User login
A fair trade-off
In the mid-90s, as a resident, I gave tissue plasminogen activator (tPA) one night to the first patient my institution registered in the study that got it approved by the Food and Drug Administration. Our director of stroke gave me a bottle of champagne the next day to thank me. That was where my career in acute inpatient neurology began.
Like many docs of my age, my hospital work has been dwindling with time, and was down to just 1-2 weekends a month in a small three-doc rotation. Not much, but it still made for some busy weekends.
The first wave of mass quarantining happened to fall just as our quarterly schedule was ending. In fact, I’d been working on writing it up for the next quarter when things began.
But then, in the course of a few days, one of us decided to retire early, and the other doc and I couldn’t agree on how to handle the rotation with only two people (somewhat naively, I told him the whole COVID thing would be over in 2-3 months; obviously I was WAY wrong).
So I finished up my last scheduled hospital call, figuring I’d be back in a few months.
So far that hasn’t happened. I’m now 17 months out since the last time I rounded on hospital patients.
And I don’t miss it at all.
This surprises me. I mean, we all start out, in medical school and residency, immersed in the hospital. It’s where the action is. Rounding, checking tests results, talking to patients, families, and nurses is ingrained into us. When I started in 1998 I hustled between four hospitals and enjoyed it (the work, not the driving).
Now I realize that my inpatient days are probably behind me, and I’m not bothered by it. That’s not to say I may not go back. Circumstances change, so, as before, I try to keep up on both inpatient and outpatient neurologic care and developments.
But for now, I’m happier without it. My weekends are my own. I don’t dread the Friday afternoon switchover where new consults suddenly start showing up on my cell phone. I don’t have to worry about running in at 2:00 a.m. to decide tPA or not tPA. My wife and I don’t have to take separate cars to go out to dinner, just in case I have to leave.
I’m sure I’ve lost some revenue because of it, but in the overall downturn of the pandemic it’s hard to know how much.
But I do know that I’ve gained time at home. With my wife, my kids, my dogs, and even just myself. My start and stop times on weekdays, and now plans for weekends, are now more predictable.
At some point those things are worth the money lost, and I’m happy to take them.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
In the mid-90s, as a resident, I gave tissue plasminogen activator (tPA) one night to the first patient my institution registered in the study that got it approved by the Food and Drug Administration. Our director of stroke gave me a bottle of champagne the next day to thank me. That was where my career in acute inpatient neurology began.
Like many docs of my age, my hospital work has been dwindling with time, and was down to just 1-2 weekends a month in a small three-doc rotation. Not much, but it still made for some busy weekends.
The first wave of mass quarantining happened to fall just as our quarterly schedule was ending. In fact, I’d been working on writing it up for the next quarter when things began.
But then, in the course of a few days, one of us decided to retire early, and the other doc and I couldn’t agree on how to handle the rotation with only two people (somewhat naively, I told him the whole COVID thing would be over in 2-3 months; obviously I was WAY wrong).
So I finished up my last scheduled hospital call, figuring I’d be back in a few months.
So far that hasn’t happened. I’m now 17 months out since the last time I rounded on hospital patients.
And I don’t miss it at all.
This surprises me. I mean, we all start out, in medical school and residency, immersed in the hospital. It’s where the action is. Rounding, checking tests results, talking to patients, families, and nurses is ingrained into us. When I started in 1998 I hustled between four hospitals and enjoyed it (the work, not the driving).
Now I realize that my inpatient days are probably behind me, and I’m not bothered by it. That’s not to say I may not go back. Circumstances change, so, as before, I try to keep up on both inpatient and outpatient neurologic care and developments.
But for now, I’m happier without it. My weekends are my own. I don’t dread the Friday afternoon switchover where new consults suddenly start showing up on my cell phone. I don’t have to worry about running in at 2:00 a.m. to decide tPA or not tPA. My wife and I don’t have to take separate cars to go out to dinner, just in case I have to leave.
I’m sure I’ve lost some revenue because of it, but in the overall downturn of the pandemic it’s hard to know how much.
But I do know that I’ve gained time at home. With my wife, my kids, my dogs, and even just myself. My start and stop times on weekdays, and now plans for weekends, are now more predictable.
At some point those things are worth the money lost, and I’m happy to take them.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
In the mid-90s, as a resident, I gave tissue plasminogen activator (tPA) one night to the first patient my institution registered in the study that got it approved by the Food and Drug Administration. Our director of stroke gave me a bottle of champagne the next day to thank me. That was where my career in acute inpatient neurology began.
Like many docs of my age, my hospital work has been dwindling with time, and was down to just 1-2 weekends a month in a small three-doc rotation. Not much, but it still made for some busy weekends.
The first wave of mass quarantining happened to fall just as our quarterly schedule was ending. In fact, I’d been working on writing it up for the next quarter when things began.
But then, in the course of a few days, one of us decided to retire early, and the other doc and I couldn’t agree on how to handle the rotation with only two people (somewhat naively, I told him the whole COVID thing would be over in 2-3 months; obviously I was WAY wrong).
So I finished up my last scheduled hospital call, figuring I’d be back in a few months.
So far that hasn’t happened. I’m now 17 months out since the last time I rounded on hospital patients.
And I don’t miss it at all.
This surprises me. I mean, we all start out, in medical school and residency, immersed in the hospital. It’s where the action is. Rounding, checking tests results, talking to patients, families, and nurses is ingrained into us. When I started in 1998 I hustled between four hospitals and enjoyed it (the work, not the driving).
Now I realize that my inpatient days are probably behind me, and I’m not bothered by it. That’s not to say I may not go back. Circumstances change, so, as before, I try to keep up on both inpatient and outpatient neurologic care and developments.
But for now, I’m happier without it. My weekends are my own. I don’t dread the Friday afternoon switchover where new consults suddenly start showing up on my cell phone. I don’t have to worry about running in at 2:00 a.m. to decide tPA or not tPA. My wife and I don’t have to take separate cars to go out to dinner, just in case I have to leave.
I’m sure I’ve lost some revenue because of it, but in the overall downturn of the pandemic it’s hard to know how much.
But I do know that I’ve gained time at home. With my wife, my kids, my dogs, and even just myself. My start and stop times on weekdays, and now plans for weekends, are now more predictable.
At some point those things are worth the money lost, and I’m happy to take them.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
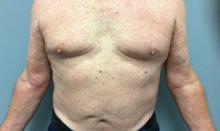
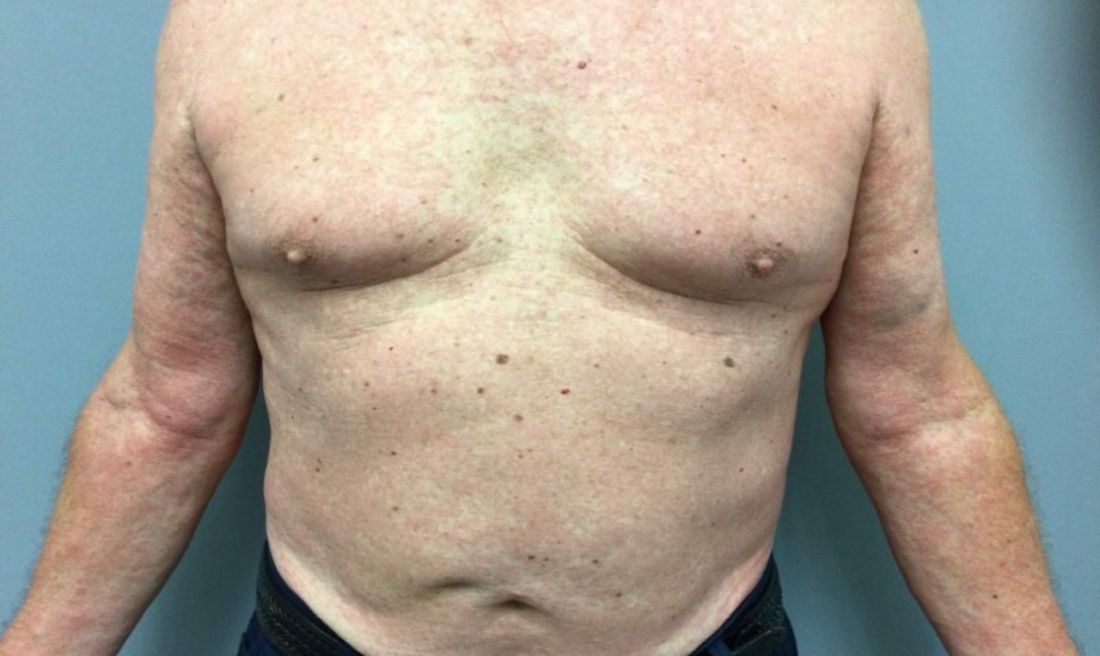
A 73-year-old White male presented with 2 days of a very pruritic rash
Reactions can occur anytime from within the first 2 weeks of treatment up to 10 days after the treatment has been discontinued. If a drug is rechallenged, eruptions may occur sooner. Pruritus is commonly seen. Clinically, erythematous papules and macules present symmetrically on the trunk and upper extremities and then become more generalized. A low-grade fever may be present.
Antibiotics are the most common causes of exanthematous drug eruptions. Penicillins and trimethoprim-sulfamethoxazole are common offenders. Cephalosporins, anticonvulsants, and allopurinol may also induce a reaction. As this condition is diagnosed clinically, skin biopsy is often not necessary. Histology is nonspecific and shows a mild perivascular lymphocytic infiltrate and few epidermal necrotic keratinocytes.
In drug reaction with eosinophilia and systemic symptoms (DRESS), symptoms present 2-6 weeks after the offending medication has been started. The cutaneous rash appears similar to an exanthematous drug eruption; however, lesions will also present on the face, and facial edema may occur. Fever is often present. Laboratory findings include a marked peripheral blood hypereosinophilia. Elevated liver function tests may be seen. Viruses such as Epstein-Barr virus, enteroviruses, adenovirus, early HIV, human herpesvirus 6, and parvovirus B19 have a similar clinical appearance to an exanthematous drug eruption. A mild eosinophilia, as seen in a drug eruption, helps to distinguish between a drug eruption and viral exanthem. In Stevens-Johnson Syndrome, mucosal membranes are involved and skin is often painful or appears dusky.
Treatment of exanthematous drug eruptions is largely supportive. Discontinuing the drug will help speed resolution and topical steroids may alleviate pruritus.
This case and photo were submitted by Dr. Bilu Martin.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to [email protected].
References
1. Bolognia J et al. “Dermatology” (St. Louis: Mosby/Elsevier, 2008).
2. James W et al. “Andrews’ Diseases of the Skin,” 13th ed. (Philadelphia: Saunders Elsevier, 2006).
Reactions can occur anytime from within the first 2 weeks of treatment up to 10 days after the treatment has been discontinued. If a drug is rechallenged, eruptions may occur sooner. Pruritus is commonly seen. Clinically, erythematous papules and macules present symmetrically on the trunk and upper extremities and then become more generalized. A low-grade fever may be present.
Antibiotics are the most common causes of exanthematous drug eruptions. Penicillins and trimethoprim-sulfamethoxazole are common offenders. Cephalosporins, anticonvulsants, and allopurinol may also induce a reaction. As this condition is diagnosed clinically, skin biopsy is often not necessary. Histology is nonspecific and shows a mild perivascular lymphocytic infiltrate and few epidermal necrotic keratinocytes.
In drug reaction with eosinophilia and systemic symptoms (DRESS), symptoms present 2-6 weeks after the offending medication has been started. The cutaneous rash appears similar to an exanthematous drug eruption; however, lesions will also present on the face, and facial edema may occur. Fever is often present. Laboratory findings include a marked peripheral blood hypereosinophilia. Elevated liver function tests may be seen. Viruses such as Epstein-Barr virus, enteroviruses, adenovirus, early HIV, human herpesvirus 6, and parvovirus B19 have a similar clinical appearance to an exanthematous drug eruption. A mild eosinophilia, as seen in a drug eruption, helps to distinguish between a drug eruption and viral exanthem. In Stevens-Johnson Syndrome, mucosal membranes are involved and skin is often painful or appears dusky.
Treatment of exanthematous drug eruptions is largely supportive. Discontinuing the drug will help speed resolution and topical steroids may alleviate pruritus.
This case and photo were submitted by Dr. Bilu Martin.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to [email protected].
References
1. Bolognia J et al. “Dermatology” (St. Louis: Mosby/Elsevier, 2008).
2. James W et al. “Andrews’ Diseases of the Skin,” 13th ed. (Philadelphia: Saunders Elsevier, 2006).
Reactions can occur anytime from within the first 2 weeks of treatment up to 10 days after the treatment has been discontinued. If a drug is rechallenged, eruptions may occur sooner. Pruritus is commonly seen. Clinically, erythematous papules and macules present symmetrically on the trunk and upper extremities and then become more generalized. A low-grade fever may be present.
Antibiotics are the most common causes of exanthematous drug eruptions. Penicillins and trimethoprim-sulfamethoxazole are common offenders. Cephalosporins, anticonvulsants, and allopurinol may also induce a reaction. As this condition is diagnosed clinically, skin biopsy is often not necessary. Histology is nonspecific and shows a mild perivascular lymphocytic infiltrate and few epidermal necrotic keratinocytes.
In drug reaction with eosinophilia and systemic symptoms (DRESS), symptoms present 2-6 weeks after the offending medication has been started. The cutaneous rash appears similar to an exanthematous drug eruption; however, lesions will also present on the face, and facial edema may occur. Fever is often present. Laboratory findings include a marked peripheral blood hypereosinophilia. Elevated liver function tests may be seen. Viruses such as Epstein-Barr virus, enteroviruses, adenovirus, early HIV, human herpesvirus 6, and parvovirus B19 have a similar clinical appearance to an exanthematous drug eruption. A mild eosinophilia, as seen in a drug eruption, helps to distinguish between a drug eruption and viral exanthem. In Stevens-Johnson Syndrome, mucosal membranes are involved and skin is often painful or appears dusky.
Treatment of exanthematous drug eruptions is largely supportive. Discontinuing the drug will help speed resolution and topical steroids may alleviate pruritus.
This case and photo were submitted by Dr. Bilu Martin.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to [email protected].
References
1. Bolognia J et al. “Dermatology” (St. Louis: Mosby/Elsevier, 2008).
2. James W et al. “Andrews’ Diseases of the Skin,” 13th ed. (Philadelphia: Saunders Elsevier, 2006).
Second woman spontaneously clears HIV: ‘We think more are out there’
It sounds like a fairy tale steeped in HIV stigma: A woman wakes up one morning and, poof, the HIV she’s been living with for 8 years is gone. But for a 30-year-old Argentinian woman from the aptly named village of Esperanza, that’s close to the truth, according to an article published in Annals of Internal Medicine.
The woman, the so-called Esperanza Patient, appears to be the second person whose immune system cleared the virus without the use of stem cell transplantation. The first was Loreen Willenberg, a California woman who, after living with HIV for 27 years, no longer had replicating HIV in her system. That case was reported last year.
“That’s the beauty of this name, right? Esperanza,” said Xu Yu, MD, principal investigator of the Ragon Institute of Massachusetts General Hospital, the Massachusetts Institute of Technology, and Harvard University, Boston, referring to the Spanish word for “hope.” “This makes us hopeful that a natural cure of HIV is actually possible.”
Two other people appear to have cleared HIV, but only after full replacement of the immune system via stem cell transplantation – the Berlin Patient, Timothy Ray Brown, and the London Patient. Another man, from Brazil, appeared to have an undetectable viral load after receiving intensified antiretroviral treatment plus supplemental vitamin B3.
The rarest of the rare
The Esperanza Patient is among a rare group of people living with HIV called elite controllers. These people’s immune systems can control HIV without antiretrovirals. Most elite controllers’ immune systems, however, can’t mount the immune attack necessary to eliminate all replicating HIV from their systems. Instead, their immune systems control the virus without affecting the reservoirs where HIV continues to make copies of itself and can spread.
The Esperanza Patient and Ms. Willenberg, however, appear to be the rarest of the rare. Their own immune systems seem not only to have stopped HIV replication outside of reservoirs but also to have stormed those reservoirs and killed all virus that might have continued to replicate.
The two women are connected in another way: At an HIV conference in 2019, Dr. Yu was presenting data on Ms. Willenberg’s case. At that conference, she met Natalia Laufer, MD, PhD, associate researcher at the Instituto de Investigaciones Biomédicas en Retrovirs y SIDA at the University of Buenos Aires. Dr. Laufer had been studying the Esperanza Patient at the time and asked Dr. Yu whether she and her team at the Ragon Institute could help her sequence the patient’s HIV genome to see whether, indeed, the virus had been spontaneously cleared from the patient’s system.
So that’s what the pair did, in collaboration with several other researchers into cures for HIV. The Esperanza Patient first acquired HIV in 2013, but in the 8 years that followed, results of 10 conventional viral load tests indicated the virus was undetectable (that is, below the level of quantification for standard technology). During that time, the woman’s boyfriend, from whom she had acquired HIV, died of AIDS-defining illnesses. She subsequently married and had a baby. Both her partner and baby are HIV negative. She only received HIV treatment for 6 months while she was pregnant.
A fossil record of HIV
Yet, there was still HIV in the woman’s system. Dr. Laufer and Dr. Yu wanted to know whether that HIV was transmissible or whether it was a relic from when HIV was still replicating and was now defective and incapable of replicating. They performed extensive genome sequencing on nearly 1.2 billion cells that Dr. Laufer had taken from the patient’s blood in 2017, 2018, 2019, and 2020, an additional 503 million cells that were from the placenta of the baby she gave birth to in 2020, and 150 million resting CD4 T cells. Proviral sequencing was undertaken of the full DNA of the HIV to detect whether the virus was still intact. The DNA was then analyzed by use of an algorithm and was tested for mutations. The investigators tested the patient’s CD4 cells to determine whether the cells still harbored any latent HIV.
In this way, they conducted a full viral workup using tests that are far more sensitive than the viral load tests the woman had undergone in the clinic. The investigators then assessed the patient’s immune system to see what the various cells of the immune system could tell them about how well her natural immune system could identify and kill HIV. They isolated the Esperanza Patient’s immune cells and subjected those cells to HIV in the lab to see whether the cells could detect and eliminate the virus.
And just to be safe, they checked to make sure there were no antiretroviral drugs in the patient’s system.
What they found was that without treatment, her CD4 count hovered around 1,000 cells – a sign of a functioning immune system. DNA sequences revealed large chunks of missing DNA, and one sequence had an immune-induced hypermutation. In total, seven proviruses were found, but none were capable of replicating. The CD4 cells they evaluated showed no evidence of latent HIV.
In other words, they had uncovered a fossil record.
“These HIV-1 DNA products clearly indicate that this person was infected with HIV-1 in the past and that active cycles of viral replication had occurred at one point,” Dr. Yu and colleagues write in their recent article.
What may be more useful to researchers looking to turn this spontaneous cure into treatment for millions of people living with active HIV was the evidence that the woman’s immune system had trained itself to attack HIV through a number of genetic mutations. What they found, the researchers write, was evidence of “an incomplete seroconversion” – that is, when the patient was acquiring HIV, the infection was stopped in its tracks.
Yet, Dr. Yu and colleagues say that they can’t prove that the woman is fully cured of HIV.
“Although this might sound unsatisfying, it reflects an intrinsic limitation of scientific research,” they write. “Scientific concepts can never be proved through empirical data collection; they can only be disproved.”
There are more out there
Are these women the only ones to have spontaneously cleared HIV? That’s the question, said Carl Dieffenbach, PhD, director of the Division of AIDS at the National Institute of Allergy and Infectious Diseases at the National Institutes of Health. Just like they can’t disprove that the women cured themselves, they can’t prove that she and Ms. Willenberg are the only two people to have experienced this cure.
“We’re all struggling with this,” Dr. Dieffenbach told this news organization. “The goal is to get enough of these people so maybe there’s a road map to how to induce, trigger, change immunity. But this could well be a unique event at the time of initiation of infection. We just don’t know.”
What is needed, Dr. Yu said, is for clinicians to reach out to them regarding cases that could mimic the cases of Ms. Willenberg and the Esperanza Patient. Elaborate testing could then be conducted to see whether these cases are similar to those of Ms. Willenberg and the Esperanza Patient.
“We do think there are more out there,” Dr. Yu said in an interview.
Asked whether we’re still far away from applying these one-off cures to the millions of people taking HIV treatment daily, Dr. Yu responded, “We might be close. That’s the beauty of scientific discovery. We don’t know, but that’s why we need more engagement of the community and care providers to help us.”
The research was funded by the Bill and Melinda Gates Foundation and the National Institutes of Health. Dr. Yu and Dr. Dieffenbach have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
It sounds like a fairy tale steeped in HIV stigma: A woman wakes up one morning and, poof, the HIV she’s been living with for 8 years is gone. But for a 30-year-old Argentinian woman from the aptly named village of Esperanza, that’s close to the truth, according to an article published in Annals of Internal Medicine.
The woman, the so-called Esperanza Patient, appears to be the second person whose immune system cleared the virus without the use of stem cell transplantation. The first was Loreen Willenberg, a California woman who, after living with HIV for 27 years, no longer had replicating HIV in her system. That case was reported last year.
“That’s the beauty of this name, right? Esperanza,” said Xu Yu, MD, principal investigator of the Ragon Institute of Massachusetts General Hospital, the Massachusetts Institute of Technology, and Harvard University, Boston, referring to the Spanish word for “hope.” “This makes us hopeful that a natural cure of HIV is actually possible.”
Two other people appear to have cleared HIV, but only after full replacement of the immune system via stem cell transplantation – the Berlin Patient, Timothy Ray Brown, and the London Patient. Another man, from Brazil, appeared to have an undetectable viral load after receiving intensified antiretroviral treatment plus supplemental vitamin B3.
The rarest of the rare
The Esperanza Patient is among a rare group of people living with HIV called elite controllers. These people’s immune systems can control HIV without antiretrovirals. Most elite controllers’ immune systems, however, can’t mount the immune attack necessary to eliminate all replicating HIV from their systems. Instead, their immune systems control the virus without affecting the reservoirs where HIV continues to make copies of itself and can spread.
The Esperanza Patient and Ms. Willenberg, however, appear to be the rarest of the rare. Their own immune systems seem not only to have stopped HIV replication outside of reservoirs but also to have stormed those reservoirs and killed all virus that might have continued to replicate.
The two women are connected in another way: At an HIV conference in 2019, Dr. Yu was presenting data on Ms. Willenberg’s case. At that conference, she met Natalia Laufer, MD, PhD, associate researcher at the Instituto de Investigaciones Biomédicas en Retrovirs y SIDA at the University of Buenos Aires. Dr. Laufer had been studying the Esperanza Patient at the time and asked Dr. Yu whether she and her team at the Ragon Institute could help her sequence the patient’s HIV genome to see whether, indeed, the virus had been spontaneously cleared from the patient’s system.
So that’s what the pair did, in collaboration with several other researchers into cures for HIV. The Esperanza Patient first acquired HIV in 2013, but in the 8 years that followed, results of 10 conventional viral load tests indicated the virus was undetectable (that is, below the level of quantification for standard technology). During that time, the woman’s boyfriend, from whom she had acquired HIV, died of AIDS-defining illnesses. She subsequently married and had a baby. Both her partner and baby are HIV negative. She only received HIV treatment for 6 months while she was pregnant.
A fossil record of HIV
Yet, there was still HIV in the woman’s system. Dr. Laufer and Dr. Yu wanted to know whether that HIV was transmissible or whether it was a relic from when HIV was still replicating and was now defective and incapable of replicating. They performed extensive genome sequencing on nearly 1.2 billion cells that Dr. Laufer had taken from the patient’s blood in 2017, 2018, 2019, and 2020, an additional 503 million cells that were from the placenta of the baby she gave birth to in 2020, and 150 million resting CD4 T cells. Proviral sequencing was undertaken of the full DNA of the HIV to detect whether the virus was still intact. The DNA was then analyzed by use of an algorithm and was tested for mutations. The investigators tested the patient’s CD4 cells to determine whether the cells still harbored any latent HIV.
In this way, they conducted a full viral workup using tests that are far more sensitive than the viral load tests the woman had undergone in the clinic. The investigators then assessed the patient’s immune system to see what the various cells of the immune system could tell them about how well her natural immune system could identify and kill HIV. They isolated the Esperanza Patient’s immune cells and subjected those cells to HIV in the lab to see whether the cells could detect and eliminate the virus.
And just to be safe, they checked to make sure there were no antiretroviral drugs in the patient’s system.
What they found was that without treatment, her CD4 count hovered around 1,000 cells – a sign of a functioning immune system. DNA sequences revealed large chunks of missing DNA, and one sequence had an immune-induced hypermutation. In total, seven proviruses were found, but none were capable of replicating. The CD4 cells they evaluated showed no evidence of latent HIV.
In other words, they had uncovered a fossil record.
“These HIV-1 DNA products clearly indicate that this person was infected with HIV-1 in the past and that active cycles of viral replication had occurred at one point,” Dr. Yu and colleagues write in their recent article.
What may be more useful to researchers looking to turn this spontaneous cure into treatment for millions of people living with active HIV was the evidence that the woman’s immune system had trained itself to attack HIV through a number of genetic mutations. What they found, the researchers write, was evidence of “an incomplete seroconversion” – that is, when the patient was acquiring HIV, the infection was stopped in its tracks.
Yet, Dr. Yu and colleagues say that they can’t prove that the woman is fully cured of HIV.
“Although this might sound unsatisfying, it reflects an intrinsic limitation of scientific research,” they write. “Scientific concepts can never be proved through empirical data collection; they can only be disproved.”
There are more out there
Are these women the only ones to have spontaneously cleared HIV? That’s the question, said Carl Dieffenbach, PhD, director of the Division of AIDS at the National Institute of Allergy and Infectious Diseases at the National Institutes of Health. Just like they can’t disprove that the women cured themselves, they can’t prove that she and Ms. Willenberg are the only two people to have experienced this cure.
“We’re all struggling with this,” Dr. Dieffenbach told this news organization. “The goal is to get enough of these people so maybe there’s a road map to how to induce, trigger, change immunity. But this could well be a unique event at the time of initiation of infection. We just don’t know.”
What is needed, Dr. Yu said, is for clinicians to reach out to them regarding cases that could mimic the cases of Ms. Willenberg and the Esperanza Patient. Elaborate testing could then be conducted to see whether these cases are similar to those of Ms. Willenberg and the Esperanza Patient.
“We do think there are more out there,” Dr. Yu said in an interview.
Asked whether we’re still far away from applying these one-off cures to the millions of people taking HIV treatment daily, Dr. Yu responded, “We might be close. That’s the beauty of scientific discovery. We don’t know, but that’s why we need more engagement of the community and care providers to help us.”
The research was funded by the Bill and Melinda Gates Foundation and the National Institutes of Health. Dr. Yu and Dr. Dieffenbach have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
It sounds like a fairy tale steeped in HIV stigma: A woman wakes up one morning and, poof, the HIV she’s been living with for 8 years is gone. But for a 30-year-old Argentinian woman from the aptly named village of Esperanza, that’s close to the truth, according to an article published in Annals of Internal Medicine.
The woman, the so-called Esperanza Patient, appears to be the second person whose immune system cleared the virus without the use of stem cell transplantation. The first was Loreen Willenberg, a California woman who, after living with HIV for 27 years, no longer had replicating HIV in her system. That case was reported last year.
“That’s the beauty of this name, right? Esperanza,” said Xu Yu, MD, principal investigator of the Ragon Institute of Massachusetts General Hospital, the Massachusetts Institute of Technology, and Harvard University, Boston, referring to the Spanish word for “hope.” “This makes us hopeful that a natural cure of HIV is actually possible.”
Two other people appear to have cleared HIV, but only after full replacement of the immune system via stem cell transplantation – the Berlin Patient, Timothy Ray Brown, and the London Patient. Another man, from Brazil, appeared to have an undetectable viral load after receiving intensified antiretroviral treatment plus supplemental vitamin B3.
The rarest of the rare
The Esperanza Patient is among a rare group of people living with HIV called elite controllers. These people’s immune systems can control HIV without antiretrovirals. Most elite controllers’ immune systems, however, can’t mount the immune attack necessary to eliminate all replicating HIV from their systems. Instead, their immune systems control the virus without affecting the reservoirs where HIV continues to make copies of itself and can spread.
The Esperanza Patient and Ms. Willenberg, however, appear to be the rarest of the rare. Their own immune systems seem not only to have stopped HIV replication outside of reservoirs but also to have stormed those reservoirs and killed all virus that might have continued to replicate.
The two women are connected in another way: At an HIV conference in 2019, Dr. Yu was presenting data on Ms. Willenberg’s case. At that conference, she met Natalia Laufer, MD, PhD, associate researcher at the Instituto de Investigaciones Biomédicas en Retrovirs y SIDA at the University of Buenos Aires. Dr. Laufer had been studying the Esperanza Patient at the time and asked Dr. Yu whether she and her team at the Ragon Institute could help her sequence the patient’s HIV genome to see whether, indeed, the virus had been spontaneously cleared from the patient’s system.
So that’s what the pair did, in collaboration with several other researchers into cures for HIV. The Esperanza Patient first acquired HIV in 2013, but in the 8 years that followed, results of 10 conventional viral load tests indicated the virus was undetectable (that is, below the level of quantification for standard technology). During that time, the woman’s boyfriend, from whom she had acquired HIV, died of AIDS-defining illnesses. She subsequently married and had a baby. Both her partner and baby are HIV negative. She only received HIV treatment for 6 months while she was pregnant.
A fossil record of HIV
Yet, there was still HIV in the woman’s system. Dr. Laufer and Dr. Yu wanted to know whether that HIV was transmissible or whether it was a relic from when HIV was still replicating and was now defective and incapable of replicating. They performed extensive genome sequencing on nearly 1.2 billion cells that Dr. Laufer had taken from the patient’s blood in 2017, 2018, 2019, and 2020, an additional 503 million cells that were from the placenta of the baby she gave birth to in 2020, and 150 million resting CD4 T cells. Proviral sequencing was undertaken of the full DNA of the HIV to detect whether the virus was still intact. The DNA was then analyzed by use of an algorithm and was tested for mutations. The investigators tested the patient’s CD4 cells to determine whether the cells still harbored any latent HIV.
In this way, they conducted a full viral workup using tests that are far more sensitive than the viral load tests the woman had undergone in the clinic. The investigators then assessed the patient’s immune system to see what the various cells of the immune system could tell them about how well her natural immune system could identify and kill HIV. They isolated the Esperanza Patient’s immune cells and subjected those cells to HIV in the lab to see whether the cells could detect and eliminate the virus.
And just to be safe, they checked to make sure there were no antiretroviral drugs in the patient’s system.
What they found was that without treatment, her CD4 count hovered around 1,000 cells – a sign of a functioning immune system. DNA sequences revealed large chunks of missing DNA, and one sequence had an immune-induced hypermutation. In total, seven proviruses were found, but none were capable of replicating. The CD4 cells they evaluated showed no evidence of latent HIV.
In other words, they had uncovered a fossil record.
“These HIV-1 DNA products clearly indicate that this person was infected with HIV-1 in the past and that active cycles of viral replication had occurred at one point,” Dr. Yu and colleagues write in their recent article.
What may be more useful to researchers looking to turn this spontaneous cure into treatment for millions of people living with active HIV was the evidence that the woman’s immune system had trained itself to attack HIV through a number of genetic mutations. What they found, the researchers write, was evidence of “an incomplete seroconversion” – that is, when the patient was acquiring HIV, the infection was stopped in its tracks.
Yet, Dr. Yu and colleagues say that they can’t prove that the woman is fully cured of HIV.
“Although this might sound unsatisfying, it reflects an intrinsic limitation of scientific research,” they write. “Scientific concepts can never be proved through empirical data collection; they can only be disproved.”
There are more out there
Are these women the only ones to have spontaneously cleared HIV? That’s the question, said Carl Dieffenbach, PhD, director of the Division of AIDS at the National Institute of Allergy and Infectious Diseases at the National Institutes of Health. Just like they can’t disprove that the women cured themselves, they can’t prove that she and Ms. Willenberg are the only two people to have experienced this cure.
“We’re all struggling with this,” Dr. Dieffenbach told this news organization. “The goal is to get enough of these people so maybe there’s a road map to how to induce, trigger, change immunity. But this could well be a unique event at the time of initiation of infection. We just don’t know.”
What is needed, Dr. Yu said, is for clinicians to reach out to them regarding cases that could mimic the cases of Ms. Willenberg and the Esperanza Patient. Elaborate testing could then be conducted to see whether these cases are similar to those of Ms. Willenberg and the Esperanza Patient.
“We do think there are more out there,” Dr. Yu said in an interview.
Asked whether we’re still far away from applying these one-off cures to the millions of people taking HIV treatment daily, Dr. Yu responded, “We might be close. That’s the beauty of scientific discovery. We don’t know, but that’s why we need more engagement of the community and care providers to help us.”
The research was funded by the Bill and Melinda Gates Foundation and the National Institutes of Health. Dr. Yu and Dr. Dieffenbach have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Coffee or tea? Drinking both tied to lower stroke, dementia risk
Drinking coffee or tea is associated with reduced risk for stroke and dementia, with the biggest benefit associated with consuming both beverages, new research suggests.
Investigators found that individuals who drank two to three cups of coffee and two to three cups of tea per day had a 30% decrease in incidence of stroke and a 28% lower risk for dementia compared with those who did not.
“From a public health perspective, because regular tea and coffee drinkers comprise such a large proportion of the population and because these beverages tend to be consumed habitually throughout adult life, even small potential health benefits or risks associated with tea and coffee intake may have important public health implications,” the investigators wrote.
The study was published online Nov. 16 in PLOS Medicine.
Synergistic effect?
Whereas earlier studies have shown significant health benefits from moderate coffee and tea intake separately, few have examined the effect of drinking both.
Researchers enrolled 365,682 participants from the UK Biobank for the analysis of coffee and tea consumption and stroke and dementia risk and 13,352 participants for the analysis of poststroke dementia.
During a median follow-up of 11.4 years, 2.8% of participants experienced a stroke and 1.4% developed dementia.
After adjustment for confounders, stroke risk was 10% lower in those who drank a half-cup to a cup of coffee per day (P < .001) and 8% lower in those who had more than two cups a day (P = .009). Tea drinkers who had more than two cups a day saw a 16% reduction in stroke (P < .001).
Those who drank both coffee and tea during the day saw the greatest benefit. Drinking two to three cups of coffee and two to three cups of tea lowered stroke risk by 32% (P < .001) and dementia risk by 28% (P = .002).
Drinking both beverages offered significantly greater benefits than drinking just coffee or tea alone, with an 11% lower risk for stroke (P < .001), an 8% lower risk for dementia (P = .001), and 18% lower risk for vascular dementia (P = .001).
Among those participants who experienced a stroke during the follow-up period, drinking two to three cups of coffee was associated with 20% lower risk for poststroke dementia (P = .044), and for those who drank both coffee and tea (half to one cup of coffee and two to three cups of tea per day) the risk for poststroke dementia was lowered by 50% (P =.006).
There was no significant association between coffee and tea consumption and risk for hemorrhagic stroke or Alzheimer’s disease.
The study was funded by the National Natural Science Foundation of China. The authors have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Drinking coffee or tea is associated with reduced risk for stroke and dementia, with the biggest benefit associated with consuming both beverages, new research suggests.
Investigators found that individuals who drank two to three cups of coffee and two to three cups of tea per day had a 30% decrease in incidence of stroke and a 28% lower risk for dementia compared with those who did not.
“From a public health perspective, because regular tea and coffee drinkers comprise such a large proportion of the population and because these beverages tend to be consumed habitually throughout adult life, even small potential health benefits or risks associated with tea and coffee intake may have important public health implications,” the investigators wrote.
The study was published online Nov. 16 in PLOS Medicine.
Synergistic effect?
Whereas earlier studies have shown significant health benefits from moderate coffee and tea intake separately, few have examined the effect of drinking both.
Researchers enrolled 365,682 participants from the UK Biobank for the analysis of coffee and tea consumption and stroke and dementia risk and 13,352 participants for the analysis of poststroke dementia.
During a median follow-up of 11.4 years, 2.8% of participants experienced a stroke and 1.4% developed dementia.
After adjustment for confounders, stroke risk was 10% lower in those who drank a half-cup to a cup of coffee per day (P < .001) and 8% lower in those who had more than two cups a day (P = .009). Tea drinkers who had more than two cups a day saw a 16% reduction in stroke (P < .001).
Those who drank both coffee and tea during the day saw the greatest benefit. Drinking two to three cups of coffee and two to three cups of tea lowered stroke risk by 32% (P < .001) and dementia risk by 28% (P = .002).
Drinking both beverages offered significantly greater benefits than drinking just coffee or tea alone, with an 11% lower risk for stroke (P < .001), an 8% lower risk for dementia (P = .001), and 18% lower risk for vascular dementia (P = .001).
Among those participants who experienced a stroke during the follow-up period, drinking two to three cups of coffee was associated with 20% lower risk for poststroke dementia (P = .044), and for those who drank both coffee and tea (half to one cup of coffee and two to three cups of tea per day) the risk for poststroke dementia was lowered by 50% (P =.006).
There was no significant association between coffee and tea consumption and risk for hemorrhagic stroke or Alzheimer’s disease.
The study was funded by the National Natural Science Foundation of China. The authors have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Drinking coffee or tea is associated with reduced risk for stroke and dementia, with the biggest benefit associated with consuming both beverages, new research suggests.
Investigators found that individuals who drank two to three cups of coffee and two to three cups of tea per day had a 30% decrease in incidence of stroke and a 28% lower risk for dementia compared with those who did not.
“From a public health perspective, because regular tea and coffee drinkers comprise such a large proportion of the population and because these beverages tend to be consumed habitually throughout adult life, even small potential health benefits or risks associated with tea and coffee intake may have important public health implications,” the investigators wrote.
The study was published online Nov. 16 in PLOS Medicine.
Synergistic effect?
Whereas earlier studies have shown significant health benefits from moderate coffee and tea intake separately, few have examined the effect of drinking both.
Researchers enrolled 365,682 participants from the UK Biobank for the analysis of coffee and tea consumption and stroke and dementia risk and 13,352 participants for the analysis of poststroke dementia.
During a median follow-up of 11.4 years, 2.8% of participants experienced a stroke and 1.4% developed dementia.
After adjustment for confounders, stroke risk was 10% lower in those who drank a half-cup to a cup of coffee per day (P < .001) and 8% lower in those who had more than two cups a day (P = .009). Tea drinkers who had more than two cups a day saw a 16% reduction in stroke (P < .001).
Those who drank both coffee and tea during the day saw the greatest benefit. Drinking two to three cups of coffee and two to three cups of tea lowered stroke risk by 32% (P < .001) and dementia risk by 28% (P = .002).
Drinking both beverages offered significantly greater benefits than drinking just coffee or tea alone, with an 11% lower risk for stroke (P < .001), an 8% lower risk for dementia (P = .001), and 18% lower risk for vascular dementia (P = .001).
Among those participants who experienced a stroke during the follow-up period, drinking two to three cups of coffee was associated with 20% lower risk for poststroke dementia (P = .044), and for those who drank both coffee and tea (half to one cup of coffee and two to three cups of tea per day) the risk for poststroke dementia was lowered by 50% (P =.006).
There was no significant association between coffee and tea consumption and risk for hemorrhagic stroke or Alzheimer’s disease.
The study was funded by the National Natural Science Foundation of China. The authors have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
St. Jude hoards billions while many of its families drain their savings
A series of sharp knocks on his driver’s side window startled Jason Burt awake.
It was the middle of the night on a Saturday in 2016. Burt was sleeping in his pickup truck in the parking lot of St. Jude Children’s Research Hospital in downtown Memphis, Tenn., where his 5-year-old daughter was being treated for brain cancer. He’d driven more than 500 miles from his home in Central Texas to visit her.
A St. Jude security guard peered into the truck and asked Burt what he was doing. Burt explained that his daughter and her mother, his ex-girlfriend, were staying in the hospital’s free patient housing. But St. Jude provides housing for only one parent. Burt, a school bus driver making $20,000 a year, told the guard he couldn’t afford a hotel. The guard let the exhausted father go back to sleep.
St. Jude would do no more to find him a place to stay.
“They were aware of the situation,” Burt said. “I didn’t push anything. I was just grateful she was getting treated and I was doing what I needed to do.”
St. Jude is the largest and most highly regarded health care charity in the country. Each year, the Memphis hospital’s fundraisers send out hundreds of millions of letters, many with heart-wrenching photographs of children left bald from battling cancer. Celebrities like Jennifer Aniston and Sofia Vergara sing the hospital’s praises in televised advertisements. This year, St. Jude’s fundraising reached outer space. The SpaceX Inspiration4 mission in September included a former St. Jude patient as a crew member.
Last year, St. Jude raised a record $2 billion. U.S. News & World Report ranked it the country’s 10th-best children’s cancer hospital, and St. Jude raised roughly as much as the nine hospitals ahead of it put together. It currently has $5.2 billion in reserves, a sum large enough to run the institution at current levels for the next four and a half years without a single additional donation.
St. Jude makes a unique promise as part of its fundraising: “Families never receive a bill from St. Jude for treatment, travel, housing or food – because all a family should worry about is helping their child live.”
But for many families, treatment at St. Jude does not relieve all the financial burdens they incur in getting care for their children, including housing, travel, and food costs that fall outside the hospital’s strict limits, a ProPublica investigation has found.
While families may not receive a bill from St. Jude, the hospital doesn’t cover what’s usually the biggest source of financial stress associated with childhood cancer: The loss of income as parents quit or take leave from jobs to be with their child during treatment. For many families, the consequence is missed payments for cars, utilities, and cellphones. Others face eviction or foreclosure because they can’t keep up with rent and mortgage payments.
Parents at St. Jude have exhausted savings and retirement accounts, borrowed from family and friends, or asked other charities for aid. ProPublica identified more than 100 St. Jude families seeking financial help through the online fundraiser GoFundMe, with half of the campaigns started in the past two years. We counted scores of other events like concerts and yard sales organized to help St. Jude families in need.
One family relied on a mixed martial arts fighter to help raise money for expenses like car repairs and cellphone bills, items that St. Jude would not cover. Another spent $10,000, originally saved to purchase a home, on costs related to treatment at St. Jude.
Only about half of the $7.3 billion St. Jude has received in contributions in the past five fiscal years went to the hospital’s research and caring for patients, according to its financial filings with the Internal Revenue Service. About 30% covered the cost of its fundraising operations, and the remaining 20%, or $1 of every $5 donated, increased its reserve fund.
Further, ProPublica found, a substantial portion of the cost for treatment is paid not by St. Jude but by families’ private insurance or by Medicaid, the government insurance program for low-income families. About 90% of patients are insured, bringing in more than $100 million in reimbursements for treatment a year. If a family shows up at St. Jude without insurance, a company hired by the charity helps them find it. St. Jude does cover copays and deductibles, an unusual benefit.
St. Jude spends about $500 million a year on patient services – a figure that includes all medical care and other assistance. Very little of what St. Jude raises from the public goes to pay for food, travel, and housing for families, the investigation found. Last year, it was 2% of the money raised, or nearly $40 million.
In written responses to ProPublica, lawyers for St. Jude and its fundraising arm, the American Lebanese Syrian Associated Charities, or ALSAC, emphasized that countless families have benefited from the charity provided since the hospital opened its doors in 1962.
“ProPublica should be celebrating St. Jude and ALSAC for their commitment to finding cures, saving children’s lives, and optimizing patient outcomes,” one of their letters said.
It is unquestioned that St. Jude has helped thousands of children and their families over the decades. Patients have offered scores of testimonials about the hospital’s generosity and care.
“This often comes as a huge relief to families who often expect to sell all their belongings just so their children can get the medical care and treatment they need to save their lives,” the hospital’s lawyers wrote. “St. Jude and ALSAC understand that this arrangement cannot cover all financial obligations of all families, nor can St. Jude or ALSAC shield families from all the financial and emotional effects” of a child’s illness.
St. Jude said it discloses the limits of its aid to families on its website and in material provided to those whose children are admitted to the hospital. That includes the rule Burt ran into, that the hospital covers the travel and housing costs of only one caregiver and one patient. For many families, the daily food budget is capped at $50. In some cases, hotel stays en route are provided only if families travel more than 500 miles to get to St. Jude.
St. Jude said its assistance is “based on guidelines to ensure fairness and responsible use of donor funds” and on remaining compliant with a federal anti-kickback statute that makes it a criminal offense to offer something of value to induce a medical referral. St. Jude declined to explain how the law affects the amount or type of financial assistance it provides to families.
“St. Jude has never promised anyone – neither patients nor the public in general – that it can solve all financial problems,” the letter said.
When parents need additional financial help, St. Jude’s social workers often send them to smaller charities or in some cases suggest that they apply for government aid.
They refer many to the Andrew McDonough B+ Foundation, which gives more than $2.5 million a year in grants to thousands of families of pediatric cancer patients at hospitals across the country to help cover rent, utilities, and other urgent expenses.
Joe McDonough, the foundation’s founder and president, said St. Jude families have the same money problems as families of patients at other children’s hospitals, even though he said St. Jude’s marketing creates the public perception that it alleviates these burdens.
“People say to me, ‘Why are you helping St. Jude families?’ ” McDonough said. “Well, what happens when a family lives in Augusta, Georgia, and they’re being treated at St. Jude? They still have to pay the rent on their apartment back in Augusta, Georgia. They still have to make their car payment. And it’s not my position to say whether St. Jude should be paying for all those expenses or not. I’m just explaining that it’s not a totally free ride.”
The help St. Jude provides to families may soon be increasing.
After ProPublica provided St. Jude with the findings of its reporting, the hospital informed families of a dramatic expansion in the assistance it will give to parents and other relatives during their kids’ treatment in Memphis.
Among the most significant changes are increasing travel benefits to two parents instead of one and covering regular trips to Memphis for siblings and other loved ones. St. Jude’s letter to parents said the changes take effect Nov. 15.
That would’ve made a big difference for Burt.
Burt’s daughter, whom ProPublica is not identifying at her mother’s request, was originally diagnosed with cancer in early 2015, when doctors discovered a tumor pressing against her brain stem. She had successful emergency surgery to remove the mass at Dell Children’s Medical Center in Austin, Texas. Medicaid and Dell Children’s covered the bill, but the family was still faced with the cost of her ongoing treatment.
“At that point I’m thinking: ‘What am I going to do? I guess I’m selling my house, whatever it takes,’” Burt recalled. “Honestly, that was probably a big deciding factor for St. Jude.”
St. Jude accepted Burt’s daughter into a clinical trial, and the family moved to the hospital’s patient housing in Memphis for several months. Both parents stopped working for a time, and people in their hometown raised cash to pay their bills.
Her cancer relapsed the following year with several new, inoperable brain tumors. Burt and his daughter’s mom broke up during that round of treatment, and financial problems piled up.
Burt said his credit score dropped so low that utility companies refused to set up service unless he first paid a deposit. One of the family’s cars was repossessed, he said. Burt’s 2005 Chevrolet Colorado pickup has 300,000 miles on it, many of them logged on trips from Texas to Memphis. When Burt’s daughter was at St. Jude for treatment or exams, he’d work all week, then visit on many weekends where he would spend Saturday night sleeping in the hospital parking lot.
He asked hospital officials if he could sleep in St. Jude’s housing, but they turned him down, he said.
Burt said he was happy with the care St. Jude provided. His daughter’s health is stable, he said, and brain scans taken during her September exam confirmed her two remaining tumors haven’t grown. But he’s still trying to recover financially.
“It’s five years now,” Burt said, “and I’m not completely caught up yet.”
A fundraising giant
St. Jude began with a fledgling entertainer praying for a career break.
When Danny Thomas, a comic and actor best known for the TV sitcom “Make Room for Daddy,” was struggling to earn a living in the late 1930s, the devout Roman Catholic went to church and asked for help from the patron saint of desperate cases, St. Jude Thaddeus. If he made it big, Thomas promised to build “a shrine where the poor and the helpless and the hopeless may come for comfort and aid,” according to a history published by ALSAC.
Within five years, Thomas became a star and worked to fulfill his promise by building a children’s hospital named after St. Jude and a fundraising organization to support it. Thomas, whose parents were Lebanese immigrants, recruited others who shared his Middle Eastern roots to help.
He used his fame to raise the hospital’s profile, appearing in ads for St. Jude and hosting fundraising events starring the likes of Elvis Presley and Sammy Davis Jr. Thomas’ daughter Marlo, herself a TV star, succeeded him in championing St. Jude.
Today, St. Jude is a specialty treatment and research center with about 5,700 employees and 73 beds. Other top children’s hospitals have more staff and beds, and they also treat more conditions.
Though St. Jude raises money across the world, most of its patients come from Tennessee and surrounding states. Patients from elsewhere are usually enrolled in clinical trials.
ALSAC, which handles St. Jude’s fundraising and investments, has 2,188 employees in Memphis and in 36 regional offices across the country. More than 400 of the fundraising arm’s employees are paid over $100,000, according to IRS filings. The charity takes in so much money each year that it regularly steers hundreds of millions of dollars in donations to reserve accounts, the filings show.
Overall, St. Jude’s reserve has grown by 58% over the past five fiscal years, during which it has added $1.9 billion to its investment accounts and shifted its portfolio toward financial products designed to generate bigger returns than stocks, bonds, and mutual funds traditionally deliver. The charity stowed more than a third of the new surplus, $688 million, in riskier private equity investments.
IRS rules do not limit the size of a nonprofit’s reserves, and experts on charitable finance differ on best practices.
St. Jude meets Better Business Bureau guidelines, which call for charities to maintain reserves of less than three times total expenses, but other experts expressed alarm that the hospital had accumulated such a large sum of money.
The size of the St. Jude reserve is “staggering,” said Laura Otten, the director of LaSalle University’s master program in nonprofit leadership. She said a typical reserve for a nonprofit the size of St. Jude is one to two years of expenses. Donors generally want to know their dollars are being put to work, she said.
The hospital said it needs a large reserve because its unique operating model relies on donations to fund annual operating costs. “[W]e are highly donor-dependent and subject to the economic driven vagaries of charitable giving,” the hospital said in a written response to ProPublica questions.
But the hospital’s reserve is already more than large enough to buffer against recessions and potential drops in donations, said Ge Bai, a professor of accounting and health policy at Johns Hopkins University. “They should be spending the money as aggressively as they raise it, but they seem to be hoarding,” Bai said.
The hospital said it is also raising billions to fund the construction of new housing and research space, although its plans do not currently include spending any of the reserve on new facilities.
St. Jude’s reserves have ballooned at a time when researchers, oncologists, advocates, and families complain about a dearth of funding for pediatric cancer studies nationally.
Dozens of other children’s hospitals across the country have research divisions devoted to pediatric cancer and enroll their patients in clinical trials for new drugs and procedures. They pay for research staff and studies in part with donations from their local communities, often competing directly against St. Jude. ALSAC has regional offices in several U.S. cities with elite pediatric cancer centers of their own, including Atlanta, Chicago, Denver, and Seattle.
Coury Shadyac, an ALSAC vice president and daughter of the organization’s CEO, Richard Shadyac Jr., oversees a team of 45 fundraisers along the West Coast “raising $300 million annually” for St. Jude, according to her LinkedIn profile. That’s $100 million more in donations than either Children’s Hospital Los Angeles or Seattle Children’s Hospital, two of the nation’s leading pediatric cancer institutions, received in fiscal year 2019, IRS disclosures show. But it’s only a small part of St. Jude’s fundraising haul.
ALSAC’s ubiquitous fundraising has led to concerns that it undercuts other hospitals’ campaigns. Some doctors interviewed by ProPublica said they have encouraged donors to give their money to hospitals closer to home.
David Clark, a pediatrician and former longtime chairperson of pediatrics at Albany Medical Center in New York, said St. Jude raises tens of thousands of dollars in his region that does little to benefit the children with cancer in his area since almost all are treated locally. ALSAC has a fundraising office located a few miles from Albany Medical.
“They think of every way they can to make money and the least amount of ways to spend it,” Clark said. “They deceive people into supporting something that is totally dishonest.”
Nearly all St. Jude solicitations feature the hospital’s patients – the children usually smiling and bald from treatment – along with the familiar promise that it never sends families a bill.
It’s a message that ALSAC has tested and researched to maximize donations. Donors appreciate the promise to never bill families, said Mary Kate Tolan, an ALSAC executive, in a podcast last year. She added that no parent should have to take out a second mortgage or lose their job because their child is being treated at St. Jude.
Alternative messaging to the no-bills promise did not “perform as well,” said Tolan, who develops emerging technologies for ALSAC. Tolan did not return requests for comment.
“Borrowing and begging”
Catherine Rainey thought she would be free of financial worry when her 2-year-old daughter Harlee was admitted to St. Jude last year.
“The first thing my dad said was: ‘Catherine, you have nothing to worry about. They raise billions of dollars. Anytime you have a problem, you tell them and they will take care of it,’ ” she said.
But like many families, the Raineys discovered that St. Jude’s charity came with limits on payments for expenses such as travel that could be bewildering.
Harlee ended up at St. Jude after first going to nearby Niswonger Children’s Hospital in Johnson City, Tenn., in October 2020. The doctors there discovered a cancerous mass attached to her right kidney. The hospital is a St. Jude affiliate, and the doctors recommended the toddler be treated in Memphis.
Rainey, a single mother of two young girls, had to leave her job as a nurse for months to be with Harlee at St. Jude. The loss of income quickly created problems. “My family, we don’t come from money,” she said. “We are not doctors and billionaires. We make it. That is it.”
St. Jude did provide food and housing on campus. But the hospital said it couldn’t help with the items that were causing Rainey to worry, including car payments, insurance, and cellphone bills.
Rainey’s boss set up a GoFundMe account to help make up some of her lost income. A small local charity, Kari’s Heart Foundation, also helped out by paying about $3,000 worth of phone bills and car payments, staving off repossession.
“It was just a bunch of borrowing and begging,” Rainey said of her experience while her daughter was treated in Memphis. “They acted like it was coming out of their own pocket.”
Harlee has checkups at St. Jude every three months that last about four days. The costs of travel to and from St. Jude put an additional strain on Rainey and Harlee. St. Jude is an eight-hour ride, without stops, from Rainey’s home in Appalachia, Va., a town of 1,432 people near the Kentucky border.
Rainey said her daughter generally can make it about two-thirds of the way, with frequent stops, before she has had enough. “When she is done, she is really done,” Rainey said. “She will scream, cry, and kick.”
In July, in advance of an August trip to Memphis, Rainey called the patient services department at St. Jude to see whether they could help pay for a hotel to break up the travel day — an expense Rainey said she could not afford.
To qualify for a hotel reimbursement, Rainey said, St. Jude told her she had to live more than 500 miles from Memphis. The ride from her home to the hospital is 530 miles (a measurement ProPublica confirmed with mapping tools). However, Rainey said, St. Jude told her it measured the trip from city limit to city limit and came up with a distance of 491 miles. Even using that metric, the distance is still more than 500 miles, ProPublica found.
When she challenged the hospital’s stance, Rainey said she was berated by a patient services representative.
“I was feeling pissed off, and I was crying,” Rainey said of the interaction. “You give up your whole life for your child, and they tell you don’t worry about anything, we will cover this and then they tell you to just push through the drive.”
Rainey did what she could to make the trip go smoothly: She configured a small table to extend across her daughter’s car seat, so Harlee could play with the coloring books, markers, and Play-Doh bought for the ride. She packed snacks and a cooler full of drinks. Since Harlee was still potty training, she brought extra towels and clothes for accidents. The final step was handing Harlee her Baby Yoda doll once she settled into her car seat. Rainey had sewed a port in the doll’s chest to mirror the one Harlee has in hers.
About three hours from Memphis, Harlee was crying inconsolably. Rainey pulled off the interstate and stopped at the first hotel she could find. She later learned it had been described in online reviews as “awful,” a “nightmare,” “disgusting,” and “horrible.”
“I didn’t know the area,” she said. “The hotel was garbage. It just made it worse.”
The drive home also required a hotel stop, but this time Rainey was able to find one that was cleaner. A $100 donation from a local charity helped to offset the cost.
Among the changes St. Jude is making is to reimburse families like Rainey’s, who live more than 400 miles from the hospital, for an overnight stay at a hotel when making the trip to Memphis.
Rainey said she was called by a St. Jude representative after ProPublica asked about her situation and was told the hospital would pay for her past hotel stays when traveling back and forth to St. Jude. The representative, Rainey said, also told her the hospital discovered the way it had been measuring mileage was inaccurate.
“I am not the only one,” Rainey said. “There are others. They should reimburse all the families.”
The anxiety of unpaid bills piling up, combined with caring for a child undergoing chemotherapy or radiation, takes a severe toll on parents and guardians, said Christopher Hope, a UPS driver who started a Memphis-based foundation after meeting St. Jude parents who were in financial crisis.
Hope’s small charity spent $12,000 last year to help families. Parents in St. Jude social media groups often refer families in need to it. The charity has helped families cover mortgage and car payments.
“I never knew anything about this until hearing about it from families,” Hope said. “All we hear is about kids and treatment, not the other side of it.”
“It’s not free”
In addition to charities like Hope’s, St. Jude families have repeatedly turned to fundraising sites and networks of their relatives, friends, and neighbors to help cover basic expenses while unable to work during their children’s treatment. Parents’ requests on fundraising sites are sometimes desperate pleas.
In January 2017, one father in North Carolina said he’d had to abandon a business venture to take time for his son to receive care at St. Jude. His income had plummeted. He asked friends to give as little as $10 to “at least make it possible to survive.”
This year, a mother in Memphis whose 1-year-old son receives care at St. Jude for sickle cell disorder ran out of medical leave and couldn’t work her shifts at a clothing distribution center. After the child had a flare-up in July requiring several days of treatment at the hospital, she said she returned home to find her power shut off. Sitting in a dark apartment, unable to pay her utility bills, she set up a GoFundMe campaign. She received less than $20 through the site; her relatives eventually pooled $350 to get her electricity restored.
Even parents with stable jobs and private health insurance often take on debt and need outside help.
When Taylr and Treg Murphy’s 17-year-old son Peyton was diagnosed with cancer and needed monthslong treatment at St. Jude in 2017, the entire family – mom, dad, sister, and brother – went with him, traveling from their home in Lafayette, La., to Memphis. Treg took a leave from his job at an oil mining company and Taylr, who works at her mother’s bakery, did the same.
“We knew that it was going to be a collective team effort,” Treg said. “Without even a discussion, we figured that if Peyton’s got to go for the surgery, we’re all going.”
Peyton had an enormous tumor that had grown out of his right femur and was crowding his knee. Rounds of chemotherapy appeared to have killed osteosarcoma cells elsewhere in his body. But he needed to undergo a procedure called limb-sparing surgery that would require weeks of recovery time at the hospital.
The hospital agreed to allow all five family members to stay for free at St. Jude if they bunked together in a single room. It assigned them a spot in Tri Delta Place, its hotel-like short-term patient residence on the campus. Tri Delta is set up for visits of up to seven days, according to the hospital’s guide for volunteers, but the Murphys were there for almost 50.
Taylr said the unit at Tri Delta had no oven or stove and St. Jude provided no grocery money, instead allotting them a $50-per-day credit at the hospital cafeteria, Kay Kafe – not enough to feed the family of five. As the weeks wore on, the Murphys split grilled cheese sandwiches and paid for food out of pocket.
After ProPublica asked about the hospital’s food allowances, St. Jude said it would increase them as part of the changes scheduled to go into effect this month. The hospital switched from a $50-a-day cap per family to providing $25 a day to each family member. For a family of four, that would double the food benefit. A weekly stipend given to families in long-term housing was increased to $150 from $125.
For the Murphys, it was the loss of their work income, more than out-of-pocket expenses, that put them into a financial hole as Peyton’s treatment went on. Treg’s employer couldn’t pay him during his long absences.
Fearful of being evicted or having their car repossessed, Taylr said she asked a St. Jude social worker for assistance. The social worker helped her apply for grants from other charities. Taylr said the B+ Foundation paid their rent one month, which ensured they’d have a home to return to.
In the years since his initial treatment, Peyton has gone back to St. Jude repeatedly for exams and surgeries to remove malignant growths in his lungs. Taylr and Treg have missed more work to bring Peyton to Memphis, costing them thousands of dollars more in income.
By the start of this year, Taylr and Treg said they were about $20,000 in debt and panicking. Dustin Poirier, a former UFC champion from their hometown, heard from a friend about Peyton and the family’s financial trouble. He donated $10,000 to them from his personal charity and in May hosted a local fundraiser that collected enough to pay off their credit cards.
St. Jude families sometimes commiserate about money problems with each other, Taylr said, but few are aware of the extent of the hospital’s unspent resources. The Murphys said they didn’t know St. Jude has more than $5 billion in reserve or that it continues to raise hundreds of millions of dollars in surplus donations each year.
“That’s just insane,” Taylr said. “That just blows my mind. When we first started getting treated, people would be like, ‘Oh, St. Jude covers everything, that’s awesome.’ That’s not how it works. People don’t understand that. I truly didn’t understand before I got into St. Jude.”
Taylr and Treg said the doctors at St. Jude are “amazing” and they’re grateful for their son’s care. But they bristled at the assumption that it was covered by the hospital’s charity. The family’s insurance paid a substantial part of the bills.
“It’s not free,” Taylr said. “My husband works very hard for the insurance we have – and they are billed.” The Murphys pay $12,000 in health insurance premiums each year.
Their struggle continues. Peyton’s cancer has relapsed, and he’s making regular trips with his mom or dad back to St. Jude for chemotherapy. The family is again applying for help from other charities.
Wiped out savings
The costs associated with care at St. Jude caused at least one family to stop going to Memphis altogether.
Last winter, Kelly Edwards was excitedly searching through Tulsa real estate listings after years of diligently saving $10,000 for a down payment on a house. She craved a permanent home for herself and the two young brothers she had taken in five years earlier at the behest of a family friend. She hoped to adopt the boys, now 13 and 9, who call her mom.
In February, the older boy, DJ, was lethargic and uninterested in his schoolwork. After several doctor visits, he was diagnosed with acute lymphoblastic leukemia at a Tulsa hospital. The cancer, referred to as ALL, is the most common type among children, with survival rates that exceed 90%. A day after his diagnosis, DJ and Edwards were driving six hours to Memphis for treatment at St. Jude, which is affiliated with the Oklahoma hospital.
The pair stayed for free at an independently operated Ronald McDonald House near St. Jude, and a weekly stipend from the hospital helped to pay for meals – aid that Edwards said was a blessing. DJ had health insurance through the Oklahoma Medicaid program.
But as with the Murphys, lost income soon put Edwards’ family into financial jeopardy. She works as a supervisor for a company that delivers packages for Amazon. After she used up two weeks of paid time off, she stopped getting paychecks. The bills, however, kept coming: rent, car payments, utilities. To that was added the $250 a week she paid a friend to stay with DJ’s younger brother and her two dogs in Tulsa.
Within four months, her house savings were wiped out. Edwards said she told her St. Jude social worker about her financial woes but got no additional help.
One of Edwards’ adult daughters started a GoFundMe campaign to help, bringing in just over $3,000. Edwards said she appreciated the aid but believes donations were kept low by the widespread perception that St. Jude families don’t have financial problems.
“Everyone hears that everything is taken care of by St. Jude,” she said. “That is not true, but everyone has that mentality.” She said someone she knew asked her “what is that money going for if St. Jude’s is paying for everything?”
DJ was scheduled to go back to St. Jude for three weeks of treatment in August, but Edwards decided she simply couldn’t afford it. “I don’t have the money to go back and forth,” she said. She worked with DJ’s local doctors and found that the hospital near her home in Tulsa could provide the same treatment he was scheduled to get in Tennessee.
The local treatment allowed her to continue working some shifts and to be at home with both of her boys. DJ is also happier when he is home, Edwards said.
Edwards and the boys are now living in a small house her brother owns just outside Tulsa. Late on a recent weekday afternoon, DJ slowly shuffled into the living room, exhausted from a day of chemotherapy treatment.
He is in the midst of a 20-week regimen where he receives the cancer-killing drugs every other day, just one phase of a nearly three-year treatment plan. He wore an orange knit hat, T-shirt, and shorts. He rubbed his eyes before asking a visitor, “How is your day going?” He smiled at the positive response. When he heard the family was eating steak for dinner, he eagerly jumped up to start helping in the kitchen. After they moved in, Edwards hung family portraits on the walls to make it feel homier. She doesn’t expect they will be moving again any time soon.
The dream of buying a home of their own is gone.
Former ProPublica reporter Marshall Allen contributed reporting. Kirsten Berg contributed research.
This story was originally published on ProPublica. ProPublica is a nonprofit newsroom that investigates abuses of power. Sign up to receive their biggest stories as soon as they’re published.
A series of sharp knocks on his driver’s side window startled Jason Burt awake.
It was the middle of the night on a Saturday in 2016. Burt was sleeping in his pickup truck in the parking lot of St. Jude Children’s Research Hospital in downtown Memphis, Tenn., where his 5-year-old daughter was being treated for brain cancer. He’d driven more than 500 miles from his home in Central Texas to visit her.
A St. Jude security guard peered into the truck and asked Burt what he was doing. Burt explained that his daughter and her mother, his ex-girlfriend, were staying in the hospital’s free patient housing. But St. Jude provides housing for only one parent. Burt, a school bus driver making $20,000 a year, told the guard he couldn’t afford a hotel. The guard let the exhausted father go back to sleep.
St. Jude would do no more to find him a place to stay.
“They were aware of the situation,” Burt said. “I didn’t push anything. I was just grateful she was getting treated and I was doing what I needed to do.”
St. Jude is the largest and most highly regarded health care charity in the country. Each year, the Memphis hospital’s fundraisers send out hundreds of millions of letters, many with heart-wrenching photographs of children left bald from battling cancer. Celebrities like Jennifer Aniston and Sofia Vergara sing the hospital’s praises in televised advertisements. This year, St. Jude’s fundraising reached outer space. The SpaceX Inspiration4 mission in September included a former St. Jude patient as a crew member.
Last year, St. Jude raised a record $2 billion. U.S. News & World Report ranked it the country’s 10th-best children’s cancer hospital, and St. Jude raised roughly as much as the nine hospitals ahead of it put together. It currently has $5.2 billion in reserves, a sum large enough to run the institution at current levels for the next four and a half years without a single additional donation.
St. Jude makes a unique promise as part of its fundraising: “Families never receive a bill from St. Jude for treatment, travel, housing or food – because all a family should worry about is helping their child live.”
But for many families, treatment at St. Jude does not relieve all the financial burdens they incur in getting care for their children, including housing, travel, and food costs that fall outside the hospital’s strict limits, a ProPublica investigation has found.
While families may not receive a bill from St. Jude, the hospital doesn’t cover what’s usually the biggest source of financial stress associated with childhood cancer: The loss of income as parents quit or take leave from jobs to be with their child during treatment. For many families, the consequence is missed payments for cars, utilities, and cellphones. Others face eviction or foreclosure because they can’t keep up with rent and mortgage payments.
Parents at St. Jude have exhausted savings and retirement accounts, borrowed from family and friends, or asked other charities for aid. ProPublica identified more than 100 St. Jude families seeking financial help through the online fundraiser GoFundMe, with half of the campaigns started in the past two years. We counted scores of other events like concerts and yard sales organized to help St. Jude families in need.
One family relied on a mixed martial arts fighter to help raise money for expenses like car repairs and cellphone bills, items that St. Jude would not cover. Another spent $10,000, originally saved to purchase a home, on costs related to treatment at St. Jude.
Only about half of the $7.3 billion St. Jude has received in contributions in the past five fiscal years went to the hospital’s research and caring for patients, according to its financial filings with the Internal Revenue Service. About 30% covered the cost of its fundraising operations, and the remaining 20%, or $1 of every $5 donated, increased its reserve fund.
Further, ProPublica found, a substantial portion of the cost for treatment is paid not by St. Jude but by families’ private insurance or by Medicaid, the government insurance program for low-income families. About 90% of patients are insured, bringing in more than $100 million in reimbursements for treatment a year. If a family shows up at St. Jude without insurance, a company hired by the charity helps them find it. St. Jude does cover copays and deductibles, an unusual benefit.
St. Jude spends about $500 million a year on patient services – a figure that includes all medical care and other assistance. Very little of what St. Jude raises from the public goes to pay for food, travel, and housing for families, the investigation found. Last year, it was 2% of the money raised, or nearly $40 million.
In written responses to ProPublica, lawyers for St. Jude and its fundraising arm, the American Lebanese Syrian Associated Charities, or ALSAC, emphasized that countless families have benefited from the charity provided since the hospital opened its doors in 1962.
“ProPublica should be celebrating St. Jude and ALSAC for their commitment to finding cures, saving children’s lives, and optimizing patient outcomes,” one of their letters said.
It is unquestioned that St. Jude has helped thousands of children and their families over the decades. Patients have offered scores of testimonials about the hospital’s generosity and care.
“This often comes as a huge relief to families who often expect to sell all their belongings just so their children can get the medical care and treatment they need to save their lives,” the hospital’s lawyers wrote. “St. Jude and ALSAC understand that this arrangement cannot cover all financial obligations of all families, nor can St. Jude or ALSAC shield families from all the financial and emotional effects” of a child’s illness.
St. Jude said it discloses the limits of its aid to families on its website and in material provided to those whose children are admitted to the hospital. That includes the rule Burt ran into, that the hospital covers the travel and housing costs of only one caregiver and one patient. For many families, the daily food budget is capped at $50. In some cases, hotel stays en route are provided only if families travel more than 500 miles to get to St. Jude.
St. Jude said its assistance is “based on guidelines to ensure fairness and responsible use of donor funds” and on remaining compliant with a federal anti-kickback statute that makes it a criminal offense to offer something of value to induce a medical referral. St. Jude declined to explain how the law affects the amount or type of financial assistance it provides to families.
“St. Jude has never promised anyone – neither patients nor the public in general – that it can solve all financial problems,” the letter said.
When parents need additional financial help, St. Jude’s social workers often send them to smaller charities or in some cases suggest that they apply for government aid.
They refer many to the Andrew McDonough B+ Foundation, which gives more than $2.5 million a year in grants to thousands of families of pediatric cancer patients at hospitals across the country to help cover rent, utilities, and other urgent expenses.
Joe McDonough, the foundation’s founder and president, said St. Jude families have the same money problems as families of patients at other children’s hospitals, even though he said St. Jude’s marketing creates the public perception that it alleviates these burdens.
“People say to me, ‘Why are you helping St. Jude families?’ ” McDonough said. “Well, what happens when a family lives in Augusta, Georgia, and they’re being treated at St. Jude? They still have to pay the rent on their apartment back in Augusta, Georgia. They still have to make their car payment. And it’s not my position to say whether St. Jude should be paying for all those expenses or not. I’m just explaining that it’s not a totally free ride.”
The help St. Jude provides to families may soon be increasing.
After ProPublica provided St. Jude with the findings of its reporting, the hospital informed families of a dramatic expansion in the assistance it will give to parents and other relatives during their kids’ treatment in Memphis.
Among the most significant changes are increasing travel benefits to two parents instead of one and covering regular trips to Memphis for siblings and other loved ones. St. Jude’s letter to parents said the changes take effect Nov. 15.
That would’ve made a big difference for Burt.
Burt’s daughter, whom ProPublica is not identifying at her mother’s request, was originally diagnosed with cancer in early 2015, when doctors discovered a tumor pressing against her brain stem. She had successful emergency surgery to remove the mass at Dell Children’s Medical Center in Austin, Texas. Medicaid and Dell Children’s covered the bill, but the family was still faced with the cost of her ongoing treatment.
“At that point I’m thinking: ‘What am I going to do? I guess I’m selling my house, whatever it takes,’” Burt recalled. “Honestly, that was probably a big deciding factor for St. Jude.”
St. Jude accepted Burt’s daughter into a clinical trial, and the family moved to the hospital’s patient housing in Memphis for several months. Both parents stopped working for a time, and people in their hometown raised cash to pay their bills.
Her cancer relapsed the following year with several new, inoperable brain tumors. Burt and his daughter’s mom broke up during that round of treatment, and financial problems piled up.
Burt said his credit score dropped so low that utility companies refused to set up service unless he first paid a deposit. One of the family’s cars was repossessed, he said. Burt’s 2005 Chevrolet Colorado pickup has 300,000 miles on it, many of them logged on trips from Texas to Memphis. When Burt’s daughter was at St. Jude for treatment or exams, he’d work all week, then visit on many weekends where he would spend Saturday night sleeping in the hospital parking lot.
He asked hospital officials if he could sleep in St. Jude’s housing, but they turned him down, he said.
Burt said he was happy with the care St. Jude provided. His daughter’s health is stable, he said, and brain scans taken during her September exam confirmed her two remaining tumors haven’t grown. But he’s still trying to recover financially.
“It’s five years now,” Burt said, “and I’m not completely caught up yet.”
A fundraising giant
St. Jude began with a fledgling entertainer praying for a career break.
When Danny Thomas, a comic and actor best known for the TV sitcom “Make Room for Daddy,” was struggling to earn a living in the late 1930s, the devout Roman Catholic went to church and asked for help from the patron saint of desperate cases, St. Jude Thaddeus. If he made it big, Thomas promised to build “a shrine where the poor and the helpless and the hopeless may come for comfort and aid,” according to a history published by ALSAC.
Within five years, Thomas became a star and worked to fulfill his promise by building a children’s hospital named after St. Jude and a fundraising organization to support it. Thomas, whose parents were Lebanese immigrants, recruited others who shared his Middle Eastern roots to help.
He used his fame to raise the hospital’s profile, appearing in ads for St. Jude and hosting fundraising events starring the likes of Elvis Presley and Sammy Davis Jr. Thomas’ daughter Marlo, herself a TV star, succeeded him in championing St. Jude.
Today, St. Jude is a specialty treatment and research center with about 5,700 employees and 73 beds. Other top children’s hospitals have more staff and beds, and they also treat more conditions.
Though St. Jude raises money across the world, most of its patients come from Tennessee and surrounding states. Patients from elsewhere are usually enrolled in clinical trials.
ALSAC, which handles St. Jude’s fundraising and investments, has 2,188 employees in Memphis and in 36 regional offices across the country. More than 400 of the fundraising arm’s employees are paid over $100,000, according to IRS filings. The charity takes in so much money each year that it regularly steers hundreds of millions of dollars in donations to reserve accounts, the filings show.
Overall, St. Jude’s reserve has grown by 58% over the past five fiscal years, during which it has added $1.9 billion to its investment accounts and shifted its portfolio toward financial products designed to generate bigger returns than stocks, bonds, and mutual funds traditionally deliver. The charity stowed more than a third of the new surplus, $688 million, in riskier private equity investments.
IRS rules do not limit the size of a nonprofit’s reserves, and experts on charitable finance differ on best practices.
St. Jude meets Better Business Bureau guidelines, which call for charities to maintain reserves of less than three times total expenses, but other experts expressed alarm that the hospital had accumulated such a large sum of money.
The size of the St. Jude reserve is “staggering,” said Laura Otten, the director of LaSalle University’s master program in nonprofit leadership. She said a typical reserve for a nonprofit the size of St. Jude is one to two years of expenses. Donors generally want to know their dollars are being put to work, she said.
The hospital said it needs a large reserve because its unique operating model relies on donations to fund annual operating costs. “[W]e are highly donor-dependent and subject to the economic driven vagaries of charitable giving,” the hospital said in a written response to ProPublica questions.
But the hospital’s reserve is already more than large enough to buffer against recessions and potential drops in donations, said Ge Bai, a professor of accounting and health policy at Johns Hopkins University. “They should be spending the money as aggressively as they raise it, but they seem to be hoarding,” Bai said.
The hospital said it is also raising billions to fund the construction of new housing and research space, although its plans do not currently include spending any of the reserve on new facilities.
St. Jude’s reserves have ballooned at a time when researchers, oncologists, advocates, and families complain about a dearth of funding for pediatric cancer studies nationally.
Dozens of other children’s hospitals across the country have research divisions devoted to pediatric cancer and enroll their patients in clinical trials for new drugs and procedures. They pay for research staff and studies in part with donations from their local communities, often competing directly against St. Jude. ALSAC has regional offices in several U.S. cities with elite pediatric cancer centers of their own, including Atlanta, Chicago, Denver, and Seattle.
Coury Shadyac, an ALSAC vice president and daughter of the organization’s CEO, Richard Shadyac Jr., oversees a team of 45 fundraisers along the West Coast “raising $300 million annually” for St. Jude, according to her LinkedIn profile. That’s $100 million more in donations than either Children’s Hospital Los Angeles or Seattle Children’s Hospital, two of the nation’s leading pediatric cancer institutions, received in fiscal year 2019, IRS disclosures show. But it’s only a small part of St. Jude’s fundraising haul.
ALSAC’s ubiquitous fundraising has led to concerns that it undercuts other hospitals’ campaigns. Some doctors interviewed by ProPublica said they have encouraged donors to give their money to hospitals closer to home.
David Clark, a pediatrician and former longtime chairperson of pediatrics at Albany Medical Center in New York, said St. Jude raises tens of thousands of dollars in his region that does little to benefit the children with cancer in his area since almost all are treated locally. ALSAC has a fundraising office located a few miles from Albany Medical.
“They think of every way they can to make money and the least amount of ways to spend it,” Clark said. “They deceive people into supporting something that is totally dishonest.”
Nearly all St. Jude solicitations feature the hospital’s patients – the children usually smiling and bald from treatment – along with the familiar promise that it never sends families a bill.
It’s a message that ALSAC has tested and researched to maximize donations. Donors appreciate the promise to never bill families, said Mary Kate Tolan, an ALSAC executive, in a podcast last year. She added that no parent should have to take out a second mortgage or lose their job because their child is being treated at St. Jude.
Alternative messaging to the no-bills promise did not “perform as well,” said Tolan, who develops emerging technologies for ALSAC. Tolan did not return requests for comment.
“Borrowing and begging”
Catherine Rainey thought she would be free of financial worry when her 2-year-old daughter Harlee was admitted to St. Jude last year.
“The first thing my dad said was: ‘Catherine, you have nothing to worry about. They raise billions of dollars. Anytime you have a problem, you tell them and they will take care of it,’ ” she said.
But like many families, the Raineys discovered that St. Jude’s charity came with limits on payments for expenses such as travel that could be bewildering.
Harlee ended up at St. Jude after first going to nearby Niswonger Children’s Hospital in Johnson City, Tenn., in October 2020. The doctors there discovered a cancerous mass attached to her right kidney. The hospital is a St. Jude affiliate, and the doctors recommended the toddler be treated in Memphis.
Rainey, a single mother of two young girls, had to leave her job as a nurse for months to be with Harlee at St. Jude. The loss of income quickly created problems. “My family, we don’t come from money,” she said. “We are not doctors and billionaires. We make it. That is it.”
St. Jude did provide food and housing on campus. But the hospital said it couldn’t help with the items that were causing Rainey to worry, including car payments, insurance, and cellphone bills.
Rainey’s boss set up a GoFundMe account to help make up some of her lost income. A small local charity, Kari’s Heart Foundation, also helped out by paying about $3,000 worth of phone bills and car payments, staving off repossession.
“It was just a bunch of borrowing and begging,” Rainey said of her experience while her daughter was treated in Memphis. “They acted like it was coming out of their own pocket.”
Harlee has checkups at St. Jude every three months that last about four days. The costs of travel to and from St. Jude put an additional strain on Rainey and Harlee. St. Jude is an eight-hour ride, without stops, from Rainey’s home in Appalachia, Va., a town of 1,432 people near the Kentucky border.
Rainey said her daughter generally can make it about two-thirds of the way, with frequent stops, before she has had enough. “When she is done, she is really done,” Rainey said. “She will scream, cry, and kick.”
In July, in advance of an August trip to Memphis, Rainey called the patient services department at St. Jude to see whether they could help pay for a hotel to break up the travel day — an expense Rainey said she could not afford.
To qualify for a hotel reimbursement, Rainey said, St. Jude told her she had to live more than 500 miles from Memphis. The ride from her home to the hospital is 530 miles (a measurement ProPublica confirmed with mapping tools). However, Rainey said, St. Jude told her it measured the trip from city limit to city limit and came up with a distance of 491 miles. Even using that metric, the distance is still more than 500 miles, ProPublica found.
When she challenged the hospital’s stance, Rainey said she was berated by a patient services representative.
“I was feeling pissed off, and I was crying,” Rainey said of the interaction. “You give up your whole life for your child, and they tell you don’t worry about anything, we will cover this and then they tell you to just push through the drive.”
Rainey did what she could to make the trip go smoothly: She configured a small table to extend across her daughter’s car seat, so Harlee could play with the coloring books, markers, and Play-Doh bought for the ride. She packed snacks and a cooler full of drinks. Since Harlee was still potty training, she brought extra towels and clothes for accidents. The final step was handing Harlee her Baby Yoda doll once she settled into her car seat. Rainey had sewed a port in the doll’s chest to mirror the one Harlee has in hers.
About three hours from Memphis, Harlee was crying inconsolably. Rainey pulled off the interstate and stopped at the first hotel she could find. She later learned it had been described in online reviews as “awful,” a “nightmare,” “disgusting,” and “horrible.”
“I didn’t know the area,” she said. “The hotel was garbage. It just made it worse.”
The drive home also required a hotel stop, but this time Rainey was able to find one that was cleaner. A $100 donation from a local charity helped to offset the cost.
Among the changes St. Jude is making is to reimburse families like Rainey’s, who live more than 400 miles from the hospital, for an overnight stay at a hotel when making the trip to Memphis.
Rainey said she was called by a St. Jude representative after ProPublica asked about her situation and was told the hospital would pay for her past hotel stays when traveling back and forth to St. Jude. The representative, Rainey said, also told her the hospital discovered the way it had been measuring mileage was inaccurate.
“I am not the only one,” Rainey said. “There are others. They should reimburse all the families.”
The anxiety of unpaid bills piling up, combined with caring for a child undergoing chemotherapy or radiation, takes a severe toll on parents and guardians, said Christopher Hope, a UPS driver who started a Memphis-based foundation after meeting St. Jude parents who were in financial crisis.
Hope’s small charity spent $12,000 last year to help families. Parents in St. Jude social media groups often refer families in need to it. The charity has helped families cover mortgage and car payments.
“I never knew anything about this until hearing about it from families,” Hope said. “All we hear is about kids and treatment, not the other side of it.”
“It’s not free”
In addition to charities like Hope’s, St. Jude families have repeatedly turned to fundraising sites and networks of their relatives, friends, and neighbors to help cover basic expenses while unable to work during their children’s treatment. Parents’ requests on fundraising sites are sometimes desperate pleas.
In January 2017, one father in North Carolina said he’d had to abandon a business venture to take time for his son to receive care at St. Jude. His income had plummeted. He asked friends to give as little as $10 to “at least make it possible to survive.”
This year, a mother in Memphis whose 1-year-old son receives care at St. Jude for sickle cell disorder ran out of medical leave and couldn’t work her shifts at a clothing distribution center. After the child had a flare-up in July requiring several days of treatment at the hospital, she said she returned home to find her power shut off. Sitting in a dark apartment, unable to pay her utility bills, she set up a GoFundMe campaign. She received less than $20 through the site; her relatives eventually pooled $350 to get her electricity restored.
Even parents with stable jobs and private health insurance often take on debt and need outside help.
When Taylr and Treg Murphy’s 17-year-old son Peyton was diagnosed with cancer and needed monthslong treatment at St. Jude in 2017, the entire family – mom, dad, sister, and brother – went with him, traveling from their home in Lafayette, La., to Memphis. Treg took a leave from his job at an oil mining company and Taylr, who works at her mother’s bakery, did the same.
“We knew that it was going to be a collective team effort,” Treg said. “Without even a discussion, we figured that if Peyton’s got to go for the surgery, we’re all going.”
Peyton had an enormous tumor that had grown out of his right femur and was crowding his knee. Rounds of chemotherapy appeared to have killed osteosarcoma cells elsewhere in his body. But he needed to undergo a procedure called limb-sparing surgery that would require weeks of recovery time at the hospital.
The hospital agreed to allow all five family members to stay for free at St. Jude if they bunked together in a single room. It assigned them a spot in Tri Delta Place, its hotel-like short-term patient residence on the campus. Tri Delta is set up for visits of up to seven days, according to the hospital’s guide for volunteers, but the Murphys were there for almost 50.
Taylr said the unit at Tri Delta had no oven or stove and St. Jude provided no grocery money, instead allotting them a $50-per-day credit at the hospital cafeteria, Kay Kafe – not enough to feed the family of five. As the weeks wore on, the Murphys split grilled cheese sandwiches and paid for food out of pocket.
After ProPublica asked about the hospital’s food allowances, St. Jude said it would increase them as part of the changes scheduled to go into effect this month. The hospital switched from a $50-a-day cap per family to providing $25 a day to each family member. For a family of four, that would double the food benefit. A weekly stipend given to families in long-term housing was increased to $150 from $125.
For the Murphys, it was the loss of their work income, more than out-of-pocket expenses, that put them into a financial hole as Peyton’s treatment went on. Treg’s employer couldn’t pay him during his long absences.
Fearful of being evicted or having their car repossessed, Taylr said she asked a St. Jude social worker for assistance. The social worker helped her apply for grants from other charities. Taylr said the B+ Foundation paid their rent one month, which ensured they’d have a home to return to.
In the years since his initial treatment, Peyton has gone back to St. Jude repeatedly for exams and surgeries to remove malignant growths in his lungs. Taylr and Treg have missed more work to bring Peyton to Memphis, costing them thousands of dollars more in income.
By the start of this year, Taylr and Treg said they were about $20,000 in debt and panicking. Dustin Poirier, a former UFC champion from their hometown, heard from a friend about Peyton and the family’s financial trouble. He donated $10,000 to them from his personal charity and in May hosted a local fundraiser that collected enough to pay off their credit cards.
St. Jude families sometimes commiserate about money problems with each other, Taylr said, but few are aware of the extent of the hospital’s unspent resources. The Murphys said they didn’t know St. Jude has more than $5 billion in reserve or that it continues to raise hundreds of millions of dollars in surplus donations each year.
“That’s just insane,” Taylr said. “That just blows my mind. When we first started getting treated, people would be like, ‘Oh, St. Jude covers everything, that’s awesome.’ That’s not how it works. People don’t understand that. I truly didn’t understand before I got into St. Jude.”
Taylr and Treg said the doctors at St. Jude are “amazing” and they’re grateful for their son’s care. But they bristled at the assumption that it was covered by the hospital’s charity. The family’s insurance paid a substantial part of the bills.
“It’s not free,” Taylr said. “My husband works very hard for the insurance we have – and they are billed.” The Murphys pay $12,000 in health insurance premiums each year.
Their struggle continues. Peyton’s cancer has relapsed, and he’s making regular trips with his mom or dad back to St. Jude for chemotherapy. The family is again applying for help from other charities.
Wiped out savings
The costs associated with care at St. Jude caused at least one family to stop going to Memphis altogether.
Last winter, Kelly Edwards was excitedly searching through Tulsa real estate listings after years of diligently saving $10,000 for a down payment on a house. She craved a permanent home for herself and the two young brothers she had taken in five years earlier at the behest of a family friend. She hoped to adopt the boys, now 13 and 9, who call her mom.
In February, the older boy, DJ, was lethargic and uninterested in his schoolwork. After several doctor visits, he was diagnosed with acute lymphoblastic leukemia at a Tulsa hospital. The cancer, referred to as ALL, is the most common type among children, with survival rates that exceed 90%. A day after his diagnosis, DJ and Edwards were driving six hours to Memphis for treatment at St. Jude, which is affiliated with the Oklahoma hospital.
The pair stayed for free at an independently operated Ronald McDonald House near St. Jude, and a weekly stipend from the hospital helped to pay for meals – aid that Edwards said was a blessing. DJ had health insurance through the Oklahoma Medicaid program.
But as with the Murphys, lost income soon put Edwards’ family into financial jeopardy. She works as a supervisor for a company that delivers packages for Amazon. After she used up two weeks of paid time off, she stopped getting paychecks. The bills, however, kept coming: rent, car payments, utilities. To that was added the $250 a week she paid a friend to stay with DJ’s younger brother and her two dogs in Tulsa.
Within four months, her house savings were wiped out. Edwards said she told her St. Jude social worker about her financial woes but got no additional help.
One of Edwards’ adult daughters started a GoFundMe campaign to help, bringing in just over $3,000. Edwards said she appreciated the aid but believes donations were kept low by the widespread perception that St. Jude families don’t have financial problems.
“Everyone hears that everything is taken care of by St. Jude,” she said. “That is not true, but everyone has that mentality.” She said someone she knew asked her “what is that money going for if St. Jude’s is paying for everything?”
DJ was scheduled to go back to St. Jude for three weeks of treatment in August, but Edwards decided she simply couldn’t afford it. “I don’t have the money to go back and forth,” she said. She worked with DJ’s local doctors and found that the hospital near her home in Tulsa could provide the same treatment he was scheduled to get in Tennessee.
The local treatment allowed her to continue working some shifts and to be at home with both of her boys. DJ is also happier when he is home, Edwards said.
Edwards and the boys are now living in a small house her brother owns just outside Tulsa. Late on a recent weekday afternoon, DJ slowly shuffled into the living room, exhausted from a day of chemotherapy treatment.
He is in the midst of a 20-week regimen where he receives the cancer-killing drugs every other day, just one phase of a nearly three-year treatment plan. He wore an orange knit hat, T-shirt, and shorts. He rubbed his eyes before asking a visitor, “How is your day going?” He smiled at the positive response. When he heard the family was eating steak for dinner, he eagerly jumped up to start helping in the kitchen. After they moved in, Edwards hung family portraits on the walls to make it feel homier. She doesn’t expect they will be moving again any time soon.
The dream of buying a home of their own is gone.
Former ProPublica reporter Marshall Allen contributed reporting. Kirsten Berg contributed research.
This story was originally published on ProPublica. ProPublica is a nonprofit newsroom that investigates abuses of power. Sign up to receive their biggest stories as soon as they’re published.
A series of sharp knocks on his driver’s side window startled Jason Burt awake.
It was the middle of the night on a Saturday in 2016. Burt was sleeping in his pickup truck in the parking lot of St. Jude Children’s Research Hospital in downtown Memphis, Tenn., where his 5-year-old daughter was being treated for brain cancer. He’d driven more than 500 miles from his home in Central Texas to visit her.
A St. Jude security guard peered into the truck and asked Burt what he was doing. Burt explained that his daughter and her mother, his ex-girlfriend, were staying in the hospital’s free patient housing. But St. Jude provides housing for only one parent. Burt, a school bus driver making $20,000 a year, told the guard he couldn’t afford a hotel. The guard let the exhausted father go back to sleep.
St. Jude would do no more to find him a place to stay.
“They were aware of the situation,” Burt said. “I didn’t push anything. I was just grateful she was getting treated and I was doing what I needed to do.”
St. Jude is the largest and most highly regarded health care charity in the country. Each year, the Memphis hospital’s fundraisers send out hundreds of millions of letters, many with heart-wrenching photographs of children left bald from battling cancer. Celebrities like Jennifer Aniston and Sofia Vergara sing the hospital’s praises in televised advertisements. This year, St. Jude’s fundraising reached outer space. The SpaceX Inspiration4 mission in September included a former St. Jude patient as a crew member.
Last year, St. Jude raised a record $2 billion. U.S. News & World Report ranked it the country’s 10th-best children’s cancer hospital, and St. Jude raised roughly as much as the nine hospitals ahead of it put together. It currently has $5.2 billion in reserves, a sum large enough to run the institution at current levels for the next four and a half years without a single additional donation.
St. Jude makes a unique promise as part of its fundraising: “Families never receive a bill from St. Jude for treatment, travel, housing or food – because all a family should worry about is helping their child live.”
But for many families, treatment at St. Jude does not relieve all the financial burdens they incur in getting care for their children, including housing, travel, and food costs that fall outside the hospital’s strict limits, a ProPublica investigation has found.
While families may not receive a bill from St. Jude, the hospital doesn’t cover what’s usually the biggest source of financial stress associated with childhood cancer: The loss of income as parents quit or take leave from jobs to be with their child during treatment. For many families, the consequence is missed payments for cars, utilities, and cellphones. Others face eviction or foreclosure because they can’t keep up with rent and mortgage payments.
Parents at St. Jude have exhausted savings and retirement accounts, borrowed from family and friends, or asked other charities for aid. ProPublica identified more than 100 St. Jude families seeking financial help through the online fundraiser GoFundMe, with half of the campaigns started in the past two years. We counted scores of other events like concerts and yard sales organized to help St. Jude families in need.
One family relied on a mixed martial arts fighter to help raise money for expenses like car repairs and cellphone bills, items that St. Jude would not cover. Another spent $10,000, originally saved to purchase a home, on costs related to treatment at St. Jude.
Only about half of the $7.3 billion St. Jude has received in contributions in the past five fiscal years went to the hospital’s research and caring for patients, according to its financial filings with the Internal Revenue Service. About 30% covered the cost of its fundraising operations, and the remaining 20%, or $1 of every $5 donated, increased its reserve fund.
Further, ProPublica found, a substantial portion of the cost for treatment is paid not by St. Jude but by families’ private insurance or by Medicaid, the government insurance program for low-income families. About 90% of patients are insured, bringing in more than $100 million in reimbursements for treatment a year. If a family shows up at St. Jude without insurance, a company hired by the charity helps them find it. St. Jude does cover copays and deductibles, an unusual benefit.
St. Jude spends about $500 million a year on patient services – a figure that includes all medical care and other assistance. Very little of what St. Jude raises from the public goes to pay for food, travel, and housing for families, the investigation found. Last year, it was 2% of the money raised, or nearly $40 million.
In written responses to ProPublica, lawyers for St. Jude and its fundraising arm, the American Lebanese Syrian Associated Charities, or ALSAC, emphasized that countless families have benefited from the charity provided since the hospital opened its doors in 1962.
“ProPublica should be celebrating St. Jude and ALSAC for their commitment to finding cures, saving children’s lives, and optimizing patient outcomes,” one of their letters said.
It is unquestioned that St. Jude has helped thousands of children and their families over the decades. Patients have offered scores of testimonials about the hospital’s generosity and care.
“This often comes as a huge relief to families who often expect to sell all their belongings just so their children can get the medical care and treatment they need to save their lives,” the hospital’s lawyers wrote. “St. Jude and ALSAC understand that this arrangement cannot cover all financial obligations of all families, nor can St. Jude or ALSAC shield families from all the financial and emotional effects” of a child’s illness.
St. Jude said it discloses the limits of its aid to families on its website and in material provided to those whose children are admitted to the hospital. That includes the rule Burt ran into, that the hospital covers the travel and housing costs of only one caregiver and one patient. For many families, the daily food budget is capped at $50. In some cases, hotel stays en route are provided only if families travel more than 500 miles to get to St. Jude.
St. Jude said its assistance is “based on guidelines to ensure fairness and responsible use of donor funds” and on remaining compliant with a federal anti-kickback statute that makes it a criminal offense to offer something of value to induce a medical referral. St. Jude declined to explain how the law affects the amount or type of financial assistance it provides to families.
“St. Jude has never promised anyone – neither patients nor the public in general – that it can solve all financial problems,” the letter said.
When parents need additional financial help, St. Jude’s social workers often send them to smaller charities or in some cases suggest that they apply for government aid.
They refer many to the Andrew McDonough B+ Foundation, which gives more than $2.5 million a year in grants to thousands of families of pediatric cancer patients at hospitals across the country to help cover rent, utilities, and other urgent expenses.
Joe McDonough, the foundation’s founder and president, said St. Jude families have the same money problems as families of patients at other children’s hospitals, even though he said St. Jude’s marketing creates the public perception that it alleviates these burdens.
“People say to me, ‘Why are you helping St. Jude families?’ ” McDonough said. “Well, what happens when a family lives in Augusta, Georgia, and they’re being treated at St. Jude? They still have to pay the rent on their apartment back in Augusta, Georgia. They still have to make their car payment. And it’s not my position to say whether St. Jude should be paying for all those expenses or not. I’m just explaining that it’s not a totally free ride.”
The help St. Jude provides to families may soon be increasing.
After ProPublica provided St. Jude with the findings of its reporting, the hospital informed families of a dramatic expansion in the assistance it will give to parents and other relatives during their kids’ treatment in Memphis.
Among the most significant changes are increasing travel benefits to two parents instead of one and covering regular trips to Memphis for siblings and other loved ones. St. Jude’s letter to parents said the changes take effect Nov. 15.
That would’ve made a big difference for Burt.
Burt’s daughter, whom ProPublica is not identifying at her mother’s request, was originally diagnosed with cancer in early 2015, when doctors discovered a tumor pressing against her brain stem. She had successful emergency surgery to remove the mass at Dell Children’s Medical Center in Austin, Texas. Medicaid and Dell Children’s covered the bill, but the family was still faced with the cost of her ongoing treatment.
“At that point I’m thinking: ‘What am I going to do? I guess I’m selling my house, whatever it takes,’” Burt recalled. “Honestly, that was probably a big deciding factor for St. Jude.”
St. Jude accepted Burt’s daughter into a clinical trial, and the family moved to the hospital’s patient housing in Memphis for several months. Both parents stopped working for a time, and people in their hometown raised cash to pay their bills.
Her cancer relapsed the following year with several new, inoperable brain tumors. Burt and his daughter’s mom broke up during that round of treatment, and financial problems piled up.
Burt said his credit score dropped so low that utility companies refused to set up service unless he first paid a deposit. One of the family’s cars was repossessed, he said. Burt’s 2005 Chevrolet Colorado pickup has 300,000 miles on it, many of them logged on trips from Texas to Memphis. When Burt’s daughter was at St. Jude for treatment or exams, he’d work all week, then visit on many weekends where he would spend Saturday night sleeping in the hospital parking lot.
He asked hospital officials if he could sleep in St. Jude’s housing, but they turned him down, he said.
Burt said he was happy with the care St. Jude provided. His daughter’s health is stable, he said, and brain scans taken during her September exam confirmed her two remaining tumors haven’t grown. But he’s still trying to recover financially.
“It’s five years now,” Burt said, “and I’m not completely caught up yet.”
A fundraising giant
St. Jude began with a fledgling entertainer praying for a career break.
When Danny Thomas, a comic and actor best known for the TV sitcom “Make Room for Daddy,” was struggling to earn a living in the late 1930s, the devout Roman Catholic went to church and asked for help from the patron saint of desperate cases, St. Jude Thaddeus. If he made it big, Thomas promised to build “a shrine where the poor and the helpless and the hopeless may come for comfort and aid,” according to a history published by ALSAC.
Within five years, Thomas became a star and worked to fulfill his promise by building a children’s hospital named after St. Jude and a fundraising organization to support it. Thomas, whose parents were Lebanese immigrants, recruited others who shared his Middle Eastern roots to help.
He used his fame to raise the hospital’s profile, appearing in ads for St. Jude and hosting fundraising events starring the likes of Elvis Presley and Sammy Davis Jr. Thomas’ daughter Marlo, herself a TV star, succeeded him in championing St. Jude.
Today, St. Jude is a specialty treatment and research center with about 5,700 employees and 73 beds. Other top children’s hospitals have more staff and beds, and they also treat more conditions.
Though St. Jude raises money across the world, most of its patients come from Tennessee and surrounding states. Patients from elsewhere are usually enrolled in clinical trials.
ALSAC, which handles St. Jude’s fundraising and investments, has 2,188 employees in Memphis and in 36 regional offices across the country. More than 400 of the fundraising arm’s employees are paid over $100,000, according to IRS filings. The charity takes in so much money each year that it regularly steers hundreds of millions of dollars in donations to reserve accounts, the filings show.
Overall, St. Jude’s reserve has grown by 58% over the past five fiscal years, during which it has added $1.9 billion to its investment accounts and shifted its portfolio toward financial products designed to generate bigger returns than stocks, bonds, and mutual funds traditionally deliver. The charity stowed more than a third of the new surplus, $688 million, in riskier private equity investments.
IRS rules do not limit the size of a nonprofit’s reserves, and experts on charitable finance differ on best practices.
St. Jude meets Better Business Bureau guidelines, which call for charities to maintain reserves of less than three times total expenses, but other experts expressed alarm that the hospital had accumulated such a large sum of money.
The size of the St. Jude reserve is “staggering,” said Laura Otten, the director of LaSalle University’s master program in nonprofit leadership. She said a typical reserve for a nonprofit the size of St. Jude is one to two years of expenses. Donors generally want to know their dollars are being put to work, she said.
The hospital said it needs a large reserve because its unique operating model relies on donations to fund annual operating costs. “[W]e are highly donor-dependent and subject to the economic driven vagaries of charitable giving,” the hospital said in a written response to ProPublica questions.
But the hospital’s reserve is already more than large enough to buffer against recessions and potential drops in donations, said Ge Bai, a professor of accounting and health policy at Johns Hopkins University. “They should be spending the money as aggressively as they raise it, but they seem to be hoarding,” Bai said.
The hospital said it is also raising billions to fund the construction of new housing and research space, although its plans do not currently include spending any of the reserve on new facilities.
St. Jude’s reserves have ballooned at a time when researchers, oncologists, advocates, and families complain about a dearth of funding for pediatric cancer studies nationally.
Dozens of other children’s hospitals across the country have research divisions devoted to pediatric cancer and enroll their patients in clinical trials for new drugs and procedures. They pay for research staff and studies in part with donations from their local communities, often competing directly against St. Jude. ALSAC has regional offices in several U.S. cities with elite pediatric cancer centers of their own, including Atlanta, Chicago, Denver, and Seattle.
Coury Shadyac, an ALSAC vice president and daughter of the organization’s CEO, Richard Shadyac Jr., oversees a team of 45 fundraisers along the West Coast “raising $300 million annually” for St. Jude, according to her LinkedIn profile. That’s $100 million more in donations than either Children’s Hospital Los Angeles or Seattle Children’s Hospital, two of the nation’s leading pediatric cancer institutions, received in fiscal year 2019, IRS disclosures show. But it’s only a small part of St. Jude’s fundraising haul.
ALSAC’s ubiquitous fundraising has led to concerns that it undercuts other hospitals’ campaigns. Some doctors interviewed by ProPublica said they have encouraged donors to give their money to hospitals closer to home.
David Clark, a pediatrician and former longtime chairperson of pediatrics at Albany Medical Center in New York, said St. Jude raises tens of thousands of dollars in his region that does little to benefit the children with cancer in his area since almost all are treated locally. ALSAC has a fundraising office located a few miles from Albany Medical.
“They think of every way they can to make money and the least amount of ways to spend it,” Clark said. “They deceive people into supporting something that is totally dishonest.”
Nearly all St. Jude solicitations feature the hospital’s patients – the children usually smiling and bald from treatment – along with the familiar promise that it never sends families a bill.
It’s a message that ALSAC has tested and researched to maximize donations. Donors appreciate the promise to never bill families, said Mary Kate Tolan, an ALSAC executive, in a podcast last year. She added that no parent should have to take out a second mortgage or lose their job because their child is being treated at St. Jude.
Alternative messaging to the no-bills promise did not “perform as well,” said Tolan, who develops emerging technologies for ALSAC. Tolan did not return requests for comment.
“Borrowing and begging”
Catherine Rainey thought she would be free of financial worry when her 2-year-old daughter Harlee was admitted to St. Jude last year.
“The first thing my dad said was: ‘Catherine, you have nothing to worry about. They raise billions of dollars. Anytime you have a problem, you tell them and they will take care of it,’ ” she said.
But like many families, the Raineys discovered that St. Jude’s charity came with limits on payments for expenses such as travel that could be bewildering.
Harlee ended up at St. Jude after first going to nearby Niswonger Children’s Hospital in Johnson City, Tenn., in October 2020. The doctors there discovered a cancerous mass attached to her right kidney. The hospital is a St. Jude affiliate, and the doctors recommended the toddler be treated in Memphis.
Rainey, a single mother of two young girls, had to leave her job as a nurse for months to be with Harlee at St. Jude. The loss of income quickly created problems. “My family, we don’t come from money,” she said. “We are not doctors and billionaires. We make it. That is it.”
St. Jude did provide food and housing on campus. But the hospital said it couldn’t help with the items that were causing Rainey to worry, including car payments, insurance, and cellphone bills.
Rainey’s boss set up a GoFundMe account to help make up some of her lost income. A small local charity, Kari’s Heart Foundation, also helped out by paying about $3,000 worth of phone bills and car payments, staving off repossession.
“It was just a bunch of borrowing and begging,” Rainey said of her experience while her daughter was treated in Memphis. “They acted like it was coming out of their own pocket.”
Harlee has checkups at St. Jude every three months that last about four days. The costs of travel to and from St. Jude put an additional strain on Rainey and Harlee. St. Jude is an eight-hour ride, without stops, from Rainey’s home in Appalachia, Va., a town of 1,432 people near the Kentucky border.
Rainey said her daughter generally can make it about two-thirds of the way, with frequent stops, before she has had enough. “When she is done, she is really done,” Rainey said. “She will scream, cry, and kick.”
In July, in advance of an August trip to Memphis, Rainey called the patient services department at St. Jude to see whether they could help pay for a hotel to break up the travel day — an expense Rainey said she could not afford.
To qualify for a hotel reimbursement, Rainey said, St. Jude told her she had to live more than 500 miles from Memphis. The ride from her home to the hospital is 530 miles (a measurement ProPublica confirmed with mapping tools). However, Rainey said, St. Jude told her it measured the trip from city limit to city limit and came up with a distance of 491 miles. Even using that metric, the distance is still more than 500 miles, ProPublica found.
When she challenged the hospital’s stance, Rainey said she was berated by a patient services representative.
“I was feeling pissed off, and I was crying,” Rainey said of the interaction. “You give up your whole life for your child, and they tell you don’t worry about anything, we will cover this and then they tell you to just push through the drive.”
Rainey did what she could to make the trip go smoothly: She configured a small table to extend across her daughter’s car seat, so Harlee could play with the coloring books, markers, and Play-Doh bought for the ride. She packed snacks and a cooler full of drinks. Since Harlee was still potty training, she brought extra towels and clothes for accidents. The final step was handing Harlee her Baby Yoda doll once she settled into her car seat. Rainey had sewed a port in the doll’s chest to mirror the one Harlee has in hers.
About three hours from Memphis, Harlee was crying inconsolably. Rainey pulled off the interstate and stopped at the first hotel she could find. She later learned it had been described in online reviews as “awful,” a “nightmare,” “disgusting,” and “horrible.”
“I didn’t know the area,” she said. “The hotel was garbage. It just made it worse.”
The drive home also required a hotel stop, but this time Rainey was able to find one that was cleaner. A $100 donation from a local charity helped to offset the cost.
Among the changes St. Jude is making is to reimburse families like Rainey’s, who live more than 400 miles from the hospital, for an overnight stay at a hotel when making the trip to Memphis.
Rainey said she was called by a St. Jude representative after ProPublica asked about her situation and was told the hospital would pay for her past hotel stays when traveling back and forth to St. Jude. The representative, Rainey said, also told her the hospital discovered the way it had been measuring mileage was inaccurate.
“I am not the only one,” Rainey said. “There are others. They should reimburse all the families.”
The anxiety of unpaid bills piling up, combined with caring for a child undergoing chemotherapy or radiation, takes a severe toll on parents and guardians, said Christopher Hope, a UPS driver who started a Memphis-based foundation after meeting St. Jude parents who were in financial crisis.
Hope’s small charity spent $12,000 last year to help families. Parents in St. Jude social media groups often refer families in need to it. The charity has helped families cover mortgage and car payments.
“I never knew anything about this until hearing about it from families,” Hope said. “All we hear is about kids and treatment, not the other side of it.”
“It’s not free”
In addition to charities like Hope’s, St. Jude families have repeatedly turned to fundraising sites and networks of their relatives, friends, and neighbors to help cover basic expenses while unable to work during their children’s treatment. Parents’ requests on fundraising sites are sometimes desperate pleas.
In January 2017, one father in North Carolina said he’d had to abandon a business venture to take time for his son to receive care at St. Jude. His income had plummeted. He asked friends to give as little as $10 to “at least make it possible to survive.”
This year, a mother in Memphis whose 1-year-old son receives care at St. Jude for sickle cell disorder ran out of medical leave and couldn’t work her shifts at a clothing distribution center. After the child had a flare-up in July requiring several days of treatment at the hospital, she said she returned home to find her power shut off. Sitting in a dark apartment, unable to pay her utility bills, she set up a GoFundMe campaign. She received less than $20 through the site; her relatives eventually pooled $350 to get her electricity restored.
Even parents with stable jobs and private health insurance often take on debt and need outside help.
When Taylr and Treg Murphy’s 17-year-old son Peyton was diagnosed with cancer and needed monthslong treatment at St. Jude in 2017, the entire family – mom, dad, sister, and brother – went with him, traveling from their home in Lafayette, La., to Memphis. Treg took a leave from his job at an oil mining company and Taylr, who works at her mother’s bakery, did the same.
“We knew that it was going to be a collective team effort,” Treg said. “Without even a discussion, we figured that if Peyton’s got to go for the surgery, we’re all going.”
Peyton had an enormous tumor that had grown out of his right femur and was crowding his knee. Rounds of chemotherapy appeared to have killed osteosarcoma cells elsewhere in his body. But he needed to undergo a procedure called limb-sparing surgery that would require weeks of recovery time at the hospital.
The hospital agreed to allow all five family members to stay for free at St. Jude if they bunked together in a single room. It assigned them a spot in Tri Delta Place, its hotel-like short-term patient residence on the campus. Tri Delta is set up for visits of up to seven days, according to the hospital’s guide for volunteers, but the Murphys were there for almost 50.
Taylr said the unit at Tri Delta had no oven or stove and St. Jude provided no grocery money, instead allotting them a $50-per-day credit at the hospital cafeteria, Kay Kafe – not enough to feed the family of five. As the weeks wore on, the Murphys split grilled cheese sandwiches and paid for food out of pocket.
After ProPublica asked about the hospital’s food allowances, St. Jude said it would increase them as part of the changes scheduled to go into effect this month. The hospital switched from a $50-a-day cap per family to providing $25 a day to each family member. For a family of four, that would double the food benefit. A weekly stipend given to families in long-term housing was increased to $150 from $125.
For the Murphys, it was the loss of their work income, more than out-of-pocket expenses, that put them into a financial hole as Peyton’s treatment went on. Treg’s employer couldn’t pay him during his long absences.
Fearful of being evicted or having their car repossessed, Taylr said she asked a St. Jude social worker for assistance. The social worker helped her apply for grants from other charities. Taylr said the B+ Foundation paid their rent one month, which ensured they’d have a home to return to.
In the years since his initial treatment, Peyton has gone back to St. Jude repeatedly for exams and surgeries to remove malignant growths in his lungs. Taylr and Treg have missed more work to bring Peyton to Memphis, costing them thousands of dollars more in income.
By the start of this year, Taylr and Treg said they were about $20,000 in debt and panicking. Dustin Poirier, a former UFC champion from their hometown, heard from a friend about Peyton and the family’s financial trouble. He donated $10,000 to them from his personal charity and in May hosted a local fundraiser that collected enough to pay off their credit cards.
St. Jude families sometimes commiserate about money problems with each other, Taylr said, but few are aware of the extent of the hospital’s unspent resources. The Murphys said they didn’t know St. Jude has more than $5 billion in reserve or that it continues to raise hundreds of millions of dollars in surplus donations each year.
“That’s just insane,” Taylr said. “That just blows my mind. When we first started getting treated, people would be like, ‘Oh, St. Jude covers everything, that’s awesome.’ That’s not how it works. People don’t understand that. I truly didn’t understand before I got into St. Jude.”
Taylr and Treg said the doctors at St. Jude are “amazing” and they’re grateful for their son’s care. But they bristled at the assumption that it was covered by the hospital’s charity. The family’s insurance paid a substantial part of the bills.
“It’s not free,” Taylr said. “My husband works very hard for the insurance we have – and they are billed.” The Murphys pay $12,000 in health insurance premiums each year.
Their struggle continues. Peyton’s cancer has relapsed, and he’s making regular trips with his mom or dad back to St. Jude for chemotherapy. The family is again applying for help from other charities.
Wiped out savings
The costs associated with care at St. Jude caused at least one family to stop going to Memphis altogether.
Last winter, Kelly Edwards was excitedly searching through Tulsa real estate listings after years of diligently saving $10,000 for a down payment on a house. She craved a permanent home for herself and the two young brothers she had taken in five years earlier at the behest of a family friend. She hoped to adopt the boys, now 13 and 9, who call her mom.
In February, the older boy, DJ, was lethargic and uninterested in his schoolwork. After several doctor visits, he was diagnosed with acute lymphoblastic leukemia at a Tulsa hospital. The cancer, referred to as ALL, is the most common type among children, with survival rates that exceed 90%. A day after his diagnosis, DJ and Edwards were driving six hours to Memphis for treatment at St. Jude, which is affiliated with the Oklahoma hospital.
The pair stayed for free at an independently operated Ronald McDonald House near St. Jude, and a weekly stipend from the hospital helped to pay for meals – aid that Edwards said was a blessing. DJ had health insurance through the Oklahoma Medicaid program.
But as with the Murphys, lost income soon put Edwards’ family into financial jeopardy. She works as a supervisor for a company that delivers packages for Amazon. After she used up two weeks of paid time off, she stopped getting paychecks. The bills, however, kept coming: rent, car payments, utilities. To that was added the $250 a week she paid a friend to stay with DJ’s younger brother and her two dogs in Tulsa.
Within four months, her house savings were wiped out. Edwards said she told her St. Jude social worker about her financial woes but got no additional help.
One of Edwards’ adult daughters started a GoFundMe campaign to help, bringing in just over $3,000. Edwards said she appreciated the aid but believes donations were kept low by the widespread perception that St. Jude families don’t have financial problems.
“Everyone hears that everything is taken care of by St. Jude,” she said. “That is not true, but everyone has that mentality.” She said someone she knew asked her “what is that money going for if St. Jude’s is paying for everything?”
DJ was scheduled to go back to St. Jude for three weeks of treatment in August, but Edwards decided she simply couldn’t afford it. “I don’t have the money to go back and forth,” she said. She worked with DJ’s local doctors and found that the hospital near her home in Tulsa could provide the same treatment he was scheduled to get in Tennessee.
The local treatment allowed her to continue working some shifts and to be at home with both of her boys. DJ is also happier when he is home, Edwards said.
Edwards and the boys are now living in a small house her brother owns just outside Tulsa. Late on a recent weekday afternoon, DJ slowly shuffled into the living room, exhausted from a day of chemotherapy treatment.
He is in the midst of a 20-week regimen where he receives the cancer-killing drugs every other day, just one phase of a nearly three-year treatment plan. He wore an orange knit hat, T-shirt, and shorts. He rubbed his eyes before asking a visitor, “How is your day going?” He smiled at the positive response. When he heard the family was eating steak for dinner, he eagerly jumped up to start helping in the kitchen. After they moved in, Edwards hung family portraits on the walls to make it feel homier. She doesn’t expect they will be moving again any time soon.
The dream of buying a home of their own is gone.
Former ProPublica reporter Marshall Allen contributed reporting. Kirsten Berg contributed research.
This story was originally published on ProPublica. ProPublica is a nonprofit newsroom that investigates abuses of power. Sign up to receive their biggest stories as soon as they’re published.
Text-based COVID monitoring system could reduce deaths, relieve ED in winter surge
COVID Watch, a text message–based remote monitoring program developed by the University of Pennsylvania Health System, was associated with a 68% reduction in the risk of death, compared with those who received usual care. This was the main finding of a paper published in the Annals of Internal Medicine.
The investigators also determined that patients who enrolled in the program were more likely to seek care in the ED and when they did, they came in on average 2 days sooner than those who received usual care.
“When our clinical team designed COVID Watch the goal was to facilitate hospital care for patients who require it, while supporting access to care for patients who can safely remain at home,” study author M. Kit Delgado, MD, MS, an assistant professor of emergency medicine and epidemiology at Penn Presbyterian Medical Center in Philadelphia, said in an interview.
Researchers had initially hoped COVID Watch would relieve pressure on EDs, Dr. Delgado said.
Significantly lower mortality seen among COVID Watch group
For the study, Dr. Delgado and colleagues enrolled 3,488 patients in COVID Watch and 4,377 in the usual care group to compare outcomes at 30 and 60 days.
“We didn’t include patients who were diagnosed with COVID in the ER or hospital, so this is a lower-risk cohort of patients who test positive in outpatient settings,” Dr. Delgado noted. “Outpatients who received usual care and COVID Watch both had relatively low mortality, but it was significantly lower in those who were in COVID Watch.”
The researchers found that 3 patients in the COVID Watch group died within 30 days of their enrollment, compared with 12 in the control group. At 60 days after enrollment, 5 people within COVID Watch died, compared with 16 not using the system. More than one-third of the deaths in the usual care group occurred outside the hospital, compared with zero deaths among those in COVID Watch.
More than half of program participants were Black or Latino
The messaging system also reduced mortality rates among “all major racial and ethnic subgroups,” the researchers said, with more than 50% of the patients enrolled in COVID Watch having been Black or Latino.
“This is important because Black and Hispanic communities have experienced higher exposure and infection rates, decreased access to care, and have had higher mortality rates,” Dr. Delgado said. “Therefore, the results imply that this type of program could play a role in decreasing disparities in COVID outcomes if scaled more broadly.”
Outside expert: COVID Watch bring new approach to digital health monitoring
The study not only highlights the efficacy and sustainment of the COVID Watch program, but it sheds light on the possibility of using text message monitoring systems on other chronic disease conditions, said Jamie Faro, PhD, who was not involved in the study.
“It brings a new approach to health monitoring using digital means, which may lessen the burden on health care providers and be more cost effective than usual care approaches,” said Dr. Faro, who is assistant professor at the department of population and quantitative health sciences at the University of Massachusetts, Worcester. “Text messaging, which is used by over 80% of Americans, can allow us to reach a large percentage of the population for remote health care monitoring.”
Researchers of the current study said the findings “reveal a model for outpatient health system management of patients with COVID-19 and possibly other conditions where the early detection of clinical declines is critical.” Dr. Faro said that COVID Watch can have a measurable impact on an outcome that is truly life or death. However, it would be critical to understand how to reach those who either “were not offered or refused to take part in the program.”
The authors of the paper and Dr. Faro had no disclosures.
COVID Watch, a text message–based remote monitoring program developed by the University of Pennsylvania Health System, was associated with a 68% reduction in the risk of death, compared with those who received usual care. This was the main finding of a paper published in the Annals of Internal Medicine.
The investigators also determined that patients who enrolled in the program were more likely to seek care in the ED and when they did, they came in on average 2 days sooner than those who received usual care.
“When our clinical team designed COVID Watch the goal was to facilitate hospital care for patients who require it, while supporting access to care for patients who can safely remain at home,” study author M. Kit Delgado, MD, MS, an assistant professor of emergency medicine and epidemiology at Penn Presbyterian Medical Center in Philadelphia, said in an interview.
Researchers had initially hoped COVID Watch would relieve pressure on EDs, Dr. Delgado said.
Significantly lower mortality seen among COVID Watch group
For the study, Dr. Delgado and colleagues enrolled 3,488 patients in COVID Watch and 4,377 in the usual care group to compare outcomes at 30 and 60 days.
“We didn’t include patients who were diagnosed with COVID in the ER or hospital, so this is a lower-risk cohort of patients who test positive in outpatient settings,” Dr. Delgado noted. “Outpatients who received usual care and COVID Watch both had relatively low mortality, but it was significantly lower in those who were in COVID Watch.”
The researchers found that 3 patients in the COVID Watch group died within 30 days of their enrollment, compared with 12 in the control group. At 60 days after enrollment, 5 people within COVID Watch died, compared with 16 not using the system. More than one-third of the deaths in the usual care group occurred outside the hospital, compared with zero deaths among those in COVID Watch.
More than half of program participants were Black or Latino
The messaging system also reduced mortality rates among “all major racial and ethnic subgroups,” the researchers said, with more than 50% of the patients enrolled in COVID Watch having been Black or Latino.
“This is important because Black and Hispanic communities have experienced higher exposure and infection rates, decreased access to care, and have had higher mortality rates,” Dr. Delgado said. “Therefore, the results imply that this type of program could play a role in decreasing disparities in COVID outcomes if scaled more broadly.”
Outside expert: COVID Watch bring new approach to digital health monitoring
The study not only highlights the efficacy and sustainment of the COVID Watch program, but it sheds light on the possibility of using text message monitoring systems on other chronic disease conditions, said Jamie Faro, PhD, who was not involved in the study.
“It brings a new approach to health monitoring using digital means, which may lessen the burden on health care providers and be more cost effective than usual care approaches,” said Dr. Faro, who is assistant professor at the department of population and quantitative health sciences at the University of Massachusetts, Worcester. “Text messaging, which is used by over 80% of Americans, can allow us to reach a large percentage of the population for remote health care monitoring.”
Researchers of the current study said the findings “reveal a model for outpatient health system management of patients with COVID-19 and possibly other conditions where the early detection of clinical declines is critical.” Dr. Faro said that COVID Watch can have a measurable impact on an outcome that is truly life or death. However, it would be critical to understand how to reach those who either “were not offered or refused to take part in the program.”
The authors of the paper and Dr. Faro had no disclosures.
COVID Watch, a text message–based remote monitoring program developed by the University of Pennsylvania Health System, was associated with a 68% reduction in the risk of death, compared with those who received usual care. This was the main finding of a paper published in the Annals of Internal Medicine.
The investigators also determined that patients who enrolled in the program were more likely to seek care in the ED and when they did, they came in on average 2 days sooner than those who received usual care.
“When our clinical team designed COVID Watch the goal was to facilitate hospital care for patients who require it, while supporting access to care for patients who can safely remain at home,” study author M. Kit Delgado, MD, MS, an assistant professor of emergency medicine and epidemiology at Penn Presbyterian Medical Center in Philadelphia, said in an interview.
Researchers had initially hoped COVID Watch would relieve pressure on EDs, Dr. Delgado said.
Significantly lower mortality seen among COVID Watch group
For the study, Dr. Delgado and colleagues enrolled 3,488 patients in COVID Watch and 4,377 in the usual care group to compare outcomes at 30 and 60 days.
“We didn’t include patients who were diagnosed with COVID in the ER or hospital, so this is a lower-risk cohort of patients who test positive in outpatient settings,” Dr. Delgado noted. “Outpatients who received usual care and COVID Watch both had relatively low mortality, but it was significantly lower in those who were in COVID Watch.”
The researchers found that 3 patients in the COVID Watch group died within 30 days of their enrollment, compared with 12 in the control group. At 60 days after enrollment, 5 people within COVID Watch died, compared with 16 not using the system. More than one-third of the deaths in the usual care group occurred outside the hospital, compared with zero deaths among those in COVID Watch.
More than half of program participants were Black or Latino
The messaging system also reduced mortality rates among “all major racial and ethnic subgroups,” the researchers said, with more than 50% of the patients enrolled in COVID Watch having been Black or Latino.
“This is important because Black and Hispanic communities have experienced higher exposure and infection rates, decreased access to care, and have had higher mortality rates,” Dr. Delgado said. “Therefore, the results imply that this type of program could play a role in decreasing disparities in COVID outcomes if scaled more broadly.”
Outside expert: COVID Watch bring new approach to digital health monitoring
The study not only highlights the efficacy and sustainment of the COVID Watch program, but it sheds light on the possibility of using text message monitoring systems on other chronic disease conditions, said Jamie Faro, PhD, who was not involved in the study.
“It brings a new approach to health monitoring using digital means, which may lessen the burden on health care providers and be more cost effective than usual care approaches,” said Dr. Faro, who is assistant professor at the department of population and quantitative health sciences at the University of Massachusetts, Worcester. “Text messaging, which is used by over 80% of Americans, can allow us to reach a large percentage of the population for remote health care monitoring.”
Researchers of the current study said the findings “reveal a model for outpatient health system management of patients with COVID-19 and possibly other conditions where the early detection of clinical declines is critical.” Dr. Faro said that COVID Watch can have a measurable impact on an outcome that is truly life or death. However, it would be critical to understand how to reach those who either “were not offered or refused to take part in the program.”
The authors of the paper and Dr. Faro had no disclosures.
FROM ANNALS OF INTERNAL MEDICINE
Texas SB8 and the future of abortion care
Texas Senate Bill 8 (SB8) is the most extreme antiabortion legislation currently in effect in the United States. SB8 was introduced by the Texas legislature on March 11, 2021, and signed into law by Governor Greg Abbott on May 19, 2021.1 The law went into effect on September 1, 2021, despite an appeal to the US Supreme Court to block the law until the courts could weigh in on its constitutionality. The bill prohibits all abortion care in the state of Texas after cardiac activity has been identified, typically at 6 weeks’ gestational age. The majority of pregnant people may be unaware at that point that they are pregnant, particularly if their menstrual cycles are irregular.2 An estimated 85% of abortions in Texas occur after the 6-week mark, leaving millions of Texans without the constitutionally protected rights assured to them in Roe v Wade.3,4 This has and will disproportionately impact communities of color and low-income people seeking abortion care.
SB8 does not contain exceptions in case of a pregnancy that results from rape, sexual assault, or incest, but it does contain an exemption for abortion care because of a medical emergency, as approved by a physician. The physician is required to note the medical emergency in the patient’s chart, stating that the “medical emergency necessitated the abortion” and “prevented compliance” with SB8.5 In practice, this exception is so vague as to leave clinicians concerned that routine management of medical conditions and complications, as in ectopic pregnancy, places them at risk of legal action against them and their colleagues should they authorize abortion care.
In Texas, abortion restrictions are nothing new. Texas patients are already subject to a 2-trip requirement: Since 2011 they have been required to have a mandatory ultrasound in one visit and schedule a second visit, 24 hours later, for the procedure.6 As of 2003, Texas law also mandates that providers discuss with patients the medical risks, adoption alternatives, and developmental stages of the pregnancy.6 There are no medical indications for either of these laws, and their impact is to delay patient care. Unfortunately, laws such as these have been increasingly common in the past decade, with 106 abortion restrictions enacted in 2021 alone.7,8
What is different about SB8?
SB8 is unique in that it deputizes private citizens to enforce the law. This represents a major change in the antichoice movement’s tactics, as previous bills have made violations a criminal offense. SB8 allows a citizen to sue anyone associated with abortion care, with a minimum penalty of $10,000. In practice, a citizen of another state, who has no connection to the patient receiving care, can sue under this Texas law.9 Anyone “aiding and abetting a violation” can be found liable for up to 4 years after the date of care, including, for example, a ride-hailing driver called to ferry the patient to the appointment, the health care team providing abortion care, or insurance companies covering the costs of care. In addition, anyone found guilty of “aiding and abetting” a violation of the bill is responsible for all costs and attorney fees associated with the civil case.5,10
Furthermore, SB8 outlines defenses that cannot be used to preempt a finding of civil liability, including “ignorance or mistake of the law,” “belief of the law’s unconstitutionality,” and “consent of the [patient] to the abortion.”5 This additional layer of restriction makes it difficult to appeal the bill and convolutes an individual’s ability to challenge the law. The law also forbids the state (Texas), a state official, a court, or a district attorney from intervening on behalf of the law—upending typical courses of appeal. This legislation also complicates both federal and state intervention regarding SB8’s constitutionality, as the state has no role in enforcing the law as it is written.5
Continue to: What has been the response?...
What has been the response?
As expected, abortion foes reacted positively to SB8, while abortion advocates expressed outrage that the law went into effect. Many were additionally confused that the Supreme Court chose not to intervene to stay the law while the courts adjudicate its constitutionality, as is typical in other cases concerning abortion restrictions.11
In a 5-4 ruling, the US Supreme Court allowed SB8 to take effect on September 1, issuing its decision on the “Shadow Docket.” As such, a decision was handed down on an expedited timeline in response to an emergency appeal without any oral arguments or a lengthy opinion explaining the ruling.11,12 The majority delivered a brief, one-paragraph order summarizing their decision, explaining that their refusal to grant the injunction was not a commentary on the law’s constitutionality. The High Court stated that they could not initially comment on the law’s constitutionality before it went into effect, citing that per the law, the state had no role in enforcement, and at the time, no private actions had yet been brought under the law. Justice Sonia Sotomayor dissented, stating, “The Court’s order is stunning. Presented with an application to enjoin a flagrantly unconstitutional law engineered to prohibit women from exercising their constitutional rights and evade judicial scrutiny, a majority of Justices have opted to bury their heads in the sand.”13
Following the Supreme Court’s refusal to act, US Attorney General Merrick Garland commented that “the Justice Department was evaluating all options to protect the constitutional rights of women and other persons.” Just one week later, the US Department of Justice filed a lawsuit against the State of Texas, arguing that SB8 was unconstitutional under the Supremacy Clause (federal law takes precedence over state law) and the Fourteenth Amendment.14,15
On October 6, in response to the Department of Justice’s challenge, District Judge Robert Pitman issued an injunction to prevent enforcement of SB8. In a 113-page ruling, Judge Pitman explained that “a person’s right under the Constitution to choose to obtain an abortion prior to fetal viability is well established.” Judge Pittman held SB8 unconstitutional, stating, “Women have been unlawfully prevented from exercising control over their lives in ways that are protected by the Constitution... Fully aware that depriving its citizens of this right by direct state action would be flagrantly unconstitutional, the State contrived an unprecedented and transparent statutory scheme to do just that.”16
Just 48 hours after the injunction issued by Judge Pitman, the Fifth Circuit Court of Appeals overturned the injunction, and SB8 went back into effect while litigation on its constitutionality proceeded.2,17 The Fifth Circuit Court of Appeals is widely considered to be one of the most conservative courts in the country.18
On October 15, 2021, the Department of Justice appealed the Fifth Circuit Court’s decision and asked the US Supreme Court to intervene, requesting that the Court issue an emergency halt to the law.19,20 On October 22, 2021, the Court declined to halt the law but scheduled oral arguments on the case for November 1, 2021. This is a stunningly fast briefing schedule for a case of such constitutional importance.
Given the legal back-and-forth, many clinicians are not providing abortion care in Texas as the litigation unfolds. SB8 permits retroactive enforcement, mandating that those “aiding and abetting” of abortion care may be civilly liable for up to 4 years after providing the care.5
Continue to: Potential outcomes, and what comes next...
Potential outcomes, and what comes next
Since the ascension of Justice Amy Coney Barrett to the High Court, there has been a nationwide increase in antiabortion legislation. Between January and July 2021, more than 90 abortion restrictions were passed, more restrictions in any single year since Roe v Wade was decided in 1973.8 In the past decade, more than 500 laws that restrict abortion have been passed across the United States, and studies indicate that 87% to 90% of American counties today are without a single abortion provider.21,22 Abortion supporters are particularly concerned about the future of Roe v Wade, with a conservative Supreme Court set to hear the challenge to SB8 on November 1, 2021, followed by a second case from Mississippi challenging the constitutionality of a 15-week ban on abortion in Dobbs v Jackson Women’s Health Organization (read about this case in “Supreme Court Case: Dobbs v Jackson Women’s Health Organization: What you need to know,” at https://www.mdedge.com/obgyn/article/245853/practice-management/supreme-court-case-dobbs-v-jackson-womens-health).23,24
At the time of this article writing, we do not know how the Supreme Court will rule on the variety of challenges to the right to privacy. That said, advocates believe it is safe to assume that the landscape of abortion access is likely to change dramatically in the coming year.
Action items: What can you do?
It is important to remember that not only does SB8 severely limit access to safe and legal abortion but also it makes pregnancy dangerous for all pregnant people in Texas and places doubt in providers’ minds on how to manage medical care for their patients.
On the federal level, many advocates are focusing on codifying the right to choose and protecting abortion care from medically unnecessary restrictions. The Women’s Health Protection Act of 2021 (WHPA) was introduced in the House of Representatives by Rep. Judy Chu (D-CA), Lois Frankel (D-FL), Ayanna Pressley (D-MA), and Veronica Escobar (D-TX), and it passed in the US House of Representatives in a 218-211 vote.25 WHPA now awaits a vote in a deeply divided US Senate. Although WHPA has wide popular support—an estimated 61% of Americans support the legislation—its future is unclear in the Senate.26 Currently, WHPA has 48 supporters, all Democrats. You can contact your legislators via the links below to encourage them to pass WHPA. If you have friends and colleagues in states in which the Senator does not support WHPA, forward these links and encourage them to sign on:
- Equal Access to Abortion, Everywhere: https://actforwomen.org/take-action/
- Physicians for Reproductive Rights: https://secure.everyaction.com/p/MOuAyW7F3Ua-FmaGtGD4Kw2
- Center for Reproductive Rights: https://reproductiverights.org/whpa-take-action/
Many also are organizing a crowdfunding campaign to support abortion providers as well as legislative resources. Additional groups to donate specifically to SB8 efforts include27:
- Equal Access to Abortion, Everywhere: https://actforwomen.org/whpa-faqs/
- Planned Parenthood of Greater Texas, Inc: https://www.plannedparenthood.org/planned-parenthood-greater-texas/senate-bill-8
- Texas Equal Access Fund: https://secure.everyaction.com/ztEh8Qeh80-k2k1Yuo5gTw2
- ActBlue Charities: https://secure.actblue.com/donate/txfunds
Furthermore, it is more important than ever to support work within states to support abortion rights. State-specific abortion advocacy groups and their efforts include:
- Avow Foundation for Abortion Access: https://avowtexas.org/support/
- Planned Parenthood of Greater Texas, Inc: https://www.plannedparenthood.org/planned-parenthood-greater-texas
- NARAL Pro-Choice Texas: https://prochoicetexas.org/
- Texas Abortion Access Network: https://txabortionaccessnetwork.org/
- ACLU Texas. Abortion in Texas. Updated October 9, 2021. Accessed November 8, 2021. https://www.aclutx.org/en/know-your-rights/abortion-texas.
- Rummler O. The 19th explains: what to know about Texas’ abortion law. The 19th. September 1, 2021; updated October 12, 2021. Accessed November 8, 2021. https://19thnews.org/2021/09/texas-new-abortion-law-what-you-need-know/.
- Kaye J, Hearron M. Even people who oppose abortion should fear Texas’s new ban. July 19, 2021. The Washington Post. Accessed November 12, 2021. https://www.washingtonpost.com/outlook/2021/07/19/texas-sb8-abortion-lawsuits/.
- Centers for Disease Control and Prevention. CDCs abortion surveillance system FAQs. November 25, 2020. Accessed November 8, 2021. https://www.cdc.gov/reproductivehealth/data_stats/abortion.htm.
- Texas Senate Bill 8. LegiScan. Accessed November 8, 2021. https://legiscan.com/TX/text/SB8/id/2395961.
- Texas abortion laws and policies. Planned Parenthood of Greater Texas, Inc. Accessed November 8, 2021. https://www.plannedparenthood.org/planned-parenthood-greater-texas/patient-resources/texas-laws-policies.
- Nash E. For the first time ever, US states enacted more than 100 abortion restrictions in a single year. October 4, 2012. Guttmacher Institute. Accessed November 12, 2021. https://www.guttmacher.org/article/2021/10/first-time-ever-us-states-enacted-more-100-abortion-restrictions-single-year.
- Nash E, Naide S. State policy trends at midyear 2021: already the worst legislative year ever for US abortion rights. July 2021. Guttmacher Institute. Accessed November 8, 2021. https://www.guttmacher.org/article/2021/07/state-policy-trends-midyear-2021-already-worst-legislative-year-ever-us-abortion.
- ACLU. Whole Women’s Health v Jackson. Updated October 7, 2021. Accessed November 8, 2021. https://www.aclu.org/cases/whole-womans-health-v-jacksonH
- Holley P, Solomon D. Your questions about Texas’s new abortion law, answered. Texas Monthly. October 7, 2021. Accessed November 8, 2021. https://www.texasmonthly.com/news-politics/texas-abortion-law-explained/.
- Millhiser I. The staggering implications of the Supreme Court’s Texas anti-abortion ruling. Vox. September 2, 2021. Accessed November 8, 2021. https://www.vox.com/22653779/supreme-court-abortion-texas-sb8-whole-womans-health-jackson-roe-wade.
- Carter S. ACLU of Texas asks US Supreme Court to stop new abortion law. Dallas Observer. August 31, 2021. Accessed November 8, 2021. https://www.dallasobserver.com/news/aclu-of-texas-asks-us-supreme-court-to-block-new-anti-abortion-law-sb-8-12314274.
- Supreme Court of the United States. Whole Women’s Health et al v Austin Reeve Jackson, Judge, et al: On application of injunction relief. September 1, 2021. Accessed November 8, 2021. https://www.supremecourt.gov/opinions/20pdf/21a24_8759.pdf.
- Lucas R. A US judge blocks enforcement of Texas’ controversial new abortion law. NPR. October 6, 2021. Accessed November 8, 2021. https://www.npr.org/2021/10/06/1040221171/a-u-s-judge-blocks-enforcement-of-texas-controversial-new-abortion-law.
- US Department of Justice. Attorney General Merrick B. Garland delivers remarks announcing lawsuit against the state of Texas to stop unconstitutional Senate Bill 8. September 8, 2021. Accessed November 8, 2021. https://www.justice.gov/opa/speech/attorney-general-merrick-b-garland-delivers-remarks-announcing-lawsuit-against-state-0.
- Barnhart T. Texas abortion law suspended by district judge hearing Biden administration challenge. Newsweek. October 6, 2021. Accessed November 8, 2021. https://www.newsweek.com/district-court-judge-issues-injunction-texas-law-banning-abortions-after-6-weeks-1636411.
- Oxner R. Appeals court allows Texas abortion law to resume, stopping federal judge’s order to block enforcement. The Texas Tribune. October 8, 2021. Accessed November 8, 2021. https://www.texastribune.org/2021/10/08/texas-abortion-appeal/.
- Oxner R. Texas’ near-total abortion ban will remain in effect as federal appeals court agrees to hear legal challenges. The Texas Tribune. October 14, 2021. Accessed November 8, 2021. https://www.texastribune.org/2021/10/14/texas-abortion-restrictions-appeal/.
- The United States District Court for the Western District of Texas, Austin Division. September 9, 2021. Accessed November 8, 2021. https://www.justsecurity.org/wp-content/uploads/2021/09/lawsuit-doj.pdf.
- Barnes R, Marimow AE. Justice Department will ask Supreme Court to block Texas abortion law while legal fights play out. Washington Post. October 15, 2021. Accessed November 8, 2021. https://www.washingtonpost.com/politics/courts_law/doj-texas-abortion-ban-supreme-court/2021/10/15/bd5762e6-2dcc-11ec-8ef6-3ca8fe943a92_story.html.
- Nash E, Bearak J, Li N, et al. Impact of Texas’ abortion ban: a 14-fold increase in driving distance to get an abortion. Guttmacher Institute. August 4, 2021; updated September 15, 2021. Accessed November 8, 2021. https://www.guttmacher.org/article/2021/08/impact-texas-abortion-ban-14-fold-increase-driving-distance-get-abortion.
- Jones RK, Jerman J. Abortion incidence and service availability in the United States, 2014. Perspect Sex Reprod Health. 2017;49:17-27. https://doi.org/10.1363/psrh.12015. Accessed November 12, 2021.
- Center for Reproductive Rights. Jackson Women’s Health Organization v Dobbs. March 19, 2018. Accessed November 8, 2021. https://reproductiverights.org/case/jackson-womens-health-organization-v-dobbs/.
- Chung A. US Supreme Court takes up Texas abortion case, lets ban remain. Oct 22, 2021. Reuters. Accessed November 8, 2021. https://www.reuters.com/world/us/us-supreme-court-hear-challenge-texas-abortion-ban-2021-10-22/.
- Equal Access to Abortion, Everywhere. Frequently asked questions. Accessed November 8, 2021. https://actforwomen.org/whpa-faqs/.
- Center for Reproductive Rights. New poll: a solid majority of voters support the Women’s Health Protection Act (WHPA). Accessed November 8, 2021. https://reproductiverights.org/wp-content/uploads/2021/06/ME-CRR_WHPA-Release-14001-June-1.pdf.
- Pardilla A, Avila A. 20 organizations fighting the Texas abortion ban. New York Magazine. September 2, 2021. Accessed November 8, 2021. https://nymag.com/strategist/2021/09/texas-abortion-ban-2021-where-to-donate.html.
Texas Senate Bill 8 (SB8) is the most extreme antiabortion legislation currently in effect in the United States. SB8 was introduced by the Texas legislature on March 11, 2021, and signed into law by Governor Greg Abbott on May 19, 2021.1 The law went into effect on September 1, 2021, despite an appeal to the US Supreme Court to block the law until the courts could weigh in on its constitutionality. The bill prohibits all abortion care in the state of Texas after cardiac activity has been identified, typically at 6 weeks’ gestational age. The majority of pregnant people may be unaware at that point that they are pregnant, particularly if their menstrual cycles are irregular.2 An estimated 85% of abortions in Texas occur after the 6-week mark, leaving millions of Texans without the constitutionally protected rights assured to them in Roe v Wade.3,4 This has and will disproportionately impact communities of color and low-income people seeking abortion care.
SB8 does not contain exceptions in case of a pregnancy that results from rape, sexual assault, or incest, but it does contain an exemption for abortion care because of a medical emergency, as approved by a physician. The physician is required to note the medical emergency in the patient’s chart, stating that the “medical emergency necessitated the abortion” and “prevented compliance” with SB8.5 In practice, this exception is so vague as to leave clinicians concerned that routine management of medical conditions and complications, as in ectopic pregnancy, places them at risk of legal action against them and their colleagues should they authorize abortion care.
In Texas, abortion restrictions are nothing new. Texas patients are already subject to a 2-trip requirement: Since 2011 they have been required to have a mandatory ultrasound in one visit and schedule a second visit, 24 hours later, for the procedure.6 As of 2003, Texas law also mandates that providers discuss with patients the medical risks, adoption alternatives, and developmental stages of the pregnancy.6 There are no medical indications for either of these laws, and their impact is to delay patient care. Unfortunately, laws such as these have been increasingly common in the past decade, with 106 abortion restrictions enacted in 2021 alone.7,8
What is different about SB8?
SB8 is unique in that it deputizes private citizens to enforce the law. This represents a major change in the antichoice movement’s tactics, as previous bills have made violations a criminal offense. SB8 allows a citizen to sue anyone associated with abortion care, with a minimum penalty of $10,000. In practice, a citizen of another state, who has no connection to the patient receiving care, can sue under this Texas law.9 Anyone “aiding and abetting a violation” can be found liable for up to 4 years after the date of care, including, for example, a ride-hailing driver called to ferry the patient to the appointment, the health care team providing abortion care, or insurance companies covering the costs of care. In addition, anyone found guilty of “aiding and abetting” a violation of the bill is responsible for all costs and attorney fees associated with the civil case.5,10
Furthermore, SB8 outlines defenses that cannot be used to preempt a finding of civil liability, including “ignorance or mistake of the law,” “belief of the law’s unconstitutionality,” and “consent of the [patient] to the abortion.”5 This additional layer of restriction makes it difficult to appeal the bill and convolutes an individual’s ability to challenge the law. The law also forbids the state (Texas), a state official, a court, or a district attorney from intervening on behalf of the law—upending typical courses of appeal. This legislation also complicates both federal and state intervention regarding SB8’s constitutionality, as the state has no role in enforcing the law as it is written.5
Continue to: What has been the response?...
What has been the response?
As expected, abortion foes reacted positively to SB8, while abortion advocates expressed outrage that the law went into effect. Many were additionally confused that the Supreme Court chose not to intervene to stay the law while the courts adjudicate its constitutionality, as is typical in other cases concerning abortion restrictions.11
In a 5-4 ruling, the US Supreme Court allowed SB8 to take effect on September 1, issuing its decision on the “Shadow Docket.” As such, a decision was handed down on an expedited timeline in response to an emergency appeal without any oral arguments or a lengthy opinion explaining the ruling.11,12 The majority delivered a brief, one-paragraph order summarizing their decision, explaining that their refusal to grant the injunction was not a commentary on the law’s constitutionality. The High Court stated that they could not initially comment on the law’s constitutionality before it went into effect, citing that per the law, the state had no role in enforcement, and at the time, no private actions had yet been brought under the law. Justice Sonia Sotomayor dissented, stating, “The Court’s order is stunning. Presented with an application to enjoin a flagrantly unconstitutional law engineered to prohibit women from exercising their constitutional rights and evade judicial scrutiny, a majority of Justices have opted to bury their heads in the sand.”13
Following the Supreme Court’s refusal to act, US Attorney General Merrick Garland commented that “the Justice Department was evaluating all options to protect the constitutional rights of women and other persons.” Just one week later, the US Department of Justice filed a lawsuit against the State of Texas, arguing that SB8 was unconstitutional under the Supremacy Clause (federal law takes precedence over state law) and the Fourteenth Amendment.14,15
On October 6, in response to the Department of Justice’s challenge, District Judge Robert Pitman issued an injunction to prevent enforcement of SB8. In a 113-page ruling, Judge Pitman explained that “a person’s right under the Constitution to choose to obtain an abortion prior to fetal viability is well established.” Judge Pittman held SB8 unconstitutional, stating, “Women have been unlawfully prevented from exercising control over their lives in ways that are protected by the Constitution... Fully aware that depriving its citizens of this right by direct state action would be flagrantly unconstitutional, the State contrived an unprecedented and transparent statutory scheme to do just that.”16
Just 48 hours after the injunction issued by Judge Pitman, the Fifth Circuit Court of Appeals overturned the injunction, and SB8 went back into effect while litigation on its constitutionality proceeded.2,17 The Fifth Circuit Court of Appeals is widely considered to be one of the most conservative courts in the country.18
On October 15, 2021, the Department of Justice appealed the Fifth Circuit Court’s decision and asked the US Supreme Court to intervene, requesting that the Court issue an emergency halt to the law.19,20 On October 22, 2021, the Court declined to halt the law but scheduled oral arguments on the case for November 1, 2021. This is a stunningly fast briefing schedule for a case of such constitutional importance.
Given the legal back-and-forth, many clinicians are not providing abortion care in Texas as the litigation unfolds. SB8 permits retroactive enforcement, mandating that those “aiding and abetting” of abortion care may be civilly liable for up to 4 years after providing the care.5
Continue to: Potential outcomes, and what comes next...
Potential outcomes, and what comes next
Since the ascension of Justice Amy Coney Barrett to the High Court, there has been a nationwide increase in antiabortion legislation. Between January and July 2021, more than 90 abortion restrictions were passed, more restrictions in any single year since Roe v Wade was decided in 1973.8 In the past decade, more than 500 laws that restrict abortion have been passed across the United States, and studies indicate that 87% to 90% of American counties today are without a single abortion provider.21,22 Abortion supporters are particularly concerned about the future of Roe v Wade, with a conservative Supreme Court set to hear the challenge to SB8 on November 1, 2021, followed by a second case from Mississippi challenging the constitutionality of a 15-week ban on abortion in Dobbs v Jackson Women’s Health Organization (read about this case in “Supreme Court Case: Dobbs v Jackson Women’s Health Organization: What you need to know,” at https://www.mdedge.com/obgyn/article/245853/practice-management/supreme-court-case-dobbs-v-jackson-womens-health).23,24
At the time of this article writing, we do not know how the Supreme Court will rule on the variety of challenges to the right to privacy. That said, advocates believe it is safe to assume that the landscape of abortion access is likely to change dramatically in the coming year.
Action items: What can you do?
It is important to remember that not only does SB8 severely limit access to safe and legal abortion but also it makes pregnancy dangerous for all pregnant people in Texas and places doubt in providers’ minds on how to manage medical care for their patients.
On the federal level, many advocates are focusing on codifying the right to choose and protecting abortion care from medically unnecessary restrictions. The Women’s Health Protection Act of 2021 (WHPA) was introduced in the House of Representatives by Rep. Judy Chu (D-CA), Lois Frankel (D-FL), Ayanna Pressley (D-MA), and Veronica Escobar (D-TX), and it passed in the US House of Representatives in a 218-211 vote.25 WHPA now awaits a vote in a deeply divided US Senate. Although WHPA has wide popular support—an estimated 61% of Americans support the legislation—its future is unclear in the Senate.26 Currently, WHPA has 48 supporters, all Democrats. You can contact your legislators via the links below to encourage them to pass WHPA. If you have friends and colleagues in states in which the Senator does not support WHPA, forward these links and encourage them to sign on:
- Equal Access to Abortion, Everywhere: https://actforwomen.org/take-action/
- Physicians for Reproductive Rights: https://secure.everyaction.com/p/MOuAyW7F3Ua-FmaGtGD4Kw2
- Center for Reproductive Rights: https://reproductiverights.org/whpa-take-action/
Many also are organizing a crowdfunding campaign to support abortion providers as well as legislative resources. Additional groups to donate specifically to SB8 efforts include27:
- Equal Access to Abortion, Everywhere: https://actforwomen.org/whpa-faqs/
- Planned Parenthood of Greater Texas, Inc: https://www.plannedparenthood.org/planned-parenthood-greater-texas/senate-bill-8
- Texas Equal Access Fund: https://secure.everyaction.com/ztEh8Qeh80-k2k1Yuo5gTw2
- ActBlue Charities: https://secure.actblue.com/donate/txfunds
Furthermore, it is more important than ever to support work within states to support abortion rights. State-specific abortion advocacy groups and their efforts include:
- Avow Foundation for Abortion Access: https://avowtexas.org/support/
- Planned Parenthood of Greater Texas, Inc: https://www.plannedparenthood.org/planned-parenthood-greater-texas
- NARAL Pro-Choice Texas: https://prochoicetexas.org/
- Texas Abortion Access Network: https://txabortionaccessnetwork.org/
Texas Senate Bill 8 (SB8) is the most extreme antiabortion legislation currently in effect in the United States. SB8 was introduced by the Texas legislature on March 11, 2021, and signed into law by Governor Greg Abbott on May 19, 2021.1 The law went into effect on September 1, 2021, despite an appeal to the US Supreme Court to block the law until the courts could weigh in on its constitutionality. The bill prohibits all abortion care in the state of Texas after cardiac activity has been identified, typically at 6 weeks’ gestational age. The majority of pregnant people may be unaware at that point that they are pregnant, particularly if their menstrual cycles are irregular.2 An estimated 85% of abortions in Texas occur after the 6-week mark, leaving millions of Texans without the constitutionally protected rights assured to them in Roe v Wade.3,4 This has and will disproportionately impact communities of color and low-income people seeking abortion care.
SB8 does not contain exceptions in case of a pregnancy that results from rape, sexual assault, or incest, but it does contain an exemption for abortion care because of a medical emergency, as approved by a physician. The physician is required to note the medical emergency in the patient’s chart, stating that the “medical emergency necessitated the abortion” and “prevented compliance” with SB8.5 In practice, this exception is so vague as to leave clinicians concerned that routine management of medical conditions and complications, as in ectopic pregnancy, places them at risk of legal action against them and their colleagues should they authorize abortion care.
In Texas, abortion restrictions are nothing new. Texas patients are already subject to a 2-trip requirement: Since 2011 they have been required to have a mandatory ultrasound in one visit and schedule a second visit, 24 hours later, for the procedure.6 As of 2003, Texas law also mandates that providers discuss with patients the medical risks, adoption alternatives, and developmental stages of the pregnancy.6 There are no medical indications for either of these laws, and their impact is to delay patient care. Unfortunately, laws such as these have been increasingly common in the past decade, with 106 abortion restrictions enacted in 2021 alone.7,8
What is different about SB8?
SB8 is unique in that it deputizes private citizens to enforce the law. This represents a major change in the antichoice movement’s tactics, as previous bills have made violations a criminal offense. SB8 allows a citizen to sue anyone associated with abortion care, with a minimum penalty of $10,000. In practice, a citizen of another state, who has no connection to the patient receiving care, can sue under this Texas law.9 Anyone “aiding and abetting a violation” can be found liable for up to 4 years after the date of care, including, for example, a ride-hailing driver called to ferry the patient to the appointment, the health care team providing abortion care, or insurance companies covering the costs of care. In addition, anyone found guilty of “aiding and abetting” a violation of the bill is responsible for all costs and attorney fees associated with the civil case.5,10
Furthermore, SB8 outlines defenses that cannot be used to preempt a finding of civil liability, including “ignorance or mistake of the law,” “belief of the law’s unconstitutionality,” and “consent of the [patient] to the abortion.”5 This additional layer of restriction makes it difficult to appeal the bill and convolutes an individual’s ability to challenge the law. The law also forbids the state (Texas), a state official, a court, or a district attorney from intervening on behalf of the law—upending typical courses of appeal. This legislation also complicates both federal and state intervention regarding SB8’s constitutionality, as the state has no role in enforcing the law as it is written.5
Continue to: What has been the response?...
What has been the response?
As expected, abortion foes reacted positively to SB8, while abortion advocates expressed outrage that the law went into effect. Many were additionally confused that the Supreme Court chose not to intervene to stay the law while the courts adjudicate its constitutionality, as is typical in other cases concerning abortion restrictions.11
In a 5-4 ruling, the US Supreme Court allowed SB8 to take effect on September 1, issuing its decision on the “Shadow Docket.” As such, a decision was handed down on an expedited timeline in response to an emergency appeal without any oral arguments or a lengthy opinion explaining the ruling.11,12 The majority delivered a brief, one-paragraph order summarizing their decision, explaining that their refusal to grant the injunction was not a commentary on the law’s constitutionality. The High Court stated that they could not initially comment on the law’s constitutionality before it went into effect, citing that per the law, the state had no role in enforcement, and at the time, no private actions had yet been brought under the law. Justice Sonia Sotomayor dissented, stating, “The Court’s order is stunning. Presented with an application to enjoin a flagrantly unconstitutional law engineered to prohibit women from exercising their constitutional rights and evade judicial scrutiny, a majority of Justices have opted to bury their heads in the sand.”13
Following the Supreme Court’s refusal to act, US Attorney General Merrick Garland commented that “the Justice Department was evaluating all options to protect the constitutional rights of women and other persons.” Just one week later, the US Department of Justice filed a lawsuit against the State of Texas, arguing that SB8 was unconstitutional under the Supremacy Clause (federal law takes precedence over state law) and the Fourteenth Amendment.14,15
On October 6, in response to the Department of Justice’s challenge, District Judge Robert Pitman issued an injunction to prevent enforcement of SB8. In a 113-page ruling, Judge Pitman explained that “a person’s right under the Constitution to choose to obtain an abortion prior to fetal viability is well established.” Judge Pittman held SB8 unconstitutional, stating, “Women have been unlawfully prevented from exercising control over their lives in ways that are protected by the Constitution... Fully aware that depriving its citizens of this right by direct state action would be flagrantly unconstitutional, the State contrived an unprecedented and transparent statutory scheme to do just that.”16
Just 48 hours after the injunction issued by Judge Pitman, the Fifth Circuit Court of Appeals overturned the injunction, and SB8 went back into effect while litigation on its constitutionality proceeded.2,17 The Fifth Circuit Court of Appeals is widely considered to be one of the most conservative courts in the country.18
On October 15, 2021, the Department of Justice appealed the Fifth Circuit Court’s decision and asked the US Supreme Court to intervene, requesting that the Court issue an emergency halt to the law.19,20 On October 22, 2021, the Court declined to halt the law but scheduled oral arguments on the case for November 1, 2021. This is a stunningly fast briefing schedule for a case of such constitutional importance.
Given the legal back-and-forth, many clinicians are not providing abortion care in Texas as the litigation unfolds. SB8 permits retroactive enforcement, mandating that those “aiding and abetting” of abortion care may be civilly liable for up to 4 years after providing the care.5
Continue to: Potential outcomes, and what comes next...
Potential outcomes, and what comes next
Since the ascension of Justice Amy Coney Barrett to the High Court, there has been a nationwide increase in antiabortion legislation. Between January and July 2021, more than 90 abortion restrictions were passed, more restrictions in any single year since Roe v Wade was decided in 1973.8 In the past decade, more than 500 laws that restrict abortion have been passed across the United States, and studies indicate that 87% to 90% of American counties today are without a single abortion provider.21,22 Abortion supporters are particularly concerned about the future of Roe v Wade, with a conservative Supreme Court set to hear the challenge to SB8 on November 1, 2021, followed by a second case from Mississippi challenging the constitutionality of a 15-week ban on abortion in Dobbs v Jackson Women’s Health Organization (read about this case in “Supreme Court Case: Dobbs v Jackson Women’s Health Organization: What you need to know,” at https://www.mdedge.com/obgyn/article/245853/practice-management/supreme-court-case-dobbs-v-jackson-womens-health).23,24
At the time of this article writing, we do not know how the Supreme Court will rule on the variety of challenges to the right to privacy. That said, advocates believe it is safe to assume that the landscape of abortion access is likely to change dramatically in the coming year.
Action items: What can you do?
It is important to remember that not only does SB8 severely limit access to safe and legal abortion but also it makes pregnancy dangerous for all pregnant people in Texas and places doubt in providers’ minds on how to manage medical care for their patients.
On the federal level, many advocates are focusing on codifying the right to choose and protecting abortion care from medically unnecessary restrictions. The Women’s Health Protection Act of 2021 (WHPA) was introduced in the House of Representatives by Rep. Judy Chu (D-CA), Lois Frankel (D-FL), Ayanna Pressley (D-MA), and Veronica Escobar (D-TX), and it passed in the US House of Representatives in a 218-211 vote.25 WHPA now awaits a vote in a deeply divided US Senate. Although WHPA has wide popular support—an estimated 61% of Americans support the legislation—its future is unclear in the Senate.26 Currently, WHPA has 48 supporters, all Democrats. You can contact your legislators via the links below to encourage them to pass WHPA. If you have friends and colleagues in states in which the Senator does not support WHPA, forward these links and encourage them to sign on:
- Equal Access to Abortion, Everywhere: https://actforwomen.org/take-action/
- Physicians for Reproductive Rights: https://secure.everyaction.com/p/MOuAyW7F3Ua-FmaGtGD4Kw2
- Center for Reproductive Rights: https://reproductiverights.org/whpa-take-action/
Many also are organizing a crowdfunding campaign to support abortion providers as well as legislative resources. Additional groups to donate specifically to SB8 efforts include27:
- Equal Access to Abortion, Everywhere: https://actforwomen.org/whpa-faqs/
- Planned Parenthood of Greater Texas, Inc: https://www.plannedparenthood.org/planned-parenthood-greater-texas/senate-bill-8
- Texas Equal Access Fund: https://secure.everyaction.com/ztEh8Qeh80-k2k1Yuo5gTw2
- ActBlue Charities: https://secure.actblue.com/donate/txfunds
Furthermore, it is more important than ever to support work within states to support abortion rights. State-specific abortion advocacy groups and their efforts include:
- Avow Foundation for Abortion Access: https://avowtexas.org/support/
- Planned Parenthood of Greater Texas, Inc: https://www.plannedparenthood.org/planned-parenthood-greater-texas
- NARAL Pro-Choice Texas: https://prochoicetexas.org/
- Texas Abortion Access Network: https://txabortionaccessnetwork.org/
- ACLU Texas. Abortion in Texas. Updated October 9, 2021. Accessed November 8, 2021. https://www.aclutx.org/en/know-your-rights/abortion-texas.
- Rummler O. The 19th explains: what to know about Texas’ abortion law. The 19th. September 1, 2021; updated October 12, 2021. Accessed November 8, 2021. https://19thnews.org/2021/09/texas-new-abortion-law-what-you-need-know/.
- Kaye J, Hearron M. Even people who oppose abortion should fear Texas’s new ban. July 19, 2021. The Washington Post. Accessed November 12, 2021. https://www.washingtonpost.com/outlook/2021/07/19/texas-sb8-abortion-lawsuits/.
- Centers for Disease Control and Prevention. CDCs abortion surveillance system FAQs. November 25, 2020. Accessed November 8, 2021. https://www.cdc.gov/reproductivehealth/data_stats/abortion.htm.
- Texas Senate Bill 8. LegiScan. Accessed November 8, 2021. https://legiscan.com/TX/text/SB8/id/2395961.
- Texas abortion laws and policies. Planned Parenthood of Greater Texas, Inc. Accessed November 8, 2021. https://www.plannedparenthood.org/planned-parenthood-greater-texas/patient-resources/texas-laws-policies.
- Nash E. For the first time ever, US states enacted more than 100 abortion restrictions in a single year. October 4, 2012. Guttmacher Institute. Accessed November 12, 2021. https://www.guttmacher.org/article/2021/10/first-time-ever-us-states-enacted-more-100-abortion-restrictions-single-year.
- Nash E, Naide S. State policy trends at midyear 2021: already the worst legislative year ever for US abortion rights. July 2021. Guttmacher Institute. Accessed November 8, 2021. https://www.guttmacher.org/article/2021/07/state-policy-trends-midyear-2021-already-worst-legislative-year-ever-us-abortion.
- ACLU. Whole Women’s Health v Jackson. Updated October 7, 2021. Accessed November 8, 2021. https://www.aclu.org/cases/whole-womans-health-v-jacksonH
- Holley P, Solomon D. Your questions about Texas’s new abortion law, answered. Texas Monthly. October 7, 2021. Accessed November 8, 2021. https://www.texasmonthly.com/news-politics/texas-abortion-law-explained/.
- Millhiser I. The staggering implications of the Supreme Court’s Texas anti-abortion ruling. Vox. September 2, 2021. Accessed November 8, 2021. https://www.vox.com/22653779/supreme-court-abortion-texas-sb8-whole-womans-health-jackson-roe-wade.
- Carter S. ACLU of Texas asks US Supreme Court to stop new abortion law. Dallas Observer. August 31, 2021. Accessed November 8, 2021. https://www.dallasobserver.com/news/aclu-of-texas-asks-us-supreme-court-to-block-new-anti-abortion-law-sb-8-12314274.
- Supreme Court of the United States. Whole Women’s Health et al v Austin Reeve Jackson, Judge, et al: On application of injunction relief. September 1, 2021. Accessed November 8, 2021. https://www.supremecourt.gov/opinions/20pdf/21a24_8759.pdf.
- Lucas R. A US judge blocks enforcement of Texas’ controversial new abortion law. NPR. October 6, 2021. Accessed November 8, 2021. https://www.npr.org/2021/10/06/1040221171/a-u-s-judge-blocks-enforcement-of-texas-controversial-new-abortion-law.
- US Department of Justice. Attorney General Merrick B. Garland delivers remarks announcing lawsuit against the state of Texas to stop unconstitutional Senate Bill 8. September 8, 2021. Accessed November 8, 2021. https://www.justice.gov/opa/speech/attorney-general-merrick-b-garland-delivers-remarks-announcing-lawsuit-against-state-0.
- Barnhart T. Texas abortion law suspended by district judge hearing Biden administration challenge. Newsweek. October 6, 2021. Accessed November 8, 2021. https://www.newsweek.com/district-court-judge-issues-injunction-texas-law-banning-abortions-after-6-weeks-1636411.
- Oxner R. Appeals court allows Texas abortion law to resume, stopping federal judge’s order to block enforcement. The Texas Tribune. October 8, 2021. Accessed November 8, 2021. https://www.texastribune.org/2021/10/08/texas-abortion-appeal/.
- Oxner R. Texas’ near-total abortion ban will remain in effect as federal appeals court agrees to hear legal challenges. The Texas Tribune. October 14, 2021. Accessed November 8, 2021. https://www.texastribune.org/2021/10/14/texas-abortion-restrictions-appeal/.
- The United States District Court for the Western District of Texas, Austin Division. September 9, 2021. Accessed November 8, 2021. https://www.justsecurity.org/wp-content/uploads/2021/09/lawsuit-doj.pdf.
- Barnes R, Marimow AE. Justice Department will ask Supreme Court to block Texas abortion law while legal fights play out. Washington Post. October 15, 2021. Accessed November 8, 2021. https://www.washingtonpost.com/politics/courts_law/doj-texas-abortion-ban-supreme-court/2021/10/15/bd5762e6-2dcc-11ec-8ef6-3ca8fe943a92_story.html.
- Nash E, Bearak J, Li N, et al. Impact of Texas’ abortion ban: a 14-fold increase in driving distance to get an abortion. Guttmacher Institute. August 4, 2021; updated September 15, 2021. Accessed November 8, 2021. https://www.guttmacher.org/article/2021/08/impact-texas-abortion-ban-14-fold-increase-driving-distance-get-abortion.
- Jones RK, Jerman J. Abortion incidence and service availability in the United States, 2014. Perspect Sex Reprod Health. 2017;49:17-27. https://doi.org/10.1363/psrh.12015. Accessed November 12, 2021.
- Center for Reproductive Rights. Jackson Women’s Health Organization v Dobbs. March 19, 2018. Accessed November 8, 2021. https://reproductiverights.org/case/jackson-womens-health-organization-v-dobbs/.
- Chung A. US Supreme Court takes up Texas abortion case, lets ban remain. Oct 22, 2021. Reuters. Accessed November 8, 2021. https://www.reuters.com/world/us/us-supreme-court-hear-challenge-texas-abortion-ban-2021-10-22/.
- Equal Access to Abortion, Everywhere. Frequently asked questions. Accessed November 8, 2021. https://actforwomen.org/whpa-faqs/.
- Center for Reproductive Rights. New poll: a solid majority of voters support the Women’s Health Protection Act (WHPA). Accessed November 8, 2021. https://reproductiverights.org/wp-content/uploads/2021/06/ME-CRR_WHPA-Release-14001-June-1.pdf.
- Pardilla A, Avila A. 20 organizations fighting the Texas abortion ban. New York Magazine. September 2, 2021. Accessed November 8, 2021. https://nymag.com/strategist/2021/09/texas-abortion-ban-2021-where-to-donate.html.
- ACLU Texas. Abortion in Texas. Updated October 9, 2021. Accessed November 8, 2021. https://www.aclutx.org/en/know-your-rights/abortion-texas.
- Rummler O. The 19th explains: what to know about Texas’ abortion law. The 19th. September 1, 2021; updated October 12, 2021. Accessed November 8, 2021. https://19thnews.org/2021/09/texas-new-abortion-law-what-you-need-know/.
- Kaye J, Hearron M. Even people who oppose abortion should fear Texas’s new ban. July 19, 2021. The Washington Post. Accessed November 12, 2021. https://www.washingtonpost.com/outlook/2021/07/19/texas-sb8-abortion-lawsuits/.
- Centers for Disease Control and Prevention. CDCs abortion surveillance system FAQs. November 25, 2020. Accessed November 8, 2021. https://www.cdc.gov/reproductivehealth/data_stats/abortion.htm.
- Texas Senate Bill 8. LegiScan. Accessed November 8, 2021. https://legiscan.com/TX/text/SB8/id/2395961.
- Texas abortion laws and policies. Planned Parenthood of Greater Texas, Inc. Accessed November 8, 2021. https://www.plannedparenthood.org/planned-parenthood-greater-texas/patient-resources/texas-laws-policies.
- Nash E. For the first time ever, US states enacted more than 100 abortion restrictions in a single year. October 4, 2012. Guttmacher Institute. Accessed November 12, 2021. https://www.guttmacher.org/article/2021/10/first-time-ever-us-states-enacted-more-100-abortion-restrictions-single-year.
- Nash E, Naide S. State policy trends at midyear 2021: already the worst legislative year ever for US abortion rights. July 2021. Guttmacher Institute. Accessed November 8, 2021. https://www.guttmacher.org/article/2021/07/state-policy-trends-midyear-2021-already-worst-legislative-year-ever-us-abortion.
- ACLU. Whole Women’s Health v Jackson. Updated October 7, 2021. Accessed November 8, 2021. https://www.aclu.org/cases/whole-womans-health-v-jacksonH
- Holley P, Solomon D. Your questions about Texas’s new abortion law, answered. Texas Monthly. October 7, 2021. Accessed November 8, 2021. https://www.texasmonthly.com/news-politics/texas-abortion-law-explained/.
- Millhiser I. The staggering implications of the Supreme Court’s Texas anti-abortion ruling. Vox. September 2, 2021. Accessed November 8, 2021. https://www.vox.com/22653779/supreme-court-abortion-texas-sb8-whole-womans-health-jackson-roe-wade.
- Carter S. ACLU of Texas asks US Supreme Court to stop new abortion law. Dallas Observer. August 31, 2021. Accessed November 8, 2021. https://www.dallasobserver.com/news/aclu-of-texas-asks-us-supreme-court-to-block-new-anti-abortion-law-sb-8-12314274.
- Supreme Court of the United States. Whole Women’s Health et al v Austin Reeve Jackson, Judge, et al: On application of injunction relief. September 1, 2021. Accessed November 8, 2021. https://www.supremecourt.gov/opinions/20pdf/21a24_8759.pdf.
- Lucas R. A US judge blocks enforcement of Texas’ controversial new abortion law. NPR. October 6, 2021. Accessed November 8, 2021. https://www.npr.org/2021/10/06/1040221171/a-u-s-judge-blocks-enforcement-of-texas-controversial-new-abortion-law.
- US Department of Justice. Attorney General Merrick B. Garland delivers remarks announcing lawsuit against the state of Texas to stop unconstitutional Senate Bill 8. September 8, 2021. Accessed November 8, 2021. https://www.justice.gov/opa/speech/attorney-general-merrick-b-garland-delivers-remarks-announcing-lawsuit-against-state-0.
- Barnhart T. Texas abortion law suspended by district judge hearing Biden administration challenge. Newsweek. October 6, 2021. Accessed November 8, 2021. https://www.newsweek.com/district-court-judge-issues-injunction-texas-law-banning-abortions-after-6-weeks-1636411.
- Oxner R. Appeals court allows Texas abortion law to resume, stopping federal judge’s order to block enforcement. The Texas Tribune. October 8, 2021. Accessed November 8, 2021. https://www.texastribune.org/2021/10/08/texas-abortion-appeal/.
- Oxner R. Texas’ near-total abortion ban will remain in effect as federal appeals court agrees to hear legal challenges. The Texas Tribune. October 14, 2021. Accessed November 8, 2021. https://www.texastribune.org/2021/10/14/texas-abortion-restrictions-appeal/.
- The United States District Court for the Western District of Texas, Austin Division. September 9, 2021. Accessed November 8, 2021. https://www.justsecurity.org/wp-content/uploads/2021/09/lawsuit-doj.pdf.
- Barnes R, Marimow AE. Justice Department will ask Supreme Court to block Texas abortion law while legal fights play out. Washington Post. October 15, 2021. Accessed November 8, 2021. https://www.washingtonpost.com/politics/courts_law/doj-texas-abortion-ban-supreme-court/2021/10/15/bd5762e6-2dcc-11ec-8ef6-3ca8fe943a92_story.html.
- Nash E, Bearak J, Li N, et al. Impact of Texas’ abortion ban: a 14-fold increase in driving distance to get an abortion. Guttmacher Institute. August 4, 2021; updated September 15, 2021. Accessed November 8, 2021. https://www.guttmacher.org/article/2021/08/impact-texas-abortion-ban-14-fold-increase-driving-distance-get-abortion.
- Jones RK, Jerman J. Abortion incidence and service availability in the United States, 2014. Perspect Sex Reprod Health. 2017;49:17-27. https://doi.org/10.1363/psrh.12015. Accessed November 12, 2021.
- Center for Reproductive Rights. Jackson Women’s Health Organization v Dobbs. March 19, 2018. Accessed November 8, 2021. https://reproductiverights.org/case/jackson-womens-health-organization-v-dobbs/.
- Chung A. US Supreme Court takes up Texas abortion case, lets ban remain. Oct 22, 2021. Reuters. Accessed November 8, 2021. https://www.reuters.com/world/us/us-supreme-court-hear-challenge-texas-abortion-ban-2021-10-22/.
- Equal Access to Abortion, Everywhere. Frequently asked questions. Accessed November 8, 2021. https://actforwomen.org/whpa-faqs/.
- Center for Reproductive Rights. New poll: a solid majority of voters support the Women’s Health Protection Act (WHPA). Accessed November 8, 2021. https://reproductiverights.org/wp-content/uploads/2021/06/ME-CRR_WHPA-Release-14001-June-1.pdf.
- Pardilla A, Avila A. 20 organizations fighting the Texas abortion ban. New York Magazine. September 2, 2021. Accessed November 8, 2021. https://nymag.com/strategist/2021/09/texas-abortion-ban-2021-where-to-donate.html.
ADVOCATE: Avacopan shows renal benefits in ANCA vasculitis
Treatment of antineutrophil cytoplasmic autoantibody (ANCA)–associated vasculitis and renal disease with the oral C5a receptor inhibitor avacopan (Tavneos, ChemoCentryx) provides significant recovery of kidney function, compared with prednisone, particularly in patients with severe kidney disease, novel research indicates.
The new analysis underscores that “the real value of avacopan is that we can now expect to get our patients steroid free,” said first author David R.W. Jayne, MD, a professor of clinical autoimmunity at the University of Cambridge (England), when presenting the findings at the American Society of Nephrology’s Kidney Week 2021.
“Whether or not we’re brave enough to initiate treatment without steroids, I think that will perhaps come with some patient experience,” he added.
The findings are from a subanalysis of renal effects in the phase 3 ADVOCATE trial, which was published in February 2021 in the New England Journal of Medicine and included 330 patients with ANCA-associated vasculitis.
The trial in large part led to the U.S. approval of avacopan by the Food and Drug Administration in October as an adjunctive treatment for adults with severe active ANCA-associated vasculitis in combination with standard therapy including glucocorticoids.
The approval was greeted with enthusiasm as suggesting a much-needed option to help reduce, or even potentially eliminate, the need for glucocorticoids and their side effects. Other agents included in treatment regimens for ANCA-associated vasculitis include cyclophosphamide and rituximab.
Dr. Jayne emphasized that, before avacopan, treatment options had been limited.
“There is nothing else new in the clinic apart from rituximab, which we have now been using for almost 20 years,” he said in an interview. “Avacopan is new, the mode of action is different from any drugs in use at the moment, and the speed of action is very quick.”
The need to more closely investigate the trial’s renal outcomes in this new analysis was important because the high mortality rates in ANCA-associated vasculitis – a rare systemic autoimmune disease causing overactivation of complement resulting in inflammation of small blood vessels – is largely driven by those with MPO and PR3 autoantibody renal vasculitis, Dr. Jayne explained.
Commenting on the study, J. Charles Jennette, MD, a professor of pathology and laboratory medicine and professor of medicine at the University of North Carolina at Chapel Hill, said the new findings on renal outcomes, such as proteinuria, may offer key insights on avacopan’s efficacy.
“To me, the most impressive outcome of the ADVOCATE Phase 3 trial was the more rapid reduction in hematuria and proteinuria with avacopan compared to conventional prednisone therapy,” he said in an interview.
Recovery of eGFR with avacopan best in those with severe renal disease
In the trial, patients with ANCA-associated vasculitis were randomized 1:1 to treatment with oral avacopan 30 mg twice daily or oral prednisone on a tapering schedule.
All patients also received background immunosuppression – about two-thirds received rituximab and a third received cyclophosphamide – followed by azathioprine.
The main study results showed similar rates of remission in both groups at week 26 and a superior remission rate with avacopan, in terms of sustained remission, at week 52 (65.7% vs. 54.9%; P < .001).
Approximately 80% of patients in the trial had renal involvement of ANCA vasculitis, the focus of the new analysis, and they had a baseline mean estimated glomerular filtration rate (eGFR) of 45 mL/min per 1.73 m2.
Among those with renal involvement, patients treated with avacopan had a significantly greater eGFR recovery, compared with the prednisone group at week 26 (P = .046) and week 52 (P < .029).
The strongest improvements were observed among patients with moderate to severe kidney damage, who had a mean eGFR of 21 mL/min per 1.73 m2 at baseline. Among those patients, the mean increase in eGFR was 13.7 mL/min per 1.73 m2 in the avacopan-treated group (n = 52) versus 8.2 mL/min per 1.73 m2 in the prednisone group (n = 48; P < .01) by week 52.
Improvements in urinary albumin:creatinine ratios (UACR) of as much as 40% were also observed in the avacopan group within the first 4 weeks of treatment, while no changes were observed in the same period in the prednisone group.
In other findings, the study also showed more rapid declines in proteinuria within 4 weeks in the avacopan group, and fewer patients had hematuria and there were greater reductions in MCP-1 in avacopan-treated patients at week 52, Dr. Jayne reported.
In terms of safety, there were no differences between the groups, with trends of fewer deaths and severe adverse events in the avacopan group.
“We found that the improved recovery of eGFR with avacopan was accentuated among those with more severe renal disease,” Dr. Jayne said.
He noted that, while the study’s aim was for the avacopan group to be steroid free, the patients received brief, reduced doses of about a third of the normal oral steroid dose early in the trial. However, using a Glucocorticoid Toxicity Index, the authors found those in the avacopan group did have fewer glucocorticoid-related adverse events.
Future issues to be examined include what happens when avacopan is discontinued and whether there will be a high relapse rate, Dr. Jayne noted.
Overall, however, “we anticipate that with longer-term follow-up, this better eGFR recovery will have a [favorable] effect on kidney failure and potentially mortality risk in these patients,” he concluded.
Targeted therapy is good for patients and doctors
Expanding upon his comments regarding the new drug, Dr. Jennette said it implies “that the C5a receptor inhibitor was targeting an event that blocks injury more quickly and effectively than prednisone.”
“This may be because prednisone has more complex pharmacodynamics and less targeted effects than a C5a receptor inhibitor,” he said.
Overall, the findings bode well for a potentially beneficial therapy, he added. “We have entered a new era of more targeted therapies, for example, targeted B-cell therapy using an anti-CD20 antibody, and targeted complement-mediated injury therapy using C5a receptor inhibitor.”
“The validation of this targeted therapy to block complement-mediated autoimmune inflammatory injury is another advance toward targeted precision therapy versus empirical therapy. This will be good for the doctors and good for the patients,” Dr. Jennette concluded.
The study was funded by ChemoCentryx. Dr. Jayne has reported receiving grants and/or consulting for AstraZeneca, ChemoCentryx, GlaxoSmithKline, MiroBio, Vifor, and Roche/Genentech. Dr. Jennette has received funding from ChemoCentryx for preclinical validation studies of avacopan in a mouse model of ANCA glomerulonephritis.
A version of this article first appeared on Medscape.com.
Treatment of antineutrophil cytoplasmic autoantibody (ANCA)–associated vasculitis and renal disease with the oral C5a receptor inhibitor avacopan (Tavneos, ChemoCentryx) provides significant recovery of kidney function, compared with prednisone, particularly in patients with severe kidney disease, novel research indicates.
The new analysis underscores that “the real value of avacopan is that we can now expect to get our patients steroid free,” said first author David R.W. Jayne, MD, a professor of clinical autoimmunity at the University of Cambridge (England), when presenting the findings at the American Society of Nephrology’s Kidney Week 2021.
“Whether or not we’re brave enough to initiate treatment without steroids, I think that will perhaps come with some patient experience,” he added.
The findings are from a subanalysis of renal effects in the phase 3 ADVOCATE trial, which was published in February 2021 in the New England Journal of Medicine and included 330 patients with ANCA-associated vasculitis.
The trial in large part led to the U.S. approval of avacopan by the Food and Drug Administration in October as an adjunctive treatment for adults with severe active ANCA-associated vasculitis in combination with standard therapy including glucocorticoids.
The approval was greeted with enthusiasm as suggesting a much-needed option to help reduce, or even potentially eliminate, the need for glucocorticoids and their side effects. Other agents included in treatment regimens for ANCA-associated vasculitis include cyclophosphamide and rituximab.
Dr. Jayne emphasized that, before avacopan, treatment options had been limited.
“There is nothing else new in the clinic apart from rituximab, which we have now been using for almost 20 years,” he said in an interview. “Avacopan is new, the mode of action is different from any drugs in use at the moment, and the speed of action is very quick.”
The need to more closely investigate the trial’s renal outcomes in this new analysis was important because the high mortality rates in ANCA-associated vasculitis – a rare systemic autoimmune disease causing overactivation of complement resulting in inflammation of small blood vessels – is largely driven by those with MPO and PR3 autoantibody renal vasculitis, Dr. Jayne explained.
Commenting on the study, J. Charles Jennette, MD, a professor of pathology and laboratory medicine and professor of medicine at the University of North Carolina at Chapel Hill, said the new findings on renal outcomes, such as proteinuria, may offer key insights on avacopan’s efficacy.
“To me, the most impressive outcome of the ADVOCATE Phase 3 trial was the more rapid reduction in hematuria and proteinuria with avacopan compared to conventional prednisone therapy,” he said in an interview.
Recovery of eGFR with avacopan best in those with severe renal disease
In the trial, patients with ANCA-associated vasculitis were randomized 1:1 to treatment with oral avacopan 30 mg twice daily or oral prednisone on a tapering schedule.
All patients also received background immunosuppression – about two-thirds received rituximab and a third received cyclophosphamide – followed by azathioprine.
The main study results showed similar rates of remission in both groups at week 26 and a superior remission rate with avacopan, in terms of sustained remission, at week 52 (65.7% vs. 54.9%; P < .001).
Approximately 80% of patients in the trial had renal involvement of ANCA vasculitis, the focus of the new analysis, and they had a baseline mean estimated glomerular filtration rate (eGFR) of 45 mL/min per 1.73 m2.
Among those with renal involvement, patients treated with avacopan had a significantly greater eGFR recovery, compared with the prednisone group at week 26 (P = .046) and week 52 (P < .029).
The strongest improvements were observed among patients with moderate to severe kidney damage, who had a mean eGFR of 21 mL/min per 1.73 m2 at baseline. Among those patients, the mean increase in eGFR was 13.7 mL/min per 1.73 m2 in the avacopan-treated group (n = 52) versus 8.2 mL/min per 1.73 m2 in the prednisone group (n = 48; P < .01) by week 52.
Improvements in urinary albumin:creatinine ratios (UACR) of as much as 40% were also observed in the avacopan group within the first 4 weeks of treatment, while no changes were observed in the same period in the prednisone group.
In other findings, the study also showed more rapid declines in proteinuria within 4 weeks in the avacopan group, and fewer patients had hematuria and there were greater reductions in MCP-1 in avacopan-treated patients at week 52, Dr. Jayne reported.
In terms of safety, there were no differences between the groups, with trends of fewer deaths and severe adverse events in the avacopan group.
“We found that the improved recovery of eGFR with avacopan was accentuated among those with more severe renal disease,” Dr. Jayne said.
He noted that, while the study’s aim was for the avacopan group to be steroid free, the patients received brief, reduced doses of about a third of the normal oral steroid dose early in the trial. However, using a Glucocorticoid Toxicity Index, the authors found those in the avacopan group did have fewer glucocorticoid-related adverse events.
Future issues to be examined include what happens when avacopan is discontinued and whether there will be a high relapse rate, Dr. Jayne noted.
Overall, however, “we anticipate that with longer-term follow-up, this better eGFR recovery will have a [favorable] effect on kidney failure and potentially mortality risk in these patients,” he concluded.
Targeted therapy is good for patients and doctors
Expanding upon his comments regarding the new drug, Dr. Jennette said it implies “that the C5a receptor inhibitor was targeting an event that blocks injury more quickly and effectively than prednisone.”
“This may be because prednisone has more complex pharmacodynamics and less targeted effects than a C5a receptor inhibitor,” he said.
Overall, the findings bode well for a potentially beneficial therapy, he added. “We have entered a new era of more targeted therapies, for example, targeted B-cell therapy using an anti-CD20 antibody, and targeted complement-mediated injury therapy using C5a receptor inhibitor.”
“The validation of this targeted therapy to block complement-mediated autoimmune inflammatory injury is another advance toward targeted precision therapy versus empirical therapy. This will be good for the doctors and good for the patients,” Dr. Jennette concluded.
The study was funded by ChemoCentryx. Dr. Jayne has reported receiving grants and/or consulting for AstraZeneca, ChemoCentryx, GlaxoSmithKline, MiroBio, Vifor, and Roche/Genentech. Dr. Jennette has received funding from ChemoCentryx for preclinical validation studies of avacopan in a mouse model of ANCA glomerulonephritis.
A version of this article first appeared on Medscape.com.
Treatment of antineutrophil cytoplasmic autoantibody (ANCA)–associated vasculitis and renal disease with the oral C5a receptor inhibitor avacopan (Tavneos, ChemoCentryx) provides significant recovery of kidney function, compared with prednisone, particularly in patients with severe kidney disease, novel research indicates.
The new analysis underscores that “the real value of avacopan is that we can now expect to get our patients steroid free,” said first author David R.W. Jayne, MD, a professor of clinical autoimmunity at the University of Cambridge (England), when presenting the findings at the American Society of Nephrology’s Kidney Week 2021.
“Whether or not we’re brave enough to initiate treatment without steroids, I think that will perhaps come with some patient experience,” he added.
The findings are from a subanalysis of renal effects in the phase 3 ADVOCATE trial, which was published in February 2021 in the New England Journal of Medicine and included 330 patients with ANCA-associated vasculitis.
The trial in large part led to the U.S. approval of avacopan by the Food and Drug Administration in October as an adjunctive treatment for adults with severe active ANCA-associated vasculitis in combination with standard therapy including glucocorticoids.
The approval was greeted with enthusiasm as suggesting a much-needed option to help reduce, or even potentially eliminate, the need for glucocorticoids and their side effects. Other agents included in treatment regimens for ANCA-associated vasculitis include cyclophosphamide and rituximab.
Dr. Jayne emphasized that, before avacopan, treatment options had been limited.
“There is nothing else new in the clinic apart from rituximab, which we have now been using for almost 20 years,” he said in an interview. “Avacopan is new, the mode of action is different from any drugs in use at the moment, and the speed of action is very quick.”
The need to more closely investigate the trial’s renal outcomes in this new analysis was important because the high mortality rates in ANCA-associated vasculitis – a rare systemic autoimmune disease causing overactivation of complement resulting in inflammation of small blood vessels – is largely driven by those with MPO and PR3 autoantibody renal vasculitis, Dr. Jayne explained.
Commenting on the study, J. Charles Jennette, MD, a professor of pathology and laboratory medicine and professor of medicine at the University of North Carolina at Chapel Hill, said the new findings on renal outcomes, such as proteinuria, may offer key insights on avacopan’s efficacy.
“To me, the most impressive outcome of the ADVOCATE Phase 3 trial was the more rapid reduction in hematuria and proteinuria with avacopan compared to conventional prednisone therapy,” he said in an interview.
Recovery of eGFR with avacopan best in those with severe renal disease
In the trial, patients with ANCA-associated vasculitis were randomized 1:1 to treatment with oral avacopan 30 mg twice daily or oral prednisone on a tapering schedule.
All patients also received background immunosuppression – about two-thirds received rituximab and a third received cyclophosphamide – followed by azathioprine.
The main study results showed similar rates of remission in both groups at week 26 and a superior remission rate with avacopan, in terms of sustained remission, at week 52 (65.7% vs. 54.9%; P < .001).
Approximately 80% of patients in the trial had renal involvement of ANCA vasculitis, the focus of the new analysis, and they had a baseline mean estimated glomerular filtration rate (eGFR) of 45 mL/min per 1.73 m2.
Among those with renal involvement, patients treated with avacopan had a significantly greater eGFR recovery, compared with the prednisone group at week 26 (P = .046) and week 52 (P < .029).
The strongest improvements were observed among patients with moderate to severe kidney damage, who had a mean eGFR of 21 mL/min per 1.73 m2 at baseline. Among those patients, the mean increase in eGFR was 13.7 mL/min per 1.73 m2 in the avacopan-treated group (n = 52) versus 8.2 mL/min per 1.73 m2 in the prednisone group (n = 48; P < .01) by week 52.
Improvements in urinary albumin:creatinine ratios (UACR) of as much as 40% were also observed in the avacopan group within the first 4 weeks of treatment, while no changes were observed in the same period in the prednisone group.
In other findings, the study also showed more rapid declines in proteinuria within 4 weeks in the avacopan group, and fewer patients had hematuria and there were greater reductions in MCP-1 in avacopan-treated patients at week 52, Dr. Jayne reported.
In terms of safety, there were no differences between the groups, with trends of fewer deaths and severe adverse events in the avacopan group.
“We found that the improved recovery of eGFR with avacopan was accentuated among those with more severe renal disease,” Dr. Jayne said.
He noted that, while the study’s aim was for the avacopan group to be steroid free, the patients received brief, reduced doses of about a third of the normal oral steroid dose early in the trial. However, using a Glucocorticoid Toxicity Index, the authors found those in the avacopan group did have fewer glucocorticoid-related adverse events.
Future issues to be examined include what happens when avacopan is discontinued and whether there will be a high relapse rate, Dr. Jayne noted.
Overall, however, “we anticipate that with longer-term follow-up, this better eGFR recovery will have a [favorable] effect on kidney failure and potentially mortality risk in these patients,” he concluded.
Targeted therapy is good for patients and doctors
Expanding upon his comments regarding the new drug, Dr. Jennette said it implies “that the C5a receptor inhibitor was targeting an event that blocks injury more quickly and effectively than prednisone.”
“This may be because prednisone has more complex pharmacodynamics and less targeted effects than a C5a receptor inhibitor,” he said.
Overall, the findings bode well for a potentially beneficial therapy, he added. “We have entered a new era of more targeted therapies, for example, targeted B-cell therapy using an anti-CD20 antibody, and targeted complement-mediated injury therapy using C5a receptor inhibitor.”
“The validation of this targeted therapy to block complement-mediated autoimmune inflammatory injury is another advance toward targeted precision therapy versus empirical therapy. This will be good for the doctors and good for the patients,” Dr. Jennette concluded.
The study was funded by ChemoCentryx. Dr. Jayne has reported receiving grants and/or consulting for AstraZeneca, ChemoCentryx, GlaxoSmithKline, MiroBio, Vifor, and Roche/Genentech. Dr. Jennette has received funding from ChemoCentryx for preclinical validation studies of avacopan in a mouse model of ANCA glomerulonephritis.
A version of this article first appeared on Medscape.com.
FROM KIDNEY WEEK 2021
High triglycerides in normal-weight men with obstructive sleep apnea
, according to results of a study published in Nature and Science of Sleep (2021:13 1771-82).
Layla B. Guscoth, MD, of the South Australian Health and Medical Research Institute and Faculty of Health and Medical Sciences, University of Adelaide, Australia, and colleagues assessed unselected male community-dwelling participants in the Men Androgen Inflammation Lifestyle Environment and Stress (MAILES) and the Florey Adelaide Male Aging Study (FAMAS) studies.
They examined the association of OSA and nocturnal hypoxemia with serum lipid profiles, and suggested that the cardiometabolic risk profiles of healthy weight individuals with OSA require clinical attention, according to the researchers.
The partial or complete obstruction of upper airways found in the OSA syndrome results in intermittent hypoxia, accompanied variably by sleep fragmentation and daytime sleepiness. While the prevalence of moderate to severe OSA was 49.7% in the Swiss HypnoLaus cohort, it was 74.7% in men aged 40 or older (or having OSA syndrome according to ICD-3 criteria). Dr. Guscoth and colleagues point out, however, that OSA is frequently underdiagnosed or unrecognized in clinical settings, and that OSA has been implicated in development of cardiovascular conditions. Furthermore, the nocturnal hypoxemia resulting from OSA during rapid eye movement (REM) sleep is longitudinally associated with cardiovascular disease and its risk factors (hypertension, insulin resistance, metabolic syndrome and carotid atherosclerosis).
Study details
Prior research suggests that intermittent hypoxemia activates the sympathetic nervous system, increases oxidative stress and systemic inflammation, and that when chronic, reduces clearance of triglyceride-rich lipoproteins and inhibits adipose tissue lipoprotein lipase activity. To clarify inconsistent results in studies investigating potential OSA-dyslipidemia associations, and to confirm research suggesting an independent association with severe OSA (apnea-hypopnea index [AHI] ≥ 30/h), the authors conducted analyses stratified by waist circumference to observe an obesity-independent association between OSA metrics and dyslipidemia.
The investigators assessed 753 MAILES participants (mean age 60.8 years) who underwent full in-home polysomnography (Embletta X100). They looked at triglycerides, high- (HDL) and low-density lipoprotein (LDL), total cholesterol, associations between lipids and continuous measures of nocturnal hypoxemia (oxygen desaturation index [ODI], AHI, and REM-AHI), and adjusted for chronic conditions, risk behavior, and sociodemographic factors.
Mean waist circumference was 99.3 cm and OSA (AHI ≥ 10) prevalence was 52.6%. No significant associations were found between OSA metrics and lipid measures in an overall analysis, nor in a sensitivity analysis excluding lipid-lowering therapies.
In a covariate adjusted analysis stratified according to waist circumference (< 95 cm, 95-100 cm, > 100 cm) to minimize the contribution of obesity to hypertriglyceridemia, triglyceride levels were positively associated with AHI, ODI and REM-AHI in the participants with a waist circumference < 95 cm (P < .05), but not in participants with waist circumferences of 95-100 cm or > 100 cm.
Worse during REM
The authors observed also that OSA during REM sleep is marked by longer obstructive events with greater oxygen desaturations. Obstructive events during REM sleep, research has shown, may be more harmful than obstructive events during non-REM sleep with respect to hypertension, cardiovascular disease, and glycemic control in type 2 diabetes.
Looking at clinical categories of OSA, Dr. Guscoth and colleagues found that severe OSA was significantly associated with higher likelihood of triglyceride levels ≥ 1.7 mmol/L (odds ratio, 4.1, 95% confidence interval, 1.1-15.5, P = .039). Analysis according to waist circumference confirmed the relationship only among men with waist circumference < 95 cm.
Clinical concern
“We therefore suggest that with our data unstratified by weight circumference, metabolic derangements associated with insulin resistance induced by intermittent hypoxia due to OSA cannot be separated from the predominant effect of visceral obesity. When stratified by weight circumference, our data show that these derangements in triglycerides are observed only in lean participants where obesity does not have a dominant effect,” the researchers concluded.
“These findings of high prevalence of metabolic risk in lean patients with OSA, I find very worrying,” coauthor Sarah Appleton, PhD, Flinders Medical Center, Adelaide, Australia, said in an interview. She cited a study showing a 61% risk of dyslipidemia in lean patients with OSA (AHI > 5/hr, body mass index < 25 kg/m2, and waist < 80 cm in women, < 90 cm in men), and two of three metabolic syndrome components in 64%. “Annual fasting blood tests would identify metabolic problems such as elevated fasting glucose and triglyceride levels,” she noted.
This work was supported by a National Health and Medical Research Council of Australia Project Grant (627227), the Hospital Research Foundation and ResMed Foundation. There were no relevant conflicts reported.
, according to results of a study published in Nature and Science of Sleep (2021:13 1771-82).
Layla B. Guscoth, MD, of the South Australian Health and Medical Research Institute and Faculty of Health and Medical Sciences, University of Adelaide, Australia, and colleagues assessed unselected male community-dwelling participants in the Men Androgen Inflammation Lifestyle Environment and Stress (MAILES) and the Florey Adelaide Male Aging Study (FAMAS) studies.
They examined the association of OSA and nocturnal hypoxemia with serum lipid profiles, and suggested that the cardiometabolic risk profiles of healthy weight individuals with OSA require clinical attention, according to the researchers.
The partial or complete obstruction of upper airways found in the OSA syndrome results in intermittent hypoxia, accompanied variably by sleep fragmentation and daytime sleepiness. While the prevalence of moderate to severe OSA was 49.7% in the Swiss HypnoLaus cohort, it was 74.7% in men aged 40 or older (or having OSA syndrome according to ICD-3 criteria). Dr. Guscoth and colleagues point out, however, that OSA is frequently underdiagnosed or unrecognized in clinical settings, and that OSA has been implicated in development of cardiovascular conditions. Furthermore, the nocturnal hypoxemia resulting from OSA during rapid eye movement (REM) sleep is longitudinally associated with cardiovascular disease and its risk factors (hypertension, insulin resistance, metabolic syndrome and carotid atherosclerosis).
Study details
Prior research suggests that intermittent hypoxemia activates the sympathetic nervous system, increases oxidative stress and systemic inflammation, and that when chronic, reduces clearance of triglyceride-rich lipoproteins and inhibits adipose tissue lipoprotein lipase activity. To clarify inconsistent results in studies investigating potential OSA-dyslipidemia associations, and to confirm research suggesting an independent association with severe OSA (apnea-hypopnea index [AHI] ≥ 30/h), the authors conducted analyses stratified by waist circumference to observe an obesity-independent association between OSA metrics and dyslipidemia.
The investigators assessed 753 MAILES participants (mean age 60.8 years) who underwent full in-home polysomnography (Embletta X100). They looked at triglycerides, high- (HDL) and low-density lipoprotein (LDL), total cholesterol, associations between lipids and continuous measures of nocturnal hypoxemia (oxygen desaturation index [ODI], AHI, and REM-AHI), and adjusted for chronic conditions, risk behavior, and sociodemographic factors.
Mean waist circumference was 99.3 cm and OSA (AHI ≥ 10) prevalence was 52.6%. No significant associations were found between OSA metrics and lipid measures in an overall analysis, nor in a sensitivity analysis excluding lipid-lowering therapies.
In a covariate adjusted analysis stratified according to waist circumference (< 95 cm, 95-100 cm, > 100 cm) to minimize the contribution of obesity to hypertriglyceridemia, triglyceride levels were positively associated with AHI, ODI and REM-AHI in the participants with a waist circumference < 95 cm (P < .05), but not in participants with waist circumferences of 95-100 cm or > 100 cm.
Worse during REM
The authors observed also that OSA during REM sleep is marked by longer obstructive events with greater oxygen desaturations. Obstructive events during REM sleep, research has shown, may be more harmful than obstructive events during non-REM sleep with respect to hypertension, cardiovascular disease, and glycemic control in type 2 diabetes.
Looking at clinical categories of OSA, Dr. Guscoth and colleagues found that severe OSA was significantly associated with higher likelihood of triglyceride levels ≥ 1.7 mmol/L (odds ratio, 4.1, 95% confidence interval, 1.1-15.5, P = .039). Analysis according to waist circumference confirmed the relationship only among men with waist circumference < 95 cm.
Clinical concern
“We therefore suggest that with our data unstratified by weight circumference, metabolic derangements associated with insulin resistance induced by intermittent hypoxia due to OSA cannot be separated from the predominant effect of visceral obesity. When stratified by weight circumference, our data show that these derangements in triglycerides are observed only in lean participants where obesity does not have a dominant effect,” the researchers concluded.
“These findings of high prevalence of metabolic risk in lean patients with OSA, I find very worrying,” coauthor Sarah Appleton, PhD, Flinders Medical Center, Adelaide, Australia, said in an interview. She cited a study showing a 61% risk of dyslipidemia in lean patients with OSA (AHI > 5/hr, body mass index < 25 kg/m2, and waist < 80 cm in women, < 90 cm in men), and two of three metabolic syndrome components in 64%. “Annual fasting blood tests would identify metabolic problems such as elevated fasting glucose and triglyceride levels,” she noted.
This work was supported by a National Health and Medical Research Council of Australia Project Grant (627227), the Hospital Research Foundation and ResMed Foundation. There were no relevant conflicts reported.
, according to results of a study published in Nature and Science of Sleep (2021:13 1771-82).
Layla B. Guscoth, MD, of the South Australian Health and Medical Research Institute and Faculty of Health and Medical Sciences, University of Adelaide, Australia, and colleagues assessed unselected male community-dwelling participants in the Men Androgen Inflammation Lifestyle Environment and Stress (MAILES) and the Florey Adelaide Male Aging Study (FAMAS) studies.
They examined the association of OSA and nocturnal hypoxemia with serum lipid profiles, and suggested that the cardiometabolic risk profiles of healthy weight individuals with OSA require clinical attention, according to the researchers.
The partial or complete obstruction of upper airways found in the OSA syndrome results in intermittent hypoxia, accompanied variably by sleep fragmentation and daytime sleepiness. While the prevalence of moderate to severe OSA was 49.7% in the Swiss HypnoLaus cohort, it was 74.7% in men aged 40 or older (or having OSA syndrome according to ICD-3 criteria). Dr. Guscoth and colleagues point out, however, that OSA is frequently underdiagnosed or unrecognized in clinical settings, and that OSA has been implicated in development of cardiovascular conditions. Furthermore, the nocturnal hypoxemia resulting from OSA during rapid eye movement (REM) sleep is longitudinally associated with cardiovascular disease and its risk factors (hypertension, insulin resistance, metabolic syndrome and carotid atherosclerosis).
Study details
Prior research suggests that intermittent hypoxemia activates the sympathetic nervous system, increases oxidative stress and systemic inflammation, and that when chronic, reduces clearance of triglyceride-rich lipoproteins and inhibits adipose tissue lipoprotein lipase activity. To clarify inconsistent results in studies investigating potential OSA-dyslipidemia associations, and to confirm research suggesting an independent association with severe OSA (apnea-hypopnea index [AHI] ≥ 30/h), the authors conducted analyses stratified by waist circumference to observe an obesity-independent association between OSA metrics and dyslipidemia.
The investigators assessed 753 MAILES participants (mean age 60.8 years) who underwent full in-home polysomnography (Embletta X100). They looked at triglycerides, high- (HDL) and low-density lipoprotein (LDL), total cholesterol, associations between lipids and continuous measures of nocturnal hypoxemia (oxygen desaturation index [ODI], AHI, and REM-AHI), and adjusted for chronic conditions, risk behavior, and sociodemographic factors.
Mean waist circumference was 99.3 cm and OSA (AHI ≥ 10) prevalence was 52.6%. No significant associations were found between OSA metrics and lipid measures in an overall analysis, nor in a sensitivity analysis excluding lipid-lowering therapies.
In a covariate adjusted analysis stratified according to waist circumference (< 95 cm, 95-100 cm, > 100 cm) to minimize the contribution of obesity to hypertriglyceridemia, triglyceride levels were positively associated with AHI, ODI and REM-AHI in the participants with a waist circumference < 95 cm (P < .05), but not in participants with waist circumferences of 95-100 cm or > 100 cm.
Worse during REM
The authors observed also that OSA during REM sleep is marked by longer obstructive events with greater oxygen desaturations. Obstructive events during REM sleep, research has shown, may be more harmful than obstructive events during non-REM sleep with respect to hypertension, cardiovascular disease, and glycemic control in type 2 diabetes.
Looking at clinical categories of OSA, Dr. Guscoth and colleagues found that severe OSA was significantly associated with higher likelihood of triglyceride levels ≥ 1.7 mmol/L (odds ratio, 4.1, 95% confidence interval, 1.1-15.5, P = .039). Analysis according to waist circumference confirmed the relationship only among men with waist circumference < 95 cm.
Clinical concern
“We therefore suggest that with our data unstratified by weight circumference, metabolic derangements associated with insulin resistance induced by intermittent hypoxia due to OSA cannot be separated from the predominant effect of visceral obesity. When stratified by weight circumference, our data show that these derangements in triglycerides are observed only in lean participants where obesity does not have a dominant effect,” the researchers concluded.
“These findings of high prevalence of metabolic risk in lean patients with OSA, I find very worrying,” coauthor Sarah Appleton, PhD, Flinders Medical Center, Adelaide, Australia, said in an interview. She cited a study showing a 61% risk of dyslipidemia in lean patients with OSA (AHI > 5/hr, body mass index < 25 kg/m2, and waist < 80 cm in women, < 90 cm in men), and two of three metabolic syndrome components in 64%. “Annual fasting blood tests would identify metabolic problems such as elevated fasting glucose and triglyceride levels,” she noted.
This work was supported by a National Health and Medical Research Council of Australia Project Grant (627227), the Hospital Research Foundation and ResMed Foundation. There were no relevant conflicts reported.
FROM NATURE AND SCIENCE OF SLEEP
Early rhythm control in atrial fibrillation (EAST-AFNET trial)
Background: Despite advances in AFib management, up to 5% of patients will have a major complication each year. Current guidelines favor rate control based on prior studies that did not show mortality benefit with rhythm control. By expanding the rhythm strategy to include catheter ablation in early AFib, this trial re-examines if implementing rhythm control leads to improved clinical outcomes.
Study design: Prospective, open blinded randomized controlled trial.
Setting: 135 centers in 11 European countries.
Synopsis: Of patients with a new AFib diagnosis (less than 1 year, median 36 days), 2,789 were randomized 1:1 to rhythm control or usual care. Patients were 75 years old or older with prior CVA or 2 or fewer cardiovascular conditions. Both arms were continued on guideline-directed treatment, including rate control medications and anticoagulation. Rhythm control involved use of antiarrhythmics, catheter ablation (8% at enrollment, 20% by 5 years), or early cardioversion. Patients assigned to rhythm control had a lower risk for primary composite outcome of CV death, stroke, or hospitalization for worsening heart failure or acute coronary syndrome (HR, 0.79; 96% confidence interval, 0.66-0.94; P = .005) at 5 years, and the trial was stopped early for efficacy. Despite the 21% relative risk reduction, the absolute risk reduction was modest at 1.1 per 100 person-years. There were no significant differences in composite rate of all-cause mortality, although more adverse events occurred in the rhythm arm (4.9% vs. 1%). Overall rates of stroke and death were relatively low in both groups, underscoring the importance of continuing guideline-directed therapy. Hospital days were similar between the two groups, suggesting that rhythm control is not associated with higher cost burden. Limitations include its open-label design, loss of patients to follow-up (9% in control arm), and lack of generalizability to patients with long-standing AFib.
Bottom line: Early initiation of rhythm control therapy was associated with improved outcomes in patients with newly diagnosed AFib compared with usual care alone.
Citation: Kirchhof P et al. Early rhythm-control therapy in patients with atrial fibrillation. N Engl J Med. 2020 Aug 29;383:1305-1316. doi: 10.1056/NEJMoa2019422.
Dr. Korovaichuk is a hospitalist at Northwestern Memorial Hospital and assistant professor of medicine, Feinberg School of Medicine, both in Chicago.
Background: Despite advances in AFib management, up to 5% of patients will have a major complication each year. Current guidelines favor rate control based on prior studies that did not show mortality benefit with rhythm control. By expanding the rhythm strategy to include catheter ablation in early AFib, this trial re-examines if implementing rhythm control leads to improved clinical outcomes.
Study design: Prospective, open blinded randomized controlled trial.
Setting: 135 centers in 11 European countries.
Synopsis: Of patients with a new AFib diagnosis (less than 1 year, median 36 days), 2,789 were randomized 1:1 to rhythm control or usual care. Patients were 75 years old or older with prior CVA or 2 or fewer cardiovascular conditions. Both arms were continued on guideline-directed treatment, including rate control medications and anticoagulation. Rhythm control involved use of antiarrhythmics, catheter ablation (8% at enrollment, 20% by 5 years), or early cardioversion. Patients assigned to rhythm control had a lower risk for primary composite outcome of CV death, stroke, or hospitalization for worsening heart failure or acute coronary syndrome (HR, 0.79; 96% confidence interval, 0.66-0.94; P = .005) at 5 years, and the trial was stopped early for efficacy. Despite the 21% relative risk reduction, the absolute risk reduction was modest at 1.1 per 100 person-years. There were no significant differences in composite rate of all-cause mortality, although more adverse events occurred in the rhythm arm (4.9% vs. 1%). Overall rates of stroke and death were relatively low in both groups, underscoring the importance of continuing guideline-directed therapy. Hospital days were similar between the two groups, suggesting that rhythm control is not associated with higher cost burden. Limitations include its open-label design, loss of patients to follow-up (9% in control arm), and lack of generalizability to patients with long-standing AFib.
Bottom line: Early initiation of rhythm control therapy was associated with improved outcomes in patients with newly diagnosed AFib compared with usual care alone.
Citation: Kirchhof P et al. Early rhythm-control therapy in patients with atrial fibrillation. N Engl J Med. 2020 Aug 29;383:1305-1316. doi: 10.1056/NEJMoa2019422.
Dr. Korovaichuk is a hospitalist at Northwestern Memorial Hospital and assistant professor of medicine, Feinberg School of Medicine, both in Chicago.
Background: Despite advances in AFib management, up to 5% of patients will have a major complication each year. Current guidelines favor rate control based on prior studies that did not show mortality benefit with rhythm control. By expanding the rhythm strategy to include catheter ablation in early AFib, this trial re-examines if implementing rhythm control leads to improved clinical outcomes.
Study design: Prospective, open blinded randomized controlled trial.
Setting: 135 centers in 11 European countries.
Synopsis: Of patients with a new AFib diagnosis (less than 1 year, median 36 days), 2,789 were randomized 1:1 to rhythm control or usual care. Patients were 75 years old or older with prior CVA or 2 or fewer cardiovascular conditions. Both arms were continued on guideline-directed treatment, including rate control medications and anticoagulation. Rhythm control involved use of antiarrhythmics, catheter ablation (8% at enrollment, 20% by 5 years), or early cardioversion. Patients assigned to rhythm control had a lower risk for primary composite outcome of CV death, stroke, or hospitalization for worsening heart failure or acute coronary syndrome (HR, 0.79; 96% confidence interval, 0.66-0.94; P = .005) at 5 years, and the trial was stopped early for efficacy. Despite the 21% relative risk reduction, the absolute risk reduction was modest at 1.1 per 100 person-years. There were no significant differences in composite rate of all-cause mortality, although more adverse events occurred in the rhythm arm (4.9% vs. 1%). Overall rates of stroke and death were relatively low in both groups, underscoring the importance of continuing guideline-directed therapy. Hospital days were similar between the two groups, suggesting that rhythm control is not associated with higher cost burden. Limitations include its open-label design, loss of patients to follow-up (9% in control arm), and lack of generalizability to patients with long-standing AFib.
Bottom line: Early initiation of rhythm control therapy was associated with improved outcomes in patients with newly diagnosed AFib compared with usual care alone.
Citation: Kirchhof P et al. Early rhythm-control therapy in patients with atrial fibrillation. N Engl J Med. 2020 Aug 29;383:1305-1316. doi: 10.1056/NEJMoa2019422.
Dr. Korovaichuk is a hospitalist at Northwestern Memorial Hospital and assistant professor of medicine, Feinberg School of Medicine, both in Chicago.