User login
Innovations in Dermatology Fall Abstract Compendium
Long-term glucocorticoids in RA linked to increased cardiovascular risk
Each month of glucocorticoid use in middle-aged patients with rheumatoid arthritis increases their odds of a major adverse cardiac event by 14%, independent of their baseline cardiovascular risk, according to a Veterans Administration study presented at the virtual annual meeting of the American College of Rheumatology. A similar study of Medicare and insurance claims data also presented at the meeting similarly found a dose-dependent increase in cardiovascular risk with long-term glucocorticoid use among patients with RA.
Up to half of patients with RA use long-term glucocorticoids, Beth Wallace, MD, an assistant professor of internal medicine at the University of Michigan, Ann Arbor, and a staff rheumatologist at the VA Ann Arbor Healthcare Center, told attendees in her presentation.
“Despite previous work suggesting they increase major [adverse] cardiovascular events, or MACE, in a dose-dependent way, prior work suggests long-term glucocorticoid use is common among RA patients with traditional basic risk factors like hyperlipidemia, diabetes, hypertension, and smoking,” Dr. Wallace said. “But we know little about the incremental effects of ongoing glucocorticoid use on MACE risk in RA, particularly as traditional predisposing comorbidities might confound its assessment.”
Christie Bartels, MD, associate professor and division head of rheumatology at the University of Wisconsin, Madison, said in an interview that these findings indicate a need to consider the risks of long-term glucocorticoid use for RA.
“The clinical implications of these studies include informed consent when using steroids in patients and when advocating for steroid-sparing therapy,” said Dr. Bartels, who was not involved in either study. ”We have never had more options for steroid-sparing medications in rheumatoid arthritis than we have right now, making it a critical time to reduce glucocorticoid use whenever possible. For short-term function and pain relief, or in some cases with many contraindications, there is still a role for glucocorticoid use, but these data show that no amount of longer-term glucocorticoid use is without risk.”
VA study details
The retrospective cohort study relied on VA administrative data for 26,239 patients with RA who had at least one rheumatology visit during 2013-2017. Only adults aged 40-90 were included (85% men), and none had other rheumatologic conditions, a previous MACE, or congestive heart failure in the preceding 5 years.
The researchers used pharmacy dispensing data to determine exposure to glucocorticoids, based on the number of days’ supply per 6 months and claims data to identify the primary outcome of MACE, defined as acute myocardial infarction, stroke, transient ischemic attack, cardiac arrest, or coronary revascularization, in the following 6 months. After a first MACE, a patient was removed from subsequent analysis so that only a participant’s initial event was considered.
The researchers adjusted their analysis for demographics, health care utilization, long-term glucocorticoid use (over 90 days), use of methotrexate or biologics, and baseline cardiac risk based on the Veterans Affairs Risk Score for Cardiovascular Disease (VARS-CVD). The VARS-CVD uses age, sex, race, tobacco use, systolic blood pressure, cholesterol, diabetes diagnosis, and use of antihypertensives to estimate the risk of a MACE in the next 5 years. A 5-year risk of less than 3% was considered low, 3%-9% medium, and above 9% high.
The population’s median 5-year MACE risk based on VARS-CVD was 5.7%, with nearly a quarter of participants (23%) having a high risk. During the first year of follow-up, 23% of patients overall, including 24% of those with high risk, received at least 90 days of glucocorticoids. An incident MACE occurred in 3.2% of overall patients and in 4.9% of high-risk patients. Median time until an incident MACE was 25 months.
After adjusting for confounders, the researchers calculated that each additional 30 days of glucocorticoid use per 6-month period was linked to a 14% increase in odds of a MACE in the subsequent 6-month period (odds ratio, 1.14). This finding remained independent of baseline cardiovascular risk, previous long-term exposure to glucocorticoids, baseline office visits, methotrexate or biologic use, and baseline Elixhauser Cormobidity Index (except rheumatoid arthritis, diabetes, hypertension, and congestive heart failure).
Dr. Wallace noted that the observational study could still include residual confounding because of factors such as rheumatic disease activity, glucocorticoid dose, and care outside the VA. They also did not distinguish between existing and incident RA and were missing some VARS-CVD data, and they did not adjust for hydroxychloroquine use, which can reduce cardiovascular risk.
Details of Medicare and private insurance claims study
In the second study, Brian Coburn, MD, a fourth-year internal medicine resident at the University of Pennsylvania, Philadelphia, presented findings on long-term glucocorticoid use and cardiovascular outcomes in patients with RA based on 2006-2015 claims data from Medicare and the Optum Clinformatics Data Mart. That study similarly found a dose-dependent increase in cardiovascular risk with increasing dosage of long-term glucocorticoids.
All the patients in the two databases had an RA diagnosis and remained on disease-modifying antirheumatic drugs (DMARDs) for at least 180 days without adding a new DMARD or stopping therapy for more than 90 days. Patients were not included if they had a history of myocardial infarction, stroke, coronary artery bypass grafting, or percutaneous coronary intervention.
Using the 180 days before and after starting DMARDs as baseline, the researchers assessed average dose of glucocorticoids during the last 90 days of the baseline period. Participants included 135,583 patients with Medicare, contributing 158,839 years at risk, and 39,272 patients in the Optum database, contributing 36,876 years at risk. The researchers then assessed composite cardiovascular events as a combination of strokes and myocardial infarctions.
A total of 2,067 cardiovascular events occurred among the Medicare patients, for a incidence of 1.3 events per 100 people per year, and 313 cardiovascular events occurred among Optum patients, for an incidence of 0.8 events per 100 people per year.
Over 1 year, a predicted 1.1% of Medicare patients not taking glucocorticoids would experience a stroke or heart attack, compared with 1.4% of those taking up to 5 mg/day of glucocorticoids, 1.7% of those taking 5-10 mg/day glucocorticoids, and 1.9% of those taking more than 10 mg/day glucocorticoids. The number needed to harm was 400 people for up to 5 mg/day, 192 people for 5-10 mg/day, and 137 people for more than 10 mg/day.
Among Optum patients, 0.7% not taking glucocorticoids would experience a stroke or heart attack over 1 year, compared with 0.9% of those taking up to 5 mg/day and 0.8% of those taking either 5-10 mg/day or more than 10 mg/day. The number needed to harm was 714 people for up to 5 mg/day of glucocorticoids, 5,000 people for 5-10 mg/day, and 1,667 for over 10 mg/day.
Dr. Bartels noted that this study “reported unadjusted rates, without controlling for traditional CVD risk factors, for instance, so it will be interesting to see that report after full analysis and peer review as well.” She added that the rates in the VA study may even be higher if there were uncounted cardiovascular events or deaths outside the VA.
“The key take away is that glucocorticoids have dose-related cardiovascular risk shown in both duration and dose of use now in these three large U.S. cohorts,” Dr. Bartels said. “Providers need to counsel patients in judicious use of glucocorticoids, favoring the role of biologic and nonbiologic DMARDs while balancing unique needs and quality-of-life considerations in our patients.”
The VA retrospective cohort study was funded by the National Institutes of Health, the American Autoimmune Related Diseases Association, the U.S. Department of Veterans Affairs, and the Michigan Institute for Clinical & Health Research. Dr. Wallace and seven other authors reported no disclosures. Several coauthors reported financial ties to multiple pharmaceutical companies. The Medicare/Optum retrospective cohort study was funded by the National Institutes of Health, the Patient-Centered Outcomes Research Institute, and the Rheumatology Research Foundation. Dr. Coburn and five coauthors had no disclosures, while several others reported financial ties to a variety of pharmaceutical companies. Dr. Bartels has received institutional grant support from Pfizer for tobacco cessation research
Each month of glucocorticoid use in middle-aged patients with rheumatoid arthritis increases their odds of a major adverse cardiac event by 14%, independent of their baseline cardiovascular risk, according to a Veterans Administration study presented at the virtual annual meeting of the American College of Rheumatology. A similar study of Medicare and insurance claims data also presented at the meeting similarly found a dose-dependent increase in cardiovascular risk with long-term glucocorticoid use among patients with RA.
Up to half of patients with RA use long-term glucocorticoids, Beth Wallace, MD, an assistant professor of internal medicine at the University of Michigan, Ann Arbor, and a staff rheumatologist at the VA Ann Arbor Healthcare Center, told attendees in her presentation.
“Despite previous work suggesting they increase major [adverse] cardiovascular events, or MACE, in a dose-dependent way, prior work suggests long-term glucocorticoid use is common among RA patients with traditional basic risk factors like hyperlipidemia, diabetes, hypertension, and smoking,” Dr. Wallace said. “But we know little about the incremental effects of ongoing glucocorticoid use on MACE risk in RA, particularly as traditional predisposing comorbidities might confound its assessment.”
Christie Bartels, MD, associate professor and division head of rheumatology at the University of Wisconsin, Madison, said in an interview that these findings indicate a need to consider the risks of long-term glucocorticoid use for RA.
“The clinical implications of these studies include informed consent when using steroids in patients and when advocating for steroid-sparing therapy,” said Dr. Bartels, who was not involved in either study. ”We have never had more options for steroid-sparing medications in rheumatoid arthritis than we have right now, making it a critical time to reduce glucocorticoid use whenever possible. For short-term function and pain relief, or in some cases with many contraindications, there is still a role for glucocorticoid use, but these data show that no amount of longer-term glucocorticoid use is without risk.”
VA study details
The retrospective cohort study relied on VA administrative data for 26,239 patients with RA who had at least one rheumatology visit during 2013-2017. Only adults aged 40-90 were included (85% men), and none had other rheumatologic conditions, a previous MACE, or congestive heart failure in the preceding 5 years.
The researchers used pharmacy dispensing data to determine exposure to glucocorticoids, based on the number of days’ supply per 6 months and claims data to identify the primary outcome of MACE, defined as acute myocardial infarction, stroke, transient ischemic attack, cardiac arrest, or coronary revascularization, in the following 6 months. After a first MACE, a patient was removed from subsequent analysis so that only a participant’s initial event was considered.
The researchers adjusted their analysis for demographics, health care utilization, long-term glucocorticoid use (over 90 days), use of methotrexate or biologics, and baseline cardiac risk based on the Veterans Affairs Risk Score for Cardiovascular Disease (VARS-CVD). The VARS-CVD uses age, sex, race, tobacco use, systolic blood pressure, cholesterol, diabetes diagnosis, and use of antihypertensives to estimate the risk of a MACE in the next 5 years. A 5-year risk of less than 3% was considered low, 3%-9% medium, and above 9% high.
The population’s median 5-year MACE risk based on VARS-CVD was 5.7%, with nearly a quarter of participants (23%) having a high risk. During the first year of follow-up, 23% of patients overall, including 24% of those with high risk, received at least 90 days of glucocorticoids. An incident MACE occurred in 3.2% of overall patients and in 4.9% of high-risk patients. Median time until an incident MACE was 25 months.
After adjusting for confounders, the researchers calculated that each additional 30 days of glucocorticoid use per 6-month period was linked to a 14% increase in odds of a MACE in the subsequent 6-month period (odds ratio, 1.14). This finding remained independent of baseline cardiovascular risk, previous long-term exposure to glucocorticoids, baseline office visits, methotrexate or biologic use, and baseline Elixhauser Cormobidity Index (except rheumatoid arthritis, diabetes, hypertension, and congestive heart failure).
Dr. Wallace noted that the observational study could still include residual confounding because of factors such as rheumatic disease activity, glucocorticoid dose, and care outside the VA. They also did not distinguish between existing and incident RA and were missing some VARS-CVD data, and they did not adjust for hydroxychloroquine use, which can reduce cardiovascular risk.
Details of Medicare and private insurance claims study
In the second study, Brian Coburn, MD, a fourth-year internal medicine resident at the University of Pennsylvania, Philadelphia, presented findings on long-term glucocorticoid use and cardiovascular outcomes in patients with RA based on 2006-2015 claims data from Medicare and the Optum Clinformatics Data Mart. That study similarly found a dose-dependent increase in cardiovascular risk with increasing dosage of long-term glucocorticoids.
All the patients in the two databases had an RA diagnosis and remained on disease-modifying antirheumatic drugs (DMARDs) for at least 180 days without adding a new DMARD or stopping therapy for more than 90 days. Patients were not included if they had a history of myocardial infarction, stroke, coronary artery bypass grafting, or percutaneous coronary intervention.
Using the 180 days before and after starting DMARDs as baseline, the researchers assessed average dose of glucocorticoids during the last 90 days of the baseline period. Participants included 135,583 patients with Medicare, contributing 158,839 years at risk, and 39,272 patients in the Optum database, contributing 36,876 years at risk. The researchers then assessed composite cardiovascular events as a combination of strokes and myocardial infarctions.
A total of 2,067 cardiovascular events occurred among the Medicare patients, for a incidence of 1.3 events per 100 people per year, and 313 cardiovascular events occurred among Optum patients, for an incidence of 0.8 events per 100 people per year.
Over 1 year, a predicted 1.1% of Medicare patients not taking glucocorticoids would experience a stroke or heart attack, compared with 1.4% of those taking up to 5 mg/day of glucocorticoids, 1.7% of those taking 5-10 mg/day glucocorticoids, and 1.9% of those taking more than 10 mg/day glucocorticoids. The number needed to harm was 400 people for up to 5 mg/day, 192 people for 5-10 mg/day, and 137 people for more than 10 mg/day.
Among Optum patients, 0.7% not taking glucocorticoids would experience a stroke or heart attack over 1 year, compared with 0.9% of those taking up to 5 mg/day and 0.8% of those taking either 5-10 mg/day or more than 10 mg/day. The number needed to harm was 714 people for up to 5 mg/day of glucocorticoids, 5,000 people for 5-10 mg/day, and 1,667 for over 10 mg/day.
Dr. Bartels noted that this study “reported unadjusted rates, without controlling for traditional CVD risk factors, for instance, so it will be interesting to see that report after full analysis and peer review as well.” She added that the rates in the VA study may even be higher if there were uncounted cardiovascular events or deaths outside the VA.
“The key take away is that glucocorticoids have dose-related cardiovascular risk shown in both duration and dose of use now in these three large U.S. cohorts,” Dr. Bartels said. “Providers need to counsel patients in judicious use of glucocorticoids, favoring the role of biologic and nonbiologic DMARDs while balancing unique needs and quality-of-life considerations in our patients.”
The VA retrospective cohort study was funded by the National Institutes of Health, the American Autoimmune Related Diseases Association, the U.S. Department of Veterans Affairs, and the Michigan Institute for Clinical & Health Research. Dr. Wallace and seven other authors reported no disclosures. Several coauthors reported financial ties to multiple pharmaceutical companies. The Medicare/Optum retrospective cohort study was funded by the National Institutes of Health, the Patient-Centered Outcomes Research Institute, and the Rheumatology Research Foundation. Dr. Coburn and five coauthors had no disclosures, while several others reported financial ties to a variety of pharmaceutical companies. Dr. Bartels has received institutional grant support from Pfizer for tobacco cessation research
Each month of glucocorticoid use in middle-aged patients with rheumatoid arthritis increases their odds of a major adverse cardiac event by 14%, independent of their baseline cardiovascular risk, according to a Veterans Administration study presented at the virtual annual meeting of the American College of Rheumatology. A similar study of Medicare and insurance claims data also presented at the meeting similarly found a dose-dependent increase in cardiovascular risk with long-term glucocorticoid use among patients with RA.
Up to half of patients with RA use long-term glucocorticoids, Beth Wallace, MD, an assistant professor of internal medicine at the University of Michigan, Ann Arbor, and a staff rheumatologist at the VA Ann Arbor Healthcare Center, told attendees in her presentation.
“Despite previous work suggesting they increase major [adverse] cardiovascular events, or MACE, in a dose-dependent way, prior work suggests long-term glucocorticoid use is common among RA patients with traditional basic risk factors like hyperlipidemia, diabetes, hypertension, and smoking,” Dr. Wallace said. “But we know little about the incremental effects of ongoing glucocorticoid use on MACE risk in RA, particularly as traditional predisposing comorbidities might confound its assessment.”
Christie Bartels, MD, associate professor and division head of rheumatology at the University of Wisconsin, Madison, said in an interview that these findings indicate a need to consider the risks of long-term glucocorticoid use for RA.
“The clinical implications of these studies include informed consent when using steroids in patients and when advocating for steroid-sparing therapy,” said Dr. Bartels, who was not involved in either study. ”We have never had more options for steroid-sparing medications in rheumatoid arthritis than we have right now, making it a critical time to reduce glucocorticoid use whenever possible. For short-term function and pain relief, or in some cases with many contraindications, there is still a role for glucocorticoid use, but these data show that no amount of longer-term glucocorticoid use is without risk.”
VA study details
The retrospective cohort study relied on VA administrative data for 26,239 patients with RA who had at least one rheumatology visit during 2013-2017. Only adults aged 40-90 were included (85% men), and none had other rheumatologic conditions, a previous MACE, or congestive heart failure in the preceding 5 years.
The researchers used pharmacy dispensing data to determine exposure to glucocorticoids, based on the number of days’ supply per 6 months and claims data to identify the primary outcome of MACE, defined as acute myocardial infarction, stroke, transient ischemic attack, cardiac arrest, or coronary revascularization, in the following 6 months. After a first MACE, a patient was removed from subsequent analysis so that only a participant’s initial event was considered.
The researchers adjusted their analysis for demographics, health care utilization, long-term glucocorticoid use (over 90 days), use of methotrexate or biologics, and baseline cardiac risk based on the Veterans Affairs Risk Score for Cardiovascular Disease (VARS-CVD). The VARS-CVD uses age, sex, race, tobacco use, systolic blood pressure, cholesterol, diabetes diagnosis, and use of antihypertensives to estimate the risk of a MACE in the next 5 years. A 5-year risk of less than 3% was considered low, 3%-9% medium, and above 9% high.
The population’s median 5-year MACE risk based on VARS-CVD was 5.7%, with nearly a quarter of participants (23%) having a high risk. During the first year of follow-up, 23% of patients overall, including 24% of those with high risk, received at least 90 days of glucocorticoids. An incident MACE occurred in 3.2% of overall patients and in 4.9% of high-risk patients. Median time until an incident MACE was 25 months.
After adjusting for confounders, the researchers calculated that each additional 30 days of glucocorticoid use per 6-month period was linked to a 14% increase in odds of a MACE in the subsequent 6-month period (odds ratio, 1.14). This finding remained independent of baseline cardiovascular risk, previous long-term exposure to glucocorticoids, baseline office visits, methotrexate or biologic use, and baseline Elixhauser Cormobidity Index (except rheumatoid arthritis, diabetes, hypertension, and congestive heart failure).
Dr. Wallace noted that the observational study could still include residual confounding because of factors such as rheumatic disease activity, glucocorticoid dose, and care outside the VA. They also did not distinguish between existing and incident RA and were missing some VARS-CVD data, and they did not adjust for hydroxychloroquine use, which can reduce cardiovascular risk.
Details of Medicare and private insurance claims study
In the second study, Brian Coburn, MD, a fourth-year internal medicine resident at the University of Pennsylvania, Philadelphia, presented findings on long-term glucocorticoid use and cardiovascular outcomes in patients with RA based on 2006-2015 claims data from Medicare and the Optum Clinformatics Data Mart. That study similarly found a dose-dependent increase in cardiovascular risk with increasing dosage of long-term glucocorticoids.
All the patients in the two databases had an RA diagnosis and remained on disease-modifying antirheumatic drugs (DMARDs) for at least 180 days without adding a new DMARD or stopping therapy for more than 90 days. Patients were not included if they had a history of myocardial infarction, stroke, coronary artery bypass grafting, or percutaneous coronary intervention.
Using the 180 days before and after starting DMARDs as baseline, the researchers assessed average dose of glucocorticoids during the last 90 days of the baseline period. Participants included 135,583 patients with Medicare, contributing 158,839 years at risk, and 39,272 patients in the Optum database, contributing 36,876 years at risk. The researchers then assessed composite cardiovascular events as a combination of strokes and myocardial infarctions.
A total of 2,067 cardiovascular events occurred among the Medicare patients, for a incidence of 1.3 events per 100 people per year, and 313 cardiovascular events occurred among Optum patients, for an incidence of 0.8 events per 100 people per year.
Over 1 year, a predicted 1.1% of Medicare patients not taking glucocorticoids would experience a stroke or heart attack, compared with 1.4% of those taking up to 5 mg/day of glucocorticoids, 1.7% of those taking 5-10 mg/day glucocorticoids, and 1.9% of those taking more than 10 mg/day glucocorticoids. The number needed to harm was 400 people for up to 5 mg/day, 192 people for 5-10 mg/day, and 137 people for more than 10 mg/day.
Among Optum patients, 0.7% not taking glucocorticoids would experience a stroke or heart attack over 1 year, compared with 0.9% of those taking up to 5 mg/day and 0.8% of those taking either 5-10 mg/day or more than 10 mg/day. The number needed to harm was 714 people for up to 5 mg/day of glucocorticoids, 5,000 people for 5-10 mg/day, and 1,667 for over 10 mg/day.
Dr. Bartels noted that this study “reported unadjusted rates, without controlling for traditional CVD risk factors, for instance, so it will be interesting to see that report after full analysis and peer review as well.” She added that the rates in the VA study may even be higher if there were uncounted cardiovascular events or deaths outside the VA.
“The key take away is that glucocorticoids have dose-related cardiovascular risk shown in both duration and dose of use now in these three large U.S. cohorts,” Dr. Bartels said. “Providers need to counsel patients in judicious use of glucocorticoids, favoring the role of biologic and nonbiologic DMARDs while balancing unique needs and quality-of-life considerations in our patients.”
The VA retrospective cohort study was funded by the National Institutes of Health, the American Autoimmune Related Diseases Association, the U.S. Department of Veterans Affairs, and the Michigan Institute for Clinical & Health Research. Dr. Wallace and seven other authors reported no disclosures. Several coauthors reported financial ties to multiple pharmaceutical companies. The Medicare/Optum retrospective cohort study was funded by the National Institutes of Health, the Patient-Centered Outcomes Research Institute, and the Rheumatology Research Foundation. Dr. Coburn and five coauthors had no disclosures, while several others reported financial ties to a variety of pharmaceutical companies. Dr. Bartels has received institutional grant support from Pfizer for tobacco cessation research
FROM ACR 2021
EMPEROR-Preserved findings confirmed in ‘true’ HFpEF patients
Main results from the landmark EMPEROR-Preserved trial, reported in August, established for the first time that treatment with a drug, the sodium-glucose cotransporter 2 inhibitor empagliflozin, could clearly benefit patients with heart failure with preserved ejection fraction (HFpEF).
The only caveat was that EMPEROR-Preserved enrolled patients with a left ventricular ejection fraction of at least 41%, while “true” HFpEF means patients with heart failure and an LVEF of at least 50%, according to recent definitions. About one-third of the 5,988 patients enrolled in EMPEROR-Preserved had an LVEF of 41%-49%, heart failure with mildly reduced ejection fraction.
Secondary analysis from the EMPEROR-Preserved trial has now resolved this ambiguity by showing that, among the 4,005 patients (67%) enrolled in the trial with an LVEF of at least 50%, treatment with empagliflozin (Jardiance) reduced the study’s primary endpoint – cardiovascular death or first hospitalization for heart failure – by a significant 17%, relative to patients who received placebo, dismissing any doubt about the relevance of the overall finding to the subgroup of patients with unmitigated HFpEF.
“This is the first large-scale trial to document meaningful and significant improvements associated with drug therapy in patients with ‘true’ HFpEF,” Stefan D. Anker, MD, said in presenting the results at the American Heart Association scientific sessions.
Streamlining heart failure treatment
The demonstration that empagliflozin is an effective – and safe – treatment for patients with HFpEF not only provides a new treatment for a disorder that until now had no evidence-based intervention, but also streamlines the management approach for treating patients with heart failure with an agent from empagliflozin’s class, the SGLT2 inhibitors, commented Mary Norine Walsh, MD, medical director of the heart failure and cardiac transplantation programs at Ascension St. Vincent Heart Center in Indianapolis.
That’s because empagliflozin has shown significant and consistent benefit across essentially the full range of LVEFs seen in patients with heart failure based on its performance in EMPEROR-Preserved as well as in a mirror-image trial, EMPEROR-Reduced, run in patients with heart failure with reduced ejection fraction.
“Clinicians do not need to stop and assess LVEF with echocardiography or other imaging before they decide on how to treat heart failure patients” with an SGLT2 inhibitor, noted Dr. Walsh, a designated discussant for the report. “Clinicians who are busy can now refer less to LVEF than to the patient’s phenotype.”
Treatment prevents hospitalization for heart failure
The more-detailed data reported by Dr. Anker also strengthened the case that the benefit from empagliflozin in patients with an LVEF of at least 50% mostly came from a reduction in hospitalizations for heart failure (HHF), which dropped following start of empagliflozin treatment by a relative 22%, compared with placebo for first HHF, a significant decline, and by a relative 17% for total HHF, a reduction that missed significance in this secondary analysis. The other half of the primary endpoint, cardiovascular death, declined by a nonsignificant 11% with empagliflozin treatment, compared with placebo in patients with clear-cut HFpEF.
The significant reduction in first HHF is, by itself, sufficient reason to use empagliflozin (or possibly a different SGLT2 inhibitor) in patients with HFpEF, maintained Clyde W. Yancy, MD, professor and chief of cardiology at Northwestern Medicine in Chicago.
“Attenuated HHF is a meaningful outcome,” stressed Dr. Yancy, also a discussant for the study. “This is the first time we’ve had evidence supporting that we can change the natural history of patients with HFpEF. While we still need to find interventions that save lives, we cannot overlook that this treatment can improve morbidity, and we cannot overlook that patient quality of life is better.”
Further benefits in patients with an LVEF of at least 50%
Dr. Anker, professor of cardiology and metabolism at Charité Medical University in Berlin, also reported results from several other analyses that further defined the effect of empagliflozin on clinical outcomes of patients with “true” HFpEF:
- The impact of empagliflozin, compared with placebo, for reducing both the study’s combined, primary outcome as well as total HHF was statistically consistent across all strata of LVEF, from 50% to greater than 70%. However, both outcome measures also showed a puzzling loss of benefit among patients with an LVEF of 65%-69%. In prior reports, a researcher on the EMPEROR-Preserved team, Milton Packer, MD, speculated that some patients in this LVEF stratum might not actually have had heart failure but instead had a different disorder that mimicked heart failure in clinical presentation, such as atrial fibrillation.
- Patients’ quality of life as measured by the Kansas City Cardiomyopathy Questionnaire showed a consistent benefit from empagliflozin treatment, compared with placebo, both in patients with an LVEF of at least 50% as well as in those with an LVEF of 41%-49%. In both subgroups the adjusted mean difference from placebo was significant and about 1.5 points.
- Patients showed a significant improvement in average New York Heart Association functional class while on treatment, and a strong trend toward less deterioration in functional class while on treatment.
- Deterioration of renal function on treatment slowed by an average 1.24 mL/min per 1.73 m2 per year in patients on empagliflozin, compared with placebo, in the subgroup with an LVEF of at least 50%.
Dr. Anker also reported the primary outcome and component results for the subgroup of patients with a baseline LVEF of 41%-49%. These patients had what looked like a “bigger magnitude” of effect from treatment, he noted, showing a significant 29% relative decline in the primary endpoint, compared with placebo-treated patients, and a significant 42% relative drop in first HHF and a significant 43% relative decline in total HHF, compared with placebo.
The primary analysis from EMPEROR-Preserved, which included all 5,988 randomized patients with heart failure and an LVEF of 41% or greater, showed a significant reduction in the combined, primary endpoint with empagliflozin treatment of 21%, compared with control patients during a median follow-up of about 26 months. The absolute rate reduction of the combined primary endpoint was 3.3% during 26-months’ follow-up. Statistical tests have shown no heterogeneity of this effect by diabetes status (49% of patients had diabetes), nor by renal function down to an estimated glomerular filtration rate at entry as low as 20 mL/min per 1.73 m2.
EMPEROR-Preserved was sponsored by Boehringer Ingelheim and Lilly, the two companies that market empagliflozin (Jardiance). Dr. Anker has been a consultant to Boehringer Ingelheim as well as to Abbott Vascular, Bayer, Brahms, Cardiac Dimensions, Cordio, Novartis, Servier, and Vifor. Dr. Walsh and Dr. Yancy had no disclosures.
Main results from the landmark EMPEROR-Preserved trial, reported in August, established for the first time that treatment with a drug, the sodium-glucose cotransporter 2 inhibitor empagliflozin, could clearly benefit patients with heart failure with preserved ejection fraction (HFpEF).
The only caveat was that EMPEROR-Preserved enrolled patients with a left ventricular ejection fraction of at least 41%, while “true” HFpEF means patients with heart failure and an LVEF of at least 50%, according to recent definitions. About one-third of the 5,988 patients enrolled in EMPEROR-Preserved had an LVEF of 41%-49%, heart failure with mildly reduced ejection fraction.
Secondary analysis from the EMPEROR-Preserved trial has now resolved this ambiguity by showing that, among the 4,005 patients (67%) enrolled in the trial with an LVEF of at least 50%, treatment with empagliflozin (Jardiance) reduced the study’s primary endpoint – cardiovascular death or first hospitalization for heart failure – by a significant 17%, relative to patients who received placebo, dismissing any doubt about the relevance of the overall finding to the subgroup of patients with unmitigated HFpEF.
“This is the first large-scale trial to document meaningful and significant improvements associated with drug therapy in patients with ‘true’ HFpEF,” Stefan D. Anker, MD, said in presenting the results at the American Heart Association scientific sessions.
Streamlining heart failure treatment
The demonstration that empagliflozin is an effective – and safe – treatment for patients with HFpEF not only provides a new treatment for a disorder that until now had no evidence-based intervention, but also streamlines the management approach for treating patients with heart failure with an agent from empagliflozin’s class, the SGLT2 inhibitors, commented Mary Norine Walsh, MD, medical director of the heart failure and cardiac transplantation programs at Ascension St. Vincent Heart Center in Indianapolis.
That’s because empagliflozin has shown significant and consistent benefit across essentially the full range of LVEFs seen in patients with heart failure based on its performance in EMPEROR-Preserved as well as in a mirror-image trial, EMPEROR-Reduced, run in patients with heart failure with reduced ejection fraction.
“Clinicians do not need to stop and assess LVEF with echocardiography or other imaging before they decide on how to treat heart failure patients” with an SGLT2 inhibitor, noted Dr. Walsh, a designated discussant for the report. “Clinicians who are busy can now refer less to LVEF than to the patient’s phenotype.”
Treatment prevents hospitalization for heart failure
The more-detailed data reported by Dr. Anker also strengthened the case that the benefit from empagliflozin in patients with an LVEF of at least 50% mostly came from a reduction in hospitalizations for heart failure (HHF), which dropped following start of empagliflozin treatment by a relative 22%, compared with placebo for first HHF, a significant decline, and by a relative 17% for total HHF, a reduction that missed significance in this secondary analysis. The other half of the primary endpoint, cardiovascular death, declined by a nonsignificant 11% with empagliflozin treatment, compared with placebo in patients with clear-cut HFpEF.
The significant reduction in first HHF is, by itself, sufficient reason to use empagliflozin (or possibly a different SGLT2 inhibitor) in patients with HFpEF, maintained Clyde W. Yancy, MD, professor and chief of cardiology at Northwestern Medicine in Chicago.
“Attenuated HHF is a meaningful outcome,” stressed Dr. Yancy, also a discussant for the study. “This is the first time we’ve had evidence supporting that we can change the natural history of patients with HFpEF. While we still need to find interventions that save lives, we cannot overlook that this treatment can improve morbidity, and we cannot overlook that patient quality of life is better.”
Further benefits in patients with an LVEF of at least 50%
Dr. Anker, professor of cardiology and metabolism at Charité Medical University in Berlin, also reported results from several other analyses that further defined the effect of empagliflozin on clinical outcomes of patients with “true” HFpEF:
- The impact of empagliflozin, compared with placebo, for reducing both the study’s combined, primary outcome as well as total HHF was statistically consistent across all strata of LVEF, from 50% to greater than 70%. However, both outcome measures also showed a puzzling loss of benefit among patients with an LVEF of 65%-69%. In prior reports, a researcher on the EMPEROR-Preserved team, Milton Packer, MD, speculated that some patients in this LVEF stratum might not actually have had heart failure but instead had a different disorder that mimicked heart failure in clinical presentation, such as atrial fibrillation.
- Patients’ quality of life as measured by the Kansas City Cardiomyopathy Questionnaire showed a consistent benefit from empagliflozin treatment, compared with placebo, both in patients with an LVEF of at least 50% as well as in those with an LVEF of 41%-49%. In both subgroups the adjusted mean difference from placebo was significant and about 1.5 points.
- Patients showed a significant improvement in average New York Heart Association functional class while on treatment, and a strong trend toward less deterioration in functional class while on treatment.
- Deterioration of renal function on treatment slowed by an average 1.24 mL/min per 1.73 m2 per year in patients on empagliflozin, compared with placebo, in the subgroup with an LVEF of at least 50%.
Dr. Anker also reported the primary outcome and component results for the subgroup of patients with a baseline LVEF of 41%-49%. These patients had what looked like a “bigger magnitude” of effect from treatment, he noted, showing a significant 29% relative decline in the primary endpoint, compared with placebo-treated patients, and a significant 42% relative drop in first HHF and a significant 43% relative decline in total HHF, compared with placebo.
The primary analysis from EMPEROR-Preserved, which included all 5,988 randomized patients with heart failure and an LVEF of 41% or greater, showed a significant reduction in the combined, primary endpoint with empagliflozin treatment of 21%, compared with control patients during a median follow-up of about 26 months. The absolute rate reduction of the combined primary endpoint was 3.3% during 26-months’ follow-up. Statistical tests have shown no heterogeneity of this effect by diabetes status (49% of patients had diabetes), nor by renal function down to an estimated glomerular filtration rate at entry as low as 20 mL/min per 1.73 m2.
EMPEROR-Preserved was sponsored by Boehringer Ingelheim and Lilly, the two companies that market empagliflozin (Jardiance). Dr. Anker has been a consultant to Boehringer Ingelheim as well as to Abbott Vascular, Bayer, Brahms, Cardiac Dimensions, Cordio, Novartis, Servier, and Vifor. Dr. Walsh and Dr. Yancy had no disclosures.
Main results from the landmark EMPEROR-Preserved trial, reported in August, established for the first time that treatment with a drug, the sodium-glucose cotransporter 2 inhibitor empagliflozin, could clearly benefit patients with heart failure with preserved ejection fraction (HFpEF).
The only caveat was that EMPEROR-Preserved enrolled patients with a left ventricular ejection fraction of at least 41%, while “true” HFpEF means patients with heart failure and an LVEF of at least 50%, according to recent definitions. About one-third of the 5,988 patients enrolled in EMPEROR-Preserved had an LVEF of 41%-49%, heart failure with mildly reduced ejection fraction.
Secondary analysis from the EMPEROR-Preserved trial has now resolved this ambiguity by showing that, among the 4,005 patients (67%) enrolled in the trial with an LVEF of at least 50%, treatment with empagliflozin (Jardiance) reduced the study’s primary endpoint – cardiovascular death or first hospitalization for heart failure – by a significant 17%, relative to patients who received placebo, dismissing any doubt about the relevance of the overall finding to the subgroup of patients with unmitigated HFpEF.
“This is the first large-scale trial to document meaningful and significant improvements associated with drug therapy in patients with ‘true’ HFpEF,” Stefan D. Anker, MD, said in presenting the results at the American Heart Association scientific sessions.
Streamlining heart failure treatment
The demonstration that empagliflozin is an effective – and safe – treatment for patients with HFpEF not only provides a new treatment for a disorder that until now had no evidence-based intervention, but also streamlines the management approach for treating patients with heart failure with an agent from empagliflozin’s class, the SGLT2 inhibitors, commented Mary Norine Walsh, MD, medical director of the heart failure and cardiac transplantation programs at Ascension St. Vincent Heart Center in Indianapolis.
That’s because empagliflozin has shown significant and consistent benefit across essentially the full range of LVEFs seen in patients with heart failure based on its performance in EMPEROR-Preserved as well as in a mirror-image trial, EMPEROR-Reduced, run in patients with heart failure with reduced ejection fraction.
“Clinicians do not need to stop and assess LVEF with echocardiography or other imaging before they decide on how to treat heart failure patients” with an SGLT2 inhibitor, noted Dr. Walsh, a designated discussant for the report. “Clinicians who are busy can now refer less to LVEF than to the patient’s phenotype.”
Treatment prevents hospitalization for heart failure
The more-detailed data reported by Dr. Anker also strengthened the case that the benefit from empagliflozin in patients with an LVEF of at least 50% mostly came from a reduction in hospitalizations for heart failure (HHF), which dropped following start of empagliflozin treatment by a relative 22%, compared with placebo for first HHF, a significant decline, and by a relative 17% for total HHF, a reduction that missed significance in this secondary analysis. The other half of the primary endpoint, cardiovascular death, declined by a nonsignificant 11% with empagliflozin treatment, compared with placebo in patients with clear-cut HFpEF.
The significant reduction in first HHF is, by itself, sufficient reason to use empagliflozin (or possibly a different SGLT2 inhibitor) in patients with HFpEF, maintained Clyde W. Yancy, MD, professor and chief of cardiology at Northwestern Medicine in Chicago.
“Attenuated HHF is a meaningful outcome,” stressed Dr. Yancy, also a discussant for the study. “This is the first time we’ve had evidence supporting that we can change the natural history of patients with HFpEF. While we still need to find interventions that save lives, we cannot overlook that this treatment can improve morbidity, and we cannot overlook that patient quality of life is better.”
Further benefits in patients with an LVEF of at least 50%
Dr. Anker, professor of cardiology and metabolism at Charité Medical University in Berlin, also reported results from several other analyses that further defined the effect of empagliflozin on clinical outcomes of patients with “true” HFpEF:
- The impact of empagliflozin, compared with placebo, for reducing both the study’s combined, primary outcome as well as total HHF was statistically consistent across all strata of LVEF, from 50% to greater than 70%. However, both outcome measures also showed a puzzling loss of benefit among patients with an LVEF of 65%-69%. In prior reports, a researcher on the EMPEROR-Preserved team, Milton Packer, MD, speculated that some patients in this LVEF stratum might not actually have had heart failure but instead had a different disorder that mimicked heart failure in clinical presentation, such as atrial fibrillation.
- Patients’ quality of life as measured by the Kansas City Cardiomyopathy Questionnaire showed a consistent benefit from empagliflozin treatment, compared with placebo, both in patients with an LVEF of at least 50% as well as in those with an LVEF of 41%-49%. In both subgroups the adjusted mean difference from placebo was significant and about 1.5 points.
- Patients showed a significant improvement in average New York Heart Association functional class while on treatment, and a strong trend toward less deterioration in functional class while on treatment.
- Deterioration of renal function on treatment slowed by an average 1.24 mL/min per 1.73 m2 per year in patients on empagliflozin, compared with placebo, in the subgroup with an LVEF of at least 50%.
Dr. Anker also reported the primary outcome and component results for the subgroup of patients with a baseline LVEF of 41%-49%. These patients had what looked like a “bigger magnitude” of effect from treatment, he noted, showing a significant 29% relative decline in the primary endpoint, compared with placebo-treated patients, and a significant 42% relative drop in first HHF and a significant 43% relative decline in total HHF, compared with placebo.
The primary analysis from EMPEROR-Preserved, which included all 5,988 randomized patients with heart failure and an LVEF of 41% or greater, showed a significant reduction in the combined, primary endpoint with empagliflozin treatment of 21%, compared with control patients during a median follow-up of about 26 months. The absolute rate reduction of the combined primary endpoint was 3.3% during 26-months’ follow-up. Statistical tests have shown no heterogeneity of this effect by diabetes status (49% of patients had diabetes), nor by renal function down to an estimated glomerular filtration rate at entry as low as 20 mL/min per 1.73 m2.
EMPEROR-Preserved was sponsored by Boehringer Ingelheim and Lilly, the two companies that market empagliflozin (Jardiance). Dr. Anker has been a consultant to Boehringer Ingelheim as well as to Abbott Vascular, Bayer, Brahms, Cardiac Dimensions, Cordio, Novartis, Servier, and Vifor. Dr. Walsh and Dr. Yancy had no disclosures.
FROM AHA2021
Easing access to DLBCL treatments: Patient study reveals racial differences
, but other “multifaceted and personalized” strategies are also needed, a new study shows.
The findings, from a survey focused on patients’ willingness to travel for treatment, offer valuable insights on DLBCL patients’ perspectives and care needs, and on racial and sociodemographic variations among their perspectives and needs, the investigators said.
Treatment decision factors
They used a choice-based conjoint analysis to assess the relative value that 302 patients with DLBCL place on clinical factors, continuity of care, and travel time. Patients were asked to select treatment plans, choosing between pairs of hypothetical options that varied in travel time, follow-up arrangement, oncologist continuity, 2-year overall survival, and intensive care unit admission rate, the authors explained.
When all follow-up care in the hypothetical scenario was provided at the treatment center, plans requiring travel time of longer than 30 minutes were less attractive, Zachary A. K. Frosch, MD, and colleagues reported in the Journal of Clinical Oncology.
Importance weights, when compared with 30-minute travel time, were –0.54, –0.57, and –0.17 for 60, 90, and 120 minute travel time, they found.
However, scenarios involving shared follow-up by the treatment center and patients’ local providers mitigated the negative impact of travel on treatment plan choice, they noted (importance weights, 0.63, 0.32, and 0.26 at 60, 90, and 120-minute travel times).
Importantly, an analysis of responses based on sociodemographic factors showed that Black participants were less likely to choose plans requiring longer travel, regardless of follow-up arrangement, the authors said.
“Black patients were also less likely than White patients to choose treatment plans that offered lower continuity with their current oncologist (importance weights, 2.50 to vs. 1.09, respectively),” they wrote.
Further, when making choices that required trade-offs, treatment efficacy was a weaker driver of treatment plan preferences for Black patient than for White patients (importance weights, 0.34 vs. 0.75 per 5% point increase in overall survival, respectively).
Why the findings matter
“Certain cancer treatments aren’t offered everywhere. Examples of this are the bone marrow transplants and [chimeric antigen receptor T-cell] therapies used to treat patients with blood cancers such as lymphoma,” Dr. Frosch said in an interview, adding that the limited geographic availability of these treatments means that patients who need them may have to travel farther and also to establish care with a new oncologist.
“These are both things that some patients may be reluctant to do,” added Dr. Frosch, who was with the Perelman School of Medicine at the University of Pennsylvania, Philadelphia, at the time of the study, but is now assistant professor at Fox Chase Cancer Center in Philadelphia.
“We wanted to better understand how patients think about these trade-offs,” he said. “We found that they were less likely to choose treatments requiring more travel, or treatments that required them to transfer care to a new oncologist. This was the case, even if it meant choosing a treatment that might be less effective against their cancer. But when patients were offered a chance to have half of their follow-up appointments locally, travel was less of a barrier.”
Importantly, not all participants valued each aspect of treatments equally, Dr. Frosch noted, referencing the responses of Black versus White patients.
He and his colleagues stressed that while collaborative follow-up may ease access to more distant treatments for some patients, the lesser willingness among Black participants to travel for cancer therapy – regardless of follow-up arrangement – means that attention must be paid to unintended consequences, to avoid worsening the existing disparities in access to cellular therapies.
These data represent a step toward better understanding of how patients considering whether or not to travel for specialized cancer care weigh trade-offs, he said.
“However, we need to dig deeper into the issues we uncovered in future research, he added. “Our findings suggest that collaborative follow-up between the hospitals that offer these treatments and the oncologists in patients’ own communities could improve access to specialized cancer treatments. But I also think it’s important to understand that this may not be the solution for everyone, and so multiple and individualized strategies are going to be needed.”
Personalized treatment strategies
The findings provide important perspective on the need to address patients’ concerns and circumstances to improve access to cellular therapies, said Ankit Kansagra, MD, the Eugene P. Frenkel, M.D. Scholar in Clinical Medicine at the University of Texas Southwestern Medical Center, Dallas.
The unique focus by Dr. Frosch and his associates on the patient perspective versus the health care system perspective underscores the need to be patient-focused, and serves as a reminder that different strategies are needed for different patients, Dr. Kansagra, who has also conducted research on access to CAR T therapies, said in an interview.
For some patients, a shared model of care is much more important than a 5% improvement in survival, he said, adding that providers shouldn’t assume that they understand a patient’s perspective.
Devising hybrid solutions that take community and individual needs into consideration would be preferable to seeking one national solution for care access, he added.
“It’s also pretty clear from this that it can be a shared model versus just an academic center or community center doing everything,” he said. “I think that’s going to be the next frontier – [determining] how we can hand over a patient, once CAR T is done, back to the community oncologist so he or she can continue following the patient and knows the survivorship plan – and keeping that model in place.”
Next steps
Further work is needed to determine the mechanisms driving the differences observed between Black and White patients in this study, the authors said, explaining that “[a]lthough the differences observed by race may reflect structural racism-driven access inequities, the relatively small subsample of Black patients and model complexity constraints limited our ability to analyze multiple factors.
“A prospective validation study to demonstrate the association of stated preferences with real-world decisions would further support our findings,” they wrote.
Dr. Frosch reported having no conflicts of interest. Dr. Kansagra is on advisory boards for Alnylam, Bristol Myers Squibb, Cota Healthcare, GSK, Janssen, Oncopeptides, and Takeda.
, but other “multifaceted and personalized” strategies are also needed, a new study shows.
The findings, from a survey focused on patients’ willingness to travel for treatment, offer valuable insights on DLBCL patients’ perspectives and care needs, and on racial and sociodemographic variations among their perspectives and needs, the investigators said.
Treatment decision factors
They used a choice-based conjoint analysis to assess the relative value that 302 patients with DLBCL place on clinical factors, continuity of care, and travel time. Patients were asked to select treatment plans, choosing between pairs of hypothetical options that varied in travel time, follow-up arrangement, oncologist continuity, 2-year overall survival, and intensive care unit admission rate, the authors explained.
When all follow-up care in the hypothetical scenario was provided at the treatment center, plans requiring travel time of longer than 30 minutes were less attractive, Zachary A. K. Frosch, MD, and colleagues reported in the Journal of Clinical Oncology.
Importance weights, when compared with 30-minute travel time, were –0.54, –0.57, and –0.17 for 60, 90, and 120 minute travel time, they found.
However, scenarios involving shared follow-up by the treatment center and patients’ local providers mitigated the negative impact of travel on treatment plan choice, they noted (importance weights, 0.63, 0.32, and 0.26 at 60, 90, and 120-minute travel times).
Importantly, an analysis of responses based on sociodemographic factors showed that Black participants were less likely to choose plans requiring longer travel, regardless of follow-up arrangement, the authors said.
“Black patients were also less likely than White patients to choose treatment plans that offered lower continuity with their current oncologist (importance weights, 2.50 to vs. 1.09, respectively),” they wrote.
Further, when making choices that required trade-offs, treatment efficacy was a weaker driver of treatment plan preferences for Black patient than for White patients (importance weights, 0.34 vs. 0.75 per 5% point increase in overall survival, respectively).
Why the findings matter
“Certain cancer treatments aren’t offered everywhere. Examples of this are the bone marrow transplants and [chimeric antigen receptor T-cell] therapies used to treat patients with blood cancers such as lymphoma,” Dr. Frosch said in an interview, adding that the limited geographic availability of these treatments means that patients who need them may have to travel farther and also to establish care with a new oncologist.
“These are both things that some patients may be reluctant to do,” added Dr. Frosch, who was with the Perelman School of Medicine at the University of Pennsylvania, Philadelphia, at the time of the study, but is now assistant professor at Fox Chase Cancer Center in Philadelphia.
“We wanted to better understand how patients think about these trade-offs,” he said. “We found that they were less likely to choose treatments requiring more travel, or treatments that required them to transfer care to a new oncologist. This was the case, even if it meant choosing a treatment that might be less effective against their cancer. But when patients were offered a chance to have half of their follow-up appointments locally, travel was less of a barrier.”
Importantly, not all participants valued each aspect of treatments equally, Dr. Frosch noted, referencing the responses of Black versus White patients.
He and his colleagues stressed that while collaborative follow-up may ease access to more distant treatments for some patients, the lesser willingness among Black participants to travel for cancer therapy – regardless of follow-up arrangement – means that attention must be paid to unintended consequences, to avoid worsening the existing disparities in access to cellular therapies.
These data represent a step toward better understanding of how patients considering whether or not to travel for specialized cancer care weigh trade-offs, he said.
“However, we need to dig deeper into the issues we uncovered in future research, he added. “Our findings suggest that collaborative follow-up between the hospitals that offer these treatments and the oncologists in patients’ own communities could improve access to specialized cancer treatments. But I also think it’s important to understand that this may not be the solution for everyone, and so multiple and individualized strategies are going to be needed.”
Personalized treatment strategies
The findings provide important perspective on the need to address patients’ concerns and circumstances to improve access to cellular therapies, said Ankit Kansagra, MD, the Eugene P. Frenkel, M.D. Scholar in Clinical Medicine at the University of Texas Southwestern Medical Center, Dallas.
The unique focus by Dr. Frosch and his associates on the patient perspective versus the health care system perspective underscores the need to be patient-focused, and serves as a reminder that different strategies are needed for different patients, Dr. Kansagra, who has also conducted research on access to CAR T therapies, said in an interview.
For some patients, a shared model of care is much more important than a 5% improvement in survival, he said, adding that providers shouldn’t assume that they understand a patient’s perspective.
Devising hybrid solutions that take community and individual needs into consideration would be preferable to seeking one national solution for care access, he added.
“It’s also pretty clear from this that it can be a shared model versus just an academic center or community center doing everything,” he said. “I think that’s going to be the next frontier – [determining] how we can hand over a patient, once CAR T is done, back to the community oncologist so he or she can continue following the patient and knows the survivorship plan – and keeping that model in place.”
Next steps
Further work is needed to determine the mechanisms driving the differences observed between Black and White patients in this study, the authors said, explaining that “[a]lthough the differences observed by race may reflect structural racism-driven access inequities, the relatively small subsample of Black patients and model complexity constraints limited our ability to analyze multiple factors.
“A prospective validation study to demonstrate the association of stated preferences with real-world decisions would further support our findings,” they wrote.
Dr. Frosch reported having no conflicts of interest. Dr. Kansagra is on advisory boards for Alnylam, Bristol Myers Squibb, Cota Healthcare, GSK, Janssen, Oncopeptides, and Takeda.
, but other “multifaceted and personalized” strategies are also needed, a new study shows.
The findings, from a survey focused on patients’ willingness to travel for treatment, offer valuable insights on DLBCL patients’ perspectives and care needs, and on racial and sociodemographic variations among their perspectives and needs, the investigators said.
Treatment decision factors
They used a choice-based conjoint analysis to assess the relative value that 302 patients with DLBCL place on clinical factors, continuity of care, and travel time. Patients were asked to select treatment plans, choosing between pairs of hypothetical options that varied in travel time, follow-up arrangement, oncologist continuity, 2-year overall survival, and intensive care unit admission rate, the authors explained.
When all follow-up care in the hypothetical scenario was provided at the treatment center, plans requiring travel time of longer than 30 minutes were less attractive, Zachary A. K. Frosch, MD, and colleagues reported in the Journal of Clinical Oncology.
Importance weights, when compared with 30-minute travel time, were –0.54, –0.57, and –0.17 for 60, 90, and 120 minute travel time, they found.
However, scenarios involving shared follow-up by the treatment center and patients’ local providers mitigated the negative impact of travel on treatment plan choice, they noted (importance weights, 0.63, 0.32, and 0.26 at 60, 90, and 120-minute travel times).
Importantly, an analysis of responses based on sociodemographic factors showed that Black participants were less likely to choose plans requiring longer travel, regardless of follow-up arrangement, the authors said.
“Black patients were also less likely than White patients to choose treatment plans that offered lower continuity with their current oncologist (importance weights, 2.50 to vs. 1.09, respectively),” they wrote.
Further, when making choices that required trade-offs, treatment efficacy was a weaker driver of treatment plan preferences for Black patient than for White patients (importance weights, 0.34 vs. 0.75 per 5% point increase in overall survival, respectively).
Why the findings matter
“Certain cancer treatments aren’t offered everywhere. Examples of this are the bone marrow transplants and [chimeric antigen receptor T-cell] therapies used to treat patients with blood cancers such as lymphoma,” Dr. Frosch said in an interview, adding that the limited geographic availability of these treatments means that patients who need them may have to travel farther and also to establish care with a new oncologist.
“These are both things that some patients may be reluctant to do,” added Dr. Frosch, who was with the Perelman School of Medicine at the University of Pennsylvania, Philadelphia, at the time of the study, but is now assistant professor at Fox Chase Cancer Center in Philadelphia.
“We wanted to better understand how patients think about these trade-offs,” he said. “We found that they were less likely to choose treatments requiring more travel, or treatments that required them to transfer care to a new oncologist. This was the case, even if it meant choosing a treatment that might be less effective against their cancer. But when patients were offered a chance to have half of their follow-up appointments locally, travel was less of a barrier.”
Importantly, not all participants valued each aspect of treatments equally, Dr. Frosch noted, referencing the responses of Black versus White patients.
He and his colleagues stressed that while collaborative follow-up may ease access to more distant treatments for some patients, the lesser willingness among Black participants to travel for cancer therapy – regardless of follow-up arrangement – means that attention must be paid to unintended consequences, to avoid worsening the existing disparities in access to cellular therapies.
These data represent a step toward better understanding of how patients considering whether or not to travel for specialized cancer care weigh trade-offs, he said.
“However, we need to dig deeper into the issues we uncovered in future research, he added. “Our findings suggest that collaborative follow-up between the hospitals that offer these treatments and the oncologists in patients’ own communities could improve access to specialized cancer treatments. But I also think it’s important to understand that this may not be the solution for everyone, and so multiple and individualized strategies are going to be needed.”
Personalized treatment strategies
The findings provide important perspective on the need to address patients’ concerns and circumstances to improve access to cellular therapies, said Ankit Kansagra, MD, the Eugene P. Frenkel, M.D. Scholar in Clinical Medicine at the University of Texas Southwestern Medical Center, Dallas.
The unique focus by Dr. Frosch and his associates on the patient perspective versus the health care system perspective underscores the need to be patient-focused, and serves as a reminder that different strategies are needed for different patients, Dr. Kansagra, who has also conducted research on access to CAR T therapies, said in an interview.
For some patients, a shared model of care is much more important than a 5% improvement in survival, he said, adding that providers shouldn’t assume that they understand a patient’s perspective.
Devising hybrid solutions that take community and individual needs into consideration would be preferable to seeking one national solution for care access, he added.
“It’s also pretty clear from this that it can be a shared model versus just an academic center or community center doing everything,” he said. “I think that’s going to be the next frontier – [determining] how we can hand over a patient, once CAR T is done, back to the community oncologist so he or she can continue following the patient and knows the survivorship plan – and keeping that model in place.”
Next steps
Further work is needed to determine the mechanisms driving the differences observed between Black and White patients in this study, the authors said, explaining that “[a]lthough the differences observed by race may reflect structural racism-driven access inequities, the relatively small subsample of Black patients and model complexity constraints limited our ability to analyze multiple factors.
“A prospective validation study to demonstrate the association of stated preferences with real-world decisions would further support our findings,” they wrote.
Dr. Frosch reported having no conflicts of interest. Dr. Kansagra is on advisory boards for Alnylam, Bristol Myers Squibb, Cota Healthcare, GSK, Janssen, Oncopeptides, and Takeda.
FROM JCO
One-quarter of lung cancer patients alive at 5 years
In recent years, the survival rate for patients with lung cancer has increased to the point where now, almost one-quarter of patients with lung cancer are alive 5 years after being diagnosed.
This new statistic is highlighted in the State of Lung Cancer report from the American Lung Association (ALA), published online on Nov. 16.
“If you look back, the 5-year survival rate has been very slowly eking up at about 1% over the years,” Andrea McKee, MD, volunteer spokesperson at the ALA, told this news organization. “To see this big jump is truly remarkable, so that is something we are all celebrating,” she added.
“But we have to change the fatalistic thinking that both patients and primary care physicians still have about lung cancer. Most people say, ‘Everybody I know who had lung cancer died,’ and that was the way it used to be,” she commented, “but that has now changed. Lung cancer is highly curable in its early stages, and even if not early-stage, there are treatments that are making an impact now.”
“So we’ve got to change that perception, as it does exist, even on the part of primary care providers, too,” Dr. McKee emphasized.
Lung cancer decreasing but still being diagnosed late
The report notes that the risk of being diagnosed with lung cancer varies considerably across the United States. For example, rates of lung cancer diagnoses are almost 2.5 times higher in Kentucky than in Utah.
Overall, the incidence is decreasing. “Over the last 5 years, the rate of new cases decreased 10% nationally,” the authors point out.
However, in almost half of the cases, the disease is diagnosed in late stages.
When diagnosed at a late stage, the 5-year survival rate for lung cancer drops to only 6%, whereas when the disease is diagnosed early, the 5-year survival rate is 60%.
At present, around 24% of cases of lung cancer are diagnosed at early stages, the report notes, but again, this varies across the United States. The highest rate (30%) is in Massachusetts, and the lowest rate (19%) is in Hawaii.
The percentage of lung cancer cases diagnosed early has been steadily increasing, presumably in part because of the introduction of low-dose CT screening for individuals at highest risk (such as smokers).
However, across the nation, only 5.7% of individuals at high risk for lung cancer underwent annual low-dose CT screening, the report notes.
“CT screening is so powerful at saving lives that even with only 5.7% of people that we’ve been able to screen, I believe it’s making a difference,” Dr. McKee commented. That small national percentage still represents a considerable number of patients, she noted, “so even with what we’ve done so far, I believe that screening is making a difference, at least within my own practice, where I’m definitely seeing it,” Dr. McKee emphasized.
Recent changes to the recommendations as to who should undergo lung cancer screening “have almost doubled the size of the screening population in the U.S.,” Dr. McKee commented. “So there are now about 15 million people who need to get screened, and it again helps that primary care physicians know that screening is very powerful at detecting early-stage lung cancer,” she said.
In her hospital’s own screening program, among the individuals who regularly undergo screening, the majority (88%) of lung cancer cases are detected at stage I or II, for which the cure rate is approximately 90%, she noted.
Another misconception of primary care physicians is that lung cancer screening has an unacceptably high false positive rate. Previous reports in the medical literature suggested the rate could be as high as 96%. “This is absolutely, positively wrong. That is not the false positive rate; the false positive rate for lung cancer screening is less than 10%,” Dr. McKee emphasized.
“So we have to change that in the minds of primary care providers as well,” she underscored.
Report highlights racial disparities
The report also highlights the racial disparities that persist in all aspects of lung cancer management – early diagnosis, surgical treatment, lack of treatment, and survival.
For example, Black Americans are 18% less likely to be diagnosed with early-stage disease and are 23% less likely to receive surgical treatment than their White counterparts. They are also 9% more likely to receive no treatment at all, and mortality from lung cancer among Black patients is 21% worse than it is for White patients.
The same trend is seen among Latinx persons, although they are just as likely as White patients to undergo surgical treatment.
First and foremost, “we have to make sure that the [Black and Latinx persons] are screened in an equal fashion,” Dr. McKee said. Providing screening for communities of color is one strategy that might improve screening rates, she suggested.
So, too, can outreach programs in which lung cancer experts work with leaders within these communities, because people are more likely to listen to their leaders regarding the importance of screening for early detection of lung cancer.
Physicians also need to emphasize that even for people who quit smoking decades ago, once those persons are in their 70s, “there is a spike again in lung cancer diagnoses, and that is true for both Black and White patients,” Dr. McKee stressed.
“Again, this is something that many doctors are not aware of,” she emphasized.
Dr. McKee has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
In recent years, the survival rate for patients with lung cancer has increased to the point where now, almost one-quarter of patients with lung cancer are alive 5 years after being diagnosed.
This new statistic is highlighted in the State of Lung Cancer report from the American Lung Association (ALA), published online on Nov. 16.
“If you look back, the 5-year survival rate has been very slowly eking up at about 1% over the years,” Andrea McKee, MD, volunteer spokesperson at the ALA, told this news organization. “To see this big jump is truly remarkable, so that is something we are all celebrating,” she added.
“But we have to change the fatalistic thinking that both patients and primary care physicians still have about lung cancer. Most people say, ‘Everybody I know who had lung cancer died,’ and that was the way it used to be,” she commented, “but that has now changed. Lung cancer is highly curable in its early stages, and even if not early-stage, there are treatments that are making an impact now.”
“So we’ve got to change that perception, as it does exist, even on the part of primary care providers, too,” Dr. McKee emphasized.
Lung cancer decreasing but still being diagnosed late
The report notes that the risk of being diagnosed with lung cancer varies considerably across the United States. For example, rates of lung cancer diagnoses are almost 2.5 times higher in Kentucky than in Utah.
Overall, the incidence is decreasing. “Over the last 5 years, the rate of new cases decreased 10% nationally,” the authors point out.
However, in almost half of the cases, the disease is diagnosed in late stages.
When diagnosed at a late stage, the 5-year survival rate for lung cancer drops to only 6%, whereas when the disease is diagnosed early, the 5-year survival rate is 60%.
At present, around 24% of cases of lung cancer are diagnosed at early stages, the report notes, but again, this varies across the United States. The highest rate (30%) is in Massachusetts, and the lowest rate (19%) is in Hawaii.
The percentage of lung cancer cases diagnosed early has been steadily increasing, presumably in part because of the introduction of low-dose CT screening for individuals at highest risk (such as smokers).
However, across the nation, only 5.7% of individuals at high risk for lung cancer underwent annual low-dose CT screening, the report notes.
“CT screening is so powerful at saving lives that even with only 5.7% of people that we’ve been able to screen, I believe it’s making a difference,” Dr. McKee commented. That small national percentage still represents a considerable number of patients, she noted, “so even with what we’ve done so far, I believe that screening is making a difference, at least within my own practice, where I’m definitely seeing it,” Dr. McKee emphasized.
Recent changes to the recommendations as to who should undergo lung cancer screening “have almost doubled the size of the screening population in the U.S.,” Dr. McKee commented. “So there are now about 15 million people who need to get screened, and it again helps that primary care physicians know that screening is very powerful at detecting early-stage lung cancer,” she said.
In her hospital’s own screening program, among the individuals who regularly undergo screening, the majority (88%) of lung cancer cases are detected at stage I or II, for which the cure rate is approximately 90%, she noted.
Another misconception of primary care physicians is that lung cancer screening has an unacceptably high false positive rate. Previous reports in the medical literature suggested the rate could be as high as 96%. “This is absolutely, positively wrong. That is not the false positive rate; the false positive rate for lung cancer screening is less than 10%,” Dr. McKee emphasized.
“So we have to change that in the minds of primary care providers as well,” she underscored.
Report highlights racial disparities
The report also highlights the racial disparities that persist in all aspects of lung cancer management – early diagnosis, surgical treatment, lack of treatment, and survival.
For example, Black Americans are 18% less likely to be diagnosed with early-stage disease and are 23% less likely to receive surgical treatment than their White counterparts. They are also 9% more likely to receive no treatment at all, and mortality from lung cancer among Black patients is 21% worse than it is for White patients.
The same trend is seen among Latinx persons, although they are just as likely as White patients to undergo surgical treatment.
First and foremost, “we have to make sure that the [Black and Latinx persons] are screened in an equal fashion,” Dr. McKee said. Providing screening for communities of color is one strategy that might improve screening rates, she suggested.
So, too, can outreach programs in which lung cancer experts work with leaders within these communities, because people are more likely to listen to their leaders regarding the importance of screening for early detection of lung cancer.
Physicians also need to emphasize that even for people who quit smoking decades ago, once those persons are in their 70s, “there is a spike again in lung cancer diagnoses, and that is true for both Black and White patients,” Dr. McKee stressed.
“Again, this is something that many doctors are not aware of,” she emphasized.
Dr. McKee has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
In recent years, the survival rate for patients with lung cancer has increased to the point where now, almost one-quarter of patients with lung cancer are alive 5 years after being diagnosed.
This new statistic is highlighted in the State of Lung Cancer report from the American Lung Association (ALA), published online on Nov. 16.
“If you look back, the 5-year survival rate has been very slowly eking up at about 1% over the years,” Andrea McKee, MD, volunteer spokesperson at the ALA, told this news organization. “To see this big jump is truly remarkable, so that is something we are all celebrating,” she added.
“But we have to change the fatalistic thinking that both patients and primary care physicians still have about lung cancer. Most people say, ‘Everybody I know who had lung cancer died,’ and that was the way it used to be,” she commented, “but that has now changed. Lung cancer is highly curable in its early stages, and even if not early-stage, there are treatments that are making an impact now.”
“So we’ve got to change that perception, as it does exist, even on the part of primary care providers, too,” Dr. McKee emphasized.
Lung cancer decreasing but still being diagnosed late
The report notes that the risk of being diagnosed with lung cancer varies considerably across the United States. For example, rates of lung cancer diagnoses are almost 2.5 times higher in Kentucky than in Utah.
Overall, the incidence is decreasing. “Over the last 5 years, the rate of new cases decreased 10% nationally,” the authors point out.
However, in almost half of the cases, the disease is diagnosed in late stages.
When diagnosed at a late stage, the 5-year survival rate for lung cancer drops to only 6%, whereas when the disease is diagnosed early, the 5-year survival rate is 60%.
At present, around 24% of cases of lung cancer are diagnosed at early stages, the report notes, but again, this varies across the United States. The highest rate (30%) is in Massachusetts, and the lowest rate (19%) is in Hawaii.
The percentage of lung cancer cases diagnosed early has been steadily increasing, presumably in part because of the introduction of low-dose CT screening for individuals at highest risk (such as smokers).
However, across the nation, only 5.7% of individuals at high risk for lung cancer underwent annual low-dose CT screening, the report notes.
“CT screening is so powerful at saving lives that even with only 5.7% of people that we’ve been able to screen, I believe it’s making a difference,” Dr. McKee commented. That small national percentage still represents a considerable number of patients, she noted, “so even with what we’ve done so far, I believe that screening is making a difference, at least within my own practice, where I’m definitely seeing it,” Dr. McKee emphasized.
Recent changes to the recommendations as to who should undergo lung cancer screening “have almost doubled the size of the screening population in the U.S.,” Dr. McKee commented. “So there are now about 15 million people who need to get screened, and it again helps that primary care physicians know that screening is very powerful at detecting early-stage lung cancer,” she said.
In her hospital’s own screening program, among the individuals who regularly undergo screening, the majority (88%) of lung cancer cases are detected at stage I or II, for which the cure rate is approximately 90%, she noted.
Another misconception of primary care physicians is that lung cancer screening has an unacceptably high false positive rate. Previous reports in the medical literature suggested the rate could be as high as 96%. “This is absolutely, positively wrong. That is not the false positive rate; the false positive rate for lung cancer screening is less than 10%,” Dr. McKee emphasized.
“So we have to change that in the minds of primary care providers as well,” she underscored.
Report highlights racial disparities
The report also highlights the racial disparities that persist in all aspects of lung cancer management – early diagnosis, surgical treatment, lack of treatment, and survival.
For example, Black Americans are 18% less likely to be diagnosed with early-stage disease and are 23% less likely to receive surgical treatment than their White counterparts. They are also 9% more likely to receive no treatment at all, and mortality from lung cancer among Black patients is 21% worse than it is for White patients.
The same trend is seen among Latinx persons, although they are just as likely as White patients to undergo surgical treatment.
First and foremost, “we have to make sure that the [Black and Latinx persons] are screened in an equal fashion,” Dr. McKee said. Providing screening for communities of color is one strategy that might improve screening rates, she suggested.
So, too, can outreach programs in which lung cancer experts work with leaders within these communities, because people are more likely to listen to their leaders regarding the importance of screening for early detection of lung cancer.
Physicians also need to emphasize that even for people who quit smoking decades ago, once those persons are in their 70s, “there is a spike again in lung cancer diagnoses, and that is true for both Black and White patients,” Dr. McKee stressed.
“Again, this is something that many doctors are not aware of,” she emphasized.
Dr. McKee has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
CHEST in the news
Creating a stronger voice for CHEST members in pulmonary, critical care, and sleep medicine, CHEST works to provide opportunities for members to serve as expert sources for both mainstream and trade media.
Below are a few highlights of media coverage from the past few months that work to expand awareness of CHEST and to promote the expertise of CHEST members in the media.
The New York Times covers the Philips recall
In August, a New York Times article published quoting incoming CHEST President, David Schulman, MD, MPH, FCCP. The article covered the recent Philips recall and its impact on the COVID-19 pandemic.
Dr. Schulman is quoted saying, “Because the number of people coming into the hospital with severe respiratory symptoms has increased as a result of COVID-19, the demand for these devices has also increased, which is problematic since available supply has decreased as a result of the Philips recall.”
The full article, Breathing Machine Recall Over Possible Cancer Risk Leaves Millions Scrambling for Substitutes, can be found on the New York Times website.
Technical expert panel on coverage determinations
Peter Gay, MD, FCCP, was quoted in an article by McKnight’s Long-Term Care News on the recent technical expert panel recommendations for national coverage determinations for optimal noninvasive ventilation.
“Centers for Medicare & Medicaid Services was wanting rigorous scientific support necessary to clarify the ‘reasonable and necessary’ role of these new mechanical therapeutic modalities where there was none in order to move forward,” said Dr. Gay. “What we have done is create a pathway to simplify the maze of regulation and perhaps most importantly, remove the obstacles that currently exist.”
The full article, Panel on Non-Invasive Ventilation Seeks to Simplify ‘Maze’ of Regulation for Device Coverage, can be found on the McKnight’s Long-Term Care News website.
Asthma and HRT
Originally appearing in HealthDay, U.S. News and World Report covered a recent journal CHEST® publication Hormone Replacement Therapy and Development of New Asthma by Erik Soeren Halvard Hansen, MD, et al.
The study included about 34,500 women who were diagnosed with asthma between 1995 and 2018, when they were 40 to 65 years of age. Each was then compared with 10 asthma-free women.
Based on that comparison, HRT use was associated with a 63% higher risk for developing asthma, according to the study.
The full article, HRT Could Raise Odds for Asthma, can be found on the U.S. News & World Report website.
Pediatric ICU admission and COVID-19
Healio Pulmonology covered a recent journal CHEST publication, Changes in Pediatric ICU Utilization and Clinical Trends During the Coronavirus Pandemic, by Janine E. Zee-Cheng, MD, et al.
“Severe infections, traumatic injuries, perioperative conditions and acute exacerbations of chronic illnesses such as asthma and diabetes are among the most common causes of admission to a pediatric ICU; thus, the epidemiology of pediatric critical illness was likely sensitive to the indirect effects of COVID-19,” Janine E. Zee-Cheng, MD, adjunct clinical assistant professor of pediatrics in the department of pediatrics at Indiana University School of Medicine, Indianapolis, and colleagues wrote.
The full article, Pediatric ICU admissions significantly decreased during COVID-19 pandemic, can be found on the Healio website.
CHEST news
CHEST also recently issued a handful of statements and press releases on a variety of topics including the spread of misinformation, support of mandatory vaccinations for health care workers, and a statement advocating for broader coverage of supplemental oxygen use.
For all recent CHEST News, including these statements, visit the CHEST Newsroom on the CHEST website and follow the hashtag #CHESTNews on Twitter.
If you have been included in a recent news article and would like it to be featured, send the coverage to [email protected].
Creating a stronger voice for CHEST members in pulmonary, critical care, and sleep medicine, CHEST works to provide opportunities for members to serve as expert sources for both mainstream and trade media.
Below are a few highlights of media coverage from the past few months that work to expand awareness of CHEST and to promote the expertise of CHEST members in the media.
The New York Times covers the Philips recall
In August, a New York Times article published quoting incoming CHEST President, David Schulman, MD, MPH, FCCP. The article covered the recent Philips recall and its impact on the COVID-19 pandemic.
Dr. Schulman is quoted saying, “Because the number of people coming into the hospital with severe respiratory symptoms has increased as a result of COVID-19, the demand for these devices has also increased, which is problematic since available supply has decreased as a result of the Philips recall.”
The full article, Breathing Machine Recall Over Possible Cancer Risk Leaves Millions Scrambling for Substitutes, can be found on the New York Times website.
Technical expert panel on coverage determinations
Peter Gay, MD, FCCP, was quoted in an article by McKnight’s Long-Term Care News on the recent technical expert panel recommendations for national coverage determinations for optimal noninvasive ventilation.
“Centers for Medicare & Medicaid Services was wanting rigorous scientific support necessary to clarify the ‘reasonable and necessary’ role of these new mechanical therapeutic modalities where there was none in order to move forward,” said Dr. Gay. “What we have done is create a pathway to simplify the maze of regulation and perhaps most importantly, remove the obstacles that currently exist.”
The full article, Panel on Non-Invasive Ventilation Seeks to Simplify ‘Maze’ of Regulation for Device Coverage, can be found on the McKnight’s Long-Term Care News website.
Asthma and HRT
Originally appearing in HealthDay, U.S. News and World Report covered a recent journal CHEST® publication Hormone Replacement Therapy and Development of New Asthma by Erik Soeren Halvard Hansen, MD, et al.
The study included about 34,500 women who were diagnosed with asthma between 1995 and 2018, when they were 40 to 65 years of age. Each was then compared with 10 asthma-free women.
Based on that comparison, HRT use was associated with a 63% higher risk for developing asthma, according to the study.
The full article, HRT Could Raise Odds for Asthma, can be found on the U.S. News & World Report website.
Pediatric ICU admission and COVID-19
Healio Pulmonology covered a recent journal CHEST publication, Changes in Pediatric ICU Utilization and Clinical Trends During the Coronavirus Pandemic, by Janine E. Zee-Cheng, MD, et al.
“Severe infections, traumatic injuries, perioperative conditions and acute exacerbations of chronic illnesses such as asthma and diabetes are among the most common causes of admission to a pediatric ICU; thus, the epidemiology of pediatric critical illness was likely sensitive to the indirect effects of COVID-19,” Janine E. Zee-Cheng, MD, adjunct clinical assistant professor of pediatrics in the department of pediatrics at Indiana University School of Medicine, Indianapolis, and colleagues wrote.
The full article, Pediatric ICU admissions significantly decreased during COVID-19 pandemic, can be found on the Healio website.
CHEST news
CHEST also recently issued a handful of statements and press releases on a variety of topics including the spread of misinformation, support of mandatory vaccinations for health care workers, and a statement advocating for broader coverage of supplemental oxygen use.
For all recent CHEST News, including these statements, visit the CHEST Newsroom on the CHEST website and follow the hashtag #CHESTNews on Twitter.
If you have been included in a recent news article and would like it to be featured, send the coverage to [email protected].
Creating a stronger voice for CHEST members in pulmonary, critical care, and sleep medicine, CHEST works to provide opportunities for members to serve as expert sources for both mainstream and trade media.
Below are a few highlights of media coverage from the past few months that work to expand awareness of CHEST and to promote the expertise of CHEST members in the media.
The New York Times covers the Philips recall
In August, a New York Times article published quoting incoming CHEST President, David Schulman, MD, MPH, FCCP. The article covered the recent Philips recall and its impact on the COVID-19 pandemic.
Dr. Schulman is quoted saying, “Because the number of people coming into the hospital with severe respiratory symptoms has increased as a result of COVID-19, the demand for these devices has also increased, which is problematic since available supply has decreased as a result of the Philips recall.”
The full article, Breathing Machine Recall Over Possible Cancer Risk Leaves Millions Scrambling for Substitutes, can be found on the New York Times website.
Technical expert panel on coverage determinations
Peter Gay, MD, FCCP, was quoted in an article by McKnight’s Long-Term Care News on the recent technical expert panel recommendations for national coverage determinations for optimal noninvasive ventilation.
“Centers for Medicare & Medicaid Services was wanting rigorous scientific support necessary to clarify the ‘reasonable and necessary’ role of these new mechanical therapeutic modalities where there was none in order to move forward,” said Dr. Gay. “What we have done is create a pathway to simplify the maze of regulation and perhaps most importantly, remove the obstacles that currently exist.”
The full article, Panel on Non-Invasive Ventilation Seeks to Simplify ‘Maze’ of Regulation for Device Coverage, can be found on the McKnight’s Long-Term Care News website.
Asthma and HRT
Originally appearing in HealthDay, U.S. News and World Report covered a recent journal CHEST® publication Hormone Replacement Therapy and Development of New Asthma by Erik Soeren Halvard Hansen, MD, et al.
The study included about 34,500 women who were diagnosed with asthma between 1995 and 2018, when they were 40 to 65 years of age. Each was then compared with 10 asthma-free women.
Based on that comparison, HRT use was associated with a 63% higher risk for developing asthma, according to the study.
The full article, HRT Could Raise Odds for Asthma, can be found on the U.S. News & World Report website.
Pediatric ICU admission and COVID-19
Healio Pulmonology covered a recent journal CHEST publication, Changes in Pediatric ICU Utilization and Clinical Trends During the Coronavirus Pandemic, by Janine E. Zee-Cheng, MD, et al.
“Severe infections, traumatic injuries, perioperative conditions and acute exacerbations of chronic illnesses such as asthma and diabetes are among the most common causes of admission to a pediatric ICU; thus, the epidemiology of pediatric critical illness was likely sensitive to the indirect effects of COVID-19,” Janine E. Zee-Cheng, MD, adjunct clinical assistant professor of pediatrics in the department of pediatrics at Indiana University School of Medicine, Indianapolis, and colleagues wrote.
The full article, Pediatric ICU admissions significantly decreased during COVID-19 pandemic, can be found on the Healio website.
CHEST news
CHEST also recently issued a handful of statements and press releases on a variety of topics including the spread of misinformation, support of mandatory vaccinations for health care workers, and a statement advocating for broader coverage of supplemental oxygen use.
For all recent CHEST News, including these statements, visit the CHEST Newsroom on the CHEST website and follow the hashtag #CHESTNews on Twitter.
If you have been included in a recent news article and would like it to be featured, send the coverage to [email protected].
Finding your passion in fellowship
(This post is part of Our Life as a Fellow blog post series. This series includes “fellow life lessons” from current trainees in leadership with CHEST.)
Finding your passion in fellowship is an integral part of career development and has a profound impact on a young professional’s personal satisfaction. This can be a difficult task, but it can be accomplished by finding a mentor, thinking about long-term career goals, and considering what re-energizes you. Entering fellowship, some may have a preconceived idea of who they would like to be upon completion of training: An asthma specialist, a physician-scientist, a critical care junkie, etc. For most of us, fellowship is a black box of opportunity with endless paths and permutations. It can be difficult to navigate this landscape, as the path may meander and a few initial interests may develop into true passions.
During my fellowship, I have been fortunate to have had many great teachers and experiences caring for patients with pulmonary hypertension, my current primary focus. Here are a few steps I have taken in pursuit of finding my passion over the past several years of post-graduate medical education. ***Disclaimer: I am still a work in progress.***
First, find a mentor. For me it was easy – I remember interviewing for fellowship with my mentor and thinking: “That is who I want to be.” I think this is hugely important. Use the insights, mistakes, and successes of someone you admire (from near or far) to help guide you. Initially, while getting to know my mentor, it was more comfortable to follow from a safe distance without making an official commitment. This was a slow process that allowed me to explore multiple clinical and research interests simultaneously. Once your mind is set, stating your professional interests in a concise way helps you and your mentor define and differentiate hobbies from passions. The practice of medicine is still very much an apprenticeship, so having someone to act as a sounding board remains important. Mentorship is also critical for networking, which is important for professional growth and life beyond fellowship. Our community is small, and “people know people.” What happens if you can’t find a perfect mentor? Don’t worry! Try out as many mentors as you can find. You can learn from every conversation and relationship. Sometimes the path taken is just as important as the destination.
Second, think about your 5- or 10-year plan. Ultimately, when training is over, we will graduate from fellowship and be released into the wild. The skills we have obtained in training are going to be the foundation for the rest of our careers. Where would you like to be a few years post-training? In a lab? Private practice? Rural medicine? Teaching? Does the energy you are spending in fellowship to develop your passion extend beyond fellowship? Part of the excitement of pursuing a passion is envisioning how it may develop over the period of coming years. I envision honing my skills as a master general pulmonary clinician and then narrowing my focus to create a pulmonary hypertension care center of excellence. I think these are important points to consider while you have the protected headspace of fellowship to experiment and explore, and while you are not constrained by contractual obligations.
Third, think about what personally and professionally energizes you. Especially in the context of an ongoing global pandemic, burnout and physician dissatisfaction are at an all-time high. Acknowledge that your job is tough, and try to identify the things that will keep the engine running. This sounds straightforward, but you have to decide what recharges you and acknowledge those things that don’t. The importance of determining things that energize me did not occur to me until I started searching for my first job. This forced me to make a list of things that contributed to my happiness and dissatisfaction. Most future employers are skilled at asking about these qualities. A happy employee is productive and effective at his or her job!
If you are in training, take some time to get creative and answer the questions above. Doodle, make lists, or journal—find a moment to reflect on your hard work and on the promise of your future.
Kevin Swiatek, DO
Dr. Swiatek is a third-year Chief Fellow in the Division of Pulmonary and Critical Care Medicine at Virginia Commonwealth University in Richmond, Virginia. Dr. Swiatek is a member of the CHEST Trainee Work Group. His clinical interests include general pulmonary medicine, care of patients with pulmonary hypertension, and using point-of-care ultrasound (POCUS) as a diagnostic tool in the medical intensive care unit. His scholarly interests include implementation of fellowship medical education, teaching POCUS, and clinical and diagnostic assessment of patients with pulmonary hypertension.
Reprinted from Thought Leader Blog. August 23, 2021. www.chestnet.org.
(This post is part of Our Life as a Fellow blog post series. This series includes “fellow life lessons” from current trainees in leadership with CHEST.)
Finding your passion in fellowship is an integral part of career development and has a profound impact on a young professional’s personal satisfaction. This can be a difficult task, but it can be accomplished by finding a mentor, thinking about long-term career goals, and considering what re-energizes you. Entering fellowship, some may have a preconceived idea of who they would like to be upon completion of training: An asthma specialist, a physician-scientist, a critical care junkie, etc. For most of us, fellowship is a black box of opportunity with endless paths and permutations. It can be difficult to navigate this landscape, as the path may meander and a few initial interests may develop into true passions.
During my fellowship, I have been fortunate to have had many great teachers and experiences caring for patients with pulmonary hypertension, my current primary focus. Here are a few steps I have taken in pursuit of finding my passion over the past several years of post-graduate medical education. ***Disclaimer: I am still a work in progress.***
First, find a mentor. For me it was easy – I remember interviewing for fellowship with my mentor and thinking: “That is who I want to be.” I think this is hugely important. Use the insights, mistakes, and successes of someone you admire (from near or far) to help guide you. Initially, while getting to know my mentor, it was more comfortable to follow from a safe distance without making an official commitment. This was a slow process that allowed me to explore multiple clinical and research interests simultaneously. Once your mind is set, stating your professional interests in a concise way helps you and your mentor define and differentiate hobbies from passions. The practice of medicine is still very much an apprenticeship, so having someone to act as a sounding board remains important. Mentorship is also critical for networking, which is important for professional growth and life beyond fellowship. Our community is small, and “people know people.” What happens if you can’t find a perfect mentor? Don’t worry! Try out as many mentors as you can find. You can learn from every conversation and relationship. Sometimes the path taken is just as important as the destination.
Second, think about your 5- or 10-year plan. Ultimately, when training is over, we will graduate from fellowship and be released into the wild. The skills we have obtained in training are going to be the foundation for the rest of our careers. Where would you like to be a few years post-training? In a lab? Private practice? Rural medicine? Teaching? Does the energy you are spending in fellowship to develop your passion extend beyond fellowship? Part of the excitement of pursuing a passion is envisioning how it may develop over the period of coming years. I envision honing my skills as a master general pulmonary clinician and then narrowing my focus to create a pulmonary hypertension care center of excellence. I think these are important points to consider while you have the protected headspace of fellowship to experiment and explore, and while you are not constrained by contractual obligations.
Third, think about what personally and professionally energizes you. Especially in the context of an ongoing global pandemic, burnout and physician dissatisfaction are at an all-time high. Acknowledge that your job is tough, and try to identify the things that will keep the engine running. This sounds straightforward, but you have to decide what recharges you and acknowledge those things that don’t. The importance of determining things that energize me did not occur to me until I started searching for my first job. This forced me to make a list of things that contributed to my happiness and dissatisfaction. Most future employers are skilled at asking about these qualities. A happy employee is productive and effective at his or her job!
If you are in training, take some time to get creative and answer the questions above. Doodle, make lists, or journal—find a moment to reflect on your hard work and on the promise of your future.
Kevin Swiatek, DO
Dr. Swiatek is a third-year Chief Fellow in the Division of Pulmonary and Critical Care Medicine at Virginia Commonwealth University in Richmond, Virginia. Dr. Swiatek is a member of the CHEST Trainee Work Group. His clinical interests include general pulmonary medicine, care of patients with pulmonary hypertension, and using point-of-care ultrasound (POCUS) as a diagnostic tool in the medical intensive care unit. His scholarly interests include implementation of fellowship medical education, teaching POCUS, and clinical and diagnostic assessment of patients with pulmonary hypertension.
Reprinted from Thought Leader Blog. August 23, 2021. www.chestnet.org.
(This post is part of Our Life as a Fellow blog post series. This series includes “fellow life lessons” from current trainees in leadership with CHEST.)
Finding your passion in fellowship is an integral part of career development and has a profound impact on a young professional’s personal satisfaction. This can be a difficult task, but it can be accomplished by finding a mentor, thinking about long-term career goals, and considering what re-energizes you. Entering fellowship, some may have a preconceived idea of who they would like to be upon completion of training: An asthma specialist, a physician-scientist, a critical care junkie, etc. For most of us, fellowship is a black box of opportunity with endless paths and permutations. It can be difficult to navigate this landscape, as the path may meander and a few initial interests may develop into true passions.
During my fellowship, I have been fortunate to have had many great teachers and experiences caring for patients with pulmonary hypertension, my current primary focus. Here are a few steps I have taken in pursuit of finding my passion over the past several years of post-graduate medical education. ***Disclaimer: I am still a work in progress.***
First, find a mentor. For me it was easy – I remember interviewing for fellowship with my mentor and thinking: “That is who I want to be.” I think this is hugely important. Use the insights, mistakes, and successes of someone you admire (from near or far) to help guide you. Initially, while getting to know my mentor, it was more comfortable to follow from a safe distance without making an official commitment. This was a slow process that allowed me to explore multiple clinical and research interests simultaneously. Once your mind is set, stating your professional interests in a concise way helps you and your mentor define and differentiate hobbies from passions. The practice of medicine is still very much an apprenticeship, so having someone to act as a sounding board remains important. Mentorship is also critical for networking, which is important for professional growth and life beyond fellowship. Our community is small, and “people know people.” What happens if you can’t find a perfect mentor? Don’t worry! Try out as many mentors as you can find. You can learn from every conversation and relationship. Sometimes the path taken is just as important as the destination.
Second, think about your 5- or 10-year plan. Ultimately, when training is over, we will graduate from fellowship and be released into the wild. The skills we have obtained in training are going to be the foundation for the rest of our careers. Where would you like to be a few years post-training? In a lab? Private practice? Rural medicine? Teaching? Does the energy you are spending in fellowship to develop your passion extend beyond fellowship? Part of the excitement of pursuing a passion is envisioning how it may develop over the period of coming years. I envision honing my skills as a master general pulmonary clinician and then narrowing my focus to create a pulmonary hypertension care center of excellence. I think these are important points to consider while you have the protected headspace of fellowship to experiment and explore, and while you are not constrained by contractual obligations.
Third, think about what personally and professionally energizes you. Especially in the context of an ongoing global pandemic, burnout and physician dissatisfaction are at an all-time high. Acknowledge that your job is tough, and try to identify the things that will keep the engine running. This sounds straightforward, but you have to decide what recharges you and acknowledge those things that don’t. The importance of determining things that energize me did not occur to me until I started searching for my first job. This forced me to make a list of things that contributed to my happiness and dissatisfaction. Most future employers are skilled at asking about these qualities. A happy employee is productive and effective at his or her job!
If you are in training, take some time to get creative and answer the questions above. Doodle, make lists, or journal—find a moment to reflect on your hard work and on the promise of your future.
Kevin Swiatek, DO
Dr. Swiatek is a third-year Chief Fellow in the Division of Pulmonary and Critical Care Medicine at Virginia Commonwealth University in Richmond, Virginia. Dr. Swiatek is a member of the CHEST Trainee Work Group. His clinical interests include general pulmonary medicine, care of patients with pulmonary hypertension, and using point-of-care ultrasound (POCUS) as a diagnostic tool in the medical intensive care unit. His scholarly interests include implementation of fellowship medical education, teaching POCUS, and clinical and diagnostic assessment of patients with pulmonary hypertension.
Reprinted from Thought Leader Blog. August 23, 2021. www.chestnet.org.
The apnea-hypopnea index: Limitations and future directions
Obstructive sleep apnea (OSA) is characterized by repetitive upper airway collapse resulting in intermittent hypoxemia and hypercapnia, large intrathoracic pressure swings, and cortical arousals. The rate of apneas and hypopneas observed during sleep, the apnea-hypopnea index (AHI), has been used for decades to diagnose OSA and to classify its severity. Despite the wide acceptance of this metric by the sleep medicine community, clinical research has found poor correlations between the AHI- and OSA-related complications or symptoms. We have come to learn that the AHI is an oversimplification of a complex and diverse disease process. (Punjabi. Chest. 2016;149[1]:16-9).
The most important features of a disease metric are reliability, and the ability to predict clinically relevant outcomes. The reliability of the AHI has been in question due to substantial night-to-night variability that can lead to missed diagnosis and disease severity misclassification (Dzierzewski et al. J Clin Sleep Med. 2020;16[4]:539-44). Furthermore, the AHI fails to reflect some important physiologic derangements resulting from respiratory events. Apart from imperfectly set thresholds for scoring, it disregards the depth and the duration of ventilatory disturbances. For example, a hypopnea lasting 30 seconds and resulting in a decrease of 10% in oxyhemoglobin saturation is considered equivalent to a hypopnea lasting 10 seconds and resulting in a decrease of 4% in oxyhemoglobin saturation. The AHI also assumes that apneas and hypopneas are equal in their biological effects regardless of when they occur during sleep (NREM vs REM), despite reports suggesting that the sequalae of OSA are sleep-stage dependent (Varga, Mokhlesi. Sleep Breath. 2019;23[2]:413-23). This is further complicated by the varying hypopnea definitions and the difficulties in differentiating obstructive vs central hypopneas. It is doubtful that these events, which differ in mechanism, would result in similar outcomes.
Over the past decade, our understanding of the different pathophysiological mechanisms leading to OSA has grown substantially, suggesting the need for a phenotype-specific treatment approach (Zinchuk, Yaggi. Chest. 2020;157[2]:403-20). The reliance on a single metric that does not capture this heterogeneity may prove detrimental to our therapeutic efforts. One extremely important dimension that is missed by the AHI is the patient. Individual response to airway obstruction varies with age, genetics, gender, and comorbidities, among other things. This may explain the difference in symptoms and outcomes experienced by patients with the same AHI. During the era of precision medicine, the concept of defining a clinical condition by a single test result, without regard to patient characteristics, is antiquated.
Several studies have attempted to propose complementary metrics that may better characterize OSA and predict outcomes. The hypoxic burden has gained a lot of attention as it is generally felt that hypoxemia is a major factor contributing to the pathogenesis of OSA-related comorbidities. Azarbarzin, et al. reported a hypoxic burden metric by measuring the area under the oxygen desaturation curve during a respiratory event (Azarbarzin et al. Eur Heart J. 2019;40[14]:1149-57). It factors the length and depth of the desaturations into a single value that expresses the average desaturation burden per hour of sleep time. The hypoxic burden was independently predictive of cardiovascular mortality in two large cohorts. Interestingly, the AHI did not have such an association. Similarly, another novel proposed parameter, the oxygen desaturation rate (ODR), outperformed the AHI in predicting cardiovascular outcomes in severe OSA patients (Wang et al. J Clin Sleep Med. 2020;16[7]:1055-62). The ODR measures the speed of an oxygen desaturation during an apnea event. Subjects with a faster ODR were found to have higher blood pressure values and variability. The authors hypothesized that slower desaturations generate hypoxemia-conditioning that may protect from exaggerated hemodynamic changes. These findings of novel hypoxemia metrics, albeit having their own limitations, recapitulate the need to move beyond the AHI to characterize OSA.
The apnea-hypopnea event duration is another overlooked feature that may impact OSA outcomes. Butler, et al. demonstrated that shorter event duration predicted a higher all-cause mortality over and beyond that predicted by AHI (Butler et al. Am J Respir Crit Care Med. 2019;199[7]:903-12). These results contrast views that early arousals in response to respiratory events may improve outcomes as they reflect a protective mechanism to prevent further hypoxemia and sympatho-excitation. For example, Ma, et al. found that higher percentage of total sleep time spent in apnea/hypopnea (AHT%) predicted worse daytime sleepiness to a higher degree than standard AHI (Ma et al. Sci Rep. 2021;11[1]:4702). However, shorter event duration may represent lower arousal thresholds (increased excitability), and ventilatory control instability (higher loop gain), predisposing patients to augmented sympathetic activity. Along similar lines, the intensity of respiratory-related arousals (as measured by EEG wavelet transformation) was found to be independent of preceding respiratory stimulus, with higher arousal intensity levels correlating with higher respiratory and heart rate responses (Amatoury et al. Sleep. 2016;39[12]:2091-100). The contribution of arousals to OSA morbidity is of particular importance for women in whom long-term outcomes of elevated AHI are poorly understood. Bearing in mind the differences in the metrics used, these results underscore the role of event duration and arousability in the pathogenesis of OSA-related morbidity.
The AHI is certainly an important piece of data that is informative and somewhat predictive. However, when used as a sole disease-defining metric, it has yielded disappointing results, especially after OSA treatment trials failed to show cardiovascular benefits despite therapies achieving a low residual AHI. As we aim to achieve a more personalized approach for diagnosing and treating OSA, we need to explore beyond the concept of a single metric to define a heterogenous and complex disorder. Instead of relying on the frequency of respiratory events, it is time to use complementary polysomnographic data that better reflect the origin and systemic effects of these disturbances. Machine-learning methods may offer sophisticated approaches to identifying polysomnographic patterns for future research. Clinical characteristics will also likely need to be considered in OSA severity scales. The identification of symptom subtypes or blood biomarkers may help identify patient groups who may be impacted differently by OSA, and consequently have a different treatment response (Malhotra et al. Sleep. 2021;44[7]:zsab030).
Almost half a century has lapsed since the original descriptions of OSA. Since then, our understanding of the disorder has improved greatly, with much still to be discovered, but our method of disease capture is unwavering. Future research requires a focus on novel measures aimed at identifying OSA endophenotypes, which will transform our understanding of disease traits and propel us into personalized therapies.
Dr. Mansour is Assistant Professor of Medicine, Division of Pulmonary, Allergy, and Critical Care Medicine, Duke University School of Medicine, Durham, North Carolina. Dr. Won is Associate Professor of Medicine, Section of Pulmonary, Critical Care, and Sleep Medicine, Yale University School of Medicine; and VA Connecticut Healthcare System, West Haven, Connecticut.
Obstructive sleep apnea (OSA) is characterized by repetitive upper airway collapse resulting in intermittent hypoxemia and hypercapnia, large intrathoracic pressure swings, and cortical arousals. The rate of apneas and hypopneas observed during sleep, the apnea-hypopnea index (AHI), has been used for decades to diagnose OSA and to classify its severity. Despite the wide acceptance of this metric by the sleep medicine community, clinical research has found poor correlations between the AHI- and OSA-related complications or symptoms. We have come to learn that the AHI is an oversimplification of a complex and diverse disease process. (Punjabi. Chest. 2016;149[1]:16-9).
The most important features of a disease metric are reliability, and the ability to predict clinically relevant outcomes. The reliability of the AHI has been in question due to substantial night-to-night variability that can lead to missed diagnosis and disease severity misclassification (Dzierzewski et al. J Clin Sleep Med. 2020;16[4]:539-44). Furthermore, the AHI fails to reflect some important physiologic derangements resulting from respiratory events. Apart from imperfectly set thresholds for scoring, it disregards the depth and the duration of ventilatory disturbances. For example, a hypopnea lasting 30 seconds and resulting in a decrease of 10% in oxyhemoglobin saturation is considered equivalent to a hypopnea lasting 10 seconds and resulting in a decrease of 4% in oxyhemoglobin saturation. The AHI also assumes that apneas and hypopneas are equal in their biological effects regardless of when they occur during sleep (NREM vs REM), despite reports suggesting that the sequalae of OSA are sleep-stage dependent (Varga, Mokhlesi. Sleep Breath. 2019;23[2]:413-23). This is further complicated by the varying hypopnea definitions and the difficulties in differentiating obstructive vs central hypopneas. It is doubtful that these events, which differ in mechanism, would result in similar outcomes.
Over the past decade, our understanding of the different pathophysiological mechanisms leading to OSA has grown substantially, suggesting the need for a phenotype-specific treatment approach (Zinchuk, Yaggi. Chest. 2020;157[2]:403-20). The reliance on a single metric that does not capture this heterogeneity may prove detrimental to our therapeutic efforts. One extremely important dimension that is missed by the AHI is the patient. Individual response to airway obstruction varies with age, genetics, gender, and comorbidities, among other things. This may explain the difference in symptoms and outcomes experienced by patients with the same AHI. During the era of precision medicine, the concept of defining a clinical condition by a single test result, without regard to patient characteristics, is antiquated.
Several studies have attempted to propose complementary metrics that may better characterize OSA and predict outcomes. The hypoxic burden has gained a lot of attention as it is generally felt that hypoxemia is a major factor contributing to the pathogenesis of OSA-related comorbidities. Azarbarzin, et al. reported a hypoxic burden metric by measuring the area under the oxygen desaturation curve during a respiratory event (Azarbarzin et al. Eur Heart J. 2019;40[14]:1149-57). It factors the length and depth of the desaturations into a single value that expresses the average desaturation burden per hour of sleep time. The hypoxic burden was independently predictive of cardiovascular mortality in two large cohorts. Interestingly, the AHI did not have such an association. Similarly, another novel proposed parameter, the oxygen desaturation rate (ODR), outperformed the AHI in predicting cardiovascular outcomes in severe OSA patients (Wang et al. J Clin Sleep Med. 2020;16[7]:1055-62). The ODR measures the speed of an oxygen desaturation during an apnea event. Subjects with a faster ODR were found to have higher blood pressure values and variability. The authors hypothesized that slower desaturations generate hypoxemia-conditioning that may protect from exaggerated hemodynamic changes. These findings of novel hypoxemia metrics, albeit having their own limitations, recapitulate the need to move beyond the AHI to characterize OSA.
The apnea-hypopnea event duration is another overlooked feature that may impact OSA outcomes. Butler, et al. demonstrated that shorter event duration predicted a higher all-cause mortality over and beyond that predicted by AHI (Butler et al. Am J Respir Crit Care Med. 2019;199[7]:903-12). These results contrast views that early arousals in response to respiratory events may improve outcomes as they reflect a protective mechanism to prevent further hypoxemia and sympatho-excitation. For example, Ma, et al. found that higher percentage of total sleep time spent in apnea/hypopnea (AHT%) predicted worse daytime sleepiness to a higher degree than standard AHI (Ma et al. Sci Rep. 2021;11[1]:4702). However, shorter event duration may represent lower arousal thresholds (increased excitability), and ventilatory control instability (higher loop gain), predisposing patients to augmented sympathetic activity. Along similar lines, the intensity of respiratory-related arousals (as measured by EEG wavelet transformation) was found to be independent of preceding respiratory stimulus, with higher arousal intensity levels correlating with higher respiratory and heart rate responses (Amatoury et al. Sleep. 2016;39[12]:2091-100). The contribution of arousals to OSA morbidity is of particular importance for women in whom long-term outcomes of elevated AHI are poorly understood. Bearing in mind the differences in the metrics used, these results underscore the role of event duration and arousability in the pathogenesis of OSA-related morbidity.
The AHI is certainly an important piece of data that is informative and somewhat predictive. However, when used as a sole disease-defining metric, it has yielded disappointing results, especially after OSA treatment trials failed to show cardiovascular benefits despite therapies achieving a low residual AHI. As we aim to achieve a more personalized approach for diagnosing and treating OSA, we need to explore beyond the concept of a single metric to define a heterogenous and complex disorder. Instead of relying on the frequency of respiratory events, it is time to use complementary polysomnographic data that better reflect the origin and systemic effects of these disturbances. Machine-learning methods may offer sophisticated approaches to identifying polysomnographic patterns for future research. Clinical characteristics will also likely need to be considered in OSA severity scales. The identification of symptom subtypes or blood biomarkers may help identify patient groups who may be impacted differently by OSA, and consequently have a different treatment response (Malhotra et al. Sleep. 2021;44[7]:zsab030).
Almost half a century has lapsed since the original descriptions of OSA. Since then, our understanding of the disorder has improved greatly, with much still to be discovered, but our method of disease capture is unwavering. Future research requires a focus on novel measures aimed at identifying OSA endophenotypes, which will transform our understanding of disease traits and propel us into personalized therapies.
Dr. Mansour is Assistant Professor of Medicine, Division of Pulmonary, Allergy, and Critical Care Medicine, Duke University School of Medicine, Durham, North Carolina. Dr. Won is Associate Professor of Medicine, Section of Pulmonary, Critical Care, and Sleep Medicine, Yale University School of Medicine; and VA Connecticut Healthcare System, West Haven, Connecticut.
Obstructive sleep apnea (OSA) is characterized by repetitive upper airway collapse resulting in intermittent hypoxemia and hypercapnia, large intrathoracic pressure swings, and cortical arousals. The rate of apneas and hypopneas observed during sleep, the apnea-hypopnea index (AHI), has been used for decades to diagnose OSA and to classify its severity. Despite the wide acceptance of this metric by the sleep medicine community, clinical research has found poor correlations between the AHI- and OSA-related complications or symptoms. We have come to learn that the AHI is an oversimplification of a complex and diverse disease process. (Punjabi. Chest. 2016;149[1]:16-9).
The most important features of a disease metric are reliability, and the ability to predict clinically relevant outcomes. The reliability of the AHI has been in question due to substantial night-to-night variability that can lead to missed diagnosis and disease severity misclassification (Dzierzewski et al. J Clin Sleep Med. 2020;16[4]:539-44). Furthermore, the AHI fails to reflect some important physiologic derangements resulting from respiratory events. Apart from imperfectly set thresholds for scoring, it disregards the depth and the duration of ventilatory disturbances. For example, a hypopnea lasting 30 seconds and resulting in a decrease of 10% in oxyhemoglobin saturation is considered equivalent to a hypopnea lasting 10 seconds and resulting in a decrease of 4% in oxyhemoglobin saturation. The AHI also assumes that apneas and hypopneas are equal in their biological effects regardless of when they occur during sleep (NREM vs REM), despite reports suggesting that the sequalae of OSA are sleep-stage dependent (Varga, Mokhlesi. Sleep Breath. 2019;23[2]:413-23). This is further complicated by the varying hypopnea definitions and the difficulties in differentiating obstructive vs central hypopneas. It is doubtful that these events, which differ in mechanism, would result in similar outcomes.
Over the past decade, our understanding of the different pathophysiological mechanisms leading to OSA has grown substantially, suggesting the need for a phenotype-specific treatment approach (Zinchuk, Yaggi. Chest. 2020;157[2]:403-20). The reliance on a single metric that does not capture this heterogeneity may prove detrimental to our therapeutic efforts. One extremely important dimension that is missed by the AHI is the patient. Individual response to airway obstruction varies with age, genetics, gender, and comorbidities, among other things. This may explain the difference in symptoms and outcomes experienced by patients with the same AHI. During the era of precision medicine, the concept of defining a clinical condition by a single test result, without regard to patient characteristics, is antiquated.
Several studies have attempted to propose complementary metrics that may better characterize OSA and predict outcomes. The hypoxic burden has gained a lot of attention as it is generally felt that hypoxemia is a major factor contributing to the pathogenesis of OSA-related comorbidities. Azarbarzin, et al. reported a hypoxic burden metric by measuring the area under the oxygen desaturation curve during a respiratory event (Azarbarzin et al. Eur Heart J. 2019;40[14]:1149-57). It factors the length and depth of the desaturations into a single value that expresses the average desaturation burden per hour of sleep time. The hypoxic burden was independently predictive of cardiovascular mortality in two large cohorts. Interestingly, the AHI did not have such an association. Similarly, another novel proposed parameter, the oxygen desaturation rate (ODR), outperformed the AHI in predicting cardiovascular outcomes in severe OSA patients (Wang et al. J Clin Sleep Med. 2020;16[7]:1055-62). The ODR measures the speed of an oxygen desaturation during an apnea event. Subjects with a faster ODR were found to have higher blood pressure values and variability. The authors hypothesized that slower desaturations generate hypoxemia-conditioning that may protect from exaggerated hemodynamic changes. These findings of novel hypoxemia metrics, albeit having their own limitations, recapitulate the need to move beyond the AHI to characterize OSA.
The apnea-hypopnea event duration is another overlooked feature that may impact OSA outcomes. Butler, et al. demonstrated that shorter event duration predicted a higher all-cause mortality over and beyond that predicted by AHI (Butler et al. Am J Respir Crit Care Med. 2019;199[7]:903-12). These results contrast views that early arousals in response to respiratory events may improve outcomes as they reflect a protective mechanism to prevent further hypoxemia and sympatho-excitation. For example, Ma, et al. found that higher percentage of total sleep time spent in apnea/hypopnea (AHT%) predicted worse daytime sleepiness to a higher degree than standard AHI (Ma et al. Sci Rep. 2021;11[1]:4702). However, shorter event duration may represent lower arousal thresholds (increased excitability), and ventilatory control instability (higher loop gain), predisposing patients to augmented sympathetic activity. Along similar lines, the intensity of respiratory-related arousals (as measured by EEG wavelet transformation) was found to be independent of preceding respiratory stimulus, with higher arousal intensity levels correlating with higher respiratory and heart rate responses (Amatoury et al. Sleep. 2016;39[12]:2091-100). The contribution of arousals to OSA morbidity is of particular importance for women in whom long-term outcomes of elevated AHI are poorly understood. Bearing in mind the differences in the metrics used, these results underscore the role of event duration and arousability in the pathogenesis of OSA-related morbidity.
The AHI is certainly an important piece of data that is informative and somewhat predictive. However, when used as a sole disease-defining metric, it has yielded disappointing results, especially after OSA treatment trials failed to show cardiovascular benefits despite therapies achieving a low residual AHI. As we aim to achieve a more personalized approach for diagnosing and treating OSA, we need to explore beyond the concept of a single metric to define a heterogenous and complex disorder. Instead of relying on the frequency of respiratory events, it is time to use complementary polysomnographic data that better reflect the origin and systemic effects of these disturbances. Machine-learning methods may offer sophisticated approaches to identifying polysomnographic patterns for future research. Clinical characteristics will also likely need to be considered in OSA severity scales. The identification of symptom subtypes or blood biomarkers may help identify patient groups who may be impacted differently by OSA, and consequently have a different treatment response (Malhotra et al. Sleep. 2021;44[7]:zsab030).
Almost half a century has lapsed since the original descriptions of OSA. Since then, our understanding of the disorder has improved greatly, with much still to be discovered, but our method of disease capture is unwavering. Future research requires a focus on novel measures aimed at identifying OSA endophenotypes, which will transform our understanding of disease traits and propel us into personalized therapies.
Dr. Mansour is Assistant Professor of Medicine, Division of Pulmonary, Allergy, and Critical Care Medicine, Duke University School of Medicine, Durham, North Carolina. Dr. Won is Associate Professor of Medicine, Section of Pulmonary, Critical Care, and Sleep Medicine, Yale University School of Medicine; and VA Connecticut Healthcare System, West Haven, Connecticut.
Seborrheic dermatitis
THE COMPARISON
A Seborrheic dermatitis in a woman with brown-gray greasy scale, as well as petaloid papules and plaques that are especially prominent in the nasolabial folds.
B Seborrheic dermatitis in a man with erythema, scale, and mild postinflammatory hypopigmentation that are especially prominent in the nasolabial folds.
C Seborrheic dermatitis in a man with erythema, faint scale, and postinflammatory hypopigmentation that are especially prominent in the nasolabial folds.
D Seborrheic dermatitis in a man with erythema and scale of the eyebrows and glabellar region.
Seborrheic dermatitis (SD) is an inflammatory condition that is thought to be part of a response to Malassezia yeast. The scalp and face are most commonly affected, particularly the nasolabial folds, eyebrows, ears, postauricular areas, and beard area. Men also may have SD on the mid upper chest in association with chest hair. In infants, the scalp and body skin folds often are affected.
Epidemiology
SD affects patients of all ages: infants, adolescents, and adults. It is among the most common dermatologic diagnoses reported in Black patients in the United States.1
Key clinical features in darker skin tones
- In those with darker skin tones, arcuate, polycyclic, or petaloid (flower petallike) plaques may be present (FIGURE A). Also, hypopigmented patches and plaques may be prominent (FIGURES B AND C). The classic description includes thin pink patches and plaques with white greasy scale on the face (FIGURE D).
- The scalp may have diffuse scale or isolated scaly plaques.
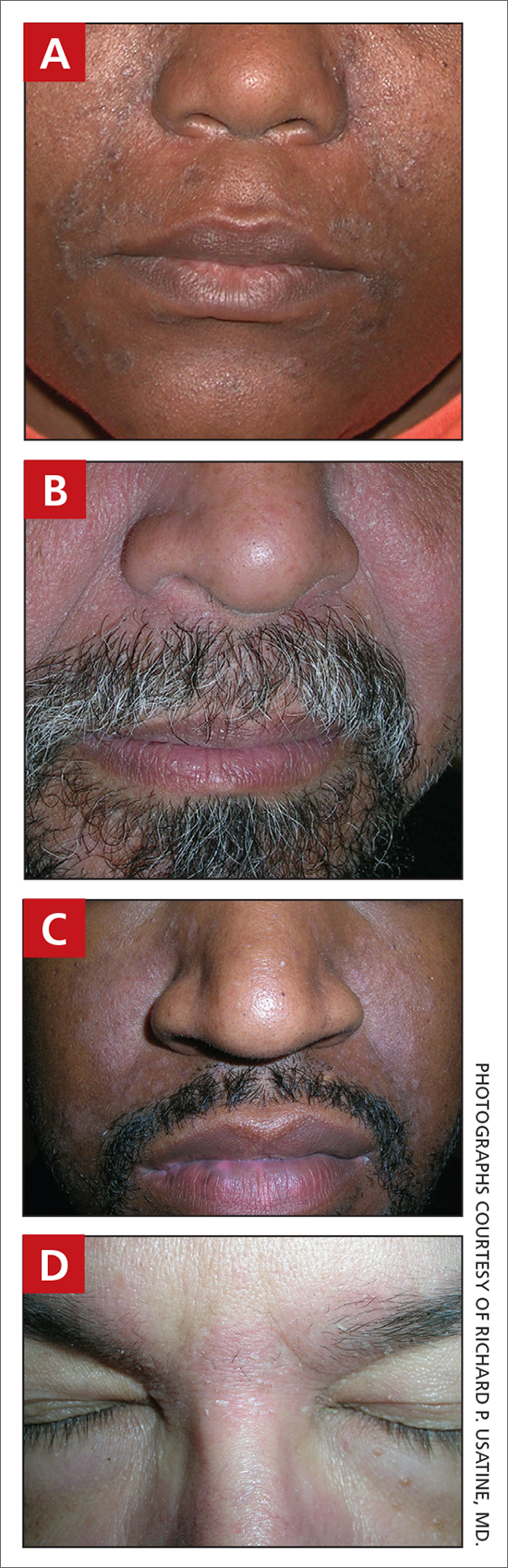
Worth noting
- In those with tightly coiled hair, there is a predisposition for dry hair and increased risk for breakage.
- Treatment plans for patients with SD often include frequent hair washing. However, in those with tightly coiled hair, the treatment plan may need to be modified due to hair texture, tendency for dryness, and washing frequency preferences. Washing the scalp at least every 1 to 2 weeks may be a preferred approach for those with tightly coiled hair at increased risk for dryness/breakage vs washing daily.2 In a sample of 201 caregivers of Black girls, Rucker Wright et al3 found that washing the hair more than once per week was not correlated with a lower prevalence of SD.
- If tightly coiled hair is temporarily straightened with heat (eg, blow-dryer, flat iron), adding a liquid-based treatment such as clobetasol solution or fluocinonide solution will cause the hair to revert to its normal curl pattern.
- It is appropriate to ask patients for their vehicle preference for medications.2 For example, if clobetasol is the treatment selected for the patient, the vehicle can reflect patient preference for a liquid, foam, cream, or ointment.
- Some antifungal/antiyeast shampoos may cause further hair dryness and breakage.
- Treatment may be delayed because patients often use various topical pomades and ointments to cover up the scale and help with pruritus.
- Diffuse scale of tinea capitis in school- aged children can be mistaken for SD, which leads to delayed diagnosis and treatment.
- Clinicians should become comfortable with scalp examinations in patients with tightly coiled hair. Patients with chief concerns related to their hair and scalp expect their clinicians to touch these areas. Avoid leaning in to examine the patient without touching the patient’s hair and scalp.2,4
Health disparity highlight
SD is among the most common cutaneous disorders diagnosed in patients with skin of color.1,5 Delay in recognition of SD in those with darker skin tones leads to delayed treatment. SD of the face can cause notable postinflammatory pigmentation alteration. Pigmentation changes in the skin further impact quality of life.
1. Alexis AF, Sergay AB, Taylor SC. Common dermatologic disorders in skin of color: a comparative practice survey. Cutis. 2007;80:387-394.
2. Grayson C, Heath C. Tips for addressing common conditions affecting pediatric and adolescent patients with skin of color [published online March 2, 2021]. Pediatr Dermatol. 2021;10.1111/ pde.14525
3. Rucker Wright D, Gathers R, Kapke A, et al. Hair care practices and their association with scalp and hair disorders in African American girls. J Am Acad Dermatol. 2011;64: 253-262. doi:10.1016/j.jaad.2010.05.037
4. Grayson C, Heath C. An approach to examining tightly coiled hair among patients with hair loss in race-discordant patientphysician interactions. JAMA Dermatol. 2021;157:505-506. doi:10.1001/jamadermatol.2021.0338
5. Gaulding JV, Gutierrez D, Bhatia BK, et al. Epidemiology of skin diseases in a diverse patient population. J Drugs Dermatol. 2018;17:1032-1036.
THE COMPARISON
A Seborrheic dermatitis in a woman with brown-gray greasy scale, as well as petaloid papules and plaques that are especially prominent in the nasolabial folds.
B Seborrheic dermatitis in a man with erythema, scale, and mild postinflammatory hypopigmentation that are especially prominent in the nasolabial folds.
C Seborrheic dermatitis in a man with erythema, faint scale, and postinflammatory hypopigmentation that are especially prominent in the nasolabial folds.
D Seborrheic dermatitis in a man with erythema and scale of the eyebrows and glabellar region.
Seborrheic dermatitis (SD) is an inflammatory condition that is thought to be part of a response to Malassezia yeast. The scalp and face are most commonly affected, particularly the nasolabial folds, eyebrows, ears, postauricular areas, and beard area. Men also may have SD on the mid upper chest in association with chest hair. In infants, the scalp and body skin folds often are affected.
Epidemiology
SD affects patients of all ages: infants, adolescents, and adults. It is among the most common dermatologic diagnoses reported in Black patients in the United States.1
Key clinical features in darker skin tones
- In those with darker skin tones, arcuate, polycyclic, or petaloid (flower petallike) plaques may be present (FIGURE A). Also, hypopigmented patches and plaques may be prominent (FIGURES B AND C). The classic description includes thin pink patches and plaques with white greasy scale on the face (FIGURE D).
- The scalp may have diffuse scale or isolated scaly plaques.

Worth noting
- In those with tightly coiled hair, there is a predisposition for dry hair and increased risk for breakage.
- Treatment plans for patients with SD often include frequent hair washing. However, in those with tightly coiled hair, the treatment plan may need to be modified due to hair texture, tendency for dryness, and washing frequency preferences. Washing the scalp at least every 1 to 2 weeks may be a preferred approach for those with tightly coiled hair at increased risk for dryness/breakage vs washing daily.2 In a sample of 201 caregivers of Black girls, Rucker Wright et al3 found that washing the hair more than once per week was not correlated with a lower prevalence of SD.
- If tightly coiled hair is temporarily straightened with heat (eg, blow-dryer, flat iron), adding a liquid-based treatment such as clobetasol solution or fluocinonide solution will cause the hair to revert to its normal curl pattern.
- It is appropriate to ask patients for their vehicle preference for medications.2 For example, if clobetasol is the treatment selected for the patient, the vehicle can reflect patient preference for a liquid, foam, cream, or ointment.
- Some antifungal/antiyeast shampoos may cause further hair dryness and breakage.
- Treatment may be delayed because patients often use various topical pomades and ointments to cover up the scale and help with pruritus.
- Diffuse scale of tinea capitis in school- aged children can be mistaken for SD, which leads to delayed diagnosis and treatment.
- Clinicians should become comfortable with scalp examinations in patients with tightly coiled hair. Patients with chief concerns related to their hair and scalp expect their clinicians to touch these areas. Avoid leaning in to examine the patient without touching the patient’s hair and scalp.2,4
Health disparity highlight
SD is among the most common cutaneous disorders diagnosed in patients with skin of color.1,5 Delay in recognition of SD in those with darker skin tones leads to delayed treatment. SD of the face can cause notable postinflammatory pigmentation alteration. Pigmentation changes in the skin further impact quality of life.
THE COMPARISON
A Seborrheic dermatitis in a woman with brown-gray greasy scale, as well as petaloid papules and plaques that are especially prominent in the nasolabial folds.
B Seborrheic dermatitis in a man with erythema, scale, and mild postinflammatory hypopigmentation that are especially prominent in the nasolabial folds.
C Seborrheic dermatitis in a man with erythema, faint scale, and postinflammatory hypopigmentation that are especially prominent in the nasolabial folds.
D Seborrheic dermatitis in a man with erythema and scale of the eyebrows and glabellar region.
Seborrheic dermatitis (SD) is an inflammatory condition that is thought to be part of a response to Malassezia yeast. The scalp and face are most commonly affected, particularly the nasolabial folds, eyebrows, ears, postauricular areas, and beard area. Men also may have SD on the mid upper chest in association with chest hair. In infants, the scalp and body skin folds often are affected.
Epidemiology
SD affects patients of all ages: infants, adolescents, and adults. It is among the most common dermatologic diagnoses reported in Black patients in the United States.1
Key clinical features in darker skin tones
- In those with darker skin tones, arcuate, polycyclic, or petaloid (flower petallike) plaques may be present (FIGURE A). Also, hypopigmented patches and plaques may be prominent (FIGURES B AND C). The classic description includes thin pink patches and plaques with white greasy scale on the face (FIGURE D).
- The scalp may have diffuse scale or isolated scaly plaques.

Worth noting
- In those with tightly coiled hair, there is a predisposition for dry hair and increased risk for breakage.
- Treatment plans for patients with SD often include frequent hair washing. However, in those with tightly coiled hair, the treatment plan may need to be modified due to hair texture, tendency for dryness, and washing frequency preferences. Washing the scalp at least every 1 to 2 weeks may be a preferred approach for those with tightly coiled hair at increased risk for dryness/breakage vs washing daily.2 In a sample of 201 caregivers of Black girls, Rucker Wright et al3 found that washing the hair more than once per week was not correlated with a lower prevalence of SD.
- If tightly coiled hair is temporarily straightened with heat (eg, blow-dryer, flat iron), adding a liquid-based treatment such as clobetasol solution or fluocinonide solution will cause the hair to revert to its normal curl pattern.
- It is appropriate to ask patients for their vehicle preference for medications.2 For example, if clobetasol is the treatment selected for the patient, the vehicle can reflect patient preference for a liquid, foam, cream, or ointment.
- Some antifungal/antiyeast shampoos may cause further hair dryness and breakage.
- Treatment may be delayed because patients often use various topical pomades and ointments to cover up the scale and help with pruritus.
- Diffuse scale of tinea capitis in school- aged children can be mistaken for SD, which leads to delayed diagnosis and treatment.
- Clinicians should become comfortable with scalp examinations in patients with tightly coiled hair. Patients with chief concerns related to their hair and scalp expect their clinicians to touch these areas. Avoid leaning in to examine the patient without touching the patient’s hair and scalp.2,4
Health disparity highlight
SD is among the most common cutaneous disorders diagnosed in patients with skin of color.1,5 Delay in recognition of SD in those with darker skin tones leads to delayed treatment. SD of the face can cause notable postinflammatory pigmentation alteration. Pigmentation changes in the skin further impact quality of life.
1. Alexis AF, Sergay AB, Taylor SC. Common dermatologic disorders in skin of color: a comparative practice survey. Cutis. 2007;80:387-394.
2. Grayson C, Heath C. Tips for addressing common conditions affecting pediatric and adolescent patients with skin of color [published online March 2, 2021]. Pediatr Dermatol. 2021;10.1111/ pde.14525
3. Rucker Wright D, Gathers R, Kapke A, et al. Hair care practices and their association with scalp and hair disorders in African American girls. J Am Acad Dermatol. 2011;64: 253-262. doi:10.1016/j.jaad.2010.05.037
4. Grayson C, Heath C. An approach to examining tightly coiled hair among patients with hair loss in race-discordant patientphysician interactions. JAMA Dermatol. 2021;157:505-506. doi:10.1001/jamadermatol.2021.0338
5. Gaulding JV, Gutierrez D, Bhatia BK, et al. Epidemiology of skin diseases in a diverse patient population. J Drugs Dermatol. 2018;17:1032-1036.
1. Alexis AF, Sergay AB, Taylor SC. Common dermatologic disorders in skin of color: a comparative practice survey. Cutis. 2007;80:387-394.
2. Grayson C, Heath C. Tips for addressing common conditions affecting pediatric and adolescent patients with skin of color [published online March 2, 2021]. Pediatr Dermatol. 2021;10.1111/ pde.14525
3. Rucker Wright D, Gathers R, Kapke A, et al. Hair care practices and their association with scalp and hair disorders in African American girls. J Am Acad Dermatol. 2011;64: 253-262. doi:10.1016/j.jaad.2010.05.037
4. Grayson C, Heath C. An approach to examining tightly coiled hair among patients with hair loss in race-discordant patientphysician interactions. JAMA Dermatol. 2021;157:505-506. doi:10.1001/jamadermatol.2021.0338
5. Gaulding JV, Gutierrez D, Bhatia BK, et al. Epidemiology of skin diseases in a diverse patient population. J Drugs Dermatol. 2018;17:1032-1036.
Ticagrelor reversal agent achieves quick hemostasis: REVERSE-IT
The experimental monoclonal antibody bentracimab, which reverses the antiplatelet effects of ticagrelor, appears to be heading toward regulatory approval, on the basis of an interim analysis of the phase 3 REVERSE-IT trial.
“Rates of effective hemostasis were adjudicated as good or excellent in more than 90% of cases with no drug-related serious adverse events or allergic or infusion-related reactions,” reported Deepak L. Bhatt, MD, at the American Heart Association scientific sessions.
The interim analysis of this nonrandomized, single-arm study was requested by the Food and Drug Administration, which is considering a conditional accelerated approval of bentracimab (formerly PB2452) if efficacy and safety are established.
Upon administration, bentracimab binds to free ticagrelor so that ticagrelor cannot bind to the P2Y12 platelet receptor. This interrupts one of the key steps in the pathway of platelet aggregation.
REVERSE-IT is still enrolling patients. This interim analysis was conducted with the first 150 patients who met eligibility criteria and were treated. Of these, 142 patients were enrolled for an urgent surgical indication and 8 for a major bleeding indication. After some exclusions for lack of urgency and reclassifications following adjudication, there were 113 surgical cases and 9 major bleeding patients evaluable for hemostasis.
Platelet function assays test reversal
On the primary reversal endpoint, which was restoration of activity on the proprietary platelet function assays Verify Now and PRUTest, a rapid restoration of platelet function was achieved in both surgical and major-bleeding patients. Platelet reactivity climbed to near normal levels within 10 minutes of administration, and peak effects were sustained through the first 24 hours after administration.
On the basis of the platelet function assays, the pattern of response to bentracimab was “very similar in the surgical and bleeding patients,” reported Dr. Bhatt, executive director of interventional cardiovascular programs at Brigham and Women’s Health, Boston.
The effect was also consistent across a broad array of prespecified subgroups, including stratifications by age, renal function, time from last dose of ticagrelor, race, and the presence of comorbidities, such as diabetes, renal dysfunction, hypertension, and history of MI.
Hemostasis documented in all but one patient
Adjudicated hemostasis was achieved in 100% of the 113 urgent surgical patients evaluated. In the nine major bleeding patients, six achieved excellent hemostasis and one achieved good hemostasis. One had poor hemostasis, and one was unevaluable.
Platelet rebound following bentracimab administration, measured by mean platelet volume, was not observed.
There were no serious adverse events, allergic reactions, or serious infusion-related reactions associated with the administration of bentracimab, Dr. Bhatt said.
While Dr. Bhatt acknowledged that the number of patients in the major-bleeding subgroup was small, he noted that the reduction in platelet reactivity relative to baseline was still significant. In addition, he characterized urgent surgery as “an excellent model of bleeding” and pointed out the consistency of results in the surgical and major-bleeding groups.
The interim results are also consistent with phase 1 data published 2 years ago, and with the subsequent phase 2 studies. All of these data are now under regulatory review both in the United States and in Europe, according to Dr. Bhatt.
No good current options for reversal
Evidence of efficacy and safety is encouraging, because current options for urgently reversing ticagrelor are “disappointing,” according to the invited discussant Gilles Montalescot, MD, PhD, professor of cardiology, Pitié-Salpêtrière Hôpital, Paris.
“Platelet transfusion has some value for clopidogrel and prasugrel, but it does not work for ticagrelor,” said Dr. Montalescot, referring to two other P2Y12 inhibitors. Substantiating the need for a reversal agent, he identified several other strategies that have proven ineffective, such as desmopressin and sorbent hemadsorption.
Overall, Dr. Montalescot acknowledged the need for a highly effective ticagrelor reversal agent, but he did have some criticisms of REVERSE-IT. For one, he was not convinced about the design.
“What was unethical in having a control group?” he asked, suggesting that it was feasible and would have addressed issues of relative efficacy and safety.
For example, the authors concluded that none of the thrombotic events were likely to be treatment related, but “four events occurred immediately after reversal without an alternate explanation,” Dr. Montalescot pointed out. “Was this a signal or background noise?”
Nevertheless, he agreed that the interim phase 3 data are consistent with the previously reported phase 2 studies, and he reiterated that a strategy to reverse ticagrelor’s effects is an important unmet need.
Dr. Bhatt has a financial relationship with a large number of pharmaceutical companies, including PhaseBio, which provided funding for the REVERSE-IT trial. Dr. Montalescot reported financial relationships with Amgen, AstraZeneca, Bayer, Boehringer Ingelheim, Boston Scientific, Bristol-Myers Squibb, Cell-Prothera, CSL-Behring, Europa, Idorsia, Servicer, Medtronic, Merck Sharpe & Dohme, Novartis, Pfizer, Quantum Genomics, and Sanofi-Aventis.
The experimental monoclonal antibody bentracimab, which reverses the antiplatelet effects of ticagrelor, appears to be heading toward regulatory approval, on the basis of an interim analysis of the phase 3 REVERSE-IT trial.
“Rates of effective hemostasis were adjudicated as good or excellent in more than 90% of cases with no drug-related serious adverse events or allergic or infusion-related reactions,” reported Deepak L. Bhatt, MD, at the American Heart Association scientific sessions.
The interim analysis of this nonrandomized, single-arm study was requested by the Food and Drug Administration, which is considering a conditional accelerated approval of bentracimab (formerly PB2452) if efficacy and safety are established.
Upon administration, bentracimab binds to free ticagrelor so that ticagrelor cannot bind to the P2Y12 platelet receptor. This interrupts one of the key steps in the pathway of platelet aggregation.
REVERSE-IT is still enrolling patients. This interim analysis was conducted with the first 150 patients who met eligibility criteria and were treated. Of these, 142 patients were enrolled for an urgent surgical indication and 8 for a major bleeding indication. After some exclusions for lack of urgency and reclassifications following adjudication, there were 113 surgical cases and 9 major bleeding patients evaluable for hemostasis.
Platelet function assays test reversal
On the primary reversal endpoint, which was restoration of activity on the proprietary platelet function assays Verify Now and PRUTest, a rapid restoration of platelet function was achieved in both surgical and major-bleeding patients. Platelet reactivity climbed to near normal levels within 10 minutes of administration, and peak effects were sustained through the first 24 hours after administration.
On the basis of the platelet function assays, the pattern of response to bentracimab was “very similar in the surgical and bleeding patients,” reported Dr. Bhatt, executive director of interventional cardiovascular programs at Brigham and Women’s Health, Boston.
The effect was also consistent across a broad array of prespecified subgroups, including stratifications by age, renal function, time from last dose of ticagrelor, race, and the presence of comorbidities, such as diabetes, renal dysfunction, hypertension, and history of MI.
Hemostasis documented in all but one patient
Adjudicated hemostasis was achieved in 100% of the 113 urgent surgical patients evaluated. In the nine major bleeding patients, six achieved excellent hemostasis and one achieved good hemostasis. One had poor hemostasis, and one was unevaluable.
Platelet rebound following bentracimab administration, measured by mean platelet volume, was not observed.
There were no serious adverse events, allergic reactions, or serious infusion-related reactions associated with the administration of bentracimab, Dr. Bhatt said.
While Dr. Bhatt acknowledged that the number of patients in the major-bleeding subgroup was small, he noted that the reduction in platelet reactivity relative to baseline was still significant. In addition, he characterized urgent surgery as “an excellent model of bleeding” and pointed out the consistency of results in the surgical and major-bleeding groups.
The interim results are also consistent with phase 1 data published 2 years ago, and with the subsequent phase 2 studies. All of these data are now under regulatory review both in the United States and in Europe, according to Dr. Bhatt.
No good current options for reversal
Evidence of efficacy and safety is encouraging, because current options for urgently reversing ticagrelor are “disappointing,” according to the invited discussant Gilles Montalescot, MD, PhD, professor of cardiology, Pitié-Salpêtrière Hôpital, Paris.
“Platelet transfusion has some value for clopidogrel and prasugrel, but it does not work for ticagrelor,” said Dr. Montalescot, referring to two other P2Y12 inhibitors. Substantiating the need for a reversal agent, he identified several other strategies that have proven ineffective, such as desmopressin and sorbent hemadsorption.
Overall, Dr. Montalescot acknowledged the need for a highly effective ticagrelor reversal agent, but he did have some criticisms of REVERSE-IT. For one, he was not convinced about the design.
“What was unethical in having a control group?” he asked, suggesting that it was feasible and would have addressed issues of relative efficacy and safety.
For example, the authors concluded that none of the thrombotic events were likely to be treatment related, but “four events occurred immediately after reversal without an alternate explanation,” Dr. Montalescot pointed out. “Was this a signal or background noise?”
Nevertheless, he agreed that the interim phase 3 data are consistent with the previously reported phase 2 studies, and he reiterated that a strategy to reverse ticagrelor’s effects is an important unmet need.
Dr. Bhatt has a financial relationship with a large number of pharmaceutical companies, including PhaseBio, which provided funding for the REVERSE-IT trial. Dr. Montalescot reported financial relationships with Amgen, AstraZeneca, Bayer, Boehringer Ingelheim, Boston Scientific, Bristol-Myers Squibb, Cell-Prothera, CSL-Behring, Europa, Idorsia, Servicer, Medtronic, Merck Sharpe & Dohme, Novartis, Pfizer, Quantum Genomics, and Sanofi-Aventis.
The experimental monoclonal antibody bentracimab, which reverses the antiplatelet effects of ticagrelor, appears to be heading toward regulatory approval, on the basis of an interim analysis of the phase 3 REVERSE-IT trial.
“Rates of effective hemostasis were adjudicated as good or excellent in more than 90% of cases with no drug-related serious adverse events or allergic or infusion-related reactions,” reported Deepak L. Bhatt, MD, at the American Heart Association scientific sessions.
The interim analysis of this nonrandomized, single-arm study was requested by the Food and Drug Administration, which is considering a conditional accelerated approval of bentracimab (formerly PB2452) if efficacy and safety are established.
Upon administration, bentracimab binds to free ticagrelor so that ticagrelor cannot bind to the P2Y12 platelet receptor. This interrupts one of the key steps in the pathway of platelet aggregation.
REVERSE-IT is still enrolling patients. This interim analysis was conducted with the first 150 patients who met eligibility criteria and were treated. Of these, 142 patients were enrolled for an urgent surgical indication and 8 for a major bleeding indication. After some exclusions for lack of urgency and reclassifications following adjudication, there were 113 surgical cases and 9 major bleeding patients evaluable for hemostasis.
Platelet function assays test reversal
On the primary reversal endpoint, which was restoration of activity on the proprietary platelet function assays Verify Now and PRUTest, a rapid restoration of platelet function was achieved in both surgical and major-bleeding patients. Platelet reactivity climbed to near normal levels within 10 minutes of administration, and peak effects were sustained through the first 24 hours after administration.
On the basis of the platelet function assays, the pattern of response to bentracimab was “very similar in the surgical and bleeding patients,” reported Dr. Bhatt, executive director of interventional cardiovascular programs at Brigham and Women’s Health, Boston.
The effect was also consistent across a broad array of prespecified subgroups, including stratifications by age, renal function, time from last dose of ticagrelor, race, and the presence of comorbidities, such as diabetes, renal dysfunction, hypertension, and history of MI.
Hemostasis documented in all but one patient
Adjudicated hemostasis was achieved in 100% of the 113 urgent surgical patients evaluated. In the nine major bleeding patients, six achieved excellent hemostasis and one achieved good hemostasis. One had poor hemostasis, and one was unevaluable.
Platelet rebound following bentracimab administration, measured by mean platelet volume, was not observed.
There were no serious adverse events, allergic reactions, or serious infusion-related reactions associated with the administration of bentracimab, Dr. Bhatt said.
While Dr. Bhatt acknowledged that the number of patients in the major-bleeding subgroup was small, he noted that the reduction in platelet reactivity relative to baseline was still significant. In addition, he characterized urgent surgery as “an excellent model of bleeding” and pointed out the consistency of results in the surgical and major-bleeding groups.
The interim results are also consistent with phase 1 data published 2 years ago, and with the subsequent phase 2 studies. All of these data are now under regulatory review both in the United States and in Europe, according to Dr. Bhatt.
No good current options for reversal
Evidence of efficacy and safety is encouraging, because current options for urgently reversing ticagrelor are “disappointing,” according to the invited discussant Gilles Montalescot, MD, PhD, professor of cardiology, Pitié-Salpêtrière Hôpital, Paris.
“Platelet transfusion has some value for clopidogrel and prasugrel, but it does not work for ticagrelor,” said Dr. Montalescot, referring to two other P2Y12 inhibitors. Substantiating the need for a reversal agent, he identified several other strategies that have proven ineffective, such as desmopressin and sorbent hemadsorption.
Overall, Dr. Montalescot acknowledged the need for a highly effective ticagrelor reversal agent, but he did have some criticisms of REVERSE-IT. For one, he was not convinced about the design.
“What was unethical in having a control group?” he asked, suggesting that it was feasible and would have addressed issues of relative efficacy and safety.
For example, the authors concluded that none of the thrombotic events were likely to be treatment related, but “four events occurred immediately after reversal without an alternate explanation,” Dr. Montalescot pointed out. “Was this a signal or background noise?”
Nevertheless, he agreed that the interim phase 3 data are consistent with the previously reported phase 2 studies, and he reiterated that a strategy to reverse ticagrelor’s effects is an important unmet need.
Dr. Bhatt has a financial relationship with a large number of pharmaceutical companies, including PhaseBio, which provided funding for the REVERSE-IT trial. Dr. Montalescot reported financial relationships with Amgen, AstraZeneca, Bayer, Boehringer Ingelheim, Boston Scientific, Bristol-Myers Squibb, Cell-Prothera, CSL-Behring, Europa, Idorsia, Servicer, Medtronic, Merck Sharpe & Dohme, Novartis, Pfizer, Quantum Genomics, and Sanofi-Aventis.
FROM AHA 2021