User login
A ‘hospitalist plus’: Grace C. Huang, MD
Editor’s note: This profile is part of SHM’s celebration of National Hospitalist Day on March 4. National Hospitalist Day occurs the first Thursday in March annually, and celebrates the fastest growing specialty in modern medicine and hospitalists’ enduring contributions to the evolving health care landscape.
Grace C. Huang, MD, is a hospitalist at Beth Israel Deaconess Medical Center and an associate professor of medicine at Harvard Medical School, both in Boston.
Dr. Huang currently serves as vice chair for career development and mentoring in the department of medicine at Beth Israel Deaconess as well as director of the Office of Academic Careers and Faculty Development, and codirector of the Beth Israel Deaconess Academy of Medical Educators. She is also director of the Rabkin Fellowship in Medical Education, a program for Harvard Medical School faculty designed to help develop the skills needed to launch or advance academic careers in medical education or academic leadership.
Additionally, Dr. Huang is the editor in chief of MedEdPORTAL, a MEDLINE-indexed, open-access journal of the Association of American Medical Colleges.
At what point in your training did you decide to practice hospital medicine, and what about it appealed to you?
I trained at a point in time where it was rare for people to aspire to go in to hospital medicine. It just wasn’t that common, and there were so few examples of what a career trajectory in hospital medicine would look like. So I don’t know that I actively chose to go into hospital medicine; I chose it because it was what I knew how to do, based on my residency experience.
But it is really easy and authentic for me now to share about what makes hospital medicine such a vibrant career choice. I’m doing a lot of things in my job other than hospital medicine, but when I am on service, it reminds me acutely what it means to stay connected to why I became a doctor. The practice of hospital medicine means to be there at the most intense time of many people’s lives, to shoulder the responsibility of knowing that what I say to my patients will be remembered forever, and to be challenged by some of medicine’s hardest problems.
Hospital medicine has a way of putting you at the nexus of individual, family, society, government, and planet. But it also means that, even while I am witness to disease, suffering, broken relationships, social injustice, and environmental issues, I get a privileged look at what it means to comfort, to identify what really matters to people, to understand what gives us dignity as human beings. Lastly, I always come back to the fact that working as a team has made my clinical job so much more enriching; it’s not trench warfare, but you do create bonds quickly with learners, colleagues, and other health professionals in such an intense, fast-paced environment.
What is your current role at Beth Israel Deaconess Medical Center?
At Beth Israel Deaconess, I’m holding four different jobs. It’s sometimes hard for me to keep track of them, but they all center on career and faculty development. I’m a vice chair for career development within the department of medicine, and I also have an institutional role for faculty development for clinicians, educators, and researchers. I provide academic promotion support for the faculty, provide ad hoc mentorship, and run professional development programming. I also direct a year-long medical education fellowship. On the side, I am the editor in chief for a medical education journal.
What are your favorite areas of clinical practice and research?
Being a generalist means I love a lot of areas of clinical practice. I’m not sure there’s a particular area that I enjoy more than others. I love teaching specific topics – antibiotics, pharmacology, direct oral anticoagulants, the microbiology of common infections. I love thinking about how the heart and kidney battle for dominance each day and being the mediator. I have a particular interest in high-value care and lab ordering (or the fact that we should do much less of it). I love complex diagnostic problems and mapping them out on paper for my team.
The research that I’ve been doing over the past 20 years has focused on how we train internists and internists-to-be to do bedside procedures. It stemmed from my own ineptitude in doing procedures, and it caused me to question the age-old approach we took in sticking needles into patients without standardized training, supervision, or safety measures.
I’ve been proud of the small role I’ve been able to play in influencing how residents are taught to do procedures, and now I’m working with others to focus on how we should teach procedures to hospitalists, who don’t do procedures on a regular basis, and aren’t under the same expectations for ongoing skill development.
What are the most challenging aspects of practicing hospital medicine, and what are the most rewarding?
The intensity is probably what’s hardest for me about hospital medicine. At this point in my career, if I’m on service for a week, it takes me just as long to recover. It’s the cognitive load of needing to keep track of details that can make a big difference, the rapidity at which patients can deteriorate, the need to change course in an instant because of new information, and wanting to be mentally present and available for my patients and my learners.
It’s also hard to see suffering up close and personal and to leave feeling helpless to change the course of severe illness or to optimize care within the constraints of the health care system. This is why I do – and have to – extract satisfaction from the smallest of wins and brief moments of connection. Like seeing a patient turn the corner after being on the brink. Or gaining trust from an initially upset family member. Getting a copy of the eulogy from the daughter of my patient. A phone call from a patient I cared for 18 months ago, thanking me for my care. Visiting patients in the hospital socially that I had gotten to know over the years.
How has COVID-19 impacted hospitalist practice, and what changes will outlast the pandemic?
What you read in the lay press has put a spotlight on hospital-based work. What has been shared resonates with my own experience – the loss of connection from visitor restrictions, the isolation patients experience when everyone is wearing personal protective equipment, the worsening of everything that was already hard to begin with, like health care disparities, mental health, access to community supports, financial challenges, the disproportionate burden on unpaid caregivers, etc.
After the pandemic is “over,” I hope that we will retain a sense of intentionality how we address limited resources, the importance of social connection, the structural racism that has disadvantaged patients and physicians of color.
How will hospital medicine as a field change in the next decade or 2?
The hospitalist model has already influenced other specialties, like ob.gyn., neurology, and cardiology, and I expect that to continue. Hospitalists have already become leaders at the highest levels, and we will see them in higher numbers throughout health care leadership.
Are there any particular mentors who have been influential in your journey as a hospitalist?
Because I’m one of the older hospitalists in my group, there were fewer mentors, other than my boss, Joe Li, MD, SFHM, [section chief in hospital medicine at Beth Israel Deaconess], who has been an amazing role model. I think also of my colleagues as peer mentors, who continue to push me to be a better doctor. Whether it means remaining curious during the physical exam, or inspiring me with their excitement about clinical cases.
Do you have any advice for students and residents interested in hospital medicine?
When I talk to trainees about career development as a hospitalist, I encourage them to think about what will make them a “Hospitalist Plus.” Whether that Plus is teaching, research, or leadership, being a hospitalist gives you an opportunity to extend your impact as a physician into related realm.
I look around at our hospital medicine group, and every person has their Plus. We have educators, quality improvement leaders, a health services researcher, a health policy expert, a textbook editor – everyone brings special expertise to the group. My Plus now is much bigger than my footprint as a hospitalist, but I would never have gotten here had I not chosen a career path that would allow me to explore the farthest reaches of my potential as a physician.
Editor’s note: This profile is part of SHM’s celebration of National Hospitalist Day on March 4. National Hospitalist Day occurs the first Thursday in March annually, and celebrates the fastest growing specialty in modern medicine and hospitalists’ enduring contributions to the evolving health care landscape.
Grace C. Huang, MD, is a hospitalist at Beth Israel Deaconess Medical Center and an associate professor of medicine at Harvard Medical School, both in Boston.
Dr. Huang currently serves as vice chair for career development and mentoring in the department of medicine at Beth Israel Deaconess as well as director of the Office of Academic Careers and Faculty Development, and codirector of the Beth Israel Deaconess Academy of Medical Educators. She is also director of the Rabkin Fellowship in Medical Education, a program for Harvard Medical School faculty designed to help develop the skills needed to launch or advance academic careers in medical education or academic leadership.
Additionally, Dr. Huang is the editor in chief of MedEdPORTAL, a MEDLINE-indexed, open-access journal of the Association of American Medical Colleges.
At what point in your training did you decide to practice hospital medicine, and what about it appealed to you?
I trained at a point in time where it was rare for people to aspire to go in to hospital medicine. It just wasn’t that common, and there were so few examples of what a career trajectory in hospital medicine would look like. So I don’t know that I actively chose to go into hospital medicine; I chose it because it was what I knew how to do, based on my residency experience.
But it is really easy and authentic for me now to share about what makes hospital medicine such a vibrant career choice. I’m doing a lot of things in my job other than hospital medicine, but when I am on service, it reminds me acutely what it means to stay connected to why I became a doctor. The practice of hospital medicine means to be there at the most intense time of many people’s lives, to shoulder the responsibility of knowing that what I say to my patients will be remembered forever, and to be challenged by some of medicine’s hardest problems.
Hospital medicine has a way of putting you at the nexus of individual, family, society, government, and planet. But it also means that, even while I am witness to disease, suffering, broken relationships, social injustice, and environmental issues, I get a privileged look at what it means to comfort, to identify what really matters to people, to understand what gives us dignity as human beings. Lastly, I always come back to the fact that working as a team has made my clinical job so much more enriching; it’s not trench warfare, but you do create bonds quickly with learners, colleagues, and other health professionals in such an intense, fast-paced environment.
What is your current role at Beth Israel Deaconess Medical Center?
At Beth Israel Deaconess, I’m holding four different jobs. It’s sometimes hard for me to keep track of them, but they all center on career and faculty development. I’m a vice chair for career development within the department of medicine, and I also have an institutional role for faculty development for clinicians, educators, and researchers. I provide academic promotion support for the faculty, provide ad hoc mentorship, and run professional development programming. I also direct a year-long medical education fellowship. On the side, I am the editor in chief for a medical education journal.
What are your favorite areas of clinical practice and research?
Being a generalist means I love a lot of areas of clinical practice. I’m not sure there’s a particular area that I enjoy more than others. I love teaching specific topics – antibiotics, pharmacology, direct oral anticoagulants, the microbiology of common infections. I love thinking about how the heart and kidney battle for dominance each day and being the mediator. I have a particular interest in high-value care and lab ordering (or the fact that we should do much less of it). I love complex diagnostic problems and mapping them out on paper for my team.
The research that I’ve been doing over the past 20 years has focused on how we train internists and internists-to-be to do bedside procedures. It stemmed from my own ineptitude in doing procedures, and it caused me to question the age-old approach we took in sticking needles into patients without standardized training, supervision, or safety measures.
I’ve been proud of the small role I’ve been able to play in influencing how residents are taught to do procedures, and now I’m working with others to focus on how we should teach procedures to hospitalists, who don’t do procedures on a regular basis, and aren’t under the same expectations for ongoing skill development.
What are the most challenging aspects of practicing hospital medicine, and what are the most rewarding?
The intensity is probably what’s hardest for me about hospital medicine. At this point in my career, if I’m on service for a week, it takes me just as long to recover. It’s the cognitive load of needing to keep track of details that can make a big difference, the rapidity at which patients can deteriorate, the need to change course in an instant because of new information, and wanting to be mentally present and available for my patients and my learners.
It’s also hard to see suffering up close and personal and to leave feeling helpless to change the course of severe illness or to optimize care within the constraints of the health care system. This is why I do – and have to – extract satisfaction from the smallest of wins and brief moments of connection. Like seeing a patient turn the corner after being on the brink. Or gaining trust from an initially upset family member. Getting a copy of the eulogy from the daughter of my patient. A phone call from a patient I cared for 18 months ago, thanking me for my care. Visiting patients in the hospital socially that I had gotten to know over the years.
How has COVID-19 impacted hospitalist practice, and what changes will outlast the pandemic?
What you read in the lay press has put a spotlight on hospital-based work. What has been shared resonates with my own experience – the loss of connection from visitor restrictions, the isolation patients experience when everyone is wearing personal protective equipment, the worsening of everything that was already hard to begin with, like health care disparities, mental health, access to community supports, financial challenges, the disproportionate burden on unpaid caregivers, etc.
After the pandemic is “over,” I hope that we will retain a sense of intentionality how we address limited resources, the importance of social connection, the structural racism that has disadvantaged patients and physicians of color.
How will hospital medicine as a field change in the next decade or 2?
The hospitalist model has already influenced other specialties, like ob.gyn., neurology, and cardiology, and I expect that to continue. Hospitalists have already become leaders at the highest levels, and we will see them in higher numbers throughout health care leadership.
Are there any particular mentors who have been influential in your journey as a hospitalist?
Because I’m one of the older hospitalists in my group, there were fewer mentors, other than my boss, Joe Li, MD, SFHM, [section chief in hospital medicine at Beth Israel Deaconess], who has been an amazing role model. I think also of my colleagues as peer mentors, who continue to push me to be a better doctor. Whether it means remaining curious during the physical exam, or inspiring me with their excitement about clinical cases.
Do you have any advice for students and residents interested in hospital medicine?
When I talk to trainees about career development as a hospitalist, I encourage them to think about what will make them a “Hospitalist Plus.” Whether that Plus is teaching, research, or leadership, being a hospitalist gives you an opportunity to extend your impact as a physician into related realm.
I look around at our hospital medicine group, and every person has their Plus. We have educators, quality improvement leaders, a health services researcher, a health policy expert, a textbook editor – everyone brings special expertise to the group. My Plus now is much bigger than my footprint as a hospitalist, but I would never have gotten here had I not chosen a career path that would allow me to explore the farthest reaches of my potential as a physician.
Editor’s note: This profile is part of SHM’s celebration of National Hospitalist Day on March 4. National Hospitalist Day occurs the first Thursday in March annually, and celebrates the fastest growing specialty in modern medicine and hospitalists’ enduring contributions to the evolving health care landscape.
Grace C. Huang, MD, is a hospitalist at Beth Israel Deaconess Medical Center and an associate professor of medicine at Harvard Medical School, both in Boston.
Dr. Huang currently serves as vice chair for career development and mentoring in the department of medicine at Beth Israel Deaconess as well as director of the Office of Academic Careers and Faculty Development, and codirector of the Beth Israel Deaconess Academy of Medical Educators. She is also director of the Rabkin Fellowship in Medical Education, a program for Harvard Medical School faculty designed to help develop the skills needed to launch or advance academic careers in medical education or academic leadership.
Additionally, Dr. Huang is the editor in chief of MedEdPORTAL, a MEDLINE-indexed, open-access journal of the Association of American Medical Colleges.
At what point in your training did you decide to practice hospital medicine, and what about it appealed to you?
I trained at a point in time where it was rare for people to aspire to go in to hospital medicine. It just wasn’t that common, and there were so few examples of what a career trajectory in hospital medicine would look like. So I don’t know that I actively chose to go into hospital medicine; I chose it because it was what I knew how to do, based on my residency experience.
But it is really easy and authentic for me now to share about what makes hospital medicine such a vibrant career choice. I’m doing a lot of things in my job other than hospital medicine, but when I am on service, it reminds me acutely what it means to stay connected to why I became a doctor. The practice of hospital medicine means to be there at the most intense time of many people’s lives, to shoulder the responsibility of knowing that what I say to my patients will be remembered forever, and to be challenged by some of medicine’s hardest problems.
Hospital medicine has a way of putting you at the nexus of individual, family, society, government, and planet. But it also means that, even while I am witness to disease, suffering, broken relationships, social injustice, and environmental issues, I get a privileged look at what it means to comfort, to identify what really matters to people, to understand what gives us dignity as human beings. Lastly, I always come back to the fact that working as a team has made my clinical job so much more enriching; it’s not trench warfare, but you do create bonds quickly with learners, colleagues, and other health professionals in such an intense, fast-paced environment.
What is your current role at Beth Israel Deaconess Medical Center?
At Beth Israel Deaconess, I’m holding four different jobs. It’s sometimes hard for me to keep track of them, but they all center on career and faculty development. I’m a vice chair for career development within the department of medicine, and I also have an institutional role for faculty development for clinicians, educators, and researchers. I provide academic promotion support for the faculty, provide ad hoc mentorship, and run professional development programming. I also direct a year-long medical education fellowship. On the side, I am the editor in chief for a medical education journal.
What are your favorite areas of clinical practice and research?
Being a generalist means I love a lot of areas of clinical practice. I’m not sure there’s a particular area that I enjoy more than others. I love teaching specific topics – antibiotics, pharmacology, direct oral anticoagulants, the microbiology of common infections. I love thinking about how the heart and kidney battle for dominance each day and being the mediator. I have a particular interest in high-value care and lab ordering (or the fact that we should do much less of it). I love complex diagnostic problems and mapping them out on paper for my team.
The research that I’ve been doing over the past 20 years has focused on how we train internists and internists-to-be to do bedside procedures. It stemmed from my own ineptitude in doing procedures, and it caused me to question the age-old approach we took in sticking needles into patients without standardized training, supervision, or safety measures.
I’ve been proud of the small role I’ve been able to play in influencing how residents are taught to do procedures, and now I’m working with others to focus on how we should teach procedures to hospitalists, who don’t do procedures on a regular basis, and aren’t under the same expectations for ongoing skill development.
What are the most challenging aspects of practicing hospital medicine, and what are the most rewarding?
The intensity is probably what’s hardest for me about hospital medicine. At this point in my career, if I’m on service for a week, it takes me just as long to recover. It’s the cognitive load of needing to keep track of details that can make a big difference, the rapidity at which patients can deteriorate, the need to change course in an instant because of new information, and wanting to be mentally present and available for my patients and my learners.
It’s also hard to see suffering up close and personal and to leave feeling helpless to change the course of severe illness or to optimize care within the constraints of the health care system. This is why I do – and have to – extract satisfaction from the smallest of wins and brief moments of connection. Like seeing a patient turn the corner after being on the brink. Or gaining trust from an initially upset family member. Getting a copy of the eulogy from the daughter of my patient. A phone call from a patient I cared for 18 months ago, thanking me for my care. Visiting patients in the hospital socially that I had gotten to know over the years.
How has COVID-19 impacted hospitalist practice, and what changes will outlast the pandemic?
What you read in the lay press has put a spotlight on hospital-based work. What has been shared resonates with my own experience – the loss of connection from visitor restrictions, the isolation patients experience when everyone is wearing personal protective equipment, the worsening of everything that was already hard to begin with, like health care disparities, mental health, access to community supports, financial challenges, the disproportionate burden on unpaid caregivers, etc.
After the pandemic is “over,” I hope that we will retain a sense of intentionality how we address limited resources, the importance of social connection, the structural racism that has disadvantaged patients and physicians of color.
How will hospital medicine as a field change in the next decade or 2?
The hospitalist model has already influenced other specialties, like ob.gyn., neurology, and cardiology, and I expect that to continue. Hospitalists have already become leaders at the highest levels, and we will see them in higher numbers throughout health care leadership.
Are there any particular mentors who have been influential in your journey as a hospitalist?
Because I’m one of the older hospitalists in my group, there were fewer mentors, other than my boss, Joe Li, MD, SFHM, [section chief in hospital medicine at Beth Israel Deaconess], who has been an amazing role model. I think also of my colleagues as peer mentors, who continue to push me to be a better doctor. Whether it means remaining curious during the physical exam, or inspiring me with their excitement about clinical cases.
Do you have any advice for students and residents interested in hospital medicine?
When I talk to trainees about career development as a hospitalist, I encourage them to think about what will make them a “Hospitalist Plus.” Whether that Plus is teaching, research, or leadership, being a hospitalist gives you an opportunity to extend your impact as a physician into related realm.
I look around at our hospital medicine group, and every person has their Plus. We have educators, quality improvement leaders, a health services researcher, a health policy expert, a textbook editor – everyone brings special expertise to the group. My Plus now is much bigger than my footprint as a hospitalist, but I would never have gotten here had I not chosen a career path that would allow me to explore the farthest reaches of my potential as a physician.
Painless Mobile Nodule on the Shoulder
The Diagnosis: Cutaneous Metaplastic Synovial Cyst
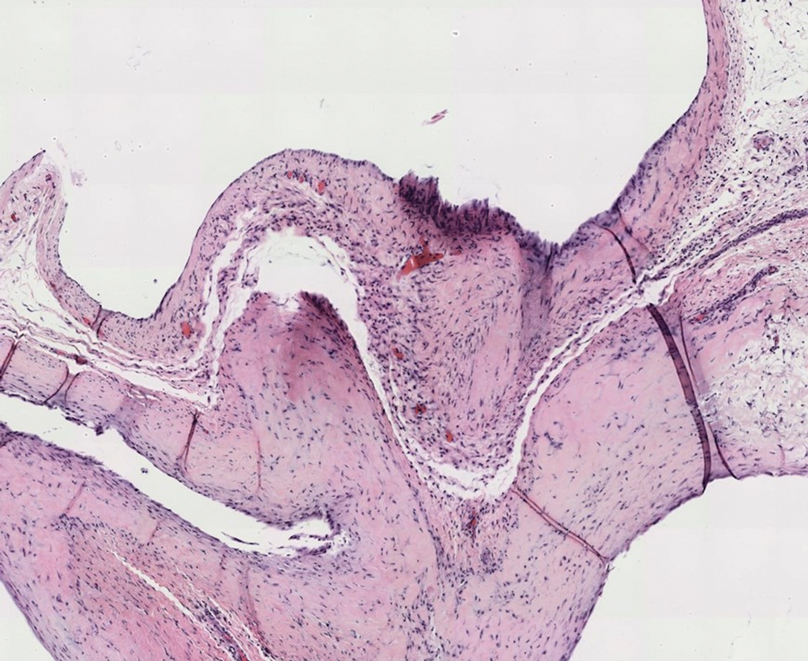
Gross examination of the excised nodule revealed a 2.5×1.2×1.0-cm, intact, gray-white, thin-walled, smooth-lined nodule filled with clear mucinouslike material. Hematoxylin and eosin-stained sections demonstrated a dermal-based cystlike structure composed of a lining of connective tissue with hyalinized material and fibrin as well as spindle and epithelioid cells with a mild mixed inflammatory infiltrate (Figure). These histopathologic findings led to the diagnosis of cutaneous metaplastic synovial cyst (CMSC).
Cutaneous metaplastic synovial cyst, also known as synovial metaplasia of the skin, is an uncommon benign cystic lesion that was first reported by Gonzalez et al1 in 1987. Histologically, CMSC lacks an epithelial lining and therefore is not a true cyst but rather a pseudocyst.2 Clinically, the lesion typically presents as a solitary subcutaneous nodule that may be tender or painless. In a literature review of CMSC cases performed by Fukuyama et al,3 distribution of reported cases according to body site varied; however, limbs were found to be the most commonly involved area. A PubMed search of articles indexed for MEDLINE as well as a Google Scholar search using the term cutaneous metaplastic synovial cyst revealed at least 37 cases reported in the English-language literature,3-9 including our present case. The pathogenesis remains uncertain; however, a majority of previously reported cases of CMSC characteristically have been associated with a pre-existing lesion, with most presentations developing at surgical scar sites secondary to operation or trauma.5 Relative tissue fragility secondary to rheumatoid arthritis10 and Ehlers-Danlos syndrome9,11,12 has been linked to CMSC in some documented reports, while a minority of cases report no antecedent events triggering formation of the lesion.13-15
As evidenced by our patient, CMSC clinically mimics several other benign entities; histopathologic examination is necessary to confirm the diagnosis. Although nodular hidradenoma also may clinically present as a solitary firm intradermal nodule, microscopy reveals a dermal-based lobulated tumor containing cystic spaces and solid areas composed of basophilic polyhedral cells and round glycogen-filled clear cells.16 Epidermoid cysts are differentiated from CMSC by the presence of a cyst wall lining composed of stratified squamous epithelium and associated laminated keratin within the lumen,17 which corresponds to its pearly white appearance on gross examination. Cutaneous ciliated cysts predominantly occur on the lower extremities of young women and are lined by simple cuboidal or columnar ciliated cells that resemble müllerian epithelium.18 Similar to CMSC, ganglion cysts are pseudocysts that lack a true epithelial lining but differ in appearance due to their mucin-filled synovial-lined sac.19 Additionally, ganglion cysts most often occur on the dorsal and volar aspects of the wrist.
Excisional biopsy is indicated as the preferred treatment of CMSC, given the lesion's benign behavior and low recurrence rate.6 Our case highlights this rare entity and reinforces its inclusion in the differential diagnosis of subcutaneous mobile nodules, especially in the setting of prior tissue injury secondary to trauma, surgical procedures, or conditions such as rheumatoid arthritis or Ehlers-Danlos syndrome. Unlike most previously reported cases, our patient reported no preceding tissue injury associated with formation of the lesion, and she was largely asymptomatic on presentation. Considering the limited number of CMSC cases demonstrated in the literature, it is important to continue reporting new cases to better understand characteristics and presentations of this uncommon lesion.
- Gonzalez JG, Ghiselli RW, Santa Cruz DJ. Synovial metaplasia of the skin. Am J Surg Pathol. 1987;11:343-350.
- Calonje E, Brenn T, Lazar A, et al. Cutaneous cysts. In: Calonje E, Brenn T, Lazar A, et al. McKee's Pathology of the Skin. 5th ed. Elsevier Limited; 2020:1680-1697.
- Fukuyama M, Sato Y, Hayakawa J, et al. Cutaneous metaplastic synovial cyst: case report and literature review from the dermatological point of view. Keio J Med. 2016;66:9-13.
- Karaytug K, Kapicioglu M, Can N, et al. Unprecedented recurrence of carpal tunnel syndrome by metaplastic synovial cyst in the carpal tunnel. Acta Orthop Traumatol Turc. 2019;53:230-232.
- Martelli SJ, Silveira FM, Carvalho PH, et al. Asymptomatic subcutaneous swelling of lower face. Oral Surg Oral Med Oral Pathol Oral Radiol. 2019;128:101-105.
- Majdi M, Saffar H, Ghanadan A. Cutaneous metaplastic synovial cyst: a case report. Iran J Pathol. 2016;11:423-426.
- Ramachandra S, Rao L, Al-Kindi M. Cutaneous metaplastic synovial cyst. Sultan Qaboos Univ Med J. 2016;16:E117-E118.
- Heidarian A, Xie Q, Banihashemi A. Cutaneous metaplastic synovial cyst presenting as an axillary mass after modified mastectomy and adjuvant radiotherapy. Am J Clin Pathol. 2016;146:S2.
- Fernandez-Flores A, Barja-Lopez JM. Cutaneous metaplastic synovial cyst in Ehlers-Danlos syndrome. J Cutan Pathol. 2020;47:729-733.
- Choonhakarn C, Tang S. Cutaneous metaplastic synovial cyst. J Dermatol. 2003;30:480-484.
- Guala A, Viglio S, Ottinetti A, et al. Cutaneous metaplastic synovial cyst in Ehlers-Danlos syndrome: report of a second case. Am J Dermatopathol. 2008;30:59-61.
- Nieto S, Buezo GF, Jones-Caballero M, et al. Cutaneous metaplastic synovial cyst in an Ehlers-Danlos patient. Am J Dermatopathol. 1997;19:407-410.
- Goiriz R, Rios-Buceta L, Alonso-Perez A, et al. Cutaneous metaplastic synovial cyst. J Am Acad Dermatol. 2005;53:180-181.
- Kim BC, Choi WJ, Park EJ, et al. Cutaneous metaplastic synovial cyst of the first metatarsal head area. Ann Dermatol. 2011;23(suppl 2):S165-S168.
- Yang HC, Tsai YJ, Hu SL, et al. Cutaneous metaplastic synovial cyst--a case report and review of literature. Dermatol Sinica. 2003;21:275-279.
- Kataria SP, Singh G, Batra A, et al. Nodular hidradenoma: a series of five cases in male subjects and review of literature. Adv Cytol Pathol. 2018;3:46-47.
- Mohamed Haflah N, Mohd Kassim A, Hassan Shukur M. Giant epidermoid cyst of the thigh. Malays Orthop J. 2011;5:17-19.
- Torisu-Itakura H, Itakura E, Horiuchi R, et al. Cutaneous ciliated cyst on the leg of a woman of menopausal age. Acta Derm Venereol. 2009;89:323-324.
- Fullen DR. Cysts and sinuses. In: Busam K, ed. Dermatopathology. Saunders; 2010:300-330.
The Diagnosis: Cutaneous Metaplastic Synovial Cyst
Gross examination of the excised nodule revealed a 2.5×1.2×1.0-cm, intact, gray-white, thin-walled, smooth-lined nodule filled with clear mucinouslike material. Hematoxylin and eosin-stained sections demonstrated a dermal-based cystlike structure composed of a lining of connective tissue with hyalinized material and fibrin as well as spindle and epithelioid cells with a mild mixed inflammatory infiltrate (Figure). These histopathologic findings led to the diagnosis of cutaneous metaplastic synovial cyst (CMSC).

Cutaneous metaplastic synovial cyst, also known as synovial metaplasia of the skin, is an uncommon benign cystic lesion that was first reported by Gonzalez et al1 in 1987. Histologically, CMSC lacks an epithelial lining and therefore is not a true cyst but rather a pseudocyst.2 Clinically, the lesion typically presents as a solitary subcutaneous nodule that may be tender or painless. In a literature review of CMSC cases performed by Fukuyama et al,3 distribution of reported cases according to body site varied; however, limbs were found to be the most commonly involved area. A PubMed search of articles indexed for MEDLINE as well as a Google Scholar search using the term cutaneous metaplastic synovial cyst revealed at least 37 cases reported in the English-language literature,3-9 including our present case. The pathogenesis remains uncertain; however, a majority of previously reported cases of CMSC characteristically have been associated with a pre-existing lesion, with most presentations developing at surgical scar sites secondary to operation or trauma.5 Relative tissue fragility secondary to rheumatoid arthritis10 and Ehlers-Danlos syndrome9,11,12 has been linked to CMSC in some documented reports, while a minority of cases report no antecedent events triggering formation of the lesion.13-15
As evidenced by our patient, CMSC clinically mimics several other benign entities; histopathologic examination is necessary to confirm the diagnosis. Although nodular hidradenoma also may clinically present as a solitary firm intradermal nodule, microscopy reveals a dermal-based lobulated tumor containing cystic spaces and solid areas composed of basophilic polyhedral cells and round glycogen-filled clear cells.16 Epidermoid cysts are differentiated from CMSC by the presence of a cyst wall lining composed of stratified squamous epithelium and associated laminated keratin within the lumen,17 which corresponds to its pearly white appearance on gross examination. Cutaneous ciliated cysts predominantly occur on the lower extremities of young women and are lined by simple cuboidal or columnar ciliated cells that resemble müllerian epithelium.18 Similar to CMSC, ganglion cysts are pseudocysts that lack a true epithelial lining but differ in appearance due to their mucin-filled synovial-lined sac.19 Additionally, ganglion cysts most often occur on the dorsal and volar aspects of the wrist.
Excisional biopsy is indicated as the preferred treatment of CMSC, given the lesion's benign behavior and low recurrence rate.6 Our case highlights this rare entity and reinforces its inclusion in the differential diagnosis of subcutaneous mobile nodules, especially in the setting of prior tissue injury secondary to trauma, surgical procedures, or conditions such as rheumatoid arthritis or Ehlers-Danlos syndrome. Unlike most previously reported cases, our patient reported no preceding tissue injury associated with formation of the lesion, and she was largely asymptomatic on presentation. Considering the limited number of CMSC cases demonstrated in the literature, it is important to continue reporting new cases to better understand characteristics and presentations of this uncommon lesion.
The Diagnosis: Cutaneous Metaplastic Synovial Cyst
Gross examination of the excised nodule revealed a 2.5×1.2×1.0-cm, intact, gray-white, thin-walled, smooth-lined nodule filled with clear mucinouslike material. Hematoxylin and eosin-stained sections demonstrated a dermal-based cystlike structure composed of a lining of connective tissue with hyalinized material and fibrin as well as spindle and epithelioid cells with a mild mixed inflammatory infiltrate (Figure). These histopathologic findings led to the diagnosis of cutaneous metaplastic synovial cyst (CMSC).

Cutaneous metaplastic synovial cyst, also known as synovial metaplasia of the skin, is an uncommon benign cystic lesion that was first reported by Gonzalez et al1 in 1987. Histologically, CMSC lacks an epithelial lining and therefore is not a true cyst but rather a pseudocyst.2 Clinically, the lesion typically presents as a solitary subcutaneous nodule that may be tender or painless. In a literature review of CMSC cases performed by Fukuyama et al,3 distribution of reported cases according to body site varied; however, limbs were found to be the most commonly involved area. A PubMed search of articles indexed for MEDLINE as well as a Google Scholar search using the term cutaneous metaplastic synovial cyst revealed at least 37 cases reported in the English-language literature,3-9 including our present case. The pathogenesis remains uncertain; however, a majority of previously reported cases of CMSC characteristically have been associated with a pre-existing lesion, with most presentations developing at surgical scar sites secondary to operation or trauma.5 Relative tissue fragility secondary to rheumatoid arthritis10 and Ehlers-Danlos syndrome9,11,12 has been linked to CMSC in some documented reports, while a minority of cases report no antecedent events triggering formation of the lesion.13-15
As evidenced by our patient, CMSC clinically mimics several other benign entities; histopathologic examination is necessary to confirm the diagnosis. Although nodular hidradenoma also may clinically present as a solitary firm intradermal nodule, microscopy reveals a dermal-based lobulated tumor containing cystic spaces and solid areas composed of basophilic polyhedral cells and round glycogen-filled clear cells.16 Epidermoid cysts are differentiated from CMSC by the presence of a cyst wall lining composed of stratified squamous epithelium and associated laminated keratin within the lumen,17 which corresponds to its pearly white appearance on gross examination. Cutaneous ciliated cysts predominantly occur on the lower extremities of young women and are lined by simple cuboidal or columnar ciliated cells that resemble müllerian epithelium.18 Similar to CMSC, ganglion cysts are pseudocysts that lack a true epithelial lining but differ in appearance due to their mucin-filled synovial-lined sac.19 Additionally, ganglion cysts most often occur on the dorsal and volar aspects of the wrist.
Excisional biopsy is indicated as the preferred treatment of CMSC, given the lesion's benign behavior and low recurrence rate.6 Our case highlights this rare entity and reinforces its inclusion in the differential diagnosis of subcutaneous mobile nodules, especially in the setting of prior tissue injury secondary to trauma, surgical procedures, or conditions such as rheumatoid arthritis or Ehlers-Danlos syndrome. Unlike most previously reported cases, our patient reported no preceding tissue injury associated with formation of the lesion, and she was largely asymptomatic on presentation. Considering the limited number of CMSC cases demonstrated in the literature, it is important to continue reporting new cases to better understand characteristics and presentations of this uncommon lesion.
- Gonzalez JG, Ghiselli RW, Santa Cruz DJ. Synovial metaplasia of the skin. Am J Surg Pathol. 1987;11:343-350.
- Calonje E, Brenn T, Lazar A, et al. Cutaneous cysts. In: Calonje E, Brenn T, Lazar A, et al. McKee's Pathology of the Skin. 5th ed. Elsevier Limited; 2020:1680-1697.
- Fukuyama M, Sato Y, Hayakawa J, et al. Cutaneous metaplastic synovial cyst: case report and literature review from the dermatological point of view. Keio J Med. 2016;66:9-13.
- Karaytug K, Kapicioglu M, Can N, et al. Unprecedented recurrence of carpal tunnel syndrome by metaplastic synovial cyst in the carpal tunnel. Acta Orthop Traumatol Turc. 2019;53:230-232.
- Martelli SJ, Silveira FM, Carvalho PH, et al. Asymptomatic subcutaneous swelling of lower face. Oral Surg Oral Med Oral Pathol Oral Radiol. 2019;128:101-105.
- Majdi M, Saffar H, Ghanadan A. Cutaneous metaplastic synovial cyst: a case report. Iran J Pathol. 2016;11:423-426.
- Ramachandra S, Rao L, Al-Kindi M. Cutaneous metaplastic synovial cyst. Sultan Qaboos Univ Med J. 2016;16:E117-E118.
- Heidarian A, Xie Q, Banihashemi A. Cutaneous metaplastic synovial cyst presenting as an axillary mass after modified mastectomy and adjuvant radiotherapy. Am J Clin Pathol. 2016;146:S2.
- Fernandez-Flores A, Barja-Lopez JM. Cutaneous metaplastic synovial cyst in Ehlers-Danlos syndrome. J Cutan Pathol. 2020;47:729-733.
- Choonhakarn C, Tang S. Cutaneous metaplastic synovial cyst. J Dermatol. 2003;30:480-484.
- Guala A, Viglio S, Ottinetti A, et al. Cutaneous metaplastic synovial cyst in Ehlers-Danlos syndrome: report of a second case. Am J Dermatopathol. 2008;30:59-61.
- Nieto S, Buezo GF, Jones-Caballero M, et al. Cutaneous metaplastic synovial cyst in an Ehlers-Danlos patient. Am J Dermatopathol. 1997;19:407-410.
- Goiriz R, Rios-Buceta L, Alonso-Perez A, et al. Cutaneous metaplastic synovial cyst. J Am Acad Dermatol. 2005;53:180-181.
- Kim BC, Choi WJ, Park EJ, et al. Cutaneous metaplastic synovial cyst of the first metatarsal head area. Ann Dermatol. 2011;23(suppl 2):S165-S168.
- Yang HC, Tsai YJ, Hu SL, et al. Cutaneous metaplastic synovial cyst--a case report and review of literature. Dermatol Sinica. 2003;21:275-279.
- Kataria SP, Singh G, Batra A, et al. Nodular hidradenoma: a series of five cases in male subjects and review of literature. Adv Cytol Pathol. 2018;3:46-47.
- Mohamed Haflah N, Mohd Kassim A, Hassan Shukur M. Giant epidermoid cyst of the thigh. Malays Orthop J. 2011;5:17-19.
- Torisu-Itakura H, Itakura E, Horiuchi R, et al. Cutaneous ciliated cyst on the leg of a woman of menopausal age. Acta Derm Venereol. 2009;89:323-324.
- Fullen DR. Cysts and sinuses. In: Busam K, ed. Dermatopathology. Saunders; 2010:300-330.
- Gonzalez JG, Ghiselli RW, Santa Cruz DJ. Synovial metaplasia of the skin. Am J Surg Pathol. 1987;11:343-350.
- Calonje E, Brenn T, Lazar A, et al. Cutaneous cysts. In: Calonje E, Brenn T, Lazar A, et al. McKee's Pathology of the Skin. 5th ed. Elsevier Limited; 2020:1680-1697.
- Fukuyama M, Sato Y, Hayakawa J, et al. Cutaneous metaplastic synovial cyst: case report and literature review from the dermatological point of view. Keio J Med. 2016;66:9-13.
- Karaytug K, Kapicioglu M, Can N, et al. Unprecedented recurrence of carpal tunnel syndrome by metaplastic synovial cyst in the carpal tunnel. Acta Orthop Traumatol Turc. 2019;53:230-232.
- Martelli SJ, Silveira FM, Carvalho PH, et al. Asymptomatic subcutaneous swelling of lower face. Oral Surg Oral Med Oral Pathol Oral Radiol. 2019;128:101-105.
- Majdi M, Saffar H, Ghanadan A. Cutaneous metaplastic synovial cyst: a case report. Iran J Pathol. 2016;11:423-426.
- Ramachandra S, Rao L, Al-Kindi M. Cutaneous metaplastic synovial cyst. Sultan Qaboos Univ Med J. 2016;16:E117-E118.
- Heidarian A, Xie Q, Banihashemi A. Cutaneous metaplastic synovial cyst presenting as an axillary mass after modified mastectomy and adjuvant radiotherapy. Am J Clin Pathol. 2016;146:S2.
- Fernandez-Flores A, Barja-Lopez JM. Cutaneous metaplastic synovial cyst in Ehlers-Danlos syndrome. J Cutan Pathol. 2020;47:729-733.
- Choonhakarn C, Tang S. Cutaneous metaplastic synovial cyst. J Dermatol. 2003;30:480-484.
- Guala A, Viglio S, Ottinetti A, et al. Cutaneous metaplastic synovial cyst in Ehlers-Danlos syndrome: report of a second case. Am J Dermatopathol. 2008;30:59-61.
- Nieto S, Buezo GF, Jones-Caballero M, et al. Cutaneous metaplastic synovial cyst in an Ehlers-Danlos patient. Am J Dermatopathol. 1997;19:407-410.
- Goiriz R, Rios-Buceta L, Alonso-Perez A, et al. Cutaneous metaplastic synovial cyst. J Am Acad Dermatol. 2005;53:180-181.
- Kim BC, Choi WJ, Park EJ, et al. Cutaneous metaplastic synovial cyst of the first metatarsal head area. Ann Dermatol. 2011;23(suppl 2):S165-S168.
- Yang HC, Tsai YJ, Hu SL, et al. Cutaneous metaplastic synovial cyst--a case report and review of literature. Dermatol Sinica. 2003;21:275-279.
- Kataria SP, Singh G, Batra A, et al. Nodular hidradenoma: a series of five cases in male subjects and review of literature. Adv Cytol Pathol. 2018;3:46-47.
- Mohamed Haflah N, Mohd Kassim A, Hassan Shukur M. Giant epidermoid cyst of the thigh. Malays Orthop J. 2011;5:17-19.
- Torisu-Itakura H, Itakura E, Horiuchi R, et al. Cutaneous ciliated cyst on the leg of a woman of menopausal age. Acta Derm Venereol. 2009;89:323-324.
- Fullen DR. Cysts and sinuses. In: Busam K, ed. Dermatopathology. Saunders; 2010:300-330.
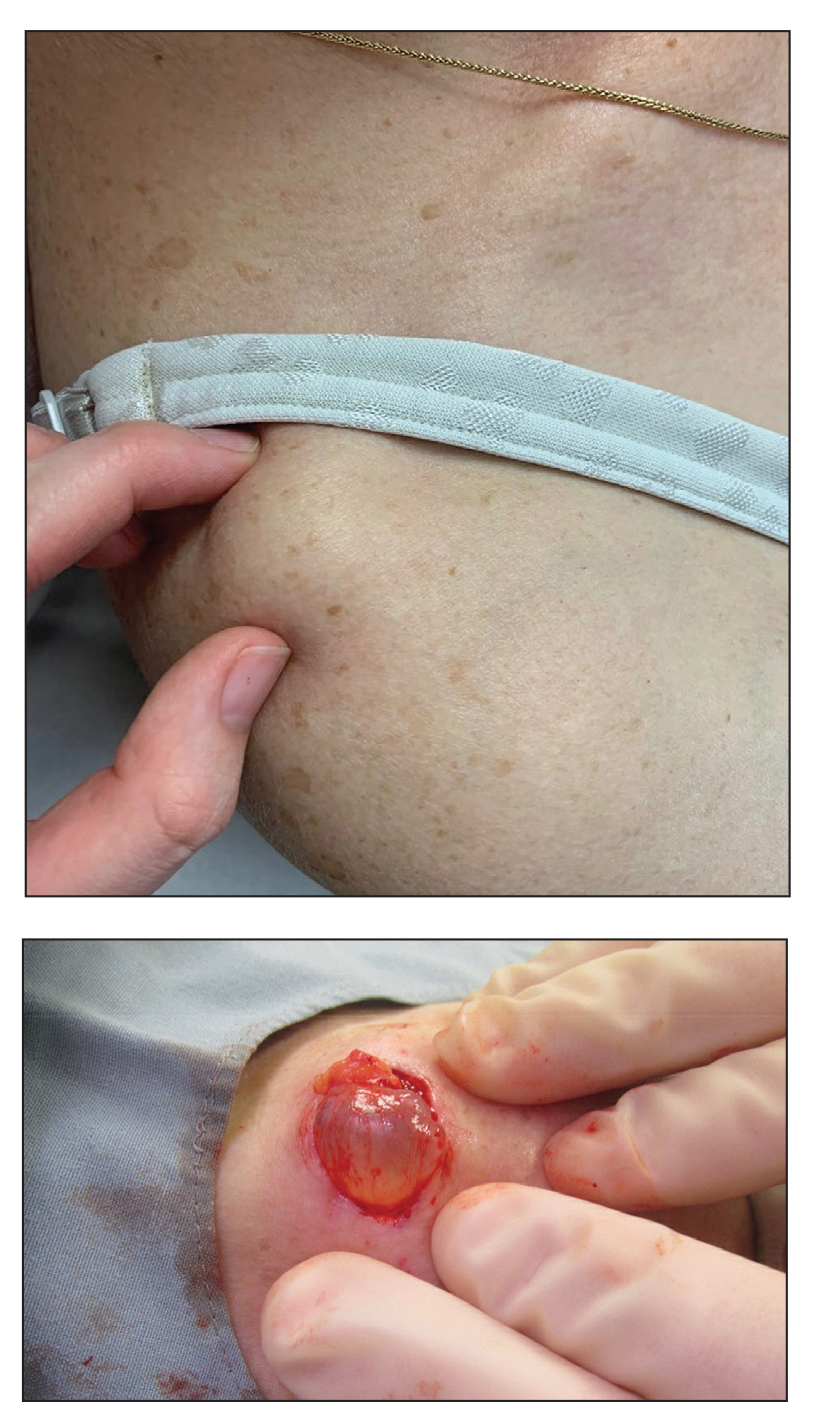
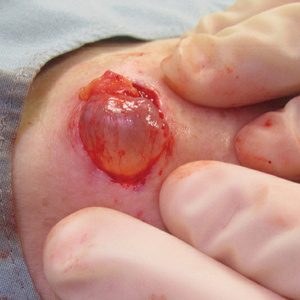
A 70-year-old woman presented to the outpatient dermatology clinic with an acute-onset lesion on the right shoulder. She first noticed a “cyst” developing in the area approximately 3 weeks prior but noted that it may have been present longer. The lesion was bothersome when her undergarments rubbed against it, but she otherwise denied pain, increase in size, or drainage from the site. Her medical history was remarkable for a proliferating trichilemmal tumor on the right parietal scalp treated with Mohs surgery approximately 13 years prior to presentation. She had no personal or family history of skin cancer. Physical examination revealed a 2.5-cm, mobile, nontender, flesh-colored subcutaneous nodule on the right shoulder (top); no ulceration, bleeding, or drainage was present. The surrounding skin demonstrated no clinical changes. The patient was scheduled for outpatient surgical excision of the nodule, which initially was suspected to be a lipoma. During the excision, a translucent cystlike nodule (bottom) was gently dissected and sent for histopathologic examination.
Mepolizumab reduced exacerbations in patients with asthma and atopy, depression comorbidities
, according to research from the annual meeting of the American Academy of Allergy, Asthma, and Immunology.
“Mepolizumab has clearly been shown to improve severe asthma control in many clinical trials, but atopy, obesity, and depression/anxiety affect patients with asthma at an increased rate,” Thomas B. Casale, MD, former AAAAI president and professor of medicine and pediatrics at the University of South Florida in Tampa, said in a presentation at the meeting. “Yet, few studies have examined whether asthma therapy with these comorbidities works.”
Dr. Casale and colleagues performed a retrospective analysis of patients in the United States from the MarketScan Commercial and Medicare Supplemental Database between November 2014 and December 2018 who had atopy, obesity, or depression/anxiety in addition to asthma and were receiving mepolizumab. Atopy in the study was defined as allergic rhinitis, anaphylaxis, atopic dermatitis, conjunctivitis, eosinophilic esophagitis, and food allergies. Patients were at least age 12 years, had at least one diagnosis for asthma, at least one diagnosis code for atopic disease, obesity, or depression/anxiety at baseline, and at least two administrations of mepolizumab within 180 days.
The researchers examined the number of exacerbations, oral corticosteroid (OCS) claims, and OCS bursts per year at 12-month follow-up, compared with baseline. They identified exacerbations by examining patients who had an emergency department or outpatient claim related to their asthma, and a claim for systemic corticosteroids made in the 4 days prior to or 5 days after a visit, or if their inpatient hospital admission contained a primary asthma diagnosis. Dr. Casale and colleagues measured OCS bursts as a pharmacy claim of at least 20 mg of prednisone per day for between 3 and 28 days plus a claim for an emergency department visit related to asthma in the 7 days prior or 6 days after the claim.
At baseline, patients across all groups were mean age 50.5-52.4 years with a Charleson Comorbidity Index score between 1.1 and 1.4, a majority were women (59.0%-72.0%) and nearly all were commercially insured (88.0%-90.0%). Patients who used biologics at baseline and/or used a biologic that wasn’t mepolizumab during the follow-up period were excluded.
Medication claims in the groups included inhaled corticosteroids (ICS) (36.8%-48.6%), ICS/long-acting beta-agonist (LABA) (60.2%-63.0%), LABA/ long-acting muscarinic antagonist (LAMA) (1.2%-3.5%), ICS/LABA/LAMA (21.2%-25.1%), short-acting beta-agonist (SABA) (83.2%-87.7%), LAMA alone (33.5%-42.1%), or leukotriene receptor antagonist (LTRA).
In the non–mutually exclusive group of patients with atopy (468 patients), 28.0% had comorbid obesity and 26.0% had comorbid depression/anxiety. For patients with obesity categorized in a non–mutually exclusive subgroup (171 patients), 79.0% had comorbid atopy and 32.0% had comorbid depression/anxiety. Among patients with non–mutually exclusive depression/anxiety (173 patients), 70.0% had comorbid atopy, while 32.0% had comorbid obesity.
The results showed the mean number of overall exacerbations decreased by 48% at 12 months in the atopic group (2.3 vs. 1.2; P < .001), 52% in the group with obesity (2.5 vs. 1.2; P < .001), and 38% in the depression/anxiety group (2.4 vs. 1.5; P < .001). The mean number of exacerbations leading to hospitalizations decreased by 64% in the atopic group (0.11 vs. 0.04; P < .001), 65% in the group with obesity (0.20 vs. 0.07; P < .001), and 68% in the group with depression/anxiety (0.22 vs. 0.07; P < .001).
The researchers also found the mean number of OCS claims and OCS bursts also significantly decreased over the 12-month follow-up period. Mean OCS claims decreased by 33% for patients in the atopic group (5.5 vs. 3.7; P < .001), by 38% in the group with obesity (6.1 vs. 3.8; P < .001), and by 31% in the group with depression/anxiety (6.2 vs. 4.3; P < .001).
The mean number of OCS bursts also significantly decreased by 40% in the atopic group (2.0 vs. 2.1; P < .001), 48% in the group with obesity (2.3 vs. 1.2; P < .001), and by 37% in the group with depression/anxiety (1.9 vs. 1.2; P < .001). In total, 69% of patients with comorbid atopy, 70.8% of patients with comorbid obesity, and 68.2% of patients with comorbid depression/anxiety experienced a mean decrease in their OCS dose over 12 months.
“These data demonstrate that patients with asthma and atopy, obesity, or depression and anxiety have significantly fewer exacerbations and reduced OCS use in a real-world setting with treatment of mepolizumab,” Dr. Casale said. “Thus, holistic patient care for severe asthma is critical, and mepolizumab provides tangible clinical benefit despite the complexities of medical comorbidities.”
This study was funded by GlaxoSmithKline, and the company also funded graphic design support of the poster. Dr. Casale reports he has received research funds from GlaxoSmithKline. Four authors report being current or former GlaxoSmithKline employees; three authors report holding stock and/or shares of GlaxoSmithKline. Three authors are IBM Watson Health employees, a company GlaxoSmithKline has provided research funding.
, according to research from the annual meeting of the American Academy of Allergy, Asthma, and Immunology.
“Mepolizumab has clearly been shown to improve severe asthma control in many clinical trials, but atopy, obesity, and depression/anxiety affect patients with asthma at an increased rate,” Thomas B. Casale, MD, former AAAAI president and professor of medicine and pediatrics at the University of South Florida in Tampa, said in a presentation at the meeting. “Yet, few studies have examined whether asthma therapy with these comorbidities works.”
Dr. Casale and colleagues performed a retrospective analysis of patients in the United States from the MarketScan Commercial and Medicare Supplemental Database between November 2014 and December 2018 who had atopy, obesity, or depression/anxiety in addition to asthma and were receiving mepolizumab. Atopy in the study was defined as allergic rhinitis, anaphylaxis, atopic dermatitis, conjunctivitis, eosinophilic esophagitis, and food allergies. Patients were at least age 12 years, had at least one diagnosis for asthma, at least one diagnosis code for atopic disease, obesity, or depression/anxiety at baseline, and at least two administrations of mepolizumab within 180 days.
The researchers examined the number of exacerbations, oral corticosteroid (OCS) claims, and OCS bursts per year at 12-month follow-up, compared with baseline. They identified exacerbations by examining patients who had an emergency department or outpatient claim related to their asthma, and a claim for systemic corticosteroids made in the 4 days prior to or 5 days after a visit, or if their inpatient hospital admission contained a primary asthma diagnosis. Dr. Casale and colleagues measured OCS bursts as a pharmacy claim of at least 20 mg of prednisone per day for between 3 and 28 days plus a claim for an emergency department visit related to asthma in the 7 days prior or 6 days after the claim.
At baseline, patients across all groups were mean age 50.5-52.4 years with a Charleson Comorbidity Index score between 1.1 and 1.4, a majority were women (59.0%-72.0%) and nearly all were commercially insured (88.0%-90.0%). Patients who used biologics at baseline and/or used a biologic that wasn’t mepolizumab during the follow-up period were excluded.
Medication claims in the groups included inhaled corticosteroids (ICS) (36.8%-48.6%), ICS/long-acting beta-agonist (LABA) (60.2%-63.0%), LABA/ long-acting muscarinic antagonist (LAMA) (1.2%-3.5%), ICS/LABA/LAMA (21.2%-25.1%), short-acting beta-agonist (SABA) (83.2%-87.7%), LAMA alone (33.5%-42.1%), or leukotriene receptor antagonist (LTRA).
In the non–mutually exclusive group of patients with atopy (468 patients), 28.0% had comorbid obesity and 26.0% had comorbid depression/anxiety. For patients with obesity categorized in a non–mutually exclusive subgroup (171 patients), 79.0% had comorbid atopy and 32.0% had comorbid depression/anxiety. Among patients with non–mutually exclusive depression/anxiety (173 patients), 70.0% had comorbid atopy, while 32.0% had comorbid obesity.
The results showed the mean number of overall exacerbations decreased by 48% at 12 months in the atopic group (2.3 vs. 1.2; P < .001), 52% in the group with obesity (2.5 vs. 1.2; P < .001), and 38% in the depression/anxiety group (2.4 vs. 1.5; P < .001). The mean number of exacerbations leading to hospitalizations decreased by 64% in the atopic group (0.11 vs. 0.04; P < .001), 65% in the group with obesity (0.20 vs. 0.07; P < .001), and 68% in the group with depression/anxiety (0.22 vs. 0.07; P < .001).
The researchers also found the mean number of OCS claims and OCS bursts also significantly decreased over the 12-month follow-up period. Mean OCS claims decreased by 33% for patients in the atopic group (5.5 vs. 3.7; P < .001), by 38% in the group with obesity (6.1 vs. 3.8; P < .001), and by 31% in the group with depression/anxiety (6.2 vs. 4.3; P < .001).
The mean number of OCS bursts also significantly decreased by 40% in the atopic group (2.0 vs. 2.1; P < .001), 48% in the group with obesity (2.3 vs. 1.2; P < .001), and by 37% in the group with depression/anxiety (1.9 vs. 1.2; P < .001). In total, 69% of patients with comorbid atopy, 70.8% of patients with comorbid obesity, and 68.2% of patients with comorbid depression/anxiety experienced a mean decrease in their OCS dose over 12 months.
“These data demonstrate that patients with asthma and atopy, obesity, or depression and anxiety have significantly fewer exacerbations and reduced OCS use in a real-world setting with treatment of mepolizumab,” Dr. Casale said. “Thus, holistic patient care for severe asthma is critical, and mepolizumab provides tangible clinical benefit despite the complexities of medical comorbidities.”
This study was funded by GlaxoSmithKline, and the company also funded graphic design support of the poster. Dr. Casale reports he has received research funds from GlaxoSmithKline. Four authors report being current or former GlaxoSmithKline employees; three authors report holding stock and/or shares of GlaxoSmithKline. Three authors are IBM Watson Health employees, a company GlaxoSmithKline has provided research funding.
, according to research from the annual meeting of the American Academy of Allergy, Asthma, and Immunology.
“Mepolizumab has clearly been shown to improve severe asthma control in many clinical trials, but atopy, obesity, and depression/anxiety affect patients with asthma at an increased rate,” Thomas B. Casale, MD, former AAAAI president and professor of medicine and pediatrics at the University of South Florida in Tampa, said in a presentation at the meeting. “Yet, few studies have examined whether asthma therapy with these comorbidities works.”
Dr. Casale and colleagues performed a retrospective analysis of patients in the United States from the MarketScan Commercial and Medicare Supplemental Database between November 2014 and December 2018 who had atopy, obesity, or depression/anxiety in addition to asthma and were receiving mepolizumab. Atopy in the study was defined as allergic rhinitis, anaphylaxis, atopic dermatitis, conjunctivitis, eosinophilic esophagitis, and food allergies. Patients were at least age 12 years, had at least one diagnosis for asthma, at least one diagnosis code for atopic disease, obesity, or depression/anxiety at baseline, and at least two administrations of mepolizumab within 180 days.
The researchers examined the number of exacerbations, oral corticosteroid (OCS) claims, and OCS bursts per year at 12-month follow-up, compared with baseline. They identified exacerbations by examining patients who had an emergency department or outpatient claim related to their asthma, and a claim for systemic corticosteroids made in the 4 days prior to or 5 days after a visit, or if their inpatient hospital admission contained a primary asthma diagnosis. Dr. Casale and colleagues measured OCS bursts as a pharmacy claim of at least 20 mg of prednisone per day for between 3 and 28 days plus a claim for an emergency department visit related to asthma in the 7 days prior or 6 days after the claim.
At baseline, patients across all groups were mean age 50.5-52.4 years with a Charleson Comorbidity Index score between 1.1 and 1.4, a majority were women (59.0%-72.0%) and nearly all were commercially insured (88.0%-90.0%). Patients who used biologics at baseline and/or used a biologic that wasn’t mepolizumab during the follow-up period were excluded.
Medication claims in the groups included inhaled corticosteroids (ICS) (36.8%-48.6%), ICS/long-acting beta-agonist (LABA) (60.2%-63.0%), LABA/ long-acting muscarinic antagonist (LAMA) (1.2%-3.5%), ICS/LABA/LAMA (21.2%-25.1%), short-acting beta-agonist (SABA) (83.2%-87.7%), LAMA alone (33.5%-42.1%), or leukotriene receptor antagonist (LTRA).
In the non–mutually exclusive group of patients with atopy (468 patients), 28.0% had comorbid obesity and 26.0% had comorbid depression/anxiety. For patients with obesity categorized in a non–mutually exclusive subgroup (171 patients), 79.0% had comorbid atopy and 32.0% had comorbid depression/anxiety. Among patients with non–mutually exclusive depression/anxiety (173 patients), 70.0% had comorbid atopy, while 32.0% had comorbid obesity.
The results showed the mean number of overall exacerbations decreased by 48% at 12 months in the atopic group (2.3 vs. 1.2; P < .001), 52% in the group with obesity (2.5 vs. 1.2; P < .001), and 38% in the depression/anxiety group (2.4 vs. 1.5; P < .001). The mean number of exacerbations leading to hospitalizations decreased by 64% in the atopic group (0.11 vs. 0.04; P < .001), 65% in the group with obesity (0.20 vs. 0.07; P < .001), and 68% in the group with depression/anxiety (0.22 vs. 0.07; P < .001).
The researchers also found the mean number of OCS claims and OCS bursts also significantly decreased over the 12-month follow-up period. Mean OCS claims decreased by 33% for patients in the atopic group (5.5 vs. 3.7; P < .001), by 38% in the group with obesity (6.1 vs. 3.8; P < .001), and by 31% in the group with depression/anxiety (6.2 vs. 4.3; P < .001).
The mean number of OCS bursts also significantly decreased by 40% in the atopic group (2.0 vs. 2.1; P < .001), 48% in the group with obesity (2.3 vs. 1.2; P < .001), and by 37% in the group with depression/anxiety (1.9 vs. 1.2; P < .001). In total, 69% of patients with comorbid atopy, 70.8% of patients with comorbid obesity, and 68.2% of patients with comorbid depression/anxiety experienced a mean decrease in their OCS dose over 12 months.
“These data demonstrate that patients with asthma and atopy, obesity, or depression and anxiety have significantly fewer exacerbations and reduced OCS use in a real-world setting with treatment of mepolizumab,” Dr. Casale said. “Thus, holistic patient care for severe asthma is critical, and mepolizumab provides tangible clinical benefit despite the complexities of medical comorbidities.”
This study was funded by GlaxoSmithKline, and the company also funded graphic design support of the poster. Dr. Casale reports he has received research funds from GlaxoSmithKline. Four authors report being current or former GlaxoSmithKline employees; three authors report holding stock and/or shares of GlaxoSmithKline. Three authors are IBM Watson Health employees, a company GlaxoSmithKline has provided research funding.
FROM AAAAI 2021
Opioid use common for pain in multiple sclerosis
, new research shows.
“This high level of opioid use supports that better pain management treatment options, including nonpharmacological options, are needed for people with MS and pain,” wrote the authors of the study, which was presented at ACTRIMS Forum 2021, held by the Americas Committee for Treatment and Research in Multiple Sclerosis.
Previous research has shown that more than 50% of people with MS report chronic pain that is serious enough to interfere with daily activities, employment, and quality of life. Many with MS report that pain is one of their worst symptoms, the authors noted.
With surprisingly few studies evaluating opioid use in the MS population, Cinda L. Hugos, PT, associate professor of neurology with the VA Portland Health Care System and the department of neurology, Oregon Health and Science University, Portland, and colleagues investigated the issue in a sample of patients participating in a U.S. multisite MS fatigue management trial conducted between 2013 and 2014.
Of the 281 participants with MS in the study, 58 patients (20.6%) reported using prescription opioids. Among them, most – 44 (76%) – reported regular daily use, 10 (17%) reported using the drugs only as needed, 3 (5%) reported only short-term use, including after recent injury or dental surgery, and 1 provided incomplete information.
Those who reported opioid use had significantly worse fatigue scores on the Modified Fatigue Impact Scale (P = .015) and worse pain scores (P < .0001).
There were no significant differences in terms of age (mean age, 53 years), gender (69% were female), or race (in both groups, about 76% were White). No significant differences were seen in disability or depression scores in the opioid users versus nonusers.
“In this sample of people with multiple sclerosis who self-reported fatigue and volunteered to join an MS fatigue management research study, more than one in five reported using prescription opioids and nearly one in six used opioids daily,” the authors wrote. “Opioid users had more pain and fatigue than nonusers.”
Commenting on the study, Jeffrey Cohen, MD, president of ACTRIMS, said that the findings are consistent with his observations that “in the general population, opioids often are used to treat chronic pain in people with MS.”
But they’re not getting the drugs from his clinic. “We do not prescribe opioids in our clinic, referring such patients to a chronic pain program,” Dr. Cohen said. “However, there clearly is need for better treatment options.”
A previous study on opioid use by people with MS, published in 2015, found even higher rates – 42% reported having ever used opioids, and 38% reported currently using opioids.
Although reports of opioid use by patients with MS have been lacking, more has been published on the emerging use of cannabis-related products. One recent study showed that nearly half of people with MS reported using a cannabis-based therapy for nerve-based pain and sleep disturbances.
Although cannabis is considered safer than opioids, the authors noted that it has its own significant drawback – a “paucity of provider guidance.”
“The range of perceived benefits and potential differential effects of THC and cannabinoid highlight the need for personalized, evidence-based guidelines regarding cannabinoid use,” they wrote.
Stretching program for spasticity shows benefits
With spasticity representing a key contributor to MS pain and affecting more than 80% of people with MS, Ms. Hugos and colleagues are developing an alternative to medication – a nonpharmacologic stretching regimen called Spasticity: Take Control” (STC).
Based on evidence-based strategies for the treatment of spasticity in MS, the program involves exercises with daily routines of 15-20 minutes over 6 months.
In a pilot study of 66 patients, also presented at the ACTRIMS meeting, the investigators reported that the program showed significant reductions in pain severity and interference, measured with the Brief Pain Inventory–Short Form, compared with a control consisting of range of motion instruction over 6 months.
The study also offered insights on the specific areas of pain. Among those who reported chronic pain (42% in the STC group and 63.3% in the range-of-motion group), the pain was most frequently reported in the lower back (74.3%), legs (68.6%), or lower back and legs (88.6%).
Ms. Hugos noted that the findings suggest a potentially important nonpharmacologic alternative to spasticity-related pain in MS.
“Stretching is the cornerstone treatment for spasticity from all causes, but there is very little information on stretching exercises in MS or any other conditions,” Ms. Hugos said. “[Our] pilot study is the first and only study using a standardized, daily stretching exercise program to treat MS spasticity,” she said.
“A fully powered study is needed to better understand the impact of different types of exercise on pain severity and interference in multiple sclerosis,” she noted.
Ms. Hugos has received consulting fees from Greenwich Biosciences, Evidera, and Techspert.io. Dr. Cohen has received personal compensation for consulting for Adamas, Atara, Bristol-Myers Squibb, Convelo, MedDay, and Mylan.
A version of this article first appeared on Medscape.com.
, new research shows.
“This high level of opioid use supports that better pain management treatment options, including nonpharmacological options, are needed for people with MS and pain,” wrote the authors of the study, which was presented at ACTRIMS Forum 2021, held by the Americas Committee for Treatment and Research in Multiple Sclerosis.
Previous research has shown that more than 50% of people with MS report chronic pain that is serious enough to interfere with daily activities, employment, and quality of life. Many with MS report that pain is one of their worst symptoms, the authors noted.
With surprisingly few studies evaluating opioid use in the MS population, Cinda L. Hugos, PT, associate professor of neurology with the VA Portland Health Care System and the department of neurology, Oregon Health and Science University, Portland, and colleagues investigated the issue in a sample of patients participating in a U.S. multisite MS fatigue management trial conducted between 2013 and 2014.
Of the 281 participants with MS in the study, 58 patients (20.6%) reported using prescription opioids. Among them, most – 44 (76%) – reported regular daily use, 10 (17%) reported using the drugs only as needed, 3 (5%) reported only short-term use, including after recent injury or dental surgery, and 1 provided incomplete information.
Those who reported opioid use had significantly worse fatigue scores on the Modified Fatigue Impact Scale (P = .015) and worse pain scores (P < .0001).
There were no significant differences in terms of age (mean age, 53 years), gender (69% were female), or race (in both groups, about 76% were White). No significant differences were seen in disability or depression scores in the opioid users versus nonusers.
“In this sample of people with multiple sclerosis who self-reported fatigue and volunteered to join an MS fatigue management research study, more than one in five reported using prescription opioids and nearly one in six used opioids daily,” the authors wrote. “Opioid users had more pain and fatigue than nonusers.”
Commenting on the study, Jeffrey Cohen, MD, president of ACTRIMS, said that the findings are consistent with his observations that “in the general population, opioids often are used to treat chronic pain in people with MS.”
But they’re not getting the drugs from his clinic. “We do not prescribe opioids in our clinic, referring such patients to a chronic pain program,” Dr. Cohen said. “However, there clearly is need for better treatment options.”
A previous study on opioid use by people with MS, published in 2015, found even higher rates – 42% reported having ever used opioids, and 38% reported currently using opioids.
Although reports of opioid use by patients with MS have been lacking, more has been published on the emerging use of cannabis-related products. One recent study showed that nearly half of people with MS reported using a cannabis-based therapy for nerve-based pain and sleep disturbances.
Although cannabis is considered safer than opioids, the authors noted that it has its own significant drawback – a “paucity of provider guidance.”
“The range of perceived benefits and potential differential effects of THC and cannabinoid highlight the need for personalized, evidence-based guidelines regarding cannabinoid use,” they wrote.
Stretching program for spasticity shows benefits
With spasticity representing a key contributor to MS pain and affecting more than 80% of people with MS, Ms. Hugos and colleagues are developing an alternative to medication – a nonpharmacologic stretching regimen called Spasticity: Take Control” (STC).
Based on evidence-based strategies for the treatment of spasticity in MS, the program involves exercises with daily routines of 15-20 minutes over 6 months.
In a pilot study of 66 patients, also presented at the ACTRIMS meeting, the investigators reported that the program showed significant reductions in pain severity and interference, measured with the Brief Pain Inventory–Short Form, compared with a control consisting of range of motion instruction over 6 months.
The study also offered insights on the specific areas of pain. Among those who reported chronic pain (42% in the STC group and 63.3% in the range-of-motion group), the pain was most frequently reported in the lower back (74.3%), legs (68.6%), or lower back and legs (88.6%).
Ms. Hugos noted that the findings suggest a potentially important nonpharmacologic alternative to spasticity-related pain in MS.
“Stretching is the cornerstone treatment for spasticity from all causes, but there is very little information on stretching exercises in MS or any other conditions,” Ms. Hugos said. “[Our] pilot study is the first and only study using a standardized, daily stretching exercise program to treat MS spasticity,” she said.
“A fully powered study is needed to better understand the impact of different types of exercise on pain severity and interference in multiple sclerosis,” she noted.
Ms. Hugos has received consulting fees from Greenwich Biosciences, Evidera, and Techspert.io. Dr. Cohen has received personal compensation for consulting for Adamas, Atara, Bristol-Myers Squibb, Convelo, MedDay, and Mylan.
A version of this article first appeared on Medscape.com.
, new research shows.
“This high level of opioid use supports that better pain management treatment options, including nonpharmacological options, are needed for people with MS and pain,” wrote the authors of the study, which was presented at ACTRIMS Forum 2021, held by the Americas Committee for Treatment and Research in Multiple Sclerosis.
Previous research has shown that more than 50% of people with MS report chronic pain that is serious enough to interfere with daily activities, employment, and quality of life. Many with MS report that pain is one of their worst symptoms, the authors noted.
With surprisingly few studies evaluating opioid use in the MS population, Cinda L. Hugos, PT, associate professor of neurology with the VA Portland Health Care System and the department of neurology, Oregon Health and Science University, Portland, and colleagues investigated the issue in a sample of patients participating in a U.S. multisite MS fatigue management trial conducted between 2013 and 2014.
Of the 281 participants with MS in the study, 58 patients (20.6%) reported using prescription opioids. Among them, most – 44 (76%) – reported regular daily use, 10 (17%) reported using the drugs only as needed, 3 (5%) reported only short-term use, including after recent injury or dental surgery, and 1 provided incomplete information.
Those who reported opioid use had significantly worse fatigue scores on the Modified Fatigue Impact Scale (P = .015) and worse pain scores (P < .0001).
There were no significant differences in terms of age (mean age, 53 years), gender (69% were female), or race (in both groups, about 76% were White). No significant differences were seen in disability or depression scores in the opioid users versus nonusers.
“In this sample of people with multiple sclerosis who self-reported fatigue and volunteered to join an MS fatigue management research study, more than one in five reported using prescription opioids and nearly one in six used opioids daily,” the authors wrote. “Opioid users had more pain and fatigue than nonusers.”
Commenting on the study, Jeffrey Cohen, MD, president of ACTRIMS, said that the findings are consistent with his observations that “in the general population, opioids often are used to treat chronic pain in people with MS.”
But they’re not getting the drugs from his clinic. “We do not prescribe opioids in our clinic, referring such patients to a chronic pain program,” Dr. Cohen said. “However, there clearly is need for better treatment options.”
A previous study on opioid use by people with MS, published in 2015, found even higher rates – 42% reported having ever used opioids, and 38% reported currently using opioids.
Although reports of opioid use by patients with MS have been lacking, more has been published on the emerging use of cannabis-related products. One recent study showed that nearly half of people with MS reported using a cannabis-based therapy for nerve-based pain and sleep disturbances.
Although cannabis is considered safer than opioids, the authors noted that it has its own significant drawback – a “paucity of provider guidance.”
“The range of perceived benefits and potential differential effects of THC and cannabinoid highlight the need for personalized, evidence-based guidelines regarding cannabinoid use,” they wrote.
Stretching program for spasticity shows benefits
With spasticity representing a key contributor to MS pain and affecting more than 80% of people with MS, Ms. Hugos and colleagues are developing an alternative to medication – a nonpharmacologic stretching regimen called Spasticity: Take Control” (STC).
Based on evidence-based strategies for the treatment of spasticity in MS, the program involves exercises with daily routines of 15-20 minutes over 6 months.
In a pilot study of 66 patients, also presented at the ACTRIMS meeting, the investigators reported that the program showed significant reductions in pain severity and interference, measured with the Brief Pain Inventory–Short Form, compared with a control consisting of range of motion instruction over 6 months.
The study also offered insights on the specific areas of pain. Among those who reported chronic pain (42% in the STC group and 63.3% in the range-of-motion group), the pain was most frequently reported in the lower back (74.3%), legs (68.6%), or lower back and legs (88.6%).
Ms. Hugos noted that the findings suggest a potentially important nonpharmacologic alternative to spasticity-related pain in MS.
“Stretching is the cornerstone treatment for spasticity from all causes, but there is very little information on stretching exercises in MS or any other conditions,” Ms. Hugos said. “[Our] pilot study is the first and only study using a standardized, daily stretching exercise program to treat MS spasticity,” she said.
“A fully powered study is needed to better understand the impact of different types of exercise on pain severity and interference in multiple sclerosis,” she noted.
Ms. Hugos has received consulting fees from Greenwich Biosciences, Evidera, and Techspert.io. Dr. Cohen has received personal compensation for consulting for Adamas, Atara, Bristol-Myers Squibb, Convelo, MedDay, and Mylan.
A version of this article first appeared on Medscape.com.
FROM ACTRIMS FORUM 2021
Neurologic disorders ubiquitous and rising in the U.S.
, according to new findings derived from the 2017 Global Burden of Disease study.
The authors of the analysis, led by Valery Feigin, MD, PhD, of New Zealand’s National Institute for Stroke and Applied Neurosciences, and published in the February 2021 issue of JAMA Neurology, looked at prevalence, incidence, mortality, and disability-adjusted life years for 14 neurological disorders across 50 states between 1990 and 2017. The diseases included in the analysis were stroke, Alzheimer’s disease and other dementias, Parkinson’s disease, epilepsy, multiple sclerosis, motor neuron disease, headaches, traumatic brain injury, spinal cord injuries, brain and other nervous system cancers, meningitis, encephalitis, and tetanus.
Tracking the burden of neurologic diseases
Dr. Feigin and colleagues estimated that a full 60% of the U.S. population lives with one or more of these disorders, a figure much greater than previous estimates for neurological disease burden nationwide. Tension-type headache and migraine were the most prevalent in the analysis by Dr. Feigin and colleagues. During the study period, they found, prevalence, incidence, and disability burden of nearly all the included disorders increased, with the exception of brain and spinal cord injuries, meningitis, and encephalitis.
The researchers attributed most of the rise in noncommunicable neurological diseases to population aging. An age-standardized analysis found trends for stroke and Alzheimer’s disease and other dementias to be declining or flat. Age-standardized stroke incidence dropped by 16% from 1990 to 2017, while stroke mortality declined by nearly a third, and stroke disability by a quarter. Age-standardized incidence of Alzheimer’s disease and other dementias dropped by 12%, and their prevalence by 13%, during the study period, though dementia mortality and disability were seen increasing.
The authors surmised that the age-standardized declines in stroke and dementias could reflect that “primary prevention of these disorders are beginning to show an influence.” With dementia, which is linked to cognitive reserve and education, “improving educational levels of cohort reaching the age groups at greatest risk of disease may also be contributing to a modest decline over time,” Dr. Feigin and his colleagues wrote.
Parkinson’s disease and multiple sclerosis, meanwhile, were both seen rising in incidence, prevalence, and disability adjusted life years (DALYs) even with age-standardized figures. The United States saw comparatively more disability in 2017 from dementias, Parkinson’s disease, epilepsy, multiple sclerosis, motor neuron disease, and headache disorders, which together comprised 6.7% of DALYs, compared with 4.4% globally; these also accounted for a higher share of mortality in the U.S. than worldwide. The authors attributed at least some of the difference to better case ascertainment in the U.S.
Regional variations
The researchers also reported variations in disease burden by state and region. While previous studies have identified a “stroke belt” concentrated in North Carolina, South Carolina, and Georgia, the new findings point to stroke disability highest in Alabama, Arkansas, and Mississippi, and mortality highest in Alabama, Mississippi, and South Carolina. The researchers noted increases in dementia mortality in these states, “likely attributable to the reciprocal association between stroke and dementia.”
Northern states saw higher burdens of multiple sclerosis compared with the rest of the country, while eastern states had higher rates of Parkinson’s disease.
Such regional and state-by state variations, Dr. Feigin and colleagues wrote in their analysis, “may be associated with differences in the case ascertainment, as well as access to health care; racial/ethnic, genetic, and socioeconomic diversity; quality and comprehensiveness of preventive strategies; and risk factor distribution.”
The researchers noted as a limitation of their study that the 14 diseases captured were not an exhaustive list of neurological conditions; chronic lower back pain, a condition included in a previous major study of the burden of neurological disease in the United States, was omitted, as were restless legs syndrome and peripheral neuropathy. The researchers cited changes to coding practice in the U.S. and accuracy of medical claims data as potential limitations of their analysis. The Global Burden of Disease study is funded by the Bill and Melinda Gates Foundation, and several of Dr. Feigin’s coauthors reported financial relationships with industry.
Time to adjust the stroke belt?
Amelia Boehme, PhD, a stroke epidemiologist at Columbia University Mailman School of Public Health in New York, said in an interview that the current study added to recent findings showing surprising local variability in stroke prevalence, incidence, and mortality. “What we had always conceptually thought of as the ‘stroke belt’ isn’t necessarily the case,” Dr. Boehme said, but is rather subject to local, county-by-county variations. “Looking at the data here in conjunction with what previous authors have found, it raises some questions as to whether or not state-level data is giving a completely accurate picture, and whether we need to start looking at the county level and adjust for populations and age.” Importantly, Dr. Boehme said, data collected in the Global Burden of Disease study tends to be exceptionally rigorous and systematic, adding weight to Dr. Feigin and colleagues’ suggestions that prevention efforts may be making a dent in stroke and dementia.
“More data is always needed before we start to say we’re seeing things change,” Dr. Boehme noted. “But any glimmer of optimism is welcome, especially with regard to interventions that have been put in place, to allow us to build on those interventions.”
Dr. Boehme disclosed no financial conflicts of interest.
, according to new findings derived from the 2017 Global Burden of Disease study.
The authors of the analysis, led by Valery Feigin, MD, PhD, of New Zealand’s National Institute for Stroke and Applied Neurosciences, and published in the February 2021 issue of JAMA Neurology, looked at prevalence, incidence, mortality, and disability-adjusted life years for 14 neurological disorders across 50 states between 1990 and 2017. The diseases included in the analysis were stroke, Alzheimer’s disease and other dementias, Parkinson’s disease, epilepsy, multiple sclerosis, motor neuron disease, headaches, traumatic brain injury, spinal cord injuries, brain and other nervous system cancers, meningitis, encephalitis, and tetanus.
Tracking the burden of neurologic diseases
Dr. Feigin and colleagues estimated that a full 60% of the U.S. population lives with one or more of these disorders, a figure much greater than previous estimates for neurological disease burden nationwide. Tension-type headache and migraine were the most prevalent in the analysis by Dr. Feigin and colleagues. During the study period, they found, prevalence, incidence, and disability burden of nearly all the included disorders increased, with the exception of brain and spinal cord injuries, meningitis, and encephalitis.
The researchers attributed most of the rise in noncommunicable neurological diseases to population aging. An age-standardized analysis found trends for stroke and Alzheimer’s disease and other dementias to be declining or flat. Age-standardized stroke incidence dropped by 16% from 1990 to 2017, while stroke mortality declined by nearly a third, and stroke disability by a quarter. Age-standardized incidence of Alzheimer’s disease and other dementias dropped by 12%, and their prevalence by 13%, during the study period, though dementia mortality and disability were seen increasing.
The authors surmised that the age-standardized declines in stroke and dementias could reflect that “primary prevention of these disorders are beginning to show an influence.” With dementia, which is linked to cognitive reserve and education, “improving educational levels of cohort reaching the age groups at greatest risk of disease may also be contributing to a modest decline over time,” Dr. Feigin and his colleagues wrote.
Parkinson’s disease and multiple sclerosis, meanwhile, were both seen rising in incidence, prevalence, and disability adjusted life years (DALYs) even with age-standardized figures. The United States saw comparatively more disability in 2017 from dementias, Parkinson’s disease, epilepsy, multiple sclerosis, motor neuron disease, and headache disorders, which together comprised 6.7% of DALYs, compared with 4.4% globally; these also accounted for a higher share of mortality in the U.S. than worldwide. The authors attributed at least some of the difference to better case ascertainment in the U.S.
Regional variations
The researchers also reported variations in disease burden by state and region. While previous studies have identified a “stroke belt” concentrated in North Carolina, South Carolina, and Georgia, the new findings point to stroke disability highest in Alabama, Arkansas, and Mississippi, and mortality highest in Alabama, Mississippi, and South Carolina. The researchers noted increases in dementia mortality in these states, “likely attributable to the reciprocal association between stroke and dementia.”
Northern states saw higher burdens of multiple sclerosis compared with the rest of the country, while eastern states had higher rates of Parkinson’s disease.
Such regional and state-by state variations, Dr. Feigin and colleagues wrote in their analysis, “may be associated with differences in the case ascertainment, as well as access to health care; racial/ethnic, genetic, and socioeconomic diversity; quality and comprehensiveness of preventive strategies; and risk factor distribution.”
The researchers noted as a limitation of their study that the 14 diseases captured were not an exhaustive list of neurological conditions; chronic lower back pain, a condition included in a previous major study of the burden of neurological disease in the United States, was omitted, as were restless legs syndrome and peripheral neuropathy. The researchers cited changes to coding practice in the U.S. and accuracy of medical claims data as potential limitations of their analysis. The Global Burden of Disease study is funded by the Bill and Melinda Gates Foundation, and several of Dr. Feigin’s coauthors reported financial relationships with industry.
Time to adjust the stroke belt?
Amelia Boehme, PhD, a stroke epidemiologist at Columbia University Mailman School of Public Health in New York, said in an interview that the current study added to recent findings showing surprising local variability in stroke prevalence, incidence, and mortality. “What we had always conceptually thought of as the ‘stroke belt’ isn’t necessarily the case,” Dr. Boehme said, but is rather subject to local, county-by-county variations. “Looking at the data here in conjunction with what previous authors have found, it raises some questions as to whether or not state-level data is giving a completely accurate picture, and whether we need to start looking at the county level and adjust for populations and age.” Importantly, Dr. Boehme said, data collected in the Global Burden of Disease study tends to be exceptionally rigorous and systematic, adding weight to Dr. Feigin and colleagues’ suggestions that prevention efforts may be making a dent in stroke and dementia.
“More data is always needed before we start to say we’re seeing things change,” Dr. Boehme noted. “But any glimmer of optimism is welcome, especially with regard to interventions that have been put in place, to allow us to build on those interventions.”
Dr. Boehme disclosed no financial conflicts of interest.
, according to new findings derived from the 2017 Global Burden of Disease study.
The authors of the analysis, led by Valery Feigin, MD, PhD, of New Zealand’s National Institute for Stroke and Applied Neurosciences, and published in the February 2021 issue of JAMA Neurology, looked at prevalence, incidence, mortality, and disability-adjusted life years for 14 neurological disorders across 50 states between 1990 and 2017. The diseases included in the analysis were stroke, Alzheimer’s disease and other dementias, Parkinson’s disease, epilepsy, multiple sclerosis, motor neuron disease, headaches, traumatic brain injury, spinal cord injuries, brain and other nervous system cancers, meningitis, encephalitis, and tetanus.
Tracking the burden of neurologic diseases
Dr. Feigin and colleagues estimated that a full 60% of the U.S. population lives with one or more of these disorders, a figure much greater than previous estimates for neurological disease burden nationwide. Tension-type headache and migraine were the most prevalent in the analysis by Dr. Feigin and colleagues. During the study period, they found, prevalence, incidence, and disability burden of nearly all the included disorders increased, with the exception of brain and spinal cord injuries, meningitis, and encephalitis.
The researchers attributed most of the rise in noncommunicable neurological diseases to population aging. An age-standardized analysis found trends for stroke and Alzheimer’s disease and other dementias to be declining or flat. Age-standardized stroke incidence dropped by 16% from 1990 to 2017, while stroke mortality declined by nearly a third, and stroke disability by a quarter. Age-standardized incidence of Alzheimer’s disease and other dementias dropped by 12%, and their prevalence by 13%, during the study period, though dementia mortality and disability were seen increasing.
The authors surmised that the age-standardized declines in stroke and dementias could reflect that “primary prevention of these disorders are beginning to show an influence.” With dementia, which is linked to cognitive reserve and education, “improving educational levels of cohort reaching the age groups at greatest risk of disease may also be contributing to a modest decline over time,” Dr. Feigin and his colleagues wrote.
Parkinson’s disease and multiple sclerosis, meanwhile, were both seen rising in incidence, prevalence, and disability adjusted life years (DALYs) even with age-standardized figures. The United States saw comparatively more disability in 2017 from dementias, Parkinson’s disease, epilepsy, multiple sclerosis, motor neuron disease, and headache disorders, which together comprised 6.7% of DALYs, compared with 4.4% globally; these also accounted for a higher share of mortality in the U.S. than worldwide. The authors attributed at least some of the difference to better case ascertainment in the U.S.
Regional variations
The researchers also reported variations in disease burden by state and region. While previous studies have identified a “stroke belt” concentrated in North Carolina, South Carolina, and Georgia, the new findings point to stroke disability highest in Alabama, Arkansas, and Mississippi, and mortality highest in Alabama, Mississippi, and South Carolina. The researchers noted increases in dementia mortality in these states, “likely attributable to the reciprocal association between stroke and dementia.”
Northern states saw higher burdens of multiple sclerosis compared with the rest of the country, while eastern states had higher rates of Parkinson’s disease.
Such regional and state-by state variations, Dr. Feigin and colleagues wrote in their analysis, “may be associated with differences in the case ascertainment, as well as access to health care; racial/ethnic, genetic, and socioeconomic diversity; quality and comprehensiveness of preventive strategies; and risk factor distribution.”
The researchers noted as a limitation of their study that the 14 diseases captured were not an exhaustive list of neurological conditions; chronic lower back pain, a condition included in a previous major study of the burden of neurological disease in the United States, was omitted, as were restless legs syndrome and peripheral neuropathy. The researchers cited changes to coding practice in the U.S. and accuracy of medical claims data as potential limitations of their analysis. The Global Burden of Disease study is funded by the Bill and Melinda Gates Foundation, and several of Dr. Feigin’s coauthors reported financial relationships with industry.
Time to adjust the stroke belt?
Amelia Boehme, PhD, a stroke epidemiologist at Columbia University Mailman School of Public Health in New York, said in an interview that the current study added to recent findings showing surprising local variability in stroke prevalence, incidence, and mortality. “What we had always conceptually thought of as the ‘stroke belt’ isn’t necessarily the case,” Dr. Boehme said, but is rather subject to local, county-by-county variations. “Looking at the data here in conjunction with what previous authors have found, it raises some questions as to whether or not state-level data is giving a completely accurate picture, and whether we need to start looking at the county level and adjust for populations and age.” Importantly, Dr. Boehme said, data collected in the Global Burden of Disease study tends to be exceptionally rigorous and systematic, adding weight to Dr. Feigin and colleagues’ suggestions that prevention efforts may be making a dent in stroke and dementia.
“More data is always needed before we start to say we’re seeing things change,” Dr. Boehme noted. “But any glimmer of optimism is welcome, especially with regard to interventions that have been put in place, to allow us to build on those interventions.”
Dr. Boehme disclosed no financial conflicts of interest.
FROM JAMA NEUROLOGY
Adalimumab earns FDA approval for ulcerative colitis in children
Adalimumab has received approval from the Food and Drug Administration for use in pediatric patients aged 5 years and older with moderately to severely active ulcerative colitis (UC), according to a press release from manufacturer AbbVie.
Approval was based on data from the phase 3 ENVISION 1 randomized, double-blind study (NCT02065557) in which 60% of patients receiving the higher induction dose showed clinical remission according to Partial May Score (PMS) at 8 weeks, 45% of whom were in remission according to Full Mayo Score (FMS) at 1 year. The approval means that children with UC and their families have the option of a subcutaneous biologic that can be administered at home.
In the ENVISION 1 study, clinical remission was defined as a PMS (based on stool frequency, rectal bleeding, and physician’s global assessment) ranging from 0 to 3 at the end of the 8-week induction period or FMS (which adds endoscopy) at the end of 52 weeks as less than or equal to 2 points total, with no individual subscore greater than 1. The study included children aged 4-17 years with active UC who were randomized to a low-dose or high-dose group.
“Through week 8, patients in both dosage groups received 2.4 mg/kg (maximum of 160 mg) at week 0, 1.2 mg/kg (maximum of 80 mg) at week 2, and 0.6 mg/kg (maximum of 40 mg) at weeks 4 and 6. The higher-dosage group also received an additional dosage of 2.4 mg/kg (maximum of 160 mg) at week 1,” according to the company press release. Between weeks 8 and 52, patients received double-blind placebo or 0.6 mg/kg adalimumab (maximum of 40 mg) every other week (maintenance standard dose) or every week (maintenance high dose).
No new adalimumab safety signals were noted in the study; headache and worsening UC were the most frequently reported treatment-emergent adverse events, and 22.6% of patients experienced a serious adverse event. “No deaths, malignancies, active tuberculosis or demyelinating disease were observed in this study,” according to the company. Full prescribing information can be found on the FDA website.
Approval expands options
“The number of FDA-approved biologics to treat pediatric UC has been limited,” said Atsushi Sakuraba, MD, PhD, an inflammatory bowel disease specialist at the University of Chicago, in an interview. “Infliximab is approved for pediatric UC and CD [Crohn’s disease], but adalimumab was only approved for CD in pediatric patients, and vedolizumab and ustekinumab are approved for neither UC nor CD in pediatric patients,” he said. “Thus, the addition of adalimumab as a treatment option for pediatric UC is of great benefit for pediatricians and patients.”
The approval will impact clinical practice in several ways, Dr. Sakuraba said. “Adalimumab may be used for those who lose response to infliximab, but may also be used as a first-line biologic because it is self-injectable,” he emphasized. Given that adalimumab is already in use for pediatric patients with Crohn’s disease, Dr. Sakuraba said he does not see any barriers to implementation of its use for children with UC. In addition, “the potential for better disease control, reduction of disease-related complications, and improved quality of life outweighs the risk of adverse reactions,” Dr. Sakuraba said. “The news of no new safety concern in the 52-week study period further supports the safety of biologic treatment in pediatric patients,” he added.
As for additional research: “It remains to be determined whether combination therapy with an immunomodulator would be more effective than monotherapy, and also whether there are any additional risks of adverse events with the addition of immunomodulators such as long-term risk of malignancy, especially lymphoproliferative disorders,” Dr. Sakuraba noted. “Therapeutic options for pediatric [inflammatory bowel disease] are limited and lagging, compared to adult patients, so studies of non-TNF [tumor necrosis factor] biologics such vedolizumab and ustekinumab are also awaited,” he said.
Dr. Sakuraba had no financial conflicts to disclose.
This article was updated March 3, 2021.
Adalimumab has received approval from the Food and Drug Administration for use in pediatric patients aged 5 years and older with moderately to severely active ulcerative colitis (UC), according to a press release from manufacturer AbbVie.
Approval was based on data from the phase 3 ENVISION 1 randomized, double-blind study (NCT02065557) in which 60% of patients receiving the higher induction dose showed clinical remission according to Partial May Score (PMS) at 8 weeks, 45% of whom were in remission according to Full Mayo Score (FMS) at 1 year. The approval means that children with UC and their families have the option of a subcutaneous biologic that can be administered at home.
In the ENVISION 1 study, clinical remission was defined as a PMS (based on stool frequency, rectal bleeding, and physician’s global assessment) ranging from 0 to 3 at the end of the 8-week induction period or FMS (which adds endoscopy) at the end of 52 weeks as less than or equal to 2 points total, with no individual subscore greater than 1. The study included children aged 4-17 years with active UC who were randomized to a low-dose or high-dose group.
“Through week 8, patients in both dosage groups received 2.4 mg/kg (maximum of 160 mg) at week 0, 1.2 mg/kg (maximum of 80 mg) at week 2, and 0.6 mg/kg (maximum of 40 mg) at weeks 4 and 6. The higher-dosage group also received an additional dosage of 2.4 mg/kg (maximum of 160 mg) at week 1,” according to the company press release. Between weeks 8 and 52, patients received double-blind placebo or 0.6 mg/kg adalimumab (maximum of 40 mg) every other week (maintenance standard dose) or every week (maintenance high dose).
No new adalimumab safety signals were noted in the study; headache and worsening UC were the most frequently reported treatment-emergent adverse events, and 22.6% of patients experienced a serious adverse event. “No deaths, malignancies, active tuberculosis or demyelinating disease were observed in this study,” according to the company. Full prescribing information can be found on the FDA website.
Approval expands options
“The number of FDA-approved biologics to treat pediatric UC has been limited,” said Atsushi Sakuraba, MD, PhD, an inflammatory bowel disease specialist at the University of Chicago, in an interview. “Infliximab is approved for pediatric UC and CD [Crohn’s disease], but adalimumab was only approved for CD in pediatric patients, and vedolizumab and ustekinumab are approved for neither UC nor CD in pediatric patients,” he said. “Thus, the addition of adalimumab as a treatment option for pediatric UC is of great benefit for pediatricians and patients.”
The approval will impact clinical practice in several ways, Dr. Sakuraba said. “Adalimumab may be used for those who lose response to infliximab, but may also be used as a first-line biologic because it is self-injectable,” he emphasized. Given that adalimumab is already in use for pediatric patients with Crohn’s disease, Dr. Sakuraba said he does not see any barriers to implementation of its use for children with UC. In addition, “the potential for better disease control, reduction of disease-related complications, and improved quality of life outweighs the risk of adverse reactions,” Dr. Sakuraba said. “The news of no new safety concern in the 52-week study period further supports the safety of biologic treatment in pediatric patients,” he added.
As for additional research: “It remains to be determined whether combination therapy with an immunomodulator would be more effective than monotherapy, and also whether there are any additional risks of adverse events with the addition of immunomodulators such as long-term risk of malignancy, especially lymphoproliferative disorders,” Dr. Sakuraba noted. “Therapeutic options for pediatric [inflammatory bowel disease] are limited and lagging, compared to adult patients, so studies of non-TNF [tumor necrosis factor] biologics such vedolizumab and ustekinumab are also awaited,” he said.
Dr. Sakuraba had no financial conflicts to disclose.
This article was updated March 3, 2021.
Adalimumab has received approval from the Food and Drug Administration for use in pediatric patients aged 5 years and older with moderately to severely active ulcerative colitis (UC), according to a press release from manufacturer AbbVie.
Approval was based on data from the phase 3 ENVISION 1 randomized, double-blind study (NCT02065557) in which 60% of patients receiving the higher induction dose showed clinical remission according to Partial May Score (PMS) at 8 weeks, 45% of whom were in remission according to Full Mayo Score (FMS) at 1 year. The approval means that children with UC and their families have the option of a subcutaneous biologic that can be administered at home.
In the ENVISION 1 study, clinical remission was defined as a PMS (based on stool frequency, rectal bleeding, and physician’s global assessment) ranging from 0 to 3 at the end of the 8-week induction period or FMS (which adds endoscopy) at the end of 52 weeks as less than or equal to 2 points total, with no individual subscore greater than 1. The study included children aged 4-17 years with active UC who were randomized to a low-dose or high-dose group.
“Through week 8, patients in both dosage groups received 2.4 mg/kg (maximum of 160 mg) at week 0, 1.2 mg/kg (maximum of 80 mg) at week 2, and 0.6 mg/kg (maximum of 40 mg) at weeks 4 and 6. The higher-dosage group also received an additional dosage of 2.4 mg/kg (maximum of 160 mg) at week 1,” according to the company press release. Between weeks 8 and 52, patients received double-blind placebo or 0.6 mg/kg adalimumab (maximum of 40 mg) every other week (maintenance standard dose) or every week (maintenance high dose).
No new adalimumab safety signals were noted in the study; headache and worsening UC were the most frequently reported treatment-emergent adverse events, and 22.6% of patients experienced a serious adverse event. “No deaths, malignancies, active tuberculosis or demyelinating disease were observed in this study,” according to the company. Full prescribing information can be found on the FDA website.
Approval expands options
“The number of FDA-approved biologics to treat pediatric UC has been limited,” said Atsushi Sakuraba, MD, PhD, an inflammatory bowel disease specialist at the University of Chicago, in an interview. “Infliximab is approved for pediatric UC and CD [Crohn’s disease], but adalimumab was only approved for CD in pediatric patients, and vedolizumab and ustekinumab are approved for neither UC nor CD in pediatric patients,” he said. “Thus, the addition of adalimumab as a treatment option for pediatric UC is of great benefit for pediatricians and patients.”
The approval will impact clinical practice in several ways, Dr. Sakuraba said. “Adalimumab may be used for those who lose response to infliximab, but may also be used as a first-line biologic because it is self-injectable,” he emphasized. Given that adalimumab is already in use for pediatric patients with Crohn’s disease, Dr. Sakuraba said he does not see any barriers to implementation of its use for children with UC. In addition, “the potential for better disease control, reduction of disease-related complications, and improved quality of life outweighs the risk of adverse reactions,” Dr. Sakuraba said. “The news of no new safety concern in the 52-week study period further supports the safety of biologic treatment in pediatric patients,” he added.
As for additional research: “It remains to be determined whether combination therapy with an immunomodulator would be more effective than monotherapy, and also whether there are any additional risks of adverse events with the addition of immunomodulators such as long-term risk of malignancy, especially lymphoproliferative disorders,” Dr. Sakuraba noted. “Therapeutic options for pediatric [inflammatory bowel disease] are limited and lagging, compared to adult patients, so studies of non-TNF [tumor necrosis factor] biologics such vedolizumab and ustekinumab are also awaited,” he said.
Dr. Sakuraba had no financial conflicts to disclose.
This article was updated March 3, 2021.
Decline in children’s COVID-19 cases slows
The number of new COVID-19 cases in children declined for the sixth consecutive week, but the drop was the smallest yet, according to a report from the American Academy of Pediatrics and the Children’s Hospital Association.
That drop of almost 6,400 cases, or 9.0%, falls short of the declines recorded in any the previous 5 weeks, which ranged from 18,000 to 46,000 cases and 15.3% to 28.7%, based on data from the heath departments of 49 states (excluding New York), as well as the District of Columbia, New York City, Puerto Rico, and Guam.
The total number of children infected with SARS-CoV-2 is up to almost 3.17 million, which represents 13.1% of cases among all age groups. That cumulative proportion was unchanged from the previous week, which has occurred only three other times over the course of the pandemic, the AAP and CHA said in their weekly COVID-19 report.
Despite the 6-week decline in new cases, however, the cumulative rate continued to climb, rising from 4,124 cases per 100,000 children to 4,209 for the week of Feb. 19-25. The states, not surprisingly, fall on both sides of that national tally. The lowest rates can be found in Hawaii (1,040 per 100,000 children), Vermont (2,111 per 100,000), and Maine (2,394), while the highest rates were recorded in North Dakota (8,580), Tennessee (7,851), and Rhode Island (7,223), the AAP and CHA said.
The number of new child deaths, nine, stayed in single digits for a second consecutive week, although it was up from six deaths reported a week earlier. Total COVID-19–related deaths in children now number 256, which represents just 0.06% of coronavirus deaths for all ages among the 43 states (along with New York City and Guam) reporting such data.
Among those jurisdictions, Texas (40), Arizona (27), and New York City (23) have reported the most deaths in children, while nine states and the District of Columbia have reported no deaths yet, the AAP and CHA noted.
The number of new COVID-19 cases in children declined for the sixth consecutive week, but the drop was the smallest yet, according to a report from the American Academy of Pediatrics and the Children’s Hospital Association.

That drop of almost 6,400 cases, or 9.0%, falls short of the declines recorded in any the previous 5 weeks, which ranged from 18,000 to 46,000 cases and 15.3% to 28.7%, based on data from the heath departments of 49 states (excluding New York), as well as the District of Columbia, New York City, Puerto Rico, and Guam.
The total number of children infected with SARS-CoV-2 is up to almost 3.17 million, which represents 13.1% of cases among all age groups. That cumulative proportion was unchanged from the previous week, which has occurred only three other times over the course of the pandemic, the AAP and CHA said in their weekly COVID-19 report.
Despite the 6-week decline in new cases, however, the cumulative rate continued to climb, rising from 4,124 cases per 100,000 children to 4,209 for the week of Feb. 19-25. The states, not surprisingly, fall on both sides of that national tally. The lowest rates can be found in Hawaii (1,040 per 100,000 children), Vermont (2,111 per 100,000), and Maine (2,394), while the highest rates were recorded in North Dakota (8,580), Tennessee (7,851), and Rhode Island (7,223), the AAP and CHA said.
The number of new child deaths, nine, stayed in single digits for a second consecutive week, although it was up from six deaths reported a week earlier. Total COVID-19–related deaths in children now number 256, which represents just 0.06% of coronavirus deaths for all ages among the 43 states (along with New York City and Guam) reporting such data.
Among those jurisdictions, Texas (40), Arizona (27), and New York City (23) have reported the most deaths in children, while nine states and the District of Columbia have reported no deaths yet, the AAP and CHA noted.
The number of new COVID-19 cases in children declined for the sixth consecutive week, but the drop was the smallest yet, according to a report from the American Academy of Pediatrics and the Children’s Hospital Association.

That drop of almost 6,400 cases, or 9.0%, falls short of the declines recorded in any the previous 5 weeks, which ranged from 18,000 to 46,000 cases and 15.3% to 28.7%, based on data from the heath departments of 49 states (excluding New York), as well as the District of Columbia, New York City, Puerto Rico, and Guam.
The total number of children infected with SARS-CoV-2 is up to almost 3.17 million, which represents 13.1% of cases among all age groups. That cumulative proportion was unchanged from the previous week, which has occurred only three other times over the course of the pandemic, the AAP and CHA said in their weekly COVID-19 report.
Despite the 6-week decline in new cases, however, the cumulative rate continued to climb, rising from 4,124 cases per 100,000 children to 4,209 for the week of Feb. 19-25. The states, not surprisingly, fall on both sides of that national tally. The lowest rates can be found in Hawaii (1,040 per 100,000 children), Vermont (2,111 per 100,000), and Maine (2,394), while the highest rates were recorded in North Dakota (8,580), Tennessee (7,851), and Rhode Island (7,223), the AAP and CHA said.
The number of new child deaths, nine, stayed in single digits for a second consecutive week, although it was up from six deaths reported a week earlier. Total COVID-19–related deaths in children now number 256, which represents just 0.06% of coronavirus deaths for all ages among the 43 states (along with New York City and Guam) reporting such data.
Among those jurisdictions, Texas (40), Arizona (27), and New York City (23) have reported the most deaths in children, while nine states and the District of Columbia have reported no deaths yet, the AAP and CHA noted.
FDA places clinical hold on sickle cell gene therapy
The Food and Drug Administration placed a clinical hold yesterday on two gene therapy trials for sickle cell disease (SCD) after two recent complications: one participant developed acute myeloid leukemia (AML) and another developed myelodysplastic syndrome (MDS). The sponsoring company, bluebird bio, suspended the trials last week upon learning of the cases.
The company has also put the brakes on a treatment for beta thalassemia already approved in the European Union and the United Kingdom, betibeglogene autotemcel (Zynteglo). The treatment hasn’t been associated with problems but uses the same gene delivery vector, a lentivirus, as that used in the SCD trials.
Overall, the company has enrolled 47 SCD patients and 63 with beta thalassemia in trials.
The gene therapy “space” is one with spectacular successes – for a form of retinal blindness and spinal muscular atrophy – rising against a backdrop of recent setbacks and failures – for Duchenne muscular dystrophy, lipoprotein lipase deficiency, and myotubular myopathy.
A lentiviral vector
The retooled lentivirus used in the SCD trials, LentiGlobin, delivers a beta-globin gene with one amino acid replacement to hematopoietic stem cells outside the patient’s body. The modified cells are then infused back into the patient. The gene therapy reshapes red blood cells, enabling them to circulate through narrow blood vessels without sickling and adhering into painful logjams.
What is worrisome is that in the patient who developed AML, and who received the gene therapy more than 5 years ago, the cancer cells contained the vector. Those test results aren’t yet available for the participant who has MDS.
The finding raises suspicion that the gene therapy had a role in the cancer, but is only correlative.
Lentiviral vectors have a good track record, but the two cases evoke memories of 2 decades ago. In 2001, five children being treated for an inherited immunodeficiency (SCID-X1) with a gamma retroviral vector developed leukemia and one died. Those viruses inserted into an oncogene. Happening 2 years after the death of 18-year-old Jesse Gelsinger in another gene therapy trial, the SCID trial had a chilling effect on the field.
Since then, lentiviral vectors have been reinvented to be “self-inactivating,” minimizing the risk for inserting willy-nilly into a genome. “Lentiviral vectors have been expressly designed to avoid insertional oncogenesis, based on prior experience with the gamma retroviruses. We don’t have evidence that the vector is causative, but our studies will shed some light on whether that’s true in these cases,” said bluebird bio chief scientific officer Philip Gregory, DPhil, on a conference call Feb. 16.
Lentiviral vectors have been successful as the backbone of chimeric antigen receptor T-cell (CAR-T) therapy, which directs modified T cells to certain blood cancers. “Among the hundreds to thousands of patients treated with CAR-T cell therapy, lentivirus vector hasn’t been associated with any malignancies,” said bluebird’s chief medical officer, Dave Davidson, MD.
Jeanne Loring, PhD, director of the Center for Regenerative Medicine at Scripps Research, agreed. “Gene therapy is having some extreme highs and lows these days. Most studies use [adeno-associated viral] vectors, which don’t integrate into the genome. But some people have antibodies to AAV vectors, and AAV is diluted out when cells divide. That’s why lentivirus, which integrates into the genome, is used for blood stem cells and T cells in CAR-T therapy.”
Pinpointing causality
At bluebird bio, investigation into the possible “genetic gymnastics” of the lentivirus vector is focusing on where it integrates into the genome – whether it harpoons an oncogene like the gamma retroviral vectors, or affects genome stability, Dr. Gregory explained. To be causative, the affected gene must be a “driver” of the cancer, and not just a “passenger.”
Another suspect is busulfan, a drug used to “condition” the recipient’s bone marrow, making room for modified stem cells. “It’s possible that busulfan is the main problem, as it is a carcinogen unto itself,” said Paul Knoepfler, PhD, a stem cell researcher at the University of California, Davis.
In addition to the two more recent reports of complications, a third trial participant, who had participated in a phase 1/2 trial, developed MDS in 2018 and died of AML in July 2020. The cancer cells from that patient did not contain viral vectors and the MDS was attributed to busulfan conditioning.
Nick Leschly, chief of bluebird, pointed out the importance of clinical context in implicating the vector. Because SCD itself stresses the bone marrow, patients already face an increased risk of developing blood cancer, he said. “Now layer on other risks of the gene therapy. It’s challenging because we’re dealing with patients who have life expectancy in the mid 40s.” Previous treatments, such the antisickling drug hydroxyurea, may also contribute to patient vulnerability.
A patient’s view
SCD affects more than 100,000 people in the United States, and about 20 million globally. Charles Hough is one of them. He can attest to the severity of the disease as well as the promise of gene therapy
Mr. Hough was diagnosed at age 2, and endured the profound fatigue, pain crises, and even coma characteristic of severe cases. He cited his “rebirth” as Sept. 25, 2018, when he received his first modified stem cells at the National Institutes of Health. Mr. Hough told his story a year ago in a webinar for the National Organization for Rare Disorders. This news organization caught up with him in light of the clinical trial hold.
Although the preparative regimens for the gene therapy were tough, his sickle cell symptoms vanished after gene therapy. Even hearing about the current hold on the clinical trial, Mr. Hough doesn’t regret his participation.
“I had a lot of friends who passed because of the complications from sickle cell. I was always worried that I wouldn’t live to see the next day. Now I don’t have that stress hanging over my head and I feel like I can live a normal life. Becoming sickle cell free was my dream.”
A version of this article first appeared on Medscape.com.
The Food and Drug Administration placed a clinical hold yesterday on two gene therapy trials for sickle cell disease (SCD) after two recent complications: one participant developed acute myeloid leukemia (AML) and another developed myelodysplastic syndrome (MDS). The sponsoring company, bluebird bio, suspended the trials last week upon learning of the cases.
The company has also put the brakes on a treatment for beta thalassemia already approved in the European Union and the United Kingdom, betibeglogene autotemcel (Zynteglo). The treatment hasn’t been associated with problems but uses the same gene delivery vector, a lentivirus, as that used in the SCD trials.
Overall, the company has enrolled 47 SCD patients and 63 with beta thalassemia in trials.
The gene therapy “space” is one with spectacular successes – for a form of retinal blindness and spinal muscular atrophy – rising against a backdrop of recent setbacks and failures – for Duchenne muscular dystrophy, lipoprotein lipase deficiency, and myotubular myopathy.
A lentiviral vector
The retooled lentivirus used in the SCD trials, LentiGlobin, delivers a beta-globin gene with one amino acid replacement to hematopoietic stem cells outside the patient’s body. The modified cells are then infused back into the patient. The gene therapy reshapes red blood cells, enabling them to circulate through narrow blood vessels without sickling and adhering into painful logjams.
What is worrisome is that in the patient who developed AML, and who received the gene therapy more than 5 years ago, the cancer cells contained the vector. Those test results aren’t yet available for the participant who has MDS.
The finding raises suspicion that the gene therapy had a role in the cancer, but is only correlative.
Lentiviral vectors have a good track record, but the two cases evoke memories of 2 decades ago. In 2001, five children being treated for an inherited immunodeficiency (SCID-X1) with a gamma retroviral vector developed leukemia and one died. Those viruses inserted into an oncogene. Happening 2 years after the death of 18-year-old Jesse Gelsinger in another gene therapy trial, the SCID trial had a chilling effect on the field.
Since then, lentiviral vectors have been reinvented to be “self-inactivating,” minimizing the risk for inserting willy-nilly into a genome. “Lentiviral vectors have been expressly designed to avoid insertional oncogenesis, based on prior experience with the gamma retroviruses. We don’t have evidence that the vector is causative, but our studies will shed some light on whether that’s true in these cases,” said bluebird bio chief scientific officer Philip Gregory, DPhil, on a conference call Feb. 16.
Lentiviral vectors have been successful as the backbone of chimeric antigen receptor T-cell (CAR-T) therapy, which directs modified T cells to certain blood cancers. “Among the hundreds to thousands of patients treated with CAR-T cell therapy, lentivirus vector hasn’t been associated with any malignancies,” said bluebird’s chief medical officer, Dave Davidson, MD.
Jeanne Loring, PhD, director of the Center for Regenerative Medicine at Scripps Research, agreed. “Gene therapy is having some extreme highs and lows these days. Most studies use [adeno-associated viral] vectors, which don’t integrate into the genome. But some people have antibodies to AAV vectors, and AAV is diluted out when cells divide. That’s why lentivirus, which integrates into the genome, is used for blood stem cells and T cells in CAR-T therapy.”
Pinpointing causality
At bluebird bio, investigation into the possible “genetic gymnastics” of the lentivirus vector is focusing on where it integrates into the genome – whether it harpoons an oncogene like the gamma retroviral vectors, or affects genome stability, Dr. Gregory explained. To be causative, the affected gene must be a “driver” of the cancer, and not just a “passenger.”
Another suspect is busulfan, a drug used to “condition” the recipient’s bone marrow, making room for modified stem cells. “It’s possible that busulfan is the main problem, as it is a carcinogen unto itself,” said Paul Knoepfler, PhD, a stem cell researcher at the University of California, Davis.
In addition to the two more recent reports of complications, a third trial participant, who had participated in a phase 1/2 trial, developed MDS in 2018 and died of AML in July 2020. The cancer cells from that patient did not contain viral vectors and the MDS was attributed to busulfan conditioning.
Nick Leschly, chief of bluebird, pointed out the importance of clinical context in implicating the vector. Because SCD itself stresses the bone marrow, patients already face an increased risk of developing blood cancer, he said. “Now layer on other risks of the gene therapy. It’s challenging because we’re dealing with patients who have life expectancy in the mid 40s.” Previous treatments, such the antisickling drug hydroxyurea, may also contribute to patient vulnerability.
A patient’s view
SCD affects more than 100,000 people in the United States, and about 20 million globally. Charles Hough is one of them. He can attest to the severity of the disease as well as the promise of gene therapy
Mr. Hough was diagnosed at age 2, and endured the profound fatigue, pain crises, and even coma characteristic of severe cases. He cited his “rebirth” as Sept. 25, 2018, when he received his first modified stem cells at the National Institutes of Health. Mr. Hough told his story a year ago in a webinar for the National Organization for Rare Disorders. This news organization caught up with him in light of the clinical trial hold.
Although the preparative regimens for the gene therapy were tough, his sickle cell symptoms vanished after gene therapy. Even hearing about the current hold on the clinical trial, Mr. Hough doesn’t regret his participation.
“I had a lot of friends who passed because of the complications from sickle cell. I was always worried that I wouldn’t live to see the next day. Now I don’t have that stress hanging over my head and I feel like I can live a normal life. Becoming sickle cell free was my dream.”
A version of this article first appeared on Medscape.com.
The Food and Drug Administration placed a clinical hold yesterday on two gene therapy trials for sickle cell disease (SCD) after two recent complications: one participant developed acute myeloid leukemia (AML) and another developed myelodysplastic syndrome (MDS). The sponsoring company, bluebird bio, suspended the trials last week upon learning of the cases.
The company has also put the brakes on a treatment for beta thalassemia already approved in the European Union and the United Kingdom, betibeglogene autotemcel (Zynteglo). The treatment hasn’t been associated with problems but uses the same gene delivery vector, a lentivirus, as that used in the SCD trials.
Overall, the company has enrolled 47 SCD patients and 63 with beta thalassemia in trials.
The gene therapy “space” is one with spectacular successes – for a form of retinal blindness and spinal muscular atrophy – rising against a backdrop of recent setbacks and failures – for Duchenne muscular dystrophy, lipoprotein lipase deficiency, and myotubular myopathy.
A lentiviral vector
The retooled lentivirus used in the SCD trials, LentiGlobin, delivers a beta-globin gene with one amino acid replacement to hematopoietic stem cells outside the patient’s body. The modified cells are then infused back into the patient. The gene therapy reshapes red blood cells, enabling them to circulate through narrow blood vessels without sickling and adhering into painful logjams.
What is worrisome is that in the patient who developed AML, and who received the gene therapy more than 5 years ago, the cancer cells contained the vector. Those test results aren’t yet available for the participant who has MDS.
The finding raises suspicion that the gene therapy had a role in the cancer, but is only correlative.
Lentiviral vectors have a good track record, but the two cases evoke memories of 2 decades ago. In 2001, five children being treated for an inherited immunodeficiency (SCID-X1) with a gamma retroviral vector developed leukemia and one died. Those viruses inserted into an oncogene. Happening 2 years after the death of 18-year-old Jesse Gelsinger in another gene therapy trial, the SCID trial had a chilling effect on the field.
Since then, lentiviral vectors have been reinvented to be “self-inactivating,” minimizing the risk for inserting willy-nilly into a genome. “Lentiviral vectors have been expressly designed to avoid insertional oncogenesis, based on prior experience with the gamma retroviruses. We don’t have evidence that the vector is causative, but our studies will shed some light on whether that’s true in these cases,” said bluebird bio chief scientific officer Philip Gregory, DPhil, on a conference call Feb. 16.
Lentiviral vectors have been successful as the backbone of chimeric antigen receptor T-cell (CAR-T) therapy, which directs modified T cells to certain blood cancers. “Among the hundreds to thousands of patients treated with CAR-T cell therapy, lentivirus vector hasn’t been associated with any malignancies,” said bluebird’s chief medical officer, Dave Davidson, MD.
Jeanne Loring, PhD, director of the Center for Regenerative Medicine at Scripps Research, agreed. “Gene therapy is having some extreme highs and lows these days. Most studies use [adeno-associated viral] vectors, which don’t integrate into the genome. But some people have antibodies to AAV vectors, and AAV is diluted out when cells divide. That’s why lentivirus, which integrates into the genome, is used for blood stem cells and T cells in CAR-T therapy.”
Pinpointing causality
At bluebird bio, investigation into the possible “genetic gymnastics” of the lentivirus vector is focusing on where it integrates into the genome – whether it harpoons an oncogene like the gamma retroviral vectors, or affects genome stability, Dr. Gregory explained. To be causative, the affected gene must be a “driver” of the cancer, and not just a “passenger.”
Another suspect is busulfan, a drug used to “condition” the recipient’s bone marrow, making room for modified stem cells. “It’s possible that busulfan is the main problem, as it is a carcinogen unto itself,” said Paul Knoepfler, PhD, a stem cell researcher at the University of California, Davis.
In addition to the two more recent reports of complications, a third trial participant, who had participated in a phase 1/2 trial, developed MDS in 2018 and died of AML in July 2020. The cancer cells from that patient did not contain viral vectors and the MDS was attributed to busulfan conditioning.
Nick Leschly, chief of bluebird, pointed out the importance of clinical context in implicating the vector. Because SCD itself stresses the bone marrow, patients already face an increased risk of developing blood cancer, he said. “Now layer on other risks of the gene therapy. It’s challenging because we’re dealing with patients who have life expectancy in the mid 40s.” Previous treatments, such the antisickling drug hydroxyurea, may also contribute to patient vulnerability.
A patient’s view
SCD affects more than 100,000 people in the United States, and about 20 million globally. Charles Hough is one of them. He can attest to the severity of the disease as well as the promise of gene therapy
Mr. Hough was diagnosed at age 2, and endured the profound fatigue, pain crises, and even coma characteristic of severe cases. He cited his “rebirth” as Sept. 25, 2018, when he received his first modified stem cells at the National Institutes of Health. Mr. Hough told his story a year ago in a webinar for the National Organization for Rare Disorders. This news organization caught up with him in light of the clinical trial hold.
Although the preparative regimens for the gene therapy were tough, his sickle cell symptoms vanished after gene therapy. Even hearing about the current hold on the clinical trial, Mr. Hough doesn’t regret his participation.
“I had a lot of friends who passed because of the complications from sickle cell. I was always worried that I wouldn’t live to see the next day. Now I don’t have that stress hanging over my head and I feel like I can live a normal life. Becoming sickle cell free was my dream.”
A version of this article first appeared on Medscape.com.
Thirteen percent of patients with type 2 diabetes have major ECG abnormalities
Major ECG abnormalities were found in 13% of more than 8,000 unselected patients with type 2 diabetes, including a 9% prevalence in the subgroup of these patients without identified cardiovascular disease (CVD) in a community-based Dutch cohort. Minor ECG abnormalities were even more prevalent.
These prevalence rates were consistent with prior findings from patients with type 2 diabetes, but the current report is notable because “it provides the most thorough description of the prevalence of ECG abnormalities in people with type 2 diabetes,” and used an “unselected and large population with comprehensive measurements,” including many without a history of CVD, said Peter P. Harms, MSc, and associates noted in a recent report in the Journal of Diabetes and Its Complications.
The analysis also identified several parameters that significantly linked with the presence of a major ECG abnormality including hypertension, male sex, older age, and higher levels of hemoglobin A1c.
“Resting ECG abnormalities might be a useful tool for CVD screening in people with type 2 diabetes,” concluded Mr. Harms, a researcher at the Amsterdam University Medical Center, and coauthors.
Findings “not unexpected”
Patients with diabetes have a higher prevalence of ECG abnormalities “because of their higher likelihood of having hypertension and other CVD risk factors,” as well as potentially having subclinical CVD, said Fred M. Kusumoto, MD, so these findings are “not unexpected. The more risk factors a patient has for structural heart disease, atrial fibrillation (AFib), or stroke from AFib, the more a physician must consider whether a baseline ECG and future surveillance is appropriate,” Dr. Kusumoto said in an interview.
But he cautioned against seeing these findings as a rationale to routinely run a resting ECG examination on every adult with diabetes.
“Patients with diabetes are very heterogeneous,” which makes it “difficult to come up with a ‘one size fits all’ recommendation” for ECG screening of patients with diabetes, he said.
While a task force of the European Society of Cardiology and the European Association for the Study of Diabetes set a class I level C guideline for resting ECG screening of patients with diabetes if they also have either hypertension or suspected CVD, the American Diabetes Association has no specific recommendations on which patients with diabetes should receive ECG screening.
“The current absence of U.S. recommendations is reasonable, as it allows patients and physicians to discuss the issues and decide on the utility of an ECG in their specific situation,” said Dr. Kusumoto, director of heart rhythm services at the Mayo Clinic in Jacksonville, Fla. But he also suggested that “the more risk factors that a patient with diabetes has for structural heart disease, AFib, or stroke from AFib the more a physician must consider whether a baseline ECG and future surveillance is appropriate.”
Data from a Dutch prospective cohort
The new study used data collected from 8,068 patients with type 2 diabetes and enrolled in the prospective Hoorn Diabetes Care System cohort, which enrolled patients newly diagnosed with type 2 diabetes in the West Friesland region of the Netherlands starting in 1996. The study includes most of these patients in the region who are under regular care of a general practitioner, and the study protocol calls for an annual resting ECG examination.
The investigators used standard, 12-lead ECG readings taken for each patient during 2018, and classified abnormalities by the Minnesota Code criteria. They divided the abnormalities into major or minor groups “in accordance with consensus between previous studies who categorised abnormalities according to perceived importance and/or severity.” The major subgroup included major QS pattern abnormalities, major ST-segment abnormalities, complete left bundle branch block or intraventricular block, or atrial fibrillation or flutter. Minor abnormalities included minor QS pattern abnormalities, minor ST-segment abnormalities, complete right bundle branch block, or premature atrial or ventricular contractions.
The prevalence of a major abnormality in the entire cohort examined was 13%, and another 16% had a minor abnormality. The most common types of abnormalities were ventricular conduction defects, in 14%; and arrhythmias, in 11%. In the subgroup of 6,494 of these patients with no history of CVD, 9% had a major abnormality and 15% a minor abnormality. Within this subgroup, 23% also had no hypertension, and their prevalence of a major abnormality was 4%, while 9% had a minor abnormality.
A multivariable analysis of potential risk factors among the entire study cohort showed that patients with hypertension had nearly triple the prevalence of a major ECG abnormality as those without hypertension, and men had double the prevalence of a major abnormality compared with women. Other markers that significantly linked with a higher rate of a major abnormality were older age, higher body mass index, higher A1c levels, and moderately depressed renal function.
“While the criteria the authors used for differentiating major and minor criteria are reasonable, in an asymptomatic patient even the presence of frequent premature atrial contractions on a baseline ECG has been associated with the development of AFib and a higher risk for stroke. The presence of left or right bundle branch block could spur additional evaluation with an echocardiogram,” said Dr. Kusumoto, president-elect of the Heart Rhythm Society.
“Generally an ECG abnormality is supplemental to clinical data in deciding the choice and timing of next therapeutic steps or additional testing. Physicians should have a fairly low threshold for obtaining ECG in patients with diabetes since it is inexpensive and can provide supplemental and potentially actionable information,” he said. “The presence of ECG abnormalities increases the possibility of underlying cardiovascular disease. When taking care of patients with diabetes at initial evaluation or without prior cardiac history or symptoms referable to the heart, two main issues are identifying the likelihood of coronary artery disease and atrial fibrillation.”
Mr. Harms and coauthors, and Dr. Kusumoto, had no disclosures.
Major ECG abnormalities were found in 13% of more than 8,000 unselected patients with type 2 diabetes, including a 9% prevalence in the subgroup of these patients without identified cardiovascular disease (CVD) in a community-based Dutch cohort. Minor ECG abnormalities were even more prevalent.
These prevalence rates were consistent with prior findings from patients with type 2 diabetes, but the current report is notable because “it provides the most thorough description of the prevalence of ECG abnormalities in people with type 2 diabetes,” and used an “unselected and large population with comprehensive measurements,” including many without a history of CVD, said Peter P. Harms, MSc, and associates noted in a recent report in the Journal of Diabetes and Its Complications.
The analysis also identified several parameters that significantly linked with the presence of a major ECG abnormality including hypertension, male sex, older age, and higher levels of hemoglobin A1c.
“Resting ECG abnormalities might be a useful tool for CVD screening in people with type 2 diabetes,” concluded Mr. Harms, a researcher at the Amsterdam University Medical Center, and coauthors.
Findings “not unexpected”
Patients with diabetes have a higher prevalence of ECG abnormalities “because of their higher likelihood of having hypertension and other CVD risk factors,” as well as potentially having subclinical CVD, said Fred M. Kusumoto, MD, so these findings are “not unexpected. The more risk factors a patient has for structural heart disease, atrial fibrillation (AFib), or stroke from AFib, the more a physician must consider whether a baseline ECG and future surveillance is appropriate,” Dr. Kusumoto said in an interview.
But he cautioned against seeing these findings as a rationale to routinely run a resting ECG examination on every adult with diabetes.
“Patients with diabetes are very heterogeneous,” which makes it “difficult to come up with a ‘one size fits all’ recommendation” for ECG screening of patients with diabetes, he said.
While a task force of the European Society of Cardiology and the European Association for the Study of Diabetes set a class I level C guideline for resting ECG screening of patients with diabetes if they also have either hypertension or suspected CVD, the American Diabetes Association has no specific recommendations on which patients with diabetes should receive ECG screening.
“The current absence of U.S. recommendations is reasonable, as it allows patients and physicians to discuss the issues and decide on the utility of an ECG in their specific situation,” said Dr. Kusumoto, director of heart rhythm services at the Mayo Clinic in Jacksonville, Fla. But he also suggested that “the more risk factors that a patient with diabetes has for structural heart disease, AFib, or stroke from AFib the more a physician must consider whether a baseline ECG and future surveillance is appropriate.”
Data from a Dutch prospective cohort
The new study used data collected from 8,068 patients with type 2 diabetes and enrolled in the prospective Hoorn Diabetes Care System cohort, which enrolled patients newly diagnosed with type 2 diabetes in the West Friesland region of the Netherlands starting in 1996. The study includes most of these patients in the region who are under regular care of a general practitioner, and the study protocol calls for an annual resting ECG examination.
The investigators used standard, 12-lead ECG readings taken for each patient during 2018, and classified abnormalities by the Minnesota Code criteria. They divided the abnormalities into major or minor groups “in accordance with consensus between previous studies who categorised abnormalities according to perceived importance and/or severity.” The major subgroup included major QS pattern abnormalities, major ST-segment abnormalities, complete left bundle branch block or intraventricular block, or atrial fibrillation or flutter. Minor abnormalities included minor QS pattern abnormalities, minor ST-segment abnormalities, complete right bundle branch block, or premature atrial or ventricular contractions.
The prevalence of a major abnormality in the entire cohort examined was 13%, and another 16% had a minor abnormality. The most common types of abnormalities were ventricular conduction defects, in 14%; and arrhythmias, in 11%. In the subgroup of 6,494 of these patients with no history of CVD, 9% had a major abnormality and 15% a minor abnormality. Within this subgroup, 23% also had no hypertension, and their prevalence of a major abnormality was 4%, while 9% had a minor abnormality.
A multivariable analysis of potential risk factors among the entire study cohort showed that patients with hypertension had nearly triple the prevalence of a major ECG abnormality as those without hypertension, and men had double the prevalence of a major abnormality compared with women. Other markers that significantly linked with a higher rate of a major abnormality were older age, higher body mass index, higher A1c levels, and moderately depressed renal function.
“While the criteria the authors used for differentiating major and minor criteria are reasonable, in an asymptomatic patient even the presence of frequent premature atrial contractions on a baseline ECG has been associated with the development of AFib and a higher risk for stroke. The presence of left or right bundle branch block could spur additional evaluation with an echocardiogram,” said Dr. Kusumoto, president-elect of the Heart Rhythm Society.
“Generally an ECG abnormality is supplemental to clinical data in deciding the choice and timing of next therapeutic steps or additional testing. Physicians should have a fairly low threshold for obtaining ECG in patients with diabetes since it is inexpensive and can provide supplemental and potentially actionable information,” he said. “The presence of ECG abnormalities increases the possibility of underlying cardiovascular disease. When taking care of patients with diabetes at initial evaluation or without prior cardiac history or symptoms referable to the heart, two main issues are identifying the likelihood of coronary artery disease and atrial fibrillation.”
Mr. Harms and coauthors, and Dr. Kusumoto, had no disclosures.
Major ECG abnormalities were found in 13% of more than 8,000 unselected patients with type 2 diabetes, including a 9% prevalence in the subgroup of these patients without identified cardiovascular disease (CVD) in a community-based Dutch cohort. Minor ECG abnormalities were even more prevalent.
These prevalence rates were consistent with prior findings from patients with type 2 diabetes, but the current report is notable because “it provides the most thorough description of the prevalence of ECG abnormalities in people with type 2 diabetes,” and used an “unselected and large population with comprehensive measurements,” including many without a history of CVD, said Peter P. Harms, MSc, and associates noted in a recent report in the Journal of Diabetes and Its Complications.
The analysis also identified several parameters that significantly linked with the presence of a major ECG abnormality including hypertension, male sex, older age, and higher levels of hemoglobin A1c.
“Resting ECG abnormalities might be a useful tool for CVD screening in people with type 2 diabetes,” concluded Mr. Harms, a researcher at the Amsterdam University Medical Center, and coauthors.
Findings “not unexpected”
Patients with diabetes have a higher prevalence of ECG abnormalities “because of their higher likelihood of having hypertension and other CVD risk factors,” as well as potentially having subclinical CVD, said Fred M. Kusumoto, MD, so these findings are “not unexpected. The more risk factors a patient has for structural heart disease, atrial fibrillation (AFib), or stroke from AFib, the more a physician must consider whether a baseline ECG and future surveillance is appropriate,” Dr. Kusumoto said in an interview.
But he cautioned against seeing these findings as a rationale to routinely run a resting ECG examination on every adult with diabetes.
“Patients with diabetes are very heterogeneous,” which makes it “difficult to come up with a ‘one size fits all’ recommendation” for ECG screening of patients with diabetes, he said.
While a task force of the European Society of Cardiology and the European Association for the Study of Diabetes set a class I level C guideline for resting ECG screening of patients with diabetes if they also have either hypertension or suspected CVD, the American Diabetes Association has no specific recommendations on which patients with diabetes should receive ECG screening.
“The current absence of U.S. recommendations is reasonable, as it allows patients and physicians to discuss the issues and decide on the utility of an ECG in their specific situation,” said Dr. Kusumoto, director of heart rhythm services at the Mayo Clinic in Jacksonville, Fla. But he also suggested that “the more risk factors that a patient with diabetes has for structural heart disease, AFib, or stroke from AFib the more a physician must consider whether a baseline ECG and future surveillance is appropriate.”
Data from a Dutch prospective cohort
The new study used data collected from 8,068 patients with type 2 diabetes and enrolled in the prospective Hoorn Diabetes Care System cohort, which enrolled patients newly diagnosed with type 2 diabetes in the West Friesland region of the Netherlands starting in 1996. The study includes most of these patients in the region who are under regular care of a general practitioner, and the study protocol calls for an annual resting ECG examination.
The investigators used standard, 12-lead ECG readings taken for each patient during 2018, and classified abnormalities by the Minnesota Code criteria. They divided the abnormalities into major or minor groups “in accordance with consensus between previous studies who categorised abnormalities according to perceived importance and/or severity.” The major subgroup included major QS pattern abnormalities, major ST-segment abnormalities, complete left bundle branch block or intraventricular block, or atrial fibrillation or flutter. Minor abnormalities included minor QS pattern abnormalities, minor ST-segment abnormalities, complete right bundle branch block, or premature atrial or ventricular contractions.
The prevalence of a major abnormality in the entire cohort examined was 13%, and another 16% had a minor abnormality. The most common types of abnormalities were ventricular conduction defects, in 14%; and arrhythmias, in 11%. In the subgroup of 6,494 of these patients with no history of CVD, 9% had a major abnormality and 15% a minor abnormality. Within this subgroup, 23% also had no hypertension, and their prevalence of a major abnormality was 4%, while 9% had a minor abnormality.
A multivariable analysis of potential risk factors among the entire study cohort showed that patients with hypertension had nearly triple the prevalence of a major ECG abnormality as those without hypertension, and men had double the prevalence of a major abnormality compared with women. Other markers that significantly linked with a higher rate of a major abnormality were older age, higher body mass index, higher A1c levels, and moderately depressed renal function.
“While the criteria the authors used for differentiating major and minor criteria are reasonable, in an asymptomatic patient even the presence of frequent premature atrial contractions on a baseline ECG has been associated with the development of AFib and a higher risk for stroke. The presence of left or right bundle branch block could spur additional evaluation with an echocardiogram,” said Dr. Kusumoto, president-elect of the Heart Rhythm Society.
“Generally an ECG abnormality is supplemental to clinical data in deciding the choice and timing of next therapeutic steps or additional testing. Physicians should have a fairly low threshold for obtaining ECG in patients with diabetes since it is inexpensive and can provide supplemental and potentially actionable information,” he said. “The presence of ECG abnormalities increases the possibility of underlying cardiovascular disease. When taking care of patients with diabetes at initial evaluation or without prior cardiac history or symptoms referable to the heart, two main issues are identifying the likelihood of coronary artery disease and atrial fibrillation.”
Mr. Harms and coauthors, and Dr. Kusumoto, had no disclosures.
FROM THE JOURNAL OF DIABETES AND ITS COMPLICATIONS
Armpit swelling after COVID-19 vaccine may mimic breast cancer
Clinicians should therefore consider recent COVID-19 vaccination history in the differential diagnosis of patients who present with unilateral axillary adenopathy, according to a new article.
“We noticed an increasing number of patients with swollen lymph nodes on just one side/one underarm who presented for routine screening mammography or ultrasound, and some women who actually felt these swollen nodes,” said author Katerina Dodelzon, MD, assistant professor of clinical radiology at Weill Cornell Medicine, New York.
“Historically, swollen lymph nodes on just one side are relatively rare and are an uncommon occurrence on screening mammography – seen only 0.02%-0.04% of the time – and is a sign that alerts a radiologist to exclude the presence of breast malignancy on that side,” she added.
In an article published in Clinical Imaging, Dr. Dodelzon and colleagues described four cases involving women who received a COVID-19 vaccine and then sought breast screening. In describing these cases, the authors sought “to inform the medical community to consider this benign and self-resolving diagnosis in the setting of what can be alarming presentation of unilateral axillary adenopathy.”
They hope they will decrease unnecessary biopsies and help reassure patients.
Adenopathy has been reported in association with other vaccines, such as the bacille Calmette-Guérin vaccine, influenza vaccines, and the human papillomavirus vaccine, commented Jessica W. T. Leung, MD, president of the Society of Breast Imaging.
“It’s too early to say if there is something different about the COVID-19 vaccines,” said Dr. Leung, who is also professor of diagnostic radiology and deputy chair of breast imaging at the University of Texas MD Anderson Cancer Center, Houston.
“The two vaccines that are currently in use – Pfizer and Moderna – are both mRNA vaccines, and it is unknown if those will give a stronger immune response,” she said. “If the Johnson & Johnson and AstraZeneca vaccines do become available, it will be interesting to see if they elicit as strong a response, since they are not mRNA vaccines. At this time, we have no data to say one way or the other.”
Dr. Leung also noted that these latest vaccine reactions may be getting more attention because “it is COVID-19 related, and everything related to COVID-19 gets more attention.
“It may also be more noticeable because of the large number of people getting vaccinated within a short period of time in an effort to contain the pandemic, and this is not the case with the other vaccines,” she said.
New recommendations from SBI
The SBI recently issued recommendations to clinicians that women who experience axillary adenopathy and who have recently been vaccinated on the same side on which the adenopathy occurs be followed for a few weeks to see whether the lymph nodes return to normal, rather than undergo biopsy.
“Many practices are now routinely inquiring about history of recent vaccination and on which side it was given,” Dr. Dodelzon said. She emphasized that women should feel empowered to share that history if they are not asked.
“Letting your mammography technologist or breast imager know that you have recently been vaccinated, and on which side, will provide the breast imager more accurate context within which to interpret the results,” she said.
In addition, the SBI recommends that, if feasible, women schedule routine screening mammography either before the first dose of the COVID-19 vaccine or 4-6 weeks after the second dose to avoid a false-positive finding.
“We want to emphasize that screening mammography is very important, and if possible, to schedule it around the vaccine,” commented Dr. Leung. “But that may not be possible, as most of us don’t have a choice when to get the vaccine.”
If it is not possible to reschedule either the mammogram or the vaccine, Dr. Leung recommends that women inform the facility that they have recently received a COVID-19 vaccine. “Currently, we recommend a follow-up in 4-12 weeks,” she said. “The swelling could subside sooner, perhaps even within 1-2 weeks, but we generally recommend waiting at least 4 weeks to capture the majority of women.”
Differences between the vaccines?
The frequency with which axillary adenopathy occurs as a side effect differs with the two COVID-19 vaccines, according to reports from the Centers for Disease Control and Prevention.
For the Moderna vaccine, axillary adenopathy ipsilateral to the vaccination arm was the second most frequently reported local reaction, with 11.6% of recipients aged 18-64 years reporting it after the first dose, and 16.0% reporting it after the second. The average duration of this adenopathy was 1-2 days.
For the Pfizer-BioNTech COVID-19 vaccine, the CDC notes that reports of adenopathy were imbalanced between the vaccine and placebo groups and concluded that adenopathy was plausibly related to the vaccine.
The average duration of adenopathy was approximately 10 days.
Adenopathy was reported within 2-4 days after vaccination for both vaccine groups, the CDC noted.
However, details from the cases reported by Dr. Dodelzon and colleagues paint a somewhat different picture. For example, in case 1, the patient self-detected unilateral axillary adenopathy 9 days after receiving the first dose of the Pfizer-BioNTech vaccine. In case 3, the time between receiving the Moderna vaccine and detection of adenopathy was 13 days.
In both of these cases, the time was much longer than the average duration of 1-2 days noted by the CDC. The authors suggest that in taking the patient’s vaccination history, radiologists understand that the side effect may occur up to several weeks following the COVID-19 vaccination.
In cases 2 and 4, the axillary adenopathy was incidentally noted during mammography, so it is unclear when the onset of this reaction occurred after receiving the COVID-19 vaccine.
The authors and Dr. Leung have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Clinicians should therefore consider recent COVID-19 vaccination history in the differential diagnosis of patients who present with unilateral axillary adenopathy, according to a new article.
“We noticed an increasing number of patients with swollen lymph nodes on just one side/one underarm who presented for routine screening mammography or ultrasound, and some women who actually felt these swollen nodes,” said author Katerina Dodelzon, MD, assistant professor of clinical radiology at Weill Cornell Medicine, New York.
“Historically, swollen lymph nodes on just one side are relatively rare and are an uncommon occurrence on screening mammography – seen only 0.02%-0.04% of the time – and is a sign that alerts a radiologist to exclude the presence of breast malignancy on that side,” she added.
In an article published in Clinical Imaging, Dr. Dodelzon and colleagues described four cases involving women who received a COVID-19 vaccine and then sought breast screening. In describing these cases, the authors sought “to inform the medical community to consider this benign and self-resolving diagnosis in the setting of what can be alarming presentation of unilateral axillary adenopathy.”
They hope they will decrease unnecessary biopsies and help reassure patients.
Adenopathy has been reported in association with other vaccines, such as the bacille Calmette-Guérin vaccine, influenza vaccines, and the human papillomavirus vaccine, commented Jessica W. T. Leung, MD, president of the Society of Breast Imaging.
“It’s too early to say if there is something different about the COVID-19 vaccines,” said Dr. Leung, who is also professor of diagnostic radiology and deputy chair of breast imaging at the University of Texas MD Anderson Cancer Center, Houston.
“The two vaccines that are currently in use – Pfizer and Moderna – are both mRNA vaccines, and it is unknown if those will give a stronger immune response,” she said. “If the Johnson & Johnson and AstraZeneca vaccines do become available, it will be interesting to see if they elicit as strong a response, since they are not mRNA vaccines. At this time, we have no data to say one way or the other.”
Dr. Leung also noted that these latest vaccine reactions may be getting more attention because “it is COVID-19 related, and everything related to COVID-19 gets more attention.
“It may also be more noticeable because of the large number of people getting vaccinated within a short period of time in an effort to contain the pandemic, and this is not the case with the other vaccines,” she said.
New recommendations from SBI
The SBI recently issued recommendations to clinicians that women who experience axillary adenopathy and who have recently been vaccinated on the same side on which the adenopathy occurs be followed for a few weeks to see whether the lymph nodes return to normal, rather than undergo biopsy.
“Many practices are now routinely inquiring about history of recent vaccination and on which side it was given,” Dr. Dodelzon said. She emphasized that women should feel empowered to share that history if they are not asked.
“Letting your mammography technologist or breast imager know that you have recently been vaccinated, and on which side, will provide the breast imager more accurate context within which to interpret the results,” she said.
In addition, the SBI recommends that, if feasible, women schedule routine screening mammography either before the first dose of the COVID-19 vaccine or 4-6 weeks after the second dose to avoid a false-positive finding.
“We want to emphasize that screening mammography is very important, and if possible, to schedule it around the vaccine,” commented Dr. Leung. “But that may not be possible, as most of us don’t have a choice when to get the vaccine.”
If it is not possible to reschedule either the mammogram or the vaccine, Dr. Leung recommends that women inform the facility that they have recently received a COVID-19 vaccine. “Currently, we recommend a follow-up in 4-12 weeks,” she said. “The swelling could subside sooner, perhaps even within 1-2 weeks, but we generally recommend waiting at least 4 weeks to capture the majority of women.”
Differences between the vaccines?
The frequency with which axillary adenopathy occurs as a side effect differs with the two COVID-19 vaccines, according to reports from the Centers for Disease Control and Prevention.
For the Moderna vaccine, axillary adenopathy ipsilateral to the vaccination arm was the second most frequently reported local reaction, with 11.6% of recipients aged 18-64 years reporting it after the first dose, and 16.0% reporting it after the second. The average duration of this adenopathy was 1-2 days.
For the Pfizer-BioNTech COVID-19 vaccine, the CDC notes that reports of adenopathy were imbalanced between the vaccine and placebo groups and concluded that adenopathy was plausibly related to the vaccine.
The average duration of adenopathy was approximately 10 days.
Adenopathy was reported within 2-4 days after vaccination for both vaccine groups, the CDC noted.
However, details from the cases reported by Dr. Dodelzon and colleagues paint a somewhat different picture. For example, in case 1, the patient self-detected unilateral axillary adenopathy 9 days after receiving the first dose of the Pfizer-BioNTech vaccine. In case 3, the time between receiving the Moderna vaccine and detection of adenopathy was 13 days.
In both of these cases, the time was much longer than the average duration of 1-2 days noted by the CDC. The authors suggest that in taking the patient’s vaccination history, radiologists understand that the side effect may occur up to several weeks following the COVID-19 vaccination.
In cases 2 and 4, the axillary adenopathy was incidentally noted during mammography, so it is unclear when the onset of this reaction occurred after receiving the COVID-19 vaccine.
The authors and Dr. Leung have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Clinicians should therefore consider recent COVID-19 vaccination history in the differential diagnosis of patients who present with unilateral axillary adenopathy, according to a new article.
“We noticed an increasing number of patients with swollen lymph nodes on just one side/one underarm who presented for routine screening mammography or ultrasound, and some women who actually felt these swollen nodes,” said author Katerina Dodelzon, MD, assistant professor of clinical radiology at Weill Cornell Medicine, New York.
“Historically, swollen lymph nodes on just one side are relatively rare and are an uncommon occurrence on screening mammography – seen only 0.02%-0.04% of the time – and is a sign that alerts a radiologist to exclude the presence of breast malignancy on that side,” she added.
In an article published in Clinical Imaging, Dr. Dodelzon and colleagues described four cases involving women who received a COVID-19 vaccine and then sought breast screening. In describing these cases, the authors sought “to inform the medical community to consider this benign and self-resolving diagnosis in the setting of what can be alarming presentation of unilateral axillary adenopathy.”
They hope they will decrease unnecessary biopsies and help reassure patients.
Adenopathy has been reported in association with other vaccines, such as the bacille Calmette-Guérin vaccine, influenza vaccines, and the human papillomavirus vaccine, commented Jessica W. T. Leung, MD, president of the Society of Breast Imaging.
“It’s too early to say if there is something different about the COVID-19 vaccines,” said Dr. Leung, who is also professor of diagnostic radiology and deputy chair of breast imaging at the University of Texas MD Anderson Cancer Center, Houston.
“The two vaccines that are currently in use – Pfizer and Moderna – are both mRNA vaccines, and it is unknown if those will give a stronger immune response,” she said. “If the Johnson & Johnson and AstraZeneca vaccines do become available, it will be interesting to see if they elicit as strong a response, since they are not mRNA vaccines. At this time, we have no data to say one way or the other.”
Dr. Leung also noted that these latest vaccine reactions may be getting more attention because “it is COVID-19 related, and everything related to COVID-19 gets more attention.
“It may also be more noticeable because of the large number of people getting vaccinated within a short period of time in an effort to contain the pandemic, and this is not the case with the other vaccines,” she said.
New recommendations from SBI
The SBI recently issued recommendations to clinicians that women who experience axillary adenopathy and who have recently been vaccinated on the same side on which the adenopathy occurs be followed for a few weeks to see whether the lymph nodes return to normal, rather than undergo biopsy.
“Many practices are now routinely inquiring about history of recent vaccination and on which side it was given,” Dr. Dodelzon said. She emphasized that women should feel empowered to share that history if they are not asked.
“Letting your mammography technologist or breast imager know that you have recently been vaccinated, and on which side, will provide the breast imager more accurate context within which to interpret the results,” she said.
In addition, the SBI recommends that, if feasible, women schedule routine screening mammography either before the first dose of the COVID-19 vaccine or 4-6 weeks after the second dose to avoid a false-positive finding.
“We want to emphasize that screening mammography is very important, and if possible, to schedule it around the vaccine,” commented Dr. Leung. “But that may not be possible, as most of us don’t have a choice when to get the vaccine.”
If it is not possible to reschedule either the mammogram or the vaccine, Dr. Leung recommends that women inform the facility that they have recently received a COVID-19 vaccine. “Currently, we recommend a follow-up in 4-12 weeks,” she said. “The swelling could subside sooner, perhaps even within 1-2 weeks, but we generally recommend waiting at least 4 weeks to capture the majority of women.”
Differences between the vaccines?
The frequency with which axillary adenopathy occurs as a side effect differs with the two COVID-19 vaccines, according to reports from the Centers for Disease Control and Prevention.
For the Moderna vaccine, axillary adenopathy ipsilateral to the vaccination arm was the second most frequently reported local reaction, with 11.6% of recipients aged 18-64 years reporting it after the first dose, and 16.0% reporting it after the second. The average duration of this adenopathy was 1-2 days.
For the Pfizer-BioNTech COVID-19 vaccine, the CDC notes that reports of adenopathy were imbalanced between the vaccine and placebo groups and concluded that adenopathy was plausibly related to the vaccine.
The average duration of adenopathy was approximately 10 days.
Adenopathy was reported within 2-4 days after vaccination for both vaccine groups, the CDC noted.
However, details from the cases reported by Dr. Dodelzon and colleagues paint a somewhat different picture. For example, in case 1, the patient self-detected unilateral axillary adenopathy 9 days after receiving the first dose of the Pfizer-BioNTech vaccine. In case 3, the time between receiving the Moderna vaccine and detection of adenopathy was 13 days.
In both of these cases, the time was much longer than the average duration of 1-2 days noted by the CDC. The authors suggest that in taking the patient’s vaccination history, radiologists understand that the side effect may occur up to several weeks following the COVID-19 vaccination.
In cases 2 and 4, the axillary adenopathy was incidentally noted during mammography, so it is unclear when the onset of this reaction occurred after receiving the COVID-19 vaccine.
The authors and Dr. Leung have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.