User login
Music and the human brain
Music has to be one of humanity’s most unique traits, and, at the same time, one of neurology’s greatest mysteries.
Where did it come from, and why?
When it started, we can only guess. The first known musical instrument is a flute carved from bear bone, made 67,000 years ago, but music is certainly older. The first instruments were probably clapped hands, then rocks and sticks.
Tens of thousands of cultures have developed over the course of human history. And, to date, not a single one is known that didn’t have music.
It takes energy to create music, too: to make and play instruments, think of songs, sing ... So at some point having music became an evolutionary advantage of some sort (one can imagine Bill and Ted saying “Dude, chicks dig it”) or it wouldn’t have lasted. Then, as people spread out, music forms got mixed and matched among cultures. Always changing, never leaving, and now somehow woven into the DNA of our brains.
The physics principles behind music are limited and simple: percussion, a vibrating string, air movement in a tube ... But from such simple things the human brain has adapted thousands of natural, and now synthetic, objects, to create an endless variety of unique sounds.
There are plenty of articles out there about how music can be relaxing or stimulating, capable of distracting you or helping you concentrate. Music can help you forget a bad day or remember a good one. They talk about PET scans and cortical activation and many other interesting things that show the effect of music on the remarkable human brain.
But at some level it doesn’t matter to me. I don’t try to understand music any more than I try to understand my dogs. I just know I couldn’t live without either. I’m not alone. Look around you: How many people on the train, or plane, or in the gym have earbuds on?
I have iTunes on my office computer, with roughly 5,000 songs covering the majority of genres from classical to rock. It’s the first program I switch on early each morning when I start the day. It gets me focused on the work at hand, and adds an enjoyable element to the day.
I’m not a musician. I took a few guitar lessons as a kid, but never really learned it. I used to joke that the only instrument I could play was the stereo (now I guess it’s iTunes). Coming from a maternal line of excellent musicians, it’s embarrassing to admit my lack of talent. But my inability to perform it myself doesn’t keep me from enjoying it.
There is no better example of the remarkable human memory than its ability to instantly recall the lyrics of songs you haven’t heard for 20, 30, 40, or more years. A few notes and it’s like you heard them yesterday. At this point, almost 30 years since my medical school graduation, I’ve likely forgotten a large portion of what I learned there. But 70s or 80s pop from my youth? Still there, and immediately recalled.
We process music everywhere – at stores, in elevators, in the car – without realizing it, like driving down the street and automatically reading signs as we pass them. But no matter where it is in our level of realization at the time, it’s a key part of our everyday lives.
Another marvel of the remarkable human brain.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
Music has to be one of humanity’s most unique traits, and, at the same time, one of neurology’s greatest mysteries.
Where did it come from, and why?
When it started, we can only guess. The first known musical instrument is a flute carved from bear bone, made 67,000 years ago, but music is certainly older. The first instruments were probably clapped hands, then rocks and sticks.
Tens of thousands of cultures have developed over the course of human history. And, to date, not a single one is known that didn’t have music.
It takes energy to create music, too: to make and play instruments, think of songs, sing ... So at some point having music became an evolutionary advantage of some sort (one can imagine Bill and Ted saying “Dude, chicks dig it”) or it wouldn’t have lasted. Then, as people spread out, music forms got mixed and matched among cultures. Always changing, never leaving, and now somehow woven into the DNA of our brains.
The physics principles behind music are limited and simple: percussion, a vibrating string, air movement in a tube ... But from such simple things the human brain has adapted thousands of natural, and now synthetic, objects, to create an endless variety of unique sounds.
There are plenty of articles out there about how music can be relaxing or stimulating, capable of distracting you or helping you concentrate. Music can help you forget a bad day or remember a good one. They talk about PET scans and cortical activation and many other interesting things that show the effect of music on the remarkable human brain.
But at some level it doesn’t matter to me. I don’t try to understand music any more than I try to understand my dogs. I just know I couldn’t live without either. I’m not alone. Look around you: How many people on the train, or plane, or in the gym have earbuds on?
I have iTunes on my office computer, with roughly 5,000 songs covering the majority of genres from classical to rock. It’s the first program I switch on early each morning when I start the day. It gets me focused on the work at hand, and adds an enjoyable element to the day.
I’m not a musician. I took a few guitar lessons as a kid, but never really learned it. I used to joke that the only instrument I could play was the stereo (now I guess it’s iTunes). Coming from a maternal line of excellent musicians, it’s embarrassing to admit my lack of talent. But my inability to perform it myself doesn’t keep me from enjoying it.
There is no better example of the remarkable human memory than its ability to instantly recall the lyrics of songs you haven’t heard for 20, 30, 40, or more years. A few notes and it’s like you heard them yesterday. At this point, almost 30 years since my medical school graduation, I’ve likely forgotten a large portion of what I learned there. But 70s or 80s pop from my youth? Still there, and immediately recalled.
We process music everywhere – at stores, in elevators, in the car – without realizing it, like driving down the street and automatically reading signs as we pass them. But no matter where it is in our level of realization at the time, it’s a key part of our everyday lives.
Another marvel of the remarkable human brain.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
Music has to be one of humanity’s most unique traits, and, at the same time, one of neurology’s greatest mysteries.
Where did it come from, and why?
When it started, we can only guess. The first known musical instrument is a flute carved from bear bone, made 67,000 years ago, but music is certainly older. The first instruments were probably clapped hands, then rocks and sticks.
Tens of thousands of cultures have developed over the course of human history. And, to date, not a single one is known that didn’t have music.
It takes energy to create music, too: to make and play instruments, think of songs, sing ... So at some point having music became an evolutionary advantage of some sort (one can imagine Bill and Ted saying “Dude, chicks dig it”) or it wouldn’t have lasted. Then, as people spread out, music forms got mixed and matched among cultures. Always changing, never leaving, and now somehow woven into the DNA of our brains.
The physics principles behind music are limited and simple: percussion, a vibrating string, air movement in a tube ... But from such simple things the human brain has adapted thousands of natural, and now synthetic, objects, to create an endless variety of unique sounds.
There are plenty of articles out there about how music can be relaxing or stimulating, capable of distracting you or helping you concentrate. Music can help you forget a bad day or remember a good one. They talk about PET scans and cortical activation and many other interesting things that show the effect of music on the remarkable human brain.
But at some level it doesn’t matter to me. I don’t try to understand music any more than I try to understand my dogs. I just know I couldn’t live without either. I’m not alone. Look around you: How many people on the train, or plane, or in the gym have earbuds on?
I have iTunes on my office computer, with roughly 5,000 songs covering the majority of genres from classical to rock. It’s the first program I switch on early each morning when I start the day. It gets me focused on the work at hand, and adds an enjoyable element to the day.
I’m not a musician. I took a few guitar lessons as a kid, but never really learned it. I used to joke that the only instrument I could play was the stereo (now I guess it’s iTunes). Coming from a maternal line of excellent musicians, it’s embarrassing to admit my lack of talent. But my inability to perform it myself doesn’t keep me from enjoying it.
There is no better example of the remarkable human memory than its ability to instantly recall the lyrics of songs you haven’t heard for 20, 30, 40, or more years. A few notes and it’s like you heard them yesterday. At this point, almost 30 years since my medical school graduation, I’ve likely forgotten a large portion of what I learned there. But 70s or 80s pop from my youth? Still there, and immediately recalled.
We process music everywhere – at stores, in elevators, in the car – without realizing it, like driving down the street and automatically reading signs as we pass them. But no matter where it is in our level of realization at the time, it’s a key part of our everyday lives.
Another marvel of the remarkable human brain.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
Red meat intake tied to higher coronary heart disease risk
Increased intake of meat was linked to the risk of coronary heart disease, and substituting plant protein for red or processed meat appeared to reduce that risk, in a study from pooled cohorts totaling more than a million persons.
“We know that red and processed meat intake has been associated with higher risks of fatal coronary heart disease,” said Laila Al-Shaar, PhD, of Penn State University, Hershey. However, very few studies have evaluated substitution of alternative protein sources for red and processed meat in relation to fatal CHD risk, she said.
In a study presented at the Epidemiology and Prevention/Lifestyle and Cardiometabolic Health meeting, Dr. Al-Shaar and colleagues reviewed individual-level data from the Pooling Project of Prospective Studies of Diet and Cancer, which included 16 prospective cohorts totaling 1,364,211 participants. The average age of the participants was 57 years, and 40% were men. Individuals with a history of cancer or cardiovascular disease were excluded. The participants were followed for 7-32 years. Diet was assessed in each cohort using baselines questionnaires, and cases were identified through medical records.
Total red meat included processed meat and unprocessed red meat; animal protein sources included seafood, poultry, eggs, and low- and high-fat dairy products; and plant protein sources included nuts and beans.
The researchers identified 51,176 fatal CHD cases during the study period. After controlling for dietary and nondietary factors, they found that an increase of 100 g per day of total red meat intake was associated with a 7% increased risk of fatal coronary heart disease (relative risk, 1.07).
However, substituting 200 calories (kcal) per day from nuts, low- and high-fat dairy products, and poultry for 200 calories per day from total red meat was associated with a 6%-14% lower risk of fatal CHD, Dr. Al-Shaar added at the meeting sponsored by the American Heart Association.
These associations were stronger when substituting the alternative protein sources for processed meat, especially among women; risk was reduced by 17%-24%, on the basis of 14,888 cases.
The researchers also found that substituting 200 calories per day from eggs for 200 calories per day for total red meat and unprocessed red meat was associated with 8% and 14% higher risk of fatal CHD, respectively; but this substitution of eggs for processed meat was not significant (4%).
“When we did the association by gender, the results were even stronger in women,” said Dr. Al-Shaar. However, “these are very preliminary results” that should be interpreted with caution, and more analysis is needed, she said. “We are planning to include other cohorts with other protein sources such as soy protein,” she noted. However, the results provide additional evidence that consumption of red and processed meat contributes to an increased risk of coronary heart disease, and that substituting some red and processed meat with nuts, dairy products, or poultry may reduce this risk, she concluded.
Women especially benefit from red meat reduction
The study is important because of the continuing interest in various sources of dietary protein intake, Linda Van Horn, PhD, RD, of Northwestern University, Chicago, said in an interview.
“The investigators studied associations of substituting other animal and plant protein sources for total red meat, unprocessed red meat, and processed meat in relation to risk of fatal CHD,” she said.
The researchers found that swapping as little as 200 calories per day of total red meat for nuts, low- or high-fat dairy products, or poultry were associated with a 6%-14% reduced risk of fatal CHD, said Dr. Van Horn. “Alternatively, if those 200 calories per day for red meat were substituted with eggs, they saw as much as 14% higher risk of fatal CHD,” she noted.
The message for both consumers and clinicians is that the findings from this large study support recommendations for plant-based and lean animal sources of protein instead of red and processed meat or eggs, as these sources “offer significantly lower risk for CHD mortality,” Dr. Van Horn said. “This may be especially true for women, but the total population is likely to benefit from this approach,” she said.
Additional research is needed, Dr. Van Horn emphasized. “Prospective lifetime data, starting in utero and over the life course, are needed to better establish recommended dietary patterns at every age and among all ethnicities and diverse socioeconomic groups,” she said.
Dr. Al-Shaar had no financial conflicts to disclose. Dr. Van Horn had no financial conflicts to disclose.
Increased intake of meat was linked to the risk of coronary heart disease, and substituting plant protein for red or processed meat appeared to reduce that risk, in a study from pooled cohorts totaling more than a million persons.
“We know that red and processed meat intake has been associated with higher risks of fatal coronary heart disease,” said Laila Al-Shaar, PhD, of Penn State University, Hershey. However, very few studies have evaluated substitution of alternative protein sources for red and processed meat in relation to fatal CHD risk, she said.
In a study presented at the Epidemiology and Prevention/Lifestyle and Cardiometabolic Health meeting, Dr. Al-Shaar and colleagues reviewed individual-level data from the Pooling Project of Prospective Studies of Diet and Cancer, which included 16 prospective cohorts totaling 1,364,211 participants. The average age of the participants was 57 years, and 40% were men. Individuals with a history of cancer or cardiovascular disease were excluded. The participants were followed for 7-32 years. Diet was assessed in each cohort using baselines questionnaires, and cases were identified through medical records.
Total red meat included processed meat and unprocessed red meat; animal protein sources included seafood, poultry, eggs, and low- and high-fat dairy products; and plant protein sources included nuts and beans.
The researchers identified 51,176 fatal CHD cases during the study period. After controlling for dietary and nondietary factors, they found that an increase of 100 g per day of total red meat intake was associated with a 7% increased risk of fatal coronary heart disease (relative risk, 1.07).
However, substituting 200 calories (kcal) per day from nuts, low- and high-fat dairy products, and poultry for 200 calories per day from total red meat was associated with a 6%-14% lower risk of fatal CHD, Dr. Al-Shaar added at the meeting sponsored by the American Heart Association.
These associations were stronger when substituting the alternative protein sources for processed meat, especially among women; risk was reduced by 17%-24%, on the basis of 14,888 cases.
The researchers also found that substituting 200 calories per day from eggs for 200 calories per day for total red meat and unprocessed red meat was associated with 8% and 14% higher risk of fatal CHD, respectively; but this substitution of eggs for processed meat was not significant (4%).
“When we did the association by gender, the results were even stronger in women,” said Dr. Al-Shaar. However, “these are very preliminary results” that should be interpreted with caution, and more analysis is needed, she said. “We are planning to include other cohorts with other protein sources such as soy protein,” she noted. However, the results provide additional evidence that consumption of red and processed meat contributes to an increased risk of coronary heart disease, and that substituting some red and processed meat with nuts, dairy products, or poultry may reduce this risk, she concluded.
Women especially benefit from red meat reduction
The study is important because of the continuing interest in various sources of dietary protein intake, Linda Van Horn, PhD, RD, of Northwestern University, Chicago, said in an interview.
“The investigators studied associations of substituting other animal and plant protein sources for total red meat, unprocessed red meat, and processed meat in relation to risk of fatal CHD,” she said.
The researchers found that swapping as little as 200 calories per day of total red meat for nuts, low- or high-fat dairy products, or poultry were associated with a 6%-14% reduced risk of fatal CHD, said Dr. Van Horn. “Alternatively, if those 200 calories per day for red meat were substituted with eggs, they saw as much as 14% higher risk of fatal CHD,” she noted.
The message for both consumers and clinicians is that the findings from this large study support recommendations for plant-based and lean animal sources of protein instead of red and processed meat or eggs, as these sources “offer significantly lower risk for CHD mortality,” Dr. Van Horn said. “This may be especially true for women, but the total population is likely to benefit from this approach,” she said.
Additional research is needed, Dr. Van Horn emphasized. “Prospective lifetime data, starting in utero and over the life course, are needed to better establish recommended dietary patterns at every age and among all ethnicities and diverse socioeconomic groups,” she said.
Dr. Al-Shaar had no financial conflicts to disclose. Dr. Van Horn had no financial conflicts to disclose.
Increased intake of meat was linked to the risk of coronary heart disease, and substituting plant protein for red or processed meat appeared to reduce that risk, in a study from pooled cohorts totaling more than a million persons.
“We know that red and processed meat intake has been associated with higher risks of fatal coronary heart disease,” said Laila Al-Shaar, PhD, of Penn State University, Hershey. However, very few studies have evaluated substitution of alternative protein sources for red and processed meat in relation to fatal CHD risk, she said.
In a study presented at the Epidemiology and Prevention/Lifestyle and Cardiometabolic Health meeting, Dr. Al-Shaar and colleagues reviewed individual-level data from the Pooling Project of Prospective Studies of Diet and Cancer, which included 16 prospective cohorts totaling 1,364,211 participants. The average age of the participants was 57 years, and 40% were men. Individuals with a history of cancer or cardiovascular disease were excluded. The participants were followed for 7-32 years. Diet was assessed in each cohort using baselines questionnaires, and cases were identified through medical records.
Total red meat included processed meat and unprocessed red meat; animal protein sources included seafood, poultry, eggs, and low- and high-fat dairy products; and plant protein sources included nuts and beans.
The researchers identified 51,176 fatal CHD cases during the study period. After controlling for dietary and nondietary factors, they found that an increase of 100 g per day of total red meat intake was associated with a 7% increased risk of fatal coronary heart disease (relative risk, 1.07).
However, substituting 200 calories (kcal) per day from nuts, low- and high-fat dairy products, and poultry for 200 calories per day from total red meat was associated with a 6%-14% lower risk of fatal CHD, Dr. Al-Shaar added at the meeting sponsored by the American Heart Association.
These associations were stronger when substituting the alternative protein sources for processed meat, especially among women; risk was reduced by 17%-24%, on the basis of 14,888 cases.
The researchers also found that substituting 200 calories per day from eggs for 200 calories per day for total red meat and unprocessed red meat was associated with 8% and 14% higher risk of fatal CHD, respectively; but this substitution of eggs for processed meat was not significant (4%).
“When we did the association by gender, the results were even stronger in women,” said Dr. Al-Shaar. However, “these are very preliminary results” that should be interpreted with caution, and more analysis is needed, she said. “We are planning to include other cohorts with other protein sources such as soy protein,” she noted. However, the results provide additional evidence that consumption of red and processed meat contributes to an increased risk of coronary heart disease, and that substituting some red and processed meat with nuts, dairy products, or poultry may reduce this risk, she concluded.
Women especially benefit from red meat reduction
The study is important because of the continuing interest in various sources of dietary protein intake, Linda Van Horn, PhD, RD, of Northwestern University, Chicago, said in an interview.
“The investigators studied associations of substituting other animal and plant protein sources for total red meat, unprocessed red meat, and processed meat in relation to risk of fatal CHD,” she said.
The researchers found that swapping as little as 200 calories per day of total red meat for nuts, low- or high-fat dairy products, or poultry were associated with a 6%-14% reduced risk of fatal CHD, said Dr. Van Horn. “Alternatively, if those 200 calories per day for red meat were substituted with eggs, they saw as much as 14% higher risk of fatal CHD,” she noted.
The message for both consumers and clinicians is that the findings from this large study support recommendations for plant-based and lean animal sources of protein instead of red and processed meat or eggs, as these sources “offer significantly lower risk for CHD mortality,” Dr. Van Horn said. “This may be especially true for women, but the total population is likely to benefit from this approach,” she said.
Additional research is needed, Dr. Van Horn emphasized. “Prospective lifetime data, starting in utero and over the life course, are needed to better establish recommended dietary patterns at every age and among all ethnicities and diverse socioeconomic groups,” she said.
Dr. Al-Shaar had no financial conflicts to disclose. Dr. Van Horn had no financial conflicts to disclose.
FROM EPI/LIFESTYLE 2021
Surgical outcomes favor addition of nivolumab to neoadjuvant chemo in resectable lung cancers
The addition of nivolumab to neoadjuvant chemotherapy did not impede the feasibility or timing of surgery in patients with resectable lung cancer, according to results from the phase 3 CheckMate 816 trial.
Adding nivolumab to chemotherapy was tolerable and did not increase the rate of surgical complications, investigator Jonathan Spicer, FRCPC, MD, PhD, of McGill University, Montreal, said in his presentation at the annual meeting of the American Society of Clinical Oncology.
His presentation comes about 2 months after the reporting of primary endpoint results of CheckMate 816 (NCT02998528). CheckMate 816 demonstrated that adding nivolumab to neoadjuvant chemotherapy significantly improved pathological complete response (pCR) in patients with resectable non–small cell lung cancer (NSCLC), according to results presented earlier at the American Association for Cancer Research annual meeting.
“The safety and surgical outcome data reported thus far from CheckMate 816, along with significant improvement in pathological complete response, support nivolumab in combination with chemotherapy as an attractive neoadjuvant option for patients with resectable NSCLC,” said Dr. Spicer (Abstract 8503).
Building on previous experience
The CheckMate 816 study builds on extensive experience in advanced NSCLC that has consistently shown better outcomes, including overall survival, with combinations of chemotherapy and immuno-oncology (IO) agents, compared to chemotherapy alone, said discussant Valerie W. Rusch, MD, of Memorial Sloan Kettering Cancer Center in New York.
Dr. Rusch called out “salient and interesting results” regarding surgical management in CheckMate 816, including a lower rate of surgery cancellations and shorter surgical duration in the chemotherapy-plus-IO arm, compared to the chemotherapy-alone arm.
Furthermore, fewer patients required a pneumonectomy and more patients had a complete resection in the chemotherapy-plus-IO arm, compared to chemotherapy alone, she noted.
“These excellent surgical results, along with the data previously presented at AACR regarding the primary endpoint, help to establish a new standard of neoadjuvant care,” Dr. Rusch said in her presentation.
Study details
CheckMate 816 included 358 patients with newly diagnosed, resectable, stage IB-IIIA NSCLC, Eastern Cooperative Oncology Group (ECOG) performance status of 0-1, and no known EGFR mutations or ALK alterations. Patients were randomized to receive nivolumab and platinum-doublet chemotherapy (nivolumab/chemotherapy) or chemotherapy alone every 3 weeks, with surgery to be performed within 6 weeks of the last dose of neoadjuvant treatment.
The median age of patients was 64 years in the nivolumab/chemotherapy arm and 65 years in the chemotherapy-alone arm. About one-third of patients had ECOG performance status of one, and about half had squamous tumor histology, Dr. Spicer said in his report. Almost two-thirds of patients had stage IIIA disease.
In the study results previously presented at the AACR meeting, both pCR and major pathologic response were significantly better following neoadjuvant chemotherapy and IO treatment, compared to chemotherapy alone.
In the intention-to-treat analysis, 24.0% of patients treated with nivolumab/chemotherapy achieved a pCR, compared to 2.2% in the chemotherapy arm, amounting to an approximate 12-fold increase in pCR, Dr. Spicer said. Similarly, the rate of major pathologic response in the intention-to-treat analysis was 36.9% and 8.9% for the nivolumab/chemotherapy and chemotherapy arms, respectively.
Surgical results
In his ASCO presentation, Dr. Spicer reported that definitive surgery was canceled in 16% of patients in the nivolumab/chemotherapy arm, and 21% of the chemotherapy arm. Reasons for surgery cancellation generally included patients declining surgery, unresectable disease, and poor lung function. “Cancellation of surgery due to neoadjuvant therapy toxicity was rare,” Dr. Spicer said in his presentation.
Among patients who did proceed to surgery, the median duration of the procedure was 184 minutes in the nivolumab/chemotherapy arm and 217 minutes in the chemotherapy arm. That half-hour difference in favor of the combination arm suggests that the complexity of surgery was not increased by the addition of nivolumab, Dr. Spicer said.
Median time to surgery was about 5 weeks in both arms, which was “well within accepted standards for a neoadjuvant therapeutic approach,” Dr. Spicer said. Most delays beyond 6 weeks were due to administrative issues, and occurred in similar proportions (21% of the nivolumab/chemotherapy arm and 18% of the chemotherapy arm).
The addition of nivolumab to chemotherapy improved pCR rates regardless of baseline stage of disease, according to Dr. Spicer. Furthermore, the depth of pathological regression in the primary tumor was “dramatically different” across stage groupings, he said. Median residual viable tumor percentage in stage IB/II patients was 28% for nivolumab/chemotherapy and 79% for chemotherapy, and in stage IIIA patients, it was 8% for nivolumab/chemotherapy and 70% for chemotherapy.
Overall, thoracotomy was the most frequent surgical approach in this international phase 3 trial, Dr. Spicer said. However, among patients with stage IIIA disease, minimally invasive approaches were used 30% of the time in the nivolumab/chemotherapy arm and 19% in the chemotherapy arm. Conversely, the rate of conversion from a minimally invasive to open approach in patients with stage IIIA disease was 11% for nivolumab/chemotherapy and 20% for chemotherapy alone.
Lobectomy was more frequent in the nivolumab/chemotherapy arm (77%) compared to the chemotherapy arm (61%), a difference that Dr. Spicer described as clinically important. He said the difference appears to be attributable to a lower rate of pneumonectomy in the nivolumab/chemotherapy arm (17%) than in the chemotherapy arm (25%).
Despite less extensive lung resection being required, the rate of R0 resection was numerically higher in the nivolumab/chemotherapy arm (83%) than in the chemotherapy arm (78%), said Dr. Spicer.
Length of hospital stay was “within expected ranges” from geographic regions represented in the trial, Dr. Spicer said. Median length of stay was 4.0 and 6.0 days, respectively, for nivolumab/chemotherapy and chemotherapy alone in North America, 9.5 and 13.0 days in Europe, and 11.0 and 13.0 days in Asia.
Likewise, 90-day surgical complications were well within expected ranges, according to the investigator, with anemia, pain, and wound complications being the most commonly reported. Rates were generally similar between study arms, other than a twofold higher rate of pain in the chemotherapy arm, possibly due to the lower rate of minimally invasive surgery or higher rate of conversion to an open procedure, compared to the nivolumab/chemotherapy arm, he said.
Awaiting survival
Rates of 30- and 90-day mortality are expected to be evaluated when survival endpoints are available, according to Dr. Spicer. Beyond pCR rate, event-free survival is also a primary endpoint of the study, while overall survival is a secondary endpoint.
The study was supported by Bristol Myers Squibb. Dr. Spicer reported disclosures related to AstraZeneca, Bristol Myers Squibb Foundation, Merck, and Roche. Dr. Rusch reported research funding with Genelux and Genentech, and travel expenses from Intuitive Surgical.
The addition of nivolumab to neoadjuvant chemotherapy did not impede the feasibility or timing of surgery in patients with resectable lung cancer, according to results from the phase 3 CheckMate 816 trial.
Adding nivolumab to chemotherapy was tolerable and did not increase the rate of surgical complications, investigator Jonathan Spicer, FRCPC, MD, PhD, of McGill University, Montreal, said in his presentation at the annual meeting of the American Society of Clinical Oncology.
His presentation comes about 2 months after the reporting of primary endpoint results of CheckMate 816 (NCT02998528). CheckMate 816 demonstrated that adding nivolumab to neoadjuvant chemotherapy significantly improved pathological complete response (pCR) in patients with resectable non–small cell lung cancer (NSCLC), according to results presented earlier at the American Association for Cancer Research annual meeting.
“The safety and surgical outcome data reported thus far from CheckMate 816, along with significant improvement in pathological complete response, support nivolumab in combination with chemotherapy as an attractive neoadjuvant option for patients with resectable NSCLC,” said Dr. Spicer (Abstract 8503).
Building on previous experience
The CheckMate 816 study builds on extensive experience in advanced NSCLC that has consistently shown better outcomes, including overall survival, with combinations of chemotherapy and immuno-oncology (IO) agents, compared to chemotherapy alone, said discussant Valerie W. Rusch, MD, of Memorial Sloan Kettering Cancer Center in New York.
Dr. Rusch called out “salient and interesting results” regarding surgical management in CheckMate 816, including a lower rate of surgery cancellations and shorter surgical duration in the chemotherapy-plus-IO arm, compared to the chemotherapy-alone arm.
Furthermore, fewer patients required a pneumonectomy and more patients had a complete resection in the chemotherapy-plus-IO arm, compared to chemotherapy alone, she noted.
“These excellent surgical results, along with the data previously presented at AACR regarding the primary endpoint, help to establish a new standard of neoadjuvant care,” Dr. Rusch said in her presentation.
Study details
CheckMate 816 included 358 patients with newly diagnosed, resectable, stage IB-IIIA NSCLC, Eastern Cooperative Oncology Group (ECOG) performance status of 0-1, and no known EGFR mutations or ALK alterations. Patients were randomized to receive nivolumab and platinum-doublet chemotherapy (nivolumab/chemotherapy) or chemotherapy alone every 3 weeks, with surgery to be performed within 6 weeks of the last dose of neoadjuvant treatment.
The median age of patients was 64 years in the nivolumab/chemotherapy arm and 65 years in the chemotherapy-alone arm. About one-third of patients had ECOG performance status of one, and about half had squamous tumor histology, Dr. Spicer said in his report. Almost two-thirds of patients had stage IIIA disease.
In the study results previously presented at the AACR meeting, both pCR and major pathologic response were significantly better following neoadjuvant chemotherapy and IO treatment, compared to chemotherapy alone.
In the intention-to-treat analysis, 24.0% of patients treated with nivolumab/chemotherapy achieved a pCR, compared to 2.2% in the chemotherapy arm, amounting to an approximate 12-fold increase in pCR, Dr. Spicer said. Similarly, the rate of major pathologic response in the intention-to-treat analysis was 36.9% and 8.9% for the nivolumab/chemotherapy and chemotherapy arms, respectively.
Surgical results
In his ASCO presentation, Dr. Spicer reported that definitive surgery was canceled in 16% of patients in the nivolumab/chemotherapy arm, and 21% of the chemotherapy arm. Reasons for surgery cancellation generally included patients declining surgery, unresectable disease, and poor lung function. “Cancellation of surgery due to neoadjuvant therapy toxicity was rare,” Dr. Spicer said in his presentation.
Among patients who did proceed to surgery, the median duration of the procedure was 184 minutes in the nivolumab/chemotherapy arm and 217 minutes in the chemotherapy arm. That half-hour difference in favor of the combination arm suggests that the complexity of surgery was not increased by the addition of nivolumab, Dr. Spicer said.
Median time to surgery was about 5 weeks in both arms, which was “well within accepted standards for a neoadjuvant therapeutic approach,” Dr. Spicer said. Most delays beyond 6 weeks were due to administrative issues, and occurred in similar proportions (21% of the nivolumab/chemotherapy arm and 18% of the chemotherapy arm).
The addition of nivolumab to chemotherapy improved pCR rates regardless of baseline stage of disease, according to Dr. Spicer. Furthermore, the depth of pathological regression in the primary tumor was “dramatically different” across stage groupings, he said. Median residual viable tumor percentage in stage IB/II patients was 28% for nivolumab/chemotherapy and 79% for chemotherapy, and in stage IIIA patients, it was 8% for nivolumab/chemotherapy and 70% for chemotherapy.
Overall, thoracotomy was the most frequent surgical approach in this international phase 3 trial, Dr. Spicer said. However, among patients with stage IIIA disease, minimally invasive approaches were used 30% of the time in the nivolumab/chemotherapy arm and 19% in the chemotherapy arm. Conversely, the rate of conversion from a minimally invasive to open approach in patients with stage IIIA disease was 11% for nivolumab/chemotherapy and 20% for chemotherapy alone.
Lobectomy was more frequent in the nivolumab/chemotherapy arm (77%) compared to the chemotherapy arm (61%), a difference that Dr. Spicer described as clinically important. He said the difference appears to be attributable to a lower rate of pneumonectomy in the nivolumab/chemotherapy arm (17%) than in the chemotherapy arm (25%).
Despite less extensive lung resection being required, the rate of R0 resection was numerically higher in the nivolumab/chemotherapy arm (83%) than in the chemotherapy arm (78%), said Dr. Spicer.
Length of hospital stay was “within expected ranges” from geographic regions represented in the trial, Dr. Spicer said. Median length of stay was 4.0 and 6.0 days, respectively, for nivolumab/chemotherapy and chemotherapy alone in North America, 9.5 and 13.0 days in Europe, and 11.0 and 13.0 days in Asia.
Likewise, 90-day surgical complications were well within expected ranges, according to the investigator, with anemia, pain, and wound complications being the most commonly reported. Rates were generally similar between study arms, other than a twofold higher rate of pain in the chemotherapy arm, possibly due to the lower rate of minimally invasive surgery or higher rate of conversion to an open procedure, compared to the nivolumab/chemotherapy arm, he said.
Awaiting survival
Rates of 30- and 90-day mortality are expected to be evaluated when survival endpoints are available, according to Dr. Spicer. Beyond pCR rate, event-free survival is also a primary endpoint of the study, while overall survival is a secondary endpoint.
The study was supported by Bristol Myers Squibb. Dr. Spicer reported disclosures related to AstraZeneca, Bristol Myers Squibb Foundation, Merck, and Roche. Dr. Rusch reported research funding with Genelux and Genentech, and travel expenses from Intuitive Surgical.
The addition of nivolumab to neoadjuvant chemotherapy did not impede the feasibility or timing of surgery in patients with resectable lung cancer, according to results from the phase 3 CheckMate 816 trial.
Adding nivolumab to chemotherapy was tolerable and did not increase the rate of surgical complications, investigator Jonathan Spicer, FRCPC, MD, PhD, of McGill University, Montreal, said in his presentation at the annual meeting of the American Society of Clinical Oncology.
His presentation comes about 2 months after the reporting of primary endpoint results of CheckMate 816 (NCT02998528). CheckMate 816 demonstrated that adding nivolumab to neoadjuvant chemotherapy significantly improved pathological complete response (pCR) in patients with resectable non–small cell lung cancer (NSCLC), according to results presented earlier at the American Association for Cancer Research annual meeting.
“The safety and surgical outcome data reported thus far from CheckMate 816, along with significant improvement in pathological complete response, support nivolumab in combination with chemotherapy as an attractive neoadjuvant option for patients with resectable NSCLC,” said Dr. Spicer (Abstract 8503).
Building on previous experience
The CheckMate 816 study builds on extensive experience in advanced NSCLC that has consistently shown better outcomes, including overall survival, with combinations of chemotherapy and immuno-oncology (IO) agents, compared to chemotherapy alone, said discussant Valerie W. Rusch, MD, of Memorial Sloan Kettering Cancer Center in New York.
Dr. Rusch called out “salient and interesting results” regarding surgical management in CheckMate 816, including a lower rate of surgery cancellations and shorter surgical duration in the chemotherapy-plus-IO arm, compared to the chemotherapy-alone arm.
Furthermore, fewer patients required a pneumonectomy and more patients had a complete resection in the chemotherapy-plus-IO arm, compared to chemotherapy alone, she noted.
“These excellent surgical results, along with the data previously presented at AACR regarding the primary endpoint, help to establish a new standard of neoadjuvant care,” Dr. Rusch said in her presentation.
Study details
CheckMate 816 included 358 patients with newly diagnosed, resectable, stage IB-IIIA NSCLC, Eastern Cooperative Oncology Group (ECOG) performance status of 0-1, and no known EGFR mutations or ALK alterations. Patients were randomized to receive nivolumab and platinum-doublet chemotherapy (nivolumab/chemotherapy) or chemotherapy alone every 3 weeks, with surgery to be performed within 6 weeks of the last dose of neoadjuvant treatment.
The median age of patients was 64 years in the nivolumab/chemotherapy arm and 65 years in the chemotherapy-alone arm. About one-third of patients had ECOG performance status of one, and about half had squamous tumor histology, Dr. Spicer said in his report. Almost two-thirds of patients had stage IIIA disease.
In the study results previously presented at the AACR meeting, both pCR and major pathologic response were significantly better following neoadjuvant chemotherapy and IO treatment, compared to chemotherapy alone.
In the intention-to-treat analysis, 24.0% of patients treated with nivolumab/chemotherapy achieved a pCR, compared to 2.2% in the chemotherapy arm, amounting to an approximate 12-fold increase in pCR, Dr. Spicer said. Similarly, the rate of major pathologic response in the intention-to-treat analysis was 36.9% and 8.9% for the nivolumab/chemotherapy and chemotherapy arms, respectively.
Surgical results
In his ASCO presentation, Dr. Spicer reported that definitive surgery was canceled in 16% of patients in the nivolumab/chemotherapy arm, and 21% of the chemotherapy arm. Reasons for surgery cancellation generally included patients declining surgery, unresectable disease, and poor lung function. “Cancellation of surgery due to neoadjuvant therapy toxicity was rare,” Dr. Spicer said in his presentation.
Among patients who did proceed to surgery, the median duration of the procedure was 184 minutes in the nivolumab/chemotherapy arm and 217 minutes in the chemotherapy arm. That half-hour difference in favor of the combination arm suggests that the complexity of surgery was not increased by the addition of nivolumab, Dr. Spicer said.
Median time to surgery was about 5 weeks in both arms, which was “well within accepted standards for a neoadjuvant therapeutic approach,” Dr. Spicer said. Most delays beyond 6 weeks were due to administrative issues, and occurred in similar proportions (21% of the nivolumab/chemotherapy arm and 18% of the chemotherapy arm).
The addition of nivolumab to chemotherapy improved pCR rates regardless of baseline stage of disease, according to Dr. Spicer. Furthermore, the depth of pathological regression in the primary tumor was “dramatically different” across stage groupings, he said. Median residual viable tumor percentage in stage IB/II patients was 28% for nivolumab/chemotherapy and 79% for chemotherapy, and in stage IIIA patients, it was 8% for nivolumab/chemotherapy and 70% for chemotherapy.
Overall, thoracotomy was the most frequent surgical approach in this international phase 3 trial, Dr. Spicer said. However, among patients with stage IIIA disease, minimally invasive approaches were used 30% of the time in the nivolumab/chemotherapy arm and 19% in the chemotherapy arm. Conversely, the rate of conversion from a minimally invasive to open approach in patients with stage IIIA disease was 11% for nivolumab/chemotherapy and 20% for chemotherapy alone.
Lobectomy was more frequent in the nivolumab/chemotherapy arm (77%) compared to the chemotherapy arm (61%), a difference that Dr. Spicer described as clinically important. He said the difference appears to be attributable to a lower rate of pneumonectomy in the nivolumab/chemotherapy arm (17%) than in the chemotherapy arm (25%).
Despite less extensive lung resection being required, the rate of R0 resection was numerically higher in the nivolumab/chemotherapy arm (83%) than in the chemotherapy arm (78%), said Dr. Spicer.
Length of hospital stay was “within expected ranges” from geographic regions represented in the trial, Dr. Spicer said. Median length of stay was 4.0 and 6.0 days, respectively, for nivolumab/chemotherapy and chemotherapy alone in North America, 9.5 and 13.0 days in Europe, and 11.0 and 13.0 days in Asia.
Likewise, 90-day surgical complications were well within expected ranges, according to the investigator, with anemia, pain, and wound complications being the most commonly reported. Rates were generally similar between study arms, other than a twofold higher rate of pain in the chemotherapy arm, possibly due to the lower rate of minimally invasive surgery or higher rate of conversion to an open procedure, compared to the nivolumab/chemotherapy arm, he said.
Awaiting survival
Rates of 30- and 90-day mortality are expected to be evaluated when survival endpoints are available, according to Dr. Spicer. Beyond pCR rate, event-free survival is also a primary endpoint of the study, while overall survival is a secondary endpoint.
The study was supported by Bristol Myers Squibb. Dr. Spicer reported disclosures related to AstraZeneca, Bristol Myers Squibb Foundation, Merck, and Roche. Dr. Rusch reported research funding with Genelux and Genentech, and travel expenses from Intuitive Surgical.
FROM ASCO 2021
Hospital-level care at home for acutely ill adults may be as safe as inpatient care
Background: Providing hospital-level care at home for select patients has proven to reduce health care cost, usage, and readmission rates, while maintaining quality and safety in other developed countries but few studies exist in the United States.
Study design: Randomized, controlled, unblinded, parallel-design trial.
Setting: Home hospital care versus inpatient care at two Boston academic hospitals, during June 2017–January 2018.
Synopsis: The study enrolled 91 adult patients from the emergency department who were deemed appropriate for non-ICU admission for treatment of prespecified diagnoses (i.e., COPD exacerbation, heart failure exacerbation, etc.). Participants were randomized to usual inpatient care or home hospital care. All home hospital patients received daily internist visits, twice-daily nursing visits, home access to additional services (physical/occupational therapy, social work, etc.), oxygen, IV medications, labs, radiology, and continuous monitoring. The authors found that home hospital care resulted in a lower total cost (P < .001), lower use of imaging and labs, and lower 30-day readmission rate, without appreciable differences in quality or safety between the two groups. Given that the study was performed at only two academic hospitals, it is unclear if these findings can be generalized to other health systems.
Bottom line: For the care of select illnesses, hospital-level care at home may be cheaper, may be just as safe, and reduced readmission rates when compared with inpatient care.
Citation: Levine D et al. Hospital-level care at home for acutely ill adults. Ann Intern Med. 2020;172:77-85.
Dr. Persaud is a hospitalist, Beth Israel Deaconess Medical Center, and instructor in medicine, Harvard Medical School, both in Boston.
Background: Providing hospital-level care at home for select patients has proven to reduce health care cost, usage, and readmission rates, while maintaining quality and safety in other developed countries but few studies exist in the United States.
Study design: Randomized, controlled, unblinded, parallel-design trial.
Setting: Home hospital care versus inpatient care at two Boston academic hospitals, during June 2017–January 2018.
Synopsis: The study enrolled 91 adult patients from the emergency department who were deemed appropriate for non-ICU admission for treatment of prespecified diagnoses (i.e., COPD exacerbation, heart failure exacerbation, etc.). Participants were randomized to usual inpatient care or home hospital care. All home hospital patients received daily internist visits, twice-daily nursing visits, home access to additional services (physical/occupational therapy, social work, etc.), oxygen, IV medications, labs, radiology, and continuous monitoring. The authors found that home hospital care resulted in a lower total cost (P < .001), lower use of imaging and labs, and lower 30-day readmission rate, without appreciable differences in quality or safety between the two groups. Given that the study was performed at only two academic hospitals, it is unclear if these findings can be generalized to other health systems.
Bottom line: For the care of select illnesses, hospital-level care at home may be cheaper, may be just as safe, and reduced readmission rates when compared with inpatient care.
Citation: Levine D et al. Hospital-level care at home for acutely ill adults. Ann Intern Med. 2020;172:77-85.
Dr. Persaud is a hospitalist, Beth Israel Deaconess Medical Center, and instructor in medicine, Harvard Medical School, both in Boston.
Background: Providing hospital-level care at home for select patients has proven to reduce health care cost, usage, and readmission rates, while maintaining quality and safety in other developed countries but few studies exist in the United States.
Study design: Randomized, controlled, unblinded, parallel-design trial.
Setting: Home hospital care versus inpatient care at two Boston academic hospitals, during June 2017–January 2018.
Synopsis: The study enrolled 91 adult patients from the emergency department who were deemed appropriate for non-ICU admission for treatment of prespecified diagnoses (i.e., COPD exacerbation, heart failure exacerbation, etc.). Participants were randomized to usual inpatient care or home hospital care. All home hospital patients received daily internist visits, twice-daily nursing visits, home access to additional services (physical/occupational therapy, social work, etc.), oxygen, IV medications, labs, radiology, and continuous monitoring. The authors found that home hospital care resulted in a lower total cost (P < .001), lower use of imaging and labs, and lower 30-day readmission rate, without appreciable differences in quality or safety between the two groups. Given that the study was performed at only two academic hospitals, it is unclear if these findings can be generalized to other health systems.
Bottom line: For the care of select illnesses, hospital-level care at home may be cheaper, may be just as safe, and reduced readmission rates when compared with inpatient care.
Citation: Levine D et al. Hospital-level care at home for acutely ill adults. Ann Intern Med. 2020;172:77-85.
Dr. Persaud is a hospitalist, Beth Israel Deaconess Medical Center, and instructor in medicine, Harvard Medical School, both in Boston.
New drug toripalimab improves survival in nasopharyngeal cancer
A new immunotherapy, toripalimab, has the potential to change practice in the treatment of nasopharyngeal carcinoma (NPC), say experts.
The drug is a monoclonal antibody that blocks programmed cell death protein 1 (PD-1), developed in China and recently approved there for the third-line treatment of NPC, among other indications. The U.S. Food and Drug Administration has granted it a breakthrough therapy designation for recurrent/metastatic NPC, as well as fast-track and orphan drug status for other tumor types.
New results show that when toripalimab was added onto chemotherapy with gemcitabine and cisplatin in the first line for recurrent or metastatic nasopharyngeal carcinoma, there was a significant improvement in both progression-free survival and overall survival.
The results come from the phase 3 trial dubbed JUPITER-02 and will be presented at the plenary session of the American Society of Clinical Oncology annual meeting this Sunday; some details were released earlier at a press briefing
The trial randomly assigned 146 patients to toripalimab and 143 to placebo on a background of gemcitabine and cisplatin, the current standard of care for recurrent/metastatic NPC.
Median progression-free survival (PFS) was 11.7 months with toripalimab vs. 8 months with placebo, a significant improvement (hazard ratio, 0.52; 95% confidence interval, 0.36-0.74. P = .0003). Overall survival was not mature at reporting but favored toripalimab with 25 deaths versus 39 in the placebo group, a 40% risk reduction (P = .0462).
The results “support the use of toripalimab in combination with [gemcitabine and cisplatin] as a new standard of care for first-line treatment of recurrent or metastatic nasopharyngeal carcinoma,” said lead investigator and medical oncologist Rui-Hua Xu, MD, PhD, of the Sun Yat-sen University Cancer Center in Guangzhou, China.
Potential to change practice
The significance of the study is that it used immunotherapy in the first-line setting for NPC instead of the second line where it’s frequently used today, commented Jared Weiss, MD, an associate professor of oncology and a head and neck cancer specialist at the University of North Carolina, Chapel Hill.
If FDA approves toripalimab for the indication, it “would change [first-line] standard of care to the triplet regimen,” he said in an interview.
The discussant for this presentation, Julie Gralow, MD, agreed. “This is one of the first studies in metastatic or recurrent NPC to show a benefit” for combining a PD-1 inhibitor with chemotherapy.
“With FDA approval, these findings should prove practice-changing,” said Dr. Gralow, a professor of breast medical oncology at the University of Washington, Seattle, and ASCO’s chief medical officer.
Toripalimab, dosed at 240 mg in the trial, or placebo were administered with gemcitabine and cisplatin every 3 weeks for up to 6 cycles, followed by toripalimab or placebo maintenance every 3 weeks until disease progression, intolerable toxicity, or completion of 2 years of treatment.
The overall response rate was 77.4% with toripalimab and 66.4% with placebo, and the median duration of response in the toripalimab group was 10 months vs. 5.7 months with placebo.
One-year PFS was 49.4% with toripalimab versus 27.9% with placebo; improved PFS was observed with toripalimab across PD-L1 subgroups.
Grade 3 or worse adverse events occurred in slightly less than 90% of both groups, with fatal adverse events occurring in slightly less than 3% in both.
Adverse events leading to discontinuation occurred in 7.5% of the study group and 4.9% on placebo. As expected with immunotherapy, immune-related adverse events such as hypothyroidism were more common with toripalimab (39.7% vs. 18.9%), as were grade 3 or worse immune-related adverse events (7.5% vs. 0.7%).
At interim analysis in May 2020, the median duration treatment was 39 weeks in the toripalimab group and 36 weeks in the placebo group.
The trial was conducted in China, Taiwan, and Singapore.
JUPITER-02 was funded by Shanghai Junshi Bioscience. Investigator disclosures weren’t reported. Dr. Weiss said he had no relevant disclosures. Dr. Gralow is an advisor for a number of companies, including Genentech, Novartis, and Roche.
A version of this article first appeared on Medscape.com.
A new immunotherapy, toripalimab, has the potential to change practice in the treatment of nasopharyngeal carcinoma (NPC), say experts.
The drug is a monoclonal antibody that blocks programmed cell death protein 1 (PD-1), developed in China and recently approved there for the third-line treatment of NPC, among other indications. The U.S. Food and Drug Administration has granted it a breakthrough therapy designation for recurrent/metastatic NPC, as well as fast-track and orphan drug status for other tumor types.
New results show that when toripalimab was added onto chemotherapy with gemcitabine and cisplatin in the first line for recurrent or metastatic nasopharyngeal carcinoma, there was a significant improvement in both progression-free survival and overall survival.
The results come from the phase 3 trial dubbed JUPITER-02 and will be presented at the plenary session of the American Society of Clinical Oncology annual meeting this Sunday; some details were released earlier at a press briefing
The trial randomly assigned 146 patients to toripalimab and 143 to placebo on a background of gemcitabine and cisplatin, the current standard of care for recurrent/metastatic NPC.
Median progression-free survival (PFS) was 11.7 months with toripalimab vs. 8 months with placebo, a significant improvement (hazard ratio, 0.52; 95% confidence interval, 0.36-0.74. P = .0003). Overall survival was not mature at reporting but favored toripalimab with 25 deaths versus 39 in the placebo group, a 40% risk reduction (P = .0462).
The results “support the use of toripalimab in combination with [gemcitabine and cisplatin] as a new standard of care for first-line treatment of recurrent or metastatic nasopharyngeal carcinoma,” said lead investigator and medical oncologist Rui-Hua Xu, MD, PhD, of the Sun Yat-sen University Cancer Center in Guangzhou, China.
Potential to change practice
The significance of the study is that it used immunotherapy in the first-line setting for NPC instead of the second line where it’s frequently used today, commented Jared Weiss, MD, an associate professor of oncology and a head and neck cancer specialist at the University of North Carolina, Chapel Hill.
If FDA approves toripalimab for the indication, it “would change [first-line] standard of care to the triplet regimen,” he said in an interview.
The discussant for this presentation, Julie Gralow, MD, agreed. “This is one of the first studies in metastatic or recurrent NPC to show a benefit” for combining a PD-1 inhibitor with chemotherapy.
“With FDA approval, these findings should prove practice-changing,” said Dr. Gralow, a professor of breast medical oncology at the University of Washington, Seattle, and ASCO’s chief medical officer.
Toripalimab, dosed at 240 mg in the trial, or placebo were administered with gemcitabine and cisplatin every 3 weeks for up to 6 cycles, followed by toripalimab or placebo maintenance every 3 weeks until disease progression, intolerable toxicity, or completion of 2 years of treatment.
The overall response rate was 77.4% with toripalimab and 66.4% with placebo, and the median duration of response in the toripalimab group was 10 months vs. 5.7 months with placebo.
One-year PFS was 49.4% with toripalimab versus 27.9% with placebo; improved PFS was observed with toripalimab across PD-L1 subgroups.
Grade 3 or worse adverse events occurred in slightly less than 90% of both groups, with fatal adverse events occurring in slightly less than 3% in both.
Adverse events leading to discontinuation occurred in 7.5% of the study group and 4.9% on placebo. As expected with immunotherapy, immune-related adverse events such as hypothyroidism were more common with toripalimab (39.7% vs. 18.9%), as were grade 3 or worse immune-related adverse events (7.5% vs. 0.7%).
At interim analysis in May 2020, the median duration treatment was 39 weeks in the toripalimab group and 36 weeks in the placebo group.
The trial was conducted in China, Taiwan, and Singapore.
JUPITER-02 was funded by Shanghai Junshi Bioscience. Investigator disclosures weren’t reported. Dr. Weiss said he had no relevant disclosures. Dr. Gralow is an advisor for a number of companies, including Genentech, Novartis, and Roche.
A version of this article first appeared on Medscape.com.
A new immunotherapy, toripalimab, has the potential to change practice in the treatment of nasopharyngeal carcinoma (NPC), say experts.
The drug is a monoclonal antibody that blocks programmed cell death protein 1 (PD-1), developed in China and recently approved there for the third-line treatment of NPC, among other indications. The U.S. Food and Drug Administration has granted it a breakthrough therapy designation for recurrent/metastatic NPC, as well as fast-track and orphan drug status for other tumor types.
New results show that when toripalimab was added onto chemotherapy with gemcitabine and cisplatin in the first line for recurrent or metastatic nasopharyngeal carcinoma, there was a significant improvement in both progression-free survival and overall survival.
The results come from the phase 3 trial dubbed JUPITER-02 and will be presented at the plenary session of the American Society of Clinical Oncology annual meeting this Sunday; some details were released earlier at a press briefing
The trial randomly assigned 146 patients to toripalimab and 143 to placebo on a background of gemcitabine and cisplatin, the current standard of care for recurrent/metastatic NPC.
Median progression-free survival (PFS) was 11.7 months with toripalimab vs. 8 months with placebo, a significant improvement (hazard ratio, 0.52; 95% confidence interval, 0.36-0.74. P = .0003). Overall survival was not mature at reporting but favored toripalimab with 25 deaths versus 39 in the placebo group, a 40% risk reduction (P = .0462).
The results “support the use of toripalimab in combination with [gemcitabine and cisplatin] as a new standard of care for first-line treatment of recurrent or metastatic nasopharyngeal carcinoma,” said lead investigator and medical oncologist Rui-Hua Xu, MD, PhD, of the Sun Yat-sen University Cancer Center in Guangzhou, China.
Potential to change practice
The significance of the study is that it used immunotherapy in the first-line setting for NPC instead of the second line where it’s frequently used today, commented Jared Weiss, MD, an associate professor of oncology and a head and neck cancer specialist at the University of North Carolina, Chapel Hill.
If FDA approves toripalimab for the indication, it “would change [first-line] standard of care to the triplet regimen,” he said in an interview.
The discussant for this presentation, Julie Gralow, MD, agreed. “This is one of the first studies in metastatic or recurrent NPC to show a benefit” for combining a PD-1 inhibitor with chemotherapy.
“With FDA approval, these findings should prove practice-changing,” said Dr. Gralow, a professor of breast medical oncology at the University of Washington, Seattle, and ASCO’s chief medical officer.
Toripalimab, dosed at 240 mg in the trial, or placebo were administered with gemcitabine and cisplatin every 3 weeks for up to 6 cycles, followed by toripalimab or placebo maintenance every 3 weeks until disease progression, intolerable toxicity, or completion of 2 years of treatment.
The overall response rate was 77.4% with toripalimab and 66.4% with placebo, and the median duration of response in the toripalimab group was 10 months vs. 5.7 months with placebo.
One-year PFS was 49.4% with toripalimab versus 27.9% with placebo; improved PFS was observed with toripalimab across PD-L1 subgroups.
Grade 3 or worse adverse events occurred in slightly less than 90% of both groups, with fatal adverse events occurring in slightly less than 3% in both.
Adverse events leading to discontinuation occurred in 7.5% of the study group and 4.9% on placebo. As expected with immunotherapy, immune-related adverse events such as hypothyroidism were more common with toripalimab (39.7% vs. 18.9%), as were grade 3 or worse immune-related adverse events (7.5% vs. 0.7%).
At interim analysis in May 2020, the median duration treatment was 39 weeks in the toripalimab group and 36 weeks in the placebo group.
The trial was conducted in China, Taiwan, and Singapore.
JUPITER-02 was funded by Shanghai Junshi Bioscience. Investigator disclosures weren’t reported. Dr. Weiss said he had no relevant disclosures. Dr. Gralow is an advisor for a number of companies, including Genentech, Novartis, and Roche.
A version of this article first appeared on Medscape.com.
Nasal swab test helps identify malignant lung nodules
A simple nasal swab may help in the diagnosis of lung cancer in smokers who have undergone CT screening and had lung nodules detected on the scan.
Only about 5% of the nearly 1.6 million lung nodules identified as incidental findings on low-dose CT screening tests will turn out to be malignant. The new test helps to distinguish between benign and malignant nodules, say researchers reporting a validation study.
The results show that the test identified those at low risk for cancer with a sensitivity of 96.3% and specificity of 41.7%, as well as identifying those as high risk, with a specificity of 90.4% and sensitivity of 58.2%.
The Percepta nasal swab is a first-of-its-kind genomic test, says the manufacturer Veracyte.
It is based on “field of injury” technology, which examines genomic changes in the lining of the respiratory tract for evidence of active cancer cells, coupled with a machine learning model that includes factors such as age, gender, and smoking history.
Veracyte hopes to begin to make the test available to a select number of sites in the second half of 2021. “The test is intended to be performed in the physician’s office on patients referred with suspicious lung nodules found on CT scans,” said Giulia C. Kennedy, PhD, chief scientific officer and chief medical officer at Veracyte. “This could include patients with nodules found through screening programs, as well as incidentally.”
“It will be made available as a laboratory developed test in the U.S. through Veracyte’s centralized CLIA laboratory,” she said in an interview. “In global markets, we will offer the test as an IVD product that can be performed on the nCounter instrument by laboratories locally. Outside of the United States, the test will require a CE mark, which we are equipped to support.”
Results with the test were presented during the American Society of Clinical Oncology (ASCO) 2021 Annual Meeting, which was held virtually this year.
It was first tested in a training set, which consisted of more than 1,100 patients. All were current or former smokers who had a lung nodule detected on chest CT scanning and were followed for up to 1 year or until a final diagnosis of lung cancer or benign disease.
Brushings of the nasal epithelium were prospectively collected in patients with lung nodules from multiple cohorts.
A total of 502 genes were used in the classifier, and performance was evaluated in an independent clinical validation set consisting of 249 patients.
The test identified true benign patients as low risk with 41.7% specificity and 96.3% sensitivity, resulting in a negative predictive value (NPV) of 97.1% in a population with a cancer prevalence of 25%. The risk of malignancy for patients in this low-risk group was less than 3% (1-NPV), and for this group, clinical guidelines recommend surveillance.
Patients with true malignancies were identified as high risk, with 58.2% sensitivity and 90.4% specificity, resulting in a positive predictive value of 67.0% in a population with 25% cancer prevalence. The risk of malignancy for patients deemed to be high risk by the classifier was 67.0%, which exceeds the current guideline threshold for consideration of surgical resection or other ablative therapy if a staging evaluation confirms early stage disease, the authors point out.
The remaining patients, who did not meet the stringent cut-offs for low or high risk, were identified as intermediate risk. In this population, the prevalence of malignancy for patients identified as intermediate risk was 20.7%, which is consistent with guidelines that provide a range for intermediate-risk patients as between 5% and 65% for whom diagnostic biopsy is recommended.
Help guide decisions, more data needed
Approached by this news organization for independent comment, Alexander Spira, MD, PhD, medical oncologist, Virginia Cancer Specialists, Fairfax, explained that the study provides an interesting way to look at a common finding and lung nodules and to predict whether further workup should be done.
“This could provide a role in reassurance that patients who fall into the low-risk category could be observed with serial imaging rather than proceeding to immediate biopsy,” he said. “It falls in under the ‘field of injury’ principle.”
Dr. Spira noted that although the low-risk group appears to have a negative predictive value of >90%, it doesn’t mean that the patient would require no further workup. “It would require CT surveillance rather than proceeding to immediate biopsy, and at this point it does appear promising, but I would want further follow-up in terms of outcomes,” he said.
“This does not apply to nonsmokers, which is of increasing prevalence, but with the increased use of CT screening for patients with a history of tobacco use, it may indeed have a role.”
He also pointed out that while the idea is to avoid biopsies, the smaller lesions are the ones that are concerning. “They are often tough to get at, and it would also depend on patient choice and anxiety as well, given the chance of being in that low percentage that the test misses,” said Dr. Spira. “Lastly, many pulmonologists are ordering PET scans in lieu of a biopsy, and this may also help.”
The bottom line is that this may help guide clinical decisions, but more data are needed. “Even in the low-risk category, 9.4% of patients had a malignancy, which is still a high miss rate,” he added.
The study was funded by Veracyte. Dr. Kennedy is employed by Veracyte. Dr. Spira has reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
A simple nasal swab may help in the diagnosis of lung cancer in smokers who have undergone CT screening and had lung nodules detected on the scan.
Only about 5% of the nearly 1.6 million lung nodules identified as incidental findings on low-dose CT screening tests will turn out to be malignant. The new test helps to distinguish between benign and malignant nodules, say researchers reporting a validation study.
The results show that the test identified those at low risk for cancer with a sensitivity of 96.3% and specificity of 41.7%, as well as identifying those as high risk, with a specificity of 90.4% and sensitivity of 58.2%.
The Percepta nasal swab is a first-of-its-kind genomic test, says the manufacturer Veracyte.
It is based on “field of injury” technology, which examines genomic changes in the lining of the respiratory tract for evidence of active cancer cells, coupled with a machine learning model that includes factors such as age, gender, and smoking history.
Veracyte hopes to begin to make the test available to a select number of sites in the second half of 2021. “The test is intended to be performed in the physician’s office on patients referred with suspicious lung nodules found on CT scans,” said Giulia C. Kennedy, PhD, chief scientific officer and chief medical officer at Veracyte. “This could include patients with nodules found through screening programs, as well as incidentally.”
“It will be made available as a laboratory developed test in the U.S. through Veracyte’s centralized CLIA laboratory,” she said in an interview. “In global markets, we will offer the test as an IVD product that can be performed on the nCounter instrument by laboratories locally. Outside of the United States, the test will require a CE mark, which we are equipped to support.”
Results with the test were presented during the American Society of Clinical Oncology (ASCO) 2021 Annual Meeting, which was held virtually this year.
It was first tested in a training set, which consisted of more than 1,100 patients. All were current or former smokers who had a lung nodule detected on chest CT scanning and were followed for up to 1 year or until a final diagnosis of lung cancer or benign disease.
Brushings of the nasal epithelium were prospectively collected in patients with lung nodules from multiple cohorts.
A total of 502 genes were used in the classifier, and performance was evaluated in an independent clinical validation set consisting of 249 patients.
The test identified true benign patients as low risk with 41.7% specificity and 96.3% sensitivity, resulting in a negative predictive value (NPV) of 97.1% in a population with a cancer prevalence of 25%. The risk of malignancy for patients in this low-risk group was less than 3% (1-NPV), and for this group, clinical guidelines recommend surveillance.
Patients with true malignancies were identified as high risk, with 58.2% sensitivity and 90.4% specificity, resulting in a positive predictive value of 67.0% in a population with 25% cancer prevalence. The risk of malignancy for patients deemed to be high risk by the classifier was 67.0%, which exceeds the current guideline threshold for consideration of surgical resection or other ablative therapy if a staging evaluation confirms early stage disease, the authors point out.
The remaining patients, who did not meet the stringent cut-offs for low or high risk, were identified as intermediate risk. In this population, the prevalence of malignancy for patients identified as intermediate risk was 20.7%, which is consistent with guidelines that provide a range for intermediate-risk patients as between 5% and 65% for whom diagnostic biopsy is recommended.
Help guide decisions, more data needed
Approached by this news organization for independent comment, Alexander Spira, MD, PhD, medical oncologist, Virginia Cancer Specialists, Fairfax, explained that the study provides an interesting way to look at a common finding and lung nodules and to predict whether further workup should be done.
“This could provide a role in reassurance that patients who fall into the low-risk category could be observed with serial imaging rather than proceeding to immediate biopsy,” he said. “It falls in under the ‘field of injury’ principle.”
Dr. Spira noted that although the low-risk group appears to have a negative predictive value of >90%, it doesn’t mean that the patient would require no further workup. “It would require CT surveillance rather than proceeding to immediate biopsy, and at this point it does appear promising, but I would want further follow-up in terms of outcomes,” he said.
“This does not apply to nonsmokers, which is of increasing prevalence, but with the increased use of CT screening for patients with a history of tobacco use, it may indeed have a role.”
He also pointed out that while the idea is to avoid biopsies, the smaller lesions are the ones that are concerning. “They are often tough to get at, and it would also depend on patient choice and anxiety as well, given the chance of being in that low percentage that the test misses,” said Dr. Spira. “Lastly, many pulmonologists are ordering PET scans in lieu of a biopsy, and this may also help.”
The bottom line is that this may help guide clinical decisions, but more data are needed. “Even in the low-risk category, 9.4% of patients had a malignancy, which is still a high miss rate,” he added.
The study was funded by Veracyte. Dr. Kennedy is employed by Veracyte. Dr. Spira has reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
A simple nasal swab may help in the diagnosis of lung cancer in smokers who have undergone CT screening and had lung nodules detected on the scan.
Only about 5% of the nearly 1.6 million lung nodules identified as incidental findings on low-dose CT screening tests will turn out to be malignant. The new test helps to distinguish between benign and malignant nodules, say researchers reporting a validation study.
The results show that the test identified those at low risk for cancer with a sensitivity of 96.3% and specificity of 41.7%, as well as identifying those as high risk, with a specificity of 90.4% and sensitivity of 58.2%.
The Percepta nasal swab is a first-of-its-kind genomic test, says the manufacturer Veracyte.
It is based on “field of injury” technology, which examines genomic changes in the lining of the respiratory tract for evidence of active cancer cells, coupled with a machine learning model that includes factors such as age, gender, and smoking history.
Veracyte hopes to begin to make the test available to a select number of sites in the second half of 2021. “The test is intended to be performed in the physician’s office on patients referred with suspicious lung nodules found on CT scans,” said Giulia C. Kennedy, PhD, chief scientific officer and chief medical officer at Veracyte. “This could include patients with nodules found through screening programs, as well as incidentally.”
“It will be made available as a laboratory developed test in the U.S. through Veracyte’s centralized CLIA laboratory,” she said in an interview. “In global markets, we will offer the test as an IVD product that can be performed on the nCounter instrument by laboratories locally. Outside of the United States, the test will require a CE mark, which we are equipped to support.”
Results with the test were presented during the American Society of Clinical Oncology (ASCO) 2021 Annual Meeting, which was held virtually this year.
It was first tested in a training set, which consisted of more than 1,100 patients. All were current or former smokers who had a lung nodule detected on chest CT scanning and were followed for up to 1 year or until a final diagnosis of lung cancer or benign disease.
Brushings of the nasal epithelium were prospectively collected in patients with lung nodules from multiple cohorts.
A total of 502 genes were used in the classifier, and performance was evaluated in an independent clinical validation set consisting of 249 patients.
The test identified true benign patients as low risk with 41.7% specificity and 96.3% sensitivity, resulting in a negative predictive value (NPV) of 97.1% in a population with a cancer prevalence of 25%. The risk of malignancy for patients in this low-risk group was less than 3% (1-NPV), and for this group, clinical guidelines recommend surveillance.
Patients with true malignancies were identified as high risk, with 58.2% sensitivity and 90.4% specificity, resulting in a positive predictive value of 67.0% in a population with 25% cancer prevalence. The risk of malignancy for patients deemed to be high risk by the classifier was 67.0%, which exceeds the current guideline threshold for consideration of surgical resection or other ablative therapy if a staging evaluation confirms early stage disease, the authors point out.
The remaining patients, who did not meet the stringent cut-offs for low or high risk, were identified as intermediate risk. In this population, the prevalence of malignancy for patients identified as intermediate risk was 20.7%, which is consistent with guidelines that provide a range for intermediate-risk patients as between 5% and 65% for whom diagnostic biopsy is recommended.
Help guide decisions, more data needed
Approached by this news organization for independent comment, Alexander Spira, MD, PhD, medical oncologist, Virginia Cancer Specialists, Fairfax, explained that the study provides an interesting way to look at a common finding and lung nodules and to predict whether further workup should be done.
“This could provide a role in reassurance that patients who fall into the low-risk category could be observed with serial imaging rather than proceeding to immediate biopsy,” he said. “It falls in under the ‘field of injury’ principle.”
Dr. Spira noted that although the low-risk group appears to have a negative predictive value of >90%, it doesn’t mean that the patient would require no further workup. “It would require CT surveillance rather than proceeding to immediate biopsy, and at this point it does appear promising, but I would want further follow-up in terms of outcomes,” he said.
“This does not apply to nonsmokers, which is of increasing prevalence, but with the increased use of CT screening for patients with a history of tobacco use, it may indeed have a role.”
He also pointed out that while the idea is to avoid biopsies, the smaller lesions are the ones that are concerning. “They are often tough to get at, and it would also depend on patient choice and anxiety as well, given the chance of being in that low percentage that the test misses,” said Dr. Spira. “Lastly, many pulmonologists are ordering PET scans in lieu of a biopsy, and this may also help.”
The bottom line is that this may help guide clinical decisions, but more data are needed. “Even in the low-risk category, 9.4% of patients had a malignancy, which is still a high miss rate,” he added.
The study was funded by Veracyte. Dr. Kennedy is employed by Veracyte. Dr. Spira has reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Urine metabolites could predict end of life in lung cancer
Lung cancer patients could soon have their risk of dying over the following 3 months accurately predicted by analyzing their urine samples, allowing them to better prepare for their end of life, say U.K. researchers.
Dr. Seamus Coyle, consultant in palliative medicine, the Clatterbridge Cancer Centre, Liverpool, and colleagues studied urine samples from more than 100 lung cancer patients, deriving a model based on their metabolite profile.
This allowed patients to be divided into high- and low-risk groups for dying over the following 3 months, with an accuracy of 88%.
The model “predicts dying … for every single day for the last 3 months of life,” Dr. Coyle said.
“That’s an outstanding prediction,” Dr. Coyle added, “based on the fact that people actively die over 2 to 3 days on average,” while “some die over a day.”
He continued: “It’s the only test that predicts dying within the last 2 weeks of life, and that’s what I’m passionate about: The earlier recognition of dying.”
The research was presented at the 2021 American Society of Clinical Oncology Annual Meeting on June 4.
‘Promising and important pilot study’
Dr. Nathan Pennell, an ASCO expert, told this news organization that “predicting the actual ‘time’ someone has left is more of an art than a science.”
“For people who may be closer to death, this would potentially allow more focus on supportive care and allow families and patients to plan more accurately for supporting their loved one through the dying process.”
He continued that “while this is a promising and important pilot study, there is more work to be done before this could be used in practice.”
For example, the treatment status of the patients was not clear.
“Were these patients all in hospice, or were some undergoing treatment which, if effective, could ‘rescue’ them from their poor prognostic state?”
Dr. Pennell continued: “Would measuring kidney function be just as good? Is this something that could be intervened upon?
“For example, if someone has a high-risk score for dying, could medical intervention to treat an infection or some other modifiable action change that ‘fate’?”
Death ‘difficult to predict’
Dr. Coyle began by saying that, while for him recognizing that a patient is dying is the start of good end of life care, “recognizing dying accurately, when someone is in the last days of life, is difficult.”
He noted that the 2019 National Audit of Care at the End of Life found that people were recognized to be dying at median of 34 hours before death, with 20% recognized in the last 8 hours.
Moreover, 50% of people who are dying “are unconscious and unable to be involved in any conversation that [is] pertinent to them.”
In an attempt to better predict the onset of dying, the researchers conducted a prospective, longitudinal study in which 424 urine samples were collected from 162 lung cancer patients from six centers.
Of those, 63 patients gave a sample within the last 28 days of life, and 29 within the last week of life.
Urine samples were analyzed using a liquid chromatography quadrupole time-of-flight mass spectrometer for 112 patients, who had a median age of 71 years and a range of 47-89 years, and 40.2% were female. The most common diagnosis was non–small cell lung cancer, in 55.4%, while 19.6% had small cell lung cancer.
Performing Cox Lasso regression analysis on the “hundreds of metabolites” identified in the urine samples, the team developed an End of Life Metabolome (ELM) that predicted an individual’s risk of dying over the following 3 months.
Kaplan-Meier analysis allowed the patients to be divided into five risk groups based on their ELM (P < .001 for trend), which showed that all patients in the lowest-risk group were still alive after more than 2 months following the urine sample.
In contrast, more than 50% of patients in the highest-risk group died within 1 week of their urine sample being taken, and 100% had died within 3 weeks.
Calculating the area under the receiver operating characteristic curve revealed that the ELM was able to predict the risk of dying for every day for the last 3 months of life with an accuracy of 88%.
ELM is being validated in a new cohort of lung cancer patients and it is being assessed in multiple cancers.
The study was funded by the Wellcome Trust UK and North West Cancer Research UK.
No relevant financial relationships were declared.
A version of this article first appeared on Medscape.com.
Lung cancer patients could soon have their risk of dying over the following 3 months accurately predicted by analyzing their urine samples, allowing them to better prepare for their end of life, say U.K. researchers.
Dr. Seamus Coyle, consultant in palliative medicine, the Clatterbridge Cancer Centre, Liverpool, and colleagues studied urine samples from more than 100 lung cancer patients, deriving a model based on their metabolite profile.
This allowed patients to be divided into high- and low-risk groups for dying over the following 3 months, with an accuracy of 88%.
The model “predicts dying … for every single day for the last 3 months of life,” Dr. Coyle said.
“That’s an outstanding prediction,” Dr. Coyle added, “based on the fact that people actively die over 2 to 3 days on average,” while “some die over a day.”
He continued: “It’s the only test that predicts dying within the last 2 weeks of life, and that’s what I’m passionate about: The earlier recognition of dying.”
The research was presented at the 2021 American Society of Clinical Oncology Annual Meeting on June 4.
‘Promising and important pilot study’
Dr. Nathan Pennell, an ASCO expert, told this news organization that “predicting the actual ‘time’ someone has left is more of an art than a science.”
“For people who may be closer to death, this would potentially allow more focus on supportive care and allow families and patients to plan more accurately for supporting their loved one through the dying process.”
He continued that “while this is a promising and important pilot study, there is more work to be done before this could be used in practice.”
For example, the treatment status of the patients was not clear.
“Were these patients all in hospice, or were some undergoing treatment which, if effective, could ‘rescue’ them from their poor prognostic state?”
Dr. Pennell continued: “Would measuring kidney function be just as good? Is this something that could be intervened upon?
“For example, if someone has a high-risk score for dying, could medical intervention to treat an infection or some other modifiable action change that ‘fate’?”
Death ‘difficult to predict’
Dr. Coyle began by saying that, while for him recognizing that a patient is dying is the start of good end of life care, “recognizing dying accurately, when someone is in the last days of life, is difficult.”
He noted that the 2019 National Audit of Care at the End of Life found that people were recognized to be dying at median of 34 hours before death, with 20% recognized in the last 8 hours.
Moreover, 50% of people who are dying “are unconscious and unable to be involved in any conversation that [is] pertinent to them.”
In an attempt to better predict the onset of dying, the researchers conducted a prospective, longitudinal study in which 424 urine samples were collected from 162 lung cancer patients from six centers.
Of those, 63 patients gave a sample within the last 28 days of life, and 29 within the last week of life.
Urine samples were analyzed using a liquid chromatography quadrupole time-of-flight mass spectrometer for 112 patients, who had a median age of 71 years and a range of 47-89 years, and 40.2% were female. The most common diagnosis was non–small cell lung cancer, in 55.4%, while 19.6% had small cell lung cancer.
Performing Cox Lasso regression analysis on the “hundreds of metabolites” identified in the urine samples, the team developed an End of Life Metabolome (ELM) that predicted an individual’s risk of dying over the following 3 months.
Kaplan-Meier analysis allowed the patients to be divided into five risk groups based on their ELM (P < .001 for trend), which showed that all patients in the lowest-risk group were still alive after more than 2 months following the urine sample.
In contrast, more than 50% of patients in the highest-risk group died within 1 week of their urine sample being taken, and 100% had died within 3 weeks.
Calculating the area under the receiver operating characteristic curve revealed that the ELM was able to predict the risk of dying for every day for the last 3 months of life with an accuracy of 88%.
ELM is being validated in a new cohort of lung cancer patients and it is being assessed in multiple cancers.
The study was funded by the Wellcome Trust UK and North West Cancer Research UK.
No relevant financial relationships were declared.
A version of this article first appeared on Medscape.com.
Lung cancer patients could soon have their risk of dying over the following 3 months accurately predicted by analyzing their urine samples, allowing them to better prepare for their end of life, say U.K. researchers.
Dr. Seamus Coyle, consultant in palliative medicine, the Clatterbridge Cancer Centre, Liverpool, and colleagues studied urine samples from more than 100 lung cancer patients, deriving a model based on their metabolite profile.
This allowed patients to be divided into high- and low-risk groups for dying over the following 3 months, with an accuracy of 88%.
The model “predicts dying … for every single day for the last 3 months of life,” Dr. Coyle said.
“That’s an outstanding prediction,” Dr. Coyle added, “based on the fact that people actively die over 2 to 3 days on average,” while “some die over a day.”
He continued: “It’s the only test that predicts dying within the last 2 weeks of life, and that’s what I’m passionate about: The earlier recognition of dying.”
The research was presented at the 2021 American Society of Clinical Oncology Annual Meeting on June 4.
‘Promising and important pilot study’
Dr. Nathan Pennell, an ASCO expert, told this news organization that “predicting the actual ‘time’ someone has left is more of an art than a science.”
“For people who may be closer to death, this would potentially allow more focus on supportive care and allow families and patients to plan more accurately for supporting their loved one through the dying process.”
He continued that “while this is a promising and important pilot study, there is more work to be done before this could be used in practice.”
For example, the treatment status of the patients was not clear.
“Were these patients all in hospice, or were some undergoing treatment which, if effective, could ‘rescue’ them from their poor prognostic state?”
Dr. Pennell continued: “Would measuring kidney function be just as good? Is this something that could be intervened upon?
“For example, if someone has a high-risk score for dying, could medical intervention to treat an infection or some other modifiable action change that ‘fate’?”
Death ‘difficult to predict’
Dr. Coyle began by saying that, while for him recognizing that a patient is dying is the start of good end of life care, “recognizing dying accurately, when someone is in the last days of life, is difficult.”
He noted that the 2019 National Audit of Care at the End of Life found that people were recognized to be dying at median of 34 hours before death, with 20% recognized in the last 8 hours.
Moreover, 50% of people who are dying “are unconscious and unable to be involved in any conversation that [is] pertinent to them.”
In an attempt to better predict the onset of dying, the researchers conducted a prospective, longitudinal study in which 424 urine samples were collected from 162 lung cancer patients from six centers.
Of those, 63 patients gave a sample within the last 28 days of life, and 29 within the last week of life.
Urine samples were analyzed using a liquid chromatography quadrupole time-of-flight mass spectrometer for 112 patients, who had a median age of 71 years and a range of 47-89 years, and 40.2% were female. The most common diagnosis was non–small cell lung cancer, in 55.4%, while 19.6% had small cell lung cancer.
Performing Cox Lasso regression analysis on the “hundreds of metabolites” identified in the urine samples, the team developed an End of Life Metabolome (ELM) that predicted an individual’s risk of dying over the following 3 months.
Kaplan-Meier analysis allowed the patients to be divided into five risk groups based on their ELM (P < .001 for trend), which showed that all patients in the lowest-risk group were still alive after more than 2 months following the urine sample.
In contrast, more than 50% of patients in the highest-risk group died within 1 week of their urine sample being taken, and 100% had died within 3 weeks.
Calculating the area under the receiver operating characteristic curve revealed that the ELM was able to predict the risk of dying for every day for the last 3 months of life with an accuracy of 88%.
ELM is being validated in a new cohort of lung cancer patients and it is being assessed in multiple cancers.
The study was funded by the Wellcome Trust UK and North West Cancer Research UK.
No relevant financial relationships were declared.
A version of this article first appeared on Medscape.com.
e-TNS device passes at-home test
The study also demonstrated that the device, manufactured by Cefaly and cleared in 2020 by the Food and Drug Administration for over-the-counter use, can be safely and effectively used at home.
The study also explored the benefits of 2 hours of use, rather than the 1 hour of use tested in a previous study. “The programming on the device is currently [set to] turn off at 1 hour. As a result of this study, I tell patients if they don’t have adequate relief, and they’re tolerating it, that they can activate it again for a second hour,” Stewart Tepper, MD, said in an interview. Dr. Tepper is a professor of neurology at Geisel School of Medicine at Dartmouth, Hanover, N.H., and a coauthor of the study that was presented by Deena Kuruvilla, MD, at the American Headache Society’s 2021 annual meeting. Dr. Kuruvilla is a neurologist and director of the Westport (Conn.) Headache Institute.
The improvements seen over the sham were significant but not overwhelming, according to Deborah Friedman, MD, MPH, professor of neurology and ophthalmology at the University of Texas, Dallas.
“The numbers are not super impressive when you compare them with other devices. I thought it was interesting that the most bothersome symptom went away in a much higher percentage of people than the headache. That was actually pretty impressive,” said Dr. Friedman, who was asked to comment on the study. She also wondered if the sham device may have inadvertently provided a small amount of stimulation, which could explain the smaller than expected efficacy difference. “It just kind of makes me wonder because I would expect to see a larger separation, even though it was statistically significant.”
The study was an overall success according to Dr. Tepper, who noted that the efficacy of pain freedom was comparable with what has been seen with calcitonin gene-related peptide receptor antagonists (gepants), as well as relieving the most bothersome symptom at 2 hours. The device failed to reduce the usage of rescue medication, suggesting that it might be a candidate to combine with rescue medications. “I think the main thing is it works. It works in a sham-controlled trial, it works at home, and it works comparably to acute medication. And it is further evidence that the lack of access is something that needs to be addressed,” said Dr. Tepper.
Access will depend on insurance companies, who have so far been reluctant to pay for the device. Dr. Tepper is not optimistic they will come around on their own. “My feeling about it is that the only way that payers will finally start to cover this is with a concerted, organized advocacy campaign by patients. The analogy is that when the disease-modifying therapies became available for multiple sclerosis, the National MS Society organized the MS patients and they demanded that the payers cover the disease modifying therapies. That’s the kind of intense focus of advocacy that needs to be done for these noninvasive neuromodulation devices,” said Dr. Tepper.
The TEAM study was a double blind, randomized, sham-controlled trial of 538 patients who were asked to use neurostimulation for a 2-hour, continuous session within 4 hours of a moderate to severe migraine accompanied by at least one migraine-associated symptom. At 2 hours, 25.5% of those using the device achieved pain freedom, compared with 18.3% of those using the sham (P < .05). Among those using the device, 56.4% had freedom from most bothersome symptom, compared with 42.3% of those using the sham (P < .01).
Pain relief at 2 hours was more common in the device group (69.5% vs. 55.2%; P < .01), as was absence of all migraine-associated symptoms at 2 hours (42.5% vs. 34.1%; P < .05), sustained pain freedom at 24 hours (22.8% vs. 15.8%; P < .05), and sustained pain relief at 24 hours (45.9% vs. 34.4%; P < .01). There was no statistically significant between-group difference in use of rescue medications.
In the device group, 8.5% of patients experienced an adverse event, versus 2.9% in the sham group (P = .004). The only adverse reaction that occurred more frequently in the device group was forehead paresthesia, discomfort, and burning (3.5% vs. 0.4%; P = .009).
The study was funded by Cefaly. Dr. Tepper and Dr. Friedman have no relevant financial disclosures.
The study also demonstrated that the device, manufactured by Cefaly and cleared in 2020 by the Food and Drug Administration for over-the-counter use, can be safely and effectively used at home.
The study also explored the benefits of 2 hours of use, rather than the 1 hour of use tested in a previous study. “The programming on the device is currently [set to] turn off at 1 hour. As a result of this study, I tell patients if they don’t have adequate relief, and they’re tolerating it, that they can activate it again for a second hour,” Stewart Tepper, MD, said in an interview. Dr. Tepper is a professor of neurology at Geisel School of Medicine at Dartmouth, Hanover, N.H., and a coauthor of the study that was presented by Deena Kuruvilla, MD, at the American Headache Society’s 2021 annual meeting. Dr. Kuruvilla is a neurologist and director of the Westport (Conn.) Headache Institute.
The improvements seen over the sham were significant but not overwhelming, according to Deborah Friedman, MD, MPH, professor of neurology and ophthalmology at the University of Texas, Dallas.
“The numbers are not super impressive when you compare them with other devices. I thought it was interesting that the most bothersome symptom went away in a much higher percentage of people than the headache. That was actually pretty impressive,” said Dr. Friedman, who was asked to comment on the study. She also wondered if the sham device may have inadvertently provided a small amount of stimulation, which could explain the smaller than expected efficacy difference. “It just kind of makes me wonder because I would expect to see a larger separation, even though it was statistically significant.”
The study was an overall success according to Dr. Tepper, who noted that the efficacy of pain freedom was comparable with what has been seen with calcitonin gene-related peptide receptor antagonists (gepants), as well as relieving the most bothersome symptom at 2 hours. The device failed to reduce the usage of rescue medication, suggesting that it might be a candidate to combine with rescue medications. “I think the main thing is it works. It works in a sham-controlled trial, it works at home, and it works comparably to acute medication. And it is further evidence that the lack of access is something that needs to be addressed,” said Dr. Tepper.
Access will depend on insurance companies, who have so far been reluctant to pay for the device. Dr. Tepper is not optimistic they will come around on their own. “My feeling about it is that the only way that payers will finally start to cover this is with a concerted, organized advocacy campaign by patients. The analogy is that when the disease-modifying therapies became available for multiple sclerosis, the National MS Society organized the MS patients and they demanded that the payers cover the disease modifying therapies. That’s the kind of intense focus of advocacy that needs to be done for these noninvasive neuromodulation devices,” said Dr. Tepper.
The TEAM study was a double blind, randomized, sham-controlled trial of 538 patients who were asked to use neurostimulation for a 2-hour, continuous session within 4 hours of a moderate to severe migraine accompanied by at least one migraine-associated symptom. At 2 hours, 25.5% of those using the device achieved pain freedom, compared with 18.3% of those using the sham (P < .05). Among those using the device, 56.4% had freedom from most bothersome symptom, compared with 42.3% of those using the sham (P < .01).
Pain relief at 2 hours was more common in the device group (69.5% vs. 55.2%; P < .01), as was absence of all migraine-associated symptoms at 2 hours (42.5% vs. 34.1%; P < .05), sustained pain freedom at 24 hours (22.8% vs. 15.8%; P < .05), and sustained pain relief at 24 hours (45.9% vs. 34.4%; P < .01). There was no statistically significant between-group difference in use of rescue medications.
In the device group, 8.5% of patients experienced an adverse event, versus 2.9% in the sham group (P = .004). The only adverse reaction that occurred more frequently in the device group was forehead paresthesia, discomfort, and burning (3.5% vs. 0.4%; P = .009).
The study was funded by Cefaly. Dr. Tepper and Dr. Friedman have no relevant financial disclosures.
The study also demonstrated that the device, manufactured by Cefaly and cleared in 2020 by the Food and Drug Administration for over-the-counter use, can be safely and effectively used at home.
The study also explored the benefits of 2 hours of use, rather than the 1 hour of use tested in a previous study. “The programming on the device is currently [set to] turn off at 1 hour. As a result of this study, I tell patients if they don’t have adequate relief, and they’re tolerating it, that they can activate it again for a second hour,” Stewart Tepper, MD, said in an interview. Dr. Tepper is a professor of neurology at Geisel School of Medicine at Dartmouth, Hanover, N.H., and a coauthor of the study that was presented by Deena Kuruvilla, MD, at the American Headache Society’s 2021 annual meeting. Dr. Kuruvilla is a neurologist and director of the Westport (Conn.) Headache Institute.
The improvements seen over the sham were significant but not overwhelming, according to Deborah Friedman, MD, MPH, professor of neurology and ophthalmology at the University of Texas, Dallas.
“The numbers are not super impressive when you compare them with other devices. I thought it was interesting that the most bothersome symptom went away in a much higher percentage of people than the headache. That was actually pretty impressive,” said Dr. Friedman, who was asked to comment on the study. She also wondered if the sham device may have inadvertently provided a small amount of stimulation, which could explain the smaller than expected efficacy difference. “It just kind of makes me wonder because I would expect to see a larger separation, even though it was statistically significant.”
The study was an overall success according to Dr. Tepper, who noted that the efficacy of pain freedom was comparable with what has been seen with calcitonin gene-related peptide receptor antagonists (gepants), as well as relieving the most bothersome symptom at 2 hours. The device failed to reduce the usage of rescue medication, suggesting that it might be a candidate to combine with rescue medications. “I think the main thing is it works. It works in a sham-controlled trial, it works at home, and it works comparably to acute medication. And it is further evidence that the lack of access is something that needs to be addressed,” said Dr. Tepper.
Access will depend on insurance companies, who have so far been reluctant to pay for the device. Dr. Tepper is not optimistic they will come around on their own. “My feeling about it is that the only way that payers will finally start to cover this is with a concerted, organized advocacy campaign by patients. The analogy is that when the disease-modifying therapies became available for multiple sclerosis, the National MS Society organized the MS patients and they demanded that the payers cover the disease modifying therapies. That’s the kind of intense focus of advocacy that needs to be done for these noninvasive neuromodulation devices,” said Dr. Tepper.
The TEAM study was a double blind, randomized, sham-controlled trial of 538 patients who were asked to use neurostimulation for a 2-hour, continuous session within 4 hours of a moderate to severe migraine accompanied by at least one migraine-associated symptom. At 2 hours, 25.5% of those using the device achieved pain freedom, compared with 18.3% of those using the sham (P < .05). Among those using the device, 56.4% had freedom from most bothersome symptom, compared with 42.3% of those using the sham (P < .01).
Pain relief at 2 hours was more common in the device group (69.5% vs. 55.2%; P < .01), as was absence of all migraine-associated symptoms at 2 hours (42.5% vs. 34.1%; P < .05), sustained pain freedom at 24 hours (22.8% vs. 15.8%; P < .05), and sustained pain relief at 24 hours (45.9% vs. 34.4%; P < .01). There was no statistically significant between-group difference in use of rescue medications.
In the device group, 8.5% of patients experienced an adverse event, versus 2.9% in the sham group (P = .004). The only adverse reaction that occurred more frequently in the device group was forehead paresthesia, discomfort, and burning (3.5% vs. 0.4%; P = .009).
The study was funded by Cefaly. Dr. Tepper and Dr. Friedman have no relevant financial disclosures.
FROM AHS 2021
As the Story Unfolds
A 78-year-old woman presented to the ambulatory care clinic for a painful tongue mass. She noticed the mass 2 months prior to presentation, and it had not grown in the interim. She had left-sided jaw pain when opening her mouth and persistent left-sided otalgia.
In the evaluation of tongue masses, ulcerations, or other surface abnormalities, exclusion of squamous cell carcinoma is the top priority. Additional tongue surface abnormalities include benign lesions such as geographic tongue, inflammatory conditions such as lichen planus, and infections such as syphilis.
The ear and jaw pain may reflect metastatic spread, neural invasion, or referred pain from the tongue. A vasculitis with predilection for the head, such as giant cell arteritis, could present with oral and ear pain. Jaw pain with mastication could reflect jaw claudication, but pain upon mouth opening is more commonly explained by temporomandibular joint dysfunction.
The patient had hypertension, hyperlipidemia, chronic kidney disease (estimated glomerular filtration rate of 42 mL/min), and diabetes mellitus. Four months prior she was diagnosed with a chronic obstructing left renal calculus on ultrasonography to evaluate chronic kidney disease. Two months prior heart failure with preserved ejection fraction was diagnosed. Stress cardiac magnetic resonance imaging (MRI) demonstrated normal ejection fraction, asymmetric septal hypertrophy, and stress-induced subendocardial perfusion defect. Her medications were metoprolol, lisinopril, simvastatin, meloxicam, and aspirin. She never used tobacco and did not consume alcohol. She was born in the Philippines and emigrated to the United States 15 years ago. She had not experienced fever, chills, hearing loss, tinnitus, cough, dysphonia, neck swelling, or joint pain. She had lost 3 kg in the previous 4 months.
The absence of tobacco and alcohol use reduces the probability of a squamous cell carcinoma, although human papillomavirus–associated squamous cell carcinoma of the tongue remains possible. Asymmetric septal hypertrophy is characteristic of hypertrophic cardiomyopathy or an infiltrative cardiomyopathy. Sarcoidosis can affect the heart and can account for the renal calculus (via hypercalcemia). Amyloid light-chain (AL) amyloidosis could involve the heart and the tongue, although in amyloidosis the cardiac MRI typically displays late gadolinium enhancement and ventricular wall thickening. The absence of tinnitus or hearing loss suggests that the left-sided otalgia is referred pain from the tongue and oral cavity rather than a primary otologic disease (eg, infection).
On physical examination, temperature was 37.2 °C, heart rate was 88 beats per minute, blood pressure was 134/62 mm Hg, and oxygen saturation was 99% while breathing ambient air. The patient’s weight was 40.4 kg (body mass index of 19.26 kg/m2). Intraoral examination revealed induration of the bilateral tongue, an erosive 1-cm pedunculated mass of the left dorsum, rough white coating on the right dorsum, and fullness of the right lateral surface with an erosion abutting tooth #2. The right submandibular salivary gland was firm. The otoscopic examination and remainder of the head and neck examination were normal. There was no cervical, supraclavicular, or axillary adenopathy. The cranial nerve, cardiovascular, pulmonary, abdominal, and skin examinations were normal.
The left-sided lingual mass and right-sided lingual erosion likely arise from the same process. Both are compatible with an infection (eg, syphilis or tuberculosis), cancer, autoimmune disease (eg, Crohn’s disease or sarcoidosis), or an infiltrative disease such as amyloidosis. Leukoplakia could reflect a candidal infection, dysplasia, squamous cell carcinoma, oral hairy leukoplakia, or hyperkeratosis. The isolated submandibular salivary gland could reflect sialadenitis from chronic salivary duct obstruction or a primary neoplasm, but more likely is caused by the same process causing the tongue abnormalities.
The white blood cell count was 8,600/μL; hemoglobin, 11.5 g/dL; mean corpuscular volume, 102.5 fL; and platelet count, 270,000/μL3. Serum sodium was 141 mEq/L; potassium, 4.1 mEq/L; chloride, 101 mEq/L; bicarbonate, 30 mEq/L; blood urea nitrogen, 19 mg/dL; creatinine, 0.7 mg/dL; and calcium, 10.4 mg/dL (reference range, 8.5-10.3). Total serum protein was 7.3 g/dL (reference range, 6.0-8.3); albumin was 3.7 g/dL. Liver biochemistry test results were normal. Serum folate and vitamin B12 levels were normal. Serum ferritin was 423 ng/mL (reference range, 11-306); transferrin saturation, 21.4% (reference range, 15.0%-50.0%); and total iron-binding capacity, 323 µg/dL (reference range, 261-478). Parathyroid hormone (PTH) was 14 pg/mL (reference range, 15-65). HIV antibody was negative.
The calcium level is at the upper range of normal, whereas the PTH level is at the lower range of normal. The differential diagnosis for PTH-independent hypercalcemia includes hypercalcemia of malignancy and granulomatous disease such as sarcoidosis. Mild hypercalcemia could contribute to the nephrolithiasis. The iron studies exclude iron deficiency and are not suggestive of anemia of chronic disease. The triad of mild hypercalcemia, cardiomyopathy, and anemia is compatible with AL amyloidosis (perhaps with associated multiple myeloma) or sarcoidosis; both disorders can present as a mass. Imaging of the head and neck and biopsy of the tongue mass are the next steps.
The left dorsal tongue mass was excised in clinic. Histopathology revealed ulcerated squamous mucosa with inflammatory changes but no malignancy. Imaging of the head and neck was scheduled.
Neither cancer or granulomas were detected, but inadequate sampling or staining must be considered. Inflammatory changes are compatible with infection, autoimmunity, and cancer; the latter can feature reactive changes that obscure the malignant cells. The absence of granulomas lowers, but does not eliminate, the possibility of sarcoidosis, tuberculosis, fungal infection, and granulomatosis with polyangiitis. Actinomycosis is an invasive orofacial infection that disregards anatomic boundaries and is characterized by inflammatory histology; although infection of the tongue is possible, infection of the jaw and face is more typical. Immunoglobulin G4–related disease can present as an inflammatory and invasive disorder; however, the characteristic histopathologic findings (lymphoplasmacytic infiltrate, fibrosis, and phlebitis) are absent.
Culture of the tissue for mycobacteria or fungi (she is at increased risk for both given her previous residency in the Philippines) could increase the diagnostic yield. Another biopsy of the tongue or an adjacent structure—guided by imaging—may provide a more diagnostic tissue sample.
MRI of the head and neck demonstrated hyperintense signal and prominence of the right lateral pterygoid muscle (Figure 1A) and slight enlargement of a right submandibular gland (Figure 1B). No tongue abnormalities were identified. Radiograph of the chest did not reveal infiltrates, masses, or lymphadenopathy.

The absence of the tongue mass on the MRI likely reflects excision of the mass at the time of biopsy. The signal enhancement in the right lateral pterygoid muscle and submandibular gland is suggestive of an infiltrative process. Infiltration of the right lateral pterygoid muscle may also explain the patient’s pain when opening her mouth. Infiltrative processes can be neoplastic (eg, salivary gland tumor, sarcoma, lymphoma), infectious (eg, mycobacterial or fungal), cellular (eg, histiocytes, mast cells, plasma cells, eosinophils, granulomas), or related to inert substances such as amyloid or iron.
Seven weeks later, the patient presented to the hospital for scheduled percutaneous nephrolithotomy of the obstructing renal calculus. The physical examination was unchanged. The complete blood count and metabolic panel were unchanged apart from hemoglobin of 9.9 g/dL and calcium of 11.5 mg/dL. Coagulation studies were within normal limits.
A percutaneous nephroureteral stent was placed under conscious sedation. The patient then underwent rapid sequence induction of general anesthesia for the nephrolithotomy with fentanyl, propofol, and rocuronium. Within minutes of initiating mechanical ventilation, severe periorbital and perioral edema, copious oral cavity bleeding, and bilateral periorbital purpura occurred. Sugammadex (neuromuscular blockade reversal) and dexamethasone were administered. Examination of the oral cavity was limited by the brisk bleeding; the right sided tongue erosion was unchanged.
Bleeding is caused by thrombocytopenia, thrombocytopathy, coagulopathy, or disruption of vessel integrity. Oral cavity bleeding could arise from the tongue ulceration, but could also reflect pulmonary, nasal, or gastrointestinal hemorrhage. Angioedema arises from mast cell– or bradykinin-mediated pathways; mast cell degranulation may have been precipitated by the anesthetic agents, opiate, or a material in the nephroureteral stent.
The edema and bleeding are temporally related to multiple medications and mechanical ventilation. A latent bleeding diathesis may have manifested in the setting of increased tissue hydrostatic pressure or vessel permeability. Amyloidosis can lead to vessel fragility and coagulopathy, and periorbital bleeding is characteristic of AL amyloidosis.
The hypercalcemia, now more pronounced, raises concern for malignancy (including multiple myeloma) and granulomatous diseases like sarcoidosis, mycobacterial infections, and fungal infections. The declining hemoglobin could be explained by chronic blood loss, hemolysis, anemia of chronic disease, or a bone marrow process.
The cardiomyopathy, bleeding disorder, and multifocal disease in the oral cavity can be explained by AL amyloidosis; the hypercalcemia suggests concomitant multiple myeloma.
At the time of the bleeding event, the partial thromboplastin time, prothrombin time, and fibrinogen were within the reference ranges. Factor X activity level was normal. No schistocytes were observed on peripheral blood smear. Immunoglobulin G level was 1,425 mg/dL (reference range, 639-1,349); IgA and IgM levels were within the reference range. Serum lambda free light chains were 151.78 mg/dL (reference range, 0.46-2.71), and the ratio of kappa to lambda light chains was 0.01 (reference range, 0.49-2.54). Serum protein electrophoresis and immunofixation demonstrated a monoclonal paraprotein (IgG lambda) level of 1.2 g/dL. Congo red staining of the previously excised left dorsal tongue mass was negative for apple-green birefringence. Reexamination of the oral cavity revealed macroglossia and scalloping of the tongue (Figure 2).
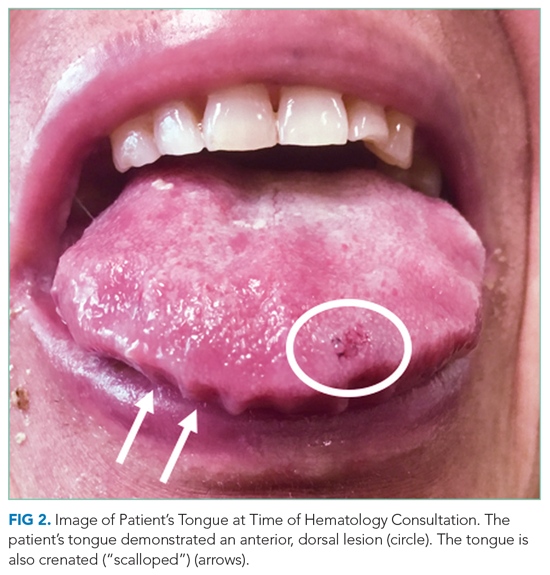
Scalloping is characteristic of an infiltrative disorder that enlarges the tongue (macroglossia) and deforms its edges, which encounter the teeth. Macroglossia is seen in AL amyloidosis, acromegaly, and hypothyroidism. A monoclonal light chain, especially a lambda light chain, is characteristic of AL amyloidosis. The Congo red stain results can support the diagnosis when positive, but it has limited sensitivity. The tongue specimen can be sent for immunohistochemistry or mass spectrometry to evaluate for light chain deposition. A bone marrow biopsy can demonstrate a clonal plasma cell population. AL amyloidosis with concomitant multiple myeloma is the most likely diagnosis.
Bone marrow aspiration and core biopsy demonstrated 30% lambda-restricted plasma cells (Figure 3A-C). Congo red staining demonstrated apple-green birefringence of the bone marrow microvasculature (Figure 3D). Skeletal survey demonstrated widespread lytic bone disease involving the calvarium (Figure 4A), left humerus (Figure 4B), and left scapula (Figure 4B). Based on the monoclonal paraprotein, more than 10% monoclonal plasma cells, skeletal lesions, and hypercalcemia, she was diagnosed with IgG lambda multiple myeloma. Based on apple-green birefringence in the bone marrow and macroglossia, she was diagnosed with AL amyloidosis. The cardiac MRI findings were compatible with AL amyloidosis. 1
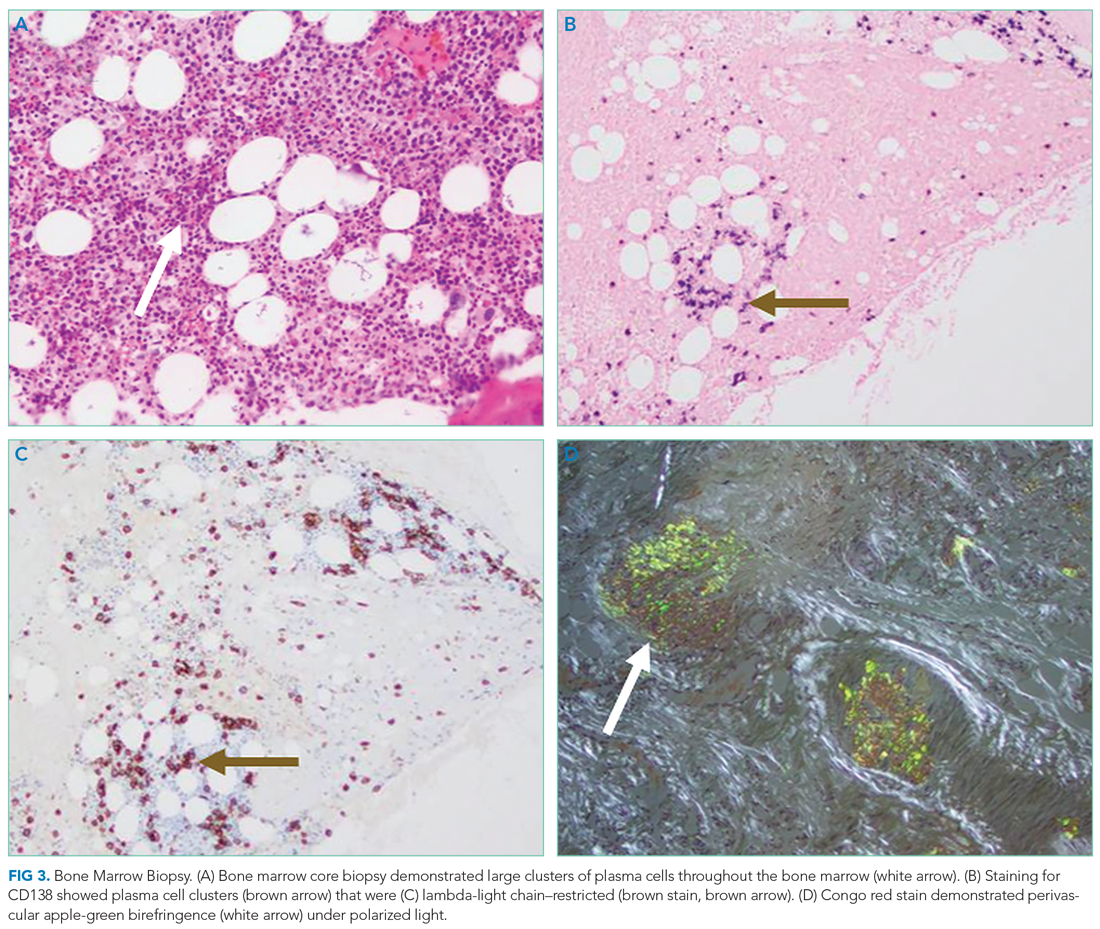
After three cycles of bortezomib and dexamethasone therapy to concurrently treat AL amyloidosis and multiple myeloma, the serum lambda light chain level decreased to 1.49 mg/dL and the monoclonal paraprotein level decreased to 0.3 g/dL. The calcium level was 9.8 mg/dL, and the hemoglobin level was 11.7 g/dL. The patient’s tongue pain resolved, allowing for improved oral intake and a 5.7-kg weight gain. The patient underwent nephrolithotomy 4 months after her initial presentation. She resumed an active lifestyle and recently traveled to visit relatives in the Philippines.
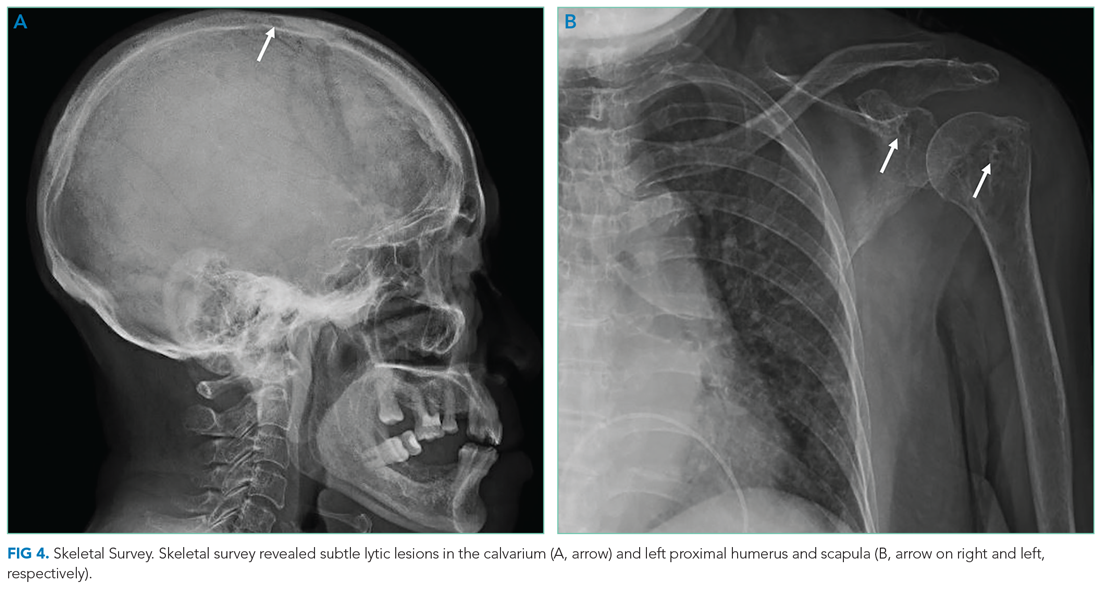
DISCUSSION
Oral diseases affect general health and quality of life and can be a harbinger of systemic disease. Tooth loss, caries, periodontal disease, and poorly fitting dentures commonly affect speech and nutrition.2 These common outpatient oral health issues can be the driving force for hospital admissions; for example, caries and periodontal disease can lead to suppurative odontogenic infection, endocarditis, brain abscess, and sepsis.
Tongue ulcerations, masses, and surface abnormalities often require consultation with a dentist or oral and maxillofacial surgeon to exclude squamous cell carcinoma.3 Other diagnostic considerations include benign neoplasms, trauma, inflammatory conditions (eg, sarcoidosis), infection (eg, syphilis, tuberculosis), and infiltrative processes such as amyloidosis.
Amyloidosis is a heterogeneous group of diseases caused by deposition of insoluble protein fibrils in tissues.4,5 The three most encountered forms of amyloidosis are AL, AA, and ATTR. Each form is named after the culprit protein.4 AL amyloidosis arises when a small clonal population of plasma cells in the bone marrow overproduces immunoglobulin light chain monomers.4,6 AA amyloidosis develops when the liver produces serum amyloid A protein (an acute phase reactant) in response to a chronic inflammatory condition such as rheumatoid arthritis or chronic intravenous drug injection.4 Transthyretin (TTR, also known as “prealbumin”) is a tetrameric protein that transports thyroxine and retinol; there are two forms of ATTR amyloidosis: hereditary and wild type. Hereditary ATTR amyloidosis develops from agglomeration of misfolded TTR monomers caused by mutations in the TTR gene. Wild-type ATTR amyloidosis is caused by age-related dissociation of the TTR tetramer into its constituent monomers that denature, misfold, and agglomerate into fibrils.5 Wild-type ATTR is now recognized as the most common form of amyloidosis, with 25% of myocardial autopsy specimens of patients 80 years or older demonstrating amyloid.7 The estimated incidence of AL amyloidosis is 10 cases per million person-years.8
Each amyloid protein homes in on specific anatomic sites.4 Characteristic combinations of organ dysfunction can suggest different forms of amyloidosis.9 Cardiac and peripheral nervous involvement (eg, carpal tunnel syndrome) is typical of both hereditary and wild-type ATTR amyloidosis; ATTR amyloidosis does not involve the kidney.4 AA amyloidosis most commonly manifests with proteinuria followed by declining glomerular filtration rate; heart failure is rare.4 The most common findings in AL amyloidosis are proteinuria, congestive heart failure, and sensory neuropathy.6 Gastrointestinal tract and hepatic involvement are each seen in nearly 20% of patients, and macroglossia is identified in approximately 10% of those with AL amyloidosis.6,10
Chronic deposition of amyloid can lead to acute presentations. Approximately 30% of patients with AL amyloidosis develop abnormal bleeding.11 Amyloid deposition in small blood vessels predisposes them to rupture. Bleeding events can be exacerbated by acquired coagulopathy due to plasma cell dyscrasia−associated thrombocytopenia, amyloid fibril adsorption of factor X, or hypofibrinogenemia.11,12 Periorbital purpura following minor trauma or transient venous hypertension is characteristic of AL amyloidosis.6,13 In this case, positive pressure ventilation and recumbent positioning increased hydrostatic pressure in the head and neck, causing rupture of the infiltrated small vessels around the eyes and in the oral cavity.14
Histological demonstration of tissue deposition of amyloid protein is the preferred method for amyloidosis diagnosis. Symptomatic sites or organs with dysfunction or radiologic changes are suitable for biopsy.6 If those sites are inaccessible or yield insufficient tissue quantity, abdominal fat pad aspiration or biopsy is indicated.15 Apple-green birefringence under polarized light of Congo red–stained tissue is characteristic, with sensitivity and specificity of approximately 80% and a positive predictive value of 85%.15 Immunoelectron microscopy is often performed simultaneously to confirm the diagnosis and determine the amyloid protein type.4,16 Immunoelectron microscopy’s sensitivity is approximately 80%, and it has specificity and positive predictive value both approaching 100%.15 Mass spectrometry is particularly useful in cases where the amyloid subtype is not clinically apparent (eg, a patient with an autoimmune condition or chronic infection as well as light chain abnormality).6 Cardiac MRI findings that suggest amyloidosis include a thickened left ventricle and late gadolinium enhancement.1 ATTR cardiac amyloidosis can be diagnosed using amyloid fibril–binding radiotracer technetium-99m-pyrophosphate scintigraphy; biopsy is often not necessary.1,4 Gene sequencing to differentiate between hereditary and wild-type forms of ATTR amyloidosis is beneficial.
The primary objectives of amyloidosis management are to control symptoms and inhibit amyloid protein production.6 Outcomes in AL amyloidosis have improved due to early diagnosis, new chemotherapeutic agents to eradicate the plasma cell clone, and autologous stem cell transplantation.6,17 Two new ATTR amyloidosis treatments are RNA interference therapies, which prevent TTR messenger RNA translation, and tafamidis, which stabilizes the TTR tetramer and prevents dissociation into its constituent monomers that precipitate in tissues.18 Both therapies can improve neuropathy-related quality of life.18 Tafamidis slows disease progression and decreases all-cause mortality in patients with hereditary and wild-type ATTR cardiac amyloidosis.19
Multiple myeloma and AL amyloidosis are both plasma cell dyscrasias involving the bone marrow, but they represent distinct disease processes with different clinical features.6 Multiple myeloma is characterized by marked expansion of a clonal plasma cell population within the bone marrow that aberrantly produces immunoglobulin. Conversely, the clonal plasma cell population responsible for producing the insoluble monoclonal light chain protein in AL amyloidosis typically constitutes less than 10% of the bone marrow.20 Multiple myeloma and AL amyloidosis may coexist in the same patient; nearly 15% of patients with multiple myeloma subsequently develop clinically overt AL amyloidosis, which portends a poor outcome.20
Amyloidosis is a rare group of diseases that arises when misfolded proteins aggregate in vital organs. The typical manifestations—congestive heart failure, neuropathy, chronic kidney disease, bleeding—are nearly always explained by more common conditions. Characteristic manifestations (like macroglossia) or associated diseases (like multiple myeloma) substantially increases the probability of AL amyloidosis. In a multisystem illness, the most common diseases must be excluded first, but this case reminds us that rare diseases, like amyloidosis, also warrant consideration as the story unfolds.
KEY TEACHING POINTS
- Different amyloid proteins precipitate in different anatomic sites, which leads to specific multiorgan combinations. The most common amyloidosis, ATTR, tends to manifest as heart failure and peripheral sensory neuropathy, while the constellation of AL amyloidosis includes heart failure, neuropathy, and proteinuria.
- Bleeding occurs in 30% of patients with AL amyloidosis. It is precipitated by fragile small blood vessels and exacerbated by acquired coagulopathy from adsorption of coagulation factors.
- Multiple myeloma and AL amyloidosis are both plasma cell dyscrasias involving the bone marrow, but they represent distinct disease processes with different clinical tempos and presentations. Multiple myeloma and AL amyloidosis may coexist in the same patient; nearly 15% of patients with multiple myeloma subsequently develop clinically overt AL amyloidosis.
Acknowledgment
The authors thank Benjamin A Derman, MD, of the University of Chicago, Chicago, Illinois, for critical review of the manuscript.
1. Witteles RM, Bokhari S, Damy T, et al. Screening for transthyretin amyloid cardiomyopathy in everyday practice. JACC Heart Fail. 2019;7(8):709-716. https://doi.org/10.1016/j.jchf.2019.04.010
2. Griffin SO, Jones JA, Brunson D, Griffin PM, Bailey WD. Burden of oral disease among older adults and implications for public health priorities. Am J Public Health. 2012;102(3):411-418. https://doi.org/10.2105/ajph.2011.300362
3. Ernster JA, Sciotto CG, O’Brien MM, et al. Rising incidence of oropharyngeal cancer and the role of oncogenic human papilloma virus. Laryngoscope. 2007;117(12):2115-2128. https://doi.org/10.1097/mlg.0b013e31813e5fbb
4. Wechalekar AD, Gillmore JD, Hawkins PN. Systemic amyloidosis. Lancet. 2016;387(10038):2641-2654. https://doi.org/10.1016/s0140-6736(15)01274-x
5. Riek R, Eisenberg DS. The activities of amyloids from a structural perspective. Nature. 2016;539(7628):227-235. https://doi.org/10.1038/nature20416
6. Gertz MA, Dispenzieri A. Systemic amyloidosis recognition, prognosis, and therapy: a systematic review. JAMA. 2020;324(1):79-89. https://doi.org/10.1001/jama.2020.5493
7. Ruberg FL, Berk JL. Transthyretin (TTR) cardiac amyloidosis. Circulation. 2012;126(10):1286-1300. https://doi.org/10.1161/circulationaha.111.078915
8. Quock TP, Yan T, Chang E, Guthrie S, Broder MS. Epidemiology of AL amyloidosis: a real-world study using US claims data. Blood Adv. 2018;2(10):1046-1053. https://doi.org/10.1182/bloodadvances.2018016402
9. Papoutsidakis N, Miller EJ, Rodonski A, Jacoby D. Time course of common clinical manifestations in patients with transthyretin cardiac amyloidosis: delay from symptom onset to diagnosis. J Card Fail. 2018;24(2):131-133. https://doi.org/10.1016/j.cardfail.2017.12.005
10. Shimazaki C, Hata H, Iida S, et al. Nationwide survey of 741 patients with systemic amyloid light-chain amyloidosis in Japan. Intern Med. 2018;57(2):181-187. https://doi.org/10.2169/internalmedicine.9206-17
11. Mumford AD, O’Donnell J, Gillmore JD, Manning RA, Hawkins PN, Laffan M. Bleeding symptoms and coagulation abnormalities in 337 patients with AL-amyloidosis. Br J Haematol. 2000;110(2):454-460. https://doi.org/10.1046/j.1365-2141.2000.02183.x
12. Choufani EB, Sanchorawala V, Ernst T, et al. Acquired factor X deficiency in patients with amyloid light-chain amyloidosis: incidence, bleeding manifestations, and response to high-dose chemotherapy. Blood. 2001;97(6):1885-1887. https://doi.org/10.1182/blood.v97.6.1885
13. Slagel GA, Lupton GP. Postproctoscopic periorbital purpura. Primary systemic amyloidosis. Arch Dermatol. 1986;122(4):464-465, 467-468.
14. Lupton GP. Pneomometry-induced purpura. Arch Dermatol. 1981;117(10):603. https://doi.org/10.1001/archderm.117.10.603a
15. Fernández de Larrea C, Verga L, Morbini P, et al. A practical approach to the diagnosis of systemic amyloidoses. Blood. 2015;125(14):2239-2244. https://doi.org/10.1182/blood-2014-11-609883
16. Vaxman I, Gertz M. Recent Advances in the diagnosis, risk stratification, and management of systemic light-chain amyloidosis. Acta Haematol. 2019;141(2):93-106. https://doi.org/10.1159/000495455
17. Muchtar E, Gertz MA, Kumar SK, et al. Improved outcomes for newly diagnosed AL amyloidosis between 2000 and 2014: cracking the glass ceiling of early death. Blood. 2017;129(15):2111-2119. https://doi.org/10.1182/blood-2016-11-751628
18. Quarta CC, Solomon SD. Stabilizing transthyretin to treat ATTR cardiomyopathy. N Engl J Med. 2018;379(11):1083-1084. https://doi.org/10.1056/nejme1810074
19. Maurer MS, Schwartz JH, Gundapaneni B, et al. Tafamidis treatment for patients with transthyretin amyloid cardiomyopathy. N Engl J Med. 2018;379(11):1007-1016. https://doi.org/10.1056/nejmoa1805689
20. Bahlis NJ, Lazarus HM. Multiple myeloma-associated AL amyloidosis: is a distinctive therapeutic approach warranted? Bone Marrow Transplant. 2006;38(1):7-15. https://doi.org/10.1038/sj.bmt.1705395
A 78-year-old woman presented to the ambulatory care clinic for a painful tongue mass. She noticed the mass 2 months prior to presentation, and it had not grown in the interim. She had left-sided jaw pain when opening her mouth and persistent left-sided otalgia.
In the evaluation of tongue masses, ulcerations, or other surface abnormalities, exclusion of squamous cell carcinoma is the top priority. Additional tongue surface abnormalities include benign lesions such as geographic tongue, inflammatory conditions such as lichen planus, and infections such as syphilis.
The ear and jaw pain may reflect metastatic spread, neural invasion, or referred pain from the tongue. A vasculitis with predilection for the head, such as giant cell arteritis, could present with oral and ear pain. Jaw pain with mastication could reflect jaw claudication, but pain upon mouth opening is more commonly explained by temporomandibular joint dysfunction.
The patient had hypertension, hyperlipidemia, chronic kidney disease (estimated glomerular filtration rate of 42 mL/min), and diabetes mellitus. Four months prior she was diagnosed with a chronic obstructing left renal calculus on ultrasonography to evaluate chronic kidney disease. Two months prior heart failure with preserved ejection fraction was diagnosed. Stress cardiac magnetic resonance imaging (MRI) demonstrated normal ejection fraction, asymmetric septal hypertrophy, and stress-induced subendocardial perfusion defect. Her medications were metoprolol, lisinopril, simvastatin, meloxicam, and aspirin. She never used tobacco and did not consume alcohol. She was born in the Philippines and emigrated to the United States 15 years ago. She had not experienced fever, chills, hearing loss, tinnitus, cough, dysphonia, neck swelling, or joint pain. She had lost 3 kg in the previous 4 months.
The absence of tobacco and alcohol use reduces the probability of a squamous cell carcinoma, although human papillomavirus–associated squamous cell carcinoma of the tongue remains possible. Asymmetric septal hypertrophy is characteristic of hypertrophic cardiomyopathy or an infiltrative cardiomyopathy. Sarcoidosis can affect the heart and can account for the renal calculus (via hypercalcemia). Amyloid light-chain (AL) amyloidosis could involve the heart and the tongue, although in amyloidosis the cardiac MRI typically displays late gadolinium enhancement and ventricular wall thickening. The absence of tinnitus or hearing loss suggests that the left-sided otalgia is referred pain from the tongue and oral cavity rather than a primary otologic disease (eg, infection).
On physical examination, temperature was 37.2 °C, heart rate was 88 beats per minute, blood pressure was 134/62 mm Hg, and oxygen saturation was 99% while breathing ambient air. The patient’s weight was 40.4 kg (body mass index of 19.26 kg/m2). Intraoral examination revealed induration of the bilateral tongue, an erosive 1-cm pedunculated mass of the left dorsum, rough white coating on the right dorsum, and fullness of the right lateral surface with an erosion abutting tooth #2. The right submandibular salivary gland was firm. The otoscopic examination and remainder of the head and neck examination were normal. There was no cervical, supraclavicular, or axillary adenopathy. The cranial nerve, cardiovascular, pulmonary, abdominal, and skin examinations were normal.
The left-sided lingual mass and right-sided lingual erosion likely arise from the same process. Both are compatible with an infection (eg, syphilis or tuberculosis), cancer, autoimmune disease (eg, Crohn’s disease or sarcoidosis), or an infiltrative disease such as amyloidosis. Leukoplakia could reflect a candidal infection, dysplasia, squamous cell carcinoma, oral hairy leukoplakia, or hyperkeratosis. The isolated submandibular salivary gland could reflect sialadenitis from chronic salivary duct obstruction or a primary neoplasm, but more likely is caused by the same process causing the tongue abnormalities.
The white blood cell count was 8,600/μL; hemoglobin, 11.5 g/dL; mean corpuscular volume, 102.5 fL; and platelet count, 270,000/μL3. Serum sodium was 141 mEq/L; potassium, 4.1 mEq/L; chloride, 101 mEq/L; bicarbonate, 30 mEq/L; blood urea nitrogen, 19 mg/dL; creatinine, 0.7 mg/dL; and calcium, 10.4 mg/dL (reference range, 8.5-10.3). Total serum protein was 7.3 g/dL (reference range, 6.0-8.3); albumin was 3.7 g/dL. Liver biochemistry test results were normal. Serum folate and vitamin B12 levels were normal. Serum ferritin was 423 ng/mL (reference range, 11-306); transferrin saturation, 21.4% (reference range, 15.0%-50.0%); and total iron-binding capacity, 323 µg/dL (reference range, 261-478). Parathyroid hormone (PTH) was 14 pg/mL (reference range, 15-65). HIV antibody was negative.
The calcium level is at the upper range of normal, whereas the PTH level is at the lower range of normal. The differential diagnosis for PTH-independent hypercalcemia includes hypercalcemia of malignancy and granulomatous disease such as sarcoidosis. Mild hypercalcemia could contribute to the nephrolithiasis. The iron studies exclude iron deficiency and are not suggestive of anemia of chronic disease. The triad of mild hypercalcemia, cardiomyopathy, and anemia is compatible with AL amyloidosis (perhaps with associated multiple myeloma) or sarcoidosis; both disorders can present as a mass. Imaging of the head and neck and biopsy of the tongue mass are the next steps.
The left dorsal tongue mass was excised in clinic. Histopathology revealed ulcerated squamous mucosa with inflammatory changes but no malignancy. Imaging of the head and neck was scheduled.
Neither cancer or granulomas were detected, but inadequate sampling or staining must be considered. Inflammatory changes are compatible with infection, autoimmunity, and cancer; the latter can feature reactive changes that obscure the malignant cells. The absence of granulomas lowers, but does not eliminate, the possibility of sarcoidosis, tuberculosis, fungal infection, and granulomatosis with polyangiitis. Actinomycosis is an invasive orofacial infection that disregards anatomic boundaries and is characterized by inflammatory histology; although infection of the tongue is possible, infection of the jaw and face is more typical. Immunoglobulin G4–related disease can present as an inflammatory and invasive disorder; however, the characteristic histopathologic findings (lymphoplasmacytic infiltrate, fibrosis, and phlebitis) are absent.
Culture of the tissue for mycobacteria or fungi (she is at increased risk for both given her previous residency in the Philippines) could increase the diagnostic yield. Another biopsy of the tongue or an adjacent structure—guided by imaging—may provide a more diagnostic tissue sample.
MRI of the head and neck demonstrated hyperintense signal and prominence of the right lateral pterygoid muscle (Figure 1A) and slight enlargement of a right submandibular gland (Figure 1B). No tongue abnormalities were identified. Radiograph of the chest did not reveal infiltrates, masses, or lymphadenopathy.

The absence of the tongue mass on the MRI likely reflects excision of the mass at the time of biopsy. The signal enhancement in the right lateral pterygoid muscle and submandibular gland is suggestive of an infiltrative process. Infiltration of the right lateral pterygoid muscle may also explain the patient’s pain when opening her mouth. Infiltrative processes can be neoplastic (eg, salivary gland tumor, sarcoma, lymphoma), infectious (eg, mycobacterial or fungal), cellular (eg, histiocytes, mast cells, plasma cells, eosinophils, granulomas), or related to inert substances such as amyloid or iron.
Seven weeks later, the patient presented to the hospital for scheduled percutaneous nephrolithotomy of the obstructing renal calculus. The physical examination was unchanged. The complete blood count and metabolic panel were unchanged apart from hemoglobin of 9.9 g/dL and calcium of 11.5 mg/dL. Coagulation studies were within normal limits.
A percutaneous nephroureteral stent was placed under conscious sedation. The patient then underwent rapid sequence induction of general anesthesia for the nephrolithotomy with fentanyl, propofol, and rocuronium. Within minutes of initiating mechanical ventilation, severe periorbital and perioral edema, copious oral cavity bleeding, and bilateral periorbital purpura occurred. Sugammadex (neuromuscular blockade reversal) and dexamethasone were administered. Examination of the oral cavity was limited by the brisk bleeding; the right sided tongue erosion was unchanged.
Bleeding is caused by thrombocytopenia, thrombocytopathy, coagulopathy, or disruption of vessel integrity. Oral cavity bleeding could arise from the tongue ulceration, but could also reflect pulmonary, nasal, or gastrointestinal hemorrhage. Angioedema arises from mast cell– or bradykinin-mediated pathways; mast cell degranulation may have been precipitated by the anesthetic agents, opiate, or a material in the nephroureteral stent.
The edema and bleeding are temporally related to multiple medications and mechanical ventilation. A latent bleeding diathesis may have manifested in the setting of increased tissue hydrostatic pressure or vessel permeability. Amyloidosis can lead to vessel fragility and coagulopathy, and periorbital bleeding is characteristic of AL amyloidosis.
The hypercalcemia, now more pronounced, raises concern for malignancy (including multiple myeloma) and granulomatous diseases like sarcoidosis, mycobacterial infections, and fungal infections. The declining hemoglobin could be explained by chronic blood loss, hemolysis, anemia of chronic disease, or a bone marrow process.
The cardiomyopathy, bleeding disorder, and multifocal disease in the oral cavity can be explained by AL amyloidosis; the hypercalcemia suggests concomitant multiple myeloma.
At the time of the bleeding event, the partial thromboplastin time, prothrombin time, and fibrinogen were within the reference ranges. Factor X activity level was normal. No schistocytes were observed on peripheral blood smear. Immunoglobulin G level was 1,425 mg/dL (reference range, 639-1,349); IgA and IgM levels were within the reference range. Serum lambda free light chains were 151.78 mg/dL (reference range, 0.46-2.71), and the ratio of kappa to lambda light chains was 0.01 (reference range, 0.49-2.54). Serum protein electrophoresis and immunofixation demonstrated a monoclonal paraprotein (IgG lambda) level of 1.2 g/dL. Congo red staining of the previously excised left dorsal tongue mass was negative for apple-green birefringence. Reexamination of the oral cavity revealed macroglossia and scalloping of the tongue (Figure 2).

Scalloping is characteristic of an infiltrative disorder that enlarges the tongue (macroglossia) and deforms its edges, which encounter the teeth. Macroglossia is seen in AL amyloidosis, acromegaly, and hypothyroidism. A monoclonal light chain, especially a lambda light chain, is characteristic of AL amyloidosis. The Congo red stain results can support the diagnosis when positive, but it has limited sensitivity. The tongue specimen can be sent for immunohistochemistry or mass spectrometry to evaluate for light chain deposition. A bone marrow biopsy can demonstrate a clonal plasma cell population. AL amyloidosis with concomitant multiple myeloma is the most likely diagnosis.
Bone marrow aspiration and core biopsy demonstrated 30% lambda-restricted plasma cells (Figure 3A-C). Congo red staining demonstrated apple-green birefringence of the bone marrow microvasculature (Figure 3D). Skeletal survey demonstrated widespread lytic bone disease involving the calvarium (Figure 4A), left humerus (Figure 4B), and left scapula (Figure 4B). Based on the monoclonal paraprotein, more than 10% monoclonal plasma cells, skeletal lesions, and hypercalcemia, she was diagnosed with IgG lambda multiple myeloma. Based on apple-green birefringence in the bone marrow and macroglossia, she was diagnosed with AL amyloidosis. The cardiac MRI findings were compatible with AL amyloidosis. 1

After three cycles of bortezomib and dexamethasone therapy to concurrently treat AL amyloidosis and multiple myeloma, the serum lambda light chain level decreased to 1.49 mg/dL and the monoclonal paraprotein level decreased to 0.3 g/dL. The calcium level was 9.8 mg/dL, and the hemoglobin level was 11.7 g/dL. The patient’s tongue pain resolved, allowing for improved oral intake and a 5.7-kg weight gain. The patient underwent nephrolithotomy 4 months after her initial presentation. She resumed an active lifestyle and recently traveled to visit relatives in the Philippines.

DISCUSSION
Oral diseases affect general health and quality of life and can be a harbinger of systemic disease. Tooth loss, caries, periodontal disease, and poorly fitting dentures commonly affect speech and nutrition.2 These common outpatient oral health issues can be the driving force for hospital admissions; for example, caries and periodontal disease can lead to suppurative odontogenic infection, endocarditis, brain abscess, and sepsis.
Tongue ulcerations, masses, and surface abnormalities often require consultation with a dentist or oral and maxillofacial surgeon to exclude squamous cell carcinoma.3 Other diagnostic considerations include benign neoplasms, trauma, inflammatory conditions (eg, sarcoidosis), infection (eg, syphilis, tuberculosis), and infiltrative processes such as amyloidosis.
Amyloidosis is a heterogeneous group of diseases caused by deposition of insoluble protein fibrils in tissues.4,5 The three most encountered forms of amyloidosis are AL, AA, and ATTR. Each form is named after the culprit protein.4 AL amyloidosis arises when a small clonal population of plasma cells in the bone marrow overproduces immunoglobulin light chain monomers.4,6 AA amyloidosis develops when the liver produces serum amyloid A protein (an acute phase reactant) in response to a chronic inflammatory condition such as rheumatoid arthritis or chronic intravenous drug injection.4 Transthyretin (TTR, also known as “prealbumin”) is a tetrameric protein that transports thyroxine and retinol; there are two forms of ATTR amyloidosis: hereditary and wild type. Hereditary ATTR amyloidosis develops from agglomeration of misfolded TTR monomers caused by mutations in the TTR gene. Wild-type ATTR amyloidosis is caused by age-related dissociation of the TTR tetramer into its constituent monomers that denature, misfold, and agglomerate into fibrils.5 Wild-type ATTR is now recognized as the most common form of amyloidosis, with 25% of myocardial autopsy specimens of patients 80 years or older demonstrating amyloid.7 The estimated incidence of AL amyloidosis is 10 cases per million person-years.8
Each amyloid protein homes in on specific anatomic sites.4 Characteristic combinations of organ dysfunction can suggest different forms of amyloidosis.9 Cardiac and peripheral nervous involvement (eg, carpal tunnel syndrome) is typical of both hereditary and wild-type ATTR amyloidosis; ATTR amyloidosis does not involve the kidney.4 AA amyloidosis most commonly manifests with proteinuria followed by declining glomerular filtration rate; heart failure is rare.4 The most common findings in AL amyloidosis are proteinuria, congestive heart failure, and sensory neuropathy.6 Gastrointestinal tract and hepatic involvement are each seen in nearly 20% of patients, and macroglossia is identified in approximately 10% of those with AL amyloidosis.6,10
Chronic deposition of amyloid can lead to acute presentations. Approximately 30% of patients with AL amyloidosis develop abnormal bleeding.11 Amyloid deposition in small blood vessels predisposes them to rupture. Bleeding events can be exacerbated by acquired coagulopathy due to plasma cell dyscrasia−associated thrombocytopenia, amyloid fibril adsorption of factor X, or hypofibrinogenemia.11,12 Periorbital purpura following minor trauma or transient venous hypertension is characteristic of AL amyloidosis.6,13 In this case, positive pressure ventilation and recumbent positioning increased hydrostatic pressure in the head and neck, causing rupture of the infiltrated small vessels around the eyes and in the oral cavity.14
Histological demonstration of tissue deposition of amyloid protein is the preferred method for amyloidosis diagnosis. Symptomatic sites or organs with dysfunction or radiologic changes are suitable for biopsy.6 If those sites are inaccessible or yield insufficient tissue quantity, abdominal fat pad aspiration or biopsy is indicated.15 Apple-green birefringence under polarized light of Congo red–stained tissue is characteristic, with sensitivity and specificity of approximately 80% and a positive predictive value of 85%.15 Immunoelectron microscopy is often performed simultaneously to confirm the diagnosis and determine the amyloid protein type.4,16 Immunoelectron microscopy’s sensitivity is approximately 80%, and it has specificity and positive predictive value both approaching 100%.15 Mass spectrometry is particularly useful in cases where the amyloid subtype is not clinically apparent (eg, a patient with an autoimmune condition or chronic infection as well as light chain abnormality).6 Cardiac MRI findings that suggest amyloidosis include a thickened left ventricle and late gadolinium enhancement.1 ATTR cardiac amyloidosis can be diagnosed using amyloid fibril–binding radiotracer technetium-99m-pyrophosphate scintigraphy; biopsy is often not necessary.1,4 Gene sequencing to differentiate between hereditary and wild-type forms of ATTR amyloidosis is beneficial.
The primary objectives of amyloidosis management are to control symptoms and inhibit amyloid protein production.6 Outcomes in AL amyloidosis have improved due to early diagnosis, new chemotherapeutic agents to eradicate the plasma cell clone, and autologous stem cell transplantation.6,17 Two new ATTR amyloidosis treatments are RNA interference therapies, which prevent TTR messenger RNA translation, and tafamidis, which stabilizes the TTR tetramer and prevents dissociation into its constituent monomers that precipitate in tissues.18 Both therapies can improve neuropathy-related quality of life.18 Tafamidis slows disease progression and decreases all-cause mortality in patients with hereditary and wild-type ATTR cardiac amyloidosis.19
Multiple myeloma and AL amyloidosis are both plasma cell dyscrasias involving the bone marrow, but they represent distinct disease processes with different clinical features.6 Multiple myeloma is characterized by marked expansion of a clonal plasma cell population within the bone marrow that aberrantly produces immunoglobulin. Conversely, the clonal plasma cell population responsible for producing the insoluble monoclonal light chain protein in AL amyloidosis typically constitutes less than 10% of the bone marrow.20 Multiple myeloma and AL amyloidosis may coexist in the same patient; nearly 15% of patients with multiple myeloma subsequently develop clinically overt AL amyloidosis, which portends a poor outcome.20
Amyloidosis is a rare group of diseases that arises when misfolded proteins aggregate in vital organs. The typical manifestations—congestive heart failure, neuropathy, chronic kidney disease, bleeding—are nearly always explained by more common conditions. Characteristic manifestations (like macroglossia) or associated diseases (like multiple myeloma) substantially increases the probability of AL amyloidosis. In a multisystem illness, the most common diseases must be excluded first, but this case reminds us that rare diseases, like amyloidosis, also warrant consideration as the story unfolds.
KEY TEACHING POINTS
- Different amyloid proteins precipitate in different anatomic sites, which leads to specific multiorgan combinations. The most common amyloidosis, ATTR, tends to manifest as heart failure and peripheral sensory neuropathy, while the constellation of AL amyloidosis includes heart failure, neuropathy, and proteinuria.
- Bleeding occurs in 30% of patients with AL amyloidosis. It is precipitated by fragile small blood vessels and exacerbated by acquired coagulopathy from adsorption of coagulation factors.
- Multiple myeloma and AL amyloidosis are both plasma cell dyscrasias involving the bone marrow, but they represent distinct disease processes with different clinical tempos and presentations. Multiple myeloma and AL amyloidosis may coexist in the same patient; nearly 15% of patients with multiple myeloma subsequently develop clinically overt AL amyloidosis.
Acknowledgment
The authors thank Benjamin A Derman, MD, of the University of Chicago, Chicago, Illinois, for critical review of the manuscript.
A 78-year-old woman presented to the ambulatory care clinic for a painful tongue mass. She noticed the mass 2 months prior to presentation, and it had not grown in the interim. She had left-sided jaw pain when opening her mouth and persistent left-sided otalgia.
In the evaluation of tongue masses, ulcerations, or other surface abnormalities, exclusion of squamous cell carcinoma is the top priority. Additional tongue surface abnormalities include benign lesions such as geographic tongue, inflammatory conditions such as lichen planus, and infections such as syphilis.
The ear and jaw pain may reflect metastatic spread, neural invasion, or referred pain from the tongue. A vasculitis with predilection for the head, such as giant cell arteritis, could present with oral and ear pain. Jaw pain with mastication could reflect jaw claudication, but pain upon mouth opening is more commonly explained by temporomandibular joint dysfunction.
The patient had hypertension, hyperlipidemia, chronic kidney disease (estimated glomerular filtration rate of 42 mL/min), and diabetes mellitus. Four months prior she was diagnosed with a chronic obstructing left renal calculus on ultrasonography to evaluate chronic kidney disease. Two months prior heart failure with preserved ejection fraction was diagnosed. Stress cardiac magnetic resonance imaging (MRI) demonstrated normal ejection fraction, asymmetric septal hypertrophy, and stress-induced subendocardial perfusion defect. Her medications were metoprolol, lisinopril, simvastatin, meloxicam, and aspirin. She never used tobacco and did not consume alcohol. She was born in the Philippines and emigrated to the United States 15 years ago. She had not experienced fever, chills, hearing loss, tinnitus, cough, dysphonia, neck swelling, or joint pain. She had lost 3 kg in the previous 4 months.
The absence of tobacco and alcohol use reduces the probability of a squamous cell carcinoma, although human papillomavirus–associated squamous cell carcinoma of the tongue remains possible. Asymmetric septal hypertrophy is characteristic of hypertrophic cardiomyopathy or an infiltrative cardiomyopathy. Sarcoidosis can affect the heart and can account for the renal calculus (via hypercalcemia). Amyloid light-chain (AL) amyloidosis could involve the heart and the tongue, although in amyloidosis the cardiac MRI typically displays late gadolinium enhancement and ventricular wall thickening. The absence of tinnitus or hearing loss suggests that the left-sided otalgia is referred pain from the tongue and oral cavity rather than a primary otologic disease (eg, infection).
On physical examination, temperature was 37.2 °C, heart rate was 88 beats per minute, blood pressure was 134/62 mm Hg, and oxygen saturation was 99% while breathing ambient air. The patient’s weight was 40.4 kg (body mass index of 19.26 kg/m2). Intraoral examination revealed induration of the bilateral tongue, an erosive 1-cm pedunculated mass of the left dorsum, rough white coating on the right dorsum, and fullness of the right lateral surface with an erosion abutting tooth #2. The right submandibular salivary gland was firm. The otoscopic examination and remainder of the head and neck examination were normal. There was no cervical, supraclavicular, or axillary adenopathy. The cranial nerve, cardiovascular, pulmonary, abdominal, and skin examinations were normal.
The left-sided lingual mass and right-sided lingual erosion likely arise from the same process. Both are compatible with an infection (eg, syphilis or tuberculosis), cancer, autoimmune disease (eg, Crohn’s disease or sarcoidosis), or an infiltrative disease such as amyloidosis. Leukoplakia could reflect a candidal infection, dysplasia, squamous cell carcinoma, oral hairy leukoplakia, or hyperkeratosis. The isolated submandibular salivary gland could reflect sialadenitis from chronic salivary duct obstruction or a primary neoplasm, but more likely is caused by the same process causing the tongue abnormalities.
The white blood cell count was 8,600/μL; hemoglobin, 11.5 g/dL; mean corpuscular volume, 102.5 fL; and platelet count, 270,000/μL3. Serum sodium was 141 mEq/L; potassium, 4.1 mEq/L; chloride, 101 mEq/L; bicarbonate, 30 mEq/L; blood urea nitrogen, 19 mg/dL; creatinine, 0.7 mg/dL; and calcium, 10.4 mg/dL (reference range, 8.5-10.3). Total serum protein was 7.3 g/dL (reference range, 6.0-8.3); albumin was 3.7 g/dL. Liver biochemistry test results were normal. Serum folate and vitamin B12 levels were normal. Serum ferritin was 423 ng/mL (reference range, 11-306); transferrin saturation, 21.4% (reference range, 15.0%-50.0%); and total iron-binding capacity, 323 µg/dL (reference range, 261-478). Parathyroid hormone (PTH) was 14 pg/mL (reference range, 15-65). HIV antibody was negative.
The calcium level is at the upper range of normal, whereas the PTH level is at the lower range of normal. The differential diagnosis for PTH-independent hypercalcemia includes hypercalcemia of malignancy and granulomatous disease such as sarcoidosis. Mild hypercalcemia could contribute to the nephrolithiasis. The iron studies exclude iron deficiency and are not suggestive of anemia of chronic disease. The triad of mild hypercalcemia, cardiomyopathy, and anemia is compatible with AL amyloidosis (perhaps with associated multiple myeloma) or sarcoidosis; both disorders can present as a mass. Imaging of the head and neck and biopsy of the tongue mass are the next steps.
The left dorsal tongue mass was excised in clinic. Histopathology revealed ulcerated squamous mucosa with inflammatory changes but no malignancy. Imaging of the head and neck was scheduled.
Neither cancer or granulomas were detected, but inadequate sampling or staining must be considered. Inflammatory changes are compatible with infection, autoimmunity, and cancer; the latter can feature reactive changes that obscure the malignant cells. The absence of granulomas lowers, but does not eliminate, the possibility of sarcoidosis, tuberculosis, fungal infection, and granulomatosis with polyangiitis. Actinomycosis is an invasive orofacial infection that disregards anatomic boundaries and is characterized by inflammatory histology; although infection of the tongue is possible, infection of the jaw and face is more typical. Immunoglobulin G4–related disease can present as an inflammatory and invasive disorder; however, the characteristic histopathologic findings (lymphoplasmacytic infiltrate, fibrosis, and phlebitis) are absent.
Culture of the tissue for mycobacteria or fungi (she is at increased risk for both given her previous residency in the Philippines) could increase the diagnostic yield. Another biopsy of the tongue or an adjacent structure—guided by imaging—may provide a more diagnostic tissue sample.
MRI of the head and neck demonstrated hyperintense signal and prominence of the right lateral pterygoid muscle (Figure 1A) and slight enlargement of a right submandibular gland (Figure 1B). No tongue abnormalities were identified. Radiograph of the chest did not reveal infiltrates, masses, or lymphadenopathy.

The absence of the tongue mass on the MRI likely reflects excision of the mass at the time of biopsy. The signal enhancement in the right lateral pterygoid muscle and submandibular gland is suggestive of an infiltrative process. Infiltration of the right lateral pterygoid muscle may also explain the patient’s pain when opening her mouth. Infiltrative processes can be neoplastic (eg, salivary gland tumor, sarcoma, lymphoma), infectious (eg, mycobacterial or fungal), cellular (eg, histiocytes, mast cells, plasma cells, eosinophils, granulomas), or related to inert substances such as amyloid or iron.
Seven weeks later, the patient presented to the hospital for scheduled percutaneous nephrolithotomy of the obstructing renal calculus. The physical examination was unchanged. The complete blood count and metabolic panel were unchanged apart from hemoglobin of 9.9 g/dL and calcium of 11.5 mg/dL. Coagulation studies were within normal limits.
A percutaneous nephroureteral stent was placed under conscious sedation. The patient then underwent rapid sequence induction of general anesthesia for the nephrolithotomy with fentanyl, propofol, and rocuronium. Within minutes of initiating mechanical ventilation, severe periorbital and perioral edema, copious oral cavity bleeding, and bilateral periorbital purpura occurred. Sugammadex (neuromuscular blockade reversal) and dexamethasone were administered. Examination of the oral cavity was limited by the brisk bleeding; the right sided tongue erosion was unchanged.
Bleeding is caused by thrombocytopenia, thrombocytopathy, coagulopathy, or disruption of vessel integrity. Oral cavity bleeding could arise from the tongue ulceration, but could also reflect pulmonary, nasal, or gastrointestinal hemorrhage. Angioedema arises from mast cell– or bradykinin-mediated pathways; mast cell degranulation may have been precipitated by the anesthetic agents, opiate, or a material in the nephroureteral stent.
The edema and bleeding are temporally related to multiple medications and mechanical ventilation. A latent bleeding diathesis may have manifested in the setting of increased tissue hydrostatic pressure or vessel permeability. Amyloidosis can lead to vessel fragility and coagulopathy, and periorbital bleeding is characteristic of AL amyloidosis.
The hypercalcemia, now more pronounced, raises concern for malignancy (including multiple myeloma) and granulomatous diseases like sarcoidosis, mycobacterial infections, and fungal infections. The declining hemoglobin could be explained by chronic blood loss, hemolysis, anemia of chronic disease, or a bone marrow process.
The cardiomyopathy, bleeding disorder, and multifocal disease in the oral cavity can be explained by AL amyloidosis; the hypercalcemia suggests concomitant multiple myeloma.
At the time of the bleeding event, the partial thromboplastin time, prothrombin time, and fibrinogen were within the reference ranges. Factor X activity level was normal. No schistocytes were observed on peripheral blood smear. Immunoglobulin G level was 1,425 mg/dL (reference range, 639-1,349); IgA and IgM levels were within the reference range. Serum lambda free light chains were 151.78 mg/dL (reference range, 0.46-2.71), and the ratio of kappa to lambda light chains was 0.01 (reference range, 0.49-2.54). Serum protein electrophoresis and immunofixation demonstrated a monoclonal paraprotein (IgG lambda) level of 1.2 g/dL. Congo red staining of the previously excised left dorsal tongue mass was negative for apple-green birefringence. Reexamination of the oral cavity revealed macroglossia and scalloping of the tongue (Figure 2).

Scalloping is characteristic of an infiltrative disorder that enlarges the tongue (macroglossia) and deforms its edges, which encounter the teeth. Macroglossia is seen in AL amyloidosis, acromegaly, and hypothyroidism. A monoclonal light chain, especially a lambda light chain, is characteristic of AL amyloidosis. The Congo red stain results can support the diagnosis when positive, but it has limited sensitivity. The tongue specimen can be sent for immunohistochemistry or mass spectrometry to evaluate for light chain deposition. A bone marrow biopsy can demonstrate a clonal plasma cell population. AL amyloidosis with concomitant multiple myeloma is the most likely diagnosis.
Bone marrow aspiration and core biopsy demonstrated 30% lambda-restricted plasma cells (Figure 3A-C). Congo red staining demonstrated apple-green birefringence of the bone marrow microvasculature (Figure 3D). Skeletal survey demonstrated widespread lytic bone disease involving the calvarium (Figure 4A), left humerus (Figure 4B), and left scapula (Figure 4B). Based on the monoclonal paraprotein, more than 10% monoclonal plasma cells, skeletal lesions, and hypercalcemia, she was diagnosed with IgG lambda multiple myeloma. Based on apple-green birefringence in the bone marrow and macroglossia, she was diagnosed with AL amyloidosis. The cardiac MRI findings were compatible with AL amyloidosis. 1

After three cycles of bortezomib and dexamethasone therapy to concurrently treat AL amyloidosis and multiple myeloma, the serum lambda light chain level decreased to 1.49 mg/dL and the monoclonal paraprotein level decreased to 0.3 g/dL. The calcium level was 9.8 mg/dL, and the hemoglobin level was 11.7 g/dL. The patient’s tongue pain resolved, allowing for improved oral intake and a 5.7-kg weight gain. The patient underwent nephrolithotomy 4 months after her initial presentation. She resumed an active lifestyle and recently traveled to visit relatives in the Philippines.

DISCUSSION
Oral diseases affect general health and quality of life and can be a harbinger of systemic disease. Tooth loss, caries, periodontal disease, and poorly fitting dentures commonly affect speech and nutrition.2 These common outpatient oral health issues can be the driving force for hospital admissions; for example, caries and periodontal disease can lead to suppurative odontogenic infection, endocarditis, brain abscess, and sepsis.
Tongue ulcerations, masses, and surface abnormalities often require consultation with a dentist or oral and maxillofacial surgeon to exclude squamous cell carcinoma.3 Other diagnostic considerations include benign neoplasms, trauma, inflammatory conditions (eg, sarcoidosis), infection (eg, syphilis, tuberculosis), and infiltrative processes such as amyloidosis.
Amyloidosis is a heterogeneous group of diseases caused by deposition of insoluble protein fibrils in tissues.4,5 The three most encountered forms of amyloidosis are AL, AA, and ATTR. Each form is named after the culprit protein.4 AL amyloidosis arises when a small clonal population of plasma cells in the bone marrow overproduces immunoglobulin light chain monomers.4,6 AA amyloidosis develops when the liver produces serum amyloid A protein (an acute phase reactant) in response to a chronic inflammatory condition such as rheumatoid arthritis or chronic intravenous drug injection.4 Transthyretin (TTR, also known as “prealbumin”) is a tetrameric protein that transports thyroxine and retinol; there are two forms of ATTR amyloidosis: hereditary and wild type. Hereditary ATTR amyloidosis develops from agglomeration of misfolded TTR monomers caused by mutations in the TTR gene. Wild-type ATTR amyloidosis is caused by age-related dissociation of the TTR tetramer into its constituent monomers that denature, misfold, and agglomerate into fibrils.5 Wild-type ATTR is now recognized as the most common form of amyloidosis, with 25% of myocardial autopsy specimens of patients 80 years or older demonstrating amyloid.7 The estimated incidence of AL amyloidosis is 10 cases per million person-years.8
Each amyloid protein homes in on specific anatomic sites.4 Characteristic combinations of organ dysfunction can suggest different forms of amyloidosis.9 Cardiac and peripheral nervous involvement (eg, carpal tunnel syndrome) is typical of both hereditary and wild-type ATTR amyloidosis; ATTR amyloidosis does not involve the kidney.4 AA amyloidosis most commonly manifests with proteinuria followed by declining glomerular filtration rate; heart failure is rare.4 The most common findings in AL amyloidosis are proteinuria, congestive heart failure, and sensory neuropathy.6 Gastrointestinal tract and hepatic involvement are each seen in nearly 20% of patients, and macroglossia is identified in approximately 10% of those with AL amyloidosis.6,10
Chronic deposition of amyloid can lead to acute presentations. Approximately 30% of patients with AL amyloidosis develop abnormal bleeding.11 Amyloid deposition in small blood vessels predisposes them to rupture. Bleeding events can be exacerbated by acquired coagulopathy due to plasma cell dyscrasia−associated thrombocytopenia, amyloid fibril adsorption of factor X, or hypofibrinogenemia.11,12 Periorbital purpura following minor trauma or transient venous hypertension is characteristic of AL amyloidosis.6,13 In this case, positive pressure ventilation and recumbent positioning increased hydrostatic pressure in the head and neck, causing rupture of the infiltrated small vessels around the eyes and in the oral cavity.14
Histological demonstration of tissue deposition of amyloid protein is the preferred method for amyloidosis diagnosis. Symptomatic sites or organs with dysfunction or radiologic changes are suitable for biopsy.6 If those sites are inaccessible or yield insufficient tissue quantity, abdominal fat pad aspiration or biopsy is indicated.15 Apple-green birefringence under polarized light of Congo red–stained tissue is characteristic, with sensitivity and specificity of approximately 80% and a positive predictive value of 85%.15 Immunoelectron microscopy is often performed simultaneously to confirm the diagnosis and determine the amyloid protein type.4,16 Immunoelectron microscopy’s sensitivity is approximately 80%, and it has specificity and positive predictive value both approaching 100%.15 Mass spectrometry is particularly useful in cases where the amyloid subtype is not clinically apparent (eg, a patient with an autoimmune condition or chronic infection as well as light chain abnormality).6 Cardiac MRI findings that suggest amyloidosis include a thickened left ventricle and late gadolinium enhancement.1 ATTR cardiac amyloidosis can be diagnosed using amyloid fibril–binding radiotracer technetium-99m-pyrophosphate scintigraphy; biopsy is often not necessary.1,4 Gene sequencing to differentiate between hereditary and wild-type forms of ATTR amyloidosis is beneficial.
The primary objectives of amyloidosis management are to control symptoms and inhibit amyloid protein production.6 Outcomes in AL amyloidosis have improved due to early diagnosis, new chemotherapeutic agents to eradicate the plasma cell clone, and autologous stem cell transplantation.6,17 Two new ATTR amyloidosis treatments are RNA interference therapies, which prevent TTR messenger RNA translation, and tafamidis, which stabilizes the TTR tetramer and prevents dissociation into its constituent monomers that precipitate in tissues.18 Both therapies can improve neuropathy-related quality of life.18 Tafamidis slows disease progression and decreases all-cause mortality in patients with hereditary and wild-type ATTR cardiac amyloidosis.19
Multiple myeloma and AL amyloidosis are both plasma cell dyscrasias involving the bone marrow, but they represent distinct disease processes with different clinical features.6 Multiple myeloma is characterized by marked expansion of a clonal plasma cell population within the bone marrow that aberrantly produces immunoglobulin. Conversely, the clonal plasma cell population responsible for producing the insoluble monoclonal light chain protein in AL amyloidosis typically constitutes less than 10% of the bone marrow.20 Multiple myeloma and AL amyloidosis may coexist in the same patient; nearly 15% of patients with multiple myeloma subsequently develop clinically overt AL amyloidosis, which portends a poor outcome.20
Amyloidosis is a rare group of diseases that arises when misfolded proteins aggregate in vital organs. The typical manifestations—congestive heart failure, neuropathy, chronic kidney disease, bleeding—are nearly always explained by more common conditions. Characteristic manifestations (like macroglossia) or associated diseases (like multiple myeloma) substantially increases the probability of AL amyloidosis. In a multisystem illness, the most common diseases must be excluded first, but this case reminds us that rare diseases, like amyloidosis, also warrant consideration as the story unfolds.
KEY TEACHING POINTS
- Different amyloid proteins precipitate in different anatomic sites, which leads to specific multiorgan combinations. The most common amyloidosis, ATTR, tends to manifest as heart failure and peripheral sensory neuropathy, while the constellation of AL amyloidosis includes heart failure, neuropathy, and proteinuria.
- Bleeding occurs in 30% of patients with AL amyloidosis. It is precipitated by fragile small blood vessels and exacerbated by acquired coagulopathy from adsorption of coagulation factors.
- Multiple myeloma and AL amyloidosis are both plasma cell dyscrasias involving the bone marrow, but they represent distinct disease processes with different clinical tempos and presentations. Multiple myeloma and AL amyloidosis may coexist in the same patient; nearly 15% of patients with multiple myeloma subsequently develop clinically overt AL amyloidosis.
Acknowledgment
The authors thank Benjamin A Derman, MD, of the University of Chicago, Chicago, Illinois, for critical review of the manuscript.
1. Witteles RM, Bokhari S, Damy T, et al. Screening for transthyretin amyloid cardiomyopathy in everyday practice. JACC Heart Fail. 2019;7(8):709-716. https://doi.org/10.1016/j.jchf.2019.04.010
2. Griffin SO, Jones JA, Brunson D, Griffin PM, Bailey WD. Burden of oral disease among older adults and implications for public health priorities. Am J Public Health. 2012;102(3):411-418. https://doi.org/10.2105/ajph.2011.300362
3. Ernster JA, Sciotto CG, O’Brien MM, et al. Rising incidence of oropharyngeal cancer and the role of oncogenic human papilloma virus. Laryngoscope. 2007;117(12):2115-2128. https://doi.org/10.1097/mlg.0b013e31813e5fbb
4. Wechalekar AD, Gillmore JD, Hawkins PN. Systemic amyloidosis. Lancet. 2016;387(10038):2641-2654. https://doi.org/10.1016/s0140-6736(15)01274-x
5. Riek R, Eisenberg DS. The activities of amyloids from a structural perspective. Nature. 2016;539(7628):227-235. https://doi.org/10.1038/nature20416
6. Gertz MA, Dispenzieri A. Systemic amyloidosis recognition, prognosis, and therapy: a systematic review. JAMA. 2020;324(1):79-89. https://doi.org/10.1001/jama.2020.5493
7. Ruberg FL, Berk JL. Transthyretin (TTR) cardiac amyloidosis. Circulation. 2012;126(10):1286-1300. https://doi.org/10.1161/circulationaha.111.078915
8. Quock TP, Yan T, Chang E, Guthrie S, Broder MS. Epidemiology of AL amyloidosis: a real-world study using US claims data. Blood Adv. 2018;2(10):1046-1053. https://doi.org/10.1182/bloodadvances.2018016402
9. Papoutsidakis N, Miller EJ, Rodonski A, Jacoby D. Time course of common clinical manifestations in patients with transthyretin cardiac amyloidosis: delay from symptom onset to diagnosis. J Card Fail. 2018;24(2):131-133. https://doi.org/10.1016/j.cardfail.2017.12.005
10. Shimazaki C, Hata H, Iida S, et al. Nationwide survey of 741 patients with systemic amyloid light-chain amyloidosis in Japan. Intern Med. 2018;57(2):181-187. https://doi.org/10.2169/internalmedicine.9206-17
11. Mumford AD, O’Donnell J, Gillmore JD, Manning RA, Hawkins PN, Laffan M. Bleeding symptoms and coagulation abnormalities in 337 patients with AL-amyloidosis. Br J Haematol. 2000;110(2):454-460. https://doi.org/10.1046/j.1365-2141.2000.02183.x
12. Choufani EB, Sanchorawala V, Ernst T, et al. Acquired factor X deficiency in patients with amyloid light-chain amyloidosis: incidence, bleeding manifestations, and response to high-dose chemotherapy. Blood. 2001;97(6):1885-1887. https://doi.org/10.1182/blood.v97.6.1885
13. Slagel GA, Lupton GP. Postproctoscopic periorbital purpura. Primary systemic amyloidosis. Arch Dermatol. 1986;122(4):464-465, 467-468.
14. Lupton GP. Pneomometry-induced purpura. Arch Dermatol. 1981;117(10):603. https://doi.org/10.1001/archderm.117.10.603a
15. Fernández de Larrea C, Verga L, Morbini P, et al. A practical approach to the diagnosis of systemic amyloidoses. Blood. 2015;125(14):2239-2244. https://doi.org/10.1182/blood-2014-11-609883
16. Vaxman I, Gertz M. Recent Advances in the diagnosis, risk stratification, and management of systemic light-chain amyloidosis. Acta Haematol. 2019;141(2):93-106. https://doi.org/10.1159/000495455
17. Muchtar E, Gertz MA, Kumar SK, et al. Improved outcomes for newly diagnosed AL amyloidosis between 2000 and 2014: cracking the glass ceiling of early death. Blood. 2017;129(15):2111-2119. https://doi.org/10.1182/blood-2016-11-751628
18. Quarta CC, Solomon SD. Stabilizing transthyretin to treat ATTR cardiomyopathy. N Engl J Med. 2018;379(11):1083-1084. https://doi.org/10.1056/nejme1810074
19. Maurer MS, Schwartz JH, Gundapaneni B, et al. Tafamidis treatment for patients with transthyretin amyloid cardiomyopathy. N Engl J Med. 2018;379(11):1007-1016. https://doi.org/10.1056/nejmoa1805689
20. Bahlis NJ, Lazarus HM. Multiple myeloma-associated AL amyloidosis: is a distinctive therapeutic approach warranted? Bone Marrow Transplant. 2006;38(1):7-15. https://doi.org/10.1038/sj.bmt.1705395
1. Witteles RM, Bokhari S, Damy T, et al. Screening for transthyretin amyloid cardiomyopathy in everyday practice. JACC Heart Fail. 2019;7(8):709-716. https://doi.org/10.1016/j.jchf.2019.04.010
2. Griffin SO, Jones JA, Brunson D, Griffin PM, Bailey WD. Burden of oral disease among older adults and implications for public health priorities. Am J Public Health. 2012;102(3):411-418. https://doi.org/10.2105/ajph.2011.300362
3. Ernster JA, Sciotto CG, O’Brien MM, et al. Rising incidence of oropharyngeal cancer and the role of oncogenic human papilloma virus. Laryngoscope. 2007;117(12):2115-2128. https://doi.org/10.1097/mlg.0b013e31813e5fbb
4. Wechalekar AD, Gillmore JD, Hawkins PN. Systemic amyloidosis. Lancet. 2016;387(10038):2641-2654. https://doi.org/10.1016/s0140-6736(15)01274-x
5. Riek R, Eisenberg DS. The activities of amyloids from a structural perspective. Nature. 2016;539(7628):227-235. https://doi.org/10.1038/nature20416
6. Gertz MA, Dispenzieri A. Systemic amyloidosis recognition, prognosis, and therapy: a systematic review. JAMA. 2020;324(1):79-89. https://doi.org/10.1001/jama.2020.5493
7. Ruberg FL, Berk JL. Transthyretin (TTR) cardiac amyloidosis. Circulation. 2012;126(10):1286-1300. https://doi.org/10.1161/circulationaha.111.078915
8. Quock TP, Yan T, Chang E, Guthrie S, Broder MS. Epidemiology of AL amyloidosis: a real-world study using US claims data. Blood Adv. 2018;2(10):1046-1053. https://doi.org/10.1182/bloodadvances.2018016402
9. Papoutsidakis N, Miller EJ, Rodonski A, Jacoby D. Time course of common clinical manifestations in patients with transthyretin cardiac amyloidosis: delay from symptom onset to diagnosis. J Card Fail. 2018;24(2):131-133. https://doi.org/10.1016/j.cardfail.2017.12.005
10. Shimazaki C, Hata H, Iida S, et al. Nationwide survey of 741 patients with systemic amyloid light-chain amyloidosis in Japan. Intern Med. 2018;57(2):181-187. https://doi.org/10.2169/internalmedicine.9206-17
11. Mumford AD, O’Donnell J, Gillmore JD, Manning RA, Hawkins PN, Laffan M. Bleeding symptoms and coagulation abnormalities in 337 patients with AL-amyloidosis. Br J Haematol. 2000;110(2):454-460. https://doi.org/10.1046/j.1365-2141.2000.02183.x
12. Choufani EB, Sanchorawala V, Ernst T, et al. Acquired factor X deficiency in patients with amyloid light-chain amyloidosis: incidence, bleeding manifestations, and response to high-dose chemotherapy. Blood. 2001;97(6):1885-1887. https://doi.org/10.1182/blood.v97.6.1885
13. Slagel GA, Lupton GP. Postproctoscopic periorbital purpura. Primary systemic amyloidosis. Arch Dermatol. 1986;122(4):464-465, 467-468.
14. Lupton GP. Pneomometry-induced purpura. Arch Dermatol. 1981;117(10):603. https://doi.org/10.1001/archderm.117.10.603a
15. Fernández de Larrea C, Verga L, Morbini P, et al. A practical approach to the diagnosis of systemic amyloidoses. Blood. 2015;125(14):2239-2244. https://doi.org/10.1182/blood-2014-11-609883
16. Vaxman I, Gertz M. Recent Advances in the diagnosis, risk stratification, and management of systemic light-chain amyloidosis. Acta Haematol. 2019;141(2):93-106. https://doi.org/10.1159/000495455
17. Muchtar E, Gertz MA, Kumar SK, et al. Improved outcomes for newly diagnosed AL amyloidosis between 2000 and 2014: cracking the glass ceiling of early death. Blood. 2017;129(15):2111-2119. https://doi.org/10.1182/blood-2016-11-751628
18. Quarta CC, Solomon SD. Stabilizing transthyretin to treat ATTR cardiomyopathy. N Engl J Med. 2018;379(11):1083-1084. https://doi.org/10.1056/nejme1810074
19. Maurer MS, Schwartz JH, Gundapaneni B, et al. Tafamidis treatment for patients with transthyretin amyloid cardiomyopathy. N Engl J Med. 2018;379(11):1007-1016. https://doi.org/10.1056/nejmoa1805689
20. Bahlis NJ, Lazarus HM. Multiple myeloma-associated AL amyloidosis: is a distinctive therapeutic approach warranted? Bone Marrow Transplant. 2006;38(1):7-15. https://doi.org/10.1038/sj.bmt.1705395
© 2021 Society of Hospital Medicine
Adoption of Hospitalist Care in Asia: Experiences From Singapore, Taiwan, Korea, and Japan
Adoption of Hospitalist Care in Asia: Experiences From Singapore, Taiwan, Korea, and Japan

This work is licensed under a Creative Commons Attribution 4.0 International License
Since its inception in the mid-1990s, the hospitalist model of care has enjoyed robust growth in the United States, increasing to around 20,000 providers by the end of its first decade.1,2 Since then, it has far outstripped early predictions of adoption, currently standing at more than 50,000 hospitalist providers.2 Although driven by numerous factors, including system-based management needs, provision of inpatient care for unassigned patients, and demands for improved patient safety and satisfaction, this meteoric growth has been driven largely by cost pressures particular to the US healthcare system.1,2 Nonetheless, the growing complexity of healthcare systems, substantial fiscal pressures, and increasing healthcare demands from aging populations are worldwide challenges to which countries outside North America also seek solutions. Countries that have initiated hospitalist care have localized adoption, evolving the model to meet their unique fiscal and system-based needs and patients’ expectations.
While there has been keen interest in the hospitalist model in Asia, there has not yet been widespread adoption, despite numerous data demonstrating that this model is associated with lower length of stay (LOS), as well as lower costs and improved patient safety.3,4 This article explores hospitalist care adoption experiences in Singapore, Taiwan, Korea, and Japan, focusing on stakeholder demand for hospitalist care, respective adoption, outcomes, and associated challenges to date.
SINGAPORE
Stakeholder Demand for Hospitalist Care
Historically in Singapore, family physicians provided primary care and internal medicine subspecialists provided inpatient care.5 Present-day trends, including an aging population, increasing rates of chronic diseases, and multisystem health issues, have stressed the historical model, leading to care fragmentation, long LOS (>9 days), and reduced patient satisfaction.5,6 Additionally, as 80% of hospital care is government funded, public hospitals are under pressure to reduce healthcare expenditures.5
Adoption of Hospitalist Care, Outcomes, and Challenges Faced
To meet patient needs and healthcare system challenges, the hospitalist model has evolved through several iterations in Singapore. The first model, implemented at Singapore General Hospital, utilized family physicians as hospitalists to coordinate inpatient care and integrate care between hospital and community settings.3,5 This model resulted in shorter LOS and reduced costs for patients cared for by family physician hospitalists.3 Despite these benefits, the family physician hospitalist model did not spread, partly due to biases favoring subspecialist care for hospitalized patients.7
The next iteration utilized general internal medicine (GIM) specialists. Traditionally, GIM specialists cared for a small number of low-acuity hospitalized patients. Recognizing the emerging need for holistic inpatient care, the Singapore Ministry of Health supported advances in generalist care, including a financial bonus and a revamped GIM training program. This spawned hospitalist-type models nationwide. At the National University Hospital (NUH), for example, GIM physicians were recruited to care for “specialty” patients in the acute medical unit and increase their ward coverage to include complex multimorbid patients. Additionally, NUH launched the enhanced complex care program, providing integrated inpatient and outpatient care to high-utilizing, complex patients. Overall, the NUH GIM division grew by 70% (faculty) and 60% (trainees) over 5 years. Currently, fueled by government enthusiasm for generalist care, hospitalist-type models are evident at newly minted hospitals across Singapore.
Although physicians act as hospitalists, the term hospitalist is not embraced in Singapore, thus limiting its potential to develop clinical- and system-improvement competencies and establish professional identity. This may be due to the strong UK-based cultural foundations and continued systemic bias favoring subspecialists.8
TAIWAN
Stakeholder Demand for Hospitalist Care
Under its national health insurance (NHI) system, Taiwan has relatively low copayments for medical services, with acute patients paying 10% of costs for a ≤30-day hospitalization, causing demand for inpatient care to remain strong.4,9 The NHI system has also led to increased numbers of patients accessing care in emergency departments (EDs), where costs may be as low as US $16 (NT $450), causing long waits for evaluation and transfer to wards.9,10 There remains an insufficient number of hospital-based physicians to manage this high patient volume, a situation exacerbated by low reimbursements.4
Adoption of Hospitalist Care, Outcomes, and Challenges Faced
In order to address rising admissions, inefficient ED management, and physician shortages, a hospitalist care program was first introduced in Taiwan in 2002, followed by the establishment of a hospitalist-run ward in National Taiwan University Hospital in 2009.11 Subsequent studies from Taiwan have found that hospitalist-run wards had lower admission costs, shorter LOS, and more do-not-resuscitate consent, and also had similar in-hospital mortality and readmission rates compared to specialist-run wards.4,12 Reflecting these successes, the Taiwan Association of Hospital Medicine (TAHM) was established in 2018, and since January 2021, the Ministry of Health and Welfare of Taiwan has mandated hospital medicine programs as an accreditation requirement for all medical centers, with a dual role of educating residents and providing inpatient care.
Despite growing opportunities, Taiwan has seen a modest increase in the number of hospitalists, rising from three in 2009 to around 300 by January 2021. An indistinct professional identity and career path are the main barriers. Given this, TAHM is trying to strengthen hospitalist professionalism by introducing both hard and soft skills, such as utilizing point-of-care ultrasonography and implementing the concepts of Choosing Wisely® and shared decision-making.
KOREA
Stakeholder Demand for Hospitalist Care
Korea has experienced a chronic physician shortage, with just 2.4 physicians per 1,000 people (World Bank, 2017), leading to significant physician burnout. Designed to protect trainee well-being, the 2015 Improvement of Training Conditions and Status of Medical Residents Act limited resident work hours while reducing internal medicine and general surgery training periods, further exacerbating physician shortages.13 In addition, Korea’s current NHI system—including its’ healthcare insurance reimbursements scheme, established in 1989 when Korea’s per capita gross domestic product was less than US $5,000—provides low reimbursements to healthcare providers.14 Along with increased attention to patient safety and healthcare-related consumer expectations, the hospitalist system in Korea aims to maintain improvements to residents’ well-being, while increasing hospital revenue and meeting patient demand for improved services.14
Adoption of Hospitalist Care, Outcomes, and Challenges Faced
Along with the Ministry of Health and Welfare, the Korean Health Insurance Review and Assessment Service launched a hospitalist pilot program in general medicine and surgery in 2016.15 Services for hospitalist-managed inpatients are charged on a new schedule covered by the NHI system, including facility fees, which are charged per diem, and separate hospitalist fees.14 New hospital medicine programs are utilized, in part, to recruit new physicians to manage a large volume of inpatients. Previous studies found that these new hospitalist care systems also improved patient safety, quality of care, and overall patient satisfaction, while being associated with shorter LOS and fewer unnecessary intensive care unit admissions.16,17 After a successful pilot, the revamped reimbursement system for hospitalist care officially started in January 2021.
Although Korea had only 250 registered hospitalists by August 2020, this is likely a substantial underestimate, as only hospital medicine teams with more than two hospitalists were allowed formal registration during the pilot period. Wider registration is currently underway for the new official reimbursement system.
JAPAN
Stakeholder Demand for Hospitalist Care
Hospitals in Japan are organized into highly compartmentalized subspecialties. Providing quality inpatient care to senior patients, who account for more than 28% of the population, and managing smooth transitions from hospital to long-term- care facilities remain challenging. In addition, given generous caps on maximum monthly out-of-pocket payments under its NHI system, LOS for Japanese hospitals are as long as 16.1 days.18 Nonetheless, given rising financial burdens associated with long-term care, hospitals are under government pressure to further shorten LOS and transition patients to local long-term-care facilities after treatment for acute symptoms.
Adoption of Hospitalist Care, Outcomes, and Challenges Faced
To meet these challenges, an increasing number of Japanese hospitals have established departments of general medicine to triage and manage patients with multiple comorbidities and to coordinate patient care across relevant specialties. The Japanese Society of Hospital General Medicine (JSHGM) was established in 2010, and currently has 1,890 members from 896 medical institutions. In 2018, general medicine was recognized by the Japanese Board of Medical Specialties as a formal specialty for certification. Currently, JSHGM is working with the Japan Primary Care Association and other organizations to establish a specialty certification system for hospitalist physicians and raise awareness of hospital medicine. A Japanese study of elderly patients with chronic aspiration pneumonia found that care by hospitalists resulted in shorter LOS and lower costs than specialist care.19 Recently, hospitalists have played a central role in COVID-19 management, opening fever intake clinics and establishing collaborative guidelines with infectious disease experts and other specialists.
Yet, different from the prototypical hospitalist first defined by Wachter and Goldman, Japanese general medicine hospitalists continue to have substantial outpatient responsibilities, albeit in the hospital setting. Out of 81 university hospitals, 69 now have a department of hospital general medicine, though only 20 have inpatient services.20 In addition, a medical culture in which patients continue to see their surgery attendings long after surgery remains strong. Clear definitions regarding hospitalists’ roles need to be established, while promoting changes toward inpatient care for both patients and subspecialists.
DISCUSSION
The four Asian countries reviewed here have all established universal access to healthcare, with Taiwan, Korea, and Japan having strong NHI systems and Singapore providing significant healthcare subsidies for those in need. Nonetheless, they also face similar challenges, including the growing complexity of healthcare systems, substantial fiscal pressures, increased healthcare demands caused by aging populations, and increased expectations regarding stakeholder well-being. As such, these countries share common driving forces that are propelling the adoption of hospitalist care models, such as lack of a sufficient physician workforce on inpatient wards; need for extra resources to shorten ED wait times prior to inpatient admission; need for providing quality care to multimorbid senior patients across highly segmented hospital departments and coordinating medical services between hospitals and outpatient care facilities; and government pressure on cutting costs, especially by shortening inpatient LOS. Some common barriers among these Asian countries include unclear definitions of hospitalists’ roles and degree of collaboration with subspecialty departments, and social and systemic biases favoring subspecialty care for inpatients.
The four Asian countries reviewed here have chosen to adopt the hospitalist model as a supplement to already established, specialty-driven inpatient care systems; as such, further comparative outcome studies focusing on cost, care quality, and patient safety and satisfaction are warranted to bolster professional hospitalist roles, further facilitate government/policy-level support for hospital care systems, and promote future training and certification systems appropriate to each country’s unique healthcare system and medical culture. Similarly, evidence-driven educational outreach programs are warranted to facilitate patient understanding of the role of hospitalists in their care.For countries interested in establishing hospital medicine programs, the adoption experiences in Singapore, Taiwan, Korea, and Japan provide valuable insights regarding how to establish hospitalist models to meet country-specific healthcare challenges while successfully functioning in the context of their unique medical-system frameworks.
1. Wachter RM, Goldman L. The hospitalist movement 5 years later. JAMA. 2002;287:487-494. https://doi.org/10.1001/jama.287.4.487
2. Wachter RM, Goldman L. Zero to 50,000—the 20th anniversary of the hospitalist. N Engl J Med. 2016;375(11):1009-1011. https://doi.org/10.1056/NEJMp1607958
3. Lee KH, Yang Y, Yang KS, et al. Bringing generalists into the hospital: outcomes of a family medicine hospitalist model in Singapore. J Hosp Med. 2011;6:115-121. https://doi.org/10.1002/jhm.821
4. Shu CC, Lin JW, Lin YF, et al. Evaluating the performance of a hospitalist system in Taiwan: a pioneer study for nationwide health insurance in Asia. J Hosp Med. 2011;6(7):378-382. https://doi.org/ 10.1002/jhm.896
5. Lee KH. The hospitalist movement—a complex adaptive response to fragmentation of care in hospitals. Ann Acad Med Singap. 2008;37(2):145-150.
6. Ge L, Ya CW, Heng BH, Tan WS. Frailty and healthcare utilization across care settings among community-dwelling older adults in Singapore. BMC Geriatrics. 2020;20:389. https://doi.org/10.1186/s12877-020-01800-8
7. Lee KH. A historical perspective of the barriers to generalism. Aust Fam Physician. 2015;44(3):154-158.
8. Choo F. Alexandra Hospital provides patients with one-stop services under new care model. Updated December 14, 2018. Accessed March 26, 2021.https://www.straitstimes.com/singapore/health/alexandra-hospital-provides-patients-with-one-stop-services-under-new-care-model
9. National Health Insurance Administration, Ministry of Health and Welfare, Taiwan. Medical services. Copayments. Updated December 28, 2020. Accessed March 26, 2021. https://www.nhi.gov.tw/English/Content_List.aspx?n=E5509C8FE29950EA&topn=1D1ECC54F86E9050
10. Tsai JCH, Chen WY, Liang YW. Nonemergent emergency department visits under the National Health Insurance in Taiwan. Health Policy. 2011;100:189-195. https://doi.org/10.1016/j.healthpol.2010.10.007
11. Taiwan Society of Hospital Medicine. The birth and growth of hospital medicine in Taiwan. Accessed March 26, 2021. https://www.hospitalist.org.tw/about_25.htm
12. Hsu NC, Huang CC, Shu CC, Yang MC. Implementation of a seven-day hospitalist program to improve the outcomes of the weekend admission: a retrospective before-after study in Taiwan. PLoS One. 2018;13(3):e0194833. https://doi.org/10.1371/journal.pone.0194833
13. Ministry of Health and Welfare, Statutes of the Republic of Korea. Act on the Improvement of Training Conditions and Status of Medical Residents. Accessed March 26, 2021. https://elaw.klri.re.kr/eng_mobile/viewer.do?hseq=49563&type=sogan&key=10
14. Chae W, Park EC, Lee KY, et al. Development and evolution of hospital medicine in Korea. J Hosp Med. 2021;16(4):247-250. https://doi.org/10.12788/jhm.3573
15. Oh SJ, Jung EJ. Prospects for the Korean model of the surgical hospitalist system. J Korean Med Assoc. 2020;63(5):236-239. https://doi.org/10.5124/jkma.2020.63.5.236
16. Ohn JH, Kim NH, Kim ES, et al. An acute medical unit in a Korean tertiary care hospital reduces the length of stay and waiting time in the emergency department. J Korean Med Sci. 2017;32:1917-1920. https://doi.org/10.3346/jkms.2017.32.12.1917
17. Lee JH, Kim AJ, Kyong TY, et al. Evaluating the outcome of multi-morbid patients cared for by hospitalists: a report of integrated medical model in Korea. J Korean Med Sci. 2019;34(25):e179. https://doi.org/10.3346/jkms.2019.34.e179
18. OECD. Length of hospital stay. Accessed March 26, 2021. https://doi.org/10.1787/8dda6b7a-en
19. Hamada O, Tsutsumi T, Miki A, et al. Impact of the hospitalist system in Japan on the quality of care and healthcare economics. Intern Med. 2019;58(23):3385-3391. https://doi.org/10.2169/internalmedicine.2872-19
20. Kawashima A. Report on general medicine’s effects on specialists and other healthcare staff in the context of inclusive local medical system. Chapter in Japanese. Accessed March 26, 2021.https://soshin.pcmed-tsukuba.jp/education/report/pdf/05_004.pdf
Since its inception in the mid-1990s, the hospitalist model of care has enjoyed robust growth in the United States, increasing to around 20,000 providers by the end of its first decade.1,2 Since then, it has far outstripped early predictions of adoption, currently standing at more than 50,000 hospitalist providers.2 Although driven by numerous factors, including system-based management needs, provision of inpatient care for unassigned patients, and demands for improved patient safety and satisfaction, this meteoric growth has been driven largely by cost pressures particular to the US healthcare system.1,2 Nonetheless, the growing complexity of healthcare systems, substantial fiscal pressures, and increasing healthcare demands from aging populations are worldwide challenges to which countries outside North America also seek solutions. Countries that have initiated hospitalist care have localized adoption, evolving the model to meet their unique fiscal and system-based needs and patients’ expectations.
While there has been keen interest in the hospitalist model in Asia, there has not yet been widespread adoption, despite numerous data demonstrating that this model is associated with lower length of stay (LOS), as well as lower costs and improved patient safety.3,4 This article explores hospitalist care adoption experiences in Singapore, Taiwan, Korea, and Japan, focusing on stakeholder demand for hospitalist care, respective adoption, outcomes, and associated challenges to date.
SINGAPORE
Stakeholder Demand for Hospitalist Care
Historically in Singapore, family physicians provided primary care and internal medicine subspecialists provided inpatient care.5 Present-day trends, including an aging population, increasing rates of chronic diseases, and multisystem health issues, have stressed the historical model, leading to care fragmentation, long LOS (>9 days), and reduced patient satisfaction.5,6 Additionally, as 80% of hospital care is government funded, public hospitals are under pressure to reduce healthcare expenditures.5
Adoption of Hospitalist Care, Outcomes, and Challenges Faced
To meet patient needs and healthcare system challenges, the hospitalist model has evolved through several iterations in Singapore. The first model, implemented at Singapore General Hospital, utilized family physicians as hospitalists to coordinate inpatient care and integrate care between hospital and community settings.3,5 This model resulted in shorter LOS and reduced costs for patients cared for by family physician hospitalists.3 Despite these benefits, the family physician hospitalist model did not spread, partly due to biases favoring subspecialist care for hospitalized patients.7
The next iteration utilized general internal medicine (GIM) specialists. Traditionally, GIM specialists cared for a small number of low-acuity hospitalized patients. Recognizing the emerging need for holistic inpatient care, the Singapore Ministry of Health supported advances in generalist care, including a financial bonus and a revamped GIM training program. This spawned hospitalist-type models nationwide. At the National University Hospital (NUH), for example, GIM physicians were recruited to care for “specialty” patients in the acute medical unit and increase their ward coverage to include complex multimorbid patients. Additionally, NUH launched the enhanced complex care program, providing integrated inpatient and outpatient care to high-utilizing, complex patients. Overall, the NUH GIM division grew by 70% (faculty) and 60% (trainees) over 5 years. Currently, fueled by government enthusiasm for generalist care, hospitalist-type models are evident at newly minted hospitals across Singapore.
Although physicians act as hospitalists, the term hospitalist is not embraced in Singapore, thus limiting its potential to develop clinical- and system-improvement competencies and establish professional identity. This may be due to the strong UK-based cultural foundations and continued systemic bias favoring subspecialists.8
TAIWAN
Stakeholder Demand for Hospitalist Care
Under its national health insurance (NHI) system, Taiwan has relatively low copayments for medical services, with acute patients paying 10% of costs for a ≤30-day hospitalization, causing demand for inpatient care to remain strong.4,9 The NHI system has also led to increased numbers of patients accessing care in emergency departments (EDs), where costs may be as low as US $16 (NT $450), causing long waits for evaluation and transfer to wards.9,10 There remains an insufficient number of hospital-based physicians to manage this high patient volume, a situation exacerbated by low reimbursements.4
Adoption of Hospitalist Care, Outcomes, and Challenges Faced
In order to address rising admissions, inefficient ED management, and physician shortages, a hospitalist care program was first introduced in Taiwan in 2002, followed by the establishment of a hospitalist-run ward in National Taiwan University Hospital in 2009.11 Subsequent studies from Taiwan have found that hospitalist-run wards had lower admission costs, shorter LOS, and more do-not-resuscitate consent, and also had similar in-hospital mortality and readmission rates compared to specialist-run wards.4,12 Reflecting these successes, the Taiwan Association of Hospital Medicine (TAHM) was established in 2018, and since January 2021, the Ministry of Health and Welfare of Taiwan has mandated hospital medicine programs as an accreditation requirement for all medical centers, with a dual role of educating residents and providing inpatient care.
Despite growing opportunities, Taiwan has seen a modest increase in the number of hospitalists, rising from three in 2009 to around 300 by January 2021. An indistinct professional identity and career path are the main barriers. Given this, TAHM is trying to strengthen hospitalist professionalism by introducing both hard and soft skills, such as utilizing point-of-care ultrasonography and implementing the concepts of Choosing Wisely® and shared decision-making.
KOREA
Stakeholder Demand for Hospitalist Care
Korea has experienced a chronic physician shortage, with just 2.4 physicians per 1,000 people (World Bank, 2017), leading to significant physician burnout. Designed to protect trainee well-being, the 2015 Improvement of Training Conditions and Status of Medical Residents Act limited resident work hours while reducing internal medicine and general surgery training periods, further exacerbating physician shortages.13 In addition, Korea’s current NHI system—including its’ healthcare insurance reimbursements scheme, established in 1989 when Korea’s per capita gross domestic product was less than US $5,000—provides low reimbursements to healthcare providers.14 Along with increased attention to patient safety and healthcare-related consumer expectations, the hospitalist system in Korea aims to maintain improvements to residents’ well-being, while increasing hospital revenue and meeting patient demand for improved services.14
Adoption of Hospitalist Care, Outcomes, and Challenges Faced
Along with the Ministry of Health and Welfare, the Korean Health Insurance Review and Assessment Service launched a hospitalist pilot program in general medicine and surgery in 2016.15 Services for hospitalist-managed inpatients are charged on a new schedule covered by the NHI system, including facility fees, which are charged per diem, and separate hospitalist fees.14 New hospital medicine programs are utilized, in part, to recruit new physicians to manage a large volume of inpatients. Previous studies found that these new hospitalist care systems also improved patient safety, quality of care, and overall patient satisfaction, while being associated with shorter LOS and fewer unnecessary intensive care unit admissions.16,17 After a successful pilot, the revamped reimbursement system for hospitalist care officially started in January 2021.
Although Korea had only 250 registered hospitalists by August 2020, this is likely a substantial underestimate, as only hospital medicine teams with more than two hospitalists were allowed formal registration during the pilot period. Wider registration is currently underway for the new official reimbursement system.
JAPAN
Stakeholder Demand for Hospitalist Care
Hospitals in Japan are organized into highly compartmentalized subspecialties. Providing quality inpatient care to senior patients, who account for more than 28% of the population, and managing smooth transitions from hospital to long-term- care facilities remain challenging. In addition, given generous caps on maximum monthly out-of-pocket payments under its NHI system, LOS for Japanese hospitals are as long as 16.1 days.18 Nonetheless, given rising financial burdens associated with long-term care, hospitals are under government pressure to further shorten LOS and transition patients to local long-term-care facilities after treatment for acute symptoms.
Adoption of Hospitalist Care, Outcomes, and Challenges Faced
To meet these challenges, an increasing number of Japanese hospitals have established departments of general medicine to triage and manage patients with multiple comorbidities and to coordinate patient care across relevant specialties. The Japanese Society of Hospital General Medicine (JSHGM) was established in 2010, and currently has 1,890 members from 896 medical institutions. In 2018, general medicine was recognized by the Japanese Board of Medical Specialties as a formal specialty for certification. Currently, JSHGM is working with the Japan Primary Care Association and other organizations to establish a specialty certification system for hospitalist physicians and raise awareness of hospital medicine. A Japanese study of elderly patients with chronic aspiration pneumonia found that care by hospitalists resulted in shorter LOS and lower costs than specialist care.19 Recently, hospitalists have played a central role in COVID-19 management, opening fever intake clinics and establishing collaborative guidelines with infectious disease experts and other specialists.
Yet, different from the prototypical hospitalist first defined by Wachter and Goldman, Japanese general medicine hospitalists continue to have substantial outpatient responsibilities, albeit in the hospital setting. Out of 81 university hospitals, 69 now have a department of hospital general medicine, though only 20 have inpatient services.20 In addition, a medical culture in which patients continue to see their surgery attendings long after surgery remains strong. Clear definitions regarding hospitalists’ roles need to be established, while promoting changes toward inpatient care for both patients and subspecialists.
DISCUSSION
The four Asian countries reviewed here have all established universal access to healthcare, with Taiwan, Korea, and Japan having strong NHI systems and Singapore providing significant healthcare subsidies for those in need. Nonetheless, they also face similar challenges, including the growing complexity of healthcare systems, substantial fiscal pressures, increased healthcare demands caused by aging populations, and increased expectations regarding stakeholder well-being. As such, these countries share common driving forces that are propelling the adoption of hospitalist care models, such as lack of a sufficient physician workforce on inpatient wards; need for extra resources to shorten ED wait times prior to inpatient admission; need for providing quality care to multimorbid senior patients across highly segmented hospital departments and coordinating medical services between hospitals and outpatient care facilities; and government pressure on cutting costs, especially by shortening inpatient LOS. Some common barriers among these Asian countries include unclear definitions of hospitalists’ roles and degree of collaboration with subspecialty departments, and social and systemic biases favoring subspecialty care for inpatients.
The four Asian countries reviewed here have chosen to adopt the hospitalist model as a supplement to already established, specialty-driven inpatient care systems; as such, further comparative outcome studies focusing on cost, care quality, and patient safety and satisfaction are warranted to bolster professional hospitalist roles, further facilitate government/policy-level support for hospital care systems, and promote future training and certification systems appropriate to each country’s unique healthcare system and medical culture. Similarly, evidence-driven educational outreach programs are warranted to facilitate patient understanding of the role of hospitalists in their care.For countries interested in establishing hospital medicine programs, the adoption experiences in Singapore, Taiwan, Korea, and Japan provide valuable insights regarding how to establish hospitalist models to meet country-specific healthcare challenges while successfully functioning in the context of their unique medical-system frameworks.
Since its inception in the mid-1990s, the hospitalist model of care has enjoyed robust growth in the United States, increasing to around 20,000 providers by the end of its first decade.1,2 Since then, it has far outstripped early predictions of adoption, currently standing at more than 50,000 hospitalist providers.2 Although driven by numerous factors, including system-based management needs, provision of inpatient care for unassigned patients, and demands for improved patient safety and satisfaction, this meteoric growth has been driven largely by cost pressures particular to the US healthcare system.1,2 Nonetheless, the growing complexity of healthcare systems, substantial fiscal pressures, and increasing healthcare demands from aging populations are worldwide challenges to which countries outside North America also seek solutions. Countries that have initiated hospitalist care have localized adoption, evolving the model to meet their unique fiscal and system-based needs and patients’ expectations.
While there has been keen interest in the hospitalist model in Asia, there has not yet been widespread adoption, despite numerous data demonstrating that this model is associated with lower length of stay (LOS), as well as lower costs and improved patient safety.3,4 This article explores hospitalist care adoption experiences in Singapore, Taiwan, Korea, and Japan, focusing on stakeholder demand for hospitalist care, respective adoption, outcomes, and associated challenges to date.
SINGAPORE
Stakeholder Demand for Hospitalist Care
Historically in Singapore, family physicians provided primary care and internal medicine subspecialists provided inpatient care.5 Present-day trends, including an aging population, increasing rates of chronic diseases, and multisystem health issues, have stressed the historical model, leading to care fragmentation, long LOS (>9 days), and reduced patient satisfaction.5,6 Additionally, as 80% of hospital care is government funded, public hospitals are under pressure to reduce healthcare expenditures.5
Adoption of Hospitalist Care, Outcomes, and Challenges Faced
To meet patient needs and healthcare system challenges, the hospitalist model has evolved through several iterations in Singapore. The first model, implemented at Singapore General Hospital, utilized family physicians as hospitalists to coordinate inpatient care and integrate care between hospital and community settings.3,5 This model resulted in shorter LOS and reduced costs for patients cared for by family physician hospitalists.3 Despite these benefits, the family physician hospitalist model did not spread, partly due to biases favoring subspecialist care for hospitalized patients.7
The next iteration utilized general internal medicine (GIM) specialists. Traditionally, GIM specialists cared for a small number of low-acuity hospitalized patients. Recognizing the emerging need for holistic inpatient care, the Singapore Ministry of Health supported advances in generalist care, including a financial bonus and a revamped GIM training program. This spawned hospitalist-type models nationwide. At the National University Hospital (NUH), for example, GIM physicians were recruited to care for “specialty” patients in the acute medical unit and increase their ward coverage to include complex multimorbid patients. Additionally, NUH launched the enhanced complex care program, providing integrated inpatient and outpatient care to high-utilizing, complex patients. Overall, the NUH GIM division grew by 70% (faculty) and 60% (trainees) over 5 years. Currently, fueled by government enthusiasm for generalist care, hospitalist-type models are evident at newly minted hospitals across Singapore.
Although physicians act as hospitalists, the term hospitalist is not embraced in Singapore, thus limiting its potential to develop clinical- and system-improvement competencies and establish professional identity. This may be due to the strong UK-based cultural foundations and continued systemic bias favoring subspecialists.8
TAIWAN
Stakeholder Demand for Hospitalist Care
Under its national health insurance (NHI) system, Taiwan has relatively low copayments for medical services, with acute patients paying 10% of costs for a ≤30-day hospitalization, causing demand for inpatient care to remain strong.4,9 The NHI system has also led to increased numbers of patients accessing care in emergency departments (EDs), where costs may be as low as US $16 (NT $450), causing long waits for evaluation and transfer to wards.9,10 There remains an insufficient number of hospital-based physicians to manage this high patient volume, a situation exacerbated by low reimbursements.4
Adoption of Hospitalist Care, Outcomes, and Challenges Faced
In order to address rising admissions, inefficient ED management, and physician shortages, a hospitalist care program was first introduced in Taiwan in 2002, followed by the establishment of a hospitalist-run ward in National Taiwan University Hospital in 2009.11 Subsequent studies from Taiwan have found that hospitalist-run wards had lower admission costs, shorter LOS, and more do-not-resuscitate consent, and also had similar in-hospital mortality and readmission rates compared to specialist-run wards.4,12 Reflecting these successes, the Taiwan Association of Hospital Medicine (TAHM) was established in 2018, and since January 2021, the Ministry of Health and Welfare of Taiwan has mandated hospital medicine programs as an accreditation requirement for all medical centers, with a dual role of educating residents and providing inpatient care.
Despite growing opportunities, Taiwan has seen a modest increase in the number of hospitalists, rising from three in 2009 to around 300 by January 2021. An indistinct professional identity and career path are the main barriers. Given this, TAHM is trying to strengthen hospitalist professionalism by introducing both hard and soft skills, such as utilizing point-of-care ultrasonography and implementing the concepts of Choosing Wisely® and shared decision-making.
KOREA
Stakeholder Demand for Hospitalist Care
Korea has experienced a chronic physician shortage, with just 2.4 physicians per 1,000 people (World Bank, 2017), leading to significant physician burnout. Designed to protect trainee well-being, the 2015 Improvement of Training Conditions and Status of Medical Residents Act limited resident work hours while reducing internal medicine and general surgery training periods, further exacerbating physician shortages.13 In addition, Korea’s current NHI system—including its’ healthcare insurance reimbursements scheme, established in 1989 when Korea’s per capita gross domestic product was less than US $5,000—provides low reimbursements to healthcare providers.14 Along with increased attention to patient safety and healthcare-related consumer expectations, the hospitalist system in Korea aims to maintain improvements to residents’ well-being, while increasing hospital revenue and meeting patient demand for improved services.14
Adoption of Hospitalist Care, Outcomes, and Challenges Faced
Along with the Ministry of Health and Welfare, the Korean Health Insurance Review and Assessment Service launched a hospitalist pilot program in general medicine and surgery in 2016.15 Services for hospitalist-managed inpatients are charged on a new schedule covered by the NHI system, including facility fees, which are charged per diem, and separate hospitalist fees.14 New hospital medicine programs are utilized, in part, to recruit new physicians to manage a large volume of inpatients. Previous studies found that these new hospitalist care systems also improved patient safety, quality of care, and overall patient satisfaction, while being associated with shorter LOS and fewer unnecessary intensive care unit admissions.16,17 After a successful pilot, the revamped reimbursement system for hospitalist care officially started in January 2021.
Although Korea had only 250 registered hospitalists by August 2020, this is likely a substantial underestimate, as only hospital medicine teams with more than two hospitalists were allowed formal registration during the pilot period. Wider registration is currently underway for the new official reimbursement system.
JAPAN
Stakeholder Demand for Hospitalist Care
Hospitals in Japan are organized into highly compartmentalized subspecialties. Providing quality inpatient care to senior patients, who account for more than 28% of the population, and managing smooth transitions from hospital to long-term- care facilities remain challenging. In addition, given generous caps on maximum monthly out-of-pocket payments under its NHI system, LOS for Japanese hospitals are as long as 16.1 days.18 Nonetheless, given rising financial burdens associated with long-term care, hospitals are under government pressure to further shorten LOS and transition patients to local long-term-care facilities after treatment for acute symptoms.
Adoption of Hospitalist Care, Outcomes, and Challenges Faced
To meet these challenges, an increasing number of Japanese hospitals have established departments of general medicine to triage and manage patients with multiple comorbidities and to coordinate patient care across relevant specialties. The Japanese Society of Hospital General Medicine (JSHGM) was established in 2010, and currently has 1,890 members from 896 medical institutions. In 2018, general medicine was recognized by the Japanese Board of Medical Specialties as a formal specialty for certification. Currently, JSHGM is working with the Japan Primary Care Association and other organizations to establish a specialty certification system for hospitalist physicians and raise awareness of hospital medicine. A Japanese study of elderly patients with chronic aspiration pneumonia found that care by hospitalists resulted in shorter LOS and lower costs than specialist care.19 Recently, hospitalists have played a central role in COVID-19 management, opening fever intake clinics and establishing collaborative guidelines with infectious disease experts and other specialists.
Yet, different from the prototypical hospitalist first defined by Wachter and Goldman, Japanese general medicine hospitalists continue to have substantial outpatient responsibilities, albeit in the hospital setting. Out of 81 university hospitals, 69 now have a department of hospital general medicine, though only 20 have inpatient services.20 In addition, a medical culture in which patients continue to see their surgery attendings long after surgery remains strong. Clear definitions regarding hospitalists’ roles need to be established, while promoting changes toward inpatient care for both patients and subspecialists.
DISCUSSION
The four Asian countries reviewed here have all established universal access to healthcare, with Taiwan, Korea, and Japan having strong NHI systems and Singapore providing significant healthcare subsidies for those in need. Nonetheless, they also face similar challenges, including the growing complexity of healthcare systems, substantial fiscal pressures, increased healthcare demands caused by aging populations, and increased expectations regarding stakeholder well-being. As such, these countries share common driving forces that are propelling the adoption of hospitalist care models, such as lack of a sufficient physician workforce on inpatient wards; need for extra resources to shorten ED wait times prior to inpatient admission; need for providing quality care to multimorbid senior patients across highly segmented hospital departments and coordinating medical services between hospitals and outpatient care facilities; and government pressure on cutting costs, especially by shortening inpatient LOS. Some common barriers among these Asian countries include unclear definitions of hospitalists’ roles and degree of collaboration with subspecialty departments, and social and systemic biases favoring subspecialty care for inpatients.
The four Asian countries reviewed here have chosen to adopt the hospitalist model as a supplement to already established, specialty-driven inpatient care systems; as such, further comparative outcome studies focusing on cost, care quality, and patient safety and satisfaction are warranted to bolster professional hospitalist roles, further facilitate government/policy-level support for hospital care systems, and promote future training and certification systems appropriate to each country’s unique healthcare system and medical culture. Similarly, evidence-driven educational outreach programs are warranted to facilitate patient understanding of the role of hospitalists in their care.For countries interested in establishing hospital medicine programs, the adoption experiences in Singapore, Taiwan, Korea, and Japan provide valuable insights regarding how to establish hospitalist models to meet country-specific healthcare challenges while successfully functioning in the context of their unique medical-system frameworks.
1. Wachter RM, Goldman L. The hospitalist movement 5 years later. JAMA. 2002;287:487-494. https://doi.org/10.1001/jama.287.4.487
2. Wachter RM, Goldman L. Zero to 50,000—the 20th anniversary of the hospitalist. N Engl J Med. 2016;375(11):1009-1011. https://doi.org/10.1056/NEJMp1607958
3. Lee KH, Yang Y, Yang KS, et al. Bringing generalists into the hospital: outcomes of a family medicine hospitalist model in Singapore. J Hosp Med. 2011;6:115-121. https://doi.org/10.1002/jhm.821
4. Shu CC, Lin JW, Lin YF, et al. Evaluating the performance of a hospitalist system in Taiwan: a pioneer study for nationwide health insurance in Asia. J Hosp Med. 2011;6(7):378-382. https://doi.org/ 10.1002/jhm.896
5. Lee KH. The hospitalist movement—a complex adaptive response to fragmentation of care in hospitals. Ann Acad Med Singap. 2008;37(2):145-150.
6. Ge L, Ya CW, Heng BH, Tan WS. Frailty and healthcare utilization across care settings among community-dwelling older adults in Singapore. BMC Geriatrics. 2020;20:389. https://doi.org/10.1186/s12877-020-01800-8
7. Lee KH. A historical perspective of the barriers to generalism. Aust Fam Physician. 2015;44(3):154-158.
8. Choo F. Alexandra Hospital provides patients with one-stop services under new care model. Updated December 14, 2018. Accessed March 26, 2021.https://www.straitstimes.com/singapore/health/alexandra-hospital-provides-patients-with-one-stop-services-under-new-care-model
9. National Health Insurance Administration, Ministry of Health and Welfare, Taiwan. Medical services. Copayments. Updated December 28, 2020. Accessed March 26, 2021. https://www.nhi.gov.tw/English/Content_List.aspx?n=E5509C8FE29950EA&topn=1D1ECC54F86E9050
10. Tsai JCH, Chen WY, Liang YW. Nonemergent emergency department visits under the National Health Insurance in Taiwan. Health Policy. 2011;100:189-195. https://doi.org/10.1016/j.healthpol.2010.10.007
11. Taiwan Society of Hospital Medicine. The birth and growth of hospital medicine in Taiwan. Accessed March 26, 2021. https://www.hospitalist.org.tw/about_25.htm
12. Hsu NC, Huang CC, Shu CC, Yang MC. Implementation of a seven-day hospitalist program to improve the outcomes of the weekend admission: a retrospective before-after study in Taiwan. PLoS One. 2018;13(3):e0194833. https://doi.org/10.1371/journal.pone.0194833
13. Ministry of Health and Welfare, Statutes of the Republic of Korea. Act on the Improvement of Training Conditions and Status of Medical Residents. Accessed March 26, 2021. https://elaw.klri.re.kr/eng_mobile/viewer.do?hseq=49563&type=sogan&key=10
14. Chae W, Park EC, Lee KY, et al. Development and evolution of hospital medicine in Korea. J Hosp Med. 2021;16(4):247-250. https://doi.org/10.12788/jhm.3573
15. Oh SJ, Jung EJ. Prospects for the Korean model of the surgical hospitalist system. J Korean Med Assoc. 2020;63(5):236-239. https://doi.org/10.5124/jkma.2020.63.5.236
16. Ohn JH, Kim NH, Kim ES, et al. An acute medical unit in a Korean tertiary care hospital reduces the length of stay and waiting time in the emergency department. J Korean Med Sci. 2017;32:1917-1920. https://doi.org/10.3346/jkms.2017.32.12.1917
17. Lee JH, Kim AJ, Kyong TY, et al. Evaluating the outcome of multi-morbid patients cared for by hospitalists: a report of integrated medical model in Korea. J Korean Med Sci. 2019;34(25):e179. https://doi.org/10.3346/jkms.2019.34.e179
18. OECD. Length of hospital stay. Accessed March 26, 2021. https://doi.org/10.1787/8dda6b7a-en
19. Hamada O, Tsutsumi T, Miki A, et al. Impact of the hospitalist system in Japan on the quality of care and healthcare economics. Intern Med. 2019;58(23):3385-3391. https://doi.org/10.2169/internalmedicine.2872-19
20. Kawashima A. Report on general medicine’s effects on specialists and other healthcare staff in the context of inclusive local medical system. Chapter in Japanese. Accessed March 26, 2021.https://soshin.pcmed-tsukuba.jp/education/report/pdf/05_004.pdf
1. Wachter RM, Goldman L. The hospitalist movement 5 years later. JAMA. 2002;287:487-494. https://doi.org/10.1001/jama.287.4.487
2. Wachter RM, Goldman L. Zero to 50,000—the 20th anniversary of the hospitalist. N Engl J Med. 2016;375(11):1009-1011. https://doi.org/10.1056/NEJMp1607958
3. Lee KH, Yang Y, Yang KS, et al. Bringing generalists into the hospital: outcomes of a family medicine hospitalist model in Singapore. J Hosp Med. 2011;6:115-121. https://doi.org/10.1002/jhm.821
4. Shu CC, Lin JW, Lin YF, et al. Evaluating the performance of a hospitalist system in Taiwan: a pioneer study for nationwide health insurance in Asia. J Hosp Med. 2011;6(7):378-382. https://doi.org/ 10.1002/jhm.896
5. Lee KH. The hospitalist movement—a complex adaptive response to fragmentation of care in hospitals. Ann Acad Med Singap. 2008;37(2):145-150.
6. Ge L, Ya CW, Heng BH, Tan WS. Frailty and healthcare utilization across care settings among community-dwelling older adults in Singapore. BMC Geriatrics. 2020;20:389. https://doi.org/10.1186/s12877-020-01800-8
7. Lee KH. A historical perspective of the barriers to generalism. Aust Fam Physician. 2015;44(3):154-158.
8. Choo F. Alexandra Hospital provides patients with one-stop services under new care model. Updated December 14, 2018. Accessed March 26, 2021.https://www.straitstimes.com/singapore/health/alexandra-hospital-provides-patients-with-one-stop-services-under-new-care-model
9. National Health Insurance Administration, Ministry of Health and Welfare, Taiwan. Medical services. Copayments. Updated December 28, 2020. Accessed March 26, 2021. https://www.nhi.gov.tw/English/Content_List.aspx?n=E5509C8FE29950EA&topn=1D1ECC54F86E9050
10. Tsai JCH, Chen WY, Liang YW. Nonemergent emergency department visits under the National Health Insurance in Taiwan. Health Policy. 2011;100:189-195. https://doi.org/10.1016/j.healthpol.2010.10.007
11. Taiwan Society of Hospital Medicine. The birth and growth of hospital medicine in Taiwan. Accessed March 26, 2021. https://www.hospitalist.org.tw/about_25.htm
12. Hsu NC, Huang CC, Shu CC, Yang MC. Implementation of a seven-day hospitalist program to improve the outcomes of the weekend admission: a retrospective before-after study in Taiwan. PLoS One. 2018;13(3):e0194833. https://doi.org/10.1371/journal.pone.0194833
13. Ministry of Health and Welfare, Statutes of the Republic of Korea. Act on the Improvement of Training Conditions and Status of Medical Residents. Accessed March 26, 2021. https://elaw.klri.re.kr/eng_mobile/viewer.do?hseq=49563&type=sogan&key=10
14. Chae W, Park EC, Lee KY, et al. Development and evolution of hospital medicine in Korea. J Hosp Med. 2021;16(4):247-250. https://doi.org/10.12788/jhm.3573
15. Oh SJ, Jung EJ. Prospects for the Korean model of the surgical hospitalist system. J Korean Med Assoc. 2020;63(5):236-239. https://doi.org/10.5124/jkma.2020.63.5.236
16. Ohn JH, Kim NH, Kim ES, et al. An acute medical unit in a Korean tertiary care hospital reduces the length of stay and waiting time in the emergency department. J Korean Med Sci. 2017;32:1917-1920. https://doi.org/10.3346/jkms.2017.32.12.1917
17. Lee JH, Kim AJ, Kyong TY, et al. Evaluating the outcome of multi-morbid patients cared for by hospitalists: a report of integrated medical model in Korea. J Korean Med Sci. 2019;34(25):e179. https://doi.org/10.3346/jkms.2019.34.e179
18. OECD. Length of hospital stay. Accessed March 26, 2021. https://doi.org/10.1787/8dda6b7a-en
19. Hamada O, Tsutsumi T, Miki A, et al. Impact of the hospitalist system in Japan on the quality of care and healthcare economics. Intern Med. 2019;58(23):3385-3391. https://doi.org/10.2169/internalmedicine.2872-19
20. Kawashima A. Report on general medicine’s effects on specialists and other healthcare staff in the context of inclusive local medical system. Chapter in Japanese. Accessed March 26, 2021.https://soshin.pcmed-tsukuba.jp/education/report/pdf/05_004.pdf
Adoption of Hospitalist Care in Asia: Experiences From Singapore, Taiwan, Korea, and Japan

This work is licensed under a Creative Commons Attribution 4.0 International License
Adoption of Hospitalist Care in Asia: Experiences From Singapore, Taiwan, Korea, and Japan

This work is licensed under a Creative Commons Attribution 4.0 International License
© 2021 Society of Hospital Medicine








