User login
Rates and Characteristics of Medical Malpractice Claims Against Hospitalists
The prospect of facing a medical malpractice claim is a source of apprehension for physicians that affects physician behavior, including leading to defensive medicine.1-3 Overall, annual defensive medicine costs have been estimated at $45.6 billion,4 and surveys of hospitalists indicate that 13.0% to 37.5% of hospitalist healthcare costs involve defensive medicine.5,6 Despite the impact of malpractice concerns on hospitalist practice and the unprecedented growth of the field of hospital medicine, relatively few studies have examined the liability environment surrounding hospitalist practice.7,8 The specific issue of malpractice claims rates faced by hospitalists has received even less attention in the medical literature.8
A better understanding of the contributing factors and other attributes of malpractice claims can help guide patient safety initiatives and inform hospitalists’ level of concern regarding liability. Although most medical errors do not result in a malpractice claim,9,10 the majority of malpractice claims in which there is an indemnity payment involve medical injury due to clinician error.11 Even malpractice claims that do not result in an indemnity payment represent opportunities to identify patient safety and risk management vulnerabilities.12
We used a national malpractice claims database to analyze the characteristics of claims made against hospitalists, including claims rates. In addition to claims rates, we also analyzed the other types of providers named in hospitalist claims given the importance of interdisciplinary collaboration to hospital medicine.13,14 To provide context for understanding hospitalist liability data, we present data on other specialties. We also describe a model to predict whether hospitalist malpractice claims will close with an indemnity payment.
METHODS
Data Sources and Elements
This analysis used a repository of malpractice claims maintained by CRICO, the captive malpractice insurer of the Harvard-affiliated medical institutions. This database, the Comparative Benchmarking System, a
Injury severity was based on a widely used scale developed for malpractice claims by the National Association of Insurance Commissioners.16 Low injury severity included emotional injury and temporary insignificant injury. Medium injury severity included temporary minor, temporary major, and permanent minor injury. High injury severity included permanent significant injury through death. Because this study used a database assembled for operational and patient safety purposes and was not human subjects research, institutional review board approval was not needed.
Study Cohort
Malpractice claims included formal lawsuits or written requests for compensation for negligent medical care. Ho
Statistical Analysis
Malpractice claims rates were treated as Poisson rates and compared using a Z-test. Malpractice claims rates are expressed as claims per 100 physician-years. Each physician-year represents 1 year of coverage of one physician by the medical malpractice carrier whose data were used. Physician-years represent the duration of time physicians practiced during which they were insured by the malpractice carrier and, as such, could have been subject to a malpractice claim that would have been included in our data. Claims rates are based on the subset of the malpractice claims in the study for which the number of physician-years of coverage is available, representing 8.2% of hospitalist claims and 11.6% of all claims.
Comparisons of the percentages of cases closing with an indemnity payment, as well as the percentages of cases in different allegation type and clinical severity categories, were made using the Fisher exact test. Indemnity payment amounts were inflation-adjusted to 2018 dollars using the Consumer Price Index. Comparisons of indemnity payment amounts between physician specialties were carried out using the Wilcoxon rank sum test given that the distribution of the payment amounts appeared nonnormal; this was confirmed with the Shapiro-Wilk test. A multivariable logistic regression model was developed to predict the binary outcome of whether a hospitalist case would close with an indemnity payment (compared with no payment), based on the 1,216 hospitalist claims. The predictors used in this regression model were chosen a priori based on hypotheses about what factors drive the likelihood that a case closes with payment. Both the unadjusted and adjusted odds ratios for the predictors are presented. The adjusted model is adjusted for all the other predictors contained in the model. All reported P values are two-sided. The statistical analysis was carried out using JMP Pro version 15 (SAS Institute Inc) and Minitab version 19 (Minitab LLC).
RESULTS
We identified 1,216 hospital medicine malpractice claims from our database. Claims rates were calculated from the subset of our data for which physician-years were available—including 5,140 physician-years encompassing 100 claims, representing 8.2% of all hospitalist claims studied. An additional 18,644 malpractice claims from five other specialties—nonhospitalist general internal medicine, internal medicine subspecialists, emergency medicine, neurosurgery, and psychiatry—were analyzed to provide context for the hospitalist claims.
The malpractice claims rate for hospitalists was significantly higher than the rate for internal medicine subspecialists (1.95 vs 1.30 claims per 100 physician-years; P < .001), though they were not significantly different from the rate for nonhospitalist general internal medicine physicians (1.95 vs 1.92 claims per 100 physician-years; P = .93) (Table 1). Compared with emergency medicine physicians, with whom hospitalists are sometimes compared due to both specialties being defined by their site of practice and the absence of longitudinal patient relationships, hospitalists had a significantly lower claims rate (1.95 vs 4.07 claims per 100 physician-years; P < .001).
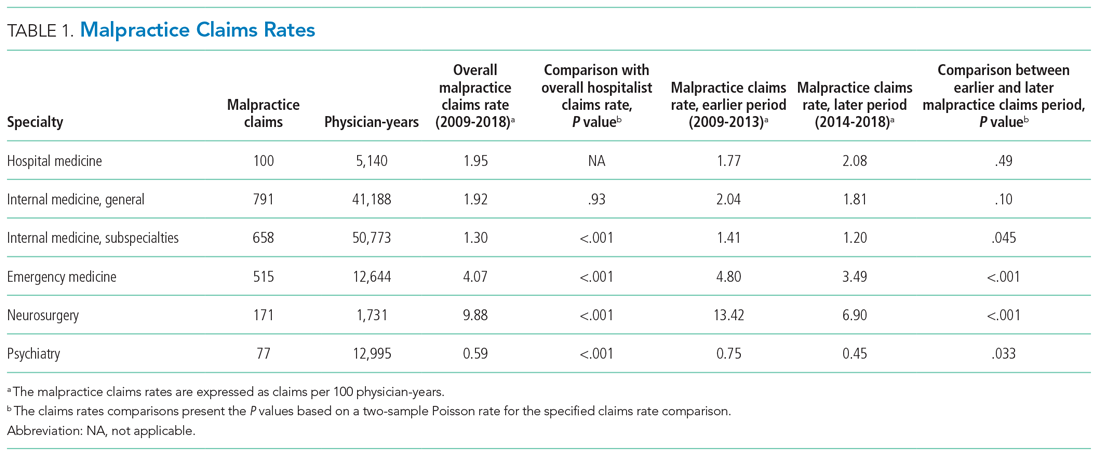
An assessment of the temporal trends in the claims rates, based on a comparison between the two halves of the study period (2014-2018 vs 2009-2013), showed that the claims rate for hospitalists was increasing, but at a rate that did not reach statistical significance (Table 1). In contrast, the claims rates for the five other specialties assessed decreased over time, and the decreases were significant for four of these five other specialties (internal medicine subspecialties, emergency medicine, neurosurgery, and psychiatry).
Multiple claims against a single physician were uncommon in our hospitalist malpractice claims data. Among the 100 claims that were used to calculate the claims rates, one physician was named in 2 claims, and all the other physicians were named in only a single claim. Among all of the 1,216 hospitalist malpractice claims we analyzed, there were eight physicians who were named in more than 1 claim, seven of whom were named in 2 claims, and one of whom was named in 3 claims.
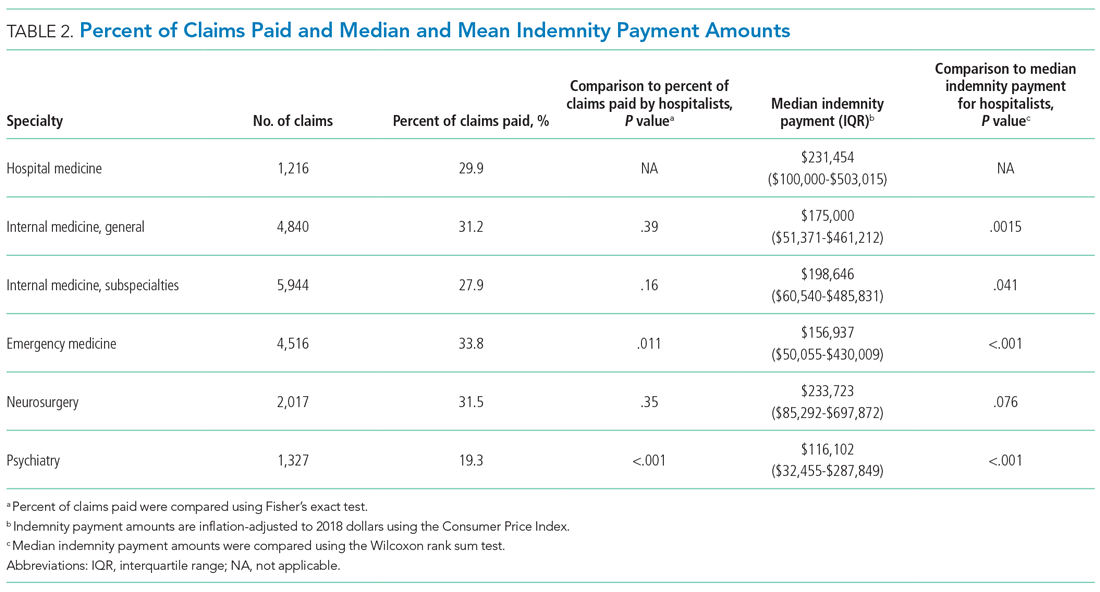
The median indemnity payment for hospitalist claims was $231,454 (interquartile range [IQR], $100,000-$503,015), similar to the median indemnity payment for neurosurgery ($233,723; IQR, $85,292-$697,872), though significantly greater than the median indemnity payment for the other four specialties studied (Table 2). Among the hospitalist claims, 29.9% resulted in an indemnity payment, not significantly different from the rate for nonhospitalist general internal medicine, internal medicine subspecialties, or neurosurgery, but significantly lower than the rate for emergency medicine (33.8%; P = .011). No
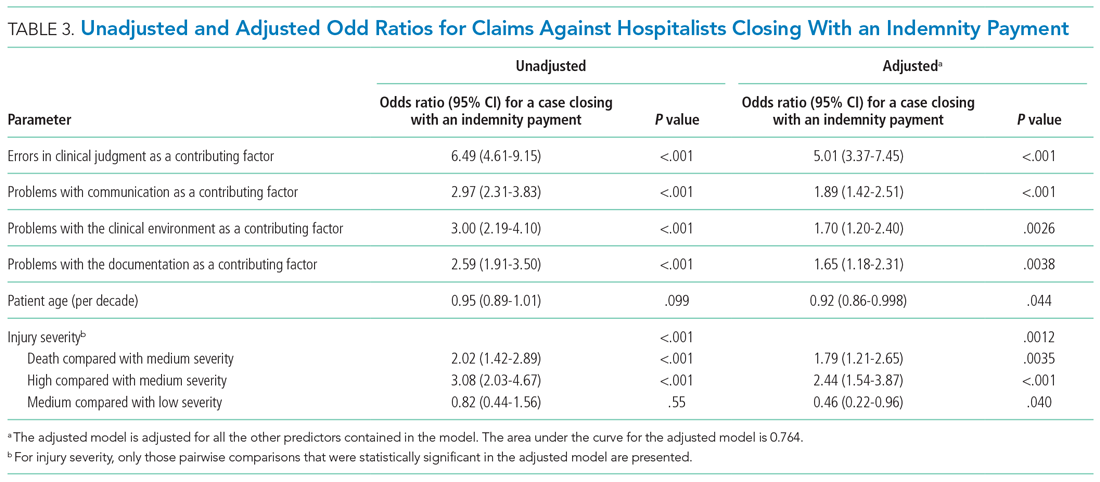
We performed a multivariable logistic regression analysis to assess the effect of different factors on the likelihood of a hospitalist case closing with an indemnity payment, compared with no payment (Table 3). In the multivariable model, the presence of an error in clinical judgment had an adjusted odds ratio (AOR) of 5.01 (95% CI, 3.37-7.45; P < .001) for a claim closing with payment, the largest effect found. The presence of problems with communication (AOR, 1.89; 95% CI, 1.42-2.51; P < .001), the clinical environment (eg, weekend/holiday or clinical busyness; AOR, 1.70; 95% CI, 1.20-2.40; P = .0026), and documentation (AOR, 1.65; 95% CI, 1.18-2.31; P = .0038) were also positive predictors of claims closing with payment. Greater patient age (per decade) was a negative predictor of the likelihood of a claim closing with payment (AOR, 0.92; 95% CI, 0.86-0.998), though it was of borderline statistical significance (P = .044).
We also assessed multiple clinical attributes of hospitalist malpractice claims, including the major allegation type and injury severity (Appendix Table). Among the 1,216 hospitalist malpractice claims studied, the most common allegation types were for errors related to medical treatment (n = 482; 39.6%), diagnosis (n = 446; 36.7%), and medications (n = 157; 12.9%). Among the hospitalist claims, 888 (73.0%) involved high-severity injury, and 674 (55.4%) involved the death of the patient. The percentages of cases involving high-severity injury and death were significantly greater for hospitalists, compared with that of the other specialties studied (P < .001 for all pairwise comparisons). Of the six specialties studied, hospital medicine was the only one in which the percentage of cases involving death exceeded 50%.
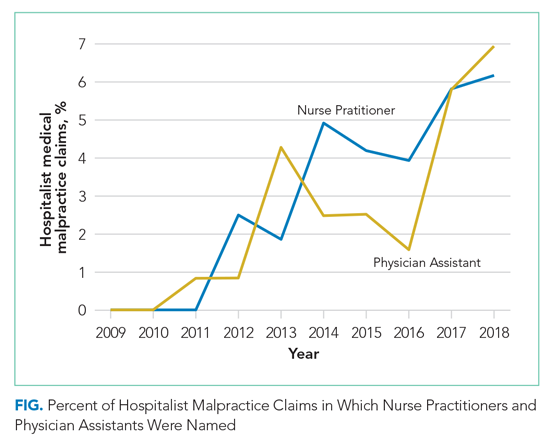
Hospital medicine is typically team-based, and we evaluated which other services were named in claims with hospital medicine as the primary responsible service. The clinician groups most commonly named in hospitalist claims were nursing (n = 269; 22.1%), followed by emergency medicine (n = 91; 7.5%), general surgery (n = 51; 4.2%), cardiology (n = 49; 4.0%), and orthopedic surgery (n = 46; 3.8%) (Appendix Figure). During the first 2 years of the study period, no physician assistants (PAs) or nurse practitioners (NPs) were named in hospitalist claims. Over the study period, the proportion of hospitalist cases also naming PAs and NPs increased steadily, reaching 6.9% and 6.2% of claims, respectively, in 2018 (Figure) (P < .001 for NPs and P = .037 for PAs based on a comparison between the two halves of the study period).
DISCUSSION
We found that the average annual claims rate for hospitalists was similar to that for nonhospitalist general internists (1.95 vs 1.92 claims per 100 physician-years) but significantly greater than that for internal medicine subspecialists (1.95 vs 1.30 claims per 100 physician-years). Hospitalist claims rates showed a notable temporal trend—a nonsignificant increase—over the study period (2009-2018). This contrasts with the five other specialties studied, all of which had decreasing claims rates, four of which were significant. An analysis of a different national malpractice claims database, the NPDB, found that the rate of paid malpractice claims overall decreased 55.7% during the period 1992-2014, again contrasting with the trend we found for hospitalist claims rates.17
We posit several explanations for why the malpractice claims rate trend for hospitalists has diverged from that of other specialties. There has been a large expansion in the number of hospitalists in the United States.18 With this increasing demand, many young physicians have entered the hospital medicine field. In a survey of general internal medicine physicians conducted by the Society of General Internal Medicine, 73% of hospitalists were aged 25 to 44 years, significantly greater than the 45% in this age range among nonhospitalist general internal medicine physicians.19 Hospitalists in their first year of practice have higher mortality rates than more experienced hospitalists.20 Therefore, the relative inexperience of hospitalists, driven by this high demand, could be putting them at increased risk of medical errors and resulting malpractice claims. The higher mortality rate among hospitalists in their first year of practice could be due to a lack of familiarity with the systems of care, such as managing test results and obtaining appropriate consults.20 This possibility suggests that enhanced training and mentorship could be valuable as a strategy to both improve the quality of care and reduce medicolegal risk. The increasing demand for hospitalists could also be affecting the qualification level of physicians entering the field.
Our analysis also showed that the severity of injury in hospitalist claims was greater than that for the other specialties studied. In addition, the percentage of claims involving death was greater for hospitalists than that for the other specialties. The increased acuity of inpatients, compared with that of outpatients—and the trend, at least for some conditions, of increased inpatient acuity over time21,22— could account for the high injury severity seen among hospitalist claims. Given the positive correlation between injury severity and the size of indemnity payments made on malpractice claims,12 the high injury severity seen in hospitalist claims was very likely a driver of the high indemnity payments observed among the hospitalist claims.
The relationship between injury severity and financial outcomes is supported by the results of our multivariable regression model (Table 3). Compared with medium-severity injury claims, both death and high-severity injury cases were significantly more likely to close with an indemnity payment (compared with no payment), with AORs of 1.79 (95% CI, 1.21-2.65) and 2.44 (95% CI, 1.54-3.87), respectively.
The most striking finding in our regression model was the magnitude of the effect of an error in clinical judgement. Cases coded with this contributing factor had five times the AOR of closing with payment (compared with no payment) (AOR, 5.01; 95% CI, 3.37-7.45). A clinical judgment call may be difficult to defend when it is ultimately associated with a bad patient outcome. The importance of clinical judgment in our analysis suggests a risk management strategy: clearly and contemporaneously documenting the rationale behind one’s clinical decision-making. This may help make a claim more defensible in the event of an adverse outcome by demonstrating that the clinician was acting reasonably based on the information available at the time. The importance of specifying a rationale for a clinical decision may be especially important in the era of electronic health records (EHRs). EHRs are not structured as chronologically linear charts, which can make it challenging during a trial to retrospectively show what information was available to the physician at the time the clinical decision was made. The importance of clinical judgment also affirms the importance of effective clinical decision support as a patient safety tool.23
More broadly, it is notable that several contributing factors, including errors in clinical judgment (as discussed previously), problems with communication, and issues with the clinical environment, were significantly associated with malpractice cases closing with payment. This demonstrates that systematically examining malpractice claims to determine the underlying contributing factors can generate predictive analytics, as well as suggest risk management and patient safety strategies.
Interdisciplinary collaboration, as a component of systems-based practice, is a core principle of hospital medicine,13 and so we analyzed the involvement of other clinicians in hospitalist claims. Of the five specialties most frequently named in claims with hospitalists, two were surgical services: general surgery (n = 51; 4.2%) and orthopedic surgery (n = 46; 3.8%). With hospitalists being asked to play an increasing role in the care of surgical patients, they may be providing care to patient populations with whom they have less experience, which could put them at risk of adverse outcomes, leading to malpractice claims.24,25 Hospitalists need to be attuned to the liability risks related to the care of patients requiring surgical management and ensure areas of responsibility are clearly delineated between the hospital medicine and surgical services.26 We also found that hospitalist claims increasingly involve PAs and NPs, likely reflecting their increasing role in providing care on hospitalist services.27,28
A prior analysis of claims rates for hospitalists that covered injury dates from 1997 to 2011 found that hospitalists had a relatively low claims rate, significantly lower than that for other internal medicine physicians.8 In addition to covering an earlier time period, that analysis based its claims rates on data from academic medical centers covered by a single insurer, and physicians at academic medical centers generally have lower claims rates, likely due, at least in part, to their spending a smaller proportion of their time on patient care, compared with nonacademic physicians.29 Another analysis of hospitalist closed claims, which shared some cases with the cohort we analyzed, was performed by The Doctors Company, a commercial liability insurer.7 That analysis astutely emphasized the importance of breakdowns in diagnostic processes as a factor underlying hospitalist claims.
Our study has several limitations. First, although our database of malpractice claims includes approximately 31% of all the claims in the country and includes claims from every state, it may not be nationally representative. Another limitation relates to calculating the claims rates for physicians. Detailed information on the number of years of clinical activity, which is necessary to calculate claims rates, was available for only a subset of our data (8.2% of the hospitalist cases and 11.6% of all cases), so claims rates are based on this subset of our data (among which academic centers are overrepresented). Therefore, the claims rates should be interpreted with caution, especially regarding their application to the community hospital setting. The institutions included in the subset of our data used for determining claims rates were stable over time, so the use of a subset of our data for calculating claims rates reduces the generalizability of our claims rates but should not be a source of bias.
Potentially offsetting strengths of our claims database and study include the availability of unpaid claims (which outnumber paid claims roughly 2:1)11,12; the presence of information on contributing factors and other case characteristics obtained through structured manual review of the cases; and the availability of the specialties of the clinicians involved. These features distinguish the database we used from the NPDB, another national database of malpractice claims, which does not include unpaid claims and which does not include information on contributing factors or physician specialty.
CONCLUSION
First described in 1996, the hospitalist field is the fastest growing specialty in modern medical history.18,30 Therefore, an understanding of the malpractice risk of hospitalists is important and can shed light on the patient safety environment in hospitals. Our analysis showed that hospitalist malpractice claims rates remain roughly stable, in contrast to most other specialties, which have seen a fall in malpractice claims rates.17 In addition, unlike a previous analysis,8 we found that claims rates for hospitalists were essentially equal to those of other general internal medicine physicians (not lower, as had been previously reported), and higher than those of the internal medicine subspecialties. Hospitalist claims also have relatively high severity of injury. Potential factors driving these trends include the increasing demand for hospitalists, which results in a higher proportion of less-experienced physicians entering the field, and the expanding clinical scope of hospitalists, which may lead to their managing patients with conditions with which they may be less comfortable. Overall, our analysis suggests that the malpractice environment for hospitalists is becoming less favorable, and therefore, hospitalists should explore opportunities for mitigating liability risk and enhancing patient safety.
1. Studdert DM, Mello MM, Sage WM, et al. Defensive medicine among high-risk specialist physicians in a volatile malpractice environment. JAMA. 2005;293(21):2609-2617. https://doi.org/10.1001/jama.293.21.2609
2. Carrier ER, Reschovsky JD, Mello MM, Mayrell RC, Katz D. Physicians’ fears of malpractice lawsuits are not assuaged by tort reforms. Health Aff (Millwood). 2010;29(9):1585-1592. https://doi.org/10.1377/hlthaff.2010.0135
3. Kachalia A, Berg A, Fagerlin A, et al. Overuse of testing in preoperative evaluation and syncope: a survey of hospitalists. Ann Intern Med. 2015;162(2):100-108. https://doi.org/10.7326/m14-0694
4. Mello MM, Chandra A, Gawande AA, Studdert DM. National costs of the medical liability system. Health Aff (Millwood). 2010;29(9):1569-1577. https://doi.org/10.1377/hlthaff.2009.0807
5. Rothberg MB, Class J, Bishop TF, Friderici J, Kleppel R, Lindenauer PK. The cost of defensive medicine on 3 hospital medicine services. JAMA Intern Med. 2014;174(11):1867-1868. https://doi.org/10.1001/jamainternmed.2014.4649
6. Saint S, Vaughn VM, Chopra V, Fowler KE, Kachalia A. Perception of resources spent on defensive medicine and history of being sued among hospitalists: results from a national survey. J Hosp Med. 2018;13(1):26-29. https://doi.org/10.12788/jhm.2800
7. Ranum D, Troxel DB, Diamond R. Hospitalist Closed Claims Study: An Expert Analysis of Medical Malpractice Allegations. The Doctors Company. 2016. https://www.thedoctors.com/siteassets/pdfs/risk-management/closed-claims-studies/10392_ccs-hospitalist_academic_single-page_version_frr.pdf
8. Schaffer AC, Puopolo AL, Raman S, Kachalia A. Liability impact of the hospitalist model of care. J Hosp Med. 2014;9(12):750-755. https://doi.org/10.1002/jhm.2244
9. Localio AR, Lawthers AG, Brennan TA, et al. Relation between malpractice claims and adverse events due to negligence. results of the Harvard Medical Practice Study III. N Engl J Med. 1991;325(4):245-251. https://doi.org/10.1056/nejm199107253250405
10. Studdert DM, Thomas EJ, Burstin HR, Zbar BI, Orav EJ, Brennan TA. Negligent care and malpractice claiming behavior in Utah and Colorado. Med Care. 2000;38(3):250-260. https://doi.org/10.1097/00005650-200003000-00002
11. Studdert DM, Mello MM, Gawande AA, et al. Claims, errors, and compensation payments in medical malpractice litigation. N Engl J Med. 2006;354(19):2024-2033. https://doi.org/10.1056/nejmsa054479
12. Medical Malpractice in America: 2018 CRICO Strategies National CBS Report. CRICO Strategies; 2018.
13. Budnitz T, McKean SC. The Core Competencies in Hospital Medicine. In: McKean SC, Ross JJ, Dressler DD, Scheurer DB, eds. Principles and Practice of Hospital Medicine, 2nd ed. McGraw-Hill Education; 2017.
14. O’Leary KJ, Haviley C, Slade ME, Shah HM, Lee J, Williams MV. Improving teamwork: impact of structured interdisciplinary rounds on a hospitalist unit. J Hosp Med. 2011;6(2):88-93. https://doi.org/10.1002/jhm.714
15. National Practitioner Data Bank: Public Use Data File. Division of Practitioner Data Banks, Bureau of Health Professions, Health Resources & Services Administration, U.S. Department of Health & Human Services; June 30, 2019. Updated August 2020.
16. Sowka MP, ed. NAIC Malpractice Claims, Final Compilation. National Association of Insurance Commissioners; 1980.
17. Schaffer AC, Jena AB, Seabury SA, Singh H, Chalasani V, Kachalia A. Rates and characteristics of paid malpractice claims among US physicians by specialty, 1992-2014. JAMA Intern Med. 2017;177(5):710-718. https://doi.org/10.1001/jamainternmed.2017.0311
18. Wachter RM, Goldman L. Zero to 50,000 - the 20th anniversary of the hospitalist. N Engl J Med. 2016;375(11):1009-1011. https://doi.org/10.1056/nejmp1607958
19. Miller CS, Fogerty RL, Gann J, Bruti CP, Klein R; The Society of General Internal Medicine Membership Committee. The growth of hospitalists and the future of the Society of General Internal Medicine: results from the 2014 membership survey. J Gen Intern Med. 2017;32(11):1179-1185. https://doi.org/10.1007/s11606-017-4126-7
20. Goodwin JS, Salameh H, Zhou J, Singh S, Kuo YF, Nattinger AB. Association of hospitalist years of experience with mortality in the hospitalized Medicare population. JAMA Intern Med. 2018;178(2):196-203. https://doi.org/10.1001/jamainternmed.2017.7049
21. Akintoye E, Briasoulis A, Egbe A, et al. National trends in admission and in-hospital mortality of patients with heart failure in the United States (2001-2014). J Am Heart Assoc. 2017;6(12):e006955. https://doi.org/10.1161/jaha.117.006955
22. Clark AV, LoPresti CM, Smith TI. Trends in inpatient admission comorbidity and electronic health data: implications for resident workload intensity. J Hosp Med. 2018;13(8):570-572. https://doi.org/10.12788/jhm.2954
23. Gilmartin HM, Liu VX, Burke RE. Annals for hospitalists inpatient notes - The role of hospitalists in the creation of learning healthcare systems. Ann Intern Med. 2020;172(2):HO2-HO3. https://doi.org/10.7326/m19-3873
24. Siegal EM. Just because you can, doesn’t mean that you should: a call for the rational application of hospitalist comanagement. J Hosp Med. 2008;3(5):398-402. https://doi.org/10.1002/jhm.361
25. Plauth WH 3rd, Pantilat SZ, Wachter RM, Fenton CL. Hospitalists’ perceptions of their residency training needs: results of a national survey. Am J Med. 2001;111(3):247-254. https://doi.org/10.1016/s0002-9343(01)00837-3
26. Thompson RE, Pfeifer K, Grant PJ, et al. Hospital medicine and perioperative care: a framework for high-quality, high-value collaborative care. J Hosp Med. 2017;12(4):277-282. https://doi.org/10.12788/jhm.2717
27. Torok H, Lackner C, Landis R, Wright S. Learning needs of physician assistants working in hospital medicine. J Hosp Med. 2012;7(3):190-194. https://doi.org/10.1002/jhm.1001
28. Kartha A, Restuccia JD, Burgess JF Jr, et al. Nurse practitioner and physician assistant scope of practice in 118 acute care hospitals. J Hosp Med. 2014;9(10):615-620. https://doi.org/10.1002/jhm.2231
29. Schaffer AC, Babayan A, Yu-Moe CW, Sato L, Einbinder JS. The effect of clinical volume on annual and per-patient encounter medical malpractice claims risk. J Patient Saf. Published online March 23, 2020. https://doi.org/10.1097/pts.0000000000000706
30. Wachter RM, Goldman L. The emerging role of “hospitalists” in the American health care system. N Engl J Med. 1996;335(7):514-517. https://doi.org/10.1056/nejm199608153350713
The prospect of facing a medical malpractice claim is a source of apprehension for physicians that affects physician behavior, including leading to defensive medicine.1-3 Overall, annual defensive medicine costs have been estimated at $45.6 billion,4 and surveys of hospitalists indicate that 13.0% to 37.5% of hospitalist healthcare costs involve defensive medicine.5,6 Despite the impact of malpractice concerns on hospitalist practice and the unprecedented growth of the field of hospital medicine, relatively few studies have examined the liability environment surrounding hospitalist practice.7,8 The specific issue of malpractice claims rates faced by hospitalists has received even less attention in the medical literature.8
A better understanding of the contributing factors and other attributes of malpractice claims can help guide patient safety initiatives and inform hospitalists’ level of concern regarding liability. Although most medical errors do not result in a malpractice claim,9,10 the majority of malpractice claims in which there is an indemnity payment involve medical injury due to clinician error.11 Even malpractice claims that do not result in an indemnity payment represent opportunities to identify patient safety and risk management vulnerabilities.12
We used a national malpractice claims database to analyze the characteristics of claims made against hospitalists, including claims rates. In addition to claims rates, we also analyzed the other types of providers named in hospitalist claims given the importance of interdisciplinary collaboration to hospital medicine.13,14 To provide context for understanding hospitalist liability data, we present data on other specialties. We also describe a model to predict whether hospitalist malpractice claims will close with an indemnity payment.
METHODS
Data Sources and Elements
This analysis used a repository of malpractice claims maintained by CRICO, the captive malpractice insurer of the Harvard-affiliated medical institutions. This database, the Comparative Benchmarking System, a
Injury severity was based on a widely used scale developed for malpractice claims by the National Association of Insurance Commissioners.16 Low injury severity included emotional injury and temporary insignificant injury. Medium injury severity included temporary minor, temporary major, and permanent minor injury. High injury severity included permanent significant injury through death. Because this study used a database assembled for operational and patient safety purposes and was not human subjects research, institutional review board approval was not needed.
Study Cohort
Malpractice claims included formal lawsuits or written requests for compensation for negligent medical care. Ho
Statistical Analysis
Malpractice claims rates were treated as Poisson rates and compared using a Z-test. Malpractice claims rates are expressed as claims per 100 physician-years. Each physician-year represents 1 year of coverage of one physician by the medical malpractice carrier whose data were used. Physician-years represent the duration of time physicians practiced during which they were insured by the malpractice carrier and, as such, could have been subject to a malpractice claim that would have been included in our data. Claims rates are based on the subset of the malpractice claims in the study for which the number of physician-years of coverage is available, representing 8.2% of hospitalist claims and 11.6% of all claims.
Comparisons of the percentages of cases closing with an indemnity payment, as well as the percentages of cases in different allegation type and clinical severity categories, were made using the Fisher exact test. Indemnity payment amounts were inflation-adjusted to 2018 dollars using the Consumer Price Index. Comparisons of indemnity payment amounts between physician specialties were carried out using the Wilcoxon rank sum test given that the distribution of the payment amounts appeared nonnormal; this was confirmed with the Shapiro-Wilk test. A multivariable logistic regression model was developed to predict the binary outcome of whether a hospitalist case would close with an indemnity payment (compared with no payment), based on the 1,216 hospitalist claims. The predictors used in this regression model were chosen a priori based on hypotheses about what factors drive the likelihood that a case closes with payment. Both the unadjusted and adjusted odds ratios for the predictors are presented. The adjusted model is adjusted for all the other predictors contained in the model. All reported P values are two-sided. The statistical analysis was carried out using JMP Pro version 15 (SAS Institute Inc) and Minitab version 19 (Minitab LLC).
RESULTS
We identified 1,216 hospital medicine malpractice claims from our database. Claims rates were calculated from the subset of our data for which physician-years were available—including 5,140 physician-years encompassing 100 claims, representing 8.2% of all hospitalist claims studied. An additional 18,644 malpractice claims from five other specialties—nonhospitalist general internal medicine, internal medicine subspecialists, emergency medicine, neurosurgery, and psychiatry—were analyzed to provide context for the hospitalist claims.
The malpractice claims rate for hospitalists was significantly higher than the rate for internal medicine subspecialists (1.95 vs 1.30 claims per 100 physician-years; P < .001), though they were not significantly different from the rate for nonhospitalist general internal medicine physicians (1.95 vs 1.92 claims per 100 physician-years; P = .93) (Table 1). Compared with emergency medicine physicians, with whom hospitalists are sometimes compared due to both specialties being defined by their site of practice and the absence of longitudinal patient relationships, hospitalists had a significantly lower claims rate (1.95 vs 4.07 claims per 100 physician-years; P < .001).

An assessment of the temporal trends in the claims rates, based on a comparison between the two halves of the study period (2014-2018 vs 2009-2013), showed that the claims rate for hospitalists was increasing, but at a rate that did not reach statistical significance (Table 1). In contrast, the claims rates for the five other specialties assessed decreased over time, and the decreases were significant for four of these five other specialties (internal medicine subspecialties, emergency medicine, neurosurgery, and psychiatry).
Multiple claims against a single physician were uncommon in our hospitalist malpractice claims data. Among the 100 claims that were used to calculate the claims rates, one physician was named in 2 claims, and all the other physicians were named in only a single claim. Among all of the 1,216 hospitalist malpractice claims we analyzed, there were eight physicians who were named in more than 1 claim, seven of whom were named in 2 claims, and one of whom was named in 3 claims.
The median indemnity payment for hospitalist claims was $231,454 (interquartile range [IQR], $100,000-$503,015), similar to the median indemnity payment for neurosurgery ($233,723; IQR, $85,292-$697,872), though significantly greater than the median indemnity payment for the other four specialties studied (Table 2). Among the hospitalist claims, 29.9% resulted in an indemnity payment, not significantly different from the rate for nonhospitalist general internal medicine, internal medicine subspecialties, or neurosurgery, but significantly lower than the rate for emergency medicine (33.8%; P = .011). No

We performed a multivariable logistic regression analysis to assess the effect of different factors on the likelihood of a hospitalist case closing with an indemnity payment, compared with no payment (Table 3). In the multivariable model, the presence of an error in clinical judgment had an adjusted odds ratio (AOR) of 5.01 (95% CI, 3.37-7.45; P < .001) for a claim closing with payment, the largest effect found. The presence of problems with communication (AOR, 1.89; 95% CI, 1.42-2.51; P < .001), the clinical environment (eg, weekend/holiday or clinical busyness; AOR, 1.70; 95% CI, 1.20-2.40; P = .0026), and documentation (AOR, 1.65; 95% CI, 1.18-2.31; P = .0038) were also positive predictors of claims closing with payment. Greater patient age (per decade) was a negative predictor of the likelihood of a claim closing with payment (AOR, 0.92; 95% CI, 0.86-0.998), though it was of borderline statistical significance (P = .044).

We also assessed multiple clinical attributes of hospitalist malpractice claims, including the major allegation type and injury severity (Appendix Table). Among the 1,216 hospitalist malpractice claims studied, the most common allegation types were for errors related to medical treatment (n = 482; 39.6%), diagnosis (n = 446; 36.7%), and medications (n = 157; 12.9%). Among the hospitalist claims, 888 (73.0%) involved high-severity injury, and 674 (55.4%) involved the death of the patient. The percentages of cases involving high-severity injury and death were significantly greater for hospitalists, compared with that of the other specialties studied (P < .001 for all pairwise comparisons). Of the six specialties studied, hospital medicine was the only one in which the percentage of cases involving death exceeded 50%.
Hospital medicine is typically team-based, and we evaluated which other services were named in claims with hospital medicine as the primary responsible service. The clinician groups most commonly named in hospitalist claims were nursing (n = 269; 22.1%), followed by emergency medicine (n = 91; 7.5%), general surgery (n = 51; 4.2%), cardiology (n = 49; 4.0%), and orthopedic surgery (n = 46; 3.8%) (Appendix Figure). During the first 2 years of the study period, no physician assistants (PAs) or nurse practitioners (NPs) were named in hospitalist claims. Over the study period, the proportion of hospitalist cases also naming PAs and NPs increased steadily, reaching 6.9% and 6.2% of claims, respectively, in 2018 (Figure) (P < .001 for NPs and P = .037 for PAs based on a comparison between the two halves of the study period).

DISCUSSION
We found that the average annual claims rate for hospitalists was similar to that for nonhospitalist general internists (1.95 vs 1.92 claims per 100 physician-years) but significantly greater than that for internal medicine subspecialists (1.95 vs 1.30 claims per 100 physician-years). Hospitalist claims rates showed a notable temporal trend—a nonsignificant increase—over the study period (2009-2018). This contrasts with the five other specialties studied, all of which had decreasing claims rates, four of which were significant. An analysis of a different national malpractice claims database, the NPDB, found that the rate of paid malpractice claims overall decreased 55.7% during the period 1992-2014, again contrasting with the trend we found for hospitalist claims rates.17
We posit several explanations for why the malpractice claims rate trend for hospitalists has diverged from that of other specialties. There has been a large expansion in the number of hospitalists in the United States.18 With this increasing demand, many young physicians have entered the hospital medicine field. In a survey of general internal medicine physicians conducted by the Society of General Internal Medicine, 73% of hospitalists were aged 25 to 44 years, significantly greater than the 45% in this age range among nonhospitalist general internal medicine physicians.19 Hospitalists in their first year of practice have higher mortality rates than more experienced hospitalists.20 Therefore, the relative inexperience of hospitalists, driven by this high demand, could be putting them at increased risk of medical errors and resulting malpractice claims. The higher mortality rate among hospitalists in their first year of practice could be due to a lack of familiarity with the systems of care, such as managing test results and obtaining appropriate consults.20 This possibility suggests that enhanced training and mentorship could be valuable as a strategy to both improve the quality of care and reduce medicolegal risk. The increasing demand for hospitalists could also be affecting the qualification level of physicians entering the field.
Our analysis also showed that the severity of injury in hospitalist claims was greater than that for the other specialties studied. In addition, the percentage of claims involving death was greater for hospitalists than that for the other specialties. The increased acuity of inpatients, compared with that of outpatients—and the trend, at least for some conditions, of increased inpatient acuity over time21,22— could account for the high injury severity seen among hospitalist claims. Given the positive correlation between injury severity and the size of indemnity payments made on malpractice claims,12 the high injury severity seen in hospitalist claims was very likely a driver of the high indemnity payments observed among the hospitalist claims.
The relationship between injury severity and financial outcomes is supported by the results of our multivariable regression model (Table 3). Compared with medium-severity injury claims, both death and high-severity injury cases were significantly more likely to close with an indemnity payment (compared with no payment), with AORs of 1.79 (95% CI, 1.21-2.65) and 2.44 (95% CI, 1.54-3.87), respectively.
The most striking finding in our regression model was the magnitude of the effect of an error in clinical judgement. Cases coded with this contributing factor had five times the AOR of closing with payment (compared with no payment) (AOR, 5.01; 95% CI, 3.37-7.45). A clinical judgment call may be difficult to defend when it is ultimately associated with a bad patient outcome. The importance of clinical judgment in our analysis suggests a risk management strategy: clearly and contemporaneously documenting the rationale behind one’s clinical decision-making. This may help make a claim more defensible in the event of an adverse outcome by demonstrating that the clinician was acting reasonably based on the information available at the time. The importance of specifying a rationale for a clinical decision may be especially important in the era of electronic health records (EHRs). EHRs are not structured as chronologically linear charts, which can make it challenging during a trial to retrospectively show what information was available to the physician at the time the clinical decision was made. The importance of clinical judgment also affirms the importance of effective clinical decision support as a patient safety tool.23
More broadly, it is notable that several contributing factors, including errors in clinical judgment (as discussed previously), problems with communication, and issues with the clinical environment, were significantly associated with malpractice cases closing with payment. This demonstrates that systematically examining malpractice claims to determine the underlying contributing factors can generate predictive analytics, as well as suggest risk management and patient safety strategies.
Interdisciplinary collaboration, as a component of systems-based practice, is a core principle of hospital medicine,13 and so we analyzed the involvement of other clinicians in hospitalist claims. Of the five specialties most frequently named in claims with hospitalists, two were surgical services: general surgery (n = 51; 4.2%) and orthopedic surgery (n = 46; 3.8%). With hospitalists being asked to play an increasing role in the care of surgical patients, they may be providing care to patient populations with whom they have less experience, which could put them at risk of adverse outcomes, leading to malpractice claims.24,25 Hospitalists need to be attuned to the liability risks related to the care of patients requiring surgical management and ensure areas of responsibility are clearly delineated between the hospital medicine and surgical services.26 We also found that hospitalist claims increasingly involve PAs and NPs, likely reflecting their increasing role in providing care on hospitalist services.27,28
A prior analysis of claims rates for hospitalists that covered injury dates from 1997 to 2011 found that hospitalists had a relatively low claims rate, significantly lower than that for other internal medicine physicians.8 In addition to covering an earlier time period, that analysis based its claims rates on data from academic medical centers covered by a single insurer, and physicians at academic medical centers generally have lower claims rates, likely due, at least in part, to their spending a smaller proportion of their time on patient care, compared with nonacademic physicians.29 Another analysis of hospitalist closed claims, which shared some cases with the cohort we analyzed, was performed by The Doctors Company, a commercial liability insurer.7 That analysis astutely emphasized the importance of breakdowns in diagnostic processes as a factor underlying hospitalist claims.
Our study has several limitations. First, although our database of malpractice claims includes approximately 31% of all the claims in the country and includes claims from every state, it may not be nationally representative. Another limitation relates to calculating the claims rates for physicians. Detailed information on the number of years of clinical activity, which is necessary to calculate claims rates, was available for only a subset of our data (8.2% of the hospitalist cases and 11.6% of all cases), so claims rates are based on this subset of our data (among which academic centers are overrepresented). Therefore, the claims rates should be interpreted with caution, especially regarding their application to the community hospital setting. The institutions included in the subset of our data used for determining claims rates were stable over time, so the use of a subset of our data for calculating claims rates reduces the generalizability of our claims rates but should not be a source of bias.
Potentially offsetting strengths of our claims database and study include the availability of unpaid claims (which outnumber paid claims roughly 2:1)11,12; the presence of information on contributing factors and other case characteristics obtained through structured manual review of the cases; and the availability of the specialties of the clinicians involved. These features distinguish the database we used from the NPDB, another national database of malpractice claims, which does not include unpaid claims and which does not include information on contributing factors or physician specialty.
CONCLUSION
First described in 1996, the hospitalist field is the fastest growing specialty in modern medical history.18,30 Therefore, an understanding of the malpractice risk of hospitalists is important and can shed light on the patient safety environment in hospitals. Our analysis showed that hospitalist malpractice claims rates remain roughly stable, in contrast to most other specialties, which have seen a fall in malpractice claims rates.17 In addition, unlike a previous analysis,8 we found that claims rates for hospitalists were essentially equal to those of other general internal medicine physicians (not lower, as had been previously reported), and higher than those of the internal medicine subspecialties. Hospitalist claims also have relatively high severity of injury. Potential factors driving these trends include the increasing demand for hospitalists, which results in a higher proportion of less-experienced physicians entering the field, and the expanding clinical scope of hospitalists, which may lead to their managing patients with conditions with which they may be less comfortable. Overall, our analysis suggests that the malpractice environment for hospitalists is becoming less favorable, and therefore, hospitalists should explore opportunities for mitigating liability risk and enhancing patient safety.
The prospect of facing a medical malpractice claim is a source of apprehension for physicians that affects physician behavior, including leading to defensive medicine.1-3 Overall, annual defensive medicine costs have been estimated at $45.6 billion,4 and surveys of hospitalists indicate that 13.0% to 37.5% of hospitalist healthcare costs involve defensive medicine.5,6 Despite the impact of malpractice concerns on hospitalist practice and the unprecedented growth of the field of hospital medicine, relatively few studies have examined the liability environment surrounding hospitalist practice.7,8 The specific issue of malpractice claims rates faced by hospitalists has received even less attention in the medical literature.8
A better understanding of the contributing factors and other attributes of malpractice claims can help guide patient safety initiatives and inform hospitalists’ level of concern regarding liability. Although most medical errors do not result in a malpractice claim,9,10 the majority of malpractice claims in which there is an indemnity payment involve medical injury due to clinician error.11 Even malpractice claims that do not result in an indemnity payment represent opportunities to identify patient safety and risk management vulnerabilities.12
We used a national malpractice claims database to analyze the characteristics of claims made against hospitalists, including claims rates. In addition to claims rates, we also analyzed the other types of providers named in hospitalist claims given the importance of interdisciplinary collaboration to hospital medicine.13,14 To provide context for understanding hospitalist liability data, we present data on other specialties. We also describe a model to predict whether hospitalist malpractice claims will close with an indemnity payment.
METHODS
Data Sources and Elements
This analysis used a repository of malpractice claims maintained by CRICO, the captive malpractice insurer of the Harvard-affiliated medical institutions. This database, the Comparative Benchmarking System, a
Injury severity was based on a widely used scale developed for malpractice claims by the National Association of Insurance Commissioners.16 Low injury severity included emotional injury and temporary insignificant injury. Medium injury severity included temporary minor, temporary major, and permanent minor injury. High injury severity included permanent significant injury through death. Because this study used a database assembled for operational and patient safety purposes and was not human subjects research, institutional review board approval was not needed.
Study Cohort
Malpractice claims included formal lawsuits or written requests for compensation for negligent medical care. Ho
Statistical Analysis
Malpractice claims rates were treated as Poisson rates and compared using a Z-test. Malpractice claims rates are expressed as claims per 100 physician-years. Each physician-year represents 1 year of coverage of one physician by the medical malpractice carrier whose data were used. Physician-years represent the duration of time physicians practiced during which they were insured by the malpractice carrier and, as such, could have been subject to a malpractice claim that would have been included in our data. Claims rates are based on the subset of the malpractice claims in the study for which the number of physician-years of coverage is available, representing 8.2% of hospitalist claims and 11.6% of all claims.
Comparisons of the percentages of cases closing with an indemnity payment, as well as the percentages of cases in different allegation type and clinical severity categories, were made using the Fisher exact test. Indemnity payment amounts were inflation-adjusted to 2018 dollars using the Consumer Price Index. Comparisons of indemnity payment amounts between physician specialties were carried out using the Wilcoxon rank sum test given that the distribution of the payment amounts appeared nonnormal; this was confirmed with the Shapiro-Wilk test. A multivariable logistic regression model was developed to predict the binary outcome of whether a hospitalist case would close with an indemnity payment (compared with no payment), based on the 1,216 hospitalist claims. The predictors used in this regression model were chosen a priori based on hypotheses about what factors drive the likelihood that a case closes with payment. Both the unadjusted and adjusted odds ratios for the predictors are presented. The adjusted model is adjusted for all the other predictors contained in the model. All reported P values are two-sided. The statistical analysis was carried out using JMP Pro version 15 (SAS Institute Inc) and Minitab version 19 (Minitab LLC).
RESULTS
We identified 1,216 hospital medicine malpractice claims from our database. Claims rates were calculated from the subset of our data for which physician-years were available—including 5,140 physician-years encompassing 100 claims, representing 8.2% of all hospitalist claims studied. An additional 18,644 malpractice claims from five other specialties—nonhospitalist general internal medicine, internal medicine subspecialists, emergency medicine, neurosurgery, and psychiatry—were analyzed to provide context for the hospitalist claims.
The malpractice claims rate for hospitalists was significantly higher than the rate for internal medicine subspecialists (1.95 vs 1.30 claims per 100 physician-years; P < .001), though they were not significantly different from the rate for nonhospitalist general internal medicine physicians (1.95 vs 1.92 claims per 100 physician-years; P = .93) (Table 1). Compared with emergency medicine physicians, with whom hospitalists are sometimes compared due to both specialties being defined by their site of practice and the absence of longitudinal patient relationships, hospitalists had a significantly lower claims rate (1.95 vs 4.07 claims per 100 physician-years; P < .001).

An assessment of the temporal trends in the claims rates, based on a comparison between the two halves of the study period (2014-2018 vs 2009-2013), showed that the claims rate for hospitalists was increasing, but at a rate that did not reach statistical significance (Table 1). In contrast, the claims rates for the five other specialties assessed decreased over time, and the decreases were significant for four of these five other specialties (internal medicine subspecialties, emergency medicine, neurosurgery, and psychiatry).
Multiple claims against a single physician were uncommon in our hospitalist malpractice claims data. Among the 100 claims that were used to calculate the claims rates, one physician was named in 2 claims, and all the other physicians were named in only a single claim. Among all of the 1,216 hospitalist malpractice claims we analyzed, there were eight physicians who were named in more than 1 claim, seven of whom were named in 2 claims, and one of whom was named in 3 claims.
The median indemnity payment for hospitalist claims was $231,454 (interquartile range [IQR], $100,000-$503,015), similar to the median indemnity payment for neurosurgery ($233,723; IQR, $85,292-$697,872), though significantly greater than the median indemnity payment for the other four specialties studied (Table 2). Among the hospitalist claims, 29.9% resulted in an indemnity payment, not significantly different from the rate for nonhospitalist general internal medicine, internal medicine subspecialties, or neurosurgery, but significantly lower than the rate for emergency medicine (33.8%; P = .011). No

We performed a multivariable logistic regression analysis to assess the effect of different factors on the likelihood of a hospitalist case closing with an indemnity payment, compared with no payment (Table 3). In the multivariable model, the presence of an error in clinical judgment had an adjusted odds ratio (AOR) of 5.01 (95% CI, 3.37-7.45; P < .001) for a claim closing with payment, the largest effect found. The presence of problems with communication (AOR, 1.89; 95% CI, 1.42-2.51; P < .001), the clinical environment (eg, weekend/holiday or clinical busyness; AOR, 1.70; 95% CI, 1.20-2.40; P = .0026), and documentation (AOR, 1.65; 95% CI, 1.18-2.31; P = .0038) were also positive predictors of claims closing with payment. Greater patient age (per decade) was a negative predictor of the likelihood of a claim closing with payment (AOR, 0.92; 95% CI, 0.86-0.998), though it was of borderline statistical significance (P = .044).

We also assessed multiple clinical attributes of hospitalist malpractice claims, including the major allegation type and injury severity (Appendix Table). Among the 1,216 hospitalist malpractice claims studied, the most common allegation types were for errors related to medical treatment (n = 482; 39.6%), diagnosis (n = 446; 36.7%), and medications (n = 157; 12.9%). Among the hospitalist claims, 888 (73.0%) involved high-severity injury, and 674 (55.4%) involved the death of the patient. The percentages of cases involving high-severity injury and death were significantly greater for hospitalists, compared with that of the other specialties studied (P < .001 for all pairwise comparisons). Of the six specialties studied, hospital medicine was the only one in which the percentage of cases involving death exceeded 50%.
Hospital medicine is typically team-based, and we evaluated which other services were named in claims with hospital medicine as the primary responsible service. The clinician groups most commonly named in hospitalist claims were nursing (n = 269; 22.1%), followed by emergency medicine (n = 91; 7.5%), general surgery (n = 51; 4.2%), cardiology (n = 49; 4.0%), and orthopedic surgery (n = 46; 3.8%) (Appendix Figure). During the first 2 years of the study period, no physician assistants (PAs) or nurse practitioners (NPs) were named in hospitalist claims. Over the study period, the proportion of hospitalist cases also naming PAs and NPs increased steadily, reaching 6.9% and 6.2% of claims, respectively, in 2018 (Figure) (P < .001 for NPs and P = .037 for PAs based on a comparison between the two halves of the study period).

DISCUSSION
We found that the average annual claims rate for hospitalists was similar to that for nonhospitalist general internists (1.95 vs 1.92 claims per 100 physician-years) but significantly greater than that for internal medicine subspecialists (1.95 vs 1.30 claims per 100 physician-years). Hospitalist claims rates showed a notable temporal trend—a nonsignificant increase—over the study period (2009-2018). This contrasts with the five other specialties studied, all of which had decreasing claims rates, four of which were significant. An analysis of a different national malpractice claims database, the NPDB, found that the rate of paid malpractice claims overall decreased 55.7% during the period 1992-2014, again contrasting with the trend we found for hospitalist claims rates.17
We posit several explanations for why the malpractice claims rate trend for hospitalists has diverged from that of other specialties. There has been a large expansion in the number of hospitalists in the United States.18 With this increasing demand, many young physicians have entered the hospital medicine field. In a survey of general internal medicine physicians conducted by the Society of General Internal Medicine, 73% of hospitalists were aged 25 to 44 years, significantly greater than the 45% in this age range among nonhospitalist general internal medicine physicians.19 Hospitalists in their first year of practice have higher mortality rates than more experienced hospitalists.20 Therefore, the relative inexperience of hospitalists, driven by this high demand, could be putting them at increased risk of medical errors and resulting malpractice claims. The higher mortality rate among hospitalists in their first year of practice could be due to a lack of familiarity with the systems of care, such as managing test results and obtaining appropriate consults.20 This possibility suggests that enhanced training and mentorship could be valuable as a strategy to both improve the quality of care and reduce medicolegal risk. The increasing demand for hospitalists could also be affecting the qualification level of physicians entering the field.
Our analysis also showed that the severity of injury in hospitalist claims was greater than that for the other specialties studied. In addition, the percentage of claims involving death was greater for hospitalists than that for the other specialties. The increased acuity of inpatients, compared with that of outpatients—and the trend, at least for some conditions, of increased inpatient acuity over time21,22— could account for the high injury severity seen among hospitalist claims. Given the positive correlation between injury severity and the size of indemnity payments made on malpractice claims,12 the high injury severity seen in hospitalist claims was very likely a driver of the high indemnity payments observed among the hospitalist claims.
The relationship between injury severity and financial outcomes is supported by the results of our multivariable regression model (Table 3). Compared with medium-severity injury claims, both death and high-severity injury cases were significantly more likely to close with an indemnity payment (compared with no payment), with AORs of 1.79 (95% CI, 1.21-2.65) and 2.44 (95% CI, 1.54-3.87), respectively.
The most striking finding in our regression model was the magnitude of the effect of an error in clinical judgement. Cases coded with this contributing factor had five times the AOR of closing with payment (compared with no payment) (AOR, 5.01; 95% CI, 3.37-7.45). A clinical judgment call may be difficult to defend when it is ultimately associated with a bad patient outcome. The importance of clinical judgment in our analysis suggests a risk management strategy: clearly and contemporaneously documenting the rationale behind one’s clinical decision-making. This may help make a claim more defensible in the event of an adverse outcome by demonstrating that the clinician was acting reasonably based on the information available at the time. The importance of specifying a rationale for a clinical decision may be especially important in the era of electronic health records (EHRs). EHRs are not structured as chronologically linear charts, which can make it challenging during a trial to retrospectively show what information was available to the physician at the time the clinical decision was made. The importance of clinical judgment also affirms the importance of effective clinical decision support as a patient safety tool.23
More broadly, it is notable that several contributing factors, including errors in clinical judgment (as discussed previously), problems with communication, and issues with the clinical environment, were significantly associated with malpractice cases closing with payment. This demonstrates that systematically examining malpractice claims to determine the underlying contributing factors can generate predictive analytics, as well as suggest risk management and patient safety strategies.
Interdisciplinary collaboration, as a component of systems-based practice, is a core principle of hospital medicine,13 and so we analyzed the involvement of other clinicians in hospitalist claims. Of the five specialties most frequently named in claims with hospitalists, two were surgical services: general surgery (n = 51; 4.2%) and orthopedic surgery (n = 46; 3.8%). With hospitalists being asked to play an increasing role in the care of surgical patients, they may be providing care to patient populations with whom they have less experience, which could put them at risk of adverse outcomes, leading to malpractice claims.24,25 Hospitalists need to be attuned to the liability risks related to the care of patients requiring surgical management and ensure areas of responsibility are clearly delineated between the hospital medicine and surgical services.26 We also found that hospitalist claims increasingly involve PAs and NPs, likely reflecting their increasing role in providing care on hospitalist services.27,28
A prior analysis of claims rates for hospitalists that covered injury dates from 1997 to 2011 found that hospitalists had a relatively low claims rate, significantly lower than that for other internal medicine physicians.8 In addition to covering an earlier time period, that analysis based its claims rates on data from academic medical centers covered by a single insurer, and physicians at academic medical centers generally have lower claims rates, likely due, at least in part, to their spending a smaller proportion of their time on patient care, compared with nonacademic physicians.29 Another analysis of hospitalist closed claims, which shared some cases with the cohort we analyzed, was performed by The Doctors Company, a commercial liability insurer.7 That analysis astutely emphasized the importance of breakdowns in diagnostic processes as a factor underlying hospitalist claims.
Our study has several limitations. First, although our database of malpractice claims includes approximately 31% of all the claims in the country and includes claims from every state, it may not be nationally representative. Another limitation relates to calculating the claims rates for physicians. Detailed information on the number of years of clinical activity, which is necessary to calculate claims rates, was available for only a subset of our data (8.2% of the hospitalist cases and 11.6% of all cases), so claims rates are based on this subset of our data (among which academic centers are overrepresented). Therefore, the claims rates should be interpreted with caution, especially regarding their application to the community hospital setting. The institutions included in the subset of our data used for determining claims rates were stable over time, so the use of a subset of our data for calculating claims rates reduces the generalizability of our claims rates but should not be a source of bias.
Potentially offsetting strengths of our claims database and study include the availability of unpaid claims (which outnumber paid claims roughly 2:1)11,12; the presence of information on contributing factors and other case characteristics obtained through structured manual review of the cases; and the availability of the specialties of the clinicians involved. These features distinguish the database we used from the NPDB, another national database of malpractice claims, which does not include unpaid claims and which does not include information on contributing factors or physician specialty.
CONCLUSION
First described in 1996, the hospitalist field is the fastest growing specialty in modern medical history.18,30 Therefore, an understanding of the malpractice risk of hospitalists is important and can shed light on the patient safety environment in hospitals. Our analysis showed that hospitalist malpractice claims rates remain roughly stable, in contrast to most other specialties, which have seen a fall in malpractice claims rates.17 In addition, unlike a previous analysis,8 we found that claims rates for hospitalists were essentially equal to those of other general internal medicine physicians (not lower, as had been previously reported), and higher than those of the internal medicine subspecialties. Hospitalist claims also have relatively high severity of injury. Potential factors driving these trends include the increasing demand for hospitalists, which results in a higher proportion of less-experienced physicians entering the field, and the expanding clinical scope of hospitalists, which may lead to their managing patients with conditions with which they may be less comfortable. Overall, our analysis suggests that the malpractice environment for hospitalists is becoming less favorable, and therefore, hospitalists should explore opportunities for mitigating liability risk and enhancing patient safety.
1. Studdert DM, Mello MM, Sage WM, et al. Defensive medicine among high-risk specialist physicians in a volatile malpractice environment. JAMA. 2005;293(21):2609-2617. https://doi.org/10.1001/jama.293.21.2609
2. Carrier ER, Reschovsky JD, Mello MM, Mayrell RC, Katz D. Physicians’ fears of malpractice lawsuits are not assuaged by tort reforms. Health Aff (Millwood). 2010;29(9):1585-1592. https://doi.org/10.1377/hlthaff.2010.0135
3. Kachalia A, Berg A, Fagerlin A, et al. Overuse of testing in preoperative evaluation and syncope: a survey of hospitalists. Ann Intern Med. 2015;162(2):100-108. https://doi.org/10.7326/m14-0694
4. Mello MM, Chandra A, Gawande AA, Studdert DM. National costs of the medical liability system. Health Aff (Millwood). 2010;29(9):1569-1577. https://doi.org/10.1377/hlthaff.2009.0807
5. Rothberg MB, Class J, Bishop TF, Friderici J, Kleppel R, Lindenauer PK. The cost of defensive medicine on 3 hospital medicine services. JAMA Intern Med. 2014;174(11):1867-1868. https://doi.org/10.1001/jamainternmed.2014.4649
6. Saint S, Vaughn VM, Chopra V, Fowler KE, Kachalia A. Perception of resources spent on defensive medicine and history of being sued among hospitalists: results from a national survey. J Hosp Med. 2018;13(1):26-29. https://doi.org/10.12788/jhm.2800
7. Ranum D, Troxel DB, Diamond R. Hospitalist Closed Claims Study: An Expert Analysis of Medical Malpractice Allegations. The Doctors Company. 2016. https://www.thedoctors.com/siteassets/pdfs/risk-management/closed-claims-studies/10392_ccs-hospitalist_academic_single-page_version_frr.pdf
8. Schaffer AC, Puopolo AL, Raman S, Kachalia A. Liability impact of the hospitalist model of care. J Hosp Med. 2014;9(12):750-755. https://doi.org/10.1002/jhm.2244
9. Localio AR, Lawthers AG, Brennan TA, et al. Relation between malpractice claims and adverse events due to negligence. results of the Harvard Medical Practice Study III. N Engl J Med. 1991;325(4):245-251. https://doi.org/10.1056/nejm199107253250405
10. Studdert DM, Thomas EJ, Burstin HR, Zbar BI, Orav EJ, Brennan TA. Negligent care and malpractice claiming behavior in Utah and Colorado. Med Care. 2000;38(3):250-260. https://doi.org/10.1097/00005650-200003000-00002
11. Studdert DM, Mello MM, Gawande AA, et al. Claims, errors, and compensation payments in medical malpractice litigation. N Engl J Med. 2006;354(19):2024-2033. https://doi.org/10.1056/nejmsa054479
12. Medical Malpractice in America: 2018 CRICO Strategies National CBS Report. CRICO Strategies; 2018.
13. Budnitz T, McKean SC. The Core Competencies in Hospital Medicine. In: McKean SC, Ross JJ, Dressler DD, Scheurer DB, eds. Principles and Practice of Hospital Medicine, 2nd ed. McGraw-Hill Education; 2017.
14. O’Leary KJ, Haviley C, Slade ME, Shah HM, Lee J, Williams MV. Improving teamwork: impact of structured interdisciplinary rounds on a hospitalist unit. J Hosp Med. 2011;6(2):88-93. https://doi.org/10.1002/jhm.714
15. National Practitioner Data Bank: Public Use Data File. Division of Practitioner Data Banks, Bureau of Health Professions, Health Resources & Services Administration, U.S. Department of Health & Human Services; June 30, 2019. Updated August 2020.
16. Sowka MP, ed. NAIC Malpractice Claims, Final Compilation. National Association of Insurance Commissioners; 1980.
17. Schaffer AC, Jena AB, Seabury SA, Singh H, Chalasani V, Kachalia A. Rates and characteristics of paid malpractice claims among US physicians by specialty, 1992-2014. JAMA Intern Med. 2017;177(5):710-718. https://doi.org/10.1001/jamainternmed.2017.0311
18. Wachter RM, Goldman L. Zero to 50,000 - the 20th anniversary of the hospitalist. N Engl J Med. 2016;375(11):1009-1011. https://doi.org/10.1056/nejmp1607958
19. Miller CS, Fogerty RL, Gann J, Bruti CP, Klein R; The Society of General Internal Medicine Membership Committee. The growth of hospitalists and the future of the Society of General Internal Medicine: results from the 2014 membership survey. J Gen Intern Med. 2017;32(11):1179-1185. https://doi.org/10.1007/s11606-017-4126-7
20. Goodwin JS, Salameh H, Zhou J, Singh S, Kuo YF, Nattinger AB. Association of hospitalist years of experience with mortality in the hospitalized Medicare population. JAMA Intern Med. 2018;178(2):196-203. https://doi.org/10.1001/jamainternmed.2017.7049
21. Akintoye E, Briasoulis A, Egbe A, et al. National trends in admission and in-hospital mortality of patients with heart failure in the United States (2001-2014). J Am Heart Assoc. 2017;6(12):e006955. https://doi.org/10.1161/jaha.117.006955
22. Clark AV, LoPresti CM, Smith TI. Trends in inpatient admission comorbidity and electronic health data: implications for resident workload intensity. J Hosp Med. 2018;13(8):570-572. https://doi.org/10.12788/jhm.2954
23. Gilmartin HM, Liu VX, Burke RE. Annals for hospitalists inpatient notes - The role of hospitalists in the creation of learning healthcare systems. Ann Intern Med. 2020;172(2):HO2-HO3. https://doi.org/10.7326/m19-3873
24. Siegal EM. Just because you can, doesn’t mean that you should: a call for the rational application of hospitalist comanagement. J Hosp Med. 2008;3(5):398-402. https://doi.org/10.1002/jhm.361
25. Plauth WH 3rd, Pantilat SZ, Wachter RM, Fenton CL. Hospitalists’ perceptions of their residency training needs: results of a national survey. Am J Med. 2001;111(3):247-254. https://doi.org/10.1016/s0002-9343(01)00837-3
26. Thompson RE, Pfeifer K, Grant PJ, et al. Hospital medicine and perioperative care: a framework for high-quality, high-value collaborative care. J Hosp Med. 2017;12(4):277-282. https://doi.org/10.12788/jhm.2717
27. Torok H, Lackner C, Landis R, Wright S. Learning needs of physician assistants working in hospital medicine. J Hosp Med. 2012;7(3):190-194. https://doi.org/10.1002/jhm.1001
28. Kartha A, Restuccia JD, Burgess JF Jr, et al. Nurse practitioner and physician assistant scope of practice in 118 acute care hospitals. J Hosp Med. 2014;9(10):615-620. https://doi.org/10.1002/jhm.2231
29. Schaffer AC, Babayan A, Yu-Moe CW, Sato L, Einbinder JS. The effect of clinical volume on annual and per-patient encounter medical malpractice claims risk. J Patient Saf. Published online March 23, 2020. https://doi.org/10.1097/pts.0000000000000706
30. Wachter RM, Goldman L. The emerging role of “hospitalists” in the American health care system. N Engl J Med. 1996;335(7):514-517. https://doi.org/10.1056/nejm199608153350713
1. Studdert DM, Mello MM, Sage WM, et al. Defensive medicine among high-risk specialist physicians in a volatile malpractice environment. JAMA. 2005;293(21):2609-2617. https://doi.org/10.1001/jama.293.21.2609
2. Carrier ER, Reschovsky JD, Mello MM, Mayrell RC, Katz D. Physicians’ fears of malpractice lawsuits are not assuaged by tort reforms. Health Aff (Millwood). 2010;29(9):1585-1592. https://doi.org/10.1377/hlthaff.2010.0135
3. Kachalia A, Berg A, Fagerlin A, et al. Overuse of testing in preoperative evaluation and syncope: a survey of hospitalists. Ann Intern Med. 2015;162(2):100-108. https://doi.org/10.7326/m14-0694
4. Mello MM, Chandra A, Gawande AA, Studdert DM. National costs of the medical liability system. Health Aff (Millwood). 2010;29(9):1569-1577. https://doi.org/10.1377/hlthaff.2009.0807
5. Rothberg MB, Class J, Bishop TF, Friderici J, Kleppel R, Lindenauer PK. The cost of defensive medicine on 3 hospital medicine services. JAMA Intern Med. 2014;174(11):1867-1868. https://doi.org/10.1001/jamainternmed.2014.4649
6. Saint S, Vaughn VM, Chopra V, Fowler KE, Kachalia A. Perception of resources spent on defensive medicine and history of being sued among hospitalists: results from a national survey. J Hosp Med. 2018;13(1):26-29. https://doi.org/10.12788/jhm.2800
7. Ranum D, Troxel DB, Diamond R. Hospitalist Closed Claims Study: An Expert Analysis of Medical Malpractice Allegations. The Doctors Company. 2016. https://www.thedoctors.com/siteassets/pdfs/risk-management/closed-claims-studies/10392_ccs-hospitalist_academic_single-page_version_frr.pdf
8. Schaffer AC, Puopolo AL, Raman S, Kachalia A. Liability impact of the hospitalist model of care. J Hosp Med. 2014;9(12):750-755. https://doi.org/10.1002/jhm.2244
9. Localio AR, Lawthers AG, Brennan TA, et al. Relation between malpractice claims and adverse events due to negligence. results of the Harvard Medical Practice Study III. N Engl J Med. 1991;325(4):245-251. https://doi.org/10.1056/nejm199107253250405
10. Studdert DM, Thomas EJ, Burstin HR, Zbar BI, Orav EJ, Brennan TA. Negligent care and malpractice claiming behavior in Utah and Colorado. Med Care. 2000;38(3):250-260. https://doi.org/10.1097/00005650-200003000-00002
11. Studdert DM, Mello MM, Gawande AA, et al. Claims, errors, and compensation payments in medical malpractice litigation. N Engl J Med. 2006;354(19):2024-2033. https://doi.org/10.1056/nejmsa054479
12. Medical Malpractice in America: 2018 CRICO Strategies National CBS Report. CRICO Strategies; 2018.
13. Budnitz T, McKean SC. The Core Competencies in Hospital Medicine. In: McKean SC, Ross JJ, Dressler DD, Scheurer DB, eds. Principles and Practice of Hospital Medicine, 2nd ed. McGraw-Hill Education; 2017.
14. O’Leary KJ, Haviley C, Slade ME, Shah HM, Lee J, Williams MV. Improving teamwork: impact of structured interdisciplinary rounds on a hospitalist unit. J Hosp Med. 2011;6(2):88-93. https://doi.org/10.1002/jhm.714
15. National Practitioner Data Bank: Public Use Data File. Division of Practitioner Data Banks, Bureau of Health Professions, Health Resources & Services Administration, U.S. Department of Health & Human Services; June 30, 2019. Updated August 2020.
16. Sowka MP, ed. NAIC Malpractice Claims, Final Compilation. National Association of Insurance Commissioners; 1980.
17. Schaffer AC, Jena AB, Seabury SA, Singh H, Chalasani V, Kachalia A. Rates and characteristics of paid malpractice claims among US physicians by specialty, 1992-2014. JAMA Intern Med. 2017;177(5):710-718. https://doi.org/10.1001/jamainternmed.2017.0311
18. Wachter RM, Goldman L. Zero to 50,000 - the 20th anniversary of the hospitalist. N Engl J Med. 2016;375(11):1009-1011. https://doi.org/10.1056/nejmp1607958
19. Miller CS, Fogerty RL, Gann J, Bruti CP, Klein R; The Society of General Internal Medicine Membership Committee. The growth of hospitalists and the future of the Society of General Internal Medicine: results from the 2014 membership survey. J Gen Intern Med. 2017;32(11):1179-1185. https://doi.org/10.1007/s11606-017-4126-7
20. Goodwin JS, Salameh H, Zhou J, Singh S, Kuo YF, Nattinger AB. Association of hospitalist years of experience with mortality in the hospitalized Medicare population. JAMA Intern Med. 2018;178(2):196-203. https://doi.org/10.1001/jamainternmed.2017.7049
21. Akintoye E, Briasoulis A, Egbe A, et al. National trends in admission and in-hospital mortality of patients with heart failure in the United States (2001-2014). J Am Heart Assoc. 2017;6(12):e006955. https://doi.org/10.1161/jaha.117.006955
22. Clark AV, LoPresti CM, Smith TI. Trends in inpatient admission comorbidity and electronic health data: implications for resident workload intensity. J Hosp Med. 2018;13(8):570-572. https://doi.org/10.12788/jhm.2954
23. Gilmartin HM, Liu VX, Burke RE. Annals for hospitalists inpatient notes - The role of hospitalists in the creation of learning healthcare systems. Ann Intern Med. 2020;172(2):HO2-HO3. https://doi.org/10.7326/m19-3873
24. Siegal EM. Just because you can, doesn’t mean that you should: a call for the rational application of hospitalist comanagement. J Hosp Med. 2008;3(5):398-402. https://doi.org/10.1002/jhm.361
25. Plauth WH 3rd, Pantilat SZ, Wachter RM, Fenton CL. Hospitalists’ perceptions of their residency training needs: results of a national survey. Am J Med. 2001;111(3):247-254. https://doi.org/10.1016/s0002-9343(01)00837-3
26. Thompson RE, Pfeifer K, Grant PJ, et al. Hospital medicine and perioperative care: a framework for high-quality, high-value collaborative care. J Hosp Med. 2017;12(4):277-282. https://doi.org/10.12788/jhm.2717
27. Torok H, Lackner C, Landis R, Wright S. Learning needs of physician assistants working in hospital medicine. J Hosp Med. 2012;7(3):190-194. https://doi.org/10.1002/jhm.1001
28. Kartha A, Restuccia JD, Burgess JF Jr, et al. Nurse practitioner and physician assistant scope of practice in 118 acute care hospitals. J Hosp Med. 2014;9(10):615-620. https://doi.org/10.1002/jhm.2231
29. Schaffer AC, Babayan A, Yu-Moe CW, Sato L, Einbinder JS. The effect of clinical volume on annual and per-patient encounter medical malpractice claims risk. J Patient Saf. Published online March 23, 2020. https://doi.org/10.1097/pts.0000000000000706
30. Wachter RM, Goldman L. The emerging role of “hospitalists” in the American health care system. N Engl J Med. 1996;335(7):514-517. https://doi.org/10.1056/nejm199608153350713
© 2021 Society of Hospital Medicine
Trauma-Informed Transformation of Evaluation and Licensure for Physicians With Mental Illness
As a physician living with bipolar disorder, I am more intimately familiar with the psychiatric ward than I would like to admit. Despite a decade of medication and weekly therapy, I live with a disease which flares, not unlike many of the patients I care for.
–Justin L Bullock, MD, MPH
Mental health conditions are common among physicians. Approximately 28% of residents experience depression or depressive symptoms during their training,1 and many will not seek mental health care due to fear of medical licensing implications.2 These fears are well-founded. Executive directors of 13 of 35 state medical boards indicated that diagnosis of mental illness alone was sufficient for sanctioning physicians.3 Another study found that two-thirds of state licensing applications pose questions about providers’ mental health that violate the American with Disabilities Act.2 What happens when a physician discloses and trusts the system to support their mental health–related needs? We address this question through the story of one trainee (JLB) recounting his experience with help-seeking and a fitness-for-duty (FFD) evaluation and conclude with recommendations to improve FFD processes.
Once again, I found myself sitting on a sheetless hospital bed with a baggy hospital gown draping my bony shoulders as I described my aborted suicide attempt to an attending psychiatrist. After a brief pause, the psychiatrist told me that they did not want to cause problems for my medical license and would drop my psychiatric hold. Even though they were the third psychiatrist to say this to me, their words caught me off guard given my recent attempt. I believe these psychiatrists factored my medical career into their clinical care because they understood that the simple act of being psychiatrically hospitalized placed my medical career in peril. After I completed a month-long outpatient treatment program, a psychiatrist and therapist cleared me to return to work.
I feel no shame about living with bipolar disorder and have always been transparent with my institution; the reason behind my absence was no secret. But before I could return to work, my case had to be reviewed by my institution’s Physician Well-being Committee. This committee was presented to me as a group that would help determine how to best support my return to work. However, before I had an opportunity to speak with the committee, I was informed that I would have to undergo a formal FFD evaluation.
FITNESS FOR DUTY
A FFD evaluation is indicated when there are credible reports of physician impairment or professional misconduct. Its purpose is to ensure that physicians can perform their essential job functions and that they are not a risk to patient safety. The Federation of State Medical Boards cautions that illness should not be conflated with impairment.4 Indeed, physicians with mental illness can function safely, thrive, and benefit patients and peers, especially when accommodations or modification reduce workplace barriers.5,6
I can best describe FFD as a 2-month-long stigmatized interrogation. I was forced to provide hair, blood, and urine samples for drug tests, complete an extensive multi-day psychiatric interview—including questions about my childhood trauma—and given a personality test. I was asked to disclose all of my private mental health records and feared I would be penalized if I refused to answer any questions. Multiple people, including my program director, confirmed that there were no performance or professionalism concerns. My suicide attempt happened outside of work; in my eyes, I had a mental illness which had been appropriately treated, not a workplace issue. I voiced my concern that the committee discriminated against me based on my mental illness. The committee told me that their decision to conduct a FFD was warranted because I had a condition that could affect my cognition, despite the lack of evidence that it actually had. My institution’s Office for Prevention of Harassment and Discrimination concurred that this was legal practice.
All of this happened despite my transparency regarding my bipolar disorder. Before I began residency, I disclosed my illness to my institution’s disability office, and I requested and received workplace accommodations. I have appropriately called-out of work whenever I felt that my bipolar could interfere with my focus on patient care. I have been openly bipolar and at the same institution for 6 years. In that time, I have received multiple teaching awards and a graduation award for exemplifying the qualities of a true physician—all while managing bipolar flares, including four hospitalizations. To destigmatize mental illness, I have discussed suicidality and getting help multiple times in front of hundreds of medical students.
The FFD evaluation found no evidence of a substance use disorder, nonadherence to treatment, or danger to my patients. Despite no adverse findings, and a record of well-managed mental illness and ongoing treatment, if I wanted to return to residency, I had to sign an agreement stipulating frequent monitoring by a “case-manager” and worksite “mentor.” I felt stigmatized and penalized for getting help. As I spoke out publicly against this process, I learned that my FFD experience is neither unique nor uncommon. Mental illness is deeply stigmatized within medicine. As a gay Black bipolar man, I hold multiple marginalized identities that inform and shape my experiences, yet the FFD committee did not have a single psychiatrist nor a single Black member. Instead, to make decisions on my case, they relied on the recommendations of the external psychiatrist, who met me for two days. In addition to the therapy and medications I had been taking for years, in order to return to work, I had to agree to a new type of therapy for the remainder of residency. I was incredulous that my employers felt it was appropriate to dictate my specific psychiatric care when I already had my own providers and my own care plan. My voice was not heard in this process, and despite my objections and the institutional mentors who spoke up for me, the FFD committee would not permit me to work without agreeing to their unmodified terms.
RE-ENVISIONING PHYSICIAN EVALUATION
Institutions face the challenging task of simultaneously navigating physician illness, patient safety, and institutional liability. In our opinion, many institutions excessively scrutinize physicians with mental illness and initiate FFD evaluations for reasons that are disproportionately skewed toward minimizing institutional liability. Moreover, in the absence of demonstrated physician workplace impairment, institutions should have systems in place to work collaboratively with the physician to ensure that they have access to professional treatment and appropriate workplace accommodations. It is possible to be simultaneously disabled and completely competent; creative and supportive accommodation processes allow physicians with disabilities to thrive, and their patients to benefit from the care of a physician with personal experience navigating disability. If a physician’s mental illness, despite accommodations, begins to impact workplace safety, a FFD evaluation may be initiated; unfortunately, the FFD evaluation itself may become a source of further harm.
FFD EVALUATIONS' POTENTIAL TO HARM PHYSICIANS WITH MENTAL ILLNESS
FFD evaluations often situate the physician as incapable of managing their own mental illness, suggesting that they must be closely monitored and restricted even when physicians come forward independently.7 These beliefs and concurrent policies can propagate harmful, inaccurate biases against physicians who live with mental illness. These biases are compounded by the structural racism endemic in healthcare and academic medicine.8 Dehumanization in medicine adds fuel to this fire, projecting the ideal physician persona as an invulnerable, infallible superhuman who can witness intense suffering and work inhumane hours without impact upon the their mental health and well-being. Altogether, these factors increase fear and discourage help-seeking among physicians with mental illness (Appendix Figure), ultimately harming physicians and patients in the process.9 Given that the FFD process involves evaluation, treatment, surveillance, and restrictions for individuals with stigmatized health conditions, these processes risk amplifying the impacts of trauma, racism, and oppression unless specifically designed to be antiracist, anti-oppressive, and trauma-informed.
TRAUMA-INFORMED APPROACH TO FFD
To encourage physician help-seeking, especially for stigmatized conditions, we must dismantle systems that traumatize physicians with mental illness (Appendix Figure) and build systems that invite and support courageous vulnerability and help-seeking.5,6 Institutions can provide evaluation and oversight of physicians while also adopting trauma-informed care principles. A trauma-informed approach to FFD would ask: How can we create systems that are informed by a genuine understanding of suffering to promote healing and avoid re-traumatization? Trauma-informed care emphasizes safety, trustworthiness, transparency, cultural humility (an antiracist, anti-oppression framework), collaboration, peer support, and patient empowerment.10 FFD evaluations differ by institution and by state, with some being performed internally and others utilizing external state physician health programs. We believe our recommendations below apply independent of context (Table).
NECESSARY CHANGES
Institutional Changes
All institutions must publish a detailed description of the FFD process, including its purpose, the definition of impairment or potential impairment, and the steps of the FFD evaluation. The FFD evaluation should be as limited in scope as possible, without invasive inquiry about the physician’s life-long history. Physicians should be invited to include a peer-support person throughout the entire process. The FFD “return to work agreement” should incorporate meaningful input from the physician as the expert in their own experience and, if already in treatment, informed by their healthcare providers. Given the stigmatization of mental health conditions and inherent power differentials in FFD processes, it is paramount that committees be diverse (including but not limited to race/ethnicity, gender, sexual orientation, and mental illness) and comprised of physicians trained in trauma-informed processes who treat the conditions affecting the individual undergoing the FFD evaluation. Finally, trustworthiness requires accountability: We recommend that all FFD systems establish an external oversight body that is equipped to effect change in real time if a physician reports a potential process violation and that collects anonymous feedback from physicians to inform required continuous quality improvement.
State and Federal Changes
It is difficult to effect meaningful change without accurate measurement of physician suicide. Therefore, we recommend mandatory reporting of physician suicide and suspected suicide to publicly available, de-identified state registries. We call for each state medical licensing board to limit licensing questions to current impairment due to mental illness, substance use disorders, or other health condition, as recommended by the Federation of State Medical Boards.4 There is a critical need for federal legislation to fund improvements in workplace safety and enhanced access to mental health treatment on demand for physicians.
Especially in these extraordinary times, as physicians are being exposed to such high burdens of stress, suffering, loss, and moral injury, we must de-stigmatize mental illness, encourage help-seeking, and provide physicians struggling with mental illness with timely and compassionate support. By creating systems that are healing and supportive for physicians, we enhance healing for all.
Bipolar is my sun and my storm. As a physician, I am not ashamed of that. For my own health and that of my patients, I must work in a system where it is safe to come forward when I am struggling.
As I fought against what I felt was a toxic and injurious process, I was fortunate to not stand alone. More than 600 residents from 18 departments at my institution signed a petition in support of reforming the FFD process informed by my experience. My institution is in the early stages of responding to this display of strength and unity with a diverse taskforce dedicated to improving the Physician Well-being Committee and FFD process.
As I accompany my patients on their healing journeys, my own experience with recovery allows me to hold the hope of healing for them. My family, friends, mentors, and providers held these rays of hope for me when I was lost in my own darkness. I now know that being cured from disease is just one form of healing. My proximity to death grounds me as some of my patients approach the end of life. Notably, some of my primary care patients have read my story online and come to their appointments to tell me that they are proud to have me as their doctor, “bipolar and all.”
1. Mata DA, Ramos MA, Bansal N, et al. Prevalence of depression and depressive symptoms among resident physicians a systematic review and meta-analysis. JAMA. 2015;314(22):2373-2383 . https://doi.org/10.1001/jama.2015.15845
2. Dyrbye LN, West CP, Sinsky CA, Goeders LE, Satele DV, Shanafelt TD. Medical Licensure questions and physician reluctance to seek care for mental health conditions. Mayo Clin Proc. 2017;92(10):1486-1493. https://doi.org/10.1016/j.mayocp.2017.06.020
3. Hendin H, Reynolds C, Fox D, et al. Licensing and physician mental health: problems and possibilities. J Med Licens Discip. 2007;93(2):6-11.
4. Federation of State Medical Boards. Physician wellness and burnout: report and recommendations of the workgroup on physician wellness and burnout. 2018. Accessed February 6, 2021. https://www.fsmb.org/siteassets/advocacy/policies/policy-on-wellness-and-burnout.pdf
5. Kirch D. Physician mental health: my personal journey and professional plea. Acad Med. 2021;96(5):618-620. https://doi.org/10.1097/ACM.0000000000003942
6. Cho HL, Huang CJ. Why mental health–related stigma matters for physician wellbeing, burnout, and patient care. J Gen Intern Med. 2020;24:1-3. https://doi.org/10.1007/s11606-019-05173-6
7. Hill AB. Breaking the stigma - a physician’s perspective on self-care and recovery. N Engl J Med. 2017;376(12):1103-1105. https://doi.org/10.1056/NEJMp1615974
8. Grubbs V. Diversity, equity, and inclusion that matter. N Engl J Med. 2020;383(4):e25. https://doi.org/10.1056/NEJMpv2022639
9. Shanafelt TD, Balch CM, Dyrbye L, et al. Special report: suicidal ideation among American surgeons. Arch Surg. 2011;146(1):54-62. https://doi.org/10.1001/archsurg.2010.292
10. Trauma-Informed Care Implementation Resource Center. Center for Healthcare Strategy. Accessed February 5, 2021. https://www.traumainformedcare.chcs.org/what-is-trauma-informed-care/
As a physician living with bipolar disorder, I am more intimately familiar with the psychiatric ward than I would like to admit. Despite a decade of medication and weekly therapy, I live with a disease which flares, not unlike many of the patients I care for.
–Justin L Bullock, MD, MPH
Mental health conditions are common among physicians. Approximately 28% of residents experience depression or depressive symptoms during their training,1 and many will not seek mental health care due to fear of medical licensing implications.2 These fears are well-founded. Executive directors of 13 of 35 state medical boards indicated that diagnosis of mental illness alone was sufficient for sanctioning physicians.3 Another study found that two-thirds of state licensing applications pose questions about providers’ mental health that violate the American with Disabilities Act.2 What happens when a physician discloses and trusts the system to support their mental health–related needs? We address this question through the story of one trainee (JLB) recounting his experience with help-seeking and a fitness-for-duty (FFD) evaluation and conclude with recommendations to improve FFD processes.
Once again, I found myself sitting on a sheetless hospital bed with a baggy hospital gown draping my bony shoulders as I described my aborted suicide attempt to an attending psychiatrist. After a brief pause, the psychiatrist told me that they did not want to cause problems for my medical license and would drop my psychiatric hold. Even though they were the third psychiatrist to say this to me, their words caught me off guard given my recent attempt. I believe these psychiatrists factored my medical career into their clinical care because they understood that the simple act of being psychiatrically hospitalized placed my medical career in peril. After I completed a month-long outpatient treatment program, a psychiatrist and therapist cleared me to return to work.
I feel no shame about living with bipolar disorder and have always been transparent with my institution; the reason behind my absence was no secret. But before I could return to work, my case had to be reviewed by my institution’s Physician Well-being Committee. This committee was presented to me as a group that would help determine how to best support my return to work. However, before I had an opportunity to speak with the committee, I was informed that I would have to undergo a formal FFD evaluation.
FITNESS FOR DUTY
A FFD evaluation is indicated when there are credible reports of physician impairment or professional misconduct. Its purpose is to ensure that physicians can perform their essential job functions and that they are not a risk to patient safety. The Federation of State Medical Boards cautions that illness should not be conflated with impairment.4 Indeed, physicians with mental illness can function safely, thrive, and benefit patients and peers, especially when accommodations or modification reduce workplace barriers.5,6
I can best describe FFD as a 2-month-long stigmatized interrogation. I was forced to provide hair, blood, and urine samples for drug tests, complete an extensive multi-day psychiatric interview—including questions about my childhood trauma—and given a personality test. I was asked to disclose all of my private mental health records and feared I would be penalized if I refused to answer any questions. Multiple people, including my program director, confirmed that there were no performance or professionalism concerns. My suicide attempt happened outside of work; in my eyes, I had a mental illness which had been appropriately treated, not a workplace issue. I voiced my concern that the committee discriminated against me based on my mental illness. The committee told me that their decision to conduct a FFD was warranted because I had a condition that could affect my cognition, despite the lack of evidence that it actually had. My institution’s Office for Prevention of Harassment and Discrimination concurred that this was legal practice.
All of this happened despite my transparency regarding my bipolar disorder. Before I began residency, I disclosed my illness to my institution’s disability office, and I requested and received workplace accommodations. I have appropriately called-out of work whenever I felt that my bipolar could interfere with my focus on patient care. I have been openly bipolar and at the same institution for 6 years. In that time, I have received multiple teaching awards and a graduation award for exemplifying the qualities of a true physician—all while managing bipolar flares, including four hospitalizations. To destigmatize mental illness, I have discussed suicidality and getting help multiple times in front of hundreds of medical students.
The FFD evaluation found no evidence of a substance use disorder, nonadherence to treatment, or danger to my patients. Despite no adverse findings, and a record of well-managed mental illness and ongoing treatment, if I wanted to return to residency, I had to sign an agreement stipulating frequent monitoring by a “case-manager” and worksite “mentor.” I felt stigmatized and penalized for getting help. As I spoke out publicly against this process, I learned that my FFD experience is neither unique nor uncommon. Mental illness is deeply stigmatized within medicine. As a gay Black bipolar man, I hold multiple marginalized identities that inform and shape my experiences, yet the FFD committee did not have a single psychiatrist nor a single Black member. Instead, to make decisions on my case, they relied on the recommendations of the external psychiatrist, who met me for two days. In addition to the therapy and medications I had been taking for years, in order to return to work, I had to agree to a new type of therapy for the remainder of residency. I was incredulous that my employers felt it was appropriate to dictate my specific psychiatric care when I already had my own providers and my own care plan. My voice was not heard in this process, and despite my objections and the institutional mentors who spoke up for me, the FFD committee would not permit me to work without agreeing to their unmodified terms.
RE-ENVISIONING PHYSICIAN EVALUATION
Institutions face the challenging task of simultaneously navigating physician illness, patient safety, and institutional liability. In our opinion, many institutions excessively scrutinize physicians with mental illness and initiate FFD evaluations for reasons that are disproportionately skewed toward minimizing institutional liability. Moreover, in the absence of demonstrated physician workplace impairment, institutions should have systems in place to work collaboratively with the physician to ensure that they have access to professional treatment and appropriate workplace accommodations. It is possible to be simultaneously disabled and completely competent; creative and supportive accommodation processes allow physicians with disabilities to thrive, and their patients to benefit from the care of a physician with personal experience navigating disability. If a physician’s mental illness, despite accommodations, begins to impact workplace safety, a FFD evaluation may be initiated; unfortunately, the FFD evaluation itself may become a source of further harm.
FFD EVALUATIONS' POTENTIAL TO HARM PHYSICIANS WITH MENTAL ILLNESS
FFD evaluations often situate the physician as incapable of managing their own mental illness, suggesting that they must be closely monitored and restricted even when physicians come forward independently.7 These beliefs and concurrent policies can propagate harmful, inaccurate biases against physicians who live with mental illness. These biases are compounded by the structural racism endemic in healthcare and academic medicine.8 Dehumanization in medicine adds fuel to this fire, projecting the ideal physician persona as an invulnerable, infallible superhuman who can witness intense suffering and work inhumane hours without impact upon the their mental health and well-being. Altogether, these factors increase fear and discourage help-seeking among physicians with mental illness (Appendix Figure), ultimately harming physicians and patients in the process.9 Given that the FFD process involves evaluation, treatment, surveillance, and restrictions for individuals with stigmatized health conditions, these processes risk amplifying the impacts of trauma, racism, and oppression unless specifically designed to be antiracist, anti-oppressive, and trauma-informed.
TRAUMA-INFORMED APPROACH TO FFD
To encourage physician help-seeking, especially for stigmatized conditions, we must dismantle systems that traumatize physicians with mental illness (Appendix Figure) and build systems that invite and support courageous vulnerability and help-seeking.5,6 Institutions can provide evaluation and oversight of physicians while also adopting trauma-informed care principles. A trauma-informed approach to FFD would ask: How can we create systems that are informed by a genuine understanding of suffering to promote healing and avoid re-traumatization? Trauma-informed care emphasizes safety, trustworthiness, transparency, cultural humility (an antiracist, anti-oppression framework), collaboration, peer support, and patient empowerment.10 FFD evaluations differ by institution and by state, with some being performed internally and others utilizing external state physician health programs. We believe our recommendations below apply independent of context (Table).

NECESSARY CHANGES
Institutional Changes
All institutions must publish a detailed description of the FFD process, including its purpose, the definition of impairment or potential impairment, and the steps of the FFD evaluation. The FFD evaluation should be as limited in scope as possible, without invasive inquiry about the physician’s life-long history. Physicians should be invited to include a peer-support person throughout the entire process. The FFD “return to work agreement” should incorporate meaningful input from the physician as the expert in their own experience and, if already in treatment, informed by their healthcare providers. Given the stigmatization of mental health conditions and inherent power differentials in FFD processes, it is paramount that committees be diverse (including but not limited to race/ethnicity, gender, sexual orientation, and mental illness) and comprised of physicians trained in trauma-informed processes who treat the conditions affecting the individual undergoing the FFD evaluation. Finally, trustworthiness requires accountability: We recommend that all FFD systems establish an external oversight body that is equipped to effect change in real time if a physician reports a potential process violation and that collects anonymous feedback from physicians to inform required continuous quality improvement.
State and Federal Changes
It is difficult to effect meaningful change without accurate measurement of physician suicide. Therefore, we recommend mandatory reporting of physician suicide and suspected suicide to publicly available, de-identified state registries. We call for each state medical licensing board to limit licensing questions to current impairment due to mental illness, substance use disorders, or other health condition, as recommended by the Federation of State Medical Boards.4 There is a critical need for federal legislation to fund improvements in workplace safety and enhanced access to mental health treatment on demand for physicians.
Especially in these extraordinary times, as physicians are being exposed to such high burdens of stress, suffering, loss, and moral injury, we must de-stigmatize mental illness, encourage help-seeking, and provide physicians struggling with mental illness with timely and compassionate support. By creating systems that are healing and supportive for physicians, we enhance healing for all.
Bipolar is my sun and my storm. As a physician, I am not ashamed of that. For my own health and that of my patients, I must work in a system where it is safe to come forward when I am struggling.
As I fought against what I felt was a toxic and injurious process, I was fortunate to not stand alone. More than 600 residents from 18 departments at my institution signed a petition in support of reforming the FFD process informed by my experience. My institution is in the early stages of responding to this display of strength and unity with a diverse taskforce dedicated to improving the Physician Well-being Committee and FFD process.
As I accompany my patients on their healing journeys, my own experience with recovery allows me to hold the hope of healing for them. My family, friends, mentors, and providers held these rays of hope for me when I was lost in my own darkness. I now know that being cured from disease is just one form of healing. My proximity to death grounds me as some of my patients approach the end of life. Notably, some of my primary care patients have read my story online and come to their appointments to tell me that they are proud to have me as their doctor, “bipolar and all.”
As a physician living with bipolar disorder, I am more intimately familiar with the psychiatric ward than I would like to admit. Despite a decade of medication and weekly therapy, I live with a disease which flares, not unlike many of the patients I care for.
–Justin L Bullock, MD, MPH
Mental health conditions are common among physicians. Approximately 28% of residents experience depression or depressive symptoms during their training,1 and many will not seek mental health care due to fear of medical licensing implications.2 These fears are well-founded. Executive directors of 13 of 35 state medical boards indicated that diagnosis of mental illness alone was sufficient for sanctioning physicians.3 Another study found that two-thirds of state licensing applications pose questions about providers’ mental health that violate the American with Disabilities Act.2 What happens when a physician discloses and trusts the system to support their mental health–related needs? We address this question through the story of one trainee (JLB) recounting his experience with help-seeking and a fitness-for-duty (FFD) evaluation and conclude with recommendations to improve FFD processes.
Once again, I found myself sitting on a sheetless hospital bed with a baggy hospital gown draping my bony shoulders as I described my aborted suicide attempt to an attending psychiatrist. After a brief pause, the psychiatrist told me that they did not want to cause problems for my medical license and would drop my psychiatric hold. Even though they were the third psychiatrist to say this to me, their words caught me off guard given my recent attempt. I believe these psychiatrists factored my medical career into their clinical care because they understood that the simple act of being psychiatrically hospitalized placed my medical career in peril. After I completed a month-long outpatient treatment program, a psychiatrist and therapist cleared me to return to work.
I feel no shame about living with bipolar disorder and have always been transparent with my institution; the reason behind my absence was no secret. But before I could return to work, my case had to be reviewed by my institution’s Physician Well-being Committee. This committee was presented to me as a group that would help determine how to best support my return to work. However, before I had an opportunity to speak with the committee, I was informed that I would have to undergo a formal FFD evaluation.
FITNESS FOR DUTY
A FFD evaluation is indicated when there are credible reports of physician impairment or professional misconduct. Its purpose is to ensure that physicians can perform their essential job functions and that they are not a risk to patient safety. The Federation of State Medical Boards cautions that illness should not be conflated with impairment.4 Indeed, physicians with mental illness can function safely, thrive, and benefit patients and peers, especially when accommodations or modification reduce workplace barriers.5,6
I can best describe FFD as a 2-month-long stigmatized interrogation. I was forced to provide hair, blood, and urine samples for drug tests, complete an extensive multi-day psychiatric interview—including questions about my childhood trauma—and given a personality test. I was asked to disclose all of my private mental health records and feared I would be penalized if I refused to answer any questions. Multiple people, including my program director, confirmed that there were no performance or professionalism concerns. My suicide attempt happened outside of work; in my eyes, I had a mental illness which had been appropriately treated, not a workplace issue. I voiced my concern that the committee discriminated against me based on my mental illness. The committee told me that their decision to conduct a FFD was warranted because I had a condition that could affect my cognition, despite the lack of evidence that it actually had. My institution’s Office for Prevention of Harassment and Discrimination concurred that this was legal practice.
All of this happened despite my transparency regarding my bipolar disorder. Before I began residency, I disclosed my illness to my institution’s disability office, and I requested and received workplace accommodations. I have appropriately called-out of work whenever I felt that my bipolar could interfere with my focus on patient care. I have been openly bipolar and at the same institution for 6 years. In that time, I have received multiple teaching awards and a graduation award for exemplifying the qualities of a true physician—all while managing bipolar flares, including four hospitalizations. To destigmatize mental illness, I have discussed suicidality and getting help multiple times in front of hundreds of medical students.
The FFD evaluation found no evidence of a substance use disorder, nonadherence to treatment, or danger to my patients. Despite no adverse findings, and a record of well-managed mental illness and ongoing treatment, if I wanted to return to residency, I had to sign an agreement stipulating frequent monitoring by a “case-manager” and worksite “mentor.” I felt stigmatized and penalized for getting help. As I spoke out publicly against this process, I learned that my FFD experience is neither unique nor uncommon. Mental illness is deeply stigmatized within medicine. As a gay Black bipolar man, I hold multiple marginalized identities that inform and shape my experiences, yet the FFD committee did not have a single psychiatrist nor a single Black member. Instead, to make decisions on my case, they relied on the recommendations of the external psychiatrist, who met me for two days. In addition to the therapy and medications I had been taking for years, in order to return to work, I had to agree to a new type of therapy for the remainder of residency. I was incredulous that my employers felt it was appropriate to dictate my specific psychiatric care when I already had my own providers and my own care plan. My voice was not heard in this process, and despite my objections and the institutional mentors who spoke up for me, the FFD committee would not permit me to work without agreeing to their unmodified terms.
RE-ENVISIONING PHYSICIAN EVALUATION
Institutions face the challenging task of simultaneously navigating physician illness, patient safety, and institutional liability. In our opinion, many institutions excessively scrutinize physicians with mental illness and initiate FFD evaluations for reasons that are disproportionately skewed toward minimizing institutional liability. Moreover, in the absence of demonstrated physician workplace impairment, institutions should have systems in place to work collaboratively with the physician to ensure that they have access to professional treatment and appropriate workplace accommodations. It is possible to be simultaneously disabled and completely competent; creative and supportive accommodation processes allow physicians with disabilities to thrive, and their patients to benefit from the care of a physician with personal experience navigating disability. If a physician’s mental illness, despite accommodations, begins to impact workplace safety, a FFD evaluation may be initiated; unfortunately, the FFD evaluation itself may become a source of further harm.
FFD EVALUATIONS' POTENTIAL TO HARM PHYSICIANS WITH MENTAL ILLNESS
FFD evaluations often situate the physician as incapable of managing their own mental illness, suggesting that they must be closely monitored and restricted even when physicians come forward independently.7 These beliefs and concurrent policies can propagate harmful, inaccurate biases against physicians who live with mental illness. These biases are compounded by the structural racism endemic in healthcare and academic medicine.8 Dehumanization in medicine adds fuel to this fire, projecting the ideal physician persona as an invulnerable, infallible superhuman who can witness intense suffering and work inhumane hours without impact upon the their mental health and well-being. Altogether, these factors increase fear and discourage help-seeking among physicians with mental illness (Appendix Figure), ultimately harming physicians and patients in the process.9 Given that the FFD process involves evaluation, treatment, surveillance, and restrictions for individuals with stigmatized health conditions, these processes risk amplifying the impacts of trauma, racism, and oppression unless specifically designed to be antiracist, anti-oppressive, and trauma-informed.
TRAUMA-INFORMED APPROACH TO FFD
To encourage physician help-seeking, especially for stigmatized conditions, we must dismantle systems that traumatize physicians with mental illness (Appendix Figure) and build systems that invite and support courageous vulnerability and help-seeking.5,6 Institutions can provide evaluation and oversight of physicians while also adopting trauma-informed care principles. A trauma-informed approach to FFD would ask: How can we create systems that are informed by a genuine understanding of suffering to promote healing and avoid re-traumatization? Trauma-informed care emphasizes safety, trustworthiness, transparency, cultural humility (an antiracist, anti-oppression framework), collaboration, peer support, and patient empowerment.10 FFD evaluations differ by institution and by state, with some being performed internally and others utilizing external state physician health programs. We believe our recommendations below apply independent of context (Table).

NECESSARY CHANGES
Institutional Changes
All institutions must publish a detailed description of the FFD process, including its purpose, the definition of impairment or potential impairment, and the steps of the FFD evaluation. The FFD evaluation should be as limited in scope as possible, without invasive inquiry about the physician’s life-long history. Physicians should be invited to include a peer-support person throughout the entire process. The FFD “return to work agreement” should incorporate meaningful input from the physician as the expert in their own experience and, if already in treatment, informed by their healthcare providers. Given the stigmatization of mental health conditions and inherent power differentials in FFD processes, it is paramount that committees be diverse (including but not limited to race/ethnicity, gender, sexual orientation, and mental illness) and comprised of physicians trained in trauma-informed processes who treat the conditions affecting the individual undergoing the FFD evaluation. Finally, trustworthiness requires accountability: We recommend that all FFD systems establish an external oversight body that is equipped to effect change in real time if a physician reports a potential process violation and that collects anonymous feedback from physicians to inform required continuous quality improvement.
State and Federal Changes
It is difficult to effect meaningful change without accurate measurement of physician suicide. Therefore, we recommend mandatory reporting of physician suicide and suspected suicide to publicly available, de-identified state registries. We call for each state medical licensing board to limit licensing questions to current impairment due to mental illness, substance use disorders, or other health condition, as recommended by the Federation of State Medical Boards.4 There is a critical need for federal legislation to fund improvements in workplace safety and enhanced access to mental health treatment on demand for physicians.
Especially in these extraordinary times, as physicians are being exposed to such high burdens of stress, suffering, loss, and moral injury, we must de-stigmatize mental illness, encourage help-seeking, and provide physicians struggling with mental illness with timely and compassionate support. By creating systems that are healing and supportive for physicians, we enhance healing for all.
Bipolar is my sun and my storm. As a physician, I am not ashamed of that. For my own health and that of my patients, I must work in a system where it is safe to come forward when I am struggling.
As I fought against what I felt was a toxic and injurious process, I was fortunate to not stand alone. More than 600 residents from 18 departments at my institution signed a petition in support of reforming the FFD process informed by my experience. My institution is in the early stages of responding to this display of strength and unity with a diverse taskforce dedicated to improving the Physician Well-being Committee and FFD process.
As I accompany my patients on their healing journeys, my own experience with recovery allows me to hold the hope of healing for them. My family, friends, mentors, and providers held these rays of hope for me when I was lost in my own darkness. I now know that being cured from disease is just one form of healing. My proximity to death grounds me as some of my patients approach the end of life. Notably, some of my primary care patients have read my story online and come to their appointments to tell me that they are proud to have me as their doctor, “bipolar and all.”
1. Mata DA, Ramos MA, Bansal N, et al. Prevalence of depression and depressive symptoms among resident physicians a systematic review and meta-analysis. JAMA. 2015;314(22):2373-2383 . https://doi.org/10.1001/jama.2015.15845
2. Dyrbye LN, West CP, Sinsky CA, Goeders LE, Satele DV, Shanafelt TD. Medical Licensure questions and physician reluctance to seek care for mental health conditions. Mayo Clin Proc. 2017;92(10):1486-1493. https://doi.org/10.1016/j.mayocp.2017.06.020
3. Hendin H, Reynolds C, Fox D, et al. Licensing and physician mental health: problems and possibilities. J Med Licens Discip. 2007;93(2):6-11.
4. Federation of State Medical Boards. Physician wellness and burnout: report and recommendations of the workgroup on physician wellness and burnout. 2018. Accessed February 6, 2021. https://www.fsmb.org/siteassets/advocacy/policies/policy-on-wellness-and-burnout.pdf
5. Kirch D. Physician mental health: my personal journey and professional plea. Acad Med. 2021;96(5):618-620. https://doi.org/10.1097/ACM.0000000000003942
6. Cho HL, Huang CJ. Why mental health–related stigma matters for physician wellbeing, burnout, and patient care. J Gen Intern Med. 2020;24:1-3. https://doi.org/10.1007/s11606-019-05173-6
7. Hill AB. Breaking the stigma - a physician’s perspective on self-care and recovery. N Engl J Med. 2017;376(12):1103-1105. https://doi.org/10.1056/NEJMp1615974
8. Grubbs V. Diversity, equity, and inclusion that matter. N Engl J Med. 2020;383(4):e25. https://doi.org/10.1056/NEJMpv2022639
9. Shanafelt TD, Balch CM, Dyrbye L, et al. Special report: suicidal ideation among American surgeons. Arch Surg. 2011;146(1):54-62. https://doi.org/10.1001/archsurg.2010.292
10. Trauma-Informed Care Implementation Resource Center. Center for Healthcare Strategy. Accessed February 5, 2021. https://www.traumainformedcare.chcs.org/what-is-trauma-informed-care/
1. Mata DA, Ramos MA, Bansal N, et al. Prevalence of depression and depressive symptoms among resident physicians a systematic review and meta-analysis. JAMA. 2015;314(22):2373-2383 . https://doi.org/10.1001/jama.2015.15845
2. Dyrbye LN, West CP, Sinsky CA, Goeders LE, Satele DV, Shanafelt TD. Medical Licensure questions and physician reluctance to seek care for mental health conditions. Mayo Clin Proc. 2017;92(10):1486-1493. https://doi.org/10.1016/j.mayocp.2017.06.020
3. Hendin H, Reynolds C, Fox D, et al. Licensing and physician mental health: problems and possibilities. J Med Licens Discip. 2007;93(2):6-11.
4. Federation of State Medical Boards. Physician wellness and burnout: report and recommendations of the workgroup on physician wellness and burnout. 2018. Accessed February 6, 2021. https://www.fsmb.org/siteassets/advocacy/policies/policy-on-wellness-and-burnout.pdf
5. Kirch D. Physician mental health: my personal journey and professional plea. Acad Med. 2021;96(5):618-620. https://doi.org/10.1097/ACM.0000000000003942
6. Cho HL, Huang CJ. Why mental health–related stigma matters for physician wellbeing, burnout, and patient care. J Gen Intern Med. 2020;24:1-3. https://doi.org/10.1007/s11606-019-05173-6
7. Hill AB. Breaking the stigma - a physician’s perspective on self-care and recovery. N Engl J Med. 2017;376(12):1103-1105. https://doi.org/10.1056/NEJMp1615974
8. Grubbs V. Diversity, equity, and inclusion that matter. N Engl J Med. 2020;383(4):e25. https://doi.org/10.1056/NEJMpv2022639
9. Shanafelt TD, Balch CM, Dyrbye L, et al. Special report: suicidal ideation among American surgeons. Arch Surg. 2011;146(1):54-62. https://doi.org/10.1001/archsurg.2010.292
10. Trauma-Informed Care Implementation Resource Center. Center for Healthcare Strategy. Accessed February 5, 2021. https://www.traumainformedcare.chcs.org/what-is-trauma-informed-care/
© 2021 Society of Hospital Medicine
Benzene was found in some sunscreens. Now what?
Just before Memorial Day, which include batches from Neutrogena, Banana Boat, CVS Health, and other brands. More than three-quarters of the products are sprays.
“We’re asking our patients to put sunscreen on from 6 months of age, telling them to do it their entire life, their whole body, multiple times a day,” Christopher G. Bunick, MD, PhD, associate professor of dermatology at Yale University, New Haven, Conn., said in an interview. If benzene-contaminated sunscreen proves to be a widespread problem, he said, “the benzene amounts can add up to a significant chronic exposure over a lifetime.”
In the Valisure statement announcing the findings, Dr. Bunick, who is also quoted in the petition, said that “it is critical that regulatory agencies address benzene contamination in sunscreens, and all topical medications at the manufacturing and final product level, so that all individuals feel safe using sunscreen products.”
The list of products that tested positive is included in the citizen petition, and a full list of products that did not show any contamination is available in an attachment.
Benzene is not an ingredient in sunscreen, and Valisure’s petition suggests that the findings are a result of contamination somewhere in the manufacturing process, not of product degradation.
“This isn’t a sunscreen issue, it’s a manufacturing issue,” said Adam Friedman, MD, professor and chief of dermatology at George Washington University, Washington. “We don’t want those things to be blurred.”
When asked to comment on Valisure’s findings, an FDA spokesperson said, “The FDA takes seriously any safety concerns raised about products we regulate, including sunscreen. While the agency evaluates the submitted citizen petition, we will continue to monitor the sunscreen marketplace and manufacturing efforts to help ensure the availability of safe sunscreens for U.S. consumers.”
Both Johnson & Johnson, Neutrogena’s parent company, and Banana Boat issued statements reiterating that benzene is not an ingredient in their products.
Assessing the risks
There is a risk of patients taking away the wrong message from these findings.
“People already have ambivalence about sunscreen, and this is just going to make that worse,” Dr. Friedman said in an interview. He pointed out that benzene is present in car exhaust, second-hand smoke, and elsewhere. Inhalation exposure has been the primary focus of toxicology investigations, as has exposure from ingesting things such as contaminated drinking water – not via topical application. “We don’t know how effectively [benzene] gets through the skin, if it gets absorbed systemically, and how that then behaves downstream,” he noted.
On the other hand, ultraviolet radiation is a well-established carcinogen. Avoiding an effective preventive measure such as sunscreen could prove more harmful than exposure to trace amounts of benzene, ultimately to be determined by the FDA.
“Just because those particular products do pose a risk, that doesn’t erase the message that sunscreens are safe and should be used,” Dr. Bunick said. “It’s not mutually exclusive.”
And then there’s the fact that the benzene contamination appears to be fairly limited. “The majority of products we tested, over 200 of them, had no detectable amounts of benzene, and uncontaminated sunscreen should certainly continue to be used,” David Light, CEO of Valisure, told this news organization.
Advising patients
With headlines blaring the news about a carcinogen in sunscreen, patients will be reaching out for advice.
“The number one question patients will have is, ‘What sunscreen do you recommend?’” said Dr. Bunick. “The answer should be to pick a sunscreen that we know wasn’t contaminated. Reassure your patient the ingredients themselves are effective and safe, and that’s not what’s leading to the contamination.”
Dr. Friedman agrees. “We need to be mindful. Dermatologists need to be armed with the facts in order to counsel patients: Sunscreen is still a very important, effective, and safe, scientifically based way to prevent the harmful effects of the sun, in addition to things like sun protective clothing and seeking shade between 10 a.m. and 4 p.m.”
As alarming as Valisure’s findings may seem initially, Dr. Bunick noted a silver lining. “The consumer, the public should feel reassured this report is out there. It shows that someone’s watching out. That’s an important safety message: These things aren’t going undetected.”
Just before Memorial Day, which include batches from Neutrogena, Banana Boat, CVS Health, and other brands. More than three-quarters of the products are sprays.
“We’re asking our patients to put sunscreen on from 6 months of age, telling them to do it their entire life, their whole body, multiple times a day,” Christopher G. Bunick, MD, PhD, associate professor of dermatology at Yale University, New Haven, Conn., said in an interview. If benzene-contaminated sunscreen proves to be a widespread problem, he said, “the benzene amounts can add up to a significant chronic exposure over a lifetime.”
In the Valisure statement announcing the findings, Dr. Bunick, who is also quoted in the petition, said that “it is critical that regulatory agencies address benzene contamination in sunscreens, and all topical medications at the manufacturing and final product level, so that all individuals feel safe using sunscreen products.”
The list of products that tested positive is included in the citizen petition, and a full list of products that did not show any contamination is available in an attachment.
Benzene is not an ingredient in sunscreen, and Valisure’s petition suggests that the findings are a result of contamination somewhere in the manufacturing process, not of product degradation.
“This isn’t a sunscreen issue, it’s a manufacturing issue,” said Adam Friedman, MD, professor and chief of dermatology at George Washington University, Washington. “We don’t want those things to be blurred.”
When asked to comment on Valisure’s findings, an FDA spokesperson said, “The FDA takes seriously any safety concerns raised about products we regulate, including sunscreen. While the agency evaluates the submitted citizen petition, we will continue to monitor the sunscreen marketplace and manufacturing efforts to help ensure the availability of safe sunscreens for U.S. consumers.”
Both Johnson & Johnson, Neutrogena’s parent company, and Banana Boat issued statements reiterating that benzene is not an ingredient in their products.
Assessing the risks
There is a risk of patients taking away the wrong message from these findings.
“People already have ambivalence about sunscreen, and this is just going to make that worse,” Dr. Friedman said in an interview. He pointed out that benzene is present in car exhaust, second-hand smoke, and elsewhere. Inhalation exposure has been the primary focus of toxicology investigations, as has exposure from ingesting things such as contaminated drinking water – not via topical application. “We don’t know how effectively [benzene] gets through the skin, if it gets absorbed systemically, and how that then behaves downstream,” he noted.
On the other hand, ultraviolet radiation is a well-established carcinogen. Avoiding an effective preventive measure such as sunscreen could prove more harmful than exposure to trace amounts of benzene, ultimately to be determined by the FDA.
“Just because those particular products do pose a risk, that doesn’t erase the message that sunscreens are safe and should be used,” Dr. Bunick said. “It’s not mutually exclusive.”
And then there’s the fact that the benzene contamination appears to be fairly limited. “The majority of products we tested, over 200 of them, had no detectable amounts of benzene, and uncontaminated sunscreen should certainly continue to be used,” David Light, CEO of Valisure, told this news organization.
Advising patients
With headlines blaring the news about a carcinogen in sunscreen, patients will be reaching out for advice.
“The number one question patients will have is, ‘What sunscreen do you recommend?’” said Dr. Bunick. “The answer should be to pick a sunscreen that we know wasn’t contaminated. Reassure your patient the ingredients themselves are effective and safe, and that’s not what’s leading to the contamination.”
Dr. Friedman agrees. “We need to be mindful. Dermatologists need to be armed with the facts in order to counsel patients: Sunscreen is still a very important, effective, and safe, scientifically based way to prevent the harmful effects of the sun, in addition to things like sun protective clothing and seeking shade between 10 a.m. and 4 p.m.”
As alarming as Valisure’s findings may seem initially, Dr. Bunick noted a silver lining. “The consumer, the public should feel reassured this report is out there. It shows that someone’s watching out. That’s an important safety message: These things aren’t going undetected.”
Just before Memorial Day, which include batches from Neutrogena, Banana Boat, CVS Health, and other brands. More than three-quarters of the products are sprays.
“We’re asking our patients to put sunscreen on from 6 months of age, telling them to do it their entire life, their whole body, multiple times a day,” Christopher G. Bunick, MD, PhD, associate professor of dermatology at Yale University, New Haven, Conn., said in an interview. If benzene-contaminated sunscreen proves to be a widespread problem, he said, “the benzene amounts can add up to a significant chronic exposure over a lifetime.”
In the Valisure statement announcing the findings, Dr. Bunick, who is also quoted in the petition, said that “it is critical that regulatory agencies address benzene contamination in sunscreens, and all topical medications at the manufacturing and final product level, so that all individuals feel safe using sunscreen products.”
The list of products that tested positive is included in the citizen petition, and a full list of products that did not show any contamination is available in an attachment.
Benzene is not an ingredient in sunscreen, and Valisure’s petition suggests that the findings are a result of contamination somewhere in the manufacturing process, not of product degradation.
“This isn’t a sunscreen issue, it’s a manufacturing issue,” said Adam Friedman, MD, professor and chief of dermatology at George Washington University, Washington. “We don’t want those things to be blurred.”
When asked to comment on Valisure’s findings, an FDA spokesperson said, “The FDA takes seriously any safety concerns raised about products we regulate, including sunscreen. While the agency evaluates the submitted citizen petition, we will continue to monitor the sunscreen marketplace and manufacturing efforts to help ensure the availability of safe sunscreens for U.S. consumers.”
Both Johnson & Johnson, Neutrogena’s parent company, and Banana Boat issued statements reiterating that benzene is not an ingredient in their products.
Assessing the risks
There is a risk of patients taking away the wrong message from these findings.
“People already have ambivalence about sunscreen, and this is just going to make that worse,” Dr. Friedman said in an interview. He pointed out that benzene is present in car exhaust, second-hand smoke, and elsewhere. Inhalation exposure has been the primary focus of toxicology investigations, as has exposure from ingesting things such as contaminated drinking water – not via topical application. “We don’t know how effectively [benzene] gets through the skin, if it gets absorbed systemically, and how that then behaves downstream,” he noted.
On the other hand, ultraviolet radiation is a well-established carcinogen. Avoiding an effective preventive measure such as sunscreen could prove more harmful than exposure to trace amounts of benzene, ultimately to be determined by the FDA.
“Just because those particular products do pose a risk, that doesn’t erase the message that sunscreens are safe and should be used,” Dr. Bunick said. “It’s not mutually exclusive.”
And then there’s the fact that the benzene contamination appears to be fairly limited. “The majority of products we tested, over 200 of them, had no detectable amounts of benzene, and uncontaminated sunscreen should certainly continue to be used,” David Light, CEO of Valisure, told this news organization.
Advising patients
With headlines blaring the news about a carcinogen in sunscreen, patients will be reaching out for advice.
“The number one question patients will have is, ‘What sunscreen do you recommend?’” said Dr. Bunick. “The answer should be to pick a sunscreen that we know wasn’t contaminated. Reassure your patient the ingredients themselves are effective and safe, and that’s not what’s leading to the contamination.”
Dr. Friedman agrees. “We need to be mindful. Dermatologists need to be armed with the facts in order to counsel patients: Sunscreen is still a very important, effective, and safe, scientifically based way to prevent the harmful effects of the sun, in addition to things like sun protective clothing and seeking shade between 10 a.m. and 4 p.m.”
As alarming as Valisure’s findings may seem initially, Dr. Bunick noted a silver lining. “The consumer, the public should feel reassured this report is out there. It shows that someone’s watching out. That’s an important safety message: These things aren’t going undetected.”
Vinorelbine survival benefit in mesothelioma overshadowed by advances in immuno-oncology
After decades of off-label use, vinorelbine finally has a randomized clinical trial supporting its efficacy as a second-line treatment of mesothelioma, an investigator reported at the annual meeting of the American Society of Clinical Oncology.
However, the development of other treatment regimens, and notably immuno-oncology approaches, are pushing this classic chemotherapy option to later lines of therapy in patients with malignant pleural mesothelioma (MPM), a speaker said at the meeting.
Adding vinorelbine to active symptom control statistically improved progression-free survival (PFS), among patients with MPM who had prior platinum-based therapy, according to results of the randomized Vinorelbine in Mesothelioma (VIM) trial.
Median PFS reached 4.2 months in the vinorelbine arm, versus 2.8 months for active symptom control alone, study results show.
That finding , coupled with safety results, supports the off-label use of vinorelbine as a treatment option for patients with relapsed MPM, according to investigator Dean Anthony Fennell, FRCP, PhD, of the University of Leicester (England).
“Vinorelbine appears to be a safe and effective treatment and could be considered as a treatment option for patients with relapsed mesothelioma,” Prof. Fennell said in his presentation at ASCO (Abstract 8507).
Changing status
While that welcome pronouncement was a long time coming, there are now other promising treatment approaches that relegate vinorelbine to a “lower priority” in the treatment algorithm, said discussant Anna K. Nowak, MBBS, FRACP, PhD, of the University of Western Australia, Nedlands.
In October 2020, the U.S. Food and Drug Administration approved the combination of ipilimumab and nivolumab for the first-line treatment of unresectable malignant pleural mesothelioma, on the basis of results from CHECKMATE-743, a randomized, open-label trial.
“Certainly, we know now that first-line ipilimumab and nivolumab is of very substantial benefit to these patients, and we still have clinical trials open in this space as well,” Prof. Nowak said in her discussion at ASCO.
There is “no doubt” that many patients with mesothelioma should at some point receive an IO agent, particularly now with recently reported randomized clinical trial evidence of an overall survival benefit, she added.
“This really pushes vinorelbine out to be a third- or fourth-line treatment,” she added, “and we know that there are usually diminishing returns from using chemotherapies further down the treatment algorithm.”
Trial details
The VIM trial described at ASCO by Prof. Fennell was a randomized, controlled phase 2 including 154 patients with MPM that had progressed after first-line chemotherapy.
“Vinorelbine has shown useful clinical activity in single-arm phase two studies, however, the specific efficacy of vinorelbine has not been evaluated in an appropriately controlled randomized trial,” Prof. Fennell said in this presentation.
Patients in the trial were randomized 2:1 to either vinorelbine plus active supportive care or active supportive care alone. Vinorelbine was given initially at 60 mg/m2 weekly every 21 days, escalated to 80 mg/m2 from cycle 2.
The median age of patients in VIM was approximately 71 years, and about 80% were male.
More partial responses were seen in the vinorelbine arm, at 3.1% of patients, compared with 1.8% for active supportive care, according to Prof. Fennell. Likewise, the rate of stable disease was higher in the vinorelbine arm, at 62.2%, versus 46.4% in the control arm.
The primary outcome of the study, PFS, was significantly improved in the vinorelbine arm, according to Dr. Fennell. The median PFS was 4.2 months in the vinorelbine arm and 2.8 months in the supportive care arm (P = .002), translating into a hazard ratio of 0.60 (95% confidence interval, 0.41-0.86), Prof. Fennell reported.
The most common grade 3-4 adverse event was neutropenia, occurring in 12.5% of the vinorelbine-treated patients and no patients managed with supportive care alone, according to the report. Other grade 3-4 adverse events occurred in fewer than 10% of patients and included dyspnea, lower respiratory infection, lymphopenia, and fatigue, among others.
Overall survival (OS) was not statistically different between vinorelbine and supportive care arms, with median OS of 9.3 months and 9.1 months, respectively.
However, a number of patients in the control arm went on to receive subsequent therapy, including 15 (or about 27%) who went into CONFIRM, a randomized phase 3 trial that, as recently reported, met its coprimary endpoints of improve OS and PFS with nivolumab vs. placebo in relapsed malignant mesothelioma.
Investigators also sought to test the hypothesis that BRCA1-negative patients might be chemoresistant, based in part on preclinical models demonstrating that BRCA1 predicted sensitivity to vinorelbine. However, there was no difference in PFS by BRCA1 expression in the VIM study, according to Prof. Fennell.
Taken together, findings of the VIM trial suggest vinorelbine is a “modestly active” agent with low cost and acceptable toxicity, according to Prof. Nowak.
“It is incumbent on us to have clear discussions with our patients on the risks and benefits of trying this as a subsequent-line therapy, in the context of this evidence that was generated as a second-line therapy,” she said in her discussant remarks on the study.
“I would say that it is a lower priority in our algorithm than cisplatin and pemetrexed, or of course, immuno-oncology agents,” she added.
Dr. Fennell reported disclosures related to AstraZeneca, Astex Therapeutics, Bayer, and multiple other pharmaceutical companies. Dr. Nowak reported disclosures with AstraZeneca, Atara Biotherapeutics, Boehringer Ingelheim, and multiple other pharmaceutical companies.
After decades of off-label use, vinorelbine finally has a randomized clinical trial supporting its efficacy as a second-line treatment of mesothelioma, an investigator reported at the annual meeting of the American Society of Clinical Oncology.
However, the development of other treatment regimens, and notably immuno-oncology approaches, are pushing this classic chemotherapy option to later lines of therapy in patients with malignant pleural mesothelioma (MPM), a speaker said at the meeting.
Adding vinorelbine to active symptom control statistically improved progression-free survival (PFS), among patients with MPM who had prior platinum-based therapy, according to results of the randomized Vinorelbine in Mesothelioma (VIM) trial.
Median PFS reached 4.2 months in the vinorelbine arm, versus 2.8 months for active symptom control alone, study results show.
That finding , coupled with safety results, supports the off-label use of vinorelbine as a treatment option for patients with relapsed MPM, according to investigator Dean Anthony Fennell, FRCP, PhD, of the University of Leicester (England).
“Vinorelbine appears to be a safe and effective treatment and could be considered as a treatment option for patients with relapsed mesothelioma,” Prof. Fennell said in his presentation at ASCO (Abstract 8507).
Changing status
While that welcome pronouncement was a long time coming, there are now other promising treatment approaches that relegate vinorelbine to a “lower priority” in the treatment algorithm, said discussant Anna K. Nowak, MBBS, FRACP, PhD, of the University of Western Australia, Nedlands.
In October 2020, the U.S. Food and Drug Administration approved the combination of ipilimumab and nivolumab for the first-line treatment of unresectable malignant pleural mesothelioma, on the basis of results from CHECKMATE-743, a randomized, open-label trial.
“Certainly, we know now that first-line ipilimumab and nivolumab is of very substantial benefit to these patients, and we still have clinical trials open in this space as well,” Prof. Nowak said in her discussion at ASCO.
There is “no doubt” that many patients with mesothelioma should at some point receive an IO agent, particularly now with recently reported randomized clinical trial evidence of an overall survival benefit, she added.
“This really pushes vinorelbine out to be a third- or fourth-line treatment,” she added, “and we know that there are usually diminishing returns from using chemotherapies further down the treatment algorithm.”
Trial details
The VIM trial described at ASCO by Prof. Fennell was a randomized, controlled phase 2 including 154 patients with MPM that had progressed after first-line chemotherapy.
“Vinorelbine has shown useful clinical activity in single-arm phase two studies, however, the specific efficacy of vinorelbine has not been evaluated in an appropriately controlled randomized trial,” Prof. Fennell said in this presentation.
Patients in the trial were randomized 2:1 to either vinorelbine plus active supportive care or active supportive care alone. Vinorelbine was given initially at 60 mg/m2 weekly every 21 days, escalated to 80 mg/m2 from cycle 2.
The median age of patients in VIM was approximately 71 years, and about 80% were male.
More partial responses were seen in the vinorelbine arm, at 3.1% of patients, compared with 1.8% for active supportive care, according to Prof. Fennell. Likewise, the rate of stable disease was higher in the vinorelbine arm, at 62.2%, versus 46.4% in the control arm.
The primary outcome of the study, PFS, was significantly improved in the vinorelbine arm, according to Dr. Fennell. The median PFS was 4.2 months in the vinorelbine arm and 2.8 months in the supportive care arm (P = .002), translating into a hazard ratio of 0.60 (95% confidence interval, 0.41-0.86), Prof. Fennell reported.
The most common grade 3-4 adverse event was neutropenia, occurring in 12.5% of the vinorelbine-treated patients and no patients managed with supportive care alone, according to the report. Other grade 3-4 adverse events occurred in fewer than 10% of patients and included dyspnea, lower respiratory infection, lymphopenia, and fatigue, among others.
Overall survival (OS) was not statistically different between vinorelbine and supportive care arms, with median OS of 9.3 months and 9.1 months, respectively.
However, a number of patients in the control arm went on to receive subsequent therapy, including 15 (or about 27%) who went into CONFIRM, a randomized phase 3 trial that, as recently reported, met its coprimary endpoints of improve OS and PFS with nivolumab vs. placebo in relapsed malignant mesothelioma.
Investigators also sought to test the hypothesis that BRCA1-negative patients might be chemoresistant, based in part on preclinical models demonstrating that BRCA1 predicted sensitivity to vinorelbine. However, there was no difference in PFS by BRCA1 expression in the VIM study, according to Prof. Fennell.
Taken together, findings of the VIM trial suggest vinorelbine is a “modestly active” agent with low cost and acceptable toxicity, according to Prof. Nowak.
“It is incumbent on us to have clear discussions with our patients on the risks and benefits of trying this as a subsequent-line therapy, in the context of this evidence that was generated as a second-line therapy,” she said in her discussant remarks on the study.
“I would say that it is a lower priority in our algorithm than cisplatin and pemetrexed, or of course, immuno-oncology agents,” she added.
Dr. Fennell reported disclosures related to AstraZeneca, Astex Therapeutics, Bayer, and multiple other pharmaceutical companies. Dr. Nowak reported disclosures with AstraZeneca, Atara Biotherapeutics, Boehringer Ingelheim, and multiple other pharmaceutical companies.
After decades of off-label use, vinorelbine finally has a randomized clinical trial supporting its efficacy as a second-line treatment of mesothelioma, an investigator reported at the annual meeting of the American Society of Clinical Oncology.
However, the development of other treatment regimens, and notably immuno-oncology approaches, are pushing this classic chemotherapy option to later lines of therapy in patients with malignant pleural mesothelioma (MPM), a speaker said at the meeting.
Adding vinorelbine to active symptom control statistically improved progression-free survival (PFS), among patients with MPM who had prior platinum-based therapy, according to results of the randomized Vinorelbine in Mesothelioma (VIM) trial.
Median PFS reached 4.2 months in the vinorelbine arm, versus 2.8 months for active symptom control alone, study results show.
That finding , coupled with safety results, supports the off-label use of vinorelbine as a treatment option for patients with relapsed MPM, according to investigator Dean Anthony Fennell, FRCP, PhD, of the University of Leicester (England).
“Vinorelbine appears to be a safe and effective treatment and could be considered as a treatment option for patients with relapsed mesothelioma,” Prof. Fennell said in his presentation at ASCO (Abstract 8507).
Changing status
While that welcome pronouncement was a long time coming, there are now other promising treatment approaches that relegate vinorelbine to a “lower priority” in the treatment algorithm, said discussant Anna K. Nowak, MBBS, FRACP, PhD, of the University of Western Australia, Nedlands.
In October 2020, the U.S. Food and Drug Administration approved the combination of ipilimumab and nivolumab for the first-line treatment of unresectable malignant pleural mesothelioma, on the basis of results from CHECKMATE-743, a randomized, open-label trial.
“Certainly, we know now that first-line ipilimumab and nivolumab is of very substantial benefit to these patients, and we still have clinical trials open in this space as well,” Prof. Nowak said in her discussion at ASCO.
There is “no doubt” that many patients with mesothelioma should at some point receive an IO agent, particularly now with recently reported randomized clinical trial evidence of an overall survival benefit, she added.
“This really pushes vinorelbine out to be a third- or fourth-line treatment,” she added, “and we know that there are usually diminishing returns from using chemotherapies further down the treatment algorithm.”
Trial details
The VIM trial described at ASCO by Prof. Fennell was a randomized, controlled phase 2 including 154 patients with MPM that had progressed after first-line chemotherapy.
“Vinorelbine has shown useful clinical activity in single-arm phase two studies, however, the specific efficacy of vinorelbine has not been evaluated in an appropriately controlled randomized trial,” Prof. Fennell said in this presentation.
Patients in the trial were randomized 2:1 to either vinorelbine plus active supportive care or active supportive care alone. Vinorelbine was given initially at 60 mg/m2 weekly every 21 days, escalated to 80 mg/m2 from cycle 2.
The median age of patients in VIM was approximately 71 years, and about 80% were male.
More partial responses were seen in the vinorelbine arm, at 3.1% of patients, compared with 1.8% for active supportive care, according to Prof. Fennell. Likewise, the rate of stable disease was higher in the vinorelbine arm, at 62.2%, versus 46.4% in the control arm.
The primary outcome of the study, PFS, was significantly improved in the vinorelbine arm, according to Dr. Fennell. The median PFS was 4.2 months in the vinorelbine arm and 2.8 months in the supportive care arm (P = .002), translating into a hazard ratio of 0.60 (95% confidence interval, 0.41-0.86), Prof. Fennell reported.
The most common grade 3-4 adverse event was neutropenia, occurring in 12.5% of the vinorelbine-treated patients and no patients managed with supportive care alone, according to the report. Other grade 3-4 adverse events occurred in fewer than 10% of patients and included dyspnea, lower respiratory infection, lymphopenia, and fatigue, among others.
Overall survival (OS) was not statistically different between vinorelbine and supportive care arms, with median OS of 9.3 months and 9.1 months, respectively.
However, a number of patients in the control arm went on to receive subsequent therapy, including 15 (or about 27%) who went into CONFIRM, a randomized phase 3 trial that, as recently reported, met its coprimary endpoints of improve OS and PFS with nivolumab vs. placebo in relapsed malignant mesothelioma.
Investigators also sought to test the hypothesis that BRCA1-negative patients might be chemoresistant, based in part on preclinical models demonstrating that BRCA1 predicted sensitivity to vinorelbine. However, there was no difference in PFS by BRCA1 expression in the VIM study, according to Prof. Fennell.
Taken together, findings of the VIM trial suggest vinorelbine is a “modestly active” agent with low cost and acceptable toxicity, according to Prof. Nowak.
“It is incumbent on us to have clear discussions with our patients on the risks and benefits of trying this as a subsequent-line therapy, in the context of this evidence that was generated as a second-line therapy,” she said in her discussant remarks on the study.
“I would say that it is a lower priority in our algorithm than cisplatin and pemetrexed, or of course, immuno-oncology agents,” she added.
Dr. Fennell reported disclosures related to AstraZeneca, Astex Therapeutics, Bayer, and multiple other pharmaceutical companies. Dr. Nowak reported disclosures with AstraZeneca, Atara Biotherapeutics, Boehringer Ingelheim, and multiple other pharmaceutical companies.
REPORTING FROM ASCO 2021
Zero-burnout practices often solo, physician-owned
according to an analysis published June 7 in Health Affairs.
The findings may have particular significance in an era when more physicians are being employed by hospitals and health systems, says lead author Samuel T. Edwards, MD, an assistant professor of medicine at Oregon Health & Science University, Portland.
“Market forces and various reform efforts have driven practices to consolidate, and we certainly see some signal here that burnout might be a potential negative consequence of that,” said Dr. Edwards, who is also a staff physician in internal medicine at the Veterans Affairs Portland Health Care System.
30% of practices reported zero burnout
Dr. Edwards told this news organization that he was surprised that 30% of the practices surveyed for this analysis reported zero burnout – meaning no member of the practice reported burnout – because reports of burnout are so pervasive in medicine.
For comparison, in 13% of practices surveyed, more than 40% of practice members reported burnout.
Burnout was assessed with a five-point measure that correlates with the emotional exhaustion scale of the Maslach Burnout Inventory.
It was also surprising, Dr. Edwards said, that practices with some of the heaviest workloads – solo practitioners juggling large numbers of patients, insurance plans, and regulatory requirements – were much more likely than larger practices to report zero burnout.
In this study, solo practices were 5.3 times as likely as practices with 6 to 10 clinicians to report zero burnout (95% confidence interval, 1.25-22.6).
The researchers found no link between burnout and patient volume or the proportion of patients with Medicaid insurance.
“People assume that working harder is associated with more burnout, and there are lots of studies that say that’s true. But in our study, it appears that people work really hard in some settings and are not burned out,” Dr. Edwards said.
He says in small offices, there may be a stronger sense of agency, a sense that everyone is on the same team, and there may be stronger relationships with patients.
The study included survey data from 715 small- to medium-size primary care practices in the United States that participated in the Agency for Healthcare Research and Quality’s EvidenceNOW quality improvement initiative between September 2015 and June 2017.
Zero-burnout practices shared several traits. They were more likely to have “a strong practice culture – one in which teamwork, communication, psychological safety, mindfulness of others, facilitative leadership, and understanding that people make and can learn from mistakes were among the key attributes,” Dr. Edwards and colleagues write.
Burnout higher with ACO participation
Organizations that participated in ACOs and other external primary care transformation projects were more likely to have high burnout rates. Specifically, 29% of these practices reported zero burnout, versus 53% that reported high rates of burnout.
Dr. Edwards said the reasons for that are unclear in this cross-sectional study, but there seemed to be an indication that getting involved in too many demonstration projects might be associated with burnout. He noted that participants in this study were already involved in the EvidenceNOW initiative.
Factors regarding electronic health records (EHRs) were not tied to burnout in this study. Dr. Edwards said they surveyed for both satisfaction with EHRs and EHR features and whether they were linked to zero burnout.
He speculates that this may indicate that by now, practices have adapted to using EHRs, though they continue to be a source of frustration for individual clinicians.
Debora Goetz Goldberg, PhD, MHA, MBA, associate professor at George Mason University, McLean, Virginia, told this news organization that she has found similar results in her research of primary care practices and burnout. She found that health system–owned practices had higher levels of burnout.
“We thought that probably was related to less autonomy and decision-making authority,” she said.
She pointed out that Dr. Edwards and colleagues found that physicians who had more “adaptive reserves” were more likely to have zero burnout. Her research found a similar association.
Such organizations, she explained, have a higher level of organizational development and a culture of innovation. They are more comfortable with change and adapt well.
“They are characterized by teamwork, strong communication, and a culture of learning,” she said.
By contrast, burnout may be higher in health system–owned practices because clinicians may feel they have less control over their work environment and feel a loss of autonomy, according to Dr. Goldberg.
“Moral distress,” which can happen when an individual’s professional values don’t line up with an organization’s values, may also play a part, she said. Physicians may be required to see more patients than they feel they can serve well in a day, for instance.
Reducing burnout may take building a more collaborative leadership style, she said.
No link between burnout and patient volume
The current research also highlighted leadership style as a potential driver of burnout.
Dr. Edwards and colleagues found that one of the strongest associations was between facilitative leadership and low burnout. Zero burnout is associated with participatory decision-making.
“Interestingly, we saw that that kind of leadership could exist in multiple settings,” he said. Health care professionals in smaller practices might know each other better and have a shared mission, but shared decision making can also exist in larger practices, he said.
Higher burnout was associated with traditional leadership models that are hierarchical and that operate with a command-and-control structure, according to the study.
The data may have implications for strategies regarding both the smallest and largest practices.
Initiatives that help small practices remain strong are valuable, especially for communities that depend on those practices, Dr. Edwards said.
The researchers give as an example the funding of primary care practice extension networks, which provide support similar to agricultural extension programs for farmers.
At the same time, “having agency at the practice level about how things work is really important in reducing burnout. So in a large system, finding ways to promote agency at the most local level possible can really help with burnout,” he said.
Dr. Edwards said his team controlled for the fact that mathematically, it’s more likely zero burnout would be reported in a solo practice than in a larger practice.
Every practice in this study, he said, had to have at least three persons who responded to the survey, and responses had to represent three roles – a clinician, a nonclinician staff member, and a clinical staff member. The response rate also had to be 50% within the practice, he explained.
All authors are supported by the Agency for Healthcare Research and Quality. Dr. Edwards was also supported by the Department of Veterans Affairs Health Services Research and Development. Dr. Goldberg has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
according to an analysis published June 7 in Health Affairs.
The findings may have particular significance in an era when more physicians are being employed by hospitals and health systems, says lead author Samuel T. Edwards, MD, an assistant professor of medicine at Oregon Health & Science University, Portland.
“Market forces and various reform efforts have driven practices to consolidate, and we certainly see some signal here that burnout might be a potential negative consequence of that,” said Dr. Edwards, who is also a staff physician in internal medicine at the Veterans Affairs Portland Health Care System.
30% of practices reported zero burnout
Dr. Edwards told this news organization that he was surprised that 30% of the practices surveyed for this analysis reported zero burnout – meaning no member of the practice reported burnout – because reports of burnout are so pervasive in medicine.
For comparison, in 13% of practices surveyed, more than 40% of practice members reported burnout.
Burnout was assessed with a five-point measure that correlates with the emotional exhaustion scale of the Maslach Burnout Inventory.
It was also surprising, Dr. Edwards said, that practices with some of the heaviest workloads – solo practitioners juggling large numbers of patients, insurance plans, and regulatory requirements – were much more likely than larger practices to report zero burnout.
In this study, solo practices were 5.3 times as likely as practices with 6 to 10 clinicians to report zero burnout (95% confidence interval, 1.25-22.6).
The researchers found no link between burnout and patient volume or the proportion of patients with Medicaid insurance.
“People assume that working harder is associated with more burnout, and there are lots of studies that say that’s true. But in our study, it appears that people work really hard in some settings and are not burned out,” Dr. Edwards said.
He says in small offices, there may be a stronger sense of agency, a sense that everyone is on the same team, and there may be stronger relationships with patients.
The study included survey data from 715 small- to medium-size primary care practices in the United States that participated in the Agency for Healthcare Research and Quality’s EvidenceNOW quality improvement initiative between September 2015 and June 2017.
Zero-burnout practices shared several traits. They were more likely to have “a strong practice culture – one in which teamwork, communication, psychological safety, mindfulness of others, facilitative leadership, and understanding that people make and can learn from mistakes were among the key attributes,” Dr. Edwards and colleagues write.
Burnout higher with ACO participation
Organizations that participated in ACOs and other external primary care transformation projects were more likely to have high burnout rates. Specifically, 29% of these practices reported zero burnout, versus 53% that reported high rates of burnout.
Dr. Edwards said the reasons for that are unclear in this cross-sectional study, but there seemed to be an indication that getting involved in too many demonstration projects might be associated with burnout. He noted that participants in this study were already involved in the EvidenceNOW initiative.
Factors regarding electronic health records (EHRs) were not tied to burnout in this study. Dr. Edwards said they surveyed for both satisfaction with EHRs and EHR features and whether they were linked to zero burnout.
He speculates that this may indicate that by now, practices have adapted to using EHRs, though they continue to be a source of frustration for individual clinicians.
Debora Goetz Goldberg, PhD, MHA, MBA, associate professor at George Mason University, McLean, Virginia, told this news organization that she has found similar results in her research of primary care practices and burnout. She found that health system–owned practices had higher levels of burnout.
“We thought that probably was related to less autonomy and decision-making authority,” she said.
She pointed out that Dr. Edwards and colleagues found that physicians who had more “adaptive reserves” were more likely to have zero burnout. Her research found a similar association.
Such organizations, she explained, have a higher level of organizational development and a culture of innovation. They are more comfortable with change and adapt well.
“They are characterized by teamwork, strong communication, and a culture of learning,” she said.
By contrast, burnout may be higher in health system–owned practices because clinicians may feel they have less control over their work environment and feel a loss of autonomy, according to Dr. Goldberg.
“Moral distress,” which can happen when an individual’s professional values don’t line up with an organization’s values, may also play a part, she said. Physicians may be required to see more patients than they feel they can serve well in a day, for instance.
Reducing burnout may take building a more collaborative leadership style, she said.
No link between burnout and patient volume
The current research also highlighted leadership style as a potential driver of burnout.
Dr. Edwards and colleagues found that one of the strongest associations was between facilitative leadership and low burnout. Zero burnout is associated with participatory decision-making.
“Interestingly, we saw that that kind of leadership could exist in multiple settings,” he said. Health care professionals in smaller practices might know each other better and have a shared mission, but shared decision making can also exist in larger practices, he said.
Higher burnout was associated with traditional leadership models that are hierarchical and that operate with a command-and-control structure, according to the study.
The data may have implications for strategies regarding both the smallest and largest practices.
Initiatives that help small practices remain strong are valuable, especially for communities that depend on those practices, Dr. Edwards said.
The researchers give as an example the funding of primary care practice extension networks, which provide support similar to agricultural extension programs for farmers.
At the same time, “having agency at the practice level about how things work is really important in reducing burnout. So in a large system, finding ways to promote agency at the most local level possible can really help with burnout,” he said.
Dr. Edwards said his team controlled for the fact that mathematically, it’s more likely zero burnout would be reported in a solo practice than in a larger practice.
Every practice in this study, he said, had to have at least three persons who responded to the survey, and responses had to represent three roles – a clinician, a nonclinician staff member, and a clinical staff member. The response rate also had to be 50% within the practice, he explained.
All authors are supported by the Agency for Healthcare Research and Quality. Dr. Edwards was also supported by the Department of Veterans Affairs Health Services Research and Development. Dr. Goldberg has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
according to an analysis published June 7 in Health Affairs.
The findings may have particular significance in an era when more physicians are being employed by hospitals and health systems, says lead author Samuel T. Edwards, MD, an assistant professor of medicine at Oregon Health & Science University, Portland.
“Market forces and various reform efforts have driven practices to consolidate, and we certainly see some signal here that burnout might be a potential negative consequence of that,” said Dr. Edwards, who is also a staff physician in internal medicine at the Veterans Affairs Portland Health Care System.
30% of practices reported zero burnout
Dr. Edwards told this news organization that he was surprised that 30% of the practices surveyed for this analysis reported zero burnout – meaning no member of the practice reported burnout – because reports of burnout are so pervasive in medicine.
For comparison, in 13% of practices surveyed, more than 40% of practice members reported burnout.
Burnout was assessed with a five-point measure that correlates with the emotional exhaustion scale of the Maslach Burnout Inventory.
It was also surprising, Dr. Edwards said, that practices with some of the heaviest workloads – solo practitioners juggling large numbers of patients, insurance plans, and regulatory requirements – were much more likely than larger practices to report zero burnout.
In this study, solo practices were 5.3 times as likely as practices with 6 to 10 clinicians to report zero burnout (95% confidence interval, 1.25-22.6).
The researchers found no link between burnout and patient volume or the proportion of patients with Medicaid insurance.
“People assume that working harder is associated with more burnout, and there are lots of studies that say that’s true. But in our study, it appears that people work really hard in some settings and are not burned out,” Dr. Edwards said.
He says in small offices, there may be a stronger sense of agency, a sense that everyone is on the same team, and there may be stronger relationships with patients.
The study included survey data from 715 small- to medium-size primary care practices in the United States that participated in the Agency for Healthcare Research and Quality’s EvidenceNOW quality improvement initiative between September 2015 and June 2017.
Zero-burnout practices shared several traits. They were more likely to have “a strong practice culture – one in which teamwork, communication, psychological safety, mindfulness of others, facilitative leadership, and understanding that people make and can learn from mistakes were among the key attributes,” Dr. Edwards and colleagues write.
Burnout higher with ACO participation
Organizations that participated in ACOs and other external primary care transformation projects were more likely to have high burnout rates. Specifically, 29% of these practices reported zero burnout, versus 53% that reported high rates of burnout.
Dr. Edwards said the reasons for that are unclear in this cross-sectional study, but there seemed to be an indication that getting involved in too many demonstration projects might be associated with burnout. He noted that participants in this study were already involved in the EvidenceNOW initiative.
Factors regarding electronic health records (EHRs) were not tied to burnout in this study. Dr. Edwards said they surveyed for both satisfaction with EHRs and EHR features and whether they were linked to zero burnout.
He speculates that this may indicate that by now, practices have adapted to using EHRs, though they continue to be a source of frustration for individual clinicians.
Debora Goetz Goldberg, PhD, MHA, MBA, associate professor at George Mason University, McLean, Virginia, told this news organization that she has found similar results in her research of primary care practices and burnout. She found that health system–owned practices had higher levels of burnout.
“We thought that probably was related to less autonomy and decision-making authority,” she said.
She pointed out that Dr. Edwards and colleagues found that physicians who had more “adaptive reserves” were more likely to have zero burnout. Her research found a similar association.
Such organizations, she explained, have a higher level of organizational development and a culture of innovation. They are more comfortable with change and adapt well.
“They are characterized by teamwork, strong communication, and a culture of learning,” she said.
By contrast, burnout may be higher in health system–owned practices because clinicians may feel they have less control over their work environment and feel a loss of autonomy, according to Dr. Goldberg.
“Moral distress,” which can happen when an individual’s professional values don’t line up with an organization’s values, may also play a part, she said. Physicians may be required to see more patients than they feel they can serve well in a day, for instance.
Reducing burnout may take building a more collaborative leadership style, she said.
No link between burnout and patient volume
The current research also highlighted leadership style as a potential driver of burnout.
Dr. Edwards and colleagues found that one of the strongest associations was between facilitative leadership and low burnout. Zero burnout is associated with participatory decision-making.
“Interestingly, we saw that that kind of leadership could exist in multiple settings,” he said. Health care professionals in smaller practices might know each other better and have a shared mission, but shared decision making can also exist in larger practices, he said.
Higher burnout was associated with traditional leadership models that are hierarchical and that operate with a command-and-control structure, according to the study.
The data may have implications for strategies regarding both the smallest and largest practices.
Initiatives that help small practices remain strong are valuable, especially for communities that depend on those practices, Dr. Edwards said.
The researchers give as an example the funding of primary care practice extension networks, which provide support similar to agricultural extension programs for farmers.
At the same time, “having agency at the practice level about how things work is really important in reducing burnout. So in a large system, finding ways to promote agency at the most local level possible can really help with burnout,” he said.
Dr. Edwards said his team controlled for the fact that mathematically, it’s more likely zero burnout would be reported in a solo practice than in a larger practice.
Every practice in this study, he said, had to have at least three persons who responded to the survey, and responses had to represent three roles – a clinician, a nonclinician staff member, and a clinical staff member. The response rate also had to be 50% within the practice, he explained.
All authors are supported by the Agency for Healthcare Research and Quality. Dr. Edwards was also supported by the Department of Veterans Affairs Health Services Research and Development. Dr. Goldberg has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Erythematous axillary plaques
Due to the condition’s persistence and the negative KOH prep, erythrasma was the most likely diagnosis.
Erythrasma is caused by a bacterial infection with Corynebacterium minutissimum. It occurs in intertriginous areas that tend to be moist and irritated by friction. The erythema is usually a dull red, rather than the bright red that one would see with yeast infections. In addition, there is typically central pallor.
Woods lamp examination can confirm the diagnosis by showing coral pink fluorescence. In this patient, however, there was no fluorescence because the patient had recently washed the area and, thus, removed the porphyrins produced by C minutissimum. Biopsy for pathology is not usually necessary.
Erythrasma is treated with topical clindamycin, fusidic acid, or mupirocin. Oral macrolides and tetracyclines are also effective.1 Due to the chronicity of the erythrasma and the discomfort it caused, this patient opted for oral doxycycline 100 mg twice daily for 10 days. At follow-up 2 weeks later, the erythrasma had resolved.
Photo and text courtesy of Daniel Stulberg, MD, FAAFP, Department of Family and Community Medicine, University of New Mexico School of Medicine, Albuquerque
Forouzan P, Cohen PR. Erythrasma revisited: diagnosis, differential diagnoses, and comprehensive review of treatment. Cureus. 2020;12:e10733. doi: 10.7759/cureus.10733

Due to the condition’s persistence and the negative KOH prep, erythrasma was the most likely diagnosis.
Erythrasma is caused by a bacterial infection with Corynebacterium minutissimum. It occurs in intertriginous areas that tend to be moist and irritated by friction. The erythema is usually a dull red, rather than the bright red that one would see with yeast infections. In addition, there is typically central pallor.
Woods lamp examination can confirm the diagnosis by showing coral pink fluorescence. In this patient, however, there was no fluorescence because the patient had recently washed the area and, thus, removed the porphyrins produced by C minutissimum. Biopsy for pathology is not usually necessary.
Erythrasma is treated with topical clindamycin, fusidic acid, or mupirocin. Oral macrolides and tetracyclines are also effective.1 Due to the chronicity of the erythrasma and the discomfort it caused, this patient opted for oral doxycycline 100 mg twice daily for 10 days. At follow-up 2 weeks later, the erythrasma had resolved.
Photo and text courtesy of Daniel Stulberg, MD, FAAFP, Department of Family and Community Medicine, University of New Mexico School of Medicine, Albuquerque

Due to the condition’s persistence and the negative KOH prep, erythrasma was the most likely diagnosis.
Erythrasma is caused by a bacterial infection with Corynebacterium minutissimum. It occurs in intertriginous areas that tend to be moist and irritated by friction. The erythema is usually a dull red, rather than the bright red that one would see with yeast infections. In addition, there is typically central pallor.
Woods lamp examination can confirm the diagnosis by showing coral pink fluorescence. In this patient, however, there was no fluorescence because the patient had recently washed the area and, thus, removed the porphyrins produced by C minutissimum. Biopsy for pathology is not usually necessary.
Erythrasma is treated with topical clindamycin, fusidic acid, or mupirocin. Oral macrolides and tetracyclines are also effective.1 Due to the chronicity of the erythrasma and the discomfort it caused, this patient opted for oral doxycycline 100 mg twice daily for 10 days. At follow-up 2 weeks later, the erythrasma had resolved.
Photo and text courtesy of Daniel Stulberg, MD, FAAFP, Department of Family and Community Medicine, University of New Mexico School of Medicine, Albuquerque
Forouzan P, Cohen PR. Erythrasma revisited: diagnosis, differential diagnoses, and comprehensive review of treatment. Cureus. 2020;12:e10733. doi: 10.7759/cureus.10733
Forouzan P, Cohen PR. Erythrasma revisited: diagnosis, differential diagnoses, and comprehensive review of treatment. Cureus. 2020;12:e10733. doi: 10.7759/cureus.10733
Looking to the future of physiologically informed sepsis resuscitation: The role of dynamic fluid-responsive measurement
Current guideline recommendations for fluid resuscitation in sepsis patients calls for an initial crystalloid fluid bolus of at least 30 mL/kg (Rhodes, et al. Intensive Care Med. 2017;43[3]:304-77) For fluid management beyond this initial bolus, recommendations had previously called for using early goal-directed therapy (EGDT) with central venous pressure (CVP) and central venous oxygen saturation to guide the use of IV fluids, vasopressors, transfusions, and dobutamine, based on the results of one single-center study that found an improvement in mortality using EGDT as compared with standard therapy.
The triad of sepsis studies
In the following years, multiple concerns were raised regarding the generalizability of this study. Three large multicenter trials were conducted in multiple countries to test the recommendations for EGDT.
PROMISE: ProMISe was a 1,260-patient randomized trial comparing the impact of EGDT vs usual care on 90-day all-cause mortality in patients with early septic shock at 56 hospitals in England. There was no significant difference in the primary study endpoint with 90-day mortality rates of 29.5% and 29.2% (RR: 1.01, 95% CI: 0.85-1.20, P =.90) (Mouncey, et al. N Engl J Med. 2015;372[14]:1301-11).
PROCESS: ProCESS was a 1,351-patient randomized trial comparing the impact of protocol-based EGDT, protocol-based standard of care, and usual care on 60 day in-hospital mortality in patients with early septic shock at 31 hospitals in the United States. There was no significant difference in the primary study endpoint with 60-day mortality rates of 21.0%, 18.2%, and 18.9% (P = .83) or in the secondary outcome of 90-day mortality with rates of 31.9%, 30.8%, and 33.7% (P = .66) (ProCESS Investigators, et al. N Engl J Med. 2014;370[18]:1683-93).
ARISE: ARISE was a 1,600-patient randomized trial comparing the impact of EGDT vs usual care on 90-day all-cause mortality in patients with early septic shock at 51 hospitals in New Zealand and Australia. There was no significant difference in the primary study end point with 90-day mortality rates of 18.6% and 18.8% (RR: 0.98, 95% CI: 0.80-1.21, P = .90). There were also no significant differences in 28-day or in-hospital mortality, duration of organ support, or length of hospital stay (ARISE Investigators, et al. N Engl J Med. 2014;371[16]:1496-506).
In summary, all three “triad” trials found no improvement with EGDT over usual care (Rowan, et al. N Engl J Med. 2017;376[23]:2223-34) calling into question the recommended methods of universally protocolized approaches to fluid and pressor resuscitation. Probable reasons for why structured EGDT was ineffective at improving outcomes over usual care in the “triad” trials was that (a) liberal fluid volume administration was the “usual care” in most enrolled patients and (b) that macrocirculatory hemodynamics, such as BP, and static intravascular pressures such as CVP and pulmonary arterial wedge pressure are poor correlates and predictors of effective circulatory volumes and the presence of fluid responsiveness.
Counterintuitively, in situations of central hypovolemia, peripheral sympathetic activity remains high in many patients while stroke volume decreases. This provides insight into why some patients appear not to benefit from fluid administration as peripheral arterial pressure may be maintained despite low central filling pressure (Convertino VA, et al. Auton Neurosci. 2004;111[2]:127-34). Many patients with sepsis and septic shock initially present in an undifferentiated state and empiric treatment decisions regarding fluid and pressor treatments are then misaligned to functional physiological status.
Novel methods and approaches are needed to differentiate these patients and provide appropriate, physiologically guided fluid resuscitation. Dynamic measurement of stroke volume (SV) after a passive leg raise (PLR) or a small IV fluid challenge is an emerging method for determining fluid responsiveness. Evidence suggests that the use of SV-guided resuscitation can reduce net fluid balance, ICU length of stay, risk of mechanical ventilation, time on vasopressors, and risk of renal replacement therapy.(Latham HE, et al. J Crit Care. 2017;42:42-6).
In addition to the lack of efficacy from administering fluid to nonfluid responsive patients, there remains a risk of over-resuscitation from excessive fluid administration. Excessive fluid administration causes hypervolemia and is associated with a variety of negative patient outcomes including tissue edema, organ dysfunction, increased ICU length of stay, prolonged ventilator dependence, and higher mortality rates (Tigabu BM, et al. J Crit Care. 2018;48:153-9). Further, unnecessary initial fluid administration necessitates a “de-resuscitative” phase that can prolong hospital stay and is associated with amplification of sepsis-associated organ failures. Specifically, a 2017 analysis of hospital discharge data found that large volume fluid resuscitation in sepsis patients during the first 24 hours of care was associated with higher rates of hospital mortality than was predicted for patients’ disease severity (Mansoori JN, et al. Crit Care. 2020;24[1]:25).
The FRESH trial
The Fluid Response Evaluation in Sepsis Hypotension and Shock (FRESH) trial was a prospective, randomized clinical trial in adults with septic shock comparing PLR-guided SV responsiveness (intervention) as a guide for fluid management with usual care. Patients presented to the ER with sepsis-associated hypotension and anticipated ICU admission. In the intervention arm, patients were assessed for fluid responsiveness (FR) before any clinically driven fluid bolus or increase in vasopressors. If a patient’s stroke volume increased by ≥10% in response to a PLR, they were considered fluid responsive and fluid was recommended as the first therapy. If a patient’s stroke volume increased by <10% then the patient was considered not to be FR and vasopressors were recommended as first-line therapy. The control arm received usual care. The primary end point was the difference in positive fluid balance at the first of either 72 hours or ICU discharge. Patients had received ~2.3 L of crystalloid fluid prior to randomization (~3.5 h from initial presentation), in keeping with 30 mL/kg recommendations. Patients treated with the PLR-guided fluid and pressor protocol had a significant lower net fluid balance (1.37 L (95% CI: 2.53-0.21, P = .021) at 72 hours or ICU discharge. In addition, the intervention group experienced significantly less frequent requirement for renal replacement therapy with a difference of 12.4% (95% CI: 27%-1%, P = .042) as well as a decreased requirement for ventilator use with a difference of 16.42% (95% CI: 33%-0%, P = .044) (Douglas IS, et al. Chest. 2020;158[4]:1431-45).
FRESH demonstrated that PLR-guided FR drove lower fluid balance in patients with septic shock who present to the ER with sepsis and creates a paradigm for future management of fluid and pressor resuscitation beyond the initial 30 mL/kg bolus. Functional evaluation for lack of FR adequately identifies a group of patients with sepsis-associated hypotension who are unlikely to benefit from additional IV fluids to establish hemodynamically stability. It facilitated physiologically informed treatment decisions for the individual patient at a specific moment in their course of treatment as opposed to relying on static measurements and goals that may ultimately not be indicative of fluid responsiveness and circulatory effectiveness. This could reduce the likelihood of fluid overload and associated organ failure and, thus, improve patient outcomes.
Microcirculatory function is significantly impacted by sepsis with a decline in capillary density and inappropriate vasodilation/constriction resulting in insufficient tissue and organ perfusion and increased oxidative stress. Such dysfunction has been found to be associated with worsened patient outcomes, including mortality. However, microcirculatory function does not correlate well with traditionally used macrohemodynamic assessments and treating to improve macrohemodynamic values does not ensure that microcirculation will improve (Charlton M, et al. J Intensive Care Soc. 2017;18(3):221-7).
Ongoing studies are exploring if dynamic fluid-guided resuscitation has the potential to improve survival in sepsis by providing insight into whether the administration of fluid will impact the microcirculation and subsequent organ perfusion of the patient.
Future directions include expanding the dynamic treatment algorithm into other settings, such as rapid response calls, or other patient populations, including those initially presenting with undifferentiated hypotension. While FRESH was not sufficiently powered to detect differences in mortality, there are currently multiple large studies being conducted aimed at determining the impact of a restricted fluid and early vasopressor strategy as compared with a large initial IV fluid bolus on mortality. The results of these studies could be used to determine if the results of FRESH will translate into patient survival outcomes.
Current guideline recommendations for fluid resuscitation in sepsis patients calls for an initial crystalloid fluid bolus of at least 30 mL/kg (Rhodes, et al. Intensive Care Med. 2017;43[3]:304-77) For fluid management beyond this initial bolus, recommendations had previously called for using early goal-directed therapy (EGDT) with central venous pressure (CVP) and central venous oxygen saturation to guide the use of IV fluids, vasopressors, transfusions, and dobutamine, based on the results of one single-center study that found an improvement in mortality using EGDT as compared with standard therapy.
The triad of sepsis studies
In the following years, multiple concerns were raised regarding the generalizability of this study. Three large multicenter trials were conducted in multiple countries to test the recommendations for EGDT.
PROMISE: ProMISe was a 1,260-patient randomized trial comparing the impact of EGDT vs usual care on 90-day all-cause mortality in patients with early septic shock at 56 hospitals in England. There was no significant difference in the primary study endpoint with 90-day mortality rates of 29.5% and 29.2% (RR: 1.01, 95% CI: 0.85-1.20, P =.90) (Mouncey, et al. N Engl J Med. 2015;372[14]:1301-11).
PROCESS: ProCESS was a 1,351-patient randomized trial comparing the impact of protocol-based EGDT, protocol-based standard of care, and usual care on 60 day in-hospital mortality in patients with early septic shock at 31 hospitals in the United States. There was no significant difference in the primary study endpoint with 60-day mortality rates of 21.0%, 18.2%, and 18.9% (P = .83) or in the secondary outcome of 90-day mortality with rates of 31.9%, 30.8%, and 33.7% (P = .66) (ProCESS Investigators, et al. N Engl J Med. 2014;370[18]:1683-93).
ARISE: ARISE was a 1,600-patient randomized trial comparing the impact of EGDT vs usual care on 90-day all-cause mortality in patients with early septic shock at 51 hospitals in New Zealand and Australia. There was no significant difference in the primary study end point with 90-day mortality rates of 18.6% and 18.8% (RR: 0.98, 95% CI: 0.80-1.21, P = .90). There were also no significant differences in 28-day or in-hospital mortality, duration of organ support, or length of hospital stay (ARISE Investigators, et al. N Engl J Med. 2014;371[16]:1496-506).
In summary, all three “triad” trials found no improvement with EGDT over usual care (Rowan, et al. N Engl J Med. 2017;376[23]:2223-34) calling into question the recommended methods of universally protocolized approaches to fluid and pressor resuscitation. Probable reasons for why structured EGDT was ineffective at improving outcomes over usual care in the “triad” trials was that (a) liberal fluid volume administration was the “usual care” in most enrolled patients and (b) that macrocirculatory hemodynamics, such as BP, and static intravascular pressures such as CVP and pulmonary arterial wedge pressure are poor correlates and predictors of effective circulatory volumes and the presence of fluid responsiveness.
Counterintuitively, in situations of central hypovolemia, peripheral sympathetic activity remains high in many patients while stroke volume decreases. This provides insight into why some patients appear not to benefit from fluid administration as peripheral arterial pressure may be maintained despite low central filling pressure (Convertino VA, et al. Auton Neurosci. 2004;111[2]:127-34). Many patients with sepsis and septic shock initially present in an undifferentiated state and empiric treatment decisions regarding fluid and pressor treatments are then misaligned to functional physiological status.
Novel methods and approaches are needed to differentiate these patients and provide appropriate, physiologically guided fluid resuscitation. Dynamic measurement of stroke volume (SV) after a passive leg raise (PLR) or a small IV fluid challenge is an emerging method for determining fluid responsiveness. Evidence suggests that the use of SV-guided resuscitation can reduce net fluid balance, ICU length of stay, risk of mechanical ventilation, time on vasopressors, and risk of renal replacement therapy.(Latham HE, et al. J Crit Care. 2017;42:42-6).
In addition to the lack of efficacy from administering fluid to nonfluid responsive patients, there remains a risk of over-resuscitation from excessive fluid administration. Excessive fluid administration causes hypervolemia and is associated with a variety of negative patient outcomes including tissue edema, organ dysfunction, increased ICU length of stay, prolonged ventilator dependence, and higher mortality rates (Tigabu BM, et al. J Crit Care. 2018;48:153-9). Further, unnecessary initial fluid administration necessitates a “de-resuscitative” phase that can prolong hospital stay and is associated with amplification of sepsis-associated organ failures. Specifically, a 2017 analysis of hospital discharge data found that large volume fluid resuscitation in sepsis patients during the first 24 hours of care was associated with higher rates of hospital mortality than was predicted for patients’ disease severity (Mansoori JN, et al. Crit Care. 2020;24[1]:25).
The FRESH trial
The Fluid Response Evaluation in Sepsis Hypotension and Shock (FRESH) trial was a prospective, randomized clinical trial in adults with septic shock comparing PLR-guided SV responsiveness (intervention) as a guide for fluid management with usual care. Patients presented to the ER with sepsis-associated hypotension and anticipated ICU admission. In the intervention arm, patients were assessed for fluid responsiveness (FR) before any clinically driven fluid bolus or increase in vasopressors. If a patient’s stroke volume increased by ≥10% in response to a PLR, they were considered fluid responsive and fluid was recommended as the first therapy. If a patient’s stroke volume increased by <10% then the patient was considered not to be FR and vasopressors were recommended as first-line therapy. The control arm received usual care. The primary end point was the difference in positive fluid balance at the first of either 72 hours or ICU discharge. Patients had received ~2.3 L of crystalloid fluid prior to randomization (~3.5 h from initial presentation), in keeping with 30 mL/kg recommendations. Patients treated with the PLR-guided fluid and pressor protocol had a significant lower net fluid balance (1.37 L (95% CI: 2.53-0.21, P = .021) at 72 hours or ICU discharge. In addition, the intervention group experienced significantly less frequent requirement for renal replacement therapy with a difference of 12.4% (95% CI: 27%-1%, P = .042) as well as a decreased requirement for ventilator use with a difference of 16.42% (95% CI: 33%-0%, P = .044) (Douglas IS, et al. Chest. 2020;158[4]:1431-45).
FRESH demonstrated that PLR-guided FR drove lower fluid balance in patients with septic shock who present to the ER with sepsis and creates a paradigm for future management of fluid and pressor resuscitation beyond the initial 30 mL/kg bolus. Functional evaluation for lack of FR adequately identifies a group of patients with sepsis-associated hypotension who are unlikely to benefit from additional IV fluids to establish hemodynamically stability. It facilitated physiologically informed treatment decisions for the individual patient at a specific moment in their course of treatment as opposed to relying on static measurements and goals that may ultimately not be indicative of fluid responsiveness and circulatory effectiveness. This could reduce the likelihood of fluid overload and associated organ failure and, thus, improve patient outcomes.
Microcirculatory function is significantly impacted by sepsis with a decline in capillary density and inappropriate vasodilation/constriction resulting in insufficient tissue and organ perfusion and increased oxidative stress. Such dysfunction has been found to be associated with worsened patient outcomes, including mortality. However, microcirculatory function does not correlate well with traditionally used macrohemodynamic assessments and treating to improve macrohemodynamic values does not ensure that microcirculation will improve (Charlton M, et al. J Intensive Care Soc. 2017;18(3):221-7).
Ongoing studies are exploring if dynamic fluid-guided resuscitation has the potential to improve survival in sepsis by providing insight into whether the administration of fluid will impact the microcirculation and subsequent organ perfusion of the patient.
Future directions include expanding the dynamic treatment algorithm into other settings, such as rapid response calls, or other patient populations, including those initially presenting with undifferentiated hypotension. While FRESH was not sufficiently powered to detect differences in mortality, there are currently multiple large studies being conducted aimed at determining the impact of a restricted fluid and early vasopressor strategy as compared with a large initial IV fluid bolus on mortality. The results of these studies could be used to determine if the results of FRESH will translate into patient survival outcomes.
Current guideline recommendations for fluid resuscitation in sepsis patients calls for an initial crystalloid fluid bolus of at least 30 mL/kg (Rhodes, et al. Intensive Care Med. 2017;43[3]:304-77) For fluid management beyond this initial bolus, recommendations had previously called for using early goal-directed therapy (EGDT) with central venous pressure (CVP) and central venous oxygen saturation to guide the use of IV fluids, vasopressors, transfusions, and dobutamine, based on the results of one single-center study that found an improvement in mortality using EGDT as compared with standard therapy.
The triad of sepsis studies
In the following years, multiple concerns were raised regarding the generalizability of this study. Three large multicenter trials were conducted in multiple countries to test the recommendations for EGDT.
PROMISE: ProMISe was a 1,260-patient randomized trial comparing the impact of EGDT vs usual care on 90-day all-cause mortality in patients with early septic shock at 56 hospitals in England. There was no significant difference in the primary study endpoint with 90-day mortality rates of 29.5% and 29.2% (RR: 1.01, 95% CI: 0.85-1.20, P =.90) (Mouncey, et al. N Engl J Med. 2015;372[14]:1301-11).
PROCESS: ProCESS was a 1,351-patient randomized trial comparing the impact of protocol-based EGDT, protocol-based standard of care, and usual care on 60 day in-hospital mortality in patients with early septic shock at 31 hospitals in the United States. There was no significant difference in the primary study endpoint with 60-day mortality rates of 21.0%, 18.2%, and 18.9% (P = .83) or in the secondary outcome of 90-day mortality with rates of 31.9%, 30.8%, and 33.7% (P = .66) (ProCESS Investigators, et al. N Engl J Med. 2014;370[18]:1683-93).
ARISE: ARISE was a 1,600-patient randomized trial comparing the impact of EGDT vs usual care on 90-day all-cause mortality in patients with early septic shock at 51 hospitals in New Zealand and Australia. There was no significant difference in the primary study end point with 90-day mortality rates of 18.6% and 18.8% (RR: 0.98, 95% CI: 0.80-1.21, P = .90). There were also no significant differences in 28-day or in-hospital mortality, duration of organ support, or length of hospital stay (ARISE Investigators, et al. N Engl J Med. 2014;371[16]:1496-506).
In summary, all three “triad” trials found no improvement with EGDT over usual care (Rowan, et al. N Engl J Med. 2017;376[23]:2223-34) calling into question the recommended methods of universally protocolized approaches to fluid and pressor resuscitation. Probable reasons for why structured EGDT was ineffective at improving outcomes over usual care in the “triad” trials was that (a) liberal fluid volume administration was the “usual care” in most enrolled patients and (b) that macrocirculatory hemodynamics, such as BP, and static intravascular pressures such as CVP and pulmonary arterial wedge pressure are poor correlates and predictors of effective circulatory volumes and the presence of fluid responsiveness.
Counterintuitively, in situations of central hypovolemia, peripheral sympathetic activity remains high in many patients while stroke volume decreases. This provides insight into why some patients appear not to benefit from fluid administration as peripheral arterial pressure may be maintained despite low central filling pressure (Convertino VA, et al. Auton Neurosci. 2004;111[2]:127-34). Many patients with sepsis and septic shock initially present in an undifferentiated state and empiric treatment decisions regarding fluid and pressor treatments are then misaligned to functional physiological status.
Novel methods and approaches are needed to differentiate these patients and provide appropriate, physiologically guided fluid resuscitation. Dynamic measurement of stroke volume (SV) after a passive leg raise (PLR) or a small IV fluid challenge is an emerging method for determining fluid responsiveness. Evidence suggests that the use of SV-guided resuscitation can reduce net fluid balance, ICU length of stay, risk of mechanical ventilation, time on vasopressors, and risk of renal replacement therapy.(Latham HE, et al. J Crit Care. 2017;42:42-6).
In addition to the lack of efficacy from administering fluid to nonfluid responsive patients, there remains a risk of over-resuscitation from excessive fluid administration. Excessive fluid administration causes hypervolemia and is associated with a variety of negative patient outcomes including tissue edema, organ dysfunction, increased ICU length of stay, prolonged ventilator dependence, and higher mortality rates (Tigabu BM, et al. J Crit Care. 2018;48:153-9). Further, unnecessary initial fluid administration necessitates a “de-resuscitative” phase that can prolong hospital stay and is associated with amplification of sepsis-associated organ failures. Specifically, a 2017 analysis of hospital discharge data found that large volume fluid resuscitation in sepsis patients during the first 24 hours of care was associated with higher rates of hospital mortality than was predicted for patients’ disease severity (Mansoori JN, et al. Crit Care. 2020;24[1]:25).
The FRESH trial
The Fluid Response Evaluation in Sepsis Hypotension and Shock (FRESH) trial was a prospective, randomized clinical trial in adults with septic shock comparing PLR-guided SV responsiveness (intervention) as a guide for fluid management with usual care. Patients presented to the ER with sepsis-associated hypotension and anticipated ICU admission. In the intervention arm, patients were assessed for fluid responsiveness (FR) before any clinically driven fluid bolus or increase in vasopressors. If a patient’s stroke volume increased by ≥10% in response to a PLR, they were considered fluid responsive and fluid was recommended as the first therapy. If a patient’s stroke volume increased by <10% then the patient was considered not to be FR and vasopressors were recommended as first-line therapy. The control arm received usual care. The primary end point was the difference in positive fluid balance at the first of either 72 hours or ICU discharge. Patients had received ~2.3 L of crystalloid fluid prior to randomization (~3.5 h from initial presentation), in keeping with 30 mL/kg recommendations. Patients treated with the PLR-guided fluid and pressor protocol had a significant lower net fluid balance (1.37 L (95% CI: 2.53-0.21, P = .021) at 72 hours or ICU discharge. In addition, the intervention group experienced significantly less frequent requirement for renal replacement therapy with a difference of 12.4% (95% CI: 27%-1%, P = .042) as well as a decreased requirement for ventilator use with a difference of 16.42% (95% CI: 33%-0%, P = .044) (Douglas IS, et al. Chest. 2020;158[4]:1431-45).
FRESH demonstrated that PLR-guided FR drove lower fluid balance in patients with septic shock who present to the ER with sepsis and creates a paradigm for future management of fluid and pressor resuscitation beyond the initial 30 mL/kg bolus. Functional evaluation for lack of FR adequately identifies a group of patients with sepsis-associated hypotension who are unlikely to benefit from additional IV fluids to establish hemodynamically stability. It facilitated physiologically informed treatment decisions for the individual patient at a specific moment in their course of treatment as opposed to relying on static measurements and goals that may ultimately not be indicative of fluid responsiveness and circulatory effectiveness. This could reduce the likelihood of fluid overload and associated organ failure and, thus, improve patient outcomes.
Microcirculatory function is significantly impacted by sepsis with a decline in capillary density and inappropriate vasodilation/constriction resulting in insufficient tissue and organ perfusion and increased oxidative stress. Such dysfunction has been found to be associated with worsened patient outcomes, including mortality. However, microcirculatory function does not correlate well with traditionally used macrohemodynamic assessments and treating to improve macrohemodynamic values does not ensure that microcirculation will improve (Charlton M, et al. J Intensive Care Soc. 2017;18(3):221-7).
Ongoing studies are exploring if dynamic fluid-guided resuscitation has the potential to improve survival in sepsis by providing insight into whether the administration of fluid will impact the microcirculation and subsequent organ perfusion of the patient.
Future directions include expanding the dynamic treatment algorithm into other settings, such as rapid response calls, or other patient populations, including those initially presenting with undifferentiated hypotension. While FRESH was not sufficiently powered to detect differences in mortality, there are currently multiple large studies being conducted aimed at determining the impact of a restricted fluid and early vasopressor strategy as compared with a large initial IV fluid bolus on mortality. The results of these studies could be used to determine if the results of FRESH will translate into patient survival outcomes.
Evolution of ECMO. COVID-19 and pulmonary aspergillus. Lung cancer screening. Food as medicine. Air pollution.
Cardiovascular medicine and surgery
Evolution of ECMO as a result of COVID
A year and a half ago, the enormity of this pandemic was only beginning to be realized. Likewise, we have never before been so well-equipped to communicate, investigate, and collaborate through modern innovations. Despite our monumental progress with diagnostics and expedited vaccine production, there remain significant challenges with management of infected individuals suffering from severe sequelae after infection such as respiratory failure. Pharmacologic therapies with steroids, antivirals, and targeted immune modulators have demonstrated modest results at best thus far.
Early intubation unsurprisingly resulted in poor outcomes and a return to other established methods using high-flow nasal cannula and noninvasive positive-pressure ventilation (NIPPV) with a goal of avoiding mechanical ventilation are again the standard of care (Rola P, et al. Clin Exp Emerg Med. 2020 Jun 10. doi: 10.15441/ceem.20.043). Furthermore, limited resources encouraged utilization of established and probably previously underutilized techniques, such as proning with expected improvements in outcomes.
When conventional lung protective mechanical ventilation strategies have been unsuccessful, we have seen improved survival with the incorporation of extracorporeal membrane oxygenation (ECMO), especially when cannulated earlier (Giraud R, et al. 2021. Phys Rep). Many centers now offer ECMO support with considerable expertise and trends toward earlier ECMO cannulation, which permit ultraprotective lung ventilation (Schmidt M, et al. Am J Respir Crit Care Med. 2019 Oct 15;200[8]:1002-12). With benefits that parallel early tracheostomy, early ECMO may permit decreased sedation and earlier mobilization, which contribute to improved outcomes (Levin NM, et al. J Clin Med. 2021 Jan 12;10[2]:25). We may be approaching a paradigm shift where ECMO is performed in lieu of mechanical ventilation (Kurihara C, et al. 2018. Crit Care Med. 2018 Nov;46[11]:e1070-e1073). Future randomized clinical trials will need to be designed to answer this question.
Robert Baeten, II, DSc, PA-C, FCCP
NetWork Steering Committee Member
Chest infections
COVID-19-associated pulmonary aspergillosis: A cause for concern?
Since the global spread of SARS-CoV-2 more than a year ago, reports of secondary infections with Aspergillus spp. have emerged. Like influenza, there has been speculation that severe COVID-19 pneumonia is a unique risk factor for invasive pulmonary aspergillosis (IPA). This entity has been dubbed CAPA, or COVID-associated pulmonary aspergillosis. While the reported incidence of CAPA has ranged from around 5% to 35% in critically ill patients, it has been difficult to distinguish reports of colonization from true infection as histopathologic evidence of disease has been limited. Using stringent diagnostic criteria, a retrospective review of 145 mechanically ventilated patients with COVID-19 found the incidence of CAPA to be 4.8% (Fekkar A, et al. Am J Respir Crit Care Med. 2021 Feb 1;203[3]:307-17) which is similar to other non-COVID ARDS series. The authors found solid organ transplant and prolonged steroid treatment to be risk factors. Like other studies, no comparator group was utilized, limiting the conclusions regarding COVID-19 as an independent risk factor for IPA. Diagnostic criteria have been adapted to assist clinicians and allow for future research: Proven infection requires temporal relation with COVID-19 ICU admission and histopathologic evidence of Aspergillus spp. invasion or positive culture from sterile sites (Koehler P, et al. Lancet Infect Dis. 2020 Dec 14;S1473-3099[20]30847-1).
Aspergillus conidia are ubiquitous in the environment, and the respiratory epithelium and associated cilia act as the first defense against IPA. Distinct from influenza pneumonia, severe COVID-19 causes diffuse alveolar damage and does not appear to cause significant damage to the respiratory epithelium (Borczuk AC, et al. Mod Pathol. 2020;33[11]:2156-68). This coupled with the lack of histopathologic evidence of invasion in most reports of CAPA raises question regarding the extent of the association between COVID-19 and IPA. Nonetheless, immune perturbation caused by COVID-19 immunomodulating therapies, such as corticosteroids and IL-6 inhibitors, may ultimately leave patients susceptible to IPA and other opportunistic infections.
Kelly M. Pennington, MD
Charles S. Dela Cruz, MD
Sebastion Kurz, MD
NetWork Steering Committee Members
Clinical pulmonary medicine
New USPSTF guidelines for lung cancer screening: A step forward
Despite lung cancer being the number one cause of cancer-related death in America, screening for lung cancer remains low, with only 2-16% eligible patients being offered screening since the US Preventive Services Task Force (USPSTF) recommendation in 2013. New guidelines published in JAMA (Krist AH, et al. JAMA. 2021;325[10]:962-970; Meza R, et al. JAMA. 2021;325[10]:988-97; Jonas DE, at al. JAMA. 2021;325[10]:971-87) have suggested broadening eligibility to those 50-80 years old, who are smokers or previously quit in the past 15 years and have a minimum 20 pack-year smoking history (Grade B recommendation). The change lowers the starting age to 50 and the smoking requirement from 30 to 20 pack-years. Based on Cancer Intervention and Surveillance Modeling Network (CISNET) modeling, utilized by the UPSTF, this change can result in 503 (vs. 381 in the prior guideline) cancer deaths averted for every 100,000 adults and an estimated 13% reduction in lung cancer mortality and 6,918 life-years gained.
This recommendation will dramatically increase the number of eligible adults for screening by 6.4 million people, an increase of 86% compared with the 2013 guidelines. Most importantly, the decrease in pack-year requirement to 20 is expected to increase eligibility for women and minimize racial disparities. African American men have a higher incidence of lung cancer with less smoke exposure compared with white men. Non-Hispanic Black, Hispanics, American Indian/Alaska Native persons are hoped to have significant benefit from these new recommendations. Original recommendations in the 2013 guideline mirrored the National Lung Screening Trial, in which 91% participants were White. Regardless of these updated recommendations, serious socioeconomic barriers may continue to limit racial/ethnic minorities from accessing high-quality lung cancer screening programs. Besides changing the screening criteria, barriers to access will need to be addressed to achieve maximal benefits of the lung cancer screening program.
Munish Luthra, MD, FCCP
Samantha D’Annunzio, MD
Steering Committee Members
Interprofessional team
Let food be thy medicine and medicine be thy food – Hippocrates
Recently an article published in The Lancet discussed malnutrition in the patient with COVID-19 infection requiring non-invasive ventilation (NIV) (Turner P, et al. Lancet. 2021 Apr 3;397[10281]:126). It is known that COVID-19 infection causes hyperinflammation and hypercatabolism, resulting in disruption of metabolic pathways leading to muscle wasting, including cardiac muscle dysfunction, muscle weakness, and prolonged fatigue (Singer PJ, et al. 2021. Intensive Med. In press).
Lipids, specifically DHA and EPA, are known to inhibit cyclooxygenase enzyme and may suppress prostaglandin production and block platelet-activating factor. Consumption of carbohydrates with high glycemic indexes can result in free radical synthesis (increasing inflammatory cytokines C reactive protein, tumor necrosis alpha and interleukin-6). Other nutrients known to have an anti-inflammatory role include vitamins A & D, selenium, and copper. Vitamin A is known to enhance an antigen-specific immune response. Probiotics may also play a role in enhancing the immune response (Turner P, et al. 2021. Lancet. 2021 Apr 3;397[10281]:1261).
Patients requiring NIV encounter impaired tolerance to oral nutrition, and enteral nutrition (EN) is prescribed (Singer PJ, et al. 2021. Intensive Med. In press). Advantages of EN are maintenance of gut integrity and intestinal permeability as well as down regulation of the inflammatory response and insulin resistance. Furthermore, negative energy balance is associated with poor outcomes. Better focus on nutrition assessment practices is needed to overcome energy deficits during treatment of COVID-19 pneumonia. An interprofessional team approach increases use of nutritional scores and optimizes nutritional interventions.
If oral nutrition is feasible, prescribing small, frequent meals and high‐protein, calorically dense foods can ensure adequate caloric intake. (Behrens S, et al. Nutr Clin Pract. 2021 Feb;36[1]:105-9). When EN is indicated, the Intensive Care Society endorses the use of fine bore feeding tubes and NIV masks with special nasogastric tube adapters to reduce mask leak. Head-of-bed elevation and avoidance of bolus feeding improve EN tolerance (Pardo T, et al. 2021. Anaesth Crit Care Pain Med. 2020 Dec;39[6]:738-9).
*Due to the novelty of this disease information is limited and further study is warranted.
David W. Unkle, MSN, APN, FCCP
Robert Baeten, DMSc, PA-C, FCCP
Nikky Keer, DO
NetWork Steering Committee Members
Occupational and environmental health
Not just COVID in the air
Particulate matter (PM) is a specific type of air pollution referred to by its size in micrometers. A direct correlation has been shown between non-accidental death and PM2.5 concentration with a 1.5% increase in daily mortality (Schwartz J, et al. J Air Waste Manag Assoc. 1996 Oct;46[10]:927-39). From 2000-2019, PM2.5 concentrations have steadily decreased over 43% (Environmental Protection Agency). Significant decline in air pollution has occurred early in the COVID-19 pandemic. PM2.5 declined in counties from states instituting early non-essential business closures in the U.S. Additionally, NASA models revealed a nearly 20% drop in global nitrogen dioxide concentrations using a COVID-19-free 2020 model to compare with actual space and ground-based observations since February 2020 (NASA Model Reveals How Much COVID-related Pollution Levels Deviated from the Norm. 2020 Nov 17. The pandemic has shown that there is a significant human behavior-driven contribution to air pollution. The historic fire season of 2020 in the western states contributed to record high air pollution with attributable mortality (Liu X, et al. medRxiv 2020.09.20197921). Additionally, the COVID-19 pandemic impeded firefighting response (Burke M, et al. PNAS. 2021;11[2]:e2011048118).
Despite the pandemic related reduction, racial-ethnic disparities continue to exist in consumption of PM2.5. In a model looking at production of PM2.5, defined as consumption by the consumer and exposure as where the product or service originated, African American and Hispanic individuals have up to 12-21% greater pollution exposure within the United States (Tessum CW, et al. Proc Natl Acad Sci USA. 2019 Mar 26;116[13]:6001-6). PM pollution increased the risk of asthma attacks corresponding to zip codes with higher poverty levels and eligibility to Medicaid (O’Lenick CR, et al. Epidemiol Community Health. 2017 Feb;71[2]:129-36). Other studies have shown people with a lower socioeconomic position, have less education, live nearer to major sources of pollution, greater reliance on public transportation and unemployment are at higher risk from effects of PM pollution (American Lung Association. Disparities in the impact of air pollution.
Disclaimer: The views expressed in this article are those of the author(s) and do not reflect the official policy of the Department of Army/Navy/Air Force, Department of Defense, or US government.
Tyler Church, DO
Fellow-in-Training Member
Bathmapriya Balakrishnan, MD
Dixie Harris, MD
NetWork Steering Committee Members
Cardiovascular medicine and surgery
Evolution of ECMO as a result of COVID
A year and a half ago, the enormity of this pandemic was only beginning to be realized. Likewise, we have never before been so well-equipped to communicate, investigate, and collaborate through modern innovations. Despite our monumental progress with diagnostics and expedited vaccine production, there remain significant challenges with management of infected individuals suffering from severe sequelae after infection such as respiratory failure. Pharmacologic therapies with steroids, antivirals, and targeted immune modulators have demonstrated modest results at best thus far.
Early intubation unsurprisingly resulted in poor outcomes and a return to other established methods using high-flow nasal cannula and noninvasive positive-pressure ventilation (NIPPV) with a goal of avoiding mechanical ventilation are again the standard of care (Rola P, et al. Clin Exp Emerg Med. 2020 Jun 10. doi: 10.15441/ceem.20.043). Furthermore, limited resources encouraged utilization of established and probably previously underutilized techniques, such as proning with expected improvements in outcomes.
When conventional lung protective mechanical ventilation strategies have been unsuccessful, we have seen improved survival with the incorporation of extracorporeal membrane oxygenation (ECMO), especially when cannulated earlier (Giraud R, et al. 2021. Phys Rep). Many centers now offer ECMO support with considerable expertise and trends toward earlier ECMO cannulation, which permit ultraprotective lung ventilation (Schmidt M, et al. Am J Respir Crit Care Med. 2019 Oct 15;200[8]:1002-12). With benefits that parallel early tracheostomy, early ECMO may permit decreased sedation and earlier mobilization, which contribute to improved outcomes (Levin NM, et al. J Clin Med. 2021 Jan 12;10[2]:25). We may be approaching a paradigm shift where ECMO is performed in lieu of mechanical ventilation (Kurihara C, et al. 2018. Crit Care Med. 2018 Nov;46[11]:e1070-e1073). Future randomized clinical trials will need to be designed to answer this question.
Robert Baeten, II, DSc, PA-C, FCCP
NetWork Steering Committee Member
Chest infections
COVID-19-associated pulmonary aspergillosis: A cause for concern?
Since the global spread of SARS-CoV-2 more than a year ago, reports of secondary infections with Aspergillus spp. have emerged. Like influenza, there has been speculation that severe COVID-19 pneumonia is a unique risk factor for invasive pulmonary aspergillosis (IPA). This entity has been dubbed CAPA, or COVID-associated pulmonary aspergillosis. While the reported incidence of CAPA has ranged from around 5% to 35% in critically ill patients, it has been difficult to distinguish reports of colonization from true infection as histopathologic evidence of disease has been limited. Using stringent diagnostic criteria, a retrospective review of 145 mechanically ventilated patients with COVID-19 found the incidence of CAPA to be 4.8% (Fekkar A, et al. Am J Respir Crit Care Med. 2021 Feb 1;203[3]:307-17) which is similar to other non-COVID ARDS series. The authors found solid organ transplant and prolonged steroid treatment to be risk factors. Like other studies, no comparator group was utilized, limiting the conclusions regarding COVID-19 as an independent risk factor for IPA. Diagnostic criteria have been adapted to assist clinicians and allow for future research: Proven infection requires temporal relation with COVID-19 ICU admission and histopathologic evidence of Aspergillus spp. invasion or positive culture from sterile sites (Koehler P, et al. Lancet Infect Dis. 2020 Dec 14;S1473-3099[20]30847-1).
Aspergillus conidia are ubiquitous in the environment, and the respiratory epithelium and associated cilia act as the first defense against IPA. Distinct from influenza pneumonia, severe COVID-19 causes diffuse alveolar damage and does not appear to cause significant damage to the respiratory epithelium (Borczuk AC, et al. Mod Pathol. 2020;33[11]:2156-68). This coupled with the lack of histopathologic evidence of invasion in most reports of CAPA raises question regarding the extent of the association between COVID-19 and IPA. Nonetheless, immune perturbation caused by COVID-19 immunomodulating therapies, such as corticosteroids and IL-6 inhibitors, may ultimately leave patients susceptible to IPA and other opportunistic infections.
Kelly M. Pennington, MD
Charles S. Dela Cruz, MD
Sebastion Kurz, MD
NetWork Steering Committee Members
Clinical pulmonary medicine
New USPSTF guidelines for lung cancer screening: A step forward
Despite lung cancer being the number one cause of cancer-related death in America, screening for lung cancer remains low, with only 2-16% eligible patients being offered screening since the US Preventive Services Task Force (USPSTF) recommendation in 2013. New guidelines published in JAMA (Krist AH, et al. JAMA. 2021;325[10]:962-970; Meza R, et al. JAMA. 2021;325[10]:988-97; Jonas DE, at al. JAMA. 2021;325[10]:971-87) have suggested broadening eligibility to those 50-80 years old, who are smokers or previously quit in the past 15 years and have a minimum 20 pack-year smoking history (Grade B recommendation). The change lowers the starting age to 50 and the smoking requirement from 30 to 20 pack-years. Based on Cancer Intervention and Surveillance Modeling Network (CISNET) modeling, utilized by the UPSTF, this change can result in 503 (vs. 381 in the prior guideline) cancer deaths averted for every 100,000 adults and an estimated 13% reduction in lung cancer mortality and 6,918 life-years gained.
This recommendation will dramatically increase the number of eligible adults for screening by 6.4 million people, an increase of 86% compared with the 2013 guidelines. Most importantly, the decrease in pack-year requirement to 20 is expected to increase eligibility for women and minimize racial disparities. African American men have a higher incidence of lung cancer with less smoke exposure compared with white men. Non-Hispanic Black, Hispanics, American Indian/Alaska Native persons are hoped to have significant benefit from these new recommendations. Original recommendations in the 2013 guideline mirrored the National Lung Screening Trial, in which 91% participants were White. Regardless of these updated recommendations, serious socioeconomic barriers may continue to limit racial/ethnic minorities from accessing high-quality lung cancer screening programs. Besides changing the screening criteria, barriers to access will need to be addressed to achieve maximal benefits of the lung cancer screening program.
Munish Luthra, MD, FCCP
Samantha D’Annunzio, MD
Steering Committee Members
Interprofessional team
Let food be thy medicine and medicine be thy food – Hippocrates
Recently an article published in The Lancet discussed malnutrition in the patient with COVID-19 infection requiring non-invasive ventilation (NIV) (Turner P, et al. Lancet. 2021 Apr 3;397[10281]:126). It is known that COVID-19 infection causes hyperinflammation and hypercatabolism, resulting in disruption of metabolic pathways leading to muscle wasting, including cardiac muscle dysfunction, muscle weakness, and prolonged fatigue (Singer PJ, et al. 2021. Intensive Med. In press).
Lipids, specifically DHA and EPA, are known to inhibit cyclooxygenase enzyme and may suppress prostaglandin production and block platelet-activating factor. Consumption of carbohydrates with high glycemic indexes can result in free radical synthesis (increasing inflammatory cytokines C reactive protein, tumor necrosis alpha and interleukin-6). Other nutrients known to have an anti-inflammatory role include vitamins A & D, selenium, and copper. Vitamin A is known to enhance an antigen-specific immune response. Probiotics may also play a role in enhancing the immune response (Turner P, et al. 2021. Lancet. 2021 Apr 3;397[10281]:1261).
Patients requiring NIV encounter impaired tolerance to oral nutrition, and enteral nutrition (EN) is prescribed (Singer PJ, et al. 2021. Intensive Med. In press). Advantages of EN are maintenance of gut integrity and intestinal permeability as well as down regulation of the inflammatory response and insulin resistance. Furthermore, negative energy balance is associated with poor outcomes. Better focus on nutrition assessment practices is needed to overcome energy deficits during treatment of COVID-19 pneumonia. An interprofessional team approach increases use of nutritional scores and optimizes nutritional interventions.
If oral nutrition is feasible, prescribing small, frequent meals and high‐protein, calorically dense foods can ensure adequate caloric intake. (Behrens S, et al. Nutr Clin Pract. 2021 Feb;36[1]:105-9). When EN is indicated, the Intensive Care Society endorses the use of fine bore feeding tubes and NIV masks with special nasogastric tube adapters to reduce mask leak. Head-of-bed elevation and avoidance of bolus feeding improve EN tolerance (Pardo T, et al. 2021. Anaesth Crit Care Pain Med. 2020 Dec;39[6]:738-9).
*Due to the novelty of this disease information is limited and further study is warranted.
David W. Unkle, MSN, APN, FCCP
Robert Baeten, DMSc, PA-C, FCCP
Nikky Keer, DO
NetWork Steering Committee Members
Occupational and environmental health
Not just COVID in the air
Particulate matter (PM) is a specific type of air pollution referred to by its size in micrometers. A direct correlation has been shown between non-accidental death and PM2.5 concentration with a 1.5% increase in daily mortality (Schwartz J, et al. J Air Waste Manag Assoc. 1996 Oct;46[10]:927-39). From 2000-2019, PM2.5 concentrations have steadily decreased over 43% (Environmental Protection Agency). Significant decline in air pollution has occurred early in the COVID-19 pandemic. PM2.5 declined in counties from states instituting early non-essential business closures in the U.S. Additionally, NASA models revealed a nearly 20% drop in global nitrogen dioxide concentrations using a COVID-19-free 2020 model to compare with actual space and ground-based observations since February 2020 (NASA Model Reveals How Much COVID-related Pollution Levels Deviated from the Norm. 2020 Nov 17. The pandemic has shown that there is a significant human behavior-driven contribution to air pollution. The historic fire season of 2020 in the western states contributed to record high air pollution with attributable mortality (Liu X, et al. medRxiv 2020.09.20197921). Additionally, the COVID-19 pandemic impeded firefighting response (Burke M, et al. PNAS. 2021;11[2]:e2011048118).
Despite the pandemic related reduction, racial-ethnic disparities continue to exist in consumption of PM2.5. In a model looking at production of PM2.5, defined as consumption by the consumer and exposure as where the product or service originated, African American and Hispanic individuals have up to 12-21% greater pollution exposure within the United States (Tessum CW, et al. Proc Natl Acad Sci USA. 2019 Mar 26;116[13]:6001-6). PM pollution increased the risk of asthma attacks corresponding to zip codes with higher poverty levels and eligibility to Medicaid (O’Lenick CR, et al. Epidemiol Community Health. 2017 Feb;71[2]:129-36). Other studies have shown people with a lower socioeconomic position, have less education, live nearer to major sources of pollution, greater reliance on public transportation and unemployment are at higher risk from effects of PM pollution (American Lung Association. Disparities in the impact of air pollution.
Disclaimer: The views expressed in this article are those of the author(s) and do not reflect the official policy of the Department of Army/Navy/Air Force, Department of Defense, or US government.
Tyler Church, DO
Fellow-in-Training Member
Bathmapriya Balakrishnan, MD
Dixie Harris, MD
NetWork Steering Committee Members
Cardiovascular medicine and surgery
Evolution of ECMO as a result of COVID
A year and a half ago, the enormity of this pandemic was only beginning to be realized. Likewise, we have never before been so well-equipped to communicate, investigate, and collaborate through modern innovations. Despite our monumental progress with diagnostics and expedited vaccine production, there remain significant challenges with management of infected individuals suffering from severe sequelae after infection such as respiratory failure. Pharmacologic therapies with steroids, antivirals, and targeted immune modulators have demonstrated modest results at best thus far.
Early intubation unsurprisingly resulted in poor outcomes and a return to other established methods using high-flow nasal cannula and noninvasive positive-pressure ventilation (NIPPV) with a goal of avoiding mechanical ventilation are again the standard of care (Rola P, et al. Clin Exp Emerg Med. 2020 Jun 10. doi: 10.15441/ceem.20.043). Furthermore, limited resources encouraged utilization of established and probably previously underutilized techniques, such as proning with expected improvements in outcomes.
When conventional lung protective mechanical ventilation strategies have been unsuccessful, we have seen improved survival with the incorporation of extracorporeal membrane oxygenation (ECMO), especially when cannulated earlier (Giraud R, et al. 2021. Phys Rep). Many centers now offer ECMO support with considerable expertise and trends toward earlier ECMO cannulation, which permit ultraprotective lung ventilation (Schmidt M, et al. Am J Respir Crit Care Med. 2019 Oct 15;200[8]:1002-12). With benefits that parallel early tracheostomy, early ECMO may permit decreased sedation and earlier mobilization, which contribute to improved outcomes (Levin NM, et al. J Clin Med. 2021 Jan 12;10[2]:25). We may be approaching a paradigm shift where ECMO is performed in lieu of mechanical ventilation (Kurihara C, et al. 2018. Crit Care Med. 2018 Nov;46[11]:e1070-e1073). Future randomized clinical trials will need to be designed to answer this question.
Robert Baeten, II, DSc, PA-C, FCCP
NetWork Steering Committee Member
Chest infections
COVID-19-associated pulmonary aspergillosis: A cause for concern?
Since the global spread of SARS-CoV-2 more than a year ago, reports of secondary infections with Aspergillus spp. have emerged. Like influenza, there has been speculation that severe COVID-19 pneumonia is a unique risk factor for invasive pulmonary aspergillosis (IPA). This entity has been dubbed CAPA, or COVID-associated pulmonary aspergillosis. While the reported incidence of CAPA has ranged from around 5% to 35% in critically ill patients, it has been difficult to distinguish reports of colonization from true infection as histopathologic evidence of disease has been limited. Using stringent diagnostic criteria, a retrospective review of 145 mechanically ventilated patients with COVID-19 found the incidence of CAPA to be 4.8% (Fekkar A, et al. Am J Respir Crit Care Med. 2021 Feb 1;203[3]:307-17) which is similar to other non-COVID ARDS series. The authors found solid organ transplant and prolonged steroid treatment to be risk factors. Like other studies, no comparator group was utilized, limiting the conclusions regarding COVID-19 as an independent risk factor for IPA. Diagnostic criteria have been adapted to assist clinicians and allow for future research: Proven infection requires temporal relation with COVID-19 ICU admission and histopathologic evidence of Aspergillus spp. invasion or positive culture from sterile sites (Koehler P, et al. Lancet Infect Dis. 2020 Dec 14;S1473-3099[20]30847-1).
Aspergillus conidia are ubiquitous in the environment, and the respiratory epithelium and associated cilia act as the first defense against IPA. Distinct from influenza pneumonia, severe COVID-19 causes diffuse alveolar damage and does not appear to cause significant damage to the respiratory epithelium (Borczuk AC, et al. Mod Pathol. 2020;33[11]:2156-68). This coupled with the lack of histopathologic evidence of invasion in most reports of CAPA raises question regarding the extent of the association between COVID-19 and IPA. Nonetheless, immune perturbation caused by COVID-19 immunomodulating therapies, such as corticosteroids and IL-6 inhibitors, may ultimately leave patients susceptible to IPA and other opportunistic infections.
Kelly M. Pennington, MD
Charles S. Dela Cruz, MD
Sebastion Kurz, MD
NetWork Steering Committee Members
Clinical pulmonary medicine
New USPSTF guidelines for lung cancer screening: A step forward
Despite lung cancer being the number one cause of cancer-related death in America, screening for lung cancer remains low, with only 2-16% eligible patients being offered screening since the US Preventive Services Task Force (USPSTF) recommendation in 2013. New guidelines published in JAMA (Krist AH, et al. JAMA. 2021;325[10]:962-970; Meza R, et al. JAMA. 2021;325[10]:988-97; Jonas DE, at al. JAMA. 2021;325[10]:971-87) have suggested broadening eligibility to those 50-80 years old, who are smokers or previously quit in the past 15 years and have a minimum 20 pack-year smoking history (Grade B recommendation). The change lowers the starting age to 50 and the smoking requirement from 30 to 20 pack-years. Based on Cancer Intervention and Surveillance Modeling Network (CISNET) modeling, utilized by the UPSTF, this change can result in 503 (vs. 381 in the prior guideline) cancer deaths averted for every 100,000 adults and an estimated 13% reduction in lung cancer mortality and 6,918 life-years gained.
This recommendation will dramatically increase the number of eligible adults for screening by 6.4 million people, an increase of 86% compared with the 2013 guidelines. Most importantly, the decrease in pack-year requirement to 20 is expected to increase eligibility for women and minimize racial disparities. African American men have a higher incidence of lung cancer with less smoke exposure compared with white men. Non-Hispanic Black, Hispanics, American Indian/Alaska Native persons are hoped to have significant benefit from these new recommendations. Original recommendations in the 2013 guideline mirrored the National Lung Screening Trial, in which 91% participants were White. Regardless of these updated recommendations, serious socioeconomic barriers may continue to limit racial/ethnic minorities from accessing high-quality lung cancer screening programs. Besides changing the screening criteria, barriers to access will need to be addressed to achieve maximal benefits of the lung cancer screening program.
Munish Luthra, MD, FCCP
Samantha D’Annunzio, MD
Steering Committee Members
Interprofessional team
Let food be thy medicine and medicine be thy food – Hippocrates
Recently an article published in The Lancet discussed malnutrition in the patient with COVID-19 infection requiring non-invasive ventilation (NIV) (Turner P, et al. Lancet. 2021 Apr 3;397[10281]:126). It is known that COVID-19 infection causes hyperinflammation and hypercatabolism, resulting in disruption of metabolic pathways leading to muscle wasting, including cardiac muscle dysfunction, muscle weakness, and prolonged fatigue (Singer PJ, et al. 2021. Intensive Med. In press).
Lipids, specifically DHA and EPA, are known to inhibit cyclooxygenase enzyme and may suppress prostaglandin production and block platelet-activating factor. Consumption of carbohydrates with high glycemic indexes can result in free radical synthesis (increasing inflammatory cytokines C reactive protein, tumor necrosis alpha and interleukin-6). Other nutrients known to have an anti-inflammatory role include vitamins A & D, selenium, and copper. Vitamin A is known to enhance an antigen-specific immune response. Probiotics may also play a role in enhancing the immune response (Turner P, et al. 2021. Lancet. 2021 Apr 3;397[10281]:1261).
Patients requiring NIV encounter impaired tolerance to oral nutrition, and enteral nutrition (EN) is prescribed (Singer PJ, et al. 2021. Intensive Med. In press). Advantages of EN are maintenance of gut integrity and intestinal permeability as well as down regulation of the inflammatory response and insulin resistance. Furthermore, negative energy balance is associated with poor outcomes. Better focus on nutrition assessment practices is needed to overcome energy deficits during treatment of COVID-19 pneumonia. An interprofessional team approach increases use of nutritional scores and optimizes nutritional interventions.
If oral nutrition is feasible, prescribing small, frequent meals and high‐protein, calorically dense foods can ensure adequate caloric intake. (Behrens S, et al. Nutr Clin Pract. 2021 Feb;36[1]:105-9). When EN is indicated, the Intensive Care Society endorses the use of fine bore feeding tubes and NIV masks with special nasogastric tube adapters to reduce mask leak. Head-of-bed elevation and avoidance of bolus feeding improve EN tolerance (Pardo T, et al. 2021. Anaesth Crit Care Pain Med. 2020 Dec;39[6]:738-9).
*Due to the novelty of this disease information is limited and further study is warranted.
David W. Unkle, MSN, APN, FCCP
Robert Baeten, DMSc, PA-C, FCCP
Nikky Keer, DO
NetWork Steering Committee Members
Occupational and environmental health
Not just COVID in the air
Particulate matter (PM) is a specific type of air pollution referred to by its size in micrometers. A direct correlation has been shown between non-accidental death and PM2.5 concentration with a 1.5% increase in daily mortality (Schwartz J, et al. J Air Waste Manag Assoc. 1996 Oct;46[10]:927-39). From 2000-2019, PM2.5 concentrations have steadily decreased over 43% (Environmental Protection Agency). Significant decline in air pollution has occurred early in the COVID-19 pandemic. PM2.5 declined in counties from states instituting early non-essential business closures in the U.S. Additionally, NASA models revealed a nearly 20% drop in global nitrogen dioxide concentrations using a COVID-19-free 2020 model to compare with actual space and ground-based observations since February 2020 (NASA Model Reveals How Much COVID-related Pollution Levels Deviated from the Norm. 2020 Nov 17. The pandemic has shown that there is a significant human behavior-driven contribution to air pollution. The historic fire season of 2020 in the western states contributed to record high air pollution with attributable mortality (Liu X, et al. medRxiv 2020.09.20197921). Additionally, the COVID-19 pandemic impeded firefighting response (Burke M, et al. PNAS. 2021;11[2]:e2011048118).
Despite the pandemic related reduction, racial-ethnic disparities continue to exist in consumption of PM2.5. In a model looking at production of PM2.5, defined as consumption by the consumer and exposure as where the product or service originated, African American and Hispanic individuals have up to 12-21% greater pollution exposure within the United States (Tessum CW, et al. Proc Natl Acad Sci USA. 2019 Mar 26;116[13]:6001-6). PM pollution increased the risk of asthma attacks corresponding to zip codes with higher poverty levels and eligibility to Medicaid (O’Lenick CR, et al. Epidemiol Community Health. 2017 Feb;71[2]:129-36). Other studies have shown people with a lower socioeconomic position, have less education, live nearer to major sources of pollution, greater reliance on public transportation and unemployment are at higher risk from effects of PM pollution (American Lung Association. Disparities in the impact of air pollution.
Disclaimer: The views expressed in this article are those of the author(s) and do not reflect the official policy of the Department of Army/Navy/Air Force, Department of Defense, or US government.
Tyler Church, DO
Fellow-in-Training Member
Bathmapriya Balakrishnan, MD
Dixie Harris, MD
NetWork Steering Committee Members
Are we there yet? Lung cancer screening – current landscape
Lung cancer is the second-most common cancer and one of the leading causes of mortality in the United States among both men and women. It accounts for almost 25% of all cancer deaths, and every year more people die of lung cancer than colon, breast, and prostate cancers combined. The American Cancer Society estimates about 235,760 new lung cancer cases and about 131,880 deaths from lung cancer in 2021.
Smoking and increasing age are the two most important risk factors for lung cancer. Lung cancer has a higher incidence among Black men than White men, and among White women compared with Black women. These differences are likely related to smoking exposure. Early diagnosis of lung cancer can improve survival, and hence screening for lung cancer in high-risk populations is desired. Among the available cancer screening tests, radiology is primarily involved in breast and lung cancer screening (LCS). In 2011, the National Lung Screening Trial (NLST) showed a benefit of annual low- dose chest CT for LCS, with about 20% reduction in lung cancer-related mortality in high-risk participants compared with chest radiographs (Aberle DR, et al. N Engl J Med. 2011 Aug 4;365[5]:395-409).
In 2013, the United States Preventative Services Task Force (USPSTF) issued a grade B recommendation in support of annual LCS by CT scan for individuals between the ages of 55-80 years with smoking history of 30 or more pack-years who are current smokers or had quit smoking in the last 15 years. Many other professional societies followed with their own recommendations with minor differences. In 2015, after the Centers for Medicare and Medicaid (CMS) decision of coverage, millions of Americans at high risk became eligible for CT LCS with no copayment or cost sharing by the patient.
The results from the European NELSON trial in 2020 augmented the NLST data showing a 24% decrease in lung cancer mortality. Nodules were measured using volume and volume doubling time rather than bidimensional axial measurements, reducing the false-positive results to 56% compared with 96% in NLST. With growing evidence of the benefits from LCS, recently USPSTF summarized with moderate certainty that annual LCS CT has moderate net benefit in people at high risk for lung cancer based on age, cumulative smoking exposure, and years since quitting smoking.
In March 2021, USPSTF has issued new recommendations with a decrease in the screening age to 50 years, and the smoking history that triggers screening to 20 pack-years (Screening for Lung Cancer: USPSTF Statement. JAMA. 2021 Mar 9;325[10]:962-70. doi: 10.1001/jama.2021.1117). These expanded eligibility criteria are projected to double the number of eligible candidates of LCS in the United States, reduce annual deaths by up to 50%, and benefit minorities and women. By widening the screening criteria to include younger individuals and who have smoked less tobacco, more lives will be saved by early detection of lung cancer. Since the NLST and NELSON trials enrolled relatively healthy people, USPSTF recommends discontinuation of screening once the person has not smoked for 15 years and in persons with any health problem that severely limits the life expectancy or the ability or willingness to undergo surgery. All screening programs must incorporate smoking cessation counseling and interventions for all the enrolled individuals who are current smokers. The USPSTF has also made recommendations on interventions to prevent the initiation of tobacco use in children and adolescents, including counseling and pharmacotherapy.
The decision to undergo LCS is inherently complex, and primary care and pulmonary physicians play a pivotal role by identifying the eligible patients, participating in shared decision-making (SDM), offering smoking cessation, ordering the CT, and managing follow-up. SDM between the patient and clinician includes a discussion of the benefits, risks, limitations, and potential harms of screening. The potential harms of screening include overdiagnosis, false-positive results, incidental findings, and the anxiety leading to further testing or follow-up. The risk of radiation exposure is markedly reduced using low-dose CT protocols compared with conventional chest CT. SDM visit also emphasizes the importance of adherence to annual screening and patient willingness and ability to undergo treatment if required. In 2015, CMS approved the addition of LCS counseling and SDM visits that are performed by physicians or qualified nonphysician practitioners (physician assistant, nurse practitioner, or clinical nurse specialist). Studies have shown that these visits improve the screening uptake rate.
To minimize the variations in the evaluation and management of screen-detected lung nodules, the American College of Radiology (ACR) developed the Lung Imaging Reporting and Data System (Lung-RADS) to be used in LCS CT reports. The latest revised version 1.1 of Lung-RADS was released in 2019. The Lung-RADS defines a positive screen and provides accepted nodule care pathways depending on their size, characteristics, and additional findings, and has been shown to decrease the rate of false-positive results in LCS. To be a designated LCS center, the department of radiology must comply with stringent requirements of technical and facility specification, with radiologist qualification, and with reporting and communication as outlined by the ACR. In addition, participation in the National LCS Registry to meet CMS quality reporting requirements is mandatory for facilities to be reimbursed by CMS.
After more than 10 years since its inception, the participation in LCS has been low. Out of 8 million eligible Americans, less than 4% have been screened (American Cancer Society, NSCLC statistics 2020) compared with breast cancer (up to 75%) (Breast Cancer: Facts and Figures 2019-2020). Adherence to annual LCS between 1-3 years in the US is only about 55%. Non-White patients, current smokers, those aged 65-73 years, and those who lack a college education are most likely to be less adherent to follow-up screening. There are hurdles at multiple levels including but not limited to patient and physician awareness, patient enrollment, adherence, follow-up, and insurance coverage. Expanding the reach of LCS in socially and economically disadvantaged, racial and ethnic minorities, and women has been even more challenging.
Significant differences exist in opinions and practices between primary care physicians (PCPs) and pulmonologists regarding referral for LCS and its benefits. Educational intervention at the PCP level aimed at awareness of USPSTF guidelines may improve utilization and adherence to screening. Increasing lung cancer awareness by community outreach programs, promoting related discussions, and providing information about available screening services to eligible population is crucial to derive the maximum benefits of LCS. Presenting decision aid tools on smartphones and online has shown to improve the participants’ knowledge of LCS, to reduce the decisional conflict, and to be acceptable among patients and providers. Implementation strategies such as involving a nonphysician provider, keeping the training on these tools brief and simple, and providing it to participants prior to the clinical encounter might be effective. Electronic medical record systems can be optimized to simplify the ordering procedure to ensure the eligibility criteria are met, to provide results to the physicians, and to direct further management of positive screen results. Most LCS programs have a nonphysician program coordinator to convey the results to the patients and physician, to send out reminders for scheduled follow up appointment, and to maintain the registry data.
In the future, newer imaging technology, and molecular biomarkers or other technologies to differentiate lung cancer more accurately from a benign nodule, and to determine its aggressiveness, will supplement the LCS to decrease false positive results. Better risk prediction models will influence screening eligibility and prognostication in a screen-detected cancer. Robust data collection from ongoing clinical programs will determine if the benefits of LCS seen in clinical trials are comparable when applied to diverse community settings.
Dr. Stowell and Dr. Sonavane are with the Mayo Clinic in Jacksonville, Fla.
Lung cancer is the second-most common cancer and one of the leading causes of mortality in the United States among both men and women. It accounts for almost 25% of all cancer deaths, and every year more people die of lung cancer than colon, breast, and prostate cancers combined. The American Cancer Society estimates about 235,760 new lung cancer cases and about 131,880 deaths from lung cancer in 2021.
Smoking and increasing age are the two most important risk factors for lung cancer. Lung cancer has a higher incidence among Black men than White men, and among White women compared with Black women. These differences are likely related to smoking exposure. Early diagnosis of lung cancer can improve survival, and hence screening for lung cancer in high-risk populations is desired. Among the available cancer screening tests, radiology is primarily involved in breast and lung cancer screening (LCS). In 2011, the National Lung Screening Trial (NLST) showed a benefit of annual low- dose chest CT for LCS, with about 20% reduction in lung cancer-related mortality in high-risk participants compared with chest radiographs (Aberle DR, et al. N Engl J Med. 2011 Aug 4;365[5]:395-409).
In 2013, the United States Preventative Services Task Force (USPSTF) issued a grade B recommendation in support of annual LCS by CT scan for individuals between the ages of 55-80 years with smoking history of 30 or more pack-years who are current smokers or had quit smoking in the last 15 years. Many other professional societies followed with their own recommendations with minor differences. In 2015, after the Centers for Medicare and Medicaid (CMS) decision of coverage, millions of Americans at high risk became eligible for CT LCS with no copayment or cost sharing by the patient.
The results from the European NELSON trial in 2020 augmented the NLST data showing a 24% decrease in lung cancer mortality. Nodules were measured using volume and volume doubling time rather than bidimensional axial measurements, reducing the false-positive results to 56% compared with 96% in NLST. With growing evidence of the benefits from LCS, recently USPSTF summarized with moderate certainty that annual LCS CT has moderate net benefit in people at high risk for lung cancer based on age, cumulative smoking exposure, and years since quitting smoking.
In March 2021, USPSTF has issued new recommendations with a decrease in the screening age to 50 years, and the smoking history that triggers screening to 20 pack-years (Screening for Lung Cancer: USPSTF Statement. JAMA. 2021 Mar 9;325[10]:962-70. doi: 10.1001/jama.2021.1117). These expanded eligibility criteria are projected to double the number of eligible candidates of LCS in the United States, reduce annual deaths by up to 50%, and benefit minorities and women. By widening the screening criteria to include younger individuals and who have smoked less tobacco, more lives will be saved by early detection of lung cancer. Since the NLST and NELSON trials enrolled relatively healthy people, USPSTF recommends discontinuation of screening once the person has not smoked for 15 years and in persons with any health problem that severely limits the life expectancy or the ability or willingness to undergo surgery. All screening programs must incorporate smoking cessation counseling and interventions for all the enrolled individuals who are current smokers. The USPSTF has also made recommendations on interventions to prevent the initiation of tobacco use in children and adolescents, including counseling and pharmacotherapy.
The decision to undergo LCS is inherently complex, and primary care and pulmonary physicians play a pivotal role by identifying the eligible patients, participating in shared decision-making (SDM), offering smoking cessation, ordering the CT, and managing follow-up. SDM between the patient and clinician includes a discussion of the benefits, risks, limitations, and potential harms of screening. The potential harms of screening include overdiagnosis, false-positive results, incidental findings, and the anxiety leading to further testing or follow-up. The risk of radiation exposure is markedly reduced using low-dose CT protocols compared with conventional chest CT. SDM visit also emphasizes the importance of adherence to annual screening and patient willingness and ability to undergo treatment if required. In 2015, CMS approved the addition of LCS counseling and SDM visits that are performed by physicians or qualified nonphysician practitioners (physician assistant, nurse practitioner, or clinical nurse specialist). Studies have shown that these visits improve the screening uptake rate.
To minimize the variations in the evaluation and management of screen-detected lung nodules, the American College of Radiology (ACR) developed the Lung Imaging Reporting and Data System (Lung-RADS) to be used in LCS CT reports. The latest revised version 1.1 of Lung-RADS was released in 2019. The Lung-RADS defines a positive screen and provides accepted nodule care pathways depending on their size, characteristics, and additional findings, and has been shown to decrease the rate of false-positive results in LCS. To be a designated LCS center, the department of radiology must comply with stringent requirements of technical and facility specification, with radiologist qualification, and with reporting and communication as outlined by the ACR. In addition, participation in the National LCS Registry to meet CMS quality reporting requirements is mandatory for facilities to be reimbursed by CMS.
After more than 10 years since its inception, the participation in LCS has been low. Out of 8 million eligible Americans, less than 4% have been screened (American Cancer Society, NSCLC statistics 2020) compared with breast cancer (up to 75%) (Breast Cancer: Facts and Figures 2019-2020). Adherence to annual LCS between 1-3 years in the US is only about 55%. Non-White patients, current smokers, those aged 65-73 years, and those who lack a college education are most likely to be less adherent to follow-up screening. There are hurdles at multiple levels including but not limited to patient and physician awareness, patient enrollment, adherence, follow-up, and insurance coverage. Expanding the reach of LCS in socially and economically disadvantaged, racial and ethnic minorities, and women has been even more challenging.
Significant differences exist in opinions and practices between primary care physicians (PCPs) and pulmonologists regarding referral for LCS and its benefits. Educational intervention at the PCP level aimed at awareness of USPSTF guidelines may improve utilization and adherence to screening. Increasing lung cancer awareness by community outreach programs, promoting related discussions, and providing information about available screening services to eligible population is crucial to derive the maximum benefits of LCS. Presenting decision aid tools on smartphones and online has shown to improve the participants’ knowledge of LCS, to reduce the decisional conflict, and to be acceptable among patients and providers. Implementation strategies such as involving a nonphysician provider, keeping the training on these tools brief and simple, and providing it to participants prior to the clinical encounter might be effective. Electronic medical record systems can be optimized to simplify the ordering procedure to ensure the eligibility criteria are met, to provide results to the physicians, and to direct further management of positive screen results. Most LCS programs have a nonphysician program coordinator to convey the results to the patients and physician, to send out reminders for scheduled follow up appointment, and to maintain the registry data.
In the future, newer imaging technology, and molecular biomarkers or other technologies to differentiate lung cancer more accurately from a benign nodule, and to determine its aggressiveness, will supplement the LCS to decrease false positive results. Better risk prediction models will influence screening eligibility and prognostication in a screen-detected cancer. Robust data collection from ongoing clinical programs will determine if the benefits of LCS seen in clinical trials are comparable when applied to diverse community settings.
Dr. Stowell and Dr. Sonavane are with the Mayo Clinic in Jacksonville, Fla.
Lung cancer is the second-most common cancer and one of the leading causes of mortality in the United States among both men and women. It accounts for almost 25% of all cancer deaths, and every year more people die of lung cancer than colon, breast, and prostate cancers combined. The American Cancer Society estimates about 235,760 new lung cancer cases and about 131,880 deaths from lung cancer in 2021.
Smoking and increasing age are the two most important risk factors for lung cancer. Lung cancer has a higher incidence among Black men than White men, and among White women compared with Black women. These differences are likely related to smoking exposure. Early diagnosis of lung cancer can improve survival, and hence screening for lung cancer in high-risk populations is desired. Among the available cancer screening tests, radiology is primarily involved in breast and lung cancer screening (LCS). In 2011, the National Lung Screening Trial (NLST) showed a benefit of annual low- dose chest CT for LCS, with about 20% reduction in lung cancer-related mortality in high-risk participants compared with chest radiographs (Aberle DR, et al. N Engl J Med. 2011 Aug 4;365[5]:395-409).
In 2013, the United States Preventative Services Task Force (USPSTF) issued a grade B recommendation in support of annual LCS by CT scan for individuals between the ages of 55-80 years with smoking history of 30 or more pack-years who are current smokers or had quit smoking in the last 15 years. Many other professional societies followed with their own recommendations with minor differences. In 2015, after the Centers for Medicare and Medicaid (CMS) decision of coverage, millions of Americans at high risk became eligible for CT LCS with no copayment or cost sharing by the patient.
The results from the European NELSON trial in 2020 augmented the NLST data showing a 24% decrease in lung cancer mortality. Nodules were measured using volume and volume doubling time rather than bidimensional axial measurements, reducing the false-positive results to 56% compared with 96% in NLST. With growing evidence of the benefits from LCS, recently USPSTF summarized with moderate certainty that annual LCS CT has moderate net benefit in people at high risk for lung cancer based on age, cumulative smoking exposure, and years since quitting smoking.
In March 2021, USPSTF has issued new recommendations with a decrease in the screening age to 50 years, and the smoking history that triggers screening to 20 pack-years (Screening for Lung Cancer: USPSTF Statement. JAMA. 2021 Mar 9;325[10]:962-70. doi: 10.1001/jama.2021.1117). These expanded eligibility criteria are projected to double the number of eligible candidates of LCS in the United States, reduce annual deaths by up to 50%, and benefit minorities and women. By widening the screening criteria to include younger individuals and who have smoked less tobacco, more lives will be saved by early detection of lung cancer. Since the NLST and NELSON trials enrolled relatively healthy people, USPSTF recommends discontinuation of screening once the person has not smoked for 15 years and in persons with any health problem that severely limits the life expectancy or the ability or willingness to undergo surgery. All screening programs must incorporate smoking cessation counseling and interventions for all the enrolled individuals who are current smokers. The USPSTF has also made recommendations on interventions to prevent the initiation of tobacco use in children and adolescents, including counseling and pharmacotherapy.
The decision to undergo LCS is inherently complex, and primary care and pulmonary physicians play a pivotal role by identifying the eligible patients, participating in shared decision-making (SDM), offering smoking cessation, ordering the CT, and managing follow-up. SDM between the patient and clinician includes a discussion of the benefits, risks, limitations, and potential harms of screening. The potential harms of screening include overdiagnosis, false-positive results, incidental findings, and the anxiety leading to further testing or follow-up. The risk of radiation exposure is markedly reduced using low-dose CT protocols compared with conventional chest CT. SDM visit also emphasizes the importance of adherence to annual screening and patient willingness and ability to undergo treatment if required. In 2015, CMS approved the addition of LCS counseling and SDM visits that are performed by physicians or qualified nonphysician practitioners (physician assistant, nurse practitioner, or clinical nurse specialist). Studies have shown that these visits improve the screening uptake rate.
To minimize the variations in the evaluation and management of screen-detected lung nodules, the American College of Radiology (ACR) developed the Lung Imaging Reporting and Data System (Lung-RADS) to be used in LCS CT reports. The latest revised version 1.1 of Lung-RADS was released in 2019. The Lung-RADS defines a positive screen and provides accepted nodule care pathways depending on their size, characteristics, and additional findings, and has been shown to decrease the rate of false-positive results in LCS. To be a designated LCS center, the department of radiology must comply with stringent requirements of technical and facility specification, with radiologist qualification, and with reporting and communication as outlined by the ACR. In addition, participation in the National LCS Registry to meet CMS quality reporting requirements is mandatory for facilities to be reimbursed by CMS.
After more than 10 years since its inception, the participation in LCS has been low. Out of 8 million eligible Americans, less than 4% have been screened (American Cancer Society, NSCLC statistics 2020) compared with breast cancer (up to 75%) (Breast Cancer: Facts and Figures 2019-2020). Adherence to annual LCS between 1-3 years in the US is only about 55%. Non-White patients, current smokers, those aged 65-73 years, and those who lack a college education are most likely to be less adherent to follow-up screening. There are hurdles at multiple levels including but not limited to patient and physician awareness, patient enrollment, adherence, follow-up, and insurance coverage. Expanding the reach of LCS in socially and economically disadvantaged, racial and ethnic minorities, and women has been even more challenging.
Significant differences exist in opinions and practices between primary care physicians (PCPs) and pulmonologists regarding referral for LCS and its benefits. Educational intervention at the PCP level aimed at awareness of USPSTF guidelines may improve utilization and adherence to screening. Increasing lung cancer awareness by community outreach programs, promoting related discussions, and providing information about available screening services to eligible population is crucial to derive the maximum benefits of LCS. Presenting decision aid tools on smartphones and online has shown to improve the participants’ knowledge of LCS, to reduce the decisional conflict, and to be acceptable among patients and providers. Implementation strategies such as involving a nonphysician provider, keeping the training on these tools brief and simple, and providing it to participants prior to the clinical encounter might be effective. Electronic medical record systems can be optimized to simplify the ordering procedure to ensure the eligibility criteria are met, to provide results to the physicians, and to direct further management of positive screen results. Most LCS programs have a nonphysician program coordinator to convey the results to the patients and physician, to send out reminders for scheduled follow up appointment, and to maintain the registry data.
In the future, newer imaging technology, and molecular biomarkers or other technologies to differentiate lung cancer more accurately from a benign nodule, and to determine its aggressiveness, will supplement the LCS to decrease false positive results. Better risk prediction models will influence screening eligibility and prognostication in a screen-detected cancer. Robust data collection from ongoing clinical programs will determine if the benefits of LCS seen in clinical trials are comparable when applied to diverse community settings.
Dr. Stowell and Dr. Sonavane are with the Mayo Clinic in Jacksonville, Fla.
CHEST 2021 safety efforts – everyone has a role
Over the past year, you’ve had to adapt to Zoom calls and socially distanced learning. It’s time to come back together, face-to-face, for our top-tier learning event in sunny Orlando, Florida.
Grab your sunscreen and book your flights – we’re ready to welcome you back to CHEST 2021 with team-focused learning sessions, immersive gaming opportunities, expert-led faculty presentations, and more. We are making the meeting as safe as possible so you can attend in person.
After careful planning, we are excited to be able at the Orange County Convention Center (OCCC) for CHEST 2021. Health and safety are our biggest concerns for the meeting, which is why we chose this location. The convention center features the extra square footage we needed to design a meeting space with ample room for social distancing.
We are committed to create a meeting experience where you can safely and effectively conduct business, network with colleagues, and experience high-quality education. With your feedback, we have implemented COVID-19 safety measures similar to what is used in your hospitals and facilities. To ensure your health and safety, there will be a few requirements asked of in-person attendees.
Preparing for CHEST 2021
As the pandemic continues and vaccines are more readily available, we are requiring all attendees – participants, vendors, and staff – to be vaccinated to attend in person in Orlando, Florida. Your second vaccination shot should take place at least 2 weeks prior to the conference start. When you complete your registration information, you will be asked to attest that you have or will have completed an FDA-approved vaccination for COVID-19 by October 17, 2021.
We also suggest scheduling extra time at your arrival to the conference site. Realize that registration, lunch lines, hotel check-in, etc, may take longer as we navigate a new way to meet in person. This year, registration will be contactless. Have your digital or print confirmation ready when you arrive – the more prepared you are, the faster registration will be.
While the venue will regularly sanitize all high touch points in the public space throughout the day, remember to pack any personal supplies you may need for individual use, especially masks. Attendees will be required to wear a mask covering the mouth and nose at all times during the meeting. There will be masks on-site in case you forget or misplace your own.
Before making your way to Orlando, complete one last health self-assessment. Are you symptom free? Consider what advice you would give your patient if they felt the way you do in that moment. When in doubt, stay home and join us online. That’s one of the benefits of CHEST 2021.
Keeping safe while experiencing CHEST 2021
Any time you are in the conference center and the Hilton Hotel, the no-contact policy is applicable. Greet your colleagues and new friends using elbow bumps, waves, and any other form of contactless gestures. We will save our handshakes and hugs for CHEST 2022!
By attending in person, you are also agreeing to perform a health status self-check every day for any onset of COVID symptoms as defined by the CDC. If you are feeling ill, immediately notify the first aid office at the meeting.
Help us deliver a high-quality experience with the lowest reasonable risk in a manner that protects us all by complying to these health and safety measures. In addition, the layout and schedule of the conference is being designed to allow time for cleaning spaces between sessions. This means more time to get to your next location, visit the exhibit hall, or spend with your colleagues.
Our commitment to your safety
CHEST is taking extra precautions to keep you safe too – it’s not just on you! Daily temperature screenings will be conducted upon entry to the convention center and Hilton Orlando for everyone.
During the meeting, floor graphics will be used to outline 6-ft social distancing. In the concession areas, seating will be properly distanced and transparent shields will be in place. The exhibit hall will have extra wide aisles, which are not only safe, but easier to move through.
Public space and public restrooms are monitored by OCCC Environmental Services. They conduct sanitizing tasks within the restroom banks throughout the day and a thorough cleaning overnight. They also regularly sanitize all high touch points in the public space throughout the day as well; ie, door handles, ATMs, escalator handrails, elevator buttons, etc.
Staff and security have been increased to provide the best customer service and information accessibility to all in-person attendees. Medical personnel will also be present on site and available to help individuals who are feeling unwell.
It’s been a long year apart from our CHEST community. We can’t wait to see you in Orlando, Florida, October 17-20, for the high-quality education you expect.
Over the past year, you’ve had to adapt to Zoom calls and socially distanced learning. It’s time to come back together, face-to-face, for our top-tier learning event in sunny Orlando, Florida.
Grab your sunscreen and book your flights – we’re ready to welcome you back to CHEST 2021 with team-focused learning sessions, immersive gaming opportunities, expert-led faculty presentations, and more. We are making the meeting as safe as possible so you can attend in person.
After careful planning, we are excited to be able at the Orange County Convention Center (OCCC) for CHEST 2021. Health and safety are our biggest concerns for the meeting, which is why we chose this location. The convention center features the extra square footage we needed to design a meeting space with ample room for social distancing.
We are committed to create a meeting experience where you can safely and effectively conduct business, network with colleagues, and experience high-quality education. With your feedback, we have implemented COVID-19 safety measures similar to what is used in your hospitals and facilities. To ensure your health and safety, there will be a few requirements asked of in-person attendees.
Preparing for CHEST 2021
As the pandemic continues and vaccines are more readily available, we are requiring all attendees – participants, vendors, and staff – to be vaccinated to attend in person in Orlando, Florida. Your second vaccination shot should take place at least 2 weeks prior to the conference start. When you complete your registration information, you will be asked to attest that you have or will have completed an FDA-approved vaccination for COVID-19 by October 17, 2021.
We also suggest scheduling extra time at your arrival to the conference site. Realize that registration, lunch lines, hotel check-in, etc, may take longer as we navigate a new way to meet in person. This year, registration will be contactless. Have your digital or print confirmation ready when you arrive – the more prepared you are, the faster registration will be.
While the venue will regularly sanitize all high touch points in the public space throughout the day, remember to pack any personal supplies you may need for individual use, especially masks. Attendees will be required to wear a mask covering the mouth and nose at all times during the meeting. There will be masks on-site in case you forget or misplace your own.
Before making your way to Orlando, complete one last health self-assessment. Are you symptom free? Consider what advice you would give your patient if they felt the way you do in that moment. When in doubt, stay home and join us online. That’s one of the benefits of CHEST 2021.
Keeping safe while experiencing CHEST 2021
Any time you are in the conference center and the Hilton Hotel, the no-contact policy is applicable. Greet your colleagues and new friends using elbow bumps, waves, and any other form of contactless gestures. We will save our handshakes and hugs for CHEST 2022!
By attending in person, you are also agreeing to perform a health status self-check every day for any onset of COVID symptoms as defined by the CDC. If you are feeling ill, immediately notify the first aid office at the meeting.
Help us deliver a high-quality experience with the lowest reasonable risk in a manner that protects us all by complying to these health and safety measures. In addition, the layout and schedule of the conference is being designed to allow time for cleaning spaces between sessions. This means more time to get to your next location, visit the exhibit hall, or spend with your colleagues.
Our commitment to your safety
CHEST is taking extra precautions to keep you safe too – it’s not just on you! Daily temperature screenings will be conducted upon entry to the convention center and Hilton Orlando for everyone.
During the meeting, floor graphics will be used to outline 6-ft social distancing. In the concession areas, seating will be properly distanced and transparent shields will be in place. The exhibit hall will have extra wide aisles, which are not only safe, but easier to move through.
Public space and public restrooms are monitored by OCCC Environmental Services. They conduct sanitizing tasks within the restroom banks throughout the day and a thorough cleaning overnight. They also regularly sanitize all high touch points in the public space throughout the day as well; ie, door handles, ATMs, escalator handrails, elevator buttons, etc.
Staff and security have been increased to provide the best customer service and information accessibility to all in-person attendees. Medical personnel will also be present on site and available to help individuals who are feeling unwell.
It’s been a long year apart from our CHEST community. We can’t wait to see you in Orlando, Florida, October 17-20, for the high-quality education you expect.
Over the past year, you’ve had to adapt to Zoom calls and socially distanced learning. It’s time to come back together, face-to-face, for our top-tier learning event in sunny Orlando, Florida.
Grab your sunscreen and book your flights – we’re ready to welcome you back to CHEST 2021 with team-focused learning sessions, immersive gaming opportunities, expert-led faculty presentations, and more. We are making the meeting as safe as possible so you can attend in person.
After careful planning, we are excited to be able at the Orange County Convention Center (OCCC) for CHEST 2021. Health and safety are our biggest concerns for the meeting, which is why we chose this location. The convention center features the extra square footage we needed to design a meeting space with ample room for social distancing.
We are committed to create a meeting experience where you can safely and effectively conduct business, network with colleagues, and experience high-quality education. With your feedback, we have implemented COVID-19 safety measures similar to what is used in your hospitals and facilities. To ensure your health and safety, there will be a few requirements asked of in-person attendees.
Preparing for CHEST 2021
As the pandemic continues and vaccines are more readily available, we are requiring all attendees – participants, vendors, and staff – to be vaccinated to attend in person in Orlando, Florida. Your second vaccination shot should take place at least 2 weeks prior to the conference start. When you complete your registration information, you will be asked to attest that you have or will have completed an FDA-approved vaccination for COVID-19 by October 17, 2021.
We also suggest scheduling extra time at your arrival to the conference site. Realize that registration, lunch lines, hotel check-in, etc, may take longer as we navigate a new way to meet in person. This year, registration will be contactless. Have your digital or print confirmation ready when you arrive – the more prepared you are, the faster registration will be.
While the venue will regularly sanitize all high touch points in the public space throughout the day, remember to pack any personal supplies you may need for individual use, especially masks. Attendees will be required to wear a mask covering the mouth and nose at all times during the meeting. There will be masks on-site in case you forget or misplace your own.
Before making your way to Orlando, complete one last health self-assessment. Are you symptom free? Consider what advice you would give your patient if they felt the way you do in that moment. When in doubt, stay home and join us online. That’s one of the benefits of CHEST 2021.
Keeping safe while experiencing CHEST 2021
Any time you are in the conference center and the Hilton Hotel, the no-contact policy is applicable. Greet your colleagues and new friends using elbow bumps, waves, and any other form of contactless gestures. We will save our handshakes and hugs for CHEST 2022!
By attending in person, you are also agreeing to perform a health status self-check every day for any onset of COVID symptoms as defined by the CDC. If you are feeling ill, immediately notify the first aid office at the meeting.
Help us deliver a high-quality experience with the lowest reasonable risk in a manner that protects us all by complying to these health and safety measures. In addition, the layout and schedule of the conference is being designed to allow time for cleaning spaces between sessions. This means more time to get to your next location, visit the exhibit hall, or spend with your colleagues.
Our commitment to your safety
CHEST is taking extra precautions to keep you safe too – it’s not just on you! Daily temperature screenings will be conducted upon entry to the convention center and Hilton Orlando for everyone.
During the meeting, floor graphics will be used to outline 6-ft social distancing. In the concession areas, seating will be properly distanced and transparent shields will be in place. The exhibit hall will have extra wide aisles, which are not only safe, but easier to move through.
Public space and public restrooms are monitored by OCCC Environmental Services. They conduct sanitizing tasks within the restroom banks throughout the day and a thorough cleaning overnight. They also regularly sanitize all high touch points in the public space throughout the day as well; ie, door handles, ATMs, escalator handrails, elevator buttons, etc.
Staff and security have been increased to provide the best customer service and information accessibility to all in-person attendees. Medical personnel will also be present on site and available to help individuals who are feeling unwell.
It’s been a long year apart from our CHEST community. We can’t wait to see you in Orlando, Florida, October 17-20, for the high-quality education you expect.