User login
Colchicine before PCI for acute MI fails to improve major outcomes
In a placebo-controlled randomized trial, a preprocedural dose of colchicine administered immediately before percutaneous coronary intervention (PCI) for an acute ST-segment elevated myocardial infarction (STEMI) did not reduce the no-reflow phenomenon or improve outcomes.
No-reflow, in which insufficient myocardial perfusion is present even though the coronary artery appears patent, was the primary outcome, and the proportion of patients experiencing this event was exactly the same (14.4%) in the colchicine and placebo groups, reported Yaser Jenab, MD, at CRT 2021 sponsored by MedStar Heart & Vascular Institute.
The hypothesis that colchicine would offer benefit in this setting was largely based on the Colchicine Cardiovascular Outcomes Trial (COLCOT). In that study, colchicine was associated with a 23% reduction in risk for major adverse cardiovascular events (MACE) relative to placebo when administered within 30 days after a myocardial infarction (hazard ratio, 0.77; P = .02).
The benefit in that trial was attributed to an anti-inflammatory effect, according to Dr. Jenab, associate professor of cardiology at Tehran (Iran) Heart Center. In particular as it relates to vascular disease, he cited experimental studies associating colchicine with a reduction in neutrophil activation and adherence to vascular endothelium.
The rationale for a preprocedural approach to colchicine was supplied by a subsequent time-to-treatment COLCOT analysis. In this study, MACE risk reduction for colchicine climbed to 48% (HR 0.52) for those treated within 3 days of the MI but largely disappeared (HR 0.96) if treatment was started at least 8 days post MI.
PodCAST-PCI trial
In the preprocedural study, called the PodCAST-PCI trial, 321 acute STEMI patients were randomized. Patients received a 1-mg dose of oral colchicine or placebo at the time PCI was scheduled. Another dose of colchicine (0.5 mg) or placebo was administered 1 hour after the procedure.
Of secondary outcomes, which included MACE at 1 month and 1 year, ST-segment resolution at 1 month, and change in inflammatory markers at 1 month, none were significant. Few even trended for significance.
For MACE, which included cardiac death, stroke, nonfatal MI, new hospitalization due to heart failure, or target vessel revascularization, the rates were lower in the colchicine group at 1 month (4.3% vs. 7.5%) and 1 year (9.3% vs. 11.2%), but neither approached significance.
For ST-segment resolution, the proportions were generally comparable among the colchicine and placebo groups, respectively, for the proportion below 50% (18.6% vs. 23.1%), between 50% and 70% (16.8% vs. 15.6%), and above 70% (64.6% vs. 61.3%).
The average troponin levels were nonsignificantly lower at 6 hours (1,847 vs. 2,883 ng/mL) in the colchicine group but higher at 48 hours (1,197 vs. 1,147 ng/mL). The average C-reactive protein (CRP) levels at 48 hours were nonsignificantly lower on colchicine (176.5 vs. 244.5 mg/L).
There were no significant differences in postprocedural perfusion, as measured with TIMI blood flow, or in the rate of stent thrombosis, which occurred in roughly 3% of each group of patients.
The small sample size was one limitation of this study, Dr. Jenab acknowledged. For this and other reasons, he cautioned that these data are not definitive and do not preclude a benefit on clinical outcomes in a study with a larger size, a different design, or different dosing.
Timing might be the issue
However, even if colchicine has a potential benefit in this setting, timing might be a major obstacle, according to Binata Shah, MD, associate director of research for the Cardiac Catheterization Laboratory at New York University.
“We have learned from our rheumatology colleagues that peak plasma levels of colchicine are not achieved for at least 1 hour after the full loading dose,” Dr. Shah said. “With us moving so quickly in a primary PCI setting, it is hard to imagine that colchicine would have had time to really kick in and exert its anti-inflammatory effect.”
Indeed, the problem might be worse than reaching the peak plasma level.
“Even though peak plasma levels occur as early as 1 hour after a full loading dose, we see that it takes about 24 hours to really see the effects translate downstream into more systemic inflammatory markers such as CRP and interleukin-6,” she added. If lowering these signals of inflammation is predictive of benefit, than this might be the biggest obstacle to benefit from colchicine in an urgent treatment setting.
Dr. Jenab and Dr. Shah reported no potential conflicts of interest.
In a placebo-controlled randomized trial, a preprocedural dose of colchicine administered immediately before percutaneous coronary intervention (PCI) for an acute ST-segment elevated myocardial infarction (STEMI) did not reduce the no-reflow phenomenon or improve outcomes.
No-reflow, in which insufficient myocardial perfusion is present even though the coronary artery appears patent, was the primary outcome, and the proportion of patients experiencing this event was exactly the same (14.4%) in the colchicine and placebo groups, reported Yaser Jenab, MD, at CRT 2021 sponsored by MedStar Heart & Vascular Institute.
The hypothesis that colchicine would offer benefit in this setting was largely based on the Colchicine Cardiovascular Outcomes Trial (COLCOT). In that study, colchicine was associated with a 23% reduction in risk for major adverse cardiovascular events (MACE) relative to placebo when administered within 30 days after a myocardial infarction (hazard ratio, 0.77; P = .02).
The benefit in that trial was attributed to an anti-inflammatory effect, according to Dr. Jenab, associate professor of cardiology at Tehran (Iran) Heart Center. In particular as it relates to vascular disease, he cited experimental studies associating colchicine with a reduction in neutrophil activation and adherence to vascular endothelium.
The rationale for a preprocedural approach to colchicine was supplied by a subsequent time-to-treatment COLCOT analysis. In this study, MACE risk reduction for colchicine climbed to 48% (HR 0.52) for those treated within 3 days of the MI but largely disappeared (HR 0.96) if treatment was started at least 8 days post MI.
PodCAST-PCI trial
In the preprocedural study, called the PodCAST-PCI trial, 321 acute STEMI patients were randomized. Patients received a 1-mg dose of oral colchicine or placebo at the time PCI was scheduled. Another dose of colchicine (0.5 mg) or placebo was administered 1 hour after the procedure.
Of secondary outcomes, which included MACE at 1 month and 1 year, ST-segment resolution at 1 month, and change in inflammatory markers at 1 month, none were significant. Few even trended for significance.
For MACE, which included cardiac death, stroke, nonfatal MI, new hospitalization due to heart failure, or target vessel revascularization, the rates were lower in the colchicine group at 1 month (4.3% vs. 7.5%) and 1 year (9.3% vs. 11.2%), but neither approached significance.
For ST-segment resolution, the proportions were generally comparable among the colchicine and placebo groups, respectively, for the proportion below 50% (18.6% vs. 23.1%), between 50% and 70% (16.8% vs. 15.6%), and above 70% (64.6% vs. 61.3%).
The average troponin levels were nonsignificantly lower at 6 hours (1,847 vs. 2,883 ng/mL) in the colchicine group but higher at 48 hours (1,197 vs. 1,147 ng/mL). The average C-reactive protein (CRP) levels at 48 hours were nonsignificantly lower on colchicine (176.5 vs. 244.5 mg/L).
There were no significant differences in postprocedural perfusion, as measured with TIMI blood flow, or in the rate of stent thrombosis, which occurred in roughly 3% of each group of patients.
The small sample size was one limitation of this study, Dr. Jenab acknowledged. For this and other reasons, he cautioned that these data are not definitive and do not preclude a benefit on clinical outcomes in a study with a larger size, a different design, or different dosing.
Timing might be the issue
However, even if colchicine has a potential benefit in this setting, timing might be a major obstacle, according to Binata Shah, MD, associate director of research for the Cardiac Catheterization Laboratory at New York University.
“We have learned from our rheumatology colleagues that peak plasma levels of colchicine are not achieved for at least 1 hour after the full loading dose,” Dr. Shah said. “With us moving so quickly in a primary PCI setting, it is hard to imagine that colchicine would have had time to really kick in and exert its anti-inflammatory effect.”
Indeed, the problem might be worse than reaching the peak plasma level.
“Even though peak plasma levels occur as early as 1 hour after a full loading dose, we see that it takes about 24 hours to really see the effects translate downstream into more systemic inflammatory markers such as CRP and interleukin-6,” she added. If lowering these signals of inflammation is predictive of benefit, than this might be the biggest obstacle to benefit from colchicine in an urgent treatment setting.
Dr. Jenab and Dr. Shah reported no potential conflicts of interest.
In a placebo-controlled randomized trial, a preprocedural dose of colchicine administered immediately before percutaneous coronary intervention (PCI) for an acute ST-segment elevated myocardial infarction (STEMI) did not reduce the no-reflow phenomenon or improve outcomes.
No-reflow, in which insufficient myocardial perfusion is present even though the coronary artery appears patent, was the primary outcome, and the proportion of patients experiencing this event was exactly the same (14.4%) in the colchicine and placebo groups, reported Yaser Jenab, MD, at CRT 2021 sponsored by MedStar Heart & Vascular Institute.
The hypothesis that colchicine would offer benefit in this setting was largely based on the Colchicine Cardiovascular Outcomes Trial (COLCOT). In that study, colchicine was associated with a 23% reduction in risk for major adverse cardiovascular events (MACE) relative to placebo when administered within 30 days after a myocardial infarction (hazard ratio, 0.77; P = .02).
The benefit in that trial was attributed to an anti-inflammatory effect, according to Dr. Jenab, associate professor of cardiology at Tehran (Iran) Heart Center. In particular as it relates to vascular disease, he cited experimental studies associating colchicine with a reduction in neutrophil activation and adherence to vascular endothelium.
The rationale for a preprocedural approach to colchicine was supplied by a subsequent time-to-treatment COLCOT analysis. In this study, MACE risk reduction for colchicine climbed to 48% (HR 0.52) for those treated within 3 days of the MI but largely disappeared (HR 0.96) if treatment was started at least 8 days post MI.
PodCAST-PCI trial
In the preprocedural study, called the PodCAST-PCI trial, 321 acute STEMI patients were randomized. Patients received a 1-mg dose of oral colchicine or placebo at the time PCI was scheduled. Another dose of colchicine (0.5 mg) or placebo was administered 1 hour after the procedure.
Of secondary outcomes, which included MACE at 1 month and 1 year, ST-segment resolution at 1 month, and change in inflammatory markers at 1 month, none were significant. Few even trended for significance.
For MACE, which included cardiac death, stroke, nonfatal MI, new hospitalization due to heart failure, or target vessel revascularization, the rates were lower in the colchicine group at 1 month (4.3% vs. 7.5%) and 1 year (9.3% vs. 11.2%), but neither approached significance.
For ST-segment resolution, the proportions were generally comparable among the colchicine and placebo groups, respectively, for the proportion below 50% (18.6% vs. 23.1%), between 50% and 70% (16.8% vs. 15.6%), and above 70% (64.6% vs. 61.3%).
The average troponin levels were nonsignificantly lower at 6 hours (1,847 vs. 2,883 ng/mL) in the colchicine group but higher at 48 hours (1,197 vs. 1,147 ng/mL). The average C-reactive protein (CRP) levels at 48 hours were nonsignificantly lower on colchicine (176.5 vs. 244.5 mg/L).
There were no significant differences in postprocedural perfusion, as measured with TIMI blood flow, or in the rate of stent thrombosis, which occurred in roughly 3% of each group of patients.
The small sample size was one limitation of this study, Dr. Jenab acknowledged. For this and other reasons, he cautioned that these data are not definitive and do not preclude a benefit on clinical outcomes in a study with a larger size, a different design, or different dosing.
Timing might be the issue
However, even if colchicine has a potential benefit in this setting, timing might be a major obstacle, according to Binata Shah, MD, associate director of research for the Cardiac Catheterization Laboratory at New York University.
“We have learned from our rheumatology colleagues that peak plasma levels of colchicine are not achieved for at least 1 hour after the full loading dose,” Dr. Shah said. “With us moving so quickly in a primary PCI setting, it is hard to imagine that colchicine would have had time to really kick in and exert its anti-inflammatory effect.”
Indeed, the problem might be worse than reaching the peak plasma level.
“Even though peak plasma levels occur as early as 1 hour after a full loading dose, we see that it takes about 24 hours to really see the effects translate downstream into more systemic inflammatory markers such as CRP and interleukin-6,” she added. If lowering these signals of inflammation is predictive of benefit, than this might be the biggest obstacle to benefit from colchicine in an urgent treatment setting.
Dr. Jenab and Dr. Shah reported no potential conflicts of interest.
FROM CRT 2021
We’re all vaccinated: Can we go back to the office (unmasked) now?
Congratulations, you’ve been vaccinated!
It’s been a year like no other, and outpatient psychiatrists turned to Zoom and other telemental health platforms to provide treatment for our patients. Offices sit empty as the dust lands and the plants wilt. Perhaps a few patients are seen in person, masked and carefully distanced, after health screening and temperature checks, with surfaces sanitized between visits, all in accordance with health department regulations. But now the vaccine offers both safety and the promise of a return to a new normal, one that is certain to look different from the normal that was left behind.
I have been vaccinated and many of my patients have also been vaccinated. I began to wonder if it was safe to start seeing patients in person; could I see fully vaccinated patients, unmasked and without temperature checks and sanitizing? I started asking this question in February, and the response I got then was that it was too soon to tell; we did not have any data on whether vaccinated people could transmit the novel coronavirus. Two vaccinated people might be at risk of transmitting the virus and then infecting others, and the question of whether the vaccines would protect against illness caused by variants remained. Preliminary data out of Israel indicated that the vaccine did reduce transmission, but no one was saying that it was fine to see patients without masks, and video-conferencing remained the safest option.
On Monday, March 8, 2021, the Centers for Disease Control and Prevention released long-awaited interim public health guidelines for fully vaccinated people. The guidelines allowed for two vaccinated people to be in a room together unmasked, and for a fully-vaccinated person to be in a room unmasked with an unvaccinated person who did not have risk factors for becoming severely ill with COVID. Was this the green light that psychiatrists were waiting for? Was there new data about transmission, or was this part of the CDC’s effort to make vaccines more desirable?
Michael Chang, MD, is a pediatric infectious disease specialist at the University of Texas Health Science Center at Houston. We spoke 2 days after the CDC interim guidelines were released. Dr. Chang was optimistic.
“, including data about variants and about transmission. At some point, however, the risk is low enough, and we should probably start thinking about going back to in-person visits,” Dr. Chang said. He said he personally would feel safe meeting unmasked with a vaccinated patient, but noted that his institution still requires doctors to wear masks. “Most vaccinations reduce transmission of illness,” Dr. Chang said, “but SARS-CoV-2 continues to surprise us in many ways.”
Katelyn Jetelina, PhD, MPH, an epidemiologist at the University of Texas School of Public Health in Dallas, distributes a newsletter, “Your Local Epidemiologist,” where she discusses data pertaining to the pandemic. In her newsletter dated March 14, 2021, Dr. Jetelina wrote, “There are now 7 sub-studies/press releases that confirm a 50-95% reduced transmission after vaccination. This is a big range, which is typical for such drastically different scientific studies. Variability is likely due to different sample sizes, locations, vaccines, genetics, cultures, etc. It will be a while until we know the ‘true’ percentage for each vaccine.”
Leslie Walker, MD, is a fully vaccinated psychiatrist in private practice in Shaker Heights, Ohio. She has recently started seeing fully vaccinated patients in person.
“So far it’s only 1 or 2 patients a day. I’m leaving it up to the patient. If they prefer masks, we stay masked. I may reverse course, depending on what information comes out.” She went on to note, “There are benefits to being able to see someone’s full facial expressions and whether they match someone’s words and body language, so the benefit of “unmasking” extends beyond comfort and convenience and must be balanced against the theoretical risk of COVID exposure in the room.”
While the CDC has now said it is safe to meet, the state health departments also have guidelines for medical practices, and everyone is still worried about vulnerable people in their households and potential spread to the community at large.
In Maryland, where I work, Aliya Jones, MD, MBA, is the head of the Behavioral Health Administration (BHA) for the Maryland Department of Health. “It remains risky to not wear masks, however, the risk is low when both individuals are vaccinated,” Dr. Jones wrote. “BHA is not recommending that providers see clients without both parties wearing a mask. All of our general practice recommendations for infection control are unchanged. People should be screened before entering clinical practices and persons who are symptomatic, whether vaccinated or not, should not be seen face-to-face, except in cases of an emergency, in which case additional precautions should be taken.”
So is it safe for a fully-vaccinated psychiatrist to have a session with a fully-vaccinated patient sitting 8 feet apart without masks? I’m left with the idea that it is for those two people, but when it comes to unvaccinated people in their households, we want more certainty than we currently have. The messaging remains unclear. The CDC’s interim guidelines offer hope for a future, but the science is still catching up, and to feel safe enough, we may want to wait a little longer for more definitive data – or herd immunity – before we reveal our smiles.
Dr. Miller is a coauthor of “Committed: The Battle Over Involuntary Psychiatric Care” (Baltimore: Johns Hopkins University Press, 2016). She has a private practice and is assistant professor of psychiatry and behavioral sciences at Johns Hopkins, both in Baltimore.
Congratulations, you’ve been vaccinated!
It’s been a year like no other, and outpatient psychiatrists turned to Zoom and other telemental health platforms to provide treatment for our patients. Offices sit empty as the dust lands and the plants wilt. Perhaps a few patients are seen in person, masked and carefully distanced, after health screening and temperature checks, with surfaces sanitized between visits, all in accordance with health department regulations. But now the vaccine offers both safety and the promise of a return to a new normal, one that is certain to look different from the normal that was left behind.
I have been vaccinated and many of my patients have also been vaccinated. I began to wonder if it was safe to start seeing patients in person; could I see fully vaccinated patients, unmasked and without temperature checks and sanitizing? I started asking this question in February, and the response I got then was that it was too soon to tell; we did not have any data on whether vaccinated people could transmit the novel coronavirus. Two vaccinated people might be at risk of transmitting the virus and then infecting others, and the question of whether the vaccines would protect against illness caused by variants remained. Preliminary data out of Israel indicated that the vaccine did reduce transmission, but no one was saying that it was fine to see patients without masks, and video-conferencing remained the safest option.
On Monday, March 8, 2021, the Centers for Disease Control and Prevention released long-awaited interim public health guidelines for fully vaccinated people. The guidelines allowed for two vaccinated people to be in a room together unmasked, and for a fully-vaccinated person to be in a room unmasked with an unvaccinated person who did not have risk factors for becoming severely ill with COVID. Was this the green light that psychiatrists were waiting for? Was there new data about transmission, or was this part of the CDC’s effort to make vaccines more desirable?
Michael Chang, MD, is a pediatric infectious disease specialist at the University of Texas Health Science Center at Houston. We spoke 2 days after the CDC interim guidelines were released. Dr. Chang was optimistic.
“, including data about variants and about transmission. At some point, however, the risk is low enough, and we should probably start thinking about going back to in-person visits,” Dr. Chang said. He said he personally would feel safe meeting unmasked with a vaccinated patient, but noted that his institution still requires doctors to wear masks. “Most vaccinations reduce transmission of illness,” Dr. Chang said, “but SARS-CoV-2 continues to surprise us in many ways.”
Katelyn Jetelina, PhD, MPH, an epidemiologist at the University of Texas School of Public Health in Dallas, distributes a newsletter, “Your Local Epidemiologist,” where she discusses data pertaining to the pandemic. In her newsletter dated March 14, 2021, Dr. Jetelina wrote, “There are now 7 sub-studies/press releases that confirm a 50-95% reduced transmission after vaccination. This is a big range, which is typical for such drastically different scientific studies. Variability is likely due to different sample sizes, locations, vaccines, genetics, cultures, etc. It will be a while until we know the ‘true’ percentage for each vaccine.”
Leslie Walker, MD, is a fully vaccinated psychiatrist in private practice in Shaker Heights, Ohio. She has recently started seeing fully vaccinated patients in person.
“So far it’s only 1 or 2 patients a day. I’m leaving it up to the patient. If they prefer masks, we stay masked. I may reverse course, depending on what information comes out.” She went on to note, “There are benefits to being able to see someone’s full facial expressions and whether they match someone’s words and body language, so the benefit of “unmasking” extends beyond comfort and convenience and must be balanced against the theoretical risk of COVID exposure in the room.”
While the CDC has now said it is safe to meet, the state health departments also have guidelines for medical practices, and everyone is still worried about vulnerable people in their households and potential spread to the community at large.
In Maryland, where I work, Aliya Jones, MD, MBA, is the head of the Behavioral Health Administration (BHA) for the Maryland Department of Health. “It remains risky to not wear masks, however, the risk is low when both individuals are vaccinated,” Dr. Jones wrote. “BHA is not recommending that providers see clients without both parties wearing a mask. All of our general practice recommendations for infection control are unchanged. People should be screened before entering clinical practices and persons who are symptomatic, whether vaccinated or not, should not be seen face-to-face, except in cases of an emergency, in which case additional precautions should be taken.”
So is it safe for a fully-vaccinated psychiatrist to have a session with a fully-vaccinated patient sitting 8 feet apart without masks? I’m left with the idea that it is for those two people, but when it comes to unvaccinated people in their households, we want more certainty than we currently have. The messaging remains unclear. The CDC’s interim guidelines offer hope for a future, but the science is still catching up, and to feel safe enough, we may want to wait a little longer for more definitive data – or herd immunity – before we reveal our smiles.
Dr. Miller is a coauthor of “Committed: The Battle Over Involuntary Psychiatric Care” (Baltimore: Johns Hopkins University Press, 2016). She has a private practice and is assistant professor of psychiatry and behavioral sciences at Johns Hopkins, both in Baltimore.
Congratulations, you’ve been vaccinated!
It’s been a year like no other, and outpatient psychiatrists turned to Zoom and other telemental health platforms to provide treatment for our patients. Offices sit empty as the dust lands and the plants wilt. Perhaps a few patients are seen in person, masked and carefully distanced, after health screening and temperature checks, with surfaces sanitized between visits, all in accordance with health department regulations. But now the vaccine offers both safety and the promise of a return to a new normal, one that is certain to look different from the normal that was left behind.
I have been vaccinated and many of my patients have also been vaccinated. I began to wonder if it was safe to start seeing patients in person; could I see fully vaccinated patients, unmasked and without temperature checks and sanitizing? I started asking this question in February, and the response I got then was that it was too soon to tell; we did not have any data on whether vaccinated people could transmit the novel coronavirus. Two vaccinated people might be at risk of transmitting the virus and then infecting others, and the question of whether the vaccines would protect against illness caused by variants remained. Preliminary data out of Israel indicated that the vaccine did reduce transmission, but no one was saying that it was fine to see patients without masks, and video-conferencing remained the safest option.
On Monday, March 8, 2021, the Centers for Disease Control and Prevention released long-awaited interim public health guidelines for fully vaccinated people. The guidelines allowed for two vaccinated people to be in a room together unmasked, and for a fully-vaccinated person to be in a room unmasked with an unvaccinated person who did not have risk factors for becoming severely ill with COVID. Was this the green light that psychiatrists were waiting for? Was there new data about transmission, or was this part of the CDC’s effort to make vaccines more desirable?
Michael Chang, MD, is a pediatric infectious disease specialist at the University of Texas Health Science Center at Houston. We spoke 2 days after the CDC interim guidelines were released. Dr. Chang was optimistic.
“, including data about variants and about transmission. At some point, however, the risk is low enough, and we should probably start thinking about going back to in-person visits,” Dr. Chang said. He said he personally would feel safe meeting unmasked with a vaccinated patient, but noted that his institution still requires doctors to wear masks. “Most vaccinations reduce transmission of illness,” Dr. Chang said, “but SARS-CoV-2 continues to surprise us in many ways.”
Katelyn Jetelina, PhD, MPH, an epidemiologist at the University of Texas School of Public Health in Dallas, distributes a newsletter, “Your Local Epidemiologist,” where she discusses data pertaining to the pandemic. In her newsletter dated March 14, 2021, Dr. Jetelina wrote, “There are now 7 sub-studies/press releases that confirm a 50-95% reduced transmission after vaccination. This is a big range, which is typical for such drastically different scientific studies. Variability is likely due to different sample sizes, locations, vaccines, genetics, cultures, etc. It will be a while until we know the ‘true’ percentage for each vaccine.”
Leslie Walker, MD, is a fully vaccinated psychiatrist in private practice in Shaker Heights, Ohio. She has recently started seeing fully vaccinated patients in person.
“So far it’s only 1 or 2 patients a day. I’m leaving it up to the patient. If they prefer masks, we stay masked. I may reverse course, depending on what information comes out.” She went on to note, “There are benefits to being able to see someone’s full facial expressions and whether they match someone’s words and body language, so the benefit of “unmasking” extends beyond comfort and convenience and must be balanced against the theoretical risk of COVID exposure in the room.”
While the CDC has now said it is safe to meet, the state health departments also have guidelines for medical practices, and everyone is still worried about vulnerable people in their households and potential spread to the community at large.
In Maryland, where I work, Aliya Jones, MD, MBA, is the head of the Behavioral Health Administration (BHA) for the Maryland Department of Health. “It remains risky to not wear masks, however, the risk is low when both individuals are vaccinated,” Dr. Jones wrote. “BHA is not recommending that providers see clients without both parties wearing a mask. All of our general practice recommendations for infection control are unchanged. People should be screened before entering clinical practices and persons who are symptomatic, whether vaccinated or not, should not be seen face-to-face, except in cases of an emergency, in which case additional precautions should be taken.”
So is it safe for a fully-vaccinated psychiatrist to have a session with a fully-vaccinated patient sitting 8 feet apart without masks? I’m left with the idea that it is for those two people, but when it comes to unvaccinated people in their households, we want more certainty than we currently have. The messaging remains unclear. The CDC’s interim guidelines offer hope for a future, but the science is still catching up, and to feel safe enough, we may want to wait a little longer for more definitive data – or herd immunity – before we reveal our smiles.
Dr. Miller is a coauthor of “Committed: The Battle Over Involuntary Psychiatric Care” (Baltimore: Johns Hopkins University Press, 2016). She has a private practice and is assistant professor of psychiatry and behavioral sciences at Johns Hopkins, both in Baltimore.
High-dose chemo no better than standard dose for B-cell lymphoma
After 10 years of follow-up, event-free survival and overall survival were similar between conventional chemotherapy treated patients with aggressive B-cell lymphoma and those receiving high-dose chemotherapy followed by autologous hematopoietic stem-cell transplantation (HSCT), according to a report published online in the Lancet Hematology.
The open-label, randomized, phase 3 trial (NCT00129090) was conducted across 61 centers in Germany on patients aged 18-60 years who had newly diagnosed, high-risk, aggressive B-cell lymphoma, according to Fabian Frontzek, MD, of the University Hospital Münster (Germany) and colleagues.
Between March 2003 and April 2009, patients were randomly assigned to eight cycles of conventional chemotherapy (cyclophosphamide, doxorubicin, vincristine, etoposide, and prednisolone) plus rituximab (R-CHOEP-14) or four cycles of high-dose chemotherapy plus rituximab followed by autologous HSCT (R-MegaCHOEP). The intention-to-treat population comprised 130 patients in the R-CHOEP-14 group and 132 patients in the R-MegaCHOEP group. The median follow-up was 9.3 years.
Similar outcomes
The 10-year event-free survival was 51% in the R-MegaCHOEP group and 57% in the R-CHOEP-14 group, a nonsignificant difference (P = .23). Similarly, the 10-year progression-free survival was 59% in the
R-MegaCHOEP group and 60% (P = .64). The 10-year overall survival was 66% in the R-MegaCHOEP group and 72% in the R-CHOEP-14 group (P = .26). Among the 190 patients who had complete remission or unconfirmed complete remission, relapse occurred in 30 (16%); 17 (17%) of 100 patients in the R-CHOEP-14 group and 13 (14%) of 90 patients in the R-MegaCHOEP group.
In terms of secondary malignancies, 22 were reported in the intention-to-treat population; comprising 12 (9%) of 127 patients in the R-CHOEP-14 group and 10 (8%) of 126 patients in the R-MegaCHOEP group.
Patients who relapsed with aggressive histology and with CNS involvement in particular had worse outcomes and “represent a group with an unmet medical need, for which new molecular and cellular therapies should be studied,” the authors stated.
“This study shows that, in the rituximab era, high-dose therapy and autologous HSCT in first-line treatment does not improve long-term survival of younger high-risk patients with aggressive B-cell lymphoma. The R-CHOEP-14 regimen led to favorable outcomes, supporting its continued use in such patients,” the researchers concluded.
In an accompanying commentary, Gita Thanarajasingam, MD, of the Mayo Clinic, Rochester, Minn., and colleagues added that the issue of long-term outcomes is critical to evaluating these new regimens.
They applauded the inclusion of secondary malignancies in the long-term follow-up, but regretted the lack of the, admittedly resource-intensive, information on long-term nonneoplastic adverse events. They added that “the burden of late adverse events such as cardiotoxicity, cumulative neuropathy, delayed infections, or lasting cognitive effects, among others that might drive substantial morbidity, does matter to lymphoma survivors.”
They also commented on the importance of considering effects on fertility in these patients, noting that R-MegaCHOEP patients would be unable to conceive naturally, but that the effect of R-CHOEP-14 was less clear.
“We encourage ongoing emphasis on this type of longitudinal follow-up of secondary malignancies and other nonneoplastic late toxicities in phase 3 studies as well as in the real world in hematological malignancies, so that after prioritizing cure in the front-line setting, we do not neglect the life we have helped survivors achieve for years and decades to come,” they concluded.
The study was sponsored by the German High-Grade Non-Hodgkin’s Lymphoma Study Group. The authors reported grants, personal fees, and non-financial support from multiple pharmaceutical and biotechnology companies. Dr. Thanarajasingam and her colleagues reported that they had no competing interests.
After 10 years of follow-up, event-free survival and overall survival were similar between conventional chemotherapy treated patients with aggressive B-cell lymphoma and those receiving high-dose chemotherapy followed by autologous hematopoietic stem-cell transplantation (HSCT), according to a report published online in the Lancet Hematology.
The open-label, randomized, phase 3 trial (NCT00129090) was conducted across 61 centers in Germany on patients aged 18-60 years who had newly diagnosed, high-risk, aggressive B-cell lymphoma, according to Fabian Frontzek, MD, of the University Hospital Münster (Germany) and colleagues.
Between March 2003 and April 2009, patients were randomly assigned to eight cycles of conventional chemotherapy (cyclophosphamide, doxorubicin, vincristine, etoposide, and prednisolone) plus rituximab (R-CHOEP-14) or four cycles of high-dose chemotherapy plus rituximab followed by autologous HSCT (R-MegaCHOEP). The intention-to-treat population comprised 130 patients in the R-CHOEP-14 group and 132 patients in the R-MegaCHOEP group. The median follow-up was 9.3 years.
Similar outcomes
The 10-year event-free survival was 51% in the R-MegaCHOEP group and 57% in the R-CHOEP-14 group, a nonsignificant difference (P = .23). Similarly, the 10-year progression-free survival was 59% in the
R-MegaCHOEP group and 60% (P = .64). The 10-year overall survival was 66% in the R-MegaCHOEP group and 72% in the R-CHOEP-14 group (P = .26). Among the 190 patients who had complete remission or unconfirmed complete remission, relapse occurred in 30 (16%); 17 (17%) of 100 patients in the R-CHOEP-14 group and 13 (14%) of 90 patients in the R-MegaCHOEP group.
In terms of secondary malignancies, 22 were reported in the intention-to-treat population; comprising 12 (9%) of 127 patients in the R-CHOEP-14 group and 10 (8%) of 126 patients in the R-MegaCHOEP group.
Patients who relapsed with aggressive histology and with CNS involvement in particular had worse outcomes and “represent a group with an unmet medical need, for which new molecular and cellular therapies should be studied,” the authors stated.
“This study shows that, in the rituximab era, high-dose therapy and autologous HSCT in first-line treatment does not improve long-term survival of younger high-risk patients with aggressive B-cell lymphoma. The R-CHOEP-14 regimen led to favorable outcomes, supporting its continued use in such patients,” the researchers concluded.
In an accompanying commentary, Gita Thanarajasingam, MD, of the Mayo Clinic, Rochester, Minn., and colleagues added that the issue of long-term outcomes is critical to evaluating these new regimens.
They applauded the inclusion of secondary malignancies in the long-term follow-up, but regretted the lack of the, admittedly resource-intensive, information on long-term nonneoplastic adverse events. They added that “the burden of late adverse events such as cardiotoxicity, cumulative neuropathy, delayed infections, or lasting cognitive effects, among others that might drive substantial morbidity, does matter to lymphoma survivors.”
They also commented on the importance of considering effects on fertility in these patients, noting that R-MegaCHOEP patients would be unable to conceive naturally, but that the effect of R-CHOEP-14 was less clear.
“We encourage ongoing emphasis on this type of longitudinal follow-up of secondary malignancies and other nonneoplastic late toxicities in phase 3 studies as well as in the real world in hematological malignancies, so that after prioritizing cure in the front-line setting, we do not neglect the life we have helped survivors achieve for years and decades to come,” they concluded.
The study was sponsored by the German High-Grade Non-Hodgkin’s Lymphoma Study Group. The authors reported grants, personal fees, and non-financial support from multiple pharmaceutical and biotechnology companies. Dr. Thanarajasingam and her colleagues reported that they had no competing interests.
After 10 years of follow-up, event-free survival and overall survival were similar between conventional chemotherapy treated patients with aggressive B-cell lymphoma and those receiving high-dose chemotherapy followed by autologous hematopoietic stem-cell transplantation (HSCT), according to a report published online in the Lancet Hematology.
The open-label, randomized, phase 3 trial (NCT00129090) was conducted across 61 centers in Germany on patients aged 18-60 years who had newly diagnosed, high-risk, aggressive B-cell lymphoma, according to Fabian Frontzek, MD, of the University Hospital Münster (Germany) and colleagues.
Between March 2003 and April 2009, patients were randomly assigned to eight cycles of conventional chemotherapy (cyclophosphamide, doxorubicin, vincristine, etoposide, and prednisolone) plus rituximab (R-CHOEP-14) or four cycles of high-dose chemotherapy plus rituximab followed by autologous HSCT (R-MegaCHOEP). The intention-to-treat population comprised 130 patients in the R-CHOEP-14 group and 132 patients in the R-MegaCHOEP group. The median follow-up was 9.3 years.
Similar outcomes
The 10-year event-free survival was 51% in the R-MegaCHOEP group and 57% in the R-CHOEP-14 group, a nonsignificant difference (P = .23). Similarly, the 10-year progression-free survival was 59% in the
R-MegaCHOEP group and 60% (P = .64). The 10-year overall survival was 66% in the R-MegaCHOEP group and 72% in the R-CHOEP-14 group (P = .26). Among the 190 patients who had complete remission or unconfirmed complete remission, relapse occurred in 30 (16%); 17 (17%) of 100 patients in the R-CHOEP-14 group and 13 (14%) of 90 patients in the R-MegaCHOEP group.
In terms of secondary malignancies, 22 were reported in the intention-to-treat population; comprising 12 (9%) of 127 patients in the R-CHOEP-14 group and 10 (8%) of 126 patients in the R-MegaCHOEP group.
Patients who relapsed with aggressive histology and with CNS involvement in particular had worse outcomes and “represent a group with an unmet medical need, for which new molecular and cellular therapies should be studied,” the authors stated.
“This study shows that, in the rituximab era, high-dose therapy and autologous HSCT in first-line treatment does not improve long-term survival of younger high-risk patients with aggressive B-cell lymphoma. The R-CHOEP-14 regimen led to favorable outcomes, supporting its continued use in such patients,” the researchers concluded.
In an accompanying commentary, Gita Thanarajasingam, MD, of the Mayo Clinic, Rochester, Minn., and colleagues added that the issue of long-term outcomes is critical to evaluating these new regimens.
They applauded the inclusion of secondary malignancies in the long-term follow-up, but regretted the lack of the, admittedly resource-intensive, information on long-term nonneoplastic adverse events. They added that “the burden of late adverse events such as cardiotoxicity, cumulative neuropathy, delayed infections, or lasting cognitive effects, among others that might drive substantial morbidity, does matter to lymphoma survivors.”
They also commented on the importance of considering effects on fertility in these patients, noting that R-MegaCHOEP patients would be unable to conceive naturally, but that the effect of R-CHOEP-14 was less clear.
“We encourage ongoing emphasis on this type of longitudinal follow-up of secondary malignancies and other nonneoplastic late toxicities in phase 3 studies as well as in the real world in hematological malignancies, so that after prioritizing cure in the front-line setting, we do not neglect the life we have helped survivors achieve for years and decades to come,” they concluded.
The study was sponsored by the German High-Grade Non-Hodgkin’s Lymphoma Study Group. The authors reported grants, personal fees, and non-financial support from multiple pharmaceutical and biotechnology companies. Dr. Thanarajasingam and her colleagues reported that they had no competing interests.
FROM THE LANCET HEMATOLOGY
Healthcare Encounter and Financial Impact of COVID-19 on Children’s Hospitals
To benefit patients and the public health of their communities, children’s hospitals across the United States prepared for and responded to COVID-19 by conserving personal protective equipment, suspending noncritical in-person healthcare encounters (including outpatient visits and elective surgeries), and implementing socially distanced essential care.1,2 These measures were promptly instituted during a time of both substantial uncertainty about the pandemic’s behavior in children—including its severity and duration—and extreme variation in local and state governments’ responses to the pandemic.
Congruent with other healthcare institutions, children’s hospitals calibrated their clinical operations to the evolving nature of the pandemic, prioritizing the safety of patients and staff while striving to maintain financial viability in the setting of increased costs and decreased revenue. In some cases, children’s hospitals aided adult hospitals and health systems by admitting young and middle-aged adult patients and by centralizing all pediatric patients requiring intensive care within a region. These efforts occurred while many children’s hospitals remained the sole source of specialized pediatric care, including care for rare complex health problems.
As the COVID-19 pandemic continues, there is a critical need to assess how the initial phase of the pandemic affected healthcare encounters and related finances in children’s hospitals. Understanding these trends will position children’s hospitals to project and prepare for subsequent COVID-19 surges, as well as future related public health crises that necessitate widespread social distancing. Therefore, we compared year-over-year trends in healthcare encounters and hospital charges across US children’s hospitals before and during the COVID-19 pandemic, focusing on the beginning of COVID-19 in the United States, which was defined as February through June 2020.
METHODS
This is a retrospective analysis of 26 children’s hospitals (22 freestanding, 4 nonfreestanding) from all US regions (12 South, 7 Midwest, 5 West, 2 Northeast) contributing encounter and financial data to the PROSPECT database (Children’s Hospital Association, Lenexa, Kansas) from February 1 to June 30 in both 2019 (before COVID-19) and 2020 (during COVID-19). In response to COVID-19, hospitals participating in PROSPECT increased the efficiency of data centralization and reporting in 2020 during the period February 1 to June 30 to expedite analysis and dissemination of findings.
The main outcome measures were the percentage of change in weekly encounters (inpatient bed-days, emergency department [ED] visits, and surgeries) and inflation-adjusted charges (categorized as inpatient care and outpatient care, such as ambulatory surgery, clinics, and ED visits) before vs during COVID-19.
RESULTS
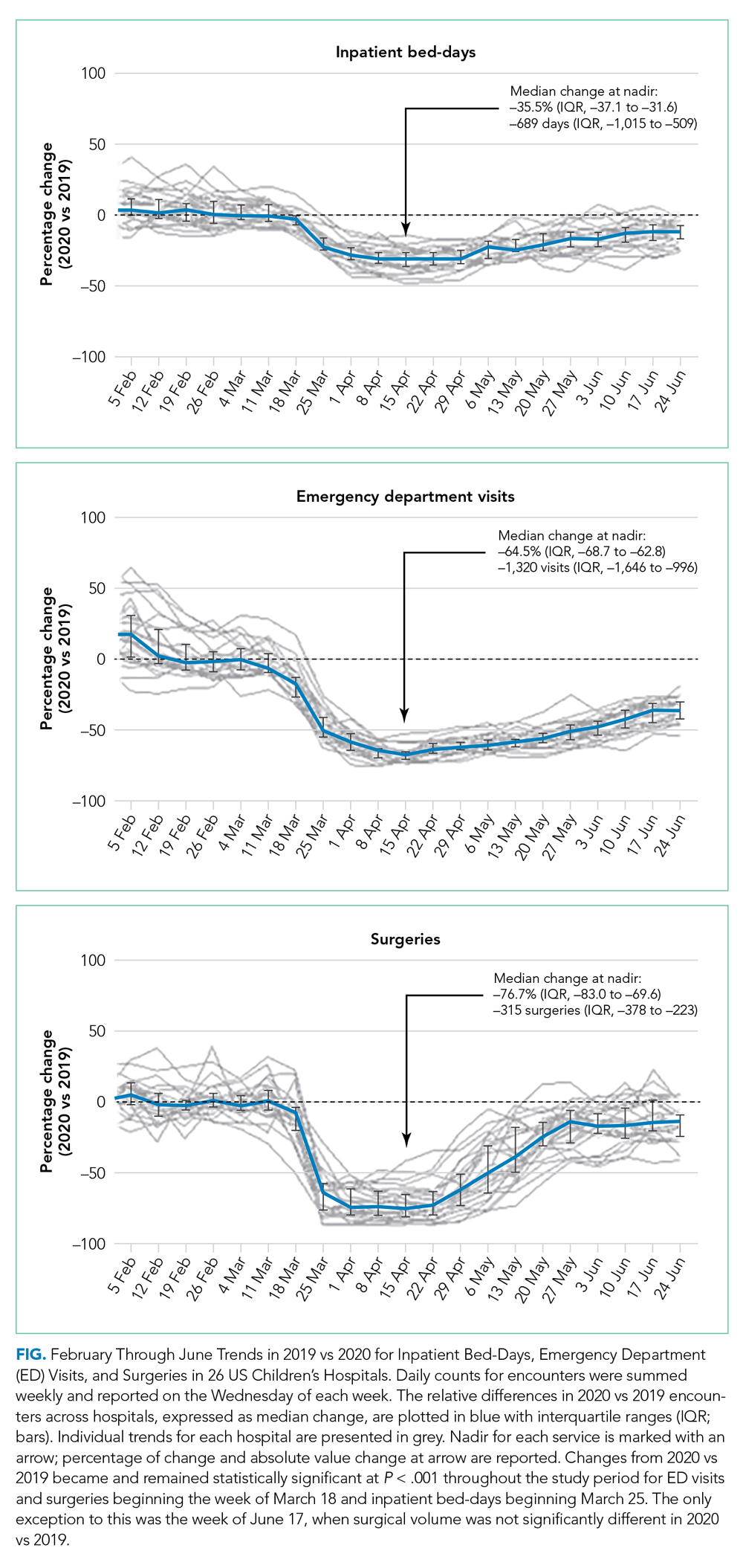
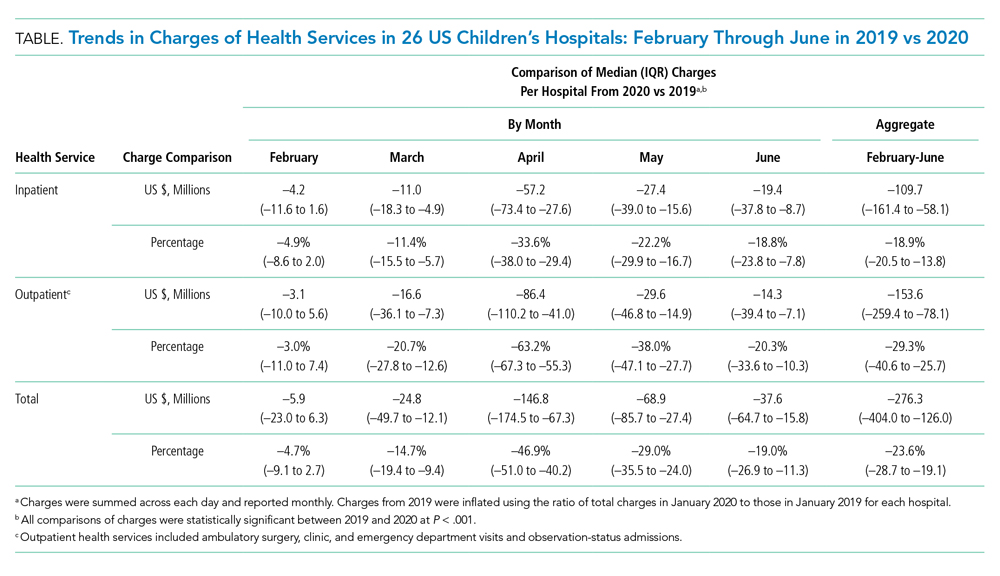
Charges that accrued from February 1 to June 30 were lower in 2020 by a median 23.6% (IQR, –28.7% to –19.1%) per children’s hospital than they were in 2019, corresponding to a median decrease of $276.3 million (IQR, $404.0-$126.0 million) in charges per hospital (Table). Forty percent of this decrease was attributable to decreased charges resulting from fewer inpatient healthcare encounters.
DISCUSSION
These findings beg the question of how well children’s hospitals are positioned to weather a recurrent surge in COVID-19. Because the severity of illness of COVID-19 has been lower to date in the pediatric vs adult populations, an increase in COVID-19-related visits to EDs and admissions to offset the decreased resource use of other pediatric healthcare problems is not anticipated. Existing hospital financial reserves as well as federal aid from the Coronavirus Aid, Relief, and Economic Security Act that helped mitigate the initial encounter and financial losses during the beginning of COVID-19 may not be readily available over time.4,5 Certainly, the findings from the current study support continued lobbying for additional state and federal funds allocated through future relief packages to children’s hospitals.
Additional approaches to financial solvency in children’s hospitals during the sustained COVID-19 pandemic include addressing surgical backlogs and sharing best practices for safe and sustained reopening of clinical operations and financial practices across institutions. Although the PROSPECT database does not contain information on the types of surgeries present within this backlog, our experiences suggest that both same-day and inpatient elective surgeries have been affected, especially lengthy procedures (eg, spinal fusion for neuromuscular scoliosis). Spread and scale of feasible and efficient solutions to reengineer and expand patient capacities and throughput for operating rooms, postanesthesia recovery areas, and intensive care and floor units are needed. Enhanced analytics that accurately predict postoperative length of hospital stay, coupled with early recovery after surgery clinical protocols, could help optimize hospital bed management. Effective ways to convert hospital rooms from single to double occupancy, to manage family visitation, and to proactively test asymptomatic patients, family, and hospital staff will mitigate continued COVID-19 penetration through children’s hospitals.
One important limitation of the current study is the measurement of hospitals’ charges. The charge data were not positioned to comprehensively measure each hospital’s financial state during the COVID-19 pandemic. However, the decrease in hospital charges reported by the children’s hospitals in the current study is comparable with the financial losses reported for many adult hospitals during the pandemic.6,7
CONCLUSION
Children’s hospitals’ ability to serve the nation’s pediatric patients depends on the success of the hospitals’ plans to manage current and future COVID-19 surges and to reopen and recover from the surges that have passed. Additional investigation is needed to identify best operational and financial practices among children’s hospitals that have enabled them to endure the COVID-19 pandemic.
1. COVID-19: ways to prepare your children’s hospital now. Children’s Hospital Association. March 12, 2020. Accessed June 30, 2020. https://www.childrenshospitals.org/Newsroom/Childrens-Hospitals-Today/Articles/2020/03/COVID-19-11-Ways-to-Prepare-Your-Hospital-Now
2. Chopra V, Toner E, Waldhorn R, Washer L. How should U.S. hospitals prepare for coronavirus disease 2019 (COVID-19)? Ann Intern Med. 2020;172(9):621-622. https://doi.org/10.7326/m20-0907
3. Oseran AS, Nash D, Kim C, et al. Changes in hospital admissions for urgent conditions during COVID-19 pandemic. Am J Manag Care. 2020;26(8):327-328. https://doi.org/10.37765/ajmc.2020.43837
4. Coronavirus Aid, Relief, and Economic Security Act or the CARES Act. 15 USC Chapter 116 (2020). Pub L No. 116-36, 134 Stat 281. https://www.congress.gov/bill/116th-congress/house-bill/748
5. The Coronavirus Aid, Relief, and Economic Security (CARES) Act Provider Relief Fund: general information. US Department of Health & Human Services. June 25, 2020. Accessed June 30, 2020. https://www.hhs.gov/coronavirus/cares-act-provider-relief-fund/general-information/index.html
6. Hospitals and health systems face unprecedented financial pressures due to COVID-19. American Hospital Association. May 2020. Accessed July 13, 2020. https://www.aha.org/system/files/media/file/2020/05/aha-covid19-financial-impact-0520-FINAL.pdf
7. Birkmeyer J, Barnato A, Birkmeyer N, Bessler R, Skinner J. The impact of the COVID-19 pandemic on hospital admissions in the United States. Health Aff (Millwood). 2020;39(11):2010-2017. https://doi.org/10.1377/hlthaff.2020.00980
To benefit patients and the public health of their communities, children’s hospitals across the United States prepared for and responded to COVID-19 by conserving personal protective equipment, suspending noncritical in-person healthcare encounters (including outpatient visits and elective surgeries), and implementing socially distanced essential care.1,2 These measures were promptly instituted during a time of both substantial uncertainty about the pandemic’s behavior in children—including its severity and duration—and extreme variation in local and state governments’ responses to the pandemic.
Congruent with other healthcare institutions, children’s hospitals calibrated their clinical operations to the evolving nature of the pandemic, prioritizing the safety of patients and staff while striving to maintain financial viability in the setting of increased costs and decreased revenue. In some cases, children’s hospitals aided adult hospitals and health systems by admitting young and middle-aged adult patients and by centralizing all pediatric patients requiring intensive care within a region. These efforts occurred while many children’s hospitals remained the sole source of specialized pediatric care, including care for rare complex health problems.
As the COVID-19 pandemic continues, there is a critical need to assess how the initial phase of the pandemic affected healthcare encounters and related finances in children’s hospitals. Understanding these trends will position children’s hospitals to project and prepare for subsequent COVID-19 surges, as well as future related public health crises that necessitate widespread social distancing. Therefore, we compared year-over-year trends in healthcare encounters and hospital charges across US children’s hospitals before and during the COVID-19 pandemic, focusing on the beginning of COVID-19 in the United States, which was defined as February through June 2020.
METHODS
This is a retrospective analysis of 26 children’s hospitals (22 freestanding, 4 nonfreestanding) from all US regions (12 South, 7 Midwest, 5 West, 2 Northeast) contributing encounter and financial data to the PROSPECT database (Children’s Hospital Association, Lenexa, Kansas) from February 1 to June 30 in both 2019 (before COVID-19) and 2020 (during COVID-19). In response to COVID-19, hospitals participating in PROSPECT increased the efficiency of data centralization and reporting in 2020 during the period February 1 to June 30 to expedite analysis and dissemination of findings.
The main outcome measures were the percentage of change in weekly encounters (inpatient bed-days, emergency department [ED] visits, and surgeries) and inflation-adjusted charges (categorized as inpatient care and outpatient care, such as ambulatory surgery, clinics, and ED visits) before vs during COVID-19.
RESULTS


Charges that accrued from February 1 to June 30 were lower in 2020 by a median 23.6% (IQR, –28.7% to –19.1%) per children’s hospital than they were in 2019, corresponding to a median decrease of $276.3 million (IQR, $404.0-$126.0 million) in charges per hospital (Table). Forty percent of this decrease was attributable to decreased charges resulting from fewer inpatient healthcare encounters.
DISCUSSION
These findings beg the question of how well children’s hospitals are positioned to weather a recurrent surge in COVID-19. Because the severity of illness of COVID-19 has been lower to date in the pediatric vs adult populations, an increase in COVID-19-related visits to EDs and admissions to offset the decreased resource use of other pediatric healthcare problems is not anticipated. Existing hospital financial reserves as well as federal aid from the Coronavirus Aid, Relief, and Economic Security Act that helped mitigate the initial encounter and financial losses during the beginning of COVID-19 may not be readily available over time.4,5 Certainly, the findings from the current study support continued lobbying for additional state and federal funds allocated through future relief packages to children’s hospitals.
Additional approaches to financial solvency in children’s hospitals during the sustained COVID-19 pandemic include addressing surgical backlogs and sharing best practices for safe and sustained reopening of clinical operations and financial practices across institutions. Although the PROSPECT database does not contain information on the types of surgeries present within this backlog, our experiences suggest that both same-day and inpatient elective surgeries have been affected, especially lengthy procedures (eg, spinal fusion for neuromuscular scoliosis). Spread and scale of feasible and efficient solutions to reengineer and expand patient capacities and throughput for operating rooms, postanesthesia recovery areas, and intensive care and floor units are needed. Enhanced analytics that accurately predict postoperative length of hospital stay, coupled with early recovery after surgery clinical protocols, could help optimize hospital bed management. Effective ways to convert hospital rooms from single to double occupancy, to manage family visitation, and to proactively test asymptomatic patients, family, and hospital staff will mitigate continued COVID-19 penetration through children’s hospitals.
One important limitation of the current study is the measurement of hospitals’ charges. The charge data were not positioned to comprehensively measure each hospital’s financial state during the COVID-19 pandemic. However, the decrease in hospital charges reported by the children’s hospitals in the current study is comparable with the financial losses reported for many adult hospitals during the pandemic.6,7
CONCLUSION
Children’s hospitals’ ability to serve the nation’s pediatric patients depends on the success of the hospitals’ plans to manage current and future COVID-19 surges and to reopen and recover from the surges that have passed. Additional investigation is needed to identify best operational and financial practices among children’s hospitals that have enabled them to endure the COVID-19 pandemic.
To benefit patients and the public health of their communities, children’s hospitals across the United States prepared for and responded to COVID-19 by conserving personal protective equipment, suspending noncritical in-person healthcare encounters (including outpatient visits and elective surgeries), and implementing socially distanced essential care.1,2 These measures were promptly instituted during a time of both substantial uncertainty about the pandemic’s behavior in children—including its severity and duration—and extreme variation in local and state governments’ responses to the pandemic.
Congruent with other healthcare institutions, children’s hospitals calibrated their clinical operations to the evolving nature of the pandemic, prioritizing the safety of patients and staff while striving to maintain financial viability in the setting of increased costs and decreased revenue. In some cases, children’s hospitals aided adult hospitals and health systems by admitting young and middle-aged adult patients and by centralizing all pediatric patients requiring intensive care within a region. These efforts occurred while many children’s hospitals remained the sole source of specialized pediatric care, including care for rare complex health problems.
As the COVID-19 pandemic continues, there is a critical need to assess how the initial phase of the pandemic affected healthcare encounters and related finances in children’s hospitals. Understanding these trends will position children’s hospitals to project and prepare for subsequent COVID-19 surges, as well as future related public health crises that necessitate widespread social distancing. Therefore, we compared year-over-year trends in healthcare encounters and hospital charges across US children’s hospitals before and during the COVID-19 pandemic, focusing on the beginning of COVID-19 in the United States, which was defined as February through June 2020.
METHODS
This is a retrospective analysis of 26 children’s hospitals (22 freestanding, 4 nonfreestanding) from all US regions (12 South, 7 Midwest, 5 West, 2 Northeast) contributing encounter and financial data to the PROSPECT database (Children’s Hospital Association, Lenexa, Kansas) from February 1 to June 30 in both 2019 (before COVID-19) and 2020 (during COVID-19). In response to COVID-19, hospitals participating in PROSPECT increased the efficiency of data centralization and reporting in 2020 during the period February 1 to June 30 to expedite analysis and dissemination of findings.
The main outcome measures were the percentage of change in weekly encounters (inpatient bed-days, emergency department [ED] visits, and surgeries) and inflation-adjusted charges (categorized as inpatient care and outpatient care, such as ambulatory surgery, clinics, and ED visits) before vs during COVID-19.
RESULTS


Charges that accrued from February 1 to June 30 were lower in 2020 by a median 23.6% (IQR, –28.7% to –19.1%) per children’s hospital than they were in 2019, corresponding to a median decrease of $276.3 million (IQR, $404.0-$126.0 million) in charges per hospital (Table). Forty percent of this decrease was attributable to decreased charges resulting from fewer inpatient healthcare encounters.
DISCUSSION
These findings beg the question of how well children’s hospitals are positioned to weather a recurrent surge in COVID-19. Because the severity of illness of COVID-19 has been lower to date in the pediatric vs adult populations, an increase in COVID-19-related visits to EDs and admissions to offset the decreased resource use of other pediatric healthcare problems is not anticipated. Existing hospital financial reserves as well as federal aid from the Coronavirus Aid, Relief, and Economic Security Act that helped mitigate the initial encounter and financial losses during the beginning of COVID-19 may not be readily available over time.4,5 Certainly, the findings from the current study support continued lobbying for additional state and federal funds allocated through future relief packages to children’s hospitals.
Additional approaches to financial solvency in children’s hospitals during the sustained COVID-19 pandemic include addressing surgical backlogs and sharing best practices for safe and sustained reopening of clinical operations and financial practices across institutions. Although the PROSPECT database does not contain information on the types of surgeries present within this backlog, our experiences suggest that both same-day and inpatient elective surgeries have been affected, especially lengthy procedures (eg, spinal fusion for neuromuscular scoliosis). Spread and scale of feasible and efficient solutions to reengineer and expand patient capacities and throughput for operating rooms, postanesthesia recovery areas, and intensive care and floor units are needed. Enhanced analytics that accurately predict postoperative length of hospital stay, coupled with early recovery after surgery clinical protocols, could help optimize hospital bed management. Effective ways to convert hospital rooms from single to double occupancy, to manage family visitation, and to proactively test asymptomatic patients, family, and hospital staff will mitigate continued COVID-19 penetration through children’s hospitals.
One important limitation of the current study is the measurement of hospitals’ charges. The charge data were not positioned to comprehensively measure each hospital’s financial state during the COVID-19 pandemic. However, the decrease in hospital charges reported by the children’s hospitals in the current study is comparable with the financial losses reported for many adult hospitals during the pandemic.6,7
CONCLUSION
Children’s hospitals’ ability to serve the nation’s pediatric patients depends on the success of the hospitals’ plans to manage current and future COVID-19 surges and to reopen and recover from the surges that have passed. Additional investigation is needed to identify best operational and financial practices among children’s hospitals that have enabled them to endure the COVID-19 pandemic.
1. COVID-19: ways to prepare your children’s hospital now. Children’s Hospital Association. March 12, 2020. Accessed June 30, 2020. https://www.childrenshospitals.org/Newsroom/Childrens-Hospitals-Today/Articles/2020/03/COVID-19-11-Ways-to-Prepare-Your-Hospital-Now
2. Chopra V, Toner E, Waldhorn R, Washer L. How should U.S. hospitals prepare for coronavirus disease 2019 (COVID-19)? Ann Intern Med. 2020;172(9):621-622. https://doi.org/10.7326/m20-0907
3. Oseran AS, Nash D, Kim C, et al. Changes in hospital admissions for urgent conditions during COVID-19 pandemic. Am J Manag Care. 2020;26(8):327-328. https://doi.org/10.37765/ajmc.2020.43837
4. Coronavirus Aid, Relief, and Economic Security Act or the CARES Act. 15 USC Chapter 116 (2020). Pub L No. 116-36, 134 Stat 281. https://www.congress.gov/bill/116th-congress/house-bill/748
5. The Coronavirus Aid, Relief, and Economic Security (CARES) Act Provider Relief Fund: general information. US Department of Health & Human Services. June 25, 2020. Accessed June 30, 2020. https://www.hhs.gov/coronavirus/cares-act-provider-relief-fund/general-information/index.html
6. Hospitals and health systems face unprecedented financial pressures due to COVID-19. American Hospital Association. May 2020. Accessed July 13, 2020. https://www.aha.org/system/files/media/file/2020/05/aha-covid19-financial-impact-0520-FINAL.pdf
7. Birkmeyer J, Barnato A, Birkmeyer N, Bessler R, Skinner J. The impact of the COVID-19 pandemic on hospital admissions in the United States. Health Aff (Millwood). 2020;39(11):2010-2017. https://doi.org/10.1377/hlthaff.2020.00980
1. COVID-19: ways to prepare your children’s hospital now. Children’s Hospital Association. March 12, 2020. Accessed June 30, 2020. https://www.childrenshospitals.org/Newsroom/Childrens-Hospitals-Today/Articles/2020/03/COVID-19-11-Ways-to-Prepare-Your-Hospital-Now
2. Chopra V, Toner E, Waldhorn R, Washer L. How should U.S. hospitals prepare for coronavirus disease 2019 (COVID-19)? Ann Intern Med. 2020;172(9):621-622. https://doi.org/10.7326/m20-0907
3. Oseran AS, Nash D, Kim C, et al. Changes in hospital admissions for urgent conditions during COVID-19 pandemic. Am J Manag Care. 2020;26(8):327-328. https://doi.org/10.37765/ajmc.2020.43837
4. Coronavirus Aid, Relief, and Economic Security Act or the CARES Act. 15 USC Chapter 116 (2020). Pub L No. 116-36, 134 Stat 281. https://www.congress.gov/bill/116th-congress/house-bill/748
5. The Coronavirus Aid, Relief, and Economic Security (CARES) Act Provider Relief Fund: general information. US Department of Health & Human Services. June 25, 2020. Accessed June 30, 2020. https://www.hhs.gov/coronavirus/cares-act-provider-relief-fund/general-information/index.html
6. Hospitals and health systems face unprecedented financial pressures due to COVID-19. American Hospital Association. May 2020. Accessed July 13, 2020. https://www.aha.org/system/files/media/file/2020/05/aha-covid19-financial-impact-0520-FINAL.pdf
7. Birkmeyer J, Barnato A, Birkmeyer N, Bessler R, Skinner J. The impact of the COVID-19 pandemic on hospital admissions in the United States. Health Aff (Millwood). 2020;39(11):2010-2017. https://doi.org/10.1377/hlthaff.2020.00980
© 2021 Society of Hospital Medicine
Variation in COVID-19 Mortality Across 117 US Hospitals in High- and Low-Burden Settings
It is clear that certain patient-level factors, such as age, sex, and comorbidities, predict outcomes of SARS-CoV-2 infection.1,2 Less is known about whether hospital-level factors, including surges of patients with COVID-19, are associated with patient outcomes.
In a multicenter cohort study of 2,215 patients with COVID-19 in 65 intensive care units (ICU) across the United States, mortality rates varied widely (6.6%-80.8%), with improved survival for patients admitted to a hospital with more (>100) rather than fewer (<50) ICU beds.3 A different study found that at the state level, COVID-19 mortality increased with increasing COVID-19 admissions.4 Together, these studies suggest that surges in COVID-19 patient volume may be associated with excess mortality. However, the first study was restricted to the ICU population, limiting generalizability, and did not consider admission volume, only ICU bed count. Meanwhile, the second study considered both hospital capacity and patient volume, but it describes a relatively small sample, did not adjust for patient-level predictors of mortality, and does not report outcomes at the hospital level.
Here, we used a large dataset to compare in-hospital mortality rates for patients with COVID-19 across US hospitals, hypothesizing that mortality would be higher in hospitals with the highest burden of COVID-19 admissions. By adjusting for patient-level predictors of mortality and normalizing admission volume for hospital size, we are able to describe residual variability in mortality that may be attributable to differences in COVID-19 patient volume.
METHODS
We included patients with an International Statistical Classification of Diseases, Tenth Revision (ICD)-10 diagnosis of COVID-19 (U07.1) who were admitted to a US hospital that contracts with CarePort Health.5 CarePort is a platform for discharge planning and care coordination that contracts with hospitals in all US regions and auto-extracts data using interface feeds.
We restricted the population to patients admitted between April 1 and April 30, 2020, after a new ICD-10 code for confirmed COVID-19 infection became available, and to hospitals that provided real-time ICD-10 data and pertinent demographic information and could be linked to Centers for Medicare & Medicaid Services (CMS) data by National Provider Identifier. We assumed that the 145 patients (1.0%) who remained hospitalized at 5 weeks all survived. For the 5.9% of patients with multiple admissions during the study period, we included only the first admission with a diagnosis code for COVID-19.
We adjusted for patient age, sex, and the 31 comorbidities in the Elixhauser index, defined by ICD-10 codes. This set of comorbidities includes those previously associated with COVID-19 survival.1,2,6 Unfortunately, inconsistent reporting of vital signs and laboratory data precluded adjusting for acute illness severity. For those patients whose residence zip code was known, we report the racial breakdown (White vs non-White) and adjusted gross income (AGI), based on linked information from the 2018 American Community Survey.7
We defined COVID-19 burden as the quotient of COVID-19 admissions in April 2020 and each hospital’s certified bed count, as reported to the CMS.8 This allowed us to normalize COVID-19 patient volume for variation in hospital size, acknowledging that admitting 10 patients with COVID-19 to a 1,000-bed hospital is different from admitting 10 patients with COVID-19 to a 20-bed hospital. Certified bed count seemed the ideal denominator because it excludes beds not readily deployable to care for patients with COVID-19 (eg, radiology suites, labor and delivery rooms).
We computed hospital-specific adjusted mortality proportions and 95% confidence intervals based on hierarchical multivariable logistic regression, adjusting for age, sex, and comorbidities, and a random effect for each hospital.9,10 Hypothesizing that there may be a threshold of burden beyond which mortality begins to rise, we compared the in-hospital mortality rate at hospitals in the highest quintile of COVID-19 burden to all other hospitals.
We conducted eight post-hoc sensitivity analyses: (1) restricting the study population to patients aged 75 years and older; (2) restricting study hospitals to those with at least 100 beds and 20 COVID-19 admissions; (3) assuming that all patients who remained hospitalized at 5 weeks had died; (4) using each patient’s last admission during the month of April rather than the first; sequentially incorporating (5) zip code–level information on race (limited to White, non-White) and (6) AGI (treated as a continuous variable) into our model; (7) computing two burdens for each hospital (one for each half of April) and using whichever was higher; and (8) treating COVID-19 burden as a continuous predictor. Analyses were performed using SAS statistical software, version 9.4 (SAS Institute Inc) using the GLIMMIX procedure. This study was deemed exempt by the University of California, San Francisco Institutional Review Board.
RESULTS
The study population included 14,226 patients with COVID-19 (median age, 66 years [range, 0-110 years]; 45.2% women) at 117 US hospitals. Based on patients’ zip code of residence, we estimate that 47.0% of patients were White and 29.1% Black, and that the mean household AGI was $61,956. Most hospitals were nonprofit (56%) or private (39%), with approximately one quarter coming from each US census region (range, 25 hospitals [21%] in Midwest to 33 hospitals [28%] in Northeast). Nine hospitals (8%) had more than 700 beds, 40 (34%) had 300 to 700 beds, and 68 (58%) had fewer than 300 beds. Thirty-six hospitals (30.8%) admitted fewer than 20 patients with COVID-19, while six hospitals (5.1%) admitted more than 500 such patients. COVID burden ranged from 0.004 to 2.03 admissions per bed.
As of June 5, 2020, 78.1% of patients had been discharged alive, 20.9% had died, and 1.0% remained hospitalized. At the hospital level, the observed mortality ranged from 0% to 44.4%, was 17.1% among hospitals in COVID-19 burden quintiles one through four, and was 22.7% in the highest burden quintile (Table). 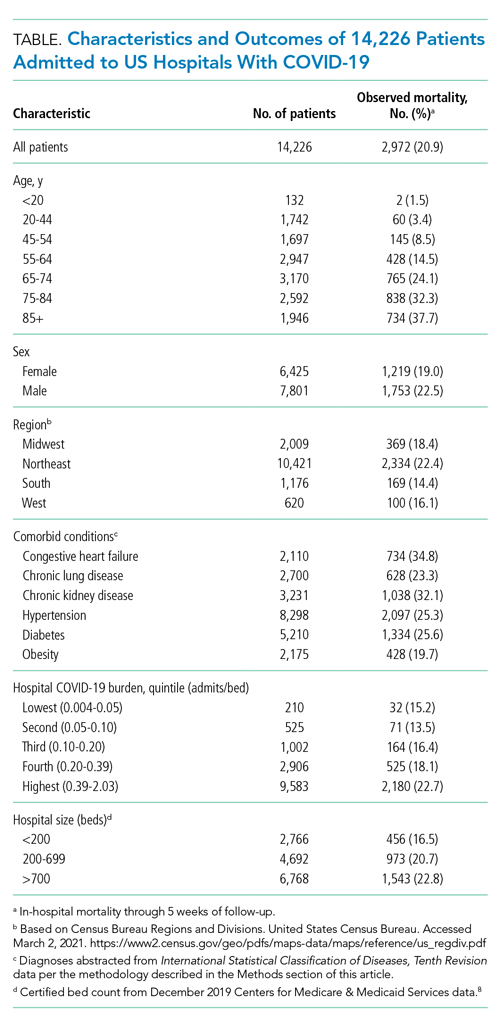

Results were similar across multiple sensitivity analyses (see Appendix Table), although the relationship between COVID-19 burden and in-hospital mortality was attenuated and not significant when the sample was restricted to hospitals with at least 100 beds and 20 COVID-19 admissions, or in analyses adjusted for race and AGI.
DISCUSSION
In this study of 14,226 patients with COVID-19 across 117 US hospitals, those patients admitted to the most burdened hospitals had a higher odds of death. This relationship, which persisted after adjusting for age, sex, and comorbid conditions, suggests that a threshold exists at which patient surges may cause excess mortality.
Notably, in sensitivity analyses adjusting for race and AGI, COVID-19 burden was no longer associated with in-hospital mortality and the point estimate was attenuated. This raises the possibility that our primary results are confounded by these factors. However, prior studies of hospitalized patients have not found race to be predictive of mortality, after adjusting for other factors.11,12
We also note that the relationship between COVID-19 burden and mortality was not significant (P = .07) when the sample was restricted to larger hospitals with more than 20 COVID-19 admissions; again, the point estimate was attenuated. This suggests that larger hospitals may be more resilient in the face of patient surges. Whether this is due to increased availability of staff who can be redeployed to patient care (as with researchers at academic centers), increased experience managing severe respiratory failure, or other factors is uncertain.
Interestingly, in-hospital mortality varied widely across study hospitals, even among the most-burdened hospitals. The reasons for this residual variability—after adjusting for age, sex, and comorbidities and stratifying by COVID-19 burden—are uncertain. To the extent that this variability reflects differences in patient management, hospital staffing, or use of investigational or advanced therapies, it will be critical to identify and disseminate any replicable best practices from high-burden hospitals with low mortality rates.
Whereas other reports have often described single-center or regional experiences,13-15 leaving open the possibility that their results were highly influenced by the local nature of the pandemic in their respective settings, our report from a large sample of hospitals across the United States in high- and low-burden settings provides a more generalizable description of mortality rates for hospitalized patients. Additional study strengths include our adjustment for comorbidities known to be associated with COVID-19 survival, the reporting of definitive outcomes for 99% of patients, and the inclusion of multiple sensitivity analyses to assess the stability of findings.
Our principal limitation is the inability to adjust for severity of acute illness due to inconsistent reporting of laboratory and vital signs data from study hospitals and missing information on interhospital transfers. While our adjusted analyses clearly suggest an association between COVID-19 burden and patient outcomes, our results may still be confounded by differences in illness severity at study hospitals. Thus, our findings should be considered hypothesis-generating and will require confirmation in future studies that include adjustment for acute illness severity.
Other limitations of our study include overrepresentation of large urban hospitals in the Northeast, although this represents the geography of the US pandemic during the study period. Our adjustment for race/ethnicity and socioeconomic status was limited in that we only had zip code-of-residence level information, did not know the zip code of residence for one quarter of study patients, and had to bifurcate the population into White/non-White categories. Finally, our definition of burden does not account for hospital resources, including staffing, ICU capacity, and the availability of advanced or investigational therapies.
CONCLUSION
In this study of 14,226 patients with COVID-19 admitted to 1 of 117 US hospitals, we found that the odds of in-hospital mortality were higher in hospitals that had the highest burden of COVID-19 admissions. This relationship, which persisted after adjustment for age, sex, and comorbid conditions, suggests that patient surges may be an independent risk factor for in-hospital death among patients with COVID-19.
ACKNOWLEGMENTS
The authors thank Bocheng Jing, MS, Senior Statistician at the UCSF Pepper Center, for providing code to identify Elixhauser conditions from ICD-10 data; and Scott Kerber, BS, and Scott Magnoni, MS, both of CarePort Health, for assistance with data extraction. They were not compensated for this work beyond their regular salaries.
1. Evidence used to update the list of underlying medical conditions that increase a person’s risk of severe illness from COVID-19. Centers for Disease Control and Prevention. Updated November 2, 2020. Accessed December 29, 2020. https://www.cdc.gov/coronavirus/2019-ncov/need-extra-precautions/evidence-table.html
2. Cummings MJ, Baldwin MR, Abrams D, et al. Epidemiology, clinical course, and outcomes of critically ill adults with COVID-19 in New York City: a prospective cohort study. Lancet. 2020;395(10239):1763-1770. https://doi.org/10.1016/S0140-6736(20)31189-2
3. Gupta S, Hayek SS, Wang W, et al. Factors associated with death in critically ill patients with coronavirus disease 2019 in the US. JAMA Intern Med. 2020;180(11):1-12. https://doi.org/10.1001/jamainternmed.2020.4568
4. Karaca-Mandic P, Sen S, Georgiou A, Zhu Y, Basu A. Association of COVID-19-related hospital use and overall covid-19 mortality in the USA. J Gen Intern Med. 2020:1-3. https://doi.org/10.1007/s11606-020-06084-7
5. ICD-10-CM official coding and reporting guidelines April 1, 2020 through September 30, 2020. Centers for Disease Control and Prevention. Accessed June 2, 2020. https://www.cdc.gov/nchs/data/icd/COVID-19-guidelines-final.pdf
6. Quan H, Sundararajan V, Halfon P, et al. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med Care. 2005;43(11):1130-1139. https://doi.org/10.1097/01.mlr.0000182534.19832.83
7. About the American Community Survey. United States Census Bureau. Updated January 4, 2021. Accessed March 2, 2021. https://www.census.gov/programs-surveys/acs/about.html
8. Provider of service files. Centers for Medicare & Medicaid Services. Revised January 15, 2020. Accessed March 2, 2021. https://www.cms.gov/research-statistics-data-systems/provider-services-current-files/2019-pos-file
9. Ash AS, Fienberg SE, Louis TA, et al. Statistical issues in assessing hospital performance. Committee of Presidents of Statistical Societies white paper. January 2012. Accessed March 1, 2021. https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/HospitalQualityInits/Downloads/Statistical-Issues-in-Assessing-Hospital-Performance.pdf
10. Bratzler DW, Normand SL, Wang Y, et al. An administrative claims model for profiling hospital 30-day mortality rates for pneumonia patients. PLoS One. 2011;12;6(4):e17401. https://doi.org/10.1371/journal.pone.0017401
11. Garibaldi BT, Fiksel J, Muschelli J, et al. Patient trajectories among persons hospitalized for COVID-19: a cohort study. Ann Intern Med. 2021;174(1):33-41. https://doi.org/10.7326/M20-3905
12. Price-Haywood EG, Burton J, Fort D, Seoane L. Hospitalization and mortality among Black patients and White patients with Covid-19. N Engl J Med. 2020;382(26):2534-2543. https://doi.org/10.1056/NEJMsa2011686
13. Bhatraju PK, Ghassemieh BJ, Nichols M, et al. Covid-19 in critically ill patients in the Seattle region - case series. N Engl J Med. 2020;382(21):2012-2022. https://doi.org/10.1056/NEJMoa2004500
14. Yang X, Yu Y, Xu J, et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med. 2020;8(5):475-481. https://doi.org/10.1016/S2213-2600(20)30079-5
15. Richardson S, Hirsch JS, Narasimhan M, et al. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City area. JAMA. 2020;323(20):2052-2059. https://doi.org/10.1001/jama.2020.6775
It is clear that certain patient-level factors, such as age, sex, and comorbidities, predict outcomes of SARS-CoV-2 infection.1,2 Less is known about whether hospital-level factors, including surges of patients with COVID-19, are associated with patient outcomes.
In a multicenter cohort study of 2,215 patients with COVID-19 in 65 intensive care units (ICU) across the United States, mortality rates varied widely (6.6%-80.8%), with improved survival for patients admitted to a hospital with more (>100) rather than fewer (<50) ICU beds.3 A different study found that at the state level, COVID-19 mortality increased with increasing COVID-19 admissions.4 Together, these studies suggest that surges in COVID-19 patient volume may be associated with excess mortality. However, the first study was restricted to the ICU population, limiting generalizability, and did not consider admission volume, only ICU bed count. Meanwhile, the second study considered both hospital capacity and patient volume, but it describes a relatively small sample, did not adjust for patient-level predictors of mortality, and does not report outcomes at the hospital level.
Here, we used a large dataset to compare in-hospital mortality rates for patients with COVID-19 across US hospitals, hypothesizing that mortality would be higher in hospitals with the highest burden of COVID-19 admissions. By adjusting for patient-level predictors of mortality and normalizing admission volume for hospital size, we are able to describe residual variability in mortality that may be attributable to differences in COVID-19 patient volume.
METHODS
We included patients with an International Statistical Classification of Diseases, Tenth Revision (ICD)-10 diagnosis of COVID-19 (U07.1) who were admitted to a US hospital that contracts with CarePort Health.5 CarePort is a platform for discharge planning and care coordination that contracts with hospitals in all US regions and auto-extracts data using interface feeds.
We restricted the population to patients admitted between April 1 and April 30, 2020, after a new ICD-10 code for confirmed COVID-19 infection became available, and to hospitals that provided real-time ICD-10 data and pertinent demographic information and could be linked to Centers for Medicare & Medicaid Services (CMS) data by National Provider Identifier. We assumed that the 145 patients (1.0%) who remained hospitalized at 5 weeks all survived. For the 5.9% of patients with multiple admissions during the study period, we included only the first admission with a diagnosis code for COVID-19.
We adjusted for patient age, sex, and the 31 comorbidities in the Elixhauser index, defined by ICD-10 codes. This set of comorbidities includes those previously associated with COVID-19 survival.1,2,6 Unfortunately, inconsistent reporting of vital signs and laboratory data precluded adjusting for acute illness severity. For those patients whose residence zip code was known, we report the racial breakdown (White vs non-White) and adjusted gross income (AGI), based on linked information from the 2018 American Community Survey.7
We defined COVID-19 burden as the quotient of COVID-19 admissions in April 2020 and each hospital’s certified bed count, as reported to the CMS.8 This allowed us to normalize COVID-19 patient volume for variation in hospital size, acknowledging that admitting 10 patients with COVID-19 to a 1,000-bed hospital is different from admitting 10 patients with COVID-19 to a 20-bed hospital. Certified bed count seemed the ideal denominator because it excludes beds not readily deployable to care for patients with COVID-19 (eg, radiology suites, labor and delivery rooms).
We computed hospital-specific adjusted mortality proportions and 95% confidence intervals based on hierarchical multivariable logistic regression, adjusting for age, sex, and comorbidities, and a random effect for each hospital.9,10 Hypothesizing that there may be a threshold of burden beyond which mortality begins to rise, we compared the in-hospital mortality rate at hospitals in the highest quintile of COVID-19 burden to all other hospitals.
We conducted eight post-hoc sensitivity analyses: (1) restricting the study population to patients aged 75 years and older; (2) restricting study hospitals to those with at least 100 beds and 20 COVID-19 admissions; (3) assuming that all patients who remained hospitalized at 5 weeks had died; (4) using each patient’s last admission during the month of April rather than the first; sequentially incorporating (5) zip code–level information on race (limited to White, non-White) and (6) AGI (treated as a continuous variable) into our model; (7) computing two burdens for each hospital (one for each half of April) and using whichever was higher; and (8) treating COVID-19 burden as a continuous predictor. Analyses were performed using SAS statistical software, version 9.4 (SAS Institute Inc) using the GLIMMIX procedure. This study was deemed exempt by the University of California, San Francisco Institutional Review Board.
RESULTS
The study population included 14,226 patients with COVID-19 (median age, 66 years [range, 0-110 years]; 45.2% women) at 117 US hospitals. Based on patients’ zip code of residence, we estimate that 47.0% of patients were White and 29.1% Black, and that the mean household AGI was $61,956. Most hospitals were nonprofit (56%) or private (39%), with approximately one quarter coming from each US census region (range, 25 hospitals [21%] in Midwest to 33 hospitals [28%] in Northeast). Nine hospitals (8%) had more than 700 beds, 40 (34%) had 300 to 700 beds, and 68 (58%) had fewer than 300 beds. Thirty-six hospitals (30.8%) admitted fewer than 20 patients with COVID-19, while six hospitals (5.1%) admitted more than 500 such patients. COVID burden ranged from 0.004 to 2.03 admissions per bed.
As of June 5, 2020, 78.1% of patients had been discharged alive, 20.9% had died, and 1.0% remained hospitalized. At the hospital level, the observed mortality ranged from 0% to 44.4%, was 17.1% among hospitals in COVID-19 burden quintiles one through four, and was 22.7% in the highest burden quintile (Table). 

Results were similar across multiple sensitivity analyses (see Appendix Table), although the relationship between COVID-19 burden and in-hospital mortality was attenuated and not significant when the sample was restricted to hospitals with at least 100 beds and 20 COVID-19 admissions, or in analyses adjusted for race and AGI.
DISCUSSION
In this study of 14,226 patients with COVID-19 across 117 US hospitals, those patients admitted to the most burdened hospitals had a higher odds of death. This relationship, which persisted after adjusting for age, sex, and comorbid conditions, suggests that a threshold exists at which patient surges may cause excess mortality.
Notably, in sensitivity analyses adjusting for race and AGI, COVID-19 burden was no longer associated with in-hospital mortality and the point estimate was attenuated. This raises the possibility that our primary results are confounded by these factors. However, prior studies of hospitalized patients have not found race to be predictive of mortality, after adjusting for other factors.11,12
We also note that the relationship between COVID-19 burden and mortality was not significant (P = .07) when the sample was restricted to larger hospitals with more than 20 COVID-19 admissions; again, the point estimate was attenuated. This suggests that larger hospitals may be more resilient in the face of patient surges. Whether this is due to increased availability of staff who can be redeployed to patient care (as with researchers at academic centers), increased experience managing severe respiratory failure, or other factors is uncertain.
Interestingly, in-hospital mortality varied widely across study hospitals, even among the most-burdened hospitals. The reasons for this residual variability—after adjusting for age, sex, and comorbidities and stratifying by COVID-19 burden—are uncertain. To the extent that this variability reflects differences in patient management, hospital staffing, or use of investigational or advanced therapies, it will be critical to identify and disseminate any replicable best practices from high-burden hospitals with low mortality rates.
Whereas other reports have often described single-center or regional experiences,13-15 leaving open the possibility that their results were highly influenced by the local nature of the pandemic in their respective settings, our report from a large sample of hospitals across the United States in high- and low-burden settings provides a more generalizable description of mortality rates for hospitalized patients. Additional study strengths include our adjustment for comorbidities known to be associated with COVID-19 survival, the reporting of definitive outcomes for 99% of patients, and the inclusion of multiple sensitivity analyses to assess the stability of findings.
Our principal limitation is the inability to adjust for severity of acute illness due to inconsistent reporting of laboratory and vital signs data from study hospitals and missing information on interhospital transfers. While our adjusted analyses clearly suggest an association between COVID-19 burden and patient outcomes, our results may still be confounded by differences in illness severity at study hospitals. Thus, our findings should be considered hypothesis-generating and will require confirmation in future studies that include adjustment for acute illness severity.
Other limitations of our study include overrepresentation of large urban hospitals in the Northeast, although this represents the geography of the US pandemic during the study period. Our adjustment for race/ethnicity and socioeconomic status was limited in that we only had zip code-of-residence level information, did not know the zip code of residence for one quarter of study patients, and had to bifurcate the population into White/non-White categories. Finally, our definition of burden does not account for hospital resources, including staffing, ICU capacity, and the availability of advanced or investigational therapies.
CONCLUSION
In this study of 14,226 patients with COVID-19 admitted to 1 of 117 US hospitals, we found that the odds of in-hospital mortality were higher in hospitals that had the highest burden of COVID-19 admissions. This relationship, which persisted after adjustment for age, sex, and comorbid conditions, suggests that patient surges may be an independent risk factor for in-hospital death among patients with COVID-19.
ACKNOWLEGMENTS
The authors thank Bocheng Jing, MS, Senior Statistician at the UCSF Pepper Center, for providing code to identify Elixhauser conditions from ICD-10 data; and Scott Kerber, BS, and Scott Magnoni, MS, both of CarePort Health, for assistance with data extraction. They were not compensated for this work beyond their regular salaries.
It is clear that certain patient-level factors, such as age, sex, and comorbidities, predict outcomes of SARS-CoV-2 infection.1,2 Less is known about whether hospital-level factors, including surges of patients with COVID-19, are associated with patient outcomes.
In a multicenter cohort study of 2,215 patients with COVID-19 in 65 intensive care units (ICU) across the United States, mortality rates varied widely (6.6%-80.8%), with improved survival for patients admitted to a hospital with more (>100) rather than fewer (<50) ICU beds.3 A different study found that at the state level, COVID-19 mortality increased with increasing COVID-19 admissions.4 Together, these studies suggest that surges in COVID-19 patient volume may be associated with excess mortality. However, the first study was restricted to the ICU population, limiting generalizability, and did not consider admission volume, only ICU bed count. Meanwhile, the second study considered both hospital capacity and patient volume, but it describes a relatively small sample, did not adjust for patient-level predictors of mortality, and does not report outcomes at the hospital level.
Here, we used a large dataset to compare in-hospital mortality rates for patients with COVID-19 across US hospitals, hypothesizing that mortality would be higher in hospitals with the highest burden of COVID-19 admissions. By adjusting for patient-level predictors of mortality and normalizing admission volume for hospital size, we are able to describe residual variability in mortality that may be attributable to differences in COVID-19 patient volume.
METHODS
We included patients with an International Statistical Classification of Diseases, Tenth Revision (ICD)-10 diagnosis of COVID-19 (U07.1) who were admitted to a US hospital that contracts with CarePort Health.5 CarePort is a platform for discharge planning and care coordination that contracts with hospitals in all US regions and auto-extracts data using interface feeds.
We restricted the population to patients admitted between April 1 and April 30, 2020, after a new ICD-10 code for confirmed COVID-19 infection became available, and to hospitals that provided real-time ICD-10 data and pertinent demographic information and could be linked to Centers for Medicare & Medicaid Services (CMS) data by National Provider Identifier. We assumed that the 145 patients (1.0%) who remained hospitalized at 5 weeks all survived. For the 5.9% of patients with multiple admissions during the study period, we included only the first admission with a diagnosis code for COVID-19.
We adjusted for patient age, sex, and the 31 comorbidities in the Elixhauser index, defined by ICD-10 codes. This set of comorbidities includes those previously associated with COVID-19 survival.1,2,6 Unfortunately, inconsistent reporting of vital signs and laboratory data precluded adjusting for acute illness severity. For those patients whose residence zip code was known, we report the racial breakdown (White vs non-White) and adjusted gross income (AGI), based on linked information from the 2018 American Community Survey.7
We defined COVID-19 burden as the quotient of COVID-19 admissions in April 2020 and each hospital’s certified bed count, as reported to the CMS.8 This allowed us to normalize COVID-19 patient volume for variation in hospital size, acknowledging that admitting 10 patients with COVID-19 to a 1,000-bed hospital is different from admitting 10 patients with COVID-19 to a 20-bed hospital. Certified bed count seemed the ideal denominator because it excludes beds not readily deployable to care for patients with COVID-19 (eg, radiology suites, labor and delivery rooms).
We computed hospital-specific adjusted mortality proportions and 95% confidence intervals based on hierarchical multivariable logistic regression, adjusting for age, sex, and comorbidities, and a random effect for each hospital.9,10 Hypothesizing that there may be a threshold of burden beyond which mortality begins to rise, we compared the in-hospital mortality rate at hospitals in the highest quintile of COVID-19 burden to all other hospitals.
We conducted eight post-hoc sensitivity analyses: (1) restricting the study population to patients aged 75 years and older; (2) restricting study hospitals to those with at least 100 beds and 20 COVID-19 admissions; (3) assuming that all patients who remained hospitalized at 5 weeks had died; (4) using each patient’s last admission during the month of April rather than the first; sequentially incorporating (5) zip code–level information on race (limited to White, non-White) and (6) AGI (treated as a continuous variable) into our model; (7) computing two burdens for each hospital (one for each half of April) and using whichever was higher; and (8) treating COVID-19 burden as a continuous predictor. Analyses were performed using SAS statistical software, version 9.4 (SAS Institute Inc) using the GLIMMIX procedure. This study was deemed exempt by the University of California, San Francisco Institutional Review Board.
RESULTS
The study population included 14,226 patients with COVID-19 (median age, 66 years [range, 0-110 years]; 45.2% women) at 117 US hospitals. Based on patients’ zip code of residence, we estimate that 47.0% of patients were White and 29.1% Black, and that the mean household AGI was $61,956. Most hospitals were nonprofit (56%) or private (39%), with approximately one quarter coming from each US census region (range, 25 hospitals [21%] in Midwest to 33 hospitals [28%] in Northeast). Nine hospitals (8%) had more than 700 beds, 40 (34%) had 300 to 700 beds, and 68 (58%) had fewer than 300 beds. Thirty-six hospitals (30.8%) admitted fewer than 20 patients with COVID-19, while six hospitals (5.1%) admitted more than 500 such patients. COVID burden ranged from 0.004 to 2.03 admissions per bed.
As of June 5, 2020, 78.1% of patients had been discharged alive, 20.9% had died, and 1.0% remained hospitalized. At the hospital level, the observed mortality ranged from 0% to 44.4%, was 17.1% among hospitals in COVID-19 burden quintiles one through four, and was 22.7% in the highest burden quintile (Table). 

Results were similar across multiple sensitivity analyses (see Appendix Table), although the relationship between COVID-19 burden and in-hospital mortality was attenuated and not significant when the sample was restricted to hospitals with at least 100 beds and 20 COVID-19 admissions, or in analyses adjusted for race and AGI.
DISCUSSION
In this study of 14,226 patients with COVID-19 across 117 US hospitals, those patients admitted to the most burdened hospitals had a higher odds of death. This relationship, which persisted after adjusting for age, sex, and comorbid conditions, suggests that a threshold exists at which patient surges may cause excess mortality.
Notably, in sensitivity analyses adjusting for race and AGI, COVID-19 burden was no longer associated with in-hospital mortality and the point estimate was attenuated. This raises the possibility that our primary results are confounded by these factors. However, prior studies of hospitalized patients have not found race to be predictive of mortality, after adjusting for other factors.11,12
We also note that the relationship between COVID-19 burden and mortality was not significant (P = .07) when the sample was restricted to larger hospitals with more than 20 COVID-19 admissions; again, the point estimate was attenuated. This suggests that larger hospitals may be more resilient in the face of patient surges. Whether this is due to increased availability of staff who can be redeployed to patient care (as with researchers at academic centers), increased experience managing severe respiratory failure, or other factors is uncertain.
Interestingly, in-hospital mortality varied widely across study hospitals, even among the most-burdened hospitals. The reasons for this residual variability—after adjusting for age, sex, and comorbidities and stratifying by COVID-19 burden—are uncertain. To the extent that this variability reflects differences in patient management, hospital staffing, or use of investigational or advanced therapies, it will be critical to identify and disseminate any replicable best practices from high-burden hospitals with low mortality rates.
Whereas other reports have often described single-center or regional experiences,13-15 leaving open the possibility that their results were highly influenced by the local nature of the pandemic in their respective settings, our report from a large sample of hospitals across the United States in high- and low-burden settings provides a more generalizable description of mortality rates for hospitalized patients. Additional study strengths include our adjustment for comorbidities known to be associated with COVID-19 survival, the reporting of definitive outcomes for 99% of patients, and the inclusion of multiple sensitivity analyses to assess the stability of findings.
Our principal limitation is the inability to adjust for severity of acute illness due to inconsistent reporting of laboratory and vital signs data from study hospitals and missing information on interhospital transfers. While our adjusted analyses clearly suggest an association between COVID-19 burden and patient outcomes, our results may still be confounded by differences in illness severity at study hospitals. Thus, our findings should be considered hypothesis-generating and will require confirmation in future studies that include adjustment for acute illness severity.
Other limitations of our study include overrepresentation of large urban hospitals in the Northeast, although this represents the geography of the US pandemic during the study period. Our adjustment for race/ethnicity and socioeconomic status was limited in that we only had zip code-of-residence level information, did not know the zip code of residence for one quarter of study patients, and had to bifurcate the population into White/non-White categories. Finally, our definition of burden does not account for hospital resources, including staffing, ICU capacity, and the availability of advanced or investigational therapies.
CONCLUSION
In this study of 14,226 patients with COVID-19 admitted to 1 of 117 US hospitals, we found that the odds of in-hospital mortality were higher in hospitals that had the highest burden of COVID-19 admissions. This relationship, which persisted after adjustment for age, sex, and comorbid conditions, suggests that patient surges may be an independent risk factor for in-hospital death among patients with COVID-19.
ACKNOWLEGMENTS
The authors thank Bocheng Jing, MS, Senior Statistician at the UCSF Pepper Center, for providing code to identify Elixhauser conditions from ICD-10 data; and Scott Kerber, BS, and Scott Magnoni, MS, both of CarePort Health, for assistance with data extraction. They were not compensated for this work beyond their regular salaries.
1. Evidence used to update the list of underlying medical conditions that increase a person’s risk of severe illness from COVID-19. Centers for Disease Control and Prevention. Updated November 2, 2020. Accessed December 29, 2020. https://www.cdc.gov/coronavirus/2019-ncov/need-extra-precautions/evidence-table.html
2. Cummings MJ, Baldwin MR, Abrams D, et al. Epidemiology, clinical course, and outcomes of critically ill adults with COVID-19 in New York City: a prospective cohort study. Lancet. 2020;395(10239):1763-1770. https://doi.org/10.1016/S0140-6736(20)31189-2
3. Gupta S, Hayek SS, Wang W, et al. Factors associated with death in critically ill patients with coronavirus disease 2019 in the US. JAMA Intern Med. 2020;180(11):1-12. https://doi.org/10.1001/jamainternmed.2020.4568
4. Karaca-Mandic P, Sen S, Georgiou A, Zhu Y, Basu A. Association of COVID-19-related hospital use and overall covid-19 mortality in the USA. J Gen Intern Med. 2020:1-3. https://doi.org/10.1007/s11606-020-06084-7
5. ICD-10-CM official coding and reporting guidelines April 1, 2020 through September 30, 2020. Centers for Disease Control and Prevention. Accessed June 2, 2020. https://www.cdc.gov/nchs/data/icd/COVID-19-guidelines-final.pdf
6. Quan H, Sundararajan V, Halfon P, et al. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med Care. 2005;43(11):1130-1139. https://doi.org/10.1097/01.mlr.0000182534.19832.83
7. About the American Community Survey. United States Census Bureau. Updated January 4, 2021. Accessed March 2, 2021. https://www.census.gov/programs-surveys/acs/about.html
8. Provider of service files. Centers for Medicare & Medicaid Services. Revised January 15, 2020. Accessed March 2, 2021. https://www.cms.gov/research-statistics-data-systems/provider-services-current-files/2019-pos-file
9. Ash AS, Fienberg SE, Louis TA, et al. Statistical issues in assessing hospital performance. Committee of Presidents of Statistical Societies white paper. January 2012. Accessed March 1, 2021. https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/HospitalQualityInits/Downloads/Statistical-Issues-in-Assessing-Hospital-Performance.pdf
10. Bratzler DW, Normand SL, Wang Y, et al. An administrative claims model for profiling hospital 30-day mortality rates for pneumonia patients. PLoS One. 2011;12;6(4):e17401. https://doi.org/10.1371/journal.pone.0017401
11. Garibaldi BT, Fiksel J, Muschelli J, et al. Patient trajectories among persons hospitalized for COVID-19: a cohort study. Ann Intern Med. 2021;174(1):33-41. https://doi.org/10.7326/M20-3905
12. Price-Haywood EG, Burton J, Fort D, Seoane L. Hospitalization and mortality among Black patients and White patients with Covid-19. N Engl J Med. 2020;382(26):2534-2543. https://doi.org/10.1056/NEJMsa2011686
13. Bhatraju PK, Ghassemieh BJ, Nichols M, et al. Covid-19 in critically ill patients in the Seattle region - case series. N Engl J Med. 2020;382(21):2012-2022. https://doi.org/10.1056/NEJMoa2004500
14. Yang X, Yu Y, Xu J, et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med. 2020;8(5):475-481. https://doi.org/10.1016/S2213-2600(20)30079-5
15. Richardson S, Hirsch JS, Narasimhan M, et al. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City area. JAMA. 2020;323(20):2052-2059. https://doi.org/10.1001/jama.2020.6775
1. Evidence used to update the list of underlying medical conditions that increase a person’s risk of severe illness from COVID-19. Centers for Disease Control and Prevention. Updated November 2, 2020. Accessed December 29, 2020. https://www.cdc.gov/coronavirus/2019-ncov/need-extra-precautions/evidence-table.html
2. Cummings MJ, Baldwin MR, Abrams D, et al. Epidemiology, clinical course, and outcomes of critically ill adults with COVID-19 in New York City: a prospective cohort study. Lancet. 2020;395(10239):1763-1770. https://doi.org/10.1016/S0140-6736(20)31189-2
3. Gupta S, Hayek SS, Wang W, et al. Factors associated with death in critically ill patients with coronavirus disease 2019 in the US. JAMA Intern Med. 2020;180(11):1-12. https://doi.org/10.1001/jamainternmed.2020.4568
4. Karaca-Mandic P, Sen S, Georgiou A, Zhu Y, Basu A. Association of COVID-19-related hospital use and overall covid-19 mortality in the USA. J Gen Intern Med. 2020:1-3. https://doi.org/10.1007/s11606-020-06084-7
5. ICD-10-CM official coding and reporting guidelines April 1, 2020 through September 30, 2020. Centers for Disease Control and Prevention. Accessed June 2, 2020. https://www.cdc.gov/nchs/data/icd/COVID-19-guidelines-final.pdf
6. Quan H, Sundararajan V, Halfon P, et al. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med Care. 2005;43(11):1130-1139. https://doi.org/10.1097/01.mlr.0000182534.19832.83
7. About the American Community Survey. United States Census Bureau. Updated January 4, 2021. Accessed March 2, 2021. https://www.census.gov/programs-surveys/acs/about.html
8. Provider of service files. Centers for Medicare & Medicaid Services. Revised January 15, 2020. Accessed March 2, 2021. https://www.cms.gov/research-statistics-data-systems/provider-services-current-files/2019-pos-file
9. Ash AS, Fienberg SE, Louis TA, et al. Statistical issues in assessing hospital performance. Committee of Presidents of Statistical Societies white paper. January 2012. Accessed March 1, 2021. https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/HospitalQualityInits/Downloads/Statistical-Issues-in-Assessing-Hospital-Performance.pdf
10. Bratzler DW, Normand SL, Wang Y, et al. An administrative claims model for profiling hospital 30-day mortality rates for pneumonia patients. PLoS One. 2011;12;6(4):e17401. https://doi.org/10.1371/journal.pone.0017401
11. Garibaldi BT, Fiksel J, Muschelli J, et al. Patient trajectories among persons hospitalized for COVID-19: a cohort study. Ann Intern Med. 2021;174(1):33-41. https://doi.org/10.7326/M20-3905
12. Price-Haywood EG, Burton J, Fort D, Seoane L. Hospitalization and mortality among Black patients and White patients with Covid-19. N Engl J Med. 2020;382(26):2534-2543. https://doi.org/10.1056/NEJMsa2011686
13. Bhatraju PK, Ghassemieh BJ, Nichols M, et al. Covid-19 in critically ill patients in the Seattle region - case series. N Engl J Med. 2020;382(21):2012-2022. https://doi.org/10.1056/NEJMoa2004500
14. Yang X, Yu Y, Xu J, et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med. 2020;8(5):475-481. https://doi.org/10.1016/S2213-2600(20)30079-5
15. Richardson S, Hirsch JS, Narasimhan M, et al. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City area. JAMA. 2020;323(20):2052-2059. https://doi.org/10.1001/jama.2020.6775
© 2021 Society of Hospital Medicine
Supine-Related Pseudoanemia in Hospitalized Patients
The World Health Organization (WHO) defines anemia as a hemoglobin value less than 12 g/dL in women and less than 13 g/dL in men.1 Hospital-acquired anemia is loosely defined as normal hemoglobin levels on admission that, at their nadir during hospitalization or on discharge, are less than WHO sex-defined cutoffs. Hospital-acquired anemia or significant decreases in hemoglobin are often identified during hospitalization.2-6 Potential causes include blood loss from phlebotomy, occult gastrointestinal bleeding, hemolysis, anemia of inflammation, and hemodilution due to fluid resuscitation. Of these causes, some are dangerous to patients, some are iatrogenic, and some are due to laboratory error.7 Physicians often evaluate decreases in hemoglobin, which could otherwise be explained by laboratory error, hemodilution, or expected decrease in hemoglobin due to hospitalization, to identify causes that may lead to potential harm.
Jacob et al8 demonstrated the effect of posture on hemoglobin concentrations in healthy volunteers, showing an average 11% relative increase in hemoglobin when going from lying to standing. This increase was attributed to shifts in plasma volume to the vascular space with recumbence. They hypothesized that the initial hemoglobin on admission is measured when patients are upright or recently upright, whereas after admission, patients are more likely to be supine, resulting in lower hemoglobin concentrations. Others have also demonstrated similar effects of patient posture on hemoglobin concentration.9-13 However, these prior results are not readily generalizable to hospitalized patients. These prior studies enrolled healthy volunteers, and most examined postural changes from the supine and standing positions; blood is rarely obtained from hospitalized patients when they are standing.
The aim of this study was to investigate whether postural changes in hemoglobin can be demonstrated in positions that patients routinely encountered during in-hospital phlebotomy: upright in a chair or recumbent in a bed. Patient position, which is not standardized during blood draws, may contribute to lower measured hemoglobin concentrations in some patients, especially sicker individuals who are recumbent more frequently. We hypothesized that going from supine to upright in a chair would result in a relative increase in hemoglobin concentration of 5% to 6%, approximately half the value of going from supine to standing.8 To investigate this, we conducted a quasi-experimental study exploring the effect of position (supine or sitting in chair) on hemoglobin concentrations in medical inpatients.
METHODS
Participants
Patients were enrolled in this single-center study between October 2017 and August 2018. Patients aged 18 years or older who were hospitalized on the general internal medicine wards were screened to determine if they met the following inclusion criteria: hospitalized for <5 days, had blood work scheduled as part of routine care (in order to decrease phlebotomy required by this study), had baseline hemoglobin >8 g/dL, and were able to remain supine without interruption overnight and able to sit in a chair for at least 1 hour the following morning. Patients were excluded from the study if they had a hematologic malignancy, were at risk of >100 mL of blood loss (eg, admitted for gastrointestinal bleeding, planned surgery), had a transfusion requirement, or received intravascular modifiers such as fluid (>100 cc/h) or intravenous diuretics. The Johns Hopkins Institutional Review Board approved this study, and all patients provided written informed consent.
Study Design
Patients enrolled in this quasi-experimental study were asked to remain supine for at least 6 hours overnight. Adherence to the recumbent position was tracked by patient self-report and by corroboration with the patient’s nurse overnight. Any interruptions to supine positioning resulted in exclusion from the study. The following morning, a member of the study team performed phlebotomy while the patient remained supine. Patients were then asked to sit comfortably in a chair for at least 1 hour with their feet on the ground; the blood draw was then repeated. All blood samples were acquired by venipuncture. Prior to each blood draw, a tourniquet was placed over the upper arm below the axilla. An antecubital vein on either arm was visualized under ultrasound guidance, and a 23-G × 3/4” butterfly needle was used for venipuncture. The vials of blood were immediately inverted after blood collection. Hemoglobin assays were processed and analyzed using Sysmex XN-10 analyzer (Sysmex Corporation). The reference range for hemoglobin in our facility was 12.0 to 15.0 g/dL for women and 13.9 to 16.3 g/dL for men. Laboratory technicians were blinded to and uninvolved in the study.
We determined, a priori, that 33 enrolled patients would provide 80% power (alpha 0.05) to detect an average hemoglobin change of 4.1%, assuming that the standard deviation of the hemoglobin change was twice the mean (ie, SD = 8.2%). The Wilcoxon signed-rank test was used to test the significance of postural hemoglobin changes. Analyses were conducted using JMP Pro 13.0 (SAS) and GraphPad Prism 8 (GraphPad Software). Significance was defined at P < .05 for all analyses.
RESULTS
Thirty-nine patients were consented and enrolled in the study; four patients were excluded prior to blood draw (two patients because of interruption of supine time, two patients because of refusal in the morning). Of the 35 patients who completed the study, 13 were women (37%); median age was 49 years (range, 25-83 years). Median supine hemoglobin concentration in our sample was 11.7 g/dL (range, 9.3-18.1 g/dL), and median baseline creatinine level was 0.70 mg/dL (range, 0.5-2.5 mg/dL). Median supine hemoglobin levels were 11.7 g/dL (range, 9.6-13.2 g/dL) in women and 11.8 g/dL (range, 9.3-18.1 g/dL) in men. In aggregate, patients had a median increase in hemoglobin concentration of 0.60 g/dL (range, –0.6 to 1.4 g/dL) with sitting, a 5.2% (range, –4.5% to 15.1%) relative change (P < .001) (Figure 1). 
DISCUSSION
International blood collection guidelines acknowledge postural changes in laboratory values and recommend standardization of patient position to either sitting in a chair or lying flat in a bed, without changes in position for 15 minutes prior to blood draw.14 When these positional accommodations cannot be met, documenting positional disruptions is recommended so that laboratory values can be interpreted accordingly. To the best of our knowledge, no hospital in the United States has standardized patient position as part of phlebotomy procedure such that patient position is documented and can be made available to interpreting providers.
Relative increases in hemoglobin or hematocrit range from 7% to 12% when patients go from supine to standing.8,9,11 The reverse relationship has also been shown, where upright-to-supine position results in decreases in hemoglobin concentrations.10,13 We found that going from supine to a seated position resulted in significant increases in hemoglobin of 0.6 g/dL and in a more than 1 g/dL increase in 29% of the patients. Although four of the 35 patients experienced either no change or a slight decrease in their hemoglobin concentration when going from supine to upright and not all patients saw a uniform effect, providers should be aware that the patient’s position can contribute to changes in hemoglobin concentration in the hospitalized setting. Providers may be able to use this information to avoid an extensive diagnostic workup when anemia is identified in hospitalized patients, although more research is needed to identify patient subsets who are at higher risk for this effect.
Until hospitals implement protocols that require phlebotomists to report patient position during phlebotomy in a standardized fashion, providers should be alert to the fact that supine positioning may result in a hemoglobin level that is significantly lower than that when drawn in a sitting position, and in almost one-third of patients, this difference may be 1.0 g/dL or greater.
Given our study criteria requiring supine positions of at least 6 hours and a baseline hemoglobin concentration >8 g/dL, our sample of patients may have been younger and healthier than the average hospitalized patient on general internal medicine wards. Since greater relative changes in plasma volume shifts and hemoglobin might be seen in patients with lower baseline hemoglobin and lower baseline plasma protein, this selection bias may underestimate the effects of position on hemoglobin changes for the average inpatient population. Additionally, we intentionally sought to obtain sitting hemoglobin levels after the supine samples to avoid the possibility of incorrectly attributing dropping hemoglobin levels to progressive hospital-acquired anemia from phlebotomy or illness. Any concomitant trend of falling hemoglobin levels in our patients would be expected to lead to a systematic underestimation of the positional change in hemoglobin we observed. We did not objectively observe adherence to supine and upright position and instead relied on patient self-reporting, which is one possible contributor to the variable effects of position on hemoglobin concentration, with some patients having no change or decreases in hemoglobin concentrations.
CONCLUSION
Posture can significantly influence hemoglobin levels in hospitalized patients on general medicine wards. Further research can determine whether it would be cost and time effective to standardize patient positions prior to phlebotomy, or at least to report patient positioning with the laboratory testing results.
1. DeMaeyer E, Adiels-Tegman M. The prevalence of anaemia in the world. World Health Stat Q. 1985;38(3):302-316.
2. Martin ND, Scantling D. Hospital-acquired anemia. J Infus Nurs. 2015;38(5):330-338. https://doi.org/10.1097/NAN.0000000000000121
3. Thavendiranathan P, Bagai A, Ebidia A, Detsky AS, Choudhry NK. Do blood tests cause anemia in hospitalized patients? The effect of diagnostic phlebotomy on hemoglobin and hematocrit levels. J Gen Intern Med. 2005;20(6):520-524. https://doi.org/10.1111/j.1525-1497.2005.0094.x
4. Salisbury AC, Reid KJ, Alexander KP, et al. Diagnostic blood loss from phlebotomy and hospital-acquired anemia during acute myocardial infarction. Arch Intern Med. 2011;171(18):1646-1653. https://doi.org/10.1001/archinternmed.2011.361
5. Languasco A, Cazap N, Marciano S, et al. Hemoglobin concentration variations over time in general medical inpatients. J Hosp Med. 2010;5(5):283-288. https://doi.org/10.1002/jhm.650
6. van der Bom JG, Cannegieter SC. Hospital-acquired anemia: the contribution of diagnostic blood loss. J Thromb Haemost. 2015;13(6):1157-1159. https://doi.org/10.1111/jth.12886
7. Berkow L. Factors affecting hemoglobin measurement. J Clin Monit Comput. 2013;27(5):499-508. https://doi.org/10.1007/s10877-013-9456-3
8. Jacob G, Raj SR, Ketch T, et al. Postural pseudoanemia: posture-dependent change in hematocrit. Mayo Clin Proc. 2005;80(5):611-614. https://doi.org/10.4065/80.5.611
9. Fawcett JK, Wynn V. Effects of posture on plasma volume and some blood constituents. J Clin Pathol. 1960;13(4):304-310. https://doi.org/10.1136/jcp.13.4.304
10. Tombridge TL. Effect of posture on hematology results. Am J ClinPathol. 1968;49(4):491-493. https://doi.org/10.1093/ajcp/49.4.491
11. Hagan RD, Diaz FJ, Horvath SM. Plasma volume changes with movement to supine and standing positions. J Appl Physiol. 1978;45(3):414-417. https://doi.org/10.1152/jappl.1978.45.3.414
12. Maw GJ, Mackenzie IL, Taylor NA. Redistribution of body fluids during postural manipulations. Acta Physiol Scand. 1995;155(2):157-163. https://doi.org/10.1111/j.1748-1716.1995.tb09960.x
13. Lima-Oliveira G, Guidi GC, Salvagno GL, Danese E, Montagnana M, Lippi G. Patient posture for blood collection by venipuncture: recall for standardization after 28 years. Rev Bras Hematol Hemoter. 2017;39(2):127-132. https://doi.org/10.1016/j.bjhh.2017.01.004
14. Simundic AM, Bölenius K, Cadamuro J, et al. Working Group for Preanalytical Phase (WG-PRE), of the European Federation of Clinical Chemistry and Laboratory Medicine (EFLM) and Latin American Working Group for Preanalytical Phase (WG-PRE-LATAM) of the Latin America Confederation of Clinical Biochemistry (COLABIOCLI). Joint EFLM-COLABIOCLI recommendation for venous blood sampling. Clin Chem Lab Med. 2018;56(12):2015-2038. https://doi.org/10.1515/cclm-2018-0602
The World Health Organization (WHO) defines anemia as a hemoglobin value less than 12 g/dL in women and less than 13 g/dL in men.1 Hospital-acquired anemia is loosely defined as normal hemoglobin levels on admission that, at their nadir during hospitalization or on discharge, are less than WHO sex-defined cutoffs. Hospital-acquired anemia or significant decreases in hemoglobin are often identified during hospitalization.2-6 Potential causes include blood loss from phlebotomy, occult gastrointestinal bleeding, hemolysis, anemia of inflammation, and hemodilution due to fluid resuscitation. Of these causes, some are dangerous to patients, some are iatrogenic, and some are due to laboratory error.7 Physicians often evaluate decreases in hemoglobin, which could otherwise be explained by laboratory error, hemodilution, or expected decrease in hemoglobin due to hospitalization, to identify causes that may lead to potential harm.
Jacob et al8 demonstrated the effect of posture on hemoglobin concentrations in healthy volunteers, showing an average 11% relative increase in hemoglobin when going from lying to standing. This increase was attributed to shifts in plasma volume to the vascular space with recumbence. They hypothesized that the initial hemoglobin on admission is measured when patients are upright or recently upright, whereas after admission, patients are more likely to be supine, resulting in lower hemoglobin concentrations. Others have also demonstrated similar effects of patient posture on hemoglobin concentration.9-13 However, these prior results are not readily generalizable to hospitalized patients. These prior studies enrolled healthy volunteers, and most examined postural changes from the supine and standing positions; blood is rarely obtained from hospitalized patients when they are standing.
The aim of this study was to investigate whether postural changes in hemoglobin can be demonstrated in positions that patients routinely encountered during in-hospital phlebotomy: upright in a chair or recumbent in a bed. Patient position, which is not standardized during blood draws, may contribute to lower measured hemoglobin concentrations in some patients, especially sicker individuals who are recumbent more frequently. We hypothesized that going from supine to upright in a chair would result in a relative increase in hemoglobin concentration of 5% to 6%, approximately half the value of going from supine to standing.8 To investigate this, we conducted a quasi-experimental study exploring the effect of position (supine or sitting in chair) on hemoglobin concentrations in medical inpatients.
METHODS
Participants
Patients were enrolled in this single-center study between October 2017 and August 2018. Patients aged 18 years or older who were hospitalized on the general internal medicine wards were screened to determine if they met the following inclusion criteria: hospitalized for <5 days, had blood work scheduled as part of routine care (in order to decrease phlebotomy required by this study), had baseline hemoglobin >8 g/dL, and were able to remain supine without interruption overnight and able to sit in a chair for at least 1 hour the following morning. Patients were excluded from the study if they had a hematologic malignancy, were at risk of >100 mL of blood loss (eg, admitted for gastrointestinal bleeding, planned surgery), had a transfusion requirement, or received intravascular modifiers such as fluid (>100 cc/h) or intravenous diuretics. The Johns Hopkins Institutional Review Board approved this study, and all patients provided written informed consent.
Study Design
Patients enrolled in this quasi-experimental study were asked to remain supine for at least 6 hours overnight. Adherence to the recumbent position was tracked by patient self-report and by corroboration with the patient’s nurse overnight. Any interruptions to supine positioning resulted in exclusion from the study. The following morning, a member of the study team performed phlebotomy while the patient remained supine. Patients were then asked to sit comfortably in a chair for at least 1 hour with their feet on the ground; the blood draw was then repeated. All blood samples were acquired by venipuncture. Prior to each blood draw, a tourniquet was placed over the upper arm below the axilla. An antecubital vein on either arm was visualized under ultrasound guidance, and a 23-G × 3/4” butterfly needle was used for venipuncture. The vials of blood were immediately inverted after blood collection. Hemoglobin assays were processed and analyzed using Sysmex XN-10 analyzer (Sysmex Corporation). The reference range for hemoglobin in our facility was 12.0 to 15.0 g/dL for women and 13.9 to 16.3 g/dL for men. Laboratory technicians were blinded to and uninvolved in the study.
We determined, a priori, that 33 enrolled patients would provide 80% power (alpha 0.05) to detect an average hemoglobin change of 4.1%, assuming that the standard deviation of the hemoglobin change was twice the mean (ie, SD = 8.2%). The Wilcoxon signed-rank test was used to test the significance of postural hemoglobin changes. Analyses were conducted using JMP Pro 13.0 (SAS) and GraphPad Prism 8 (GraphPad Software). Significance was defined at P < .05 for all analyses.
RESULTS
Thirty-nine patients were consented and enrolled in the study; four patients were excluded prior to blood draw (two patients because of interruption of supine time, two patients because of refusal in the morning). Of the 35 patients who completed the study, 13 were women (37%); median age was 49 years (range, 25-83 years). Median supine hemoglobin concentration in our sample was 11.7 g/dL (range, 9.3-18.1 g/dL), and median baseline creatinine level was 0.70 mg/dL (range, 0.5-2.5 mg/dL). Median supine hemoglobin levels were 11.7 g/dL (range, 9.6-13.2 g/dL) in women and 11.8 g/dL (range, 9.3-18.1 g/dL) in men. In aggregate, patients had a median increase in hemoglobin concentration of 0.60 g/dL (range, –0.6 to 1.4 g/dL) with sitting, a 5.2% (range, –4.5% to 15.1%) relative change (P < .001) (Figure 1). 

DISCUSSION
International blood collection guidelines acknowledge postural changes in laboratory values and recommend standardization of patient position to either sitting in a chair or lying flat in a bed, without changes in position for 15 minutes prior to blood draw.14 When these positional accommodations cannot be met, documenting positional disruptions is recommended so that laboratory values can be interpreted accordingly. To the best of our knowledge, no hospital in the United States has standardized patient position as part of phlebotomy procedure such that patient position is documented and can be made available to interpreting providers.
Relative increases in hemoglobin or hematocrit range from 7% to 12% when patients go from supine to standing.8,9,11 The reverse relationship has also been shown, where upright-to-supine position results in decreases in hemoglobin concentrations.10,13 We found that going from supine to a seated position resulted in significant increases in hemoglobin of 0.6 g/dL and in a more than 1 g/dL increase in 29% of the patients. Although four of the 35 patients experienced either no change or a slight decrease in their hemoglobin concentration when going from supine to upright and not all patients saw a uniform effect, providers should be aware that the patient’s position can contribute to changes in hemoglobin concentration in the hospitalized setting. Providers may be able to use this information to avoid an extensive diagnostic workup when anemia is identified in hospitalized patients, although more research is needed to identify patient subsets who are at higher risk for this effect.
Until hospitals implement protocols that require phlebotomists to report patient position during phlebotomy in a standardized fashion, providers should be alert to the fact that supine positioning may result in a hemoglobin level that is significantly lower than that when drawn in a sitting position, and in almost one-third of patients, this difference may be 1.0 g/dL or greater.
Given our study criteria requiring supine positions of at least 6 hours and a baseline hemoglobin concentration >8 g/dL, our sample of patients may have been younger and healthier than the average hospitalized patient on general internal medicine wards. Since greater relative changes in plasma volume shifts and hemoglobin might be seen in patients with lower baseline hemoglobin and lower baseline plasma protein, this selection bias may underestimate the effects of position on hemoglobin changes for the average inpatient population. Additionally, we intentionally sought to obtain sitting hemoglobin levels after the supine samples to avoid the possibility of incorrectly attributing dropping hemoglobin levels to progressive hospital-acquired anemia from phlebotomy or illness. Any concomitant trend of falling hemoglobin levels in our patients would be expected to lead to a systematic underestimation of the positional change in hemoglobin we observed. We did not objectively observe adherence to supine and upright position and instead relied on patient self-reporting, which is one possible contributor to the variable effects of position on hemoglobin concentration, with some patients having no change or decreases in hemoglobin concentrations.
CONCLUSION
Posture can significantly influence hemoglobin levels in hospitalized patients on general medicine wards. Further research can determine whether it would be cost and time effective to standardize patient positions prior to phlebotomy, or at least to report patient positioning with the laboratory testing results.
The World Health Organization (WHO) defines anemia as a hemoglobin value less than 12 g/dL in women and less than 13 g/dL in men.1 Hospital-acquired anemia is loosely defined as normal hemoglobin levels on admission that, at their nadir during hospitalization or on discharge, are less than WHO sex-defined cutoffs. Hospital-acquired anemia or significant decreases in hemoglobin are often identified during hospitalization.2-6 Potential causes include blood loss from phlebotomy, occult gastrointestinal bleeding, hemolysis, anemia of inflammation, and hemodilution due to fluid resuscitation. Of these causes, some are dangerous to patients, some are iatrogenic, and some are due to laboratory error.7 Physicians often evaluate decreases in hemoglobin, which could otherwise be explained by laboratory error, hemodilution, or expected decrease in hemoglobin due to hospitalization, to identify causes that may lead to potential harm.
Jacob et al8 demonstrated the effect of posture on hemoglobin concentrations in healthy volunteers, showing an average 11% relative increase in hemoglobin when going from lying to standing. This increase was attributed to shifts in plasma volume to the vascular space with recumbence. They hypothesized that the initial hemoglobin on admission is measured when patients are upright or recently upright, whereas after admission, patients are more likely to be supine, resulting in lower hemoglobin concentrations. Others have also demonstrated similar effects of patient posture on hemoglobin concentration.9-13 However, these prior results are not readily generalizable to hospitalized patients. These prior studies enrolled healthy volunteers, and most examined postural changes from the supine and standing positions; blood is rarely obtained from hospitalized patients when they are standing.
The aim of this study was to investigate whether postural changes in hemoglobin can be demonstrated in positions that patients routinely encountered during in-hospital phlebotomy: upright in a chair or recumbent in a bed. Patient position, which is not standardized during blood draws, may contribute to lower measured hemoglobin concentrations in some patients, especially sicker individuals who are recumbent more frequently. We hypothesized that going from supine to upright in a chair would result in a relative increase in hemoglobin concentration of 5% to 6%, approximately half the value of going from supine to standing.8 To investigate this, we conducted a quasi-experimental study exploring the effect of position (supine or sitting in chair) on hemoglobin concentrations in medical inpatients.
METHODS
Participants
Patients were enrolled in this single-center study between October 2017 and August 2018. Patients aged 18 years or older who were hospitalized on the general internal medicine wards were screened to determine if they met the following inclusion criteria: hospitalized for <5 days, had blood work scheduled as part of routine care (in order to decrease phlebotomy required by this study), had baseline hemoglobin >8 g/dL, and were able to remain supine without interruption overnight and able to sit in a chair for at least 1 hour the following morning. Patients were excluded from the study if they had a hematologic malignancy, were at risk of >100 mL of blood loss (eg, admitted for gastrointestinal bleeding, planned surgery), had a transfusion requirement, or received intravascular modifiers such as fluid (>100 cc/h) or intravenous diuretics. The Johns Hopkins Institutional Review Board approved this study, and all patients provided written informed consent.
Study Design
Patients enrolled in this quasi-experimental study were asked to remain supine for at least 6 hours overnight. Adherence to the recumbent position was tracked by patient self-report and by corroboration with the patient’s nurse overnight. Any interruptions to supine positioning resulted in exclusion from the study. The following morning, a member of the study team performed phlebotomy while the patient remained supine. Patients were then asked to sit comfortably in a chair for at least 1 hour with their feet on the ground; the blood draw was then repeated. All blood samples were acquired by venipuncture. Prior to each blood draw, a tourniquet was placed over the upper arm below the axilla. An antecubital vein on either arm was visualized under ultrasound guidance, and a 23-G × 3/4” butterfly needle was used for venipuncture. The vials of blood were immediately inverted after blood collection. Hemoglobin assays were processed and analyzed using Sysmex XN-10 analyzer (Sysmex Corporation). The reference range for hemoglobin in our facility was 12.0 to 15.0 g/dL for women and 13.9 to 16.3 g/dL for men. Laboratory technicians were blinded to and uninvolved in the study.
We determined, a priori, that 33 enrolled patients would provide 80% power (alpha 0.05) to detect an average hemoglobin change of 4.1%, assuming that the standard deviation of the hemoglobin change was twice the mean (ie, SD = 8.2%). The Wilcoxon signed-rank test was used to test the significance of postural hemoglobin changes. Analyses were conducted using JMP Pro 13.0 (SAS) and GraphPad Prism 8 (GraphPad Software). Significance was defined at P < .05 for all analyses.
RESULTS
Thirty-nine patients were consented and enrolled in the study; four patients were excluded prior to blood draw (two patients because of interruption of supine time, two patients because of refusal in the morning). Of the 35 patients who completed the study, 13 were women (37%); median age was 49 years (range, 25-83 years). Median supine hemoglobin concentration in our sample was 11.7 g/dL (range, 9.3-18.1 g/dL), and median baseline creatinine level was 0.70 mg/dL (range, 0.5-2.5 mg/dL). Median supine hemoglobin levels were 11.7 g/dL (range, 9.6-13.2 g/dL) in women and 11.8 g/dL (range, 9.3-18.1 g/dL) in men. In aggregate, patients had a median increase in hemoglobin concentration of 0.60 g/dL (range, –0.6 to 1.4 g/dL) with sitting, a 5.2% (range, –4.5% to 15.1%) relative change (P < .001) (Figure 1). 

DISCUSSION
International blood collection guidelines acknowledge postural changes in laboratory values and recommend standardization of patient position to either sitting in a chair or lying flat in a bed, without changes in position for 15 minutes prior to blood draw.14 When these positional accommodations cannot be met, documenting positional disruptions is recommended so that laboratory values can be interpreted accordingly. To the best of our knowledge, no hospital in the United States has standardized patient position as part of phlebotomy procedure such that patient position is documented and can be made available to interpreting providers.
Relative increases in hemoglobin or hematocrit range from 7% to 12% when patients go from supine to standing.8,9,11 The reverse relationship has also been shown, where upright-to-supine position results in decreases in hemoglobin concentrations.10,13 We found that going from supine to a seated position resulted in significant increases in hemoglobin of 0.6 g/dL and in a more than 1 g/dL increase in 29% of the patients. Although four of the 35 patients experienced either no change or a slight decrease in their hemoglobin concentration when going from supine to upright and not all patients saw a uniform effect, providers should be aware that the patient’s position can contribute to changes in hemoglobin concentration in the hospitalized setting. Providers may be able to use this information to avoid an extensive diagnostic workup when anemia is identified in hospitalized patients, although more research is needed to identify patient subsets who are at higher risk for this effect.
Until hospitals implement protocols that require phlebotomists to report patient position during phlebotomy in a standardized fashion, providers should be alert to the fact that supine positioning may result in a hemoglobin level that is significantly lower than that when drawn in a sitting position, and in almost one-third of patients, this difference may be 1.0 g/dL or greater.
Given our study criteria requiring supine positions of at least 6 hours and a baseline hemoglobin concentration >8 g/dL, our sample of patients may have been younger and healthier than the average hospitalized patient on general internal medicine wards. Since greater relative changes in plasma volume shifts and hemoglobin might be seen in patients with lower baseline hemoglobin and lower baseline plasma protein, this selection bias may underestimate the effects of position on hemoglobin changes for the average inpatient population. Additionally, we intentionally sought to obtain sitting hemoglobin levels after the supine samples to avoid the possibility of incorrectly attributing dropping hemoglobin levels to progressive hospital-acquired anemia from phlebotomy or illness. Any concomitant trend of falling hemoglobin levels in our patients would be expected to lead to a systematic underestimation of the positional change in hemoglobin we observed. We did not objectively observe adherence to supine and upright position and instead relied on patient self-reporting, which is one possible contributor to the variable effects of position on hemoglobin concentration, with some patients having no change or decreases in hemoglobin concentrations.
CONCLUSION
Posture can significantly influence hemoglobin levels in hospitalized patients on general medicine wards. Further research can determine whether it would be cost and time effective to standardize patient positions prior to phlebotomy, or at least to report patient positioning with the laboratory testing results.
1. DeMaeyer E, Adiels-Tegman M. The prevalence of anaemia in the world. World Health Stat Q. 1985;38(3):302-316.
2. Martin ND, Scantling D. Hospital-acquired anemia. J Infus Nurs. 2015;38(5):330-338. https://doi.org/10.1097/NAN.0000000000000121
3. Thavendiranathan P, Bagai A, Ebidia A, Detsky AS, Choudhry NK. Do blood tests cause anemia in hospitalized patients? The effect of diagnostic phlebotomy on hemoglobin and hematocrit levels. J Gen Intern Med. 2005;20(6):520-524. https://doi.org/10.1111/j.1525-1497.2005.0094.x
4. Salisbury AC, Reid KJ, Alexander KP, et al. Diagnostic blood loss from phlebotomy and hospital-acquired anemia during acute myocardial infarction. Arch Intern Med. 2011;171(18):1646-1653. https://doi.org/10.1001/archinternmed.2011.361
5. Languasco A, Cazap N, Marciano S, et al. Hemoglobin concentration variations over time in general medical inpatients. J Hosp Med. 2010;5(5):283-288. https://doi.org/10.1002/jhm.650
6. van der Bom JG, Cannegieter SC. Hospital-acquired anemia: the contribution of diagnostic blood loss. J Thromb Haemost. 2015;13(6):1157-1159. https://doi.org/10.1111/jth.12886
7. Berkow L. Factors affecting hemoglobin measurement. J Clin Monit Comput. 2013;27(5):499-508. https://doi.org/10.1007/s10877-013-9456-3
8. Jacob G, Raj SR, Ketch T, et al. Postural pseudoanemia: posture-dependent change in hematocrit. Mayo Clin Proc. 2005;80(5):611-614. https://doi.org/10.4065/80.5.611
9. Fawcett JK, Wynn V. Effects of posture on plasma volume and some blood constituents. J Clin Pathol. 1960;13(4):304-310. https://doi.org/10.1136/jcp.13.4.304
10. Tombridge TL. Effect of posture on hematology results. Am J ClinPathol. 1968;49(4):491-493. https://doi.org/10.1093/ajcp/49.4.491
11. Hagan RD, Diaz FJ, Horvath SM. Plasma volume changes with movement to supine and standing positions. J Appl Physiol. 1978;45(3):414-417. https://doi.org/10.1152/jappl.1978.45.3.414
12. Maw GJ, Mackenzie IL, Taylor NA. Redistribution of body fluids during postural manipulations. Acta Physiol Scand. 1995;155(2):157-163. https://doi.org/10.1111/j.1748-1716.1995.tb09960.x
13. Lima-Oliveira G, Guidi GC, Salvagno GL, Danese E, Montagnana M, Lippi G. Patient posture for blood collection by venipuncture: recall for standardization after 28 years. Rev Bras Hematol Hemoter. 2017;39(2):127-132. https://doi.org/10.1016/j.bjhh.2017.01.004
14. Simundic AM, Bölenius K, Cadamuro J, et al. Working Group for Preanalytical Phase (WG-PRE), of the European Federation of Clinical Chemistry and Laboratory Medicine (EFLM) and Latin American Working Group for Preanalytical Phase (WG-PRE-LATAM) of the Latin America Confederation of Clinical Biochemistry (COLABIOCLI). Joint EFLM-COLABIOCLI recommendation for venous blood sampling. Clin Chem Lab Med. 2018;56(12):2015-2038. https://doi.org/10.1515/cclm-2018-0602
1. DeMaeyer E, Adiels-Tegman M. The prevalence of anaemia in the world. World Health Stat Q. 1985;38(3):302-316.
2. Martin ND, Scantling D. Hospital-acquired anemia. J Infus Nurs. 2015;38(5):330-338. https://doi.org/10.1097/NAN.0000000000000121
3. Thavendiranathan P, Bagai A, Ebidia A, Detsky AS, Choudhry NK. Do blood tests cause anemia in hospitalized patients? The effect of diagnostic phlebotomy on hemoglobin and hematocrit levels. J Gen Intern Med. 2005;20(6):520-524. https://doi.org/10.1111/j.1525-1497.2005.0094.x
4. Salisbury AC, Reid KJ, Alexander KP, et al. Diagnostic blood loss from phlebotomy and hospital-acquired anemia during acute myocardial infarction. Arch Intern Med. 2011;171(18):1646-1653. https://doi.org/10.1001/archinternmed.2011.361
5. Languasco A, Cazap N, Marciano S, et al. Hemoglobin concentration variations over time in general medical inpatients. J Hosp Med. 2010;5(5):283-288. https://doi.org/10.1002/jhm.650
6. van der Bom JG, Cannegieter SC. Hospital-acquired anemia: the contribution of diagnostic blood loss. J Thromb Haemost. 2015;13(6):1157-1159. https://doi.org/10.1111/jth.12886
7. Berkow L. Factors affecting hemoglobin measurement. J Clin Monit Comput. 2013;27(5):499-508. https://doi.org/10.1007/s10877-013-9456-3
8. Jacob G, Raj SR, Ketch T, et al. Postural pseudoanemia: posture-dependent change in hematocrit. Mayo Clin Proc. 2005;80(5):611-614. https://doi.org/10.4065/80.5.611
9. Fawcett JK, Wynn V. Effects of posture on plasma volume and some blood constituents. J Clin Pathol. 1960;13(4):304-310. https://doi.org/10.1136/jcp.13.4.304
10. Tombridge TL. Effect of posture on hematology results. Am J ClinPathol. 1968;49(4):491-493. https://doi.org/10.1093/ajcp/49.4.491
11. Hagan RD, Diaz FJ, Horvath SM. Plasma volume changes with movement to supine and standing positions. J Appl Physiol. 1978;45(3):414-417. https://doi.org/10.1152/jappl.1978.45.3.414
12. Maw GJ, Mackenzie IL, Taylor NA. Redistribution of body fluids during postural manipulations. Acta Physiol Scand. 1995;155(2):157-163. https://doi.org/10.1111/j.1748-1716.1995.tb09960.x
13. Lima-Oliveira G, Guidi GC, Salvagno GL, Danese E, Montagnana M, Lippi G. Patient posture for blood collection by venipuncture: recall for standardization after 28 years. Rev Bras Hematol Hemoter. 2017;39(2):127-132. https://doi.org/10.1016/j.bjhh.2017.01.004
14. Simundic AM, Bölenius K, Cadamuro J, et al. Working Group for Preanalytical Phase (WG-PRE), of the European Federation of Clinical Chemistry and Laboratory Medicine (EFLM) and Latin American Working Group for Preanalytical Phase (WG-PRE-LATAM) of the Latin America Confederation of Clinical Biochemistry (COLABIOCLI). Joint EFLM-COLABIOCLI recommendation for venous blood sampling. Clin Chem Lab Med. 2018;56(12):2015-2038. https://doi.org/10.1515/cclm-2018-0602
© 2021 Society of Hospital Medicine
Clinical Guideline Highlights for the Hospitalist: Management of Acute and Chronic Pain in Sickle Cell Disease
Sickle cell disease (SCD) affects an estimated 100,000 people in the United States.1 Pain is the most common complication of SCD and the primary reason patients with SCD seek medical attention.2 In 2016, three-fourths of the approximately 130,000 SCD-related hospitalizations in the United States involved pain crises.3 When managing patients with SCD and chronic pain, an individualized and interdisciplinary approach is crucial. In 2020, the American Society of Hematology (ASH) developed guidelines reflecting the latest evidence in managing acute and chronic pain in adult and pediatric patients with SCD. The ASH guidelines provide 18 recommendations; here, we highlight the 8 recommendations most pertinent to the hospitalist.
KEY RECOMMENDATIONS FOR THE HOSPITALIST
Acute Pain
Acute pain in the guideline is defined as pain that results in an unplanned visit to an acute care center for treatment.
Recommendation 1. For adult and pediatric patients presenting to an acute care setting with SCD-related acute pain, the ASH guideline panel recommends rapid (ie, within 1 hour of arrival at the emergency department [ED]) assessment and administration of analgesia, with reassessments every 30-60 minutes to optimize pain control (Strong recommendation; low certainty in the evidence about effects).
Although the perceived benefits are unclear due to insufficient evidence, the panel agrees that delaying pain management results in undeniable harm to patients. Hence, this recommendation was deemed both acceptable and ethical. Rapid evaluation also allows for earlier identification and treatment of other potential SCD-related complications.
Recommendation 2. For adult and pediatric patients presenting to an acute care setting with SCD-associated pain for whom opioid therapy is indicated, the ASH guideline panel suggests tailored opioid dosing based on consideration of baseline opioid therapy and prior effective therapy. (For adults: conditional recommendation; moderate certainty in the evidence about effects. For children: conditional recommendation; low certainty in the evidence about effects).
One randomized controlled trial examined patient-specific opioid dosing (based on current chronic opioid therapy [COT] and previously known effective acute pain management) vs weight-based dosing in the ED and found that participants randomized into the patient-specific protocol had a greater reduction in pain and decreased rate of hospital admission.4
The panel acknowledges that intravenous patient-controlled opioid analgesia is generally the standard of care at most institutions. However, no clear data address whether continuous opioid infusion in addition to on-demand dosing is beneficial.
Recommendation 3. For adult and pediatric patients with acute pain related to SCD, the ASH guideline panel suggests a short course (5 to 7 days) of nonsteroidal anti-inflammatory drugs (NSAIDs) in addition to opioids (Conditional recommendation; very low certainty in the evidence about effects).
The use of NSAIDs for managing pain in hospitalized patients with SCD has been associated with a reduction in the use of opioids in the inpatient setting and decreased lengths of stay.5 The potential harms of NSAIDs, including renal and gastrointestinal toxicity, however, should be factored into the decision-making as the risks may outweigh the potential benefits.
Recommendation 4. For adult and pediatric patients with SCD hospitalized for acute pain, the ASH guideline panel suggests a subanesthetic (analgesic) infusion of ketamine as adjunctive treatment of pain refractory or not effectively treated with opioids alone (Conditional recommendation; very low certainty in the evidence about effects). The guideline panel also suggests regional anesthesia for localized pain refractory or not effectively treated with opioids alone (Conditional recommendation; very low certainty in the evidence about effects).
Studies have demonstrated reduced pain and opioid utilization in individuals who received adjuvant ketamine infusions6 or regional anesthesia (ie, epidural).7 Feasibility, however, is limited to centers that have the appropriate experience and expertise with these interventions.
Recommendation 5. For adult and pediatric patients who have recurrent acute pain associated with SCD, the ASH guideline panel suggests against chronic monthly transfusion therapy as a first-line strategy to prevent or reduce recurrent acute pain episodes (Conditional recommendation; low certainty in the evidence about effects). The evidence for monthly transfusions in preventing recurrent pain is limited. There is, however, a moderate risk of harm, including iron overload and transfusion reactions, in addition to substantial burden and costs.
Chronic Pain
Chronic pain in the guideline is defined as ongoing pain present on most days over the past 6 months.
Recommendation 6. For adult patients with SCD who have chronic pain from the SCD-related identifiable cause avascular necrosis (AVN) of the bone, the ASH guideline panel suggests the use of serotonin-norepinephrine reuptake inhibitors (SNRIs) or NSAIDs in the context of a comprehensive disease and pain management plan (Conditional recommendation; very low certainty in the evidence about effects). For patients with no identifiable cause beyond SCD, the guideline panel suggests SNRIs, tricyclic antidepressants, or gabapentinoids for pain management (Conditional recommendation; very low certainty in the evidence about effects). Given the lack of direct evidence, indirect evidence was used to formulate these recommendations. For pain associated with AVN, data were extrapolated from literature on osteoarthritis, a form of degenerative arthropathy. For pain without an identifiable cause, evidence was taken from studies on fibromyalgia, a condition the panel felt most closely aligned with chronic pain related to SCD.
No recommendations were made for pediatric patients as the indirect evidence base only addressed adult patients.
Recommendation 7. For adult and pediatric patients with SCD and emerging and/or recently developed chronic pain, the ASH guideline panel does not recommend initiating COT unless pain is refractory to multiple other treatment modalities (Conditional recommendation; very low certainty in the evidence about effects). For patients receiving COT who are functioning well and have perceived benefit, the ASH guideline panel suggests shared decision-making for continuation of COT (Conditional recommendation; very low certainty in the evidence about effects).
High-quality data on the benefit of long-term COT in individuals with chronic noncancer pain are lacking. The panel maintains that the decision to initiate or continue COT should be individualized after weighing appropriate risks and benefits.
Recommendation 8. For adult and pediatric patients with chronic pain related to SCD, the panel suggests cognitive and behavioral pain management strategies in the context of a comprehensive disease and pain management plan (Conditional recommendation; very low certainty in the evidence about effects). Cognitive behavioral therapy may decrease overall pain intensity and improve coping skills.8 The panel agrees that medications alone may not be effective in reducing the burden of chronic pain in adult and pediatric patients with SCD.
CRITIQUE
The guidelines were created by a multidisciplinary panel that included physicians from hematology, pain medicine, psychiatry, and emergency medicine, a doctoral nurse practitioner, and two patient representatives. The Mayo Evidence-Based Practice Research Program supported the guideline-development process. The GRADE (Grading of Recommendations Assessment, Development, and Evaluation) approach was used to assess evidence and make recommendations.
High-quality data in treating acute and chronic pain in both adult and pediatric patients with SCD are limited. As such, the majority of recommendations in these guidelines are conditional. The panel included studies that were indirectly related to SCD based on consensus (eg, inferred data from disease processes thought to be similar to SCD). One panelist disclosed receiving direct payments from a company that could be affected by these guidelines; however, it was deemed that the conflict was unlikely to have influenced any recommendations.
AREAS IN NEED OF FUTURE STUDY
The panel acknowledges that further investigation is needed for both nonpharmacologic and pharmacologic modalities in treating acute and chronic pain related to SCD. Examples include evaluating the comparative-effectiveness of COT vs nonopioid pharmacotherapy, the benefits and harms of continuous opioid infusions in acute pain crises, and the impact of chronic transfusions on acute and chronic pain.
1. Data & statistics on sickle cell disease. Centers for Disease Control and Prevention. Accessed August 23, 2020. https://www.cdc.gov/ncbddd/sicklecell/data.html
2. Complications and treatments of sickle cell disease. Centers for Disease Control and Prevention. Accessed August 23, 2020. https://www.cdc.gov/ncbddd/sicklecell/treatments.html
3. Fingar KR, Owens PL, Reid LD, Mistry KB, Barrett ML. Characteristics of Inpatient Hospital Stays Involving Sickle Cell Disease, 2000-2016. Agency for Healthcare Research and Quality. Healthcare Cost and Utilization Project. Statistical Brief 251. September 2019. Accessed August 23, 2020. www.hcup-us.ahrq.gov/reports/statbriefs/sb251-Sickle-Cell-Disease-Stays-2016.pdf
4. Tanabe P, Silva S, Bosworth HB, et al. A randomized controlled trial comparing two vaso-occlusive episode (VOE) protocols in sickle cell disease (SCD). Am J Hematol. 2018;93(2):159-168. https://doi.org/10.1002/ajh.24948
5. Perlin E, Finke H, Castro O, et al. Enhancement of pain control with ketorolac tromethamine in patients with sickle cell vaso-occlusive crisis. Am J Hematol. 1994;46(1):43-47. https://doi.org/10.1002/ajh.2830460108
6. Sheehy KA, Lippold C, Rice AL, et al. Subanesthetic ketamine for pain management in hospitalized children, adolescents, and young adults: a single-center cohort study. J Pain Res. 2017;10:787-795. https://doi.org/10.2147/jpr.s131156
7. New T, Venable C, Fraser L, et al. Management of refractory pain in hospitalized adolescents with sickle cell disease: changing from intravenous opioids to continuous infusion epidural analgesia. J Pediatr Hematol Oncol. 2014;36(6):e398-e402. https://doi.org/10.1097/mph.0000000000000026
8. Schatz J, Schlenz AM, McClellan CB, et al. Changes in coping, pain, and activity after cognitive-behavioral training: a randomized clinical trial for pediatric sickle cell disease using smartphones. Clin J Pain. 2015;31(6):536-547. https://doi.org/10.1097/ajp.0000000000000183
Sickle cell disease (SCD) affects an estimated 100,000 people in the United States.1 Pain is the most common complication of SCD and the primary reason patients with SCD seek medical attention.2 In 2016, three-fourths of the approximately 130,000 SCD-related hospitalizations in the United States involved pain crises.3 When managing patients with SCD and chronic pain, an individualized and interdisciplinary approach is crucial. In 2020, the American Society of Hematology (ASH) developed guidelines reflecting the latest evidence in managing acute and chronic pain in adult and pediatric patients with SCD. The ASH guidelines provide 18 recommendations; here, we highlight the 8 recommendations most pertinent to the hospitalist.
KEY RECOMMENDATIONS FOR THE HOSPITALIST
Acute Pain
Acute pain in the guideline is defined as pain that results in an unplanned visit to an acute care center for treatment.
Recommendation 1. For adult and pediatric patients presenting to an acute care setting with SCD-related acute pain, the ASH guideline panel recommends rapid (ie, within 1 hour of arrival at the emergency department [ED]) assessment and administration of analgesia, with reassessments every 30-60 minutes to optimize pain control (Strong recommendation; low certainty in the evidence about effects).
Although the perceived benefits are unclear due to insufficient evidence, the panel agrees that delaying pain management results in undeniable harm to patients. Hence, this recommendation was deemed both acceptable and ethical. Rapid evaluation also allows for earlier identification and treatment of other potential SCD-related complications.
Recommendation 2. For adult and pediatric patients presenting to an acute care setting with SCD-associated pain for whom opioid therapy is indicated, the ASH guideline panel suggests tailored opioid dosing based on consideration of baseline opioid therapy and prior effective therapy. (For adults: conditional recommendation; moderate certainty in the evidence about effects. For children: conditional recommendation; low certainty in the evidence about effects).
One randomized controlled trial examined patient-specific opioid dosing (based on current chronic opioid therapy [COT] and previously known effective acute pain management) vs weight-based dosing in the ED and found that participants randomized into the patient-specific protocol had a greater reduction in pain and decreased rate of hospital admission.4
The panel acknowledges that intravenous patient-controlled opioid analgesia is generally the standard of care at most institutions. However, no clear data address whether continuous opioid infusion in addition to on-demand dosing is beneficial.
Recommendation 3. For adult and pediatric patients with acute pain related to SCD, the ASH guideline panel suggests a short course (5 to 7 days) of nonsteroidal anti-inflammatory drugs (NSAIDs) in addition to opioids (Conditional recommendation; very low certainty in the evidence about effects).
The use of NSAIDs for managing pain in hospitalized patients with SCD has been associated with a reduction in the use of opioids in the inpatient setting and decreased lengths of stay.5 The potential harms of NSAIDs, including renal and gastrointestinal toxicity, however, should be factored into the decision-making as the risks may outweigh the potential benefits.
Recommendation 4. For adult and pediatric patients with SCD hospitalized for acute pain, the ASH guideline panel suggests a subanesthetic (analgesic) infusion of ketamine as adjunctive treatment of pain refractory or not effectively treated with opioids alone (Conditional recommendation; very low certainty in the evidence about effects). The guideline panel also suggests regional anesthesia for localized pain refractory or not effectively treated with opioids alone (Conditional recommendation; very low certainty in the evidence about effects).
Studies have demonstrated reduced pain and opioid utilization in individuals who received adjuvant ketamine infusions6 or regional anesthesia (ie, epidural).7 Feasibility, however, is limited to centers that have the appropriate experience and expertise with these interventions.
Recommendation 5. For adult and pediatric patients who have recurrent acute pain associated with SCD, the ASH guideline panel suggests against chronic monthly transfusion therapy as a first-line strategy to prevent or reduce recurrent acute pain episodes (Conditional recommendation; low certainty in the evidence about effects). The evidence for monthly transfusions in preventing recurrent pain is limited. There is, however, a moderate risk of harm, including iron overload and transfusion reactions, in addition to substantial burden and costs.
Chronic Pain
Chronic pain in the guideline is defined as ongoing pain present on most days over the past 6 months.
Recommendation 6. For adult patients with SCD who have chronic pain from the SCD-related identifiable cause avascular necrosis (AVN) of the bone, the ASH guideline panel suggests the use of serotonin-norepinephrine reuptake inhibitors (SNRIs) or NSAIDs in the context of a comprehensive disease and pain management plan (Conditional recommendation; very low certainty in the evidence about effects). For patients with no identifiable cause beyond SCD, the guideline panel suggests SNRIs, tricyclic antidepressants, or gabapentinoids for pain management (Conditional recommendation; very low certainty in the evidence about effects). Given the lack of direct evidence, indirect evidence was used to formulate these recommendations. For pain associated with AVN, data were extrapolated from literature on osteoarthritis, a form of degenerative arthropathy. For pain without an identifiable cause, evidence was taken from studies on fibromyalgia, a condition the panel felt most closely aligned with chronic pain related to SCD.
No recommendations were made for pediatric patients as the indirect evidence base only addressed adult patients.
Recommendation 7. For adult and pediatric patients with SCD and emerging and/or recently developed chronic pain, the ASH guideline panel does not recommend initiating COT unless pain is refractory to multiple other treatment modalities (Conditional recommendation; very low certainty in the evidence about effects). For patients receiving COT who are functioning well and have perceived benefit, the ASH guideline panel suggests shared decision-making for continuation of COT (Conditional recommendation; very low certainty in the evidence about effects).
High-quality data on the benefit of long-term COT in individuals with chronic noncancer pain are lacking. The panel maintains that the decision to initiate or continue COT should be individualized after weighing appropriate risks and benefits.
Recommendation 8. For adult and pediatric patients with chronic pain related to SCD, the panel suggests cognitive and behavioral pain management strategies in the context of a comprehensive disease and pain management plan (Conditional recommendation; very low certainty in the evidence about effects). Cognitive behavioral therapy may decrease overall pain intensity and improve coping skills.8 The panel agrees that medications alone may not be effective in reducing the burden of chronic pain in adult and pediatric patients with SCD.
CRITIQUE
The guidelines were created by a multidisciplinary panel that included physicians from hematology, pain medicine, psychiatry, and emergency medicine, a doctoral nurse practitioner, and two patient representatives. The Mayo Evidence-Based Practice Research Program supported the guideline-development process. The GRADE (Grading of Recommendations Assessment, Development, and Evaluation) approach was used to assess evidence and make recommendations.
High-quality data in treating acute and chronic pain in both adult and pediatric patients with SCD are limited. As such, the majority of recommendations in these guidelines are conditional. The panel included studies that were indirectly related to SCD based on consensus (eg, inferred data from disease processes thought to be similar to SCD). One panelist disclosed receiving direct payments from a company that could be affected by these guidelines; however, it was deemed that the conflict was unlikely to have influenced any recommendations.
AREAS IN NEED OF FUTURE STUDY
The panel acknowledges that further investigation is needed for both nonpharmacologic and pharmacologic modalities in treating acute and chronic pain related to SCD. Examples include evaluating the comparative-effectiveness of COT vs nonopioid pharmacotherapy, the benefits and harms of continuous opioid infusions in acute pain crises, and the impact of chronic transfusions on acute and chronic pain.
Sickle cell disease (SCD) affects an estimated 100,000 people in the United States.1 Pain is the most common complication of SCD and the primary reason patients with SCD seek medical attention.2 In 2016, three-fourths of the approximately 130,000 SCD-related hospitalizations in the United States involved pain crises.3 When managing patients with SCD and chronic pain, an individualized and interdisciplinary approach is crucial. In 2020, the American Society of Hematology (ASH) developed guidelines reflecting the latest evidence in managing acute and chronic pain in adult and pediatric patients with SCD. The ASH guidelines provide 18 recommendations; here, we highlight the 8 recommendations most pertinent to the hospitalist.
KEY RECOMMENDATIONS FOR THE HOSPITALIST
Acute Pain
Acute pain in the guideline is defined as pain that results in an unplanned visit to an acute care center for treatment.
Recommendation 1. For adult and pediatric patients presenting to an acute care setting with SCD-related acute pain, the ASH guideline panel recommends rapid (ie, within 1 hour of arrival at the emergency department [ED]) assessment and administration of analgesia, with reassessments every 30-60 minutes to optimize pain control (Strong recommendation; low certainty in the evidence about effects).
Although the perceived benefits are unclear due to insufficient evidence, the panel agrees that delaying pain management results in undeniable harm to patients. Hence, this recommendation was deemed both acceptable and ethical. Rapid evaluation also allows for earlier identification and treatment of other potential SCD-related complications.
Recommendation 2. For adult and pediatric patients presenting to an acute care setting with SCD-associated pain for whom opioid therapy is indicated, the ASH guideline panel suggests tailored opioid dosing based on consideration of baseline opioid therapy and prior effective therapy. (For adults: conditional recommendation; moderate certainty in the evidence about effects. For children: conditional recommendation; low certainty in the evidence about effects).
One randomized controlled trial examined patient-specific opioid dosing (based on current chronic opioid therapy [COT] and previously known effective acute pain management) vs weight-based dosing in the ED and found that participants randomized into the patient-specific protocol had a greater reduction in pain and decreased rate of hospital admission.4
The panel acknowledges that intravenous patient-controlled opioid analgesia is generally the standard of care at most institutions. However, no clear data address whether continuous opioid infusion in addition to on-demand dosing is beneficial.
Recommendation 3. For adult and pediatric patients with acute pain related to SCD, the ASH guideline panel suggests a short course (5 to 7 days) of nonsteroidal anti-inflammatory drugs (NSAIDs) in addition to opioids (Conditional recommendation; very low certainty in the evidence about effects).
The use of NSAIDs for managing pain in hospitalized patients with SCD has been associated with a reduction in the use of opioids in the inpatient setting and decreased lengths of stay.5 The potential harms of NSAIDs, including renal and gastrointestinal toxicity, however, should be factored into the decision-making as the risks may outweigh the potential benefits.
Recommendation 4. For adult and pediatric patients with SCD hospitalized for acute pain, the ASH guideline panel suggests a subanesthetic (analgesic) infusion of ketamine as adjunctive treatment of pain refractory or not effectively treated with opioids alone (Conditional recommendation; very low certainty in the evidence about effects). The guideline panel also suggests regional anesthesia for localized pain refractory or not effectively treated with opioids alone (Conditional recommendation; very low certainty in the evidence about effects).
Studies have demonstrated reduced pain and opioid utilization in individuals who received adjuvant ketamine infusions6 or regional anesthesia (ie, epidural).7 Feasibility, however, is limited to centers that have the appropriate experience and expertise with these interventions.
Recommendation 5. For adult and pediatric patients who have recurrent acute pain associated with SCD, the ASH guideline panel suggests against chronic monthly transfusion therapy as a first-line strategy to prevent or reduce recurrent acute pain episodes (Conditional recommendation; low certainty in the evidence about effects). The evidence for monthly transfusions in preventing recurrent pain is limited. There is, however, a moderate risk of harm, including iron overload and transfusion reactions, in addition to substantial burden and costs.
Chronic Pain
Chronic pain in the guideline is defined as ongoing pain present on most days over the past 6 months.
Recommendation 6. For adult patients with SCD who have chronic pain from the SCD-related identifiable cause avascular necrosis (AVN) of the bone, the ASH guideline panel suggests the use of serotonin-norepinephrine reuptake inhibitors (SNRIs) or NSAIDs in the context of a comprehensive disease and pain management plan (Conditional recommendation; very low certainty in the evidence about effects). For patients with no identifiable cause beyond SCD, the guideline panel suggests SNRIs, tricyclic antidepressants, or gabapentinoids for pain management (Conditional recommendation; very low certainty in the evidence about effects). Given the lack of direct evidence, indirect evidence was used to formulate these recommendations. For pain associated with AVN, data were extrapolated from literature on osteoarthritis, a form of degenerative arthropathy. For pain without an identifiable cause, evidence was taken from studies on fibromyalgia, a condition the panel felt most closely aligned with chronic pain related to SCD.
No recommendations were made for pediatric patients as the indirect evidence base only addressed adult patients.
Recommendation 7. For adult and pediatric patients with SCD and emerging and/or recently developed chronic pain, the ASH guideline panel does not recommend initiating COT unless pain is refractory to multiple other treatment modalities (Conditional recommendation; very low certainty in the evidence about effects). For patients receiving COT who are functioning well and have perceived benefit, the ASH guideline panel suggests shared decision-making for continuation of COT (Conditional recommendation; very low certainty in the evidence about effects).
High-quality data on the benefit of long-term COT in individuals with chronic noncancer pain are lacking. The panel maintains that the decision to initiate or continue COT should be individualized after weighing appropriate risks and benefits.
Recommendation 8. For adult and pediatric patients with chronic pain related to SCD, the panel suggests cognitive and behavioral pain management strategies in the context of a comprehensive disease and pain management plan (Conditional recommendation; very low certainty in the evidence about effects). Cognitive behavioral therapy may decrease overall pain intensity and improve coping skills.8 The panel agrees that medications alone may not be effective in reducing the burden of chronic pain in adult and pediatric patients with SCD.
CRITIQUE
The guidelines were created by a multidisciplinary panel that included physicians from hematology, pain medicine, psychiatry, and emergency medicine, a doctoral nurse practitioner, and two patient representatives. The Mayo Evidence-Based Practice Research Program supported the guideline-development process. The GRADE (Grading of Recommendations Assessment, Development, and Evaluation) approach was used to assess evidence and make recommendations.
High-quality data in treating acute and chronic pain in both adult and pediatric patients with SCD are limited. As such, the majority of recommendations in these guidelines are conditional. The panel included studies that were indirectly related to SCD based on consensus (eg, inferred data from disease processes thought to be similar to SCD). One panelist disclosed receiving direct payments from a company that could be affected by these guidelines; however, it was deemed that the conflict was unlikely to have influenced any recommendations.
AREAS IN NEED OF FUTURE STUDY
The panel acknowledges that further investigation is needed for both nonpharmacologic and pharmacologic modalities in treating acute and chronic pain related to SCD. Examples include evaluating the comparative-effectiveness of COT vs nonopioid pharmacotherapy, the benefits and harms of continuous opioid infusions in acute pain crises, and the impact of chronic transfusions on acute and chronic pain.
1. Data & statistics on sickle cell disease. Centers for Disease Control and Prevention. Accessed August 23, 2020. https://www.cdc.gov/ncbddd/sicklecell/data.html
2. Complications and treatments of sickle cell disease. Centers for Disease Control and Prevention. Accessed August 23, 2020. https://www.cdc.gov/ncbddd/sicklecell/treatments.html
3. Fingar KR, Owens PL, Reid LD, Mistry KB, Barrett ML. Characteristics of Inpatient Hospital Stays Involving Sickle Cell Disease, 2000-2016. Agency for Healthcare Research and Quality. Healthcare Cost and Utilization Project. Statistical Brief 251. September 2019. Accessed August 23, 2020. www.hcup-us.ahrq.gov/reports/statbriefs/sb251-Sickle-Cell-Disease-Stays-2016.pdf
4. Tanabe P, Silva S, Bosworth HB, et al. A randomized controlled trial comparing two vaso-occlusive episode (VOE) protocols in sickle cell disease (SCD). Am J Hematol. 2018;93(2):159-168. https://doi.org/10.1002/ajh.24948
5. Perlin E, Finke H, Castro O, et al. Enhancement of pain control with ketorolac tromethamine in patients with sickle cell vaso-occlusive crisis. Am J Hematol. 1994;46(1):43-47. https://doi.org/10.1002/ajh.2830460108
6. Sheehy KA, Lippold C, Rice AL, et al. Subanesthetic ketamine for pain management in hospitalized children, adolescents, and young adults: a single-center cohort study. J Pain Res. 2017;10:787-795. https://doi.org/10.2147/jpr.s131156
7. New T, Venable C, Fraser L, et al. Management of refractory pain in hospitalized adolescents with sickle cell disease: changing from intravenous opioids to continuous infusion epidural analgesia. J Pediatr Hematol Oncol. 2014;36(6):e398-e402. https://doi.org/10.1097/mph.0000000000000026
8. Schatz J, Schlenz AM, McClellan CB, et al. Changes in coping, pain, and activity after cognitive-behavioral training: a randomized clinical trial for pediatric sickle cell disease using smartphones. Clin J Pain. 2015;31(6):536-547. https://doi.org/10.1097/ajp.0000000000000183
1. Data & statistics on sickle cell disease. Centers for Disease Control and Prevention. Accessed August 23, 2020. https://www.cdc.gov/ncbddd/sicklecell/data.html
2. Complications and treatments of sickle cell disease. Centers for Disease Control and Prevention. Accessed August 23, 2020. https://www.cdc.gov/ncbddd/sicklecell/treatments.html
3. Fingar KR, Owens PL, Reid LD, Mistry KB, Barrett ML. Characteristics of Inpatient Hospital Stays Involving Sickle Cell Disease, 2000-2016. Agency for Healthcare Research and Quality. Healthcare Cost and Utilization Project. Statistical Brief 251. September 2019. Accessed August 23, 2020. www.hcup-us.ahrq.gov/reports/statbriefs/sb251-Sickle-Cell-Disease-Stays-2016.pdf
4. Tanabe P, Silva S, Bosworth HB, et al. A randomized controlled trial comparing two vaso-occlusive episode (VOE) protocols in sickle cell disease (SCD). Am J Hematol. 2018;93(2):159-168. https://doi.org/10.1002/ajh.24948
5. Perlin E, Finke H, Castro O, et al. Enhancement of pain control with ketorolac tromethamine in patients with sickle cell vaso-occlusive crisis. Am J Hematol. 1994;46(1):43-47. https://doi.org/10.1002/ajh.2830460108
6. Sheehy KA, Lippold C, Rice AL, et al. Subanesthetic ketamine for pain management in hospitalized children, adolescents, and young adults: a single-center cohort study. J Pain Res. 2017;10:787-795. https://doi.org/10.2147/jpr.s131156
7. New T, Venable C, Fraser L, et al. Management of refractory pain in hospitalized adolescents with sickle cell disease: changing from intravenous opioids to continuous infusion epidural analgesia. J Pediatr Hematol Oncol. 2014;36(6):e398-e402. https://doi.org/10.1097/mph.0000000000000026
8. Schatz J, Schlenz AM, McClellan CB, et al. Changes in coping, pain, and activity after cognitive-behavioral training: a randomized clinical trial for pediatric sickle cell disease using smartphones. Clin J Pain. 2015;31(6):536-547. https://doi.org/10.1097/ajp.0000000000000183
© 2021 Society of Hospital Medicine
Procedural Competency Among Hospitalists: A Literature Review and Future Considerations
Over the past 20 years, hospitalists have served as the primary workforce for the clinical care of medical inpatients in the United States.1,2 Core competencies1 state that hospitalists should be able to perform the following bedside procedures: lumbar puncture, paracentesis, thoracentesis, arthrocentesis, and central venous catheter placement. More recently, standard of care has dictated that these procedures be performed under ultrasound guidance,3-6 and thus hospitalists are also expected to be adept at point-of-care ultrasound (POCUS).7
However, no current national standard exists for ensuring basic competency among hospitalists performing bedside procedures. In addition, hospitalists’ procedural volumes are declining,8,9 and standards for procedural training during internal medicine residency have been reduced.10 As a result, many residents who intend to become hospitalists are no longer prepared to perform these procedures.
The ramifications of the loss of procedural competency for hospitalists are manifold. Technical errors are a significant source of patient morbidity and mortality,11-15 and complications arising specifically from nonoperative procedures range from 0 to 19%,16 although these data do not distinguish technical errors from unpreventable adverse events nor the degree to which hospitalists contributed to these complications. Second, hospitalists in academic medical centers might be ill equipped to function as supervisors of trainees performing procedures, which could perpetuate a cycle of suboptimal technical skills.17 Finally, the discrepancy between consensus guidelines for hospitalists and their scope of practice represents a significant area of risk management for institutions that base their credentialing policies on published competencies.
There are many compelling reasons for why hospitalists should maintain—in fact reclaim—a primary role in bedside procedures.18 Hospitalists in community and rural settings might not have easy access to procedural specialists. In academic institutions, hospitalists are the primary instructors and supervisors of procedures performed by internal medicine residents. The increased availability of POCUS allows formally trained hospitalists to perform procedures more safely under imaging guidance.16
The literature on procedures performed by hospitalists, although limited, has focused on POCUS, systems innovations such as medical procedure services (MPS), and policy recommendations for procedural credentialing. Most studies on effective procedural instructional approaches have been conducted among trainees, who are procedural novices. This research does not sufficiently address the dilemma that hospitalists face as independent physicians for whom procedures are not a significant component of their practice, yet are expected to perform invasive procedures occasionally. The purpose of our literature review is to synthesize the available research to characterize contributors to hospitalists’ procedural competency. We conclude with considerations for hospital medicine practice.
METHODS
We performed a structured literature search for peer-reviewed articles related to hospitalists conducting procedures, being trained in procedures, or related to hospitalist-run MPS. We focused our search on the core hospitalist procedures with the highest potential morbidity (ie, lumbar puncture, abdominal paracentesis, thoracentesis, and central venous catheterization). We searched PubMed and Google Scholar for articles published since 1996 (when the term “hospitalists” was first coined) using keyword searches for [hospitalist OR hospital medicine] AND [procedur* OR medical procedur* OR medical procedure service] OR [(procedur* AND (train* OR educat* OR teach OR instruct*)] OR abdominal paracentes* OR thoracentes* OR lumbar puncture OR central venous catheter* OR ultrasound OR point-of-care. We included original research, brief research reports, perspectives, guidelines, and consensus statements. Exclusion criteria were articles that focused on nonhospitalists and conference abstracts. We used pearling to identify secondary sources from included articles’ bibliographies, without limits on year of publication.
RESULTS
Trends Towards Specialist Referrals
Between 1986 and 2007, the number and variety of procedures performed by internists decreased by half.19 Hospitalists still completed procedures in greater volume and variety than nonhospitalists,8 with approximately 50% of hospitalists performing lumbar punctures (50%), abdominal paracenteses (49%), and thoracenteses (44%) compared with less than 25% for all three procedures for nonhospitalists. Additionally, only 11% of surveyed hospitalists8 performed all nine core procedures, although these included procedures that are largely cognitive in nature (eg, electrocardiogram interpretation, chest X-ray interpretation) or procedures that have been relegated to other specialists (eg, endotracheal intubation, ventilator management, or joint injection/aspiration).
Surveys showed that, especially in larger cities and academic centers, procedural specialists have taken over a disproportionate share of procedures even as the number of procedures performed continued to rise.20 Between 1993 and 2008, the number of paracenteses and thoracenteses increased by 133% and decreased by 14%, respectively, but the share of procedures performed by radiologists increased by 964% and 358%, respectively, as evident in an analysis of Medicare billing data.20 A more recent study of Medicare claims from 2004 to 2016 similarly revealed that the percentage of paracenteses performed by radiologists compared with nonradiologists rose from 70% to 80% and thoracenteses from 47% to 66%, respectively.21 Comparable trends were apparent in claims data for lumbar punctures; between 1991 and 2011, the share of lumbar punctures performed by radiologists rose from 11% to 48%.22
In academic medical centers, hospitalists might have the opportunity to pursue other activities (eg, education, administration, research) as they progress in their careers, resulting in less clinical activity. Although hospitalists who are more clinically active in hospital care tended to perform more procedures,8 those with smaller clinical footprints reported lower levels of comfort with performing procedures8 and might have less available time to maintain procedural competency or train in new technologies such as POCUS.17
Additionally, hospitalists in both academic and community settings cited efficiency as a major reason for procedural referral. Hospitalists tended to perform more procedures if they had fixed salaries or if less than 50% of their income was based on clinical productivity, although this trend was not significant.8 Further, they also might be motivated by competing opportunity costs such as time lost caring for other patients or length of shift, which influences the amount of time spent at work.23
Notably, speculation that hospitalists referred more complex cases to specialists was not borne out by studies examining referral patterns.21,24,25
Procedural Outcomes for Hospitalists vs Nonhospitalists
No convincing data exist that procedures performed by specialists have better outcomes than those completed at the bedside by well-trained generalists, although studies were limited to the inpatient setting, to generalists who have some exposure to procedures, and to internal medicine residents on inpatient rotations. In one retrospective review, interventional radiology (IR) referrals were associated with more platelet or plasma transfusions and intensive care unit transfers than those performed at the bedside by internal medicine residents, findings that remained significant after accounting for complexity (eg, Model for End-stage Liver Disease score, need for dialysis, and platelet count).24 Similarly, a prospective audit of 529 bedside procedures did not show any differences in complication rates between generalists and pulmonologists, once generalists underwent standardized training and used pleural safety checklists and ultrasound guidance.26 An administrative database review of 130,000 inpatient thoracenteses across several university hospitals between 2010 and 2013 found that the risk of iatrogenic pneumothorax was similar among operators from IR, medicine, and pulmonary (2.8%, 2.9%, and 3.1%, respectively)27; these findings have been reproduced in other studies.28 Finally, the increasing adoption of procedural ultrasound permits procedures to be conducted more safely at the bedside, without the need to refer to radiology for imaging guidance.3-5
IR procedures also are associated with increased healthcare costs compared with bedside procedures. One study showed that hospital costs for admissions when paracenteses were performed by radiologists were higher than those in which the procedure was completed at the bedside by gastroenterologists or hepatologists.25 A chart review examining 399 paracenteses, thoracenteses, and lumbar punctures found that the average procedure cost increased by 38% for referred procedures and 56% for radiology-performed procedures, as compared with bedside procedures.29 Needing ancillary staffing in dedicated suites to perform procedures contributed to the excess cost.9 Moreover, referred procedures resulted in increased length of stay, which can incur additional costs. However, the data were conflicting; two studies did not show a statistical difference,25,28 while others found an increased length of stay,24,27,29 which might be due to the unavailability of specialists during off hours, thereby delaying nonemergent procedures.21 Detailed cost analyses have controlled for the use of procedural facilities and blood transfusions among IR specialists and simulation training among generalists, showing that total costs were $663 per patient undergoing IR procedures compared with $134 per patient undergoing bedside procedures.30
Lack of Standardized Procedural Training or Assessment
A robust body of primary studies and systematic reviews supports the use of simulation for procedural training to improve comfort and skill as well as reduce complication rates and costs.31,32 A systematic review that investigated the impact of four paradigms of procedural training found that MPS and quality improvement/patient safety approaches led to the most active learning compared with apprenticeship (ie, “see one, do one”) and approaches based on educational theories.33 Nevertheless, the vast majority of the research has been conducted in trainees,32,34 with sparse evidence among practicing physicians. One cohort study of attending physicians’ central venous catheter insertion skills on simulators found low and variable short-term performance, showing overall poor adherence to checklists.35 One article suggested that hospitalists’ procedural skills were below established thresholds of competency at baseline and that simulation-based training did not result in sustained skills, but the small sample size and high attrition limited meaningful conclusions.36 Although continuing medical education courses are available to hospitalists, there is no published evidence supporting their effectiveness.
Proxies for procedural skill have included comfort and experience, yet these markers have broadly been shown to be inadequate.34,36,37 Additionally, the natural decline of skill over time has invoked the need for periodic reassessment of proficiency.36,38 Credentialing has been equally inconstant; a survey of the Society of Hospital Medicine’s (SHM) POCUS task force revealed that only half of respondents reported their hospitals required a minimum number of procedures for initial credentialing and recredentialing.39 In short, periodic assessment of procedural skills among hospitalists has not been a routine process at many institutions.
Role of Hospitalist-Run Medical Procedure Services
It might not be necessary for all hospitalists to be proficient and credentialed in a given procedure,1 and a trend has emerged in the creation of MPS staffed by hospitalists as proceduralists. The primary aim of these MPS has been to recapture the procedures—and associated revenue—that would otherwise be referred to specialists. Moreover, concentrating procedures among a core group of hospitalists endeavors to support patient safety through several principles: (1) to increase technical proficiency through higher procedural volumes, (2) to facilitate rigorous training and assessment among dedicated individuals, and (3) to systematize best practices of operator performance, communication, and documentation.
MPS have been implemented around the country and have demonstrated several advantages. In one institution, medical firms that were offered the use of an MPS had 48% more procedural attempts by nonspecialists, without significant differences in the proportions of successful attempts or complications compared with the firms who more often referred to specialists.40 A retrospective study analyzed outcomes of 1,707 bedside procedures, of which 548 were performed by an MPS, and found that procedures done by the MPS were more likely to result in lower rates of unsuccessful procedures and to use best-practice safety processes (ie, to involve attending physicians, to use ultrasound guidance, and to avoid femoral sites for catheterization).12 Satisfaction was high among patients who underwent bedside procedures performed by a hospitalist-supervised, intern-based procedure service with a focus on bedside communication.41 From a workforce perspective, MPS have also allowed surgical or radiological subspecialties to focus on more complex cases with higher reimbursement rates,18,42 for proceduralists to expand beyond core procedures (eg, bone marrow biopsies43), and to train advanced practice providers.44 Although studies have not shown that the outcomes of procedures completed by an MPS are better than the outcomes of procedures performed by other specialists,45 one can potentially extrapolate from earlier data that procedures done at the bedside by nonradiologists would have comparable outcomes.
DISCUSSION
A myriad of factors is influencing hospitalists’ scope of practice with respect to bedside procedures. Some evidence suggests that procedures performed by specialists are not superior to those done by generalists and might be associated with increased costs. The most promising developments in the past few decades include simulation-based training, which has demonstrated effectiveness across an array of clinical outcomes but has not been sufficiently evaluated in hospitalists to draw conclusions, and hospitalist-led MPS, which promote safe and productive procedural clinical practices. However, decreasing procedural volume, increasing referrals to specialists, dwindling hospitalist interest and/or confidence, time constraints, limited training opportunities, nonuniform credentialing policies, and lack of standardized assessment are cumulatively contributing to a loss of procedural competency among hospitalists.
Taken together, these forces should compel hospital medicine groups that expect their hospitalists to perform their own procedures to identify necessary steps for ensuring the safety of hospitalized patients under their care. The following considerations derive from the available—albeit modest—evidence on procedural performance in hospital medicine (Table).
1. Create MPS to establish a core set of hospitalists to perform procedures and train them using evidence-based practices. Creation of an MPS places the responsibility of core bedside procedures in the hands of a select group of proceduralists. This strategy streamlines training and assessment of individual procedural competency to meet standards set by SHM36,46 and improves educational outcomes.47-49 MPS could improve clinical outcomes,12,42,50-52 including length of stay and cost, while maintaining patient satisfaction,41 as well as recoup lost revenue from referrals by increasing the volume of procedures done by generalists,40,49 although no robust data supporting the latter point exists. Implementing an MPS requires full-time equivalent (FTE) support for proceduralists and administrative support for data collection and tracking complications. Furthermore, a well-functioning MPS will require investment in portable ultrasound machines and training in POCUS, which has been shown to decrease complications and increase success of invasive bedside procedures.3-7 Hospital medicine groups should be aware that staffing an MPS can divert hospitalist labor and resources from other needed clinical areas, especially during the initial, low-volume phases of implementation. Strategies to offset relative value unit (RVU) loss include combining the MPS with existing clinical roles such as medical consults, code triage, and rapid response teams; or with services with lower patient caps, which might work particularly well in community hospitals. In many institutions, hospitalists can bill for procedural consults in addition to the procedures when the consult involves nonmedical patients, which is relevant when the procedure ultimately cannot be performed (eg, too little ascites to safely perform a paracentesis). Further research should establish best practices of MPS to ensure maximum procedural productivity and safety, because there are no rigorous prospective studies that evaluate strategies to create this service. Such strategies include determining the optimal ratio of proceduralists to general hospitalists, hospital characteristics that benefit most from MPS (eg, referral centers, urban-based settings), volume and type of procedures performed, and the proportion and type of referrals that are most cost-effective.
2. Establish policies with procedural specialists to arrange coverage for off-hours procedures and delineate thresholds for procedures that specialists should perform. Expanding hospitalists’ capabilities in performing procedures should trigger reconsideration of the medical center’s approach to procedural safety. A goal would be to have hospital medicine groups work collaboratively with specialists and other disciplines (eg, surgery, emergency medicine, anesthesia, or radiology) to ensure 24-hour, 7-day a week coverage of urgent bedside procedures. The potential to decrease length of stay and improve off-hour procedural quality might be a compelling rationale for hospital administration, whether or not an MPS is used. That said, we recognize that other services might be unable or unwilling to provide such coverage and that specialist off-hour coverage would incur increased costs and could reduce exposure opportunities for internal medicine residents.
A hospital-level procedures committee might be required to support an institutional imperative for procedural safety and to oversee the implementation of approaches that are practical, financially sustainable, and equitable for all service lines, especially because hospitalist groups might bear the early costs of training and retraining.
3. Hospitalist–proceduralists should collaborate with internal medicine residency programs to offer intensive procedural training experiences to residents who want these skills to be part of their future practice. Robust procedural training for trainees promotes better outcomes for the current workforce and helps to populate the future workforce with procedurally competent practitioners. Simulation-based training is a well-established procedural instruction method that is safe, authentic, and effective in terms of clinical outcomes.34 As the primary teachers of residents in many institutions, hospitalists often are the ones who impart procedural skills to residents, despite uneven skill sets. It is in the interest of internal medicine residency program directors to advocate for a core group of hospitalist–proceduralists, as MPS offer an infrastructure for training that has been shown to increase procedural volume and improve skills.47,48,50 Program directors could therefore be incentivized to sponsor some of these procedural roles with teaching and administration funds, as a trade-off for securing higher-quality procedural training and closer supervision for their trainees. The dual necessity of teaching procedural skills to residents and attending physicians alike offers economies of scale for the use of facilities, personnel, and equipment, and gives faculty an opportunity to extend their clinical teaching skills into the domain of procedural supervision.
4. Hospital medicine groups should re-evaluate credentialing and privileging to ensure procedural competency. Given the lack of published data that characterizes how many hospital medicine groups credential hospitalists to perform procedures and what practices they use to assess competency, hospital medicine groups might be signing off on procedures without verifying hospitalists’ proficiency in core procedures. SHM’s position statement on credentialing for ultrasound-guided procedures46 describes standards that could be applied to other procedures. It proposes that credentialing processes should be grounded in simulation- and patient-based assessments of cognitive and psychomotor skills, using published checklists and global ratings for feedback. Simulation training could support provisional certification, but hospitalists should reach minimum thresholds of supervised patient-based experience before initial credentialing, with continuous reassessment of competency to mitigate skill decay. Prospectively tracking procedural metrics, such as procedural volume and complication rates, also will support systematic skill assessment. Finally, similar to any other medical error, near misses and complications should trigger periprocedural safety reviews.
Limitations
The modest body of research on hospitalists and procedures is the central limitation of our synthesis. Much of the literature consisted of consensus statements, retrospective studies, and small prospective educational studies. As a result, we did not adopt all strategies considered standard in a scoping or systematic review. The literature on MPS specifically was insufficient to draw conclusions about their operational and financial impact or effects on procedure quality. Our primary recommendation to implement MPS requires significant fiscal investment and infrastructure. It also entails risks that must be proactively addressed, including the potential for net financial loss and decreased educational opportunities for residents.
CONCLUSIONS
Hospitalists regularly face the predicament of being expected to independently perform procedures, with little access to training, minimal experience, and no ongoing assessment to ensure their proficiency or the safety of their patients. Past assumptions about hospitalists’ responsibility do not reflect realities in practice patterns and have not translated to widespread adoption of procedural training, monitoring, and assessment mechanisms. Our work summarizes a body of literature that, although limited in empiric studies of hospitalists themselves, offers insights with recommendations for hospital medicine groups wishing to uphold procedural skills as part of their providers’ professional identity.
1. Dressler DD, Pistoria MJ, Budnitz TL, McKean SCW, Amin AN. Core competencies in hospital medicine: Development and methodology. J Hosp Med. 2006;1(1):48-56. https://doi.org/10.1002/jhm.6
2. Wachter RM, Goldman L. Zero to 50,000 — The 20th anniversary of the hospitalist. N Engl J Med. 2016;375(11):1009-1011. https://doi.org/10.1056/NEJMp1607958
3. Cho J, Jensen TP, Reierson K, et al. Recommendations on the use of ultrasound guidance for adult abdominal paracentesis: a position statement of the Society of Hospital Medicine. J Hosp Med. 2019;14:E7-E15. https://doi.org/10.12788/jhm.3095
4. Soni NJ, Franco-Sadud R, Kobaidze K, et al. Recommendations on the use of ultrasound guidance for adult lumbar puncture: a position statement of the Society of Hospital Medicine. J Hosp Med. 2019;14(10):591-601. https://doi.org/10.12788/jhm.3197
5. Dancel R, Schnobrich D, Puri N, et al. Recommendations on the use of ultrasound guidance for adult thoracentesis: a position statement of the Society of Hospital Medicine. J Hosp Med. 2018;13(2):126-135. https://doi.org/10.12788/jhm.2940
6. Franco-Sadud R, Schnobrich D, Mathews BK, et al. Recommendations on the use of ultrasound guidance for central and peripheral vascular access in adults: a position statement of the Society of Hospital Medicine. J Hosp Med. 2019;14:E1-E22. https://doi.org/10.12788/jhm.3287
7. Soni NJ, Schnobrich D, Mathews BK, et al. Point-of-care ultrasound for hospitalists: a position statement of the Society of Hospital Medicine. J Hosp Med. 2019;14:E1-E6. https://doi.org/10.12788/jhm.3079
8. Thakkar R, Wright SM, Alguire P, Wigton RS, Boonyasai RT. Procedures performed by hospitalist and non-hospitalist general internists. J Gen Intern Med. 2010;25(5):448-452. https://doi.org/10.1007/s11606-010-1284-2
9. Lucas BP, Asbury JK, Franco-Sadud R. Training future hospitalists with simulators: a needed step toward accessible, expertly performed bedside procedures. J Hosp Med. 2009;4(7):395-396. https://doi.org/10.1002/jhm.602
10. American Board of Internal Medicine. Policies and procedures for certification. Accessed December 3, 2020. https://www.abim.org/~/media/ABIM%20Public/Files/pdf/publications/certification-guides/policies-and-procedures.pdf
11. Myers LC. Toward preventing medical malpractice claims related to chest procedures. Ann Am Thorac Soc. 2020;17(6):776-779. https://doi.org/10.1513/AnnalsATS.201912-863RL
12. Tukey MH, Wiener RS. The impact of a medical procedure service on patient safety, procedure quality and resident training opportunities. J Gen Intern Med. 2014;29(3):485-490. https://doi.org/10.1007/s11606-013-2709-5
13. Brennan TA, Leape LL, Laird NM, et al. Incidence of adverse events and negligence in hospitalized patients. N Engl J Med. 1991;324(6):370-376. https://doi.org/10.1056/NEJM199102073240604
14. Leape LL, Brennan TA, Laird N, et al. The nature of adverse events in hospitalized patients. N Engl J Med. 1991;324(6):377-384. https://doi.org/10.1056/NEJM199102073240605
15. Myers LC, Gartland RM, Skillings J, et al. An examination of medical malpractice claims involving physician trainees. Acad Med. 2020;95(8):1215-1222. https://doi.org/10.1097/ACM.0000000000003117
16. Mercaldi CJ, Lanes SF. Ultrasound guidance decreases complications and improves the cost of care among patients undergoing thoracentesis and paracentesis. Chest. 2013;143(2):532-538. https://doi.org/10.1378/chest.12-0447
17. Vaisman A, Cram P. Procedural competence among faculty in academic health centers: challenges and future directions. Acad Med. 2017;92(1):31-34. https://doi.org/10.1097/ACM.0000000000001327
18. Nelson B. Hospitalists try to reclaim lead role in bedside procedures. The Hospitalist. March 2015. Accessed June 27, 2020. https://www.the-hospitalist.org/hospitalist/article/122571/hospitalists-try-reclaim-lead-role-bedside-procedures
19. Wigton RS, Alguire P; American College of Physicians. The declining number and variety of procedures done by general internists: a resurvey of members of the American College of Physicians. Ann Intern Med. 2007;146(5):355-360. https://doi.org/10.7326/0003-4819-146-5-200703060-00007
20. Duszak R Jr, Chatterjee AR, Schneider DA. National fluid shifts: fifteen-year trends in paracentesis and thoracentesis procedures. J Am Coll Radiol. 2010;7(11):859-864. https://doi.org/10.1016/j.jacr.2010.04.013
21. Gottumukkala RV, Prabhakar AM, Hemingway J, Hughes DR, Duszak R Jr. Disparities over time in volume, day of the week, and patient complexity between paracentesis and thoracentesis procedures performed by radiologists versus those performed by nonradiologists. J Vasc Interv Radiol. 2019;30(11):1769-1778.e1. https://doi.org/10.1016/j.jvir.2019.04.015
22. Kroll H, Duszak R Jr, Nsiah E, Hughes DR, Sumer S, Wintermark M. Trends in lumbar puncture over 2 decades: a dramatic shift to radiology. Am J Roentgenol. 2014;204(1):15-19. https://doi.org/10.2214/AJR.14.12622
23. Jensen T, Lai A, Mourad M. Can lessons from systems-based mastery learning for thoracentesis be translated to hospitalists? J Hosp Med. 2016;11(11):811-812. https://doi.org/10.1002/jhm.2655
24. Barsuk JH, Cohen ER, Feinglass J, McGaghie WC, Wayne DB. Clinical outcomes after bedside and interventional radiology paracentesis procedures. Am J Med. 2013;126(4):349-356. https://doi.org/10.1016/j.amjmed.2012.09.016
25. Barsuk JH, Feinglass J, Kozmic SE, Hohmann SF, Ganger D, Wayne DB. Specialties performing paracentesis procedures at university hospitals: implications for training and certification. J Hosp Med. 2014;9(3):162-168. https://doi.org/10.1002/jhm.2153
26. See KC, Ong V, Teoh CM, et al. Bedside pleural procedures by pulmonologists and non-pulmonologists: a 3-year safety audit. Respirology. 2014;19(3):396-402. https://doi.org/10.1111/resp.12244
27. Kozmic SE, Wayne DB, Feinglass J, Hohmann SF, Barsuk JH. Factors associated with inpatient thoracentesis procedure quality at university hospitals. Jt Comm J Qual Patient Saf. 2016;42(1):34-40. https://doi.org/10.1016/S1553-7250(16)42004-0
28. Berger MS, Divilov V, Paredes H, Sun E. Abdominal paracentesis: safety and efficacy comparing medicine resident bedside paracentesis vs. paracentesis performed by interventional radiology. J Clin Gastroenterol Hepatol. 2018;2(4). https://doi.org/10.21767/2575-7733.1000050
29. Kay C, Wozniak EM, Szabo A, Jackson JL. Examining invasive bedside procedure performance at an academic medical center. South Med J. 2016;109(7):402-407. https://doi.org/10.14423/SMJ.0000000000000485
30. Barsuk JH, Cohen ER, Feinglass J, et al. Cost savings of performing paracentesis procedures at the bedside after simulation-based education. Simul Healthc. 2014;9(5):312-318. https://doi.org/10.1097/SIH.0000000000000040
31. Barsuk JH, Cohen ER, Williams MV, et al. Simulation-based mastery learning for thoracentesis skills improves patient outcomes: a randomized trial. Acad Med. 2018;93(5):729-735. https://doi.org/10.1097/ACM.0000000000001965
32. Huang GC, McSparron JI, Balk EM, et al. Procedural instruction in invasive bedside procedures: a systematic review and meta-analysis of effective teaching approaches. BMJ Qual Saf. 2016;25(4):281-294. https://doi.org/10.1136/bmjqs-2014-003518
33. Brydges R, Stroud L, Wong BM, Holmboe ES, Imrie K, Hatala R. Core competencies or a competent core? a scoping review and realist synthesis of invasive bedside procedural skills training in internal medicine. Acad Med. 2017;92(11):1632-1643. https://doi.org/10.1097/ACM.0000000000001726
34. Brydges R, Hatala R, Zendejas B, Erwin PJ, Cook DA. Linking simulation-based educational assessments and patient-related outcomes: a systematic review and meta-analysis. Acad Med. 2015;90(2):246-256. https://doi.org/10.1097/ACM.0000000000000549
35. Barsuk JH, Cohen ER, Nguyen D, et al. Attending physician adherence to a 29-component central venous catheter bundle checklist during simulated procedures. Crit Care Med. 2016;44(10):1871-1881. https://doi.org/10.1097/CCM.0000000000001831
36. Crocker JT, Hale CP, Vanka A, Ricotta DN, McSparron JI, Huang GC. Raising the bar for procedural competency among hospitalists. Ann Intern Med. 2019;170(9):654-655. https://doi.org/10.7326/M18-3007
37. Barsuk JH, Cohen ER, Feinglass J, McGaghie WC, Wayne DB. Residents’ procedural experience does not ensure competence: a research synthesis. J Grad Med Educ. 2017;9(2):201-208. https://doi.org/10.4300/JGME-D-16-00426.1
38. Sawyer T, White M, Zaveri P, et al. Learn, see, practice, prove, do, maintain: an evidence-based pedagogical framework for procedural skill training in medicine. Acad Med. 2015;90(8):1025-1033. https://doi.org/10.1097/ACM.0000000000000734
39. Jensen T, Soni N, Tierney D, Lucas B. Hospital privileging practices for bedside procedures: a survey of hospitalist experts. J Hosp Med. 2017;12(10):836-839. https://doi.org/10.12788/jhm.2837
40. Lucas BP, Asbury JK, Wang Y, et al. Impact of a bedside procedure service on general medicine inpatients: a firm-based trial. J Hosp Med. 2007;2(3):143-149. https://doi.org/10.1002/jhm.159
41. Mourad M, Auerbach AD, Maselli J, Sliwka D. Patient satisfaction with a hospitalist procedure service: Is bedside procedure teaching reassuring to patients? J Hosp Med. 2011;6(4):219-224. https://doi.org/10.1002/jhm.856
42. Ault MJ, Rosen BT. Proceduralists — leading patient-safety initiatives. N Engl J Med. 2007;356(17):1789-1790. https://doi.org/10.1056/NEJMc063239
43. Obasi JU, Umpierrez De Reguero AP. Safety profile of bone marrow aspiration and biopsies performed by the hospitalist procedure service at an academic center: an observational study. Blood. 2019;134(suppl 1): 5848. https://doi.org/10.1182/blood-2019-121444
44. Gisondi MA, Regan L, Branzetti J, Hopson LR. More learners, finite resources, and the changing landscape of procedural training at the bedside. Acad Med. 2018;93(5):699-704. https://doi.org/10.1097/ACM.0000000000002062
45. McCormack J. The new proceduralists: Have they found their niche? American Medical News. September 17, 2007. Accessed August 30, 2020. https://amednews.com/article/20070917/business/309179994/4/
46. Lucas BP, Tierney DM, Jensen TP, et al; Society of Hospital Medicine Point-of-Care Ultrasound Task Force. Credentialing of hospitalists in ultrasound-guided bedside procedures: a position statement of the Society of Hospital Medicine. J Hosp Med. 2018;13(2):117-125. https://doi.org/10.12788/jhm.2917
47. Lenhard A, Moallem M, Marrie RA, Becker J, Garland A. An intervention to improve procedure education for internal medicine residents. J Gen Intern Med. 2008;23(3):288-293. https://doi.org/10.1007/s11606-008-0513-4
48. Mourad M, Ranji S, Sliwka D. A randomized controlled trial of the impact of a teaching procedure service on the training of internal medicine residents. J Grad Med Educ. 2012;4(2):170-175. https://doi.org/10.4300/JGME-D-11-00136.1
49. Montuno A, Hunt BR, Lee MM. Potential impact of a bedside procedure service on training procedurally competent hospitalists in a community-based residency program. J Community Hosp Intern Med Perspect. 2016;6(3):31054. https://doi.org/10.3402/jchimp.v6.31054
50. Smith CC, Gordon CE, Feller‐Kopman D, et al. Creation of an innovative inpatient medical procedure service and a method to evaluate house staff competency. J Gen Intern Med. 2004;19(5p2):510-513. https://doi.org/10.1111/j.1525-1497.2004.30161.x
51. Mourad M. Capsule commentary on Tukey et al., the impact of a medical procedure service on patient safety, procedure quality and resident training opportunities. J Gen Intern Med. 2014;29(3):518. https://doi.org/10.1007/s11606-013-2740-6
52. Halm EA, Lee C, Chassin MR. Is volume related to outcome in health care: a systematic review and methodologic critique of the literature. 2002. Database of Abstracts of Reviews of Effects (DARE): Quality-assessed Reviews . Accessed June 26, 2020. https://www.ncbi.nlm.nih.gov/books/NBK69189/
Over the past 20 years, hospitalists have served as the primary workforce for the clinical care of medical inpatients in the United States.1,2 Core competencies1 state that hospitalists should be able to perform the following bedside procedures: lumbar puncture, paracentesis, thoracentesis, arthrocentesis, and central venous catheter placement. More recently, standard of care has dictated that these procedures be performed under ultrasound guidance,3-6 and thus hospitalists are also expected to be adept at point-of-care ultrasound (POCUS).7
However, no current national standard exists for ensuring basic competency among hospitalists performing bedside procedures. In addition, hospitalists’ procedural volumes are declining,8,9 and standards for procedural training during internal medicine residency have been reduced.10 As a result, many residents who intend to become hospitalists are no longer prepared to perform these procedures.
The ramifications of the loss of procedural competency for hospitalists are manifold. Technical errors are a significant source of patient morbidity and mortality,11-15 and complications arising specifically from nonoperative procedures range from 0 to 19%,16 although these data do not distinguish technical errors from unpreventable adverse events nor the degree to which hospitalists contributed to these complications. Second, hospitalists in academic medical centers might be ill equipped to function as supervisors of trainees performing procedures, which could perpetuate a cycle of suboptimal technical skills.17 Finally, the discrepancy between consensus guidelines for hospitalists and their scope of practice represents a significant area of risk management for institutions that base their credentialing policies on published competencies.
There are many compelling reasons for why hospitalists should maintain—in fact reclaim—a primary role in bedside procedures.18 Hospitalists in community and rural settings might not have easy access to procedural specialists. In academic institutions, hospitalists are the primary instructors and supervisors of procedures performed by internal medicine residents. The increased availability of POCUS allows formally trained hospitalists to perform procedures more safely under imaging guidance.16
The literature on procedures performed by hospitalists, although limited, has focused on POCUS, systems innovations such as medical procedure services (MPS), and policy recommendations for procedural credentialing. Most studies on effective procedural instructional approaches have been conducted among trainees, who are procedural novices. This research does not sufficiently address the dilemma that hospitalists face as independent physicians for whom procedures are not a significant component of their practice, yet are expected to perform invasive procedures occasionally. The purpose of our literature review is to synthesize the available research to characterize contributors to hospitalists’ procedural competency. We conclude with considerations for hospital medicine practice.
METHODS
We performed a structured literature search for peer-reviewed articles related to hospitalists conducting procedures, being trained in procedures, or related to hospitalist-run MPS. We focused our search on the core hospitalist procedures with the highest potential morbidity (ie, lumbar puncture, abdominal paracentesis, thoracentesis, and central venous catheterization). We searched PubMed and Google Scholar for articles published since 1996 (when the term “hospitalists” was first coined) using keyword searches for [hospitalist OR hospital medicine] AND [procedur* OR medical procedur* OR medical procedure service] OR [(procedur* AND (train* OR educat* OR teach OR instruct*)] OR abdominal paracentes* OR thoracentes* OR lumbar puncture OR central venous catheter* OR ultrasound OR point-of-care. We included original research, brief research reports, perspectives, guidelines, and consensus statements. Exclusion criteria were articles that focused on nonhospitalists and conference abstracts. We used pearling to identify secondary sources from included articles’ bibliographies, without limits on year of publication.
RESULTS
Trends Towards Specialist Referrals
Between 1986 and 2007, the number and variety of procedures performed by internists decreased by half.19 Hospitalists still completed procedures in greater volume and variety than nonhospitalists,8 with approximately 50% of hospitalists performing lumbar punctures (50%), abdominal paracenteses (49%), and thoracenteses (44%) compared with less than 25% for all three procedures for nonhospitalists. Additionally, only 11% of surveyed hospitalists8 performed all nine core procedures, although these included procedures that are largely cognitive in nature (eg, electrocardiogram interpretation, chest X-ray interpretation) or procedures that have been relegated to other specialists (eg, endotracheal intubation, ventilator management, or joint injection/aspiration).
Surveys showed that, especially in larger cities and academic centers, procedural specialists have taken over a disproportionate share of procedures even as the number of procedures performed continued to rise.20 Between 1993 and 2008, the number of paracenteses and thoracenteses increased by 133% and decreased by 14%, respectively, but the share of procedures performed by radiologists increased by 964% and 358%, respectively, as evident in an analysis of Medicare billing data.20 A more recent study of Medicare claims from 2004 to 2016 similarly revealed that the percentage of paracenteses performed by radiologists compared with nonradiologists rose from 70% to 80% and thoracenteses from 47% to 66%, respectively.21 Comparable trends were apparent in claims data for lumbar punctures; between 1991 and 2011, the share of lumbar punctures performed by radiologists rose from 11% to 48%.22
In academic medical centers, hospitalists might have the opportunity to pursue other activities (eg, education, administration, research) as they progress in their careers, resulting in less clinical activity. Although hospitalists who are more clinically active in hospital care tended to perform more procedures,8 those with smaller clinical footprints reported lower levels of comfort with performing procedures8 and might have less available time to maintain procedural competency or train in new technologies such as POCUS.17
Additionally, hospitalists in both academic and community settings cited efficiency as a major reason for procedural referral. Hospitalists tended to perform more procedures if they had fixed salaries or if less than 50% of their income was based on clinical productivity, although this trend was not significant.8 Further, they also might be motivated by competing opportunity costs such as time lost caring for other patients or length of shift, which influences the amount of time spent at work.23
Notably, speculation that hospitalists referred more complex cases to specialists was not borne out by studies examining referral patterns.21,24,25
Procedural Outcomes for Hospitalists vs Nonhospitalists
No convincing data exist that procedures performed by specialists have better outcomes than those completed at the bedside by well-trained generalists, although studies were limited to the inpatient setting, to generalists who have some exposure to procedures, and to internal medicine residents on inpatient rotations. In one retrospective review, interventional radiology (IR) referrals were associated with more platelet or plasma transfusions and intensive care unit transfers than those performed at the bedside by internal medicine residents, findings that remained significant after accounting for complexity (eg, Model for End-stage Liver Disease score, need for dialysis, and platelet count).24 Similarly, a prospective audit of 529 bedside procedures did not show any differences in complication rates between generalists and pulmonologists, once generalists underwent standardized training and used pleural safety checklists and ultrasound guidance.26 An administrative database review of 130,000 inpatient thoracenteses across several university hospitals between 2010 and 2013 found that the risk of iatrogenic pneumothorax was similar among operators from IR, medicine, and pulmonary (2.8%, 2.9%, and 3.1%, respectively)27; these findings have been reproduced in other studies.28 Finally, the increasing adoption of procedural ultrasound permits procedures to be conducted more safely at the bedside, without the need to refer to radiology for imaging guidance.3-5
IR procedures also are associated with increased healthcare costs compared with bedside procedures. One study showed that hospital costs for admissions when paracenteses were performed by radiologists were higher than those in which the procedure was completed at the bedside by gastroenterologists or hepatologists.25 A chart review examining 399 paracenteses, thoracenteses, and lumbar punctures found that the average procedure cost increased by 38% for referred procedures and 56% for radiology-performed procedures, as compared with bedside procedures.29 Needing ancillary staffing in dedicated suites to perform procedures contributed to the excess cost.9 Moreover, referred procedures resulted in increased length of stay, which can incur additional costs. However, the data were conflicting; two studies did not show a statistical difference,25,28 while others found an increased length of stay,24,27,29 which might be due to the unavailability of specialists during off hours, thereby delaying nonemergent procedures.21 Detailed cost analyses have controlled for the use of procedural facilities and blood transfusions among IR specialists and simulation training among generalists, showing that total costs were $663 per patient undergoing IR procedures compared with $134 per patient undergoing bedside procedures.30
Lack of Standardized Procedural Training or Assessment
A robust body of primary studies and systematic reviews supports the use of simulation for procedural training to improve comfort and skill as well as reduce complication rates and costs.31,32 A systematic review that investigated the impact of four paradigms of procedural training found that MPS and quality improvement/patient safety approaches led to the most active learning compared with apprenticeship (ie, “see one, do one”) and approaches based on educational theories.33 Nevertheless, the vast majority of the research has been conducted in trainees,32,34 with sparse evidence among practicing physicians. One cohort study of attending physicians’ central venous catheter insertion skills on simulators found low and variable short-term performance, showing overall poor adherence to checklists.35 One article suggested that hospitalists’ procedural skills were below established thresholds of competency at baseline and that simulation-based training did not result in sustained skills, but the small sample size and high attrition limited meaningful conclusions.36 Although continuing medical education courses are available to hospitalists, there is no published evidence supporting their effectiveness.
Proxies for procedural skill have included comfort and experience, yet these markers have broadly been shown to be inadequate.34,36,37 Additionally, the natural decline of skill over time has invoked the need for periodic reassessment of proficiency.36,38 Credentialing has been equally inconstant; a survey of the Society of Hospital Medicine’s (SHM) POCUS task force revealed that only half of respondents reported their hospitals required a minimum number of procedures for initial credentialing and recredentialing.39 In short, periodic assessment of procedural skills among hospitalists has not been a routine process at many institutions.
Role of Hospitalist-Run Medical Procedure Services
It might not be necessary for all hospitalists to be proficient and credentialed in a given procedure,1 and a trend has emerged in the creation of MPS staffed by hospitalists as proceduralists. The primary aim of these MPS has been to recapture the procedures—and associated revenue—that would otherwise be referred to specialists. Moreover, concentrating procedures among a core group of hospitalists endeavors to support patient safety through several principles: (1) to increase technical proficiency through higher procedural volumes, (2) to facilitate rigorous training and assessment among dedicated individuals, and (3) to systematize best practices of operator performance, communication, and documentation.
MPS have been implemented around the country and have demonstrated several advantages. In one institution, medical firms that were offered the use of an MPS had 48% more procedural attempts by nonspecialists, without significant differences in the proportions of successful attempts or complications compared with the firms who more often referred to specialists.40 A retrospective study analyzed outcomes of 1,707 bedside procedures, of which 548 were performed by an MPS, and found that procedures done by the MPS were more likely to result in lower rates of unsuccessful procedures and to use best-practice safety processes (ie, to involve attending physicians, to use ultrasound guidance, and to avoid femoral sites for catheterization).12 Satisfaction was high among patients who underwent bedside procedures performed by a hospitalist-supervised, intern-based procedure service with a focus on bedside communication.41 From a workforce perspective, MPS have also allowed surgical or radiological subspecialties to focus on more complex cases with higher reimbursement rates,18,42 for proceduralists to expand beyond core procedures (eg, bone marrow biopsies43), and to train advanced practice providers.44 Although studies have not shown that the outcomes of procedures completed by an MPS are better than the outcomes of procedures performed by other specialists,45 one can potentially extrapolate from earlier data that procedures done at the bedside by nonradiologists would have comparable outcomes.
DISCUSSION
A myriad of factors is influencing hospitalists’ scope of practice with respect to bedside procedures. Some evidence suggests that procedures performed by specialists are not superior to those done by generalists and might be associated with increased costs. The most promising developments in the past few decades include simulation-based training, which has demonstrated effectiveness across an array of clinical outcomes but has not been sufficiently evaluated in hospitalists to draw conclusions, and hospitalist-led MPS, which promote safe and productive procedural clinical practices. However, decreasing procedural volume, increasing referrals to specialists, dwindling hospitalist interest and/or confidence, time constraints, limited training opportunities, nonuniform credentialing policies, and lack of standardized assessment are cumulatively contributing to a loss of procedural competency among hospitalists.
Taken together, these forces should compel hospital medicine groups that expect their hospitalists to perform their own procedures to identify necessary steps for ensuring the safety of hospitalized patients under their care. The following considerations derive from the available—albeit modest—evidence on procedural performance in hospital medicine (Table).

1. Create MPS to establish a core set of hospitalists to perform procedures and train them using evidence-based practices. Creation of an MPS places the responsibility of core bedside procedures in the hands of a select group of proceduralists. This strategy streamlines training and assessment of individual procedural competency to meet standards set by SHM36,46 and improves educational outcomes.47-49 MPS could improve clinical outcomes,12,42,50-52 including length of stay and cost, while maintaining patient satisfaction,41 as well as recoup lost revenue from referrals by increasing the volume of procedures done by generalists,40,49 although no robust data supporting the latter point exists. Implementing an MPS requires full-time equivalent (FTE) support for proceduralists and administrative support for data collection and tracking complications. Furthermore, a well-functioning MPS will require investment in portable ultrasound machines and training in POCUS, which has been shown to decrease complications and increase success of invasive bedside procedures.3-7 Hospital medicine groups should be aware that staffing an MPS can divert hospitalist labor and resources from other needed clinical areas, especially during the initial, low-volume phases of implementation. Strategies to offset relative value unit (RVU) loss include combining the MPS with existing clinical roles such as medical consults, code triage, and rapid response teams; or with services with lower patient caps, which might work particularly well in community hospitals. In many institutions, hospitalists can bill for procedural consults in addition to the procedures when the consult involves nonmedical patients, which is relevant when the procedure ultimately cannot be performed (eg, too little ascites to safely perform a paracentesis). Further research should establish best practices of MPS to ensure maximum procedural productivity and safety, because there are no rigorous prospective studies that evaluate strategies to create this service. Such strategies include determining the optimal ratio of proceduralists to general hospitalists, hospital characteristics that benefit most from MPS (eg, referral centers, urban-based settings), volume and type of procedures performed, and the proportion and type of referrals that are most cost-effective.
2. Establish policies with procedural specialists to arrange coverage for off-hours procedures and delineate thresholds for procedures that specialists should perform. Expanding hospitalists’ capabilities in performing procedures should trigger reconsideration of the medical center’s approach to procedural safety. A goal would be to have hospital medicine groups work collaboratively with specialists and other disciplines (eg, surgery, emergency medicine, anesthesia, or radiology) to ensure 24-hour, 7-day a week coverage of urgent bedside procedures. The potential to decrease length of stay and improve off-hour procedural quality might be a compelling rationale for hospital administration, whether or not an MPS is used. That said, we recognize that other services might be unable or unwilling to provide such coverage and that specialist off-hour coverage would incur increased costs and could reduce exposure opportunities for internal medicine residents.
A hospital-level procedures committee might be required to support an institutional imperative for procedural safety and to oversee the implementation of approaches that are practical, financially sustainable, and equitable for all service lines, especially because hospitalist groups might bear the early costs of training and retraining.
3. Hospitalist–proceduralists should collaborate with internal medicine residency programs to offer intensive procedural training experiences to residents who want these skills to be part of their future practice. Robust procedural training for trainees promotes better outcomes for the current workforce and helps to populate the future workforce with procedurally competent practitioners. Simulation-based training is a well-established procedural instruction method that is safe, authentic, and effective in terms of clinical outcomes.34 As the primary teachers of residents in many institutions, hospitalists often are the ones who impart procedural skills to residents, despite uneven skill sets. It is in the interest of internal medicine residency program directors to advocate for a core group of hospitalist–proceduralists, as MPS offer an infrastructure for training that has been shown to increase procedural volume and improve skills.47,48,50 Program directors could therefore be incentivized to sponsor some of these procedural roles with teaching and administration funds, as a trade-off for securing higher-quality procedural training and closer supervision for their trainees. The dual necessity of teaching procedural skills to residents and attending physicians alike offers economies of scale for the use of facilities, personnel, and equipment, and gives faculty an opportunity to extend their clinical teaching skills into the domain of procedural supervision.
4. Hospital medicine groups should re-evaluate credentialing and privileging to ensure procedural competency. Given the lack of published data that characterizes how many hospital medicine groups credential hospitalists to perform procedures and what practices they use to assess competency, hospital medicine groups might be signing off on procedures without verifying hospitalists’ proficiency in core procedures. SHM’s position statement on credentialing for ultrasound-guided procedures46 describes standards that could be applied to other procedures. It proposes that credentialing processes should be grounded in simulation- and patient-based assessments of cognitive and psychomotor skills, using published checklists and global ratings for feedback. Simulation training could support provisional certification, but hospitalists should reach minimum thresholds of supervised patient-based experience before initial credentialing, with continuous reassessment of competency to mitigate skill decay. Prospectively tracking procedural metrics, such as procedural volume and complication rates, also will support systematic skill assessment. Finally, similar to any other medical error, near misses and complications should trigger periprocedural safety reviews.
Limitations
The modest body of research on hospitalists and procedures is the central limitation of our synthesis. Much of the literature consisted of consensus statements, retrospective studies, and small prospective educational studies. As a result, we did not adopt all strategies considered standard in a scoping or systematic review. The literature on MPS specifically was insufficient to draw conclusions about their operational and financial impact or effects on procedure quality. Our primary recommendation to implement MPS requires significant fiscal investment and infrastructure. It also entails risks that must be proactively addressed, including the potential for net financial loss and decreased educational opportunities for residents.
CONCLUSIONS
Hospitalists regularly face the predicament of being expected to independently perform procedures, with little access to training, minimal experience, and no ongoing assessment to ensure their proficiency or the safety of their patients. Past assumptions about hospitalists’ responsibility do not reflect realities in practice patterns and have not translated to widespread adoption of procedural training, monitoring, and assessment mechanisms. Our work summarizes a body of literature that, although limited in empiric studies of hospitalists themselves, offers insights with recommendations for hospital medicine groups wishing to uphold procedural skills as part of their providers’ professional identity.
Over the past 20 years, hospitalists have served as the primary workforce for the clinical care of medical inpatients in the United States.1,2 Core competencies1 state that hospitalists should be able to perform the following bedside procedures: lumbar puncture, paracentesis, thoracentesis, arthrocentesis, and central venous catheter placement. More recently, standard of care has dictated that these procedures be performed under ultrasound guidance,3-6 and thus hospitalists are also expected to be adept at point-of-care ultrasound (POCUS).7
However, no current national standard exists for ensuring basic competency among hospitalists performing bedside procedures. In addition, hospitalists’ procedural volumes are declining,8,9 and standards for procedural training during internal medicine residency have been reduced.10 As a result, many residents who intend to become hospitalists are no longer prepared to perform these procedures.
The ramifications of the loss of procedural competency for hospitalists are manifold. Technical errors are a significant source of patient morbidity and mortality,11-15 and complications arising specifically from nonoperative procedures range from 0 to 19%,16 although these data do not distinguish technical errors from unpreventable adverse events nor the degree to which hospitalists contributed to these complications. Second, hospitalists in academic medical centers might be ill equipped to function as supervisors of trainees performing procedures, which could perpetuate a cycle of suboptimal technical skills.17 Finally, the discrepancy between consensus guidelines for hospitalists and their scope of practice represents a significant area of risk management for institutions that base their credentialing policies on published competencies.
There are many compelling reasons for why hospitalists should maintain—in fact reclaim—a primary role in bedside procedures.18 Hospitalists in community and rural settings might not have easy access to procedural specialists. In academic institutions, hospitalists are the primary instructors and supervisors of procedures performed by internal medicine residents. The increased availability of POCUS allows formally trained hospitalists to perform procedures more safely under imaging guidance.16
The literature on procedures performed by hospitalists, although limited, has focused on POCUS, systems innovations such as medical procedure services (MPS), and policy recommendations for procedural credentialing. Most studies on effective procedural instructional approaches have been conducted among trainees, who are procedural novices. This research does not sufficiently address the dilemma that hospitalists face as independent physicians for whom procedures are not a significant component of their practice, yet are expected to perform invasive procedures occasionally. The purpose of our literature review is to synthesize the available research to characterize contributors to hospitalists’ procedural competency. We conclude with considerations for hospital medicine practice.
METHODS
We performed a structured literature search for peer-reviewed articles related to hospitalists conducting procedures, being trained in procedures, or related to hospitalist-run MPS. We focused our search on the core hospitalist procedures with the highest potential morbidity (ie, lumbar puncture, abdominal paracentesis, thoracentesis, and central venous catheterization). We searched PubMed and Google Scholar for articles published since 1996 (when the term “hospitalists” was first coined) using keyword searches for [hospitalist OR hospital medicine] AND [procedur* OR medical procedur* OR medical procedure service] OR [(procedur* AND (train* OR educat* OR teach OR instruct*)] OR abdominal paracentes* OR thoracentes* OR lumbar puncture OR central venous catheter* OR ultrasound OR point-of-care. We included original research, brief research reports, perspectives, guidelines, and consensus statements. Exclusion criteria were articles that focused on nonhospitalists and conference abstracts. We used pearling to identify secondary sources from included articles’ bibliographies, without limits on year of publication.
RESULTS
Trends Towards Specialist Referrals
Between 1986 and 2007, the number and variety of procedures performed by internists decreased by half.19 Hospitalists still completed procedures in greater volume and variety than nonhospitalists,8 with approximately 50% of hospitalists performing lumbar punctures (50%), abdominal paracenteses (49%), and thoracenteses (44%) compared with less than 25% for all three procedures for nonhospitalists. Additionally, only 11% of surveyed hospitalists8 performed all nine core procedures, although these included procedures that are largely cognitive in nature (eg, electrocardiogram interpretation, chest X-ray interpretation) or procedures that have been relegated to other specialists (eg, endotracheal intubation, ventilator management, or joint injection/aspiration).
Surveys showed that, especially in larger cities and academic centers, procedural specialists have taken over a disproportionate share of procedures even as the number of procedures performed continued to rise.20 Between 1993 and 2008, the number of paracenteses and thoracenteses increased by 133% and decreased by 14%, respectively, but the share of procedures performed by radiologists increased by 964% and 358%, respectively, as evident in an analysis of Medicare billing data.20 A more recent study of Medicare claims from 2004 to 2016 similarly revealed that the percentage of paracenteses performed by radiologists compared with nonradiologists rose from 70% to 80% and thoracenteses from 47% to 66%, respectively.21 Comparable trends were apparent in claims data for lumbar punctures; between 1991 and 2011, the share of lumbar punctures performed by radiologists rose from 11% to 48%.22
In academic medical centers, hospitalists might have the opportunity to pursue other activities (eg, education, administration, research) as they progress in their careers, resulting in less clinical activity. Although hospitalists who are more clinically active in hospital care tended to perform more procedures,8 those with smaller clinical footprints reported lower levels of comfort with performing procedures8 and might have less available time to maintain procedural competency or train in new technologies such as POCUS.17
Additionally, hospitalists in both academic and community settings cited efficiency as a major reason for procedural referral. Hospitalists tended to perform more procedures if they had fixed salaries or if less than 50% of their income was based on clinical productivity, although this trend was not significant.8 Further, they also might be motivated by competing opportunity costs such as time lost caring for other patients or length of shift, which influences the amount of time spent at work.23
Notably, speculation that hospitalists referred more complex cases to specialists was not borne out by studies examining referral patterns.21,24,25
Procedural Outcomes for Hospitalists vs Nonhospitalists
No convincing data exist that procedures performed by specialists have better outcomes than those completed at the bedside by well-trained generalists, although studies were limited to the inpatient setting, to generalists who have some exposure to procedures, and to internal medicine residents on inpatient rotations. In one retrospective review, interventional radiology (IR) referrals were associated with more platelet or plasma transfusions and intensive care unit transfers than those performed at the bedside by internal medicine residents, findings that remained significant after accounting for complexity (eg, Model for End-stage Liver Disease score, need for dialysis, and platelet count).24 Similarly, a prospective audit of 529 bedside procedures did not show any differences in complication rates between generalists and pulmonologists, once generalists underwent standardized training and used pleural safety checklists and ultrasound guidance.26 An administrative database review of 130,000 inpatient thoracenteses across several university hospitals between 2010 and 2013 found that the risk of iatrogenic pneumothorax was similar among operators from IR, medicine, and pulmonary (2.8%, 2.9%, and 3.1%, respectively)27; these findings have been reproduced in other studies.28 Finally, the increasing adoption of procedural ultrasound permits procedures to be conducted more safely at the bedside, without the need to refer to radiology for imaging guidance.3-5
IR procedures also are associated with increased healthcare costs compared with bedside procedures. One study showed that hospital costs for admissions when paracenteses were performed by radiologists were higher than those in which the procedure was completed at the bedside by gastroenterologists or hepatologists.25 A chart review examining 399 paracenteses, thoracenteses, and lumbar punctures found that the average procedure cost increased by 38% for referred procedures and 56% for radiology-performed procedures, as compared with bedside procedures.29 Needing ancillary staffing in dedicated suites to perform procedures contributed to the excess cost.9 Moreover, referred procedures resulted in increased length of stay, which can incur additional costs. However, the data were conflicting; two studies did not show a statistical difference,25,28 while others found an increased length of stay,24,27,29 which might be due to the unavailability of specialists during off hours, thereby delaying nonemergent procedures.21 Detailed cost analyses have controlled for the use of procedural facilities and blood transfusions among IR specialists and simulation training among generalists, showing that total costs were $663 per patient undergoing IR procedures compared with $134 per patient undergoing bedside procedures.30
Lack of Standardized Procedural Training or Assessment
A robust body of primary studies and systematic reviews supports the use of simulation for procedural training to improve comfort and skill as well as reduce complication rates and costs.31,32 A systematic review that investigated the impact of four paradigms of procedural training found that MPS and quality improvement/patient safety approaches led to the most active learning compared with apprenticeship (ie, “see one, do one”) and approaches based on educational theories.33 Nevertheless, the vast majority of the research has been conducted in trainees,32,34 with sparse evidence among practicing physicians. One cohort study of attending physicians’ central venous catheter insertion skills on simulators found low and variable short-term performance, showing overall poor adherence to checklists.35 One article suggested that hospitalists’ procedural skills were below established thresholds of competency at baseline and that simulation-based training did not result in sustained skills, but the small sample size and high attrition limited meaningful conclusions.36 Although continuing medical education courses are available to hospitalists, there is no published evidence supporting their effectiveness.
Proxies for procedural skill have included comfort and experience, yet these markers have broadly been shown to be inadequate.34,36,37 Additionally, the natural decline of skill over time has invoked the need for periodic reassessment of proficiency.36,38 Credentialing has been equally inconstant; a survey of the Society of Hospital Medicine’s (SHM) POCUS task force revealed that only half of respondents reported their hospitals required a minimum number of procedures for initial credentialing and recredentialing.39 In short, periodic assessment of procedural skills among hospitalists has not been a routine process at many institutions.
Role of Hospitalist-Run Medical Procedure Services
It might not be necessary for all hospitalists to be proficient and credentialed in a given procedure,1 and a trend has emerged in the creation of MPS staffed by hospitalists as proceduralists. The primary aim of these MPS has been to recapture the procedures—and associated revenue—that would otherwise be referred to specialists. Moreover, concentrating procedures among a core group of hospitalists endeavors to support patient safety through several principles: (1) to increase technical proficiency through higher procedural volumes, (2) to facilitate rigorous training and assessment among dedicated individuals, and (3) to systematize best practices of operator performance, communication, and documentation.
MPS have been implemented around the country and have demonstrated several advantages. In one institution, medical firms that were offered the use of an MPS had 48% more procedural attempts by nonspecialists, without significant differences in the proportions of successful attempts or complications compared with the firms who more often referred to specialists.40 A retrospective study analyzed outcomes of 1,707 bedside procedures, of which 548 were performed by an MPS, and found that procedures done by the MPS were more likely to result in lower rates of unsuccessful procedures and to use best-practice safety processes (ie, to involve attending physicians, to use ultrasound guidance, and to avoid femoral sites for catheterization).12 Satisfaction was high among patients who underwent bedside procedures performed by a hospitalist-supervised, intern-based procedure service with a focus on bedside communication.41 From a workforce perspective, MPS have also allowed surgical or radiological subspecialties to focus on more complex cases with higher reimbursement rates,18,42 for proceduralists to expand beyond core procedures (eg, bone marrow biopsies43), and to train advanced practice providers.44 Although studies have not shown that the outcomes of procedures completed by an MPS are better than the outcomes of procedures performed by other specialists,45 one can potentially extrapolate from earlier data that procedures done at the bedside by nonradiologists would have comparable outcomes.
DISCUSSION
A myriad of factors is influencing hospitalists’ scope of practice with respect to bedside procedures. Some evidence suggests that procedures performed by specialists are not superior to those done by generalists and might be associated with increased costs. The most promising developments in the past few decades include simulation-based training, which has demonstrated effectiveness across an array of clinical outcomes but has not been sufficiently evaluated in hospitalists to draw conclusions, and hospitalist-led MPS, which promote safe and productive procedural clinical practices. However, decreasing procedural volume, increasing referrals to specialists, dwindling hospitalist interest and/or confidence, time constraints, limited training opportunities, nonuniform credentialing policies, and lack of standardized assessment are cumulatively contributing to a loss of procedural competency among hospitalists.
Taken together, these forces should compel hospital medicine groups that expect their hospitalists to perform their own procedures to identify necessary steps for ensuring the safety of hospitalized patients under their care. The following considerations derive from the available—albeit modest—evidence on procedural performance in hospital medicine (Table).

1. Create MPS to establish a core set of hospitalists to perform procedures and train them using evidence-based practices. Creation of an MPS places the responsibility of core bedside procedures in the hands of a select group of proceduralists. This strategy streamlines training and assessment of individual procedural competency to meet standards set by SHM36,46 and improves educational outcomes.47-49 MPS could improve clinical outcomes,12,42,50-52 including length of stay and cost, while maintaining patient satisfaction,41 as well as recoup lost revenue from referrals by increasing the volume of procedures done by generalists,40,49 although no robust data supporting the latter point exists. Implementing an MPS requires full-time equivalent (FTE) support for proceduralists and administrative support for data collection and tracking complications. Furthermore, a well-functioning MPS will require investment in portable ultrasound machines and training in POCUS, which has been shown to decrease complications and increase success of invasive bedside procedures.3-7 Hospital medicine groups should be aware that staffing an MPS can divert hospitalist labor and resources from other needed clinical areas, especially during the initial, low-volume phases of implementation. Strategies to offset relative value unit (RVU) loss include combining the MPS with existing clinical roles such as medical consults, code triage, and rapid response teams; or with services with lower patient caps, which might work particularly well in community hospitals. In many institutions, hospitalists can bill for procedural consults in addition to the procedures when the consult involves nonmedical patients, which is relevant when the procedure ultimately cannot be performed (eg, too little ascites to safely perform a paracentesis). Further research should establish best practices of MPS to ensure maximum procedural productivity and safety, because there are no rigorous prospective studies that evaluate strategies to create this service. Such strategies include determining the optimal ratio of proceduralists to general hospitalists, hospital characteristics that benefit most from MPS (eg, referral centers, urban-based settings), volume and type of procedures performed, and the proportion and type of referrals that are most cost-effective.
2. Establish policies with procedural specialists to arrange coverage for off-hours procedures and delineate thresholds for procedures that specialists should perform. Expanding hospitalists’ capabilities in performing procedures should trigger reconsideration of the medical center’s approach to procedural safety. A goal would be to have hospital medicine groups work collaboratively with specialists and other disciplines (eg, surgery, emergency medicine, anesthesia, or radiology) to ensure 24-hour, 7-day a week coverage of urgent bedside procedures. The potential to decrease length of stay and improve off-hour procedural quality might be a compelling rationale for hospital administration, whether or not an MPS is used. That said, we recognize that other services might be unable or unwilling to provide such coverage and that specialist off-hour coverage would incur increased costs and could reduce exposure opportunities for internal medicine residents.
A hospital-level procedures committee might be required to support an institutional imperative for procedural safety and to oversee the implementation of approaches that are practical, financially sustainable, and equitable for all service lines, especially because hospitalist groups might bear the early costs of training and retraining.
3. Hospitalist–proceduralists should collaborate with internal medicine residency programs to offer intensive procedural training experiences to residents who want these skills to be part of their future practice. Robust procedural training for trainees promotes better outcomes for the current workforce and helps to populate the future workforce with procedurally competent practitioners. Simulation-based training is a well-established procedural instruction method that is safe, authentic, and effective in terms of clinical outcomes.34 As the primary teachers of residents in many institutions, hospitalists often are the ones who impart procedural skills to residents, despite uneven skill sets. It is in the interest of internal medicine residency program directors to advocate for a core group of hospitalist–proceduralists, as MPS offer an infrastructure for training that has been shown to increase procedural volume and improve skills.47,48,50 Program directors could therefore be incentivized to sponsor some of these procedural roles with teaching and administration funds, as a trade-off for securing higher-quality procedural training and closer supervision for their trainees. The dual necessity of teaching procedural skills to residents and attending physicians alike offers economies of scale for the use of facilities, personnel, and equipment, and gives faculty an opportunity to extend their clinical teaching skills into the domain of procedural supervision.
4. Hospital medicine groups should re-evaluate credentialing and privileging to ensure procedural competency. Given the lack of published data that characterizes how many hospital medicine groups credential hospitalists to perform procedures and what practices they use to assess competency, hospital medicine groups might be signing off on procedures without verifying hospitalists’ proficiency in core procedures. SHM’s position statement on credentialing for ultrasound-guided procedures46 describes standards that could be applied to other procedures. It proposes that credentialing processes should be grounded in simulation- and patient-based assessments of cognitive and psychomotor skills, using published checklists and global ratings for feedback. Simulation training could support provisional certification, but hospitalists should reach minimum thresholds of supervised patient-based experience before initial credentialing, with continuous reassessment of competency to mitigate skill decay. Prospectively tracking procedural metrics, such as procedural volume and complication rates, also will support systematic skill assessment. Finally, similar to any other medical error, near misses and complications should trigger periprocedural safety reviews.
Limitations
The modest body of research on hospitalists and procedures is the central limitation of our synthesis. Much of the literature consisted of consensus statements, retrospective studies, and small prospective educational studies. As a result, we did not adopt all strategies considered standard in a scoping or systematic review. The literature on MPS specifically was insufficient to draw conclusions about their operational and financial impact or effects on procedure quality. Our primary recommendation to implement MPS requires significant fiscal investment and infrastructure. It also entails risks that must be proactively addressed, including the potential for net financial loss and decreased educational opportunities for residents.
CONCLUSIONS
Hospitalists regularly face the predicament of being expected to independently perform procedures, with little access to training, minimal experience, and no ongoing assessment to ensure their proficiency or the safety of their patients. Past assumptions about hospitalists’ responsibility do not reflect realities in practice patterns and have not translated to widespread adoption of procedural training, monitoring, and assessment mechanisms. Our work summarizes a body of literature that, although limited in empiric studies of hospitalists themselves, offers insights with recommendations for hospital medicine groups wishing to uphold procedural skills as part of their providers’ professional identity.
1. Dressler DD, Pistoria MJ, Budnitz TL, McKean SCW, Amin AN. Core competencies in hospital medicine: Development and methodology. J Hosp Med. 2006;1(1):48-56. https://doi.org/10.1002/jhm.6
2. Wachter RM, Goldman L. Zero to 50,000 — The 20th anniversary of the hospitalist. N Engl J Med. 2016;375(11):1009-1011. https://doi.org/10.1056/NEJMp1607958
3. Cho J, Jensen TP, Reierson K, et al. Recommendations on the use of ultrasound guidance for adult abdominal paracentesis: a position statement of the Society of Hospital Medicine. J Hosp Med. 2019;14:E7-E15. https://doi.org/10.12788/jhm.3095
4. Soni NJ, Franco-Sadud R, Kobaidze K, et al. Recommendations on the use of ultrasound guidance for adult lumbar puncture: a position statement of the Society of Hospital Medicine. J Hosp Med. 2019;14(10):591-601. https://doi.org/10.12788/jhm.3197
5. Dancel R, Schnobrich D, Puri N, et al. Recommendations on the use of ultrasound guidance for adult thoracentesis: a position statement of the Society of Hospital Medicine. J Hosp Med. 2018;13(2):126-135. https://doi.org/10.12788/jhm.2940
6. Franco-Sadud R, Schnobrich D, Mathews BK, et al. Recommendations on the use of ultrasound guidance for central and peripheral vascular access in adults: a position statement of the Society of Hospital Medicine. J Hosp Med. 2019;14:E1-E22. https://doi.org/10.12788/jhm.3287
7. Soni NJ, Schnobrich D, Mathews BK, et al. Point-of-care ultrasound for hospitalists: a position statement of the Society of Hospital Medicine. J Hosp Med. 2019;14:E1-E6. https://doi.org/10.12788/jhm.3079
8. Thakkar R, Wright SM, Alguire P, Wigton RS, Boonyasai RT. Procedures performed by hospitalist and non-hospitalist general internists. J Gen Intern Med. 2010;25(5):448-452. https://doi.org/10.1007/s11606-010-1284-2
9. Lucas BP, Asbury JK, Franco-Sadud R. Training future hospitalists with simulators: a needed step toward accessible, expertly performed bedside procedures. J Hosp Med. 2009;4(7):395-396. https://doi.org/10.1002/jhm.602
10. American Board of Internal Medicine. Policies and procedures for certification. Accessed December 3, 2020. https://www.abim.org/~/media/ABIM%20Public/Files/pdf/publications/certification-guides/policies-and-procedures.pdf
11. Myers LC. Toward preventing medical malpractice claims related to chest procedures. Ann Am Thorac Soc. 2020;17(6):776-779. https://doi.org/10.1513/AnnalsATS.201912-863RL
12. Tukey MH, Wiener RS. The impact of a medical procedure service on patient safety, procedure quality and resident training opportunities. J Gen Intern Med. 2014;29(3):485-490. https://doi.org/10.1007/s11606-013-2709-5
13. Brennan TA, Leape LL, Laird NM, et al. Incidence of adverse events and negligence in hospitalized patients. N Engl J Med. 1991;324(6):370-376. https://doi.org/10.1056/NEJM199102073240604
14. Leape LL, Brennan TA, Laird N, et al. The nature of adverse events in hospitalized patients. N Engl J Med. 1991;324(6):377-384. https://doi.org/10.1056/NEJM199102073240605
15. Myers LC, Gartland RM, Skillings J, et al. An examination of medical malpractice claims involving physician trainees. Acad Med. 2020;95(8):1215-1222. https://doi.org/10.1097/ACM.0000000000003117
16. Mercaldi CJ, Lanes SF. Ultrasound guidance decreases complications and improves the cost of care among patients undergoing thoracentesis and paracentesis. Chest. 2013;143(2):532-538. https://doi.org/10.1378/chest.12-0447
17. Vaisman A, Cram P. Procedural competence among faculty in academic health centers: challenges and future directions. Acad Med. 2017;92(1):31-34. https://doi.org/10.1097/ACM.0000000000001327
18. Nelson B. Hospitalists try to reclaim lead role in bedside procedures. The Hospitalist. March 2015. Accessed June 27, 2020. https://www.the-hospitalist.org/hospitalist/article/122571/hospitalists-try-reclaim-lead-role-bedside-procedures
19. Wigton RS, Alguire P; American College of Physicians. The declining number and variety of procedures done by general internists: a resurvey of members of the American College of Physicians. Ann Intern Med. 2007;146(5):355-360. https://doi.org/10.7326/0003-4819-146-5-200703060-00007
20. Duszak R Jr, Chatterjee AR, Schneider DA. National fluid shifts: fifteen-year trends in paracentesis and thoracentesis procedures. J Am Coll Radiol. 2010;7(11):859-864. https://doi.org/10.1016/j.jacr.2010.04.013
21. Gottumukkala RV, Prabhakar AM, Hemingway J, Hughes DR, Duszak R Jr. Disparities over time in volume, day of the week, and patient complexity between paracentesis and thoracentesis procedures performed by radiologists versus those performed by nonradiologists. J Vasc Interv Radiol. 2019;30(11):1769-1778.e1. https://doi.org/10.1016/j.jvir.2019.04.015
22. Kroll H, Duszak R Jr, Nsiah E, Hughes DR, Sumer S, Wintermark M. Trends in lumbar puncture over 2 decades: a dramatic shift to radiology. Am J Roentgenol. 2014;204(1):15-19. https://doi.org/10.2214/AJR.14.12622
23. Jensen T, Lai A, Mourad M. Can lessons from systems-based mastery learning for thoracentesis be translated to hospitalists? J Hosp Med. 2016;11(11):811-812. https://doi.org/10.1002/jhm.2655
24. Barsuk JH, Cohen ER, Feinglass J, McGaghie WC, Wayne DB. Clinical outcomes after bedside and interventional radiology paracentesis procedures. Am J Med. 2013;126(4):349-356. https://doi.org/10.1016/j.amjmed.2012.09.016
25. Barsuk JH, Feinglass J, Kozmic SE, Hohmann SF, Ganger D, Wayne DB. Specialties performing paracentesis procedures at university hospitals: implications for training and certification. J Hosp Med. 2014;9(3):162-168. https://doi.org/10.1002/jhm.2153
26. See KC, Ong V, Teoh CM, et al. Bedside pleural procedures by pulmonologists and non-pulmonologists: a 3-year safety audit. Respirology. 2014;19(3):396-402. https://doi.org/10.1111/resp.12244
27. Kozmic SE, Wayne DB, Feinglass J, Hohmann SF, Barsuk JH. Factors associated with inpatient thoracentesis procedure quality at university hospitals. Jt Comm J Qual Patient Saf. 2016;42(1):34-40. https://doi.org/10.1016/S1553-7250(16)42004-0
28. Berger MS, Divilov V, Paredes H, Sun E. Abdominal paracentesis: safety and efficacy comparing medicine resident bedside paracentesis vs. paracentesis performed by interventional radiology. J Clin Gastroenterol Hepatol. 2018;2(4). https://doi.org/10.21767/2575-7733.1000050
29. Kay C, Wozniak EM, Szabo A, Jackson JL. Examining invasive bedside procedure performance at an academic medical center. South Med J. 2016;109(7):402-407. https://doi.org/10.14423/SMJ.0000000000000485
30. Barsuk JH, Cohen ER, Feinglass J, et al. Cost savings of performing paracentesis procedures at the bedside after simulation-based education. Simul Healthc. 2014;9(5):312-318. https://doi.org/10.1097/SIH.0000000000000040
31. Barsuk JH, Cohen ER, Williams MV, et al. Simulation-based mastery learning for thoracentesis skills improves patient outcomes: a randomized trial. Acad Med. 2018;93(5):729-735. https://doi.org/10.1097/ACM.0000000000001965
32. Huang GC, McSparron JI, Balk EM, et al. Procedural instruction in invasive bedside procedures: a systematic review and meta-analysis of effective teaching approaches. BMJ Qual Saf. 2016;25(4):281-294. https://doi.org/10.1136/bmjqs-2014-003518
33. Brydges R, Stroud L, Wong BM, Holmboe ES, Imrie K, Hatala R. Core competencies or a competent core? a scoping review and realist synthesis of invasive bedside procedural skills training in internal medicine. Acad Med. 2017;92(11):1632-1643. https://doi.org/10.1097/ACM.0000000000001726
34. Brydges R, Hatala R, Zendejas B, Erwin PJ, Cook DA. Linking simulation-based educational assessments and patient-related outcomes: a systematic review and meta-analysis. Acad Med. 2015;90(2):246-256. https://doi.org/10.1097/ACM.0000000000000549
35. Barsuk JH, Cohen ER, Nguyen D, et al. Attending physician adherence to a 29-component central venous catheter bundle checklist during simulated procedures. Crit Care Med. 2016;44(10):1871-1881. https://doi.org/10.1097/CCM.0000000000001831
36. Crocker JT, Hale CP, Vanka A, Ricotta DN, McSparron JI, Huang GC. Raising the bar for procedural competency among hospitalists. Ann Intern Med. 2019;170(9):654-655. https://doi.org/10.7326/M18-3007
37. Barsuk JH, Cohen ER, Feinglass J, McGaghie WC, Wayne DB. Residents’ procedural experience does not ensure competence: a research synthesis. J Grad Med Educ. 2017;9(2):201-208. https://doi.org/10.4300/JGME-D-16-00426.1
38. Sawyer T, White M, Zaveri P, et al. Learn, see, practice, prove, do, maintain: an evidence-based pedagogical framework for procedural skill training in medicine. Acad Med. 2015;90(8):1025-1033. https://doi.org/10.1097/ACM.0000000000000734
39. Jensen T, Soni N, Tierney D, Lucas B. Hospital privileging practices for bedside procedures: a survey of hospitalist experts. J Hosp Med. 2017;12(10):836-839. https://doi.org/10.12788/jhm.2837
40. Lucas BP, Asbury JK, Wang Y, et al. Impact of a bedside procedure service on general medicine inpatients: a firm-based trial. J Hosp Med. 2007;2(3):143-149. https://doi.org/10.1002/jhm.159
41. Mourad M, Auerbach AD, Maselli J, Sliwka D. Patient satisfaction with a hospitalist procedure service: Is bedside procedure teaching reassuring to patients? J Hosp Med. 2011;6(4):219-224. https://doi.org/10.1002/jhm.856
42. Ault MJ, Rosen BT. Proceduralists — leading patient-safety initiatives. N Engl J Med. 2007;356(17):1789-1790. https://doi.org/10.1056/NEJMc063239
43. Obasi JU, Umpierrez De Reguero AP. Safety profile of bone marrow aspiration and biopsies performed by the hospitalist procedure service at an academic center: an observational study. Blood. 2019;134(suppl 1): 5848. https://doi.org/10.1182/blood-2019-121444
44. Gisondi MA, Regan L, Branzetti J, Hopson LR. More learners, finite resources, and the changing landscape of procedural training at the bedside. Acad Med. 2018;93(5):699-704. https://doi.org/10.1097/ACM.0000000000002062
45. McCormack J. The new proceduralists: Have they found their niche? American Medical News. September 17, 2007. Accessed August 30, 2020. https://amednews.com/article/20070917/business/309179994/4/
46. Lucas BP, Tierney DM, Jensen TP, et al; Society of Hospital Medicine Point-of-Care Ultrasound Task Force. Credentialing of hospitalists in ultrasound-guided bedside procedures: a position statement of the Society of Hospital Medicine. J Hosp Med. 2018;13(2):117-125. https://doi.org/10.12788/jhm.2917
47. Lenhard A, Moallem M, Marrie RA, Becker J, Garland A. An intervention to improve procedure education for internal medicine residents. J Gen Intern Med. 2008;23(3):288-293. https://doi.org/10.1007/s11606-008-0513-4
48. Mourad M, Ranji S, Sliwka D. A randomized controlled trial of the impact of a teaching procedure service on the training of internal medicine residents. J Grad Med Educ. 2012;4(2):170-175. https://doi.org/10.4300/JGME-D-11-00136.1
49. Montuno A, Hunt BR, Lee MM. Potential impact of a bedside procedure service on training procedurally competent hospitalists in a community-based residency program. J Community Hosp Intern Med Perspect. 2016;6(3):31054. https://doi.org/10.3402/jchimp.v6.31054
50. Smith CC, Gordon CE, Feller‐Kopman D, et al. Creation of an innovative inpatient medical procedure service and a method to evaluate house staff competency. J Gen Intern Med. 2004;19(5p2):510-513. https://doi.org/10.1111/j.1525-1497.2004.30161.x
51. Mourad M. Capsule commentary on Tukey et al., the impact of a medical procedure service on patient safety, procedure quality and resident training opportunities. J Gen Intern Med. 2014;29(3):518. https://doi.org/10.1007/s11606-013-2740-6
52. Halm EA, Lee C, Chassin MR. Is volume related to outcome in health care: a systematic review and methodologic critique of the literature. 2002. Database of Abstracts of Reviews of Effects (DARE): Quality-assessed Reviews . Accessed June 26, 2020. https://www.ncbi.nlm.nih.gov/books/NBK69189/
1. Dressler DD, Pistoria MJ, Budnitz TL, McKean SCW, Amin AN. Core competencies in hospital medicine: Development and methodology. J Hosp Med. 2006;1(1):48-56. https://doi.org/10.1002/jhm.6
2. Wachter RM, Goldman L. Zero to 50,000 — The 20th anniversary of the hospitalist. N Engl J Med. 2016;375(11):1009-1011. https://doi.org/10.1056/NEJMp1607958
3. Cho J, Jensen TP, Reierson K, et al. Recommendations on the use of ultrasound guidance for adult abdominal paracentesis: a position statement of the Society of Hospital Medicine. J Hosp Med. 2019;14:E7-E15. https://doi.org/10.12788/jhm.3095
4. Soni NJ, Franco-Sadud R, Kobaidze K, et al. Recommendations on the use of ultrasound guidance for adult lumbar puncture: a position statement of the Society of Hospital Medicine. J Hosp Med. 2019;14(10):591-601. https://doi.org/10.12788/jhm.3197
5. Dancel R, Schnobrich D, Puri N, et al. Recommendations on the use of ultrasound guidance for adult thoracentesis: a position statement of the Society of Hospital Medicine. J Hosp Med. 2018;13(2):126-135. https://doi.org/10.12788/jhm.2940
6. Franco-Sadud R, Schnobrich D, Mathews BK, et al. Recommendations on the use of ultrasound guidance for central and peripheral vascular access in adults: a position statement of the Society of Hospital Medicine. J Hosp Med. 2019;14:E1-E22. https://doi.org/10.12788/jhm.3287
7. Soni NJ, Schnobrich D, Mathews BK, et al. Point-of-care ultrasound for hospitalists: a position statement of the Society of Hospital Medicine. J Hosp Med. 2019;14:E1-E6. https://doi.org/10.12788/jhm.3079
8. Thakkar R, Wright SM, Alguire P, Wigton RS, Boonyasai RT. Procedures performed by hospitalist and non-hospitalist general internists. J Gen Intern Med. 2010;25(5):448-452. https://doi.org/10.1007/s11606-010-1284-2
9. Lucas BP, Asbury JK, Franco-Sadud R. Training future hospitalists with simulators: a needed step toward accessible, expertly performed bedside procedures. J Hosp Med. 2009;4(7):395-396. https://doi.org/10.1002/jhm.602
10. American Board of Internal Medicine. Policies and procedures for certification. Accessed December 3, 2020. https://www.abim.org/~/media/ABIM%20Public/Files/pdf/publications/certification-guides/policies-and-procedures.pdf
11. Myers LC. Toward preventing medical malpractice claims related to chest procedures. Ann Am Thorac Soc. 2020;17(6):776-779. https://doi.org/10.1513/AnnalsATS.201912-863RL
12. Tukey MH, Wiener RS. The impact of a medical procedure service on patient safety, procedure quality and resident training opportunities. J Gen Intern Med. 2014;29(3):485-490. https://doi.org/10.1007/s11606-013-2709-5
13. Brennan TA, Leape LL, Laird NM, et al. Incidence of adverse events and negligence in hospitalized patients. N Engl J Med. 1991;324(6):370-376. https://doi.org/10.1056/NEJM199102073240604
14. Leape LL, Brennan TA, Laird N, et al. The nature of adverse events in hospitalized patients. N Engl J Med. 1991;324(6):377-384. https://doi.org/10.1056/NEJM199102073240605
15. Myers LC, Gartland RM, Skillings J, et al. An examination of medical malpractice claims involving physician trainees. Acad Med. 2020;95(8):1215-1222. https://doi.org/10.1097/ACM.0000000000003117
16. Mercaldi CJ, Lanes SF. Ultrasound guidance decreases complications and improves the cost of care among patients undergoing thoracentesis and paracentesis. Chest. 2013;143(2):532-538. https://doi.org/10.1378/chest.12-0447
17. Vaisman A, Cram P. Procedural competence among faculty in academic health centers: challenges and future directions. Acad Med. 2017;92(1):31-34. https://doi.org/10.1097/ACM.0000000000001327
18. Nelson B. Hospitalists try to reclaim lead role in bedside procedures. The Hospitalist. March 2015. Accessed June 27, 2020. https://www.the-hospitalist.org/hospitalist/article/122571/hospitalists-try-reclaim-lead-role-bedside-procedures
19. Wigton RS, Alguire P; American College of Physicians. The declining number and variety of procedures done by general internists: a resurvey of members of the American College of Physicians. Ann Intern Med. 2007;146(5):355-360. https://doi.org/10.7326/0003-4819-146-5-200703060-00007
20. Duszak R Jr, Chatterjee AR, Schneider DA. National fluid shifts: fifteen-year trends in paracentesis and thoracentesis procedures. J Am Coll Radiol. 2010;7(11):859-864. https://doi.org/10.1016/j.jacr.2010.04.013
21. Gottumukkala RV, Prabhakar AM, Hemingway J, Hughes DR, Duszak R Jr. Disparities over time in volume, day of the week, and patient complexity between paracentesis and thoracentesis procedures performed by radiologists versus those performed by nonradiologists. J Vasc Interv Radiol. 2019;30(11):1769-1778.e1. https://doi.org/10.1016/j.jvir.2019.04.015
22. Kroll H, Duszak R Jr, Nsiah E, Hughes DR, Sumer S, Wintermark M. Trends in lumbar puncture over 2 decades: a dramatic shift to radiology. Am J Roentgenol. 2014;204(1):15-19. https://doi.org/10.2214/AJR.14.12622
23. Jensen T, Lai A, Mourad M. Can lessons from systems-based mastery learning for thoracentesis be translated to hospitalists? J Hosp Med. 2016;11(11):811-812. https://doi.org/10.1002/jhm.2655
24. Barsuk JH, Cohen ER, Feinglass J, McGaghie WC, Wayne DB. Clinical outcomes after bedside and interventional radiology paracentesis procedures. Am J Med. 2013;126(4):349-356. https://doi.org/10.1016/j.amjmed.2012.09.016
25. Barsuk JH, Feinglass J, Kozmic SE, Hohmann SF, Ganger D, Wayne DB. Specialties performing paracentesis procedures at university hospitals: implications for training and certification. J Hosp Med. 2014;9(3):162-168. https://doi.org/10.1002/jhm.2153
26. See KC, Ong V, Teoh CM, et al. Bedside pleural procedures by pulmonologists and non-pulmonologists: a 3-year safety audit. Respirology. 2014;19(3):396-402. https://doi.org/10.1111/resp.12244
27. Kozmic SE, Wayne DB, Feinglass J, Hohmann SF, Barsuk JH. Factors associated with inpatient thoracentesis procedure quality at university hospitals. Jt Comm J Qual Patient Saf. 2016;42(1):34-40. https://doi.org/10.1016/S1553-7250(16)42004-0
28. Berger MS, Divilov V, Paredes H, Sun E. Abdominal paracentesis: safety and efficacy comparing medicine resident bedside paracentesis vs. paracentesis performed by interventional radiology. J Clin Gastroenterol Hepatol. 2018;2(4). https://doi.org/10.21767/2575-7733.1000050
29. Kay C, Wozniak EM, Szabo A, Jackson JL. Examining invasive bedside procedure performance at an academic medical center. South Med J. 2016;109(7):402-407. https://doi.org/10.14423/SMJ.0000000000000485
30. Barsuk JH, Cohen ER, Feinglass J, et al. Cost savings of performing paracentesis procedures at the bedside after simulation-based education. Simul Healthc. 2014;9(5):312-318. https://doi.org/10.1097/SIH.0000000000000040
31. Barsuk JH, Cohen ER, Williams MV, et al. Simulation-based mastery learning for thoracentesis skills improves patient outcomes: a randomized trial. Acad Med. 2018;93(5):729-735. https://doi.org/10.1097/ACM.0000000000001965
32. Huang GC, McSparron JI, Balk EM, et al. Procedural instruction in invasive bedside procedures: a systematic review and meta-analysis of effective teaching approaches. BMJ Qual Saf. 2016;25(4):281-294. https://doi.org/10.1136/bmjqs-2014-003518
33. Brydges R, Stroud L, Wong BM, Holmboe ES, Imrie K, Hatala R. Core competencies or a competent core? a scoping review and realist synthesis of invasive bedside procedural skills training in internal medicine. Acad Med. 2017;92(11):1632-1643. https://doi.org/10.1097/ACM.0000000000001726
34. Brydges R, Hatala R, Zendejas B, Erwin PJ, Cook DA. Linking simulation-based educational assessments and patient-related outcomes: a systematic review and meta-analysis. Acad Med. 2015;90(2):246-256. https://doi.org/10.1097/ACM.0000000000000549
35. Barsuk JH, Cohen ER, Nguyen D, et al. Attending physician adherence to a 29-component central venous catheter bundle checklist during simulated procedures. Crit Care Med. 2016;44(10):1871-1881. https://doi.org/10.1097/CCM.0000000000001831
36. Crocker JT, Hale CP, Vanka A, Ricotta DN, McSparron JI, Huang GC. Raising the bar for procedural competency among hospitalists. Ann Intern Med. 2019;170(9):654-655. https://doi.org/10.7326/M18-3007
37. Barsuk JH, Cohen ER, Feinglass J, McGaghie WC, Wayne DB. Residents’ procedural experience does not ensure competence: a research synthesis. J Grad Med Educ. 2017;9(2):201-208. https://doi.org/10.4300/JGME-D-16-00426.1
38. Sawyer T, White M, Zaveri P, et al. Learn, see, practice, prove, do, maintain: an evidence-based pedagogical framework for procedural skill training in medicine. Acad Med. 2015;90(8):1025-1033. https://doi.org/10.1097/ACM.0000000000000734
39. Jensen T, Soni N, Tierney D, Lucas B. Hospital privileging practices for bedside procedures: a survey of hospitalist experts. J Hosp Med. 2017;12(10):836-839. https://doi.org/10.12788/jhm.2837
40. Lucas BP, Asbury JK, Wang Y, et al. Impact of a bedside procedure service on general medicine inpatients: a firm-based trial. J Hosp Med. 2007;2(3):143-149. https://doi.org/10.1002/jhm.159
41. Mourad M, Auerbach AD, Maselli J, Sliwka D. Patient satisfaction with a hospitalist procedure service: Is bedside procedure teaching reassuring to patients? J Hosp Med. 2011;6(4):219-224. https://doi.org/10.1002/jhm.856
42. Ault MJ, Rosen BT. Proceduralists — leading patient-safety initiatives. N Engl J Med. 2007;356(17):1789-1790. https://doi.org/10.1056/NEJMc063239
43. Obasi JU, Umpierrez De Reguero AP. Safety profile of bone marrow aspiration and biopsies performed by the hospitalist procedure service at an academic center: an observational study. Blood. 2019;134(suppl 1): 5848. https://doi.org/10.1182/blood-2019-121444
44. Gisondi MA, Regan L, Branzetti J, Hopson LR. More learners, finite resources, and the changing landscape of procedural training at the bedside. Acad Med. 2018;93(5):699-704. https://doi.org/10.1097/ACM.0000000000002062
45. McCormack J. The new proceduralists: Have they found their niche? American Medical News. September 17, 2007. Accessed August 30, 2020. https://amednews.com/article/20070917/business/309179994/4/
46. Lucas BP, Tierney DM, Jensen TP, et al; Society of Hospital Medicine Point-of-Care Ultrasound Task Force. Credentialing of hospitalists in ultrasound-guided bedside procedures: a position statement of the Society of Hospital Medicine. J Hosp Med. 2018;13(2):117-125. https://doi.org/10.12788/jhm.2917
47. Lenhard A, Moallem M, Marrie RA, Becker J, Garland A. An intervention to improve procedure education for internal medicine residents. J Gen Intern Med. 2008;23(3):288-293. https://doi.org/10.1007/s11606-008-0513-4
48. Mourad M, Ranji S, Sliwka D. A randomized controlled trial of the impact of a teaching procedure service on the training of internal medicine residents. J Grad Med Educ. 2012;4(2):170-175. https://doi.org/10.4300/JGME-D-11-00136.1
49. Montuno A, Hunt BR, Lee MM. Potential impact of a bedside procedure service on training procedurally competent hospitalists in a community-based residency program. J Community Hosp Intern Med Perspect. 2016;6(3):31054. https://doi.org/10.3402/jchimp.v6.31054
50. Smith CC, Gordon CE, Feller‐Kopman D, et al. Creation of an innovative inpatient medical procedure service and a method to evaluate house staff competency. J Gen Intern Med. 2004;19(5p2):510-513. https://doi.org/10.1111/j.1525-1497.2004.30161.x
51. Mourad M. Capsule commentary on Tukey et al., the impact of a medical procedure service on patient safety, procedure quality and resident training opportunities. J Gen Intern Med. 2014;29(3):518. https://doi.org/10.1007/s11606-013-2740-6
52. Halm EA, Lee C, Chassin MR. Is volume related to outcome in health care: a systematic review and methodologic critique of the literature. 2002. Database of Abstracts of Reviews of Effects (DARE): Quality-assessed Reviews . Accessed June 26, 2020. https://www.ncbi.nlm.nih.gov/books/NBK69189/
© 2021 Society of Hospital Medicine
Things We Do for No Reason™: Ova and Parasite Testing in Patients With Acute Diarrhea Arising During Hospitalization
Inspired by the ABIM Foundation’s Choosing Wisely® campaign, the “Things We Do for No Reason™” (TWDFNR) series reviews practices that have become common parts of hospital care but may provide little value to our patients. Practices reviewed in the TWDFNR series do not represent clear-cut conclusions or clinical practice standards but are meant as a starting place for research and active discussions among hospitalists and patients. We invite you to be part of that discussion.
CLINICAL SCENARIO
A 54-year-old immunocompetent man admitted to the hospital for non–ST-segment elevation myocardial infarction develops profuse watery diarrhea after his third day of admission. He denies prior episodes of diarrhea. He does not have any fevers, blood in the stool, recent travel, or antibiotic use. Vital signs include a blood pressure of 128/82 mm Hg, heart rate of 120 beats per minute, respiratory rate of 16 breaths per min, oxygen saturation of 100% on room air, and temperature of 36.9 °C. His physical examination is normal, without signs of abdominal tenderness, rebound, or guarding. Complete blood count is normal, without eosinophilia. The comprehensive metabolic panel shows mild hypokalemia of 3.3 mmol/L. The hospitalist resuscitates him with normal saline, provides oral potassium repletion, and orders a stool culture, Clostridioides difficile test, and an ova and parasite (O&P) test. Loperamide and time resolve his symptoms in 2 days. Results of his stool culture, C difficile, and O&P tests return negative in 3 days.
BACKGROUND
Acute diarrhea is a common complaint in both inpatient and outpatient settings. It is defined as the passage of three or more liquid or poorly formed stools in a 24-hour period lasting less than 14 days. Persistent diarrhea lasts from 14 to 29 days, while chronic diarrhea lasts longer than 30 days. There are 47.8 million cases of acute diarrhea per year in the United States, costing $150 million in US health expenditures.1 Viral pathogens remain the most common cause of acute diarrhea in the United States.1,2 Standard O&P testing consists of applying a stool sample to a slide with either saline or iodine (wet mount) and evaluating the specimen with a microscope.
WHY YOU MIGHT THINK O&P TESTING IS HELPFUL
Giardia and Cryptosporidium remain the most commonly implicated parasitic pathogens in acute diarrheal episodes in the United States.3 Cryptosporidium cases in the United States range from 2.2 to 3.9 per 100,000 persons,4 and Giardia cases in the United States range from 5.8 to 6.4 per 100,000 persons.5 To avoid missing potentially treatable causes, providers often order O&P tests reflexively as part of a standard workup for acute diarrhea. From 2001 to 2007, Associated Regional and University Pathologists Laboratories experienced a 379% increase in O&P testing.6 Many providers ordering these tests assume that standard O&P testing covers most, if not all, parasites and that a negative test will rule out a parasitic cause of disease. Furthermore, providers are unaware that more sensitive tests to detect certain parasites have replaced standard O&P microscopy.3
WHY O&P TESTING IS USUALLY UNNECESSARY
Most hospitalized patients do not have a parasitic infection
In a review of 5,681 completed O&P tests from a tertiary care medical center in Canada over a 5-year period, only 1.4% of tests were positive.7 In a 3-year retrospective analysis of stool samples obtained after 3 days of hospitalization, positive results were found in only 1 of 191 stool cultures and in 0 of 90 O&P samples.8 Current practice guidelines suggest not testing patients with stool studies in cases of acute diarrhea lasting less than 7 days in the absence of significant risk factors for parasitic disease because it has been shown to be a rare event and most cases will self-resolve with supportive care only.1,9
The stool O&P test has low sensitivity
Classically ordered stool O&P tests have low sensitivity for the detection of Giardia and Cryptosporidium, the two most common parasites in the United States.6,10,11 O&P studies detect Giardia in only 66% to 79% of specimen samples and Cryptosporidium in less than 5% of specimens. Diagnostic yields can be improved with the use of special stains such as modified acid-fast stain (MAF).6 Despite use of MAF staining, though, sensitivity for Cryptosporidium detection has remained at only 55%.12 Additionally, several studies have shown that physicians are generally unaware of the test characteristics of stool O&P tests and they do not know to order the newer more sensitive enzyme immunoassays (EIA) or direct fluorescent antibody (DFA) tests even in situations when testing for a parasitic infection is appropriate.10,11,13,14 As stated earlier, a parasitic infection without significant risk factors is a rare event. A negative test with low or moderate sensitivity is not additive to such a low clinical suspicion because it does not significantly change posttest probability.
Testing can have adverse consequences
In addition to the low yield, O&P testing is technically complex, is time intensive, and requires an experienced technician’s interpretation. Inappropriate testing increases the cost of care and staff workload without much benefit.6 As such, some institutions have opted to send the O&P tests to labs with experienced technicians. Other institutions have adopted a restrictive stool O&P testing approach that reduces healthcare time and costs and improves the rate of positive tests.13,15 A study at a single tertiary care medical center demonstrated an estimated cost savings of $21,931 annually by implementing a computer-based algorithm to restrict testing for stool cultures and O&P tests to patients with higher probabilities of infection.15 The algorithm directed clinicians to provide further information when attempting to order stool culture, O&P, or other specific stool tests. For patients hospitalized for more than 3 days, the system did not allow certain testing. For patients with worrisome features like severe symptoms or an immunocompromised state, the algorithm directed the clinician to place an infectious disease or gastroenterology consult rather than order stool tests. Decreased laboratory costs of all stool studies (including O&P) in adult inpatient locations led to the cost savings. Additionally, the study authors felt that they likely underestimated the cost savings because they did not account for other expenses in the analysis, such as nursing workload and supplies.15
WHAT YOU SHOULD DO INSTEAD
Clinicians should evaluate patients on a case-by-case basis and determine the need to test based on the presence of high-risk features (Table). 
When performing parasitic testing in patients without a recent travel history but with other high-risk features, test for the most prevalent parasites in the United States (ie, Giardia, Cryptosporidium, and Entamoeba histolytica) with DFA or EIA tests.3 DFA testing for Giardia is 99% sensitive.12 In patients with symptoms lasting more than 7 days and recent travel, in addition to the above DFA/EIA tests, perform O&P testing with wet mount, modified acid-fast bacilli stain to detect rare parasites such as helminths, Strongyloides, Cyclospora, and Cystoisospora.3 In patients who live or travel to endemic areas (about 10% of traveler’s diarrhea is caused by parasitic infections), have unexplained eosinophilia, or are part of a community outbreak (eg, childcare institutions or drinking water/food outbreaks), test for Giardia, Cryptosporidium, Cyclospora, Cystoisospora, Entamoeba histolytica, and Isospora belli.9 In addition, among patients with AIDS or immunosuppression, testing should include assays for Microsporidia, Strongyloides, and Mycobacterium avium complex (Figure).9,16 Newer tests, such as the multiplex real-time polymerase chain reaction assay, can also simultaneously detect Entamoeba histolytica, Giardia lamblia, and Cryptosporidium parvum. For more information on parasitic testing, we suggest reading the review article “Beyond O&P times three.”3 It is important to familiarize yourself with the parasitic tests available at your respective clinics/hospital so the optimal test can be used.
RECOMMENDATIONS
- Prescribe a trial of “wait and see” for patients without high-risk features for parasitic disease.
- Test patients with high-risk features for parasitic disease by utilizing targeted testing.
- For patients with high-risk features but no travel history, first perform DFA, EIA, or multiplex real-time polymerase chain reaction testing to evaluate for Giardia, Cryptosporidium, and Entamoeba histolytica.
- If DFA/EIA testing is negative, obtain O&P tests with and without stains, such as acid-fast bacilli, for detection of other rare parasites.
CONCLUSION
Hospitalists should risk-stratify patients to determine when O&P testing is appropriate. Employ more targeted testing, especially use of DFA/EIA tests when evaluating for parasites. Avoid parasitic testing if symptoms have lasted less than 7 days and the patient has no other high-risk features. Become familiar with the tests available at your institution and their sensitivities. As in our clinical scenario, most acute cases of diarrhea resolve without intervention and should be managed and treated conservatively.
Do you think this is a low-value practice? Is this truly a “Thing We Do for No Reason”? Share what you do in your practice and join in the conversation online by retweeting it on Twitter (#TWDFNR) and liking it on Facebook. We invite you to propose ideas for other “Things We Do for No Reason ™ ” topics by emailing [email protected] .
Acknowledgments
The authors thank Dr Lenny Feldman for his assistance with editing the manuscript.
1. Riddle MS, DuPont HL, Connor BA. ACG clinical guideline: diagnosis, treatment, and prevention of acute diarrheal infections in adults. Am J Gastroenterol. 2016;111(5):602-622. https://doi.org/10.1038/ajg.2016.126
2. DuPont HL. Acute infectious diarrhea in immunocompetent adults. N Engl J Med. 2014;370(16):1532-1540. https://doi.org/10.1056/nejmra1301069
3. Mohapatra S, Singh DP, Alcid D, Pitchumoni CS. Beyond O&P times three. Am J Gastroenterol. 2018;113(6):805-818. https://doi.org/10.1038/s41395-018-0083-y
4. Painter JE, Hlavsa MC, Collier SA, Xiao L, Yoder JS. Cryptosporidiosis surveillance -- United States, 2011-2012. MMWR Suppl. 2015;64(3):1-14.
5. Painter JE, Gargano JW, Collier SA, Yoder JS. Giardiasis surveillance -- United States, 2011-2012. MMWR Suppl. 2015;64(3):15-25.
6. Polage CR, Stoddard GJ, Rolfs RT, Petti CA. Physician use of parasite tests in the United States from 1997 to 2006 and in a Utah Cryptosporidium outbreak in 2007. J Clin Microbiol. 2011;49(2):591-596. https://doi.org/10.1128/jcm.01806-10
7. Mosli M, Gregor J, Chande N, Lannigan R. Nonutility of routine testing of stool for ova and parasites in a tertiary care Canadian centre. Can J Microbiol. 2012;58(5):653-659. https://doi.org/10.1139/w2012-039
8. Siegel DL, Edelstein PH, Nachamkin I. Inappropriate testing for diarrheal diseases in the hospital. JAMA. 1990;263(7):979-982.
9. Shane AL, Mody RK, Crump JA, et al. 2017 Infectious Diseases Society of America clinical practice guidelines for the diagnosis and management of infectious diarrhea. Clin Infect Dis. 2017;65(12):e45-e80. https://doi.org/10.1093/cid/cix669
10. Hennessy TW, Marcus R, Deneen V, et al. Survey of physician diagnostic practices for patients with acute diarrhea: clinical and public health implications. Clin Infect Dis. 2004;38 (Suppl 3):S203-S211. https://doi.org/10.1086/381588
11. Morin CA, Roberts CL, Mshar PA, Addiss DG, Hadler JL. What do physicians know about cryptosporidiosis? a survey of Connecticut physicians. Arch Intern Med. 1997;157(9):1017-1022.
12. McHardy IH, Wu M, Shimizu-Cohen R, Couturier MR, Humphries RM. Detection of intestinal protozoa in the clinical laboratory. J Clin Microbiol. 2014;52(3):712-720. https://doi.org/10.1128/jcm.02877-13
13. Valenstein P, Pfaller M, Yungbluth M. The use and abuse of routine stool microbiology: a College of American Pathologists Q-probes study of 601 institutions. Arch Pathol Lab Med. 1996;120(2):206-211.
14. Jones JL, Lopez A, Wahlquist SP, Nadle J, Wilson M; Emerging Infections Program FoodNet Working Group. Survey of clinical laboratory practices for parasitic diseases. Clin Infect Dis. 2004;38(Suppl 3):S198-S202. https://doi.org/10.1086/381587
15. Tewell CE, Talbot TR, Nelson GE, et al. Reducing inappropriate testing for the evaluation of diarrhea among hospitalized patients. Am J Med. 2018;131(2):193-199.e1. https://doi.org/10.1016/j.amjmed.2017.10.006
16. Thielman NM, Guerrant RL. Clinical practice. acute infectious diarrhea. N Engl J Med. 2004;350(1):38-47. https://doi.org/10.1056/nejmcp031534
17. Marti H, Koella JC. Multiple stool examinations for ova and parasites and rate of false-negative results. J Clin Microbiol. 1993;31(11):3044-3045. https://doi.org/10.1128/jcm.31.11.3044-3045.1993
Inspired by the ABIM Foundation’s Choosing Wisely® campaign, the “Things We Do for No Reason™” (TWDFNR) series reviews practices that have become common parts of hospital care but may provide little value to our patients. Practices reviewed in the TWDFNR series do not represent clear-cut conclusions or clinical practice standards but are meant as a starting place for research and active discussions among hospitalists and patients. We invite you to be part of that discussion.
CLINICAL SCENARIO
A 54-year-old immunocompetent man admitted to the hospital for non–ST-segment elevation myocardial infarction develops profuse watery diarrhea after his third day of admission. He denies prior episodes of diarrhea. He does not have any fevers, blood in the stool, recent travel, or antibiotic use. Vital signs include a blood pressure of 128/82 mm Hg, heart rate of 120 beats per minute, respiratory rate of 16 breaths per min, oxygen saturation of 100% on room air, and temperature of 36.9 °C. His physical examination is normal, without signs of abdominal tenderness, rebound, or guarding. Complete blood count is normal, without eosinophilia. The comprehensive metabolic panel shows mild hypokalemia of 3.3 mmol/L. The hospitalist resuscitates him with normal saline, provides oral potassium repletion, and orders a stool culture, Clostridioides difficile test, and an ova and parasite (O&P) test. Loperamide and time resolve his symptoms in 2 days. Results of his stool culture, C difficile, and O&P tests return negative in 3 days.
BACKGROUND
Acute diarrhea is a common complaint in both inpatient and outpatient settings. It is defined as the passage of three or more liquid or poorly formed stools in a 24-hour period lasting less than 14 days. Persistent diarrhea lasts from 14 to 29 days, while chronic diarrhea lasts longer than 30 days. There are 47.8 million cases of acute diarrhea per year in the United States, costing $150 million in US health expenditures.1 Viral pathogens remain the most common cause of acute diarrhea in the United States.1,2 Standard O&P testing consists of applying a stool sample to a slide with either saline or iodine (wet mount) and evaluating the specimen with a microscope.
WHY YOU MIGHT THINK O&P TESTING IS HELPFUL
Giardia and Cryptosporidium remain the most commonly implicated parasitic pathogens in acute diarrheal episodes in the United States.3 Cryptosporidium cases in the United States range from 2.2 to 3.9 per 100,000 persons,4 and Giardia cases in the United States range from 5.8 to 6.4 per 100,000 persons.5 To avoid missing potentially treatable causes, providers often order O&P tests reflexively as part of a standard workup for acute diarrhea. From 2001 to 2007, Associated Regional and University Pathologists Laboratories experienced a 379% increase in O&P testing.6 Many providers ordering these tests assume that standard O&P testing covers most, if not all, parasites and that a negative test will rule out a parasitic cause of disease. Furthermore, providers are unaware that more sensitive tests to detect certain parasites have replaced standard O&P microscopy.3
WHY O&P TESTING IS USUALLY UNNECESSARY
Most hospitalized patients do not have a parasitic infection
In a review of 5,681 completed O&P tests from a tertiary care medical center in Canada over a 5-year period, only 1.4% of tests were positive.7 In a 3-year retrospective analysis of stool samples obtained after 3 days of hospitalization, positive results were found in only 1 of 191 stool cultures and in 0 of 90 O&P samples.8 Current practice guidelines suggest not testing patients with stool studies in cases of acute diarrhea lasting less than 7 days in the absence of significant risk factors for parasitic disease because it has been shown to be a rare event and most cases will self-resolve with supportive care only.1,9
The stool O&P test has low sensitivity
Classically ordered stool O&P tests have low sensitivity for the detection of Giardia and Cryptosporidium, the two most common parasites in the United States.6,10,11 O&P studies detect Giardia in only 66% to 79% of specimen samples and Cryptosporidium in less than 5% of specimens. Diagnostic yields can be improved with the use of special stains such as modified acid-fast stain (MAF).6 Despite use of MAF staining, though, sensitivity for Cryptosporidium detection has remained at only 55%.12 Additionally, several studies have shown that physicians are generally unaware of the test characteristics of stool O&P tests and they do not know to order the newer more sensitive enzyme immunoassays (EIA) or direct fluorescent antibody (DFA) tests even in situations when testing for a parasitic infection is appropriate.10,11,13,14 As stated earlier, a parasitic infection without significant risk factors is a rare event. A negative test with low or moderate sensitivity is not additive to such a low clinical suspicion because it does not significantly change posttest probability.
Testing can have adverse consequences
In addition to the low yield, O&P testing is technically complex, is time intensive, and requires an experienced technician’s interpretation. Inappropriate testing increases the cost of care and staff workload without much benefit.6 As such, some institutions have opted to send the O&P tests to labs with experienced technicians. Other institutions have adopted a restrictive stool O&P testing approach that reduces healthcare time and costs and improves the rate of positive tests.13,15 A study at a single tertiary care medical center demonstrated an estimated cost savings of $21,931 annually by implementing a computer-based algorithm to restrict testing for stool cultures and O&P tests to patients with higher probabilities of infection.15 The algorithm directed clinicians to provide further information when attempting to order stool culture, O&P, or other specific stool tests. For patients hospitalized for more than 3 days, the system did not allow certain testing. For patients with worrisome features like severe symptoms or an immunocompromised state, the algorithm directed the clinician to place an infectious disease or gastroenterology consult rather than order stool tests. Decreased laboratory costs of all stool studies (including O&P) in adult inpatient locations led to the cost savings. Additionally, the study authors felt that they likely underestimated the cost savings because they did not account for other expenses in the analysis, such as nursing workload and supplies.15
WHAT YOU SHOULD DO INSTEAD
Clinicians should evaluate patients on a case-by-case basis and determine the need to test based on the presence of high-risk features (Table). 
When performing parasitic testing in patients without a recent travel history but with other high-risk features, test for the most prevalent parasites in the United States (ie, Giardia, Cryptosporidium, and Entamoeba histolytica) with DFA or EIA tests.3 DFA testing for Giardia is 99% sensitive.12 In patients with symptoms lasting more than 7 days and recent travel, in addition to the above DFA/EIA tests, perform O&P testing with wet mount, modified acid-fast bacilli stain to detect rare parasites such as helminths, Strongyloides, Cyclospora, and Cystoisospora.3 In patients who live or travel to endemic areas (about 10% of traveler’s diarrhea is caused by parasitic infections), have unexplained eosinophilia, or are part of a community outbreak (eg, childcare institutions or drinking water/food outbreaks), test for Giardia, Cryptosporidium, Cyclospora, Cystoisospora, Entamoeba histolytica, and Isospora belli.9 In addition, among patients with AIDS or immunosuppression, testing should include assays for Microsporidia, Strongyloides, and Mycobacterium avium complex (Figure).9,16 Newer tests, such as the multiplex real-time polymerase chain reaction assay, can also simultaneously detect Entamoeba histolytica, Giardia lamblia, and Cryptosporidium parvum. For more information on parasitic testing, we suggest reading the review article “Beyond O&P times three.”3 It is important to familiarize yourself with the parasitic tests available at your respective clinics/hospital so the optimal test can be used.

RECOMMENDATIONS
- Prescribe a trial of “wait and see” for patients without high-risk features for parasitic disease.
- Test patients with high-risk features for parasitic disease by utilizing targeted testing.
- For patients with high-risk features but no travel history, first perform DFA, EIA, or multiplex real-time polymerase chain reaction testing to evaluate for Giardia, Cryptosporidium, and Entamoeba histolytica.
- If DFA/EIA testing is negative, obtain O&P tests with and without stains, such as acid-fast bacilli, for detection of other rare parasites.
CONCLUSION
Hospitalists should risk-stratify patients to determine when O&P testing is appropriate. Employ more targeted testing, especially use of DFA/EIA tests when evaluating for parasites. Avoid parasitic testing if symptoms have lasted less than 7 days and the patient has no other high-risk features. Become familiar with the tests available at your institution and their sensitivities. As in our clinical scenario, most acute cases of diarrhea resolve without intervention and should be managed and treated conservatively.
Do you think this is a low-value practice? Is this truly a “Thing We Do for No Reason”? Share what you do in your practice and join in the conversation online by retweeting it on Twitter (#TWDFNR) and liking it on Facebook. We invite you to propose ideas for other “Things We Do for No Reason ™ ” topics by emailing [email protected] .
Acknowledgments
The authors thank Dr Lenny Feldman for his assistance with editing the manuscript.
Inspired by the ABIM Foundation’s Choosing Wisely® campaign, the “Things We Do for No Reason™” (TWDFNR) series reviews practices that have become common parts of hospital care but may provide little value to our patients. Practices reviewed in the TWDFNR series do not represent clear-cut conclusions or clinical practice standards but are meant as a starting place for research and active discussions among hospitalists and patients. We invite you to be part of that discussion.
CLINICAL SCENARIO
A 54-year-old immunocompetent man admitted to the hospital for non–ST-segment elevation myocardial infarction develops profuse watery diarrhea after his third day of admission. He denies prior episodes of diarrhea. He does not have any fevers, blood in the stool, recent travel, or antibiotic use. Vital signs include a blood pressure of 128/82 mm Hg, heart rate of 120 beats per minute, respiratory rate of 16 breaths per min, oxygen saturation of 100% on room air, and temperature of 36.9 °C. His physical examination is normal, without signs of abdominal tenderness, rebound, or guarding. Complete blood count is normal, without eosinophilia. The comprehensive metabolic panel shows mild hypokalemia of 3.3 mmol/L. The hospitalist resuscitates him with normal saline, provides oral potassium repletion, and orders a stool culture, Clostridioides difficile test, and an ova and parasite (O&P) test. Loperamide and time resolve his symptoms in 2 days. Results of his stool culture, C difficile, and O&P tests return negative in 3 days.
BACKGROUND
Acute diarrhea is a common complaint in both inpatient and outpatient settings. It is defined as the passage of three or more liquid or poorly formed stools in a 24-hour period lasting less than 14 days. Persistent diarrhea lasts from 14 to 29 days, while chronic diarrhea lasts longer than 30 days. There are 47.8 million cases of acute diarrhea per year in the United States, costing $150 million in US health expenditures.1 Viral pathogens remain the most common cause of acute diarrhea in the United States.1,2 Standard O&P testing consists of applying a stool sample to a slide with either saline or iodine (wet mount) and evaluating the specimen with a microscope.
WHY YOU MIGHT THINK O&P TESTING IS HELPFUL
Giardia and Cryptosporidium remain the most commonly implicated parasitic pathogens in acute diarrheal episodes in the United States.3 Cryptosporidium cases in the United States range from 2.2 to 3.9 per 100,000 persons,4 and Giardia cases in the United States range from 5.8 to 6.4 per 100,000 persons.5 To avoid missing potentially treatable causes, providers often order O&P tests reflexively as part of a standard workup for acute diarrhea. From 2001 to 2007, Associated Regional and University Pathologists Laboratories experienced a 379% increase in O&P testing.6 Many providers ordering these tests assume that standard O&P testing covers most, if not all, parasites and that a negative test will rule out a parasitic cause of disease. Furthermore, providers are unaware that more sensitive tests to detect certain parasites have replaced standard O&P microscopy.3
WHY O&P TESTING IS USUALLY UNNECESSARY
Most hospitalized patients do not have a parasitic infection
In a review of 5,681 completed O&P tests from a tertiary care medical center in Canada over a 5-year period, only 1.4% of tests were positive.7 In a 3-year retrospective analysis of stool samples obtained after 3 days of hospitalization, positive results were found in only 1 of 191 stool cultures and in 0 of 90 O&P samples.8 Current practice guidelines suggest not testing patients with stool studies in cases of acute diarrhea lasting less than 7 days in the absence of significant risk factors for parasitic disease because it has been shown to be a rare event and most cases will self-resolve with supportive care only.1,9
The stool O&P test has low sensitivity
Classically ordered stool O&P tests have low sensitivity for the detection of Giardia and Cryptosporidium, the two most common parasites in the United States.6,10,11 O&P studies detect Giardia in only 66% to 79% of specimen samples and Cryptosporidium in less than 5% of specimens. Diagnostic yields can be improved with the use of special stains such as modified acid-fast stain (MAF).6 Despite use of MAF staining, though, sensitivity for Cryptosporidium detection has remained at only 55%.12 Additionally, several studies have shown that physicians are generally unaware of the test characteristics of stool O&P tests and they do not know to order the newer more sensitive enzyme immunoassays (EIA) or direct fluorescent antibody (DFA) tests even in situations when testing for a parasitic infection is appropriate.10,11,13,14 As stated earlier, a parasitic infection without significant risk factors is a rare event. A negative test with low or moderate sensitivity is not additive to such a low clinical suspicion because it does not significantly change posttest probability.
Testing can have adverse consequences
In addition to the low yield, O&P testing is technically complex, is time intensive, and requires an experienced technician’s interpretation. Inappropriate testing increases the cost of care and staff workload without much benefit.6 As such, some institutions have opted to send the O&P tests to labs with experienced technicians. Other institutions have adopted a restrictive stool O&P testing approach that reduces healthcare time and costs and improves the rate of positive tests.13,15 A study at a single tertiary care medical center demonstrated an estimated cost savings of $21,931 annually by implementing a computer-based algorithm to restrict testing for stool cultures and O&P tests to patients with higher probabilities of infection.15 The algorithm directed clinicians to provide further information when attempting to order stool culture, O&P, or other specific stool tests. For patients hospitalized for more than 3 days, the system did not allow certain testing. For patients with worrisome features like severe symptoms or an immunocompromised state, the algorithm directed the clinician to place an infectious disease or gastroenterology consult rather than order stool tests. Decreased laboratory costs of all stool studies (including O&P) in adult inpatient locations led to the cost savings. Additionally, the study authors felt that they likely underestimated the cost savings because they did not account for other expenses in the analysis, such as nursing workload and supplies.15
WHAT YOU SHOULD DO INSTEAD
Clinicians should evaluate patients on a case-by-case basis and determine the need to test based on the presence of high-risk features (Table). 
When performing parasitic testing in patients without a recent travel history but with other high-risk features, test for the most prevalent parasites in the United States (ie, Giardia, Cryptosporidium, and Entamoeba histolytica) with DFA or EIA tests.3 DFA testing for Giardia is 99% sensitive.12 In patients with symptoms lasting more than 7 days and recent travel, in addition to the above DFA/EIA tests, perform O&P testing with wet mount, modified acid-fast bacilli stain to detect rare parasites such as helminths, Strongyloides, Cyclospora, and Cystoisospora.3 In patients who live or travel to endemic areas (about 10% of traveler’s diarrhea is caused by parasitic infections), have unexplained eosinophilia, or are part of a community outbreak (eg, childcare institutions or drinking water/food outbreaks), test for Giardia, Cryptosporidium, Cyclospora, Cystoisospora, Entamoeba histolytica, and Isospora belli.9 In addition, among patients with AIDS or immunosuppression, testing should include assays for Microsporidia, Strongyloides, and Mycobacterium avium complex (Figure).9,16 Newer tests, such as the multiplex real-time polymerase chain reaction assay, can also simultaneously detect Entamoeba histolytica, Giardia lamblia, and Cryptosporidium parvum. For more information on parasitic testing, we suggest reading the review article “Beyond O&P times three.”3 It is important to familiarize yourself with the parasitic tests available at your respective clinics/hospital so the optimal test can be used.

RECOMMENDATIONS
- Prescribe a trial of “wait and see” for patients without high-risk features for parasitic disease.
- Test patients with high-risk features for parasitic disease by utilizing targeted testing.
- For patients with high-risk features but no travel history, first perform DFA, EIA, or multiplex real-time polymerase chain reaction testing to evaluate for Giardia, Cryptosporidium, and Entamoeba histolytica.
- If DFA/EIA testing is negative, obtain O&P tests with and without stains, such as acid-fast bacilli, for detection of other rare parasites.
CONCLUSION
Hospitalists should risk-stratify patients to determine when O&P testing is appropriate. Employ more targeted testing, especially use of DFA/EIA tests when evaluating for parasites. Avoid parasitic testing if symptoms have lasted less than 7 days and the patient has no other high-risk features. Become familiar with the tests available at your institution and their sensitivities. As in our clinical scenario, most acute cases of diarrhea resolve without intervention and should be managed and treated conservatively.
Do you think this is a low-value practice? Is this truly a “Thing We Do for No Reason”? Share what you do in your practice and join in the conversation online by retweeting it on Twitter (#TWDFNR) and liking it on Facebook. We invite you to propose ideas for other “Things We Do for No Reason ™ ” topics by emailing [email protected] .
Acknowledgments
The authors thank Dr Lenny Feldman for his assistance with editing the manuscript.
1. Riddle MS, DuPont HL, Connor BA. ACG clinical guideline: diagnosis, treatment, and prevention of acute diarrheal infections in adults. Am J Gastroenterol. 2016;111(5):602-622. https://doi.org/10.1038/ajg.2016.126
2. DuPont HL. Acute infectious diarrhea in immunocompetent adults. N Engl J Med. 2014;370(16):1532-1540. https://doi.org/10.1056/nejmra1301069
3. Mohapatra S, Singh DP, Alcid D, Pitchumoni CS. Beyond O&P times three. Am J Gastroenterol. 2018;113(6):805-818. https://doi.org/10.1038/s41395-018-0083-y
4. Painter JE, Hlavsa MC, Collier SA, Xiao L, Yoder JS. Cryptosporidiosis surveillance -- United States, 2011-2012. MMWR Suppl. 2015;64(3):1-14.
5. Painter JE, Gargano JW, Collier SA, Yoder JS. Giardiasis surveillance -- United States, 2011-2012. MMWR Suppl. 2015;64(3):15-25.
6. Polage CR, Stoddard GJ, Rolfs RT, Petti CA. Physician use of parasite tests in the United States from 1997 to 2006 and in a Utah Cryptosporidium outbreak in 2007. J Clin Microbiol. 2011;49(2):591-596. https://doi.org/10.1128/jcm.01806-10
7. Mosli M, Gregor J, Chande N, Lannigan R. Nonutility of routine testing of stool for ova and parasites in a tertiary care Canadian centre. Can J Microbiol. 2012;58(5):653-659. https://doi.org/10.1139/w2012-039
8. Siegel DL, Edelstein PH, Nachamkin I. Inappropriate testing for diarrheal diseases in the hospital. JAMA. 1990;263(7):979-982.
9. Shane AL, Mody RK, Crump JA, et al. 2017 Infectious Diseases Society of America clinical practice guidelines for the diagnosis and management of infectious diarrhea. Clin Infect Dis. 2017;65(12):e45-e80. https://doi.org/10.1093/cid/cix669
10. Hennessy TW, Marcus R, Deneen V, et al. Survey of physician diagnostic practices for patients with acute diarrhea: clinical and public health implications. Clin Infect Dis. 2004;38 (Suppl 3):S203-S211. https://doi.org/10.1086/381588
11. Morin CA, Roberts CL, Mshar PA, Addiss DG, Hadler JL. What do physicians know about cryptosporidiosis? a survey of Connecticut physicians. Arch Intern Med. 1997;157(9):1017-1022.
12. McHardy IH, Wu M, Shimizu-Cohen R, Couturier MR, Humphries RM. Detection of intestinal protozoa in the clinical laboratory. J Clin Microbiol. 2014;52(3):712-720. https://doi.org/10.1128/jcm.02877-13
13. Valenstein P, Pfaller M, Yungbluth M. The use and abuse of routine stool microbiology: a College of American Pathologists Q-probes study of 601 institutions. Arch Pathol Lab Med. 1996;120(2):206-211.
14. Jones JL, Lopez A, Wahlquist SP, Nadle J, Wilson M; Emerging Infections Program FoodNet Working Group. Survey of clinical laboratory practices for parasitic diseases. Clin Infect Dis. 2004;38(Suppl 3):S198-S202. https://doi.org/10.1086/381587
15. Tewell CE, Talbot TR, Nelson GE, et al. Reducing inappropriate testing for the evaluation of diarrhea among hospitalized patients. Am J Med. 2018;131(2):193-199.e1. https://doi.org/10.1016/j.amjmed.2017.10.006
16. Thielman NM, Guerrant RL. Clinical practice. acute infectious diarrhea. N Engl J Med. 2004;350(1):38-47. https://doi.org/10.1056/nejmcp031534
17. Marti H, Koella JC. Multiple stool examinations for ova and parasites and rate of false-negative results. J Clin Microbiol. 1993;31(11):3044-3045. https://doi.org/10.1128/jcm.31.11.3044-3045.1993
1. Riddle MS, DuPont HL, Connor BA. ACG clinical guideline: diagnosis, treatment, and prevention of acute diarrheal infections in adults. Am J Gastroenterol. 2016;111(5):602-622. https://doi.org/10.1038/ajg.2016.126
2. DuPont HL. Acute infectious diarrhea in immunocompetent adults. N Engl J Med. 2014;370(16):1532-1540. https://doi.org/10.1056/nejmra1301069
3. Mohapatra S, Singh DP, Alcid D, Pitchumoni CS. Beyond O&P times three. Am J Gastroenterol. 2018;113(6):805-818. https://doi.org/10.1038/s41395-018-0083-y
4. Painter JE, Hlavsa MC, Collier SA, Xiao L, Yoder JS. Cryptosporidiosis surveillance -- United States, 2011-2012. MMWR Suppl. 2015;64(3):1-14.
5. Painter JE, Gargano JW, Collier SA, Yoder JS. Giardiasis surveillance -- United States, 2011-2012. MMWR Suppl. 2015;64(3):15-25.
6. Polage CR, Stoddard GJ, Rolfs RT, Petti CA. Physician use of parasite tests in the United States from 1997 to 2006 and in a Utah Cryptosporidium outbreak in 2007. J Clin Microbiol. 2011;49(2):591-596. https://doi.org/10.1128/jcm.01806-10
7. Mosli M, Gregor J, Chande N, Lannigan R. Nonutility of routine testing of stool for ova and parasites in a tertiary care Canadian centre. Can J Microbiol. 2012;58(5):653-659. https://doi.org/10.1139/w2012-039
8. Siegel DL, Edelstein PH, Nachamkin I. Inappropriate testing for diarrheal diseases in the hospital. JAMA. 1990;263(7):979-982.
9. Shane AL, Mody RK, Crump JA, et al. 2017 Infectious Diseases Society of America clinical practice guidelines for the diagnosis and management of infectious diarrhea. Clin Infect Dis. 2017;65(12):e45-e80. https://doi.org/10.1093/cid/cix669
10. Hennessy TW, Marcus R, Deneen V, et al. Survey of physician diagnostic practices for patients with acute diarrhea: clinical and public health implications. Clin Infect Dis. 2004;38 (Suppl 3):S203-S211. https://doi.org/10.1086/381588
11. Morin CA, Roberts CL, Mshar PA, Addiss DG, Hadler JL. What do physicians know about cryptosporidiosis? a survey of Connecticut physicians. Arch Intern Med. 1997;157(9):1017-1022.
12. McHardy IH, Wu M, Shimizu-Cohen R, Couturier MR, Humphries RM. Detection of intestinal protozoa in the clinical laboratory. J Clin Microbiol. 2014;52(3):712-720. https://doi.org/10.1128/jcm.02877-13
13. Valenstein P, Pfaller M, Yungbluth M. The use and abuse of routine stool microbiology: a College of American Pathologists Q-probes study of 601 institutions. Arch Pathol Lab Med. 1996;120(2):206-211.
14. Jones JL, Lopez A, Wahlquist SP, Nadle J, Wilson M; Emerging Infections Program FoodNet Working Group. Survey of clinical laboratory practices for parasitic diseases. Clin Infect Dis. 2004;38(Suppl 3):S198-S202. https://doi.org/10.1086/381587
15. Tewell CE, Talbot TR, Nelson GE, et al. Reducing inappropriate testing for the evaluation of diarrhea among hospitalized patients. Am J Med. 2018;131(2):193-199.e1. https://doi.org/10.1016/j.amjmed.2017.10.006
16. Thielman NM, Guerrant RL. Clinical practice. acute infectious diarrhea. N Engl J Med. 2004;350(1):38-47. https://doi.org/10.1056/nejmcp031534
17. Marti H, Koella JC. Multiple stool examinations for ova and parasites and rate of false-negative results. J Clin Microbiol. 1993;31(11):3044-3045. https://doi.org/10.1128/jcm.31.11.3044-3045.1993
© 2021 Society of Hospital Medicine
Limiting Patient Autonomy: Mandatory COVID-19 Diagnostic Testing
Despite the important clinical and public health implications of a COVID-19 diagnosis, respect for autonomy allows patients to decline testing without explanation and with impunity. Whether physicians believe a test is indicated for clinical care of an individual patient, prevention of nosocomial transmission, or the greater public health, patients may refuse. Such refusals may be increasing due to quarantine requirements, concerns regarding contact tracing, and the persistent absence of a curative treatment.1,2 Mass screening of all healthcare workers (HCWs) is being considered to prevent hospital transmission,3 and universal screening in nursing homes has thwarted outbreaks while providing data to facilitate resource allocation.4 Given these circumstances, patients’ absolute right to refuse a noninvasive test with the potential for multifaceted downstream benefit is worthy of reconsideration, in favor of mandatory testing. Mandatory testing confers numerous benefits, including mitigating risk to other patients and HCWs, who play a central role in pandemic response. Because infected HCWs may transmit the virus to patients, they also should undergo mandatory testing,3 particularly in the presence of symptoms, since nasal secretions increase the diagnostic yield of testing.5 Although pretest probability (as an estimate of disease prevalence) typically determines the testing strategy for admitted patients, model-based analyses suggest that testing every 3 days for HCWs or continuously hospitalized patients would nearly eliminate infectivity.6
Tools for assisting frustrated HCWs navigating patients’ right to refuse testing have been developed that incorporate education, clear communication, and conflict resolution.7 Such approaches are, however, only moderately successful, making the use of personal protective equipment (PPE) based on a default assumption of COVID-19 positivity common.8 The burden and disheartening waste created by low-yield PPE use among patients unwilling to be tested becomes particularly evident in the context of shortages. Such vexing, stressful shortages, as well as the dual responsibilities of hospitals as stewards of both individual patient and population health, serve as reminders that efficient allocation of resources must be valued alongside the autonomous rights of patients.9 Moreover, recent reports suggest that test avoidance is a growing problem.1,2 Refusal to accept testing may be rooted in anxiety, concerns about the consequences of a positive result (eg, inability to attend school or work), or a desire for self-determination.1,2 The hesitancy that leads to refusal may also arise from misinformation, poor public health messaging, distrust in the establishment, and unproductive considerations related to conscientious objection without foundation.2 Concepts of individual liberty that often underlie steadfast adherence to the principles of self-determination created opposition to masks that antagonized public health efforts to limit the spread of COVID-19. Although influencing inpatients’ behavior to benefit both the public and HCWs may be distinct from community settings, the attitudes that lead to test refusal and defiance of mask-related ordinances likely have substantial commonalities.
THE PATIENT ROLE IN HEALTHCARE DECISIONS
As a pillar of ethical
ANALOGOUS EXPERIENCES: ETHICAL LESSONS AND PRACTICAL IMPLICATIONS
In non-healthcare settings, the controversies surrounding vaccination and access to schools for unvaccinated children are perhaps the public and professional debate most analogous to COVID-19 testing refusal.12 Although policymakers may distinguish between testing and vaccination, these interventions similarly hold the potential to limit disease incidence and mitigate health impact. To preserve public health, most states prevent (with varied exemptions) unvaccinated children from attending schools. COVID-19 testing may in the future become a requirement for participation in group social activities, athletic competitions, or physical presence in the workplace to facilitate quarantining and/or targeted use of PPE for transmission risk reduction. Given the dramatic mitigation benefits accruable on college campuses,13 required testing for in-person learning has become common.
There are also parallels, and therefore lessons, to be drawn from experience in testing for HIV, although HIV-related stigma and devalued status of the marginalized populations initially infected impacted the broader societal view of HIV compared with COVID-19. For example, antenatal HIV screening of pregnant women is strongly recommended to facilitate interventions that reduce the chance of vertical transmission.14 The limitations of purely elective testing are one justification for the current standard of opt-out screening. However, in this case, the health complications of refusal are largely the burden of the fetus, over whose future the mother holds a great deal of choice and responsibility, irrespective of HIV status. The public health implications of HIV test refusal are far less immediate than for COVID-19 infection because there is no effective curative therapy for COVID-19 and spread occurs through nonintimate, unintentional, and unpredictable exposure.
Translating societal attitudes and practices into the healthcare setting to consider mandated COVID-19 testing requires additional considerations related to both patients and providers: (1) HCWs have committed to a set of values and professional obligations that include tasks requiring risks15; (2) the public expects HCWs to perform their duties according to a social contract that has few restrictions16; (3) limiting patient access to hospital care due to COVID-19 testing refusal would contradict and create conflicts related to professional conceptions of hospitals and physicians as patient agents15; and (4) patients who conscientiously object to testing may seek healthcare less diligently, which may lead to health decrements. The associated postponement of essential care may unduly burden the healthcare system, particularly in situations such as ambulatory care–sensitive conditions.
HEALTHCARE WORKER PROTECTION, PATIENT ACCESS, AND THE VALUE OF PARSIMONY
The extent to which the public health justification for mandatory testing extends to hospitalized patients to protect HCWs is ambiguous. HCWs are of enormous instrumental value and are therefore essential for the pandemic response and health of the broader population. Their protection may therefore justify curtailing informed consent for diagnostic testing. Downstream effects on the supply of frontline HCWs may be realized. Poor control over working conditions may negatively impact motivation among HCWs. In addition, they may feel disenfranchised while obligatorily taking personal risks in caring for patients unwilling to commit to the common good through diagnostic test consent. Hospitalized patients who refuse testing may remain patients under investigation (PUIs), thus requiring special respiratory precautions (SRP) throughout their hospitalization, thereby placing a persistent burden on those with responsibilities requiring patient contact.17 Repeatedly donning and doffing PPE may remind at-risk HCWs that a myriad of benefits may accrue from frequent, ubiquitous testing. Their motivation may be tempered by the demoralizing requirement to care for patients who will not consent to a simple test, knowing that an opportunity to diminish the burdens of this communicable disease that has taken the lives of many HCWs is being relinquished.
Although HCWs could use SRP universally, their selective application in rooms of known COVID-19–positive patients and those with temporary PUI status has several advantages.17 First, we learned that HIV testing on patients was helpful in enabling surgeons to selectively implement special precautions among infected patients rather than universally applied intensive precautions. Even in the setting of high rates of HIV infection and educational interventions, HCWs do not reliably apply protective measures included in universal precautions.18 In keeping with these experiences, limiting the number of patients on SRP will minimize the “precautions fatigue” that drives nonadherent behavior among HCWs.17 As a result, minimizing the proportion of patients on SRP through testing (and liberation from unnecessary precautions in most cases) will improve uptake of crucial hand hygiene practices and adoption of vigilant PPE use. Second, definitive knowledge of COVID-19 status will increase patient access to care because, whether by personal choice or policy, many HCWs limit in-person contact with patients who are or may be COVID-19 positive. For example, many inpatient dialysis units do not accept patients without a negative COVID-19 nasal swab. Physical therapists may delay or avoid seeing a PUI, which will pose challenges for efficient determination of discharge disposition. Third, selective use of SRP will limit the environmental impact of disposed PPE, which is neither recyclable nor biodegradable. Infectious or regulated biomedical waste products are a significant source of environmental pollution, and the World Health Organization has recommended parsimonious, selective use of PPE to minimize the adverse environmental consequences of biomedical waste products.
CONCLUSION
In summary, there are substantial justifications for mandatory testing for COVID-19 in the hospital for HCWs and patients, as has been successfully piloted in selected long-term care facilities. Patients who refuse to allow testing may have to accept that their care may be compromised. For preservation of HCW supply and maintenance of HCW morale, hospital policies should make explicit, without punishment or coercion, that HCWs may modify the care they provide to patients who refuse to consent to COVID-19 testing.
1. Morris NP. Refusing testing during a pandemic. Am J Public Health. 2020;110(9):1354-1355. https://doi.org/10.2105/AJPH.2020.305810
2. Rubin R. First it was masks; now some refuse testing for SARS-CoV-2. JAMA. 2020;324(20):2015-2016. https://doi.org/10.1001/jama.2020.22003
3. Black JRM, Bailey C, Przewrocka J, Dijkstra KK, Swanton C. COVID-19: the case for health-care worker screening to prevent hospital transmission. Lancet. 2020;395(10234):1418-1420. https://doi.org/10.1016/S0140-6736(20)30917-X
4. McBee SM, Thomasson ED, Scott MA, et al. Notes from the field: universal statewide laboratory testing for SARS-CoV-2 in nursing homes—West Virginia, April 21–May 8, 2020. MMWR Morb Mortal Wkly Rep. 2020;69(34):1177-1179. http://dx.doi.org/10.15585/mmwr.mm6934a4
5. Long DR, Gombar S, Hogan CA, et al. Occurrence and timing of subsequent severe acute respiratory syndrome coronavirus 2 reverse-transcription polymerase chain reaction positivity among initially negative patients. Clin Infect Dis. 2021;72(2):323-326. https://doi.org/10.1093/cid/ciaa722
6. Larremore DB, Wilder B, Lester E, et al. Test sensitivity is secondary to frequency and turnaround time for COVID-19 screening. Sci Adv. 2021;7(1):eabd5393. https://advances.sciencemag.org/content/7/1/eabd5393
7. Lu AC, Burgart AM. Elective surgery and COVID-19: a framework for the untested patient. Ann Surg. 2020;272(6):e291-e295. https://doi.org/10.1097/SLA.0000000000004474
8. Podboy A, Cholankeril G, Cianfichi L, Guzman E Jr, Ahmed A, Banerjee S. Implementation and impact of universal preprocedure testing of patients for COVID-19 before endoscopy. Gastroenterology. 2020;159(4):1586-1588. https://doi.org/10.1053/j.gastro.2020.06.022
9. O’Neill O. Some limits of informed consent. J Med Ethics. 2003;29(1):4-7. https://doi.org/10.1136/jme.29.1.4
10. Will JF. A brief historical and theoretical perspective on patient autonomy and medical decision making: part II: the autonomy model. Chest. 2011;139(6):1491-1497. https://doi.org/10.1378/chest.11-0516
11. Will JF. A brief historical and theoretical perspective on patient autonomy and medical decision making: part I: the beneficence model. Chest. 2011;139(3):669-673. https://doi.org/10.1378/chest.10-2532
12. Hendrix KS, Sturm LA, Zimet GD, Meslin EM. Ethics and childhood vaccination policy in the United States. Am J Public Health. 2016;106(2):273-278. https://doi.org/10.2105/AJPH.2015.302952
13. Losina E, Leifer V, Millham L, et al. College campuses and COVID-19 mitigation: clinical and economic value. Ann Intern Med. Published online December 21, 2020. https://doi.org/10.7326/M20-6558
14. Selph SS, Bougatsos C, Dana T, Grusing S, Chou R. Screening for HIV infection in pregnant women: updated evidence report and systematic review for the US Preventive Services Task Force. JAMA. 2019;321(23):2349-2360. https://doi.org/10.1001/jama.2019.2593
15. Dranove D, White WD. Agency and the organization of health care delivery. Inquiry. 1987;24(4):405-415.
16. Huber SJ, Wynia MK. When pestilence prevails...physician responsibilities in epidemics. Am J Bioeth. 2004;4(1):W5-W11. https://www.tandfonline.com/doi/abs/10.1162/152651604773067497
17. Ruhnke GW. COVID-19 diagnostic testing and the psychology of precautions fatigue. Cleve Clin J Med. 2020;88(1):19-21. https://doi.org/10.3949/ccjm.88a.20086
18. Freeman SW, Chambers CV. Compliance with universal precautions in a medical practice with a high rate of HIV infection. J Am Board Fam Pract. 1992;5(3):313-318.
Despite the important clinical and public health implications of a COVID-19 diagnosis, respect for autonomy allows patients to decline testing without explanation and with impunity. Whether physicians believe a test is indicated for clinical care of an individual patient, prevention of nosocomial transmission, or the greater public health, patients may refuse. Such refusals may be increasing due to quarantine requirements, concerns regarding contact tracing, and the persistent absence of a curative treatment.1,2 Mass screening of all healthcare workers (HCWs) is being considered to prevent hospital transmission,3 and universal screening in nursing homes has thwarted outbreaks while providing data to facilitate resource allocation.4 Given these circumstances, patients’ absolute right to refuse a noninvasive test with the potential for multifaceted downstream benefit is worthy of reconsideration, in favor of mandatory testing. Mandatory testing confers numerous benefits, including mitigating risk to other patients and HCWs, who play a central role in pandemic response. Because infected HCWs may transmit the virus to patients, they also should undergo mandatory testing,3 particularly in the presence of symptoms, since nasal secretions increase the diagnostic yield of testing.5 Although pretest probability (as an estimate of disease prevalence) typically determines the testing strategy for admitted patients, model-based analyses suggest that testing every 3 days for HCWs or continuously hospitalized patients would nearly eliminate infectivity.6
Tools for assisting frustrated HCWs navigating patients’ right to refuse testing have been developed that incorporate education, clear communication, and conflict resolution.7 Such approaches are, however, only moderately successful, making the use of personal protective equipment (PPE) based on a default assumption of COVID-19 positivity common.8 The burden and disheartening waste created by low-yield PPE use among patients unwilling to be tested becomes particularly evident in the context of shortages. Such vexing, stressful shortages, as well as the dual responsibilities of hospitals as stewards of both individual patient and population health, serve as reminders that efficient allocation of resources must be valued alongside the autonomous rights of patients.9 Moreover, recent reports suggest that test avoidance is a growing problem.1,2 Refusal to accept testing may be rooted in anxiety, concerns about the consequences of a positive result (eg, inability to attend school or work), or a desire for self-determination.1,2 The hesitancy that leads to refusal may also arise from misinformation, poor public health messaging, distrust in the establishment, and unproductive considerations related to conscientious objection without foundation.2 Concepts of individual liberty that often underlie steadfast adherence to the principles of self-determination created opposition to masks that antagonized public health efforts to limit the spread of COVID-19. Although influencing inpatients’ behavior to benefit both the public and HCWs may be distinct from community settings, the attitudes that lead to test refusal and defiance of mask-related ordinances likely have substantial commonalities.
THE PATIENT ROLE IN HEALTHCARE DECISIONS
As a pillar of ethical
ANALOGOUS EXPERIENCES: ETHICAL LESSONS AND PRACTICAL IMPLICATIONS
In non-healthcare settings, the controversies surrounding vaccination and access to schools for unvaccinated children are perhaps the public and professional debate most analogous to COVID-19 testing refusal.12 Although policymakers may distinguish between testing and vaccination, these interventions similarly hold the potential to limit disease incidence and mitigate health impact. To preserve public health, most states prevent (with varied exemptions) unvaccinated children from attending schools. COVID-19 testing may in the future become a requirement for participation in group social activities, athletic competitions, or physical presence in the workplace to facilitate quarantining and/or targeted use of PPE for transmission risk reduction. Given the dramatic mitigation benefits accruable on college campuses,13 required testing for in-person learning has become common.
There are also parallels, and therefore lessons, to be drawn from experience in testing for HIV, although HIV-related stigma and devalued status of the marginalized populations initially infected impacted the broader societal view of HIV compared with COVID-19. For example, antenatal HIV screening of pregnant women is strongly recommended to facilitate interventions that reduce the chance of vertical transmission.14 The limitations of purely elective testing are one justification for the current standard of opt-out screening. However, in this case, the health complications of refusal are largely the burden of the fetus, over whose future the mother holds a great deal of choice and responsibility, irrespective of HIV status. The public health implications of HIV test refusal are far less immediate than for COVID-19 infection because there is no effective curative therapy for COVID-19 and spread occurs through nonintimate, unintentional, and unpredictable exposure.
Translating societal attitudes and practices into the healthcare setting to consider mandated COVID-19 testing requires additional considerations related to both patients and providers: (1) HCWs have committed to a set of values and professional obligations that include tasks requiring risks15; (2) the public expects HCWs to perform their duties according to a social contract that has few restrictions16; (3) limiting patient access to hospital care due to COVID-19 testing refusal would contradict and create conflicts related to professional conceptions of hospitals and physicians as patient agents15; and (4) patients who conscientiously object to testing may seek healthcare less diligently, which may lead to health decrements. The associated postponement of essential care may unduly burden the healthcare system, particularly in situations such as ambulatory care–sensitive conditions.
HEALTHCARE WORKER PROTECTION, PATIENT ACCESS, AND THE VALUE OF PARSIMONY
The extent to which the public health justification for mandatory testing extends to hospitalized patients to protect HCWs is ambiguous. HCWs are of enormous instrumental value and are therefore essential for the pandemic response and health of the broader population. Their protection may therefore justify curtailing informed consent for diagnostic testing. Downstream effects on the supply of frontline HCWs may be realized. Poor control over working conditions may negatively impact motivation among HCWs. In addition, they may feel disenfranchised while obligatorily taking personal risks in caring for patients unwilling to commit to the common good through diagnostic test consent. Hospitalized patients who refuse testing may remain patients under investigation (PUIs), thus requiring special respiratory precautions (SRP) throughout their hospitalization, thereby placing a persistent burden on those with responsibilities requiring patient contact.17 Repeatedly donning and doffing PPE may remind at-risk HCWs that a myriad of benefits may accrue from frequent, ubiquitous testing. Their motivation may be tempered by the demoralizing requirement to care for patients who will not consent to a simple test, knowing that an opportunity to diminish the burdens of this communicable disease that has taken the lives of many HCWs is being relinquished.
Although HCWs could use SRP universally, their selective application in rooms of known COVID-19–positive patients and those with temporary PUI status has several advantages.17 First, we learned that HIV testing on patients was helpful in enabling surgeons to selectively implement special precautions among infected patients rather than universally applied intensive precautions. Even in the setting of high rates of HIV infection and educational interventions, HCWs do not reliably apply protective measures included in universal precautions.18 In keeping with these experiences, limiting the number of patients on SRP will minimize the “precautions fatigue” that drives nonadherent behavior among HCWs.17 As a result, minimizing the proportion of patients on SRP through testing (and liberation from unnecessary precautions in most cases) will improve uptake of crucial hand hygiene practices and adoption of vigilant PPE use. Second, definitive knowledge of COVID-19 status will increase patient access to care because, whether by personal choice or policy, many HCWs limit in-person contact with patients who are or may be COVID-19 positive. For example, many inpatient dialysis units do not accept patients without a negative COVID-19 nasal swab. Physical therapists may delay or avoid seeing a PUI, which will pose challenges for efficient determination of discharge disposition. Third, selective use of SRP will limit the environmental impact of disposed PPE, which is neither recyclable nor biodegradable. Infectious or regulated biomedical waste products are a significant source of environmental pollution, and the World Health Organization has recommended parsimonious, selective use of PPE to minimize the adverse environmental consequences of biomedical waste products.
CONCLUSION
In summary, there are substantial justifications for mandatory testing for COVID-19 in the hospital for HCWs and patients, as has been successfully piloted in selected long-term care facilities. Patients who refuse to allow testing may have to accept that their care may be compromised. For preservation of HCW supply and maintenance of HCW morale, hospital policies should make explicit, without punishment or coercion, that HCWs may modify the care they provide to patients who refuse to consent to COVID-19 testing.
Despite the important clinical and public health implications of a COVID-19 diagnosis, respect for autonomy allows patients to decline testing without explanation and with impunity. Whether physicians believe a test is indicated for clinical care of an individual patient, prevention of nosocomial transmission, or the greater public health, patients may refuse. Such refusals may be increasing due to quarantine requirements, concerns regarding contact tracing, and the persistent absence of a curative treatment.1,2 Mass screening of all healthcare workers (HCWs) is being considered to prevent hospital transmission,3 and universal screening in nursing homes has thwarted outbreaks while providing data to facilitate resource allocation.4 Given these circumstances, patients’ absolute right to refuse a noninvasive test with the potential for multifaceted downstream benefit is worthy of reconsideration, in favor of mandatory testing. Mandatory testing confers numerous benefits, including mitigating risk to other patients and HCWs, who play a central role in pandemic response. Because infected HCWs may transmit the virus to patients, they also should undergo mandatory testing,3 particularly in the presence of symptoms, since nasal secretions increase the diagnostic yield of testing.5 Although pretest probability (as an estimate of disease prevalence) typically determines the testing strategy for admitted patients, model-based analyses suggest that testing every 3 days for HCWs or continuously hospitalized patients would nearly eliminate infectivity.6
Tools for assisting frustrated HCWs navigating patients’ right to refuse testing have been developed that incorporate education, clear communication, and conflict resolution.7 Such approaches are, however, only moderately successful, making the use of personal protective equipment (PPE) based on a default assumption of COVID-19 positivity common.8 The burden and disheartening waste created by low-yield PPE use among patients unwilling to be tested becomes particularly evident in the context of shortages. Such vexing, stressful shortages, as well as the dual responsibilities of hospitals as stewards of both individual patient and population health, serve as reminders that efficient allocation of resources must be valued alongside the autonomous rights of patients.9 Moreover, recent reports suggest that test avoidance is a growing problem.1,2 Refusal to accept testing may be rooted in anxiety, concerns about the consequences of a positive result (eg, inability to attend school or work), or a desire for self-determination.1,2 The hesitancy that leads to refusal may also arise from misinformation, poor public health messaging, distrust in the establishment, and unproductive considerations related to conscientious objection without foundation.2 Concepts of individual liberty that often underlie steadfast adherence to the principles of self-determination created opposition to masks that antagonized public health efforts to limit the spread of COVID-19. Although influencing inpatients’ behavior to benefit both the public and HCWs may be distinct from community settings, the attitudes that lead to test refusal and defiance of mask-related ordinances likely have substantial commonalities.
THE PATIENT ROLE IN HEALTHCARE DECISIONS
As a pillar of ethical
ANALOGOUS EXPERIENCES: ETHICAL LESSONS AND PRACTICAL IMPLICATIONS
In non-healthcare settings, the controversies surrounding vaccination and access to schools for unvaccinated children are perhaps the public and professional debate most analogous to COVID-19 testing refusal.12 Although policymakers may distinguish between testing and vaccination, these interventions similarly hold the potential to limit disease incidence and mitigate health impact. To preserve public health, most states prevent (with varied exemptions) unvaccinated children from attending schools. COVID-19 testing may in the future become a requirement for participation in group social activities, athletic competitions, or physical presence in the workplace to facilitate quarantining and/or targeted use of PPE for transmission risk reduction. Given the dramatic mitigation benefits accruable on college campuses,13 required testing for in-person learning has become common.
There are also parallels, and therefore lessons, to be drawn from experience in testing for HIV, although HIV-related stigma and devalued status of the marginalized populations initially infected impacted the broader societal view of HIV compared with COVID-19. For example, antenatal HIV screening of pregnant women is strongly recommended to facilitate interventions that reduce the chance of vertical transmission.14 The limitations of purely elective testing are one justification for the current standard of opt-out screening. However, in this case, the health complications of refusal are largely the burden of the fetus, over whose future the mother holds a great deal of choice and responsibility, irrespective of HIV status. The public health implications of HIV test refusal are far less immediate than for COVID-19 infection because there is no effective curative therapy for COVID-19 and spread occurs through nonintimate, unintentional, and unpredictable exposure.
Translating societal attitudes and practices into the healthcare setting to consider mandated COVID-19 testing requires additional considerations related to both patients and providers: (1) HCWs have committed to a set of values and professional obligations that include tasks requiring risks15; (2) the public expects HCWs to perform their duties according to a social contract that has few restrictions16; (3) limiting patient access to hospital care due to COVID-19 testing refusal would contradict and create conflicts related to professional conceptions of hospitals and physicians as patient agents15; and (4) patients who conscientiously object to testing may seek healthcare less diligently, which may lead to health decrements. The associated postponement of essential care may unduly burden the healthcare system, particularly in situations such as ambulatory care–sensitive conditions.
HEALTHCARE WORKER PROTECTION, PATIENT ACCESS, AND THE VALUE OF PARSIMONY
The extent to which the public health justification for mandatory testing extends to hospitalized patients to protect HCWs is ambiguous. HCWs are of enormous instrumental value and are therefore essential for the pandemic response and health of the broader population. Their protection may therefore justify curtailing informed consent for diagnostic testing. Downstream effects on the supply of frontline HCWs may be realized. Poor control over working conditions may negatively impact motivation among HCWs. In addition, they may feel disenfranchised while obligatorily taking personal risks in caring for patients unwilling to commit to the common good through diagnostic test consent. Hospitalized patients who refuse testing may remain patients under investigation (PUIs), thus requiring special respiratory precautions (SRP) throughout their hospitalization, thereby placing a persistent burden on those with responsibilities requiring patient contact.17 Repeatedly donning and doffing PPE may remind at-risk HCWs that a myriad of benefits may accrue from frequent, ubiquitous testing. Their motivation may be tempered by the demoralizing requirement to care for patients who will not consent to a simple test, knowing that an opportunity to diminish the burdens of this communicable disease that has taken the lives of many HCWs is being relinquished.
Although HCWs could use SRP universally, their selective application in rooms of known COVID-19–positive patients and those with temporary PUI status has several advantages.17 First, we learned that HIV testing on patients was helpful in enabling surgeons to selectively implement special precautions among infected patients rather than universally applied intensive precautions. Even in the setting of high rates of HIV infection and educational interventions, HCWs do not reliably apply protective measures included in universal precautions.18 In keeping with these experiences, limiting the number of patients on SRP will minimize the “precautions fatigue” that drives nonadherent behavior among HCWs.17 As a result, minimizing the proportion of patients on SRP through testing (and liberation from unnecessary precautions in most cases) will improve uptake of crucial hand hygiene practices and adoption of vigilant PPE use. Second, definitive knowledge of COVID-19 status will increase patient access to care because, whether by personal choice or policy, many HCWs limit in-person contact with patients who are or may be COVID-19 positive. For example, many inpatient dialysis units do not accept patients without a negative COVID-19 nasal swab. Physical therapists may delay or avoid seeing a PUI, which will pose challenges for efficient determination of discharge disposition. Third, selective use of SRP will limit the environmental impact of disposed PPE, which is neither recyclable nor biodegradable. Infectious or regulated biomedical waste products are a significant source of environmental pollution, and the World Health Organization has recommended parsimonious, selective use of PPE to minimize the adverse environmental consequences of biomedical waste products.
CONCLUSION
In summary, there are substantial justifications for mandatory testing for COVID-19 in the hospital for HCWs and patients, as has been successfully piloted in selected long-term care facilities. Patients who refuse to allow testing may have to accept that their care may be compromised. For preservation of HCW supply and maintenance of HCW morale, hospital policies should make explicit, without punishment or coercion, that HCWs may modify the care they provide to patients who refuse to consent to COVID-19 testing.
1. Morris NP. Refusing testing during a pandemic. Am J Public Health. 2020;110(9):1354-1355. https://doi.org/10.2105/AJPH.2020.305810
2. Rubin R. First it was masks; now some refuse testing for SARS-CoV-2. JAMA. 2020;324(20):2015-2016. https://doi.org/10.1001/jama.2020.22003
3. Black JRM, Bailey C, Przewrocka J, Dijkstra KK, Swanton C. COVID-19: the case for health-care worker screening to prevent hospital transmission. Lancet. 2020;395(10234):1418-1420. https://doi.org/10.1016/S0140-6736(20)30917-X
4. McBee SM, Thomasson ED, Scott MA, et al. Notes from the field: universal statewide laboratory testing for SARS-CoV-2 in nursing homes—West Virginia, April 21–May 8, 2020. MMWR Morb Mortal Wkly Rep. 2020;69(34):1177-1179. http://dx.doi.org/10.15585/mmwr.mm6934a4
5. Long DR, Gombar S, Hogan CA, et al. Occurrence and timing of subsequent severe acute respiratory syndrome coronavirus 2 reverse-transcription polymerase chain reaction positivity among initially negative patients. Clin Infect Dis. 2021;72(2):323-326. https://doi.org/10.1093/cid/ciaa722
6. Larremore DB, Wilder B, Lester E, et al. Test sensitivity is secondary to frequency and turnaround time for COVID-19 screening. Sci Adv. 2021;7(1):eabd5393. https://advances.sciencemag.org/content/7/1/eabd5393
7. Lu AC, Burgart AM. Elective surgery and COVID-19: a framework for the untested patient. Ann Surg. 2020;272(6):e291-e295. https://doi.org/10.1097/SLA.0000000000004474
8. Podboy A, Cholankeril G, Cianfichi L, Guzman E Jr, Ahmed A, Banerjee S. Implementation and impact of universal preprocedure testing of patients for COVID-19 before endoscopy. Gastroenterology. 2020;159(4):1586-1588. https://doi.org/10.1053/j.gastro.2020.06.022
9. O’Neill O. Some limits of informed consent. J Med Ethics. 2003;29(1):4-7. https://doi.org/10.1136/jme.29.1.4
10. Will JF. A brief historical and theoretical perspective on patient autonomy and medical decision making: part II: the autonomy model. Chest. 2011;139(6):1491-1497. https://doi.org/10.1378/chest.11-0516
11. Will JF. A brief historical and theoretical perspective on patient autonomy and medical decision making: part I: the beneficence model. Chest. 2011;139(3):669-673. https://doi.org/10.1378/chest.10-2532
12. Hendrix KS, Sturm LA, Zimet GD, Meslin EM. Ethics and childhood vaccination policy in the United States. Am J Public Health. 2016;106(2):273-278. https://doi.org/10.2105/AJPH.2015.302952
13. Losina E, Leifer V, Millham L, et al. College campuses and COVID-19 mitigation: clinical and economic value. Ann Intern Med. Published online December 21, 2020. https://doi.org/10.7326/M20-6558
14. Selph SS, Bougatsos C, Dana T, Grusing S, Chou R. Screening for HIV infection in pregnant women: updated evidence report and systematic review for the US Preventive Services Task Force. JAMA. 2019;321(23):2349-2360. https://doi.org/10.1001/jama.2019.2593
15. Dranove D, White WD. Agency and the organization of health care delivery. Inquiry. 1987;24(4):405-415.
16. Huber SJ, Wynia MK. When pestilence prevails...physician responsibilities in epidemics. Am J Bioeth. 2004;4(1):W5-W11. https://www.tandfonline.com/doi/abs/10.1162/152651604773067497
17. Ruhnke GW. COVID-19 diagnostic testing and the psychology of precautions fatigue. Cleve Clin J Med. 2020;88(1):19-21. https://doi.org/10.3949/ccjm.88a.20086
18. Freeman SW, Chambers CV. Compliance with universal precautions in a medical practice with a high rate of HIV infection. J Am Board Fam Pract. 1992;5(3):313-318.
1. Morris NP. Refusing testing during a pandemic. Am J Public Health. 2020;110(9):1354-1355. https://doi.org/10.2105/AJPH.2020.305810
2. Rubin R. First it was masks; now some refuse testing for SARS-CoV-2. JAMA. 2020;324(20):2015-2016. https://doi.org/10.1001/jama.2020.22003
3. Black JRM, Bailey C, Przewrocka J, Dijkstra KK, Swanton C. COVID-19: the case for health-care worker screening to prevent hospital transmission. Lancet. 2020;395(10234):1418-1420. https://doi.org/10.1016/S0140-6736(20)30917-X
4. McBee SM, Thomasson ED, Scott MA, et al. Notes from the field: universal statewide laboratory testing for SARS-CoV-2 in nursing homes—West Virginia, April 21–May 8, 2020. MMWR Morb Mortal Wkly Rep. 2020;69(34):1177-1179. http://dx.doi.org/10.15585/mmwr.mm6934a4
5. Long DR, Gombar S, Hogan CA, et al. Occurrence and timing of subsequent severe acute respiratory syndrome coronavirus 2 reverse-transcription polymerase chain reaction positivity among initially negative patients. Clin Infect Dis. 2021;72(2):323-326. https://doi.org/10.1093/cid/ciaa722
6. Larremore DB, Wilder B, Lester E, et al. Test sensitivity is secondary to frequency and turnaround time for COVID-19 screening. Sci Adv. 2021;7(1):eabd5393. https://advances.sciencemag.org/content/7/1/eabd5393
7. Lu AC, Burgart AM. Elective surgery and COVID-19: a framework for the untested patient. Ann Surg. 2020;272(6):e291-e295. https://doi.org/10.1097/SLA.0000000000004474
8. Podboy A, Cholankeril G, Cianfichi L, Guzman E Jr, Ahmed A, Banerjee S. Implementation and impact of universal preprocedure testing of patients for COVID-19 before endoscopy. Gastroenterology. 2020;159(4):1586-1588. https://doi.org/10.1053/j.gastro.2020.06.022
9. O’Neill O. Some limits of informed consent. J Med Ethics. 2003;29(1):4-7. https://doi.org/10.1136/jme.29.1.4
10. Will JF. A brief historical and theoretical perspective on patient autonomy and medical decision making: part II: the autonomy model. Chest. 2011;139(6):1491-1497. https://doi.org/10.1378/chest.11-0516
11. Will JF. A brief historical and theoretical perspective on patient autonomy and medical decision making: part I: the beneficence model. Chest. 2011;139(3):669-673. https://doi.org/10.1378/chest.10-2532
12. Hendrix KS, Sturm LA, Zimet GD, Meslin EM. Ethics and childhood vaccination policy in the United States. Am J Public Health. 2016;106(2):273-278. https://doi.org/10.2105/AJPH.2015.302952
13. Losina E, Leifer V, Millham L, et al. College campuses and COVID-19 mitigation: clinical and economic value. Ann Intern Med. Published online December 21, 2020. https://doi.org/10.7326/M20-6558
14. Selph SS, Bougatsos C, Dana T, Grusing S, Chou R. Screening for HIV infection in pregnant women: updated evidence report and systematic review for the US Preventive Services Task Force. JAMA. 2019;321(23):2349-2360. https://doi.org/10.1001/jama.2019.2593
15. Dranove D, White WD. Agency and the organization of health care delivery. Inquiry. 1987;24(4):405-415.
16. Huber SJ, Wynia MK. When pestilence prevails...physician responsibilities in epidemics. Am J Bioeth. 2004;4(1):W5-W11. https://www.tandfonline.com/doi/abs/10.1162/152651604773067497
17. Ruhnke GW. COVID-19 diagnostic testing and the psychology of precautions fatigue. Cleve Clin J Med. 2020;88(1):19-21. https://doi.org/10.3949/ccjm.88a.20086
18. Freeman SW, Chambers CV. Compliance with universal precautions in a medical practice with a high rate of HIV infection. J Am Board Fam Pract. 1992;5(3):313-318.
© 2021 Society of Hospital Medicine