User login
Controversial Alzheimer’s drug unlikely to get the OK in Europe
At its November meeting, , making it highly unlikely the drug will be recommended for approval at its December meeting.
In a news release issued Nov. 17, Biogen said the company received a “negative trend vote” on the aducanumab marketing authorization application in Europe.
“While we are disappointed with the trend vote, we strongly believe in the strength of our data and that aducanumab has the potential to make a positive and meaningful difference for people and families affected by Alzheimer’s disease [AD],” Priya Singhal, MD, MPH, head of global safety and regulatory sciences and interim head of research and development at Biogen, said in the release.
The EMA committee is expected to adopt a formal opinion on the marketing application at its December meeting (Dec. 13-16, 2021).
“Biogen will continue to engage with the EMA and CHMP as it considers next steps towards the goal of providing access to aducanumab to patients in Europe,” the company said.
At the recent Clinical Trials on Alzheimer’s Disease conference, Biogen announced new phase 3 findings that “provide further evidence of aducanumab’s effect on lowering amyloid beta plaque and downstream tau pathology, the two defining pathologies of Alzheimer’s disease,” the company said.
No clinically meaningful effect
In a statement from the nonprofit U.K. Science Media Centre, Prof. Robert Howard, from University College London, said the result of the CHMP vote “is absolutely the decision that we should have expected from the EMA’s expert advisory panel and is consistent with the FDA’s [U.S. Food and Drug Administration’s] Advisory Committee who voted unanimously 12 months ago against approval of aducanumab because of a lack of demonstrable efficacy in the pivotal phase 3 trials ENGAGE and EMERGE.”
“The FDA’s accelerated approval of aducanumab, solely on the grounds that it was reasonable to expect that reduction in amyloid would lead to improvement in the course of Alzheimer’s disease, despite all the evidence indicating no meaningful correlation between amyloid reduction and symptom improvement, has been highly controversial and has called into question the impartiality of the FDA and its staff,” Prof. Howard noted.
He anticipates that when the EMA panel meets in December they will not grant a license to aducanumab.
“Aducanumab is a treatment without convincing efficacy, with serious associated adverse effects and a high financial cost. On the basis of the available evidence and in the best interests of people with Alzheimer’s disease, their families and those who care for them, EMA and MHRA [Medicines and Healthcare products Regulatory Agency] should not approve a license for aducanumab,” Prof. Howard said.
Also weighing in, David Thomas, head of policy at Alzheimer’s Research UK, said the need for new AD treatments is “urgent,” but added that “it’s vital that regulators judge that any new treatment is safe and effective.”
“Results of aducanumab’s phase 3 trials, EMERGE and ENGAGE, have sparked much debate among the research community about how to judge the effectiveness of any new Alzheimer’s treatment,” Mr. Thomas noted.
“The FDA’s approval of aducanumab in the U.S. was based on the drug’s ability to clear the hallmark Alzheimer’s protein amyloid from the brain. As part of this approval the regulator now requires further trials to be carried out to ensure that aducanumab brings long-term improvement to people’s memory, thinking and day-to-day lives,” Mr. Thomas said.
EMA is now undertaking its own review of the data and “it’s important that we wait for the committee’s official recommendation, which is expected next month. In the meantime, we must continue to work at pace to ensure researchers are developing a broad pipeline of potential new treatments for diseases like Alzheimer’s, and that health systems like the NHS [National Health Service] will be ready to deliver them in the years ahead,” he added.
‘Reckless’ FDA decision
In related news, the Centers for Medicare and Medicaid Services (CMS) has announced that the Medicare Part B standard premium would rise to $170 per month for all enrollees, a 15% spike over the 2021 premium level.
“All Part B Medicare beneficiaries soon will be forced to bear significant financial burden as a direct result of the FDA’s reckless decision to approve aducanumab, a drug that has not been proven to provide any clinically meaningful benefit to Alzheimer’s patients but nevertheless carries an indefensible annual price tag set by Biogen at $56,000 per year for just the drug alone,” Michael Carome, MD, director of Public Citizen’s Health Research Group, said in a statement.
“To protect the many Medicare beneficiaries who cannot afford the unacceptable 15% jump in Part B premiums, CMS must promptly announce that it will exclude aducanumab from coverage under the Medicare program until there is definitive evidence that the drug provides substantial evidence of cognitive benefit to Alzheimer’s disease patients,” Dr. Carome said.
A version of this article first appeared on Medscape.com.
At its November meeting, , making it highly unlikely the drug will be recommended for approval at its December meeting.
In a news release issued Nov. 17, Biogen said the company received a “negative trend vote” on the aducanumab marketing authorization application in Europe.
“While we are disappointed with the trend vote, we strongly believe in the strength of our data and that aducanumab has the potential to make a positive and meaningful difference for people and families affected by Alzheimer’s disease [AD],” Priya Singhal, MD, MPH, head of global safety and regulatory sciences and interim head of research and development at Biogen, said in the release.
The EMA committee is expected to adopt a formal opinion on the marketing application at its December meeting (Dec. 13-16, 2021).
“Biogen will continue to engage with the EMA and CHMP as it considers next steps towards the goal of providing access to aducanumab to patients in Europe,” the company said.
At the recent Clinical Trials on Alzheimer’s Disease conference, Biogen announced new phase 3 findings that “provide further evidence of aducanumab’s effect on lowering amyloid beta plaque and downstream tau pathology, the two defining pathologies of Alzheimer’s disease,” the company said.
No clinically meaningful effect
In a statement from the nonprofit U.K. Science Media Centre, Prof. Robert Howard, from University College London, said the result of the CHMP vote “is absolutely the decision that we should have expected from the EMA’s expert advisory panel and is consistent with the FDA’s [U.S. Food and Drug Administration’s] Advisory Committee who voted unanimously 12 months ago against approval of aducanumab because of a lack of demonstrable efficacy in the pivotal phase 3 trials ENGAGE and EMERGE.”
“The FDA’s accelerated approval of aducanumab, solely on the grounds that it was reasonable to expect that reduction in amyloid would lead to improvement in the course of Alzheimer’s disease, despite all the evidence indicating no meaningful correlation between amyloid reduction and symptom improvement, has been highly controversial and has called into question the impartiality of the FDA and its staff,” Prof. Howard noted.
He anticipates that when the EMA panel meets in December they will not grant a license to aducanumab.
“Aducanumab is a treatment without convincing efficacy, with serious associated adverse effects and a high financial cost. On the basis of the available evidence and in the best interests of people with Alzheimer’s disease, their families and those who care for them, EMA and MHRA [Medicines and Healthcare products Regulatory Agency] should not approve a license for aducanumab,” Prof. Howard said.
Also weighing in, David Thomas, head of policy at Alzheimer’s Research UK, said the need for new AD treatments is “urgent,” but added that “it’s vital that regulators judge that any new treatment is safe and effective.”
“Results of aducanumab’s phase 3 trials, EMERGE and ENGAGE, have sparked much debate among the research community about how to judge the effectiveness of any new Alzheimer’s treatment,” Mr. Thomas noted.
“The FDA’s approval of aducanumab in the U.S. was based on the drug’s ability to clear the hallmark Alzheimer’s protein amyloid from the brain. As part of this approval the regulator now requires further trials to be carried out to ensure that aducanumab brings long-term improvement to people’s memory, thinking and day-to-day lives,” Mr. Thomas said.
EMA is now undertaking its own review of the data and “it’s important that we wait for the committee’s official recommendation, which is expected next month. In the meantime, we must continue to work at pace to ensure researchers are developing a broad pipeline of potential new treatments for diseases like Alzheimer’s, and that health systems like the NHS [National Health Service] will be ready to deliver them in the years ahead,” he added.
‘Reckless’ FDA decision
In related news, the Centers for Medicare and Medicaid Services (CMS) has announced that the Medicare Part B standard premium would rise to $170 per month for all enrollees, a 15% spike over the 2021 premium level.
“All Part B Medicare beneficiaries soon will be forced to bear significant financial burden as a direct result of the FDA’s reckless decision to approve aducanumab, a drug that has not been proven to provide any clinically meaningful benefit to Alzheimer’s patients but nevertheless carries an indefensible annual price tag set by Biogen at $56,000 per year for just the drug alone,” Michael Carome, MD, director of Public Citizen’s Health Research Group, said in a statement.
“To protect the many Medicare beneficiaries who cannot afford the unacceptable 15% jump in Part B premiums, CMS must promptly announce that it will exclude aducanumab from coverage under the Medicare program until there is definitive evidence that the drug provides substantial evidence of cognitive benefit to Alzheimer’s disease patients,” Dr. Carome said.
A version of this article first appeared on Medscape.com.
At its November meeting, , making it highly unlikely the drug will be recommended for approval at its December meeting.
In a news release issued Nov. 17, Biogen said the company received a “negative trend vote” on the aducanumab marketing authorization application in Europe.
“While we are disappointed with the trend vote, we strongly believe in the strength of our data and that aducanumab has the potential to make a positive and meaningful difference for people and families affected by Alzheimer’s disease [AD],” Priya Singhal, MD, MPH, head of global safety and regulatory sciences and interim head of research and development at Biogen, said in the release.
The EMA committee is expected to adopt a formal opinion on the marketing application at its December meeting (Dec. 13-16, 2021).
“Biogen will continue to engage with the EMA and CHMP as it considers next steps towards the goal of providing access to aducanumab to patients in Europe,” the company said.
At the recent Clinical Trials on Alzheimer’s Disease conference, Biogen announced new phase 3 findings that “provide further evidence of aducanumab’s effect on lowering amyloid beta plaque and downstream tau pathology, the two defining pathologies of Alzheimer’s disease,” the company said.
No clinically meaningful effect
In a statement from the nonprofit U.K. Science Media Centre, Prof. Robert Howard, from University College London, said the result of the CHMP vote “is absolutely the decision that we should have expected from the EMA’s expert advisory panel and is consistent with the FDA’s [U.S. Food and Drug Administration’s] Advisory Committee who voted unanimously 12 months ago against approval of aducanumab because of a lack of demonstrable efficacy in the pivotal phase 3 trials ENGAGE and EMERGE.”
“The FDA’s accelerated approval of aducanumab, solely on the grounds that it was reasonable to expect that reduction in amyloid would lead to improvement in the course of Alzheimer’s disease, despite all the evidence indicating no meaningful correlation between amyloid reduction and symptom improvement, has been highly controversial and has called into question the impartiality of the FDA and its staff,” Prof. Howard noted.
He anticipates that when the EMA panel meets in December they will not grant a license to aducanumab.
“Aducanumab is a treatment without convincing efficacy, with serious associated adverse effects and a high financial cost. On the basis of the available evidence and in the best interests of people with Alzheimer’s disease, their families and those who care for them, EMA and MHRA [Medicines and Healthcare products Regulatory Agency] should not approve a license for aducanumab,” Prof. Howard said.
Also weighing in, David Thomas, head of policy at Alzheimer’s Research UK, said the need for new AD treatments is “urgent,” but added that “it’s vital that regulators judge that any new treatment is safe and effective.”
“Results of aducanumab’s phase 3 trials, EMERGE and ENGAGE, have sparked much debate among the research community about how to judge the effectiveness of any new Alzheimer’s treatment,” Mr. Thomas noted.
“The FDA’s approval of aducanumab in the U.S. was based on the drug’s ability to clear the hallmark Alzheimer’s protein amyloid from the brain. As part of this approval the regulator now requires further trials to be carried out to ensure that aducanumab brings long-term improvement to people’s memory, thinking and day-to-day lives,” Mr. Thomas said.
EMA is now undertaking its own review of the data and “it’s important that we wait for the committee’s official recommendation, which is expected next month. In the meantime, we must continue to work at pace to ensure researchers are developing a broad pipeline of potential new treatments for diseases like Alzheimer’s, and that health systems like the NHS [National Health Service] will be ready to deliver them in the years ahead,” he added.
‘Reckless’ FDA decision
In related news, the Centers for Medicare and Medicaid Services (CMS) has announced that the Medicare Part B standard premium would rise to $170 per month for all enrollees, a 15% spike over the 2021 premium level.
“All Part B Medicare beneficiaries soon will be forced to bear significant financial burden as a direct result of the FDA’s reckless decision to approve aducanumab, a drug that has not been proven to provide any clinically meaningful benefit to Alzheimer’s patients but nevertheless carries an indefensible annual price tag set by Biogen at $56,000 per year for just the drug alone,” Michael Carome, MD, director of Public Citizen’s Health Research Group, said in a statement.
“To protect the many Medicare beneficiaries who cannot afford the unacceptable 15% jump in Part B premiums, CMS must promptly announce that it will exclude aducanumab from coverage under the Medicare program until there is definitive evidence that the drug provides substantial evidence of cognitive benefit to Alzheimer’s disease patients,” Dr. Carome said.
A version of this article first appeared on Medscape.com.
U.S. overdose deaths hit an all-time high
a 28.5% increase from the previous year.
Deaths in some states rose even more precipitously. Vermont saw an almost 70% increase, and drug overdose deaths in West Virginia increased by 62%. Many states, including Alabama, California, Kansas, Kentucky, Louisiana, Tennessee, and Washington, had a 45%-50% rise in overdose deaths.
The data released by the CDC was provisional, as there is generally a lag between a reported overdose and confirmation of the death to the National Vital Statistics System. The agency uses statistical models that render the counts almost 100% accurate, the CDC says.
The vast majority (73,757) of overdose deaths involved opioids – with most of those (62,338) involving synthetic opioids such as fentanyl. Federal officials said that one American died every 5 minutes from an overdose, or 265 a day.
“We have to acknowledge what this is – it is a crisis,” Department of Health & Human Services Secretary Xavier Becerra told reporters on a call.
“As much as the numbers speak so vividly, they don’t tell the whole story. We see it in the faces of grieving families and all those overworked caregivers. You hear it every time you get that panicked 911 phone call, you read it in obituaries of sons and daughters who left us way too soon,” Mr. Becerra said.
Rahul Gupta, MD, director of the White House Office of National Drug Control Policy, said that “this is unacceptable, and it requires an unprecedented response.”
Dr. Gupta, who noted that he has a waiver to treat substance use disorder patients with buprenorphine, said he’s seen “first-hand the heartbreak of the overdose epidemic,” adding that, with 23 years in practice, “I’ve learned that an overdose is a cry for help and for far too many people that cry goes unanswered.”
Both Mr. Becerra and Dr. Gupta called on Congress to pass President Joe Biden’s fiscal 2022 budget request, noting that it calls for $41 billion – a $669 million increase from fiscal year 2021 – to go to agencies working on drug interdiction and substance use prevention, treatment, and recovery support.
Dr. Gupta also announced that the administration was releasing a model law that could be used by state legislatures to help standardize policies on making the overdose antidote naloxone more accessible. Currently, such policies are a patchwork across the nation.
In addition, the federal government is newly supporting harm reduction, Mr. Becerra said. This means federal money can be used by clinics and outreach programs to buy fentanyl test strips, which they can then distribute to drug users.
“It’s important for Americans to have the ability to make sure that they can test for fentanyl in the substance,” Dr. Gupta said.
Fake pills, fentanyl a huge issue
Federal officials said that both fentanyl and methamphetamine are contributing to rising numbers of fatalities.
“Drug cartels in Mexico are mass-producing fentanyl and methamphetamine largely sourced from chemicals in China and they are distributing these substances throughout the United States,” Anne Milgram, administrator of the Drug Enforcement Administration, said on the call.
Ms. Milgram said the agency had seized 12,000 pounds of fentanyl in 2021, enough to provide every American with a lethal dose. Fentanyl is also mixed in with cocaine, heroin, methamphetamine, and marijuana – often in counterfeit pills, Ms. Milgram said.
The DEA and other law enforcement agencies have seized more than 14 million such pills in 2021. “These types of pills are easily accessible today on social media and e-commerce platforms, Ms. Milgram said.
“Drug dealers are now in our homes,” she said. “Wherever there is a smart phone or a computer, a dealer is one click away,” Ms. Milgram said.
National Institute on Drug Abuse Director Nora D. Volkow, MD, said that dealers will continue to push both fentanyl and methamphetamine because they are among the most addictive substances. They also are more profitable because they don’t require cultivation and harvesting, she said on the call.
Dr. Volkow also noted that naloxone is not as effective in reversing fentanyl overdoses because fentanyl is more potent than heroin and other opioids, and “it gets into the brain extremely rapidly.”
Ongoing research is aimed at developing a faster delivery mechanism and a longer-lasting formulation to counter overdoses, Dr. Volkow said.
A version of this article first appeared on Medscape.com.
a 28.5% increase from the previous year.
Deaths in some states rose even more precipitously. Vermont saw an almost 70% increase, and drug overdose deaths in West Virginia increased by 62%. Many states, including Alabama, California, Kansas, Kentucky, Louisiana, Tennessee, and Washington, had a 45%-50% rise in overdose deaths.
The data released by the CDC was provisional, as there is generally a lag between a reported overdose and confirmation of the death to the National Vital Statistics System. The agency uses statistical models that render the counts almost 100% accurate, the CDC says.
The vast majority (73,757) of overdose deaths involved opioids – with most of those (62,338) involving synthetic opioids such as fentanyl. Federal officials said that one American died every 5 minutes from an overdose, or 265 a day.
“We have to acknowledge what this is – it is a crisis,” Department of Health & Human Services Secretary Xavier Becerra told reporters on a call.
“As much as the numbers speak so vividly, they don’t tell the whole story. We see it in the faces of grieving families and all those overworked caregivers. You hear it every time you get that panicked 911 phone call, you read it in obituaries of sons and daughters who left us way too soon,” Mr. Becerra said.
Rahul Gupta, MD, director of the White House Office of National Drug Control Policy, said that “this is unacceptable, and it requires an unprecedented response.”
Dr. Gupta, who noted that he has a waiver to treat substance use disorder patients with buprenorphine, said he’s seen “first-hand the heartbreak of the overdose epidemic,” adding that, with 23 years in practice, “I’ve learned that an overdose is a cry for help and for far too many people that cry goes unanswered.”
Both Mr. Becerra and Dr. Gupta called on Congress to pass President Joe Biden’s fiscal 2022 budget request, noting that it calls for $41 billion – a $669 million increase from fiscal year 2021 – to go to agencies working on drug interdiction and substance use prevention, treatment, and recovery support.
Dr. Gupta also announced that the administration was releasing a model law that could be used by state legislatures to help standardize policies on making the overdose antidote naloxone more accessible. Currently, such policies are a patchwork across the nation.
In addition, the federal government is newly supporting harm reduction, Mr. Becerra said. This means federal money can be used by clinics and outreach programs to buy fentanyl test strips, which they can then distribute to drug users.
“It’s important for Americans to have the ability to make sure that they can test for fentanyl in the substance,” Dr. Gupta said.
Fake pills, fentanyl a huge issue
Federal officials said that both fentanyl and methamphetamine are contributing to rising numbers of fatalities.
“Drug cartels in Mexico are mass-producing fentanyl and methamphetamine largely sourced from chemicals in China and they are distributing these substances throughout the United States,” Anne Milgram, administrator of the Drug Enforcement Administration, said on the call.
Ms. Milgram said the agency had seized 12,000 pounds of fentanyl in 2021, enough to provide every American with a lethal dose. Fentanyl is also mixed in with cocaine, heroin, methamphetamine, and marijuana – often in counterfeit pills, Ms. Milgram said.
The DEA and other law enforcement agencies have seized more than 14 million such pills in 2021. “These types of pills are easily accessible today on social media and e-commerce platforms, Ms. Milgram said.
“Drug dealers are now in our homes,” she said. “Wherever there is a smart phone or a computer, a dealer is one click away,” Ms. Milgram said.
National Institute on Drug Abuse Director Nora D. Volkow, MD, said that dealers will continue to push both fentanyl and methamphetamine because they are among the most addictive substances. They also are more profitable because they don’t require cultivation and harvesting, she said on the call.
Dr. Volkow also noted that naloxone is not as effective in reversing fentanyl overdoses because fentanyl is more potent than heroin and other opioids, and “it gets into the brain extremely rapidly.”
Ongoing research is aimed at developing a faster delivery mechanism and a longer-lasting formulation to counter overdoses, Dr. Volkow said.
A version of this article first appeared on Medscape.com.
a 28.5% increase from the previous year.
Deaths in some states rose even more precipitously. Vermont saw an almost 70% increase, and drug overdose deaths in West Virginia increased by 62%. Many states, including Alabama, California, Kansas, Kentucky, Louisiana, Tennessee, and Washington, had a 45%-50% rise in overdose deaths.
The data released by the CDC was provisional, as there is generally a lag between a reported overdose and confirmation of the death to the National Vital Statistics System. The agency uses statistical models that render the counts almost 100% accurate, the CDC says.
The vast majority (73,757) of overdose deaths involved opioids – with most of those (62,338) involving synthetic opioids such as fentanyl. Federal officials said that one American died every 5 minutes from an overdose, or 265 a day.
“We have to acknowledge what this is – it is a crisis,” Department of Health & Human Services Secretary Xavier Becerra told reporters on a call.
“As much as the numbers speak so vividly, they don’t tell the whole story. We see it in the faces of grieving families and all those overworked caregivers. You hear it every time you get that panicked 911 phone call, you read it in obituaries of sons and daughters who left us way too soon,” Mr. Becerra said.
Rahul Gupta, MD, director of the White House Office of National Drug Control Policy, said that “this is unacceptable, and it requires an unprecedented response.”
Dr. Gupta, who noted that he has a waiver to treat substance use disorder patients with buprenorphine, said he’s seen “first-hand the heartbreak of the overdose epidemic,” adding that, with 23 years in practice, “I’ve learned that an overdose is a cry for help and for far too many people that cry goes unanswered.”
Both Mr. Becerra and Dr. Gupta called on Congress to pass President Joe Biden’s fiscal 2022 budget request, noting that it calls for $41 billion – a $669 million increase from fiscal year 2021 – to go to agencies working on drug interdiction and substance use prevention, treatment, and recovery support.
Dr. Gupta also announced that the administration was releasing a model law that could be used by state legislatures to help standardize policies on making the overdose antidote naloxone more accessible. Currently, such policies are a patchwork across the nation.
In addition, the federal government is newly supporting harm reduction, Mr. Becerra said. This means federal money can be used by clinics and outreach programs to buy fentanyl test strips, which they can then distribute to drug users.
“It’s important for Americans to have the ability to make sure that they can test for fentanyl in the substance,” Dr. Gupta said.
Fake pills, fentanyl a huge issue
Federal officials said that both fentanyl and methamphetamine are contributing to rising numbers of fatalities.
“Drug cartels in Mexico are mass-producing fentanyl and methamphetamine largely sourced from chemicals in China and they are distributing these substances throughout the United States,” Anne Milgram, administrator of the Drug Enforcement Administration, said on the call.
Ms. Milgram said the agency had seized 12,000 pounds of fentanyl in 2021, enough to provide every American with a lethal dose. Fentanyl is also mixed in with cocaine, heroin, methamphetamine, and marijuana – often in counterfeit pills, Ms. Milgram said.
The DEA and other law enforcement agencies have seized more than 14 million such pills in 2021. “These types of pills are easily accessible today on social media and e-commerce platforms, Ms. Milgram said.
“Drug dealers are now in our homes,” she said. “Wherever there is a smart phone or a computer, a dealer is one click away,” Ms. Milgram said.
National Institute on Drug Abuse Director Nora D. Volkow, MD, said that dealers will continue to push both fentanyl and methamphetamine because they are among the most addictive substances. They also are more profitable because they don’t require cultivation and harvesting, she said on the call.
Dr. Volkow also noted that naloxone is not as effective in reversing fentanyl overdoses because fentanyl is more potent than heroin and other opioids, and “it gets into the brain extremely rapidly.”
Ongoing research is aimed at developing a faster delivery mechanism and a longer-lasting formulation to counter overdoses, Dr. Volkow said.
A version of this article first appeared on Medscape.com.
Timing of endoscopy for acute upper GI bleeding
Background: Prior studies have failed to show a benefit to earlier endoscopic intervention in acute GI bleeding. However, those studies were performed in all-comers without attention to the varying risk within the patient population.
Study design: Randomized controlled trial.
Setting: Single center in Hong Kong.
Synopsis: Patients at high risk for further bleeding or death by clinical score were randomized to endoscopy within 6 hours (“urgent endoscopy”), vs. the following day (“early endoscopy”), of GI consultation. Those who required immediate endoscopic intervention because of hemodynamic instability were excluded. All were prescribed proton-pump inhibitor drip, with the addition of vasoactive drugs and antibiotics if there was a suspected variceal bleed. There was no difference in 30-day mortality between the two groups – 8.9% with urgent endoscopy and 6.6% with early endoscopy (HR, 1.35; 95% CI, 0.72-2.54). There was no difference in length of hospital stay or the number of transfusions. Earlier endoscopy within 6 hours was associated with a higher number of actively bleeding lesions requiring intervention and a nonstatistical increase in recurrent bleeding within 30 days. It is believed that more time on proton-pump inhibitor infusion prior to endoscopy allows for stabilization of bleeds, thus requiring less intervention when endoscopy does occur.
Bottom line: Early endoscopy within 6 hours was not beneficial for those at high risk for rebleeding and death from upper GI bleed.
Citation: Lau JYW et al. Timing of endoscopy for acute upper gastrointestinal bleeding. N Engl J Med. 2020;382:1299-308. doi:10.1056/NEJMoa1912484.
Dr. Lee is a hospitalist at Northwestern Memorial Hospital and Lurie Children’s Hospital and assistant professor of medicine, Feinberg School of Medicine, all in Chicago.
Background: Prior studies have failed to show a benefit to earlier endoscopic intervention in acute GI bleeding. However, those studies were performed in all-comers without attention to the varying risk within the patient population.
Study design: Randomized controlled trial.
Setting: Single center in Hong Kong.
Synopsis: Patients at high risk for further bleeding or death by clinical score were randomized to endoscopy within 6 hours (“urgent endoscopy”), vs. the following day (“early endoscopy”), of GI consultation. Those who required immediate endoscopic intervention because of hemodynamic instability were excluded. All were prescribed proton-pump inhibitor drip, with the addition of vasoactive drugs and antibiotics if there was a suspected variceal bleed. There was no difference in 30-day mortality between the two groups – 8.9% with urgent endoscopy and 6.6% with early endoscopy (HR, 1.35; 95% CI, 0.72-2.54). There was no difference in length of hospital stay or the number of transfusions. Earlier endoscopy within 6 hours was associated with a higher number of actively bleeding lesions requiring intervention and a nonstatistical increase in recurrent bleeding within 30 days. It is believed that more time on proton-pump inhibitor infusion prior to endoscopy allows for stabilization of bleeds, thus requiring less intervention when endoscopy does occur.
Bottom line: Early endoscopy within 6 hours was not beneficial for those at high risk for rebleeding and death from upper GI bleed.
Citation: Lau JYW et al. Timing of endoscopy for acute upper gastrointestinal bleeding. N Engl J Med. 2020;382:1299-308. doi:10.1056/NEJMoa1912484.
Dr. Lee is a hospitalist at Northwestern Memorial Hospital and Lurie Children’s Hospital and assistant professor of medicine, Feinberg School of Medicine, all in Chicago.
Background: Prior studies have failed to show a benefit to earlier endoscopic intervention in acute GI bleeding. However, those studies were performed in all-comers without attention to the varying risk within the patient population.
Study design: Randomized controlled trial.
Setting: Single center in Hong Kong.
Synopsis: Patients at high risk for further bleeding or death by clinical score were randomized to endoscopy within 6 hours (“urgent endoscopy”), vs. the following day (“early endoscopy”), of GI consultation. Those who required immediate endoscopic intervention because of hemodynamic instability were excluded. All were prescribed proton-pump inhibitor drip, with the addition of vasoactive drugs and antibiotics if there was a suspected variceal bleed. There was no difference in 30-day mortality between the two groups – 8.9% with urgent endoscopy and 6.6% with early endoscopy (HR, 1.35; 95% CI, 0.72-2.54). There was no difference in length of hospital stay or the number of transfusions. Earlier endoscopy within 6 hours was associated with a higher number of actively bleeding lesions requiring intervention and a nonstatistical increase in recurrent bleeding within 30 days. It is believed that more time on proton-pump inhibitor infusion prior to endoscopy allows for stabilization of bleeds, thus requiring less intervention when endoscopy does occur.
Bottom line: Early endoscopy within 6 hours was not beneficial for those at high risk for rebleeding and death from upper GI bleed.
Citation: Lau JYW et al. Timing of endoscopy for acute upper gastrointestinal bleeding. N Engl J Med. 2020;382:1299-308. doi:10.1056/NEJMoa1912484.
Dr. Lee is a hospitalist at Northwestern Memorial Hospital and Lurie Children’s Hospital and assistant professor of medicine, Feinberg School of Medicine, all in Chicago.
Retiform Purpura on the Buttocks in 6 Critically Ill COVID-19 Patients
To the Editor:
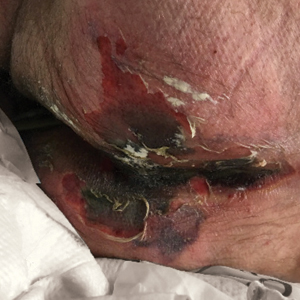
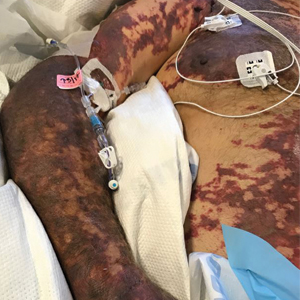
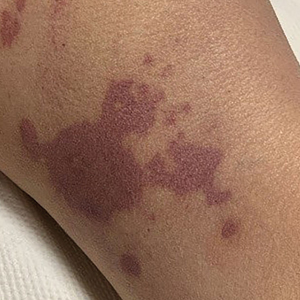
There is emerging evidence of skin findings in patients with COVID-19, including perniolike changes of the toes as well as urticarial and vesicular eruptions.1 Magro et al2 reported 3 cases of livedoid and purpuric skin eruptions in critically ill COVID-19 patients with evidence of thrombotic vasculopathy on skin biopsy, including a 32-year-old man with striking buttocks retiform purpura. Histopathologic analysis revealed thrombotic vasculopathy and pressure-induced ischemic necrosis. Since that patient was first evaluated (March 2020), we identified 6 more cases of critically ill COVID-19 patients from a single academic hospital in New York City with essentially identical clinical findings. Herein, we report those 6 cases of critically ill and intubated patients with COVID-19 who developed retiform purpura on the buttocks only, approximately 11 to 21 days after onset of COVID-19 symptoms.
We provided consultation for 5 men and 1 woman (age range, 42–78 years) who were critically ill with COVID-19 and developed retiform purpura on the buttocks (Figures 1 and 2). All had an elevated D-dimer concentration: 2 patients, >700 ng/mL; 2 patients, >2000 ng/mL; 2 patients, >6000 ng/mL (reference, 229 ng/mL). Three patients experienced a peak D-dimer concentration on the day retiform purpura was reported.
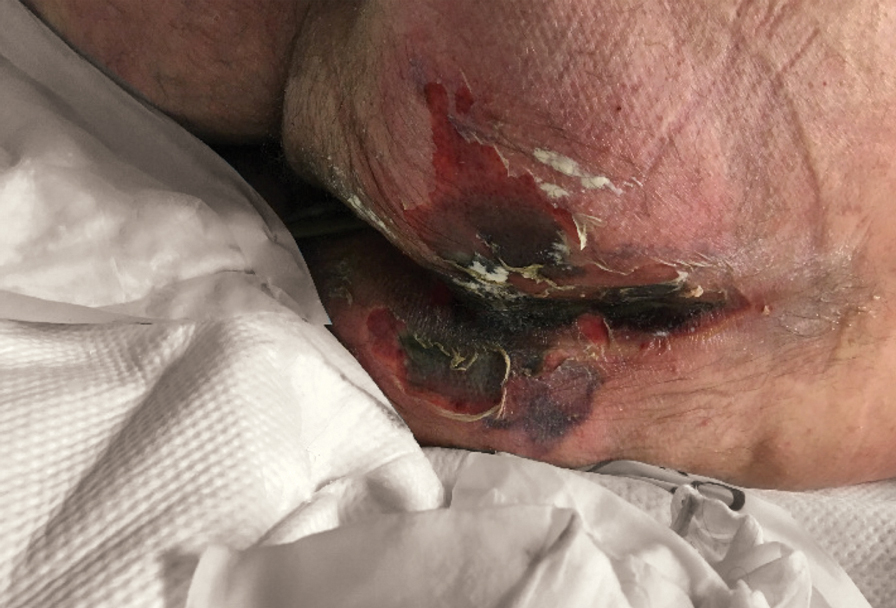
Further evidence of coagulopathy in these patients included 1 patient with a newly diagnosed left popliteal deep vein thrombosis and 1 patient with a known history of protein C deficiency and deep vein thromboses. Five patients were receiving anticoagulation on the day the skin changes were documented; anticoagulation was contraindicated in the sixth patient because of oropharyngeal bleeding. Anticoagulation was continued at the treatment dosage (enoxaparin 80 mg twice daily) in 3 patients, and in 2 patients receiving a prophylactic dose (enoxaparin 40 mg daily), anticoagulation was escalated to treatment dose due to rising D-dimer levels and newly diagnosed retiform purpura. Skin biopsy was deferred for all patients due to positional and ventilatory restrictions. At that point in their care, 3 patients remained admitted on medicine floors, 2 were in the intensive care unit, and 1 had died.
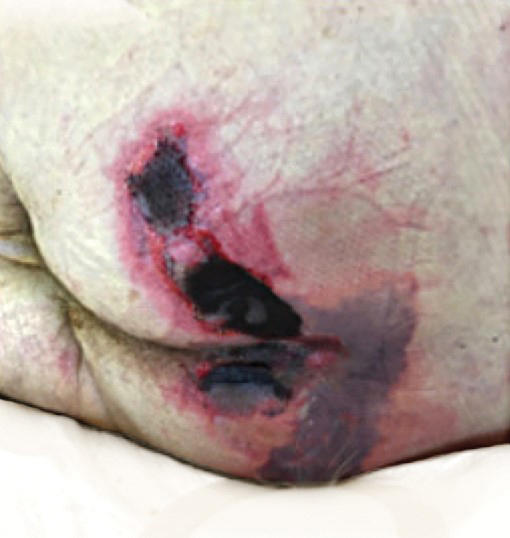
Although the differential diagnosis for retiform purpura is broad and should be fully considered in any patient with this finding, based on the elevated D-dimer concentration, critical illness secondary to COVID-19, and striking similarity to earlier reported case of buttocks retiform purpura with thrombotic vasculopathy and pressure injury noted histopathologically,2 we suspect the buttocks retiform purpura in our 6 cases also represent a combination of cutaneous thrombosis and pressure injury. In addition to acral livedoid eruptions (also reported by Magro and colleagues2), we suspect that this cutaneous manifestation might be associated with a hypercoagulable state in some patients, especially in the setting of a rising D-dimer concentration. One study found that 31% of 184 patients with severe COVID-19 had thrombotic complications,3 a clinical picture that portends a poor prognosis.4
COVID-19 patients presenting with retiform purpura should be fully evaluated based on the broad differential for this morphology. We present 6 cases of buttocks retiform purpura in critically ill COVID-19 patients—all with strikingly similar morphologic findings, an elevated D-dimer concentration, and critical illness due to COVID-19—to alert clinicians to this constellation of findings and propose that this cutaneous manifestation could indicate an associated hypercoaguable state and should prompt a hematology consultation. Additionally, biopsy of this skin finding should be considered, especially if biopsy results might serve to guide management; however, obtaining a biopsy specimen can be technically difficult because of ventilatory requirements.
Given the magnitude of the COVID-19 pandemic and the propensity of these patients to experience thrombotic events, recognition of this skin finding in COVID-19 is important and might allow timely intervention.
- Recalcati S. Cutaneous manifestations in COVID-19: a first perspective. J Eur Acad Dermatol Venereol. 2020;34:e212-e213. doi:10.1111/jdv.16387
- Magro C, Mulvey JJ, Berlin D, et al. Complement associated microvascular injury and thrombosis in the pathogenesis of severe COVID-19 infection: a report of five cases. Transl Res. 2020;220:1-13. doi:10.1016/j.trsl.2020.04.007
- Klok FA, Kruip MJHA, van der Meer NJM, et al. Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Thromb Res. 2020;191:145-147. doi:10.1016/j.thromres.2020.04.013
- Tang N, Li D, Wang X, et al. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J Thromb Haemost. 2020;18:844-847. doi:10.1111/jth.14768
To the Editor:
There is emerging evidence of skin findings in patients with COVID-19, including perniolike changes of the toes as well as urticarial and vesicular eruptions.1 Magro et al2 reported 3 cases of livedoid and purpuric skin eruptions in critically ill COVID-19 patients with evidence of thrombotic vasculopathy on skin biopsy, including a 32-year-old man with striking buttocks retiform purpura. Histopathologic analysis revealed thrombotic vasculopathy and pressure-induced ischemic necrosis. Since that patient was first evaluated (March 2020), we identified 6 more cases of critically ill COVID-19 patients from a single academic hospital in New York City with essentially identical clinical findings. Herein, we report those 6 cases of critically ill and intubated patients with COVID-19 who developed retiform purpura on the buttocks only, approximately 11 to 21 days after onset of COVID-19 symptoms.
We provided consultation for 5 men and 1 woman (age range, 42–78 years) who were critically ill with COVID-19 and developed retiform purpura on the buttocks (Figures 1 and 2). All had an elevated D-dimer concentration: 2 patients, >700 ng/mL; 2 patients, >2000 ng/mL; 2 patients, >6000 ng/mL (reference, 229 ng/mL). Three patients experienced a peak D-dimer concentration on the day retiform purpura was reported.

Further evidence of coagulopathy in these patients included 1 patient with a newly diagnosed left popliteal deep vein thrombosis and 1 patient with a known history of protein C deficiency and deep vein thromboses. Five patients were receiving anticoagulation on the day the skin changes were documented; anticoagulation was contraindicated in the sixth patient because of oropharyngeal bleeding. Anticoagulation was continued at the treatment dosage (enoxaparin 80 mg twice daily) in 3 patients, and in 2 patients receiving a prophylactic dose (enoxaparin 40 mg daily), anticoagulation was escalated to treatment dose due to rising D-dimer levels and newly diagnosed retiform purpura. Skin biopsy was deferred for all patients due to positional and ventilatory restrictions. At that point in their care, 3 patients remained admitted on medicine floors, 2 were in the intensive care unit, and 1 had died.

Although the differential diagnosis for retiform purpura is broad and should be fully considered in any patient with this finding, based on the elevated D-dimer concentration, critical illness secondary to COVID-19, and striking similarity to earlier reported case of buttocks retiform purpura with thrombotic vasculopathy and pressure injury noted histopathologically,2 we suspect the buttocks retiform purpura in our 6 cases also represent a combination of cutaneous thrombosis and pressure injury. In addition to acral livedoid eruptions (also reported by Magro and colleagues2), we suspect that this cutaneous manifestation might be associated with a hypercoagulable state in some patients, especially in the setting of a rising D-dimer concentration. One study found that 31% of 184 patients with severe COVID-19 had thrombotic complications,3 a clinical picture that portends a poor prognosis.4
COVID-19 patients presenting with retiform purpura should be fully evaluated based on the broad differential for this morphology. We present 6 cases of buttocks retiform purpura in critically ill COVID-19 patients—all with strikingly similar morphologic findings, an elevated D-dimer concentration, and critical illness due to COVID-19—to alert clinicians to this constellation of findings and propose that this cutaneous manifestation could indicate an associated hypercoaguable state and should prompt a hematology consultation. Additionally, biopsy of this skin finding should be considered, especially if biopsy results might serve to guide management; however, obtaining a biopsy specimen can be technically difficult because of ventilatory requirements.
Given the magnitude of the COVID-19 pandemic and the propensity of these patients to experience thrombotic events, recognition of this skin finding in COVID-19 is important and might allow timely intervention.
To the Editor:
There is emerging evidence of skin findings in patients with COVID-19, including perniolike changes of the toes as well as urticarial and vesicular eruptions.1 Magro et al2 reported 3 cases of livedoid and purpuric skin eruptions in critically ill COVID-19 patients with evidence of thrombotic vasculopathy on skin biopsy, including a 32-year-old man with striking buttocks retiform purpura. Histopathologic analysis revealed thrombotic vasculopathy and pressure-induced ischemic necrosis. Since that patient was first evaluated (March 2020), we identified 6 more cases of critically ill COVID-19 patients from a single academic hospital in New York City with essentially identical clinical findings. Herein, we report those 6 cases of critically ill and intubated patients with COVID-19 who developed retiform purpura on the buttocks only, approximately 11 to 21 days after onset of COVID-19 symptoms.
We provided consultation for 5 men and 1 woman (age range, 42–78 years) who were critically ill with COVID-19 and developed retiform purpura on the buttocks (Figures 1 and 2). All had an elevated D-dimer concentration: 2 patients, >700 ng/mL; 2 patients, >2000 ng/mL; 2 patients, >6000 ng/mL (reference, 229 ng/mL). Three patients experienced a peak D-dimer concentration on the day retiform purpura was reported.

Further evidence of coagulopathy in these patients included 1 patient with a newly diagnosed left popliteal deep vein thrombosis and 1 patient with a known history of protein C deficiency and deep vein thromboses. Five patients were receiving anticoagulation on the day the skin changes were documented; anticoagulation was contraindicated in the sixth patient because of oropharyngeal bleeding. Anticoagulation was continued at the treatment dosage (enoxaparin 80 mg twice daily) in 3 patients, and in 2 patients receiving a prophylactic dose (enoxaparin 40 mg daily), anticoagulation was escalated to treatment dose due to rising D-dimer levels and newly diagnosed retiform purpura. Skin biopsy was deferred for all patients due to positional and ventilatory restrictions. At that point in their care, 3 patients remained admitted on medicine floors, 2 were in the intensive care unit, and 1 had died.

Although the differential diagnosis for retiform purpura is broad and should be fully considered in any patient with this finding, based on the elevated D-dimer concentration, critical illness secondary to COVID-19, and striking similarity to earlier reported case of buttocks retiform purpura with thrombotic vasculopathy and pressure injury noted histopathologically,2 we suspect the buttocks retiform purpura in our 6 cases also represent a combination of cutaneous thrombosis and pressure injury. In addition to acral livedoid eruptions (also reported by Magro and colleagues2), we suspect that this cutaneous manifestation might be associated with a hypercoagulable state in some patients, especially in the setting of a rising D-dimer concentration. One study found that 31% of 184 patients with severe COVID-19 had thrombotic complications,3 a clinical picture that portends a poor prognosis.4
COVID-19 patients presenting with retiform purpura should be fully evaluated based on the broad differential for this morphology. We present 6 cases of buttocks retiform purpura in critically ill COVID-19 patients—all with strikingly similar morphologic findings, an elevated D-dimer concentration, and critical illness due to COVID-19—to alert clinicians to this constellation of findings and propose that this cutaneous manifestation could indicate an associated hypercoaguable state and should prompt a hematology consultation. Additionally, biopsy of this skin finding should be considered, especially if biopsy results might serve to guide management; however, obtaining a biopsy specimen can be technically difficult because of ventilatory requirements.
Given the magnitude of the COVID-19 pandemic and the propensity of these patients to experience thrombotic events, recognition of this skin finding in COVID-19 is important and might allow timely intervention.
- Recalcati S. Cutaneous manifestations in COVID-19: a first perspective. J Eur Acad Dermatol Venereol. 2020;34:e212-e213. doi:10.1111/jdv.16387
- Magro C, Mulvey JJ, Berlin D, et al. Complement associated microvascular injury and thrombosis in the pathogenesis of severe COVID-19 infection: a report of five cases. Transl Res. 2020;220:1-13. doi:10.1016/j.trsl.2020.04.007
- Klok FA, Kruip MJHA, van der Meer NJM, et al. Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Thromb Res. 2020;191:145-147. doi:10.1016/j.thromres.2020.04.013
- Tang N, Li D, Wang X, et al. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J Thromb Haemost. 2020;18:844-847. doi:10.1111/jth.14768
- Recalcati S. Cutaneous manifestations in COVID-19: a first perspective. J Eur Acad Dermatol Venereol. 2020;34:e212-e213. doi:10.1111/jdv.16387
- Magro C, Mulvey JJ, Berlin D, et al. Complement associated microvascular injury and thrombosis in the pathogenesis of severe COVID-19 infection: a report of five cases. Transl Res. 2020;220:1-13. doi:10.1016/j.trsl.2020.04.007
- Klok FA, Kruip MJHA, van der Meer NJM, et al. Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Thromb Res. 2020;191:145-147. doi:10.1016/j.thromres.2020.04.013
- Tang N, Li D, Wang X, et al. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J Thromb Haemost. 2020;18:844-847. doi:10.1111/jth.14768
Practice Points
- Retiform purpura in a severely ill patient with COVID-19 and a markedly elevated D-dimer concentration might be a cutaneous sign of systemic coagulopathy.
- This constellation of findings should prompt consideration of skin biopsy and hematology consultation.
Purpura Fulminans in an Asplenic Intravenous Drug User
To the Editor:
A 56-year-old man with a history of opioid abuse and splenectomy decades prior due to a motor vehicle accident was brought to an outside emergency department with confusion, slurred speech, and difficulty breathing. Over the next few days, he became febrile and hypotensive, requiring vasopressors. Clinical laboratory testing revealed a urine drug screen positive for opioids and a low platelet count in the setting of a rapidly evolving retiform purpuric rash.
The patient was transferred to our institution 6 days after initial presentation with primary diagnoses of septic shock with multiorgan failure and disseminated intravascular coagulation (DIC). Blood cultures were positive for gram-negative rods. After several days of broad-spectrum antibiotics and supportive care, cultures were reported as positive for Capnocytophaga canimorsus. Upon further questioning, the patient’s wife reported that the couple had a new puppy and that the patient often allowed the dog to bite him playfully and lick abrasions on his hands and legs. He had not received medical treatment for any of the dog’s bites.
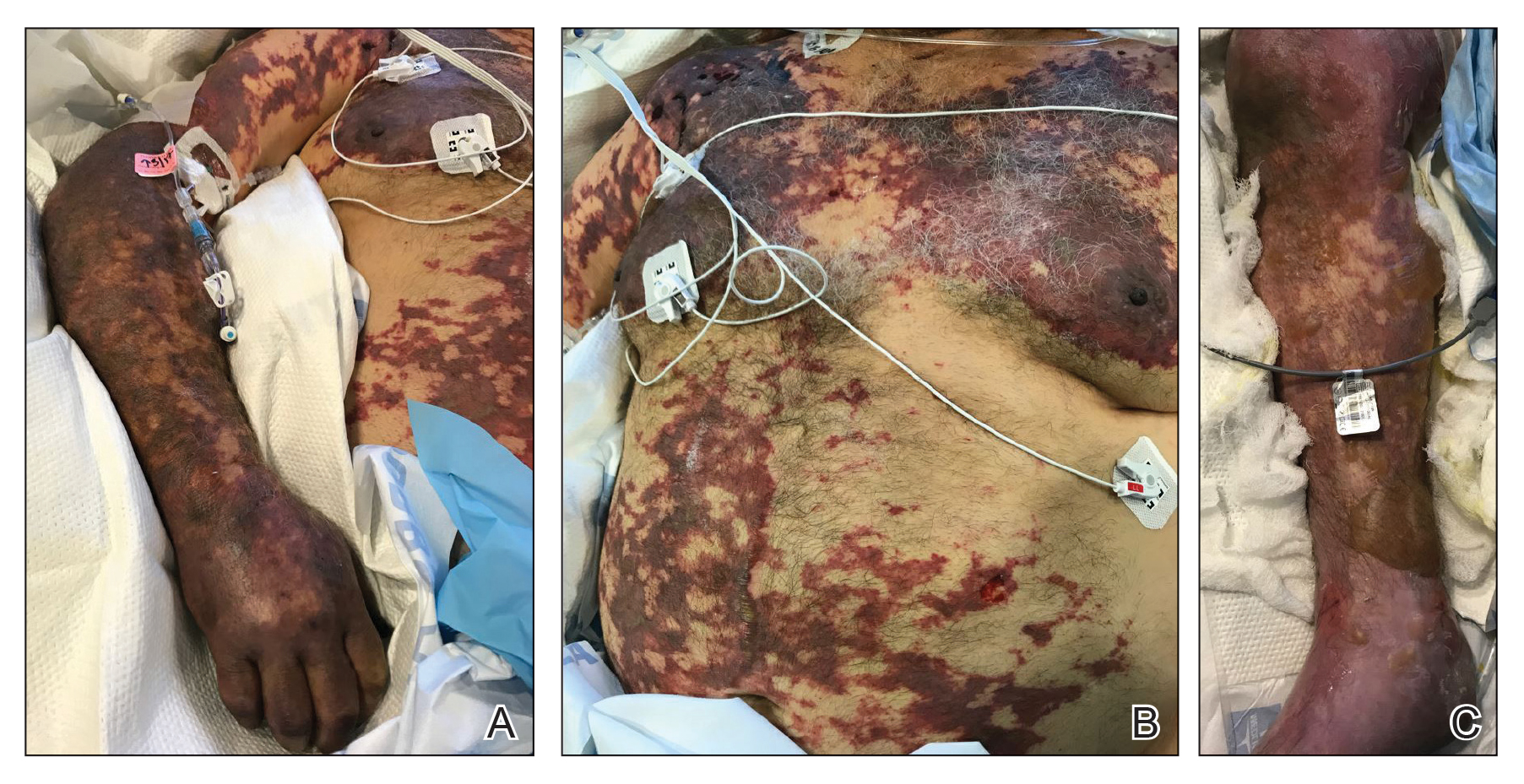
On initial examination at the time of transfer, the patient’s skin was remarkable for diffuse areas of stellate and retiform purpura with dusky centers and necrosis of the nasal tip and earlobes. Both hands were purpuric, with necrosis of the fingertips (Figure 1A). The flank was marked by large areas of full-thickness sloughing of the skin (Figure 1B). The lower extremities were edematous, with some areas of stellate purpura and numerous large bullae that drained straw-colored fluid (Figure 1C). Lower extremity pulses were found with Doppler ultrasonography.
Given the presence of rapidly developing retiform purpura in the clinical context of severe sepsis, purpura fulminans (PF) was the primary consideration in the differential diagnosis. Levamisole-induced necrosis syndrome also was considered because of necrosis of the ears and nose as well as the history of substance use; however, the patient was not known to have a history of cocaine abuse, and a test of antineutrophil cytoplasmic antibody was negative.
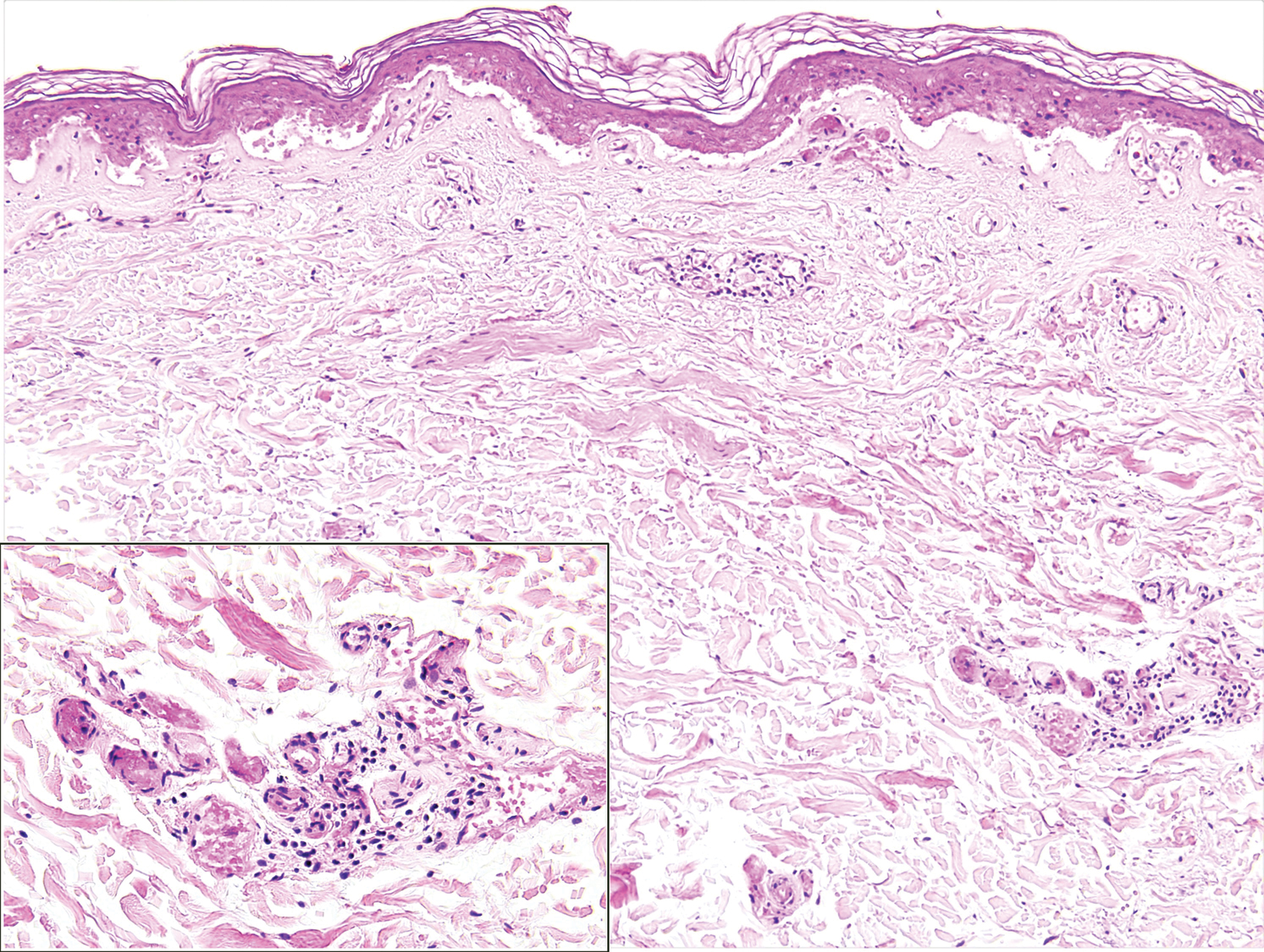
A punch biopsy of the abdomen revealed intravascular thrombi with epidermal and sweat gland necrosis, consistent with PF (Figure 2). Gram, Giemsa, and Gomori methenamine-silver stains were negative for organisms. Tissue culture remained negative. Repeat blood cultures demonstrated Candida parapsilosis fungemia. Respiratory culture was positive for budding yeast.
The patient was treated with antimicrobials, intravenous argatroban, and subcutaneous heparin. Purpura and bullae on the trunk slowly resolved with systemic therapy and wound care with petrolatum and nonadherent dressings. However, lesions on the nasal tip, all fingers of both hands, and several toes evolved into dry gangrene. The hospital course was complicated by renal failure requiring continuous renal replacement therapy; respiratory failure requiring ventilator support; and elevated levels of liver enzymes, consistent with involvement of the hepatic microvasculature.
The patient was in the medical intensive care unit at our institution for 2 weeks and was transferred to a burn center for specialized wound care. At transfer, he was still on a ventilator and receiving continuous renal replacement therapy. Subsequently, the patient required a left above-the-knee amputation, right below-the-knee amputation, and amputation of several digits of the upper extremities. In the months after the amputations, he required multiple stump revisions and experienced surgical site infections that complicated healing.
Purpura fulminans is an uncommon syndrome characterized by intravascular thrombosis and hemorrhagic infarction of the skin. The condition commonly is associated with septic shock, causing vascular collapse and DIC. It often develops rapidly.
Because of associated high mortality, it is important to differentiate PF from other causes of cutaneous retiform purpura, including other causes of thrombosis and large vessel vasculitis. Leading causes of PF include infection and hereditary or acquired deficiency of protein C, protein S, or antithrombin III. Regardless of cause, biopsy results demonstrate vascular thrombosis out of proportion to vasculitis. The mortality rate is 42% to 50%. The incidence of postinfectious sepsis sequelae in PF is higher than in survivors of sepsis only, especially amputation.1-3 Most patients do not die from complications of sepsis but from sequelae of the hypercoagulable and prothrombotic state associated with PF.4 Hemorrhagic infarction can affect the kidneys, brain, lungs, heart, eyes, and adrenal glands (ie, necrosis, namely Waterhouse-Friderichsen syndrome).5
The most common infectious cause of PF is sepsis secondary to Neisseria meningitidis, with as many as 25% of infected patients developing PF.6Streptococcus pneumoniae is another common cause. Other important causative organisms include Streptococcus pyogenes; Staphylococcus aureus (in the setting of intravenous substance use); Klebsiella oxytoca; Klebsiella aerogenes; rickettsial organisms; and viruses, including cytomegalovirus and varicella-zoster virus.2,7-13 Two earlier cases associated with Capnocytophaga were characterized by concomitant renal failure, metabolic acidosis, hemolytic anemia, and DIC.14
It is estimated that Capnocytophaga causes 11% to 46% of all cases of sepsis15; sepsis resulting from Capnocytophaga has extremely poor outcomes, with mortality reaching as high as 60%. The organism is part of the normal oral flora of cats and dogs, and a bite (less often, a scratch) is the cause of most Capnocytophaga infections. The clinical spectrum of C canimorsus infection associated with dog saliva exposure more commonly includes cellulitis at or around the site of inoculation, meningitis, and endocarditis.16
Although patients affected by PF can be young and healthy, several risk factors for PF have been identified2,6,16: asplenia, an immunocompromised state, systemic corticosteroid use, cirrhosis, and alcoholism. Asplenic patients have been shown to be particularly susceptible to systemic Capnocytophaga infection; when bitten by a dog, they should be treated with prophylactic antibiotics to cover Capnocytophaga.17 Immunocompetent patients rarely develop severe infection with Capnocytophaga.16,18,19 The complement system in particular is critically important in defending against C canimorsus.20
The underlying pathophysiology of acute infectious PF is multifactorial, encompassing increased expression of procoagulant tissue factor by monocytes and endothelial cells in the presence of bacterial pathogens. Dysfunction of protein C, an anticoagulant component of the coagulation cascade, often is cited as a crucial derangement leading to the development of a prothrombotic state in acute infectious PF.21 Serum protein S and antithrombin deficiency also can play a role.22 Specific in vitro examination of C canimorsus has revealed a protease that catalyzes N-terminal cleavage of procoagulant factor X, resulting in loss of function.15
Retiform purpura is a hallmark feature of PF, often beginning as nonblanching erythema with localized edema and petechiae before evolving into the characteristic stellate lesions with hemorrhagic bullae and subsequent necrosis.23 Pathologic examination reveals microthrombi involving arterioles and smaller vessels.24 There typically is laboratory evidence of DIC in PF, including elevated prothrombin time and partial thromboplastin time, thrombocytopenia, elevated D-dimer, and a decreased fibrinogen level.6,23
Capnocytophaga bacteria are challenging to grow on standard culture media. Optimal media for growth include 5% sheep’s blood and chocolate agar.16 Polymerase chain reaction can identify Capnocytophaga; in cases in which blood culture does not produce growth, 16S ribosomal RNA gene sequencing of tissue from skin biopsy has identified the pathogen.25
Some Capnocytophaga isolates have been shown to produce beta-lactamase; individual strains can be resistant to penicillins, cephalosporins, and imipenem.26 Factors associated with an increased risk for death include decreased leukocyte and platelet counts and an increased level of arterial lactate.27
Empiric antibiotic therapy for Capnocytophaga sepsis should include a beta-lactam and beta-lactamase inhibitor, such as piperacillin-tazobactam. Management of DIC can include therapeutic heparin or low-molecular-weight heparin and prophylactic platelet transfusion to maintain a pre-established value.28-30 Debridement should be conservative; it is important to wait for definite delineation between viable and necrotic tissue,31 which might take several months.32 Human skin allografts, in addition to artificial skin, are utilized as supplemental therapy for more rapid wound closure after removal of necrotic tissue.33,34 Hyperoxygenated fatty acids have been noted to aid in more rapid wound healing in infants with PF.35
Fresh frozen plasma is one method to replace missing factors, but it contains little protein C.36 Outcomes with recombinant human activated protein C (drotrecogin alfa) are mixed, and studies have shown no benefit in reducing the risk for death.37,38 Protein C concentrate has shown therapeutic benefit in some case reports and small retrospective studies.4 In one case report, protein C concentrate and heparin were utilized in combination with antithrombin III.21
Hyperbaric O2 might be of benefit when initiated within 5 days after onset of PF. However, hyperbaric O2 does carry risk; O2 toxicity, barotrauma, and barriers to timely resuscitation when the patient is inside the pressurized chamber can occur.2
There is a single report of successful use of the vasodilator iloprost for meningococcal PF without need for surgical intervention; the team also utilized topical nitroglycerin patches on the fingers to avoid digital amputation.39 Epoprostenol, tissue plasminogen activator, and antithrombin have been utilized in cases of extensive PF. Fibrinolytic therapy might have some utility, but only in a setting of malignancy-associated DIC.40
Treatment of acute infectious PF lacks a high level of evidence. Options include replacement of anticoagulant factors, anticoagulant therapy, hyperbaric O2, topical and systemic vasodilators, and, in the setting of underlying cancer, fibrinolytics. Even with therapy, prognosis is guarded.
- Ghosh SK, Bandyopadhyay D, Dutta A. Purpura fulminans: a cutaneous marker of disseminated intravascular coagulation. West J Emerg Med. 2009;10:41.
- Ursin Rein P, Jacobsen D, Ormaasen V, et al. Pneumococcal sepsis requiring mechanical ventilation: cohort study in 38 patients with rapid progression to septic shock. Acta Anaesthesiol Scand. 2018;62:1428-1435. doi:10.1111/aas
- Contou D, Canoui-Poitrine F, Coudroy R, et al; Hopeful Study Group. Long-term quality of life in adult patients surviving purpura fulminans: an exposed-unexposed multicenter cohort study. Clin Infect Dis. 2019;69:332-340. doi:10.1093/cid/ciy901
- Chalmers E, Cooper P, Forman K, et al. Purpura fulminans: recognition, diagnosis and management. Arch Dis Child. 2011;96:1066-1071. doi:10.1136/adc.2010.199919
- Karimi K, Odhav A, Kollipara R, et al. Acute cutaneous necrosis: a guide to early diagnosis and treatment. J Cutan Med Surg. 2017;21:425-437. doi:10.1177/1203475417708164
- Colling ME, Bendapudi PK. Purpura fulminans: mechanism and management of dysregulated hemostasis. Transfus Med Rev. 2018;32:69-76. doi:10.1016/j.tmrv.2017.10.001
- Kankeu Fonkoua L, Zhang S, Canty E, et al. Purpura fulminans from reduced protein S following cytomegalovirus and varicella infection. Am J Hematol. 2019;94:491-495. doi:10.1002/ajh.25386
- Okuzono S, Ishimura M, Kanno S, et al. Streptococcus pyogenes-purpura fulminans as an invasive form of group A streptococcal infection. Ann Clin Microbiol Antimicrob. 2018;17:31. doi:10.1186/s12941-018-0282-9
- Gupta D, Chandrashekar L, Srinivas BH, et al. Acute infectious purpura fulminans caused by group A β-hemolytic Streptococcus: an uncommon organism. Indian Dermatol Online J. 2016;7:132-133. doi:10.4103/2229-5178.178093
- Saini S, Duncan RA. Sloughing skin in intravenous drug user. IDCases. 2018;12:74-75. doi:10.1016/j.idcr.2018.03.007
- Tsubouchi N, Tsurukiri J, Numata J, et al. Acute infectious purpura fulminans caused by Klebsiella oxytoca. Intern Med. 2019;58:1801-1802. doi:10.2169/internalmedicine.2350-18
- Yamamoto S, Ito R. Acute infectious purpura fulminans with Enterobacter aerogenes post-neurosurgery. IDCases. 2019;15:e00514. doi:10.1016/j.idcr.2019.e00514
- Dalugama C, Gawarammana IB. Rare presentation of rickettsial infection as purpura fulminans: a case report. J Med Case Rep. 2018;12:145. doi:10.1186/s13256-018-1672-5
- Kazandjieva J, Antonov D, Kamarashev J, et al. Acrally distributed dermatoses: vascular dermatoses (purpura and vasculitis). Clin Dermatol. 2017;35:68-80. doi:10.1016/j.clindermatol.2016.09.013
- Hack K, Renzi F, Hess E, et al. Inactivation of human coagulation factor X by a protease of the pathogen Capnocytophaga canimorsus. J Thromb Haemost. 2017;15:487-499. doi:10.1111/jth.13605
- Zajkowska J, M, Falkowski D, et al. Capnocytophaga canimorsus—an underestimated danger after dog or cat bite - review of literature. Przegl Epidemiol. 2016;70:289-295.
- Di Sabatino A, Carsetti R, Corazza GR. Post-splenectomy and hyposplenic states. Lancet. 2011;378:86-97. doi:10.1016/S0140-6736(10)61493-6
- Behrend Christiansen C, Berg RMG, Plovsing RR, et al. Two cases of infectious purpura fulminans and septic shock caused by Capnocytophaga canimorsus transmitted from dogs. Scand J Infect Dis. 2012;44:635-639. doi:10.3109/00365548.2012.672765
- Ruddock TL, Rindler JM, Bergfeld WF. Capnocytophaga canimorsus septicemia in an asplenic patient. Cutis. 1997;60:95-97.
- Mantovani E, Busani S, Biagioni E, et al. Purpura fulminans and septic shock due to Capnocytophaga canimorsus after dog bite: a case report and review of the literature. Case Rep Crit Care. 2018;2018:7090268. doi:10.1155/2018/7090268
- Bendapudi PK, Robbins A, LeBoeuf N, et al. Persistence of endothelial thrombomodulin in a patient with infectious purpura fulminans treated with protein C concentrate. Blood Adv. 2018;2:2917-2921. doi:10.1182/bloodadvances.2018024430
- Lerolle N, Carlotti A, Melican K, et al. Assessment of the interplay between blood and skin vascular abnormalities in adult purpura fulminans. Am J Respir Crit Care Med. 2013;188:684-692. doi:10.1164/rccm.201302-0228OC.
- Thornsberry LA, LoSicco KI, English JC III. The skin and hypercoagulable states. J Am Acad Dermatol. 2013;69:450-462. doi:10.1016/j.jaad.2013.01.043
- Adcock DM, Hicks MJ. Dermatopathology of skin necrosis associated with purpura fulminans. Semin Thromb Hemost. 1990;16:283-292. doi:10.1055/s-2007-1002681
- Dautzenberg KHW, Polderman FN, van Suylen RJ, et al. Purpura fulminans mimicking toxic epidermal necrolysis—additional value of 16S rRNA sequencing and skin biopsy. Neth J Med. 2017;75:165-168.
- Zangenah S, Andersson AF, V, et al. Genomic analysis reveals the presence of a class D beta-lactamase with broad substrate specificity in animal bite associated Capnocytophaga species. Eur J Clin Microbiol Infect Dis. 2017;36:657-662. doi:10.1007/s10096-016-2842-2
- Contou D, Sonneville R, Canoui-Poitrine F, et al; Hopeful Study Group. Clinical spectrum and short-term outcome of adult patients with purpura fulminans: a French multicenter retrospective cohort study. Intensive Care Med. 2018;44:1502-1511. doi:10.1007/s00134-018-5341-3
- Zenz W, Zoehrer B, Levin M, et al; . Use of recombinant tissue plasminogen activator in children with meningococcal purpura fulminans: a retrospective study. Crit Care Med. 2004;32:1777-1780. doi:10.1097/01.ccm.0000133667.86429.5d
- Wallace JS, Hall JC. Use of drug therapy to manage acute cutaneous necrosis of the skin. J Drugs Dermatol. 2010;9:341-349.
- Squizzato A, Hunt BJ, Kinasewitz GT, et al. Supportive management strategies for disseminated intravascular coagulation. an international consensus. Thromb Haemost. 2016;115:896-904. doi:10.1160/TH15-09-0740
- Herrera R, Hobar PC, Ginsburg CM. Surgical intervention for the complications of meningococcal-induced purpura fulminans. Pediatr Infect Dis J. 1994;13:734-737. doi:10.1097/00006454-199408000-00011
- Pino PA, JA, F. Delayed surgical debridement and use of semiocclusive dressings for salvage of fingers after purpura fulminans. Hand (N Y). 2016;11:NP34-NP37. doi:10.1177/1558944716661996
- Gaucher S, J, Jarraya M. Human skin allografts as a useful adjunct in the treatment of purpura fulminans. J Wound Care. 2010;19:355-358. doi:10.12968/jowc.2010.19.8.77714
- Mazzone L, Schiestl C. Management of septic skin necroses. Eur J Pediatr Surg. 2013;23:349-358. doi:10.1055/s-0033-1352530
- G, Torra-Bou JE, Manzano-Canillas ML, et al. Management of purpura fulminans skin lesions in a premature neonate with sepsis: a case study. J Wound Care. 2019;28:198-203. doi:10.12968/jowc.2019.28.4.198
- Kizilocak H, Ozdemir N, Dikme G, et al. Homozygous protein C deficiency presenting as neonatal purpura fulminans: management with fresh frozen plasma, low molecular weight heparin and protein C concentrate. J Thromb Thrombolysis. 2018;45:315-318. doi:10.1007/s11239-017-1606-x
- Ranieri VM, Thompson BT, Barie PS, et al; . Drotrecogin alfa (activated) in adults with septic shock. N Engl J Med. 2012;366:2055-2064. doi:10.1056/NEJMoa1202290
- Bernard GR, Vincent J-L, Laterre P-F, et al; . Efficacy and safety of recombinant human activated protein C for severe sepsis. N Engl J Med. 2001;344:699-709. doi:10.1056/NEJM200103083441001
- Hage-Sleiman M, Derre N, Verdet C, et al. Meningococcal purpura fulminans and severe myocarditis with clinical meningitis but no meningeal inflammation: a case report. BMC Infect Dis. 2019;19:252. doi:10.1186/s12879-019-3866-x
- Levi M, Toh CH, Thachil J, et al. Guidelines for the diagnosis and management of disseminated intravascular coagulation. British Committee for Standards in Haematology. Br J Haematol. 2009;145:24-33. doi:10.1111/j.1365-2141.2009.07600.x
To the Editor:
A 56-year-old man with a history of opioid abuse and splenectomy decades prior due to a motor vehicle accident was brought to an outside emergency department with confusion, slurred speech, and difficulty breathing. Over the next few days, he became febrile and hypotensive, requiring vasopressors. Clinical laboratory testing revealed a urine drug screen positive for opioids and a low platelet count in the setting of a rapidly evolving retiform purpuric rash.
The patient was transferred to our institution 6 days after initial presentation with primary diagnoses of septic shock with multiorgan failure and disseminated intravascular coagulation (DIC). Blood cultures were positive for gram-negative rods. After several days of broad-spectrum antibiotics and supportive care, cultures were reported as positive for Capnocytophaga canimorsus. Upon further questioning, the patient’s wife reported that the couple had a new puppy and that the patient often allowed the dog to bite him playfully and lick abrasions on his hands and legs. He had not received medical treatment for any of the dog’s bites.
On initial examination at the time of transfer, the patient’s skin was remarkable for diffuse areas of stellate and retiform purpura with dusky centers and necrosis of the nasal tip and earlobes. Both hands were purpuric, with necrosis of the fingertips (Figure 1A). The flank was marked by large areas of full-thickness sloughing of the skin (Figure 1B). The lower extremities were edematous, with some areas of stellate purpura and numerous large bullae that drained straw-colored fluid (Figure 1C). Lower extremity pulses were found with Doppler ultrasonography.

Given the presence of rapidly developing retiform purpura in the clinical context of severe sepsis, purpura fulminans (PF) was the primary consideration in the differential diagnosis. Levamisole-induced necrosis syndrome also was considered because of necrosis of the ears and nose as well as the history of substance use; however, the patient was not known to have a history of cocaine abuse, and a test of antineutrophil cytoplasmic antibody was negative.
A punch biopsy of the abdomen revealed intravascular thrombi with epidermal and sweat gland necrosis, consistent with PF (Figure 2). Gram, Giemsa, and Gomori methenamine-silver stains were negative for organisms. Tissue culture remained negative. Repeat blood cultures demonstrated Candida parapsilosis fungemia. Respiratory culture was positive for budding yeast.

The patient was treated with antimicrobials, intravenous argatroban, and subcutaneous heparin. Purpura and bullae on the trunk slowly resolved with systemic therapy and wound care with petrolatum and nonadherent dressings. However, lesions on the nasal tip, all fingers of both hands, and several toes evolved into dry gangrene. The hospital course was complicated by renal failure requiring continuous renal replacement therapy; respiratory failure requiring ventilator support; and elevated levels of liver enzymes, consistent with involvement of the hepatic microvasculature.
The patient was in the medical intensive care unit at our institution for 2 weeks and was transferred to a burn center for specialized wound care. At transfer, he was still on a ventilator and receiving continuous renal replacement therapy. Subsequently, the patient required a left above-the-knee amputation, right below-the-knee amputation, and amputation of several digits of the upper extremities. In the months after the amputations, he required multiple stump revisions and experienced surgical site infections that complicated healing.
Purpura fulminans is an uncommon syndrome characterized by intravascular thrombosis and hemorrhagic infarction of the skin. The condition commonly is associated with septic shock, causing vascular collapse and DIC. It often develops rapidly.
Because of associated high mortality, it is important to differentiate PF from other causes of cutaneous retiform purpura, including other causes of thrombosis and large vessel vasculitis. Leading causes of PF include infection and hereditary or acquired deficiency of protein C, protein S, or antithrombin III. Regardless of cause, biopsy results demonstrate vascular thrombosis out of proportion to vasculitis. The mortality rate is 42% to 50%. The incidence of postinfectious sepsis sequelae in PF is higher than in survivors of sepsis only, especially amputation.1-3 Most patients do not die from complications of sepsis but from sequelae of the hypercoagulable and prothrombotic state associated with PF.4 Hemorrhagic infarction can affect the kidneys, brain, lungs, heart, eyes, and adrenal glands (ie, necrosis, namely Waterhouse-Friderichsen syndrome).5
The most common infectious cause of PF is sepsis secondary to Neisseria meningitidis, with as many as 25% of infected patients developing PF.6Streptococcus pneumoniae is another common cause. Other important causative organisms include Streptococcus pyogenes; Staphylococcus aureus (in the setting of intravenous substance use); Klebsiella oxytoca; Klebsiella aerogenes; rickettsial organisms; and viruses, including cytomegalovirus and varicella-zoster virus.2,7-13 Two earlier cases associated with Capnocytophaga were characterized by concomitant renal failure, metabolic acidosis, hemolytic anemia, and DIC.14
It is estimated that Capnocytophaga causes 11% to 46% of all cases of sepsis15; sepsis resulting from Capnocytophaga has extremely poor outcomes, with mortality reaching as high as 60%. The organism is part of the normal oral flora of cats and dogs, and a bite (less often, a scratch) is the cause of most Capnocytophaga infections. The clinical spectrum of C canimorsus infection associated with dog saliva exposure more commonly includes cellulitis at or around the site of inoculation, meningitis, and endocarditis.16
Although patients affected by PF can be young and healthy, several risk factors for PF have been identified2,6,16: asplenia, an immunocompromised state, systemic corticosteroid use, cirrhosis, and alcoholism. Asplenic patients have been shown to be particularly susceptible to systemic Capnocytophaga infection; when bitten by a dog, they should be treated with prophylactic antibiotics to cover Capnocytophaga.17 Immunocompetent patients rarely develop severe infection with Capnocytophaga.16,18,19 The complement system in particular is critically important in defending against C canimorsus.20
The underlying pathophysiology of acute infectious PF is multifactorial, encompassing increased expression of procoagulant tissue factor by monocytes and endothelial cells in the presence of bacterial pathogens. Dysfunction of protein C, an anticoagulant component of the coagulation cascade, often is cited as a crucial derangement leading to the development of a prothrombotic state in acute infectious PF.21 Serum protein S and antithrombin deficiency also can play a role.22 Specific in vitro examination of C canimorsus has revealed a protease that catalyzes N-terminal cleavage of procoagulant factor X, resulting in loss of function.15
Retiform purpura is a hallmark feature of PF, often beginning as nonblanching erythema with localized edema and petechiae before evolving into the characteristic stellate lesions with hemorrhagic bullae and subsequent necrosis.23 Pathologic examination reveals microthrombi involving arterioles and smaller vessels.24 There typically is laboratory evidence of DIC in PF, including elevated prothrombin time and partial thromboplastin time, thrombocytopenia, elevated D-dimer, and a decreased fibrinogen level.6,23
Capnocytophaga bacteria are challenging to grow on standard culture media. Optimal media for growth include 5% sheep’s blood and chocolate agar.16 Polymerase chain reaction can identify Capnocytophaga; in cases in which blood culture does not produce growth, 16S ribosomal RNA gene sequencing of tissue from skin biopsy has identified the pathogen.25
Some Capnocytophaga isolates have been shown to produce beta-lactamase; individual strains can be resistant to penicillins, cephalosporins, and imipenem.26 Factors associated with an increased risk for death include decreased leukocyte and platelet counts and an increased level of arterial lactate.27
Empiric antibiotic therapy for Capnocytophaga sepsis should include a beta-lactam and beta-lactamase inhibitor, such as piperacillin-tazobactam. Management of DIC can include therapeutic heparin or low-molecular-weight heparin and prophylactic platelet transfusion to maintain a pre-established value.28-30 Debridement should be conservative; it is important to wait for definite delineation between viable and necrotic tissue,31 which might take several months.32 Human skin allografts, in addition to artificial skin, are utilized as supplemental therapy for more rapid wound closure after removal of necrotic tissue.33,34 Hyperoxygenated fatty acids have been noted to aid in more rapid wound healing in infants with PF.35
Fresh frozen plasma is one method to replace missing factors, but it contains little protein C.36 Outcomes with recombinant human activated protein C (drotrecogin alfa) are mixed, and studies have shown no benefit in reducing the risk for death.37,38 Protein C concentrate has shown therapeutic benefit in some case reports and small retrospective studies.4 In one case report, protein C concentrate and heparin were utilized in combination with antithrombin III.21
Hyperbaric O2 might be of benefit when initiated within 5 days after onset of PF. However, hyperbaric O2 does carry risk; O2 toxicity, barotrauma, and barriers to timely resuscitation when the patient is inside the pressurized chamber can occur.2
There is a single report of successful use of the vasodilator iloprost for meningococcal PF without need for surgical intervention; the team also utilized topical nitroglycerin patches on the fingers to avoid digital amputation.39 Epoprostenol, tissue plasminogen activator, and antithrombin have been utilized in cases of extensive PF. Fibrinolytic therapy might have some utility, but only in a setting of malignancy-associated DIC.40
Treatment of acute infectious PF lacks a high level of evidence. Options include replacement of anticoagulant factors, anticoagulant therapy, hyperbaric O2, topical and systemic vasodilators, and, in the setting of underlying cancer, fibrinolytics. Even with therapy, prognosis is guarded.
To the Editor:
A 56-year-old man with a history of opioid abuse and splenectomy decades prior due to a motor vehicle accident was brought to an outside emergency department with confusion, slurred speech, and difficulty breathing. Over the next few days, he became febrile and hypotensive, requiring vasopressors. Clinical laboratory testing revealed a urine drug screen positive for opioids and a low platelet count in the setting of a rapidly evolving retiform purpuric rash.
The patient was transferred to our institution 6 days after initial presentation with primary diagnoses of septic shock with multiorgan failure and disseminated intravascular coagulation (DIC). Blood cultures were positive for gram-negative rods. After several days of broad-spectrum antibiotics and supportive care, cultures were reported as positive for Capnocytophaga canimorsus. Upon further questioning, the patient’s wife reported that the couple had a new puppy and that the patient often allowed the dog to bite him playfully and lick abrasions on his hands and legs. He had not received medical treatment for any of the dog’s bites.
On initial examination at the time of transfer, the patient’s skin was remarkable for diffuse areas of stellate and retiform purpura with dusky centers and necrosis of the nasal tip and earlobes. Both hands were purpuric, with necrosis of the fingertips (Figure 1A). The flank was marked by large areas of full-thickness sloughing of the skin (Figure 1B). The lower extremities were edematous, with some areas of stellate purpura and numerous large bullae that drained straw-colored fluid (Figure 1C). Lower extremity pulses were found with Doppler ultrasonography.

Given the presence of rapidly developing retiform purpura in the clinical context of severe sepsis, purpura fulminans (PF) was the primary consideration in the differential diagnosis. Levamisole-induced necrosis syndrome also was considered because of necrosis of the ears and nose as well as the history of substance use; however, the patient was not known to have a history of cocaine abuse, and a test of antineutrophil cytoplasmic antibody was negative.
A punch biopsy of the abdomen revealed intravascular thrombi with epidermal and sweat gland necrosis, consistent with PF (Figure 2). Gram, Giemsa, and Gomori methenamine-silver stains were negative for organisms. Tissue culture remained negative. Repeat blood cultures demonstrated Candida parapsilosis fungemia. Respiratory culture was positive for budding yeast.

The patient was treated with antimicrobials, intravenous argatroban, and subcutaneous heparin. Purpura and bullae on the trunk slowly resolved with systemic therapy and wound care with petrolatum and nonadherent dressings. However, lesions on the nasal tip, all fingers of both hands, and several toes evolved into dry gangrene. The hospital course was complicated by renal failure requiring continuous renal replacement therapy; respiratory failure requiring ventilator support; and elevated levels of liver enzymes, consistent with involvement of the hepatic microvasculature.
The patient was in the medical intensive care unit at our institution for 2 weeks and was transferred to a burn center for specialized wound care. At transfer, he was still on a ventilator and receiving continuous renal replacement therapy. Subsequently, the patient required a left above-the-knee amputation, right below-the-knee amputation, and amputation of several digits of the upper extremities. In the months after the amputations, he required multiple stump revisions and experienced surgical site infections that complicated healing.
Purpura fulminans is an uncommon syndrome characterized by intravascular thrombosis and hemorrhagic infarction of the skin. The condition commonly is associated with septic shock, causing vascular collapse and DIC. It often develops rapidly.
Because of associated high mortality, it is important to differentiate PF from other causes of cutaneous retiform purpura, including other causes of thrombosis and large vessel vasculitis. Leading causes of PF include infection and hereditary or acquired deficiency of protein C, protein S, or antithrombin III. Regardless of cause, biopsy results demonstrate vascular thrombosis out of proportion to vasculitis. The mortality rate is 42% to 50%. The incidence of postinfectious sepsis sequelae in PF is higher than in survivors of sepsis only, especially amputation.1-3 Most patients do not die from complications of sepsis but from sequelae of the hypercoagulable and prothrombotic state associated with PF.4 Hemorrhagic infarction can affect the kidneys, brain, lungs, heart, eyes, and adrenal glands (ie, necrosis, namely Waterhouse-Friderichsen syndrome).5
The most common infectious cause of PF is sepsis secondary to Neisseria meningitidis, with as many as 25% of infected patients developing PF.6Streptococcus pneumoniae is another common cause. Other important causative organisms include Streptococcus pyogenes; Staphylococcus aureus (in the setting of intravenous substance use); Klebsiella oxytoca; Klebsiella aerogenes; rickettsial organisms; and viruses, including cytomegalovirus and varicella-zoster virus.2,7-13 Two earlier cases associated with Capnocytophaga were characterized by concomitant renal failure, metabolic acidosis, hemolytic anemia, and DIC.14
It is estimated that Capnocytophaga causes 11% to 46% of all cases of sepsis15; sepsis resulting from Capnocytophaga has extremely poor outcomes, with mortality reaching as high as 60%. The organism is part of the normal oral flora of cats and dogs, and a bite (less often, a scratch) is the cause of most Capnocytophaga infections. The clinical spectrum of C canimorsus infection associated with dog saliva exposure more commonly includes cellulitis at or around the site of inoculation, meningitis, and endocarditis.16
Although patients affected by PF can be young and healthy, several risk factors for PF have been identified2,6,16: asplenia, an immunocompromised state, systemic corticosteroid use, cirrhosis, and alcoholism. Asplenic patients have been shown to be particularly susceptible to systemic Capnocytophaga infection; when bitten by a dog, they should be treated with prophylactic antibiotics to cover Capnocytophaga.17 Immunocompetent patients rarely develop severe infection with Capnocytophaga.16,18,19 The complement system in particular is critically important in defending against C canimorsus.20
The underlying pathophysiology of acute infectious PF is multifactorial, encompassing increased expression of procoagulant tissue factor by monocytes and endothelial cells in the presence of bacterial pathogens. Dysfunction of protein C, an anticoagulant component of the coagulation cascade, often is cited as a crucial derangement leading to the development of a prothrombotic state in acute infectious PF.21 Serum protein S and antithrombin deficiency also can play a role.22 Specific in vitro examination of C canimorsus has revealed a protease that catalyzes N-terminal cleavage of procoagulant factor X, resulting in loss of function.15
Retiform purpura is a hallmark feature of PF, often beginning as nonblanching erythema with localized edema and petechiae before evolving into the characteristic stellate lesions with hemorrhagic bullae and subsequent necrosis.23 Pathologic examination reveals microthrombi involving arterioles and smaller vessels.24 There typically is laboratory evidence of DIC in PF, including elevated prothrombin time and partial thromboplastin time, thrombocytopenia, elevated D-dimer, and a decreased fibrinogen level.6,23
Capnocytophaga bacteria are challenging to grow on standard culture media. Optimal media for growth include 5% sheep’s blood and chocolate agar.16 Polymerase chain reaction can identify Capnocytophaga; in cases in which blood culture does not produce growth, 16S ribosomal RNA gene sequencing of tissue from skin biopsy has identified the pathogen.25
Some Capnocytophaga isolates have been shown to produce beta-lactamase; individual strains can be resistant to penicillins, cephalosporins, and imipenem.26 Factors associated with an increased risk for death include decreased leukocyte and platelet counts and an increased level of arterial lactate.27
Empiric antibiotic therapy for Capnocytophaga sepsis should include a beta-lactam and beta-lactamase inhibitor, such as piperacillin-tazobactam. Management of DIC can include therapeutic heparin or low-molecular-weight heparin and prophylactic platelet transfusion to maintain a pre-established value.28-30 Debridement should be conservative; it is important to wait for definite delineation between viable and necrotic tissue,31 which might take several months.32 Human skin allografts, in addition to artificial skin, are utilized as supplemental therapy for more rapid wound closure after removal of necrotic tissue.33,34 Hyperoxygenated fatty acids have been noted to aid in more rapid wound healing in infants with PF.35
Fresh frozen plasma is one method to replace missing factors, but it contains little protein C.36 Outcomes with recombinant human activated protein C (drotrecogin alfa) are mixed, and studies have shown no benefit in reducing the risk for death.37,38 Protein C concentrate has shown therapeutic benefit in some case reports and small retrospective studies.4 In one case report, protein C concentrate and heparin were utilized in combination with antithrombin III.21
Hyperbaric O2 might be of benefit when initiated within 5 days after onset of PF. However, hyperbaric O2 does carry risk; O2 toxicity, barotrauma, and barriers to timely resuscitation when the patient is inside the pressurized chamber can occur.2
There is a single report of successful use of the vasodilator iloprost for meningococcal PF without need for surgical intervention; the team also utilized topical nitroglycerin patches on the fingers to avoid digital amputation.39 Epoprostenol, tissue plasminogen activator, and antithrombin have been utilized in cases of extensive PF. Fibrinolytic therapy might have some utility, but only in a setting of malignancy-associated DIC.40
Treatment of acute infectious PF lacks a high level of evidence. Options include replacement of anticoagulant factors, anticoagulant therapy, hyperbaric O2, topical and systemic vasodilators, and, in the setting of underlying cancer, fibrinolytics. Even with therapy, prognosis is guarded.
- Ghosh SK, Bandyopadhyay D, Dutta A. Purpura fulminans: a cutaneous marker of disseminated intravascular coagulation. West J Emerg Med. 2009;10:41.
- Ursin Rein P, Jacobsen D, Ormaasen V, et al. Pneumococcal sepsis requiring mechanical ventilation: cohort study in 38 patients with rapid progression to septic shock. Acta Anaesthesiol Scand. 2018;62:1428-1435. doi:10.1111/aas
- Contou D, Canoui-Poitrine F, Coudroy R, et al; Hopeful Study Group. Long-term quality of life in adult patients surviving purpura fulminans: an exposed-unexposed multicenter cohort study. Clin Infect Dis. 2019;69:332-340. doi:10.1093/cid/ciy901
- Chalmers E, Cooper P, Forman K, et al. Purpura fulminans: recognition, diagnosis and management. Arch Dis Child. 2011;96:1066-1071. doi:10.1136/adc.2010.199919
- Karimi K, Odhav A, Kollipara R, et al. Acute cutaneous necrosis: a guide to early diagnosis and treatment. J Cutan Med Surg. 2017;21:425-437. doi:10.1177/1203475417708164
- Colling ME, Bendapudi PK. Purpura fulminans: mechanism and management of dysregulated hemostasis. Transfus Med Rev. 2018;32:69-76. doi:10.1016/j.tmrv.2017.10.001
- Kankeu Fonkoua L, Zhang S, Canty E, et al. Purpura fulminans from reduced protein S following cytomegalovirus and varicella infection. Am J Hematol. 2019;94:491-495. doi:10.1002/ajh.25386
- Okuzono S, Ishimura M, Kanno S, et al. Streptococcus pyogenes-purpura fulminans as an invasive form of group A streptococcal infection. Ann Clin Microbiol Antimicrob. 2018;17:31. doi:10.1186/s12941-018-0282-9
- Gupta D, Chandrashekar L, Srinivas BH, et al. Acute infectious purpura fulminans caused by group A β-hemolytic Streptococcus: an uncommon organism. Indian Dermatol Online J. 2016;7:132-133. doi:10.4103/2229-5178.178093
- Saini S, Duncan RA. Sloughing skin in intravenous drug user. IDCases. 2018;12:74-75. doi:10.1016/j.idcr.2018.03.007
- Tsubouchi N, Tsurukiri J, Numata J, et al. Acute infectious purpura fulminans caused by Klebsiella oxytoca. Intern Med. 2019;58:1801-1802. doi:10.2169/internalmedicine.2350-18
- Yamamoto S, Ito R. Acute infectious purpura fulminans with Enterobacter aerogenes post-neurosurgery. IDCases. 2019;15:e00514. doi:10.1016/j.idcr.2019.e00514
- Dalugama C, Gawarammana IB. Rare presentation of rickettsial infection as purpura fulminans: a case report. J Med Case Rep. 2018;12:145. doi:10.1186/s13256-018-1672-5
- Kazandjieva J, Antonov D, Kamarashev J, et al. Acrally distributed dermatoses: vascular dermatoses (purpura and vasculitis). Clin Dermatol. 2017;35:68-80. doi:10.1016/j.clindermatol.2016.09.013
- Hack K, Renzi F, Hess E, et al. Inactivation of human coagulation factor X by a protease of the pathogen Capnocytophaga canimorsus. J Thromb Haemost. 2017;15:487-499. doi:10.1111/jth.13605
- Zajkowska J, M, Falkowski D, et al. Capnocytophaga canimorsus—an underestimated danger after dog or cat bite - review of literature. Przegl Epidemiol. 2016;70:289-295.
- Di Sabatino A, Carsetti R, Corazza GR. Post-splenectomy and hyposplenic states. Lancet. 2011;378:86-97. doi:10.1016/S0140-6736(10)61493-6
- Behrend Christiansen C, Berg RMG, Plovsing RR, et al. Two cases of infectious purpura fulminans and septic shock caused by Capnocytophaga canimorsus transmitted from dogs. Scand J Infect Dis. 2012;44:635-639. doi:10.3109/00365548.2012.672765
- Ruddock TL, Rindler JM, Bergfeld WF. Capnocytophaga canimorsus septicemia in an asplenic patient. Cutis. 1997;60:95-97.
- Mantovani E, Busani S, Biagioni E, et al. Purpura fulminans and septic shock due to Capnocytophaga canimorsus after dog bite: a case report and review of the literature. Case Rep Crit Care. 2018;2018:7090268. doi:10.1155/2018/7090268
- Bendapudi PK, Robbins A, LeBoeuf N, et al. Persistence of endothelial thrombomodulin in a patient with infectious purpura fulminans treated with protein C concentrate. Blood Adv. 2018;2:2917-2921. doi:10.1182/bloodadvances.2018024430
- Lerolle N, Carlotti A, Melican K, et al. Assessment of the interplay between blood and skin vascular abnormalities in adult purpura fulminans. Am J Respir Crit Care Med. 2013;188:684-692. doi:10.1164/rccm.201302-0228OC.
- Thornsberry LA, LoSicco KI, English JC III. The skin and hypercoagulable states. J Am Acad Dermatol. 2013;69:450-462. doi:10.1016/j.jaad.2013.01.043
- Adcock DM, Hicks MJ. Dermatopathology of skin necrosis associated with purpura fulminans. Semin Thromb Hemost. 1990;16:283-292. doi:10.1055/s-2007-1002681
- Dautzenberg KHW, Polderman FN, van Suylen RJ, et al. Purpura fulminans mimicking toxic epidermal necrolysis—additional value of 16S rRNA sequencing and skin biopsy. Neth J Med. 2017;75:165-168.
- Zangenah S, Andersson AF, V, et al. Genomic analysis reveals the presence of a class D beta-lactamase with broad substrate specificity in animal bite associated Capnocytophaga species. Eur J Clin Microbiol Infect Dis. 2017;36:657-662. doi:10.1007/s10096-016-2842-2
- Contou D, Sonneville R, Canoui-Poitrine F, et al; Hopeful Study Group. Clinical spectrum and short-term outcome of adult patients with purpura fulminans: a French multicenter retrospective cohort study. Intensive Care Med. 2018;44:1502-1511. doi:10.1007/s00134-018-5341-3
- Zenz W, Zoehrer B, Levin M, et al; . Use of recombinant tissue plasminogen activator in children with meningococcal purpura fulminans: a retrospective study. Crit Care Med. 2004;32:1777-1780. doi:10.1097/01.ccm.0000133667.86429.5d
- Wallace JS, Hall JC. Use of drug therapy to manage acute cutaneous necrosis of the skin. J Drugs Dermatol. 2010;9:341-349.
- Squizzato A, Hunt BJ, Kinasewitz GT, et al. Supportive management strategies for disseminated intravascular coagulation. an international consensus. Thromb Haemost. 2016;115:896-904. doi:10.1160/TH15-09-0740
- Herrera R, Hobar PC, Ginsburg CM. Surgical intervention for the complications of meningococcal-induced purpura fulminans. Pediatr Infect Dis J. 1994;13:734-737. doi:10.1097/00006454-199408000-00011
- Pino PA, JA, F. Delayed surgical debridement and use of semiocclusive dressings for salvage of fingers after purpura fulminans. Hand (N Y). 2016;11:NP34-NP37. doi:10.1177/1558944716661996
- Gaucher S, J, Jarraya M. Human skin allografts as a useful adjunct in the treatment of purpura fulminans. J Wound Care. 2010;19:355-358. doi:10.12968/jowc.2010.19.8.77714
- Mazzone L, Schiestl C. Management of septic skin necroses. Eur J Pediatr Surg. 2013;23:349-358. doi:10.1055/s-0033-1352530
- G, Torra-Bou JE, Manzano-Canillas ML, et al. Management of purpura fulminans skin lesions in a premature neonate with sepsis: a case study. J Wound Care. 2019;28:198-203. doi:10.12968/jowc.2019.28.4.198
- Kizilocak H, Ozdemir N, Dikme G, et al. Homozygous protein C deficiency presenting as neonatal purpura fulminans: management with fresh frozen plasma, low molecular weight heparin and protein C concentrate. J Thromb Thrombolysis. 2018;45:315-318. doi:10.1007/s11239-017-1606-x
- Ranieri VM, Thompson BT, Barie PS, et al; . Drotrecogin alfa (activated) in adults with septic shock. N Engl J Med. 2012;366:2055-2064. doi:10.1056/NEJMoa1202290
- Bernard GR, Vincent J-L, Laterre P-F, et al; . Efficacy and safety of recombinant human activated protein C for severe sepsis. N Engl J Med. 2001;344:699-709. doi:10.1056/NEJM200103083441001
- Hage-Sleiman M, Derre N, Verdet C, et al. Meningococcal purpura fulminans and severe myocarditis with clinical meningitis but no meningeal inflammation: a case report. BMC Infect Dis. 2019;19:252. doi:10.1186/s12879-019-3866-x
- Levi M, Toh CH, Thachil J, et al. Guidelines for the diagnosis and management of disseminated intravascular coagulation. British Committee for Standards in Haematology. Br J Haematol. 2009;145:24-33. doi:10.1111/j.1365-2141.2009.07600.x
- Ghosh SK, Bandyopadhyay D, Dutta A. Purpura fulminans: a cutaneous marker of disseminated intravascular coagulation. West J Emerg Med. 2009;10:41.
- Ursin Rein P, Jacobsen D, Ormaasen V, et al. Pneumococcal sepsis requiring mechanical ventilation: cohort study in 38 patients with rapid progression to septic shock. Acta Anaesthesiol Scand. 2018;62:1428-1435. doi:10.1111/aas
- Contou D, Canoui-Poitrine F, Coudroy R, et al; Hopeful Study Group. Long-term quality of life in adult patients surviving purpura fulminans: an exposed-unexposed multicenter cohort study. Clin Infect Dis. 2019;69:332-340. doi:10.1093/cid/ciy901
- Chalmers E, Cooper P, Forman K, et al. Purpura fulminans: recognition, diagnosis and management. Arch Dis Child. 2011;96:1066-1071. doi:10.1136/adc.2010.199919
- Karimi K, Odhav A, Kollipara R, et al. Acute cutaneous necrosis: a guide to early diagnosis and treatment. J Cutan Med Surg. 2017;21:425-437. doi:10.1177/1203475417708164
- Colling ME, Bendapudi PK. Purpura fulminans: mechanism and management of dysregulated hemostasis. Transfus Med Rev. 2018;32:69-76. doi:10.1016/j.tmrv.2017.10.001
- Kankeu Fonkoua L, Zhang S, Canty E, et al. Purpura fulminans from reduced protein S following cytomegalovirus and varicella infection. Am J Hematol. 2019;94:491-495. doi:10.1002/ajh.25386
- Okuzono S, Ishimura M, Kanno S, et al. Streptococcus pyogenes-purpura fulminans as an invasive form of group A streptococcal infection. Ann Clin Microbiol Antimicrob. 2018;17:31. doi:10.1186/s12941-018-0282-9
- Gupta D, Chandrashekar L, Srinivas BH, et al. Acute infectious purpura fulminans caused by group A β-hemolytic Streptococcus: an uncommon organism. Indian Dermatol Online J. 2016;7:132-133. doi:10.4103/2229-5178.178093
- Saini S, Duncan RA. Sloughing skin in intravenous drug user. IDCases. 2018;12:74-75. doi:10.1016/j.idcr.2018.03.007
- Tsubouchi N, Tsurukiri J, Numata J, et al. Acute infectious purpura fulminans caused by Klebsiella oxytoca. Intern Med. 2019;58:1801-1802. doi:10.2169/internalmedicine.2350-18
- Yamamoto S, Ito R. Acute infectious purpura fulminans with Enterobacter aerogenes post-neurosurgery. IDCases. 2019;15:e00514. doi:10.1016/j.idcr.2019.e00514
- Dalugama C, Gawarammana IB. Rare presentation of rickettsial infection as purpura fulminans: a case report. J Med Case Rep. 2018;12:145. doi:10.1186/s13256-018-1672-5
- Kazandjieva J, Antonov D, Kamarashev J, et al. Acrally distributed dermatoses: vascular dermatoses (purpura and vasculitis). Clin Dermatol. 2017;35:68-80. doi:10.1016/j.clindermatol.2016.09.013
- Hack K, Renzi F, Hess E, et al. Inactivation of human coagulation factor X by a protease of the pathogen Capnocytophaga canimorsus. J Thromb Haemost. 2017;15:487-499. doi:10.1111/jth.13605
- Zajkowska J, M, Falkowski D, et al. Capnocytophaga canimorsus—an underestimated danger after dog or cat bite - review of literature. Przegl Epidemiol. 2016;70:289-295.
- Di Sabatino A, Carsetti R, Corazza GR. Post-splenectomy and hyposplenic states. Lancet. 2011;378:86-97. doi:10.1016/S0140-6736(10)61493-6
- Behrend Christiansen C, Berg RMG, Plovsing RR, et al. Two cases of infectious purpura fulminans and septic shock caused by Capnocytophaga canimorsus transmitted from dogs. Scand J Infect Dis. 2012;44:635-639. doi:10.3109/00365548.2012.672765
- Ruddock TL, Rindler JM, Bergfeld WF. Capnocytophaga canimorsus septicemia in an asplenic patient. Cutis. 1997;60:95-97.
- Mantovani E, Busani S, Biagioni E, et al. Purpura fulminans and septic shock due to Capnocytophaga canimorsus after dog bite: a case report and review of the literature. Case Rep Crit Care. 2018;2018:7090268. doi:10.1155/2018/7090268
- Bendapudi PK, Robbins A, LeBoeuf N, et al. Persistence of endothelial thrombomodulin in a patient with infectious purpura fulminans treated with protein C concentrate. Blood Adv. 2018;2:2917-2921. doi:10.1182/bloodadvances.2018024430
- Lerolle N, Carlotti A, Melican K, et al. Assessment of the interplay between blood and skin vascular abnormalities in adult purpura fulminans. Am J Respir Crit Care Med. 2013;188:684-692. doi:10.1164/rccm.201302-0228OC.
- Thornsberry LA, LoSicco KI, English JC III. The skin and hypercoagulable states. J Am Acad Dermatol. 2013;69:450-462. doi:10.1016/j.jaad.2013.01.043
- Adcock DM, Hicks MJ. Dermatopathology of skin necrosis associated with purpura fulminans. Semin Thromb Hemost. 1990;16:283-292. doi:10.1055/s-2007-1002681
- Dautzenberg KHW, Polderman FN, van Suylen RJ, et al. Purpura fulminans mimicking toxic epidermal necrolysis—additional value of 16S rRNA sequencing and skin biopsy. Neth J Med. 2017;75:165-168.
- Zangenah S, Andersson AF, V, et al. Genomic analysis reveals the presence of a class D beta-lactamase with broad substrate specificity in animal bite associated Capnocytophaga species. Eur J Clin Microbiol Infect Dis. 2017;36:657-662. doi:10.1007/s10096-016-2842-2
- Contou D, Sonneville R, Canoui-Poitrine F, et al; Hopeful Study Group. Clinical spectrum and short-term outcome of adult patients with purpura fulminans: a French multicenter retrospective cohort study. Intensive Care Med. 2018;44:1502-1511. doi:10.1007/s00134-018-5341-3
- Zenz W, Zoehrer B, Levin M, et al; . Use of recombinant tissue plasminogen activator in children with meningococcal purpura fulminans: a retrospective study. Crit Care Med. 2004;32:1777-1780. doi:10.1097/01.ccm.0000133667.86429.5d
- Wallace JS, Hall JC. Use of drug therapy to manage acute cutaneous necrosis of the skin. J Drugs Dermatol. 2010;9:341-349.
- Squizzato A, Hunt BJ, Kinasewitz GT, et al. Supportive management strategies for disseminated intravascular coagulation. an international consensus. Thromb Haemost. 2016;115:896-904. doi:10.1160/TH15-09-0740
- Herrera R, Hobar PC, Ginsburg CM. Surgical intervention for the complications of meningococcal-induced purpura fulminans. Pediatr Infect Dis J. 1994;13:734-737. doi:10.1097/00006454-199408000-00011
- Pino PA, JA, F. Delayed surgical debridement and use of semiocclusive dressings for salvage of fingers after purpura fulminans. Hand (N Y). 2016;11:NP34-NP37. doi:10.1177/1558944716661996
- Gaucher S, J, Jarraya M. Human skin allografts as a useful adjunct in the treatment of purpura fulminans. J Wound Care. 2010;19:355-358. doi:10.12968/jowc.2010.19.8.77714
- Mazzone L, Schiestl C. Management of septic skin necroses. Eur J Pediatr Surg. 2013;23:349-358. doi:10.1055/s-0033-1352530
- G, Torra-Bou JE, Manzano-Canillas ML, et al. Management of purpura fulminans skin lesions in a premature neonate with sepsis: a case study. J Wound Care. 2019;28:198-203. doi:10.12968/jowc.2019.28.4.198
- Kizilocak H, Ozdemir N, Dikme G, et al. Homozygous protein C deficiency presenting as neonatal purpura fulminans: management with fresh frozen plasma, low molecular weight heparin and protein C concentrate. J Thromb Thrombolysis. 2018;45:315-318. doi:10.1007/s11239-017-1606-x
- Ranieri VM, Thompson BT, Barie PS, et al; . Drotrecogin alfa (activated) in adults with septic shock. N Engl J Med. 2012;366:2055-2064. doi:10.1056/NEJMoa1202290
- Bernard GR, Vincent J-L, Laterre P-F, et al; . Efficacy and safety of recombinant human activated protein C for severe sepsis. N Engl J Med. 2001;344:699-709. doi:10.1056/NEJM200103083441001
- Hage-Sleiman M, Derre N, Verdet C, et al. Meningococcal purpura fulminans and severe myocarditis with clinical meningitis but no meningeal inflammation: a case report. BMC Infect Dis. 2019;19:252. doi:10.1186/s12879-019-3866-x
- Levi M, Toh CH, Thachil J, et al. Guidelines for the diagnosis and management of disseminated intravascular coagulation. British Committee for Standards in Haematology. Br J Haematol. 2009;145:24-33. doi:10.1111/j.1365-2141.2009.07600.x
Practice Points
- Capnocytophaga species are fastidious, slow-growing microorganisms. It is important, therefore, to maintain a high degree of suspicion and alertthe microbiology laboratory to increase the likelihood of isolation.
- Patients should be cautioned regarding the need for prophylactic antibiotics in the event of an animal bite; asplenic patients are at particular risk for infection.
- In patients with severe purpura fulminans and a gangrenous limb, it is important to allow adequate time for demarcation of gangrene and not rush to amputation.
Purpura Fulminans Induced by Vibrio vulnificus
To the Editor:
Purpura fulminans (PF) is an acute, life-threatening condition characterized by intravascular thrombosis and hemorrhagic necrosis of the skin. It classically presents as retiform purpura with branched or angular purpuric lesions. Purpura fulminans often occurs in the setting of disseminated intravascular coagulation, secondary to sepsis, trauma, malignancy, autoimmune disease, and congenital or acquired protein C or S deficiency, among other abnormalities.1 Rapid identification and treatment of the underlying cause are mainstays of management. We report a case of PF secondary to Vibrio vulnificus infection and highlight the importance of timely consideration of this etiologic agent due to the high mortality rate and specific treatment required.
A 58-year-old man with liver cirrhosis and hepatitis B virus presented with pain, swelling, and localized erythema affecting both legs as well as a fever. He reported vomiting blood and an episode of bloody diarrhea over the preceding 24 hours. He denied exposure to sick contacts or a history of autoimmune disease. At initial presentation to the emergency department, physical examination revealed few scattered, sharply demarcated, erythematous to violaceous patches that rapidly progressed overnight to hemorrhagic bullae and widespread retiform purpuric patches on both legs (Figure 1). As the patient’s skin condition worsened, he had a blood pressure of 80/50 mm Hg and a pulse rate of 110/min. Serum analysis was notable for mild leukocytosis (10.74×109/L [reference range, 4.8–10.8×109/L), thrombocytopenia (39×109/L [reference range, 150–450×109/L]), and decreased C3 (25 mg/dL [reference range, 81–157 mg/dL]) and C4 (8 mg/dL [reference range, 13–39 mg/dL]). Laboratory findings also were remarkable for prothrombin time (23.3 seconds [reference range, 8.8–12.3 seconds]), partial thromboplastin time (52.5 seconds [reference range, 23.6–35.8 seconds]), and international normalized ratio (2.01 [reference range, 0.8–1.13]). Aspartate transaminase (237 U/L [reference range, 11–39 U/L]) and alanine transaminase (80 U/L [reference range, 11–35 U/L]) were elevated, while antineutrophil cytoplasmic antibodies, serum immunoglobulin, and cryoglobulins were unremarkable. Punch biopsies of the left thigh were performed, and histopathology revealed small vessel thrombosis and ischemic changes consistent with PF (Figure 2). Vancomycin, clindamycin, cefepime injection, and piperacillin-tazobactam were administered intravenously for empiric broad-spectrum sepsis coverage. Within hours, the patient experienced refractory septic shock with disseminated intravascular coagulation and died from pulmonary embolism and subsequent cardiac arrest. Tissue and blood cultures grew V vulnificus.
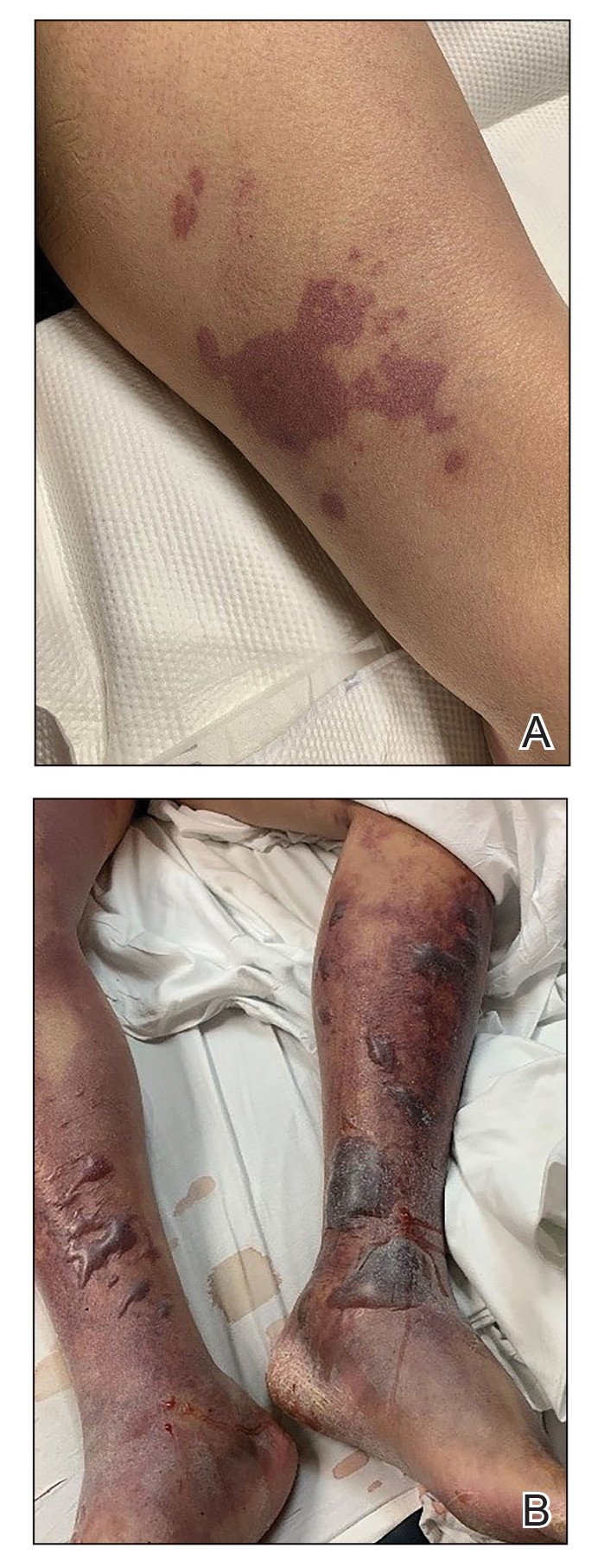
Vibrio vulnificus is a gram-negative bacillus and a rare cause of primary septicemia following consumption of shellfish, especially oysters. Wounds exposed to saltwater or brackish water contaminated with the microorganism can produce soft-tissue infections. Individuals with chronic liver disease are at greater risk for V vulnificus infection.2 The clinical presentation of V vulnificus includes early cellulitislike patches, late purpura with hemorrhagic bullae, and rapidly progressing shock.3
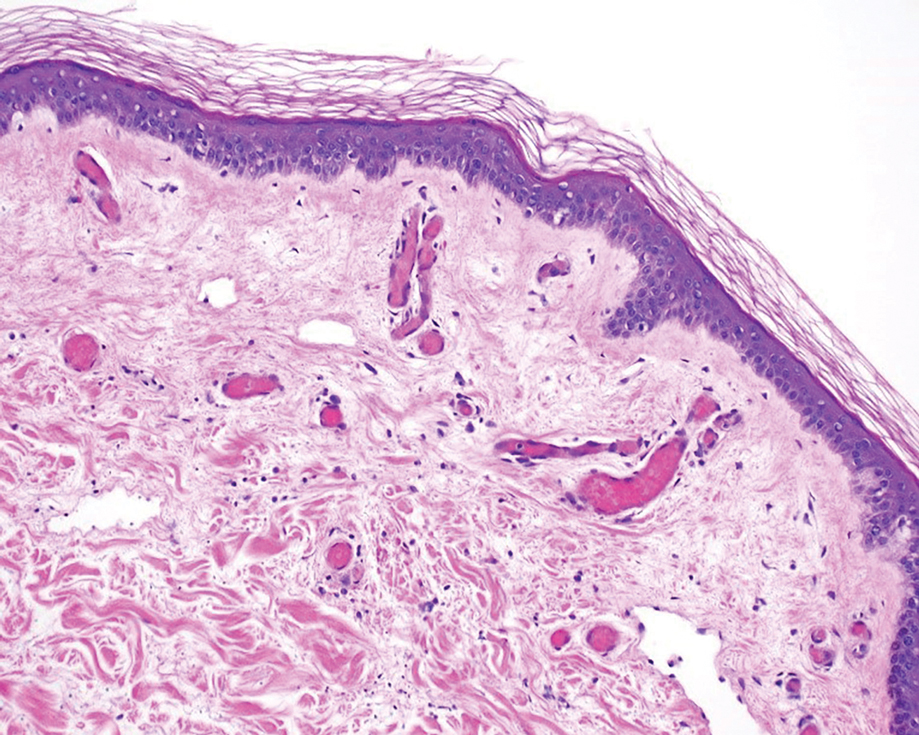
Mortality rates from V vulnificus infection are high.4 Therefore, it is recommended to presumptively diagnose V vulnificus septicemia in any individual at risk for infection who presents with the characteristic history in the setting of hypotension, fever, or septic shock. It is crucial for providers to be aware that broad-spectrum antibiotics commonly used for sepsis are inadequate for the treatment of V vulnificus. Immediate treatment with tetracycline (minocycline or doxycycline) and a third-generation cephalosporin (cefotaxime or ceftriaxone injection) or in combination with ciprofloxacin has been proven effective.4,5
Vibrio vulnificus rarely is described in the literature as inducing PF. In one previously reported case, the patient was otherwise healthy and managed to recover following antibiotic therapy and wound debridement,6 whereas in another case the patient had undiagnosed liver cirrhosis and died from the infection.6,7 In the latter case, the patient presented to the emergency department in a coma. Our patient did not have the clinical signs of sepsis upon initial presentation to the emergency department. It is possible the infection rapidly progressed because of his underlying liver disease. Genotyping analysis of V vulnificus has shown that strains with low pathogenicity can cause primary septicemia in humans.7
Our case reinforces the need to quickly recognize V vulnificus as a rare underlying cause of PF and administer the appropriate treatment.
- Levi M, Ten Cate H. Disseminated intravascular coagulation. N Engl J Med. 1999;341:586-592.
- Tacket CO, Brenner F, Blake PA. Clinical features and an epidemiological study of Vibrio vulnificus infections. J Infect Dis. 1984;149:558-561.
- Blake PA, Merson MH, Weaver RE et al. Disease caused by a marine Vibrio: clinical characteristics and epidemiology. N Engl J Med. 1979;300:1-5.
- Liu JW, Lee IK, Tang HJ, et al. Prognostic factors and antibiotics in Vibrio vulnificus septicemia. Arch Intern Med. 2006;166:2117-2123.
- Chen SC, Lee YT, Tsai SJ, et al. Antibiotic therapy for necrotizing fasciitis caused by Vibrio vulnificus: retrospective analysis of an 8 year period.J Antimicrob Chemother. 2012;67:488-493.
- Choi HJ, Lee DK, Lee MW et al. Vibrio vulnificus septicemia presenting as purpura fulminans. J Dermatol. 2005;32:48-51.
- Hori M, Nakayama A, Kitagawa D et al. A case of Vibrio vulnificus infection complicated with fulminant purpura: gene and biotype analysis of the pathogen. JMM Case Rep. 2017;4:e005096.
To the Editor:
Purpura fulminans (PF) is an acute, life-threatening condition characterized by intravascular thrombosis and hemorrhagic necrosis of the skin. It classically presents as retiform purpura with branched or angular purpuric lesions. Purpura fulminans often occurs in the setting of disseminated intravascular coagulation, secondary to sepsis, trauma, malignancy, autoimmune disease, and congenital or acquired protein C or S deficiency, among other abnormalities.1 Rapid identification and treatment of the underlying cause are mainstays of management. We report a case of PF secondary to Vibrio vulnificus infection and highlight the importance of timely consideration of this etiologic agent due to the high mortality rate and specific treatment required.
A 58-year-old man with liver cirrhosis and hepatitis B virus presented with pain, swelling, and localized erythema affecting both legs as well as a fever. He reported vomiting blood and an episode of bloody diarrhea over the preceding 24 hours. He denied exposure to sick contacts or a history of autoimmune disease. At initial presentation to the emergency department, physical examination revealed few scattered, sharply demarcated, erythematous to violaceous patches that rapidly progressed overnight to hemorrhagic bullae and widespread retiform purpuric patches on both legs (Figure 1). As the patient’s skin condition worsened, he had a blood pressure of 80/50 mm Hg and a pulse rate of 110/min. Serum analysis was notable for mild leukocytosis (10.74×109/L [reference range, 4.8–10.8×109/L), thrombocytopenia (39×109/L [reference range, 150–450×109/L]), and decreased C3 (25 mg/dL [reference range, 81–157 mg/dL]) and C4 (8 mg/dL [reference range, 13–39 mg/dL]). Laboratory findings also were remarkable for prothrombin time (23.3 seconds [reference range, 8.8–12.3 seconds]), partial thromboplastin time (52.5 seconds [reference range, 23.6–35.8 seconds]), and international normalized ratio (2.01 [reference range, 0.8–1.13]). Aspartate transaminase (237 U/L [reference range, 11–39 U/L]) and alanine transaminase (80 U/L [reference range, 11–35 U/L]) were elevated, while antineutrophil cytoplasmic antibodies, serum immunoglobulin, and cryoglobulins were unremarkable. Punch biopsies of the left thigh were performed, and histopathology revealed small vessel thrombosis and ischemic changes consistent with PF (Figure 2). Vancomycin, clindamycin, cefepime injection, and piperacillin-tazobactam were administered intravenously for empiric broad-spectrum sepsis coverage. Within hours, the patient experienced refractory septic shock with disseminated intravascular coagulation and died from pulmonary embolism and subsequent cardiac arrest. Tissue and blood cultures grew V vulnificus.

Vibrio vulnificus is a gram-negative bacillus and a rare cause of primary septicemia following consumption of shellfish, especially oysters. Wounds exposed to saltwater or brackish water contaminated with the microorganism can produce soft-tissue infections. Individuals with chronic liver disease are at greater risk for V vulnificus infection.2 The clinical presentation of V vulnificus includes early cellulitislike patches, late purpura with hemorrhagic bullae, and rapidly progressing shock.3

Mortality rates from V vulnificus infection are high.4 Therefore, it is recommended to presumptively diagnose V vulnificus septicemia in any individual at risk for infection who presents with the characteristic history in the setting of hypotension, fever, or septic shock. It is crucial for providers to be aware that broad-spectrum antibiotics commonly used for sepsis are inadequate for the treatment of V vulnificus. Immediate treatment with tetracycline (minocycline or doxycycline) and a third-generation cephalosporin (cefotaxime or ceftriaxone injection) or in combination with ciprofloxacin has been proven effective.4,5
Vibrio vulnificus rarely is described in the literature as inducing PF. In one previously reported case, the patient was otherwise healthy and managed to recover following antibiotic therapy and wound debridement,6 whereas in another case the patient had undiagnosed liver cirrhosis and died from the infection.6,7 In the latter case, the patient presented to the emergency department in a coma. Our patient did not have the clinical signs of sepsis upon initial presentation to the emergency department. It is possible the infection rapidly progressed because of his underlying liver disease. Genotyping analysis of V vulnificus has shown that strains with low pathogenicity can cause primary septicemia in humans.7
Our case reinforces the need to quickly recognize V vulnificus as a rare underlying cause of PF and administer the appropriate treatment.
To the Editor:
Purpura fulminans (PF) is an acute, life-threatening condition characterized by intravascular thrombosis and hemorrhagic necrosis of the skin. It classically presents as retiform purpura with branched or angular purpuric lesions. Purpura fulminans often occurs in the setting of disseminated intravascular coagulation, secondary to sepsis, trauma, malignancy, autoimmune disease, and congenital or acquired protein C or S deficiency, among other abnormalities.1 Rapid identification and treatment of the underlying cause are mainstays of management. We report a case of PF secondary to Vibrio vulnificus infection and highlight the importance of timely consideration of this etiologic agent due to the high mortality rate and specific treatment required.
A 58-year-old man with liver cirrhosis and hepatitis B virus presented with pain, swelling, and localized erythema affecting both legs as well as a fever. He reported vomiting blood and an episode of bloody diarrhea over the preceding 24 hours. He denied exposure to sick contacts or a history of autoimmune disease. At initial presentation to the emergency department, physical examination revealed few scattered, sharply demarcated, erythematous to violaceous patches that rapidly progressed overnight to hemorrhagic bullae and widespread retiform purpuric patches on both legs (Figure 1). As the patient’s skin condition worsened, he had a blood pressure of 80/50 mm Hg and a pulse rate of 110/min. Serum analysis was notable for mild leukocytosis (10.74×109/L [reference range, 4.8–10.8×109/L), thrombocytopenia (39×109/L [reference range, 150–450×109/L]), and decreased C3 (25 mg/dL [reference range, 81–157 mg/dL]) and C4 (8 mg/dL [reference range, 13–39 mg/dL]). Laboratory findings also were remarkable for prothrombin time (23.3 seconds [reference range, 8.8–12.3 seconds]), partial thromboplastin time (52.5 seconds [reference range, 23.6–35.8 seconds]), and international normalized ratio (2.01 [reference range, 0.8–1.13]). Aspartate transaminase (237 U/L [reference range, 11–39 U/L]) and alanine transaminase (80 U/L [reference range, 11–35 U/L]) were elevated, while antineutrophil cytoplasmic antibodies, serum immunoglobulin, and cryoglobulins were unremarkable. Punch biopsies of the left thigh were performed, and histopathology revealed small vessel thrombosis and ischemic changes consistent with PF (Figure 2). Vancomycin, clindamycin, cefepime injection, and piperacillin-tazobactam were administered intravenously for empiric broad-spectrum sepsis coverage. Within hours, the patient experienced refractory septic shock with disseminated intravascular coagulation and died from pulmonary embolism and subsequent cardiac arrest. Tissue and blood cultures grew V vulnificus.

Vibrio vulnificus is a gram-negative bacillus and a rare cause of primary septicemia following consumption of shellfish, especially oysters. Wounds exposed to saltwater or brackish water contaminated with the microorganism can produce soft-tissue infections. Individuals with chronic liver disease are at greater risk for V vulnificus infection.2 The clinical presentation of V vulnificus includes early cellulitislike patches, late purpura with hemorrhagic bullae, and rapidly progressing shock.3

Mortality rates from V vulnificus infection are high.4 Therefore, it is recommended to presumptively diagnose V vulnificus septicemia in any individual at risk for infection who presents with the characteristic history in the setting of hypotension, fever, or septic shock. It is crucial for providers to be aware that broad-spectrum antibiotics commonly used for sepsis are inadequate for the treatment of V vulnificus. Immediate treatment with tetracycline (minocycline or doxycycline) and a third-generation cephalosporin (cefotaxime or ceftriaxone injection) or in combination with ciprofloxacin has been proven effective.4,5
Vibrio vulnificus rarely is described in the literature as inducing PF. In one previously reported case, the patient was otherwise healthy and managed to recover following antibiotic therapy and wound debridement,6 whereas in another case the patient had undiagnosed liver cirrhosis and died from the infection.6,7 In the latter case, the patient presented to the emergency department in a coma. Our patient did not have the clinical signs of sepsis upon initial presentation to the emergency department. It is possible the infection rapidly progressed because of his underlying liver disease. Genotyping analysis of V vulnificus has shown that strains with low pathogenicity can cause primary septicemia in humans.7
Our case reinforces the need to quickly recognize V vulnificus as a rare underlying cause of PF and administer the appropriate treatment.
- Levi M, Ten Cate H. Disseminated intravascular coagulation. N Engl J Med. 1999;341:586-592.
- Tacket CO, Brenner F, Blake PA. Clinical features and an epidemiological study of Vibrio vulnificus infections. J Infect Dis. 1984;149:558-561.
- Blake PA, Merson MH, Weaver RE et al. Disease caused by a marine Vibrio: clinical characteristics and epidemiology. N Engl J Med. 1979;300:1-5.
- Liu JW, Lee IK, Tang HJ, et al. Prognostic factors and antibiotics in Vibrio vulnificus septicemia. Arch Intern Med. 2006;166:2117-2123.
- Chen SC, Lee YT, Tsai SJ, et al. Antibiotic therapy for necrotizing fasciitis caused by Vibrio vulnificus: retrospective analysis of an 8 year period.J Antimicrob Chemother. 2012;67:488-493.
- Choi HJ, Lee DK, Lee MW et al. Vibrio vulnificus septicemia presenting as purpura fulminans. J Dermatol. 2005;32:48-51.
- Hori M, Nakayama A, Kitagawa D et al. A case of Vibrio vulnificus infection complicated with fulminant purpura: gene and biotype analysis of the pathogen. JMM Case Rep. 2017;4:e005096.
- Levi M, Ten Cate H. Disseminated intravascular coagulation. N Engl J Med. 1999;341:586-592.
- Tacket CO, Brenner F, Blake PA. Clinical features and an epidemiological study of Vibrio vulnificus infections. J Infect Dis. 1984;149:558-561.
- Blake PA, Merson MH, Weaver RE et al. Disease caused by a marine Vibrio: clinical characteristics and epidemiology. N Engl J Med. 1979;300:1-5.
- Liu JW, Lee IK, Tang HJ, et al. Prognostic factors and antibiotics in Vibrio vulnificus septicemia. Arch Intern Med. 2006;166:2117-2123.
- Chen SC, Lee YT, Tsai SJ, et al. Antibiotic therapy for necrotizing fasciitis caused by Vibrio vulnificus: retrospective analysis of an 8 year period.J Antimicrob Chemother. 2012;67:488-493.
- Choi HJ, Lee DK, Lee MW et al. Vibrio vulnificus septicemia presenting as purpura fulminans. J Dermatol. 2005;32:48-51.
- Hori M, Nakayama A, Kitagawa D et al. A case of Vibrio vulnificus infection complicated with fulminant purpura: gene and biotype analysis of the pathogen. JMM Case Rep. 2017;4:e005096.
Practice Points
- Purpura fulminans (PF) is a life-threatening condition characterized by intravascular coagulation and skin necrosis.
- Patients with underlying liver disease are at greater risk for PF secondary to Vibrio vulnificus infection.
- Given the high mortality rate, rapid identification of the etiologic agent and timely antibiotic treatment are necessary.
COVID-19 vaccines: Lower serologic response among IBD, rheumatic diseases
Patients with immune-mediated inflammatory diseases (IMIDs), such as inflammatory bowel disease and rheumatic conditions, have a reduced serologic response to a 2-dose vaccination regimen with mRNA COVID-19 vaccines, according to the findings of a meta-analysis.
“These results suggest that IMID patients receiving mRNA vaccines should complete the vaccine series without delay and support the strategy of providing a third dose of the vaccine,” wrote study authors Atsushi Sakuraba, MD, of the University of Chicago Medicine, and colleagues in Gastroenterology.
During the COVID-19 pandemic, concerns were raised about the susceptibility of patients with pre-existing conditions to infection with the novel coronavirus, the authors noted. Likewise, ongoing concerns have centered on the risk of worse COVID-19–related outcomes among patients with IMIDs who are treated with immunosuppressive agents.
Since the onset of the pandemic, several registries have been established to gauge the incidence and prognosis of COVID-19 in patients with IMID, including the Surveillance Epidemiology of Coronavirus Under Research Exclusion (SECURE)–Inflammatory Bowel Disease (IBD) registry and the COVID-19 Global Rheumatology Alliance 75 (C19-GRA), which includes patients with rheumatic diseases.
Authorization of COVID-19 mRNA vaccines provided hope that the COVID-19 pandemic could soon come to an end given the overwhelming safety and efficacy data supporting the use of these vaccines for preventing hospitalization and death. Despite these data, little is known regarding the efficacy of mRNA COVID-19 vaccines in patients with IMIDs and/or patients treated with immunosuppressive therapies, as these patients were excluded from the regulatory vaccine studies.
The study by Dr. Sakuraba and colleagues was a meta-analysis of 25 observational studies that reported serologic response rates to COVID-19 vaccination in a pooled cohort of 5,360 patients with IMIDs. Data regarding the reference population, medications, vaccination, and proportion of patients who achieved a serologic response were extracted from the observational studies and included in the meta-analysis.
In the analyzed studies, serologic response was evaluated separately after one or two vaccine doses. The researchers also examined the post-vaccine serologic response rate in patients with IMIDs versus controls without IMIDs.
A total of 23 studies used the BNT162b2 or mRNA-1273 vaccines, while 3 studies reported that 50% to 75.9% of patients received the AZD1222 vaccine. Some studies also included patients who received other COVID-19 vaccines, including CoronaVac, BBV152, and Ad26.COV2.S.
While 6 studies assessed serologic response to COVID-19 after just 1 dose, 20 studies assessed the post-vaccination serologic response following 2 doses. In most cases, researchers evaluated serologic response at 2 to 3 weeks after the first dose. After the second vaccine dose, most studies examined serologic response at 1 to 3 weeks.
The serologic response after 1 dose of the mRNA vaccines was 73.2% (95% CI 65.7-79.5). In a multivariate meta-regression analysis, the researchers found that a significantly greater proportion of patients with IMIDs who took anti-tumor necrosis factor (anti-TNF) therapies had a lower serologic response rate (coefficient, –2.60; 95% CI –4.49 to –0.72; P =.0069). The investigators indicated this “likely contributed to the difference in serologic response rates and overall heterogeneity.”
Studies with patients with IBD reported a lower serologic response rate compared with studies that included patients with rheumatoid arthritis (49.2% vs. 65.0%, respectively), which the investigators explained was likely reflective of the increased use of anti-TNF agents in patients with IBD.
After 2 doses of the mRNA vaccines, the pooled serologic response was 83.4% (95% CI, 76.8%-88.4%). Multivariate meta-regression found that a significantly greater proportion of patients who took anti-CD20 treatments had a lower serologic response (coefficient, -6.08; 95% CI -9.40 to -2.76; P <.001). The investigators found that older age was significantly associated with lower serologic response after 2 doses (coefficient, -0.044; 95% CI -0.083 to -0.0050; P =.027).
For the non-mRNA COVID-19 vaccines, the rates of serologic response after 2 doses were 93.5% with AZD1222, 22.9% with CoronaVac, and 55.6% with BBV152.
Compared with controls without IMIDs, those with IMIDs were significantly less likely to achieve a serologic response following 2 mRNA vaccine doses (odds ratio, 0.086; 95% CI 0.036-0.206; P <.001). The investigators noted that there were not enough studies to examine and compare serologic response rates to adenoviral or inactivated vaccines between patients and controls.
In terms of limitations, the researchers wrote that additional studies examining humoral and cellular immunity to COVID-19 vaccines are needed to determine vaccine efficacy and durability in patients with IMIDs. Additionally, there is a need for studies with larger patient populations to determine serologic response to COVID-19 vaccines in the broader IMID population.
The researchers reported no funding for the study and no relevant conflicts of interest with the pharmaceutical industry.
Patients with immune-mediated inflammatory diseases (IMIDs), such as inflammatory bowel disease and rheumatic conditions, have a reduced serologic response to a 2-dose vaccination regimen with mRNA COVID-19 vaccines, according to the findings of a meta-analysis.
“These results suggest that IMID patients receiving mRNA vaccines should complete the vaccine series without delay and support the strategy of providing a third dose of the vaccine,” wrote study authors Atsushi Sakuraba, MD, of the University of Chicago Medicine, and colleagues in Gastroenterology.
During the COVID-19 pandemic, concerns were raised about the susceptibility of patients with pre-existing conditions to infection with the novel coronavirus, the authors noted. Likewise, ongoing concerns have centered on the risk of worse COVID-19–related outcomes among patients with IMIDs who are treated with immunosuppressive agents.
Since the onset of the pandemic, several registries have been established to gauge the incidence and prognosis of COVID-19 in patients with IMID, including the Surveillance Epidemiology of Coronavirus Under Research Exclusion (SECURE)–Inflammatory Bowel Disease (IBD) registry and the COVID-19 Global Rheumatology Alliance 75 (C19-GRA), which includes patients with rheumatic diseases.
Authorization of COVID-19 mRNA vaccines provided hope that the COVID-19 pandemic could soon come to an end given the overwhelming safety and efficacy data supporting the use of these vaccines for preventing hospitalization and death. Despite these data, little is known regarding the efficacy of mRNA COVID-19 vaccines in patients with IMIDs and/or patients treated with immunosuppressive therapies, as these patients were excluded from the regulatory vaccine studies.
The study by Dr. Sakuraba and colleagues was a meta-analysis of 25 observational studies that reported serologic response rates to COVID-19 vaccination in a pooled cohort of 5,360 patients with IMIDs. Data regarding the reference population, medications, vaccination, and proportion of patients who achieved a serologic response were extracted from the observational studies and included in the meta-analysis.
In the analyzed studies, serologic response was evaluated separately after one or two vaccine doses. The researchers also examined the post-vaccine serologic response rate in patients with IMIDs versus controls without IMIDs.
A total of 23 studies used the BNT162b2 or mRNA-1273 vaccines, while 3 studies reported that 50% to 75.9% of patients received the AZD1222 vaccine. Some studies also included patients who received other COVID-19 vaccines, including CoronaVac, BBV152, and Ad26.COV2.S.
While 6 studies assessed serologic response to COVID-19 after just 1 dose, 20 studies assessed the post-vaccination serologic response following 2 doses. In most cases, researchers evaluated serologic response at 2 to 3 weeks after the first dose. After the second vaccine dose, most studies examined serologic response at 1 to 3 weeks.
The serologic response after 1 dose of the mRNA vaccines was 73.2% (95% CI 65.7-79.5). In a multivariate meta-regression analysis, the researchers found that a significantly greater proportion of patients with IMIDs who took anti-tumor necrosis factor (anti-TNF) therapies had a lower serologic response rate (coefficient, –2.60; 95% CI –4.49 to –0.72; P =.0069). The investigators indicated this “likely contributed to the difference in serologic response rates and overall heterogeneity.”
Studies with patients with IBD reported a lower serologic response rate compared with studies that included patients with rheumatoid arthritis (49.2% vs. 65.0%, respectively), which the investigators explained was likely reflective of the increased use of anti-TNF agents in patients with IBD.
After 2 doses of the mRNA vaccines, the pooled serologic response was 83.4% (95% CI, 76.8%-88.4%). Multivariate meta-regression found that a significantly greater proportion of patients who took anti-CD20 treatments had a lower serologic response (coefficient, -6.08; 95% CI -9.40 to -2.76; P <.001). The investigators found that older age was significantly associated with lower serologic response after 2 doses (coefficient, -0.044; 95% CI -0.083 to -0.0050; P =.027).
For the non-mRNA COVID-19 vaccines, the rates of serologic response after 2 doses were 93.5% with AZD1222, 22.9% with CoronaVac, and 55.6% with BBV152.
Compared with controls without IMIDs, those with IMIDs were significantly less likely to achieve a serologic response following 2 mRNA vaccine doses (odds ratio, 0.086; 95% CI 0.036-0.206; P <.001). The investigators noted that there were not enough studies to examine and compare serologic response rates to adenoviral or inactivated vaccines between patients and controls.
In terms of limitations, the researchers wrote that additional studies examining humoral and cellular immunity to COVID-19 vaccines are needed to determine vaccine efficacy and durability in patients with IMIDs. Additionally, there is a need for studies with larger patient populations to determine serologic response to COVID-19 vaccines in the broader IMID population.
The researchers reported no funding for the study and no relevant conflicts of interest with the pharmaceutical industry.
Patients with immune-mediated inflammatory diseases (IMIDs), such as inflammatory bowel disease and rheumatic conditions, have a reduced serologic response to a 2-dose vaccination regimen with mRNA COVID-19 vaccines, according to the findings of a meta-analysis.
“These results suggest that IMID patients receiving mRNA vaccines should complete the vaccine series without delay and support the strategy of providing a third dose of the vaccine,” wrote study authors Atsushi Sakuraba, MD, of the University of Chicago Medicine, and colleagues in Gastroenterology.
During the COVID-19 pandemic, concerns were raised about the susceptibility of patients with pre-existing conditions to infection with the novel coronavirus, the authors noted. Likewise, ongoing concerns have centered on the risk of worse COVID-19–related outcomes among patients with IMIDs who are treated with immunosuppressive agents.
Since the onset of the pandemic, several registries have been established to gauge the incidence and prognosis of COVID-19 in patients with IMID, including the Surveillance Epidemiology of Coronavirus Under Research Exclusion (SECURE)–Inflammatory Bowel Disease (IBD) registry and the COVID-19 Global Rheumatology Alliance 75 (C19-GRA), which includes patients with rheumatic diseases.
Authorization of COVID-19 mRNA vaccines provided hope that the COVID-19 pandemic could soon come to an end given the overwhelming safety and efficacy data supporting the use of these vaccines for preventing hospitalization and death. Despite these data, little is known regarding the efficacy of mRNA COVID-19 vaccines in patients with IMIDs and/or patients treated with immunosuppressive therapies, as these patients were excluded from the regulatory vaccine studies.
The study by Dr. Sakuraba and colleagues was a meta-analysis of 25 observational studies that reported serologic response rates to COVID-19 vaccination in a pooled cohort of 5,360 patients with IMIDs. Data regarding the reference population, medications, vaccination, and proportion of patients who achieved a serologic response were extracted from the observational studies and included in the meta-analysis.
In the analyzed studies, serologic response was evaluated separately after one or two vaccine doses. The researchers also examined the post-vaccine serologic response rate in patients with IMIDs versus controls without IMIDs.
A total of 23 studies used the BNT162b2 or mRNA-1273 vaccines, while 3 studies reported that 50% to 75.9% of patients received the AZD1222 vaccine. Some studies also included patients who received other COVID-19 vaccines, including CoronaVac, BBV152, and Ad26.COV2.S.
While 6 studies assessed serologic response to COVID-19 after just 1 dose, 20 studies assessed the post-vaccination serologic response following 2 doses. In most cases, researchers evaluated serologic response at 2 to 3 weeks after the first dose. After the second vaccine dose, most studies examined serologic response at 1 to 3 weeks.
The serologic response after 1 dose of the mRNA vaccines was 73.2% (95% CI 65.7-79.5). In a multivariate meta-regression analysis, the researchers found that a significantly greater proportion of patients with IMIDs who took anti-tumor necrosis factor (anti-TNF) therapies had a lower serologic response rate (coefficient, –2.60; 95% CI –4.49 to –0.72; P =.0069). The investigators indicated this “likely contributed to the difference in serologic response rates and overall heterogeneity.”
Studies with patients with IBD reported a lower serologic response rate compared with studies that included patients with rheumatoid arthritis (49.2% vs. 65.0%, respectively), which the investigators explained was likely reflective of the increased use of anti-TNF agents in patients with IBD.
After 2 doses of the mRNA vaccines, the pooled serologic response was 83.4% (95% CI, 76.8%-88.4%). Multivariate meta-regression found that a significantly greater proportion of patients who took anti-CD20 treatments had a lower serologic response (coefficient, -6.08; 95% CI -9.40 to -2.76; P <.001). The investigators found that older age was significantly associated with lower serologic response after 2 doses (coefficient, -0.044; 95% CI -0.083 to -0.0050; P =.027).
For the non-mRNA COVID-19 vaccines, the rates of serologic response after 2 doses were 93.5% with AZD1222, 22.9% with CoronaVac, and 55.6% with BBV152.
Compared with controls without IMIDs, those with IMIDs were significantly less likely to achieve a serologic response following 2 mRNA vaccine doses (odds ratio, 0.086; 95% CI 0.036-0.206; P <.001). The investigators noted that there were not enough studies to examine and compare serologic response rates to adenoviral or inactivated vaccines between patients and controls.
In terms of limitations, the researchers wrote that additional studies examining humoral and cellular immunity to COVID-19 vaccines are needed to determine vaccine efficacy and durability in patients with IMIDs. Additionally, there is a need for studies with larger patient populations to determine serologic response to COVID-19 vaccines in the broader IMID population.
The researchers reported no funding for the study and no relevant conflicts of interest with the pharmaceutical industry.
FROM GASTROENTEROLOGY
Case: Older patient with T2D has recurrent flushing
He has had no other symptoms. His only abnormalities on physical exam are a blood pressure of 160/100 and mild peripheral edema.
His current medications include: Famotidine 20 mg b.i.d., Pseudoephedrine/guaifenesin SR b.i.d., Metformin 1,000 mg twice a day, Nifedipine 60 mg XL once a day, and Atorvastatin 20 mg once a day.
His laboratory work up includes: blood urea nitrogen: 20, creatinine: 1.3, sodium: 140, Chloride: 104, potassium: 3.9, glucose: 205, white blood cell count: 6,000, hematocrit: 41, 24-hour urine 5-hydroxyindoleacetic acid (5HIAA) test: 12 mg/day (normal 2-8 mg/day), free catecholamines: 80 mg/24 hours (normal less than 100 mg/24 hours).
What is the most likely diagnosis?
A. Drug effect
B. Pheochromocytoma
C. Carcinoid syndrome
D. Mastocytosis
E. Medullary thyroid cancer
The most likely diagnosis is a drug effect. His flushing is likely caused by nifedipine.
Flushing is one of the most common side effects of this drug.1 This patient had lab testing done for carcinoid (urine 5HIAA), presumably because he had flushing. This lab test result was a false positive, likely because of guaifenesin ingestion, which can cause false-positive 5HIAA results.2
Carcinoid syndrome is very rare (estimates from less than 1 patient/100,000), and the vast majority of patients who have it present with metastatic disease at presentation. Drug side effects are common, and usually are much more likely than rare diseases.
Four principles for assisting with making a diagnosis
This case points out the following four principles that I will touch on to help us make diagnoses in challenging cases.
1. Trigger symptoms: These are symptoms that make us think of a rare disease. In this case, the symptom is flushing, which may make you think of carcinoid syndrome.
Another good example of a trigger symptom is night sweats, where you may think of tuberculosis or lymphoma. These symptoms almost always have a much more common and likely cause, which in this case is a common drug side effect.
Trigger symptoms are great to pay attention to, but do not jump to working up the rare diagnosis without more evidence that it is a plausible diagnosis. Working up rare diseases without a reasonable pretest probability will lead to significant false-positive results.
2. Distinguishing features: These are findings, or combinations of findings, that make rarer diseases more likely. For example, flushing, although seen in many patients with carcinoid syndrome, is much more commonly caused by rosacea, medications, or estrogen/testosterone deficiency.
If a patient presents with flushing plus diarrhea, carcinoid syndrome becomes more likely in differentials. An example of a specific distinguishing feature is transient visual obstructions in patients with idiopathic intracranial hypertension (IIH or pseudotumor cerebri).
Sudden transient visual loss is not a symptom we see often, but headaches and obesity are problems we see every day. A patient with headaches and obesity is very likely to have IIH if they have transient visual obstructions along with headaches and obesity.
3. Intentional physical exams: Do the physical exam focusing on what findings will change your diagnostic probabilities. For example, in this case, if you are considering carcinoid, do a careful abdominal exam, with close attention to the liver, as 75% of patients with carcinoid syndrome have liver metastases.
If you are thinking about IIH, a fundoscopic exam is mandatory, as papilledema is a key feature of this diagnosis.
Read about the rare diagnosis you are considering, this will help with targeting your exam.
4. Remember the unusual presentation of a common disease is more common than the common presentation of a rare disease: Good examples of this are sleep apnea and gastroesophageal reflux disease causing night sweats more commonly than finding lymphomas or active tuberculosis (in the United States) as the cause.3
Pearl: Trigger symptoms help us think of rare diseases, but distinguishing features are most helpful in including or excluding the diagnosis.
Dr. Paauw is professor of medicine in the division of general internal medicine at the University of Washington, Seattle, and serves as third-year medical student clerkship director at the University of Washington. He is a member of the editorial advisory board of Internal Medicine News. Dr. Paauw has no conflicts to disclose. Contact him at [email protected].
References
1. Gueret P et al. Drugs. 1990;39 Suppl 2:67-72.
2. Corcuff J et al. Endocr Connect. 2017;6:R87.
3. Smith CS and Paauw DS. J Am Board Fam Pract. 2000;13:424-9.
He has had no other symptoms. His only abnormalities on physical exam are a blood pressure of 160/100 and mild peripheral edema.
His current medications include: Famotidine 20 mg b.i.d., Pseudoephedrine/guaifenesin SR b.i.d., Metformin 1,000 mg twice a day, Nifedipine 60 mg XL once a day, and Atorvastatin 20 mg once a day.
His laboratory work up includes: blood urea nitrogen: 20, creatinine: 1.3, sodium: 140, Chloride: 104, potassium: 3.9, glucose: 205, white blood cell count: 6,000, hematocrit: 41, 24-hour urine 5-hydroxyindoleacetic acid (5HIAA) test: 12 mg/day (normal 2-8 mg/day), free catecholamines: 80 mg/24 hours (normal less than 100 mg/24 hours).
What is the most likely diagnosis?
A. Drug effect
B. Pheochromocytoma
C. Carcinoid syndrome
D. Mastocytosis
E. Medullary thyroid cancer
The most likely diagnosis is a drug effect. His flushing is likely caused by nifedipine.
Flushing is one of the most common side effects of this drug.1 This patient had lab testing done for carcinoid (urine 5HIAA), presumably because he had flushing. This lab test result was a false positive, likely because of guaifenesin ingestion, which can cause false-positive 5HIAA results.2
Carcinoid syndrome is very rare (estimates from less than 1 patient/100,000), and the vast majority of patients who have it present with metastatic disease at presentation. Drug side effects are common, and usually are much more likely than rare diseases.
Four principles for assisting with making a diagnosis
This case points out the following four principles that I will touch on to help us make diagnoses in challenging cases.
1. Trigger symptoms: These are symptoms that make us think of a rare disease. In this case, the symptom is flushing, which may make you think of carcinoid syndrome.
Another good example of a trigger symptom is night sweats, where you may think of tuberculosis or lymphoma. These symptoms almost always have a much more common and likely cause, which in this case is a common drug side effect.
Trigger symptoms are great to pay attention to, but do not jump to working up the rare diagnosis without more evidence that it is a plausible diagnosis. Working up rare diseases without a reasonable pretest probability will lead to significant false-positive results.
2. Distinguishing features: These are findings, or combinations of findings, that make rarer diseases more likely. For example, flushing, although seen in many patients with carcinoid syndrome, is much more commonly caused by rosacea, medications, or estrogen/testosterone deficiency.
If a patient presents with flushing plus diarrhea, carcinoid syndrome becomes more likely in differentials. An example of a specific distinguishing feature is transient visual obstructions in patients with idiopathic intracranial hypertension (IIH or pseudotumor cerebri).
Sudden transient visual loss is not a symptom we see often, but headaches and obesity are problems we see every day. A patient with headaches and obesity is very likely to have IIH if they have transient visual obstructions along with headaches and obesity.
3. Intentional physical exams: Do the physical exam focusing on what findings will change your diagnostic probabilities. For example, in this case, if you are considering carcinoid, do a careful abdominal exam, with close attention to the liver, as 75% of patients with carcinoid syndrome have liver metastases.
If you are thinking about IIH, a fundoscopic exam is mandatory, as papilledema is a key feature of this diagnosis.
Read about the rare diagnosis you are considering, this will help with targeting your exam.
4. Remember the unusual presentation of a common disease is more common than the common presentation of a rare disease: Good examples of this are sleep apnea and gastroesophageal reflux disease causing night sweats more commonly than finding lymphomas or active tuberculosis (in the United States) as the cause.3
Pearl: Trigger symptoms help us think of rare diseases, but distinguishing features are most helpful in including or excluding the diagnosis.
Dr. Paauw is professor of medicine in the division of general internal medicine at the University of Washington, Seattle, and serves as third-year medical student clerkship director at the University of Washington. He is a member of the editorial advisory board of Internal Medicine News. Dr. Paauw has no conflicts to disclose. Contact him at [email protected].
References
1. Gueret P et al. Drugs. 1990;39 Suppl 2:67-72.
2. Corcuff J et al. Endocr Connect. 2017;6:R87.
3. Smith CS and Paauw DS. J Am Board Fam Pract. 2000;13:424-9.
He has had no other symptoms. His only abnormalities on physical exam are a blood pressure of 160/100 and mild peripheral edema.
His current medications include: Famotidine 20 mg b.i.d., Pseudoephedrine/guaifenesin SR b.i.d., Metformin 1,000 mg twice a day, Nifedipine 60 mg XL once a day, and Atorvastatin 20 mg once a day.
His laboratory work up includes: blood urea nitrogen: 20, creatinine: 1.3, sodium: 140, Chloride: 104, potassium: 3.9, glucose: 205, white blood cell count: 6,000, hematocrit: 41, 24-hour urine 5-hydroxyindoleacetic acid (5HIAA) test: 12 mg/day (normal 2-8 mg/day), free catecholamines: 80 mg/24 hours (normal less than 100 mg/24 hours).
What is the most likely diagnosis?
A. Drug effect
B. Pheochromocytoma
C. Carcinoid syndrome
D. Mastocytosis
E. Medullary thyroid cancer
The most likely diagnosis is a drug effect. His flushing is likely caused by nifedipine.
Flushing is one of the most common side effects of this drug.1 This patient had lab testing done for carcinoid (urine 5HIAA), presumably because he had flushing. This lab test result was a false positive, likely because of guaifenesin ingestion, which can cause false-positive 5HIAA results.2
Carcinoid syndrome is very rare (estimates from less than 1 patient/100,000), and the vast majority of patients who have it present with metastatic disease at presentation. Drug side effects are common, and usually are much more likely than rare diseases.
Four principles for assisting with making a diagnosis
This case points out the following four principles that I will touch on to help us make diagnoses in challenging cases.
1. Trigger symptoms: These are symptoms that make us think of a rare disease. In this case, the symptom is flushing, which may make you think of carcinoid syndrome.
Another good example of a trigger symptom is night sweats, where you may think of tuberculosis or lymphoma. These symptoms almost always have a much more common and likely cause, which in this case is a common drug side effect.
Trigger symptoms are great to pay attention to, but do not jump to working up the rare diagnosis without more evidence that it is a plausible diagnosis. Working up rare diseases without a reasonable pretest probability will lead to significant false-positive results.
2. Distinguishing features: These are findings, or combinations of findings, that make rarer diseases more likely. For example, flushing, although seen in many patients with carcinoid syndrome, is much more commonly caused by rosacea, medications, or estrogen/testosterone deficiency.
If a patient presents with flushing plus diarrhea, carcinoid syndrome becomes more likely in differentials. An example of a specific distinguishing feature is transient visual obstructions in patients with idiopathic intracranial hypertension (IIH or pseudotumor cerebri).
Sudden transient visual loss is not a symptom we see often, but headaches and obesity are problems we see every day. A patient with headaches and obesity is very likely to have IIH if they have transient visual obstructions along with headaches and obesity.
3. Intentional physical exams: Do the physical exam focusing on what findings will change your diagnostic probabilities. For example, in this case, if you are considering carcinoid, do a careful abdominal exam, with close attention to the liver, as 75% of patients with carcinoid syndrome have liver metastases.
If you are thinking about IIH, a fundoscopic exam is mandatory, as papilledema is a key feature of this diagnosis.
Read about the rare diagnosis you are considering, this will help with targeting your exam.
4. Remember the unusual presentation of a common disease is more common than the common presentation of a rare disease: Good examples of this are sleep apnea and gastroesophageal reflux disease causing night sweats more commonly than finding lymphomas or active tuberculosis (in the United States) as the cause.3
Pearl: Trigger symptoms help us think of rare diseases, but distinguishing features are most helpful in including or excluding the diagnosis.
Dr. Paauw is professor of medicine in the division of general internal medicine at the University of Washington, Seattle, and serves as third-year medical student clerkship director at the University of Washington. He is a member of the editorial advisory board of Internal Medicine News. Dr. Paauw has no conflicts to disclose. Contact him at [email protected].
References
1. Gueret P et al. Drugs. 1990;39 Suppl 2:67-72.
2. Corcuff J et al. Endocr Connect. 2017;6:R87.
3. Smith CS and Paauw DS. J Am Board Fam Pract. 2000;13:424-9.
Perinatal research and the Tooth Fairy
How much did you get per tooth from the Tooth Fairy? How much do your children or grandchildren receive each time they lose a baby tooth? In my family the Tooth Fairy seems to be more than keeping with inflation. Has she ever been caught in the act of swapping cash for enamel in your home? Has she every slipped up one night but managed to resurrect her credibility the following night by doubling the reward? And, by the way, what does the Tooth Fairy do with all those teeth, and who’s funding her nocturnal switcheroos?
A recent study from the Center for Genomic Medicine at the Massachusetts General Hospital in Boston may provide an answer to at least one of those questions. It turns out some researchers have been collecting baby teeth in hopes of assessing prenatal and perinatal stress in infants.
Not surprisingly, teeth are like trees, preserving a history of the environment in their growth rings. The Boston researchers hypothesized that the thickness of one particular growth line referred to as the neonatal line (NNL) might reflect prenatal and immediate postnatal environmental stress. Using data and naturally shed teeth collected in an English longitudinal study, the authors discovered that the teeth of children whose mothers had a long history of severe depression or other psychiatric problems and children of mothers who at 32 weeks’ gestation experienced anxiety and/or depression were more likely to have thicker NNLs. On the other hand, the teeth of children whose mothers had received “significant social support” in the immediate postnatal period exhibited thinner NNLs.
Based on anecdotal observations, I think most of us already suspected that the children whose mothers had significant psychiatric illness began life with a challenge, but it is nice to know that we may now have a tool to document one small bit of evidence of the structural damage that occurred during this period of stress. Of course, the prior owners of these baby teeth won’t benefit from the findings in this study; however, the evidence that social support during the critical perinatal period can ameliorate the damage might stimulate more robust prenatal programs for mother and infants at risk in the future.
It will be interesting to see if this investigative tool becomes more widely used to determine the degree to which a variety of potential perinatal stressors are manifesting themselves in structural change in newborns. For example, collecting baby teeth from neonatal ICU graduates may answer some questions about how certain environmental conditions such as sound, vibration, bright light, and temperature may result in long-term damage to the infants. Most of us suspect that skin-to-skin contact with mother and kangaroo care are beneficial. A study that includes a survey of NNLs might go a long way toward supporting our suspicions.
I can even imagine that a deep retrospective study of NNLs in baby teeth collected over the last 100 years might demonstrate the effect of phenomena such as wars, natural disasters, forced migration, and pandemics, to name a few.
It may be time to put out a nationwide call to all Tooth Fairies both active and retired to dig deep in their top bureau drawers. Those little bits of long-forgotten enamel may hold the answers to a plethora of unanswered questions about those critical months surrounding the birth of a child.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at [email protected].
How much did you get per tooth from the Tooth Fairy? How much do your children or grandchildren receive each time they lose a baby tooth? In my family the Tooth Fairy seems to be more than keeping with inflation. Has she ever been caught in the act of swapping cash for enamel in your home? Has she every slipped up one night but managed to resurrect her credibility the following night by doubling the reward? And, by the way, what does the Tooth Fairy do with all those teeth, and who’s funding her nocturnal switcheroos?
A recent study from the Center for Genomic Medicine at the Massachusetts General Hospital in Boston may provide an answer to at least one of those questions. It turns out some researchers have been collecting baby teeth in hopes of assessing prenatal and perinatal stress in infants.
Not surprisingly, teeth are like trees, preserving a history of the environment in their growth rings. The Boston researchers hypothesized that the thickness of one particular growth line referred to as the neonatal line (NNL) might reflect prenatal and immediate postnatal environmental stress. Using data and naturally shed teeth collected in an English longitudinal study, the authors discovered that the teeth of children whose mothers had a long history of severe depression or other psychiatric problems and children of mothers who at 32 weeks’ gestation experienced anxiety and/or depression were more likely to have thicker NNLs. On the other hand, the teeth of children whose mothers had received “significant social support” in the immediate postnatal period exhibited thinner NNLs.
Based on anecdotal observations, I think most of us already suspected that the children whose mothers had significant psychiatric illness began life with a challenge, but it is nice to know that we may now have a tool to document one small bit of evidence of the structural damage that occurred during this period of stress. Of course, the prior owners of these baby teeth won’t benefit from the findings in this study; however, the evidence that social support during the critical perinatal period can ameliorate the damage might stimulate more robust prenatal programs for mother and infants at risk in the future.
It will be interesting to see if this investigative tool becomes more widely used to determine the degree to which a variety of potential perinatal stressors are manifesting themselves in structural change in newborns. For example, collecting baby teeth from neonatal ICU graduates may answer some questions about how certain environmental conditions such as sound, vibration, bright light, and temperature may result in long-term damage to the infants. Most of us suspect that skin-to-skin contact with mother and kangaroo care are beneficial. A study that includes a survey of NNLs might go a long way toward supporting our suspicions.
I can even imagine that a deep retrospective study of NNLs in baby teeth collected over the last 100 years might demonstrate the effect of phenomena such as wars, natural disasters, forced migration, and pandemics, to name a few.
It may be time to put out a nationwide call to all Tooth Fairies both active and retired to dig deep in their top bureau drawers. Those little bits of long-forgotten enamel may hold the answers to a plethora of unanswered questions about those critical months surrounding the birth of a child.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at [email protected].
How much did you get per tooth from the Tooth Fairy? How much do your children or grandchildren receive each time they lose a baby tooth? In my family the Tooth Fairy seems to be more than keeping with inflation. Has she ever been caught in the act of swapping cash for enamel in your home? Has she every slipped up one night but managed to resurrect her credibility the following night by doubling the reward? And, by the way, what does the Tooth Fairy do with all those teeth, and who’s funding her nocturnal switcheroos?
A recent study from the Center for Genomic Medicine at the Massachusetts General Hospital in Boston may provide an answer to at least one of those questions. It turns out some researchers have been collecting baby teeth in hopes of assessing prenatal and perinatal stress in infants.
Not surprisingly, teeth are like trees, preserving a history of the environment in their growth rings. The Boston researchers hypothesized that the thickness of one particular growth line referred to as the neonatal line (NNL) might reflect prenatal and immediate postnatal environmental stress. Using data and naturally shed teeth collected in an English longitudinal study, the authors discovered that the teeth of children whose mothers had a long history of severe depression or other psychiatric problems and children of mothers who at 32 weeks’ gestation experienced anxiety and/or depression were more likely to have thicker NNLs. On the other hand, the teeth of children whose mothers had received “significant social support” in the immediate postnatal period exhibited thinner NNLs.
Based on anecdotal observations, I think most of us already suspected that the children whose mothers had significant psychiatric illness began life with a challenge, but it is nice to know that we may now have a tool to document one small bit of evidence of the structural damage that occurred during this period of stress. Of course, the prior owners of these baby teeth won’t benefit from the findings in this study; however, the evidence that social support during the critical perinatal period can ameliorate the damage might stimulate more robust prenatal programs for mother and infants at risk in the future.
It will be interesting to see if this investigative tool becomes more widely used to determine the degree to which a variety of potential perinatal stressors are manifesting themselves in structural change in newborns. For example, collecting baby teeth from neonatal ICU graduates may answer some questions about how certain environmental conditions such as sound, vibration, bright light, and temperature may result in long-term damage to the infants. Most of us suspect that skin-to-skin contact with mother and kangaroo care are beneficial. A study that includes a survey of NNLs might go a long way toward supporting our suspicions.
I can even imagine that a deep retrospective study of NNLs in baby teeth collected over the last 100 years might demonstrate the effect of phenomena such as wars, natural disasters, forced migration, and pandemics, to name a few.
It may be time to put out a nationwide call to all Tooth Fairies both active and retired to dig deep in their top bureau drawers. Those little bits of long-forgotten enamel may hold the answers to a plethora of unanswered questions about those critical months surrounding the birth of a child.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at [email protected].
Largest ever review of new daily persistent headache assesses its clinical features
according to a new retrospective chart review.
“Future prospective studies are needed to better understand this disabling disorder,” wrote Randolph W. Evans, MD, of Baylor College of Medicine of Houston, and Dana P. Turner, PhD, of Massachusetts General Hospital and Harvard Medical School in Boston. Their study was published Oct. 28 in Headache.
To categorize the infrequently reported clinical features of NDPH, the researchers launched a retrospective study of patients who were provisionally diagnosed with NDPH by Dr. Evans at an outpatient clinic in Houston from Sept. 1, 2011, to Feb. 28, 2020. Of the 328 patients whose diagnosis ultimately matched the ICHD-3 criteria, the average age at onset was 40.3 years (range 12-87 years). Approximately 70% were White, and nearly 66% were women. Two hundred and sixty were diagnosed with the migraine phenotype and 68 were diagnosed with the tension-type phenotype.
Key features
The median duration of NDPH at the time of the initial consult with Dr. Evans was 0.7 years, and it was 1.9 years at the time of the last visit. Almost 33% of patients with the migraine phenotype had a history of episodic migraine compared with 16.2% with the tension-type phenotype. Headaches were side-locked unilateral in 8.5% (n = 28) of all patients, and 3.6% (n = 12) had a thunderclap onset.
The most common clinical features across all patients included noise sensitivity (72.1%), light sensitivity (71%), moderate pain at the time of initial consult (57.9%), pressure pain (54.9%), and throbbing pain (50.9%). Nausea was reported in 157 patients and vomiting was reported in 48 patients, all of whom were in the migraine phenotype group. Thunderclap onset was far more prevalent in the migraine phenotype group (11 patients) compared with the tension-type phenotype group (1 patient), as was vertigo (19 patients compared with 1) and visual aura (21 compared with 0).
The top precipitating factors across all patients included stressful life events (20.4%), an antecedent upper respiratory infection or flu-like illness (10.1%), and antecedent extracranial surgery (1.5%). Exacerbating or aggravating factors were far more prevalent in the migraine phenotype group compared with the tension-type phenotype group, with stress (14.6% vs. 5.9%), bright or flashing light (10.4% vs. 1.5%), loud noise (8.5% vs. 0%), and lack of sleep (6.5% vs. 4.4%) leading the way.
The months with the most onsets were June (8.5%), January (7.6%), and February (7.6%); there was no clear seasonal or cyclical variation. The most common prognostic type across all patients was persisting (refractory) at 93%, followed by remitting (self-limiting) at 4.3% and relapsing-remitting at 2.7%.
Unlocking a medical mystery
“This is the largest case review study ever published on NDPH, especially because most people think it’s a fairly rare disorder when it’s actually not,” Herbert G. Markley, MD, of the New England Regional Headache Center in Worcester, Mass., said in an interview.
“The thing people need to understand is that they may have a lot of these patients in their practice and not realize it,” he added. “They keep trying one medication after another, and the patients are giving up, and the doctors are giving up. It’s terrible. We don’t know what causes it, and we don’t know how to treat it. It’s one of the biggest mysteries left in medical science.”
“My idea about this condition, and this is shared by others, is that NDPH is not a diagnosis that describes a cohesive group of patients but rather a group of people who share certain features,” Morris Levin, MD, director of the Headache Center at the University of California, San Francisco, said in an interview. “And they would be better served if this diagnosis was split into different categories.”
While praising Dr. Evans and Dr. Turner for their categorization and classification work, Dr. Levin asked, “Let’s say you diagnose someone with NDPH; does that in any way help you with management of this person? The answer is no. Some might say, ‘If you put the patients in the migraine phenotype group, then you can use migraine treatments.’ My point would be: then call it migraine.
“I believe another way to approach NDPH might be to create subcategories of migraine and tension-type headaches,” he added. “A migraine that is either intermittent or nonexistent suddenly becomes daily. That could be a subcategory; rather than being called NDPH, call it ‘new persistent chronic migraine.’ Or ‘new persistent chronic tension-type headache.’ Perhaps that would serve us better in terms of grasping the underlying mechanisms and the best treatment for these patients.”
Along the same lines, Dr. Markley echoed Dr. Evans’ call for more prospective studies and more research on possible medication, hoping to fuel further understanding of this debilitating disorder.
“I think this will be a landmark study for people to look back on,” he said, “especially for anyone going into the headache specialty who has never heard of this type of headache and keeps wondering why they can’t help certain patients, no matter how many medications they try.”
The authors acknowledged their study’s limitations, including its single-center nature and the data abstraction process being performed by just one person. They added, however, that Dr. Evans is a “very experienced researcher with more than 30 years of experience in headache medicine who was abstracting his own patients, data that were very familiar to him.”
Dr. Evans and Dr. Turner declared no potential conflicts of interest.
according to a new retrospective chart review.
“Future prospective studies are needed to better understand this disabling disorder,” wrote Randolph W. Evans, MD, of Baylor College of Medicine of Houston, and Dana P. Turner, PhD, of Massachusetts General Hospital and Harvard Medical School in Boston. Their study was published Oct. 28 in Headache.
To categorize the infrequently reported clinical features of NDPH, the researchers launched a retrospective study of patients who were provisionally diagnosed with NDPH by Dr. Evans at an outpatient clinic in Houston from Sept. 1, 2011, to Feb. 28, 2020. Of the 328 patients whose diagnosis ultimately matched the ICHD-3 criteria, the average age at onset was 40.3 years (range 12-87 years). Approximately 70% were White, and nearly 66% were women. Two hundred and sixty were diagnosed with the migraine phenotype and 68 were diagnosed with the tension-type phenotype.
Key features
The median duration of NDPH at the time of the initial consult with Dr. Evans was 0.7 years, and it was 1.9 years at the time of the last visit. Almost 33% of patients with the migraine phenotype had a history of episodic migraine compared with 16.2% with the tension-type phenotype. Headaches were side-locked unilateral in 8.5% (n = 28) of all patients, and 3.6% (n = 12) had a thunderclap onset.
The most common clinical features across all patients included noise sensitivity (72.1%), light sensitivity (71%), moderate pain at the time of initial consult (57.9%), pressure pain (54.9%), and throbbing pain (50.9%). Nausea was reported in 157 patients and vomiting was reported in 48 patients, all of whom were in the migraine phenotype group. Thunderclap onset was far more prevalent in the migraine phenotype group (11 patients) compared with the tension-type phenotype group (1 patient), as was vertigo (19 patients compared with 1) and visual aura (21 compared with 0).
The top precipitating factors across all patients included stressful life events (20.4%), an antecedent upper respiratory infection or flu-like illness (10.1%), and antecedent extracranial surgery (1.5%). Exacerbating or aggravating factors were far more prevalent in the migraine phenotype group compared with the tension-type phenotype group, with stress (14.6% vs. 5.9%), bright or flashing light (10.4% vs. 1.5%), loud noise (8.5% vs. 0%), and lack of sleep (6.5% vs. 4.4%) leading the way.
The months with the most onsets were June (8.5%), January (7.6%), and February (7.6%); there was no clear seasonal or cyclical variation. The most common prognostic type across all patients was persisting (refractory) at 93%, followed by remitting (self-limiting) at 4.3% and relapsing-remitting at 2.7%.
Unlocking a medical mystery
“This is the largest case review study ever published on NDPH, especially because most people think it’s a fairly rare disorder when it’s actually not,” Herbert G. Markley, MD, of the New England Regional Headache Center in Worcester, Mass., said in an interview.
“The thing people need to understand is that they may have a lot of these patients in their practice and not realize it,” he added. “They keep trying one medication after another, and the patients are giving up, and the doctors are giving up. It’s terrible. We don’t know what causes it, and we don’t know how to treat it. It’s one of the biggest mysteries left in medical science.”
“My idea about this condition, and this is shared by others, is that NDPH is not a diagnosis that describes a cohesive group of patients but rather a group of people who share certain features,” Morris Levin, MD, director of the Headache Center at the University of California, San Francisco, said in an interview. “And they would be better served if this diagnosis was split into different categories.”
While praising Dr. Evans and Dr. Turner for their categorization and classification work, Dr. Levin asked, “Let’s say you diagnose someone with NDPH; does that in any way help you with management of this person? The answer is no. Some might say, ‘If you put the patients in the migraine phenotype group, then you can use migraine treatments.’ My point would be: then call it migraine.
“I believe another way to approach NDPH might be to create subcategories of migraine and tension-type headaches,” he added. “A migraine that is either intermittent or nonexistent suddenly becomes daily. That could be a subcategory; rather than being called NDPH, call it ‘new persistent chronic migraine.’ Or ‘new persistent chronic tension-type headache.’ Perhaps that would serve us better in terms of grasping the underlying mechanisms and the best treatment for these patients.”
Along the same lines, Dr. Markley echoed Dr. Evans’ call for more prospective studies and more research on possible medication, hoping to fuel further understanding of this debilitating disorder.
“I think this will be a landmark study for people to look back on,” he said, “especially for anyone going into the headache specialty who has never heard of this type of headache and keeps wondering why they can’t help certain patients, no matter how many medications they try.”
The authors acknowledged their study’s limitations, including its single-center nature and the data abstraction process being performed by just one person. They added, however, that Dr. Evans is a “very experienced researcher with more than 30 years of experience in headache medicine who was abstracting his own patients, data that were very familiar to him.”
Dr. Evans and Dr. Turner declared no potential conflicts of interest.
according to a new retrospective chart review.
“Future prospective studies are needed to better understand this disabling disorder,” wrote Randolph W. Evans, MD, of Baylor College of Medicine of Houston, and Dana P. Turner, PhD, of Massachusetts General Hospital and Harvard Medical School in Boston. Their study was published Oct. 28 in Headache.
To categorize the infrequently reported clinical features of NDPH, the researchers launched a retrospective study of patients who were provisionally diagnosed with NDPH by Dr. Evans at an outpatient clinic in Houston from Sept. 1, 2011, to Feb. 28, 2020. Of the 328 patients whose diagnosis ultimately matched the ICHD-3 criteria, the average age at onset was 40.3 years (range 12-87 years). Approximately 70% were White, and nearly 66% were women. Two hundred and sixty were diagnosed with the migraine phenotype and 68 were diagnosed with the tension-type phenotype.
Key features
The median duration of NDPH at the time of the initial consult with Dr. Evans was 0.7 years, and it was 1.9 years at the time of the last visit. Almost 33% of patients with the migraine phenotype had a history of episodic migraine compared with 16.2% with the tension-type phenotype. Headaches were side-locked unilateral in 8.5% (n = 28) of all patients, and 3.6% (n = 12) had a thunderclap onset.
The most common clinical features across all patients included noise sensitivity (72.1%), light sensitivity (71%), moderate pain at the time of initial consult (57.9%), pressure pain (54.9%), and throbbing pain (50.9%). Nausea was reported in 157 patients and vomiting was reported in 48 patients, all of whom were in the migraine phenotype group. Thunderclap onset was far more prevalent in the migraine phenotype group (11 patients) compared with the tension-type phenotype group (1 patient), as was vertigo (19 patients compared with 1) and visual aura (21 compared with 0).
The top precipitating factors across all patients included stressful life events (20.4%), an antecedent upper respiratory infection or flu-like illness (10.1%), and antecedent extracranial surgery (1.5%). Exacerbating or aggravating factors were far more prevalent in the migraine phenotype group compared with the tension-type phenotype group, with stress (14.6% vs. 5.9%), bright or flashing light (10.4% vs. 1.5%), loud noise (8.5% vs. 0%), and lack of sleep (6.5% vs. 4.4%) leading the way.
The months with the most onsets were June (8.5%), January (7.6%), and February (7.6%); there was no clear seasonal or cyclical variation. The most common prognostic type across all patients was persisting (refractory) at 93%, followed by remitting (self-limiting) at 4.3% and relapsing-remitting at 2.7%.
Unlocking a medical mystery
“This is the largest case review study ever published on NDPH, especially because most people think it’s a fairly rare disorder when it’s actually not,” Herbert G. Markley, MD, of the New England Regional Headache Center in Worcester, Mass., said in an interview.
“The thing people need to understand is that they may have a lot of these patients in their practice and not realize it,” he added. “They keep trying one medication after another, and the patients are giving up, and the doctors are giving up. It’s terrible. We don’t know what causes it, and we don’t know how to treat it. It’s one of the biggest mysteries left in medical science.”
“My idea about this condition, and this is shared by others, is that NDPH is not a diagnosis that describes a cohesive group of patients but rather a group of people who share certain features,” Morris Levin, MD, director of the Headache Center at the University of California, San Francisco, said in an interview. “And they would be better served if this diagnosis was split into different categories.”
While praising Dr. Evans and Dr. Turner for their categorization and classification work, Dr. Levin asked, “Let’s say you diagnose someone with NDPH; does that in any way help you with management of this person? The answer is no. Some might say, ‘If you put the patients in the migraine phenotype group, then you can use migraine treatments.’ My point would be: then call it migraine.
“I believe another way to approach NDPH might be to create subcategories of migraine and tension-type headaches,” he added. “A migraine that is either intermittent or nonexistent suddenly becomes daily. That could be a subcategory; rather than being called NDPH, call it ‘new persistent chronic migraine.’ Or ‘new persistent chronic tension-type headache.’ Perhaps that would serve us better in terms of grasping the underlying mechanisms and the best treatment for these patients.”
Along the same lines, Dr. Markley echoed Dr. Evans’ call for more prospective studies and more research on possible medication, hoping to fuel further understanding of this debilitating disorder.
“I think this will be a landmark study for people to look back on,” he said, “especially for anyone going into the headache specialty who has never heard of this type of headache and keeps wondering why they can’t help certain patients, no matter how many medications they try.”
The authors acknowledged their study’s limitations, including its single-center nature and the data abstraction process being performed by just one person. They added, however, that Dr. Evans is a “very experienced researcher with more than 30 years of experience in headache medicine who was abstracting his own patients, data that were very familiar to him.”
Dr. Evans and Dr. Turner declared no potential conflicts of interest.
FROM HEADACHE