User login
PTSD prevalent in survivors of severe COVID-19
Posttraumatic stress disorder may occur in up to a third of patients who recover from severe COVID-19 infection, new research suggests.
A study of more than 300 patients who presented to the emergency department with the virus showed a 30.2% prevalence for PTSD 30-120 days after COVID recovery.
or having persistent medical symptoms after hospitalization.
Additional diagnoses, such as depressive and hypomanic episodes and generalized anxiety disorder (GAD), were also present in some of the survivors.
“Previous coronavirus epidemics were associated with PTSD diagnoses in postillness stages, with meta-analytic findings indicating a prevalence of 32.2%,” write the investigators, led by Delfina Janiri, MD, department of psychiatry, Fondazione Policlinico Universitario Agostino Gemelli IRCCS, Rome.
However, data focused specifically on COVID-19 have been “piecemeal,” they add.
The findings were published online Feb. 18 in a research letter in JAMA Psychiatry.
A traumatic event
From April to October 2020, the researchers assessed 381 consecutive patients (100% white; 56.4% men; mean age, 55.3 years) who presented to the ED and subsequently participated in a health check at the Fondazione Policlinico Universitario Agostino Gemelli.
The mean length of stay for the 309 patients hospitalized with severe COVID-19 was 18.4 days.
Results showed that 115 participants (30.2%) had PTSD, based on DSM-5 criteria, and 55.7% of the women had the disorder. Additional diagnoses found in the full patient population included:
- Depressive episodes (17.3%).
- GAD (7%).
- Hypomanic episodes (0.7%).
- Psychotic disorders (0.2%).
Patients with PTSD had higher rates than those without PTSD of a previous history of psychiatric disorders (34.8% vs. 20.7%; P = .003) and of delirium or agitation during hospitalization, as assessed with the Confusion Assessment Method (16.5% vs. 6.4%; P = .002).
In addition, 62.6% of those with PTSD had three or more persistent COVID-19 symptoms vs. 37.2% of their counterparts without PTSD (P < .001).
After logistic regression analyses, significant factors associated with a PTSD diagnosis were persistent medical symptoms (P = .002), delirium or agitation (P = .02), and being female (P = .02).
The investigators note that their results are “in line” with findings reported in research examining other traumatic events. This includes about 30% of Hurricane Katrina survivors who experienced PTSD, as did around 25% of survivors of the 2011 “Great Japan Earthquake and Tsunami.”
Study limitations cited include the “relatively small” size of the patient population, that it focused on only one participating center, and that it didn’t include a control group of non-COVID patients who reported to the ED.
“Further longitudinal studies are needed to tailor therapeutic interventions and prevention strategies,” the researchers write.
Dr. Janiri and four of the five other authors have disclosed no relevant financial relationships. The other author, Gabriele Sani, MD, reported having received personal fees from Angelini Spa, Janssen, and Lundbeck outside the submitted work.
A version of this article first appeared on Medscape.com.
Posttraumatic stress disorder may occur in up to a third of patients who recover from severe COVID-19 infection, new research suggests.
A study of more than 300 patients who presented to the emergency department with the virus showed a 30.2% prevalence for PTSD 30-120 days after COVID recovery.
or having persistent medical symptoms after hospitalization.
Additional diagnoses, such as depressive and hypomanic episodes and generalized anxiety disorder (GAD), were also present in some of the survivors.
“Previous coronavirus epidemics were associated with PTSD diagnoses in postillness stages, with meta-analytic findings indicating a prevalence of 32.2%,” write the investigators, led by Delfina Janiri, MD, department of psychiatry, Fondazione Policlinico Universitario Agostino Gemelli IRCCS, Rome.
However, data focused specifically on COVID-19 have been “piecemeal,” they add.
The findings were published online Feb. 18 in a research letter in JAMA Psychiatry.
A traumatic event
From April to October 2020, the researchers assessed 381 consecutive patients (100% white; 56.4% men; mean age, 55.3 years) who presented to the ED and subsequently participated in a health check at the Fondazione Policlinico Universitario Agostino Gemelli.
The mean length of stay for the 309 patients hospitalized with severe COVID-19 was 18.4 days.
Results showed that 115 participants (30.2%) had PTSD, based on DSM-5 criteria, and 55.7% of the women had the disorder. Additional diagnoses found in the full patient population included:
- Depressive episodes (17.3%).
- GAD (7%).
- Hypomanic episodes (0.7%).
- Psychotic disorders (0.2%).
Patients with PTSD had higher rates than those without PTSD of a previous history of psychiatric disorders (34.8% vs. 20.7%; P = .003) and of delirium or agitation during hospitalization, as assessed with the Confusion Assessment Method (16.5% vs. 6.4%; P = .002).
In addition, 62.6% of those with PTSD had three or more persistent COVID-19 symptoms vs. 37.2% of their counterparts without PTSD (P < .001).
After logistic regression analyses, significant factors associated with a PTSD diagnosis were persistent medical symptoms (P = .002), delirium or agitation (P = .02), and being female (P = .02).
The investigators note that their results are “in line” with findings reported in research examining other traumatic events. This includes about 30% of Hurricane Katrina survivors who experienced PTSD, as did around 25% of survivors of the 2011 “Great Japan Earthquake and Tsunami.”
Study limitations cited include the “relatively small” size of the patient population, that it focused on only one participating center, and that it didn’t include a control group of non-COVID patients who reported to the ED.
“Further longitudinal studies are needed to tailor therapeutic interventions and prevention strategies,” the researchers write.
Dr. Janiri and four of the five other authors have disclosed no relevant financial relationships. The other author, Gabriele Sani, MD, reported having received personal fees from Angelini Spa, Janssen, and Lundbeck outside the submitted work.
A version of this article first appeared on Medscape.com.
Posttraumatic stress disorder may occur in up to a third of patients who recover from severe COVID-19 infection, new research suggests.
A study of more than 300 patients who presented to the emergency department with the virus showed a 30.2% prevalence for PTSD 30-120 days after COVID recovery.
or having persistent medical symptoms after hospitalization.
Additional diagnoses, such as depressive and hypomanic episodes and generalized anxiety disorder (GAD), were also present in some of the survivors.
“Previous coronavirus epidemics were associated with PTSD diagnoses in postillness stages, with meta-analytic findings indicating a prevalence of 32.2%,” write the investigators, led by Delfina Janiri, MD, department of psychiatry, Fondazione Policlinico Universitario Agostino Gemelli IRCCS, Rome.
However, data focused specifically on COVID-19 have been “piecemeal,” they add.
The findings were published online Feb. 18 in a research letter in JAMA Psychiatry.
A traumatic event
From April to October 2020, the researchers assessed 381 consecutive patients (100% white; 56.4% men; mean age, 55.3 years) who presented to the ED and subsequently participated in a health check at the Fondazione Policlinico Universitario Agostino Gemelli.
The mean length of stay for the 309 patients hospitalized with severe COVID-19 was 18.4 days.
Results showed that 115 participants (30.2%) had PTSD, based on DSM-5 criteria, and 55.7% of the women had the disorder. Additional diagnoses found in the full patient population included:
- Depressive episodes (17.3%).
- GAD (7%).
- Hypomanic episodes (0.7%).
- Psychotic disorders (0.2%).
Patients with PTSD had higher rates than those without PTSD of a previous history of psychiatric disorders (34.8% vs. 20.7%; P = .003) and of delirium or agitation during hospitalization, as assessed with the Confusion Assessment Method (16.5% vs. 6.4%; P = .002).
In addition, 62.6% of those with PTSD had three or more persistent COVID-19 symptoms vs. 37.2% of their counterparts without PTSD (P < .001).
After logistic regression analyses, significant factors associated with a PTSD diagnosis were persistent medical symptoms (P = .002), delirium or agitation (P = .02), and being female (P = .02).
The investigators note that their results are “in line” with findings reported in research examining other traumatic events. This includes about 30% of Hurricane Katrina survivors who experienced PTSD, as did around 25% of survivors of the 2011 “Great Japan Earthquake and Tsunami.”
Study limitations cited include the “relatively small” size of the patient population, that it focused on only one participating center, and that it didn’t include a control group of non-COVID patients who reported to the ED.
“Further longitudinal studies are needed to tailor therapeutic interventions and prevention strategies,” the researchers write.
Dr. Janiri and four of the five other authors have disclosed no relevant financial relationships. The other author, Gabriele Sani, MD, reported having received personal fees from Angelini Spa, Janssen, and Lundbeck outside the submitted work.
A version of this article first appeared on Medscape.com.
Identifying Acute Migraine Triggers
To read a digital version of the print supplement, click here.
To gain a full understanding of the challenges faced by neurologists, headache specialists, and other clinicians when managing triggers that prompt migraine headache attacks, consider the case of Lola H, a 35-year-old woman with a history of recurrent headaches that was diagnosed 12 months earlier as migraine without aura. Lola is taking oral sumatriptan, 100 mg, for acute migraine episodes, which she reports experiencing 3 or 4 times a month. She also has up to 3 headaches a week that are less severe. Lola’s insurance carrier limits the number of sumatriptan tablets covered in a month, forcing her use the medication only when she has severe headaches. However, the rationing strategy adds stress to her life that could be contributing to additional headache episodes.
Lola raised this issue during a recent visit with her neurologist, at which time she also presented a lengthy list of things she felt were triggering her attacks: stress, her menstrual cycle, chocolate, the odor of gasoline, and the weather, among other potential causes. Regarding the weather, she reported experiencing headaches—sometimes severe—whenever barometric pressure drops. Lola told her clinician that she believes she has gained control of 2 triggers—chocolate and gasoline fumes—through avoidance. She no longer consumes chocolate and other sweets and relies on family members and friends to fill her automobile fuel tank. However, Lola said she cannot control other triggers and needs her clinician’s help. She insists on leaving the appointment with answers she feels satisfied with. More than anything, she wants to feel secure that she has enough medication to meet her needs. (We will reveal how Lola fared later in this article.)
Heterogeneity of triggers: A daunting challenge
Lola’s circumstances are not uncommon among the nearly 40 million migraine sufferers in the United States.1 For clinicians seeking to better understand how potential triggers cause migraine attacks, it is important to appreciate the fine lines that exist between (1) perceived triggers, (2) their apparent frequency and strength, (3) patients’ efforts to control these factors, and (4) the distinction between real migraine triggers and premonitory symptoms. Moreover, recognizing how each of these 4 issues influence the patient’s experience enables the clinician to optimally manage individuals who experience migraine headache.
A diverse array of potential stimuli
Perhaps the most daunting challenge when trying to uncover what is triggering Lola’s and other migraine patients’ headaches is the realization that triggers and perceived triggers comprise a diverse array of potential stimuli. A meta-analysis of 85 studies conducted over a 60-year period and involving more than 27,000 participants revealed 420 unique headache triggers categorized to 1 of 15 categories.2 Characterizing these triggers as vastly heterogenous is an understatement, considering that they include factors such as raising the arms overhead, sneezing, abstaining from coffee, using a low pillow, weekend sleep habits, use of shower gels, relaxing after stressful events, bus journeys, road stripes, and more.2 The authors of the meta-analysis note that the variability observed likely is because of methodology differences among the studies included in their analysis. For this reason, the authors advised caution in interpreting the results.2
Nevertheless, the investigators concluded that (1) most of those who experience frequent headaches—including migraine sufferers—believe they have at least 1 trigger, and (2) stress is the trigger most frequently cited by patients, followed by sleep and environmental triggers.2
Perceived differences in trigger frequency and strength
Heterogeneity extends beyond the triggers and perceived triggers; incidence and strength also can vary greatly. In a cross-sectional observational study, 300 headache sufferers were asked to rate 33 triggers by frequency and potency.3 Triggers that were perceived to be encountered most days per month were air conditioning and caffeine (both 30 days). Red wine was perceived to be encountered least frequently (0 days). Importantly, interperson variability for frequency of encounter was significant—most ranges for each trigger spanned
15 days or more.3
Triggers that were perceived to increase the chance of a headache were stress (75%), missing a meal, and dehydration (both 60%). Nuts and salty food were least perceived to increase the likelihood of a headache (both 0%). Similar to frequency, perceptions about trigger strengths varied widely, with most ranges spreading by more than 50% for each trigger.3
The authors noted that the heterogeneity seen in frequency of encounters with triggers remained even when 2 patients had matching perceptions about the importance of the trigger in precipitating a headache. The fact that this was observed for nearly every perceived trigger is an important aspect of understanding trigger frequency at the patient-care level.3 Additionally, most headache sufferers do not believe that triggers are simply present or absent. Rather, they have diverse beliefs about the strength of perceived triggers.3 These broad differences among patients make it difficult to predict how an individual headache sufferer will rate triggers.3
The patient’s trigger belief system
Layered onto this challenge is the observation that many migraine sufferers use the so-called trigger belief system to create a safe and controlled environment to stave off headache attacks. Indeed, perception often dictates how headache triggers are experienced, and this adds to the complexity of managing these patients. This is borne out in 2 studies. One, involving more than 200 individuals, revealed that perceived control over and impact of headaches were related to headache severity.4 Another trial involving more than 300 patients seeking treatment for headache showed that participants who were confident they could avoid and better manage their headaches also believed that they could control factors impacting their headaches. Although this led to the use of positive coping strategies, it also triggered more anxiety.5
Trigger avoidance can also lead to reduced quality of life. Consider, for example, physical activity, which is recommended to prevent and manage migraine: As many as 8 in 10 migraine sufferers avoid physical activity because they fear triggering an episode.6 In a recent study, investigators examined the potential correlation between anxiety sensitivity and physical activity avoidance among 100 women with migraine. Participants completed an anonymous survey about migraine and avoiding physical activity. The survey included a tool that measured anxiety sensitivity. Migraine attacks in the previous 30 days were self-reported.6 Higher anxiety scores were linked with more frequent physical activity avoidance. Participants who cited physical concerns were nearly 8 times more likely to avoid vigorous physical activity. Those who mentioned cognitive concerns were more than 5 times more likely to avoid moderate physical activity.6 The authors noted that even though avoiding triggers often is recommended, in some situations it can lead to more migraines because of increased anxiety. For this reason, although avoidance is probably best for certain triggers that have true biologic onset mechanisms, triggers that work through anticipatory anxiety or fearful expectations might best be handled via gradual exposure and desensitization.6
Distinguishing between real triggers and premonitory symptoms
Once clinicians gain an understanding of these factors, they may consider themselves adequately prepared to optimally manage migraine sufferers. Yet, there is one more factor to consider: premonitory symptoms. Separating premonitory symptoms from actual migraine triggers gets the clinician closer to optimal management. Yet, it is not always easy to make the distinction.
Premonitory symptoms typically present 2 to 48 hours (or more) before migraine headache onset. Symptoms include changes in mood and activity, irritability, fatigue, food cravings, repetitive yawning, stiff neck, and photophobia. Because these symptoms can continue even past the premonitory stage, they often can be misinterpreted as actual migraine triggers.7 Premonitory symptoms have been found to accompany 30% to 80% of migraine attacks. Signs that often are confused with actual migraine triggers include neck pain, photophobia, and food cravings.8
Timing of symptoms: Potential clues
It is critical to understand the interval between a symptom and migraine onset, which can help determine whether it is an actual trigger, premonitory symptom, or other manifestation. For this, it helps to review the timeline of a migraine attack. The 4 phases of a migraine attack—premonitory/prodrome, aura, headache, and postdrome—are well known. According to the American Migraine Foundation, the 4 phases are marked by specific symptoms that typically—but not always—occur along a general timeline (Figure 1).9
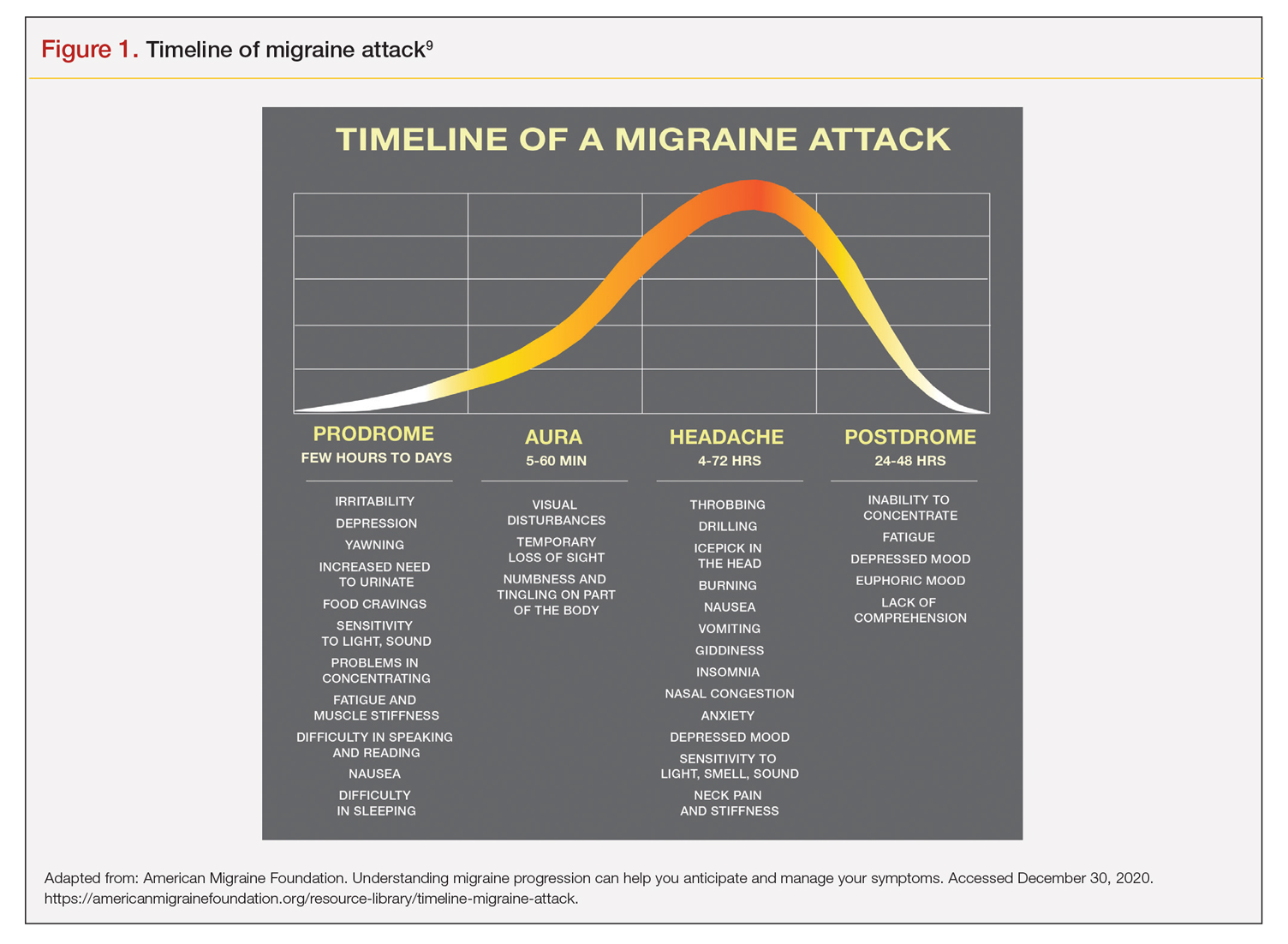
Although this symptom timeline can help guide clinicians and migraine sufferers, it is important to understand that these phases do not always occur linearly, and there often is significant overlap, which can mislead both the clinician and patient.7
Common triggers
Stress: Stress is the most frequently self-reported migraine trigger, and numerous studies show a link between stress and migraine. However, retrospective and prospective diary studies have not been able to confirm a link between stressful days and acute migraine attacks. Moreover, although 1 study demonstrated that migraines were less likely to occur on holidays or days off, relaxation did not play a role. Fatigue was more common premigraine than mental exhaustion or insomnia, leading investigators to surmise it is a premonitory symptom and not a migraine cause.8 Further confounding is the finding that reduced stress from one day to the next is linked with migraine onset the next day, making it a possible predictor of a migraine attack because of the so-called letdown effect.10
Menstruation: Menstruation appears to be the most common migraine trigger in women. A large 3-month study showed an increase of migraine prevalence and persistence up to 96%. Additionally, diary studies demonstrate menstruation as a trigger, and in a population-based trial, more than half of female migraine sufferers reported an increased rate
of migraine. However, only 4% actually had a migraine during menstruation.8,11
Diet: Studies that seek to find a link between specific foods and migraine are open to question because they do not have a placebo component, and it is difficult to tell the difference between premonitory food cravings and actual triggers. Moreover, in most patients food appears to be 1 of several contributors to migraine headache, and not the only factor.8 Fasting is among the most studied and consistent migraine triggers and seems to be more common during longer fasts. Dehydration is not thought to be a cause; rather, it could be because of hypoglycemia.8 A trial involving more than 3000 women revealed that those with migraine had a lower diet quality than their counterparts who did not experience migraine. Other smaller studies show benefit from low-fat vegan and ketogenic diets. Also, patients with irritable bowel syndrome and migraine might benefit from eliminating such foods that cause irritation to improve both migraine frequency and bowel symptoms.8
Weather: A few small studies have sought to evaluate the link between weather and migraines. One involving 77 individuals with migraine showed that slightly more than half were sensitive to at least 1 weather factor (i.e., barometric changes, lightning, temperature, and precipitation). Another involving 238 participants demonstrated a nonstatistically significant trend toward increased frequency of migraine attacks on days with high pressure, low wind speed, and increased sunshine, but they called the correlation questionable. A study of 90 individuals found lightning to be an independent risk factor for migraine.8
Sensory stimuli: Although visual, aural, olfactory, and other sensory stimuli have been shown to cause migraine in susceptible individuals and worsen migraine intensity, it is difficult to determine if these are true triggers or premonitory symptoms. Indeed, the discomfort thresholds of various sensory stimuli are lower in migraine sufferers.8 Odors are the most commonly reported triggers among all sensory stimuli. In 1 study, 7 in every 10 migraine sufferers reported that odors triggered their attack. Common offenders include perfumes, paint, gasoline, bleach, and rancid odors. Smoke and exhaust fumes were also found to be problematic.8
Best evidence on migraine triggers
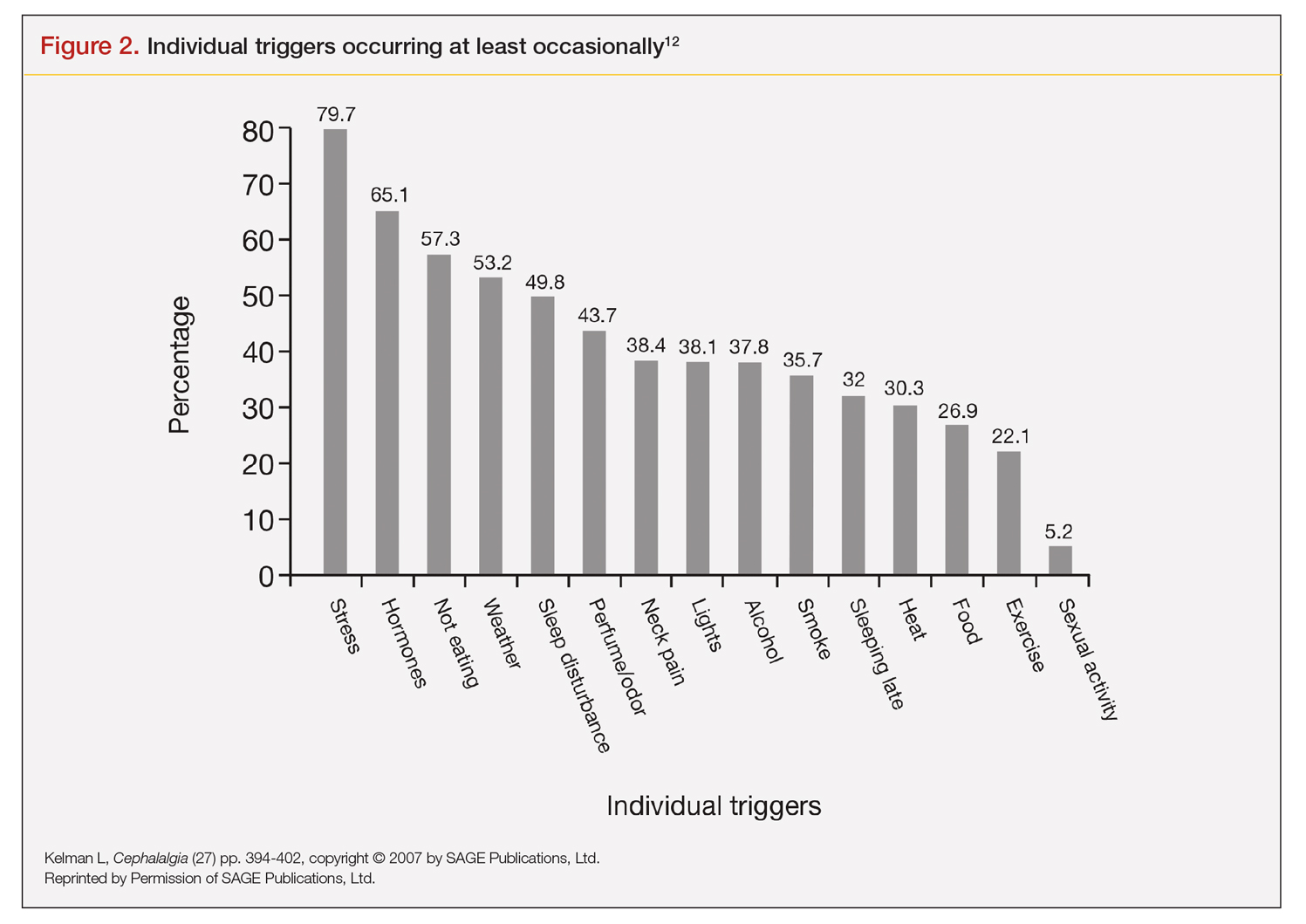
A large retrospective survey looked at acute migraine trigger factors in 1750 individuals treated in a clinical practice. A detailed headache evaluation was conducted that included neurologic history, structured headache interview, and a neurologic exam.12 More than three-fourths of patients experienced triggers. Approximately 6 in every 10 patients had between 4 and 9 triggers. Nearly one-fourth experienced 1 to 3 triggers.12 As shown in Figure 2, the 5 triggers that occurred occasionally in at least half of patients were stress (80%), hormone (65%), fasting (57%), weather (53%), and sleep disturbance (50%). The most common triggers, occurring very frequently, were hormone (33%) and stress (26%).12
Trials that use randomized and blinded exposure to possible triggers can—at face value—appear most useful. Potent compounds such as glyceryl trinitrite, prostaglandin I2 and E2, calcitonin-gene related peptide, and pituitary adenylate cyclase-activating polypeptide have been shown to be dependable triggers in the experimental setting. However, trial designs that do not closely mimic real-world experience lack usefulness in clinical practice. Moreover, studies of low intensity migraine triggers that are common in the real world—chocolate and aspartame, for instance—have produced mixed results.13 So-called N-of-1 studies—in which a single patient is repeatedly exposed in blinded fashion to a trigger or placebo—might be the best way to uncover actual triggers. However, migraine sufferers are, understandably, not interested in deliberately inducing an attack.13
This leaves results of diary studies as the most reflective of clinical practice. These evaluations limit the chance of bias that retrospective studies could carry and often provide a significant amount of real-world data that can be used to test hypotheses. Moreover, the advent of smartphone technology enables researchers to capture data in real time, which can help distinguish premonitory symptoms from actual triggers.13 A high-quality paper diary study (PAMINA) involving 327 individuals showed strong evidence that a migraine was likely to occur with the following factors: menstruation, muscle tension/neck pain, psychic tension, and sunshine that lasted 3 or more hours per day.14
Diary studies are also able to assess the ability of migraine sufferers to predict a headache. In an evaluation that used an electronic diary design, researchers looked at self-prediction through use of a single question asked daily: “How likely are you to have a headache today?” When participants reported at the beginning of a particular day that headache was “almost certain,” actual headache occurred more than 90% of the time on that day.13
Leveraging best evidence into best practices
The clinician–patient conversation
A clinician’s detective skills are put to the test when evaluating patients with migraine. Avoiding dead-ends and fruitless pursuits can spare providers and patients frustration and help patients avoid needless suffering. Yet, as noted, the list of potential triggers appears endless, duration and frequency are variable, patients often try to control their environment with mixed results, and it is difficult to tell the difference between actual triggers and premonitory symptoms.
Which brings us back to Lola H. She desperately wanted to leave her appointment with confidence that she had enough medication to meet her needs each month. She might have expected to go home with access to more medication, but she did not. Rather, she left her clinician’s office with a plan to reduce the number of monthly migraine episodes she experienced, enabling her to use medication as needed, rather than only when her attacks were severe.
Her clinician confirmed that avoiding gasoline odor would reduce the number of attacks. Eliminating chocolates and sweets might, but it is not known whether chocolate is a trigger. He suggested that she try allowing herself chocolate now and then, which would probably serve as a stress reducer. The net result would likely be no change in number of episodes, while allowing for a small improvement in quality of life.
Perhaps most striking, Lola left the appointment certain that her issue with lower barometric pressure was resolved because her clinician took the time to ask how she knew about the daily barometric pressure levels.
Her smartwatch, she told him. He strongly suggested that she delete the watch’s barometric pressure app, which she did on the spot. At her next appointment, Lola was happy to report that barometric pressure no longer played a significant role in her episodes. Moreover, the other strategies she and her clinician discussed have reduced the number of severe and less severe episodes. She no longer lives with the stress of pill-rationing and looks forward to enjoying a small amount of chocolate ice cream each weekend with her family.
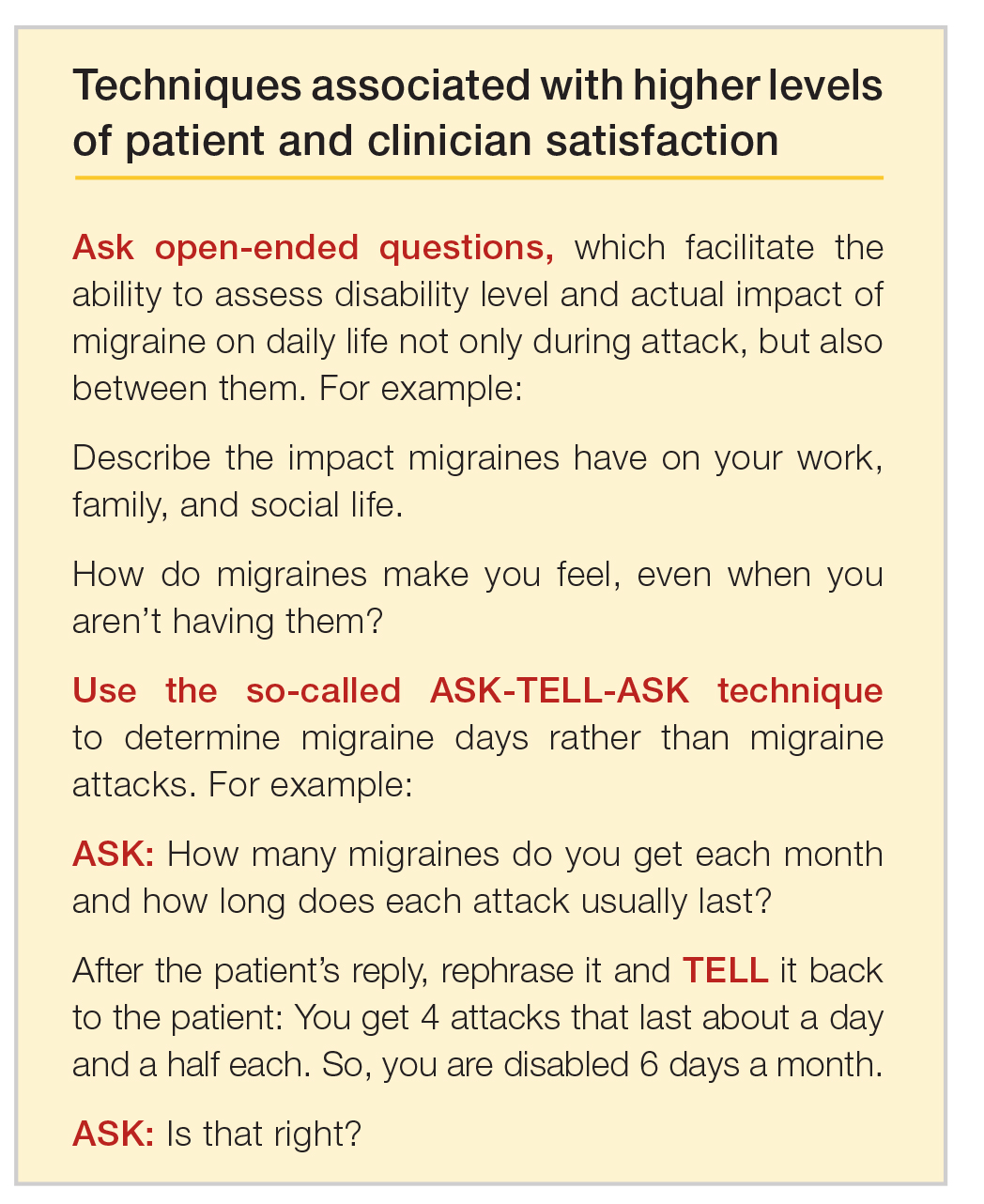
Lola’s case demonstrates that in clinical practice the best way to elicit meaningful responses from migraine sufferers is to ask open-ended questions. Yet, clinicians often fail to do so. In a study evaluating communication strategies between clinicians and 60 long-term migraineurs (average 14 years) who experienced frequent headaches (average 5 per month), 90% of migraine-specific questions asked during an appointment were either close-ended or consisted of a short answer. Postvisit interviews with both clinicians and patients revealed these pairs misaligned on migraine frequency (55% of the time) and impairment (51%). One-third of patients were candidates to receive preventive medication, yet only 20% of them did so. Prevention was not discussed in half of the visits with patients who were candidates to receive preventive therapy but did not get it.10
A panel with extensive experience in migraine reviewed questions from migraine diaries and noted that migraine sufferers find value in capturing wide-ranging information about their condition, whereas clinicians prefer small amounts of data they perceive as relevant to managing migraine.15 It also is reported that the number of questions patients ask is inversely related to the amount of information a clinician gives.16
Recommended strategies
In his review article, Mamura reinforces the concept of open 2-way communication, which involves patients in their care more deeply and reinforces positive habits.8 It is important to be mindful that discussing triggers exclusively can be counterproductive and frustrating for patients, particularly if the conversation is about trigger avoidance. Patients might feel stigmatized by triggers, come away thinking nothing can be done to control them, and become more anxious about impending episodes.8 In fact, a better strategy could be to work with the patient to train their brain to become acclimated to certain triggers or premonitory symptoms, rather than avoiding them.17
Mamura provides a concise summary of 4 best-practice strategies.8 The first 2 should be factored into advice given to patients about migraine triggers and premonitory symptoms; the remaining 2 help frame the treatment approach:
1. Ask patients to keep a headache diary, which eliminates faulty recall and provides a clearer picture of migraine frequency and severity. Include symptoms and other important factors—missed time from work, canceled family activities, nausea and vomiting, etc. Journaling might uncover triggers that can be eliminated or acclimated to via behavior modification. It also can reveal to the patient that certain triggers might not be as useful in predicting migraine as originally thought.
2. Rather than trigger avoidance, suggest that patients concentrate on healthy lifestyle choices, including maintaining healthy weight, getting sufficient sleep, and exercising regularly. The latter has been shown in some studies to work better than preventive medications. Although a behavior change, such as acclimating to certain noises, is not thought to be a healthy lifestyle habit, it ends up being just that if it improves tolerance and reduces migraine episodes.
3. Recognize that many patients have a fear of experiencing a migraine between episodes. If observed in a patient, treat it aggressively. Left unmanaged, this condition could lead to chronic migraine and medication overuse. The right acute medication might be sufficient, but sometimes cognitive-behavioral therapy and biofeedback techniques could be necessary to address behavioral issues that might be interfering with successful treatment.
4. If attacks are frequent and making it difficult to identify distinctive triggers, consider preventive therapy. Patients might resist using preventive medications because they feel eliminating triggers is sufficient, or they might be concerned about medication cost and adverse effects. Eliminating a stressful event alone likely will not help, so it’s important to review with patients—using plain language—how preventive medication treats the underlying cause of migraine and decreases brain excitability, making the patient less prone to attacks.8
Emerging medications
New treatments offer increased options for the acute and preventive treatment of migraine attacks. New acute pharmacologic treatments include gepants and ditans. The gepants target calcitonin gene-related peptide (CGRP), a potent vasodilator and neurotransmitter. During migraine episodes, serum levels of CGRP increase as the peptide is released, modulating the transmission of nociceptive signals from peripheral trigeminal sensory afferents into the central trigeminal system.19 In clinical trials of acute migraine treatment, the gepants provided pain freedom at 2 hours in approximately 20% of attacks, pain relief at 2 hours in 58%, and freedom from the most bothersome symptom at 2 hours in 36% with significant benefit over placebo.20,21 As these oral formulations became available in 2020, there are not enough data on potential long-term adverse events, particularly cardiovascular effects.18 However, the gepants are not associated with medication overuse headache and there is no contraindication to their use in patients with cardiovascular disease. These agents, currently available for oral administration, also show benefit as preventive agents and parenteral formulations are in development.
Ditans target the serotonin 5HT-1F receptor. Triptans target this receptor as well as the 5HT-1B and 5HT-1D receptors. The 5HT-1B receptor action is responsible for constriction of cerebral and peripheral blood vessels that occurs with triptans. Thus, ditans are also options for treating patients with vascular disease. Lasmiditan, the only compound in this class thus far, yielded pain freedom in 2 hours in 31%, pain relief 2 hours in 64%, and relief of the most bothersome symptom at 2 hours in 41%.22 Lasmiditan crosses the blood-brain barrier. Thus, the most common side effects are dizziness, somnolence, and paresthesias, which are generally mild to moderate in severity. Based on driving simulation studies performed in the absence of migraine, patients should not drive within 8 hours of dosing.
Neuromodulation devices are effective options that avoid potential adverse events of systemic treatments. They include external trigeminal nerve stimulation, noninvasive vagus nerve stimulation, remote electrical neuromodulation, caloric vestibular stimulation, and single-pulse transcranial magnetic stimulation. Other treatments in development for the acute treatment of migraine include improved parenteral delivery systems and combination oral preparations of existing medications.
Monoclonal antibodies that block the CGRP ligand or its receptor were introduced in 2018, with 4 medications currently available. This is the first class of migraine medications specifically designed for migraine prevention. They are administered parenterally (subcutaneously or intravenously) either monthly or quarterly, depending on the medication. The anti-CGRP monoclonal antibodies are indicated for the prevention of episodic and chronic migraine and are eliminated by proteolytic degradation, not involving the liver and reducing the potential for hepatotoxicity and drug-drug interactions.23 A meta-analysis evaluated the subcutaneous formulations studied for chronic migraine, enrolling participants with at least 15 headache days monthly with migraine occurring on at least 8 of those days. In this cohort with a large disease burden, the pooled 50% responder rate 9-12 weeks after administration of the first dose was 37.4% and the 75% responder rate was 12.7%.24 There was also improvement in the number of migraine days, number of days using acute treatment, and total headache hours. The most common side effects were injection site reactions and constipation, with post-marketing reports of hypertension in the compound targeting the CGRP receptor, with or without pre-existing hypertension.25 The safety profile for use during pregnancy was not studied in any of the medications discussed in this section.
Summary
Acute migraine headache is a significant management challenge for neurologists, headache specialists, and other clinicians involved in its treatment. Addressing potential migraine triggers can be a particularly overwhelming task because multiple components and factors play into the disorder. Perceived triggers; their frequency and strength; the effort by patients to control their environment; and the blurred line between real triggers and premonitory symptoms present the clinician with an amalgam of components that must be sorted through, defined, prioritized, and managed. Even though migraine symptoms generally appear along a 4-phase timeline, some symptoms stretch across multiple phases and not all symptoms play out in a linear fashion.
There are specific steps clinicians can take to simplify the process, optimize management, and improve patient outcomes and quality of life. Start by gaining an understanding of common triggers and the best evidence regarding triggers and premonitory symptoms. Use this evidence to modify your approach in practice, if necessary. Specifically:
• Improve the information you gather from patients by asking open-ended questions about their experience during a migraine headache, as well as the period between attacks.
• Rather than having patients tell you how many episodes they experience each month, try to elicit from them how many days per month they feel disabled.
• Ask your patients to keep a headache diary.
• Talk less about trigger avoidance and more about making healthy lifestyle choices.
• Address potential cephalgiaphobia via cognitive-behavioral therapy and biofeedback techniques.
• Consider managing frequent migraine attacks with preventive treatment, which now includes CGRP-targeted medications that are as effective as other agents but have fewer adverse effects and might avoid medication overuse headache.
Dr. Friedman reports being an Advisory Board member for Allergan, Biohaven Pharmaceuticals, Eli Lilly, Impel NeuroPharma, Invex, Lundbeck, Revance Therapeutics, Satsuma Pharmaceuticals, Teva Pharmaceuticals, Theranica, and Zosano Pharma. She has received grant support from Allergan, Eli Lilly, Merck, and Zosano Pharma. She is on the Medical Advisory Board for Spinal CSF Leak Foundation, HealthyWomen, and the Board of Directors of the Southern Headache Society. She currently sits on the Editorial Board of Neurology Reviews and has been a contributing author for MedLink Neurology and Medscape.
1. Migraine Research Foundation. Migraine facts. Accessed January 24, 2021. https://migraineresearchfoundation.org/about-migraine/migraine-facts.
2. Pellegrino A, Davis-Martin R, Houle T, Turner D, Smitherman T. Perceived triggers of primary headache disorders: a meta-analysis. Cephalalgia. 2018;38(6):1188-1198.
3. Turner D, Houle T. Influences on headache trigger beliefs and perceptions. Cephalalgia. 2018;38(9):1545-1553.
4. Scharff L, Turk D, Marcus D. The relationship of locus of control and psychosocial‐behavioral response in chronic headache. Headache. 1995;35(9):527-533.
5. French D, Holroyd K, Pinell C, Malinoski P, O’Donnell F, Hill K. Perceived self‐efficacy and headache‐related disability. Headache. 2000;40(8):647-656.
6. Farris S, Thomas J, Abrantes A, et al. Anxiety sensitivity and intentional avoidance of physical activity in women with probable migraine. Cephalalgia. 2019;39(11):1465-1469.
7. Goadsby P, Holland P, Martins-Oliveira M, Hoffman J, Schankin C, Akerman S. Pathophysiology of migraine: A disorder of sensory processing. Physiol Rev. 2017;97(2):553-622.
8. Mamura M. Triggers, protectors, and predictors in episodic migraine. Curr Pain Headache Rep. 2018;22:81.
9. American Migraine Foundation. Understanding migraine progression can help you anticipate and manage your symptoms. Accessed December 30, 2020. https://americanmigrainefoundation.org/resource-library/timeline-migraine-attack.
10. Lipton R, Buse D, Hall C, et al. Reduction in perceived stress as a migraine trigger: Testing the “let-down headache” hypothesis. Neurology. 2014;82:1395-1401.
11. Loder E. Prophylaxis of menstrual migraine with triptans: Problems and possibilities. Neurology. 2002;59(11):1677-1681.
12. Kelman L. The triggers or precipitants of the acute migraine attack. Cephalalgia. 2007;27:394-402.
13. Pavolic J, Buse D, Sollars M, Haut S, Lipton R. Trigger factors and premonitory features of migraine attacks: Summary of studies. Headache. 2014;54(10):1670-1679.
14. Wöber C, Brannath W, Schmidt K, et al; PAMINA Study Group. Prospective analysis of factors related to migraine attacks: The PAMINA study. Cephalalgia. 2007;27:304-314.
15. Dodick D, Tepper S, Lipton R, et al. Improving medical communication in migraine management: A modified Delphi study to develop a digital migraine tracker. Headache. 2018;58(9):1358-1372.
16. Sheftell F. Communicating the right therapy for the right patient at the right time: Acute therapy. Can J Neurol Sci. 2002;29(suppl 2):S33-S39.
17. Goadsby P, Silberstein S. Migraine triggers: Harnessing the messages of clinical practice. Neurology. 2013;80:434-425.
18. Woo M. Refining a marvel. Nature. 2020;586:s4-s6.
19. Dodick DW. Migraine. Lancet. 2018;10(127):1315-30.
20. Croop R, Goadsby P. J., Stock D. A. et al. Efficacy and safety of rimegepant for the acute treatment of migraine: Evidence from randomized controlled trials. Front Pharmacol. 2020;24 https://doi.org/10.3389/fphar.2019.01577.
21. Yang Y, Chen C, Sun Y et al. Safety and efficacy of ubrogepant for acute treatment of episodic migraine: A meta-analysis of randomized clinical trials. CNS Drugs. 2020;34:463-71.
22. Curto M, Cipolla F, Cisale GY et al. Profiling lasmiditan as a treatment option for migraine. Exp Rev Pharmacother. 2020;21(2):147-53.
23. Raffaelli B, Reuter U. The biology of monoclonal antibodies: Focus on calcitonin gene-related peptide for prophylactic migraine therapy. Neurotherapeutics. 2018;15(2): 324-35.
24. Han L, Lui Y, Xiong H et al. CRP monoclonal antibody for preventive treatment of chronic migraine: An update of meta-analysis. Brain Behav. 2019;9(2):e01215.
25. Saely S, Croteau D, Jawidzik L et al. Hypertension: A new safety risk for patients treated with erenumab. Headache. 2021 (epub ahead of print) https://doi.org/10.1111/head.14051.
To read a digital version of the print supplement, click here.
To gain a full understanding of the challenges faced by neurologists, headache specialists, and other clinicians when managing triggers that prompt migraine headache attacks, consider the case of Lola H, a 35-year-old woman with a history of recurrent headaches that was diagnosed 12 months earlier as migraine without aura. Lola is taking oral sumatriptan, 100 mg, for acute migraine episodes, which she reports experiencing 3 or 4 times a month. She also has up to 3 headaches a week that are less severe. Lola’s insurance carrier limits the number of sumatriptan tablets covered in a month, forcing her use the medication only when she has severe headaches. However, the rationing strategy adds stress to her life that could be contributing to additional headache episodes.
Lola raised this issue during a recent visit with her neurologist, at which time she also presented a lengthy list of things she felt were triggering her attacks: stress, her menstrual cycle, chocolate, the odor of gasoline, and the weather, among other potential causes. Regarding the weather, she reported experiencing headaches—sometimes severe—whenever barometric pressure drops. Lola told her clinician that she believes she has gained control of 2 triggers—chocolate and gasoline fumes—through avoidance. She no longer consumes chocolate and other sweets and relies on family members and friends to fill her automobile fuel tank. However, Lola said she cannot control other triggers and needs her clinician’s help. She insists on leaving the appointment with answers she feels satisfied with. More than anything, she wants to feel secure that she has enough medication to meet her needs. (We will reveal how Lola fared later in this article.)
Heterogeneity of triggers: A daunting challenge
Lola’s circumstances are not uncommon among the nearly 40 million migraine sufferers in the United States.1 For clinicians seeking to better understand how potential triggers cause migraine attacks, it is important to appreciate the fine lines that exist between (1) perceived triggers, (2) their apparent frequency and strength, (3) patients’ efforts to control these factors, and (4) the distinction between real migraine triggers and premonitory symptoms. Moreover, recognizing how each of these 4 issues influence the patient’s experience enables the clinician to optimally manage individuals who experience migraine headache.
A diverse array of potential stimuli
Perhaps the most daunting challenge when trying to uncover what is triggering Lola’s and other migraine patients’ headaches is the realization that triggers and perceived triggers comprise a diverse array of potential stimuli. A meta-analysis of 85 studies conducted over a 60-year period and involving more than 27,000 participants revealed 420 unique headache triggers categorized to 1 of 15 categories.2 Characterizing these triggers as vastly heterogenous is an understatement, considering that they include factors such as raising the arms overhead, sneezing, abstaining from coffee, using a low pillow, weekend sleep habits, use of shower gels, relaxing after stressful events, bus journeys, road stripes, and more.2 The authors of the meta-analysis note that the variability observed likely is because of methodology differences among the studies included in their analysis. For this reason, the authors advised caution in interpreting the results.2
Nevertheless, the investigators concluded that (1) most of those who experience frequent headaches—including migraine sufferers—believe they have at least 1 trigger, and (2) stress is the trigger most frequently cited by patients, followed by sleep and environmental triggers.2
Perceived differences in trigger frequency and strength
Heterogeneity extends beyond the triggers and perceived triggers; incidence and strength also can vary greatly. In a cross-sectional observational study, 300 headache sufferers were asked to rate 33 triggers by frequency and potency.3 Triggers that were perceived to be encountered most days per month were air conditioning and caffeine (both 30 days). Red wine was perceived to be encountered least frequently (0 days). Importantly, interperson variability for frequency of encounter was significant—most ranges for each trigger spanned
15 days or more.3
Triggers that were perceived to increase the chance of a headache were stress (75%), missing a meal, and dehydration (both 60%). Nuts and salty food were least perceived to increase the likelihood of a headache (both 0%). Similar to frequency, perceptions about trigger strengths varied widely, with most ranges spreading by more than 50% for each trigger.3
The authors noted that the heterogeneity seen in frequency of encounters with triggers remained even when 2 patients had matching perceptions about the importance of the trigger in precipitating a headache. The fact that this was observed for nearly every perceived trigger is an important aspect of understanding trigger frequency at the patient-care level.3 Additionally, most headache sufferers do not believe that triggers are simply present or absent. Rather, they have diverse beliefs about the strength of perceived triggers.3 These broad differences among patients make it difficult to predict how an individual headache sufferer will rate triggers.3
The patient’s trigger belief system
Layered onto this challenge is the observation that many migraine sufferers use the so-called trigger belief system to create a safe and controlled environment to stave off headache attacks. Indeed, perception often dictates how headache triggers are experienced, and this adds to the complexity of managing these patients. This is borne out in 2 studies. One, involving more than 200 individuals, revealed that perceived control over and impact of headaches were related to headache severity.4 Another trial involving more than 300 patients seeking treatment for headache showed that participants who were confident they could avoid and better manage their headaches also believed that they could control factors impacting their headaches. Although this led to the use of positive coping strategies, it also triggered more anxiety.5
Trigger avoidance can also lead to reduced quality of life. Consider, for example, physical activity, which is recommended to prevent and manage migraine: As many as 8 in 10 migraine sufferers avoid physical activity because they fear triggering an episode.6 In a recent study, investigators examined the potential correlation between anxiety sensitivity and physical activity avoidance among 100 women with migraine. Participants completed an anonymous survey about migraine and avoiding physical activity. The survey included a tool that measured anxiety sensitivity. Migraine attacks in the previous 30 days were self-reported.6 Higher anxiety scores were linked with more frequent physical activity avoidance. Participants who cited physical concerns were nearly 8 times more likely to avoid vigorous physical activity. Those who mentioned cognitive concerns were more than 5 times more likely to avoid moderate physical activity.6 The authors noted that even though avoiding triggers often is recommended, in some situations it can lead to more migraines because of increased anxiety. For this reason, although avoidance is probably best for certain triggers that have true biologic onset mechanisms, triggers that work through anticipatory anxiety or fearful expectations might best be handled via gradual exposure and desensitization.6
Distinguishing between real triggers and premonitory symptoms
Once clinicians gain an understanding of these factors, they may consider themselves adequately prepared to optimally manage migraine sufferers. Yet, there is one more factor to consider: premonitory symptoms. Separating premonitory symptoms from actual migraine triggers gets the clinician closer to optimal management. Yet, it is not always easy to make the distinction.
Premonitory symptoms typically present 2 to 48 hours (or more) before migraine headache onset. Symptoms include changes in mood and activity, irritability, fatigue, food cravings, repetitive yawning, stiff neck, and photophobia. Because these symptoms can continue even past the premonitory stage, they often can be misinterpreted as actual migraine triggers.7 Premonitory symptoms have been found to accompany 30% to 80% of migraine attacks. Signs that often are confused with actual migraine triggers include neck pain, photophobia, and food cravings.8
Timing of symptoms: Potential clues
It is critical to understand the interval between a symptom and migraine onset, which can help determine whether it is an actual trigger, premonitory symptom, or other manifestation. For this, it helps to review the timeline of a migraine attack. The 4 phases of a migraine attack—premonitory/prodrome, aura, headache, and postdrome—are well known. According to the American Migraine Foundation, the 4 phases are marked by specific symptoms that typically—but not always—occur along a general timeline (Figure 1).9

Although this symptom timeline can help guide clinicians and migraine sufferers, it is important to understand that these phases do not always occur linearly, and there often is significant overlap, which can mislead both the clinician and patient.7
Common triggers
Stress: Stress is the most frequently self-reported migraine trigger, and numerous studies show a link between stress and migraine. However, retrospective and prospective diary studies have not been able to confirm a link between stressful days and acute migraine attacks. Moreover, although 1 study demonstrated that migraines were less likely to occur on holidays or days off, relaxation did not play a role. Fatigue was more common premigraine than mental exhaustion or insomnia, leading investigators to surmise it is a premonitory symptom and not a migraine cause.8 Further confounding is the finding that reduced stress from one day to the next is linked with migraine onset the next day, making it a possible predictor of a migraine attack because of the so-called letdown effect.10
Menstruation: Menstruation appears to be the most common migraine trigger in women. A large 3-month study showed an increase of migraine prevalence and persistence up to 96%. Additionally, diary studies demonstrate menstruation as a trigger, and in a population-based trial, more than half of female migraine sufferers reported an increased rate
of migraine. However, only 4% actually had a migraine during menstruation.8,11
Diet: Studies that seek to find a link between specific foods and migraine are open to question because they do not have a placebo component, and it is difficult to tell the difference between premonitory food cravings and actual triggers. Moreover, in most patients food appears to be 1 of several contributors to migraine headache, and not the only factor.8 Fasting is among the most studied and consistent migraine triggers and seems to be more common during longer fasts. Dehydration is not thought to be a cause; rather, it could be because of hypoglycemia.8 A trial involving more than 3000 women revealed that those with migraine had a lower diet quality than their counterparts who did not experience migraine. Other smaller studies show benefit from low-fat vegan and ketogenic diets. Also, patients with irritable bowel syndrome and migraine might benefit from eliminating such foods that cause irritation to improve both migraine frequency and bowel symptoms.8
Weather: A few small studies have sought to evaluate the link between weather and migraines. One involving 77 individuals with migraine showed that slightly more than half were sensitive to at least 1 weather factor (i.e., barometric changes, lightning, temperature, and precipitation). Another involving 238 participants demonstrated a nonstatistically significant trend toward increased frequency of migraine attacks on days with high pressure, low wind speed, and increased sunshine, but they called the correlation questionable. A study of 90 individuals found lightning to be an independent risk factor for migraine.8
Sensory stimuli: Although visual, aural, olfactory, and other sensory stimuli have been shown to cause migraine in susceptible individuals and worsen migraine intensity, it is difficult to determine if these are true triggers or premonitory symptoms. Indeed, the discomfort thresholds of various sensory stimuli are lower in migraine sufferers.8 Odors are the most commonly reported triggers among all sensory stimuli. In 1 study, 7 in every 10 migraine sufferers reported that odors triggered their attack. Common offenders include perfumes, paint, gasoline, bleach, and rancid odors. Smoke and exhaust fumes were also found to be problematic.8
Best evidence on migraine triggers
A large retrospective survey looked at acute migraine trigger factors in 1750 individuals treated in a clinical practice. A detailed headache evaluation was conducted that included neurologic history, structured headache interview, and a neurologic exam.12 More than three-fourths of patients experienced triggers. Approximately 6 in every 10 patients had between 4 and 9 triggers. Nearly one-fourth experienced 1 to 3 triggers.12 As shown in Figure 2, the 5 triggers that occurred occasionally in at least half of patients were stress (80%), hormone (65%), fasting (57%), weather (53%), and sleep disturbance (50%). The most common triggers, occurring very frequently, were hormone (33%) and stress (26%).12

Trials that use randomized and blinded exposure to possible triggers can—at face value—appear most useful. Potent compounds such as glyceryl trinitrite, prostaglandin I2 and E2, calcitonin-gene related peptide, and pituitary adenylate cyclase-activating polypeptide have been shown to be dependable triggers in the experimental setting. However, trial designs that do not closely mimic real-world experience lack usefulness in clinical practice. Moreover, studies of low intensity migraine triggers that are common in the real world—chocolate and aspartame, for instance—have produced mixed results.13 So-called N-of-1 studies—in which a single patient is repeatedly exposed in blinded fashion to a trigger or placebo—might be the best way to uncover actual triggers. However, migraine sufferers are, understandably, not interested in deliberately inducing an attack.13
This leaves results of diary studies as the most reflective of clinical practice. These evaluations limit the chance of bias that retrospective studies could carry and often provide a significant amount of real-world data that can be used to test hypotheses. Moreover, the advent of smartphone technology enables researchers to capture data in real time, which can help distinguish premonitory symptoms from actual triggers.13 A high-quality paper diary study (PAMINA) involving 327 individuals showed strong evidence that a migraine was likely to occur with the following factors: menstruation, muscle tension/neck pain, psychic tension, and sunshine that lasted 3 or more hours per day.14
Diary studies are also able to assess the ability of migraine sufferers to predict a headache. In an evaluation that used an electronic diary design, researchers looked at self-prediction through use of a single question asked daily: “How likely are you to have a headache today?” When participants reported at the beginning of a particular day that headache was “almost certain,” actual headache occurred more than 90% of the time on that day.13
Leveraging best evidence into best practices
The clinician–patient conversation
A clinician’s detective skills are put to the test when evaluating patients with migraine. Avoiding dead-ends and fruitless pursuits can spare providers and patients frustration and help patients avoid needless suffering. Yet, as noted, the list of potential triggers appears endless, duration and frequency are variable, patients often try to control their environment with mixed results, and it is difficult to tell the difference between actual triggers and premonitory symptoms.
Which brings us back to Lola H. She desperately wanted to leave her appointment with confidence that she had enough medication to meet her needs each month. She might have expected to go home with access to more medication, but she did not. Rather, she left her clinician’s office with a plan to reduce the number of monthly migraine episodes she experienced, enabling her to use medication as needed, rather than only when her attacks were severe.
Her clinician confirmed that avoiding gasoline odor would reduce the number of attacks. Eliminating chocolates and sweets might, but it is not known whether chocolate is a trigger. He suggested that she try allowing herself chocolate now and then, which would probably serve as a stress reducer. The net result would likely be no change in number of episodes, while allowing for a small improvement in quality of life.
Perhaps most striking, Lola left the appointment certain that her issue with lower barometric pressure was resolved because her clinician took the time to ask how she knew about the daily barometric pressure levels.
Her smartwatch, she told him. He strongly suggested that she delete the watch’s barometric pressure app, which she did on the spot. At her next appointment, Lola was happy to report that barometric pressure no longer played a significant role in her episodes. Moreover, the other strategies she and her clinician discussed have reduced the number of severe and less severe episodes. She no longer lives with the stress of pill-rationing and looks forward to enjoying a small amount of chocolate ice cream each weekend with her family.
Lola’s case demonstrates that in clinical practice the best way to elicit meaningful responses from migraine sufferers is to ask open-ended questions. Yet, clinicians often fail to do so. In a study evaluating communication strategies between clinicians and 60 long-term migraineurs (average 14 years) who experienced frequent headaches (average 5 per month), 90% of migraine-specific questions asked during an appointment were either close-ended or consisted of a short answer. Postvisit interviews with both clinicians and patients revealed these pairs misaligned on migraine frequency (55% of the time) and impairment (51%). One-third of patients were candidates to receive preventive medication, yet only 20% of them did so. Prevention was not discussed in half of the visits with patients who were candidates to receive preventive therapy but did not get it.10
A panel with extensive experience in migraine reviewed questions from migraine diaries and noted that migraine sufferers find value in capturing wide-ranging information about their condition, whereas clinicians prefer small amounts of data they perceive as relevant to managing migraine.15 It also is reported that the number of questions patients ask is inversely related to the amount of information a clinician gives.16
Recommended strategies
In his review article, Mamura reinforces the concept of open 2-way communication, which involves patients in their care more deeply and reinforces positive habits.8 It is important to be mindful that discussing triggers exclusively can be counterproductive and frustrating for patients, particularly if the conversation is about trigger avoidance. Patients might feel stigmatized by triggers, come away thinking nothing can be done to control them, and become more anxious about impending episodes.8 In fact, a better strategy could be to work with the patient to train their brain to become acclimated to certain triggers or premonitory symptoms, rather than avoiding them.17
Mamura provides a concise summary of 4 best-practice strategies.8 The first 2 should be factored into advice given to patients about migraine triggers and premonitory symptoms; the remaining 2 help frame the treatment approach:
1. Ask patients to keep a headache diary, which eliminates faulty recall and provides a clearer picture of migraine frequency and severity. Include symptoms and other important factors—missed time from work, canceled family activities, nausea and vomiting, etc. Journaling might uncover triggers that can be eliminated or acclimated to via behavior modification. It also can reveal to the patient that certain triggers might not be as useful in predicting migraine as originally thought.
2. Rather than trigger avoidance, suggest that patients concentrate on healthy lifestyle choices, including maintaining healthy weight, getting sufficient sleep, and exercising regularly. The latter has been shown in some studies to work better than preventive medications. Although a behavior change, such as acclimating to certain noises, is not thought to be a healthy lifestyle habit, it ends up being just that if it improves tolerance and reduces migraine episodes.
3. Recognize that many patients have a fear of experiencing a migraine between episodes. If observed in a patient, treat it aggressively. Left unmanaged, this condition could lead to chronic migraine and medication overuse. The right acute medication might be sufficient, but sometimes cognitive-behavioral therapy and biofeedback techniques could be necessary to address behavioral issues that might be interfering with successful treatment.
4. If attacks are frequent and making it difficult to identify distinctive triggers, consider preventive therapy. Patients might resist using preventive medications because they feel eliminating triggers is sufficient, or they might be concerned about medication cost and adverse effects. Eliminating a stressful event alone likely will not help, so it’s important to review with patients—using plain language—how preventive medication treats the underlying cause of migraine and decreases brain excitability, making the patient less prone to attacks.8

Emerging medications
New treatments offer increased options for the acute and preventive treatment of migraine attacks. New acute pharmacologic treatments include gepants and ditans. The gepants target calcitonin gene-related peptide (CGRP), a potent vasodilator and neurotransmitter. During migraine episodes, serum levels of CGRP increase as the peptide is released, modulating the transmission of nociceptive signals from peripheral trigeminal sensory afferents into the central trigeminal system.19 In clinical trials of acute migraine treatment, the gepants provided pain freedom at 2 hours in approximately 20% of attacks, pain relief at 2 hours in 58%, and freedom from the most bothersome symptom at 2 hours in 36% with significant benefit over placebo.20,21 As these oral formulations became available in 2020, there are not enough data on potential long-term adverse events, particularly cardiovascular effects.18 However, the gepants are not associated with medication overuse headache and there is no contraindication to their use in patients with cardiovascular disease. These agents, currently available for oral administration, also show benefit as preventive agents and parenteral formulations are in development.
Ditans target the serotonin 5HT-1F receptor. Triptans target this receptor as well as the 5HT-1B and 5HT-1D receptors. The 5HT-1B receptor action is responsible for constriction of cerebral and peripheral blood vessels that occurs with triptans. Thus, ditans are also options for treating patients with vascular disease. Lasmiditan, the only compound in this class thus far, yielded pain freedom in 2 hours in 31%, pain relief 2 hours in 64%, and relief of the most bothersome symptom at 2 hours in 41%.22 Lasmiditan crosses the blood-brain barrier. Thus, the most common side effects are dizziness, somnolence, and paresthesias, which are generally mild to moderate in severity. Based on driving simulation studies performed in the absence of migraine, patients should not drive within 8 hours of dosing.
Neuromodulation devices are effective options that avoid potential adverse events of systemic treatments. They include external trigeminal nerve stimulation, noninvasive vagus nerve stimulation, remote electrical neuromodulation, caloric vestibular stimulation, and single-pulse transcranial magnetic stimulation. Other treatments in development for the acute treatment of migraine include improved parenteral delivery systems and combination oral preparations of existing medications.
Monoclonal antibodies that block the CGRP ligand or its receptor were introduced in 2018, with 4 medications currently available. This is the first class of migraine medications specifically designed for migraine prevention. They are administered parenterally (subcutaneously or intravenously) either monthly or quarterly, depending on the medication. The anti-CGRP monoclonal antibodies are indicated for the prevention of episodic and chronic migraine and are eliminated by proteolytic degradation, not involving the liver and reducing the potential for hepatotoxicity and drug-drug interactions.23 A meta-analysis evaluated the subcutaneous formulations studied for chronic migraine, enrolling participants with at least 15 headache days monthly with migraine occurring on at least 8 of those days. In this cohort with a large disease burden, the pooled 50% responder rate 9-12 weeks after administration of the first dose was 37.4% and the 75% responder rate was 12.7%.24 There was also improvement in the number of migraine days, number of days using acute treatment, and total headache hours. The most common side effects were injection site reactions and constipation, with post-marketing reports of hypertension in the compound targeting the CGRP receptor, with or without pre-existing hypertension.25 The safety profile for use during pregnancy was not studied in any of the medications discussed in this section.
Summary
Acute migraine headache is a significant management challenge for neurologists, headache specialists, and other clinicians involved in its treatment. Addressing potential migraine triggers can be a particularly overwhelming task because multiple components and factors play into the disorder. Perceived triggers; their frequency and strength; the effort by patients to control their environment; and the blurred line between real triggers and premonitory symptoms present the clinician with an amalgam of components that must be sorted through, defined, prioritized, and managed. Even though migraine symptoms generally appear along a 4-phase timeline, some symptoms stretch across multiple phases and not all symptoms play out in a linear fashion.
There are specific steps clinicians can take to simplify the process, optimize management, and improve patient outcomes and quality of life. Start by gaining an understanding of common triggers and the best evidence regarding triggers and premonitory symptoms. Use this evidence to modify your approach in practice, if necessary. Specifically:
• Improve the information you gather from patients by asking open-ended questions about their experience during a migraine headache, as well as the period between attacks.
• Rather than having patients tell you how many episodes they experience each month, try to elicit from them how many days per month they feel disabled.
• Ask your patients to keep a headache diary.
• Talk less about trigger avoidance and more about making healthy lifestyle choices.
• Address potential cephalgiaphobia via cognitive-behavioral therapy and biofeedback techniques.
• Consider managing frequent migraine attacks with preventive treatment, which now includes CGRP-targeted medications that are as effective as other agents but have fewer adverse effects and might avoid medication overuse headache.
Dr. Friedman reports being an Advisory Board member for Allergan, Biohaven Pharmaceuticals, Eli Lilly, Impel NeuroPharma, Invex, Lundbeck, Revance Therapeutics, Satsuma Pharmaceuticals, Teva Pharmaceuticals, Theranica, and Zosano Pharma. She has received grant support from Allergan, Eli Lilly, Merck, and Zosano Pharma. She is on the Medical Advisory Board for Spinal CSF Leak Foundation, HealthyWomen, and the Board of Directors of the Southern Headache Society. She currently sits on the Editorial Board of Neurology Reviews and has been a contributing author for MedLink Neurology and Medscape.
To read a digital version of the print supplement, click here.
To gain a full understanding of the challenges faced by neurologists, headache specialists, and other clinicians when managing triggers that prompt migraine headache attacks, consider the case of Lola H, a 35-year-old woman with a history of recurrent headaches that was diagnosed 12 months earlier as migraine without aura. Lola is taking oral sumatriptan, 100 mg, for acute migraine episodes, which she reports experiencing 3 or 4 times a month. She also has up to 3 headaches a week that are less severe. Lola’s insurance carrier limits the number of sumatriptan tablets covered in a month, forcing her use the medication only when she has severe headaches. However, the rationing strategy adds stress to her life that could be contributing to additional headache episodes.
Lola raised this issue during a recent visit with her neurologist, at which time she also presented a lengthy list of things she felt were triggering her attacks: stress, her menstrual cycle, chocolate, the odor of gasoline, and the weather, among other potential causes. Regarding the weather, she reported experiencing headaches—sometimes severe—whenever barometric pressure drops. Lola told her clinician that she believes she has gained control of 2 triggers—chocolate and gasoline fumes—through avoidance. She no longer consumes chocolate and other sweets and relies on family members and friends to fill her automobile fuel tank. However, Lola said she cannot control other triggers and needs her clinician’s help. She insists on leaving the appointment with answers she feels satisfied with. More than anything, she wants to feel secure that she has enough medication to meet her needs. (We will reveal how Lola fared later in this article.)
Heterogeneity of triggers: A daunting challenge
Lola’s circumstances are not uncommon among the nearly 40 million migraine sufferers in the United States.1 For clinicians seeking to better understand how potential triggers cause migraine attacks, it is important to appreciate the fine lines that exist between (1) perceived triggers, (2) their apparent frequency and strength, (3) patients’ efforts to control these factors, and (4) the distinction between real migraine triggers and premonitory symptoms. Moreover, recognizing how each of these 4 issues influence the patient’s experience enables the clinician to optimally manage individuals who experience migraine headache.
A diverse array of potential stimuli
Perhaps the most daunting challenge when trying to uncover what is triggering Lola’s and other migraine patients’ headaches is the realization that triggers and perceived triggers comprise a diverse array of potential stimuli. A meta-analysis of 85 studies conducted over a 60-year period and involving more than 27,000 participants revealed 420 unique headache triggers categorized to 1 of 15 categories.2 Characterizing these triggers as vastly heterogenous is an understatement, considering that they include factors such as raising the arms overhead, sneezing, abstaining from coffee, using a low pillow, weekend sleep habits, use of shower gels, relaxing after stressful events, bus journeys, road stripes, and more.2 The authors of the meta-analysis note that the variability observed likely is because of methodology differences among the studies included in their analysis. For this reason, the authors advised caution in interpreting the results.2
Nevertheless, the investigators concluded that (1) most of those who experience frequent headaches—including migraine sufferers—believe they have at least 1 trigger, and (2) stress is the trigger most frequently cited by patients, followed by sleep and environmental triggers.2
Perceived differences in trigger frequency and strength
Heterogeneity extends beyond the triggers and perceived triggers; incidence and strength also can vary greatly. In a cross-sectional observational study, 300 headache sufferers were asked to rate 33 triggers by frequency and potency.3 Triggers that were perceived to be encountered most days per month were air conditioning and caffeine (both 30 days). Red wine was perceived to be encountered least frequently (0 days). Importantly, interperson variability for frequency of encounter was significant—most ranges for each trigger spanned
15 days or more.3
Triggers that were perceived to increase the chance of a headache were stress (75%), missing a meal, and dehydration (both 60%). Nuts and salty food were least perceived to increase the likelihood of a headache (both 0%). Similar to frequency, perceptions about trigger strengths varied widely, with most ranges spreading by more than 50% for each trigger.3
The authors noted that the heterogeneity seen in frequency of encounters with triggers remained even when 2 patients had matching perceptions about the importance of the trigger in precipitating a headache. The fact that this was observed for nearly every perceived trigger is an important aspect of understanding trigger frequency at the patient-care level.3 Additionally, most headache sufferers do not believe that triggers are simply present or absent. Rather, they have diverse beliefs about the strength of perceived triggers.3 These broad differences among patients make it difficult to predict how an individual headache sufferer will rate triggers.3
The patient’s trigger belief system
Layered onto this challenge is the observation that many migraine sufferers use the so-called trigger belief system to create a safe and controlled environment to stave off headache attacks. Indeed, perception often dictates how headache triggers are experienced, and this adds to the complexity of managing these patients. This is borne out in 2 studies. One, involving more than 200 individuals, revealed that perceived control over and impact of headaches were related to headache severity.4 Another trial involving more than 300 patients seeking treatment for headache showed that participants who were confident they could avoid and better manage their headaches also believed that they could control factors impacting their headaches. Although this led to the use of positive coping strategies, it also triggered more anxiety.5
Trigger avoidance can also lead to reduced quality of life. Consider, for example, physical activity, which is recommended to prevent and manage migraine: As many as 8 in 10 migraine sufferers avoid physical activity because they fear triggering an episode.6 In a recent study, investigators examined the potential correlation between anxiety sensitivity and physical activity avoidance among 100 women with migraine. Participants completed an anonymous survey about migraine and avoiding physical activity. The survey included a tool that measured anxiety sensitivity. Migraine attacks in the previous 30 days were self-reported.6 Higher anxiety scores were linked with more frequent physical activity avoidance. Participants who cited physical concerns were nearly 8 times more likely to avoid vigorous physical activity. Those who mentioned cognitive concerns were more than 5 times more likely to avoid moderate physical activity.6 The authors noted that even though avoiding triggers often is recommended, in some situations it can lead to more migraines because of increased anxiety. For this reason, although avoidance is probably best for certain triggers that have true biologic onset mechanisms, triggers that work through anticipatory anxiety or fearful expectations might best be handled via gradual exposure and desensitization.6
Distinguishing between real triggers and premonitory symptoms
Once clinicians gain an understanding of these factors, they may consider themselves adequately prepared to optimally manage migraine sufferers. Yet, there is one more factor to consider: premonitory symptoms. Separating premonitory symptoms from actual migraine triggers gets the clinician closer to optimal management. Yet, it is not always easy to make the distinction.
Premonitory symptoms typically present 2 to 48 hours (or more) before migraine headache onset. Symptoms include changes in mood and activity, irritability, fatigue, food cravings, repetitive yawning, stiff neck, and photophobia. Because these symptoms can continue even past the premonitory stage, they often can be misinterpreted as actual migraine triggers.7 Premonitory symptoms have been found to accompany 30% to 80% of migraine attacks. Signs that often are confused with actual migraine triggers include neck pain, photophobia, and food cravings.8
Timing of symptoms: Potential clues
It is critical to understand the interval between a symptom and migraine onset, which can help determine whether it is an actual trigger, premonitory symptom, or other manifestation. For this, it helps to review the timeline of a migraine attack. The 4 phases of a migraine attack—premonitory/prodrome, aura, headache, and postdrome—are well known. According to the American Migraine Foundation, the 4 phases are marked by specific symptoms that typically—but not always—occur along a general timeline (Figure 1).9

Although this symptom timeline can help guide clinicians and migraine sufferers, it is important to understand that these phases do not always occur linearly, and there often is significant overlap, which can mislead both the clinician and patient.7
Common triggers
Stress: Stress is the most frequently self-reported migraine trigger, and numerous studies show a link between stress and migraine. However, retrospective and prospective diary studies have not been able to confirm a link between stressful days and acute migraine attacks. Moreover, although 1 study demonstrated that migraines were less likely to occur on holidays or days off, relaxation did not play a role. Fatigue was more common premigraine than mental exhaustion or insomnia, leading investigators to surmise it is a premonitory symptom and not a migraine cause.8 Further confounding is the finding that reduced stress from one day to the next is linked with migraine onset the next day, making it a possible predictor of a migraine attack because of the so-called letdown effect.10
Menstruation: Menstruation appears to be the most common migraine trigger in women. A large 3-month study showed an increase of migraine prevalence and persistence up to 96%. Additionally, diary studies demonstrate menstruation as a trigger, and in a population-based trial, more than half of female migraine sufferers reported an increased rate
of migraine. However, only 4% actually had a migraine during menstruation.8,11
Diet: Studies that seek to find a link between specific foods and migraine are open to question because they do not have a placebo component, and it is difficult to tell the difference between premonitory food cravings and actual triggers. Moreover, in most patients food appears to be 1 of several contributors to migraine headache, and not the only factor.8 Fasting is among the most studied and consistent migraine triggers and seems to be more common during longer fasts. Dehydration is not thought to be a cause; rather, it could be because of hypoglycemia.8 A trial involving more than 3000 women revealed that those with migraine had a lower diet quality than their counterparts who did not experience migraine. Other smaller studies show benefit from low-fat vegan and ketogenic diets. Also, patients with irritable bowel syndrome and migraine might benefit from eliminating such foods that cause irritation to improve both migraine frequency and bowel symptoms.8
Weather: A few small studies have sought to evaluate the link between weather and migraines. One involving 77 individuals with migraine showed that slightly more than half were sensitive to at least 1 weather factor (i.e., barometric changes, lightning, temperature, and precipitation). Another involving 238 participants demonstrated a nonstatistically significant trend toward increased frequency of migraine attacks on days with high pressure, low wind speed, and increased sunshine, but they called the correlation questionable. A study of 90 individuals found lightning to be an independent risk factor for migraine.8
Sensory stimuli: Although visual, aural, olfactory, and other sensory stimuli have been shown to cause migraine in susceptible individuals and worsen migraine intensity, it is difficult to determine if these are true triggers or premonitory symptoms. Indeed, the discomfort thresholds of various sensory stimuli are lower in migraine sufferers.8 Odors are the most commonly reported triggers among all sensory stimuli. In 1 study, 7 in every 10 migraine sufferers reported that odors triggered their attack. Common offenders include perfumes, paint, gasoline, bleach, and rancid odors. Smoke and exhaust fumes were also found to be problematic.8
Best evidence on migraine triggers
A large retrospective survey looked at acute migraine trigger factors in 1750 individuals treated in a clinical practice. A detailed headache evaluation was conducted that included neurologic history, structured headache interview, and a neurologic exam.12 More than three-fourths of patients experienced triggers. Approximately 6 in every 10 patients had between 4 and 9 triggers. Nearly one-fourth experienced 1 to 3 triggers.12 As shown in Figure 2, the 5 triggers that occurred occasionally in at least half of patients were stress (80%), hormone (65%), fasting (57%), weather (53%), and sleep disturbance (50%). The most common triggers, occurring very frequently, were hormone (33%) and stress (26%).12

Trials that use randomized and blinded exposure to possible triggers can—at face value—appear most useful. Potent compounds such as glyceryl trinitrite, prostaglandin I2 and E2, calcitonin-gene related peptide, and pituitary adenylate cyclase-activating polypeptide have been shown to be dependable triggers in the experimental setting. However, trial designs that do not closely mimic real-world experience lack usefulness in clinical practice. Moreover, studies of low intensity migraine triggers that are common in the real world—chocolate and aspartame, for instance—have produced mixed results.13 So-called N-of-1 studies—in which a single patient is repeatedly exposed in blinded fashion to a trigger or placebo—might be the best way to uncover actual triggers. However, migraine sufferers are, understandably, not interested in deliberately inducing an attack.13
This leaves results of diary studies as the most reflective of clinical practice. These evaluations limit the chance of bias that retrospective studies could carry and often provide a significant amount of real-world data that can be used to test hypotheses. Moreover, the advent of smartphone technology enables researchers to capture data in real time, which can help distinguish premonitory symptoms from actual triggers.13 A high-quality paper diary study (PAMINA) involving 327 individuals showed strong evidence that a migraine was likely to occur with the following factors: menstruation, muscle tension/neck pain, psychic tension, and sunshine that lasted 3 or more hours per day.14
Diary studies are also able to assess the ability of migraine sufferers to predict a headache. In an evaluation that used an electronic diary design, researchers looked at self-prediction through use of a single question asked daily: “How likely are you to have a headache today?” When participants reported at the beginning of a particular day that headache was “almost certain,” actual headache occurred more than 90% of the time on that day.13
Leveraging best evidence into best practices
The clinician–patient conversation
A clinician’s detective skills are put to the test when evaluating patients with migraine. Avoiding dead-ends and fruitless pursuits can spare providers and patients frustration and help patients avoid needless suffering. Yet, as noted, the list of potential triggers appears endless, duration and frequency are variable, patients often try to control their environment with mixed results, and it is difficult to tell the difference between actual triggers and premonitory symptoms.
Which brings us back to Lola H. She desperately wanted to leave her appointment with confidence that she had enough medication to meet her needs each month. She might have expected to go home with access to more medication, but she did not. Rather, she left her clinician’s office with a plan to reduce the number of monthly migraine episodes she experienced, enabling her to use medication as needed, rather than only when her attacks were severe.
Her clinician confirmed that avoiding gasoline odor would reduce the number of attacks. Eliminating chocolates and sweets might, but it is not known whether chocolate is a trigger. He suggested that she try allowing herself chocolate now and then, which would probably serve as a stress reducer. The net result would likely be no change in number of episodes, while allowing for a small improvement in quality of life.
Perhaps most striking, Lola left the appointment certain that her issue with lower barometric pressure was resolved because her clinician took the time to ask how she knew about the daily barometric pressure levels.
Her smartwatch, she told him. He strongly suggested that she delete the watch’s barometric pressure app, which she did on the spot. At her next appointment, Lola was happy to report that barometric pressure no longer played a significant role in her episodes. Moreover, the other strategies she and her clinician discussed have reduced the number of severe and less severe episodes. She no longer lives with the stress of pill-rationing and looks forward to enjoying a small amount of chocolate ice cream each weekend with her family.
Lola’s case demonstrates that in clinical practice the best way to elicit meaningful responses from migraine sufferers is to ask open-ended questions. Yet, clinicians often fail to do so. In a study evaluating communication strategies between clinicians and 60 long-term migraineurs (average 14 years) who experienced frequent headaches (average 5 per month), 90% of migraine-specific questions asked during an appointment were either close-ended or consisted of a short answer. Postvisit interviews with both clinicians and patients revealed these pairs misaligned on migraine frequency (55% of the time) and impairment (51%). One-third of patients were candidates to receive preventive medication, yet only 20% of them did so. Prevention was not discussed in half of the visits with patients who were candidates to receive preventive therapy but did not get it.10
A panel with extensive experience in migraine reviewed questions from migraine diaries and noted that migraine sufferers find value in capturing wide-ranging information about their condition, whereas clinicians prefer small amounts of data they perceive as relevant to managing migraine.15 It also is reported that the number of questions patients ask is inversely related to the amount of information a clinician gives.16
Recommended strategies
In his review article, Mamura reinforces the concept of open 2-way communication, which involves patients in their care more deeply and reinforces positive habits.8 It is important to be mindful that discussing triggers exclusively can be counterproductive and frustrating for patients, particularly if the conversation is about trigger avoidance. Patients might feel stigmatized by triggers, come away thinking nothing can be done to control them, and become more anxious about impending episodes.8 In fact, a better strategy could be to work with the patient to train their brain to become acclimated to certain triggers or premonitory symptoms, rather than avoiding them.17
Mamura provides a concise summary of 4 best-practice strategies.8 The first 2 should be factored into advice given to patients about migraine triggers and premonitory symptoms; the remaining 2 help frame the treatment approach:
1. Ask patients to keep a headache diary, which eliminates faulty recall and provides a clearer picture of migraine frequency and severity. Include symptoms and other important factors—missed time from work, canceled family activities, nausea and vomiting, etc. Journaling might uncover triggers that can be eliminated or acclimated to via behavior modification. It also can reveal to the patient that certain triggers might not be as useful in predicting migraine as originally thought.
2. Rather than trigger avoidance, suggest that patients concentrate on healthy lifestyle choices, including maintaining healthy weight, getting sufficient sleep, and exercising regularly. The latter has been shown in some studies to work better than preventive medications. Although a behavior change, such as acclimating to certain noises, is not thought to be a healthy lifestyle habit, it ends up being just that if it improves tolerance and reduces migraine episodes.
3. Recognize that many patients have a fear of experiencing a migraine between episodes. If observed in a patient, treat it aggressively. Left unmanaged, this condition could lead to chronic migraine and medication overuse. The right acute medication might be sufficient, but sometimes cognitive-behavioral therapy and biofeedback techniques could be necessary to address behavioral issues that might be interfering with successful treatment.
4. If attacks are frequent and making it difficult to identify distinctive triggers, consider preventive therapy. Patients might resist using preventive medications because they feel eliminating triggers is sufficient, or they might be concerned about medication cost and adverse effects. Eliminating a stressful event alone likely will not help, so it’s important to review with patients—using plain language—how preventive medication treats the underlying cause of migraine and decreases brain excitability, making the patient less prone to attacks.8

Emerging medications
New treatments offer increased options for the acute and preventive treatment of migraine attacks. New acute pharmacologic treatments include gepants and ditans. The gepants target calcitonin gene-related peptide (CGRP), a potent vasodilator and neurotransmitter. During migraine episodes, serum levels of CGRP increase as the peptide is released, modulating the transmission of nociceptive signals from peripheral trigeminal sensory afferents into the central trigeminal system.19 In clinical trials of acute migraine treatment, the gepants provided pain freedom at 2 hours in approximately 20% of attacks, pain relief at 2 hours in 58%, and freedom from the most bothersome symptom at 2 hours in 36% with significant benefit over placebo.20,21 As these oral formulations became available in 2020, there are not enough data on potential long-term adverse events, particularly cardiovascular effects.18 However, the gepants are not associated with medication overuse headache and there is no contraindication to their use in patients with cardiovascular disease. These agents, currently available for oral administration, also show benefit as preventive agents and parenteral formulations are in development.
Ditans target the serotonin 5HT-1F receptor. Triptans target this receptor as well as the 5HT-1B and 5HT-1D receptors. The 5HT-1B receptor action is responsible for constriction of cerebral and peripheral blood vessels that occurs with triptans. Thus, ditans are also options for treating patients with vascular disease. Lasmiditan, the only compound in this class thus far, yielded pain freedom in 2 hours in 31%, pain relief 2 hours in 64%, and relief of the most bothersome symptom at 2 hours in 41%.22 Lasmiditan crosses the blood-brain barrier. Thus, the most common side effects are dizziness, somnolence, and paresthesias, which are generally mild to moderate in severity. Based on driving simulation studies performed in the absence of migraine, patients should not drive within 8 hours of dosing.
Neuromodulation devices are effective options that avoid potential adverse events of systemic treatments. They include external trigeminal nerve stimulation, noninvasive vagus nerve stimulation, remote electrical neuromodulation, caloric vestibular stimulation, and single-pulse transcranial magnetic stimulation. Other treatments in development for the acute treatment of migraine include improved parenteral delivery systems and combination oral preparations of existing medications.
Monoclonal antibodies that block the CGRP ligand or its receptor were introduced in 2018, with 4 medications currently available. This is the first class of migraine medications specifically designed for migraine prevention. They are administered parenterally (subcutaneously or intravenously) either monthly or quarterly, depending on the medication. The anti-CGRP monoclonal antibodies are indicated for the prevention of episodic and chronic migraine and are eliminated by proteolytic degradation, not involving the liver and reducing the potential for hepatotoxicity and drug-drug interactions.23 A meta-analysis evaluated the subcutaneous formulations studied for chronic migraine, enrolling participants with at least 15 headache days monthly with migraine occurring on at least 8 of those days. In this cohort with a large disease burden, the pooled 50% responder rate 9-12 weeks after administration of the first dose was 37.4% and the 75% responder rate was 12.7%.24 There was also improvement in the number of migraine days, number of days using acute treatment, and total headache hours. The most common side effects were injection site reactions and constipation, with post-marketing reports of hypertension in the compound targeting the CGRP receptor, with or without pre-existing hypertension.25 The safety profile for use during pregnancy was not studied in any of the medications discussed in this section.
Summary
Acute migraine headache is a significant management challenge for neurologists, headache specialists, and other clinicians involved in its treatment. Addressing potential migraine triggers can be a particularly overwhelming task because multiple components and factors play into the disorder. Perceived triggers; their frequency and strength; the effort by patients to control their environment; and the blurred line between real triggers and premonitory symptoms present the clinician with an amalgam of components that must be sorted through, defined, prioritized, and managed. Even though migraine symptoms generally appear along a 4-phase timeline, some symptoms stretch across multiple phases and not all symptoms play out in a linear fashion.
There are specific steps clinicians can take to simplify the process, optimize management, and improve patient outcomes and quality of life. Start by gaining an understanding of common triggers and the best evidence regarding triggers and premonitory symptoms. Use this evidence to modify your approach in practice, if necessary. Specifically:
• Improve the information you gather from patients by asking open-ended questions about their experience during a migraine headache, as well as the period between attacks.
• Rather than having patients tell you how many episodes they experience each month, try to elicit from them how many days per month they feel disabled.
• Ask your patients to keep a headache diary.
• Talk less about trigger avoidance and more about making healthy lifestyle choices.
• Address potential cephalgiaphobia via cognitive-behavioral therapy and biofeedback techniques.
• Consider managing frequent migraine attacks with preventive treatment, which now includes CGRP-targeted medications that are as effective as other agents but have fewer adverse effects and might avoid medication overuse headache.
Dr. Friedman reports being an Advisory Board member for Allergan, Biohaven Pharmaceuticals, Eli Lilly, Impel NeuroPharma, Invex, Lundbeck, Revance Therapeutics, Satsuma Pharmaceuticals, Teva Pharmaceuticals, Theranica, and Zosano Pharma. She has received grant support from Allergan, Eli Lilly, Merck, and Zosano Pharma. She is on the Medical Advisory Board for Spinal CSF Leak Foundation, HealthyWomen, and the Board of Directors of the Southern Headache Society. She currently sits on the Editorial Board of Neurology Reviews and has been a contributing author for MedLink Neurology and Medscape.
1. Migraine Research Foundation. Migraine facts. Accessed January 24, 2021. https://migraineresearchfoundation.org/about-migraine/migraine-facts.
2. Pellegrino A, Davis-Martin R, Houle T, Turner D, Smitherman T. Perceived triggers of primary headache disorders: a meta-analysis. Cephalalgia. 2018;38(6):1188-1198.
3. Turner D, Houle T. Influences on headache trigger beliefs and perceptions. Cephalalgia. 2018;38(9):1545-1553.
4. Scharff L, Turk D, Marcus D. The relationship of locus of control and psychosocial‐behavioral response in chronic headache. Headache. 1995;35(9):527-533.
5. French D, Holroyd K, Pinell C, Malinoski P, O’Donnell F, Hill K. Perceived self‐efficacy and headache‐related disability. Headache. 2000;40(8):647-656.
6. Farris S, Thomas J, Abrantes A, et al. Anxiety sensitivity and intentional avoidance of physical activity in women with probable migraine. Cephalalgia. 2019;39(11):1465-1469.
7. Goadsby P, Holland P, Martins-Oliveira M, Hoffman J, Schankin C, Akerman S. Pathophysiology of migraine: A disorder of sensory processing. Physiol Rev. 2017;97(2):553-622.
8. Mamura M. Triggers, protectors, and predictors in episodic migraine. Curr Pain Headache Rep. 2018;22:81.
9. American Migraine Foundation. Understanding migraine progression can help you anticipate and manage your symptoms. Accessed December 30, 2020. https://americanmigrainefoundation.org/resource-library/timeline-migraine-attack.
10. Lipton R, Buse D, Hall C, et al. Reduction in perceived stress as a migraine trigger: Testing the “let-down headache” hypothesis. Neurology. 2014;82:1395-1401.
11. Loder E. Prophylaxis of menstrual migraine with triptans: Problems and possibilities. Neurology. 2002;59(11):1677-1681.
12. Kelman L. The triggers or precipitants of the acute migraine attack. Cephalalgia. 2007;27:394-402.
13. Pavolic J, Buse D, Sollars M, Haut S, Lipton R. Trigger factors and premonitory features of migraine attacks: Summary of studies. Headache. 2014;54(10):1670-1679.
14. Wöber C, Brannath W, Schmidt K, et al; PAMINA Study Group. Prospective analysis of factors related to migraine attacks: The PAMINA study. Cephalalgia. 2007;27:304-314.
15. Dodick D, Tepper S, Lipton R, et al. Improving medical communication in migraine management: A modified Delphi study to develop a digital migraine tracker. Headache. 2018;58(9):1358-1372.
16. Sheftell F. Communicating the right therapy for the right patient at the right time: Acute therapy. Can J Neurol Sci. 2002;29(suppl 2):S33-S39.
17. Goadsby P, Silberstein S. Migraine triggers: Harnessing the messages of clinical practice. Neurology. 2013;80:434-425.
18. Woo M. Refining a marvel. Nature. 2020;586:s4-s6.
19. Dodick DW. Migraine. Lancet. 2018;10(127):1315-30.
20. Croop R, Goadsby P. J., Stock D. A. et al. Efficacy and safety of rimegepant for the acute treatment of migraine: Evidence from randomized controlled trials. Front Pharmacol. 2020;24 https://doi.org/10.3389/fphar.2019.01577.
21. Yang Y, Chen C, Sun Y et al. Safety and efficacy of ubrogepant for acute treatment of episodic migraine: A meta-analysis of randomized clinical trials. CNS Drugs. 2020;34:463-71.
22. Curto M, Cipolla F, Cisale GY et al. Profiling lasmiditan as a treatment option for migraine. Exp Rev Pharmacother. 2020;21(2):147-53.
23. Raffaelli B, Reuter U. The biology of monoclonal antibodies: Focus on calcitonin gene-related peptide for prophylactic migraine therapy. Neurotherapeutics. 2018;15(2): 324-35.
24. Han L, Lui Y, Xiong H et al. CRP monoclonal antibody for preventive treatment of chronic migraine: An update of meta-analysis. Brain Behav. 2019;9(2):e01215.
25. Saely S, Croteau D, Jawidzik L et al. Hypertension: A new safety risk for patients treated with erenumab. Headache. 2021 (epub ahead of print) https://doi.org/10.1111/head.14051.
1. Migraine Research Foundation. Migraine facts. Accessed January 24, 2021. https://migraineresearchfoundation.org/about-migraine/migraine-facts.
2. Pellegrino A, Davis-Martin R, Houle T, Turner D, Smitherman T. Perceived triggers of primary headache disorders: a meta-analysis. Cephalalgia. 2018;38(6):1188-1198.
3. Turner D, Houle T. Influences on headache trigger beliefs and perceptions. Cephalalgia. 2018;38(9):1545-1553.
4. Scharff L, Turk D, Marcus D. The relationship of locus of control and psychosocial‐behavioral response in chronic headache. Headache. 1995;35(9):527-533.
5. French D, Holroyd K, Pinell C, Malinoski P, O’Donnell F, Hill K. Perceived self‐efficacy and headache‐related disability. Headache. 2000;40(8):647-656.
6. Farris S, Thomas J, Abrantes A, et al. Anxiety sensitivity and intentional avoidance of physical activity in women with probable migraine. Cephalalgia. 2019;39(11):1465-1469.
7. Goadsby P, Holland P, Martins-Oliveira M, Hoffman J, Schankin C, Akerman S. Pathophysiology of migraine: A disorder of sensory processing. Physiol Rev. 2017;97(2):553-622.
8. Mamura M. Triggers, protectors, and predictors in episodic migraine. Curr Pain Headache Rep. 2018;22:81.
9. American Migraine Foundation. Understanding migraine progression can help you anticipate and manage your symptoms. Accessed December 30, 2020. https://americanmigrainefoundation.org/resource-library/timeline-migraine-attack.
10. Lipton R, Buse D, Hall C, et al. Reduction in perceived stress as a migraine trigger: Testing the “let-down headache” hypothesis. Neurology. 2014;82:1395-1401.
11. Loder E. Prophylaxis of menstrual migraine with triptans: Problems and possibilities. Neurology. 2002;59(11):1677-1681.
12. Kelman L. The triggers or precipitants of the acute migraine attack. Cephalalgia. 2007;27:394-402.
13. Pavolic J, Buse D, Sollars M, Haut S, Lipton R. Trigger factors and premonitory features of migraine attacks: Summary of studies. Headache. 2014;54(10):1670-1679.
14. Wöber C, Brannath W, Schmidt K, et al; PAMINA Study Group. Prospective analysis of factors related to migraine attacks: The PAMINA study. Cephalalgia. 2007;27:304-314.
15. Dodick D, Tepper S, Lipton R, et al. Improving medical communication in migraine management: A modified Delphi study to develop a digital migraine tracker. Headache. 2018;58(9):1358-1372.
16. Sheftell F. Communicating the right therapy for the right patient at the right time: Acute therapy. Can J Neurol Sci. 2002;29(suppl 2):S33-S39.
17. Goadsby P, Silberstein S. Migraine triggers: Harnessing the messages of clinical practice. Neurology. 2013;80:434-425.
18. Woo M. Refining a marvel. Nature. 2020;586:s4-s6.
19. Dodick DW. Migraine. Lancet. 2018;10(127):1315-30.
20. Croop R, Goadsby P. J., Stock D. A. et al. Efficacy and safety of rimegepant for the acute treatment of migraine: Evidence from randomized controlled trials. Front Pharmacol. 2020;24 https://doi.org/10.3389/fphar.2019.01577.
21. Yang Y, Chen C, Sun Y et al. Safety and efficacy of ubrogepant for acute treatment of episodic migraine: A meta-analysis of randomized clinical trials. CNS Drugs. 2020;34:463-71.
22. Curto M, Cipolla F, Cisale GY et al. Profiling lasmiditan as a treatment option for migraine. Exp Rev Pharmacother. 2020;21(2):147-53.
23. Raffaelli B, Reuter U. The biology of monoclonal antibodies: Focus on calcitonin gene-related peptide for prophylactic migraine therapy. Neurotherapeutics. 2018;15(2): 324-35.
24. Han L, Lui Y, Xiong H et al. CRP monoclonal antibody for preventive treatment of chronic migraine: An update of meta-analysis. Brain Behav. 2019;9(2):e01215.
25. Saely S, Croteau D, Jawidzik L et al. Hypertension: A new safety risk for patients treated with erenumab. Headache. 2021 (epub ahead of print) https://doi.org/10.1111/head.14051.
How has COVID-19 affected metastatic breast cancer treatment decisions?
Patients with metastatic breast cancer (MBC) face an elevated risk of severe illness or dying from COVID-19. Given the slow rollout of the Moderna and Pfizer COVID vaccines and new, more infectious viral variants circulating in the United States, oncologists will face a challenging balancing act for the foreseeable future: sustaining patients› MBC care while safeguarding them from COVID. The scale leans heavily toward continuing treatment, experts say.
“If we stop treatment for metastatic breast cancer, death will occur quickly,” Fatima F. Cardoso, MD, director of the breast unit at Champalimaud Clinical Centre in Lisbon, Portugal, stated this past August in a Medscape perspective.
Joanne Mortimer, MD, director of Women’s Cancer Programs at City of Hope, a comprehensive cancer center near Los Angeles, expressed a similar sentiment. “Having MBC is worse than getting COVID,” she told Medscape. “We can’t stop treating patients with MBC because of concerns of exposure.”
But maintaining treatment does not mean business as usual. Oncologists have had to modify their pre-pandemic practices to some degree, and that degree largely depends on local COVID conditions.
“Weighing the risk of treatment with the risk of contracting the virus means that many places have carried on treating metastatic breast cancer in a more thoughtful, careful way,” said Jill Dietz, MD, president of the American Society of Breast Surgeons. “That means focusing on high-value treatments for patients with the goal of maximizing their outcomes and quality of life while limiting in-person visits and potentially unnecessary elements of care.”
To guide this more careful approach, Dr. Dietz and colleagues from the recently formed COVID-19 Pandemic Breast Cancer Consortium published recommendations in April 2020 to account for different disease types and severities. These recommendations align closely with those from Cardoso and colleagues in Europe, also published last April.
Although issued early in the pandemic when there was greater uncertainty about viral transmission, adverse outcomes, and treatment for COVID-19, these recommendations still hold almost a year later.
“The framework has proven to be timeless in that it can help institutions where they are in the pandemic,” Dr. Dietz said.
The recommendations at play
For MBC, in particular, Dr. Dietz and colleagues focused on patients who need systemic care but whose treatment can be modified to keep them home more. The modifications include prescribing oral agents such as capecitabine, vinorelbine, and cyclophosphamide to minimize visits to the hospital or infusion suite.
To limit adverse events associated with these oral drugs, Dr. Dietz and colleagues also recommended reducing the dose when possible. For instance, research shows that lowering the dose of the CDK4/6 inhibitor palbociclib in patients with HR+/HER2-negative MBC does not diminish efficacy.
When oral agents are not an option, Dr. Dietz and colleagues suggested stretching out the intervals for chemotherapy infusions or injections. Data show that trastuzumab and pertuzumab injections for metastatic HER2-positive tumors «may reasonably be administered at longer intervals,» such as 4 weeks instead of 3 weeks.
The extent to which oncologists have applied these recommendations hinges on two factors: the local severity of COVID-19 cases and institution-specific policies.
For some oncologists, the pandemic has largely left treatment decisions untouched. “COVID-19 has only minimally impacted my practice,” said Rita Nanda, MD, director of the Breast Oncology Program and associate professor of medicine at University of Chicago Medicine.
Dr. Nanda recalled her concerns in the early days of the pandemic. As the nation watched COVID cases surge across New York City, Chicagoans prepared for the worst, fashioning the McCormick Place Convention Center into a field hospital for COVID patients.
“But fortunately, we were never overwhelmed at the University of Chicago and never needed to use the convention center,” Dr. Nanda said. “We did not have to alter or limit the type of therapy patients with MBC could receive.”
The main changes described by Dr. Nanda at the University of Chicago have centered around limiting the flow of traffic within the infusion suite or hospital by implementing prescreening checks to catch patients with COVID symptoms, keeping waiting rooms empty and infusion centers socially distanced, and having patients come in for appointments solo. The university’s home phlebotomy service also came in handy. Implemented before the pandemic, this service allowed patients with MBC to get their labs done at home before coming in for treatment.
“Overall, with social distancing, mask wearing, and limiting who comes in to the clinic, we have been able to keep patients and staff safe without altering treatment-specific decisions,” Dr. Nanda said.
Streamlining the foot traffic in the cancer center also worked well for Lisa A. Carey, MD, chief of the Division of Hematology/Oncology and deputy director of clinical sciences at University of North Carolina-Chapel Hill. “The truth is, oncological principles of care are still in place,” said Dr. Carey, the Richardson and Marilyn Jacobs Preyer Distinguished Professor in Breast Cancer Research. “COVID hasn’t altered those; it has only thrown a little wrench in how we deliver that care.”
Kelly McCann, MD, PhD, a hematologist/oncologist in the Department of Medicine at the David Geffen School of Medicine, University of California, Los Angeles, has had to walk a tighter rope to protect her patients with MBC, as COVID cases began to soar in LA county last fall.
To keep patients with MBC home more, Dr. McCann said many chose oral cytotoxic chemotherapies over infusional therapies. Some patients with HER2-positive MBC, for instance, opted for the oral combination neratinib + capecitabine over a trastuzumab-deruxtecan infusion, and others with triple-negative tumors chose capecitabine over a taxane.
At City of Hope, Mortimer has had a different experience of LA county’s COVID surge. Because the center only treats patients with cancer, “we have not had huge numbers of patients with COVID or had to significantly modify our practice outside of screening patients who come in and doing fewer scans compared to pre-COVID,” she said. With these precautions, “we have had no internal transmission of COVID within our institution.”
Still, patients with MBC have gotten COVID, and treating both illnesses does complicate decision-making. In some cases, Mortimer has postponed cancer treatment for patients who can delay for a few weeks while they recover from COVID. But patients who need to continue MBC treatment receive care in a separate unit, and Mortimer considers prescribing Eli Lilly’s recently approved antibody therapy bamlanivimab to treat COVID symptoms. “For each situation, we can always page our infectious disease experts to address any concerns or questions,” Dr. Mortimer said.
The MBC and COVID toll
The biggest hurdle for patients with MBC has been less about treatment decisions and more about handling the psychological toll of the pandemic, according to Charles Shapiro, MD, medical oncologist, Icahn School of Medicine at Mount Sinai in New York City.
“These are my personal observations, but I’ve seen how much more stressful it is to have metastatic breast cancer during the pandemic,” said Dr. Shapiro, who worries that fear of COVID may fuel or exacerbate patients’ depression and anxiety. “Patients can’t have family and friends by their side during infusions or appointments, and many feel isolated because of the risk of exposure.”
Because most patients with MBC still need in-person care such as exams, blood draws, or chemotherapy infusions, Dr. McCann has found that many of her patients “are afraid to come to a medical center and have been delaying appointments, imaging, and procedures.”
The psychological toll of treating breast cancer during the pandemic has touched oncologists as well. A recent survey found that burnout scores were significantly higher among physicians whose patients experienced delays in care, including chemotherapy or specialty consultations.
Getting patients vaccinated will improve protection and hopefully lessen fears surrounding COVID infection and transmission. Preliminary recommendations from the National Comprehensive Cancer Network›s COVID-19 Vaccination Advisory Committee state that patients with cancer «should be prioritized for vaccination.»
Dr. McCann agreed. “I’ve recommended COVID-19 vaccination to all of my patients with MBC,” she said. But because a lot of these therapies suppress the immune system to some degree, “I’ll recommend a period of time for vaccination in which the immune system is expected to have recovered, such as in the days prior to a dose of chemotherapy.”
Overall, according to Dr. Carey, institutional responses to treating MBC during the pandemic have been very similar: The key has been that “no one is keeping secrets,” she said. “Our global oncology community is sharing and adopting best practices. Our focus has been doing right by our patients.”
A version of this article first appeared on Medscape.com.
Patients with metastatic breast cancer (MBC) face an elevated risk of severe illness or dying from COVID-19. Given the slow rollout of the Moderna and Pfizer COVID vaccines and new, more infectious viral variants circulating in the United States, oncologists will face a challenging balancing act for the foreseeable future: sustaining patients› MBC care while safeguarding them from COVID. The scale leans heavily toward continuing treatment, experts say.
“If we stop treatment for metastatic breast cancer, death will occur quickly,” Fatima F. Cardoso, MD, director of the breast unit at Champalimaud Clinical Centre in Lisbon, Portugal, stated this past August in a Medscape perspective.
Joanne Mortimer, MD, director of Women’s Cancer Programs at City of Hope, a comprehensive cancer center near Los Angeles, expressed a similar sentiment. “Having MBC is worse than getting COVID,” she told Medscape. “We can’t stop treating patients with MBC because of concerns of exposure.”
But maintaining treatment does not mean business as usual. Oncologists have had to modify their pre-pandemic practices to some degree, and that degree largely depends on local COVID conditions.
“Weighing the risk of treatment with the risk of contracting the virus means that many places have carried on treating metastatic breast cancer in a more thoughtful, careful way,” said Jill Dietz, MD, president of the American Society of Breast Surgeons. “That means focusing on high-value treatments for patients with the goal of maximizing their outcomes and quality of life while limiting in-person visits and potentially unnecessary elements of care.”
To guide this more careful approach, Dr. Dietz and colleagues from the recently formed COVID-19 Pandemic Breast Cancer Consortium published recommendations in April 2020 to account for different disease types and severities. These recommendations align closely with those from Cardoso and colleagues in Europe, also published last April.
Although issued early in the pandemic when there was greater uncertainty about viral transmission, adverse outcomes, and treatment for COVID-19, these recommendations still hold almost a year later.
“The framework has proven to be timeless in that it can help institutions where they are in the pandemic,” Dr. Dietz said.
The recommendations at play
For MBC, in particular, Dr. Dietz and colleagues focused on patients who need systemic care but whose treatment can be modified to keep them home more. The modifications include prescribing oral agents such as capecitabine, vinorelbine, and cyclophosphamide to minimize visits to the hospital or infusion suite.
To limit adverse events associated with these oral drugs, Dr. Dietz and colleagues also recommended reducing the dose when possible. For instance, research shows that lowering the dose of the CDK4/6 inhibitor palbociclib in patients with HR+/HER2-negative MBC does not diminish efficacy.
When oral agents are not an option, Dr. Dietz and colleagues suggested stretching out the intervals for chemotherapy infusions or injections. Data show that trastuzumab and pertuzumab injections for metastatic HER2-positive tumors «may reasonably be administered at longer intervals,» such as 4 weeks instead of 3 weeks.
The extent to which oncologists have applied these recommendations hinges on two factors: the local severity of COVID-19 cases and institution-specific policies.
For some oncologists, the pandemic has largely left treatment decisions untouched. “COVID-19 has only minimally impacted my practice,” said Rita Nanda, MD, director of the Breast Oncology Program and associate professor of medicine at University of Chicago Medicine.
Dr. Nanda recalled her concerns in the early days of the pandemic. As the nation watched COVID cases surge across New York City, Chicagoans prepared for the worst, fashioning the McCormick Place Convention Center into a field hospital for COVID patients.
“But fortunately, we were never overwhelmed at the University of Chicago and never needed to use the convention center,” Dr. Nanda said. “We did not have to alter or limit the type of therapy patients with MBC could receive.”
The main changes described by Dr. Nanda at the University of Chicago have centered around limiting the flow of traffic within the infusion suite or hospital by implementing prescreening checks to catch patients with COVID symptoms, keeping waiting rooms empty and infusion centers socially distanced, and having patients come in for appointments solo. The university’s home phlebotomy service also came in handy. Implemented before the pandemic, this service allowed patients with MBC to get their labs done at home before coming in for treatment.
“Overall, with social distancing, mask wearing, and limiting who comes in to the clinic, we have been able to keep patients and staff safe without altering treatment-specific decisions,” Dr. Nanda said.
Streamlining the foot traffic in the cancer center also worked well for Lisa A. Carey, MD, chief of the Division of Hematology/Oncology and deputy director of clinical sciences at University of North Carolina-Chapel Hill. “The truth is, oncological principles of care are still in place,” said Dr. Carey, the Richardson and Marilyn Jacobs Preyer Distinguished Professor in Breast Cancer Research. “COVID hasn’t altered those; it has only thrown a little wrench in how we deliver that care.”
Kelly McCann, MD, PhD, a hematologist/oncologist in the Department of Medicine at the David Geffen School of Medicine, University of California, Los Angeles, has had to walk a tighter rope to protect her patients with MBC, as COVID cases began to soar in LA county last fall.
To keep patients with MBC home more, Dr. McCann said many chose oral cytotoxic chemotherapies over infusional therapies. Some patients with HER2-positive MBC, for instance, opted for the oral combination neratinib + capecitabine over a trastuzumab-deruxtecan infusion, and others with triple-negative tumors chose capecitabine over a taxane.
At City of Hope, Mortimer has had a different experience of LA county’s COVID surge. Because the center only treats patients with cancer, “we have not had huge numbers of patients with COVID or had to significantly modify our practice outside of screening patients who come in and doing fewer scans compared to pre-COVID,” she said. With these precautions, “we have had no internal transmission of COVID within our institution.”
Still, patients with MBC have gotten COVID, and treating both illnesses does complicate decision-making. In some cases, Mortimer has postponed cancer treatment for patients who can delay for a few weeks while they recover from COVID. But patients who need to continue MBC treatment receive care in a separate unit, and Mortimer considers prescribing Eli Lilly’s recently approved antibody therapy bamlanivimab to treat COVID symptoms. “For each situation, we can always page our infectious disease experts to address any concerns or questions,” Dr. Mortimer said.
The MBC and COVID toll
The biggest hurdle for patients with MBC has been less about treatment decisions and more about handling the psychological toll of the pandemic, according to Charles Shapiro, MD, medical oncologist, Icahn School of Medicine at Mount Sinai in New York City.
“These are my personal observations, but I’ve seen how much more stressful it is to have metastatic breast cancer during the pandemic,” said Dr. Shapiro, who worries that fear of COVID may fuel or exacerbate patients’ depression and anxiety. “Patients can’t have family and friends by their side during infusions or appointments, and many feel isolated because of the risk of exposure.”
Because most patients with MBC still need in-person care such as exams, blood draws, or chemotherapy infusions, Dr. McCann has found that many of her patients “are afraid to come to a medical center and have been delaying appointments, imaging, and procedures.”
The psychological toll of treating breast cancer during the pandemic has touched oncologists as well. A recent survey found that burnout scores were significantly higher among physicians whose patients experienced delays in care, including chemotherapy or specialty consultations.
Getting patients vaccinated will improve protection and hopefully lessen fears surrounding COVID infection and transmission. Preliminary recommendations from the National Comprehensive Cancer Network›s COVID-19 Vaccination Advisory Committee state that patients with cancer «should be prioritized for vaccination.»
Dr. McCann agreed. “I’ve recommended COVID-19 vaccination to all of my patients with MBC,” she said. But because a lot of these therapies suppress the immune system to some degree, “I’ll recommend a period of time for vaccination in which the immune system is expected to have recovered, such as in the days prior to a dose of chemotherapy.”
Overall, according to Dr. Carey, institutional responses to treating MBC during the pandemic have been very similar: The key has been that “no one is keeping secrets,” she said. “Our global oncology community is sharing and adopting best practices. Our focus has been doing right by our patients.”
A version of this article first appeared on Medscape.com.
Patients with metastatic breast cancer (MBC) face an elevated risk of severe illness or dying from COVID-19. Given the slow rollout of the Moderna and Pfizer COVID vaccines and new, more infectious viral variants circulating in the United States, oncologists will face a challenging balancing act for the foreseeable future: sustaining patients› MBC care while safeguarding them from COVID. The scale leans heavily toward continuing treatment, experts say.
“If we stop treatment for metastatic breast cancer, death will occur quickly,” Fatima F. Cardoso, MD, director of the breast unit at Champalimaud Clinical Centre in Lisbon, Portugal, stated this past August in a Medscape perspective.
Joanne Mortimer, MD, director of Women’s Cancer Programs at City of Hope, a comprehensive cancer center near Los Angeles, expressed a similar sentiment. “Having MBC is worse than getting COVID,” she told Medscape. “We can’t stop treating patients with MBC because of concerns of exposure.”
But maintaining treatment does not mean business as usual. Oncologists have had to modify their pre-pandemic practices to some degree, and that degree largely depends on local COVID conditions.
“Weighing the risk of treatment with the risk of contracting the virus means that many places have carried on treating metastatic breast cancer in a more thoughtful, careful way,” said Jill Dietz, MD, president of the American Society of Breast Surgeons. “That means focusing on high-value treatments for patients with the goal of maximizing their outcomes and quality of life while limiting in-person visits and potentially unnecessary elements of care.”
To guide this more careful approach, Dr. Dietz and colleagues from the recently formed COVID-19 Pandemic Breast Cancer Consortium published recommendations in April 2020 to account for different disease types and severities. These recommendations align closely with those from Cardoso and colleagues in Europe, also published last April.
Although issued early in the pandemic when there was greater uncertainty about viral transmission, adverse outcomes, and treatment for COVID-19, these recommendations still hold almost a year later.
“The framework has proven to be timeless in that it can help institutions where they are in the pandemic,” Dr. Dietz said.
The recommendations at play
For MBC, in particular, Dr. Dietz and colleagues focused on patients who need systemic care but whose treatment can be modified to keep them home more. The modifications include prescribing oral agents such as capecitabine, vinorelbine, and cyclophosphamide to minimize visits to the hospital or infusion suite.
To limit adverse events associated with these oral drugs, Dr. Dietz and colleagues also recommended reducing the dose when possible. For instance, research shows that lowering the dose of the CDK4/6 inhibitor palbociclib in patients with HR+/HER2-negative MBC does not diminish efficacy.
When oral agents are not an option, Dr. Dietz and colleagues suggested stretching out the intervals for chemotherapy infusions or injections. Data show that trastuzumab and pertuzumab injections for metastatic HER2-positive tumors «may reasonably be administered at longer intervals,» such as 4 weeks instead of 3 weeks.
The extent to which oncologists have applied these recommendations hinges on two factors: the local severity of COVID-19 cases and institution-specific policies.
For some oncologists, the pandemic has largely left treatment decisions untouched. “COVID-19 has only minimally impacted my practice,” said Rita Nanda, MD, director of the Breast Oncology Program and associate professor of medicine at University of Chicago Medicine.
Dr. Nanda recalled her concerns in the early days of the pandemic. As the nation watched COVID cases surge across New York City, Chicagoans prepared for the worst, fashioning the McCormick Place Convention Center into a field hospital for COVID patients.
“But fortunately, we were never overwhelmed at the University of Chicago and never needed to use the convention center,” Dr. Nanda said. “We did not have to alter or limit the type of therapy patients with MBC could receive.”
The main changes described by Dr. Nanda at the University of Chicago have centered around limiting the flow of traffic within the infusion suite or hospital by implementing prescreening checks to catch patients with COVID symptoms, keeping waiting rooms empty and infusion centers socially distanced, and having patients come in for appointments solo. The university’s home phlebotomy service also came in handy. Implemented before the pandemic, this service allowed patients with MBC to get their labs done at home before coming in for treatment.
“Overall, with social distancing, mask wearing, and limiting who comes in to the clinic, we have been able to keep patients and staff safe without altering treatment-specific decisions,” Dr. Nanda said.
Streamlining the foot traffic in the cancer center also worked well for Lisa A. Carey, MD, chief of the Division of Hematology/Oncology and deputy director of clinical sciences at University of North Carolina-Chapel Hill. “The truth is, oncological principles of care are still in place,” said Dr. Carey, the Richardson and Marilyn Jacobs Preyer Distinguished Professor in Breast Cancer Research. “COVID hasn’t altered those; it has only thrown a little wrench in how we deliver that care.”
Kelly McCann, MD, PhD, a hematologist/oncologist in the Department of Medicine at the David Geffen School of Medicine, University of California, Los Angeles, has had to walk a tighter rope to protect her patients with MBC, as COVID cases began to soar in LA county last fall.
To keep patients with MBC home more, Dr. McCann said many chose oral cytotoxic chemotherapies over infusional therapies. Some patients with HER2-positive MBC, for instance, opted for the oral combination neratinib + capecitabine over a trastuzumab-deruxtecan infusion, and others with triple-negative tumors chose capecitabine over a taxane.
At City of Hope, Mortimer has had a different experience of LA county’s COVID surge. Because the center only treats patients with cancer, “we have not had huge numbers of patients with COVID or had to significantly modify our practice outside of screening patients who come in and doing fewer scans compared to pre-COVID,” she said. With these precautions, “we have had no internal transmission of COVID within our institution.”
Still, patients with MBC have gotten COVID, and treating both illnesses does complicate decision-making. In some cases, Mortimer has postponed cancer treatment for patients who can delay for a few weeks while they recover from COVID. But patients who need to continue MBC treatment receive care in a separate unit, and Mortimer considers prescribing Eli Lilly’s recently approved antibody therapy bamlanivimab to treat COVID symptoms. “For each situation, we can always page our infectious disease experts to address any concerns or questions,” Dr. Mortimer said.
The MBC and COVID toll
The biggest hurdle for patients with MBC has been less about treatment decisions and more about handling the psychological toll of the pandemic, according to Charles Shapiro, MD, medical oncologist, Icahn School of Medicine at Mount Sinai in New York City.
“These are my personal observations, but I’ve seen how much more stressful it is to have metastatic breast cancer during the pandemic,” said Dr. Shapiro, who worries that fear of COVID may fuel or exacerbate patients’ depression and anxiety. “Patients can’t have family and friends by their side during infusions or appointments, and many feel isolated because of the risk of exposure.”
Because most patients with MBC still need in-person care such as exams, blood draws, or chemotherapy infusions, Dr. McCann has found that many of her patients “are afraid to come to a medical center and have been delaying appointments, imaging, and procedures.”
The psychological toll of treating breast cancer during the pandemic has touched oncologists as well. A recent survey found that burnout scores were significantly higher among physicians whose patients experienced delays in care, including chemotherapy or specialty consultations.
Getting patients vaccinated will improve protection and hopefully lessen fears surrounding COVID infection and transmission. Preliminary recommendations from the National Comprehensive Cancer Network›s COVID-19 Vaccination Advisory Committee state that patients with cancer «should be prioritized for vaccination.»
Dr. McCann agreed. “I’ve recommended COVID-19 vaccination to all of my patients with MBC,” she said. But because a lot of these therapies suppress the immune system to some degree, “I’ll recommend a period of time for vaccination in which the immune system is expected to have recovered, such as in the days prior to a dose of chemotherapy.”
Overall, according to Dr. Carey, institutional responses to treating MBC during the pandemic have been very similar: The key has been that “no one is keeping secrets,” she said. “Our global oncology community is sharing and adopting best practices. Our focus has been doing right by our patients.”
A version of this article first appeared on Medscape.com.
Age should not be a barrier to aggressive esophageal cancer treatment
Neoadjuvant chemoradiation plus esophagectomy can be performed safely in well-selected older patients with locally advanced esophageal or esophagogastric junction cancer, according to a review 282 patients treated from 2004 through 2019 at Ochsner Medical Center, New Orleans.
Although guidelines recommend curative-intent neoadjuvant chemoradiation (NACR) followed by surgical resection, it’s been demonstrated in several studies that “older patients with potentially curable stage II and III disease are often not considered” for the approach out of concern that they will not tolerate it, said investigators led by W. Peter Sawyer, MD, a surgery resident at Ochsner.
Outcomes, however, were comparable in the study when 188 patients aged younger than 70 years were compared with 94 patients aged 70 years or older, including 4 who were over 80 years old. the investigators concluded.
The patients had NACR followed by esophagectomy mostly for stage 2 disease. The average age was 59 years in the younger group and 74 years in the older group.
Older patients had a higher prevalence of cardiac, vascular, and pulmonary comorbidities and were more likely to have postoperative atrial arrhythmia and urinary retention.
However, there were no statistically significant differences in hospital length of stay (about 10 days in both groups), operative mortality (4.3% in the older group versus 3.8% in the younger group), or the incidence of postoperative grade 3 or higher complications (27.7% older versus 38.3% younger). Age-adjusted survival was 44.8% at 5 years among patients 70 years and older versus 39% among younger patients.
Comorbidity scores, clinically positive nodes, and clinical T3 tumors predicted worse survival on multivariate analysis, but age did not.
“Age itself doesn’t represent a contraindication to aggressive treatment. It is the patient’s comorbidities and functional status which are more important to predict the risk of complications after esophagectomy,” said Daniela Molena, MD, director of the esophageal surgery program at Memorial Sloan Kettering Cancer Center, New York, when asked for a comment.
The team noted that the results “reflect careful patient selection as well as thorough preoperative evaluation and preparation.” Patients with unstable or severe chronic heart disease, moderate to severe chronic liver disease, or severe chronic pulmonary disease were ineligible for surgery. Eligible patients had cardiac stress testing and were strongly encouraged to begin daily exercise. Nutritional deficiencies were addressed before surgery.
The Ochsner team is one of several in recent years that have pushed back on age limits for aggressive esophageal cancer treatment.
A British team, for example, reviewed 992 transthoracic esophagectomies, including 330 in patients 70 years or older, and found lower in-hospital mortality and pulmonary and cardiac morbidity for older patients than previously reported.
They concluded that “age should not be a discriminating factor when determining the treatment strategy for patients presenting with curative esophageal cancer.”
Even so, undertreatment remains “a big problem for elderly patients, and since the median age at diagnoses is 68 years, this is a problem for a large portion of patients with esophageal cancer,” Dr. Molena said.
“Patients can be cured of this disease” with aggressive treatment, but “unfortunately, patients often are not evaluated by a surgeon or referred to a high-volume center and are discouraged from undergoing surgery after an apparent good response to chemoradiation,” partly because esophagectomy has “unfairly gained a bad reputation over the years,” she said.
“There are clear data that outcomes of esophagectomy are very good at high volume centers with minimally invasive techniques and the ability to promptly identify and treat complications,” Dr. Molena said.
Overall, 52% of patients aged 70 years or older with stage II and III disease underwent NACR plus surgery at Ochsner, suggesting that “optimal, curative intent triple modality therapy [chemo, radiation, and esophagectomy] can be used successfully in a sizable segment of the elderly population,” the investigators said.
Treatment has changed significantly at Ochsner since the start of the review period in 2004, including a shift away from a fluorouracil and cisplatin doublet in favor of carboplatin and paclitaxel, which is less toxic, and greater use of minimally invasive surgery. The proportion of people 70 years or older undergoing triple modality treatment has been steadily increasing.
There was no outside funding. Investigator disclosures weren’t reported. Dr. Molena had no relevant disclosures.
Neoadjuvant chemoradiation plus esophagectomy can be performed safely in well-selected older patients with locally advanced esophageal or esophagogastric junction cancer, according to a review 282 patients treated from 2004 through 2019 at Ochsner Medical Center, New Orleans.
Although guidelines recommend curative-intent neoadjuvant chemoradiation (NACR) followed by surgical resection, it’s been demonstrated in several studies that “older patients with potentially curable stage II and III disease are often not considered” for the approach out of concern that they will not tolerate it, said investigators led by W. Peter Sawyer, MD, a surgery resident at Ochsner.
Outcomes, however, were comparable in the study when 188 patients aged younger than 70 years were compared with 94 patients aged 70 years or older, including 4 who were over 80 years old. the investigators concluded.
The patients had NACR followed by esophagectomy mostly for stage 2 disease. The average age was 59 years in the younger group and 74 years in the older group.
Older patients had a higher prevalence of cardiac, vascular, and pulmonary comorbidities and were more likely to have postoperative atrial arrhythmia and urinary retention.
However, there were no statistically significant differences in hospital length of stay (about 10 days in both groups), operative mortality (4.3% in the older group versus 3.8% in the younger group), or the incidence of postoperative grade 3 or higher complications (27.7% older versus 38.3% younger). Age-adjusted survival was 44.8% at 5 years among patients 70 years and older versus 39% among younger patients.
Comorbidity scores, clinically positive nodes, and clinical T3 tumors predicted worse survival on multivariate analysis, but age did not.
“Age itself doesn’t represent a contraindication to aggressive treatment. It is the patient’s comorbidities and functional status which are more important to predict the risk of complications after esophagectomy,” said Daniela Molena, MD, director of the esophageal surgery program at Memorial Sloan Kettering Cancer Center, New York, when asked for a comment.
The team noted that the results “reflect careful patient selection as well as thorough preoperative evaluation and preparation.” Patients with unstable or severe chronic heart disease, moderate to severe chronic liver disease, or severe chronic pulmonary disease were ineligible for surgery. Eligible patients had cardiac stress testing and were strongly encouraged to begin daily exercise. Nutritional deficiencies were addressed before surgery.
The Ochsner team is one of several in recent years that have pushed back on age limits for aggressive esophageal cancer treatment.
A British team, for example, reviewed 992 transthoracic esophagectomies, including 330 in patients 70 years or older, and found lower in-hospital mortality and pulmonary and cardiac morbidity for older patients than previously reported.
They concluded that “age should not be a discriminating factor when determining the treatment strategy for patients presenting with curative esophageal cancer.”
Even so, undertreatment remains “a big problem for elderly patients, and since the median age at diagnoses is 68 years, this is a problem for a large portion of patients with esophageal cancer,” Dr. Molena said.
“Patients can be cured of this disease” with aggressive treatment, but “unfortunately, patients often are not evaluated by a surgeon or referred to a high-volume center and are discouraged from undergoing surgery after an apparent good response to chemoradiation,” partly because esophagectomy has “unfairly gained a bad reputation over the years,” she said.
“There are clear data that outcomes of esophagectomy are very good at high volume centers with minimally invasive techniques and the ability to promptly identify and treat complications,” Dr. Molena said.
Overall, 52% of patients aged 70 years or older with stage II and III disease underwent NACR plus surgery at Ochsner, suggesting that “optimal, curative intent triple modality therapy [chemo, radiation, and esophagectomy] can be used successfully in a sizable segment of the elderly population,” the investigators said.
Treatment has changed significantly at Ochsner since the start of the review period in 2004, including a shift away from a fluorouracil and cisplatin doublet in favor of carboplatin and paclitaxel, which is less toxic, and greater use of minimally invasive surgery. The proportion of people 70 years or older undergoing triple modality treatment has been steadily increasing.
There was no outside funding. Investigator disclosures weren’t reported. Dr. Molena had no relevant disclosures.
Neoadjuvant chemoradiation plus esophagectomy can be performed safely in well-selected older patients with locally advanced esophageal or esophagogastric junction cancer, according to a review 282 patients treated from 2004 through 2019 at Ochsner Medical Center, New Orleans.
Although guidelines recommend curative-intent neoadjuvant chemoradiation (NACR) followed by surgical resection, it’s been demonstrated in several studies that “older patients with potentially curable stage II and III disease are often not considered” for the approach out of concern that they will not tolerate it, said investigators led by W. Peter Sawyer, MD, a surgery resident at Ochsner.
Outcomes, however, were comparable in the study when 188 patients aged younger than 70 years were compared with 94 patients aged 70 years or older, including 4 who were over 80 years old. the investigators concluded.
The patients had NACR followed by esophagectomy mostly for stage 2 disease. The average age was 59 years in the younger group and 74 years in the older group.
Older patients had a higher prevalence of cardiac, vascular, and pulmonary comorbidities and were more likely to have postoperative atrial arrhythmia and urinary retention.
However, there were no statistically significant differences in hospital length of stay (about 10 days in both groups), operative mortality (4.3% in the older group versus 3.8% in the younger group), or the incidence of postoperative grade 3 or higher complications (27.7% older versus 38.3% younger). Age-adjusted survival was 44.8% at 5 years among patients 70 years and older versus 39% among younger patients.
Comorbidity scores, clinically positive nodes, and clinical T3 tumors predicted worse survival on multivariate analysis, but age did not.
“Age itself doesn’t represent a contraindication to aggressive treatment. It is the patient’s comorbidities and functional status which are more important to predict the risk of complications after esophagectomy,” said Daniela Molena, MD, director of the esophageal surgery program at Memorial Sloan Kettering Cancer Center, New York, when asked for a comment.
The team noted that the results “reflect careful patient selection as well as thorough preoperative evaluation and preparation.” Patients with unstable or severe chronic heart disease, moderate to severe chronic liver disease, or severe chronic pulmonary disease were ineligible for surgery. Eligible patients had cardiac stress testing and were strongly encouraged to begin daily exercise. Nutritional deficiencies were addressed before surgery.
The Ochsner team is one of several in recent years that have pushed back on age limits for aggressive esophageal cancer treatment.
A British team, for example, reviewed 992 transthoracic esophagectomies, including 330 in patients 70 years or older, and found lower in-hospital mortality and pulmonary and cardiac morbidity for older patients than previously reported.
They concluded that “age should not be a discriminating factor when determining the treatment strategy for patients presenting with curative esophageal cancer.”
Even so, undertreatment remains “a big problem for elderly patients, and since the median age at diagnoses is 68 years, this is a problem for a large portion of patients with esophageal cancer,” Dr. Molena said.
“Patients can be cured of this disease” with aggressive treatment, but “unfortunately, patients often are not evaluated by a surgeon or referred to a high-volume center and are discouraged from undergoing surgery after an apparent good response to chemoradiation,” partly because esophagectomy has “unfairly gained a bad reputation over the years,” she said.
“There are clear data that outcomes of esophagectomy are very good at high volume centers with minimally invasive techniques and the ability to promptly identify and treat complications,” Dr. Molena said.
Overall, 52% of patients aged 70 years or older with stage II and III disease underwent NACR plus surgery at Ochsner, suggesting that “optimal, curative intent triple modality therapy [chemo, radiation, and esophagectomy] can be used successfully in a sizable segment of the elderly population,” the investigators said.
Treatment has changed significantly at Ochsner since the start of the review period in 2004, including a shift away from a fluorouracil and cisplatin doublet in favor of carboplatin and paclitaxel, which is less toxic, and greater use of minimally invasive surgery. The proportion of people 70 years or older undergoing triple modality treatment has been steadily increasing.
There was no outside funding. Investigator disclosures weren’t reported. Dr. Molena had no relevant disclosures.
FROM THE JOURNAL OF THE AMERICAN COLLEGE OF SURGEONS
Seeking the next generation of antibiotics
Crispr drugs can be effective
Globally, some 700,000 people die from antibiotic-resistant infections ever year; by 2050, that number could be 10 million, according to the United Nations. To find new ways to fight pathogenic microorganisms, scientists are looking to Crispr, the gene-editing tool, according to the New York Times.
“Crispr is a specialized region of DNA that creates what amount to genetic scissors – enzymes that allow the cell (or a scientist) to precisely edit other DNA or its sister molecule, RNA…Crispr was originally discovered in bacteria, where it helps keep track of past injury. When a virus attacks, the bacterium stores small chunks of the viral genome within its own DNA. This helps the bacterium recognize viral infections when they occur again. Then, using Crispr-associated enzymes, it can disarm the virus and prevent the infection from spreading…today researchers are looking to Crispr to edit bacteria and viruses that infect humans and create new treatments.”
In a recent study, researchers successfully used a Crispr-associated enzyme called Cas9 to eliminate a species of Salmonella. They programmed the Cas9 to view the bacterium as the enemy and forced Salmonella to make lethal cuts to its own genome.
Some companies are now exploring Crispr-based antibiotics that might be delivered through viruses engineered so that they cannot reproduce or cause infections themselves, to name just one approach.
“Now researchers face the challenge of demonstrating that Crispr antibacterial and antiviral drugs are effective in living animals and in humans, not just in the lab, and that they will be cheaper than conventional therapies.”
Reference
1. Sheikh K. Is Crispr the Next Antibiotic? The New York Times. Oct 28, 2019.
https://www.nytimes.com/2019/10/28/health/crispr-genetics-antibiotic-resistance.html. Accessed Dec 3, 2019.
Crispr drugs can be effective
Crispr drugs can be effective
Globally, some 700,000 people die from antibiotic-resistant infections ever year; by 2050, that number could be 10 million, according to the United Nations. To find new ways to fight pathogenic microorganisms, scientists are looking to Crispr, the gene-editing tool, according to the New York Times.
“Crispr is a specialized region of DNA that creates what amount to genetic scissors – enzymes that allow the cell (or a scientist) to precisely edit other DNA or its sister molecule, RNA…Crispr was originally discovered in bacteria, where it helps keep track of past injury. When a virus attacks, the bacterium stores small chunks of the viral genome within its own DNA. This helps the bacterium recognize viral infections when they occur again. Then, using Crispr-associated enzymes, it can disarm the virus and prevent the infection from spreading…today researchers are looking to Crispr to edit bacteria and viruses that infect humans and create new treatments.”
In a recent study, researchers successfully used a Crispr-associated enzyme called Cas9 to eliminate a species of Salmonella. They programmed the Cas9 to view the bacterium as the enemy and forced Salmonella to make lethal cuts to its own genome.
Some companies are now exploring Crispr-based antibiotics that might be delivered through viruses engineered so that they cannot reproduce or cause infections themselves, to name just one approach.
“Now researchers face the challenge of demonstrating that Crispr antibacterial and antiviral drugs are effective in living animals and in humans, not just in the lab, and that they will be cheaper than conventional therapies.”
Reference
1. Sheikh K. Is Crispr the Next Antibiotic? The New York Times. Oct 28, 2019.
https://www.nytimes.com/2019/10/28/health/crispr-genetics-antibiotic-resistance.html. Accessed Dec 3, 2019.
Globally, some 700,000 people die from antibiotic-resistant infections ever year; by 2050, that number could be 10 million, according to the United Nations. To find new ways to fight pathogenic microorganisms, scientists are looking to Crispr, the gene-editing tool, according to the New York Times.
“Crispr is a specialized region of DNA that creates what amount to genetic scissors – enzymes that allow the cell (or a scientist) to precisely edit other DNA or its sister molecule, RNA…Crispr was originally discovered in bacteria, where it helps keep track of past injury. When a virus attacks, the bacterium stores small chunks of the viral genome within its own DNA. This helps the bacterium recognize viral infections when they occur again. Then, using Crispr-associated enzymes, it can disarm the virus and prevent the infection from spreading…today researchers are looking to Crispr to edit bacteria and viruses that infect humans and create new treatments.”
In a recent study, researchers successfully used a Crispr-associated enzyme called Cas9 to eliminate a species of Salmonella. They programmed the Cas9 to view the bacterium as the enemy and forced Salmonella to make lethal cuts to its own genome.
Some companies are now exploring Crispr-based antibiotics that might be delivered through viruses engineered so that they cannot reproduce or cause infections themselves, to name just one approach.
“Now researchers face the challenge of demonstrating that Crispr antibacterial and antiviral drugs are effective in living animals and in humans, not just in the lab, and that they will be cheaper than conventional therapies.”
Reference
1. Sheikh K. Is Crispr the Next Antibiotic? The New York Times. Oct 28, 2019.
https://www.nytimes.com/2019/10/28/health/crispr-genetics-antibiotic-resistance.html. Accessed Dec 3, 2019.
COVID-19 pandemic hinders access to contraception
The pandemic has affected reproductive health because of barriers to contraception access, potentially increasing unwanted pregnancies, reported Tracy Kuo Lin, PhD, of the University of California, San Francisco, and associates.
During the pandemic, women have experienced an increased desire to avoid pregnancy, and when that desire is coupled with loss of income, accessing contraception becomes more difficult, Dr. Lin and colleagues observed in a cross-sectional survey published in the journal Contraception.
The study aimed to quantify the effect of COVID-19 on women’s economic status and reproductive health decisions related to childbearing and pregnancy. Women aged 18-49 who resided in the U.S. were targeted via Facebook and Instagram advertisements from May 16 to June 16, 2020. In all, 554 racially diverse respondents from 43 states were selected: 41% aged 18-24, 37% aged 25-34, and 23% aged 35-49.
Income losses affect nearly half of respondents
In determining risk of unwanted pregnancy, the researchers considered the influence of COVID-19 on a number of factors related to economic well-being as well as respondents’ sexual activity, intimate partner violence, overall desire for pregnancy, and access to contraception as issues affecting their interest in becoming pregnant and their ability to access medical care and contraception. Overall, 46% experienced a reduction in income, 43% reported no change, and 10% experienced an increase in income.
Difficulty in being able to afford food, transportation, and housing doubled among respondents from 8% to 16% as a result of the pandemic. The study authors cited education, race/ethnicity, federal poverty level, and change in income as predictors of inability to provide for these basic needs.
A total of 83% of respondents reported having sex within the past month; 54% of those had sex with someone they lived with, compared with 29% who had sex with someone they did not live with. The pandemic had no impact on sexual desire for 37% of respondents, compared with 32% who experienced a decrease in desire, and 29% who experienced more desire for sex. The presence of shelter-in-place orders had no effect on frequency of or desire for sex. Among the respondents, 4% noted intimate partner violence, which increased slightly from 3% before the pandemic.
Among respondents using contraception, the study authors noted that 17% reported greater difficulty accessing birth control during the pandemic compared with 4% who felt access had become easier. Of those citing increased difficulties, 9% noted increasing challenges getting to a pharmacy, 4% were less able to afford birth control, 3% said it had become harder to obtain a prescription, and 1% cited difficulties having long-acting reversible contraceptives removed.
Despite the pandemic’s overall impact on quality of life, 41% of respondents reported a stronger desire to become pregnant, compared with 25% who had a reduced desire, and 34% whose interest in pregnancy was unchanged by the pandemic.
More than one-third of respondents (37%) admitted that COVID-19 contributed to their fears of becoming pregnant while 13% indicated their fear of pregnancy stemmed from concerns over being able to afford the cost of having a child. Not surprisingly, the decrease in desire for pregnancy was twice as high in those who reported they were unable to afford food, transportation, and/or housing compared with those who saw no change in their ability to afford basic needs.
“In these uncertain economic times, it is of utmost importance to create policies that will ensure access to and comprehensive coverage of core sexual and reproductive health services,” Dr. Lin and colleagues urged. “By doing so, we safeguard people’s ability to make decisions that support their reproductive health goals.”
Will COVID-19 drive needed practice and policy changes?
“This study highlights the economic and reproductive health toll of COVID-19 and the pressing need for improved contraception access,” Eve Espey, MD, MPH, said in an interview.
“Ob.gyns. and other practitioners can use this information to consider evidence-based practice changes that incorporate telemedicine visits, extended refills on contraceptive methods, and a focus on postpartum and postabortion initiation of contraception,” noted Dr. Espey, of the University of New Mexico, Albuquerque. For women who are experiencing economic hardship, consulting with state-based programs that offer pharmacy access and online access to contraceptives may offer a reasonable alternative, she added.
The study was funded by the University of California, San Francisco’s National Center of Excellence in Women’s Health. Dr. Lin received funding from Lazarex Cancer Foundation. The remaining authors had no conflicts of interest and reported no disclosures.
The pandemic has affected reproductive health because of barriers to contraception access, potentially increasing unwanted pregnancies, reported Tracy Kuo Lin, PhD, of the University of California, San Francisco, and associates.
During the pandemic, women have experienced an increased desire to avoid pregnancy, and when that desire is coupled with loss of income, accessing contraception becomes more difficult, Dr. Lin and colleagues observed in a cross-sectional survey published in the journal Contraception.
The study aimed to quantify the effect of COVID-19 on women’s economic status and reproductive health decisions related to childbearing and pregnancy. Women aged 18-49 who resided in the U.S. were targeted via Facebook and Instagram advertisements from May 16 to June 16, 2020. In all, 554 racially diverse respondents from 43 states were selected: 41% aged 18-24, 37% aged 25-34, and 23% aged 35-49.
Income losses affect nearly half of respondents
In determining risk of unwanted pregnancy, the researchers considered the influence of COVID-19 on a number of factors related to economic well-being as well as respondents’ sexual activity, intimate partner violence, overall desire for pregnancy, and access to contraception as issues affecting their interest in becoming pregnant and their ability to access medical care and contraception. Overall, 46% experienced a reduction in income, 43% reported no change, and 10% experienced an increase in income.
Difficulty in being able to afford food, transportation, and housing doubled among respondents from 8% to 16% as a result of the pandemic. The study authors cited education, race/ethnicity, federal poverty level, and change in income as predictors of inability to provide for these basic needs.
A total of 83% of respondents reported having sex within the past month; 54% of those had sex with someone they lived with, compared with 29% who had sex with someone they did not live with. The pandemic had no impact on sexual desire for 37% of respondents, compared with 32% who experienced a decrease in desire, and 29% who experienced more desire for sex. The presence of shelter-in-place orders had no effect on frequency of or desire for sex. Among the respondents, 4% noted intimate partner violence, which increased slightly from 3% before the pandemic.
Among respondents using contraception, the study authors noted that 17% reported greater difficulty accessing birth control during the pandemic compared with 4% who felt access had become easier. Of those citing increased difficulties, 9% noted increasing challenges getting to a pharmacy, 4% were less able to afford birth control, 3% said it had become harder to obtain a prescription, and 1% cited difficulties having long-acting reversible contraceptives removed.
Despite the pandemic’s overall impact on quality of life, 41% of respondents reported a stronger desire to become pregnant, compared with 25% who had a reduced desire, and 34% whose interest in pregnancy was unchanged by the pandemic.
More than one-third of respondents (37%) admitted that COVID-19 contributed to their fears of becoming pregnant while 13% indicated their fear of pregnancy stemmed from concerns over being able to afford the cost of having a child. Not surprisingly, the decrease in desire for pregnancy was twice as high in those who reported they were unable to afford food, transportation, and/or housing compared with those who saw no change in their ability to afford basic needs.
“In these uncertain economic times, it is of utmost importance to create policies that will ensure access to and comprehensive coverage of core sexual and reproductive health services,” Dr. Lin and colleagues urged. “By doing so, we safeguard people’s ability to make decisions that support their reproductive health goals.”
Will COVID-19 drive needed practice and policy changes?
“This study highlights the economic and reproductive health toll of COVID-19 and the pressing need for improved contraception access,” Eve Espey, MD, MPH, said in an interview.
“Ob.gyns. and other practitioners can use this information to consider evidence-based practice changes that incorporate telemedicine visits, extended refills on contraceptive methods, and a focus on postpartum and postabortion initiation of contraception,” noted Dr. Espey, of the University of New Mexico, Albuquerque. For women who are experiencing economic hardship, consulting with state-based programs that offer pharmacy access and online access to contraceptives may offer a reasonable alternative, she added.
The study was funded by the University of California, San Francisco’s National Center of Excellence in Women’s Health. Dr. Lin received funding from Lazarex Cancer Foundation. The remaining authors had no conflicts of interest and reported no disclosures.
The pandemic has affected reproductive health because of barriers to contraception access, potentially increasing unwanted pregnancies, reported Tracy Kuo Lin, PhD, of the University of California, San Francisco, and associates.
During the pandemic, women have experienced an increased desire to avoid pregnancy, and when that desire is coupled with loss of income, accessing contraception becomes more difficult, Dr. Lin and colleagues observed in a cross-sectional survey published in the journal Contraception.
The study aimed to quantify the effect of COVID-19 on women’s economic status and reproductive health decisions related to childbearing and pregnancy. Women aged 18-49 who resided in the U.S. were targeted via Facebook and Instagram advertisements from May 16 to June 16, 2020. In all, 554 racially diverse respondents from 43 states were selected: 41% aged 18-24, 37% aged 25-34, and 23% aged 35-49.
Income losses affect nearly half of respondents
In determining risk of unwanted pregnancy, the researchers considered the influence of COVID-19 on a number of factors related to economic well-being as well as respondents’ sexual activity, intimate partner violence, overall desire for pregnancy, and access to contraception as issues affecting their interest in becoming pregnant and their ability to access medical care and contraception. Overall, 46% experienced a reduction in income, 43% reported no change, and 10% experienced an increase in income.
Difficulty in being able to afford food, transportation, and housing doubled among respondents from 8% to 16% as a result of the pandemic. The study authors cited education, race/ethnicity, federal poverty level, and change in income as predictors of inability to provide for these basic needs.
A total of 83% of respondents reported having sex within the past month; 54% of those had sex with someone they lived with, compared with 29% who had sex with someone they did not live with. The pandemic had no impact on sexual desire for 37% of respondents, compared with 32% who experienced a decrease in desire, and 29% who experienced more desire for sex. The presence of shelter-in-place orders had no effect on frequency of or desire for sex. Among the respondents, 4% noted intimate partner violence, which increased slightly from 3% before the pandemic.
Among respondents using contraception, the study authors noted that 17% reported greater difficulty accessing birth control during the pandemic compared with 4% who felt access had become easier. Of those citing increased difficulties, 9% noted increasing challenges getting to a pharmacy, 4% were less able to afford birth control, 3% said it had become harder to obtain a prescription, and 1% cited difficulties having long-acting reversible contraceptives removed.
Despite the pandemic’s overall impact on quality of life, 41% of respondents reported a stronger desire to become pregnant, compared with 25% who had a reduced desire, and 34% whose interest in pregnancy was unchanged by the pandemic.
More than one-third of respondents (37%) admitted that COVID-19 contributed to their fears of becoming pregnant while 13% indicated their fear of pregnancy stemmed from concerns over being able to afford the cost of having a child. Not surprisingly, the decrease in desire for pregnancy was twice as high in those who reported they were unable to afford food, transportation, and/or housing compared with those who saw no change in their ability to afford basic needs.
“In these uncertain economic times, it is of utmost importance to create policies that will ensure access to and comprehensive coverage of core sexual and reproductive health services,” Dr. Lin and colleagues urged. “By doing so, we safeguard people’s ability to make decisions that support their reproductive health goals.”
Will COVID-19 drive needed practice and policy changes?
“This study highlights the economic and reproductive health toll of COVID-19 and the pressing need for improved contraception access,” Eve Espey, MD, MPH, said in an interview.
“Ob.gyns. and other practitioners can use this information to consider evidence-based practice changes that incorporate telemedicine visits, extended refills on contraceptive methods, and a focus on postpartum and postabortion initiation of contraception,” noted Dr. Espey, of the University of New Mexico, Albuquerque. For women who are experiencing economic hardship, consulting with state-based programs that offer pharmacy access and online access to contraceptives may offer a reasonable alternative, she added.
The study was funded by the University of California, San Francisco’s National Center of Excellence in Women’s Health. Dr. Lin received funding from Lazarex Cancer Foundation. The remaining authors had no conflicts of interest and reported no disclosures.
FROM CONTRACEPTION
'Living brain implants' may restore stroke mobility
Researchers behind the ongoing Cortimo trial successfully performed a procedure on a patient 2 years removed from a stroke, in which microelectrode arrays were implanted into his brain to decode signals driving motor function. These signals then allowed him to operate a powered brace worn on his paralyzed arm.
This news organization spoke with the trial’s principal investigator, Mijail D. Serruya, MD, PhD, an assistant professor of neurology at Thomas Jefferson University Hospital, Philadelphia, about the trial’s initial findings, what this technology may ultimately look like, and the implications for stroke patients in knowing that restorative interventions may be on the horizon.
How did you first get involved with implanting electrodes to help stroke patients with recovery?
I was involved in the first human application of a microelectrode array in a young man who had quadriplegia because of a spinal cord injury. We showed that we could record signal directly from his motor cortex and use it to move a cursor on the screen, and open and close a prosthetic hand and arm.
I was naive and thought that this would soon be a widely available clinical medical device. Now it’s nearly 15 years later, and while it certainly has been safely used in multiple labs to record signals from people with spinal cord injury, amyotrophic lateral sclerosis (ALS), or locked-in syndrome from a brain stem stroke, it still requires a team of technicians and a percutaneous connector. It really has not gotten out of the university.
A few years ago I spoke with Robert Rosenwasser, MD, chairman of the department of neurosurgery at Thomas Jefferson, who runs a very busy stroke center and performed the surgery in this trial. We put our heads together and said: “Maybe the time is now to see whether we can move this technology to this much more prevalent condition of a hemispheric stroke.” And that’s what we did.
How did the idea of using computer brain electrode interfaces begin?
Around 20 years ago, if you had someone who had severe paralysis and you wanted to restore movement, the question was, where can you get a good control signal from? Obviously, if someone can talk, they can use a voice-actuated system with speech recognition and maybe you can track their eye gaze. But if they’re trying to move their limbs, you want a motor control signal.
In someone who has end-stage ALS or a brain stem stroke, you can’t even record residual muscle activity; you have almost nothing to work with. The only thing left is to try to record directly from the brain itself.
It’s important to clarify that brain-computer interfaces are not necessarily stimulating the brain to inject the signal. They’re just recording the endogenous activity that the brain makes. In comparison, a deep brain stimulator is usually not recording anything; it’s just delivering energy to the brain and hoping for the best.
But what we’re doing is asking, if the person is trying to move the paralyzed limb but can’t, can we get to the source of the signal and then do something with it?
What’s the process for measuring that in, for example, someone who has a localized lesion in the motor cortex?
The first step is a scan. People have been doing functional MRI on patients who have had a stroke as long as we’ve had fMRI. We know that people can actually activate on MRI areas of their brain around the stroke, but obviously not in the stroke because it’s been lesioned. However, we do know that the circuit adjacent to it and other regions do appear able to be modulated.
So by having a person either imagine trying to do what they want to do or doing what they can do, if they have some tiny residual movement, you can then identify a kind of hot spot on the fMRI where the brain gobbles up all the oxygen because it’s so active. Then that gives you an anatomical target for the surgeon to place the electrode arrays.
The Cortimo trial’s enticing findings
What are the most striking results that you’ve seen so far with the device?
The first thing is that we were able to get such recordings at all. We knew from fMRIs that there were fluctuations in oxygen changing when the person was trying to do something they couldn’t do. But nobody knew that you would see this whole population of individual neurons chattering away when you place these electrode arrays in the motor cortex right next to the stroke, and make sense of what we’re recording.
Obviously, that’s very encouraging and gives us hope that many months or years after a stroke, people’s brains are able to maintain this representation of all these different movements and plans. It’s almost like it’s trapped on the other side of the stroke and some of the signals can’t get out.
The other discovery we’re pleased with is that we can actually decode signals in real time and the person can use it to do something, such as trigger the brain to open and close the hand. That’s very different from all the prior research with brain array interfaces.
Furthermore, the gentleman who participated actually had strokes in other parts of his brain affecting his vision; he had homonymous hemianopia. That raised the question of what happens if you affect parts of the brain that have to do with attention and visual processing. Could a system like this work? And again, the answer appears to be yes.
What are the next steps for this technology before it can potentially become available in the clinic?
For this to work, the system clearly has to be fully implantable. What we used was percutaneous. The risk-benefit may be acceptable for someone who has quadriplegia because of, for example, spinal cord injury or end-stage ALS who may already have a tracheostomy and a percutaneous endoscopic gastrostomy. But for someone who is hemiparetic and ambulatory, that may not be acceptable. And a fully implantable system would also have much better patient compliance.
Also, when you’re recording from lots and lots of individual brain cells at many, many samples a second on many, many channels, it’s certainly an engineering challenge. It’s not just a single channel that you occasionally query; it’s hundreds of thousands of channels of this complicated data stream.
But these are solvable challenges. People have been making a lot of progress. It’s really a matter of funding and the engineering expertise, rather than some sort of fundamental scientific breakthrough.
With that said, I think it could be within the next 5-10 years that we could actually have a product that expands the toolbox of what can be done for patients who’ve had a stroke, if they’re motivated and there’s no real contraindication.
Creating a novel device
On that point, are you partnering with engineering and technology companies?
The hope is that we and other groups working on this can do for the interface sort of what Celera Genomics did for the Human Genome Project. By having enough interest and investment, you may be able to propel the field forward to widespread use rather than just a purely academic, lab-science type of project.
We are in discussion with different companies to see how we can move ahead with this, and we would be pleased to work with whomever is interested. It may be that different companies have different pieces of the puzzle – a better sensor or a better wireless transmitter.
The plan is to move as quickly as we can to a fully implantable system. And then the benchmark for any kind of clinical advancement is to do a prospective trial. With devices, if you can get a big enough effect size, then you sometimes don’t need quite as many patients to prove it. If paralysis is striking enough and you can reverse that, then you can convince the Food and Drug Administration of its safety and efficacy, and the various insurance companies, that it’s actually reasonable and necessary.
How long will an implantable device last?
That’s a key question and concern. If you have someone like our participant, who’s in his early 40s, will it keep working 10, 20, 30, 40 years? For the rest of his life? Deep brain stimulators and cochlear implants do function for those long durations, but their designs are quite different. There’s a macroelectrode that’s just delivering current, which is very different from listening in on this microscopic scale. There are different technical considerations.
One possible solution is to make the device out of living tissue, which is something I just wrote about with my colleague D. Kacy Cullen. Living electrodes and amplifiers may seem a bit like science fiction, but on the other hand, we have over a century of plastic surgeons, neurosurgeons, and orthopedic surgeons doing all kinds of complicated modifications of the body, moving nerves and vessels around. It makes you realize that, in a sense, they’ve already done living electrodes by doing a nerve transfer. So the question becomes whether we can refine that living electrode technology, which could then open up more possibilities.
Are there any final messages you’d like to share with clinician audience of this news organization?
Regardless of our specialty, we’re always telling our patients about the benefits of things like eating healthy, exercise, and sleep. Now we can point to the fact that, 2 years after stroke, all of these brain areas are still active, and devices that can potentially reverse and unparalyze your limbs may be available in the coming 5- or 10-plus years. That gives clinicians more justification to tell their patients to really stay on top of those things so that they can be in as optimal brain-mind health as possible to someday benefit from them.
Patients and their families need to be part of the conversation of where this is all going. That’s one thing that’s totally different for brain devices versus other devices, where a person’s psychological state doesn’t necessarily matter. But with a brain device, your mental state, psychosocial situation, exercise, sleep – the way you think about and approach it – actually changes to the structure of the brain pretty dramatically.
I don’t want to cause unreasonable hope that we’re going to snap our fingers and it’s going to be cured. But I do think it’s fair to raise a possibility as a way to say that keeping oneself really healthy is justified.
A version of this article first appeared on Medscape.com.
Researchers behind the ongoing Cortimo trial successfully performed a procedure on a patient 2 years removed from a stroke, in which microelectrode arrays were implanted into his brain to decode signals driving motor function. These signals then allowed him to operate a powered brace worn on his paralyzed arm.
This news organization spoke with the trial’s principal investigator, Mijail D. Serruya, MD, PhD, an assistant professor of neurology at Thomas Jefferson University Hospital, Philadelphia, about the trial’s initial findings, what this technology may ultimately look like, and the implications for stroke patients in knowing that restorative interventions may be on the horizon.
How did you first get involved with implanting electrodes to help stroke patients with recovery?
I was involved in the first human application of a microelectrode array in a young man who had quadriplegia because of a spinal cord injury. We showed that we could record signal directly from his motor cortex and use it to move a cursor on the screen, and open and close a prosthetic hand and arm.
I was naive and thought that this would soon be a widely available clinical medical device. Now it’s nearly 15 years later, and while it certainly has been safely used in multiple labs to record signals from people with spinal cord injury, amyotrophic lateral sclerosis (ALS), or locked-in syndrome from a brain stem stroke, it still requires a team of technicians and a percutaneous connector. It really has not gotten out of the university.
A few years ago I spoke with Robert Rosenwasser, MD, chairman of the department of neurosurgery at Thomas Jefferson, who runs a very busy stroke center and performed the surgery in this trial. We put our heads together and said: “Maybe the time is now to see whether we can move this technology to this much more prevalent condition of a hemispheric stroke.” And that’s what we did.
How did the idea of using computer brain electrode interfaces begin?
Around 20 years ago, if you had someone who had severe paralysis and you wanted to restore movement, the question was, where can you get a good control signal from? Obviously, if someone can talk, they can use a voice-actuated system with speech recognition and maybe you can track their eye gaze. But if they’re trying to move their limbs, you want a motor control signal.
In someone who has end-stage ALS or a brain stem stroke, you can’t even record residual muscle activity; you have almost nothing to work with. The only thing left is to try to record directly from the brain itself.
It’s important to clarify that brain-computer interfaces are not necessarily stimulating the brain to inject the signal. They’re just recording the endogenous activity that the brain makes. In comparison, a deep brain stimulator is usually not recording anything; it’s just delivering energy to the brain and hoping for the best.
But what we’re doing is asking, if the person is trying to move the paralyzed limb but can’t, can we get to the source of the signal and then do something with it?
What’s the process for measuring that in, for example, someone who has a localized lesion in the motor cortex?
The first step is a scan. People have been doing functional MRI on patients who have had a stroke as long as we’ve had fMRI. We know that people can actually activate on MRI areas of their brain around the stroke, but obviously not in the stroke because it’s been lesioned. However, we do know that the circuit adjacent to it and other regions do appear able to be modulated.
So by having a person either imagine trying to do what they want to do or doing what they can do, if they have some tiny residual movement, you can then identify a kind of hot spot on the fMRI where the brain gobbles up all the oxygen because it’s so active. Then that gives you an anatomical target for the surgeon to place the electrode arrays.
The Cortimo trial’s enticing findings
What are the most striking results that you’ve seen so far with the device?
The first thing is that we were able to get such recordings at all. We knew from fMRIs that there were fluctuations in oxygen changing when the person was trying to do something they couldn’t do. But nobody knew that you would see this whole population of individual neurons chattering away when you place these electrode arrays in the motor cortex right next to the stroke, and make sense of what we’re recording.
Obviously, that’s very encouraging and gives us hope that many months or years after a stroke, people’s brains are able to maintain this representation of all these different movements and plans. It’s almost like it’s trapped on the other side of the stroke and some of the signals can’t get out.
The other discovery we’re pleased with is that we can actually decode signals in real time and the person can use it to do something, such as trigger the brain to open and close the hand. That’s very different from all the prior research with brain array interfaces.
Furthermore, the gentleman who participated actually had strokes in other parts of his brain affecting his vision; he had homonymous hemianopia. That raised the question of what happens if you affect parts of the brain that have to do with attention and visual processing. Could a system like this work? And again, the answer appears to be yes.
What are the next steps for this technology before it can potentially become available in the clinic?
For this to work, the system clearly has to be fully implantable. What we used was percutaneous. The risk-benefit may be acceptable for someone who has quadriplegia because of, for example, spinal cord injury or end-stage ALS who may already have a tracheostomy and a percutaneous endoscopic gastrostomy. But for someone who is hemiparetic and ambulatory, that may not be acceptable. And a fully implantable system would also have much better patient compliance.
Also, when you’re recording from lots and lots of individual brain cells at many, many samples a second on many, many channels, it’s certainly an engineering challenge. It’s not just a single channel that you occasionally query; it’s hundreds of thousands of channels of this complicated data stream.
But these are solvable challenges. People have been making a lot of progress. It’s really a matter of funding and the engineering expertise, rather than some sort of fundamental scientific breakthrough.
With that said, I think it could be within the next 5-10 years that we could actually have a product that expands the toolbox of what can be done for patients who’ve had a stroke, if they’re motivated and there’s no real contraindication.
Creating a novel device
On that point, are you partnering with engineering and technology companies?
The hope is that we and other groups working on this can do for the interface sort of what Celera Genomics did for the Human Genome Project. By having enough interest and investment, you may be able to propel the field forward to widespread use rather than just a purely academic, lab-science type of project.
We are in discussion with different companies to see how we can move ahead with this, and we would be pleased to work with whomever is interested. It may be that different companies have different pieces of the puzzle – a better sensor or a better wireless transmitter.
The plan is to move as quickly as we can to a fully implantable system. And then the benchmark for any kind of clinical advancement is to do a prospective trial. With devices, if you can get a big enough effect size, then you sometimes don’t need quite as many patients to prove it. If paralysis is striking enough and you can reverse that, then you can convince the Food and Drug Administration of its safety and efficacy, and the various insurance companies, that it’s actually reasonable and necessary.
How long will an implantable device last?
That’s a key question and concern. If you have someone like our participant, who’s in his early 40s, will it keep working 10, 20, 30, 40 years? For the rest of his life? Deep brain stimulators and cochlear implants do function for those long durations, but their designs are quite different. There’s a macroelectrode that’s just delivering current, which is very different from listening in on this microscopic scale. There are different technical considerations.
One possible solution is to make the device out of living tissue, which is something I just wrote about with my colleague D. Kacy Cullen. Living electrodes and amplifiers may seem a bit like science fiction, but on the other hand, we have over a century of plastic surgeons, neurosurgeons, and orthopedic surgeons doing all kinds of complicated modifications of the body, moving nerves and vessels around. It makes you realize that, in a sense, they’ve already done living electrodes by doing a nerve transfer. So the question becomes whether we can refine that living electrode technology, which could then open up more possibilities.
Are there any final messages you’d like to share with clinician audience of this news organization?
Regardless of our specialty, we’re always telling our patients about the benefits of things like eating healthy, exercise, and sleep. Now we can point to the fact that, 2 years after stroke, all of these brain areas are still active, and devices that can potentially reverse and unparalyze your limbs may be available in the coming 5- or 10-plus years. That gives clinicians more justification to tell their patients to really stay on top of those things so that they can be in as optimal brain-mind health as possible to someday benefit from them.
Patients and their families need to be part of the conversation of where this is all going. That’s one thing that’s totally different for brain devices versus other devices, where a person’s psychological state doesn’t necessarily matter. But with a brain device, your mental state, psychosocial situation, exercise, sleep – the way you think about and approach it – actually changes to the structure of the brain pretty dramatically.
I don’t want to cause unreasonable hope that we’re going to snap our fingers and it’s going to be cured. But I do think it’s fair to raise a possibility as a way to say that keeping oneself really healthy is justified.
A version of this article first appeared on Medscape.com.
Researchers behind the ongoing Cortimo trial successfully performed a procedure on a patient 2 years removed from a stroke, in which microelectrode arrays were implanted into his brain to decode signals driving motor function. These signals then allowed him to operate a powered brace worn on his paralyzed arm.
This news organization spoke with the trial’s principal investigator, Mijail D. Serruya, MD, PhD, an assistant professor of neurology at Thomas Jefferson University Hospital, Philadelphia, about the trial’s initial findings, what this technology may ultimately look like, and the implications for stroke patients in knowing that restorative interventions may be on the horizon.
How did you first get involved with implanting electrodes to help stroke patients with recovery?
I was involved in the first human application of a microelectrode array in a young man who had quadriplegia because of a spinal cord injury. We showed that we could record signal directly from his motor cortex and use it to move a cursor on the screen, and open and close a prosthetic hand and arm.
I was naive and thought that this would soon be a widely available clinical medical device. Now it’s nearly 15 years later, and while it certainly has been safely used in multiple labs to record signals from people with spinal cord injury, amyotrophic lateral sclerosis (ALS), or locked-in syndrome from a brain stem stroke, it still requires a team of technicians and a percutaneous connector. It really has not gotten out of the university.
A few years ago I spoke with Robert Rosenwasser, MD, chairman of the department of neurosurgery at Thomas Jefferson, who runs a very busy stroke center and performed the surgery in this trial. We put our heads together and said: “Maybe the time is now to see whether we can move this technology to this much more prevalent condition of a hemispheric stroke.” And that’s what we did.
How did the idea of using computer brain electrode interfaces begin?
Around 20 years ago, if you had someone who had severe paralysis and you wanted to restore movement, the question was, where can you get a good control signal from? Obviously, if someone can talk, they can use a voice-actuated system with speech recognition and maybe you can track their eye gaze. But if they’re trying to move their limbs, you want a motor control signal.
In someone who has end-stage ALS or a brain stem stroke, you can’t even record residual muscle activity; you have almost nothing to work with. The only thing left is to try to record directly from the brain itself.
It’s important to clarify that brain-computer interfaces are not necessarily stimulating the brain to inject the signal. They’re just recording the endogenous activity that the brain makes. In comparison, a deep brain stimulator is usually not recording anything; it’s just delivering energy to the brain and hoping for the best.
But what we’re doing is asking, if the person is trying to move the paralyzed limb but can’t, can we get to the source of the signal and then do something with it?
What’s the process for measuring that in, for example, someone who has a localized lesion in the motor cortex?
The first step is a scan. People have been doing functional MRI on patients who have had a stroke as long as we’ve had fMRI. We know that people can actually activate on MRI areas of their brain around the stroke, but obviously not in the stroke because it’s been lesioned. However, we do know that the circuit adjacent to it and other regions do appear able to be modulated.
So by having a person either imagine trying to do what they want to do or doing what they can do, if they have some tiny residual movement, you can then identify a kind of hot spot on the fMRI where the brain gobbles up all the oxygen because it’s so active. Then that gives you an anatomical target for the surgeon to place the electrode arrays.
The Cortimo trial’s enticing findings
What are the most striking results that you’ve seen so far with the device?
The first thing is that we were able to get such recordings at all. We knew from fMRIs that there were fluctuations in oxygen changing when the person was trying to do something they couldn’t do. But nobody knew that you would see this whole population of individual neurons chattering away when you place these electrode arrays in the motor cortex right next to the stroke, and make sense of what we’re recording.
Obviously, that’s very encouraging and gives us hope that many months or years after a stroke, people’s brains are able to maintain this representation of all these different movements and plans. It’s almost like it’s trapped on the other side of the stroke and some of the signals can’t get out.
The other discovery we’re pleased with is that we can actually decode signals in real time and the person can use it to do something, such as trigger the brain to open and close the hand. That’s very different from all the prior research with brain array interfaces.
Furthermore, the gentleman who participated actually had strokes in other parts of his brain affecting his vision; he had homonymous hemianopia. That raised the question of what happens if you affect parts of the brain that have to do with attention and visual processing. Could a system like this work? And again, the answer appears to be yes.
What are the next steps for this technology before it can potentially become available in the clinic?
For this to work, the system clearly has to be fully implantable. What we used was percutaneous. The risk-benefit may be acceptable for someone who has quadriplegia because of, for example, spinal cord injury or end-stage ALS who may already have a tracheostomy and a percutaneous endoscopic gastrostomy. But for someone who is hemiparetic and ambulatory, that may not be acceptable. And a fully implantable system would also have much better patient compliance.
Also, when you’re recording from lots and lots of individual brain cells at many, many samples a second on many, many channels, it’s certainly an engineering challenge. It’s not just a single channel that you occasionally query; it’s hundreds of thousands of channels of this complicated data stream.
But these are solvable challenges. People have been making a lot of progress. It’s really a matter of funding and the engineering expertise, rather than some sort of fundamental scientific breakthrough.
With that said, I think it could be within the next 5-10 years that we could actually have a product that expands the toolbox of what can be done for patients who’ve had a stroke, if they’re motivated and there’s no real contraindication.
Creating a novel device
On that point, are you partnering with engineering and technology companies?
The hope is that we and other groups working on this can do for the interface sort of what Celera Genomics did for the Human Genome Project. By having enough interest and investment, you may be able to propel the field forward to widespread use rather than just a purely academic, lab-science type of project.
We are in discussion with different companies to see how we can move ahead with this, and we would be pleased to work with whomever is interested. It may be that different companies have different pieces of the puzzle – a better sensor or a better wireless transmitter.
The plan is to move as quickly as we can to a fully implantable system. And then the benchmark for any kind of clinical advancement is to do a prospective trial. With devices, if you can get a big enough effect size, then you sometimes don’t need quite as many patients to prove it. If paralysis is striking enough and you can reverse that, then you can convince the Food and Drug Administration of its safety and efficacy, and the various insurance companies, that it’s actually reasonable and necessary.
How long will an implantable device last?
That’s a key question and concern. If you have someone like our participant, who’s in his early 40s, will it keep working 10, 20, 30, 40 years? For the rest of his life? Deep brain stimulators and cochlear implants do function for those long durations, but their designs are quite different. There’s a macroelectrode that’s just delivering current, which is very different from listening in on this microscopic scale. There are different technical considerations.
One possible solution is to make the device out of living tissue, which is something I just wrote about with my colleague D. Kacy Cullen. Living electrodes and amplifiers may seem a bit like science fiction, but on the other hand, we have over a century of plastic surgeons, neurosurgeons, and orthopedic surgeons doing all kinds of complicated modifications of the body, moving nerves and vessels around. It makes you realize that, in a sense, they’ve already done living electrodes by doing a nerve transfer. So the question becomes whether we can refine that living electrode technology, which could then open up more possibilities.
Are there any final messages you’d like to share with clinician audience of this news organization?
Regardless of our specialty, we’re always telling our patients about the benefits of things like eating healthy, exercise, and sleep. Now we can point to the fact that, 2 years after stroke, all of these brain areas are still active, and devices that can potentially reverse and unparalyze your limbs may be available in the coming 5- or 10-plus years. That gives clinicians more justification to tell their patients to really stay on top of those things so that they can be in as optimal brain-mind health as possible to someday benefit from them.
Patients and their families need to be part of the conversation of where this is all going. That’s one thing that’s totally different for brain devices versus other devices, where a person’s psychological state doesn’t necessarily matter. But with a brain device, your mental state, psychosocial situation, exercise, sleep – the way you think about and approach it – actually changes to the structure of the brain pretty dramatically.
I don’t want to cause unreasonable hope that we’re going to snap our fingers and it’s going to be cured. But I do think it’s fair to raise a possibility as a way to say that keeping oneself really healthy is justified.
A version of this article first appeared on Medscape.com.
The fax that got under my skin
I got an interesting fax recently.
It started with how tough things have been for small practices during the pandemic (like I need reminding) and suggests it has solutions for my practice to stay afloat.
I’m used to all kinds of these approaches, and was going to toss the fax, but decided to read on out of curiosity. I assumed it was an advertisement for a loan company, or to sell vitamins out of my office.
This one, surprisingly, suggested I buy gadgets that would allow me to “balance uneven skin tones,” “shrink pores,” “eliminate freckles and stretch marks,” and do “laser vaginal resurfacing”
Are you kidding me?
First of all, I try very hard to stay in my lane. I’m a neurologist, hopefully a competent one, and have no desire to go beyond that. Imagine how bad this would look in a legal case: I’d be pretty hard pressed to convince a malpractice lawyer and jury that “eliminating stretch marks” and “laser vaginal resurfacing” are within the scope and training of your average neurologist.
Second, I don’t see this sort of thing as reflecting well on me. Patients come here to be treated for Parkinson’s disease, strokes, and epilepsy. If I tried to change the appointment’s topic to “those issues are minor, let’s talk about your stretch marks” I’m pretty sure they’d be looking for a new neurologist. And, when it got back to the physician who referred them, so would she.
Third, my patients are tightening their belts like everyone else in this pandemic-associated economic downturn. Suddenly trying to sell them on a pricey cash-pay procedure, let alone one that’s pretty far out of my field, isn’t going to fly. Like my own family they’re watching every penny right now and shrinking pores is at the bottom of their financial priorities. If they really want that done I’d to happy to refer them to a dermatologist.
Not surprisingly, I tossed the fax. Caring for my patients is challenging enough when I stick to what I do best.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
I got an interesting fax recently.
It started with how tough things have been for small practices during the pandemic (like I need reminding) and suggests it has solutions for my practice to stay afloat.
I’m used to all kinds of these approaches, and was going to toss the fax, but decided to read on out of curiosity. I assumed it was an advertisement for a loan company, or to sell vitamins out of my office.
This one, surprisingly, suggested I buy gadgets that would allow me to “balance uneven skin tones,” “shrink pores,” “eliminate freckles and stretch marks,” and do “laser vaginal resurfacing”
Are you kidding me?
First of all, I try very hard to stay in my lane. I’m a neurologist, hopefully a competent one, and have no desire to go beyond that. Imagine how bad this would look in a legal case: I’d be pretty hard pressed to convince a malpractice lawyer and jury that “eliminating stretch marks” and “laser vaginal resurfacing” are within the scope and training of your average neurologist.
Second, I don’t see this sort of thing as reflecting well on me. Patients come here to be treated for Parkinson’s disease, strokes, and epilepsy. If I tried to change the appointment’s topic to “those issues are minor, let’s talk about your stretch marks” I’m pretty sure they’d be looking for a new neurologist. And, when it got back to the physician who referred them, so would she.
Third, my patients are tightening their belts like everyone else in this pandemic-associated economic downturn. Suddenly trying to sell them on a pricey cash-pay procedure, let alone one that’s pretty far out of my field, isn’t going to fly. Like my own family they’re watching every penny right now and shrinking pores is at the bottom of their financial priorities. If they really want that done I’d to happy to refer them to a dermatologist.
Not surprisingly, I tossed the fax. Caring for my patients is challenging enough when I stick to what I do best.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
I got an interesting fax recently.
It started with how tough things have been for small practices during the pandemic (like I need reminding) and suggests it has solutions for my practice to stay afloat.
I’m used to all kinds of these approaches, and was going to toss the fax, but decided to read on out of curiosity. I assumed it was an advertisement for a loan company, or to sell vitamins out of my office.
This one, surprisingly, suggested I buy gadgets that would allow me to “balance uneven skin tones,” “shrink pores,” “eliminate freckles and stretch marks,” and do “laser vaginal resurfacing”
Are you kidding me?
First of all, I try very hard to stay in my lane. I’m a neurologist, hopefully a competent one, and have no desire to go beyond that. Imagine how bad this would look in a legal case: I’d be pretty hard pressed to convince a malpractice lawyer and jury that “eliminating stretch marks” and “laser vaginal resurfacing” are within the scope and training of your average neurologist.
Second, I don’t see this sort of thing as reflecting well on me. Patients come here to be treated for Parkinson’s disease, strokes, and epilepsy. If I tried to change the appointment’s topic to “those issues are minor, let’s talk about your stretch marks” I’m pretty sure they’d be looking for a new neurologist. And, when it got back to the physician who referred them, so would she.
Third, my patients are tightening their belts like everyone else in this pandemic-associated economic downturn. Suddenly trying to sell them on a pricey cash-pay procedure, let alone one that’s pretty far out of my field, isn’t going to fly. Like my own family they’re watching every penny right now and shrinking pores is at the bottom of their financial priorities. If they really want that done I’d to happy to refer them to a dermatologist.
Not surprisingly, I tossed the fax. Caring for my patients is challenging enough when I stick to what I do best.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
FDA approves first targeted treatment for rare DMD mutation
, the agency has announced.
This particular mutation of the DMD gene “is amenable to exon 45 skipping,” the FDA noted in a press release. The agency added that this is its first approval of a targeted treatment for patients with the mutation.
“Developing drugs designed for patients with specific mutations is a critical part of personalized medicine,” Eric Bastings, MD, deputy director of the Office of Neuroscience at the FDA’s Center for Drug Evaluation and Research, said in a statement.
The approval was based on results from a 43-person randomized controlled trial. Patients who received casimersen had a greater increase in production of the muscle-fiber protein dystrophin compared with their counterparts who received placebo.
Approved – with cautions
The FDA noted that DMD prevalence worldwide is about 1 in 3,600 boys – although it can also affect girls in rare cases. Symptoms of the disorder are commonly first observed around age 3 years but worsen steadily over time. DMD gene mutations lead to a decrease in dystrophin.
As reported by Medscape Medical News in August, the FDA approved viltolarsen (Viltepso, NS Pharma) for the treatment of DMD in patients with a confirmed mutation amenable to exon 53 skipping, following approval of golodirsen injection (Vyondys 53, Sarepta Therapeutics) for the same indication in December 2019.
The DMD gene mutation that is amenable to exon 45 skipping is present in about 8% of patients with DMD.
The trial that carried weight with the FDA included 43 male participants with DMD aged 7-20 years. All were confirmed to have the exon 45-skipping gene mutation and all were randomly assigned 2:1 to received IV casimersen 30 mg/kg or matching placebo.
Results showed that, between baseline and 48 weeks post treatment, the casimersen group showed a significantly higher increase in levels of dystrophin protein than in the placebo group.
Upper respiratory tract infections, fever, joint and throat pain, headache, and cough were the most common adverse events experienced by the active-treatment group.
Although the clinical studies assessing casimersen did not show any reports of kidney toxicity, the adverse event was observed in some nonclinical studies. Therefore, clinicians should monitor kidney function in any patient receiving this treatment, the FDA recommended.
Overall, “the FDA has concluded that the data submitted by the applicant demonstrated an increase in dystrophin production that is reasonably likely to predict clinical benefit” in this patient population, the agency said in its press release.
However, it noted that definitive clinical benefits such as improved motor function were not “established.”
“In making this decision, the FDA considered the potential risks associated with the drug, the life-threatening and debilitating nature of the disease, and the lack of [other] available therapy,” the agency said.
It added that the manufacturer is currently conducting a multicenter study focused on the safety and efficacy of the drug in ambulatory patients with DMD.
The FDA approved casimersen using its Accelerated Approval pathway, granted Fast Track and Priority Review designations to its applications, and gave the treatment Orphan Drug designation.
A version of this article first appeared on Medscape.com.
, the agency has announced.
This particular mutation of the DMD gene “is amenable to exon 45 skipping,” the FDA noted in a press release. The agency added that this is its first approval of a targeted treatment for patients with the mutation.
“Developing drugs designed for patients with specific mutations is a critical part of personalized medicine,” Eric Bastings, MD, deputy director of the Office of Neuroscience at the FDA’s Center for Drug Evaluation and Research, said in a statement.
The approval was based on results from a 43-person randomized controlled trial. Patients who received casimersen had a greater increase in production of the muscle-fiber protein dystrophin compared with their counterparts who received placebo.
Approved – with cautions
The FDA noted that DMD prevalence worldwide is about 1 in 3,600 boys – although it can also affect girls in rare cases. Symptoms of the disorder are commonly first observed around age 3 years but worsen steadily over time. DMD gene mutations lead to a decrease in dystrophin.
As reported by Medscape Medical News in August, the FDA approved viltolarsen (Viltepso, NS Pharma) for the treatment of DMD in patients with a confirmed mutation amenable to exon 53 skipping, following approval of golodirsen injection (Vyondys 53, Sarepta Therapeutics) for the same indication in December 2019.
The DMD gene mutation that is amenable to exon 45 skipping is present in about 8% of patients with DMD.
The trial that carried weight with the FDA included 43 male participants with DMD aged 7-20 years. All were confirmed to have the exon 45-skipping gene mutation and all were randomly assigned 2:1 to received IV casimersen 30 mg/kg or matching placebo.
Results showed that, between baseline and 48 weeks post treatment, the casimersen group showed a significantly higher increase in levels of dystrophin protein than in the placebo group.
Upper respiratory tract infections, fever, joint and throat pain, headache, and cough were the most common adverse events experienced by the active-treatment group.
Although the clinical studies assessing casimersen did not show any reports of kidney toxicity, the adverse event was observed in some nonclinical studies. Therefore, clinicians should monitor kidney function in any patient receiving this treatment, the FDA recommended.
Overall, “the FDA has concluded that the data submitted by the applicant demonstrated an increase in dystrophin production that is reasonably likely to predict clinical benefit” in this patient population, the agency said in its press release.
However, it noted that definitive clinical benefits such as improved motor function were not “established.”
“In making this decision, the FDA considered the potential risks associated with the drug, the life-threatening and debilitating nature of the disease, and the lack of [other] available therapy,” the agency said.
It added that the manufacturer is currently conducting a multicenter study focused on the safety and efficacy of the drug in ambulatory patients with DMD.
The FDA approved casimersen using its Accelerated Approval pathway, granted Fast Track and Priority Review designations to its applications, and gave the treatment Orphan Drug designation.
A version of this article first appeared on Medscape.com.
, the agency has announced.
This particular mutation of the DMD gene “is amenable to exon 45 skipping,” the FDA noted in a press release. The agency added that this is its first approval of a targeted treatment for patients with the mutation.
“Developing drugs designed for patients with specific mutations is a critical part of personalized medicine,” Eric Bastings, MD, deputy director of the Office of Neuroscience at the FDA’s Center for Drug Evaluation and Research, said in a statement.
The approval was based on results from a 43-person randomized controlled trial. Patients who received casimersen had a greater increase in production of the muscle-fiber protein dystrophin compared with their counterparts who received placebo.
Approved – with cautions
The FDA noted that DMD prevalence worldwide is about 1 in 3,600 boys – although it can also affect girls in rare cases. Symptoms of the disorder are commonly first observed around age 3 years but worsen steadily over time. DMD gene mutations lead to a decrease in dystrophin.
As reported by Medscape Medical News in August, the FDA approved viltolarsen (Viltepso, NS Pharma) for the treatment of DMD in patients with a confirmed mutation amenable to exon 53 skipping, following approval of golodirsen injection (Vyondys 53, Sarepta Therapeutics) for the same indication in December 2019.
The DMD gene mutation that is amenable to exon 45 skipping is present in about 8% of patients with DMD.
The trial that carried weight with the FDA included 43 male participants with DMD aged 7-20 years. All were confirmed to have the exon 45-skipping gene mutation and all were randomly assigned 2:1 to received IV casimersen 30 mg/kg or matching placebo.
Results showed that, between baseline and 48 weeks post treatment, the casimersen group showed a significantly higher increase in levels of dystrophin protein than in the placebo group.
Upper respiratory tract infections, fever, joint and throat pain, headache, and cough were the most common adverse events experienced by the active-treatment group.
Although the clinical studies assessing casimersen did not show any reports of kidney toxicity, the adverse event was observed in some nonclinical studies. Therefore, clinicians should monitor kidney function in any patient receiving this treatment, the FDA recommended.
Overall, “the FDA has concluded that the data submitted by the applicant demonstrated an increase in dystrophin production that is reasonably likely to predict clinical benefit” in this patient population, the agency said in its press release.
However, it noted that definitive clinical benefits such as improved motor function were not “established.”
“In making this decision, the FDA considered the potential risks associated with the drug, the life-threatening and debilitating nature of the disease, and the lack of [other] available therapy,” the agency said.
It added that the manufacturer is currently conducting a multicenter study focused on the safety and efficacy of the drug in ambulatory patients with DMD.
The FDA approved casimersen using its Accelerated Approval pathway, granted Fast Track and Priority Review designations to its applications, and gave the treatment Orphan Drug designation.
A version of this article first appeared on Medscape.com.
New-onset arrhythmias low in COVID-19 and flu
Among 3,970 patients treated during the early months of the pandemic, new onset AF/AFL was seen in 4%, matching the 4% incidence found in a historic cohort of patients hospitalized with influenza.
On the other hand, mortality was similarly high in both groups of patients studied with AF/AFL, showing a 77% increased risk of death in COVID-19 and a 78% increased risk in influenza, a team from Icahn School of Medicine at Mount Sinai in New York reported.
“We saw new onset Afib and flutter in a minority of patients and it was associated with much higher mortality, but the point is that this increase is basically the same as what you see in influenza, which we feel is an indication that this is more of a generalized response to the inflammatory milieu of such a severe viral illness, as opposed to something specific to COVID,” Vivek Y. Reddy, MD, said in the report, published online Feb. 25 in JACC: Clinical Electrophysiology.
“Here we see, with a similar respiratory virus used as controls, that the results are exactly what I would have expected to see, which is that where there is a lot of inflammation, we see Afib,” said John Mandrola, MD, of Baptist Medical Associates, Louisville, Ky., who was not involved with the study.
“We need more studies like this one because we know SARS-CoV-2 is a bad virus that may have important effects on the heart, but all the of research done so far has been problematic because it didn’t include controls.”
Atrial arrhythmias in COVID and flu
Dr. Reddy and coinvestigators performed a retrospective analysis of a large cohort of patients admitted with laboratory-confirmed COVID-19 during Feb. 4-April 22, 2020, to one of five hospitals within the Mount Sinai Health System.
Their comparator arm included 1,420 patients with confirmed influenza A or B hospitalized between Jan. 1, 2017, and Jan. 1, 2020. For both cohorts, automated electronic record abstraction was used and all patient data were de-identified prior to analysis. In the COVID-19 cohort, a manual review of 1,110 charts was also performed.
Compared with those who did not develop AF/AFL, COVID-19 patients with newly detected AF/AFL and COVID-19 were older (74 vs. 66 years; P < .01) and had higher levels of inflammatory markers, including C-reactive protein and interleukin-6, and higher troponin and D-dimer levels (all P < .01).
Overall, including those with a history of atrial arrhythmias, 10% of patients with hospitalized COVID-19 (13% in the manual review) and 12% of those with influenza had AF/AFL detected during their hospitalization.
Mortality at 30 days was higher in COVID-19 patients with AF/AFL compared to those without (46% vs. 26%; P < .01), as were the rates of intubation (27% vs. 15%; relative risk, 1.8; P < .01), and stroke (1.6% vs. 0.6%, RR, 2.7; P = .05).
Despite having more comorbidities, in-hospital mortality was significantly lower in the influenza cohort overall, compared to the COVID-19 cohort (9% vs. 29%; P < .01), reflecting the higher case fatality rate in COVID-19, Dr. Reddy, director of cardiac arrhythmia services at Mount Sinai Hospital, said in an interview.
But as with COVID-19, those influenza patients who had in-hospital AF/AFL were more likely to require intubation (14% vs. 7%; P = .004) or die (16% vs. 10%; P = .003).
“The data are not perfect and there are always limitations when doing an observational study using historic controls, but my guess would be that if we looked at other databases and other populations hospitalized for severe illness, we’d likely see something similar because when the body is inflamed, you’re more likely to see Afib,” said Dr. Mandrola.
Dr. Reddy concurred, noting that they considered comparing other populations to COVID-19 patients, including those with “just generalized severe illness,” but in the end felt there were many similarities between influenza and COVID-19, even though mortality in the latter is higher.
“It would be interesting for people to look at other illnesses and see if they find the same thing,” he said.
Dr. Reddy reported having no disclosures relevant to COVID-19. Dr. Mandrola is chief cardiology correspondent for Medscape.com. He reported having no relevant disclosures. MDedge is a member of the Medscape Professional Network.
Among 3,970 patients treated during the early months of the pandemic, new onset AF/AFL was seen in 4%, matching the 4% incidence found in a historic cohort of patients hospitalized with influenza.
On the other hand, mortality was similarly high in both groups of patients studied with AF/AFL, showing a 77% increased risk of death in COVID-19 and a 78% increased risk in influenza, a team from Icahn School of Medicine at Mount Sinai in New York reported.
“We saw new onset Afib and flutter in a minority of patients and it was associated with much higher mortality, but the point is that this increase is basically the same as what you see in influenza, which we feel is an indication that this is more of a generalized response to the inflammatory milieu of such a severe viral illness, as opposed to something specific to COVID,” Vivek Y. Reddy, MD, said in the report, published online Feb. 25 in JACC: Clinical Electrophysiology.
“Here we see, with a similar respiratory virus used as controls, that the results are exactly what I would have expected to see, which is that where there is a lot of inflammation, we see Afib,” said John Mandrola, MD, of Baptist Medical Associates, Louisville, Ky., who was not involved with the study.
“We need more studies like this one because we know SARS-CoV-2 is a bad virus that may have important effects on the heart, but all the of research done so far has been problematic because it didn’t include controls.”
Atrial arrhythmias in COVID and flu
Dr. Reddy and coinvestigators performed a retrospective analysis of a large cohort of patients admitted with laboratory-confirmed COVID-19 during Feb. 4-April 22, 2020, to one of five hospitals within the Mount Sinai Health System.
Their comparator arm included 1,420 patients with confirmed influenza A or B hospitalized between Jan. 1, 2017, and Jan. 1, 2020. For both cohorts, automated electronic record abstraction was used and all patient data were de-identified prior to analysis. In the COVID-19 cohort, a manual review of 1,110 charts was also performed.
Compared with those who did not develop AF/AFL, COVID-19 patients with newly detected AF/AFL and COVID-19 were older (74 vs. 66 years; P < .01) and had higher levels of inflammatory markers, including C-reactive protein and interleukin-6, and higher troponin and D-dimer levels (all P < .01).
Overall, including those with a history of atrial arrhythmias, 10% of patients with hospitalized COVID-19 (13% in the manual review) and 12% of those with influenza had AF/AFL detected during their hospitalization.
Mortality at 30 days was higher in COVID-19 patients with AF/AFL compared to those without (46% vs. 26%; P < .01), as were the rates of intubation (27% vs. 15%; relative risk, 1.8; P < .01), and stroke (1.6% vs. 0.6%, RR, 2.7; P = .05).
Despite having more comorbidities, in-hospital mortality was significantly lower in the influenza cohort overall, compared to the COVID-19 cohort (9% vs. 29%; P < .01), reflecting the higher case fatality rate in COVID-19, Dr. Reddy, director of cardiac arrhythmia services at Mount Sinai Hospital, said in an interview.
But as with COVID-19, those influenza patients who had in-hospital AF/AFL were more likely to require intubation (14% vs. 7%; P = .004) or die (16% vs. 10%; P = .003).
“The data are not perfect and there are always limitations when doing an observational study using historic controls, but my guess would be that if we looked at other databases and other populations hospitalized for severe illness, we’d likely see something similar because when the body is inflamed, you’re more likely to see Afib,” said Dr. Mandrola.
Dr. Reddy concurred, noting that they considered comparing other populations to COVID-19 patients, including those with “just generalized severe illness,” but in the end felt there were many similarities between influenza and COVID-19, even though mortality in the latter is higher.
“It would be interesting for people to look at other illnesses and see if they find the same thing,” he said.
Dr. Reddy reported having no disclosures relevant to COVID-19. Dr. Mandrola is chief cardiology correspondent for Medscape.com. He reported having no relevant disclosures. MDedge is a member of the Medscape Professional Network.
Among 3,970 patients treated during the early months of the pandemic, new onset AF/AFL was seen in 4%, matching the 4% incidence found in a historic cohort of patients hospitalized with influenza.
On the other hand, mortality was similarly high in both groups of patients studied with AF/AFL, showing a 77% increased risk of death in COVID-19 and a 78% increased risk in influenza, a team from Icahn School of Medicine at Mount Sinai in New York reported.
“We saw new onset Afib and flutter in a minority of patients and it was associated with much higher mortality, but the point is that this increase is basically the same as what you see in influenza, which we feel is an indication that this is more of a generalized response to the inflammatory milieu of such a severe viral illness, as opposed to something specific to COVID,” Vivek Y. Reddy, MD, said in the report, published online Feb. 25 in JACC: Clinical Electrophysiology.
“Here we see, with a similar respiratory virus used as controls, that the results are exactly what I would have expected to see, which is that where there is a lot of inflammation, we see Afib,” said John Mandrola, MD, of Baptist Medical Associates, Louisville, Ky., who was not involved with the study.
“We need more studies like this one because we know SARS-CoV-2 is a bad virus that may have important effects on the heart, but all the of research done so far has been problematic because it didn’t include controls.”
Atrial arrhythmias in COVID and flu
Dr. Reddy and coinvestigators performed a retrospective analysis of a large cohort of patients admitted with laboratory-confirmed COVID-19 during Feb. 4-April 22, 2020, to one of five hospitals within the Mount Sinai Health System.
Their comparator arm included 1,420 patients with confirmed influenza A or B hospitalized between Jan. 1, 2017, and Jan. 1, 2020. For both cohorts, automated electronic record abstraction was used and all patient data were de-identified prior to analysis. In the COVID-19 cohort, a manual review of 1,110 charts was also performed.
Compared with those who did not develop AF/AFL, COVID-19 patients with newly detected AF/AFL and COVID-19 were older (74 vs. 66 years; P < .01) and had higher levels of inflammatory markers, including C-reactive protein and interleukin-6, and higher troponin and D-dimer levels (all P < .01).
Overall, including those with a history of atrial arrhythmias, 10% of patients with hospitalized COVID-19 (13% in the manual review) and 12% of those with influenza had AF/AFL detected during their hospitalization.
Mortality at 30 days was higher in COVID-19 patients with AF/AFL compared to those without (46% vs. 26%; P < .01), as were the rates of intubation (27% vs. 15%; relative risk, 1.8; P < .01), and stroke (1.6% vs. 0.6%, RR, 2.7; P = .05).
Despite having more comorbidities, in-hospital mortality was significantly lower in the influenza cohort overall, compared to the COVID-19 cohort (9% vs. 29%; P < .01), reflecting the higher case fatality rate in COVID-19, Dr. Reddy, director of cardiac arrhythmia services at Mount Sinai Hospital, said in an interview.
But as with COVID-19, those influenza patients who had in-hospital AF/AFL were more likely to require intubation (14% vs. 7%; P = .004) or die (16% vs. 10%; P = .003).
“The data are not perfect and there are always limitations when doing an observational study using historic controls, but my guess would be that if we looked at other databases and other populations hospitalized for severe illness, we’d likely see something similar because when the body is inflamed, you’re more likely to see Afib,” said Dr. Mandrola.
Dr. Reddy concurred, noting that they considered comparing other populations to COVID-19 patients, including those with “just generalized severe illness,” but in the end felt there were many similarities between influenza and COVID-19, even though mortality in the latter is higher.
“It would be interesting for people to look at other illnesses and see if they find the same thing,” he said.
Dr. Reddy reported having no disclosures relevant to COVID-19. Dr. Mandrola is chief cardiology correspondent for Medscape.com. He reported having no relevant disclosures. MDedge is a member of the Medscape Professional Network.
FROM JACC: CLINICAL ELECTROPHYSIOLOGY