User login
Clofarabine/cladribine with LDAC alternating with decitabine safe and effective in older patients with AML
Key clinical point: Low-intensity chemotherapy with clofarabine or cladribine and low-dose cytarabine (LDAC) alternating with decitabine was effective and safe for the treatment of older patients with newly diagnosed acute myeloid leukemia (AML).
Major finding: Overall, response was observed in 66% of patients, with complete remission (CR) and CR with incomplete count recovery in 59% and 7% of patients, respectively. The 4- and 8-week mortality was low at 2% and 11%, respectively. During median follow-up of 60 months, the median overall survival was 12.5 months.
Study details: This study assessed 248 older patients (median age, 69 years) with newly diagnosed AML who were treated with either clofarabine (n=119) or cladribine (n=129) combined with LDAC alternating with decitabine.
Disclosures: This study was funded by the MD Anderson Cancer Center Support and the Charif Souki Cancer Research Fund. Some investigators including the lead author reported ties with various pharmaceutical companies.
Source: Kadia TM et al. Am J Hematol. 2021 Apr 26. doi: 10.1002/ajh.26206.
Key clinical point: Low-intensity chemotherapy with clofarabine or cladribine and low-dose cytarabine (LDAC) alternating with decitabine was effective and safe for the treatment of older patients with newly diagnosed acute myeloid leukemia (AML).
Major finding: Overall, response was observed in 66% of patients, with complete remission (CR) and CR with incomplete count recovery in 59% and 7% of patients, respectively. The 4- and 8-week mortality was low at 2% and 11%, respectively. During median follow-up of 60 months, the median overall survival was 12.5 months.
Study details: This study assessed 248 older patients (median age, 69 years) with newly diagnosed AML who were treated with either clofarabine (n=119) or cladribine (n=129) combined with LDAC alternating with decitabine.
Disclosures: This study was funded by the MD Anderson Cancer Center Support and the Charif Souki Cancer Research Fund. Some investigators including the lead author reported ties with various pharmaceutical companies.
Source: Kadia TM et al. Am J Hematol. 2021 Apr 26. doi: 10.1002/ajh.26206.
Key clinical point: Low-intensity chemotherapy with clofarabine or cladribine and low-dose cytarabine (LDAC) alternating with decitabine was effective and safe for the treatment of older patients with newly diagnosed acute myeloid leukemia (AML).
Major finding: Overall, response was observed in 66% of patients, with complete remission (CR) and CR with incomplete count recovery in 59% and 7% of patients, respectively. The 4- and 8-week mortality was low at 2% and 11%, respectively. During median follow-up of 60 months, the median overall survival was 12.5 months.
Study details: This study assessed 248 older patients (median age, 69 years) with newly diagnosed AML who were treated with either clofarabine (n=119) or cladribine (n=129) combined with LDAC alternating with decitabine.
Disclosures: This study was funded by the MD Anderson Cancer Center Support and the Charif Souki Cancer Research Fund. Some investigators including the lead author reported ties with various pharmaceutical companies.
Source: Kadia TM et al. Am J Hematol. 2021 Apr 26. doi: 10.1002/ajh.26206.
Relapsed/refractory AML: MIV alone or in combination with venetoclax shows promise in phase 1
Key clinical point: In patients with relapsed/refractory acute myeloid leukemia (AML), mivebresib (MIV) was tolerated and showed antileukemic activity as monotherapy (MIV-mono) and in combination with venetoclax (MIV-Ven).
Major finding: In the MIV-mono cohort, response included complete remission with incomplete blood count recovery (5%) and resistant disease (79%). In patients receiving MIV-Ven, responses were complete remission (7%), partial remission (7%), leukemia-free state (7%), resistant disease (40%), and aplasia (3%). Treatment-emergent adverse events (TEAEs) were reported in 100% of patients, with serious TEAEs in 74%, 88%, and 40% of patients in MIV-mono, MIV-Ven, and patients who switched from MIV-mono to MIV-Ven groups, respectively.
Study details: Findings are from phase 1 study including 44 adult patients with relapsed/refractory AML who received either MIV-mono (n=19) or MIV-Ven (n=25). Because of disease progression, 5 patients switched from MIV-mono to MIV-Ven.
Disclosures: This study was funded by AbbVie. Investigators including the lead author reported ties with various pharmaceutical companies including AbbVie.
Source: Borthakur G et al. Cancer. 2021 May 2. doi: 10.1002/cncr.33590.
Key clinical point: In patients with relapsed/refractory acute myeloid leukemia (AML), mivebresib (MIV) was tolerated and showed antileukemic activity as monotherapy (MIV-mono) and in combination with venetoclax (MIV-Ven).
Major finding: In the MIV-mono cohort, response included complete remission with incomplete blood count recovery (5%) and resistant disease (79%). In patients receiving MIV-Ven, responses were complete remission (7%), partial remission (7%), leukemia-free state (7%), resistant disease (40%), and aplasia (3%). Treatment-emergent adverse events (TEAEs) were reported in 100% of patients, with serious TEAEs in 74%, 88%, and 40% of patients in MIV-mono, MIV-Ven, and patients who switched from MIV-mono to MIV-Ven groups, respectively.
Study details: Findings are from phase 1 study including 44 adult patients with relapsed/refractory AML who received either MIV-mono (n=19) or MIV-Ven (n=25). Because of disease progression, 5 patients switched from MIV-mono to MIV-Ven.
Disclosures: This study was funded by AbbVie. Investigators including the lead author reported ties with various pharmaceutical companies including AbbVie.
Source: Borthakur G et al. Cancer. 2021 May 2. doi: 10.1002/cncr.33590.
Key clinical point: In patients with relapsed/refractory acute myeloid leukemia (AML), mivebresib (MIV) was tolerated and showed antileukemic activity as monotherapy (MIV-mono) and in combination with venetoclax (MIV-Ven).
Major finding: In the MIV-mono cohort, response included complete remission with incomplete blood count recovery (5%) and resistant disease (79%). In patients receiving MIV-Ven, responses were complete remission (7%), partial remission (7%), leukemia-free state (7%), resistant disease (40%), and aplasia (3%). Treatment-emergent adverse events (TEAEs) were reported in 100% of patients, with serious TEAEs in 74%, 88%, and 40% of patients in MIV-mono, MIV-Ven, and patients who switched from MIV-mono to MIV-Ven groups, respectively.
Study details: Findings are from phase 1 study including 44 adult patients with relapsed/refractory AML who received either MIV-mono (n=19) or MIV-Ven (n=25). Because of disease progression, 5 patients switched from MIV-mono to MIV-Ven.
Disclosures: This study was funded by AbbVie. Investigators including the lead author reported ties with various pharmaceutical companies including AbbVie.
Source: Borthakur G et al. Cancer. 2021 May 2. doi: 10.1002/cncr.33590.
No survival benefit of adding tosedostat to LDAC vs. LDAC alone in older patients with AML
Key clinical point: Addition of tosedostat to low-dose cytosine arabinoside (LDAC) did not confer any additional clinical benefit compared with LDAC alone in older patients with acute myeloid leukemia (AML) unfit for intensive chemotherapy.
Major finding: There was no significant benefit of adding tosedostat to LDAC vs. LDAC alone on overall response (25% vs. 18%; P = .22) and 2-year overall survival (16% vs. 12%; P = .8). Overall rates of grade 3 or higher toxicity were low, but diarrhea and cardiac toxicity were significantly higher with tosedostat.
Study details: Findings are from LI-1 trial including 243 older (age, 60 years or older) patients with de novo or secondary AML or high-risk myelodysplastic syndrome who were unfit for intensive chemotherapy. Patients were randomly allocated to receive either LDAC (n=121) or LDAC+tosedostat (n=122).
Disclosures: This study was supported by CTI Biopharma, Blood Cancer UK, the Haematology Clinical Trials Unit, and the Centre for Trials Research, Cardiff University. No author disclosures were reported.
Source: Dennis M et al. Br J Haematol. 2021 May 7. doi: 10.1111/bjh.17501.
Key clinical point: Addition of tosedostat to low-dose cytosine arabinoside (LDAC) did not confer any additional clinical benefit compared with LDAC alone in older patients with acute myeloid leukemia (AML) unfit for intensive chemotherapy.
Major finding: There was no significant benefit of adding tosedostat to LDAC vs. LDAC alone on overall response (25% vs. 18%; P = .22) and 2-year overall survival (16% vs. 12%; P = .8). Overall rates of grade 3 or higher toxicity were low, but diarrhea and cardiac toxicity were significantly higher with tosedostat.
Study details: Findings are from LI-1 trial including 243 older (age, 60 years or older) patients with de novo or secondary AML or high-risk myelodysplastic syndrome who were unfit for intensive chemotherapy. Patients were randomly allocated to receive either LDAC (n=121) or LDAC+tosedostat (n=122).
Disclosures: This study was supported by CTI Biopharma, Blood Cancer UK, the Haematology Clinical Trials Unit, and the Centre for Trials Research, Cardiff University. No author disclosures were reported.
Source: Dennis M et al. Br J Haematol. 2021 May 7. doi: 10.1111/bjh.17501.
Key clinical point: Addition of tosedostat to low-dose cytosine arabinoside (LDAC) did not confer any additional clinical benefit compared with LDAC alone in older patients with acute myeloid leukemia (AML) unfit for intensive chemotherapy.
Major finding: There was no significant benefit of adding tosedostat to LDAC vs. LDAC alone on overall response (25% vs. 18%; P = .22) and 2-year overall survival (16% vs. 12%; P = .8). Overall rates of grade 3 or higher toxicity were low, but diarrhea and cardiac toxicity were significantly higher with tosedostat.
Study details: Findings are from LI-1 trial including 243 older (age, 60 years or older) patients with de novo or secondary AML or high-risk myelodysplastic syndrome who were unfit for intensive chemotherapy. Patients were randomly allocated to receive either LDAC (n=121) or LDAC+tosedostat (n=122).
Disclosures: This study was supported by CTI Biopharma, Blood Cancer UK, the Haematology Clinical Trials Unit, and the Centre for Trials Research, Cardiff University. No author disclosures were reported.
Source: Dennis M et al. Br J Haematol. 2021 May 7. doi: 10.1111/bjh.17501.
AML: Mismatched unrelated donor with PTCy outscores CBT in absence of matched donors
Key clinical point: In the absence of human leukocyte antigen-matched donors, allogeneic hematopoietic cell transplantation (allo-HCT) using a mismatched unrelated donor (MMUD) with posttransplant cyclophosphamide (PTCy) yielded better survival outcomes vs. cord blood transplantation (CBT) in patients with acute myeloid leukemia (AML).
Major finding: CBT was associated with a significantly higher risk for nonrelapse mortality (adjusted hazard ratio [aHR], 2.09), worse leukemia-free survival (aHR, 1.68), and overall survival (aHR, 1.70) vs. MMUD (all P less than .0001).
Study details: This study included adult patients with AML who underwent a first allo-HCT using CBT (n=902) or single-allele MMUD with PTCy (n=280) between 2010 and 2019.
Disclosures: No funding source was disclosed. The authors declared no conflicts of interest.
Source: Dholaria B et al. J Hematol Oncol. 2021 May 3. doi: 10.1186/s13045-021-01086-2.
Key clinical point: In the absence of human leukocyte antigen-matched donors, allogeneic hematopoietic cell transplantation (allo-HCT) using a mismatched unrelated donor (MMUD) with posttransplant cyclophosphamide (PTCy) yielded better survival outcomes vs. cord blood transplantation (CBT) in patients with acute myeloid leukemia (AML).
Major finding: CBT was associated with a significantly higher risk for nonrelapse mortality (adjusted hazard ratio [aHR], 2.09), worse leukemia-free survival (aHR, 1.68), and overall survival (aHR, 1.70) vs. MMUD (all P less than .0001).
Study details: This study included adult patients with AML who underwent a first allo-HCT using CBT (n=902) or single-allele MMUD with PTCy (n=280) between 2010 and 2019.
Disclosures: No funding source was disclosed. The authors declared no conflicts of interest.
Source: Dholaria B et al. J Hematol Oncol. 2021 May 3. doi: 10.1186/s13045-021-01086-2.
Key clinical point: In the absence of human leukocyte antigen-matched donors, allogeneic hematopoietic cell transplantation (allo-HCT) using a mismatched unrelated donor (MMUD) with posttransplant cyclophosphamide (PTCy) yielded better survival outcomes vs. cord blood transplantation (CBT) in patients with acute myeloid leukemia (AML).
Major finding: CBT was associated with a significantly higher risk for nonrelapse mortality (adjusted hazard ratio [aHR], 2.09), worse leukemia-free survival (aHR, 1.68), and overall survival (aHR, 1.70) vs. MMUD (all P less than .0001).
Study details: This study included adult patients with AML who underwent a first allo-HCT using CBT (n=902) or single-allele MMUD with PTCy (n=280) between 2010 and 2019.
Disclosures: No funding source was disclosed. The authors declared no conflicts of interest.
Source: Dholaria B et al. J Hematol Oncol. 2021 May 3. doi: 10.1186/s13045-021-01086-2.
The pandemic changed smokers, but farming didn’t change humans
Pandemic smoking: More or less?
The COVID-19 pandemic has changed a lot of habits in people, for better or worse. Some people may have turned to food and alcohol for comfort, while others started on health kicks to emerge from the ordeal as new people. Well, the same can be said about smokers.
New evidence comes from a survey conducted from May to July 2020 of 694 current and former smokers with an average age of 53 years. All had been hospitalized prior to the pandemic and had previously participated in clinical trials to for smoking cessation in Boston, Nashville, and Pittsburgh hospitals.
Researchers found that 32% of participants smoked more, 37% smoked less, and 31% made no change in their smoking habits. By the time of the survey, 28% of former smokers had relapsed. Although 68% of the participants believed smoking increased the risk of getting COVID-19, that still didn’t stop some people from smoking more. Why?
Respondents “might have increased their smoking due to stress and boredom. On the other hand, the fear of catching COVID might have led them to cut down or quit smoking,” said lead author Nancy A. Rigotti, MD. “Even before the pandemic, tobacco smoking was the leading preventable cause of death in the United States. COVID-19 has given smokers yet another good reason to stop smoking.”
This creates an opportunity for physicians to preach the gospel to smokers about their vulnerability to respiratory disease in hopes of getting them to quit for good. We just wish the same could be said for all of our excessive pandemic online shopping.
3,000 years and just one pair of genomes to wear
Men and women are different. We’ll give you a moment to pick your jaw off the ground.
It makes sense though, the sexes being different, especially when you look at the broader animal kingdom. The males and females of many species are slightly different when it comes to size and shape, but there’s a big question that literally only anthropologists have asked: Were human males and females more different in the past than they are today?
To be more specific, some scientists believe that males and females grew more similar when humans shifted from a hunter-gatherer lifestyle to a farming-based lifestyle, as agriculture encouraged a more equitable division of labor. Others believe that the differences come down to random chance.
Researchers from Penn State University analyzed genomic data from over 350,000 males and females stored in the UK Biobank and looked at the recent (within the last ~3,000 years; post-agriculture adoption in Britain) evolutionary histories of these loci. Height, body mass, hip circumference, body fat percentage, and waist circumference were analyzed, and while there were thousands of differences in the genomes, only one trait occurred more frequently during that time period: Females gained a significantly higher body fat content than males.
It’s a sad day then for the millions of people who were big fans of the “farming caused men and women to become more similar” theory. Count the LOTME crew among them. Be honest: Wouldn’t life be so much simpler if men and women were exactly the same? Just think about it, no more arguments about leaving the toilet seat up. It’d be worth it just for that.
Proteins don’t lie
Research published in Open Biology shows that the human brain contains 14,315 different proteins. The team conducting that study wanted to find out which organ was the most similar to the old brain box, so they did protein counts for the 32 other major tissue types, including heart, salivary gland, lung, spleen, and endometrium.
The tissue with the most proteins in common with the center of human intelligence? You’re thinking it has to be colon at this point, right? We were sure it was going to be colon, but it’s not.
The winner, with 13,442 shared proteins, is the testes. The testes have 15,687 proteins, of which 85.7% are shared with the brain. The researchers, sadly, did not provide protein counts for the other tissue types, but we bet colon was a close second.
Dreaming about COVID?
We thought we were the only ones who have been having crazy dreams lately. Each one seems crazier and more vivid than the one before. Have you been having weird dreams lately?
This is likely your brain’s coping mechanism to handle your pandemic stress, according to Dr. Erik Hoel of Tufts University. Dreams that are crazy and scary might make real life seem lighter and simpler. He calls it the “overfitted brain hypothesis.”
“It is their very strangeness that gives them their biological function,” Dr. Hoel said. It literally makes you feel like COVID-19 and lockdowns aren’t as scary as they seem.
We always knew our minds were powerful things. Apparently, your brain gets tired of everyday familiarity just like you do, and it creates crazy dreams to keep things interesting.
Just remember: That recurring dream that you’re back in college and missing 10 assignments is there to help you, not scare you! Even though it is pretty scary.
Pandemic smoking: More or less?
The COVID-19 pandemic has changed a lot of habits in people, for better or worse. Some people may have turned to food and alcohol for comfort, while others started on health kicks to emerge from the ordeal as new people. Well, the same can be said about smokers.
New evidence comes from a survey conducted from May to July 2020 of 694 current and former smokers with an average age of 53 years. All had been hospitalized prior to the pandemic and had previously participated in clinical trials to for smoking cessation in Boston, Nashville, and Pittsburgh hospitals.
Researchers found that 32% of participants smoked more, 37% smoked less, and 31% made no change in their smoking habits. By the time of the survey, 28% of former smokers had relapsed. Although 68% of the participants believed smoking increased the risk of getting COVID-19, that still didn’t stop some people from smoking more. Why?
Respondents “might have increased their smoking due to stress and boredom. On the other hand, the fear of catching COVID might have led them to cut down or quit smoking,” said lead author Nancy A. Rigotti, MD. “Even before the pandemic, tobacco smoking was the leading preventable cause of death in the United States. COVID-19 has given smokers yet another good reason to stop smoking.”
This creates an opportunity for physicians to preach the gospel to smokers about their vulnerability to respiratory disease in hopes of getting them to quit for good. We just wish the same could be said for all of our excessive pandemic online shopping.
3,000 years and just one pair of genomes to wear
Men and women are different. We’ll give you a moment to pick your jaw off the ground.
It makes sense though, the sexes being different, especially when you look at the broader animal kingdom. The males and females of many species are slightly different when it comes to size and shape, but there’s a big question that literally only anthropologists have asked: Were human males and females more different in the past than they are today?
To be more specific, some scientists believe that males and females grew more similar when humans shifted from a hunter-gatherer lifestyle to a farming-based lifestyle, as agriculture encouraged a more equitable division of labor. Others believe that the differences come down to random chance.
Researchers from Penn State University analyzed genomic data from over 350,000 males and females stored in the UK Biobank and looked at the recent (within the last ~3,000 years; post-agriculture adoption in Britain) evolutionary histories of these loci. Height, body mass, hip circumference, body fat percentage, and waist circumference were analyzed, and while there were thousands of differences in the genomes, only one trait occurred more frequently during that time period: Females gained a significantly higher body fat content than males.
It’s a sad day then for the millions of people who were big fans of the “farming caused men and women to become more similar” theory. Count the LOTME crew among them. Be honest: Wouldn’t life be so much simpler if men and women were exactly the same? Just think about it, no more arguments about leaving the toilet seat up. It’d be worth it just for that.
Proteins don’t lie
Research published in Open Biology shows that the human brain contains 14,315 different proteins. The team conducting that study wanted to find out which organ was the most similar to the old brain box, so they did protein counts for the 32 other major tissue types, including heart, salivary gland, lung, spleen, and endometrium.
The tissue with the most proteins in common with the center of human intelligence? You’re thinking it has to be colon at this point, right? We were sure it was going to be colon, but it’s not.
The winner, with 13,442 shared proteins, is the testes. The testes have 15,687 proteins, of which 85.7% are shared with the brain. The researchers, sadly, did not provide protein counts for the other tissue types, but we bet colon was a close second.
Dreaming about COVID?
We thought we were the only ones who have been having crazy dreams lately. Each one seems crazier and more vivid than the one before. Have you been having weird dreams lately?
This is likely your brain’s coping mechanism to handle your pandemic stress, according to Dr. Erik Hoel of Tufts University. Dreams that are crazy and scary might make real life seem lighter and simpler. He calls it the “overfitted brain hypothesis.”
“It is their very strangeness that gives them their biological function,” Dr. Hoel said. It literally makes you feel like COVID-19 and lockdowns aren’t as scary as they seem.
We always knew our minds were powerful things. Apparently, your brain gets tired of everyday familiarity just like you do, and it creates crazy dreams to keep things interesting.
Just remember: That recurring dream that you’re back in college and missing 10 assignments is there to help you, not scare you! Even though it is pretty scary.
Pandemic smoking: More or less?
The COVID-19 pandemic has changed a lot of habits in people, for better or worse. Some people may have turned to food and alcohol for comfort, while others started on health kicks to emerge from the ordeal as new people. Well, the same can be said about smokers.
New evidence comes from a survey conducted from May to July 2020 of 694 current and former smokers with an average age of 53 years. All had been hospitalized prior to the pandemic and had previously participated in clinical trials to for smoking cessation in Boston, Nashville, and Pittsburgh hospitals.
Researchers found that 32% of participants smoked more, 37% smoked less, and 31% made no change in their smoking habits. By the time of the survey, 28% of former smokers had relapsed. Although 68% of the participants believed smoking increased the risk of getting COVID-19, that still didn’t stop some people from smoking more. Why?
Respondents “might have increased their smoking due to stress and boredom. On the other hand, the fear of catching COVID might have led them to cut down or quit smoking,” said lead author Nancy A. Rigotti, MD. “Even before the pandemic, tobacco smoking was the leading preventable cause of death in the United States. COVID-19 has given smokers yet another good reason to stop smoking.”
This creates an opportunity for physicians to preach the gospel to smokers about their vulnerability to respiratory disease in hopes of getting them to quit for good. We just wish the same could be said for all of our excessive pandemic online shopping.
3,000 years and just one pair of genomes to wear
Men and women are different. We’ll give you a moment to pick your jaw off the ground.
It makes sense though, the sexes being different, especially when you look at the broader animal kingdom. The males and females of many species are slightly different when it comes to size and shape, but there’s a big question that literally only anthropologists have asked: Were human males and females more different in the past than they are today?
To be more specific, some scientists believe that males and females grew more similar when humans shifted from a hunter-gatherer lifestyle to a farming-based lifestyle, as agriculture encouraged a more equitable division of labor. Others believe that the differences come down to random chance.
Researchers from Penn State University analyzed genomic data from over 350,000 males and females stored in the UK Biobank and looked at the recent (within the last ~3,000 years; post-agriculture adoption in Britain) evolutionary histories of these loci. Height, body mass, hip circumference, body fat percentage, and waist circumference were analyzed, and while there were thousands of differences in the genomes, only one trait occurred more frequently during that time period: Females gained a significantly higher body fat content than males.
It’s a sad day then for the millions of people who were big fans of the “farming caused men and women to become more similar” theory. Count the LOTME crew among them. Be honest: Wouldn’t life be so much simpler if men and women were exactly the same? Just think about it, no more arguments about leaving the toilet seat up. It’d be worth it just for that.
Proteins don’t lie
Research published in Open Biology shows that the human brain contains 14,315 different proteins. The team conducting that study wanted to find out which organ was the most similar to the old brain box, so they did protein counts for the 32 other major tissue types, including heart, salivary gland, lung, spleen, and endometrium.
The tissue with the most proteins in common with the center of human intelligence? You’re thinking it has to be colon at this point, right? We were sure it was going to be colon, but it’s not.
The winner, with 13,442 shared proteins, is the testes. The testes have 15,687 proteins, of which 85.7% are shared with the brain. The researchers, sadly, did not provide protein counts for the other tissue types, but we bet colon was a close second.
Dreaming about COVID?
We thought we were the only ones who have been having crazy dreams lately. Each one seems crazier and more vivid than the one before. Have you been having weird dreams lately?
This is likely your brain’s coping mechanism to handle your pandemic stress, according to Dr. Erik Hoel of Tufts University. Dreams that are crazy and scary might make real life seem lighter and simpler. He calls it the “overfitted brain hypothesis.”
“It is their very strangeness that gives them their biological function,” Dr. Hoel said. It literally makes you feel like COVID-19 and lockdowns aren’t as scary as they seem.
We always knew our minds were powerful things. Apparently, your brain gets tired of everyday familiarity just like you do, and it creates crazy dreams to keep things interesting.
Just remember: That recurring dream that you’re back in college and missing 10 assignments is there to help you, not scare you! Even though it is pretty scary.
Clinical Edge Journal Scan Commentary: AML June 2021
Several studies published this month have shed some more light on the treatment and prognosis of certain molecular subgroups such as FLT3, spliceasome mutations and IDH mutation. A study from MD Anderson Cancer Center (MDACC) demonstrated the efficacy of FLT33 inhibitors in the treatment of patients with very low allelic burden. Patients who recived both a FMS-like tyrosine kinase inhibitor (FLT3i) during induction and an allogeneic HCT at time of first remission had better 5 year overall survival (OS) (100%) than those who received neither (27%). The 5-year OS rate among patients who did not receive FLT3i-based induction but underwent allo-SCT in CR1 was 71%. None of the patients who received a FLT3i had detectable FLT3 at time of relapse, compared to 67% for those who did not. The main weakness of the study is the small number of patients (total of 50 patients). This study however does provide some provocative data regarding the validity of the FLT3 allelic ratio in decisions regarding treatment and prognosis. The results of this study need to be validated in a larger cohort of patients.
A study by Lachowiez et al, further refined the prognostic significance of spliceosome mutations in patients treated with ven + HMA. Of the 119 patients, 39 had spliceosome mutations. The overall response and prognosis was not different between patients with and without spliceosome mutations. However, SRSF2 was associated with IDH2 mutation and the median OS was not reached vs, patient with U2AF1 who had a higher association with RAS mutations (OS 8 months). The prognostic role of standard cytotoxic chemotherapy in improving the outcome of patients with an IDH mutation was studied by the group. In a re-evaluation of the randomized trial comparing the addition of fludarabine or cladrbaine to standard 7 +3, 50 patients with an IDH mutation were evaluated. Patients with an IDH mutation who received cladribine. IDH2 mutation had a positive impact on OS in patients treated with DAC regimen (54% vs. 33%; P = .0087) but not in those treated with daunorubicin+cytarabine (DA; 21% vs. 23%; P = .22) regimen. Moreover, DAC induction was independently associated with a reduced risk for death when the observations were censored at allogeneic hematopoietic stem cell transplant (hazard ratio, 0.21; P = .02).
Although hypomethylating agents + venetoclax (HMA +ven) have shown efficacy in multiple patient subgroups, the outcome of patients with post-MPN acute myeloid leukemia (AML) remains poor even with HMA + ven. In a retrospective study, 31 patients with post MPN AML were treated with HMA + ven. The overall survival for patients with relapsed post MPN AML was 3 months with none of the 9 patients achieving a CR/CRi. As for the patients with previously untreated post MPN-AML, the median survival was still poor at 8 months with 43% achieving CR/CRi. This study highlights the large unmet need in this patient population. In comparison, in a retrospective study by Piccini et al, of the 47 patients with relapsed/refractory (R/R) AML treated with venetoclax based regimens the composite CR rate was 55%. These outcomes are similar to other studies of venetoclax based regimens in patients with R/R AML.
In a retrospective study from the European Society for Blood and Marrow Transplantation (EBMT) registry, the outcomes of patients receiving mismatched unrelated donor (MMUD) with post transplant cyclophosphamide (PTCy) had better outcomes compared to patients receiving cord blood transplantation (CBT). The 2 year leukemia free survival (LFS) for CBT vs MMUD was 42.8% vs 60.5% and OS was 46.85 vs 62.8%. This retrospective study demonstrated that PTCy can improve the outcomes of patients receiving of MMUD (Dholaria B et al. J Hematol Oncol. 2021 May).
Several studies published this month have shed some more light on the treatment and prognosis of certain molecular subgroups such as FLT3, spliceasome mutations and IDH mutation. A study from MD Anderson Cancer Center (MDACC) demonstrated the efficacy of FLT33 inhibitors in the treatment of patients with very low allelic burden. Patients who recived both a FMS-like tyrosine kinase inhibitor (FLT3i) during induction and an allogeneic HCT at time of first remission had better 5 year overall survival (OS) (100%) than those who received neither (27%). The 5-year OS rate among patients who did not receive FLT3i-based induction but underwent allo-SCT in CR1 was 71%. None of the patients who received a FLT3i had detectable FLT3 at time of relapse, compared to 67% for those who did not. The main weakness of the study is the small number of patients (total of 50 patients). This study however does provide some provocative data regarding the validity of the FLT3 allelic ratio in decisions regarding treatment and prognosis. The results of this study need to be validated in a larger cohort of patients.
A study by Lachowiez et al, further refined the prognostic significance of spliceosome mutations in patients treated with ven + HMA. Of the 119 patients, 39 had spliceosome mutations. The overall response and prognosis was not different between patients with and without spliceosome mutations. However, SRSF2 was associated with IDH2 mutation and the median OS was not reached vs, patient with U2AF1 who had a higher association with RAS mutations (OS 8 months). The prognostic role of standard cytotoxic chemotherapy in improving the outcome of patients with an IDH mutation was studied by the group. In a re-evaluation of the randomized trial comparing the addition of fludarabine or cladrbaine to standard 7 +3, 50 patients with an IDH mutation were evaluated. Patients with an IDH mutation who received cladribine. IDH2 mutation had a positive impact on OS in patients treated with DAC regimen (54% vs. 33%; P = .0087) but not in those treated with daunorubicin+cytarabine (DA; 21% vs. 23%; P = .22) regimen. Moreover, DAC induction was independently associated with a reduced risk for death when the observations were censored at allogeneic hematopoietic stem cell transplant (hazard ratio, 0.21; P = .02).
Although hypomethylating agents + venetoclax (HMA +ven) have shown efficacy in multiple patient subgroups, the outcome of patients with post-MPN acute myeloid leukemia (AML) remains poor even with HMA + ven. In a retrospective study, 31 patients with post MPN AML were treated with HMA + ven. The overall survival for patients with relapsed post MPN AML was 3 months with none of the 9 patients achieving a CR/CRi. As for the patients with previously untreated post MPN-AML, the median survival was still poor at 8 months with 43% achieving CR/CRi. This study highlights the large unmet need in this patient population. In comparison, in a retrospective study by Piccini et al, of the 47 patients with relapsed/refractory (R/R) AML treated with venetoclax based regimens the composite CR rate was 55%. These outcomes are similar to other studies of venetoclax based regimens in patients with R/R AML.
In a retrospective study from the European Society for Blood and Marrow Transplantation (EBMT) registry, the outcomes of patients receiving mismatched unrelated donor (MMUD) with post transplant cyclophosphamide (PTCy) had better outcomes compared to patients receiving cord blood transplantation (CBT). The 2 year leukemia free survival (LFS) for CBT vs MMUD was 42.8% vs 60.5% and OS was 46.85 vs 62.8%. This retrospective study demonstrated that PTCy can improve the outcomes of patients receiving of MMUD (Dholaria B et al. J Hematol Oncol. 2021 May).
Several studies published this month have shed some more light on the treatment and prognosis of certain molecular subgroups such as FLT3, spliceasome mutations and IDH mutation. A study from MD Anderson Cancer Center (MDACC) demonstrated the efficacy of FLT33 inhibitors in the treatment of patients with very low allelic burden. Patients who recived both a FMS-like tyrosine kinase inhibitor (FLT3i) during induction and an allogeneic HCT at time of first remission had better 5 year overall survival (OS) (100%) than those who received neither (27%). The 5-year OS rate among patients who did not receive FLT3i-based induction but underwent allo-SCT in CR1 was 71%. None of the patients who received a FLT3i had detectable FLT3 at time of relapse, compared to 67% for those who did not. The main weakness of the study is the small number of patients (total of 50 patients). This study however does provide some provocative data regarding the validity of the FLT3 allelic ratio in decisions regarding treatment and prognosis. The results of this study need to be validated in a larger cohort of patients.
A study by Lachowiez et al, further refined the prognostic significance of spliceosome mutations in patients treated with ven + HMA. Of the 119 patients, 39 had spliceosome mutations. The overall response and prognosis was not different between patients with and without spliceosome mutations. However, SRSF2 was associated with IDH2 mutation and the median OS was not reached vs, patient with U2AF1 who had a higher association with RAS mutations (OS 8 months). The prognostic role of standard cytotoxic chemotherapy in improving the outcome of patients with an IDH mutation was studied by the group. In a re-evaluation of the randomized trial comparing the addition of fludarabine or cladrbaine to standard 7 +3, 50 patients with an IDH mutation were evaluated. Patients with an IDH mutation who received cladribine. IDH2 mutation had a positive impact on OS in patients treated with DAC regimen (54% vs. 33%; P = .0087) but not in those treated with daunorubicin+cytarabine (DA; 21% vs. 23%; P = .22) regimen. Moreover, DAC induction was independently associated with a reduced risk for death when the observations were censored at allogeneic hematopoietic stem cell transplant (hazard ratio, 0.21; P = .02).
Although hypomethylating agents + venetoclax (HMA +ven) have shown efficacy in multiple patient subgroups, the outcome of patients with post-MPN acute myeloid leukemia (AML) remains poor even with HMA + ven. In a retrospective study, 31 patients with post MPN AML were treated with HMA + ven. The overall survival for patients with relapsed post MPN AML was 3 months with none of the 9 patients achieving a CR/CRi. As for the patients with previously untreated post MPN-AML, the median survival was still poor at 8 months with 43% achieving CR/CRi. This study highlights the large unmet need in this patient population. In comparison, in a retrospective study by Piccini et al, of the 47 patients with relapsed/refractory (R/R) AML treated with venetoclax based regimens the composite CR rate was 55%. These outcomes are similar to other studies of venetoclax based regimens in patients with R/R AML.
In a retrospective study from the European Society for Blood and Marrow Transplantation (EBMT) registry, the outcomes of patients receiving mismatched unrelated donor (MMUD) with post transplant cyclophosphamide (PTCy) had better outcomes compared to patients receiving cord blood transplantation (CBT). The 2 year leukemia free survival (LFS) for CBT vs MMUD was 42.8% vs 60.5% and OS was 46.85 vs 62.8%. This retrospective study demonstrated that PTCy can improve the outcomes of patients receiving of MMUD (Dholaria B et al. J Hematol Oncol. 2021 May).
Treating Hepatitis C Virus Reinfection With 8 Weeks of Ledipasvir/Sofosbuvir Achieves Sustained Virologic Response
Three patients reinfected with hepatitis C virus after a sustained virologic response were considered treatment naïve and treated with a short-course direct acting antiviral regimen.
To decrease the incidence and prevalence of hepatitis C virus (HCV) in the United States, hepatology experts, public health officials, and patient advocates agree that linkage to care is essential for treatment of people who inject drugs (PWID). The most recent surveillance report from the Centers for Disease Control and Prevention (CDC) estimates that injection drug use accounts for the transmission of approximately 72% of new HCV infections.1,2
Although recent studies of direct-acting antiviral (DAA) agents have not been designed to investigate the long-term rates of reinfection in this population, various population-based studies in multiple countries have attempted to describe the rate of reinfection for this cohort.3-7 This rate varies widely based on the defined population of PWID, definition of reinfection, and the prevalence of HCV in a given PWID population. However, studies have consistently shown a relatively low historic rate of reinfection, which varies from 1 to 5 per 100 person-years in patients who have ever injected drugs, to 3 to 33 per 100 person-years in patients who continue injection drug use (IDU). Higher rates are found in those who engage in high-risk behaviors such as needle sharing.3-7 Yet, the US opioid crisis is attributable to a recent rise in both overall incidence and reinfections, highlighting the importance of determining the best treatment strategy for those who become reinfected.1
Current HCV guidelines from the American Association for the Study of Liver Diseases AASLD) and Infectious Diseases Society of America (IDSA) encourage access to retreatment for PWID who become reinfected, stating that new reinfections should follow treatment-naïve therapy recommendations.8 However, to date this recommendation has not been validated by published clinical trials or patient case reports. This is likely due in part both to the small number of reinfections among PWID requiring retreatment and barriers to payment for treatment, particularly for individuals with substance use disorders.9 While this recommendation can be found under the key population section for the “Identification and Management of HCV in People Who Inject Drugs,” health care providers (HCPs) may easily miss this statement if they alternatively refer to the “Treatment-Experienced” section that recommends escalation to either sofosbuvir/velpatasvir/voxilaprevir or glecaprevir/pibrentasvir in patients who are NS5A inhibitor DAA-experienced.8 Anecdotally, the first instinct for many HCPs when considering a treatment regimen for a reinfected patient is to refer to treatment-experienced regimen recommendations rather than appreciating the reinfected virus to be treatment naïve.
A treatment-escalation approach could have the consequence of limiting the number of times a patient could undergo treatment on successive reinfections. Additionally, these retreatment regimens often are more expensive, resulting in further cost barriers for payors approving retreatment for individuals with HCV reinfection. In contrast, demonstrating efficacy of a less costly short-course regimen would support increased access to initial and retreatment courses for PWID. The implications of enabling improved access to care is essential in the setting of the ongoing opioid epidemic in the United States.
Given the perspective that the virus should be considered treatment naïve for patients who become reinfected, we describe here 3 cases of patients previously achieving sustained virologic response (SVR) being retreated with the cost-effective 8-week regimen of ledipasvir/sofosbuvir following reinfection.
Case Reports
Case 1
A 59-year-old male presented for his third treatment course for HCV genotype 1a. The patient initially underwent 76 weeks of interferon-based HCV treatment in 2007 and 2008, from which he was determined to have achieved SVR in 24 weeks (SVR24) in April 2009. His viral load remained undetected through February 2010 but subsequently had detectable virus again in 2011 following relapsed use of alcohol, cocaine, and injection drugs. The patient elected to await approval of DAAs and eventually completed an 8-week regimen of ledipasvir/sofosbuvir from May to July 2016, achieving SVR24 in December 2016. The patient’s viral load was rechecked in October 2018 and he was again viremic following recent IDU, suggesting a second reinfection.
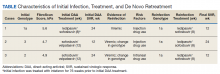
In preparation for his third HCV treatment, the patient was included in shared decision making to consider retreating his de novo infection as treatment naïve to provide a briefer (ie, 8 weeks) and more cost-effective treatment given his low likelihood of advanced fibrotic liver disease—his FibroScan score was 6.5 kPa, whereas scores ≥ 12.5 kPa in patients with chronic HCV suggest a higher likelihood of cirrhosis.10 At week 4, the patient’s viral load was undetected, he completed his 8-week regimen of ledipasvir/sofosbuvir as planned and achieved SVR12 (Table). He had reported excellent adherence throughout treatment with assistance of a pill box and validated by a reported pill count.
Case 2
A 32-year-old male presented with HCV genotype 1a. Like case 1, this patient had a low FibroScan score of 4.7 kPa. He was previously infected with genotype 3 and completed a 12-week course of sofosbuvir/velpatasvir in November 2016. He achieved SVR12 as evidenced by an undetected viral load in February 2017 despite questionable adherence throughout and relapsed use of heroin by the end of his regimen. He continued intermittent IDU and presented in October 2018 with a detectable viral load, now with genotype 1a. The patient similarly agreed to undergo an 8-week regimen of ledipasvir/sofosbuvir, considering his de novo infection to be treatment naïve. His viral load at treatment week 3 was quantitatively negative while qualitatively detectable at < 15 U/mL. He completed his treatment course in March 2019 and was determined to have achieved SVR24 in September 2019.
Case 3
A 51-year-old male presented with a history of HCV genotype 1a and a low FibroScan score (4.9 kPa ). The patient was previously infected with genotype 2 and had achieved SVR24 following a 12-week regimen of sofosbuvir/velpatasvir in 2017. The patient subsequently was reinfected with genotype 1a and completed an 8-week course of ledipasvir/sofosbuvir in May 2019. The patient had his SVR12 lab drawn 9 days early and was undetectable at that time. He reported 0 missed doses during treatment and achieved an undetected viral load by treatment week 4.
Discussion
We demonstrate that HCV reinfection after treatment with previous interferon and/or DAA-based regimens can be treated with less costly 8-week treatment regimens. Current guidelines include a statement allowing for reinfected patients to follow initial treatment guidelines, but this statement has previously lacked published evidence and may be overlooked by HCPs who refer to recommendations for treatment-experienced patients. Given the increasing likelihood of HCPs encountering patients who have become reinfected with HCV after achieving SVR from a DAA regimen, further delineation may be needed in the recommendations for treatment-experienced patients to highlight the important nuance of recognizing that reinfections should follow initial treatment guidance.
While all 3 of these cases met criteria for the least costly and simplest 1 pill once daily 8-week regimen of ledipasvir/sofosbuvir, patients requiring retreatment with alternative genotypes or evidence of advanced fibrotic liver disease could benefit from a similar approach of using the least expensive and/or shortest duration regimen for which they meet eligibility. With this approach, coverage could be further expanded to the PWID population to help limit HCV transmission amid the opioid crisis.1
Studies have established that PWID are able to achieve similar SVR efficacy rates similar to that of the general population when treated in the setting of an interdisciplinary treatment team that offers collaborative management of complex psychosocial comorbidities and harm reduction strategies.11,12 These integrative patient-centric strategies may include personalized behavioral health pretreatment evaluations, access to substance use treatment, harm reduction counseling, needle exchange programs, and close follow-up by a case manager.2,13 Current DAA regimens combined with 1 or more of these strategies have demonstrated SVR12 rates of 90 to 95% for initial treatment regimens.11 These high SVR12 rates were even achieved in a recent study in which 74% (76/103) of participants had self-reported IDU within 30 days of HCV treatment start and similar IDU rates throughout treatment.12 A meta-analysis, including real-world studies of DAA treatment outcomes yielded a pooled SVR of 88% (95% CI, 83‐92%) for recent PWID and 91% (95% CI, 88‐95%) for individuals using opiate substitution therapy (OST).14 Additionally, linking PWID with OST also reduces risk for reinfection.14,15
For any patient with detectable HCV after completing the initial DAA regimen, it is important to distinguish between relapse and reinfection. SVR12 is generally synonymous with a clinical cure. Patients with ongoing risk factors posttreatment should continue to have their HCV viral load monitored for evidence of reinfection. Patients without known risk factors may benefit from repeat viral load only if there is clinical concern for reinfection, for example, a rise in liver enzymes.
We have shown that patients with ongoing risk factors who are reinfected can be treated successfully with cost-effective 8-week regimens. For comparison this 8-week regimen of ledipasvir/sofosbuvir has an average wholesale price (AWP) of $28,800, while alternative regimens approved for treatment-naïve patients vary in AWP from $31,680 to $43,200, and regimens approved for retreatment of DAA failures have an AWP as high as $89,712.
An 8-week treatment regimen for both initial and reinfection regimens affords many advantages in medication adherence and both medication and provider resource cost-effectiveness. First, new HCV reinfections are disproportionally younger individuals often with complex psychosocial issues that impact retention in treatment. An 8-week course of treatment can be initiated concurrently with substance abuse treatment programs, including intensive outpatient programs and residential treatment programs that are usually at least 28 days. Many of these programs provide aftercare options that would extend the entire course of treatment. These opportunities afford individuals to receive HCV treatment in a setting that supports medication adherence, sobriety efforts, and education on harm reduction to reduce risk for reinfection.
Finally, statistical models indicate eradication of HCV will require scaling up the treatment of PWID in conjunction with harm reduction strategies such as OST and needle exchange programs.16 In contrast, there are low risks associated with retreatment given these medications are well-tolerated, treatment of PWID lowers the risk of further HCV transmission, and the understanding of these reinfections being treatment naïve disavows concerns of these patients having resistance to regimens that cleared their prior infections. The opportunity to provide retreatment without escalating regimen complexity or cost increases access to care for a vulnerable population while aiding in the eradication of HCV.
1. Centers for Disease Control and Prevention. Viral Hepatitis Surveillance - United States, 2018. Updated August 28, 2020. Accessed May 18, 2021. https://www.cdc.gov/hepatitis/statistics/2018surveillance/HepC.htm 2. Grebely J, Robaeys G, Bruggmann P, et al; International Network for Hepatitis in Substance Users. Recommendations for the management of hepatitis C virus infection among people who inject drugs. Int J Drug Policy. 2015;26(10):1028-1038. doi:10.1016/j.drugpo.2015.07.005
3. Marco A, Esteban JI, Solé C, et al. Hepatitis C virus reinfection among prisoners with sustained virological response after treatment for chronic hepatitis C. J Hepatol. 2013;59(1):45-51. doi:10.1016/j.jhep.2013.03.008
4. Midgard H, Bjøro B, Mæland A, et al. Hepatitis C reinfection after sustained virological response. J Hepatol. 2016;64(5):1020-1026. doi:10.1016/j.jhep.2016.01.001
5. Currie SL, Ryan JC, Tracy D, et al. A prospective study to examine persistent HCV reinfection in injection drug users who have previously cleared the virus [published correction appears in Drug Alcohol Depend. 2008 Jul;96(1-2):192]. Drug Alcohol Depend. 2008;93(1-2):148-154. doi:10.1016/j.drugalcdep.2007.09.011
6. Grady BP, Vanhommerig JW, Schinkel J, et al. Low incidence of reinfection with the hepatitis C virus following treatment in active drug users in Amsterdam. Eur J Gastroenterol Hepatol. 2012;24(11):1302-1307. doi:10.1097/MEG.0b013e32835702a8
7. Grebely J, Pham ST, Matthews GV, et al; ATAHC Study Group. Hepatitis C virus reinfection and superinfection among treated and untreated participants with recent infection. Hepatology. 2012;55(4):1058-1069. doi:10.1002/hep.24754
8. American Association for the Study of Liver Diseases and the Infectious Diseases Society of America. HCV Guidance: Recommendations for Testing, Managing, and Treating Hepatitis C. Accessed May 26, 2021. https://www.hcvguidelines.org
9. National Viral Hepatitis Roundtable, Center for Health Law and Policy Innovation, Harvard Law School. Hepatitis C: The State of Medicaid Access. 2017 National Summary Report. Updated October 23, 2017. Accessed May 26, 2021. https://hepcstage.wpengine.com/wp-content/uploads/2017/10/State-of-HepC_2017_FINAL.pdf
10. Singh S, Muir AJ, Dieterich DT, Falck-Ytter YT. American Gastroenterological Association Institute technical review on the role of elastography in chronic liver diseases. Gastroenterology. 2017;152(6):1544-1577. doi:10.1053/j.gastro.2017.03.016
11. Dore GJ, Altice F, Litwin AH, et al; C-EDGE CO-STAR Study Group. Elbasvir-grazoprevir to treat hepatitis C virus infection in persons receiving opioid agonist therapy: a randomized trial. Ann Intern Med. 2016;165(9):625-634. doi:10.7326/M16-0816
12. Grebely J, Dalgard O, Conway B, et al; SIMPLIFY Study Group. Sofosbuvir and velpatasvir for hepatitis C virus infection in people with recent injection drug use (SIMPLIFY): an open-label, single-arm, phase 4, multicentre trial. Lancet Gastroenterol Hepatol. 2018;3(3):153-161. doi:10.1016/S2468-1253(17)30404-1
13. Cos TA, Bartholomew TS, Huynh, KJ. Role of behavioral health providers in treating hepatitis C. Professional Psychol Res Pract. 2019;50(4):246–254. doi:10.1037/pro0000243
14. Latham NH, Doyle JS, Palmer AY, et al. Staying hepatitis C negative: a systematic review and meta-analysis of cure and reinfection in people who inject drugs. Liver Int. 2019;39(12):2244-2260. doi:10.1111/liv.14152
15. Platt L, Minozzi S, Reed J, et al. Needle syringe programmes and opioid substitution therapy for preventing hepatitis C transmission in people who inject drugs. Cochrane Database Syst Rev. 2017;9(9):CD012021. Published 2017 Sep 18. doi:10.1002/14651858.CD012021.pub2
16. Fraser H, Martin NK, Brummer-Korvenkontio H, et al. Model projections on the impact of HCV treatment in the prevention of HCV transmission among people who inject drugs in Europe. J Hepatol. 2018;68(3):402-411. doi:10.1016/j.jhep.2017.10.010
Three patients reinfected with hepatitis C virus after a sustained virologic response were considered treatment naïve and treated with a short-course direct acting antiviral regimen.
Three patients reinfected with hepatitis C virus after a sustained virologic response were considered treatment naïve and treated with a short-course direct acting antiviral regimen.
To decrease the incidence and prevalence of hepatitis C virus (HCV) in the United States, hepatology experts, public health officials, and patient advocates agree that linkage to care is essential for treatment of people who inject drugs (PWID). The most recent surveillance report from the Centers for Disease Control and Prevention (CDC) estimates that injection drug use accounts for the transmission of approximately 72% of new HCV infections.1,2
Although recent studies of direct-acting antiviral (DAA) agents have not been designed to investigate the long-term rates of reinfection in this population, various population-based studies in multiple countries have attempted to describe the rate of reinfection for this cohort.3-7 This rate varies widely based on the defined population of PWID, definition of reinfection, and the prevalence of HCV in a given PWID population. However, studies have consistently shown a relatively low historic rate of reinfection, which varies from 1 to 5 per 100 person-years in patients who have ever injected drugs, to 3 to 33 per 100 person-years in patients who continue injection drug use (IDU). Higher rates are found in those who engage in high-risk behaviors such as needle sharing.3-7 Yet, the US opioid crisis is attributable to a recent rise in both overall incidence and reinfections, highlighting the importance of determining the best treatment strategy for those who become reinfected.1
Current HCV guidelines from the American Association for the Study of Liver Diseases AASLD) and Infectious Diseases Society of America (IDSA) encourage access to retreatment for PWID who become reinfected, stating that new reinfections should follow treatment-naïve therapy recommendations.8 However, to date this recommendation has not been validated by published clinical trials or patient case reports. This is likely due in part both to the small number of reinfections among PWID requiring retreatment and barriers to payment for treatment, particularly for individuals with substance use disorders.9 While this recommendation can be found under the key population section for the “Identification and Management of HCV in People Who Inject Drugs,” health care providers (HCPs) may easily miss this statement if they alternatively refer to the “Treatment-Experienced” section that recommends escalation to either sofosbuvir/velpatasvir/voxilaprevir or glecaprevir/pibrentasvir in patients who are NS5A inhibitor DAA-experienced.8 Anecdotally, the first instinct for many HCPs when considering a treatment regimen for a reinfected patient is to refer to treatment-experienced regimen recommendations rather than appreciating the reinfected virus to be treatment naïve.
A treatment-escalation approach could have the consequence of limiting the number of times a patient could undergo treatment on successive reinfections. Additionally, these retreatment regimens often are more expensive, resulting in further cost barriers for payors approving retreatment for individuals with HCV reinfection. In contrast, demonstrating efficacy of a less costly short-course regimen would support increased access to initial and retreatment courses for PWID. The implications of enabling improved access to care is essential in the setting of the ongoing opioid epidemic in the United States.
Given the perspective that the virus should be considered treatment naïve for patients who become reinfected, we describe here 3 cases of patients previously achieving sustained virologic response (SVR) being retreated with the cost-effective 8-week regimen of ledipasvir/sofosbuvir following reinfection.
Case Reports
Case 1
A 59-year-old male presented for his third treatment course for HCV genotype 1a. The patient initially underwent 76 weeks of interferon-based HCV treatment in 2007 and 2008, from which he was determined to have achieved SVR in 24 weeks (SVR24) in April 2009. His viral load remained undetected through February 2010 but subsequently had detectable virus again in 2011 following relapsed use of alcohol, cocaine, and injection drugs. The patient elected to await approval of DAAs and eventually completed an 8-week regimen of ledipasvir/sofosbuvir from May to July 2016, achieving SVR24 in December 2016. The patient’s viral load was rechecked in October 2018 and he was again viremic following recent IDU, suggesting a second reinfection.
In preparation for his third HCV treatment, the patient was included in shared decision making to consider retreating his de novo infection as treatment naïve to provide a briefer (ie, 8 weeks) and more cost-effective treatment given his low likelihood of advanced fibrotic liver disease—his FibroScan score was 6.5 kPa, whereas scores ≥ 12.5 kPa in patients with chronic HCV suggest a higher likelihood of cirrhosis.10 At week 4, the patient’s viral load was undetected, he completed his 8-week regimen of ledipasvir/sofosbuvir as planned and achieved SVR12 (Table). He had reported excellent adherence throughout treatment with assistance of a pill box and validated by a reported pill count.
Case 2
A 32-year-old male presented with HCV genotype 1a. Like case 1, this patient had a low FibroScan score of 4.7 kPa. He was previously infected with genotype 3 and completed a 12-week course of sofosbuvir/velpatasvir in November 2016. He achieved SVR12 as evidenced by an undetected viral load in February 2017 despite questionable adherence throughout and relapsed use of heroin by the end of his regimen. He continued intermittent IDU and presented in October 2018 with a detectable viral load, now with genotype 1a. The patient similarly agreed to undergo an 8-week regimen of ledipasvir/sofosbuvir, considering his de novo infection to be treatment naïve. His viral load at treatment week 3 was quantitatively negative while qualitatively detectable at < 15 U/mL. He completed his treatment course in March 2019 and was determined to have achieved SVR24 in September 2019.
Case 3
A 51-year-old male presented with a history of HCV genotype 1a and a low FibroScan score (4.9 kPa ). The patient was previously infected with genotype 2 and had achieved SVR24 following a 12-week regimen of sofosbuvir/velpatasvir in 2017. The patient subsequently was reinfected with genotype 1a and completed an 8-week course of ledipasvir/sofosbuvir in May 2019. The patient had his SVR12 lab drawn 9 days early and was undetectable at that time. He reported 0 missed doses during treatment and achieved an undetected viral load by treatment week 4.
Discussion
We demonstrate that HCV reinfection after treatment with previous interferon and/or DAA-based regimens can be treated with less costly 8-week treatment regimens. Current guidelines include a statement allowing for reinfected patients to follow initial treatment guidelines, but this statement has previously lacked published evidence and may be overlooked by HCPs who refer to recommendations for treatment-experienced patients. Given the increasing likelihood of HCPs encountering patients who have become reinfected with HCV after achieving SVR from a DAA regimen, further delineation may be needed in the recommendations for treatment-experienced patients to highlight the important nuance of recognizing that reinfections should follow initial treatment guidance.
While all 3 of these cases met criteria for the least costly and simplest 1 pill once daily 8-week regimen of ledipasvir/sofosbuvir, patients requiring retreatment with alternative genotypes or evidence of advanced fibrotic liver disease could benefit from a similar approach of using the least expensive and/or shortest duration regimen for which they meet eligibility. With this approach, coverage could be further expanded to the PWID population to help limit HCV transmission amid the opioid crisis.1
Studies have established that PWID are able to achieve similar SVR efficacy rates similar to that of the general population when treated in the setting of an interdisciplinary treatment team that offers collaborative management of complex psychosocial comorbidities and harm reduction strategies.11,12 These integrative patient-centric strategies may include personalized behavioral health pretreatment evaluations, access to substance use treatment, harm reduction counseling, needle exchange programs, and close follow-up by a case manager.2,13 Current DAA regimens combined with 1 or more of these strategies have demonstrated SVR12 rates of 90 to 95% for initial treatment regimens.11 These high SVR12 rates were even achieved in a recent study in which 74% (76/103) of participants had self-reported IDU within 30 days of HCV treatment start and similar IDU rates throughout treatment.12 A meta-analysis, including real-world studies of DAA treatment outcomes yielded a pooled SVR of 88% (95% CI, 83‐92%) for recent PWID and 91% (95% CI, 88‐95%) for individuals using opiate substitution therapy (OST).14 Additionally, linking PWID with OST also reduces risk for reinfection.14,15
For any patient with detectable HCV after completing the initial DAA regimen, it is important to distinguish between relapse and reinfection. SVR12 is generally synonymous with a clinical cure. Patients with ongoing risk factors posttreatment should continue to have their HCV viral load monitored for evidence of reinfection. Patients without known risk factors may benefit from repeat viral load only if there is clinical concern for reinfection, for example, a rise in liver enzymes.
We have shown that patients with ongoing risk factors who are reinfected can be treated successfully with cost-effective 8-week regimens. For comparison this 8-week regimen of ledipasvir/sofosbuvir has an average wholesale price (AWP) of $28,800, while alternative regimens approved for treatment-naïve patients vary in AWP from $31,680 to $43,200, and regimens approved for retreatment of DAA failures have an AWP as high as $89,712.
An 8-week treatment regimen for both initial and reinfection regimens affords many advantages in medication adherence and both medication and provider resource cost-effectiveness. First, new HCV reinfections are disproportionally younger individuals often with complex psychosocial issues that impact retention in treatment. An 8-week course of treatment can be initiated concurrently with substance abuse treatment programs, including intensive outpatient programs and residential treatment programs that are usually at least 28 days. Many of these programs provide aftercare options that would extend the entire course of treatment. These opportunities afford individuals to receive HCV treatment in a setting that supports medication adherence, sobriety efforts, and education on harm reduction to reduce risk for reinfection.
Finally, statistical models indicate eradication of HCV will require scaling up the treatment of PWID in conjunction with harm reduction strategies such as OST and needle exchange programs.16 In contrast, there are low risks associated with retreatment given these medications are well-tolerated, treatment of PWID lowers the risk of further HCV transmission, and the understanding of these reinfections being treatment naïve disavows concerns of these patients having resistance to regimens that cleared their prior infections. The opportunity to provide retreatment without escalating regimen complexity or cost increases access to care for a vulnerable population while aiding in the eradication of HCV.
To decrease the incidence and prevalence of hepatitis C virus (HCV) in the United States, hepatology experts, public health officials, and patient advocates agree that linkage to care is essential for treatment of people who inject drugs (PWID). The most recent surveillance report from the Centers for Disease Control and Prevention (CDC) estimates that injection drug use accounts for the transmission of approximately 72% of new HCV infections.1,2
Although recent studies of direct-acting antiviral (DAA) agents have not been designed to investigate the long-term rates of reinfection in this population, various population-based studies in multiple countries have attempted to describe the rate of reinfection for this cohort.3-7 This rate varies widely based on the defined population of PWID, definition of reinfection, and the prevalence of HCV in a given PWID population. However, studies have consistently shown a relatively low historic rate of reinfection, which varies from 1 to 5 per 100 person-years in patients who have ever injected drugs, to 3 to 33 per 100 person-years in patients who continue injection drug use (IDU). Higher rates are found in those who engage in high-risk behaviors such as needle sharing.3-7 Yet, the US opioid crisis is attributable to a recent rise in both overall incidence and reinfections, highlighting the importance of determining the best treatment strategy for those who become reinfected.1
Current HCV guidelines from the American Association for the Study of Liver Diseases AASLD) and Infectious Diseases Society of America (IDSA) encourage access to retreatment for PWID who become reinfected, stating that new reinfections should follow treatment-naïve therapy recommendations.8 However, to date this recommendation has not been validated by published clinical trials or patient case reports. This is likely due in part both to the small number of reinfections among PWID requiring retreatment and barriers to payment for treatment, particularly for individuals with substance use disorders.9 While this recommendation can be found under the key population section for the “Identification and Management of HCV in People Who Inject Drugs,” health care providers (HCPs) may easily miss this statement if they alternatively refer to the “Treatment-Experienced” section that recommends escalation to either sofosbuvir/velpatasvir/voxilaprevir or glecaprevir/pibrentasvir in patients who are NS5A inhibitor DAA-experienced.8 Anecdotally, the first instinct for many HCPs when considering a treatment regimen for a reinfected patient is to refer to treatment-experienced regimen recommendations rather than appreciating the reinfected virus to be treatment naïve.
A treatment-escalation approach could have the consequence of limiting the number of times a patient could undergo treatment on successive reinfections. Additionally, these retreatment regimens often are more expensive, resulting in further cost barriers for payors approving retreatment for individuals with HCV reinfection. In contrast, demonstrating efficacy of a less costly short-course regimen would support increased access to initial and retreatment courses for PWID. The implications of enabling improved access to care is essential in the setting of the ongoing opioid epidemic in the United States.
Given the perspective that the virus should be considered treatment naïve for patients who become reinfected, we describe here 3 cases of patients previously achieving sustained virologic response (SVR) being retreated with the cost-effective 8-week regimen of ledipasvir/sofosbuvir following reinfection.
Case Reports
Case 1
A 59-year-old male presented for his third treatment course for HCV genotype 1a. The patient initially underwent 76 weeks of interferon-based HCV treatment in 2007 and 2008, from which he was determined to have achieved SVR in 24 weeks (SVR24) in April 2009. His viral load remained undetected through February 2010 but subsequently had detectable virus again in 2011 following relapsed use of alcohol, cocaine, and injection drugs. The patient elected to await approval of DAAs and eventually completed an 8-week regimen of ledipasvir/sofosbuvir from May to July 2016, achieving SVR24 in December 2016. The patient’s viral load was rechecked in October 2018 and he was again viremic following recent IDU, suggesting a second reinfection.
In preparation for his third HCV treatment, the patient was included in shared decision making to consider retreating his de novo infection as treatment naïve to provide a briefer (ie, 8 weeks) and more cost-effective treatment given his low likelihood of advanced fibrotic liver disease—his FibroScan score was 6.5 kPa, whereas scores ≥ 12.5 kPa in patients with chronic HCV suggest a higher likelihood of cirrhosis.10 At week 4, the patient’s viral load was undetected, he completed his 8-week regimen of ledipasvir/sofosbuvir as planned and achieved SVR12 (Table). He had reported excellent adherence throughout treatment with assistance of a pill box and validated by a reported pill count.
Case 2
A 32-year-old male presented with HCV genotype 1a. Like case 1, this patient had a low FibroScan score of 4.7 kPa. He was previously infected with genotype 3 and completed a 12-week course of sofosbuvir/velpatasvir in November 2016. He achieved SVR12 as evidenced by an undetected viral load in February 2017 despite questionable adherence throughout and relapsed use of heroin by the end of his regimen. He continued intermittent IDU and presented in October 2018 with a detectable viral load, now with genotype 1a. The patient similarly agreed to undergo an 8-week regimen of ledipasvir/sofosbuvir, considering his de novo infection to be treatment naïve. His viral load at treatment week 3 was quantitatively negative while qualitatively detectable at < 15 U/mL. He completed his treatment course in March 2019 and was determined to have achieved SVR24 in September 2019.
Case 3
A 51-year-old male presented with a history of HCV genotype 1a and a low FibroScan score (4.9 kPa ). The patient was previously infected with genotype 2 and had achieved SVR24 following a 12-week regimen of sofosbuvir/velpatasvir in 2017. The patient subsequently was reinfected with genotype 1a and completed an 8-week course of ledipasvir/sofosbuvir in May 2019. The patient had his SVR12 lab drawn 9 days early and was undetectable at that time. He reported 0 missed doses during treatment and achieved an undetected viral load by treatment week 4.
Discussion
We demonstrate that HCV reinfection after treatment with previous interferon and/or DAA-based regimens can be treated with less costly 8-week treatment regimens. Current guidelines include a statement allowing for reinfected patients to follow initial treatment guidelines, but this statement has previously lacked published evidence and may be overlooked by HCPs who refer to recommendations for treatment-experienced patients. Given the increasing likelihood of HCPs encountering patients who have become reinfected with HCV after achieving SVR from a DAA regimen, further delineation may be needed in the recommendations for treatment-experienced patients to highlight the important nuance of recognizing that reinfections should follow initial treatment guidance.
While all 3 of these cases met criteria for the least costly and simplest 1 pill once daily 8-week regimen of ledipasvir/sofosbuvir, patients requiring retreatment with alternative genotypes or evidence of advanced fibrotic liver disease could benefit from a similar approach of using the least expensive and/or shortest duration regimen for which they meet eligibility. With this approach, coverage could be further expanded to the PWID population to help limit HCV transmission amid the opioid crisis.1
Studies have established that PWID are able to achieve similar SVR efficacy rates similar to that of the general population when treated in the setting of an interdisciplinary treatment team that offers collaborative management of complex psychosocial comorbidities and harm reduction strategies.11,12 These integrative patient-centric strategies may include personalized behavioral health pretreatment evaluations, access to substance use treatment, harm reduction counseling, needle exchange programs, and close follow-up by a case manager.2,13 Current DAA regimens combined with 1 or more of these strategies have demonstrated SVR12 rates of 90 to 95% for initial treatment regimens.11 These high SVR12 rates were even achieved in a recent study in which 74% (76/103) of participants had self-reported IDU within 30 days of HCV treatment start and similar IDU rates throughout treatment.12 A meta-analysis, including real-world studies of DAA treatment outcomes yielded a pooled SVR of 88% (95% CI, 83‐92%) for recent PWID and 91% (95% CI, 88‐95%) for individuals using opiate substitution therapy (OST).14 Additionally, linking PWID with OST also reduces risk for reinfection.14,15
For any patient with detectable HCV after completing the initial DAA regimen, it is important to distinguish between relapse and reinfection. SVR12 is generally synonymous with a clinical cure. Patients with ongoing risk factors posttreatment should continue to have their HCV viral load monitored for evidence of reinfection. Patients without known risk factors may benefit from repeat viral load only if there is clinical concern for reinfection, for example, a rise in liver enzymes.
We have shown that patients with ongoing risk factors who are reinfected can be treated successfully with cost-effective 8-week regimens. For comparison this 8-week regimen of ledipasvir/sofosbuvir has an average wholesale price (AWP) of $28,800, while alternative regimens approved for treatment-naïve patients vary in AWP from $31,680 to $43,200, and regimens approved for retreatment of DAA failures have an AWP as high as $89,712.
An 8-week treatment regimen for both initial and reinfection regimens affords many advantages in medication adherence and both medication and provider resource cost-effectiveness. First, new HCV reinfections are disproportionally younger individuals often with complex psychosocial issues that impact retention in treatment. An 8-week course of treatment can be initiated concurrently with substance abuse treatment programs, including intensive outpatient programs and residential treatment programs that are usually at least 28 days. Many of these programs provide aftercare options that would extend the entire course of treatment. These opportunities afford individuals to receive HCV treatment in a setting that supports medication adherence, sobriety efforts, and education on harm reduction to reduce risk for reinfection.
Finally, statistical models indicate eradication of HCV will require scaling up the treatment of PWID in conjunction with harm reduction strategies such as OST and needle exchange programs.16 In contrast, there are low risks associated with retreatment given these medications are well-tolerated, treatment of PWID lowers the risk of further HCV transmission, and the understanding of these reinfections being treatment naïve disavows concerns of these patients having resistance to regimens that cleared their prior infections. The opportunity to provide retreatment without escalating regimen complexity or cost increases access to care for a vulnerable population while aiding in the eradication of HCV.
1. Centers for Disease Control and Prevention. Viral Hepatitis Surveillance - United States, 2018. Updated August 28, 2020. Accessed May 18, 2021. https://www.cdc.gov/hepatitis/statistics/2018surveillance/HepC.htm 2. Grebely J, Robaeys G, Bruggmann P, et al; International Network for Hepatitis in Substance Users. Recommendations for the management of hepatitis C virus infection among people who inject drugs. Int J Drug Policy. 2015;26(10):1028-1038. doi:10.1016/j.drugpo.2015.07.005
3. Marco A, Esteban JI, Solé C, et al. Hepatitis C virus reinfection among prisoners with sustained virological response after treatment for chronic hepatitis C. J Hepatol. 2013;59(1):45-51. doi:10.1016/j.jhep.2013.03.008
4. Midgard H, Bjøro B, Mæland A, et al. Hepatitis C reinfection after sustained virological response. J Hepatol. 2016;64(5):1020-1026. doi:10.1016/j.jhep.2016.01.001
5. Currie SL, Ryan JC, Tracy D, et al. A prospective study to examine persistent HCV reinfection in injection drug users who have previously cleared the virus [published correction appears in Drug Alcohol Depend. 2008 Jul;96(1-2):192]. Drug Alcohol Depend. 2008;93(1-2):148-154. doi:10.1016/j.drugalcdep.2007.09.011
6. Grady BP, Vanhommerig JW, Schinkel J, et al. Low incidence of reinfection with the hepatitis C virus following treatment in active drug users in Amsterdam. Eur J Gastroenterol Hepatol. 2012;24(11):1302-1307. doi:10.1097/MEG.0b013e32835702a8
7. Grebely J, Pham ST, Matthews GV, et al; ATAHC Study Group. Hepatitis C virus reinfection and superinfection among treated and untreated participants with recent infection. Hepatology. 2012;55(4):1058-1069. doi:10.1002/hep.24754
8. American Association for the Study of Liver Diseases and the Infectious Diseases Society of America. HCV Guidance: Recommendations for Testing, Managing, and Treating Hepatitis C. Accessed May 26, 2021. https://www.hcvguidelines.org
9. National Viral Hepatitis Roundtable, Center for Health Law and Policy Innovation, Harvard Law School. Hepatitis C: The State of Medicaid Access. 2017 National Summary Report. Updated October 23, 2017. Accessed May 26, 2021. https://hepcstage.wpengine.com/wp-content/uploads/2017/10/State-of-HepC_2017_FINAL.pdf
10. Singh S, Muir AJ, Dieterich DT, Falck-Ytter YT. American Gastroenterological Association Institute technical review on the role of elastography in chronic liver diseases. Gastroenterology. 2017;152(6):1544-1577. doi:10.1053/j.gastro.2017.03.016
11. Dore GJ, Altice F, Litwin AH, et al; C-EDGE CO-STAR Study Group. Elbasvir-grazoprevir to treat hepatitis C virus infection in persons receiving opioid agonist therapy: a randomized trial. Ann Intern Med. 2016;165(9):625-634. doi:10.7326/M16-0816
12. Grebely J, Dalgard O, Conway B, et al; SIMPLIFY Study Group. Sofosbuvir and velpatasvir for hepatitis C virus infection in people with recent injection drug use (SIMPLIFY): an open-label, single-arm, phase 4, multicentre trial. Lancet Gastroenterol Hepatol. 2018;3(3):153-161. doi:10.1016/S2468-1253(17)30404-1
13. Cos TA, Bartholomew TS, Huynh, KJ. Role of behavioral health providers in treating hepatitis C. Professional Psychol Res Pract. 2019;50(4):246–254. doi:10.1037/pro0000243
14. Latham NH, Doyle JS, Palmer AY, et al. Staying hepatitis C negative: a systematic review and meta-analysis of cure and reinfection in people who inject drugs. Liver Int. 2019;39(12):2244-2260. doi:10.1111/liv.14152
15. Platt L, Minozzi S, Reed J, et al. Needle syringe programmes and opioid substitution therapy for preventing hepatitis C transmission in people who inject drugs. Cochrane Database Syst Rev. 2017;9(9):CD012021. Published 2017 Sep 18. doi:10.1002/14651858.CD012021.pub2
16. Fraser H, Martin NK, Brummer-Korvenkontio H, et al. Model projections on the impact of HCV treatment in the prevention of HCV transmission among people who inject drugs in Europe. J Hepatol. 2018;68(3):402-411. doi:10.1016/j.jhep.2017.10.010
1. Centers for Disease Control and Prevention. Viral Hepatitis Surveillance - United States, 2018. Updated August 28, 2020. Accessed May 18, 2021. https://www.cdc.gov/hepatitis/statistics/2018surveillance/HepC.htm 2. Grebely J, Robaeys G, Bruggmann P, et al; International Network for Hepatitis in Substance Users. Recommendations for the management of hepatitis C virus infection among people who inject drugs. Int J Drug Policy. 2015;26(10):1028-1038. doi:10.1016/j.drugpo.2015.07.005
3. Marco A, Esteban JI, Solé C, et al. Hepatitis C virus reinfection among prisoners with sustained virological response after treatment for chronic hepatitis C. J Hepatol. 2013;59(1):45-51. doi:10.1016/j.jhep.2013.03.008
4. Midgard H, Bjøro B, Mæland A, et al. Hepatitis C reinfection after sustained virological response. J Hepatol. 2016;64(5):1020-1026. doi:10.1016/j.jhep.2016.01.001
5. Currie SL, Ryan JC, Tracy D, et al. A prospective study to examine persistent HCV reinfection in injection drug users who have previously cleared the virus [published correction appears in Drug Alcohol Depend. 2008 Jul;96(1-2):192]. Drug Alcohol Depend. 2008;93(1-2):148-154. doi:10.1016/j.drugalcdep.2007.09.011
6. Grady BP, Vanhommerig JW, Schinkel J, et al. Low incidence of reinfection with the hepatitis C virus following treatment in active drug users in Amsterdam. Eur J Gastroenterol Hepatol. 2012;24(11):1302-1307. doi:10.1097/MEG.0b013e32835702a8
7. Grebely J, Pham ST, Matthews GV, et al; ATAHC Study Group. Hepatitis C virus reinfection and superinfection among treated and untreated participants with recent infection. Hepatology. 2012;55(4):1058-1069. doi:10.1002/hep.24754
8. American Association for the Study of Liver Diseases and the Infectious Diseases Society of America. HCV Guidance: Recommendations for Testing, Managing, and Treating Hepatitis C. Accessed May 26, 2021. https://www.hcvguidelines.org
9. National Viral Hepatitis Roundtable, Center for Health Law and Policy Innovation, Harvard Law School. Hepatitis C: The State of Medicaid Access. 2017 National Summary Report. Updated October 23, 2017. Accessed May 26, 2021. https://hepcstage.wpengine.com/wp-content/uploads/2017/10/State-of-HepC_2017_FINAL.pdf
10. Singh S, Muir AJ, Dieterich DT, Falck-Ytter YT. American Gastroenterological Association Institute technical review on the role of elastography in chronic liver diseases. Gastroenterology. 2017;152(6):1544-1577. doi:10.1053/j.gastro.2017.03.016
11. Dore GJ, Altice F, Litwin AH, et al; C-EDGE CO-STAR Study Group. Elbasvir-grazoprevir to treat hepatitis C virus infection in persons receiving opioid agonist therapy: a randomized trial. Ann Intern Med. 2016;165(9):625-634. doi:10.7326/M16-0816
12. Grebely J, Dalgard O, Conway B, et al; SIMPLIFY Study Group. Sofosbuvir and velpatasvir for hepatitis C virus infection in people with recent injection drug use (SIMPLIFY): an open-label, single-arm, phase 4, multicentre trial. Lancet Gastroenterol Hepatol. 2018;3(3):153-161. doi:10.1016/S2468-1253(17)30404-1
13. Cos TA, Bartholomew TS, Huynh, KJ. Role of behavioral health providers in treating hepatitis C. Professional Psychol Res Pract. 2019;50(4):246–254. doi:10.1037/pro0000243
14. Latham NH, Doyle JS, Palmer AY, et al. Staying hepatitis C negative: a systematic review and meta-analysis of cure and reinfection in people who inject drugs. Liver Int. 2019;39(12):2244-2260. doi:10.1111/liv.14152
15. Platt L, Minozzi S, Reed J, et al. Needle syringe programmes and opioid substitution therapy for preventing hepatitis C transmission in people who inject drugs. Cochrane Database Syst Rev. 2017;9(9):CD012021. Published 2017 Sep 18. doi:10.1002/14651858.CD012021.pub2
16. Fraser H, Martin NK, Brummer-Korvenkontio H, et al. Model projections on the impact of HCV treatment in the prevention of HCV transmission among people who inject drugs in Europe. J Hepatol. 2018;68(3):402-411. doi:10.1016/j.jhep.2017.10.010
Thinking Outside the ‘Cage’
A 74-year-old male veteran presented at an urgent care clinic in Aguadilla, Puerto Rico, with a sharp, nonradiating, left-sided precordial chest pain that started while cleaning his house and gardening. The patient described the pain as 9 on the 10-point Wong-Baker FACES Pain Rating Scale, lasting about 5 to 10 minutes and was alleviated with rest. The patient’s medical history consisted of multiple comorbidities, including a mitral valve replacement with a Star-Edwards valve (ball in cage) in 1987. The electrocardiogram performed at the clinic showed no acute ischemic changes. Due to the persistent pain, the patient was transferred to Veterans Affairs Caribbean Healthcare System in San Juan, Puerto Rico, for further evaluation and management. On arrival, the patient had an international normalized ratio (INR) of 2.22; elevated high-sensitive troponin enzyme readings of 56 ng/L at 6:38 PM (0h); 61 ng/L at 7:38 PM (1h); and 83 ng/L at 9:47 PM (3h), reference range, 0-22 ng/L, and changes that prompted admission to the cardiac critical care unit. Two days later, a follow-up enzyme level was 52 ng/L. Cardiac catheterization revealed an acute filling defect at mid-left anterior descending artery and remaining coronary arteries with < 25% atherosclerosis (Figure). A myocardial perfusion study was performed for myocardial viability. The results showed a small, reversible perfusion defect involving the apical-septal wall with the remaining left ventricular myocardium appearing viable. Aspirin was added to the patient’s anticoagulation regimen of warfarin. Once target INR was reached, the patient was discharged home without recurrence of angina.
- What is your diagnosis?
- How would you treat this patient?
Acute coronary syndrome (ACS) consists of clinical suspicion of myocardial ischemia or laboratory confirmation of myocardial infarction (MI). ACS includes 3 major entities: non-ST elevation MI (NSTEMI), unstable angina, and ST-elevation MI (STEMI). ACS usually occurs as a result of a reduced supply of oxygenated blood to the myocardium, which is caused by restriction or occlusion of at least 1 of the coronary arteries. This alteration in blood flow is commonly secondary to a rupture of an atherosclerotic plaque or spontaneous dissection of a coronary artery. In rare cases, this reduction in blood flow is caused by a coronary embolism (CE) arising from a prosthetic heart valve.1,2
One of the first descriptions of CE was provided by Rudolf Virchow in the 1850s from postmortem autopsy findings.3 At that time, these coronary findings were associated with intracardiac mural thrombus or infective endocarditis. During the 1940s, CE was described in living patients who had survived a MI, and outcomes were not as catastrophic as originally believed. In the 1960s, a higher than usual association between prosthetic valves and CE was suspected and later confirmed by the invention and implementation of coronary angiography. Multiple studies have been published that confirm the association between prosthetic valves (especially in the mitral position), atrial fibrillation (AF), and a higher than usual rate of CEs.4,5
Discussion
The prevalence of this disease has varied during the years. Data from autopsies of patients with ACS and evidence of thromboembolic material in coronary arteries originally estimated a prevalence as high as 13%.6,7 After the invention of diagnostic angiography, consensus studies have established the prevalence to be approximately 3% in patient with ACS.1 The prevalence may be higher in patient with significant risk factors that may increase the probability of CEs, like prosthetic heart valves and AF.2
In 2015 Shibata and colleagues proposed a scoring system for the diagnosis of CE. The scoring system consisted of major and minor criteria.6 Diagnosis of CE is established by ≥ 2 major criteria; 1 major and 2 minor; or ≥ 3 minor criteria. This scoring system increases the diagnostic probability of the disease.1,6
The major criteria are angiographic evidence of coronary artery embolism and thrombosis without atherosclerotic components (met by this patient); concomitant coronary emboli in multiple coronary vascular territories; concomitant systemic embolization without left ventricular thrombus attributable to acute MI; histological evidence of venous origin of coronary embolic material; and evidence of an embolic source based on transthoracic echocardiography, transesophageal echocardiography, computed tomography, or magnetic resonance imaging.1,6 The minor criteria are 25% stenosis on coronary angiography except for the culprit lesion (met by this patient); presence of emboli risk factors, such as prosthetic heart valve (met by this patient); and AF.1,6
Management of CE remains controversial; aspiration of thrombus may be considered in the acute setting and with evidence of a heavy thrombus formation. This may allow for restoration of flow and retrieval of thrombus formation for histopathologic evaluation. However, it is important to mention that in the setting of STEMI, aspiration has been shown to increase risk of stroke and lead to increased morbidity. If aspiration of thrombus provides good restoration of flow, there is no need for further percutaneous intervention. Benefits of aspiration in low thrombus burden are not well established and do not provide any additional benefit compared with those of anticoagulation.6-11
Anticoagulation should be initiated in patients with AF and low bleeding risk, even when CHA2DS2-VASc (congestive heart failure, hypertension, aged ≥ 75 years, diabetes mellitus, stroke or transient ischemic attack, vascular disease, aged 65 to 74 years, sex category) score is low. In patients with prolonged immobilization, recent surgery, pregnancy, use of oral contraceptives/tamoxifen, or other reversible risks, 3 months of anticoagulation has been shown to be sufficient. In the setting of active cancer or known thrombophilia, prolonged anticoagulation is recommended. Thrombophilia testing is not recommended in the setting of CE.1
The America College of Cardiology/American Heart Association guidelines for valvular heart disease recommend that patients with mechanical prosthetic aortic valves should be started on a vitamin K antagonist with a target INR of 2 to 3. (Class 1A). Prosthetic mitral and high thromboembolic valves require a higher INR target above 3.0. The addition of antiplatelet agents, such as aspirin in doses of 75 to 100 mg, should be started to decrease risk of thromboembolic disease in all patients with prosthetic heart valves.12
CE is not a common cause of ACS. Nevertheless, it was considered in the differential diagnosis of this patient, and diagnostic criteria were reviewed. This patient met the diagnostic criteria for a definitive diagnosis of CE. These included 1 major and 2 minor criteria: angiographic evidence of coronary artery embolism and thrombosis without atherosclerotic components; < 25% stenosis on coronary angiography except for the culprit lesion; and presence of emboli risk factors (prosthetic heart valve).
CE is rare, and review of the literature reveals that it accounts for < 3% of all ACS cases. Despite its rarity, it is important to recognize its risk factors, which include prosthetic heart valves, valvuloplasty, vasculitis, AF, left ventricular aneurysm, and endocarditis. The difference in treatment between CE and the most frequently encountered etiologies of ACS reveals the importance in recognizing this syndrome. Management of CE remains controversial. Nevertheless, when the culprit lesion is located in a distal portion of the vessel involved, as was seen in our patient, and in cases where there is a low thrombi burden, anticoagulation instead of thrombectomy is usually preferred. Patients with prosthetic mechanical valves have a high incidence of thromboembolism. This sometimes leads to thrombi formation in uncommon locations. Guidelines of therapy in these patients recommend that all prosthetic mechanical valves should be treated with both antiplatelet and anticoagulation therapies to reduce the risk of thrombi formation.
Conclusion
Physicians involved in diagnosing ACS should be aware of the risk factors for CE and always consider it while evaluating patients and developing the differential diagnosis.
1. Raphael CE, Heit JA, Reeder GS, et al. Coronary embolus: an underappreciated cause of acute coronary syndromes. JACC Cardiovasc Interv. 2018;11(2):172-180. doi:10.1016/j.jcin.2017.08.057
2. Popovic B, Agrinier N, Bouchahda N, et al. Coronary embolism among ST-segment-elevation myocardial infarction patients: mechanisms and management. Circ Cardiovasc Interv. 2018;11(1):e005587. doi:10.1161/CIRCINTERVENTIONS.117.005587
3. Oakley C, Yusuf R, Hollman A. Coronary embolism and angina in mitral stenosis. Br Heart J. 1961;23(4):357-369. doi:10.1136/hrt.23.4.357
4. Charles RG, Epstein EJ. Diagnosis of coronary embolism: a review. J R Soc Med. 1983;76(10):863-869.
5. Bawell MB, Moragues V, Shrader EL. Coronary embolism. Circulation. 1956;14(6):1159-1163. doi:10.1161/01.cir.14.6.1159
6. Shibata T, Kawakami S, Noguchi T, et al. Prevalence, clinical features, and prognosis of acute myocardial infarction attributable to coronary artery embolism. Circulation. 2015;132(4):241-250. doi:10.1161/CIRCULATIONAHA.114.015134
7. Prizel KR, Hutchins GM, Bulkley BH. Coronary artery embolism and myocardial infarction. Ann Intern Med. 1978;88(2):155-161. doi:10.7326/0003-4819-88-2-155
8. Lacunza-Ruiz FJ, Muñoz-Esparza C, García-de-Lara J. Coronary embolism and thrombosis of prosthetic mitral valve. JACC Cardiovasc Interv. 2014;7(10):e127-e128. doi:10.1016/j.jcin.2014.02.025
9. Jolly SS, Cairns JA, Yusuf S, et al. Outcomes after thrombus aspiration for ST elevation myocardial infarction: 1-year follow-up of the prospective randomised TOTAL trial. Lancet. 2016;387(10014):127-135. doi:10.1016/S0140-6736(15)00448-1
10. Fröbert O, Lagerqvist B, Olivecrona GK, et al. Thrombus aspiration during ST-segment elevation myocardial infarction [published correction appears in N Engl J Med. 2014 Aug 21;371(8):786]. N Engl J Med. 2013;369(17):1587-1597. doi:10.1056/NEJMoa1308789
11. Kalçık M, Yesin M, Gürsoy MO, Karakoyun S, Özkan M. Treatment strategies for prosthetic valve thrombosis-derived coronary embolism. JACC Cardiovasc Interv. 2015;8(5):756-757. doi:10.1016/j.jcin.2014.11.019
12. Nishimura RA, Otto CM, Bonow RO, et al. 2017 AHA/ACC focused update of the 2014 AHA/ACC Guideline for the Management of Patients With Valvular Heart Disease: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2017;135(25):e1159-e1195. doi:10.1161/CIR.0000000000000503
A 74-year-old male veteran presented at an urgent care clinic in Aguadilla, Puerto Rico, with a sharp, nonradiating, left-sided precordial chest pain that started while cleaning his house and gardening. The patient described the pain as 9 on the 10-point Wong-Baker FACES Pain Rating Scale, lasting about 5 to 10 minutes and was alleviated with rest. The patient’s medical history consisted of multiple comorbidities, including a mitral valve replacement with a Star-Edwards valve (ball in cage) in 1987. The electrocardiogram performed at the clinic showed no acute ischemic changes. Due to the persistent pain, the patient was transferred to Veterans Affairs Caribbean Healthcare System in San Juan, Puerto Rico, for further evaluation and management. On arrival, the patient had an international normalized ratio (INR) of 2.22; elevated high-sensitive troponin enzyme readings of 56 ng/L at 6:38 PM (0h); 61 ng/L at 7:38 PM (1h); and 83 ng/L at 9:47 PM (3h), reference range, 0-22 ng/L, and changes that prompted admission to the cardiac critical care unit. Two days later, a follow-up enzyme level was 52 ng/L. Cardiac catheterization revealed an acute filling defect at mid-left anterior descending artery and remaining coronary arteries with < 25% atherosclerosis (Figure). A myocardial perfusion study was performed for myocardial viability. The results showed a small, reversible perfusion defect involving the apical-septal wall with the remaining left ventricular myocardium appearing viable. Aspirin was added to the patient’s anticoagulation regimen of warfarin. Once target INR was reached, the patient was discharged home without recurrence of angina.
- What is your diagnosis?
- How would you treat this patient?
Acute coronary syndrome (ACS) consists of clinical suspicion of myocardial ischemia or laboratory confirmation of myocardial infarction (MI). ACS includes 3 major entities: non-ST elevation MI (NSTEMI), unstable angina, and ST-elevation MI (STEMI). ACS usually occurs as a result of a reduced supply of oxygenated blood to the myocardium, which is caused by restriction or occlusion of at least 1 of the coronary arteries. This alteration in blood flow is commonly secondary to a rupture of an atherosclerotic plaque or spontaneous dissection of a coronary artery. In rare cases, this reduction in blood flow is caused by a coronary embolism (CE) arising from a prosthetic heart valve.1,2
One of the first descriptions of CE was provided by Rudolf Virchow in the 1850s from postmortem autopsy findings.3 At that time, these coronary findings were associated with intracardiac mural thrombus or infective endocarditis. During the 1940s, CE was described in living patients who had survived a MI, and outcomes were not as catastrophic as originally believed. In the 1960s, a higher than usual association between prosthetic valves and CE was suspected and later confirmed by the invention and implementation of coronary angiography. Multiple studies have been published that confirm the association between prosthetic valves (especially in the mitral position), atrial fibrillation (AF), and a higher than usual rate of CEs.4,5
Discussion
The prevalence of this disease has varied during the years. Data from autopsies of patients with ACS and evidence of thromboembolic material in coronary arteries originally estimated a prevalence as high as 13%.6,7 After the invention of diagnostic angiography, consensus studies have established the prevalence to be approximately 3% in patient with ACS.1 The prevalence may be higher in patient with significant risk factors that may increase the probability of CEs, like prosthetic heart valves and AF.2
In 2015 Shibata and colleagues proposed a scoring system for the diagnosis of CE. The scoring system consisted of major and minor criteria.6 Diagnosis of CE is established by ≥ 2 major criteria; 1 major and 2 minor; or ≥ 3 minor criteria. This scoring system increases the diagnostic probability of the disease.1,6
The major criteria are angiographic evidence of coronary artery embolism and thrombosis without atherosclerotic components (met by this patient); concomitant coronary emboli in multiple coronary vascular territories; concomitant systemic embolization without left ventricular thrombus attributable to acute MI; histological evidence of venous origin of coronary embolic material; and evidence of an embolic source based on transthoracic echocardiography, transesophageal echocardiography, computed tomography, or magnetic resonance imaging.1,6 The minor criteria are 25% stenosis on coronary angiography except for the culprit lesion (met by this patient); presence of emboli risk factors, such as prosthetic heart valve (met by this patient); and AF.1,6
Management of CE remains controversial; aspiration of thrombus may be considered in the acute setting and with evidence of a heavy thrombus formation. This may allow for restoration of flow and retrieval of thrombus formation for histopathologic evaluation. However, it is important to mention that in the setting of STEMI, aspiration has been shown to increase risk of stroke and lead to increased morbidity. If aspiration of thrombus provides good restoration of flow, there is no need for further percutaneous intervention. Benefits of aspiration in low thrombus burden are not well established and do not provide any additional benefit compared with those of anticoagulation.6-11
Anticoagulation should be initiated in patients with AF and low bleeding risk, even when CHA2DS2-VASc (congestive heart failure, hypertension, aged ≥ 75 years, diabetes mellitus, stroke or transient ischemic attack, vascular disease, aged 65 to 74 years, sex category) score is low. In patients with prolonged immobilization, recent surgery, pregnancy, use of oral contraceptives/tamoxifen, or other reversible risks, 3 months of anticoagulation has been shown to be sufficient. In the setting of active cancer or known thrombophilia, prolonged anticoagulation is recommended. Thrombophilia testing is not recommended in the setting of CE.1
The America College of Cardiology/American Heart Association guidelines for valvular heart disease recommend that patients with mechanical prosthetic aortic valves should be started on a vitamin K antagonist with a target INR of 2 to 3. (Class 1A). Prosthetic mitral and high thromboembolic valves require a higher INR target above 3.0. The addition of antiplatelet agents, such as aspirin in doses of 75 to 100 mg, should be started to decrease risk of thromboembolic disease in all patients with prosthetic heart valves.12
CE is not a common cause of ACS. Nevertheless, it was considered in the differential diagnosis of this patient, and diagnostic criteria were reviewed. This patient met the diagnostic criteria for a definitive diagnosis of CE. These included 1 major and 2 minor criteria: angiographic evidence of coronary artery embolism and thrombosis without atherosclerotic components; < 25% stenosis on coronary angiography except for the culprit lesion; and presence of emboli risk factors (prosthetic heart valve).
CE is rare, and review of the literature reveals that it accounts for < 3% of all ACS cases. Despite its rarity, it is important to recognize its risk factors, which include prosthetic heart valves, valvuloplasty, vasculitis, AF, left ventricular aneurysm, and endocarditis. The difference in treatment between CE and the most frequently encountered etiologies of ACS reveals the importance in recognizing this syndrome. Management of CE remains controversial. Nevertheless, when the culprit lesion is located in a distal portion of the vessel involved, as was seen in our patient, and in cases where there is a low thrombi burden, anticoagulation instead of thrombectomy is usually preferred. Patients with prosthetic mechanical valves have a high incidence of thromboembolism. This sometimes leads to thrombi formation in uncommon locations. Guidelines of therapy in these patients recommend that all prosthetic mechanical valves should be treated with both antiplatelet and anticoagulation therapies to reduce the risk of thrombi formation.
Conclusion
Physicians involved in diagnosing ACS should be aware of the risk factors for CE and always consider it while evaluating patients and developing the differential diagnosis.
A 74-year-old male veteran presented at an urgent care clinic in Aguadilla, Puerto Rico, with a sharp, nonradiating, left-sided precordial chest pain that started while cleaning his house and gardening. The patient described the pain as 9 on the 10-point Wong-Baker FACES Pain Rating Scale, lasting about 5 to 10 minutes and was alleviated with rest. The patient’s medical history consisted of multiple comorbidities, including a mitral valve replacement with a Star-Edwards valve (ball in cage) in 1987. The electrocardiogram performed at the clinic showed no acute ischemic changes. Due to the persistent pain, the patient was transferred to Veterans Affairs Caribbean Healthcare System in San Juan, Puerto Rico, for further evaluation and management. On arrival, the patient had an international normalized ratio (INR) of 2.22; elevated high-sensitive troponin enzyme readings of 56 ng/L at 6:38 PM (0h); 61 ng/L at 7:38 PM (1h); and 83 ng/L at 9:47 PM (3h), reference range, 0-22 ng/L, and changes that prompted admission to the cardiac critical care unit. Two days later, a follow-up enzyme level was 52 ng/L. Cardiac catheterization revealed an acute filling defect at mid-left anterior descending artery and remaining coronary arteries with < 25% atherosclerosis (Figure). A myocardial perfusion study was performed for myocardial viability. The results showed a small, reversible perfusion defect involving the apical-septal wall with the remaining left ventricular myocardium appearing viable. Aspirin was added to the patient’s anticoagulation regimen of warfarin. Once target INR was reached, the patient was discharged home without recurrence of angina.
- What is your diagnosis?
- How would you treat this patient?
Acute coronary syndrome (ACS) consists of clinical suspicion of myocardial ischemia or laboratory confirmation of myocardial infarction (MI). ACS includes 3 major entities: non-ST elevation MI (NSTEMI), unstable angina, and ST-elevation MI (STEMI). ACS usually occurs as a result of a reduced supply of oxygenated blood to the myocardium, which is caused by restriction or occlusion of at least 1 of the coronary arteries. This alteration in blood flow is commonly secondary to a rupture of an atherosclerotic plaque or spontaneous dissection of a coronary artery. In rare cases, this reduction in blood flow is caused by a coronary embolism (CE) arising from a prosthetic heart valve.1,2
One of the first descriptions of CE was provided by Rudolf Virchow in the 1850s from postmortem autopsy findings.3 At that time, these coronary findings were associated with intracardiac mural thrombus or infective endocarditis. During the 1940s, CE was described in living patients who had survived a MI, and outcomes were not as catastrophic as originally believed. In the 1960s, a higher than usual association between prosthetic valves and CE was suspected and later confirmed by the invention and implementation of coronary angiography. Multiple studies have been published that confirm the association between prosthetic valves (especially in the mitral position), atrial fibrillation (AF), and a higher than usual rate of CEs.4,5
Discussion
The prevalence of this disease has varied during the years. Data from autopsies of patients with ACS and evidence of thromboembolic material in coronary arteries originally estimated a prevalence as high as 13%.6,7 After the invention of diagnostic angiography, consensus studies have established the prevalence to be approximately 3% in patient with ACS.1 The prevalence may be higher in patient with significant risk factors that may increase the probability of CEs, like prosthetic heart valves and AF.2
In 2015 Shibata and colleagues proposed a scoring system for the diagnosis of CE. The scoring system consisted of major and minor criteria.6 Diagnosis of CE is established by ≥ 2 major criteria; 1 major and 2 minor; or ≥ 3 minor criteria. This scoring system increases the diagnostic probability of the disease.1,6
The major criteria are angiographic evidence of coronary artery embolism and thrombosis without atherosclerotic components (met by this patient); concomitant coronary emboli in multiple coronary vascular territories; concomitant systemic embolization without left ventricular thrombus attributable to acute MI; histological evidence of venous origin of coronary embolic material; and evidence of an embolic source based on transthoracic echocardiography, transesophageal echocardiography, computed tomography, or magnetic resonance imaging.1,6 The minor criteria are 25% stenosis on coronary angiography except for the culprit lesion (met by this patient); presence of emboli risk factors, such as prosthetic heart valve (met by this patient); and AF.1,6
Management of CE remains controversial; aspiration of thrombus may be considered in the acute setting and with evidence of a heavy thrombus formation. This may allow for restoration of flow and retrieval of thrombus formation for histopathologic evaluation. However, it is important to mention that in the setting of STEMI, aspiration has been shown to increase risk of stroke and lead to increased morbidity. If aspiration of thrombus provides good restoration of flow, there is no need for further percutaneous intervention. Benefits of aspiration in low thrombus burden are not well established and do not provide any additional benefit compared with those of anticoagulation.6-11
Anticoagulation should be initiated in patients with AF and low bleeding risk, even when CHA2DS2-VASc (congestive heart failure, hypertension, aged ≥ 75 years, diabetes mellitus, stroke or transient ischemic attack, vascular disease, aged 65 to 74 years, sex category) score is low. In patients with prolonged immobilization, recent surgery, pregnancy, use of oral contraceptives/tamoxifen, or other reversible risks, 3 months of anticoagulation has been shown to be sufficient. In the setting of active cancer or known thrombophilia, prolonged anticoagulation is recommended. Thrombophilia testing is not recommended in the setting of CE.1
The America College of Cardiology/American Heart Association guidelines for valvular heart disease recommend that patients with mechanical prosthetic aortic valves should be started on a vitamin K antagonist with a target INR of 2 to 3. (Class 1A). Prosthetic mitral and high thromboembolic valves require a higher INR target above 3.0. The addition of antiplatelet agents, such as aspirin in doses of 75 to 100 mg, should be started to decrease risk of thromboembolic disease in all patients with prosthetic heart valves.12
CE is not a common cause of ACS. Nevertheless, it was considered in the differential diagnosis of this patient, and diagnostic criteria were reviewed. This patient met the diagnostic criteria for a definitive diagnosis of CE. These included 1 major and 2 minor criteria: angiographic evidence of coronary artery embolism and thrombosis without atherosclerotic components; < 25% stenosis on coronary angiography except for the culprit lesion; and presence of emboli risk factors (prosthetic heart valve).
CE is rare, and review of the literature reveals that it accounts for < 3% of all ACS cases. Despite its rarity, it is important to recognize its risk factors, which include prosthetic heart valves, valvuloplasty, vasculitis, AF, left ventricular aneurysm, and endocarditis. The difference in treatment between CE and the most frequently encountered etiologies of ACS reveals the importance in recognizing this syndrome. Management of CE remains controversial. Nevertheless, when the culprit lesion is located in a distal portion of the vessel involved, as was seen in our patient, and in cases where there is a low thrombi burden, anticoagulation instead of thrombectomy is usually preferred. Patients with prosthetic mechanical valves have a high incidence of thromboembolism. This sometimes leads to thrombi formation in uncommon locations. Guidelines of therapy in these patients recommend that all prosthetic mechanical valves should be treated with both antiplatelet and anticoagulation therapies to reduce the risk of thrombi formation.
Conclusion
Physicians involved in diagnosing ACS should be aware of the risk factors for CE and always consider it while evaluating patients and developing the differential diagnosis.
1. Raphael CE, Heit JA, Reeder GS, et al. Coronary embolus: an underappreciated cause of acute coronary syndromes. JACC Cardiovasc Interv. 2018;11(2):172-180. doi:10.1016/j.jcin.2017.08.057
2. Popovic B, Agrinier N, Bouchahda N, et al. Coronary embolism among ST-segment-elevation myocardial infarction patients: mechanisms and management. Circ Cardiovasc Interv. 2018;11(1):e005587. doi:10.1161/CIRCINTERVENTIONS.117.005587
3. Oakley C, Yusuf R, Hollman A. Coronary embolism and angina in mitral stenosis. Br Heart J. 1961;23(4):357-369. doi:10.1136/hrt.23.4.357
4. Charles RG, Epstein EJ. Diagnosis of coronary embolism: a review. J R Soc Med. 1983;76(10):863-869.
5. Bawell MB, Moragues V, Shrader EL. Coronary embolism. Circulation. 1956;14(6):1159-1163. doi:10.1161/01.cir.14.6.1159
6. Shibata T, Kawakami S, Noguchi T, et al. Prevalence, clinical features, and prognosis of acute myocardial infarction attributable to coronary artery embolism. Circulation. 2015;132(4):241-250. doi:10.1161/CIRCULATIONAHA.114.015134
7. Prizel KR, Hutchins GM, Bulkley BH. Coronary artery embolism and myocardial infarction. Ann Intern Med. 1978;88(2):155-161. doi:10.7326/0003-4819-88-2-155
8. Lacunza-Ruiz FJ, Muñoz-Esparza C, García-de-Lara J. Coronary embolism and thrombosis of prosthetic mitral valve. JACC Cardiovasc Interv. 2014;7(10):e127-e128. doi:10.1016/j.jcin.2014.02.025
9. Jolly SS, Cairns JA, Yusuf S, et al. Outcomes after thrombus aspiration for ST elevation myocardial infarction: 1-year follow-up of the prospective randomised TOTAL trial. Lancet. 2016;387(10014):127-135. doi:10.1016/S0140-6736(15)00448-1
10. Fröbert O, Lagerqvist B, Olivecrona GK, et al. Thrombus aspiration during ST-segment elevation myocardial infarction [published correction appears in N Engl J Med. 2014 Aug 21;371(8):786]. N Engl J Med. 2013;369(17):1587-1597. doi:10.1056/NEJMoa1308789
11. Kalçık M, Yesin M, Gürsoy MO, Karakoyun S, Özkan M. Treatment strategies for prosthetic valve thrombosis-derived coronary embolism. JACC Cardiovasc Interv. 2015;8(5):756-757. doi:10.1016/j.jcin.2014.11.019
12. Nishimura RA, Otto CM, Bonow RO, et al. 2017 AHA/ACC focused update of the 2014 AHA/ACC Guideline for the Management of Patients With Valvular Heart Disease: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2017;135(25):e1159-e1195. doi:10.1161/CIR.0000000000000503
1. Raphael CE, Heit JA, Reeder GS, et al. Coronary embolus: an underappreciated cause of acute coronary syndromes. JACC Cardiovasc Interv. 2018;11(2):172-180. doi:10.1016/j.jcin.2017.08.057
2. Popovic B, Agrinier N, Bouchahda N, et al. Coronary embolism among ST-segment-elevation myocardial infarction patients: mechanisms and management. Circ Cardiovasc Interv. 2018;11(1):e005587. doi:10.1161/CIRCINTERVENTIONS.117.005587
3. Oakley C, Yusuf R, Hollman A. Coronary embolism and angina in mitral stenosis. Br Heart J. 1961;23(4):357-369. doi:10.1136/hrt.23.4.357
4. Charles RG, Epstein EJ. Diagnosis of coronary embolism: a review. J R Soc Med. 1983;76(10):863-869.
5. Bawell MB, Moragues V, Shrader EL. Coronary embolism. Circulation. 1956;14(6):1159-1163. doi:10.1161/01.cir.14.6.1159
6. Shibata T, Kawakami S, Noguchi T, et al. Prevalence, clinical features, and prognosis of acute myocardial infarction attributable to coronary artery embolism. Circulation. 2015;132(4):241-250. doi:10.1161/CIRCULATIONAHA.114.015134
7. Prizel KR, Hutchins GM, Bulkley BH. Coronary artery embolism and myocardial infarction. Ann Intern Med. 1978;88(2):155-161. doi:10.7326/0003-4819-88-2-155
8. Lacunza-Ruiz FJ, Muñoz-Esparza C, García-de-Lara J. Coronary embolism and thrombosis of prosthetic mitral valve. JACC Cardiovasc Interv. 2014;7(10):e127-e128. doi:10.1016/j.jcin.2014.02.025
9. Jolly SS, Cairns JA, Yusuf S, et al. Outcomes after thrombus aspiration for ST elevation myocardial infarction: 1-year follow-up of the prospective randomised TOTAL trial. Lancet. 2016;387(10014):127-135. doi:10.1016/S0140-6736(15)00448-1
10. Fröbert O, Lagerqvist B, Olivecrona GK, et al. Thrombus aspiration during ST-segment elevation myocardial infarction [published correction appears in N Engl J Med. 2014 Aug 21;371(8):786]. N Engl J Med. 2013;369(17):1587-1597. doi:10.1056/NEJMoa1308789
11. Kalçık M, Yesin M, Gürsoy MO, Karakoyun S, Özkan M. Treatment strategies for prosthetic valve thrombosis-derived coronary embolism. JACC Cardiovasc Interv. 2015;8(5):756-757. doi:10.1016/j.jcin.2014.11.019
12. Nishimura RA, Otto CM, Bonow RO, et al. 2017 AHA/ACC focused update of the 2014 AHA/ACC Guideline for the Management of Patients With Valvular Heart Disease: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2017;135(25):e1159-e1195. doi:10.1161/CIR.0000000000000503
Left-Sided Amyand Hernia: Case Report and Review of the Literature
Left-sided Amyand hernia is a rare condition that requires a high degree of clinical suspicion to correctly diagnose.
The presence of the vermiform appendix within an inguinal hernia sac is termed an Amyand hernia. While the incidence of Amyand hernia in the general population is thought to be exceedingly rare, the presence of a left-sided Amyand hernia is even more rare due to the normal anatomical position of the appendix on the right side. Left-sided Amyand hernia presents a novel diagnosis that necessitates a high degree of clinical suspicion and special consideration during patient workup and operative treatment. We describe such a case and provide a review of all reports in the literature of this rare finding.
Case Presentation
A male aged 62 years presented to the emergency department of the Michael E. DeBakey Veterans Affairs Medical Center in Houston, Texas, in acute distress after experiencing 5 days of nausea and pain in his lower abdomen. The patient’s history was significant for cocaine abuse and a left-sided inguinal hernia that was repaired about 15 years prior to this visit. He reported having no bowel movements for the past 5 days and no other symptoms, including vomiting, hematemesis, and trauma to the abdomen. The patient’s abdominal pain was located in the suprapubic and periumbilical regions. Upon palpation of the lower abdomen, a firm, protruding mass was identified in the left lower quadrant and suspected to be a left-sided inguinal hernia.
A scout film and computed tomography (CT) scan of the abdomen taken on the same day that the patient presented to the emergency department confirmed the presence of a large left-sided inguinal hernia with possible bowel strangulation involving the colon (Figures 1, 2, and 3). The patient was diagnosed with an incarcerated recurrent left inguinal hernia and was taken emergently to the operating suite. General anesthesia and an ilioinguinal nerve block were performed. An inguinal incision was made on the left side, and the large hernia sac was identified and separated from the scrotum and spermatic cord structures.
On visual inspection, the hernia was identified as both a direct and an indirect inguinal hernia, making it a pantaloon hernia. The hernia sac was opened, and contents of the herniated sac were found to include the omentum, a loop of transverse colon, as well as the entire cecum and appendix, confirming the diagnosis of an Amyand hernia (Figure 4). Though the bowel was initially dusky, all the bowel became pink and appeared to be viable after detorsion of the bowel. Diagnostic laparoscopy through a 5-mm port was performed to assess the remainder of the bowel located intra-abdominally. The remaining intra-abdominal bowel appeared healthy and without obvious signs of ischemia, twisting, or malrotation. The large hernia defect was repaired with a polypropylene mesh.
Discussion
An Amyand hernia is an inguinal hernia in which the vermiform appendix is located within the hernial sac. Named after the French surgeon Claudius Amyand who first documented such a case during an appendectomy in 1735, the Amyand hernia is rare and is thought to occur in < 1% of inguinal hernias.1 Given the normal anatomical position of the appendix on the right side of the body, most Amyand hernias occur in a right-sided inguinal hernia.
A literature review yielded 25 reported instances of a left-sided Amyand hernia (Table 1) including this report. The true age of incidence of Amyand hernia for each patient is difficult to determine, as many patients do not present until pain or discomfort reaches high levels, often many years after hernia formation. Additionally, some cases of left-sided Amyand hernia described herein, including our case, are recurrent cases of a previous hernia that have been surgically repaired.2-20
Presentation of Amyand hernia often resembles that of a complicated inguinal hernia, acute appendicitis, or both. Hence, clinicians should consider this a possibility when patients present with signs and symptoms that could otherwise be thought to be originating from an incarcerated, strangulated, or recurrent hernia. Specifically, these signs and symptoms include a tender, nonreducible mass in the inguinal region, acute lower abdominal pain, nausea, or signs of intestinal obstruction such as failure to produce bowel movements.4,17 Because of the unusual anatomy in patients presenting with left-sided Amyand hernia, tenderness at the McBurney point usually is absent and not a useful diagnostic tool to rule out acute appendicitis.
A literature review indicates that an Amyand hernia on either side tends to occur in males more often than it does in females. The rate of diagnosis of Amyand hernia also has been reported to be 3 times higher in children than it is in adults due to failure of the processus vaginalis to obliterate during development.21 Our literature review supports this finding, as 16 of the documented 25 cases of left-sided Amyand hernia were reported in males. Additionally, information regarding gender was not found in 6 cases, suggesting a potential for an even higher prevalence in males.
Explanations as to why the appendix is on the left side in these patients include developmental anomalies, such as situs inversus, intestinal rotation, mobile cecum, or an abnormally long appendix.3,8 In our case, the likely causative culprit was a mobile cecum, as there was neither indication of intestinal malformation, rotation, nor of an abnormally long appendix during surgery. Additionally, pre-operative radiologic studies, clinical evaluation, and electrocardiogram did not suggest the presence of situs inversus.
Treatment of Amyand hernia usually follows the landmark classification algorithm set forth in 2007 by Losanoff and Basson (Table 2).22 This system stratifies treatment based on intraoperative findings of the appendix and surrounding structures, ranging from type 1, which involves a normal appendix within the hernia, to type 4, which includes acute appendicitis with additional abdominal pathology. Our patient presented with a type 1 Amyand hernia and appendectomy was foregone as per the guidelines; however, there have been numerous reported cases of surgeons opting for prophylactic appendectomy in the case of a normal appearing appendix and surrounding structures. The decision to act independent of the Losanoff and Basson classification underscores the lack of true standardization, namely, when it comes to a treatment approach for type 1 Amyand hernias. Nonetheless, many contend that indiscriminately performing appendectomies in all cases of left-sided Amyand hernia is useful as a prophylactic measure, as cases of future appendicitis in these patients will have atypical presentations based on the contralateral location of the appendix.6,11,17
Others disagree, citing that prophylactic appendectomy in the case of a normal looking appendix is unnecessary and complicates an otherwise sterile surgery (clean wound classification) with the removal of an appendix containing fecal matter and gut microbiota (converted into a clean contaminated or a contaminated wound classification).17 Additionally, it is thought that in the cases of middle-aged or geriatric patients where the chances of appendicitis are far less, the risks of detriment from prophylactic appendectomy may outweigh the benefits. In these cases, a macroscopic view of the appendix based on visual examination during the operation should guide decision making.4
While the decision to remove a healthy-appearing appendix remains contentious, the decision for or against placement of a heterogenous hernia mesh has proven to be binary, with near universally accepted criteria. If signs of perforation or infection are present in the hernia sac, then surgeons will forego hernioplasty with mesh for simple herniorrhaphy. This contraindication for mesh placement is due to the increased risk of mesh infection, wound infection, and fistulae associated with the introduction of a foreign structure to an active infection site.2
While most cases of Amyand hernia are diagnosed intraoperatively, there have been documented cases of preoperative diagnosis using ultrasonography and CT imaging modalities.19,23,24 In all cases, the presence of the vermiform appendix within the hernia sac can complicate diagnosis and treatment, and preoperative knowledge of this condition may help to guide physician decision making. Identifying Amyand hernia via CT scan is not only useful for alerting physicians of a potentially inflamed appendix within the hernia sac, but also may create opportunities for the use of other treatment modalities. For example, laparoscopic Amyand hernia reduction, an approach that was performed successfully and documented for the first time by Vermillion and colleagues, was made possible by preoperative diagnosis and can potentially result in improved patient outcomes.25
Regardless, while standardization of treatment for Amyand hernia has not yet occurred, it is clear that improved preoperative diagnosis, especially in the case of an unanticipated left-sided Amyand hernia, can allow for better planning and use of a wider variety of treatment modalities. The main impediment to this approach is that suspected cases of appendicitis and inguinal hernias (the most common preoperative diagnoses of Amyand hernia) usually are diagnosed clinically without the need of additional imaging studies like CT or ultrasound. In accordance with the guiding principle of radiation safety of exposing patients to “as low as reasonably achievable” (ALARA) radiation and with consideration of expediency of care and cost efficiency, we recommend physicians continue to screen for and treat cases of potentially emergent appendicitis and/or inguinal hernia as per the conventional methodology. The best approach may involve increasing preoperative diagnoses of left-sided Amyand hernias via physician awareness of this rare finding, as well as evaluating imaging studies that have previously been obtained in order to narrow a broad differential diagnosis.
Conclusions
Left-sided Amyand hernia is an exceptionally rare condition whose preoperative diagnosis remains difficult to establish but whose treatment decision tree is significantly impacted by the condition.
1. Franko J, Raftopoulos I, Sulkowski R. A rare variation of Amyand’s hernia. Am J Gastroenterol. 2002;97(10):2684-2685. doi:10.1111/j.1572-0241.2002.06060.x
2. Carey LC. Acute appendicitis occurring in hernias: a report of 10 cases. Surgery. 1967;61(2):236-238.
3. Kaymakci A, Akillioglu I, Akkoyun I, Guven S, Ozdemir A, Gulen S. Amyand’s hernia: a series of 30 cases in children. Hernia. 2009;13(6):609-612. doi:10.1007/s10029-009-0528-8
4. Cankorkmaz L, Ozer H, Guney C, Atalar MH, Arslan MS, Koyluoglu G. Amyand’s hernia in the children: a single center experience. Surgery. 2010;147(1):140-143. doi:10.1016/j.surg.2009.09.038
5. Yasumoto R, Kawano M, Kawanishi H, et al. Left acute scrotum associated with appendicitis. Int J Urol. 1998;5(1):108-110. doi:10.1111/j.1442-2042.1998.tb00254.x
6. Bakhshi GD, Bhandarwar AH, Govila AA. Acute appendicitis in left scrotum. Indian J Gastroenterol. 2004;23(5):195.
7. Breitenstein S, Eisenbach C, Wille G, Decurtins M. Incarcerated vermiform appendix in a left-sided inguinal hernia. Hernia. 2005;9(1):100-102. doi:10.1007/s10029-004-0263-0
8. Gupta S, Sharma R, Kaushik R. Left-sided Amyand’s hernia. Singapore Med J. 2005;46(8):424-425.
9. Gupta N, Wilkinson TV, Wilkinson A, Akhtar M. Left-sided incarcerated Amyand’s hernia. Indian J Surg. 2007;69(1):17-18.
10. Tayade, MB, Bakhshi GD, Borisa AD, Deshpande G, Joshi N. A rare combination of left sided Amyand’s and Richter’s hernia. Bombay Hosp J. 2008;50(4): 644-645
11. Johari HG, Paydar S, Davani SZ, Eskandari S, Johari MG. Left-sided Amyand hernia. Ann Saudi Med. 2009;29(4):321-322. doi:10.4103/0256-4947.55305
12. Ali SM, Malik KA, Al-Qadhi H. Amyand’s Hernia: Study of four cases and literature review. Sultan Qaboos Univ Med J. 2012;12(2):232-236. doi:10.12816/0003119
13. Ravishankaran P, Mohan G, Srinivasan A, Ravindran G, Ramalingam A. Left sided amyand’s hernia, a rare occurrence: A Case Report. Indian J Surg. 2013;75(3):247-248. doi:10.1007/s12262-010-0223-0
14. Singh K, Singh RR, Kaur S. Amyand’s hernia. J Indian Assoc Pediatr Surg. 2011;16(4):171-172. doi:10.4103/0971-9261.86890
15. Khan TS, Wani ML, Bijli AH, et al. Amyand’s hernia: a rare occurrence. Ann Nigerian Med. 2011;5(2):62-64.doi:10.4103/0331-3131.92955
16. Ghafouri A, Anbara T, Foroutankia R. A rare case report of appendix and cecum in the sac of left inguinal hernia (left Amyand’s hernia). Med J Islam Repub Iran. 2012;26(2):94-95.
17. Al-Mayoof AF, Al-Ani BH. Left-sided amyand hernia: report of two cases with review of literature. European J Pediatr Surg Rep. 2014;2(1):63-66. doi:10.1055/s-0033-1347131
18. Unver M, Ozturk S, Karaman K, Turgut E. Left sided Amyand’s hernia. World J Gastrointest Surg. 2013;5(10):285-286. doi:10.4240/wjgs.v5.i10.285
19. Maeda K, Kunieda K, Kawai M, et al. Giant left-sided inguinoscrotal hernia containing the cecum and appendix (giant left-sided Amyand’s hernia). Clin Case Rep. 2014;2(6):254-257. doi:10.1002/ccr3.104
20. Mongardini M, Maturo A, De Anna L, et al. Appendiceal abscess in a giant left-sided inguinoscrotal hernia: a rare case of Amyand hernia. Springerplus. 2015;4:378. Published 2015 Jul 26. doi:10.1186/s40064-015-1162-9
21. Ivanschuk G, Cesmebasi A, Sorenson EP, Blaak C, Loukas M, Tubbs SR. Amyand’s hernia: a review. Med Sci Monit. 2014;20:140-146. Published 2014 Jan 28. doi:10.12659/MSM.889873
22. Losanoff JE, Basson MD. Amyand hernia: what lies beneath--a proposed classification scheme to determine management. Am Surg. 2007;73(12):1288-1290.
23. Coulier B, Pacary J, Broze B. Sonographic diagnosis of appendicitis within a right inguinal hernia (Amyand’s hernia). J Clin Ultrasound. 2006;34(9):454-457. doi:10.1002/jcu.20266
24. Vehbi H, Agirgun C, Agirgun F, Dogan Y. Preoperative diagnosis of Amyand’s hernia by ultrasound and computed tomography. Turk J Emerg Med. 2016;16(2):72-74. Published 2016 May 8. doi:10.1016/j.tjem.2015.11.014
25. Vermillion JM, Abernathy SW, Snyder SK. Laparoscopic reduction of Amyand’s hernia. Hernia. 1999;3:159-160. doi:10.1007/BF01195318
Left-sided Amyand hernia is a rare condition that requires a high degree of clinical suspicion to correctly diagnose.
Left-sided Amyand hernia is a rare condition that requires a high degree of clinical suspicion to correctly diagnose.
The presence of the vermiform appendix within an inguinal hernia sac is termed an Amyand hernia. While the incidence of Amyand hernia in the general population is thought to be exceedingly rare, the presence of a left-sided Amyand hernia is even more rare due to the normal anatomical position of the appendix on the right side. Left-sided Amyand hernia presents a novel diagnosis that necessitates a high degree of clinical suspicion and special consideration during patient workup and operative treatment. We describe such a case and provide a review of all reports in the literature of this rare finding.
Case Presentation
A male aged 62 years presented to the emergency department of the Michael E. DeBakey Veterans Affairs Medical Center in Houston, Texas, in acute distress after experiencing 5 days of nausea and pain in his lower abdomen. The patient’s history was significant for cocaine abuse and a left-sided inguinal hernia that was repaired about 15 years prior to this visit. He reported having no bowel movements for the past 5 days and no other symptoms, including vomiting, hematemesis, and trauma to the abdomen. The patient’s abdominal pain was located in the suprapubic and periumbilical regions. Upon palpation of the lower abdomen, a firm, protruding mass was identified in the left lower quadrant and suspected to be a left-sided inguinal hernia.
A scout film and computed tomography (CT) scan of the abdomen taken on the same day that the patient presented to the emergency department confirmed the presence of a large left-sided inguinal hernia with possible bowel strangulation involving the colon (Figures 1, 2, and 3). The patient was diagnosed with an incarcerated recurrent left inguinal hernia and was taken emergently to the operating suite. General anesthesia and an ilioinguinal nerve block were performed. An inguinal incision was made on the left side, and the large hernia sac was identified and separated from the scrotum and spermatic cord structures.
On visual inspection, the hernia was identified as both a direct and an indirect inguinal hernia, making it a pantaloon hernia. The hernia sac was opened, and contents of the herniated sac were found to include the omentum, a loop of transverse colon, as well as the entire cecum and appendix, confirming the diagnosis of an Amyand hernia (Figure 4). Though the bowel was initially dusky, all the bowel became pink and appeared to be viable after detorsion of the bowel. Diagnostic laparoscopy through a 5-mm port was performed to assess the remainder of the bowel located intra-abdominally. The remaining intra-abdominal bowel appeared healthy and without obvious signs of ischemia, twisting, or malrotation. The large hernia defect was repaired with a polypropylene mesh.
Discussion
An Amyand hernia is an inguinal hernia in which the vermiform appendix is located within the hernial sac. Named after the French surgeon Claudius Amyand who first documented such a case during an appendectomy in 1735, the Amyand hernia is rare and is thought to occur in < 1% of inguinal hernias.1 Given the normal anatomical position of the appendix on the right side of the body, most Amyand hernias occur in a right-sided inguinal hernia.
A literature review yielded 25 reported instances of a left-sided Amyand hernia (Table 1) including this report. The true age of incidence of Amyand hernia for each patient is difficult to determine, as many patients do not present until pain or discomfort reaches high levels, often many years after hernia formation. Additionally, some cases of left-sided Amyand hernia described herein, including our case, are recurrent cases of a previous hernia that have been surgically repaired.2-20
Presentation of Amyand hernia often resembles that of a complicated inguinal hernia, acute appendicitis, or both. Hence, clinicians should consider this a possibility when patients present with signs and symptoms that could otherwise be thought to be originating from an incarcerated, strangulated, or recurrent hernia. Specifically, these signs and symptoms include a tender, nonreducible mass in the inguinal region, acute lower abdominal pain, nausea, or signs of intestinal obstruction such as failure to produce bowel movements.4,17 Because of the unusual anatomy in patients presenting with left-sided Amyand hernia, tenderness at the McBurney point usually is absent and not a useful diagnostic tool to rule out acute appendicitis.
A literature review indicates that an Amyand hernia on either side tends to occur in males more often than it does in females. The rate of diagnosis of Amyand hernia also has been reported to be 3 times higher in children than it is in adults due to failure of the processus vaginalis to obliterate during development.21 Our literature review supports this finding, as 16 of the documented 25 cases of left-sided Amyand hernia were reported in males. Additionally, information regarding gender was not found in 6 cases, suggesting a potential for an even higher prevalence in males.
Explanations as to why the appendix is on the left side in these patients include developmental anomalies, such as situs inversus, intestinal rotation, mobile cecum, or an abnormally long appendix.3,8 In our case, the likely causative culprit was a mobile cecum, as there was neither indication of intestinal malformation, rotation, nor of an abnormally long appendix during surgery. Additionally, pre-operative radiologic studies, clinical evaluation, and electrocardiogram did not suggest the presence of situs inversus.
Treatment of Amyand hernia usually follows the landmark classification algorithm set forth in 2007 by Losanoff and Basson (Table 2).22 This system stratifies treatment based on intraoperative findings of the appendix and surrounding structures, ranging from type 1, which involves a normal appendix within the hernia, to type 4, which includes acute appendicitis with additional abdominal pathology. Our patient presented with a type 1 Amyand hernia and appendectomy was foregone as per the guidelines; however, there have been numerous reported cases of surgeons opting for prophylactic appendectomy in the case of a normal appearing appendix and surrounding structures. The decision to act independent of the Losanoff and Basson classification underscores the lack of true standardization, namely, when it comes to a treatment approach for type 1 Amyand hernias. Nonetheless, many contend that indiscriminately performing appendectomies in all cases of left-sided Amyand hernia is useful as a prophylactic measure, as cases of future appendicitis in these patients will have atypical presentations based on the contralateral location of the appendix.6,11,17
Others disagree, citing that prophylactic appendectomy in the case of a normal looking appendix is unnecessary and complicates an otherwise sterile surgery (clean wound classification) with the removal of an appendix containing fecal matter and gut microbiota (converted into a clean contaminated or a contaminated wound classification).17 Additionally, it is thought that in the cases of middle-aged or geriatric patients where the chances of appendicitis are far less, the risks of detriment from prophylactic appendectomy may outweigh the benefits. In these cases, a macroscopic view of the appendix based on visual examination during the operation should guide decision making.4
While the decision to remove a healthy-appearing appendix remains contentious, the decision for or against placement of a heterogenous hernia mesh has proven to be binary, with near universally accepted criteria. If signs of perforation or infection are present in the hernia sac, then surgeons will forego hernioplasty with mesh for simple herniorrhaphy. This contraindication for mesh placement is due to the increased risk of mesh infection, wound infection, and fistulae associated with the introduction of a foreign structure to an active infection site.2
While most cases of Amyand hernia are diagnosed intraoperatively, there have been documented cases of preoperative diagnosis using ultrasonography and CT imaging modalities.19,23,24 In all cases, the presence of the vermiform appendix within the hernia sac can complicate diagnosis and treatment, and preoperative knowledge of this condition may help to guide physician decision making. Identifying Amyand hernia via CT scan is not only useful for alerting physicians of a potentially inflamed appendix within the hernia sac, but also may create opportunities for the use of other treatment modalities. For example, laparoscopic Amyand hernia reduction, an approach that was performed successfully and documented for the first time by Vermillion and colleagues, was made possible by preoperative diagnosis and can potentially result in improved patient outcomes.25
Regardless, while standardization of treatment for Amyand hernia has not yet occurred, it is clear that improved preoperative diagnosis, especially in the case of an unanticipated left-sided Amyand hernia, can allow for better planning and use of a wider variety of treatment modalities. The main impediment to this approach is that suspected cases of appendicitis and inguinal hernias (the most common preoperative diagnoses of Amyand hernia) usually are diagnosed clinically without the need of additional imaging studies like CT or ultrasound. In accordance with the guiding principle of radiation safety of exposing patients to “as low as reasonably achievable” (ALARA) radiation and with consideration of expediency of care and cost efficiency, we recommend physicians continue to screen for and treat cases of potentially emergent appendicitis and/or inguinal hernia as per the conventional methodology. The best approach may involve increasing preoperative diagnoses of left-sided Amyand hernias via physician awareness of this rare finding, as well as evaluating imaging studies that have previously been obtained in order to narrow a broad differential diagnosis.
Conclusions
Left-sided Amyand hernia is an exceptionally rare condition whose preoperative diagnosis remains difficult to establish but whose treatment decision tree is significantly impacted by the condition.
The presence of the vermiform appendix within an inguinal hernia sac is termed an Amyand hernia. While the incidence of Amyand hernia in the general population is thought to be exceedingly rare, the presence of a left-sided Amyand hernia is even more rare due to the normal anatomical position of the appendix on the right side. Left-sided Amyand hernia presents a novel diagnosis that necessitates a high degree of clinical suspicion and special consideration during patient workup and operative treatment. We describe such a case and provide a review of all reports in the literature of this rare finding.
Case Presentation
A male aged 62 years presented to the emergency department of the Michael E. DeBakey Veterans Affairs Medical Center in Houston, Texas, in acute distress after experiencing 5 days of nausea and pain in his lower abdomen. The patient’s history was significant for cocaine abuse and a left-sided inguinal hernia that was repaired about 15 years prior to this visit. He reported having no bowel movements for the past 5 days and no other symptoms, including vomiting, hematemesis, and trauma to the abdomen. The patient’s abdominal pain was located in the suprapubic and periumbilical regions. Upon palpation of the lower abdomen, a firm, protruding mass was identified in the left lower quadrant and suspected to be a left-sided inguinal hernia.
A scout film and computed tomography (CT) scan of the abdomen taken on the same day that the patient presented to the emergency department confirmed the presence of a large left-sided inguinal hernia with possible bowel strangulation involving the colon (Figures 1, 2, and 3). The patient was diagnosed with an incarcerated recurrent left inguinal hernia and was taken emergently to the operating suite. General anesthesia and an ilioinguinal nerve block were performed. An inguinal incision was made on the left side, and the large hernia sac was identified and separated from the scrotum and spermatic cord structures.
On visual inspection, the hernia was identified as both a direct and an indirect inguinal hernia, making it a pantaloon hernia. The hernia sac was opened, and contents of the herniated sac were found to include the omentum, a loop of transverse colon, as well as the entire cecum and appendix, confirming the diagnosis of an Amyand hernia (Figure 4). Though the bowel was initially dusky, all the bowel became pink and appeared to be viable after detorsion of the bowel. Diagnostic laparoscopy through a 5-mm port was performed to assess the remainder of the bowel located intra-abdominally. The remaining intra-abdominal bowel appeared healthy and without obvious signs of ischemia, twisting, or malrotation. The large hernia defect was repaired with a polypropylene mesh.
Discussion
An Amyand hernia is an inguinal hernia in which the vermiform appendix is located within the hernial sac. Named after the French surgeon Claudius Amyand who first documented such a case during an appendectomy in 1735, the Amyand hernia is rare and is thought to occur in < 1% of inguinal hernias.1 Given the normal anatomical position of the appendix on the right side of the body, most Amyand hernias occur in a right-sided inguinal hernia.
A literature review yielded 25 reported instances of a left-sided Amyand hernia (Table 1) including this report. The true age of incidence of Amyand hernia for each patient is difficult to determine, as many patients do not present until pain or discomfort reaches high levels, often many years after hernia formation. Additionally, some cases of left-sided Amyand hernia described herein, including our case, are recurrent cases of a previous hernia that have been surgically repaired.2-20
Presentation of Amyand hernia often resembles that of a complicated inguinal hernia, acute appendicitis, or both. Hence, clinicians should consider this a possibility when patients present with signs and symptoms that could otherwise be thought to be originating from an incarcerated, strangulated, or recurrent hernia. Specifically, these signs and symptoms include a tender, nonreducible mass in the inguinal region, acute lower abdominal pain, nausea, or signs of intestinal obstruction such as failure to produce bowel movements.4,17 Because of the unusual anatomy in patients presenting with left-sided Amyand hernia, tenderness at the McBurney point usually is absent and not a useful diagnostic tool to rule out acute appendicitis.
A literature review indicates that an Amyand hernia on either side tends to occur in males more often than it does in females. The rate of diagnosis of Amyand hernia also has been reported to be 3 times higher in children than it is in adults due to failure of the processus vaginalis to obliterate during development.21 Our literature review supports this finding, as 16 of the documented 25 cases of left-sided Amyand hernia were reported in males. Additionally, information regarding gender was not found in 6 cases, suggesting a potential for an even higher prevalence in males.
Explanations as to why the appendix is on the left side in these patients include developmental anomalies, such as situs inversus, intestinal rotation, mobile cecum, or an abnormally long appendix.3,8 In our case, the likely causative culprit was a mobile cecum, as there was neither indication of intestinal malformation, rotation, nor of an abnormally long appendix during surgery. Additionally, pre-operative radiologic studies, clinical evaluation, and electrocardiogram did not suggest the presence of situs inversus.
Treatment of Amyand hernia usually follows the landmark classification algorithm set forth in 2007 by Losanoff and Basson (Table 2).22 This system stratifies treatment based on intraoperative findings of the appendix and surrounding structures, ranging from type 1, which involves a normal appendix within the hernia, to type 4, which includes acute appendicitis with additional abdominal pathology. Our patient presented with a type 1 Amyand hernia and appendectomy was foregone as per the guidelines; however, there have been numerous reported cases of surgeons opting for prophylactic appendectomy in the case of a normal appearing appendix and surrounding structures. The decision to act independent of the Losanoff and Basson classification underscores the lack of true standardization, namely, when it comes to a treatment approach for type 1 Amyand hernias. Nonetheless, many contend that indiscriminately performing appendectomies in all cases of left-sided Amyand hernia is useful as a prophylactic measure, as cases of future appendicitis in these patients will have atypical presentations based on the contralateral location of the appendix.6,11,17
Others disagree, citing that prophylactic appendectomy in the case of a normal looking appendix is unnecessary and complicates an otherwise sterile surgery (clean wound classification) with the removal of an appendix containing fecal matter and gut microbiota (converted into a clean contaminated or a contaminated wound classification).17 Additionally, it is thought that in the cases of middle-aged or geriatric patients where the chances of appendicitis are far less, the risks of detriment from prophylactic appendectomy may outweigh the benefits. In these cases, a macroscopic view of the appendix based on visual examination during the operation should guide decision making.4
While the decision to remove a healthy-appearing appendix remains contentious, the decision for or against placement of a heterogenous hernia mesh has proven to be binary, with near universally accepted criteria. If signs of perforation or infection are present in the hernia sac, then surgeons will forego hernioplasty with mesh for simple herniorrhaphy. This contraindication for mesh placement is due to the increased risk of mesh infection, wound infection, and fistulae associated with the introduction of a foreign structure to an active infection site.2
While most cases of Amyand hernia are diagnosed intraoperatively, there have been documented cases of preoperative diagnosis using ultrasonography and CT imaging modalities.19,23,24 In all cases, the presence of the vermiform appendix within the hernia sac can complicate diagnosis and treatment, and preoperative knowledge of this condition may help to guide physician decision making. Identifying Amyand hernia via CT scan is not only useful for alerting physicians of a potentially inflamed appendix within the hernia sac, but also may create opportunities for the use of other treatment modalities. For example, laparoscopic Amyand hernia reduction, an approach that was performed successfully and documented for the first time by Vermillion and colleagues, was made possible by preoperative diagnosis and can potentially result in improved patient outcomes.25
Regardless, while standardization of treatment for Amyand hernia has not yet occurred, it is clear that improved preoperative diagnosis, especially in the case of an unanticipated left-sided Amyand hernia, can allow for better planning and use of a wider variety of treatment modalities. The main impediment to this approach is that suspected cases of appendicitis and inguinal hernias (the most common preoperative diagnoses of Amyand hernia) usually are diagnosed clinically without the need of additional imaging studies like CT or ultrasound. In accordance with the guiding principle of radiation safety of exposing patients to “as low as reasonably achievable” (ALARA) radiation and with consideration of expediency of care and cost efficiency, we recommend physicians continue to screen for and treat cases of potentially emergent appendicitis and/or inguinal hernia as per the conventional methodology. The best approach may involve increasing preoperative diagnoses of left-sided Amyand hernias via physician awareness of this rare finding, as well as evaluating imaging studies that have previously been obtained in order to narrow a broad differential diagnosis.
Conclusions
Left-sided Amyand hernia is an exceptionally rare condition whose preoperative diagnosis remains difficult to establish but whose treatment decision tree is significantly impacted by the condition.
1. Franko J, Raftopoulos I, Sulkowski R. A rare variation of Amyand’s hernia. Am J Gastroenterol. 2002;97(10):2684-2685. doi:10.1111/j.1572-0241.2002.06060.x
2. Carey LC. Acute appendicitis occurring in hernias: a report of 10 cases. Surgery. 1967;61(2):236-238.
3. Kaymakci A, Akillioglu I, Akkoyun I, Guven S, Ozdemir A, Gulen S. Amyand’s hernia: a series of 30 cases in children. Hernia. 2009;13(6):609-612. doi:10.1007/s10029-009-0528-8
4. Cankorkmaz L, Ozer H, Guney C, Atalar MH, Arslan MS, Koyluoglu G. Amyand’s hernia in the children: a single center experience. Surgery. 2010;147(1):140-143. doi:10.1016/j.surg.2009.09.038
5. Yasumoto R, Kawano M, Kawanishi H, et al. Left acute scrotum associated with appendicitis. Int J Urol. 1998;5(1):108-110. doi:10.1111/j.1442-2042.1998.tb00254.x
6. Bakhshi GD, Bhandarwar AH, Govila AA. Acute appendicitis in left scrotum. Indian J Gastroenterol. 2004;23(5):195.
7. Breitenstein S, Eisenbach C, Wille G, Decurtins M. Incarcerated vermiform appendix in a left-sided inguinal hernia. Hernia. 2005;9(1):100-102. doi:10.1007/s10029-004-0263-0
8. Gupta S, Sharma R, Kaushik R. Left-sided Amyand’s hernia. Singapore Med J. 2005;46(8):424-425.
9. Gupta N, Wilkinson TV, Wilkinson A, Akhtar M. Left-sided incarcerated Amyand’s hernia. Indian J Surg. 2007;69(1):17-18.
10. Tayade, MB, Bakhshi GD, Borisa AD, Deshpande G, Joshi N. A rare combination of left sided Amyand’s and Richter’s hernia. Bombay Hosp J. 2008;50(4): 644-645
11. Johari HG, Paydar S, Davani SZ, Eskandari S, Johari MG. Left-sided Amyand hernia. Ann Saudi Med. 2009;29(4):321-322. doi:10.4103/0256-4947.55305
12. Ali SM, Malik KA, Al-Qadhi H. Amyand’s Hernia: Study of four cases and literature review. Sultan Qaboos Univ Med J. 2012;12(2):232-236. doi:10.12816/0003119
13. Ravishankaran P, Mohan G, Srinivasan A, Ravindran G, Ramalingam A. Left sided amyand’s hernia, a rare occurrence: A Case Report. Indian J Surg. 2013;75(3):247-248. doi:10.1007/s12262-010-0223-0
14. Singh K, Singh RR, Kaur S. Amyand’s hernia. J Indian Assoc Pediatr Surg. 2011;16(4):171-172. doi:10.4103/0971-9261.86890
15. Khan TS, Wani ML, Bijli AH, et al. Amyand’s hernia: a rare occurrence. Ann Nigerian Med. 2011;5(2):62-64.doi:10.4103/0331-3131.92955
16. Ghafouri A, Anbara T, Foroutankia R. A rare case report of appendix and cecum in the sac of left inguinal hernia (left Amyand’s hernia). Med J Islam Repub Iran. 2012;26(2):94-95.
17. Al-Mayoof AF, Al-Ani BH. Left-sided amyand hernia: report of two cases with review of literature. European J Pediatr Surg Rep. 2014;2(1):63-66. doi:10.1055/s-0033-1347131
18. Unver M, Ozturk S, Karaman K, Turgut E. Left sided Amyand’s hernia. World J Gastrointest Surg. 2013;5(10):285-286. doi:10.4240/wjgs.v5.i10.285
19. Maeda K, Kunieda K, Kawai M, et al. Giant left-sided inguinoscrotal hernia containing the cecum and appendix (giant left-sided Amyand’s hernia). Clin Case Rep. 2014;2(6):254-257. doi:10.1002/ccr3.104
20. Mongardini M, Maturo A, De Anna L, et al. Appendiceal abscess in a giant left-sided inguinoscrotal hernia: a rare case of Amyand hernia. Springerplus. 2015;4:378. Published 2015 Jul 26. doi:10.1186/s40064-015-1162-9
21. Ivanschuk G, Cesmebasi A, Sorenson EP, Blaak C, Loukas M, Tubbs SR. Amyand’s hernia: a review. Med Sci Monit. 2014;20:140-146. Published 2014 Jan 28. doi:10.12659/MSM.889873
22. Losanoff JE, Basson MD. Amyand hernia: what lies beneath--a proposed classification scheme to determine management. Am Surg. 2007;73(12):1288-1290.
23. Coulier B, Pacary J, Broze B. Sonographic diagnosis of appendicitis within a right inguinal hernia (Amyand’s hernia). J Clin Ultrasound. 2006;34(9):454-457. doi:10.1002/jcu.20266
24. Vehbi H, Agirgun C, Agirgun F, Dogan Y. Preoperative diagnosis of Amyand’s hernia by ultrasound and computed tomography. Turk J Emerg Med. 2016;16(2):72-74. Published 2016 May 8. doi:10.1016/j.tjem.2015.11.014
25. Vermillion JM, Abernathy SW, Snyder SK. Laparoscopic reduction of Amyand’s hernia. Hernia. 1999;3:159-160. doi:10.1007/BF01195318
1. Franko J, Raftopoulos I, Sulkowski R. A rare variation of Amyand’s hernia. Am J Gastroenterol. 2002;97(10):2684-2685. doi:10.1111/j.1572-0241.2002.06060.x
2. Carey LC. Acute appendicitis occurring in hernias: a report of 10 cases. Surgery. 1967;61(2):236-238.
3. Kaymakci A, Akillioglu I, Akkoyun I, Guven S, Ozdemir A, Gulen S. Amyand’s hernia: a series of 30 cases in children. Hernia. 2009;13(6):609-612. doi:10.1007/s10029-009-0528-8
4. Cankorkmaz L, Ozer H, Guney C, Atalar MH, Arslan MS, Koyluoglu G. Amyand’s hernia in the children: a single center experience. Surgery. 2010;147(1):140-143. doi:10.1016/j.surg.2009.09.038
5. Yasumoto R, Kawano M, Kawanishi H, et al. Left acute scrotum associated with appendicitis. Int J Urol. 1998;5(1):108-110. doi:10.1111/j.1442-2042.1998.tb00254.x
6. Bakhshi GD, Bhandarwar AH, Govila AA. Acute appendicitis in left scrotum. Indian J Gastroenterol. 2004;23(5):195.
7. Breitenstein S, Eisenbach C, Wille G, Decurtins M. Incarcerated vermiform appendix in a left-sided inguinal hernia. Hernia. 2005;9(1):100-102. doi:10.1007/s10029-004-0263-0
8. Gupta S, Sharma R, Kaushik R. Left-sided Amyand’s hernia. Singapore Med J. 2005;46(8):424-425.
9. Gupta N, Wilkinson TV, Wilkinson A, Akhtar M. Left-sided incarcerated Amyand’s hernia. Indian J Surg. 2007;69(1):17-18.
10. Tayade, MB, Bakhshi GD, Borisa AD, Deshpande G, Joshi N. A rare combination of left sided Amyand’s and Richter’s hernia. Bombay Hosp J. 2008;50(4): 644-645
11. Johari HG, Paydar S, Davani SZ, Eskandari S, Johari MG. Left-sided Amyand hernia. Ann Saudi Med. 2009;29(4):321-322. doi:10.4103/0256-4947.55305
12. Ali SM, Malik KA, Al-Qadhi H. Amyand’s Hernia: Study of four cases and literature review. Sultan Qaboos Univ Med J. 2012;12(2):232-236. doi:10.12816/0003119
13. Ravishankaran P, Mohan G, Srinivasan A, Ravindran G, Ramalingam A. Left sided amyand’s hernia, a rare occurrence: A Case Report. Indian J Surg. 2013;75(3):247-248. doi:10.1007/s12262-010-0223-0
14. Singh K, Singh RR, Kaur S. Amyand’s hernia. J Indian Assoc Pediatr Surg. 2011;16(4):171-172. doi:10.4103/0971-9261.86890
15. Khan TS, Wani ML, Bijli AH, et al. Amyand’s hernia: a rare occurrence. Ann Nigerian Med. 2011;5(2):62-64.doi:10.4103/0331-3131.92955
16. Ghafouri A, Anbara T, Foroutankia R. A rare case report of appendix and cecum in the sac of left inguinal hernia (left Amyand’s hernia). Med J Islam Repub Iran. 2012;26(2):94-95.
17. Al-Mayoof AF, Al-Ani BH. Left-sided amyand hernia: report of two cases with review of literature. European J Pediatr Surg Rep. 2014;2(1):63-66. doi:10.1055/s-0033-1347131
18. Unver M, Ozturk S, Karaman K, Turgut E. Left sided Amyand’s hernia. World J Gastrointest Surg. 2013;5(10):285-286. doi:10.4240/wjgs.v5.i10.285
19. Maeda K, Kunieda K, Kawai M, et al. Giant left-sided inguinoscrotal hernia containing the cecum and appendix (giant left-sided Amyand’s hernia). Clin Case Rep. 2014;2(6):254-257. doi:10.1002/ccr3.104
20. Mongardini M, Maturo A, De Anna L, et al. Appendiceal abscess in a giant left-sided inguinoscrotal hernia: a rare case of Amyand hernia. Springerplus. 2015;4:378. Published 2015 Jul 26. doi:10.1186/s40064-015-1162-9
21. Ivanschuk G, Cesmebasi A, Sorenson EP, Blaak C, Loukas M, Tubbs SR. Amyand’s hernia: a review. Med Sci Monit. 2014;20:140-146. Published 2014 Jan 28. doi:10.12659/MSM.889873
22. Losanoff JE, Basson MD. Amyand hernia: what lies beneath--a proposed classification scheme to determine management. Am Surg. 2007;73(12):1288-1290.
23. Coulier B, Pacary J, Broze B. Sonographic diagnosis of appendicitis within a right inguinal hernia (Amyand’s hernia). J Clin Ultrasound. 2006;34(9):454-457. doi:10.1002/jcu.20266
24. Vehbi H, Agirgun C, Agirgun F, Dogan Y. Preoperative diagnosis of Amyand’s hernia by ultrasound and computed tomography. Turk J Emerg Med. 2016;16(2):72-74. Published 2016 May 8. doi:10.1016/j.tjem.2015.11.014
25. Vermillion JM, Abernathy SW, Snyder SK. Laparoscopic reduction of Amyand’s hernia. Hernia. 1999;3:159-160. doi:10.1007/BF01195318
Obesity amplifies harmful effects of alcohol on the liver
Being overweight or having obesity significantly increases the risk for liver disease and the likelihood of dying from it compared with being of normal weight, regardless of level of alcohol consumption, new research shows.
"People in the overweight or obese range who drank were found to be at greater risk of liver diseases compared with participants within a healthy weight range who consumed alcohol at the same level," senior author Emmanuel Stamatakis, PhD, of the Charles Perkins Centre and the Faculty of Medicine and Health, Sydney, said in a press statement.
"Even for people who drank within alcohol guidelines, participants classified as obese were at over 50% greater risk of liver disease," he said.
"Obesity is an independent risk factor for steatosis, acute alcoholic hepatitis, and cirrhosis in alcoholic liver disease (ALD), which may increase the risk of mortality in ALD patients," the study's first author, Elif Inan-Eroglu, PhD, a postdoctoral research fellow at the Charles Perkins Centre, said in an interview.
Further prospective studies are needed to better understand the underlying mechanisms behind the association between alcohol consumption and liver disease across different adiposity levels, the authors say.
Meanwhile, the take-home message from the findings should be that "clinicians should consider the presence of overweight and obesity when they discuss defining safe alcohol levels for their patients, keeping in mind that there is no 'safe' level of alcohol," Dr. Inan-Eroglu said.
"Alcohol drinking guidelines need to acknowledge that two-thirds of the adult population are overweight or obese and consider specific recommendations for this majority population group," he said.
First and largest study of its kind
Obesity, well-known to be an independent risk factor for nonalcoholic fatty liver disease (NAFLD), is also known to worsen outcomes in ALD. And likewise, alcohol consumption, the cause of ALD, can promote obesity and therefore increase the risk of NAFLD.
Dr. Stamatakis and colleagues sought to evaluate the roles of the combined factors in terms of incidence and mortality in both ALD and NAFLD.
For the study, published online May 31 in the European Journal of Clinical Nutrition, they evaluated data from 465,437 participants in the U.K. Biobank. The study is said to be the first and largest of its kind.
In the cohort, a total of 1,090 liver disease deaths were recorded, including 230 deaths from ALD and 192 from NAFLD over an average follow-up of 10.5 years.
After a multivariate adjustment, the overall risk of ALD, NAFLD, and liver disease incidence and mortality were significantly higher in participants who were overweight or had obesity, compared with those of normal weight, at all levels of alcohol consumption.
For instance, among those with alcohol use exceeding guidelines, the risk of ALD was significantly increased in normal weight individuals versus never-drinkers (hazard ratio [HR], 5.38), and the risk was even higher among those who were also overweight or had obesity (HR, 8.58).
In terms of the risk of death related to ALD, among those reporting alcohol consumption above guidelines, the risk was nearly double among those who were overweight or had obesity (HR, 10.29) versus those with normal weight (HR, 5.84), when each group was compared to those drinking within guidelines.
Regarding NAFLD, consistent with evidence that low to moderate alcohol consumption is, in fact, linked to a reduced risk, those in the study who reported alcohol consumption within guidelines and normal weight did show a reduced risk of NAFLD compared with an index group of never-drinkers (HR, 0.85).
However, being overweight or having obesity increased the risk of NAFLD in those participants (HR, 1.51).
Furthermore, even those reporting alcohol consumption above guidelines who were of normal weight had a reduced risk of NAFLD compared with never drinkers of normal weight (HR, 0.89).
Regarding the risk of liver disease among those reporting alcohol consumption above guidelines compared with never-drinkers, the risk was again lower among those of normal weight versus those who were overweight or had obesity (HR, 0.95 vs. 1.52), as were the risks of mortality (HR, 1.24 vs. 2.20).
Overall, "we found evidence that being overweight/[having obesity] amplified the harmful effect of alcohol on the liver disease incidence and mortality," the authors conclude.
The authors have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com
Being overweight or having obesity significantly increases the risk for liver disease and the likelihood of dying from it compared with being of normal weight, regardless of level of alcohol consumption, new research shows.
"People in the overweight or obese range who drank were found to be at greater risk of liver diseases compared with participants within a healthy weight range who consumed alcohol at the same level," senior author Emmanuel Stamatakis, PhD, of the Charles Perkins Centre and the Faculty of Medicine and Health, Sydney, said in a press statement.
"Even for people who drank within alcohol guidelines, participants classified as obese were at over 50% greater risk of liver disease," he said.
"Obesity is an independent risk factor for steatosis, acute alcoholic hepatitis, and cirrhosis in alcoholic liver disease (ALD), which may increase the risk of mortality in ALD patients," the study's first author, Elif Inan-Eroglu, PhD, a postdoctoral research fellow at the Charles Perkins Centre, said in an interview.
Further prospective studies are needed to better understand the underlying mechanisms behind the association between alcohol consumption and liver disease across different adiposity levels, the authors say.
Meanwhile, the take-home message from the findings should be that "clinicians should consider the presence of overweight and obesity when they discuss defining safe alcohol levels for their patients, keeping in mind that there is no 'safe' level of alcohol," Dr. Inan-Eroglu said.
"Alcohol drinking guidelines need to acknowledge that two-thirds of the adult population are overweight or obese and consider specific recommendations for this majority population group," he said.
First and largest study of its kind
Obesity, well-known to be an independent risk factor for nonalcoholic fatty liver disease (NAFLD), is also known to worsen outcomes in ALD. And likewise, alcohol consumption, the cause of ALD, can promote obesity and therefore increase the risk of NAFLD.
Dr. Stamatakis and colleagues sought to evaluate the roles of the combined factors in terms of incidence and mortality in both ALD and NAFLD.
For the study, published online May 31 in the European Journal of Clinical Nutrition, they evaluated data from 465,437 participants in the U.K. Biobank. The study is said to be the first and largest of its kind.
In the cohort, a total of 1,090 liver disease deaths were recorded, including 230 deaths from ALD and 192 from NAFLD over an average follow-up of 10.5 years.
After a multivariate adjustment, the overall risk of ALD, NAFLD, and liver disease incidence and mortality were significantly higher in participants who were overweight or had obesity, compared with those of normal weight, at all levels of alcohol consumption.
For instance, among those with alcohol use exceeding guidelines, the risk of ALD was significantly increased in normal weight individuals versus never-drinkers (hazard ratio [HR], 5.38), and the risk was even higher among those who were also overweight or had obesity (HR, 8.58).
In terms of the risk of death related to ALD, among those reporting alcohol consumption above guidelines, the risk was nearly double among those who were overweight or had obesity (HR, 10.29) versus those with normal weight (HR, 5.84), when each group was compared to those drinking within guidelines.
Regarding NAFLD, consistent with evidence that low to moderate alcohol consumption is, in fact, linked to a reduced risk, those in the study who reported alcohol consumption within guidelines and normal weight did show a reduced risk of NAFLD compared with an index group of never-drinkers (HR, 0.85).
However, being overweight or having obesity increased the risk of NAFLD in those participants (HR, 1.51).
Furthermore, even those reporting alcohol consumption above guidelines who were of normal weight had a reduced risk of NAFLD compared with never drinkers of normal weight (HR, 0.89).
Regarding the risk of liver disease among those reporting alcohol consumption above guidelines compared with never-drinkers, the risk was again lower among those of normal weight versus those who were overweight or had obesity (HR, 0.95 vs. 1.52), as were the risks of mortality (HR, 1.24 vs. 2.20).
Overall, "we found evidence that being overweight/[having obesity] amplified the harmful effect of alcohol on the liver disease incidence and mortality," the authors conclude.
The authors have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com
Being overweight or having obesity significantly increases the risk for liver disease and the likelihood of dying from it compared with being of normal weight, regardless of level of alcohol consumption, new research shows.
"People in the overweight or obese range who drank were found to be at greater risk of liver diseases compared with participants within a healthy weight range who consumed alcohol at the same level," senior author Emmanuel Stamatakis, PhD, of the Charles Perkins Centre and the Faculty of Medicine and Health, Sydney, said in a press statement.
"Even for people who drank within alcohol guidelines, participants classified as obese were at over 50% greater risk of liver disease," he said.
"Obesity is an independent risk factor for steatosis, acute alcoholic hepatitis, and cirrhosis in alcoholic liver disease (ALD), which may increase the risk of mortality in ALD patients," the study's first author, Elif Inan-Eroglu, PhD, a postdoctoral research fellow at the Charles Perkins Centre, said in an interview.
Further prospective studies are needed to better understand the underlying mechanisms behind the association between alcohol consumption and liver disease across different adiposity levels, the authors say.
Meanwhile, the take-home message from the findings should be that "clinicians should consider the presence of overweight and obesity when they discuss defining safe alcohol levels for their patients, keeping in mind that there is no 'safe' level of alcohol," Dr. Inan-Eroglu said.
"Alcohol drinking guidelines need to acknowledge that two-thirds of the adult population are overweight or obese and consider specific recommendations for this majority population group," he said.
First and largest study of its kind
Obesity, well-known to be an independent risk factor for nonalcoholic fatty liver disease (NAFLD), is also known to worsen outcomes in ALD. And likewise, alcohol consumption, the cause of ALD, can promote obesity and therefore increase the risk of NAFLD.
Dr. Stamatakis and colleagues sought to evaluate the roles of the combined factors in terms of incidence and mortality in both ALD and NAFLD.
For the study, published online May 31 in the European Journal of Clinical Nutrition, they evaluated data from 465,437 participants in the U.K. Biobank. The study is said to be the first and largest of its kind.
In the cohort, a total of 1,090 liver disease deaths were recorded, including 230 deaths from ALD and 192 from NAFLD over an average follow-up of 10.5 years.
After a multivariate adjustment, the overall risk of ALD, NAFLD, and liver disease incidence and mortality were significantly higher in participants who were overweight or had obesity, compared with those of normal weight, at all levels of alcohol consumption.
For instance, among those with alcohol use exceeding guidelines, the risk of ALD was significantly increased in normal weight individuals versus never-drinkers (hazard ratio [HR], 5.38), and the risk was even higher among those who were also overweight or had obesity (HR, 8.58).
In terms of the risk of death related to ALD, among those reporting alcohol consumption above guidelines, the risk was nearly double among those who were overweight or had obesity (HR, 10.29) versus those with normal weight (HR, 5.84), when each group was compared to those drinking within guidelines.
Regarding NAFLD, consistent with evidence that low to moderate alcohol consumption is, in fact, linked to a reduced risk, those in the study who reported alcohol consumption within guidelines and normal weight did show a reduced risk of NAFLD compared with an index group of never-drinkers (HR, 0.85).
However, being overweight or having obesity increased the risk of NAFLD in those participants (HR, 1.51).
Furthermore, even those reporting alcohol consumption above guidelines who were of normal weight had a reduced risk of NAFLD compared with never drinkers of normal weight (HR, 0.89).
Regarding the risk of liver disease among those reporting alcohol consumption above guidelines compared with never-drinkers, the risk was again lower among those of normal weight versus those who were overweight or had obesity (HR, 0.95 vs. 1.52), as were the risks of mortality (HR, 1.24 vs. 2.20).
Overall, "we found evidence that being overweight/[having obesity] amplified the harmful effect of alcohol on the liver disease incidence and mortality," the authors conclude.
The authors have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com