligrams per week), some experts have suggested avoiding breastfeeding for 24 hours following a dose.57,78 It is the practice of this author to recommend not breastfeeding at all on methotrexate.
5-ASA therapies are low risk for breastfeeding, but alternatives to sulfasalazine are preferred. The sulfapyridine metabolite transfers to breast milk and may cause hemolysis in infants born with a glucose-6-phosphate dehydrogenase deficiency.78
With regards to calcineurin inhibitors, tacrolimus appears in breast milk in low quantities, while cyclosporine levels are variable. Data from the National Transplantation Pregnancy Registry suggest that these medications can be used at the time of breastfeeding with close monitoring.78
There is not enough data on small molecule therapies at this time to support breastfeeding safety, and it is our practice to not recommend breastfeeding in this scenario.
The transfer of steroids to the child via breast milk does occur but at subtherapeutic levels.16 Budesonide has high first pass metabolism and is low risk during breastfeeding.83-84 As far as is known, IBD maintenance medications do not suppress lactation. The use of intravenous corticosteroids can, however, temporarily decrease milk production.16,85
Vaccines
Vaccination of infants can proceed as indicated by the Center for Disease Control and Prevention guidelines, with one exception. If the child’s mother was exposed to any biologic agents (not including certolizumab) during the third trimester, any live vaccines should be withheld in the first 6 months of life. In the United States, this restriction currently only applies to the rotavirus vaccine, which is administered starting at the age of 2 months.16,86 Notably, inadvertent administration of the rotavirus vaccine in the biologic-exposed child does not appear to result in any adverse effects.87 Immunity is achieved even if the child is exposed to IBD therapies through breast milk.88
Developmental milestones
Infant exposure to biologics and thiopurines has not been shown to result in any developmental delays. The PIANO study measured developmental milestones at 48 months from birth and found no differences when compared with validated population norms.39 A separate study observing childhood development up to 7 years of age in patients born to mothers with IBD found similar cognitive scores and motor development when compared with those born to mothers without IBD.89
Conclusion
Women considering conception should be optimized prior to pregnancy and maintained on appropriate medications throughout pregnancy and lactation to achieve a healthy pregnancy for both mother and baby. To date, biologics and thiopurines are not associated with adverse pregnancy outcomes. More data are needed for small molecules.
Dr. Chugh is an advanced inflammatory bowel disease fellow in the division of gastroenterology at the University of California San Francisco. Dr. Mahadevan is professor of medicine and codirector at the Center for Colitis and Crohn’s Disease in the division of gastroenterology at the University of California San Francisco. Dr. Mahadevan has potential conflicts related to AbbVie, Janssen, BMS, Takeda, Pfizer, Lilly, Gilead, Arena, and Prometheus Biosciences.
References
1. Ye Y et al. Inflamm Bowel Dis. 2020;26:619-25.
2. Sykora J et al. World J Gastroenterol. 2018;24:2741-63.
3. Murakami Y et al. J Gastroenterol 2019;54:1070-7.
4. Hashash JG and Kane S. Gastroenterol Hepatol. (N Y) 2015;11:96-102.
5. Miller JP. J R Soc Med. 1986;79:221-5.
6. Cornish J et al. Gut. 2007;56:830-7.
7. Leung KK et al. Inflamm Bowel Dis. 2021;27:550-62.
8. O’Toole A et al. Dig Dis Sci. 2015;60:2750-61.
9. Nguyen GC et al. Inflamm Bowel Dis. 2008;14:1105-11.
10. Lee HH et al. Aliment Pharmacol Ther. 2020;51:861-9.
11. Kim MA et al. J Crohns Colitis. 2021;15:719-32.
12. Conradt E et al. Pediatrics. 2019;144.
13. ACOG Committee Opinion No. 762: Prepregnancy Counseling. Obstet Gynecol. 2019;133:e78-e89.
14. Farraye FA et al. Am J Gastroenterol. 2017;112:241-58.
15. Lee S et al. J Crohns Colitis. 2018;12:702-9.
16. Mahadevan U et al. Inflamm Bowel Dis. 2019;25:627-41.
17. Ward MG et al. Inflamm. Bowel Dis 2015;21:2839-47.
18. Battat R et al. Inflamm Bowel Dis. 2014;20:1120-8.
19. Pedersen N et al. Aliment Pharmacol Ther. 2013;38:501-12.
20. Annese V. Pharmacol Res. 2020;159:104892.
21. Bennett RA et al. Gastroenterology. 1991;100:1638-43.
22. Turpin W et al. Inflamm Bowel Dis. 2018;24:1133-48.
23. de Lima A et al. Clin Gastroenterol Hepatol. 2016;14:1285-92 e1.
24. Selinger C et al. Frontline Gastroenterol. 2021;12:182-7.
25. Mahadevan U et al. Gastroenterology. 2007;133:1106-12.
26. Hatch Q et al. Dis Colon Rectum. 2014;57:174-8.
27. Foulon A et al. Inflamm Bowel Dis. 2017;23:712-20.
28. Norgard B et al. Am J Gastroenterol. 2007;102:1947-54.
29. Broms G et al. Scand J Gastroenterol 2016;51:1462-9.
30. Meyer A et al. Aliment Pharmacol Ther. 2020;52:1480-90.
31. Kammerlander H et al. Inflamm Bowel Dis. 2017;23:1011-8.
32. Tandon P et al. J Clin Gastroenterol. 2019;53:574-81.
33. Kammerlander H et al. Inflamm Bowel Dis. 2018;24:839-48.
34. Julsgaard M et al. Inflamm Bowel Dis. 2017;23:1240-6.
35. Ko MS et al. Dig Dis Sci. 2020;65:2979-85.
36. Cappell MS et al. J Reprod Med. 2010;55:115-23.
37. Committee ASoP et al. Gastrointest Endosc. 2012;76:18-24.
38. Aboubakr A et al. Dig Dis Sci. 2021;66:1829-35.
39. Mahadevan U et al. Gastroenterology. 2021;160:1131-9.
40. Diav-Citrin O et al. Gastroenterology. 1998;114:23-8.
41. Rahimi R et al. Reprod Toxicol. 2008;25:271-5.
42. Norgard B et al. Aliment Pharmacol Ther. 2001;15:483-6.
43. Leung YP et al. J Crohns Colitis. 2015;9:223-30.
44. Schulze H et al. Aliment Pharmacol Ther. 2014;40:991-1008.
45. Szymanska E et al. J Gynecol Obstet Hum Reprod. 2021;50:101777.
46. Weber-Schoendorfer C et al. Arthritis Rheumatol. 2014;66:1101-10.
47. Nielsen OH et al. Clin Gastroenterol Hepatol. 2022 Jan;20(1):74-87.e3.
48. Coelho J et al. Gut. 2011;60:198-203.
49. Sheikh M et al. J Crohns Colitis. 2015;9:680-4.
50. Kanis SL et al. Clin Gastroenterol Hepatol. 2017;15:1232-41 e1.
51. Mahadevan U et al. Inflamm Bowel Dis. 2018;24:2494-500.
52. Rosen MH et al. Inflamm Bowel Dis. 2020;26:971-3.
53. Porter C et al. J Reprod Immunol. 2016;116:7-12.
54. Mahadevan U et al. Clin Gastroenterol Hepatol. 2013;11:286-92; quiz e24.
55. Picardo S and Seow CH. Best Pract Res Clin Gastroenterol. 2020;44-5:101670.
56. Flanagan E et al. Aliment Pharmacol Ther. 2020;52:1551-62.
57. Singh S et al. Gastroenterology. 2021;160:2512-56 e9.
58. de Lima A et al. Gut. 2016;65:1261-8.
59. Julsgaard M et al. Inflamm Bowel Dis. 2020;26:93-102.
60. Wils P et al. Aliment Pharmacol Ther. 2021;53:460-70.
61. Mahadevan U et al. Aliment Pharmacol Ther. 2017;45:941-50.
62. Bar-Gil Shitrit A et al. Am J Gastroenterol. 2019;114:1172-5.
63. Klenske E et al. J Crohns Colitis. 2019;13:267-9.
64. Matro R et al. Gastroenterology. 2018;155:696-704.
65. Feuerstein JD et al. Gastroenterology. 2020;158:1450-61.
66. Sandborn WJ et al. J Crohns Colitis. 2021 Jul 5;15(7):1120-1129.
67. Lamb CA et al. Gut. 2019;68:s1-s106.
68. Nguyen GC et al. Gastroenterology. 2016;150:734-57 e1.
69. Ravid A et al. Dis Colon Rectum. 2002;45:1283-8.
70. Seligman NS et al. J Matern Fetal Neonatal Med. 2011;24:525-30.
71. Kim YH et al. Medicine (Baltimore). 2019;98:e17309.
72. Hansen AT et al. J Thromb Haemost. 2017;15:702-8.
73. Bates SM et al. J Thromb Thrombolysis. 2016;41:92-128.
74. Bennett A et al. Inflamm Bowel Dis. 2021 May 17;izab104.
75. Yu A et al. Inflamm Bowel Dis. 2020;26:1926-32.
76. Mahadevan U et al. Gastroenterology. 2017;152:451-62 e2.
77. Long MD et al. J Clin Gastroenterol. 2016;50:152-6.
78. Drugs and Lactation Database (LactMed). 2006 ed. Bethesda, MD: National Library of Medicine (US), 2006-2021.
79. Khalili H et al. Gut. 2013;62:1153-9.
80. Long MD and Hutfless S. Gastroenterology. 2016;150:1518-20.
81. Centers for Disease Control and Prevention. U S. Medical Eligibility Criteria for Contraceptive Use, 2010. MMWR Recomm Rep. 2010;59:1-86.
82. Angelberger S et al. J Crohns Colitis. 2011;5:95-100.
83. Vestergaard T et al. Scand J Gastroenterol. 2018;53:1459-62.
84. Beaulieu DB et al. Inflamm Bowel Dis. 2009;15:25-8.
85. Anderson PO. Breastfeed Med. 2017;12:199-201.
86. Wodi AP et al. MMWR Morb Mortal Wkly Rep. 2021;70:189-92.
87. Chiarella-Redfern H et al. Inflamm Bowel Dis. 2022 Jan 5;28(1):79-86.
88. Beaulieu DB et al. Clin Gastroenterol Hepatol. 2018;16:99-105.
89. Friedman S et al. J Crohns Colitis. 2020 Dec 2;14(12):1709-1716.
Inflammatory bowel disease (IBD) incidence is rising globally.1-3 In the United States, we have seen a 123% increase in prevalence of IBD among adults and a 133% increase among children from 2007 to 2016, with an annual percentage change of 9.9%.1 The rise of IBD in young people, and the overall higher prevalence in women compared with men, make pregnancy and IBD a topic of increasing importance for gastroenterologists.1 Here, we will discuss management and expectations in women with IBD before conception, during pregnancy, and post partum.
Preconception
Disease activity
Achieving both clinical and endoscopic remission of disease prior to conception is the key to ensuring the best maternal and fetal outcomes. Patients with IBD who conceive while in remission remain in remission 80% of the time.4,5 On the other hand, those who conceive while their disease is active may continue to have active or worsening disease in nearly 70% of cases.4 Active disease has been associated with an increased incidence of preterm birth, low birth weight, and small-for-gestational-age birth.6-8 Active disease can also exacerbate malnutrition and result in poor maternal weight gain, which is associated with intrauterine growth restriction.9,7 Pregnancy outcomes in patients with IBD and quiescent disease are similar to those in the general population.10,11
Health care maintenance
Optimizing maternal health prior to conception is critical. Alcohol, tobacco, recreational drugs, and marijuana should all be avoided. Opioids should be tapered off prior to conception, as continued use may result in neonatal opioid withdrawal syndrome and long-term neurodevelopmental consequences.12,13 In addition, aiming for a healthy body mass index between 18 and 25 months prior to conception allows for better overall pregnancy outcomes.13 Appropriate cancer screening includes colon cancer screening in those with more than 8 years of colitis, regular pap smear for cervical cancer, and annual total body skin cancer examinations for patients on thiopurines and biologic therapies.14
Nutrition
Folic acid supplementation with at least 400 micrograms (mcg) daily is necessary for all women planning pregnancy. Patients with small bowel involvement or history of small bowel resection should have a folate intake of a minimum of 2 grams per day. Adequate vitamin D levels (at least 20 ng/mL) are recommended in all women with IBD. Those with malabsorption should be screened for deficiencies in vitamin B12, folate, and iron.13 These nutritional markers should be evaluated prepregnancy, during the first trimester, and thereafter as needed.15-18
Preconception counseling
Steroid-free remission for at least 3 months prior to conception is recommended and is associated with reduced risk of flare during pregnancy.16,19 IBD medications needed to control disease activity are generally safe preconception and during pregnancy, with some exception (Table).

Misconceptions regarding heritability of IBD have sometimes discouraged men and women from having children. While genetics may increase susceptibility, environmental and other factors are involved as well. The concordance rates for monozygotic twins range from 33.3%-58.3% for Crohn’s disease and 13.4%-27.9% for ulcerative colitis (UC).20 The risk of a child developing IBD is higher in those who have multiple relatives with IBD and whose parents had IBD at the time of conception.21 While genetic testing for IBD loci is available, it is not commonly performed at this time as many genes are involved.22
Pregnancy
Coordinated care
A complete team of specialists with coordinated care among all providers is needed for optimal maternal and fetal outcomes.23,24 A gastroenterologist, ideally an IBD specialist, should follow the patient throughout pregnancy, seeing the patient at least once during the first or second trimester and as needed during pregnancy.16 A high-risk obstetrician or maternal-fetal medicine specialist should be involved early in pregnancy, as well. Open communication among all disciplines ensures that a common message is conveyed to the patient.16,24 A nutritionist, mental health provider, and lactation specialist knowledgeable about IBD drugs may be of assistance, as well.16
Disease activity
While women with IBD are at increased risk of spontaneous abortion, preterm birth, and labor complications, this risk is mitigated by controlling disease activity.25 The risk of preterm birth, small-for-gestational-age birth, and delivery via C-section is much higher in women with moderate-to-high disease activity, compared with those with low disease activity.26 The presence of active perianal disease mandates C-section over vaginal delivery. Fourth-degree lacerations following vaginal delivery are most common among those patients with perianal disease.26,27 Stillbirths were shown to be increased only in those with active IBD when compared with non-IBD comparators and inactive IBD.28-31;11
Noninvasive methods for disease monitoring are preferred in pregnancy, but serum markers such as erythrocyte sedimentation rate and C-reactive protein may not be reliable in the pregnant patient (Figure).32 Fecal calprotectin does rise in correlation with disease activity, but exact thresholds have not been validated in pregnancy.33,34

An unsedated, unprepped flexible sigmoidoscopy can be safely performed throughout pregnancy.35 When there is a strong indication, a complete colonoscopy can be performed in the pregnant patient as well.36 Current American Society for Gastrointestinal Endoscopy (ASGE) guidelines suggest placing the patient in the left lateral tilt position to avoid decreased maternal and placental perfusion via compression of the aorta or inferior vena cava and performing endoscopy during the second trimester, although trimester-specific timing is not always feasible by indication.37
Medication use and safety
IBD medications are a priority topic of concern among pregnant patients or those considering conception.38 Comprehensive data from the PIANO (Pregnancy in Inflammatory Bowel Disease and Neonatal Outcomes) registry has shown that most IBD drugs do not result in adverse pregnancy outcomes and should be continued.39 The use of biologics and thiopurines, either in combination or alone, is not related to an increased risk of congenital malformations, spontaneous abortion, preterm birth, low birth weight, or infections during the child’s first year of life.7,39 Developmental milestones also remain unaffected.39 Here, we will discuss safety considerations during pregnancy (see Table).
5-aminosalycylic acid. 5-aminosalicylic acid (5-ASA) agents are generally low risk during pregnancy and should be continued.40-41 Sulfasalazine does interfere with folate metabolism, but by increasing folic acid supplementation to 2 grams per day, sulfasalazine can be continued throughout pregnancy, as well.42
Corticosteroids. Intrapartum corticosteroid use is associated with an increased risk of gestational diabetes and adrenal insufficiency when used long term.43-45 Short-term use may, however, be necessary to control an acute flare. The lowest dose for the shortest duration possible is recommended. Because of its high first-pass metabolism, budesonide is considered low risk in pregnancy.
Methotrexate. Methotrexate needs to be stopped at least 3 months prior to conception and should be avoided throughout pregnancy. Use during pregnancy can result in spontaneous abortions, as well as embryotoxicity.46
Thiopurines (6-mercaptopurine and azathioprine). Patients who are taking thiopurines prior to conception to maintain remission can continue to do so. Data on thiopurines from the PIANO registry has shown no increase in spontaneous abortions, congenital malformations, low birth weight, preterm birth, rates of infection in the child, or developmental delays.47-51
Calcineurin inhibitors (cyclosporine and tacrolimus). Calcineurin inhibitors are reserved for the management of acute severe UC. Safety data on calcineurin inhibitors is conflicting, and there is not enough information at this time to identify risk during pregnancy. Cyclosporine can be used for salvage therapy if absolutely needed, and there are case reports of its successful using during pregnancy.16,52
Biologic therapies. With the exception of certolizumab, all of the currently used biologics are actively transported across the placenta.39,53,54 Intrapartum use of biologic therapies does not worsen pregnancy or neonatal outcomes, including the risk for intensive care unit admission, infections, and developmental milestones.39,47
While drug concentrations may vary slightly during pregnancy, these changes are not substantial enough to warrant more frequent monitoring or dose adjustments, and prepregnancy weight should be used for dosing.55,56
Antitumor necrosis factor agents used in IBD include infliximab, adalimumab, certolizumab, and golimumab.57 All are low risk for pregnant patients and their offspring. Dosage timings can be adjusted, but not stopped, to minimize exposure to the child; however, it cannot be adjusted for certolizumab pegol because of its lack of placental transfer.58-59
Natalizumab and vedolizumab are integrin receptor antagonists and are also low risk in pregnancy.57;60-62;39
Ustekinumab, an interleukin-12/23 antagonist, can be found in infant serum and cord blood, as well. Health outcomes are similar in the exposed mother and child, however, compared with those of the general population.39;63-64
Small molecule drugs. Unlike monoclonal antibodies, which do not cross the placenta in large amounts until early in the second trimester, small molecules can cross in the first trimester during the critical period of organogenesis.
The two small molecule agents currently approved for use in UC are tofacitinib, a janus kinase inhibitor, and ozanimod, a sphingosine-1-phosphate receptor agonist.65-66 Further data are still needed to make recommendations on the use of tofacitinib and ozanimod in pregnancy. At this time, we recommend weighing the risks (unknown risk to human pregnancy) vs. benefits (controlled disease activity with clear risk of harm to mother and baby from flare) in the individual patient before counseling on use in pregnancy.
Delivery
Mode of delivery
The mode of delivery should be determined by the obstetrician. C-section is recommended for patients with active perianal disease or, in some cases, a history of ileal pouch anal anastomosis (IPAA).67-68 Vaginal delivery in the setting of perianal disease has been shown to increase the risk of fourth-degree laceration and anal sphincter dysfunction in the future.26-27 Anorectal motility may be impacted by IPAA construction and vaginal delivery independently of each other. It is therefore suggested that vaginal delivery be avoided in patients with a history of IPAA to avoid compounding the risk. Some studies do not show clear harm from vaginal delivery in the setting of IPAA, however, and informed decision making among all stakeholders should be had.27;69-70
Anticoagulation
The incidence of venous thromboembolism (VTE) is elevated in patients with IBD during pregnancy, and up to 12 weeks postpartum, compared with pregnant patients without IBD.71-72 VTE for prophylaxis is indicated in the pregnant patient while hospitalized and potentially thereafter depending on the patient’s risk factors, which may include obesity, prior personal history of VTE, heart failure, and prolonged immobility. Unfractionated heparin, low molecular weight heparin, and warfarin are safe for breastfeeding women.16,73
Postpartum care of mother
There is a risk of postpartum flare, occurring in about one third of patients in the first 6 months postpartum.74-75 De-escalating therapy during delivery or immediately postpartum is a predictor of a postpartum flare.75 If no infection is present and the timing interval is appropriate, biologic therapies should be continued and can be resumed 24 hours after a vaginal delivery and 48 hours after a C-section.16,76
NSAIDs and opioids can be used for pain relief but should be avoided in the long-term to prevent flares (NSAIDs) and infant sedation (associated with opioids) when used while breastfeeding.77 The LactMed database is an excellent resource for clarification on risk of medication use while breastfeeding.78
In particular, contraception should be addressed postpartum. Exogenous estrogen use increases the risk of VTE, which is already increased in IBD; nonestrogen containing, long-acting reversible contraception is preferred.79-80 Progestin-only implants or intrauterine devices may be used first line. The efficacy of oral contraceptives is theoretically reduced in those with rapid bowel transit, active small bowel inflammation, and prior small bowel resection, so adding another form of contraception is recommended.16,81
Source: American Gastroenterological Association
Postdelivery care of baby
Breastfeeding
Guidelines regarding medication use during breastfeeding are similar to those in pregnancy (see Table). Breastfeeding on biologics and thiopurines can continue without interruption in the child. Thiopurine concentrations in breast milk are low or undetectable.82,78 TNF receptor antagonists, anti-integrin therapies, and ustekinumab are found in low to undetectable levels in breast milk, as well.78
On the other hand, the active metabolite of methotrexate is detectable in breast milk and most sources recommend not breastfeeding on methotrexate. At doses used in IBD (15-25 milligrams per week), some experts have suggested avoiding breastfeeding for 24 hours following a dose.57,78 It is the practice of this author to recommend not breastfeeding at all on methotrexate.
5-ASA therapies are low risk for breastfeeding, but alternatives to sulfasalazine are preferred. The sulfapyridine metabolite transfers to breast milk and may cause hemolysis in infants born with a glucose-6-phosphate dehydrogenase deficiency.78
With regards to calcineurin inhibitors, tacrolimus appears in breast milk in low quantities, while cyclosporine levels are variable. Data from the National Transplantation Pregnancy Registry suggest that these medications can be used at the time of breastfeeding with close monitoring.78
There is not enough data on small molecule therapies at this time to support breastfeeding safety, and it is our practice to not recommend breastfeeding in this scenario.
The transfer of steroids to the child via breast milk does occur but at subtherapeutic levels.16 Budesonide has high first pass metabolism and is low risk during breastfeeding.83-84 As far as is known, IBD maintenance medications do not suppress lactation. The use of intravenous corticosteroids can, however, temporarily decrease milk production.16,85
Vaccines
Vaccination of infants can proceed as indicated by the Center for Disease Control and Prevention guidelines, with one exception. If the child’s mother was exposed to any biologic agents (not including certolizumab) during the third trimester, any live vaccines should be withheld in the first 6 months of life. In the United States, this restriction currently only applies to the rotavirus vaccine, which is administered starting at the age of 2 months.16,86 Notably, inadvertent administration of the rotavirus vaccine in the biologic-exposed child does not appear to result in any adverse effects.87 Immunity is achieved even if the child is exposed to IBD therapies through breast milk.88
Developmental milestones
Infant exposure to biologics and thiopurines has not been shown to result in any developmental delays. The PIANO study measured developmental milestones at 48 months from birth and found no differences when compared with validated population norms.39 A separate study observing childhood development up to 7 years of age in patients born to mothers with IBD found similar cognitive scores and motor development when compared with those born to mothers without IBD.89
Conclusion
Women considering conception should be optimized prior to pregnancy and maintained on appropriate medications throughout pregnancy and lactation to achieve a healthy pregnancy for both mother and baby. To date, biologics and thiopurines are not associated with adverse pregnancy outcomes. More data are needed for small molecules.
Dr. Chugh is an advanced inflammatory bowel disease fellow in the division of gastroenterology at the University of California San Francisco. Dr. Mahadevan is professor of medicine and codirector at the Center for Colitis and Crohn’s Disease in the division of gastroenterology at the University of California San Francisco. Dr. Mahadevan has potential conflicts related to AbbVie, Janssen, BMS, Takeda, Pfizer, Lilly, Gilead, Arena, and Prometheus Biosciences.
References
1. Ye Y et al. Inflamm Bowel Dis. 2020;26:619-25.
2. Sykora J et al. World J Gastroenterol. 2018;24:2741-63.
3. Murakami Y et al. J Gastroenterol 2019;54:1070-7.
4. Hashash JG and Kane S. Gastroenterol Hepatol. (N Y) 2015;11:96-102.
5. Miller JP. J R Soc Med. 1986;79:221-5.
6. Cornish J et al. Gut. 2007;56:830-7.
7. Leung KK et al. Inflamm Bowel Dis. 2021;27:550-62.
8. O’Toole A et al. Dig Dis Sci. 2015;60:2750-61.
9. Nguyen GC et al. Inflamm Bowel Dis. 2008;14:1105-11.
10. Lee HH et al. Aliment Pharmacol Ther. 2020;51:861-9.
11. Kim MA et al. J Crohns Colitis. 2021;15:719-32.
12. Conradt E et al. Pediatrics. 2019;144.
13. ACOG Committee Opinion No. 762: Prepregnancy Counseling. Obstet Gynecol. 2019;133:e78-e89.
14. Farraye FA et al. Am J Gastroenterol. 2017;112:241-58.
15. Lee S et al. J Crohns Colitis. 2018;12:702-9.
16. Mahadevan U et al. Inflamm Bowel Dis. 2019;25:627-41.
17. Ward MG et al. Inflamm. Bowel Dis 2015;21:2839-47.
18. Battat R et al. Inflamm Bowel Dis. 2014;20:1120-8.
19. Pedersen N et al. Aliment Pharmacol Ther. 2013;38:501-12.
20. Annese V. Pharmacol Res. 2020;159:104892.
21. Bennett RA et al. Gastroenterology. 1991;100:1638-43.
22. Turpin W et al. Inflamm Bowel Dis. 2018;24:1133-48.
23. de Lima A et al. Clin Gastroenterol Hepatol. 2016;14:1285-92 e1.
24. Selinger C et al. Frontline Gastroenterol. 2021;12:182-7.
25. Mahadevan U et al. Gastroenterology. 2007;133:1106-12.
26. Hatch Q et al. Dis Colon Rectum. 2014;57:174-8.
27. Foulon A et al. Inflamm Bowel Dis. 2017;23:712-20.
28. Norgard B et al. Am J Gastroenterol. 2007;102:1947-54.
29. Broms G et al. Scand J Gastroenterol 2016;51:1462-9.
30. Meyer A et al. Aliment Pharmacol Ther. 2020;52:1480-90.
31. Kammerlander H et al. Inflamm Bowel Dis. 2017;23:1011-8.
32. Tandon P et al. J Clin Gastroenterol. 2019;53:574-81.
33. Kammerlander H et al. Inflamm Bowel Dis. 2018;24:839-48.
34. Julsgaard M et al. Inflamm Bowel Dis. 2017;23:1240-6.
35. Ko MS et al. Dig Dis Sci. 2020;65:2979-85.
36. Cappell MS et al. J Reprod Med. 2010;55:115-23.
37. Committee ASoP et al. Gastrointest Endosc. 2012;76:18-24.
38. Aboubakr A et al. Dig Dis Sci. 2021;66:1829-35.
39. Mahadevan U et al. Gastroenterology. 2021;160:1131-9.
40. Diav-Citrin O et al. Gastroenterology. 1998;114:23-8.
41. Rahimi R et al. Reprod Toxicol. 2008;25:271-5.
42. Norgard B et al. Aliment Pharmacol Ther. 2001;15:483-6.
43. Leung YP et al. J Crohns Colitis. 2015;9:223-30.
44. Schulze H et al. Aliment Pharmacol Ther. 2014;40:991-1008.
45. Szymanska E et al. J Gynecol Obstet Hum Reprod. 2021;50:101777.
46. Weber-Schoendorfer C et al. Arthritis Rheumatol. 2014;66:1101-10.
47. Nielsen OH et al. Clin Gastroenterol Hepatol. 2022 Jan;20(1):74-87.e3.
48. Coelho J et al. Gut. 2011;60:198-203.
49. Sheikh M et al. J Crohns Colitis. 2015;9:680-4.
50. Kanis SL et al. Clin Gastroenterol Hepatol. 2017;15:1232-41 e1.
51. Mahadevan U et al. Inflamm Bowel Dis. 2018;24:2494-500.
52. Rosen MH et al. Inflamm Bowel Dis. 2020;26:971-3.
53. Porter C et al. J Reprod Immunol. 2016;116:7-12.
54. Mahadevan U et al. Clin Gastroenterol Hepatol. 2013;11:286-92; quiz e24.
55. Picardo S and Seow CH. Best Pract Res Clin Gastroenterol. 2020;44-5:101670.
56. Flanagan E et al. Aliment Pharmacol Ther. 2020;52:1551-62.
57. Singh S et al. Gastroenterology. 2021;160:2512-56 e9.
58. de Lima A et al. Gut. 2016;65:1261-8.
59. Julsgaard M et al. Inflamm Bowel Dis. 2020;26:93-102.
60. Wils P et al. Aliment Pharmacol Ther. 2021;53:460-70.
61. Mahadevan U et al. Aliment Pharmacol Ther. 2017;45:941-50.
62. Bar-Gil Shitrit A et al. Am J Gastroenterol. 2019;114:1172-5.
63. Klenske E et al. J Crohns Colitis. 2019;13:267-9.
64. Matro R et al. Gastroenterology. 2018;155:696-704.
65. Feuerstein JD et al. Gastroenterology. 2020;158:1450-61.
66. Sandborn WJ et al. J Crohns Colitis. 2021 Jul 5;15(7):1120-1129.
67. Lamb CA et al. Gut. 2019;68:s1-s106.
68. Nguyen GC et al. Gastroenterology. 2016;150:734-57 e1.
69. Ravid A et al. Dis Colon Rectum. 2002;45:1283-8.
70. Seligman NS et al. J Matern Fetal Neonatal Med. 2011;24:525-30.
71. Kim YH et al. Medicine (Baltimore). 2019;98:e17309.
72. Hansen AT et al. J Thromb Haemost. 2017;15:702-8.
73. Bates SM et al. J Thromb Thrombolysis. 2016;41:92-128.
74. Bennett A et al. Inflamm Bowel Dis. 2021 May 17;izab104.
75. Yu A et al. Inflamm Bowel Dis. 2020;26:1926-32.
76. Mahadevan U et al. Gastroenterology. 2017;152:451-62 e2.
77. Long MD et al. J Clin Gastroenterol. 2016;50:152-6.
78. Drugs and Lactation Database (LactMed). 2006 ed. Bethesda, MD: National Library of Medicine (US), 2006-2021.
79. Khalili H et al. Gut. 2013;62:1153-9.
80. Long MD and Hutfless S. Gastroenterology. 2016;150:1518-20.
81. Centers for Disease Control and Prevention. U S. Medical Eligibility Criteria for Contraceptive Use, 2010. MMWR Recomm Rep. 2010;59:1-86.
82. Angelberger S et al. J Crohns Colitis. 2011;5:95-100.
83. Vestergaard T et al. Scand J Gastroenterol. 2018;53:1459-62.
84. Beaulieu DB et al. Inflamm Bowel Dis. 2009;15:25-8.
85. Anderson PO. Breastfeed Med. 2017;12:199-201.
86. Wodi AP et al. MMWR Morb Mortal Wkly Rep. 2021;70:189-92.
87. Chiarella-Redfern H et al. Inflamm Bowel Dis. 2022 Jan 5;28(1):79-86.
88. Beaulieu DB et al. Clin Gastroenterol Hepatol. 2018;16:99-105.
89. Friedman S et al. J Crohns Colitis. 2020 Dec 2;14(12):1709-1716.






