User login
Decline in child COVID-19 cases picks up after 2-week slowdown
, according to data gathered by the American Academy of Pediatrics and the Children’s Hospital Association.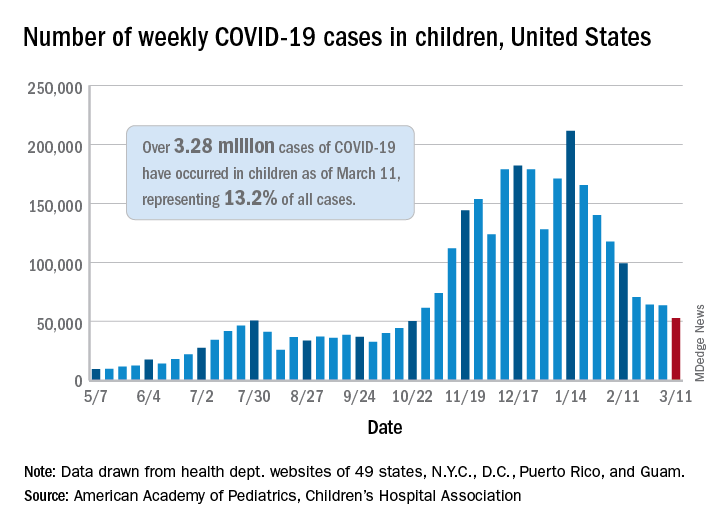
From Feb. 19 to March 4, the drop in new cases averaged just 5% each week, compared with 13.3% per week over the 5-week period from Jan. 15 to Feb. 18. For the week of March 5-11, a total of 52,695 COVID-19 cases were reported in children, down from 63,562 the previous week and the lowest number since late October, based on data from 49 states (excluding New York), the District of Columbia, New York City, Puerto Rico, and Guam.
In those jurisdictions, 3.28 million children have been infected with SARS-CoV-2, representing 13.2% of all cases since the beginning of the pandemic. The cumulative rate of COVID-19 has now risen to 4,364 cases per 100,000 children nationally, with state rates ranging from 1,062 per 100,000 in Hawaii to 8,692 per 100,000 in North Dakota, the AAP and CHA said in their weekly COVID-19 report.
Hospitalization data are more limited – 24 states and New York City – but continue to show that serious illness is much less common in younger individuals: Children represent just 1.9% of all hospitalizations, and only 0.8% of the children who have been infected were hospitalized. Neither rate has changed since early February, the AAP and CHA said.
The number of deaths in children, however, rose from 253 to 266, the largest 1-week increase since early February in the 43 states (along with New York City, Puerto Rico, and Guam) that are tracking mortality data by age, the AAP and CHA reported.
Among those 46 jurisdictions, there are 10 (9 states and the District of Columbia) that have not yet reported a COVID-19–related child death, while Texas has almost twice as many deaths, 47, as the next state, Arizona, which has 24. Meanwhile, California’s total of 452,000 cases is almost 2½ times higher than the 183,000 recorded by Illinois, according to the report.
, according to data gathered by the American Academy of Pediatrics and the Children’s Hospital Association.
From Feb. 19 to March 4, the drop in new cases averaged just 5% each week, compared with 13.3% per week over the 5-week period from Jan. 15 to Feb. 18. For the week of March 5-11, a total of 52,695 COVID-19 cases were reported in children, down from 63,562 the previous week and the lowest number since late October, based on data from 49 states (excluding New York), the District of Columbia, New York City, Puerto Rico, and Guam.
In those jurisdictions, 3.28 million children have been infected with SARS-CoV-2, representing 13.2% of all cases since the beginning of the pandemic. The cumulative rate of COVID-19 has now risen to 4,364 cases per 100,000 children nationally, with state rates ranging from 1,062 per 100,000 in Hawaii to 8,692 per 100,000 in North Dakota, the AAP and CHA said in their weekly COVID-19 report.
Hospitalization data are more limited – 24 states and New York City – but continue to show that serious illness is much less common in younger individuals: Children represent just 1.9% of all hospitalizations, and only 0.8% of the children who have been infected were hospitalized. Neither rate has changed since early February, the AAP and CHA said.
The number of deaths in children, however, rose from 253 to 266, the largest 1-week increase since early February in the 43 states (along with New York City, Puerto Rico, and Guam) that are tracking mortality data by age, the AAP and CHA reported.
Among those 46 jurisdictions, there are 10 (9 states and the District of Columbia) that have not yet reported a COVID-19–related child death, while Texas has almost twice as many deaths, 47, as the next state, Arizona, which has 24. Meanwhile, California’s total of 452,000 cases is almost 2½ times higher than the 183,000 recorded by Illinois, according to the report.
, according to data gathered by the American Academy of Pediatrics and the Children’s Hospital Association.
From Feb. 19 to March 4, the drop in new cases averaged just 5% each week, compared with 13.3% per week over the 5-week period from Jan. 15 to Feb. 18. For the week of March 5-11, a total of 52,695 COVID-19 cases were reported in children, down from 63,562 the previous week and the lowest number since late October, based on data from 49 states (excluding New York), the District of Columbia, New York City, Puerto Rico, and Guam.
In those jurisdictions, 3.28 million children have been infected with SARS-CoV-2, representing 13.2% of all cases since the beginning of the pandemic. The cumulative rate of COVID-19 has now risen to 4,364 cases per 100,000 children nationally, with state rates ranging from 1,062 per 100,000 in Hawaii to 8,692 per 100,000 in North Dakota, the AAP and CHA said in their weekly COVID-19 report.
Hospitalization data are more limited – 24 states and New York City – but continue to show that serious illness is much less common in younger individuals: Children represent just 1.9% of all hospitalizations, and only 0.8% of the children who have been infected were hospitalized. Neither rate has changed since early February, the AAP and CHA said.
The number of deaths in children, however, rose from 253 to 266, the largest 1-week increase since early February in the 43 states (along with New York City, Puerto Rico, and Guam) that are tracking mortality data by age, the AAP and CHA reported.
Among those 46 jurisdictions, there are 10 (9 states and the District of Columbia) that have not yet reported a COVID-19–related child death, while Texas has almost twice as many deaths, 47, as the next state, Arizona, which has 24. Meanwhile, California’s total of 452,000 cases is almost 2½ times higher than the 183,000 recorded by Illinois, according to the report.
The Zoom effect on cosmetic procedures
As clinics were allowed to reopen under local government guidelines several months into the COVID-19 pandemic, many cosmetic dermatologists and aesthetic surgeons had no idea what our schedules would be like. .
While scheduled appointments, no shows, cancellations, and rebookings seem to wax and wane with surges in COVID-19 cases locally and with associated media coverage, there appear to be several reasons why demand has increased. Because people are wearing masks, they can easily hide signs of recovery or “something new” in their appearance. Patients aren’t typically around as many people and have more time to recover in private. There is also the positive effect a procedure can have on mood and self-esteem during what has been a difficult year. And people have had more time to read beauty and self-care articles, as well as advertisements for skin and hair care on social media.
The Zoom effect
One reason I did not anticipate is the Zoom effect. I don’t intend to single out Zoom – as there are other videoconferencing options available – but it seems to be the one patients bring up the most. Virtual meetings, conferences, and social events, and video calls with loved ones have become a part of daily routines for many, who are now seeing themselves on camera during these interactions as they never did before. It has created a strange new phenomenon.
Patients have literally said to me “I don’t like the way I look on Zoom” and ask about options to improve what they are seeing. They are often surprised to see that their appearance on virtual meetings, for example, does not reflect the way they feel inside, or how they think they should look. Even medical dermatology patients who have had no interest in cosmetic procedures previously have been coming in for this specific reason – both female and male patients.
Since photography is a hobby, I counsel patients that lighting and shadows play a huge role in how they appear on screen. Depending on the lighting, camera angle, and camera quality, suboptimal lighting can highlight shadows and wrinkles not normally seen in natural or optimal light. In a recent interview on KCRW, the Los Angeles NPR affiliate station, the founding director of the Virtual Human Interaction Lab (VHIL) at Stanford University highlighted work on the effect that Zoom and virtual interactions have had on people during the COVID-19 pandemic. He notes that during a normal in-person meeting or conference, attention is usually on the person speaking, but now with everyone on camera at once, people have the pressure and subsequent feelings of exhaustion (a different type of exhaustion than being there in person) of being seen at all times. To address “Zoom Fatigue,” the VHIL’s recommendations include turning off the camera periodically, or changing the settings so your image is not seen. Another option is to use background filters, including some face filters (a cat for example), which Zoom has created to ease some of the stress of these meetings.
Back to the actual in-person office visits: In my experience, all cosmetic procedures across the board, including injectables, skin resurfacing, and lasers have increased. In Dr. Talakoub’s practice, she has noted a tenfold increase in the use of deoxycholic acid (Kybella) and neck procedures attributed to the unflattering angle of the neck as people look down on their computer screens.There has also been an increase in the use of other injectables, such as Botox of the glabella to address scowling at the screen, facial fillers to address the dark shadows cast on the tear troughs, and lip fillers (noted to be 10-20 times higher) because of masks that can hide healing downtime. Similarly, increased use of Coolsculpting has been noted, as some patients have the flexibility of being able to take their Zoom meetings during the procedure, when they otherwise may not have had the time. Some patients have told me that the appointment with me is the only visit they’ve made outside of their home during the pandemic. Once the consultations or procedures are completed, patients often show gratitude and their self-esteem is increased. Some patients have said they even feel better and more productive at work, or note more positive interactions with their loved ones after the work has been done, likely because they feel better about themselves.There have been discussions about the benefits people have in being able to use Zoom and other videoconferencing platforms to gather and create, as well as see people and communicate in a way that can sometimes be more effective than a phone call. As physicians, these virtual tools have also allowed us to provide telehealth visits, a flexible, safe, and comfortable option for both the patient and practitioner. If done in a safe place, the ability to see each other without wearing a mask is also a nice treat.
The gratification and improvement in psyche that patients experience after our visits during this unprecedented, challenging time has been evident. Perhaps it’s the social interaction with their trusted physician, the outcome of the procedure itself, or a combination of both, which has a net positive effect on the physician-patient relationship.
While cosmetic procedures are appropriately deemed elective by hospital facilities and practitioners and should be of lower importance with regard to use of available facilities and PPE than those related to COVID-19 and other life-threatening scenarios, the longevity of this pandemic has surprisingly highlighted the numerous ways in which cosmetic visits can help patients, and the importance of being able to be there for patients – in a safe manner for all involved.
Dr. Wesley and Dr. Talakoub are cocontributors to this column. Dr. Wesley practices dermatology in Beverly Hills, Calif. Dr. Talakoub is in private practice in McLean, Va. This month’s column is by Dr. Wesley. Write to them at [email protected]. They had no relevant disclosures.
As clinics were allowed to reopen under local government guidelines several months into the COVID-19 pandemic, many cosmetic dermatologists and aesthetic surgeons had no idea what our schedules would be like. .
While scheduled appointments, no shows, cancellations, and rebookings seem to wax and wane with surges in COVID-19 cases locally and with associated media coverage, there appear to be several reasons why demand has increased. Because people are wearing masks, they can easily hide signs of recovery or “something new” in their appearance. Patients aren’t typically around as many people and have more time to recover in private. There is also the positive effect a procedure can have on mood and self-esteem during what has been a difficult year. And people have had more time to read beauty and self-care articles, as well as advertisements for skin and hair care on social media.
The Zoom effect
One reason I did not anticipate is the Zoom effect. I don’t intend to single out Zoom – as there are other videoconferencing options available – but it seems to be the one patients bring up the most. Virtual meetings, conferences, and social events, and video calls with loved ones have become a part of daily routines for many, who are now seeing themselves on camera during these interactions as they never did before. It has created a strange new phenomenon.
Patients have literally said to me “I don’t like the way I look on Zoom” and ask about options to improve what they are seeing. They are often surprised to see that their appearance on virtual meetings, for example, does not reflect the way they feel inside, or how they think they should look. Even medical dermatology patients who have had no interest in cosmetic procedures previously have been coming in for this specific reason – both female and male patients.
Since photography is a hobby, I counsel patients that lighting and shadows play a huge role in how they appear on screen. Depending on the lighting, camera angle, and camera quality, suboptimal lighting can highlight shadows and wrinkles not normally seen in natural or optimal light. In a recent interview on KCRW, the Los Angeles NPR affiliate station, the founding director of the Virtual Human Interaction Lab (VHIL) at Stanford University highlighted work on the effect that Zoom and virtual interactions have had on people during the COVID-19 pandemic. He notes that during a normal in-person meeting or conference, attention is usually on the person speaking, but now with everyone on camera at once, people have the pressure and subsequent feelings of exhaustion (a different type of exhaustion than being there in person) of being seen at all times. To address “Zoom Fatigue,” the VHIL’s recommendations include turning off the camera periodically, or changing the settings so your image is not seen. Another option is to use background filters, including some face filters (a cat for example), which Zoom has created to ease some of the stress of these meetings.
Back to the actual in-person office visits: In my experience, all cosmetic procedures across the board, including injectables, skin resurfacing, and lasers have increased. In Dr. Talakoub’s practice, she has noted a tenfold increase in the use of deoxycholic acid (Kybella) and neck procedures attributed to the unflattering angle of the neck as people look down on their computer screens.There has also been an increase in the use of other injectables, such as Botox of the glabella to address scowling at the screen, facial fillers to address the dark shadows cast on the tear troughs, and lip fillers (noted to be 10-20 times higher) because of masks that can hide healing downtime. Similarly, increased use of Coolsculpting has been noted, as some patients have the flexibility of being able to take their Zoom meetings during the procedure, when they otherwise may not have had the time. Some patients have told me that the appointment with me is the only visit they’ve made outside of their home during the pandemic. Once the consultations or procedures are completed, patients often show gratitude and their self-esteem is increased. Some patients have said they even feel better and more productive at work, or note more positive interactions with their loved ones after the work has been done, likely because they feel better about themselves.There have been discussions about the benefits people have in being able to use Zoom and other videoconferencing platforms to gather and create, as well as see people and communicate in a way that can sometimes be more effective than a phone call. As physicians, these virtual tools have also allowed us to provide telehealth visits, a flexible, safe, and comfortable option for both the patient and practitioner. If done in a safe place, the ability to see each other without wearing a mask is also a nice treat.
The gratification and improvement in psyche that patients experience after our visits during this unprecedented, challenging time has been evident. Perhaps it’s the social interaction with their trusted physician, the outcome of the procedure itself, or a combination of both, which has a net positive effect on the physician-patient relationship.
While cosmetic procedures are appropriately deemed elective by hospital facilities and practitioners and should be of lower importance with regard to use of available facilities and PPE than those related to COVID-19 and other life-threatening scenarios, the longevity of this pandemic has surprisingly highlighted the numerous ways in which cosmetic visits can help patients, and the importance of being able to be there for patients – in a safe manner for all involved.
Dr. Wesley and Dr. Talakoub are cocontributors to this column. Dr. Wesley practices dermatology in Beverly Hills, Calif. Dr. Talakoub is in private practice in McLean, Va. This month’s column is by Dr. Wesley. Write to them at [email protected]. They had no relevant disclosures.
As clinics were allowed to reopen under local government guidelines several months into the COVID-19 pandemic, many cosmetic dermatologists and aesthetic surgeons had no idea what our schedules would be like. .
While scheduled appointments, no shows, cancellations, and rebookings seem to wax and wane with surges in COVID-19 cases locally and with associated media coverage, there appear to be several reasons why demand has increased. Because people are wearing masks, they can easily hide signs of recovery or “something new” in their appearance. Patients aren’t typically around as many people and have more time to recover in private. There is also the positive effect a procedure can have on mood and self-esteem during what has been a difficult year. And people have had more time to read beauty and self-care articles, as well as advertisements for skin and hair care on social media.
The Zoom effect
One reason I did not anticipate is the Zoom effect. I don’t intend to single out Zoom – as there are other videoconferencing options available – but it seems to be the one patients bring up the most. Virtual meetings, conferences, and social events, and video calls with loved ones have become a part of daily routines for many, who are now seeing themselves on camera during these interactions as they never did before. It has created a strange new phenomenon.
Patients have literally said to me “I don’t like the way I look on Zoom” and ask about options to improve what they are seeing. They are often surprised to see that their appearance on virtual meetings, for example, does not reflect the way they feel inside, or how they think they should look. Even medical dermatology patients who have had no interest in cosmetic procedures previously have been coming in for this specific reason – both female and male patients.
Since photography is a hobby, I counsel patients that lighting and shadows play a huge role in how they appear on screen. Depending on the lighting, camera angle, and camera quality, suboptimal lighting can highlight shadows and wrinkles not normally seen in natural or optimal light. In a recent interview on KCRW, the Los Angeles NPR affiliate station, the founding director of the Virtual Human Interaction Lab (VHIL) at Stanford University highlighted work on the effect that Zoom and virtual interactions have had on people during the COVID-19 pandemic. He notes that during a normal in-person meeting or conference, attention is usually on the person speaking, but now with everyone on camera at once, people have the pressure and subsequent feelings of exhaustion (a different type of exhaustion than being there in person) of being seen at all times. To address “Zoom Fatigue,” the VHIL’s recommendations include turning off the camera periodically, or changing the settings so your image is not seen. Another option is to use background filters, including some face filters (a cat for example), which Zoom has created to ease some of the stress of these meetings.
Back to the actual in-person office visits: In my experience, all cosmetic procedures across the board, including injectables, skin resurfacing, and lasers have increased. In Dr. Talakoub’s practice, she has noted a tenfold increase in the use of deoxycholic acid (Kybella) and neck procedures attributed to the unflattering angle of the neck as people look down on their computer screens.There has also been an increase in the use of other injectables, such as Botox of the glabella to address scowling at the screen, facial fillers to address the dark shadows cast on the tear troughs, and lip fillers (noted to be 10-20 times higher) because of masks that can hide healing downtime. Similarly, increased use of Coolsculpting has been noted, as some patients have the flexibility of being able to take their Zoom meetings during the procedure, when they otherwise may not have had the time. Some patients have told me that the appointment with me is the only visit they’ve made outside of their home during the pandemic. Once the consultations or procedures are completed, patients often show gratitude and their self-esteem is increased. Some patients have said they even feel better and more productive at work, or note more positive interactions with their loved ones after the work has been done, likely because they feel better about themselves.There have been discussions about the benefits people have in being able to use Zoom and other videoconferencing platforms to gather and create, as well as see people and communicate in a way that can sometimes be more effective than a phone call. As physicians, these virtual tools have also allowed us to provide telehealth visits, a flexible, safe, and comfortable option for both the patient and practitioner. If done in a safe place, the ability to see each other without wearing a mask is also a nice treat.
The gratification and improvement in psyche that patients experience after our visits during this unprecedented, challenging time has been evident. Perhaps it’s the social interaction with their trusted physician, the outcome of the procedure itself, or a combination of both, which has a net positive effect on the physician-patient relationship.
While cosmetic procedures are appropriately deemed elective by hospital facilities and practitioners and should be of lower importance with regard to use of available facilities and PPE than those related to COVID-19 and other life-threatening scenarios, the longevity of this pandemic has surprisingly highlighted the numerous ways in which cosmetic visits can help patients, and the importance of being able to be there for patients – in a safe manner for all involved.
Dr. Wesley and Dr. Talakoub are cocontributors to this column. Dr. Wesley practices dermatology in Beverly Hills, Calif. Dr. Talakoub is in private practice in McLean, Va. This month’s column is by Dr. Wesley. Write to them at [email protected]. They had no relevant disclosures.
Nota bene: Don’t change a note
A man I saw earlier this year called my office recently. He’d read my note, and after discussing it with his attorney, wanted me to make changes. He said that the way I’d described his symptoms would be damaging to a legal action he was involved in, and so he wanted it reworded.
Obviously, I said “no.” I’m not a fiction writer. My notes are what people tell me happened. I don’t make them up.
He wasn’t happy about this, and a few days later I got a request to send his records to another neurologist, which I gladly did.
In this business a large measure of what we do is based on the patient’s symptoms. what they report to us, and how they describe it. When I put their story into a written note, my goal is to be as accurate to what they told me as possible. I’m not here to embellish, disparage, or minimize their history.
To do so is to look bad at the very least and open yourself up to malpractice and legal charges at worst.
This isn’t to say errors can’t be modified. I’m far from perfect. Sometimes I might misunderstand something a patient tells me, or get information crossed up. When that happens there’s nothing wrong with writing an addendum, clarifying or correcting what my earlier note said – but not changing the original note.
I asked the unhappy patient to write down what he thought was incorrect, and I’d be willing to include that in his chart. He wasn’t willing to do that, and there was no way I would ever change a note. I tried to politely explain the reasons why, but he wasn’t listening.
So I lost him as a patient. After 23 years of practice, that doesn’t bother me. I learned a long time ago that I can’t please everyone, nor can I be everyone’s doctor. Saying “no” is just as important as saying “yes,” though at times more difficult.
This time, though, the answer was pretty obvious, and will be the same next time I get the same request from a patient.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
A man I saw earlier this year called my office recently. He’d read my note, and after discussing it with his attorney, wanted me to make changes. He said that the way I’d described his symptoms would be damaging to a legal action he was involved in, and so he wanted it reworded.
Obviously, I said “no.” I’m not a fiction writer. My notes are what people tell me happened. I don’t make them up.
He wasn’t happy about this, and a few days later I got a request to send his records to another neurologist, which I gladly did.
In this business a large measure of what we do is based on the patient’s symptoms. what they report to us, and how they describe it. When I put their story into a written note, my goal is to be as accurate to what they told me as possible. I’m not here to embellish, disparage, or minimize their history.
To do so is to look bad at the very least and open yourself up to malpractice and legal charges at worst.
This isn’t to say errors can’t be modified. I’m far from perfect. Sometimes I might misunderstand something a patient tells me, or get information crossed up. When that happens there’s nothing wrong with writing an addendum, clarifying or correcting what my earlier note said – but not changing the original note.
I asked the unhappy patient to write down what he thought was incorrect, and I’d be willing to include that in his chart. He wasn’t willing to do that, and there was no way I would ever change a note. I tried to politely explain the reasons why, but he wasn’t listening.
So I lost him as a patient. After 23 years of practice, that doesn’t bother me. I learned a long time ago that I can’t please everyone, nor can I be everyone’s doctor. Saying “no” is just as important as saying “yes,” though at times more difficult.
This time, though, the answer was pretty obvious, and will be the same next time I get the same request from a patient.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
A man I saw earlier this year called my office recently. He’d read my note, and after discussing it with his attorney, wanted me to make changes. He said that the way I’d described his symptoms would be damaging to a legal action he was involved in, and so he wanted it reworded.
Obviously, I said “no.” I’m not a fiction writer. My notes are what people tell me happened. I don’t make them up.
He wasn’t happy about this, and a few days later I got a request to send his records to another neurologist, which I gladly did.
In this business a large measure of what we do is based on the patient’s symptoms. what they report to us, and how they describe it. When I put their story into a written note, my goal is to be as accurate to what they told me as possible. I’m not here to embellish, disparage, or minimize their history.
To do so is to look bad at the very least and open yourself up to malpractice and legal charges at worst.
This isn’t to say errors can’t be modified. I’m far from perfect. Sometimes I might misunderstand something a patient tells me, or get information crossed up. When that happens there’s nothing wrong with writing an addendum, clarifying or correcting what my earlier note said – but not changing the original note.
I asked the unhappy patient to write down what he thought was incorrect, and I’d be willing to include that in his chart. He wasn’t willing to do that, and there was no way I would ever change a note. I tried to politely explain the reasons why, but he wasn’t listening.
So I lost him as a patient. After 23 years of practice, that doesn’t bother me. I learned a long time ago that I can’t please everyone, nor can I be everyone’s doctor. Saying “no” is just as important as saying “yes,” though at times more difficult.
This time, though, the answer was pretty obvious, and will be the same next time I get the same request from a patient.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
Blood cancer patients, survivors hesitate over COVID-19 vaccine
Nearly one in three patients with blood cancer, and survivors, say they are unlikely to get a COVID-19 vaccine or unsure about getting it if one were available. The findings come from a nationwide survey by The Leukemia & Lymphoma Society, which collected 6,517 responses.
“These findings are worrisome, to say the least,” Gwen Nichols, MD, chief medical officer of the society, said in a statement.
“We know cancer patients – and blood cancer patients in particular – are susceptible to the worst effects of the virus [and] all of us in the medical community need to help cancer patients understand the importance of getting vaccinated,” she added.
The survey – the largest ever done in which cancer patients and survivors were asked about their attitudes toward COVID-19 vaccines – was published online March 8 by The Leukemia & Lymphoma Society.
Survey sample
The survey asked patients with blood cancer, and survivors, about their attitudes regarding COVID-19 and COVID-19 vaccines.
“The main outcome [was] vaccine attitudes,” noted the authors, headed by Rena Conti, PhD, dean’s research scholar, Boston University.
Respondents were asked: “How likely are you to choose to get the vaccine?” Participants could indicate they were very unlikely, unlikely, neither likely nor unlikely, likely, or very likely to get vaccinated.
“We found that 17% of respondents indicate[d] that they [were] unlikely or very unlikely to take a vaccine,” Dr. Conti and colleagues observed.
Among the 17% – deemed to be “vaccine hesitant” – slightly over half (54%) stated they had concerns about the side effects associated with COVID-19 vaccination and believed neither of the two newly approved vaccines had been or would ever be tested properly.
The survey authors noted that there is no reason to believe COVID-19 vaccines are any less safe in patients with blood cancers, but concerns have been expressed that patients with some forms of blood cancer or those undergoing certain treatments may not achieve the same immune response to the vaccine as would noncancer controls.
Importantly, the survey was conducted Dec. 1-21, 2020, and responses differed depending on whether respondents answered the survey before or after the Pfizer-BioNTech and Moderna vaccines had been given emergency use authorization by the Food and Drug Administration starting Dec. 10, 2020.
There was a slight increase in positive responses after the vaccines were granted regulatory approval. (One-third of those who responded to the survey after the approval were 3.7% more likely to indicate they would get vaccinated). “This suggests that hesitancy may be influenced by emerging information dissemination, government action, and vaccine availability, transforming the hypothetical opportunity of vaccination to a real one,” the survey authors speculated.
Survey respondents who were vaccine hesitant were also over 14% more likely to indicate that they didn’t think they would require hospitalization should they contract COVID-19. But clinical data have suggested that approximately half of patients with a hematological malignancy who required hospitalization for COVID-19 die from the infection, the authors noted.
“Vaccine hesitant respondents [were] also significantly less likely to engage in protective health behaviors,” the survey authors pointed out. For example, they were almost 4% less likely to have worn a face mask and 1.6% less likely to have taken other protective measures to guard against COVID-19 infection.
Need for clear messaging
To counter vaccine hesitancy, the authors suggest there is a need for clear, consistent messaging targeting patients with cancer that emphasize the risks of COVID-19 and underscore vaccine benefits.
Dr. Conti pointed out that patients with blood cancer are, in fact, being given preferential access to vaccines in many communities, although this clearly doesn’t mean patients are willing to get vaccinated, as she also noted.
“We need both adequate supply and strong demand to keep this vulnerable population safe,” Dr. Conti emphasized.
The Leukemia & Lymphoma Society plans to repeat the survey in the near future to assess patients’ and survivors’ access to vaccines as well as their willingness to get vaccinated.
The authors have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Nearly one in three patients with blood cancer, and survivors, say they are unlikely to get a COVID-19 vaccine or unsure about getting it if one were available. The findings come from a nationwide survey by The Leukemia & Lymphoma Society, which collected 6,517 responses.
“These findings are worrisome, to say the least,” Gwen Nichols, MD, chief medical officer of the society, said in a statement.
“We know cancer patients – and blood cancer patients in particular – are susceptible to the worst effects of the virus [and] all of us in the medical community need to help cancer patients understand the importance of getting vaccinated,” she added.
The survey – the largest ever done in which cancer patients and survivors were asked about their attitudes toward COVID-19 vaccines – was published online March 8 by The Leukemia & Lymphoma Society.
Survey sample
The survey asked patients with blood cancer, and survivors, about their attitudes regarding COVID-19 and COVID-19 vaccines.
“The main outcome [was] vaccine attitudes,” noted the authors, headed by Rena Conti, PhD, dean’s research scholar, Boston University.
Respondents were asked: “How likely are you to choose to get the vaccine?” Participants could indicate they were very unlikely, unlikely, neither likely nor unlikely, likely, or very likely to get vaccinated.
“We found that 17% of respondents indicate[d] that they [were] unlikely or very unlikely to take a vaccine,” Dr. Conti and colleagues observed.
Among the 17% – deemed to be “vaccine hesitant” – slightly over half (54%) stated they had concerns about the side effects associated with COVID-19 vaccination and believed neither of the two newly approved vaccines had been or would ever be tested properly.
The survey authors noted that there is no reason to believe COVID-19 vaccines are any less safe in patients with blood cancers, but concerns have been expressed that patients with some forms of blood cancer or those undergoing certain treatments may not achieve the same immune response to the vaccine as would noncancer controls.
Importantly, the survey was conducted Dec. 1-21, 2020, and responses differed depending on whether respondents answered the survey before or after the Pfizer-BioNTech and Moderna vaccines had been given emergency use authorization by the Food and Drug Administration starting Dec. 10, 2020.
There was a slight increase in positive responses after the vaccines were granted regulatory approval. (One-third of those who responded to the survey after the approval were 3.7% more likely to indicate they would get vaccinated). “This suggests that hesitancy may be influenced by emerging information dissemination, government action, and vaccine availability, transforming the hypothetical opportunity of vaccination to a real one,” the survey authors speculated.
Survey respondents who were vaccine hesitant were also over 14% more likely to indicate that they didn’t think they would require hospitalization should they contract COVID-19. But clinical data have suggested that approximately half of patients with a hematological malignancy who required hospitalization for COVID-19 die from the infection, the authors noted.
“Vaccine hesitant respondents [were] also significantly less likely to engage in protective health behaviors,” the survey authors pointed out. For example, they were almost 4% less likely to have worn a face mask and 1.6% less likely to have taken other protective measures to guard against COVID-19 infection.
Need for clear messaging
To counter vaccine hesitancy, the authors suggest there is a need for clear, consistent messaging targeting patients with cancer that emphasize the risks of COVID-19 and underscore vaccine benefits.
Dr. Conti pointed out that patients with blood cancer are, in fact, being given preferential access to vaccines in many communities, although this clearly doesn’t mean patients are willing to get vaccinated, as she also noted.
“We need both adequate supply and strong demand to keep this vulnerable population safe,” Dr. Conti emphasized.
The Leukemia & Lymphoma Society plans to repeat the survey in the near future to assess patients’ and survivors’ access to vaccines as well as their willingness to get vaccinated.
The authors have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Nearly one in three patients with blood cancer, and survivors, say they are unlikely to get a COVID-19 vaccine or unsure about getting it if one were available. The findings come from a nationwide survey by The Leukemia & Lymphoma Society, which collected 6,517 responses.
“These findings are worrisome, to say the least,” Gwen Nichols, MD, chief medical officer of the society, said in a statement.
“We know cancer patients – and blood cancer patients in particular – are susceptible to the worst effects of the virus [and] all of us in the medical community need to help cancer patients understand the importance of getting vaccinated,” she added.
The survey – the largest ever done in which cancer patients and survivors were asked about their attitudes toward COVID-19 vaccines – was published online March 8 by The Leukemia & Lymphoma Society.
Survey sample
The survey asked patients with blood cancer, and survivors, about their attitudes regarding COVID-19 and COVID-19 vaccines.
“The main outcome [was] vaccine attitudes,” noted the authors, headed by Rena Conti, PhD, dean’s research scholar, Boston University.
Respondents were asked: “How likely are you to choose to get the vaccine?” Participants could indicate they were very unlikely, unlikely, neither likely nor unlikely, likely, or very likely to get vaccinated.
“We found that 17% of respondents indicate[d] that they [were] unlikely or very unlikely to take a vaccine,” Dr. Conti and colleagues observed.
Among the 17% – deemed to be “vaccine hesitant” – slightly over half (54%) stated they had concerns about the side effects associated with COVID-19 vaccination and believed neither of the two newly approved vaccines had been or would ever be tested properly.
The survey authors noted that there is no reason to believe COVID-19 vaccines are any less safe in patients with blood cancers, but concerns have been expressed that patients with some forms of blood cancer or those undergoing certain treatments may not achieve the same immune response to the vaccine as would noncancer controls.
Importantly, the survey was conducted Dec. 1-21, 2020, and responses differed depending on whether respondents answered the survey before or after the Pfizer-BioNTech and Moderna vaccines had been given emergency use authorization by the Food and Drug Administration starting Dec. 10, 2020.
There was a slight increase in positive responses after the vaccines were granted regulatory approval. (One-third of those who responded to the survey after the approval were 3.7% more likely to indicate they would get vaccinated). “This suggests that hesitancy may be influenced by emerging information dissemination, government action, and vaccine availability, transforming the hypothetical opportunity of vaccination to a real one,” the survey authors speculated.
Survey respondents who were vaccine hesitant were also over 14% more likely to indicate that they didn’t think they would require hospitalization should they contract COVID-19. But clinical data have suggested that approximately half of patients with a hematological malignancy who required hospitalization for COVID-19 die from the infection, the authors noted.
“Vaccine hesitant respondents [were] also significantly less likely to engage in protective health behaviors,” the survey authors pointed out. For example, they were almost 4% less likely to have worn a face mask and 1.6% less likely to have taken other protective measures to guard against COVID-19 infection.
Need for clear messaging
To counter vaccine hesitancy, the authors suggest there is a need for clear, consistent messaging targeting patients with cancer that emphasize the risks of COVID-19 and underscore vaccine benefits.
Dr. Conti pointed out that patients with blood cancer are, in fact, being given preferential access to vaccines in many communities, although this clearly doesn’t mean patients are willing to get vaccinated, as she also noted.
“We need both adequate supply and strong demand to keep this vulnerable population safe,” Dr. Conti emphasized.
The Leukemia & Lymphoma Society plans to repeat the survey in the near future to assess patients’ and survivors’ access to vaccines as well as their willingness to get vaccinated.
The authors have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Novel Alzheimer’s drug slows cognitive decline in phase 2 trial
Results from the TRAILBLAZER-ALZ trial were presented at the 2021 International Conference on Alzheimer’s and Parkinson’s Diseases (AD/PD) and were simultaneously published online March 13 in the New England Journal of Medicine.
As previously reported by Medscape Medical News, topline results showed that donanemab slowed cognitive decline by 32% on the Integrated AD Rating Scale (iADRS) from baseline to 76 weeks relative to placebo.
The newly released detailed findings showed that “the use of donanemab resulted in a better composite score for cognition and for the ability to perform activities of daily living than placebo at 76 weeks, although results for secondary outcomes were mixed,” the investigators, with first author Mark A. Mintun, MD, an employee of Eli Lilly, reported.
Results revealed improvement in scores on the Clinical Dementia Rating Scale-Sum of Boxes (CDR-SB) and the 13-item cognitive subscale of the AD Assessment Scale (ADAS-Cog13), but the differences between the two treatment groups were not significant. In addition, score changes on the AD Cooperative Study–Instrumental Activities of Daily Inventory (ADCS-iADL) and the Mini-Mental State Examination (MMSE) were not “substantial.”
However, the donanemab group did show an 85-centiloid greater reduction in amyloid plaque level at 76 weeks, as shown on PET, compared with the placebo group.
Proof of concept?
The humanized antibody donanemab, which was previously known as LY3002813, targets a modified form of deposited amyloid-beta (A-beta) peptide called N3pG.
The randomized, placebo-controlled, double-blind TRAILBLAZER-ALZ trial, which was described as a “phase 2 proof of concept trial” in the AD/PD program, was conducted at 56 sites in the United States and Canada and included 257 patients between the ages of 60 and 85 years (52% were women). PET confirmed tau and amyloid deposition in all participants.
The active treatment group (n = 131) was randomly assigned to receive donanemab 700 mg for three doses; after that, treatment was bumped up to 1,400 mg. Both the donanemab and placebo groups (n = 126) received treatment intravenously every 4 weeks for up to 72 weeks.
Participants also underwent F-florbetapir and F-flortaucipir PET scans at various timepoints and completed a slew of cognitive tests.
The study’s primary outcome measure was change between baseline and 76 weeks post treatment on composite score for cognition, as measured by the iADRS. The iADRS combines the ADAS-Cog13 and the ADCS-iADL.
This measure ranges from 0 to 144, with lower scores associated with greater cognitive impairment. Both treatment groups had an iADRS score of 106 at baseline.
More research needed
Results showed that the score change from baseline on the iADRS was –6.86 for the active treatment group vs –10.06 for the placebo group (group difference, 3.2; 95% confidence interval [CI], 0.12-6.27; P = .04). Although significant, “the trial was powered to show a 6-point difference,” which was not met, the investigators note.
Differences in score changes from baseline to 76 weeks for the treatment vs. placebo groups on the following secondary outcome measures were:
- CDR-SB: –0.36 (95% CI, –0.83 to –0.12).
- ADAS-Cog13: –1.86 (95% CI, –3.63 to –0.09).
- ADCS-iADL: 1.21 (95% CI, –0.77 to 3.2).
- MMSE: 0.64 (95% CI, –0.4 to 1.67).
The CDR-SB was designated as the first secondary outcome, and because it did not show a significant between-group difference, “the hierarchy failed and no definite conclusions can be drawn from data regarding the differences between groups in the change in the ADAS-Cog13,” the investigators wrote.
In addition, the differences in scores on the latter two secondary outcomes were not “substantial,” they reported.
However, at 76 weeks, the donanemab group showed a reduction of 84.13 centiloids in amyloid plaque level vs. an increase of 0.93 centiloids in the placebo group (between-group difference, 85.06 centiloids). At 24 weeks, the active-treatment group had a 67.83-centiloids greater reduction vs. the placebo group.
In addition, 40%, 59.8%, and 67.8% of the donanemab group achieved “amyloid-negative status” at 24, 52, and 76 weeks, respectively. Amyloid-negative status was defined as an amyloid plaque level of less than 24.1 centiloids.
Total incidence of death or serious adverse events did not differ significantly between the groups. However, the donanemab group had significantly more reports of ARIA-E compared with the placebo group (26.7% vs. 0.8%).
Overall, the researchers noted that more trials of longer duration with larger patient numbers are warranted “to further determine the efficacy and safety of donanemab” in AD.
Positive signal?
In a statement, Maria Carrillo, PhD, chief science officer for the Alzheimer’s Association, said the organization “is encouraged by this promising data.
“It is the first phase 2 Alzheimer’s trial to show positive results on a primary outcome measure related to memory and thinking,” Dr. Carrillo said. However, “more work needs to be done on this experimental drug therapy.”
Dr. Carrillo noted that because the trial was moderately sized and only 180 participants completed the study, “we look forward to the results of a second, larger phase 2 trial of this drug.”
Still, she added, there were several “novel and innovative aspects” in the way the study was conducted noting that it showcases the evolution of AD research.
“I’m hopeful for the future,” Dr. Carrillo said.
Also commenting on the results, Howard Fillit, MD, neuroscientist and founding executive director and chief science officer of the Alzheimer’s Drug Discovery Foundation, said the study showed “the pharmacology works” and that the drug did what it was supposed to do in terms of removing A-beta plaque.
“It also gave us a signal in a relatively small phase 2 study that there might be a modest cognitive benefit,” said Dr. Fillit, who was not involved with the research.
He noted that although the rate of decline slowing was statistically significant it remains to be seen whether this is clinically meaningful, particularly in light of the fact that the secondary outcome results were mixed.
“Basically, it was a positive study that probably needs to be followed by another, much larger study to get us to really see the benefit,” Dr. Fillit said.
Dr. Mintun is an employee of Eli Lilly, which funded the study. Dr. Carrillo and Dr. Fillit have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Results from the TRAILBLAZER-ALZ trial were presented at the 2021 International Conference on Alzheimer’s and Parkinson’s Diseases (AD/PD) and were simultaneously published online March 13 in the New England Journal of Medicine.
As previously reported by Medscape Medical News, topline results showed that donanemab slowed cognitive decline by 32% on the Integrated AD Rating Scale (iADRS) from baseline to 76 weeks relative to placebo.
The newly released detailed findings showed that “the use of donanemab resulted in a better composite score for cognition and for the ability to perform activities of daily living than placebo at 76 weeks, although results for secondary outcomes were mixed,” the investigators, with first author Mark A. Mintun, MD, an employee of Eli Lilly, reported.
Results revealed improvement in scores on the Clinical Dementia Rating Scale-Sum of Boxes (CDR-SB) and the 13-item cognitive subscale of the AD Assessment Scale (ADAS-Cog13), but the differences between the two treatment groups were not significant. In addition, score changes on the AD Cooperative Study–Instrumental Activities of Daily Inventory (ADCS-iADL) and the Mini-Mental State Examination (MMSE) were not “substantial.”
However, the donanemab group did show an 85-centiloid greater reduction in amyloid plaque level at 76 weeks, as shown on PET, compared with the placebo group.
Proof of concept?
The humanized antibody donanemab, which was previously known as LY3002813, targets a modified form of deposited amyloid-beta (A-beta) peptide called N3pG.
The randomized, placebo-controlled, double-blind TRAILBLAZER-ALZ trial, which was described as a “phase 2 proof of concept trial” in the AD/PD program, was conducted at 56 sites in the United States and Canada and included 257 patients between the ages of 60 and 85 years (52% were women). PET confirmed tau and amyloid deposition in all participants.
The active treatment group (n = 131) was randomly assigned to receive donanemab 700 mg for three doses; after that, treatment was bumped up to 1,400 mg. Both the donanemab and placebo groups (n = 126) received treatment intravenously every 4 weeks for up to 72 weeks.
Participants also underwent F-florbetapir and F-flortaucipir PET scans at various timepoints and completed a slew of cognitive tests.
The study’s primary outcome measure was change between baseline and 76 weeks post treatment on composite score for cognition, as measured by the iADRS. The iADRS combines the ADAS-Cog13 and the ADCS-iADL.
This measure ranges from 0 to 144, with lower scores associated with greater cognitive impairment. Both treatment groups had an iADRS score of 106 at baseline.
More research needed
Results showed that the score change from baseline on the iADRS was –6.86 for the active treatment group vs –10.06 for the placebo group (group difference, 3.2; 95% confidence interval [CI], 0.12-6.27; P = .04). Although significant, “the trial was powered to show a 6-point difference,” which was not met, the investigators note.
Differences in score changes from baseline to 76 weeks for the treatment vs. placebo groups on the following secondary outcome measures were:
- CDR-SB: –0.36 (95% CI, –0.83 to –0.12).
- ADAS-Cog13: –1.86 (95% CI, –3.63 to –0.09).
- ADCS-iADL: 1.21 (95% CI, –0.77 to 3.2).
- MMSE: 0.64 (95% CI, –0.4 to 1.67).
The CDR-SB was designated as the first secondary outcome, and because it did not show a significant between-group difference, “the hierarchy failed and no definite conclusions can be drawn from data regarding the differences between groups in the change in the ADAS-Cog13,” the investigators wrote.
In addition, the differences in scores on the latter two secondary outcomes were not “substantial,” they reported.
However, at 76 weeks, the donanemab group showed a reduction of 84.13 centiloids in amyloid plaque level vs. an increase of 0.93 centiloids in the placebo group (between-group difference, 85.06 centiloids). At 24 weeks, the active-treatment group had a 67.83-centiloids greater reduction vs. the placebo group.
In addition, 40%, 59.8%, and 67.8% of the donanemab group achieved “amyloid-negative status” at 24, 52, and 76 weeks, respectively. Amyloid-negative status was defined as an amyloid plaque level of less than 24.1 centiloids.
Total incidence of death or serious adverse events did not differ significantly between the groups. However, the donanemab group had significantly more reports of ARIA-E compared with the placebo group (26.7% vs. 0.8%).
Overall, the researchers noted that more trials of longer duration with larger patient numbers are warranted “to further determine the efficacy and safety of donanemab” in AD.
Positive signal?
In a statement, Maria Carrillo, PhD, chief science officer for the Alzheimer’s Association, said the organization “is encouraged by this promising data.
“It is the first phase 2 Alzheimer’s trial to show positive results on a primary outcome measure related to memory and thinking,” Dr. Carrillo said. However, “more work needs to be done on this experimental drug therapy.”
Dr. Carrillo noted that because the trial was moderately sized and only 180 participants completed the study, “we look forward to the results of a second, larger phase 2 trial of this drug.”
Still, she added, there were several “novel and innovative aspects” in the way the study was conducted noting that it showcases the evolution of AD research.
“I’m hopeful for the future,” Dr. Carrillo said.
Also commenting on the results, Howard Fillit, MD, neuroscientist and founding executive director and chief science officer of the Alzheimer’s Drug Discovery Foundation, said the study showed “the pharmacology works” and that the drug did what it was supposed to do in terms of removing A-beta plaque.
“It also gave us a signal in a relatively small phase 2 study that there might be a modest cognitive benefit,” said Dr. Fillit, who was not involved with the research.
He noted that although the rate of decline slowing was statistically significant it remains to be seen whether this is clinically meaningful, particularly in light of the fact that the secondary outcome results were mixed.
“Basically, it was a positive study that probably needs to be followed by another, much larger study to get us to really see the benefit,” Dr. Fillit said.
Dr. Mintun is an employee of Eli Lilly, which funded the study. Dr. Carrillo and Dr. Fillit have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Results from the TRAILBLAZER-ALZ trial were presented at the 2021 International Conference on Alzheimer’s and Parkinson’s Diseases (AD/PD) and were simultaneously published online March 13 in the New England Journal of Medicine.
As previously reported by Medscape Medical News, topline results showed that donanemab slowed cognitive decline by 32% on the Integrated AD Rating Scale (iADRS) from baseline to 76 weeks relative to placebo.
The newly released detailed findings showed that “the use of donanemab resulted in a better composite score for cognition and for the ability to perform activities of daily living than placebo at 76 weeks, although results for secondary outcomes were mixed,” the investigators, with first author Mark A. Mintun, MD, an employee of Eli Lilly, reported.
Results revealed improvement in scores on the Clinical Dementia Rating Scale-Sum of Boxes (CDR-SB) and the 13-item cognitive subscale of the AD Assessment Scale (ADAS-Cog13), but the differences between the two treatment groups were not significant. In addition, score changes on the AD Cooperative Study–Instrumental Activities of Daily Inventory (ADCS-iADL) and the Mini-Mental State Examination (MMSE) were not “substantial.”
However, the donanemab group did show an 85-centiloid greater reduction in amyloid plaque level at 76 weeks, as shown on PET, compared with the placebo group.
Proof of concept?
The humanized antibody donanemab, which was previously known as LY3002813, targets a modified form of deposited amyloid-beta (A-beta) peptide called N3pG.
The randomized, placebo-controlled, double-blind TRAILBLAZER-ALZ trial, which was described as a “phase 2 proof of concept trial” in the AD/PD program, was conducted at 56 sites in the United States and Canada and included 257 patients between the ages of 60 and 85 years (52% were women). PET confirmed tau and amyloid deposition in all participants.
The active treatment group (n = 131) was randomly assigned to receive donanemab 700 mg for three doses; after that, treatment was bumped up to 1,400 mg. Both the donanemab and placebo groups (n = 126) received treatment intravenously every 4 weeks for up to 72 weeks.
Participants also underwent F-florbetapir and F-flortaucipir PET scans at various timepoints and completed a slew of cognitive tests.
The study’s primary outcome measure was change between baseline and 76 weeks post treatment on composite score for cognition, as measured by the iADRS. The iADRS combines the ADAS-Cog13 and the ADCS-iADL.
This measure ranges from 0 to 144, with lower scores associated with greater cognitive impairment. Both treatment groups had an iADRS score of 106 at baseline.
More research needed
Results showed that the score change from baseline on the iADRS was –6.86 for the active treatment group vs –10.06 for the placebo group (group difference, 3.2; 95% confidence interval [CI], 0.12-6.27; P = .04). Although significant, “the trial was powered to show a 6-point difference,” which was not met, the investigators note.
Differences in score changes from baseline to 76 weeks for the treatment vs. placebo groups on the following secondary outcome measures were:
- CDR-SB: –0.36 (95% CI, –0.83 to –0.12).
- ADAS-Cog13: –1.86 (95% CI, –3.63 to –0.09).
- ADCS-iADL: 1.21 (95% CI, –0.77 to 3.2).
- MMSE: 0.64 (95% CI, –0.4 to 1.67).
The CDR-SB was designated as the first secondary outcome, and because it did not show a significant between-group difference, “the hierarchy failed and no definite conclusions can be drawn from data regarding the differences between groups in the change in the ADAS-Cog13,” the investigators wrote.
In addition, the differences in scores on the latter two secondary outcomes were not “substantial,” they reported.
However, at 76 weeks, the donanemab group showed a reduction of 84.13 centiloids in amyloid plaque level vs. an increase of 0.93 centiloids in the placebo group (between-group difference, 85.06 centiloids). At 24 weeks, the active-treatment group had a 67.83-centiloids greater reduction vs. the placebo group.
In addition, 40%, 59.8%, and 67.8% of the donanemab group achieved “amyloid-negative status” at 24, 52, and 76 weeks, respectively. Amyloid-negative status was defined as an amyloid plaque level of less than 24.1 centiloids.
Total incidence of death or serious adverse events did not differ significantly between the groups. However, the donanemab group had significantly more reports of ARIA-E compared with the placebo group (26.7% vs. 0.8%).
Overall, the researchers noted that more trials of longer duration with larger patient numbers are warranted “to further determine the efficacy and safety of donanemab” in AD.
Positive signal?
In a statement, Maria Carrillo, PhD, chief science officer for the Alzheimer’s Association, said the organization “is encouraged by this promising data.
“It is the first phase 2 Alzheimer’s trial to show positive results on a primary outcome measure related to memory and thinking,” Dr. Carrillo said. However, “more work needs to be done on this experimental drug therapy.”
Dr. Carrillo noted that because the trial was moderately sized and only 180 participants completed the study, “we look forward to the results of a second, larger phase 2 trial of this drug.”
Still, she added, there were several “novel and innovative aspects” in the way the study was conducted noting that it showcases the evolution of AD research.
“I’m hopeful for the future,” Dr. Carrillo said.
Also commenting on the results, Howard Fillit, MD, neuroscientist and founding executive director and chief science officer of the Alzheimer’s Drug Discovery Foundation, said the study showed “the pharmacology works” and that the drug did what it was supposed to do in terms of removing A-beta plaque.
“It also gave us a signal in a relatively small phase 2 study that there might be a modest cognitive benefit,” said Dr. Fillit, who was not involved with the research.
He noted that although the rate of decline slowing was statistically significant it remains to be seen whether this is clinically meaningful, particularly in light of the fact that the secondary outcome results were mixed.
“Basically, it was a positive study that probably needs to be followed by another, much larger study to get us to really see the benefit,” Dr. Fillit said.
Dr. Mintun is an employee of Eli Lilly, which funded the study. Dr. Carrillo and Dr. Fillit have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
‘Major update’ of BP guidance for kidney disease; treat to 120 mm Hg
The new 2021 Kidney Disease: Improving Global Outcomes (KDIGO) clinical practice guideline for blood pressure management for adults with chronic kidney disease (CKD) who are not receiving dialysis advises treating to a target systolic blood pressure of less than 120 mm Hg, provided measurements are “standardized” and that blood pressure is “measured properly.”
This blood pressure target – largely based on evidence from the Systolic Blood Pressure Intervention Trial (SPRINT) – represents “a major update” from the 2012 KDIGO guideline, which advised clinicians to treat to a target blood pressure of less than or equal to 130/80 mm Hg for patients with albuminuria or less than or equal to 140/90 mm Hg for patients without albuminuria.
The new goal is also lower than the less than 130/80 mm Hg target in the 2017 American College of Cardiology/American Heart Association guideline.
In a study of the public health implications of the guideline, Kathryn Foti, PhD, and colleagues determined that 70% of U.S. adults with CKD would now be eligible for treatment to lower blood pressure, as opposed to 50% under the previous KDIGO guideline and 56% under the ACC/AHA guideline.
“This is a major update of an influential set of guidelines for chronic kidney disease patients” at a time when blood pressure control is worsening in the United States, Dr. Foti, a postdoctoral researcher in the department of epidemiology at Johns Hopkins Bloomberg School of Public Health, Baltimore, said in a statement from her institution.
The 2021 KDIGO blood pressure guideline and executive summary and the public health implications study are published online in Kidney International.
First, ‘take blood pressure well’
The cochair of the new KDIGO guidelines, Alfred K. Cheung, MD, from the University of Utah, Salt Lake City, said in an interview that the guideline has “two important points.”
First, “take that blood pressure well,” he said. “That has a lot to do with patient preparation rather than any fancy instrument,” he emphasized.
Second, the guideline proposes a systolic blood pressure target of less than 120 mm Hg for most people with CKD not receiving dialysis, except for children and kidney transplant recipients. This target is “contingent on ‘standardized’ blood pressure measurement.”
The document provides a checklist for obtaining a standardized blood pressure measurement, adapted from the 2017 ACC/AHA blood pressure guidelines. It starts with the patient relaxed and sitting on a chair for more than 5 minutes.
In contrast to this measurement, a “routine” or “casual” office blood pressure measurement could be off by plus or minus 10 mm Hg, Dr. Cheung noted.
In a typical scenario, he continued, a patient cannot find a place to park, rushes into the clinic, and has his or her blood pressure checked right away, which would provide a “totally unreliable” reading. Adding a “fudge factor” (correction factor) would not provide an accurate reading.
Clinicians “would not settle for a potassium measurement that is 5.0 mmol/L plus or minus a few decimal points” to guide treatment, he pointed out.
Second, target 120, properly measured
“The very first chapter of the guidelines is devoted to blood pressure measurement, because we recognize if we’re going to do 120 [mm Hg] – the emphasis is on 120 measured properly – so we try to drive that point home,” Tara I. Chang, MD, guideline second author and a coauthor of the public health implications study, pointed out in an interview.
“There are a lot of other things that we base clinical decisions on where we really require some degree of precision, and blood pressure is important enough that to us it’s kind of in the same boat,” said Dr. Chang, from Stanford (Calif.) University.
“In SPRINT, people were randomized to less than less than 120 vs. less than 140 (they weren’t randomized to <130),” she noted.
“The recommendation should be widely adopted in clinical practice,” the guideline authors write, “since accurate measurements will ensure that proper guidance is being applied to the management of BP, as it is to the management of other risk factors.”
Still need individual treatment
Nevertheless, patients still need individualized treatment, the document stresses. “Not every patient with CKD will be appropriate to target to less than 120,” Dr. Chang said. However, “we want people to at least consider less than 120,” she added, to avoid therapeutic inertia.
“If you take the blood pressure in a standardized manner – such as in the ACCORD trial and in the SPRINT trial – even patients over 75 years old, or people over 80 years old, they have very little side effects,” Dr. Cheung noted.
“In the overall cohort,” he continued, “they do not have a significant increase in serious adverse events, do not have adverse events of postural hypotension, syncope, bradycardia, injurious falls – so people are worried about it, but it’s not borne out by the data.
“That said, I have two cautions,” Dr. Cheung noted. “One. If you drop somebody’s blood pressure rapidly over a week, you may be more likely to get in trouble. If you drop the blood pressure gradually over several weeks, several months, you’re much less likely to get into trouble.”
“Two. If the patient is old, you know the patient has carotid stenosis and already has postural dizziness, you may not want to try on that patient – but just because the patient is old is not the reason not to target 120.”
ACE inhibitors and ARBs beneficial in albuminuria, underused
“How do you get to less than 120? The short answer is, use whatever medications you need to – there is no necessarily right cocktail,” Dr. Chang said.
“We’ve known that angiotensin-converting enzyme (ACE) inhibitors and ARBs [angiotensin II receptor blockers] are beneficial in patients with CKD and in particular those with heavier albuminuria,” she continued. “We’ve known this for over 20 years.”
Yet, the study identified underutilization – “a persistent gap, just like blood pressure control and awareness,” she noted. “We’re just not making much headway.
“We are not recommending ACE inhibitors or ARBs for all the patients,” Dr. Cheung clarified. “If you are diabetic and have heavy proteinuria, that’s when the use of ACE inhibitors and ARBs are most indicated.”
Public health implications
SPRINT showed that treating to a systolic blood pressure of less than 120 mm Hg vs. less than 140 mm Hg reduced the risk for cardiovascular disease by 25% and all-cause mortality by 27% for participants with and those without CKD, Dr. Foti and colleagues stress.
They aimed to estimate how the new guideline would affect (1) the number of U.S. patients with CKD who would be eligible for blood pressure lowering treatment, and (2) the proportion of those with albuminuria who would be eligible for an ACE inhibitor or an ARB.
The researchers analyzed data from 1,699 adults with CKD (estimated glomerular filtration rate, 15-59 mL/min/1.73 m2 or a urinary albumin-to-creatinine ratio of ≥30 mg/g) who participated in the 2015-2018 National Health and Nutrition Examination Survey.
Both the 2021 and 2012 KDIGO guidelines recommend that patients with albuminuria and blood pressure higher than the target value who are not kidney transplant recipients should be treated with an ACE inhibitor or an ARB.
On the basis of the new target, 78% of patients with CKD and albuminuria were eligible for ACE inhibitor/ARB treatment by the 2021 KDIGO guideline, compared with 71% by the 2012 KDIGO guideline. However, only 39% were taking one of these drugs.
These findings show that “with the new guideline and with the lower blood pressure target, you potentially have an even larger pool of people who have blood pressure that’s not under control, and a potential larger group of people who may benefit from ACE inhibitors and ARBs,” Dr. Chang said.
“Our paper is not the only one to show that we haven’t made a whole lot of progress,” she said, “and now that the bar has been lowered, there [have] to be some renewed efforts on controlling blood pressure, because we know that blood pressure control is such an important risk factor for cardiovascular outcomes.”
Dr. Foti is supported by an NIH/National Heart, Lung, and Blood Institute grant. Dr. Cheung has received consultancy fees from Amgen, Bard, Boehringer Ingelheim, Calliditas, Tricida, and UpToDate, and grant/research support from the National Institutes of Health for SPRINT (monies paid to institution). Dr. Chang has received consultancy fees from Bayer, Gilead, Janssen Research and Development, Novo Nordisk, Tricida, and Vascular Dynamics; grant/research support from AstraZeneca and Satellite Healthcare (monies paid to institution), the NIH, and the American Heart Association; is on advisory boards for AstraZeneca and Fresenius Medical Care Renal Therapies Group; and has received workshop honoraria from Fresenius. Disclosures of relevant financial relationships of the other authors are listed in the original articles.
A version of this article first appeared on Medscape.com.
The new 2021 Kidney Disease: Improving Global Outcomes (KDIGO) clinical practice guideline for blood pressure management for adults with chronic kidney disease (CKD) who are not receiving dialysis advises treating to a target systolic blood pressure of less than 120 mm Hg, provided measurements are “standardized” and that blood pressure is “measured properly.”
This blood pressure target – largely based on evidence from the Systolic Blood Pressure Intervention Trial (SPRINT) – represents “a major update” from the 2012 KDIGO guideline, which advised clinicians to treat to a target blood pressure of less than or equal to 130/80 mm Hg for patients with albuminuria or less than or equal to 140/90 mm Hg for patients without albuminuria.
The new goal is also lower than the less than 130/80 mm Hg target in the 2017 American College of Cardiology/American Heart Association guideline.
In a study of the public health implications of the guideline, Kathryn Foti, PhD, and colleagues determined that 70% of U.S. adults with CKD would now be eligible for treatment to lower blood pressure, as opposed to 50% under the previous KDIGO guideline and 56% under the ACC/AHA guideline.
“This is a major update of an influential set of guidelines for chronic kidney disease patients” at a time when blood pressure control is worsening in the United States, Dr. Foti, a postdoctoral researcher in the department of epidemiology at Johns Hopkins Bloomberg School of Public Health, Baltimore, said in a statement from her institution.
The 2021 KDIGO blood pressure guideline and executive summary and the public health implications study are published online in Kidney International.
First, ‘take blood pressure well’
The cochair of the new KDIGO guidelines, Alfred K. Cheung, MD, from the University of Utah, Salt Lake City, said in an interview that the guideline has “two important points.”
First, “take that blood pressure well,” he said. “That has a lot to do with patient preparation rather than any fancy instrument,” he emphasized.
Second, the guideline proposes a systolic blood pressure target of less than 120 mm Hg for most people with CKD not receiving dialysis, except for children and kidney transplant recipients. This target is “contingent on ‘standardized’ blood pressure measurement.”
The document provides a checklist for obtaining a standardized blood pressure measurement, adapted from the 2017 ACC/AHA blood pressure guidelines. It starts with the patient relaxed and sitting on a chair for more than 5 minutes.
In contrast to this measurement, a “routine” or “casual” office blood pressure measurement could be off by plus or minus 10 mm Hg, Dr. Cheung noted.
In a typical scenario, he continued, a patient cannot find a place to park, rushes into the clinic, and has his or her blood pressure checked right away, which would provide a “totally unreliable” reading. Adding a “fudge factor” (correction factor) would not provide an accurate reading.
Clinicians “would not settle for a potassium measurement that is 5.0 mmol/L plus or minus a few decimal points” to guide treatment, he pointed out.
Second, target 120, properly measured
“The very first chapter of the guidelines is devoted to blood pressure measurement, because we recognize if we’re going to do 120 [mm Hg] – the emphasis is on 120 measured properly – so we try to drive that point home,” Tara I. Chang, MD, guideline second author and a coauthor of the public health implications study, pointed out in an interview.
“There are a lot of other things that we base clinical decisions on where we really require some degree of precision, and blood pressure is important enough that to us it’s kind of in the same boat,” said Dr. Chang, from Stanford (Calif.) University.
“In SPRINT, people were randomized to less than less than 120 vs. less than 140 (they weren’t randomized to <130),” she noted.
“The recommendation should be widely adopted in clinical practice,” the guideline authors write, “since accurate measurements will ensure that proper guidance is being applied to the management of BP, as it is to the management of other risk factors.”
Still need individual treatment
Nevertheless, patients still need individualized treatment, the document stresses. “Not every patient with CKD will be appropriate to target to less than 120,” Dr. Chang said. However, “we want people to at least consider less than 120,” she added, to avoid therapeutic inertia.
“If you take the blood pressure in a standardized manner – such as in the ACCORD trial and in the SPRINT trial – even patients over 75 years old, or people over 80 years old, they have very little side effects,” Dr. Cheung noted.
“In the overall cohort,” he continued, “they do not have a significant increase in serious adverse events, do not have adverse events of postural hypotension, syncope, bradycardia, injurious falls – so people are worried about it, but it’s not borne out by the data.
“That said, I have two cautions,” Dr. Cheung noted. “One. If you drop somebody’s blood pressure rapidly over a week, you may be more likely to get in trouble. If you drop the blood pressure gradually over several weeks, several months, you’re much less likely to get into trouble.”
“Two. If the patient is old, you know the patient has carotid stenosis and already has postural dizziness, you may not want to try on that patient – but just because the patient is old is not the reason not to target 120.”
ACE inhibitors and ARBs beneficial in albuminuria, underused
“How do you get to less than 120? The short answer is, use whatever medications you need to – there is no necessarily right cocktail,” Dr. Chang said.
“We’ve known that angiotensin-converting enzyme (ACE) inhibitors and ARBs [angiotensin II receptor blockers] are beneficial in patients with CKD and in particular those with heavier albuminuria,” she continued. “We’ve known this for over 20 years.”
Yet, the study identified underutilization – “a persistent gap, just like blood pressure control and awareness,” she noted. “We’re just not making much headway.
“We are not recommending ACE inhibitors or ARBs for all the patients,” Dr. Cheung clarified. “If you are diabetic and have heavy proteinuria, that’s when the use of ACE inhibitors and ARBs are most indicated.”
Public health implications
SPRINT showed that treating to a systolic blood pressure of less than 120 mm Hg vs. less than 140 mm Hg reduced the risk for cardiovascular disease by 25% and all-cause mortality by 27% for participants with and those without CKD, Dr. Foti and colleagues stress.
They aimed to estimate how the new guideline would affect (1) the number of U.S. patients with CKD who would be eligible for blood pressure lowering treatment, and (2) the proportion of those with albuminuria who would be eligible for an ACE inhibitor or an ARB.
The researchers analyzed data from 1,699 adults with CKD (estimated glomerular filtration rate, 15-59 mL/min/1.73 m2 or a urinary albumin-to-creatinine ratio of ≥30 mg/g) who participated in the 2015-2018 National Health and Nutrition Examination Survey.
Both the 2021 and 2012 KDIGO guidelines recommend that patients with albuminuria and blood pressure higher than the target value who are not kidney transplant recipients should be treated with an ACE inhibitor or an ARB.
On the basis of the new target, 78% of patients with CKD and albuminuria were eligible for ACE inhibitor/ARB treatment by the 2021 KDIGO guideline, compared with 71% by the 2012 KDIGO guideline. However, only 39% were taking one of these drugs.
These findings show that “with the new guideline and with the lower blood pressure target, you potentially have an even larger pool of people who have blood pressure that’s not under control, and a potential larger group of people who may benefit from ACE inhibitors and ARBs,” Dr. Chang said.
“Our paper is not the only one to show that we haven’t made a whole lot of progress,” she said, “and now that the bar has been lowered, there [have] to be some renewed efforts on controlling blood pressure, because we know that blood pressure control is such an important risk factor for cardiovascular outcomes.”
Dr. Foti is supported by an NIH/National Heart, Lung, and Blood Institute grant. Dr. Cheung has received consultancy fees from Amgen, Bard, Boehringer Ingelheim, Calliditas, Tricida, and UpToDate, and grant/research support from the National Institutes of Health for SPRINT (monies paid to institution). Dr. Chang has received consultancy fees from Bayer, Gilead, Janssen Research and Development, Novo Nordisk, Tricida, and Vascular Dynamics; grant/research support from AstraZeneca and Satellite Healthcare (monies paid to institution), the NIH, and the American Heart Association; is on advisory boards for AstraZeneca and Fresenius Medical Care Renal Therapies Group; and has received workshop honoraria from Fresenius. Disclosures of relevant financial relationships of the other authors are listed in the original articles.
A version of this article first appeared on Medscape.com.
The new 2021 Kidney Disease: Improving Global Outcomes (KDIGO) clinical practice guideline for blood pressure management for adults with chronic kidney disease (CKD) who are not receiving dialysis advises treating to a target systolic blood pressure of less than 120 mm Hg, provided measurements are “standardized” and that blood pressure is “measured properly.”
This blood pressure target – largely based on evidence from the Systolic Blood Pressure Intervention Trial (SPRINT) – represents “a major update” from the 2012 KDIGO guideline, which advised clinicians to treat to a target blood pressure of less than or equal to 130/80 mm Hg for patients with albuminuria or less than or equal to 140/90 mm Hg for patients without albuminuria.
The new goal is also lower than the less than 130/80 mm Hg target in the 2017 American College of Cardiology/American Heart Association guideline.
In a study of the public health implications of the guideline, Kathryn Foti, PhD, and colleagues determined that 70% of U.S. adults with CKD would now be eligible for treatment to lower blood pressure, as opposed to 50% under the previous KDIGO guideline and 56% under the ACC/AHA guideline.
“This is a major update of an influential set of guidelines for chronic kidney disease patients” at a time when blood pressure control is worsening in the United States, Dr. Foti, a postdoctoral researcher in the department of epidemiology at Johns Hopkins Bloomberg School of Public Health, Baltimore, said in a statement from her institution.
The 2021 KDIGO blood pressure guideline and executive summary and the public health implications study are published online in Kidney International.
First, ‘take blood pressure well’
The cochair of the new KDIGO guidelines, Alfred K. Cheung, MD, from the University of Utah, Salt Lake City, said in an interview that the guideline has “two important points.”
First, “take that blood pressure well,” he said. “That has a lot to do with patient preparation rather than any fancy instrument,” he emphasized.
Second, the guideline proposes a systolic blood pressure target of less than 120 mm Hg for most people with CKD not receiving dialysis, except for children and kidney transplant recipients. This target is “contingent on ‘standardized’ blood pressure measurement.”
The document provides a checklist for obtaining a standardized blood pressure measurement, adapted from the 2017 ACC/AHA blood pressure guidelines. It starts with the patient relaxed and sitting on a chair for more than 5 minutes.
In contrast to this measurement, a “routine” or “casual” office blood pressure measurement could be off by plus or minus 10 mm Hg, Dr. Cheung noted.
In a typical scenario, he continued, a patient cannot find a place to park, rushes into the clinic, and has his or her blood pressure checked right away, which would provide a “totally unreliable” reading. Adding a “fudge factor” (correction factor) would not provide an accurate reading.
Clinicians “would not settle for a potassium measurement that is 5.0 mmol/L plus or minus a few decimal points” to guide treatment, he pointed out.
Second, target 120, properly measured
“The very first chapter of the guidelines is devoted to blood pressure measurement, because we recognize if we’re going to do 120 [mm Hg] – the emphasis is on 120 measured properly – so we try to drive that point home,” Tara I. Chang, MD, guideline second author and a coauthor of the public health implications study, pointed out in an interview.
“There are a lot of other things that we base clinical decisions on where we really require some degree of precision, and blood pressure is important enough that to us it’s kind of in the same boat,” said Dr. Chang, from Stanford (Calif.) University.
“In SPRINT, people were randomized to less than less than 120 vs. less than 140 (they weren’t randomized to <130),” she noted.
“The recommendation should be widely adopted in clinical practice,” the guideline authors write, “since accurate measurements will ensure that proper guidance is being applied to the management of BP, as it is to the management of other risk factors.”
Still need individual treatment
Nevertheless, patients still need individualized treatment, the document stresses. “Not every patient with CKD will be appropriate to target to less than 120,” Dr. Chang said. However, “we want people to at least consider less than 120,” she added, to avoid therapeutic inertia.
“If you take the blood pressure in a standardized manner – such as in the ACCORD trial and in the SPRINT trial – even patients over 75 years old, or people over 80 years old, they have very little side effects,” Dr. Cheung noted.
“In the overall cohort,” he continued, “they do not have a significant increase in serious adverse events, do not have adverse events of postural hypotension, syncope, bradycardia, injurious falls – so people are worried about it, but it’s not borne out by the data.
“That said, I have two cautions,” Dr. Cheung noted. “One. If you drop somebody’s blood pressure rapidly over a week, you may be more likely to get in trouble. If you drop the blood pressure gradually over several weeks, several months, you’re much less likely to get into trouble.”
“Two. If the patient is old, you know the patient has carotid stenosis and already has postural dizziness, you may not want to try on that patient – but just because the patient is old is not the reason not to target 120.”
ACE inhibitors and ARBs beneficial in albuminuria, underused
“How do you get to less than 120? The short answer is, use whatever medications you need to – there is no necessarily right cocktail,” Dr. Chang said.
“We’ve known that angiotensin-converting enzyme (ACE) inhibitors and ARBs [angiotensin II receptor blockers] are beneficial in patients with CKD and in particular those with heavier albuminuria,” she continued. “We’ve known this for over 20 years.”
Yet, the study identified underutilization – “a persistent gap, just like blood pressure control and awareness,” she noted. “We’re just not making much headway.
“We are not recommending ACE inhibitors or ARBs for all the patients,” Dr. Cheung clarified. “If you are diabetic and have heavy proteinuria, that’s when the use of ACE inhibitors and ARBs are most indicated.”
Public health implications
SPRINT showed that treating to a systolic blood pressure of less than 120 mm Hg vs. less than 140 mm Hg reduced the risk for cardiovascular disease by 25% and all-cause mortality by 27% for participants with and those without CKD, Dr. Foti and colleagues stress.
They aimed to estimate how the new guideline would affect (1) the number of U.S. patients with CKD who would be eligible for blood pressure lowering treatment, and (2) the proportion of those with albuminuria who would be eligible for an ACE inhibitor or an ARB.
The researchers analyzed data from 1,699 adults with CKD (estimated glomerular filtration rate, 15-59 mL/min/1.73 m2 or a urinary albumin-to-creatinine ratio of ≥30 mg/g) who participated in the 2015-2018 National Health and Nutrition Examination Survey.
Both the 2021 and 2012 KDIGO guidelines recommend that patients with albuminuria and blood pressure higher than the target value who are not kidney transplant recipients should be treated with an ACE inhibitor or an ARB.
On the basis of the new target, 78% of patients with CKD and albuminuria were eligible for ACE inhibitor/ARB treatment by the 2021 KDIGO guideline, compared with 71% by the 2012 KDIGO guideline. However, only 39% were taking one of these drugs.
These findings show that “with the new guideline and with the lower blood pressure target, you potentially have an even larger pool of people who have blood pressure that’s not under control, and a potential larger group of people who may benefit from ACE inhibitors and ARBs,” Dr. Chang said.
“Our paper is not the only one to show that we haven’t made a whole lot of progress,” she said, “and now that the bar has been lowered, there [have] to be some renewed efforts on controlling blood pressure, because we know that blood pressure control is such an important risk factor for cardiovascular outcomes.”
Dr. Foti is supported by an NIH/National Heart, Lung, and Blood Institute grant. Dr. Cheung has received consultancy fees from Amgen, Bard, Boehringer Ingelheim, Calliditas, Tricida, and UpToDate, and grant/research support from the National Institutes of Health for SPRINT (monies paid to institution). Dr. Chang has received consultancy fees from Bayer, Gilead, Janssen Research and Development, Novo Nordisk, Tricida, and Vascular Dynamics; grant/research support from AstraZeneca and Satellite Healthcare (monies paid to institution), the NIH, and the American Heart Association; is on advisory boards for AstraZeneca and Fresenius Medical Care Renal Therapies Group; and has received workshop honoraria from Fresenius. Disclosures of relevant financial relationships of the other authors are listed in the original articles.
A version of this article first appeared on Medscape.com.
CDC’s new gonorrhea treatment recs: What’s changed, and when to retest
REFERENCES
- CDC. Expedited partner therapy. Accessed March 15, 2021. www.cdc.gov/std/ept/default.htm
- St. Cyr S, Barbee L, Workowski KA, et al. Update to CDC's treatment guidelines for gonococcal infection, 2020. MMWR Morbid Mortal Wkly Rep. 2020;69:1911-1916. Accessed March 15, 2021. https://www.cdc.gov/mmwr/volumes/69/wr/mm6950a6.htm
- CDC. Gonococcal infections. Accessed March 15, 2021. www.cdc.gov/std/tg2015/gonorrhea.htm
REFERENCES
- CDC. Expedited partner therapy. Accessed March 15, 2021. www.cdc.gov/std/ept/default.htm
- St. Cyr S, Barbee L, Workowski KA, et al. Update to CDC's treatment guidelines for gonococcal infection, 2020. MMWR Morbid Mortal Wkly Rep. 2020;69:1911-1916. Accessed March 15, 2021. https://www.cdc.gov/mmwr/volumes/69/wr/mm6950a6.htm
- CDC. Gonococcal infections. Accessed March 15, 2021. www.cdc.gov/std/tg2015/gonorrhea.htm
REFERENCES
- CDC. Expedited partner therapy. Accessed March 15, 2021. www.cdc.gov/std/ept/default.htm
- St. Cyr S, Barbee L, Workowski KA, et al. Update to CDC's treatment guidelines for gonococcal infection, 2020. MMWR Morbid Mortal Wkly Rep. 2020;69:1911-1916. Accessed March 15, 2021. https://www.cdc.gov/mmwr/volumes/69/wr/mm6950a6.htm
- CDC. Gonococcal infections. Accessed March 15, 2021. www.cdc.gov/std/tg2015/gonorrhea.htm
Don’t delay: Cancer patients need both doses of COVID vaccine
The new findings, which are soon to be published as a preprint, cast doubt on the current U.K. policy of delaying the second dose of the vaccine.
Delaying the second dose can leave most patients with cancer wholly or partially unprotected, according to the researchers. Moreover, such a delay has implications for transmission of SARS-CoV-2 in the cancer patient’s environs as well as for the evolution of virus variants that could be of concern, the researchers concluded.
The data come from a British study that included 151 patients with cancer and 54 healthy control persons. All participants received the COVID-19 mRNA BNT162b2 vaccine (Pfizer-BioNTech).
This vaccine requires two doses. The first few participants in this study were given the second dose 21 days after they had received the first dose, but then national guidelines changed, and the remaining participants had to wait 12 weeks to receive their second dose.
The researchers reported that, among health controls, the immune efficacy of the first dose was very high (97% efficacious). By contrast, among patients with solid tumors, the immune efficacy of a single dose was strikingly low (39%), and it was even lower in patients with hematologic malignancies (13%).
The second dose of vaccine greatly and rapidly increased the immune efficacy in patients with solid tumors (95% within 2 weeks of receiving the second dose), the researchers added.
Too few patients with hematologic cancers had received the second dose before the study ended for clear conclusions to be drawn. Nevertheless, the available data suggest that 50% of patients with hematologic cancers who had received the booster at day 21 were seropositive at 5 weeks vs. only 8% of those who had not received the booster.
“Our data provide the first real-world evidence of immune efficacy following one dose of the Pfizer vaccine in immunocompromised patient populations [and] clearly show that the poor one-dose efficacy in cancer patients can be rescued with an early booster at day 21,” commented senior author Sheeba Irshad, MD, senior clinical lecturer, King’s College London.
“Based on our findings, we would recommend an urgent review of the vaccine strategy for clinically extremely vulnerable groups. Until then, it is important that cancer patients continue to observe all public health measures in place, such as social distancing and shielding when attending hospitals, even after vaccination,” Dr. Irshad added.
The paper, with first author Leticia Monin-Aldama, PhD, is scheduled to appear on the preprint server medRxiv. It has not undergone peer review. The paper was distributed to journalists, with comments from experts not involved in the study, by the UK Science Media Centre.
These data are “of immediate importance” to patients with cancer, commented Shoba Amarnath, PhD, Newcastle University research fellow, Laboratory of T-cell Regulation, Newcastle University Center for Cancer, Newcastle upon Tyne, England.
“These findings are consistent with our understanding. … We know that the immune system within cancer patients is compromised as compared to healthy controls,” Dr. Amarnath said. “The data in the study support the notion that, in solid cancer patients, a considerable delay in second dose will extend the period when cancer patients are at risk of SARS-CoV-2 infection.”
Although more data are required, “this study does raise the issue of whether patients with cancer, other diseases, or those undergoing therapies that affect the body’s immune response should be fast-tracked for their second vaccine dose,” commented Lawrence Young, PhD, professor of molecular oncology and director of the Warwick Cancer Research Center, University of Warwick, Coventry, England.
Stephen Evans, MSc, professor of pharmacoepidemiology, London School of Hygiene and Tropical Medicine, underlined that the study is “essentially” observational and “inevitable limitations must be taken into account.
“Nevertheless, these results do suggest that the vaccines may well not protect those patients with cancer as well as those without cancer,” Mr. Evans said. He added that it is “important that this population continues to observe all COVID-19–associated measures, such as social distancing and shielding when attending hospitals, even after vaccination.”
Study details
Previous studies have shown that some patients with cancer have prolonged responses to SARS-CoV-2 infection, with ongoing immune dysregulation, inefficient seroconversion, and prolonged viral shedding.
There are few data, however, on how these patients respond to COVID-19 vaccination. The authors point out that, among the 18,860 individuals who received the Pfizer vaccine during its development trials, “none with an active oncological diagnosis was included.”
To investigate this issue, they launched the SARS-CoV-2 for Cancer Patients (SOAP-02) study.
The 151 patients with cancer who participated in this study were mostly elderly, the authors noted (75% were older than 65 years; the median age was 73 years). The majority (63%) had solid-tumor malignancies. Of those, 8% had late-stage disease and had been living with their cancer for more than 24 months.
The healthy control persons were vaccine-eligible primary health care workers who were not age matched to the cancer patients.
All participants received the first dose of vaccine; 31 (of 151) patients with cancer and 16 (of 54) healthy control persons received the second dose on day 21.
The remaining participants were scheduled to receive their second dose 12 weeks later (after the study ended), in line with the changes in the national guidelines.
The team reported that, approximately 21 days after receiving the first vaccine dose, the immune efficacy of the vaccine was estimated to be 97% among healthy control persons vs. 39% for patients with solid tumors and only 13% for those with hematologic malignancies (P < .0001 for both).
T-cell responses, as assessed via interferon-gamma and/or interleukin-2 production, were observed in 82% of healthy control persons, 71% of patients with solid tumors, and 50% of those with hematologic cancers.
Vaccine boosting at day 21 resulted in immune efficacy of 100% for healthy control persons and 95% for patients with solid tumors. In contrast, only 43% of those who did not receive the second dose were seropositive 2 weeks later.
Further analysis suggested that participants who did not have a serologic response were “spread evenly” across different cancer types, but the reduced responses were more frequent among patients who had received the vaccine within 15 days of cancer treatment, especially chemotherapy, and had undergone intensive treatments.
The SOAP study is sponsored by King’s College London and Guy’s and St. Thomas Trust Foundation NHS Trust. It is funded from grants from the KCL Charity, Cancer Research UK, and program grants from Breast Cancer Now. The investigators have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The new findings, which are soon to be published as a preprint, cast doubt on the current U.K. policy of delaying the second dose of the vaccine.
Delaying the second dose can leave most patients with cancer wholly or partially unprotected, according to the researchers. Moreover, such a delay has implications for transmission of SARS-CoV-2 in the cancer patient’s environs as well as for the evolution of virus variants that could be of concern, the researchers concluded.
The data come from a British study that included 151 patients with cancer and 54 healthy control persons. All participants received the COVID-19 mRNA BNT162b2 vaccine (Pfizer-BioNTech).
This vaccine requires two doses. The first few participants in this study were given the second dose 21 days after they had received the first dose, but then national guidelines changed, and the remaining participants had to wait 12 weeks to receive their second dose.
The researchers reported that, among health controls, the immune efficacy of the first dose was very high (97% efficacious). By contrast, among patients with solid tumors, the immune efficacy of a single dose was strikingly low (39%), and it was even lower in patients with hematologic malignancies (13%).
The second dose of vaccine greatly and rapidly increased the immune efficacy in patients with solid tumors (95% within 2 weeks of receiving the second dose), the researchers added.
Too few patients with hematologic cancers had received the second dose before the study ended for clear conclusions to be drawn. Nevertheless, the available data suggest that 50% of patients with hematologic cancers who had received the booster at day 21 were seropositive at 5 weeks vs. only 8% of those who had not received the booster.
“Our data provide the first real-world evidence of immune efficacy following one dose of the Pfizer vaccine in immunocompromised patient populations [and] clearly show that the poor one-dose efficacy in cancer patients can be rescued with an early booster at day 21,” commented senior author Sheeba Irshad, MD, senior clinical lecturer, King’s College London.
“Based on our findings, we would recommend an urgent review of the vaccine strategy for clinically extremely vulnerable groups. Until then, it is important that cancer patients continue to observe all public health measures in place, such as social distancing and shielding when attending hospitals, even after vaccination,” Dr. Irshad added.
The paper, with first author Leticia Monin-Aldama, PhD, is scheduled to appear on the preprint server medRxiv. It has not undergone peer review. The paper was distributed to journalists, with comments from experts not involved in the study, by the UK Science Media Centre.
These data are “of immediate importance” to patients with cancer, commented Shoba Amarnath, PhD, Newcastle University research fellow, Laboratory of T-cell Regulation, Newcastle University Center for Cancer, Newcastle upon Tyne, England.
“These findings are consistent with our understanding. … We know that the immune system within cancer patients is compromised as compared to healthy controls,” Dr. Amarnath said. “The data in the study support the notion that, in solid cancer patients, a considerable delay in second dose will extend the period when cancer patients are at risk of SARS-CoV-2 infection.”
Although more data are required, “this study does raise the issue of whether patients with cancer, other diseases, or those undergoing therapies that affect the body’s immune response should be fast-tracked for their second vaccine dose,” commented Lawrence Young, PhD, professor of molecular oncology and director of the Warwick Cancer Research Center, University of Warwick, Coventry, England.
Stephen Evans, MSc, professor of pharmacoepidemiology, London School of Hygiene and Tropical Medicine, underlined that the study is “essentially” observational and “inevitable limitations must be taken into account.
“Nevertheless, these results do suggest that the vaccines may well not protect those patients with cancer as well as those without cancer,” Mr. Evans said. He added that it is “important that this population continues to observe all COVID-19–associated measures, such as social distancing and shielding when attending hospitals, even after vaccination.”
Study details
Previous studies have shown that some patients with cancer have prolonged responses to SARS-CoV-2 infection, with ongoing immune dysregulation, inefficient seroconversion, and prolonged viral shedding.
There are few data, however, on how these patients respond to COVID-19 vaccination. The authors point out that, among the 18,860 individuals who received the Pfizer vaccine during its development trials, “none with an active oncological diagnosis was included.”
To investigate this issue, they launched the SARS-CoV-2 for Cancer Patients (SOAP-02) study.
The 151 patients with cancer who participated in this study were mostly elderly, the authors noted (75% were older than 65 years; the median age was 73 years). The majority (63%) had solid-tumor malignancies. Of those, 8% had late-stage disease and had been living with their cancer for more than 24 months.
The healthy control persons were vaccine-eligible primary health care workers who were not age matched to the cancer patients.
All participants received the first dose of vaccine; 31 (of 151) patients with cancer and 16 (of 54) healthy control persons received the second dose on day 21.
The remaining participants were scheduled to receive their second dose 12 weeks later (after the study ended), in line with the changes in the national guidelines.
The team reported that, approximately 21 days after receiving the first vaccine dose, the immune efficacy of the vaccine was estimated to be 97% among healthy control persons vs. 39% for patients with solid tumors and only 13% for those with hematologic malignancies (P < .0001 for both).
T-cell responses, as assessed via interferon-gamma and/or interleukin-2 production, were observed in 82% of healthy control persons, 71% of patients with solid tumors, and 50% of those with hematologic cancers.
Vaccine boosting at day 21 resulted in immune efficacy of 100% for healthy control persons and 95% for patients with solid tumors. In contrast, only 43% of those who did not receive the second dose were seropositive 2 weeks later.
Further analysis suggested that participants who did not have a serologic response were “spread evenly” across different cancer types, but the reduced responses were more frequent among patients who had received the vaccine within 15 days of cancer treatment, especially chemotherapy, and had undergone intensive treatments.
The SOAP study is sponsored by King’s College London and Guy’s and St. Thomas Trust Foundation NHS Trust. It is funded from grants from the KCL Charity, Cancer Research UK, and program grants from Breast Cancer Now. The investigators have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The new findings, which are soon to be published as a preprint, cast doubt on the current U.K. policy of delaying the second dose of the vaccine.
Delaying the second dose can leave most patients with cancer wholly or partially unprotected, according to the researchers. Moreover, such a delay has implications for transmission of SARS-CoV-2 in the cancer patient’s environs as well as for the evolution of virus variants that could be of concern, the researchers concluded.
The data come from a British study that included 151 patients with cancer and 54 healthy control persons. All participants received the COVID-19 mRNA BNT162b2 vaccine (Pfizer-BioNTech).
This vaccine requires two doses. The first few participants in this study were given the second dose 21 days after they had received the first dose, but then national guidelines changed, and the remaining participants had to wait 12 weeks to receive their second dose.
The researchers reported that, among health controls, the immune efficacy of the first dose was very high (97% efficacious). By contrast, among patients with solid tumors, the immune efficacy of a single dose was strikingly low (39%), and it was even lower in patients with hematologic malignancies (13%).
The second dose of vaccine greatly and rapidly increased the immune efficacy in patients with solid tumors (95% within 2 weeks of receiving the second dose), the researchers added.
Too few patients with hematologic cancers had received the second dose before the study ended for clear conclusions to be drawn. Nevertheless, the available data suggest that 50% of patients with hematologic cancers who had received the booster at day 21 were seropositive at 5 weeks vs. only 8% of those who had not received the booster.
“Our data provide the first real-world evidence of immune efficacy following one dose of the Pfizer vaccine in immunocompromised patient populations [and] clearly show that the poor one-dose efficacy in cancer patients can be rescued with an early booster at day 21,” commented senior author Sheeba Irshad, MD, senior clinical lecturer, King’s College London.
“Based on our findings, we would recommend an urgent review of the vaccine strategy for clinically extremely vulnerable groups. Until then, it is important that cancer patients continue to observe all public health measures in place, such as social distancing and shielding when attending hospitals, even after vaccination,” Dr. Irshad added.
The paper, with first author Leticia Monin-Aldama, PhD, is scheduled to appear on the preprint server medRxiv. It has not undergone peer review. The paper was distributed to journalists, with comments from experts not involved in the study, by the UK Science Media Centre.
These data are “of immediate importance” to patients with cancer, commented Shoba Amarnath, PhD, Newcastle University research fellow, Laboratory of T-cell Regulation, Newcastle University Center for Cancer, Newcastle upon Tyne, England.
“These findings are consistent with our understanding. … We know that the immune system within cancer patients is compromised as compared to healthy controls,” Dr. Amarnath said. “The data in the study support the notion that, in solid cancer patients, a considerable delay in second dose will extend the period when cancer patients are at risk of SARS-CoV-2 infection.”
Although more data are required, “this study does raise the issue of whether patients with cancer, other diseases, or those undergoing therapies that affect the body’s immune response should be fast-tracked for their second vaccine dose,” commented Lawrence Young, PhD, professor of molecular oncology and director of the Warwick Cancer Research Center, University of Warwick, Coventry, England.
Stephen Evans, MSc, professor of pharmacoepidemiology, London School of Hygiene and Tropical Medicine, underlined that the study is “essentially” observational and “inevitable limitations must be taken into account.
“Nevertheless, these results do suggest that the vaccines may well not protect those patients with cancer as well as those without cancer,” Mr. Evans said. He added that it is “important that this population continues to observe all COVID-19–associated measures, such as social distancing and shielding when attending hospitals, even after vaccination.”
Study details
Previous studies have shown that some patients with cancer have prolonged responses to SARS-CoV-2 infection, with ongoing immune dysregulation, inefficient seroconversion, and prolonged viral shedding.
There are few data, however, on how these patients respond to COVID-19 vaccination. The authors point out that, among the 18,860 individuals who received the Pfizer vaccine during its development trials, “none with an active oncological diagnosis was included.”
To investigate this issue, they launched the SARS-CoV-2 for Cancer Patients (SOAP-02) study.
The 151 patients with cancer who participated in this study were mostly elderly, the authors noted (75% were older than 65 years; the median age was 73 years). The majority (63%) had solid-tumor malignancies. Of those, 8% had late-stage disease and had been living with their cancer for more than 24 months.
The healthy control persons were vaccine-eligible primary health care workers who were not age matched to the cancer patients.
All participants received the first dose of vaccine; 31 (of 151) patients with cancer and 16 (of 54) healthy control persons received the second dose on day 21.
The remaining participants were scheduled to receive their second dose 12 weeks later (after the study ended), in line with the changes in the national guidelines.
The team reported that, approximately 21 days after receiving the first vaccine dose, the immune efficacy of the vaccine was estimated to be 97% among healthy control persons vs. 39% for patients with solid tumors and only 13% for those with hematologic malignancies (P < .0001 for both).
T-cell responses, as assessed via interferon-gamma and/or interleukin-2 production, were observed in 82% of healthy control persons, 71% of patients with solid tumors, and 50% of those with hematologic cancers.
Vaccine boosting at day 21 resulted in immune efficacy of 100% for healthy control persons and 95% for patients with solid tumors. In contrast, only 43% of those who did not receive the second dose were seropositive 2 weeks later.
Further analysis suggested that participants who did not have a serologic response were “spread evenly” across different cancer types, but the reduced responses were more frequent among patients who had received the vaccine within 15 days of cancer treatment, especially chemotherapy, and had undergone intensive treatments.
The SOAP study is sponsored by King’s College London and Guy’s and St. Thomas Trust Foundation NHS Trust. It is funded from grants from the KCL Charity, Cancer Research UK, and program grants from Breast Cancer Now. The investigators have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Cannabinoids may pose death risk for older patients with COPD
, compared with nonusers, findings from a large study have shown.
Synthetic cannabinoids drugs, such as nabilone and dronabinol, have been approved by the Food and Drug Administration for nausea and vomiting caused by chemotherapy. But their off-label use by adults with COPD to help manage chronic musculoskeletal pain, insomnia, and refractory dyspnea is on the rise, wrote Nicholas T. Vozoris, MD, of the University of Toronto and colleagues.
Cannabinoids may actually contribute to negative respiratory outcomes among individuals with COPD through several possible mechanisms including causing sedation, inducing anxiety, and provoking respiratory muscle weakness, they said.
“Possible adverse respiratory effects of cannabinoids may occur with greater likelihood among older adults (in whom COPD is more prevalent), as this group is known to less efficiently metabolise drugs,” they noted.
In a retrospective, population-based cohort study published in Thorax the researchers identified 185,876 adults aged 66 years and older with COPD using health administrative database information from 2006 to 2016. New cannabinoid users (those starting nabilone or dronabinol) were matched with control nonusers (defined as new users of noncannabinoid drugs). Individuals receiving palliative care, or having a diagnosis of cancer or HIV, were excluded because these are settings where synthetic cannabinoids may be prescribed for nausea or vomiting, and these patients are more likely to be in a poorer state of health.
Overall, new cannabinoid users had significantly higher all-cause mortality rates, compared with nonusers (hazard ratio, 1.64). The effects was greater in high-dose users.
Daniel R. Ouellette, MD, associate professor of medicine at Wayne State University and a senior staff physician at Henry Ford Hospital, both in Detroit, commented that this study has value for clinicians. “Many states are liberalizing cannabinoid use, and it is important to know the health effects of this type of drug on patients with chronic respiratory disease,” he noted. “The study is somewhat surprising. While one might have expected adverse consequences in patients with COPD who inhaled smoke from cannabinoids, it is somewhat unexpected that oral use would be associated with adverse consequences.” He added, “Pain in older adults is a complex problem. Cannabinoids are often recommended for pain in the general community, but pain per se is not a primary symptom for most patients with COPD from their respiratory problems. Physicians treating patients with COPD should diagnose the cause of the pain and provide appropriate treatment.”
Dose makes a difference
All-cause mortality increased by 231% and hospitalization for COPD or pneumonia increased by 178% among new users of higher-dose cannabinoids, compared with nonusers. Higher dose was defined in this study as more than 1.5mg/day of nabilone. No significant differences appeared in new users vs. nonusers in hospitalization for COPD or pneumonia at lower doses, and no significant differences appeared overall in outpatient respiratory exacerbations, emergency department visits for COPD or pneumonia, or COPD- or pneumonia-related mortality.
Potential limitations and implications
“The fact that COPD- or pneumonia-related mortality was not observed to occur with significantly greater rates among cannabinoid users with COPD may suggest that the increased all-cause mortality finding was not being driven by adverse respiratory-related drug effects, as we hypothesized, and instead was possibly a result of unresolved confounding,” the researchers noted.
The study findings were limited by several factors including the inability to prove causation in an observational study, and the potential for confounding based on unmeasured differences between cannabinoid users and nonusers, the researchers said. “Our findings may not be generalizable to all individuals with COPD, as our study included only those aged 66 years and older, and our COPD identification algorithm, while highly specific, had modest sensitivity,” they added. However, the results were strengthened by the large study population and suggest that cannabinoids are not contraindicated for older adults with COPD, the researchers said. “There can be legitimate reasons for using cannabinoids in this population, such as to help treat chemotherapy-related nausea and vomiting, and possibly for end-of-life care,” they emphasized.
The study findings serve to inform clinicians of the significantly increased mortality risk when older adults with COPD initiate cannabinoids, and “this information should be discussed with patients and incorporated in prescribing decision-making and management plans,” along with consideration of using lower doses when possible to minimize adverse events, they concluded.
The study was supported by The Lung Association – Ontario Grant Review/Grant-In-Aid. The researchers had no financial conflicts to disclose.
, compared with nonusers, findings from a large study have shown.
Synthetic cannabinoids drugs, such as nabilone and dronabinol, have been approved by the Food and Drug Administration for nausea and vomiting caused by chemotherapy. But their off-label use by adults with COPD to help manage chronic musculoskeletal pain, insomnia, and refractory dyspnea is on the rise, wrote Nicholas T. Vozoris, MD, of the University of Toronto and colleagues.
Cannabinoids may actually contribute to negative respiratory outcomes among individuals with COPD through several possible mechanisms including causing sedation, inducing anxiety, and provoking respiratory muscle weakness, they said.
“Possible adverse respiratory effects of cannabinoids may occur with greater likelihood among older adults (in whom COPD is more prevalent), as this group is known to less efficiently metabolise drugs,” they noted.
In a retrospective, population-based cohort study published in Thorax the researchers identified 185,876 adults aged 66 years and older with COPD using health administrative database information from 2006 to 2016. New cannabinoid users (those starting nabilone or dronabinol) were matched with control nonusers (defined as new users of noncannabinoid drugs). Individuals receiving palliative care, or having a diagnosis of cancer or HIV, were excluded because these are settings where synthetic cannabinoids may be prescribed for nausea or vomiting, and these patients are more likely to be in a poorer state of health.
Overall, new cannabinoid users had significantly higher all-cause mortality rates, compared with nonusers (hazard ratio, 1.64). The effects was greater in high-dose users.
Daniel R. Ouellette, MD, associate professor of medicine at Wayne State University and a senior staff physician at Henry Ford Hospital, both in Detroit, commented that this study has value for clinicians. “Many states are liberalizing cannabinoid use, and it is important to know the health effects of this type of drug on patients with chronic respiratory disease,” he noted. “The study is somewhat surprising. While one might have expected adverse consequences in patients with COPD who inhaled smoke from cannabinoids, it is somewhat unexpected that oral use would be associated with adverse consequences.” He added, “Pain in older adults is a complex problem. Cannabinoids are often recommended for pain in the general community, but pain per se is not a primary symptom for most patients with COPD from their respiratory problems. Physicians treating patients with COPD should diagnose the cause of the pain and provide appropriate treatment.”
Dose makes a difference
All-cause mortality increased by 231% and hospitalization for COPD or pneumonia increased by 178% among new users of higher-dose cannabinoids, compared with nonusers. Higher dose was defined in this study as more than 1.5mg/day of nabilone. No significant differences appeared in new users vs. nonusers in hospitalization for COPD or pneumonia at lower doses, and no significant differences appeared overall in outpatient respiratory exacerbations, emergency department visits for COPD or pneumonia, or COPD- or pneumonia-related mortality.
Potential limitations and implications
“The fact that COPD- or pneumonia-related mortality was not observed to occur with significantly greater rates among cannabinoid users with COPD may suggest that the increased all-cause mortality finding was not being driven by adverse respiratory-related drug effects, as we hypothesized, and instead was possibly a result of unresolved confounding,” the researchers noted.
The study findings were limited by several factors including the inability to prove causation in an observational study, and the potential for confounding based on unmeasured differences between cannabinoid users and nonusers, the researchers said. “Our findings may not be generalizable to all individuals with COPD, as our study included only those aged 66 years and older, and our COPD identification algorithm, while highly specific, had modest sensitivity,” they added. However, the results were strengthened by the large study population and suggest that cannabinoids are not contraindicated for older adults with COPD, the researchers said. “There can be legitimate reasons for using cannabinoids in this population, such as to help treat chemotherapy-related nausea and vomiting, and possibly for end-of-life care,” they emphasized.
The study findings serve to inform clinicians of the significantly increased mortality risk when older adults with COPD initiate cannabinoids, and “this information should be discussed with patients and incorporated in prescribing decision-making and management plans,” along with consideration of using lower doses when possible to minimize adverse events, they concluded.
The study was supported by The Lung Association – Ontario Grant Review/Grant-In-Aid. The researchers had no financial conflicts to disclose.
, compared with nonusers, findings from a large study have shown.
Synthetic cannabinoids drugs, such as nabilone and dronabinol, have been approved by the Food and Drug Administration for nausea and vomiting caused by chemotherapy. But their off-label use by adults with COPD to help manage chronic musculoskeletal pain, insomnia, and refractory dyspnea is on the rise, wrote Nicholas T. Vozoris, MD, of the University of Toronto and colleagues.
Cannabinoids may actually contribute to negative respiratory outcomes among individuals with COPD through several possible mechanisms including causing sedation, inducing anxiety, and provoking respiratory muscle weakness, they said.
“Possible adverse respiratory effects of cannabinoids may occur with greater likelihood among older adults (in whom COPD is more prevalent), as this group is known to less efficiently metabolise drugs,” they noted.
In a retrospective, population-based cohort study published in Thorax the researchers identified 185,876 adults aged 66 years and older with COPD using health administrative database information from 2006 to 2016. New cannabinoid users (those starting nabilone or dronabinol) were matched with control nonusers (defined as new users of noncannabinoid drugs). Individuals receiving palliative care, or having a diagnosis of cancer or HIV, were excluded because these are settings where synthetic cannabinoids may be prescribed for nausea or vomiting, and these patients are more likely to be in a poorer state of health.
Overall, new cannabinoid users had significantly higher all-cause mortality rates, compared with nonusers (hazard ratio, 1.64). The effects was greater in high-dose users.
Daniel R. Ouellette, MD, associate professor of medicine at Wayne State University and a senior staff physician at Henry Ford Hospital, both in Detroit, commented that this study has value for clinicians. “Many states are liberalizing cannabinoid use, and it is important to know the health effects of this type of drug on patients with chronic respiratory disease,” he noted. “The study is somewhat surprising. While one might have expected adverse consequences in patients with COPD who inhaled smoke from cannabinoids, it is somewhat unexpected that oral use would be associated with adverse consequences.” He added, “Pain in older adults is a complex problem. Cannabinoids are often recommended for pain in the general community, but pain per se is not a primary symptom for most patients with COPD from their respiratory problems. Physicians treating patients with COPD should diagnose the cause of the pain and provide appropriate treatment.”
Dose makes a difference
All-cause mortality increased by 231% and hospitalization for COPD or pneumonia increased by 178% among new users of higher-dose cannabinoids, compared with nonusers. Higher dose was defined in this study as more than 1.5mg/day of nabilone. No significant differences appeared in new users vs. nonusers in hospitalization for COPD or pneumonia at lower doses, and no significant differences appeared overall in outpatient respiratory exacerbations, emergency department visits for COPD or pneumonia, or COPD- or pneumonia-related mortality.
Potential limitations and implications
“The fact that COPD- or pneumonia-related mortality was not observed to occur with significantly greater rates among cannabinoid users with COPD may suggest that the increased all-cause mortality finding was not being driven by adverse respiratory-related drug effects, as we hypothesized, and instead was possibly a result of unresolved confounding,” the researchers noted.
The study findings were limited by several factors including the inability to prove causation in an observational study, and the potential for confounding based on unmeasured differences between cannabinoid users and nonusers, the researchers said. “Our findings may not be generalizable to all individuals with COPD, as our study included only those aged 66 years and older, and our COPD identification algorithm, while highly specific, had modest sensitivity,” they added. However, the results were strengthened by the large study population and suggest that cannabinoids are not contraindicated for older adults with COPD, the researchers said. “There can be legitimate reasons for using cannabinoids in this population, such as to help treat chemotherapy-related nausea and vomiting, and possibly for end-of-life care,” they emphasized.
The study findings serve to inform clinicians of the significantly increased mortality risk when older adults with COPD initiate cannabinoids, and “this information should be discussed with patients and incorporated in prescribing decision-making and management plans,” along with consideration of using lower doses when possible to minimize adverse events, they concluded.
The study was supported by The Lung Association – Ontario Grant Review/Grant-In-Aid. The researchers had no financial conflicts to disclose.
FROM THORAX
Expert recommendations for targeted therapies in advanced NSCLC
The guidelines, jointly released by the American Society of Clinical Oncology (ASCO) and Ontario Health (OH), were published in the Journal of Clinical Oncology. The recommendations are based on results from 54 studies published or presented from Dec. 2015 to May 2020.
The new guidelines supplant ASCO’s 2017 guidelines on stage IV NSCLC. Several driver mutations were touched upon in the 2017 document, but their corresponding targeted therapies were not recommended as first-line treatment.
With substantial progress in targeted therapies since 2017, treatment decision-making in 2021 focuses on the molecular signatures of tumors and PD-L1 score, according to the authors of the current guidelines, Nasser Hanna, MD, of Indiana University, Indianapolis, and colleagues.
“All patients with nonsquamous NSCLC should have the results of testing for potentially targetable mutations (alterations) before implementing therapy for advanced lung cancer, regardless of smoking status recommendations,” the authors wrote.
They noted that about a third of patients with NSCLC have known targetable genetic alterations. The Food and Drug Administration has approved therapeutics targeting seven alterations: EGFR and ALK alterations, ROS-1 fusions, BRAF V600e mutations, RET fusions, MET exon 14 skipping mutations, and NTRK fusions.
EGFR-mutant NSCLC
The authors’ recommendation for osimertinib as first-line therapy applies to patients who have EGFR-activating mutations in exon 19 (deletion), exon 21 L858R, or exon 20 T790M.
The authors also said osimertinib is an option for patients with other EGFR mutations. Alternatively, these patients can receive afatinib or treatments outlined in the ASCO/OH nondriver mutation guideline, which was published in the Journal of Clinical Oncology in 2020.
If osimertinib is not available for first-line treatment, other options include gefitinib, erlotinib, icotinib, gefitinib plus chemotherapy, dacomitinib, afatinib, erlotinib plus bevacizumab, or erlotinib plus ramucirumab.
The authors recommend osimertinib in the second-line setting for patients who did not receive osimertinib initially and who have a T790M mutation at the time of progression. For patients who have progressed on EGFR tyrosine kinase inhibitors and have no T790M mutation or if their disease has progressed on osimertinib, second-line treatment should be based on the ASCO/OH nondriver mutation guideline, according to Dr. Hanna and colleagues.
ALK-mutant NSCLC
For patients with ALK alterations, the authors recommend alectinib or brigatinib as first-line treatment. If these agents are not available, ceritinib or crizotinib should be offered.
In the second-line setting, if alectinib or brigatinib were given initially, lorlatinib may be offered. If crizotinib was given as first-line therapy, then alectinib, brigatinib, or ceritinib should be offered.
If crizotinib was given in the first-line setting and alectinib, brigatinib, or ceritinib were given in the second-line setting, third-line treatment should be lorlatinib or standard treatment based on the ASCO/OH nondriver mutation guideline.
Other mutations
For stage IV NSCLC patients with alterations in ROS1, BRAF, RET, MET, or NTRK, the authors recommend either targeted or standard nontargeted therapy upfront, with the approach not given first-line used in the second line.
“It is unknown if improved outcomes would be seen when comparing standard nondriver mutation treatment with using the targeted therapy in the first- or second-line setting,” the authors wrote.
They noted that the recommendations for EGFR-activating mutations and ALK fusions are based on results from phase 3 trials, but recommendations for other targetable mutations are supported by phase 2 single-arm data.
The authors also noted promising reports for agents aimed at other molecular targets, including aberrations in KRAS, HER2, and NRG-1.
“Although there are insufficient data to recommend targeted therapy in these and other subgroups at the time of this guideline update, we anticipate rapid evolution of the evidence and availability of targeted therapies in these subgroups of patients soon,” the authors wrote.
Cost considerations
The authors noted that cost is a consideration when deciding on treatment, and costs can vary widely. According to 2020 Medicare drug prices, the monthly cost of ramucirumab was $61, while the monthly cost of ceritinib was $21,107.
“Increasingly, individuals with cancer are required to pay a larger proportion of their treatment costs through deductibles and coinsurance. Higher patient out-of-pocket costs have been shown to be a barrier to initiating and adhering to recommended cancer treatments,” the authors wrote.
“Discussion of cost can be an important part of shared decision-making. Clinicians should discuss with patients the use of less expensive alternatives when it is practical and feasible for treatment of the patient’s disease,” they added.
The guidelines were funded by ASCO. The authors had numerous disclosures, including Dr. Hanna, who disclosed relationships with UpToDate, Merck KGaA, Bristol-Myers Squibb, AstraZeneca/MedImmune, Genentech, and BeyondSpring Pharmaceuticals.
The guidelines, jointly released by the American Society of Clinical Oncology (ASCO) and Ontario Health (OH), were published in the Journal of Clinical Oncology. The recommendations are based on results from 54 studies published or presented from Dec. 2015 to May 2020.
The new guidelines supplant ASCO’s 2017 guidelines on stage IV NSCLC. Several driver mutations were touched upon in the 2017 document, but their corresponding targeted therapies were not recommended as first-line treatment.
With substantial progress in targeted therapies since 2017, treatment decision-making in 2021 focuses on the molecular signatures of tumors and PD-L1 score, according to the authors of the current guidelines, Nasser Hanna, MD, of Indiana University, Indianapolis, and colleagues.
“All patients with nonsquamous NSCLC should have the results of testing for potentially targetable mutations (alterations) before implementing therapy for advanced lung cancer, regardless of smoking status recommendations,” the authors wrote.
They noted that about a third of patients with NSCLC have known targetable genetic alterations. The Food and Drug Administration has approved therapeutics targeting seven alterations: EGFR and ALK alterations, ROS-1 fusions, BRAF V600e mutations, RET fusions, MET exon 14 skipping mutations, and NTRK fusions.
EGFR-mutant NSCLC
The authors’ recommendation for osimertinib as first-line therapy applies to patients who have EGFR-activating mutations in exon 19 (deletion), exon 21 L858R, or exon 20 T790M.
The authors also said osimertinib is an option for patients with other EGFR mutations. Alternatively, these patients can receive afatinib or treatments outlined in the ASCO/OH nondriver mutation guideline, which was published in the Journal of Clinical Oncology in 2020.
If osimertinib is not available for first-line treatment, other options include gefitinib, erlotinib, icotinib, gefitinib plus chemotherapy, dacomitinib, afatinib, erlotinib plus bevacizumab, or erlotinib plus ramucirumab.
The authors recommend osimertinib in the second-line setting for patients who did not receive osimertinib initially and who have a T790M mutation at the time of progression. For patients who have progressed on EGFR tyrosine kinase inhibitors and have no T790M mutation or if their disease has progressed on osimertinib, second-line treatment should be based on the ASCO/OH nondriver mutation guideline, according to Dr. Hanna and colleagues.
ALK-mutant NSCLC
For patients with ALK alterations, the authors recommend alectinib or brigatinib as first-line treatment. If these agents are not available, ceritinib or crizotinib should be offered.
In the second-line setting, if alectinib or brigatinib were given initially, lorlatinib may be offered. If crizotinib was given as first-line therapy, then alectinib, brigatinib, or ceritinib should be offered.
If crizotinib was given in the first-line setting and alectinib, brigatinib, or ceritinib were given in the second-line setting, third-line treatment should be lorlatinib or standard treatment based on the ASCO/OH nondriver mutation guideline.
Other mutations
For stage IV NSCLC patients with alterations in ROS1, BRAF, RET, MET, or NTRK, the authors recommend either targeted or standard nontargeted therapy upfront, with the approach not given first-line used in the second line.
“It is unknown if improved outcomes would be seen when comparing standard nondriver mutation treatment with using the targeted therapy in the first- or second-line setting,” the authors wrote.
They noted that the recommendations for EGFR-activating mutations and ALK fusions are based on results from phase 3 trials, but recommendations for other targetable mutations are supported by phase 2 single-arm data.
The authors also noted promising reports for agents aimed at other molecular targets, including aberrations in KRAS, HER2, and NRG-1.
“Although there are insufficient data to recommend targeted therapy in these and other subgroups at the time of this guideline update, we anticipate rapid evolution of the evidence and availability of targeted therapies in these subgroups of patients soon,” the authors wrote.
Cost considerations
The authors noted that cost is a consideration when deciding on treatment, and costs can vary widely. According to 2020 Medicare drug prices, the monthly cost of ramucirumab was $61, while the monthly cost of ceritinib was $21,107.
“Increasingly, individuals with cancer are required to pay a larger proportion of their treatment costs through deductibles and coinsurance. Higher patient out-of-pocket costs have been shown to be a barrier to initiating and adhering to recommended cancer treatments,” the authors wrote.
“Discussion of cost can be an important part of shared decision-making. Clinicians should discuss with patients the use of less expensive alternatives when it is practical and feasible for treatment of the patient’s disease,” they added.
The guidelines were funded by ASCO. The authors had numerous disclosures, including Dr. Hanna, who disclosed relationships with UpToDate, Merck KGaA, Bristol-Myers Squibb, AstraZeneca/MedImmune, Genentech, and BeyondSpring Pharmaceuticals.
The guidelines, jointly released by the American Society of Clinical Oncology (ASCO) and Ontario Health (OH), were published in the Journal of Clinical Oncology. The recommendations are based on results from 54 studies published or presented from Dec. 2015 to May 2020.
The new guidelines supplant ASCO’s 2017 guidelines on stage IV NSCLC. Several driver mutations were touched upon in the 2017 document, but their corresponding targeted therapies were not recommended as first-line treatment.
With substantial progress in targeted therapies since 2017, treatment decision-making in 2021 focuses on the molecular signatures of tumors and PD-L1 score, according to the authors of the current guidelines, Nasser Hanna, MD, of Indiana University, Indianapolis, and colleagues.
“All patients with nonsquamous NSCLC should have the results of testing for potentially targetable mutations (alterations) before implementing therapy for advanced lung cancer, regardless of smoking status recommendations,” the authors wrote.
They noted that about a third of patients with NSCLC have known targetable genetic alterations. The Food and Drug Administration has approved therapeutics targeting seven alterations: EGFR and ALK alterations, ROS-1 fusions, BRAF V600e mutations, RET fusions, MET exon 14 skipping mutations, and NTRK fusions.
EGFR-mutant NSCLC
The authors’ recommendation for osimertinib as first-line therapy applies to patients who have EGFR-activating mutations in exon 19 (deletion), exon 21 L858R, or exon 20 T790M.
The authors also said osimertinib is an option for patients with other EGFR mutations. Alternatively, these patients can receive afatinib or treatments outlined in the ASCO/OH nondriver mutation guideline, which was published in the Journal of Clinical Oncology in 2020.
If osimertinib is not available for first-line treatment, other options include gefitinib, erlotinib, icotinib, gefitinib plus chemotherapy, dacomitinib, afatinib, erlotinib plus bevacizumab, or erlotinib plus ramucirumab.
The authors recommend osimertinib in the second-line setting for patients who did not receive osimertinib initially and who have a T790M mutation at the time of progression. For patients who have progressed on EGFR tyrosine kinase inhibitors and have no T790M mutation or if their disease has progressed on osimertinib, second-line treatment should be based on the ASCO/OH nondriver mutation guideline, according to Dr. Hanna and colleagues.
ALK-mutant NSCLC
For patients with ALK alterations, the authors recommend alectinib or brigatinib as first-line treatment. If these agents are not available, ceritinib or crizotinib should be offered.
In the second-line setting, if alectinib or brigatinib were given initially, lorlatinib may be offered. If crizotinib was given as first-line therapy, then alectinib, brigatinib, or ceritinib should be offered.
If crizotinib was given in the first-line setting and alectinib, brigatinib, or ceritinib were given in the second-line setting, third-line treatment should be lorlatinib or standard treatment based on the ASCO/OH nondriver mutation guideline.
Other mutations
For stage IV NSCLC patients with alterations in ROS1, BRAF, RET, MET, or NTRK, the authors recommend either targeted or standard nontargeted therapy upfront, with the approach not given first-line used in the second line.
“It is unknown if improved outcomes would be seen when comparing standard nondriver mutation treatment with using the targeted therapy in the first- or second-line setting,” the authors wrote.
They noted that the recommendations for EGFR-activating mutations and ALK fusions are based on results from phase 3 trials, but recommendations for other targetable mutations are supported by phase 2 single-arm data.
The authors also noted promising reports for agents aimed at other molecular targets, including aberrations in KRAS, HER2, and NRG-1.
“Although there are insufficient data to recommend targeted therapy in these and other subgroups at the time of this guideline update, we anticipate rapid evolution of the evidence and availability of targeted therapies in these subgroups of patients soon,” the authors wrote.
Cost considerations
The authors noted that cost is a consideration when deciding on treatment, and costs can vary widely. According to 2020 Medicare drug prices, the monthly cost of ramucirumab was $61, while the monthly cost of ceritinib was $21,107.
“Increasingly, individuals with cancer are required to pay a larger proportion of their treatment costs through deductibles and coinsurance. Higher patient out-of-pocket costs have been shown to be a barrier to initiating and adhering to recommended cancer treatments,” the authors wrote.
“Discussion of cost can be an important part of shared decision-making. Clinicians should discuss with patients the use of less expensive alternatives when it is practical and feasible for treatment of the patient’s disease,” they added.
The guidelines were funded by ASCO. The authors had numerous disclosures, including Dr. Hanna, who disclosed relationships with UpToDate, Merck KGaA, Bristol-Myers Squibb, AstraZeneca/MedImmune, Genentech, and BeyondSpring Pharmaceuticals.
FROM JOURNAL OF CLINICAL ONCOLOGY








