User login
2020 left many GIs unhappy in life outside work
A year ago, 81% of gastroenterologists were happy outside of work. Not anymore.
In these COVID-19–pandemic times, that number is down to 54%, according to a survey of more than 12,000 physicians in 29 specialties that was conducted by Medscape.
“Whether on the front lines of treating COVID-19 patients, pivoting from in-person to virtual care, or even having to shutter their practices, physicians faced an onslaught of crises, while political tensions, social unrest, and environmental concerns probably affected their lives outside of medicine,” Keith L. Martin and Mary Lyn Koval of Medscape wrote in the Gastroenterologist Lifestyle, Happiness & Burnout Report 2021.
Surprisingly, perhaps, the proportion of GIs who say that they’re burned out or are both burned out and depressed now is only a little higher (40%) than in last year’s survey (36%). It’s also just under this year’s burnout rate of 42% for all physicians, which has not changed since last year.
COVID-19 may have had some effect on burnout, though. Among the gastroenterologists with burnout, 15% said it began after the pandemic started, which was, again, less than physicians overall, who had a distribution of 79% before and 21% after. The GIs were slightly less likely to report that their burnout had a severe impact on their everyday lives than physicians overall – 44% versus 47% – but more likely to say that it was bad enough to consider leaving medicine – 15% versus 10%.
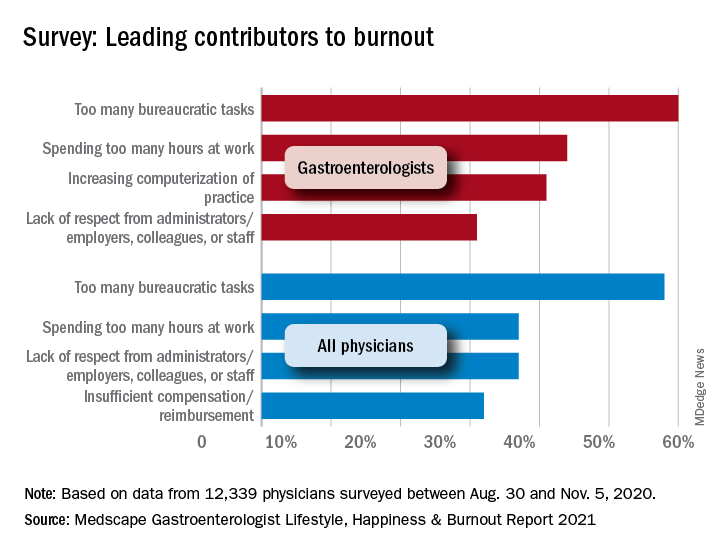
“The chief causes of burnout remain consistent from past years and are pushing physicians to the breaking point,” the Medscape report noted, citing one physician who called it “death by 1,000 cuts.” The biggest contributor to burnout over this past year was, for 60% of gastroenterologists, the excessive number of bureaucratic tasks, followed by spending too much time at work (44%) and increasing computerization (41%).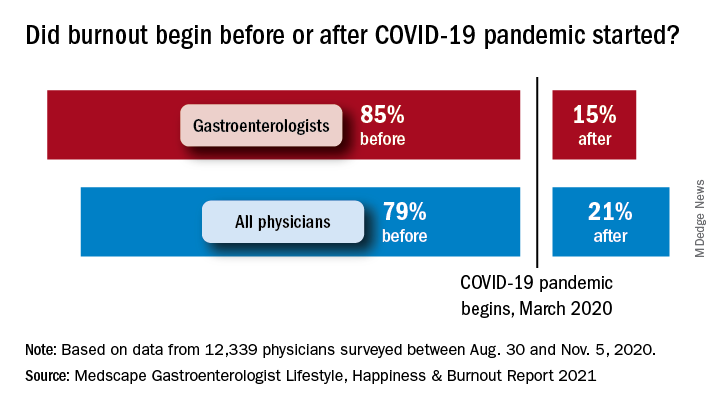
The two pandemic-related contributors included in the survey were near the bottom of the list for gastroenterologists: stress from social distancing/societal issues (15%) and stress related to treating COVID-19 patients (8%), based on data for the 12,339 physicians – of whom about 2% were GIs – polled from Aug. 30 to Nov. 5, 2020.
To deal with their burnout, many gastroenterologists are exercising – at least 51% of them, anyway. Other popular coping mechanisms include talking with family members and close friends (39%), playing or listening to music (38%), isolating themselves from others (36%), and sleeping (26%). For all physicians, the top choices were exercise (48%), talking with family members/friends (43%), and isolation (43%).
When the subject of professional help was raised, a large majority (84%) of GIs planned to forgo such care. That information was not available for physicians as a group, but 70% of internists agreed, as did 83% of nephrologists, 80% of cardiologists, 80% of oncologists, 89% of urologists, and 80% of general surgeons.
A majority of gastroenterologists (58%) said that their symptoms weren’t severe enough to warrant such help, but 38% said they were too busy, and 11% didn’t want to risk disclosure. Some physicians commented on their own situations:
- “I have no energy when I get home and I feel like I’m ignoring my family, but I need to decompress and process what I dealt with during the day” (oncologist).
- “I can’t do the things that I enjoy to relieve stress, such as traveling. My hair is falling out because I can’t destress” (ob.gyn.).
- “I’m tired and discouraged. It stresses my marriage. I have a hard time getting out of bed in the morning. I count the days until Friday” (psychiatrist).
A year ago, 81% of gastroenterologists were happy outside of work. Not anymore.

In these COVID-19–pandemic times, that number is down to 54%, according to a survey of more than 12,000 physicians in 29 specialties that was conducted by Medscape.
“Whether on the front lines of treating COVID-19 patients, pivoting from in-person to virtual care, or even having to shutter their practices, physicians faced an onslaught of crises, while political tensions, social unrest, and environmental concerns probably affected their lives outside of medicine,” Keith L. Martin and Mary Lyn Koval of Medscape wrote in the Gastroenterologist Lifestyle, Happiness & Burnout Report 2021.
Surprisingly, perhaps, the proportion of GIs who say that they’re burned out or are both burned out and depressed now is only a little higher (40%) than in last year’s survey (36%). It’s also just under this year’s burnout rate of 42% for all physicians, which has not changed since last year.
COVID-19 may have had some effect on burnout, though. Among the gastroenterologists with burnout, 15% said it began after the pandemic started, which was, again, less than physicians overall, who had a distribution of 79% before and 21% after. The GIs were slightly less likely to report that their burnout had a severe impact on their everyday lives than physicians overall – 44% versus 47% – but more likely to say that it was bad enough to consider leaving medicine – 15% versus 10%.
“The chief causes of burnout remain consistent from past years and are pushing physicians to the breaking point,” the Medscape report noted, citing one physician who called it “death by 1,000 cuts.” The biggest contributor to burnout over this past year was, for 60% of gastroenterologists, the excessive number of bureaucratic tasks, followed by spending too much time at work (44%) and increasing computerization (41%).
The two pandemic-related contributors included in the survey were near the bottom of the list for gastroenterologists: stress from social distancing/societal issues (15%) and stress related to treating COVID-19 patients (8%), based on data for the 12,339 physicians – of whom about 2% were GIs – polled from Aug. 30 to Nov. 5, 2020.
To deal with their burnout, many gastroenterologists are exercising – at least 51% of them, anyway. Other popular coping mechanisms include talking with family members and close friends (39%), playing or listening to music (38%), isolating themselves from others (36%), and sleeping (26%). For all physicians, the top choices were exercise (48%), talking with family members/friends (43%), and isolation (43%).
When the subject of professional help was raised, a large majority (84%) of GIs planned to forgo such care. That information was not available for physicians as a group, but 70% of internists agreed, as did 83% of nephrologists, 80% of cardiologists, 80% of oncologists, 89% of urologists, and 80% of general surgeons.
A majority of gastroenterologists (58%) said that their symptoms weren’t severe enough to warrant such help, but 38% said they were too busy, and 11% didn’t want to risk disclosure. Some physicians commented on their own situations:
- “I have no energy when I get home and I feel like I’m ignoring my family, but I need to decompress and process what I dealt with during the day” (oncologist).
- “I can’t do the things that I enjoy to relieve stress, such as traveling. My hair is falling out because I can’t destress” (ob.gyn.).
- “I’m tired and discouraged. It stresses my marriage. I have a hard time getting out of bed in the morning. I count the days until Friday” (psychiatrist).
A year ago, 81% of gastroenterologists were happy outside of work. Not anymore.

In these COVID-19–pandemic times, that number is down to 54%, according to a survey of more than 12,000 physicians in 29 specialties that was conducted by Medscape.
“Whether on the front lines of treating COVID-19 patients, pivoting from in-person to virtual care, or even having to shutter their practices, physicians faced an onslaught of crises, while political tensions, social unrest, and environmental concerns probably affected their lives outside of medicine,” Keith L. Martin and Mary Lyn Koval of Medscape wrote in the Gastroenterologist Lifestyle, Happiness & Burnout Report 2021.
Surprisingly, perhaps, the proportion of GIs who say that they’re burned out or are both burned out and depressed now is only a little higher (40%) than in last year’s survey (36%). It’s also just under this year’s burnout rate of 42% for all physicians, which has not changed since last year.
COVID-19 may have had some effect on burnout, though. Among the gastroenterologists with burnout, 15% said it began after the pandemic started, which was, again, less than physicians overall, who had a distribution of 79% before and 21% after. The GIs were slightly less likely to report that their burnout had a severe impact on their everyday lives than physicians overall – 44% versus 47% – but more likely to say that it was bad enough to consider leaving medicine – 15% versus 10%.
“The chief causes of burnout remain consistent from past years and are pushing physicians to the breaking point,” the Medscape report noted, citing one physician who called it “death by 1,000 cuts.” The biggest contributor to burnout over this past year was, for 60% of gastroenterologists, the excessive number of bureaucratic tasks, followed by spending too much time at work (44%) and increasing computerization (41%).
The two pandemic-related contributors included in the survey were near the bottom of the list for gastroenterologists: stress from social distancing/societal issues (15%) and stress related to treating COVID-19 patients (8%), based on data for the 12,339 physicians – of whom about 2% were GIs – polled from Aug. 30 to Nov. 5, 2020.
To deal with their burnout, many gastroenterologists are exercising – at least 51% of them, anyway. Other popular coping mechanisms include talking with family members and close friends (39%), playing or listening to music (38%), isolating themselves from others (36%), and sleeping (26%). For all physicians, the top choices were exercise (48%), talking with family members/friends (43%), and isolation (43%).
When the subject of professional help was raised, a large majority (84%) of GIs planned to forgo such care. That information was not available for physicians as a group, but 70% of internists agreed, as did 83% of nephrologists, 80% of cardiologists, 80% of oncologists, 89% of urologists, and 80% of general surgeons.
A majority of gastroenterologists (58%) said that their symptoms weren’t severe enough to warrant such help, but 38% said they were too busy, and 11% didn’t want to risk disclosure. Some physicians commented on their own situations:
- “I have no energy when I get home and I feel like I’m ignoring my family, but I need to decompress and process what I dealt with during the day” (oncologist).
- “I can’t do the things that I enjoy to relieve stress, such as traveling. My hair is falling out because I can’t destress” (ob.gyn.).
- “I’m tired and discouraged. It stresses my marriage. I have a hard time getting out of bed in the morning. I count the days until Friday” (psychiatrist).
Fired for good judgment a sign of physicians’ lost respect
What happened to Hasan Gokal, MD, should stick painfully in the craws of all physicians. It should serve as a call to action, because Dr. Gokal is sitting at home today without a job and under threat of further legal action while we continue about our day.
Dr. Gokal’s “crime” is that he vaccinated 10 strangers and acquaintances with soon-to-expire doses of the Moderna COVID-19 vaccine. He drove to the homes of some in the dark of night and injected others on his Sugar Land, Texas, lawn. He spent hours in a frantic search for willing recipients to beat the expiration clock. With minutes to spare, he gave the last dose to his at-risk wife, who has symptomatic pulmonary sarcoidosis, but whose age meant she did not fall into a vaccine priority tier.
According to the New York Times, Dr. Gokal’s wife was hesitant, afraid he might get into trouble. But why would she be hesitant? He wasn’t doing anything immoral. Perhaps she knew how far physicians have fallen and how bitterly they both could suffer.
In Barren County, Ky., where I live, a state of emergency was declared by our judge executive because of inclement weather. This directive allows our emergency management to “waive procedures and formalities otherwise required by the law.” It’s too bad that the same courtesy was not afforded to Dr. Gokal in Texas. It’s a shame that ice and snow didn’t drive his actions. Perhaps that would have protected him against the harsh criticism. Rather, it was his oath to patients and dedication to his fellow humans that motivated him, and for that, he was made to suffer.
Dr. Gokal was right to think that pouring the last 10 vaccine doses down the toilet would be an egregious act. But he was wrong in thinking his decision to find takers for the vaccine would be viewed as expedient. Instead, he was accused of graft and even nepotism. And there is the rub. That he was fired and charged with the theft of $137 worth of vaccines says everything about how physicians are treated in the year 2021. Dr. Gokal’s lawyer says the charge carried a maximum penalty of 1 year in prison and a fine of nearly $4,000.
Thank God a sage judge threw out the case and “rebuked” the office of District Attorney Kim Ogg. That hasn’t stopped her from threatening to bring the case to a grand jury. That threat invites anyone faced with the same scenario to flush the extra vaccine doses into the septic system. It encourages us to choose the toilet handle to avoid a mug shot.
And we can’t ignore the racial slant to this story. The Times reported that Dr. Gokal asked the officials, “Are you suggesting that there were too many Indian names in this group?”
“Exactly” was the answer. Let that sink in.
None of this would have happened 20 years ago. Back then, no one would have questioned the wisdom a physician gains from all our years of training and residency. In an age when anyone who conducts an office visit is now called “doctor,” respect for the letters “MD” has been leveled. We physicians have lost our autonomy and been cowed into submission.
But whatever his profession, Hasan Gokal was fired for being a good human. Today, the sun rose on 10 individuals who now enjoy better protection against a deadly pandemic. They include a bed-bound nonagenarian. A woman in her 80s with dementia. A mother with a child who uses a ventilator. All now have antibodies against SARS-CoV2 because of the tireless actions of Dr. Gokal.
Yet Dr. Gokal’s future is uncertain. Will we help him, or will we leave him to the wolves? In an email exchange with his lawyer’s office, I learned that Dr. Gokal has received offers of employment but is unable to entertain them because the actions by the Harris County District Attorney triggered an automatic review by the Texas Medical Board. A GoFundMe page was launched, but an appreciative Dr. Gokal stated publicly that he’d rather the money go to a needy charity.
In the last paragraph of the Times article, Dr. Gokal asks, “How can I take it back?” referencing stories about “the Pakistani doctor in Houston who stole all those vaccines.”
Let’s help him take back his story. In helping him, perhaps we can take back a little control. We could start with letters of support that could be mailed to his lawyer, Paul Doyle, Esq., of Houston, or tweet, respectfully of course, to the district attorney @Kimoggforda.
We can also let the Harris County Public Health Department in Houston know what we think of their actions.
On Martin Luther King Day, Kim Ogg, the district attorney who charged Dr. Gokal, tweeted MLK’s famous quote: “Injustice anywhere is a threat to justice everywhere.”
Let that motivate us to action.
Melissa Walton-Shirley, MD, is a native Kentuckian who retired from full-time invasive cardiology. She enjoys locums work in Montana and is a champion of physician rights and patient safety. In addition to opinion writing, she enjoys spending time with her husband, daughters and parents, and sidelines as a backing vocalist for local rock bands. A version of this article first appeared on Medscape.com.
What happened to Hasan Gokal, MD, should stick painfully in the craws of all physicians. It should serve as a call to action, because Dr. Gokal is sitting at home today without a job and under threat of further legal action while we continue about our day.
Dr. Gokal’s “crime” is that he vaccinated 10 strangers and acquaintances with soon-to-expire doses of the Moderna COVID-19 vaccine. He drove to the homes of some in the dark of night and injected others on his Sugar Land, Texas, lawn. He spent hours in a frantic search for willing recipients to beat the expiration clock. With minutes to spare, he gave the last dose to his at-risk wife, who has symptomatic pulmonary sarcoidosis, but whose age meant she did not fall into a vaccine priority tier.
According to the New York Times, Dr. Gokal’s wife was hesitant, afraid he might get into trouble. But why would she be hesitant? He wasn’t doing anything immoral. Perhaps she knew how far physicians have fallen and how bitterly they both could suffer.
In Barren County, Ky., where I live, a state of emergency was declared by our judge executive because of inclement weather. This directive allows our emergency management to “waive procedures and formalities otherwise required by the law.” It’s too bad that the same courtesy was not afforded to Dr. Gokal in Texas. It’s a shame that ice and snow didn’t drive his actions. Perhaps that would have protected him against the harsh criticism. Rather, it was his oath to patients and dedication to his fellow humans that motivated him, and for that, he was made to suffer.
Dr. Gokal was right to think that pouring the last 10 vaccine doses down the toilet would be an egregious act. But he was wrong in thinking his decision to find takers for the vaccine would be viewed as expedient. Instead, he was accused of graft and even nepotism. And there is the rub. That he was fired and charged with the theft of $137 worth of vaccines says everything about how physicians are treated in the year 2021. Dr. Gokal’s lawyer says the charge carried a maximum penalty of 1 year in prison and a fine of nearly $4,000.
Thank God a sage judge threw out the case and “rebuked” the office of District Attorney Kim Ogg. That hasn’t stopped her from threatening to bring the case to a grand jury. That threat invites anyone faced with the same scenario to flush the extra vaccine doses into the septic system. It encourages us to choose the toilet handle to avoid a mug shot.
And we can’t ignore the racial slant to this story. The Times reported that Dr. Gokal asked the officials, “Are you suggesting that there were too many Indian names in this group?”
“Exactly” was the answer. Let that sink in.
None of this would have happened 20 years ago. Back then, no one would have questioned the wisdom a physician gains from all our years of training and residency. In an age when anyone who conducts an office visit is now called “doctor,” respect for the letters “MD” has been leveled. We physicians have lost our autonomy and been cowed into submission.
But whatever his profession, Hasan Gokal was fired for being a good human. Today, the sun rose on 10 individuals who now enjoy better protection against a deadly pandemic. They include a bed-bound nonagenarian. A woman in her 80s with dementia. A mother with a child who uses a ventilator. All now have antibodies against SARS-CoV2 because of the tireless actions of Dr. Gokal.
Yet Dr. Gokal’s future is uncertain. Will we help him, or will we leave him to the wolves? In an email exchange with his lawyer’s office, I learned that Dr. Gokal has received offers of employment but is unable to entertain them because the actions by the Harris County District Attorney triggered an automatic review by the Texas Medical Board. A GoFundMe page was launched, but an appreciative Dr. Gokal stated publicly that he’d rather the money go to a needy charity.
In the last paragraph of the Times article, Dr. Gokal asks, “How can I take it back?” referencing stories about “the Pakistani doctor in Houston who stole all those vaccines.”
Let’s help him take back his story. In helping him, perhaps we can take back a little control. We could start with letters of support that could be mailed to his lawyer, Paul Doyle, Esq., of Houston, or tweet, respectfully of course, to the district attorney @Kimoggforda.
We can also let the Harris County Public Health Department in Houston know what we think of their actions.
On Martin Luther King Day, Kim Ogg, the district attorney who charged Dr. Gokal, tweeted MLK’s famous quote: “Injustice anywhere is a threat to justice everywhere.”
Let that motivate us to action.
Melissa Walton-Shirley, MD, is a native Kentuckian who retired from full-time invasive cardiology. She enjoys locums work in Montana and is a champion of physician rights and patient safety. In addition to opinion writing, she enjoys spending time with her husband, daughters and parents, and sidelines as a backing vocalist for local rock bands. A version of this article first appeared on Medscape.com.
What happened to Hasan Gokal, MD, should stick painfully in the craws of all physicians. It should serve as a call to action, because Dr. Gokal is sitting at home today without a job and under threat of further legal action while we continue about our day.
Dr. Gokal’s “crime” is that he vaccinated 10 strangers and acquaintances with soon-to-expire doses of the Moderna COVID-19 vaccine. He drove to the homes of some in the dark of night and injected others on his Sugar Land, Texas, lawn. He spent hours in a frantic search for willing recipients to beat the expiration clock. With minutes to spare, he gave the last dose to his at-risk wife, who has symptomatic pulmonary sarcoidosis, but whose age meant she did not fall into a vaccine priority tier.
According to the New York Times, Dr. Gokal’s wife was hesitant, afraid he might get into trouble. But why would she be hesitant? He wasn’t doing anything immoral. Perhaps she knew how far physicians have fallen and how bitterly they both could suffer.
In Barren County, Ky., where I live, a state of emergency was declared by our judge executive because of inclement weather. This directive allows our emergency management to “waive procedures and formalities otherwise required by the law.” It’s too bad that the same courtesy was not afforded to Dr. Gokal in Texas. It’s a shame that ice and snow didn’t drive his actions. Perhaps that would have protected him against the harsh criticism. Rather, it was his oath to patients and dedication to his fellow humans that motivated him, and for that, he was made to suffer.
Dr. Gokal was right to think that pouring the last 10 vaccine doses down the toilet would be an egregious act. But he was wrong in thinking his decision to find takers for the vaccine would be viewed as expedient. Instead, he was accused of graft and even nepotism. And there is the rub. That he was fired and charged with the theft of $137 worth of vaccines says everything about how physicians are treated in the year 2021. Dr. Gokal’s lawyer says the charge carried a maximum penalty of 1 year in prison and a fine of nearly $4,000.
Thank God a sage judge threw out the case and “rebuked” the office of District Attorney Kim Ogg. That hasn’t stopped her from threatening to bring the case to a grand jury. That threat invites anyone faced with the same scenario to flush the extra vaccine doses into the septic system. It encourages us to choose the toilet handle to avoid a mug shot.
And we can’t ignore the racial slant to this story. The Times reported that Dr. Gokal asked the officials, “Are you suggesting that there were too many Indian names in this group?”
“Exactly” was the answer. Let that sink in.
None of this would have happened 20 years ago. Back then, no one would have questioned the wisdom a physician gains from all our years of training and residency. In an age when anyone who conducts an office visit is now called “doctor,” respect for the letters “MD” has been leveled. We physicians have lost our autonomy and been cowed into submission.
But whatever his profession, Hasan Gokal was fired for being a good human. Today, the sun rose on 10 individuals who now enjoy better protection against a deadly pandemic. They include a bed-bound nonagenarian. A woman in her 80s with dementia. A mother with a child who uses a ventilator. All now have antibodies against SARS-CoV2 because of the tireless actions of Dr. Gokal.
Yet Dr. Gokal’s future is uncertain. Will we help him, or will we leave him to the wolves? In an email exchange with his lawyer’s office, I learned that Dr. Gokal has received offers of employment but is unable to entertain them because the actions by the Harris County District Attorney triggered an automatic review by the Texas Medical Board. A GoFundMe page was launched, but an appreciative Dr. Gokal stated publicly that he’d rather the money go to a needy charity.
In the last paragraph of the Times article, Dr. Gokal asks, “How can I take it back?” referencing stories about “the Pakistani doctor in Houston who stole all those vaccines.”
Let’s help him take back his story. In helping him, perhaps we can take back a little control. We could start with letters of support that could be mailed to his lawyer, Paul Doyle, Esq., of Houston, or tweet, respectfully of course, to the district attorney @Kimoggforda.
We can also let the Harris County Public Health Department in Houston know what we think of their actions.
On Martin Luther King Day, Kim Ogg, the district attorney who charged Dr. Gokal, tweeted MLK’s famous quote: “Injustice anywhere is a threat to justice everywhere.”
Let that motivate us to action.
Melissa Walton-Shirley, MD, is a native Kentuckian who retired from full-time invasive cardiology. She enjoys locums work in Montana and is a champion of physician rights and patient safety. In addition to opinion writing, she enjoys spending time with her husband, daughters and parents, and sidelines as a backing vocalist for local rock bands. A version of this article first appeared on Medscape.com.
ACR, AAD, AAO, RDS issue joint statement on safe use of hydroxychloroquine
Hydroxychloroquine can be used safely and effectively with attention to dosing, risk factors, and screening, but communication among physicians, patients, and eye care specialists is key to optimizing outcomes and preventing complications, according to a joint statement from four medical societies.
The American College of Rheumatology, American Academy of Dermatology, Rheumatologic Dermatology Society, and the American Academy of Ophthalmology have produced a statement, published in Arthritis & Rheumatology, “to emphasize points of agreement that should be recognized by practitioners in all specialties,” lead author James T. Rosenbaum, MD, of Oregon Health & Science University, Portland, and colleagues wrote.
The statement was developed by a working group that included rheumatologists, ophthalmologists, and dermatologists with records of published studies on the use of hydroxychloroquine (HCQ) and its toxicity. The statement updated elements of the 2016 American Academy of Ophthalmology guidelines for monitoring patients for retinal toxicity when using HCQ.
“The need for collaborative management has triggered this joint statement, which applies only to managing the risk of HCQ retinopathy and does not include consideration of cardiac, muscle, dermatologic, or other toxicities,” the authors noted.
The authors emphasized that HCQ plays a valuable role in controlling many rheumatic diseases, and should not be abandoned out of fear of retinopathy. However, proper dosing, recognition of risk factors, and screening strategies are essential.
Dosing data
Data on HCQ dosing and retinopathy are limited, but the authors cited a study of 2,361 rheumatic disease patients with an average HCQ dosing regimen of 5.0 mg/kg per day or less in which the toxicity risk was less than 2% for up to 10 years of use. Although data show some increase in risk with duration of use, “for a patient with a normal screening exam in a given year, the risk of developing retinopathy in the ensuing year is low (e.g., less than 5%), even after 20 years of use,” the authors said.
Risk factor recognition
“High daily [HCQ] dosage relative to body weight and cumulative dose are the primary risk factors for retinopathy,” the authors noted. Reduced renal function is an additional risk factor, and patients with renal insufficiency should be monitored and may need lower doses.
In addition, patients with a phenotype of initial parafoveal toxicity may be at increased risk for advanced disease evidenced by damage to the foveal center. “The phenotype of initial parafoveal toxicity is not universal, and in many patients (East Asians particularly) the retinal changes may appear initially along the pericentral vascular arcades,” so these patients should be screened with additional tests beyond the central macula, they emphasized.
Screening strategies
Patients should receive a baseline retinal exam within a few months of starting HCQ to rule out underlying retinal disease, according to the statement. The goal of screening is “to detect early retinopathy before a bullseye becomes visible on ophthalmoscopy, since at that severe stage the damage tends to progress even after discontinuing the medication and may eventually threaten central vision,” the authors said.
In the absence of risk factors, patients can defer screening for 5 years, but should be screened annually from 5 years and forward, they said. Examples of underlying retinal disease include “significant macular degeneration, severe diabetic retinopathy, or hereditary disorders of retinal function, but these are judgments best made by the ophthalmologist since mild and stable abnormalities that do not interfere with interpretation of critical diagnostic tests may not be a contraindication” to use of HCQ.
The consensus opinion statement has limitations, notably the shortage of data on optimum HCQ dosage and the lack of prospective studies of toxicity, including the need for studies of the impact of blood levels on toxicity and studies of pharmacogenomics to stratify risk, the authors noted.
“It is important that the drug is not stopped prematurely, but also that it is not continued in the face of definitive evidence of retinal toxicity except in some situations with unusual medical need,” they said.
“Suggestive or uncertain findings should be discussed with the patient and prescribing physician to justify further examinations, but the drug need not be stopped until evidence for retinopathy is definitive, in particular for patients with active rheumatic or cutaneous disease,” and the overall risk of retinopathy remains low if the principles described in the statement are followed, they concluded.
First author Dr. Rosenbaum disclosed financial relationships with AbbVie, UCB, Gilead, Novartis, Horizon, Roche, Eyevensys, Santen, Corvus, Affibody, Kyverna, Pfizer, Horizon, and UpToDate. Another 5 of the study’s 11 authors also disclosed relationships with multiple companies.
Hydroxychloroquine can be used safely and effectively with attention to dosing, risk factors, and screening, but communication among physicians, patients, and eye care specialists is key to optimizing outcomes and preventing complications, according to a joint statement from four medical societies.
The American College of Rheumatology, American Academy of Dermatology, Rheumatologic Dermatology Society, and the American Academy of Ophthalmology have produced a statement, published in Arthritis & Rheumatology, “to emphasize points of agreement that should be recognized by practitioners in all specialties,” lead author James T. Rosenbaum, MD, of Oregon Health & Science University, Portland, and colleagues wrote.
The statement was developed by a working group that included rheumatologists, ophthalmologists, and dermatologists with records of published studies on the use of hydroxychloroquine (HCQ) and its toxicity. The statement updated elements of the 2016 American Academy of Ophthalmology guidelines for monitoring patients for retinal toxicity when using HCQ.
“The need for collaborative management has triggered this joint statement, which applies only to managing the risk of HCQ retinopathy and does not include consideration of cardiac, muscle, dermatologic, or other toxicities,” the authors noted.
The authors emphasized that HCQ plays a valuable role in controlling many rheumatic diseases, and should not be abandoned out of fear of retinopathy. However, proper dosing, recognition of risk factors, and screening strategies are essential.
Dosing data
Data on HCQ dosing and retinopathy are limited, but the authors cited a study of 2,361 rheumatic disease patients with an average HCQ dosing regimen of 5.0 mg/kg per day or less in which the toxicity risk was less than 2% for up to 10 years of use. Although data show some increase in risk with duration of use, “for a patient with a normal screening exam in a given year, the risk of developing retinopathy in the ensuing year is low (e.g., less than 5%), even after 20 years of use,” the authors said.
Risk factor recognition
“High daily [HCQ] dosage relative to body weight and cumulative dose are the primary risk factors for retinopathy,” the authors noted. Reduced renal function is an additional risk factor, and patients with renal insufficiency should be monitored and may need lower doses.
In addition, patients with a phenotype of initial parafoveal toxicity may be at increased risk for advanced disease evidenced by damage to the foveal center. “The phenotype of initial parafoveal toxicity is not universal, and in many patients (East Asians particularly) the retinal changes may appear initially along the pericentral vascular arcades,” so these patients should be screened with additional tests beyond the central macula, they emphasized.
Screening strategies
Patients should receive a baseline retinal exam within a few months of starting HCQ to rule out underlying retinal disease, according to the statement. The goal of screening is “to detect early retinopathy before a bullseye becomes visible on ophthalmoscopy, since at that severe stage the damage tends to progress even after discontinuing the medication and may eventually threaten central vision,” the authors said.
In the absence of risk factors, patients can defer screening for 5 years, but should be screened annually from 5 years and forward, they said. Examples of underlying retinal disease include “significant macular degeneration, severe diabetic retinopathy, or hereditary disorders of retinal function, but these are judgments best made by the ophthalmologist since mild and stable abnormalities that do not interfere with interpretation of critical diagnostic tests may not be a contraindication” to use of HCQ.
The consensus opinion statement has limitations, notably the shortage of data on optimum HCQ dosage and the lack of prospective studies of toxicity, including the need for studies of the impact of blood levels on toxicity and studies of pharmacogenomics to stratify risk, the authors noted.
“It is important that the drug is not stopped prematurely, but also that it is not continued in the face of definitive evidence of retinal toxicity except in some situations with unusual medical need,” they said.
“Suggestive or uncertain findings should be discussed with the patient and prescribing physician to justify further examinations, but the drug need not be stopped until evidence for retinopathy is definitive, in particular for patients with active rheumatic or cutaneous disease,” and the overall risk of retinopathy remains low if the principles described in the statement are followed, they concluded.
First author Dr. Rosenbaum disclosed financial relationships with AbbVie, UCB, Gilead, Novartis, Horizon, Roche, Eyevensys, Santen, Corvus, Affibody, Kyverna, Pfizer, Horizon, and UpToDate. Another 5 of the study’s 11 authors also disclosed relationships with multiple companies.
Hydroxychloroquine can be used safely and effectively with attention to dosing, risk factors, and screening, but communication among physicians, patients, and eye care specialists is key to optimizing outcomes and preventing complications, according to a joint statement from four medical societies.
The American College of Rheumatology, American Academy of Dermatology, Rheumatologic Dermatology Society, and the American Academy of Ophthalmology have produced a statement, published in Arthritis & Rheumatology, “to emphasize points of agreement that should be recognized by practitioners in all specialties,” lead author James T. Rosenbaum, MD, of Oregon Health & Science University, Portland, and colleagues wrote.
The statement was developed by a working group that included rheumatologists, ophthalmologists, and dermatologists with records of published studies on the use of hydroxychloroquine (HCQ) and its toxicity. The statement updated elements of the 2016 American Academy of Ophthalmology guidelines for monitoring patients for retinal toxicity when using HCQ.
“The need for collaborative management has triggered this joint statement, which applies only to managing the risk of HCQ retinopathy and does not include consideration of cardiac, muscle, dermatologic, or other toxicities,” the authors noted.
The authors emphasized that HCQ plays a valuable role in controlling many rheumatic diseases, and should not be abandoned out of fear of retinopathy. However, proper dosing, recognition of risk factors, and screening strategies are essential.
Dosing data
Data on HCQ dosing and retinopathy are limited, but the authors cited a study of 2,361 rheumatic disease patients with an average HCQ dosing regimen of 5.0 mg/kg per day or less in which the toxicity risk was less than 2% for up to 10 years of use. Although data show some increase in risk with duration of use, “for a patient with a normal screening exam in a given year, the risk of developing retinopathy in the ensuing year is low (e.g., less than 5%), even after 20 years of use,” the authors said.
Risk factor recognition
“High daily [HCQ] dosage relative to body weight and cumulative dose are the primary risk factors for retinopathy,” the authors noted. Reduced renal function is an additional risk factor, and patients with renal insufficiency should be monitored and may need lower doses.
In addition, patients with a phenotype of initial parafoveal toxicity may be at increased risk for advanced disease evidenced by damage to the foveal center. “The phenotype of initial parafoveal toxicity is not universal, and in many patients (East Asians particularly) the retinal changes may appear initially along the pericentral vascular arcades,” so these patients should be screened with additional tests beyond the central macula, they emphasized.
Screening strategies
Patients should receive a baseline retinal exam within a few months of starting HCQ to rule out underlying retinal disease, according to the statement. The goal of screening is “to detect early retinopathy before a bullseye becomes visible on ophthalmoscopy, since at that severe stage the damage tends to progress even after discontinuing the medication and may eventually threaten central vision,” the authors said.
In the absence of risk factors, patients can defer screening for 5 years, but should be screened annually from 5 years and forward, they said. Examples of underlying retinal disease include “significant macular degeneration, severe diabetic retinopathy, or hereditary disorders of retinal function, but these are judgments best made by the ophthalmologist since mild and stable abnormalities that do not interfere with interpretation of critical diagnostic tests may not be a contraindication” to use of HCQ.
The consensus opinion statement has limitations, notably the shortage of data on optimum HCQ dosage and the lack of prospective studies of toxicity, including the need for studies of the impact of blood levels on toxicity and studies of pharmacogenomics to stratify risk, the authors noted.
“It is important that the drug is not stopped prematurely, but also that it is not continued in the face of definitive evidence of retinal toxicity except in some situations with unusual medical need,” they said.
“Suggestive or uncertain findings should be discussed with the patient and prescribing physician to justify further examinations, but the drug need not be stopped until evidence for retinopathy is definitive, in particular for patients with active rheumatic or cutaneous disease,” and the overall risk of retinopathy remains low if the principles described in the statement are followed, they concluded.
First author Dr. Rosenbaum disclosed financial relationships with AbbVie, UCB, Gilead, Novartis, Horizon, Roche, Eyevensys, Santen, Corvus, Affibody, Kyverna, Pfizer, Horizon, and UpToDate. Another 5 of the study’s 11 authors also disclosed relationships with multiple companies.
FROM ARTHRITIS & rHEUMATOLOGY
Mindfulness can help patients manage ‘good’ change – and relief
Two themes have emerged recently in my psychotherapy practice, and in the mirror: relief and exhaustion. Some peace in the public discourse, or at least a pause in the ominous discord, has had the effect of a lightening, an unburdening. Some release from a contracted sense of tension around the specifics of violence and a broader sense of civil fracture has been palpable like a big, deep breath, exhaled. No sensible person would mistake this for being out of the metaphoric woods. A virus menaces and mutates, economic woes follow, and lots of us don’t get along. But, yes, there is some relief, some good change.
But even good change, even a downshift into relief, can pose some challenges to look for and overcome.
Consider for a moment the notion that every change represents a loss, a metaphoric “death” of the prior state of things. This is true of big, painful losses, like the death of a loved one, and small ones, like finding an empty cookie jar. It’s also true in changes we associate with benefit or relief: a refund check, a job promotion, a resolving migraine, or the breaking out of some civility.
In changes of all sorts, the world outside of one’s mind has shifted – at odds, momentarily, with our inner, now obsolete understanding of that changed world. The inside of the head does not match the outside. How we make that adjustment, so “inside = outside,” is a clinically familiar process: it’s grieving, with a sequence famously elaborated upon by Elisabeth Kübler-Ross, MD,1 and others.
We all likely know the steps: shock/denial, anger, “bargaining,” depression, and acceptance. A quick review: Our initial anxious/threat reaction leads to grievous judgment, to rationalizing “woulda/coulda/shoulda’s,” then to truly landing in the disappointment of a loss or change, and the accepting of a new steady state. Inside proceeds to match outside.
So, what then of relief? How do we process “good” change? I think we still must move from “in ≠ out” to “in = out,” navigating some pitfalls along the way.
Initial threat often remains; apprehension of the “new” still can generate energy, and even a sense of threat, regardless of a kiss or a shove. Our brainstems run roughshod over this first phase.
Step two is about judgment. We can move past the threat to, “How do I feel about it?” Here’s where grievous feeling gets swapped out for something more peak-positive – joy, or relief if the change represents an ending of a state of suffering, tension, or uncertainty.
The “bargaining” step still happens, but often around a kind of testing regimen: Is this too good to be true? Is it really different? We run scenarios.
The thud of disappointment also gets a makeover. It’s a settling into the beneficial change and its associations: gratitude, a sense of energy shifting.
The bookend “OK” seems anodyne here – why would anyone not accept relief, some good change?2 But it can nevertheless represent a challenge for many. The receding tension of the last year could open into a burst of energy, but I’m finding that exhaustion is just as or more common. That’s not illness, but a weary exhaling from the longest of held breaths.
One other twist: What happens when one of those steps is an individual obstacle, trigger, or hard-to-hold state? Especially for those with deep experience in disappointment or even trauma, buying into acceptance of a new normal can feel like a fool’s game. This is an especially complex spot for individuals who won’t quite allow for joyful acceptance to break out, lest it reveals itself as a humiliating trick or a too-brief respite from the “usual.”
Mindfulness practices, such as meditation, are helpful in managing this process. Committed time and optimal conditions to witness and adapt to the various inner states that ebb and flow generate a clear therapeutic benefit. Patients improve their identification of somatic manifestations, emotional reactions, and cycling ruminations of thought. What generates distraction and loss of mindful attention becomes better recognized. Contemplative work in between sessions becomes more productive.
What else do I advise?3 Patience, and some compassion for ourselves in this unusual time. Grief, and relief, are complex but truly human processes that generate not just one state of experience, but a cascade of them. While that cascade can hurt, it’s actually normal, not illness. But it can be exhausting.
Dr. Sazima is a Northern California psychiatrist, educator, and author. He is senior behavioral faculty at the Stanford-O’Connor Family Medicine Residency Program in San José, Calif. His latest book is “Practical Mindfulness: A Physician’s No-Nonsense Guide to Meditation for Beginners,” Miami: Mango Publishing, 2021. Dr. Sazima disclosed no relevant financial relationships.
References
1. Kübler-Ross E. “On Death And Dying,” New York: Simon & Schuster, 1969.
2. Selye H. “Stress Without Distress,” New York: Lippincott, Williams & Wilkins, 1974.
3. Sazima G. “Practical Mindfulness: A Physician’s No-Nonsense Guide to Meditation for Beginners,” Miami: Mango Publishing, 2021.
Two themes have emerged recently in my psychotherapy practice, and in the mirror: relief and exhaustion. Some peace in the public discourse, or at least a pause in the ominous discord, has had the effect of a lightening, an unburdening. Some release from a contracted sense of tension around the specifics of violence and a broader sense of civil fracture has been palpable like a big, deep breath, exhaled. No sensible person would mistake this for being out of the metaphoric woods. A virus menaces and mutates, economic woes follow, and lots of us don’t get along. But, yes, there is some relief, some good change.
But even good change, even a downshift into relief, can pose some challenges to look for and overcome.
Consider for a moment the notion that every change represents a loss, a metaphoric “death” of the prior state of things. This is true of big, painful losses, like the death of a loved one, and small ones, like finding an empty cookie jar. It’s also true in changes we associate with benefit or relief: a refund check, a job promotion, a resolving migraine, or the breaking out of some civility.
In changes of all sorts, the world outside of one’s mind has shifted – at odds, momentarily, with our inner, now obsolete understanding of that changed world. The inside of the head does not match the outside. How we make that adjustment, so “inside = outside,” is a clinically familiar process: it’s grieving, with a sequence famously elaborated upon by Elisabeth Kübler-Ross, MD,1 and others.
We all likely know the steps: shock/denial, anger, “bargaining,” depression, and acceptance. A quick review: Our initial anxious/threat reaction leads to grievous judgment, to rationalizing “woulda/coulda/shoulda’s,” then to truly landing in the disappointment of a loss or change, and the accepting of a new steady state. Inside proceeds to match outside.
So, what then of relief? How do we process “good” change? I think we still must move from “in ≠ out” to “in = out,” navigating some pitfalls along the way.
Initial threat often remains; apprehension of the “new” still can generate energy, and even a sense of threat, regardless of a kiss or a shove. Our brainstems run roughshod over this first phase.
Step two is about judgment. We can move past the threat to, “How do I feel about it?” Here’s where grievous feeling gets swapped out for something more peak-positive – joy, or relief if the change represents an ending of a state of suffering, tension, or uncertainty.
The “bargaining” step still happens, but often around a kind of testing regimen: Is this too good to be true? Is it really different? We run scenarios.
The thud of disappointment also gets a makeover. It’s a settling into the beneficial change and its associations: gratitude, a sense of energy shifting.
The bookend “OK” seems anodyne here – why would anyone not accept relief, some good change?2 But it can nevertheless represent a challenge for many. The receding tension of the last year could open into a burst of energy, but I’m finding that exhaustion is just as or more common. That’s not illness, but a weary exhaling from the longest of held breaths.
One other twist: What happens when one of those steps is an individual obstacle, trigger, or hard-to-hold state? Especially for those with deep experience in disappointment or even trauma, buying into acceptance of a new normal can feel like a fool’s game. This is an especially complex spot for individuals who won’t quite allow for joyful acceptance to break out, lest it reveals itself as a humiliating trick or a too-brief respite from the “usual.”
Mindfulness practices, such as meditation, are helpful in managing this process. Committed time and optimal conditions to witness and adapt to the various inner states that ebb and flow generate a clear therapeutic benefit. Patients improve their identification of somatic manifestations, emotional reactions, and cycling ruminations of thought. What generates distraction and loss of mindful attention becomes better recognized. Contemplative work in between sessions becomes more productive.
What else do I advise?3 Patience, and some compassion for ourselves in this unusual time. Grief, and relief, are complex but truly human processes that generate not just one state of experience, but a cascade of them. While that cascade can hurt, it’s actually normal, not illness. But it can be exhausting.
Dr. Sazima is a Northern California psychiatrist, educator, and author. He is senior behavioral faculty at the Stanford-O’Connor Family Medicine Residency Program in San José, Calif. His latest book is “Practical Mindfulness: A Physician’s No-Nonsense Guide to Meditation for Beginners,” Miami: Mango Publishing, 2021. Dr. Sazima disclosed no relevant financial relationships.
References
1. Kübler-Ross E. “On Death And Dying,” New York: Simon & Schuster, 1969.
2. Selye H. “Stress Without Distress,” New York: Lippincott, Williams & Wilkins, 1974.
3. Sazima G. “Practical Mindfulness: A Physician’s No-Nonsense Guide to Meditation for Beginners,” Miami: Mango Publishing, 2021.
Two themes have emerged recently in my psychotherapy practice, and in the mirror: relief and exhaustion. Some peace in the public discourse, or at least a pause in the ominous discord, has had the effect of a lightening, an unburdening. Some release from a contracted sense of tension around the specifics of violence and a broader sense of civil fracture has been palpable like a big, deep breath, exhaled. No sensible person would mistake this for being out of the metaphoric woods. A virus menaces and mutates, economic woes follow, and lots of us don’t get along. But, yes, there is some relief, some good change.
But even good change, even a downshift into relief, can pose some challenges to look for and overcome.
Consider for a moment the notion that every change represents a loss, a metaphoric “death” of the prior state of things. This is true of big, painful losses, like the death of a loved one, and small ones, like finding an empty cookie jar. It’s also true in changes we associate with benefit or relief: a refund check, a job promotion, a resolving migraine, or the breaking out of some civility.
In changes of all sorts, the world outside of one’s mind has shifted – at odds, momentarily, with our inner, now obsolete understanding of that changed world. The inside of the head does not match the outside. How we make that adjustment, so “inside = outside,” is a clinically familiar process: it’s grieving, with a sequence famously elaborated upon by Elisabeth Kübler-Ross, MD,1 and others.
We all likely know the steps: shock/denial, anger, “bargaining,” depression, and acceptance. A quick review: Our initial anxious/threat reaction leads to grievous judgment, to rationalizing “woulda/coulda/shoulda’s,” then to truly landing in the disappointment of a loss or change, and the accepting of a new steady state. Inside proceeds to match outside.
So, what then of relief? How do we process “good” change? I think we still must move from “in ≠ out” to “in = out,” navigating some pitfalls along the way.
Initial threat often remains; apprehension of the “new” still can generate energy, and even a sense of threat, regardless of a kiss or a shove. Our brainstems run roughshod over this first phase.
Step two is about judgment. We can move past the threat to, “How do I feel about it?” Here’s where grievous feeling gets swapped out for something more peak-positive – joy, or relief if the change represents an ending of a state of suffering, tension, or uncertainty.
The “bargaining” step still happens, but often around a kind of testing regimen: Is this too good to be true? Is it really different? We run scenarios.
The thud of disappointment also gets a makeover. It’s a settling into the beneficial change and its associations: gratitude, a sense of energy shifting.
The bookend “OK” seems anodyne here – why would anyone not accept relief, some good change?2 But it can nevertheless represent a challenge for many. The receding tension of the last year could open into a burst of energy, but I’m finding that exhaustion is just as or more common. That’s not illness, but a weary exhaling from the longest of held breaths.
One other twist: What happens when one of those steps is an individual obstacle, trigger, or hard-to-hold state? Especially for those with deep experience in disappointment or even trauma, buying into acceptance of a new normal can feel like a fool’s game. This is an especially complex spot for individuals who won’t quite allow for joyful acceptance to break out, lest it reveals itself as a humiliating trick or a too-brief respite from the “usual.”
Mindfulness practices, such as meditation, are helpful in managing this process. Committed time and optimal conditions to witness and adapt to the various inner states that ebb and flow generate a clear therapeutic benefit. Patients improve their identification of somatic manifestations, emotional reactions, and cycling ruminations of thought. What generates distraction and loss of mindful attention becomes better recognized. Contemplative work in between sessions becomes more productive.
What else do I advise?3 Patience, and some compassion for ourselves in this unusual time. Grief, and relief, are complex but truly human processes that generate not just one state of experience, but a cascade of them. While that cascade can hurt, it’s actually normal, not illness. But it can be exhausting.
Dr. Sazima is a Northern California psychiatrist, educator, and author. He is senior behavioral faculty at the Stanford-O’Connor Family Medicine Residency Program in San José, Calif. His latest book is “Practical Mindfulness: A Physician’s No-Nonsense Guide to Meditation for Beginners,” Miami: Mango Publishing, 2021. Dr. Sazima disclosed no relevant financial relationships.
References
1. Kübler-Ross E. “On Death And Dying,” New York: Simon & Schuster, 1969.
2. Selye H. “Stress Without Distress,” New York: Lippincott, Williams & Wilkins, 1974.
3. Sazima G. “Practical Mindfulness: A Physician’s No-Nonsense Guide to Meditation for Beginners,” Miami: Mango Publishing, 2021.
Sudden Cardiac Death in a Young Patient With Psoriasis
To the Editor:
The evolution in the understanding of psoriasis and psoriatic arthritis has unfolded many new facets of this immune-mediated inflammatory disease. Once considered to be just a cutaneous disease, psoriasis is not actually confined to skin but can involve almost any other system of the body. Cardiovascular morbidity and mortality are the major concerns in patients with psoriasis. We report the sudden death of a young man with severe psoriasis.
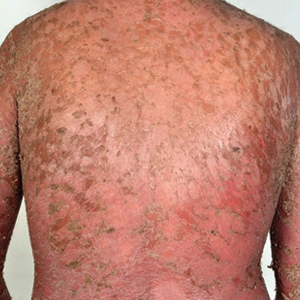
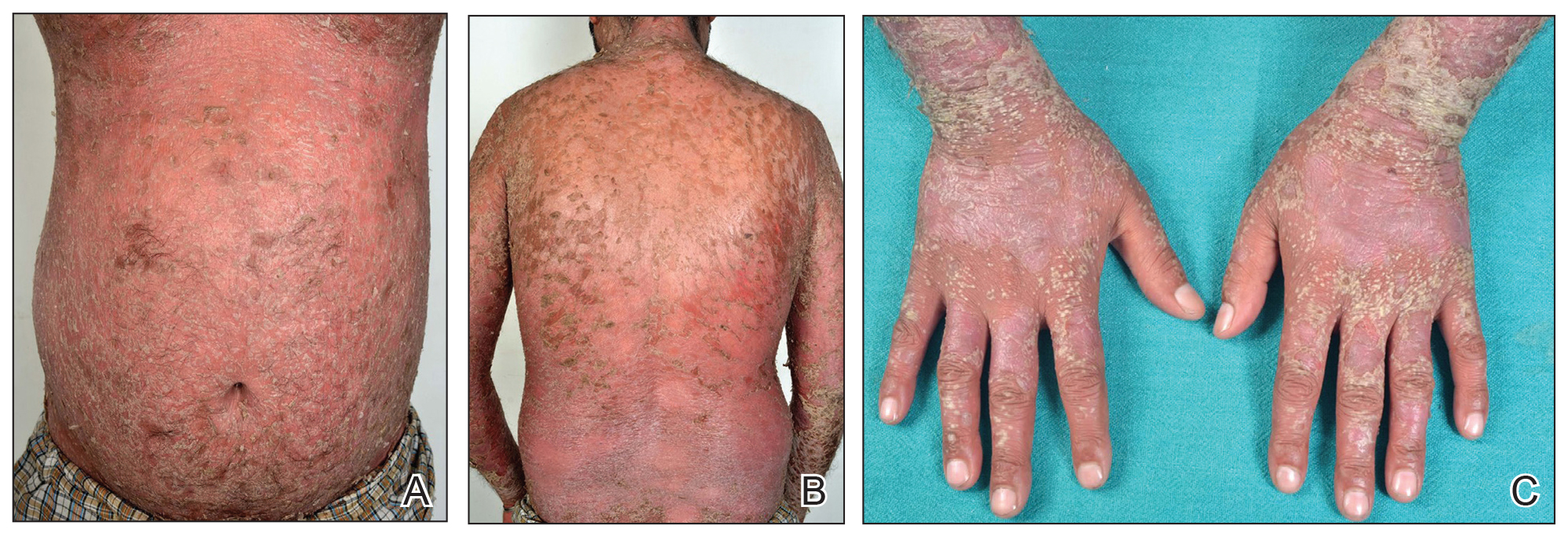
A 31-year-old man was admitted for severe psoriasis with pustular exacerbation (Figures 1A and 1B). He had moderate to severe unstable disease during the last 8 years and was managed with oral methotrexate (0.3–0.5 mg/kg/wk). He was not compliant with treatment, which led to multiple relapses. There was no personal or family history of risk factors for cardiovascular events (CVEs). At the time of present hospitalization, his vital parameters were normal. Physical examination revealed erythematous scaly plaques on more than 75% of the body surface area. Multiple pustules also were noted, often coalescing to form plaques (Figure 1C). Baseline investigations consisting of complete blood cell count, lipid profile, liver and renal functions, and chest radiography were within reference range. Baseline electrocardiogram (ECG) at admission was unremarkable (Figure 2A), except for sinus tachycardia. Low-voltage complexes in limb leads were appreciated as well as a corrected QT interval of 420 milliseconds (within reference range). Echocardiography was normal (visual ejection fraction of 60%).
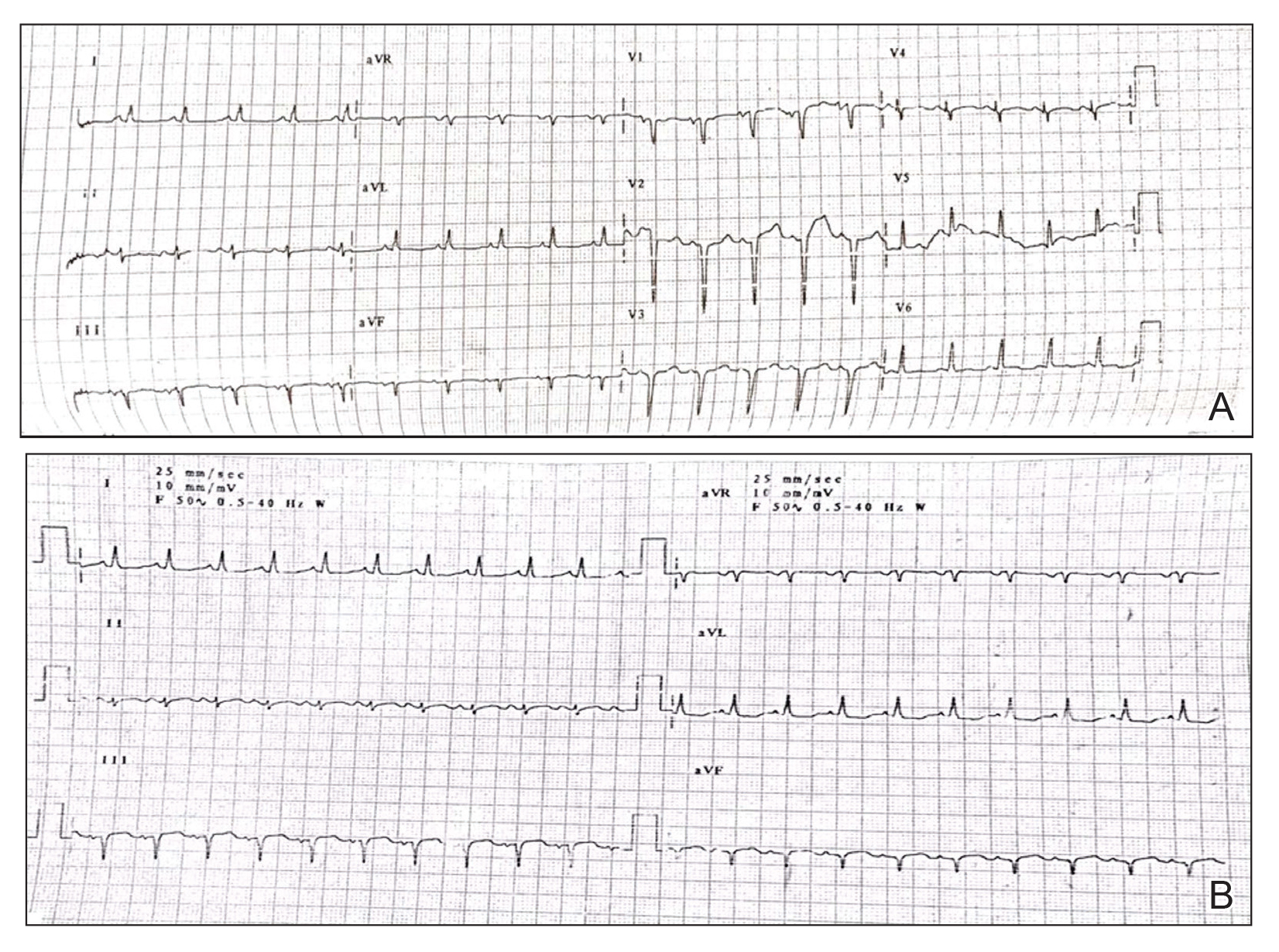
The patient was unable to tolerate methotrexate due to excessive nausea; he was started on oral acitretin 25 mg once daily. There was no improvement in psoriasis over the following week, and he reported mild upper abdominal discomfort. He did not have any chest pain or dyspnea, and his pulse and blood pressure were normal. Serum electrolytes, liver function, lipid profile, and an ultrasound of the abdomen revealed no abnormalities. A repeat ECG showed no changes, and cardiac biomarkers were not elevated. Two days later, the patient collapsed while still in the hospital. A cardiac monitor and ECG showed ventricular tachycardia (VT)(Figure 2B); however, serum electrolytes, calcium, magnesium, and phosphorus levels were within reference range. Aggressive resuscitative measures including multiple attempts at cardioversion with up to 200 J (biphasic) and intravenous amiodarone infusion failed to revive the patient, and he died.
Proinflammatory cytokines such as IL-6 and tumor necrosis factor α are increased in young people with ventricular arrhythmias who have no evidence of myocardial injury (MI), suggesting an inflammatory background is involved.1 Psoriasis, a common immune-mediated inflammatory disease, has a chronic state of systemic inflammation with notably higher serum levels of tumor necrosis factor α, IFN-γ, IL-6, IL-8, IL-12, and IL-18 compared to controls.2 This inflammation is not confined to skin but can involve blood vessels, joints, and the liver, as demonstrated by increased fluorodeoxyglucose uptake.3 It also seems to exert its influence on supraventricular beat development in patients with psoriasis who do not have a history of CVEs.4 Tumor necrosis factor α is one of the major cytokines playing a role in the inflammatory process of psoriasis. Studies have shown serum levels of tumor necrosis factor α to correlate with the clinical symptoms of heart failure and to supraventricular arrhythmia in animal models.4 Various extreme CVEs can be an expression of this ongoing dynamic process. It would be interesting to know which specific factors among these inflammatory cytokines lead to rhythm irregularities.
Another theory is that young patients may experience micro-MI during the disease course. These small infarcted areas may act as aberrant pulse generators or lead to conduction disturbances. One study found increased correct QT interval dispersion, a predictor of ventricular arrhythmias, to be associated with psoriasis.5 A nationwide population-based matched cohort study by Chiu et al6 revealed that patients with psoriasis have a higher risk for arrhythmia independent of traditional cardiovascular risk factors. Our patient also had severe unstable psoriasis for 8 years that may have led to increased accumulation of proarrhythmogenic cytokines in the heart and could have led to VT.
Acitretin as a potential cause of sudden cardiac death remains a possibility in our case; however, the exact mechanism leading to such sudden arrhythmia is lacking. Acitretin is known to increase serum triglycerides and cholesterol, specifically by shifting high-density lipoproteins to low-density lipoproteins, thereby increasing the risk for CVE. However, it takes time for such derangement to occur, eventually leading to CVE. Mittal et al7 reported a psoriasis patient who died secondary to MI after 5 days of low-dose acitretin. Lack of evidence makes acitretin a less likely cause of mortality.
We present a case of sudden cardiac death secondary to VT in a young patient with psoriasis and no other traditional cardiovascular risk factors. This case highlights the importance of being vigilant for adverse CVEs such as arrhythmia in psoriatic patients, especially in younger patients with severe unstable disease.
- Kowalewski M, Urban M, Mroczko B, et al. Proinflammatory cytokines (IL-6, TNF-alpha) and cardiac troponin I (cTnI) in serum of young people with ventricular arrhythmias. Pol Arch Med Wewn. 2002;108:647-651.
- Arican O, Aral M, Sasmaz S, et al. Serum levels of TNF-alpha, IFN-gamma, IL-6, IL-8, IL-12, IL-17, and IL-18 in patients with active psoriasis and correlation with disease severity. Mediators Inflamm. 2005;2005:273-279.
- Mehta NN, Yu Y, Saboury B, et al. Systemic and vascular inflammation in patients with moderate to severe psoriasis as measured by [18F]-fluorodeoxyglucose positron emission tomography-computed tomography (FDG-PET/CT): a pilot study. Arch Dermatol. 2011;147:1031-1039.
- Markuszeski L, Bissinger A, Janusz I, et al. Heart rate and arrhythmia in patients with psoriasis vulgaris. Arch Med Res. 2007;38:64-69.
- Simsek H, Sahin M, Akyol A, et al. Increased risk of atrial and ventricular arrhythmia in long-lasting psoriasis patients. ScientificWorldJournal. 2013;2013:901215.
- Chiu HY, Chang WL, Huang WF, et al. Increased risk of arrhythmia in patients with psoriatic disease: a nationwide population-based matched cohort study. J Am Acad Dermatol. 2015;73:429-438.
- Mittal R, Malhotra S, Pandhi P, et al. Efficacy and safety of combination acitretin and pioglitazone therapy in patients with moderate to severe chronic plaque-type psoriasis: a randomized, double-blind, placebo-controlled clinical trial. Arch Dermatol. 2009;145:387-393.
To the Editor:
The evolution in the understanding of psoriasis and psoriatic arthritis has unfolded many new facets of this immune-mediated inflammatory disease. Once considered to be just a cutaneous disease, psoriasis is not actually confined to skin but can involve almost any other system of the body. Cardiovascular morbidity and mortality are the major concerns in patients with psoriasis. We report the sudden death of a young man with severe psoriasis.
A 31-year-old man was admitted for severe psoriasis with pustular exacerbation (Figures 1A and 1B). He had moderate to severe unstable disease during the last 8 years and was managed with oral methotrexate (0.3–0.5 mg/kg/wk). He was not compliant with treatment, which led to multiple relapses. There was no personal or family history of risk factors for cardiovascular events (CVEs). At the time of present hospitalization, his vital parameters were normal. Physical examination revealed erythematous scaly plaques on more than 75% of the body surface area. Multiple pustules also were noted, often coalescing to form plaques (Figure 1C). Baseline investigations consisting of complete blood cell count, lipid profile, liver and renal functions, and chest radiography were within reference range. Baseline electrocardiogram (ECG) at admission was unremarkable (Figure 2A), except for sinus tachycardia. Low-voltage complexes in limb leads were appreciated as well as a corrected QT interval of 420 milliseconds (within reference range). Echocardiography was normal (visual ejection fraction of 60%).


The patient was unable to tolerate methotrexate due to excessive nausea; he was started on oral acitretin 25 mg once daily. There was no improvement in psoriasis over the following week, and he reported mild upper abdominal discomfort. He did not have any chest pain or dyspnea, and his pulse and blood pressure were normal. Serum electrolytes, liver function, lipid profile, and an ultrasound of the abdomen revealed no abnormalities. A repeat ECG showed no changes, and cardiac biomarkers were not elevated. Two days later, the patient collapsed while still in the hospital. A cardiac monitor and ECG showed ventricular tachycardia (VT)(Figure 2B); however, serum electrolytes, calcium, magnesium, and phosphorus levels were within reference range. Aggressive resuscitative measures including multiple attempts at cardioversion with up to 200 J (biphasic) and intravenous amiodarone infusion failed to revive the patient, and he died.
Proinflammatory cytokines such as IL-6 and tumor necrosis factor α are increased in young people with ventricular arrhythmias who have no evidence of myocardial injury (MI), suggesting an inflammatory background is involved.1 Psoriasis, a common immune-mediated inflammatory disease, has a chronic state of systemic inflammation with notably higher serum levels of tumor necrosis factor α, IFN-γ, IL-6, IL-8, IL-12, and IL-18 compared to controls.2 This inflammation is not confined to skin but can involve blood vessels, joints, and the liver, as demonstrated by increased fluorodeoxyglucose uptake.3 It also seems to exert its influence on supraventricular beat development in patients with psoriasis who do not have a history of CVEs.4 Tumor necrosis factor α is one of the major cytokines playing a role in the inflammatory process of psoriasis. Studies have shown serum levels of tumor necrosis factor α to correlate with the clinical symptoms of heart failure and to supraventricular arrhythmia in animal models.4 Various extreme CVEs can be an expression of this ongoing dynamic process. It would be interesting to know which specific factors among these inflammatory cytokines lead to rhythm irregularities.
Another theory is that young patients may experience micro-MI during the disease course. These small infarcted areas may act as aberrant pulse generators or lead to conduction disturbances. One study found increased correct QT interval dispersion, a predictor of ventricular arrhythmias, to be associated with psoriasis.5 A nationwide population-based matched cohort study by Chiu et al6 revealed that patients with psoriasis have a higher risk for arrhythmia independent of traditional cardiovascular risk factors. Our patient also had severe unstable psoriasis for 8 years that may have led to increased accumulation of proarrhythmogenic cytokines in the heart and could have led to VT.
Acitretin as a potential cause of sudden cardiac death remains a possibility in our case; however, the exact mechanism leading to such sudden arrhythmia is lacking. Acitretin is known to increase serum triglycerides and cholesterol, specifically by shifting high-density lipoproteins to low-density lipoproteins, thereby increasing the risk for CVE. However, it takes time for such derangement to occur, eventually leading to CVE. Mittal et al7 reported a psoriasis patient who died secondary to MI after 5 days of low-dose acitretin. Lack of evidence makes acitretin a less likely cause of mortality.
We present a case of sudden cardiac death secondary to VT in a young patient with psoriasis and no other traditional cardiovascular risk factors. This case highlights the importance of being vigilant for adverse CVEs such as arrhythmia in psoriatic patients, especially in younger patients with severe unstable disease.
To the Editor:
The evolution in the understanding of psoriasis and psoriatic arthritis has unfolded many new facets of this immune-mediated inflammatory disease. Once considered to be just a cutaneous disease, psoriasis is not actually confined to skin but can involve almost any other system of the body. Cardiovascular morbidity and mortality are the major concerns in patients with psoriasis. We report the sudden death of a young man with severe psoriasis.
A 31-year-old man was admitted for severe psoriasis with pustular exacerbation (Figures 1A and 1B). He had moderate to severe unstable disease during the last 8 years and was managed with oral methotrexate (0.3–0.5 mg/kg/wk). He was not compliant with treatment, which led to multiple relapses. There was no personal or family history of risk factors for cardiovascular events (CVEs). At the time of present hospitalization, his vital parameters were normal. Physical examination revealed erythematous scaly plaques on more than 75% of the body surface area. Multiple pustules also were noted, often coalescing to form plaques (Figure 1C). Baseline investigations consisting of complete blood cell count, lipid profile, liver and renal functions, and chest radiography were within reference range. Baseline electrocardiogram (ECG) at admission was unremarkable (Figure 2A), except for sinus tachycardia. Low-voltage complexes in limb leads were appreciated as well as a corrected QT interval of 420 milliseconds (within reference range). Echocardiography was normal (visual ejection fraction of 60%).


The patient was unable to tolerate methotrexate due to excessive nausea; he was started on oral acitretin 25 mg once daily. There was no improvement in psoriasis over the following week, and he reported mild upper abdominal discomfort. He did not have any chest pain or dyspnea, and his pulse and blood pressure were normal. Serum electrolytes, liver function, lipid profile, and an ultrasound of the abdomen revealed no abnormalities. A repeat ECG showed no changes, and cardiac biomarkers were not elevated. Two days later, the patient collapsed while still in the hospital. A cardiac monitor and ECG showed ventricular tachycardia (VT)(Figure 2B); however, serum electrolytes, calcium, magnesium, and phosphorus levels were within reference range. Aggressive resuscitative measures including multiple attempts at cardioversion with up to 200 J (biphasic) and intravenous amiodarone infusion failed to revive the patient, and he died.
Proinflammatory cytokines such as IL-6 and tumor necrosis factor α are increased in young people with ventricular arrhythmias who have no evidence of myocardial injury (MI), suggesting an inflammatory background is involved.1 Psoriasis, a common immune-mediated inflammatory disease, has a chronic state of systemic inflammation with notably higher serum levels of tumor necrosis factor α, IFN-γ, IL-6, IL-8, IL-12, and IL-18 compared to controls.2 This inflammation is not confined to skin but can involve blood vessels, joints, and the liver, as demonstrated by increased fluorodeoxyglucose uptake.3 It also seems to exert its influence on supraventricular beat development in patients with psoriasis who do not have a history of CVEs.4 Tumor necrosis factor α is one of the major cytokines playing a role in the inflammatory process of psoriasis. Studies have shown serum levels of tumor necrosis factor α to correlate with the clinical symptoms of heart failure and to supraventricular arrhythmia in animal models.4 Various extreme CVEs can be an expression of this ongoing dynamic process. It would be interesting to know which specific factors among these inflammatory cytokines lead to rhythm irregularities.
Another theory is that young patients may experience micro-MI during the disease course. These small infarcted areas may act as aberrant pulse generators or lead to conduction disturbances. One study found increased correct QT interval dispersion, a predictor of ventricular arrhythmias, to be associated with psoriasis.5 A nationwide population-based matched cohort study by Chiu et al6 revealed that patients with psoriasis have a higher risk for arrhythmia independent of traditional cardiovascular risk factors. Our patient also had severe unstable psoriasis for 8 years that may have led to increased accumulation of proarrhythmogenic cytokines in the heart and could have led to VT.
Acitretin as a potential cause of sudden cardiac death remains a possibility in our case; however, the exact mechanism leading to such sudden arrhythmia is lacking. Acitretin is known to increase serum triglycerides and cholesterol, specifically by shifting high-density lipoproteins to low-density lipoproteins, thereby increasing the risk for CVE. However, it takes time for such derangement to occur, eventually leading to CVE. Mittal et al7 reported a psoriasis patient who died secondary to MI after 5 days of low-dose acitretin. Lack of evidence makes acitretin a less likely cause of mortality.
We present a case of sudden cardiac death secondary to VT in a young patient with psoriasis and no other traditional cardiovascular risk factors. This case highlights the importance of being vigilant for adverse CVEs such as arrhythmia in psoriatic patients, especially in younger patients with severe unstable disease.
- Kowalewski M, Urban M, Mroczko B, et al. Proinflammatory cytokines (IL-6, TNF-alpha) and cardiac troponin I (cTnI) in serum of young people with ventricular arrhythmias. Pol Arch Med Wewn. 2002;108:647-651.
- Arican O, Aral M, Sasmaz S, et al. Serum levels of TNF-alpha, IFN-gamma, IL-6, IL-8, IL-12, IL-17, and IL-18 in patients with active psoriasis and correlation with disease severity. Mediators Inflamm. 2005;2005:273-279.
- Mehta NN, Yu Y, Saboury B, et al. Systemic and vascular inflammation in patients with moderate to severe psoriasis as measured by [18F]-fluorodeoxyglucose positron emission tomography-computed tomography (FDG-PET/CT): a pilot study. Arch Dermatol. 2011;147:1031-1039.
- Markuszeski L, Bissinger A, Janusz I, et al. Heart rate and arrhythmia in patients with psoriasis vulgaris. Arch Med Res. 2007;38:64-69.
- Simsek H, Sahin M, Akyol A, et al. Increased risk of atrial and ventricular arrhythmia in long-lasting psoriasis patients. ScientificWorldJournal. 2013;2013:901215.
- Chiu HY, Chang WL, Huang WF, et al. Increased risk of arrhythmia in patients with psoriatic disease: a nationwide population-based matched cohort study. J Am Acad Dermatol. 2015;73:429-438.
- Mittal R, Malhotra S, Pandhi P, et al. Efficacy and safety of combination acitretin and pioglitazone therapy in patients with moderate to severe chronic plaque-type psoriasis: a randomized, double-blind, placebo-controlled clinical trial. Arch Dermatol. 2009;145:387-393.
- Kowalewski M, Urban M, Mroczko B, et al. Proinflammatory cytokines (IL-6, TNF-alpha) and cardiac troponin I (cTnI) in serum of young people with ventricular arrhythmias. Pol Arch Med Wewn. 2002;108:647-651.
- Arican O, Aral M, Sasmaz S, et al. Serum levels of TNF-alpha, IFN-gamma, IL-6, IL-8, IL-12, IL-17, and IL-18 in patients with active psoriasis and correlation with disease severity. Mediators Inflamm. 2005;2005:273-279.
- Mehta NN, Yu Y, Saboury B, et al. Systemic and vascular inflammation in patients with moderate to severe psoriasis as measured by [18F]-fluorodeoxyglucose positron emission tomography-computed tomography (FDG-PET/CT): a pilot study. Arch Dermatol. 2011;147:1031-1039.
- Markuszeski L, Bissinger A, Janusz I, et al. Heart rate and arrhythmia in patients with psoriasis vulgaris. Arch Med Res. 2007;38:64-69.
- Simsek H, Sahin M, Akyol A, et al. Increased risk of atrial and ventricular arrhythmia in long-lasting psoriasis patients. ScientificWorldJournal. 2013;2013:901215.
- Chiu HY, Chang WL, Huang WF, et al. Increased risk of arrhythmia in patients with psoriatic disease: a nationwide population-based matched cohort study. J Am Acad Dermatol. 2015;73:429-438.
- Mittal R, Malhotra S, Pandhi P, et al. Efficacy and safety of combination acitretin and pioglitazone therapy in patients with moderate to severe chronic plaque-type psoriasis: a randomized, double-blind, placebo-controlled clinical trial. Arch Dermatol. 2009;145:387-393.
Practice Points
- Low-grade chronic inflammation in patients with psoriasis can lead to vascular inflammation, which can further lead to the development of major adverse cardiovascular events (CVEs) and arrhythmia.
- The need for a multidisciplinary approach and close monitoring of cardiovascular risk factors in patients with psoriasis to prevent a CVE is vital.
- Baseline electrocardiogram and biomarkers for cardiovascular disease also should be performed in young patients with severe or unstable psoriasis.
ACOG advises on care for transgender patients
Transgender patients have unique needs regarding obstetric and gynecologic care as well as preventive care, and ob.gyns. can help by providing support, education, and understanding, according to new guidance from the American College of Obstetricians and Gynecologists.
“The American College of Obstetricians and Gynecologists opposes discrimination on the basis of gender identity, urges public and private health insurance plans to cover necessary services for individuals with gender dysphoria, and advocates for inclusive, thoughtful, and affirming care for transgender individuals,” according to the committee opinion, published in the March issue of Obstetrics & Gynecology. The opinion was developed jointly by ACOG’s Committee on Gynecologic Practice and Committee on Health Care for Underserved Women, led by Beth Cronin, MD, of Brown University, Providence, R.I., and Colleen K, Stockdale, MD, of the University of Iowa, Iowa City.
“Lack of awareness, knowledge, and sensitivity, as well as bias from health care professionals leads to inadequate access to, underuse of, and inequities within the health care system for transgender patients,” the authors wrote.
The committee opinion provides guidance for ob.gyns. on topics including inclusivity, routine screening, fertility and reproductive issues, hormone therapy, medication use, and surgery.
“One of the most incredible things about being an ob.gyn. is that this field is a hybrid of primary care and surgical practice,” said K. Ashley Brandt, DO, in an interview. “Many patients seek out care from ob.gyns. for routine screening such as a Pap test, for initiation of hormone therapy, or for postoperative management,” said Dr. Brandt, an ob.gyn. and a plastic surgeon at Reading Hospital/Tower Health System in West Reading, Pa. “Many of my colleagues are starting to see an increase in transgender and gender-nonconforming individuals and do not know where to access resources or information on basic care needs. I think ACOG issuing this guidance is a great first step in providing an overview for the ob.gyn., who otherwise haven’t had formal training in transgender medicine,” she emphasized.
Dr. Brandt said she was not surprised by any of the recommendations. “These recommendations, while evolving and updating as new data emerge, have been in place by WPATH (the World Professional Association for Transgender Health) and the Endocrine Society for quite some time,” she noted. “However, this updated committee opinion is a summary of recommendations that are relevant to the clinical practice of an ob.gyn.”
“Since the publication of Care for Transgender Adolescents (2017) and Healthcare for Transgender Individuals (2011), there has been an exponential increase in data that have helped to improve and guide best practices for this patient population including better defining risks, needs, therapy, and follow-up,” said Nancy Sokkary, MD, a specialist in pediatric and adolescent gynecology in Macon, Ga., in an interview. “This document also served as an opportunity for ACOG to educate ob.gyns. about health inequities and emphasize need for gender-affirming and inclusive care,” she said.
“These recommendations are consistent with literature that has been published over the last several years,” she added. “It is certainly important for ob.gyns. to have a document unequivocally supporting hysterectomies and bilateral salpingo-oophorectomy as medically necessary for transgender patients that desire these procedures for their transition.”
Inclusive environment
Approximately 1.4 million adults and 150,000 youth aged 13-17 years in the United States identify as transgender, but these individuals are often marginalized socially and economically, which can lead to worse health outcomes, according to the committee. “Creating a safe and affirming health care environment for all patients, including transgender individuals, is essential,” the authors said. Steps to create a supportive office setting include educating staff to avoid assumptions about sex and gender, and ask appropriately about choice of pronouns and orientation. Use patient forms that reflect a full range of options and places for patients to write in a response. Also, use electronic medical records to track information on use of names other than legal names. “Ob.gyns. play an important role in caring for gender-nonconforming people,” said Dr. Sokkary. “Ob.gyn. providers may have varying levels of participation in gender-affirming hormone or surgery provision, but they can universally conduct routine health maintenance, contraceptive and fertility counseling, and obstetric care in a respectful and inclusive environment,” she said.
Track transition issues
The opinion notes that many gender-transition medications can be prescribed not only by ob.gyns., but by a range of health care professionals with training and education. When it comes to medication and surgery, neither medication nor surgery is required for legally changing one’s name or gender, but patient desires vary from those seeking only letters of support for such legal changes to those who want to pursue hormone therapy or procedures such as chest surgery, hysterectomy, or phalloplasty.
Transgender patients seeking care from ob.gyns. include transmasculine and transfeminine individuals who are seeking various degrees of masculinizing or feminizing therapies.
Masculinizing therapies may result in development of facial hair, deepening voice, and changes in muscle mass, but patients undergoing masculinizing therapies should be reminded of the potential for continued ovulation, according to the opinion. “The only absolute contraindications to masculinizing hormone therapy are current pregnancy, unstable coronary artery disease, and polycythemia (hematocrit greater than 55%),” the authors wrote.
Feminizing therapies have no absolute contraindications, but “risks include venous thromboembolism (VTE), hypertriglyceridemia, development of gallstones, and elevated liver enzymes,” they noted.
Talk about sex and fertility
Clinicians treating transgender patients should discuss fertility and parenting early in the process of any gender transition, ideally before the patient undergoes hormone therapy or surgery, according to the opinion. Fertility preservation options for transgender patients are the same as for cisgender patients who wish to preserve fertility for various reasons, and include “sperm banking, oocyte preservation, embryo preservation, and in some cases, ovarian or testicular tissue cryopreservation,” the authors noted.
However, patients who do not desire pregnancy but may have the potential to become pregnant or impregnate others should be counseled on contraceptive options and reminded that gender-affirming hormone therapy alone does not provide effective contraception, they emphasized. In addition, “all patients should be counseled on barrier use for prevention of sexually transmitted diseases,” they said.
Consistent routine screening and preventive care
The committee opinion also states that transgender patients should undergo routine screening for any anatomical structures that are present, such as breast cancer screening for transmasculine individuals with breast tissue, and cervical cancer screening for those with a cervix. Transfeminine individuals should undergo prostate cancer screening in accordance with the recommendations for cisgender men, the authors said.
“As for all patients, transgender individuals should be counseled about the importance of routine preventive health care,” according to the opinion. “All individuals should be routinely screened for intimate partner violence, depression, substance use, cancer, and other health care needs and should be screened for sexually transmitted infections and counseled about appropriate immunizations based on age and risk factors, including HPV vaccination,” the authors said.
“We continue to see patient discrimination and discomfort with the medical system as a barrier to preventive care among gender-nonconforming individuals,” said Dr. Sokkary. “[Ensuring] that your clinic is a safe, inclusive place is a good start. Also, having providers such as ob.gyns. and family medicine physicians provide gender-affirming care in addition to routine screening and testing is helpful,” she said.
One of the ongoing challenges of counseling transgender patients across a range of age groups, from youth through menopause, is a lack of data on the long-term effects of hormone therapy or surgical intervention, Dr. Brandt noted. “Since there is a paucity of this information, many of the screening recommendations fall in line with that of cisgender patients; however, this is not always the case as screening is determined by hormonal usage, risk factors, and surgical state. It is important for clinicians to be aware of evolutions in screening that will continue to occur as more evidence becomes available,” she emphasized.
In addition, “This document did not include specific guidance for transgender and gender-diverse adolescents, and there are many factors and recommendations that are unique to this population,” Dr. Sokkary said.
Barriers and overcoming them
The main barrier to care with transgender and gender-nonconfirming patients is access to care and finding providers who are competent in gender-affirming health, Dr. Brandt noted. “Another significant barrier involves caring for transgender male patients in a traditionally ‘women’s health’ specialty,” she said. “While the office of an ob.gyn. can be very affirming for transgender women, it has the potential to exacerbate discomfort in transgender male patients,” she noted. “Having gender-affirming posters and pamphlets in the waiting area are ways to make patients feel more at ease. Another of the ways to overcome this barrier is education of the staff and health care providers,” added Dr. Brandt. “Fortunately, this is starting to occur at medical school and residency levels. For ob.gyns. already in practice, articles such as this committee opinion can serve as a resource for providers seeking to understand health care needs of this community,” she said.
“Cost and insurance coverage continue to be barriers, but this has improved immensely: There are now several local and national resources that can help with this depending on the issue,” said Dr. Sokkary. “Additionally, we still lack robust data that define cancer risk among transgender individuals, and until we have more evidence-based recommendations providers should follow screening outlined in this document,” she said.
Use the ACOG opinion as a starting point
“This committee opinion is a great introduction and summary for ob.gyns. seeking to understand basic care needs for gender-nonconforming individuals,” said Dr. Brandt. “However, I strongly encourage ob.gyns. who wish to truly incorporate gender-affirming care as part of their routine clinical practice to participate in continuing education, read the WPATH standards of care among many of the resources provided in the committee opinion, and attend conferences that are specific to transgender health and medicine,” she said.
The opinion received no outside funding. The authors were vetted by ACOG and had no relevant financial conflicts to disclose. Dr. Brandt had no financial conflicts to disclose. Dr. Sokkary had no financial conflicts to disclose.
Transgender patients have unique needs regarding obstetric and gynecologic care as well as preventive care, and ob.gyns. can help by providing support, education, and understanding, according to new guidance from the American College of Obstetricians and Gynecologists.
“The American College of Obstetricians and Gynecologists opposes discrimination on the basis of gender identity, urges public and private health insurance plans to cover necessary services for individuals with gender dysphoria, and advocates for inclusive, thoughtful, and affirming care for transgender individuals,” according to the committee opinion, published in the March issue of Obstetrics & Gynecology. The opinion was developed jointly by ACOG’s Committee on Gynecologic Practice and Committee on Health Care for Underserved Women, led by Beth Cronin, MD, of Brown University, Providence, R.I., and Colleen K, Stockdale, MD, of the University of Iowa, Iowa City.
“Lack of awareness, knowledge, and sensitivity, as well as bias from health care professionals leads to inadequate access to, underuse of, and inequities within the health care system for transgender patients,” the authors wrote.
The committee opinion provides guidance for ob.gyns. on topics including inclusivity, routine screening, fertility and reproductive issues, hormone therapy, medication use, and surgery.
“One of the most incredible things about being an ob.gyn. is that this field is a hybrid of primary care and surgical practice,” said K. Ashley Brandt, DO, in an interview. “Many patients seek out care from ob.gyns. for routine screening such as a Pap test, for initiation of hormone therapy, or for postoperative management,” said Dr. Brandt, an ob.gyn. and a plastic surgeon at Reading Hospital/Tower Health System in West Reading, Pa. “Many of my colleagues are starting to see an increase in transgender and gender-nonconforming individuals and do not know where to access resources or information on basic care needs. I think ACOG issuing this guidance is a great first step in providing an overview for the ob.gyn., who otherwise haven’t had formal training in transgender medicine,” she emphasized.
Dr. Brandt said she was not surprised by any of the recommendations. “These recommendations, while evolving and updating as new data emerge, have been in place by WPATH (the World Professional Association for Transgender Health) and the Endocrine Society for quite some time,” she noted. “However, this updated committee opinion is a summary of recommendations that are relevant to the clinical practice of an ob.gyn.”
“Since the publication of Care for Transgender Adolescents (2017) and Healthcare for Transgender Individuals (2011), there has been an exponential increase in data that have helped to improve and guide best practices for this patient population including better defining risks, needs, therapy, and follow-up,” said Nancy Sokkary, MD, a specialist in pediatric and adolescent gynecology in Macon, Ga., in an interview. “This document also served as an opportunity for ACOG to educate ob.gyns. about health inequities and emphasize need for gender-affirming and inclusive care,” she said.
“These recommendations are consistent with literature that has been published over the last several years,” she added. “It is certainly important for ob.gyns. to have a document unequivocally supporting hysterectomies and bilateral salpingo-oophorectomy as medically necessary for transgender patients that desire these procedures for their transition.”
Inclusive environment
Approximately 1.4 million adults and 150,000 youth aged 13-17 years in the United States identify as transgender, but these individuals are often marginalized socially and economically, which can lead to worse health outcomes, according to the committee. “Creating a safe and affirming health care environment for all patients, including transgender individuals, is essential,” the authors said. Steps to create a supportive office setting include educating staff to avoid assumptions about sex and gender, and ask appropriately about choice of pronouns and orientation. Use patient forms that reflect a full range of options and places for patients to write in a response. Also, use electronic medical records to track information on use of names other than legal names. “Ob.gyns. play an important role in caring for gender-nonconforming people,” said Dr. Sokkary. “Ob.gyn. providers may have varying levels of participation in gender-affirming hormone or surgery provision, but they can universally conduct routine health maintenance, contraceptive and fertility counseling, and obstetric care in a respectful and inclusive environment,” she said.
Track transition issues
The opinion notes that many gender-transition medications can be prescribed not only by ob.gyns., but by a range of health care professionals with training and education. When it comes to medication and surgery, neither medication nor surgery is required for legally changing one’s name or gender, but patient desires vary from those seeking only letters of support for such legal changes to those who want to pursue hormone therapy or procedures such as chest surgery, hysterectomy, or phalloplasty.
Transgender patients seeking care from ob.gyns. include transmasculine and transfeminine individuals who are seeking various degrees of masculinizing or feminizing therapies.
Masculinizing therapies may result in development of facial hair, deepening voice, and changes in muscle mass, but patients undergoing masculinizing therapies should be reminded of the potential for continued ovulation, according to the opinion. “The only absolute contraindications to masculinizing hormone therapy are current pregnancy, unstable coronary artery disease, and polycythemia (hematocrit greater than 55%),” the authors wrote.
Feminizing therapies have no absolute contraindications, but “risks include venous thromboembolism (VTE), hypertriglyceridemia, development of gallstones, and elevated liver enzymes,” they noted.
Talk about sex and fertility
Clinicians treating transgender patients should discuss fertility and parenting early in the process of any gender transition, ideally before the patient undergoes hormone therapy or surgery, according to the opinion. Fertility preservation options for transgender patients are the same as for cisgender patients who wish to preserve fertility for various reasons, and include “sperm banking, oocyte preservation, embryo preservation, and in some cases, ovarian or testicular tissue cryopreservation,” the authors noted.
However, patients who do not desire pregnancy but may have the potential to become pregnant or impregnate others should be counseled on contraceptive options and reminded that gender-affirming hormone therapy alone does not provide effective contraception, they emphasized. In addition, “all patients should be counseled on barrier use for prevention of sexually transmitted diseases,” they said.
Consistent routine screening and preventive care
The committee opinion also states that transgender patients should undergo routine screening for any anatomical structures that are present, such as breast cancer screening for transmasculine individuals with breast tissue, and cervical cancer screening for those with a cervix. Transfeminine individuals should undergo prostate cancer screening in accordance with the recommendations for cisgender men, the authors said.
“As for all patients, transgender individuals should be counseled about the importance of routine preventive health care,” according to the opinion. “All individuals should be routinely screened for intimate partner violence, depression, substance use, cancer, and other health care needs and should be screened for sexually transmitted infections and counseled about appropriate immunizations based on age and risk factors, including HPV vaccination,” the authors said.
“We continue to see patient discrimination and discomfort with the medical system as a barrier to preventive care among gender-nonconforming individuals,” said Dr. Sokkary. “[Ensuring] that your clinic is a safe, inclusive place is a good start. Also, having providers such as ob.gyns. and family medicine physicians provide gender-affirming care in addition to routine screening and testing is helpful,” she said.
One of the ongoing challenges of counseling transgender patients across a range of age groups, from youth through menopause, is a lack of data on the long-term effects of hormone therapy or surgical intervention, Dr. Brandt noted. “Since there is a paucity of this information, many of the screening recommendations fall in line with that of cisgender patients; however, this is not always the case as screening is determined by hormonal usage, risk factors, and surgical state. It is important for clinicians to be aware of evolutions in screening that will continue to occur as more evidence becomes available,” she emphasized.
In addition, “This document did not include specific guidance for transgender and gender-diverse adolescents, and there are many factors and recommendations that are unique to this population,” Dr. Sokkary said.
Barriers and overcoming them
The main barrier to care with transgender and gender-nonconfirming patients is access to care and finding providers who are competent in gender-affirming health, Dr. Brandt noted. “Another significant barrier involves caring for transgender male patients in a traditionally ‘women’s health’ specialty,” she said. “While the office of an ob.gyn. can be very affirming for transgender women, it has the potential to exacerbate discomfort in transgender male patients,” she noted. “Having gender-affirming posters and pamphlets in the waiting area are ways to make patients feel more at ease. Another of the ways to overcome this barrier is education of the staff and health care providers,” added Dr. Brandt. “Fortunately, this is starting to occur at medical school and residency levels. For ob.gyns. already in practice, articles such as this committee opinion can serve as a resource for providers seeking to understand health care needs of this community,” she said.
“Cost and insurance coverage continue to be barriers, but this has improved immensely: There are now several local and national resources that can help with this depending on the issue,” said Dr. Sokkary. “Additionally, we still lack robust data that define cancer risk among transgender individuals, and until we have more evidence-based recommendations providers should follow screening outlined in this document,” she said.
Use the ACOG opinion as a starting point
“This committee opinion is a great introduction and summary for ob.gyns. seeking to understand basic care needs for gender-nonconforming individuals,” said Dr. Brandt. “However, I strongly encourage ob.gyns. who wish to truly incorporate gender-affirming care as part of their routine clinical practice to participate in continuing education, read the WPATH standards of care among many of the resources provided in the committee opinion, and attend conferences that are specific to transgender health and medicine,” she said.
The opinion received no outside funding. The authors were vetted by ACOG and had no relevant financial conflicts to disclose. Dr. Brandt had no financial conflicts to disclose. Dr. Sokkary had no financial conflicts to disclose.
Transgender patients have unique needs regarding obstetric and gynecologic care as well as preventive care, and ob.gyns. can help by providing support, education, and understanding, according to new guidance from the American College of Obstetricians and Gynecologists.
“The American College of Obstetricians and Gynecologists opposes discrimination on the basis of gender identity, urges public and private health insurance plans to cover necessary services for individuals with gender dysphoria, and advocates for inclusive, thoughtful, and affirming care for transgender individuals,” according to the committee opinion, published in the March issue of Obstetrics & Gynecology. The opinion was developed jointly by ACOG’s Committee on Gynecologic Practice and Committee on Health Care for Underserved Women, led by Beth Cronin, MD, of Brown University, Providence, R.I., and Colleen K, Stockdale, MD, of the University of Iowa, Iowa City.
“Lack of awareness, knowledge, and sensitivity, as well as bias from health care professionals leads to inadequate access to, underuse of, and inequities within the health care system for transgender patients,” the authors wrote.
The committee opinion provides guidance for ob.gyns. on topics including inclusivity, routine screening, fertility and reproductive issues, hormone therapy, medication use, and surgery.
“One of the most incredible things about being an ob.gyn. is that this field is a hybrid of primary care and surgical practice,” said K. Ashley Brandt, DO, in an interview. “Many patients seek out care from ob.gyns. for routine screening such as a Pap test, for initiation of hormone therapy, or for postoperative management,” said Dr. Brandt, an ob.gyn. and a plastic surgeon at Reading Hospital/Tower Health System in West Reading, Pa. “Many of my colleagues are starting to see an increase in transgender and gender-nonconforming individuals and do not know where to access resources or information on basic care needs. I think ACOG issuing this guidance is a great first step in providing an overview for the ob.gyn., who otherwise haven’t had formal training in transgender medicine,” she emphasized.
Dr. Brandt said she was not surprised by any of the recommendations. “These recommendations, while evolving and updating as new data emerge, have been in place by WPATH (the World Professional Association for Transgender Health) and the Endocrine Society for quite some time,” she noted. “However, this updated committee opinion is a summary of recommendations that are relevant to the clinical practice of an ob.gyn.”
“Since the publication of Care for Transgender Adolescents (2017) and Healthcare for Transgender Individuals (2011), there has been an exponential increase in data that have helped to improve and guide best practices for this patient population including better defining risks, needs, therapy, and follow-up,” said Nancy Sokkary, MD, a specialist in pediatric and adolescent gynecology in Macon, Ga., in an interview. “This document also served as an opportunity for ACOG to educate ob.gyns. about health inequities and emphasize need for gender-affirming and inclusive care,” she said.
“These recommendations are consistent with literature that has been published over the last several years,” she added. “It is certainly important for ob.gyns. to have a document unequivocally supporting hysterectomies and bilateral salpingo-oophorectomy as medically necessary for transgender patients that desire these procedures for their transition.”
Inclusive environment
Approximately 1.4 million adults and 150,000 youth aged 13-17 years in the United States identify as transgender, but these individuals are often marginalized socially and economically, which can lead to worse health outcomes, according to the committee. “Creating a safe and affirming health care environment for all patients, including transgender individuals, is essential,” the authors said. Steps to create a supportive office setting include educating staff to avoid assumptions about sex and gender, and ask appropriately about choice of pronouns and orientation. Use patient forms that reflect a full range of options and places for patients to write in a response. Also, use electronic medical records to track information on use of names other than legal names. “Ob.gyns. play an important role in caring for gender-nonconforming people,” said Dr. Sokkary. “Ob.gyn. providers may have varying levels of participation in gender-affirming hormone or surgery provision, but they can universally conduct routine health maintenance, contraceptive and fertility counseling, and obstetric care in a respectful and inclusive environment,” she said.
Track transition issues
The opinion notes that many gender-transition medications can be prescribed not only by ob.gyns., but by a range of health care professionals with training and education. When it comes to medication and surgery, neither medication nor surgery is required for legally changing one’s name or gender, but patient desires vary from those seeking only letters of support for such legal changes to those who want to pursue hormone therapy or procedures such as chest surgery, hysterectomy, or phalloplasty.
Transgender patients seeking care from ob.gyns. include transmasculine and transfeminine individuals who are seeking various degrees of masculinizing or feminizing therapies.
Masculinizing therapies may result in development of facial hair, deepening voice, and changes in muscle mass, but patients undergoing masculinizing therapies should be reminded of the potential for continued ovulation, according to the opinion. “The only absolute contraindications to masculinizing hormone therapy are current pregnancy, unstable coronary artery disease, and polycythemia (hematocrit greater than 55%),” the authors wrote.
Feminizing therapies have no absolute contraindications, but “risks include venous thromboembolism (VTE), hypertriglyceridemia, development of gallstones, and elevated liver enzymes,” they noted.
Talk about sex and fertility
Clinicians treating transgender patients should discuss fertility and parenting early in the process of any gender transition, ideally before the patient undergoes hormone therapy or surgery, according to the opinion. Fertility preservation options for transgender patients are the same as for cisgender patients who wish to preserve fertility for various reasons, and include “sperm banking, oocyte preservation, embryo preservation, and in some cases, ovarian or testicular tissue cryopreservation,” the authors noted.
However, patients who do not desire pregnancy but may have the potential to become pregnant or impregnate others should be counseled on contraceptive options and reminded that gender-affirming hormone therapy alone does not provide effective contraception, they emphasized. In addition, “all patients should be counseled on barrier use for prevention of sexually transmitted diseases,” they said.
Consistent routine screening and preventive care
The committee opinion also states that transgender patients should undergo routine screening for any anatomical structures that are present, such as breast cancer screening for transmasculine individuals with breast tissue, and cervical cancer screening for those with a cervix. Transfeminine individuals should undergo prostate cancer screening in accordance with the recommendations for cisgender men, the authors said.
“As for all patients, transgender individuals should be counseled about the importance of routine preventive health care,” according to the opinion. “All individuals should be routinely screened for intimate partner violence, depression, substance use, cancer, and other health care needs and should be screened for sexually transmitted infections and counseled about appropriate immunizations based on age and risk factors, including HPV vaccination,” the authors said.
“We continue to see patient discrimination and discomfort with the medical system as a barrier to preventive care among gender-nonconforming individuals,” said Dr. Sokkary. “[Ensuring] that your clinic is a safe, inclusive place is a good start. Also, having providers such as ob.gyns. and family medicine physicians provide gender-affirming care in addition to routine screening and testing is helpful,” she said.
One of the ongoing challenges of counseling transgender patients across a range of age groups, from youth through menopause, is a lack of data on the long-term effects of hormone therapy or surgical intervention, Dr. Brandt noted. “Since there is a paucity of this information, many of the screening recommendations fall in line with that of cisgender patients; however, this is not always the case as screening is determined by hormonal usage, risk factors, and surgical state. It is important for clinicians to be aware of evolutions in screening that will continue to occur as more evidence becomes available,” she emphasized.
In addition, “This document did not include specific guidance for transgender and gender-diverse adolescents, and there are many factors and recommendations that are unique to this population,” Dr. Sokkary said.
Barriers and overcoming them
The main barrier to care with transgender and gender-nonconfirming patients is access to care and finding providers who are competent in gender-affirming health, Dr. Brandt noted. “Another significant barrier involves caring for transgender male patients in a traditionally ‘women’s health’ specialty,” she said. “While the office of an ob.gyn. can be very affirming for transgender women, it has the potential to exacerbate discomfort in transgender male patients,” she noted. “Having gender-affirming posters and pamphlets in the waiting area are ways to make patients feel more at ease. Another of the ways to overcome this barrier is education of the staff and health care providers,” added Dr. Brandt. “Fortunately, this is starting to occur at medical school and residency levels. For ob.gyns. already in practice, articles such as this committee opinion can serve as a resource for providers seeking to understand health care needs of this community,” she said.
“Cost and insurance coverage continue to be barriers, but this has improved immensely: There are now several local and national resources that can help with this depending on the issue,” said Dr. Sokkary. “Additionally, we still lack robust data that define cancer risk among transgender individuals, and until we have more evidence-based recommendations providers should follow screening outlined in this document,” she said.
Use the ACOG opinion as a starting point
“This committee opinion is a great introduction and summary for ob.gyns. seeking to understand basic care needs for gender-nonconforming individuals,” said Dr. Brandt. “However, I strongly encourage ob.gyns. who wish to truly incorporate gender-affirming care as part of their routine clinical practice to participate in continuing education, read the WPATH standards of care among many of the resources provided in the committee opinion, and attend conferences that are specific to transgender health and medicine,” she said.
The opinion received no outside funding. The authors were vetted by ACOG and had no relevant financial conflicts to disclose. Dr. Brandt had no financial conflicts to disclose. Dr. Sokkary had no financial conflicts to disclose.
SHM CEO Eric Howell likes to fix things
Engineering provided a foundation for hospital medicine
Editor’s note: This profile is part of SHM’s celebration of National Hospitalist Day on March 4. National Hospitalist Day occurs the first Thursday in March annually, and celebrates the fastest growing specialty in modern medicine and hospitalists’ enduring contributions to the evolving health care landscape.
For Eric E. Howell, MD, MHM, CEO since July 2020 for the Society of Hospital Medicine, an undergraduate degree in electrical engineering and a lifelong proclivity for figuring out puzzles, solving problems, and taking things apart to see how they fit back together were building blocks for an exemplary career as a hospitalist, group administrator, and medical educator.
When he was growing up in historic Annapolis, Md., near the shores of Chesapeake Bay, things to put back together included remote control airplanes, small boat engines, and cars. As a hospitalist, his interest in solving problems and facility with numbers and systems led him to become an expert on quality improvement, transitions of care, and conflict management.
“One thing about engineering, you’re always having to fix things. It helps you learn to assess complex situations,” said Dr. Howell, who is 52. “It was helpful for me to bring an engineering approach into the hospital. One of my earliest successes was reengineering admissions processes to dramatically reduce the amount of time patients were spending in the emergency room before they could be admitted to the hospital.”
But his career path in hospital medicine came about by a lucky chance, following residency and a year as chief resident at Johns Hopkins Bayview Medical Center in Baltimore. “One of my duties as chief resident was taking care of hospitalized patients. I didn’t know it but I was becoming a de facto hospitalist,” he recalled.
At the time, he thought he might end up choosing to specialize in something like cardiology or critical care medicine, but in 2000 he was invited to join the new “non-house-staff” medical service at Bayview. Also called a general medicine inpatient service, it eventually evolved into the hospitalist service.
His residency program director, Roy Ziegelstein, MD, a cardiologist and now the vice dean of education at Johns Hopkins, created a job for him.
“I was one of the first four doctors hired. I thought I’d just do it for a year, but I loved inpatient work, so I stayed,” Dr. Howell said. “Roy mentored me for the next 20 years and helped me to become an above average hospitalist.”
Early on, Dr. Howell’s department chair, David Hellman, MD, who had worked at the University of California–San Francisco with hospital medicine pioneer Robert Wachter, MD, MHM, sent Dr. Howell to San Francisco to be mentored by Dr. Wachter, since there were few hospital mentors on the East Coast at that time.
“What I took away from that experience was how important it was to professionalize hospital medicine – in order to develop specialized expertise,” Dr. Howell recalled. “Dr. Wachter taught me that hospitalists need to have a professional focus. Quality improvement, systems-based improvement, and value all became part of that,” he said.
“Many people thought to be a hospitalist all you had to know was basic medicine. But it turns out medicine in the hospital is just as specialized as any other specialty. The hospital itself requires specialized knowledge that didn’t even exist 20 years ago.” Because of complicated disease states and clinical systems, hospitalists have to be better at navigating the software of today’s hospital.
New job opportunities
Dr. Howell describes his career path as a new job focus opening up every 5 years or so, redefining what he does and trying something new and exciting with better pay. His first was a focus on clinical hospital medicine and learning how to be a better doctor. Then in 2005 he began work as a teacher at Johns Hopkins School of Medicine. There he mastered the teaching of medical trainees, winning awards as an instructor, including SHM’s award for excellence in teaching.
In 2010 he again changed his focus to program building, leading the expansion of the hospitalist service for Bayview and three other hospitals in the Johns Hopkins system. Dr. Howell helped grow the service to nearly 200 clinicians while becoming skilled at operational and program development.
His fourth job incarnation, starting in 2015, was the obsessive pursuit of quality improvement, marshaling data to measure and improve clinical and other outcomes on the quality dashboard – mortality, length of stay, readmissions, rates of adverse events – and putting quality improvement strategies in place.
“Our mortality rates at Bayview were well below national standards. We came up with an amazing program. A lot of hospital medicine programs pursue improvement, but we really measured it. We benchmarked ourselves against other programs at Hopkins,” he said. “I set up a dedicated conference room, as many QI programs do. We called it True North, and each wall had a different QI focus, with updates on the reported metrics. Every other week we met there to talk about the metrics,” he said.
That experience led to working with SHM, which he had joined as a member early in his career and for which he had previously served as president. He became SHM’s quality improvement liaison and a co-principal investigator on Project BOOST (Better Outcomes for Older adults through Safe Transitions), SHM’s pioneering, national mentored-implementation model aimed at improving transitions of care from participating hospitals to reduce readmissions. “BOOST really established SHM’s reputation as a quality improvement-oriented organization. It was a stake in the ground for quality and led to SHM receiving the Joint Commission’s 2011 John M. Eisenberg Award for Innovation in Patient Safety and Quality,” he said.
Dr. Howell’s fifth career phase, medical society management, emerged when he was recruited to apply for the SHM chief executive position – held since its inception by retiring CEO Larry Wellikson, MD, MHM. Dr. Howell started work at SHM in the midst of the pandemic, spending much of his time working from home – especially when Philadelphia implemented stricter COVID-19 restrictions. Once pandemic restrictions are loosened, he expects to do a lot of traveling. But for now, the external-facing part of his job is mainly on Zoom.
Making the world a better place
Dr. Howell said he has held fast to three mottos in life, which have guided his career path as well as his personal life: (1) to make the world a better place; (2) to be ethical and transparent; and (3) to invest in people. His wife of 19 years, Heather Howell, an Annapolis realtor, says making the world a better place is what they taught their children, Mason, 18, who starts college at Rice University in fall 2021 with an interest in premed, and Anna, 16, a competitive sailor. “We always had a poster hanging in our house extolling that message,” Ms. Howell said.
Dr. Howell grew up in a nautical family, with many of his relatives working in the maritime business. His kids grew up on the water, learning to pilot a powerboat before driving a car, as he did. “We boat all the time on the bay” in his lobster boat, which he often works on to keep it seaworthy, Ms. Howell said.
“There’s nothing like taking care of hospitalized patients to make you feel you’re making the world a better place,” Dr. Howell observed. “Very often you can make a huge difference for the patients you do care for, and that is incredibly rewarding.” Although the demands of his SHM leadership position required relinquishing most of his responsibilities at Johns Hopkins, he continues to see patients and teach residents there 2-4 weeks a year on a teaching service.
“Why do I still see patients? I find it so rewarding. And I get to teach, which I love,” he said. “To be honest, I don’t think you truly need to see patients to be head of a professional medical society like SHM. Maybe someday I’ll give that up. But only if it’s necessary to make the society more successful.”
Half of Dr. Howell’s Society work now is planned and half is “putting out fires” – while learning members’ needs in real time. “Right now, we’re worried about burnout and PTSD, because frankly it’s stressful to take care of COVID patients. It’s scary for a lot of clinicians. I’m working with our members to make sure they have what they need to be clinically prepared, including resources to be more resilient professionally.”
Every step of his career, Dr. Howell said, has seemed like the best job he ever had. “Making the world a better place is still important to me. I tell SHM members that it’s important to know they are making a difference. What they’re doing is really important, especially with COVID, and it needs to be sustainable,” he said.
“SHM has such a powerful mission – it’s about making patient care better, and making hospitalists better clinicians. I know the Society is having a powerful impact, and that’s good enough for me. I’m into teams. Hospital medicine is a team sport, but so is SHM, interacting with its members, staff, and board.”
Initiating another new program
One of Dr. Howell’s last major projects for Hopkins was to launch and be chief medical officer for the Joint Commission–accredited Baltimore Civic Center Field Hospital for COVID-19 patients, opened in March 2020.
With a surge capacity of 250 beds, and a negative pressure ward set up in the center’s exhibit hall, it is jointly operated by the University of Maryland Medical System and Johns Hopkins Hospital. The field hospital’s mission has since expanded to include viral tests, infusions of monoclonal antibodies, and COVID-19 vaccinations.
Planning for a smooth transition, Dr. Howell brought Melinda E. Kantsiper, MD, director of clinical operations, Division of Hospital Medicine at Johns Hopkins Bayview, on board as associate medical officer, to eventually replace him as CMO after a few months working alongside him. “Eric brings that logical engineering eye to problem solving,” Dr. Kantsiper said.
“We wanted to build a very safe, high-quality hospital setting but had to do it very quickly. Watching him once again do what he does best, initiating a new program, building things carefully and thoughtfully, without being overly cautious, I could see his years of experience and good judgment about how hospitals run. He’s very logical but very caring. He’s also good at spotting young leaders and their talents.”
Some people have a knack for solving problems, added Dr. Ziegelstein, Dr. Howell’s mentor from his early days at Bayview. “Eric is different. He’s someone who’s able to identify gaps, problem areas, and vulnerabilities within an organization and then come up with a potential menu of solutions, think about which would be most likely to succeed, implement it, and assess the outcome. That’s the difference between a skilled manager and a true leader, and I’d say Eric had that ability while still in training,” Dr. Ziegelstein said.
“Eric understood early on not only what the field of hospital medicine could offer, he also understood how to catalyze change, without taking on too much change at one time,” Dr. Ziegelstein said. “He understood people’s sensibilities and concerns about this new service, and he catalyzed its growth through incremental change.”
Engineering provided a foundation for hospital medicine
Engineering provided a foundation for hospital medicine
Editor’s note: This profile is part of SHM’s celebration of National Hospitalist Day on March 4. National Hospitalist Day occurs the first Thursday in March annually, and celebrates the fastest growing specialty in modern medicine and hospitalists’ enduring contributions to the evolving health care landscape.
For Eric E. Howell, MD, MHM, CEO since July 2020 for the Society of Hospital Medicine, an undergraduate degree in electrical engineering and a lifelong proclivity for figuring out puzzles, solving problems, and taking things apart to see how they fit back together were building blocks for an exemplary career as a hospitalist, group administrator, and medical educator.
When he was growing up in historic Annapolis, Md., near the shores of Chesapeake Bay, things to put back together included remote control airplanes, small boat engines, and cars. As a hospitalist, his interest in solving problems and facility with numbers and systems led him to become an expert on quality improvement, transitions of care, and conflict management.
“One thing about engineering, you’re always having to fix things. It helps you learn to assess complex situations,” said Dr. Howell, who is 52. “It was helpful for me to bring an engineering approach into the hospital. One of my earliest successes was reengineering admissions processes to dramatically reduce the amount of time patients were spending in the emergency room before they could be admitted to the hospital.”
But his career path in hospital medicine came about by a lucky chance, following residency and a year as chief resident at Johns Hopkins Bayview Medical Center in Baltimore. “One of my duties as chief resident was taking care of hospitalized patients. I didn’t know it but I was becoming a de facto hospitalist,” he recalled.
At the time, he thought he might end up choosing to specialize in something like cardiology or critical care medicine, but in 2000 he was invited to join the new “non-house-staff” medical service at Bayview. Also called a general medicine inpatient service, it eventually evolved into the hospitalist service.
His residency program director, Roy Ziegelstein, MD, a cardiologist and now the vice dean of education at Johns Hopkins, created a job for him.
“I was one of the first four doctors hired. I thought I’d just do it for a year, but I loved inpatient work, so I stayed,” Dr. Howell said. “Roy mentored me for the next 20 years and helped me to become an above average hospitalist.”
Early on, Dr. Howell’s department chair, David Hellman, MD, who had worked at the University of California–San Francisco with hospital medicine pioneer Robert Wachter, MD, MHM, sent Dr. Howell to San Francisco to be mentored by Dr. Wachter, since there were few hospital mentors on the East Coast at that time.
“What I took away from that experience was how important it was to professionalize hospital medicine – in order to develop specialized expertise,” Dr. Howell recalled. “Dr. Wachter taught me that hospitalists need to have a professional focus. Quality improvement, systems-based improvement, and value all became part of that,” he said.
“Many people thought to be a hospitalist all you had to know was basic medicine. But it turns out medicine in the hospital is just as specialized as any other specialty. The hospital itself requires specialized knowledge that didn’t even exist 20 years ago.” Because of complicated disease states and clinical systems, hospitalists have to be better at navigating the software of today’s hospital.
New job opportunities
Dr. Howell describes his career path as a new job focus opening up every 5 years or so, redefining what he does and trying something new and exciting with better pay. His first was a focus on clinical hospital medicine and learning how to be a better doctor. Then in 2005 he began work as a teacher at Johns Hopkins School of Medicine. There he mastered the teaching of medical trainees, winning awards as an instructor, including SHM’s award for excellence in teaching.
In 2010 he again changed his focus to program building, leading the expansion of the hospitalist service for Bayview and three other hospitals in the Johns Hopkins system. Dr. Howell helped grow the service to nearly 200 clinicians while becoming skilled at operational and program development.
His fourth job incarnation, starting in 2015, was the obsessive pursuit of quality improvement, marshaling data to measure and improve clinical and other outcomes on the quality dashboard – mortality, length of stay, readmissions, rates of adverse events – and putting quality improvement strategies in place.
“Our mortality rates at Bayview were well below national standards. We came up with an amazing program. A lot of hospital medicine programs pursue improvement, but we really measured it. We benchmarked ourselves against other programs at Hopkins,” he said. “I set up a dedicated conference room, as many QI programs do. We called it True North, and each wall had a different QI focus, with updates on the reported metrics. Every other week we met there to talk about the metrics,” he said.
That experience led to working with SHM, which he had joined as a member early in his career and for which he had previously served as president. He became SHM’s quality improvement liaison and a co-principal investigator on Project BOOST (Better Outcomes for Older adults through Safe Transitions), SHM’s pioneering, national mentored-implementation model aimed at improving transitions of care from participating hospitals to reduce readmissions. “BOOST really established SHM’s reputation as a quality improvement-oriented organization. It was a stake in the ground for quality and led to SHM receiving the Joint Commission’s 2011 John M. Eisenberg Award for Innovation in Patient Safety and Quality,” he said.
Dr. Howell’s fifth career phase, medical society management, emerged when he was recruited to apply for the SHM chief executive position – held since its inception by retiring CEO Larry Wellikson, MD, MHM. Dr. Howell started work at SHM in the midst of the pandemic, spending much of his time working from home – especially when Philadelphia implemented stricter COVID-19 restrictions. Once pandemic restrictions are loosened, he expects to do a lot of traveling. But for now, the external-facing part of his job is mainly on Zoom.
Making the world a better place
Dr. Howell said he has held fast to three mottos in life, which have guided his career path as well as his personal life: (1) to make the world a better place; (2) to be ethical and transparent; and (3) to invest in people. His wife of 19 years, Heather Howell, an Annapolis realtor, says making the world a better place is what they taught their children, Mason, 18, who starts college at Rice University in fall 2021 with an interest in premed, and Anna, 16, a competitive sailor. “We always had a poster hanging in our house extolling that message,” Ms. Howell said.
Dr. Howell grew up in a nautical family, with many of his relatives working in the maritime business. His kids grew up on the water, learning to pilot a powerboat before driving a car, as he did. “We boat all the time on the bay” in his lobster boat, which he often works on to keep it seaworthy, Ms. Howell said.
“There’s nothing like taking care of hospitalized patients to make you feel you’re making the world a better place,” Dr. Howell observed. “Very often you can make a huge difference for the patients you do care for, and that is incredibly rewarding.” Although the demands of his SHM leadership position required relinquishing most of his responsibilities at Johns Hopkins, he continues to see patients and teach residents there 2-4 weeks a year on a teaching service.
“Why do I still see patients? I find it so rewarding. And I get to teach, which I love,” he said. “To be honest, I don’t think you truly need to see patients to be head of a professional medical society like SHM. Maybe someday I’ll give that up. But only if it’s necessary to make the society more successful.”
Half of Dr. Howell’s Society work now is planned and half is “putting out fires” – while learning members’ needs in real time. “Right now, we’re worried about burnout and PTSD, because frankly it’s stressful to take care of COVID patients. It’s scary for a lot of clinicians. I’m working with our members to make sure they have what they need to be clinically prepared, including resources to be more resilient professionally.”
Every step of his career, Dr. Howell said, has seemed like the best job he ever had. “Making the world a better place is still important to me. I tell SHM members that it’s important to know they are making a difference. What they’re doing is really important, especially with COVID, and it needs to be sustainable,” he said.
“SHM has such a powerful mission – it’s about making patient care better, and making hospitalists better clinicians. I know the Society is having a powerful impact, and that’s good enough for me. I’m into teams. Hospital medicine is a team sport, but so is SHM, interacting with its members, staff, and board.”
Initiating another new program
One of Dr. Howell’s last major projects for Hopkins was to launch and be chief medical officer for the Joint Commission–accredited Baltimore Civic Center Field Hospital for COVID-19 patients, opened in March 2020.
With a surge capacity of 250 beds, and a negative pressure ward set up in the center’s exhibit hall, it is jointly operated by the University of Maryland Medical System and Johns Hopkins Hospital. The field hospital’s mission has since expanded to include viral tests, infusions of monoclonal antibodies, and COVID-19 vaccinations.
Planning for a smooth transition, Dr. Howell brought Melinda E. Kantsiper, MD, director of clinical operations, Division of Hospital Medicine at Johns Hopkins Bayview, on board as associate medical officer, to eventually replace him as CMO after a few months working alongside him. “Eric brings that logical engineering eye to problem solving,” Dr. Kantsiper said.
“We wanted to build a very safe, high-quality hospital setting but had to do it very quickly. Watching him once again do what he does best, initiating a new program, building things carefully and thoughtfully, without being overly cautious, I could see his years of experience and good judgment about how hospitals run. He’s very logical but very caring. He’s also good at spotting young leaders and their talents.”
Some people have a knack for solving problems, added Dr. Ziegelstein, Dr. Howell’s mentor from his early days at Bayview. “Eric is different. He’s someone who’s able to identify gaps, problem areas, and vulnerabilities within an organization and then come up with a potential menu of solutions, think about which would be most likely to succeed, implement it, and assess the outcome. That’s the difference between a skilled manager and a true leader, and I’d say Eric had that ability while still in training,” Dr. Ziegelstein said.
“Eric understood early on not only what the field of hospital medicine could offer, he also understood how to catalyze change, without taking on too much change at one time,” Dr. Ziegelstein said. “He understood people’s sensibilities and concerns about this new service, and he catalyzed its growth through incremental change.”
Editor’s note: This profile is part of SHM’s celebration of National Hospitalist Day on March 4. National Hospitalist Day occurs the first Thursday in March annually, and celebrates the fastest growing specialty in modern medicine and hospitalists’ enduring contributions to the evolving health care landscape.
For Eric E. Howell, MD, MHM, CEO since July 2020 for the Society of Hospital Medicine, an undergraduate degree in electrical engineering and a lifelong proclivity for figuring out puzzles, solving problems, and taking things apart to see how they fit back together were building blocks for an exemplary career as a hospitalist, group administrator, and medical educator.
When he was growing up in historic Annapolis, Md., near the shores of Chesapeake Bay, things to put back together included remote control airplanes, small boat engines, and cars. As a hospitalist, his interest in solving problems and facility with numbers and systems led him to become an expert on quality improvement, transitions of care, and conflict management.
“One thing about engineering, you’re always having to fix things. It helps you learn to assess complex situations,” said Dr. Howell, who is 52. “It was helpful for me to bring an engineering approach into the hospital. One of my earliest successes was reengineering admissions processes to dramatically reduce the amount of time patients were spending in the emergency room before they could be admitted to the hospital.”
But his career path in hospital medicine came about by a lucky chance, following residency and a year as chief resident at Johns Hopkins Bayview Medical Center in Baltimore. “One of my duties as chief resident was taking care of hospitalized patients. I didn’t know it but I was becoming a de facto hospitalist,” he recalled.
At the time, he thought he might end up choosing to specialize in something like cardiology or critical care medicine, but in 2000 he was invited to join the new “non-house-staff” medical service at Bayview. Also called a general medicine inpatient service, it eventually evolved into the hospitalist service.
His residency program director, Roy Ziegelstein, MD, a cardiologist and now the vice dean of education at Johns Hopkins, created a job for him.
“I was one of the first four doctors hired. I thought I’d just do it for a year, but I loved inpatient work, so I stayed,” Dr. Howell said. “Roy mentored me for the next 20 years and helped me to become an above average hospitalist.”
Early on, Dr. Howell’s department chair, David Hellman, MD, who had worked at the University of California–San Francisco with hospital medicine pioneer Robert Wachter, MD, MHM, sent Dr. Howell to San Francisco to be mentored by Dr. Wachter, since there were few hospital mentors on the East Coast at that time.
“What I took away from that experience was how important it was to professionalize hospital medicine – in order to develop specialized expertise,” Dr. Howell recalled. “Dr. Wachter taught me that hospitalists need to have a professional focus. Quality improvement, systems-based improvement, and value all became part of that,” he said.
“Many people thought to be a hospitalist all you had to know was basic medicine. But it turns out medicine in the hospital is just as specialized as any other specialty. The hospital itself requires specialized knowledge that didn’t even exist 20 years ago.” Because of complicated disease states and clinical systems, hospitalists have to be better at navigating the software of today’s hospital.
New job opportunities
Dr. Howell describes his career path as a new job focus opening up every 5 years or so, redefining what he does and trying something new and exciting with better pay. His first was a focus on clinical hospital medicine and learning how to be a better doctor. Then in 2005 he began work as a teacher at Johns Hopkins School of Medicine. There he mastered the teaching of medical trainees, winning awards as an instructor, including SHM’s award for excellence in teaching.
In 2010 he again changed his focus to program building, leading the expansion of the hospitalist service for Bayview and three other hospitals in the Johns Hopkins system. Dr. Howell helped grow the service to nearly 200 clinicians while becoming skilled at operational and program development.
His fourth job incarnation, starting in 2015, was the obsessive pursuit of quality improvement, marshaling data to measure and improve clinical and other outcomes on the quality dashboard – mortality, length of stay, readmissions, rates of adverse events – and putting quality improvement strategies in place.
“Our mortality rates at Bayview were well below national standards. We came up with an amazing program. A lot of hospital medicine programs pursue improvement, but we really measured it. We benchmarked ourselves against other programs at Hopkins,” he said. “I set up a dedicated conference room, as many QI programs do. We called it True North, and each wall had a different QI focus, with updates on the reported metrics. Every other week we met there to talk about the metrics,” he said.
That experience led to working with SHM, which he had joined as a member early in his career and for which he had previously served as president. He became SHM’s quality improvement liaison and a co-principal investigator on Project BOOST (Better Outcomes for Older adults through Safe Transitions), SHM’s pioneering, national mentored-implementation model aimed at improving transitions of care from participating hospitals to reduce readmissions. “BOOST really established SHM’s reputation as a quality improvement-oriented organization. It was a stake in the ground for quality and led to SHM receiving the Joint Commission’s 2011 John M. Eisenberg Award for Innovation in Patient Safety and Quality,” he said.
Dr. Howell’s fifth career phase, medical society management, emerged when he was recruited to apply for the SHM chief executive position – held since its inception by retiring CEO Larry Wellikson, MD, MHM. Dr. Howell started work at SHM in the midst of the pandemic, spending much of his time working from home – especially when Philadelphia implemented stricter COVID-19 restrictions. Once pandemic restrictions are loosened, he expects to do a lot of traveling. But for now, the external-facing part of his job is mainly on Zoom.
Making the world a better place
Dr. Howell said he has held fast to three mottos in life, which have guided his career path as well as his personal life: (1) to make the world a better place; (2) to be ethical and transparent; and (3) to invest in people. His wife of 19 years, Heather Howell, an Annapolis realtor, says making the world a better place is what they taught their children, Mason, 18, who starts college at Rice University in fall 2021 with an interest in premed, and Anna, 16, a competitive sailor. “We always had a poster hanging in our house extolling that message,” Ms. Howell said.
Dr. Howell grew up in a nautical family, with many of his relatives working in the maritime business. His kids grew up on the water, learning to pilot a powerboat before driving a car, as he did. “We boat all the time on the bay” in his lobster boat, which he often works on to keep it seaworthy, Ms. Howell said.
“There’s nothing like taking care of hospitalized patients to make you feel you’re making the world a better place,” Dr. Howell observed. “Very often you can make a huge difference for the patients you do care for, and that is incredibly rewarding.” Although the demands of his SHM leadership position required relinquishing most of his responsibilities at Johns Hopkins, he continues to see patients and teach residents there 2-4 weeks a year on a teaching service.
“Why do I still see patients? I find it so rewarding. And I get to teach, which I love,” he said. “To be honest, I don’t think you truly need to see patients to be head of a professional medical society like SHM. Maybe someday I’ll give that up. But only if it’s necessary to make the society more successful.”
Half of Dr. Howell’s Society work now is planned and half is “putting out fires” – while learning members’ needs in real time. “Right now, we’re worried about burnout and PTSD, because frankly it’s stressful to take care of COVID patients. It’s scary for a lot of clinicians. I’m working with our members to make sure they have what they need to be clinically prepared, including resources to be more resilient professionally.”
Every step of his career, Dr. Howell said, has seemed like the best job he ever had. “Making the world a better place is still important to me. I tell SHM members that it’s important to know they are making a difference. What they’re doing is really important, especially with COVID, and it needs to be sustainable,” he said.
“SHM has such a powerful mission – it’s about making patient care better, and making hospitalists better clinicians. I know the Society is having a powerful impact, and that’s good enough for me. I’m into teams. Hospital medicine is a team sport, but so is SHM, interacting with its members, staff, and board.”
Initiating another new program
One of Dr. Howell’s last major projects for Hopkins was to launch and be chief medical officer for the Joint Commission–accredited Baltimore Civic Center Field Hospital for COVID-19 patients, opened in March 2020.
With a surge capacity of 250 beds, and a negative pressure ward set up in the center’s exhibit hall, it is jointly operated by the University of Maryland Medical System and Johns Hopkins Hospital. The field hospital’s mission has since expanded to include viral tests, infusions of monoclonal antibodies, and COVID-19 vaccinations.
Planning for a smooth transition, Dr. Howell brought Melinda E. Kantsiper, MD, director of clinical operations, Division of Hospital Medicine at Johns Hopkins Bayview, on board as associate medical officer, to eventually replace him as CMO after a few months working alongside him. “Eric brings that logical engineering eye to problem solving,” Dr. Kantsiper said.
“We wanted to build a very safe, high-quality hospital setting but had to do it very quickly. Watching him once again do what he does best, initiating a new program, building things carefully and thoughtfully, without being overly cautious, I could see his years of experience and good judgment about how hospitals run. He’s very logical but very caring. He’s also good at spotting young leaders and their talents.”
Some people have a knack for solving problems, added Dr. Ziegelstein, Dr. Howell’s mentor from his early days at Bayview. “Eric is different. He’s someone who’s able to identify gaps, problem areas, and vulnerabilities within an organization and then come up with a potential menu of solutions, think about which would be most likely to succeed, implement it, and assess the outcome. That’s the difference between a skilled manager and a true leader, and I’d say Eric had that ability while still in training,” Dr. Ziegelstein said.
“Eric understood early on not only what the field of hospital medicine could offer, he also understood how to catalyze change, without taking on too much change at one time,” Dr. Ziegelstein said. “He understood people’s sensibilities and concerns about this new service, and he catalyzed its growth through incremental change.”
Patients with asthma say most doctors don’t ask about cannabis use
Among individuals with asthma and allergies who use cannabis, more than half said they aren’t willing to discuss their use of cannabis with their doctor and their doctor doesn’t ask, according to recent research at the annual meeting of the American Academy of Allergy, Asthma, and Immunology, held virtually this year.
In an online survey of respondents with asthma and allergies in the Allergy & Asthma Network, 88 of 489 (18.0%) reported cannabis use. Of these respondents, 37.5% said they wanted to discuss their cannabis use with their doctor, 51.1% said they would not want to, and 11.4% reported they were unsure. In addition, 40.9% of respondents said their doctor inquired about cannabis use, while 51.1% said their doctor did not bring up cannabis use at all, either through a verbal discussion or on an intake form.
To date, there has not been much research on use of cannabis among patients with allergies and asthma, Joanna S. Zeiger, MS, PhD, of the Canna Research Foundation in Boulder, Colo., said in her presentation. “This is a group with whom route of administration could have broad adverse effects. Smoking or vaping cannabis in this population could lead to increased symptoms of cough and wheeze, as well as increased use of asthma medications and exacerbations of their disease.”
Dr. Zeiger and colleagues recruited 489 respondents for the AAN Pain, Exercise, and Cannabis Experience Survey study through social media channels between May 2020 and September 2020. In the survey, the researchers asked questions about the nature of the respondent’s cannabis use (medical, recreational, or both), the types of cannabinoids used (tetrahydrocannabinol [THC], cannabidiol [CBD], or both), the route of administration (capsule, edible, oil/tincture, smoke, spray, topical, or vaporizer), and subjective effects. Most of the respondents reported using both THC and CBD, with smoking, edibles, and vaping being the most comment route of administration.
Of the 88 respondents who said they currently used cannabis, 60.2% were aged less than 50 years, 72.4% were women, and 71.6% were White. A majority of respondents had been using cannabis for 3 or more years (54.5%) , used it less than one time per day (60.2%), and used it for pain (68.2%). Current asthma was reported in 51 respondents (58.0%), and 39.2% had uncontrolled asthma. Half of those respondents with uncontrolled asthma reported smoking cannabis, and 25.0% reported coughing because of cannabis. Both THC and CBD were used by 47.7% of respondents; 33% reported THC use alone, while 19.3% used CBD alone.
Reported effects of cannabis use
The most common positive effects of using cannabis reported among respondents were that it helped with sleep (66 respondents), calmed them down (60 respondents), reduced pain (60 respondents), or decreased anxiety (59 respondents). Many respondents who reported positive effects were using both THC and CBD. For example, respondents who reported using cannabinoids for calming, 46.7% reported using both, compared with 36.7% who used THC only and 16.7% who used CBD only. Among respondents who reported that cannabis helped them sleep, 51.5% used both THC and CBD.
Regarding adverse effects, there were no significant differences based on use of THC or CBD, but 31.9% of respondents who said they smoked cannabis and 4.9% of respondents who used cannabis through a route of administration that wasn’t smoking reported they coughed with their cannabis use (P < .001). No respondents reported anaphyalaxis, although, among individuals who did not use cannabis, 2.5% reported a cannabis allergy.
‘Cannabis allergy is real’
Commenting on the research, Gordon L. Sussman MD, allergist, clinical immunologist, and clinical professor of medicine at the University of Toronto, said the survey is a thorough questionnaire that is likely representative of attitudes about cannabis in the United States and countries where cannabis is not broadly legalized.
Cannabis allergy, however, is not uncommon, and “is something that people should be aware of,” he said. “Cannabis IgE allergy is real, is probably fairly common, and is something that [clinicians] should be asking about routinely.”
One limitation of the research was not knowing the number of people who declined to answer the survey, as there may be a bias in the results toward people who want to answer the questions, compared with those who did not want to answer. “When you do a survey, only a certain number of people are going to answer, and [you also want input from] people that don’t answer,” Dr. Sussman said.
Dr. Sussman acknowledged it can be difficult to get patients to admit cannabis use, even in countries like Canada where it is legal. Surveys like the one administered by Dr. Zeiger and colleagues are “the first step” to getting updated assessments of cannabis attitudes and recommendations. “The next step is doing an international survey, so you get different countries’ viewpoints and perspectives,” he said.
This study was supported by the Allergy & Asthma Network and the Canna Research Foundation. Three authors are affiliated with the Canna Research Foundation. Dr. Sussman reported no financial conflicts of interest. Dr. Sussman participates in the International Cannabis Allergy KAP Collaboration, a group founded by one of the coauthors, William Silvers, MD, but Dr. Sussman was not involved with this study.
Among individuals with asthma and allergies who use cannabis, more than half said they aren’t willing to discuss their use of cannabis with their doctor and their doctor doesn’t ask, according to recent research at the annual meeting of the American Academy of Allergy, Asthma, and Immunology, held virtually this year.
In an online survey of respondents with asthma and allergies in the Allergy & Asthma Network, 88 of 489 (18.0%) reported cannabis use. Of these respondents, 37.5% said they wanted to discuss their cannabis use with their doctor, 51.1% said they would not want to, and 11.4% reported they were unsure. In addition, 40.9% of respondents said their doctor inquired about cannabis use, while 51.1% said their doctor did not bring up cannabis use at all, either through a verbal discussion or on an intake form.
To date, there has not been much research on use of cannabis among patients with allergies and asthma, Joanna S. Zeiger, MS, PhD, of the Canna Research Foundation in Boulder, Colo., said in her presentation. “This is a group with whom route of administration could have broad adverse effects. Smoking or vaping cannabis in this population could lead to increased symptoms of cough and wheeze, as well as increased use of asthma medications and exacerbations of their disease.”
Dr. Zeiger and colleagues recruited 489 respondents for the AAN Pain, Exercise, and Cannabis Experience Survey study through social media channels between May 2020 and September 2020. In the survey, the researchers asked questions about the nature of the respondent’s cannabis use (medical, recreational, or both), the types of cannabinoids used (tetrahydrocannabinol [THC], cannabidiol [CBD], or both), the route of administration (capsule, edible, oil/tincture, smoke, spray, topical, or vaporizer), and subjective effects. Most of the respondents reported using both THC and CBD, with smoking, edibles, and vaping being the most comment route of administration.
Of the 88 respondents who said they currently used cannabis, 60.2% were aged less than 50 years, 72.4% were women, and 71.6% were White. A majority of respondents had been using cannabis for 3 or more years (54.5%) , used it less than one time per day (60.2%), and used it for pain (68.2%). Current asthma was reported in 51 respondents (58.0%), and 39.2% had uncontrolled asthma. Half of those respondents with uncontrolled asthma reported smoking cannabis, and 25.0% reported coughing because of cannabis. Both THC and CBD were used by 47.7% of respondents; 33% reported THC use alone, while 19.3% used CBD alone.
Reported effects of cannabis use
The most common positive effects of using cannabis reported among respondents were that it helped with sleep (66 respondents), calmed them down (60 respondents), reduced pain (60 respondents), or decreased anxiety (59 respondents). Many respondents who reported positive effects were using both THC and CBD. For example, respondents who reported using cannabinoids for calming, 46.7% reported using both, compared with 36.7% who used THC only and 16.7% who used CBD only. Among respondents who reported that cannabis helped them sleep, 51.5% used both THC and CBD.
Regarding adverse effects, there were no significant differences based on use of THC or CBD, but 31.9% of respondents who said they smoked cannabis and 4.9% of respondents who used cannabis through a route of administration that wasn’t smoking reported they coughed with their cannabis use (P < .001). No respondents reported anaphyalaxis, although, among individuals who did not use cannabis, 2.5% reported a cannabis allergy.
‘Cannabis allergy is real’
Commenting on the research, Gordon L. Sussman MD, allergist, clinical immunologist, and clinical professor of medicine at the University of Toronto, said the survey is a thorough questionnaire that is likely representative of attitudes about cannabis in the United States and countries where cannabis is not broadly legalized.
Cannabis allergy, however, is not uncommon, and “is something that people should be aware of,” he said. “Cannabis IgE allergy is real, is probably fairly common, and is something that [clinicians] should be asking about routinely.”
One limitation of the research was not knowing the number of people who declined to answer the survey, as there may be a bias in the results toward people who want to answer the questions, compared with those who did not want to answer. “When you do a survey, only a certain number of people are going to answer, and [you also want input from] people that don’t answer,” Dr. Sussman said.
Dr. Sussman acknowledged it can be difficult to get patients to admit cannabis use, even in countries like Canada where it is legal. Surveys like the one administered by Dr. Zeiger and colleagues are “the first step” to getting updated assessments of cannabis attitudes and recommendations. “The next step is doing an international survey, so you get different countries’ viewpoints and perspectives,” he said.
This study was supported by the Allergy & Asthma Network and the Canna Research Foundation. Three authors are affiliated with the Canna Research Foundation. Dr. Sussman reported no financial conflicts of interest. Dr. Sussman participates in the International Cannabis Allergy KAP Collaboration, a group founded by one of the coauthors, William Silvers, MD, but Dr. Sussman was not involved with this study.
Among individuals with asthma and allergies who use cannabis, more than half said they aren’t willing to discuss their use of cannabis with their doctor and their doctor doesn’t ask, according to recent research at the annual meeting of the American Academy of Allergy, Asthma, and Immunology, held virtually this year.
In an online survey of respondents with asthma and allergies in the Allergy & Asthma Network, 88 of 489 (18.0%) reported cannabis use. Of these respondents, 37.5% said they wanted to discuss their cannabis use with their doctor, 51.1% said they would not want to, and 11.4% reported they were unsure. In addition, 40.9% of respondents said their doctor inquired about cannabis use, while 51.1% said their doctor did not bring up cannabis use at all, either through a verbal discussion or on an intake form.
To date, there has not been much research on use of cannabis among patients with allergies and asthma, Joanna S. Zeiger, MS, PhD, of the Canna Research Foundation in Boulder, Colo., said in her presentation. “This is a group with whom route of administration could have broad adverse effects. Smoking or vaping cannabis in this population could lead to increased symptoms of cough and wheeze, as well as increased use of asthma medications and exacerbations of their disease.”
Dr. Zeiger and colleagues recruited 489 respondents for the AAN Pain, Exercise, and Cannabis Experience Survey study through social media channels between May 2020 and September 2020. In the survey, the researchers asked questions about the nature of the respondent’s cannabis use (medical, recreational, or both), the types of cannabinoids used (tetrahydrocannabinol [THC], cannabidiol [CBD], or both), the route of administration (capsule, edible, oil/tincture, smoke, spray, topical, or vaporizer), and subjective effects. Most of the respondents reported using both THC and CBD, with smoking, edibles, and vaping being the most comment route of administration.
Of the 88 respondents who said they currently used cannabis, 60.2% were aged less than 50 years, 72.4% were women, and 71.6% were White. A majority of respondents had been using cannabis for 3 or more years (54.5%) , used it less than one time per day (60.2%), and used it for pain (68.2%). Current asthma was reported in 51 respondents (58.0%), and 39.2% had uncontrolled asthma. Half of those respondents with uncontrolled asthma reported smoking cannabis, and 25.0% reported coughing because of cannabis. Both THC and CBD were used by 47.7% of respondents; 33% reported THC use alone, while 19.3% used CBD alone.
Reported effects of cannabis use
The most common positive effects of using cannabis reported among respondents were that it helped with sleep (66 respondents), calmed them down (60 respondents), reduced pain (60 respondents), or decreased anxiety (59 respondents). Many respondents who reported positive effects were using both THC and CBD. For example, respondents who reported using cannabinoids for calming, 46.7% reported using both, compared with 36.7% who used THC only and 16.7% who used CBD only. Among respondents who reported that cannabis helped them sleep, 51.5% used both THC and CBD.
Regarding adverse effects, there were no significant differences based on use of THC or CBD, but 31.9% of respondents who said they smoked cannabis and 4.9% of respondents who used cannabis through a route of administration that wasn’t smoking reported they coughed with their cannabis use (P < .001). No respondents reported anaphyalaxis, although, among individuals who did not use cannabis, 2.5% reported a cannabis allergy.
‘Cannabis allergy is real’
Commenting on the research, Gordon L. Sussman MD, allergist, clinical immunologist, and clinical professor of medicine at the University of Toronto, said the survey is a thorough questionnaire that is likely representative of attitudes about cannabis in the United States and countries where cannabis is not broadly legalized.
Cannabis allergy, however, is not uncommon, and “is something that people should be aware of,” he said. “Cannabis IgE allergy is real, is probably fairly common, and is something that [clinicians] should be asking about routinely.”
One limitation of the research was not knowing the number of people who declined to answer the survey, as there may be a bias in the results toward people who want to answer the questions, compared with those who did not want to answer. “When you do a survey, only a certain number of people are going to answer, and [you also want input from] people that don’t answer,” Dr. Sussman said.
Dr. Sussman acknowledged it can be difficult to get patients to admit cannabis use, even in countries like Canada where it is legal. Surveys like the one administered by Dr. Zeiger and colleagues are “the first step” to getting updated assessments of cannabis attitudes and recommendations. “The next step is doing an international survey, so you get different countries’ viewpoints and perspectives,” he said.
This study was supported by the Allergy & Asthma Network and the Canna Research Foundation. Three authors are affiliated with the Canna Research Foundation. Dr. Sussman reported no financial conflicts of interest. Dr. Sussman participates in the International Cannabis Allergy KAP Collaboration, a group founded by one of the coauthors, William Silvers, MD, but Dr. Sussman was not involved with this study.
FROM AAAAI 2021