User login
New world order: Reflecting on a year of COVID
I remember sitting at the pool in San Diego. I had been there before many years prior – one of my first medical conferences. I remember the clinking of metal sail stays in the morning breeze.
Flying out this time I packed a few surgical masks. I guiltily picked up an N95 from the hospital floors the day before leaving, but then left it at home thinking it overkill. I still have it in a ziplock bag a year later – it’s our emergency “what-if-we-have-to-care-for-one-another-with-COVID-in-this-tiny-house-full-of-kids” N95. Not that my husband has been fit tested. At the time, neither was I.
I returned after the conference to befuddlement over how we might fit test thousands of people, racing COVID to the front door. An overly complicated task, as we didn’t even know who was supposed to be responsible for orchestrating such an effort. We didn’t even know if we could spare the N95s.
Still in California, I sat by the pool wondering if anyone would acknowledge the impending new reality. At the conference we were told “don’t shake hands, don’t touch your face, wash your hands a lot.” I gave a workshop without a mask. I ate dinner in an actual restaurant worried only about gluten free soy sauce. I sat in a lecture hall with almost 5,000 people. I started to have a conversation with a friend from Seattle, but he needed to leave because they found a positive patient in his hospital. I listened to a prerecorded webinar by the pool from our chief safety officer saying there was a plan. I was not reassured.
When we flew home the world had already changed. There were patients in New York now. Masks had appeared in the airport news stand. Yet we breathed the air in the closed space of the red eye and forgot to be concerned. At work that Monday I asked my team – fist to 5, how worried are you about this? Brave faces and side eyes at each other and a lot of 1s or 2s held up in the air. My job this week, I told them, is to get you all to a 5.
I was working with a resident who 2 months prior I had told, as we worked together in the lounge, I don’t think you’re going to China on vacation. She hadn’t gone, of course. I wasn’t going on spring break either. On one of my last train rides a commuter friend (remember those?) told me we’ll all feel a lot better once we realize that none of us are going to get to do any of the things we want to do.
The med students were still there, helping the team and hanging onto their education. I told everyone not to see any patient with a respiratory complaint until we first discussed the case. On the third day of service I had to call infection control because a hypoxic febrile patient had come to the floor without isolation orders. “Are we testing?” No, I was informed, she hadn’t had exposures, hadn’t travelled. Speechless that we were screening for travel to Italy while living with one tiny state between us and the American epicenter, I can now recall thinking that our infection control officer did not sound well rested.
My N95 was still in a baggy at home. The PAPRS hadn’t appeared yet. Literally no one could agree what kind of mask the CDC or infection control or the ID consultant of the day recommended – today we are using surgical masks, I was told. Thursday will likely be different. “Anyway, she doesn’t sound like she has it.” I walked to the floors.
My med student started presenting our septic viral pneumonia patient including the very well done exam that I previously forbade him from obtaining. What happened to not seeing respiratory patients, I asked. Oh, they said, well night float said it didn’t sound like COVID. Insufficiently convinced by our second year resident’s unjustifiably overconfident, though ultimately correct, assessment – I held my head in my hands and give my first hallway COVID chalk talk of the new era. Complete with telling the team to question everything they thought they knew now including everything I said except “be careful.” That was about when Philadelphia ran out of toilet paper.
That weekend I sat in front of a bay of computers as our Medical Officer of the Day. Air traffic control for ED patients coming in for a landing on medical teams, I watched the new biohazard warnings line up indicating respiratory isolation patients waiting for a bed. I watched CRPs and D-dimers, and looked for leukopenia. I vowed I would follow up on tests to hone my COVID illness script. I soon realized that tests lie anyway.
By the end of that week we’d fallen through the looking glass. The old rules didn’t apply. We weren’t going to China, or Arizona; we didn’t know when the med students were coming back; the jobs we had were not the jobs we signed up for but were those that the world needed us to do; we couldn’t trust our intuition or our tests; we had no experts – and yet we started to grow the humble beginnings of expertise like spring garden sprouts.
In a chaotic world, seeds of order take shape and then scatter like a screensaver. The skills needed to manage chaos are different from those that leaders use in simple ordered times. Order cannot be pulled from chaos by force of will or cleverness, nor can it be delegated, cascaded, demanded, or launched. Order emerges when communities that are receptive to learning see patterns through noise, and slowly, lovingly, coax moments of stability into being.
Dr. Jaffe is division director for hospital medicine in the Department of Medicine at Thomas Jefferson University Hospital in Philadelphia.
I remember sitting at the pool in San Diego. I had been there before many years prior – one of my first medical conferences. I remember the clinking of metal sail stays in the morning breeze.
Flying out this time I packed a few surgical masks. I guiltily picked up an N95 from the hospital floors the day before leaving, but then left it at home thinking it overkill. I still have it in a ziplock bag a year later – it’s our emergency “what-if-we-have-to-care-for-one-another-with-COVID-in-this-tiny-house-full-of-kids” N95. Not that my husband has been fit tested. At the time, neither was I.
I returned after the conference to befuddlement over how we might fit test thousands of people, racing COVID to the front door. An overly complicated task, as we didn’t even know who was supposed to be responsible for orchestrating such an effort. We didn’t even know if we could spare the N95s.
Still in California, I sat by the pool wondering if anyone would acknowledge the impending new reality. At the conference we were told “don’t shake hands, don’t touch your face, wash your hands a lot.” I gave a workshop without a mask. I ate dinner in an actual restaurant worried only about gluten free soy sauce. I sat in a lecture hall with almost 5,000 people. I started to have a conversation with a friend from Seattle, but he needed to leave because they found a positive patient in his hospital. I listened to a prerecorded webinar by the pool from our chief safety officer saying there was a plan. I was not reassured.
When we flew home the world had already changed. There were patients in New York now. Masks had appeared in the airport news stand. Yet we breathed the air in the closed space of the red eye and forgot to be concerned. At work that Monday I asked my team – fist to 5, how worried are you about this? Brave faces and side eyes at each other and a lot of 1s or 2s held up in the air. My job this week, I told them, is to get you all to a 5.
I was working with a resident who 2 months prior I had told, as we worked together in the lounge, I don’t think you’re going to China on vacation. She hadn’t gone, of course. I wasn’t going on spring break either. On one of my last train rides a commuter friend (remember those?) told me we’ll all feel a lot better once we realize that none of us are going to get to do any of the things we want to do.
The med students were still there, helping the team and hanging onto their education. I told everyone not to see any patient with a respiratory complaint until we first discussed the case. On the third day of service I had to call infection control because a hypoxic febrile patient had come to the floor without isolation orders. “Are we testing?” No, I was informed, she hadn’t had exposures, hadn’t travelled. Speechless that we were screening for travel to Italy while living with one tiny state between us and the American epicenter, I can now recall thinking that our infection control officer did not sound well rested.
My N95 was still in a baggy at home. The PAPRS hadn’t appeared yet. Literally no one could agree what kind of mask the CDC or infection control or the ID consultant of the day recommended – today we are using surgical masks, I was told. Thursday will likely be different. “Anyway, she doesn’t sound like she has it.” I walked to the floors.
My med student started presenting our septic viral pneumonia patient including the very well done exam that I previously forbade him from obtaining. What happened to not seeing respiratory patients, I asked. Oh, they said, well night float said it didn’t sound like COVID. Insufficiently convinced by our second year resident’s unjustifiably overconfident, though ultimately correct, assessment – I held my head in my hands and give my first hallway COVID chalk talk of the new era. Complete with telling the team to question everything they thought they knew now including everything I said except “be careful.” That was about when Philadelphia ran out of toilet paper.
That weekend I sat in front of a bay of computers as our Medical Officer of the Day. Air traffic control for ED patients coming in for a landing on medical teams, I watched the new biohazard warnings line up indicating respiratory isolation patients waiting for a bed. I watched CRPs and D-dimers, and looked for leukopenia. I vowed I would follow up on tests to hone my COVID illness script. I soon realized that tests lie anyway.
By the end of that week we’d fallen through the looking glass. The old rules didn’t apply. We weren’t going to China, or Arizona; we didn’t know when the med students were coming back; the jobs we had were not the jobs we signed up for but were those that the world needed us to do; we couldn’t trust our intuition or our tests; we had no experts – and yet we started to grow the humble beginnings of expertise like spring garden sprouts.
In a chaotic world, seeds of order take shape and then scatter like a screensaver. The skills needed to manage chaos are different from those that leaders use in simple ordered times. Order cannot be pulled from chaos by force of will or cleverness, nor can it be delegated, cascaded, demanded, or launched. Order emerges when communities that are receptive to learning see patterns through noise, and slowly, lovingly, coax moments of stability into being.
Dr. Jaffe is division director for hospital medicine in the Department of Medicine at Thomas Jefferson University Hospital in Philadelphia.
I remember sitting at the pool in San Diego. I had been there before many years prior – one of my first medical conferences. I remember the clinking of metal sail stays in the morning breeze.
Flying out this time I packed a few surgical masks. I guiltily picked up an N95 from the hospital floors the day before leaving, but then left it at home thinking it overkill. I still have it in a ziplock bag a year later – it’s our emergency “what-if-we-have-to-care-for-one-another-with-COVID-in-this-tiny-house-full-of-kids” N95. Not that my husband has been fit tested. At the time, neither was I.
I returned after the conference to befuddlement over how we might fit test thousands of people, racing COVID to the front door. An overly complicated task, as we didn’t even know who was supposed to be responsible for orchestrating such an effort. We didn’t even know if we could spare the N95s.
Still in California, I sat by the pool wondering if anyone would acknowledge the impending new reality. At the conference we were told “don’t shake hands, don’t touch your face, wash your hands a lot.” I gave a workshop without a mask. I ate dinner in an actual restaurant worried only about gluten free soy sauce. I sat in a lecture hall with almost 5,000 people. I started to have a conversation with a friend from Seattle, but he needed to leave because they found a positive patient in his hospital. I listened to a prerecorded webinar by the pool from our chief safety officer saying there was a plan. I was not reassured.
When we flew home the world had already changed. There were patients in New York now. Masks had appeared in the airport news stand. Yet we breathed the air in the closed space of the red eye and forgot to be concerned. At work that Monday I asked my team – fist to 5, how worried are you about this? Brave faces and side eyes at each other and a lot of 1s or 2s held up in the air. My job this week, I told them, is to get you all to a 5.
I was working with a resident who 2 months prior I had told, as we worked together in the lounge, I don’t think you’re going to China on vacation. She hadn’t gone, of course. I wasn’t going on spring break either. On one of my last train rides a commuter friend (remember those?) told me we’ll all feel a lot better once we realize that none of us are going to get to do any of the things we want to do.
The med students were still there, helping the team and hanging onto their education. I told everyone not to see any patient with a respiratory complaint until we first discussed the case. On the third day of service I had to call infection control because a hypoxic febrile patient had come to the floor without isolation orders. “Are we testing?” No, I was informed, she hadn’t had exposures, hadn’t travelled. Speechless that we were screening for travel to Italy while living with one tiny state between us and the American epicenter, I can now recall thinking that our infection control officer did not sound well rested.
My N95 was still in a baggy at home. The PAPRS hadn’t appeared yet. Literally no one could agree what kind of mask the CDC or infection control or the ID consultant of the day recommended – today we are using surgical masks, I was told. Thursday will likely be different. “Anyway, she doesn’t sound like she has it.” I walked to the floors.
My med student started presenting our septic viral pneumonia patient including the very well done exam that I previously forbade him from obtaining. What happened to not seeing respiratory patients, I asked. Oh, they said, well night float said it didn’t sound like COVID. Insufficiently convinced by our second year resident’s unjustifiably overconfident, though ultimately correct, assessment – I held my head in my hands and give my first hallway COVID chalk talk of the new era. Complete with telling the team to question everything they thought they knew now including everything I said except “be careful.” That was about when Philadelphia ran out of toilet paper.
That weekend I sat in front of a bay of computers as our Medical Officer of the Day. Air traffic control for ED patients coming in for a landing on medical teams, I watched the new biohazard warnings line up indicating respiratory isolation patients waiting for a bed. I watched CRPs and D-dimers, and looked for leukopenia. I vowed I would follow up on tests to hone my COVID illness script. I soon realized that tests lie anyway.
By the end of that week we’d fallen through the looking glass. The old rules didn’t apply. We weren’t going to China, or Arizona; we didn’t know when the med students were coming back; the jobs we had were not the jobs we signed up for but were those that the world needed us to do; we couldn’t trust our intuition or our tests; we had no experts – and yet we started to grow the humble beginnings of expertise like spring garden sprouts.
In a chaotic world, seeds of order take shape and then scatter like a screensaver. The skills needed to manage chaos are different from those that leaders use in simple ordered times. Order cannot be pulled from chaos by force of will or cleverness, nor can it be delegated, cascaded, demanded, or launched. Order emerges when communities that are receptive to learning see patterns through noise, and slowly, lovingly, coax moments of stability into being.
Dr. Jaffe is division director for hospital medicine in the Department of Medicine at Thomas Jefferson University Hospital in Philadelphia.
Role of 3D Printing and Modeling to Aid in Neuroradiology Education for Medical Trainees
Applications of 3-dimensional (3D) printing in medical imaging and health care are expanding. 3D printing may serve a variety of roles and is used increasingly in the context of presurgical planning, as specific medical models may be created using individual patient imaging data.1 These patient-specific models may assist in medical trainee education, decrease operating room time, improve patient education for potential planned surgery, and guide clinicians for optimizing therapy.1,2 This article discusses the utility of 3D printing at a single institution to serve in enhancing specifically neuroradiology education.
Background
As digital imaging and 3D printing have increased in popularity, the potential application of using imaging data to guide patient therapy has shown significant promise. Computed tomography (CT) is a commonly used modality that can be used to create 3D anatomical models, as it is frequently used in the medical setting, demonstrates excellent resolution to the millimeter scale, and can readily pinpoint pathology on imaging.
Image Acquisition
CT scans can be rapidly obtained, which adds significant value, particularly in the context of point-of-care 3D printing. Another modality commonly used for 3D printing is magnetic resonance imaging (MRI), which unlike CT, does not expose the patient to ionizing radiation. The 3D printing process is initiated with patient-specific CT or MRI data stored in the digital imaging and communications in medicine (DICOM) format, which is the international standard for communication and management of medical imaging information and related data. DICOM allows for faster and robust collaboration among imaging professionals.3
Image Processing
To print 3D anatomical models, patient-specific data must be converted from DICOM into standard tessellation language (STL) format, which can be created and edited with a variety of softwares.3 At James A. Haley Veterans’ Hospital in Tampa, Florida, we use an image processing package that includes the Materialise 3-matic and interactive medical image control system. Image quality is essential; therefore, careful attention to details such as pixel dimensions, slice thickness, and slice increments must be considered.3,4
An STL file creates a 3D image from triangle approximations. The entire 3D shape will be made of numerous large or small triangles, depending on the slice thickness, therefore, quality of the original radiologic image. The size and position of the triangles used to make the model can be varied to approximate the object’s shape. The smaller the triangles, the better the image quality and vice versa. This concept is analogous to approximating a circle using straight lines of equal length—more, smaller lines will result in better approximation of a circle (Figure 1).5,6 Similarly, using smaller triangles allows for better approximation of the image. As the human body is a complex structure, mimicking the body requires a system able to create nongeometrical shapes, which is made possible via these triangle approximations in a 3D STL file.
The creation of an STL file from DICOM data starts with a threshold-based segmentation process followed by additional fine-tuning and edits, and ends in the creation of a 3D part. The initial segmentation can be created with the threshold tool, using a Hounsfield unit range based on the area of interest desired (eg, bone, blood, fat). This is used to create an initial mask, which can be further optimized. The region grow tool allows the user to focus the segmentation by discarding areas that are not directly connected to the region of interest. In contrast, the split mask tool divides areas that are connected. Next, fine-tuning the segmentation using tools such as multiple slice edit helps to optimize the model. After all edits are made, the calculate part tool converts the mask into a 3D component that can be used in downstream applications. For the purposes of demonstration and proof of concept, the models provided in this article were created via open-source hardware designs under free or open licenses.7-9
3D Printing in Neuroradiology Education
Neuroradiologists focus on diagnosing pathology related to the brain, head and neck, and spine. CT and MRI scans are the primary modalities used to diagnose these conditions. 3D printing is a useful tool for the trainee who wishes to fully understand neuroanatomy and obtain further appreciation of imaging pathology as it relates to 3D anatomy. Head and neck imaging are a complex subdiscipline of neuroradiology that often require further training beyond radiology residency. A neuroradiology fellowship that focuses on head and neck imaging extends the training.
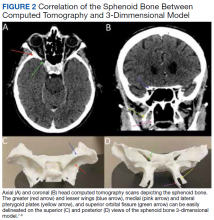
3D printing has the potential to improve the understanding of various imaging pathologies by providing the trainee with a more in-depth appreciation of the anterior, middle, and posterior cranial fossa, the skull base foramina (ie, foramen ovale, spinosum, rotundum), and complex 3D areas, such as the pterygopalatine fossa, which are all critical areas to investigate on imaging. Figure 2 highlights how a complex anatomical structure, such as the sphenoid bone when printed in 3D, can be correlated with CT cross-sectional images to supplement the educational experience.
Furthermore, the various lobes, sulci, and gyri of the brain and cerebellum and how they interrelate to nearby vasculature and bony structures can be difficult to conceptualize for early trainees. A 3D-printed cerebellum and its relation to the brainstem is illustrated in Figure 3A. Additional complex head and neck structures of the middle ear membranous and bony labyrinth and ossicles and multiple views of the mandible are shown in Figures 3B through 3E.
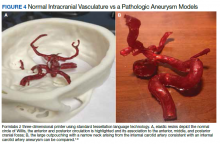
3D printing in the context of neurovascular pathology holds great promise, particularly as these models may provide the trainee, patient, and proceduralist essential details such as appearance and morphology of an intracranial aneurysm, relationship and size of the neck of aneurysm, incorporation of vessels emanating from the aneurysmal sac, and details of the dome of the aneurysm. For example, the normal circle of Willis in Figure 4A is juxtaposed with an example of a saccular internal carotid artery aneurysm (Figure 4B).
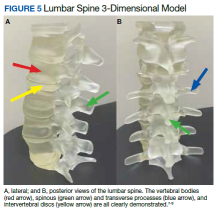
A variety of conditions can affect the bony spine from degenerative, trauma, neoplastic, and inflammatory etiologies. A CT scan of the spine is readily used to detect these different conditions and often is used in the initial evaluation of trauma as indicated in the American College of Radiology appropriateness criteria.10 In addition, MRI is used to evaluate the spinal cord and to further define spinal stenosis as well as evaluate radiculopathy. An appreciation of the bony and soft tissue structures within the spine can be garnered with the use of 3D models (Figure 5).
Trainees can further their understanding of approaches in spinal procedures, including lumbar puncture, myelography, and facet injections. A variety of approaches to access the spinal canal have been documented, such as interspinous, paraspinous, and interlaminar oblique; 3D-printed models can aid in practicing these procedures.11 For example, a water-filled tube can be inserted into the vertebral canal to provide realistic tactile feedback for simulation of a lumbar puncture. An appreciation of the 3D anatomy can guide the clinician on the optimal approach, which can help limit time and potentially improve outcomes.
Future Directions
Artificial Intelligence (AI) offers the ability to teach computers to perform tasks that ordinarily require human intelligence. In the context of 3D printing, the ability to use AI to readily convert and process DICOM data into printable STL models holds significant promise. Currently, the manual conversion of a DICOM file into a segmented 3D model may take several days, necessitating a number of productive hours even from the imaging and engineering champion. If machines could aid in this process, the ability to readily scale clinical 3D printing and promote widespread adoption would be feasible. Several studies already are looking into this concept to determine how deep learning networks may automatically recognize lesions on medical imaging to assist a human operator, potentially cutting hours from the clinical 3D printing workflow.12,13
Furthermore, there are several applications for AI in the context of 3D printing upstream or before the creation of a 3D model. A number of AI tools are already in use at the CT and MRI scanner. Current strategies leverage deep learning and advances in neural networks to improve image quality and create thin section DICOM data, which can be converted into printable 3D files. Additionally, the ability to automate tasks using AI can improve production capacity by assessing material costs and ensuring cost efficiency, which will be critical as point-of-care 3D printing develops widespread adoption. AI also can reduce printing errors by using automated adaptive feedback, using machine learning to search for possible print errors, and sending feedback to the computer to ensure appropriate settings (eg, temperature settings/environmental conditions).
Conclusions
Based on this single-institution experience, 3D-printed complex neuroanatomical structures seems feasible and may enhance resident education and patient safety. Interested trainees may have the opportunity to learn and be involved in the printing process of new and innovative ideas. Further studies may involve printing various pathologic processes and applying these same steps and principles to other subspecialties of radiology. Finally, AI has the potential to advance the 3D printing process in the future.
1. Rengier F, Mehndiratta A, von Tengg-Kobligk H, et al. 3D printing based on imaging data: review of medical applications. Int J Comput Assist Radiol Surg. 2010;5(4):335-341. doi:10.1007/s11548-010-0476-x
2. Perica E, Sun Z. Patient-specific three-dimensional printing for pre-surgical planning in hepatocellular carcinoma treatment. Quant Imaging Med Surg. 2017;7(6):668-677. doi:10.21037/qims.2017.11.02
3. Hwang JJ, Jung Y-H, Cho B-H. The need for DICOM encapsulation of 3D scanning STL data. Imaging Sci Dent. 2018;48(4):301-302. doi:10.5624/isd.2018.48.4.301
4. Whyms BJ, Vorperian HK, Gentry LR, Schimek EM, Bersu ET, Chung MK. The effect of computed tomographic scanner parameters and 3-dimensional volume rendering techniques on the accuracy of linear, angular, and volumetric measurements of the mandible. Oral Surg Oral Med, Oral Pathol Oral Radiol. 2013;115(5):682-691. doi:10.1016/j.oooo.2013.02.008
5. Materialise Cloud. Triangle reduction. Accessed May 20, 2021. https://cloud.materialise.com/tools/triangle-reduction
6. Comaneanu RM, Tarcolea M, Vlasceanu D, Cotrut MC. Virtual 3D reconstruction, diagnosis and surgical planning with Mimics software. Int J Nano Biomaterials. 2012;4(1);69-77.
7. Thingiverse: Digital designs for physical objects. Accessed May 20, 2021. https://www.thingiverse.com
8. Cults. Download for free 3D models for 3D printers. Accessed May 20, 2021. https://cults3d.com/en
9. yeggi. Search engine for 3D printer models. Accessed May 20, 2021. https://www.yeggi.com
10. Expert Panel on Neurological Imaging and Musculoskeletal Imaging; Beckmann NM, West OC, Nunez D, et al. ACR appropriateness criteria suspected spine trauma. J Am Coll Radiol. 2919;16(5):S264-285. doi:10.1016/j.jacr.2019.02.002
11. McKinney AM. Normal variants of the lumbar and sacral spine. In: Atlas of Head/Neck and Spine Normal Imaging Variants. Springer; 2018:263-321.
12. Sollini M, Bartoli F, Marciano A, et al. Artificial intelligence and hybrid imaging: the best match for personalized medicine in oncology. Eur J Hybrid Imaging. 2020;4(1):24. doi:10.1186/s41824-020-00094-8
13. Küstner T, Hepp T, Fischer M, et al. Fully automated and standardized segmentation of adipose tissue compartments via deep learning in 3D whole-body MRI of epidemiologic cohort studies. Radiol Artif Intell.2020;2(6):e200010. doi:10.1148/ryai.2020200010
Applications of 3-dimensional (3D) printing in medical imaging and health care are expanding. 3D printing may serve a variety of roles and is used increasingly in the context of presurgical planning, as specific medical models may be created using individual patient imaging data.1 These patient-specific models may assist in medical trainee education, decrease operating room time, improve patient education for potential planned surgery, and guide clinicians for optimizing therapy.1,2 This article discusses the utility of 3D printing at a single institution to serve in enhancing specifically neuroradiology education.
Background
As digital imaging and 3D printing have increased in popularity, the potential application of using imaging data to guide patient therapy has shown significant promise. Computed tomography (CT) is a commonly used modality that can be used to create 3D anatomical models, as it is frequently used in the medical setting, demonstrates excellent resolution to the millimeter scale, and can readily pinpoint pathology on imaging.
Image Acquisition
CT scans can be rapidly obtained, which adds significant value, particularly in the context of point-of-care 3D printing. Another modality commonly used for 3D printing is magnetic resonance imaging (MRI), which unlike CT, does not expose the patient to ionizing radiation. The 3D printing process is initiated with patient-specific CT or MRI data stored in the digital imaging and communications in medicine (DICOM) format, which is the international standard for communication and management of medical imaging information and related data. DICOM allows for faster and robust collaboration among imaging professionals.3
Image Processing
To print 3D anatomical models, patient-specific data must be converted from DICOM into standard tessellation language (STL) format, which can be created and edited with a variety of softwares.3 At James A. Haley Veterans’ Hospital in Tampa, Florida, we use an image processing package that includes the Materialise 3-matic and interactive medical image control system. Image quality is essential; therefore, careful attention to details such as pixel dimensions, slice thickness, and slice increments must be considered.3,4
An STL file creates a 3D image from triangle approximations. The entire 3D shape will be made of numerous large or small triangles, depending on the slice thickness, therefore, quality of the original radiologic image. The size and position of the triangles used to make the model can be varied to approximate the object’s shape. The smaller the triangles, the better the image quality and vice versa. This concept is analogous to approximating a circle using straight lines of equal length—more, smaller lines will result in better approximation of a circle (Figure 1).5,6 Similarly, using smaller triangles allows for better approximation of the image. As the human body is a complex structure, mimicking the body requires a system able to create nongeometrical shapes, which is made possible via these triangle approximations in a 3D STL file.
The creation of an STL file from DICOM data starts with a threshold-based segmentation process followed by additional fine-tuning and edits, and ends in the creation of a 3D part. The initial segmentation can be created with the threshold tool, using a Hounsfield unit range based on the area of interest desired (eg, bone, blood, fat). This is used to create an initial mask, which can be further optimized. The region grow tool allows the user to focus the segmentation by discarding areas that are not directly connected to the region of interest. In contrast, the split mask tool divides areas that are connected. Next, fine-tuning the segmentation using tools such as multiple slice edit helps to optimize the model. After all edits are made, the calculate part tool converts the mask into a 3D component that can be used in downstream applications. For the purposes of demonstration and proof of concept, the models provided in this article were created via open-source hardware designs under free or open licenses.7-9
3D Printing in Neuroradiology Education
Neuroradiologists focus on diagnosing pathology related to the brain, head and neck, and spine. CT and MRI scans are the primary modalities used to diagnose these conditions. 3D printing is a useful tool for the trainee who wishes to fully understand neuroanatomy and obtain further appreciation of imaging pathology as it relates to 3D anatomy. Head and neck imaging are a complex subdiscipline of neuroradiology that often require further training beyond radiology residency. A neuroradiology fellowship that focuses on head and neck imaging extends the training.
3D printing has the potential to improve the understanding of various imaging pathologies by providing the trainee with a more in-depth appreciation of the anterior, middle, and posterior cranial fossa, the skull base foramina (ie, foramen ovale, spinosum, rotundum), and complex 3D areas, such as the pterygopalatine fossa, which are all critical areas to investigate on imaging. Figure 2 highlights how a complex anatomical structure, such as the sphenoid bone when printed in 3D, can be correlated with CT cross-sectional images to supplement the educational experience.
Furthermore, the various lobes, sulci, and gyri of the brain and cerebellum and how they interrelate to nearby vasculature and bony structures can be difficult to conceptualize for early trainees. A 3D-printed cerebellum and its relation to the brainstem is illustrated in Figure 3A. Additional complex head and neck structures of the middle ear membranous and bony labyrinth and ossicles and multiple views of the mandible are shown in Figures 3B through 3E.
3D printing in the context of neurovascular pathology holds great promise, particularly as these models may provide the trainee, patient, and proceduralist essential details such as appearance and morphology of an intracranial aneurysm, relationship and size of the neck of aneurysm, incorporation of vessels emanating from the aneurysmal sac, and details of the dome of the aneurysm. For example, the normal circle of Willis in Figure 4A is juxtaposed with an example of a saccular internal carotid artery aneurysm (Figure 4B).
A variety of conditions can affect the bony spine from degenerative, trauma, neoplastic, and inflammatory etiologies. A CT scan of the spine is readily used to detect these different conditions and often is used in the initial evaluation of trauma as indicated in the American College of Radiology appropriateness criteria.10 In addition, MRI is used to evaluate the spinal cord and to further define spinal stenosis as well as evaluate radiculopathy. An appreciation of the bony and soft tissue structures within the spine can be garnered with the use of 3D models (Figure 5).
Trainees can further their understanding of approaches in spinal procedures, including lumbar puncture, myelography, and facet injections. A variety of approaches to access the spinal canal have been documented, such as interspinous, paraspinous, and interlaminar oblique; 3D-printed models can aid in practicing these procedures.11 For example, a water-filled tube can be inserted into the vertebral canal to provide realistic tactile feedback for simulation of a lumbar puncture. An appreciation of the 3D anatomy can guide the clinician on the optimal approach, which can help limit time and potentially improve outcomes.
Future Directions
Artificial Intelligence (AI) offers the ability to teach computers to perform tasks that ordinarily require human intelligence. In the context of 3D printing, the ability to use AI to readily convert and process DICOM data into printable STL models holds significant promise. Currently, the manual conversion of a DICOM file into a segmented 3D model may take several days, necessitating a number of productive hours even from the imaging and engineering champion. If machines could aid in this process, the ability to readily scale clinical 3D printing and promote widespread adoption would be feasible. Several studies already are looking into this concept to determine how deep learning networks may automatically recognize lesions on medical imaging to assist a human operator, potentially cutting hours from the clinical 3D printing workflow.12,13
Furthermore, there are several applications for AI in the context of 3D printing upstream or before the creation of a 3D model. A number of AI tools are already in use at the CT and MRI scanner. Current strategies leverage deep learning and advances in neural networks to improve image quality and create thin section DICOM data, which can be converted into printable 3D files. Additionally, the ability to automate tasks using AI can improve production capacity by assessing material costs and ensuring cost efficiency, which will be critical as point-of-care 3D printing develops widespread adoption. AI also can reduce printing errors by using automated adaptive feedback, using machine learning to search for possible print errors, and sending feedback to the computer to ensure appropriate settings (eg, temperature settings/environmental conditions).
Conclusions
Based on this single-institution experience, 3D-printed complex neuroanatomical structures seems feasible and may enhance resident education and patient safety. Interested trainees may have the opportunity to learn and be involved in the printing process of new and innovative ideas. Further studies may involve printing various pathologic processes and applying these same steps and principles to other subspecialties of radiology. Finally, AI has the potential to advance the 3D printing process in the future.
Applications of 3-dimensional (3D) printing in medical imaging and health care are expanding. 3D printing may serve a variety of roles and is used increasingly in the context of presurgical planning, as specific medical models may be created using individual patient imaging data.1 These patient-specific models may assist in medical trainee education, decrease operating room time, improve patient education for potential planned surgery, and guide clinicians for optimizing therapy.1,2 This article discusses the utility of 3D printing at a single institution to serve in enhancing specifically neuroradiology education.
Background
As digital imaging and 3D printing have increased in popularity, the potential application of using imaging data to guide patient therapy has shown significant promise. Computed tomography (CT) is a commonly used modality that can be used to create 3D anatomical models, as it is frequently used in the medical setting, demonstrates excellent resolution to the millimeter scale, and can readily pinpoint pathology on imaging.
Image Acquisition
CT scans can be rapidly obtained, which adds significant value, particularly in the context of point-of-care 3D printing. Another modality commonly used for 3D printing is magnetic resonance imaging (MRI), which unlike CT, does not expose the patient to ionizing radiation. The 3D printing process is initiated with patient-specific CT or MRI data stored in the digital imaging and communications in medicine (DICOM) format, which is the international standard for communication and management of medical imaging information and related data. DICOM allows for faster and robust collaboration among imaging professionals.3
Image Processing
To print 3D anatomical models, patient-specific data must be converted from DICOM into standard tessellation language (STL) format, which can be created and edited with a variety of softwares.3 At James A. Haley Veterans’ Hospital in Tampa, Florida, we use an image processing package that includes the Materialise 3-matic and interactive medical image control system. Image quality is essential; therefore, careful attention to details such as pixel dimensions, slice thickness, and slice increments must be considered.3,4
An STL file creates a 3D image from triangle approximations. The entire 3D shape will be made of numerous large or small triangles, depending on the slice thickness, therefore, quality of the original radiologic image. The size and position of the triangles used to make the model can be varied to approximate the object’s shape. The smaller the triangles, the better the image quality and vice versa. This concept is analogous to approximating a circle using straight lines of equal length—more, smaller lines will result in better approximation of a circle (Figure 1).5,6 Similarly, using smaller triangles allows for better approximation of the image. As the human body is a complex structure, mimicking the body requires a system able to create nongeometrical shapes, which is made possible via these triangle approximations in a 3D STL file.
The creation of an STL file from DICOM data starts with a threshold-based segmentation process followed by additional fine-tuning and edits, and ends in the creation of a 3D part. The initial segmentation can be created with the threshold tool, using a Hounsfield unit range based on the area of interest desired (eg, bone, blood, fat). This is used to create an initial mask, which can be further optimized. The region grow tool allows the user to focus the segmentation by discarding areas that are not directly connected to the region of interest. In contrast, the split mask tool divides areas that are connected. Next, fine-tuning the segmentation using tools such as multiple slice edit helps to optimize the model. After all edits are made, the calculate part tool converts the mask into a 3D component that can be used in downstream applications. For the purposes of demonstration and proof of concept, the models provided in this article were created via open-source hardware designs under free or open licenses.7-9
3D Printing in Neuroradiology Education
Neuroradiologists focus on diagnosing pathology related to the brain, head and neck, and spine. CT and MRI scans are the primary modalities used to diagnose these conditions. 3D printing is a useful tool for the trainee who wishes to fully understand neuroanatomy and obtain further appreciation of imaging pathology as it relates to 3D anatomy. Head and neck imaging are a complex subdiscipline of neuroradiology that often require further training beyond radiology residency. A neuroradiology fellowship that focuses on head and neck imaging extends the training.
3D printing has the potential to improve the understanding of various imaging pathologies by providing the trainee with a more in-depth appreciation of the anterior, middle, and posterior cranial fossa, the skull base foramina (ie, foramen ovale, spinosum, rotundum), and complex 3D areas, such as the pterygopalatine fossa, which are all critical areas to investigate on imaging. Figure 2 highlights how a complex anatomical structure, such as the sphenoid bone when printed in 3D, can be correlated with CT cross-sectional images to supplement the educational experience.
Furthermore, the various lobes, sulci, and gyri of the brain and cerebellum and how they interrelate to nearby vasculature and bony structures can be difficult to conceptualize for early trainees. A 3D-printed cerebellum and its relation to the brainstem is illustrated in Figure 3A. Additional complex head and neck structures of the middle ear membranous and bony labyrinth and ossicles and multiple views of the mandible are shown in Figures 3B through 3E.
3D printing in the context of neurovascular pathology holds great promise, particularly as these models may provide the trainee, patient, and proceduralist essential details such as appearance and morphology of an intracranial aneurysm, relationship and size of the neck of aneurysm, incorporation of vessels emanating from the aneurysmal sac, and details of the dome of the aneurysm. For example, the normal circle of Willis in Figure 4A is juxtaposed with an example of a saccular internal carotid artery aneurysm (Figure 4B).
A variety of conditions can affect the bony spine from degenerative, trauma, neoplastic, and inflammatory etiologies. A CT scan of the spine is readily used to detect these different conditions and often is used in the initial evaluation of trauma as indicated in the American College of Radiology appropriateness criteria.10 In addition, MRI is used to evaluate the spinal cord and to further define spinal stenosis as well as evaluate radiculopathy. An appreciation of the bony and soft tissue structures within the spine can be garnered with the use of 3D models (Figure 5).
Trainees can further their understanding of approaches in spinal procedures, including lumbar puncture, myelography, and facet injections. A variety of approaches to access the spinal canal have been documented, such as interspinous, paraspinous, and interlaminar oblique; 3D-printed models can aid in practicing these procedures.11 For example, a water-filled tube can be inserted into the vertebral canal to provide realistic tactile feedback for simulation of a lumbar puncture. An appreciation of the 3D anatomy can guide the clinician on the optimal approach, which can help limit time and potentially improve outcomes.
Future Directions
Artificial Intelligence (AI) offers the ability to teach computers to perform tasks that ordinarily require human intelligence. In the context of 3D printing, the ability to use AI to readily convert and process DICOM data into printable STL models holds significant promise. Currently, the manual conversion of a DICOM file into a segmented 3D model may take several days, necessitating a number of productive hours even from the imaging and engineering champion. If machines could aid in this process, the ability to readily scale clinical 3D printing and promote widespread adoption would be feasible. Several studies already are looking into this concept to determine how deep learning networks may automatically recognize lesions on medical imaging to assist a human operator, potentially cutting hours from the clinical 3D printing workflow.12,13
Furthermore, there are several applications for AI in the context of 3D printing upstream or before the creation of a 3D model. A number of AI tools are already in use at the CT and MRI scanner. Current strategies leverage deep learning and advances in neural networks to improve image quality and create thin section DICOM data, which can be converted into printable 3D files. Additionally, the ability to automate tasks using AI can improve production capacity by assessing material costs and ensuring cost efficiency, which will be critical as point-of-care 3D printing develops widespread adoption. AI also can reduce printing errors by using automated adaptive feedback, using machine learning to search for possible print errors, and sending feedback to the computer to ensure appropriate settings (eg, temperature settings/environmental conditions).
Conclusions
Based on this single-institution experience, 3D-printed complex neuroanatomical structures seems feasible and may enhance resident education and patient safety. Interested trainees may have the opportunity to learn and be involved in the printing process of new and innovative ideas. Further studies may involve printing various pathologic processes and applying these same steps and principles to other subspecialties of radiology. Finally, AI has the potential to advance the 3D printing process in the future.
1. Rengier F, Mehndiratta A, von Tengg-Kobligk H, et al. 3D printing based on imaging data: review of medical applications. Int J Comput Assist Radiol Surg. 2010;5(4):335-341. doi:10.1007/s11548-010-0476-x
2. Perica E, Sun Z. Patient-specific three-dimensional printing for pre-surgical planning in hepatocellular carcinoma treatment. Quant Imaging Med Surg. 2017;7(6):668-677. doi:10.21037/qims.2017.11.02
3. Hwang JJ, Jung Y-H, Cho B-H. The need for DICOM encapsulation of 3D scanning STL data. Imaging Sci Dent. 2018;48(4):301-302. doi:10.5624/isd.2018.48.4.301
4. Whyms BJ, Vorperian HK, Gentry LR, Schimek EM, Bersu ET, Chung MK. The effect of computed tomographic scanner parameters and 3-dimensional volume rendering techniques on the accuracy of linear, angular, and volumetric measurements of the mandible. Oral Surg Oral Med, Oral Pathol Oral Radiol. 2013;115(5):682-691. doi:10.1016/j.oooo.2013.02.008
5. Materialise Cloud. Triangle reduction. Accessed May 20, 2021. https://cloud.materialise.com/tools/triangle-reduction
6. Comaneanu RM, Tarcolea M, Vlasceanu D, Cotrut MC. Virtual 3D reconstruction, diagnosis and surgical planning with Mimics software. Int J Nano Biomaterials. 2012;4(1);69-77.
7. Thingiverse: Digital designs for physical objects. Accessed May 20, 2021. https://www.thingiverse.com
8. Cults. Download for free 3D models for 3D printers. Accessed May 20, 2021. https://cults3d.com/en
9. yeggi. Search engine for 3D printer models. Accessed May 20, 2021. https://www.yeggi.com
10. Expert Panel on Neurological Imaging and Musculoskeletal Imaging; Beckmann NM, West OC, Nunez D, et al. ACR appropriateness criteria suspected spine trauma. J Am Coll Radiol. 2919;16(5):S264-285. doi:10.1016/j.jacr.2019.02.002
11. McKinney AM. Normal variants of the lumbar and sacral spine. In: Atlas of Head/Neck and Spine Normal Imaging Variants. Springer; 2018:263-321.
12. Sollini M, Bartoli F, Marciano A, et al. Artificial intelligence and hybrid imaging: the best match for personalized medicine in oncology. Eur J Hybrid Imaging. 2020;4(1):24. doi:10.1186/s41824-020-00094-8
13. Küstner T, Hepp T, Fischer M, et al. Fully automated and standardized segmentation of adipose tissue compartments via deep learning in 3D whole-body MRI of epidemiologic cohort studies. Radiol Artif Intell.2020;2(6):e200010. doi:10.1148/ryai.2020200010
1. Rengier F, Mehndiratta A, von Tengg-Kobligk H, et al. 3D printing based on imaging data: review of medical applications. Int J Comput Assist Radiol Surg. 2010;5(4):335-341. doi:10.1007/s11548-010-0476-x
2. Perica E, Sun Z. Patient-specific three-dimensional printing for pre-surgical planning in hepatocellular carcinoma treatment. Quant Imaging Med Surg. 2017;7(6):668-677. doi:10.21037/qims.2017.11.02
3. Hwang JJ, Jung Y-H, Cho B-H. The need for DICOM encapsulation of 3D scanning STL data. Imaging Sci Dent. 2018;48(4):301-302. doi:10.5624/isd.2018.48.4.301
4. Whyms BJ, Vorperian HK, Gentry LR, Schimek EM, Bersu ET, Chung MK. The effect of computed tomographic scanner parameters and 3-dimensional volume rendering techniques on the accuracy of linear, angular, and volumetric measurements of the mandible. Oral Surg Oral Med, Oral Pathol Oral Radiol. 2013;115(5):682-691. doi:10.1016/j.oooo.2013.02.008
5. Materialise Cloud. Triangle reduction. Accessed May 20, 2021. https://cloud.materialise.com/tools/triangle-reduction
6. Comaneanu RM, Tarcolea M, Vlasceanu D, Cotrut MC. Virtual 3D reconstruction, diagnosis and surgical planning with Mimics software. Int J Nano Biomaterials. 2012;4(1);69-77.
7. Thingiverse: Digital designs for physical objects. Accessed May 20, 2021. https://www.thingiverse.com
8. Cults. Download for free 3D models for 3D printers. Accessed May 20, 2021. https://cults3d.com/en
9. yeggi. Search engine for 3D printer models. Accessed May 20, 2021. https://www.yeggi.com
10. Expert Panel on Neurological Imaging and Musculoskeletal Imaging; Beckmann NM, West OC, Nunez D, et al. ACR appropriateness criteria suspected spine trauma. J Am Coll Radiol. 2919;16(5):S264-285. doi:10.1016/j.jacr.2019.02.002
11. McKinney AM. Normal variants of the lumbar and sacral spine. In: Atlas of Head/Neck and Spine Normal Imaging Variants. Springer; 2018:263-321.
12. Sollini M, Bartoli F, Marciano A, et al. Artificial intelligence and hybrid imaging: the best match for personalized medicine in oncology. Eur J Hybrid Imaging. 2020;4(1):24. doi:10.1186/s41824-020-00094-8
13. Küstner T, Hepp T, Fischer M, et al. Fully automated and standardized segmentation of adipose tissue compartments via deep learning in 3D whole-body MRI of epidemiologic cohort studies. Radiol Artif Intell.2020;2(6):e200010. doi:10.1148/ryai.2020200010
Free U.K. tool could help guide COVID-19 care for cancer patients
An online support tool for health care professionals that recommends whether to admit or discharge a cancer patient with COVID-19, based on their risk of a severe complication, has been developed by researchers from Manchester.
The team used machine learning on data from more than 900 cancer patients with COVID-19, conducting multiple analyses to arrive at a set of features that could accurately predict the need for admission or oxygen therapy, as well as the risk of death.
Dr. Rebecca Lee, The Christie NHS Foundation Trust, Manchester, and colleagues then developed thresholds to derive a score that recommended admission in 95% of patients who went on to need oxygen and an even greater proportion of those who later died.
The research was presented at the 2021 American Society of Clinical Oncology (ASCO) Annual Meeting on June 4.
CORONET
The resulting COVID-19 Risk in Oncology Evaluation Tool (CORONET) model “performs very well at predicting admission and severity of COVID-19 in patients with cancer,” Dr. Lee said. “We have set pragmatic and clinically relevant thresholds that focus on the safety regarding an admission versus discharge decision.”
To help health care professionals, the researchers have built a free online support tool that allows them to enter data and receive a recommendation “as to whether their patient should be considered for discharge, considered for admission, or is at high risk of having a severe outcome of coronavirus,” Dr. Lee explained.
“The health care professional can then explore the recommendation by seeing how their patient … compares with the rest of the cohort.”
The tool also includes a “diagram showing which features are most important to recommend a discharge decision versus an admission decision for each individual patient.”
Clinically intuitive
Dr. Alexi Wright, associate professor, Dana-Faber Cancer Institute, Boston, who was not involved in the study, commented that there were many things that were “really nice about the study.”
“First and foremost that they were establishing a tool to efficiently triage [patients] presenting with COVID,” she said, adding that it was “clinically intuitive” that the team made “pragmatic choices,” and the use of a random forest algorithm means the results are “very interpretable.”
However, Dr. Wright wondered whether the results can be replicated.
Alongside a lack of information on the deaths in the cohort, she pointed out that “ideally you have three data sets, with a training set, a testing set, and a validation set.”
The CORONET model was, however, trained and evaluated on the same dataset, “so it really needs external validation before it would be ready for direct clinical application.”
She continued that there is a “critical need to establish that studies can both be reproduced and replicated,” noting that a recent review showed that 85% of machine-learning studies that were used to detect COVID-19 using chest radiographs “failed fundamental reproducibility and quality checks.”
Risk factors
Dr. Lee began her presentation by reminding the audience that cancer patients are at increased risk of severe COVID-19 and death, with older age, male sex, nosocomial infection, higher ECOG performance status, and active cancer among the risk factors for mortality.
“However, outcomes are very heterogeneous, ranging from patients without symptoms at all to cases with multi-organ failure and death,” she said.
It is consequently “very important for the treating clinician to determine which patients could be safely discharged to the community versus those who need additional support in being admitted to hospital.”
To develop a tool that could distinguish between those two groups of patients, the researchers collected data on 1,743 cancer patients, which was reduced down to 920 patients after excluding those without laboratory confirmed COVID-19 and those with missing data.
Using recursive feature elimination, they selected 10 key patient features associated with prognosis, then compared a lasso regression model with a random forest model, with the latter performing the best.
The team then divided their patients into four cohorts, with the model trained on three cohorts and tested on the fourth. This resulted in the CORONET score, with the final model determined by testing it against the entire patient population.
Next, thresholds were determined for assessing patients for admission versus discharge, as well as for severity of illness, giving the final CORONET model, from which the online tool was developed.
Checking performance
The results showed that the model was able to predict admission with an area under the receiver operating characteristics curve (AUROC) of 0.82 for admission, 0.85 for oxygen requirement, and 0.79 for death.
Further analysis revealed that the most important feature at the time of presentation for determining outcome was the National Early Warning Score 2 (NEWS2), “which is a composite score of heart rate, respiratory rate, saturations and confusion level,” Dr. Lee said.
In addition, C-reactive protein levels, albumin, age, and platelet counts “were also very important features,” she continued, “and these have also been shown in a number of different studies to be important at determining the outcome from coronavirus.”
To examine the performance of the CORONET score further, they applied it to a European hospital dataset, ESMO-CoCARE registry data, and a U.S. cohort, the COVID-19 and Cancer Consortium Registry (CCC19). They found that the score discriminated between patients, but it did so with some degree of heterogeneity.
This was largely driven by higher patient age among the U.S. patients, a higher NEWS2 score, and lower albumin levels, Dr. Lee said.
To ensure the score’s applicability to clinical practice, the team set pragmatic thresholds to determine whether or not a patient required admission or whether they were at risk of dying.
For admission, they set a sensitivity of 85% and a specificity of 56%, while for mortality they set a sensitivity of 43% and a specificity of 92%.
When this was converted into a decision support tool, the model recommended hospital admission for 95% of patients who eventually required oxygen and 97% of patients who died.
The study was funded by The Christie Charitable Foundation. Dr. Lee declares relationships with AstraZeneca and Bristol-Myers Squibb (Inst). Dr. Wright declares relationships with NCCN/AstraZeneca (Inst).
A version of this article first appeared on Medscape.com.
An online support tool for health care professionals that recommends whether to admit or discharge a cancer patient with COVID-19, based on their risk of a severe complication, has been developed by researchers from Manchester.
The team used machine learning on data from more than 900 cancer patients with COVID-19, conducting multiple analyses to arrive at a set of features that could accurately predict the need for admission or oxygen therapy, as well as the risk of death.
Dr. Rebecca Lee, The Christie NHS Foundation Trust, Manchester, and colleagues then developed thresholds to derive a score that recommended admission in 95% of patients who went on to need oxygen and an even greater proportion of those who later died.
The research was presented at the 2021 American Society of Clinical Oncology (ASCO) Annual Meeting on June 4.
CORONET
The resulting COVID-19 Risk in Oncology Evaluation Tool (CORONET) model “performs very well at predicting admission and severity of COVID-19 in patients with cancer,” Dr. Lee said. “We have set pragmatic and clinically relevant thresholds that focus on the safety regarding an admission versus discharge decision.”
To help health care professionals, the researchers have built a free online support tool that allows them to enter data and receive a recommendation “as to whether their patient should be considered for discharge, considered for admission, or is at high risk of having a severe outcome of coronavirus,” Dr. Lee explained.
“The health care professional can then explore the recommendation by seeing how their patient … compares with the rest of the cohort.”
The tool also includes a “diagram showing which features are most important to recommend a discharge decision versus an admission decision for each individual patient.”
Clinically intuitive
Dr. Alexi Wright, associate professor, Dana-Faber Cancer Institute, Boston, who was not involved in the study, commented that there were many things that were “really nice about the study.”
“First and foremost that they were establishing a tool to efficiently triage [patients] presenting with COVID,” she said, adding that it was “clinically intuitive” that the team made “pragmatic choices,” and the use of a random forest algorithm means the results are “very interpretable.”
However, Dr. Wright wondered whether the results can be replicated.
Alongside a lack of information on the deaths in the cohort, she pointed out that “ideally you have three data sets, with a training set, a testing set, and a validation set.”
The CORONET model was, however, trained and evaluated on the same dataset, “so it really needs external validation before it would be ready for direct clinical application.”
She continued that there is a “critical need to establish that studies can both be reproduced and replicated,” noting that a recent review showed that 85% of machine-learning studies that were used to detect COVID-19 using chest radiographs “failed fundamental reproducibility and quality checks.”
Risk factors
Dr. Lee began her presentation by reminding the audience that cancer patients are at increased risk of severe COVID-19 and death, with older age, male sex, nosocomial infection, higher ECOG performance status, and active cancer among the risk factors for mortality.
“However, outcomes are very heterogeneous, ranging from patients without symptoms at all to cases with multi-organ failure and death,” she said.
It is consequently “very important for the treating clinician to determine which patients could be safely discharged to the community versus those who need additional support in being admitted to hospital.”
To develop a tool that could distinguish between those two groups of patients, the researchers collected data on 1,743 cancer patients, which was reduced down to 920 patients after excluding those without laboratory confirmed COVID-19 and those with missing data.
Using recursive feature elimination, they selected 10 key patient features associated with prognosis, then compared a lasso regression model with a random forest model, with the latter performing the best.
The team then divided their patients into four cohorts, with the model trained on three cohorts and tested on the fourth. This resulted in the CORONET score, with the final model determined by testing it against the entire patient population.
Next, thresholds were determined for assessing patients for admission versus discharge, as well as for severity of illness, giving the final CORONET model, from which the online tool was developed.
Checking performance
The results showed that the model was able to predict admission with an area under the receiver operating characteristics curve (AUROC) of 0.82 for admission, 0.85 for oxygen requirement, and 0.79 for death.
Further analysis revealed that the most important feature at the time of presentation for determining outcome was the National Early Warning Score 2 (NEWS2), “which is a composite score of heart rate, respiratory rate, saturations and confusion level,” Dr. Lee said.
In addition, C-reactive protein levels, albumin, age, and platelet counts “were also very important features,” she continued, “and these have also been shown in a number of different studies to be important at determining the outcome from coronavirus.”
To examine the performance of the CORONET score further, they applied it to a European hospital dataset, ESMO-CoCARE registry data, and a U.S. cohort, the COVID-19 and Cancer Consortium Registry (CCC19). They found that the score discriminated between patients, but it did so with some degree of heterogeneity.
This was largely driven by higher patient age among the U.S. patients, a higher NEWS2 score, and lower albumin levels, Dr. Lee said.
To ensure the score’s applicability to clinical practice, the team set pragmatic thresholds to determine whether or not a patient required admission or whether they were at risk of dying.
For admission, they set a sensitivity of 85% and a specificity of 56%, while for mortality they set a sensitivity of 43% and a specificity of 92%.
When this was converted into a decision support tool, the model recommended hospital admission for 95% of patients who eventually required oxygen and 97% of patients who died.
The study was funded by The Christie Charitable Foundation. Dr. Lee declares relationships with AstraZeneca and Bristol-Myers Squibb (Inst). Dr. Wright declares relationships with NCCN/AstraZeneca (Inst).
A version of this article first appeared on Medscape.com.
An online support tool for health care professionals that recommends whether to admit or discharge a cancer patient with COVID-19, based on their risk of a severe complication, has been developed by researchers from Manchester.
The team used machine learning on data from more than 900 cancer patients with COVID-19, conducting multiple analyses to arrive at a set of features that could accurately predict the need for admission or oxygen therapy, as well as the risk of death.
Dr. Rebecca Lee, The Christie NHS Foundation Trust, Manchester, and colleagues then developed thresholds to derive a score that recommended admission in 95% of patients who went on to need oxygen and an even greater proportion of those who later died.
The research was presented at the 2021 American Society of Clinical Oncology (ASCO) Annual Meeting on June 4.
CORONET
The resulting COVID-19 Risk in Oncology Evaluation Tool (CORONET) model “performs very well at predicting admission and severity of COVID-19 in patients with cancer,” Dr. Lee said. “We have set pragmatic and clinically relevant thresholds that focus on the safety regarding an admission versus discharge decision.”
To help health care professionals, the researchers have built a free online support tool that allows them to enter data and receive a recommendation “as to whether their patient should be considered for discharge, considered for admission, or is at high risk of having a severe outcome of coronavirus,” Dr. Lee explained.
“The health care professional can then explore the recommendation by seeing how their patient … compares with the rest of the cohort.”
The tool also includes a “diagram showing which features are most important to recommend a discharge decision versus an admission decision for each individual patient.”
Clinically intuitive
Dr. Alexi Wright, associate professor, Dana-Faber Cancer Institute, Boston, who was not involved in the study, commented that there were many things that were “really nice about the study.”
“First and foremost that they were establishing a tool to efficiently triage [patients] presenting with COVID,” she said, adding that it was “clinically intuitive” that the team made “pragmatic choices,” and the use of a random forest algorithm means the results are “very interpretable.”
However, Dr. Wright wondered whether the results can be replicated.
Alongside a lack of information on the deaths in the cohort, she pointed out that “ideally you have three data sets, with a training set, a testing set, and a validation set.”
The CORONET model was, however, trained and evaluated on the same dataset, “so it really needs external validation before it would be ready for direct clinical application.”
She continued that there is a “critical need to establish that studies can both be reproduced and replicated,” noting that a recent review showed that 85% of machine-learning studies that were used to detect COVID-19 using chest radiographs “failed fundamental reproducibility and quality checks.”
Risk factors
Dr. Lee began her presentation by reminding the audience that cancer patients are at increased risk of severe COVID-19 and death, with older age, male sex, nosocomial infection, higher ECOG performance status, and active cancer among the risk factors for mortality.
“However, outcomes are very heterogeneous, ranging from patients without symptoms at all to cases with multi-organ failure and death,” she said.
It is consequently “very important for the treating clinician to determine which patients could be safely discharged to the community versus those who need additional support in being admitted to hospital.”
To develop a tool that could distinguish between those two groups of patients, the researchers collected data on 1,743 cancer patients, which was reduced down to 920 patients after excluding those without laboratory confirmed COVID-19 and those with missing data.
Using recursive feature elimination, they selected 10 key patient features associated with prognosis, then compared a lasso regression model with a random forest model, with the latter performing the best.
The team then divided their patients into four cohorts, with the model trained on three cohorts and tested on the fourth. This resulted in the CORONET score, with the final model determined by testing it against the entire patient population.
Next, thresholds were determined for assessing patients for admission versus discharge, as well as for severity of illness, giving the final CORONET model, from which the online tool was developed.
Checking performance
The results showed that the model was able to predict admission with an area under the receiver operating characteristics curve (AUROC) of 0.82 for admission, 0.85 for oxygen requirement, and 0.79 for death.
Further analysis revealed that the most important feature at the time of presentation for determining outcome was the National Early Warning Score 2 (NEWS2), “which is a composite score of heart rate, respiratory rate, saturations and confusion level,” Dr. Lee said.
In addition, C-reactive protein levels, albumin, age, and platelet counts “were also very important features,” she continued, “and these have also been shown in a number of different studies to be important at determining the outcome from coronavirus.”
To examine the performance of the CORONET score further, they applied it to a European hospital dataset, ESMO-CoCARE registry data, and a U.S. cohort, the COVID-19 and Cancer Consortium Registry (CCC19). They found that the score discriminated between patients, but it did so with some degree of heterogeneity.
This was largely driven by higher patient age among the U.S. patients, a higher NEWS2 score, and lower albumin levels, Dr. Lee said.
To ensure the score’s applicability to clinical practice, the team set pragmatic thresholds to determine whether or not a patient required admission or whether they were at risk of dying.
For admission, they set a sensitivity of 85% and a specificity of 56%, while for mortality they set a sensitivity of 43% and a specificity of 92%.
When this was converted into a decision support tool, the model recommended hospital admission for 95% of patients who eventually required oxygen and 97% of patients who died.
The study was funded by The Christie Charitable Foundation. Dr. Lee declares relationships with AstraZeneca and Bristol-Myers Squibb (Inst). Dr. Wright declares relationships with NCCN/AstraZeneca (Inst).
A version of this article first appeared on Medscape.com.
Procalcitonin-Guided Antibiotic Prescribing for Acute Exacerbations of Chronic Obstructive Pulmonary Disease in the Emergency Department
The Global Initiative for Chronic Obstructive Lung Disease (GOLD) guidelines define acute exacerbations of chronic obstructive pulmonary disease (AECOPD) as a sudden worsening of respiratory symptoms that require additional interventions. Exacerbations are classified as mild (treated with short-acting bronchodilators only), moderate (treated with antibiotics and/or oral corticosteroids), or severe (treatment requiring hospitalization). Exacerbations must include increased dyspnea, and other symptoms may involve increased sputum volume and purulence, cough, and a change in sputum color. These symptoms can be due to viral, bacterial, or environmental causes, with viral respiratory infections being the most common cause.1-4 However, determining the etiology of an exacerbation can be difficult based on symptoms alone and can lead to an excessive and unnecessary use of antibiotics. Only the change in sputum color is considered highly sensitive and specific for bacterial causes.1 As a result, there has been an increased interest in the use of acute biomarkers to determine whether antibiotics are necessary.
Procalcitonin (PCT) is an acute phase reactant that increases in response to inflammation, especially inflammation caused by a bacterial infection. Recent studies have suggested that PCT may be used in patients experiencing an AECOPD to reduce antibiotic use without impacting rates of treatment failure.5-9 A majority of these studies have been in the inpatient setting or a combination of inpatient and outpatient settings.
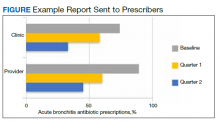
The purpose of this study was to create and to evaluate the efficacy and practicality of a PCT-based algorithm to aid emergency department (ED) clinicians in the evaluation of patients with AECOPD who do not require hospitalization. The primary outcome of this project was the rate of antibiotic prescriptions before and after the initiation of the algorithm.
Methods
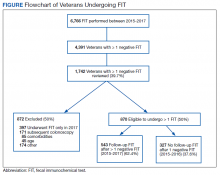
This was an observational, retrospective, pre/post assessment at the Phoenix Veterans Affairs Health Care System (PVAHCS) in Arizona. Patients who were discharged from the ED with a diagnosis of an AECOPD were identified using International Classification of Diseases, Tenth Revision (ICD-10) codes. Patient charts were reviewed from November 2018 to March 2019 for the preimplementation group and from November 2019 for March 2020 in the postimplementation group. The periods were chosen to reflect similar seasons for both the pre- and postimplementation interventions. Patients were excluded from analysis if they required hospital admission, were immunocompromised, on chronic antimicrobial therapy, had no documented medical history of COPD, or if they were presenting primarily for medication refills. Information collected included the rate of antibiotic prescriptions, procalcitonin test orders, COPD GOLD classification, and 30-, 60-, and 90-day reexacerbation rates.
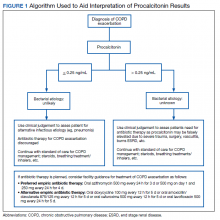
A PCT-based algorithm (Figure 1) was developed and approved by the PVAHCS Antimicrobial Stewardship Program, the Pharmacy and Therapeutics committee, and ED leadership. PCT threshold values were based on values approved by the US Food and Drug Administration and previous studies—antibiotics were discouraged for PCT levels ≤ 0.25 ng/mL but could be considered for PCT levels > 0.25 ng/mL.5,8,9 Clinicians were not required to use the algorithm, and the use of clinical judgement was encouraged. The recommended antibiotic therapies were based on previously approved PVAHCS antimicrobial stewardship guidance. To promote utilization, a PCT quick order option was added to the ED laboratory order menu.
ED clinicians were individually educated by the antimicrobial stewardship and emergency medicine pharmacists, an infectious disease physician champion, and the pharmacy resident. Clinicians were educated about PCT and its use in the setting of AECOPD to aid in the determination of bacterial infections. Each clinician received an electronic copy the algorithm and summary of the study protocol before implementation and 3 months after implementation for follow-up education. In addition, a printed copy of the algorithm was posted in multiple clinician workstations within the ED. For the first month of implementation, the project lead was available full-time in the ED to encourage algorithm use and to field questions or concerns from clinicians.
Outcome Measures
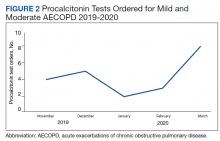
The primary outcome was the rate of antibiotic prescriptions pre- and postintervention. The safety endpoints were 30-, 60-, and 90-day reexacerbation rates. Reexacerbation rates were defined by ICD-10 codes and documentation from a primary care visit or subsequent ED visit. The secondary outcomes were the rate of PCT tests ordered and used for treatment decisions. Other areas of interest were antibiotic prescribing trends, duration of therapy, and patient COPD GOLD classification.
Statistical analysis
It was estimated that a sample size of 146 patients (73 patients/group) would provide 80% power to detect a between-group difference of 10% in the percentage of patients who were prescribed antibiotics. Categorical variables were expressed using estimates of frequency and percentages. Percentages were compared using Fisher exact tests. For all tests, the significance level was set at 0.05.
Results
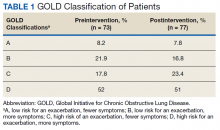
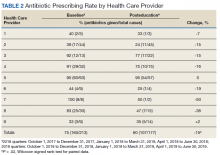
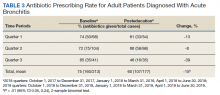
Seventy-three patients were included in the preintervention group and 77 in the postintervention group. The GOLD classification rates were similar between the groups (Table 1). In addition, > 90% of patients were White males and all patients were aged ≥ 50 years, which is characteristic of the US Department of Veterans Affairs (VA) population.
The percentage of antibiotic prescriptions decreased by 20% after implementation, falling from 83.6% before to 63.6% after the implementation (P =.01). The documented change in sputum color remained low compared with antibiotic prescriptions: 17.8% preimplementation and 16.9% postimplementation. The reduction in antibiotic prescriptions was associated with limited differences observed in 30-, 60-, and 90-day reexacerbation rates pre- and postintervention: 19.2% vs 23.4%, 12.3% vs 11.7%, and 4.1% vs 9.1%, respectively.
Prior to the education, introduction of the algorithm, and implementation of the PCT quick-order menu, PCT was ordered for 1.4% of AECOPD cases. Postintervention, PCT was ordered for 28.6% of mild-to-moderate AECOPD cases and used in clinical decision making per clinical documentation 81.8% of the time. PCT was used in 5 GOLD group B patients, 5 GOLD group C patients, and 7 GOLD group D patients. In all cases, PCT was < 0.25 ng/mL. In 4 cases PCT was ordered but not used: 1 GOLD group D patient refused traditional treatment with oral corticosteroids, which resulted in the clinician prescribing antibiotics, and the other 3 cases did not use PCT based on clinical decision making. The rate of PCT tests ordered for mild-to-moderate AECOPD over time is depicted in Figure 2.
The average duration of antibiotic therapy was about 6 days pre- and postintervention. This is longer than the PVAHCS recommended duration of 5 days but is consistent with the GOLD guidelines recommended duration of 5 to 7 days.1 Azithromycin is recommended as a first-line treatment option at the PVAHCS based on the local antibiogram, and it remained the most commonly prescribed antibiotic pre- and postintervention. Outcomes of interest are detailed in Table 2.
Discussion
The implementation of PCT-guided antibiotic prescribing for patients with mild and moderate AECOPD who presented to the ED resulted in a 20% reduction in antibiotic prescriptions, falling from 83.6% before the intervention to 63.6% afterward (P = .01). The measured decrease in antibiotic prescriptions is consistent with other studies evaluating the use of acute phase reactants to guide antibiotic prescribing for AECOPD.10,11 In addition, there was no observed difference in reexacerbation rates. This adds to the increasing body of evidence that antibiotics are overprescribed in mild and moderate AECOPD.12 This is exemplified in our data by the low percentage of patients who had a documented change in sputum color; symptoms that are well known to be highly specific and sensitive for a bacterial infection in AECOPD.
Many health care providers (HCPs) in the ED were unfamiliar with PCT prior to implementation. A primary concern with this study was its impact on diagnostic stewardship. Preimplementation, ED clinicians ordered PCT 8 times for any cause. Postintervention, ED clinicians ordered PCT 180 times for any cause: 36% of these orders were for patients with AECOPD who were discharged from the ED or who required hospital admission. The other orders were for other respiratory conditions, including asthma exacerbations, pneumonia, bronchitis, sinusitis, pharyngitis, nonspecific respiratory infections, and respiratory failure.
The early phase of the COVID-19 pandemic coincided with the postintervention phase of this project. PVAHCS started preparing for the pandemic in March 2020, and the first confirmed diagnosis at the facility occurred mid-March. COVID-19 may have contributed to the sharp increase in PCT tests. There is currently no well-defined role for PCT in the diagnosis or management of COVID-19, but ED clinicians may have increased their use of PCT tests to help characterize the etiology of the large influx of patients presenting with respiratory symptoms.13
Strengths
Strengths of this project include its multimodal implementation and overall pragmatic design, which reflects real-world utilization of procalcitonin by ED HCPs. The HCPs were not mandated to follow the procalcitonin algorithm, and the use of clinical judgment was strongly encouraged. This project occurred concomitantly with the VA Infectious Disease Academic Detailing education program. The program focused on clinician education for the proper diagnosis and treatment of respiratory tract infections. In addition, viral illness packs were introduced as part of this initiative to reduce unnecessary antibiotic prescribing. The viral illness pack included standard items for symptom relief, such as saline nasal spray, cough drops, and hand sanitizer, as well as an explanation card of why the patient was not receiving antibiotics. Several studies have suggested that patients expect a prescription for an antibiotic when they present with respiratory tract symptoms, and HCPs often are compelled to maintain patient satisfaction, thus leading to unnecessary antibiotic prescriptions.14 The viral illness pack helped fulfill the patient’s expectation to receive treatment after seeking care. In addition, the project lead was available full time during the first month of PCT algorithm implementation to address questions and concerns, which may have improved HCPs overall confidence in using PCT.
Limitations
Limitations of this project include its population and its retrospective nature. The PVAHCS patient population is predominantly older, more White, and more male compared with the general civilian population, and results may not be generalizable to other populations. Data were limited to documentation in the electronic health record. The population was based on data extraction by the ICD-10 code, which may not be an accurate capture of the total population as HCPs may not select the most accurate ICD-10 code on documentation. Another potential limitation was the COVID-19 pandemic which may have resulted in HCPs ordering PCT more frequently as more patients presented to the ED with undifferentiated respiratory symptoms. Finally, there were minimal differences observed in reexacerbation rates; however, although the sample size was powered to detect a difference in antibiotic prescriptions, the sample size was not powered to detect a statistically significant difference in the primary safety outcome.
Conclusions
PCT-guided antibiotic prescribing significantly reduced the number of antibiotic prescriptions without an observable increase in reexacerbation rates for patients with mild and moderate AECOPD in the ED. This study provides a pragmatic evaluation of PCT-guided antibiotic prescribing for patients with AECOPD solely in the outpatient setting. Acute phase reactants like PCT can play a role in the management of AECOPD to reduce unnecessary antibiotic prescriptions.
1. Global Initiative for Chronic Obstructive Lung Disease. Global strategy for the diagnosis, management and prevention of chronic obstructive pulmonary disease: 2020 report. Accessd June 2, 2021. http://www.goldcopd.org/wp-content/uploads/2019/12/GOLD-2020-FINAL-ver1.2-03Dec19_WMV.pdf
2. George SN, Garcha DS, Mackay AJ, et al. Human rhinovirus infection during naturally occurring COPD exacerbations. Eur Respir J. 2014;44(1):87-96. doi:10.1183/09031936.00223113
3. Seemungal T, Harper-Owen R, Bhowmik A, et al. Respiratory viruses, symptoms, and inflammatory markers in acute exacerbations and stable chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2001;164(9):1618-1623. doi:10.1164/ajrccm.164.9.2105011
4. Rohde G, Wiethege A, Borg I, et al. Respiratory viruses in exacerbations of chronic obstructive pulmonary disease requiring hospitalisation: a case-control study. Thorax. 2003;58(1):37-42. doi:10.1136/thorax.58.1.37
5. Bremmer DN, Moffa MA, Ma K, et al. Acute exacerbations of chronic obstructive pulmonary disease with a low procalcitonin concentration: impact of antibiotic therapy. Clin Infect Dis. 2019;68(5):725-730. doi:10.1093/cid/ciy552
6. Mathioudakis AG, Chatzimavridou-Grigoriadou V, Corlateanu A, Vestbo J. Procalcitonin to guide antibiotic administration in COPD exacerbations: a meta-analysis. Eur Respir Rev. 2017;26(143):160073. Published 2017 Jan 31. doi:10.1183/16000617.0073-2016
7. van der Does Y, Rood PP, Haagsma JA, Patka P, van Gorp EC, Limper M. Procalcitonin-guided therapy for the initiation of antibiotics in the ED: a systematic review. Am J Emerg Med. 2016;34(7):1286-1293. doi:10.1016/j.ajem.2016.03.065
8. Huang DT, Yealy DM, Filbin MR, et al. Procalcitonin-guided use of antibiotics for lower respiratory tract infection. N Engl J Med. 2018;379(3):236-249. doi:10.1056/NEJMoa1802670
9. Picart J, Moiton MP, Gaüzère BA, Gazaille V, Combes X, DiBernardo S. Introduction of a PCT-based algorithm to guide antibiotic prescription in COPD exacerbation. Med Mal Infect. 2016;46(8):429-435. doi:10.1016/j.medmal.2016.07.008
10. Schuetz P, Chiappa V, Briel M, Greenwald JL. Procalcitonin algorithms for antibiotic therapy decisions: a systematic review of randomized controlled trials and recommendations for clinical algorithms. Arch Intern Med. 2011;171(15):1322-1331. doi:10.1001/archinternmed.2011.318
11. Butler CC, Gillespie D, White P, et al. C-reactive protein testing to guide antibiotic prescribing for COPD exacerbations. N Engl J Med. 2019;381(2):111-120. |doi:10.1056/NEJMoa1803185
12. Vollenweider DJ, Frei A, Steurer-Stey CA, Garcia-Aymerich J, Puhan MA. Antibiotics for exacerbations of chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2018;10(10):CD010257. Published 2018 Oct 29. doi:10.1002/14651858.CD010257.pub2
13. Centers for Disease Control and Prevention. Interim clinical guidance for management of patients with confirmed coronavirus disease (COVID-19). Updated February 16, 2021. Accessed May 14, 2021. https://www.cdc.gov/coronavirus/2019ncov/hcp/clinical-guidance-management-patients.html
14. Gaarslev C, Yee M, Chan G, Fletcher-Lartey S, Khan R. A mixed methods study to understand patient expectations for antibiotics for an upper respiratory tract infection. Antimicrob Resist Infect Control. 2016;5:39. Published 2016 Oct 20. doi:10.1186/s13756-016-0134-3
The Global Initiative for Chronic Obstructive Lung Disease (GOLD) guidelines define acute exacerbations of chronic obstructive pulmonary disease (AECOPD) as a sudden worsening of respiratory symptoms that require additional interventions. Exacerbations are classified as mild (treated with short-acting bronchodilators only), moderate (treated with antibiotics and/or oral corticosteroids), or severe (treatment requiring hospitalization). Exacerbations must include increased dyspnea, and other symptoms may involve increased sputum volume and purulence, cough, and a change in sputum color. These symptoms can be due to viral, bacterial, or environmental causes, with viral respiratory infections being the most common cause.1-4 However, determining the etiology of an exacerbation can be difficult based on symptoms alone and can lead to an excessive and unnecessary use of antibiotics. Only the change in sputum color is considered highly sensitive and specific for bacterial causes.1 As a result, there has been an increased interest in the use of acute biomarkers to determine whether antibiotics are necessary.
Procalcitonin (PCT) is an acute phase reactant that increases in response to inflammation, especially inflammation caused by a bacterial infection. Recent studies have suggested that PCT may be used in patients experiencing an AECOPD to reduce antibiotic use without impacting rates of treatment failure.5-9 A majority of these studies have been in the inpatient setting or a combination of inpatient and outpatient settings.
The purpose of this study was to create and to evaluate the efficacy and practicality of a PCT-based algorithm to aid emergency department (ED) clinicians in the evaluation of patients with AECOPD who do not require hospitalization. The primary outcome of this project was the rate of antibiotic prescriptions before and after the initiation of the algorithm.
Methods
This was an observational, retrospective, pre/post assessment at the Phoenix Veterans Affairs Health Care System (PVAHCS) in Arizona. Patients who were discharged from the ED with a diagnosis of an AECOPD were identified using International Classification of Diseases, Tenth Revision (ICD-10) codes. Patient charts were reviewed from November 2018 to March 2019 for the preimplementation group and from November 2019 for March 2020 in the postimplementation group. The periods were chosen to reflect similar seasons for both the pre- and postimplementation interventions. Patients were excluded from analysis if they required hospital admission, were immunocompromised, on chronic antimicrobial therapy, had no documented medical history of COPD, or if they were presenting primarily for medication refills. Information collected included the rate of antibiotic prescriptions, procalcitonin test orders, COPD GOLD classification, and 30-, 60-, and 90-day reexacerbation rates.
A PCT-based algorithm (Figure 1) was developed and approved by the PVAHCS Antimicrobial Stewardship Program, the Pharmacy and Therapeutics committee, and ED leadership. PCT threshold values were based on values approved by the US Food and Drug Administration and previous studies—antibiotics were discouraged for PCT levels ≤ 0.25 ng/mL but could be considered for PCT levels > 0.25 ng/mL.5,8,9 Clinicians were not required to use the algorithm, and the use of clinical judgement was encouraged. The recommended antibiotic therapies were based on previously approved PVAHCS antimicrobial stewardship guidance. To promote utilization, a PCT quick order option was added to the ED laboratory order menu.
ED clinicians were individually educated by the antimicrobial stewardship and emergency medicine pharmacists, an infectious disease physician champion, and the pharmacy resident. Clinicians were educated about PCT and its use in the setting of AECOPD to aid in the determination of bacterial infections. Each clinician received an electronic copy the algorithm and summary of the study protocol before implementation and 3 months after implementation for follow-up education. In addition, a printed copy of the algorithm was posted in multiple clinician workstations within the ED. For the first month of implementation, the project lead was available full-time in the ED to encourage algorithm use and to field questions or concerns from clinicians.
Outcome Measures
The primary outcome was the rate of antibiotic prescriptions pre- and postintervention. The safety endpoints were 30-, 60-, and 90-day reexacerbation rates. Reexacerbation rates were defined by ICD-10 codes and documentation from a primary care visit or subsequent ED visit. The secondary outcomes were the rate of PCT tests ordered and used for treatment decisions. Other areas of interest were antibiotic prescribing trends, duration of therapy, and patient COPD GOLD classification.
Statistical analysis
It was estimated that a sample size of 146 patients (73 patients/group) would provide 80% power to detect a between-group difference of 10% in the percentage of patients who were prescribed antibiotics. Categorical variables were expressed using estimates of frequency and percentages. Percentages were compared using Fisher exact tests. For all tests, the significance level was set at 0.05.
Results
Seventy-three patients were included in the preintervention group and 77 in the postintervention group. The GOLD classification rates were similar between the groups (Table 1). In addition, > 90% of patients were White males and all patients were aged ≥ 50 years, which is characteristic of the US Department of Veterans Affairs (VA) population.
The percentage of antibiotic prescriptions decreased by 20% after implementation, falling from 83.6% before to 63.6% after the implementation (P =.01). The documented change in sputum color remained low compared with antibiotic prescriptions: 17.8% preimplementation and 16.9% postimplementation. The reduction in antibiotic prescriptions was associated with limited differences observed in 30-, 60-, and 90-day reexacerbation rates pre- and postintervention: 19.2% vs 23.4%, 12.3% vs 11.7%, and 4.1% vs 9.1%, respectively.
Prior to the education, introduction of the algorithm, and implementation of the PCT quick-order menu, PCT was ordered for 1.4% of AECOPD cases. Postintervention, PCT was ordered for 28.6% of mild-to-moderate AECOPD cases and used in clinical decision making per clinical documentation 81.8% of the time. PCT was used in 5 GOLD group B patients, 5 GOLD group C patients, and 7 GOLD group D patients. In all cases, PCT was < 0.25 ng/mL. In 4 cases PCT was ordered but not used: 1 GOLD group D patient refused traditional treatment with oral corticosteroids, which resulted in the clinician prescribing antibiotics, and the other 3 cases did not use PCT based on clinical decision making. The rate of PCT tests ordered for mild-to-moderate AECOPD over time is depicted in Figure 2.
The average duration of antibiotic therapy was about 6 days pre- and postintervention. This is longer than the PVAHCS recommended duration of 5 days but is consistent with the GOLD guidelines recommended duration of 5 to 7 days.1 Azithromycin is recommended as a first-line treatment option at the PVAHCS based on the local antibiogram, and it remained the most commonly prescribed antibiotic pre- and postintervention. Outcomes of interest are detailed in Table 2.
Discussion
The implementation of PCT-guided antibiotic prescribing for patients with mild and moderate AECOPD who presented to the ED resulted in a 20% reduction in antibiotic prescriptions, falling from 83.6% before the intervention to 63.6% afterward (P = .01). The measured decrease in antibiotic prescriptions is consistent with other studies evaluating the use of acute phase reactants to guide antibiotic prescribing for AECOPD.10,11 In addition, there was no observed difference in reexacerbation rates. This adds to the increasing body of evidence that antibiotics are overprescribed in mild and moderate AECOPD.12 This is exemplified in our data by the low percentage of patients who had a documented change in sputum color; symptoms that are well known to be highly specific and sensitive for a bacterial infection in AECOPD.
Many health care providers (HCPs) in the ED were unfamiliar with PCT prior to implementation. A primary concern with this study was its impact on diagnostic stewardship. Preimplementation, ED clinicians ordered PCT 8 times for any cause. Postintervention, ED clinicians ordered PCT 180 times for any cause: 36% of these orders were for patients with AECOPD who were discharged from the ED or who required hospital admission. The other orders were for other respiratory conditions, including asthma exacerbations, pneumonia, bronchitis, sinusitis, pharyngitis, nonspecific respiratory infections, and respiratory failure.
The early phase of the COVID-19 pandemic coincided with the postintervention phase of this project. PVAHCS started preparing for the pandemic in March 2020, and the first confirmed diagnosis at the facility occurred mid-March. COVID-19 may have contributed to the sharp increase in PCT tests. There is currently no well-defined role for PCT in the diagnosis or management of COVID-19, but ED clinicians may have increased their use of PCT tests to help characterize the etiology of the large influx of patients presenting with respiratory symptoms.13
Strengths
Strengths of this project include its multimodal implementation and overall pragmatic design, which reflects real-world utilization of procalcitonin by ED HCPs. The HCPs were not mandated to follow the procalcitonin algorithm, and the use of clinical judgment was strongly encouraged. This project occurred concomitantly with the VA Infectious Disease Academic Detailing education program. The program focused on clinician education for the proper diagnosis and treatment of respiratory tract infections. In addition, viral illness packs were introduced as part of this initiative to reduce unnecessary antibiotic prescribing. The viral illness pack included standard items for symptom relief, such as saline nasal spray, cough drops, and hand sanitizer, as well as an explanation card of why the patient was not receiving antibiotics. Several studies have suggested that patients expect a prescription for an antibiotic when they present with respiratory tract symptoms, and HCPs often are compelled to maintain patient satisfaction, thus leading to unnecessary antibiotic prescriptions.14 The viral illness pack helped fulfill the patient’s expectation to receive treatment after seeking care. In addition, the project lead was available full time during the first month of PCT algorithm implementation to address questions and concerns, which may have improved HCPs overall confidence in using PCT.
Limitations
Limitations of this project include its population and its retrospective nature. The PVAHCS patient population is predominantly older, more White, and more male compared with the general civilian population, and results may not be generalizable to other populations. Data were limited to documentation in the electronic health record. The population was based on data extraction by the ICD-10 code, which may not be an accurate capture of the total population as HCPs may not select the most accurate ICD-10 code on documentation. Another potential limitation was the COVID-19 pandemic which may have resulted in HCPs ordering PCT more frequently as more patients presented to the ED with undifferentiated respiratory symptoms. Finally, there were minimal differences observed in reexacerbation rates; however, although the sample size was powered to detect a difference in antibiotic prescriptions, the sample size was not powered to detect a statistically significant difference in the primary safety outcome.
Conclusions
PCT-guided antibiotic prescribing significantly reduced the number of antibiotic prescriptions without an observable increase in reexacerbation rates for patients with mild and moderate AECOPD in the ED. This study provides a pragmatic evaluation of PCT-guided antibiotic prescribing for patients with AECOPD solely in the outpatient setting. Acute phase reactants like PCT can play a role in the management of AECOPD to reduce unnecessary antibiotic prescriptions.
The Global Initiative for Chronic Obstructive Lung Disease (GOLD) guidelines define acute exacerbations of chronic obstructive pulmonary disease (AECOPD) as a sudden worsening of respiratory symptoms that require additional interventions. Exacerbations are classified as mild (treated with short-acting bronchodilators only), moderate (treated with antibiotics and/or oral corticosteroids), or severe (treatment requiring hospitalization). Exacerbations must include increased dyspnea, and other symptoms may involve increased sputum volume and purulence, cough, and a change in sputum color. These symptoms can be due to viral, bacterial, or environmental causes, with viral respiratory infections being the most common cause.1-4 However, determining the etiology of an exacerbation can be difficult based on symptoms alone and can lead to an excessive and unnecessary use of antibiotics. Only the change in sputum color is considered highly sensitive and specific for bacterial causes.1 As a result, there has been an increased interest in the use of acute biomarkers to determine whether antibiotics are necessary.
Procalcitonin (PCT) is an acute phase reactant that increases in response to inflammation, especially inflammation caused by a bacterial infection. Recent studies have suggested that PCT may be used in patients experiencing an AECOPD to reduce antibiotic use without impacting rates of treatment failure.5-9 A majority of these studies have been in the inpatient setting or a combination of inpatient and outpatient settings.
The purpose of this study was to create and to evaluate the efficacy and practicality of a PCT-based algorithm to aid emergency department (ED) clinicians in the evaluation of patients with AECOPD who do not require hospitalization. The primary outcome of this project was the rate of antibiotic prescriptions before and after the initiation of the algorithm.
Methods
This was an observational, retrospective, pre/post assessment at the Phoenix Veterans Affairs Health Care System (PVAHCS) in Arizona. Patients who were discharged from the ED with a diagnosis of an AECOPD were identified using International Classification of Diseases, Tenth Revision (ICD-10) codes. Patient charts were reviewed from November 2018 to March 2019 for the preimplementation group and from November 2019 for March 2020 in the postimplementation group. The periods were chosen to reflect similar seasons for both the pre- and postimplementation interventions. Patients were excluded from analysis if they required hospital admission, were immunocompromised, on chronic antimicrobial therapy, had no documented medical history of COPD, or if they were presenting primarily for medication refills. Information collected included the rate of antibiotic prescriptions, procalcitonin test orders, COPD GOLD classification, and 30-, 60-, and 90-day reexacerbation rates.
A PCT-based algorithm (Figure 1) was developed and approved by the PVAHCS Antimicrobial Stewardship Program, the Pharmacy and Therapeutics committee, and ED leadership. PCT threshold values were based on values approved by the US Food and Drug Administration and previous studies—antibiotics were discouraged for PCT levels ≤ 0.25 ng/mL but could be considered for PCT levels > 0.25 ng/mL.5,8,9 Clinicians were not required to use the algorithm, and the use of clinical judgement was encouraged. The recommended antibiotic therapies were based on previously approved PVAHCS antimicrobial stewardship guidance. To promote utilization, a PCT quick order option was added to the ED laboratory order menu.
ED clinicians were individually educated by the antimicrobial stewardship and emergency medicine pharmacists, an infectious disease physician champion, and the pharmacy resident. Clinicians were educated about PCT and its use in the setting of AECOPD to aid in the determination of bacterial infections. Each clinician received an electronic copy the algorithm and summary of the study protocol before implementation and 3 months after implementation for follow-up education. In addition, a printed copy of the algorithm was posted in multiple clinician workstations within the ED. For the first month of implementation, the project lead was available full-time in the ED to encourage algorithm use and to field questions or concerns from clinicians.
Outcome Measures
The primary outcome was the rate of antibiotic prescriptions pre- and postintervention. The safety endpoints were 30-, 60-, and 90-day reexacerbation rates. Reexacerbation rates were defined by ICD-10 codes and documentation from a primary care visit or subsequent ED visit. The secondary outcomes were the rate of PCT tests ordered and used for treatment decisions. Other areas of interest were antibiotic prescribing trends, duration of therapy, and patient COPD GOLD classification.
Statistical analysis
It was estimated that a sample size of 146 patients (73 patients/group) would provide 80% power to detect a between-group difference of 10% in the percentage of patients who were prescribed antibiotics. Categorical variables were expressed using estimates of frequency and percentages. Percentages were compared using Fisher exact tests. For all tests, the significance level was set at 0.05.
Results
Seventy-three patients were included in the preintervention group and 77 in the postintervention group. The GOLD classification rates were similar between the groups (Table 1). In addition, > 90% of patients were White males and all patients were aged ≥ 50 years, which is characteristic of the US Department of Veterans Affairs (VA) population.
The percentage of antibiotic prescriptions decreased by 20% after implementation, falling from 83.6% before to 63.6% after the implementation (P =.01). The documented change in sputum color remained low compared with antibiotic prescriptions: 17.8% preimplementation and 16.9% postimplementation. The reduction in antibiotic prescriptions was associated with limited differences observed in 30-, 60-, and 90-day reexacerbation rates pre- and postintervention: 19.2% vs 23.4%, 12.3% vs 11.7%, and 4.1% vs 9.1%, respectively.
Prior to the education, introduction of the algorithm, and implementation of the PCT quick-order menu, PCT was ordered for 1.4% of AECOPD cases. Postintervention, PCT was ordered for 28.6% of mild-to-moderate AECOPD cases and used in clinical decision making per clinical documentation 81.8% of the time. PCT was used in 5 GOLD group B patients, 5 GOLD group C patients, and 7 GOLD group D patients. In all cases, PCT was < 0.25 ng/mL. In 4 cases PCT was ordered but not used: 1 GOLD group D patient refused traditional treatment with oral corticosteroids, which resulted in the clinician prescribing antibiotics, and the other 3 cases did not use PCT based on clinical decision making. The rate of PCT tests ordered for mild-to-moderate AECOPD over time is depicted in Figure 2.
The average duration of antibiotic therapy was about 6 days pre- and postintervention. This is longer than the PVAHCS recommended duration of 5 days but is consistent with the GOLD guidelines recommended duration of 5 to 7 days.1 Azithromycin is recommended as a first-line treatment option at the PVAHCS based on the local antibiogram, and it remained the most commonly prescribed antibiotic pre- and postintervention. Outcomes of interest are detailed in Table 2.
Discussion
The implementation of PCT-guided antibiotic prescribing for patients with mild and moderate AECOPD who presented to the ED resulted in a 20% reduction in antibiotic prescriptions, falling from 83.6% before the intervention to 63.6% afterward (P = .01). The measured decrease in antibiotic prescriptions is consistent with other studies evaluating the use of acute phase reactants to guide antibiotic prescribing for AECOPD.10,11 In addition, there was no observed difference in reexacerbation rates. This adds to the increasing body of evidence that antibiotics are overprescribed in mild and moderate AECOPD.12 This is exemplified in our data by the low percentage of patients who had a documented change in sputum color; symptoms that are well known to be highly specific and sensitive for a bacterial infection in AECOPD.
Many health care providers (HCPs) in the ED were unfamiliar with PCT prior to implementation. A primary concern with this study was its impact on diagnostic stewardship. Preimplementation, ED clinicians ordered PCT 8 times for any cause. Postintervention, ED clinicians ordered PCT 180 times for any cause: 36% of these orders were for patients with AECOPD who were discharged from the ED or who required hospital admission. The other orders were for other respiratory conditions, including asthma exacerbations, pneumonia, bronchitis, sinusitis, pharyngitis, nonspecific respiratory infections, and respiratory failure.
The early phase of the COVID-19 pandemic coincided with the postintervention phase of this project. PVAHCS started preparing for the pandemic in March 2020, and the first confirmed diagnosis at the facility occurred mid-March. COVID-19 may have contributed to the sharp increase in PCT tests. There is currently no well-defined role for PCT in the diagnosis or management of COVID-19, but ED clinicians may have increased their use of PCT tests to help characterize the etiology of the large influx of patients presenting with respiratory symptoms.13
Strengths
Strengths of this project include its multimodal implementation and overall pragmatic design, which reflects real-world utilization of procalcitonin by ED HCPs. The HCPs were not mandated to follow the procalcitonin algorithm, and the use of clinical judgment was strongly encouraged. This project occurred concomitantly with the VA Infectious Disease Academic Detailing education program. The program focused on clinician education for the proper diagnosis and treatment of respiratory tract infections. In addition, viral illness packs were introduced as part of this initiative to reduce unnecessary antibiotic prescribing. The viral illness pack included standard items for symptom relief, such as saline nasal spray, cough drops, and hand sanitizer, as well as an explanation card of why the patient was not receiving antibiotics. Several studies have suggested that patients expect a prescription for an antibiotic when they present with respiratory tract symptoms, and HCPs often are compelled to maintain patient satisfaction, thus leading to unnecessary antibiotic prescriptions.14 The viral illness pack helped fulfill the patient’s expectation to receive treatment after seeking care. In addition, the project lead was available full time during the first month of PCT algorithm implementation to address questions and concerns, which may have improved HCPs overall confidence in using PCT.
Limitations
Limitations of this project include its population and its retrospective nature. The PVAHCS patient population is predominantly older, more White, and more male compared with the general civilian population, and results may not be generalizable to other populations. Data were limited to documentation in the electronic health record. The population was based on data extraction by the ICD-10 code, which may not be an accurate capture of the total population as HCPs may not select the most accurate ICD-10 code on documentation. Another potential limitation was the COVID-19 pandemic which may have resulted in HCPs ordering PCT more frequently as more patients presented to the ED with undifferentiated respiratory symptoms. Finally, there were minimal differences observed in reexacerbation rates; however, although the sample size was powered to detect a difference in antibiotic prescriptions, the sample size was not powered to detect a statistically significant difference in the primary safety outcome.
Conclusions
PCT-guided antibiotic prescribing significantly reduced the number of antibiotic prescriptions without an observable increase in reexacerbation rates for patients with mild and moderate AECOPD in the ED. This study provides a pragmatic evaluation of PCT-guided antibiotic prescribing for patients with AECOPD solely in the outpatient setting. Acute phase reactants like PCT can play a role in the management of AECOPD to reduce unnecessary antibiotic prescriptions.
1. Global Initiative for Chronic Obstructive Lung Disease. Global strategy for the diagnosis, management and prevention of chronic obstructive pulmonary disease: 2020 report. Accessd June 2, 2021. http://www.goldcopd.org/wp-content/uploads/2019/12/GOLD-2020-FINAL-ver1.2-03Dec19_WMV.pdf
2. George SN, Garcha DS, Mackay AJ, et al. Human rhinovirus infection during naturally occurring COPD exacerbations. Eur Respir J. 2014;44(1):87-96. doi:10.1183/09031936.00223113
3. Seemungal T, Harper-Owen R, Bhowmik A, et al. Respiratory viruses, symptoms, and inflammatory markers in acute exacerbations and stable chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2001;164(9):1618-1623. doi:10.1164/ajrccm.164.9.2105011
4. Rohde G, Wiethege A, Borg I, et al. Respiratory viruses in exacerbations of chronic obstructive pulmonary disease requiring hospitalisation: a case-control study. Thorax. 2003;58(1):37-42. doi:10.1136/thorax.58.1.37
5. Bremmer DN, Moffa MA, Ma K, et al. Acute exacerbations of chronic obstructive pulmonary disease with a low procalcitonin concentration: impact of antibiotic therapy. Clin Infect Dis. 2019;68(5):725-730. doi:10.1093/cid/ciy552
6. Mathioudakis AG, Chatzimavridou-Grigoriadou V, Corlateanu A, Vestbo J. Procalcitonin to guide antibiotic administration in COPD exacerbations: a meta-analysis. Eur Respir Rev. 2017;26(143):160073. Published 2017 Jan 31. doi:10.1183/16000617.0073-2016
7. van der Does Y, Rood PP, Haagsma JA, Patka P, van Gorp EC, Limper M. Procalcitonin-guided therapy for the initiation of antibiotics in the ED: a systematic review. Am J Emerg Med. 2016;34(7):1286-1293. doi:10.1016/j.ajem.2016.03.065
8. Huang DT, Yealy DM, Filbin MR, et al. Procalcitonin-guided use of antibiotics for lower respiratory tract infection. N Engl J Med. 2018;379(3):236-249. doi:10.1056/NEJMoa1802670
9. Picart J, Moiton MP, Gaüzère BA, Gazaille V, Combes X, DiBernardo S. Introduction of a PCT-based algorithm to guide antibiotic prescription in COPD exacerbation. Med Mal Infect. 2016;46(8):429-435. doi:10.1016/j.medmal.2016.07.008
10. Schuetz P, Chiappa V, Briel M, Greenwald JL. Procalcitonin algorithms for antibiotic therapy decisions: a systematic review of randomized controlled trials and recommendations for clinical algorithms. Arch Intern Med. 2011;171(15):1322-1331. doi:10.1001/archinternmed.2011.318
11. Butler CC, Gillespie D, White P, et al. C-reactive protein testing to guide antibiotic prescribing for COPD exacerbations. N Engl J Med. 2019;381(2):111-120. |doi:10.1056/NEJMoa1803185
12. Vollenweider DJ, Frei A, Steurer-Stey CA, Garcia-Aymerich J, Puhan MA. Antibiotics for exacerbations of chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2018;10(10):CD010257. Published 2018 Oct 29. doi:10.1002/14651858.CD010257.pub2
13. Centers for Disease Control and Prevention. Interim clinical guidance for management of patients with confirmed coronavirus disease (COVID-19). Updated February 16, 2021. Accessed May 14, 2021. https://www.cdc.gov/coronavirus/2019ncov/hcp/clinical-guidance-management-patients.html
14. Gaarslev C, Yee M, Chan G, Fletcher-Lartey S, Khan R. A mixed methods study to understand patient expectations for antibiotics for an upper respiratory tract infection. Antimicrob Resist Infect Control. 2016;5:39. Published 2016 Oct 20. doi:10.1186/s13756-016-0134-3
1. Global Initiative for Chronic Obstructive Lung Disease. Global strategy for the diagnosis, management and prevention of chronic obstructive pulmonary disease: 2020 report. Accessd June 2, 2021. http://www.goldcopd.org/wp-content/uploads/2019/12/GOLD-2020-FINAL-ver1.2-03Dec19_WMV.pdf
2. George SN, Garcha DS, Mackay AJ, et al. Human rhinovirus infection during naturally occurring COPD exacerbations. Eur Respir J. 2014;44(1):87-96. doi:10.1183/09031936.00223113
3. Seemungal T, Harper-Owen R, Bhowmik A, et al. Respiratory viruses, symptoms, and inflammatory markers in acute exacerbations and stable chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2001;164(9):1618-1623. doi:10.1164/ajrccm.164.9.2105011
4. Rohde G, Wiethege A, Borg I, et al. Respiratory viruses in exacerbations of chronic obstructive pulmonary disease requiring hospitalisation: a case-control study. Thorax. 2003;58(1):37-42. doi:10.1136/thorax.58.1.37
5. Bremmer DN, Moffa MA, Ma K, et al. Acute exacerbations of chronic obstructive pulmonary disease with a low procalcitonin concentration: impact of antibiotic therapy. Clin Infect Dis. 2019;68(5):725-730. doi:10.1093/cid/ciy552
6. Mathioudakis AG, Chatzimavridou-Grigoriadou V, Corlateanu A, Vestbo J. Procalcitonin to guide antibiotic administration in COPD exacerbations: a meta-analysis. Eur Respir Rev. 2017;26(143):160073. Published 2017 Jan 31. doi:10.1183/16000617.0073-2016
7. van der Does Y, Rood PP, Haagsma JA, Patka P, van Gorp EC, Limper M. Procalcitonin-guided therapy for the initiation of antibiotics in the ED: a systematic review. Am J Emerg Med. 2016;34(7):1286-1293. doi:10.1016/j.ajem.2016.03.065
8. Huang DT, Yealy DM, Filbin MR, et al. Procalcitonin-guided use of antibiotics for lower respiratory tract infection. N Engl J Med. 2018;379(3):236-249. doi:10.1056/NEJMoa1802670
9. Picart J, Moiton MP, Gaüzère BA, Gazaille V, Combes X, DiBernardo S. Introduction of a PCT-based algorithm to guide antibiotic prescription in COPD exacerbation. Med Mal Infect. 2016;46(8):429-435. doi:10.1016/j.medmal.2016.07.008
10. Schuetz P, Chiappa V, Briel M, Greenwald JL. Procalcitonin algorithms for antibiotic therapy decisions: a systematic review of randomized controlled trials and recommendations for clinical algorithms. Arch Intern Med. 2011;171(15):1322-1331. doi:10.1001/archinternmed.2011.318
11. Butler CC, Gillespie D, White P, et al. C-reactive protein testing to guide antibiotic prescribing for COPD exacerbations. N Engl J Med. 2019;381(2):111-120. |doi:10.1056/NEJMoa1803185
12. Vollenweider DJ, Frei A, Steurer-Stey CA, Garcia-Aymerich J, Puhan MA. Antibiotics for exacerbations of chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2018;10(10):CD010257. Published 2018 Oct 29. doi:10.1002/14651858.CD010257.pub2
13. Centers for Disease Control and Prevention. Interim clinical guidance for management of patients with confirmed coronavirus disease (COVID-19). Updated February 16, 2021. Accessed May 14, 2021. https://www.cdc.gov/coronavirus/2019ncov/hcp/clinical-guidance-management-patients.html
14. Gaarslev C, Yee M, Chan G, Fletcher-Lartey S, Khan R. A mixed methods study to understand patient expectations for antibiotics for an upper respiratory tract infection. Antimicrob Resist Infect Control. 2016;5:39. Published 2016 Oct 20. doi:10.1186/s13756-016-0134-3
Audit and Feedback: A Quality Improvement Study to Improve Antimicrobial Stewardship
Antibiotics are commonly overused for several viral respiratory conditions where antibiotic treatment is not clinically indicated. For example, a 2016 study by Fleming-Dutra and colleagues showed that at least 30% of all antibiotics prescribed in an outpatient setting were inappropriate and for acute bronchitis, antibiotic prescriptions were inappropriate in 50% of cases.1 Acute bronchitis is predominantly a viral illness where antibiotics should be rarely used.2-8 The Healthcare Effectiveness Data and Information Set has measured the avoidance of antibiotic treatment in adults with acute bronchitis since 2006. The National Committee for Quality Assurance reported in 2018 that about 75% of adults received antibiotics for acute bronchitis.9 Inappropriate antibiotic use contributes to antimicrobial resistance, resulting in the increase of morbidity and mortality of treatable infections.10 Reducing inappropriate antibiotic use in outpatient settings is a high-priority public health issue and is a Healthy People 2030 objective.11
Antimicrobial Stewardship
Antimicrobial stewardship programs measure and track how antibiotics are prescribed by health care providers (HCPs) and used by patients. The Centers for Disease Control and Prevention (CDC) created a framework for outpatient antimicrobial stewardship programs by outlining 4 core elements: (1) commitment from every person involved in patient care to act as an antibiotic steward; (2) policies and interventions to promote appropriate antibiotic prescribing practices; (3) antibiotic prescription tracking and reporting; and (4) appropriate antibiotic use education.12
Audit and feedback (A&F) is a form of antibiotic prescription tracking and reporting that involves measuring and comparing a HCP’s performance (ie, antibiotic prescribing) with a standard, and the results of this audit are shared with the HCP. This strategy is based on the belief that a HCP is motivated to modify practice habits when given feedback showing that his or her performance is inconsistent with targeted expectations. A&F is most effective when feedback is provided by a supervisor or respected peer, presented more than once, individualized, delivered in both verbal and written formats, and includes explicit targets and an action plan.13,14
This study focuses on an antimicrobial stewardship program implemented in an outpatient Indian Health Service ambulatory care clinic in the Pacific Northwest. The clinic was staffed by 9 HCPs serving about 12,000 American Indian and Alaskan Native patients. The clinic includes a full-service pharmacy where nearly all prescriptions issued by in-house HCPs are filled. The clinic’s antibiotic prescribing rate for adult patients with acute bronchitis was similar to the national mean in 2018 (75%).9 The study objective was to reduce the rate of potentially inappropriate (not guideline-concordant) antibiotic prescribing in patients with acute bronchitis without underlying chronic lung disease or evidence of bacterial infection through A&F.
Methods
The antimicrobial stewardship program was implemented by 3 pharmacists, including a pharmacy resident. HCPs received education by pharmacy staff on evidence-based prescribing for adult acute bronchitis and quarterly feedback on antibiotic prescribing rates. All prescribing and dispensing records necessary for the program were available in the clinic electronic health record. The rate of potentially inappropriate antibiotic prescribing was calculated as the proportion of eligible bronchitis cases who received antibiotics.
In October 2018, a 60-minute educational session was provided by 2 pharmacists to HCPs. The material covered an overview of acute bronchitis presentation, diagnosis, treatment (Table 1), and a comparison of national and local prescribing data (baseline audit).2-4 The educational session concluded with prescription strategies to reduce inappropriate antibiotic prescribing, including but not limited to: delayed prescriptions, patient and caregiver education, use of nonantibiotic medications to control symptoms, and use of A&F reports.5-8 At the conclusion of the session, HCPs committed to engage in the antimicrobial stewardship program.
Audit
To determine the total number of eligible bronchitis cases (denominator), a visit report was generated by a pharmacist for a primary diagnosis of acute bronchitis using International Statistical Classification of Diseases, Tenth Revision (ICD 10) codes (J20.3 - J20.9) for the review period. Only adults aged ≥ 18 years were included. Patients with a chronic lung disease (eg, chronic obstructive pulmonary disease, asthma) and those who had a concomitant bacterial infection (eg, urinary tract infection, cellulitis) were excluded. A visit for acute bronchitis that included additional ICD 10 codes indicating the patient had a chronic lung disease or concomitant bacterial infection were used to determine exclusion. The remaining patients who received a potentially inappropriate antibiotic prescription (numerator) were those who were prescribed or dispensed antibiotics on the date of service.
Feedback
Baseline data were presented to HCPs during the educational session in October 2018. Prospective audits were performed quarterly thereafter (January, April, and July) by the pharmacy resident using the criteria described above. Audit data were compiled into personalized reports and provided to HCPs by the pharmacy resident with written and verbal individual feedback. Written feedback was sent by email to each HCP containing the HCP’s rate, the clinic rate in aggregate, rates from the prior year and quarter(s) for comparison, and clinical pearls from the guidelines (Figure). Verbal feedback included a review of the written feedback and answering any questions concerning the report.
Implementation
Study periods were chosen to coincide with the pharmacy residency training year, which starts in July and ends in June. The start date of October 2018 differed from the start of the residency year (July 2018) owing to delays in obtaining permissions. A&F and analysis of prescribing rates continued through the end of the residency year, for total duration of 9 months (October 1, 2018 to June 30, 2019). For ease of reporting, quarterly reports followed the federal government’s fiscal year (FY) which runs from October 1 of the prior calendar year through September 30 of the year being described. HCPs received 4 feedback reports: baseline (October 1, 2018 - June 30, 2018) in October 2018, quarter 1 (October 1, 2018 - December 31, 2018) in January 2019, quarter 2 (January 1, 2019 - March 31, 2019) in April 2019, and quarter 3 (April 1, 2019 - June 30, 2019) in July 2019.
Statistical Analysis
Prescribing rates were compared between identical 9 -month periods. A 2-sample binomial test for proportions was used to derive an approximate CI of prescribing rates at the patient level. However, to account for clustering of patients within HCP panels and dependence of observations over study periods stemming from examining the same HCPs within each of the periods, the Wilcoxon signed rank test for paired data was used to evaluate prescribing rates at the HCP level. Statistical analysis was performed using R statistical software version 4.0.3. Differences were considered significant at P < .05 set a priori.
This study was approved by the Portland Area Indian Health Service Institutional Review Board (Study ID: 1316730).
Results
All 9 HCPs who see adult patients at the clinic agreed to participate and were all fully present in each study period. Among HCPs, there were 5 physicians and 4 physician assistants or nurse practitioners. There was a total of 213 visits that met study criteria during the baseline period (October 1, 2017 to June 30, 2018) and 177 visits in the posteducation period (October 1, 2018 to June 30, 2019). The total number of acute bronchitis encounters varied by HCP (Ranges, 5-63 [baseline] and 2-57 [posteducation]); however, the relative number of encounters each HCP contributed was similar in each study period (Table 2). The pharmacy resident spent about 2 hours each quarter to generate 9 feedback reports, 1 for each HCP.
Antibiotic Prescribing
Antibiotic prescribing rates decreased from 75% at baseline to 60% at posteducation month 9 (absolute difference, -15% [95% CI, 5 - 24%]; P ≤ .01) (Table 3). The clinic rate was lower for each quarter in FY 2019 (posteducation) compared with the same quarter of FY 2018 (baseline), with the lowest rate observed in the final quarter of the study. Comparing pre- and post- A&F, the rates for HCPs prescribing antibiotics were lower for 7 HCPs, unchanged for 1 HCP, and slightly increased for 1 HCP(P = .02).
Discussion
Acute bronchitis remains a common diagnosis where antibiotics are prescribed despite being a predominately viral illness. Guidelines and evidence-based practices advise against antibiotics for this diagnosis. According to the American Academy of Family Physicians, antibiotics are reserved for cases where chronic lung disease is present as these patients are at a high risk of developing pneumonia.3 The decision to prescribe antibiotics is complex and driven by several interdependent factors, such as patient expectations, health system limitations, clinician training, and specialty.15 HCPs may more aggressively treat acute bronchitis among American Indian/Alaskan Native (AI/AN) people due to a high risk of developing serious complications from respiratory illnesses.16 A clinician’s background, usual patient cohort (ie, mostly pediatric or geriatric), and time spent in urgent care or in activities outside of patient care (administration) may account for the difference in patient encounters by HCP for acute bronchitis.
Following the CDC framework, this antimicrobial stewardship program helped empower people involved in patient care (eg, pharmacists, HCPs), educate staff on proper use of antibiotics for acute bronchitis, and track and report antibiotic prescribing through the A&F process. Educational interventions coupled with ongoing A&F are reproducible by other health care facilities and are not usually time consuming. This study showcases a successful example of implementing A&F in an antimicrobial stewardship quality improvement project that could be translated toward other conditions (eg, sinusitis, urinary tract infection, community-acquired pneumonia).
In a similar study, Meeker and colleagues used a variation of an A&F intervention using a monthly email showing peer comparisons to notify clinicians who were prescribing too many unnecessary antibiotics for common respiratory illnesses that did not require antibiotics, such as the common cold.17 The peer comparison intervention arm emailed a rank order that listed prescribers by the number of prescriptions for common respiratory illnesses. This intervention demonstrated a reduction of 5.2% in inappropriate antibiotic prescribing.
Limitations
This quality improvement study had several limitations. The study did not account for the duration of symptoms as a factor to judge appropriateness. Although this was identified early in the study, it was unavoidable since there was no report that could extract the duration of symptoms in the electronic health record. Future studies should consider a manual review of each encounter to overcome this limitation. Another limitation was that only three-quarters of the year and not the entire year were reviewed. Future studies should include longer time frames to measure the durability of changes to antibiotic prescriptions. Lastly, the study did not assess diagnosis shifting (the practice of changing the proportion of antibiotic-appropriate acute respiratory tract infection diagnosis over time), effects of patient demographics (patient age and sex were not recorded), or any sustained effect on prescribing rates after the study ended.
Conclusions
Clinician education coupled with A&F are components of the CDC’s framework for an effective antimicrobial stewardship program. The intervention seem to be an effective means toward reducing inappropriate antibiotic prescribing for acute bronchitis and has the potential for application to other antimicrobial stewardship initiatives. The present study adds to the growing body of evidence on the importance and impact an antimicrobial stewardship program has on a clinic or health system.
Acknowledgment
The results of this study have been reported at the 2019 IHS Southwest Regional Pharmacy Continuing Education Seminar, April 12-14, 2019.
1. Fleming-Dutra KE, Hersh AL, Shapiro DJ, et al. Prevalence of inappropriate antibiotic prescriptions among US ambulatory care visits, 2010-2011. JAMA. 2016;315(17):1864-1873. doi:10.1001/jama.2016.4151
2. Barnett ML, Linder JA. Antibiotic prescribing for adults with acute bronchitis in the United States, 1996-2010. JAMA. 2014;311(19):2020-2022. doi:10.1001/jama.2013.286141
3. Kinkade S, Long NA. Acute bronchitis. Am Fam Physician. 2016;94(7):560-565.
4. Harris AM, Hicks LA, Qaseem A; High Value Care Task Force of the American College of Physicians and for the Centers for Disease Control and Prevention. Appropriate antibiotic use for acute respiratory tract infection in adults: advice for high-value care from the American College of Physicians and the Centers for Disease Control and Prevention. Ann Intern Med. 2016;164(6):425-434. doi:10.7326/M15-1840
5. Gonzales R, Bartlett JG, Besser RE, et al. Principles of appropriate antibiotic use for treatment of uncomplicated acute bronchitis: background. Ann Intern Med. 2001;134(6):521-529. doi:10.7326/0003-4819-134-6-200103200-00021
6. Centers for Disease Control and Prevention. Adult outpatient treatment recommendations. Updated October 3, 2017. Accessed May 19, 2021. www.cdc.gov/antibiotic-use/community/for-hcp/outpatient-hcp/adult-treatment-rec.html
7. Braman SS. Chronic cough due to chronic bronchitis: ACCP evidence-based clinical practice guidelines. Chest. 2006;129(1 suppl):104S-115S. doi:10.1378/chest.129.1_suppl.104S
8. Petersen I, Johnson AM, Islam A, Duckworth G, Livermore DM, Hayward AC. Protective effect of antibiotics against serious complications of common respiratory tract infections: retrospective cohort study with the UK General Practice Research Database. BMJ. 2007;335(7627):982. doi:10.1136/bmj.39345.405243.BE
9. National Committee for Quality Assurance. Avoidance of antibiotic treatment in adults with acute bronchitis (AAB). Accessed May 19, 2021. https://www.ncqa.org/hedis/measures/avoidance-of-antibiotic-treatment-in-adults-with-acute-bronchitis
10. Centers for Disease Control and Prevention. Antibiotic resistance threats in the United States, 2013. Published April 23, 2013. Accessed May 19, 2021. https://www.cdc.gov/drugresistance/threat-report-2013/pdf/ar-threats-2013-508.pdf
11. US Department of Health and Human Services, Office of Disease Prevention and Health Promotion. Healthy People 2030: reduce inappropriate antibiotic use in outpatient settings — HAI‑D01. Accessed May 19, 2021. https://health.gov/healthypeople/objectives-and-data/browse-objectives/healthcare-associated-infections/reduce-inappropriate-antibiotic-use-outpatient-settings-hai-d01
12. Sanchez GV, Fleming-Dutra KE, Roberts RM, Hicks LA. Core elements of outpatient antibiotic stewardship. MMWR Recomm Rep. 2016;65(6):1-12. Published 2016 Nov 11. doi:10.15585/mmwr.rr6506a1
13. Ivers N, Jamtvedt G, Flottorp S, et al. Audit and feedback: effects on professional practice and healthcare outcomes. Cochrane Database Syst Rev. 2012;(6):CD000259. Published 2012 Jun 13. doi:10.1002/14651858.CD000259.pub3
14. Ivers NM, Grimshaw JM, Jamtvedt G, et al. Growing literature, stagnant science? Systematic review, meta-regression and cumulative analysis of audit and feedback interventions in health care. J Gen Intern Med. 2014;29(11):1534-1541. doi:10.1007/s11606-014-2913-y
15. Ranji SR, Steinman MA, Shojania KG, et al. Closing the Quality Gap: A Critical Analysis of Quality Improvement Strategies. Vol. 4: Antibiotic Prescribing Behavior. Agency for Healthcare Research and Quality (US); 2006. Accessed May 20, 2021. https://www.ncbi.nlm.nih.gov/books/NBK43956/
16. Groom AV, Hennessy TW, Singleton RJ, Butler JC, Holve S, Cheek JE. Pneumonia and influenza mortality among American Indian and Alaska Native people, 1990-2009. Am J Public Health. 2014;104 Suppl 3(suppl 3):S460-S469. doi:10.2105/AJPH.2013.301740
17. Meeker D, Linder JA, Fox CR, et al. Effect of behavioral interventions on inappropriate antibiotic prescribing among primary care practices: a randomized clinical trial. JAMA. 2016;315(6):562-570. doi:10.1001/jama.2016.0275
Antibiotics are commonly overused for several viral respiratory conditions where antibiotic treatment is not clinically indicated. For example, a 2016 study by Fleming-Dutra and colleagues showed that at least 30% of all antibiotics prescribed in an outpatient setting were inappropriate and for acute bronchitis, antibiotic prescriptions were inappropriate in 50% of cases.1 Acute bronchitis is predominantly a viral illness where antibiotics should be rarely used.2-8 The Healthcare Effectiveness Data and Information Set has measured the avoidance of antibiotic treatment in adults with acute bronchitis since 2006. The National Committee for Quality Assurance reported in 2018 that about 75% of adults received antibiotics for acute bronchitis.9 Inappropriate antibiotic use contributes to antimicrobial resistance, resulting in the increase of morbidity and mortality of treatable infections.10 Reducing inappropriate antibiotic use in outpatient settings is a high-priority public health issue and is a Healthy People 2030 objective.11
Antimicrobial Stewardship
Antimicrobial stewardship programs measure and track how antibiotics are prescribed by health care providers (HCPs) and used by patients. The Centers for Disease Control and Prevention (CDC) created a framework for outpatient antimicrobial stewardship programs by outlining 4 core elements: (1) commitment from every person involved in patient care to act as an antibiotic steward; (2) policies and interventions to promote appropriate antibiotic prescribing practices; (3) antibiotic prescription tracking and reporting; and (4) appropriate antibiotic use education.12
Audit and feedback (A&F) is a form of antibiotic prescription tracking and reporting that involves measuring and comparing a HCP’s performance (ie, antibiotic prescribing) with a standard, and the results of this audit are shared with the HCP. This strategy is based on the belief that a HCP is motivated to modify practice habits when given feedback showing that his or her performance is inconsistent with targeted expectations. A&F is most effective when feedback is provided by a supervisor or respected peer, presented more than once, individualized, delivered in both verbal and written formats, and includes explicit targets and an action plan.13,14
This study focuses on an antimicrobial stewardship program implemented in an outpatient Indian Health Service ambulatory care clinic in the Pacific Northwest. The clinic was staffed by 9 HCPs serving about 12,000 American Indian and Alaskan Native patients. The clinic includes a full-service pharmacy where nearly all prescriptions issued by in-house HCPs are filled. The clinic’s antibiotic prescribing rate for adult patients with acute bronchitis was similar to the national mean in 2018 (75%).9 The study objective was to reduce the rate of potentially inappropriate (not guideline-concordant) antibiotic prescribing in patients with acute bronchitis without underlying chronic lung disease or evidence of bacterial infection through A&F.
Methods
The antimicrobial stewardship program was implemented by 3 pharmacists, including a pharmacy resident. HCPs received education by pharmacy staff on evidence-based prescribing for adult acute bronchitis and quarterly feedback on antibiotic prescribing rates. All prescribing and dispensing records necessary for the program were available in the clinic electronic health record. The rate of potentially inappropriate antibiotic prescribing was calculated as the proportion of eligible bronchitis cases who received antibiotics.
In October 2018, a 60-minute educational session was provided by 2 pharmacists to HCPs. The material covered an overview of acute bronchitis presentation, diagnosis, treatment (Table 1), and a comparison of national and local prescribing data (baseline audit).2-4 The educational session concluded with prescription strategies to reduce inappropriate antibiotic prescribing, including but not limited to: delayed prescriptions, patient and caregiver education, use of nonantibiotic medications to control symptoms, and use of A&F reports.5-8 At the conclusion of the session, HCPs committed to engage in the antimicrobial stewardship program.
Audit
To determine the total number of eligible bronchitis cases (denominator), a visit report was generated by a pharmacist for a primary diagnosis of acute bronchitis using International Statistical Classification of Diseases, Tenth Revision (ICD 10) codes (J20.3 - J20.9) for the review period. Only adults aged ≥ 18 years were included. Patients with a chronic lung disease (eg, chronic obstructive pulmonary disease, asthma) and those who had a concomitant bacterial infection (eg, urinary tract infection, cellulitis) were excluded. A visit for acute bronchitis that included additional ICD 10 codes indicating the patient had a chronic lung disease or concomitant bacterial infection were used to determine exclusion. The remaining patients who received a potentially inappropriate antibiotic prescription (numerator) were those who were prescribed or dispensed antibiotics on the date of service.
Feedback
Baseline data were presented to HCPs during the educational session in October 2018. Prospective audits were performed quarterly thereafter (January, April, and July) by the pharmacy resident using the criteria described above. Audit data were compiled into personalized reports and provided to HCPs by the pharmacy resident with written and verbal individual feedback. Written feedback was sent by email to each HCP containing the HCP’s rate, the clinic rate in aggregate, rates from the prior year and quarter(s) for comparison, and clinical pearls from the guidelines (Figure). Verbal feedback included a review of the written feedback and answering any questions concerning the report.
Implementation
Study periods were chosen to coincide with the pharmacy residency training year, which starts in July and ends in June. The start date of October 2018 differed from the start of the residency year (July 2018) owing to delays in obtaining permissions. A&F and analysis of prescribing rates continued through the end of the residency year, for total duration of 9 months (October 1, 2018 to June 30, 2019). For ease of reporting, quarterly reports followed the federal government’s fiscal year (FY) which runs from October 1 of the prior calendar year through September 30 of the year being described. HCPs received 4 feedback reports: baseline (October 1, 2018 - June 30, 2018) in October 2018, quarter 1 (October 1, 2018 - December 31, 2018) in January 2019, quarter 2 (January 1, 2019 - March 31, 2019) in April 2019, and quarter 3 (April 1, 2019 - June 30, 2019) in July 2019.
Statistical Analysis
Prescribing rates were compared between identical 9 -month periods. A 2-sample binomial test for proportions was used to derive an approximate CI of prescribing rates at the patient level. However, to account for clustering of patients within HCP panels and dependence of observations over study periods stemming from examining the same HCPs within each of the periods, the Wilcoxon signed rank test for paired data was used to evaluate prescribing rates at the HCP level. Statistical analysis was performed using R statistical software version 4.0.3. Differences were considered significant at P < .05 set a priori.
This study was approved by the Portland Area Indian Health Service Institutional Review Board (Study ID: 1316730).
Results
All 9 HCPs who see adult patients at the clinic agreed to participate and were all fully present in each study period. Among HCPs, there were 5 physicians and 4 physician assistants or nurse practitioners. There was a total of 213 visits that met study criteria during the baseline period (October 1, 2017 to June 30, 2018) and 177 visits in the posteducation period (October 1, 2018 to June 30, 2019). The total number of acute bronchitis encounters varied by HCP (Ranges, 5-63 [baseline] and 2-57 [posteducation]); however, the relative number of encounters each HCP contributed was similar in each study period (Table 2). The pharmacy resident spent about 2 hours each quarter to generate 9 feedback reports, 1 for each HCP.
Antibiotic Prescribing
Antibiotic prescribing rates decreased from 75% at baseline to 60% at posteducation month 9 (absolute difference, -15% [95% CI, 5 - 24%]; P ≤ .01) (Table 3). The clinic rate was lower for each quarter in FY 2019 (posteducation) compared with the same quarter of FY 2018 (baseline), with the lowest rate observed in the final quarter of the study. Comparing pre- and post- A&F, the rates for HCPs prescribing antibiotics were lower for 7 HCPs, unchanged for 1 HCP, and slightly increased for 1 HCP(P = .02).
Discussion
Acute bronchitis remains a common diagnosis where antibiotics are prescribed despite being a predominately viral illness. Guidelines and evidence-based practices advise against antibiotics for this diagnosis. According to the American Academy of Family Physicians, antibiotics are reserved for cases where chronic lung disease is present as these patients are at a high risk of developing pneumonia.3 The decision to prescribe antibiotics is complex and driven by several interdependent factors, such as patient expectations, health system limitations, clinician training, and specialty.15 HCPs may more aggressively treat acute bronchitis among American Indian/Alaskan Native (AI/AN) people due to a high risk of developing serious complications from respiratory illnesses.16 A clinician’s background, usual patient cohort (ie, mostly pediatric or geriatric), and time spent in urgent care or in activities outside of patient care (administration) may account for the difference in patient encounters by HCP for acute bronchitis.
Following the CDC framework, this antimicrobial stewardship program helped empower people involved in patient care (eg, pharmacists, HCPs), educate staff on proper use of antibiotics for acute bronchitis, and track and report antibiotic prescribing through the A&F process. Educational interventions coupled with ongoing A&F are reproducible by other health care facilities and are not usually time consuming. This study showcases a successful example of implementing A&F in an antimicrobial stewardship quality improvement project that could be translated toward other conditions (eg, sinusitis, urinary tract infection, community-acquired pneumonia).
In a similar study, Meeker and colleagues used a variation of an A&F intervention using a monthly email showing peer comparisons to notify clinicians who were prescribing too many unnecessary antibiotics for common respiratory illnesses that did not require antibiotics, such as the common cold.17 The peer comparison intervention arm emailed a rank order that listed prescribers by the number of prescriptions for common respiratory illnesses. This intervention demonstrated a reduction of 5.2% in inappropriate antibiotic prescribing.
Limitations
This quality improvement study had several limitations. The study did not account for the duration of symptoms as a factor to judge appropriateness. Although this was identified early in the study, it was unavoidable since there was no report that could extract the duration of symptoms in the electronic health record. Future studies should consider a manual review of each encounter to overcome this limitation. Another limitation was that only three-quarters of the year and not the entire year were reviewed. Future studies should include longer time frames to measure the durability of changes to antibiotic prescriptions. Lastly, the study did not assess diagnosis shifting (the practice of changing the proportion of antibiotic-appropriate acute respiratory tract infection diagnosis over time), effects of patient demographics (patient age and sex were not recorded), or any sustained effect on prescribing rates after the study ended.
Conclusions
Clinician education coupled with A&F are components of the CDC’s framework for an effective antimicrobial stewardship program. The intervention seem to be an effective means toward reducing inappropriate antibiotic prescribing for acute bronchitis and has the potential for application to other antimicrobial stewardship initiatives. The present study adds to the growing body of evidence on the importance and impact an antimicrobial stewardship program has on a clinic or health system.
Acknowledgment
The results of this study have been reported at the 2019 IHS Southwest Regional Pharmacy Continuing Education Seminar, April 12-14, 2019.
Antibiotics are commonly overused for several viral respiratory conditions where antibiotic treatment is not clinically indicated. For example, a 2016 study by Fleming-Dutra and colleagues showed that at least 30% of all antibiotics prescribed in an outpatient setting were inappropriate and for acute bronchitis, antibiotic prescriptions were inappropriate in 50% of cases.1 Acute bronchitis is predominantly a viral illness where antibiotics should be rarely used.2-8 The Healthcare Effectiveness Data and Information Set has measured the avoidance of antibiotic treatment in adults with acute bronchitis since 2006. The National Committee for Quality Assurance reported in 2018 that about 75% of adults received antibiotics for acute bronchitis.9 Inappropriate antibiotic use contributes to antimicrobial resistance, resulting in the increase of morbidity and mortality of treatable infections.10 Reducing inappropriate antibiotic use in outpatient settings is a high-priority public health issue and is a Healthy People 2030 objective.11
Antimicrobial Stewardship
Antimicrobial stewardship programs measure and track how antibiotics are prescribed by health care providers (HCPs) and used by patients. The Centers for Disease Control and Prevention (CDC) created a framework for outpatient antimicrobial stewardship programs by outlining 4 core elements: (1) commitment from every person involved in patient care to act as an antibiotic steward; (2) policies and interventions to promote appropriate antibiotic prescribing practices; (3) antibiotic prescription tracking and reporting; and (4) appropriate antibiotic use education.12
Audit and feedback (A&F) is a form of antibiotic prescription tracking and reporting that involves measuring and comparing a HCP’s performance (ie, antibiotic prescribing) with a standard, and the results of this audit are shared with the HCP. This strategy is based on the belief that a HCP is motivated to modify practice habits when given feedback showing that his or her performance is inconsistent with targeted expectations. A&F is most effective when feedback is provided by a supervisor or respected peer, presented more than once, individualized, delivered in both verbal and written formats, and includes explicit targets and an action plan.13,14
This study focuses on an antimicrobial stewardship program implemented in an outpatient Indian Health Service ambulatory care clinic in the Pacific Northwest. The clinic was staffed by 9 HCPs serving about 12,000 American Indian and Alaskan Native patients. The clinic includes a full-service pharmacy where nearly all prescriptions issued by in-house HCPs are filled. The clinic’s antibiotic prescribing rate for adult patients with acute bronchitis was similar to the national mean in 2018 (75%).9 The study objective was to reduce the rate of potentially inappropriate (not guideline-concordant) antibiotic prescribing in patients with acute bronchitis without underlying chronic lung disease or evidence of bacterial infection through A&F.
Methods
The antimicrobial stewardship program was implemented by 3 pharmacists, including a pharmacy resident. HCPs received education by pharmacy staff on evidence-based prescribing for adult acute bronchitis and quarterly feedback on antibiotic prescribing rates. All prescribing and dispensing records necessary for the program were available in the clinic electronic health record. The rate of potentially inappropriate antibiotic prescribing was calculated as the proportion of eligible bronchitis cases who received antibiotics.
In October 2018, a 60-minute educational session was provided by 2 pharmacists to HCPs. The material covered an overview of acute bronchitis presentation, diagnosis, treatment (Table 1), and a comparison of national and local prescribing data (baseline audit).2-4 The educational session concluded with prescription strategies to reduce inappropriate antibiotic prescribing, including but not limited to: delayed prescriptions, patient and caregiver education, use of nonantibiotic medications to control symptoms, and use of A&F reports.5-8 At the conclusion of the session, HCPs committed to engage in the antimicrobial stewardship program.
Audit
To determine the total number of eligible bronchitis cases (denominator), a visit report was generated by a pharmacist for a primary diagnosis of acute bronchitis using International Statistical Classification of Diseases, Tenth Revision (ICD 10) codes (J20.3 - J20.9) for the review period. Only adults aged ≥ 18 years were included. Patients with a chronic lung disease (eg, chronic obstructive pulmonary disease, asthma) and those who had a concomitant bacterial infection (eg, urinary tract infection, cellulitis) were excluded. A visit for acute bronchitis that included additional ICD 10 codes indicating the patient had a chronic lung disease or concomitant bacterial infection were used to determine exclusion. The remaining patients who received a potentially inappropriate antibiotic prescription (numerator) were those who were prescribed or dispensed antibiotics on the date of service.
Feedback
Baseline data were presented to HCPs during the educational session in October 2018. Prospective audits were performed quarterly thereafter (January, April, and July) by the pharmacy resident using the criteria described above. Audit data were compiled into personalized reports and provided to HCPs by the pharmacy resident with written and verbal individual feedback. Written feedback was sent by email to each HCP containing the HCP’s rate, the clinic rate in aggregate, rates from the prior year and quarter(s) for comparison, and clinical pearls from the guidelines (Figure). Verbal feedback included a review of the written feedback and answering any questions concerning the report.
Implementation
Study periods were chosen to coincide with the pharmacy residency training year, which starts in July and ends in June. The start date of October 2018 differed from the start of the residency year (July 2018) owing to delays in obtaining permissions. A&F and analysis of prescribing rates continued through the end of the residency year, for total duration of 9 months (October 1, 2018 to June 30, 2019). For ease of reporting, quarterly reports followed the federal government’s fiscal year (FY) which runs from October 1 of the prior calendar year through September 30 of the year being described. HCPs received 4 feedback reports: baseline (October 1, 2018 - June 30, 2018) in October 2018, quarter 1 (October 1, 2018 - December 31, 2018) in January 2019, quarter 2 (January 1, 2019 - March 31, 2019) in April 2019, and quarter 3 (April 1, 2019 - June 30, 2019) in July 2019.
Statistical Analysis
Prescribing rates were compared between identical 9 -month periods. A 2-sample binomial test for proportions was used to derive an approximate CI of prescribing rates at the patient level. However, to account for clustering of patients within HCP panels and dependence of observations over study periods stemming from examining the same HCPs within each of the periods, the Wilcoxon signed rank test for paired data was used to evaluate prescribing rates at the HCP level. Statistical analysis was performed using R statistical software version 4.0.3. Differences were considered significant at P < .05 set a priori.
This study was approved by the Portland Area Indian Health Service Institutional Review Board (Study ID: 1316730).
Results
All 9 HCPs who see adult patients at the clinic agreed to participate and were all fully present in each study period. Among HCPs, there were 5 physicians and 4 physician assistants or nurse practitioners. There was a total of 213 visits that met study criteria during the baseline period (October 1, 2017 to June 30, 2018) and 177 visits in the posteducation period (October 1, 2018 to June 30, 2019). The total number of acute bronchitis encounters varied by HCP (Ranges, 5-63 [baseline] and 2-57 [posteducation]); however, the relative number of encounters each HCP contributed was similar in each study period (Table 2). The pharmacy resident spent about 2 hours each quarter to generate 9 feedback reports, 1 for each HCP.
Antibiotic Prescribing
Antibiotic prescribing rates decreased from 75% at baseline to 60% at posteducation month 9 (absolute difference, -15% [95% CI, 5 - 24%]; P ≤ .01) (Table 3). The clinic rate was lower for each quarter in FY 2019 (posteducation) compared with the same quarter of FY 2018 (baseline), with the lowest rate observed in the final quarter of the study. Comparing pre- and post- A&F, the rates for HCPs prescribing antibiotics were lower for 7 HCPs, unchanged for 1 HCP, and slightly increased for 1 HCP(P = .02).
Discussion
Acute bronchitis remains a common diagnosis where antibiotics are prescribed despite being a predominately viral illness. Guidelines and evidence-based practices advise against antibiotics for this diagnosis. According to the American Academy of Family Physicians, antibiotics are reserved for cases where chronic lung disease is present as these patients are at a high risk of developing pneumonia.3 The decision to prescribe antibiotics is complex and driven by several interdependent factors, such as patient expectations, health system limitations, clinician training, and specialty.15 HCPs may more aggressively treat acute bronchitis among American Indian/Alaskan Native (AI/AN) people due to a high risk of developing serious complications from respiratory illnesses.16 A clinician’s background, usual patient cohort (ie, mostly pediatric or geriatric), and time spent in urgent care or in activities outside of patient care (administration) may account for the difference in patient encounters by HCP for acute bronchitis.
Following the CDC framework, this antimicrobial stewardship program helped empower people involved in patient care (eg, pharmacists, HCPs), educate staff on proper use of antibiotics for acute bronchitis, and track and report antibiotic prescribing through the A&F process. Educational interventions coupled with ongoing A&F are reproducible by other health care facilities and are not usually time consuming. This study showcases a successful example of implementing A&F in an antimicrobial stewardship quality improvement project that could be translated toward other conditions (eg, sinusitis, urinary tract infection, community-acquired pneumonia).
In a similar study, Meeker and colleagues used a variation of an A&F intervention using a monthly email showing peer comparisons to notify clinicians who were prescribing too many unnecessary antibiotics for common respiratory illnesses that did not require antibiotics, such as the common cold.17 The peer comparison intervention arm emailed a rank order that listed prescribers by the number of prescriptions for common respiratory illnesses. This intervention demonstrated a reduction of 5.2% in inappropriate antibiotic prescribing.
Limitations
This quality improvement study had several limitations. The study did not account for the duration of symptoms as a factor to judge appropriateness. Although this was identified early in the study, it was unavoidable since there was no report that could extract the duration of symptoms in the electronic health record. Future studies should consider a manual review of each encounter to overcome this limitation. Another limitation was that only three-quarters of the year and not the entire year were reviewed. Future studies should include longer time frames to measure the durability of changes to antibiotic prescriptions. Lastly, the study did not assess diagnosis shifting (the practice of changing the proportion of antibiotic-appropriate acute respiratory tract infection diagnosis over time), effects of patient demographics (patient age and sex were not recorded), or any sustained effect on prescribing rates after the study ended.
Conclusions
Clinician education coupled with A&F are components of the CDC’s framework for an effective antimicrobial stewardship program. The intervention seem to be an effective means toward reducing inappropriate antibiotic prescribing for acute bronchitis and has the potential for application to other antimicrobial stewardship initiatives. The present study adds to the growing body of evidence on the importance and impact an antimicrobial stewardship program has on a clinic or health system.
Acknowledgment
The results of this study have been reported at the 2019 IHS Southwest Regional Pharmacy Continuing Education Seminar, April 12-14, 2019.
1. Fleming-Dutra KE, Hersh AL, Shapiro DJ, et al. Prevalence of inappropriate antibiotic prescriptions among US ambulatory care visits, 2010-2011. JAMA. 2016;315(17):1864-1873. doi:10.1001/jama.2016.4151
2. Barnett ML, Linder JA. Antibiotic prescribing for adults with acute bronchitis in the United States, 1996-2010. JAMA. 2014;311(19):2020-2022. doi:10.1001/jama.2013.286141
3. Kinkade S, Long NA. Acute bronchitis. Am Fam Physician. 2016;94(7):560-565.
4. Harris AM, Hicks LA, Qaseem A; High Value Care Task Force of the American College of Physicians and for the Centers for Disease Control and Prevention. Appropriate antibiotic use for acute respiratory tract infection in adults: advice for high-value care from the American College of Physicians and the Centers for Disease Control and Prevention. Ann Intern Med. 2016;164(6):425-434. doi:10.7326/M15-1840
5. Gonzales R, Bartlett JG, Besser RE, et al. Principles of appropriate antibiotic use for treatment of uncomplicated acute bronchitis: background. Ann Intern Med. 2001;134(6):521-529. doi:10.7326/0003-4819-134-6-200103200-00021
6. Centers for Disease Control and Prevention. Adult outpatient treatment recommendations. Updated October 3, 2017. Accessed May 19, 2021. www.cdc.gov/antibiotic-use/community/for-hcp/outpatient-hcp/adult-treatment-rec.html
7. Braman SS. Chronic cough due to chronic bronchitis: ACCP evidence-based clinical practice guidelines. Chest. 2006;129(1 suppl):104S-115S. doi:10.1378/chest.129.1_suppl.104S
8. Petersen I, Johnson AM, Islam A, Duckworth G, Livermore DM, Hayward AC. Protective effect of antibiotics against serious complications of common respiratory tract infections: retrospective cohort study with the UK General Practice Research Database. BMJ. 2007;335(7627):982. doi:10.1136/bmj.39345.405243.BE
9. National Committee for Quality Assurance. Avoidance of antibiotic treatment in adults with acute bronchitis (AAB). Accessed May 19, 2021. https://www.ncqa.org/hedis/measures/avoidance-of-antibiotic-treatment-in-adults-with-acute-bronchitis
10. Centers for Disease Control and Prevention. Antibiotic resistance threats in the United States, 2013. Published April 23, 2013. Accessed May 19, 2021. https://www.cdc.gov/drugresistance/threat-report-2013/pdf/ar-threats-2013-508.pdf
11. US Department of Health and Human Services, Office of Disease Prevention and Health Promotion. Healthy People 2030: reduce inappropriate antibiotic use in outpatient settings — HAI‑D01. Accessed May 19, 2021. https://health.gov/healthypeople/objectives-and-data/browse-objectives/healthcare-associated-infections/reduce-inappropriate-antibiotic-use-outpatient-settings-hai-d01
12. Sanchez GV, Fleming-Dutra KE, Roberts RM, Hicks LA. Core elements of outpatient antibiotic stewardship. MMWR Recomm Rep. 2016;65(6):1-12. Published 2016 Nov 11. doi:10.15585/mmwr.rr6506a1
13. Ivers N, Jamtvedt G, Flottorp S, et al. Audit and feedback: effects on professional practice and healthcare outcomes. Cochrane Database Syst Rev. 2012;(6):CD000259. Published 2012 Jun 13. doi:10.1002/14651858.CD000259.pub3
14. Ivers NM, Grimshaw JM, Jamtvedt G, et al. Growing literature, stagnant science? Systematic review, meta-regression and cumulative analysis of audit and feedback interventions in health care. J Gen Intern Med. 2014;29(11):1534-1541. doi:10.1007/s11606-014-2913-y
15. Ranji SR, Steinman MA, Shojania KG, et al. Closing the Quality Gap: A Critical Analysis of Quality Improvement Strategies. Vol. 4: Antibiotic Prescribing Behavior. Agency for Healthcare Research and Quality (US); 2006. Accessed May 20, 2021. https://www.ncbi.nlm.nih.gov/books/NBK43956/
16. Groom AV, Hennessy TW, Singleton RJ, Butler JC, Holve S, Cheek JE. Pneumonia and influenza mortality among American Indian and Alaska Native people, 1990-2009. Am J Public Health. 2014;104 Suppl 3(suppl 3):S460-S469. doi:10.2105/AJPH.2013.301740
17. Meeker D, Linder JA, Fox CR, et al. Effect of behavioral interventions on inappropriate antibiotic prescribing among primary care practices: a randomized clinical trial. JAMA. 2016;315(6):562-570. doi:10.1001/jama.2016.0275
1. Fleming-Dutra KE, Hersh AL, Shapiro DJ, et al. Prevalence of inappropriate antibiotic prescriptions among US ambulatory care visits, 2010-2011. JAMA. 2016;315(17):1864-1873. doi:10.1001/jama.2016.4151
2. Barnett ML, Linder JA. Antibiotic prescribing for adults with acute bronchitis in the United States, 1996-2010. JAMA. 2014;311(19):2020-2022. doi:10.1001/jama.2013.286141
3. Kinkade S, Long NA. Acute bronchitis. Am Fam Physician. 2016;94(7):560-565.
4. Harris AM, Hicks LA, Qaseem A; High Value Care Task Force of the American College of Physicians and for the Centers for Disease Control and Prevention. Appropriate antibiotic use for acute respiratory tract infection in adults: advice for high-value care from the American College of Physicians and the Centers for Disease Control and Prevention. Ann Intern Med. 2016;164(6):425-434. doi:10.7326/M15-1840
5. Gonzales R, Bartlett JG, Besser RE, et al. Principles of appropriate antibiotic use for treatment of uncomplicated acute bronchitis: background. Ann Intern Med. 2001;134(6):521-529. doi:10.7326/0003-4819-134-6-200103200-00021
6. Centers for Disease Control and Prevention. Adult outpatient treatment recommendations. Updated October 3, 2017. Accessed May 19, 2021. www.cdc.gov/antibiotic-use/community/for-hcp/outpatient-hcp/adult-treatment-rec.html
7. Braman SS. Chronic cough due to chronic bronchitis: ACCP evidence-based clinical practice guidelines. Chest. 2006;129(1 suppl):104S-115S. doi:10.1378/chest.129.1_suppl.104S
8. Petersen I, Johnson AM, Islam A, Duckworth G, Livermore DM, Hayward AC. Protective effect of antibiotics against serious complications of common respiratory tract infections: retrospective cohort study with the UK General Practice Research Database. BMJ. 2007;335(7627):982. doi:10.1136/bmj.39345.405243.BE
9. National Committee for Quality Assurance. Avoidance of antibiotic treatment in adults with acute bronchitis (AAB). Accessed May 19, 2021. https://www.ncqa.org/hedis/measures/avoidance-of-antibiotic-treatment-in-adults-with-acute-bronchitis
10. Centers for Disease Control and Prevention. Antibiotic resistance threats in the United States, 2013. Published April 23, 2013. Accessed May 19, 2021. https://www.cdc.gov/drugresistance/threat-report-2013/pdf/ar-threats-2013-508.pdf
11. US Department of Health and Human Services, Office of Disease Prevention and Health Promotion. Healthy People 2030: reduce inappropriate antibiotic use in outpatient settings — HAI‑D01. Accessed May 19, 2021. https://health.gov/healthypeople/objectives-and-data/browse-objectives/healthcare-associated-infections/reduce-inappropriate-antibiotic-use-outpatient-settings-hai-d01
12. Sanchez GV, Fleming-Dutra KE, Roberts RM, Hicks LA. Core elements of outpatient antibiotic stewardship. MMWR Recomm Rep. 2016;65(6):1-12. Published 2016 Nov 11. doi:10.15585/mmwr.rr6506a1
13. Ivers N, Jamtvedt G, Flottorp S, et al. Audit and feedback: effects on professional practice and healthcare outcomes. Cochrane Database Syst Rev. 2012;(6):CD000259. Published 2012 Jun 13. doi:10.1002/14651858.CD000259.pub3
14. Ivers NM, Grimshaw JM, Jamtvedt G, et al. Growing literature, stagnant science? Systematic review, meta-regression and cumulative analysis of audit and feedback interventions in health care. J Gen Intern Med. 2014;29(11):1534-1541. doi:10.1007/s11606-014-2913-y
15. Ranji SR, Steinman MA, Shojania KG, et al. Closing the Quality Gap: A Critical Analysis of Quality Improvement Strategies. Vol. 4: Antibiotic Prescribing Behavior. Agency for Healthcare Research and Quality (US); 2006. Accessed May 20, 2021. https://www.ncbi.nlm.nih.gov/books/NBK43956/
16. Groom AV, Hennessy TW, Singleton RJ, Butler JC, Holve S, Cheek JE. Pneumonia and influenza mortality among American Indian and Alaska Native people, 1990-2009. Am J Public Health. 2014;104 Suppl 3(suppl 3):S460-S469. doi:10.2105/AJPH.2013.301740
17. Meeker D, Linder JA, Fox CR, et al. Effect of behavioral interventions on inappropriate antibiotic prescribing among primary care practices: a randomized clinical trial. JAMA. 2016;315(6):562-570. doi:10.1001/jama.2016.0275
A large proportion of migraine patients are not offered preventive treatment
, new research suggests. Investigators found that among patients with migraine who are eligible for preventive therapy, more than a third were not offered this option. In addition, fewer than 10% were currently taking preventive medication, and an additional 10% had discontinued preventive therapy.
“We confirmed that as of 2012 to 2013 – the years these data were collected from a large, comprehensive survey – gaps in care remained,” said study investigator Stephanie J. Nahas, MD, director of the headache medicine fellowship program, Thomas Jefferson University, Philadelphia. “In this preventive-eligible population, 35% reported never even being offered preventive medication.”
Furthermore, only 28% of patients taking preventive medication experienced a reduction in headache frequency to less than 4 days per month, which is a primary goal of treatment, said Dr. Nahas. Disease burden, as measured with scales of disability and affective comorbidities, remained substantial.
The findings were presented at the American Headache Society’s 2021 annual meeting.
Lack of efficacy?
In 2019, the American Headache Society published a position statement recommending that preventive treatment be considered for patients who have migraine and four or more monthly headache days (MHDs), regardless of their level of associated disability. However, previous data suggest few patients who are eligible for preventive treatment receive it. In addition, many who have used preventive medications do not adhere to their regimens because of problems with tolerability, efficacy, or both.
To identify treatment gaps and characterize self-reported use of preventive medications for migraine, the investigators examined data from the Chronic Migraine Epidemiology and Outcomes (CaMEO) study, a web-based survey conducted in a representative U.S. sample from September 2012 through November 2013.
The survey identified and characterized patients who met modified criteria for migraine consistent with those in ICHD-3. The researchers classified respondents who had migraine and four or more MHDs as potentially eligible for migraine preventive treatment.
The investigators assessed the study population’s use of oral preventive medications, migraine-related disability and burden, willingness to take preventive treatment, and reasons for discontinuation.
Assessments included the Migraine Disability Assessment Questionnaire, the Patient Health Questionnaire–9 for depression, the Generalized Anxiety Disorder 7-Item Scale, the Migraine Specific Quality of Life questionnaire, and the Migraine Symptom Severity Scale.
In all, 16,789 respondents met criteria for migraine, and 6,579 (39.2%) reported having at least four MHDs. The median age of this subgroup that was eligible for preventive treatment was 40.3 years, and approximately 79% were women.
Only 9.8% of respondents who were eligible for preventive medications were currently using an oral preventive medication. Among those who had ever tried an oral preventive medication, 53.6% discontinued it. Efficacy for patients who used medications appeared to be inadequate. Among all current users of preventive treatment, 68.4% continued to have at least four MHDs.
The researchers assessed treatment eligibility among patients not taking preventive medication. Among respondents who had never used a preventive treatment, 35.7% were eligible to receive it. Among all users who had discontinued preventive medication, 61.0% were still eligible to receive it.
Attitudes toward injectables
Among respondents who had never used a preventive treatment, 64.3% had zero to three MHDs. The remaining 35.7% had 4-7, 8-14, or 15 or more MHDs. Among current users of preventive treatments, 68.4% had four or more MHDs. Among those who had discontinued preventive treatment, 61.0% had four or more MHDs.
Patients who have never used preventive medication “have substantial management gaps,” said Dr. Nahas. High proportions of these patients have moderate or severe disability (64.7%), depression (43%), and anxiety (39%). The rates of these outcomes are higher in users who discontinued treatment, likely because of confounding by indication, she added.
The prevalence of anxiety was similar between those who currently used, formerly used, or never used preventive medications. However, there were differences between never-users and current or former users with respect to moderate to severe depression (never-users, 43%; current users, 49.4%; discontinued users, 46.5%) and moderate to severe disability (never-users, 64.7%; current users, 80.4%; discontinued users, 78.9%).
In all, 44.6% of those who discontinued preventive therapy reported safety and tolerability problems as reasons for stopping treatment. In addition, 39.7% reported that these medications did not prevent enough headaches. Some patients reported partial or temporary efficacy as a reason for discontinuation. Other reasons were related to health care costs and access and personal preferences. Only 9.2% of patients who discontinued treatment said that their headaches improved enough to stop medication.
The investigators also analyzed respondents’ interest in preventive therapies. Among respondents who had never used preventive therapies, 61.8% of those who were eligible to use them were somewhat or very interested in trying an oral prescription medication for migraine prevention. However, 59.1% of never-users who were eligible for preventive medications were not at all interested, not sure, or needed more information about trying an injectable preventive medication. About 40% were not at all interested in injectables. In general, current users and those who had discontinued medication were more interested in preventive medication, including injectables.
‘Disheartening’ discontinuation rates
There are likely multiple reasons for the low rate of migraine prevention treatment, said Dr. Nahas. Many people with migraine never consult a clinician, owing to factors such as stigma, cost, lack of access, and lack of awareness. In addition, patients with migraine are frequently misdiagnosed, she added.
“Other data suggest that only about a quarter of people with episodic migraine and under 5% of people with chronic migraine consult a clinician, receive an accurate diagnosis, and are prescribed appropriate therapy,” said Dr. Nahas.
When the data in this analysis were gathered, public awareness of migraine was much lower than it is today, and injectable migraine therapies had not gained broad acceptance, she noted. Dr. Nahas added it is possible that attitudes toward injectable preventive medications have changed.
“Would people still prefer daily oral medications? We can’t know for sure until we start asking,” she said. In addition, scientific advances and educational outreach have increased clinicians’ awareness, interest, and skill regarding injectable medications, she said.
“I would certainly hope to see that a much greater proportion of preventive-eligible persons with migraine were at least offered, if not currently taking, preventive medication,” said Dr. Nahas. “But there’s no pleasing everyone, so I think we would still see somewhat disheartening discontinuation rates. The reasons for discontinuation, however, might be less typified by concerns about safety and tolerability.”
Still relevant
Commenting on the study, Mia Tova Minen, MD, chief of headache research and associate professor of neurology and population health at NYU Langone Health, New York, noted that although CaMEO is an older study, its results are still highly relevant.
“Unfortunately, primary care providers are still uncomfortable prescribing migraine preventive medications, and this accounts for the large percentage [of patients] with migraine who, while eligible for migraine preventive therapy, are not offered it,” she said.
Although the public and primary care physicians are now more aware of preventive treatments for migraine, “the number of people offered migraine preventive medication still needs to increase dramatically,” said Dr. Minen.
The American Academy of Neurology’s guidelines for migraine prevention were published in 2012 and are currently being updated. The updated guidelines may include new evidence for candesartan and emerging treatments, such as melatonin and aerobic exercise.
“It is my hope that primary care providers will become more comfortable prescribing migraine preventive medications sooner,” said Dr. Minen.
The current findings suggest a need for additional ways of educating patients with migraine who are eligible for preventive therapies so that they can advocate for themselves, she added. They also suggest the idea of demanding more insurance coverage of behavioral therapies for migraine, because data indicate that these treatments have long-term efficacy and good safety profiles, said Dr. Minen.
An ‘invisible’ disorder
Also commenting on the study, Barbara L. Nye, MD, director of the headache fellowship and codirector of the headache clinic at Dartmouth-Hitchcock Medical Center, Lebanon, N.H., said the CaMEO cohort likely is representative of the general population of patients with migraine.
She noted that a significant weakness of the current study is that it examined data collected before the Food and Drug Administration approved monoclonal antibodies and therefore does not reflect patients’ current experience with medications.
“I believe that the attitudes and fears surrounding the use of injectable medication are now likely far less than previously reported, given the positive track record the new generation of once-a-month injectable medications has,” said Dr. Nye.
The findings reinforce the idea that either patients are not talking to their primary care physicians about their headaches and disability or that clinicians are not asking about them, she added. “Both issues are likely linked to the stigma that this disease state has surrounding it. This is an invisible neurological disorder to most,” Dr. Nye said.
The study was sponsored by Allergan before it was acquired by AbbVie. Dr. Nahas has served as a consultant, advisory board member, or speaker for AbbVie/Allergan, Alder/Lundbeck, Amgen/Novartis, Biohaven, Eli Lilly, Impel, Nesos Corp, Supernus, Teva, Theranica, and Zosano. She has not received and will not receive monetary compensation for this research. Dr. Minen has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, new research suggests. Investigators found that among patients with migraine who are eligible for preventive therapy, more than a third were not offered this option. In addition, fewer than 10% were currently taking preventive medication, and an additional 10% had discontinued preventive therapy.
“We confirmed that as of 2012 to 2013 – the years these data were collected from a large, comprehensive survey – gaps in care remained,” said study investigator Stephanie J. Nahas, MD, director of the headache medicine fellowship program, Thomas Jefferson University, Philadelphia. “In this preventive-eligible population, 35% reported never even being offered preventive medication.”
Furthermore, only 28% of patients taking preventive medication experienced a reduction in headache frequency to less than 4 days per month, which is a primary goal of treatment, said Dr. Nahas. Disease burden, as measured with scales of disability and affective comorbidities, remained substantial.
The findings were presented at the American Headache Society’s 2021 annual meeting.
Lack of efficacy?
In 2019, the American Headache Society published a position statement recommending that preventive treatment be considered for patients who have migraine and four or more monthly headache days (MHDs), regardless of their level of associated disability. However, previous data suggest few patients who are eligible for preventive treatment receive it. In addition, many who have used preventive medications do not adhere to their regimens because of problems with tolerability, efficacy, or both.
To identify treatment gaps and characterize self-reported use of preventive medications for migraine, the investigators examined data from the Chronic Migraine Epidemiology and Outcomes (CaMEO) study, a web-based survey conducted in a representative U.S. sample from September 2012 through November 2013.
The survey identified and characterized patients who met modified criteria for migraine consistent with those in ICHD-3. The researchers classified respondents who had migraine and four or more MHDs as potentially eligible for migraine preventive treatment.
The investigators assessed the study population’s use of oral preventive medications, migraine-related disability and burden, willingness to take preventive treatment, and reasons for discontinuation.
Assessments included the Migraine Disability Assessment Questionnaire, the Patient Health Questionnaire–9 for depression, the Generalized Anxiety Disorder 7-Item Scale, the Migraine Specific Quality of Life questionnaire, and the Migraine Symptom Severity Scale.
In all, 16,789 respondents met criteria for migraine, and 6,579 (39.2%) reported having at least four MHDs. The median age of this subgroup that was eligible for preventive treatment was 40.3 years, and approximately 79% were women.
Only 9.8% of respondents who were eligible for preventive medications were currently using an oral preventive medication. Among those who had ever tried an oral preventive medication, 53.6% discontinued it. Efficacy for patients who used medications appeared to be inadequate. Among all current users of preventive treatment, 68.4% continued to have at least four MHDs.
The researchers assessed treatment eligibility among patients not taking preventive medication. Among respondents who had never used a preventive treatment, 35.7% were eligible to receive it. Among all users who had discontinued preventive medication, 61.0% were still eligible to receive it.
Attitudes toward injectables
Among respondents who had never used a preventive treatment, 64.3% had zero to three MHDs. The remaining 35.7% had 4-7, 8-14, or 15 or more MHDs. Among current users of preventive treatments, 68.4% had four or more MHDs. Among those who had discontinued preventive treatment, 61.0% had four or more MHDs.
Patients who have never used preventive medication “have substantial management gaps,” said Dr. Nahas. High proportions of these patients have moderate or severe disability (64.7%), depression (43%), and anxiety (39%). The rates of these outcomes are higher in users who discontinued treatment, likely because of confounding by indication, she added.
The prevalence of anxiety was similar between those who currently used, formerly used, or never used preventive medications. However, there were differences between never-users and current or former users with respect to moderate to severe depression (never-users, 43%; current users, 49.4%; discontinued users, 46.5%) and moderate to severe disability (never-users, 64.7%; current users, 80.4%; discontinued users, 78.9%).
In all, 44.6% of those who discontinued preventive therapy reported safety and tolerability problems as reasons for stopping treatment. In addition, 39.7% reported that these medications did not prevent enough headaches. Some patients reported partial or temporary efficacy as a reason for discontinuation. Other reasons were related to health care costs and access and personal preferences. Only 9.2% of patients who discontinued treatment said that their headaches improved enough to stop medication.
The investigators also analyzed respondents’ interest in preventive therapies. Among respondents who had never used preventive therapies, 61.8% of those who were eligible to use them were somewhat or very interested in trying an oral prescription medication for migraine prevention. However, 59.1% of never-users who were eligible for preventive medications were not at all interested, not sure, or needed more information about trying an injectable preventive medication. About 40% were not at all interested in injectables. In general, current users and those who had discontinued medication were more interested in preventive medication, including injectables.
‘Disheartening’ discontinuation rates
There are likely multiple reasons for the low rate of migraine prevention treatment, said Dr. Nahas. Many people with migraine never consult a clinician, owing to factors such as stigma, cost, lack of access, and lack of awareness. In addition, patients with migraine are frequently misdiagnosed, she added.
“Other data suggest that only about a quarter of people with episodic migraine and under 5% of people with chronic migraine consult a clinician, receive an accurate diagnosis, and are prescribed appropriate therapy,” said Dr. Nahas.
When the data in this analysis were gathered, public awareness of migraine was much lower than it is today, and injectable migraine therapies had not gained broad acceptance, she noted. Dr. Nahas added it is possible that attitudes toward injectable preventive medications have changed.
“Would people still prefer daily oral medications? We can’t know for sure until we start asking,” she said. In addition, scientific advances and educational outreach have increased clinicians’ awareness, interest, and skill regarding injectable medications, she said.
“I would certainly hope to see that a much greater proportion of preventive-eligible persons with migraine were at least offered, if not currently taking, preventive medication,” said Dr. Nahas. “But there’s no pleasing everyone, so I think we would still see somewhat disheartening discontinuation rates. The reasons for discontinuation, however, might be less typified by concerns about safety and tolerability.”
Still relevant
Commenting on the study, Mia Tova Minen, MD, chief of headache research and associate professor of neurology and population health at NYU Langone Health, New York, noted that although CaMEO is an older study, its results are still highly relevant.
“Unfortunately, primary care providers are still uncomfortable prescribing migraine preventive medications, and this accounts for the large percentage [of patients] with migraine who, while eligible for migraine preventive therapy, are not offered it,” she said.
Although the public and primary care physicians are now more aware of preventive treatments for migraine, “the number of people offered migraine preventive medication still needs to increase dramatically,” said Dr. Minen.
The American Academy of Neurology’s guidelines for migraine prevention were published in 2012 and are currently being updated. The updated guidelines may include new evidence for candesartan and emerging treatments, such as melatonin and aerobic exercise.
“It is my hope that primary care providers will become more comfortable prescribing migraine preventive medications sooner,” said Dr. Minen.
The current findings suggest a need for additional ways of educating patients with migraine who are eligible for preventive therapies so that they can advocate for themselves, she added. They also suggest the idea of demanding more insurance coverage of behavioral therapies for migraine, because data indicate that these treatments have long-term efficacy and good safety profiles, said Dr. Minen.
An ‘invisible’ disorder
Also commenting on the study, Barbara L. Nye, MD, director of the headache fellowship and codirector of the headache clinic at Dartmouth-Hitchcock Medical Center, Lebanon, N.H., said the CaMEO cohort likely is representative of the general population of patients with migraine.
She noted that a significant weakness of the current study is that it examined data collected before the Food and Drug Administration approved monoclonal antibodies and therefore does not reflect patients’ current experience with medications.
“I believe that the attitudes and fears surrounding the use of injectable medication are now likely far less than previously reported, given the positive track record the new generation of once-a-month injectable medications has,” said Dr. Nye.
The findings reinforce the idea that either patients are not talking to their primary care physicians about their headaches and disability or that clinicians are not asking about them, she added. “Both issues are likely linked to the stigma that this disease state has surrounding it. This is an invisible neurological disorder to most,” Dr. Nye said.
The study was sponsored by Allergan before it was acquired by AbbVie. Dr. Nahas has served as a consultant, advisory board member, or speaker for AbbVie/Allergan, Alder/Lundbeck, Amgen/Novartis, Biohaven, Eli Lilly, Impel, Nesos Corp, Supernus, Teva, Theranica, and Zosano. She has not received and will not receive monetary compensation for this research. Dr. Minen has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, new research suggests. Investigators found that among patients with migraine who are eligible for preventive therapy, more than a third were not offered this option. In addition, fewer than 10% were currently taking preventive medication, and an additional 10% had discontinued preventive therapy.
“We confirmed that as of 2012 to 2013 – the years these data were collected from a large, comprehensive survey – gaps in care remained,” said study investigator Stephanie J. Nahas, MD, director of the headache medicine fellowship program, Thomas Jefferson University, Philadelphia. “In this preventive-eligible population, 35% reported never even being offered preventive medication.”
Furthermore, only 28% of patients taking preventive medication experienced a reduction in headache frequency to less than 4 days per month, which is a primary goal of treatment, said Dr. Nahas. Disease burden, as measured with scales of disability and affective comorbidities, remained substantial.
The findings were presented at the American Headache Society’s 2021 annual meeting.
Lack of efficacy?
In 2019, the American Headache Society published a position statement recommending that preventive treatment be considered for patients who have migraine and four or more monthly headache days (MHDs), regardless of their level of associated disability. However, previous data suggest few patients who are eligible for preventive treatment receive it. In addition, many who have used preventive medications do not adhere to their regimens because of problems with tolerability, efficacy, or both.
To identify treatment gaps and characterize self-reported use of preventive medications for migraine, the investigators examined data from the Chronic Migraine Epidemiology and Outcomes (CaMEO) study, a web-based survey conducted in a representative U.S. sample from September 2012 through November 2013.
The survey identified and characterized patients who met modified criteria for migraine consistent with those in ICHD-3. The researchers classified respondents who had migraine and four or more MHDs as potentially eligible for migraine preventive treatment.
The investigators assessed the study population’s use of oral preventive medications, migraine-related disability and burden, willingness to take preventive treatment, and reasons for discontinuation.
Assessments included the Migraine Disability Assessment Questionnaire, the Patient Health Questionnaire–9 for depression, the Generalized Anxiety Disorder 7-Item Scale, the Migraine Specific Quality of Life questionnaire, and the Migraine Symptom Severity Scale.
In all, 16,789 respondents met criteria for migraine, and 6,579 (39.2%) reported having at least four MHDs. The median age of this subgroup that was eligible for preventive treatment was 40.3 years, and approximately 79% were women.
Only 9.8% of respondents who were eligible for preventive medications were currently using an oral preventive medication. Among those who had ever tried an oral preventive medication, 53.6% discontinued it. Efficacy for patients who used medications appeared to be inadequate. Among all current users of preventive treatment, 68.4% continued to have at least four MHDs.
The researchers assessed treatment eligibility among patients not taking preventive medication. Among respondents who had never used a preventive treatment, 35.7% were eligible to receive it. Among all users who had discontinued preventive medication, 61.0% were still eligible to receive it.
Attitudes toward injectables
Among respondents who had never used a preventive treatment, 64.3% had zero to three MHDs. The remaining 35.7% had 4-7, 8-14, or 15 or more MHDs. Among current users of preventive treatments, 68.4% had four or more MHDs. Among those who had discontinued preventive treatment, 61.0% had four or more MHDs.
Patients who have never used preventive medication “have substantial management gaps,” said Dr. Nahas. High proportions of these patients have moderate or severe disability (64.7%), depression (43%), and anxiety (39%). The rates of these outcomes are higher in users who discontinued treatment, likely because of confounding by indication, she added.
The prevalence of anxiety was similar between those who currently used, formerly used, or never used preventive medications. However, there were differences between never-users and current or former users with respect to moderate to severe depression (never-users, 43%; current users, 49.4%; discontinued users, 46.5%) and moderate to severe disability (never-users, 64.7%; current users, 80.4%; discontinued users, 78.9%).
In all, 44.6% of those who discontinued preventive therapy reported safety and tolerability problems as reasons for stopping treatment. In addition, 39.7% reported that these medications did not prevent enough headaches. Some patients reported partial or temporary efficacy as a reason for discontinuation. Other reasons were related to health care costs and access and personal preferences. Only 9.2% of patients who discontinued treatment said that their headaches improved enough to stop medication.
The investigators also analyzed respondents’ interest in preventive therapies. Among respondents who had never used preventive therapies, 61.8% of those who were eligible to use them were somewhat or very interested in trying an oral prescription medication for migraine prevention. However, 59.1% of never-users who were eligible for preventive medications were not at all interested, not sure, or needed more information about trying an injectable preventive medication. About 40% were not at all interested in injectables. In general, current users and those who had discontinued medication were more interested in preventive medication, including injectables.
‘Disheartening’ discontinuation rates
There are likely multiple reasons for the low rate of migraine prevention treatment, said Dr. Nahas. Many people with migraine never consult a clinician, owing to factors such as stigma, cost, lack of access, and lack of awareness. In addition, patients with migraine are frequently misdiagnosed, she added.
“Other data suggest that only about a quarter of people with episodic migraine and under 5% of people with chronic migraine consult a clinician, receive an accurate diagnosis, and are prescribed appropriate therapy,” said Dr. Nahas.
When the data in this analysis were gathered, public awareness of migraine was much lower than it is today, and injectable migraine therapies had not gained broad acceptance, she noted. Dr. Nahas added it is possible that attitudes toward injectable preventive medications have changed.
“Would people still prefer daily oral medications? We can’t know for sure until we start asking,” she said. In addition, scientific advances and educational outreach have increased clinicians’ awareness, interest, and skill regarding injectable medications, she said.
“I would certainly hope to see that a much greater proportion of preventive-eligible persons with migraine were at least offered, if not currently taking, preventive medication,” said Dr. Nahas. “But there’s no pleasing everyone, so I think we would still see somewhat disheartening discontinuation rates. The reasons for discontinuation, however, might be less typified by concerns about safety and tolerability.”
Still relevant
Commenting on the study, Mia Tova Minen, MD, chief of headache research and associate professor of neurology and population health at NYU Langone Health, New York, noted that although CaMEO is an older study, its results are still highly relevant.
“Unfortunately, primary care providers are still uncomfortable prescribing migraine preventive medications, and this accounts for the large percentage [of patients] with migraine who, while eligible for migraine preventive therapy, are not offered it,” she said.
Although the public and primary care physicians are now more aware of preventive treatments for migraine, “the number of people offered migraine preventive medication still needs to increase dramatically,” said Dr. Minen.
The American Academy of Neurology’s guidelines for migraine prevention were published in 2012 and are currently being updated. The updated guidelines may include new evidence for candesartan and emerging treatments, such as melatonin and aerobic exercise.
“It is my hope that primary care providers will become more comfortable prescribing migraine preventive medications sooner,” said Dr. Minen.
The current findings suggest a need for additional ways of educating patients with migraine who are eligible for preventive therapies so that they can advocate for themselves, she added. They also suggest the idea of demanding more insurance coverage of behavioral therapies for migraine, because data indicate that these treatments have long-term efficacy and good safety profiles, said Dr. Minen.
An ‘invisible’ disorder
Also commenting on the study, Barbara L. Nye, MD, director of the headache fellowship and codirector of the headache clinic at Dartmouth-Hitchcock Medical Center, Lebanon, N.H., said the CaMEO cohort likely is representative of the general population of patients with migraine.
She noted that a significant weakness of the current study is that it examined data collected before the Food and Drug Administration approved monoclonal antibodies and therefore does not reflect patients’ current experience with medications.
“I believe that the attitudes and fears surrounding the use of injectable medication are now likely far less than previously reported, given the positive track record the new generation of once-a-month injectable medications has,” said Dr. Nye.
The findings reinforce the idea that either patients are not talking to their primary care physicians about their headaches and disability or that clinicians are not asking about them, she added. “Both issues are likely linked to the stigma that this disease state has surrounding it. This is an invisible neurological disorder to most,” Dr. Nye said.
The study was sponsored by Allergan before it was acquired by AbbVie. Dr. Nahas has served as a consultant, advisory board member, or speaker for AbbVie/Allergan, Alder/Lundbeck, Amgen/Novartis, Biohaven, Eli Lilly, Impel, Nesos Corp, Supernus, Teva, Theranica, and Zosano. She has not received and will not receive monetary compensation for this research. Dr. Minen has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM AHS 2021
High Rate of Inappropriate Fecal Immunochemical Testing at a Large Veterans Affairs Health Care System
Colonoscopies and annual fecal immunochemical tests (FITs), are 2 of the preferred modalities for colorectal cancer (CRC) screening endorsed by the US Preventive Services Task Forces as well as the US Multi-Society Task Force of Colorectal Cancer, which represents the American Gastroenterological Association, American College of Gastroenterology, and the American Society of Gastrointestinal Endoscopy.1,2 The recommendations include proper patient selection (patients aged 50 - 75 years with a life expectancy of at least 10 years), and a discussion with the patient regarding both options.
Background
It is known that patients with a positive FIT are at an increased risk for CRC. Lee and colleagues found that patients who do not undergo subsequent colonoscopy after a positive FIT have a 1.64 relative risk of death from colon cancer compared with those who undergo follow-up colonoscopy.3 Studies also have shown that longer wait times (10 months vs 1 month) between a positive FIT and colonoscopy also are associated with a higher risk of CRC.4 FIT utilize antibodies specific for the globin moiety of human hemoglobin and measure the development of antibody-globin complexes using immunoassay techniques. FIT has largely replaced the fecal occult blood test (FOBT), which depends on the detection of heme in feces through oxidation.
A US Department of Veterans Affairs (VA) study found that a longer time to colonoscopy was associated with a higher risk of neoplasia in veterans with a positive FOBT (odds ratio [OR], 1.10).5 It is thus crucial that a positive FOBT or FIT be investigated with follow-up colonoscopy. However, a retrospective study at a single safety-net hospital in San Francisco found that only 55.6% of patients with a positive FIT completed colonoscopy within 1 year.6 Importantly, almost half the patients examined in this study lacked documentation of the result of the FIT or counseling regarding the significance of the positive FIT by the patient’s primary care provider who ordered the test. A VA study looked at veterans aged > 70 years at 4 VA medical centers who did not receive a follow-up colonoscopy within 1 year and reported that 26% of patients studied had a documented refusal to undergo colonoscopy.7
It also is clear that FOBT is used inappropriately for colon cancer screening in some patients. A 2005 single-center VA study looked at inappropriate fecal occult blood tests and found that 18% of veterans for whom FOBTs were ordered had a severe comorbid illness, 13% had signs or symptoms of gastrointestinal (GI) blood loss, and 7% had a history of colorectal neoplasia or inflammatory bowel disease.8 An additional national VA study looked at all veterans aged ≥ 50 years who underwent FOBT or screening colonoscopy between 2009 and 2011 and found 26% to be inappropriate (13.9% of veterans not due for screening, 7.8% with limited life expectancy, and 11% receiving a FOBT when colonoscopy was indicated).9
An often-misunderstood additional requirement in utilizing FIT for CRC screening is that negative tests should be repeated annually.2 A study from Kaiser Permanente in California found that 75.3 to 86.1% of eligible patients underwent yearly FIT.10 In this study, programmatic FIT detected 80.4% of all patients with CRC detected within 1 year of testing.
Since most of the VA-specific studies are based on inappropriate or inadequate use of FOBT, we feel it is essential that further data be gained on appropriate and inappropriate testing. The aim of this study is to determine the frequency at which improper FIT occurs because of failure to obtain serial FIT over time with a negative result, failure to follow-up a positive FIT result with a diagnostic colonoscopy, or performance of FIT in veterans undergoing a recent colonoscopy with adequate bowel preparation. This quality assurance study received an institutional review board exemption from the VA Pittsburgh Healthcare System (VAPHS) in Pennsylvania.
Methods
VAPHS has a data repository of all veterans served within the health care system, which was queried for all veterans who underwent a FIT in the system from January 1, 2015 through December 31, 2017 as well as the number and results of FITs during the interval. In addition, the data repository was also queried specifically for veterans who had at least 1 colonoscopy as well as FIT between 2015 and 2017. The ordering location for each FIT also was queried.
We made 3 calculations for this study. First, we measured the rate of a negative initial FIT in 2015 and/or 2016 followed by a second FIT in 2016 and/or 2017 in a random selection of veterans (3% SE, 95% CI). Demographics were compared in an equal random number of veterans who did and did not have a follow up FIT (5% SE, 95% CI of all negative FIT). Second, we measured the rate of completing colonoscopy following a positive FIT in a random selection of veterans (3% SE, 95% SI). Finally, we calculated FITs following a colonoscopy for all veterans.
Using a power analysis with a 3% SE and 95% CI for sample size calculation and accounting for the approximate 50% exclusion rate from the final eligible population of veterans with at least 1 negative FIT, a random sample of 1,742 patient charts with a negative FIT in the interval were then reviewed to determine the frequency with which they underwent multiple FITs in the interval as well as for the presence of exclusionary factors. Because of the large number of veterans involved in this category, a more detailed demographics review was performed of a subset of these patients using a 95% CI and 5% SE. Using a 95% CI and 3% SE, 445 veterans with a positive FIT in the interval were reviewed to determine the frequency at which they underwent a follow-up diagnostic colonoscopy.
Because of a relatively small sample size, all 108 veterans who underwent a colonoscopy followed by a FIT were reviewed to determine the reason for follow-up FIT. In addition, in veterans who then went on to have a subsequent repeat colonoscopy, the examination findings were recorded.
Results
From January 1, 2015 to December 31, 2017, 6,766 FIT, were ordered at VAPHS. Of these, 4,391 unique veterans had at least 1 negative FIT during the period and 709 unique veterans had a positive FIT. There were 832 veterans who had both a FIT and colonoscopy during the study period. Of these, 108 had a colonoscopy with a subsequent FIT (Figure).
Of 1,742 randomly selected veterans with at least 1 negative FIT in the study interval, 870 were eligible for multiple FITs during this period as they were in the appropriate screening age (50-75 years or 85 years based on an assessment of life expectancy by the ordering health care provider [HCP]), did not have exclusionary comorbidities to multiple FIT, were not lost to follow-up, and had at least 1 negative FIT collected from 2015 to 2016 (veterans who only had a FIT in 2017 were excluded from this aim to avoid confounding). Of these 870 veterans, 543 (62.4%) underwent at least 2 FITs during the study period. In a demographic comparison of 110 veterans with 1 FIT and 110 veterans with > 1 FIT, there were no statistically significant differences in demographics (Table 1).
In a random chart review of 410 veterans with a positive FIT, 113 (27.5%) veterans did not undergo a subsequent colonoscopy within 1 year due to patient refusal, failure to schedule, or failure to keep colonoscopy appointment. There were no differences in demographics between those that underwent a diagnostic colonoscopy and those that did not (Table 2).
Of the 108 patients with a FIT following colonoscopy in the study interval, 97 FITs were negative. Ninety-five of the 108 FITs (88%) were judged to be inappropriate, having been performed for indications, including 38 for colon cancer screening, 23 for anemia, 32 for GI symptoms (eg, diarrhea, rectal bleeding, possible GI bleeding), and 2 for unclear indications. Thirteen FITs were deemed appropriate, as they were performed on veterans who refused to have a repeat colonoscopy following an examination with inadequate bowel preparation (Table 3). There was no difference in age or race between these 2 groups, although there was a statistically significant difference in gender (Table 4).
There were 19 patients who had a colonoscopy following a prior colonoscopy and subsequent positive FIT in the interval. Eight patients had no significant findings, 10 had nonadvanced adenomas, and 1 had an advanced adenoma (this patient had inadequate preparation with recommendation to repeat colonoscopy in 1 year).
While not a specific aim of the study we were able to identify certain HCPs by clinic location who systematically performed inappropriate or appropriate FIT. There were 47 separate ordering locations for the 95 inappropriate FIT following recent colonoscopy. Of these, 1 location was responsible for ordering 20 (21%) inappropriate FIT. Eight locations accounted for 51% of all the inappropriately ordered FIT. Two clinics seemed to be high performers in regard to overall appropriate vs inappropriate FIT use. The appropriate FIT rate for these locations was 30 of 33 (90.9%) and 26 of 28 (92.8%), respectively.
Discussion
In this retrospective study, we found that a large percentage of veterans eligible for colon cancer screening utilizing FIT did not undergo appropriate screening. Almost 40% of veterans in a 3-year interval received only 1 FIT. This seemed to occur due to a combination of patient refusal and inadequate education by HCPs regarding how to screen appropriately for CRC using FIT. This occurred despite a reminder in the VA Computerized Patient Record System regarding CRC screening.
There did not seem to be significant differences in demographics between those who were screened appropriately vs inappropriately. While there was a statistically significant difference in gender between those who had an appropriate FIT following recent colonoscopy (2 of 13 were female) and those who had an inappropriate FIT after recent colonoscopy (1 of 95 was a female), we are uncertain of the significance of this finding given the small number of female veterans in the analysis.
We do believe that the ratio of veterans in our study with a single FIT likely underestimates the true prevalence. To avoid confounding from factors such as inadequate prior follow-up in the study interval, we excluded veterans who underwent FIT only in 2017 for this analysis. As such, a significant percentage of these veterans were actually eligible to be screened throughout the study interval.
In spite of recommendations regarding the need for diagnostic colonoscopy following a positive FIT, we found that more than one-quarter of patients did not undergo colonoscopy. Although this number is an improvement over previously published literature that found almost half of patients at a safety-net hospital did not undergo diagnostic colonoscopy following a positive FIT, this is still clearly suboptimal.6
VAPHS has a mandate that all patients with a positive FIT be scheduled for colonoscopy within 30 days, either at VAPHS or in the community. An alert is sent to both ordering HCP regarding the positive FIT as well as to the GI department. In addition to contact from the ordering HCP, all veterans also are contacted by either a physician or nurse practitioner GI provider to provide test results and an explanation of its clinical significance and to facilitate colonoscopy scheduling. If a patient cannot be reached by telephone, the patient is sent a certified letter from the GI department regarding the significance of a positive FIT and instructions for scheduling a colonoscopy.
Despite this outreach, 27.5% of veterans did not have a diagnostic colonoscopy following a positive FIT. This suggests that there may be inadequate education and counseling of veterans at the time of the FIT order about the subsequent series of events and need for diagnostic colonoscopy following a positive FIT. If a patient refuses to undergo a colonoscopy under any circumstances (including after a positive FIT), the utility of placing a FIT order is questionable.
There is also a need for more education of ordering HCPs on appropriate indications for FITs. We found that 35% of FIT ordered after a recent colonoscopy were done for the purpose of CRC screening, despite clear guidelines recommending against this. In addition, another 50% of FIT ordered after recent colonoscopy was done either for evaluation of GI symptoms like diarrhea and rectal bleeding or in the evaluation of anemia, both of which are inappropriate uses for FIT. Since FIT is an antibody test against globin, the protein component of hemoglobin that degrades during passage through the small bowel, it is not a useful test for the evaluation of upper GI or small bowel bleeding. A relatively recent database study in the Netherlands looking at the diagnosis of upper GI malignancies within 3 years of a positive FIT found a < 1% rate.11
In our study, albeit limited by the small number of veterans undergoing a repeat colonoscopy following a prior colonoscopy and subsequent positive FIT, there were few significant findings. Only 1 veteran had an advanced adenoma detected, and this veteran had already been recommended a repeat colonoscopy in 1 year due to an inadequate bowel preparation on the last examination.
Lastly, we found that certain HCPs (based on ordering clinic location) systematically performed improper FIT compared with other HCPs. This presumably is due to a lack of education on appropriate FIT usage and suggests opportunity for educational and/or systems interventions.
Limitations
While our study strengths include a relatively large number of veterans and detailed review of individual patient data, it has multiple limitations. As a retrospective chart review-based study, incomplete or inaccurate data are a possibility. It is possible that patients underwent repeat FIT or underwent colonoscopy outside of the VA system and never recorded into the VA records. In addition, there is likely a sampling bias in this study as only veterans who underwent at least 1 FIT in the interval were included. These patients may be different from those who choose to undergo colonoscopy for CRC screening or from those who do not undergo screening at all.
Conclusions
A large percentage of patients underwent improper FIT at a tertiary referral academic VA medical center. Additional education and systems interventions are necessary to improve both provider and patient adherence to appropriate CRC screening. For example, one measure may include providing HCPs with a list of their patients not up-to-date with CRC screening that was shown to increase patient participation in FIT screening compared with patients who received usual care in a 2017 study.12 In addition, a 2018 study showed that a digital health intervention that allows patients to self-order tests (eg, on an iPad) can increase CRC screening rates.13
Author Contributions
Adam Gluskin: Study concept and design; acquisition of data; analysis and interpretation of data; drafting of the manuscript. Jeffrey Dueker: Study concept and design; analysis and interpretation of data; statistical analysis; critical revision of the manuscript for important intellectual content. Asif Khalid: Study concept and design; analysis and interpretation of data; drafting of the manuscripts; critical revision of the manuscript for important intellectual content; study supervision.
1. US Preventive Services Task Force, Bibbins-Domingo K, Grossman DC, et al. Screening for Colorectal Cancer: US Preventive Services Task Force recommendation statement [published correction appears in JAMA. 2016 Aug 2;316(5):545] [published correction appears in JAMA. 2017 Jun 6;317(21):2239]. JAMA. 2016;315(23):2564-2575. doi:10.1001/jama.2016.5989
2. Rex DK, Boland CR, Dominitz JA, et al. Colorectal cancer screening: recommendations for physicians and patients from the U.S. Multi-Society Task Force on Colorectal Cancer. Gastroenterology. 2017;153(1):307-323. doi:10.1053/j.gastro.2017.05.013
3. Lee YC, Li-Sheng Chen S, Ming-Fang Yen A, et al. Association between colorectal cancer mortality and gradient fecal hemoglobin concentration in colonoscopy noncompliers. J Natl Cancer Inst. 2017;109(5):djw269. doi:10.1093/jnci/djw269
4. Corley DA, Jensen CD, Quinn VP, et al. Association between time to colonoscopy after a positive fecal test result and risk of colorectal cancer and cancer stage at diagnosis. JAMA. 2017;317(16):1631-1641. doi:10.1001/jama.2017.3634
5. Gellad ZF, Almirall D, Provenzale D, Fisher DA. Time from positive screening fecal occult blood test to colonoscopy and risk of neoplasia. Dig Dis Sci. 2009;54(11):2497-2502. doi:10.1007/s10620-008-0653-8
6. Issaka RB, Singh MH, Oshima SM, et al. Inadequate utilization of diagnostic colonoscopy following abnormal FIT results in an integrated safety-net System. Am J Gastroenterol. 2017;112(2):375-382. doi:10.1038/ajg.2016.555
7. Carlson CM, Kirby KA, Casadei MA, Partin MR, Kistler CE, Walter LC. Lack of follow-up after fecal occult blood testing in older adults: inappropriate screening or failure to follow up?. Arch Intern Med. 2011;171(3):249-256. doi:10.1001/archinternmed.2010.372
8. Fisher DA, Judd L, Sanford NS. Inappropriate colorectal cancer screening: findings and implications. Am J Gastroenterol. 2005;100(11):2526-2530. doi:10.1111/j.1572-0241.2005.00322.x
9. Powell AA, Saini SD, Breitenstein MK, Noorbaloochi S, Cutting A, Fisher DA, Bloomfield HE, Halek K, Partin MR. Rates and correlates of potentially inappropriate colorectal cancer screening in the Veterans Health Administration. J Gen Intern Med. 2015 Jun;30(6):732-41. doi: 10.1007/s11606-014-3163-8
10. Jensen CD, Corley DA, Quinn VP, et al. Fecal immunochemical test program performance over 4 rounds of annual screening: a retrospective cohort study. Ann Intern Med. 2016;164(7):456-463. doi:10.7326/M15-0983
11. van der Vlugt M, Grobbee EJ, Bossuyt PM, et al. Risk of oral and upper gastrointestinal cancers in persons with positive results from a fecal immunochemical test in a colorectal cancer screening program. Clin Gastroenterol Hepatol. 2018;16(8):1237-1243.e2. doi:10.1016/j.cgh.2018.01.037
12. Rat C, Pogu C, Le Donné D, et al. Effect of physician notification regarding nonadherence to colorectal cancer screening on patient participation in fecal immunochemical test cancer screening: a randomized clinical trial. JAMA. 2017;318(9):816-824. doi:10.1001/jama.2017.11387
13. Miller DP Jr, Denizard-Thompson N, Weaver KE, et al. Effect of a digital health intervention on receipt of colorectal cancer screening in vulnerable patients: a randomized controlled trial. Ann Intern Med. 2018;168(8):550-557. doi:10.7326/M17-2315
Colonoscopies and annual fecal immunochemical tests (FITs), are 2 of the preferred modalities for colorectal cancer (CRC) screening endorsed by the US Preventive Services Task Forces as well as the US Multi-Society Task Force of Colorectal Cancer, which represents the American Gastroenterological Association, American College of Gastroenterology, and the American Society of Gastrointestinal Endoscopy.1,2 The recommendations include proper patient selection (patients aged 50 - 75 years with a life expectancy of at least 10 years), and a discussion with the patient regarding both options.
Background
It is known that patients with a positive FIT are at an increased risk for CRC. Lee and colleagues found that patients who do not undergo subsequent colonoscopy after a positive FIT have a 1.64 relative risk of death from colon cancer compared with those who undergo follow-up colonoscopy.3 Studies also have shown that longer wait times (10 months vs 1 month) between a positive FIT and colonoscopy also are associated with a higher risk of CRC.4 FIT utilize antibodies specific for the globin moiety of human hemoglobin and measure the development of antibody-globin complexes using immunoassay techniques. FIT has largely replaced the fecal occult blood test (FOBT), which depends on the detection of heme in feces through oxidation.
A US Department of Veterans Affairs (VA) study found that a longer time to colonoscopy was associated with a higher risk of neoplasia in veterans with a positive FOBT (odds ratio [OR], 1.10).5 It is thus crucial that a positive FOBT or FIT be investigated with follow-up colonoscopy. However, a retrospective study at a single safety-net hospital in San Francisco found that only 55.6% of patients with a positive FIT completed colonoscopy within 1 year.6 Importantly, almost half the patients examined in this study lacked documentation of the result of the FIT or counseling regarding the significance of the positive FIT by the patient’s primary care provider who ordered the test. A VA study looked at veterans aged > 70 years at 4 VA medical centers who did not receive a follow-up colonoscopy within 1 year and reported that 26% of patients studied had a documented refusal to undergo colonoscopy.7
It also is clear that FOBT is used inappropriately for colon cancer screening in some patients. A 2005 single-center VA study looked at inappropriate fecal occult blood tests and found that 18% of veterans for whom FOBTs were ordered had a severe comorbid illness, 13% had signs or symptoms of gastrointestinal (GI) blood loss, and 7% had a history of colorectal neoplasia or inflammatory bowel disease.8 An additional national VA study looked at all veterans aged ≥ 50 years who underwent FOBT or screening colonoscopy between 2009 and 2011 and found 26% to be inappropriate (13.9% of veterans not due for screening, 7.8% with limited life expectancy, and 11% receiving a FOBT when colonoscopy was indicated).9
An often-misunderstood additional requirement in utilizing FIT for CRC screening is that negative tests should be repeated annually.2 A study from Kaiser Permanente in California found that 75.3 to 86.1% of eligible patients underwent yearly FIT.10 In this study, programmatic FIT detected 80.4% of all patients with CRC detected within 1 year of testing.
Since most of the VA-specific studies are based on inappropriate or inadequate use of FOBT, we feel it is essential that further data be gained on appropriate and inappropriate testing. The aim of this study is to determine the frequency at which improper FIT occurs because of failure to obtain serial FIT over time with a negative result, failure to follow-up a positive FIT result with a diagnostic colonoscopy, or performance of FIT in veterans undergoing a recent colonoscopy with adequate bowel preparation. This quality assurance study received an institutional review board exemption from the VA Pittsburgh Healthcare System (VAPHS) in Pennsylvania.
Methods
VAPHS has a data repository of all veterans served within the health care system, which was queried for all veterans who underwent a FIT in the system from January 1, 2015 through December 31, 2017 as well as the number and results of FITs during the interval. In addition, the data repository was also queried specifically for veterans who had at least 1 colonoscopy as well as FIT between 2015 and 2017. The ordering location for each FIT also was queried.
We made 3 calculations for this study. First, we measured the rate of a negative initial FIT in 2015 and/or 2016 followed by a second FIT in 2016 and/or 2017 in a random selection of veterans (3% SE, 95% CI). Demographics were compared in an equal random number of veterans who did and did not have a follow up FIT (5% SE, 95% CI of all negative FIT). Second, we measured the rate of completing colonoscopy following a positive FIT in a random selection of veterans (3% SE, 95% SI). Finally, we calculated FITs following a colonoscopy for all veterans.
Using a power analysis with a 3% SE and 95% CI for sample size calculation and accounting for the approximate 50% exclusion rate from the final eligible population of veterans with at least 1 negative FIT, a random sample of 1,742 patient charts with a negative FIT in the interval were then reviewed to determine the frequency with which they underwent multiple FITs in the interval as well as for the presence of exclusionary factors. Because of the large number of veterans involved in this category, a more detailed demographics review was performed of a subset of these patients using a 95% CI and 5% SE. Using a 95% CI and 3% SE, 445 veterans with a positive FIT in the interval were reviewed to determine the frequency at which they underwent a follow-up diagnostic colonoscopy.
Because of a relatively small sample size, all 108 veterans who underwent a colonoscopy followed by a FIT were reviewed to determine the reason for follow-up FIT. In addition, in veterans who then went on to have a subsequent repeat colonoscopy, the examination findings were recorded.
Results
From January 1, 2015 to December 31, 2017, 6,766 FIT, were ordered at VAPHS. Of these, 4,391 unique veterans had at least 1 negative FIT during the period and 709 unique veterans had a positive FIT. There were 832 veterans who had both a FIT and colonoscopy during the study period. Of these, 108 had a colonoscopy with a subsequent FIT (Figure).
Of 1,742 randomly selected veterans with at least 1 negative FIT in the study interval, 870 were eligible for multiple FITs during this period as they were in the appropriate screening age (50-75 years or 85 years based on an assessment of life expectancy by the ordering health care provider [HCP]), did not have exclusionary comorbidities to multiple FIT, were not lost to follow-up, and had at least 1 negative FIT collected from 2015 to 2016 (veterans who only had a FIT in 2017 were excluded from this aim to avoid confounding). Of these 870 veterans, 543 (62.4%) underwent at least 2 FITs during the study period. In a demographic comparison of 110 veterans with 1 FIT and 110 veterans with > 1 FIT, there were no statistically significant differences in demographics (Table 1).
In a random chart review of 410 veterans with a positive FIT, 113 (27.5%) veterans did not undergo a subsequent colonoscopy within 1 year due to patient refusal, failure to schedule, or failure to keep colonoscopy appointment. There were no differences in demographics between those that underwent a diagnostic colonoscopy and those that did not (Table 2).
Of the 108 patients with a FIT following colonoscopy in the study interval, 97 FITs were negative. Ninety-five of the 108 FITs (88%) were judged to be inappropriate, having been performed for indications, including 38 for colon cancer screening, 23 for anemia, 32 for GI symptoms (eg, diarrhea, rectal bleeding, possible GI bleeding), and 2 for unclear indications. Thirteen FITs were deemed appropriate, as they were performed on veterans who refused to have a repeat colonoscopy following an examination with inadequate bowel preparation (Table 3). There was no difference in age or race between these 2 groups, although there was a statistically significant difference in gender (Table 4).
There were 19 patients who had a colonoscopy following a prior colonoscopy and subsequent positive FIT in the interval. Eight patients had no significant findings, 10 had nonadvanced adenomas, and 1 had an advanced adenoma (this patient had inadequate preparation with recommendation to repeat colonoscopy in 1 year).
While not a specific aim of the study we were able to identify certain HCPs by clinic location who systematically performed inappropriate or appropriate FIT. There were 47 separate ordering locations for the 95 inappropriate FIT following recent colonoscopy. Of these, 1 location was responsible for ordering 20 (21%) inappropriate FIT. Eight locations accounted for 51% of all the inappropriately ordered FIT. Two clinics seemed to be high performers in regard to overall appropriate vs inappropriate FIT use. The appropriate FIT rate for these locations was 30 of 33 (90.9%) and 26 of 28 (92.8%), respectively.
Discussion
In this retrospective study, we found that a large percentage of veterans eligible for colon cancer screening utilizing FIT did not undergo appropriate screening. Almost 40% of veterans in a 3-year interval received only 1 FIT. This seemed to occur due to a combination of patient refusal and inadequate education by HCPs regarding how to screen appropriately for CRC using FIT. This occurred despite a reminder in the VA Computerized Patient Record System regarding CRC screening.
There did not seem to be significant differences in demographics between those who were screened appropriately vs inappropriately. While there was a statistically significant difference in gender between those who had an appropriate FIT following recent colonoscopy (2 of 13 were female) and those who had an inappropriate FIT after recent colonoscopy (1 of 95 was a female), we are uncertain of the significance of this finding given the small number of female veterans in the analysis.
We do believe that the ratio of veterans in our study with a single FIT likely underestimates the true prevalence. To avoid confounding from factors such as inadequate prior follow-up in the study interval, we excluded veterans who underwent FIT only in 2017 for this analysis. As such, a significant percentage of these veterans were actually eligible to be screened throughout the study interval.
In spite of recommendations regarding the need for diagnostic colonoscopy following a positive FIT, we found that more than one-quarter of patients did not undergo colonoscopy. Although this number is an improvement over previously published literature that found almost half of patients at a safety-net hospital did not undergo diagnostic colonoscopy following a positive FIT, this is still clearly suboptimal.6
VAPHS has a mandate that all patients with a positive FIT be scheduled for colonoscopy within 30 days, either at VAPHS or in the community. An alert is sent to both ordering HCP regarding the positive FIT as well as to the GI department. In addition to contact from the ordering HCP, all veterans also are contacted by either a physician or nurse practitioner GI provider to provide test results and an explanation of its clinical significance and to facilitate colonoscopy scheduling. If a patient cannot be reached by telephone, the patient is sent a certified letter from the GI department regarding the significance of a positive FIT and instructions for scheduling a colonoscopy.
Despite this outreach, 27.5% of veterans did not have a diagnostic colonoscopy following a positive FIT. This suggests that there may be inadequate education and counseling of veterans at the time of the FIT order about the subsequent series of events and need for diagnostic colonoscopy following a positive FIT. If a patient refuses to undergo a colonoscopy under any circumstances (including after a positive FIT), the utility of placing a FIT order is questionable.
There is also a need for more education of ordering HCPs on appropriate indications for FITs. We found that 35% of FIT ordered after a recent colonoscopy were done for the purpose of CRC screening, despite clear guidelines recommending against this. In addition, another 50% of FIT ordered after recent colonoscopy was done either for evaluation of GI symptoms like diarrhea and rectal bleeding or in the evaluation of anemia, both of which are inappropriate uses for FIT. Since FIT is an antibody test against globin, the protein component of hemoglobin that degrades during passage through the small bowel, it is not a useful test for the evaluation of upper GI or small bowel bleeding. A relatively recent database study in the Netherlands looking at the diagnosis of upper GI malignancies within 3 years of a positive FIT found a < 1% rate.11
In our study, albeit limited by the small number of veterans undergoing a repeat colonoscopy following a prior colonoscopy and subsequent positive FIT, there were few significant findings. Only 1 veteran had an advanced adenoma detected, and this veteran had already been recommended a repeat colonoscopy in 1 year due to an inadequate bowel preparation on the last examination.
Lastly, we found that certain HCPs (based on ordering clinic location) systematically performed improper FIT compared with other HCPs. This presumably is due to a lack of education on appropriate FIT usage and suggests opportunity for educational and/or systems interventions.
Limitations
While our study strengths include a relatively large number of veterans and detailed review of individual patient data, it has multiple limitations. As a retrospective chart review-based study, incomplete or inaccurate data are a possibility. It is possible that patients underwent repeat FIT or underwent colonoscopy outside of the VA system and never recorded into the VA records. In addition, there is likely a sampling bias in this study as only veterans who underwent at least 1 FIT in the interval were included. These patients may be different from those who choose to undergo colonoscopy for CRC screening or from those who do not undergo screening at all.
Conclusions
A large percentage of patients underwent improper FIT at a tertiary referral academic VA medical center. Additional education and systems interventions are necessary to improve both provider and patient adherence to appropriate CRC screening. For example, one measure may include providing HCPs with a list of their patients not up-to-date with CRC screening that was shown to increase patient participation in FIT screening compared with patients who received usual care in a 2017 study.12 In addition, a 2018 study showed that a digital health intervention that allows patients to self-order tests (eg, on an iPad) can increase CRC screening rates.13
Author Contributions
Adam Gluskin: Study concept and design; acquisition of data; analysis and interpretation of data; drafting of the manuscript. Jeffrey Dueker: Study concept and design; analysis and interpretation of data; statistical analysis; critical revision of the manuscript for important intellectual content. Asif Khalid: Study concept and design; analysis and interpretation of data; drafting of the manuscripts; critical revision of the manuscript for important intellectual content; study supervision.
Colonoscopies and annual fecal immunochemical tests (FITs), are 2 of the preferred modalities for colorectal cancer (CRC) screening endorsed by the US Preventive Services Task Forces as well as the US Multi-Society Task Force of Colorectal Cancer, which represents the American Gastroenterological Association, American College of Gastroenterology, and the American Society of Gastrointestinal Endoscopy.1,2 The recommendations include proper patient selection (patients aged 50 - 75 years with a life expectancy of at least 10 years), and a discussion with the patient regarding both options.
Background
It is known that patients with a positive FIT are at an increased risk for CRC. Lee and colleagues found that patients who do not undergo subsequent colonoscopy after a positive FIT have a 1.64 relative risk of death from colon cancer compared with those who undergo follow-up colonoscopy.3 Studies also have shown that longer wait times (10 months vs 1 month) between a positive FIT and colonoscopy also are associated with a higher risk of CRC.4 FIT utilize antibodies specific for the globin moiety of human hemoglobin and measure the development of antibody-globin complexes using immunoassay techniques. FIT has largely replaced the fecal occult blood test (FOBT), which depends on the detection of heme in feces through oxidation.
A US Department of Veterans Affairs (VA) study found that a longer time to colonoscopy was associated with a higher risk of neoplasia in veterans with a positive FOBT (odds ratio [OR], 1.10).5 It is thus crucial that a positive FOBT or FIT be investigated with follow-up colonoscopy. However, a retrospective study at a single safety-net hospital in San Francisco found that only 55.6% of patients with a positive FIT completed colonoscopy within 1 year.6 Importantly, almost half the patients examined in this study lacked documentation of the result of the FIT or counseling regarding the significance of the positive FIT by the patient’s primary care provider who ordered the test. A VA study looked at veterans aged > 70 years at 4 VA medical centers who did not receive a follow-up colonoscopy within 1 year and reported that 26% of patients studied had a documented refusal to undergo colonoscopy.7
It also is clear that FOBT is used inappropriately for colon cancer screening in some patients. A 2005 single-center VA study looked at inappropriate fecal occult blood tests and found that 18% of veterans for whom FOBTs were ordered had a severe comorbid illness, 13% had signs or symptoms of gastrointestinal (GI) blood loss, and 7% had a history of colorectal neoplasia or inflammatory bowel disease.8 An additional national VA study looked at all veterans aged ≥ 50 years who underwent FOBT or screening colonoscopy between 2009 and 2011 and found 26% to be inappropriate (13.9% of veterans not due for screening, 7.8% with limited life expectancy, and 11% receiving a FOBT when colonoscopy was indicated).9
An often-misunderstood additional requirement in utilizing FIT for CRC screening is that negative tests should be repeated annually.2 A study from Kaiser Permanente in California found that 75.3 to 86.1% of eligible patients underwent yearly FIT.10 In this study, programmatic FIT detected 80.4% of all patients with CRC detected within 1 year of testing.
Since most of the VA-specific studies are based on inappropriate or inadequate use of FOBT, we feel it is essential that further data be gained on appropriate and inappropriate testing. The aim of this study is to determine the frequency at which improper FIT occurs because of failure to obtain serial FIT over time with a negative result, failure to follow-up a positive FIT result with a diagnostic colonoscopy, or performance of FIT in veterans undergoing a recent colonoscopy with adequate bowel preparation. This quality assurance study received an institutional review board exemption from the VA Pittsburgh Healthcare System (VAPHS) in Pennsylvania.
Methods
VAPHS has a data repository of all veterans served within the health care system, which was queried for all veterans who underwent a FIT in the system from January 1, 2015 through December 31, 2017 as well as the number and results of FITs during the interval. In addition, the data repository was also queried specifically for veterans who had at least 1 colonoscopy as well as FIT between 2015 and 2017. The ordering location for each FIT also was queried.
We made 3 calculations for this study. First, we measured the rate of a negative initial FIT in 2015 and/or 2016 followed by a second FIT in 2016 and/or 2017 in a random selection of veterans (3% SE, 95% CI). Demographics were compared in an equal random number of veterans who did and did not have a follow up FIT (5% SE, 95% CI of all negative FIT). Second, we measured the rate of completing colonoscopy following a positive FIT in a random selection of veterans (3% SE, 95% SI). Finally, we calculated FITs following a colonoscopy for all veterans.
Using a power analysis with a 3% SE and 95% CI for sample size calculation and accounting for the approximate 50% exclusion rate from the final eligible population of veterans with at least 1 negative FIT, a random sample of 1,742 patient charts with a negative FIT in the interval were then reviewed to determine the frequency with which they underwent multiple FITs in the interval as well as for the presence of exclusionary factors. Because of the large number of veterans involved in this category, a more detailed demographics review was performed of a subset of these patients using a 95% CI and 5% SE. Using a 95% CI and 3% SE, 445 veterans with a positive FIT in the interval were reviewed to determine the frequency at which they underwent a follow-up diagnostic colonoscopy.
Because of a relatively small sample size, all 108 veterans who underwent a colonoscopy followed by a FIT were reviewed to determine the reason for follow-up FIT. In addition, in veterans who then went on to have a subsequent repeat colonoscopy, the examination findings were recorded.
Results
From January 1, 2015 to December 31, 2017, 6,766 FIT, were ordered at VAPHS. Of these, 4,391 unique veterans had at least 1 negative FIT during the period and 709 unique veterans had a positive FIT. There were 832 veterans who had both a FIT and colonoscopy during the study period. Of these, 108 had a colonoscopy with a subsequent FIT (Figure).
Of 1,742 randomly selected veterans with at least 1 negative FIT in the study interval, 870 were eligible for multiple FITs during this period as they were in the appropriate screening age (50-75 years or 85 years based on an assessment of life expectancy by the ordering health care provider [HCP]), did not have exclusionary comorbidities to multiple FIT, were not lost to follow-up, and had at least 1 negative FIT collected from 2015 to 2016 (veterans who only had a FIT in 2017 were excluded from this aim to avoid confounding). Of these 870 veterans, 543 (62.4%) underwent at least 2 FITs during the study period. In a demographic comparison of 110 veterans with 1 FIT and 110 veterans with > 1 FIT, there were no statistically significant differences in demographics (Table 1).
In a random chart review of 410 veterans with a positive FIT, 113 (27.5%) veterans did not undergo a subsequent colonoscopy within 1 year due to patient refusal, failure to schedule, or failure to keep colonoscopy appointment. There were no differences in demographics between those that underwent a diagnostic colonoscopy and those that did not (Table 2).
Of the 108 patients with a FIT following colonoscopy in the study interval, 97 FITs were negative. Ninety-five of the 108 FITs (88%) were judged to be inappropriate, having been performed for indications, including 38 for colon cancer screening, 23 for anemia, 32 for GI symptoms (eg, diarrhea, rectal bleeding, possible GI bleeding), and 2 for unclear indications. Thirteen FITs were deemed appropriate, as they were performed on veterans who refused to have a repeat colonoscopy following an examination with inadequate bowel preparation (Table 3). There was no difference in age or race between these 2 groups, although there was a statistically significant difference in gender (Table 4).
There were 19 patients who had a colonoscopy following a prior colonoscopy and subsequent positive FIT in the interval. Eight patients had no significant findings, 10 had nonadvanced adenomas, and 1 had an advanced adenoma (this patient had inadequate preparation with recommendation to repeat colonoscopy in 1 year).
While not a specific aim of the study we were able to identify certain HCPs by clinic location who systematically performed inappropriate or appropriate FIT. There were 47 separate ordering locations for the 95 inappropriate FIT following recent colonoscopy. Of these, 1 location was responsible for ordering 20 (21%) inappropriate FIT. Eight locations accounted for 51% of all the inappropriately ordered FIT. Two clinics seemed to be high performers in regard to overall appropriate vs inappropriate FIT use. The appropriate FIT rate for these locations was 30 of 33 (90.9%) and 26 of 28 (92.8%), respectively.
Discussion
In this retrospective study, we found that a large percentage of veterans eligible for colon cancer screening utilizing FIT did not undergo appropriate screening. Almost 40% of veterans in a 3-year interval received only 1 FIT. This seemed to occur due to a combination of patient refusal and inadequate education by HCPs regarding how to screen appropriately for CRC using FIT. This occurred despite a reminder in the VA Computerized Patient Record System regarding CRC screening.
There did not seem to be significant differences in demographics between those who were screened appropriately vs inappropriately. While there was a statistically significant difference in gender between those who had an appropriate FIT following recent colonoscopy (2 of 13 were female) and those who had an inappropriate FIT after recent colonoscopy (1 of 95 was a female), we are uncertain of the significance of this finding given the small number of female veterans in the analysis.
We do believe that the ratio of veterans in our study with a single FIT likely underestimates the true prevalence. To avoid confounding from factors such as inadequate prior follow-up in the study interval, we excluded veterans who underwent FIT only in 2017 for this analysis. As such, a significant percentage of these veterans were actually eligible to be screened throughout the study interval.
In spite of recommendations regarding the need for diagnostic colonoscopy following a positive FIT, we found that more than one-quarter of patients did not undergo colonoscopy. Although this number is an improvement over previously published literature that found almost half of patients at a safety-net hospital did not undergo diagnostic colonoscopy following a positive FIT, this is still clearly suboptimal.6
VAPHS has a mandate that all patients with a positive FIT be scheduled for colonoscopy within 30 days, either at VAPHS or in the community. An alert is sent to both ordering HCP regarding the positive FIT as well as to the GI department. In addition to contact from the ordering HCP, all veterans also are contacted by either a physician or nurse practitioner GI provider to provide test results and an explanation of its clinical significance and to facilitate colonoscopy scheduling. If a patient cannot be reached by telephone, the patient is sent a certified letter from the GI department regarding the significance of a positive FIT and instructions for scheduling a colonoscopy.
Despite this outreach, 27.5% of veterans did not have a diagnostic colonoscopy following a positive FIT. This suggests that there may be inadequate education and counseling of veterans at the time of the FIT order about the subsequent series of events and need for diagnostic colonoscopy following a positive FIT. If a patient refuses to undergo a colonoscopy under any circumstances (including after a positive FIT), the utility of placing a FIT order is questionable.
There is also a need for more education of ordering HCPs on appropriate indications for FITs. We found that 35% of FIT ordered after a recent colonoscopy were done for the purpose of CRC screening, despite clear guidelines recommending against this. In addition, another 50% of FIT ordered after recent colonoscopy was done either for evaluation of GI symptoms like diarrhea and rectal bleeding or in the evaluation of anemia, both of which are inappropriate uses for FIT. Since FIT is an antibody test against globin, the protein component of hemoglobin that degrades during passage through the small bowel, it is not a useful test for the evaluation of upper GI or small bowel bleeding. A relatively recent database study in the Netherlands looking at the diagnosis of upper GI malignancies within 3 years of a positive FIT found a < 1% rate.11
In our study, albeit limited by the small number of veterans undergoing a repeat colonoscopy following a prior colonoscopy and subsequent positive FIT, there were few significant findings. Only 1 veteran had an advanced adenoma detected, and this veteran had already been recommended a repeat colonoscopy in 1 year due to an inadequate bowel preparation on the last examination.
Lastly, we found that certain HCPs (based on ordering clinic location) systematically performed improper FIT compared with other HCPs. This presumably is due to a lack of education on appropriate FIT usage and suggests opportunity for educational and/or systems interventions.
Limitations
While our study strengths include a relatively large number of veterans and detailed review of individual patient data, it has multiple limitations. As a retrospective chart review-based study, incomplete or inaccurate data are a possibility. It is possible that patients underwent repeat FIT or underwent colonoscopy outside of the VA system and never recorded into the VA records. In addition, there is likely a sampling bias in this study as only veterans who underwent at least 1 FIT in the interval were included. These patients may be different from those who choose to undergo colonoscopy for CRC screening or from those who do not undergo screening at all.
Conclusions
A large percentage of patients underwent improper FIT at a tertiary referral academic VA medical center. Additional education and systems interventions are necessary to improve both provider and patient adherence to appropriate CRC screening. For example, one measure may include providing HCPs with a list of their patients not up-to-date with CRC screening that was shown to increase patient participation in FIT screening compared with patients who received usual care in a 2017 study.12 In addition, a 2018 study showed that a digital health intervention that allows patients to self-order tests (eg, on an iPad) can increase CRC screening rates.13
Author Contributions
Adam Gluskin: Study concept and design; acquisition of data; analysis and interpretation of data; drafting of the manuscript. Jeffrey Dueker: Study concept and design; analysis and interpretation of data; statistical analysis; critical revision of the manuscript for important intellectual content. Asif Khalid: Study concept and design; analysis and interpretation of data; drafting of the manuscripts; critical revision of the manuscript for important intellectual content; study supervision.
1. US Preventive Services Task Force, Bibbins-Domingo K, Grossman DC, et al. Screening for Colorectal Cancer: US Preventive Services Task Force recommendation statement [published correction appears in JAMA. 2016 Aug 2;316(5):545] [published correction appears in JAMA. 2017 Jun 6;317(21):2239]. JAMA. 2016;315(23):2564-2575. doi:10.1001/jama.2016.5989
2. Rex DK, Boland CR, Dominitz JA, et al. Colorectal cancer screening: recommendations for physicians and patients from the U.S. Multi-Society Task Force on Colorectal Cancer. Gastroenterology. 2017;153(1):307-323. doi:10.1053/j.gastro.2017.05.013
3. Lee YC, Li-Sheng Chen S, Ming-Fang Yen A, et al. Association between colorectal cancer mortality and gradient fecal hemoglobin concentration in colonoscopy noncompliers. J Natl Cancer Inst. 2017;109(5):djw269. doi:10.1093/jnci/djw269
4. Corley DA, Jensen CD, Quinn VP, et al. Association between time to colonoscopy after a positive fecal test result and risk of colorectal cancer and cancer stage at diagnosis. JAMA. 2017;317(16):1631-1641. doi:10.1001/jama.2017.3634
5. Gellad ZF, Almirall D, Provenzale D, Fisher DA. Time from positive screening fecal occult blood test to colonoscopy and risk of neoplasia. Dig Dis Sci. 2009;54(11):2497-2502. doi:10.1007/s10620-008-0653-8
6. Issaka RB, Singh MH, Oshima SM, et al. Inadequate utilization of diagnostic colonoscopy following abnormal FIT results in an integrated safety-net System. Am J Gastroenterol. 2017;112(2):375-382. doi:10.1038/ajg.2016.555
7. Carlson CM, Kirby KA, Casadei MA, Partin MR, Kistler CE, Walter LC. Lack of follow-up after fecal occult blood testing in older adults: inappropriate screening or failure to follow up?. Arch Intern Med. 2011;171(3):249-256. doi:10.1001/archinternmed.2010.372
8. Fisher DA, Judd L, Sanford NS. Inappropriate colorectal cancer screening: findings and implications. Am J Gastroenterol. 2005;100(11):2526-2530. doi:10.1111/j.1572-0241.2005.00322.x
9. Powell AA, Saini SD, Breitenstein MK, Noorbaloochi S, Cutting A, Fisher DA, Bloomfield HE, Halek K, Partin MR. Rates and correlates of potentially inappropriate colorectal cancer screening in the Veterans Health Administration. J Gen Intern Med. 2015 Jun;30(6):732-41. doi: 10.1007/s11606-014-3163-8
10. Jensen CD, Corley DA, Quinn VP, et al. Fecal immunochemical test program performance over 4 rounds of annual screening: a retrospective cohort study. Ann Intern Med. 2016;164(7):456-463. doi:10.7326/M15-0983
11. van der Vlugt M, Grobbee EJ, Bossuyt PM, et al. Risk of oral and upper gastrointestinal cancers in persons with positive results from a fecal immunochemical test in a colorectal cancer screening program. Clin Gastroenterol Hepatol. 2018;16(8):1237-1243.e2. doi:10.1016/j.cgh.2018.01.037
12. Rat C, Pogu C, Le Donné D, et al. Effect of physician notification regarding nonadherence to colorectal cancer screening on patient participation in fecal immunochemical test cancer screening: a randomized clinical trial. JAMA. 2017;318(9):816-824. doi:10.1001/jama.2017.11387
13. Miller DP Jr, Denizard-Thompson N, Weaver KE, et al. Effect of a digital health intervention on receipt of colorectal cancer screening in vulnerable patients: a randomized controlled trial. Ann Intern Med. 2018;168(8):550-557. doi:10.7326/M17-2315
1. US Preventive Services Task Force, Bibbins-Domingo K, Grossman DC, et al. Screening for Colorectal Cancer: US Preventive Services Task Force recommendation statement [published correction appears in JAMA. 2016 Aug 2;316(5):545] [published correction appears in JAMA. 2017 Jun 6;317(21):2239]. JAMA. 2016;315(23):2564-2575. doi:10.1001/jama.2016.5989
2. Rex DK, Boland CR, Dominitz JA, et al. Colorectal cancer screening: recommendations for physicians and patients from the U.S. Multi-Society Task Force on Colorectal Cancer. Gastroenterology. 2017;153(1):307-323. doi:10.1053/j.gastro.2017.05.013
3. Lee YC, Li-Sheng Chen S, Ming-Fang Yen A, et al. Association between colorectal cancer mortality and gradient fecal hemoglobin concentration in colonoscopy noncompliers. J Natl Cancer Inst. 2017;109(5):djw269. doi:10.1093/jnci/djw269
4. Corley DA, Jensen CD, Quinn VP, et al. Association between time to colonoscopy after a positive fecal test result and risk of colorectal cancer and cancer stage at diagnosis. JAMA. 2017;317(16):1631-1641. doi:10.1001/jama.2017.3634
5. Gellad ZF, Almirall D, Provenzale D, Fisher DA. Time from positive screening fecal occult blood test to colonoscopy and risk of neoplasia. Dig Dis Sci. 2009;54(11):2497-2502. doi:10.1007/s10620-008-0653-8
6. Issaka RB, Singh MH, Oshima SM, et al. Inadequate utilization of diagnostic colonoscopy following abnormal FIT results in an integrated safety-net System. Am J Gastroenterol. 2017;112(2):375-382. doi:10.1038/ajg.2016.555
7. Carlson CM, Kirby KA, Casadei MA, Partin MR, Kistler CE, Walter LC. Lack of follow-up after fecal occult blood testing in older adults: inappropriate screening or failure to follow up?. Arch Intern Med. 2011;171(3):249-256. doi:10.1001/archinternmed.2010.372
8. Fisher DA, Judd L, Sanford NS. Inappropriate colorectal cancer screening: findings and implications. Am J Gastroenterol. 2005;100(11):2526-2530. doi:10.1111/j.1572-0241.2005.00322.x
9. Powell AA, Saini SD, Breitenstein MK, Noorbaloochi S, Cutting A, Fisher DA, Bloomfield HE, Halek K, Partin MR. Rates and correlates of potentially inappropriate colorectal cancer screening in the Veterans Health Administration. J Gen Intern Med. 2015 Jun;30(6):732-41. doi: 10.1007/s11606-014-3163-8
10. Jensen CD, Corley DA, Quinn VP, et al. Fecal immunochemical test program performance over 4 rounds of annual screening: a retrospective cohort study. Ann Intern Med. 2016;164(7):456-463. doi:10.7326/M15-0983
11. van der Vlugt M, Grobbee EJ, Bossuyt PM, et al. Risk of oral and upper gastrointestinal cancers in persons with positive results from a fecal immunochemical test in a colorectal cancer screening program. Clin Gastroenterol Hepatol. 2018;16(8):1237-1243.e2. doi:10.1016/j.cgh.2018.01.037
12. Rat C, Pogu C, Le Donné D, et al. Effect of physician notification regarding nonadherence to colorectal cancer screening on patient participation in fecal immunochemical test cancer screening: a randomized clinical trial. JAMA. 2017;318(9):816-824. doi:10.1001/jama.2017.11387
13. Miller DP Jr, Denizard-Thompson N, Weaver KE, et al. Effect of a digital health intervention on receipt of colorectal cancer screening in vulnerable patients: a randomized controlled trial. Ann Intern Med. 2018;168(8):550-557. doi:10.7326/M17-2315
Mistrust and Mandates: COVID-19 Vaccination in the Military
It is June and most of us are looking forward to a more normal summer than the one we had in 2020. Many Americans have been vaccinated and states are rolling back some (or all) masking requirements and restrictions on gatherings. In many sectors, including the US Department of Defense (DoD) and the US Department of Veterans Affairs (VA), worries from public health officials about vaccine supply and how to ethically allocate demand have given way to a new set of concerns: We have the shots, but for widespread protection we have to get them into arms.
The reluctance to roll up the sleeve is known as vaccine hesitancy. The National Academies of Science comments on vaccine hesitancy in its report on COVID-19 vaccination allocation. “Potential consequences of vaccine hesitancy—which the committee views as an attitude, preference, or motivational state—are the behaviors of vaccine refusal or delay.”2
On that count, there was encouraging albeit unexpected news in waning days of May. Media reported a sharp increase in the COVID-vaccination of military personnel. Unnamed DoD officials indicated, they had seen a 55% increase in the vaccination of active-duty service members over the previous month. This news represents a dramatic turnaround in a trend of vaccine hesitancy among military members that has persisted since the vaccine became available.3 Even last month, this would have been a very different column. The DoD has not disclosed the exact number of service members who have declined COVID-19 vaccination but multiple news outlets have documented that there was widespread and significant vaccine hesitancy among military personnel. In February, Military News reported that one-third of troops who were offered the vaccine declined it; and in April, USA Today stated that 40% of Marines had refused vaccination.4,5
Still, it is worth examining the data on vaccination among active duty service members. From December 2020 through March 2021, the military conducted the first study to evaluate rates of vaccine initiation and completion in the military in general and for service members from racial/ethnic minorities in particular. Black military personnel were 28% less likely than non-Hispanic White service members to initiate vaccination against coronavirus even after adjusting for other possible confounders. Just 29% of White, 25.5% of Hispanic, and 18.7% of Black service members had initiated the vaccine process in the survey.6
The authors suggest that in part, vaccine hesitancy explains the findings.4 Vaccine hesitancy among racial and ethnic minorities is even more tragic because these same already disadvantaged cohorts have disproportionately suffered from COVID-19 throughout the pandemic with higher rates of infection, serious illness requiring hospitalization, and infection-related morbidity.7
Vaccine hesitancy, delay, or refusal in Black Americans whether military or civilian often is attributed to the historical abuses like the Tuskegee syphilis experiments or the more recent example of cancer cell lines taken from Henrietta Lacks without consent.8 Such government sponsored betrayals no doubt are the soil in which hesitancy grows but recent commentators have opined that focusing solely on these infamous examples may ignore current systemic racism that is pervasively feeding Black Americans reluctance to consider or accept COVID-19 vaccination.9 Blaming infamous research also provides a convenient excuse for confronting contemporary racial discrimination in health care and taking responsibility as health care practitioners for reversing it. “Framing the conversation about distrust in COVID vaccines in terms of everyday racism rather than historical atrocities may increase underserved communities’ willingness to be vaccinated,” Bajaj and Stanford wrote in a recent recent New England Journal of Medicine commentary. “When we hyperfocus on Sims, Lacks, and Tuskegee, we ascribe the current Black health experience to past racism, rooting our present in immovable historical occurrences and undermining efforts to combat mistrust. Everyday racism, by contrast, can be tackled in the present.”9
The study of racial/ethnic disparities in COVID-19 vaccination in active-duty service members was a work product of the Armed Forces Health Surveillance Division. The authors underscore several factors that support the connection between discrimination and vaccine hesitancy in the military. Lack of access to and ability to obtain COVID-19 vaccination continues to be a major barrier that disadvantaged populations must overcome.10 The COVID-19 vaccine is widely available, easily obtained, and free of charge for all military personnel. Yet the vaccine hesitancy in the military parallels that of the civilian sector. This led the study authors to opine that, “forces external to the U.S. Military, such as interpersonal and societal factors also contribute to vaccine hesitancy among military service members.”6
Obviously, any unvaccinated active-duty service member reduces the combat readiness of the fighting force a consideration that led some in Congress to call for mandating vaccination. The vaccine is currently being administered under an emergency use authorization (EUA), which prevents even the military from mandating it.11 Even if President Joseph Biden obtained a waiver to make the vaccine mandatory, the implications of forcing service members who have volunteered to serve their country is ethically problematic. Those problems are exponentially amplified when applied to members of ethnic and racial minorities who have a past and present of health disparities and discrimination. Respecting the decision of those in uniform to decline COVID-19 vaccination is the first and perhaps most important step to rebuilding the trust that is the most promising means of reducing vaccine hesitancy.
Part of the accountability we all bear for health care inequity and racism is to continue the work of this landmark study to better understand vaccine hesitancy among military and veteran cohorts, develop counseling and education that target those attitudes, beliefs, and motivations with education, counseling, and support. All of us can in some small measure follow the ethical mandate “to dispel rumors and provide facts to people” of Secretary Austin, a Black retired 4-star Army general.1
1. Garmone J. Secretary of Defense Addresses Vaccine Hesitancy in the Military. Published February 25, 2021. Accessed May 26, 2021. https://www.defense.gov/Explore/News/Article/Article/2516511/secretary-of-defense-addresses-vaccine-hesitancy-in-military/
2. National Academies of Sciences, Engineering, and Medicine. Framework for Equitable Allocation of COVID-19 Vaccine . The National Academies of Science; 2020:188. doi:10.17226/25917
3. Liebermann O. US military sees 55% jump in COVID-19 vaccinations over last month. Published May 20, 2021. Accessed May 26, 2021. https://www.cnn.com/2021/05/20/politics/us-military-covid-vaccinations/index.html
4. Kime P. Almost one-third of us troops are refusing COVID-19 vaccines, officials Say. Published February 17, 2021. Accessed May 26, 2021. https://www.military.com/daily-news/2021/02/17/almost-one-third-of-us-troops-are-refusing-covid-vaccines-officials-say.html
5. Elbeshbishi S. Nearly 40% of Marines decline COVID-19 vaccine, prompting some Democrats to urge Biden to set mandate for the military. USA Today. April 10, 2021. Accessed May 26, 2021. https://www.usatoday.com/story/news/politics/2021/04/10/covid-vaccine-nearly-forty-percent-us-marines-decline/7173918002/
6. Lang MA, Stahlman S, Wells NY, et al. Disparities in COVID-19 vaccine initiation and completion among active component service members and health care personnel, 11 December 2020-12 March 2021. MSMR. 2021;28(4):2-9.
7. Webb Hooper M, Nápoles AM, Pérez-Stable EJ. COVID-19 and racial/ethnic disparities. JAMA . 2020;323(24):2466-2467. doi:10.1001/jama.2020.8598
8. Kum D. Fueled by a history of mistreatment, Black Americans distrust the new COVID-19 vaccines. TIME. December 8, 2020. Accessed May 26, 2021.https://time.com/5925074/black-americans-covid-19-vaccine-distrust/
9. Bajaj SS, Stanford FC. Beyond Tuskegee - Vaccine Distrust and Everyday Racism. N Engl J Med. 2021;384(5):e12. doi:10.1056/NEJMpv2035827
10. Feldman N. Why Black and Latino people still lag on COVID-19 vaccines-and how to fix it. NPR. April 26, 2021. Accessed May 26, 2021. https://www.npr.org/sections/health-shots/2021/04/26/989962041/why-black-and-latino-people-still-lag-on-covid-vaccines-and-how-to-fix-it
11. Kaufman E. Lawmakers ask Biden to issue waiver to make COVID-19 vaccination mandatory of members of the military. Updated March 24, 2021. Accessed May 26, 2021. https://www.cnn.com/2021/03/24/politics/congress-letter-military-vaccine/index.html
It is June and most of us are looking forward to a more normal summer than the one we had in 2020. Many Americans have been vaccinated and states are rolling back some (or all) masking requirements and restrictions on gatherings. In many sectors, including the US Department of Defense (DoD) and the US Department of Veterans Affairs (VA), worries from public health officials about vaccine supply and how to ethically allocate demand have given way to a new set of concerns: We have the shots, but for widespread protection we have to get them into arms.
The reluctance to roll up the sleeve is known as vaccine hesitancy. The National Academies of Science comments on vaccine hesitancy in its report on COVID-19 vaccination allocation. “Potential consequences of vaccine hesitancy—which the committee views as an attitude, preference, or motivational state—are the behaviors of vaccine refusal or delay.”2
On that count, there was encouraging albeit unexpected news in waning days of May. Media reported a sharp increase in the COVID-vaccination of military personnel. Unnamed DoD officials indicated, they had seen a 55% increase in the vaccination of active-duty service members over the previous month. This news represents a dramatic turnaround in a trend of vaccine hesitancy among military members that has persisted since the vaccine became available.3 Even last month, this would have been a very different column. The DoD has not disclosed the exact number of service members who have declined COVID-19 vaccination but multiple news outlets have documented that there was widespread and significant vaccine hesitancy among military personnel. In February, Military News reported that one-third of troops who were offered the vaccine declined it; and in April, USA Today stated that 40% of Marines had refused vaccination.4,5
Still, it is worth examining the data on vaccination among active duty service members. From December 2020 through March 2021, the military conducted the first study to evaluate rates of vaccine initiation and completion in the military in general and for service members from racial/ethnic minorities in particular. Black military personnel were 28% less likely than non-Hispanic White service members to initiate vaccination against coronavirus even after adjusting for other possible confounders. Just 29% of White, 25.5% of Hispanic, and 18.7% of Black service members had initiated the vaccine process in the survey.6
The authors suggest that in part, vaccine hesitancy explains the findings.4 Vaccine hesitancy among racial and ethnic minorities is even more tragic because these same already disadvantaged cohorts have disproportionately suffered from COVID-19 throughout the pandemic with higher rates of infection, serious illness requiring hospitalization, and infection-related morbidity.7
Vaccine hesitancy, delay, or refusal in Black Americans whether military or civilian often is attributed to the historical abuses like the Tuskegee syphilis experiments or the more recent example of cancer cell lines taken from Henrietta Lacks without consent.8 Such government sponsored betrayals no doubt are the soil in which hesitancy grows but recent commentators have opined that focusing solely on these infamous examples may ignore current systemic racism that is pervasively feeding Black Americans reluctance to consider or accept COVID-19 vaccination.9 Blaming infamous research also provides a convenient excuse for confronting contemporary racial discrimination in health care and taking responsibility as health care practitioners for reversing it. “Framing the conversation about distrust in COVID vaccines in terms of everyday racism rather than historical atrocities may increase underserved communities’ willingness to be vaccinated,” Bajaj and Stanford wrote in a recent recent New England Journal of Medicine commentary. “When we hyperfocus on Sims, Lacks, and Tuskegee, we ascribe the current Black health experience to past racism, rooting our present in immovable historical occurrences and undermining efforts to combat mistrust. Everyday racism, by contrast, can be tackled in the present.”9
The study of racial/ethnic disparities in COVID-19 vaccination in active-duty service members was a work product of the Armed Forces Health Surveillance Division. The authors underscore several factors that support the connection between discrimination and vaccine hesitancy in the military. Lack of access to and ability to obtain COVID-19 vaccination continues to be a major barrier that disadvantaged populations must overcome.10 The COVID-19 vaccine is widely available, easily obtained, and free of charge for all military personnel. Yet the vaccine hesitancy in the military parallels that of the civilian sector. This led the study authors to opine that, “forces external to the U.S. Military, such as interpersonal and societal factors also contribute to vaccine hesitancy among military service members.”6
Obviously, any unvaccinated active-duty service member reduces the combat readiness of the fighting force a consideration that led some in Congress to call for mandating vaccination. The vaccine is currently being administered under an emergency use authorization (EUA), which prevents even the military from mandating it.11 Even if President Joseph Biden obtained a waiver to make the vaccine mandatory, the implications of forcing service members who have volunteered to serve their country is ethically problematic. Those problems are exponentially amplified when applied to members of ethnic and racial minorities who have a past and present of health disparities and discrimination. Respecting the decision of those in uniform to decline COVID-19 vaccination is the first and perhaps most important step to rebuilding the trust that is the most promising means of reducing vaccine hesitancy.
Part of the accountability we all bear for health care inequity and racism is to continue the work of this landmark study to better understand vaccine hesitancy among military and veteran cohorts, develop counseling and education that target those attitudes, beliefs, and motivations with education, counseling, and support. All of us can in some small measure follow the ethical mandate “to dispel rumors and provide facts to people” of Secretary Austin, a Black retired 4-star Army general.1
It is June and most of us are looking forward to a more normal summer than the one we had in 2020. Many Americans have been vaccinated and states are rolling back some (or all) masking requirements and restrictions on gatherings. In many sectors, including the US Department of Defense (DoD) and the US Department of Veterans Affairs (VA), worries from public health officials about vaccine supply and how to ethically allocate demand have given way to a new set of concerns: We have the shots, but for widespread protection we have to get them into arms.
The reluctance to roll up the sleeve is known as vaccine hesitancy. The National Academies of Science comments on vaccine hesitancy in its report on COVID-19 vaccination allocation. “Potential consequences of vaccine hesitancy—which the committee views as an attitude, preference, or motivational state—are the behaviors of vaccine refusal or delay.”2
On that count, there was encouraging albeit unexpected news in waning days of May. Media reported a sharp increase in the COVID-vaccination of military personnel. Unnamed DoD officials indicated, they had seen a 55% increase in the vaccination of active-duty service members over the previous month. This news represents a dramatic turnaround in a trend of vaccine hesitancy among military members that has persisted since the vaccine became available.3 Even last month, this would have been a very different column. The DoD has not disclosed the exact number of service members who have declined COVID-19 vaccination but multiple news outlets have documented that there was widespread and significant vaccine hesitancy among military personnel. In February, Military News reported that one-third of troops who were offered the vaccine declined it; and in April, USA Today stated that 40% of Marines had refused vaccination.4,5
Still, it is worth examining the data on vaccination among active duty service members. From December 2020 through March 2021, the military conducted the first study to evaluate rates of vaccine initiation and completion in the military in general and for service members from racial/ethnic minorities in particular. Black military personnel were 28% less likely than non-Hispanic White service members to initiate vaccination against coronavirus even after adjusting for other possible confounders. Just 29% of White, 25.5% of Hispanic, and 18.7% of Black service members had initiated the vaccine process in the survey.6
The authors suggest that in part, vaccine hesitancy explains the findings.4 Vaccine hesitancy among racial and ethnic minorities is even more tragic because these same already disadvantaged cohorts have disproportionately suffered from COVID-19 throughout the pandemic with higher rates of infection, serious illness requiring hospitalization, and infection-related morbidity.7
Vaccine hesitancy, delay, or refusal in Black Americans whether military or civilian often is attributed to the historical abuses like the Tuskegee syphilis experiments or the more recent example of cancer cell lines taken from Henrietta Lacks without consent.8 Such government sponsored betrayals no doubt are the soil in which hesitancy grows but recent commentators have opined that focusing solely on these infamous examples may ignore current systemic racism that is pervasively feeding Black Americans reluctance to consider or accept COVID-19 vaccination.9 Blaming infamous research also provides a convenient excuse for confronting contemporary racial discrimination in health care and taking responsibility as health care practitioners for reversing it. “Framing the conversation about distrust in COVID vaccines in terms of everyday racism rather than historical atrocities may increase underserved communities’ willingness to be vaccinated,” Bajaj and Stanford wrote in a recent recent New England Journal of Medicine commentary. “When we hyperfocus on Sims, Lacks, and Tuskegee, we ascribe the current Black health experience to past racism, rooting our present in immovable historical occurrences and undermining efforts to combat mistrust. Everyday racism, by contrast, can be tackled in the present.”9
The study of racial/ethnic disparities in COVID-19 vaccination in active-duty service members was a work product of the Armed Forces Health Surveillance Division. The authors underscore several factors that support the connection between discrimination and vaccine hesitancy in the military. Lack of access to and ability to obtain COVID-19 vaccination continues to be a major barrier that disadvantaged populations must overcome.10 The COVID-19 vaccine is widely available, easily obtained, and free of charge for all military personnel. Yet the vaccine hesitancy in the military parallels that of the civilian sector. This led the study authors to opine that, “forces external to the U.S. Military, such as interpersonal and societal factors also contribute to vaccine hesitancy among military service members.”6
Obviously, any unvaccinated active-duty service member reduces the combat readiness of the fighting force a consideration that led some in Congress to call for mandating vaccination. The vaccine is currently being administered under an emergency use authorization (EUA), which prevents even the military from mandating it.11 Even if President Joseph Biden obtained a waiver to make the vaccine mandatory, the implications of forcing service members who have volunteered to serve their country is ethically problematic. Those problems are exponentially amplified when applied to members of ethnic and racial minorities who have a past and present of health disparities and discrimination. Respecting the decision of those in uniform to decline COVID-19 vaccination is the first and perhaps most important step to rebuilding the trust that is the most promising means of reducing vaccine hesitancy.
Part of the accountability we all bear for health care inequity and racism is to continue the work of this landmark study to better understand vaccine hesitancy among military and veteran cohorts, develop counseling and education that target those attitudes, beliefs, and motivations with education, counseling, and support. All of us can in some small measure follow the ethical mandate “to dispel rumors and provide facts to people” of Secretary Austin, a Black retired 4-star Army general.1
1. Garmone J. Secretary of Defense Addresses Vaccine Hesitancy in the Military. Published February 25, 2021. Accessed May 26, 2021. https://www.defense.gov/Explore/News/Article/Article/2516511/secretary-of-defense-addresses-vaccine-hesitancy-in-military/
2. National Academies of Sciences, Engineering, and Medicine. Framework for Equitable Allocation of COVID-19 Vaccine . The National Academies of Science; 2020:188. doi:10.17226/25917
3. Liebermann O. US military sees 55% jump in COVID-19 vaccinations over last month. Published May 20, 2021. Accessed May 26, 2021. https://www.cnn.com/2021/05/20/politics/us-military-covid-vaccinations/index.html
4. Kime P. Almost one-third of us troops are refusing COVID-19 vaccines, officials Say. Published February 17, 2021. Accessed May 26, 2021. https://www.military.com/daily-news/2021/02/17/almost-one-third-of-us-troops-are-refusing-covid-vaccines-officials-say.html
5. Elbeshbishi S. Nearly 40% of Marines decline COVID-19 vaccine, prompting some Democrats to urge Biden to set mandate for the military. USA Today. April 10, 2021. Accessed May 26, 2021. https://www.usatoday.com/story/news/politics/2021/04/10/covid-vaccine-nearly-forty-percent-us-marines-decline/7173918002/
6. Lang MA, Stahlman S, Wells NY, et al. Disparities in COVID-19 vaccine initiation and completion among active component service members and health care personnel, 11 December 2020-12 March 2021. MSMR. 2021;28(4):2-9.
7. Webb Hooper M, Nápoles AM, Pérez-Stable EJ. COVID-19 and racial/ethnic disparities. JAMA . 2020;323(24):2466-2467. doi:10.1001/jama.2020.8598
8. Kum D. Fueled by a history of mistreatment, Black Americans distrust the new COVID-19 vaccines. TIME. December 8, 2020. Accessed May 26, 2021.https://time.com/5925074/black-americans-covid-19-vaccine-distrust/
9. Bajaj SS, Stanford FC. Beyond Tuskegee - Vaccine Distrust and Everyday Racism. N Engl J Med. 2021;384(5):e12. doi:10.1056/NEJMpv2035827
10. Feldman N. Why Black and Latino people still lag on COVID-19 vaccines-and how to fix it. NPR. April 26, 2021. Accessed May 26, 2021. https://www.npr.org/sections/health-shots/2021/04/26/989962041/why-black-and-latino-people-still-lag-on-covid-vaccines-and-how-to-fix-it
11. Kaufman E. Lawmakers ask Biden to issue waiver to make COVID-19 vaccination mandatory of members of the military. Updated March 24, 2021. Accessed May 26, 2021. https://www.cnn.com/2021/03/24/politics/congress-letter-military-vaccine/index.html
1. Garmone J. Secretary of Defense Addresses Vaccine Hesitancy in the Military. Published February 25, 2021. Accessed May 26, 2021. https://www.defense.gov/Explore/News/Article/Article/2516511/secretary-of-defense-addresses-vaccine-hesitancy-in-military/
2. National Academies of Sciences, Engineering, and Medicine. Framework for Equitable Allocation of COVID-19 Vaccine . The National Academies of Science; 2020:188. doi:10.17226/25917
3. Liebermann O. US military sees 55% jump in COVID-19 vaccinations over last month. Published May 20, 2021. Accessed May 26, 2021. https://www.cnn.com/2021/05/20/politics/us-military-covid-vaccinations/index.html
4. Kime P. Almost one-third of us troops are refusing COVID-19 vaccines, officials Say. Published February 17, 2021. Accessed May 26, 2021. https://www.military.com/daily-news/2021/02/17/almost-one-third-of-us-troops-are-refusing-covid-vaccines-officials-say.html
5. Elbeshbishi S. Nearly 40% of Marines decline COVID-19 vaccine, prompting some Democrats to urge Biden to set mandate for the military. USA Today. April 10, 2021. Accessed May 26, 2021. https://www.usatoday.com/story/news/politics/2021/04/10/covid-vaccine-nearly-forty-percent-us-marines-decline/7173918002/
6. Lang MA, Stahlman S, Wells NY, et al. Disparities in COVID-19 vaccine initiation and completion among active component service members and health care personnel, 11 December 2020-12 March 2021. MSMR. 2021;28(4):2-9.
7. Webb Hooper M, Nápoles AM, Pérez-Stable EJ. COVID-19 and racial/ethnic disparities. JAMA . 2020;323(24):2466-2467. doi:10.1001/jama.2020.8598
8. Kum D. Fueled by a history of mistreatment, Black Americans distrust the new COVID-19 vaccines. TIME. December 8, 2020. Accessed May 26, 2021.https://time.com/5925074/black-americans-covid-19-vaccine-distrust/
9. Bajaj SS, Stanford FC. Beyond Tuskegee - Vaccine Distrust and Everyday Racism. N Engl J Med. 2021;384(5):e12. doi:10.1056/NEJMpv2035827
10. Feldman N. Why Black and Latino people still lag on COVID-19 vaccines-and how to fix it. NPR. April 26, 2021. Accessed May 26, 2021. https://www.npr.org/sections/health-shots/2021/04/26/989962041/why-black-and-latino-people-still-lag-on-covid-vaccines-and-how-to-fix-it
11. Kaufman E. Lawmakers ask Biden to issue waiver to make COVID-19 vaccination mandatory of members of the military. Updated March 24, 2021. Accessed May 26, 2021. https://www.cnn.com/2021/03/24/politics/congress-letter-military-vaccine/index.html
Protein tyrosine phosphatase receptor gamma, a novel biomarker for TKI response in CML
Key clinical point: Protein tyrosine phosphatase receptor gamma (PTPRG), a tumor suppressor gene, could serve as a new biomarker to evaluate therapeutic response to tyrosine kinase inhibitors (TKIs) in patients with chronic myeloid leukemia (CML).
Major finding: In patients with CML, PTPRG expression was significantly lower at diagnosis vs. follow-up (P less than .001). Patients with optimal response to TKI had significantly higher PTPRG expression during follow-up vs. diagnosis (P less than .0005); however, no difference was observed in patients with a failed response to TKI (P = .312).
Study details: This study assessed PTPRG expression in 21 patients with CML (chronic phase, n=18; accelerated phase, n=3) treated with imatinib (n=12) or nilotinib (n=9) and 7 healthy individuals.
Disclosures: This study was funded by the Qatar National Research Fund, and open access funding was enabled by the Qatar National Library. The authors declared no conflicts of interest.
Source: Ismail MA et al. Sci Rep. 2021 Apr 23. doi: 10.1038/s41598-021-86875-y.
Key clinical point: Protein tyrosine phosphatase receptor gamma (PTPRG), a tumor suppressor gene, could serve as a new biomarker to evaluate therapeutic response to tyrosine kinase inhibitors (TKIs) in patients with chronic myeloid leukemia (CML).
Major finding: In patients with CML, PTPRG expression was significantly lower at diagnosis vs. follow-up (P less than .001). Patients with optimal response to TKI had significantly higher PTPRG expression during follow-up vs. diagnosis (P less than .0005); however, no difference was observed in patients with a failed response to TKI (P = .312).
Study details: This study assessed PTPRG expression in 21 patients with CML (chronic phase, n=18; accelerated phase, n=3) treated with imatinib (n=12) or nilotinib (n=9) and 7 healthy individuals.
Disclosures: This study was funded by the Qatar National Research Fund, and open access funding was enabled by the Qatar National Library. The authors declared no conflicts of interest.
Source: Ismail MA et al. Sci Rep. 2021 Apr 23. doi: 10.1038/s41598-021-86875-y.
Key clinical point: Protein tyrosine phosphatase receptor gamma (PTPRG), a tumor suppressor gene, could serve as a new biomarker to evaluate therapeutic response to tyrosine kinase inhibitors (TKIs) in patients with chronic myeloid leukemia (CML).
Major finding: In patients with CML, PTPRG expression was significantly lower at diagnosis vs. follow-up (P less than .001). Patients with optimal response to TKI had significantly higher PTPRG expression during follow-up vs. diagnosis (P less than .0005); however, no difference was observed in patients with a failed response to TKI (P = .312).
Study details: This study assessed PTPRG expression in 21 patients with CML (chronic phase, n=18; accelerated phase, n=3) treated with imatinib (n=12) or nilotinib (n=9) and 7 healthy individuals.
Disclosures: This study was funded by the Qatar National Research Fund, and open access funding was enabled by the Qatar National Library. The authors declared no conflicts of interest.
Source: Ismail MA et al. Sci Rep. 2021 Apr 23. doi: 10.1038/s41598-021-86875-y.
CIP2A is a potential biomarker for disease progression and treatment failure in CML-CP
Key clinical point: High cancerous inhibitor of protein phosphatase 2A (CIP2A) levels at diagnosis predicts subsequent disease progression and treatment failure in patients with chronic-phase chronic myeloid leukemia (CML-CP) treated with imatinib or dasatinib.
Major finding: High vs. low CIP2A levels were associated with poor progression-free survival (P = .04) and freedom from progression (P = .03). Patients with high vs. low CIP2A levels had a higher chance of treatment failure at 5 years (41% vs. 7.5%; P = .002) in both imatinib- (45% vs. 11%; P = .02) and dasatinib-treated (36% vs. 4%; P = .007) patients.
Study details: Data come from an analysis of 172 patients with newly diagnosed CML-CP enrolled in phase 3 SPIRIT2 trial who were randomly allocated to frontline imatinib (n=90) or dasatinib (n=82).
Disclosures: This study was funded by Bristol Myers Squibb (BMS). The lead author reported research support and/or honoraria from Novartis, BMS, and Pfizer. Other authors declared no conflicts of interest.
Source: Clark RE et al. Cancers (Basel). 2021 Apr 29. doi: 10.3390/cancers13092155.
Key clinical point: High cancerous inhibitor of protein phosphatase 2A (CIP2A) levels at diagnosis predicts subsequent disease progression and treatment failure in patients with chronic-phase chronic myeloid leukemia (CML-CP) treated with imatinib or dasatinib.
Major finding: High vs. low CIP2A levels were associated with poor progression-free survival (P = .04) and freedom from progression (P = .03). Patients with high vs. low CIP2A levels had a higher chance of treatment failure at 5 years (41% vs. 7.5%; P = .002) in both imatinib- (45% vs. 11%; P = .02) and dasatinib-treated (36% vs. 4%; P = .007) patients.
Study details: Data come from an analysis of 172 patients with newly diagnosed CML-CP enrolled in phase 3 SPIRIT2 trial who were randomly allocated to frontline imatinib (n=90) or dasatinib (n=82).
Disclosures: This study was funded by Bristol Myers Squibb (BMS). The lead author reported research support and/or honoraria from Novartis, BMS, and Pfizer. Other authors declared no conflicts of interest.
Source: Clark RE et al. Cancers (Basel). 2021 Apr 29. doi: 10.3390/cancers13092155.
Key clinical point: High cancerous inhibitor of protein phosphatase 2A (CIP2A) levels at diagnosis predicts subsequent disease progression and treatment failure in patients with chronic-phase chronic myeloid leukemia (CML-CP) treated with imatinib or dasatinib.
Major finding: High vs. low CIP2A levels were associated with poor progression-free survival (P = .04) and freedom from progression (P = .03). Patients with high vs. low CIP2A levels had a higher chance of treatment failure at 5 years (41% vs. 7.5%; P = .002) in both imatinib- (45% vs. 11%; P = .02) and dasatinib-treated (36% vs. 4%; P = .007) patients.
Study details: Data come from an analysis of 172 patients with newly diagnosed CML-CP enrolled in phase 3 SPIRIT2 trial who were randomly allocated to frontline imatinib (n=90) or dasatinib (n=82).
Disclosures: This study was funded by Bristol Myers Squibb (BMS). The lead author reported research support and/or honoraria from Novartis, BMS, and Pfizer. Other authors declared no conflicts of interest.
Source: Clark RE et al. Cancers (Basel). 2021 Apr 29. doi: 10.3390/cancers13092155.