User login
Let’s not criticize off-label prescribing
The public health crisis sparked by COVID-19 has engendered much debate in the realm where politics, journalism, law, and medicine meet.
Doctors have used the media to name other doctors as sources of harmful misinformation, in some cases going so far as to invoke medical practice board oversight as a potential intervention when doctors make public statements deemed too far out of bounds scientifically. Over the past year, some physicians have been harshly criticized for speaking about off-label prescribing, a widely accepted part of everyday medical practice.
The science and ethics of off-label prescribing have not changed; what has changed is the quality of dialogue around it. As psychiatrists, it does not fall within our scope of practice to offer definitive public opinions on the treatment of COVID-19, nor is that our purpose here. However, we can speak to a process that damages patients and doctors alike by undermining trust. All of this heat around bad medical information, in our opinion, amounts to using the methods of other fields to evaluate science and clinical practice. A remedy, then, to improve the quality of public medical intelligence would be to clarify the rules of scientific debate and to once again clearly state that off-label prescribing is part and parcel of the good practice of clinical medicine.
Physicians who work in the field of professional discipline have thought about the limits of propriety in making charges of impropriety. We (R.S.E. and R.S.K.) asked the American Psychiatric Association’s Ethics Committee to expand upon its existing commentary on innovative practice and making allegations of professional misconduct. We used the committee’s answers to our questions as the basis for the arguments we are making in this piece.
The APA’s Ethics Committee uses clear-cut benchmarks to define innovative medical care: “The standards of care ... evolve with evidence from research and observations of practice. Among the expected supports for innovative practice are scientific testing, peer-reviewed publication, replication, and broad or widespread acceptance within a relevant scientific or professional community.” When it comes to off-label prescribing for any medical condition, it is easy enough to ascertain whether clinical reports have appeared in peer-reviewed journals.
Two of the biggest blockbusters in psychiatry, chlorpromazine and lithium, began as drugs used for other conditions almost since the inception of our field. In other words, the use of these drugs for mental illness began, in today’s jargon, as off-label. We practitioners of psychiatry live in the land of off-label prescribing and have always comfortably done so. In fact, almost all of medicine does. The key in today’s world of best-practice medicine is obtaining a truly informed consent.
For COVID-19, our incredible psychotropic molecules may once again be doing some trail-blazing off-label work. Late last year, Eric J. Lenze, MD, professor of psychiatry and director of the Healthy Mind Lab at Washington University in St. Louis, reported in a preliminary study of adult outpatients with symptomatic COVID-19 that those treated with fluvoxamine “had a lower likelihood of clinical deterioration over 15 days,” compared with those on placebo (JAMA. 2020;324[22]:2292-300). We were heartened to see Dr. Lenze discuss his work on a recent “60 Minutes” segment. David Seftel, MD, MBA, a clinician who administered fluvoxamine as early treatment for a COVID-19 outbreak that occurred in a community of racetrack employees and their families in the San Francisco Bay Area, also was featured. Rather than waiting for the results of large clinical trials, Dr. Lenze and Dr. Seftel proceeded, based on reports published in peer-reviewed journals, to treat patients whose lives were at risk.
If we find ourselves strongly disagreeing about the science of off-label prescribing, the proper response is to critique methodologies, not the character or competence of colleagues. The APA Ethics Committee discourages use of the media as a forum for making allegations of incompetent or unethical practice: “Judgments regarding violations of established norms of ethical or professional conduct should be made not by individuals but by bodies authorized to take evidence and make informed decisions.”
At least one state legislature is taking action to protect patients’ access to the doctors they trust. In Arizona, SB 1416 passed in the Senate and is now working its way through the House. This bill would prohibit medical boards from disciplining doctors for speaking out about or prescribing off-label drugs when a reasonable basis for use exists.
Psychological research on the “backfire effect” suggests that heavy-handed campaigns to enforce medical consensus will only harden minds in ways that neither advance science nor improve the quality of clinical decision-making.
Medical disciplinary boards and the news media were neither designed nor are they equipped to adjudicate scientific debates. Science is never settled: Hypothesis and theory are always open to testing and revision as new evidence emerges. There is a place in medicine for formal disciplinary processes, as well-delineated by professional bodies such as the APA Ethics Committee. Another important part of protecting the public is to support an environment of scientific inquiry in which diversity of opinion is welcomed. As physicians, we translate science into excellent clinical care every day in our practices, and we advance science by sharing what we learn through friendly collegial communication and collaboration.
Dr. Emmons is part-time clinical associate professor in the department of psychiatry at the University of Vermont, Burlington, and is a past chair of the Ethics Committee for the Vermont District Branch of the American Psychiatric Association. He is in private practice in Moretown, Vt., and disclosed no relevant financial relationships. Dr. Kohanski is in private practice in Dayton, N.J., and is a diplomate of the American Board of Psychiatry & Neurology. She also is the host and author of Clinical Correlation, a series of the Psychcast. Dr. Kohanski disclosed no relevant financial relationships.
The public health crisis sparked by COVID-19 has engendered much debate in the realm where politics, journalism, law, and medicine meet.
Doctors have used the media to name other doctors as sources of harmful misinformation, in some cases going so far as to invoke medical practice board oversight as a potential intervention when doctors make public statements deemed too far out of bounds scientifically. Over the past year, some physicians have been harshly criticized for speaking about off-label prescribing, a widely accepted part of everyday medical practice.
The science and ethics of off-label prescribing have not changed; what has changed is the quality of dialogue around it. As psychiatrists, it does not fall within our scope of practice to offer definitive public opinions on the treatment of COVID-19, nor is that our purpose here. However, we can speak to a process that damages patients and doctors alike by undermining trust. All of this heat around bad medical information, in our opinion, amounts to using the methods of other fields to evaluate science and clinical practice. A remedy, then, to improve the quality of public medical intelligence would be to clarify the rules of scientific debate and to once again clearly state that off-label prescribing is part and parcel of the good practice of clinical medicine.
Physicians who work in the field of professional discipline have thought about the limits of propriety in making charges of impropriety. We (R.S.E. and R.S.K.) asked the American Psychiatric Association’s Ethics Committee to expand upon its existing commentary on innovative practice and making allegations of professional misconduct. We used the committee’s answers to our questions as the basis for the arguments we are making in this piece.
The APA’s Ethics Committee uses clear-cut benchmarks to define innovative medical care: “The standards of care ... evolve with evidence from research and observations of practice. Among the expected supports for innovative practice are scientific testing, peer-reviewed publication, replication, and broad or widespread acceptance within a relevant scientific or professional community.” When it comes to off-label prescribing for any medical condition, it is easy enough to ascertain whether clinical reports have appeared in peer-reviewed journals.
Two of the biggest blockbusters in psychiatry, chlorpromazine and lithium, began as drugs used for other conditions almost since the inception of our field. In other words, the use of these drugs for mental illness began, in today’s jargon, as off-label. We practitioners of psychiatry live in the land of off-label prescribing and have always comfortably done so. In fact, almost all of medicine does. The key in today’s world of best-practice medicine is obtaining a truly informed consent.
For COVID-19, our incredible psychotropic molecules may once again be doing some trail-blazing off-label work. Late last year, Eric J. Lenze, MD, professor of psychiatry and director of the Healthy Mind Lab at Washington University in St. Louis, reported in a preliminary study of adult outpatients with symptomatic COVID-19 that those treated with fluvoxamine “had a lower likelihood of clinical deterioration over 15 days,” compared with those on placebo (JAMA. 2020;324[22]:2292-300). We were heartened to see Dr. Lenze discuss his work on a recent “60 Minutes” segment. David Seftel, MD, MBA, a clinician who administered fluvoxamine as early treatment for a COVID-19 outbreak that occurred in a community of racetrack employees and their families in the San Francisco Bay Area, also was featured. Rather than waiting for the results of large clinical trials, Dr. Lenze and Dr. Seftel proceeded, based on reports published in peer-reviewed journals, to treat patients whose lives were at risk.
If we find ourselves strongly disagreeing about the science of off-label prescribing, the proper response is to critique methodologies, not the character or competence of colleagues. The APA Ethics Committee discourages use of the media as a forum for making allegations of incompetent or unethical practice: “Judgments regarding violations of established norms of ethical or professional conduct should be made not by individuals but by bodies authorized to take evidence and make informed decisions.”
At least one state legislature is taking action to protect patients’ access to the doctors they trust. In Arizona, SB 1416 passed in the Senate and is now working its way through the House. This bill would prohibit medical boards from disciplining doctors for speaking out about or prescribing off-label drugs when a reasonable basis for use exists.
Psychological research on the “backfire effect” suggests that heavy-handed campaigns to enforce medical consensus will only harden minds in ways that neither advance science nor improve the quality of clinical decision-making.
Medical disciplinary boards and the news media were neither designed nor are they equipped to adjudicate scientific debates. Science is never settled: Hypothesis and theory are always open to testing and revision as new evidence emerges. There is a place in medicine for formal disciplinary processes, as well-delineated by professional bodies such as the APA Ethics Committee. Another important part of protecting the public is to support an environment of scientific inquiry in which diversity of opinion is welcomed. As physicians, we translate science into excellent clinical care every day in our practices, and we advance science by sharing what we learn through friendly collegial communication and collaboration.
Dr. Emmons is part-time clinical associate professor in the department of psychiatry at the University of Vermont, Burlington, and is a past chair of the Ethics Committee for the Vermont District Branch of the American Psychiatric Association. He is in private practice in Moretown, Vt., and disclosed no relevant financial relationships. Dr. Kohanski is in private practice in Dayton, N.J., and is a diplomate of the American Board of Psychiatry & Neurology. She also is the host and author of Clinical Correlation, a series of the Psychcast. Dr. Kohanski disclosed no relevant financial relationships.
The public health crisis sparked by COVID-19 has engendered much debate in the realm where politics, journalism, law, and medicine meet.
Doctors have used the media to name other doctors as sources of harmful misinformation, in some cases going so far as to invoke medical practice board oversight as a potential intervention when doctors make public statements deemed too far out of bounds scientifically. Over the past year, some physicians have been harshly criticized for speaking about off-label prescribing, a widely accepted part of everyday medical practice.
The science and ethics of off-label prescribing have not changed; what has changed is the quality of dialogue around it. As psychiatrists, it does not fall within our scope of practice to offer definitive public opinions on the treatment of COVID-19, nor is that our purpose here. However, we can speak to a process that damages patients and doctors alike by undermining trust. All of this heat around bad medical information, in our opinion, amounts to using the methods of other fields to evaluate science and clinical practice. A remedy, then, to improve the quality of public medical intelligence would be to clarify the rules of scientific debate and to once again clearly state that off-label prescribing is part and parcel of the good practice of clinical medicine.
Physicians who work in the field of professional discipline have thought about the limits of propriety in making charges of impropriety. We (R.S.E. and R.S.K.) asked the American Psychiatric Association’s Ethics Committee to expand upon its existing commentary on innovative practice and making allegations of professional misconduct. We used the committee’s answers to our questions as the basis for the arguments we are making in this piece.
The APA’s Ethics Committee uses clear-cut benchmarks to define innovative medical care: “The standards of care ... evolve with evidence from research and observations of practice. Among the expected supports for innovative practice are scientific testing, peer-reviewed publication, replication, and broad or widespread acceptance within a relevant scientific or professional community.” When it comes to off-label prescribing for any medical condition, it is easy enough to ascertain whether clinical reports have appeared in peer-reviewed journals.
Two of the biggest blockbusters in psychiatry, chlorpromazine and lithium, began as drugs used for other conditions almost since the inception of our field. In other words, the use of these drugs for mental illness began, in today’s jargon, as off-label. We practitioners of psychiatry live in the land of off-label prescribing and have always comfortably done so. In fact, almost all of medicine does. The key in today’s world of best-practice medicine is obtaining a truly informed consent.
For COVID-19, our incredible psychotropic molecules may once again be doing some trail-blazing off-label work. Late last year, Eric J. Lenze, MD, professor of psychiatry and director of the Healthy Mind Lab at Washington University in St. Louis, reported in a preliminary study of adult outpatients with symptomatic COVID-19 that those treated with fluvoxamine “had a lower likelihood of clinical deterioration over 15 days,” compared with those on placebo (JAMA. 2020;324[22]:2292-300). We were heartened to see Dr. Lenze discuss his work on a recent “60 Minutes” segment. David Seftel, MD, MBA, a clinician who administered fluvoxamine as early treatment for a COVID-19 outbreak that occurred in a community of racetrack employees and their families in the San Francisco Bay Area, also was featured. Rather than waiting for the results of large clinical trials, Dr. Lenze and Dr. Seftel proceeded, based on reports published in peer-reviewed journals, to treat patients whose lives were at risk.
If we find ourselves strongly disagreeing about the science of off-label prescribing, the proper response is to critique methodologies, not the character or competence of colleagues. The APA Ethics Committee discourages use of the media as a forum for making allegations of incompetent or unethical practice: “Judgments regarding violations of established norms of ethical or professional conduct should be made not by individuals but by bodies authorized to take evidence and make informed decisions.”
At least one state legislature is taking action to protect patients’ access to the doctors they trust. In Arizona, SB 1416 passed in the Senate and is now working its way through the House. This bill would prohibit medical boards from disciplining doctors for speaking out about or prescribing off-label drugs when a reasonable basis for use exists.
Psychological research on the “backfire effect” suggests that heavy-handed campaigns to enforce medical consensus will only harden minds in ways that neither advance science nor improve the quality of clinical decision-making.
Medical disciplinary boards and the news media were neither designed nor are they equipped to adjudicate scientific debates. Science is never settled: Hypothesis and theory are always open to testing and revision as new evidence emerges. There is a place in medicine for formal disciplinary processes, as well-delineated by professional bodies such as the APA Ethics Committee. Another important part of protecting the public is to support an environment of scientific inquiry in which diversity of opinion is welcomed. As physicians, we translate science into excellent clinical care every day in our practices, and we advance science by sharing what we learn through friendly collegial communication and collaboration.
Dr. Emmons is part-time clinical associate professor in the department of psychiatry at the University of Vermont, Burlington, and is a past chair of the Ethics Committee for the Vermont District Branch of the American Psychiatric Association. He is in private practice in Moretown, Vt., and disclosed no relevant financial relationships. Dr. Kohanski is in private practice in Dayton, N.J., and is a diplomate of the American Board of Psychiatry & Neurology. She also is the host and author of Clinical Correlation, a series of the Psychcast. Dr. Kohanski disclosed no relevant financial relationships.
Updated recommendations released on COVID-19 and pediatric ALL
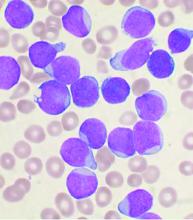
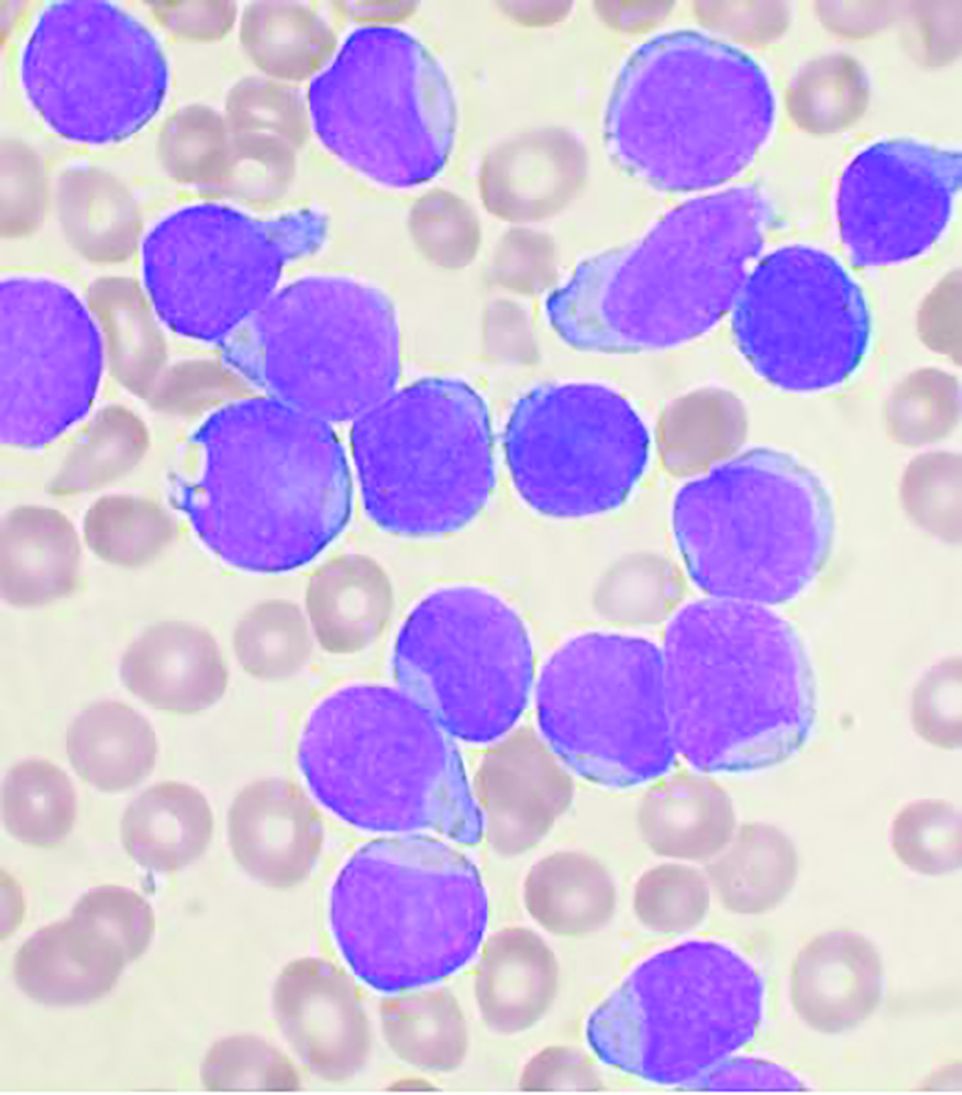
The main threat to the vast majority of children with acute lymphoblastic leukemia still remains the ALL itself, according to updated recommendations released by the Leukemia Committee of the French Society for the Fight Against Cancers and Leukemias in Children and Adolescents (SFCE).
“The situation of the current COVID-19 pandemic is continuously evolving. We thus have taken the more recent knowledge into account to update the previous recommendations from the Leukemia Committee,” Jérémie Rouger-Gaudichon, MD, of Pediatric Hemato-Immuno-Oncology Unit, Centre Hospitalier Universitaire, Caen (France), and colleagues wrote on behalf of the SFCE.
The updated recommendations are based on data collected in a real-time prospective survey among the 30 SFCE centers since April 2020. As of December 2020, 127 cases of COVID-19 were reported, most of them being enrolled in the PEDONCOVID study (NCT04433871) according to the report. Of these, eight patients required hospitalization in intensive care unit and one patient with relapsed acute lymphoblastic leukemia (ALL) died from ARDS with multiorgan failure. This confirms earlier reports that SARS-CoV-2 infection can be severe in some children with cancer and/or having hematopoietic stem cell transplant (HSCT), according to the report, which was published online in Bulletin du Cancer.
Recommendations
General recommendations were provided in the report, including the following:
- Test for SARS-CoV-2 (preferably by PCR or at least by immunological tests, on nasopharyngeal swab) before starting intensive induction chemotherapy or other intensive phase of treatment, for ALL patients, with or without symptoms.
- Delay systemic treatment if possible (e.g., absence of major hyperleukocytosis) in positive patients. During later phases, if patients test positive, tests should be repeated over time until negativity, especially before the beginning of an intensive course.
- Isolate any COVID-19–negative child or adolescent to allow treatment to continue (facial mask, social distancing, barrier measures, no contact with individuals suspected of COVID-19 or COVID-19–positive), in particular for patients to be allografted.
- Limit visitation to parents and potentially siblings in patients slated for HSCT and follow all necessary sanitary procedures for those visits.
The report provides a lengthy discussion of more detailed recommendations, including the following for first-line treatment of ALL:
- For patients with high-risk ALL, an individualized decision regarding transplantation and its timing should weigh the risks of transplantation in an epidemic context of COVID-19 against the risk linked to ALL.
- Minimizing hospital visits by the use of home blood tests and partial use of telemedicine may be considered.
- A physical examination should be performed regularly to avoid any delay in the diagnosis of treatment complications or relapse and preventative measures for SARS-CoV-2 should be applied in the home.
Patients with relapsed ALL may be at more risk from the effects of COVID-19 disease, according to the others, so for ALL patients receiving second-line or more treatment the recommendations include the following:
- Testing must be performed before starting a chemotherapy block, and postponing chemotherapy in case of positive test should be discussed in accordance with each specific situation and benefits/risks ratio regarding the leukemia.
- First-relapse patients should follow the INTREALL treatment protocol as much as possible and those who reach appropriate complete remission should be considered promptly for allogeneic transplantation, despite the pandemic.
- Second relapse and refractory relapses require testing and negative results for inclusion in phase I-II trials being conducted by most if not all academic or industrial promoters.
- The indication for treatment with CAR-T cells must be weighed with the center that would perform the procedure to determine the feasibility of performing all necessary procedures including apheresis and manufacturing.
In the case of a SARS-CoV-2 infection diagnosis during the treatment of ALL, discussions should occur with regard to stopping and/or postponing all chemotherapies, according to the severity of the ALL, the stage of treatment and the severity of clinical and/or radiological signs. In addition, any specific anti-COVID-19 treatment must be discussed with the infectious diseases team, according to the report.
“Fortunately, SARS-CoV-2 infection appears nevertheless to be mild in most children with cancer/ALL. Thus, the main threat to the vast majority of children with ALL still remains the ALL itself. Long-term data including well-matched case-control studies will tell if treatment delays/modifications due to COVID-19 have impacted the outcome if children with ALL,” the authors stated. However, “despite extremely rapid advances obtained in less than one year, our knowledge of SARS-CoV-2 and its complications is still incomplete,” they concluded, adding that the recommendations will likely need to be updated within another few months.
The authors reported that they had no conflicts of interest.
The main threat to the vast majority of children with acute lymphoblastic leukemia still remains the ALL itself, according to updated recommendations released by the Leukemia Committee of the French Society for the Fight Against Cancers and Leukemias in Children and Adolescents (SFCE).
“The situation of the current COVID-19 pandemic is continuously evolving. We thus have taken the more recent knowledge into account to update the previous recommendations from the Leukemia Committee,” Jérémie Rouger-Gaudichon, MD, of Pediatric Hemato-Immuno-Oncology Unit, Centre Hospitalier Universitaire, Caen (France), and colleagues wrote on behalf of the SFCE.
The updated recommendations are based on data collected in a real-time prospective survey among the 30 SFCE centers since April 2020. As of December 2020, 127 cases of COVID-19 were reported, most of them being enrolled in the PEDONCOVID study (NCT04433871) according to the report. Of these, eight patients required hospitalization in intensive care unit and one patient with relapsed acute lymphoblastic leukemia (ALL) died from ARDS with multiorgan failure. This confirms earlier reports that SARS-CoV-2 infection can be severe in some children with cancer and/or having hematopoietic stem cell transplant (HSCT), according to the report, which was published online in Bulletin du Cancer.
Recommendations
General recommendations were provided in the report, including the following:
- Test for SARS-CoV-2 (preferably by PCR or at least by immunological tests, on nasopharyngeal swab) before starting intensive induction chemotherapy or other intensive phase of treatment, for ALL patients, with or without symptoms.
- Delay systemic treatment if possible (e.g., absence of major hyperleukocytosis) in positive patients. During later phases, if patients test positive, tests should be repeated over time until negativity, especially before the beginning of an intensive course.
- Isolate any COVID-19–negative child or adolescent to allow treatment to continue (facial mask, social distancing, barrier measures, no contact with individuals suspected of COVID-19 or COVID-19–positive), in particular for patients to be allografted.
- Limit visitation to parents and potentially siblings in patients slated for HSCT and follow all necessary sanitary procedures for those visits.
The report provides a lengthy discussion of more detailed recommendations, including the following for first-line treatment of ALL:
- For patients with high-risk ALL, an individualized decision regarding transplantation and its timing should weigh the risks of transplantation in an epidemic context of COVID-19 against the risk linked to ALL.
- Minimizing hospital visits by the use of home blood tests and partial use of telemedicine may be considered.
- A physical examination should be performed regularly to avoid any delay in the diagnosis of treatment complications or relapse and preventative measures for SARS-CoV-2 should be applied in the home.
Patients with relapsed ALL may be at more risk from the effects of COVID-19 disease, according to the others, so for ALL patients receiving second-line or more treatment the recommendations include the following:
- Testing must be performed before starting a chemotherapy block, and postponing chemotherapy in case of positive test should be discussed in accordance with each specific situation and benefits/risks ratio regarding the leukemia.
- First-relapse patients should follow the INTREALL treatment protocol as much as possible and those who reach appropriate complete remission should be considered promptly for allogeneic transplantation, despite the pandemic.
- Second relapse and refractory relapses require testing and negative results for inclusion in phase I-II trials being conducted by most if not all academic or industrial promoters.
- The indication for treatment with CAR-T cells must be weighed with the center that would perform the procedure to determine the feasibility of performing all necessary procedures including apheresis and manufacturing.
In the case of a SARS-CoV-2 infection diagnosis during the treatment of ALL, discussions should occur with regard to stopping and/or postponing all chemotherapies, according to the severity of the ALL, the stage of treatment and the severity of clinical and/or radiological signs. In addition, any specific anti-COVID-19 treatment must be discussed with the infectious diseases team, according to the report.
“Fortunately, SARS-CoV-2 infection appears nevertheless to be mild in most children with cancer/ALL. Thus, the main threat to the vast majority of children with ALL still remains the ALL itself. Long-term data including well-matched case-control studies will tell if treatment delays/modifications due to COVID-19 have impacted the outcome if children with ALL,” the authors stated. However, “despite extremely rapid advances obtained in less than one year, our knowledge of SARS-CoV-2 and its complications is still incomplete,” they concluded, adding that the recommendations will likely need to be updated within another few months.
The authors reported that they had no conflicts of interest.
The main threat to the vast majority of children with acute lymphoblastic leukemia still remains the ALL itself, according to updated recommendations released by the Leukemia Committee of the French Society for the Fight Against Cancers and Leukemias in Children and Adolescents (SFCE).
“The situation of the current COVID-19 pandemic is continuously evolving. We thus have taken the more recent knowledge into account to update the previous recommendations from the Leukemia Committee,” Jérémie Rouger-Gaudichon, MD, of Pediatric Hemato-Immuno-Oncology Unit, Centre Hospitalier Universitaire, Caen (France), and colleagues wrote on behalf of the SFCE.
The updated recommendations are based on data collected in a real-time prospective survey among the 30 SFCE centers since April 2020. As of December 2020, 127 cases of COVID-19 were reported, most of them being enrolled in the PEDONCOVID study (NCT04433871) according to the report. Of these, eight patients required hospitalization in intensive care unit and one patient with relapsed acute lymphoblastic leukemia (ALL) died from ARDS with multiorgan failure. This confirms earlier reports that SARS-CoV-2 infection can be severe in some children with cancer and/or having hematopoietic stem cell transplant (HSCT), according to the report, which was published online in Bulletin du Cancer.
Recommendations
General recommendations were provided in the report, including the following:
- Test for SARS-CoV-2 (preferably by PCR or at least by immunological tests, on nasopharyngeal swab) before starting intensive induction chemotherapy or other intensive phase of treatment, for ALL patients, with or without symptoms.
- Delay systemic treatment if possible (e.g., absence of major hyperleukocytosis) in positive patients. During later phases, if patients test positive, tests should be repeated over time until negativity, especially before the beginning of an intensive course.
- Isolate any COVID-19–negative child or adolescent to allow treatment to continue (facial mask, social distancing, barrier measures, no contact with individuals suspected of COVID-19 or COVID-19–positive), in particular for patients to be allografted.
- Limit visitation to parents and potentially siblings in patients slated for HSCT and follow all necessary sanitary procedures for those visits.
The report provides a lengthy discussion of more detailed recommendations, including the following for first-line treatment of ALL:
- For patients with high-risk ALL, an individualized decision regarding transplantation and its timing should weigh the risks of transplantation in an epidemic context of COVID-19 against the risk linked to ALL.
- Minimizing hospital visits by the use of home blood tests and partial use of telemedicine may be considered.
- A physical examination should be performed regularly to avoid any delay in the diagnosis of treatment complications or relapse and preventative measures for SARS-CoV-2 should be applied in the home.
Patients with relapsed ALL may be at more risk from the effects of COVID-19 disease, according to the others, so for ALL patients receiving second-line or more treatment the recommendations include the following:
- Testing must be performed before starting a chemotherapy block, and postponing chemotherapy in case of positive test should be discussed in accordance with each specific situation and benefits/risks ratio regarding the leukemia.
- First-relapse patients should follow the INTREALL treatment protocol as much as possible and those who reach appropriate complete remission should be considered promptly for allogeneic transplantation, despite the pandemic.
- Second relapse and refractory relapses require testing and negative results for inclusion in phase I-II trials being conducted by most if not all academic or industrial promoters.
- The indication for treatment with CAR-T cells must be weighed with the center that would perform the procedure to determine the feasibility of performing all necessary procedures including apheresis and manufacturing.
In the case of a SARS-CoV-2 infection diagnosis during the treatment of ALL, discussions should occur with regard to stopping and/or postponing all chemotherapies, according to the severity of the ALL, the stage of treatment and the severity of clinical and/or radiological signs. In addition, any specific anti-COVID-19 treatment must be discussed with the infectious diseases team, according to the report.
“Fortunately, SARS-CoV-2 infection appears nevertheless to be mild in most children with cancer/ALL. Thus, the main threat to the vast majority of children with ALL still remains the ALL itself. Long-term data including well-matched case-control studies will tell if treatment delays/modifications due to COVID-19 have impacted the outcome if children with ALL,” the authors stated. However, “despite extremely rapid advances obtained in less than one year, our knowledge of SARS-CoV-2 and its complications is still incomplete,” they concluded, adding that the recommendations will likely need to be updated within another few months.
The authors reported that they had no conflicts of interest.
FROM BULLETIN DU CANCER
Climate change: Dermatologists address impact on health, and mobilize to increase awareness
Climate change will increasingly affect the distribution and frequency of insect-borne diseases, cutaneous leishmaniasis, skin cancer, fungal diseases, and a host of other illnesses that have cutaneous manifestations or involve the skin – and dermatologists are being urged to be ready to diagnose clinical findings, counsel patients about risk mitigation, and decrease the carbon footprint of their practices and medical organizations.
“Climate change is not a far-off threat but an urgent health issue,” Misha Rosenbach, MD, associate professor of dermatology at the University of Pennsylvania, Philadelphia, wrote in an editorial with coauthor Mary Sun, a student at Icahn School of Medicine at Mount Sinai, New York. It was first published online in the British Journal of Dermatology last year, titled, “The climate emergency: Why should dermatologists care and how can they act?”.
. Some of the 150-plus members of the ERG have been writing about the dermatologic impacts of climate change – including content that filled the January issue of the International Journal of Women’s Dermatology – and speaking about the issues.
A session at the AAD’s virtual annual meeting in April will address climate change and dermatology – the second such session at an annual meeting – and the first two of three planned virtual symposia led by Dr. Rosenbach and his colleagues, have been hosted by the Association of Professors of Dermatology. The ERG encouraged the AAD’s adoption of a position statement in 2018 about climate change and dermatology and its membership in the Medical Society Consortium on Climate and Health.
“There’s been a lot of conversation in the medical community about the health effects of climate change, but most people leave out the skin,” said Mary L. Williams, MD, clinical professor of dermatology at the University of California, San Francisco, who is a cofounder and coleader with Dr. Rosenbach of the climate change ERG.
“That’s interesting because the skin is the most environmental of all our organs. Of course it will be impacted by all that’s going on,” she said. “We want to bring the dermatologic community and the wider medical community along with us [in appreciating and acting on this knowledge].”
Changing disease patterns
Dr. Rosenbach did not think much about how climate change could affect his patients and his clinical practice until he saw a severe case of hand, foot, and mouth disease in a hospitalized adult in Philadelphia about 10 years ago.
A presentation of the case at an infectious disease conference spurred discussion of how the preceding winters had been warmer and of correlations reported by researchers in China between the incidence of hand, foot, and mouth disease – historically a mild infection in children – and average temperature and other meteorological factors. “I knew about climate change, but I never knew we’d see different diseases in our clinical practice, or old diseases affecting new hosts,” Dr. Rosenbach said in an interview.
He pored over the literature to deepen his understanding of climate change science and the impact of climate change on medicine, and found an “emerging focus” on climate change in some medical journals, but “very little in dermatology.” In collaboration with Benjamin Kaffenberger, MD, a dermatologist at The Ohio State University, and colleagues, including an entomologist, Dr. Rosenbach wrote a review of publications relating to climate change and skin disease in North America.
Published in 2017 in the Journal of the American Academy of Dermatology, the review details how bacteria, viruses, fungi, and parasites are responding to changing weather patterns in North America, and why dermatologists should be able to recognize changing patterns of disease. Globalization plays a role in changing disease and vector patterns, but “climate change allows expansion of the natural range of pathogens, hosts, reservoirs, and vectors that allow diseases to appear in immunologically naive populations,” they wrote.
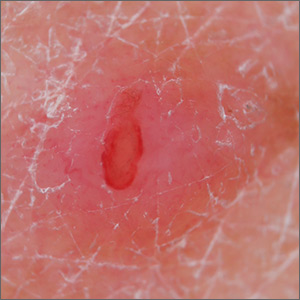
Patterns of infectious diseases with cutaneous manifestations are already changing. The geographic range of coccidioidomycosis, or valley fever, for instance, “has basically doubled in the Southwest U.S., extending up the entire West Coast,” Dr. Rosenbach said, as the result of longer dry seasons and more frequent wind storms that aerosolize the mycosis-causing, soil-dwelling fungal spores.
Lyme disease and associated tick-borne infections continue to expand northward as Ixodes tick vectors move and breed “exactly in sync with a warming world,” Dr. Rosenbach said. “We’re seeing Lyme in Philadelphia in February, whereas in the past we may not have seen it until May ... There are derms in Maine [whose patients have Lyme disease] who may never have seen a case before, and derms in Canada who are making diagnoses of Lyme [for the first time].”
And locally acquired cases of dengue are being reported in Hawaii, Texas, and Florida – and even North Carolina, according to a review of infectious diseases with cutaneous manifestations in the issue of the International Journal of Women’s Dermatology dedicated to climate change. As with Ixodes ticks, which transmit Lyme disease, rising temperatures lead to longer breeding seasons for Aedes mosquitoes, which transmit dengue. Increased endemicity of dengue is concerning because severe illness is significantly more likely in individuals previously infected with a different serotype.
“Dermatologists should be ready to identify and diagnose these mosquito-borne diseases that we think of as occurring in Central America or tropical regions,” Dr. Rosenbach said. “In my children’s lifetime there will be tropical diseases in New York, Philadelphia, Boston and other such places.”
In his articles and talks, Dr. Rosenbach lays out the science of climate change – for instance, the change in average global temperatures above preindustrial levels (an approximate 1° C rise) , the threshold beyond which the Earth will become less hospitable (1.5° C of warming according to United Nation’s Intergovernmental Panel on Climate Change), the current projections for future warming (an increase of about 3° Celsius by 2100), and the “gold-standard” level of scientific certainty that climate change is human-caused.
Mathematical climate modeling, he emphasized in the interview, can accurately project changes in infection rates. Researchers predicted 10 years ago in a published paper, for instance, that based on global warming patterns, the sand fly vector responsible for cutaneous leishmaniasis would live in the Southern United States and cause endemic infections within 10 years.
And in 2018, Dr. Rosenbach said, a paper in JAMA Dermatology described how more than half – 59% – of the cases of cutaneous leishmaniasis diagnosed in Texas were endemic, all occurring in people with no prior travel outside the United States.
Dr. Williams’ devotion to climate change and dermatology and to the climate change ERG was inspired in large part by Dr. Rosenbach’s 2017 paper in JAAD. She had long been concerned about climate change, she said, but “the review article was really the impetus for me to think, this is really within my specialty.”
Extreme weather events, and the climate-driven migration expected to increasingly occur, have clear relevance to dermatology, Dr. Williams said. “Often, the most vexing problems that people have when they’re forced out of their homes ... are dermatologic,” she said, like infections from contaminated waters after flooding and the spread of scabies and other communicable diseases due to crowding and unsanitary conditions.
But there are other less obvious ramifications of a changing climate that affect dermatology. Dr. Williams has delved into the literature on heat-related illness, for instance, and found that most research has been in the realm of sports medicine and military health. “Most of us don’t treat serious heat-related illnesses, but our skin is responsible for keeping us cool and there’s an important role for dermatologists to play in knowing how the skin does that and who is at risk for heat illness because the skin is unable to do the full job,” she said.
Research is needed to identify which medications can interfere with the skin’s thermoregulatory responses and put patients at risk, she noted. “And a lot of the work on sweat gland physiology is probably 30 years old now. We should bring to bear contemporary research techniques.”
Dermatology is also “in the early stages of understanding the role that air pollution plays in skin disease,” Dr. Williams said. “Most of the medical literature focuses on the effects of pollution on the lungs and in cardiovascular disease.”
There is evidence linking small particulate matter found in wood smoke and other air pollutants to exacerbations of atopic dermatitis and other inflammatory skin conditions, she noted, but mechanisms need to be explored and health disparities examined. “While we know that there are health disparities in terms of [exposure to] pollution and respiratory illness, we have no idea if this is the case with our skin diseases like atopic dermatitis,” said Dr. Williams.
In general, according to the AAD position statement, low-income and minority communities, in addition to the very young and the very old, “are and will continue to be disproportionately affected by climate change.”
Education and the carbon footprint
Viewing climate change as a social determinant of health (SDH ) – and integrating it into medical training as such – is a topic of active discussion. At UCSF, Sarah J. Coates, MD, a fellow in pediatric dermatology, is working with colleagues to integrate climate change into formal resident education. “We know that climate change affects housing, food security, migration ... and certain populations are and will be especially vulnerable,” she said in an interview. “The effects of climate change fit squarely into the social determinant of health curriculum that we’re building here.”
Dr. Coates began to appreciate the link between climate and infectious diseases – a topic she now writes and speaks about – when she saw several patients with coccidioidomycosis as a dermatology resident at UCSF and learned that the cases represented an epidemic in the Central Valley “resulting from several years of drought.”
Her medical school and residency training were otherwise devoid of any discussion of climate change. At UCSF and nearby Stanford (Calif.) University, this is no longer the case, she and Dr. Williams said. “The medical students here have been quite active and are requesting education,” noted Dr. Williams. “The desire to know more is coming from the bottom.”
Mary E. Maloney, MD, professor of medicine and director of dermatologic surgery at the University of Massachusetts, Worcester, sees the same interest from physicians-in-training in the Boston area. They want education about climate science, the impact of climate changes on health and risk mitigation, and ways to reduce medicine’s carbon footprint. “We need to teach them and charge them to lead in their communities,” she said in an interview.
Dr. Maloney joined the AAD’s climate change resource group soon after its inception, having realized the urgency of climate change and feeling that she needed “to get passionate and not just do small things.” As a Mohs surgeon, she expects an “explosion” of skin cancer as temperatures and sun exposure continue to increase.
She urges dermatologists to work to decrease the carbon footprint of their practices and to advocate for local hospitals and other clinical institutions to do so. On the AAD website, members now have free access to tools provided by the nonprofit organization My Green Doctor for outpatient offices to lighten their carbon footprints in a cost-effective – or even cost-saving – manner.
Dr. Maloney’s institution has moved to automated lighting systems and the use of LED lights, she said, and has encouraged ride sharing (prior to the pandemic) and computer switch-offs at night. And in her practice, she and a colleague have been working to reduce the purchasing and use of disposable plastics.
Educating patients about the effects of climate change on the health of their skin is another of the missions listed in the AAD’s position statement, and it’s something that Dr. Coates is currently researching. “It seems similar to talking about other social determinants of health,” she said. “Saying to a patient, for instance, ‘we’ve had some really terrible wildfires lately. They’re getting worse as the seasons go on and we know that’s because of climate change. How do you think your current rash relates to the current air quality? How you think the air quality affects your skin?’ ”
Dr. Rosenbach emphasizes that physicians are a broadly trusted group. “I’d tell a patient, ‘you’re the fourth patient I’ve seen with Lyme – we think that’s because it’s been a warmer year due to climate change,’” he said. “I don’t think that bringing up climate change has ever been a source of friction.”
Climate change will increasingly affect the distribution and frequency of insect-borne diseases, cutaneous leishmaniasis, skin cancer, fungal diseases, and a host of other illnesses that have cutaneous manifestations or involve the skin – and dermatologists are being urged to be ready to diagnose clinical findings, counsel patients about risk mitigation, and decrease the carbon footprint of their practices and medical organizations.
“Climate change is not a far-off threat but an urgent health issue,” Misha Rosenbach, MD, associate professor of dermatology at the University of Pennsylvania, Philadelphia, wrote in an editorial with coauthor Mary Sun, a student at Icahn School of Medicine at Mount Sinai, New York. It was first published online in the British Journal of Dermatology last year, titled, “The climate emergency: Why should dermatologists care and how can they act?”.
. Some of the 150-plus members of the ERG have been writing about the dermatologic impacts of climate change – including content that filled the January issue of the International Journal of Women’s Dermatology – and speaking about the issues.
A session at the AAD’s virtual annual meeting in April will address climate change and dermatology – the second such session at an annual meeting – and the first two of three planned virtual symposia led by Dr. Rosenbach and his colleagues, have been hosted by the Association of Professors of Dermatology. The ERG encouraged the AAD’s adoption of a position statement in 2018 about climate change and dermatology and its membership in the Medical Society Consortium on Climate and Health.
“There’s been a lot of conversation in the medical community about the health effects of climate change, but most people leave out the skin,” said Mary L. Williams, MD, clinical professor of dermatology at the University of California, San Francisco, who is a cofounder and coleader with Dr. Rosenbach of the climate change ERG.
“That’s interesting because the skin is the most environmental of all our organs. Of course it will be impacted by all that’s going on,” she said. “We want to bring the dermatologic community and the wider medical community along with us [in appreciating and acting on this knowledge].”
Changing disease patterns
Dr. Rosenbach did not think much about how climate change could affect his patients and his clinical practice until he saw a severe case of hand, foot, and mouth disease in a hospitalized adult in Philadelphia about 10 years ago.
A presentation of the case at an infectious disease conference spurred discussion of how the preceding winters had been warmer and of correlations reported by researchers in China between the incidence of hand, foot, and mouth disease – historically a mild infection in children – and average temperature and other meteorological factors. “I knew about climate change, but I never knew we’d see different diseases in our clinical practice, or old diseases affecting new hosts,” Dr. Rosenbach said in an interview.
He pored over the literature to deepen his understanding of climate change science and the impact of climate change on medicine, and found an “emerging focus” on climate change in some medical journals, but “very little in dermatology.” In collaboration with Benjamin Kaffenberger, MD, a dermatologist at The Ohio State University, and colleagues, including an entomologist, Dr. Rosenbach wrote a review of publications relating to climate change and skin disease in North America.
Published in 2017 in the Journal of the American Academy of Dermatology, the review details how bacteria, viruses, fungi, and parasites are responding to changing weather patterns in North America, and why dermatologists should be able to recognize changing patterns of disease. Globalization plays a role in changing disease and vector patterns, but “climate change allows expansion of the natural range of pathogens, hosts, reservoirs, and vectors that allow diseases to appear in immunologically naive populations,” they wrote.
Patterns of infectious diseases with cutaneous manifestations are already changing. The geographic range of coccidioidomycosis, or valley fever, for instance, “has basically doubled in the Southwest U.S., extending up the entire West Coast,” Dr. Rosenbach said, as the result of longer dry seasons and more frequent wind storms that aerosolize the mycosis-causing, soil-dwelling fungal spores.
Lyme disease and associated tick-borne infections continue to expand northward as Ixodes tick vectors move and breed “exactly in sync with a warming world,” Dr. Rosenbach said. “We’re seeing Lyme in Philadelphia in February, whereas in the past we may not have seen it until May ... There are derms in Maine [whose patients have Lyme disease] who may never have seen a case before, and derms in Canada who are making diagnoses of Lyme [for the first time].”
And locally acquired cases of dengue are being reported in Hawaii, Texas, and Florida – and even North Carolina, according to a review of infectious diseases with cutaneous manifestations in the issue of the International Journal of Women’s Dermatology dedicated to climate change. As with Ixodes ticks, which transmit Lyme disease, rising temperatures lead to longer breeding seasons for Aedes mosquitoes, which transmit dengue. Increased endemicity of dengue is concerning because severe illness is significantly more likely in individuals previously infected with a different serotype.
“Dermatologists should be ready to identify and diagnose these mosquito-borne diseases that we think of as occurring in Central America or tropical regions,” Dr. Rosenbach said. “In my children’s lifetime there will be tropical diseases in New York, Philadelphia, Boston and other such places.”
In his articles and talks, Dr. Rosenbach lays out the science of climate change – for instance, the change in average global temperatures above preindustrial levels (an approximate 1° C rise) , the threshold beyond which the Earth will become less hospitable (1.5° C of warming according to United Nation’s Intergovernmental Panel on Climate Change), the current projections for future warming (an increase of about 3° Celsius by 2100), and the “gold-standard” level of scientific certainty that climate change is human-caused.
Mathematical climate modeling, he emphasized in the interview, can accurately project changes in infection rates. Researchers predicted 10 years ago in a published paper, for instance, that based on global warming patterns, the sand fly vector responsible for cutaneous leishmaniasis would live in the Southern United States and cause endemic infections within 10 years.
And in 2018, Dr. Rosenbach said, a paper in JAMA Dermatology described how more than half – 59% – of the cases of cutaneous leishmaniasis diagnosed in Texas were endemic, all occurring in people with no prior travel outside the United States.
Dr. Williams’ devotion to climate change and dermatology and to the climate change ERG was inspired in large part by Dr. Rosenbach’s 2017 paper in JAAD. She had long been concerned about climate change, she said, but “the review article was really the impetus for me to think, this is really within my specialty.”
Extreme weather events, and the climate-driven migration expected to increasingly occur, have clear relevance to dermatology, Dr. Williams said. “Often, the most vexing problems that people have when they’re forced out of their homes ... are dermatologic,” she said, like infections from contaminated waters after flooding and the spread of scabies and other communicable diseases due to crowding and unsanitary conditions.
But there are other less obvious ramifications of a changing climate that affect dermatology. Dr. Williams has delved into the literature on heat-related illness, for instance, and found that most research has been in the realm of sports medicine and military health. “Most of us don’t treat serious heat-related illnesses, but our skin is responsible for keeping us cool and there’s an important role for dermatologists to play in knowing how the skin does that and who is at risk for heat illness because the skin is unable to do the full job,” she said.
Research is needed to identify which medications can interfere with the skin’s thermoregulatory responses and put patients at risk, she noted. “And a lot of the work on sweat gland physiology is probably 30 years old now. We should bring to bear contemporary research techniques.”
Dermatology is also “in the early stages of understanding the role that air pollution plays in skin disease,” Dr. Williams said. “Most of the medical literature focuses on the effects of pollution on the lungs and in cardiovascular disease.”
There is evidence linking small particulate matter found in wood smoke and other air pollutants to exacerbations of atopic dermatitis and other inflammatory skin conditions, she noted, but mechanisms need to be explored and health disparities examined. “While we know that there are health disparities in terms of [exposure to] pollution and respiratory illness, we have no idea if this is the case with our skin diseases like atopic dermatitis,” said Dr. Williams.
In general, according to the AAD position statement, low-income and minority communities, in addition to the very young and the very old, “are and will continue to be disproportionately affected by climate change.”
Education and the carbon footprint
Viewing climate change as a social determinant of health (SDH ) – and integrating it into medical training as such – is a topic of active discussion. At UCSF, Sarah J. Coates, MD, a fellow in pediatric dermatology, is working with colleagues to integrate climate change into formal resident education. “We know that climate change affects housing, food security, migration ... and certain populations are and will be especially vulnerable,” she said in an interview. “The effects of climate change fit squarely into the social determinant of health curriculum that we’re building here.”
Dr. Coates began to appreciate the link between climate and infectious diseases – a topic she now writes and speaks about – when she saw several patients with coccidioidomycosis as a dermatology resident at UCSF and learned that the cases represented an epidemic in the Central Valley “resulting from several years of drought.”
Her medical school and residency training were otherwise devoid of any discussion of climate change. At UCSF and nearby Stanford (Calif.) University, this is no longer the case, she and Dr. Williams said. “The medical students here have been quite active and are requesting education,” noted Dr. Williams. “The desire to know more is coming from the bottom.”
Mary E. Maloney, MD, professor of medicine and director of dermatologic surgery at the University of Massachusetts, Worcester, sees the same interest from physicians-in-training in the Boston area. They want education about climate science, the impact of climate changes on health and risk mitigation, and ways to reduce medicine’s carbon footprint. “We need to teach them and charge them to lead in their communities,” she said in an interview.
Dr. Maloney joined the AAD’s climate change resource group soon after its inception, having realized the urgency of climate change and feeling that she needed “to get passionate and not just do small things.” As a Mohs surgeon, she expects an “explosion” of skin cancer as temperatures and sun exposure continue to increase.
She urges dermatologists to work to decrease the carbon footprint of their practices and to advocate for local hospitals and other clinical institutions to do so. On the AAD website, members now have free access to tools provided by the nonprofit organization My Green Doctor for outpatient offices to lighten their carbon footprints in a cost-effective – or even cost-saving – manner.
Dr. Maloney’s institution has moved to automated lighting systems and the use of LED lights, she said, and has encouraged ride sharing (prior to the pandemic) and computer switch-offs at night. And in her practice, she and a colleague have been working to reduce the purchasing and use of disposable plastics.
Educating patients about the effects of climate change on the health of their skin is another of the missions listed in the AAD’s position statement, and it’s something that Dr. Coates is currently researching. “It seems similar to talking about other social determinants of health,” she said. “Saying to a patient, for instance, ‘we’ve had some really terrible wildfires lately. They’re getting worse as the seasons go on and we know that’s because of climate change. How do you think your current rash relates to the current air quality? How you think the air quality affects your skin?’ ”
Dr. Rosenbach emphasizes that physicians are a broadly trusted group. “I’d tell a patient, ‘you’re the fourth patient I’ve seen with Lyme – we think that’s because it’s been a warmer year due to climate change,’” he said. “I don’t think that bringing up climate change has ever been a source of friction.”
Climate change will increasingly affect the distribution and frequency of insect-borne diseases, cutaneous leishmaniasis, skin cancer, fungal diseases, and a host of other illnesses that have cutaneous manifestations or involve the skin – and dermatologists are being urged to be ready to diagnose clinical findings, counsel patients about risk mitigation, and decrease the carbon footprint of their practices and medical organizations.
“Climate change is not a far-off threat but an urgent health issue,” Misha Rosenbach, MD, associate professor of dermatology at the University of Pennsylvania, Philadelphia, wrote in an editorial with coauthor Mary Sun, a student at Icahn School of Medicine at Mount Sinai, New York. It was first published online in the British Journal of Dermatology last year, titled, “The climate emergency: Why should dermatologists care and how can they act?”.
. Some of the 150-plus members of the ERG have been writing about the dermatologic impacts of climate change – including content that filled the January issue of the International Journal of Women’s Dermatology – and speaking about the issues.
A session at the AAD’s virtual annual meeting in April will address climate change and dermatology – the second such session at an annual meeting – and the first two of three planned virtual symposia led by Dr. Rosenbach and his colleagues, have been hosted by the Association of Professors of Dermatology. The ERG encouraged the AAD’s adoption of a position statement in 2018 about climate change and dermatology and its membership in the Medical Society Consortium on Climate and Health.
“There’s been a lot of conversation in the medical community about the health effects of climate change, but most people leave out the skin,” said Mary L. Williams, MD, clinical professor of dermatology at the University of California, San Francisco, who is a cofounder and coleader with Dr. Rosenbach of the climate change ERG.
“That’s interesting because the skin is the most environmental of all our organs. Of course it will be impacted by all that’s going on,” she said. “We want to bring the dermatologic community and the wider medical community along with us [in appreciating and acting on this knowledge].”
Changing disease patterns
Dr. Rosenbach did not think much about how climate change could affect his patients and his clinical practice until he saw a severe case of hand, foot, and mouth disease in a hospitalized adult in Philadelphia about 10 years ago.
A presentation of the case at an infectious disease conference spurred discussion of how the preceding winters had been warmer and of correlations reported by researchers in China between the incidence of hand, foot, and mouth disease – historically a mild infection in children – and average temperature and other meteorological factors. “I knew about climate change, but I never knew we’d see different diseases in our clinical practice, or old diseases affecting new hosts,” Dr. Rosenbach said in an interview.
He pored over the literature to deepen his understanding of climate change science and the impact of climate change on medicine, and found an “emerging focus” on climate change in some medical journals, but “very little in dermatology.” In collaboration with Benjamin Kaffenberger, MD, a dermatologist at The Ohio State University, and colleagues, including an entomologist, Dr. Rosenbach wrote a review of publications relating to climate change and skin disease in North America.
Published in 2017 in the Journal of the American Academy of Dermatology, the review details how bacteria, viruses, fungi, and parasites are responding to changing weather patterns in North America, and why dermatologists should be able to recognize changing patterns of disease. Globalization plays a role in changing disease and vector patterns, but “climate change allows expansion of the natural range of pathogens, hosts, reservoirs, and vectors that allow diseases to appear in immunologically naive populations,” they wrote.
Patterns of infectious diseases with cutaneous manifestations are already changing. The geographic range of coccidioidomycosis, or valley fever, for instance, “has basically doubled in the Southwest U.S., extending up the entire West Coast,” Dr. Rosenbach said, as the result of longer dry seasons and more frequent wind storms that aerosolize the mycosis-causing, soil-dwelling fungal spores.
Lyme disease and associated tick-borne infections continue to expand northward as Ixodes tick vectors move and breed “exactly in sync with a warming world,” Dr. Rosenbach said. “We’re seeing Lyme in Philadelphia in February, whereas in the past we may not have seen it until May ... There are derms in Maine [whose patients have Lyme disease] who may never have seen a case before, and derms in Canada who are making diagnoses of Lyme [for the first time].”
And locally acquired cases of dengue are being reported in Hawaii, Texas, and Florida – and even North Carolina, according to a review of infectious diseases with cutaneous manifestations in the issue of the International Journal of Women’s Dermatology dedicated to climate change. As with Ixodes ticks, which transmit Lyme disease, rising temperatures lead to longer breeding seasons for Aedes mosquitoes, which transmit dengue. Increased endemicity of dengue is concerning because severe illness is significantly more likely in individuals previously infected with a different serotype.
“Dermatologists should be ready to identify and diagnose these mosquito-borne diseases that we think of as occurring in Central America or tropical regions,” Dr. Rosenbach said. “In my children’s lifetime there will be tropical diseases in New York, Philadelphia, Boston and other such places.”
In his articles and talks, Dr. Rosenbach lays out the science of climate change – for instance, the change in average global temperatures above preindustrial levels (an approximate 1° C rise) , the threshold beyond which the Earth will become less hospitable (1.5° C of warming according to United Nation’s Intergovernmental Panel on Climate Change), the current projections for future warming (an increase of about 3° Celsius by 2100), and the “gold-standard” level of scientific certainty that climate change is human-caused.
Mathematical climate modeling, he emphasized in the interview, can accurately project changes in infection rates. Researchers predicted 10 years ago in a published paper, for instance, that based on global warming patterns, the sand fly vector responsible for cutaneous leishmaniasis would live in the Southern United States and cause endemic infections within 10 years.
And in 2018, Dr. Rosenbach said, a paper in JAMA Dermatology described how more than half – 59% – of the cases of cutaneous leishmaniasis diagnosed in Texas were endemic, all occurring in people with no prior travel outside the United States.
Dr. Williams’ devotion to climate change and dermatology and to the climate change ERG was inspired in large part by Dr. Rosenbach’s 2017 paper in JAAD. She had long been concerned about climate change, she said, but “the review article was really the impetus for me to think, this is really within my specialty.”
Extreme weather events, and the climate-driven migration expected to increasingly occur, have clear relevance to dermatology, Dr. Williams said. “Often, the most vexing problems that people have when they’re forced out of their homes ... are dermatologic,” she said, like infections from contaminated waters after flooding and the spread of scabies and other communicable diseases due to crowding and unsanitary conditions.
But there are other less obvious ramifications of a changing climate that affect dermatology. Dr. Williams has delved into the literature on heat-related illness, for instance, and found that most research has been in the realm of sports medicine and military health. “Most of us don’t treat serious heat-related illnesses, but our skin is responsible for keeping us cool and there’s an important role for dermatologists to play in knowing how the skin does that and who is at risk for heat illness because the skin is unable to do the full job,” she said.
Research is needed to identify which medications can interfere with the skin’s thermoregulatory responses and put patients at risk, she noted. “And a lot of the work on sweat gland physiology is probably 30 years old now. We should bring to bear contemporary research techniques.”
Dermatology is also “in the early stages of understanding the role that air pollution plays in skin disease,” Dr. Williams said. “Most of the medical literature focuses on the effects of pollution on the lungs and in cardiovascular disease.”
There is evidence linking small particulate matter found in wood smoke and other air pollutants to exacerbations of atopic dermatitis and other inflammatory skin conditions, she noted, but mechanisms need to be explored and health disparities examined. “While we know that there are health disparities in terms of [exposure to] pollution and respiratory illness, we have no idea if this is the case with our skin diseases like atopic dermatitis,” said Dr. Williams.
In general, according to the AAD position statement, low-income and minority communities, in addition to the very young and the very old, “are and will continue to be disproportionately affected by climate change.”
Education and the carbon footprint
Viewing climate change as a social determinant of health (SDH ) – and integrating it into medical training as such – is a topic of active discussion. At UCSF, Sarah J. Coates, MD, a fellow in pediatric dermatology, is working with colleagues to integrate climate change into formal resident education. “We know that climate change affects housing, food security, migration ... and certain populations are and will be especially vulnerable,” she said in an interview. “The effects of climate change fit squarely into the social determinant of health curriculum that we’re building here.”
Dr. Coates began to appreciate the link between climate and infectious diseases – a topic she now writes and speaks about – when she saw several patients with coccidioidomycosis as a dermatology resident at UCSF and learned that the cases represented an epidemic in the Central Valley “resulting from several years of drought.”
Her medical school and residency training were otherwise devoid of any discussion of climate change. At UCSF and nearby Stanford (Calif.) University, this is no longer the case, she and Dr. Williams said. “The medical students here have been quite active and are requesting education,” noted Dr. Williams. “The desire to know more is coming from the bottom.”
Mary E. Maloney, MD, professor of medicine and director of dermatologic surgery at the University of Massachusetts, Worcester, sees the same interest from physicians-in-training in the Boston area. They want education about climate science, the impact of climate changes on health and risk mitigation, and ways to reduce medicine’s carbon footprint. “We need to teach them and charge them to lead in their communities,” she said in an interview.
Dr. Maloney joined the AAD’s climate change resource group soon after its inception, having realized the urgency of climate change and feeling that she needed “to get passionate and not just do small things.” As a Mohs surgeon, she expects an “explosion” of skin cancer as temperatures and sun exposure continue to increase.
She urges dermatologists to work to decrease the carbon footprint of their practices and to advocate for local hospitals and other clinical institutions to do so. On the AAD website, members now have free access to tools provided by the nonprofit organization My Green Doctor for outpatient offices to lighten their carbon footprints in a cost-effective – or even cost-saving – manner.
Dr. Maloney’s institution has moved to automated lighting systems and the use of LED lights, she said, and has encouraged ride sharing (prior to the pandemic) and computer switch-offs at night. And in her practice, she and a colleague have been working to reduce the purchasing and use of disposable plastics.
Educating patients about the effects of climate change on the health of their skin is another of the missions listed in the AAD’s position statement, and it’s something that Dr. Coates is currently researching. “It seems similar to talking about other social determinants of health,” she said. “Saying to a patient, for instance, ‘we’ve had some really terrible wildfires lately. They’re getting worse as the seasons go on and we know that’s because of climate change. How do you think your current rash relates to the current air quality? How you think the air quality affects your skin?’ ”
Dr. Rosenbach emphasizes that physicians are a broadly trusted group. “I’d tell a patient, ‘you’re the fourth patient I’ve seen with Lyme – we think that’s because it’s been a warmer year due to climate change,’” he said. “I don’t think that bringing up climate change has ever been a source of friction.”
Do you want to become a hospitalist leader?
Learn how or even whether you should
Have you ever thought you could be a leader, in your hospitalist group, in hospital administration, or at another institution? The reasons to seek a leadership role as a hospitalist are many, but there are also many drawbacks. According to hospitalists who have reached high rungs on the leadership ladder, you will need a blend of desire, enthusiasm, education, and experience if you want to succeed in leadership.
The right reasons
“People who make good leaders have a sense of purpose and want to make a difference,” said Eric Howell, MD, MHM, CEO of the Society of Hospital Medicine, and former chief of medical units at Johns Hopkins Bayview in Baltimore. “I think most hospitalists have that sense of wanting to help patients and society, so that’s a strong mission in itself. Just by training and the very design of our health care system, hospitalists are often natural leaders, and in leadership roles, because they run teams of clinicians and train medical students.”
Danielle Scheurer, MD, SFHM, chief quality officer and professor of medicine at the Medical University of South Carolina, and current president of SHM, said some hospitalists end up in leadership roles almost by accident – because there is a leadership “void” in the health system where they work, and no one else wants to step up. Others disconnect from the leadership track and are happy to simply be part of a team.
“If you are yearning to make a difference and that’s your motivation then you will find leadership is more fulfilling than difficult,” she said. “But if you take a leadership role to fill a void or think you just want to take some nonclinical time, it’s probably not a good idea. Some people think administrative leadership is easier than being a hospitalist, but it is not. Leadership should not be about getting away from something else. It should be a thoughtful career move, and if it is, being a leader can be meaningful and fulfilling.”
Nancy Spector, MD, the vice dean for faculty and executive director of the Executive Leadership in Academic Medicine program at Drexel University College of Medicine in Philadelphia, said a willingness to fail is vital for a leader. “You have to be open to successes, yes, but also to making mistakes,” she said. “It’s about honing the skills that leadership requires and be open to development and change.”
Kierstin Cates Kennedy, MD, SFHM, chief of hospital medicine at the University of Alabama at Birmingham, said that a hospitalist fresh out of residency will gain insight into whether leadership is the right path by acquiring a deeper understanding of how health care institutions work.
“When you are new to the hospital, you see how things work, how people interact with each other, and learn the politics,” she said. “One of the easiest ways to do it is get involved in a committee and be a part of meetings. You can have some input and get exposure to other leaders and they can learn more about you. Once you get an organizational understanding under your belt, then you can start taking on projects to gain even more understanding.”
Still up for it?
If you think you have the commitment and desire for leadership as an early career hospitalist, how would you continue down the leadership path?
“A great way is to find a person you want to be like, who could be a mentor. Find a successful leader that you admire, and one who is willing to guide you,” Dr. Howell said. “Books are helpful as well, and I still find I’m learning today – I have a list that includes Drive by Daniel Pink and Good to Great by Jim Collins. There are Malcolm Gladwell books that also have terrific knowledge to impart.”
Mark W. Shen, MD, SFHM, associate professor at Dell Medical School at the University of Texas at Austin and former president of St. Louis Children’s Hospital, said potential hospitalist leaders must be aware of their fellow clinicians.
“Pay attention to the needs of the hospitalist group as they are articulated by the lead hospitalist, the administration, and the patients,” he said. “There are so many activities that come up on a day-in, day-out basis. You should jump in and volunteer to take the lead on some of those activities. Leading your peers is often one of the most challenging parts of leadership. I think taking on even just a small activity like, say, working on a clinical pathway for the group, will result in a lot of preparation for future leadership roles.”
An example of an early career activity that Dr. Shen felt was valuable to future leaders was helping in the development of a hospitalist core curriculum. “We would use the core curriculum to educate students and residents coming through our rotation and have some degree of commonality or standardization,” he said. “So even though I wasn’t an explicit leader of the hospitalist group at the time, I’d say that helping develop the core curriculum aided me in understanding what leadership was all about.”
Getting started in a leadership role, Dr. Spector said, can be helped by embracing a knowledge of the business of medicine. “Business and finance are a reality you shouldn’t avoid,” she said. “Another way to learn is to partner with your local administrators or whoever is running your division or your department. There are business managers and business partners in every institution, and you can learn a great deal from them. It’s important to network and get to know people because we’re a people business, and opportunity comes when people know who you are.”
Dr. Howell noted that advocating for yourself is sometimes hard, and it can be a red flag in some circumstances, but you should tell your bosses where you want to go professionally.
“You can say that you want to grow professionally, and let’s face it, there are naturally-inclined leaders. We all need to be transparent in goal paths,” he said. “But if you want a leadership role for power, money, and prestige then you’re not applying the right thinking. If you want to help others and you have a mission you believe in, then communicate that to your bosses.”
Dr. Scheurer believes choosing between clinical and administrative leadership is not so clear cut, because in the health care setting they tend to morph into each other. “Many times clinicians will end up taking on a leadership role that has a significant administrative component to it,” she said. “I do think if clinicians make a career move and get the right training then they can be exemplary leaders in health care, but I do worry a little about clinicians going into leadership roles without any formal training. They are usually well-intentioned but that’s not enough. It’s not any different than medical training. If you want to be a good leader you need training to develop your skills, and a lot of those skills do not come naturally or easily. We thrust good clinicians into leadership roles because they are good clinicians, but if they don’t have the right skills, being a leader can be a problem.”
How do leaders improve?
If you have made it to a leadership position, and have been in that role for a while, you might start to feel you are stuck in your growth trajectory. If so, how do you continue to improve?
According to Dr. Kennedy, whether you are looking to get into leadership or want to improve, focusing on emotional intelligence is important. “A book like Emotional Intelligence 2.0 by Travis Bradbury is a great introduction,” she said. “With my leadership team, we did a book club where we read Primal Leadership, which is focused on emotional intelligence and on aspects like setting a culture.”
Dr. Howell said that to grow as a leader, be careful what you say no to. “I used to talk about having a tag line that was ‘just say yes,’” he said. “At least try to say yes most of the time because it opens up opportunities and shows you are looking to do more, not less.”
Also, Dr. Howell recommends that leaders look for tools that minimize blind spots and get information from staff through survey assessments. “Get the input of others on your strengths and weaknesses,” he said. “Nurses, doctors, and sometimes patients can give you good information that will help you grow as a leader. Don’t be afraid of feedback.”
Never stop learning
Dr. Scheurer said it is important to recognize that you are never finished learning when you are a leader.
“See leadership as a continuous learning journey. You can never be too good of a leader in medicine,” she said. “Never stop learning, because the field keeps changing and you have to constantly learn and find pleasure in that learning. You should look at leadership the same way. A lot of leadership theories change with the times and you should always try to get good advice. You don’t take every piece of advice – just like in medicine when you read an article and you try to apply it to patients in your practice. Take some advice, leave some advice, and develop a leadership style that is genuine and authentic.”
Dr. Kennedy believes that a hospitalist’s leadership potential may be limited if you see continued learning as a chore, rather than an opportunity.
“If you resent it learning about leadership, then is it really for you?” she asked. “I find myself reading on the topic or talking about it, and it’s fun. How do you make an environment work better, how do you inspire people, how do you help them grow? These are some of the most important questions leaders face. Isn’t it fun if you can find some answers?”
Learn how or even whether you should
Learn how or even whether you should
Have you ever thought you could be a leader, in your hospitalist group, in hospital administration, or at another institution? The reasons to seek a leadership role as a hospitalist are many, but there are also many drawbacks. According to hospitalists who have reached high rungs on the leadership ladder, you will need a blend of desire, enthusiasm, education, and experience if you want to succeed in leadership.
The right reasons
“People who make good leaders have a sense of purpose and want to make a difference,” said Eric Howell, MD, MHM, CEO of the Society of Hospital Medicine, and former chief of medical units at Johns Hopkins Bayview in Baltimore. “I think most hospitalists have that sense of wanting to help patients and society, so that’s a strong mission in itself. Just by training and the very design of our health care system, hospitalists are often natural leaders, and in leadership roles, because they run teams of clinicians and train medical students.”
Danielle Scheurer, MD, SFHM, chief quality officer and professor of medicine at the Medical University of South Carolina, and current president of SHM, said some hospitalists end up in leadership roles almost by accident – because there is a leadership “void” in the health system where they work, and no one else wants to step up. Others disconnect from the leadership track and are happy to simply be part of a team.
“If you are yearning to make a difference and that’s your motivation then you will find leadership is more fulfilling than difficult,” she said. “But if you take a leadership role to fill a void or think you just want to take some nonclinical time, it’s probably not a good idea. Some people think administrative leadership is easier than being a hospitalist, but it is not. Leadership should not be about getting away from something else. It should be a thoughtful career move, and if it is, being a leader can be meaningful and fulfilling.”
Nancy Spector, MD, the vice dean for faculty and executive director of the Executive Leadership in Academic Medicine program at Drexel University College of Medicine in Philadelphia, said a willingness to fail is vital for a leader. “You have to be open to successes, yes, but also to making mistakes,” she said. “It’s about honing the skills that leadership requires and be open to development and change.”
Kierstin Cates Kennedy, MD, SFHM, chief of hospital medicine at the University of Alabama at Birmingham, said that a hospitalist fresh out of residency will gain insight into whether leadership is the right path by acquiring a deeper understanding of how health care institutions work.
“When you are new to the hospital, you see how things work, how people interact with each other, and learn the politics,” she said. “One of the easiest ways to do it is get involved in a committee and be a part of meetings. You can have some input and get exposure to other leaders and they can learn more about you. Once you get an organizational understanding under your belt, then you can start taking on projects to gain even more understanding.”
Still up for it?
If you think you have the commitment and desire for leadership as an early career hospitalist, how would you continue down the leadership path?
“A great way is to find a person you want to be like, who could be a mentor. Find a successful leader that you admire, and one who is willing to guide you,” Dr. Howell said. “Books are helpful as well, and I still find I’m learning today – I have a list that includes Drive by Daniel Pink and Good to Great by Jim Collins. There are Malcolm Gladwell books that also have terrific knowledge to impart.”
Mark W. Shen, MD, SFHM, associate professor at Dell Medical School at the University of Texas at Austin and former president of St. Louis Children’s Hospital, said potential hospitalist leaders must be aware of their fellow clinicians.
“Pay attention to the needs of the hospitalist group as they are articulated by the lead hospitalist, the administration, and the patients,” he said. “There are so many activities that come up on a day-in, day-out basis. You should jump in and volunteer to take the lead on some of those activities. Leading your peers is often one of the most challenging parts of leadership. I think taking on even just a small activity like, say, working on a clinical pathway for the group, will result in a lot of preparation for future leadership roles.”
An example of an early career activity that Dr. Shen felt was valuable to future leaders was helping in the development of a hospitalist core curriculum. “We would use the core curriculum to educate students and residents coming through our rotation and have some degree of commonality or standardization,” he said. “So even though I wasn’t an explicit leader of the hospitalist group at the time, I’d say that helping develop the core curriculum aided me in understanding what leadership was all about.”
Getting started in a leadership role, Dr. Spector said, can be helped by embracing a knowledge of the business of medicine. “Business and finance are a reality you shouldn’t avoid,” she said. “Another way to learn is to partner with your local administrators or whoever is running your division or your department. There are business managers and business partners in every institution, and you can learn a great deal from them. It’s important to network and get to know people because we’re a people business, and opportunity comes when people know who you are.”
Dr. Howell noted that advocating for yourself is sometimes hard, and it can be a red flag in some circumstances, but you should tell your bosses where you want to go professionally.
“You can say that you want to grow professionally, and let’s face it, there are naturally-inclined leaders. We all need to be transparent in goal paths,” he said. “But if you want a leadership role for power, money, and prestige then you’re not applying the right thinking. If you want to help others and you have a mission you believe in, then communicate that to your bosses.”
Dr. Scheurer believes choosing between clinical and administrative leadership is not so clear cut, because in the health care setting they tend to morph into each other. “Many times clinicians will end up taking on a leadership role that has a significant administrative component to it,” she said. “I do think if clinicians make a career move and get the right training then they can be exemplary leaders in health care, but I do worry a little about clinicians going into leadership roles without any formal training. They are usually well-intentioned but that’s not enough. It’s not any different than medical training. If you want to be a good leader you need training to develop your skills, and a lot of those skills do not come naturally or easily. We thrust good clinicians into leadership roles because they are good clinicians, but if they don’t have the right skills, being a leader can be a problem.”
How do leaders improve?
If you have made it to a leadership position, and have been in that role for a while, you might start to feel you are stuck in your growth trajectory. If so, how do you continue to improve?
According to Dr. Kennedy, whether you are looking to get into leadership or want to improve, focusing on emotional intelligence is important. “A book like Emotional Intelligence 2.0 by Travis Bradbury is a great introduction,” she said. “With my leadership team, we did a book club where we read Primal Leadership, which is focused on emotional intelligence and on aspects like setting a culture.”
Dr. Howell said that to grow as a leader, be careful what you say no to. “I used to talk about having a tag line that was ‘just say yes,’” he said. “At least try to say yes most of the time because it opens up opportunities and shows you are looking to do more, not less.”
Also, Dr. Howell recommends that leaders look for tools that minimize blind spots and get information from staff through survey assessments. “Get the input of others on your strengths and weaknesses,” he said. “Nurses, doctors, and sometimes patients can give you good information that will help you grow as a leader. Don’t be afraid of feedback.”
Never stop learning
Dr. Scheurer said it is important to recognize that you are never finished learning when you are a leader.
“See leadership as a continuous learning journey. You can never be too good of a leader in medicine,” she said. “Never stop learning, because the field keeps changing and you have to constantly learn and find pleasure in that learning. You should look at leadership the same way. A lot of leadership theories change with the times and you should always try to get good advice. You don’t take every piece of advice – just like in medicine when you read an article and you try to apply it to patients in your practice. Take some advice, leave some advice, and develop a leadership style that is genuine and authentic.”
Dr. Kennedy believes that a hospitalist’s leadership potential may be limited if you see continued learning as a chore, rather than an opportunity.
“If you resent it learning about leadership, then is it really for you?” she asked. “I find myself reading on the topic or talking about it, and it’s fun. How do you make an environment work better, how do you inspire people, how do you help them grow? These are some of the most important questions leaders face. Isn’t it fun if you can find some answers?”
Have you ever thought you could be a leader, in your hospitalist group, in hospital administration, or at another institution? The reasons to seek a leadership role as a hospitalist are many, but there are also many drawbacks. According to hospitalists who have reached high rungs on the leadership ladder, you will need a blend of desire, enthusiasm, education, and experience if you want to succeed in leadership.
The right reasons
“People who make good leaders have a sense of purpose and want to make a difference,” said Eric Howell, MD, MHM, CEO of the Society of Hospital Medicine, and former chief of medical units at Johns Hopkins Bayview in Baltimore. “I think most hospitalists have that sense of wanting to help patients and society, so that’s a strong mission in itself. Just by training and the very design of our health care system, hospitalists are often natural leaders, and in leadership roles, because they run teams of clinicians and train medical students.”
Danielle Scheurer, MD, SFHM, chief quality officer and professor of medicine at the Medical University of South Carolina, and current president of SHM, said some hospitalists end up in leadership roles almost by accident – because there is a leadership “void” in the health system where they work, and no one else wants to step up. Others disconnect from the leadership track and are happy to simply be part of a team.
“If you are yearning to make a difference and that’s your motivation then you will find leadership is more fulfilling than difficult,” she said. “But if you take a leadership role to fill a void or think you just want to take some nonclinical time, it’s probably not a good idea. Some people think administrative leadership is easier than being a hospitalist, but it is not. Leadership should not be about getting away from something else. It should be a thoughtful career move, and if it is, being a leader can be meaningful and fulfilling.”
Nancy Spector, MD, the vice dean for faculty and executive director of the Executive Leadership in Academic Medicine program at Drexel University College of Medicine in Philadelphia, said a willingness to fail is vital for a leader. “You have to be open to successes, yes, but also to making mistakes,” she said. “It’s about honing the skills that leadership requires and be open to development and change.”
Kierstin Cates Kennedy, MD, SFHM, chief of hospital medicine at the University of Alabama at Birmingham, said that a hospitalist fresh out of residency will gain insight into whether leadership is the right path by acquiring a deeper understanding of how health care institutions work.
“When you are new to the hospital, you see how things work, how people interact with each other, and learn the politics,” she said. “One of the easiest ways to do it is get involved in a committee and be a part of meetings. You can have some input and get exposure to other leaders and they can learn more about you. Once you get an organizational understanding under your belt, then you can start taking on projects to gain even more understanding.”
Still up for it?
If you think you have the commitment and desire for leadership as an early career hospitalist, how would you continue down the leadership path?
“A great way is to find a person you want to be like, who could be a mentor. Find a successful leader that you admire, and one who is willing to guide you,” Dr. Howell said. “Books are helpful as well, and I still find I’m learning today – I have a list that includes Drive by Daniel Pink and Good to Great by Jim Collins. There are Malcolm Gladwell books that also have terrific knowledge to impart.”
Mark W. Shen, MD, SFHM, associate professor at Dell Medical School at the University of Texas at Austin and former president of St. Louis Children’s Hospital, said potential hospitalist leaders must be aware of their fellow clinicians.
“Pay attention to the needs of the hospitalist group as they are articulated by the lead hospitalist, the administration, and the patients,” he said. “There are so many activities that come up on a day-in, day-out basis. You should jump in and volunteer to take the lead on some of those activities. Leading your peers is often one of the most challenging parts of leadership. I think taking on even just a small activity like, say, working on a clinical pathway for the group, will result in a lot of preparation for future leadership roles.”
An example of an early career activity that Dr. Shen felt was valuable to future leaders was helping in the development of a hospitalist core curriculum. “We would use the core curriculum to educate students and residents coming through our rotation and have some degree of commonality or standardization,” he said. “So even though I wasn’t an explicit leader of the hospitalist group at the time, I’d say that helping develop the core curriculum aided me in understanding what leadership was all about.”
Getting started in a leadership role, Dr. Spector said, can be helped by embracing a knowledge of the business of medicine. “Business and finance are a reality you shouldn’t avoid,” she said. “Another way to learn is to partner with your local administrators or whoever is running your division or your department. There are business managers and business partners in every institution, and you can learn a great deal from them. It’s important to network and get to know people because we’re a people business, and opportunity comes when people know who you are.”
Dr. Howell noted that advocating for yourself is sometimes hard, and it can be a red flag in some circumstances, but you should tell your bosses where you want to go professionally.
“You can say that you want to grow professionally, and let’s face it, there are naturally-inclined leaders. We all need to be transparent in goal paths,” he said. “But if you want a leadership role for power, money, and prestige then you’re not applying the right thinking. If you want to help others and you have a mission you believe in, then communicate that to your bosses.”
Dr. Scheurer believes choosing between clinical and administrative leadership is not so clear cut, because in the health care setting they tend to morph into each other. “Many times clinicians will end up taking on a leadership role that has a significant administrative component to it,” she said. “I do think if clinicians make a career move and get the right training then they can be exemplary leaders in health care, but I do worry a little about clinicians going into leadership roles without any formal training. They are usually well-intentioned but that’s not enough. It’s not any different than medical training. If you want to be a good leader you need training to develop your skills, and a lot of those skills do not come naturally or easily. We thrust good clinicians into leadership roles because they are good clinicians, but if they don’t have the right skills, being a leader can be a problem.”
How do leaders improve?
If you have made it to a leadership position, and have been in that role for a while, you might start to feel you are stuck in your growth trajectory. If so, how do you continue to improve?
According to Dr. Kennedy, whether you are looking to get into leadership or want to improve, focusing on emotional intelligence is important. “A book like Emotional Intelligence 2.0 by Travis Bradbury is a great introduction,” she said. “With my leadership team, we did a book club where we read Primal Leadership, which is focused on emotional intelligence and on aspects like setting a culture.”
Dr. Howell said that to grow as a leader, be careful what you say no to. “I used to talk about having a tag line that was ‘just say yes,’” he said. “At least try to say yes most of the time because it opens up opportunities and shows you are looking to do more, not less.”
Also, Dr. Howell recommends that leaders look for tools that minimize blind spots and get information from staff through survey assessments. “Get the input of others on your strengths and weaknesses,” he said. “Nurses, doctors, and sometimes patients can give you good information that will help you grow as a leader. Don’t be afraid of feedback.”
Never stop learning
Dr. Scheurer said it is important to recognize that you are never finished learning when you are a leader.
“See leadership as a continuous learning journey. You can never be too good of a leader in medicine,” she said. “Never stop learning, because the field keeps changing and you have to constantly learn and find pleasure in that learning. You should look at leadership the same way. A lot of leadership theories change with the times and you should always try to get good advice. You don’t take every piece of advice – just like in medicine when you read an article and you try to apply it to patients in your practice. Take some advice, leave some advice, and develop a leadership style that is genuine and authentic.”
Dr. Kennedy believes that a hospitalist’s leadership potential may be limited if you see continued learning as a chore, rather than an opportunity.
“If you resent it learning about leadership, then is it really for you?” she asked. “I find myself reading on the topic or talking about it, and it’s fun. How do you make an environment work better, how do you inspire people, how do you help them grow? These are some of the most important questions leaders face. Isn’t it fun if you can find some answers?”
Applying lessons from Oprah to your practice
In my last column, I explained how I’m like Tom Brady. I’m not really. Brady is a Super Bowl–winning quarterback worth over $200 million. No, I’m like Oprah. Well, trying anyway.
Brady and Oprah, in addition to being gazillionaires, have in common that they’re arguably the GOATs (Greatest Of All Time) in their fields. Watching Oprah interview Meghan Markle and Prince Harry was like watching Tom Brady on the jumbotron – she made it look easy. Her ability to create conversation and coax information from guests is hall-of-fame good. But although they are both admirable, trying to be like Brady is useful only for next Thanksgiving when you’re trying to beat your cousins from Massachusetts in touch football. .
1. Prepare ahead. It’s clear that Oprah has binders of notes about her guests and thoroughly reviewed them before she invites them to sit down. We should do the same. Open the chart and read as much as you can before you open the door. Have important information in your head so you don’t have to break from your interview to refer to it.
2. Sprinkle pleasantry. She’d never start an interview with: So why are you here? Nor should we. Even one nonscripted question or comment can help build a little rapport before getting to the work.
3. Be brief. Oprah gets her question out fast, then gets out of the way. And as a bonus, this is the easiest place to shave a few minutes from your appointments from your own end. Think for a second before you speak and try to find the shortest route to your question. Try to keep your questions to just a sentence or two.
4. Stay on it. Once you’ve discovered something relevant, stay with it, resisting the urge to finish the review of symptoms. This is not just to make a diagnosis, but as importantly, trying to diagnose “the real reason” for the visit. Then, when the question is done, own the transition. Oprah uses: “Let’s move on.” This is a bit abrupt for us, but it can be helpful if used sparingly and gently. I might soften this a little by adding “I want to be sure we have enough time to get through everything for you.”
5. Wait. A few seconds seems an eternity on the air (and in clinic), but sometimes the silent pause is just what’s needed to help the patient expand and share.
6. Be nonjudgmental. Most of us believe we’re pretty good at this, yet, it’s sometimes a blind spot. It’s easy to blame the obese patient for his stasis dermatitis or the hidradenitis patient who hasn’t stop smoking for her cysts. It also helps to be nontransactional. If you make patients feel that you’re asking questions only to extract information, you’ll never reach Oprah level.
7. Be in the moment. It is difficult, but when possible, avoid typing notes while you’re still interviewing. We’re not just there to get the facts, we’re also trying to get the story and that sometimes takes really listening.
I’m no more like Oprah than Brady, of course. But it is more fun to close my eyes and imagine myself being her when I see my next patient. That is, until Thanksgiving. Watch out, Bedards from Attleboro.
Dr. Benabio is director of Healthcare Transformation and chief of dermatology at Kaiser Permanente San Diego. The opinions expressed in this column are his own and do not represent those of Kaiser Permanente. Dr. Benabio is @Dermdoc on Twitter. Write to him at [email protected].
In my last column, I explained how I’m like Tom Brady. I’m not really. Brady is a Super Bowl–winning quarterback worth over $200 million. No, I’m like Oprah. Well, trying anyway.
Brady and Oprah, in addition to being gazillionaires, have in common that they’re arguably the GOATs (Greatest Of All Time) in their fields. Watching Oprah interview Meghan Markle and Prince Harry was like watching Tom Brady on the jumbotron – she made it look easy. Her ability to create conversation and coax information from guests is hall-of-fame good. But although they are both admirable, trying to be like Brady is useful only for next Thanksgiving when you’re trying to beat your cousins from Massachusetts in touch football. .
1. Prepare ahead. It’s clear that Oprah has binders of notes about her guests and thoroughly reviewed them before she invites them to sit down. We should do the same. Open the chart and read as much as you can before you open the door. Have important information in your head so you don’t have to break from your interview to refer to it.
2. Sprinkle pleasantry. She’d never start an interview with: So why are you here? Nor should we. Even one nonscripted question or comment can help build a little rapport before getting to the work.
3. Be brief. Oprah gets her question out fast, then gets out of the way. And as a bonus, this is the easiest place to shave a few minutes from your appointments from your own end. Think for a second before you speak and try to find the shortest route to your question. Try to keep your questions to just a sentence or two.
4. Stay on it. Once you’ve discovered something relevant, stay with it, resisting the urge to finish the review of symptoms. This is not just to make a diagnosis, but as importantly, trying to diagnose “the real reason” for the visit. Then, when the question is done, own the transition. Oprah uses: “Let’s move on.” This is a bit abrupt for us, but it can be helpful if used sparingly and gently. I might soften this a little by adding “I want to be sure we have enough time to get through everything for you.”
5. Wait. A few seconds seems an eternity on the air (and in clinic), but sometimes the silent pause is just what’s needed to help the patient expand and share.
6. Be nonjudgmental. Most of us believe we’re pretty good at this, yet, it’s sometimes a blind spot. It’s easy to blame the obese patient for his stasis dermatitis or the hidradenitis patient who hasn’t stop smoking for her cysts. It also helps to be nontransactional. If you make patients feel that you’re asking questions only to extract information, you’ll never reach Oprah level.
7. Be in the moment. It is difficult, but when possible, avoid typing notes while you’re still interviewing. We’re not just there to get the facts, we’re also trying to get the story and that sometimes takes really listening.
I’m no more like Oprah than Brady, of course. But it is more fun to close my eyes and imagine myself being her when I see my next patient. That is, until Thanksgiving. Watch out, Bedards from Attleboro.
Dr. Benabio is director of Healthcare Transformation and chief of dermatology at Kaiser Permanente San Diego. The opinions expressed in this column are his own and do not represent those of Kaiser Permanente. Dr. Benabio is @Dermdoc on Twitter. Write to him at [email protected].
In my last column, I explained how I’m like Tom Brady. I’m not really. Brady is a Super Bowl–winning quarterback worth over $200 million. No, I’m like Oprah. Well, trying anyway.
Brady and Oprah, in addition to being gazillionaires, have in common that they’re arguably the GOATs (Greatest Of All Time) in their fields. Watching Oprah interview Meghan Markle and Prince Harry was like watching Tom Brady on the jumbotron – she made it look easy. Her ability to create conversation and coax information from guests is hall-of-fame good. But although they are both admirable, trying to be like Brady is useful only for next Thanksgiving when you’re trying to beat your cousins from Massachusetts in touch football. .
1. Prepare ahead. It’s clear that Oprah has binders of notes about her guests and thoroughly reviewed them before she invites them to sit down. We should do the same. Open the chart and read as much as you can before you open the door. Have important information in your head so you don’t have to break from your interview to refer to it.
2. Sprinkle pleasantry. She’d never start an interview with: So why are you here? Nor should we. Even one nonscripted question or comment can help build a little rapport before getting to the work.
3. Be brief. Oprah gets her question out fast, then gets out of the way. And as a bonus, this is the easiest place to shave a few minutes from your appointments from your own end. Think for a second before you speak and try to find the shortest route to your question. Try to keep your questions to just a sentence or two.
4. Stay on it. Once you’ve discovered something relevant, stay with it, resisting the urge to finish the review of symptoms. This is not just to make a diagnosis, but as importantly, trying to diagnose “the real reason” for the visit. Then, when the question is done, own the transition. Oprah uses: “Let’s move on.” This is a bit abrupt for us, but it can be helpful if used sparingly and gently. I might soften this a little by adding “I want to be sure we have enough time to get through everything for you.”
5. Wait. A few seconds seems an eternity on the air (and in clinic), but sometimes the silent pause is just what’s needed to help the patient expand and share.
6. Be nonjudgmental. Most of us believe we’re pretty good at this, yet, it’s sometimes a blind spot. It’s easy to blame the obese patient for his stasis dermatitis or the hidradenitis patient who hasn’t stop smoking for her cysts. It also helps to be nontransactional. If you make patients feel that you’re asking questions only to extract information, you’ll never reach Oprah level.
7. Be in the moment. It is difficult, but when possible, avoid typing notes while you’re still interviewing. We’re not just there to get the facts, we’re also trying to get the story and that sometimes takes really listening.
I’m no more like Oprah than Brady, of course. But it is more fun to close my eyes and imagine myself being her when I see my next patient. That is, until Thanksgiving. Watch out, Bedards from Attleboro.
Dr. Benabio is director of Healthcare Transformation and chief of dermatology at Kaiser Permanente San Diego. The opinions expressed in this column are his own and do not represent those of Kaiser Permanente. Dr. Benabio is @Dermdoc on Twitter. Write to him at [email protected].
School refusal and COVID-19: The pediatrician's role
Hooray for back to school! But not for everyone. ... what to do with those who have trouble transitioning back?
As we have now passed a year since COVID-19–related shutdowns were implemented throughout the United States; and with returns to in-person schooling continuing to vary based on location, many of us either in our personal lives, or through conversations with patients and families, are experiencing a yearning for the “good old days” of fully in-person schooling. As the place where children and adolescents spend a good portion of their waking hours, school is integral to not just children’s academic development, but to emotional and social development as well. One interesting phenomenon I’ve seen working with many children and families is that the strong desire to go back to school is not universal. Some of my patients are perfectly happy to be doing “remote schooling”, as it reduces the stress that they were experiencing in this setting before the pandemic.1 These families find themselves wondering – how will I get my child to return to school? As we (hopefully) turn the corner toward a return to normalcy, I believe many of us may find ourselves counseling families on whether a return to in-person schooling is in their child’s best interest. Even when a family decides it is best for their child to return, we might encounter scenarios in which children and adolescents outright refuse to go to school, or engage in avoidant behavior, which is broadly known as “school refusal.” Discussion of a treatment approach to this often challenging clinical scenario is warranted.
The first step in addressing the issue is defining it. School refusal is not a “diagnosis” in psychiatric lexicon, rather it describes a behavior which may be a symptom or manifestation of any number of underlying factors. One helpful definition proposed is (a) missing 25% of total school time for at least 2 weeks or (b) experiencing difficulty attending school such that there is significant interference in the child’s or family’s daily routine for at least 2 weeks, or (c) missing at least 10 days of school over a period of 15 weeks.2 The common thread of this, and any other definition, is sustained absenteeism or avoidance with significant impact to education, family life, or both. It is estimated that the prevalence of this phenomenon is between 1% and 2% of school-aged children.
Next to consider is what might be prompting or underlying the behavior. A comprehensive evaluation approach should include consideration of environmental factors such as bullying and learning difficulties, as well as presence of an anxiety or depressive disorder. Awareness of whether the child/adolescent has a 504 plan or individualized education program (IEP) is vital, as these can be marshaled for additional support. Family factors, including parental illness (medical and/or psychiatric), should also be considered. As school avoidance behaviors often include somatic symptoms of anxiety such as palpitations, shortness of breath, and abdominal pain; a rule out of medical etiology is recommended, as well as a caution to consider both medical and behavioral factors simultaneously, as focus on either separately can lead to missing the other.
Separation anxiety and social anxiety disorders are two specific conditions that may manifest in school refusal and should be evaluated for specifically. Separation anxiety is characterized by developmentally inappropriate, excessive worry or distress associated with separation from a primary caregiver or major attachment figure. Social anxiety is characterized by excessive fear or worry about being negatively evaluated by others in social situations.3 One publicly available tool that can be helpful for screening for a variety of anxiety disorders in children and adolescents is the SCARED.4 The PHQ-9 Adolescent5 is one such screening instrument for depression, which can be a driving factor or co-occur in children with school refusal.
When it comes to treatment, the best evidence out there is for a cognitive-behavioral therapy (CBT)–based approach motivated toward a return to the school setting as soon as possible.6,7 This will involve looking at how thoughts, behaviors, and feelings are interacting with each other in the clinical scenario and how these might be challenged or changed in a positive manner. Coping and problem-solving skills are often incorporated. This approach may also involve gradual exposure to the anxiety-producing situation in a hierarchical fashion starting with less anxiety-provoking scenarios and moving toward increasingly challenging ones. CBT for school refusal is likely most effective when including both school and family involvement to ensure consistency across settings. Making sure that there are not inadvertent reinforcing factors motivating staying home (for instance unrestricted access to electronic devices) is an important step to consider. If anxiety or depression is moderately to severely impairing – which is frequently the case when school refusal comes to clinical attention, consider use of medication as part of the treatment strategy. Selective serotonin reuptake inhibitors as a class are the most commonly used medications and deserve strong consideration.
To summarize, school refusal can occur for a variety of reasons. Early identification and comprehensive treatment taking into account child and family preference and using a multimodal approach to encourage and support a quick return to the school environment is considered best practice.
Dr. Hoffnung is a pediatric psychiatrist at the University of Vermont Children’s Hospital and an assistant professor of psychiatry at the Robert Larner, M.D. College of Medicine at the University of Vermont, both in Burlington. He has no relevant financial disclosures. Email him at [email protected].
References
1. See, for example: www.npr.org/2021/03/08/971457441/as-many-parents-fret-over-remote-learning-some-find-their-kids-are-thriving.
2. Kearney CA. Educ Psychol Rev. 2008;20:257-82.
3. Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition. Arlington, Va.: American Psychiatric Association, 2013.
4. Available at: www.pediatricbipolar.pitt.edu/resources/instruments.
5. Available at: www.aacap.org/App_Themes/AACAP/docs/member_resources/toolbox_for_clinical_practice_and_outcomes/symptoms/GLAD-PC_PHQ-9.pdf.
6. Elliott JG and Place M. J Child Psychol Psychiatry. 2019;60(1):4-15.
7. Prabhuswamy M. J Paed Child Health. 2018;54(10):1117-20.
Hooray for back to school! But not for everyone. ... what to do with those who have trouble transitioning back?
As we have now passed a year since COVID-19–related shutdowns were implemented throughout the United States; and with returns to in-person schooling continuing to vary based on location, many of us either in our personal lives, or through conversations with patients and families, are experiencing a yearning for the “good old days” of fully in-person schooling. As the place where children and adolescents spend a good portion of their waking hours, school is integral to not just children’s academic development, but to emotional and social development as well. One interesting phenomenon I’ve seen working with many children and families is that the strong desire to go back to school is not universal. Some of my patients are perfectly happy to be doing “remote schooling”, as it reduces the stress that they were experiencing in this setting before the pandemic.1 These families find themselves wondering – how will I get my child to return to school? As we (hopefully) turn the corner toward a return to normalcy, I believe many of us may find ourselves counseling families on whether a return to in-person schooling is in their child’s best interest. Even when a family decides it is best for their child to return, we might encounter scenarios in which children and adolescents outright refuse to go to school, or engage in avoidant behavior, which is broadly known as “school refusal.” Discussion of a treatment approach to this often challenging clinical scenario is warranted.
The first step in addressing the issue is defining it. School refusal is not a “diagnosis” in psychiatric lexicon, rather it describes a behavior which may be a symptom or manifestation of any number of underlying factors. One helpful definition proposed is (a) missing 25% of total school time for at least 2 weeks or (b) experiencing difficulty attending school such that there is significant interference in the child’s or family’s daily routine for at least 2 weeks, or (c) missing at least 10 days of school over a period of 15 weeks.2 The common thread of this, and any other definition, is sustained absenteeism or avoidance with significant impact to education, family life, or both. It is estimated that the prevalence of this phenomenon is between 1% and 2% of school-aged children.
Next to consider is what might be prompting or underlying the behavior. A comprehensive evaluation approach should include consideration of environmental factors such as bullying and learning difficulties, as well as presence of an anxiety or depressive disorder. Awareness of whether the child/adolescent has a 504 plan or individualized education program (IEP) is vital, as these can be marshaled for additional support. Family factors, including parental illness (medical and/or psychiatric), should also be considered. As school avoidance behaviors often include somatic symptoms of anxiety such as palpitations, shortness of breath, and abdominal pain; a rule out of medical etiology is recommended, as well as a caution to consider both medical and behavioral factors simultaneously, as focus on either separately can lead to missing the other.
Separation anxiety and social anxiety disorders are two specific conditions that may manifest in school refusal and should be evaluated for specifically. Separation anxiety is characterized by developmentally inappropriate, excessive worry or distress associated with separation from a primary caregiver or major attachment figure. Social anxiety is characterized by excessive fear or worry about being negatively evaluated by others in social situations.3 One publicly available tool that can be helpful for screening for a variety of anxiety disorders in children and adolescents is the SCARED.4 The PHQ-9 Adolescent5 is one such screening instrument for depression, which can be a driving factor or co-occur in children with school refusal.
When it comes to treatment, the best evidence out there is for a cognitive-behavioral therapy (CBT)–based approach motivated toward a return to the school setting as soon as possible.6,7 This will involve looking at how thoughts, behaviors, and feelings are interacting with each other in the clinical scenario and how these might be challenged or changed in a positive manner. Coping and problem-solving skills are often incorporated. This approach may also involve gradual exposure to the anxiety-producing situation in a hierarchical fashion starting with less anxiety-provoking scenarios and moving toward increasingly challenging ones. CBT for school refusal is likely most effective when including both school and family involvement to ensure consistency across settings. Making sure that there are not inadvertent reinforcing factors motivating staying home (for instance unrestricted access to electronic devices) is an important step to consider. If anxiety or depression is moderately to severely impairing – which is frequently the case when school refusal comes to clinical attention, consider use of medication as part of the treatment strategy. Selective serotonin reuptake inhibitors as a class are the most commonly used medications and deserve strong consideration.
To summarize, school refusal can occur for a variety of reasons. Early identification and comprehensive treatment taking into account child and family preference and using a multimodal approach to encourage and support a quick return to the school environment is considered best practice.
Dr. Hoffnung is a pediatric psychiatrist at the University of Vermont Children’s Hospital and an assistant professor of psychiatry at the Robert Larner, M.D. College of Medicine at the University of Vermont, both in Burlington. He has no relevant financial disclosures. Email him at [email protected].
References
1. See, for example: www.npr.org/2021/03/08/971457441/as-many-parents-fret-over-remote-learning-some-find-their-kids-are-thriving.
2. Kearney CA. Educ Psychol Rev. 2008;20:257-82.
3. Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition. Arlington, Va.: American Psychiatric Association, 2013.
4. Available at: www.pediatricbipolar.pitt.edu/resources/instruments.
5. Available at: www.aacap.org/App_Themes/AACAP/docs/member_resources/toolbox_for_clinical_practice_and_outcomes/symptoms/GLAD-PC_PHQ-9.pdf.
6. Elliott JG and Place M. J Child Psychol Psychiatry. 2019;60(1):4-15.
7. Prabhuswamy M. J Paed Child Health. 2018;54(10):1117-20.
Hooray for back to school! But not for everyone. ... what to do with those who have trouble transitioning back?
As we have now passed a year since COVID-19–related shutdowns were implemented throughout the United States; and with returns to in-person schooling continuing to vary based on location, many of us either in our personal lives, or through conversations with patients and families, are experiencing a yearning for the “good old days” of fully in-person schooling. As the place where children and adolescents spend a good portion of their waking hours, school is integral to not just children’s academic development, but to emotional and social development as well. One interesting phenomenon I’ve seen working with many children and families is that the strong desire to go back to school is not universal. Some of my patients are perfectly happy to be doing “remote schooling”, as it reduces the stress that they were experiencing in this setting before the pandemic.1 These families find themselves wondering – how will I get my child to return to school? As we (hopefully) turn the corner toward a return to normalcy, I believe many of us may find ourselves counseling families on whether a return to in-person schooling is in their child’s best interest. Even when a family decides it is best for their child to return, we might encounter scenarios in which children and adolescents outright refuse to go to school, or engage in avoidant behavior, which is broadly known as “school refusal.” Discussion of a treatment approach to this often challenging clinical scenario is warranted.
The first step in addressing the issue is defining it. School refusal is not a “diagnosis” in psychiatric lexicon, rather it describes a behavior which may be a symptom or manifestation of any number of underlying factors. One helpful definition proposed is (a) missing 25% of total school time for at least 2 weeks or (b) experiencing difficulty attending school such that there is significant interference in the child’s or family’s daily routine for at least 2 weeks, or (c) missing at least 10 days of school over a period of 15 weeks.2 The common thread of this, and any other definition, is sustained absenteeism or avoidance with significant impact to education, family life, or both. It is estimated that the prevalence of this phenomenon is between 1% and 2% of school-aged children.
Next to consider is what might be prompting or underlying the behavior. A comprehensive evaluation approach should include consideration of environmental factors such as bullying and learning difficulties, as well as presence of an anxiety or depressive disorder. Awareness of whether the child/adolescent has a 504 plan or individualized education program (IEP) is vital, as these can be marshaled for additional support. Family factors, including parental illness (medical and/or psychiatric), should also be considered. As school avoidance behaviors often include somatic symptoms of anxiety such as palpitations, shortness of breath, and abdominal pain; a rule out of medical etiology is recommended, as well as a caution to consider both medical and behavioral factors simultaneously, as focus on either separately can lead to missing the other.
Separation anxiety and social anxiety disorders are two specific conditions that may manifest in school refusal and should be evaluated for specifically. Separation anxiety is characterized by developmentally inappropriate, excessive worry or distress associated with separation from a primary caregiver or major attachment figure. Social anxiety is characterized by excessive fear or worry about being negatively evaluated by others in social situations.3 One publicly available tool that can be helpful for screening for a variety of anxiety disorders in children and adolescents is the SCARED.4 The PHQ-9 Adolescent5 is one such screening instrument for depression, which can be a driving factor or co-occur in children with school refusal.
When it comes to treatment, the best evidence out there is for a cognitive-behavioral therapy (CBT)–based approach motivated toward a return to the school setting as soon as possible.6,7 This will involve looking at how thoughts, behaviors, and feelings are interacting with each other in the clinical scenario and how these might be challenged or changed in a positive manner. Coping and problem-solving skills are often incorporated. This approach may also involve gradual exposure to the anxiety-producing situation in a hierarchical fashion starting with less anxiety-provoking scenarios and moving toward increasingly challenging ones. CBT for school refusal is likely most effective when including both school and family involvement to ensure consistency across settings. Making sure that there are not inadvertent reinforcing factors motivating staying home (for instance unrestricted access to electronic devices) is an important step to consider. If anxiety or depression is moderately to severely impairing – which is frequently the case when school refusal comes to clinical attention, consider use of medication as part of the treatment strategy. Selective serotonin reuptake inhibitors as a class are the most commonly used medications and deserve strong consideration.
To summarize, school refusal can occur for a variety of reasons. Early identification and comprehensive treatment taking into account child and family preference and using a multimodal approach to encourage and support a quick return to the school environment is considered best practice.
Dr. Hoffnung is a pediatric psychiatrist at the University of Vermont Children’s Hospital and an assistant professor of psychiatry at the Robert Larner, M.D. College of Medicine at the University of Vermont, both in Burlington. He has no relevant financial disclosures. Email him at [email protected].
References
1. See, for example: www.npr.org/2021/03/08/971457441/as-many-parents-fret-over-remote-learning-some-find-their-kids-are-thriving.
2. Kearney CA. Educ Psychol Rev. 2008;20:257-82.
3. Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition. Arlington, Va.: American Psychiatric Association, 2013.
4. Available at: www.pediatricbipolar.pitt.edu/resources/instruments.
5. Available at: www.aacap.org/App_Themes/AACAP/docs/member_resources/toolbox_for_clinical_practice_and_outcomes/symptoms/GLAD-PC_PHQ-9.pdf.
6. Elliott JG and Place M. J Child Psychol Psychiatry. 2019;60(1):4-15.
7. Prabhuswamy M. J Paed Child Health. 2018;54(10):1117-20.
Overshadowed by COVID, HIV Epidemic Rages On
How many epidemics can one country contain? Nearly all of our national attention has been focused on the COVID-19 epidemic but, lest we forget, there’s still another one to be reckoned with: the HIV epidemic is still going strong. In fact, it’s gathering strength, in part, because of the economic and health devastation wrought by COVID.
Over nearly 4 decades, the epidemiology of HIV has changed, according to The Lancet’s HIV in the USA series. Current data, the report says, “illustrate an epidemic defined by stark health inequities that largely fall along lines of disadvantages in economic opportunity and social capital.” Moreover, the US, the authors say, “continues to lag behind other G-7 nations when it comes to controlling its HIV epidemic and is the only high-income country among the top 10 countries most affected by HIV.”
The 6-paper series’ release comes 2 years after the launch of the US Department of Health and Human Services’ (HHS) announcement of its goal to reduce HIV transmissions by at least 90% by 2030.
The authors analyzed publicly available HIV surveillance and census data to describe current prevalence and new HIV diagnoses by region, race, ethnicity, and age, as well as trends in those categories over time. They also reviewed literature to “explore the reasons” for the distribution of cases and important disparities in prevalence. Among other things, the researchers found “pronounced” racial, sexual, and gender disparities, “substantial” gaps in domestic program funding, and a “patchwork healthcare system” that limited access to treatment and prevention services.
Although when it began, the HIV epidemic was focused largely on the bicoastal big cities, mainly New York and San Francisco, in recent years the South has been hit particularly hard, with 52% of new HIV transmissions in 2018, despite representing only 37% of the US population. Six Southern states (Florida, Georgia, Louisiana, Maryland, Mississippi, and Tennessee) and the District of Columbia had the highest annual HIV diagnosis rates between 2010 and 2018, likely reflecting the higher burden of infection among black residents: In 2018, 38% of all new HIV diagnoses among men who have sex with men (MSM) were in the black population, and 63% of those were in the South.
The South’s HIV problem is intensified by disparities, the report says, that are probably driven by the restricted expansion of Medicaid, health care provider shortages, low health literacy, and stigma. The South also has the lowest number of pre-exposure prophylaxis (PrEP) users per new HIV diagnosis, in part because of the longer distances to PrEP services relative to other regions. More than half of MSM who live at least 60 minutes away from PrEP services live in the South. While HIV in the rural South largely is due to sexual transmission, the researchers note, the largest clusters of the concurrent opioid epidemic have been detected in rural and periurban counties of West Virginia and Indiana.
Identifying HIV transmission clusters and outbreaks has traditionally been challenging for several reasons, the researchers say, including delays between infection and diagnosis, mobility of populations, and limitations in tracing sex and drug partners. They suggest that analysis of molecular data can help overcome some of those barriers, making it possible to identify clusters of ongoing HIV transmission.
The report also recommends other approaches to better understand and respond to ongoing HIV infections, such as mapping and data visualization, telemedicine, and automated data systems to facilitate linkage to care. However, the authors add, gaps in data and data systems remain that prevent full understanding of some key impacts of the epidemic. But any interventions to promote HIV prevention and treatment adherence, the authors suggested, should take a multifaceted approach and address the whole individual.
Chris Beyrer, MD, MPH, investigator at the Johns Hopkins Bloomberg School of Public Health, Baltimore, and a lead author on the series, says, “We have incredible tools to prevent and treat HIV, but people may not fully utilize them if they are facing personal or structural issues that pose more immediate hardship, like substance use and mental health disorders. You may struggle to take a daily medication if you are facing food insecurity or cannot find affordable treatment for your substance use disorder.”
Anthony Fauci, MD, director of the National Institute of Allergy and Infectious Diseases, and now also Chief Medical Advisor to President Biden, says, “To end the HIV epidemic, we must continue to develop and deploy novel HIV treatment and prevention strategies suited to the different needs and preferences of diverse populations disproportionately affected by HIV. It is also essential that HIV health services continue during the COVID-19 pandemic.”
How many epidemics can one country contain? Nearly all of our national attention has been focused on the COVID-19 epidemic but, lest we forget, there’s still another one to be reckoned with: the HIV epidemic is still going strong. In fact, it’s gathering strength, in part, because of the economic and health devastation wrought by COVID.
Over nearly 4 decades, the epidemiology of HIV has changed, according to The Lancet’s HIV in the USA series. Current data, the report says, “illustrate an epidemic defined by stark health inequities that largely fall along lines of disadvantages in economic opportunity and social capital.” Moreover, the US, the authors say, “continues to lag behind other G-7 nations when it comes to controlling its HIV epidemic and is the only high-income country among the top 10 countries most affected by HIV.”
The 6-paper series’ release comes 2 years after the launch of the US Department of Health and Human Services’ (HHS) announcement of its goal to reduce HIV transmissions by at least 90% by 2030.
The authors analyzed publicly available HIV surveillance and census data to describe current prevalence and new HIV diagnoses by region, race, ethnicity, and age, as well as trends in those categories over time. They also reviewed literature to “explore the reasons” for the distribution of cases and important disparities in prevalence. Among other things, the researchers found “pronounced” racial, sexual, and gender disparities, “substantial” gaps in domestic program funding, and a “patchwork healthcare system” that limited access to treatment and prevention services.
Although when it began, the HIV epidemic was focused largely on the bicoastal big cities, mainly New York and San Francisco, in recent years the South has been hit particularly hard, with 52% of new HIV transmissions in 2018, despite representing only 37% of the US population. Six Southern states (Florida, Georgia, Louisiana, Maryland, Mississippi, and Tennessee) and the District of Columbia had the highest annual HIV diagnosis rates between 2010 and 2018, likely reflecting the higher burden of infection among black residents: In 2018, 38% of all new HIV diagnoses among men who have sex with men (MSM) were in the black population, and 63% of those were in the South.
The South’s HIV problem is intensified by disparities, the report says, that are probably driven by the restricted expansion of Medicaid, health care provider shortages, low health literacy, and stigma. The South also has the lowest number of pre-exposure prophylaxis (PrEP) users per new HIV diagnosis, in part because of the longer distances to PrEP services relative to other regions. More than half of MSM who live at least 60 minutes away from PrEP services live in the South. While HIV in the rural South largely is due to sexual transmission, the researchers note, the largest clusters of the concurrent opioid epidemic have been detected in rural and periurban counties of West Virginia and Indiana.
Identifying HIV transmission clusters and outbreaks has traditionally been challenging for several reasons, the researchers say, including delays between infection and diagnosis, mobility of populations, and limitations in tracing sex and drug partners. They suggest that analysis of molecular data can help overcome some of those barriers, making it possible to identify clusters of ongoing HIV transmission.
The report also recommends other approaches to better understand and respond to ongoing HIV infections, such as mapping and data visualization, telemedicine, and automated data systems to facilitate linkage to care. However, the authors add, gaps in data and data systems remain that prevent full understanding of some key impacts of the epidemic. But any interventions to promote HIV prevention and treatment adherence, the authors suggested, should take a multifaceted approach and address the whole individual.
Chris Beyrer, MD, MPH, investigator at the Johns Hopkins Bloomberg School of Public Health, Baltimore, and a lead author on the series, says, “We have incredible tools to prevent and treat HIV, but people may not fully utilize them if they are facing personal or structural issues that pose more immediate hardship, like substance use and mental health disorders. You may struggle to take a daily medication if you are facing food insecurity or cannot find affordable treatment for your substance use disorder.”
Anthony Fauci, MD, director of the National Institute of Allergy and Infectious Diseases, and now also Chief Medical Advisor to President Biden, says, “To end the HIV epidemic, we must continue to develop and deploy novel HIV treatment and prevention strategies suited to the different needs and preferences of diverse populations disproportionately affected by HIV. It is also essential that HIV health services continue during the COVID-19 pandemic.”
How many epidemics can one country contain? Nearly all of our national attention has been focused on the COVID-19 epidemic but, lest we forget, there’s still another one to be reckoned with: the HIV epidemic is still going strong. In fact, it’s gathering strength, in part, because of the economic and health devastation wrought by COVID.
Over nearly 4 decades, the epidemiology of HIV has changed, according to The Lancet’s HIV in the USA series. Current data, the report says, “illustrate an epidemic defined by stark health inequities that largely fall along lines of disadvantages in economic opportunity and social capital.” Moreover, the US, the authors say, “continues to lag behind other G-7 nations when it comes to controlling its HIV epidemic and is the only high-income country among the top 10 countries most affected by HIV.”
The 6-paper series’ release comes 2 years after the launch of the US Department of Health and Human Services’ (HHS) announcement of its goal to reduce HIV transmissions by at least 90% by 2030.
The authors analyzed publicly available HIV surveillance and census data to describe current prevalence and new HIV diagnoses by region, race, ethnicity, and age, as well as trends in those categories over time. They also reviewed literature to “explore the reasons” for the distribution of cases and important disparities in prevalence. Among other things, the researchers found “pronounced” racial, sexual, and gender disparities, “substantial” gaps in domestic program funding, and a “patchwork healthcare system” that limited access to treatment and prevention services.
Although when it began, the HIV epidemic was focused largely on the bicoastal big cities, mainly New York and San Francisco, in recent years the South has been hit particularly hard, with 52% of new HIV transmissions in 2018, despite representing only 37% of the US population. Six Southern states (Florida, Georgia, Louisiana, Maryland, Mississippi, and Tennessee) and the District of Columbia had the highest annual HIV diagnosis rates between 2010 and 2018, likely reflecting the higher burden of infection among black residents: In 2018, 38% of all new HIV diagnoses among men who have sex with men (MSM) were in the black population, and 63% of those were in the South.
The South’s HIV problem is intensified by disparities, the report says, that are probably driven by the restricted expansion of Medicaid, health care provider shortages, low health literacy, and stigma. The South also has the lowest number of pre-exposure prophylaxis (PrEP) users per new HIV diagnosis, in part because of the longer distances to PrEP services relative to other regions. More than half of MSM who live at least 60 minutes away from PrEP services live in the South. While HIV in the rural South largely is due to sexual transmission, the researchers note, the largest clusters of the concurrent opioid epidemic have been detected in rural and periurban counties of West Virginia and Indiana.
Identifying HIV transmission clusters and outbreaks has traditionally been challenging for several reasons, the researchers say, including delays between infection and diagnosis, mobility of populations, and limitations in tracing sex and drug partners. They suggest that analysis of molecular data can help overcome some of those barriers, making it possible to identify clusters of ongoing HIV transmission.
The report also recommends other approaches to better understand and respond to ongoing HIV infections, such as mapping and data visualization, telemedicine, and automated data systems to facilitate linkage to care. However, the authors add, gaps in data and data systems remain that prevent full understanding of some key impacts of the epidemic. But any interventions to promote HIV prevention and treatment adherence, the authors suggested, should take a multifaceted approach and address the whole individual.
Chris Beyrer, MD, MPH, investigator at the Johns Hopkins Bloomberg School of Public Health, Baltimore, and a lead author on the series, says, “We have incredible tools to prevent and treat HIV, but people may not fully utilize them if they are facing personal or structural issues that pose more immediate hardship, like substance use and mental health disorders. You may struggle to take a daily medication if you are facing food insecurity or cannot find affordable treatment for your substance use disorder.”
Anthony Fauci, MD, director of the National Institute of Allergy and Infectious Diseases, and now also Chief Medical Advisor to President Biden, says, “To end the HIV epidemic, we must continue to develop and deploy novel HIV treatment and prevention strategies suited to the different needs and preferences of diverse populations disproportionately affected by HIV. It is also essential that HIV health services continue during the COVID-19 pandemic.”
STD Prevention: We’ve Come Far, but not Far Enough
On any given day in 2018, one in five people had a sexually transmitted infection (STI), according to the US Centers for Disease Control and Prevention’s (CDC) recently released Sexually Transmitted Disease (STD) Surveillance Report, 2018. There were nearly 68 million infections in the US—and 26 million STIs were acquired in that year.
“The CDC report is an important reminder that infectious diseases continue to do what they do best, which is to cause illness and spread from person to person,” says David Aronoff, MD, director, Division of Infectious Diseases, Department of Medicine, Vanderbilt University Medical Center. “Sexually transmitted infections are persistent threats to human health.”
Most of the infections on the CDC’s watchlist were due to the human papillomavirus (HPV), herpes simplex virus-2 (HSV-2), and trichomoniasis. Chlamydia, gonorrhea, HIV, hepatitis B virus, and syphilis followed. Although lower on the list, gonorrhea and syphilis numbers are on the rise to a disquieting degree. Since 2014, gonorrhea cases have increased 63% and syphilis cases, 71%.
Syphilis is still treatable with penicillin. But “the tragedy of poor STI [sexually transmitted infection] control is compounded by the fact that many of the germs that cause STIs are gradually developing more and more resistance to available treatments,” Dr. Aronoff says.
The rise in gonorrhea cases is particularly concerning to many health care providers. “There’s a very limited pipeline of new antibiotics to use if we’re confronted with antibiotic-resistant STIs,” says Ina Park, MD, assistant professor at University of California San Francisco School of Medicine; medical director, California Prevention Training Center; and author of Strange Bedfellows: Adventures in the Science, History and Surprising Secrets of STDs. In the case of gonorrhea, she warns, we’re down to one class of antibiotics. When all conventional therapies fail in cases of multidrug-resistant gonorrhea, patients have to be hospitalized and treated with broad-spectrum IV antibiotics, such as ertapenem. “We really don’t want to have to resort to that for an infection as common as gonorrhea,” she says.
Syphilis’ resurgence in new populations also is a concern. In the ’80s, says Michelle Collins-Ogle, MD, there was an epidemic of syphilis in pregnant women and newborns. Then it “sort of quieted down,” she says, in part because obstetricians and gynecologists and other health care providers did a better job of screening, diagnosing, and treating in that demographic. The latest resurgence is in young men of color who have sex with men—“we didn’t see that coming.”
Women and babies are still vulnerable, though. In one year, according to the CDC, syphilis cases among women of childbearing age leaped 36%. And, alarmingly, since 2014, cases of congenital syphilis have increased 185%. Between 2017 and 2018 alone, newborn deaths due to syphilis increased 22%—a “startling” number, says Gail Bolan, MD, the CDC director of STD prevention, in a release about the surveillance report. “Too many babies are needlessly dying. Every single instance of congenital syphilis is one too many when we have the tools to prevent it.”
Can all STIs be prevented? Can the rising tides be turned? Dr. Aronoff says, “As with the COVID-19 pandemic, STIs provide an important opportunity for us to understand how multiple factors can contribute to their spread and difficulty controlling.” Drug use, poverty, unstable housing, and stigma can all reduce access to STD prevention and care, he says. “And, as we’ve seen with COVID-19, under-resourced public health programs can also foster epidemics and pandemics of STIs.” Moreover, he adds, many public health programs at the state and local level have been subjected to budget cuts, which translates into less control of disease.
Some STI rates have been reduced with, for instance, antiretrovirals for HIV/AIDS and the HPV vaccine. But there’s still ground to cover, and new patient groups to protect. Nearly half of all new infections in 2018 were in young people aged 15 to 24 years. Not only is it another dangerous trend, it is an expensive one. Chlamydia, gonorrhea, and syphilis combined accounted for $1.1 billion in direct medical costs in 2018, the CDC report says, and care for young people aged 15 to 24 made up about 60% of those costs.
“Low or decreasing rates of condom use among vulnerable groups, including young people and gay and bisexual men, play important roles in driving ongoing STI rates,” Aronoff says. In part, that’s due to lack of comprehensive sex education, a lack that’s taking a huge toll.
“Remember now, we basically cut out a lot of the sex education. It doesn’t exist,” says Dr. Collins-Ogle. She has run clinics for several decades, and says she continually sees young male patients who don’t know how to use a condom. We know more now, though, she points out. “Back in the ‘80s, we didn’t have a direct correlation between STIs and AIDS. Now we know that having syphilis, for example, predisposes you to HIV acquisition. We also know that having HSV2, for example, predisposes you to HIV.”
It’s an ongoing battle, though, with each new generation of pathogens—and people. And as the CDC report shows, it’s like fighting a Hydra: When one infection is wrestled to the ground, another rears its head. There’s no time to rest on laurels. “Having highly contagious infections caused by difficult or impossible-to-treat microbes,” says David Aronoff, “is not a future I would wish on anyone
On any given day in 2018, one in five people had a sexually transmitted infection (STI), according to the US Centers for Disease Control and Prevention’s (CDC) recently released Sexually Transmitted Disease (STD) Surveillance Report, 2018. There were nearly 68 million infections in the US—and 26 million STIs were acquired in that year.
“The CDC report is an important reminder that infectious diseases continue to do what they do best, which is to cause illness and spread from person to person,” says David Aronoff, MD, director, Division of Infectious Diseases, Department of Medicine, Vanderbilt University Medical Center. “Sexually transmitted infections are persistent threats to human health.”
Most of the infections on the CDC’s watchlist were due to the human papillomavirus (HPV), herpes simplex virus-2 (HSV-2), and trichomoniasis. Chlamydia, gonorrhea, HIV, hepatitis B virus, and syphilis followed. Although lower on the list, gonorrhea and syphilis numbers are on the rise to a disquieting degree. Since 2014, gonorrhea cases have increased 63% and syphilis cases, 71%.
Syphilis is still treatable with penicillin. But “the tragedy of poor STI [sexually transmitted infection] control is compounded by the fact that many of the germs that cause STIs are gradually developing more and more resistance to available treatments,” Dr. Aronoff says.
The rise in gonorrhea cases is particularly concerning to many health care providers. “There’s a very limited pipeline of new antibiotics to use if we’re confronted with antibiotic-resistant STIs,” says Ina Park, MD, assistant professor at University of California San Francisco School of Medicine; medical director, California Prevention Training Center; and author of Strange Bedfellows: Adventures in the Science, History and Surprising Secrets of STDs. In the case of gonorrhea, she warns, we’re down to one class of antibiotics. When all conventional therapies fail in cases of multidrug-resistant gonorrhea, patients have to be hospitalized and treated with broad-spectrum IV antibiotics, such as ertapenem. “We really don’t want to have to resort to that for an infection as common as gonorrhea,” she says.
Syphilis’ resurgence in new populations also is a concern. In the ’80s, says Michelle Collins-Ogle, MD, there was an epidemic of syphilis in pregnant women and newborns. Then it “sort of quieted down,” she says, in part because obstetricians and gynecologists and other health care providers did a better job of screening, diagnosing, and treating in that demographic. The latest resurgence is in young men of color who have sex with men—“we didn’t see that coming.”
Women and babies are still vulnerable, though. In one year, according to the CDC, syphilis cases among women of childbearing age leaped 36%. And, alarmingly, since 2014, cases of congenital syphilis have increased 185%. Between 2017 and 2018 alone, newborn deaths due to syphilis increased 22%—a “startling” number, says Gail Bolan, MD, the CDC director of STD prevention, in a release about the surveillance report. “Too many babies are needlessly dying. Every single instance of congenital syphilis is one too many when we have the tools to prevent it.”
Can all STIs be prevented? Can the rising tides be turned? Dr. Aronoff says, “As with the COVID-19 pandemic, STIs provide an important opportunity for us to understand how multiple factors can contribute to their spread and difficulty controlling.” Drug use, poverty, unstable housing, and stigma can all reduce access to STD prevention and care, he says. “And, as we’ve seen with COVID-19, under-resourced public health programs can also foster epidemics and pandemics of STIs.” Moreover, he adds, many public health programs at the state and local level have been subjected to budget cuts, which translates into less control of disease.
Some STI rates have been reduced with, for instance, antiretrovirals for HIV/AIDS and the HPV vaccine. But there’s still ground to cover, and new patient groups to protect. Nearly half of all new infections in 2018 were in young people aged 15 to 24 years. Not only is it another dangerous trend, it is an expensive one. Chlamydia, gonorrhea, and syphilis combined accounted for $1.1 billion in direct medical costs in 2018, the CDC report says, and care for young people aged 15 to 24 made up about 60% of those costs.
“Low or decreasing rates of condom use among vulnerable groups, including young people and gay and bisexual men, play important roles in driving ongoing STI rates,” Aronoff says. In part, that’s due to lack of comprehensive sex education, a lack that’s taking a huge toll.
“Remember now, we basically cut out a lot of the sex education. It doesn’t exist,” says Dr. Collins-Ogle. She has run clinics for several decades, and says she continually sees young male patients who don’t know how to use a condom. We know more now, though, she points out. “Back in the ‘80s, we didn’t have a direct correlation between STIs and AIDS. Now we know that having syphilis, for example, predisposes you to HIV acquisition. We also know that having HSV2, for example, predisposes you to HIV.”
It’s an ongoing battle, though, with each new generation of pathogens—and people. And as the CDC report shows, it’s like fighting a Hydra: When one infection is wrestled to the ground, another rears its head. There’s no time to rest on laurels. “Having highly contagious infections caused by difficult or impossible-to-treat microbes,” says David Aronoff, “is not a future I would wish on anyone
On any given day in 2018, one in five people had a sexually transmitted infection (STI), according to the US Centers for Disease Control and Prevention’s (CDC) recently released Sexually Transmitted Disease (STD) Surveillance Report, 2018. There were nearly 68 million infections in the US—and 26 million STIs were acquired in that year.
“The CDC report is an important reminder that infectious diseases continue to do what they do best, which is to cause illness and spread from person to person,” says David Aronoff, MD, director, Division of Infectious Diseases, Department of Medicine, Vanderbilt University Medical Center. “Sexually transmitted infections are persistent threats to human health.”
Most of the infections on the CDC’s watchlist were due to the human papillomavirus (HPV), herpes simplex virus-2 (HSV-2), and trichomoniasis. Chlamydia, gonorrhea, HIV, hepatitis B virus, and syphilis followed. Although lower on the list, gonorrhea and syphilis numbers are on the rise to a disquieting degree. Since 2014, gonorrhea cases have increased 63% and syphilis cases, 71%.
Syphilis is still treatable with penicillin. But “the tragedy of poor STI [sexually transmitted infection] control is compounded by the fact that many of the germs that cause STIs are gradually developing more and more resistance to available treatments,” Dr. Aronoff says.
The rise in gonorrhea cases is particularly concerning to many health care providers. “There’s a very limited pipeline of new antibiotics to use if we’re confronted with antibiotic-resistant STIs,” says Ina Park, MD, assistant professor at University of California San Francisco School of Medicine; medical director, California Prevention Training Center; and author of Strange Bedfellows: Adventures in the Science, History and Surprising Secrets of STDs. In the case of gonorrhea, she warns, we’re down to one class of antibiotics. When all conventional therapies fail in cases of multidrug-resistant gonorrhea, patients have to be hospitalized and treated with broad-spectrum IV antibiotics, such as ertapenem. “We really don’t want to have to resort to that for an infection as common as gonorrhea,” she says.
Syphilis’ resurgence in new populations also is a concern. In the ’80s, says Michelle Collins-Ogle, MD, there was an epidemic of syphilis in pregnant women and newborns. Then it “sort of quieted down,” she says, in part because obstetricians and gynecologists and other health care providers did a better job of screening, diagnosing, and treating in that demographic. The latest resurgence is in young men of color who have sex with men—“we didn’t see that coming.”
Women and babies are still vulnerable, though. In one year, according to the CDC, syphilis cases among women of childbearing age leaped 36%. And, alarmingly, since 2014, cases of congenital syphilis have increased 185%. Between 2017 and 2018 alone, newborn deaths due to syphilis increased 22%—a “startling” number, says Gail Bolan, MD, the CDC director of STD prevention, in a release about the surveillance report. “Too many babies are needlessly dying. Every single instance of congenital syphilis is one too many when we have the tools to prevent it.”
Can all STIs be prevented? Can the rising tides be turned? Dr. Aronoff says, “As with the COVID-19 pandemic, STIs provide an important opportunity for us to understand how multiple factors can contribute to their spread and difficulty controlling.” Drug use, poverty, unstable housing, and stigma can all reduce access to STD prevention and care, he says. “And, as we’ve seen with COVID-19, under-resourced public health programs can also foster epidemics and pandemics of STIs.” Moreover, he adds, many public health programs at the state and local level have been subjected to budget cuts, which translates into less control of disease.
Some STI rates have been reduced with, for instance, antiretrovirals for HIV/AIDS and the HPV vaccine. But there’s still ground to cover, and new patient groups to protect. Nearly half of all new infections in 2018 were in young people aged 15 to 24 years. Not only is it another dangerous trend, it is an expensive one. Chlamydia, gonorrhea, and syphilis combined accounted for $1.1 billion in direct medical costs in 2018, the CDC report says, and care for young people aged 15 to 24 made up about 60% of those costs.
“Low or decreasing rates of condom use among vulnerable groups, including young people and gay and bisexual men, play important roles in driving ongoing STI rates,” Aronoff says. In part, that’s due to lack of comprehensive sex education, a lack that’s taking a huge toll.
“Remember now, we basically cut out a lot of the sex education. It doesn’t exist,” says Dr. Collins-Ogle. She has run clinics for several decades, and says she continually sees young male patients who don’t know how to use a condom. We know more now, though, she points out. “Back in the ‘80s, we didn’t have a direct correlation between STIs and AIDS. Now we know that having syphilis, for example, predisposes you to HIV acquisition. We also know that having HSV2, for example, predisposes you to HIV.”
It’s an ongoing battle, though, with each new generation of pathogens—and people. And as the CDC report shows, it’s like fighting a Hydra: When one infection is wrestled to the ground, another rears its head. There’s no time to rest on laurels. “Having highly contagious infections caused by difficult or impossible-to-treat microbes,” says David Aronoff, “is not a future I would wish on anyone
Assessing Risk for Amputation Patients During a Pandemic
Risk assessment becomes more complex during a pandemic—but even more necessary. Researchers from Virginia Commonwealth University and Hunter Holmes McGuire Veterans Afffairs Medical Center who studied a population of veterans who underwent leg amputation found that “preoperative testing may not be a feasible and well-applied standard, making risk assessment in the setting of a pandemic even more crucial for surgeons undertaking lower extremity amputations in this high-risk population.”
In their study, the researchers found that a majority of the patients had one or more risk factor from the list published by the European Centre for Disease Prevention and Control (ECDC). What’s more, based on their data, the researchers say veteran amputees are at a much higher risk for complications and negative outcomes if infected with COVID-19, compared with the general population.
Of 50,083 veterans who needed nontraumatic lower extremity amputations between 1999 and 2018, 82% of those with above-knee amputations and 89% of those with below-knee amputations had at least one ECDC risk factor comorbidity. Hypertension and diabetes were the two most prevalent conditions in all cohorts, regardless of race.
Between 40% and 50% of the patients studied were current or past smokers, “well beyond the prevalence of smoking” in the general US population,” the researchers say. One quarter of the veterans were Black. That also is a greater proportion than the proportion of Black patients in the national male veteran population; race is an “especially concerning” potential COVID-19 progression factor, the researchers say.
A year after the COVID-19 pandemic began, the researchers examined the association of Risk Analysis Index scores with postoperative outcomes in 47,197 patients who underwent lower extremity amputation: 27,098 below the knee and 20,099 above the knee amputations.
Frailty was associated with increased rates of major cardiac, pulmonary, and renal complications, as well as sepsis, intubation greater than 48 hours, reintubation, and increased length of stay. Higher frailty scores were associated with up to triple the likelihood of a postoperative complication and up to 32 times likelihood of death within 30 days.
In a previous study, the researchers concluded that standardized frailty indicators might be particularly relevant in a pandemic that has a heavy impact in elderly patients with comorbidities. The risk factors for COVID-19, they note, are similar to many of the factors assessed in surgical frailty scores. Surgical frailty and its assessment, they add, have become “essential considerations” in perioperative management for aging patients.
Risk assessment becomes more complex during a pandemic—but even more necessary. Researchers from Virginia Commonwealth University and Hunter Holmes McGuire Veterans Afffairs Medical Center who studied a population of veterans who underwent leg amputation found that “preoperative testing may not be a feasible and well-applied standard, making risk assessment in the setting of a pandemic even more crucial for surgeons undertaking lower extremity amputations in this high-risk population.”
In their study, the researchers found that a majority of the patients had one or more risk factor from the list published by the European Centre for Disease Prevention and Control (ECDC). What’s more, based on their data, the researchers say veteran amputees are at a much higher risk for complications and negative outcomes if infected with COVID-19, compared with the general population.
Of 50,083 veterans who needed nontraumatic lower extremity amputations between 1999 and 2018, 82% of those with above-knee amputations and 89% of those with below-knee amputations had at least one ECDC risk factor comorbidity. Hypertension and diabetes were the two most prevalent conditions in all cohorts, regardless of race.
Between 40% and 50% of the patients studied were current or past smokers, “well beyond the prevalence of smoking” in the general US population,” the researchers say. One quarter of the veterans were Black. That also is a greater proportion than the proportion of Black patients in the national male veteran population; race is an “especially concerning” potential COVID-19 progression factor, the researchers say.
A year after the COVID-19 pandemic began, the researchers examined the association of Risk Analysis Index scores with postoperative outcomes in 47,197 patients who underwent lower extremity amputation: 27,098 below the knee and 20,099 above the knee amputations.
Frailty was associated with increased rates of major cardiac, pulmonary, and renal complications, as well as sepsis, intubation greater than 48 hours, reintubation, and increased length of stay. Higher frailty scores were associated with up to triple the likelihood of a postoperative complication and up to 32 times likelihood of death within 30 days.
In a previous study, the researchers concluded that standardized frailty indicators might be particularly relevant in a pandemic that has a heavy impact in elderly patients with comorbidities. The risk factors for COVID-19, they note, are similar to many of the factors assessed in surgical frailty scores. Surgical frailty and its assessment, they add, have become “essential considerations” in perioperative management for aging patients.
Risk assessment becomes more complex during a pandemic—but even more necessary. Researchers from Virginia Commonwealth University and Hunter Holmes McGuire Veterans Afffairs Medical Center who studied a population of veterans who underwent leg amputation found that “preoperative testing may not be a feasible and well-applied standard, making risk assessment in the setting of a pandemic even more crucial for surgeons undertaking lower extremity amputations in this high-risk population.”
In their study, the researchers found that a majority of the patients had one or more risk factor from the list published by the European Centre for Disease Prevention and Control (ECDC). What’s more, based on their data, the researchers say veteran amputees are at a much higher risk for complications and negative outcomes if infected with COVID-19, compared with the general population.
Of 50,083 veterans who needed nontraumatic lower extremity amputations between 1999 and 2018, 82% of those with above-knee amputations and 89% of those with below-knee amputations had at least one ECDC risk factor comorbidity. Hypertension and diabetes were the two most prevalent conditions in all cohorts, regardless of race.
Between 40% and 50% of the patients studied were current or past smokers, “well beyond the prevalence of smoking” in the general US population,” the researchers say. One quarter of the veterans were Black. That also is a greater proportion than the proportion of Black patients in the national male veteran population; race is an “especially concerning” potential COVID-19 progression factor, the researchers say.
A year after the COVID-19 pandemic began, the researchers examined the association of Risk Analysis Index scores with postoperative outcomes in 47,197 patients who underwent lower extremity amputation: 27,098 below the knee and 20,099 above the knee amputations.
Frailty was associated with increased rates of major cardiac, pulmonary, and renal complications, as well as sepsis, intubation greater than 48 hours, reintubation, and increased length of stay. Higher frailty scores were associated with up to triple the likelihood of a postoperative complication and up to 32 times likelihood of death within 30 days.
In a previous study, the researchers concluded that standardized frailty indicators might be particularly relevant in a pandemic that has a heavy impact in elderly patients with comorbidities. The risk factors for COVID-19, they note, are similar to many of the factors assessed in surgical frailty scores. Surgical frailty and its assessment, they add, have become “essential considerations” in perioperative management for aging patients.
Pink papule on thigh

A deep-shave biopsy indicated that this was an inflamed/irritated solitary neurofibroma. Basal cell carcinoma, inflamed nevus, and Merkel cell carcinoma were also considered.
Most often manifesting in adults, solitary neurofibromas are common nonencapsulated, soft to firm papules that range in size from 2 mm to 2 cm. Solitary neurofibromas are benign and work-up for systemic neurofibromatosis is not indicated. However, if a patient presents with multiple neurofibromas, axillary freckling, or multiple café au lait macules, systemic disease should be considered, followed by molecular testing and/or referral to a medical geneticist or neurofibromatosis clinic.
Although both the triage amalgamated diagnostic algorithm and the 2-step dermoscopy algorithm suggested this lesion was higher risk, it was ultimately found to be benign. This case highlights areas in which dermoscopy and physical exam lack specificity, but this trade-off increases the sensitivity of an algorithmic approach. Solitary pink papules can include some subtle, but fearsome, diagnoses and deserve close attention. In this case, the biopsy not only helped confirm the diagnosis, but it also alleviated the discomfort caused by the neurofibroma.
Text courtesy of Jonathan Karnes, MD, medical director, MDFMR Dermatology Services, Augusta, ME. Photos courtesy of Jonathan Karnes, MD (copyright retained).
1. Geller S, Pulitzer M, Brady MS, et al. Dermoscopic assessment of vascular structures in solitary small pink lesions—differentiating between good and evil. Dermatol Pract Concept. 2017;7:47-50. doi: 10.5826/dpc.0703a10

A deep-shave biopsy indicated that this was an inflamed/irritated solitary neurofibroma. Basal cell carcinoma, inflamed nevus, and Merkel cell carcinoma were also considered.
Most often manifesting in adults, solitary neurofibromas are common nonencapsulated, soft to firm papules that range in size from 2 mm to 2 cm. Solitary neurofibromas are benign and work-up for systemic neurofibromatosis is not indicated. However, if a patient presents with multiple neurofibromas, axillary freckling, or multiple café au lait macules, systemic disease should be considered, followed by molecular testing and/or referral to a medical geneticist or neurofibromatosis clinic.
Although both the triage amalgamated diagnostic algorithm and the 2-step dermoscopy algorithm suggested this lesion was higher risk, it was ultimately found to be benign. This case highlights areas in which dermoscopy and physical exam lack specificity, but this trade-off increases the sensitivity of an algorithmic approach. Solitary pink papules can include some subtle, but fearsome, diagnoses and deserve close attention. In this case, the biopsy not only helped confirm the diagnosis, but it also alleviated the discomfort caused by the neurofibroma.
Text courtesy of Jonathan Karnes, MD, medical director, MDFMR Dermatology Services, Augusta, ME. Photos courtesy of Jonathan Karnes, MD (copyright retained).

A deep-shave biopsy indicated that this was an inflamed/irritated solitary neurofibroma. Basal cell carcinoma, inflamed nevus, and Merkel cell carcinoma were also considered.
Most often manifesting in adults, solitary neurofibromas are common nonencapsulated, soft to firm papules that range in size from 2 mm to 2 cm. Solitary neurofibromas are benign and work-up for systemic neurofibromatosis is not indicated. However, if a patient presents with multiple neurofibromas, axillary freckling, or multiple café au lait macules, systemic disease should be considered, followed by molecular testing and/or referral to a medical geneticist or neurofibromatosis clinic.
Although both the triage amalgamated diagnostic algorithm and the 2-step dermoscopy algorithm suggested this lesion was higher risk, it was ultimately found to be benign. This case highlights areas in which dermoscopy and physical exam lack specificity, but this trade-off increases the sensitivity of an algorithmic approach. Solitary pink papules can include some subtle, but fearsome, diagnoses and deserve close attention. In this case, the biopsy not only helped confirm the diagnosis, but it also alleviated the discomfort caused by the neurofibroma.
Text courtesy of Jonathan Karnes, MD, medical director, MDFMR Dermatology Services, Augusta, ME. Photos courtesy of Jonathan Karnes, MD (copyright retained).
1. Geller S, Pulitzer M, Brady MS, et al. Dermoscopic assessment of vascular structures in solitary small pink lesions—differentiating between good and evil. Dermatol Pract Concept. 2017;7:47-50. doi: 10.5826/dpc.0703a10
1. Geller S, Pulitzer M, Brady MS, et al. Dermoscopic assessment of vascular structures in solitary small pink lesions—differentiating between good and evil. Dermatol Pract Concept. 2017;7:47-50. doi: 10.5826/dpc.0703a10