User login
SNP chips deemed ‘extremely unreliable’ for identifying rare variants
In fact, SNP chips are “extremely unreliable for genotyping very rare pathogenic variants,” and a positive result for such a variant “is more likely to be wrong than right,” researchers reported in the BMJ.
The authors explained that SNP chips are “DNA microarrays that test genetic variation at many hundreds of thousands of specific locations across the genome.” Although SNP chips have proven accurate in identifying common variants, past reports have suggested that SNP chips perform poorly for genotyping rare variants.
To gain more insight, Caroline Wright, PhD, of the University of Exeter (England) and colleagues conducted a large study.
The researchers analyzed data on 49,908 people from the UK Biobank who had SNP chip and next-generation sequencing results, as well as an additional 21 people who purchased consumer genetic tests and shared their data online via the Personal Genome Project.
The researchers compared the SNP chip and sequencing results. They also selected rare pathogenic variants in BRCA1 and BRCA2 for detailed analysis of clinically actionable variants in the UK Biobank, and they assessed BRCA-related cancers in participants using cancer registry data.
Largest evaluation of SNP chips
SNP chips performed well for common variants, the researchers found. Sensitivity, specificity, positive-predictive value, and negative-predictive value all exceeded 99% for 108,574 common variants.
For rare variants, SNP chips performed poorly, with a positive-predictive value of 16% for variants with a frequency below 0.001% in the UK Biobank.
“The study provides the largest evaluation of the performance of SNP chips for genotyping genetic variants at different frequencies in the population, particularly focusing on very rare variants,” Dr. Wright said. “The biggest surprise was how poorly the SNP chips we evaluated performed for rare variants.”
Dr. Wright noted that there is an inherent problem built into using SNP chip technology to genotype very rare variants.
“The SNP chip technology relies on clustering data from multiple individuals in order to determine what genotype each individual has at a specific position in their genome,” Dr. Wright explained. “Although this method works very well for common variants, the rarer the variant, the harder it is to distinguish from experimental noise.”
False positives and cancer: ‘Don’t trust the results’
The researchers found that, for rare BRCA variants (frequency below 0.01%), SNP chips had a sensitivity of 34.6%, specificity of 98.3%, negative-predictive value of 99.9%, and positive-predictive value of 4.2%.
Rates of BRCA-related cancers in patients with positive SNP chip results were similar to rates in age-matched control subjects because “the vast majority of variants were false positives,” the researchers noted.
“If these variants are incorrectly genotyped – that is, false positives detected – a woman could be offered screening or even prophylactic surgery inappropriately when she is more likely to be at population background risk [for BRCA-related cancers],” Dr. Wright said.
“For very-rare-disease–causing genetic variants, don’t trust the results from SNP chips; for example, those from direct-to-consumer genetic tests. Never use them to guide clinical action without diagnostic validation,” she added.
Heather Hampel, a genetic counselor and researcher at the Ohio State University Comprehensive Cancer Center in Columbus, agreed.
“Positive results on SNP-based tests need to be confirmed by medical-grade genetic testing using a sequencing technology,” she said. “Negative results on an SNP- based test cannot be considered to rule out mutations in BRCA1/2 or other cancer-susceptibility genes, so individuals with strong personal and family histories of cancer should be seen by a genetic counselor to consider medical-grade genetic testing using a sequencing technology.”
Practicing oncologists can trust patients’ prior germline genetic test results if the testing was performed in a cancer genetics clinic, which uses sequencing-based technologies, Ms. Hampel noted.
“If the test was performed before 2013, there are likely new genes that have been discovered for which their patient was not tested, and repeat testing may be warranted,” Ms. Hampel said. “A referral to a cancer genetic counselor would be appropriate.”
Ms. Hampel disclosed relationships with Genome Medical, GI OnDemand, Invitae Genetics, and Promega. Dr. Wright and her coauthors disclosed no conflicts of interest. The group’s research was conducted using the UK Biobank and the University of Exeter High-Performance Computing, with funding from the Wellcome Trust and the National Institute for Health Research.
In fact, SNP chips are “extremely unreliable for genotyping very rare pathogenic variants,” and a positive result for such a variant “is more likely to be wrong than right,” researchers reported in the BMJ.
The authors explained that SNP chips are “DNA microarrays that test genetic variation at many hundreds of thousands of specific locations across the genome.” Although SNP chips have proven accurate in identifying common variants, past reports have suggested that SNP chips perform poorly for genotyping rare variants.
To gain more insight, Caroline Wright, PhD, of the University of Exeter (England) and colleagues conducted a large study.
The researchers analyzed data on 49,908 people from the UK Biobank who had SNP chip and next-generation sequencing results, as well as an additional 21 people who purchased consumer genetic tests and shared their data online via the Personal Genome Project.
The researchers compared the SNP chip and sequencing results. They also selected rare pathogenic variants in BRCA1 and BRCA2 for detailed analysis of clinically actionable variants in the UK Biobank, and they assessed BRCA-related cancers in participants using cancer registry data.
Largest evaluation of SNP chips
SNP chips performed well for common variants, the researchers found. Sensitivity, specificity, positive-predictive value, and negative-predictive value all exceeded 99% for 108,574 common variants.
For rare variants, SNP chips performed poorly, with a positive-predictive value of 16% for variants with a frequency below 0.001% in the UK Biobank.
“The study provides the largest evaluation of the performance of SNP chips for genotyping genetic variants at different frequencies in the population, particularly focusing on very rare variants,” Dr. Wright said. “The biggest surprise was how poorly the SNP chips we evaluated performed for rare variants.”
Dr. Wright noted that there is an inherent problem built into using SNP chip technology to genotype very rare variants.
“The SNP chip technology relies on clustering data from multiple individuals in order to determine what genotype each individual has at a specific position in their genome,” Dr. Wright explained. “Although this method works very well for common variants, the rarer the variant, the harder it is to distinguish from experimental noise.”
False positives and cancer: ‘Don’t trust the results’
The researchers found that, for rare BRCA variants (frequency below 0.01%), SNP chips had a sensitivity of 34.6%, specificity of 98.3%, negative-predictive value of 99.9%, and positive-predictive value of 4.2%.
Rates of BRCA-related cancers in patients with positive SNP chip results were similar to rates in age-matched control subjects because “the vast majority of variants were false positives,” the researchers noted.
“If these variants are incorrectly genotyped – that is, false positives detected – a woman could be offered screening or even prophylactic surgery inappropriately when she is more likely to be at population background risk [for BRCA-related cancers],” Dr. Wright said.
“For very-rare-disease–causing genetic variants, don’t trust the results from SNP chips; for example, those from direct-to-consumer genetic tests. Never use them to guide clinical action without diagnostic validation,” she added.
Heather Hampel, a genetic counselor and researcher at the Ohio State University Comprehensive Cancer Center in Columbus, agreed.
“Positive results on SNP-based tests need to be confirmed by medical-grade genetic testing using a sequencing technology,” she said. “Negative results on an SNP- based test cannot be considered to rule out mutations in BRCA1/2 or other cancer-susceptibility genes, so individuals with strong personal and family histories of cancer should be seen by a genetic counselor to consider medical-grade genetic testing using a sequencing technology.”
Practicing oncologists can trust patients’ prior germline genetic test results if the testing was performed in a cancer genetics clinic, which uses sequencing-based technologies, Ms. Hampel noted.
“If the test was performed before 2013, there are likely new genes that have been discovered for which their patient was not tested, and repeat testing may be warranted,” Ms. Hampel said. “A referral to a cancer genetic counselor would be appropriate.”
Ms. Hampel disclosed relationships with Genome Medical, GI OnDemand, Invitae Genetics, and Promega. Dr. Wright and her coauthors disclosed no conflicts of interest. The group’s research was conducted using the UK Biobank and the University of Exeter High-Performance Computing, with funding from the Wellcome Trust and the National Institute for Health Research.
In fact, SNP chips are “extremely unreliable for genotyping very rare pathogenic variants,” and a positive result for such a variant “is more likely to be wrong than right,” researchers reported in the BMJ.
The authors explained that SNP chips are “DNA microarrays that test genetic variation at many hundreds of thousands of specific locations across the genome.” Although SNP chips have proven accurate in identifying common variants, past reports have suggested that SNP chips perform poorly for genotyping rare variants.
To gain more insight, Caroline Wright, PhD, of the University of Exeter (England) and colleagues conducted a large study.
The researchers analyzed data on 49,908 people from the UK Biobank who had SNP chip and next-generation sequencing results, as well as an additional 21 people who purchased consumer genetic tests and shared their data online via the Personal Genome Project.
The researchers compared the SNP chip and sequencing results. They also selected rare pathogenic variants in BRCA1 and BRCA2 for detailed analysis of clinically actionable variants in the UK Biobank, and they assessed BRCA-related cancers in participants using cancer registry data.
Largest evaluation of SNP chips
SNP chips performed well for common variants, the researchers found. Sensitivity, specificity, positive-predictive value, and negative-predictive value all exceeded 99% for 108,574 common variants.
For rare variants, SNP chips performed poorly, with a positive-predictive value of 16% for variants with a frequency below 0.001% in the UK Biobank.
“The study provides the largest evaluation of the performance of SNP chips for genotyping genetic variants at different frequencies in the population, particularly focusing on very rare variants,” Dr. Wright said. “The biggest surprise was how poorly the SNP chips we evaluated performed for rare variants.”
Dr. Wright noted that there is an inherent problem built into using SNP chip technology to genotype very rare variants.
“The SNP chip technology relies on clustering data from multiple individuals in order to determine what genotype each individual has at a specific position in their genome,” Dr. Wright explained. “Although this method works very well for common variants, the rarer the variant, the harder it is to distinguish from experimental noise.”
False positives and cancer: ‘Don’t trust the results’
The researchers found that, for rare BRCA variants (frequency below 0.01%), SNP chips had a sensitivity of 34.6%, specificity of 98.3%, negative-predictive value of 99.9%, and positive-predictive value of 4.2%.
Rates of BRCA-related cancers in patients with positive SNP chip results were similar to rates in age-matched control subjects because “the vast majority of variants were false positives,” the researchers noted.
“If these variants are incorrectly genotyped – that is, false positives detected – a woman could be offered screening or even prophylactic surgery inappropriately when she is more likely to be at population background risk [for BRCA-related cancers],” Dr. Wright said.
“For very-rare-disease–causing genetic variants, don’t trust the results from SNP chips; for example, those from direct-to-consumer genetic tests. Never use them to guide clinical action without diagnostic validation,” she added.
Heather Hampel, a genetic counselor and researcher at the Ohio State University Comprehensive Cancer Center in Columbus, agreed.
“Positive results on SNP-based tests need to be confirmed by medical-grade genetic testing using a sequencing technology,” she said. “Negative results on an SNP- based test cannot be considered to rule out mutations in BRCA1/2 or other cancer-susceptibility genes, so individuals with strong personal and family histories of cancer should be seen by a genetic counselor to consider medical-grade genetic testing using a sequencing technology.”
Practicing oncologists can trust patients’ prior germline genetic test results if the testing was performed in a cancer genetics clinic, which uses sequencing-based technologies, Ms. Hampel noted.
“If the test was performed before 2013, there are likely new genes that have been discovered for which their patient was not tested, and repeat testing may be warranted,” Ms. Hampel said. “A referral to a cancer genetic counselor would be appropriate.”
Ms. Hampel disclosed relationships with Genome Medical, GI OnDemand, Invitae Genetics, and Promega. Dr. Wright and her coauthors disclosed no conflicts of interest. The group’s research was conducted using the UK Biobank and the University of Exeter High-Performance Computing, with funding from the Wellcome Trust and the National Institute for Health Research.
FROM BMJ
Survey explores impact of pandemic on dermatologist happiness, burnout
, according to Medscape’s 2021 Dermatologist Lifestyle, Happiness & Burnout Report.
In addition, 15% reported being burned out, and 3% reported being depressed, yet about half reported being too busy to seek help for burnout and/or depression.
Those are among the key findings from the Medscape report, which was published online on Feb. 19, 2021. More than 12,000 physicians from 29 specialties, including dermatology, participated in the survey, which explores how physicians are coping with burnout, maintaining their personal wellness, and viewing their workplaces and futures amid the COVID-19 pandemic.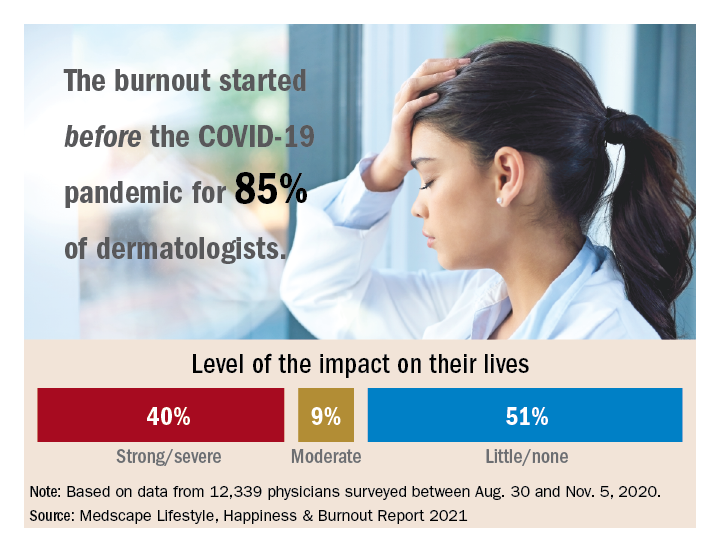
Among dermatologists who reported burnout, 85% said that it started prior to the pandemic, but 15% said it began with the pandemic. That finding resonates with Diane L. Whitaker-Worth, MD, a dermatologist with the University of Connecticut Health Center, Farmington. “A lot of dermatology practices closed down for a while, which was a huge economic hit,” she said in an interview. “I work for a university, so the stress wasn’t quite as bad. We shut down for about a week, but we canceled a lot of visits. We ramped up quickly, and I would say by the summer more people were coming in. Then we got backlogged. We’re still drowning in the number of patients who want to get in sooner, who can’t get an appointment, who need to be seen. It’s unbelievable, and it’s unrelenting.”
Dermatology trainees were also upended, with many residency programs going virtual. “We had to quickly figure out how to continue educating our residents,” said Dr. Whitaker-Worth, who also directs the university’s dermatology residency program. “What’s reasonable to expect them to be doing in clinic? There were fears about becoming infected [with the] virus. Every week, I had double the amount of work in the bureaucratic realm, trying to figure out how we run our clinic and keep our residents safe but learning. That was hard and the residents were really stressed. They were afraid they were going to get pulled to the ICUs. At that time, we didn’t have adequate PPE, and patients and doctors were dying.”
According to the dermatologists who responded to the Medscape survey and reported burnout, the seven chief contributors to burnout were too many bureaucratic tasks (70%); increasing computerization of practice (47%); insufficient compensation/reimbursement (31%); lack of respect from patients (27%); government regulations (26%); lack of respect from administrators/employers, colleagues, or staff (23%); and stress from social distancing/societal issues related to COVID-19 (15%).
“Even though dermatologists seemingly have such a nice schedule, compared to a lot of other doctors, it’s still a very stressful occupation,” said Dr. Whitaker-Worth, who coauthored a study on the topic of burnout among female dermatologists. “It is harder to practice now because there are so many people telling us how we have to do things. That will burn you out over time, when control is taken away, when tasks are handed to you randomly by different entities – insurance companies, the government, the electronic medical record.”
Among dermatologists who self-reported burnout on the survey, 51% said it had no impact on their life, 9% said the impact was moderate, while 40% indicated that it had a strong/severe impact. About half (49%) use exercise to cope with burnout, while other key coping strategies include talking with family members/close friends (40%), playing or listening to music (39%), isolating themselves from others (35%), eating junk food (35%), and drinking alcohol (30%). At the same time, only 6% indicated that they are currently seeking professional health for their burnout and/or depression, and 3% indicated that they are planning to seek professional help. When asked why they hadn’t sought help for their burnout and/or depression, 51% of respondents said they were too busy and 36% said their symptoms weren’t severe enough.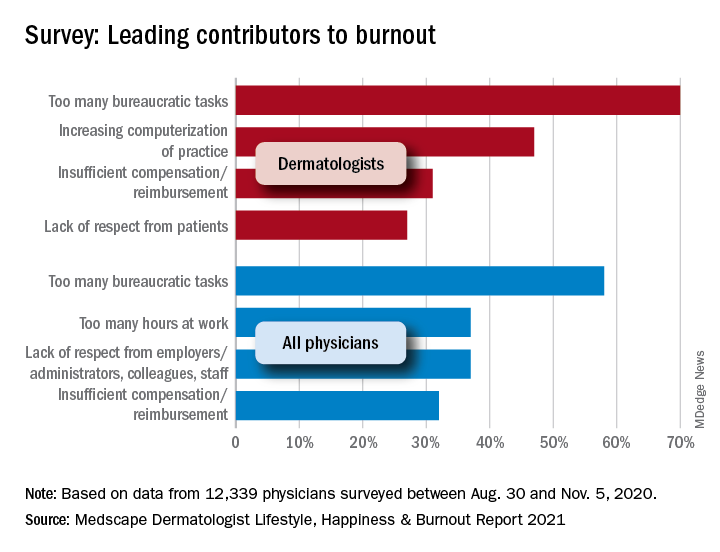
Dr. Whitaker-Worth characterized bureaucratic tasks as “a huge cause” of her burnout, but the larger contributor, she said, is managing her role as wife and mother of four children who are currently at home attending online school classes or working remotely, while she juggles her own work responsibilities. “They were stressed,” she said of her children. “The whole world was stressed. There are exceptions, but I still think that women are mostly shouldering the tasks at home. Even if they’re not doing them, they’re still feeling responsible for them. During the pandemic, every aspect of life became harder. Work was harder. Getting kids focused on school was harder. Doing basic tasks like errands was harder.”
Despite the stress and uncertainty generated by the pandemic, Dr. Whitaker-Worth considers dermatology as one of the happier specialties in medicine. “We still have a little more control of our time,” she said. “We are lucky in that we have reasonable hours, not as much in-house call, and a little more control over our day. I think work-life balance is the main thing that drives burnout – over bureaucracy, over everything.”
, according to Medscape’s 2021 Dermatologist Lifestyle, Happiness & Burnout Report.
In addition, 15% reported being burned out, and 3% reported being depressed, yet about half reported being too busy to seek help for burnout and/or depression.
Those are among the key findings from the Medscape report, which was published online on Feb. 19, 2021. More than 12,000 physicians from 29 specialties, including dermatology, participated in the survey, which explores how physicians are coping with burnout, maintaining their personal wellness, and viewing their workplaces and futures amid the COVID-19 pandemic.
Among dermatologists who reported burnout, 85% said that it started prior to the pandemic, but 15% said it began with the pandemic. That finding resonates with Diane L. Whitaker-Worth, MD, a dermatologist with the University of Connecticut Health Center, Farmington. “A lot of dermatology practices closed down for a while, which was a huge economic hit,” she said in an interview. “I work for a university, so the stress wasn’t quite as bad. We shut down for about a week, but we canceled a lot of visits. We ramped up quickly, and I would say by the summer more people were coming in. Then we got backlogged. We’re still drowning in the number of patients who want to get in sooner, who can’t get an appointment, who need to be seen. It’s unbelievable, and it’s unrelenting.”
Dermatology trainees were also upended, with many residency programs going virtual. “We had to quickly figure out how to continue educating our residents,” said Dr. Whitaker-Worth, who also directs the university’s dermatology residency program. “What’s reasonable to expect them to be doing in clinic? There were fears about becoming infected [with the] virus. Every week, I had double the amount of work in the bureaucratic realm, trying to figure out how we run our clinic and keep our residents safe but learning. That was hard and the residents were really stressed. They were afraid they were going to get pulled to the ICUs. At that time, we didn’t have adequate PPE, and patients and doctors were dying.”
According to the dermatologists who responded to the Medscape survey and reported burnout, the seven chief contributors to burnout were too many bureaucratic tasks (70%); increasing computerization of practice (47%); insufficient compensation/reimbursement (31%); lack of respect from patients (27%); government regulations (26%); lack of respect from administrators/employers, colleagues, or staff (23%); and stress from social distancing/societal issues related to COVID-19 (15%).
“Even though dermatologists seemingly have such a nice schedule, compared to a lot of other doctors, it’s still a very stressful occupation,” said Dr. Whitaker-Worth, who coauthored a study on the topic of burnout among female dermatologists. “It is harder to practice now because there are so many people telling us how we have to do things. That will burn you out over time, when control is taken away, when tasks are handed to you randomly by different entities – insurance companies, the government, the electronic medical record.”
Among dermatologists who self-reported burnout on the survey, 51% said it had no impact on their life, 9% said the impact was moderate, while 40% indicated that it had a strong/severe impact. About half (49%) use exercise to cope with burnout, while other key coping strategies include talking with family members/close friends (40%), playing or listening to music (39%), isolating themselves from others (35%), eating junk food (35%), and drinking alcohol (30%). At the same time, only 6% indicated that they are currently seeking professional health for their burnout and/or depression, and 3% indicated that they are planning to seek professional help. When asked why they hadn’t sought help for their burnout and/or depression, 51% of respondents said they were too busy and 36% said their symptoms weren’t severe enough.
Dr. Whitaker-Worth characterized bureaucratic tasks as “a huge cause” of her burnout, but the larger contributor, she said, is managing her role as wife and mother of four children who are currently at home attending online school classes or working remotely, while she juggles her own work responsibilities. “They were stressed,” she said of her children. “The whole world was stressed. There are exceptions, but I still think that women are mostly shouldering the tasks at home. Even if they’re not doing them, they’re still feeling responsible for them. During the pandemic, every aspect of life became harder. Work was harder. Getting kids focused on school was harder. Doing basic tasks like errands was harder.”
Despite the stress and uncertainty generated by the pandemic, Dr. Whitaker-Worth considers dermatology as one of the happier specialties in medicine. “We still have a little more control of our time,” she said. “We are lucky in that we have reasonable hours, not as much in-house call, and a little more control over our day. I think work-life balance is the main thing that drives burnout – over bureaucracy, over everything.”
, according to Medscape’s 2021 Dermatologist Lifestyle, Happiness & Burnout Report.
In addition, 15% reported being burned out, and 3% reported being depressed, yet about half reported being too busy to seek help for burnout and/or depression.
Those are among the key findings from the Medscape report, which was published online on Feb. 19, 2021. More than 12,000 physicians from 29 specialties, including dermatology, participated in the survey, which explores how physicians are coping with burnout, maintaining their personal wellness, and viewing their workplaces and futures amid the COVID-19 pandemic.
Among dermatologists who reported burnout, 85% said that it started prior to the pandemic, but 15% said it began with the pandemic. That finding resonates with Diane L. Whitaker-Worth, MD, a dermatologist with the University of Connecticut Health Center, Farmington. “A lot of dermatology practices closed down for a while, which was a huge economic hit,” she said in an interview. “I work for a university, so the stress wasn’t quite as bad. We shut down for about a week, but we canceled a lot of visits. We ramped up quickly, and I would say by the summer more people were coming in. Then we got backlogged. We’re still drowning in the number of patients who want to get in sooner, who can’t get an appointment, who need to be seen. It’s unbelievable, and it’s unrelenting.”
Dermatology trainees were also upended, with many residency programs going virtual. “We had to quickly figure out how to continue educating our residents,” said Dr. Whitaker-Worth, who also directs the university’s dermatology residency program. “What’s reasonable to expect them to be doing in clinic? There were fears about becoming infected [with the] virus. Every week, I had double the amount of work in the bureaucratic realm, trying to figure out how we run our clinic and keep our residents safe but learning. That was hard and the residents were really stressed. They were afraid they were going to get pulled to the ICUs. At that time, we didn’t have adequate PPE, and patients and doctors were dying.”
According to the dermatologists who responded to the Medscape survey and reported burnout, the seven chief contributors to burnout were too many bureaucratic tasks (70%); increasing computerization of practice (47%); insufficient compensation/reimbursement (31%); lack of respect from patients (27%); government regulations (26%); lack of respect from administrators/employers, colleagues, or staff (23%); and stress from social distancing/societal issues related to COVID-19 (15%).
“Even though dermatologists seemingly have such a nice schedule, compared to a lot of other doctors, it’s still a very stressful occupation,” said Dr. Whitaker-Worth, who coauthored a study on the topic of burnout among female dermatologists. “It is harder to practice now because there are so many people telling us how we have to do things. That will burn you out over time, when control is taken away, when tasks are handed to you randomly by different entities – insurance companies, the government, the electronic medical record.”
Among dermatologists who self-reported burnout on the survey, 51% said it had no impact on their life, 9% said the impact was moderate, while 40% indicated that it had a strong/severe impact. About half (49%) use exercise to cope with burnout, while other key coping strategies include talking with family members/close friends (40%), playing or listening to music (39%), isolating themselves from others (35%), eating junk food (35%), and drinking alcohol (30%). At the same time, only 6% indicated that they are currently seeking professional health for their burnout and/or depression, and 3% indicated that they are planning to seek professional help. When asked why they hadn’t sought help for their burnout and/or depression, 51% of respondents said they were too busy and 36% said their symptoms weren’t severe enough.
Dr. Whitaker-Worth characterized bureaucratic tasks as “a huge cause” of her burnout, but the larger contributor, she said, is managing her role as wife and mother of four children who are currently at home attending online school classes or working remotely, while she juggles her own work responsibilities. “They were stressed,” she said of her children. “The whole world was stressed. There are exceptions, but I still think that women are mostly shouldering the tasks at home. Even if they’re not doing them, they’re still feeling responsible for them. During the pandemic, every aspect of life became harder. Work was harder. Getting kids focused on school was harder. Doing basic tasks like errands was harder.”
Despite the stress and uncertainty generated by the pandemic, Dr. Whitaker-Worth considers dermatology as one of the happier specialties in medicine. “We still have a little more control of our time,” she said. “We are lucky in that we have reasonable hours, not as much in-house call, and a little more control over our day. I think work-life balance is the main thing that drives burnout – over bureaucracy, over everything.”
Office etiquette: Answering patient phone calls
In my office, one of the many consequences of the COVID-19 pandemic has been a dramatic increase in telephone traffic. I’m sure there are multiple reasons for this, but a major one is calls from patients who remain reluctant to visit our office in person.
Our veteran front-office staff members were adept at handling phone traffic at any level, but most of them retired because of the pandemic. The young folks who replaced them have struggled at times. You would think that millennials, who spend so much time on phones, would have little to learn in that department – until you remember that Twitter, Twitch, and TikTok do not demand polished interpersonal skills.
To address this issue, I have a memo in my office, which I have written, that establishes clear rules for proper professional telephone etiquette. If you want to adapt it for your own office, feel free to do so:
1. You only have one chance to make a first impression. The way we answer it determines, to a significant extent, how the community thinks of us, as people and as health care providers.
2. Answer all incoming calls before the third ring.
3. Answer warmly, enthusiastically, and professionally. Since the caller cannot see you, your voice is the only impression of our office a first-time caller will get.
4. Identify yourself and our office immediately. “Good morning, Doctor Eastern’s office. This is _____. How may I help you?” No one should ever have to ask what office they have reached, or to whom they are speaking.
5. Speak softly. This is to ensure confidentiality (more on that next), and because most people find loud telephone voices unpleasant.
6. Maintaining patient confidentiality is a top priority. It makes patients feel secure about being treated in our office, and it is also the law. Keep in mind that patients and others in the office may be able to overhear your phone conversations. Keep your voice down; never use the phone’s hands-free “speaker” function.
Be cautious about all information that is given over the phone. Don’t disclose any personal information unless you are absolutely certain you are talking to the correct patient. If the caller is not the patient, never discuss personal information without the patient’s permission.
7. Adopt a positive vocabulary – one that focuses on helping people. For example, rather than saying, “I don’t know,” say, “Let me find out for you,” or “I’ll find out who can help you with that.”
8. Offer to take a message if the caller has a question or issue you cannot address. Assure the patient that the appropriate staffer will call back later that day. That way, office workflow is not interrupted, and the patient still receives a prompt (and correct) answer.
9. All messages left overnight with the answering service must be returned as early as possible the very next business day. This is a top priority each morning. Few things annoy callers trying to reach their doctors more than unreturned calls. If the office will be closed for a holiday, or a response will be delayed for any other reason, make sure the service knows, and passes it on to patients.
10. Everyone in the office must answer calls when necessary. If you notice that a phone is ringing and the receptionists are swamped, please answer it; an incoming call must never go unanswered.
11. If the phone rings while you are dealing with a patient in person, the patient in front of you is your first priority. Put the caller on hold, but always ask permission before doing so, and wait for an answer. Never leave a caller on hold for more than a minute or two unless absolutely unavoidable.
12. NEVER answer, “Doctor’s office, please hold.” To a patient, that is even worse than not answering at all. No matter how often your hold message tells callers how important they are, they know they are being ignored. Such encounters never end well: Those who wait will be grumpy and rude when you get back to them; those who hang up will be even more grumpy and rude when they call back. Worst of all are those who don’t call back and seek care elsewhere – often leaving a nasty comment on social media besides.
Dr. Eastern practices dermatology and dermatologic surgery in Belleville, N.J. He is the author of numerous articles and textbook chapters, and is a long-time monthly columnist for Dermatology News. Write to him at [email protected].
In my office, one of the many consequences of the COVID-19 pandemic has been a dramatic increase in telephone traffic. I’m sure there are multiple reasons for this, but a major one is calls from patients who remain reluctant to visit our office in person.
Our veteran front-office staff members were adept at handling phone traffic at any level, but most of them retired because of the pandemic. The young folks who replaced them have struggled at times. You would think that millennials, who spend so much time on phones, would have little to learn in that department – until you remember that Twitter, Twitch, and TikTok do not demand polished interpersonal skills.
To address this issue, I have a memo in my office, which I have written, that establishes clear rules for proper professional telephone etiquette. If you want to adapt it for your own office, feel free to do so:
1. You only have one chance to make a first impression. The way we answer it determines, to a significant extent, how the community thinks of us, as people and as health care providers.
2. Answer all incoming calls before the third ring.
3. Answer warmly, enthusiastically, and professionally. Since the caller cannot see you, your voice is the only impression of our office a first-time caller will get.
4. Identify yourself and our office immediately. “Good morning, Doctor Eastern’s office. This is _____. How may I help you?” No one should ever have to ask what office they have reached, or to whom they are speaking.
5. Speak softly. This is to ensure confidentiality (more on that next), and because most people find loud telephone voices unpleasant.
6. Maintaining patient confidentiality is a top priority. It makes patients feel secure about being treated in our office, and it is also the law. Keep in mind that patients and others in the office may be able to overhear your phone conversations. Keep your voice down; never use the phone’s hands-free “speaker” function.
Be cautious about all information that is given over the phone. Don’t disclose any personal information unless you are absolutely certain you are talking to the correct patient. If the caller is not the patient, never discuss personal information without the patient’s permission.
7. Adopt a positive vocabulary – one that focuses on helping people. For example, rather than saying, “I don’t know,” say, “Let me find out for you,” or “I’ll find out who can help you with that.”
8. Offer to take a message if the caller has a question or issue you cannot address. Assure the patient that the appropriate staffer will call back later that day. That way, office workflow is not interrupted, and the patient still receives a prompt (and correct) answer.
9. All messages left overnight with the answering service must be returned as early as possible the very next business day. This is a top priority each morning. Few things annoy callers trying to reach their doctors more than unreturned calls. If the office will be closed for a holiday, or a response will be delayed for any other reason, make sure the service knows, and passes it on to patients.
10. Everyone in the office must answer calls when necessary. If you notice that a phone is ringing and the receptionists are swamped, please answer it; an incoming call must never go unanswered.
11. If the phone rings while you are dealing with a patient in person, the patient in front of you is your first priority. Put the caller on hold, but always ask permission before doing so, and wait for an answer. Never leave a caller on hold for more than a minute or two unless absolutely unavoidable.
12. NEVER answer, “Doctor’s office, please hold.” To a patient, that is even worse than not answering at all. No matter how often your hold message tells callers how important they are, they know they are being ignored. Such encounters never end well: Those who wait will be grumpy and rude when you get back to them; those who hang up will be even more grumpy and rude when they call back. Worst of all are those who don’t call back and seek care elsewhere – often leaving a nasty comment on social media besides.
Dr. Eastern practices dermatology and dermatologic surgery in Belleville, N.J. He is the author of numerous articles and textbook chapters, and is a long-time monthly columnist for Dermatology News. Write to him at [email protected].
In my office, one of the many consequences of the COVID-19 pandemic has been a dramatic increase in telephone traffic. I’m sure there are multiple reasons for this, but a major one is calls from patients who remain reluctant to visit our office in person.
Our veteran front-office staff members were adept at handling phone traffic at any level, but most of them retired because of the pandemic. The young folks who replaced them have struggled at times. You would think that millennials, who spend so much time on phones, would have little to learn in that department – until you remember that Twitter, Twitch, and TikTok do not demand polished interpersonal skills.
To address this issue, I have a memo in my office, which I have written, that establishes clear rules for proper professional telephone etiquette. If you want to adapt it for your own office, feel free to do so:
1. You only have one chance to make a first impression. The way we answer it determines, to a significant extent, how the community thinks of us, as people and as health care providers.
2. Answer all incoming calls before the third ring.
3. Answer warmly, enthusiastically, and professionally. Since the caller cannot see you, your voice is the only impression of our office a first-time caller will get.
4. Identify yourself and our office immediately. “Good morning, Doctor Eastern’s office. This is _____. How may I help you?” No one should ever have to ask what office they have reached, or to whom they are speaking.
5. Speak softly. This is to ensure confidentiality (more on that next), and because most people find loud telephone voices unpleasant.
6. Maintaining patient confidentiality is a top priority. It makes patients feel secure about being treated in our office, and it is also the law. Keep in mind that patients and others in the office may be able to overhear your phone conversations. Keep your voice down; never use the phone’s hands-free “speaker” function.
Be cautious about all information that is given over the phone. Don’t disclose any personal information unless you are absolutely certain you are talking to the correct patient. If the caller is not the patient, never discuss personal information without the patient’s permission.
7. Adopt a positive vocabulary – one that focuses on helping people. For example, rather than saying, “I don’t know,” say, “Let me find out for you,” or “I’ll find out who can help you with that.”
8. Offer to take a message if the caller has a question or issue you cannot address. Assure the patient that the appropriate staffer will call back later that day. That way, office workflow is not interrupted, and the patient still receives a prompt (and correct) answer.
9. All messages left overnight with the answering service must be returned as early as possible the very next business day. This is a top priority each morning. Few things annoy callers trying to reach their doctors more than unreturned calls. If the office will be closed for a holiday, or a response will be delayed for any other reason, make sure the service knows, and passes it on to patients.
10. Everyone in the office must answer calls when necessary. If you notice that a phone is ringing and the receptionists are swamped, please answer it; an incoming call must never go unanswered.
11. If the phone rings while you are dealing with a patient in person, the patient in front of you is your first priority. Put the caller on hold, but always ask permission before doing so, and wait for an answer. Never leave a caller on hold for more than a minute or two unless absolutely unavoidable.
12. NEVER answer, “Doctor’s office, please hold.” To a patient, that is even worse than not answering at all. No matter how often your hold message tells callers how important they are, they know they are being ignored. Such encounters never end well: Those who wait will be grumpy and rude when you get back to them; those who hang up will be even more grumpy and rude when they call back. Worst of all are those who don’t call back and seek care elsewhere – often leaving a nasty comment on social media besides.
Dr. Eastern practices dermatology and dermatologic surgery in Belleville, N.J. He is the author of numerous articles and textbook chapters, and is a long-time monthly columnist for Dermatology News. Write to him at [email protected].
Can supplementary estrogen relieve MS symptoms in menopausal women?
, a neurologist told colleagues at the meeting held by the Americas Committee for Treatment and Research in Multiple Sclerosis.
This kind of research should explore the effects of aging, including in the brain, and “focus on what is preventable – this dramatic and abrupt loss of estrogen in women with MS,” said Rhonda Voskuhl, MD, of the Brain Research Institute at the University of California, Los Angeles.
“This is a call to action. There’s a huge gap that needs to be filled,” she added in an interview. “Not enough attention has been paid to menopause and cognitive issues in MS and even in healthy women.”
Research has found that many women with MS experience a decline in function during menopause, she said. “They’re having a worsening of their preexisting disabilities,” she noted, due to neurodegeneration.
Dr. Voskuhl highlighted a 2016 study, for instance, that found postmenopausal women with MS on hormone replacement therapy reported better physical function and quality of life than did their counterparts after adjustment for covariates. She also pointed to a 2019 study that concluded that “natural menopause seems to be a turning point to a more progressive phase of MS.”
Estrogen appears to play a significant role. “It’s involved in synaptic plasticity,” she said. “That’s why the disabilities are worsening.”
Dr. Voskuhl supports a year-long, randomized and controlled study of estrogen supplementation in 150-200 participants. The goal, she said, is “not just to prevent loss and bad things from happening but also make improvements.”
In healthy patients, she said, outcomes should include cognitive decline in menopause, cognitive domain outcomes, and region-specific biomarkers in the frontal cortex and hippocampus instead of global cognition and global brain volume. In patients with MS, she said, the focus should be on worsening of disability with emphasis on specific disabilities such as walking and region-specific biomarkers for the motor cortex and spinal cord.
“We need to be looking at cortical gray matter, which we know is responsive to estrogen,” Dr. Voskuhl said. She led a 2018 placebo-controlled study that found women with MS who took estrogen supplements appeared to experience localized sparing of progressive gray matter, which the researchers linked to improved results in cognitive testing. The findings, the study authors wrote, suggest “a clinically relevant, disability-specific biomarker for clinical trials of candidate neuroprotective treatments in MS.”
What about men? Does hormone loss worsen their MS? Dr. Voskuhl said there seems to be a connection between lower levels of testosterone and more disability in men with MS. But their situation is different. Loss of testosterone in men is gradual and happens over decades instead of over the short period of menopause in women, she said.
Jennifer Graves, MD, a neurologist at the University of California, San Diego, agreed that it’s time for further research into estrogen supplementation in MS. As she noted, “we don’t know the exact biological mechanism that might link perimenopause with developing a more progressive type of MS.”
She added: “An overall decrease in estrogen may be at play but there are other biological changes around menopause. We must also take care in studies to try to separate out what might be due to ovarian aging versus other types of aging processes that might be happening at the same time.”
Dr. Voskuhl disclosed that she is an inventor on university patents for use of estriol and estrogen receptor–beta ligands as treatments. Dr. Graves reports no relevant disclosures.
, a neurologist told colleagues at the meeting held by the Americas Committee for Treatment and Research in Multiple Sclerosis.
This kind of research should explore the effects of aging, including in the brain, and “focus on what is preventable – this dramatic and abrupt loss of estrogen in women with MS,” said Rhonda Voskuhl, MD, of the Brain Research Institute at the University of California, Los Angeles.
“This is a call to action. There’s a huge gap that needs to be filled,” she added in an interview. “Not enough attention has been paid to menopause and cognitive issues in MS and even in healthy women.”
Research has found that many women with MS experience a decline in function during menopause, she said. “They’re having a worsening of their preexisting disabilities,” she noted, due to neurodegeneration.
Dr. Voskuhl highlighted a 2016 study, for instance, that found postmenopausal women with MS on hormone replacement therapy reported better physical function and quality of life than did their counterparts after adjustment for covariates. She also pointed to a 2019 study that concluded that “natural menopause seems to be a turning point to a more progressive phase of MS.”
Estrogen appears to play a significant role. “It’s involved in synaptic plasticity,” she said. “That’s why the disabilities are worsening.”
Dr. Voskuhl supports a year-long, randomized and controlled study of estrogen supplementation in 150-200 participants. The goal, she said, is “not just to prevent loss and bad things from happening but also make improvements.”
In healthy patients, she said, outcomes should include cognitive decline in menopause, cognitive domain outcomes, and region-specific biomarkers in the frontal cortex and hippocampus instead of global cognition and global brain volume. In patients with MS, she said, the focus should be on worsening of disability with emphasis on specific disabilities such as walking and region-specific biomarkers for the motor cortex and spinal cord.
“We need to be looking at cortical gray matter, which we know is responsive to estrogen,” Dr. Voskuhl said. She led a 2018 placebo-controlled study that found women with MS who took estrogen supplements appeared to experience localized sparing of progressive gray matter, which the researchers linked to improved results in cognitive testing. The findings, the study authors wrote, suggest “a clinically relevant, disability-specific biomarker for clinical trials of candidate neuroprotective treatments in MS.”
What about men? Does hormone loss worsen their MS? Dr. Voskuhl said there seems to be a connection between lower levels of testosterone and more disability in men with MS. But their situation is different. Loss of testosterone in men is gradual and happens over decades instead of over the short period of menopause in women, she said.
Jennifer Graves, MD, a neurologist at the University of California, San Diego, agreed that it’s time for further research into estrogen supplementation in MS. As she noted, “we don’t know the exact biological mechanism that might link perimenopause with developing a more progressive type of MS.”
She added: “An overall decrease in estrogen may be at play but there are other biological changes around menopause. We must also take care in studies to try to separate out what might be due to ovarian aging versus other types of aging processes that might be happening at the same time.”
Dr. Voskuhl disclosed that she is an inventor on university patents for use of estriol and estrogen receptor–beta ligands as treatments. Dr. Graves reports no relevant disclosures.
, a neurologist told colleagues at the meeting held by the Americas Committee for Treatment and Research in Multiple Sclerosis.
This kind of research should explore the effects of aging, including in the brain, and “focus on what is preventable – this dramatic and abrupt loss of estrogen in women with MS,” said Rhonda Voskuhl, MD, of the Brain Research Institute at the University of California, Los Angeles.
“This is a call to action. There’s a huge gap that needs to be filled,” she added in an interview. “Not enough attention has been paid to menopause and cognitive issues in MS and even in healthy women.”
Research has found that many women with MS experience a decline in function during menopause, she said. “They’re having a worsening of their preexisting disabilities,” she noted, due to neurodegeneration.
Dr. Voskuhl highlighted a 2016 study, for instance, that found postmenopausal women with MS on hormone replacement therapy reported better physical function and quality of life than did their counterparts after adjustment for covariates. She also pointed to a 2019 study that concluded that “natural menopause seems to be a turning point to a more progressive phase of MS.”
Estrogen appears to play a significant role. “It’s involved in synaptic plasticity,” she said. “That’s why the disabilities are worsening.”
Dr. Voskuhl supports a year-long, randomized and controlled study of estrogen supplementation in 150-200 participants. The goal, she said, is “not just to prevent loss and bad things from happening but also make improvements.”
In healthy patients, she said, outcomes should include cognitive decline in menopause, cognitive domain outcomes, and region-specific biomarkers in the frontal cortex and hippocampus instead of global cognition and global brain volume. In patients with MS, she said, the focus should be on worsening of disability with emphasis on specific disabilities such as walking and region-specific biomarkers for the motor cortex and spinal cord.
“We need to be looking at cortical gray matter, which we know is responsive to estrogen,” Dr. Voskuhl said. She led a 2018 placebo-controlled study that found women with MS who took estrogen supplements appeared to experience localized sparing of progressive gray matter, which the researchers linked to improved results in cognitive testing. The findings, the study authors wrote, suggest “a clinically relevant, disability-specific biomarker for clinical trials of candidate neuroprotective treatments in MS.”
What about men? Does hormone loss worsen their MS? Dr. Voskuhl said there seems to be a connection between lower levels of testosterone and more disability in men with MS. But their situation is different. Loss of testosterone in men is gradual and happens over decades instead of over the short period of menopause in women, she said.
Jennifer Graves, MD, a neurologist at the University of California, San Diego, agreed that it’s time for further research into estrogen supplementation in MS. As she noted, “we don’t know the exact biological mechanism that might link perimenopause with developing a more progressive type of MS.”
She added: “An overall decrease in estrogen may be at play but there are other biological changes around menopause. We must also take care in studies to try to separate out what might be due to ovarian aging versus other types of aging processes that might be happening at the same time.”
Dr. Voskuhl disclosed that she is an inventor on university patents for use of estriol and estrogen receptor–beta ligands as treatments. Dr. Graves reports no relevant disclosures.
FROM ACTRIMS FORUM 2021
Reinstating in-person mifepristone administration requirements is harmful to patients and providers
In May 2020, the American College of Obstetricians and Gynecologists (ACOG), along with other organizations and physicians (Council of University Chairs of Obstetrics and Gynecology, New York State Academy of Family Physicians, SisterSong Women of Color Reproductive Justice Collective, Honor MacNaughton, MD), filed a civil action against the US Food and Drug Administration (FDA) and the Department of Health and Human Services (HHS) challenging the requirements of in-person mifepristone dispensing, which was one of the 3 restrictions placed on the medicine as part of mifepristone’s risk evaluation and mitigation strategy (REMS). The requirements, which also include provider certification and patient signatures on specified consent forms, specifically target dosages of mifepristone for use related to abortions and miscarriages but do not apply when prescribing mifepristone for other medical conditions, even with higher doses. During the pandemic, the FDA suspended the REMS requirements for many other medications, including those more toxic than mifepristone. Additionally, the HHS activated a “telemedicine exception” that allows physicians to use telemedicine to satisfy mandatory requirements for prescribing controlled substances, including opioids, while minimizing the patient’s and provider’s risk of exposure to COVID-19 with in-person appointments. Notably, mifepristone for abortion and miscarriage management was excluded from this relaxation of the REMS requirement.
On July 13, 2020, a Federal District Court concluded that the in-person requirements were a “substantial obstacle” for women seeking abortions during the COVID-19 pandemic and granted a preliminary injunction to temporarily stop the FDA’s enforcement of the in-person requirements for mifepristone. We wrote about what that decision meant for ObGyns and urged clinicians to advocate to make the injunction permanent (OBG Manag. 2020;32(12):13-14, 23, 38. doi: 10.12788/obgm.0034.)
From there, however, the FDA worked to reverse that decision, which included applications to the District Court and to the Supreme Court for a stay of the injunction. If successful, this would suspend the injunction while the case was pending. In October, after the Supreme Court deferred review of the application (preferring a review by the lower courts), the District Court upheld the injunction of the in-person requirements citing the worsening pandemic crisis.
In-person requirement re-instated
On January 12, 2021, the United States Supreme Court granted the stay of the District Court’s injunction, which allowed the federal government to enforce the in-person requirement for mifepristone once again. The decision came down to a vote of 6 to 3. As is typical for decisions on stay orders, the court did not release a majority opinion explaining the reasoning behind this decision. In a concurring opinion, Chief Justice John Roberts wrote that the decision was not a judgment of if the requirements for in-person dispensing of mifepristone imposed an undue burden on women seeking an abortion. Instead, the Chief Justice explained that the decision came down to if a District Court could order the FDA to change their regulations based on “the court’s own evaluations of the COVID-19 pandemic,” maintaining that the court could not overrule “the politically accountable entities with the ‘background, competence, and expertise to assess public health.’”1 No other justices joined his opinion.
A worrisome pattern of a conservative supermajority
In her dissent, Justice Sonia Sotomayor criticized the government’s “statistically insignificant, cherry-picked data” and argued that the government did not provide any explanation from an FDA or HHS official explaining why mifepristone’s in-person requirement is more important than the in-person requirements of other drugs that have been waived during the pandemic.2 Therefore, she explained, there is “no reasoned decision” by any health official anywhere on which they can base the decision to grant the stay.
This ruling was the Supreme Court’s first major decision on reproductive health since the confirmation of Justice Amy Coney Barrett and may be an insight into future decisions of the new conservative supermajority on abortion and reproductive health issues. Particularly worrisome is what this decision could mean for stays in abortion cases that dictate whether or not the regulation is enforced during an active case. Even if cases are ruled in favor of patients and abortion providers, if the courts continue to allow enforcement of abortion restrictions during litigation, this could result in permanent closure of abortion clinics and prevent many individuals from accessing safe and legal abortion.
Looking toward the future
In the setting of almost 29 million cases of COVID-19 and more than 526,000 deaths, this stay order requires women seeking a medication abortion to make an appointment at a clinic, risking possible exposure to COVID-19, in order to access mifepristone.3,4 The Biden administration can and should remove the FDA requirement for in-person delivery of mifepristone, which would mitigate the effects of the stay order and allow women to obtain medication abortions during the pandemic.
Take action
- Contact your local ACLU (find them here) or lawyer in your area for assistance navigating the legal landscape to prescribe mifepristone after this stay order
- Minimize a patient’s wait time for mifepristone administration by blocking time in your weekly schedule for patients seeking abortion care
- Work with other providers and health care professionals in your area to submit petitions to the FDA
- FDA v American College of Obstetricians and Gynecologists, 592 US __ (2021)(Roberts, CJ, concurring).
- FDA v American College of Obstetricians and Gynecologists, 592 US __ (2021)(Sotomayor, J, dissenting).
- COVID data tracker. Centers for Disease Control and Prevention website. https://covid.cdc.gov/covid-data-tracker. Accessed March 9, 2021.
- Fulcer IR, Neill S, Bharadwa S, et al. State and federal abortion restrictions increase risk of COVID-19 exposure by mandating unnecessary clinic visits. Contraception. 2020;102:385-391.
In May 2020, the American College of Obstetricians and Gynecologists (ACOG), along with other organizations and physicians (Council of University Chairs of Obstetrics and Gynecology, New York State Academy of Family Physicians, SisterSong Women of Color Reproductive Justice Collective, Honor MacNaughton, MD), filed a civil action against the US Food and Drug Administration (FDA) and the Department of Health and Human Services (HHS) challenging the requirements of in-person mifepristone dispensing, which was one of the 3 restrictions placed on the medicine as part of mifepristone’s risk evaluation and mitigation strategy (REMS). The requirements, which also include provider certification and patient signatures on specified consent forms, specifically target dosages of mifepristone for use related to abortions and miscarriages but do not apply when prescribing mifepristone for other medical conditions, even with higher doses. During the pandemic, the FDA suspended the REMS requirements for many other medications, including those more toxic than mifepristone. Additionally, the HHS activated a “telemedicine exception” that allows physicians to use telemedicine to satisfy mandatory requirements for prescribing controlled substances, including opioids, while minimizing the patient’s and provider’s risk of exposure to COVID-19 with in-person appointments. Notably, mifepristone for abortion and miscarriage management was excluded from this relaxation of the REMS requirement.
On July 13, 2020, a Federal District Court concluded that the in-person requirements were a “substantial obstacle” for women seeking abortions during the COVID-19 pandemic and granted a preliminary injunction to temporarily stop the FDA’s enforcement of the in-person requirements for mifepristone. We wrote about what that decision meant for ObGyns and urged clinicians to advocate to make the injunction permanent (OBG Manag. 2020;32(12):13-14, 23, 38. doi: 10.12788/obgm.0034.)
From there, however, the FDA worked to reverse that decision, which included applications to the District Court and to the Supreme Court for a stay of the injunction. If successful, this would suspend the injunction while the case was pending. In October, after the Supreme Court deferred review of the application (preferring a review by the lower courts), the District Court upheld the injunction of the in-person requirements citing the worsening pandemic crisis.
In-person requirement re-instated
On January 12, 2021, the United States Supreme Court granted the stay of the District Court’s injunction, which allowed the federal government to enforce the in-person requirement for mifepristone once again. The decision came down to a vote of 6 to 3. As is typical for decisions on stay orders, the court did not release a majority opinion explaining the reasoning behind this decision. In a concurring opinion, Chief Justice John Roberts wrote that the decision was not a judgment of if the requirements for in-person dispensing of mifepristone imposed an undue burden on women seeking an abortion. Instead, the Chief Justice explained that the decision came down to if a District Court could order the FDA to change their regulations based on “the court’s own evaluations of the COVID-19 pandemic,” maintaining that the court could not overrule “the politically accountable entities with the ‘background, competence, and expertise to assess public health.’”1 No other justices joined his opinion.
A worrisome pattern of a conservative supermajority
In her dissent, Justice Sonia Sotomayor criticized the government’s “statistically insignificant, cherry-picked data” and argued that the government did not provide any explanation from an FDA or HHS official explaining why mifepristone’s in-person requirement is more important than the in-person requirements of other drugs that have been waived during the pandemic.2 Therefore, she explained, there is “no reasoned decision” by any health official anywhere on which they can base the decision to grant the stay.
This ruling was the Supreme Court’s first major decision on reproductive health since the confirmation of Justice Amy Coney Barrett and may be an insight into future decisions of the new conservative supermajority on abortion and reproductive health issues. Particularly worrisome is what this decision could mean for stays in abortion cases that dictate whether or not the regulation is enforced during an active case. Even if cases are ruled in favor of patients and abortion providers, if the courts continue to allow enforcement of abortion restrictions during litigation, this could result in permanent closure of abortion clinics and prevent many individuals from accessing safe and legal abortion.
Looking toward the future
In the setting of almost 29 million cases of COVID-19 and more than 526,000 deaths, this stay order requires women seeking a medication abortion to make an appointment at a clinic, risking possible exposure to COVID-19, in order to access mifepristone.3,4 The Biden administration can and should remove the FDA requirement for in-person delivery of mifepristone, which would mitigate the effects of the stay order and allow women to obtain medication abortions during the pandemic.
Take action
- Contact your local ACLU (find them here) or lawyer in your area for assistance navigating the legal landscape to prescribe mifepristone after this stay order
- Minimize a patient’s wait time for mifepristone administration by blocking time in your weekly schedule for patients seeking abortion care
- Work with other providers and health care professionals in your area to submit petitions to the FDA
In May 2020, the American College of Obstetricians and Gynecologists (ACOG), along with other organizations and physicians (Council of University Chairs of Obstetrics and Gynecology, New York State Academy of Family Physicians, SisterSong Women of Color Reproductive Justice Collective, Honor MacNaughton, MD), filed a civil action against the US Food and Drug Administration (FDA) and the Department of Health and Human Services (HHS) challenging the requirements of in-person mifepristone dispensing, which was one of the 3 restrictions placed on the medicine as part of mifepristone’s risk evaluation and mitigation strategy (REMS). The requirements, which also include provider certification and patient signatures on specified consent forms, specifically target dosages of mifepristone for use related to abortions and miscarriages but do not apply when prescribing mifepristone for other medical conditions, even with higher doses. During the pandemic, the FDA suspended the REMS requirements for many other medications, including those more toxic than mifepristone. Additionally, the HHS activated a “telemedicine exception” that allows physicians to use telemedicine to satisfy mandatory requirements for prescribing controlled substances, including opioids, while minimizing the patient’s and provider’s risk of exposure to COVID-19 with in-person appointments. Notably, mifepristone for abortion and miscarriage management was excluded from this relaxation of the REMS requirement.
On July 13, 2020, a Federal District Court concluded that the in-person requirements were a “substantial obstacle” for women seeking abortions during the COVID-19 pandemic and granted a preliminary injunction to temporarily stop the FDA’s enforcement of the in-person requirements for mifepristone. We wrote about what that decision meant for ObGyns and urged clinicians to advocate to make the injunction permanent (OBG Manag. 2020;32(12):13-14, 23, 38. doi: 10.12788/obgm.0034.)
From there, however, the FDA worked to reverse that decision, which included applications to the District Court and to the Supreme Court for a stay of the injunction. If successful, this would suspend the injunction while the case was pending. In October, after the Supreme Court deferred review of the application (preferring a review by the lower courts), the District Court upheld the injunction of the in-person requirements citing the worsening pandemic crisis.
In-person requirement re-instated
On January 12, 2021, the United States Supreme Court granted the stay of the District Court’s injunction, which allowed the federal government to enforce the in-person requirement for mifepristone once again. The decision came down to a vote of 6 to 3. As is typical for decisions on stay orders, the court did not release a majority opinion explaining the reasoning behind this decision. In a concurring opinion, Chief Justice John Roberts wrote that the decision was not a judgment of if the requirements for in-person dispensing of mifepristone imposed an undue burden on women seeking an abortion. Instead, the Chief Justice explained that the decision came down to if a District Court could order the FDA to change their regulations based on “the court’s own evaluations of the COVID-19 pandemic,” maintaining that the court could not overrule “the politically accountable entities with the ‘background, competence, and expertise to assess public health.’”1 No other justices joined his opinion.
A worrisome pattern of a conservative supermajority
In her dissent, Justice Sonia Sotomayor criticized the government’s “statistically insignificant, cherry-picked data” and argued that the government did not provide any explanation from an FDA or HHS official explaining why mifepristone’s in-person requirement is more important than the in-person requirements of other drugs that have been waived during the pandemic.2 Therefore, she explained, there is “no reasoned decision” by any health official anywhere on which they can base the decision to grant the stay.
This ruling was the Supreme Court’s first major decision on reproductive health since the confirmation of Justice Amy Coney Barrett and may be an insight into future decisions of the new conservative supermajority on abortion and reproductive health issues. Particularly worrisome is what this decision could mean for stays in abortion cases that dictate whether or not the regulation is enforced during an active case. Even if cases are ruled in favor of patients and abortion providers, if the courts continue to allow enforcement of abortion restrictions during litigation, this could result in permanent closure of abortion clinics and prevent many individuals from accessing safe and legal abortion.
Looking toward the future
In the setting of almost 29 million cases of COVID-19 and more than 526,000 deaths, this stay order requires women seeking a medication abortion to make an appointment at a clinic, risking possible exposure to COVID-19, in order to access mifepristone.3,4 The Biden administration can and should remove the FDA requirement for in-person delivery of mifepristone, which would mitigate the effects of the stay order and allow women to obtain medication abortions during the pandemic.
Take action
- Contact your local ACLU (find them here) or lawyer in your area for assistance navigating the legal landscape to prescribe mifepristone after this stay order
- Minimize a patient’s wait time for mifepristone administration by blocking time in your weekly schedule for patients seeking abortion care
- Work with other providers and health care professionals in your area to submit petitions to the FDA
- FDA v American College of Obstetricians and Gynecologists, 592 US __ (2021)(Roberts, CJ, concurring).
- FDA v American College of Obstetricians and Gynecologists, 592 US __ (2021)(Sotomayor, J, dissenting).
- COVID data tracker. Centers for Disease Control and Prevention website. https://covid.cdc.gov/covid-data-tracker. Accessed March 9, 2021.
- Fulcer IR, Neill S, Bharadwa S, et al. State and federal abortion restrictions increase risk of COVID-19 exposure by mandating unnecessary clinic visits. Contraception. 2020;102:385-391.
- FDA v American College of Obstetricians and Gynecologists, 592 US __ (2021)(Roberts, CJ, concurring).
- FDA v American College of Obstetricians and Gynecologists, 592 US __ (2021)(Sotomayor, J, dissenting).
- COVID data tracker. Centers for Disease Control and Prevention website. https://covid.cdc.gov/covid-data-tracker. Accessed March 9, 2021.
- Fulcer IR, Neill S, Bharadwa S, et al. State and federal abortion restrictions increase risk of COVID-19 exposure by mandating unnecessary clinic visits. Contraception. 2020;102:385-391.
Type 2 diabetes linked to increased risk for Parkinson’s
New analyses of both observational and genetic data have provided “convincing evidence” that type 2 diabetes is associated with an increased risk for Parkinson’s disease.
“The fact that we see the same effects in both types of analysis separately makes it more likely that these results are real – that type 2 diabetes really is a driver of Parkinson’s disease risk,” Alastair Noyce, PhD, senior author of the new studies, said in an interview.
The two analyses are reported in one paper published online March 8 in the journal Movement Disorders.
Dr. Noyce, clinical senior lecturer in the preventive neurology unit at the Wolfson Institute of Preventive Medicine, Queen Mary University of London, explained that his group is interested in risk factors for Parkinson’s disease, particularly those relevant at the population level and which might be modifiable.
“Several studies have looked at diabetes as a risk factor for Parkinson’s but very few have focused on type 2 diabetes, and, as this is such a growing health issue, we wanted to look at that in more detail,” he said.
The researchers performed two different analyses: a meta-analysis of observational studies investigating an association between type 2 diabetes and Parkinson’s; and a separate Mendelian randomization analysis of genetic data on the two conditions.
They found similar results in both studies, with the observational data suggesting type 2 diabetes was associated with a 21% increased risk for Parkinson’s disease and the genetic data suggesting an 8% increased risk. There were also hints that type 2 diabetes might also be associated with faster progression of Parkinson’s symptoms.
“I don’t think type 2 diabetes is a major cause of Parkinson’s, but it probably makes some contribution and may increase the risk of a more aggressive form of the condition,” Dr. Noyce said.
“I would say the increased risk of Parkinson’s disease attributable to type 2 diabetes may be similar to that of head injury or pesticide exposure, but it is important, as type 2 diabetes is very prevalent and is increasing,” he added. “As we see the growth in type 2 diabetes, this could lead to a later increase in Parkinson’s, which is already one of the fastest-growing diseases worldwide.”
For the meta-analysis of observational data, the researchers included nine studies that investigated preceding type 2 diabetes specifically and its effect on the risk for Parkinson’s disease and progression.
The pooled effect estimates showed that type 2 diabetes was associated with an increased risk for Parkinson’s disease (odds ratio, 1.21; 95% confidence interval, 1.07-1.36), and there was some evidence that type 2 diabetes was associated with faster progression of motor symptoms (standardized mean difference [SMD], 0.55) and cognitive decline (SMD, −0.92).
The observational meta-analysis included seven cohort studies and two case-control studies, and these different types of studies showed different results in regard to the association between diabetes and Parkinson’s. While the cohort studies showed a detrimental effect of diabetes on Parkinson’s risk (OR, 1.29), the case-control studies suggested protective effect (OR, 0.51).
Addressing this, Dr. Noyce noted that the case-control studies may be less reliable as they suffered more from survivor bias. “Diabetes may cause deaths in mid-life before people go on to develop Parkinson’s, and this would cause a protective effect to be seen, but we believe this to be a spurious result. Cohort studies are generally more reliable and are less susceptible to survivor bias,” he said.
For the genetic analysis, the researchers combined results from two large publicly available genome-wide association studies – one for type 2 diabetes and one for Parkinson’s disease to assess whether individuals with a genetic tendency to type 2 diabetes had a higher risk of developing Parkinson’s.
Results showed an increased risk for Parkinson’s in those individuals with genetic variants associated with type 2 diabetes, with an odds ratio of 1.08 (P = .010). There was also some evidence of an effect on motor progression (OR, 1.10; P = .032) but not on cognitive progression.
On the possible mechanism behind this observation, Dr. Noyce noted type 2 diabetes and Parkinson’s have some similarities in biology, including abnormal protein aggregation.
In the study, the authors also suggest that circulating insulin may have a neuroprotective role, whereas systemic and local insulin resistance can influence pathways known to be important in Parkinson’s pathogenesis, including those that relate to mitochondrial dysfunction, neuroinflammation, synaptic plasticity, and mitochondrial dysfunction.
Dr. Noyce further pointed out that several drugs used for the treatment of type 2 diabetes have been repurposed as possible treatments for Parkinson’s disease and are now being tested for this new indication. “Our results support that approach and raise the idea that some of these drugs may even prevent Parkinson’s in people at risk,” he said.
Most people who have type 2 diabetes won’t get Parkinson’s disease, he added. Other outcomes such as heart disease, kidney disease, and microvascular complications are far more likely, and the main aim of preventing and treating type 2 diabetes is to prevent these far more common outcomes. “But our data suggests that this could also have a possible benefit in reducing future Parkinson’s risk,” he said.
Not on the horizon at present is the possibility of screening patients with type 2 diabetes for signs of early Parkinson’s, Dr. Noyce said.
“There isn’t a test for identifying presymptomatic neurodegenerative diseases such as Parkinson’s yet, but perhaps in the future there will be, and type 2 diabetes may be one risk factor to take into account when considering such screening,” he added.
This work was financially supported by grants from The Michael J. Fox Foundation; the Canadian Consortium on Neurodegeneration in Aging (CCNA); the Canada First Research Excellence Fund (CFREF), awarded to McGill University for the Healthy Brains for Healthy Lives (HBHL) initiative; and Parkinson Canada, and the Intramural Research Program of the NIH, National Institute on Aging.
Dr. Noyce reports grants from the Barts Charity, Parkinson’s UK, Aligning Science Across Parkinson’s and Michael J. Fox Foundation, and the Virginia Keiley Benefaction; and personal fees/honoraria from Britannia, BIAL, AbbVie, Global Kinetics Corporation, Profile, Biogen, Roche, and UCB outside of the submitted work.
A version of this article first appeared on Medscape.com.
New analyses of both observational and genetic data have provided “convincing evidence” that type 2 diabetes is associated with an increased risk for Parkinson’s disease.
“The fact that we see the same effects in both types of analysis separately makes it more likely that these results are real – that type 2 diabetes really is a driver of Parkinson’s disease risk,” Alastair Noyce, PhD, senior author of the new studies, said in an interview.
The two analyses are reported in one paper published online March 8 in the journal Movement Disorders.
Dr. Noyce, clinical senior lecturer in the preventive neurology unit at the Wolfson Institute of Preventive Medicine, Queen Mary University of London, explained that his group is interested in risk factors for Parkinson’s disease, particularly those relevant at the population level and which might be modifiable.
“Several studies have looked at diabetes as a risk factor for Parkinson’s but very few have focused on type 2 diabetes, and, as this is such a growing health issue, we wanted to look at that in more detail,” he said.
The researchers performed two different analyses: a meta-analysis of observational studies investigating an association between type 2 diabetes and Parkinson’s; and a separate Mendelian randomization analysis of genetic data on the two conditions.
They found similar results in both studies, with the observational data suggesting type 2 diabetes was associated with a 21% increased risk for Parkinson’s disease and the genetic data suggesting an 8% increased risk. There were also hints that type 2 diabetes might also be associated with faster progression of Parkinson’s symptoms.
“I don’t think type 2 diabetes is a major cause of Parkinson’s, but it probably makes some contribution and may increase the risk of a more aggressive form of the condition,” Dr. Noyce said.
“I would say the increased risk of Parkinson’s disease attributable to type 2 diabetes may be similar to that of head injury or pesticide exposure, but it is important, as type 2 diabetes is very prevalent and is increasing,” he added. “As we see the growth in type 2 diabetes, this could lead to a later increase in Parkinson’s, which is already one of the fastest-growing diseases worldwide.”
For the meta-analysis of observational data, the researchers included nine studies that investigated preceding type 2 diabetes specifically and its effect on the risk for Parkinson’s disease and progression.
The pooled effect estimates showed that type 2 diabetes was associated with an increased risk for Parkinson’s disease (odds ratio, 1.21; 95% confidence interval, 1.07-1.36), and there was some evidence that type 2 diabetes was associated with faster progression of motor symptoms (standardized mean difference [SMD], 0.55) and cognitive decline (SMD, −0.92).
The observational meta-analysis included seven cohort studies and two case-control studies, and these different types of studies showed different results in regard to the association between diabetes and Parkinson’s. While the cohort studies showed a detrimental effect of diabetes on Parkinson’s risk (OR, 1.29), the case-control studies suggested protective effect (OR, 0.51).
Addressing this, Dr. Noyce noted that the case-control studies may be less reliable as they suffered more from survivor bias. “Diabetes may cause deaths in mid-life before people go on to develop Parkinson’s, and this would cause a protective effect to be seen, but we believe this to be a spurious result. Cohort studies are generally more reliable and are less susceptible to survivor bias,” he said.
For the genetic analysis, the researchers combined results from two large publicly available genome-wide association studies – one for type 2 diabetes and one for Parkinson’s disease to assess whether individuals with a genetic tendency to type 2 diabetes had a higher risk of developing Parkinson’s.
Results showed an increased risk for Parkinson’s in those individuals with genetic variants associated with type 2 diabetes, with an odds ratio of 1.08 (P = .010). There was also some evidence of an effect on motor progression (OR, 1.10; P = .032) but not on cognitive progression.
On the possible mechanism behind this observation, Dr. Noyce noted type 2 diabetes and Parkinson’s have some similarities in biology, including abnormal protein aggregation.
In the study, the authors also suggest that circulating insulin may have a neuroprotective role, whereas systemic and local insulin resistance can influence pathways known to be important in Parkinson’s pathogenesis, including those that relate to mitochondrial dysfunction, neuroinflammation, synaptic plasticity, and mitochondrial dysfunction.
Dr. Noyce further pointed out that several drugs used for the treatment of type 2 diabetes have been repurposed as possible treatments for Parkinson’s disease and are now being tested for this new indication. “Our results support that approach and raise the idea that some of these drugs may even prevent Parkinson’s in people at risk,” he said.
Most people who have type 2 diabetes won’t get Parkinson’s disease, he added. Other outcomes such as heart disease, kidney disease, and microvascular complications are far more likely, and the main aim of preventing and treating type 2 diabetes is to prevent these far more common outcomes. “But our data suggests that this could also have a possible benefit in reducing future Parkinson’s risk,” he said.
Not on the horizon at present is the possibility of screening patients with type 2 diabetes for signs of early Parkinson’s, Dr. Noyce said.
“There isn’t a test for identifying presymptomatic neurodegenerative diseases such as Parkinson’s yet, but perhaps in the future there will be, and type 2 diabetes may be one risk factor to take into account when considering such screening,” he added.
This work was financially supported by grants from The Michael J. Fox Foundation; the Canadian Consortium on Neurodegeneration in Aging (CCNA); the Canada First Research Excellence Fund (CFREF), awarded to McGill University for the Healthy Brains for Healthy Lives (HBHL) initiative; and Parkinson Canada, and the Intramural Research Program of the NIH, National Institute on Aging.
Dr. Noyce reports grants from the Barts Charity, Parkinson’s UK, Aligning Science Across Parkinson’s and Michael J. Fox Foundation, and the Virginia Keiley Benefaction; and personal fees/honoraria from Britannia, BIAL, AbbVie, Global Kinetics Corporation, Profile, Biogen, Roche, and UCB outside of the submitted work.
A version of this article first appeared on Medscape.com.
New analyses of both observational and genetic data have provided “convincing evidence” that type 2 diabetes is associated with an increased risk for Parkinson’s disease.
“The fact that we see the same effects in both types of analysis separately makes it more likely that these results are real – that type 2 diabetes really is a driver of Parkinson’s disease risk,” Alastair Noyce, PhD, senior author of the new studies, said in an interview.
The two analyses are reported in one paper published online March 8 in the journal Movement Disorders.
Dr. Noyce, clinical senior lecturer in the preventive neurology unit at the Wolfson Institute of Preventive Medicine, Queen Mary University of London, explained that his group is interested in risk factors for Parkinson’s disease, particularly those relevant at the population level and which might be modifiable.
“Several studies have looked at diabetes as a risk factor for Parkinson’s but very few have focused on type 2 diabetes, and, as this is such a growing health issue, we wanted to look at that in more detail,” he said.
The researchers performed two different analyses: a meta-analysis of observational studies investigating an association between type 2 diabetes and Parkinson’s; and a separate Mendelian randomization analysis of genetic data on the two conditions.
They found similar results in both studies, with the observational data suggesting type 2 diabetes was associated with a 21% increased risk for Parkinson’s disease and the genetic data suggesting an 8% increased risk. There were also hints that type 2 diabetes might also be associated with faster progression of Parkinson’s symptoms.
“I don’t think type 2 diabetes is a major cause of Parkinson’s, but it probably makes some contribution and may increase the risk of a more aggressive form of the condition,” Dr. Noyce said.
“I would say the increased risk of Parkinson’s disease attributable to type 2 diabetes may be similar to that of head injury or pesticide exposure, but it is important, as type 2 diabetes is very prevalent and is increasing,” he added. “As we see the growth in type 2 diabetes, this could lead to a later increase in Parkinson’s, which is already one of the fastest-growing diseases worldwide.”
For the meta-analysis of observational data, the researchers included nine studies that investigated preceding type 2 diabetes specifically and its effect on the risk for Parkinson’s disease and progression.
The pooled effect estimates showed that type 2 diabetes was associated with an increased risk for Parkinson’s disease (odds ratio, 1.21; 95% confidence interval, 1.07-1.36), and there was some evidence that type 2 diabetes was associated with faster progression of motor symptoms (standardized mean difference [SMD], 0.55) and cognitive decline (SMD, −0.92).
The observational meta-analysis included seven cohort studies and two case-control studies, and these different types of studies showed different results in regard to the association between diabetes and Parkinson’s. While the cohort studies showed a detrimental effect of diabetes on Parkinson’s risk (OR, 1.29), the case-control studies suggested protective effect (OR, 0.51).
Addressing this, Dr. Noyce noted that the case-control studies may be less reliable as they suffered more from survivor bias. “Diabetes may cause deaths in mid-life before people go on to develop Parkinson’s, and this would cause a protective effect to be seen, but we believe this to be a spurious result. Cohort studies are generally more reliable and are less susceptible to survivor bias,” he said.
For the genetic analysis, the researchers combined results from two large publicly available genome-wide association studies – one for type 2 diabetes and one for Parkinson’s disease to assess whether individuals with a genetic tendency to type 2 diabetes had a higher risk of developing Parkinson’s.
Results showed an increased risk for Parkinson’s in those individuals with genetic variants associated with type 2 diabetes, with an odds ratio of 1.08 (P = .010). There was also some evidence of an effect on motor progression (OR, 1.10; P = .032) but not on cognitive progression.
On the possible mechanism behind this observation, Dr. Noyce noted type 2 diabetes and Parkinson’s have some similarities in biology, including abnormal protein aggregation.
In the study, the authors also suggest that circulating insulin may have a neuroprotective role, whereas systemic and local insulin resistance can influence pathways known to be important in Parkinson’s pathogenesis, including those that relate to mitochondrial dysfunction, neuroinflammation, synaptic plasticity, and mitochondrial dysfunction.
Dr. Noyce further pointed out that several drugs used for the treatment of type 2 diabetes have been repurposed as possible treatments for Parkinson’s disease and are now being tested for this new indication. “Our results support that approach and raise the idea that some of these drugs may even prevent Parkinson’s in people at risk,” he said.
Most people who have type 2 diabetes won’t get Parkinson’s disease, he added. Other outcomes such as heart disease, kidney disease, and microvascular complications are far more likely, and the main aim of preventing and treating type 2 diabetes is to prevent these far more common outcomes. “But our data suggests that this could also have a possible benefit in reducing future Parkinson’s risk,” he said.
Not on the horizon at present is the possibility of screening patients with type 2 diabetes for signs of early Parkinson’s, Dr. Noyce said.
“There isn’t a test for identifying presymptomatic neurodegenerative diseases such as Parkinson’s yet, but perhaps in the future there will be, and type 2 diabetes may be one risk factor to take into account when considering such screening,” he added.
This work was financially supported by grants from The Michael J. Fox Foundation; the Canadian Consortium on Neurodegeneration in Aging (CCNA); the Canada First Research Excellence Fund (CFREF), awarded to McGill University for the Healthy Brains for Healthy Lives (HBHL) initiative; and Parkinson Canada, and the Intramural Research Program of the NIH, National Institute on Aging.
Dr. Noyce reports grants from the Barts Charity, Parkinson’s UK, Aligning Science Across Parkinson’s and Michael J. Fox Foundation, and the Virginia Keiley Benefaction; and personal fees/honoraria from Britannia, BIAL, AbbVie, Global Kinetics Corporation, Profile, Biogen, Roche, and UCB outside of the submitted work.
A version of this article first appeared on Medscape.com.
Increase in thyroid cancer rates points to overscreening
The researchers say this is “likely to be due to the effect of intense scrutiny of the thyroid gland ... in middle-aged individuals” with sensitive imaging such as ultrasonography or CT.
There is “a lot of evidence” to suggest that this is leading to overdiagnosis, lead author Adalberto Miranda-Filho, PhD, told this news organization. The degree of overdiagnosis depends on the “diagnostic pressure” resulting from opportunistic screening.
The potential for cancer overdiagnosis, including thyroid cancer, was recently estimated to be about 20% for five common types, and there have been calls to relabel low-risk lesions as something other than cancer.
Dr. Miranda-Filho does not believe that subclinical papillary thyroid cancer should be renamed, because that would “require agreement of pathologists and other researchers,” although he said it is “important to explain that not all ‘cancers’ can evolve into a life-threatening disease.”
Screening for thyroid cancer is not currently recommended because it could increase the risk for patient harms without improving outcomes, explained Dr. Miranda-Filho of the Cancer Surveillance Branch, International Agency for Research on Cancer, Lyon, France.
The research was published on March 1 in The Lancet Diabetes and Endocrinology.
Papillary thyroid cancers increased in all countries
Thyroid cancer, which includes a range of histologic subtypes, accounts for 3% of cancer diagnoses globally, the team writes. It is estimated that there were 586,000 new patients in 2020.
Recent evidence suggests the incidence has risen substantially in many high- and medium-income countries over the past 30 years, although there is wide variation between and within populations. Moreover, mortality rates for thyroid cancer have decreased or remained stable.
To investigate this further, the French researchers examined data on thyroid cancer incidence collated by the IARC for the period 1998-2012. They focused on 25 countries from Europe, the Americas, Asia, and Oceania, which have cancer registries that cover more than 2 million of their population.
The analyses were restricted to individuals aged 20-84 years, and the proportion of cases of unspecified thyroid cancer had to be less than 10%.
Data on almost 150,000 thyroid cancer cases were examined, including 59,499 from South Korea, 15,535 from the United States, 15,158 from Canada, 8,684 from the United Kingdom, 8,106 from Australia, and 3,806 from China.
Across all countries, papillary thyroid cancer was the main contributor to thyroid cancer cases. Despite wide variations between countries, it was the only histologic subtype that increased in incidence in all countries.
For the period 2008-2012, the age-standardized incidence rate for papillary thyroid cancer in women ranged from 4.3 to 5.3 per 100,000 person-years in the Netherlands, the United Kingdom, and Denmark to 143.3 cases per 100,000 person-years in South Korea.
Among men, the age-standardized incidence rate ranged from 1.2 to 1.6 per 100,000 person-years in Thailand, Bulgaria, and the Netherlands to 30.7 per 100,000 person-years in South Korea.
Over the whole study period, rates of papillary thyroid cancer increased in both men and women, with large variability between countries. The increases in women were rapid and exceeded 20 cases per 100,000 in several countries, most notably, South Korea.
Interestingly, incidence rates in South Korea, China, Japan, and Turkey were low and stable until the 2000s and then rose markedly, whereas the increase in incidence in the United States, Austria, Croatia, Germany, Slovenia, Spain, Lithuania, and Bulgaria stabilized around 2009.
Not much change in incidence rates for other subtypes of thyroid cancer
For other histologic subtypes of thyroid cancer, the trends in incidence rates were relatively stable and low.
However, some countries, such as the United States, China, South Korea, Turkey, and some Northern European countries, saw increases in follicular thyroid cancer, albeit at much lower rates than for papillary thyroid cancer.
Overall, age-standardized rates for follicular thyroid cancer ranged from 0.5 to 2.5 per 100,000 person-years among women and from 0.3 to 1.5 per 100,000 person-years among men. Rates for medullary thyroid cancer were less than one per 100,000 person-years among men and women. For the anaplastic subtype, rates were less than 0.2 per 100,000 person-years.
The team notes that small decreases in anaplastic thyroid cancer rates across the study period were recorded in 21 countries, including Colombia, Lithuania, Bulgaria, Slovenia, Germany, and Norway.
However, Dr. Miranda-Filho said that overall, the incidence rate of anaplastic thyroid cancer “seems to be not affected by the intensity of screening,” whereas the “lack of evidence” on changing trends for follicular disease suggests that opportunistic screening allows only non–life-threatening tumors to emerge “from a large reservoir of subclinical asymptomatic neoplasms in the thyroid glands.”
The study was supported by the French Institut National du Cancer, the Italian Association for Cancer Research, and the Italian Ministry of Health to Centro di Riferimento Oncologico di Aviano. The authors have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The researchers say this is “likely to be due to the effect of intense scrutiny of the thyroid gland ... in middle-aged individuals” with sensitive imaging such as ultrasonography or CT.
There is “a lot of evidence” to suggest that this is leading to overdiagnosis, lead author Adalberto Miranda-Filho, PhD, told this news organization. The degree of overdiagnosis depends on the “diagnostic pressure” resulting from opportunistic screening.
The potential for cancer overdiagnosis, including thyroid cancer, was recently estimated to be about 20% for five common types, and there have been calls to relabel low-risk lesions as something other than cancer.
Dr. Miranda-Filho does not believe that subclinical papillary thyroid cancer should be renamed, because that would “require agreement of pathologists and other researchers,” although he said it is “important to explain that not all ‘cancers’ can evolve into a life-threatening disease.”
Screening for thyroid cancer is not currently recommended because it could increase the risk for patient harms without improving outcomes, explained Dr. Miranda-Filho of the Cancer Surveillance Branch, International Agency for Research on Cancer, Lyon, France.
The research was published on March 1 in The Lancet Diabetes and Endocrinology.
Papillary thyroid cancers increased in all countries
Thyroid cancer, which includes a range of histologic subtypes, accounts for 3% of cancer diagnoses globally, the team writes. It is estimated that there were 586,000 new patients in 2020.
Recent evidence suggests the incidence has risen substantially in many high- and medium-income countries over the past 30 years, although there is wide variation between and within populations. Moreover, mortality rates for thyroid cancer have decreased or remained stable.
To investigate this further, the French researchers examined data on thyroid cancer incidence collated by the IARC for the period 1998-2012. They focused on 25 countries from Europe, the Americas, Asia, and Oceania, which have cancer registries that cover more than 2 million of their population.
The analyses were restricted to individuals aged 20-84 years, and the proportion of cases of unspecified thyroid cancer had to be less than 10%.
Data on almost 150,000 thyroid cancer cases were examined, including 59,499 from South Korea, 15,535 from the United States, 15,158 from Canada, 8,684 from the United Kingdom, 8,106 from Australia, and 3,806 from China.
Across all countries, papillary thyroid cancer was the main contributor to thyroid cancer cases. Despite wide variations between countries, it was the only histologic subtype that increased in incidence in all countries.
For the period 2008-2012, the age-standardized incidence rate for papillary thyroid cancer in women ranged from 4.3 to 5.3 per 100,000 person-years in the Netherlands, the United Kingdom, and Denmark to 143.3 cases per 100,000 person-years in South Korea.
Among men, the age-standardized incidence rate ranged from 1.2 to 1.6 per 100,000 person-years in Thailand, Bulgaria, and the Netherlands to 30.7 per 100,000 person-years in South Korea.
Over the whole study period, rates of papillary thyroid cancer increased in both men and women, with large variability between countries. The increases in women were rapid and exceeded 20 cases per 100,000 in several countries, most notably, South Korea.
Interestingly, incidence rates in South Korea, China, Japan, and Turkey were low and stable until the 2000s and then rose markedly, whereas the increase in incidence in the United States, Austria, Croatia, Germany, Slovenia, Spain, Lithuania, and Bulgaria stabilized around 2009.
Not much change in incidence rates for other subtypes of thyroid cancer
For other histologic subtypes of thyroid cancer, the trends in incidence rates were relatively stable and low.
However, some countries, such as the United States, China, South Korea, Turkey, and some Northern European countries, saw increases in follicular thyroid cancer, albeit at much lower rates than for papillary thyroid cancer.
Overall, age-standardized rates for follicular thyroid cancer ranged from 0.5 to 2.5 per 100,000 person-years among women and from 0.3 to 1.5 per 100,000 person-years among men. Rates for medullary thyroid cancer were less than one per 100,000 person-years among men and women. For the anaplastic subtype, rates were less than 0.2 per 100,000 person-years.
The team notes that small decreases in anaplastic thyroid cancer rates across the study period were recorded in 21 countries, including Colombia, Lithuania, Bulgaria, Slovenia, Germany, and Norway.
However, Dr. Miranda-Filho said that overall, the incidence rate of anaplastic thyroid cancer “seems to be not affected by the intensity of screening,” whereas the “lack of evidence” on changing trends for follicular disease suggests that opportunistic screening allows only non–life-threatening tumors to emerge “from a large reservoir of subclinical asymptomatic neoplasms in the thyroid glands.”
The study was supported by the French Institut National du Cancer, the Italian Association for Cancer Research, and the Italian Ministry of Health to Centro di Riferimento Oncologico di Aviano. The authors have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The researchers say this is “likely to be due to the effect of intense scrutiny of the thyroid gland ... in middle-aged individuals” with sensitive imaging such as ultrasonography or CT.
There is “a lot of evidence” to suggest that this is leading to overdiagnosis, lead author Adalberto Miranda-Filho, PhD, told this news organization. The degree of overdiagnosis depends on the “diagnostic pressure” resulting from opportunistic screening.
The potential for cancer overdiagnosis, including thyroid cancer, was recently estimated to be about 20% for five common types, and there have been calls to relabel low-risk lesions as something other than cancer.
Dr. Miranda-Filho does not believe that subclinical papillary thyroid cancer should be renamed, because that would “require agreement of pathologists and other researchers,” although he said it is “important to explain that not all ‘cancers’ can evolve into a life-threatening disease.”
Screening for thyroid cancer is not currently recommended because it could increase the risk for patient harms without improving outcomes, explained Dr. Miranda-Filho of the Cancer Surveillance Branch, International Agency for Research on Cancer, Lyon, France.
The research was published on March 1 in The Lancet Diabetes and Endocrinology.
Papillary thyroid cancers increased in all countries
Thyroid cancer, which includes a range of histologic subtypes, accounts for 3% of cancer diagnoses globally, the team writes. It is estimated that there were 586,000 new patients in 2020.
Recent evidence suggests the incidence has risen substantially in many high- and medium-income countries over the past 30 years, although there is wide variation between and within populations. Moreover, mortality rates for thyroid cancer have decreased or remained stable.
To investigate this further, the French researchers examined data on thyroid cancer incidence collated by the IARC for the period 1998-2012. They focused on 25 countries from Europe, the Americas, Asia, and Oceania, which have cancer registries that cover more than 2 million of their population.
The analyses were restricted to individuals aged 20-84 years, and the proportion of cases of unspecified thyroid cancer had to be less than 10%.
Data on almost 150,000 thyroid cancer cases were examined, including 59,499 from South Korea, 15,535 from the United States, 15,158 from Canada, 8,684 from the United Kingdom, 8,106 from Australia, and 3,806 from China.
Across all countries, papillary thyroid cancer was the main contributor to thyroid cancer cases. Despite wide variations between countries, it was the only histologic subtype that increased in incidence in all countries.
For the period 2008-2012, the age-standardized incidence rate for papillary thyroid cancer in women ranged from 4.3 to 5.3 per 100,000 person-years in the Netherlands, the United Kingdom, and Denmark to 143.3 cases per 100,000 person-years in South Korea.
Among men, the age-standardized incidence rate ranged from 1.2 to 1.6 per 100,000 person-years in Thailand, Bulgaria, and the Netherlands to 30.7 per 100,000 person-years in South Korea.
Over the whole study period, rates of papillary thyroid cancer increased in both men and women, with large variability between countries. The increases in women were rapid and exceeded 20 cases per 100,000 in several countries, most notably, South Korea.
Interestingly, incidence rates in South Korea, China, Japan, and Turkey were low and stable until the 2000s and then rose markedly, whereas the increase in incidence in the United States, Austria, Croatia, Germany, Slovenia, Spain, Lithuania, and Bulgaria stabilized around 2009.
Not much change in incidence rates for other subtypes of thyroid cancer
For other histologic subtypes of thyroid cancer, the trends in incidence rates were relatively stable and low.
However, some countries, such as the United States, China, South Korea, Turkey, and some Northern European countries, saw increases in follicular thyroid cancer, albeit at much lower rates than for papillary thyroid cancer.
Overall, age-standardized rates for follicular thyroid cancer ranged from 0.5 to 2.5 per 100,000 person-years among women and from 0.3 to 1.5 per 100,000 person-years among men. Rates for medullary thyroid cancer were less than one per 100,000 person-years among men and women. For the anaplastic subtype, rates were less than 0.2 per 100,000 person-years.
The team notes that small decreases in anaplastic thyroid cancer rates across the study period were recorded in 21 countries, including Colombia, Lithuania, Bulgaria, Slovenia, Germany, and Norway.
However, Dr. Miranda-Filho said that overall, the incidence rate of anaplastic thyroid cancer “seems to be not affected by the intensity of screening,” whereas the “lack of evidence” on changing trends for follicular disease suggests that opportunistic screening allows only non–life-threatening tumors to emerge “from a large reservoir of subclinical asymptomatic neoplasms in the thyroid glands.”
The study was supported by the French Institut National du Cancer, the Italian Association for Cancer Research, and the Italian Ministry of Health to Centro di Riferimento Oncologico di Aviano. The authors have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Some with long COVID see relief after vaccination
Several weeks after getting his second dose of an mRNA vaccine, Aaron Goyang thinks his long bout with COVID-19 has finally come to an end.
Mr. Goyang, who is 33 and is a radiology technician in Austin, Tex., thinks he got COVID-19 from some of the coughing, gasping patients he treated last spring.
At the time, testing was scarce, and by the time he was tested – several weeks into his illness – it came back negative. He fought off the initial symptoms but experienced relapse a week later.
Mr. Goyang says that, for the next 8 or 9 months, he was on a roller coaster with extreme shortness of breath and chest tightness that could be so severe it would send him to the emergency department. He had to use an inhaler to get through his workdays.
“Even if I was just sitting around, it would come and take me,” he says. “It almost felt like someone was bear-hugging me constantly, and I just couldn’t get in a good enough breath.”
On his best days, he would walk around his neighborhood, being careful not to overdo it. He tried running once, and it nearly sent him to the hospital.
“Very honestly, I didn’t know if I would ever be able to do it again,” he says.
But Mr. Goyang says that, several weeks after getting the Pfizer vaccine, he was able to run a mile again with no problems. “I was very thankful for that,” he says.
Mr. Goyang is not alone. Some social media groups are dedicated to patients who are living with a condition that’s been known as long COVID and that was recently termed postacute sequelae of SARS-CoV-2 infection (PASC). These patients are sometimes referred to as long haulers.
On social media, patients with PASC are eagerly and anxiously quizzing each other about the vaccines and their effects.
Survivor Corps, which has a public Facebook group with 159,000 members, recently took a poll to see whether there was any substance to rumors that those with long COVID were feeling better after being vaccinated.
“Out of 400 people, 36% showed an improvement in symptoms, anywhere between a mild improvement to complete resolution of symptoms,” said Diana Berrent, a long-COVID patient who founded the group. Survivor Corps has become active in patient advocacy and is a resource for researchers studying the new condition.
Ms. Berrent has become such a trusted voice during the pandemic. She interviewed Anthony Fauci, MD, head of the National Institutes of Allergy and Infectious Diseases, last October.
“The implications are huge,” she says.
“Some of this damage is permanent damage. It’s not going to cure the scarring of your heart tissue, it’s not going to cure the irreparable damage to your lungs, but if it’s making people feel better, then that’s an indication there’s viral persistence going on,” says Ms. Berrent.
“I’ve been saying for months and months, we shouldn’t be calling this postacute anything,” she adds.
Patients report improvement
Daniel Griffin, MD, PhD, is equally excited. He’s an infectious disease specialist at Columbia University, New York. He says about one in five patients he treated for COVID-19 last year never got better. Many of them, such as Mr. Goyang, were health care workers.
“I don’t know if people actually catch this, but a lot of our coworkers are either permanently disabled or died,” Dr. Griffin says.
Health care workers were also among the first to be vaccinated. Dr. Griffin says many of his patients began reaching out to him about a week or two after being vaccinated “and saying, ‘You know, I actually feel better.’ And some of them were saying, ‘I feel all better,’ after being sick – a lot of them – for a year.”
Then he was getting calls and texts from other doctors, asking, “Hey, are you seeing this?”
The benefits of vaccination for some long-haulers came as a surprise. Dr. Griffin says that, before the vaccines came out, many of his patients were worried that getting vaccinated might overstimulate their immune systems and cause symptoms to get worse.
Indeed, a small percentage of people – about 3%-5%, based on informal polls on social media – report that they do experience worsening of symptoms after getting the shot. It’s not clear why.
Dr. Griffin estimates that between 30% and 50% of patients’ symptoms improve after they receive the mRNA vaccines. “I’m seeing this chunk of people – they tell me their brain fog has improved, their fatigue is gone, the fevers that wouldn’t resolve have now gone,” he says. “I’m seeing that personally, and I’m hearing it from my colleagues.”
Dr. Griffin says the observation has launched several studies and that there are several theories about how the vaccines might be affecting long COVID.
An immune system boost?
One possibility is that the virus continues to stimulate the immune system, which continues to fight the virus for months. If that is the case, Dr. Griffin says, the vaccine may be giving the immune system the boost it needs to finally clear the virus away.
Donna Farber, PhD, a professor of microbiology and immunology at Columbia University, has heard the stories, too.
“It is possible that the persisting virus in long COVID-19 may be at a low level – not enough to stimulate a potent immune response to clear the virus, but enough to cause symptoms. Activating the immune response therefore is therapeutic in directing viral clearance,” she says.
Dr. Farber explains that long COVID may be a bit like Lyme disease. Some patients with Lyme disease must take antibiotics for months before their symptoms disappear.
Dr. Griffin says there’s another possibility. Several studies have now shown that people with lingering COVID-19 symptoms develop autoantibodies. There’s a theory that SARS-CoV-2 may create an autoimmune condition that leads to long-term symptoms.
If that is the case, Dr. Griffin says, the vaccine may be helping the body to reset its tolerance to itself, “so maybe now you’re getting a healthy immune response.”
More studies are needed to know for sure.
Either way, the vaccines are a much-needed bit of hope for the long-COVID community, and Dr. Griffin tells his patients who are still worried that, at the very least, they’ll be protected from another SARS-CoV-2 infection.
A version of this article first appeared on Medscape.com.
Several weeks after getting his second dose of an mRNA vaccine, Aaron Goyang thinks his long bout with COVID-19 has finally come to an end.
Mr. Goyang, who is 33 and is a radiology technician in Austin, Tex., thinks he got COVID-19 from some of the coughing, gasping patients he treated last spring.
At the time, testing was scarce, and by the time he was tested – several weeks into his illness – it came back negative. He fought off the initial symptoms but experienced relapse a week later.
Mr. Goyang says that, for the next 8 or 9 months, he was on a roller coaster with extreme shortness of breath and chest tightness that could be so severe it would send him to the emergency department. He had to use an inhaler to get through his workdays.
“Even if I was just sitting around, it would come and take me,” he says. “It almost felt like someone was bear-hugging me constantly, and I just couldn’t get in a good enough breath.”
On his best days, he would walk around his neighborhood, being careful not to overdo it. He tried running once, and it nearly sent him to the hospital.
“Very honestly, I didn’t know if I would ever be able to do it again,” he says.
But Mr. Goyang says that, several weeks after getting the Pfizer vaccine, he was able to run a mile again with no problems. “I was very thankful for that,” he says.
Mr. Goyang is not alone. Some social media groups are dedicated to patients who are living with a condition that’s been known as long COVID and that was recently termed postacute sequelae of SARS-CoV-2 infection (PASC). These patients are sometimes referred to as long haulers.
On social media, patients with PASC are eagerly and anxiously quizzing each other about the vaccines and their effects.
Survivor Corps, which has a public Facebook group with 159,000 members, recently took a poll to see whether there was any substance to rumors that those with long COVID were feeling better after being vaccinated.
“Out of 400 people, 36% showed an improvement in symptoms, anywhere between a mild improvement to complete resolution of symptoms,” said Diana Berrent, a long-COVID patient who founded the group. Survivor Corps has become active in patient advocacy and is a resource for researchers studying the new condition.
Ms. Berrent has become such a trusted voice during the pandemic. She interviewed Anthony Fauci, MD, head of the National Institutes of Allergy and Infectious Diseases, last October.
“The implications are huge,” she says.
“Some of this damage is permanent damage. It’s not going to cure the scarring of your heart tissue, it’s not going to cure the irreparable damage to your lungs, but if it’s making people feel better, then that’s an indication there’s viral persistence going on,” says Ms. Berrent.
“I’ve been saying for months and months, we shouldn’t be calling this postacute anything,” she adds.
Patients report improvement
Daniel Griffin, MD, PhD, is equally excited. He’s an infectious disease specialist at Columbia University, New York. He says about one in five patients he treated for COVID-19 last year never got better. Many of them, such as Mr. Goyang, were health care workers.
“I don’t know if people actually catch this, but a lot of our coworkers are either permanently disabled or died,” Dr. Griffin says.
Health care workers were also among the first to be vaccinated. Dr. Griffin says many of his patients began reaching out to him about a week or two after being vaccinated “and saying, ‘You know, I actually feel better.’ And some of them were saying, ‘I feel all better,’ after being sick – a lot of them – for a year.”
Then he was getting calls and texts from other doctors, asking, “Hey, are you seeing this?”
The benefits of vaccination for some long-haulers came as a surprise. Dr. Griffin says that, before the vaccines came out, many of his patients were worried that getting vaccinated might overstimulate their immune systems and cause symptoms to get worse.
Indeed, a small percentage of people – about 3%-5%, based on informal polls on social media – report that they do experience worsening of symptoms after getting the shot. It’s not clear why.
Dr. Griffin estimates that between 30% and 50% of patients’ symptoms improve after they receive the mRNA vaccines. “I’m seeing this chunk of people – they tell me their brain fog has improved, their fatigue is gone, the fevers that wouldn’t resolve have now gone,” he says. “I’m seeing that personally, and I’m hearing it from my colleagues.”
Dr. Griffin says the observation has launched several studies and that there are several theories about how the vaccines might be affecting long COVID.
An immune system boost?
One possibility is that the virus continues to stimulate the immune system, which continues to fight the virus for months. If that is the case, Dr. Griffin says, the vaccine may be giving the immune system the boost it needs to finally clear the virus away.
Donna Farber, PhD, a professor of microbiology and immunology at Columbia University, has heard the stories, too.
“It is possible that the persisting virus in long COVID-19 may be at a low level – not enough to stimulate a potent immune response to clear the virus, but enough to cause symptoms. Activating the immune response therefore is therapeutic in directing viral clearance,” she says.
Dr. Farber explains that long COVID may be a bit like Lyme disease. Some patients with Lyme disease must take antibiotics for months before their symptoms disappear.
Dr. Griffin says there’s another possibility. Several studies have now shown that people with lingering COVID-19 symptoms develop autoantibodies. There’s a theory that SARS-CoV-2 may create an autoimmune condition that leads to long-term symptoms.
If that is the case, Dr. Griffin says, the vaccine may be helping the body to reset its tolerance to itself, “so maybe now you’re getting a healthy immune response.”
More studies are needed to know for sure.
Either way, the vaccines are a much-needed bit of hope for the long-COVID community, and Dr. Griffin tells his patients who are still worried that, at the very least, they’ll be protected from another SARS-CoV-2 infection.
A version of this article first appeared on Medscape.com.
Several weeks after getting his second dose of an mRNA vaccine, Aaron Goyang thinks his long bout with COVID-19 has finally come to an end.
Mr. Goyang, who is 33 and is a radiology technician in Austin, Tex., thinks he got COVID-19 from some of the coughing, gasping patients he treated last spring.
At the time, testing was scarce, and by the time he was tested – several weeks into his illness – it came back negative. He fought off the initial symptoms but experienced relapse a week later.
Mr. Goyang says that, for the next 8 or 9 months, he was on a roller coaster with extreme shortness of breath and chest tightness that could be so severe it would send him to the emergency department. He had to use an inhaler to get through his workdays.
“Even if I was just sitting around, it would come and take me,” he says. “It almost felt like someone was bear-hugging me constantly, and I just couldn’t get in a good enough breath.”
On his best days, he would walk around his neighborhood, being careful not to overdo it. He tried running once, and it nearly sent him to the hospital.
“Very honestly, I didn’t know if I would ever be able to do it again,” he says.
But Mr. Goyang says that, several weeks after getting the Pfizer vaccine, he was able to run a mile again with no problems. “I was very thankful for that,” he says.
Mr. Goyang is not alone. Some social media groups are dedicated to patients who are living with a condition that’s been known as long COVID and that was recently termed postacute sequelae of SARS-CoV-2 infection (PASC). These patients are sometimes referred to as long haulers.
On social media, patients with PASC are eagerly and anxiously quizzing each other about the vaccines and their effects.
Survivor Corps, which has a public Facebook group with 159,000 members, recently took a poll to see whether there was any substance to rumors that those with long COVID were feeling better after being vaccinated.
“Out of 400 people, 36% showed an improvement in symptoms, anywhere between a mild improvement to complete resolution of symptoms,” said Diana Berrent, a long-COVID patient who founded the group. Survivor Corps has become active in patient advocacy and is a resource for researchers studying the new condition.
Ms. Berrent has become such a trusted voice during the pandemic. She interviewed Anthony Fauci, MD, head of the National Institutes of Allergy and Infectious Diseases, last October.
“The implications are huge,” she says.
“Some of this damage is permanent damage. It’s not going to cure the scarring of your heart tissue, it’s not going to cure the irreparable damage to your lungs, but if it’s making people feel better, then that’s an indication there’s viral persistence going on,” says Ms. Berrent.
“I’ve been saying for months and months, we shouldn’t be calling this postacute anything,” she adds.
Patients report improvement
Daniel Griffin, MD, PhD, is equally excited. He’s an infectious disease specialist at Columbia University, New York. He says about one in five patients he treated for COVID-19 last year never got better. Many of them, such as Mr. Goyang, were health care workers.
“I don’t know if people actually catch this, but a lot of our coworkers are either permanently disabled or died,” Dr. Griffin says.
Health care workers were also among the first to be vaccinated. Dr. Griffin says many of his patients began reaching out to him about a week or two after being vaccinated “and saying, ‘You know, I actually feel better.’ And some of them were saying, ‘I feel all better,’ after being sick – a lot of them – for a year.”
Then he was getting calls and texts from other doctors, asking, “Hey, are you seeing this?”
The benefits of vaccination for some long-haulers came as a surprise. Dr. Griffin says that, before the vaccines came out, many of his patients were worried that getting vaccinated might overstimulate their immune systems and cause symptoms to get worse.
Indeed, a small percentage of people – about 3%-5%, based on informal polls on social media – report that they do experience worsening of symptoms after getting the shot. It’s not clear why.
Dr. Griffin estimates that between 30% and 50% of patients’ symptoms improve after they receive the mRNA vaccines. “I’m seeing this chunk of people – they tell me their brain fog has improved, their fatigue is gone, the fevers that wouldn’t resolve have now gone,” he says. “I’m seeing that personally, and I’m hearing it from my colleagues.”
Dr. Griffin says the observation has launched several studies and that there are several theories about how the vaccines might be affecting long COVID.
An immune system boost?
One possibility is that the virus continues to stimulate the immune system, which continues to fight the virus for months. If that is the case, Dr. Griffin says, the vaccine may be giving the immune system the boost it needs to finally clear the virus away.
Donna Farber, PhD, a professor of microbiology and immunology at Columbia University, has heard the stories, too.
“It is possible that the persisting virus in long COVID-19 may be at a low level – not enough to stimulate a potent immune response to clear the virus, but enough to cause symptoms. Activating the immune response therefore is therapeutic in directing viral clearance,” she says.
Dr. Farber explains that long COVID may be a bit like Lyme disease. Some patients with Lyme disease must take antibiotics for months before their symptoms disappear.
Dr. Griffin says there’s another possibility. Several studies have now shown that people with lingering COVID-19 symptoms develop autoantibodies. There’s a theory that SARS-CoV-2 may create an autoimmune condition that leads to long-term symptoms.
If that is the case, Dr. Griffin says, the vaccine may be helping the body to reset its tolerance to itself, “so maybe now you’re getting a healthy immune response.”
More studies are needed to know for sure.
Either way, the vaccines are a much-needed bit of hope for the long-COVID community, and Dr. Griffin tells his patients who are still worried that, at the very least, they’ll be protected from another SARS-CoV-2 infection.
A version of this article first appeared on Medscape.com.
The skill set of the ‘pluripotent’ hospitalist
Editor’s note: National Hospitalist Day occurs the first Thursday in March annually, and serves to celebrate the fastest growing specialty in modern medicine and hospitalists’ enduring contributions to the evolving health care landscape. On National Hospitalist Day in 2021, SHM convened a virtual roundtable with a diverse group of hospitalists to discuss skill set, wellness, and other key issues for hospitalists. To listen to the entire roundtable discussion, visit this Explore The Space podcast episode.
A hospitalist isn’t just a physician who happens to work in a hospital. They are medical professionals with a robust skill set that they use both inside and outside the hospital setting. But what skill sets do hospitalists need to become successful in their careers? And what skill sets does a “pluripotent” hospitalist need in their armamentarium?
These were the issues discussed by participants of a virtual roundtable discussion on National Hospitalist Day – March 4, 2021 – as part of a joint effort of the Society of Hospital Medicine and the Explore the Space podcast.
Maylyn S. Martinez, MD, clinician-researcher and clinical associate at the University of Chicago, sees her hospitalist and research skill sets as two “buckets” of skills she can sort through, with diagnostic, knowledge-based care coordination, and interpersonal skills as lanes where she can focus and improve. “I’m always trying to work in, and sharpen, and find ways to get better at something in each of those every day,” she said.
For Anika Kumar, MD, FHM, pediatric editor of the Hospitalist and clinical assistant professor of pediatrics at the Cleveland Clinic Lerner College of Medicine, much of her work is focused on problem solving. “I approach that as: ‘How do I come up with my differential diagnosis, and how do I diagnose the patient?’ I think that the lanes are a little bit different, but there is some overlap.”
Adaptability is another important part of the skill set for the hospitalist, Ndidi Unaka, MD, MEd, associate professor in the division of hospital medicine at Cincinnati Children’s Hospital Medical Center, said during the discussion. “I think we all really value teamwork, and we take on the role of being the coordinator and making sure things are getting done in a seamless and thoughtful manner. Communicating with families, communicating with our research team, communicating with primary care physicians. I think that is something we’re very used to doing, and I think we do it well. I think we don’t shy away from difficult conversations with consultants. And I think that’s what makes being a hospitalist so amazing.”
Achieving wellness as a hospitalist
Another topic discussed during the roundtable was “comprehensive care for the hospitalist” and how they can achieve a sense of wellness for themselves. Gurpreet Dhaliwal, MD, clinician-educator and professor of medicine at the University of California, San Francisco, said long-term satisfaction in one’s career is less about compensation and more about autonomy, mastery, and purpose.
“Autonomy is shrinking a little bit in health care. But if we connect to our purpose – ‘what are we doing here and how do we connect?’ – it’s either learning about patients and their stories, being with a team of people that you work with, that really builds that purpose,” he said.
Regarding mastery, there’s “tremendous joy if you’re in an environment where people value your mastery, whether it is working in a team or communicating or diagnosing or doing a procedure. If you think of setting up the work environment and those things are in place, I think a lot of wellness can actually happen at work, even though another component, of course, is balancing your life outside of work,” Dr. Dhaliwal said.
This may seem out of reach during COVID-19, but wellness is still achievable during the pandemic, Dr. Martinez said. Her time is spent 75% as a researcher and 25% as a clinician, which is her ideal balance. “I enjoy doing my research, doing my own statistics and writing grants and just learning about this problem that I’ve developed an interest in,” she said. “I just think that’s an important piece for people to focus on as far as health care for the hospitalist, is that there’s no no-one-size-fits-all, that’s for sure.”
Dr. Kumar noted that her clinical time gives her energy for nonclinical work. “I love my clinical time. It’s one of my favorite things that I do,” she said. Although she is tired at the end of the week, “I feel like I am not only giving back to my patients and my team, but I’m also giving back to myself and reminding myself why it is I do what I do every day,” she said.
Wellness for Dr. Unaka meant remembering what drew her to medicine. “It was definitely the opportunity to build strong relationships with patients and families,” she said. While these encounters can sometimes be heavy and stay with a hospitalist, “the fact that we’re in it with them is something that gives a lot of us purpose. I think that when I reflect on all of those things, I’m so happy that I’m in the role that I am.”
Unique skills during COVID-19
Mark Shapiro, MD, hospitalist and host of the roundtable and the Explore the Space podcast, also asked the panelists what skills they unexpectedly leveraged during the pandemic. Communication – with colleagues and with the community they serve – was a universal answer among the panelists.
“I learned – really from seeing some of our senior leaders here do it so well – the importance of being visible, particularly at a time when people were not together and more isolated,” Dr. Unaka said. “I think being able to be visible when you can, in order to deliver really complicated or tough news or communicate about uncertainty, for instance. Being here for our residents – many of our interns moved here sight unseen. I think they needed to feel like they had some sense of normalcy and a sense of community. I really learned how important it was to be visible, and available, and how important the little things mattered.”
Dr. Martinez said that worrying about her patients with COVID-19 in the hospital and the uncertainty around the disease kept her up at night. “I think we always have a hard time leaving work at work and getting a good night’s sleep. I just could not let go of worrying about these patients and having terrible insomnia, trying to leave work at work and I couldn’t – even after they were discharged.”
Dr. Shapiro said the skill he most needed to work on during the pandemic was his courage. “I remember the first time I took care of COVID patients. I was scared. I have no problems saying that out loud. That was a scary experience.”
The demeanor of the nurses on his unit, who had already seen patients with COVID-19, helped ground him during those moments and gave him the courage to move forward. “They’d already been doing it and they were the same. Same affect, same jokes, same everything,” he said. “That actually really helped, and I’ve leaned on that every time I’ve been back on our COVID service.”
Importance of mental health
The COVID-19 pandemic has also shined a light on the importance of mental health. “I think it is important to acknowledge that as hospitalists who have been out on the bleeding edge for a year, mental health is critically important, and we know that we face shortages in that space for the public at large and also for our profession,” Dr. Shapiro said.
When asked about what mental health and self-care looks like for her, Dr. Kumar referenced the need for exercise, meditation, and yoga. “My mental health was better knowing that the people closest to me – whether they be colleagues or friends or family – their mental health was also in a good place and they were also in a good place. And that helped to build me up,” she said.
Dr. Unaka called attention to the stigma around mental health, particularly among physicians, and the lack of resources to address the issue. “It’s a real problem,” she said. “I think it’s at a point where we as a profession need to advocate on behalf of each other and on behalf of our trainees. And honestly, I think we need to view mental health as just ‘health’ and stop separating it out in order for us to move to a place where people feel like they can access what they need without feeling shame about it.”
Editor’s note: National Hospitalist Day occurs the first Thursday in March annually, and serves to celebrate the fastest growing specialty in modern medicine and hospitalists’ enduring contributions to the evolving health care landscape. On National Hospitalist Day in 2021, SHM convened a virtual roundtable with a diverse group of hospitalists to discuss skill set, wellness, and other key issues for hospitalists. To listen to the entire roundtable discussion, visit this Explore The Space podcast episode.
A hospitalist isn’t just a physician who happens to work in a hospital. They are medical professionals with a robust skill set that they use both inside and outside the hospital setting. But what skill sets do hospitalists need to become successful in their careers? And what skill sets does a “pluripotent” hospitalist need in their armamentarium?
These were the issues discussed by participants of a virtual roundtable discussion on National Hospitalist Day – March 4, 2021 – as part of a joint effort of the Society of Hospital Medicine and the Explore the Space podcast.
Maylyn S. Martinez, MD, clinician-researcher and clinical associate at the University of Chicago, sees her hospitalist and research skill sets as two “buckets” of skills she can sort through, with diagnostic, knowledge-based care coordination, and interpersonal skills as lanes where she can focus and improve. “I’m always trying to work in, and sharpen, and find ways to get better at something in each of those every day,” she said.
For Anika Kumar, MD, FHM, pediatric editor of the Hospitalist and clinical assistant professor of pediatrics at the Cleveland Clinic Lerner College of Medicine, much of her work is focused on problem solving. “I approach that as: ‘How do I come up with my differential diagnosis, and how do I diagnose the patient?’ I think that the lanes are a little bit different, but there is some overlap.”
Adaptability is another important part of the skill set for the hospitalist, Ndidi Unaka, MD, MEd, associate professor in the division of hospital medicine at Cincinnati Children’s Hospital Medical Center, said during the discussion. “I think we all really value teamwork, and we take on the role of being the coordinator and making sure things are getting done in a seamless and thoughtful manner. Communicating with families, communicating with our research team, communicating with primary care physicians. I think that is something we’re very used to doing, and I think we do it well. I think we don’t shy away from difficult conversations with consultants. And I think that’s what makes being a hospitalist so amazing.”
Achieving wellness as a hospitalist
Another topic discussed during the roundtable was “comprehensive care for the hospitalist” and how they can achieve a sense of wellness for themselves. Gurpreet Dhaliwal, MD, clinician-educator and professor of medicine at the University of California, San Francisco, said long-term satisfaction in one’s career is less about compensation and more about autonomy, mastery, and purpose.
“Autonomy is shrinking a little bit in health care. But if we connect to our purpose – ‘what are we doing here and how do we connect?’ – it’s either learning about patients and their stories, being with a team of people that you work with, that really builds that purpose,” he said.
Regarding mastery, there’s “tremendous joy if you’re in an environment where people value your mastery, whether it is working in a team or communicating or diagnosing or doing a procedure. If you think of setting up the work environment and those things are in place, I think a lot of wellness can actually happen at work, even though another component, of course, is balancing your life outside of work,” Dr. Dhaliwal said.
This may seem out of reach during COVID-19, but wellness is still achievable during the pandemic, Dr. Martinez said. Her time is spent 75% as a researcher and 25% as a clinician, which is her ideal balance. “I enjoy doing my research, doing my own statistics and writing grants and just learning about this problem that I’ve developed an interest in,” she said. “I just think that’s an important piece for people to focus on as far as health care for the hospitalist, is that there’s no no-one-size-fits-all, that’s for sure.”
Dr. Kumar noted that her clinical time gives her energy for nonclinical work. “I love my clinical time. It’s one of my favorite things that I do,” she said. Although she is tired at the end of the week, “I feel like I am not only giving back to my patients and my team, but I’m also giving back to myself and reminding myself why it is I do what I do every day,” she said.
Wellness for Dr. Unaka meant remembering what drew her to medicine. “It was definitely the opportunity to build strong relationships with patients and families,” she said. While these encounters can sometimes be heavy and stay with a hospitalist, “the fact that we’re in it with them is something that gives a lot of us purpose. I think that when I reflect on all of those things, I’m so happy that I’m in the role that I am.”
Unique skills during COVID-19
Mark Shapiro, MD, hospitalist and host of the roundtable and the Explore the Space podcast, also asked the panelists what skills they unexpectedly leveraged during the pandemic. Communication – with colleagues and with the community they serve – was a universal answer among the panelists.
“I learned – really from seeing some of our senior leaders here do it so well – the importance of being visible, particularly at a time when people were not together and more isolated,” Dr. Unaka said. “I think being able to be visible when you can, in order to deliver really complicated or tough news or communicate about uncertainty, for instance. Being here for our residents – many of our interns moved here sight unseen. I think they needed to feel like they had some sense of normalcy and a sense of community. I really learned how important it was to be visible, and available, and how important the little things mattered.”
Dr. Martinez said that worrying about her patients with COVID-19 in the hospital and the uncertainty around the disease kept her up at night. “I think we always have a hard time leaving work at work and getting a good night’s sleep. I just could not let go of worrying about these patients and having terrible insomnia, trying to leave work at work and I couldn’t – even after they were discharged.”
Dr. Shapiro said the skill he most needed to work on during the pandemic was his courage. “I remember the first time I took care of COVID patients. I was scared. I have no problems saying that out loud. That was a scary experience.”
The demeanor of the nurses on his unit, who had already seen patients with COVID-19, helped ground him during those moments and gave him the courage to move forward. “They’d already been doing it and they were the same. Same affect, same jokes, same everything,” he said. “That actually really helped, and I’ve leaned on that every time I’ve been back on our COVID service.”
Importance of mental health
The COVID-19 pandemic has also shined a light on the importance of mental health. “I think it is important to acknowledge that as hospitalists who have been out on the bleeding edge for a year, mental health is critically important, and we know that we face shortages in that space for the public at large and also for our profession,” Dr. Shapiro said.
When asked about what mental health and self-care looks like for her, Dr. Kumar referenced the need for exercise, meditation, and yoga. “My mental health was better knowing that the people closest to me – whether they be colleagues or friends or family – their mental health was also in a good place and they were also in a good place. And that helped to build me up,” she said.
Dr. Unaka called attention to the stigma around mental health, particularly among physicians, and the lack of resources to address the issue. “It’s a real problem,” she said. “I think it’s at a point where we as a profession need to advocate on behalf of each other and on behalf of our trainees. And honestly, I think we need to view mental health as just ‘health’ and stop separating it out in order for us to move to a place where people feel like they can access what they need without feeling shame about it.”
Editor’s note: National Hospitalist Day occurs the first Thursday in March annually, and serves to celebrate the fastest growing specialty in modern medicine and hospitalists’ enduring contributions to the evolving health care landscape. On National Hospitalist Day in 2021, SHM convened a virtual roundtable with a diverse group of hospitalists to discuss skill set, wellness, and other key issues for hospitalists. To listen to the entire roundtable discussion, visit this Explore The Space podcast episode.
A hospitalist isn’t just a physician who happens to work in a hospital. They are medical professionals with a robust skill set that they use both inside and outside the hospital setting. But what skill sets do hospitalists need to become successful in their careers? And what skill sets does a “pluripotent” hospitalist need in their armamentarium?
These were the issues discussed by participants of a virtual roundtable discussion on National Hospitalist Day – March 4, 2021 – as part of a joint effort of the Society of Hospital Medicine and the Explore the Space podcast.
Maylyn S. Martinez, MD, clinician-researcher and clinical associate at the University of Chicago, sees her hospitalist and research skill sets as two “buckets” of skills she can sort through, with diagnostic, knowledge-based care coordination, and interpersonal skills as lanes where she can focus and improve. “I’m always trying to work in, and sharpen, and find ways to get better at something in each of those every day,” she said.
For Anika Kumar, MD, FHM, pediatric editor of the Hospitalist and clinical assistant professor of pediatrics at the Cleveland Clinic Lerner College of Medicine, much of her work is focused on problem solving. “I approach that as: ‘How do I come up with my differential diagnosis, and how do I diagnose the patient?’ I think that the lanes are a little bit different, but there is some overlap.”
Adaptability is another important part of the skill set for the hospitalist, Ndidi Unaka, MD, MEd, associate professor in the division of hospital medicine at Cincinnati Children’s Hospital Medical Center, said during the discussion. “I think we all really value teamwork, and we take on the role of being the coordinator and making sure things are getting done in a seamless and thoughtful manner. Communicating with families, communicating with our research team, communicating with primary care physicians. I think that is something we’re very used to doing, and I think we do it well. I think we don’t shy away from difficult conversations with consultants. And I think that’s what makes being a hospitalist so amazing.”
Achieving wellness as a hospitalist
Another topic discussed during the roundtable was “comprehensive care for the hospitalist” and how they can achieve a sense of wellness for themselves. Gurpreet Dhaliwal, MD, clinician-educator and professor of medicine at the University of California, San Francisco, said long-term satisfaction in one’s career is less about compensation and more about autonomy, mastery, and purpose.
“Autonomy is shrinking a little bit in health care. But if we connect to our purpose – ‘what are we doing here and how do we connect?’ – it’s either learning about patients and their stories, being with a team of people that you work with, that really builds that purpose,” he said.
Regarding mastery, there’s “tremendous joy if you’re in an environment where people value your mastery, whether it is working in a team or communicating or diagnosing or doing a procedure. If you think of setting up the work environment and those things are in place, I think a lot of wellness can actually happen at work, even though another component, of course, is balancing your life outside of work,” Dr. Dhaliwal said.
This may seem out of reach during COVID-19, but wellness is still achievable during the pandemic, Dr. Martinez said. Her time is spent 75% as a researcher and 25% as a clinician, which is her ideal balance. “I enjoy doing my research, doing my own statistics and writing grants and just learning about this problem that I’ve developed an interest in,” she said. “I just think that’s an important piece for people to focus on as far as health care for the hospitalist, is that there’s no no-one-size-fits-all, that’s for sure.”
Dr. Kumar noted that her clinical time gives her energy for nonclinical work. “I love my clinical time. It’s one of my favorite things that I do,” she said. Although she is tired at the end of the week, “I feel like I am not only giving back to my patients and my team, but I’m also giving back to myself and reminding myself why it is I do what I do every day,” she said.
Wellness for Dr. Unaka meant remembering what drew her to medicine. “It was definitely the opportunity to build strong relationships with patients and families,” she said. While these encounters can sometimes be heavy and stay with a hospitalist, “the fact that we’re in it with them is something that gives a lot of us purpose. I think that when I reflect on all of those things, I’m so happy that I’m in the role that I am.”
Unique skills during COVID-19
Mark Shapiro, MD, hospitalist and host of the roundtable and the Explore the Space podcast, also asked the panelists what skills they unexpectedly leveraged during the pandemic. Communication – with colleagues and with the community they serve – was a universal answer among the panelists.
“I learned – really from seeing some of our senior leaders here do it so well – the importance of being visible, particularly at a time when people were not together and more isolated,” Dr. Unaka said. “I think being able to be visible when you can, in order to deliver really complicated or tough news or communicate about uncertainty, for instance. Being here for our residents – many of our interns moved here sight unseen. I think they needed to feel like they had some sense of normalcy and a sense of community. I really learned how important it was to be visible, and available, and how important the little things mattered.”
Dr. Martinez said that worrying about her patients with COVID-19 in the hospital and the uncertainty around the disease kept her up at night. “I think we always have a hard time leaving work at work and getting a good night’s sleep. I just could not let go of worrying about these patients and having terrible insomnia, trying to leave work at work and I couldn’t – even after they were discharged.”
Dr. Shapiro said the skill he most needed to work on during the pandemic was his courage. “I remember the first time I took care of COVID patients. I was scared. I have no problems saying that out loud. That was a scary experience.”
The demeanor of the nurses on his unit, who had already seen patients with COVID-19, helped ground him during those moments and gave him the courage to move forward. “They’d already been doing it and they were the same. Same affect, same jokes, same everything,” he said. “That actually really helped, and I’ve leaned on that every time I’ve been back on our COVID service.”
Importance of mental health
The COVID-19 pandemic has also shined a light on the importance of mental health. “I think it is important to acknowledge that as hospitalists who have been out on the bleeding edge for a year, mental health is critically important, and we know that we face shortages in that space for the public at large and also for our profession,” Dr. Shapiro said.
When asked about what mental health and self-care looks like for her, Dr. Kumar referenced the need for exercise, meditation, and yoga. “My mental health was better knowing that the people closest to me – whether they be colleagues or friends or family – their mental health was also in a good place and they were also in a good place. And that helped to build me up,” she said.
Dr. Unaka called attention to the stigma around mental health, particularly among physicians, and the lack of resources to address the issue. “It’s a real problem,” she said. “I think it’s at a point where we as a profession need to advocate on behalf of each other and on behalf of our trainees. And honestly, I think we need to view mental health as just ‘health’ and stop separating it out in order for us to move to a place where people feel like they can access what they need without feeling shame about it.”
An 80-year-old patient presents with an asymptomatic firm pink plaque on his shoulder
Melanoma is a type of skin cancer that arises from melanocytes. According to the American Cancer Society, about 106,110 new melanomas will be diagnosed in the United States in 2021.The risk for developing melanoma increases with age. There are multiple clinical forms of cutaneous melanoma. The four main types are superficial spreading melanoma, nodular melanoma, melanoma in situ (lentigo maligna), and acral lentiginous melanoma. Rare variants include amelanotic melanoma, nevoid melanoma, spitzoid melanoma, and desmoplastic melanoma (DM). Melanoma can also rarely affect parts of the eye and mucosa.
, according to the Memorial Sloan Kettering Cancer Center. It typically presents as a subtle pigmented, pink, red, or skin colored patch, papule or plaque on sun-exposed skin (head and neck most frequently). Chronic UV exposure has been linked to DM. It may be mistaken for a scar or dermatofibroma. DM tends to grow locally and has less risk for nodal metastasis.1
Histologic diagnosis may be challenging. Two histologic variants in desmoplastic melanoma have been described: pure and mixed, depending on the degree of desmoplasia and cellularity present in the tumor.1 Pure DM tends to have a less aggressive course. Melanocytes can appear spindled in a fibrotic stroma. Patchy lymphocyte aggregates may be seen. Perineural invasion is more common in desmoplastic melanoma. Histologically, the differential includes spindle cell carcinoma and sarcoma. Immunostaining is helpful in differentiation.
Our patient had no lymphadenopathy on physical examination. Biopsy revealed a desmoplastic melanoma, 3.6 mm in depth, no ulceration, no regression, mitotic rate 1/mm2. He was referred to surgical oncology. The patient underwent wide excision. Sentinel lymph node biopsy was deferred.
It is imperative for dermatologists to be cognizant of this challenging subtype of melanoma when evaluating patients.
This case and photo were submitted by Dr. Bilu Martin.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to [email protected].
References
1. Chen L et al. J Am Acad Dermatol. 2013 May;68(5):825-33.
Melanoma is a type of skin cancer that arises from melanocytes. According to the American Cancer Society, about 106,110 new melanomas will be diagnosed in the United States in 2021.The risk for developing melanoma increases with age. There are multiple clinical forms of cutaneous melanoma. The four main types are superficial spreading melanoma, nodular melanoma, melanoma in situ (lentigo maligna), and acral lentiginous melanoma. Rare variants include amelanotic melanoma, nevoid melanoma, spitzoid melanoma, and desmoplastic melanoma (DM). Melanoma can also rarely affect parts of the eye and mucosa.
, according to the Memorial Sloan Kettering Cancer Center. It typically presents as a subtle pigmented, pink, red, or skin colored patch, papule or plaque on sun-exposed skin (head and neck most frequently). Chronic UV exposure has been linked to DM. It may be mistaken for a scar or dermatofibroma. DM tends to grow locally and has less risk for nodal metastasis.1
Histologic diagnosis may be challenging. Two histologic variants in desmoplastic melanoma have been described: pure and mixed, depending on the degree of desmoplasia and cellularity present in the tumor.1 Pure DM tends to have a less aggressive course. Melanocytes can appear spindled in a fibrotic stroma. Patchy lymphocyte aggregates may be seen. Perineural invasion is more common in desmoplastic melanoma. Histologically, the differential includes spindle cell carcinoma and sarcoma. Immunostaining is helpful in differentiation.
Our patient had no lymphadenopathy on physical examination. Biopsy revealed a desmoplastic melanoma, 3.6 mm in depth, no ulceration, no regression, mitotic rate 1/mm2. He was referred to surgical oncology. The patient underwent wide excision. Sentinel lymph node biopsy was deferred.
It is imperative for dermatologists to be cognizant of this challenging subtype of melanoma when evaluating patients.
This case and photo were submitted by Dr. Bilu Martin.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to [email protected].
References
1. Chen L et al. J Am Acad Dermatol. 2013 May;68(5):825-33.
Melanoma is a type of skin cancer that arises from melanocytes. According to the American Cancer Society, about 106,110 new melanomas will be diagnosed in the United States in 2021.The risk for developing melanoma increases with age. There are multiple clinical forms of cutaneous melanoma. The four main types are superficial spreading melanoma, nodular melanoma, melanoma in situ (lentigo maligna), and acral lentiginous melanoma. Rare variants include amelanotic melanoma, nevoid melanoma, spitzoid melanoma, and desmoplastic melanoma (DM). Melanoma can also rarely affect parts of the eye and mucosa.
, according to the Memorial Sloan Kettering Cancer Center. It typically presents as a subtle pigmented, pink, red, or skin colored patch, papule or plaque on sun-exposed skin (head and neck most frequently). Chronic UV exposure has been linked to DM. It may be mistaken for a scar or dermatofibroma. DM tends to grow locally and has less risk for nodal metastasis.1
Histologic diagnosis may be challenging. Two histologic variants in desmoplastic melanoma have been described: pure and mixed, depending on the degree of desmoplasia and cellularity present in the tumor.1 Pure DM tends to have a less aggressive course. Melanocytes can appear spindled in a fibrotic stroma. Patchy lymphocyte aggregates may be seen. Perineural invasion is more common in desmoplastic melanoma. Histologically, the differential includes spindle cell carcinoma and sarcoma. Immunostaining is helpful in differentiation.
Our patient had no lymphadenopathy on physical examination. Biopsy revealed a desmoplastic melanoma, 3.6 mm in depth, no ulceration, no regression, mitotic rate 1/mm2. He was referred to surgical oncology. The patient underwent wide excision. Sentinel lymph node biopsy was deferred.
It is imperative for dermatologists to be cognizant of this challenging subtype of melanoma when evaluating patients.
This case and photo were submitted by Dr. Bilu Martin.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to [email protected].
References
1. Chen L et al. J Am Acad Dermatol. 2013 May;68(5):825-33.