User login
The ABCs of successful vaccinations: A role for psychiatry
While the implementation of mass vaccinations is a public health task, individual clinicians are critical for the success of any vaccination campaign. Psychiatrists may be well positioned to help increase vaccine uptake among psychiatric patients. They see their patients more frequently than primary care physicians do, which allows for patient engagement over time regarding vaccinations. Also, as physicians, psychiatrists are a trusted source of medical information, and they are well-versed in using the tools of nudging and motivational interviewing to manage ambivalence about receiving a vaccine (vaccine hesitancy).1
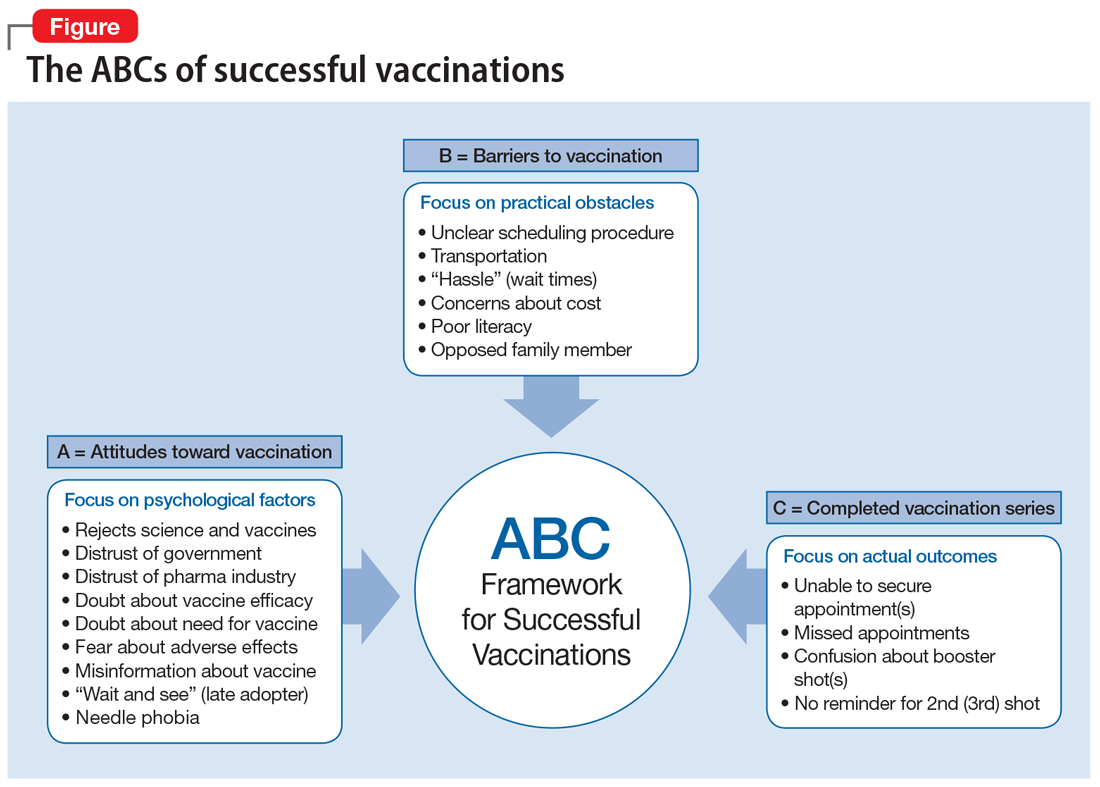
The “ABCs of successful vaccinations” (Figure) provide a framework that psychiatrists can use when speaking with their patients about vaccinations. The ABCs assess psychological factors that hinder acceptance of vaccination (A = Attitudes toward vaccination), practical challenges in vaccine access for patients who are willing to get vaccinated (B = Barriers to vaccination), and the actual outcome of “shot in the arm” (C = Completed vaccination series). The Figure provides examples of each area of focus.
How to talk to patients about vaccines
“Attitudes toward vaccination” is an area in which psychiatrists can potentially move patients from hesitancy to vaccine confidence and acceptance. First, express confidence in the vaccine (ie, make a clear statement: “You are an excellent candidate for this vaccine.”). Then, begin a discussion using presumptive language: “You must be ready to receive the vaccine.” In individuals who hesitate, elicit their concern: “What would make vaccination more acceptable?” In those who agree in principle about the benefits of vaccinations, ask about any impediments: “What would get in the way of getting vaccinated?” While some patients may require more information about the vaccine, others may need more time or mostly concrete help, such as assistance with scheduling a vaccine appointment. Do not to forget to follow up to see if a planned and complete vaccination series has taken place. The CDC offers an excellent online toolkit to help clinicians discuss vaccinations with their patients.2
Psychiatric patients, particularly those from disadvantaged and marginalized populations, have much to gain if psychiatrists are involved in preventive health care, including the coronavirus vaccination drive or the annual flu vaccination campaign.
1. McClure CC, Cataldi JR, O’Leary ST. Vaccine hesitancy: where we are and where we are going. Clin Ther. 2017;39(8):1550-1562.
2. Centers for Disease Control and Prevention. COVID-19 vaccination toolkits. Accessed February 8, 2021. https://www.cdc.gov/coronavirus/2019-ncov/vaccines/toolkits.html
While the implementation of mass vaccinations is a public health task, individual clinicians are critical for the success of any vaccination campaign. Psychiatrists may be well positioned to help increase vaccine uptake among psychiatric patients. They see their patients more frequently than primary care physicians do, which allows for patient engagement over time regarding vaccinations. Also, as physicians, psychiatrists are a trusted source of medical information, and they are well-versed in using the tools of nudging and motivational interviewing to manage ambivalence about receiving a vaccine (vaccine hesitancy).1
The “ABCs of successful vaccinations” (Figure) provide a framework that psychiatrists can use when speaking with their patients about vaccinations. The ABCs assess psychological factors that hinder acceptance of vaccination (A = Attitudes toward vaccination), practical challenges in vaccine access for patients who are willing to get vaccinated (B = Barriers to vaccination), and the actual outcome of “shot in the arm” (C = Completed vaccination series). The Figure provides examples of each area of focus.

How to talk to patients about vaccines
“Attitudes toward vaccination” is an area in which psychiatrists can potentially move patients from hesitancy to vaccine confidence and acceptance. First, express confidence in the vaccine (ie, make a clear statement: “You are an excellent candidate for this vaccine.”). Then, begin a discussion using presumptive language: “You must be ready to receive the vaccine.” In individuals who hesitate, elicit their concern: “What would make vaccination more acceptable?” In those who agree in principle about the benefits of vaccinations, ask about any impediments: “What would get in the way of getting vaccinated?” While some patients may require more information about the vaccine, others may need more time or mostly concrete help, such as assistance with scheduling a vaccine appointment. Do not to forget to follow up to see if a planned and complete vaccination series has taken place. The CDC offers an excellent online toolkit to help clinicians discuss vaccinations with their patients.2
Psychiatric patients, particularly those from disadvantaged and marginalized populations, have much to gain if psychiatrists are involved in preventive health care, including the coronavirus vaccination drive or the annual flu vaccination campaign.
While the implementation of mass vaccinations is a public health task, individual clinicians are critical for the success of any vaccination campaign. Psychiatrists may be well positioned to help increase vaccine uptake among psychiatric patients. They see their patients more frequently than primary care physicians do, which allows for patient engagement over time regarding vaccinations. Also, as physicians, psychiatrists are a trusted source of medical information, and they are well-versed in using the tools of nudging and motivational interviewing to manage ambivalence about receiving a vaccine (vaccine hesitancy).1
The “ABCs of successful vaccinations” (Figure) provide a framework that psychiatrists can use when speaking with their patients about vaccinations. The ABCs assess psychological factors that hinder acceptance of vaccination (A = Attitudes toward vaccination), practical challenges in vaccine access for patients who are willing to get vaccinated (B = Barriers to vaccination), and the actual outcome of “shot in the arm” (C = Completed vaccination series). The Figure provides examples of each area of focus.

How to talk to patients about vaccines
“Attitudes toward vaccination” is an area in which psychiatrists can potentially move patients from hesitancy to vaccine confidence and acceptance. First, express confidence in the vaccine (ie, make a clear statement: “You are an excellent candidate for this vaccine.”). Then, begin a discussion using presumptive language: “You must be ready to receive the vaccine.” In individuals who hesitate, elicit their concern: “What would make vaccination more acceptable?” In those who agree in principle about the benefits of vaccinations, ask about any impediments: “What would get in the way of getting vaccinated?” While some patients may require more information about the vaccine, others may need more time or mostly concrete help, such as assistance with scheduling a vaccine appointment. Do not to forget to follow up to see if a planned and complete vaccination series has taken place. The CDC offers an excellent online toolkit to help clinicians discuss vaccinations with their patients.2
Psychiatric patients, particularly those from disadvantaged and marginalized populations, have much to gain if psychiatrists are involved in preventive health care, including the coronavirus vaccination drive or the annual flu vaccination campaign.
1. McClure CC, Cataldi JR, O’Leary ST. Vaccine hesitancy: where we are and where we are going. Clin Ther. 2017;39(8):1550-1562.
2. Centers for Disease Control and Prevention. COVID-19 vaccination toolkits. Accessed February 8, 2021. https://www.cdc.gov/coronavirus/2019-ncov/vaccines/toolkits.html
1. McClure CC, Cataldi JR, O’Leary ST. Vaccine hesitancy: where we are and where we are going. Clin Ther. 2017;39(8):1550-1562.
2. Centers for Disease Control and Prevention. COVID-19 vaccination toolkits. Accessed February 8, 2021. https://www.cdc.gov/coronavirus/2019-ncov/vaccines/toolkits.html
COVID concerns, private equities, and virtual realities
I am hopeful that we are beginning to see a sustained decline in COVID-19 cases and hospitalizations. Although, total COVID-19 cases and deaths continue to rise (more than 460,000 deaths in the United States), vaccinations and treatment options have reduced the prevalence of severe disease, hospitalizations, and mortality rates. Worries about variants continue, but we now will enter a prolonged phase before we finally subdue COVID-19 and fully open our economies.
Health systems and practices are looking ahead and beginning to focus on how practice will look after COVID-19. From a business standpoint, we are seeing an accelerating consolidation of community practices. We anticipate the first resale of a private equity (PE)–acquired GI practice: Gastro Health was the first practice to join with a PE firm in 2016. Published rumors suggest a sale of the (now larger, multistate) practice at 15-times-plus EBITDA (earnings before interest, taxes, depreciation, and amortization) could begin as early as this quarter. It would not be a surprise to see 40% of independent gastroenterologists employed in a PE-backed model within a few years. Health systems and payers (especially United Health Group) continue to scoop up practices as well.
Clinical care has been changed forever. I expect fully 30% of visits will remain virtual, and innovative health systems will capitalize on that fact to right-size their brick-and-mortar facilities. Start-up companies will virtualize care and develop new models that allow board-certified gastroenterologist to focus on care they only can provide, resulting in substantial cost savings and (hopefully) similar or better outcomes. Remote patient monitoring (both reactive and predictive) is now firmly entrenched in our care armamentarium.
As you will see in this issue, we must create more effective interventions for NAFLD. Obesity will play an increasingly important role in the development of digestive and liver disease, so gastroenterologists must develop better tools and processes to combat root causes.
Begin thinking about DDW. While it again will be a virtual meeting, the content will be rich. Virtual meetings open up additional possibilities to gain new knowledge, although those personal connections over cocktails will be sorely missed.
John I. Allen, MD, MBA, AGAF
Editor in Chief
I am hopeful that we are beginning to see a sustained decline in COVID-19 cases and hospitalizations. Although, total COVID-19 cases and deaths continue to rise (more than 460,000 deaths in the United States), vaccinations and treatment options have reduced the prevalence of severe disease, hospitalizations, and mortality rates. Worries about variants continue, but we now will enter a prolonged phase before we finally subdue COVID-19 and fully open our economies.
Health systems and practices are looking ahead and beginning to focus on how practice will look after COVID-19. From a business standpoint, we are seeing an accelerating consolidation of community practices. We anticipate the first resale of a private equity (PE)–acquired GI practice: Gastro Health was the first practice to join with a PE firm in 2016. Published rumors suggest a sale of the (now larger, multistate) practice at 15-times-plus EBITDA (earnings before interest, taxes, depreciation, and amortization) could begin as early as this quarter. It would not be a surprise to see 40% of independent gastroenterologists employed in a PE-backed model within a few years. Health systems and payers (especially United Health Group) continue to scoop up practices as well.
Clinical care has been changed forever. I expect fully 30% of visits will remain virtual, and innovative health systems will capitalize on that fact to right-size their brick-and-mortar facilities. Start-up companies will virtualize care and develop new models that allow board-certified gastroenterologist to focus on care they only can provide, resulting in substantial cost savings and (hopefully) similar or better outcomes. Remote patient monitoring (both reactive and predictive) is now firmly entrenched in our care armamentarium.
As you will see in this issue, we must create more effective interventions for NAFLD. Obesity will play an increasingly important role in the development of digestive and liver disease, so gastroenterologists must develop better tools and processes to combat root causes.
Begin thinking about DDW. While it again will be a virtual meeting, the content will be rich. Virtual meetings open up additional possibilities to gain new knowledge, although those personal connections over cocktails will be sorely missed.
John I. Allen, MD, MBA, AGAF
Editor in Chief
I am hopeful that we are beginning to see a sustained decline in COVID-19 cases and hospitalizations. Although, total COVID-19 cases and deaths continue to rise (more than 460,000 deaths in the United States), vaccinations and treatment options have reduced the prevalence of severe disease, hospitalizations, and mortality rates. Worries about variants continue, but we now will enter a prolonged phase before we finally subdue COVID-19 and fully open our economies.
Health systems and practices are looking ahead and beginning to focus on how practice will look after COVID-19. From a business standpoint, we are seeing an accelerating consolidation of community practices. We anticipate the first resale of a private equity (PE)–acquired GI practice: Gastro Health was the first practice to join with a PE firm in 2016. Published rumors suggest a sale of the (now larger, multistate) practice at 15-times-plus EBITDA (earnings before interest, taxes, depreciation, and amortization) could begin as early as this quarter. It would not be a surprise to see 40% of independent gastroenterologists employed in a PE-backed model within a few years. Health systems and payers (especially United Health Group) continue to scoop up practices as well.
Clinical care has been changed forever. I expect fully 30% of visits will remain virtual, and innovative health systems will capitalize on that fact to right-size their brick-and-mortar facilities. Start-up companies will virtualize care and develop new models that allow board-certified gastroenterologist to focus on care they only can provide, resulting in substantial cost savings and (hopefully) similar or better outcomes. Remote patient monitoring (both reactive and predictive) is now firmly entrenched in our care armamentarium.
As you will see in this issue, we must create more effective interventions for NAFLD. Obesity will play an increasingly important role in the development of digestive and liver disease, so gastroenterologists must develop better tools and processes to combat root causes.
Begin thinking about DDW. While it again will be a virtual meeting, the content will be rich. Virtual meetings open up additional possibilities to gain new knowledge, although those personal connections over cocktails will be sorely missed.
John I. Allen, MD, MBA, AGAF
Editor in Chief
Treatment Options for Atopic Dermatitis in Children
Until recently, atopic dermatitis was considered a childhood disease that was self-limited over a few years. Emerging studies have shown that the burden of atopic dermatitis includes potential cardiac disease in adulthood, comorbidities including allergy and psychological disorders, and possible superinfection complications.
Dr Lawrence F. Eichenfield, chief of the department of pediatric and adolescent dermatology at Rady Children's Hospital, reports on biological, systemic, and topical treatments either currently in use or being studied for children suffering from atopic dermatitis. These studies include both steroid and steroid-sparing topical agents, a novel AhR modulating agent, as well as JAK inhibitors that are under active investigation.
--
Lawrence F. Eichenfield, MD, Distinguished Professor; Vice Chair, Department of Dermatology and Pediatrics, University of California, San Diego; Chief, Department of Pediatric and Adolescent Dermatology, Rady Children's Hospital, San Diego, California.
Lawrence F. Eichenfield, MD, has disclosed the following relevant financial relationships:
Serve(d) as a director, officer, partner, employee, advisor, consultant, or trustee for: AbbVie; Dermavant; Dermira; Forte Biosciences; Galderma Laboratories; Incyte; Leo Pharma; Eli Lilly and Company; Otsuka; Novartis; Pfizer. Serve(d) as a speaker or a member of a speakers bureau for: Regeneron; Sanofi-Genzyme; Pfizer. Received research grant from: AbbVie; Regeneron; Sanofi Genzyme; Ortho Dermatology. Serve(d) on the data safety monitoring board for: Asana; Glenmark/Ichnos.
Until recently, atopic dermatitis was considered a childhood disease that was self-limited over a few years. Emerging studies have shown that the burden of atopic dermatitis includes potential cardiac disease in adulthood, comorbidities including allergy and psychological disorders, and possible superinfection complications.
Dr Lawrence F. Eichenfield, chief of the department of pediatric and adolescent dermatology at Rady Children's Hospital, reports on biological, systemic, and topical treatments either currently in use or being studied for children suffering from atopic dermatitis. These studies include both steroid and steroid-sparing topical agents, a novel AhR modulating agent, as well as JAK inhibitors that are under active investigation.
--
Lawrence F. Eichenfield, MD, Distinguished Professor; Vice Chair, Department of Dermatology and Pediatrics, University of California, San Diego; Chief, Department of Pediatric and Adolescent Dermatology, Rady Children's Hospital, San Diego, California.
Lawrence F. Eichenfield, MD, has disclosed the following relevant financial relationships:
Serve(d) as a director, officer, partner, employee, advisor, consultant, or trustee for: AbbVie; Dermavant; Dermira; Forte Biosciences; Galderma Laboratories; Incyte; Leo Pharma; Eli Lilly and Company; Otsuka; Novartis; Pfizer. Serve(d) as a speaker or a member of a speakers bureau for: Regeneron; Sanofi-Genzyme; Pfizer. Received research grant from: AbbVie; Regeneron; Sanofi Genzyme; Ortho Dermatology. Serve(d) on the data safety monitoring board for: Asana; Glenmark/Ichnos.
Until recently, atopic dermatitis was considered a childhood disease that was self-limited over a few years. Emerging studies have shown that the burden of atopic dermatitis includes potential cardiac disease in adulthood, comorbidities including allergy and psychological disorders, and possible superinfection complications.
Dr Lawrence F. Eichenfield, chief of the department of pediatric and adolescent dermatology at Rady Children's Hospital, reports on biological, systemic, and topical treatments either currently in use or being studied for children suffering from atopic dermatitis. These studies include both steroid and steroid-sparing topical agents, a novel AhR modulating agent, as well as JAK inhibitors that are under active investigation.
--
Lawrence F. Eichenfield, MD, Distinguished Professor; Vice Chair, Department of Dermatology and Pediatrics, University of California, San Diego; Chief, Department of Pediatric and Adolescent Dermatology, Rady Children's Hospital, San Diego, California.
Lawrence F. Eichenfield, MD, has disclosed the following relevant financial relationships:
Serve(d) as a director, officer, partner, employee, advisor, consultant, or trustee for: AbbVie; Dermavant; Dermira; Forte Biosciences; Galderma Laboratories; Incyte; Leo Pharma; Eli Lilly and Company; Otsuka; Novartis; Pfizer. Serve(d) as a speaker or a member of a speakers bureau for: Regeneron; Sanofi-Genzyme; Pfizer. Received research grant from: AbbVie; Regeneron; Sanofi Genzyme; Ortho Dermatology. Serve(d) on the data safety monitoring board for: Asana; Glenmark/Ichnos.

Diagnosis and assessment of patients with systemic sclerosis
Systemic sclerosis, also known as scleroderma, is a rare autoimmune disease characterized by thickening of the skin, which often causes significant disability or physical distress. Early on in the disease course, it is commonly misdiagnosed as other diseases such as lupus or rheumatoid arthritis.
Patients are classified as having either limited or diffuse cutaneous disease depending on where it presents on the body. All patients with systemic sclerosis are susceptible to internal organ involvement regardless of whether they have limited or diffuse disease.
Dr. Elizabeth Volkmann, Director of the UCLA Scleroderma Program, discusses key defining symptoms that can signal early presentation of systemic sclerosis. She also reviews how to properly screen patients with a high resolution chest CT scan, as interstitial lung disease is the leading cause of death among these patients.
Elizabeth Volkmann, MD, MS, Assistant Professor of Medicine, Director, UCLA Scleroderma Program, Co-Director, CTD-ILD Program, Division of Rheumatology, Department of Medicine, University of California, Los Angeles
Dr. Volkmann has disclosed the following financial relationships:
Grants: Corbus, Forbius. Consulting: Boehringer Ingelheim.
Systemic sclerosis, also known as scleroderma, is a rare autoimmune disease characterized by thickening of the skin, which often causes significant disability or physical distress. Early on in the disease course, it is commonly misdiagnosed as other diseases such as lupus or rheumatoid arthritis.
Patients are classified as having either limited or diffuse cutaneous disease depending on where it presents on the body. All patients with systemic sclerosis are susceptible to internal organ involvement regardless of whether they have limited or diffuse disease.
Dr. Elizabeth Volkmann, Director of the UCLA Scleroderma Program, discusses key defining symptoms that can signal early presentation of systemic sclerosis. She also reviews how to properly screen patients with a high resolution chest CT scan, as interstitial lung disease is the leading cause of death among these patients.
Elizabeth Volkmann, MD, MS, Assistant Professor of Medicine, Director, UCLA Scleroderma Program, Co-Director, CTD-ILD Program, Division of Rheumatology, Department of Medicine, University of California, Los Angeles
Dr. Volkmann has disclosed the following financial relationships:
Grants: Corbus, Forbius. Consulting: Boehringer Ingelheim.
Systemic sclerosis, also known as scleroderma, is a rare autoimmune disease characterized by thickening of the skin, which often causes significant disability or physical distress. Early on in the disease course, it is commonly misdiagnosed as other diseases such as lupus or rheumatoid arthritis.
Patients are classified as having either limited or diffuse cutaneous disease depending on where it presents on the body. All patients with systemic sclerosis are susceptible to internal organ involvement regardless of whether they have limited or diffuse disease.
Dr. Elizabeth Volkmann, Director of the UCLA Scleroderma Program, discusses key defining symptoms that can signal early presentation of systemic sclerosis. She also reviews how to properly screen patients with a high resolution chest CT scan, as interstitial lung disease is the leading cause of death among these patients.
Elizabeth Volkmann, MD, MS, Assistant Professor of Medicine, Director, UCLA Scleroderma Program, Co-Director, CTD-ILD Program, Division of Rheumatology, Department of Medicine, University of California, Los Angeles
Dr. Volkmann has disclosed the following financial relationships:
Grants: Corbus, Forbius. Consulting: Boehringer Ingelheim.

Latest Treatment Options in HR+/HER2- Advanced Breast Cancer in Postmenopausal Women
Hormone-positive (HR+)/human epidermal growth factor receptor 2–negative (HER2-) breast cancer is not curable, but it can have an indolent course that can be controlled for many years with effective treatment.
For postmenopausal women with HR+ breast cancers, the standard of care is endocrine therapy such as exemestane, anastrozole, tamoxifen, or fulvestrant.
In the first-line setting, endocrine therapy may be given alone. In advanced or metastatic disease, endocrine therapy may be combined with one of several newer treatment options, most notably CDK4/6 inhibitors.
Dr Peter Kaufman, of the University of Vermont Cancer Center, takes us through the latest evidence underlining the benefit of CDK4/6 inhibitors in terms of both progression-free and overall survival.
He also outlines the key research questions relating to the use of these drugs, including whether biomarkers can be identified to allow better patient selection.
Finally, Dr Kaufman discusses other therapeutic options for HR+/HER2- advanced breast cancer, such as CDK4/6 inhibitors combined with alpelisib or everolimus, and the emerging use of selective estrogen receptor degraders.
--
Professor, Department of Medicine, Division of Hematology and Oncology, The Robert Larner, M.D. College of Medicine, University of Vermont
Attending Physician, Department of Medicine, Division of Hematology and Oncology, University of Vermont Cancer Center, Burlington, Vermont.
Peter A. Kaufman, MD, has disclosed the following relevant financial relationships:
Serve(d) as a speaker or a member of a speakers bureau for: Eli Lilly and Company
Received research grant from: Eli Lilly and Company; Eisai; Pfizer; Macrogenics; Polyphor; Sanofi
Received income in an amount equal to or greater than $250 from: Eli Lilly and Company; Eisai; Pfizer; Macrogenics; Polyphor; Sanofi; Amgen; Puma
Hormone-positive (HR+)/human epidermal growth factor receptor 2–negative (HER2-) breast cancer is not curable, but it can have an indolent course that can be controlled for many years with effective treatment.
For postmenopausal women with HR+ breast cancers, the standard of care is endocrine therapy such as exemestane, anastrozole, tamoxifen, or fulvestrant.
In the first-line setting, endocrine therapy may be given alone. In advanced or metastatic disease, endocrine therapy may be combined with one of several newer treatment options, most notably CDK4/6 inhibitors.
Dr Peter Kaufman, of the University of Vermont Cancer Center, takes us through the latest evidence underlining the benefit of CDK4/6 inhibitors in terms of both progression-free and overall survival.
He also outlines the key research questions relating to the use of these drugs, including whether biomarkers can be identified to allow better patient selection.
Finally, Dr Kaufman discusses other therapeutic options for HR+/HER2- advanced breast cancer, such as CDK4/6 inhibitors combined with alpelisib or everolimus, and the emerging use of selective estrogen receptor degraders.
--
Professor, Department of Medicine, Division of Hematology and Oncology, The Robert Larner, M.D. College of Medicine, University of Vermont
Attending Physician, Department of Medicine, Division of Hematology and Oncology, University of Vermont Cancer Center, Burlington, Vermont.
Peter A. Kaufman, MD, has disclosed the following relevant financial relationships:
Serve(d) as a speaker or a member of a speakers bureau for: Eli Lilly and Company
Received research grant from: Eli Lilly and Company; Eisai; Pfizer; Macrogenics; Polyphor; Sanofi
Received income in an amount equal to or greater than $250 from: Eli Lilly and Company; Eisai; Pfizer; Macrogenics; Polyphor; Sanofi; Amgen; Puma
Hormone-positive (HR+)/human epidermal growth factor receptor 2–negative (HER2-) breast cancer is not curable, but it can have an indolent course that can be controlled for many years with effective treatment.
For postmenopausal women with HR+ breast cancers, the standard of care is endocrine therapy such as exemestane, anastrozole, tamoxifen, or fulvestrant.
In the first-line setting, endocrine therapy may be given alone. In advanced or metastatic disease, endocrine therapy may be combined with one of several newer treatment options, most notably CDK4/6 inhibitors.
Dr Peter Kaufman, of the University of Vermont Cancer Center, takes us through the latest evidence underlining the benefit of CDK4/6 inhibitors in terms of both progression-free and overall survival.
He also outlines the key research questions relating to the use of these drugs, including whether biomarkers can be identified to allow better patient selection.
Finally, Dr Kaufman discusses other therapeutic options for HR+/HER2- advanced breast cancer, such as CDK4/6 inhibitors combined with alpelisib or everolimus, and the emerging use of selective estrogen receptor degraders.
--
Professor, Department of Medicine, Division of Hematology and Oncology, The Robert Larner, M.D. College of Medicine, University of Vermont
Attending Physician, Department of Medicine, Division of Hematology and Oncology, University of Vermont Cancer Center, Burlington, Vermont.
Peter A. Kaufman, MD, has disclosed the following relevant financial relationships:
Serve(d) as a speaker or a member of a speakers bureau for: Eli Lilly and Company
Received research grant from: Eli Lilly and Company; Eisai; Pfizer; Macrogenics; Polyphor; Sanofi
Received income in an amount equal to or greater than $250 from: Eli Lilly and Company; Eisai; Pfizer; Macrogenics; Polyphor; Sanofi; Amgen; Puma

Asthma not an independent risk factor for severe COVID-19, hospitalization
Asthma is not an independent risk factor for more severe disease or hospitalization due to COVID-19, according to recent research presented at the annual meeting of the American Academy of Allergy, Asthma, and Immunology, held virtually this year.
“In our cohort of patients tested for SARS-CoV-2 at Stanford between March and September, asthma was not an independent risk factor in and of itself for hospitalization or more severe disease from COVID,” Lauren E. Eggert, MD, of the Sean N. Parker Center for Allergy and Asthma Research at Stanford (Calif.) University, said in a poster presentation at the meeting. “What’s more, allergic asthma actually decreased the risk of hospitalization by nearly half.”
Dr. Eggert noted that there have been conflicting data on whether comorbid asthma is or is not a risk factor for more severe COVID-19. “The general thought at the beginning of the pandemic was that because COVID-19 is predominantly a viral respiratory illness, and viral illnesses are known to cause asthma exacerbations, that patients with asthma may be at higher risk if they got COVID infection,” she explained. “But some of the data also showed that Th2 inflammation downregulates ACE2 receptor [expression], which has been shown to be the port of entry for the SARS-CoV-2 virus, so maybe allergy might have a protective effect.”
The researchers at Stanford University identified 168,190 patients at Stanford Health Care who had a positive real-time reverse transcriptase polymerase chain reaction (RT-PCR) test for SARS-CoV-2 between March and September 2020 and collected data from their electronic medical records on their history of asthma, if they were hospitalized, comorbid conditions, and laboratory values. Patients who had no other data available except for a positive SARS-CoV-2 result, or were younger than 28 days, were excluded from the study. Dr. Eggert and colleagues used COVID-19 treatment guidelines from the National Institutes of Health to assess disease severity, which grades COVID-19 severity as asymptomatic or presymptomatic infection, mild illness, moderate illness, severe illness, and critical illness.
In total, the researchers analyzed 5,596 patients who were SARS-CoV-2 positive, with 605 patients (10.8%) hospitalized within 14 days of receiving a positive test. Of these, 100 patients (16.5%) were patients with asthma. There were no significant differences between groups hospitalized and not hospitalized due to COVID-19 in patients with asthma and with no asthma.
Among patients with asthma and COVID-19, 28.0% had asymptomatic illness, 19.0% had moderate disease, 33.0% had severe disease, and 20.0% had critical COVID-19, compared with 36.0% of patients without asthma who had asymptomatic illness, 12.0% with moderate disease, 30.0% with severe disease, and 21.0% with critical COVID-19. Dr. Eggert and colleagues performed a univariate analysis, which showed a significant association between asthma and COVID-19 related hospitalization (odds ratio, 1.53; 95% confidence interval, 1.2-1.93; P < .001), but when adjusting for factors such as diabetes, obesity coronary heart disease, and hypertension, they found there was not a significant association between asthma and hospitalization due to COVID-19 (OR, 1.12; 95% CI, 0.86-1.45; P < .40).
In a univariate analysis, asthma was associated with more severe disease in patients hospitalized for COVID-19, but the results were not significant (OR, 1.21; 95% CI, 0.8-1.85; P = .37). When analyzing allergic asthma alone in a univariate analysis, the researchers found a significant association between allergic asthma and lower hospitalization risk, compared with patients who had nonallergic asthma (OR, 0.55; 95% CI, 0.31-0.92; P = .029), and this association remained after they performed a multivariate analysis as well.
“When we stratified by allergic asthma versus nonallergic asthma, we found that having a diagnosis of allergic asthma actually conferred a protective effect, and there was almost half the risk of hospitalization in asthmatics with allergic asthma as compared to others, which we thought was very interesting,” Dr. Eggert said.
“Eosinophil levels during hospitalization, even when adjusted for systemic steroid use – and we followed patients out through September, when dexamethasone was standard of care – also correlated with better outcomes,” she explained. “This is independent of asthmatic status.”
The researchers noted that confirmation of these results are needed through large, multicenter cohort studies, particularly with regard to how allergic asthma might have a protective effect against SARS-CoV-2 infection. “I think going forward, these findings are very interesting and need to be looked at further to explain the mechanism behind them better,” Dr. Eggert said.
“I think there is also a lot of interest in how this might affect our patients on biologics, which deplete the eosinophils and get rid of that allergic phenotype,” she added. “Does that have any effect on disease severity? Unfortunately, the number of patents on biologics was very small in our cohort, but I do think this is an interesting area for exploration.”
This study was funded in part by the Sean N. Parker Center for Allergy & Asthma Research, Stanford University, Sunshine Foundation, Crown Foundation, and the Parker Foundation.
Asthma is not an independent risk factor for more severe disease or hospitalization due to COVID-19, according to recent research presented at the annual meeting of the American Academy of Allergy, Asthma, and Immunology, held virtually this year.
“In our cohort of patients tested for SARS-CoV-2 at Stanford between March and September, asthma was not an independent risk factor in and of itself for hospitalization or more severe disease from COVID,” Lauren E. Eggert, MD, of the Sean N. Parker Center for Allergy and Asthma Research at Stanford (Calif.) University, said in a poster presentation at the meeting. “What’s more, allergic asthma actually decreased the risk of hospitalization by nearly half.”
Dr. Eggert noted that there have been conflicting data on whether comorbid asthma is or is not a risk factor for more severe COVID-19. “The general thought at the beginning of the pandemic was that because COVID-19 is predominantly a viral respiratory illness, and viral illnesses are known to cause asthma exacerbations, that patients with asthma may be at higher risk if they got COVID infection,” she explained. “But some of the data also showed that Th2 inflammation downregulates ACE2 receptor [expression], which has been shown to be the port of entry for the SARS-CoV-2 virus, so maybe allergy might have a protective effect.”
The researchers at Stanford University identified 168,190 patients at Stanford Health Care who had a positive real-time reverse transcriptase polymerase chain reaction (RT-PCR) test for SARS-CoV-2 between March and September 2020 and collected data from their electronic medical records on their history of asthma, if they were hospitalized, comorbid conditions, and laboratory values. Patients who had no other data available except for a positive SARS-CoV-2 result, or were younger than 28 days, were excluded from the study. Dr. Eggert and colleagues used COVID-19 treatment guidelines from the National Institutes of Health to assess disease severity, which grades COVID-19 severity as asymptomatic or presymptomatic infection, mild illness, moderate illness, severe illness, and critical illness.
In total, the researchers analyzed 5,596 patients who were SARS-CoV-2 positive, with 605 patients (10.8%) hospitalized within 14 days of receiving a positive test. Of these, 100 patients (16.5%) were patients with asthma. There were no significant differences between groups hospitalized and not hospitalized due to COVID-19 in patients with asthma and with no asthma.
Among patients with asthma and COVID-19, 28.0% had asymptomatic illness, 19.0% had moderate disease, 33.0% had severe disease, and 20.0% had critical COVID-19, compared with 36.0% of patients without asthma who had asymptomatic illness, 12.0% with moderate disease, 30.0% with severe disease, and 21.0% with critical COVID-19. Dr. Eggert and colleagues performed a univariate analysis, which showed a significant association between asthma and COVID-19 related hospitalization (odds ratio, 1.53; 95% confidence interval, 1.2-1.93; P < .001), but when adjusting for factors such as diabetes, obesity coronary heart disease, and hypertension, they found there was not a significant association between asthma and hospitalization due to COVID-19 (OR, 1.12; 95% CI, 0.86-1.45; P < .40).
In a univariate analysis, asthma was associated with more severe disease in patients hospitalized for COVID-19, but the results were not significant (OR, 1.21; 95% CI, 0.8-1.85; P = .37). When analyzing allergic asthma alone in a univariate analysis, the researchers found a significant association between allergic asthma and lower hospitalization risk, compared with patients who had nonallergic asthma (OR, 0.55; 95% CI, 0.31-0.92; P = .029), and this association remained after they performed a multivariate analysis as well.
“When we stratified by allergic asthma versus nonallergic asthma, we found that having a diagnosis of allergic asthma actually conferred a protective effect, and there was almost half the risk of hospitalization in asthmatics with allergic asthma as compared to others, which we thought was very interesting,” Dr. Eggert said.
“Eosinophil levels during hospitalization, even when adjusted for systemic steroid use – and we followed patients out through September, when dexamethasone was standard of care – also correlated with better outcomes,” she explained. “This is independent of asthmatic status.”
The researchers noted that confirmation of these results are needed through large, multicenter cohort studies, particularly with regard to how allergic asthma might have a protective effect against SARS-CoV-2 infection. “I think going forward, these findings are very interesting and need to be looked at further to explain the mechanism behind them better,” Dr. Eggert said.
“I think there is also a lot of interest in how this might affect our patients on biologics, which deplete the eosinophils and get rid of that allergic phenotype,” she added. “Does that have any effect on disease severity? Unfortunately, the number of patents on biologics was very small in our cohort, but I do think this is an interesting area for exploration.”
This study was funded in part by the Sean N. Parker Center for Allergy & Asthma Research, Stanford University, Sunshine Foundation, Crown Foundation, and the Parker Foundation.
Asthma is not an independent risk factor for more severe disease or hospitalization due to COVID-19, according to recent research presented at the annual meeting of the American Academy of Allergy, Asthma, and Immunology, held virtually this year.
“In our cohort of patients tested for SARS-CoV-2 at Stanford between March and September, asthma was not an independent risk factor in and of itself for hospitalization or more severe disease from COVID,” Lauren E. Eggert, MD, of the Sean N. Parker Center for Allergy and Asthma Research at Stanford (Calif.) University, said in a poster presentation at the meeting. “What’s more, allergic asthma actually decreased the risk of hospitalization by nearly half.”
Dr. Eggert noted that there have been conflicting data on whether comorbid asthma is or is not a risk factor for more severe COVID-19. “The general thought at the beginning of the pandemic was that because COVID-19 is predominantly a viral respiratory illness, and viral illnesses are known to cause asthma exacerbations, that patients with asthma may be at higher risk if they got COVID infection,” she explained. “But some of the data also showed that Th2 inflammation downregulates ACE2 receptor [expression], which has been shown to be the port of entry for the SARS-CoV-2 virus, so maybe allergy might have a protective effect.”
The researchers at Stanford University identified 168,190 patients at Stanford Health Care who had a positive real-time reverse transcriptase polymerase chain reaction (RT-PCR) test for SARS-CoV-2 between March and September 2020 and collected data from their electronic medical records on their history of asthma, if they were hospitalized, comorbid conditions, and laboratory values. Patients who had no other data available except for a positive SARS-CoV-2 result, or were younger than 28 days, were excluded from the study. Dr. Eggert and colleagues used COVID-19 treatment guidelines from the National Institutes of Health to assess disease severity, which grades COVID-19 severity as asymptomatic or presymptomatic infection, mild illness, moderate illness, severe illness, and critical illness.
In total, the researchers analyzed 5,596 patients who were SARS-CoV-2 positive, with 605 patients (10.8%) hospitalized within 14 days of receiving a positive test. Of these, 100 patients (16.5%) were patients with asthma. There were no significant differences between groups hospitalized and not hospitalized due to COVID-19 in patients with asthma and with no asthma.
Among patients with asthma and COVID-19, 28.0% had asymptomatic illness, 19.0% had moderate disease, 33.0% had severe disease, and 20.0% had critical COVID-19, compared with 36.0% of patients without asthma who had asymptomatic illness, 12.0% with moderate disease, 30.0% with severe disease, and 21.0% with critical COVID-19. Dr. Eggert and colleagues performed a univariate analysis, which showed a significant association between asthma and COVID-19 related hospitalization (odds ratio, 1.53; 95% confidence interval, 1.2-1.93; P < .001), but when adjusting for factors such as diabetes, obesity coronary heart disease, and hypertension, they found there was not a significant association between asthma and hospitalization due to COVID-19 (OR, 1.12; 95% CI, 0.86-1.45; P < .40).
In a univariate analysis, asthma was associated with more severe disease in patients hospitalized for COVID-19, but the results were not significant (OR, 1.21; 95% CI, 0.8-1.85; P = .37). When analyzing allergic asthma alone in a univariate analysis, the researchers found a significant association between allergic asthma and lower hospitalization risk, compared with patients who had nonallergic asthma (OR, 0.55; 95% CI, 0.31-0.92; P = .029), and this association remained after they performed a multivariate analysis as well.
“When we stratified by allergic asthma versus nonallergic asthma, we found that having a diagnosis of allergic asthma actually conferred a protective effect, and there was almost half the risk of hospitalization in asthmatics with allergic asthma as compared to others, which we thought was very interesting,” Dr. Eggert said.
“Eosinophil levels during hospitalization, even when adjusted for systemic steroid use – and we followed patients out through September, when dexamethasone was standard of care – also correlated with better outcomes,” she explained. “This is independent of asthmatic status.”
The researchers noted that confirmation of these results are needed through large, multicenter cohort studies, particularly with regard to how allergic asthma might have a protective effect against SARS-CoV-2 infection. “I think going forward, these findings are very interesting and need to be looked at further to explain the mechanism behind them better,” Dr. Eggert said.
“I think there is also a lot of interest in how this might affect our patients on biologics, which deplete the eosinophils and get rid of that allergic phenotype,” she added. “Does that have any effect on disease severity? Unfortunately, the number of patents on biologics was very small in our cohort, but I do think this is an interesting area for exploration.”
This study was funded in part by the Sean N. Parker Center for Allergy & Asthma Research, Stanford University, Sunshine Foundation, Crown Foundation, and the Parker Foundation.
FROM AAAAI
Frequent medication refills show some patients not achieving asthma control
While most commercially insured patients with asthma have good disease control, some patients may not, according to a recent review of U.S. administrative claims data.
The results of the retrospective analysis, presented at the annual meeting of the American Academy of Allergy, Asthma, and Immunology, held virtually this year, showed some patients with asthma had two or more refills for prescribed systemic corticosteroids (SCS) or short-acting beta agonists (SABA) within a period of 12 months.
“,” Randall Brown, MD, MPH, pulmonologist and senior director of Global Respiratory Medical Affairs at Teva Pharmaceuticals in West Chester, Penn., said in a presentation at the meeting. “Understanding the extent of systemic steroid and SABA prescriptions among patients with asthma and the distribution of those prescriptions across disease severity can be useful in determining the degree of disease control.”
Global Initiative for Asthma (GINA) guidelines consider factors such as symptom control and risk of exacerbation when determining asthma severity, but uncontrolled asthma can still be difficult to assess. Dr. Brown and colleagues set out to determine the prevalence of uncontrolled asthma for patients in the IBM/Watson MarketScan U.S. claims database as well as the rate of uncontrolled asthma by GINA classification. In total, 597,955 patients who had an asthma diagnosis between 12 months before or up to 3 months after the index data of filling a SABA prescription were included for analysis. Patients were at least 12 years old with commercial insurance for at least 12 months, and had no other respiratory diseases other than asthma during the 12 months prior to the index date and during the study period.
The researchers then measured each patient’s 2018 GINA classification of asthma severity based on the number of SCS and SABA prescription claims made between January and December 2017. Overall, 54.3% patients were GINA Step 1, 14.6% were Step 2, 10.2% were Step 3, 19.8% were Step 4, and 1.1% were Step 5.
Dr. Brown and colleagues found that, regardless of GINA disease severity, 18.8% of patients filled two or more SCS prescriptions in 1 year, 27.4% filled three or more SABA prescriptions in 1 year, and 38.7% filled two or more SCS and/or three or more SABA prescriptions in 1 year. “[A] large proportion of these patients did not meet the GINA goal of disease control,” Dr. Brown said.
The researchers found 13% of patients with uncontrolled asthma categorized as GINA Step 1, 20% of patients categorized as GINA Step 2, 19% of patients who were GINA Step 3, 31% of patients who were GINA Step 4, and 54% of patients categorized as GINA Step 5 filled two or more two or more SCS prescriptions per year.
The proportion of patients with uncontrolled asthma who filled three or more SABA prescriptions per year included 19% in GINA Step 1, 29% in GINA Step 2, 35% in GINA Step 3, 44% in GINA Step 4, and 57% in GINA Step 5 groups. For patients who filled both two or more SCS and/or three or more SABA prescriptions per year, the proportion of patients with uncontrolled asthma by GINA category was 29% in GINA Step 1, 42% in GINA Step 2, 46% in GINA Step 3, 58% in GINA Step 4, and 76% of patients in GINA Step 5.
While “poor control was seen across all of the GINA disease severity classifications, the greatest proportion of uncontrolled disease was seen at the highest disease severity, which was also true when we used a stricter definition of uncontrolled disease,” Dr. Brown said. When the researchers applied stricter criteria for patients categorized as GINA Step 5, 39% of patients filled three or more SCS, 41% filled four or more SABA, and 60% filled three or more SCS and/or four or more SABA prescriptions over 12 months.
Dr. Brown said that the analysis “highlights the need for improved asthma management strategies within each of the asthma GINA classification steps.”
“While this population that was studied may be reflective of the wider insured U.S. population, the proportions of uncontrolled asthma may be even greater in non–commercially insured patients within the United States,” he said. “Updates to GINA guidelines incorporate recent consensus [and] recent scientific information and therapies, but many patients in the U.S. are not meeting the GINA goal of disease control. Newer paradigms for systemic corticosteroid-free asthma control as a target of disease ‘remission’ are becoming more commonplace. Such changes and goals may lead to improved asthma management strategies and advancement in treatment.”
This study was funded in part by Teva Branded Pharmaceutical Products R&D, which also provided funding for medical writing assistance from Ashfield MedComms. The authors report being employees of Teva Pharmaceuticals.
While most commercially insured patients with asthma have good disease control, some patients may not, according to a recent review of U.S. administrative claims data.
The results of the retrospective analysis, presented at the annual meeting of the American Academy of Allergy, Asthma, and Immunology, held virtually this year, showed some patients with asthma had two or more refills for prescribed systemic corticosteroids (SCS) or short-acting beta agonists (SABA) within a period of 12 months.
“,” Randall Brown, MD, MPH, pulmonologist and senior director of Global Respiratory Medical Affairs at Teva Pharmaceuticals in West Chester, Penn., said in a presentation at the meeting. “Understanding the extent of systemic steroid and SABA prescriptions among patients with asthma and the distribution of those prescriptions across disease severity can be useful in determining the degree of disease control.”
Global Initiative for Asthma (GINA) guidelines consider factors such as symptom control and risk of exacerbation when determining asthma severity, but uncontrolled asthma can still be difficult to assess. Dr. Brown and colleagues set out to determine the prevalence of uncontrolled asthma for patients in the IBM/Watson MarketScan U.S. claims database as well as the rate of uncontrolled asthma by GINA classification. In total, 597,955 patients who had an asthma diagnosis between 12 months before or up to 3 months after the index data of filling a SABA prescription were included for analysis. Patients were at least 12 years old with commercial insurance for at least 12 months, and had no other respiratory diseases other than asthma during the 12 months prior to the index date and during the study period.
The researchers then measured each patient’s 2018 GINA classification of asthma severity based on the number of SCS and SABA prescription claims made between January and December 2017. Overall, 54.3% patients were GINA Step 1, 14.6% were Step 2, 10.2% were Step 3, 19.8% were Step 4, and 1.1% were Step 5.
Dr. Brown and colleagues found that, regardless of GINA disease severity, 18.8% of patients filled two or more SCS prescriptions in 1 year, 27.4% filled three or more SABA prescriptions in 1 year, and 38.7% filled two or more SCS and/or three or more SABA prescriptions in 1 year. “[A] large proportion of these patients did not meet the GINA goal of disease control,” Dr. Brown said.
The researchers found 13% of patients with uncontrolled asthma categorized as GINA Step 1, 20% of patients categorized as GINA Step 2, 19% of patients who were GINA Step 3, 31% of patients who were GINA Step 4, and 54% of patients categorized as GINA Step 5 filled two or more two or more SCS prescriptions per year.
The proportion of patients with uncontrolled asthma who filled three or more SABA prescriptions per year included 19% in GINA Step 1, 29% in GINA Step 2, 35% in GINA Step 3, 44% in GINA Step 4, and 57% in GINA Step 5 groups. For patients who filled both two or more SCS and/or three or more SABA prescriptions per year, the proportion of patients with uncontrolled asthma by GINA category was 29% in GINA Step 1, 42% in GINA Step 2, 46% in GINA Step 3, 58% in GINA Step 4, and 76% of patients in GINA Step 5.
While “poor control was seen across all of the GINA disease severity classifications, the greatest proportion of uncontrolled disease was seen at the highest disease severity, which was also true when we used a stricter definition of uncontrolled disease,” Dr. Brown said. When the researchers applied stricter criteria for patients categorized as GINA Step 5, 39% of patients filled three or more SCS, 41% filled four or more SABA, and 60% filled three or more SCS and/or four or more SABA prescriptions over 12 months.
Dr. Brown said that the analysis “highlights the need for improved asthma management strategies within each of the asthma GINA classification steps.”
“While this population that was studied may be reflective of the wider insured U.S. population, the proportions of uncontrolled asthma may be even greater in non–commercially insured patients within the United States,” he said. “Updates to GINA guidelines incorporate recent consensus [and] recent scientific information and therapies, but many patients in the U.S. are not meeting the GINA goal of disease control. Newer paradigms for systemic corticosteroid-free asthma control as a target of disease ‘remission’ are becoming more commonplace. Such changes and goals may lead to improved asthma management strategies and advancement in treatment.”
This study was funded in part by Teva Branded Pharmaceutical Products R&D, which also provided funding for medical writing assistance from Ashfield MedComms. The authors report being employees of Teva Pharmaceuticals.
While most commercially insured patients with asthma have good disease control, some patients may not, according to a recent review of U.S. administrative claims data.
The results of the retrospective analysis, presented at the annual meeting of the American Academy of Allergy, Asthma, and Immunology, held virtually this year, showed some patients with asthma had two or more refills for prescribed systemic corticosteroids (SCS) or short-acting beta agonists (SABA) within a period of 12 months.
“,” Randall Brown, MD, MPH, pulmonologist and senior director of Global Respiratory Medical Affairs at Teva Pharmaceuticals in West Chester, Penn., said in a presentation at the meeting. “Understanding the extent of systemic steroid and SABA prescriptions among patients with asthma and the distribution of those prescriptions across disease severity can be useful in determining the degree of disease control.”
Global Initiative for Asthma (GINA) guidelines consider factors such as symptom control and risk of exacerbation when determining asthma severity, but uncontrolled asthma can still be difficult to assess. Dr. Brown and colleagues set out to determine the prevalence of uncontrolled asthma for patients in the IBM/Watson MarketScan U.S. claims database as well as the rate of uncontrolled asthma by GINA classification. In total, 597,955 patients who had an asthma diagnosis between 12 months before or up to 3 months after the index data of filling a SABA prescription were included for analysis. Patients were at least 12 years old with commercial insurance for at least 12 months, and had no other respiratory diseases other than asthma during the 12 months prior to the index date and during the study period.
The researchers then measured each patient’s 2018 GINA classification of asthma severity based on the number of SCS and SABA prescription claims made between January and December 2017. Overall, 54.3% patients were GINA Step 1, 14.6% were Step 2, 10.2% were Step 3, 19.8% were Step 4, and 1.1% were Step 5.
Dr. Brown and colleagues found that, regardless of GINA disease severity, 18.8% of patients filled two or more SCS prescriptions in 1 year, 27.4% filled three or more SABA prescriptions in 1 year, and 38.7% filled two or more SCS and/or three or more SABA prescriptions in 1 year. “[A] large proportion of these patients did not meet the GINA goal of disease control,” Dr. Brown said.
The researchers found 13% of patients with uncontrolled asthma categorized as GINA Step 1, 20% of patients categorized as GINA Step 2, 19% of patients who were GINA Step 3, 31% of patients who were GINA Step 4, and 54% of patients categorized as GINA Step 5 filled two or more two or more SCS prescriptions per year.
The proportion of patients with uncontrolled asthma who filled three or more SABA prescriptions per year included 19% in GINA Step 1, 29% in GINA Step 2, 35% in GINA Step 3, 44% in GINA Step 4, and 57% in GINA Step 5 groups. For patients who filled both two or more SCS and/or three or more SABA prescriptions per year, the proportion of patients with uncontrolled asthma by GINA category was 29% in GINA Step 1, 42% in GINA Step 2, 46% in GINA Step 3, 58% in GINA Step 4, and 76% of patients in GINA Step 5.
While “poor control was seen across all of the GINA disease severity classifications, the greatest proportion of uncontrolled disease was seen at the highest disease severity, which was also true when we used a stricter definition of uncontrolled disease,” Dr. Brown said. When the researchers applied stricter criteria for patients categorized as GINA Step 5, 39% of patients filled three or more SCS, 41% filled four or more SABA, and 60% filled three or more SCS and/or four or more SABA prescriptions over 12 months.
Dr. Brown said that the analysis “highlights the need for improved asthma management strategies within each of the asthma GINA classification steps.”
“While this population that was studied may be reflective of the wider insured U.S. population, the proportions of uncontrolled asthma may be even greater in non–commercially insured patients within the United States,” he said. “Updates to GINA guidelines incorporate recent consensus [and] recent scientific information and therapies, but many patients in the U.S. are not meeting the GINA goal of disease control. Newer paradigms for systemic corticosteroid-free asthma control as a target of disease ‘remission’ are becoming more commonplace. Such changes and goals may lead to improved asthma management strategies and advancement in treatment.”
This study was funded in part by Teva Branded Pharmaceutical Products R&D, which also provided funding for medical writing assistance from Ashfield MedComms. The authors report being employees of Teva Pharmaceuticals.
FROM AAAAI
Masks don’t affect oxygen saturation in people with asthma
Wearing a mask to protect against transmission of COVID-19 does not decrease oxygen saturation, according to a new study.
Oxygen saturation did not decline in more than 200 mask-wearing individuals attending an asthma and allergy clinic, regardless of the type of mask they were wearing and how long they had been wearing the mask.
The study was presented in a late breaking poster session by Marisa Hodges, MD, University of Michigan, Ann Arbor, at the virtual annual meeting of the American Academy of Allergy, Asthma, and Immunology.
“In patients with or without asthma, wearing a mask does not decrease your oxygen level,” coauthor Alan P. Baptist, MD, MPH, director of the University of Michigan Comprehensive Asthma Program, said in an interview.
“Some of my asthma patients had called me requesting an exemption from wearing a mask because they feared that their oxygen intake may be affected, and that got me thinking,” said Malika Gupta, MD, assistant professor, division of allergy and immunology, University of Michigan, Ann Arbor, and the study’s lead investigator.
“We say masks are safe, but I couldn’t find any data to support that statement, and we wanted to provide them with evidence, so they could feel comfortable about wearing their masks,” Dr. Gupta added.
The study collected 223 surveys from adult and pediatric patients presenting to the University of Michigan Medicine Allergy Clinic between Sept. 10 and Oct. 23, 2020.
The patients were asked whether they had a diagnosis of asthma, their degree of perceived control if they did have asthma, the type of mask they were wearing, and how long they had been wearing it.
Investigators obtained resting pulse oximetry readings to measure oxygen saturation (SpO2) from all study participants.
Forty percent of the participants were male, 46% reported having asthma, and 27% were age 19 years or younger.
Overall, the mean SpO2 was 98% (range, 93%-100%) in both asthma and nonasthma groups.
The study also looked at SpO2 with 3 different types of masks: fabric, surgical, and N95.
The mean SpO2 for a fabric mask was 98% (119 patients), for a surgical mask it was also 98% (83 patients), and for the N95 mask it was 99% (3 patients).
Similar results were found with duration of mask use, with the mean SpO2 98% in those wearing a mask for 1 hour or less and 99% in those wearing a mask for 1 hour or longer.
People with asthma who reported they were well controlled showed similar mean SpO2 levels (98%) compared with those who reported they were not well controlled (96.5%)
“No effect on oxygen saturation was noted in any patients, whether they had asthma or not, whether it was well controlled or not, and this was also true regardless of what masks they wore and how long they wore the masks for. So our data reinforce that wearing a mask, whether it be a surgical mask, cloth mask, or N95, is completely safe,” Dr. Baptist said.
“We know wearing a mask is an essential step we can all take to reduce the spread of COVID-19, and we hope these data will give peace of mind to individuals who fear that wearing a mask will adversely affect their oxygen levels,” Dr. Gupta added.
Leonard B. Bacharier, MD, professor of pediatrics and director of the Center for Pediatric Asthma, Monroe Carell Jr. Children’s Hospital at Vanderbilt University Medical Center, Nashville, Tenn., agreed with the investigators’ conclusions.
“The authors found no differences in oxygen saturations between asthmatic and nonasthmatic patients, nor was there a difference based upon mask use or type,” Dr. Bacharier, who was not part of the study, said in an interview.
“These findings provide reassurance that patients, including those with stable asthma, do not experience impaired oxygenation while wearing a mask.”
Dr. Hodges, Dr. Baptist, and Dr. Bacharier have disclosed no relevant financial relationships.
This article was updated 3/11/21.
A version of this article first appeared on Medscape.com.
Wearing a mask to protect against transmission of COVID-19 does not decrease oxygen saturation, according to a new study.
Oxygen saturation did not decline in more than 200 mask-wearing individuals attending an asthma and allergy clinic, regardless of the type of mask they were wearing and how long they had been wearing the mask.
The study was presented in a late breaking poster session by Marisa Hodges, MD, University of Michigan, Ann Arbor, at the virtual annual meeting of the American Academy of Allergy, Asthma, and Immunology.
“In patients with or without asthma, wearing a mask does not decrease your oxygen level,” coauthor Alan P. Baptist, MD, MPH, director of the University of Michigan Comprehensive Asthma Program, said in an interview.
“Some of my asthma patients had called me requesting an exemption from wearing a mask because they feared that their oxygen intake may be affected, and that got me thinking,” said Malika Gupta, MD, assistant professor, division of allergy and immunology, University of Michigan, Ann Arbor, and the study’s lead investigator.
“We say masks are safe, but I couldn’t find any data to support that statement, and we wanted to provide them with evidence, so they could feel comfortable about wearing their masks,” Dr. Gupta added.
The study collected 223 surveys from adult and pediatric patients presenting to the University of Michigan Medicine Allergy Clinic between Sept. 10 and Oct. 23, 2020.
The patients were asked whether they had a diagnosis of asthma, their degree of perceived control if they did have asthma, the type of mask they were wearing, and how long they had been wearing it.
Investigators obtained resting pulse oximetry readings to measure oxygen saturation (SpO2) from all study participants.
Forty percent of the participants were male, 46% reported having asthma, and 27% were age 19 years or younger.
Overall, the mean SpO2 was 98% (range, 93%-100%) in both asthma and nonasthma groups.
The study also looked at SpO2 with 3 different types of masks: fabric, surgical, and N95.
The mean SpO2 for a fabric mask was 98% (119 patients), for a surgical mask it was also 98% (83 patients), and for the N95 mask it was 99% (3 patients).
Similar results were found with duration of mask use, with the mean SpO2 98% in those wearing a mask for 1 hour or less and 99% in those wearing a mask for 1 hour or longer.
People with asthma who reported they were well controlled showed similar mean SpO2 levels (98%) compared with those who reported they were not well controlled (96.5%)
“No effect on oxygen saturation was noted in any patients, whether they had asthma or not, whether it was well controlled or not, and this was also true regardless of what masks they wore and how long they wore the masks for. So our data reinforce that wearing a mask, whether it be a surgical mask, cloth mask, or N95, is completely safe,” Dr. Baptist said.
“We know wearing a mask is an essential step we can all take to reduce the spread of COVID-19, and we hope these data will give peace of mind to individuals who fear that wearing a mask will adversely affect their oxygen levels,” Dr. Gupta added.
Leonard B. Bacharier, MD, professor of pediatrics and director of the Center for Pediatric Asthma, Monroe Carell Jr. Children’s Hospital at Vanderbilt University Medical Center, Nashville, Tenn., agreed with the investigators’ conclusions.
“The authors found no differences in oxygen saturations between asthmatic and nonasthmatic patients, nor was there a difference based upon mask use or type,” Dr. Bacharier, who was not part of the study, said in an interview.
“These findings provide reassurance that patients, including those with stable asthma, do not experience impaired oxygenation while wearing a mask.”
Dr. Hodges, Dr. Baptist, and Dr. Bacharier have disclosed no relevant financial relationships.
This article was updated 3/11/21.
A version of this article first appeared on Medscape.com.
Wearing a mask to protect against transmission of COVID-19 does not decrease oxygen saturation, according to a new study.
Oxygen saturation did not decline in more than 200 mask-wearing individuals attending an asthma and allergy clinic, regardless of the type of mask they were wearing and how long they had been wearing the mask.
The study was presented in a late breaking poster session by Marisa Hodges, MD, University of Michigan, Ann Arbor, at the virtual annual meeting of the American Academy of Allergy, Asthma, and Immunology.
“In patients with or without asthma, wearing a mask does not decrease your oxygen level,” coauthor Alan P. Baptist, MD, MPH, director of the University of Michigan Comprehensive Asthma Program, said in an interview.
“Some of my asthma patients had called me requesting an exemption from wearing a mask because they feared that their oxygen intake may be affected, and that got me thinking,” said Malika Gupta, MD, assistant professor, division of allergy and immunology, University of Michigan, Ann Arbor, and the study’s lead investigator.
“We say masks are safe, but I couldn’t find any data to support that statement, and we wanted to provide them with evidence, so they could feel comfortable about wearing their masks,” Dr. Gupta added.
The study collected 223 surveys from adult and pediatric patients presenting to the University of Michigan Medicine Allergy Clinic between Sept. 10 and Oct. 23, 2020.
The patients were asked whether they had a diagnosis of asthma, their degree of perceived control if they did have asthma, the type of mask they were wearing, and how long they had been wearing it.
Investigators obtained resting pulse oximetry readings to measure oxygen saturation (SpO2) from all study participants.
Forty percent of the participants were male, 46% reported having asthma, and 27% were age 19 years or younger.
Overall, the mean SpO2 was 98% (range, 93%-100%) in both asthma and nonasthma groups.
The study also looked at SpO2 with 3 different types of masks: fabric, surgical, and N95.
The mean SpO2 for a fabric mask was 98% (119 patients), for a surgical mask it was also 98% (83 patients), and for the N95 mask it was 99% (3 patients).
Similar results were found with duration of mask use, with the mean SpO2 98% in those wearing a mask for 1 hour or less and 99% in those wearing a mask for 1 hour or longer.
People with asthma who reported they were well controlled showed similar mean SpO2 levels (98%) compared with those who reported they were not well controlled (96.5%)
“No effect on oxygen saturation was noted in any patients, whether they had asthma or not, whether it was well controlled or not, and this was also true regardless of what masks they wore and how long they wore the masks for. So our data reinforce that wearing a mask, whether it be a surgical mask, cloth mask, or N95, is completely safe,” Dr. Baptist said.
“We know wearing a mask is an essential step we can all take to reduce the spread of COVID-19, and we hope these data will give peace of mind to individuals who fear that wearing a mask will adversely affect their oxygen levels,” Dr. Gupta added.
Leonard B. Bacharier, MD, professor of pediatrics and director of the Center for Pediatric Asthma, Monroe Carell Jr. Children’s Hospital at Vanderbilt University Medical Center, Nashville, Tenn., agreed with the investigators’ conclusions.
“The authors found no differences in oxygen saturations between asthmatic and nonasthmatic patients, nor was there a difference based upon mask use or type,” Dr. Bacharier, who was not part of the study, said in an interview.
“These findings provide reassurance that patients, including those with stable asthma, do not experience impaired oxygenation while wearing a mask.”
Dr. Hodges, Dr. Baptist, and Dr. Bacharier have disclosed no relevant financial relationships.
This article was updated 3/11/21.
A version of this article first appeared on Medscape.com.
FROM AAAAI
FDA grants emergency use authorization to Johnson & Johnson COVID-19 vaccine
And then there were three.
More vaccine availability at a time of high demand and limited supply could help officials vaccinate more Americans, more quickly. In addition, the J&J vaccine offers one-dose convenience and storage at conventional refrigeration temperatures.
Initial reactions to the EUA for the J&J vaccine have been positive.
“The advantages of having a third vaccine, especially one that is a single shot and can be stored without special refrigeration requirements, will be a major contribution in getting the general public vaccinated sooner, both in the U.S. and around the world,” Phyllis Tien, MD, professor of medicine in the division of infectious diseases at the University of California, San Francisco, told Medscape Medical News.
“It’s great news. We have yet a third vaccine that is highly effective at preventing COVID, and even more effective at preventing severe COVID,” said Paul Goepfert, MD. It’s a “tremendous boon for our country and other countries as well.”
“This vaccine has also been shown to be effective against the B.1.351 strain that was first described in South Africa,” added Dr. Goepfert, director of the Alabama Vaccine Research Clinic and infectious disease specialist at the University of Alabama at Birmingham.
The EUA “is indeed exciting news,” Colleen Kraft, MD, associate chief medical officer at Emory University Hospital and associate professor at Emory University School of Medicine in Atlanta, said during a February 25 media briefing.
One recent concern centers on people aged 60 years and older. Documents the FDA released earlier this week suggest a lower efficacy, 42%, for the J&J immunization among people in this age group with certain relevant comorbidities. In contrast, without underlying conditions like heart disease or diabetes, efficacy in this cohort was 72%.
The more the merrier
The scope and urgency of the COVID-19 pandemic necessitates as many protective measures as possible, said Raj Shah, MD, geriatrician, and associate professor of family medicine and codirector of the Center for Community Health Equity at Rush University in Chicago.
“Trying to vaccinate as many individuals living in the United States to prevent the spread of COVID is such a big project that no one company or one vaccine was going to be able to ramp up fast enough on its own,” Dr. Shah told Medscape Medical News.“This has been the hope for us,” he added, “to get to multiple vaccines with slightly different properties that will provide more options.”
Experience with the J&J vaccine so far suggests reactions are less severe. “The nice thing about the Johnson and Johnson [vaccine] is that it definitely has less side effects,” Dr. Kraft said.
On the other hand, low-grade fever, chills, or fatigue after vaccination can be considered a positive because they can reflect how well the immune system is responding, she added.
One and done?
Single-dose administration could be more than a convenience — it could also help clinicians vaccinate members of underserved communities and rural locations, where returning for a second dose could be more difficult for some people.
“In a controlled setting, in a clinical trial, we do a lot to make sure people get all the treatment they need,” Dr. Shah said. “We’re not seeing it right now, but we’re always worried when we have more than one dose that has to be administered, that some people will drop off and not come back for the second vaccine.”
This group could include the needle-phobic, he added. “For them, having it done once alleviates a lot of the anxiety.”
Looking beyond the numbers
The phase 3 ENSEMBLE study of the J&J vaccine revealed a 72% efficacy for preventing moderate-to-severe COVID-19 among U.S. participants. In contrast, researchers reported 94% to 95% efficacy for the Pfizer/BioNTech and Moderna vaccines.
However, experts agreed that focusing solely on these numbers can miss more important points. For example, no participants who received the J&J vaccine in the phase 3 trial died from COVID-19-related illness. There were five such deaths in the placebo cohort.
“One of the things that these vaccines do very well is they minimize severe disease,” Dr. Kraft said. “As somebody that has spent an inordinate time in the hospital taking care of patients with severe disease from COVID, this is very much a welcome addition to our armamentarium to fight this virus.”
“If you can give something that prevents people from dying, that is a true path to normalcy,” Dr. Goepfert added.
More work to do
“The demand is strong from all groups right now. We just have to work on getting more vaccines out there,” Dr. Shah said.
“We are at a point in this country where we are getting better with the distribution of the vaccine,” he added, “but we are nowhere close to achieving that distribution of vaccines to get to everybody.”
Dr. Goepfert, Dr. Shah, and Dr. Kraft disclosed no relevant financial relationships. Dr. Tien received support from Johnson & Johnson to conduct the J&J COVID-19 vaccine trial in the San Francisco VA Health Care System.
A version of this article first appeared on Medscape.com.
And then there were three.
More vaccine availability at a time of high demand and limited supply could help officials vaccinate more Americans, more quickly. In addition, the J&J vaccine offers one-dose convenience and storage at conventional refrigeration temperatures.
Initial reactions to the EUA for the J&J vaccine have been positive.
“The advantages of having a third vaccine, especially one that is a single shot and can be stored without special refrigeration requirements, will be a major contribution in getting the general public vaccinated sooner, both in the U.S. and around the world,” Phyllis Tien, MD, professor of medicine in the division of infectious diseases at the University of California, San Francisco, told Medscape Medical News.
“It’s great news. We have yet a third vaccine that is highly effective at preventing COVID, and even more effective at preventing severe COVID,” said Paul Goepfert, MD. It’s a “tremendous boon for our country and other countries as well.”
“This vaccine has also been shown to be effective against the B.1.351 strain that was first described in South Africa,” added Dr. Goepfert, director of the Alabama Vaccine Research Clinic and infectious disease specialist at the University of Alabama at Birmingham.
The EUA “is indeed exciting news,” Colleen Kraft, MD, associate chief medical officer at Emory University Hospital and associate professor at Emory University School of Medicine in Atlanta, said during a February 25 media briefing.
One recent concern centers on people aged 60 years and older. Documents the FDA released earlier this week suggest a lower efficacy, 42%, for the J&J immunization among people in this age group with certain relevant comorbidities. In contrast, without underlying conditions like heart disease or diabetes, efficacy in this cohort was 72%.
The more the merrier
The scope and urgency of the COVID-19 pandemic necessitates as many protective measures as possible, said Raj Shah, MD, geriatrician, and associate professor of family medicine and codirector of the Center for Community Health Equity at Rush University in Chicago.
“Trying to vaccinate as many individuals living in the United States to prevent the spread of COVID is such a big project that no one company or one vaccine was going to be able to ramp up fast enough on its own,” Dr. Shah told Medscape Medical News.“This has been the hope for us,” he added, “to get to multiple vaccines with slightly different properties that will provide more options.”
Experience with the J&J vaccine so far suggests reactions are less severe. “The nice thing about the Johnson and Johnson [vaccine] is that it definitely has less side effects,” Dr. Kraft said.
On the other hand, low-grade fever, chills, or fatigue after vaccination can be considered a positive because they can reflect how well the immune system is responding, she added.
One and done?
Single-dose administration could be more than a convenience — it could also help clinicians vaccinate members of underserved communities and rural locations, where returning for a second dose could be more difficult for some people.
“In a controlled setting, in a clinical trial, we do a lot to make sure people get all the treatment they need,” Dr. Shah said. “We’re not seeing it right now, but we’re always worried when we have more than one dose that has to be administered, that some people will drop off and not come back for the second vaccine.”
This group could include the needle-phobic, he added. “For them, having it done once alleviates a lot of the anxiety.”
Looking beyond the numbers
The phase 3 ENSEMBLE study of the J&J vaccine revealed a 72% efficacy for preventing moderate-to-severe COVID-19 among U.S. participants. In contrast, researchers reported 94% to 95% efficacy for the Pfizer/BioNTech and Moderna vaccines.
However, experts agreed that focusing solely on these numbers can miss more important points. For example, no participants who received the J&J vaccine in the phase 3 trial died from COVID-19-related illness. There were five such deaths in the placebo cohort.
“One of the things that these vaccines do very well is they minimize severe disease,” Dr. Kraft said. “As somebody that has spent an inordinate time in the hospital taking care of patients with severe disease from COVID, this is very much a welcome addition to our armamentarium to fight this virus.”
“If you can give something that prevents people from dying, that is a true path to normalcy,” Dr. Goepfert added.
More work to do
“The demand is strong from all groups right now. We just have to work on getting more vaccines out there,” Dr. Shah said.
“We are at a point in this country where we are getting better with the distribution of the vaccine,” he added, “but we are nowhere close to achieving that distribution of vaccines to get to everybody.”
Dr. Goepfert, Dr. Shah, and Dr. Kraft disclosed no relevant financial relationships. Dr. Tien received support from Johnson & Johnson to conduct the J&J COVID-19 vaccine trial in the San Francisco VA Health Care System.
A version of this article first appeared on Medscape.com.
And then there were three.
More vaccine availability at a time of high demand and limited supply could help officials vaccinate more Americans, more quickly. In addition, the J&J vaccine offers one-dose convenience and storage at conventional refrigeration temperatures.
Initial reactions to the EUA for the J&J vaccine have been positive.
“The advantages of having a third vaccine, especially one that is a single shot and can be stored without special refrigeration requirements, will be a major contribution in getting the general public vaccinated sooner, both in the U.S. and around the world,” Phyllis Tien, MD, professor of medicine in the division of infectious diseases at the University of California, San Francisco, told Medscape Medical News.
“It’s great news. We have yet a third vaccine that is highly effective at preventing COVID, and even more effective at preventing severe COVID,” said Paul Goepfert, MD. It’s a “tremendous boon for our country and other countries as well.”
“This vaccine has also been shown to be effective against the B.1.351 strain that was first described in South Africa,” added Dr. Goepfert, director of the Alabama Vaccine Research Clinic and infectious disease specialist at the University of Alabama at Birmingham.
The EUA “is indeed exciting news,” Colleen Kraft, MD, associate chief medical officer at Emory University Hospital and associate professor at Emory University School of Medicine in Atlanta, said during a February 25 media briefing.
One recent concern centers on people aged 60 years and older. Documents the FDA released earlier this week suggest a lower efficacy, 42%, for the J&J immunization among people in this age group with certain relevant comorbidities. In contrast, without underlying conditions like heart disease or diabetes, efficacy in this cohort was 72%.
The more the merrier
The scope and urgency of the COVID-19 pandemic necessitates as many protective measures as possible, said Raj Shah, MD, geriatrician, and associate professor of family medicine and codirector of the Center for Community Health Equity at Rush University in Chicago.
“Trying to vaccinate as many individuals living in the United States to prevent the spread of COVID is such a big project that no one company or one vaccine was going to be able to ramp up fast enough on its own,” Dr. Shah told Medscape Medical News.“This has been the hope for us,” he added, “to get to multiple vaccines with slightly different properties that will provide more options.”
Experience with the J&J vaccine so far suggests reactions are less severe. “The nice thing about the Johnson and Johnson [vaccine] is that it definitely has less side effects,” Dr. Kraft said.
On the other hand, low-grade fever, chills, or fatigue after vaccination can be considered a positive because they can reflect how well the immune system is responding, she added.
One and done?
Single-dose administration could be more than a convenience — it could also help clinicians vaccinate members of underserved communities and rural locations, where returning for a second dose could be more difficult for some people.
“In a controlled setting, in a clinical trial, we do a lot to make sure people get all the treatment they need,” Dr. Shah said. “We’re not seeing it right now, but we’re always worried when we have more than one dose that has to be administered, that some people will drop off and not come back for the second vaccine.”
This group could include the needle-phobic, he added. “For them, having it done once alleviates a lot of the anxiety.”
Looking beyond the numbers
The phase 3 ENSEMBLE study of the J&J vaccine revealed a 72% efficacy for preventing moderate-to-severe COVID-19 among U.S. participants. In contrast, researchers reported 94% to 95% efficacy for the Pfizer/BioNTech and Moderna vaccines.
However, experts agreed that focusing solely on these numbers can miss more important points. For example, no participants who received the J&J vaccine in the phase 3 trial died from COVID-19-related illness. There were five such deaths in the placebo cohort.
“One of the things that these vaccines do very well is they minimize severe disease,” Dr. Kraft said. “As somebody that has spent an inordinate time in the hospital taking care of patients with severe disease from COVID, this is very much a welcome addition to our armamentarium to fight this virus.”
“If you can give something that prevents people from dying, that is a true path to normalcy,” Dr. Goepfert added.
More work to do
“The demand is strong from all groups right now. We just have to work on getting more vaccines out there,” Dr. Shah said.
“We are at a point in this country where we are getting better with the distribution of the vaccine,” he added, “but we are nowhere close to achieving that distribution of vaccines to get to everybody.”
Dr. Goepfert, Dr. Shah, and Dr. Kraft disclosed no relevant financial relationships. Dr. Tien received support from Johnson & Johnson to conduct the J&J COVID-19 vaccine trial in the San Francisco VA Health Care System.
A version of this article first appeared on Medscape.com.
J&J COVID-19 vaccine wins unanimous backing of FDA panel
The Food and Drug Administration (FDA) is expected to quickly provide an emergency use authorization (EUA) for the vaccine following the recommendation by the panel. The FDA’s Vaccines and Related Biological Products Advisory Committee voted 22-0 on this question: Based on the totality of scientific evidence available, do the benefits of the Johnson & Johnson COVID-19 Vaccine outweigh its risks for use in individuals 18 years of age and older?
The Johnson & Johnson vaccine is expected to offer more convenient dosing and be easier to distribute than the two rival products already available in the United States. Janssen’s vaccine is intended to be given in a single dose. In December, the FDA granted EUAs for the Pfizer/BioNTech and Moderna COVID-19 vaccines, which are each two-dose regimens.
Johnson & Johnson’s vaccine can be stored for at least 3 months at normal refrigerator temperatures of 2°C to 8°C (36°F to 46°F). Its shipping and storage fits into the existing medical supply infrastructure, the company said in its briefing materials for the FDA advisory committee meeting. In contrast, Pfizer’s vaccine is stored in ultracold freezers at temperatures between -80°C and -60°C (-112°F and -76°F), according to the Centers for Disease Control and Prevention. Moderna’s vaccine may be stored in a freezer between -25°C and -15°C (-13°F and 5°F).
But FDA advisers focused more in their deliberations on concerns about Janssen’s vaccine, including emerging reports of allergic reactions.
The advisers also discussed how patients might respond to the widely reported gap between Johnson & Johnson’s topline efficacy rates compared with rivals. The company’s initial unveiling last month of key results for its vaccine caused an initial wave of disappointment, with its overall efficacy against moderate-to-severe COVID-19 28 days postvaccination first reported at about 66% globally. By contrast, results for the Pfizer and Moderna vaccines suggest they have efficacy rates of 95% and 94%.
But in concluding, the advisers spoke of the Janssen vaccine as a much-needed tool to address the COVID-19 pandemic. The death toll in the United States attributed to the virus has reached 501,414, according to the World Health Organization.
“Despite the concerns that were raised during the discussion. I think what we have to keep in mind is that we’re still in the midst of this deadly pandemic,” said FDA adviser Archana Chatterjee, MD, PhD, from Rosalind Franklin University. “There is a shortage of vaccines that are currently authorized, and I think authorization of this vaccine will help meet the needs at the moment.”
The FDA is not bound to accept the recommendations of its advisers, but it often does so.
Anaphylaxis case
FDA advisers raised only a few questions for Johnson & Johnson and FDA staff ahead of their vote. The committee’s deliberations were less contentious and heated than had been during its December reviews of the Pfizer and Moderna vaccines. In those meetings, the panel voted 17-4, with one abstention, in favor of Pfizer’s vaccine and 20-0, with one abstention, on the Moderna vaccine.
“We are very comfortable now with the procedure, as well as the vaccines,” said Arnold Monto, MD, after the Feb. 26 vote on the Janssen vaccine. Dr. Monto, from the University of Michigan School of Public Health in Ann Arbor, has served as the chairman of the FDA panel through its review of all three COVID-19 vaccines.
Among the issues noted in the deliberations was the emergence of a concern about anaphylaxis with the vaccine.
This serious allergic reaction has been seen in people who have taken the Pfizer and Moderna vaccines. Before the week of the panel meeting, though, there had not been reports of anaphylaxis with the Johnson & Johnson vaccine, said Macaya Douoguih, MD, MPH, head of clinical development and medical affairs for Janssen/ Johnson & Johnson’s vaccines division.
However, on February 24, Johnson & Johnson received preliminary reports about two cases of severe allergic reaction from an open-label study in South Africa, with one of these being anaphylaxis, Dr. Douoguih said. The company will continue to closely monitor for these events as outlined in their pharmacovigilance plan, Dr. Douoguih said.
Federal health officials have sought to make clinicians aware of the rare risk for anaphylaxis with COVID vaccines, while reminding the public that this reaction can be managed.
The FDA had Tom Shimabukuro, MD, MPH, MBA, from the CDC, give an update on postmarketing surveillance for the Pfizer and Moderna vaccines as part of the review of the Johnson & Johnson application. Dr. Shimabukuro and CDC colleagues published a report in JAMA on February 14 that looked at an anaphylaxis case reported connected with COVID vaccines between December 14, 2020, and January 18, 2021.
The CDC identified 66 case reports received that met Brighton Collaboration case definition criteria for anaphylaxis (levels 1, 2, or 3): 47 following Pfizer/BioNTech vaccine, for a reporting rate of 4.7 cases/million doses administered, and 19 following Moderna vaccine, for a reporting rate of 2.5 cases/million doses administered, Dr. Shimabukuro and CDC colleagues wrote.
The CDC has published materials to help clinicians prepare for the possibility of this rare event, Dr. Shimabukuro told the FDA advisers.
“The take-home message here is that these are rare events and anaphylaxis, although clinically serious, is treatable,” Dr. Shimabukuro said.
At the conclusion of the meeting, FDA panelist Patrick Moore, MD, MPH, from the University of Pittsburgh in Pennsylvania, stressed the need to convey to the public that the COVID vaccines appear so far to be safe. Many people earlier had doubts about how the FDA could both safely and quickly review the applications for EUAs for these products.
“As of February 26, things are looking good. That could change tomorrow,” Dr. Moore said. But “this whole EUA process does seem to have worked, despite my own personal concerns about it.”
No second-class vaccines
The Johnson & Johnson vaccine, known as Ad26.COV2.S, is composed of a recombinant, replication-incompetent human adenovirus type 26 (Ad26) vector. It’s intended to encode a stabilized form of SARS-CoV-2 spike (S) protein. The Pfizer and Moderna vaccines use a different mechanism. They rely on mRNA.
The FDA advisers also discussed how patients might respond to the widely reported gap between Janssen’s topline efficacy rates compared with rivals. They urged against people parsing study details too finely and seeking to pick and choose their shots.
“It’s important that people do not think that one vaccine is better than another,” said FDA adviser H. Cody Meissner, MD, from Tufts University School of Medicine in Boston.
Dr. Monto agreed, noting that many people in the United States are still waiting for their turn to get COVID vaccines because of the limited early supply.
Trying to game the system to get one vaccine instead of another would not be wise. “In this environment, whatever you can get, get,” Dr. Monto said.
During an open public hearing, Sarah Christopherson, policy advocacy director of the National Women’s Health Network, said that press reports are fueling a damaging impression in the public that there are “first and second-class” vaccines.
“That has the potential to exacerbate existing mistrust” in vaccines, she said. “Public health authorities must address these perceptions head on.”
She urged against attempts to compare the Janssen vaccine to others, noting the potential effects of emerging variants of the virus.
“It’s difficult to make an apples-to-apples comparison between vaccines,” she said.
Johnson & Johnson’s efficacy results, which are lower than those of the mRNA vaccines, may be a reflection of the ways in which SARS-Co-V-2 is mutating and thus becoming more of a threat, according to the company. A key study of the new vaccine, involving about 44,000 people, coincided with the emergence of new SARS-CoV-2 variants, which were emerging in some of the countries where the pivotal COV3001 study was being conducted, the company said.
At least 14 days after vaccination, the Johnson & Johnson COVID vaccine efficacy (95% confidence interval) was 72.0% (58.2, 81.7) in the United States, 68.1% (48.8, 80.7) in Brazil, and 64.0% (41.2, 78.7) in South Africa.
Weakened standards?
Several researchers called on the FDA to maintain a critical attitude when assessing Johnson & Johnson’s application for the EUA, warning of a potential for a permanent erosion of agency rules due to hasty action on COVID vaccines.
They raised concerns about the FDA demanding too little in terms of follow-up studies on COVID vaccines and with persisting murkiness resulting in attempts to determine how well these treatments work beyond the initial study period.
“I worry about FDA lowering its approval standards,” said Peter Doshi, PhD, from The BMJ and a faculty member at the University of Maryland School of Medicine in Baltimore, during an open public hearing at the meeting.
“There’s a real urgency to stand back right now and look at the forest here, as well as the trees, and I urge the committee to consider the effects FDA decisions may have on the entire regulatory approval process,” Dr. Doshi said.
Dr. Doshi asked why Johnson & Johnson did not seek a standard full approval — a biologics license application (BLA) — instead of aiming for the lower bar of an EUA. The FDA already has allowed wide distribution of the Pfizer/BioNTech and Moderna vaccines through EUAs. That removes the sense of urgency that FDA faced last year in his view.
The FDA’s June 2020 guidance on the development of COVID vaccines had asked drugmakers to plan on following participants in COVID vaccine trials for “ideally at least one to two years.” Yet people who got placebo in Moderna and Pfizer trials already are being vaccinated, Dr. Doshi said. And Johnson & Johnson said in its presentation to the FDA that if the Ad26.COV2.S vaccine were granted an EUA, the COV3001 study design would be amended to “facilitate cross-over of placebo participants in all participating countries to receive one dose of active study vaccine as fast as operationally feasible.”
“I’m nervous about the prospect of there never being a COVID vaccine that meets the FDA’s approval standard” for a BLA instead of the more limited EUA, Dr. Doshi said.
Diana Zuckerman, PhD, president of the nonprofit National Center for Health Research, noted that the FDA’s subsequent guidance tailored for EUAs for COVID vaccines “drastically shortened” the follow-up time to a median of 2 months. Dr. Zuckerman said that a crossover design would be “a reasonable compromise, but only if the placebo group has at least 6 months of data.” Dr. Zuckerman opened her remarks in the open public hearing by saying she had inherited Johnson & Johnson stock, so was speaking at the meeting against her own financial interest.
“As soon as a vaccine is authorized, we start losing the placebo group. If FDA lets that happen, that’s a huge loss for public health and a huge loss of information about how we can all stay safe,” Dr. Zuckerman said.
A version of this article first appeared on Medscape.com.
The Food and Drug Administration (FDA) is expected to quickly provide an emergency use authorization (EUA) for the vaccine following the recommendation by the panel. The FDA’s Vaccines and Related Biological Products Advisory Committee voted 22-0 on this question: Based on the totality of scientific evidence available, do the benefits of the Johnson & Johnson COVID-19 Vaccine outweigh its risks for use in individuals 18 years of age and older?
The Johnson & Johnson vaccine is expected to offer more convenient dosing and be easier to distribute than the two rival products already available in the United States. Janssen’s vaccine is intended to be given in a single dose. In December, the FDA granted EUAs for the Pfizer/BioNTech and Moderna COVID-19 vaccines, which are each two-dose regimens.
Johnson & Johnson’s vaccine can be stored for at least 3 months at normal refrigerator temperatures of 2°C to 8°C (36°F to 46°F). Its shipping and storage fits into the existing medical supply infrastructure, the company said in its briefing materials for the FDA advisory committee meeting. In contrast, Pfizer’s vaccine is stored in ultracold freezers at temperatures between -80°C and -60°C (-112°F and -76°F), according to the Centers for Disease Control and Prevention. Moderna’s vaccine may be stored in a freezer between -25°C and -15°C (-13°F and 5°F).
But FDA advisers focused more in their deliberations on concerns about Janssen’s vaccine, including emerging reports of allergic reactions.
The advisers also discussed how patients might respond to the widely reported gap between Johnson & Johnson’s topline efficacy rates compared with rivals. The company’s initial unveiling last month of key results for its vaccine caused an initial wave of disappointment, with its overall efficacy against moderate-to-severe COVID-19 28 days postvaccination first reported at about 66% globally. By contrast, results for the Pfizer and Moderna vaccines suggest they have efficacy rates of 95% and 94%.
But in concluding, the advisers spoke of the Janssen vaccine as a much-needed tool to address the COVID-19 pandemic. The death toll in the United States attributed to the virus has reached 501,414, according to the World Health Organization.
“Despite the concerns that were raised during the discussion. I think what we have to keep in mind is that we’re still in the midst of this deadly pandemic,” said FDA adviser Archana Chatterjee, MD, PhD, from Rosalind Franklin University. “There is a shortage of vaccines that are currently authorized, and I think authorization of this vaccine will help meet the needs at the moment.”
The FDA is not bound to accept the recommendations of its advisers, but it often does so.
Anaphylaxis case
FDA advisers raised only a few questions for Johnson & Johnson and FDA staff ahead of their vote. The committee’s deliberations were less contentious and heated than had been during its December reviews of the Pfizer and Moderna vaccines. In those meetings, the panel voted 17-4, with one abstention, in favor of Pfizer’s vaccine and 20-0, with one abstention, on the Moderna vaccine.
“We are very comfortable now with the procedure, as well as the vaccines,” said Arnold Monto, MD, after the Feb. 26 vote on the Janssen vaccine. Dr. Monto, from the University of Michigan School of Public Health in Ann Arbor, has served as the chairman of the FDA panel through its review of all three COVID-19 vaccines.
Among the issues noted in the deliberations was the emergence of a concern about anaphylaxis with the vaccine.
This serious allergic reaction has been seen in people who have taken the Pfizer and Moderna vaccines. Before the week of the panel meeting, though, there had not been reports of anaphylaxis with the Johnson & Johnson vaccine, said Macaya Douoguih, MD, MPH, head of clinical development and medical affairs for Janssen/ Johnson & Johnson’s vaccines division.
However, on February 24, Johnson & Johnson received preliminary reports about two cases of severe allergic reaction from an open-label study in South Africa, with one of these being anaphylaxis, Dr. Douoguih said. The company will continue to closely monitor for these events as outlined in their pharmacovigilance plan, Dr. Douoguih said.
Federal health officials have sought to make clinicians aware of the rare risk for anaphylaxis with COVID vaccines, while reminding the public that this reaction can be managed.
The FDA had Tom Shimabukuro, MD, MPH, MBA, from the CDC, give an update on postmarketing surveillance for the Pfizer and Moderna vaccines as part of the review of the Johnson & Johnson application. Dr. Shimabukuro and CDC colleagues published a report in JAMA on February 14 that looked at an anaphylaxis case reported connected with COVID vaccines between December 14, 2020, and January 18, 2021.
The CDC identified 66 case reports received that met Brighton Collaboration case definition criteria for anaphylaxis (levels 1, 2, or 3): 47 following Pfizer/BioNTech vaccine, for a reporting rate of 4.7 cases/million doses administered, and 19 following Moderna vaccine, for a reporting rate of 2.5 cases/million doses administered, Dr. Shimabukuro and CDC colleagues wrote.
The CDC has published materials to help clinicians prepare for the possibility of this rare event, Dr. Shimabukuro told the FDA advisers.
“The take-home message here is that these are rare events and anaphylaxis, although clinically serious, is treatable,” Dr. Shimabukuro said.
At the conclusion of the meeting, FDA panelist Patrick Moore, MD, MPH, from the University of Pittsburgh in Pennsylvania, stressed the need to convey to the public that the COVID vaccines appear so far to be safe. Many people earlier had doubts about how the FDA could both safely and quickly review the applications for EUAs for these products.
“As of February 26, things are looking good. That could change tomorrow,” Dr. Moore said. But “this whole EUA process does seem to have worked, despite my own personal concerns about it.”
No second-class vaccines
The Johnson & Johnson vaccine, known as Ad26.COV2.S, is composed of a recombinant, replication-incompetent human adenovirus type 26 (Ad26) vector. It’s intended to encode a stabilized form of SARS-CoV-2 spike (S) protein. The Pfizer and Moderna vaccines use a different mechanism. They rely on mRNA.
The FDA advisers also discussed how patients might respond to the widely reported gap between Janssen’s topline efficacy rates compared with rivals. They urged against people parsing study details too finely and seeking to pick and choose their shots.
“It’s important that people do not think that one vaccine is better than another,” said FDA adviser H. Cody Meissner, MD, from Tufts University School of Medicine in Boston.
Dr. Monto agreed, noting that many people in the United States are still waiting for their turn to get COVID vaccines because of the limited early supply.
Trying to game the system to get one vaccine instead of another would not be wise. “In this environment, whatever you can get, get,” Dr. Monto said.
During an open public hearing, Sarah Christopherson, policy advocacy director of the National Women’s Health Network, said that press reports are fueling a damaging impression in the public that there are “first and second-class” vaccines.
“That has the potential to exacerbate existing mistrust” in vaccines, she said. “Public health authorities must address these perceptions head on.”
She urged against attempts to compare the Janssen vaccine to others, noting the potential effects of emerging variants of the virus.
“It’s difficult to make an apples-to-apples comparison between vaccines,” she said.
Johnson & Johnson’s efficacy results, which are lower than those of the mRNA vaccines, may be a reflection of the ways in which SARS-Co-V-2 is mutating and thus becoming more of a threat, according to the company. A key study of the new vaccine, involving about 44,000 people, coincided with the emergence of new SARS-CoV-2 variants, which were emerging in some of the countries where the pivotal COV3001 study was being conducted, the company said.
At least 14 days after vaccination, the Johnson & Johnson COVID vaccine efficacy (95% confidence interval) was 72.0% (58.2, 81.7) in the United States, 68.1% (48.8, 80.7) in Brazil, and 64.0% (41.2, 78.7) in South Africa.
Weakened standards?
Several researchers called on the FDA to maintain a critical attitude when assessing Johnson & Johnson’s application for the EUA, warning of a potential for a permanent erosion of agency rules due to hasty action on COVID vaccines.
They raised concerns about the FDA demanding too little in terms of follow-up studies on COVID vaccines and with persisting murkiness resulting in attempts to determine how well these treatments work beyond the initial study period.
“I worry about FDA lowering its approval standards,” said Peter Doshi, PhD, from The BMJ and a faculty member at the University of Maryland School of Medicine in Baltimore, during an open public hearing at the meeting.
“There’s a real urgency to stand back right now and look at the forest here, as well as the trees, and I urge the committee to consider the effects FDA decisions may have on the entire regulatory approval process,” Dr. Doshi said.
Dr. Doshi asked why Johnson & Johnson did not seek a standard full approval — a biologics license application (BLA) — instead of aiming for the lower bar of an EUA. The FDA already has allowed wide distribution of the Pfizer/BioNTech and Moderna vaccines through EUAs. That removes the sense of urgency that FDA faced last year in his view.
The FDA’s June 2020 guidance on the development of COVID vaccines had asked drugmakers to plan on following participants in COVID vaccine trials for “ideally at least one to two years.” Yet people who got placebo in Moderna and Pfizer trials already are being vaccinated, Dr. Doshi said. And Johnson & Johnson said in its presentation to the FDA that if the Ad26.COV2.S vaccine were granted an EUA, the COV3001 study design would be amended to “facilitate cross-over of placebo participants in all participating countries to receive one dose of active study vaccine as fast as operationally feasible.”
“I’m nervous about the prospect of there never being a COVID vaccine that meets the FDA’s approval standard” for a BLA instead of the more limited EUA, Dr. Doshi said.
Diana Zuckerman, PhD, president of the nonprofit National Center for Health Research, noted that the FDA’s subsequent guidance tailored for EUAs for COVID vaccines “drastically shortened” the follow-up time to a median of 2 months. Dr. Zuckerman said that a crossover design would be “a reasonable compromise, but only if the placebo group has at least 6 months of data.” Dr. Zuckerman opened her remarks in the open public hearing by saying she had inherited Johnson & Johnson stock, so was speaking at the meeting against her own financial interest.
“As soon as a vaccine is authorized, we start losing the placebo group. If FDA lets that happen, that’s a huge loss for public health and a huge loss of information about how we can all stay safe,” Dr. Zuckerman said.
A version of this article first appeared on Medscape.com.
The Food and Drug Administration (FDA) is expected to quickly provide an emergency use authorization (EUA) for the vaccine following the recommendation by the panel. The FDA’s Vaccines and Related Biological Products Advisory Committee voted 22-0 on this question: Based on the totality of scientific evidence available, do the benefits of the Johnson & Johnson COVID-19 Vaccine outweigh its risks for use in individuals 18 years of age and older?
The Johnson & Johnson vaccine is expected to offer more convenient dosing and be easier to distribute than the two rival products already available in the United States. Janssen’s vaccine is intended to be given in a single dose. In December, the FDA granted EUAs for the Pfizer/BioNTech and Moderna COVID-19 vaccines, which are each two-dose regimens.
Johnson & Johnson’s vaccine can be stored for at least 3 months at normal refrigerator temperatures of 2°C to 8°C (36°F to 46°F). Its shipping and storage fits into the existing medical supply infrastructure, the company said in its briefing materials for the FDA advisory committee meeting. In contrast, Pfizer’s vaccine is stored in ultracold freezers at temperatures between -80°C and -60°C (-112°F and -76°F), according to the Centers for Disease Control and Prevention. Moderna’s vaccine may be stored in a freezer between -25°C and -15°C (-13°F and 5°F).
But FDA advisers focused more in their deliberations on concerns about Janssen’s vaccine, including emerging reports of allergic reactions.
The advisers also discussed how patients might respond to the widely reported gap between Johnson & Johnson’s topline efficacy rates compared with rivals. The company’s initial unveiling last month of key results for its vaccine caused an initial wave of disappointment, with its overall efficacy against moderate-to-severe COVID-19 28 days postvaccination first reported at about 66% globally. By contrast, results for the Pfizer and Moderna vaccines suggest they have efficacy rates of 95% and 94%.
But in concluding, the advisers spoke of the Janssen vaccine as a much-needed tool to address the COVID-19 pandemic. The death toll in the United States attributed to the virus has reached 501,414, according to the World Health Organization.
“Despite the concerns that were raised during the discussion. I think what we have to keep in mind is that we’re still in the midst of this deadly pandemic,” said FDA adviser Archana Chatterjee, MD, PhD, from Rosalind Franklin University. “There is a shortage of vaccines that are currently authorized, and I think authorization of this vaccine will help meet the needs at the moment.”
The FDA is not bound to accept the recommendations of its advisers, but it often does so.
Anaphylaxis case
FDA advisers raised only a few questions for Johnson & Johnson and FDA staff ahead of their vote. The committee’s deliberations were less contentious and heated than had been during its December reviews of the Pfizer and Moderna vaccines. In those meetings, the panel voted 17-4, with one abstention, in favor of Pfizer’s vaccine and 20-0, with one abstention, on the Moderna vaccine.
“We are very comfortable now with the procedure, as well as the vaccines,” said Arnold Monto, MD, after the Feb. 26 vote on the Janssen vaccine. Dr. Monto, from the University of Michigan School of Public Health in Ann Arbor, has served as the chairman of the FDA panel through its review of all three COVID-19 vaccines.
Among the issues noted in the deliberations was the emergence of a concern about anaphylaxis with the vaccine.
This serious allergic reaction has been seen in people who have taken the Pfizer and Moderna vaccines. Before the week of the panel meeting, though, there had not been reports of anaphylaxis with the Johnson & Johnson vaccine, said Macaya Douoguih, MD, MPH, head of clinical development and medical affairs for Janssen/ Johnson & Johnson’s vaccines division.
However, on February 24, Johnson & Johnson received preliminary reports about two cases of severe allergic reaction from an open-label study in South Africa, with one of these being anaphylaxis, Dr. Douoguih said. The company will continue to closely monitor for these events as outlined in their pharmacovigilance plan, Dr. Douoguih said.
Federal health officials have sought to make clinicians aware of the rare risk for anaphylaxis with COVID vaccines, while reminding the public that this reaction can be managed.
The FDA had Tom Shimabukuro, MD, MPH, MBA, from the CDC, give an update on postmarketing surveillance for the Pfizer and Moderna vaccines as part of the review of the Johnson & Johnson application. Dr. Shimabukuro and CDC colleagues published a report in JAMA on February 14 that looked at an anaphylaxis case reported connected with COVID vaccines between December 14, 2020, and January 18, 2021.
The CDC identified 66 case reports received that met Brighton Collaboration case definition criteria for anaphylaxis (levels 1, 2, or 3): 47 following Pfizer/BioNTech vaccine, for a reporting rate of 4.7 cases/million doses administered, and 19 following Moderna vaccine, for a reporting rate of 2.5 cases/million doses administered, Dr. Shimabukuro and CDC colleagues wrote.
The CDC has published materials to help clinicians prepare for the possibility of this rare event, Dr. Shimabukuro told the FDA advisers.
“The take-home message here is that these are rare events and anaphylaxis, although clinically serious, is treatable,” Dr. Shimabukuro said.
At the conclusion of the meeting, FDA panelist Patrick Moore, MD, MPH, from the University of Pittsburgh in Pennsylvania, stressed the need to convey to the public that the COVID vaccines appear so far to be safe. Many people earlier had doubts about how the FDA could both safely and quickly review the applications for EUAs for these products.
“As of February 26, things are looking good. That could change tomorrow,” Dr. Moore said. But “this whole EUA process does seem to have worked, despite my own personal concerns about it.”
No second-class vaccines
The Johnson & Johnson vaccine, known as Ad26.COV2.S, is composed of a recombinant, replication-incompetent human adenovirus type 26 (Ad26) vector. It’s intended to encode a stabilized form of SARS-CoV-2 spike (S) protein. The Pfizer and Moderna vaccines use a different mechanism. They rely on mRNA.
The FDA advisers also discussed how patients might respond to the widely reported gap between Janssen’s topline efficacy rates compared with rivals. They urged against people parsing study details too finely and seeking to pick and choose their shots.
“It’s important that people do not think that one vaccine is better than another,” said FDA adviser H. Cody Meissner, MD, from Tufts University School of Medicine in Boston.
Dr. Monto agreed, noting that many people in the United States are still waiting for their turn to get COVID vaccines because of the limited early supply.
Trying to game the system to get one vaccine instead of another would not be wise. “In this environment, whatever you can get, get,” Dr. Monto said.
During an open public hearing, Sarah Christopherson, policy advocacy director of the National Women’s Health Network, said that press reports are fueling a damaging impression in the public that there are “first and second-class” vaccines.
“That has the potential to exacerbate existing mistrust” in vaccines, she said. “Public health authorities must address these perceptions head on.”
She urged against attempts to compare the Janssen vaccine to others, noting the potential effects of emerging variants of the virus.
“It’s difficult to make an apples-to-apples comparison between vaccines,” she said.
Johnson & Johnson’s efficacy results, which are lower than those of the mRNA vaccines, may be a reflection of the ways in which SARS-Co-V-2 is mutating and thus becoming more of a threat, according to the company. A key study of the new vaccine, involving about 44,000 people, coincided with the emergence of new SARS-CoV-2 variants, which were emerging in some of the countries where the pivotal COV3001 study was being conducted, the company said.
At least 14 days after vaccination, the Johnson & Johnson COVID vaccine efficacy (95% confidence interval) was 72.0% (58.2, 81.7) in the United States, 68.1% (48.8, 80.7) in Brazil, and 64.0% (41.2, 78.7) in South Africa.
Weakened standards?
Several researchers called on the FDA to maintain a critical attitude when assessing Johnson & Johnson’s application for the EUA, warning of a potential for a permanent erosion of agency rules due to hasty action on COVID vaccines.
They raised concerns about the FDA demanding too little in terms of follow-up studies on COVID vaccines and with persisting murkiness resulting in attempts to determine how well these treatments work beyond the initial study period.
“I worry about FDA lowering its approval standards,” said Peter Doshi, PhD, from The BMJ and a faculty member at the University of Maryland School of Medicine in Baltimore, during an open public hearing at the meeting.
“There’s a real urgency to stand back right now and look at the forest here, as well as the trees, and I urge the committee to consider the effects FDA decisions may have on the entire regulatory approval process,” Dr. Doshi said.
Dr. Doshi asked why Johnson & Johnson did not seek a standard full approval — a biologics license application (BLA) — instead of aiming for the lower bar of an EUA. The FDA already has allowed wide distribution of the Pfizer/BioNTech and Moderna vaccines through EUAs. That removes the sense of urgency that FDA faced last year in his view.
The FDA’s June 2020 guidance on the development of COVID vaccines had asked drugmakers to plan on following participants in COVID vaccine trials for “ideally at least one to two years.” Yet people who got placebo in Moderna and Pfizer trials already are being vaccinated, Dr. Doshi said. And Johnson & Johnson said in its presentation to the FDA that if the Ad26.COV2.S vaccine were granted an EUA, the COV3001 study design would be amended to “facilitate cross-over of placebo participants in all participating countries to receive one dose of active study vaccine as fast as operationally feasible.”
“I’m nervous about the prospect of there never being a COVID vaccine that meets the FDA’s approval standard” for a BLA instead of the more limited EUA, Dr. Doshi said.
Diana Zuckerman, PhD, president of the nonprofit National Center for Health Research, noted that the FDA’s subsequent guidance tailored for EUAs for COVID vaccines “drastically shortened” the follow-up time to a median of 2 months. Dr. Zuckerman said that a crossover design would be “a reasonable compromise, but only if the placebo group has at least 6 months of data.” Dr. Zuckerman opened her remarks in the open public hearing by saying she had inherited Johnson & Johnson stock, so was speaking at the meeting against her own financial interest.
“As soon as a vaccine is authorized, we start losing the placebo group. If FDA lets that happen, that’s a huge loss for public health and a huge loss of information about how we can all stay safe,” Dr. Zuckerman said.
A version of this article first appeared on Medscape.com.