User login
Striking While the Iron Is Hot: Using the Updated PHM Competencies in Time-Variable Training
In July 2020, the revision of The Pediatric Hospital Medicine Core Competencies was published, bringing to fruition three years of meticulous work.1 The working group produced 66 chapters outlining the knowledge, skills, and attitudes needed for competent pediatric hospitalist practice. The arrival of these competencies is especially prescient given pediatric hospital medicine’s (PHM’s) relatively new standing as an American Board of Medical Specialties certified subspecialty, as the competencies can serve as a guide for improvement of fellowship curricula, assessment systems, and faculty development. The competencies also represent an opportunity for PHM to take a bold step forward in the world of graduate medical education (GME) by realizing a key tenet of competency-based medical education (CBME)—competency-based, time-variable training (CBTVT), in which learners train until competence is achieved rather than for a predetermined duration.2,3 In this perspective, we describe how medical education in the United States adopted a time-based training paradigm (in which time-in-training is a surrogate for competence), how CBME has brought time-variable training to the fore, and how PHM has an opportunity to be on the leading edge of education innovation.
TIME-BASED TRAINING IN THE UNITED STATES
In the 1800s, during the time of the “Wild West,” medical education in the United States matched this moniker. There was little standardization across the multiple training pathways to become a practicing physician, including apprenticeships, lecture series, and university courses.4 Predictably, this led to significant heterogeneity in the quality of medical care that a patient of the day received. This problem became clearer as Americans traveled to Europe and witnessed more structured and rigorous training programs, only to return to the comparatively poor state of medical education back home.5 There was a clear need for curricular standardization.
In 1876, the American Medical College Association (which later became the Association of American Medical Colleges [AAMC]) was founded to meet this need, and in 1905 the Association adopted a set of minimum standards for medical training that included the now-familiar two years of basic sciences and two years of clinical training.6 Two subsequent national surveys in the United States were commissioned to evaluate whether medical schools met this new standard, with both surveys finding that roughly half of existing programs passed muster.7,8 As a result, nearly half of US medical schools had closed by 1920 in a crusade to standardize curricula and produce competent physicians. By the time the American Medical Association established initial standards for internship (an archetype of GME),4 time-based medical training was the dominant paradigm. This historical perspective highlights the rationale for standardization of education processes and curricula, particularly in terms of accountability to the American public. But heralded by the 1978 landmark paper by McGaghie et al,9 the paradigm began to shift in the late twentieth century from a focus on the process of physician training to outcomes.
CBME AND TIME VARIABILITY
In contrast to the process-focused model of the early 1900s, CBME starts by identifying patient and healthcare system needs, defining competencies required to meet those needs, and then designing curricular and assessment processes to help learners achieve those competencies.2 This outcomes-based approach grew as a response to calls for greater accountability to the public due to evidence that some graduates were unprepared for unsupervised practice, raising concerns that strictly time-based training was no longer defensible.10 CBME aims to mitigate these concerns by starting with desired outcomes of training and working backward to ensure those outcomes are met.
While many programs have attempted to implement CBME, most still rely heavily on time-in-training to determine competence. Learners participate in structured curricula and, unless they are extreme outliers, are deemed ready for unsupervised practice after a predetermined duration. This model presumes that competence and time are related in a fixed, predictable manner and that learners gain competence at a uniform rate. However, learners do not, in fact, progress uniformly. A study by Schumacher et al11 involving 23 pediatric residency programs showed significant interlearner variability in rates of entrustment (used as a surrogate for competence), leading the authors to call for time-variable training in GME. Significant interlearner variation in rates of competence attainment have been shown in other specialties as well.12 As more CBME studies on training outcomes emerge, the evidence is mounting that not all learners need the same duration of training to become competent providers. Time-in-training and competence attainment are not related in a fixed manner. As Dr Jason13 wrote in 1969, “By making time a constant, we make achievement a variable.” Variable achievement (competence, outcomes) was the very driver for medical education’s shift to a competency-based approach. If variable competence was not acceptable then, why should it be now? The goal of CBTVT is not shorter training, but rather flexible, individualized training both in terms of content and duration. While this also means some learners may need to extend their training, this should already be part of GME programs that are required to have remediation policies for learners who are not progressing as expected.
AN OPPORTUNITY FOR PHM
Time variability is an oft-cited tenet of CBME,2,3 but one that is being piloted by relatively few programs in the United States, mostly in undergraduate medical education (UME).14-16 The Education in Pediatrics Across the Continuum (EPAC), a consortium consisting of four institutions piloting CBTVT in UME,14 has shown early evidence of feasibility17 and that UME graduates from CBTVT programs enter residency with levels of competence similar to those of graduates of traditional time-based programs.18 We believe that PHM can take a step toward truly realizing CBME by implementing CBTVT in fellowship programs.
There are multiple reasons why this is an opportune time for PHM fellowships to consider CBTVT. First, PHM is a relatively new board-certified subspecialty with a recently revised set of core competencies1 that are likely to catalyze programmatic innovation. A key step in change management is building on previous efforts to generate more change.19 Programs can leverage the momentum from current and impending change initiatives to innovate and implement CBTVT. Second, the revised PHM competencies provide the first crucial step in implementing a CBME program by defining desired training outcomes necessary to deliver high-quality patient care. With PHM competencies now well defined, programs can focus on developing programs of assessment and corresponding faculty development, which can help deliver valid, defensible decisions about fellow competence.
Finally, PHM has a workforce that can support CBTVT. A major barrier to time-variable training in GME is the need for trainees-as-workforce. In many GME programs, residents and fellows provide a relatively inexpensive, renewable workforce. Trainees’ clinical rotations are often scheduled up to 1 year in advance to ensure care teams are fully staffed, particularly in the inpatient setting, creating a system where flexibility in training is impossible without creating gaps in clinical coverage. However, many PHM fellowships do not completely rely on fellows to cover clinical service lines. PHM fellows spend 32 weeks over 2 years in core clinical rotations with faculty supervision, in accordance with the Accreditation Council for Graduate Medical Education program requirements, both for 2- and 3-year programs. Some CBME experts estimate (based on previous and ongoing CBTVT pilots) that training duration is likely to vary by roughly 20% from current time-based practices when CBTVT is initially implemented.20 Thus, only a small number of clinical service weeks are likely to be affected. If a fellow were deemed ready for unsupervised practice before finishing 2 years of fellowship in a CBTVT program, the corresponding faculty supervisor could use the time previously assigned for supervision to pursue other priorities, such as education, scholarship, or quality improvement. Why provide supervision if a clinical competency committee has deemed a fellow ready for unsupervised practice? Some level of observation and formative feedback could continue, but full supervision would be redundant and unnecessary. CBTVT would allow for some fellows to experience the uncertainty that comes with unsupervised decision-making while still in an environment with trusted fellowship mentors and advisors.
STEPS TOWARD CHANGE
PHM fellowship programs likely cannot flip a switch to “turn on” CBTVT immediately, but they can take steps toward making the transition. Validity, or defensibility of decisions, will be crucial for assessment in CBTVT systems. Programs will need to develop robust assessment systems that collect myriad data to answer the question, “When is this learner competent to deliver high-quality care without supervision?” Programs can align assessment instruments, faculty-development initiatives, and clinical competency committee (CCC) processes with the 2020 PHM competencies to provide a defensible answer. Program leaders should then seek validity evidence, either in existing literature or through novel scholarly initiatives, to support these summative decisions. Engaging all fellowship stakeholders in transitions to CBTVT will be important and should include fellows, program directors, CCC members, clinical leadership, and members from accrediting and credentialing bodies.
CONCLUSION
As fellowship programs review and revise curricula and assessment systems around the updated PHM core competencies, they should also consider what changes are necessary to implement CBTVT. Time variability is not a novelty but, rather, is a corollary to the outcomes-based approach of CBME. PHM fellowships should strike while the iron is hot and build on current change initiatives prompted by the growth of our specialty to be leaders in CBTVT.
1. Maniscalco J, Gage S, Sofia Teferi M, Fisher ES. The Pediatric Hospital Medicine Core Competencies: 2020 Revision. J Hosp Med. 2020;15(7):389-394. https://doi.org/10.12788/jhm.3391
2. Frank JR, Snell LS, Cate OT, et al. Competency-based medical education: theory to practice. Med Teach. 2010;32(8):638-645. https://doi.org/10.3109/0142159X.2010.501190
3. Lucey CR, Thibault GE, Ten Cate O. Competency-based, tme-variable education in the health professions: crossroads. Acad Med. 2018;93(3S Competency-Based, Time-Variable Education in the Health Professions):S1-S5. https://doi.org/10.1097/ACM.0000000000002080
4. Custers EJFM, Ten Cate O. The history of medical education in Europe and the United States, with respect to time and proficiency. Acad Med. 2018;93(3S Competency-Based, Time-Variable Education in the Health Professions):S49-S54. https://doi.org/10.1097/ACM.0000000000002079
5. Barr DA. Revolution or evolution? Putting the Flexner Report in context. Med Educ. 2011;45(1):17-22. https://doi.org/10.1111/j.1365-2923.2010.03850.x
6. Association of American Medical Colleges. Minutes of the Fifteenth Annual Meeting. April 10, 1905; Chicago, IL.
7. Bevan A. Council on Medical Education of the American Medical Association. JAMA. 1907;48(20):1701-1707.
8. Flexner A. Medical education in the United States and Canada. From the Carnegie Foundation for the Advancement of Teaching, Bulletin Number Four, 1910. Bull World Health Organ. 2002;80(7):594-602.
9. McGaghie WC, Sajid AW, Miller GE, et al. Competency-based curriculum development in medical education: an introduction. Public Health Pap. 1978;(68):11-91.
10. Frank JR, Snell L, Englander R, Holmboe ES, ICBME Collaborators. Implementing competency-based medical education: moving forward. Med Teach. 2017;39(6):568-573. https://doi.org/10.1080/0142159X.2017.1315069
11. Schumacher DJ, West DC, Schwartz A, et al. Longitudinal assessment of resident performance using entrustable professional activities. JAMA Netw Open. 2020;3(1):e1919316. https://doi.org/10.1001/jamanetworkopen.2019.19316
12. Warm EJ, Held J, Hellman M, et al. Entrusting observable practice activities and milestones over the 36 months of an internal medicine residency. Acad Med. 2016;91(10):1398-1405. https://doi.org/10.1097/ACM.0000000000001292
13. Jason H. Effective medical instruction: requirements and possibilities. In: Proceedings of a 1969 International Symposium on Medical Education. Medica; 1970:5-8.
14. Andrews JS, Bale JF Jr, Soep JB, et al. Education in Pediatrics Across the Continuum (EPAC): first steps toward realizing the dream of competency-based education. Acad Med. 2018;93(3):414-420. https://doi.org/10.1097/ACM.0000000000002020
15. Mejicano GC, Bumsted TN. Describing the journey and lessons learned implementing a competency-based, time-variable undergraduate medical education curriculum. Acad Med. 2018;93(3S Competency-Based, Time-Variable Education in the Health Professions):S42-S48. https://doi.org/10.1097/ACM.0000000000002068
16. Goldhamer MEJ, Pusic MV, Co JPT, Weinstein DF. Can COVID catalyze an educational transformation? Competency-based advancement in a crisis. N Engl J Med. 2020;383(11):1003-1005. https://doi.org/10.1056/NEJMp2018570
17. Murray KE, Lane JL, Carraccio C, et al. Crossing the gap: using competency-based assessment to determine whether learners are ready for the undergraduate-to-graduate transition. Acad Med. 2019;94(3):338-345. https://doi.org/10.1097/ACM.0000000000002535
18. Schwartz A, Balmer DF, Borman-Shoap E, et al. Shared mental models among clinical competency committees in the context of time-variable, competency-based advancement to residency. Acad Med. 2020;95(11S Association of American Medical Colleges Learn Serve Lead: Proceedings of the 59th Annual Research in Medical Education Presentations):S95-S102. https://doi.org/10.1097/ACM.0000000000003638
19. Kotter JP. Leading change: why transformation efforts fail. Harvard Business Review. May-June 1995. Accessed March 1, 2021. https://hbr.org/1995/05/leading-change-why-transformation-efforts-fail-2
20. Schumacher DJ, Caretta-Weyer H, Busari J, et al. Competency-based time-variable training internationally: ensuring practical next steps. Med Teach. Forthcoming.
In July 2020, the revision of The Pediatric Hospital Medicine Core Competencies was published, bringing to fruition three years of meticulous work.1 The working group produced 66 chapters outlining the knowledge, skills, and attitudes needed for competent pediatric hospitalist practice. The arrival of these competencies is especially prescient given pediatric hospital medicine’s (PHM’s) relatively new standing as an American Board of Medical Specialties certified subspecialty, as the competencies can serve as a guide for improvement of fellowship curricula, assessment systems, and faculty development. The competencies also represent an opportunity for PHM to take a bold step forward in the world of graduate medical education (GME) by realizing a key tenet of competency-based medical education (CBME)—competency-based, time-variable training (CBTVT), in which learners train until competence is achieved rather than for a predetermined duration.2,3 In this perspective, we describe how medical education in the United States adopted a time-based training paradigm (in which time-in-training is a surrogate for competence), how CBME has brought time-variable training to the fore, and how PHM has an opportunity to be on the leading edge of education innovation.
TIME-BASED TRAINING IN THE UNITED STATES
In the 1800s, during the time of the “Wild West,” medical education in the United States matched this moniker. There was little standardization across the multiple training pathways to become a practicing physician, including apprenticeships, lecture series, and university courses.4 Predictably, this led to significant heterogeneity in the quality of medical care that a patient of the day received. This problem became clearer as Americans traveled to Europe and witnessed more structured and rigorous training programs, only to return to the comparatively poor state of medical education back home.5 There was a clear need for curricular standardization.
In 1876, the American Medical College Association (which later became the Association of American Medical Colleges [AAMC]) was founded to meet this need, and in 1905 the Association adopted a set of minimum standards for medical training that included the now-familiar two years of basic sciences and two years of clinical training.6 Two subsequent national surveys in the United States were commissioned to evaluate whether medical schools met this new standard, with both surveys finding that roughly half of existing programs passed muster.7,8 As a result, nearly half of US medical schools had closed by 1920 in a crusade to standardize curricula and produce competent physicians. By the time the American Medical Association established initial standards for internship (an archetype of GME),4 time-based medical training was the dominant paradigm. This historical perspective highlights the rationale for standardization of education processes and curricula, particularly in terms of accountability to the American public. But heralded by the 1978 landmark paper by McGaghie et al,9 the paradigm began to shift in the late twentieth century from a focus on the process of physician training to outcomes.
CBME AND TIME VARIABILITY
In contrast to the process-focused model of the early 1900s, CBME starts by identifying patient and healthcare system needs, defining competencies required to meet those needs, and then designing curricular and assessment processes to help learners achieve those competencies.2 This outcomes-based approach grew as a response to calls for greater accountability to the public due to evidence that some graduates were unprepared for unsupervised practice, raising concerns that strictly time-based training was no longer defensible.10 CBME aims to mitigate these concerns by starting with desired outcomes of training and working backward to ensure those outcomes are met.
While many programs have attempted to implement CBME, most still rely heavily on time-in-training to determine competence. Learners participate in structured curricula and, unless they are extreme outliers, are deemed ready for unsupervised practice after a predetermined duration. This model presumes that competence and time are related in a fixed, predictable manner and that learners gain competence at a uniform rate. However, learners do not, in fact, progress uniformly. A study by Schumacher et al11 involving 23 pediatric residency programs showed significant interlearner variability in rates of entrustment (used as a surrogate for competence), leading the authors to call for time-variable training in GME. Significant interlearner variation in rates of competence attainment have been shown in other specialties as well.12 As more CBME studies on training outcomes emerge, the evidence is mounting that not all learners need the same duration of training to become competent providers. Time-in-training and competence attainment are not related in a fixed manner. As Dr Jason13 wrote in 1969, “By making time a constant, we make achievement a variable.” Variable achievement (competence, outcomes) was the very driver for medical education’s shift to a competency-based approach. If variable competence was not acceptable then, why should it be now? The goal of CBTVT is not shorter training, but rather flexible, individualized training both in terms of content and duration. While this also means some learners may need to extend their training, this should already be part of GME programs that are required to have remediation policies for learners who are not progressing as expected.
AN OPPORTUNITY FOR PHM
Time variability is an oft-cited tenet of CBME,2,3 but one that is being piloted by relatively few programs in the United States, mostly in undergraduate medical education (UME).14-16 The Education in Pediatrics Across the Continuum (EPAC), a consortium consisting of four institutions piloting CBTVT in UME,14 has shown early evidence of feasibility17 and that UME graduates from CBTVT programs enter residency with levels of competence similar to those of graduates of traditional time-based programs.18 We believe that PHM can take a step toward truly realizing CBME by implementing CBTVT in fellowship programs.
There are multiple reasons why this is an opportune time for PHM fellowships to consider CBTVT. First, PHM is a relatively new board-certified subspecialty with a recently revised set of core competencies1 that are likely to catalyze programmatic innovation. A key step in change management is building on previous efforts to generate more change.19 Programs can leverage the momentum from current and impending change initiatives to innovate and implement CBTVT. Second, the revised PHM competencies provide the first crucial step in implementing a CBME program by defining desired training outcomes necessary to deliver high-quality patient care. With PHM competencies now well defined, programs can focus on developing programs of assessment and corresponding faculty development, which can help deliver valid, defensible decisions about fellow competence.
Finally, PHM has a workforce that can support CBTVT. A major barrier to time-variable training in GME is the need for trainees-as-workforce. In many GME programs, residents and fellows provide a relatively inexpensive, renewable workforce. Trainees’ clinical rotations are often scheduled up to 1 year in advance to ensure care teams are fully staffed, particularly in the inpatient setting, creating a system where flexibility in training is impossible without creating gaps in clinical coverage. However, many PHM fellowships do not completely rely on fellows to cover clinical service lines. PHM fellows spend 32 weeks over 2 years in core clinical rotations with faculty supervision, in accordance with the Accreditation Council for Graduate Medical Education program requirements, both for 2- and 3-year programs. Some CBME experts estimate (based on previous and ongoing CBTVT pilots) that training duration is likely to vary by roughly 20% from current time-based practices when CBTVT is initially implemented.20 Thus, only a small number of clinical service weeks are likely to be affected. If a fellow were deemed ready for unsupervised practice before finishing 2 years of fellowship in a CBTVT program, the corresponding faculty supervisor could use the time previously assigned for supervision to pursue other priorities, such as education, scholarship, or quality improvement. Why provide supervision if a clinical competency committee has deemed a fellow ready for unsupervised practice? Some level of observation and formative feedback could continue, but full supervision would be redundant and unnecessary. CBTVT would allow for some fellows to experience the uncertainty that comes with unsupervised decision-making while still in an environment with trusted fellowship mentors and advisors.
STEPS TOWARD CHANGE
PHM fellowship programs likely cannot flip a switch to “turn on” CBTVT immediately, but they can take steps toward making the transition. Validity, or defensibility of decisions, will be crucial for assessment in CBTVT systems. Programs will need to develop robust assessment systems that collect myriad data to answer the question, “When is this learner competent to deliver high-quality care without supervision?” Programs can align assessment instruments, faculty-development initiatives, and clinical competency committee (CCC) processes with the 2020 PHM competencies to provide a defensible answer. Program leaders should then seek validity evidence, either in existing literature or through novel scholarly initiatives, to support these summative decisions. Engaging all fellowship stakeholders in transitions to CBTVT will be important and should include fellows, program directors, CCC members, clinical leadership, and members from accrediting and credentialing bodies.
CONCLUSION
As fellowship programs review and revise curricula and assessment systems around the updated PHM core competencies, they should also consider what changes are necessary to implement CBTVT. Time variability is not a novelty but, rather, is a corollary to the outcomes-based approach of CBME. PHM fellowships should strike while the iron is hot and build on current change initiatives prompted by the growth of our specialty to be leaders in CBTVT.
In July 2020, the revision of The Pediatric Hospital Medicine Core Competencies was published, bringing to fruition three years of meticulous work.1 The working group produced 66 chapters outlining the knowledge, skills, and attitudes needed for competent pediatric hospitalist practice. The arrival of these competencies is especially prescient given pediatric hospital medicine’s (PHM’s) relatively new standing as an American Board of Medical Specialties certified subspecialty, as the competencies can serve as a guide for improvement of fellowship curricula, assessment systems, and faculty development. The competencies also represent an opportunity for PHM to take a bold step forward in the world of graduate medical education (GME) by realizing a key tenet of competency-based medical education (CBME)—competency-based, time-variable training (CBTVT), in which learners train until competence is achieved rather than for a predetermined duration.2,3 In this perspective, we describe how medical education in the United States adopted a time-based training paradigm (in which time-in-training is a surrogate for competence), how CBME has brought time-variable training to the fore, and how PHM has an opportunity to be on the leading edge of education innovation.
TIME-BASED TRAINING IN THE UNITED STATES
In the 1800s, during the time of the “Wild West,” medical education in the United States matched this moniker. There was little standardization across the multiple training pathways to become a practicing physician, including apprenticeships, lecture series, and university courses.4 Predictably, this led to significant heterogeneity in the quality of medical care that a patient of the day received. This problem became clearer as Americans traveled to Europe and witnessed more structured and rigorous training programs, only to return to the comparatively poor state of medical education back home.5 There was a clear need for curricular standardization.
In 1876, the American Medical College Association (which later became the Association of American Medical Colleges [AAMC]) was founded to meet this need, and in 1905 the Association adopted a set of minimum standards for medical training that included the now-familiar two years of basic sciences and two years of clinical training.6 Two subsequent national surveys in the United States were commissioned to evaluate whether medical schools met this new standard, with both surveys finding that roughly half of existing programs passed muster.7,8 As a result, nearly half of US medical schools had closed by 1920 in a crusade to standardize curricula and produce competent physicians. By the time the American Medical Association established initial standards for internship (an archetype of GME),4 time-based medical training was the dominant paradigm. This historical perspective highlights the rationale for standardization of education processes and curricula, particularly in terms of accountability to the American public. But heralded by the 1978 landmark paper by McGaghie et al,9 the paradigm began to shift in the late twentieth century from a focus on the process of physician training to outcomes.
CBME AND TIME VARIABILITY
In contrast to the process-focused model of the early 1900s, CBME starts by identifying patient and healthcare system needs, defining competencies required to meet those needs, and then designing curricular and assessment processes to help learners achieve those competencies.2 This outcomes-based approach grew as a response to calls for greater accountability to the public due to evidence that some graduates were unprepared for unsupervised practice, raising concerns that strictly time-based training was no longer defensible.10 CBME aims to mitigate these concerns by starting with desired outcomes of training and working backward to ensure those outcomes are met.
While many programs have attempted to implement CBME, most still rely heavily on time-in-training to determine competence. Learners participate in structured curricula and, unless they are extreme outliers, are deemed ready for unsupervised practice after a predetermined duration. This model presumes that competence and time are related in a fixed, predictable manner and that learners gain competence at a uniform rate. However, learners do not, in fact, progress uniformly. A study by Schumacher et al11 involving 23 pediatric residency programs showed significant interlearner variability in rates of entrustment (used as a surrogate for competence), leading the authors to call for time-variable training in GME. Significant interlearner variation in rates of competence attainment have been shown in other specialties as well.12 As more CBME studies on training outcomes emerge, the evidence is mounting that not all learners need the same duration of training to become competent providers. Time-in-training and competence attainment are not related in a fixed manner. As Dr Jason13 wrote in 1969, “By making time a constant, we make achievement a variable.” Variable achievement (competence, outcomes) was the very driver for medical education’s shift to a competency-based approach. If variable competence was not acceptable then, why should it be now? The goal of CBTVT is not shorter training, but rather flexible, individualized training both in terms of content and duration. While this also means some learners may need to extend their training, this should already be part of GME programs that are required to have remediation policies for learners who are not progressing as expected.
AN OPPORTUNITY FOR PHM
Time variability is an oft-cited tenet of CBME,2,3 but one that is being piloted by relatively few programs in the United States, mostly in undergraduate medical education (UME).14-16 The Education in Pediatrics Across the Continuum (EPAC), a consortium consisting of four institutions piloting CBTVT in UME,14 has shown early evidence of feasibility17 and that UME graduates from CBTVT programs enter residency with levels of competence similar to those of graduates of traditional time-based programs.18 We believe that PHM can take a step toward truly realizing CBME by implementing CBTVT in fellowship programs.
There are multiple reasons why this is an opportune time for PHM fellowships to consider CBTVT. First, PHM is a relatively new board-certified subspecialty with a recently revised set of core competencies1 that are likely to catalyze programmatic innovation. A key step in change management is building on previous efforts to generate more change.19 Programs can leverage the momentum from current and impending change initiatives to innovate and implement CBTVT. Second, the revised PHM competencies provide the first crucial step in implementing a CBME program by defining desired training outcomes necessary to deliver high-quality patient care. With PHM competencies now well defined, programs can focus on developing programs of assessment and corresponding faculty development, which can help deliver valid, defensible decisions about fellow competence.
Finally, PHM has a workforce that can support CBTVT. A major barrier to time-variable training in GME is the need for trainees-as-workforce. In many GME programs, residents and fellows provide a relatively inexpensive, renewable workforce. Trainees’ clinical rotations are often scheduled up to 1 year in advance to ensure care teams are fully staffed, particularly in the inpatient setting, creating a system where flexibility in training is impossible without creating gaps in clinical coverage. However, many PHM fellowships do not completely rely on fellows to cover clinical service lines. PHM fellows spend 32 weeks over 2 years in core clinical rotations with faculty supervision, in accordance with the Accreditation Council for Graduate Medical Education program requirements, both for 2- and 3-year programs. Some CBME experts estimate (based on previous and ongoing CBTVT pilots) that training duration is likely to vary by roughly 20% from current time-based practices when CBTVT is initially implemented.20 Thus, only a small number of clinical service weeks are likely to be affected. If a fellow were deemed ready for unsupervised practice before finishing 2 years of fellowship in a CBTVT program, the corresponding faculty supervisor could use the time previously assigned for supervision to pursue other priorities, such as education, scholarship, or quality improvement. Why provide supervision if a clinical competency committee has deemed a fellow ready for unsupervised practice? Some level of observation and formative feedback could continue, but full supervision would be redundant and unnecessary. CBTVT would allow for some fellows to experience the uncertainty that comes with unsupervised decision-making while still in an environment with trusted fellowship mentors and advisors.
STEPS TOWARD CHANGE
PHM fellowship programs likely cannot flip a switch to “turn on” CBTVT immediately, but they can take steps toward making the transition. Validity, or defensibility of decisions, will be crucial for assessment in CBTVT systems. Programs will need to develop robust assessment systems that collect myriad data to answer the question, “When is this learner competent to deliver high-quality care without supervision?” Programs can align assessment instruments, faculty-development initiatives, and clinical competency committee (CCC) processes with the 2020 PHM competencies to provide a defensible answer. Program leaders should then seek validity evidence, either in existing literature or through novel scholarly initiatives, to support these summative decisions. Engaging all fellowship stakeholders in transitions to CBTVT will be important and should include fellows, program directors, CCC members, clinical leadership, and members from accrediting and credentialing bodies.
CONCLUSION
As fellowship programs review and revise curricula and assessment systems around the updated PHM core competencies, they should also consider what changes are necessary to implement CBTVT. Time variability is not a novelty but, rather, is a corollary to the outcomes-based approach of CBME. PHM fellowships should strike while the iron is hot and build on current change initiatives prompted by the growth of our specialty to be leaders in CBTVT.
1. Maniscalco J, Gage S, Sofia Teferi M, Fisher ES. The Pediatric Hospital Medicine Core Competencies: 2020 Revision. J Hosp Med. 2020;15(7):389-394. https://doi.org/10.12788/jhm.3391
2. Frank JR, Snell LS, Cate OT, et al. Competency-based medical education: theory to practice. Med Teach. 2010;32(8):638-645. https://doi.org/10.3109/0142159X.2010.501190
3. Lucey CR, Thibault GE, Ten Cate O. Competency-based, tme-variable education in the health professions: crossroads. Acad Med. 2018;93(3S Competency-Based, Time-Variable Education in the Health Professions):S1-S5. https://doi.org/10.1097/ACM.0000000000002080
4. Custers EJFM, Ten Cate O. The history of medical education in Europe and the United States, with respect to time and proficiency. Acad Med. 2018;93(3S Competency-Based, Time-Variable Education in the Health Professions):S49-S54. https://doi.org/10.1097/ACM.0000000000002079
5. Barr DA. Revolution or evolution? Putting the Flexner Report in context. Med Educ. 2011;45(1):17-22. https://doi.org/10.1111/j.1365-2923.2010.03850.x
6. Association of American Medical Colleges. Minutes of the Fifteenth Annual Meeting. April 10, 1905; Chicago, IL.
7. Bevan A. Council on Medical Education of the American Medical Association. JAMA. 1907;48(20):1701-1707.
8. Flexner A. Medical education in the United States and Canada. From the Carnegie Foundation for the Advancement of Teaching, Bulletin Number Four, 1910. Bull World Health Organ. 2002;80(7):594-602.
9. McGaghie WC, Sajid AW, Miller GE, et al. Competency-based curriculum development in medical education: an introduction. Public Health Pap. 1978;(68):11-91.
10. Frank JR, Snell L, Englander R, Holmboe ES, ICBME Collaborators. Implementing competency-based medical education: moving forward. Med Teach. 2017;39(6):568-573. https://doi.org/10.1080/0142159X.2017.1315069
11. Schumacher DJ, West DC, Schwartz A, et al. Longitudinal assessment of resident performance using entrustable professional activities. JAMA Netw Open. 2020;3(1):e1919316. https://doi.org/10.1001/jamanetworkopen.2019.19316
12. Warm EJ, Held J, Hellman M, et al. Entrusting observable practice activities and milestones over the 36 months of an internal medicine residency. Acad Med. 2016;91(10):1398-1405. https://doi.org/10.1097/ACM.0000000000001292
13. Jason H. Effective medical instruction: requirements and possibilities. In: Proceedings of a 1969 International Symposium on Medical Education. Medica; 1970:5-8.
14. Andrews JS, Bale JF Jr, Soep JB, et al. Education in Pediatrics Across the Continuum (EPAC): first steps toward realizing the dream of competency-based education. Acad Med. 2018;93(3):414-420. https://doi.org/10.1097/ACM.0000000000002020
15. Mejicano GC, Bumsted TN. Describing the journey and lessons learned implementing a competency-based, time-variable undergraduate medical education curriculum. Acad Med. 2018;93(3S Competency-Based, Time-Variable Education in the Health Professions):S42-S48. https://doi.org/10.1097/ACM.0000000000002068
16. Goldhamer MEJ, Pusic MV, Co JPT, Weinstein DF. Can COVID catalyze an educational transformation? Competency-based advancement in a crisis. N Engl J Med. 2020;383(11):1003-1005. https://doi.org/10.1056/NEJMp2018570
17. Murray KE, Lane JL, Carraccio C, et al. Crossing the gap: using competency-based assessment to determine whether learners are ready for the undergraduate-to-graduate transition. Acad Med. 2019;94(3):338-345. https://doi.org/10.1097/ACM.0000000000002535
18. Schwartz A, Balmer DF, Borman-Shoap E, et al. Shared mental models among clinical competency committees in the context of time-variable, competency-based advancement to residency. Acad Med. 2020;95(11S Association of American Medical Colleges Learn Serve Lead: Proceedings of the 59th Annual Research in Medical Education Presentations):S95-S102. https://doi.org/10.1097/ACM.0000000000003638
19. Kotter JP. Leading change: why transformation efforts fail. Harvard Business Review. May-June 1995. Accessed March 1, 2021. https://hbr.org/1995/05/leading-change-why-transformation-efforts-fail-2
20. Schumacher DJ, Caretta-Weyer H, Busari J, et al. Competency-based time-variable training internationally: ensuring practical next steps. Med Teach. Forthcoming.
1. Maniscalco J, Gage S, Sofia Teferi M, Fisher ES. The Pediatric Hospital Medicine Core Competencies: 2020 Revision. J Hosp Med. 2020;15(7):389-394. https://doi.org/10.12788/jhm.3391
2. Frank JR, Snell LS, Cate OT, et al. Competency-based medical education: theory to practice. Med Teach. 2010;32(8):638-645. https://doi.org/10.3109/0142159X.2010.501190
3. Lucey CR, Thibault GE, Ten Cate O. Competency-based, tme-variable education in the health professions: crossroads. Acad Med. 2018;93(3S Competency-Based, Time-Variable Education in the Health Professions):S1-S5. https://doi.org/10.1097/ACM.0000000000002080
4. Custers EJFM, Ten Cate O. The history of medical education in Europe and the United States, with respect to time and proficiency. Acad Med. 2018;93(3S Competency-Based, Time-Variable Education in the Health Professions):S49-S54. https://doi.org/10.1097/ACM.0000000000002079
5. Barr DA. Revolution or evolution? Putting the Flexner Report in context. Med Educ. 2011;45(1):17-22. https://doi.org/10.1111/j.1365-2923.2010.03850.x
6. Association of American Medical Colleges. Minutes of the Fifteenth Annual Meeting. April 10, 1905; Chicago, IL.
7. Bevan A. Council on Medical Education of the American Medical Association. JAMA. 1907;48(20):1701-1707.
8. Flexner A. Medical education in the United States and Canada. From the Carnegie Foundation for the Advancement of Teaching, Bulletin Number Four, 1910. Bull World Health Organ. 2002;80(7):594-602.
9. McGaghie WC, Sajid AW, Miller GE, et al. Competency-based curriculum development in medical education: an introduction. Public Health Pap. 1978;(68):11-91.
10. Frank JR, Snell L, Englander R, Holmboe ES, ICBME Collaborators. Implementing competency-based medical education: moving forward. Med Teach. 2017;39(6):568-573. https://doi.org/10.1080/0142159X.2017.1315069
11. Schumacher DJ, West DC, Schwartz A, et al. Longitudinal assessment of resident performance using entrustable professional activities. JAMA Netw Open. 2020;3(1):e1919316. https://doi.org/10.1001/jamanetworkopen.2019.19316
12. Warm EJ, Held J, Hellman M, et al. Entrusting observable practice activities and milestones over the 36 months of an internal medicine residency. Acad Med. 2016;91(10):1398-1405. https://doi.org/10.1097/ACM.0000000000001292
13. Jason H. Effective medical instruction: requirements and possibilities. In: Proceedings of a 1969 International Symposium on Medical Education. Medica; 1970:5-8.
14. Andrews JS, Bale JF Jr, Soep JB, et al. Education in Pediatrics Across the Continuum (EPAC): first steps toward realizing the dream of competency-based education. Acad Med. 2018;93(3):414-420. https://doi.org/10.1097/ACM.0000000000002020
15. Mejicano GC, Bumsted TN. Describing the journey and lessons learned implementing a competency-based, time-variable undergraduate medical education curriculum. Acad Med. 2018;93(3S Competency-Based, Time-Variable Education in the Health Professions):S42-S48. https://doi.org/10.1097/ACM.0000000000002068
16. Goldhamer MEJ, Pusic MV, Co JPT, Weinstein DF. Can COVID catalyze an educational transformation? Competency-based advancement in a crisis. N Engl J Med. 2020;383(11):1003-1005. https://doi.org/10.1056/NEJMp2018570
17. Murray KE, Lane JL, Carraccio C, et al. Crossing the gap: using competency-based assessment to determine whether learners are ready for the undergraduate-to-graduate transition. Acad Med. 2019;94(3):338-345. https://doi.org/10.1097/ACM.0000000000002535
18. Schwartz A, Balmer DF, Borman-Shoap E, et al. Shared mental models among clinical competency committees in the context of time-variable, competency-based advancement to residency. Acad Med. 2020;95(11S Association of American Medical Colleges Learn Serve Lead: Proceedings of the 59th Annual Research in Medical Education Presentations):S95-S102. https://doi.org/10.1097/ACM.0000000000003638
19. Kotter JP. Leading change: why transformation efforts fail. Harvard Business Review. May-June 1995. Accessed March 1, 2021. https://hbr.org/1995/05/leading-change-why-transformation-efforts-fail-2
20. Schumacher DJ, Caretta-Weyer H, Busari J, et al. Competency-based time-variable training internationally: ensuring practical next steps. Med Teach. Forthcoming.
© 2021 Society of Hospital Medicine
Development and Evolution of Hospital Medicine in Korea
The healthcare system in South Korea (Korea) is evolving. Korea began developing a national health insurance system for the entire population in 1989, and implementation took 12 years. The healthcare insurance premium was set in 1989 when the Korean gross domestic product (GDP) per capita was less than $5,000 USD. Since then, the incremental rise in healthcare insurance premiums, approximately 6% of the typical Korean income, has been relatively small considering the economic growth of Korea.1-3 The success and rapid adoption of national health insurance in Korea revealed unanticipated problems in three specific areas: low individual contributions relative to costs of care, low levels of reimbursement to providers, and incomplete coverage of medical services, which then require out-of-pocket payments (typically 20% of the inpatient care fee).4 Additionally, while little attention has been paid to quality and safety in the past, the nation has come to recognize the importance of these considerations,5 particularly with regard to patient expectations for and consumption of medical care and the consequent demand for better services from the medical community and government.6
Healthcare constitutes about 7.5% of Korea’s GDP. In contrast, healthcare spending accounts for about 17.7% of US GDP and about 8.8% of the GDP of member nations of the Organisation for Economic Co-operation and Development (OECD) in aggregate. Additionally, Korea has a longer length of hospital stay (16.5 days vs 7.3 days for OECD countries) and fewer practicing physicians (1.1 per 1,000 persons vs 1.9 per 1,000 for OECD countries). Therefore, the average cumulative annual patient hospital days per physician is 2,394 days in Korea, three times higher than that in the OECD countries.7 Furthermore, the number of physicians providing hospital care in Korea has remained relatively low.8
Despite recent growth in the total number of practicing physicians, the number of hospital-based physicians remains insufficient to cover admitted patients. The pressure for hospital-based physicians to serve large numbers of patients to generate sufficient revenue has deterred growth of more physicians focused on inpatient care.6 In order to operate the hospital, doctors must see as many patients as possible because physicians are reimbursed primarily by an inpatient care fee rather than by type or extent of care provided. Therefore, this low fee schedule makes it difficult to provide good quality inpatient care while simultaneously trying to increase accessibility. The costs of testing and treatment are reimbursed through a separate fee schedule.
THE NEED FOR A HOSPITALIST SYSTEM IN KOREA
The Korean government enacted two new policies regarding medical school graduates and residents that led to implementation of a hospitalist system. The first policy created a quota of medical residents to match the total number of medical school graduates.9 Before the new regulation, the number of medical resident positions exceeded the number of medical school graduates by 20%, which led to a shortage of resident applicants in “unpopular” medical specialties or departments. These specialties (eg, general surgery, obstetrics and gynecology, urology) have a lower fee schedule, which leads to lower wages while having very high workloads. The decrease in the number of available medical resident positions results in greater workloads for physicians providing inpatient care. These changes, including the shortage of a resident workforce, led to a burgeoning hospital-based attending physician workforce to care for hospitalized patients.
The other policy, created in December 2015 and known as the Act on the Improvement of Training Conditions and Status of Medical Residents, was enacted to improve working conditions for residents by limiting their working hours to 80 or fewer per week.10 These work-hour restrictions made it nearly impossible to provide 24-hour inpatient care depending solely on residents.10-12 This policy increased the need for hospitalists in Korea, similar to that of the US Accreditation Council for Graduate Medical Education in 2003, which limited resident work hours. Because of these changes, the hospitalist model was introduced to manage the growing volume of inpatients at teaching hospitals.13
The institutional implementation of the Korean hospitalist system was catalyzed by the need to solve current problems such as patient safety, healthcare quality, and residents’ well-being.8,14 Therefore, the hospitalist system was designed and implemented to meet the needs of patients, medical professionals, and hospitals.6,8,14 Furthermore, under the universal health insurance system in Korea, institutional implementation also meant creating a new set of fee schedules for hospitalists. The Korean hospitalist system reflects the nuances and challenges faced by the Korean healthcare system.5
DESIGN, IMPLEMENTATION, AND EVALUATION
A council was formed to implement a hospitalist model suitable for Korea. The council was composed of five groups: the Korean Association of Internal Medicine, Korean Surgical Society, Korean Medical Association, Korean Hospital Association, and Korean Academy of Medical Sciences. The Korean Association of Internal Medicine and the Korean Surgical Society were involved in creating a new medical discipline because their members provided a disproportionate amount of inpatient care and were most often responsible for hospital patient safety and quality care. Along with the support of the council, the Ministry of Health and Welfare began to realize the high demand on inpatient care and requested an official proposal to broaden the hospitalist model, with two conditions. First, the government requested a unified proposal from the medical community that reflected the collective voices of individual stakeholders, with an expectation that the new system would focus on patient safety and healthcare quality across all specialties. Second, the proposal had to be detailed and include a specific fee schedule for hospitalist services.
Before implementing a national hospitalist system, we conducted a pilot study supported by funding from the council. This first study, the Korean Hospitalist System Operation and Evaluation Research (September 2015 to August 2016)14, was designed to (a) determine hospitalist needs (eg, rotation schedule, salary, working conditions) for this new profession, (b) determine the necessary number of hospitalists and appropriate fee schedules to cover salary and benefits, and (c) provide Korean hospitalist models with operational methods to facilitate implementation at individual institutions. The council and research team selected four hospitals for the privately funded pilot study (Table). These hospitals already had an inpatient care system managed by specialists prior to the pilot study, so major changes in patient care and hospital operations were not required.
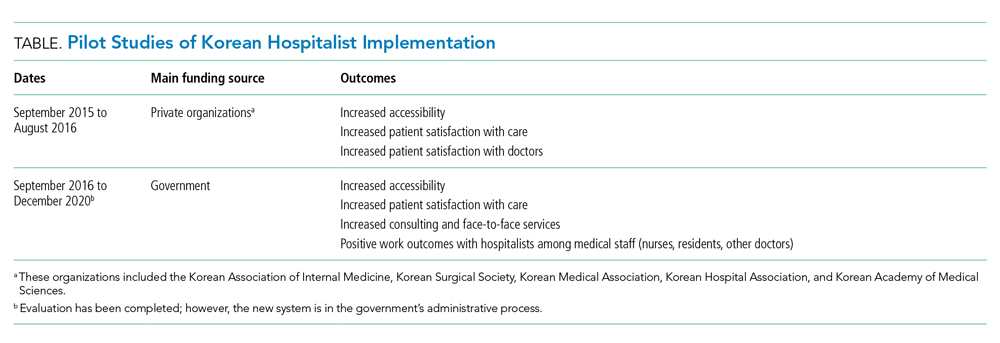
The pilot study demonstrated improvements in patient satisfaction, medical service quality, and patient safety.8,14,15 As a result, fees billed from hospitalist services were now covered by national health insurance; previously, they were not covered by any inpatient care fee or bundled service fee. The next study (phase 2) was then conducted to evaluate the national implementation of the hospitalist system and its outcomes. The first phase defined criteria for monitoring and evaluating the institutionalized hospitalist system in Korea. The second phase, initiated in September 2016, evaluated how the implemented system could be expanded nationwide. The government was actively involved in the study; 31 of 344 (9%) general hospitals were selected and able to apply the hospitalist fee schedule for care of patients who were admitted to the hospitalist ward.
To investigate the effect of the hospitalist system, we measured the satisfaction of patients and medical providers (eg, specialists, residents, and nurses). The outcomes were number of doctors’ calls to hospitalists and to other specialties, duration of time required to address medical problems, number of contacts with the hospitalist (residents or other physicians in the control group), and time spent with hospitalized patients. In addition, we analyzed claims costs and the operating costs for implementing the system within each hospital (Table).
The new set of fee schedules was created specifically for hospitalist care services so hospitals could now claim separate inpatient care fees and hospitalist fees. The standard hospitalist fee schedule is applied only to patients who are on hospitalist-led wards. The fee schedule has two criteria: The hospitalist ward must have 50 beds which are assigned to hospitalists only, and each hospitalist ward has a team of five hospitalists. A maximum of 25 patients could be assigned to an individual hospitalist. However, fees can be adjusted based on individual hospital operation and management after government approval.
Under the national insurance system, the fee schedule is essential to obtain reimbursement for provided services. As a new medical profession, it is important to have the institutional implementation of the hospitalist system. In contrast to those in the United States, inpatients in Korea pay inpatient care fees only, which are charged per day. Therefore, reducing the length of stay without increasing patient volume does not financially benefit the hospital.
DEFINITION OF A KOREAN HOSPITALIST AND SCOPE OF PRACTICE
The definition of a Korean hospitalist was carefully developed, and a reimbursement system for hospitalists was created within the Korean healthcare system. All hospitalists are medical specialists who have completed advanced education and clinical training in their specialty area (internal medicine or surgery). Creating a definition of a hospitalist and a standard fee schedule was necessary for national incorporation of this new approach. The model must also be accepted by related parties, including healthcare professionals such as hospital executives, nonhospitalist doctors, and nurses. Therefore, the system has a minimum of two requirements to operate at the hospital level, and the remaining factors can be modified by individual hospitals to protect the new discipline within the healthcare system. First, hospitalist services are provided only to hospitalist patients. To establish the new system, limiting the patient range to a specific group was necessary to prevent abuse of human-based resources within the hospital. Second, hospitalists must be stationed near the hospitalist ward to enhance accessibility, patient safety, and healthcare quality. In other words, a hospitalist provides services to hospitalized patients who paid the hospitalist fee, and the hospitalist does not provide care beyond the hospitalist ward (eg, they cannot provide outpatient consultation or provide care on other wards).
INPATIENT CARE REIMBURSEMENT BEFORE AND AFTER IMPLEMENTATION
Korean national health insurance is the universal health insurance under a single insurer (ie, the government). Therefore, all Korean citizens have national health insurance. Individuals can choose to have additional health insurance (private insurance) if they wish to pay an out-of-pocket fee with their additional private insurance. In Korea, the inpatient care fee includes all fees to provide care for the patient during hospital stays, such as the physician fee, facility fee, and consultation fee. The inpatient care fee schedule is charged per day including all the inpatient care composition. The claim and reimbursement system is different in Korea in that the hospital as a whole submits the claim for reimbursement. There is no separate claim from physicians, lab technicians, or facilities. Doctors provide care to patients and are then paid for the services in terms of salaries. Since the original inpatient care fee schedule was low and insufficient, it was difficult to provide high-quality care. Also, resources for spending on inpatient care management was limited. So a new system to improve inpatient care was needed. Implementing the hospitalist system in Korea led to the creation of a new fee schedule specifically for hospitalists so that hospitals could claim hospitalist fees on top of inpatient care fees when the patient was cared for by the hospitalist. The additional fees in the hospitalist fee schedule allow safe and high-quality care to be provided for patients during hospital stays.
KOREAN HOSPITAL MEDICINE TODAY
The Korean hospitalist system has been implemented for 3 years with the government’s active involvement, with approximately 250 specialists working as hospitalists as of August 2020. Importantly, Korean hospitalists are not limited to internal medicine specialists; in fact, several different specialties practice as hospitalists, including surgeons and other medical specialists, who account for 20% and 30% of the hospitalist workforce, respectively. Since the system’s implementation, all inpatient care and management is now transferred to specialists from residents in hospitalist wards. This change has increased patient safety and care quality. At the beginning of the pilot study, the concept of a “hospitalist” was new in Korea and the public did not know who a hospitalist was nor what a hospitalist did. However, after 3 years, patients now seek hospitalist care.
CONCLUSION
National implementation of the hospitalist model represents a key paradigm shift in the Korean healthcare system. The Korean hospitalist system is the result of the hospitals’, doctors’, and patients’ desire for higher-quality care. We expect to see growth of hospitalists in Korea and provision of better, safer, and more efficient inpatient care across specialties, payers, and government. Since we are at the early stage of the system, further efforts to support implementation are required. We hope our implementation process of a new medical system could serve as a model for other countries who are seeking to adopt hospitalist systems within their current healthcare paradigms.
Acknowledgments
The authors thank the medical professionals, government officers, and other professionals who put great effort into the implementation of the Korean Hospitalist System.
1. Kwon S. Thirty years of national health insurance in South Korea: lessons for achieving universal health care coverage. Health Policy Plan. 2009;24(1):63-71. https://doi.org/10.1093/heapol/czn037
2. Song YJ. The South Korean health care system. JMAJ. 2009;52(3):206-209.
3. Park YH, Park E-C. Healthcare policy. In: Preventive Medicine and Public Health. Vol 3.Gyechuk; 2017:821-833.
4. Park E-C, Lee TJ, Jun BY, Jung SH, Jeong HS. Health security. In: Preventive Medicine and Public Health. Vol 3. Gyechuk; 2017:888-897.
5. Jang S-I, Jang S-y, Park E-C. Trends of US hospitalist and suggestions for introduction of Korean hospitalist. Korean J Med. 2015;89(1):1-5. http://doi.org/10.3904/kjm.2015.89.1.1
6. Jang S-I. Korean hospitalist system implementation and development strategies based on pilot studies. J Korean Med Assoc. 2019;62(11):558-563. http://doi.org/10.5124/jkma.2019.62.11.558
7. OECD. Health at a Glance 2017: OECD indicators. OECD iLibrary. 2017. Accessed September 27, 2019. https://doi.org/10.1787/health_glance-2017-en
8. Jang S-I, Park E-C, Nam JM, et al. A study on the implementation and the evaluation of Korean Hospitalist System to improve the quality of hospitalization (Phase 2). Institute of Health Services Research, Yonsei University; 2018.
9. Koh DY. Political strategies to enhance medical residents. Health Policy. Vol 37. Seoul National University Hospital; 2014.
10. Act on the Improvement of Training Conditions and Status of Medical Residents. Vol No. 16260: Ministry of Health and Welfare; 2015.
11. Eom JS. Operating the hospitalist system. J Korean Med Assoc. 2016;59(5):342-344. http://doi.org/10.5124/jkma.2016.59.5.342
12. Kim S-S. Working conditions of interns/residents and patient safety: Painful training might not be authentic. J Korean Med Assoc. 2016;59(2):82-84. http://dx.doi.org/10.5124/jkma.2016.59.2.82
13. Oshimura J, Sperring J, Bauer BD, Rauch DA. Inpatient staffing within pediatric residency programs: work hour restrictions and the evolving role of the pediatric hospitalist. J Hosp Med. 2012;7(4):299-303. https://doi.org/10.1002/jhm.952
14. Park E-C, Lee SG, Kim T-H, et al. A study on the implementation and the evaluation of Korean Hospitalist System to improve the quality of hospitalization (Phase 1). Institute of Health Services Research, Yonsei University; 2016.
15. Jang S-I, Jung E-J, Park SK, Chae W, Kim Y-K. A study on the feasibility of the Korean hospitalist system and cost estimation. Ministry of Health and Welfare, Institute of Health Service Research Yonsei University; 2019.
The healthcare system in South Korea (Korea) is evolving. Korea began developing a national health insurance system for the entire population in 1989, and implementation took 12 years. The healthcare insurance premium was set in 1989 when the Korean gross domestic product (GDP) per capita was less than $5,000 USD. Since then, the incremental rise in healthcare insurance premiums, approximately 6% of the typical Korean income, has been relatively small considering the economic growth of Korea.1-3 The success and rapid adoption of national health insurance in Korea revealed unanticipated problems in three specific areas: low individual contributions relative to costs of care, low levels of reimbursement to providers, and incomplete coverage of medical services, which then require out-of-pocket payments (typically 20% of the inpatient care fee).4 Additionally, while little attention has been paid to quality and safety in the past, the nation has come to recognize the importance of these considerations,5 particularly with regard to patient expectations for and consumption of medical care and the consequent demand for better services from the medical community and government.6
Healthcare constitutes about 7.5% of Korea’s GDP. In contrast, healthcare spending accounts for about 17.7% of US GDP and about 8.8% of the GDP of member nations of the Organisation for Economic Co-operation and Development (OECD) in aggregate. Additionally, Korea has a longer length of hospital stay (16.5 days vs 7.3 days for OECD countries) and fewer practicing physicians (1.1 per 1,000 persons vs 1.9 per 1,000 for OECD countries). Therefore, the average cumulative annual patient hospital days per physician is 2,394 days in Korea, three times higher than that in the OECD countries.7 Furthermore, the number of physicians providing hospital care in Korea has remained relatively low.8
Despite recent growth in the total number of practicing physicians, the number of hospital-based physicians remains insufficient to cover admitted patients. The pressure for hospital-based physicians to serve large numbers of patients to generate sufficient revenue has deterred growth of more physicians focused on inpatient care.6 In order to operate the hospital, doctors must see as many patients as possible because physicians are reimbursed primarily by an inpatient care fee rather than by type or extent of care provided. Therefore, this low fee schedule makes it difficult to provide good quality inpatient care while simultaneously trying to increase accessibility. The costs of testing and treatment are reimbursed through a separate fee schedule.
THE NEED FOR A HOSPITALIST SYSTEM IN KOREA
The Korean government enacted two new policies regarding medical school graduates and residents that led to implementation of a hospitalist system. The first policy created a quota of medical residents to match the total number of medical school graduates.9 Before the new regulation, the number of medical resident positions exceeded the number of medical school graduates by 20%, which led to a shortage of resident applicants in “unpopular” medical specialties or departments. These specialties (eg, general surgery, obstetrics and gynecology, urology) have a lower fee schedule, which leads to lower wages while having very high workloads. The decrease in the number of available medical resident positions results in greater workloads for physicians providing inpatient care. These changes, including the shortage of a resident workforce, led to a burgeoning hospital-based attending physician workforce to care for hospitalized patients.
The other policy, created in December 2015 and known as the Act on the Improvement of Training Conditions and Status of Medical Residents, was enacted to improve working conditions for residents by limiting their working hours to 80 or fewer per week.10 These work-hour restrictions made it nearly impossible to provide 24-hour inpatient care depending solely on residents.10-12 This policy increased the need for hospitalists in Korea, similar to that of the US Accreditation Council for Graduate Medical Education in 2003, which limited resident work hours. Because of these changes, the hospitalist model was introduced to manage the growing volume of inpatients at teaching hospitals.13
The institutional implementation of the Korean hospitalist system was catalyzed by the need to solve current problems such as patient safety, healthcare quality, and residents’ well-being.8,14 Therefore, the hospitalist system was designed and implemented to meet the needs of patients, medical professionals, and hospitals.6,8,14 Furthermore, under the universal health insurance system in Korea, institutional implementation also meant creating a new set of fee schedules for hospitalists. The Korean hospitalist system reflects the nuances and challenges faced by the Korean healthcare system.5
DESIGN, IMPLEMENTATION, AND EVALUATION
A council was formed to implement a hospitalist model suitable for Korea. The council was composed of five groups: the Korean Association of Internal Medicine, Korean Surgical Society, Korean Medical Association, Korean Hospital Association, and Korean Academy of Medical Sciences. The Korean Association of Internal Medicine and the Korean Surgical Society were involved in creating a new medical discipline because their members provided a disproportionate amount of inpatient care and were most often responsible for hospital patient safety and quality care. Along with the support of the council, the Ministry of Health and Welfare began to realize the high demand on inpatient care and requested an official proposal to broaden the hospitalist model, with two conditions. First, the government requested a unified proposal from the medical community that reflected the collective voices of individual stakeholders, with an expectation that the new system would focus on patient safety and healthcare quality across all specialties. Second, the proposal had to be detailed and include a specific fee schedule for hospitalist services.
Before implementing a national hospitalist system, we conducted a pilot study supported by funding from the council. This first study, the Korean Hospitalist System Operation and Evaluation Research (September 2015 to August 2016)14, was designed to (a) determine hospitalist needs (eg, rotation schedule, salary, working conditions) for this new profession, (b) determine the necessary number of hospitalists and appropriate fee schedules to cover salary and benefits, and (c) provide Korean hospitalist models with operational methods to facilitate implementation at individual institutions. The council and research team selected four hospitals for the privately funded pilot study (Table). These hospitals already had an inpatient care system managed by specialists prior to the pilot study, so major changes in patient care and hospital operations were not required.

The pilot study demonstrated improvements in patient satisfaction, medical service quality, and patient safety.8,14,15 As a result, fees billed from hospitalist services were now covered by national health insurance; previously, they were not covered by any inpatient care fee or bundled service fee. The next study (phase 2) was then conducted to evaluate the national implementation of the hospitalist system and its outcomes. The first phase defined criteria for monitoring and evaluating the institutionalized hospitalist system in Korea. The second phase, initiated in September 2016, evaluated how the implemented system could be expanded nationwide. The government was actively involved in the study; 31 of 344 (9%) general hospitals were selected and able to apply the hospitalist fee schedule for care of patients who were admitted to the hospitalist ward.
To investigate the effect of the hospitalist system, we measured the satisfaction of patients and medical providers (eg, specialists, residents, and nurses). The outcomes were number of doctors’ calls to hospitalists and to other specialties, duration of time required to address medical problems, number of contacts with the hospitalist (residents or other physicians in the control group), and time spent with hospitalized patients. In addition, we analyzed claims costs and the operating costs for implementing the system within each hospital (Table).
The new set of fee schedules was created specifically for hospitalist care services so hospitals could now claim separate inpatient care fees and hospitalist fees. The standard hospitalist fee schedule is applied only to patients who are on hospitalist-led wards. The fee schedule has two criteria: The hospitalist ward must have 50 beds which are assigned to hospitalists only, and each hospitalist ward has a team of five hospitalists. A maximum of 25 patients could be assigned to an individual hospitalist. However, fees can be adjusted based on individual hospital operation and management after government approval.
Under the national insurance system, the fee schedule is essential to obtain reimbursement for provided services. As a new medical profession, it is important to have the institutional implementation of the hospitalist system. In contrast to those in the United States, inpatients in Korea pay inpatient care fees only, which are charged per day. Therefore, reducing the length of stay without increasing patient volume does not financially benefit the hospital.
DEFINITION OF A KOREAN HOSPITALIST AND SCOPE OF PRACTICE
The definition of a Korean hospitalist was carefully developed, and a reimbursement system for hospitalists was created within the Korean healthcare system. All hospitalists are medical specialists who have completed advanced education and clinical training in their specialty area (internal medicine or surgery). Creating a definition of a hospitalist and a standard fee schedule was necessary for national incorporation of this new approach. The model must also be accepted by related parties, including healthcare professionals such as hospital executives, nonhospitalist doctors, and nurses. Therefore, the system has a minimum of two requirements to operate at the hospital level, and the remaining factors can be modified by individual hospitals to protect the new discipline within the healthcare system. First, hospitalist services are provided only to hospitalist patients. To establish the new system, limiting the patient range to a specific group was necessary to prevent abuse of human-based resources within the hospital. Second, hospitalists must be stationed near the hospitalist ward to enhance accessibility, patient safety, and healthcare quality. In other words, a hospitalist provides services to hospitalized patients who paid the hospitalist fee, and the hospitalist does not provide care beyond the hospitalist ward (eg, they cannot provide outpatient consultation or provide care on other wards).
INPATIENT CARE REIMBURSEMENT BEFORE AND AFTER IMPLEMENTATION
Korean national health insurance is the universal health insurance under a single insurer (ie, the government). Therefore, all Korean citizens have national health insurance. Individuals can choose to have additional health insurance (private insurance) if they wish to pay an out-of-pocket fee with their additional private insurance. In Korea, the inpatient care fee includes all fees to provide care for the patient during hospital stays, such as the physician fee, facility fee, and consultation fee. The inpatient care fee schedule is charged per day including all the inpatient care composition. The claim and reimbursement system is different in Korea in that the hospital as a whole submits the claim for reimbursement. There is no separate claim from physicians, lab technicians, or facilities. Doctors provide care to patients and are then paid for the services in terms of salaries. Since the original inpatient care fee schedule was low and insufficient, it was difficult to provide high-quality care. Also, resources for spending on inpatient care management was limited. So a new system to improve inpatient care was needed. Implementing the hospitalist system in Korea led to the creation of a new fee schedule specifically for hospitalists so that hospitals could claim hospitalist fees on top of inpatient care fees when the patient was cared for by the hospitalist. The additional fees in the hospitalist fee schedule allow safe and high-quality care to be provided for patients during hospital stays.
KOREAN HOSPITAL MEDICINE TODAY
The Korean hospitalist system has been implemented for 3 years with the government’s active involvement, with approximately 250 specialists working as hospitalists as of August 2020. Importantly, Korean hospitalists are not limited to internal medicine specialists; in fact, several different specialties practice as hospitalists, including surgeons and other medical specialists, who account for 20% and 30% of the hospitalist workforce, respectively. Since the system’s implementation, all inpatient care and management is now transferred to specialists from residents in hospitalist wards. This change has increased patient safety and care quality. At the beginning of the pilot study, the concept of a “hospitalist” was new in Korea and the public did not know who a hospitalist was nor what a hospitalist did. However, after 3 years, patients now seek hospitalist care.
CONCLUSION
National implementation of the hospitalist model represents a key paradigm shift in the Korean healthcare system. The Korean hospitalist system is the result of the hospitals’, doctors’, and patients’ desire for higher-quality care. We expect to see growth of hospitalists in Korea and provision of better, safer, and more efficient inpatient care across specialties, payers, and government. Since we are at the early stage of the system, further efforts to support implementation are required. We hope our implementation process of a new medical system could serve as a model for other countries who are seeking to adopt hospitalist systems within their current healthcare paradigms.
Acknowledgments
The authors thank the medical professionals, government officers, and other professionals who put great effort into the implementation of the Korean Hospitalist System.
The healthcare system in South Korea (Korea) is evolving. Korea began developing a national health insurance system for the entire population in 1989, and implementation took 12 years. The healthcare insurance premium was set in 1989 when the Korean gross domestic product (GDP) per capita was less than $5,000 USD. Since then, the incremental rise in healthcare insurance premiums, approximately 6% of the typical Korean income, has been relatively small considering the economic growth of Korea.1-3 The success and rapid adoption of national health insurance in Korea revealed unanticipated problems in three specific areas: low individual contributions relative to costs of care, low levels of reimbursement to providers, and incomplete coverage of medical services, which then require out-of-pocket payments (typically 20% of the inpatient care fee).4 Additionally, while little attention has been paid to quality and safety in the past, the nation has come to recognize the importance of these considerations,5 particularly with regard to patient expectations for and consumption of medical care and the consequent demand for better services from the medical community and government.6
Healthcare constitutes about 7.5% of Korea’s GDP. In contrast, healthcare spending accounts for about 17.7% of US GDP and about 8.8% of the GDP of member nations of the Organisation for Economic Co-operation and Development (OECD) in aggregate. Additionally, Korea has a longer length of hospital stay (16.5 days vs 7.3 days for OECD countries) and fewer practicing physicians (1.1 per 1,000 persons vs 1.9 per 1,000 for OECD countries). Therefore, the average cumulative annual patient hospital days per physician is 2,394 days in Korea, three times higher than that in the OECD countries.7 Furthermore, the number of physicians providing hospital care in Korea has remained relatively low.8
Despite recent growth in the total number of practicing physicians, the number of hospital-based physicians remains insufficient to cover admitted patients. The pressure for hospital-based physicians to serve large numbers of patients to generate sufficient revenue has deterred growth of more physicians focused on inpatient care.6 In order to operate the hospital, doctors must see as many patients as possible because physicians are reimbursed primarily by an inpatient care fee rather than by type or extent of care provided. Therefore, this low fee schedule makes it difficult to provide good quality inpatient care while simultaneously trying to increase accessibility. The costs of testing and treatment are reimbursed through a separate fee schedule.
THE NEED FOR A HOSPITALIST SYSTEM IN KOREA
The Korean government enacted two new policies regarding medical school graduates and residents that led to implementation of a hospitalist system. The first policy created a quota of medical residents to match the total number of medical school graduates.9 Before the new regulation, the number of medical resident positions exceeded the number of medical school graduates by 20%, which led to a shortage of resident applicants in “unpopular” medical specialties or departments. These specialties (eg, general surgery, obstetrics and gynecology, urology) have a lower fee schedule, which leads to lower wages while having very high workloads. The decrease in the number of available medical resident positions results in greater workloads for physicians providing inpatient care. These changes, including the shortage of a resident workforce, led to a burgeoning hospital-based attending physician workforce to care for hospitalized patients.
The other policy, created in December 2015 and known as the Act on the Improvement of Training Conditions and Status of Medical Residents, was enacted to improve working conditions for residents by limiting their working hours to 80 or fewer per week.10 These work-hour restrictions made it nearly impossible to provide 24-hour inpatient care depending solely on residents.10-12 This policy increased the need for hospitalists in Korea, similar to that of the US Accreditation Council for Graduate Medical Education in 2003, which limited resident work hours. Because of these changes, the hospitalist model was introduced to manage the growing volume of inpatients at teaching hospitals.13
The institutional implementation of the Korean hospitalist system was catalyzed by the need to solve current problems such as patient safety, healthcare quality, and residents’ well-being.8,14 Therefore, the hospitalist system was designed and implemented to meet the needs of patients, medical professionals, and hospitals.6,8,14 Furthermore, under the universal health insurance system in Korea, institutional implementation also meant creating a new set of fee schedules for hospitalists. The Korean hospitalist system reflects the nuances and challenges faced by the Korean healthcare system.5
DESIGN, IMPLEMENTATION, AND EVALUATION
A council was formed to implement a hospitalist model suitable for Korea. The council was composed of five groups: the Korean Association of Internal Medicine, Korean Surgical Society, Korean Medical Association, Korean Hospital Association, and Korean Academy of Medical Sciences. The Korean Association of Internal Medicine and the Korean Surgical Society were involved in creating a new medical discipline because their members provided a disproportionate amount of inpatient care and were most often responsible for hospital patient safety and quality care. Along with the support of the council, the Ministry of Health and Welfare began to realize the high demand on inpatient care and requested an official proposal to broaden the hospitalist model, with two conditions. First, the government requested a unified proposal from the medical community that reflected the collective voices of individual stakeholders, with an expectation that the new system would focus on patient safety and healthcare quality across all specialties. Second, the proposal had to be detailed and include a specific fee schedule for hospitalist services.
Before implementing a national hospitalist system, we conducted a pilot study supported by funding from the council. This first study, the Korean Hospitalist System Operation and Evaluation Research (September 2015 to August 2016)14, was designed to (a) determine hospitalist needs (eg, rotation schedule, salary, working conditions) for this new profession, (b) determine the necessary number of hospitalists and appropriate fee schedules to cover salary and benefits, and (c) provide Korean hospitalist models with operational methods to facilitate implementation at individual institutions. The council and research team selected four hospitals for the privately funded pilot study (Table). These hospitals already had an inpatient care system managed by specialists prior to the pilot study, so major changes in patient care and hospital operations were not required.

The pilot study demonstrated improvements in patient satisfaction, medical service quality, and patient safety.8,14,15 As a result, fees billed from hospitalist services were now covered by national health insurance; previously, they were not covered by any inpatient care fee or bundled service fee. The next study (phase 2) was then conducted to evaluate the national implementation of the hospitalist system and its outcomes. The first phase defined criteria for monitoring and evaluating the institutionalized hospitalist system in Korea. The second phase, initiated in September 2016, evaluated how the implemented system could be expanded nationwide. The government was actively involved in the study; 31 of 344 (9%) general hospitals were selected and able to apply the hospitalist fee schedule for care of patients who were admitted to the hospitalist ward.
To investigate the effect of the hospitalist system, we measured the satisfaction of patients and medical providers (eg, specialists, residents, and nurses). The outcomes were number of doctors’ calls to hospitalists and to other specialties, duration of time required to address medical problems, number of contacts with the hospitalist (residents or other physicians in the control group), and time spent with hospitalized patients. In addition, we analyzed claims costs and the operating costs for implementing the system within each hospital (Table).
The new set of fee schedules was created specifically for hospitalist care services so hospitals could now claim separate inpatient care fees and hospitalist fees. The standard hospitalist fee schedule is applied only to patients who are on hospitalist-led wards. The fee schedule has two criteria: The hospitalist ward must have 50 beds which are assigned to hospitalists only, and each hospitalist ward has a team of five hospitalists. A maximum of 25 patients could be assigned to an individual hospitalist. However, fees can be adjusted based on individual hospital operation and management after government approval.
Under the national insurance system, the fee schedule is essential to obtain reimbursement for provided services. As a new medical profession, it is important to have the institutional implementation of the hospitalist system. In contrast to those in the United States, inpatients in Korea pay inpatient care fees only, which are charged per day. Therefore, reducing the length of stay without increasing patient volume does not financially benefit the hospital.
DEFINITION OF A KOREAN HOSPITALIST AND SCOPE OF PRACTICE
The definition of a Korean hospitalist was carefully developed, and a reimbursement system for hospitalists was created within the Korean healthcare system. All hospitalists are medical specialists who have completed advanced education and clinical training in their specialty area (internal medicine or surgery). Creating a definition of a hospitalist and a standard fee schedule was necessary for national incorporation of this new approach. The model must also be accepted by related parties, including healthcare professionals such as hospital executives, nonhospitalist doctors, and nurses. Therefore, the system has a minimum of two requirements to operate at the hospital level, and the remaining factors can be modified by individual hospitals to protect the new discipline within the healthcare system. First, hospitalist services are provided only to hospitalist patients. To establish the new system, limiting the patient range to a specific group was necessary to prevent abuse of human-based resources within the hospital. Second, hospitalists must be stationed near the hospitalist ward to enhance accessibility, patient safety, and healthcare quality. In other words, a hospitalist provides services to hospitalized patients who paid the hospitalist fee, and the hospitalist does not provide care beyond the hospitalist ward (eg, they cannot provide outpatient consultation or provide care on other wards).
INPATIENT CARE REIMBURSEMENT BEFORE AND AFTER IMPLEMENTATION
Korean national health insurance is the universal health insurance under a single insurer (ie, the government). Therefore, all Korean citizens have national health insurance. Individuals can choose to have additional health insurance (private insurance) if they wish to pay an out-of-pocket fee with their additional private insurance. In Korea, the inpatient care fee includes all fees to provide care for the patient during hospital stays, such as the physician fee, facility fee, and consultation fee. The inpatient care fee schedule is charged per day including all the inpatient care composition. The claim and reimbursement system is different in Korea in that the hospital as a whole submits the claim for reimbursement. There is no separate claim from physicians, lab technicians, or facilities. Doctors provide care to patients and are then paid for the services in terms of salaries. Since the original inpatient care fee schedule was low and insufficient, it was difficult to provide high-quality care. Also, resources for spending on inpatient care management was limited. So a new system to improve inpatient care was needed. Implementing the hospitalist system in Korea led to the creation of a new fee schedule specifically for hospitalists so that hospitals could claim hospitalist fees on top of inpatient care fees when the patient was cared for by the hospitalist. The additional fees in the hospitalist fee schedule allow safe and high-quality care to be provided for patients during hospital stays.
KOREAN HOSPITAL MEDICINE TODAY
The Korean hospitalist system has been implemented for 3 years with the government’s active involvement, with approximately 250 specialists working as hospitalists as of August 2020. Importantly, Korean hospitalists are not limited to internal medicine specialists; in fact, several different specialties practice as hospitalists, including surgeons and other medical specialists, who account for 20% and 30% of the hospitalist workforce, respectively. Since the system’s implementation, all inpatient care and management is now transferred to specialists from residents in hospitalist wards. This change has increased patient safety and care quality. At the beginning of the pilot study, the concept of a “hospitalist” was new in Korea and the public did not know who a hospitalist was nor what a hospitalist did. However, after 3 years, patients now seek hospitalist care.
CONCLUSION
National implementation of the hospitalist model represents a key paradigm shift in the Korean healthcare system. The Korean hospitalist system is the result of the hospitals’, doctors’, and patients’ desire for higher-quality care. We expect to see growth of hospitalists in Korea and provision of better, safer, and more efficient inpatient care across specialties, payers, and government. Since we are at the early stage of the system, further efforts to support implementation are required. We hope our implementation process of a new medical system could serve as a model for other countries who are seeking to adopt hospitalist systems within their current healthcare paradigms.
Acknowledgments
The authors thank the medical professionals, government officers, and other professionals who put great effort into the implementation of the Korean Hospitalist System.
1. Kwon S. Thirty years of national health insurance in South Korea: lessons for achieving universal health care coverage. Health Policy Plan. 2009;24(1):63-71. https://doi.org/10.1093/heapol/czn037
2. Song YJ. The South Korean health care system. JMAJ. 2009;52(3):206-209.
3. Park YH, Park E-C. Healthcare policy. In: Preventive Medicine and Public Health. Vol 3.Gyechuk; 2017:821-833.
4. Park E-C, Lee TJ, Jun BY, Jung SH, Jeong HS. Health security. In: Preventive Medicine and Public Health. Vol 3. Gyechuk; 2017:888-897.
5. Jang S-I, Jang S-y, Park E-C. Trends of US hospitalist and suggestions for introduction of Korean hospitalist. Korean J Med. 2015;89(1):1-5. http://doi.org/10.3904/kjm.2015.89.1.1
6. Jang S-I. Korean hospitalist system implementation and development strategies based on pilot studies. J Korean Med Assoc. 2019;62(11):558-563. http://doi.org/10.5124/jkma.2019.62.11.558
7. OECD. Health at a Glance 2017: OECD indicators. OECD iLibrary. 2017. Accessed September 27, 2019. https://doi.org/10.1787/health_glance-2017-en
8. Jang S-I, Park E-C, Nam JM, et al. A study on the implementation and the evaluation of Korean Hospitalist System to improve the quality of hospitalization (Phase 2). Institute of Health Services Research, Yonsei University; 2018.
9. Koh DY. Political strategies to enhance medical residents. Health Policy. Vol 37. Seoul National University Hospital; 2014.
10. Act on the Improvement of Training Conditions and Status of Medical Residents. Vol No. 16260: Ministry of Health and Welfare; 2015.
11. Eom JS. Operating the hospitalist system. J Korean Med Assoc. 2016;59(5):342-344. http://doi.org/10.5124/jkma.2016.59.5.342
12. Kim S-S. Working conditions of interns/residents and patient safety: Painful training might not be authentic. J Korean Med Assoc. 2016;59(2):82-84. http://dx.doi.org/10.5124/jkma.2016.59.2.82
13. Oshimura J, Sperring J, Bauer BD, Rauch DA. Inpatient staffing within pediatric residency programs: work hour restrictions and the evolving role of the pediatric hospitalist. J Hosp Med. 2012;7(4):299-303. https://doi.org/10.1002/jhm.952
14. Park E-C, Lee SG, Kim T-H, et al. A study on the implementation and the evaluation of Korean Hospitalist System to improve the quality of hospitalization (Phase 1). Institute of Health Services Research, Yonsei University; 2016.
15. Jang S-I, Jung E-J, Park SK, Chae W, Kim Y-K. A study on the feasibility of the Korean hospitalist system and cost estimation. Ministry of Health and Welfare, Institute of Health Service Research Yonsei University; 2019.
1. Kwon S. Thirty years of national health insurance in South Korea: lessons for achieving universal health care coverage. Health Policy Plan. 2009;24(1):63-71. https://doi.org/10.1093/heapol/czn037
2. Song YJ. The South Korean health care system. JMAJ. 2009;52(3):206-209.
3. Park YH, Park E-C. Healthcare policy. In: Preventive Medicine and Public Health. Vol 3.Gyechuk; 2017:821-833.
4. Park E-C, Lee TJ, Jun BY, Jung SH, Jeong HS. Health security. In: Preventive Medicine and Public Health. Vol 3. Gyechuk; 2017:888-897.
5. Jang S-I, Jang S-y, Park E-C. Trends of US hospitalist and suggestions for introduction of Korean hospitalist. Korean J Med. 2015;89(1):1-5. http://doi.org/10.3904/kjm.2015.89.1.1
6. Jang S-I. Korean hospitalist system implementation and development strategies based on pilot studies. J Korean Med Assoc. 2019;62(11):558-563. http://doi.org/10.5124/jkma.2019.62.11.558
7. OECD. Health at a Glance 2017: OECD indicators. OECD iLibrary. 2017. Accessed September 27, 2019. https://doi.org/10.1787/health_glance-2017-en
8. Jang S-I, Park E-C, Nam JM, et al. A study on the implementation and the evaluation of Korean Hospitalist System to improve the quality of hospitalization (Phase 2). Institute of Health Services Research, Yonsei University; 2018.
9. Koh DY. Political strategies to enhance medical residents. Health Policy. Vol 37. Seoul National University Hospital; 2014.
10. Act on the Improvement of Training Conditions and Status of Medical Residents. Vol No. 16260: Ministry of Health and Welfare; 2015.
11. Eom JS. Operating the hospitalist system. J Korean Med Assoc. 2016;59(5):342-344. http://doi.org/10.5124/jkma.2016.59.5.342
12. Kim S-S. Working conditions of interns/residents and patient safety: Painful training might not be authentic. J Korean Med Assoc. 2016;59(2):82-84. http://dx.doi.org/10.5124/jkma.2016.59.2.82
13. Oshimura J, Sperring J, Bauer BD, Rauch DA. Inpatient staffing within pediatric residency programs: work hour restrictions and the evolving role of the pediatric hospitalist. J Hosp Med. 2012;7(4):299-303. https://doi.org/10.1002/jhm.952
14. Park E-C, Lee SG, Kim T-H, et al. A study on the implementation and the evaluation of Korean Hospitalist System to improve the quality of hospitalization (Phase 1). Institute of Health Services Research, Yonsei University; 2016.
15. Jang S-I, Jung E-J, Park SK, Chae W, Kim Y-K. A study on the feasibility of the Korean hospitalist system and cost estimation. Ministry of Health and Welfare, Institute of Health Service Research Yonsei University; 2019.
© 2021 Society of Hospital Medicine
Could pollen be driving COVID-19 infections?
Some scientists say they’ve noticed a pattern to the recurring waves of SARS-CoV-2 infections around the globe: As pollen levels increased in outdoor air in 31 countries, COVID-19 cases accelerated.
Yet other recent studies point in the opposite direction, suggesting that peaks in pollen seasons coincide with a fall-off in the spread of some respiratory viruses, like COVID-19 and influenza. There’s even some evidence that pollen may compete with the virus that causes COVID-19 and may even help prevent infection.
So which is it? The answer may still be up in the air.
Doctors don’t fully understand what makes some viruses – like the ones that cause the flu – circulate in seasonal patterns.
There are, of course, many theories. These revolve around things like temperature and humidity – viruses tend to prefer colder, drier air – something that’s thought to help them spread more easily in the winter months. People are exposed to less sunlight during the winter, as they spend more time indoors, and the earth points away from the sun, providing some natural shielding. That may play a role because ultraviolet light from the sun acts like a natural disinfectant and may help keep circulating viral levels down.
In addition, exposure to sunlight helps the body make vitamin D, which may help keep our immune responses strong. Extreme temperatures – both cold and hot – also change our behavior, so that we spend more time cloistered indoors, where we can more easily cough and sneeze on each other and generally swap more germs.
Spike in pollen, jump in infections
The new study, published in the Proceedings of the National Academy of Sciences, adds a new variable to this mix – pollen. It relies on data from 248 airborne pollen–monitoring sites in 31 countries. The study also took into account other effects, such as population density, temperature, humidity, and lockdown orders. The study authors found that, when pollen in an area spiked, so did infections, after an average lag of about 4 days. The study authors say pollen seemed to account for, on average, 44% of the infection rate variability between countries.
The study authors say pollen could be a culprit in respiratory infections, not because the viruses hitch a ride on pollen grains and travel into our mouth, eyes, and nose, but because pollen seems to perturb our immune defenses, even if a person isn’t allergic to it.
“When we inhale pollen, they end up on our nasal mucosa, and here they diminish the expression of genes that are important for the defense against airborne viruses,” study author Stefanie Gilles, PhD, chair of environmental medicine at the Technical University of Munich, said in a press conference.
In a study published last year, Dr. Gilles found that mice exposed to pollen made less interferon and other protective chemical signals to the immune system. Those then infected with respiratory syncytial virus had more virus in their bodies, compared with mice not exposed to pollen. She seemed to see the same effect in human volunteers.
The study authors think pollen may cause the body to drop its defenses against the airborne virus that causes COVID-19, too.
“If you’re in a crowded room, and other people are there that are asymptomatic, and you’ve just been breathing in pollen all day long, chances are that you’re going to be more susceptible to the virus,” says Lewis Ziska, PhD, a plant physiologist who studies pollen, climate change, and health at Columbia University’s Mailman School of Public Health in New York. “Having a mask is obviously really critical in that regard.”
Masks do a great job of blocking pollen, so wearing one is even more important when pollen and viruses are floating around, he says.
Other researchers, however, say that, while the study raises some interesting questions, it can’t prove that pollen is increasing COVID-19 infections.
“Just because two things happen at the same time doesn’t mean that one causes the other,” says Martijn Hoogeveen, PhD, a professor of technical sciences and environment at the Open University in the Netherlands.
Dr. Hoogeveen’s recent study, published in Science of the Total Environment, found that the arrival of pollen season in the Netherlands coincides with the end of flu season, and that COVID-19 infection peaks tend to follow a similar pattern – exactly the opposite of the PNAS study.
Another preprint study, which focused on the Chicago area, found the same thing – as pollen climbs, flu cases drop. The researchers behind that study think pollen may actually compete with viruses in our airways, helping to block them from infecting our cells.
Patterns may be hard to nail down
Why did these studies reach such different conclusions?
Dr. Hoogeveen’s paper focused on a single country and looked at the incidence of flu infections over four seasons, from 2016 to 2020, while the PNAS study collected data on pollen from January through the first week of April 2020.
He thinks that a single season, or really part of a season, may not be long enough to see meaningful patterns, especially considering that this new-to-humans virus was spreading quickly at nearly the same time. He says it will be interesting to follow what happens with COVID-19 infections and pollen in the coming months and years.
Dr. Hoogeveen says that in a large study spanning so many countries it would have been nearly impossible to account for differences in pandemic control strategies. Some countries embraced the use of masks, stay-at-home orders, and social distancing, for example, while others took less stringent measures in order to let the virus run its course in pursuit of herd immunity.
Limiting the study area to a single country or city, he says, helps researchers better understand all the variables that might have been in play along with pollen.
“There is no scientific consensus yet, about what it is driving, and that’s what makes it such an interesting field,” he says.
A version of this article first appeared on Medscape.com.
Some scientists say they’ve noticed a pattern to the recurring waves of SARS-CoV-2 infections around the globe: As pollen levels increased in outdoor air in 31 countries, COVID-19 cases accelerated.
Yet other recent studies point in the opposite direction, suggesting that peaks in pollen seasons coincide with a fall-off in the spread of some respiratory viruses, like COVID-19 and influenza. There’s even some evidence that pollen may compete with the virus that causes COVID-19 and may even help prevent infection.
So which is it? The answer may still be up in the air.
Doctors don’t fully understand what makes some viruses – like the ones that cause the flu – circulate in seasonal patterns.
There are, of course, many theories. These revolve around things like temperature and humidity – viruses tend to prefer colder, drier air – something that’s thought to help them spread more easily in the winter months. People are exposed to less sunlight during the winter, as they spend more time indoors, and the earth points away from the sun, providing some natural shielding. That may play a role because ultraviolet light from the sun acts like a natural disinfectant and may help keep circulating viral levels down.
In addition, exposure to sunlight helps the body make vitamin D, which may help keep our immune responses strong. Extreme temperatures – both cold and hot – also change our behavior, so that we spend more time cloistered indoors, where we can more easily cough and sneeze on each other and generally swap more germs.
Spike in pollen, jump in infections
The new study, published in the Proceedings of the National Academy of Sciences, adds a new variable to this mix – pollen. It relies on data from 248 airborne pollen–monitoring sites in 31 countries. The study also took into account other effects, such as population density, temperature, humidity, and lockdown orders. The study authors found that, when pollen in an area spiked, so did infections, after an average lag of about 4 days. The study authors say pollen seemed to account for, on average, 44% of the infection rate variability between countries.
The study authors say pollen could be a culprit in respiratory infections, not because the viruses hitch a ride on pollen grains and travel into our mouth, eyes, and nose, but because pollen seems to perturb our immune defenses, even if a person isn’t allergic to it.
“When we inhale pollen, they end up on our nasal mucosa, and here they diminish the expression of genes that are important for the defense against airborne viruses,” study author Stefanie Gilles, PhD, chair of environmental medicine at the Technical University of Munich, said in a press conference.
In a study published last year, Dr. Gilles found that mice exposed to pollen made less interferon and other protective chemical signals to the immune system. Those then infected with respiratory syncytial virus had more virus in their bodies, compared with mice not exposed to pollen. She seemed to see the same effect in human volunteers.
The study authors think pollen may cause the body to drop its defenses against the airborne virus that causes COVID-19, too.
“If you’re in a crowded room, and other people are there that are asymptomatic, and you’ve just been breathing in pollen all day long, chances are that you’re going to be more susceptible to the virus,” says Lewis Ziska, PhD, a plant physiologist who studies pollen, climate change, and health at Columbia University’s Mailman School of Public Health in New York. “Having a mask is obviously really critical in that regard.”
Masks do a great job of blocking pollen, so wearing one is even more important when pollen and viruses are floating around, he says.
Other researchers, however, say that, while the study raises some interesting questions, it can’t prove that pollen is increasing COVID-19 infections.
“Just because two things happen at the same time doesn’t mean that one causes the other,” says Martijn Hoogeveen, PhD, a professor of technical sciences and environment at the Open University in the Netherlands.
Dr. Hoogeveen’s recent study, published in Science of the Total Environment, found that the arrival of pollen season in the Netherlands coincides with the end of flu season, and that COVID-19 infection peaks tend to follow a similar pattern – exactly the opposite of the PNAS study.
Another preprint study, which focused on the Chicago area, found the same thing – as pollen climbs, flu cases drop. The researchers behind that study think pollen may actually compete with viruses in our airways, helping to block them from infecting our cells.
Patterns may be hard to nail down
Why did these studies reach such different conclusions?
Dr. Hoogeveen’s paper focused on a single country and looked at the incidence of flu infections over four seasons, from 2016 to 2020, while the PNAS study collected data on pollen from January through the first week of April 2020.
He thinks that a single season, or really part of a season, may not be long enough to see meaningful patterns, especially considering that this new-to-humans virus was spreading quickly at nearly the same time. He says it will be interesting to follow what happens with COVID-19 infections and pollen in the coming months and years.
Dr. Hoogeveen says that in a large study spanning so many countries it would have been nearly impossible to account for differences in pandemic control strategies. Some countries embraced the use of masks, stay-at-home orders, and social distancing, for example, while others took less stringent measures in order to let the virus run its course in pursuit of herd immunity.
Limiting the study area to a single country or city, he says, helps researchers better understand all the variables that might have been in play along with pollen.
“There is no scientific consensus yet, about what it is driving, and that’s what makes it such an interesting field,” he says.
A version of this article first appeared on Medscape.com.
Some scientists say they’ve noticed a pattern to the recurring waves of SARS-CoV-2 infections around the globe: As pollen levels increased in outdoor air in 31 countries, COVID-19 cases accelerated.
Yet other recent studies point in the opposite direction, suggesting that peaks in pollen seasons coincide with a fall-off in the spread of some respiratory viruses, like COVID-19 and influenza. There’s even some evidence that pollen may compete with the virus that causes COVID-19 and may even help prevent infection.
So which is it? The answer may still be up in the air.
Doctors don’t fully understand what makes some viruses – like the ones that cause the flu – circulate in seasonal patterns.
There are, of course, many theories. These revolve around things like temperature and humidity – viruses tend to prefer colder, drier air – something that’s thought to help them spread more easily in the winter months. People are exposed to less sunlight during the winter, as they spend more time indoors, and the earth points away from the sun, providing some natural shielding. That may play a role because ultraviolet light from the sun acts like a natural disinfectant and may help keep circulating viral levels down.
In addition, exposure to sunlight helps the body make vitamin D, which may help keep our immune responses strong. Extreme temperatures – both cold and hot – also change our behavior, so that we spend more time cloistered indoors, where we can more easily cough and sneeze on each other and generally swap more germs.
Spike in pollen, jump in infections
The new study, published in the Proceedings of the National Academy of Sciences, adds a new variable to this mix – pollen. It relies on data from 248 airborne pollen–monitoring sites in 31 countries. The study also took into account other effects, such as population density, temperature, humidity, and lockdown orders. The study authors found that, when pollen in an area spiked, so did infections, after an average lag of about 4 days. The study authors say pollen seemed to account for, on average, 44% of the infection rate variability between countries.
The study authors say pollen could be a culprit in respiratory infections, not because the viruses hitch a ride on pollen grains and travel into our mouth, eyes, and nose, but because pollen seems to perturb our immune defenses, even if a person isn’t allergic to it.
“When we inhale pollen, they end up on our nasal mucosa, and here they diminish the expression of genes that are important for the defense against airborne viruses,” study author Stefanie Gilles, PhD, chair of environmental medicine at the Technical University of Munich, said in a press conference.
In a study published last year, Dr. Gilles found that mice exposed to pollen made less interferon and other protective chemical signals to the immune system. Those then infected with respiratory syncytial virus had more virus in their bodies, compared with mice not exposed to pollen. She seemed to see the same effect in human volunteers.
The study authors think pollen may cause the body to drop its defenses against the airborne virus that causes COVID-19, too.
“If you’re in a crowded room, and other people are there that are asymptomatic, and you’ve just been breathing in pollen all day long, chances are that you’re going to be more susceptible to the virus,” says Lewis Ziska, PhD, a plant physiologist who studies pollen, climate change, and health at Columbia University’s Mailman School of Public Health in New York. “Having a mask is obviously really critical in that regard.”
Masks do a great job of blocking pollen, so wearing one is even more important when pollen and viruses are floating around, he says.
Other researchers, however, say that, while the study raises some interesting questions, it can’t prove that pollen is increasing COVID-19 infections.
“Just because two things happen at the same time doesn’t mean that one causes the other,” says Martijn Hoogeveen, PhD, a professor of technical sciences and environment at the Open University in the Netherlands.
Dr. Hoogeveen’s recent study, published in Science of the Total Environment, found that the arrival of pollen season in the Netherlands coincides with the end of flu season, and that COVID-19 infection peaks tend to follow a similar pattern – exactly the opposite of the PNAS study.
Another preprint study, which focused on the Chicago area, found the same thing – as pollen climbs, flu cases drop. The researchers behind that study think pollen may actually compete with viruses in our airways, helping to block them from infecting our cells.
Patterns may be hard to nail down
Why did these studies reach such different conclusions?
Dr. Hoogeveen’s paper focused on a single country and looked at the incidence of flu infections over four seasons, from 2016 to 2020, while the PNAS study collected data on pollen from January through the first week of April 2020.
He thinks that a single season, or really part of a season, may not be long enough to see meaningful patterns, especially considering that this new-to-humans virus was spreading quickly at nearly the same time. He says it will be interesting to follow what happens with COVID-19 infections and pollen in the coming months and years.
Dr. Hoogeveen says that in a large study spanning so many countries it would have been nearly impossible to account for differences in pandemic control strategies. Some countries embraced the use of masks, stay-at-home orders, and social distancing, for example, while others took less stringent measures in order to let the virus run its course in pursuit of herd immunity.
Limiting the study area to a single country or city, he says, helps researchers better understand all the variables that might have been in play along with pollen.
“There is no scientific consensus yet, about what it is driving, and that’s what makes it such an interesting field,” he says.
A version of this article first appeared on Medscape.com.
Neurologic drug prices jump 50% in five years
, new research shows. Results of the retrospective study also showed that most of the increased costs for these agents were due to rising costs for neuroimmunology drugs, mainly for those used to treat multiple sclerosis (MS).
“The same brand name medication in 2017 cost approximately 50% more than in 2013,” said Adam de Havenon, MD, assistant professor of neurology, University of Utah, Salt Lake City.
“An analogy would be if you bought an iPhone 5 in 2013 for $500, and then in 2017, you were asked to pay $750 for the exact same iPhone 5,” Dr. de Havenon added.
The study findings were published online March 10 in the journal Neurology.
$26 billion in payments
Both neurologists and patients are concerned about the high cost of prescription drugs for neurologic diseases, and Medicare Part D data indicate that these drugs are the most expensive component of neurologic care, the researchers noted. In addition, out-of-pocket costs have increased significantly for patients with neurologic disease such as Parkinson’s disease, epilepsy, and MS.
To understand trends in payments for neurologic drugs, Dr. de Havenon and colleagues analyzed Medicare Part D claims filed from 2013 to 2017. The payments include costs paid by Medicare, the patient, government subsidies, and other third-party payers.
In addition to examining more current Medicare Part D data than previous studies, the current analysis examined all medications prescribed by neurologists that consistently remained branded or generic during the 5-year study period, said Dr. de Havenon. This approach resulted in a large number of claims and a large total cost.
To calculate the percentage change in annual payment claims, the researchers used 2013 prices as a reference point. They identified drugs named in 2013 claims and classified them as generic, brand-name only, or brand-name with generic equivalent. Researchers also divided the drugs by neurologic subspecialty.
The analysis included 520 drugs, all of which were available in each year of the study period. Of these drugs, 322 were generic, 61 were brand-name only, and 137 were brand-name with a generic equivalent. There were 90.7 million total claims.
Results showed total payments amounted to $26.65 billion. Yearly total payments increased from $4.05 billion in 2013 to $6.09 billion in 2017, representing a 50.4% increase, even after adjusting for inflation. Total claims increased by 7.6% – from 17.1 million in 2013 to 18.4 million in 2017.
From 2013 to 2017, claim payments increased by 0.6% for generic drugs, 42.4% for brand-name only drugs, and 45% for brand-name drugs with generic equivalents. The proportion of claims increased from 81.9% to 88% for generic drugs and from 4.9% to 6.2% for brand-name only drugs.
However, the proportion of claims for brand-name drugs with generic equivalents decreased from 13.3% to 5.8%.
Treatment barrier
Neuroimmunologic drugs, most of which were prescribed for MS, had exceptional cost, the researchers noted. These drugs accounted for more than 50% of payments but only 4.3% of claims. Claim payment for these drugs increased by 46.9% during the study period, from $3,337 to $4,902.
When neuroimmunologic drugs were removed from the analysis there was still significant increase in claim payments for brand-name only drugs (50.4%) and brand-name drugs with generic equivalents (45.6%).
Although neuroimmunologic medicines, including monoclonal antibodies, are more expensive to produce, this factor alone does not explain their exceptional cost, said Dr. de Havenon. “The high cost of brand-name drugs in this speciality is likely because the market bears it,” he added. “In other words, MS is a disabling disease and the medications work, so historically the Centers for Medicare & Medicaid Services have been willing to tolerate the high cost of these primarily brand-name medications.”
Several countries have controlled drug costs by negotiating with pharmaceutical companies and through legislation, Dr. de Havenon noted.
“My intent with this article was to raise awareness on the topic, which I struggle with frequently as a clinician. I know I want my patients to have a medication, but the cost prevents it,” he said.
‘Unfettered’ price-setting
Commenting on the findings, Robert J. Fox, MD, vice chair for research at the Neurological Institute of the Cleveland Clinic, said the study “brings into clear light” what neurologists, particularly those who treat MS, have long suspected but did not really know. These neurologists “are typically distanced from the payment aspects of the medications they prescribe,” said Dr. Fox, who was not involved with the research.
Although a particular strength of the study was its comprehensiveness, the researchers excluded infusion claims – which account for a large portion of total patient care costs for many disorders, he noted.
Drugs for MS historically have been expensive, ostensibly because of their high cost of development. In addition, the large and continued price increase that occurs long after these drugs have been approved remains unexplained, said Dr. Fox.
He noted that the study findings might not directly affect clinical practice because neurologists will continue prescribing medications they think are best for their patients. “Instead, I think this is a lesson to lawmakers about the massive error in the Medicare Modernization Act of 2003, where the federal government was prohibited from negotiating drug prices. If the seller is unfettered in setting a price, then no one should be surprised when the price rises,” Dr. Fox said.
Because many new drugs and new generic formulations for treating MS have become available during the past year, “repeating these types of economic studies for the period 2020-2025 will help us understand if generic competition – as well as new laws if they are passed – alter price,” he concluded.
The study was funded by the American Academy of Neurology, which publishes Neurology. Dr. de Havenon has received clinical research funding from AMAG Pharmaceuticals and Regeneron Pharmaceuticals. Dr. Fox receives consulting fees from many pharmaceutical companies involved in the development of therapies for MS.
A version of this article first appeared on Medscape.com.
, new research shows. Results of the retrospective study also showed that most of the increased costs for these agents were due to rising costs for neuroimmunology drugs, mainly for those used to treat multiple sclerosis (MS).
“The same brand name medication in 2017 cost approximately 50% more than in 2013,” said Adam de Havenon, MD, assistant professor of neurology, University of Utah, Salt Lake City.
“An analogy would be if you bought an iPhone 5 in 2013 for $500, and then in 2017, you were asked to pay $750 for the exact same iPhone 5,” Dr. de Havenon added.
The study findings were published online March 10 in the journal Neurology.
$26 billion in payments
Both neurologists and patients are concerned about the high cost of prescription drugs for neurologic diseases, and Medicare Part D data indicate that these drugs are the most expensive component of neurologic care, the researchers noted. In addition, out-of-pocket costs have increased significantly for patients with neurologic disease such as Parkinson’s disease, epilepsy, and MS.
To understand trends in payments for neurologic drugs, Dr. de Havenon and colleagues analyzed Medicare Part D claims filed from 2013 to 2017. The payments include costs paid by Medicare, the patient, government subsidies, and other third-party payers.
In addition to examining more current Medicare Part D data than previous studies, the current analysis examined all medications prescribed by neurologists that consistently remained branded or generic during the 5-year study period, said Dr. de Havenon. This approach resulted in a large number of claims and a large total cost.
To calculate the percentage change in annual payment claims, the researchers used 2013 prices as a reference point. They identified drugs named in 2013 claims and classified them as generic, brand-name only, or brand-name with generic equivalent. Researchers also divided the drugs by neurologic subspecialty.
The analysis included 520 drugs, all of which were available in each year of the study period. Of these drugs, 322 were generic, 61 were brand-name only, and 137 were brand-name with a generic equivalent. There were 90.7 million total claims.
Results showed total payments amounted to $26.65 billion. Yearly total payments increased from $4.05 billion in 2013 to $6.09 billion in 2017, representing a 50.4% increase, even after adjusting for inflation. Total claims increased by 7.6% – from 17.1 million in 2013 to 18.4 million in 2017.
From 2013 to 2017, claim payments increased by 0.6% for generic drugs, 42.4% for brand-name only drugs, and 45% for brand-name drugs with generic equivalents. The proportion of claims increased from 81.9% to 88% for generic drugs and from 4.9% to 6.2% for brand-name only drugs.
However, the proportion of claims for brand-name drugs with generic equivalents decreased from 13.3% to 5.8%.
Treatment barrier
Neuroimmunologic drugs, most of which were prescribed for MS, had exceptional cost, the researchers noted. These drugs accounted for more than 50% of payments but only 4.3% of claims. Claim payment for these drugs increased by 46.9% during the study period, from $3,337 to $4,902.
When neuroimmunologic drugs were removed from the analysis there was still significant increase in claim payments for brand-name only drugs (50.4%) and brand-name drugs with generic equivalents (45.6%).
Although neuroimmunologic medicines, including monoclonal antibodies, are more expensive to produce, this factor alone does not explain their exceptional cost, said Dr. de Havenon. “The high cost of brand-name drugs in this speciality is likely because the market bears it,” he added. “In other words, MS is a disabling disease and the medications work, so historically the Centers for Medicare & Medicaid Services have been willing to tolerate the high cost of these primarily brand-name medications.”
Several countries have controlled drug costs by negotiating with pharmaceutical companies and through legislation, Dr. de Havenon noted.
“My intent with this article was to raise awareness on the topic, which I struggle with frequently as a clinician. I know I want my patients to have a medication, but the cost prevents it,” he said.
‘Unfettered’ price-setting
Commenting on the findings, Robert J. Fox, MD, vice chair for research at the Neurological Institute of the Cleveland Clinic, said the study “brings into clear light” what neurologists, particularly those who treat MS, have long suspected but did not really know. These neurologists “are typically distanced from the payment aspects of the medications they prescribe,” said Dr. Fox, who was not involved with the research.
Although a particular strength of the study was its comprehensiveness, the researchers excluded infusion claims – which account for a large portion of total patient care costs for many disorders, he noted.
Drugs for MS historically have been expensive, ostensibly because of their high cost of development. In addition, the large and continued price increase that occurs long after these drugs have been approved remains unexplained, said Dr. Fox.
He noted that the study findings might not directly affect clinical practice because neurologists will continue prescribing medications they think are best for their patients. “Instead, I think this is a lesson to lawmakers about the massive error in the Medicare Modernization Act of 2003, where the federal government was prohibited from negotiating drug prices. If the seller is unfettered in setting a price, then no one should be surprised when the price rises,” Dr. Fox said.
Because many new drugs and new generic formulations for treating MS have become available during the past year, “repeating these types of economic studies for the period 2020-2025 will help us understand if generic competition – as well as new laws if they are passed – alter price,” he concluded.
The study was funded by the American Academy of Neurology, which publishes Neurology. Dr. de Havenon has received clinical research funding from AMAG Pharmaceuticals and Regeneron Pharmaceuticals. Dr. Fox receives consulting fees from many pharmaceutical companies involved in the development of therapies for MS.
A version of this article first appeared on Medscape.com.
, new research shows. Results of the retrospective study also showed that most of the increased costs for these agents were due to rising costs for neuroimmunology drugs, mainly for those used to treat multiple sclerosis (MS).
“The same brand name medication in 2017 cost approximately 50% more than in 2013,” said Adam de Havenon, MD, assistant professor of neurology, University of Utah, Salt Lake City.
“An analogy would be if you bought an iPhone 5 in 2013 for $500, and then in 2017, you were asked to pay $750 for the exact same iPhone 5,” Dr. de Havenon added.
The study findings were published online March 10 in the journal Neurology.
$26 billion in payments
Both neurologists and patients are concerned about the high cost of prescription drugs for neurologic diseases, and Medicare Part D data indicate that these drugs are the most expensive component of neurologic care, the researchers noted. In addition, out-of-pocket costs have increased significantly for patients with neurologic disease such as Parkinson’s disease, epilepsy, and MS.
To understand trends in payments for neurologic drugs, Dr. de Havenon and colleagues analyzed Medicare Part D claims filed from 2013 to 2017. The payments include costs paid by Medicare, the patient, government subsidies, and other third-party payers.
In addition to examining more current Medicare Part D data than previous studies, the current analysis examined all medications prescribed by neurologists that consistently remained branded or generic during the 5-year study period, said Dr. de Havenon. This approach resulted in a large number of claims and a large total cost.
To calculate the percentage change in annual payment claims, the researchers used 2013 prices as a reference point. They identified drugs named in 2013 claims and classified them as generic, brand-name only, or brand-name with generic equivalent. Researchers also divided the drugs by neurologic subspecialty.
The analysis included 520 drugs, all of which were available in each year of the study period. Of these drugs, 322 were generic, 61 were brand-name only, and 137 were brand-name with a generic equivalent. There were 90.7 million total claims.
Results showed total payments amounted to $26.65 billion. Yearly total payments increased from $4.05 billion in 2013 to $6.09 billion in 2017, representing a 50.4% increase, even after adjusting for inflation. Total claims increased by 7.6% – from 17.1 million in 2013 to 18.4 million in 2017.
From 2013 to 2017, claim payments increased by 0.6% for generic drugs, 42.4% for brand-name only drugs, and 45% for brand-name drugs with generic equivalents. The proportion of claims increased from 81.9% to 88% for generic drugs and from 4.9% to 6.2% for brand-name only drugs.
However, the proportion of claims for brand-name drugs with generic equivalents decreased from 13.3% to 5.8%.
Treatment barrier
Neuroimmunologic drugs, most of which were prescribed for MS, had exceptional cost, the researchers noted. These drugs accounted for more than 50% of payments but only 4.3% of claims. Claim payment for these drugs increased by 46.9% during the study period, from $3,337 to $4,902.
When neuroimmunologic drugs were removed from the analysis there was still significant increase in claim payments for brand-name only drugs (50.4%) and brand-name drugs with generic equivalents (45.6%).
Although neuroimmunologic medicines, including monoclonal antibodies, are more expensive to produce, this factor alone does not explain their exceptional cost, said Dr. de Havenon. “The high cost of brand-name drugs in this speciality is likely because the market bears it,” he added. “In other words, MS is a disabling disease and the medications work, so historically the Centers for Medicare & Medicaid Services have been willing to tolerate the high cost of these primarily brand-name medications.”
Several countries have controlled drug costs by negotiating with pharmaceutical companies and through legislation, Dr. de Havenon noted.
“My intent with this article was to raise awareness on the topic, which I struggle with frequently as a clinician. I know I want my patients to have a medication, but the cost prevents it,” he said.
‘Unfettered’ price-setting
Commenting on the findings, Robert J. Fox, MD, vice chair for research at the Neurological Institute of the Cleveland Clinic, said the study “brings into clear light” what neurologists, particularly those who treat MS, have long suspected but did not really know. These neurologists “are typically distanced from the payment aspects of the medications they prescribe,” said Dr. Fox, who was not involved with the research.
Although a particular strength of the study was its comprehensiveness, the researchers excluded infusion claims – which account for a large portion of total patient care costs for many disorders, he noted.
Drugs for MS historically have been expensive, ostensibly because of their high cost of development. In addition, the large and continued price increase that occurs long after these drugs have been approved remains unexplained, said Dr. Fox.
He noted that the study findings might not directly affect clinical practice because neurologists will continue prescribing medications they think are best for their patients. “Instead, I think this is a lesson to lawmakers about the massive error in the Medicare Modernization Act of 2003, where the federal government was prohibited from negotiating drug prices. If the seller is unfettered in setting a price, then no one should be surprised when the price rises,” Dr. Fox said.
Because many new drugs and new generic formulations for treating MS have become available during the past year, “repeating these types of economic studies for the period 2020-2025 will help us understand if generic competition – as well as new laws if they are passed – alter price,” he concluded.
The study was funded by the American Academy of Neurology, which publishes Neurology. Dr. de Havenon has received clinical research funding from AMAG Pharmaceuticals and Regeneron Pharmaceuticals. Dr. Fox receives consulting fees from many pharmaceutical companies involved in the development of therapies for MS.
A version of this article first appeared on Medscape.com.
FROM NEUROLOGY
AAP issues five recommendations for common dermatologic problems
The American Academy of Pediatrics recently issued five recommendations for the most common dermatologic problems in primary care pediatrics.
Topics include diagnostic and management strategies for a variety of conditions, including atopic dermatitis, fungal infections, and autoimmune conditions.
The AAP Section on Dermatology created the recommendations, which were then reviewed and approved by “more than a dozen relevant AAP committees, councils, and sections,” before final approval by the AAP executive committee and board of directors.
The final list represents a collaborative effort with the Choosing Wisely initiative of the American Board of Internal Medicine Foundation, which aims “to promote conversations between clinicians and patients by helping patients choose care that is supported by evidence, not duplicative of other tests or procedures already received, free from harm, [and] truly necessary.”
Lawrence Eichenfield, MD, professor of dermatology and pediatrics at the University of California, San Diego, and chief of pediatric and adolescent dermatology at Rady Children’s Hospital, San Diego, said that the recommendations are “a fine set of suggestions to help health care providers with some of their pediatric dermatology issues.”
• To begin, the AAP recommended against use of combination topical steroid antifungals for candida skin infections, diaper dermatitis, and tinea corporis, despite approvals for these indications.
“Many providers are unaware that the combination products contain a relatively high-potency topical steroid,” the AAP wrote, noting that “combination products are also often expensive and not covered by pharmacy plans.”
Diaper dermatitis responds best to barrier creams and ointments alone, according to the AAP. If needed, a topical, low-potency steroid may be used no more than twice a day, and tapered with improvement. Similarly, the AAP recommended a separate, low-potency steroid for tinea corporis if pruritus is severe.
• In contrast with this call for minimal treatment intensity, the AAP recommended a more intensive approach to tinea capitis, advising against topical medications alone.
“Topical treatments cannot penetrate the hair shaft itself, which is where the infection lies; thus, monotherapy with topical medications is insufficient to effectively treat the infection,” the AAP wrote. “This insufficient treatment can lead to increased health care costs resulting from multiple visits and the prescribing of ineffective medications.”
While medicated shampoos may still be used as adjunctive treatments for tinea capitis, the AAP recommended primary therapy with either griseofulvin or terbinafine, slightly favoring terbinafine because of adequate efficacy, lesser expense, and shorter regimen.
According to Dr. Eichenfield, a more thorough workup should also be considered.
“Consider culturing possible tinea capitis, so that oral antifungals can be used judiciously and not used for other scaling scalp diagnoses,” he said.
• For most cases of atopic dermatitis, the AAP advised against oral or injected corticosteroids, despite rapid efficacy, because of potential for adverse events, such as adrenal suppression, growth retardation, and disease worsening upon discontinuation. Instead, they recommended topical therapies, “good skin care practices,” and if necessary, “phototherapy and/or steroid-sparing systemic agents.”
“Systemic corticosteroids should only be prescribed for severe flares once all other treatment options have been exhausted and should be limited to a short course for the purpose of bridging to a steroid-sparing agent,” the AAP wrote.
Dr. Eichenfield emphasized this point, noting that new therapies have expanded treatment options.
“Be aware of the advances in atopic dermatitis,” he said, “with newer topical medications and with a new systemic biologic agent approved for moderate to severe refractory atopic dermatitis for ages 6 and older.”
• Turning to diagnostic strategies, the AAP recommended against routine laboratory testing for associated autoimmune diseases among patients with vitiligo, unless clinical signs and/or symptoms of such diseases are present.
“There is no convincing evidence that extensive workups in the absence of specific clinical suspicion improves outcomes for patients and may in fact beget additional costs and harms,” the AAP wrote. “Although many studies suggest ordering these tests, it is based largely on the increased cosegregation of vitiligo and thyroid disease and not on improved outcomes from having identified an abnormal laboratory test result.”
• Similarly, the AAP advised practitioners to avoid routinely testing patients with alopecia areata for other diseases if relevant symptoms and signs aren’t present.
“As in the case of vitiligo, it is more common to find thyroid autoantibodies or subclinical hypothyroidism than overt thyroid disease, unless there are clinically suspicious findings,” the AAP wrote. “Patients identified as having subclinical hypothyroidism are not currently treated and may even have resolution of the abnormal TSH.”
Before drawing blood, Dr. Eichenfield suggested that clinicians first ask the right questions.
“Be comfortable with screening questions about growth, weight, or activity changes to assist with decisions for thyroid screening in a patient with vitiligo or alopecia areata,” he said.
Choosing Wisely is an initiative of the American Board of Internal Medicine. The AAP and Dr. Eichenfield reported no conflicts of interest.
The American Academy of Pediatrics recently issued five recommendations for the most common dermatologic problems in primary care pediatrics.
Topics include diagnostic and management strategies for a variety of conditions, including atopic dermatitis, fungal infections, and autoimmune conditions.
The AAP Section on Dermatology created the recommendations, which were then reviewed and approved by “more than a dozen relevant AAP committees, councils, and sections,” before final approval by the AAP executive committee and board of directors.
The final list represents a collaborative effort with the Choosing Wisely initiative of the American Board of Internal Medicine Foundation, which aims “to promote conversations between clinicians and patients by helping patients choose care that is supported by evidence, not duplicative of other tests or procedures already received, free from harm, [and] truly necessary.”
Lawrence Eichenfield, MD, professor of dermatology and pediatrics at the University of California, San Diego, and chief of pediatric and adolescent dermatology at Rady Children’s Hospital, San Diego, said that the recommendations are “a fine set of suggestions to help health care providers with some of their pediatric dermatology issues.”
• To begin, the AAP recommended against use of combination topical steroid antifungals for candida skin infections, diaper dermatitis, and tinea corporis, despite approvals for these indications.
“Many providers are unaware that the combination products contain a relatively high-potency topical steroid,” the AAP wrote, noting that “combination products are also often expensive and not covered by pharmacy plans.”
Diaper dermatitis responds best to barrier creams and ointments alone, according to the AAP. If needed, a topical, low-potency steroid may be used no more than twice a day, and tapered with improvement. Similarly, the AAP recommended a separate, low-potency steroid for tinea corporis if pruritus is severe.
• In contrast with this call for minimal treatment intensity, the AAP recommended a more intensive approach to tinea capitis, advising against topical medications alone.
“Topical treatments cannot penetrate the hair shaft itself, which is where the infection lies; thus, monotherapy with topical medications is insufficient to effectively treat the infection,” the AAP wrote. “This insufficient treatment can lead to increased health care costs resulting from multiple visits and the prescribing of ineffective medications.”
While medicated shampoos may still be used as adjunctive treatments for tinea capitis, the AAP recommended primary therapy with either griseofulvin or terbinafine, slightly favoring terbinafine because of adequate efficacy, lesser expense, and shorter regimen.
According to Dr. Eichenfield, a more thorough workup should also be considered.
“Consider culturing possible tinea capitis, so that oral antifungals can be used judiciously and not used for other scaling scalp diagnoses,” he said.
• For most cases of atopic dermatitis, the AAP advised against oral or injected corticosteroids, despite rapid efficacy, because of potential for adverse events, such as adrenal suppression, growth retardation, and disease worsening upon discontinuation. Instead, they recommended topical therapies, “good skin care practices,” and if necessary, “phototherapy and/or steroid-sparing systemic agents.”
“Systemic corticosteroids should only be prescribed for severe flares once all other treatment options have been exhausted and should be limited to a short course for the purpose of bridging to a steroid-sparing agent,” the AAP wrote.
Dr. Eichenfield emphasized this point, noting that new therapies have expanded treatment options.
“Be aware of the advances in atopic dermatitis,” he said, “with newer topical medications and with a new systemic biologic agent approved for moderate to severe refractory atopic dermatitis for ages 6 and older.”
• Turning to diagnostic strategies, the AAP recommended against routine laboratory testing for associated autoimmune diseases among patients with vitiligo, unless clinical signs and/or symptoms of such diseases are present.
“There is no convincing evidence that extensive workups in the absence of specific clinical suspicion improves outcomes for patients and may in fact beget additional costs and harms,” the AAP wrote. “Although many studies suggest ordering these tests, it is based largely on the increased cosegregation of vitiligo and thyroid disease and not on improved outcomes from having identified an abnormal laboratory test result.”
• Similarly, the AAP advised practitioners to avoid routinely testing patients with alopecia areata for other diseases if relevant symptoms and signs aren’t present.
“As in the case of vitiligo, it is more common to find thyroid autoantibodies or subclinical hypothyroidism than overt thyroid disease, unless there are clinically suspicious findings,” the AAP wrote. “Patients identified as having subclinical hypothyroidism are not currently treated and may even have resolution of the abnormal TSH.”
Before drawing blood, Dr. Eichenfield suggested that clinicians first ask the right questions.
“Be comfortable with screening questions about growth, weight, or activity changes to assist with decisions for thyroid screening in a patient with vitiligo or alopecia areata,” he said.
Choosing Wisely is an initiative of the American Board of Internal Medicine. The AAP and Dr. Eichenfield reported no conflicts of interest.
The American Academy of Pediatrics recently issued five recommendations for the most common dermatologic problems in primary care pediatrics.
Topics include diagnostic and management strategies for a variety of conditions, including atopic dermatitis, fungal infections, and autoimmune conditions.
The AAP Section on Dermatology created the recommendations, which were then reviewed and approved by “more than a dozen relevant AAP committees, councils, and sections,” before final approval by the AAP executive committee and board of directors.
The final list represents a collaborative effort with the Choosing Wisely initiative of the American Board of Internal Medicine Foundation, which aims “to promote conversations between clinicians and patients by helping patients choose care that is supported by evidence, not duplicative of other tests or procedures already received, free from harm, [and] truly necessary.”
Lawrence Eichenfield, MD, professor of dermatology and pediatrics at the University of California, San Diego, and chief of pediatric and adolescent dermatology at Rady Children’s Hospital, San Diego, said that the recommendations are “a fine set of suggestions to help health care providers with some of their pediatric dermatology issues.”
• To begin, the AAP recommended against use of combination topical steroid antifungals for candida skin infections, diaper dermatitis, and tinea corporis, despite approvals for these indications.
“Many providers are unaware that the combination products contain a relatively high-potency topical steroid,” the AAP wrote, noting that “combination products are also often expensive and not covered by pharmacy plans.”
Diaper dermatitis responds best to barrier creams and ointments alone, according to the AAP. If needed, a topical, low-potency steroid may be used no more than twice a day, and tapered with improvement. Similarly, the AAP recommended a separate, low-potency steroid for tinea corporis if pruritus is severe.
• In contrast with this call for minimal treatment intensity, the AAP recommended a more intensive approach to tinea capitis, advising against topical medications alone.
“Topical treatments cannot penetrate the hair shaft itself, which is where the infection lies; thus, monotherapy with topical medications is insufficient to effectively treat the infection,” the AAP wrote. “This insufficient treatment can lead to increased health care costs resulting from multiple visits and the prescribing of ineffective medications.”
While medicated shampoos may still be used as adjunctive treatments for tinea capitis, the AAP recommended primary therapy with either griseofulvin or terbinafine, slightly favoring terbinafine because of adequate efficacy, lesser expense, and shorter regimen.
According to Dr. Eichenfield, a more thorough workup should also be considered.
“Consider culturing possible tinea capitis, so that oral antifungals can be used judiciously and not used for other scaling scalp diagnoses,” he said.
• For most cases of atopic dermatitis, the AAP advised against oral or injected corticosteroids, despite rapid efficacy, because of potential for adverse events, such as adrenal suppression, growth retardation, and disease worsening upon discontinuation. Instead, they recommended topical therapies, “good skin care practices,” and if necessary, “phototherapy and/or steroid-sparing systemic agents.”
“Systemic corticosteroids should only be prescribed for severe flares once all other treatment options have been exhausted and should be limited to a short course for the purpose of bridging to a steroid-sparing agent,” the AAP wrote.
Dr. Eichenfield emphasized this point, noting that new therapies have expanded treatment options.
“Be aware of the advances in atopic dermatitis,” he said, “with newer topical medications and with a new systemic biologic agent approved for moderate to severe refractory atopic dermatitis for ages 6 and older.”
• Turning to diagnostic strategies, the AAP recommended against routine laboratory testing for associated autoimmune diseases among patients with vitiligo, unless clinical signs and/or symptoms of such diseases are present.
“There is no convincing evidence that extensive workups in the absence of specific clinical suspicion improves outcomes for patients and may in fact beget additional costs and harms,” the AAP wrote. “Although many studies suggest ordering these tests, it is based largely on the increased cosegregation of vitiligo and thyroid disease and not on improved outcomes from having identified an abnormal laboratory test result.”
• Similarly, the AAP advised practitioners to avoid routinely testing patients with alopecia areata for other diseases if relevant symptoms and signs aren’t present.
“As in the case of vitiligo, it is more common to find thyroid autoantibodies or subclinical hypothyroidism than overt thyroid disease, unless there are clinically suspicious findings,” the AAP wrote. “Patients identified as having subclinical hypothyroidism are not currently treated and may even have resolution of the abnormal TSH.”
Before drawing blood, Dr. Eichenfield suggested that clinicians first ask the right questions.
“Be comfortable with screening questions about growth, weight, or activity changes to assist with decisions for thyroid screening in a patient with vitiligo or alopecia areata,” he said.
Choosing Wisely is an initiative of the American Board of Internal Medicine. The AAP and Dr. Eichenfield reported no conflicts of interest.
FROM CHOOSING WISELY AND THE AAP
Virtual is the new real
Why did we fall short on maximizing telehealth’s value in the COVID-19 pandemic?
The COVID-19 pandemic catalyzed the transformation of Internet-based, remotely accessible innovative technologies. Internet-based customer service delivery technology was rapidly adopted and utilized by several services industries, but health care systems in most of the countries across the world faced unique challenges in adopting the technology for the delivery of health care services. The health care ecosystem of the United States was not immune to such challenges, and several significant barriers surfaced while the pandemic was underway.
Complexly structured, fragmented, unprepared, and overly burnt-out health systems in the United States arguably have fallen short of maximizing the value of telehealth in delivering safe, easily accessible, comprehensive, and cost-effective health care services. In this essay, we examine the reasons for such a suboptimal performance and discuss a few important strategies that may be useful in maximizing the value of telehealth value in several, appropriate health care services.
Hospitals and telehealth
Are hospitalists preparing ourselves “not to see” patients in a hospital-based health care delivery setting? If you have not yet started yet, now may be the right time! Yes, a certain percentage of doctor-patient encounters in hospital settings will remain virtual forever.
A well-established telehealth infrastructure is rarely found in most U.S. hospitals, although the COVID-19 pandemic has unexpectedly boosted the rapid growth of telehealth in the country.1 Public health emergency declarations in the United States in the face of the COVID-19 crisis have facilitated two important initiatives to restore health care delivery amidst formal and informal lockdowns that brought states to a grinding halt. These extend from expansion of virtual services, including telehealth, virtual check-ins, and e-visits, to the decision by the Department of Health & Human Services Office of Civil Rights to exercise enforcement discretion and waive penalties for the use of relatively inexpensive, non–public-facing mobile and other audiovisual technology tools.2
Hospital-based care in the United States taps nearly 33% of national health expenditure. An additional 30% of national health expenditure that is related to physicians, prescriptions, and other facilities is indirectly influenced by care delivered at health care facilities.3 Studies show that about 20% of ED visits could potentially be avoided via virtual urgent care offerings.4 A rapidly changing health care ecosystem is proving formidable for most hospital systems, and a test for their resilience and agility. Not just the implementation of telehealth is challenging, but getting it right is the key success factor.
Hospital-based telehealth
Expansion of telehealth coverage by the Centers for Medicare & Medicaid Services and most commercial payers did not quite ride the pandemic-induced momentum across the care continuum. Hospitals are lagging far behind ambulatory care in implementing telehealth. As illustrated in the “4-T Matrix” (see graphic) we would like to examine four key reasons for such a sluggish initial uptake and try to propose four important strategies that may help us to maximize the value created by telehealth technologies.
1. Timing
The health care system has always lagged far behind other service industries in terms of technology adaptation. Because of the unique nature of health care services, face-to-face interaction supersedes all other forms of communication. A rapidly evolving pandemic was not matched by simultaneous technology education for patients and providers. The enormous choice of hard-to-navigate telehealth tools; time and labor-intensive implementation; and uncertainty around payer, policy, and regulatory expectations might have precluded providers from the rapid adoption of telehealth in the hospital setting. Patients’ specific characteristics, such as the absence of technology-centered education, information, age, comorbidities, lack of technical literacy, and dependency on caregivers contributed to the suboptimal response from patients and families.
Deploying simple, ubiquitous, user-friendly, and technologically less challenging telehealth solutions may be a better approach to increase the adoption of such solutions by providers and patients. Hospitals need to develop and distribute telehealth user guides in all possible modes of communication. Provider-centric in-service sessions, workshops, and live support by “superuser teams” often work well in reducing end-user resistance.
2. Technical
Current electronic medical records vary widely in their features and offerings, and their ability to interact with third-party software and platforms. Dissatisfaction of end users with EMRs is well known, as is their likely relationship to burnout. Recent research continues to show a strong relationship between EMR usability and the odds of burnout among physicians.5 In the current climate, administrators and health informaticists have the responsibility to avoid adding increased burdens to end users.
Another issue is the limited connectivity in many remote/rural areas that would impact implementation of telehealth platforms. Studies indicate that 33% of rural Americans lack access to high-speed broadband Internet to support video visits.6 The recent successful implementation of telehealth across 530 providers in 75 ambulatory practices operated by Munson Healthcare, a rural health system in northern Michigan, sheds light on the technology’s enormous potential in providing safe access to rural populations.6,7
Privacy and safety of patient data is of paramount importance. According to a national poll on healthy aging by the University of Michigan in May 2019, targeting older adults, 47% of survey responders expressed difficulty using technology and 49% of survey responders were concerned about privacy.8 Use of certification and other tools offered by the Office of the National Coordinator for Health Information Technology would help reassure users, and the ability to capture and share images between providers would be of immense benefit in facilitating e-consults.
The need of the hour is redesigned work flow, to help providers adopt and use virtual care/telehealth efficiently. Work flow redesign must be coupled with technological advances to allow seamless integration of third-party telehealth platforms into existing EMR systems or built directly into EMRs. Use of quality metrics and analytical tools specific to telehealth would help measure the technology’s impact on patient care, outcomes, and end-user/provider experience.
3. Teams and training
Outcomes of health care interventions are often determined by the effectiveness of teams. Irrespective of how robust health care systems may have been initially, rapidly spreading infectious diseases like COVID-19 can quickly derail the system, bringing the workforce and patients to a breaking point.5 Decentralized, uncoordinated, and siloed efforts by individual teams across the care continuum were contributing factors for the partial success of telehealth care delivery pathways. The hospital systems with telehealth-ready teams at the start of the COVID-19 pandemic were so rare that the knowledge and technical training opportunities for innovators grew severalfold during the pandemic.
As per the American Medical Association, telehealth success is massively dependent on building the right team. Core, leadership, advisory, and implementation teams comprised of clinical representatives, end users, administrative personnel, executive members of the organization, technical experts, and payment/policy experts should be put together before implementing a telehealth strategy.9 Seamless integration of hospital-based care with ambulatory care via a telehealth platform is only complete when care managers are trained and deployed to fulfill the needs of a diverse group of patients. Deriving overall value from telehealth is only possible when there is a skill development, training and mentoring team put in place.
4. Thinking
In most U.S. hospitals, inpatient health care is equally distributed between nonprocedure and procedure-based services. Hospitals resorted to suspension of nonemergent procedures to mitigate the risk of spreading COVID-19. This was further compounded by many patients’ self-selection to defer care, an abrupt reduction in the influx of patients from the referral base because of suboptimally operating ambulatory care services, leading to low hospital occupancy.
Hospitals across the nation have gone through a massive short-term financial crunch and unfavorable cash-flow forecast, which prompted a paradoxical work-force reduction. While some argue that it may be akin to strategic myopia, the authors believed that such a response is strategically imperative to keep the hospital afloat. It is reasonable to attribute the paucity of innovation to constrained resources, and health systems are simply staying overly optimistic about “weathering the storm” and reverting soon to “business as usual.” The technological framework necessary for deploying a telehealth solution often comes with a price. Financially challenged hospital systems rarely exercise any capital-intensive activities. By contrast, telehealth adoption by ambulatory care can result in quicker resumption of patient care in community settings. A lack of operational and infrastructure synchrony between ambulatory and in-hospital systems has failed to capture telehealth-driven inpatient volume. For example, direct admissions from ambulatory telehealth referrals was a missed opportunity in several places. Referrals for labs, diagnostic tests, and other allied services could have helped hospitals offset their fixed costs. Similarly, work flows related to discharge and postdischarge follow up rarely embrace telehealth tools or telehealth care pathways. A brisk change in the health care ecosystem is partly responsible for this.
Digital strategy needs to be incorporated into business strategy. For the reasons already discussed, telehealth technology is not a “nice to have” anymore, but a “must have.” At present, providers are of the opinion that about 20% of their patient services can be delivered via a telehealth platform. Similar trends are observed among patients, as a new modality of access to care is increasingly beneficial to them. Telehealth must be incorporated in standardized hospital work flows. Use of telehealth for preoperative clearance will greatly minimize same-day surgery cancellations. Given the potential shortage in resources, telehealth adoption for inpatient consultations will help systems conserve personal protective equipment, minimize the risk of staff exposure to COVID-19, and improve efficiency.
Digital strategy also prompts the reengineering of care delivery.10 Excessive and unused physical capacity can be converted into digital care hubs. Health maintenance, prevention, health promotion, health education, and chronic disease management not only can serve a variety of patient groups but can also help address the “last-mile problem” in health care. A successful digital strategy usually has three important components – Commitment: Hospital leadership is committed to include digital transformation as a strategic objective; Cost: Digital strategy is added as a line item in the budget; and Control: Measurable metrics are put in place to monitor the performance, impact, and influence of the digital strategy.
Conclusion
For decades, most U.S. health systems occupied the periphery of technological transformation when compared to the rest of the service industry. While most health systems took a heroic approach to the adoption of telehealth during COVID-19, despite being unprepared, the need for a systematic telehealth deployment is far from being adequately fulfilled. The COVID-19 pandemic brought permanent changes to several business disciplines globally. Given the impact of the pandemic on the health and overall wellbeing of American society, the U.S. health care industry must leave no stone unturned in its quest for transformation.
Dr. Lingisetty is a hospitalist and physician executive at Baptist Health System, Little Rock, Ark, and is cofounder/president of SHM’s Arkansas chapter. Dr. Prasad is medical director of care management and a hospitalist at Advocate Aurora Health in Milwaukee. He is cochair of SHM’s IT Special Interest Group, sits on the HQPS committee, and is president of SHM’s Wisconsin chapter. Dr. Palabindala is the medical director, utilization management, and physician advisory services at the University of Mississippi Medical Center and an associate professor of medicine and academic hospitalist at the University of Mississippi, both in Jackson.
References
1. Finnegan M. “Telehealth booms amid COVID-19 crisis.” Computerworld. 2020 Apr 27. www.computerworld.com/article/3540315/telehealth-booms-amid-covid-19-crisis-virtual-care-is-here-to-stay.html. Accessed 2020 Sep 12.
2. Department of Health & Human Services. “OCR Announces Notification of Enforcement Discretion for Telehealth Remote Communications During the COVID-19 Nationwide Public Health Emergency.” 2020 Mar 17. www.hhs.gov/about/news/2020/03/17/ocr-announces-notification-of-enforcement-discretion-for-telehealth-remote-communications-during-the-covid-19.html. Accessed 2020 Sep 12.
3. National Center for Health Statistics. “Health Expenditures.” www.cdc.gov/nchs/fastats/health-expenditures.htm. Accessed 2020 Sep 12.
4. Bestsennyy O et al. “Telehealth: A post–COVID-19 reality?” McKinsey & Company. 2020 May 29. www.mckinsey.com/industries/healthcare-systems-and-services/our-insights/telehealth-a-quarter-trillion-dollar-post-covid-19-reality. Accessed 2020 Sep 12.
5. Melnick ER et al. The Association Between Perceived Electronic Health Record Usability and Professional Burnout Among U.S. Physicians. Mayo Clin Proc. 2020 March;95(3):476-87.
6. Hirko KA et al. Telehealth in response to the COVID-19 pandemic: Implications for rural health disparities. J Am Med Inform Assoc. 2020 Nov;27(11):1816-8. .
7. American Academy of Family Physicians. “Study Examines Telehealth, Rural Disparities in Pandemic.” 2020 July 30. www.aafp.org/news/practice-professional-issues/20200730ruraltelehealth.html. Accessed 2020 Dec 15.
8. Kurlander J et al. “Virtual Visits: Telehealth and Older Adults.” National Poll on Healthy Aging. 2019 Oct. hdl.handle.net/2027.42/151376.
9. American Medical Association. Telehealth Implementation Playbook. 2019. www.ama-assn.org/system/files/2020-04/ama-telehealth-implementation-playbook.pdf.
10. Smith AC et al. Telehealth for global emergencies: Implications for coronavirus disease 2019 (COVID-19). J Telemed Telecare. 2020 Jun;26(5):309-13.
Why did we fall short on maximizing telehealth’s value in the COVID-19 pandemic?
Why did we fall short on maximizing telehealth’s value in the COVID-19 pandemic?
The COVID-19 pandemic catalyzed the transformation of Internet-based, remotely accessible innovative technologies. Internet-based customer service delivery technology was rapidly adopted and utilized by several services industries, but health care systems in most of the countries across the world faced unique challenges in adopting the technology for the delivery of health care services. The health care ecosystem of the United States was not immune to such challenges, and several significant barriers surfaced while the pandemic was underway.
Complexly structured, fragmented, unprepared, and overly burnt-out health systems in the United States arguably have fallen short of maximizing the value of telehealth in delivering safe, easily accessible, comprehensive, and cost-effective health care services. In this essay, we examine the reasons for such a suboptimal performance and discuss a few important strategies that may be useful in maximizing the value of telehealth value in several, appropriate health care services.
Hospitals and telehealth
Are hospitalists preparing ourselves “not to see” patients in a hospital-based health care delivery setting? If you have not yet started yet, now may be the right time! Yes, a certain percentage of doctor-patient encounters in hospital settings will remain virtual forever.
A well-established telehealth infrastructure is rarely found in most U.S. hospitals, although the COVID-19 pandemic has unexpectedly boosted the rapid growth of telehealth in the country.1 Public health emergency declarations in the United States in the face of the COVID-19 crisis have facilitated two important initiatives to restore health care delivery amidst formal and informal lockdowns that brought states to a grinding halt. These extend from expansion of virtual services, including telehealth, virtual check-ins, and e-visits, to the decision by the Department of Health & Human Services Office of Civil Rights to exercise enforcement discretion and waive penalties for the use of relatively inexpensive, non–public-facing mobile and other audiovisual technology tools.2
Hospital-based care in the United States taps nearly 33% of national health expenditure. An additional 30% of national health expenditure that is related to physicians, prescriptions, and other facilities is indirectly influenced by care delivered at health care facilities.3 Studies show that about 20% of ED visits could potentially be avoided via virtual urgent care offerings.4 A rapidly changing health care ecosystem is proving formidable for most hospital systems, and a test for their resilience and agility. Not just the implementation of telehealth is challenging, but getting it right is the key success factor.
Hospital-based telehealth

Expansion of telehealth coverage by the Centers for Medicare & Medicaid Services and most commercial payers did not quite ride the pandemic-induced momentum across the care continuum. Hospitals are lagging far behind ambulatory care in implementing telehealth. As illustrated in the “4-T Matrix” (see graphic) we would like to examine four key reasons for such a sluggish initial uptake and try to propose four important strategies that may help us to maximize the value created by telehealth technologies.
1. Timing
The health care system has always lagged far behind other service industries in terms of technology adaptation. Because of the unique nature of health care services, face-to-face interaction supersedes all other forms of communication. A rapidly evolving pandemic was not matched by simultaneous technology education for patients and providers. The enormous choice of hard-to-navigate telehealth tools; time and labor-intensive implementation; and uncertainty around payer, policy, and regulatory expectations might have precluded providers from the rapid adoption of telehealth in the hospital setting. Patients’ specific characteristics, such as the absence of technology-centered education, information, age, comorbidities, lack of technical literacy, and dependency on caregivers contributed to the suboptimal response from patients and families.
Deploying simple, ubiquitous, user-friendly, and technologically less challenging telehealth solutions may be a better approach to increase the adoption of such solutions by providers and patients. Hospitals need to develop and distribute telehealth user guides in all possible modes of communication. Provider-centric in-service sessions, workshops, and live support by “superuser teams” often work well in reducing end-user resistance.
2. Technical
Current electronic medical records vary widely in their features and offerings, and their ability to interact with third-party software and platforms. Dissatisfaction of end users with EMRs is well known, as is their likely relationship to burnout. Recent research continues to show a strong relationship between EMR usability and the odds of burnout among physicians.5 In the current climate, administrators and health informaticists have the responsibility to avoid adding increased burdens to end users.
Another issue is the limited connectivity in many remote/rural areas that would impact implementation of telehealth platforms. Studies indicate that 33% of rural Americans lack access to high-speed broadband Internet to support video visits.6 The recent successful implementation of telehealth across 530 providers in 75 ambulatory practices operated by Munson Healthcare, a rural health system in northern Michigan, sheds light on the technology’s enormous potential in providing safe access to rural populations.6,7
Privacy and safety of patient data is of paramount importance. According to a national poll on healthy aging by the University of Michigan in May 2019, targeting older adults, 47% of survey responders expressed difficulty using technology and 49% of survey responders were concerned about privacy.8 Use of certification and other tools offered by the Office of the National Coordinator for Health Information Technology would help reassure users, and the ability to capture and share images between providers would be of immense benefit in facilitating e-consults.
The need of the hour is redesigned work flow, to help providers adopt and use virtual care/telehealth efficiently. Work flow redesign must be coupled with technological advances to allow seamless integration of third-party telehealth platforms into existing EMR systems or built directly into EMRs. Use of quality metrics and analytical tools specific to telehealth would help measure the technology’s impact on patient care, outcomes, and end-user/provider experience.
3. Teams and training
Outcomes of health care interventions are often determined by the effectiveness of teams. Irrespective of how robust health care systems may have been initially, rapidly spreading infectious diseases like COVID-19 can quickly derail the system, bringing the workforce and patients to a breaking point.5 Decentralized, uncoordinated, and siloed efforts by individual teams across the care continuum were contributing factors for the partial success of telehealth care delivery pathways. The hospital systems with telehealth-ready teams at the start of the COVID-19 pandemic were so rare that the knowledge and technical training opportunities for innovators grew severalfold during the pandemic.
As per the American Medical Association, telehealth success is massively dependent on building the right team. Core, leadership, advisory, and implementation teams comprised of clinical representatives, end users, administrative personnel, executive members of the organization, technical experts, and payment/policy experts should be put together before implementing a telehealth strategy.9 Seamless integration of hospital-based care with ambulatory care via a telehealth platform is only complete when care managers are trained and deployed to fulfill the needs of a diverse group of patients. Deriving overall value from telehealth is only possible when there is a skill development, training and mentoring team put in place.
4. Thinking
In most U.S. hospitals, inpatient health care is equally distributed between nonprocedure and procedure-based services. Hospitals resorted to suspension of nonemergent procedures to mitigate the risk of spreading COVID-19. This was further compounded by many patients’ self-selection to defer care, an abrupt reduction in the influx of patients from the referral base because of suboptimally operating ambulatory care services, leading to low hospital occupancy.
Hospitals across the nation have gone through a massive short-term financial crunch and unfavorable cash-flow forecast, which prompted a paradoxical work-force reduction. While some argue that it may be akin to strategic myopia, the authors believed that such a response is strategically imperative to keep the hospital afloat. It is reasonable to attribute the paucity of innovation to constrained resources, and health systems are simply staying overly optimistic about “weathering the storm” and reverting soon to “business as usual.” The technological framework necessary for deploying a telehealth solution often comes with a price. Financially challenged hospital systems rarely exercise any capital-intensive activities. By contrast, telehealth adoption by ambulatory care can result in quicker resumption of patient care in community settings. A lack of operational and infrastructure synchrony between ambulatory and in-hospital systems has failed to capture telehealth-driven inpatient volume. For example, direct admissions from ambulatory telehealth referrals was a missed opportunity in several places. Referrals for labs, diagnostic tests, and other allied services could have helped hospitals offset their fixed costs. Similarly, work flows related to discharge and postdischarge follow up rarely embrace telehealth tools or telehealth care pathways. A brisk change in the health care ecosystem is partly responsible for this.
Digital strategy needs to be incorporated into business strategy. For the reasons already discussed, telehealth technology is not a “nice to have” anymore, but a “must have.” At present, providers are of the opinion that about 20% of their patient services can be delivered via a telehealth platform. Similar trends are observed among patients, as a new modality of access to care is increasingly beneficial to them. Telehealth must be incorporated in standardized hospital work flows. Use of telehealth for preoperative clearance will greatly minimize same-day surgery cancellations. Given the potential shortage in resources, telehealth adoption for inpatient consultations will help systems conserve personal protective equipment, minimize the risk of staff exposure to COVID-19, and improve efficiency.
Digital strategy also prompts the reengineering of care delivery.10 Excessive and unused physical capacity can be converted into digital care hubs. Health maintenance, prevention, health promotion, health education, and chronic disease management not only can serve a variety of patient groups but can also help address the “last-mile problem” in health care. A successful digital strategy usually has three important components – Commitment: Hospital leadership is committed to include digital transformation as a strategic objective; Cost: Digital strategy is added as a line item in the budget; and Control: Measurable metrics are put in place to monitor the performance, impact, and influence of the digital strategy.
Conclusion
For decades, most U.S. health systems occupied the periphery of technological transformation when compared to the rest of the service industry. While most health systems took a heroic approach to the adoption of telehealth during COVID-19, despite being unprepared, the need for a systematic telehealth deployment is far from being adequately fulfilled. The COVID-19 pandemic brought permanent changes to several business disciplines globally. Given the impact of the pandemic on the health and overall wellbeing of American society, the U.S. health care industry must leave no stone unturned in its quest for transformation.
Dr. Lingisetty is a hospitalist and physician executive at Baptist Health System, Little Rock, Ark, and is cofounder/president of SHM’s Arkansas chapter. Dr. Prasad is medical director of care management and a hospitalist at Advocate Aurora Health in Milwaukee. He is cochair of SHM’s IT Special Interest Group, sits on the HQPS committee, and is president of SHM’s Wisconsin chapter. Dr. Palabindala is the medical director, utilization management, and physician advisory services at the University of Mississippi Medical Center and an associate professor of medicine and academic hospitalist at the University of Mississippi, both in Jackson.
References
1. Finnegan M. “Telehealth booms amid COVID-19 crisis.” Computerworld. 2020 Apr 27. www.computerworld.com/article/3540315/telehealth-booms-amid-covid-19-crisis-virtual-care-is-here-to-stay.html. Accessed 2020 Sep 12.
2. Department of Health & Human Services. “OCR Announces Notification of Enforcement Discretion for Telehealth Remote Communications During the COVID-19 Nationwide Public Health Emergency.” 2020 Mar 17. www.hhs.gov/about/news/2020/03/17/ocr-announces-notification-of-enforcement-discretion-for-telehealth-remote-communications-during-the-covid-19.html. Accessed 2020 Sep 12.
3. National Center for Health Statistics. “Health Expenditures.” www.cdc.gov/nchs/fastats/health-expenditures.htm. Accessed 2020 Sep 12.
4. Bestsennyy O et al. “Telehealth: A post–COVID-19 reality?” McKinsey & Company. 2020 May 29. www.mckinsey.com/industries/healthcare-systems-and-services/our-insights/telehealth-a-quarter-trillion-dollar-post-covid-19-reality. Accessed 2020 Sep 12.
5. Melnick ER et al. The Association Between Perceived Electronic Health Record Usability and Professional Burnout Among U.S. Physicians. Mayo Clin Proc. 2020 March;95(3):476-87.
6. Hirko KA et al. Telehealth in response to the COVID-19 pandemic: Implications for rural health disparities. J Am Med Inform Assoc. 2020 Nov;27(11):1816-8. .
7. American Academy of Family Physicians. “Study Examines Telehealth, Rural Disparities in Pandemic.” 2020 July 30. www.aafp.org/news/practice-professional-issues/20200730ruraltelehealth.html. Accessed 2020 Dec 15.
8. Kurlander J et al. “Virtual Visits: Telehealth and Older Adults.” National Poll on Healthy Aging. 2019 Oct. hdl.handle.net/2027.42/151376.
9. American Medical Association. Telehealth Implementation Playbook. 2019. www.ama-assn.org/system/files/2020-04/ama-telehealth-implementation-playbook.pdf.
10. Smith AC et al. Telehealth for global emergencies: Implications for coronavirus disease 2019 (COVID-19). J Telemed Telecare. 2020 Jun;26(5):309-13.
The COVID-19 pandemic catalyzed the transformation of Internet-based, remotely accessible innovative technologies. Internet-based customer service delivery technology was rapidly adopted and utilized by several services industries, but health care systems in most of the countries across the world faced unique challenges in adopting the technology for the delivery of health care services. The health care ecosystem of the United States was not immune to such challenges, and several significant barriers surfaced while the pandemic was underway.
Complexly structured, fragmented, unprepared, and overly burnt-out health systems in the United States arguably have fallen short of maximizing the value of telehealth in delivering safe, easily accessible, comprehensive, and cost-effective health care services. In this essay, we examine the reasons for such a suboptimal performance and discuss a few important strategies that may be useful in maximizing the value of telehealth value in several, appropriate health care services.
Hospitals and telehealth
Are hospitalists preparing ourselves “not to see” patients in a hospital-based health care delivery setting? If you have not yet started yet, now may be the right time! Yes, a certain percentage of doctor-patient encounters in hospital settings will remain virtual forever.
A well-established telehealth infrastructure is rarely found in most U.S. hospitals, although the COVID-19 pandemic has unexpectedly boosted the rapid growth of telehealth in the country.1 Public health emergency declarations in the United States in the face of the COVID-19 crisis have facilitated two important initiatives to restore health care delivery amidst formal and informal lockdowns that brought states to a grinding halt. These extend from expansion of virtual services, including telehealth, virtual check-ins, and e-visits, to the decision by the Department of Health & Human Services Office of Civil Rights to exercise enforcement discretion and waive penalties for the use of relatively inexpensive, non–public-facing mobile and other audiovisual technology tools.2
Hospital-based care in the United States taps nearly 33% of national health expenditure. An additional 30% of national health expenditure that is related to physicians, prescriptions, and other facilities is indirectly influenced by care delivered at health care facilities.3 Studies show that about 20% of ED visits could potentially be avoided via virtual urgent care offerings.4 A rapidly changing health care ecosystem is proving formidable for most hospital systems, and a test for their resilience and agility. Not just the implementation of telehealth is challenging, but getting it right is the key success factor.
Hospital-based telehealth

Expansion of telehealth coverage by the Centers for Medicare & Medicaid Services and most commercial payers did not quite ride the pandemic-induced momentum across the care continuum. Hospitals are lagging far behind ambulatory care in implementing telehealth. As illustrated in the “4-T Matrix” (see graphic) we would like to examine four key reasons for such a sluggish initial uptake and try to propose four important strategies that may help us to maximize the value created by telehealth technologies.
1. Timing
The health care system has always lagged far behind other service industries in terms of technology adaptation. Because of the unique nature of health care services, face-to-face interaction supersedes all other forms of communication. A rapidly evolving pandemic was not matched by simultaneous technology education for patients and providers. The enormous choice of hard-to-navigate telehealth tools; time and labor-intensive implementation; and uncertainty around payer, policy, and regulatory expectations might have precluded providers from the rapid adoption of telehealth in the hospital setting. Patients’ specific characteristics, such as the absence of technology-centered education, information, age, comorbidities, lack of technical literacy, and dependency on caregivers contributed to the suboptimal response from patients and families.
Deploying simple, ubiquitous, user-friendly, and technologically less challenging telehealth solutions may be a better approach to increase the adoption of such solutions by providers and patients. Hospitals need to develop and distribute telehealth user guides in all possible modes of communication. Provider-centric in-service sessions, workshops, and live support by “superuser teams” often work well in reducing end-user resistance.
2. Technical
Current electronic medical records vary widely in their features and offerings, and their ability to interact with third-party software and platforms. Dissatisfaction of end users with EMRs is well known, as is their likely relationship to burnout. Recent research continues to show a strong relationship between EMR usability and the odds of burnout among physicians.5 In the current climate, administrators and health informaticists have the responsibility to avoid adding increased burdens to end users.
Another issue is the limited connectivity in many remote/rural areas that would impact implementation of telehealth platforms. Studies indicate that 33% of rural Americans lack access to high-speed broadband Internet to support video visits.6 The recent successful implementation of telehealth across 530 providers in 75 ambulatory practices operated by Munson Healthcare, a rural health system in northern Michigan, sheds light on the technology’s enormous potential in providing safe access to rural populations.6,7
Privacy and safety of patient data is of paramount importance. According to a national poll on healthy aging by the University of Michigan in May 2019, targeting older adults, 47% of survey responders expressed difficulty using technology and 49% of survey responders were concerned about privacy.8 Use of certification and other tools offered by the Office of the National Coordinator for Health Information Technology would help reassure users, and the ability to capture and share images between providers would be of immense benefit in facilitating e-consults.
The need of the hour is redesigned work flow, to help providers adopt and use virtual care/telehealth efficiently. Work flow redesign must be coupled with technological advances to allow seamless integration of third-party telehealth platforms into existing EMR systems or built directly into EMRs. Use of quality metrics and analytical tools specific to telehealth would help measure the technology’s impact on patient care, outcomes, and end-user/provider experience.
3. Teams and training
Outcomes of health care interventions are often determined by the effectiveness of teams. Irrespective of how robust health care systems may have been initially, rapidly spreading infectious diseases like COVID-19 can quickly derail the system, bringing the workforce and patients to a breaking point.5 Decentralized, uncoordinated, and siloed efforts by individual teams across the care continuum were contributing factors for the partial success of telehealth care delivery pathways. The hospital systems with telehealth-ready teams at the start of the COVID-19 pandemic were so rare that the knowledge and technical training opportunities for innovators grew severalfold during the pandemic.
As per the American Medical Association, telehealth success is massively dependent on building the right team. Core, leadership, advisory, and implementation teams comprised of clinical representatives, end users, administrative personnel, executive members of the organization, technical experts, and payment/policy experts should be put together before implementing a telehealth strategy.9 Seamless integration of hospital-based care with ambulatory care via a telehealth platform is only complete when care managers are trained and deployed to fulfill the needs of a diverse group of patients. Deriving overall value from telehealth is only possible when there is a skill development, training and mentoring team put in place.
4. Thinking
In most U.S. hospitals, inpatient health care is equally distributed between nonprocedure and procedure-based services. Hospitals resorted to suspension of nonemergent procedures to mitigate the risk of spreading COVID-19. This was further compounded by many patients’ self-selection to defer care, an abrupt reduction in the influx of patients from the referral base because of suboptimally operating ambulatory care services, leading to low hospital occupancy.
Hospitals across the nation have gone through a massive short-term financial crunch and unfavorable cash-flow forecast, which prompted a paradoxical work-force reduction. While some argue that it may be akin to strategic myopia, the authors believed that such a response is strategically imperative to keep the hospital afloat. It is reasonable to attribute the paucity of innovation to constrained resources, and health systems are simply staying overly optimistic about “weathering the storm” and reverting soon to “business as usual.” The technological framework necessary for deploying a telehealth solution often comes with a price. Financially challenged hospital systems rarely exercise any capital-intensive activities. By contrast, telehealth adoption by ambulatory care can result in quicker resumption of patient care in community settings. A lack of operational and infrastructure synchrony between ambulatory and in-hospital systems has failed to capture telehealth-driven inpatient volume. For example, direct admissions from ambulatory telehealth referrals was a missed opportunity in several places. Referrals for labs, diagnostic tests, and other allied services could have helped hospitals offset their fixed costs. Similarly, work flows related to discharge and postdischarge follow up rarely embrace telehealth tools or telehealth care pathways. A brisk change in the health care ecosystem is partly responsible for this.
Digital strategy needs to be incorporated into business strategy. For the reasons already discussed, telehealth technology is not a “nice to have” anymore, but a “must have.” At present, providers are of the opinion that about 20% of their patient services can be delivered via a telehealth platform. Similar trends are observed among patients, as a new modality of access to care is increasingly beneficial to them. Telehealth must be incorporated in standardized hospital work flows. Use of telehealth for preoperative clearance will greatly minimize same-day surgery cancellations. Given the potential shortage in resources, telehealth adoption for inpatient consultations will help systems conserve personal protective equipment, minimize the risk of staff exposure to COVID-19, and improve efficiency.
Digital strategy also prompts the reengineering of care delivery.10 Excessive and unused physical capacity can be converted into digital care hubs. Health maintenance, prevention, health promotion, health education, and chronic disease management not only can serve a variety of patient groups but can also help address the “last-mile problem” in health care. A successful digital strategy usually has three important components – Commitment: Hospital leadership is committed to include digital transformation as a strategic objective; Cost: Digital strategy is added as a line item in the budget; and Control: Measurable metrics are put in place to monitor the performance, impact, and influence of the digital strategy.
Conclusion
For decades, most U.S. health systems occupied the periphery of technological transformation when compared to the rest of the service industry. While most health systems took a heroic approach to the adoption of telehealth during COVID-19, despite being unprepared, the need for a systematic telehealth deployment is far from being adequately fulfilled. The COVID-19 pandemic brought permanent changes to several business disciplines globally. Given the impact of the pandemic on the health and overall wellbeing of American society, the U.S. health care industry must leave no stone unturned in its quest for transformation.
Dr. Lingisetty is a hospitalist and physician executive at Baptist Health System, Little Rock, Ark, and is cofounder/president of SHM’s Arkansas chapter. Dr. Prasad is medical director of care management and a hospitalist at Advocate Aurora Health in Milwaukee. He is cochair of SHM’s IT Special Interest Group, sits on the HQPS committee, and is president of SHM’s Wisconsin chapter. Dr. Palabindala is the medical director, utilization management, and physician advisory services at the University of Mississippi Medical Center and an associate professor of medicine and academic hospitalist at the University of Mississippi, both in Jackson.
References
1. Finnegan M. “Telehealth booms amid COVID-19 crisis.” Computerworld. 2020 Apr 27. www.computerworld.com/article/3540315/telehealth-booms-amid-covid-19-crisis-virtual-care-is-here-to-stay.html. Accessed 2020 Sep 12.
2. Department of Health & Human Services. “OCR Announces Notification of Enforcement Discretion for Telehealth Remote Communications During the COVID-19 Nationwide Public Health Emergency.” 2020 Mar 17. www.hhs.gov/about/news/2020/03/17/ocr-announces-notification-of-enforcement-discretion-for-telehealth-remote-communications-during-the-covid-19.html. Accessed 2020 Sep 12.
3. National Center for Health Statistics. “Health Expenditures.” www.cdc.gov/nchs/fastats/health-expenditures.htm. Accessed 2020 Sep 12.
4. Bestsennyy O et al. “Telehealth: A post–COVID-19 reality?” McKinsey & Company. 2020 May 29. www.mckinsey.com/industries/healthcare-systems-and-services/our-insights/telehealth-a-quarter-trillion-dollar-post-covid-19-reality. Accessed 2020 Sep 12.
5. Melnick ER et al. The Association Between Perceived Electronic Health Record Usability and Professional Burnout Among U.S. Physicians. Mayo Clin Proc. 2020 March;95(3):476-87.
6. Hirko KA et al. Telehealth in response to the COVID-19 pandemic: Implications for rural health disparities. J Am Med Inform Assoc. 2020 Nov;27(11):1816-8. .
7. American Academy of Family Physicians. “Study Examines Telehealth, Rural Disparities in Pandemic.” 2020 July 30. www.aafp.org/news/practice-professional-issues/20200730ruraltelehealth.html. Accessed 2020 Dec 15.
8. Kurlander J et al. “Virtual Visits: Telehealth and Older Adults.” National Poll on Healthy Aging. 2019 Oct. hdl.handle.net/2027.42/151376.
9. American Medical Association. Telehealth Implementation Playbook. 2019. www.ama-assn.org/system/files/2020-04/ama-telehealth-implementation-playbook.pdf.
10. Smith AC et al. Telehealth for global emergencies: Implications for coronavirus disease 2019 (COVID-19). J Telemed Telecare. 2020 Jun;26(5):309-13.
Fauci worries about possible post–COVID-19 ‘mental health pandemic’
Anthony Fauci, MD, says he’s concerned about how Americans will react once the coronavirus pandemic is brought under control, CBS News reports.
Noting that an American Psychological Association survey showed people reporting high stress levels because of the pandemic, CBS’s Norah O’Donnell asked if Dr. Fauci was concerned about a possible “mental health pandemic.”
“Very much so,” Dr. Fauci, director of the National Institute of Allergy and Infectious Diseases and a top White House coronavirus adviser, replied.
“That’s the reason why I want to get the virological aspect of this pandemic behind us as quickly as we possibly can because the long-term ravages of this are so multifaceted,” Dr. Fauci said.
, he said.
“And then the other things: Not only the mental health effects, but many people have put off routine types of medical examinations that they normally would have done,” Dr. Fauci said.
“I hope we don’t see an increase in some preventable situations that would not have happened if people had the normal access to medical care, which clearly was interrupted by the shutdown associated with COVID-19,” he added.
The American Psychological Association released the survey results March 11 in what many people consider the 1-year anniversary of the start of the coronavirus pandemic.
“The prolonged stress experienced by adults, especially the high levels of stress reported by Americans directly linked to the pandemic, is seriously affecting mental and physical health, including changes to weight, sleep and alcohol use,” the APA said in a news release.
Some of the key findings of the survey include:
- 61% of respondents reported experiencing undesired weight changes since the start of the pandemic.
- 67% said their sleep habits changed, with 35% saying they slept more and 31% less.
- 23% reported drinking more alcohol to cope with stress.
- 47% said they delayed or canceled health care services because of the pandemic.
- 48% said their stress levels had increased.
A version of this article first appeared on Medscape.com.
Anthony Fauci, MD, says he’s concerned about how Americans will react once the coronavirus pandemic is brought under control, CBS News reports.
Noting that an American Psychological Association survey showed people reporting high stress levels because of the pandemic, CBS’s Norah O’Donnell asked if Dr. Fauci was concerned about a possible “mental health pandemic.”
“Very much so,” Dr. Fauci, director of the National Institute of Allergy and Infectious Diseases and a top White House coronavirus adviser, replied.
“That’s the reason why I want to get the virological aspect of this pandemic behind us as quickly as we possibly can because the long-term ravages of this are so multifaceted,” Dr. Fauci said.
, he said.
“And then the other things: Not only the mental health effects, but many people have put off routine types of medical examinations that they normally would have done,” Dr. Fauci said.
“I hope we don’t see an increase in some preventable situations that would not have happened if people had the normal access to medical care, which clearly was interrupted by the shutdown associated with COVID-19,” he added.
The American Psychological Association released the survey results March 11 in what many people consider the 1-year anniversary of the start of the coronavirus pandemic.
“The prolonged stress experienced by adults, especially the high levels of stress reported by Americans directly linked to the pandemic, is seriously affecting mental and physical health, including changes to weight, sleep and alcohol use,” the APA said in a news release.
Some of the key findings of the survey include:
- 61% of respondents reported experiencing undesired weight changes since the start of the pandemic.
- 67% said their sleep habits changed, with 35% saying they slept more and 31% less.
- 23% reported drinking more alcohol to cope with stress.
- 47% said they delayed or canceled health care services because of the pandemic.
- 48% said their stress levels had increased.
A version of this article first appeared on Medscape.com.
Anthony Fauci, MD, says he’s concerned about how Americans will react once the coronavirus pandemic is brought under control, CBS News reports.
Noting that an American Psychological Association survey showed people reporting high stress levels because of the pandemic, CBS’s Norah O’Donnell asked if Dr. Fauci was concerned about a possible “mental health pandemic.”
“Very much so,” Dr. Fauci, director of the National Institute of Allergy and Infectious Diseases and a top White House coronavirus adviser, replied.
“That’s the reason why I want to get the virological aspect of this pandemic behind us as quickly as we possibly can because the long-term ravages of this are so multifaceted,” Dr. Fauci said.
, he said.
“And then the other things: Not only the mental health effects, but many people have put off routine types of medical examinations that they normally would have done,” Dr. Fauci said.
“I hope we don’t see an increase in some preventable situations that would not have happened if people had the normal access to medical care, which clearly was interrupted by the shutdown associated with COVID-19,” he added.
The American Psychological Association released the survey results March 11 in what many people consider the 1-year anniversary of the start of the coronavirus pandemic.
“The prolonged stress experienced by adults, especially the high levels of stress reported by Americans directly linked to the pandemic, is seriously affecting mental and physical health, including changes to weight, sleep and alcohol use,” the APA said in a news release.
Some of the key findings of the survey include:
- 61% of respondents reported experiencing undesired weight changes since the start of the pandemic.
- 67% said their sleep habits changed, with 35% saying they slept more and 31% less.
- 23% reported drinking more alcohol to cope with stress.
- 47% said they delayed or canceled health care services because of the pandemic.
- 48% said their stress levels had increased.
A version of this article first appeared on Medscape.com.
Despite risks and warnings, CNS polypharmacy is prevalent among patients with dementia
, new research suggests.
Investigators found that 14% of these individuals were receiving CNS-active polypharmacy, defined as combinations of multiple psychotropic and opioid medications taken for more than 30 days.
“For most patients, the risks of these medications, particularly in combination, are almost certainly greater than the potential benefits,” said Donovan Maust, MD, associate director of the geriatric psychiatry program, University of Michigan, Ann Arbor.
The study was published online March 9 in JAMA.
Serious risks
Memory impairment is the cardinal feature of dementia, but behavioral and psychological symptoms, which can include apathy, delusions, and agitation, are common during all stages of illness and cause significant caregiver distress, the researchers noted.
They noted that there is a dearth of high-quality evidence to support prescribing these medications in this patient population, yet “clinicians regularly prescribe psychotropic medications to community-dwelling persons with dementia in rates that far exceed use in the general older adult population.”
The Beers Criteria, from the American Geriatrics Society, advise against the practice of CNS polypharmacy because of the significant increase in risk for falls as well as impaired cognition, cardiac conduction abnormalities, respiratory suppression, and death when polypharmacy involves opioids.
They note that previous studies from Europe of polypharmacy for patients with dementia have not included antiepileptic medications or opioids, so the true extent of CNS-active polypharmacy may be “significantly” underestimated.
To determine the prevalence of polypharmacy with CNS-active medications among community-dwelling older adults with dementia, the researchers analyzed data on prescription fills for nearly 1.2 million community-dwelling Medicare patients with dementia.
The primary outcome was the prevalence of CNS-active polypharmacy in 2018. They defined CNS-active polypharmacy as exposure to three or more medications for more than 30 consecutive days from the following drug classes: antidepressants, antipsychotics, antiepileptics, benzodiazepines, nonbenzodiazepines, benzodiazepine receptor agonist hypnotics, and opioids.
They found that roughly one in seven (13.9%) patients met criteria for CNS-active polypharmacy. Of those receiving a CNS-active polypharmacy regimen, 57.8% had been doing so for longer than 180 days, and 6.8% had been doing so for a year. Nearly 30% of patients were exposed to five or more medications, and 5.2% were exposed to five or more medication classes.
Conservative approach warranted
Nearly all (92%) patients taking three or more CNS-active medications were taking an antidepressant, “consistent with their place as the psychotropic class most commonly prescribed both to older adults overall and those with dementia,” the investigators noted.
There is minimal high-quality evidence to support the efficacy of antidepressants for the treatment of depression for patients with dementia, they pointed out.
Nearly half (47%) of patients who were taking three or more CNS-active medications received at least one antipsychotic, most often quetiapine. Antipsychotics are not approved for people with dementia but are often prescribed off label for agitation, anxiety, and sleep problems, the researchers noted.
Nearly two thirds (62%) of patients with dementia who were taking three or more CNS drugs were taking an antiepileptic (most commonly, gabapentin); 41%, benzodiazepines; 32%, opioids; and 6%, Z-drugs.
The most common polypharmacy class combination included at least one antidepressant, one antiepileptic, and one antipsychotic. These accounted for 12.9% of polypharmacy days.
Despite limited high-quality evidence of efficacy, the prescribing of psychotropic medications and opioids is “pervasive” for adults with dementia in the United States, the investigators noted.
“Especially given that older adults with dementia might not be able to convey side effects they are experiencing, I think clinicians should be more conservative in how they are prescribing these medications and skeptical about the potential for benefit,” said Dr. Maust.
Regarding study limitations, the researchers noted that prescription medication claims may have led to an overestimation of the exposure to polypharmacy, insofar as the prescriptions may have been filled but not taken or were taken only on an as-needed basis.
In addition, the investigators were unable to determine the appropriateness of the particular combinations used or to examine the specific harms associated with CNS-active polypharmacy.
A major clinical challenge
Weighing in on the results, Howard Fillit, MD, founding executive director and chief science officer of the Alzheimer’s Drug Discovery Foundation, said the study is important because polypharmacy is one of the “geriatric giants, and the question is, what do you do about it?”
Dr. Fillit said it is important to conduct a careful medication review for all older patients, “making sure that the use of each drug is appropriate. The most important thing is to define what is the appropriate utilization of these kinds of drugs. That goes for both overutilization or misuse of these drugs and underutilization, where people are undertreated for symptoms that can’t be managed by behavioral management, for example,” Dr. Fillit said.
Dr. Fillit also said the finding that about 14% of dementia patients were receiving three or more of these drugs “may not be an outrageous number, because these patients, especially as they get into moderate and severe stages of disease, can be incredibly difficult to manage.
“Very often, dementia patients have depression, and up to 90% will have agitation and even psychosis during the course of dementia. And many of these patients need these types of drugs,” said Dr. Fillit.
Echoing the authors, Dr. Fillit said a key limitation of the study is not knowing whether the prescribing was appropriate or not.
The study was supported by a grant from the National Institute on Aging. Dr. Maust and Dr. Fillit have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, new research suggests.
Investigators found that 14% of these individuals were receiving CNS-active polypharmacy, defined as combinations of multiple psychotropic and opioid medications taken for more than 30 days.
“For most patients, the risks of these medications, particularly in combination, are almost certainly greater than the potential benefits,” said Donovan Maust, MD, associate director of the geriatric psychiatry program, University of Michigan, Ann Arbor.
The study was published online March 9 in JAMA.
Serious risks
Memory impairment is the cardinal feature of dementia, but behavioral and psychological symptoms, which can include apathy, delusions, and agitation, are common during all stages of illness and cause significant caregiver distress, the researchers noted.
They noted that there is a dearth of high-quality evidence to support prescribing these medications in this patient population, yet “clinicians regularly prescribe psychotropic medications to community-dwelling persons with dementia in rates that far exceed use in the general older adult population.”
The Beers Criteria, from the American Geriatrics Society, advise against the practice of CNS polypharmacy because of the significant increase in risk for falls as well as impaired cognition, cardiac conduction abnormalities, respiratory suppression, and death when polypharmacy involves opioids.
They note that previous studies from Europe of polypharmacy for patients with dementia have not included antiepileptic medications or opioids, so the true extent of CNS-active polypharmacy may be “significantly” underestimated.
To determine the prevalence of polypharmacy with CNS-active medications among community-dwelling older adults with dementia, the researchers analyzed data on prescription fills for nearly 1.2 million community-dwelling Medicare patients with dementia.
The primary outcome was the prevalence of CNS-active polypharmacy in 2018. They defined CNS-active polypharmacy as exposure to three or more medications for more than 30 consecutive days from the following drug classes: antidepressants, antipsychotics, antiepileptics, benzodiazepines, nonbenzodiazepines, benzodiazepine receptor agonist hypnotics, and opioids.
They found that roughly one in seven (13.9%) patients met criteria for CNS-active polypharmacy. Of those receiving a CNS-active polypharmacy regimen, 57.8% had been doing so for longer than 180 days, and 6.8% had been doing so for a year. Nearly 30% of patients were exposed to five or more medications, and 5.2% were exposed to five or more medication classes.
Conservative approach warranted
Nearly all (92%) patients taking three or more CNS-active medications were taking an antidepressant, “consistent with their place as the psychotropic class most commonly prescribed both to older adults overall and those with dementia,” the investigators noted.
There is minimal high-quality evidence to support the efficacy of antidepressants for the treatment of depression for patients with dementia, they pointed out.
Nearly half (47%) of patients who were taking three or more CNS-active medications received at least one antipsychotic, most often quetiapine. Antipsychotics are not approved for people with dementia but are often prescribed off label for agitation, anxiety, and sleep problems, the researchers noted.
Nearly two thirds (62%) of patients with dementia who were taking three or more CNS drugs were taking an antiepileptic (most commonly, gabapentin); 41%, benzodiazepines; 32%, opioids; and 6%, Z-drugs.
The most common polypharmacy class combination included at least one antidepressant, one antiepileptic, and one antipsychotic. These accounted for 12.9% of polypharmacy days.
Despite limited high-quality evidence of efficacy, the prescribing of psychotropic medications and opioids is “pervasive” for adults with dementia in the United States, the investigators noted.
“Especially given that older adults with dementia might not be able to convey side effects they are experiencing, I think clinicians should be more conservative in how they are prescribing these medications and skeptical about the potential for benefit,” said Dr. Maust.
Regarding study limitations, the researchers noted that prescription medication claims may have led to an overestimation of the exposure to polypharmacy, insofar as the prescriptions may have been filled but not taken or were taken only on an as-needed basis.
In addition, the investigators were unable to determine the appropriateness of the particular combinations used or to examine the specific harms associated with CNS-active polypharmacy.
A major clinical challenge
Weighing in on the results, Howard Fillit, MD, founding executive director and chief science officer of the Alzheimer’s Drug Discovery Foundation, said the study is important because polypharmacy is one of the “geriatric giants, and the question is, what do you do about it?”
Dr. Fillit said it is important to conduct a careful medication review for all older patients, “making sure that the use of each drug is appropriate. The most important thing is to define what is the appropriate utilization of these kinds of drugs. That goes for both overutilization or misuse of these drugs and underutilization, where people are undertreated for symptoms that can’t be managed by behavioral management, for example,” Dr. Fillit said.
Dr. Fillit also said the finding that about 14% of dementia patients were receiving three or more of these drugs “may not be an outrageous number, because these patients, especially as they get into moderate and severe stages of disease, can be incredibly difficult to manage.
“Very often, dementia patients have depression, and up to 90% will have agitation and even psychosis during the course of dementia. And many of these patients need these types of drugs,” said Dr. Fillit.
Echoing the authors, Dr. Fillit said a key limitation of the study is not knowing whether the prescribing was appropriate or not.
The study was supported by a grant from the National Institute on Aging. Dr. Maust and Dr. Fillit have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, new research suggests.
Investigators found that 14% of these individuals were receiving CNS-active polypharmacy, defined as combinations of multiple psychotropic and opioid medications taken for more than 30 days.
“For most patients, the risks of these medications, particularly in combination, are almost certainly greater than the potential benefits,” said Donovan Maust, MD, associate director of the geriatric psychiatry program, University of Michigan, Ann Arbor.
The study was published online March 9 in JAMA.
Serious risks
Memory impairment is the cardinal feature of dementia, but behavioral and psychological symptoms, which can include apathy, delusions, and agitation, are common during all stages of illness and cause significant caregiver distress, the researchers noted.
They noted that there is a dearth of high-quality evidence to support prescribing these medications in this patient population, yet “clinicians regularly prescribe psychotropic medications to community-dwelling persons with dementia in rates that far exceed use in the general older adult population.”
The Beers Criteria, from the American Geriatrics Society, advise against the practice of CNS polypharmacy because of the significant increase in risk for falls as well as impaired cognition, cardiac conduction abnormalities, respiratory suppression, and death when polypharmacy involves opioids.
They note that previous studies from Europe of polypharmacy for patients with dementia have not included antiepileptic medications or opioids, so the true extent of CNS-active polypharmacy may be “significantly” underestimated.
To determine the prevalence of polypharmacy with CNS-active medications among community-dwelling older adults with dementia, the researchers analyzed data on prescription fills for nearly 1.2 million community-dwelling Medicare patients with dementia.
The primary outcome was the prevalence of CNS-active polypharmacy in 2018. They defined CNS-active polypharmacy as exposure to three or more medications for more than 30 consecutive days from the following drug classes: antidepressants, antipsychotics, antiepileptics, benzodiazepines, nonbenzodiazepines, benzodiazepine receptor agonist hypnotics, and opioids.
They found that roughly one in seven (13.9%) patients met criteria for CNS-active polypharmacy. Of those receiving a CNS-active polypharmacy regimen, 57.8% had been doing so for longer than 180 days, and 6.8% had been doing so for a year. Nearly 30% of patients were exposed to five or more medications, and 5.2% were exposed to five or more medication classes.
Conservative approach warranted
Nearly all (92%) patients taking three or more CNS-active medications were taking an antidepressant, “consistent with their place as the psychotropic class most commonly prescribed both to older adults overall and those with dementia,” the investigators noted.
There is minimal high-quality evidence to support the efficacy of antidepressants for the treatment of depression for patients with dementia, they pointed out.
Nearly half (47%) of patients who were taking three or more CNS-active medications received at least one antipsychotic, most often quetiapine. Antipsychotics are not approved for people with dementia but are often prescribed off label for agitation, anxiety, and sleep problems, the researchers noted.
Nearly two thirds (62%) of patients with dementia who were taking three or more CNS drugs were taking an antiepileptic (most commonly, gabapentin); 41%, benzodiazepines; 32%, opioids; and 6%, Z-drugs.
The most common polypharmacy class combination included at least one antidepressant, one antiepileptic, and one antipsychotic. These accounted for 12.9% of polypharmacy days.
Despite limited high-quality evidence of efficacy, the prescribing of psychotropic medications and opioids is “pervasive” for adults with dementia in the United States, the investigators noted.
“Especially given that older adults with dementia might not be able to convey side effects they are experiencing, I think clinicians should be more conservative in how they are prescribing these medications and skeptical about the potential for benefit,” said Dr. Maust.
Regarding study limitations, the researchers noted that prescription medication claims may have led to an overestimation of the exposure to polypharmacy, insofar as the prescriptions may have been filled but not taken or were taken only on an as-needed basis.
In addition, the investigators were unable to determine the appropriateness of the particular combinations used or to examine the specific harms associated with CNS-active polypharmacy.
A major clinical challenge
Weighing in on the results, Howard Fillit, MD, founding executive director and chief science officer of the Alzheimer’s Drug Discovery Foundation, said the study is important because polypharmacy is one of the “geriatric giants, and the question is, what do you do about it?”
Dr. Fillit said it is important to conduct a careful medication review for all older patients, “making sure that the use of each drug is appropriate. The most important thing is to define what is the appropriate utilization of these kinds of drugs. That goes for both overutilization or misuse of these drugs and underutilization, where people are undertreated for symptoms that can’t be managed by behavioral management, for example,” Dr. Fillit said.
Dr. Fillit also said the finding that about 14% of dementia patients were receiving three or more of these drugs “may not be an outrageous number, because these patients, especially as they get into moderate and severe stages of disease, can be incredibly difficult to manage.
“Very often, dementia patients have depression, and up to 90% will have agitation and even psychosis during the course of dementia. And many of these patients need these types of drugs,” said Dr. Fillit.
Echoing the authors, Dr. Fillit said a key limitation of the study is not knowing whether the prescribing was appropriate or not.
The study was supported by a grant from the National Institute on Aging. Dr. Maust and Dr. Fillit have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM JAMA
Telerheumatology will thrive post pandemic
Telemedicine has had a profound effect upon the practice of rheumatology during the COVID-19 pandemic and will continue to do so afterward, speakers predicted at the 2021 Rheumatology Winter Clinical Symposium.
“Telemedicine will change the way we do business. It already has,” observed Eric M. Ruderman, MD, professor of medicine (rheumatology) at Northwestern University in Chicago.
“All of a sudden in March of last year we all turned on a dime and went 100% remote, and we made it work. And it has worked well. It’s not the same as seeing people in person, but I’m pretty sure that going forward probably somewhere in the range of 30% of our visits are going to be telemedicine. It’s an incredible way to deal with people who are stable and are driving in from an hour-and-a-half away to get their prescription refilled,” he said.
Conditions well suited for video patient visits are those where the physical exam isn’t informative or necessary, such as polymyalgia rheumatica, axial spondyloarthritis with axial disease only, childhood periodic fever syndromes, and even many cases of rheumatoid arthritis, in Dr. Ruderman’s view.
“People who are stable – maybe not in remission, but we’ve decided they’re at that their target – a lot of those visits can be done remotely. It’s way more efficient. Everybody loves it: We like it, the patients like it. But we have to get to where we can do it better. The technology is clumsy right now,” he said.
“We do need better and smarter platforms,” agreed Alvin F. Wells, MD, PhD, a telerheumatology pioneer who has been involved in digital/video communication with his patients for nearly 6 years. “But the biggest issue is connectivity. Not all of our patients can get on the Internet.”
The telerheumatology paradigm he has used during the pandemic and will continue to use afterward is to see every new patient in the office, then do the follow-up visits virtually.
“They don’t need to come back into the office in 4 weeks. I’ve done my physical exam, ordered the x-rays and lab work. At the virtual 4-week follow-up we go over everything and I tell them if they need to come in for training in giving their injections,” explained Dr. Wells, a rheumatologist in Franklin, Wisc.
“The telemedicine visit doesn’t take the place of an in-person visit, but it allows you to stratify, to say who needs to be seen sooner rather than later,” he added.
While he anticipates that physician-patient virtual visits will continue to be an important part of clinical practice post pandemic, he predicted the major growth areas for telerheumatology once COVID-19 is squashed will be in clinician-to-clinician interactions and remote patient monitoring using smart devices.
Dr. Wells hasn’t gone into the hospital once since the pandemic began. Initially, that was because he didn’t want to deal with the personal protective equipment shortage or expose himself to the virus. Now, it’s because it’s just a more efficient use of his time to conduct virtual – and billable – 15-minute e-consults with clinicians in the hospital.
“I’ve had a lot of appropriate consults with the hospitalists,” he said. He can walk a hospitalist through a real-time physical exam at a gout patient’s bedside and order the right laboratory tests.
“I don’t need to go into the hospital. The interventional radiologist can tap an ankle or toe as well as I can,” the rheumatologist said.
Dermatologist George Martin, MD, rose from the audience to say that while he recognizes that pandemic telemedicine has been a good fit for rheumatologists, it’s been a very different story in dermatology.
“I realize telemedicine works really well when you don’t have to lay your hands on a patient, or when you’re just doing a stable follow-up and talking about test results. But we in dermatology have found as a group that telemedicine is pretty worthless. When patients are trying to send you a video stream of what their melanoma looks like, or maybe it’s a benign seborrheic keratosis, you’re going to hang their life on that? Dermatology is a very hands-on, visual thing, and unless the camera work becomes better telemedicine is worthless, with the exception of a laboratory follow-up or a stable visit where a physical exam is not required,” declared Dr. Martin, who is in private practice in Maui.
Dr. Wells reported serving as a consultant to MiCare Path, a remote health and monitoring company.
Telemedicine has had a profound effect upon the practice of rheumatology during the COVID-19 pandemic and will continue to do so afterward, speakers predicted at the 2021 Rheumatology Winter Clinical Symposium.
“Telemedicine will change the way we do business. It already has,” observed Eric M. Ruderman, MD, professor of medicine (rheumatology) at Northwestern University in Chicago.
“All of a sudden in March of last year we all turned on a dime and went 100% remote, and we made it work. And it has worked well. It’s not the same as seeing people in person, but I’m pretty sure that going forward probably somewhere in the range of 30% of our visits are going to be telemedicine. It’s an incredible way to deal with people who are stable and are driving in from an hour-and-a-half away to get their prescription refilled,” he said.
Conditions well suited for video patient visits are those where the physical exam isn’t informative or necessary, such as polymyalgia rheumatica, axial spondyloarthritis with axial disease only, childhood periodic fever syndromes, and even many cases of rheumatoid arthritis, in Dr. Ruderman’s view.
“People who are stable – maybe not in remission, but we’ve decided they’re at that their target – a lot of those visits can be done remotely. It’s way more efficient. Everybody loves it: We like it, the patients like it. But we have to get to where we can do it better. The technology is clumsy right now,” he said.
“We do need better and smarter platforms,” agreed Alvin F. Wells, MD, PhD, a telerheumatology pioneer who has been involved in digital/video communication with his patients for nearly 6 years. “But the biggest issue is connectivity. Not all of our patients can get on the Internet.”
The telerheumatology paradigm he has used during the pandemic and will continue to use afterward is to see every new patient in the office, then do the follow-up visits virtually.
“They don’t need to come back into the office in 4 weeks. I’ve done my physical exam, ordered the x-rays and lab work. At the virtual 4-week follow-up we go over everything and I tell them if they need to come in for training in giving their injections,” explained Dr. Wells, a rheumatologist in Franklin, Wisc.
“The telemedicine visit doesn’t take the place of an in-person visit, but it allows you to stratify, to say who needs to be seen sooner rather than later,” he added.
While he anticipates that physician-patient virtual visits will continue to be an important part of clinical practice post pandemic, he predicted the major growth areas for telerheumatology once COVID-19 is squashed will be in clinician-to-clinician interactions and remote patient monitoring using smart devices.
Dr. Wells hasn’t gone into the hospital once since the pandemic began. Initially, that was because he didn’t want to deal with the personal protective equipment shortage or expose himself to the virus. Now, it’s because it’s just a more efficient use of his time to conduct virtual – and billable – 15-minute e-consults with clinicians in the hospital.
“I’ve had a lot of appropriate consults with the hospitalists,” he said. He can walk a hospitalist through a real-time physical exam at a gout patient’s bedside and order the right laboratory tests.
“I don’t need to go into the hospital. The interventional radiologist can tap an ankle or toe as well as I can,” the rheumatologist said.
Dermatologist George Martin, MD, rose from the audience to say that while he recognizes that pandemic telemedicine has been a good fit for rheumatologists, it’s been a very different story in dermatology.
“I realize telemedicine works really well when you don’t have to lay your hands on a patient, or when you’re just doing a stable follow-up and talking about test results. But we in dermatology have found as a group that telemedicine is pretty worthless. When patients are trying to send you a video stream of what their melanoma looks like, or maybe it’s a benign seborrheic keratosis, you’re going to hang their life on that? Dermatology is a very hands-on, visual thing, and unless the camera work becomes better telemedicine is worthless, with the exception of a laboratory follow-up or a stable visit where a physical exam is not required,” declared Dr. Martin, who is in private practice in Maui.
Dr. Wells reported serving as a consultant to MiCare Path, a remote health and monitoring company.
Telemedicine has had a profound effect upon the practice of rheumatology during the COVID-19 pandemic and will continue to do so afterward, speakers predicted at the 2021 Rheumatology Winter Clinical Symposium.
“Telemedicine will change the way we do business. It already has,” observed Eric M. Ruderman, MD, professor of medicine (rheumatology) at Northwestern University in Chicago.
“All of a sudden in March of last year we all turned on a dime and went 100% remote, and we made it work. And it has worked well. It’s not the same as seeing people in person, but I’m pretty sure that going forward probably somewhere in the range of 30% of our visits are going to be telemedicine. It’s an incredible way to deal with people who are stable and are driving in from an hour-and-a-half away to get their prescription refilled,” he said.
Conditions well suited for video patient visits are those where the physical exam isn’t informative or necessary, such as polymyalgia rheumatica, axial spondyloarthritis with axial disease only, childhood periodic fever syndromes, and even many cases of rheumatoid arthritis, in Dr. Ruderman’s view.
“People who are stable – maybe not in remission, but we’ve decided they’re at that their target – a lot of those visits can be done remotely. It’s way more efficient. Everybody loves it: We like it, the patients like it. But we have to get to where we can do it better. The technology is clumsy right now,” he said.
“We do need better and smarter platforms,” agreed Alvin F. Wells, MD, PhD, a telerheumatology pioneer who has been involved in digital/video communication with his patients for nearly 6 years. “But the biggest issue is connectivity. Not all of our patients can get on the Internet.”
The telerheumatology paradigm he has used during the pandemic and will continue to use afterward is to see every new patient in the office, then do the follow-up visits virtually.
“They don’t need to come back into the office in 4 weeks. I’ve done my physical exam, ordered the x-rays and lab work. At the virtual 4-week follow-up we go over everything and I tell them if they need to come in for training in giving their injections,” explained Dr. Wells, a rheumatologist in Franklin, Wisc.
“The telemedicine visit doesn’t take the place of an in-person visit, but it allows you to stratify, to say who needs to be seen sooner rather than later,” he added.
While he anticipates that physician-patient virtual visits will continue to be an important part of clinical practice post pandemic, he predicted the major growth areas for telerheumatology once COVID-19 is squashed will be in clinician-to-clinician interactions and remote patient monitoring using smart devices.
Dr. Wells hasn’t gone into the hospital once since the pandemic began. Initially, that was because he didn’t want to deal with the personal protective equipment shortage or expose himself to the virus. Now, it’s because it’s just a more efficient use of his time to conduct virtual – and billable – 15-minute e-consults with clinicians in the hospital.
“I’ve had a lot of appropriate consults with the hospitalists,” he said. He can walk a hospitalist through a real-time physical exam at a gout patient’s bedside and order the right laboratory tests.
“I don’t need to go into the hospital. The interventional radiologist can tap an ankle or toe as well as I can,” the rheumatologist said.
Dermatologist George Martin, MD, rose from the audience to say that while he recognizes that pandemic telemedicine has been a good fit for rheumatologists, it’s been a very different story in dermatology.
“I realize telemedicine works really well when you don’t have to lay your hands on a patient, or when you’re just doing a stable follow-up and talking about test results. But we in dermatology have found as a group that telemedicine is pretty worthless. When patients are trying to send you a video stream of what their melanoma looks like, or maybe it’s a benign seborrheic keratosis, you’re going to hang their life on that? Dermatology is a very hands-on, visual thing, and unless the camera work becomes better telemedicine is worthless, with the exception of a laboratory follow-up or a stable visit where a physical exam is not required,” declared Dr. Martin, who is in private practice in Maui.
Dr. Wells reported serving as a consultant to MiCare Path, a remote health and monitoring company.
FROM RWCS 2021
The siesta solution
Are you a napper? Unless you’re retired that may sound like a ridiculous question. When could you possibly fit in the time to doze off for even 20 minutes? I suspect there may be one or two of you who, although you are still working, have found a way to schedule a nap into your schedules. The rest of us must wait until we no longer have clinical responsibilities.
In my experience, you regular nappers seem to be the lucky few who have discovered the art of nodding off after lunch and waking up refreshed and ready to take on a full afternoon of patients. We in the unlucky majority may have tried taking a nap but run the risk of its flowing into a deep slumber the length of which we can’t control. Or, more likely, we find that we wake feeling groggy and disoriented and, even worse, the daytime nod off has messed up our nighttime schedule.
Well, it turns out the ability to take daytime naps and reap their cardiometabolic benefits is not just luck but has a significant genetic component. Investigators at Massachusetts General Hospital in Boston have recently published a study in which they report finding more than a score of gene regions that determine a person’s propensity to take daytime naps.. The researchers have also unearthed preliminary evidence supporting a link between daytime napping and cardiometabolic health. My mother began napping when my sister and I were infants and never gave it up. Unfortunately, I seem to have ended up on the wrong side of the genomic shuffle.
Although this new research is interesting, I don’t think the investigators have enough information to answer one of the questions that every pediatrician fields multiple times each week. “When should my toddler grow out of his afternoon nap?” Although it looks like we may be getting closer to a gene-based answer, I have always couched my reply in terms of behavior modification and the fostering of habit-forming associations.
As a child begins to transition from multiple short naps interspersed with feedings to a pattern of two distinct naps, I suggest to parents that they begin to think of the afternoon nap as a siesta. In other words, the nap is something that always comes immediately after lunch with no intervening shenanigans. No playtime, no Teletubbies videos, no quick trips to the grocery store, nothing, nada, zip.
At least for me, lunch has always been soporific. And I suspect we will learn eventually that association cuts across the entire genetic landscape to one degree or another. It makes sense to take advantage of that association and remove all other distractions. For some parents, that means creating the illusion that they too are taking a siesta: No TV, no phone calls. Imagine that the whole household has suddenly moved to Spain for the next hour or two. If you’ve ever been a tourist in rural Spain and tried to do anything, buy anything, or visit a museum between 2 and 4 p.m. you’ve got the idea.
When the child is young he or she will probably fall asleep as long as his parents have been reasonably successful at maintaining sleep hygiene practices. As the child is gaining more stamina and gives up the morning nap, the siesta will remain as a quiet time because that’s the way it’s always been in the household. The child may sleep or play quietly, or be read a sleep-inducing story because no other options will be available until some predetermined time. An hour is usually reasonable. If sleep hasn’t overtaken them, an earlier bedtime will probably be in order. The child will outgrow the napping part of the siesta when his or her sleep need is gone. But, the siesta/quiet time can remain as an option until all-day school intervenes. This scheme works if you can get parents to appropriately prioritize their child’s sleep needs. That’s not always an easy sell.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at [email protected].
Are you a napper? Unless you’re retired that may sound like a ridiculous question. When could you possibly fit in the time to doze off for even 20 minutes? I suspect there may be one or two of you who, although you are still working, have found a way to schedule a nap into your schedules. The rest of us must wait until we no longer have clinical responsibilities.
In my experience, you regular nappers seem to be the lucky few who have discovered the art of nodding off after lunch and waking up refreshed and ready to take on a full afternoon of patients. We in the unlucky majority may have tried taking a nap but run the risk of its flowing into a deep slumber the length of which we can’t control. Or, more likely, we find that we wake feeling groggy and disoriented and, even worse, the daytime nod off has messed up our nighttime schedule.
Well, it turns out the ability to take daytime naps and reap their cardiometabolic benefits is not just luck but has a significant genetic component. Investigators at Massachusetts General Hospital in Boston have recently published a study in which they report finding more than a score of gene regions that determine a person’s propensity to take daytime naps.. The researchers have also unearthed preliminary evidence supporting a link between daytime napping and cardiometabolic health. My mother began napping when my sister and I were infants and never gave it up. Unfortunately, I seem to have ended up on the wrong side of the genomic shuffle.
Although this new research is interesting, I don’t think the investigators have enough information to answer one of the questions that every pediatrician fields multiple times each week. “When should my toddler grow out of his afternoon nap?” Although it looks like we may be getting closer to a gene-based answer, I have always couched my reply in terms of behavior modification and the fostering of habit-forming associations.
As a child begins to transition from multiple short naps interspersed with feedings to a pattern of two distinct naps, I suggest to parents that they begin to think of the afternoon nap as a siesta. In other words, the nap is something that always comes immediately after lunch with no intervening shenanigans. No playtime, no Teletubbies videos, no quick trips to the grocery store, nothing, nada, zip.
At least for me, lunch has always been soporific. And I suspect we will learn eventually that association cuts across the entire genetic landscape to one degree or another. It makes sense to take advantage of that association and remove all other distractions. For some parents, that means creating the illusion that they too are taking a siesta: No TV, no phone calls. Imagine that the whole household has suddenly moved to Spain for the next hour or two. If you’ve ever been a tourist in rural Spain and tried to do anything, buy anything, or visit a museum between 2 and 4 p.m. you’ve got the idea.
When the child is young he or she will probably fall asleep as long as his parents have been reasonably successful at maintaining sleep hygiene practices. As the child is gaining more stamina and gives up the morning nap, the siesta will remain as a quiet time because that’s the way it’s always been in the household. The child may sleep or play quietly, or be read a sleep-inducing story because no other options will be available until some predetermined time. An hour is usually reasonable. If sleep hasn’t overtaken them, an earlier bedtime will probably be in order. The child will outgrow the napping part of the siesta when his or her sleep need is gone. But, the siesta/quiet time can remain as an option until all-day school intervenes. This scheme works if you can get parents to appropriately prioritize their child’s sleep needs. That’s not always an easy sell.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at [email protected].
Are you a napper? Unless you’re retired that may sound like a ridiculous question. When could you possibly fit in the time to doze off for even 20 minutes? I suspect there may be one or two of you who, although you are still working, have found a way to schedule a nap into your schedules. The rest of us must wait until we no longer have clinical responsibilities.
In my experience, you regular nappers seem to be the lucky few who have discovered the art of nodding off after lunch and waking up refreshed and ready to take on a full afternoon of patients. We in the unlucky majority may have tried taking a nap but run the risk of its flowing into a deep slumber the length of which we can’t control. Or, more likely, we find that we wake feeling groggy and disoriented and, even worse, the daytime nod off has messed up our nighttime schedule.
Well, it turns out the ability to take daytime naps and reap their cardiometabolic benefits is not just luck but has a significant genetic component. Investigators at Massachusetts General Hospital in Boston have recently published a study in which they report finding more than a score of gene regions that determine a person’s propensity to take daytime naps.. The researchers have also unearthed preliminary evidence supporting a link between daytime napping and cardiometabolic health. My mother began napping when my sister and I were infants and never gave it up. Unfortunately, I seem to have ended up on the wrong side of the genomic shuffle.
Although this new research is interesting, I don’t think the investigators have enough information to answer one of the questions that every pediatrician fields multiple times each week. “When should my toddler grow out of his afternoon nap?” Although it looks like we may be getting closer to a gene-based answer, I have always couched my reply in terms of behavior modification and the fostering of habit-forming associations.
As a child begins to transition from multiple short naps interspersed with feedings to a pattern of two distinct naps, I suggest to parents that they begin to think of the afternoon nap as a siesta. In other words, the nap is something that always comes immediately after lunch with no intervening shenanigans. No playtime, no Teletubbies videos, no quick trips to the grocery store, nothing, nada, zip.
At least for me, lunch has always been soporific. And I suspect we will learn eventually that association cuts across the entire genetic landscape to one degree or another. It makes sense to take advantage of that association and remove all other distractions. For some parents, that means creating the illusion that they too are taking a siesta: No TV, no phone calls. Imagine that the whole household has suddenly moved to Spain for the next hour or two. If you’ve ever been a tourist in rural Spain and tried to do anything, buy anything, or visit a museum between 2 and 4 p.m. you’ve got the idea.
When the child is young he or she will probably fall asleep as long as his parents have been reasonably successful at maintaining sleep hygiene practices. As the child is gaining more stamina and gives up the morning nap, the siesta will remain as a quiet time because that’s the way it’s always been in the household. The child may sleep or play quietly, or be read a sleep-inducing story because no other options will be available until some predetermined time. An hour is usually reasonable. If sleep hasn’t overtaken them, an earlier bedtime will probably be in order. The child will outgrow the napping part of the siesta when his or her sleep need is gone. But, the siesta/quiet time can remain as an option until all-day school intervenes. This scheme works if you can get parents to appropriately prioritize their child’s sleep needs. That’s not always an easy sell.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at [email protected].