User login
Introducing Point-Counterpoint Perspectives in the Journal of Hospital Medicine
Providing high-quality, efficient, and evidence-based healthcare is a complicated and complex process. The right approach or path forward is not always clear. In medicine, decision-making inherently involves uncertainty; evidence may be lacking, or values or context may differ, and thus, reasonable clinicians may choose to make different decisions based on the same data.
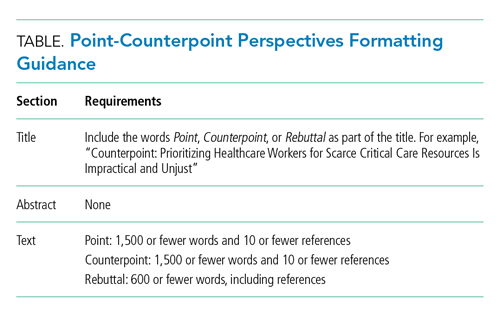
In this spirit of fostering education and healthy debate to improve understanding of challenges relevant to the field of hospital medicine, we are pleased to introduce our Point-Counterpoint series within the Perspectives in Hospital Medicine section of the journal. Point-Counterpoint Perspectives presents a debate by content experts. Each provides an interpretation of evidence regarding patient management or other controversial issues relating to hospital-based care. The format consists of an overview of the topic with an original point followed by a counterpoint response and, finally, a rebuttal (Table). We ask contributors to be as outspoken in their points and counterpoints as the evidence allows in order to fully elaborate the questions and uncertainties that may otherwise be familiar only to experts in the field or to those in other disciplines.
Our inaugural point-counterpoint articles address whether healthcare workers should receive priority for scarce drugs and therapies during the coronavirus disease 2019 (COVID-19) pandemic. The intermittent shortage of medical supplies and protective equipment has made it not only difficult but also at times dangerous for healthcare workers to care for infected patients.1 The risks of developing COVID-19 and fear of transmitting it to loved ones has led to stress, fatigue, and burnout among healthcare workers, leading some to quit and even attempt suicide. The downstream effects of this stress may adversely affect patients and exacerbate staffing challenges in an already taxed healthcare system.2 Do we have a special obligation to those on the front lines? We are grateful to Drs Kirk R Daffner, Armand Antommaria, and Ndidi I Unaka, for addressing this controversial topic.3-5
1. Lagu T, Artenstein AW, Werner RM. Fool me twice: the role for hospitals and health systems in fixing the broken PPE supply chain. J Hosp Med. 2020;15(9):570-571. https://doi.org/10.12788/jhm.3489
2. Ali SS. Why some nurses have quit during the coronavirus pandemic. NBC News. May,10, 2020. Accessed January 18, 2021. https://www.nbcnews.com/news/us-news/why-some-nurses-have-quit-during-coronavirus-pandemic-n1201796
3. Daffner KR. Point: healthcare providers should receive treatment priority during a pandemic. J Hosp Med. 2021;16(3):180-181. https://doi.org/10.12788/jhm.3596
4. Antommaria A, Unaka NI. Counterpoint: prioritizing healthcare workers for scarce critical resources is impractical and unjust. J Hosp Med. 2021;16(3):182-183. https://doi.org/10.12788/jhm.3597
5. Daffner KR. Rebuttal: accounting for the community’s reciprocal obligations during a pandemic. J Hosp Med. 2021;16(3):184. https://doi.org/10.12788/jhm.3600
Providing high-quality, efficient, and evidence-based healthcare is a complicated and complex process. The right approach or path forward is not always clear. In medicine, decision-making inherently involves uncertainty; evidence may be lacking, or values or context may differ, and thus, reasonable clinicians may choose to make different decisions based on the same data.
In this spirit of fostering education and healthy debate to improve understanding of challenges relevant to the field of hospital medicine, we are pleased to introduce our Point-Counterpoint series within the Perspectives in Hospital Medicine section of the journal. Point-Counterpoint Perspectives presents a debate by content experts. Each provides an interpretation of evidence regarding patient management or other controversial issues relating to hospital-based care. The format consists of an overview of the topic with an original point followed by a counterpoint response and, finally, a rebuttal (Table). We ask contributors to be as outspoken in their points and counterpoints as the evidence allows in order to fully elaborate the questions and uncertainties that may otherwise be familiar only to experts in the field or to those in other disciplines.

Our inaugural point-counterpoint articles address whether healthcare workers should receive priority for scarce drugs and therapies during the coronavirus disease 2019 (COVID-19) pandemic. The intermittent shortage of medical supplies and protective equipment has made it not only difficult but also at times dangerous for healthcare workers to care for infected patients.1 The risks of developing COVID-19 and fear of transmitting it to loved ones has led to stress, fatigue, and burnout among healthcare workers, leading some to quit and even attempt suicide. The downstream effects of this stress may adversely affect patients and exacerbate staffing challenges in an already taxed healthcare system.2 Do we have a special obligation to those on the front lines? We are grateful to Drs Kirk R Daffner, Armand Antommaria, and Ndidi I Unaka, for addressing this controversial topic.3-5
Providing high-quality, efficient, and evidence-based healthcare is a complicated and complex process. The right approach or path forward is not always clear. In medicine, decision-making inherently involves uncertainty; evidence may be lacking, or values or context may differ, and thus, reasonable clinicians may choose to make different decisions based on the same data.
In this spirit of fostering education and healthy debate to improve understanding of challenges relevant to the field of hospital medicine, we are pleased to introduce our Point-Counterpoint series within the Perspectives in Hospital Medicine section of the journal. Point-Counterpoint Perspectives presents a debate by content experts. Each provides an interpretation of evidence regarding patient management or other controversial issues relating to hospital-based care. The format consists of an overview of the topic with an original point followed by a counterpoint response and, finally, a rebuttal (Table). We ask contributors to be as outspoken in their points and counterpoints as the evidence allows in order to fully elaborate the questions and uncertainties that may otherwise be familiar only to experts in the field or to those in other disciplines.

Our inaugural point-counterpoint articles address whether healthcare workers should receive priority for scarce drugs and therapies during the coronavirus disease 2019 (COVID-19) pandemic. The intermittent shortage of medical supplies and protective equipment has made it not only difficult but also at times dangerous for healthcare workers to care for infected patients.1 The risks of developing COVID-19 and fear of transmitting it to loved ones has led to stress, fatigue, and burnout among healthcare workers, leading some to quit and even attempt suicide. The downstream effects of this stress may adversely affect patients and exacerbate staffing challenges in an already taxed healthcare system.2 Do we have a special obligation to those on the front lines? We are grateful to Drs Kirk R Daffner, Armand Antommaria, and Ndidi I Unaka, for addressing this controversial topic.3-5
1. Lagu T, Artenstein AW, Werner RM. Fool me twice: the role for hospitals and health systems in fixing the broken PPE supply chain. J Hosp Med. 2020;15(9):570-571. https://doi.org/10.12788/jhm.3489
2. Ali SS. Why some nurses have quit during the coronavirus pandemic. NBC News. May,10, 2020. Accessed January 18, 2021. https://www.nbcnews.com/news/us-news/why-some-nurses-have-quit-during-coronavirus-pandemic-n1201796
3. Daffner KR. Point: healthcare providers should receive treatment priority during a pandemic. J Hosp Med. 2021;16(3):180-181. https://doi.org/10.12788/jhm.3596
4. Antommaria A, Unaka NI. Counterpoint: prioritizing healthcare workers for scarce critical resources is impractical and unjust. J Hosp Med. 2021;16(3):182-183. https://doi.org/10.12788/jhm.3597
5. Daffner KR. Rebuttal: accounting for the community’s reciprocal obligations during a pandemic. J Hosp Med. 2021;16(3):184. https://doi.org/10.12788/jhm.3600
1. Lagu T, Artenstein AW, Werner RM. Fool me twice: the role for hospitals and health systems in fixing the broken PPE supply chain. J Hosp Med. 2020;15(9):570-571. https://doi.org/10.12788/jhm.3489
2. Ali SS. Why some nurses have quit during the coronavirus pandemic. NBC News. May,10, 2020. Accessed January 18, 2021. https://www.nbcnews.com/news/us-news/why-some-nurses-have-quit-during-coronavirus-pandemic-n1201796
3. Daffner KR. Point: healthcare providers should receive treatment priority during a pandemic. J Hosp Med. 2021;16(3):180-181. https://doi.org/10.12788/jhm.3596
4. Antommaria A, Unaka NI. Counterpoint: prioritizing healthcare workers for scarce critical resources is impractical and unjust. J Hosp Med. 2021;16(3):182-183. https://doi.org/10.12788/jhm.3597
5. Daffner KR. Rebuttal: accounting for the community’s reciprocal obligations during a pandemic. J Hosp Med. 2021;16(3):184. https://doi.org/10.12788/jhm.3600
© 2021 Society of Hospital Medicine
Readmissions Following Hospitalization for Infection in Children With or Without Medical Complexity
Hospitalizations for infections are common in children, with respiratory illnesses, including pneumonia and bronchiolitis, among the most prevalent indications for hospitalization.1,2 Infections are also among the most frequent indications for all-cause readmissions and for potentially preventable readmissions in children.3-5 Beyond hospital resource use, infection hospitalizations and readmissions represent a considerable cause of life disruption for patients and their families.6,7 While emerging evidence supports shortened durations of parenteral antibiotics before transitioning to oral therapy for some infections (eg, pyelonephritis, osteomyelitis),8-10 other infections may require extended treatment courses for weeks. The risk of adverse outcomes (eg, complications of medical treatment, readmission risk) and burdens placed on patients and their families may therefore differ across infection types and extend well beyond the immediate hospitalization.
Although infections are common and pediatric providers are expected to have proficiency in managing infections, substantial variation in the management of common pediatric infections exists and is associated with adverse hospitalization outcomes, including increased readmission risk and healthcare costs.11-18 Potentially avoidable resource use associated with hospital readmission from infection has led to adoption of hospital-level readmission metrics as indicators of the quality of healthcare delivery. For example, the Pediatric Quality Measures Program, established by the Children’s Health Insurance Program Reauthorization Act of 2009, has prioritized measurement of readmissions following hospitalization for lower respiratory tract infection.19 With government agencies increasingly using readmission metrics to assess quality of healthcare delivery, developing metrics that focus on these resource-intensive conditions is essential.
Because infections are a common and costly indication for hospital resource use and because substantial variation in management has been observed, promoting a broader understanding of infection-specific readmission rates is important for prioritizing readmission-reduction opportunities in children. This study’s objectives were the following: (1) to describe the prevalence and characteristics of infection hospitalizations in children and their associated readmissions and (2) to estimate the number of readmissions avoided and costs saved if all hospitals achieved the 10th percentile of the hospitals’ risk-adjusted readmission rate (ie, readmission benchmark).
METHODS
Study Design and Data Source
We performed a retrospective cohort analysis using the 2014 Agency for Healthcare Research and Quality (AHRQ) Nationwide Readmissions Database (NRD).20 The 2014 NRD is an administrative database that contains information on inpatient stays from January 1, 2014, to December 31, 2014, for all payers and allows for weighted national estimates of readmissions for all US individuals. Data within NRD are aggregated from 22 geographically diverse states representing approximately one-half of the US population. NRD contains deidentified patient-level data with unique verified patient identifiers to track individuals within and across hospitals in a state. However, AHRQ guidelines specify that NRD cannot be used for reporting hospital-specific readmission rates. Thus, for the current study, the Inpatient Essentials (Children’s Hospital Association), or IE, database was used to measure hospital-level readmission rates and to distinguish benchmark readmission rates for individual infection diagnoses.21 The IE database is composed of 90 children’s hospitals distributed throughout all regions of the United States. The inclusion of free-standing children’s hospitals and children’s hospitals within adult hospitals allows for comparisons and benchmarking across hospitals on multiple metrics, including readmissions.
Study Population
Children 0 to 17 years of age with a primary diagnosis at the index admission for infection between January 1, 2014, and November 30, 2014, were included. The end date of November 30, 2014, allowed for a full 30-day readmission window for all index admissions. We excluded index admissions that resulted in transfer to another acute care hospital or in-hospital mortality. Additionally, we excluded index admissions of children who had hematologic or immunologic conditions, malignancy, or history of bone marrow and solid-organ transplant, using the classification system for complex chronic conditions (CCCs) from Feudtner et al.22 Due to the high likelihood of immunosuppression in patients with these conditions, children may have nuanced experiences with illness severity, trajectory, and treatment associated with infection that place them at increased risk for nonpreventable readmission.
Main Exposure
The main exposure was infection type during the index admission. Condition-specific index admissions were identified using AHRQ’s Clinical Classifications Software (CCS) categories.23 CCS is a classification schema that categorizes the greater than 14,000 International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) diagnosis codes and 3,900 ICD-9-CM procedure codes into clinically meaningful categories of 295 diagnosis (including mental health codes and E-codes) and 231 procedural groupings. Twenty-two groupings indicative of infection were distinguished and used for the current study. Examples of infections included aspiration pneumonia, pneumonia, bronchiolitis, and sexually transmitted infection. We combined related CCS categories when possible for ease of interpretation and presentation of data (Appendix Table 1).
Main Outcome Measure
The main outcome measure was 30-day hospital readmission. Readmission was defined as all-cause, unplanned admission within 30 days following discharge from a preceding hospitalization. Planned hospital readmissions were identified and excluded using methods from AHRQ’s Pediatric All-Condition Readmission Measure.24 We defined a same-cause return as a return with the same CCS infection category as the index admission. Costs associated with readmissions were estimated from charges using hospital-specific cost-to-charge ratios provided with NRD.
Patient Demographic and Clinical Characteristics
Patient demographic characteristics included age at index admission (<1 year, 1-5 years, 6-9 years, 10-14 years, and 15-18 years), sex, payer (ie, government, private, other), and discharge disposition (ie, routine, home health, other). We assessed all patients for medical complexity, as defined by the presence of at least one CCC, and we reported the categories of CCCs by organ system involved.22 Otherwise, patients were identified as without medical complexity.
Statistical Analysis
We summarized continuous variables with medians and interquartile ranges and categorical variables with frequencies and percentages. To develop benchmark readmission rates for each infection type, we used generalized linear mixed models with random intercepts for each hospital in the IE database. For each infection type, the benchmark readmission rate was defined as the 10th percentile of hospitals’ risk-adjusted readmission rates. The 10th percentile was chosen to identify the best performing 10% of hospitals (ie, hospitals with the lowest readmission rates). Because children with medical complexity account for a large proportion of hospital resource use and are at high risk for readmission,4,25 we developed benchmarks stratified by presence/absence of a CCC (ie, with complexity vs without complexity). Models were adjusted for severity of illness using the Hospitalization Resource Intensity Score for Kids (H-RISK),26 a scoring system that assigns relative weights for each All Patient Refined Diagnosis-Related Group (3M Corporation) and severity of illness level, and each hospital’s risk-adjusted readmission rate was determined.
With use of weights to achieve national estimates of index admissions and readmissions, we determined the number of potentially avoidable readmissions by calculating the number of readmissions observed in the NRD that would not occur if all hospitals achieved readmission rates equal to the 10th percentile. Avoidable costs were estimated by multiplying the number of potentially avoidable readmissions by the mean cost of a readmission for infections of that type. Estimates of avoidable readmissions and costs were stratified by medical complexity. In addition to describing estimates at the 10th percentile benchmark, we similarly developed estimates of potentially avoidable readmissions and avoidable costs for the 5th and 25th percentiles, which are presented within Appendix Table 2 (children without complexity) and Appendix Table 3 (children with complexity).
All statistical analyses were performed using SAS version 9.4 (SAS Institute), and P values <.001 were considered statistically significant due to the large sample size. The Office of Research Integrity at Children’s Mercy Hospital deemed this study exempt from institutional board review.
RESULTS
Characteristics of the Study Population
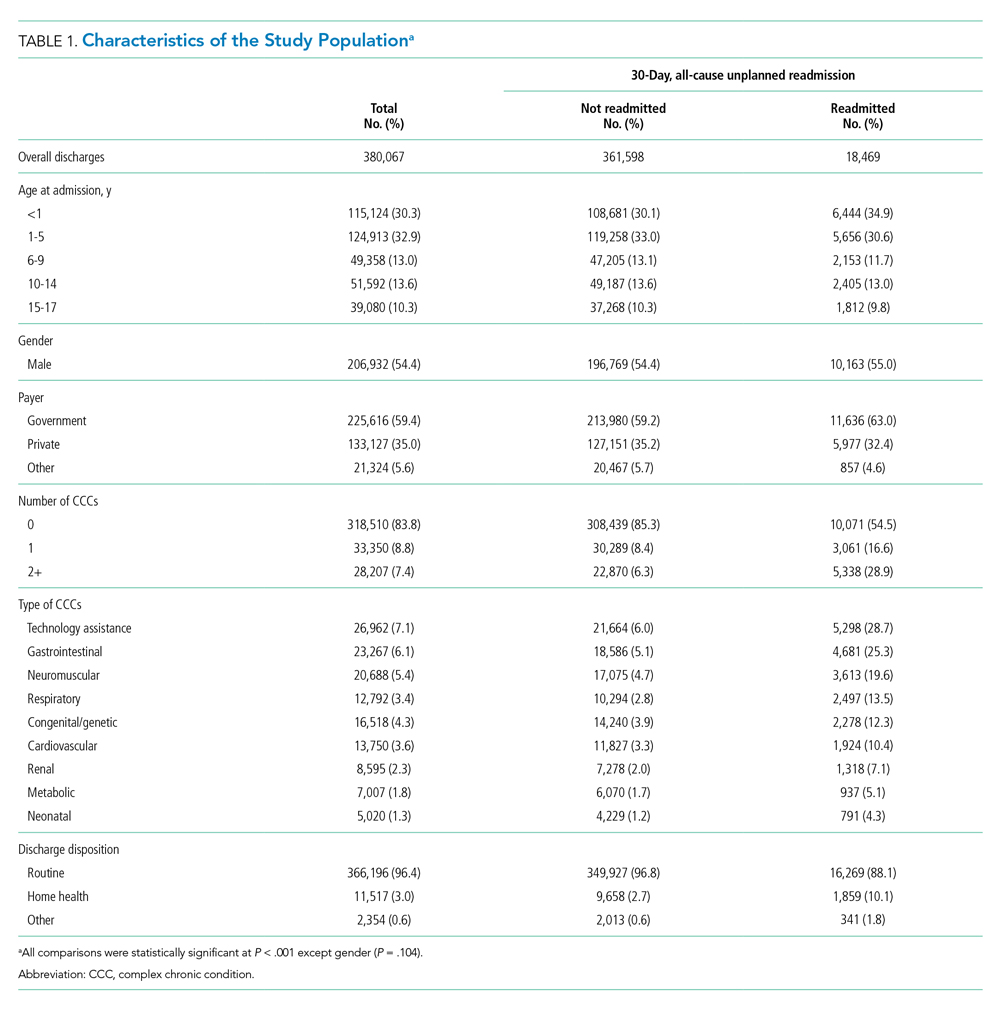
The study included 380,067 index admissions for infection and an accompanying 18,469 unplanned all-cause readmissions over the 30 days following discharge (readmission rate, 4.9%; Table 1). Children ages 1 to 5 years accounted for the largest percentage (32.9%) of index hospitalizations, followed by infants younger than 1 year (30.3%). The readmission rate by age group was highest for infants younger than 1 year, compared with rates among all other age groups (5.6% among infants < 1 year vs 4.4%-4.7% for other age groups; P < .001). In the overall cohort, 16.2% of admissions included patients with a CCC. Children with medical complexity had higher readmission rates than those without medical complexity (no CCC, 3.2%; 1 CCC, 9.2%; 2+ CCCs, 18.9%). A greater percentage of children experiencing a readmission had government insurance (63.0% vs 59.2%; P < .001) and received home health nursing (10.1% vs 2.7%; P < .001) following the index encounter.
Children Without Complexity
Index Admissions and 30-day Readmissions
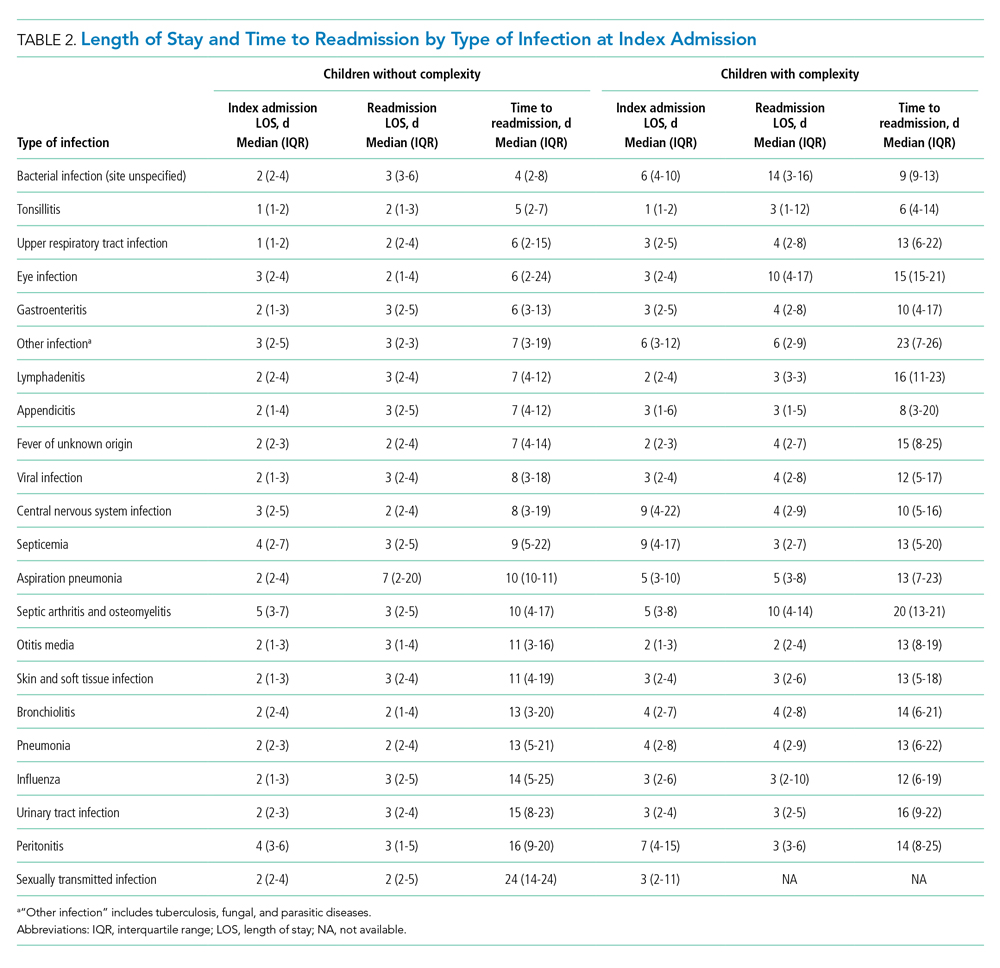
Among patients without medical complexity, index admissions occurred most frequently for pneumonia (n = 54,717), bronchiolitis (n = 53,959), and appendicitis (n = 45,036) (Figure 1). The median length of stay (LOS) for index admissions ranged from 1 to 5 days (Table 2), with septic arthritis and osteomyelitis having the longest median LOS at 5 (IQR, 3-7) days.
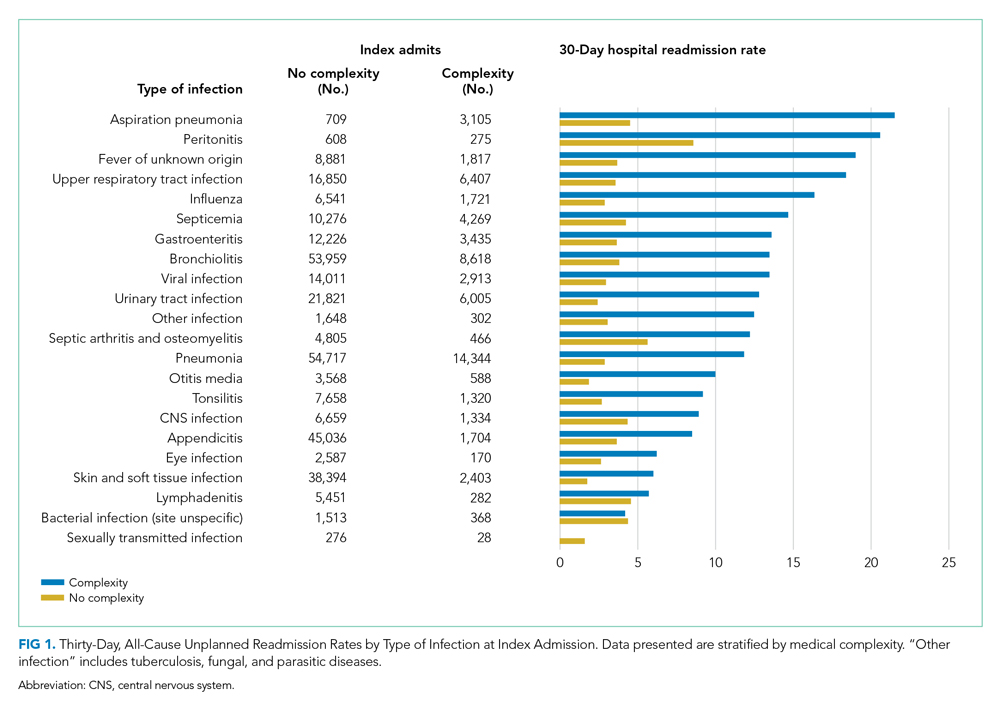
Thirty-day readmission rates varied substantially by infection at the index admission (range, 1.5% for sexually transmitted infection to 8.6% for peritonitis) (Figure 1). The median LOS for 30-day readmissions varied from 2 to 7 days (Table 2), while the median number of days to readmission varied substantially by infection type (range, 4 days for bacterial infection [site unspecified] to 24 days for sexually transmitted infections). Among the top five indications for admission for children without complexity, 14.9% to 52.8% of readmissions were for the same cause as the index admission; however, many additional returns were likely related to the index admission (Appendix Table 4). For example, among other return reasons, an additional 992 (61.7%) readmissions following appendicitis hospitalizations were for complications of surgical procedures or medical care, peritonitis, intestinal obstruction, and abdominal pain.
Impact of Achieving Readmission Benchmarks
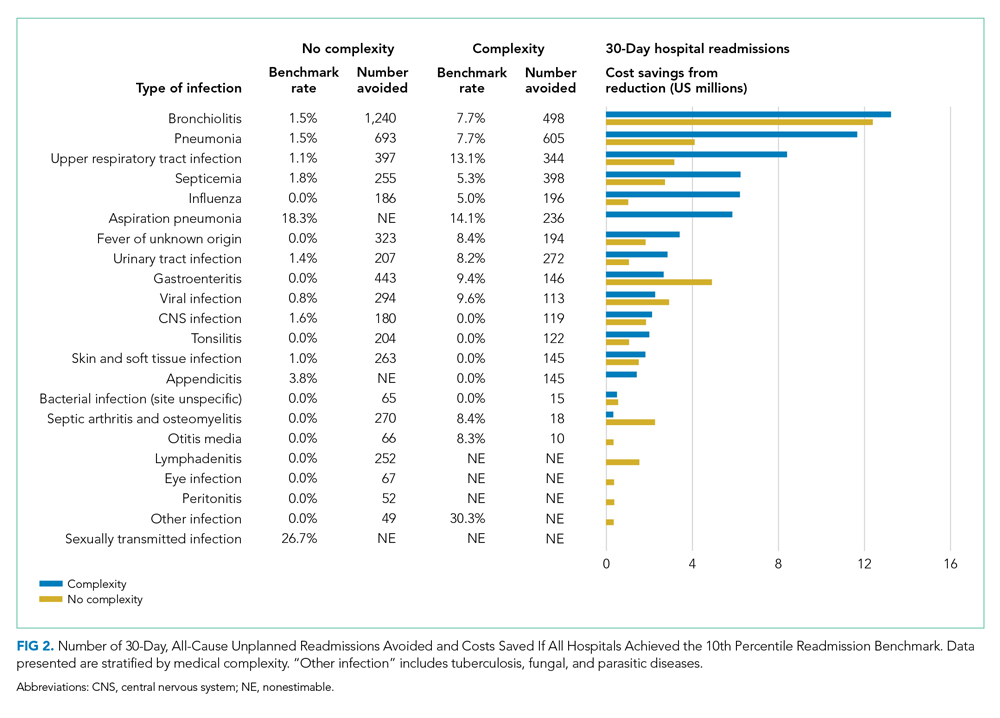
Among children without complexity, readmission benchmarks (ie, the 10th percentile of readmission rates across hospitals) ranged from 0% to 26.7% (Figure 2). An estimated 54.7% of readmissions (n = 5,507) could potentially be reduced if hospitals achieved infection-specific benchmark readmission rates, which could result in an estimated $44.5 million in savings. Pneumonia, bronchiolitis, gastroenteritis, and upper respiratory tract infections were among conditions with the greatest potential reductions in readmissions and costs if a 10th percentile benchmark was achieved.
Children With Medical Complexity
Index Admissions and 30-day Readmissions
Among patients with complexity, index admissions occurred most frequently for pneumonia (n = 14,344), bronchiolitis (n = 8,618), and upper respiratory tract infection (n = 6,407) (Figure 1). The median LOS for index admissions ranged from 1 to 9 days (Table 2), with septicemia and CNS infections having the longest median LOS at 9 days.
Thirty-day readmission rates varied substantially by the type of infection at the index admission (range, 0% for sexually transmitted infection to 21.6% for aspiration pneumonia) (Figure 1). The median LOS for 30-day readmissions varied from 2 to 14 days (Table 2), and the median number of days to readmission varied substantially by infection type (range, 6 days for tonsillitis to 23 days for other infection). Among the top five indications for admission for medically complex children, 8% to 40.4% of readmissions were for the same cause as the index admission (Appendix Table 4). As with the children without complexity, additional returns were likely related to the index admission.
Impact of Achieving Readmission Benchmarks
Among children with medical complexity, readmission benchmarks ranged from 0% to 30.3% (Figure 2). An estimated 42.6% of readmissions (n = 3,576) could potentially be reduced if hospitals achieved infection-specific benchmark readmission rates, which could result in an estimated $70.8 million in savings. Pneumonia, bronchiolitis, septicemia, and upper respiratory tract infections were among conditions with the greatest potential reductions in readmissions and costs if the benchmarks were achieved.
DISCUSSION
The current study uncovered new findings regarding unplanned readmissions following index infection hospitalizations for children. In particular, readmission rates and time to readmission varied substantially by infection subtype. The study also identified priority infections and unique considerations for children with CCCs, all of which may help maximize the value of readmission metrics aimed at advancing hospital quality and reducing costs for infection hospitalizations in children. If all hospitals achieved the readmission rates of the best performing hospitals, 42.6% to 54.7% fewer readmissions could be realized with associated cost savings.
Nationally, studies have reported 30-day, all-cause unplanned readmission rates of 6.2% to 10.3%.5,27 In our current study we observed an overall readmission rate of 4.9% across all infectious conditions; however, readmission rates varied substantially by condition, with upper and lower respiratory tract infections, septicemia, and gastroenteritis among infections with the greatest number of potentially avoidable readmissions based on achievement of readmission benchmarks. Currently, pediatric-specific all-cause and lower respiratory tract infection readmission metrics have been developed with the aim of improving quality of care and reducing healthcare expenditures.28 Future readmission studies and metrics may prioritize conditions such as septicemia, gastroenteritis, and other respiratory tract infections. Our current study demonstrated that many readmissions following infection hospitalizations were associated with the same CCS category or within a related CCS category (eg, complications of surgical procedures or medical care, appendicitis, peritonitis, intestinal obstruction, and abdominal pain constituted the top five indications for readmission for appendicitis, whereas respiratory illnesses constituted the top five indications for readmissions for pneumonia). While this current study cannot clarify this relationship further, and the “avoidability” of unplanned readmissions is debated,29-31 our findings suggest that future investigations might focus on identifying whether condition-specific interventions during the index admission could mitigate some readmissions.
While the LOS of the index admission and the readmission were similar for most infection subtypes, we observed substantial variability in the temporal risk for readmission by infection subtype. Our observations complement studies exploring the timing of readmissions for other pediatric conditions.32-34 In particular, our findings further highlight that the composition and interaction of factors influencing infection readmissions may vary by condition. Infections represent a diverse group of conditions, with treatment courses that vary in need for parenteral antibiotics, ability to tailor treatment based on organism and susceptibilities, and length of treatment. While treatment for some infections may be accomplished, or nearly accomplished, prior to discharge, other infections (eg, osteomyelitis) may require prolonged treatment, shifting the burden of management and monitoring to patients and their families, which along with the timeliness and adequacy of outpatient follow-up, may modify an individual’s readmission risk. Consequently, a “one-size fits all” approach to discharge counseling may not be successful across all conditions. Our study lays the groundwork for how these temporal relationships may be used by clinicians to counsel families regarding the likely readmission timeframe, based on infection, and to establish follow-up appointments ahead of the infection-specific “readmission window,” which may allow outpatient providers to intervene when readmission risk is greatest.
Consistent with prior literature, children with medical complexity in our study had increased frequency of 30-day, all-cause unplanned readmissions and LOS, compared with peers who did not have complexity.5 Readmission rates following hospitalizations for aspiration pneumonia were comparable to those reported by Thompson et al in their study examining rates of pneumonia in children with neurologic impairment.35 In our current study, nearly 96% of readmissions following aspiration pneumonia hospitalizations were for children with medical complexity, and more than 58% of these readmissions were for aspiration pneumonia or respiratory illness. Future investigations should seek to explore factors contributing to readmissions in children with medical complexity and to evaluate whether interventions such as postdischarge coaching or discharge bundles could assist with reductions in healthcare resource use.36,37
Despite a lack of clear association between readmissions and quality of care for children,38 readmissions rates continue to be used as a quality measure for hospitalized patients. Within our present study, we found that achieving benchmark readmission rates for infection hospitalizations could lead to substantial reductions in readmissions; however, these reductions were seen across this relatively similar group of infection diagnoses, and hospitals may face greater challenges when attempting to achieve a 10th percentile readmission benchmark across a more expansive set of diagnoses. Despite these challenges, understanding the intricacies of readmissions may lead to improved readmission metrics and the systematic identification of avoidable readmissions, the goal of which is to enhance the quality of healthcare for hospitalized children.
Our findings should be interpreted in the context of several limitations. We defined our readmission benchmark at the 10th percentile using the IE database. Because an estimated 70% of hospitalizations for children occur within general hospitals,39 we believe that our use of the IE database allowed for increased generalizability, though the broadening of our findings to nonacademic hospital settings may be less reliable. While we describe the 10th percentile readmission benchmark here, alternative benchmarks would result in different estimates of avoidable readmissions and associated readmission costs (Appendix Tables 2 and 3). The IE and NRD databases do not distinguish intensive care use. We tried to address this limitation through model adjustments using H-RISK, which is particularly helpful for adjusting for NICU admissions through use of the 27 All Patient Refined Diagnosis-Related Groups for neonatal conditions. Additionally, the NRD uses state-level data to derive national estimates and is not equipped to measure readmissions to hospitals in a different state, distinguish patient deaths occurring after discharge, or to examine the specific indication for readmission (ie, unable to assess if the readmission is related to a complication of the treatment plan, progression of the illness course, or for an unrelated reason). While sociodemographic and socioeconomic factors may affect readmissions, the NRD does not contain information on patients’ race/ethnicity, family/social attributes, or postdischarge follow-up health services, which are known to influence the need for readmission.
Despite these limitations, this study highlights future areas for research and potential opportunities for reducing readmission following infection hospitalizations. First, identifying hospital- and systems-based factors that contribute to readmission reductions at the best-performing hospitals may identify opportunities for hospitals with the highest readmission rates to achieve the rates of the best-performing hospitals. Second, conditions such as upper and lower respiratory tract infections, along with septicemia and gastroenteritis, may serve as prime targets for future investigation based on the estimated number of avoidable readmissions and potential cost savings associated with these conditions. Finally, future investigations that explore discharge counseling and follow-up tailored to the infection-specific temporal risk and patient complexity may identify opportunities for further readmission reductions.
CONCLUSION
Our study provides a broad look at readmissions following infection hospitalizations and highlights substantial variation in readmissions based on infection type. To improve hospital resource use for infections, future preventive measures could prioritize children with complex chronic conditions and those with specific diagnoses (eg, upper and lower respiratory tract infections).
Disclaimer
This information or content and conclusions are those of the authors and should not be construed as the official position or policy of, nor should any endorsements be inferred by, NIH or the US government.
1. Keren R, Luan X, Localio R, et al; Pediatric Research in Inpatient Settings (PRIS) Network. Prioritization of comparative effectiveness research topics in hospital pediatrics. Arch Pediatr Adolesc Med. 2012;166(12):1155-1164. https://doi.org/10.1001/archpediatrics.2012.1266
2. Van Horne B, Netherton E, Helton J, Fu M, Greeley C. The scope and trends of pediatric hospitalizations in Texas, 2004-2010. Hosp Pediatr. 2015;5(7):390-398. https://doi.org/10.1542/hpeds.2014-0105
3. Neuman MI, Hall M, Gay JC, et al. Readmissions among children previously hospitalized with pneumonia. Pediatrics. 2014;134(1):100-109. https://doi.org/10.1542/peds.2014-0331
4. Gay JC, Hain PD, Grantham JA, Saville BR. Epidemiology of 15-day readmissions to a children’s hospital. Pediatrics. 2011;127(6):e1505-e1512. https://doi.org/10.1542/peds.2010-1737
5. Berry JG, Toomey SL, Zaslavsky AM, et al. Pediatric readmission prevalence and variability across hospitals. JAMA. 2013;309(4):372-380. https://doi.org/10.1001/jama.2012.188351
6. Shudy M, de Almeida ML, Ly S, et al. Impact of pediatric critical illness and injury on families: a systematic literature review. Pediatrics. 2006;118(suppl 3):S203-S218. https://doi.org/10.1542/peds.2006-0951b
7. Rennick JE, Johnston CC, Dougherty G, Platt R, Ritchie JA. Children’s psychological responses after critical illness and exposure to invasive technology. J Dev Behav Pediatr. 2002;23(3):133-144. https://doi.org/10.1097/00004703-200206000-00002
8. Michael M, Hodson EM, Craig JC, Martin S, Moyer VA. Short versus standard duration oral antibiotic therapy for acute urinary tract infection in children. Cochrane Database Syst Rev. 2003;(1):CD003966. https://doi.org/10.1002/14651858.cd003966
9. Greenberg D, Givon-Lavi N, Sadaka Y, Ben-Shimol S, Bar-Ziv J, Dagan R. Short-course antibiotic treatment for community-acquired alveolar pneumonia in ambulatory children: a double-blind, randomized, placebo-controlled trial. Pediatr Infect Dis J. 2014;33(2):136-142. https://doi.org/10.1097/inf.0000000000000023
10. Keren R, Shah SS, Srivastava R, et al; Pediatric Research in Inpatient Settings Network. Comparative effectiveness of intravenous vs oral antibiotics for postdischarge treatment of acute osteomyelitis in children. JAMA Pediatr. 2015;169(2):120-128. https://doi.org/10.1001/jamapediatrics.2014.2822
11. Markham JL, Hall M, Bettenhausen JL, Myers AL, Puls HT, McCulloh RJ. Variation in care and clinical outcomes in children hospitalized with orbital cellulitis. Hosp Pediatr. 2018;8(1):28-35. https://doi.org/10.1542/hpeds.2017-0040
12. Neubauer HC, Hall M, Wallace SS, Cruz AT, Queen MA, Foradori DM, Aronson PL, Markham JL, Nead JA, Hester GZ, McCulloh RJ, Lopez MA. Variation in diagnostic test use and associated outcomes in staphylococcal scalded skin syndrome at children’s hospitals. Hosp Pediatr. 2018;8(9):530-537. https://doi.org/10.1542/hpeds.2018-0032
13. Aronson PL, Thurm C, Alpern ER, et al; Febrile Young Infant Research Collaborative. Variation in care of the febrile young infant <90 days in US pediatric emergency departments. Pediatrics. 2014;134(4):667-677. https://doi.org/10.1542/peds.2014-1382
14. Florin TA, French B, Zorc JJ, Alpern ER, Shah SS. Variation in emergency department diagnostic testing and disposition outcomes in pneumonia. Pediatrics. 2013;132(2):237-244. https://doi.org/10.1542/peds.2013-0179
15. Brogan TV, Hall M, Williams DJ, et al. Variability in processes of care and outcomes among children hospitalized with community-acquired pneumonia. Pediatr Infect Dis J. 2012;31(10):1036-1041. https://doi.org/10.1097/inf.0b013e31825f2b10
16. Leyenaar JK, Lagu T, Shieh MS, Pekow PS, Lindenauer PK. Variation in resource utilization for the management of uncomplicated community-acquired pneumonia across community and children’s hospitals. J Pediatr. 2014;165(3):585-591. https://doi.org/10.1016/j.jpeds.2014.04.062
17. Knapp JF, Simon SD, Sharma V. Variation and trends in ED use of radiographs for asthma, bronchiolitis, and croup in children. Pediatrics. 2013;132(2):245-252. https://doi.org/10.1542/peds.2012-2830
18. Rice-Townsend S, Barnes JN, Hall M, Baxter JL, Rangel SJ. Variation in practice and resource utilization associated with the diagnosis and management of appendicitis at freestanding children’s hospitals: implications for value-based comparative analysis. Ann Surg. 2014;259(6):1228-1234. https://doi.org/10.1097/SLA.0000000000000246
19. Pediatric Quality Measures Program (PQMP). Agency for Healthcare Research and Quality. Accessed March 1, 2019. https://www.ahrq.gov/pqmp/index.html
20. NRD Database Documentation. Accessed June 1, 2019. https://www.hcup-us.ahrq.gov/db/nation/nrd/nrddbdocumentation.jsp
21. Inpatient Essentials. Children’s Hospitals Association. Accessed August 1, 2018. https://www.childrenshospitals.org/Programs-and-Services/Data-Analytics-and-Research/Pediatric-Analytic-Solutions/Inpatient-Essentials
22. Feudtner C, Feinstein JA, Zhong W, Hall M, Dai D. Pediatric complex chronic conditions classification system version 2: updated for ICD-10 and complex medical technology dependence and transplantation. BMC Pediatr. 2014;14:199. https://doi.org/10.1186/1471-2431-14-199
23. Clinical Classifications Software (CCS) for ICD-9-CM. Healthcare Cost and Utilization Project. March 2017. Accessed August 2, 2018. https://www.hcup-us.ahrq.gov/toolssoftware/ccs/ccs.jsp
24. NQF: Quality Positioning System. National Quality Forum. Accessed September 3, 2018. http://www.qualityforum.org/QPS/QPSTool.aspx
25. Berry JG, Ash AS, Cohen E, Hasan F, Feudtner C, Hall M. Contributions of children with multiple chronic conditions to pediatric hospitalizations in the United States: a retrospective cohort analysis. Hosp Pediatr. 2017;7(7):365-372. https://doi.org/10.1542/hpeds.2016-0179
26. Richardson T, Rodean J, Harris M, Berry J, Gay JC, Hall M. Development of Hospitalization Resource Intensity Scores for Kids (H-RISK) and comparison across pediatric populations. J Hosp Med. 2018;13(9):602-608. https://doi.org/10.12788/jhm.2948
27. Auger KA, Mueller EL, Weinberg SH, et al. A validated method for identifying unplanned pediatric readmission. J Pediatr. 2016;170:105-12.e122. https://doi.org/10.1016/j.jpeds.2015.11.051
28. NQF: Pediatric Measures Final Report. National Quality Forum. June 2016. Accessed January 24, 2019. https://www.qualityforum.org/Publications/2016/06/Pediatric_Measures_Final_Report.aspx
29. Hain PD, Gay JC, Berutti TW, Whitney GM, Wang W, Saville BR. Preventability of early readmissions at a children’s hospital. Pediatrics. 2013;131(1):e171-e181. https://doi.org/10.1542/peds.2012-0820
30. Toomey SL, Peltz A, Loren S, et al. Potentially preventable 30-day hospital readmissions at a children’s hospital. Pediatrics. 2016;138(2):e20154182. https://doi.org/10.1542/peds.2015-4182
31. Jonas JA, Devon EP, Ronan JC, et al. Determining preventability of pediatric readmissions using fault tree analysis. J Hosp Med. 2016;11(5):329-335. https://doi.org/10.1002/jhm.2555
32. Bucholz EM, Gay JC, Hall M, Harris M, Berry JG. Timing and causes of common pediatric readmissions. J Pediatr. 2018;200:240-248.e1. https://doi.org/10.1016/j.jpeds.2018.04.044
33. Morse RB, Hall M, Fieldston ES, et al. Children’s hospitals with shorter lengths of stay do not have higher readmission rates. J Pediatr. 2013;163(4):1034-8.e1. https://doi.org/10.1016/j.jpeds.2013.03.083
34. Kenyon CC, Melvin PR, Chiang VW, Elliott MN, Schuster MA, Berry JG. Rehospitalization for childhood asthma: timing, variation, and opportunities for intervention. J Pediatr. 2014;164(2):300-305. https://doi.org/10.1016/j.jpeds.2013.10.003
35. Thomson J, Hall M, Ambroggio L, et al. Aspiration and non-aspiration pneumonia in hospitalized children with neurologic impairment. Pediatrics. 2016;137(2):e20151612. https://doi.org/10.1542/peds.2015-1612
36. Coller RJ, Klitzner TS, Lerner CF, et al. Complex Care hospital use and postdischarge coaching: a randomized controlled trial. Pediatrics. 2018;142(2):e20174278. https://doi.org/10.1542/peds.2017-4278
37. Stephens JR, Kimple KS, Steiner MJ, Berry JG. Discharge interventions and modifiable risk factors for preventing hospital readmissions in children with medical complexity. Rev Recent Clin Trials. 2017;12(4):290-297. https://doi.org/10.2174/1574887112666170816144455
38. Bardach NS, Vittinghoff E, Asteria-Peñaloza R, et al. Measuring hospital quality using pediatric readmission and revisit rates. Pediatrics. 2013;132(3):429-436. https://doi.org/10.1542/peds.2012-3527
39. Leyenaar JK, Ralston SL, Shieh MS, Pekow PS, Mangione-Smith R, Lindenauer PK. Epidemiology of pediatric hospitalizations at general hospitals and freestanding children’s hospitals in the United States. J Hosp Med. 2016;11(11):743-749. https://doi.org/10.1002/jhm.2624
Hospitalizations for infections are common in children, with respiratory illnesses, including pneumonia and bronchiolitis, among the most prevalent indications for hospitalization.1,2 Infections are also among the most frequent indications for all-cause readmissions and for potentially preventable readmissions in children.3-5 Beyond hospital resource use, infection hospitalizations and readmissions represent a considerable cause of life disruption for patients and their families.6,7 While emerging evidence supports shortened durations of parenteral antibiotics before transitioning to oral therapy for some infections (eg, pyelonephritis, osteomyelitis),8-10 other infections may require extended treatment courses for weeks. The risk of adverse outcomes (eg, complications of medical treatment, readmission risk) and burdens placed on patients and their families may therefore differ across infection types and extend well beyond the immediate hospitalization.
Although infections are common and pediatric providers are expected to have proficiency in managing infections, substantial variation in the management of common pediatric infections exists and is associated with adverse hospitalization outcomes, including increased readmission risk and healthcare costs.11-18 Potentially avoidable resource use associated with hospital readmission from infection has led to adoption of hospital-level readmission metrics as indicators of the quality of healthcare delivery. For example, the Pediatric Quality Measures Program, established by the Children’s Health Insurance Program Reauthorization Act of 2009, has prioritized measurement of readmissions following hospitalization for lower respiratory tract infection.19 With government agencies increasingly using readmission metrics to assess quality of healthcare delivery, developing metrics that focus on these resource-intensive conditions is essential.
Because infections are a common and costly indication for hospital resource use and because substantial variation in management has been observed, promoting a broader understanding of infection-specific readmission rates is important for prioritizing readmission-reduction opportunities in children. This study’s objectives were the following: (1) to describe the prevalence and characteristics of infection hospitalizations in children and their associated readmissions and (2) to estimate the number of readmissions avoided and costs saved if all hospitals achieved the 10th percentile of the hospitals’ risk-adjusted readmission rate (ie, readmission benchmark).
METHODS
Study Design and Data Source
We performed a retrospective cohort analysis using the 2014 Agency for Healthcare Research and Quality (AHRQ) Nationwide Readmissions Database (NRD).20 The 2014 NRD is an administrative database that contains information on inpatient stays from January 1, 2014, to December 31, 2014, for all payers and allows for weighted national estimates of readmissions for all US individuals. Data within NRD are aggregated from 22 geographically diverse states representing approximately one-half of the US population. NRD contains deidentified patient-level data with unique verified patient identifiers to track individuals within and across hospitals in a state. However, AHRQ guidelines specify that NRD cannot be used for reporting hospital-specific readmission rates. Thus, for the current study, the Inpatient Essentials (Children’s Hospital Association), or IE, database was used to measure hospital-level readmission rates and to distinguish benchmark readmission rates for individual infection diagnoses.21 The IE database is composed of 90 children’s hospitals distributed throughout all regions of the United States. The inclusion of free-standing children’s hospitals and children’s hospitals within adult hospitals allows for comparisons and benchmarking across hospitals on multiple metrics, including readmissions.
Study Population
Children 0 to 17 years of age with a primary diagnosis at the index admission for infection between January 1, 2014, and November 30, 2014, were included. The end date of November 30, 2014, allowed for a full 30-day readmission window for all index admissions. We excluded index admissions that resulted in transfer to another acute care hospital or in-hospital mortality. Additionally, we excluded index admissions of children who had hematologic or immunologic conditions, malignancy, or history of bone marrow and solid-organ transplant, using the classification system for complex chronic conditions (CCCs) from Feudtner et al.22 Due to the high likelihood of immunosuppression in patients with these conditions, children may have nuanced experiences with illness severity, trajectory, and treatment associated with infection that place them at increased risk for nonpreventable readmission.
Main Exposure
The main exposure was infection type during the index admission. Condition-specific index admissions were identified using AHRQ’s Clinical Classifications Software (CCS) categories.23 CCS is a classification schema that categorizes the greater than 14,000 International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) diagnosis codes and 3,900 ICD-9-CM procedure codes into clinically meaningful categories of 295 diagnosis (including mental health codes and E-codes) and 231 procedural groupings. Twenty-two groupings indicative of infection were distinguished and used for the current study. Examples of infections included aspiration pneumonia, pneumonia, bronchiolitis, and sexually transmitted infection. We combined related CCS categories when possible for ease of interpretation and presentation of data (Appendix Table 1).
Main Outcome Measure
The main outcome measure was 30-day hospital readmission. Readmission was defined as all-cause, unplanned admission within 30 days following discharge from a preceding hospitalization. Planned hospital readmissions were identified and excluded using methods from AHRQ’s Pediatric All-Condition Readmission Measure.24 We defined a same-cause return as a return with the same CCS infection category as the index admission. Costs associated with readmissions were estimated from charges using hospital-specific cost-to-charge ratios provided with NRD.
Patient Demographic and Clinical Characteristics
Patient demographic characteristics included age at index admission (<1 year, 1-5 years, 6-9 years, 10-14 years, and 15-18 years), sex, payer (ie, government, private, other), and discharge disposition (ie, routine, home health, other). We assessed all patients for medical complexity, as defined by the presence of at least one CCC, and we reported the categories of CCCs by organ system involved.22 Otherwise, patients were identified as without medical complexity.
Statistical Analysis
We summarized continuous variables with medians and interquartile ranges and categorical variables with frequencies and percentages. To develop benchmark readmission rates for each infection type, we used generalized linear mixed models with random intercepts for each hospital in the IE database. For each infection type, the benchmark readmission rate was defined as the 10th percentile of hospitals’ risk-adjusted readmission rates. The 10th percentile was chosen to identify the best performing 10% of hospitals (ie, hospitals with the lowest readmission rates). Because children with medical complexity account for a large proportion of hospital resource use and are at high risk for readmission,4,25 we developed benchmarks stratified by presence/absence of a CCC (ie, with complexity vs without complexity). Models were adjusted for severity of illness using the Hospitalization Resource Intensity Score for Kids (H-RISK),26 a scoring system that assigns relative weights for each All Patient Refined Diagnosis-Related Group (3M Corporation) and severity of illness level, and each hospital’s risk-adjusted readmission rate was determined.
With use of weights to achieve national estimates of index admissions and readmissions, we determined the number of potentially avoidable readmissions by calculating the number of readmissions observed in the NRD that would not occur if all hospitals achieved readmission rates equal to the 10th percentile. Avoidable costs were estimated by multiplying the number of potentially avoidable readmissions by the mean cost of a readmission for infections of that type. Estimates of avoidable readmissions and costs were stratified by medical complexity. In addition to describing estimates at the 10th percentile benchmark, we similarly developed estimates of potentially avoidable readmissions and avoidable costs for the 5th and 25th percentiles, which are presented within Appendix Table 2 (children without complexity) and Appendix Table 3 (children with complexity).
All statistical analyses were performed using SAS version 9.4 (SAS Institute), and P values <.001 were considered statistically significant due to the large sample size. The Office of Research Integrity at Children’s Mercy Hospital deemed this study exempt from institutional board review.
RESULTS
Characteristics of the Study Population
The study included 380,067 index admissions for infection and an accompanying 18,469 unplanned all-cause readmissions over the 30 days following discharge (readmission rate, 4.9%; Table 1). Children ages 1 to 5 years accounted for the largest percentage (32.9%) of index hospitalizations, followed by infants younger than 1 year (30.3%). The readmission rate by age group was highest for infants younger than 1 year, compared with rates among all other age groups (5.6% among infants < 1 year vs 4.4%-4.7% for other age groups; P < .001). In the overall cohort, 16.2% of admissions included patients with a CCC. Children with medical complexity had higher readmission rates than those without medical complexity (no CCC, 3.2%; 1 CCC, 9.2%; 2+ CCCs, 18.9%). A greater percentage of children experiencing a readmission had government insurance (63.0% vs 59.2%; P < .001) and received home health nursing (10.1% vs 2.7%; P < .001) following the index encounter.

Children Without Complexity
Index Admissions and 30-day Readmissions
Among patients without medical complexity, index admissions occurred most frequently for pneumonia (n = 54,717), bronchiolitis (n = 53,959), and appendicitis (n = 45,036) (Figure 1). The median length of stay (LOS) for index admissions ranged from 1 to 5 days (Table 2), with septic arthritis and osteomyelitis having the longest median LOS at 5 (IQR, 3-7) days.

Thirty-day readmission rates varied substantially by infection at the index admission (range, 1.5% for sexually transmitted infection to 8.6% for peritonitis) (Figure 1). The median LOS for 30-day readmissions varied from 2 to 7 days (Table 2), while the median number of days to readmission varied substantially by infection type (range, 4 days for bacterial infection [site unspecified] to 24 days for sexually transmitted infections). Among the top five indications for admission for children without complexity, 14.9% to 52.8% of readmissions were for the same cause as the index admission; however, many additional returns were likely related to the index admission (Appendix Table 4). For example, among other return reasons, an additional 992 (61.7%) readmissions following appendicitis hospitalizations were for complications of surgical procedures or medical care, peritonitis, intestinal obstruction, and abdominal pain.

Impact of Achieving Readmission Benchmarks
Among children without complexity, readmission benchmarks (ie, the 10th percentile of readmission rates across hospitals) ranged from 0% to 26.7% (Figure 2). An estimated 54.7% of readmissions (n = 5,507) could potentially be reduced if hospitals achieved infection-specific benchmark readmission rates, which could result in an estimated $44.5 million in savings. Pneumonia, bronchiolitis, gastroenteritis, and upper respiratory tract infections were among conditions with the greatest potential reductions in readmissions and costs if a 10th percentile benchmark was achieved.

Children With Medical Complexity
Index Admissions and 30-day Readmissions
Among patients with complexity, index admissions occurred most frequently for pneumonia (n = 14,344), bronchiolitis (n = 8,618), and upper respiratory tract infection (n = 6,407) (Figure 1). The median LOS for index admissions ranged from 1 to 9 days (Table 2), with septicemia and CNS infections having the longest median LOS at 9 days.
Thirty-day readmission rates varied substantially by the type of infection at the index admission (range, 0% for sexually transmitted infection to 21.6% for aspiration pneumonia) (Figure 1). The median LOS for 30-day readmissions varied from 2 to 14 days (Table 2), and the median number of days to readmission varied substantially by infection type (range, 6 days for tonsillitis to 23 days for other infection). Among the top five indications for admission for medically complex children, 8% to 40.4% of readmissions were for the same cause as the index admission (Appendix Table 4). As with the children without complexity, additional returns were likely related to the index admission.
Impact of Achieving Readmission Benchmarks
Among children with medical complexity, readmission benchmarks ranged from 0% to 30.3% (Figure 2). An estimated 42.6% of readmissions (n = 3,576) could potentially be reduced if hospitals achieved infection-specific benchmark readmission rates, which could result in an estimated $70.8 million in savings. Pneumonia, bronchiolitis, septicemia, and upper respiratory tract infections were among conditions with the greatest potential reductions in readmissions and costs if the benchmarks were achieved.
DISCUSSION
The current study uncovered new findings regarding unplanned readmissions following index infection hospitalizations for children. In particular, readmission rates and time to readmission varied substantially by infection subtype. The study also identified priority infections and unique considerations for children with CCCs, all of which may help maximize the value of readmission metrics aimed at advancing hospital quality and reducing costs for infection hospitalizations in children. If all hospitals achieved the readmission rates of the best performing hospitals, 42.6% to 54.7% fewer readmissions could be realized with associated cost savings.
Nationally, studies have reported 30-day, all-cause unplanned readmission rates of 6.2% to 10.3%.5,27 In our current study we observed an overall readmission rate of 4.9% across all infectious conditions; however, readmission rates varied substantially by condition, with upper and lower respiratory tract infections, septicemia, and gastroenteritis among infections with the greatest number of potentially avoidable readmissions based on achievement of readmission benchmarks. Currently, pediatric-specific all-cause and lower respiratory tract infection readmission metrics have been developed with the aim of improving quality of care and reducing healthcare expenditures.28 Future readmission studies and metrics may prioritize conditions such as septicemia, gastroenteritis, and other respiratory tract infections. Our current study demonstrated that many readmissions following infection hospitalizations were associated with the same CCS category or within a related CCS category (eg, complications of surgical procedures or medical care, appendicitis, peritonitis, intestinal obstruction, and abdominal pain constituted the top five indications for readmission for appendicitis, whereas respiratory illnesses constituted the top five indications for readmissions for pneumonia). While this current study cannot clarify this relationship further, and the “avoidability” of unplanned readmissions is debated,29-31 our findings suggest that future investigations might focus on identifying whether condition-specific interventions during the index admission could mitigate some readmissions.
While the LOS of the index admission and the readmission were similar for most infection subtypes, we observed substantial variability in the temporal risk for readmission by infection subtype. Our observations complement studies exploring the timing of readmissions for other pediatric conditions.32-34 In particular, our findings further highlight that the composition and interaction of factors influencing infection readmissions may vary by condition. Infections represent a diverse group of conditions, with treatment courses that vary in need for parenteral antibiotics, ability to tailor treatment based on organism and susceptibilities, and length of treatment. While treatment for some infections may be accomplished, or nearly accomplished, prior to discharge, other infections (eg, osteomyelitis) may require prolonged treatment, shifting the burden of management and monitoring to patients and their families, which along with the timeliness and adequacy of outpatient follow-up, may modify an individual’s readmission risk. Consequently, a “one-size fits all” approach to discharge counseling may not be successful across all conditions. Our study lays the groundwork for how these temporal relationships may be used by clinicians to counsel families regarding the likely readmission timeframe, based on infection, and to establish follow-up appointments ahead of the infection-specific “readmission window,” which may allow outpatient providers to intervene when readmission risk is greatest.
Consistent with prior literature, children with medical complexity in our study had increased frequency of 30-day, all-cause unplanned readmissions and LOS, compared with peers who did not have complexity.5 Readmission rates following hospitalizations for aspiration pneumonia were comparable to those reported by Thompson et al in their study examining rates of pneumonia in children with neurologic impairment.35 In our current study, nearly 96% of readmissions following aspiration pneumonia hospitalizations were for children with medical complexity, and more than 58% of these readmissions were for aspiration pneumonia or respiratory illness. Future investigations should seek to explore factors contributing to readmissions in children with medical complexity and to evaluate whether interventions such as postdischarge coaching or discharge bundles could assist with reductions in healthcare resource use.36,37
Despite a lack of clear association between readmissions and quality of care for children,38 readmissions rates continue to be used as a quality measure for hospitalized patients. Within our present study, we found that achieving benchmark readmission rates for infection hospitalizations could lead to substantial reductions in readmissions; however, these reductions were seen across this relatively similar group of infection diagnoses, and hospitals may face greater challenges when attempting to achieve a 10th percentile readmission benchmark across a more expansive set of diagnoses. Despite these challenges, understanding the intricacies of readmissions may lead to improved readmission metrics and the systematic identification of avoidable readmissions, the goal of which is to enhance the quality of healthcare for hospitalized children.
Our findings should be interpreted in the context of several limitations. We defined our readmission benchmark at the 10th percentile using the IE database. Because an estimated 70% of hospitalizations for children occur within general hospitals,39 we believe that our use of the IE database allowed for increased generalizability, though the broadening of our findings to nonacademic hospital settings may be less reliable. While we describe the 10th percentile readmission benchmark here, alternative benchmarks would result in different estimates of avoidable readmissions and associated readmission costs (Appendix Tables 2 and 3). The IE and NRD databases do not distinguish intensive care use. We tried to address this limitation through model adjustments using H-RISK, which is particularly helpful for adjusting for NICU admissions through use of the 27 All Patient Refined Diagnosis-Related Groups for neonatal conditions. Additionally, the NRD uses state-level data to derive national estimates and is not equipped to measure readmissions to hospitals in a different state, distinguish patient deaths occurring after discharge, or to examine the specific indication for readmission (ie, unable to assess if the readmission is related to a complication of the treatment plan, progression of the illness course, or for an unrelated reason). While sociodemographic and socioeconomic factors may affect readmissions, the NRD does not contain information on patients’ race/ethnicity, family/social attributes, or postdischarge follow-up health services, which are known to influence the need for readmission.
Despite these limitations, this study highlights future areas for research and potential opportunities for reducing readmission following infection hospitalizations. First, identifying hospital- and systems-based factors that contribute to readmission reductions at the best-performing hospitals may identify opportunities for hospitals with the highest readmission rates to achieve the rates of the best-performing hospitals. Second, conditions such as upper and lower respiratory tract infections, along with septicemia and gastroenteritis, may serve as prime targets for future investigation based on the estimated number of avoidable readmissions and potential cost savings associated with these conditions. Finally, future investigations that explore discharge counseling and follow-up tailored to the infection-specific temporal risk and patient complexity may identify opportunities for further readmission reductions.
CONCLUSION
Our study provides a broad look at readmissions following infection hospitalizations and highlights substantial variation in readmissions based on infection type. To improve hospital resource use for infections, future preventive measures could prioritize children with complex chronic conditions and those with specific diagnoses (eg, upper and lower respiratory tract infections).
Disclaimer
This information or content and conclusions are those of the authors and should not be construed as the official position or policy of, nor should any endorsements be inferred by, NIH or the US government.
Hospitalizations for infections are common in children, with respiratory illnesses, including pneumonia and bronchiolitis, among the most prevalent indications for hospitalization.1,2 Infections are also among the most frequent indications for all-cause readmissions and for potentially preventable readmissions in children.3-5 Beyond hospital resource use, infection hospitalizations and readmissions represent a considerable cause of life disruption for patients and their families.6,7 While emerging evidence supports shortened durations of parenteral antibiotics before transitioning to oral therapy for some infections (eg, pyelonephritis, osteomyelitis),8-10 other infections may require extended treatment courses for weeks. The risk of adverse outcomes (eg, complications of medical treatment, readmission risk) and burdens placed on patients and their families may therefore differ across infection types and extend well beyond the immediate hospitalization.
Although infections are common and pediatric providers are expected to have proficiency in managing infections, substantial variation in the management of common pediatric infections exists and is associated with adverse hospitalization outcomes, including increased readmission risk and healthcare costs.11-18 Potentially avoidable resource use associated with hospital readmission from infection has led to adoption of hospital-level readmission metrics as indicators of the quality of healthcare delivery. For example, the Pediatric Quality Measures Program, established by the Children’s Health Insurance Program Reauthorization Act of 2009, has prioritized measurement of readmissions following hospitalization for lower respiratory tract infection.19 With government agencies increasingly using readmission metrics to assess quality of healthcare delivery, developing metrics that focus on these resource-intensive conditions is essential.
Because infections are a common and costly indication for hospital resource use and because substantial variation in management has been observed, promoting a broader understanding of infection-specific readmission rates is important for prioritizing readmission-reduction opportunities in children. This study’s objectives were the following: (1) to describe the prevalence and characteristics of infection hospitalizations in children and their associated readmissions and (2) to estimate the number of readmissions avoided and costs saved if all hospitals achieved the 10th percentile of the hospitals’ risk-adjusted readmission rate (ie, readmission benchmark).
METHODS
Study Design and Data Source
We performed a retrospective cohort analysis using the 2014 Agency for Healthcare Research and Quality (AHRQ) Nationwide Readmissions Database (NRD).20 The 2014 NRD is an administrative database that contains information on inpatient stays from January 1, 2014, to December 31, 2014, for all payers and allows for weighted national estimates of readmissions for all US individuals. Data within NRD are aggregated from 22 geographically diverse states representing approximately one-half of the US population. NRD contains deidentified patient-level data with unique verified patient identifiers to track individuals within and across hospitals in a state. However, AHRQ guidelines specify that NRD cannot be used for reporting hospital-specific readmission rates. Thus, for the current study, the Inpatient Essentials (Children’s Hospital Association), or IE, database was used to measure hospital-level readmission rates and to distinguish benchmark readmission rates for individual infection diagnoses.21 The IE database is composed of 90 children’s hospitals distributed throughout all regions of the United States. The inclusion of free-standing children’s hospitals and children’s hospitals within adult hospitals allows for comparisons and benchmarking across hospitals on multiple metrics, including readmissions.
Study Population
Children 0 to 17 years of age with a primary diagnosis at the index admission for infection between January 1, 2014, and November 30, 2014, were included. The end date of November 30, 2014, allowed for a full 30-day readmission window for all index admissions. We excluded index admissions that resulted in transfer to another acute care hospital or in-hospital mortality. Additionally, we excluded index admissions of children who had hematologic or immunologic conditions, malignancy, or history of bone marrow and solid-organ transplant, using the classification system for complex chronic conditions (CCCs) from Feudtner et al.22 Due to the high likelihood of immunosuppression in patients with these conditions, children may have nuanced experiences with illness severity, trajectory, and treatment associated with infection that place them at increased risk for nonpreventable readmission.
Main Exposure
The main exposure was infection type during the index admission. Condition-specific index admissions were identified using AHRQ’s Clinical Classifications Software (CCS) categories.23 CCS is a classification schema that categorizes the greater than 14,000 International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) diagnosis codes and 3,900 ICD-9-CM procedure codes into clinically meaningful categories of 295 diagnosis (including mental health codes and E-codes) and 231 procedural groupings. Twenty-two groupings indicative of infection were distinguished and used for the current study. Examples of infections included aspiration pneumonia, pneumonia, bronchiolitis, and sexually transmitted infection. We combined related CCS categories when possible for ease of interpretation and presentation of data (Appendix Table 1).
Main Outcome Measure
The main outcome measure was 30-day hospital readmission. Readmission was defined as all-cause, unplanned admission within 30 days following discharge from a preceding hospitalization. Planned hospital readmissions were identified and excluded using methods from AHRQ’s Pediatric All-Condition Readmission Measure.24 We defined a same-cause return as a return with the same CCS infection category as the index admission. Costs associated with readmissions were estimated from charges using hospital-specific cost-to-charge ratios provided with NRD.
Patient Demographic and Clinical Characteristics
Patient demographic characteristics included age at index admission (<1 year, 1-5 years, 6-9 years, 10-14 years, and 15-18 years), sex, payer (ie, government, private, other), and discharge disposition (ie, routine, home health, other). We assessed all patients for medical complexity, as defined by the presence of at least one CCC, and we reported the categories of CCCs by organ system involved.22 Otherwise, patients were identified as without medical complexity.
Statistical Analysis
We summarized continuous variables with medians and interquartile ranges and categorical variables with frequencies and percentages. To develop benchmark readmission rates for each infection type, we used generalized linear mixed models with random intercepts for each hospital in the IE database. For each infection type, the benchmark readmission rate was defined as the 10th percentile of hospitals’ risk-adjusted readmission rates. The 10th percentile was chosen to identify the best performing 10% of hospitals (ie, hospitals with the lowest readmission rates). Because children with medical complexity account for a large proportion of hospital resource use and are at high risk for readmission,4,25 we developed benchmarks stratified by presence/absence of a CCC (ie, with complexity vs without complexity). Models were adjusted for severity of illness using the Hospitalization Resource Intensity Score for Kids (H-RISK),26 a scoring system that assigns relative weights for each All Patient Refined Diagnosis-Related Group (3M Corporation) and severity of illness level, and each hospital’s risk-adjusted readmission rate was determined.
With use of weights to achieve national estimates of index admissions and readmissions, we determined the number of potentially avoidable readmissions by calculating the number of readmissions observed in the NRD that would not occur if all hospitals achieved readmission rates equal to the 10th percentile. Avoidable costs were estimated by multiplying the number of potentially avoidable readmissions by the mean cost of a readmission for infections of that type. Estimates of avoidable readmissions and costs were stratified by medical complexity. In addition to describing estimates at the 10th percentile benchmark, we similarly developed estimates of potentially avoidable readmissions and avoidable costs for the 5th and 25th percentiles, which are presented within Appendix Table 2 (children without complexity) and Appendix Table 3 (children with complexity).
All statistical analyses were performed using SAS version 9.4 (SAS Institute), and P values <.001 were considered statistically significant due to the large sample size. The Office of Research Integrity at Children’s Mercy Hospital deemed this study exempt from institutional board review.
RESULTS
Characteristics of the Study Population
The study included 380,067 index admissions for infection and an accompanying 18,469 unplanned all-cause readmissions over the 30 days following discharge (readmission rate, 4.9%; Table 1). Children ages 1 to 5 years accounted for the largest percentage (32.9%) of index hospitalizations, followed by infants younger than 1 year (30.3%). The readmission rate by age group was highest for infants younger than 1 year, compared with rates among all other age groups (5.6% among infants < 1 year vs 4.4%-4.7% for other age groups; P < .001). In the overall cohort, 16.2% of admissions included patients with a CCC. Children with medical complexity had higher readmission rates than those without medical complexity (no CCC, 3.2%; 1 CCC, 9.2%; 2+ CCCs, 18.9%). A greater percentage of children experiencing a readmission had government insurance (63.0% vs 59.2%; P < .001) and received home health nursing (10.1% vs 2.7%; P < .001) following the index encounter.

Children Without Complexity
Index Admissions and 30-day Readmissions
Among patients without medical complexity, index admissions occurred most frequently for pneumonia (n = 54,717), bronchiolitis (n = 53,959), and appendicitis (n = 45,036) (Figure 1). The median length of stay (LOS) for index admissions ranged from 1 to 5 days (Table 2), with septic arthritis and osteomyelitis having the longest median LOS at 5 (IQR, 3-7) days.

Thirty-day readmission rates varied substantially by infection at the index admission (range, 1.5% for sexually transmitted infection to 8.6% for peritonitis) (Figure 1). The median LOS for 30-day readmissions varied from 2 to 7 days (Table 2), while the median number of days to readmission varied substantially by infection type (range, 4 days for bacterial infection [site unspecified] to 24 days for sexually transmitted infections). Among the top five indications for admission for children without complexity, 14.9% to 52.8% of readmissions were for the same cause as the index admission; however, many additional returns were likely related to the index admission (Appendix Table 4). For example, among other return reasons, an additional 992 (61.7%) readmissions following appendicitis hospitalizations were for complications of surgical procedures or medical care, peritonitis, intestinal obstruction, and abdominal pain.

Impact of Achieving Readmission Benchmarks
Among children without complexity, readmission benchmarks (ie, the 10th percentile of readmission rates across hospitals) ranged from 0% to 26.7% (Figure 2). An estimated 54.7% of readmissions (n = 5,507) could potentially be reduced if hospitals achieved infection-specific benchmark readmission rates, which could result in an estimated $44.5 million in savings. Pneumonia, bronchiolitis, gastroenteritis, and upper respiratory tract infections were among conditions with the greatest potential reductions in readmissions and costs if a 10th percentile benchmark was achieved.

Children With Medical Complexity
Index Admissions and 30-day Readmissions
Among patients with complexity, index admissions occurred most frequently for pneumonia (n = 14,344), bronchiolitis (n = 8,618), and upper respiratory tract infection (n = 6,407) (Figure 1). The median LOS for index admissions ranged from 1 to 9 days (Table 2), with septicemia and CNS infections having the longest median LOS at 9 days.
Thirty-day readmission rates varied substantially by the type of infection at the index admission (range, 0% for sexually transmitted infection to 21.6% for aspiration pneumonia) (Figure 1). The median LOS for 30-day readmissions varied from 2 to 14 days (Table 2), and the median number of days to readmission varied substantially by infection type (range, 6 days for tonsillitis to 23 days for other infection). Among the top five indications for admission for medically complex children, 8% to 40.4% of readmissions were for the same cause as the index admission (Appendix Table 4). As with the children without complexity, additional returns were likely related to the index admission.
Impact of Achieving Readmission Benchmarks
Among children with medical complexity, readmission benchmarks ranged from 0% to 30.3% (Figure 2). An estimated 42.6% of readmissions (n = 3,576) could potentially be reduced if hospitals achieved infection-specific benchmark readmission rates, which could result in an estimated $70.8 million in savings. Pneumonia, bronchiolitis, septicemia, and upper respiratory tract infections were among conditions with the greatest potential reductions in readmissions and costs if the benchmarks were achieved.
DISCUSSION
The current study uncovered new findings regarding unplanned readmissions following index infection hospitalizations for children. In particular, readmission rates and time to readmission varied substantially by infection subtype. The study also identified priority infections and unique considerations for children with CCCs, all of which may help maximize the value of readmission metrics aimed at advancing hospital quality and reducing costs for infection hospitalizations in children. If all hospitals achieved the readmission rates of the best performing hospitals, 42.6% to 54.7% fewer readmissions could be realized with associated cost savings.
Nationally, studies have reported 30-day, all-cause unplanned readmission rates of 6.2% to 10.3%.5,27 In our current study we observed an overall readmission rate of 4.9% across all infectious conditions; however, readmission rates varied substantially by condition, with upper and lower respiratory tract infections, septicemia, and gastroenteritis among infections with the greatest number of potentially avoidable readmissions based on achievement of readmission benchmarks. Currently, pediatric-specific all-cause and lower respiratory tract infection readmission metrics have been developed with the aim of improving quality of care and reducing healthcare expenditures.28 Future readmission studies and metrics may prioritize conditions such as septicemia, gastroenteritis, and other respiratory tract infections. Our current study demonstrated that many readmissions following infection hospitalizations were associated with the same CCS category or within a related CCS category (eg, complications of surgical procedures or medical care, appendicitis, peritonitis, intestinal obstruction, and abdominal pain constituted the top five indications for readmission for appendicitis, whereas respiratory illnesses constituted the top five indications for readmissions for pneumonia). While this current study cannot clarify this relationship further, and the “avoidability” of unplanned readmissions is debated,29-31 our findings suggest that future investigations might focus on identifying whether condition-specific interventions during the index admission could mitigate some readmissions.
While the LOS of the index admission and the readmission were similar for most infection subtypes, we observed substantial variability in the temporal risk for readmission by infection subtype. Our observations complement studies exploring the timing of readmissions for other pediatric conditions.32-34 In particular, our findings further highlight that the composition and interaction of factors influencing infection readmissions may vary by condition. Infections represent a diverse group of conditions, with treatment courses that vary in need for parenteral antibiotics, ability to tailor treatment based on organism and susceptibilities, and length of treatment. While treatment for some infections may be accomplished, or nearly accomplished, prior to discharge, other infections (eg, osteomyelitis) may require prolonged treatment, shifting the burden of management and monitoring to patients and their families, which along with the timeliness and adequacy of outpatient follow-up, may modify an individual’s readmission risk. Consequently, a “one-size fits all” approach to discharge counseling may not be successful across all conditions. Our study lays the groundwork for how these temporal relationships may be used by clinicians to counsel families regarding the likely readmission timeframe, based on infection, and to establish follow-up appointments ahead of the infection-specific “readmission window,” which may allow outpatient providers to intervene when readmission risk is greatest.
Consistent with prior literature, children with medical complexity in our study had increased frequency of 30-day, all-cause unplanned readmissions and LOS, compared with peers who did not have complexity.5 Readmission rates following hospitalizations for aspiration pneumonia were comparable to those reported by Thompson et al in their study examining rates of pneumonia in children with neurologic impairment.35 In our current study, nearly 96% of readmissions following aspiration pneumonia hospitalizations were for children with medical complexity, and more than 58% of these readmissions were for aspiration pneumonia or respiratory illness. Future investigations should seek to explore factors contributing to readmissions in children with medical complexity and to evaluate whether interventions such as postdischarge coaching or discharge bundles could assist with reductions in healthcare resource use.36,37
Despite a lack of clear association between readmissions and quality of care for children,38 readmissions rates continue to be used as a quality measure for hospitalized patients. Within our present study, we found that achieving benchmark readmission rates for infection hospitalizations could lead to substantial reductions in readmissions; however, these reductions were seen across this relatively similar group of infection diagnoses, and hospitals may face greater challenges when attempting to achieve a 10th percentile readmission benchmark across a more expansive set of diagnoses. Despite these challenges, understanding the intricacies of readmissions may lead to improved readmission metrics and the systematic identification of avoidable readmissions, the goal of which is to enhance the quality of healthcare for hospitalized children.
Our findings should be interpreted in the context of several limitations. We defined our readmission benchmark at the 10th percentile using the IE database. Because an estimated 70% of hospitalizations for children occur within general hospitals,39 we believe that our use of the IE database allowed for increased generalizability, though the broadening of our findings to nonacademic hospital settings may be less reliable. While we describe the 10th percentile readmission benchmark here, alternative benchmarks would result in different estimates of avoidable readmissions and associated readmission costs (Appendix Tables 2 and 3). The IE and NRD databases do not distinguish intensive care use. We tried to address this limitation through model adjustments using H-RISK, which is particularly helpful for adjusting for NICU admissions through use of the 27 All Patient Refined Diagnosis-Related Groups for neonatal conditions. Additionally, the NRD uses state-level data to derive national estimates and is not equipped to measure readmissions to hospitals in a different state, distinguish patient deaths occurring after discharge, or to examine the specific indication for readmission (ie, unable to assess if the readmission is related to a complication of the treatment plan, progression of the illness course, or for an unrelated reason). While sociodemographic and socioeconomic factors may affect readmissions, the NRD does not contain information on patients’ race/ethnicity, family/social attributes, or postdischarge follow-up health services, which are known to influence the need for readmission.
Despite these limitations, this study highlights future areas for research and potential opportunities for reducing readmission following infection hospitalizations. First, identifying hospital- and systems-based factors that contribute to readmission reductions at the best-performing hospitals may identify opportunities for hospitals with the highest readmission rates to achieve the rates of the best-performing hospitals. Second, conditions such as upper and lower respiratory tract infections, along with septicemia and gastroenteritis, may serve as prime targets for future investigation based on the estimated number of avoidable readmissions and potential cost savings associated with these conditions. Finally, future investigations that explore discharge counseling and follow-up tailored to the infection-specific temporal risk and patient complexity may identify opportunities for further readmission reductions.
CONCLUSION
Our study provides a broad look at readmissions following infection hospitalizations and highlights substantial variation in readmissions based on infection type. To improve hospital resource use for infections, future preventive measures could prioritize children with complex chronic conditions and those with specific diagnoses (eg, upper and lower respiratory tract infections).
Disclaimer
This information or content and conclusions are those of the authors and should not be construed as the official position or policy of, nor should any endorsements be inferred by, NIH or the US government.
1. Keren R, Luan X, Localio R, et al; Pediatric Research in Inpatient Settings (PRIS) Network. Prioritization of comparative effectiveness research topics in hospital pediatrics. Arch Pediatr Adolesc Med. 2012;166(12):1155-1164. https://doi.org/10.1001/archpediatrics.2012.1266
2. Van Horne B, Netherton E, Helton J, Fu M, Greeley C. The scope and trends of pediatric hospitalizations in Texas, 2004-2010. Hosp Pediatr. 2015;5(7):390-398. https://doi.org/10.1542/hpeds.2014-0105
3. Neuman MI, Hall M, Gay JC, et al. Readmissions among children previously hospitalized with pneumonia. Pediatrics. 2014;134(1):100-109. https://doi.org/10.1542/peds.2014-0331
4. Gay JC, Hain PD, Grantham JA, Saville BR. Epidemiology of 15-day readmissions to a children’s hospital. Pediatrics. 2011;127(6):e1505-e1512. https://doi.org/10.1542/peds.2010-1737
5. Berry JG, Toomey SL, Zaslavsky AM, et al. Pediatric readmission prevalence and variability across hospitals. JAMA. 2013;309(4):372-380. https://doi.org/10.1001/jama.2012.188351
6. Shudy M, de Almeida ML, Ly S, et al. Impact of pediatric critical illness and injury on families: a systematic literature review. Pediatrics. 2006;118(suppl 3):S203-S218. https://doi.org/10.1542/peds.2006-0951b
7. Rennick JE, Johnston CC, Dougherty G, Platt R, Ritchie JA. Children’s psychological responses after critical illness and exposure to invasive technology. J Dev Behav Pediatr. 2002;23(3):133-144. https://doi.org/10.1097/00004703-200206000-00002
8. Michael M, Hodson EM, Craig JC, Martin S, Moyer VA. Short versus standard duration oral antibiotic therapy for acute urinary tract infection in children. Cochrane Database Syst Rev. 2003;(1):CD003966. https://doi.org/10.1002/14651858.cd003966
9. Greenberg D, Givon-Lavi N, Sadaka Y, Ben-Shimol S, Bar-Ziv J, Dagan R. Short-course antibiotic treatment for community-acquired alveolar pneumonia in ambulatory children: a double-blind, randomized, placebo-controlled trial. Pediatr Infect Dis J. 2014;33(2):136-142. https://doi.org/10.1097/inf.0000000000000023
10. Keren R, Shah SS, Srivastava R, et al; Pediatric Research in Inpatient Settings Network. Comparative effectiveness of intravenous vs oral antibiotics for postdischarge treatment of acute osteomyelitis in children. JAMA Pediatr. 2015;169(2):120-128. https://doi.org/10.1001/jamapediatrics.2014.2822
11. Markham JL, Hall M, Bettenhausen JL, Myers AL, Puls HT, McCulloh RJ. Variation in care and clinical outcomes in children hospitalized with orbital cellulitis. Hosp Pediatr. 2018;8(1):28-35. https://doi.org/10.1542/hpeds.2017-0040
12. Neubauer HC, Hall M, Wallace SS, Cruz AT, Queen MA, Foradori DM, Aronson PL, Markham JL, Nead JA, Hester GZ, McCulloh RJ, Lopez MA. Variation in diagnostic test use and associated outcomes in staphylococcal scalded skin syndrome at children’s hospitals. Hosp Pediatr. 2018;8(9):530-537. https://doi.org/10.1542/hpeds.2018-0032
13. Aronson PL, Thurm C, Alpern ER, et al; Febrile Young Infant Research Collaborative. Variation in care of the febrile young infant <90 days in US pediatric emergency departments. Pediatrics. 2014;134(4):667-677. https://doi.org/10.1542/peds.2014-1382
14. Florin TA, French B, Zorc JJ, Alpern ER, Shah SS. Variation in emergency department diagnostic testing and disposition outcomes in pneumonia. Pediatrics. 2013;132(2):237-244. https://doi.org/10.1542/peds.2013-0179
15. Brogan TV, Hall M, Williams DJ, et al. Variability in processes of care and outcomes among children hospitalized with community-acquired pneumonia. Pediatr Infect Dis J. 2012;31(10):1036-1041. https://doi.org/10.1097/inf.0b013e31825f2b10
16. Leyenaar JK, Lagu T, Shieh MS, Pekow PS, Lindenauer PK. Variation in resource utilization for the management of uncomplicated community-acquired pneumonia across community and children’s hospitals. J Pediatr. 2014;165(3):585-591. https://doi.org/10.1016/j.jpeds.2014.04.062
17. Knapp JF, Simon SD, Sharma V. Variation and trends in ED use of radiographs for asthma, bronchiolitis, and croup in children. Pediatrics. 2013;132(2):245-252. https://doi.org/10.1542/peds.2012-2830
18. Rice-Townsend S, Barnes JN, Hall M, Baxter JL, Rangel SJ. Variation in practice and resource utilization associated with the diagnosis and management of appendicitis at freestanding children’s hospitals: implications for value-based comparative analysis. Ann Surg. 2014;259(6):1228-1234. https://doi.org/10.1097/SLA.0000000000000246
19. Pediatric Quality Measures Program (PQMP). Agency for Healthcare Research and Quality. Accessed March 1, 2019. https://www.ahrq.gov/pqmp/index.html
20. NRD Database Documentation. Accessed June 1, 2019. https://www.hcup-us.ahrq.gov/db/nation/nrd/nrddbdocumentation.jsp
21. Inpatient Essentials. Children’s Hospitals Association. Accessed August 1, 2018. https://www.childrenshospitals.org/Programs-and-Services/Data-Analytics-and-Research/Pediatric-Analytic-Solutions/Inpatient-Essentials
22. Feudtner C, Feinstein JA, Zhong W, Hall M, Dai D. Pediatric complex chronic conditions classification system version 2: updated for ICD-10 and complex medical technology dependence and transplantation. BMC Pediatr. 2014;14:199. https://doi.org/10.1186/1471-2431-14-199
23. Clinical Classifications Software (CCS) for ICD-9-CM. Healthcare Cost and Utilization Project. March 2017. Accessed August 2, 2018. https://www.hcup-us.ahrq.gov/toolssoftware/ccs/ccs.jsp
24. NQF: Quality Positioning System. National Quality Forum. Accessed September 3, 2018. http://www.qualityforum.org/QPS/QPSTool.aspx
25. Berry JG, Ash AS, Cohen E, Hasan F, Feudtner C, Hall M. Contributions of children with multiple chronic conditions to pediatric hospitalizations in the United States: a retrospective cohort analysis. Hosp Pediatr. 2017;7(7):365-372. https://doi.org/10.1542/hpeds.2016-0179
26. Richardson T, Rodean J, Harris M, Berry J, Gay JC, Hall M. Development of Hospitalization Resource Intensity Scores for Kids (H-RISK) and comparison across pediatric populations. J Hosp Med. 2018;13(9):602-608. https://doi.org/10.12788/jhm.2948
27. Auger KA, Mueller EL, Weinberg SH, et al. A validated method for identifying unplanned pediatric readmission. J Pediatr. 2016;170:105-12.e122. https://doi.org/10.1016/j.jpeds.2015.11.051
28. NQF: Pediatric Measures Final Report. National Quality Forum. June 2016. Accessed January 24, 2019. https://www.qualityforum.org/Publications/2016/06/Pediatric_Measures_Final_Report.aspx
29. Hain PD, Gay JC, Berutti TW, Whitney GM, Wang W, Saville BR. Preventability of early readmissions at a children’s hospital. Pediatrics. 2013;131(1):e171-e181. https://doi.org/10.1542/peds.2012-0820
30. Toomey SL, Peltz A, Loren S, et al. Potentially preventable 30-day hospital readmissions at a children’s hospital. Pediatrics. 2016;138(2):e20154182. https://doi.org/10.1542/peds.2015-4182
31. Jonas JA, Devon EP, Ronan JC, et al. Determining preventability of pediatric readmissions using fault tree analysis. J Hosp Med. 2016;11(5):329-335. https://doi.org/10.1002/jhm.2555
32. Bucholz EM, Gay JC, Hall M, Harris M, Berry JG. Timing and causes of common pediatric readmissions. J Pediatr. 2018;200:240-248.e1. https://doi.org/10.1016/j.jpeds.2018.04.044
33. Morse RB, Hall M, Fieldston ES, et al. Children’s hospitals with shorter lengths of stay do not have higher readmission rates. J Pediatr. 2013;163(4):1034-8.e1. https://doi.org/10.1016/j.jpeds.2013.03.083
34. Kenyon CC, Melvin PR, Chiang VW, Elliott MN, Schuster MA, Berry JG. Rehospitalization for childhood asthma: timing, variation, and opportunities for intervention. J Pediatr. 2014;164(2):300-305. https://doi.org/10.1016/j.jpeds.2013.10.003
35. Thomson J, Hall M, Ambroggio L, et al. Aspiration and non-aspiration pneumonia in hospitalized children with neurologic impairment. Pediatrics. 2016;137(2):e20151612. https://doi.org/10.1542/peds.2015-1612
36. Coller RJ, Klitzner TS, Lerner CF, et al. Complex Care hospital use and postdischarge coaching: a randomized controlled trial. Pediatrics. 2018;142(2):e20174278. https://doi.org/10.1542/peds.2017-4278
37. Stephens JR, Kimple KS, Steiner MJ, Berry JG. Discharge interventions and modifiable risk factors for preventing hospital readmissions in children with medical complexity. Rev Recent Clin Trials. 2017;12(4):290-297. https://doi.org/10.2174/1574887112666170816144455
38. Bardach NS, Vittinghoff E, Asteria-Peñaloza R, et al. Measuring hospital quality using pediatric readmission and revisit rates. Pediatrics. 2013;132(3):429-436. https://doi.org/10.1542/peds.2012-3527
39. Leyenaar JK, Ralston SL, Shieh MS, Pekow PS, Mangione-Smith R, Lindenauer PK. Epidemiology of pediatric hospitalizations at general hospitals and freestanding children’s hospitals in the United States. J Hosp Med. 2016;11(11):743-749. https://doi.org/10.1002/jhm.2624
1. Keren R, Luan X, Localio R, et al; Pediatric Research in Inpatient Settings (PRIS) Network. Prioritization of comparative effectiveness research topics in hospital pediatrics. Arch Pediatr Adolesc Med. 2012;166(12):1155-1164. https://doi.org/10.1001/archpediatrics.2012.1266
2. Van Horne B, Netherton E, Helton J, Fu M, Greeley C. The scope and trends of pediatric hospitalizations in Texas, 2004-2010. Hosp Pediatr. 2015;5(7):390-398. https://doi.org/10.1542/hpeds.2014-0105
3. Neuman MI, Hall M, Gay JC, et al. Readmissions among children previously hospitalized with pneumonia. Pediatrics. 2014;134(1):100-109. https://doi.org/10.1542/peds.2014-0331
4. Gay JC, Hain PD, Grantham JA, Saville BR. Epidemiology of 15-day readmissions to a children’s hospital. Pediatrics. 2011;127(6):e1505-e1512. https://doi.org/10.1542/peds.2010-1737
5. Berry JG, Toomey SL, Zaslavsky AM, et al. Pediatric readmission prevalence and variability across hospitals. JAMA. 2013;309(4):372-380. https://doi.org/10.1001/jama.2012.188351
6. Shudy M, de Almeida ML, Ly S, et al. Impact of pediatric critical illness and injury on families: a systematic literature review. Pediatrics. 2006;118(suppl 3):S203-S218. https://doi.org/10.1542/peds.2006-0951b
7. Rennick JE, Johnston CC, Dougherty G, Platt R, Ritchie JA. Children’s psychological responses after critical illness and exposure to invasive technology. J Dev Behav Pediatr. 2002;23(3):133-144. https://doi.org/10.1097/00004703-200206000-00002
8. Michael M, Hodson EM, Craig JC, Martin S, Moyer VA. Short versus standard duration oral antibiotic therapy for acute urinary tract infection in children. Cochrane Database Syst Rev. 2003;(1):CD003966. https://doi.org/10.1002/14651858.cd003966
9. Greenberg D, Givon-Lavi N, Sadaka Y, Ben-Shimol S, Bar-Ziv J, Dagan R. Short-course antibiotic treatment for community-acquired alveolar pneumonia in ambulatory children: a double-blind, randomized, placebo-controlled trial. Pediatr Infect Dis J. 2014;33(2):136-142. https://doi.org/10.1097/inf.0000000000000023
10. Keren R, Shah SS, Srivastava R, et al; Pediatric Research in Inpatient Settings Network. Comparative effectiveness of intravenous vs oral antibiotics for postdischarge treatment of acute osteomyelitis in children. JAMA Pediatr. 2015;169(2):120-128. https://doi.org/10.1001/jamapediatrics.2014.2822
11. Markham JL, Hall M, Bettenhausen JL, Myers AL, Puls HT, McCulloh RJ. Variation in care and clinical outcomes in children hospitalized with orbital cellulitis. Hosp Pediatr. 2018;8(1):28-35. https://doi.org/10.1542/hpeds.2017-0040
12. Neubauer HC, Hall M, Wallace SS, Cruz AT, Queen MA, Foradori DM, Aronson PL, Markham JL, Nead JA, Hester GZ, McCulloh RJ, Lopez MA. Variation in diagnostic test use and associated outcomes in staphylococcal scalded skin syndrome at children’s hospitals. Hosp Pediatr. 2018;8(9):530-537. https://doi.org/10.1542/hpeds.2018-0032
13. Aronson PL, Thurm C, Alpern ER, et al; Febrile Young Infant Research Collaborative. Variation in care of the febrile young infant <90 days in US pediatric emergency departments. Pediatrics. 2014;134(4):667-677. https://doi.org/10.1542/peds.2014-1382
14. Florin TA, French B, Zorc JJ, Alpern ER, Shah SS. Variation in emergency department diagnostic testing and disposition outcomes in pneumonia. Pediatrics. 2013;132(2):237-244. https://doi.org/10.1542/peds.2013-0179
15. Brogan TV, Hall M, Williams DJ, et al. Variability in processes of care and outcomes among children hospitalized with community-acquired pneumonia. Pediatr Infect Dis J. 2012;31(10):1036-1041. https://doi.org/10.1097/inf.0b013e31825f2b10
16. Leyenaar JK, Lagu T, Shieh MS, Pekow PS, Lindenauer PK. Variation in resource utilization for the management of uncomplicated community-acquired pneumonia across community and children’s hospitals. J Pediatr. 2014;165(3):585-591. https://doi.org/10.1016/j.jpeds.2014.04.062
17. Knapp JF, Simon SD, Sharma V. Variation and trends in ED use of radiographs for asthma, bronchiolitis, and croup in children. Pediatrics. 2013;132(2):245-252. https://doi.org/10.1542/peds.2012-2830
18. Rice-Townsend S, Barnes JN, Hall M, Baxter JL, Rangel SJ. Variation in practice and resource utilization associated with the diagnosis and management of appendicitis at freestanding children’s hospitals: implications for value-based comparative analysis. Ann Surg. 2014;259(6):1228-1234. https://doi.org/10.1097/SLA.0000000000000246
19. Pediatric Quality Measures Program (PQMP). Agency for Healthcare Research and Quality. Accessed March 1, 2019. https://www.ahrq.gov/pqmp/index.html
20. NRD Database Documentation. Accessed June 1, 2019. https://www.hcup-us.ahrq.gov/db/nation/nrd/nrddbdocumentation.jsp
21. Inpatient Essentials. Children’s Hospitals Association. Accessed August 1, 2018. https://www.childrenshospitals.org/Programs-and-Services/Data-Analytics-and-Research/Pediatric-Analytic-Solutions/Inpatient-Essentials
22. Feudtner C, Feinstein JA, Zhong W, Hall M, Dai D. Pediatric complex chronic conditions classification system version 2: updated for ICD-10 and complex medical technology dependence and transplantation. BMC Pediatr. 2014;14:199. https://doi.org/10.1186/1471-2431-14-199
23. Clinical Classifications Software (CCS) for ICD-9-CM. Healthcare Cost and Utilization Project. March 2017. Accessed August 2, 2018. https://www.hcup-us.ahrq.gov/toolssoftware/ccs/ccs.jsp
24. NQF: Quality Positioning System. National Quality Forum. Accessed September 3, 2018. http://www.qualityforum.org/QPS/QPSTool.aspx
25. Berry JG, Ash AS, Cohen E, Hasan F, Feudtner C, Hall M. Contributions of children with multiple chronic conditions to pediatric hospitalizations in the United States: a retrospective cohort analysis. Hosp Pediatr. 2017;7(7):365-372. https://doi.org/10.1542/hpeds.2016-0179
26. Richardson T, Rodean J, Harris M, Berry J, Gay JC, Hall M. Development of Hospitalization Resource Intensity Scores for Kids (H-RISK) and comparison across pediatric populations. J Hosp Med. 2018;13(9):602-608. https://doi.org/10.12788/jhm.2948
27. Auger KA, Mueller EL, Weinberg SH, et al. A validated method for identifying unplanned pediatric readmission. J Pediatr. 2016;170:105-12.e122. https://doi.org/10.1016/j.jpeds.2015.11.051
28. NQF: Pediatric Measures Final Report. National Quality Forum. June 2016. Accessed January 24, 2019. https://www.qualityforum.org/Publications/2016/06/Pediatric_Measures_Final_Report.aspx
29. Hain PD, Gay JC, Berutti TW, Whitney GM, Wang W, Saville BR. Preventability of early readmissions at a children’s hospital. Pediatrics. 2013;131(1):e171-e181. https://doi.org/10.1542/peds.2012-0820
30. Toomey SL, Peltz A, Loren S, et al. Potentially preventable 30-day hospital readmissions at a children’s hospital. Pediatrics. 2016;138(2):e20154182. https://doi.org/10.1542/peds.2015-4182
31. Jonas JA, Devon EP, Ronan JC, et al. Determining preventability of pediatric readmissions using fault tree analysis. J Hosp Med. 2016;11(5):329-335. https://doi.org/10.1002/jhm.2555
32. Bucholz EM, Gay JC, Hall M, Harris M, Berry JG. Timing and causes of common pediatric readmissions. J Pediatr. 2018;200:240-248.e1. https://doi.org/10.1016/j.jpeds.2018.04.044
33. Morse RB, Hall M, Fieldston ES, et al. Children’s hospitals with shorter lengths of stay do not have higher readmission rates. J Pediatr. 2013;163(4):1034-8.e1. https://doi.org/10.1016/j.jpeds.2013.03.083
34. Kenyon CC, Melvin PR, Chiang VW, Elliott MN, Schuster MA, Berry JG. Rehospitalization for childhood asthma: timing, variation, and opportunities for intervention. J Pediatr. 2014;164(2):300-305. https://doi.org/10.1016/j.jpeds.2013.10.003
35. Thomson J, Hall M, Ambroggio L, et al. Aspiration and non-aspiration pneumonia in hospitalized children with neurologic impairment. Pediatrics. 2016;137(2):e20151612. https://doi.org/10.1542/peds.2015-1612
36. Coller RJ, Klitzner TS, Lerner CF, et al. Complex Care hospital use and postdischarge coaching: a randomized controlled trial. Pediatrics. 2018;142(2):e20174278. https://doi.org/10.1542/peds.2017-4278
37. Stephens JR, Kimple KS, Steiner MJ, Berry JG. Discharge interventions and modifiable risk factors for preventing hospital readmissions in children with medical complexity. Rev Recent Clin Trials. 2017;12(4):290-297. https://doi.org/10.2174/1574887112666170816144455
38. Bardach NS, Vittinghoff E, Asteria-Peñaloza R, et al. Measuring hospital quality using pediatric readmission and revisit rates. Pediatrics. 2013;132(3):429-436. https://doi.org/10.1542/peds.2012-3527
39. Leyenaar JK, Ralston SL, Shieh MS, Pekow PS, Mangione-Smith R, Lindenauer PK. Epidemiology of pediatric hospitalizations at general hospitals and freestanding children’s hospitals in the United States. J Hosp Med. 2016;11(11):743-749. https://doi.org/10.1002/jhm.2624
© 2021 Society of Hospital Medicine
Early and Significant Reduction in C-Reactive Protein Levels After Corticosteroid Therapy Is Associated With Reduced Mortality in Patients With COVID-19
Confirmed cases of coronavirus disease 2019 (COVID-19) exceed 111 million, and the disease is responsible for approximately 2.4 million deaths worldwide.1 In the United States, 28 million cases of COVID-19 have been reported, and the disease has caused more than 497,000 deaths.2 The clinical presentation of COVID-19 varies widely, with the most severe presentation characterized by acute respiratory distress syndrome and a marked systemic inflammatory response. Corticosteroids have emerged as a potential therapeutic option in a subset of patients. Results from the recently published RECOVERY trial suggest a substantial mortality benefit of dexamethasone in patients who require mechanical ventilation, with a risk reduction of approximately 33%.3 In addition, a recent large retrospective study demonstrated a reduction in the risk of mechanical ventilation or mortality with corticosteroids in a prespecified subset of patients with C-reactive protein (CRP) ≥20 mg/dL, which indicates a high burden of inflammation.4
Some patients with severe COVID-19 experience a positive feedback cascade of proinflammatory cytokines, called the cytokine storm, which can worsen lung injury and, in some cases, progress to vasodilatory shock and multiorgan failure.5 This complication’s cytokine cascade includes interleukin (IL) 6, IL-1β, and CC chemokine ligand 3 (CCL3), which are released by airway macrophages and all of which are heavily implicated in the maladaptive forms of immune response to COVID-19.6,7 The cytokine IL-6 is the primary signal for the production of CRP, and corticosteroids have been shown, both in vitro and in vivo, to reduce the production of IL-6 and other cytokines by airway macrophages.6 Levels of CRP have been shown to correlate with outcomes in COVID-19 and bacterial pneumonias.7,8 Reduction in CRP levels following the institution of therapy, known as CRP response, has been shown to predict outcomes in other inflammatory conditions, such as osteomyelitis, hidradenitis suppurativa, and some cases of bacterial pneumonia.8-10 Similar CRP response in hemophagocytic lymphohistiocytosis, an entity which closely resembles cytokine storm syndrome, has been shown to correlate with disease activity in patients following treatment with an IL-1 antagonist.11 Whether the CRP response as a response to therapeutics in COVID-19 is associated with improved outcomes remains unknown.
Laboratory measurement of CRP levels offers several advantages over the measurement of interleukins. Notably, the half-life of CRP is approximately 19 hours, which is comparable across different age groups and inflammatory conditions because its concentration depends primarily on synthesis in the liver, and a decreased level suggests decreased stimulus for synthesis.8 This makes CRP a useful biomarker to assess response to therapy, in contrast to interleukins, which have short half-lives, are variable in heterogeneous populations, and can be difficult to measure. In addition, CRP measurement is rapid and relatively inexpensive.
We hypothesized that reduction in CRP levels by 50% or more within 72 hours after the initiation of corticosteroids in patients with COVID-19 is associated with reduced inpatient mortality and may be an early indicator of therapeutic response.
METHODS
Study Participants
In this retrospective cohort study, we reviewed all adult patients admitted to Montefiore Medical Center (Bronx, New York) for COVID-19 between March 10, 2020, and May 2, 2020. Patients must have been discharged (alive or deceased) by the administrative censor date (May 2, 2020) to be included. Patients who died within the first 48 hours of admission were excluded to allow sufficient time for corticosteroid treatment to take effect. For inclusion in the corticosteroid group, patients needed to have received at least 2 consecutive days of corticosteroid treatment beginning within the first 48 hours of admission with a total daily dose of 0.5 mg/kg prednisone equivalent or greater. Patients who received treatment-dose corticosteroids later in the hospital course were excluded (Appendix Figure).
Comparison Group and Outcome
We examined trends in CRP levels for patients who received corticosteroids vs trends among patients who did not receive corticosteroids. In addition, among patients who were treated with corticosteroids, we compared the inpatient mortality of those who did have a reduction in CRP level after treatment with inpatient mortality of those who did not have a reduction in CRP level after treatment. First, CRP level trends over time were examined in all patients, and compared between those who received corticosteroid treatment and those who did not. Then, patients who received corticosteroids were categorized based on changes in CRP levels after beginning corticosteroids. The first CRP level obtained during the first 48 hours of admission was used as the initial CRP level. For each patient, the last CRP level within the 72 hours after initiation of treatment was used to calculate the change in CRP level from admission. A patient was considered to be a “CRP responder” if their CRP level decreased by 50% or more within 72 hours after treatment and a “CRP nonresponder” if their CRP level did not drop by at least 50% within 72 hours of treatment. Patients who did not have a CRP level within the initial 48 hours of admission or a subsequent CRP measured in the 72 hours after treatment were considered to have an “undetermined CRP response” and excluded from the mortality analysis.
We observed a rise in CRP starting around day 6 among patients treated with corticosteroids and performed a post hoc analysis to determine if this was due to a selection effect whereby patients staying in the hospital longer had higher CRP levels or represented actual rise. In order to address this, we performed a stratified analysis comparing the trends in CRP levels among patients with a length of stay (LOS) of 7 or more days with trends among those with an LOS less than 7 days.
Statistical Analysis
To characterize differences in patients who received corticosteroids and those who did not, we examined their demographic, clinical characteristics, and admission laboratory values, using chi-square test for categorical variables and Kruskal-Wallis test for continuous variables (Table 1). The change in CRP levels from day 0 (presentation to the hospital) in both groups was plotted in a time-series analysis. For each day in the time series, the 95% CIs for the changes in CRP were computed using the t statistic for the corresponding distribution. The Kruskal-Wallis test was used to assess the significance of differences between groups at 72 hours after initiation of treatment.
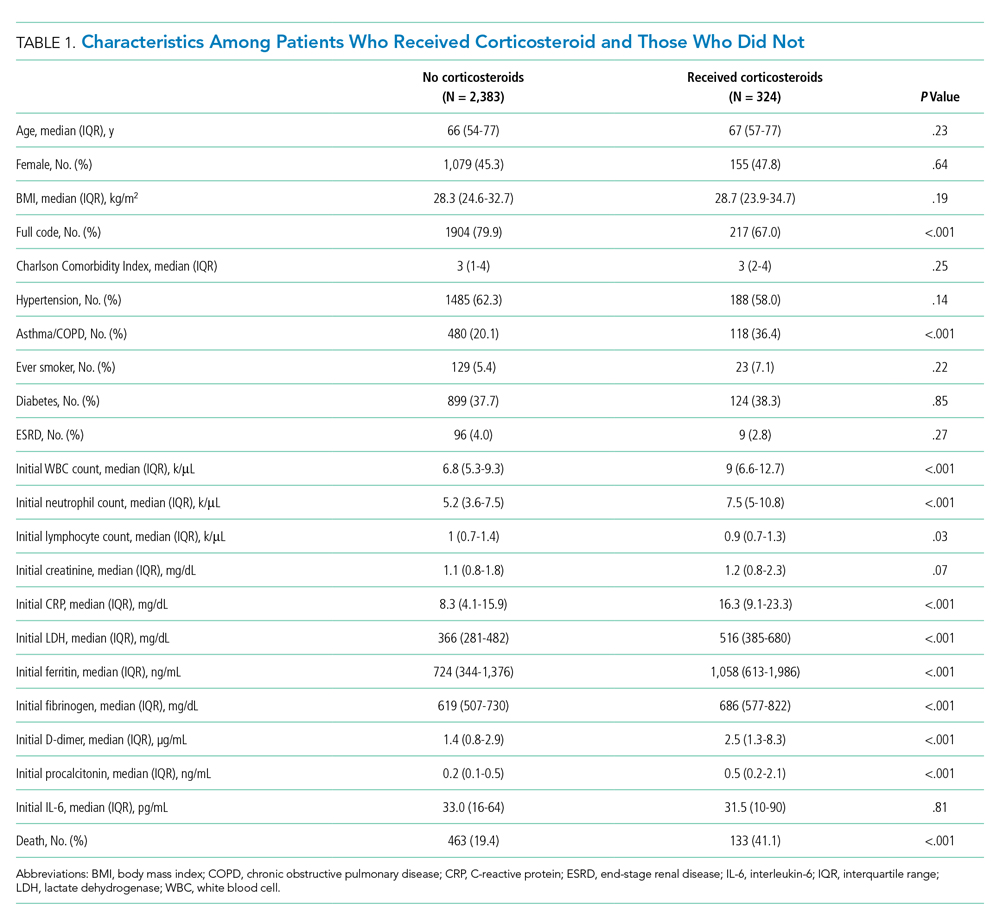
After categorizing patients by CRP response, we compared demographic, clinical, and laboratory characteristics of patients who were CRP responsive with those of patients who were not, using the same tests of statistical inference mentioned above. To compare time to inpatient mortality differences between CRP response groups, Kaplan-Meier survival curves were generated and statistical significance determined via log-rank test. Univariable logistic regression was used to estimate the odds ratio of inpatient mortality between comparison groups in an unadjusted analysis. Last, to examine the independent association between CRP response and mortality, we constructed a multivariate model that included variables that were significantly associated with mortality in univariable analysis and considered to be important potential confounders by the authors. Details on variable selection for the model are listed in Appendix Table 1.
Data Collection
Data were directly extracted from our center’s electronic health record system. Data processing and recoding was performed using the Python programming language (version 2.7.17) and data analysis was done using Stata 12 (StataCorp LLC; 2011). This study was approved by the institutional review board of the Albert Einstein College of Medicine.
RESULTS
Corticosteroids vs No Corticosteroids
Between March 10, 2020, and May 2, 2020, a total of 3,382 adult patients were admitted for COVID-19 at Montefiore Medical Center. Of these, 2,707 patients met the study inclusion criteria, and 324 of those received corticosteroid treatment. Their demographic characteristics, comorbidities, and admission lab values are shown in Table 1. Patients who received corticosteroids were older, had higher comorbidity scores, were more likely to have asthma or chronic obstructive pulmonary disease, and were less likely to be full code status, compared with patients who did not receive corticosteroids. Patients who received corticosteroids also had higher initial white blood cell (WBC) and neutrophil counts but lower lymphocyte count. The two groups were comparable in initial creatinine level. Additional patient characteristics and addmission lab values are shown in Appendix Table 2.
Average change in CRP levels by hospital day for those who received corticosteroids and those who did not are shown in Figure 1A. Among patients who received corticosteroid treatment, there was a significant decrease in CRP level at 72 hours of treatment (P < .001). In the post hoc analysis of trends in CRP levels, we found that CRP levels among those treated with corticosteroids started to rise around day 6 after the initial drop. This trend was observed even after removing patients with shorter LOS (<7 days) (Figure 1B). The median durations of corticosteroid therapy were 3 days among patients whose LOS was less than 7 days and 6 days among those whose LOS was 7 days or greater. The rise in CRP level was seen at day 5 and day 7 within each group, respectively. Crude death rate was 41.7% among patients with LOS of less than 7 days and 40.6% in those with LOS of 7 days or greater.
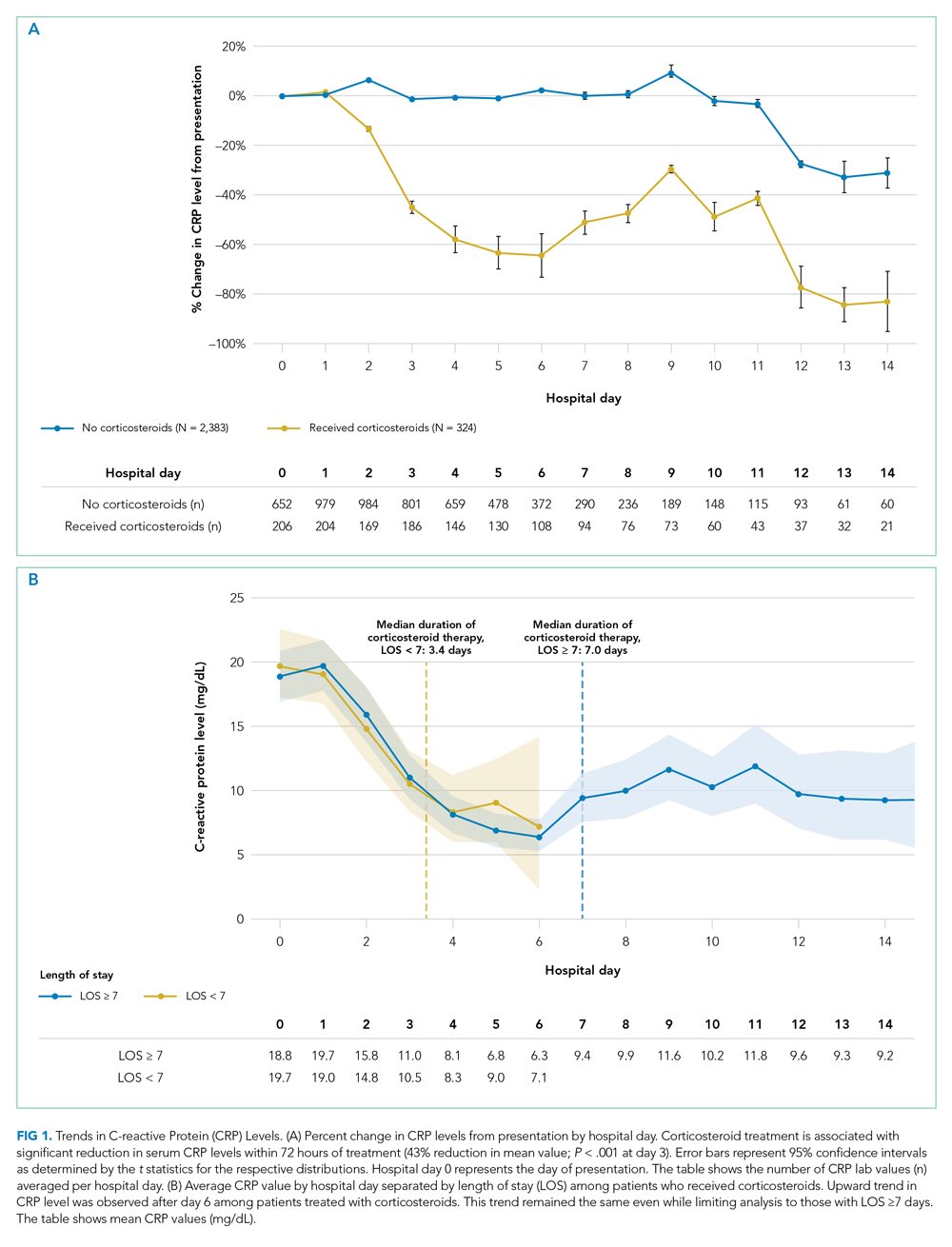
CRP Responders vs Nonresponders
Among the 324 patients who received corticosteroids, 131 (40.4%) were classified as responders, 92 (28.4%) were classified as nonresponders, and 101 (31.2%) were undetermined. Characteristics of CRP responders and CRP nonresponders are shown in Table 2 and Appendix Table 3. CRP responders were more likely to have dementia, higher median admission platelet count, and fibrinogen level compared with CRP nonresponders. Patients whose CRP response was undetermined were excluded from the analysis. Their characteristics are shown in Appendix Table 4.
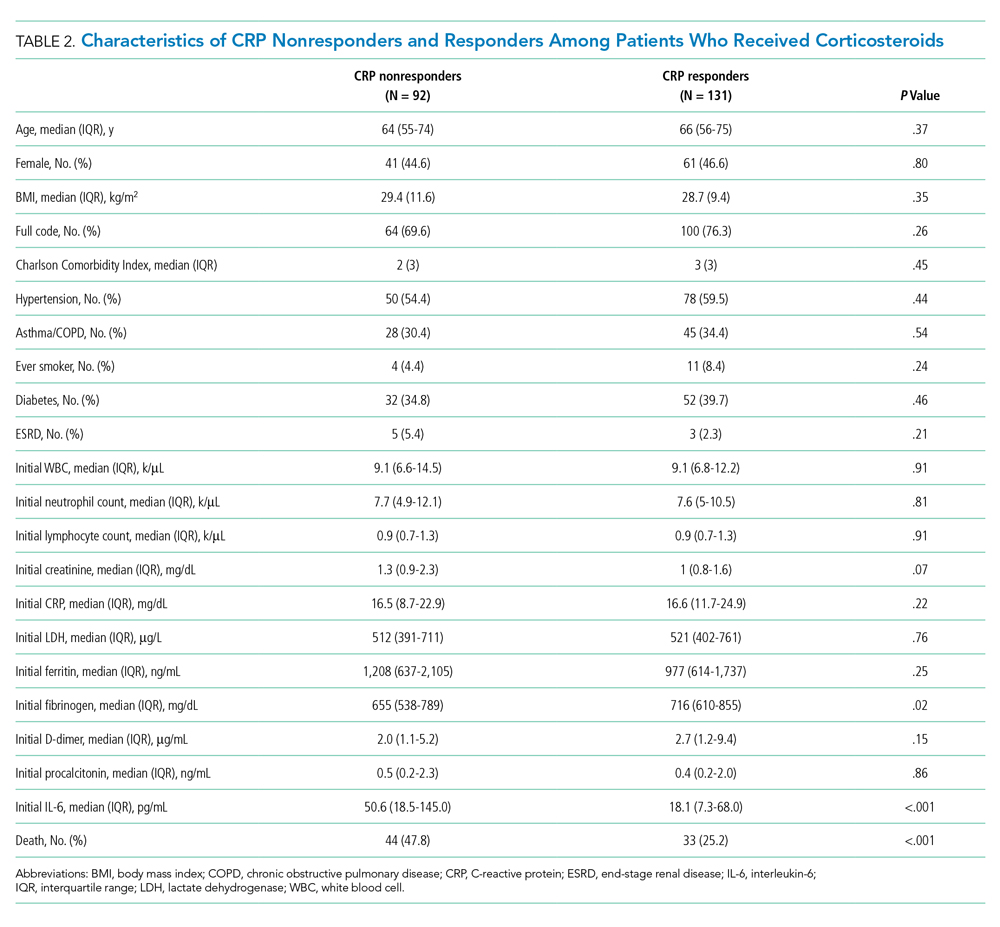
The observed inpatient mortality rate was 25.2% among CRP responders and 47.8% among CRP nonresponders. This was also demonstrated in the Kaplan-Meier survival curve (Figure 2). The odds of inpatient mortality among CRP responders was strongly and significantly reduced compared with those among nonresponders in an unadjusted analysis (odds ratio [OR], 0.37; 95% CI, 0.21-0.65; P = .001) and after adjustment for demographic and clinical characteristics including age, Charlson Comorbidity Index, initial WBC count, initial CRP level, and initial fibrinogen level (OR, 0.27; 95% CI, 0.14-0.54; P < .001). Details on how variables were operationalized and information on missing data are included in Appendix Table 1.
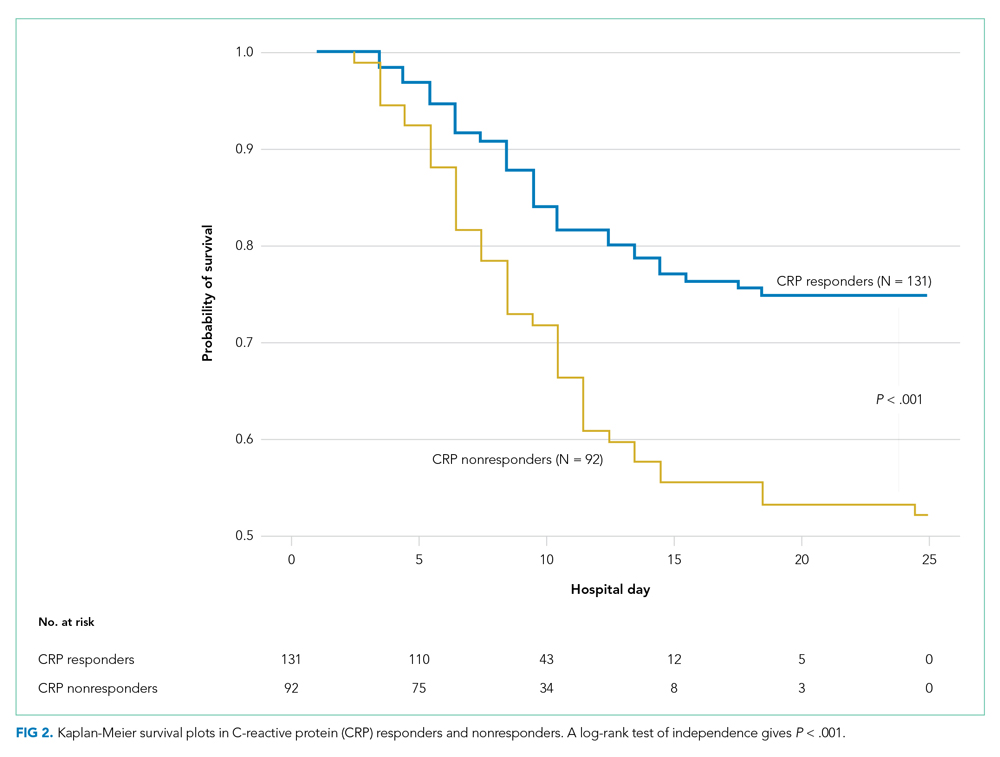
To explore whether this observed effect differed depending on severity of the respiratory illness, we examined the association between CRP response and mortality in subgroups stratified by intubation status. Within our cohort of 223 patients (92 CRP responders and 131 CRP nonresponders), 166 patients were never intubated, 50 patients were intubated in the first 48 hours, and 7 patients were intubated later on during the admission. The odds ratios for death among CRP responders vs nonresponders were 0.50 (P = .07) among patients never intubated and 0.46 (P = .2) among patients intubated within the initial 48 hours of admission.
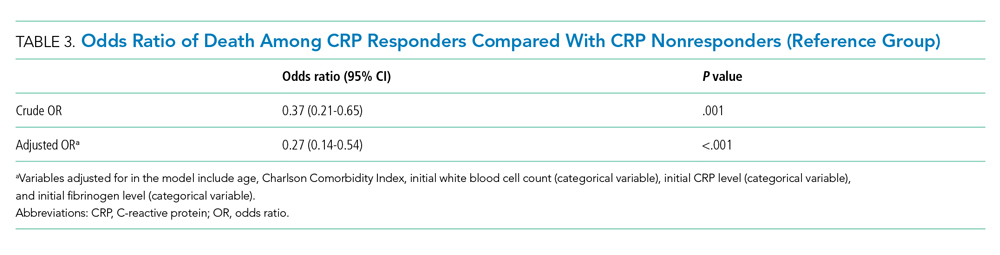
DISCUSSION
In this retrospective study, we found that, on average, patients treated with corticosteroids had a swift and marked reduction in serum CRP. In addition, among patients treated with corticosteroids, those whose CRP was reduced by 50% or more within 72 hours after treatment had a dramatically reduced risk of inpatient mortality compared with the risk among nonresponders. This study contributes to a growing body of evidence that suggests that corticosteroids may be an efficacious treatment to reduce adverse events in patients with COVID-19 who have evidence of high levels of inflammation as measured by CRP level.3,4,12,13
It remains unclear whether CRP is simply a biomarker of disease activity or if it plays a role in mediating inflammation. While CRP is commonly understood to be an acute phase reactant, it has been suggested that, after undergoing proteolysis, it functions as a chemoattractant for monocytes.14 In addition, it is now known that the inflammatory CD14+/CD16+ monocytes that express high levels of IL-6 are key drivers of the cytokine storm in COVID-19.15 Therefore, it may be possible that the high levels of circulating CRP in patients with cytokine storm recruits monocytes to the lungs, which leads to further lung injury.
Other mechanisms of immune dysregulation that may contribute to lung injury and respiratory failure in COVID-19, such as cytokine-induced T-cell suppression, have been proposed.7,16 The related markers, such as levels of T-cells or specific cytokines, may therefore represent different but related underlying immune mechanisms affecting the clinical course of COVID-19 that may respond to different therapeutic modalities such as direct IL-6 blockade or chemokine receptor blockade, among others that are currently under investigation.17,18
Regardless of the underlying mechanism of immune regulation, our study shows that serial measurement of CRP may serve as an early indicator of response to corticosteroids that correlates with decreased mortality. The association between CRP response and reduced risk of mortality was present in both subgroups, those requiring mechanical ventilation and those who did not. The risk reduction was similar in magnitude to the overall effect but was not statistically significant in either group. Interestingly, our time series analysis demonstrated a rise in CRP around day 6 among patients treated with corticosteroids (notably, most patients were treated for 5 to 7 days). Our post hoc analysis suggests that this may represent a “rebound” in inflammation after discontinuation of corticosteroids. However, the clinical significance of this rebound and whether a longer course of steroids would improve outcomes is not known. Because corticosteroid therapy may be associated with adverse effects in some patients,4 it is possible that CRP nonresponders represent a subset of patients in whom corticosteroids are not effective and for whom alternative therapies should be considered. In one study looking at the usefulness of IL-1 inhibition for severe COVID-19 infection, patients who received IL-1 inhibitor therapy had improved mortality and a significant decrease in CRP concentration as compared with the historical group.19 Finally, it is worth noting that, in one large retrospective study, there was harm associated with corticosteroid therapy in patients with low levels of CRP, and in the RECOVERY trial there was a trend toward harm for patients with no oxygen requirement.3,4 Serial measurement of CRP may further identify the subset of patients in whom corticosteroid therapy might be harmful.
This study has several limitations. First, the retrospective nature of this study is inherently prone to selection bias, and despite the large number of clinical variables accounted for, unmeasured confounders may still exist. This study was also conducted at a single clinical center operating under emergency circumstances at a time during which healthcare resources were limited. Overall in-hospital mortality was high but similar to mortality rates reported at other hospitals in the New York City area during the same months.20 The strengths of this study include a large cohort of COVID-19 patients from New York City, an epicenter of COVID-19, who received corticosteroids.
CONCLUSION
We found that therapy with corticosteroids in patients with COVID-19 is associated with a substantial reduction in CRP levels within 72 hours of therapy, and for those patients in whom CRP levels decrease by 50% or more, there is a significantly lower risk of inpatient mortality. Future studies are needed to validate these findings in other cohorts and to determine if markers other than CRP levels may be predictors of a therapeutic response or if CRP nonresponders would benefit from other targeted therapies.
1. WHO coronavirus disease (COVID-19) dashboard. World Health Organization. Updated February 22, 2021. Accessed February 22, 2021. https://covid19.who.int/
2. COVID Data Tracker: United States COVID-19 Cases and Deaths by State. Centers for Disease Control and Prevention. Updated February 22, 2021. Accessed February 22, 2021. https://covid.cdc.gov/covid-data-tracker/#cases_casesper100klast7days
3. Horby P, Lim WS, Emberson JR, et al; RECOVERY Collaborative Group. Dexamethasone in hospitalized patients with Covid-19 - preliminary report. N Engl J Med. Published online July 17, 2020. https://doi.org/10.1056/NEJMoa2021436
4. Keller MJ, Kitsis EA, Arora S, et al. Effect of systemic glucocorticoids on mortality or mechanical ventilation in patients with COVID-19. J Hosp Med. 2020;15(8);489-493. https://doi.org/10.12788/jhm.3497
5. Tay MZ, Poh CM, Rénia L, MacAry PA, Ng LFP. The trinity of COVID-19: immunity, inflammation and intervention. Nat Rev Immunol. 2020;20(6):363-374. https://doi.org/10.1038/s41577-020-0311-8
6. Goleva E, Hauk PJ, Hall CF, et al. Corticosteroid-resistant asthma is associated with classical antimicrobial activation of airway macrophages. J Allergy Clin Immunol. 2008;122(3):550-559.e3. https://doi.org/10.1016/j.jaci.2008.07.007
7. Giamarellos-Bourboulis EJ, Netea MG, Rovina N. Complex immune dysregulation in COVID-19 patients with severe respiratory failure. Cell Host Microbe. 2020;27(6):992-1000.e3. https://doi.org/10.1016/j.chom.2020.04.009
8. Luna CM. C-reactive protein in pneumonia: let me try again. Chest. 2004;125(4):1192-1195. https://doi.org/10.1378/chest.125.4.1192
9. Montaudié H, Seitz-Polski B, Cornille A, Benzaken S, Lacour JP, Passeron T. Interleukin 6 and high-sensitivity C-reactive protein are potential predictive markers of response to infliximab in hidradenitis suppurativa. J Am Acad Dermatol. 2017;76(1):156-158. https://doi.org/10.1016/j.jaad.2016.08.036
10. Menéndez R, Martínez R, Reyes S, et al. Biomarkers improve mortality prediction by prognostic scales in community-acquired pneumonia. Thorax. 2009;64(7):587-591. https://doi.org/10.1136/thx.2008.105312
11. Rajasekaran S, Kruse K, Kovey K, et al. Therapeutic role of anakinra, an interleukin-1 receptor antagonist, in the management of secondary hemophagocytic lymphohistiocytosis/sepsis/multiple organ dysfunction/macrophage activating syndrome in critically ill children. Pediatr Crit Care Med. 2014;15(5):401-408. https://doi.org/10.1097/pcc.0000000000000078
12. Wang Y, Jiang W, He Q, et al. A retrospective cohort study of methylprednisolone therapy in severe patients with COVID-19 pneumonia. Signal Transduct Target Ther. 2020;5(1):57. https://doi.org/10.1038/s41392-020-0158-2
13. Fadel R, Morrison AR, Vahia A, et al. Early short course corticosteroids in hospitalized patients with COVID-19. Clin Infect Dis. Published online May 19, 2020. https://doi.org/10.1093/cid/ciaa601
14. Robey FA, Ohura K, Futaki S, et al. Proteolysis of human c-reactive protein produces peptides with potent immunomodulating activity. J Biol Chem. 1987;262(15):7053-7057.
15. Zhou Y, Fu B, Zheng X, et al. Pathogenic T cells and inflammatory monocytes incite inflammatory storm in severe COVID-19 patients. Natl Sci Rev. Published online March 13, 2020. https://doi.org/10.1093/nsr/nwaa041
16. Zhang X, Tan Y, Ling Y, et al. Viral and host factors related to the clinical outcome of COVID-19. Nature. 2020;583(7816):437-440. https://doi/10.1038/s41586-020-2355-0(2020).
17. Tocilizumab in COVID-19 Pneumonia (TOCIVID-19). ClinicalTrials.gov identifier: NCT04317092. Updated October 22, 2020. Accessed October 22, 2020. https://www.clinicaltrials.gov/ct2/show/NCT04317092
18. Study to Evaluate the Efficacy and Safety of Leronlimab for Patients With Severe or Critical Coronavirus Disease 2019 (COVID-19). ClinicalTrials.gov identifier: NCT04347239. Updated October 19, 2020. Accessed November 16, 2020.https://www.clinicaltrials.gov/ct2/show/NCT04347239
19. Huet T, Beaussier H, Voisin O, et al. Anakinra for severe forms of COVID-19: a cohort study. Lancet Rheumatol. 2020;2(7):e393-e400. https://doi.org/10.1016/s2665-9913(20)30164-8
20. Richardson S, Hirsch JS, Narasimhan M, et al. Presenting characteristics, comorbidities and outcomes among 5700 patients hospitalized with COVID-19 in the New York City area. JAMA. 2020;323(20):2052-2059. https://doi.org/10.1001/jama.2020.6775
Confirmed cases of coronavirus disease 2019 (COVID-19) exceed 111 million, and the disease is responsible for approximately 2.4 million deaths worldwide.1 In the United States, 28 million cases of COVID-19 have been reported, and the disease has caused more than 497,000 deaths.2 The clinical presentation of COVID-19 varies widely, with the most severe presentation characterized by acute respiratory distress syndrome and a marked systemic inflammatory response. Corticosteroids have emerged as a potential therapeutic option in a subset of patients. Results from the recently published RECOVERY trial suggest a substantial mortality benefit of dexamethasone in patients who require mechanical ventilation, with a risk reduction of approximately 33%.3 In addition, a recent large retrospective study demonstrated a reduction in the risk of mechanical ventilation or mortality with corticosteroids in a prespecified subset of patients with C-reactive protein (CRP) ≥20 mg/dL, which indicates a high burden of inflammation.4
Some patients with severe COVID-19 experience a positive feedback cascade of proinflammatory cytokines, called the cytokine storm, which can worsen lung injury and, in some cases, progress to vasodilatory shock and multiorgan failure.5 This complication’s cytokine cascade includes interleukin (IL) 6, IL-1β, and CC chemokine ligand 3 (CCL3), which are released by airway macrophages and all of which are heavily implicated in the maladaptive forms of immune response to COVID-19.6,7 The cytokine IL-6 is the primary signal for the production of CRP, and corticosteroids have been shown, both in vitro and in vivo, to reduce the production of IL-6 and other cytokines by airway macrophages.6 Levels of CRP have been shown to correlate with outcomes in COVID-19 and bacterial pneumonias.7,8 Reduction in CRP levels following the institution of therapy, known as CRP response, has been shown to predict outcomes in other inflammatory conditions, such as osteomyelitis, hidradenitis suppurativa, and some cases of bacterial pneumonia.8-10 Similar CRP response in hemophagocytic lymphohistiocytosis, an entity which closely resembles cytokine storm syndrome, has been shown to correlate with disease activity in patients following treatment with an IL-1 antagonist.11 Whether the CRP response as a response to therapeutics in COVID-19 is associated with improved outcomes remains unknown.
Laboratory measurement of CRP levels offers several advantages over the measurement of interleukins. Notably, the half-life of CRP is approximately 19 hours, which is comparable across different age groups and inflammatory conditions because its concentration depends primarily on synthesis in the liver, and a decreased level suggests decreased stimulus for synthesis.8 This makes CRP a useful biomarker to assess response to therapy, in contrast to interleukins, which have short half-lives, are variable in heterogeneous populations, and can be difficult to measure. In addition, CRP measurement is rapid and relatively inexpensive.
We hypothesized that reduction in CRP levels by 50% or more within 72 hours after the initiation of corticosteroids in patients with COVID-19 is associated with reduced inpatient mortality and may be an early indicator of therapeutic response.
METHODS
Study Participants
In this retrospective cohort study, we reviewed all adult patients admitted to Montefiore Medical Center (Bronx, New York) for COVID-19 between March 10, 2020, and May 2, 2020. Patients must have been discharged (alive or deceased) by the administrative censor date (May 2, 2020) to be included. Patients who died within the first 48 hours of admission were excluded to allow sufficient time for corticosteroid treatment to take effect. For inclusion in the corticosteroid group, patients needed to have received at least 2 consecutive days of corticosteroid treatment beginning within the first 48 hours of admission with a total daily dose of 0.5 mg/kg prednisone equivalent or greater. Patients who received treatment-dose corticosteroids later in the hospital course were excluded (Appendix Figure).
Comparison Group and Outcome
We examined trends in CRP levels for patients who received corticosteroids vs trends among patients who did not receive corticosteroids. In addition, among patients who were treated with corticosteroids, we compared the inpatient mortality of those who did have a reduction in CRP level after treatment with inpatient mortality of those who did not have a reduction in CRP level after treatment. First, CRP level trends over time were examined in all patients, and compared between those who received corticosteroid treatment and those who did not. Then, patients who received corticosteroids were categorized based on changes in CRP levels after beginning corticosteroids. The first CRP level obtained during the first 48 hours of admission was used as the initial CRP level. For each patient, the last CRP level within the 72 hours after initiation of treatment was used to calculate the change in CRP level from admission. A patient was considered to be a “CRP responder” if their CRP level decreased by 50% or more within 72 hours after treatment and a “CRP nonresponder” if their CRP level did not drop by at least 50% within 72 hours of treatment. Patients who did not have a CRP level within the initial 48 hours of admission or a subsequent CRP measured in the 72 hours after treatment were considered to have an “undetermined CRP response” and excluded from the mortality analysis.
We observed a rise in CRP starting around day 6 among patients treated with corticosteroids and performed a post hoc analysis to determine if this was due to a selection effect whereby patients staying in the hospital longer had higher CRP levels or represented actual rise. In order to address this, we performed a stratified analysis comparing the trends in CRP levels among patients with a length of stay (LOS) of 7 or more days with trends among those with an LOS less than 7 days.
Statistical Analysis
To characterize differences in patients who received corticosteroids and those who did not, we examined their demographic, clinical characteristics, and admission laboratory values, using chi-square test for categorical variables and Kruskal-Wallis test for continuous variables (Table 1). The change in CRP levels from day 0 (presentation to the hospital) in both groups was plotted in a time-series analysis. For each day in the time series, the 95% CIs for the changes in CRP were computed using the t statistic for the corresponding distribution. The Kruskal-Wallis test was used to assess the significance of differences between groups at 72 hours after initiation of treatment.

After categorizing patients by CRP response, we compared demographic, clinical, and laboratory characteristics of patients who were CRP responsive with those of patients who were not, using the same tests of statistical inference mentioned above. To compare time to inpatient mortality differences between CRP response groups, Kaplan-Meier survival curves were generated and statistical significance determined via log-rank test. Univariable logistic regression was used to estimate the odds ratio of inpatient mortality between comparison groups in an unadjusted analysis. Last, to examine the independent association between CRP response and mortality, we constructed a multivariate model that included variables that were significantly associated with mortality in univariable analysis and considered to be important potential confounders by the authors. Details on variable selection for the model are listed in Appendix Table 1.
Data Collection
Data were directly extracted from our center’s electronic health record system. Data processing and recoding was performed using the Python programming language (version 2.7.17) and data analysis was done using Stata 12 (StataCorp LLC; 2011). This study was approved by the institutional review board of the Albert Einstein College of Medicine.
RESULTS
Corticosteroids vs No Corticosteroids
Between March 10, 2020, and May 2, 2020, a total of 3,382 adult patients were admitted for COVID-19 at Montefiore Medical Center. Of these, 2,707 patients met the study inclusion criteria, and 324 of those received corticosteroid treatment. Their demographic characteristics, comorbidities, and admission lab values are shown in Table 1. Patients who received corticosteroids were older, had higher comorbidity scores, were more likely to have asthma or chronic obstructive pulmonary disease, and were less likely to be full code status, compared with patients who did not receive corticosteroids. Patients who received corticosteroids also had higher initial white blood cell (WBC) and neutrophil counts but lower lymphocyte count. The two groups were comparable in initial creatinine level. Additional patient characteristics and addmission lab values are shown in Appendix Table 2.
Average change in CRP levels by hospital day for those who received corticosteroids and those who did not are shown in Figure 1A. Among patients who received corticosteroid treatment, there was a significant decrease in CRP level at 72 hours of treatment (P < .001). In the post hoc analysis of trends in CRP levels, we found that CRP levels among those treated with corticosteroids started to rise around day 6 after the initial drop. This trend was observed even after removing patients with shorter LOS (<7 days) (Figure 1B). The median durations of corticosteroid therapy were 3 days among patients whose LOS was less than 7 days and 6 days among those whose LOS was 7 days or greater. The rise in CRP level was seen at day 5 and day 7 within each group, respectively. Crude death rate was 41.7% among patients with LOS of less than 7 days and 40.6% in those with LOS of 7 days or greater.

CRP Responders vs Nonresponders
Among the 324 patients who received corticosteroids, 131 (40.4%) were classified as responders, 92 (28.4%) were classified as nonresponders, and 101 (31.2%) were undetermined. Characteristics of CRP responders and CRP nonresponders are shown in Table 2 and Appendix Table 3. CRP responders were more likely to have dementia, higher median admission platelet count, and fibrinogen level compared with CRP nonresponders. Patients whose CRP response was undetermined were excluded from the analysis. Their characteristics are shown in Appendix Table 4.

The observed inpatient mortality rate was 25.2% among CRP responders and 47.8% among CRP nonresponders. This was also demonstrated in the Kaplan-Meier survival curve (Figure 2). The odds of inpatient mortality among CRP responders was strongly and significantly reduced compared with those among nonresponders in an unadjusted analysis (odds ratio [OR], 0.37; 95% CI, 0.21-0.65; P = .001) and after adjustment for demographic and clinical characteristics including age, Charlson Comorbidity Index, initial WBC count, initial CRP level, and initial fibrinogen level (OR, 0.27; 95% CI, 0.14-0.54; P < .001). Details on how variables were operationalized and information on missing data are included in Appendix Table 1.

To explore whether this observed effect differed depending on severity of the respiratory illness, we examined the association between CRP response and mortality in subgroups stratified by intubation status. Within our cohort of 223 patients (92 CRP responders and 131 CRP nonresponders), 166 patients were never intubated, 50 patients were intubated in the first 48 hours, and 7 patients were intubated later on during the admission. The odds ratios for death among CRP responders vs nonresponders were 0.50 (P = .07) among patients never intubated and 0.46 (P = .2) among patients intubated within the initial 48 hours of admission.

DISCUSSION
In this retrospective study, we found that, on average, patients treated with corticosteroids had a swift and marked reduction in serum CRP. In addition, among patients treated with corticosteroids, those whose CRP was reduced by 50% or more within 72 hours after treatment had a dramatically reduced risk of inpatient mortality compared with the risk among nonresponders. This study contributes to a growing body of evidence that suggests that corticosteroids may be an efficacious treatment to reduce adverse events in patients with COVID-19 who have evidence of high levels of inflammation as measured by CRP level.3,4,12,13
It remains unclear whether CRP is simply a biomarker of disease activity or if it plays a role in mediating inflammation. While CRP is commonly understood to be an acute phase reactant, it has been suggested that, after undergoing proteolysis, it functions as a chemoattractant for monocytes.14 In addition, it is now known that the inflammatory CD14+/CD16+ monocytes that express high levels of IL-6 are key drivers of the cytokine storm in COVID-19.15 Therefore, it may be possible that the high levels of circulating CRP in patients with cytokine storm recruits monocytes to the lungs, which leads to further lung injury.
Other mechanisms of immune dysregulation that may contribute to lung injury and respiratory failure in COVID-19, such as cytokine-induced T-cell suppression, have been proposed.7,16 The related markers, such as levels of T-cells or specific cytokines, may therefore represent different but related underlying immune mechanisms affecting the clinical course of COVID-19 that may respond to different therapeutic modalities such as direct IL-6 blockade or chemokine receptor blockade, among others that are currently under investigation.17,18
Regardless of the underlying mechanism of immune regulation, our study shows that serial measurement of CRP may serve as an early indicator of response to corticosteroids that correlates with decreased mortality. The association between CRP response and reduced risk of mortality was present in both subgroups, those requiring mechanical ventilation and those who did not. The risk reduction was similar in magnitude to the overall effect but was not statistically significant in either group. Interestingly, our time series analysis demonstrated a rise in CRP around day 6 among patients treated with corticosteroids (notably, most patients were treated for 5 to 7 days). Our post hoc analysis suggests that this may represent a “rebound” in inflammation after discontinuation of corticosteroids. However, the clinical significance of this rebound and whether a longer course of steroids would improve outcomes is not known. Because corticosteroid therapy may be associated with adverse effects in some patients,4 it is possible that CRP nonresponders represent a subset of patients in whom corticosteroids are not effective and for whom alternative therapies should be considered. In one study looking at the usefulness of IL-1 inhibition for severe COVID-19 infection, patients who received IL-1 inhibitor therapy had improved mortality and a significant decrease in CRP concentration as compared with the historical group.19 Finally, it is worth noting that, in one large retrospective study, there was harm associated with corticosteroid therapy in patients with low levels of CRP, and in the RECOVERY trial there was a trend toward harm for patients with no oxygen requirement.3,4 Serial measurement of CRP may further identify the subset of patients in whom corticosteroid therapy might be harmful.
This study has several limitations. First, the retrospective nature of this study is inherently prone to selection bias, and despite the large number of clinical variables accounted for, unmeasured confounders may still exist. This study was also conducted at a single clinical center operating under emergency circumstances at a time during which healthcare resources were limited. Overall in-hospital mortality was high but similar to mortality rates reported at other hospitals in the New York City area during the same months.20 The strengths of this study include a large cohort of COVID-19 patients from New York City, an epicenter of COVID-19, who received corticosteroids.
CONCLUSION
We found that therapy with corticosteroids in patients with COVID-19 is associated with a substantial reduction in CRP levels within 72 hours of therapy, and for those patients in whom CRP levels decrease by 50% or more, there is a significantly lower risk of inpatient mortality. Future studies are needed to validate these findings in other cohorts and to determine if markers other than CRP levels may be predictors of a therapeutic response or if CRP nonresponders would benefit from other targeted therapies.
Confirmed cases of coronavirus disease 2019 (COVID-19) exceed 111 million, and the disease is responsible for approximately 2.4 million deaths worldwide.1 In the United States, 28 million cases of COVID-19 have been reported, and the disease has caused more than 497,000 deaths.2 The clinical presentation of COVID-19 varies widely, with the most severe presentation characterized by acute respiratory distress syndrome and a marked systemic inflammatory response. Corticosteroids have emerged as a potential therapeutic option in a subset of patients. Results from the recently published RECOVERY trial suggest a substantial mortality benefit of dexamethasone in patients who require mechanical ventilation, with a risk reduction of approximately 33%.3 In addition, a recent large retrospective study demonstrated a reduction in the risk of mechanical ventilation or mortality with corticosteroids in a prespecified subset of patients with C-reactive protein (CRP) ≥20 mg/dL, which indicates a high burden of inflammation.4
Some patients with severe COVID-19 experience a positive feedback cascade of proinflammatory cytokines, called the cytokine storm, which can worsen lung injury and, in some cases, progress to vasodilatory shock and multiorgan failure.5 This complication’s cytokine cascade includes interleukin (IL) 6, IL-1β, and CC chemokine ligand 3 (CCL3), which are released by airway macrophages and all of which are heavily implicated in the maladaptive forms of immune response to COVID-19.6,7 The cytokine IL-6 is the primary signal for the production of CRP, and corticosteroids have been shown, both in vitro and in vivo, to reduce the production of IL-6 and other cytokines by airway macrophages.6 Levels of CRP have been shown to correlate with outcomes in COVID-19 and bacterial pneumonias.7,8 Reduction in CRP levels following the institution of therapy, known as CRP response, has been shown to predict outcomes in other inflammatory conditions, such as osteomyelitis, hidradenitis suppurativa, and some cases of bacterial pneumonia.8-10 Similar CRP response in hemophagocytic lymphohistiocytosis, an entity which closely resembles cytokine storm syndrome, has been shown to correlate with disease activity in patients following treatment with an IL-1 antagonist.11 Whether the CRP response as a response to therapeutics in COVID-19 is associated with improved outcomes remains unknown.
Laboratory measurement of CRP levels offers several advantages over the measurement of interleukins. Notably, the half-life of CRP is approximately 19 hours, which is comparable across different age groups and inflammatory conditions because its concentration depends primarily on synthesis in the liver, and a decreased level suggests decreased stimulus for synthesis.8 This makes CRP a useful biomarker to assess response to therapy, in contrast to interleukins, which have short half-lives, are variable in heterogeneous populations, and can be difficult to measure. In addition, CRP measurement is rapid and relatively inexpensive.
We hypothesized that reduction in CRP levels by 50% or more within 72 hours after the initiation of corticosteroids in patients with COVID-19 is associated with reduced inpatient mortality and may be an early indicator of therapeutic response.
METHODS
Study Participants
In this retrospective cohort study, we reviewed all adult patients admitted to Montefiore Medical Center (Bronx, New York) for COVID-19 between March 10, 2020, and May 2, 2020. Patients must have been discharged (alive or deceased) by the administrative censor date (May 2, 2020) to be included. Patients who died within the first 48 hours of admission were excluded to allow sufficient time for corticosteroid treatment to take effect. For inclusion in the corticosteroid group, patients needed to have received at least 2 consecutive days of corticosteroid treatment beginning within the first 48 hours of admission with a total daily dose of 0.5 mg/kg prednisone equivalent or greater. Patients who received treatment-dose corticosteroids later in the hospital course were excluded (Appendix Figure).
Comparison Group and Outcome
We examined trends in CRP levels for patients who received corticosteroids vs trends among patients who did not receive corticosteroids. In addition, among patients who were treated with corticosteroids, we compared the inpatient mortality of those who did have a reduction in CRP level after treatment with inpatient mortality of those who did not have a reduction in CRP level after treatment. First, CRP level trends over time were examined in all patients, and compared between those who received corticosteroid treatment and those who did not. Then, patients who received corticosteroids were categorized based on changes in CRP levels after beginning corticosteroids. The first CRP level obtained during the first 48 hours of admission was used as the initial CRP level. For each patient, the last CRP level within the 72 hours after initiation of treatment was used to calculate the change in CRP level from admission. A patient was considered to be a “CRP responder” if their CRP level decreased by 50% or more within 72 hours after treatment and a “CRP nonresponder” if their CRP level did not drop by at least 50% within 72 hours of treatment. Patients who did not have a CRP level within the initial 48 hours of admission or a subsequent CRP measured in the 72 hours after treatment were considered to have an “undetermined CRP response” and excluded from the mortality analysis.
We observed a rise in CRP starting around day 6 among patients treated with corticosteroids and performed a post hoc analysis to determine if this was due to a selection effect whereby patients staying in the hospital longer had higher CRP levels or represented actual rise. In order to address this, we performed a stratified analysis comparing the trends in CRP levels among patients with a length of stay (LOS) of 7 or more days with trends among those with an LOS less than 7 days.
Statistical Analysis
To characterize differences in patients who received corticosteroids and those who did not, we examined their demographic, clinical characteristics, and admission laboratory values, using chi-square test for categorical variables and Kruskal-Wallis test for continuous variables (Table 1). The change in CRP levels from day 0 (presentation to the hospital) in both groups was plotted in a time-series analysis. For each day in the time series, the 95% CIs for the changes in CRP were computed using the t statistic for the corresponding distribution. The Kruskal-Wallis test was used to assess the significance of differences between groups at 72 hours after initiation of treatment.

After categorizing patients by CRP response, we compared demographic, clinical, and laboratory characteristics of patients who were CRP responsive with those of patients who were not, using the same tests of statistical inference mentioned above. To compare time to inpatient mortality differences between CRP response groups, Kaplan-Meier survival curves were generated and statistical significance determined via log-rank test. Univariable logistic regression was used to estimate the odds ratio of inpatient mortality between comparison groups in an unadjusted analysis. Last, to examine the independent association between CRP response and mortality, we constructed a multivariate model that included variables that were significantly associated with mortality in univariable analysis and considered to be important potential confounders by the authors. Details on variable selection for the model are listed in Appendix Table 1.
Data Collection
Data were directly extracted from our center’s electronic health record system. Data processing and recoding was performed using the Python programming language (version 2.7.17) and data analysis was done using Stata 12 (StataCorp LLC; 2011). This study was approved by the institutional review board of the Albert Einstein College of Medicine.
RESULTS
Corticosteroids vs No Corticosteroids
Between March 10, 2020, and May 2, 2020, a total of 3,382 adult patients were admitted for COVID-19 at Montefiore Medical Center. Of these, 2,707 patients met the study inclusion criteria, and 324 of those received corticosteroid treatment. Their demographic characteristics, comorbidities, and admission lab values are shown in Table 1. Patients who received corticosteroids were older, had higher comorbidity scores, were more likely to have asthma or chronic obstructive pulmonary disease, and were less likely to be full code status, compared with patients who did not receive corticosteroids. Patients who received corticosteroids also had higher initial white blood cell (WBC) and neutrophil counts but lower lymphocyte count. The two groups were comparable in initial creatinine level. Additional patient characteristics and addmission lab values are shown in Appendix Table 2.
Average change in CRP levels by hospital day for those who received corticosteroids and those who did not are shown in Figure 1A. Among patients who received corticosteroid treatment, there was a significant decrease in CRP level at 72 hours of treatment (P < .001). In the post hoc analysis of trends in CRP levels, we found that CRP levels among those treated with corticosteroids started to rise around day 6 after the initial drop. This trend was observed even after removing patients with shorter LOS (<7 days) (Figure 1B). The median durations of corticosteroid therapy were 3 days among patients whose LOS was less than 7 days and 6 days among those whose LOS was 7 days or greater. The rise in CRP level was seen at day 5 and day 7 within each group, respectively. Crude death rate was 41.7% among patients with LOS of less than 7 days and 40.6% in those with LOS of 7 days or greater.

CRP Responders vs Nonresponders
Among the 324 patients who received corticosteroids, 131 (40.4%) were classified as responders, 92 (28.4%) were classified as nonresponders, and 101 (31.2%) were undetermined. Characteristics of CRP responders and CRP nonresponders are shown in Table 2 and Appendix Table 3. CRP responders were more likely to have dementia, higher median admission platelet count, and fibrinogen level compared with CRP nonresponders. Patients whose CRP response was undetermined were excluded from the analysis. Their characteristics are shown in Appendix Table 4.

The observed inpatient mortality rate was 25.2% among CRP responders and 47.8% among CRP nonresponders. This was also demonstrated in the Kaplan-Meier survival curve (Figure 2). The odds of inpatient mortality among CRP responders was strongly and significantly reduced compared with those among nonresponders in an unadjusted analysis (odds ratio [OR], 0.37; 95% CI, 0.21-0.65; P = .001) and after adjustment for demographic and clinical characteristics including age, Charlson Comorbidity Index, initial WBC count, initial CRP level, and initial fibrinogen level (OR, 0.27; 95% CI, 0.14-0.54; P < .001). Details on how variables were operationalized and information on missing data are included in Appendix Table 1.

To explore whether this observed effect differed depending on severity of the respiratory illness, we examined the association between CRP response and mortality in subgroups stratified by intubation status. Within our cohort of 223 patients (92 CRP responders and 131 CRP nonresponders), 166 patients were never intubated, 50 patients were intubated in the first 48 hours, and 7 patients were intubated later on during the admission. The odds ratios for death among CRP responders vs nonresponders were 0.50 (P = .07) among patients never intubated and 0.46 (P = .2) among patients intubated within the initial 48 hours of admission.

DISCUSSION
In this retrospective study, we found that, on average, patients treated with corticosteroids had a swift and marked reduction in serum CRP. In addition, among patients treated with corticosteroids, those whose CRP was reduced by 50% or more within 72 hours after treatment had a dramatically reduced risk of inpatient mortality compared with the risk among nonresponders. This study contributes to a growing body of evidence that suggests that corticosteroids may be an efficacious treatment to reduce adverse events in patients with COVID-19 who have evidence of high levels of inflammation as measured by CRP level.3,4,12,13
It remains unclear whether CRP is simply a biomarker of disease activity or if it plays a role in mediating inflammation. While CRP is commonly understood to be an acute phase reactant, it has been suggested that, after undergoing proteolysis, it functions as a chemoattractant for monocytes.14 In addition, it is now known that the inflammatory CD14+/CD16+ monocytes that express high levels of IL-6 are key drivers of the cytokine storm in COVID-19.15 Therefore, it may be possible that the high levels of circulating CRP in patients with cytokine storm recruits monocytes to the lungs, which leads to further lung injury.
Other mechanisms of immune dysregulation that may contribute to lung injury and respiratory failure in COVID-19, such as cytokine-induced T-cell suppression, have been proposed.7,16 The related markers, such as levels of T-cells or specific cytokines, may therefore represent different but related underlying immune mechanisms affecting the clinical course of COVID-19 that may respond to different therapeutic modalities such as direct IL-6 blockade or chemokine receptor blockade, among others that are currently under investigation.17,18
Regardless of the underlying mechanism of immune regulation, our study shows that serial measurement of CRP may serve as an early indicator of response to corticosteroids that correlates with decreased mortality. The association between CRP response and reduced risk of mortality was present in both subgroups, those requiring mechanical ventilation and those who did not. The risk reduction was similar in magnitude to the overall effect but was not statistically significant in either group. Interestingly, our time series analysis demonstrated a rise in CRP around day 6 among patients treated with corticosteroids (notably, most patients were treated for 5 to 7 days). Our post hoc analysis suggests that this may represent a “rebound” in inflammation after discontinuation of corticosteroids. However, the clinical significance of this rebound and whether a longer course of steroids would improve outcomes is not known. Because corticosteroid therapy may be associated with adverse effects in some patients,4 it is possible that CRP nonresponders represent a subset of patients in whom corticosteroids are not effective and for whom alternative therapies should be considered. In one study looking at the usefulness of IL-1 inhibition for severe COVID-19 infection, patients who received IL-1 inhibitor therapy had improved mortality and a significant decrease in CRP concentration as compared with the historical group.19 Finally, it is worth noting that, in one large retrospective study, there was harm associated with corticosteroid therapy in patients with low levels of CRP, and in the RECOVERY trial there was a trend toward harm for patients with no oxygen requirement.3,4 Serial measurement of CRP may further identify the subset of patients in whom corticosteroid therapy might be harmful.
This study has several limitations. First, the retrospective nature of this study is inherently prone to selection bias, and despite the large number of clinical variables accounted for, unmeasured confounders may still exist. This study was also conducted at a single clinical center operating under emergency circumstances at a time during which healthcare resources were limited. Overall in-hospital mortality was high but similar to mortality rates reported at other hospitals in the New York City area during the same months.20 The strengths of this study include a large cohort of COVID-19 patients from New York City, an epicenter of COVID-19, who received corticosteroids.
CONCLUSION
We found that therapy with corticosteroids in patients with COVID-19 is associated with a substantial reduction in CRP levels within 72 hours of therapy, and for those patients in whom CRP levels decrease by 50% or more, there is a significantly lower risk of inpatient mortality. Future studies are needed to validate these findings in other cohorts and to determine if markers other than CRP levels may be predictors of a therapeutic response or if CRP nonresponders would benefit from other targeted therapies.
1. WHO coronavirus disease (COVID-19) dashboard. World Health Organization. Updated February 22, 2021. Accessed February 22, 2021. https://covid19.who.int/
2. COVID Data Tracker: United States COVID-19 Cases and Deaths by State. Centers for Disease Control and Prevention. Updated February 22, 2021. Accessed February 22, 2021. https://covid.cdc.gov/covid-data-tracker/#cases_casesper100klast7days
3. Horby P, Lim WS, Emberson JR, et al; RECOVERY Collaborative Group. Dexamethasone in hospitalized patients with Covid-19 - preliminary report. N Engl J Med. Published online July 17, 2020. https://doi.org/10.1056/NEJMoa2021436
4. Keller MJ, Kitsis EA, Arora S, et al. Effect of systemic glucocorticoids on mortality or mechanical ventilation in patients with COVID-19. J Hosp Med. 2020;15(8);489-493. https://doi.org/10.12788/jhm.3497
5. Tay MZ, Poh CM, Rénia L, MacAry PA, Ng LFP. The trinity of COVID-19: immunity, inflammation and intervention. Nat Rev Immunol. 2020;20(6):363-374. https://doi.org/10.1038/s41577-020-0311-8
6. Goleva E, Hauk PJ, Hall CF, et al. Corticosteroid-resistant asthma is associated with classical antimicrobial activation of airway macrophages. J Allergy Clin Immunol. 2008;122(3):550-559.e3. https://doi.org/10.1016/j.jaci.2008.07.007
7. Giamarellos-Bourboulis EJ, Netea MG, Rovina N. Complex immune dysregulation in COVID-19 patients with severe respiratory failure. Cell Host Microbe. 2020;27(6):992-1000.e3. https://doi.org/10.1016/j.chom.2020.04.009
8. Luna CM. C-reactive protein in pneumonia: let me try again. Chest. 2004;125(4):1192-1195. https://doi.org/10.1378/chest.125.4.1192
9. Montaudié H, Seitz-Polski B, Cornille A, Benzaken S, Lacour JP, Passeron T. Interleukin 6 and high-sensitivity C-reactive protein are potential predictive markers of response to infliximab in hidradenitis suppurativa. J Am Acad Dermatol. 2017;76(1):156-158. https://doi.org/10.1016/j.jaad.2016.08.036
10. Menéndez R, Martínez R, Reyes S, et al. Biomarkers improve mortality prediction by prognostic scales in community-acquired pneumonia. Thorax. 2009;64(7):587-591. https://doi.org/10.1136/thx.2008.105312
11. Rajasekaran S, Kruse K, Kovey K, et al. Therapeutic role of anakinra, an interleukin-1 receptor antagonist, in the management of secondary hemophagocytic lymphohistiocytosis/sepsis/multiple organ dysfunction/macrophage activating syndrome in critically ill children. Pediatr Crit Care Med. 2014;15(5):401-408. https://doi.org/10.1097/pcc.0000000000000078
12. Wang Y, Jiang W, He Q, et al. A retrospective cohort study of methylprednisolone therapy in severe patients with COVID-19 pneumonia. Signal Transduct Target Ther. 2020;5(1):57. https://doi.org/10.1038/s41392-020-0158-2
13. Fadel R, Morrison AR, Vahia A, et al. Early short course corticosteroids in hospitalized patients with COVID-19. Clin Infect Dis. Published online May 19, 2020. https://doi.org/10.1093/cid/ciaa601
14. Robey FA, Ohura K, Futaki S, et al. Proteolysis of human c-reactive protein produces peptides with potent immunomodulating activity. J Biol Chem. 1987;262(15):7053-7057.
15. Zhou Y, Fu B, Zheng X, et al. Pathogenic T cells and inflammatory monocytes incite inflammatory storm in severe COVID-19 patients. Natl Sci Rev. Published online March 13, 2020. https://doi.org/10.1093/nsr/nwaa041
16. Zhang X, Tan Y, Ling Y, et al. Viral and host factors related to the clinical outcome of COVID-19. Nature. 2020;583(7816):437-440. https://doi/10.1038/s41586-020-2355-0(2020).
17. Tocilizumab in COVID-19 Pneumonia (TOCIVID-19). ClinicalTrials.gov identifier: NCT04317092. Updated October 22, 2020. Accessed October 22, 2020. https://www.clinicaltrials.gov/ct2/show/NCT04317092
18. Study to Evaluate the Efficacy and Safety of Leronlimab for Patients With Severe or Critical Coronavirus Disease 2019 (COVID-19). ClinicalTrials.gov identifier: NCT04347239. Updated October 19, 2020. Accessed November 16, 2020.https://www.clinicaltrials.gov/ct2/show/NCT04347239
19. Huet T, Beaussier H, Voisin O, et al. Anakinra for severe forms of COVID-19: a cohort study. Lancet Rheumatol. 2020;2(7):e393-e400. https://doi.org/10.1016/s2665-9913(20)30164-8
20. Richardson S, Hirsch JS, Narasimhan M, et al. Presenting characteristics, comorbidities and outcomes among 5700 patients hospitalized with COVID-19 in the New York City area. JAMA. 2020;323(20):2052-2059. https://doi.org/10.1001/jama.2020.6775
1. WHO coronavirus disease (COVID-19) dashboard. World Health Organization. Updated February 22, 2021. Accessed February 22, 2021. https://covid19.who.int/
2. COVID Data Tracker: United States COVID-19 Cases and Deaths by State. Centers for Disease Control and Prevention. Updated February 22, 2021. Accessed February 22, 2021. https://covid.cdc.gov/covid-data-tracker/#cases_casesper100klast7days
3. Horby P, Lim WS, Emberson JR, et al; RECOVERY Collaborative Group. Dexamethasone in hospitalized patients with Covid-19 - preliminary report. N Engl J Med. Published online July 17, 2020. https://doi.org/10.1056/NEJMoa2021436
4. Keller MJ, Kitsis EA, Arora S, et al. Effect of systemic glucocorticoids on mortality or mechanical ventilation in patients with COVID-19. J Hosp Med. 2020;15(8);489-493. https://doi.org/10.12788/jhm.3497
5. Tay MZ, Poh CM, Rénia L, MacAry PA, Ng LFP. The trinity of COVID-19: immunity, inflammation and intervention. Nat Rev Immunol. 2020;20(6):363-374. https://doi.org/10.1038/s41577-020-0311-8
6. Goleva E, Hauk PJ, Hall CF, et al. Corticosteroid-resistant asthma is associated with classical antimicrobial activation of airway macrophages. J Allergy Clin Immunol. 2008;122(3):550-559.e3. https://doi.org/10.1016/j.jaci.2008.07.007
7. Giamarellos-Bourboulis EJ, Netea MG, Rovina N. Complex immune dysregulation in COVID-19 patients with severe respiratory failure. Cell Host Microbe. 2020;27(6):992-1000.e3. https://doi.org/10.1016/j.chom.2020.04.009
8. Luna CM. C-reactive protein in pneumonia: let me try again. Chest. 2004;125(4):1192-1195. https://doi.org/10.1378/chest.125.4.1192
9. Montaudié H, Seitz-Polski B, Cornille A, Benzaken S, Lacour JP, Passeron T. Interleukin 6 and high-sensitivity C-reactive protein are potential predictive markers of response to infliximab in hidradenitis suppurativa. J Am Acad Dermatol. 2017;76(1):156-158. https://doi.org/10.1016/j.jaad.2016.08.036
10. Menéndez R, Martínez R, Reyes S, et al. Biomarkers improve mortality prediction by prognostic scales in community-acquired pneumonia. Thorax. 2009;64(7):587-591. https://doi.org/10.1136/thx.2008.105312
11. Rajasekaran S, Kruse K, Kovey K, et al. Therapeutic role of anakinra, an interleukin-1 receptor antagonist, in the management of secondary hemophagocytic lymphohistiocytosis/sepsis/multiple organ dysfunction/macrophage activating syndrome in critically ill children. Pediatr Crit Care Med. 2014;15(5):401-408. https://doi.org/10.1097/pcc.0000000000000078
12. Wang Y, Jiang W, He Q, et al. A retrospective cohort study of methylprednisolone therapy in severe patients with COVID-19 pneumonia. Signal Transduct Target Ther. 2020;5(1):57. https://doi.org/10.1038/s41392-020-0158-2
13. Fadel R, Morrison AR, Vahia A, et al. Early short course corticosteroids in hospitalized patients with COVID-19. Clin Infect Dis. Published online May 19, 2020. https://doi.org/10.1093/cid/ciaa601
14. Robey FA, Ohura K, Futaki S, et al. Proteolysis of human c-reactive protein produces peptides with potent immunomodulating activity. J Biol Chem. 1987;262(15):7053-7057.
15. Zhou Y, Fu B, Zheng X, et al. Pathogenic T cells and inflammatory monocytes incite inflammatory storm in severe COVID-19 patients. Natl Sci Rev. Published online March 13, 2020. https://doi.org/10.1093/nsr/nwaa041
16. Zhang X, Tan Y, Ling Y, et al. Viral and host factors related to the clinical outcome of COVID-19. Nature. 2020;583(7816):437-440. https://doi/10.1038/s41586-020-2355-0(2020).
17. Tocilizumab in COVID-19 Pneumonia (TOCIVID-19). ClinicalTrials.gov identifier: NCT04317092. Updated October 22, 2020. Accessed October 22, 2020. https://www.clinicaltrials.gov/ct2/show/NCT04317092
18. Study to Evaluate the Efficacy and Safety of Leronlimab for Patients With Severe or Critical Coronavirus Disease 2019 (COVID-19). ClinicalTrials.gov identifier: NCT04347239. Updated October 19, 2020. Accessed November 16, 2020.https://www.clinicaltrials.gov/ct2/show/NCT04347239
19. Huet T, Beaussier H, Voisin O, et al. Anakinra for severe forms of COVID-19: a cohort study. Lancet Rheumatol. 2020;2(7):e393-e400. https://doi.org/10.1016/s2665-9913(20)30164-8
20. Richardson S, Hirsch JS, Narasimhan M, et al. Presenting characteristics, comorbidities and outcomes among 5700 patients hospitalized with COVID-19 in the New York City area. JAMA. 2020;323(20):2052-2059. https://doi.org/10.1001/jama.2020.6775
© 2021 Society of Hospital Medicine
Advancing Diversity, Equity, and Inclusion in Hospital Medicine
Studies continue to demonstrate persistent gaps in equity for women and underrepresented minorities (URMs)1 throughout nearly all aspects of academic medicine, including rank,2-4 tenure,5 authorship,6,7 funding opportunities,8,9 awards,10 speakership,11 leadership,12,13 and salaries.2,14,15
In this article, we report DEI efforts within our division, focusing on the development of our strategic plan and specific outcomes related to compensation, recruitment, and policies.
METHODS
Our Division’s Framework to DEI—“It Takes a Village”
Our Division of Hospital Medicine (DHM), previously within the Division of General Internal Medicine, was founded in October 2017. The DHM at the University of Colorado Hospital (UCH) is composed of 100 faculty members (70 physicians and 30 advanced-practice providers; 58% women and 42% men). In 2018, we implemented a stepwise approach to critically assess DEI within our group and to build a strategic plan to address the issues.

As a new division, we sought stakeholder feedback from division members. All faculty within the division were invited to attend a meeting in which issues related to DEI were discussed. A literature review that spanned both medical and nonmedical fields was also completed. Search terms included salary equity, gender equity, diverse teams, diversity recruitment and retention, diversifying leadership, and diverse speakers. Salaries, internally funded time, and other processes, such as recruitment, promotion, and hiring for leadership positions, were evaluated during the first year we became a division.
TThrough this work, and with stakeholder engagement, we developed a divisional strategic plan to address DEI globally. Our strategic plan included developing a DEI director role to assist with overseeing DEI efforts. We have highlighted the various methods utilized for each component (Figure 1). This work occurred from October 2017 to December 2018.
Our institutional structures
Using best practices from both medical and nonmedical fields, we developed evidence-based approaches to
Compensation: transparent and consistent approaches based upon benchmarking with a framework of equal pay for equal work and similar advanced training/academic rank. In conjunction with efforts within the School of Medicine (SOM), Department of Medicine (DOM), and the UCH, our division sought to study salaries across DHM faculty members. We had an open call for faculty to participate in a newly developed DHM Compensation Committee, with the intent of rigorously examining our compensation practices and goals. Through faculty feedback and committee work, salary equity was defined as equal pay (ie, base salary for one clinical full-time equivalent [FTE]) for equal work based on academic rank and/or years of practice/advanced training. We also compared DHM salaries to regional academic hospital medicine groups and concluded that DHM salaries were lower than local and national benchmarks. This information was used to create a two-phase approach to increasing salaries for all individuals below the American Association of Medical Colleges (AAMC) benchmarks33 for academic hospitalists. We also developed a stipend system for external roles that came with additional compensation and roles within our own division that came with additional pay (ie, nocturnist). Phase 1 focused on those whose salaries were furthest away from and below benchmark, and phase 2 targeted all remaining individuals below benchmark.
A similar review of FTEs (based on required number of shifts for a full-time hospitalist) tied to our internal DHM leadership positions was completed by the division head and director of DEI. Specifically, the mission for each of our internally funded roles, job descriptions, and responsibilities was reviewed to ensure equity in funding.
Recruitment and advancement: processes to ensure equity and diversity in recruitment, tracking, and reporting, working to eliminate/mitigate bias. In collaboration with members of the AAMC Group on Women in Medicine and Science (GWIMS) and coauthors from various institutions, we developed toolkits and checklists aimed at achieving equity and diversity within candidate pools and on major committees, including, but not limited to, search and promotion committees.32 Additionally, a checklist was developed to help recruit more diverse speakers, including women and URMs, for local, regional, and national conferences.
Policies: evidence-based approaches, tracking and reporting, standardized approaches to eliminate/mitigate bias, embracing nontraditional paths. In partnership with our departmental efforts, members of our team led data collection and reporting for salary benchmarking, leadership roles, and committee membership. This included developing surveys and reporting templates that can be used to identify disparities and inform future efforts. We worked to ensure that we have faculty representing our field at the department and SOM levels. Specifically, we made sure to nominate division members during open calls for departmental and schoolwide committees, including the promotions committee.
Our People
The faculty and staff within our division have been instrumental in moving efforts forward in the following important areas.
Leadership: develop the position of director of DEI as well as leadership structures to support and increase DEI. One of the first steps in our strategic plan was creating a director of DEI leadership role (Appendix Figure 2). The director is responsible for researching, applying, and promoting a broad scope of DEI initiatives and best practices within the DHM, DOM, and SOM (in collaboration with their leaders), including recruitment, retention, and promotion of medical students, residents, and faculty; educational program development; health disparities research; and community-engaged scholarship.
Support: develop family leave policies/develop flexible work policies. Several members of our division worked on departmental committees and served in leadership roles on staff and faculty council. Estimated costs were assessed. Through collective efforts of department leadership and division head support, the department approved parental leave to employees following the birth of an employee’s child or the placement of a child with an employee in connection with adoption or permanent foster care.
Mentorship/sponsorship: enhance faculty advancement programs/develop pipeline and trainings/collaborate with student groups and organizations/invest in all of our people. Faculty across our divisional sites have held important roles in developing pipeline programs for undergraduate students bound for health professions, as well as programs developed specifically for medical students and internal medicine residents. This includes two programs, the CU Hospitalist Scholars Program (CUHSP) and Leadership Education for Aspiring Doctors (LEAD), in which undergraduate students have the opportunity to round with hospital medicine teams, work on quality-improvement projects, and receive extensive mentorship and advising from a diverse faculty team. Additionally, our faculty advancement team within the DHM has grown and been restructured to include more defined goals and to ensure each faculty member has at least one mentor in their area of interest.
Supportive: lactation space and support/diverse space options/inclusive and diverse environments. We worked closely with hospital leadership to advocate for adequately equipped lactation spaces, including equipment such as pumps, refrigerators, and computer workstations.
Measures
Our measures focused on (1) development and implementation of our DEI strategic plan, including new policies, processes, and practices related to key components of the DEI program; and (2) assessment of specific DEI programs, including pre-post salary data disparities based on rank and pre-post disparities for protected time for similar roles.
Analysis
Through rapid PDSA cycles, we evaluated salary equity, equity in leadership allotment, and committee membership. We have developed a tracking board to track progress of the multiple projects in the strategic plan.
RESULTS
Strategic Plan Development and Tracking
From October 2017 to December 2018, we developed a robust strategic plan and stepwise approach to DEI (Figure 1 and Figure 2). The director of DEI position was developed (see Appendix Figure 2 for job description) to help oversee these efforts. Figure 3 highlights the specific efforts and the progress made on implementation (ie, high-level dashboard or “tracking board”). While outcomes are still pending in the areas of recruitment and advancement and environment, we have made measurable improvements in compensation, as outlined in the following section.

Compensation
One year after the salary-equity interventions, all of our physician faculty’s salaries were at the goal benchmark (Table), and differences in salary for those in similar years of rank were nearly eliminated. Similarly, after implementing an internally consistent approach to assigning FTE for new and established positions within the division (ie, those that fall within the purview of the division), all faculty in similar types of roles had similar amounts of protected time.
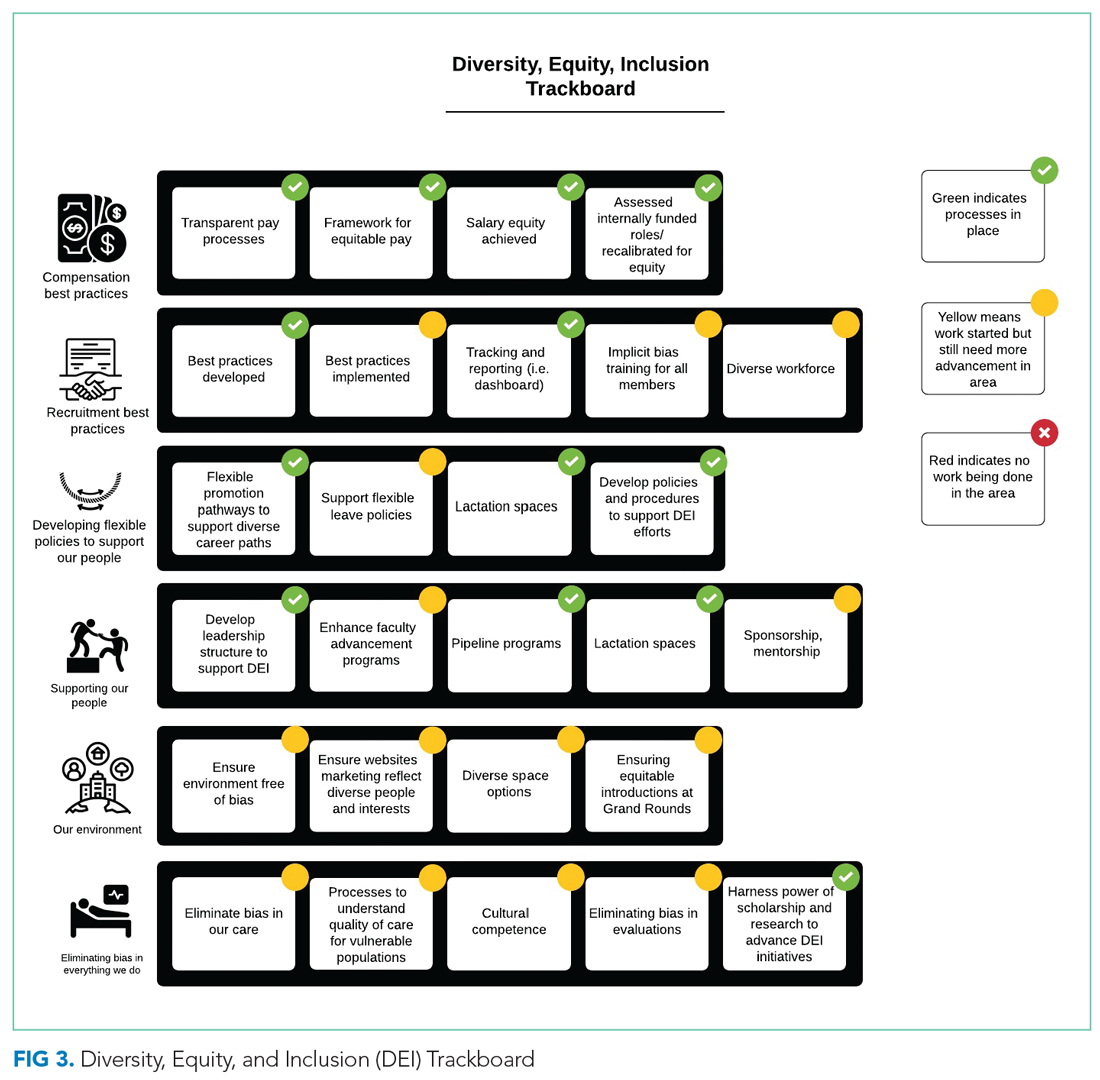
Recruitment and Advancement
Toolkits32 and committee recommendations have been incorporated into division goals, though some aspects are still in implementation phases, as division-wide implicit bias training was delayed secondary to the COVID-19 pandemic. Key goals include: (1) implicit bias training for all members of major committees; (2) aiming for a goal of at least 40% representation of women and 40% URMs on committees; (3) having a diversity expert serve on each committee in order to identify and discuss any potential bias in the search and candidate-selection processes; and (4) careful tracking of diversity metrics in regard to diversity of candidates at each step of the interview and selection process.
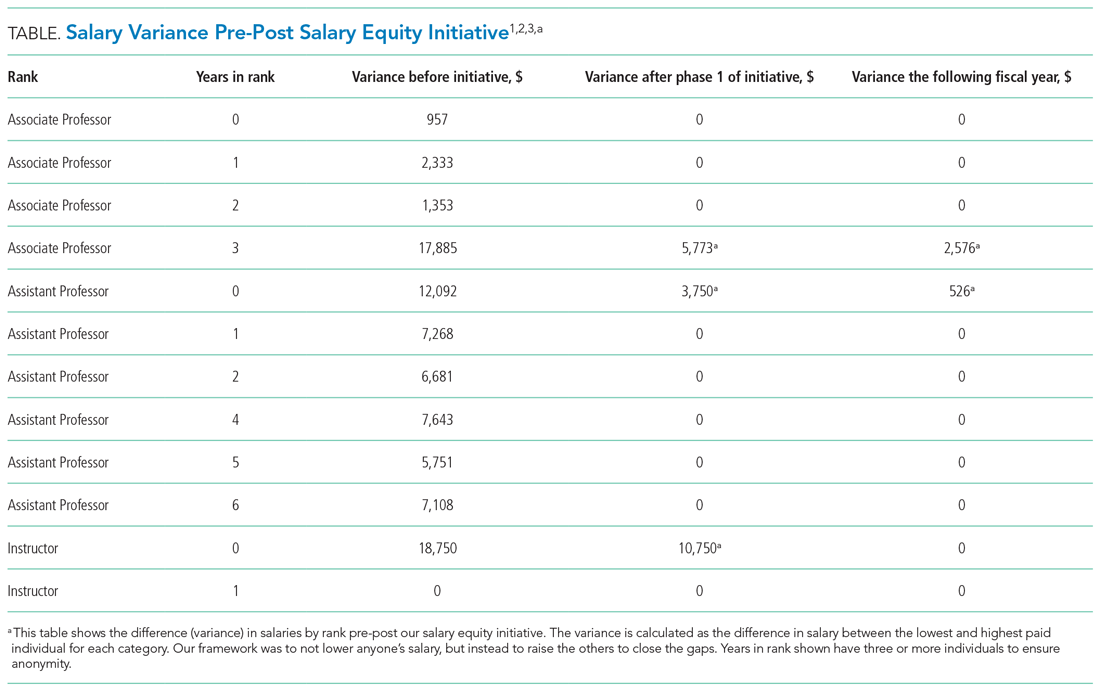
Surveys and reporting templates for equity on committees and leadership positions have been developed and deployed. Data dashboards for our division have been developed as well (for compensation, leadership, and committee membership). A divisional dashboard to report recruitment efforts is in progress.
We have successfully nominated several faculty members to the SOM promotions committee and departmental committees during open calls for these positions. At the division level, we have also adapted internal policies to ensure promotion occurs on time and offers alternative pathways for faculty that may primarily focus on clinical pathways. All faculty who have gone up for promotion thus far have been successfully promoted in their desired pathway.
Environment
We successfully advocated and achieved adequately equipped lactation spaces, including equipment such as pumps, refrigerators, and computer workstations. This achievement was possible because of our hospital partners. Our efforts helped us acquire sufficient space and facilities such that nursing mothers can pump and still be able to answer phones, enter orders, and document visits.
Our team members conducted environmental scans and raised concerns when the environment was not inclusive, such as conference rooms with portraits of leadership that do not show diversity. The all-male pictures were removed from one frequently used departmental conference room, which will eventually house a diverse group of pictures and achievements.
We aim to eliminate bias by offering implicit bias training for our faculty. While this is presently required for those who serve on committees, in leadership positions, or those involved in recruitment and interviewing for the DOM, our goal is to eventually provide this training to all faculty and staff in the division. We have also incorporated DEI topics into our educational conferences for faculty, including sessions on recognizing bias in medicine, how to be an upstander/ally, and the impact of race and racism on medicine.
DISCUSSION
The important findings of this work are: (1) that successes in DEI can be achieved with strategic planning and stakeholder engagement; (2) through simple modification of processes, we can improve equity in compensation and FTE allotted to leadership; (3) though it takes time, diversity recruitment can be improved using sound, sustainable, evidence-based processes; (4) this work is time-intensive and challenging, requiring ongoing efforts to improve, modify, and enhance current efforts and future successes.
We have certainly made some progress with DEI initiatives within our division and have also learned a great deal from this experience. First, change is difficult for all parties involved, including those leading change and those affected by the changes. We purposely made an effort to facilitate discussions with all of the DHM faculty and staff to ensure that everyone felt included in this work and that everyone’s voice was heard. This was exemplified by inviting all faculty members to a feedback session in which we discussed DEI within our division and areas that we wanted to improve on. Early on, we were able to define what diversity, equity, and inclusion meant to us as a division and then use these definitions to develop tangible goals for all the areas of highest importance to the group.
By increasing faculty presence on key committees, such as the promotions committee, we now have faculty members who are well versed in promotions processes. We are fortunate to have a promotions process that supports faculty advancement for faculty with diverse interests that spans from supporting highly clinical faculty, clinician educators, as well as more traditional researchers.34 By having hospitalists serve in these roles, we help to add to the diverse perspectives on these committees, including emphasizing the scholarship that is associated with quality improvement, as well as DEI efforts which can often be viewed as service as opposed to scholarship.
Clear communication and transparency were key to all of our DEI initiatives. We had monthly updates on our DEI efforts during business meetings and also held impromptu meetings (also known as flash mobs35) to answer questions and discuss concerns in real time. As with all DEI work, it is important to know where you are starting (having accurate data and a clear understanding of the data) and be able to communicate that data to the group. For example, using AAMC salary benchmarking33 as well as other benchmarks allowed us to accurately calculate variance among salaries and identify the appropriate goal salary for each of our faculty members. Likewise, by completing an in-depth inventory on the work being done by all of our faculty in leadership roles, we were able to standardize the compensation/FTE for each of these roles. Tracking these changes over time, via the use of dashboards in our case, allows for real-time measurements and accountability for all of those involved. Our end goal will be to have all of these initiatives feed into one large dashboard.
Collaborating with leadership and stakeholders in the DOM, SOM, and hospital helped to make our DEI initiatives successful. Much too often, we work in silos when it comes to DEI work. However, we tend to have similar goals and can achieve much more if we work together. Collaboration with multiple stakeholders allowed for wider dissemination and resulted in a larger impact to the campus and community at large. This has been exemplified by the committee composition guidance that has been utilized by the DOM, as well as implementation of campus-wide policies, specifically the parental leave policy, which our faculty members played an important role in creating. Likewise, it is important to look outside of our institutions and work with other hospital medicine groups around the country who are interested in promoting DEI.
We still have much work ahead of us. We are continuing to measure outcomes status postimplementation of the toolkit and checklists being used for diversity recruitment and committee composition. Additionally, we are actively working on several initiatives, including:
- Instituting implicit bias training for all of our faculty
- Partnering with national leaders and our hospital systems to develop zero-tolerance policies regarding abusive behaviors (verbal, physical, and other), racism, and sexism in the hospital and other work settings
- Development of specific recruitment strategies as a means of diversifying our healthcare workforce (of note, based on a 2020 survey of our faculty, in which there was a 70% response rate, 8.5% of our faculty identified as URMs)
- Completion of a diversity dashboard to track our progress in all of these efforts over time
- Development of a more robust pipeline to promotion and leadership for our URM faculty
This study has several strengths. Many of the plans and strategies described here can be used to guide others interested in implementing this work. Figure 2 provides a stepwise
approach to addressing DEI in hospital medicine groups and divisions. We conducted this work at a large academic medical center, and while it may not be generalizable, it does offer some ideas for others to consider in their own work to advance DEI at their institutions. There are also several limitations to this work. Eliminating salary inequities with our approach did take resources. We took advantage of already lower salaries and the need to increase salaries closer to benchmark and paired this effort with our DEI efforts to achieve salary equity. This required partnerships with the department and hospital. Efforts to advance DEI also take a lot of time and effort, and thus commitment from the division, department, and institution as a whole is key. While we have outcomes for our efforts related to salary equity, recruitment efforts should be realized over time, as currently it is too early to tell. We have highlighted the efforts that have been put in place at this time.
CONCLUSION
Using a systematic evidence-based approach with key stakeholder involvement, a division-wide DEI strategy was developed and implemented. While this work is still ongoing, short-term wins are possible, in particular around salary equity and development of policies and structures to promote DEI.
1. Underrepresented racial and ethnic groups. National Institutes of Health website. Accessed December 26, 2020. https://extramural-diversity.nih.gov/diversity-matters/underrepresented-groups
2. Ash AS, Carr PL, Goldstein R, Friedman RH. Compensation and advancement of women in academic medicine: is there equity? Ann Intern Med. 2004;141(3):205-212. https://doi.org/10.7326/0003-4819-141-3-200408030-00009
3. Jena AB, Khullar D, Ho O, Olenski AR, Blumenthal DM. Sex differences in academic rank in US medical schools in 2014. JAMA. 2015;314(11):1149-1158. https://doi.org/10.1001/jama.2015.10680
4. Fang D, Moy E, Colburn L, Hurley J. Racial and ethnic disparities in faculty promotion in academic medicine. JAMA. 2000;284(9):1085-1092. https://doi.org/10.1001/jama.284.9.1085
5. Baptiste D, Fecher AM, Dolejs SC, et al. Gender differences in academic surgery, work-life balance, and satisfaction. J Surg Res. 2017;218:99-107. https://doi.org/10.1016/j.jss.2017.05.075
6. Hart KL, Perlis RH. Trends in proportion of women as authors of medical journal articles, 2008-2018. JAMA Intern Med. 2019;179:1285-1287. https://doi.org/10.1001/jamainternmed.2019.0907
7. Thomas EG, Jayabalasingham B, Collins T, Geertzen J, Bui C, Dominici F. Gender disparities in invited commentary authorship in 2459 medical journals. JAMA Netw Open. 2019;2(10):e1913682. https://doi.org/10.1001/jamanetworkopen.2019.13682
8. Hechtman LA, Moore NP, Schulkey CE, et al. NIH funding longevity by gender. Proc Natl Acad Sci U S A. 2018;115(31):7943-7948. https://doi.org/10.1073/pnas.1800615115
9. Sege R, Nykiel-Bub L, Selk S. Sex differences in institutional support for junior biomedical researchers. JAMA. 2015;314(11):1175-1177. https://doi.org/10.1001/jama.2015.8517
10. Silver JK, Slocum CS, Bank AM, et al. Where are the women? The underrepresentation of women physicians among recognition award recipients from medical specialty societies. PM R. 2017;9(8):804-815. https://doi.org/10.1016/j.pmrj.2017.06.001
11. Ruzycki SM, Fletcher S, Earp M, Bharwani A, Lithgow KC. Trends in the proportion of female speakers at medical conferences in the United States and in Canada, 2007 to 2017. JAMA Netw Open. 2019;2(4):e192103. https://doi.org/10.1001/jamanetworkopen.2019.2103
12. Carr PL, Raj A, Kaplan SE, Terrin N, Breeze JL, Freund KM. Gender differences in academic medicine: retention, rank, and leadership comparisons from the National Faculty Survey. Acad Med. 2018;93(11):1694-1699. https://doi.org/10.1097/ACM.0000000000002146
13. Carr PL, Gunn C, Raj A, Kaplan S, Freund KM. Recruitment, promotion, and retention of women in academic medicine: how institutions are addressing gender disparities. Womens Health Issues. 2017;27(3):374-381. https://doi.org/10.1016/j.whi.2016.11.003
14. Jena AB, Olenski AR, Blumenthal DM. Sex differences in physician salary in US public medical schools. JAMA Intern Med. 2016;176(9):1294-1304. https://doi.org/10.1001/jamainternmed.2016.3284
15. Lo Sasso AT, Richards MR, Chou CF, Gerber SE. The $16,819 pay gap for newly trained physicians: the unexplained trend of men earning more than women. Health Aff (Millwood). 2011;30(2):193-201. https://doi.org/10.1377/hlthaff.2010.0597
16. Wachter RM, Goldman L. The emerging role of “hospitalists” in the American health care system. N Engl J Med. 1996;335(7):514-517. https://doi.org/10.1056/NEJM199608153350713
17. Weaver AC, Wetterneck TB, Whelan CT, Hinami K. A matter of priorities? Exploring the persistent gender pay gap in hospital medicine. J Hosp Med. 2015;10(8):486-490. https://doi.org/10.1002/jhm.2400
18. Burden M, Frank MG, Keniston A, et al. Gender disparities in leadership and scholarly productivity of academic hospitalists. J Hosp Med. 2015;10(8):481-485. https://doi.org/10.1002/jhm.2340
19. Northcutt N, Papp S, Keniston A, et al, Society of Hospital Medicine Diversity, Equity and Inclusion Special Interest Group. SPEAKers at the National Society of Hospital Medicine Meeting: a follow-up study of gender equity for conference speakers from 2015 to 2019. The SPEAK UP Study. J Hosp Med. 2020;15(4):228-231. https://doi.org/10.12788/jhm.3401
20. Shah SS, Shaughnessy EE, Spector ND. Leading by example: how medical journals can improve representation in academic medicine. J Hosp Med. 2019;14(7):393. https://doi.org/10.12788/jhm.3247
21. Shah SS, Shaughnessy EE, Spector ND. Promoting gender equity at the Journal of Hospital Medicine [editorial]. J Hosp Med. 2020;15(9):517. https://doi.org/10.12788/jhm.3522
22. Sheehy AM, Kolehmainen C, Carnes M. We specialize in change leadership: a call for hospitalists to lead the quest for workforce gender equity [editorial]. J Hosp Med. 2015;10(8):551-552. https://doi.org/10.1002/jhm.2399
23. Evans MK, Rosenbaum L, Malina D, Morrissey S, Rubin EJ. Diagnosing and treating systemic racism [editorial]. N Engl J Med. 2020;383(3):274-276. https://doi.org/10.1056/NEJMe2021693
24. Rock D, Grant H. Why diverse teams are smarter. Harvard Business Review. Published November 4, 2016. Accessed July 24, 2019. https://hbr.org/2016/11/why-diverse-teams-are-smarter
25. Johnson RL, Saha S, Arbelaez JJ, Beach MC, Cooper LA. Racial and ethnic differences in patient perceptions of bias and cultural competence in health care. J Gen Intern Med. 2004;19(2):101-110. https://doi.org/10.1111/j.1525-1497.2004.30262.x
26. Betancourt JR, Green AR, Carrillo JE, Park ER. Cultural competence and health care disparities: key perspectives and trends. Health Aff (Millwood). 2005;24(2):499-505. https://doi.org/10.1377/hlthaff.24.2.499
27. Acosta D, Ackerman-Barger K. Breaking the silence: time to talk about race and racism [comment]. Acad Med. 2017;92(3):285-288. https://doi.org/10.1097/ACM.0000000000001416
28. Cohen JJ, Gabriel BA, Terrell C. The case for diversity in the health care workforce. Health Aff (Millwood). 2002;21(5):90-102. https://doi.org/10.1377/hlthaff.21.5.90
29. Chang E, Simon M, Dong X. Integrating cultural humility into health care professional education and training. Adv Health Sci Educ Theory Pract. 2012;17(2):269-278. https://doi.org/10.1007/s10459-010-9264-1
30. Foronda C, Baptiste DL, Reinholdt MM, Ousman K. Cultural humility: a concept analysis. J Transcult Nurs. 2016;27(3):210-217. https://doi.org/10.1177/1043659615592677
31. Butkus R, Serchen J, Moyer DV, et al; Health and Public Policy Committee of the American College of Physicians. Achieving gender equity in physician compensation and career advancement: a position paper of the American College of Physicians. Ann Intern Med. 2018;168(10):721-723. https://doi.org/10.7326/M17-3438
32. Burden M, del Pino-Jones A, Shafer M, Sheth S, Rexrode K. GWIMS Equity Recruitment Toolkit. Accessed July 27, 2019. https://www.aamc.org/download/492864/data/equityinrecruitmenttoolkit.pdf
33. AAMC Faculty Salary Report. AAMC website. Accessed September 6, 2020. https://www.aamc.org/data-reports/workforce/report/aamc-faculty-salary-report
34. Promotion process. University of Colorado Anschutz Medical Campus website. Accessed September 7, 2020. https://medschool.cuanschutz.edu/faculty-affairs/for-faculty/promotion-and-tenure/promotion-process
35. Pierce RG, Diaz M, Kneeland P. Optimizing well-being, practice culture, and professional thriving in an era of turbulence. J Hosp Med. 2019;14(2):126-128. https://doi.org/10.12788/jhm.3101
Studies continue to demonstrate persistent gaps in equity for women and underrepresented minorities (URMs)1 throughout nearly all aspects of academic medicine, including rank,2-4 tenure,5 authorship,6,7 funding opportunities,8,9 awards,10 speakership,11 leadership,12,13 and salaries.2,14,15
In this article, we report DEI efforts within our division, focusing on the development of our strategic plan and specific outcomes related to compensation, recruitment, and policies.
METHODS
Our Division’s Framework to DEI—“It Takes a Village”
Our Division of Hospital Medicine (DHM), previously within the Division of General Internal Medicine, was founded in October 2017. The DHM at the University of Colorado Hospital (UCH) is composed of 100 faculty members (70 physicians and 30 advanced-practice providers; 58% women and 42% men). In 2018, we implemented a stepwise approach to critically assess DEI within our group and to build a strategic plan to address the issues.

As a new division, we sought stakeholder feedback from division members. All faculty within the division were invited to attend a meeting in which issues related to DEI were discussed. A literature review that spanned both medical and nonmedical fields was also completed. Search terms included salary equity, gender equity, diverse teams, diversity recruitment and retention, diversifying leadership, and diverse speakers. Salaries, internally funded time, and other processes, such as recruitment, promotion, and hiring for leadership positions, were evaluated during the first year we became a division.
TThrough this work, and with stakeholder engagement, we developed a divisional strategic plan to address DEI globally. Our strategic plan included developing a DEI director role to assist with overseeing DEI efforts. We have highlighted the various methods utilized for each component (Figure 1). This work occurred from October 2017 to December 2018.
Our institutional structures
Using best practices from both medical and nonmedical fields, we developed evidence-based approaches to
Compensation: transparent and consistent approaches based upon benchmarking with a framework of equal pay for equal work and similar advanced training/academic rank. In conjunction with efforts within the School of Medicine (SOM), Department of Medicine (DOM), and the UCH, our division sought to study salaries across DHM faculty members. We had an open call for faculty to participate in a newly developed DHM Compensation Committee, with the intent of rigorously examining our compensation practices and goals. Through faculty feedback and committee work, salary equity was defined as equal pay (ie, base salary for one clinical full-time equivalent [FTE]) for equal work based on academic rank and/or years of practice/advanced training. We also compared DHM salaries to regional academic hospital medicine groups and concluded that DHM salaries were lower than local and national benchmarks. This information was used to create a two-phase approach to increasing salaries for all individuals below the American Association of Medical Colleges (AAMC) benchmarks33 for academic hospitalists. We also developed a stipend system for external roles that came with additional compensation and roles within our own division that came with additional pay (ie, nocturnist). Phase 1 focused on those whose salaries were furthest away from and below benchmark, and phase 2 targeted all remaining individuals below benchmark.
A similar review of FTEs (based on required number of shifts for a full-time hospitalist) tied to our internal DHM leadership positions was completed by the division head and director of DEI. Specifically, the mission for each of our internally funded roles, job descriptions, and responsibilities was reviewed to ensure equity in funding.
Recruitment and advancement: processes to ensure equity and diversity in recruitment, tracking, and reporting, working to eliminate/mitigate bias. In collaboration with members of the AAMC Group on Women in Medicine and Science (GWIMS) and coauthors from various institutions, we developed toolkits and checklists aimed at achieving equity and diversity within candidate pools and on major committees, including, but not limited to, search and promotion committees.32 Additionally, a checklist was developed to help recruit more diverse speakers, including women and URMs, for local, regional, and national conferences.
Policies: evidence-based approaches, tracking and reporting, standardized approaches to eliminate/mitigate bias, embracing nontraditional paths. In partnership with our departmental efforts, members of our team led data collection and reporting for salary benchmarking, leadership roles, and committee membership. This included developing surveys and reporting templates that can be used to identify disparities and inform future efforts. We worked to ensure that we have faculty representing our field at the department and SOM levels. Specifically, we made sure to nominate division members during open calls for departmental and schoolwide committees, including the promotions committee.
Our People
The faculty and staff within our division have been instrumental in moving efforts forward in the following important areas.
Leadership: develop the position of director of DEI as well as leadership structures to support and increase DEI. One of the first steps in our strategic plan was creating a director of DEI leadership role (Appendix Figure 2). The director is responsible for researching, applying, and promoting a broad scope of DEI initiatives and best practices within the DHM, DOM, and SOM (in collaboration with their leaders), including recruitment, retention, and promotion of medical students, residents, and faculty; educational program development; health disparities research; and community-engaged scholarship.
Support: develop family leave policies/develop flexible work policies. Several members of our division worked on departmental committees and served in leadership roles on staff and faculty council. Estimated costs were assessed. Through collective efforts of department leadership and division head support, the department approved parental leave to employees following the birth of an employee’s child or the placement of a child with an employee in connection with adoption or permanent foster care.
Mentorship/sponsorship: enhance faculty advancement programs/develop pipeline and trainings/collaborate with student groups and organizations/invest in all of our people. Faculty across our divisional sites have held important roles in developing pipeline programs for undergraduate students bound for health professions, as well as programs developed specifically for medical students and internal medicine residents. This includes two programs, the CU Hospitalist Scholars Program (CUHSP) and Leadership Education for Aspiring Doctors (LEAD), in which undergraduate students have the opportunity to round with hospital medicine teams, work on quality-improvement projects, and receive extensive mentorship and advising from a diverse faculty team. Additionally, our faculty advancement team within the DHM has grown and been restructured to include more defined goals and to ensure each faculty member has at least one mentor in their area of interest.
Supportive: lactation space and support/diverse space options/inclusive and diverse environments. We worked closely with hospital leadership to advocate for adequately equipped lactation spaces, including equipment such as pumps, refrigerators, and computer workstations.
Measures
Our measures focused on (1) development and implementation of our DEI strategic plan, including new policies, processes, and practices related to key components of the DEI program; and (2) assessment of specific DEI programs, including pre-post salary data disparities based on rank and pre-post disparities for protected time for similar roles.
Analysis
Through rapid PDSA cycles, we evaluated salary equity, equity in leadership allotment, and committee membership. We have developed a tracking board to track progress of the multiple projects in the strategic plan.
RESULTS
Strategic Plan Development and Tracking
From October 2017 to December 2018, we developed a robust strategic plan and stepwise approach to DEI (Figure 1 and Figure 2). The director of DEI position was developed (see Appendix Figure 2 for job description) to help oversee these efforts. Figure 3 highlights the specific efforts and the progress made on implementation (ie, high-level dashboard or “tracking board”). While outcomes are still pending in the areas of recruitment and advancement and environment, we have made measurable improvements in compensation, as outlined in the following section.

Compensation
One year after the salary-equity interventions, all of our physician faculty’s salaries were at the goal benchmark (Table), and differences in salary for those in similar years of rank were nearly eliminated. Similarly, after implementing an internally consistent approach to assigning FTE for new and established positions within the division (ie, those that fall within the purview of the division), all faculty in similar types of roles had similar amounts of protected time.

Recruitment and Advancement
Toolkits32 and committee recommendations have been incorporated into division goals, though some aspects are still in implementation phases, as division-wide implicit bias training was delayed secondary to the COVID-19 pandemic. Key goals include: (1) implicit bias training for all members of major committees; (2) aiming for a goal of at least 40% representation of women and 40% URMs on committees; (3) having a diversity expert serve on each committee in order to identify and discuss any potential bias in the search and candidate-selection processes; and (4) careful tracking of diversity metrics in regard to diversity of candidates at each step of the interview and selection process.

Surveys and reporting templates for equity on committees and leadership positions have been developed and deployed. Data dashboards for our division have been developed as well (for compensation, leadership, and committee membership). A divisional dashboard to report recruitment efforts is in progress.
We have successfully nominated several faculty members to the SOM promotions committee and departmental committees during open calls for these positions. At the division level, we have also adapted internal policies to ensure promotion occurs on time and offers alternative pathways for faculty that may primarily focus on clinical pathways. All faculty who have gone up for promotion thus far have been successfully promoted in their desired pathway.
Environment
We successfully advocated and achieved adequately equipped lactation spaces, including equipment such as pumps, refrigerators, and computer workstations. This achievement was possible because of our hospital partners. Our efforts helped us acquire sufficient space and facilities such that nursing mothers can pump and still be able to answer phones, enter orders, and document visits.
Our team members conducted environmental scans and raised concerns when the environment was not inclusive, such as conference rooms with portraits of leadership that do not show diversity. The all-male pictures were removed from one frequently used departmental conference room, which will eventually house a diverse group of pictures and achievements.
We aim to eliminate bias by offering implicit bias training for our faculty. While this is presently required for those who serve on committees, in leadership positions, or those involved in recruitment and interviewing for the DOM, our goal is to eventually provide this training to all faculty and staff in the division. We have also incorporated DEI topics into our educational conferences for faculty, including sessions on recognizing bias in medicine, how to be an upstander/ally, and the impact of race and racism on medicine.
DISCUSSION
The important findings of this work are: (1) that successes in DEI can be achieved with strategic planning and stakeholder engagement; (2) through simple modification of processes, we can improve equity in compensation and FTE allotted to leadership; (3) though it takes time, diversity recruitment can be improved using sound, sustainable, evidence-based processes; (4) this work is time-intensive and challenging, requiring ongoing efforts to improve, modify, and enhance current efforts and future successes.
We have certainly made some progress with DEI initiatives within our division and have also learned a great deal from this experience. First, change is difficult for all parties involved, including those leading change and those affected by the changes. We purposely made an effort to facilitate discussions with all of the DHM faculty and staff to ensure that everyone felt included in this work and that everyone’s voice was heard. This was exemplified by inviting all faculty members to a feedback session in which we discussed DEI within our division and areas that we wanted to improve on. Early on, we were able to define what diversity, equity, and inclusion meant to us as a division and then use these definitions to develop tangible goals for all the areas of highest importance to the group.
By increasing faculty presence on key committees, such as the promotions committee, we now have faculty members who are well versed in promotions processes. We are fortunate to have a promotions process that supports faculty advancement for faculty with diverse interests that spans from supporting highly clinical faculty, clinician educators, as well as more traditional researchers.34 By having hospitalists serve in these roles, we help to add to the diverse perspectives on these committees, including emphasizing the scholarship that is associated with quality improvement, as well as DEI efforts which can often be viewed as service as opposed to scholarship.
Clear communication and transparency were key to all of our DEI initiatives. We had monthly updates on our DEI efforts during business meetings and also held impromptu meetings (also known as flash mobs35) to answer questions and discuss concerns in real time. As with all DEI work, it is important to know where you are starting (having accurate data and a clear understanding of the data) and be able to communicate that data to the group. For example, using AAMC salary benchmarking33 as well as other benchmarks allowed us to accurately calculate variance among salaries and identify the appropriate goal salary for each of our faculty members. Likewise, by completing an in-depth inventory on the work being done by all of our faculty in leadership roles, we were able to standardize the compensation/FTE for each of these roles. Tracking these changes over time, via the use of dashboards in our case, allows for real-time measurements and accountability for all of those involved. Our end goal will be to have all of these initiatives feed into one large dashboard.
Collaborating with leadership and stakeholders in the DOM, SOM, and hospital helped to make our DEI initiatives successful. Much too often, we work in silos when it comes to DEI work. However, we tend to have similar goals and can achieve much more if we work together. Collaboration with multiple stakeholders allowed for wider dissemination and resulted in a larger impact to the campus and community at large. This has been exemplified by the committee composition guidance that has been utilized by the DOM, as well as implementation of campus-wide policies, specifically the parental leave policy, which our faculty members played an important role in creating. Likewise, it is important to look outside of our institutions and work with other hospital medicine groups around the country who are interested in promoting DEI.
We still have much work ahead of us. We are continuing to measure outcomes status postimplementation of the toolkit and checklists being used for diversity recruitment and committee composition. Additionally, we are actively working on several initiatives, including:
- Instituting implicit bias training for all of our faculty
- Partnering with national leaders and our hospital systems to develop zero-tolerance policies regarding abusive behaviors (verbal, physical, and other), racism, and sexism in the hospital and other work settings
- Development of specific recruitment strategies as a means of diversifying our healthcare workforce (of note, based on a 2020 survey of our faculty, in which there was a 70% response rate, 8.5% of our faculty identified as URMs)
- Completion of a diversity dashboard to track our progress in all of these efforts over time
- Development of a more robust pipeline to promotion and leadership for our URM faculty
This study has several strengths. Many of the plans and strategies described here can be used to guide others interested in implementing this work. Figure 2 provides a stepwise
approach to addressing DEI in hospital medicine groups and divisions. We conducted this work at a large academic medical center, and while it may not be generalizable, it does offer some ideas for others to consider in their own work to advance DEI at their institutions. There are also several limitations to this work. Eliminating salary inequities with our approach did take resources. We took advantage of already lower salaries and the need to increase salaries closer to benchmark and paired this effort with our DEI efforts to achieve salary equity. This required partnerships with the department and hospital. Efforts to advance DEI also take a lot of time and effort, and thus commitment from the division, department, and institution as a whole is key. While we have outcomes for our efforts related to salary equity, recruitment efforts should be realized over time, as currently it is too early to tell. We have highlighted the efforts that have been put in place at this time.
CONCLUSION
Using a systematic evidence-based approach with key stakeholder involvement, a division-wide DEI strategy was developed and implemented. While this work is still ongoing, short-term wins are possible, in particular around salary equity and development of policies and structures to promote DEI.
Studies continue to demonstrate persistent gaps in equity for women and underrepresented minorities (URMs)1 throughout nearly all aspects of academic medicine, including rank,2-4 tenure,5 authorship,6,7 funding opportunities,8,9 awards,10 speakership,11 leadership,12,13 and salaries.2,14,15
In this article, we report DEI efforts within our division, focusing on the development of our strategic plan and specific outcomes related to compensation, recruitment, and policies.
METHODS
Our Division’s Framework to DEI—“It Takes a Village”
Our Division of Hospital Medicine (DHM), previously within the Division of General Internal Medicine, was founded in October 2017. The DHM at the University of Colorado Hospital (UCH) is composed of 100 faculty members (70 physicians and 30 advanced-practice providers; 58% women and 42% men). In 2018, we implemented a stepwise approach to critically assess DEI within our group and to build a strategic plan to address the issues.

As a new division, we sought stakeholder feedback from division members. All faculty within the division were invited to attend a meeting in which issues related to DEI were discussed. A literature review that spanned both medical and nonmedical fields was also completed. Search terms included salary equity, gender equity, diverse teams, diversity recruitment and retention, diversifying leadership, and diverse speakers. Salaries, internally funded time, and other processes, such as recruitment, promotion, and hiring for leadership positions, were evaluated during the first year we became a division.
TThrough this work, and with stakeholder engagement, we developed a divisional strategic plan to address DEI globally. Our strategic plan included developing a DEI director role to assist with overseeing DEI efforts. We have highlighted the various methods utilized for each component (Figure 1). This work occurred from October 2017 to December 2018.
Our institutional structures
Using best practices from both medical and nonmedical fields, we developed evidence-based approaches to
Compensation: transparent and consistent approaches based upon benchmarking with a framework of equal pay for equal work and similar advanced training/academic rank. In conjunction with efforts within the School of Medicine (SOM), Department of Medicine (DOM), and the UCH, our division sought to study salaries across DHM faculty members. We had an open call for faculty to participate in a newly developed DHM Compensation Committee, with the intent of rigorously examining our compensation practices and goals. Through faculty feedback and committee work, salary equity was defined as equal pay (ie, base salary for one clinical full-time equivalent [FTE]) for equal work based on academic rank and/or years of practice/advanced training. We also compared DHM salaries to regional academic hospital medicine groups and concluded that DHM salaries were lower than local and national benchmarks. This information was used to create a two-phase approach to increasing salaries for all individuals below the American Association of Medical Colleges (AAMC) benchmarks33 for academic hospitalists. We also developed a stipend system for external roles that came with additional compensation and roles within our own division that came with additional pay (ie, nocturnist). Phase 1 focused on those whose salaries were furthest away from and below benchmark, and phase 2 targeted all remaining individuals below benchmark.
A similar review of FTEs (based on required number of shifts for a full-time hospitalist) tied to our internal DHM leadership positions was completed by the division head and director of DEI. Specifically, the mission for each of our internally funded roles, job descriptions, and responsibilities was reviewed to ensure equity in funding.
Recruitment and advancement: processes to ensure equity and diversity in recruitment, tracking, and reporting, working to eliminate/mitigate bias. In collaboration with members of the AAMC Group on Women in Medicine and Science (GWIMS) and coauthors from various institutions, we developed toolkits and checklists aimed at achieving equity and diversity within candidate pools and on major committees, including, but not limited to, search and promotion committees.32 Additionally, a checklist was developed to help recruit more diverse speakers, including women and URMs, for local, regional, and national conferences.
Policies: evidence-based approaches, tracking and reporting, standardized approaches to eliminate/mitigate bias, embracing nontraditional paths. In partnership with our departmental efforts, members of our team led data collection and reporting for salary benchmarking, leadership roles, and committee membership. This included developing surveys and reporting templates that can be used to identify disparities and inform future efforts. We worked to ensure that we have faculty representing our field at the department and SOM levels. Specifically, we made sure to nominate division members during open calls for departmental and schoolwide committees, including the promotions committee.
Our People
The faculty and staff within our division have been instrumental in moving efforts forward in the following important areas.
Leadership: develop the position of director of DEI as well as leadership structures to support and increase DEI. One of the first steps in our strategic plan was creating a director of DEI leadership role (Appendix Figure 2). The director is responsible for researching, applying, and promoting a broad scope of DEI initiatives and best practices within the DHM, DOM, and SOM (in collaboration with their leaders), including recruitment, retention, and promotion of medical students, residents, and faculty; educational program development; health disparities research; and community-engaged scholarship.
Support: develop family leave policies/develop flexible work policies. Several members of our division worked on departmental committees and served in leadership roles on staff and faculty council. Estimated costs were assessed. Through collective efforts of department leadership and division head support, the department approved parental leave to employees following the birth of an employee’s child or the placement of a child with an employee in connection with adoption or permanent foster care.
Mentorship/sponsorship: enhance faculty advancement programs/develop pipeline and trainings/collaborate with student groups and organizations/invest in all of our people. Faculty across our divisional sites have held important roles in developing pipeline programs for undergraduate students bound for health professions, as well as programs developed specifically for medical students and internal medicine residents. This includes two programs, the CU Hospitalist Scholars Program (CUHSP) and Leadership Education for Aspiring Doctors (LEAD), in which undergraduate students have the opportunity to round with hospital medicine teams, work on quality-improvement projects, and receive extensive mentorship and advising from a diverse faculty team. Additionally, our faculty advancement team within the DHM has grown and been restructured to include more defined goals and to ensure each faculty member has at least one mentor in their area of interest.
Supportive: lactation space and support/diverse space options/inclusive and diverse environments. We worked closely with hospital leadership to advocate for adequately equipped lactation spaces, including equipment such as pumps, refrigerators, and computer workstations.
Measures
Our measures focused on (1) development and implementation of our DEI strategic plan, including new policies, processes, and practices related to key components of the DEI program; and (2) assessment of specific DEI programs, including pre-post salary data disparities based on rank and pre-post disparities for protected time for similar roles.
Analysis
Through rapid PDSA cycles, we evaluated salary equity, equity in leadership allotment, and committee membership. We have developed a tracking board to track progress of the multiple projects in the strategic plan.
RESULTS
Strategic Plan Development and Tracking
From October 2017 to December 2018, we developed a robust strategic plan and stepwise approach to DEI (Figure 1 and Figure 2). The director of DEI position was developed (see Appendix Figure 2 for job description) to help oversee these efforts. Figure 3 highlights the specific efforts and the progress made on implementation (ie, high-level dashboard or “tracking board”). While outcomes are still pending in the areas of recruitment and advancement and environment, we have made measurable improvements in compensation, as outlined in the following section.

Compensation
One year after the salary-equity interventions, all of our physician faculty’s salaries were at the goal benchmark (Table), and differences in salary for those in similar years of rank were nearly eliminated. Similarly, after implementing an internally consistent approach to assigning FTE for new and established positions within the division (ie, those that fall within the purview of the division), all faculty in similar types of roles had similar amounts of protected time.

Recruitment and Advancement
Toolkits32 and committee recommendations have been incorporated into division goals, though some aspects are still in implementation phases, as division-wide implicit bias training was delayed secondary to the COVID-19 pandemic. Key goals include: (1) implicit bias training for all members of major committees; (2) aiming for a goal of at least 40% representation of women and 40% URMs on committees; (3) having a diversity expert serve on each committee in order to identify and discuss any potential bias in the search and candidate-selection processes; and (4) careful tracking of diversity metrics in regard to diversity of candidates at each step of the interview and selection process.

Surveys and reporting templates for equity on committees and leadership positions have been developed and deployed. Data dashboards for our division have been developed as well (for compensation, leadership, and committee membership). A divisional dashboard to report recruitment efforts is in progress.
We have successfully nominated several faculty members to the SOM promotions committee and departmental committees during open calls for these positions. At the division level, we have also adapted internal policies to ensure promotion occurs on time and offers alternative pathways for faculty that may primarily focus on clinical pathways. All faculty who have gone up for promotion thus far have been successfully promoted in their desired pathway.
Environment
We successfully advocated and achieved adequately equipped lactation spaces, including equipment such as pumps, refrigerators, and computer workstations. This achievement was possible because of our hospital partners. Our efforts helped us acquire sufficient space and facilities such that nursing mothers can pump and still be able to answer phones, enter orders, and document visits.
Our team members conducted environmental scans and raised concerns when the environment was not inclusive, such as conference rooms with portraits of leadership that do not show diversity. The all-male pictures were removed from one frequently used departmental conference room, which will eventually house a diverse group of pictures and achievements.
We aim to eliminate bias by offering implicit bias training for our faculty. While this is presently required for those who serve on committees, in leadership positions, or those involved in recruitment and interviewing for the DOM, our goal is to eventually provide this training to all faculty and staff in the division. We have also incorporated DEI topics into our educational conferences for faculty, including sessions on recognizing bias in medicine, how to be an upstander/ally, and the impact of race and racism on medicine.
DISCUSSION
The important findings of this work are: (1) that successes in DEI can be achieved with strategic planning and stakeholder engagement; (2) through simple modification of processes, we can improve equity in compensation and FTE allotted to leadership; (3) though it takes time, diversity recruitment can be improved using sound, sustainable, evidence-based processes; (4) this work is time-intensive and challenging, requiring ongoing efforts to improve, modify, and enhance current efforts and future successes.
We have certainly made some progress with DEI initiatives within our division and have also learned a great deal from this experience. First, change is difficult for all parties involved, including those leading change and those affected by the changes. We purposely made an effort to facilitate discussions with all of the DHM faculty and staff to ensure that everyone felt included in this work and that everyone’s voice was heard. This was exemplified by inviting all faculty members to a feedback session in which we discussed DEI within our division and areas that we wanted to improve on. Early on, we were able to define what diversity, equity, and inclusion meant to us as a division and then use these definitions to develop tangible goals for all the areas of highest importance to the group.
By increasing faculty presence on key committees, such as the promotions committee, we now have faculty members who are well versed in promotions processes. We are fortunate to have a promotions process that supports faculty advancement for faculty with diverse interests that spans from supporting highly clinical faculty, clinician educators, as well as more traditional researchers.34 By having hospitalists serve in these roles, we help to add to the diverse perspectives on these committees, including emphasizing the scholarship that is associated with quality improvement, as well as DEI efforts which can often be viewed as service as opposed to scholarship.
Clear communication and transparency were key to all of our DEI initiatives. We had monthly updates on our DEI efforts during business meetings and also held impromptu meetings (also known as flash mobs35) to answer questions and discuss concerns in real time. As with all DEI work, it is important to know where you are starting (having accurate data and a clear understanding of the data) and be able to communicate that data to the group. For example, using AAMC salary benchmarking33 as well as other benchmarks allowed us to accurately calculate variance among salaries and identify the appropriate goal salary for each of our faculty members. Likewise, by completing an in-depth inventory on the work being done by all of our faculty in leadership roles, we were able to standardize the compensation/FTE for each of these roles. Tracking these changes over time, via the use of dashboards in our case, allows for real-time measurements and accountability for all of those involved. Our end goal will be to have all of these initiatives feed into one large dashboard.
Collaborating with leadership and stakeholders in the DOM, SOM, and hospital helped to make our DEI initiatives successful. Much too often, we work in silos when it comes to DEI work. However, we tend to have similar goals and can achieve much more if we work together. Collaboration with multiple stakeholders allowed for wider dissemination and resulted in a larger impact to the campus and community at large. This has been exemplified by the committee composition guidance that has been utilized by the DOM, as well as implementation of campus-wide policies, specifically the parental leave policy, which our faculty members played an important role in creating. Likewise, it is important to look outside of our institutions and work with other hospital medicine groups around the country who are interested in promoting DEI.
We still have much work ahead of us. We are continuing to measure outcomes status postimplementation of the toolkit and checklists being used for diversity recruitment and committee composition. Additionally, we are actively working on several initiatives, including:
- Instituting implicit bias training for all of our faculty
- Partnering with national leaders and our hospital systems to develop zero-tolerance policies regarding abusive behaviors (verbal, physical, and other), racism, and sexism in the hospital and other work settings
- Development of specific recruitment strategies as a means of diversifying our healthcare workforce (of note, based on a 2020 survey of our faculty, in which there was a 70% response rate, 8.5% of our faculty identified as URMs)
- Completion of a diversity dashboard to track our progress in all of these efforts over time
- Development of a more robust pipeline to promotion and leadership for our URM faculty
This study has several strengths. Many of the plans and strategies described here can be used to guide others interested in implementing this work. Figure 2 provides a stepwise
approach to addressing DEI in hospital medicine groups and divisions. We conducted this work at a large academic medical center, and while it may not be generalizable, it does offer some ideas for others to consider in their own work to advance DEI at their institutions. There are also several limitations to this work. Eliminating salary inequities with our approach did take resources. We took advantage of already lower salaries and the need to increase salaries closer to benchmark and paired this effort with our DEI efforts to achieve salary equity. This required partnerships with the department and hospital. Efforts to advance DEI also take a lot of time and effort, and thus commitment from the division, department, and institution as a whole is key. While we have outcomes for our efforts related to salary equity, recruitment efforts should be realized over time, as currently it is too early to tell. We have highlighted the efforts that have been put in place at this time.
CONCLUSION
Using a systematic evidence-based approach with key stakeholder involvement, a division-wide DEI strategy was developed and implemented. While this work is still ongoing, short-term wins are possible, in particular around salary equity and development of policies and structures to promote DEI.
1. Underrepresented racial and ethnic groups. National Institutes of Health website. Accessed December 26, 2020. https://extramural-diversity.nih.gov/diversity-matters/underrepresented-groups
2. Ash AS, Carr PL, Goldstein R, Friedman RH. Compensation and advancement of women in academic medicine: is there equity? Ann Intern Med. 2004;141(3):205-212. https://doi.org/10.7326/0003-4819-141-3-200408030-00009
3. Jena AB, Khullar D, Ho O, Olenski AR, Blumenthal DM. Sex differences in academic rank in US medical schools in 2014. JAMA. 2015;314(11):1149-1158. https://doi.org/10.1001/jama.2015.10680
4. Fang D, Moy E, Colburn L, Hurley J. Racial and ethnic disparities in faculty promotion in academic medicine. JAMA. 2000;284(9):1085-1092. https://doi.org/10.1001/jama.284.9.1085
5. Baptiste D, Fecher AM, Dolejs SC, et al. Gender differences in academic surgery, work-life balance, and satisfaction. J Surg Res. 2017;218:99-107. https://doi.org/10.1016/j.jss.2017.05.075
6. Hart KL, Perlis RH. Trends in proportion of women as authors of medical journal articles, 2008-2018. JAMA Intern Med. 2019;179:1285-1287. https://doi.org/10.1001/jamainternmed.2019.0907
7. Thomas EG, Jayabalasingham B, Collins T, Geertzen J, Bui C, Dominici F. Gender disparities in invited commentary authorship in 2459 medical journals. JAMA Netw Open. 2019;2(10):e1913682. https://doi.org/10.1001/jamanetworkopen.2019.13682
8. Hechtman LA, Moore NP, Schulkey CE, et al. NIH funding longevity by gender. Proc Natl Acad Sci U S A. 2018;115(31):7943-7948. https://doi.org/10.1073/pnas.1800615115
9. Sege R, Nykiel-Bub L, Selk S. Sex differences in institutional support for junior biomedical researchers. JAMA. 2015;314(11):1175-1177. https://doi.org/10.1001/jama.2015.8517
10. Silver JK, Slocum CS, Bank AM, et al. Where are the women? The underrepresentation of women physicians among recognition award recipients from medical specialty societies. PM R. 2017;9(8):804-815. https://doi.org/10.1016/j.pmrj.2017.06.001
11. Ruzycki SM, Fletcher S, Earp M, Bharwani A, Lithgow KC. Trends in the proportion of female speakers at medical conferences in the United States and in Canada, 2007 to 2017. JAMA Netw Open. 2019;2(4):e192103. https://doi.org/10.1001/jamanetworkopen.2019.2103
12. Carr PL, Raj A, Kaplan SE, Terrin N, Breeze JL, Freund KM. Gender differences in academic medicine: retention, rank, and leadership comparisons from the National Faculty Survey. Acad Med. 2018;93(11):1694-1699. https://doi.org/10.1097/ACM.0000000000002146
13. Carr PL, Gunn C, Raj A, Kaplan S, Freund KM. Recruitment, promotion, and retention of women in academic medicine: how institutions are addressing gender disparities. Womens Health Issues. 2017;27(3):374-381. https://doi.org/10.1016/j.whi.2016.11.003
14. Jena AB, Olenski AR, Blumenthal DM. Sex differences in physician salary in US public medical schools. JAMA Intern Med. 2016;176(9):1294-1304. https://doi.org/10.1001/jamainternmed.2016.3284
15. Lo Sasso AT, Richards MR, Chou CF, Gerber SE. The $16,819 pay gap for newly trained physicians: the unexplained trend of men earning more than women. Health Aff (Millwood). 2011;30(2):193-201. https://doi.org/10.1377/hlthaff.2010.0597
16. Wachter RM, Goldman L. The emerging role of “hospitalists” in the American health care system. N Engl J Med. 1996;335(7):514-517. https://doi.org/10.1056/NEJM199608153350713
17. Weaver AC, Wetterneck TB, Whelan CT, Hinami K. A matter of priorities? Exploring the persistent gender pay gap in hospital medicine. J Hosp Med. 2015;10(8):486-490. https://doi.org/10.1002/jhm.2400
18. Burden M, Frank MG, Keniston A, et al. Gender disparities in leadership and scholarly productivity of academic hospitalists. J Hosp Med. 2015;10(8):481-485. https://doi.org/10.1002/jhm.2340
19. Northcutt N, Papp S, Keniston A, et al, Society of Hospital Medicine Diversity, Equity and Inclusion Special Interest Group. SPEAKers at the National Society of Hospital Medicine Meeting: a follow-up study of gender equity for conference speakers from 2015 to 2019. The SPEAK UP Study. J Hosp Med. 2020;15(4):228-231. https://doi.org/10.12788/jhm.3401
20. Shah SS, Shaughnessy EE, Spector ND. Leading by example: how medical journals can improve representation in academic medicine. J Hosp Med. 2019;14(7):393. https://doi.org/10.12788/jhm.3247
21. Shah SS, Shaughnessy EE, Spector ND. Promoting gender equity at the Journal of Hospital Medicine [editorial]. J Hosp Med. 2020;15(9):517. https://doi.org/10.12788/jhm.3522
22. Sheehy AM, Kolehmainen C, Carnes M. We specialize in change leadership: a call for hospitalists to lead the quest for workforce gender equity [editorial]. J Hosp Med. 2015;10(8):551-552. https://doi.org/10.1002/jhm.2399
23. Evans MK, Rosenbaum L, Malina D, Morrissey S, Rubin EJ. Diagnosing and treating systemic racism [editorial]. N Engl J Med. 2020;383(3):274-276. https://doi.org/10.1056/NEJMe2021693
24. Rock D, Grant H. Why diverse teams are smarter. Harvard Business Review. Published November 4, 2016. Accessed July 24, 2019. https://hbr.org/2016/11/why-diverse-teams-are-smarter
25. Johnson RL, Saha S, Arbelaez JJ, Beach MC, Cooper LA. Racial and ethnic differences in patient perceptions of bias and cultural competence in health care. J Gen Intern Med. 2004;19(2):101-110. https://doi.org/10.1111/j.1525-1497.2004.30262.x
26. Betancourt JR, Green AR, Carrillo JE, Park ER. Cultural competence and health care disparities: key perspectives and trends. Health Aff (Millwood). 2005;24(2):499-505. https://doi.org/10.1377/hlthaff.24.2.499
27. Acosta D, Ackerman-Barger K. Breaking the silence: time to talk about race and racism [comment]. Acad Med. 2017;92(3):285-288. https://doi.org/10.1097/ACM.0000000000001416
28. Cohen JJ, Gabriel BA, Terrell C. The case for diversity in the health care workforce. Health Aff (Millwood). 2002;21(5):90-102. https://doi.org/10.1377/hlthaff.21.5.90
29. Chang E, Simon M, Dong X. Integrating cultural humility into health care professional education and training. Adv Health Sci Educ Theory Pract. 2012;17(2):269-278. https://doi.org/10.1007/s10459-010-9264-1
30. Foronda C, Baptiste DL, Reinholdt MM, Ousman K. Cultural humility: a concept analysis. J Transcult Nurs. 2016;27(3):210-217. https://doi.org/10.1177/1043659615592677
31. Butkus R, Serchen J, Moyer DV, et al; Health and Public Policy Committee of the American College of Physicians. Achieving gender equity in physician compensation and career advancement: a position paper of the American College of Physicians. Ann Intern Med. 2018;168(10):721-723. https://doi.org/10.7326/M17-3438
32. Burden M, del Pino-Jones A, Shafer M, Sheth S, Rexrode K. GWIMS Equity Recruitment Toolkit. Accessed July 27, 2019. https://www.aamc.org/download/492864/data/equityinrecruitmenttoolkit.pdf
33. AAMC Faculty Salary Report. AAMC website. Accessed September 6, 2020. https://www.aamc.org/data-reports/workforce/report/aamc-faculty-salary-report
34. Promotion process. University of Colorado Anschutz Medical Campus website. Accessed September 7, 2020. https://medschool.cuanschutz.edu/faculty-affairs/for-faculty/promotion-and-tenure/promotion-process
35. Pierce RG, Diaz M, Kneeland P. Optimizing well-being, practice culture, and professional thriving in an era of turbulence. J Hosp Med. 2019;14(2):126-128. https://doi.org/10.12788/jhm.3101
1. Underrepresented racial and ethnic groups. National Institutes of Health website. Accessed December 26, 2020. https://extramural-diversity.nih.gov/diversity-matters/underrepresented-groups
2. Ash AS, Carr PL, Goldstein R, Friedman RH. Compensation and advancement of women in academic medicine: is there equity? Ann Intern Med. 2004;141(3):205-212. https://doi.org/10.7326/0003-4819-141-3-200408030-00009
3. Jena AB, Khullar D, Ho O, Olenski AR, Blumenthal DM. Sex differences in academic rank in US medical schools in 2014. JAMA. 2015;314(11):1149-1158. https://doi.org/10.1001/jama.2015.10680
4. Fang D, Moy E, Colburn L, Hurley J. Racial and ethnic disparities in faculty promotion in academic medicine. JAMA. 2000;284(9):1085-1092. https://doi.org/10.1001/jama.284.9.1085
5. Baptiste D, Fecher AM, Dolejs SC, et al. Gender differences in academic surgery, work-life balance, and satisfaction. J Surg Res. 2017;218:99-107. https://doi.org/10.1016/j.jss.2017.05.075
6. Hart KL, Perlis RH. Trends in proportion of women as authors of medical journal articles, 2008-2018. JAMA Intern Med. 2019;179:1285-1287. https://doi.org/10.1001/jamainternmed.2019.0907
7. Thomas EG, Jayabalasingham B, Collins T, Geertzen J, Bui C, Dominici F. Gender disparities in invited commentary authorship in 2459 medical journals. JAMA Netw Open. 2019;2(10):e1913682. https://doi.org/10.1001/jamanetworkopen.2019.13682
8. Hechtman LA, Moore NP, Schulkey CE, et al. NIH funding longevity by gender. Proc Natl Acad Sci U S A. 2018;115(31):7943-7948. https://doi.org/10.1073/pnas.1800615115
9. Sege R, Nykiel-Bub L, Selk S. Sex differences in institutional support for junior biomedical researchers. JAMA. 2015;314(11):1175-1177. https://doi.org/10.1001/jama.2015.8517
10. Silver JK, Slocum CS, Bank AM, et al. Where are the women? The underrepresentation of women physicians among recognition award recipients from medical specialty societies. PM R. 2017;9(8):804-815. https://doi.org/10.1016/j.pmrj.2017.06.001
11. Ruzycki SM, Fletcher S, Earp M, Bharwani A, Lithgow KC. Trends in the proportion of female speakers at medical conferences in the United States and in Canada, 2007 to 2017. JAMA Netw Open. 2019;2(4):e192103. https://doi.org/10.1001/jamanetworkopen.2019.2103
12. Carr PL, Raj A, Kaplan SE, Terrin N, Breeze JL, Freund KM. Gender differences in academic medicine: retention, rank, and leadership comparisons from the National Faculty Survey. Acad Med. 2018;93(11):1694-1699. https://doi.org/10.1097/ACM.0000000000002146
13. Carr PL, Gunn C, Raj A, Kaplan S, Freund KM. Recruitment, promotion, and retention of women in academic medicine: how institutions are addressing gender disparities. Womens Health Issues. 2017;27(3):374-381. https://doi.org/10.1016/j.whi.2016.11.003
14. Jena AB, Olenski AR, Blumenthal DM. Sex differences in physician salary in US public medical schools. JAMA Intern Med. 2016;176(9):1294-1304. https://doi.org/10.1001/jamainternmed.2016.3284
15. Lo Sasso AT, Richards MR, Chou CF, Gerber SE. The $16,819 pay gap for newly trained physicians: the unexplained trend of men earning more than women. Health Aff (Millwood). 2011;30(2):193-201. https://doi.org/10.1377/hlthaff.2010.0597
16. Wachter RM, Goldman L. The emerging role of “hospitalists” in the American health care system. N Engl J Med. 1996;335(7):514-517. https://doi.org/10.1056/NEJM199608153350713
17. Weaver AC, Wetterneck TB, Whelan CT, Hinami K. A matter of priorities? Exploring the persistent gender pay gap in hospital medicine. J Hosp Med. 2015;10(8):486-490. https://doi.org/10.1002/jhm.2400
18. Burden M, Frank MG, Keniston A, et al. Gender disparities in leadership and scholarly productivity of academic hospitalists. J Hosp Med. 2015;10(8):481-485. https://doi.org/10.1002/jhm.2340
19. Northcutt N, Papp S, Keniston A, et al, Society of Hospital Medicine Diversity, Equity and Inclusion Special Interest Group. SPEAKers at the National Society of Hospital Medicine Meeting: a follow-up study of gender equity for conference speakers from 2015 to 2019. The SPEAK UP Study. J Hosp Med. 2020;15(4):228-231. https://doi.org/10.12788/jhm.3401
20. Shah SS, Shaughnessy EE, Spector ND. Leading by example: how medical journals can improve representation in academic medicine. J Hosp Med. 2019;14(7):393. https://doi.org/10.12788/jhm.3247
21. Shah SS, Shaughnessy EE, Spector ND. Promoting gender equity at the Journal of Hospital Medicine [editorial]. J Hosp Med. 2020;15(9):517. https://doi.org/10.12788/jhm.3522
22. Sheehy AM, Kolehmainen C, Carnes M. We specialize in change leadership: a call for hospitalists to lead the quest for workforce gender equity [editorial]. J Hosp Med. 2015;10(8):551-552. https://doi.org/10.1002/jhm.2399
23. Evans MK, Rosenbaum L, Malina D, Morrissey S, Rubin EJ. Diagnosing and treating systemic racism [editorial]. N Engl J Med. 2020;383(3):274-276. https://doi.org/10.1056/NEJMe2021693
24. Rock D, Grant H. Why diverse teams are smarter. Harvard Business Review. Published November 4, 2016. Accessed July 24, 2019. https://hbr.org/2016/11/why-diverse-teams-are-smarter
25. Johnson RL, Saha S, Arbelaez JJ, Beach MC, Cooper LA. Racial and ethnic differences in patient perceptions of bias and cultural competence in health care. J Gen Intern Med. 2004;19(2):101-110. https://doi.org/10.1111/j.1525-1497.2004.30262.x
26. Betancourt JR, Green AR, Carrillo JE, Park ER. Cultural competence and health care disparities: key perspectives and trends. Health Aff (Millwood). 2005;24(2):499-505. https://doi.org/10.1377/hlthaff.24.2.499
27. Acosta D, Ackerman-Barger K. Breaking the silence: time to talk about race and racism [comment]. Acad Med. 2017;92(3):285-288. https://doi.org/10.1097/ACM.0000000000001416
28. Cohen JJ, Gabriel BA, Terrell C. The case for diversity in the health care workforce. Health Aff (Millwood). 2002;21(5):90-102. https://doi.org/10.1377/hlthaff.21.5.90
29. Chang E, Simon M, Dong X. Integrating cultural humility into health care professional education and training. Adv Health Sci Educ Theory Pract. 2012;17(2):269-278. https://doi.org/10.1007/s10459-010-9264-1
30. Foronda C, Baptiste DL, Reinholdt MM, Ousman K. Cultural humility: a concept analysis. J Transcult Nurs. 2016;27(3):210-217. https://doi.org/10.1177/1043659615592677
31. Butkus R, Serchen J, Moyer DV, et al; Health and Public Policy Committee of the American College of Physicians. Achieving gender equity in physician compensation and career advancement: a position paper of the American College of Physicians. Ann Intern Med. 2018;168(10):721-723. https://doi.org/10.7326/M17-3438
32. Burden M, del Pino-Jones A, Shafer M, Sheth S, Rexrode K. GWIMS Equity Recruitment Toolkit. Accessed July 27, 2019. https://www.aamc.org/download/492864/data/equityinrecruitmenttoolkit.pdf
33. AAMC Faculty Salary Report. AAMC website. Accessed September 6, 2020. https://www.aamc.org/data-reports/workforce/report/aamc-faculty-salary-report
34. Promotion process. University of Colorado Anschutz Medical Campus website. Accessed September 7, 2020. https://medschool.cuanschutz.edu/faculty-affairs/for-faculty/promotion-and-tenure/promotion-process
35. Pierce RG, Diaz M, Kneeland P. Optimizing well-being, practice culture, and professional thriving in an era of turbulence. J Hosp Med. 2019;14(2):126-128. https://doi.org/10.12788/jhm.3101
© 2021 Society of Hospital Medicine
Antibiotic Regimens and Associated Outcomes in Children Hospitalized With Staphylococcal Scalded Skin Syndrome
Staphylococcal scalded skin syndrome (SSSS) is an exfoliative toxin-mediated dermatitis that predominantly occurs in young children. Multiple recent reports indicate a rising incidence of this disease.1-4 Recommended treatment for SSSS includes antistaphylococcal antibiotics and supportive care measures.5,6 Elimination or reduction of the toxin-producing Staphylococcus aureus is thought to help limit disease progression and promote recovery. Experts advocate for the use of antibiotics even when there is no apparent focal source of infection, such as an abscess.6,7
Several factors may affect antibiotic selection, including the desire to inhibit toxin production and to target the causative pathogen in a bactericidal fashion. Because SSSS is toxin mediated, clindamycin is often recommended because of its inhibition of toxin synthesis.5,8 The clinical utility of adding other antibiotics to clindamycin for coverage of methicillin-sensitive S aureus (MSSA) or methicillin-resistant S aureus (MRSA) is uncertain. Several studies report MSSA to be the predominant pathogen identified by culture2,9; however, SSSS caused by MRSA has been reported.9-11 Additionally, bactericidal antibiotics (eg, nafcillin) have been considered to hold potential clinical advantage as compared with bacteriostatic antibiotics (eg, clindamycin), even though clinical studies have not clearly demonstrated this advantage in the general population.12,13 Some experts recommend additional MRSA or MSSA coverage (such as vancomycin or nafcillin) in patients with high illness severity or nonresponse to therapy, or in areas where there is high prevalence of staphylococcal resistance to clindamycin.5,7,9,14 Alternatively, for areas with low MRSA prevalence, monotherapy with an anti-MSSA antibiotic is another potential option. No recent studies have compared patient outcomes among antibiotic regimens in children with SSSS.
Knowledge of the outcomes associated with different antibiotic regimens for children hospitalized with SSSS is needed and could be used to improve patient outcomes and potentially promote antibiotic stewardship. In this study, our objectives were to (1) describe antibiotic regimens given to children hospitalized with SSSS, and (2) examine the association of three antibiotic regimens commonly used for SSSS (clindamycin monotherapy, clindamycin plus additional MSSA coverage, and clindamycin plus additional MRSA coverage) with patient outcomes of length of stay (LOS), treatment failure, and cost in a large cohort of children at US children’s hospitals.
METHODS
We conducted a multicenter, retrospective cohort study utilizing data within the Pediatric Health Information System (PHIS) database from July 1, 2011, to June 30, 2016. Thirty-five free-standing tertiary care US children’s hospitals within 24 states were included. The Children’s Hospital Association (Lenexa, Kansas) maintains the PHIS database, which contains de-identified patient information, including diagnoses (with International Classification of Diseases, Ninth and Tenth Revision, Clinical Modification [ICD-9-CM, ICD-10-CM]), demographics, procedures, and daily billing records. Data quality and reliability are confirmed by participating institutions and the Children’s Hospital Association.15 The local institutional review board (IRB) deemed the study exempt from formal IRB review, as patient information was de-identified.
Study Population
We included hospitalized children aged newborn to 18 years with a primary or secondary diagnosis of SSSS (ICD-9, 695.81; ICD-10, L00). Children whose primary presentation and admission were to a PHIS hospital were included; children transferred from another hospital were excluded. The following exclusion criteria were based on previously published methodology.16 Children with complex chronic medical conditions as classified by Feudtner et al17 were excluded, since these children may require a different treatment approach than the general pediatric population. In order to decrease diagnostic ambiguity, we excluded children if an alternative dermatologic diagnosis was recorded as a principal or secondary diagnosis (eg, Stevens-Johnson syndrome or scarlet fever).16 Finally, hospitals with fewer than 10 children with SSSS during the study period were excluded.
Antibiotic Regimen Groups
We used PHIS daily billing codes to determine the antibiotics received by the study population. Children were classified into antibiotic regimen groups based on whether they received specific antibiotic combinations. Antibiotics received on any day during the hospitalization, including in the emergency department (ED), were used to assign patients to regimen groups. Antibiotics were classified into regimen groups based on consensus among study investigators, which included two board-certified pediatric infectious diseases specialists (A.C., R.M.). Antibiotic group definitions are listed in Table 1. Oral and intravenous (IV) therapies were grouped together for clindamycin, cephalexin/cefazolin, and linezolid because of good oral bioavailability in most situations.18 The three most common antistaphylococcal groups were chosen for further analysis: clindamycin alone, clindamycin plus MSSA coverage, and clindamycin plus MRSA coverage. The clindamycin group was defined as children with receipt of oral or IV clindamycin. Children who received clindamycin with additional MSSA coverage, such as cefazolin or nafcillin, were categorized as the clindamycin plus MSSA group. Children who received clindamycin with additional MRSA coverage, such as vancomycin or linezolid, were categorized as the clindamycin plus MRSA group. We chose not to include children who received the above regimens plus other antibiotics with partial antistaphylococcal activity, such as ampicillin, gentamicin, or ceftriaxone, in the clindamycin plus MSSA and clindamycin plus MRSA groups. We excluded these antibiotics to decrease the heterogeneity in the definition of regimen groups and allow a more direct comparison for effectiveness.

Covariates
Covariates included age, sex, ethnicity and/or race, payer type, level of care, illness severity, and region. The variable definitions below are in keeping with a prior study of SSSS.16 Age was categorized as: birth to 59 days, 2 to 11 months, 1 to 4 years (preschool age), 5 to 10 years (school age), and 11 to 18 years (adolescent). We examined infants younger than 60 days separately from older infants because this population may warrant additional treatment considerations. Race and ethnicity were categorized as White (non-Hispanic), African American (non-Hispanic), Hispanic, or other. Payer types included government, private, or other. Level of care was assigned as either intensive care or acute care. Illness severity was assigned using the All Patient Refined Diagnosis Related Group (APR-DRG; 3M Corporation, St. Paul, Minnesota) severity levels.19 In line with a prior study,16 we defined “low illness severity” as the APR-DRG minor (1) classification. The moderate (2), major (3), and extreme (4) classifications were defined as “moderate to high illness severity,” since there were very few classifications of major or extreme (<5%) illness severity. We categorized hospitals into the following US regions: Northeast, Midwest, South, and West.
Outcome Measures
The primary outcome was hospital LOS in days, and secondary outcomes were treatment failure and hospital costs. Hospital LOS was chosen as the primary outcome to represent the time needed for the child to show clinical improvement. Treatment failure was defined as a same-cause 14-day ED revisit or hospital readmission, and these were determined to be same-cause if a diagnosis for SSSS (ICD-9, 695.81; ICD-10, L00) was documented for the return encounter. The 14-day interval for readmission and ED revisit was chosen to measure any relapse of symptoms after completion of antibiotic therapy, similar to a prior study of treatment failure in skin and soft tissue infections.20 Total costs of the hospitalization were estimated from charges using hospital- and year-specific cost-to-charge ratios. Subcategories of cost, including clinical, pharmacy, imaging, laboratory, supply, and other, were also compared among the three groups.
Statistical Analysis
Demographic and clinical characteristics of children were summarized using frequencies and percentages for categorical variables and medians with interquartile ranges (IQRs) for continuous variables. These were compared across antibiotic groups using chi-square and Kruskal–Wallis tests, respectively. In unadjusted analyses, outcomes were compared across antibiotic regimen groups using these same statistical tests. In order to account for patient clustering within hospitals, generalized linear mixed-effects models were used to model outcomes with a random intercept for each hospital. Models were adjusted for SSSS being listed as a principal or secondary diagnosis, race, illness severity, and level of care. We log-transformed LOS and cost data prior to modeling because of the nonnormal distributions for these data. Owing to the inability to measure the number of antibiotic doses, and to reduce the possibility of including children who received few regimen-defined combination antibiotics, a post hoc sensitivity analysis was performed. This analysis used an alternative definition for antibiotic regimen groups, for which children admitted for 2 or more calendar days must have received regimen-specified antibiotics on at least 2 days of the admission. Additionally, outcomes were stratified by low and moderate/high illness severity and compared across the three antibiotic regimen groups. All analyses were performed with SAS (SAS 9.4; SAS Institute, Cary, North Carolina), and P values of less than .05 were considered statistically significant.
RESULTS
Overall, 1,815 hospitalized children with SSSS were identified in the PHIS database, and after application of the exclusion criteria, 1,259 children remained, with 1,247 (99%) receiving antibiotics (Figure). The antibiotic regimens received by these children are described in Table 1. Of these, 828 children (66%) received one of the three most common antistaphylococcal regimens (clindamycin, clindamycin + MSSA, and clindamycin + MRSA) and were included for further analysis.
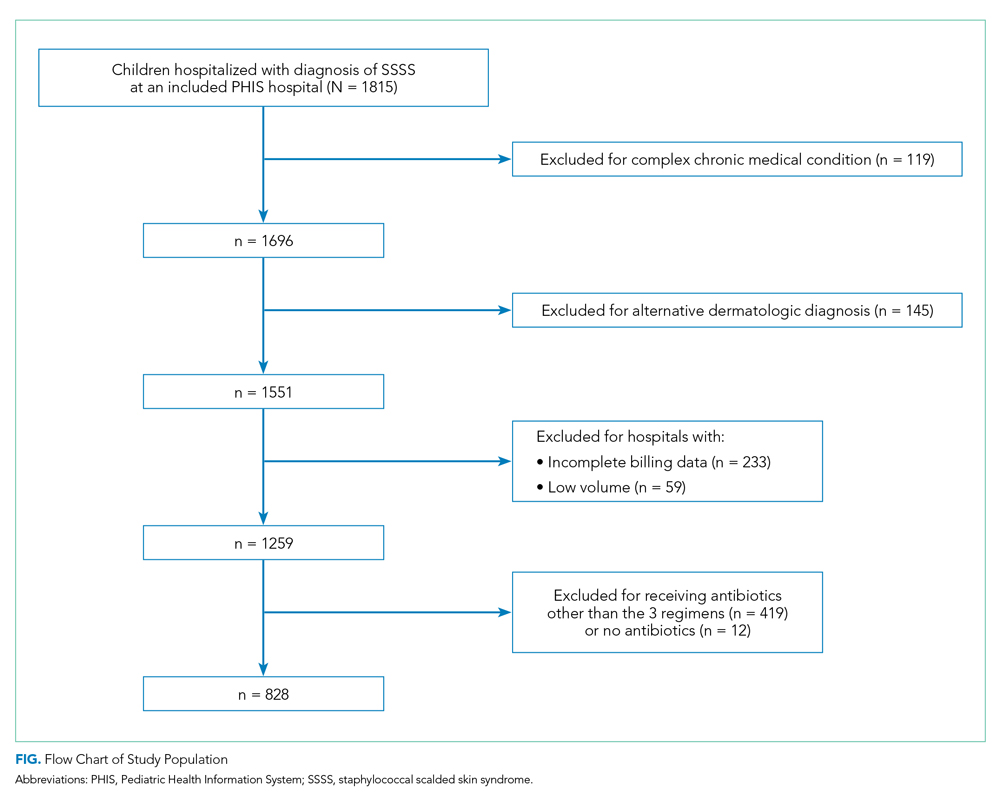
Characteristics of the 828 children are presented in Table 2. Most children (82%) were aged 4 years or younger, and distributions of age, sex, and insurance payer were similar among children receiving the three regimens. Thirty-two percent had moderate to high illness severity, and 3.5% required management in the intensive care setting. Of the three antibiotic regimens, clindamycin monotherapy was most common (47%), followed by clindamycin plus MSSA coverage (33%), and clindamycin plus MRSA coverage (20%). A higher proportion of children in the clindamycin plus MRSA group were African American and were hospitalized in the South. Children receiving clindamycin plus MRSA coverage had higher illness severity (44%) as compared with clindamycin monotherapy (28%) and clindamycin plus MSSA coverage (32%) (P = .001). Additionally, a larger proportion of children treated with clindamycin plus MRSA coverage were managed in the intensive care setting as compared with the clindamycin plus MSSA or clindamycin monotherapy groups.
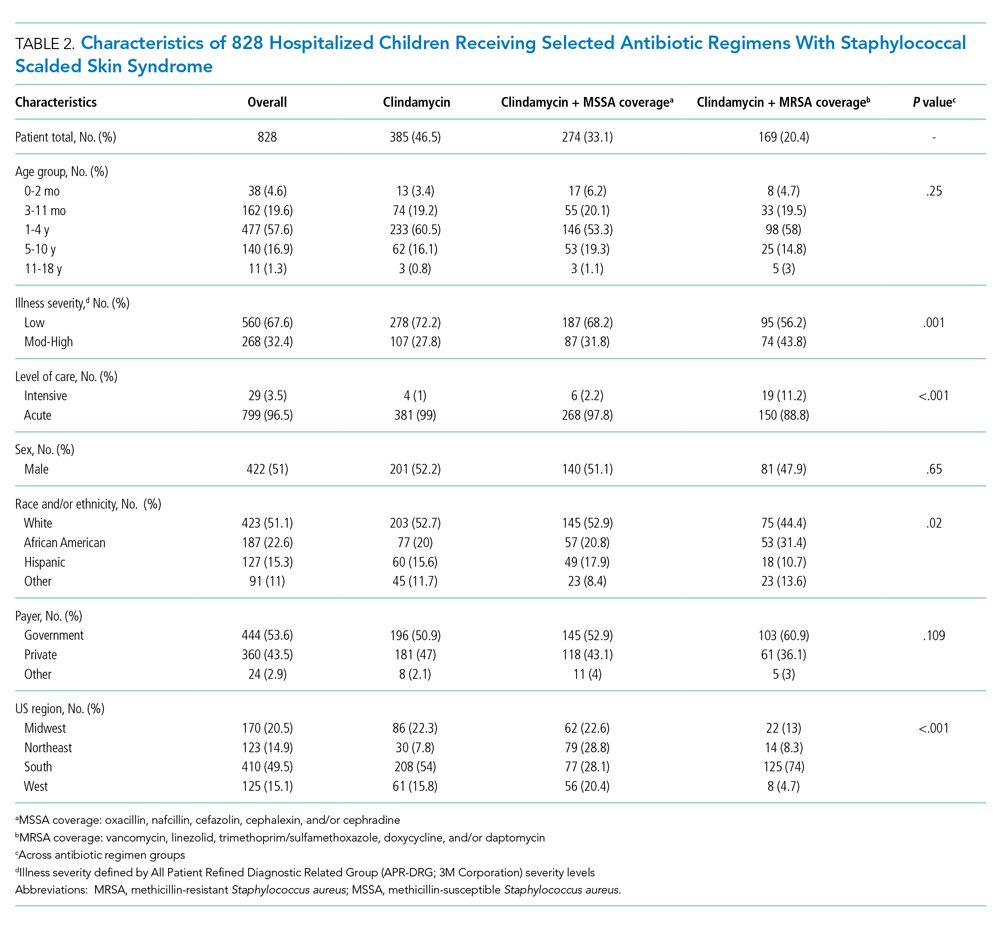
Among the 828 children with SSSS, the median LOS was 2 days (IQR, 2-3), and treatment failure was 1.1% (95% CI, 0.4-1.8). After adjustment for illness severity, race, payer, and region (Table 3), the three antibiotic regimens were not associated with significant differences in LOS or treatment failure. Costs were significantly different among the three antibiotic regimens. Clindamycin plus MRSA coverage was associated with the greatest costs, whereas clindamycin monotherapy was associated with the lowest costs (mean, $5,348 vs $4,839, respectively; P < .001) (Table 3). In a sensitivity analysis using an alternative antibiotic regimen definition, we found results in line with the primary analysis, with no statistically significant differences in LOS (P = .44) or treatment failure (P = .54), but significant differences in cost (P < .001). Additionally, the same findings were present for LOS, treatment failure, and cost when outcomes were stratified by illness severity (Appendix Table). However, significant contributors to the higher cost in the clindamycin plus MRSA group did vary by illness severity stratification. Laboratory, supply, and pharmacy cost categories differed significantly among antibiotic groups for the low illness severity strata, whereas pharmacy was the only significant cost category difference in moderate/high illness severity.
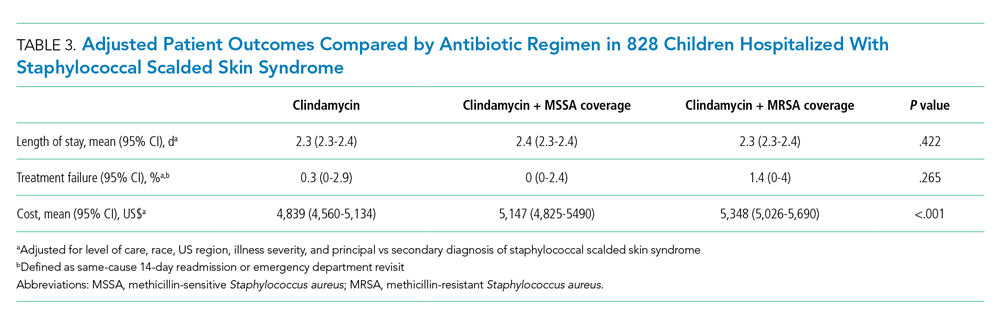
DISCUSSION
Clindamycin monotherapy, clindamycin plus MSSA coverage, and clindamycin plus MRSA coverage are the most commonly administered antistaphylococcal antibiotic regimens for children hospitalized with SSSS at US children’s hospitals. Our multicenter study found that, across these antistaphylococcal antibiotic regimens, there were no associated differences in hospital LOS or treatment failure. However, the antibiotic regimens were associated with significant differences in overall hospital costs. These findings suggest that the use of clindamycin with additional MSSA or MRSA antibiotic coverage for children with SSSS may not be associated with additional clinical benefit, as compared with clindamycin monotherapy, and could potentially be more costly.
Prior literature describing LOS in relation to antibiotic use for children with SSSS is limited. Authors of a recent case series of 21 children in Philadelphia reported approximately 50% of children received clindamycin monotherapy or combination therapy, but patient outcomes such as LOS were not described.9 Clindamycin use and outcomes have been described in smaller studies and case reports of SSSS, which reported positive outcomes such as patient recovery and lack of disease recurrence.2,9,21 A small retrospective, comparative effectiveness study of 30 neonates with SSSS examined beta-lactamase–resistant penicillin use with and without cephalosporins. They found no effect on LOS, but findings were limited by a small sample size.22 Our study cohort included relatively few neonates, and thus our findings may not be applicable to this population subgroup. We chose not to include regimens with third-generation cephalosporins or ampicillin, which may have limited the number of included neonates, because these antibiotics are frequently administered during evaluation for invasive bacterial infections.23 We found a very low occurrence of treatment failure in our study cohort across all three groups, which is consistent with other studies of SSSS that report an overall good prognosis and low recurrence and/or readmission rates.6,16,24 The low prevalence of treatment failure, however, precluded our ability to detect small differences among antibiotic regimen groups that may exist.
We observed that cost differed significantly across antibiotic regimen groups, with lowest cost associated with clindamycin monotherapy in adjusted analysis despite similar LOS. Even with our illness-severity adjustment, there may have been other unmeasured factors resulting in the higher cost associated with the combination groups. Hence, we also examined cost breakdown with a stratified analysis by illness severity. We found that pharmacy costs were significantly different among antibiotic groups in both illness severity strata, whereas those with low illness severity also differed by laboratory and supply costs. Thus, pharmacy cost differences may be the largest driver in the cost differential among groups. Lower cost in the clindamycin monotherapy group is likely due to administration of a single antibiotic. The reason for supply and laboratory cost differences is uncertain, but higher cost in the clindamycin plus MRSA group could possibly be from laboratory testing related to drug monitoring (eg, renal function testing or drug levels). While other studies have reported costs for hospitalized children with SSSS associated with different patient characteristics or diagnostic testing,1,16 to our knowledge, no other studies have reported cost related to antibiotic regimens for SSSS. As healthcare reimbursements shift to value-based models, identifying treatment regimens with equal efficacy but lower cost will become increasingly important. Future studies should also examine other covariates and outcomes, such as oral vs parenteral antibiotic use, use of monitoring laboratories related to antibiotic choice, and adverse drug effects.
Several strengths and additional limitations apply to our study. Our study is one of the few to describe outcomes associated with antibiotic regimens for children with SSSS. With the PHIS database, we were able to include a large number of children with SSSS from children’s hospitals across the United States. Although the PHIS database affords these strengths, there are limitations inherent to administrative data. Children with SSSS were identified by documented ICD-9 and ICD-10 diagnostic codes, which might lead to misclassification. However, misclassification is less likely because only one ICD-9 and ICD-10 code exists for SSSS, and the characteristics of this condition are specific. Also, diagnostic codes for other dermatologic conditions (eg, scarlet fever) were excluded to further reduce the chance of misclassification. A limitation to our use of PHIS billing codes was the inability to confirm the dosage of antibiotics given, the number of doses, or whether antibiotics were prescribed upon discharge. Another limitation is that children whose antibiotic therapy was changed during hospitalization (eg, from clindamycin monotherapy to cefazolin monotherapy) were categorized into the combination groups. However, the sensitivity analysis performed based on a stricter antibiotic group definition (receipt of both antibiotics on at least 2 calendar days) did not alter the outcomes, which is reassuring. We were unable to assess the use of targeted antibiotic therapy because clinical data (eg, microbiology results) were not available. However, this may be less important because some literature suggests that cultures for S aureus are obtained infrequently2 and may be difficult to interpret when obtained,25 since culture growth can represent colonization rather than causative strains. An additional limitation is that administrative data do not include certain clinical outcomes, such as fever duration or degree of skin involvement, which could have differed among the groups. Last, the PHIS database only captures revisits or readmissions to PHIS hospitals, and so we are unable to exclude the possibility of a child being seen at or readmitted to another hospital.
Due to the observational design of this study and potential for incomplete measurement of illness severity, we recommend a future prospective trial with randomization to confirm these findings. One possible reason that LOS did not differ among groups is that the burden of clindamycin-resistant strains in our cohort could be low, and the addition of MSSA or MRSA coverage does not result in a clinically important increase in S aureus coverage. However, pooled pediatric hospital antibiogram data suggest the overall rate of clindamycin resistance is close to 20% in hospitals located in all US regions.26 Limited studies also suggest that MSSA may be the predominant pathogen associated with SSSS.2,9 To address this, future randomized trials could compare the effectiveness of clindamycin monotherapy to MSSA-specific agents like cefazolin or nafcillin. Unfortunately, anti-MSSA monotherapy was not evaluated in our study because very few children received this treatment. Using monotherapy as opposed to multiple antibiotics has the potential to promote antibiotic stewardship for antistaphylococcal antibiotics in the management of SSSS. Reducing unnecessary antibiotic use not only potentially affects antibiotic resistance, but could also benefit patients in reducing possible side effects, cost, and IV catheter complications.27 However, acknowledging our study limitations, our findings should be applied cautiously in clinical settings, in the context of local antibiogram data, individual culture results, and specific patient factors. The local clindamycin resistance rate for both MSSA and MRSA should be considered. Many antibiotics chosen to treat MRSA—such as vancomycin and trimethoprim/sulfamethoxazole—will also have anti-MSSA activity and may have lower local resistance rates than clindamycin. Practitioners may also consider how each antibiotic kills bacteria; for example, beta-lactams rely on bacterial replication, but clindamycin does not. Each factor should influence how empiric treatment, whether monotherapy or combination, is chosen for children with SSSS.
CONCLUSION
In this large, multicenter cohort of hospitalized children with SSSS, we found that the addition of MSSA or MRSA coverage to clindamycin monotherapy was not associated with differences in outcomes of hospital LOS and treatment failure. Furthermore, clindamycin monotherapy was associated with lower overall cost. Prospective randomized studies are needed to confirm these findings and assess whether clindamycin monotherapy, monotherapy with an anti-MSSA antibiotic, or alternative regimens are most effective for treatment of children with SSSS.
1. Staiman A, Hsu DY, Silverberg JI. Epidemiology of staphylococcal scalded skin syndrome in United States children. Br J Dermatol. 2018;178(3):704-708. https://doi.org/10.1111/bjd.16097
2. Hulten KG, Kok M, King KE, Lamberth LB, Kaplan SL. Increasing numbers of staphylococcal scalded skin syndrome cases caused by ST121 in Houston, TX. Pediatr Infect Dis J. 2020;39(1):30-34. https://doi.org/10.1097/INF.0000000000002499
3. Arnold JD, Hoek SN, Kirkorian AY. Epidemiology of staphylococcal scalded skin syndrome in the United States: A cross-sectional study, 2010-2014. J Am Acad Dermatol. 2018;78(2):404-406. https://doi.org/10.1016/j.jaad.2017.09.023
4. Hayward A, Knott F, Petersen I, et al. Increasing hospitalizations and general practice prescriptions for community-onset staphylococcal disease, England. Emerg Infect Dis. 2008;14(5):720-726. https://doi.org/10.3201/eid1405.070153
5. Berk DR, Bayliss SJ. MRSA, staphylococcal scalded skin syndrome, and other cutaneous bacterial emergencies. Pediatr Ann. 2010;39(10):627-633. https://doi.org/10.3928/00904481-20100922-02
6. Ladhani S, Joannou CL, Lochrie DP, Evans RW, Poston SM. Clinical, microbial, and biochemical aspects of the exfoliative toxins causing staphylococcal scalded-skin syndrome. Clin Microbiol Rev. 1999;12(2):224-242.
7. Handler MZ, Schwartz RA. Staphylococcal scalded skin syndrome: diagnosis and management in children and adults. J Eur Acad Dermatol Venereol. 2014;28(11):1418-1423. https://doi.org/10.1111/jdv.12541
8. Hodille E, Rose W, Diep BA, Goutelle S, Lina G, Dumitrescu O. The role of antibiotics in modulating virulence in Staphylococcus aureus. Clin Microbiol Rev. 2017;30(4):887-917. https://doi.org/10.1128/CMR.00120-16
9. Braunstein I, Wanat KA, Abuabara K, McGowan KL, Yan AC, Treat JR. Antibiotic sensitivity and resistance patterns in pediatric staphylococcal scalded skin syndrome. Pediatr Dermatol. 2014;31(3):305-308. https://doi.org/10.1111/pde.12195
10. Yamaguchi T, Yokota Y, Terajima J, et al. Clonal association of Staphylococcus aureus causing bullous impetigo and the emergence of new methicillin-resistant clonal groups in Kansai district in Japan. J Infect Dis. 2002;185(10):1511-1516. https://doi.org/10.1086/340212
11. Noguchi N, Nakaminami H, Nishijima S, Kurokawa I, So H, Sasatsu M. Antimicrobial agent of susceptibilities and antiseptic resistance gene distribution among methicillin-resistant Staphylococcus aureus isolates from patients with impetigo and staphylococcal scalded skin syndrome. J Clin Microbiol. 2006;44(6):2119-2125. https://doi.org/10.1128/JCM.02690-05
12. Pankey GA, Sabath LD. Clinical relevance of bacteriostatic versus bactericidal mechanisms of action in the treatment of Gram-positive bacterial infections. Clin Infect Dis. 2004;38(6):864-870. https://doi.org/10.1086/381972
13. Wald-Dickler N, Holtom P, Spellberg B. Busting the myth of “static vs cidal”: a systemic literature review. Clin Infect Dis. 2018;66(9):1470-1474. https://doi.org/10.1093/cid/cix1127
14. Ladhani S, Joannou CL. Difficulties in diagnosis and management of the staphylococcal scalded skin syndrome. Pediatr Infect Dis J. 2000;19(9):819-821. https://doi.org/10.1097/00006454-200009000-00002
15. Mongelluzzo J, Mohamad Z, Ten Have TR, Shah SS. Corticosteroids and mortality in children with bacterial meningitis. JAMA. 2008;299(17):2048-2055. https://doi.org/10.1001/jama.299.17.2048
16. Neubauer HC, Hall M, Wallace SS, et al. Variation in diagnostic test use and associated outcomes in staphylococcal scalded skin syndrome at children’s hospitals. Hosp Pediatr. 2018;8(9):530-537. https://doi.org/10.1542/hpeds.2018-0032
17. Feudtner C, Feinstein JA, Zhong W, Hall M, Dai D. Pediatric complex chronic conditions classification system version 2: updated for ICD-10 and complex medical technology dependence and transplantation. BMC Pediatr. 2014;14:199. https://doi.org/10.1186/1471-2431-14-199
18. Sauberan JS, Bradley JS. Antimicrobial agents. In: Long SS, ed. Principles and Practice of Pediatric Infectious Diseases. Elsevier; 2018:1499-1531.
19. Sedman AB, Bahl V, Bunting E, et al. Clinical redesign using all patient refined diagnosis related groups. Pediatrics. 2004;114(4):965-969. https://doi.org/10.1542/peds.2004-0650
20. Williams DJ, Cooper WO, Kaltenbach LA, et al. Comparative effectiveness of antibiotic treatment strategies for pediatric skin and soft-tissue infections. Pediatrics. 2011;128(3):e479-487. https://doi.org/10.1542/peds.2010-3681
21. Haasnoot PJ, De Vries A. Staphylococcal scalded skin syndrome in a 4-year-old child: a case report. J Med Case Rep. 2018;12(1):20. https://doi.org/ 10.1186/s13256-017-1533-7
22. Li MY, Hua Y, Wei GH, Qiu L. Staphylococcal scalded skin syndrome in neonates: an 8-year retrospective study in a single institution. Pediatr Dermatol. 2014;31(1):43-47. https://doi.org/10.1111/pde.12114
23. Markham JL, Hall M, Queen MA, et al. Variation in antibiotic selection and clinical outcomes in infants <60 days hospitalized with skin and soft tissue infections. Hosp Pediatr. 2019;9(1):30-38. https://doi.org/10.1542/hpeds.2017-0237
24. Davidson J, Polly S, Hayes PJ, Fisher KR, Talati AJ, Patel T. Recurrent staphylococcal scalded skin syndrome in an extremely low-birth-weight neonate. AJP Rep. 2017;7(2):e134-e137. https://doi.org/10.1055/s-0037-1603971
25. Ladhani S, Robbie S, Chapple DS, Joannou CL, Evans RW. Isolating Staphylococcus aureus from children with suspected Staphylococcal scalded skin syndrome is not clinically useful. Pediatr Infect Dis J. 2003;22(3):284-286.
26. Tamma PD, Robinson GL, Gerber JS, et al. Pediatric antimicrobial susceptibility trends across the United States. Infect Control Hosp Epidemiol. 2013;34(12):1244-1251. https://doi.org/10.1086/673974
27. Unbeck M, Forberg U, Ygge BM, Ehrenberg A, Petzold M, Johansson E. Peripheral venous catheter related complications are common among paediatric and neonatal patients. Acta Paediatr. 2015;104(6):566-574. https://doi.org/10.1111/apa.12963
Staphylococcal scalded skin syndrome (SSSS) is an exfoliative toxin-mediated dermatitis that predominantly occurs in young children. Multiple recent reports indicate a rising incidence of this disease.1-4 Recommended treatment for SSSS includes antistaphylococcal antibiotics and supportive care measures.5,6 Elimination or reduction of the toxin-producing Staphylococcus aureus is thought to help limit disease progression and promote recovery. Experts advocate for the use of antibiotics even when there is no apparent focal source of infection, such as an abscess.6,7
Several factors may affect antibiotic selection, including the desire to inhibit toxin production and to target the causative pathogen in a bactericidal fashion. Because SSSS is toxin mediated, clindamycin is often recommended because of its inhibition of toxin synthesis.5,8 The clinical utility of adding other antibiotics to clindamycin for coverage of methicillin-sensitive S aureus (MSSA) or methicillin-resistant S aureus (MRSA) is uncertain. Several studies report MSSA to be the predominant pathogen identified by culture2,9; however, SSSS caused by MRSA has been reported.9-11 Additionally, bactericidal antibiotics (eg, nafcillin) have been considered to hold potential clinical advantage as compared with bacteriostatic antibiotics (eg, clindamycin), even though clinical studies have not clearly demonstrated this advantage in the general population.12,13 Some experts recommend additional MRSA or MSSA coverage (such as vancomycin or nafcillin) in patients with high illness severity or nonresponse to therapy, or in areas where there is high prevalence of staphylococcal resistance to clindamycin.5,7,9,14 Alternatively, for areas with low MRSA prevalence, monotherapy with an anti-MSSA antibiotic is another potential option. No recent studies have compared patient outcomes among antibiotic regimens in children with SSSS.
Knowledge of the outcomes associated with different antibiotic regimens for children hospitalized with SSSS is needed and could be used to improve patient outcomes and potentially promote antibiotic stewardship. In this study, our objectives were to (1) describe antibiotic regimens given to children hospitalized with SSSS, and (2) examine the association of three antibiotic regimens commonly used for SSSS (clindamycin monotherapy, clindamycin plus additional MSSA coverage, and clindamycin plus additional MRSA coverage) with patient outcomes of length of stay (LOS), treatment failure, and cost in a large cohort of children at US children’s hospitals.
METHODS
We conducted a multicenter, retrospective cohort study utilizing data within the Pediatric Health Information System (PHIS) database from July 1, 2011, to June 30, 2016. Thirty-five free-standing tertiary care US children’s hospitals within 24 states were included. The Children’s Hospital Association (Lenexa, Kansas) maintains the PHIS database, which contains de-identified patient information, including diagnoses (with International Classification of Diseases, Ninth and Tenth Revision, Clinical Modification [ICD-9-CM, ICD-10-CM]), demographics, procedures, and daily billing records. Data quality and reliability are confirmed by participating institutions and the Children’s Hospital Association.15 The local institutional review board (IRB) deemed the study exempt from formal IRB review, as patient information was de-identified.
Study Population
We included hospitalized children aged newborn to 18 years with a primary or secondary diagnosis of SSSS (ICD-9, 695.81; ICD-10, L00). Children whose primary presentation and admission were to a PHIS hospital were included; children transferred from another hospital were excluded. The following exclusion criteria were based on previously published methodology.16 Children with complex chronic medical conditions as classified by Feudtner et al17 were excluded, since these children may require a different treatment approach than the general pediatric population. In order to decrease diagnostic ambiguity, we excluded children if an alternative dermatologic diagnosis was recorded as a principal or secondary diagnosis (eg, Stevens-Johnson syndrome or scarlet fever).16 Finally, hospitals with fewer than 10 children with SSSS during the study period were excluded.
Antibiotic Regimen Groups
We used PHIS daily billing codes to determine the antibiotics received by the study population. Children were classified into antibiotic regimen groups based on whether they received specific antibiotic combinations. Antibiotics received on any day during the hospitalization, including in the emergency department (ED), were used to assign patients to regimen groups. Antibiotics were classified into regimen groups based on consensus among study investigators, which included two board-certified pediatric infectious diseases specialists (A.C., R.M.). Antibiotic group definitions are listed in Table 1. Oral and intravenous (IV) therapies were grouped together for clindamycin, cephalexin/cefazolin, and linezolid because of good oral bioavailability in most situations.18 The three most common antistaphylococcal groups were chosen for further analysis: clindamycin alone, clindamycin plus MSSA coverage, and clindamycin plus MRSA coverage. The clindamycin group was defined as children with receipt of oral or IV clindamycin. Children who received clindamycin with additional MSSA coverage, such as cefazolin or nafcillin, were categorized as the clindamycin plus MSSA group. Children who received clindamycin with additional MRSA coverage, such as vancomycin or linezolid, were categorized as the clindamycin plus MRSA group. We chose not to include children who received the above regimens plus other antibiotics with partial antistaphylococcal activity, such as ampicillin, gentamicin, or ceftriaxone, in the clindamycin plus MSSA and clindamycin plus MRSA groups. We excluded these antibiotics to decrease the heterogeneity in the definition of regimen groups and allow a more direct comparison for effectiveness.

Covariates
Covariates included age, sex, ethnicity and/or race, payer type, level of care, illness severity, and region. The variable definitions below are in keeping with a prior study of SSSS.16 Age was categorized as: birth to 59 days, 2 to 11 months, 1 to 4 years (preschool age), 5 to 10 years (school age), and 11 to 18 years (adolescent). We examined infants younger than 60 days separately from older infants because this population may warrant additional treatment considerations. Race and ethnicity were categorized as White (non-Hispanic), African American (non-Hispanic), Hispanic, or other. Payer types included government, private, or other. Level of care was assigned as either intensive care or acute care. Illness severity was assigned using the All Patient Refined Diagnosis Related Group (APR-DRG; 3M Corporation, St. Paul, Minnesota) severity levels.19 In line with a prior study,16 we defined “low illness severity” as the APR-DRG minor (1) classification. The moderate (2), major (3), and extreme (4) classifications were defined as “moderate to high illness severity,” since there were very few classifications of major or extreme (<5%) illness severity. We categorized hospitals into the following US regions: Northeast, Midwest, South, and West.
Outcome Measures
The primary outcome was hospital LOS in days, and secondary outcomes were treatment failure and hospital costs. Hospital LOS was chosen as the primary outcome to represent the time needed for the child to show clinical improvement. Treatment failure was defined as a same-cause 14-day ED revisit or hospital readmission, and these were determined to be same-cause if a diagnosis for SSSS (ICD-9, 695.81; ICD-10, L00) was documented for the return encounter. The 14-day interval for readmission and ED revisit was chosen to measure any relapse of symptoms after completion of antibiotic therapy, similar to a prior study of treatment failure in skin and soft tissue infections.20 Total costs of the hospitalization were estimated from charges using hospital- and year-specific cost-to-charge ratios. Subcategories of cost, including clinical, pharmacy, imaging, laboratory, supply, and other, were also compared among the three groups.
Statistical Analysis
Demographic and clinical characteristics of children were summarized using frequencies and percentages for categorical variables and medians with interquartile ranges (IQRs) for continuous variables. These were compared across antibiotic groups using chi-square and Kruskal–Wallis tests, respectively. In unadjusted analyses, outcomes were compared across antibiotic regimen groups using these same statistical tests. In order to account for patient clustering within hospitals, generalized linear mixed-effects models were used to model outcomes with a random intercept for each hospital. Models were adjusted for SSSS being listed as a principal or secondary diagnosis, race, illness severity, and level of care. We log-transformed LOS and cost data prior to modeling because of the nonnormal distributions for these data. Owing to the inability to measure the number of antibiotic doses, and to reduce the possibility of including children who received few regimen-defined combination antibiotics, a post hoc sensitivity analysis was performed. This analysis used an alternative definition for antibiotic regimen groups, for which children admitted for 2 or more calendar days must have received regimen-specified antibiotics on at least 2 days of the admission. Additionally, outcomes were stratified by low and moderate/high illness severity and compared across the three antibiotic regimen groups. All analyses were performed with SAS (SAS 9.4; SAS Institute, Cary, North Carolina), and P values of less than .05 were considered statistically significant.
RESULTS
Overall, 1,815 hospitalized children with SSSS were identified in the PHIS database, and after application of the exclusion criteria, 1,259 children remained, with 1,247 (99%) receiving antibiotics (Figure). The antibiotic regimens received by these children are described in Table 1. Of these, 828 children (66%) received one of the three most common antistaphylococcal regimens (clindamycin, clindamycin + MSSA, and clindamycin + MRSA) and were included for further analysis.

Characteristics of the 828 children are presented in Table 2. Most children (82%) were aged 4 years or younger, and distributions of age, sex, and insurance payer were similar among children receiving the three regimens. Thirty-two percent had moderate to high illness severity, and 3.5% required management in the intensive care setting. Of the three antibiotic regimens, clindamycin monotherapy was most common (47%), followed by clindamycin plus MSSA coverage (33%), and clindamycin plus MRSA coverage (20%). A higher proportion of children in the clindamycin plus MRSA group were African American and were hospitalized in the South. Children receiving clindamycin plus MRSA coverage had higher illness severity (44%) as compared with clindamycin monotherapy (28%) and clindamycin plus MSSA coverage (32%) (P = .001). Additionally, a larger proportion of children treated with clindamycin plus MRSA coverage were managed in the intensive care setting as compared with the clindamycin plus MSSA or clindamycin monotherapy groups.

Among the 828 children with SSSS, the median LOS was 2 days (IQR, 2-3), and treatment failure was 1.1% (95% CI, 0.4-1.8). After adjustment for illness severity, race, payer, and region (Table 3), the three antibiotic regimens were not associated with significant differences in LOS or treatment failure. Costs were significantly different among the three antibiotic regimens. Clindamycin plus MRSA coverage was associated with the greatest costs, whereas clindamycin monotherapy was associated with the lowest costs (mean, $5,348 vs $4,839, respectively; P < .001) (Table 3). In a sensitivity analysis using an alternative antibiotic regimen definition, we found results in line with the primary analysis, with no statistically significant differences in LOS (P = .44) or treatment failure (P = .54), but significant differences in cost (P < .001). Additionally, the same findings were present for LOS, treatment failure, and cost when outcomes were stratified by illness severity (Appendix Table). However, significant contributors to the higher cost in the clindamycin plus MRSA group did vary by illness severity stratification. Laboratory, supply, and pharmacy cost categories differed significantly among antibiotic groups for the low illness severity strata, whereas pharmacy was the only significant cost category difference in moderate/high illness severity.

DISCUSSION
Clindamycin monotherapy, clindamycin plus MSSA coverage, and clindamycin plus MRSA coverage are the most commonly administered antistaphylococcal antibiotic regimens for children hospitalized with SSSS at US children’s hospitals. Our multicenter study found that, across these antistaphylococcal antibiotic regimens, there were no associated differences in hospital LOS or treatment failure. However, the antibiotic regimens were associated with significant differences in overall hospital costs. These findings suggest that the use of clindamycin with additional MSSA or MRSA antibiotic coverage for children with SSSS may not be associated with additional clinical benefit, as compared with clindamycin monotherapy, and could potentially be more costly.
Prior literature describing LOS in relation to antibiotic use for children with SSSS is limited. Authors of a recent case series of 21 children in Philadelphia reported approximately 50% of children received clindamycin monotherapy or combination therapy, but patient outcomes such as LOS were not described.9 Clindamycin use and outcomes have been described in smaller studies and case reports of SSSS, which reported positive outcomes such as patient recovery and lack of disease recurrence.2,9,21 A small retrospective, comparative effectiveness study of 30 neonates with SSSS examined beta-lactamase–resistant penicillin use with and without cephalosporins. They found no effect on LOS, but findings were limited by a small sample size.22 Our study cohort included relatively few neonates, and thus our findings may not be applicable to this population subgroup. We chose not to include regimens with third-generation cephalosporins or ampicillin, which may have limited the number of included neonates, because these antibiotics are frequently administered during evaluation for invasive bacterial infections.23 We found a very low occurrence of treatment failure in our study cohort across all three groups, which is consistent with other studies of SSSS that report an overall good prognosis and low recurrence and/or readmission rates.6,16,24 The low prevalence of treatment failure, however, precluded our ability to detect small differences among antibiotic regimen groups that may exist.
We observed that cost differed significantly across antibiotic regimen groups, with lowest cost associated with clindamycin monotherapy in adjusted analysis despite similar LOS. Even with our illness-severity adjustment, there may have been other unmeasured factors resulting in the higher cost associated with the combination groups. Hence, we also examined cost breakdown with a stratified analysis by illness severity. We found that pharmacy costs were significantly different among antibiotic groups in both illness severity strata, whereas those with low illness severity also differed by laboratory and supply costs. Thus, pharmacy cost differences may be the largest driver in the cost differential among groups. Lower cost in the clindamycin monotherapy group is likely due to administration of a single antibiotic. The reason for supply and laboratory cost differences is uncertain, but higher cost in the clindamycin plus MRSA group could possibly be from laboratory testing related to drug monitoring (eg, renal function testing or drug levels). While other studies have reported costs for hospitalized children with SSSS associated with different patient characteristics or diagnostic testing,1,16 to our knowledge, no other studies have reported cost related to antibiotic regimens for SSSS. As healthcare reimbursements shift to value-based models, identifying treatment regimens with equal efficacy but lower cost will become increasingly important. Future studies should also examine other covariates and outcomes, such as oral vs parenteral antibiotic use, use of monitoring laboratories related to antibiotic choice, and adverse drug effects.
Several strengths and additional limitations apply to our study. Our study is one of the few to describe outcomes associated with antibiotic regimens for children with SSSS. With the PHIS database, we were able to include a large number of children with SSSS from children’s hospitals across the United States. Although the PHIS database affords these strengths, there are limitations inherent to administrative data. Children with SSSS were identified by documented ICD-9 and ICD-10 diagnostic codes, which might lead to misclassification. However, misclassification is less likely because only one ICD-9 and ICD-10 code exists for SSSS, and the characteristics of this condition are specific. Also, diagnostic codes for other dermatologic conditions (eg, scarlet fever) were excluded to further reduce the chance of misclassification. A limitation to our use of PHIS billing codes was the inability to confirm the dosage of antibiotics given, the number of doses, or whether antibiotics were prescribed upon discharge. Another limitation is that children whose antibiotic therapy was changed during hospitalization (eg, from clindamycin monotherapy to cefazolin monotherapy) were categorized into the combination groups. However, the sensitivity analysis performed based on a stricter antibiotic group definition (receipt of both antibiotics on at least 2 calendar days) did not alter the outcomes, which is reassuring. We were unable to assess the use of targeted antibiotic therapy because clinical data (eg, microbiology results) were not available. However, this may be less important because some literature suggests that cultures for S aureus are obtained infrequently2 and may be difficult to interpret when obtained,25 since culture growth can represent colonization rather than causative strains. An additional limitation is that administrative data do not include certain clinical outcomes, such as fever duration or degree of skin involvement, which could have differed among the groups. Last, the PHIS database only captures revisits or readmissions to PHIS hospitals, and so we are unable to exclude the possibility of a child being seen at or readmitted to another hospital.
Due to the observational design of this study and potential for incomplete measurement of illness severity, we recommend a future prospective trial with randomization to confirm these findings. One possible reason that LOS did not differ among groups is that the burden of clindamycin-resistant strains in our cohort could be low, and the addition of MSSA or MRSA coverage does not result in a clinically important increase in S aureus coverage. However, pooled pediatric hospital antibiogram data suggest the overall rate of clindamycin resistance is close to 20% in hospitals located in all US regions.26 Limited studies also suggest that MSSA may be the predominant pathogen associated with SSSS.2,9 To address this, future randomized trials could compare the effectiveness of clindamycin monotherapy to MSSA-specific agents like cefazolin or nafcillin. Unfortunately, anti-MSSA monotherapy was not evaluated in our study because very few children received this treatment. Using monotherapy as opposed to multiple antibiotics has the potential to promote antibiotic stewardship for antistaphylococcal antibiotics in the management of SSSS. Reducing unnecessary antibiotic use not only potentially affects antibiotic resistance, but could also benefit patients in reducing possible side effects, cost, and IV catheter complications.27 However, acknowledging our study limitations, our findings should be applied cautiously in clinical settings, in the context of local antibiogram data, individual culture results, and specific patient factors. The local clindamycin resistance rate for both MSSA and MRSA should be considered. Many antibiotics chosen to treat MRSA—such as vancomycin and trimethoprim/sulfamethoxazole—will also have anti-MSSA activity and may have lower local resistance rates than clindamycin. Practitioners may also consider how each antibiotic kills bacteria; for example, beta-lactams rely on bacterial replication, but clindamycin does not. Each factor should influence how empiric treatment, whether monotherapy or combination, is chosen for children with SSSS.
CONCLUSION
In this large, multicenter cohort of hospitalized children with SSSS, we found that the addition of MSSA or MRSA coverage to clindamycin monotherapy was not associated with differences in outcomes of hospital LOS and treatment failure. Furthermore, clindamycin monotherapy was associated with lower overall cost. Prospective randomized studies are needed to confirm these findings and assess whether clindamycin monotherapy, monotherapy with an anti-MSSA antibiotic, or alternative regimens are most effective for treatment of children with SSSS.
Staphylococcal scalded skin syndrome (SSSS) is an exfoliative toxin-mediated dermatitis that predominantly occurs in young children. Multiple recent reports indicate a rising incidence of this disease.1-4 Recommended treatment for SSSS includes antistaphylococcal antibiotics and supportive care measures.5,6 Elimination or reduction of the toxin-producing Staphylococcus aureus is thought to help limit disease progression and promote recovery. Experts advocate for the use of antibiotics even when there is no apparent focal source of infection, such as an abscess.6,7
Several factors may affect antibiotic selection, including the desire to inhibit toxin production and to target the causative pathogen in a bactericidal fashion. Because SSSS is toxin mediated, clindamycin is often recommended because of its inhibition of toxin synthesis.5,8 The clinical utility of adding other antibiotics to clindamycin for coverage of methicillin-sensitive S aureus (MSSA) or methicillin-resistant S aureus (MRSA) is uncertain. Several studies report MSSA to be the predominant pathogen identified by culture2,9; however, SSSS caused by MRSA has been reported.9-11 Additionally, bactericidal antibiotics (eg, nafcillin) have been considered to hold potential clinical advantage as compared with bacteriostatic antibiotics (eg, clindamycin), even though clinical studies have not clearly demonstrated this advantage in the general population.12,13 Some experts recommend additional MRSA or MSSA coverage (such as vancomycin or nafcillin) in patients with high illness severity or nonresponse to therapy, or in areas where there is high prevalence of staphylococcal resistance to clindamycin.5,7,9,14 Alternatively, for areas with low MRSA prevalence, monotherapy with an anti-MSSA antibiotic is another potential option. No recent studies have compared patient outcomes among antibiotic regimens in children with SSSS.
Knowledge of the outcomes associated with different antibiotic regimens for children hospitalized with SSSS is needed and could be used to improve patient outcomes and potentially promote antibiotic stewardship. In this study, our objectives were to (1) describe antibiotic regimens given to children hospitalized with SSSS, and (2) examine the association of three antibiotic regimens commonly used for SSSS (clindamycin monotherapy, clindamycin plus additional MSSA coverage, and clindamycin plus additional MRSA coverage) with patient outcomes of length of stay (LOS), treatment failure, and cost in a large cohort of children at US children’s hospitals.
METHODS
We conducted a multicenter, retrospective cohort study utilizing data within the Pediatric Health Information System (PHIS) database from July 1, 2011, to June 30, 2016. Thirty-five free-standing tertiary care US children’s hospitals within 24 states were included. The Children’s Hospital Association (Lenexa, Kansas) maintains the PHIS database, which contains de-identified patient information, including diagnoses (with International Classification of Diseases, Ninth and Tenth Revision, Clinical Modification [ICD-9-CM, ICD-10-CM]), demographics, procedures, and daily billing records. Data quality and reliability are confirmed by participating institutions and the Children’s Hospital Association.15 The local institutional review board (IRB) deemed the study exempt from formal IRB review, as patient information was de-identified.
Study Population
We included hospitalized children aged newborn to 18 years with a primary or secondary diagnosis of SSSS (ICD-9, 695.81; ICD-10, L00). Children whose primary presentation and admission were to a PHIS hospital were included; children transferred from another hospital were excluded. The following exclusion criteria were based on previously published methodology.16 Children with complex chronic medical conditions as classified by Feudtner et al17 were excluded, since these children may require a different treatment approach than the general pediatric population. In order to decrease diagnostic ambiguity, we excluded children if an alternative dermatologic diagnosis was recorded as a principal or secondary diagnosis (eg, Stevens-Johnson syndrome or scarlet fever).16 Finally, hospitals with fewer than 10 children with SSSS during the study period were excluded.
Antibiotic Regimen Groups
We used PHIS daily billing codes to determine the antibiotics received by the study population. Children were classified into antibiotic regimen groups based on whether they received specific antibiotic combinations. Antibiotics received on any day during the hospitalization, including in the emergency department (ED), were used to assign patients to regimen groups. Antibiotics were classified into regimen groups based on consensus among study investigators, which included two board-certified pediatric infectious diseases specialists (A.C., R.M.). Antibiotic group definitions are listed in Table 1. Oral and intravenous (IV) therapies were grouped together for clindamycin, cephalexin/cefazolin, and linezolid because of good oral bioavailability in most situations.18 The three most common antistaphylococcal groups were chosen for further analysis: clindamycin alone, clindamycin plus MSSA coverage, and clindamycin plus MRSA coverage. The clindamycin group was defined as children with receipt of oral or IV clindamycin. Children who received clindamycin with additional MSSA coverage, such as cefazolin or nafcillin, were categorized as the clindamycin plus MSSA group. Children who received clindamycin with additional MRSA coverage, such as vancomycin or linezolid, were categorized as the clindamycin plus MRSA group. We chose not to include children who received the above regimens plus other antibiotics with partial antistaphylococcal activity, such as ampicillin, gentamicin, or ceftriaxone, in the clindamycin plus MSSA and clindamycin plus MRSA groups. We excluded these antibiotics to decrease the heterogeneity in the definition of regimen groups and allow a more direct comparison for effectiveness.

Covariates
Covariates included age, sex, ethnicity and/or race, payer type, level of care, illness severity, and region. The variable definitions below are in keeping with a prior study of SSSS.16 Age was categorized as: birth to 59 days, 2 to 11 months, 1 to 4 years (preschool age), 5 to 10 years (school age), and 11 to 18 years (adolescent). We examined infants younger than 60 days separately from older infants because this population may warrant additional treatment considerations. Race and ethnicity were categorized as White (non-Hispanic), African American (non-Hispanic), Hispanic, or other. Payer types included government, private, or other. Level of care was assigned as either intensive care or acute care. Illness severity was assigned using the All Patient Refined Diagnosis Related Group (APR-DRG; 3M Corporation, St. Paul, Minnesota) severity levels.19 In line with a prior study,16 we defined “low illness severity” as the APR-DRG minor (1) classification. The moderate (2), major (3), and extreme (4) classifications were defined as “moderate to high illness severity,” since there were very few classifications of major or extreme (<5%) illness severity. We categorized hospitals into the following US regions: Northeast, Midwest, South, and West.
Outcome Measures
The primary outcome was hospital LOS in days, and secondary outcomes were treatment failure and hospital costs. Hospital LOS was chosen as the primary outcome to represent the time needed for the child to show clinical improvement. Treatment failure was defined as a same-cause 14-day ED revisit or hospital readmission, and these were determined to be same-cause if a diagnosis for SSSS (ICD-9, 695.81; ICD-10, L00) was documented for the return encounter. The 14-day interval for readmission and ED revisit was chosen to measure any relapse of symptoms after completion of antibiotic therapy, similar to a prior study of treatment failure in skin and soft tissue infections.20 Total costs of the hospitalization were estimated from charges using hospital- and year-specific cost-to-charge ratios. Subcategories of cost, including clinical, pharmacy, imaging, laboratory, supply, and other, were also compared among the three groups.
Statistical Analysis
Demographic and clinical characteristics of children were summarized using frequencies and percentages for categorical variables and medians with interquartile ranges (IQRs) for continuous variables. These were compared across antibiotic groups using chi-square and Kruskal–Wallis tests, respectively. In unadjusted analyses, outcomes were compared across antibiotic regimen groups using these same statistical tests. In order to account for patient clustering within hospitals, generalized linear mixed-effects models were used to model outcomes with a random intercept for each hospital. Models were adjusted for SSSS being listed as a principal or secondary diagnosis, race, illness severity, and level of care. We log-transformed LOS and cost data prior to modeling because of the nonnormal distributions for these data. Owing to the inability to measure the number of antibiotic doses, and to reduce the possibility of including children who received few regimen-defined combination antibiotics, a post hoc sensitivity analysis was performed. This analysis used an alternative definition for antibiotic regimen groups, for which children admitted for 2 or more calendar days must have received regimen-specified antibiotics on at least 2 days of the admission. Additionally, outcomes were stratified by low and moderate/high illness severity and compared across the three antibiotic regimen groups. All analyses were performed with SAS (SAS 9.4; SAS Institute, Cary, North Carolina), and P values of less than .05 were considered statistically significant.
RESULTS
Overall, 1,815 hospitalized children with SSSS were identified in the PHIS database, and after application of the exclusion criteria, 1,259 children remained, with 1,247 (99%) receiving antibiotics (Figure). The antibiotic regimens received by these children are described in Table 1. Of these, 828 children (66%) received one of the three most common antistaphylococcal regimens (clindamycin, clindamycin + MSSA, and clindamycin + MRSA) and were included for further analysis.

Characteristics of the 828 children are presented in Table 2. Most children (82%) were aged 4 years or younger, and distributions of age, sex, and insurance payer were similar among children receiving the three regimens. Thirty-two percent had moderate to high illness severity, and 3.5% required management in the intensive care setting. Of the three antibiotic regimens, clindamycin monotherapy was most common (47%), followed by clindamycin plus MSSA coverage (33%), and clindamycin plus MRSA coverage (20%). A higher proportion of children in the clindamycin plus MRSA group were African American and were hospitalized in the South. Children receiving clindamycin plus MRSA coverage had higher illness severity (44%) as compared with clindamycin monotherapy (28%) and clindamycin plus MSSA coverage (32%) (P = .001). Additionally, a larger proportion of children treated with clindamycin plus MRSA coverage were managed in the intensive care setting as compared with the clindamycin plus MSSA or clindamycin monotherapy groups.

Among the 828 children with SSSS, the median LOS was 2 days (IQR, 2-3), and treatment failure was 1.1% (95% CI, 0.4-1.8). After adjustment for illness severity, race, payer, and region (Table 3), the three antibiotic regimens were not associated with significant differences in LOS or treatment failure. Costs were significantly different among the three antibiotic regimens. Clindamycin plus MRSA coverage was associated with the greatest costs, whereas clindamycin monotherapy was associated with the lowest costs (mean, $5,348 vs $4,839, respectively; P < .001) (Table 3). In a sensitivity analysis using an alternative antibiotic regimen definition, we found results in line with the primary analysis, with no statistically significant differences in LOS (P = .44) or treatment failure (P = .54), but significant differences in cost (P < .001). Additionally, the same findings were present for LOS, treatment failure, and cost when outcomes were stratified by illness severity (Appendix Table). However, significant contributors to the higher cost in the clindamycin plus MRSA group did vary by illness severity stratification. Laboratory, supply, and pharmacy cost categories differed significantly among antibiotic groups for the low illness severity strata, whereas pharmacy was the only significant cost category difference in moderate/high illness severity.

DISCUSSION
Clindamycin monotherapy, clindamycin plus MSSA coverage, and clindamycin plus MRSA coverage are the most commonly administered antistaphylococcal antibiotic regimens for children hospitalized with SSSS at US children’s hospitals. Our multicenter study found that, across these antistaphylococcal antibiotic regimens, there were no associated differences in hospital LOS or treatment failure. However, the antibiotic regimens were associated with significant differences in overall hospital costs. These findings suggest that the use of clindamycin with additional MSSA or MRSA antibiotic coverage for children with SSSS may not be associated with additional clinical benefit, as compared with clindamycin monotherapy, and could potentially be more costly.
Prior literature describing LOS in relation to antibiotic use for children with SSSS is limited. Authors of a recent case series of 21 children in Philadelphia reported approximately 50% of children received clindamycin monotherapy or combination therapy, but patient outcomes such as LOS were not described.9 Clindamycin use and outcomes have been described in smaller studies and case reports of SSSS, which reported positive outcomes such as patient recovery and lack of disease recurrence.2,9,21 A small retrospective, comparative effectiveness study of 30 neonates with SSSS examined beta-lactamase–resistant penicillin use with and without cephalosporins. They found no effect on LOS, but findings were limited by a small sample size.22 Our study cohort included relatively few neonates, and thus our findings may not be applicable to this population subgroup. We chose not to include regimens with third-generation cephalosporins or ampicillin, which may have limited the number of included neonates, because these antibiotics are frequently administered during evaluation for invasive bacterial infections.23 We found a very low occurrence of treatment failure in our study cohort across all three groups, which is consistent with other studies of SSSS that report an overall good prognosis and low recurrence and/or readmission rates.6,16,24 The low prevalence of treatment failure, however, precluded our ability to detect small differences among antibiotic regimen groups that may exist.
We observed that cost differed significantly across antibiotic regimen groups, with lowest cost associated with clindamycin monotherapy in adjusted analysis despite similar LOS. Even with our illness-severity adjustment, there may have been other unmeasured factors resulting in the higher cost associated with the combination groups. Hence, we also examined cost breakdown with a stratified analysis by illness severity. We found that pharmacy costs were significantly different among antibiotic groups in both illness severity strata, whereas those with low illness severity also differed by laboratory and supply costs. Thus, pharmacy cost differences may be the largest driver in the cost differential among groups. Lower cost in the clindamycin monotherapy group is likely due to administration of a single antibiotic. The reason for supply and laboratory cost differences is uncertain, but higher cost in the clindamycin plus MRSA group could possibly be from laboratory testing related to drug monitoring (eg, renal function testing or drug levels). While other studies have reported costs for hospitalized children with SSSS associated with different patient characteristics or diagnostic testing,1,16 to our knowledge, no other studies have reported cost related to antibiotic regimens for SSSS. As healthcare reimbursements shift to value-based models, identifying treatment regimens with equal efficacy but lower cost will become increasingly important. Future studies should also examine other covariates and outcomes, such as oral vs parenteral antibiotic use, use of monitoring laboratories related to antibiotic choice, and adverse drug effects.
Several strengths and additional limitations apply to our study. Our study is one of the few to describe outcomes associated with antibiotic regimens for children with SSSS. With the PHIS database, we were able to include a large number of children with SSSS from children’s hospitals across the United States. Although the PHIS database affords these strengths, there are limitations inherent to administrative data. Children with SSSS were identified by documented ICD-9 and ICD-10 diagnostic codes, which might lead to misclassification. However, misclassification is less likely because only one ICD-9 and ICD-10 code exists for SSSS, and the characteristics of this condition are specific. Also, diagnostic codes for other dermatologic conditions (eg, scarlet fever) were excluded to further reduce the chance of misclassification. A limitation to our use of PHIS billing codes was the inability to confirm the dosage of antibiotics given, the number of doses, or whether antibiotics were prescribed upon discharge. Another limitation is that children whose antibiotic therapy was changed during hospitalization (eg, from clindamycin monotherapy to cefazolin monotherapy) were categorized into the combination groups. However, the sensitivity analysis performed based on a stricter antibiotic group definition (receipt of both antibiotics on at least 2 calendar days) did not alter the outcomes, which is reassuring. We were unable to assess the use of targeted antibiotic therapy because clinical data (eg, microbiology results) were not available. However, this may be less important because some literature suggests that cultures for S aureus are obtained infrequently2 and may be difficult to interpret when obtained,25 since culture growth can represent colonization rather than causative strains. An additional limitation is that administrative data do not include certain clinical outcomes, such as fever duration or degree of skin involvement, which could have differed among the groups. Last, the PHIS database only captures revisits or readmissions to PHIS hospitals, and so we are unable to exclude the possibility of a child being seen at or readmitted to another hospital.
Due to the observational design of this study and potential for incomplete measurement of illness severity, we recommend a future prospective trial with randomization to confirm these findings. One possible reason that LOS did not differ among groups is that the burden of clindamycin-resistant strains in our cohort could be low, and the addition of MSSA or MRSA coverage does not result in a clinically important increase in S aureus coverage. However, pooled pediatric hospital antibiogram data suggest the overall rate of clindamycin resistance is close to 20% in hospitals located in all US regions.26 Limited studies also suggest that MSSA may be the predominant pathogen associated with SSSS.2,9 To address this, future randomized trials could compare the effectiveness of clindamycin monotherapy to MSSA-specific agents like cefazolin or nafcillin. Unfortunately, anti-MSSA monotherapy was not evaluated in our study because very few children received this treatment. Using monotherapy as opposed to multiple antibiotics has the potential to promote antibiotic stewardship for antistaphylococcal antibiotics in the management of SSSS. Reducing unnecessary antibiotic use not only potentially affects antibiotic resistance, but could also benefit patients in reducing possible side effects, cost, and IV catheter complications.27 However, acknowledging our study limitations, our findings should be applied cautiously in clinical settings, in the context of local antibiogram data, individual culture results, and specific patient factors. The local clindamycin resistance rate for both MSSA and MRSA should be considered. Many antibiotics chosen to treat MRSA—such as vancomycin and trimethoprim/sulfamethoxazole—will also have anti-MSSA activity and may have lower local resistance rates than clindamycin. Practitioners may also consider how each antibiotic kills bacteria; for example, beta-lactams rely on bacterial replication, but clindamycin does not. Each factor should influence how empiric treatment, whether monotherapy or combination, is chosen for children with SSSS.
CONCLUSION
In this large, multicenter cohort of hospitalized children with SSSS, we found that the addition of MSSA or MRSA coverage to clindamycin monotherapy was not associated with differences in outcomes of hospital LOS and treatment failure. Furthermore, clindamycin monotherapy was associated with lower overall cost. Prospective randomized studies are needed to confirm these findings and assess whether clindamycin monotherapy, monotherapy with an anti-MSSA antibiotic, or alternative regimens are most effective for treatment of children with SSSS.
1. Staiman A, Hsu DY, Silverberg JI. Epidemiology of staphylococcal scalded skin syndrome in United States children. Br J Dermatol. 2018;178(3):704-708. https://doi.org/10.1111/bjd.16097
2. Hulten KG, Kok M, King KE, Lamberth LB, Kaplan SL. Increasing numbers of staphylococcal scalded skin syndrome cases caused by ST121 in Houston, TX. Pediatr Infect Dis J. 2020;39(1):30-34. https://doi.org/10.1097/INF.0000000000002499
3. Arnold JD, Hoek SN, Kirkorian AY. Epidemiology of staphylococcal scalded skin syndrome in the United States: A cross-sectional study, 2010-2014. J Am Acad Dermatol. 2018;78(2):404-406. https://doi.org/10.1016/j.jaad.2017.09.023
4. Hayward A, Knott F, Petersen I, et al. Increasing hospitalizations and general practice prescriptions for community-onset staphylococcal disease, England. Emerg Infect Dis. 2008;14(5):720-726. https://doi.org/10.3201/eid1405.070153
5. Berk DR, Bayliss SJ. MRSA, staphylococcal scalded skin syndrome, and other cutaneous bacterial emergencies. Pediatr Ann. 2010;39(10):627-633. https://doi.org/10.3928/00904481-20100922-02
6. Ladhani S, Joannou CL, Lochrie DP, Evans RW, Poston SM. Clinical, microbial, and biochemical aspects of the exfoliative toxins causing staphylococcal scalded-skin syndrome. Clin Microbiol Rev. 1999;12(2):224-242.
7. Handler MZ, Schwartz RA. Staphylococcal scalded skin syndrome: diagnosis and management in children and adults. J Eur Acad Dermatol Venereol. 2014;28(11):1418-1423. https://doi.org/10.1111/jdv.12541
8. Hodille E, Rose W, Diep BA, Goutelle S, Lina G, Dumitrescu O. The role of antibiotics in modulating virulence in Staphylococcus aureus. Clin Microbiol Rev. 2017;30(4):887-917. https://doi.org/10.1128/CMR.00120-16
9. Braunstein I, Wanat KA, Abuabara K, McGowan KL, Yan AC, Treat JR. Antibiotic sensitivity and resistance patterns in pediatric staphylococcal scalded skin syndrome. Pediatr Dermatol. 2014;31(3):305-308. https://doi.org/10.1111/pde.12195
10. Yamaguchi T, Yokota Y, Terajima J, et al. Clonal association of Staphylococcus aureus causing bullous impetigo and the emergence of new methicillin-resistant clonal groups in Kansai district in Japan. J Infect Dis. 2002;185(10):1511-1516. https://doi.org/10.1086/340212
11. Noguchi N, Nakaminami H, Nishijima S, Kurokawa I, So H, Sasatsu M. Antimicrobial agent of susceptibilities and antiseptic resistance gene distribution among methicillin-resistant Staphylococcus aureus isolates from patients with impetigo and staphylococcal scalded skin syndrome. J Clin Microbiol. 2006;44(6):2119-2125. https://doi.org/10.1128/JCM.02690-05
12. Pankey GA, Sabath LD. Clinical relevance of bacteriostatic versus bactericidal mechanisms of action in the treatment of Gram-positive bacterial infections. Clin Infect Dis. 2004;38(6):864-870. https://doi.org/10.1086/381972
13. Wald-Dickler N, Holtom P, Spellberg B. Busting the myth of “static vs cidal”: a systemic literature review. Clin Infect Dis. 2018;66(9):1470-1474. https://doi.org/10.1093/cid/cix1127
14. Ladhani S, Joannou CL. Difficulties in diagnosis and management of the staphylococcal scalded skin syndrome. Pediatr Infect Dis J. 2000;19(9):819-821. https://doi.org/10.1097/00006454-200009000-00002
15. Mongelluzzo J, Mohamad Z, Ten Have TR, Shah SS. Corticosteroids and mortality in children with bacterial meningitis. JAMA. 2008;299(17):2048-2055. https://doi.org/10.1001/jama.299.17.2048
16. Neubauer HC, Hall M, Wallace SS, et al. Variation in diagnostic test use and associated outcomes in staphylococcal scalded skin syndrome at children’s hospitals. Hosp Pediatr. 2018;8(9):530-537. https://doi.org/10.1542/hpeds.2018-0032
17. Feudtner C, Feinstein JA, Zhong W, Hall M, Dai D. Pediatric complex chronic conditions classification system version 2: updated for ICD-10 and complex medical technology dependence and transplantation. BMC Pediatr. 2014;14:199. https://doi.org/10.1186/1471-2431-14-199
18. Sauberan JS, Bradley JS. Antimicrobial agents. In: Long SS, ed. Principles and Practice of Pediatric Infectious Diseases. Elsevier; 2018:1499-1531.
19. Sedman AB, Bahl V, Bunting E, et al. Clinical redesign using all patient refined diagnosis related groups. Pediatrics. 2004;114(4):965-969. https://doi.org/10.1542/peds.2004-0650
20. Williams DJ, Cooper WO, Kaltenbach LA, et al. Comparative effectiveness of antibiotic treatment strategies for pediatric skin and soft-tissue infections. Pediatrics. 2011;128(3):e479-487. https://doi.org/10.1542/peds.2010-3681
21. Haasnoot PJ, De Vries A. Staphylococcal scalded skin syndrome in a 4-year-old child: a case report. J Med Case Rep. 2018;12(1):20. https://doi.org/ 10.1186/s13256-017-1533-7
22. Li MY, Hua Y, Wei GH, Qiu L. Staphylococcal scalded skin syndrome in neonates: an 8-year retrospective study in a single institution. Pediatr Dermatol. 2014;31(1):43-47. https://doi.org/10.1111/pde.12114
23. Markham JL, Hall M, Queen MA, et al. Variation in antibiotic selection and clinical outcomes in infants <60 days hospitalized with skin and soft tissue infections. Hosp Pediatr. 2019;9(1):30-38. https://doi.org/10.1542/hpeds.2017-0237
24. Davidson J, Polly S, Hayes PJ, Fisher KR, Talati AJ, Patel T. Recurrent staphylococcal scalded skin syndrome in an extremely low-birth-weight neonate. AJP Rep. 2017;7(2):e134-e137. https://doi.org/10.1055/s-0037-1603971
25. Ladhani S, Robbie S, Chapple DS, Joannou CL, Evans RW. Isolating Staphylococcus aureus from children with suspected Staphylococcal scalded skin syndrome is not clinically useful. Pediatr Infect Dis J. 2003;22(3):284-286.
26. Tamma PD, Robinson GL, Gerber JS, et al. Pediatric antimicrobial susceptibility trends across the United States. Infect Control Hosp Epidemiol. 2013;34(12):1244-1251. https://doi.org/10.1086/673974
27. Unbeck M, Forberg U, Ygge BM, Ehrenberg A, Petzold M, Johansson E. Peripheral venous catheter related complications are common among paediatric and neonatal patients. Acta Paediatr. 2015;104(6):566-574. https://doi.org/10.1111/apa.12963
1. Staiman A, Hsu DY, Silverberg JI. Epidemiology of staphylococcal scalded skin syndrome in United States children. Br J Dermatol. 2018;178(3):704-708. https://doi.org/10.1111/bjd.16097
2. Hulten KG, Kok M, King KE, Lamberth LB, Kaplan SL. Increasing numbers of staphylococcal scalded skin syndrome cases caused by ST121 in Houston, TX. Pediatr Infect Dis J. 2020;39(1):30-34. https://doi.org/10.1097/INF.0000000000002499
3. Arnold JD, Hoek SN, Kirkorian AY. Epidemiology of staphylococcal scalded skin syndrome in the United States: A cross-sectional study, 2010-2014. J Am Acad Dermatol. 2018;78(2):404-406. https://doi.org/10.1016/j.jaad.2017.09.023
4. Hayward A, Knott F, Petersen I, et al. Increasing hospitalizations and general practice prescriptions for community-onset staphylococcal disease, England. Emerg Infect Dis. 2008;14(5):720-726. https://doi.org/10.3201/eid1405.070153
5. Berk DR, Bayliss SJ. MRSA, staphylococcal scalded skin syndrome, and other cutaneous bacterial emergencies. Pediatr Ann. 2010;39(10):627-633. https://doi.org/10.3928/00904481-20100922-02
6. Ladhani S, Joannou CL, Lochrie DP, Evans RW, Poston SM. Clinical, microbial, and biochemical aspects of the exfoliative toxins causing staphylococcal scalded-skin syndrome. Clin Microbiol Rev. 1999;12(2):224-242.
7. Handler MZ, Schwartz RA. Staphylococcal scalded skin syndrome: diagnosis and management in children and adults. J Eur Acad Dermatol Venereol. 2014;28(11):1418-1423. https://doi.org/10.1111/jdv.12541
8. Hodille E, Rose W, Diep BA, Goutelle S, Lina G, Dumitrescu O. The role of antibiotics in modulating virulence in Staphylococcus aureus. Clin Microbiol Rev. 2017;30(4):887-917. https://doi.org/10.1128/CMR.00120-16
9. Braunstein I, Wanat KA, Abuabara K, McGowan KL, Yan AC, Treat JR. Antibiotic sensitivity and resistance patterns in pediatric staphylococcal scalded skin syndrome. Pediatr Dermatol. 2014;31(3):305-308. https://doi.org/10.1111/pde.12195
10. Yamaguchi T, Yokota Y, Terajima J, et al. Clonal association of Staphylococcus aureus causing bullous impetigo and the emergence of new methicillin-resistant clonal groups in Kansai district in Japan. J Infect Dis. 2002;185(10):1511-1516. https://doi.org/10.1086/340212
11. Noguchi N, Nakaminami H, Nishijima S, Kurokawa I, So H, Sasatsu M. Antimicrobial agent of susceptibilities and antiseptic resistance gene distribution among methicillin-resistant Staphylococcus aureus isolates from patients with impetigo and staphylococcal scalded skin syndrome. J Clin Microbiol. 2006;44(6):2119-2125. https://doi.org/10.1128/JCM.02690-05
12. Pankey GA, Sabath LD. Clinical relevance of bacteriostatic versus bactericidal mechanisms of action in the treatment of Gram-positive bacterial infections. Clin Infect Dis. 2004;38(6):864-870. https://doi.org/10.1086/381972
13. Wald-Dickler N, Holtom P, Spellberg B. Busting the myth of “static vs cidal”: a systemic literature review. Clin Infect Dis. 2018;66(9):1470-1474. https://doi.org/10.1093/cid/cix1127
14. Ladhani S, Joannou CL. Difficulties in diagnosis and management of the staphylococcal scalded skin syndrome. Pediatr Infect Dis J. 2000;19(9):819-821. https://doi.org/10.1097/00006454-200009000-00002
15. Mongelluzzo J, Mohamad Z, Ten Have TR, Shah SS. Corticosteroids and mortality in children with bacterial meningitis. JAMA. 2008;299(17):2048-2055. https://doi.org/10.1001/jama.299.17.2048
16. Neubauer HC, Hall M, Wallace SS, et al. Variation in diagnostic test use and associated outcomes in staphylococcal scalded skin syndrome at children’s hospitals. Hosp Pediatr. 2018;8(9):530-537. https://doi.org/10.1542/hpeds.2018-0032
17. Feudtner C, Feinstein JA, Zhong W, Hall M, Dai D. Pediatric complex chronic conditions classification system version 2: updated for ICD-10 and complex medical technology dependence and transplantation. BMC Pediatr. 2014;14:199. https://doi.org/10.1186/1471-2431-14-199
18. Sauberan JS, Bradley JS. Antimicrobial agents. In: Long SS, ed. Principles and Practice of Pediatric Infectious Diseases. Elsevier; 2018:1499-1531.
19. Sedman AB, Bahl V, Bunting E, et al. Clinical redesign using all patient refined diagnosis related groups. Pediatrics. 2004;114(4):965-969. https://doi.org/10.1542/peds.2004-0650
20. Williams DJ, Cooper WO, Kaltenbach LA, et al. Comparative effectiveness of antibiotic treatment strategies for pediatric skin and soft-tissue infections. Pediatrics. 2011;128(3):e479-487. https://doi.org/10.1542/peds.2010-3681
21. Haasnoot PJ, De Vries A. Staphylococcal scalded skin syndrome in a 4-year-old child: a case report. J Med Case Rep. 2018;12(1):20. https://doi.org/ 10.1186/s13256-017-1533-7
22. Li MY, Hua Y, Wei GH, Qiu L. Staphylococcal scalded skin syndrome in neonates: an 8-year retrospective study in a single institution. Pediatr Dermatol. 2014;31(1):43-47. https://doi.org/10.1111/pde.12114
23. Markham JL, Hall M, Queen MA, et al. Variation in antibiotic selection and clinical outcomes in infants <60 days hospitalized with skin and soft tissue infections. Hosp Pediatr. 2019;9(1):30-38. https://doi.org/10.1542/hpeds.2017-0237
24. Davidson J, Polly S, Hayes PJ, Fisher KR, Talati AJ, Patel T. Recurrent staphylococcal scalded skin syndrome in an extremely low-birth-weight neonate. AJP Rep. 2017;7(2):e134-e137. https://doi.org/10.1055/s-0037-1603971
25. Ladhani S, Robbie S, Chapple DS, Joannou CL, Evans RW. Isolating Staphylococcus aureus from children with suspected Staphylococcal scalded skin syndrome is not clinically useful. Pediatr Infect Dis J. 2003;22(3):284-286.
26. Tamma PD, Robinson GL, Gerber JS, et al. Pediatric antimicrobial susceptibility trends across the United States. Infect Control Hosp Epidemiol. 2013;34(12):1244-1251. https://doi.org/10.1086/673974
27. Unbeck M, Forberg U, Ygge BM, Ehrenberg A, Petzold M, Johansson E. Peripheral venous catheter related complications are common among paediatric and neonatal patients. Acta Paediatr. 2015;104(6):566-574. https://doi.org/10.1111/apa.12963
©2021 Society of Hospital Medicine
Implementing a Telehospitalist Program Between Veterans Health Administration Hospitals: Outcomes, Acceptance, and Barriers to Implementation
Healthcare in rural areas faces increasing challenges due to community hospital closures, physician shortages, and a more concentrated population of older adults with higher rates of comorbid conditions than their urban counterparts.1-3 Critical access hospitals (CAHs), which primarily serve rural areas, have fewer clinical capabilities, worse process-of-care measures, and higher mortality rates for some conditions when compared to non-CAHs.4 As such, CAHs are closing at record numbers across the United States,5 resulting in loss of available hospital beds and patient access to timely emergency services,6 which can worsen outcomes, further widening the rural-urban healthcare gap.7,8 Furthermore, this strain on an overwhelmed health system in the most vulnerable areas restricts the ability to respond to healthcare crises like the coronavirus disease 2019 pandemic.9
Providing adequate staff for currently available hospital beds is also a problem in rural areas. Studies demonstrating improved outcomes, decreased length of stay (LOS), and increased quality with hospitalist services have resulted in a high demand for hospitalists nationwide.10-12 Recruiting hospitalists to work in rural areas, however, has become increasingly challenging due to low-patient volumes, financial viability of hospitalist-model adoption, and provider shortages.13,14 Recently, the Veterans Health Administration (VHA) reported a 28% nationwide shortage of hospitalists,15 which disproportionally affects rural VHA hospitals. Staffing difficulties and reliance on intermittent providers were reported by more than 80% of rural and low-complexity VHA facilities.16
Telehospitalist services (THS) can help deliver high-quality care to rural residents locally, decrease travel expenses, support hospital volume, and increase healthcare capacity in response to a pandemic.14,17,18 Only a few studies have described THS (mostly with overnight or cross-coverage models directed to CAHs), and clinical outcomes have been inconsistently reported.17,19-21 Furthermore, no program has been conducted within an integrated health system akin to the VHA. The primary objective of this quality improvement (QI) initiative was to perform a mixed-methods evaluation of THS between VHA hospitals to compare clinical outcomes and patient and staff satisfaction. Secondary outcomes included description of the implementation process, unexpected challenges, and subsequent QI initiatives. These results will expand the knowledge on feasibility of THS and provide implementation guidance.
METHODS
A mixed-methods approach was used to evaluate outcomes of this QI project. Reporting follows the revised Standards for Quality Improvement Reporting Excellence (SQUIRE 2.0).22
Context
The VHA is the largest integrated healthcare system in the United States, with more than 8 million veterans enrolled, more than 30% of whom reside in a rural area. The VHA comprises more than 1,000 outpatient clinics and 170 acute care VA Medical Centers,23,24 including more than 35 rural and low-complexity hospitals.25 Low-complexity hospitals are those with the lowest volume and levels of patient complexity and minimal or no teaching programs, research, intensive care unit (ICU) beds, and subspecialists. Lack of reimbursement and interstate licensing, often cited as barriers to telemedicine, do not apply to the VHA. The hub site was a large tertiary care (high-complexity) VHA hospital located in Iowa City, Iowa. The spoke site was a low-complexity (10-bed acute inpatient unit with no ICU) rural VA hospital located in Tomah, Wisconsin.
Study Population
The preimplementation cohort for comparison included all patients admitted between January 1, 2018, and January 6, 2019. The postimplementation study cohort included all observation and acute care admissions during the pilot phase (January 7 to May 3, 2019) and sustainability phase (July 15 to December 31, 2019). The postimplementation analysis excluded the time period of May 4 to July 14, 2019, due to an interruption (gap) in THS. The gap period allowed for preliminary data analysis, optimization of the telecommunication system, and the recruitment and training of additional providers who could provide long-term staffing to the service.
Intervention
Preimplementation
Prior to THS implementation, Tomah’s inpatient ward was staffed by one physician per shift, who could be a hospitalist, medical officer of the day (MOD), or an intermittent provider (locum tenens). Hospitalists covering the acute inpatient ward prior to the THS transitioned to cover weekends, nights, and urgent care service shifts.
We visited the spoke site and held information-sharing sessions with key stakeholders (administrators, clinician leaders, nurses, and ancillary staff) prior to kick-off. Recurrent phone meetings addressed anticipated and emerging challenges. Telehospitalist and local providers underwent technology and service training.
Technology and Connectivity
A low-cost technology system using tablet computers provided Health Insurance Portability and Accountability Act–compliant videoconferencing with a telehospitalist at the hub site. An Eko-Core digital stethoscope® with a web-based audio stream was available. Telehospitalists conducted encounters from a private office space with telehealth capabilities. A total of $9,000 was spent on equipment at both sites. Due to connectivity problems and data limits, the tablets were switched to mobile computer-on-wheels workstations and hospital-based Wi-Fi for the sustainability phase.
THS Description
An experienced hub hospitalist, together with an advanced practice provider (APP; nurse practitioner [NP] or physician assistant [PA]), cared for all patients admitted to the 10-bed inpatient unit at the spoke site, Monday through Friday from 8:00 AM to 4:30 PM. The APP had limited or no prior experience in acute inpatient medicine. The telehospitalist worked as a team with the APP. The APP was the main point of contact for nurses, performed physical examinations, and directed patient care to their level of comfort (in a similar manner as a teaching team). The telehospitalist conducted bedside patient rounds, participated in multidisciplinary huddles, and shared clinical documentation and administrative duties with the APP. The telehospitalist was the primary staff for admitted patients and had full access to the electronic health record (EHR). The THS was staffed by 10 hospitalists during the study period. Overnight and weekend cross-coverage and admissions were performed by MODs, who also covered the urgent care and cross-covered other nonmedical units.
Quantitative Evaluation Methods
Workload and Clinical Outcomes
An EHR query identified all patients admitted during the pre- and postimplementation periods. Demographic data, clinical Nosos risk scores,26,27 and top admission diagnoses were reported. Workload was evaluated using the average number of encounters per day and self-reported telehospitalist worksheets, which were cross-referenced with EHR data. Clinical outcomes included LOS, 30-day hospital readmission rate, 30-day standardized mortality (SMR30), in-hospital mortality, and VHA-specific inpatient quality metrics. Independent sample t tests for continuous variables and chi-square tests or Fisher’s exact test (for patient class) for categorical variables were used to compare pre- and postimplementation groups. Statistical process control (SPC) charts evaluated changes over time. All analyses were conducted using Microsoft Excel and R.28
Anonymous surveys were distributed to spoke-site inpatient and administrative staff at 1 month and 12 months postimplementation, assessing satisfaction, technology/connectivity, communication, and challenges (Appendix Figure 1). Satisfaction of the telehospitalist physicians at the hub site was measured 12 months postimplementation by a 26-question survey assessing the same domains, plus quality of care (Appendix Figure 2).
Patient Satisfaction
The VHA Survey of Healthcare Experiences of Patients (SHEP), a version of the Hospital Consumer Assessment of Healthcare Providers and Systems Survey,29,30 was mailed to all patients after discharge. Survey responses concerning inpatient provider care (eg, care coordination, communication, hospital rating, willingness to recommend the hospital) during the pre- and postimplementation phases were compared using a two-sample test of independent proportions. Responses obtained during May and June 2019 were excluded.
Qualitative Evaluation Methods
The authors had full access to, and took full responsibility for, the integrity of the data. The project was evaluated by the University of Iowa Institutional Review Board and the Iowa City VA Research and Development Committee and was determined to be a non–human-subjects QI project.
RESULTS
Quantitative Workload and Clinical Outcomes
There were 822 admissions during the preimplementation period and 550 admissions during the postimplementation period (253 during the pilot and 297 during sustainability phase). Patient characteristics pre- and postimplementation were not significantly different (Table 1). The median patient age was 65 years; 96% of patients were male, and 83% were rural residents. The most common admission diagnosis was alcohol-related (36%); regarding patient disposition, 78% of admissions were discharged home.
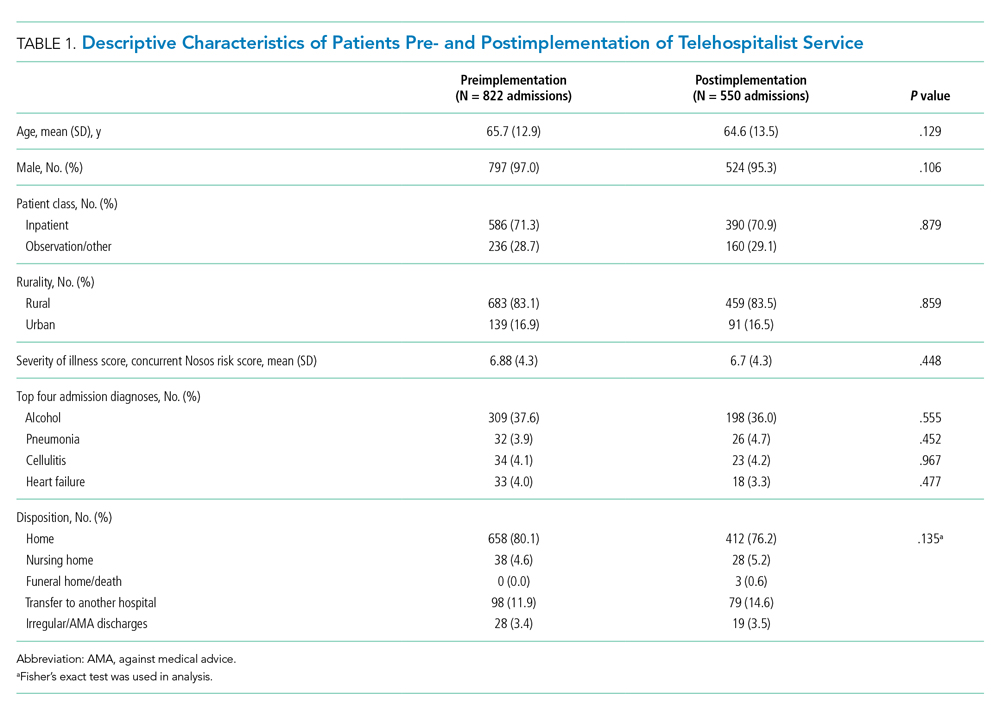
Workload
There were 502 patient encounters staffed by the telehospitalist in the pilot phase, with an average of 6.25 encounters per day, and a telehospitalist-reported workload of 7 hours per day. There were 538 patient encounters, with an average of 4.67 encounters per day and a workload of 5.6 hours per day in the sustainability phase. The average daily census decreased from 5.0 (SD, 1.1) patients per day during preimplementation to 3.1 (SD, 0.5) patients per day during postimplementation (Table 2). In some of the months during the study period, admissions decreased below the lower SPC limit, suggesting a significant change (Figure). Adjusted LOS was significantly lower, with 3.0 (SD, 0.7) days vs 2.3 (SD, 0.3) days in the pre- and postimplementation periods, respectively. Bed occupancy rates were significantly lower in the sustainability phase compared with the pilot phase and the preimplementation period. Readmission rates varied, ranging from <10% to >30%, not significantly different but slightly higher in the postimplementation period. Readmission rates for heart failure, chronic obstructive pulmonary disease, and pneumonia remained unchanged; other medical readmissions (mostly alcohol-related) were slightly higher in the postimplementation period.
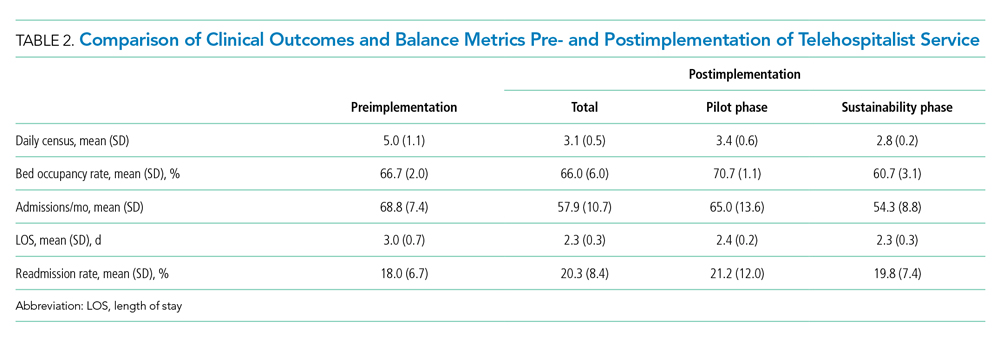
In-hospital mortality and SMR30 did not change significantly, but there was improvement in the 12-month rolling average of the observed/expected SMR30 from 1.40 to 1.08. Additional VHA-specific quality metrics were monitored and showed either small improvements or no change (data not shown).
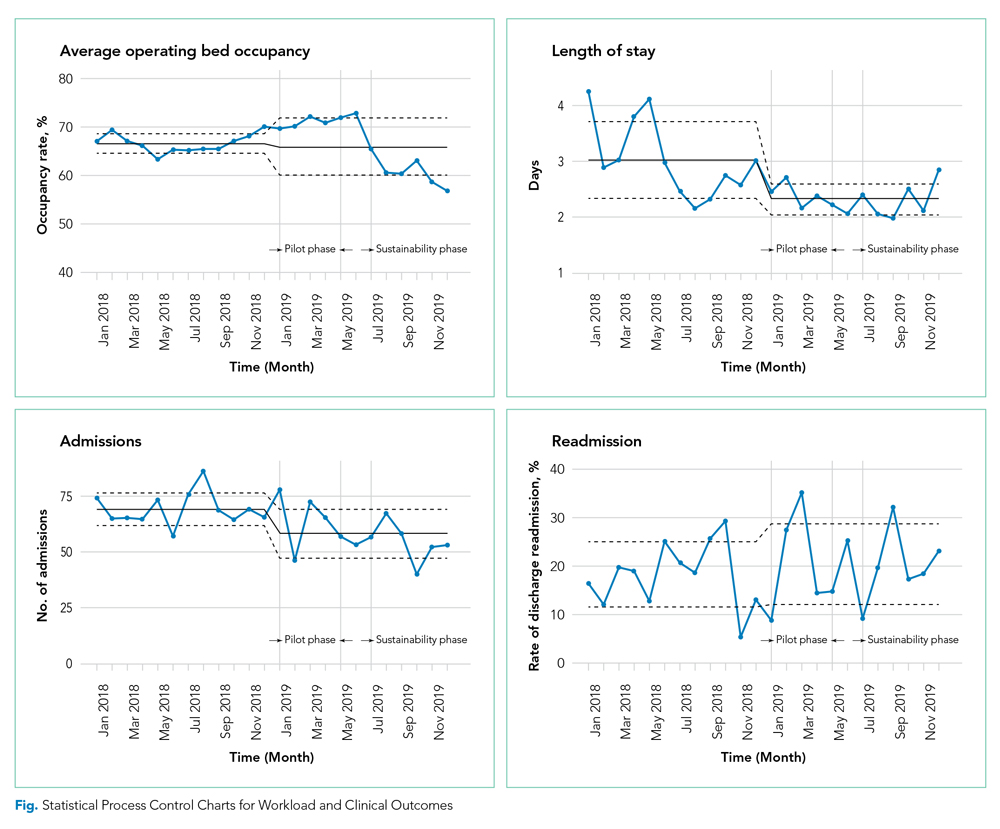
Satisfaction at Hub and Spoke Sites
After sending two reminder communications via email, the telehospitalist satisfaction survey had a total response rate of 90% (9/10). Telehospitalists were satisfied or very satisfied (89%) with the program and the local providers (88.9%), rating their experience as good or excellent (100%) (Table 3). Communication with patients, families, and local staff was noted as being “positive” or “mostly positive.” Telehospitalists reported confidence in the accuracy of their diagnoses and rated the quality of care as being equal to that of a face-to-face encounter. Connectivity problems were prevalent, although most providers were able to resort to a back-up plan. Other challenges included differences in culture and concerns about liability. We received 27 responses from the spoke-site satisfaction survey; the response rate could not be determined because the survey was distributed by the spoke site for anonymity. Of the respondents, 37% identified as nurses, 25.9% as healthcare providers (APPs or physicians), and 33.3% as other staff (eg, social worker, nutritionist, physical therapist, utilization management, administrators); 3.7% did not respond. Among the participants, 88% had personally interacted with the THS. Most providers and other staff perceived THS as valuable (57.1% and 77.8%, respectively) and were satisfied or highly satisfied with THS (57.1% and 55.6%, respectively). On average, nurses provided lower ratings across all survey items than providers and other staff. Challenges noted by all staff included issues with communication, workflow, and technology/connectivity.
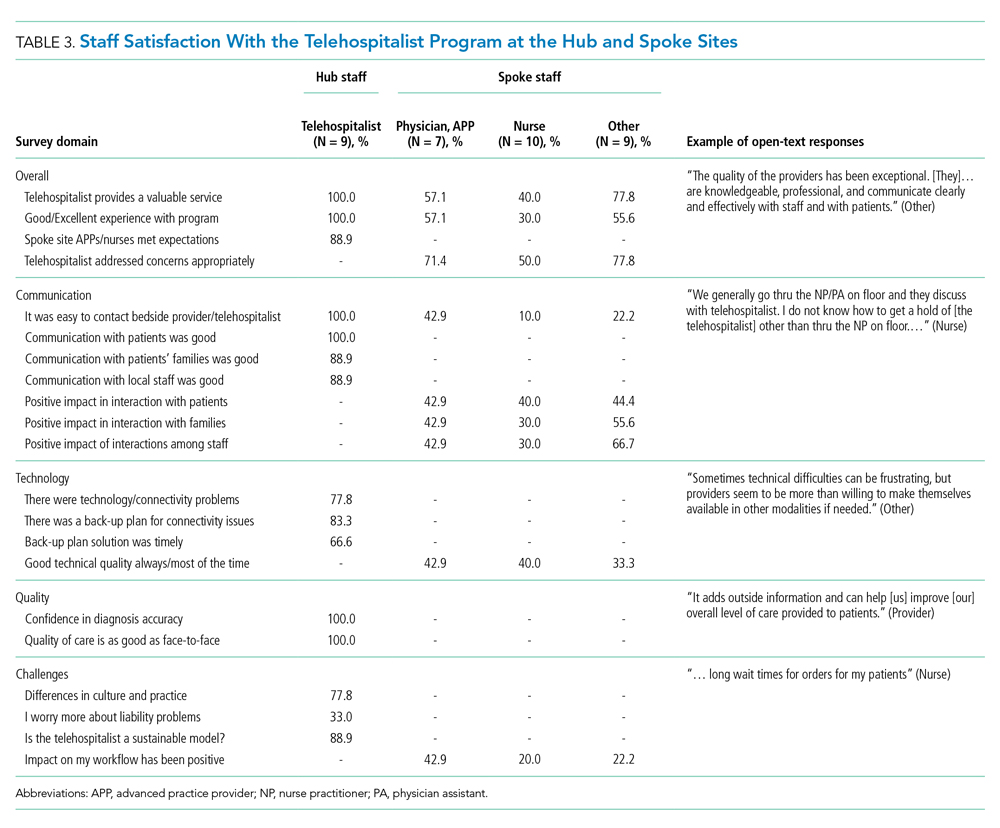
Qualitative Strengths
Our process evaluation identified high quality of care and teamwork as contributors to the success of the program. Overall, staff credited perceived improvements in quality of care to the quality of providers staffing the THS, including the local APPs. Noting the telehospitalists’ knowledge base and level of engagement as key attributes, one staff member commented: “I prefer a telehospitalist that really care[s] about patients than some provider that is physically here but does not engage.” Staff perceived improvements in the continuity of care, as well as care processes such as handoffs and transitions of care.
Improvements in teamwork were perceived compared with the previous model of care. Telehospitalists were lauded for their professionalism and communication skills. Overall, nurses felt providers in the THS listened more to their views. In addition, nurse respondents felt they could learn from several providers and said they enjoyed the telehospitalists’ disposition to teach and discuss patient care. The responsiveness of the THS staff was instrumental in building teamwork and acceptance. A bedside interdisciplinary protocol was established for appropriate patients. Local staff felt this was crucial for teamwork and patient satisfaction. Telehospitalists reported high-value in interdisciplinary rounds, facilitating interaction with nurses and ancillary staff. Handoff problems were identified, leading to QI initiatives to mitigate those issues.
Challenges
The survey identified administrative barriers, technical difficulties, workflow constraints, and clinical concerns. The credentialing process was complicated, delaying the onboarding of telehospitalists. Internet connectivity was inconsistent, leading to disruption in video communications; however, during the sustainability phase, updated technology improved communications. The communication workflow was resisted by some nurses, who wanted to phone the telehospitalist directly rather than having the local APP as the first contact. Secure messaging was enabled to allow nurses direct contact during the sustainability phase.
Workload was a concern among telehospitalists and local staff. Telehospitalists perceived the documentation requirements and administrative workload to be two to three times higher than at other hospitals—despite the lower number of encounters. Finally, clinical concerns from spoke-site clinicians included a perceived rise in the acuity of patients (which was not evident by the Nosos score) and delayed decisions to transfer-out patients. These concerns were addressed with educational sessions for telehospitalists during the sustainability phase.
Additional Quality Improvement Projects
The implementation of THS resulted in QI initiatives at the spoke site, including an EHR-integrated handoff tool; a documentation evaluation that led to the elimination of duplicative, inefficient, and error-prone templates; and a revision of the alcohol withdrawal treatment protocol during the sustainability phase to reduce the use of intravenous benzodiazepines. A more comprehensive benzodiazepine-sparing alcohol withdrawal treatment protocol was also developed but was not implemented until after the study period (January 2020).
DISCUSSION
Our pre-post study evaluation found implementation of a THS to be noninferior to face-to-face care, with no significant change in mortality, readmission rate, or patient satisfaction. The significant improvement observed in LOS is consistent with the adoption of hospitalist models in other medical care settings,11 but had not been reported by previous telehospitalist studies. For example, in their retrospective chart review comparing an NP-supported telehospitalist model to locum tenens hospitalists, Boltz et al found no difference in LOS.31 Moreover, as in our study, they found no differences in readmissions, mortality, and patient satisfaction.31 Similarly, Kuperman et al reported unchanged daily census, LOS, and transfer rates from a CAH with their virtual hospitalist program, but a decrease in the percentage of patients transferred-out from the emergency department, suggesting that more patients were treated locally.19
Reduction in LOS is one of the primary measures of efficiency in hospital care31; reducing LOS while maintaining the quality of care lowers hospital costs. The reduction in LOS in our study could be attributed to greater continuity of care, engagement/experience of the telehospitalists, or other factors. This decrease in LOS and slight reduction in admissions resulted in an overall lower daily census during the study period and impacted efficiency. Our study was unable to determine the cause for the reduction in admissions; however, several concurrent events, including the expansion of community-care options for veterans under the MISSION ACT (Maintaining Internal Systems and Strengthening Integrated Outside Networks Act) in June 2019, a nationwide smoking ban at VA facilities (October 2019), and a modification in the alcohol withdrawal treatment protocol might have influenced veterans’ choice of hospital.
Readmission rates were slightly higher, though nonsignificant, in the postimplementation period. Alcohol-related readmissions accounted for most readmissions; some of the protocol changes, such as admitting all patients with alcohol withdrawal to inpatient class instead of admitting some to the observation class, accounted for part of the increase in readmission rates. Readmission rates for other conditions such as chronic obstructive pulmonary disease, chronic heart failure, or pneumonia were not significantly different, suggesting that the reduction in LOS did not result in an unintended increased readmission rate for those conditions.
Rural hospitals are struggling with staffing and finances. Resorting to locum tenens staffing is costly and can result in variable quality of care.32,33 APPs are increasingly taking on hospitalist positions, with 65% of adult hospitalist programs, including half of all VHA hospitals, employing NPs and PAs.34,35 In response to this expanded scope of practice, hospitals employing APPs in hospitalist roles must comply with state and federal laws, which often require that APPs be supervised by or work in collaboration with an on-site or off-site physician. The THS is a great model to support APPs and address staffing and cost challenges in low-volume rural facilities, while maintaining quality of care. Some APP-telehospitalist programs similar to ours have reported cost reductions of up 58% compared to programs that employ locum tenens physicians.31 In our model, we assume that a single telehospitalist hub could provide coverage to two or three spoke sites with APP support, reducing staffing costs.
Hub telehospitalists reported satisfaction with the program, and they perceived the quality of care to be comparable to face-to-face encounters; their responses were consistent with those previously reported in an evaluation of telemedicine acute care by JaKa et al.
This study has several limitations. First, the VHA is an integrated health system, one that serves an older, predominantly male patient population. Also, the lack of reimbursement and interstate licensing restrictions limit generalizability of these results to other CAHs or healthcare systems. Furthermore, the intervention was limited to a single rural site; while this allowed for a detailed evaluation, unique barriers or facilitators might exist that limit its applicability. In addition, QI initiatives implemented by the VHA during the project period might have confounded some of our results. Last, patient satisfaction survey data are overall limited in their ability to fully assess patient’s experience and satisfaction with the program. Further qualitative studies are needed to gain deeper insight into patient perspectives with the THS and whether modality of care delivery influences patients’ care decisions. Future studies should consider a multisite design with one or more hubs and multiple spoke sites.
CONCLUSION
Telehospitalist services are a feasible and safe approach to provide inpatient services and address staffing needs of rural hospitals. To enhance program performance, it is essential to ensure adequate technological quality, clearly delineate and define roles and responsibilities of the care team, and address communication issues or staff concerns in a timely manner.
Acknowledgments
The authors thank the staff, administration, and leadership at the Tomah and Iowa City VA Medical Centers for working with us on this project. They offer special thanks to Kevin Glenn, MD, MS, Ethan Kuperman, MD, MS, FHM, and Jennifer Chapin, MSN, RN, for sharing their expertise, and the telehealth team, including Nathaniel Samuelson, Angela McDowell, and Katrin Metcalf.
Disclaimer
The views expressed in this article are those of the authors and do not necessarily represent the views of the Department of Veterans Affairs.
1. O’Connor A, Wellenius G. Rural-urban disparities in the prevalence of diabetes and coronary heart disease. Public Health. 2012;126(10):813-820. https://doi.org/10.1016/j.puhe.2012.05.029
2. Kaufman BG, Thomas SR, Randolph RK, et al. The rising rate of rural hospital closures. J Rural Health. 2016;32(1):35-43. https://doi.org/10.1111/jrh.12128
3. MacDowell M, Glasser M, Fitts M, Nielsen K, Hunsaker M. A national view of rural health workforce issues in the USA. Rural Remote Health. 2010;10(3):1531.
4. Joynt KE, Harris Y, Orav EJ, Jha AK. Quality of care and patient outcomes in critical access rural hospitals. JAMA. 2011;306(1):45-52. https://doi.org/10.1001/jama.2011.902
5. The Chartis Group. Chartis Center for Rural Health. The Rural Health Safety Net Under Pressure: Rural Hospital Vulnerability. Published February 2020. Accessed May 07, 2020. https://www.chartis.com/forum/wp-content/uploads/2020/02/CCRH_Vulnerability-Research_FiNAL-02.14.20.pdf
6. Miller KEM, James HJ, Holmes GM, Van Houtven CH. The effect of rural hospital closures on emergency medical service response and transport times. Health Serv Res. 2020;55(2):288-300. https://doi.org/10.1111/1475-6773.13254
7. Buchmueller TC, Jacobson M, Wold C. How far to the hospital? The effect of hospital closures on access to care. J Health Econ. 2006;25(4):740-761. https://doi.org/10.1016/j.jhealeco.2005.10.006
8. Mohr NM, Harland KK, Shane DM, et al. Rural patients with severe sepsis or septic shock who bypass rural hospitals have increased mortality: an instrumental variables approach. Crit Care Med. 2017;45(1):85-93. https://doi.org/10.1097/ccm.0000000000002026
9. Gutierrez J, Kuperman E, Kaboli PJ. Using telehealth as a tool for rural hospitals in the COVID-19 pandemic response. J Rural Health. 2020;10.1111/jrh.12443. https://doi.org/10.1111/jrh.12443
10. Kisuule F, Howell EE. Hospitalists and their impact on quality, patient safety, and satisfaction. Obstet Gynecol Clin North Am. 2015;42(3):433-446. https://doi.org/10.1016/j.ogc.2015.05.003
11. Peterson MC. A systematic review of outcomes and quality measures in adult patients cared for by hospitalists vs nonhospitalists. Mayo Clin Proc. 2009;84(3):248-254. https://doi.org/10.4065/84.3.248
12. Auerbach AD, Wachter RM, Katz P, Showstack J, Baron RB, Goldman L. Implementation of a voluntary hospitalist service at a community teaching hospital: improved clinical efficiency and patient outcomes. Ann Intern Med. 2002;137(11):859-865. https://doi.org/10.7326/0003-4819-137-11-200212030-00006
13. Casey MM, Hung P, Moscovice I, Prasad S. The use of hospitalists by small rural hospitals: results of a national survey. Med Care Res Rev. 2014;71(4):356-366. https://doi.org/10.1177/1077558714533822
14. Sanders RB, Simpson KN, Kazley AS, Giarrizzi DP. New hospital telemedicine services: potential market for a nighttime telehospitalist service. Telemed J E Health. 2014;20(10):902-908. https://doi.org/10.1089/tmj.2013.0344
15. Department of Veterans Affairs. Office of Inspector General. OIG Determination of Veterans Health Administration’s Occupational Staffing Shortages. Published September 30, 2019. Accessed June 15, 2020. https://www.va.gov/oig/pubs/VAOIG-19-00346-241.pdf
16. Gutierrez J, Moeckli J, McAdams N, Kaboli PJ. Perceptions of telehospitalist services to address staffing needs in rural and low complexity hospitals in the Veterans Health Administration. J Rural Health. 2019;36(3):355-359. https://doi.org/10.1111/jrh.12403
17. Eagle Telemedicine. EAGLE TELEMEDICINE NIGHT COVERAGE SOLUTIONS: Why They Work for Hospitals and Physicians. Accessed May 28, 2018. http://www.eagletelemedicine.com/wp-content/uploads/2016/11/EHP_WP_Telenocturnist_FINAL.pdf
18. Gujral J, Antoine C, Chandra S. The role of telehospitalist in COVID-19 response: Hospitalist caring remotely for New York patients explain their role. ACP Hospitalist. 2020; May 2020.
19. Kuperman EF, Linson EL, Klefstad K, Perry E, Glenn K. The virtual hospitalist: a single-site implementation bringing hospitalist coverage to critical access hospitals. J Hosp Med. 2018;13(11):759-763. https://doi.org/10.12788/jhm.3061
20. JaKa MM, Dinh JM, Ziegenfuss JY, et al. Patient and care team perspectives of telemedicine in critical access hospitals. J Hosp Med. 2020;15(6):345-348. https://doi.org/10.12788/jhm.3412
21. Khanal S, Burgon J, Leonard S, Griffiths M, Eddowes LA. Recommendations for the improved effectiveness and reporting of telemedicine programs in developing countries: results of a systematic literature review. Telemed J E Health. 2015;21(11):903-915. https://doi.org/10.1089/tmj.2014.0194
22. Ogrinc G, Davies L, Goodman D, Batalden P, Davidoff F, Stevens D. SQUIRE 2.0 (Standards for QUality Improvement Reporting Excellence): revised publication guidelines from a detailed consensus process. BMJ Qual Saf. 2016;25(12):986-992. https://doi.org/10.1136/bmjqs-2015-004411
23. VHA Office of Rural Health. ORH 2020-2024 STRATEGIC PLAN. In: U.S. Department of Veterans Affairs, ed 2020. Accessed January 18, 2021 https://www.ruralhealth.va.gov/aboutus/index.asp
24. Veterans Health Administration. About VHA. In: U.S. Department of Veterans Affairs, ed. 2019. Accessed January 18, 2021.https://www.va.gov/health/aboutvha.asp
25. GeoSpatial Outcomes Division. VHA Office of Rural Health. U.S. Department of Veterans Affairs. Rural Veterans Health Care Atlas. 2nd ed - FY-2015. Accessed July 30, 2020. https://www.ruralhealth.va.gov/docs/atlas/CHAPTER_02_RHRI_Pts_treated_at_VAMCs.pdf
26. Wagner TH, Upadhyay A, Cowgill E, et al. Risk adjustment tools for learning health systems: a comparison of DxCG and CMS-HCC V21. Health Serv Res. 2016;51(5):2002-2019. https://doi.org/10.1111/1475-6773.12454
27. Wagner T, Stefos T, Moran E, et al. Technical Report 30: Risk Adjustment: Guide to the V21 and Nosos Risk Score Programs. Updated February 8, 2016. Accessed July 30, 2020. https://www.herc.research.va.gov/include/page.asp?id=technical-report-risk-adjustment
28. The R Foundation. The R Project for Statistical Computing. Accessed August 10, 2020. https://www.R-project.org/
29. Cleary PD, Meterko M, Wright SM, Zaslavsky AM. Are comparisons of patient experiences across hospitals fair? A study in Veterans Health Administration hospitals. Med Care. 2014;52(7):619-625. https://doi.org/10.1097/mlr.0000000000000144
30. Giordano LA, Elliott MN, Goldstein E, Lehrman WG, Spencer PA. Development, implementation, and public reporting of the HCAHPS survey. Med Care Res Rev. 2010;67(1):27-37. doi:10.1177/1077558709341065
31. Boltz M, Cuellar NG, Cole C, Pistorese B. Comparing an on-site nurse practitioner with telemedicine physician support hospitalist programme with a traditional physician hospitalist programme. J Telemed and Telecare. 2019;25(4):213-220. https://doi.org/10.1177%2F1357633X18758744
32. Quinn R. The pros and cons of locum tenens for hospitalists. The Hospitalist. 2012(12). Accessed May 29, 2018. https://www.the-hospitalist.org/hospitalist/article/124988/pros-and-cons-locum-tenens-hospitalists
33. Blumenthal DM, Olenski AR, Tsugawa Y, Jena AB. Association between treatment by locum tenens internal medicine physicians and 30-day mortality among hospitalized Medicare beneficiaries. JAMA. 2017;318(21):2119-2129. https://doi.org/10.1001/jama.2017.17925
34. Butcher L. Nurses as hospitalists | AHA Trustee Services. American Hospital Association. Accessed July 14, 2020 https://trustees.aha.org/articles/1238-nurses-as-hospitalists
35. Kartha A, Restuccia JD, Burgess JF, Jr, et al. Nurse practitioner and physician assistant scope of practice in 118 acute care hospitals. J Hosp Med. 2014;9(10):615-620. https://doi.org/10.1002/jhm.2231
Healthcare in rural areas faces increasing challenges due to community hospital closures, physician shortages, and a more concentrated population of older adults with higher rates of comorbid conditions than their urban counterparts.1-3 Critical access hospitals (CAHs), which primarily serve rural areas, have fewer clinical capabilities, worse process-of-care measures, and higher mortality rates for some conditions when compared to non-CAHs.4 As such, CAHs are closing at record numbers across the United States,5 resulting in loss of available hospital beds and patient access to timely emergency services,6 which can worsen outcomes, further widening the rural-urban healthcare gap.7,8 Furthermore, this strain on an overwhelmed health system in the most vulnerable areas restricts the ability to respond to healthcare crises like the coronavirus disease 2019 pandemic.9
Providing adequate staff for currently available hospital beds is also a problem in rural areas. Studies demonstrating improved outcomes, decreased length of stay (LOS), and increased quality with hospitalist services have resulted in a high demand for hospitalists nationwide.10-12 Recruiting hospitalists to work in rural areas, however, has become increasingly challenging due to low-patient volumes, financial viability of hospitalist-model adoption, and provider shortages.13,14 Recently, the Veterans Health Administration (VHA) reported a 28% nationwide shortage of hospitalists,15 which disproportionally affects rural VHA hospitals. Staffing difficulties and reliance on intermittent providers were reported by more than 80% of rural and low-complexity VHA facilities.16
Telehospitalist services (THS) can help deliver high-quality care to rural residents locally, decrease travel expenses, support hospital volume, and increase healthcare capacity in response to a pandemic.14,17,18 Only a few studies have described THS (mostly with overnight or cross-coverage models directed to CAHs), and clinical outcomes have been inconsistently reported.17,19-21 Furthermore, no program has been conducted within an integrated health system akin to the VHA. The primary objective of this quality improvement (QI) initiative was to perform a mixed-methods evaluation of THS between VHA hospitals to compare clinical outcomes and patient and staff satisfaction. Secondary outcomes included description of the implementation process, unexpected challenges, and subsequent QI initiatives. These results will expand the knowledge on feasibility of THS and provide implementation guidance.
METHODS
A mixed-methods approach was used to evaluate outcomes of this QI project. Reporting follows the revised Standards for Quality Improvement Reporting Excellence (SQUIRE 2.0).22
Context
The VHA is the largest integrated healthcare system in the United States, with more than 8 million veterans enrolled, more than 30% of whom reside in a rural area. The VHA comprises more than 1,000 outpatient clinics and 170 acute care VA Medical Centers,23,24 including more than 35 rural and low-complexity hospitals.25 Low-complexity hospitals are those with the lowest volume and levels of patient complexity and minimal or no teaching programs, research, intensive care unit (ICU) beds, and subspecialists. Lack of reimbursement and interstate licensing, often cited as barriers to telemedicine, do not apply to the VHA. The hub site was a large tertiary care (high-complexity) VHA hospital located in Iowa City, Iowa. The spoke site was a low-complexity (10-bed acute inpatient unit with no ICU) rural VA hospital located in Tomah, Wisconsin.
Study Population
The preimplementation cohort for comparison included all patients admitted between January 1, 2018, and January 6, 2019. The postimplementation study cohort included all observation and acute care admissions during the pilot phase (January 7 to May 3, 2019) and sustainability phase (July 15 to December 31, 2019). The postimplementation analysis excluded the time period of May 4 to July 14, 2019, due to an interruption (gap) in THS. The gap period allowed for preliminary data analysis, optimization of the telecommunication system, and the recruitment and training of additional providers who could provide long-term staffing to the service.
Intervention
Preimplementation
Prior to THS implementation, Tomah’s inpatient ward was staffed by one physician per shift, who could be a hospitalist, medical officer of the day (MOD), or an intermittent provider (locum tenens). Hospitalists covering the acute inpatient ward prior to the THS transitioned to cover weekends, nights, and urgent care service shifts.
We visited the spoke site and held information-sharing sessions with key stakeholders (administrators, clinician leaders, nurses, and ancillary staff) prior to kick-off. Recurrent phone meetings addressed anticipated and emerging challenges. Telehospitalist and local providers underwent technology and service training.
Technology and Connectivity
A low-cost technology system using tablet computers provided Health Insurance Portability and Accountability Act–compliant videoconferencing with a telehospitalist at the hub site. An Eko-Core digital stethoscope® with a web-based audio stream was available. Telehospitalists conducted encounters from a private office space with telehealth capabilities. A total of $9,000 was spent on equipment at both sites. Due to connectivity problems and data limits, the tablets were switched to mobile computer-on-wheels workstations and hospital-based Wi-Fi for the sustainability phase.
THS Description
An experienced hub hospitalist, together with an advanced practice provider (APP; nurse practitioner [NP] or physician assistant [PA]), cared for all patients admitted to the 10-bed inpatient unit at the spoke site, Monday through Friday from 8:00 AM to 4:30 PM. The APP had limited or no prior experience in acute inpatient medicine. The telehospitalist worked as a team with the APP. The APP was the main point of contact for nurses, performed physical examinations, and directed patient care to their level of comfort (in a similar manner as a teaching team). The telehospitalist conducted bedside patient rounds, participated in multidisciplinary huddles, and shared clinical documentation and administrative duties with the APP. The telehospitalist was the primary staff for admitted patients and had full access to the electronic health record (EHR). The THS was staffed by 10 hospitalists during the study period. Overnight and weekend cross-coverage and admissions were performed by MODs, who also covered the urgent care and cross-covered other nonmedical units.
Quantitative Evaluation Methods
Workload and Clinical Outcomes
An EHR query identified all patients admitted during the pre- and postimplementation periods. Demographic data, clinical Nosos risk scores,26,27 and top admission diagnoses were reported. Workload was evaluated using the average number of encounters per day and self-reported telehospitalist worksheets, which were cross-referenced with EHR data. Clinical outcomes included LOS, 30-day hospital readmission rate, 30-day standardized mortality (SMR30), in-hospital mortality, and VHA-specific inpatient quality metrics. Independent sample t tests for continuous variables and chi-square tests or Fisher’s exact test (for patient class) for categorical variables were used to compare pre- and postimplementation groups. Statistical process control (SPC) charts evaluated changes over time. All analyses were conducted using Microsoft Excel and R.28
Anonymous surveys were distributed to spoke-site inpatient and administrative staff at 1 month and 12 months postimplementation, assessing satisfaction, technology/connectivity, communication, and challenges (Appendix Figure 1). Satisfaction of the telehospitalist physicians at the hub site was measured 12 months postimplementation by a 26-question survey assessing the same domains, plus quality of care (Appendix Figure 2).
Patient Satisfaction
The VHA Survey of Healthcare Experiences of Patients (SHEP), a version of the Hospital Consumer Assessment of Healthcare Providers and Systems Survey,29,30 was mailed to all patients after discharge. Survey responses concerning inpatient provider care (eg, care coordination, communication, hospital rating, willingness to recommend the hospital) during the pre- and postimplementation phases were compared using a two-sample test of independent proportions. Responses obtained during May and June 2019 were excluded.
Qualitative Evaluation Methods
The authors had full access to, and took full responsibility for, the integrity of the data. The project was evaluated by the University of Iowa Institutional Review Board and the Iowa City VA Research and Development Committee and was determined to be a non–human-subjects QI project.
RESULTS
Quantitative Workload and Clinical Outcomes
There were 822 admissions during the preimplementation period and 550 admissions during the postimplementation period (253 during the pilot and 297 during sustainability phase). Patient characteristics pre- and postimplementation were not significantly different (Table 1). The median patient age was 65 years; 96% of patients were male, and 83% were rural residents. The most common admission diagnosis was alcohol-related (36%); regarding patient disposition, 78% of admissions were discharged home.

Workload
There were 502 patient encounters staffed by the telehospitalist in the pilot phase, with an average of 6.25 encounters per day, and a telehospitalist-reported workload of 7 hours per day. There were 538 patient encounters, with an average of 4.67 encounters per day and a workload of 5.6 hours per day in the sustainability phase. The average daily census decreased from 5.0 (SD, 1.1) patients per day during preimplementation to 3.1 (SD, 0.5) patients per day during postimplementation (Table 2). In some of the months during the study period, admissions decreased below the lower SPC limit, suggesting a significant change (Figure). Adjusted LOS was significantly lower, with 3.0 (SD, 0.7) days vs 2.3 (SD, 0.3) days in the pre- and postimplementation periods, respectively. Bed occupancy rates were significantly lower in the sustainability phase compared with the pilot phase and the preimplementation period. Readmission rates varied, ranging from <10% to >30%, not significantly different but slightly higher in the postimplementation period. Readmission rates for heart failure, chronic obstructive pulmonary disease, and pneumonia remained unchanged; other medical readmissions (mostly alcohol-related) were slightly higher in the postimplementation period.

In-hospital mortality and SMR30 did not change significantly, but there was improvement in the 12-month rolling average of the observed/expected SMR30 from 1.40 to 1.08. Additional VHA-specific quality metrics were monitored and showed either small improvements or no change (data not shown).

Satisfaction at Hub and Spoke Sites
After sending two reminder communications via email, the telehospitalist satisfaction survey had a total response rate of 90% (9/10). Telehospitalists were satisfied or very satisfied (89%) with the program and the local providers (88.9%), rating their experience as good or excellent (100%) (Table 3). Communication with patients, families, and local staff was noted as being “positive” or “mostly positive.” Telehospitalists reported confidence in the accuracy of their diagnoses and rated the quality of care as being equal to that of a face-to-face encounter. Connectivity problems were prevalent, although most providers were able to resort to a back-up plan. Other challenges included differences in culture and concerns about liability. We received 27 responses from the spoke-site satisfaction survey; the response rate could not be determined because the survey was distributed by the spoke site for anonymity. Of the respondents, 37% identified as nurses, 25.9% as healthcare providers (APPs or physicians), and 33.3% as other staff (eg, social worker, nutritionist, physical therapist, utilization management, administrators); 3.7% did not respond. Among the participants, 88% had personally interacted with the THS. Most providers and other staff perceived THS as valuable (57.1% and 77.8%, respectively) and were satisfied or highly satisfied with THS (57.1% and 55.6%, respectively). On average, nurses provided lower ratings across all survey items than providers and other staff. Challenges noted by all staff included issues with communication, workflow, and technology/connectivity.

Qualitative Strengths
Our process evaluation identified high quality of care and teamwork as contributors to the success of the program. Overall, staff credited perceived improvements in quality of care to the quality of providers staffing the THS, including the local APPs. Noting the telehospitalists’ knowledge base and level of engagement as key attributes, one staff member commented: “I prefer a telehospitalist that really care[s] about patients than some provider that is physically here but does not engage.” Staff perceived improvements in the continuity of care, as well as care processes such as handoffs and transitions of care.
Improvements in teamwork were perceived compared with the previous model of care. Telehospitalists were lauded for their professionalism and communication skills. Overall, nurses felt providers in the THS listened more to their views. In addition, nurse respondents felt they could learn from several providers and said they enjoyed the telehospitalists’ disposition to teach and discuss patient care. The responsiveness of the THS staff was instrumental in building teamwork and acceptance. A bedside interdisciplinary protocol was established for appropriate patients. Local staff felt this was crucial for teamwork and patient satisfaction. Telehospitalists reported high-value in interdisciplinary rounds, facilitating interaction with nurses and ancillary staff. Handoff problems were identified, leading to QI initiatives to mitigate those issues.
Challenges
The survey identified administrative barriers, technical difficulties, workflow constraints, and clinical concerns. The credentialing process was complicated, delaying the onboarding of telehospitalists. Internet connectivity was inconsistent, leading to disruption in video communications; however, during the sustainability phase, updated technology improved communications. The communication workflow was resisted by some nurses, who wanted to phone the telehospitalist directly rather than having the local APP as the first contact. Secure messaging was enabled to allow nurses direct contact during the sustainability phase.
Workload was a concern among telehospitalists and local staff. Telehospitalists perceived the documentation requirements and administrative workload to be two to three times higher than at other hospitals—despite the lower number of encounters. Finally, clinical concerns from spoke-site clinicians included a perceived rise in the acuity of patients (which was not evident by the Nosos score) and delayed decisions to transfer-out patients. These concerns were addressed with educational sessions for telehospitalists during the sustainability phase.
Additional Quality Improvement Projects
The implementation of THS resulted in QI initiatives at the spoke site, including an EHR-integrated handoff tool; a documentation evaluation that led to the elimination of duplicative, inefficient, and error-prone templates; and a revision of the alcohol withdrawal treatment protocol during the sustainability phase to reduce the use of intravenous benzodiazepines. A more comprehensive benzodiazepine-sparing alcohol withdrawal treatment protocol was also developed but was not implemented until after the study period (January 2020).
DISCUSSION
Our pre-post study evaluation found implementation of a THS to be noninferior to face-to-face care, with no significant change in mortality, readmission rate, or patient satisfaction. The significant improvement observed in LOS is consistent with the adoption of hospitalist models in other medical care settings,11 but had not been reported by previous telehospitalist studies. For example, in their retrospective chart review comparing an NP-supported telehospitalist model to locum tenens hospitalists, Boltz et al found no difference in LOS.31 Moreover, as in our study, they found no differences in readmissions, mortality, and patient satisfaction.31 Similarly, Kuperman et al reported unchanged daily census, LOS, and transfer rates from a CAH with their virtual hospitalist program, but a decrease in the percentage of patients transferred-out from the emergency department, suggesting that more patients were treated locally.19
Reduction in LOS is one of the primary measures of efficiency in hospital care31; reducing LOS while maintaining the quality of care lowers hospital costs. The reduction in LOS in our study could be attributed to greater continuity of care, engagement/experience of the telehospitalists, or other factors. This decrease in LOS and slight reduction in admissions resulted in an overall lower daily census during the study period and impacted efficiency. Our study was unable to determine the cause for the reduction in admissions; however, several concurrent events, including the expansion of community-care options for veterans under the MISSION ACT (Maintaining Internal Systems and Strengthening Integrated Outside Networks Act) in June 2019, a nationwide smoking ban at VA facilities (October 2019), and a modification in the alcohol withdrawal treatment protocol might have influenced veterans’ choice of hospital.
Readmission rates were slightly higher, though nonsignificant, in the postimplementation period. Alcohol-related readmissions accounted for most readmissions; some of the protocol changes, such as admitting all patients with alcohol withdrawal to inpatient class instead of admitting some to the observation class, accounted for part of the increase in readmission rates. Readmission rates for other conditions such as chronic obstructive pulmonary disease, chronic heart failure, or pneumonia were not significantly different, suggesting that the reduction in LOS did not result in an unintended increased readmission rate for those conditions.
Rural hospitals are struggling with staffing and finances. Resorting to locum tenens staffing is costly and can result in variable quality of care.32,33 APPs are increasingly taking on hospitalist positions, with 65% of adult hospitalist programs, including half of all VHA hospitals, employing NPs and PAs.34,35 In response to this expanded scope of practice, hospitals employing APPs in hospitalist roles must comply with state and federal laws, which often require that APPs be supervised by or work in collaboration with an on-site or off-site physician. The THS is a great model to support APPs and address staffing and cost challenges in low-volume rural facilities, while maintaining quality of care. Some APP-telehospitalist programs similar to ours have reported cost reductions of up 58% compared to programs that employ locum tenens physicians.31 In our model, we assume that a single telehospitalist hub could provide coverage to two or three spoke sites with APP support, reducing staffing costs.
Hub telehospitalists reported satisfaction with the program, and they perceived the quality of care to be comparable to face-to-face encounters; their responses were consistent with those previously reported in an evaluation of telemedicine acute care by JaKa et al.
This study has several limitations. First, the VHA is an integrated health system, one that serves an older, predominantly male patient population. Also, the lack of reimbursement and interstate licensing restrictions limit generalizability of these results to other CAHs or healthcare systems. Furthermore, the intervention was limited to a single rural site; while this allowed for a detailed evaluation, unique barriers or facilitators might exist that limit its applicability. In addition, QI initiatives implemented by the VHA during the project period might have confounded some of our results. Last, patient satisfaction survey data are overall limited in their ability to fully assess patient’s experience and satisfaction with the program. Further qualitative studies are needed to gain deeper insight into patient perspectives with the THS and whether modality of care delivery influences patients’ care decisions. Future studies should consider a multisite design with one or more hubs and multiple spoke sites.
CONCLUSION
Telehospitalist services are a feasible and safe approach to provide inpatient services and address staffing needs of rural hospitals. To enhance program performance, it is essential to ensure adequate technological quality, clearly delineate and define roles and responsibilities of the care team, and address communication issues or staff concerns in a timely manner.
Acknowledgments
The authors thank the staff, administration, and leadership at the Tomah and Iowa City VA Medical Centers for working with us on this project. They offer special thanks to Kevin Glenn, MD, MS, Ethan Kuperman, MD, MS, FHM, and Jennifer Chapin, MSN, RN, for sharing their expertise, and the telehealth team, including Nathaniel Samuelson, Angela McDowell, and Katrin Metcalf.
Disclaimer
The views expressed in this article are those of the authors and do not necessarily represent the views of the Department of Veterans Affairs.
Healthcare in rural areas faces increasing challenges due to community hospital closures, physician shortages, and a more concentrated population of older adults with higher rates of comorbid conditions than their urban counterparts.1-3 Critical access hospitals (CAHs), which primarily serve rural areas, have fewer clinical capabilities, worse process-of-care measures, and higher mortality rates for some conditions when compared to non-CAHs.4 As such, CAHs are closing at record numbers across the United States,5 resulting in loss of available hospital beds and patient access to timely emergency services,6 which can worsen outcomes, further widening the rural-urban healthcare gap.7,8 Furthermore, this strain on an overwhelmed health system in the most vulnerable areas restricts the ability to respond to healthcare crises like the coronavirus disease 2019 pandemic.9
Providing adequate staff for currently available hospital beds is also a problem in rural areas. Studies demonstrating improved outcomes, decreased length of stay (LOS), and increased quality with hospitalist services have resulted in a high demand for hospitalists nationwide.10-12 Recruiting hospitalists to work in rural areas, however, has become increasingly challenging due to low-patient volumes, financial viability of hospitalist-model adoption, and provider shortages.13,14 Recently, the Veterans Health Administration (VHA) reported a 28% nationwide shortage of hospitalists,15 which disproportionally affects rural VHA hospitals. Staffing difficulties and reliance on intermittent providers were reported by more than 80% of rural and low-complexity VHA facilities.16
Telehospitalist services (THS) can help deliver high-quality care to rural residents locally, decrease travel expenses, support hospital volume, and increase healthcare capacity in response to a pandemic.14,17,18 Only a few studies have described THS (mostly with overnight or cross-coverage models directed to CAHs), and clinical outcomes have been inconsistently reported.17,19-21 Furthermore, no program has been conducted within an integrated health system akin to the VHA. The primary objective of this quality improvement (QI) initiative was to perform a mixed-methods evaluation of THS between VHA hospitals to compare clinical outcomes and patient and staff satisfaction. Secondary outcomes included description of the implementation process, unexpected challenges, and subsequent QI initiatives. These results will expand the knowledge on feasibility of THS and provide implementation guidance.
METHODS
A mixed-methods approach was used to evaluate outcomes of this QI project. Reporting follows the revised Standards for Quality Improvement Reporting Excellence (SQUIRE 2.0).22
Context
The VHA is the largest integrated healthcare system in the United States, with more than 8 million veterans enrolled, more than 30% of whom reside in a rural area. The VHA comprises more than 1,000 outpatient clinics and 170 acute care VA Medical Centers,23,24 including more than 35 rural and low-complexity hospitals.25 Low-complexity hospitals are those with the lowest volume and levels of patient complexity and minimal or no teaching programs, research, intensive care unit (ICU) beds, and subspecialists. Lack of reimbursement and interstate licensing, often cited as barriers to telemedicine, do not apply to the VHA. The hub site was a large tertiary care (high-complexity) VHA hospital located in Iowa City, Iowa. The spoke site was a low-complexity (10-bed acute inpatient unit with no ICU) rural VA hospital located in Tomah, Wisconsin.
Study Population
The preimplementation cohort for comparison included all patients admitted between January 1, 2018, and January 6, 2019. The postimplementation study cohort included all observation and acute care admissions during the pilot phase (January 7 to May 3, 2019) and sustainability phase (July 15 to December 31, 2019). The postimplementation analysis excluded the time period of May 4 to July 14, 2019, due to an interruption (gap) in THS. The gap period allowed for preliminary data analysis, optimization of the telecommunication system, and the recruitment and training of additional providers who could provide long-term staffing to the service.
Intervention
Preimplementation
Prior to THS implementation, Tomah’s inpatient ward was staffed by one physician per shift, who could be a hospitalist, medical officer of the day (MOD), or an intermittent provider (locum tenens). Hospitalists covering the acute inpatient ward prior to the THS transitioned to cover weekends, nights, and urgent care service shifts.
We visited the spoke site and held information-sharing sessions with key stakeholders (administrators, clinician leaders, nurses, and ancillary staff) prior to kick-off. Recurrent phone meetings addressed anticipated and emerging challenges. Telehospitalist and local providers underwent technology and service training.
Technology and Connectivity
A low-cost technology system using tablet computers provided Health Insurance Portability and Accountability Act–compliant videoconferencing with a telehospitalist at the hub site. An Eko-Core digital stethoscope® with a web-based audio stream was available. Telehospitalists conducted encounters from a private office space with telehealth capabilities. A total of $9,000 was spent on equipment at both sites. Due to connectivity problems and data limits, the tablets were switched to mobile computer-on-wheels workstations and hospital-based Wi-Fi for the sustainability phase.
THS Description
An experienced hub hospitalist, together with an advanced practice provider (APP; nurse practitioner [NP] or physician assistant [PA]), cared for all patients admitted to the 10-bed inpatient unit at the spoke site, Monday through Friday from 8:00 AM to 4:30 PM. The APP had limited or no prior experience in acute inpatient medicine. The telehospitalist worked as a team with the APP. The APP was the main point of contact for nurses, performed physical examinations, and directed patient care to their level of comfort (in a similar manner as a teaching team). The telehospitalist conducted bedside patient rounds, participated in multidisciplinary huddles, and shared clinical documentation and administrative duties with the APP. The telehospitalist was the primary staff for admitted patients and had full access to the electronic health record (EHR). The THS was staffed by 10 hospitalists during the study period. Overnight and weekend cross-coverage and admissions were performed by MODs, who also covered the urgent care and cross-covered other nonmedical units.
Quantitative Evaluation Methods
Workload and Clinical Outcomes
An EHR query identified all patients admitted during the pre- and postimplementation periods. Demographic data, clinical Nosos risk scores,26,27 and top admission diagnoses were reported. Workload was evaluated using the average number of encounters per day and self-reported telehospitalist worksheets, which were cross-referenced with EHR data. Clinical outcomes included LOS, 30-day hospital readmission rate, 30-day standardized mortality (SMR30), in-hospital mortality, and VHA-specific inpatient quality metrics. Independent sample t tests for continuous variables and chi-square tests or Fisher’s exact test (for patient class) for categorical variables were used to compare pre- and postimplementation groups. Statistical process control (SPC) charts evaluated changes over time. All analyses were conducted using Microsoft Excel and R.28
Anonymous surveys were distributed to spoke-site inpatient and administrative staff at 1 month and 12 months postimplementation, assessing satisfaction, technology/connectivity, communication, and challenges (Appendix Figure 1). Satisfaction of the telehospitalist physicians at the hub site was measured 12 months postimplementation by a 26-question survey assessing the same domains, plus quality of care (Appendix Figure 2).
Patient Satisfaction
The VHA Survey of Healthcare Experiences of Patients (SHEP), a version of the Hospital Consumer Assessment of Healthcare Providers and Systems Survey,29,30 was mailed to all patients after discharge. Survey responses concerning inpatient provider care (eg, care coordination, communication, hospital rating, willingness to recommend the hospital) during the pre- and postimplementation phases were compared using a two-sample test of independent proportions. Responses obtained during May and June 2019 were excluded.
Qualitative Evaluation Methods
The authors had full access to, and took full responsibility for, the integrity of the data. The project was evaluated by the University of Iowa Institutional Review Board and the Iowa City VA Research and Development Committee and was determined to be a non–human-subjects QI project.
RESULTS
Quantitative Workload and Clinical Outcomes
There were 822 admissions during the preimplementation period and 550 admissions during the postimplementation period (253 during the pilot and 297 during sustainability phase). Patient characteristics pre- and postimplementation were not significantly different (Table 1). The median patient age was 65 years; 96% of patients were male, and 83% were rural residents. The most common admission diagnosis was alcohol-related (36%); regarding patient disposition, 78% of admissions were discharged home.

Workload
There were 502 patient encounters staffed by the telehospitalist in the pilot phase, with an average of 6.25 encounters per day, and a telehospitalist-reported workload of 7 hours per day. There were 538 patient encounters, with an average of 4.67 encounters per day and a workload of 5.6 hours per day in the sustainability phase. The average daily census decreased from 5.0 (SD, 1.1) patients per day during preimplementation to 3.1 (SD, 0.5) patients per day during postimplementation (Table 2). In some of the months during the study period, admissions decreased below the lower SPC limit, suggesting a significant change (Figure). Adjusted LOS was significantly lower, with 3.0 (SD, 0.7) days vs 2.3 (SD, 0.3) days in the pre- and postimplementation periods, respectively. Bed occupancy rates were significantly lower in the sustainability phase compared with the pilot phase and the preimplementation period. Readmission rates varied, ranging from <10% to >30%, not significantly different but slightly higher in the postimplementation period. Readmission rates for heart failure, chronic obstructive pulmonary disease, and pneumonia remained unchanged; other medical readmissions (mostly alcohol-related) were slightly higher in the postimplementation period.

In-hospital mortality and SMR30 did not change significantly, but there was improvement in the 12-month rolling average of the observed/expected SMR30 from 1.40 to 1.08. Additional VHA-specific quality metrics were monitored and showed either small improvements or no change (data not shown).

Satisfaction at Hub and Spoke Sites
After sending two reminder communications via email, the telehospitalist satisfaction survey had a total response rate of 90% (9/10). Telehospitalists were satisfied or very satisfied (89%) with the program and the local providers (88.9%), rating their experience as good or excellent (100%) (Table 3). Communication with patients, families, and local staff was noted as being “positive” or “mostly positive.” Telehospitalists reported confidence in the accuracy of their diagnoses and rated the quality of care as being equal to that of a face-to-face encounter. Connectivity problems were prevalent, although most providers were able to resort to a back-up plan. Other challenges included differences in culture and concerns about liability. We received 27 responses from the spoke-site satisfaction survey; the response rate could not be determined because the survey was distributed by the spoke site for anonymity. Of the respondents, 37% identified as nurses, 25.9% as healthcare providers (APPs or physicians), and 33.3% as other staff (eg, social worker, nutritionist, physical therapist, utilization management, administrators); 3.7% did not respond. Among the participants, 88% had personally interacted with the THS. Most providers and other staff perceived THS as valuable (57.1% and 77.8%, respectively) and were satisfied or highly satisfied with THS (57.1% and 55.6%, respectively). On average, nurses provided lower ratings across all survey items than providers and other staff. Challenges noted by all staff included issues with communication, workflow, and technology/connectivity.

Qualitative Strengths
Our process evaluation identified high quality of care and teamwork as contributors to the success of the program. Overall, staff credited perceived improvements in quality of care to the quality of providers staffing the THS, including the local APPs. Noting the telehospitalists’ knowledge base and level of engagement as key attributes, one staff member commented: “I prefer a telehospitalist that really care[s] about patients than some provider that is physically here but does not engage.” Staff perceived improvements in the continuity of care, as well as care processes such as handoffs and transitions of care.
Improvements in teamwork were perceived compared with the previous model of care. Telehospitalists were lauded for their professionalism and communication skills. Overall, nurses felt providers in the THS listened more to their views. In addition, nurse respondents felt they could learn from several providers and said they enjoyed the telehospitalists’ disposition to teach and discuss patient care. The responsiveness of the THS staff was instrumental in building teamwork and acceptance. A bedside interdisciplinary protocol was established for appropriate patients. Local staff felt this was crucial for teamwork and patient satisfaction. Telehospitalists reported high-value in interdisciplinary rounds, facilitating interaction with nurses and ancillary staff. Handoff problems were identified, leading to QI initiatives to mitigate those issues.
Challenges
The survey identified administrative barriers, technical difficulties, workflow constraints, and clinical concerns. The credentialing process was complicated, delaying the onboarding of telehospitalists. Internet connectivity was inconsistent, leading to disruption in video communications; however, during the sustainability phase, updated technology improved communications. The communication workflow was resisted by some nurses, who wanted to phone the telehospitalist directly rather than having the local APP as the first contact. Secure messaging was enabled to allow nurses direct contact during the sustainability phase.
Workload was a concern among telehospitalists and local staff. Telehospitalists perceived the documentation requirements and administrative workload to be two to three times higher than at other hospitals—despite the lower number of encounters. Finally, clinical concerns from spoke-site clinicians included a perceived rise in the acuity of patients (which was not evident by the Nosos score) and delayed decisions to transfer-out patients. These concerns were addressed with educational sessions for telehospitalists during the sustainability phase.
Additional Quality Improvement Projects
The implementation of THS resulted in QI initiatives at the spoke site, including an EHR-integrated handoff tool; a documentation evaluation that led to the elimination of duplicative, inefficient, and error-prone templates; and a revision of the alcohol withdrawal treatment protocol during the sustainability phase to reduce the use of intravenous benzodiazepines. A more comprehensive benzodiazepine-sparing alcohol withdrawal treatment protocol was also developed but was not implemented until after the study period (January 2020).
DISCUSSION
Our pre-post study evaluation found implementation of a THS to be noninferior to face-to-face care, with no significant change in mortality, readmission rate, or patient satisfaction. The significant improvement observed in LOS is consistent with the adoption of hospitalist models in other medical care settings,11 but had not been reported by previous telehospitalist studies. For example, in their retrospective chart review comparing an NP-supported telehospitalist model to locum tenens hospitalists, Boltz et al found no difference in LOS.31 Moreover, as in our study, they found no differences in readmissions, mortality, and patient satisfaction.31 Similarly, Kuperman et al reported unchanged daily census, LOS, and transfer rates from a CAH with their virtual hospitalist program, but a decrease in the percentage of patients transferred-out from the emergency department, suggesting that more patients were treated locally.19
Reduction in LOS is one of the primary measures of efficiency in hospital care31; reducing LOS while maintaining the quality of care lowers hospital costs. The reduction in LOS in our study could be attributed to greater continuity of care, engagement/experience of the telehospitalists, or other factors. This decrease in LOS and slight reduction in admissions resulted in an overall lower daily census during the study period and impacted efficiency. Our study was unable to determine the cause for the reduction in admissions; however, several concurrent events, including the expansion of community-care options for veterans under the MISSION ACT (Maintaining Internal Systems and Strengthening Integrated Outside Networks Act) in June 2019, a nationwide smoking ban at VA facilities (October 2019), and a modification in the alcohol withdrawal treatment protocol might have influenced veterans’ choice of hospital.
Readmission rates were slightly higher, though nonsignificant, in the postimplementation period. Alcohol-related readmissions accounted for most readmissions; some of the protocol changes, such as admitting all patients with alcohol withdrawal to inpatient class instead of admitting some to the observation class, accounted for part of the increase in readmission rates. Readmission rates for other conditions such as chronic obstructive pulmonary disease, chronic heart failure, or pneumonia were not significantly different, suggesting that the reduction in LOS did not result in an unintended increased readmission rate for those conditions.
Rural hospitals are struggling with staffing and finances. Resorting to locum tenens staffing is costly and can result in variable quality of care.32,33 APPs are increasingly taking on hospitalist positions, with 65% of adult hospitalist programs, including half of all VHA hospitals, employing NPs and PAs.34,35 In response to this expanded scope of practice, hospitals employing APPs in hospitalist roles must comply with state and federal laws, which often require that APPs be supervised by or work in collaboration with an on-site or off-site physician. The THS is a great model to support APPs and address staffing and cost challenges in low-volume rural facilities, while maintaining quality of care. Some APP-telehospitalist programs similar to ours have reported cost reductions of up 58% compared to programs that employ locum tenens physicians.31 In our model, we assume that a single telehospitalist hub could provide coverage to two or three spoke sites with APP support, reducing staffing costs.
Hub telehospitalists reported satisfaction with the program, and they perceived the quality of care to be comparable to face-to-face encounters; their responses were consistent with those previously reported in an evaluation of telemedicine acute care by JaKa et al.
This study has several limitations. First, the VHA is an integrated health system, one that serves an older, predominantly male patient population. Also, the lack of reimbursement and interstate licensing restrictions limit generalizability of these results to other CAHs or healthcare systems. Furthermore, the intervention was limited to a single rural site; while this allowed for a detailed evaluation, unique barriers or facilitators might exist that limit its applicability. In addition, QI initiatives implemented by the VHA during the project period might have confounded some of our results. Last, patient satisfaction survey data are overall limited in their ability to fully assess patient’s experience and satisfaction with the program. Further qualitative studies are needed to gain deeper insight into patient perspectives with the THS and whether modality of care delivery influences patients’ care decisions. Future studies should consider a multisite design with one or more hubs and multiple spoke sites.
CONCLUSION
Telehospitalist services are a feasible and safe approach to provide inpatient services and address staffing needs of rural hospitals. To enhance program performance, it is essential to ensure adequate technological quality, clearly delineate and define roles and responsibilities of the care team, and address communication issues or staff concerns in a timely manner.
Acknowledgments
The authors thank the staff, administration, and leadership at the Tomah and Iowa City VA Medical Centers for working with us on this project. They offer special thanks to Kevin Glenn, MD, MS, Ethan Kuperman, MD, MS, FHM, and Jennifer Chapin, MSN, RN, for sharing their expertise, and the telehealth team, including Nathaniel Samuelson, Angela McDowell, and Katrin Metcalf.
Disclaimer
The views expressed in this article are those of the authors and do not necessarily represent the views of the Department of Veterans Affairs.
1. O’Connor A, Wellenius G. Rural-urban disparities in the prevalence of diabetes and coronary heart disease. Public Health. 2012;126(10):813-820. https://doi.org/10.1016/j.puhe.2012.05.029
2. Kaufman BG, Thomas SR, Randolph RK, et al. The rising rate of rural hospital closures. J Rural Health. 2016;32(1):35-43. https://doi.org/10.1111/jrh.12128
3. MacDowell M, Glasser M, Fitts M, Nielsen K, Hunsaker M. A national view of rural health workforce issues in the USA. Rural Remote Health. 2010;10(3):1531.
4. Joynt KE, Harris Y, Orav EJ, Jha AK. Quality of care and patient outcomes in critical access rural hospitals. JAMA. 2011;306(1):45-52. https://doi.org/10.1001/jama.2011.902
5. The Chartis Group. Chartis Center for Rural Health. The Rural Health Safety Net Under Pressure: Rural Hospital Vulnerability. Published February 2020. Accessed May 07, 2020. https://www.chartis.com/forum/wp-content/uploads/2020/02/CCRH_Vulnerability-Research_FiNAL-02.14.20.pdf
6. Miller KEM, James HJ, Holmes GM, Van Houtven CH. The effect of rural hospital closures on emergency medical service response and transport times. Health Serv Res. 2020;55(2):288-300. https://doi.org/10.1111/1475-6773.13254
7. Buchmueller TC, Jacobson M, Wold C. How far to the hospital? The effect of hospital closures on access to care. J Health Econ. 2006;25(4):740-761. https://doi.org/10.1016/j.jhealeco.2005.10.006
8. Mohr NM, Harland KK, Shane DM, et al. Rural patients with severe sepsis or septic shock who bypass rural hospitals have increased mortality: an instrumental variables approach. Crit Care Med. 2017;45(1):85-93. https://doi.org/10.1097/ccm.0000000000002026
9. Gutierrez J, Kuperman E, Kaboli PJ. Using telehealth as a tool for rural hospitals in the COVID-19 pandemic response. J Rural Health. 2020;10.1111/jrh.12443. https://doi.org/10.1111/jrh.12443
10. Kisuule F, Howell EE. Hospitalists and their impact on quality, patient safety, and satisfaction. Obstet Gynecol Clin North Am. 2015;42(3):433-446. https://doi.org/10.1016/j.ogc.2015.05.003
11. Peterson MC. A systematic review of outcomes and quality measures in adult patients cared for by hospitalists vs nonhospitalists. Mayo Clin Proc. 2009;84(3):248-254. https://doi.org/10.4065/84.3.248
12. Auerbach AD, Wachter RM, Katz P, Showstack J, Baron RB, Goldman L. Implementation of a voluntary hospitalist service at a community teaching hospital: improved clinical efficiency and patient outcomes. Ann Intern Med. 2002;137(11):859-865. https://doi.org/10.7326/0003-4819-137-11-200212030-00006
13. Casey MM, Hung P, Moscovice I, Prasad S. The use of hospitalists by small rural hospitals: results of a national survey. Med Care Res Rev. 2014;71(4):356-366. https://doi.org/10.1177/1077558714533822
14. Sanders RB, Simpson KN, Kazley AS, Giarrizzi DP. New hospital telemedicine services: potential market for a nighttime telehospitalist service. Telemed J E Health. 2014;20(10):902-908. https://doi.org/10.1089/tmj.2013.0344
15. Department of Veterans Affairs. Office of Inspector General. OIG Determination of Veterans Health Administration’s Occupational Staffing Shortages. Published September 30, 2019. Accessed June 15, 2020. https://www.va.gov/oig/pubs/VAOIG-19-00346-241.pdf
16. Gutierrez J, Moeckli J, McAdams N, Kaboli PJ. Perceptions of telehospitalist services to address staffing needs in rural and low complexity hospitals in the Veterans Health Administration. J Rural Health. 2019;36(3):355-359. https://doi.org/10.1111/jrh.12403
17. Eagle Telemedicine. EAGLE TELEMEDICINE NIGHT COVERAGE SOLUTIONS: Why They Work for Hospitals and Physicians. Accessed May 28, 2018. http://www.eagletelemedicine.com/wp-content/uploads/2016/11/EHP_WP_Telenocturnist_FINAL.pdf
18. Gujral J, Antoine C, Chandra S. The role of telehospitalist in COVID-19 response: Hospitalist caring remotely for New York patients explain their role. ACP Hospitalist. 2020; May 2020.
19. Kuperman EF, Linson EL, Klefstad K, Perry E, Glenn K. The virtual hospitalist: a single-site implementation bringing hospitalist coverage to critical access hospitals. J Hosp Med. 2018;13(11):759-763. https://doi.org/10.12788/jhm.3061
20. JaKa MM, Dinh JM, Ziegenfuss JY, et al. Patient and care team perspectives of telemedicine in critical access hospitals. J Hosp Med. 2020;15(6):345-348. https://doi.org/10.12788/jhm.3412
21. Khanal S, Burgon J, Leonard S, Griffiths M, Eddowes LA. Recommendations for the improved effectiveness and reporting of telemedicine programs in developing countries: results of a systematic literature review. Telemed J E Health. 2015;21(11):903-915. https://doi.org/10.1089/tmj.2014.0194
22. Ogrinc G, Davies L, Goodman D, Batalden P, Davidoff F, Stevens D. SQUIRE 2.0 (Standards for QUality Improvement Reporting Excellence): revised publication guidelines from a detailed consensus process. BMJ Qual Saf. 2016;25(12):986-992. https://doi.org/10.1136/bmjqs-2015-004411
23. VHA Office of Rural Health. ORH 2020-2024 STRATEGIC PLAN. In: U.S. Department of Veterans Affairs, ed 2020. Accessed January 18, 2021 https://www.ruralhealth.va.gov/aboutus/index.asp
24. Veterans Health Administration. About VHA. In: U.S. Department of Veterans Affairs, ed. 2019. Accessed January 18, 2021.https://www.va.gov/health/aboutvha.asp
25. GeoSpatial Outcomes Division. VHA Office of Rural Health. U.S. Department of Veterans Affairs. Rural Veterans Health Care Atlas. 2nd ed - FY-2015. Accessed July 30, 2020. https://www.ruralhealth.va.gov/docs/atlas/CHAPTER_02_RHRI_Pts_treated_at_VAMCs.pdf
26. Wagner TH, Upadhyay A, Cowgill E, et al. Risk adjustment tools for learning health systems: a comparison of DxCG and CMS-HCC V21. Health Serv Res. 2016;51(5):2002-2019. https://doi.org/10.1111/1475-6773.12454
27. Wagner T, Stefos T, Moran E, et al. Technical Report 30: Risk Adjustment: Guide to the V21 and Nosos Risk Score Programs. Updated February 8, 2016. Accessed July 30, 2020. https://www.herc.research.va.gov/include/page.asp?id=technical-report-risk-adjustment
28. The R Foundation. The R Project for Statistical Computing. Accessed August 10, 2020. https://www.R-project.org/
29. Cleary PD, Meterko M, Wright SM, Zaslavsky AM. Are comparisons of patient experiences across hospitals fair? A study in Veterans Health Administration hospitals. Med Care. 2014;52(7):619-625. https://doi.org/10.1097/mlr.0000000000000144
30. Giordano LA, Elliott MN, Goldstein E, Lehrman WG, Spencer PA. Development, implementation, and public reporting of the HCAHPS survey. Med Care Res Rev. 2010;67(1):27-37. doi:10.1177/1077558709341065
31. Boltz M, Cuellar NG, Cole C, Pistorese B. Comparing an on-site nurse practitioner with telemedicine physician support hospitalist programme with a traditional physician hospitalist programme. J Telemed and Telecare. 2019;25(4):213-220. https://doi.org/10.1177%2F1357633X18758744
32. Quinn R. The pros and cons of locum tenens for hospitalists. The Hospitalist. 2012(12). Accessed May 29, 2018. https://www.the-hospitalist.org/hospitalist/article/124988/pros-and-cons-locum-tenens-hospitalists
33. Blumenthal DM, Olenski AR, Tsugawa Y, Jena AB. Association between treatment by locum tenens internal medicine physicians and 30-day mortality among hospitalized Medicare beneficiaries. JAMA. 2017;318(21):2119-2129. https://doi.org/10.1001/jama.2017.17925
34. Butcher L. Nurses as hospitalists | AHA Trustee Services. American Hospital Association. Accessed July 14, 2020 https://trustees.aha.org/articles/1238-nurses-as-hospitalists
35. Kartha A, Restuccia JD, Burgess JF, Jr, et al. Nurse practitioner and physician assistant scope of practice in 118 acute care hospitals. J Hosp Med. 2014;9(10):615-620. https://doi.org/10.1002/jhm.2231
1. O’Connor A, Wellenius G. Rural-urban disparities in the prevalence of diabetes and coronary heart disease. Public Health. 2012;126(10):813-820. https://doi.org/10.1016/j.puhe.2012.05.029
2. Kaufman BG, Thomas SR, Randolph RK, et al. The rising rate of rural hospital closures. J Rural Health. 2016;32(1):35-43. https://doi.org/10.1111/jrh.12128
3. MacDowell M, Glasser M, Fitts M, Nielsen K, Hunsaker M. A national view of rural health workforce issues in the USA. Rural Remote Health. 2010;10(3):1531.
4. Joynt KE, Harris Y, Orav EJ, Jha AK. Quality of care and patient outcomes in critical access rural hospitals. JAMA. 2011;306(1):45-52. https://doi.org/10.1001/jama.2011.902
5. The Chartis Group. Chartis Center for Rural Health. The Rural Health Safety Net Under Pressure: Rural Hospital Vulnerability. Published February 2020. Accessed May 07, 2020. https://www.chartis.com/forum/wp-content/uploads/2020/02/CCRH_Vulnerability-Research_FiNAL-02.14.20.pdf
6. Miller KEM, James HJ, Holmes GM, Van Houtven CH. The effect of rural hospital closures on emergency medical service response and transport times. Health Serv Res. 2020;55(2):288-300. https://doi.org/10.1111/1475-6773.13254
7. Buchmueller TC, Jacobson M, Wold C. How far to the hospital? The effect of hospital closures on access to care. J Health Econ. 2006;25(4):740-761. https://doi.org/10.1016/j.jhealeco.2005.10.006
8. Mohr NM, Harland KK, Shane DM, et al. Rural patients with severe sepsis or septic shock who bypass rural hospitals have increased mortality: an instrumental variables approach. Crit Care Med. 2017;45(1):85-93. https://doi.org/10.1097/ccm.0000000000002026
9. Gutierrez J, Kuperman E, Kaboli PJ. Using telehealth as a tool for rural hospitals in the COVID-19 pandemic response. J Rural Health. 2020;10.1111/jrh.12443. https://doi.org/10.1111/jrh.12443
10. Kisuule F, Howell EE. Hospitalists and their impact on quality, patient safety, and satisfaction. Obstet Gynecol Clin North Am. 2015;42(3):433-446. https://doi.org/10.1016/j.ogc.2015.05.003
11. Peterson MC. A systematic review of outcomes and quality measures in adult patients cared for by hospitalists vs nonhospitalists. Mayo Clin Proc. 2009;84(3):248-254. https://doi.org/10.4065/84.3.248
12. Auerbach AD, Wachter RM, Katz P, Showstack J, Baron RB, Goldman L. Implementation of a voluntary hospitalist service at a community teaching hospital: improved clinical efficiency and patient outcomes. Ann Intern Med. 2002;137(11):859-865. https://doi.org/10.7326/0003-4819-137-11-200212030-00006
13. Casey MM, Hung P, Moscovice I, Prasad S. The use of hospitalists by small rural hospitals: results of a national survey. Med Care Res Rev. 2014;71(4):356-366. https://doi.org/10.1177/1077558714533822
14. Sanders RB, Simpson KN, Kazley AS, Giarrizzi DP. New hospital telemedicine services: potential market for a nighttime telehospitalist service. Telemed J E Health. 2014;20(10):902-908. https://doi.org/10.1089/tmj.2013.0344
15. Department of Veterans Affairs. Office of Inspector General. OIG Determination of Veterans Health Administration’s Occupational Staffing Shortages. Published September 30, 2019. Accessed June 15, 2020. https://www.va.gov/oig/pubs/VAOIG-19-00346-241.pdf
16. Gutierrez J, Moeckli J, McAdams N, Kaboli PJ. Perceptions of telehospitalist services to address staffing needs in rural and low complexity hospitals in the Veterans Health Administration. J Rural Health. 2019;36(3):355-359. https://doi.org/10.1111/jrh.12403
17. Eagle Telemedicine. EAGLE TELEMEDICINE NIGHT COVERAGE SOLUTIONS: Why They Work for Hospitals and Physicians. Accessed May 28, 2018. http://www.eagletelemedicine.com/wp-content/uploads/2016/11/EHP_WP_Telenocturnist_FINAL.pdf
18. Gujral J, Antoine C, Chandra S. The role of telehospitalist in COVID-19 response: Hospitalist caring remotely for New York patients explain their role. ACP Hospitalist. 2020; May 2020.
19. Kuperman EF, Linson EL, Klefstad K, Perry E, Glenn K. The virtual hospitalist: a single-site implementation bringing hospitalist coverage to critical access hospitals. J Hosp Med. 2018;13(11):759-763. https://doi.org/10.12788/jhm.3061
20. JaKa MM, Dinh JM, Ziegenfuss JY, et al. Patient and care team perspectives of telemedicine in critical access hospitals. J Hosp Med. 2020;15(6):345-348. https://doi.org/10.12788/jhm.3412
21. Khanal S, Burgon J, Leonard S, Griffiths M, Eddowes LA. Recommendations for the improved effectiveness and reporting of telemedicine programs in developing countries: results of a systematic literature review. Telemed J E Health. 2015;21(11):903-915. https://doi.org/10.1089/tmj.2014.0194
22. Ogrinc G, Davies L, Goodman D, Batalden P, Davidoff F, Stevens D. SQUIRE 2.0 (Standards for QUality Improvement Reporting Excellence): revised publication guidelines from a detailed consensus process. BMJ Qual Saf. 2016;25(12):986-992. https://doi.org/10.1136/bmjqs-2015-004411
23. VHA Office of Rural Health. ORH 2020-2024 STRATEGIC PLAN. In: U.S. Department of Veterans Affairs, ed 2020. Accessed January 18, 2021 https://www.ruralhealth.va.gov/aboutus/index.asp
24. Veterans Health Administration. About VHA. In: U.S. Department of Veterans Affairs, ed. 2019. Accessed January 18, 2021.https://www.va.gov/health/aboutvha.asp
25. GeoSpatial Outcomes Division. VHA Office of Rural Health. U.S. Department of Veterans Affairs. Rural Veterans Health Care Atlas. 2nd ed - FY-2015. Accessed July 30, 2020. https://www.ruralhealth.va.gov/docs/atlas/CHAPTER_02_RHRI_Pts_treated_at_VAMCs.pdf
26. Wagner TH, Upadhyay A, Cowgill E, et al. Risk adjustment tools for learning health systems: a comparison of DxCG and CMS-HCC V21. Health Serv Res. 2016;51(5):2002-2019. https://doi.org/10.1111/1475-6773.12454
27. Wagner T, Stefos T, Moran E, et al. Technical Report 30: Risk Adjustment: Guide to the V21 and Nosos Risk Score Programs. Updated February 8, 2016. Accessed July 30, 2020. https://www.herc.research.va.gov/include/page.asp?id=technical-report-risk-adjustment
28. The R Foundation. The R Project for Statistical Computing. Accessed August 10, 2020. https://www.R-project.org/
29. Cleary PD, Meterko M, Wright SM, Zaslavsky AM. Are comparisons of patient experiences across hospitals fair? A study in Veterans Health Administration hospitals. Med Care. 2014;52(7):619-625. https://doi.org/10.1097/mlr.0000000000000144
30. Giordano LA, Elliott MN, Goldstein E, Lehrman WG, Spencer PA. Development, implementation, and public reporting of the HCAHPS survey. Med Care Res Rev. 2010;67(1):27-37. doi:10.1177/1077558709341065
31. Boltz M, Cuellar NG, Cole C, Pistorese B. Comparing an on-site nurse practitioner with telemedicine physician support hospitalist programme with a traditional physician hospitalist programme. J Telemed and Telecare. 2019;25(4):213-220. https://doi.org/10.1177%2F1357633X18758744
32. Quinn R. The pros and cons of locum tenens for hospitalists. The Hospitalist. 2012(12). Accessed May 29, 2018. https://www.the-hospitalist.org/hospitalist/article/124988/pros-and-cons-locum-tenens-hospitalists
33. Blumenthal DM, Olenski AR, Tsugawa Y, Jena AB. Association between treatment by locum tenens internal medicine physicians and 30-day mortality among hospitalized Medicare beneficiaries. JAMA. 2017;318(21):2119-2129. https://doi.org/10.1001/jama.2017.17925
34. Butcher L. Nurses as hospitalists | AHA Trustee Services. American Hospital Association. Accessed July 14, 2020 https://trustees.aha.org/articles/1238-nurses-as-hospitalists
35. Kartha A, Restuccia JD, Burgess JF, Jr, et al. Nurse practitioner and physician assistant scope of practice in 118 acute care hospitals. J Hosp Med. 2014;9(10):615-620. https://doi.org/10.1002/jhm.2231
© 2021 Society of Hospital Medicine
Clinical Progress Note: Direct Oral Anticoagulants for Treatment of Venous Thromboembolism in Children
Venous thromboembolism (VTE) is a life-threatening event occurring with increasing frequency in hospitalized children and an incidence of more than 58 events per 10,000 hospitalizations.1 In pediatric patients, VTEs occur less often than in adults, have bimodal peaks in neonates and adolescents, and are typically provoked, with central venous access as the most common risk factor.1,
Treatment of pediatric VTE includes unfractionated heparin (UFH), low-molecular-weight heparin (LMWH), and vitamin K antagonists (ie, warfarin). These agents have limitations, including parenteral administration, frequent lab monitoring, and drug/dietary interactions complicating use. Only recently have there been pediatric studies to assess these agents’ pharmacokinetics, pharmacodynamics, safety, and efficacy.2
Direct oral anticoagulants (DOACs) commonly used to treat VTE in adults have two mechanisms of action: direct thrombin (activated factor II) inhibition (ie, dabigatran) and activated factor X (Xa) inhibition (ie, rivaroxaban, apixaban, edoxaban, betrixaban). DOACs offer practical advantages over and efficacy similar to that of warfarin and heparin products, including oral administration, predictable pharmacology, no required lab monitoring, and fewer drug/dietary interactions. DOACs are already approved for VTE treatment in patients 18 years and older.3
This clinical practice update synthesizes 6 years (2014-2020) of literature regarding DOACs for treatment of VTE, focusing on their current role in patients 18 years and older and their emerging role in pediatric patients.
USE IN ADULTS
DOACs are approved by the US Food and Drug Administration (FDA) for multiple anticoagulation indications in adults, including treatment and prevention of acute VTE and prevention of stroke in nonvalvular atrial fibrillation (Table). DOACs are well tolerated by most adults; however, use in certain populations, including patients with liver disease with coagulopathy, advanced renal disease (creatinine clearance <30 mL/min), and class III obesity (body mass index [BMI] >40 kg/m2), requires caution.4,5 For adult patients with VTE without contraindications, DOACs are considered equivalent to warfarin; current CHEST guidelines even suggest preference of DOACs over warfarin.5 While it is prudent to exercise caution when extrapolating adult data to children, these data have informed ongoing pediatric DOAC clinical trials.
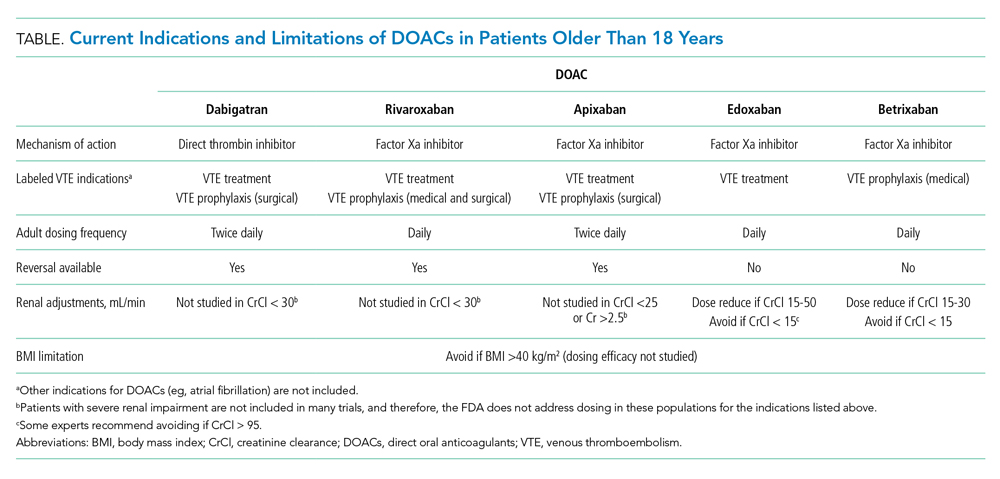
The efficacy and safety of each of the DOACs (aside from betrixaban, which is indicated only for prophylaxis) have compared with warfarin for treatment of VTE in adults.6 A meta-analysis of six clinical trials determined DOACs are noninferior to warfarin for VTE treatment.3 Only two of six trials included patients with provoked VTEs. The meta-analysis found no difference in rates of recurrent symptomatic VTE (primary outcome; relative risk [RR], 0.91; 95% CI, 0.79-1.06) or all-cause mortality (secondary outcome; RR, 0.98; 95% CI, 0.84-1.14). Additionally, DOACs were shown as possibly safer than warfarin due to fewer major bleeding events, particularly fatal bleeding (RR, 0.36; 95% CI, 0.15-0.84) and intracranial bleeding (RR, 0.34; 95% CI, 0.17-0.69). For clinically relevant nonmajor bleeding (eg, gastrointestinal bleeding requiring <2 U packed red blood cells), results were similar (RR, 0.73; 95% CI, 0.58-0.93).
DOACs appear to have effectiveness comparable with that of warfarin. A retrospective matched cohort study of 59,525 patients with acute VTE compared outcomes of patients on DOACs (95% on rivaroxaban) with those of patients on warfarin.6 There were no differences in all-cause mortality or major bleeding. Another retrospective cohort study of 62,431 patients with acute VTE compared rivaroxaban and apixaban with warfarin, as well as rivaroxaban and apixaban with each other.7 There were no differences in 3- and 6-month mortality between warfarin and DOAC users or between rivaroxaban and apixaban users.
Initial approval of DOACs brought concerns about reversibility in the setting of bleeding or urgent procedural need. Clinical practice guidelines, primarily based on observational studies and laboratory parameters in vitro or in healthy volunteers, recommend activated prothrombin complex concentrates as a first-line intervention.8 However, specific agents have now been FDA-approved for DOAC reversal.
Idarucizumab is an FDA-approved (2015) monoclonal antibody with high affinity for dabigatran. Approval was based on a multicenter prospective cohort study of 503 patients taking dabigatran who presented with major bleeding (301 patients) or requiring an urgent surgery (202 patients).9 Idarucizumab resulted in a median time to bleeding cessation of 2.5 hours for those 134 patients in whom time to bleeding cessation could be assessed. Patients with intracranial bleeding were excluded from the timed portion because follow up imaging was not mandated. For those requiring surgery, 93% had normal periprocedural hemostasis.
Andexanet alfa is an FDA-approved (2018) drug for reversal of apixaban and rivaroxaban that acts as a catalytically inactive decoy Xa molecule, binding Xa inhibitors with high affinity. A multicenter prospective cohort study of 352 patients on Xa inhibitors with major bleeding found administration of andexanet alfa resulted in excellent or good hemostasis in 82% of patients (204/249 patients) at 12 hours.10 There was no difference between rivaroxaban and apixaban patients. Both idarucizumab and andexanet alfa remain expensive and not universally available, but availability and use will likely increase with time.
EVIDENCE FOR USE IN CHILDREN
In pediatric patients, most VTEs are provoked, with the most common risk factor being presence of a central line. Frequency of this risk factor varies based on age (>60% of cases in older children and nearly 90% in neonates).1 The most recent American Society of Hematology guidelines recommend treating pediatric symptomatic VTE with anticoagulation and treating asymptomatic VTE instead of observation.2 These recommendations rely on evidence in adult patients due to the current paucity of evidence in pediatrics.
“Pediatric investigation plans” are the cornerstone for ongoing clinical trials of DOACs in pediatrics. While studies evaluating safety and efficacy of standard anticoagulants (UFH, LMWH, and warfarin) in pediatrics exist, clinical trials at the time of drug development did not include pediatric patients. This means none of the currently used anticoagulants were initially developed or approved for children.1 Under the Pediatric Research Equity Act of 2007, the FDA requires pharmaceutical companies to submit a New Drug Application to perform pediatric studies of drugs deemed likely for use in pediatric patients. Pediatric investigation plans allow for establishing safety, efficacy, dosing, and administration routes in pediatric populations. All four DOACs currently approved for treatment of VTE in adults have ongoing efficacy and safety clinical trials for children.
The first and only published clinical trial of DOAC efficacy and safety in pediatrics compared rivaroxaban to standard treatment of acute VTE (Appendix Table).11 The industry-sponsored, open-label EINSTEIN-Jr trial randomized patients aged 0 to 17 years 2:1 to weight-based rivaroxaban or standard treatment after receiving initial parenteral therapy for 5 to 9 days. While most patients were treated for at least 3 months, patients younger than 2 years with line-related thrombosis were treated for only 1 month. The study population mostly consisted of patients with initial, symptomatic, provoked VTE, with types ranging from cerebral venous sinus thrombosis to catheter-associated thrombosis. VTE risk factors, which varied by age, included presence of a central line, major infection, surgery, or trauma. While most VTEs in pediatric patients are expected to be central-line related, in the EINSTEIN-Jr trial only 25.2% of VTEs were central line–associated. The study evaluated symptomatic recurrent VTE (primary efficacy outcome) and clinically relevant bleeding (safety outcome). No significant difference was found between treatment groups in efficacy or safety outcomes, and there were no treatment-related deaths. While the trial was not powered to assess noninferiority due to low incidence of VTE in pediatrics, the absolute number of symptomatic recurrent VTEs was lower in the rivaroxaban group compared with the standard-care group (1% vs 3%). The investigators concluded that rivaroxaban is similarly efficacious and safe in children as compared with adults. FDA approval of rivaroxaban in pediatrics is expected given the trial’s favorable results. Clinicians may wish to consider whether the studied population is comparable with their own patients because the trial had a lower percentage of line-associated VTE than previously reported in the pediatric population.
Multiple clinical trials evaluating the efficacy and safety of other DOACs in pediatric patients are currently underway (Appendix Table).12-14 Apixaban and edoxaban have active multicenter, randomized, open-label clinical trials recruiting patients up to age 17 who have imaging-confirmed acute VTE. A similar trial for dabigatran has recently completed recruitment. Outcome measures include recurrent VTE, VTE-related mortality, and major or clinically relevant non-major bleeding. Like EINSTEIN-Jr, patients in the dabigatran and edoxaban trials were treated with parenteral therapy for at least 5 days prior to randomization.12,14 In the apixaban trial, participants can be randomized without initial parenteral treatment.13 Betrixaban, the newest DOAC approved in adults, does not currently have any open pediatric trials.
AREAS IN NEED OF FUTURE STUDY
Lack of approved reversal agents may initially limit DOAC use in children. An open-label study examining idarucizumab safety has completed enrollment, but it has not yet published results.15 To date, there are no pediatric clinical trials examining andexanet alpha. Future work will need to establish efficacy and safety of reversal agents in pediatrics.
DOACs have not been adequately studied in populations of patients with comorbidities, such as liver disease, renal disease, altered enteral absorption, and BMI higher than 40. Physiologic differences in children with cancer and in neonates merit further evaluation of DOAC safety and efficacy. While ongoing trials established weight-based dosing regimens for children, longitudinal studies will need to ensure adequate anticoagulation, especially in the populations listed here.
The safety outcomes in most DOAC studies include clinically relevant bleeding and VTE-related mortality. These outcomes are much less common in pediatric patients than they are in adults, and future studies may need to expand safety outcomes to those more frequently seen in children. Primary and secondary endpoint variability in pediatric DOAC clinical trials presents challenges interpreting and comparing study results.
SUMMARY
VTE is an increasingly common complication in hospitalized children contributing to significant morbidity.1 For decades, the only treatment options have been UFH, LMWH, or warfarin. DOACs offer many advantages compared with standard anticoagulation options. The only clinical trial evaluating efficacy and safety of DOACs published to date demonstrates that pediatric patients taking rivaroxaban have outcomes similar to those of patients receiving standard care. It is expected that DOACs will gain FDA approval for treatment of VTE in pediatric patients in the near future; therefore, hospitalists should understand indications for use of these medications.
1. Monagle P, Newall F. Management of thrombosis in children and neonates: practical use of anticoagulants in children. Hematology Am Soc Hematol Educ Program. 2018;2018(1):399-404. https://doi.org/10.1182/asheducation-2018.1.399
2. Monagle P, Cuello CA, Augustine C, et al. American Society of Hematology 2018 guidelines for management of venous thromboembolism: treatment of pediatric venous thromboembolism. Blood Adv. 2018;2(22):3292-3316. https://doi.org/10.1182/bloodadvances.2018024786
3. Gómez-Outes A, Terleira-Fernández AI, Lecumberri R, Suárez-Gea ML, Vargas-Castrillón E. Direct oral anticoagulants in the treatment of acute venous thromboembolism: a systematic review and meta-analysis. Thromb Res. 2014;134(4):774-782. https://doi.org/10.1016/j.thromres.2014.06.020
4. Martin K, Beyer-Westendorf J, Davidson BL, Huisman MV, Sandset PM, Moll S. Use of the direct oral anticoagulants in obese patients: guidance from the SSC of the ISTH. J Thromb Haemost. 2016;14(6):1308-1313. https://doi.org/10.1111/jth.13323
5. Kearon C, Akl EA, Ornelas J, et al. Antithrombotic therapy for VTE disease: CHEST guideline and expert panel report. Chest. 2016;149(2):315-352. https://doi.org/10.1016/j.chest.2015.11.026
6. Jun M, Lix LM, Durand M, et al. Comparative safety of direct oral anticoagulants and warfarin in venous thromboembolism: multicentre, population based, observational study. BMJ. 2017;359:j4323. https://doi.org/10.1136/bmj.j4323
7. Roetker NS, Lutsey PL, Zakai NA, Alonso A, Adam TJ, MacLehose RF. All-cause mortality risk with direct oral anticoagulants and warfarin in the primary treatment of venous thromboembolism. Thromb Haemost. 2018;118(9):1637-1645. https://doi.org/10.1055/s-0038-1668521
8. Hoffman M, Goldstein JN, Levy JH. The impact of prothrombin complex concentrates when treating DOAC-associated bleeding: a review. Int J Emerg Med. 2018;11(1):55. https://doi.org/10.1186/s12245-018-0215-6
9. Pollack CV Jr, Reilly PA, van Ryn J, et al. Idarucizumab for dabigatran reversal - full cohort analysis. N Engl J Med. 2017;377(5):431-441. https://doi.org/10.1056/nejmoa1707278
10. Connolly SJ, Crowther M, Eikelboom JW, et al. Full study report of andexanet alfa for bleeding associated with factor Xa inhibitors. N Engl J Med. 2019;380(14):1326-1335. https://doi.org/10.1056/nejmoa1814051
11. Male C, Lensing AWA, Palumbo JS, et al. Rivaroxaban compared with standard anticoagulants for the treatment of acute venous thromboembolism in children: a randomised, controlled, phase 3 trial. Lancet Haematol. 2020;7(1):e18-e27. https://doi.org/10.1016/s2352-3026(19)30219-4
12. Open label study comparing efficacy and safety of dabigatran etexilate to standard of care in paediatric patients with venous thromboembolism (VTE). ClinicalTrials.gov identifier: NCT01895777. Posted July 11, 2013. Updated July 7, 2020. Accessed September 23, 2020. https://clinicaltrials.gov/ct2/show/NCT01895777
13. Apixaban for the acute treatment of venous thromboembolism in children. ClinicalTrials.gov identifier: NCT02464969. Posted June 8, 2015. Updated September 10, 2020. Accessed September 23, 2020. https://clinicaltrials.gov/ct2/show/NCT02464969
14. Hokusai study in pediatric patients with confirmed venous thromboembolism (VTE). ClinicalTrials.gov identifier: NCT02798471. Posted June 14, 2016. Update March 6, 2020. Accessed September 23, 2020. https://clinicaltrials.gov/ct2/show/NCT02798471
15. Reversal dabigatran anticoagulant effect with idarucizumab. ClinicalTrials.gov Identifier: NCT02815670. Posted June 28, 2016. Updated April 14, 2020. Accessed September 23, 2020. https://clinicaltrials.gov/ct2/show/NCT02815670
Venous thromboembolism (VTE) is a life-threatening event occurring with increasing frequency in hospitalized children and an incidence of more than 58 events per 10,000 hospitalizations.1 In pediatric patients, VTEs occur less often than in adults, have bimodal peaks in neonates and adolescents, and are typically provoked, with central venous access as the most common risk factor.1,
Treatment of pediatric VTE includes unfractionated heparin (UFH), low-molecular-weight heparin (LMWH), and vitamin K antagonists (ie, warfarin). These agents have limitations, including parenteral administration, frequent lab monitoring, and drug/dietary interactions complicating use. Only recently have there been pediatric studies to assess these agents’ pharmacokinetics, pharmacodynamics, safety, and efficacy.2
Direct oral anticoagulants (DOACs) commonly used to treat VTE in adults have two mechanisms of action: direct thrombin (activated factor II) inhibition (ie, dabigatran) and activated factor X (Xa) inhibition (ie, rivaroxaban, apixaban, edoxaban, betrixaban). DOACs offer practical advantages over and efficacy similar to that of warfarin and heparin products, including oral administration, predictable pharmacology, no required lab monitoring, and fewer drug/dietary interactions. DOACs are already approved for VTE treatment in patients 18 years and older.3
This clinical practice update synthesizes 6 years (2014-2020) of literature regarding DOACs for treatment of VTE, focusing on their current role in patients 18 years and older and their emerging role in pediatric patients.
USE IN ADULTS
DOACs are approved by the US Food and Drug Administration (FDA) for multiple anticoagulation indications in adults, including treatment and prevention of acute VTE and prevention of stroke in nonvalvular atrial fibrillation (Table). DOACs are well tolerated by most adults; however, use in certain populations, including patients with liver disease with coagulopathy, advanced renal disease (creatinine clearance <30 mL/min), and class III obesity (body mass index [BMI] >40 kg/m2), requires caution.4,5 For adult patients with VTE without contraindications, DOACs are considered equivalent to warfarin; current CHEST guidelines even suggest preference of DOACs over warfarin.5 While it is prudent to exercise caution when extrapolating adult data to children, these data have informed ongoing pediatric DOAC clinical trials.

The efficacy and safety of each of the DOACs (aside from betrixaban, which is indicated only for prophylaxis) have compared with warfarin for treatment of VTE in adults.6 A meta-analysis of six clinical trials determined DOACs are noninferior to warfarin for VTE treatment.3 Only two of six trials included patients with provoked VTEs. The meta-analysis found no difference in rates of recurrent symptomatic VTE (primary outcome; relative risk [RR], 0.91; 95% CI, 0.79-1.06) or all-cause mortality (secondary outcome; RR, 0.98; 95% CI, 0.84-1.14). Additionally, DOACs were shown as possibly safer than warfarin due to fewer major bleeding events, particularly fatal bleeding (RR, 0.36; 95% CI, 0.15-0.84) and intracranial bleeding (RR, 0.34; 95% CI, 0.17-0.69). For clinically relevant nonmajor bleeding (eg, gastrointestinal bleeding requiring <2 U packed red blood cells), results were similar (RR, 0.73; 95% CI, 0.58-0.93).
DOACs appear to have effectiveness comparable with that of warfarin. A retrospective matched cohort study of 59,525 patients with acute VTE compared outcomes of patients on DOACs (95% on rivaroxaban) with those of patients on warfarin.6 There were no differences in all-cause mortality or major bleeding. Another retrospective cohort study of 62,431 patients with acute VTE compared rivaroxaban and apixaban with warfarin, as well as rivaroxaban and apixaban with each other.7 There were no differences in 3- and 6-month mortality between warfarin and DOAC users or between rivaroxaban and apixaban users.
Initial approval of DOACs brought concerns about reversibility in the setting of bleeding or urgent procedural need. Clinical practice guidelines, primarily based on observational studies and laboratory parameters in vitro or in healthy volunteers, recommend activated prothrombin complex concentrates as a first-line intervention.8 However, specific agents have now been FDA-approved for DOAC reversal.
Idarucizumab is an FDA-approved (2015) monoclonal antibody with high affinity for dabigatran. Approval was based on a multicenter prospective cohort study of 503 patients taking dabigatran who presented with major bleeding (301 patients) or requiring an urgent surgery (202 patients).9 Idarucizumab resulted in a median time to bleeding cessation of 2.5 hours for those 134 patients in whom time to bleeding cessation could be assessed. Patients with intracranial bleeding were excluded from the timed portion because follow up imaging was not mandated. For those requiring surgery, 93% had normal periprocedural hemostasis.
Andexanet alfa is an FDA-approved (2018) drug for reversal of apixaban and rivaroxaban that acts as a catalytically inactive decoy Xa molecule, binding Xa inhibitors with high affinity. A multicenter prospective cohort study of 352 patients on Xa inhibitors with major bleeding found administration of andexanet alfa resulted in excellent or good hemostasis in 82% of patients (204/249 patients) at 12 hours.10 There was no difference between rivaroxaban and apixaban patients. Both idarucizumab and andexanet alfa remain expensive and not universally available, but availability and use will likely increase with time.
EVIDENCE FOR USE IN CHILDREN
In pediatric patients, most VTEs are provoked, with the most common risk factor being presence of a central line. Frequency of this risk factor varies based on age (>60% of cases in older children and nearly 90% in neonates).1 The most recent American Society of Hematology guidelines recommend treating pediatric symptomatic VTE with anticoagulation and treating asymptomatic VTE instead of observation.2 These recommendations rely on evidence in adult patients due to the current paucity of evidence in pediatrics.
“Pediatric investigation plans” are the cornerstone for ongoing clinical trials of DOACs in pediatrics. While studies evaluating safety and efficacy of standard anticoagulants (UFH, LMWH, and warfarin) in pediatrics exist, clinical trials at the time of drug development did not include pediatric patients. This means none of the currently used anticoagulants were initially developed or approved for children.1 Under the Pediatric Research Equity Act of 2007, the FDA requires pharmaceutical companies to submit a New Drug Application to perform pediatric studies of drugs deemed likely for use in pediatric patients. Pediatric investigation plans allow for establishing safety, efficacy, dosing, and administration routes in pediatric populations. All four DOACs currently approved for treatment of VTE in adults have ongoing efficacy and safety clinical trials for children.
The first and only published clinical trial of DOAC efficacy and safety in pediatrics compared rivaroxaban to standard treatment of acute VTE (Appendix Table).11 The industry-sponsored, open-label EINSTEIN-Jr trial randomized patients aged 0 to 17 years 2:1 to weight-based rivaroxaban or standard treatment after receiving initial parenteral therapy for 5 to 9 days. While most patients were treated for at least 3 months, patients younger than 2 years with line-related thrombosis were treated for only 1 month. The study population mostly consisted of patients with initial, symptomatic, provoked VTE, with types ranging from cerebral venous sinus thrombosis to catheter-associated thrombosis. VTE risk factors, which varied by age, included presence of a central line, major infection, surgery, or trauma. While most VTEs in pediatric patients are expected to be central-line related, in the EINSTEIN-Jr trial only 25.2% of VTEs were central line–associated. The study evaluated symptomatic recurrent VTE (primary efficacy outcome) and clinically relevant bleeding (safety outcome). No significant difference was found between treatment groups in efficacy or safety outcomes, and there were no treatment-related deaths. While the trial was not powered to assess noninferiority due to low incidence of VTE in pediatrics, the absolute number of symptomatic recurrent VTEs was lower in the rivaroxaban group compared with the standard-care group (1% vs 3%). The investigators concluded that rivaroxaban is similarly efficacious and safe in children as compared with adults. FDA approval of rivaroxaban in pediatrics is expected given the trial’s favorable results. Clinicians may wish to consider whether the studied population is comparable with their own patients because the trial had a lower percentage of line-associated VTE than previously reported in the pediatric population.
Multiple clinical trials evaluating the efficacy and safety of other DOACs in pediatric patients are currently underway (Appendix Table).12-14 Apixaban and edoxaban have active multicenter, randomized, open-label clinical trials recruiting patients up to age 17 who have imaging-confirmed acute VTE. A similar trial for dabigatran has recently completed recruitment. Outcome measures include recurrent VTE, VTE-related mortality, and major or clinically relevant non-major bleeding. Like EINSTEIN-Jr, patients in the dabigatran and edoxaban trials were treated with parenteral therapy for at least 5 days prior to randomization.12,14 In the apixaban trial, participants can be randomized without initial parenteral treatment.13 Betrixaban, the newest DOAC approved in adults, does not currently have any open pediatric trials.
AREAS IN NEED OF FUTURE STUDY
Lack of approved reversal agents may initially limit DOAC use in children. An open-label study examining idarucizumab safety has completed enrollment, but it has not yet published results.15 To date, there are no pediatric clinical trials examining andexanet alpha. Future work will need to establish efficacy and safety of reversal agents in pediatrics.
DOACs have not been adequately studied in populations of patients with comorbidities, such as liver disease, renal disease, altered enteral absorption, and BMI higher than 40. Physiologic differences in children with cancer and in neonates merit further evaluation of DOAC safety and efficacy. While ongoing trials established weight-based dosing regimens for children, longitudinal studies will need to ensure adequate anticoagulation, especially in the populations listed here.
The safety outcomes in most DOAC studies include clinically relevant bleeding and VTE-related mortality. These outcomes are much less common in pediatric patients than they are in adults, and future studies may need to expand safety outcomes to those more frequently seen in children. Primary and secondary endpoint variability in pediatric DOAC clinical trials presents challenges interpreting and comparing study results.
SUMMARY
VTE is an increasingly common complication in hospitalized children contributing to significant morbidity.1 For decades, the only treatment options have been UFH, LMWH, or warfarin. DOACs offer many advantages compared with standard anticoagulation options. The only clinical trial evaluating efficacy and safety of DOACs published to date demonstrates that pediatric patients taking rivaroxaban have outcomes similar to those of patients receiving standard care. It is expected that DOACs will gain FDA approval for treatment of VTE in pediatric patients in the near future; therefore, hospitalists should understand indications for use of these medications.
Venous thromboembolism (VTE) is a life-threatening event occurring with increasing frequency in hospitalized children and an incidence of more than 58 events per 10,000 hospitalizations.1 In pediatric patients, VTEs occur less often than in adults, have bimodal peaks in neonates and adolescents, and are typically provoked, with central venous access as the most common risk factor.1,
Treatment of pediatric VTE includes unfractionated heparin (UFH), low-molecular-weight heparin (LMWH), and vitamin K antagonists (ie, warfarin). These agents have limitations, including parenteral administration, frequent lab monitoring, and drug/dietary interactions complicating use. Only recently have there been pediatric studies to assess these agents’ pharmacokinetics, pharmacodynamics, safety, and efficacy.2
Direct oral anticoagulants (DOACs) commonly used to treat VTE in adults have two mechanisms of action: direct thrombin (activated factor II) inhibition (ie, dabigatran) and activated factor X (Xa) inhibition (ie, rivaroxaban, apixaban, edoxaban, betrixaban). DOACs offer practical advantages over and efficacy similar to that of warfarin and heparin products, including oral administration, predictable pharmacology, no required lab monitoring, and fewer drug/dietary interactions. DOACs are already approved for VTE treatment in patients 18 years and older.3
This clinical practice update synthesizes 6 years (2014-2020) of literature regarding DOACs for treatment of VTE, focusing on their current role in patients 18 years and older and their emerging role in pediatric patients.
USE IN ADULTS
DOACs are approved by the US Food and Drug Administration (FDA) for multiple anticoagulation indications in adults, including treatment and prevention of acute VTE and prevention of stroke in nonvalvular atrial fibrillation (Table). DOACs are well tolerated by most adults; however, use in certain populations, including patients with liver disease with coagulopathy, advanced renal disease (creatinine clearance <30 mL/min), and class III obesity (body mass index [BMI] >40 kg/m2), requires caution.4,5 For adult patients with VTE without contraindications, DOACs are considered equivalent to warfarin; current CHEST guidelines even suggest preference of DOACs over warfarin.5 While it is prudent to exercise caution when extrapolating adult data to children, these data have informed ongoing pediatric DOAC clinical trials.

The efficacy and safety of each of the DOACs (aside from betrixaban, which is indicated only for prophylaxis) have compared with warfarin for treatment of VTE in adults.6 A meta-analysis of six clinical trials determined DOACs are noninferior to warfarin for VTE treatment.3 Only two of six trials included patients with provoked VTEs. The meta-analysis found no difference in rates of recurrent symptomatic VTE (primary outcome; relative risk [RR], 0.91; 95% CI, 0.79-1.06) or all-cause mortality (secondary outcome; RR, 0.98; 95% CI, 0.84-1.14). Additionally, DOACs were shown as possibly safer than warfarin due to fewer major bleeding events, particularly fatal bleeding (RR, 0.36; 95% CI, 0.15-0.84) and intracranial bleeding (RR, 0.34; 95% CI, 0.17-0.69). For clinically relevant nonmajor bleeding (eg, gastrointestinal bleeding requiring <2 U packed red blood cells), results were similar (RR, 0.73; 95% CI, 0.58-0.93).
DOACs appear to have effectiveness comparable with that of warfarin. A retrospective matched cohort study of 59,525 patients with acute VTE compared outcomes of patients on DOACs (95% on rivaroxaban) with those of patients on warfarin.6 There were no differences in all-cause mortality or major bleeding. Another retrospective cohort study of 62,431 patients with acute VTE compared rivaroxaban and apixaban with warfarin, as well as rivaroxaban and apixaban with each other.7 There were no differences in 3- and 6-month mortality between warfarin and DOAC users or between rivaroxaban and apixaban users.
Initial approval of DOACs brought concerns about reversibility in the setting of bleeding or urgent procedural need. Clinical practice guidelines, primarily based on observational studies and laboratory parameters in vitro or in healthy volunteers, recommend activated prothrombin complex concentrates as a first-line intervention.8 However, specific agents have now been FDA-approved for DOAC reversal.
Idarucizumab is an FDA-approved (2015) monoclonal antibody with high affinity for dabigatran. Approval was based on a multicenter prospective cohort study of 503 patients taking dabigatran who presented with major bleeding (301 patients) or requiring an urgent surgery (202 patients).9 Idarucizumab resulted in a median time to bleeding cessation of 2.5 hours for those 134 patients in whom time to bleeding cessation could be assessed. Patients with intracranial bleeding were excluded from the timed portion because follow up imaging was not mandated. For those requiring surgery, 93% had normal periprocedural hemostasis.
Andexanet alfa is an FDA-approved (2018) drug for reversal of apixaban and rivaroxaban that acts as a catalytically inactive decoy Xa molecule, binding Xa inhibitors with high affinity. A multicenter prospective cohort study of 352 patients on Xa inhibitors with major bleeding found administration of andexanet alfa resulted in excellent or good hemostasis in 82% of patients (204/249 patients) at 12 hours.10 There was no difference between rivaroxaban and apixaban patients. Both idarucizumab and andexanet alfa remain expensive and not universally available, but availability and use will likely increase with time.
EVIDENCE FOR USE IN CHILDREN
In pediatric patients, most VTEs are provoked, with the most common risk factor being presence of a central line. Frequency of this risk factor varies based on age (>60% of cases in older children and nearly 90% in neonates).1 The most recent American Society of Hematology guidelines recommend treating pediatric symptomatic VTE with anticoagulation and treating asymptomatic VTE instead of observation.2 These recommendations rely on evidence in adult patients due to the current paucity of evidence in pediatrics.
“Pediatric investigation plans” are the cornerstone for ongoing clinical trials of DOACs in pediatrics. While studies evaluating safety and efficacy of standard anticoagulants (UFH, LMWH, and warfarin) in pediatrics exist, clinical trials at the time of drug development did not include pediatric patients. This means none of the currently used anticoagulants were initially developed or approved for children.1 Under the Pediatric Research Equity Act of 2007, the FDA requires pharmaceutical companies to submit a New Drug Application to perform pediatric studies of drugs deemed likely for use in pediatric patients. Pediatric investigation plans allow for establishing safety, efficacy, dosing, and administration routes in pediatric populations. All four DOACs currently approved for treatment of VTE in adults have ongoing efficacy and safety clinical trials for children.
The first and only published clinical trial of DOAC efficacy and safety in pediatrics compared rivaroxaban to standard treatment of acute VTE (Appendix Table).11 The industry-sponsored, open-label EINSTEIN-Jr trial randomized patients aged 0 to 17 years 2:1 to weight-based rivaroxaban or standard treatment after receiving initial parenteral therapy for 5 to 9 days. While most patients were treated for at least 3 months, patients younger than 2 years with line-related thrombosis were treated for only 1 month. The study population mostly consisted of patients with initial, symptomatic, provoked VTE, with types ranging from cerebral venous sinus thrombosis to catheter-associated thrombosis. VTE risk factors, which varied by age, included presence of a central line, major infection, surgery, or trauma. While most VTEs in pediatric patients are expected to be central-line related, in the EINSTEIN-Jr trial only 25.2% of VTEs were central line–associated. The study evaluated symptomatic recurrent VTE (primary efficacy outcome) and clinically relevant bleeding (safety outcome). No significant difference was found between treatment groups in efficacy or safety outcomes, and there were no treatment-related deaths. While the trial was not powered to assess noninferiority due to low incidence of VTE in pediatrics, the absolute number of symptomatic recurrent VTEs was lower in the rivaroxaban group compared with the standard-care group (1% vs 3%). The investigators concluded that rivaroxaban is similarly efficacious and safe in children as compared with adults. FDA approval of rivaroxaban in pediatrics is expected given the trial’s favorable results. Clinicians may wish to consider whether the studied population is comparable with their own patients because the trial had a lower percentage of line-associated VTE than previously reported in the pediatric population.
Multiple clinical trials evaluating the efficacy and safety of other DOACs in pediatric patients are currently underway (Appendix Table).12-14 Apixaban and edoxaban have active multicenter, randomized, open-label clinical trials recruiting patients up to age 17 who have imaging-confirmed acute VTE. A similar trial for dabigatran has recently completed recruitment. Outcome measures include recurrent VTE, VTE-related mortality, and major or clinically relevant non-major bleeding. Like EINSTEIN-Jr, patients in the dabigatran and edoxaban trials were treated with parenteral therapy for at least 5 days prior to randomization.12,14 In the apixaban trial, participants can be randomized without initial parenteral treatment.13 Betrixaban, the newest DOAC approved in adults, does not currently have any open pediatric trials.
AREAS IN NEED OF FUTURE STUDY
Lack of approved reversal agents may initially limit DOAC use in children. An open-label study examining idarucizumab safety has completed enrollment, but it has not yet published results.15 To date, there are no pediatric clinical trials examining andexanet alpha. Future work will need to establish efficacy and safety of reversal agents in pediatrics.
DOACs have not been adequately studied in populations of patients with comorbidities, such as liver disease, renal disease, altered enteral absorption, and BMI higher than 40. Physiologic differences in children with cancer and in neonates merit further evaluation of DOAC safety and efficacy. While ongoing trials established weight-based dosing regimens for children, longitudinal studies will need to ensure adequate anticoagulation, especially in the populations listed here.
The safety outcomes in most DOAC studies include clinically relevant bleeding and VTE-related mortality. These outcomes are much less common in pediatric patients than they are in adults, and future studies may need to expand safety outcomes to those more frequently seen in children. Primary and secondary endpoint variability in pediatric DOAC clinical trials presents challenges interpreting and comparing study results.
SUMMARY
VTE is an increasingly common complication in hospitalized children contributing to significant morbidity.1 For decades, the only treatment options have been UFH, LMWH, or warfarin. DOACs offer many advantages compared with standard anticoagulation options. The only clinical trial evaluating efficacy and safety of DOACs published to date demonstrates that pediatric patients taking rivaroxaban have outcomes similar to those of patients receiving standard care. It is expected that DOACs will gain FDA approval for treatment of VTE in pediatric patients in the near future; therefore, hospitalists should understand indications for use of these medications.
1. Monagle P, Newall F. Management of thrombosis in children and neonates: practical use of anticoagulants in children. Hematology Am Soc Hematol Educ Program. 2018;2018(1):399-404. https://doi.org/10.1182/asheducation-2018.1.399
2. Monagle P, Cuello CA, Augustine C, et al. American Society of Hematology 2018 guidelines for management of venous thromboembolism: treatment of pediatric venous thromboembolism. Blood Adv. 2018;2(22):3292-3316. https://doi.org/10.1182/bloodadvances.2018024786
3. Gómez-Outes A, Terleira-Fernández AI, Lecumberri R, Suárez-Gea ML, Vargas-Castrillón E. Direct oral anticoagulants in the treatment of acute venous thromboembolism: a systematic review and meta-analysis. Thromb Res. 2014;134(4):774-782. https://doi.org/10.1016/j.thromres.2014.06.020
4. Martin K, Beyer-Westendorf J, Davidson BL, Huisman MV, Sandset PM, Moll S. Use of the direct oral anticoagulants in obese patients: guidance from the SSC of the ISTH. J Thromb Haemost. 2016;14(6):1308-1313. https://doi.org/10.1111/jth.13323
5. Kearon C, Akl EA, Ornelas J, et al. Antithrombotic therapy for VTE disease: CHEST guideline and expert panel report. Chest. 2016;149(2):315-352. https://doi.org/10.1016/j.chest.2015.11.026
6. Jun M, Lix LM, Durand M, et al. Comparative safety of direct oral anticoagulants and warfarin in venous thromboembolism: multicentre, population based, observational study. BMJ. 2017;359:j4323. https://doi.org/10.1136/bmj.j4323
7. Roetker NS, Lutsey PL, Zakai NA, Alonso A, Adam TJ, MacLehose RF. All-cause mortality risk with direct oral anticoagulants and warfarin in the primary treatment of venous thromboembolism. Thromb Haemost. 2018;118(9):1637-1645. https://doi.org/10.1055/s-0038-1668521
8. Hoffman M, Goldstein JN, Levy JH. The impact of prothrombin complex concentrates when treating DOAC-associated bleeding: a review. Int J Emerg Med. 2018;11(1):55. https://doi.org/10.1186/s12245-018-0215-6
9. Pollack CV Jr, Reilly PA, van Ryn J, et al. Idarucizumab for dabigatran reversal - full cohort analysis. N Engl J Med. 2017;377(5):431-441. https://doi.org/10.1056/nejmoa1707278
10. Connolly SJ, Crowther M, Eikelboom JW, et al. Full study report of andexanet alfa for bleeding associated with factor Xa inhibitors. N Engl J Med. 2019;380(14):1326-1335. https://doi.org/10.1056/nejmoa1814051
11. Male C, Lensing AWA, Palumbo JS, et al. Rivaroxaban compared with standard anticoagulants for the treatment of acute venous thromboembolism in children: a randomised, controlled, phase 3 trial. Lancet Haematol. 2020;7(1):e18-e27. https://doi.org/10.1016/s2352-3026(19)30219-4
12. Open label study comparing efficacy and safety of dabigatran etexilate to standard of care in paediatric patients with venous thromboembolism (VTE). ClinicalTrials.gov identifier: NCT01895777. Posted July 11, 2013. Updated July 7, 2020. Accessed September 23, 2020. https://clinicaltrials.gov/ct2/show/NCT01895777
13. Apixaban for the acute treatment of venous thromboembolism in children. ClinicalTrials.gov identifier: NCT02464969. Posted June 8, 2015. Updated September 10, 2020. Accessed September 23, 2020. https://clinicaltrials.gov/ct2/show/NCT02464969
14. Hokusai study in pediatric patients with confirmed venous thromboembolism (VTE). ClinicalTrials.gov identifier: NCT02798471. Posted June 14, 2016. Update March 6, 2020. Accessed September 23, 2020. https://clinicaltrials.gov/ct2/show/NCT02798471
15. Reversal dabigatran anticoagulant effect with idarucizumab. ClinicalTrials.gov Identifier: NCT02815670. Posted June 28, 2016. Updated April 14, 2020. Accessed September 23, 2020. https://clinicaltrials.gov/ct2/show/NCT02815670
1. Monagle P, Newall F. Management of thrombosis in children and neonates: practical use of anticoagulants in children. Hematology Am Soc Hematol Educ Program. 2018;2018(1):399-404. https://doi.org/10.1182/asheducation-2018.1.399
2. Monagle P, Cuello CA, Augustine C, et al. American Society of Hematology 2018 guidelines for management of venous thromboembolism: treatment of pediatric venous thromboembolism. Blood Adv. 2018;2(22):3292-3316. https://doi.org/10.1182/bloodadvances.2018024786
3. Gómez-Outes A, Terleira-Fernández AI, Lecumberri R, Suárez-Gea ML, Vargas-Castrillón E. Direct oral anticoagulants in the treatment of acute venous thromboembolism: a systematic review and meta-analysis. Thromb Res. 2014;134(4):774-782. https://doi.org/10.1016/j.thromres.2014.06.020
4. Martin K, Beyer-Westendorf J, Davidson BL, Huisman MV, Sandset PM, Moll S. Use of the direct oral anticoagulants in obese patients: guidance from the SSC of the ISTH. J Thromb Haemost. 2016;14(6):1308-1313. https://doi.org/10.1111/jth.13323
5. Kearon C, Akl EA, Ornelas J, et al. Antithrombotic therapy for VTE disease: CHEST guideline and expert panel report. Chest. 2016;149(2):315-352. https://doi.org/10.1016/j.chest.2015.11.026
6. Jun M, Lix LM, Durand M, et al. Comparative safety of direct oral anticoagulants and warfarin in venous thromboembolism: multicentre, population based, observational study. BMJ. 2017;359:j4323. https://doi.org/10.1136/bmj.j4323
7. Roetker NS, Lutsey PL, Zakai NA, Alonso A, Adam TJ, MacLehose RF. All-cause mortality risk with direct oral anticoagulants and warfarin in the primary treatment of venous thromboembolism. Thromb Haemost. 2018;118(9):1637-1645. https://doi.org/10.1055/s-0038-1668521
8. Hoffman M, Goldstein JN, Levy JH. The impact of prothrombin complex concentrates when treating DOAC-associated bleeding: a review. Int J Emerg Med. 2018;11(1):55. https://doi.org/10.1186/s12245-018-0215-6
9. Pollack CV Jr, Reilly PA, van Ryn J, et al. Idarucizumab for dabigatran reversal - full cohort analysis. N Engl J Med. 2017;377(5):431-441. https://doi.org/10.1056/nejmoa1707278
10. Connolly SJ, Crowther M, Eikelboom JW, et al. Full study report of andexanet alfa for bleeding associated with factor Xa inhibitors. N Engl J Med. 2019;380(14):1326-1335. https://doi.org/10.1056/nejmoa1814051
11. Male C, Lensing AWA, Palumbo JS, et al. Rivaroxaban compared with standard anticoagulants for the treatment of acute venous thromboembolism in children: a randomised, controlled, phase 3 trial. Lancet Haematol. 2020;7(1):e18-e27. https://doi.org/10.1016/s2352-3026(19)30219-4
12. Open label study comparing efficacy and safety of dabigatran etexilate to standard of care in paediatric patients with venous thromboembolism (VTE). ClinicalTrials.gov identifier: NCT01895777. Posted July 11, 2013. Updated July 7, 2020. Accessed September 23, 2020. https://clinicaltrials.gov/ct2/show/NCT01895777
13. Apixaban for the acute treatment of venous thromboembolism in children. ClinicalTrials.gov identifier: NCT02464969. Posted June 8, 2015. Updated September 10, 2020. Accessed September 23, 2020. https://clinicaltrials.gov/ct2/show/NCT02464969
14. Hokusai study in pediatric patients with confirmed venous thromboembolism (VTE). ClinicalTrials.gov identifier: NCT02798471. Posted June 14, 2016. Update March 6, 2020. Accessed September 23, 2020. https://clinicaltrials.gov/ct2/show/NCT02798471
15. Reversal dabigatran anticoagulant effect with idarucizumab. ClinicalTrials.gov Identifier: NCT02815670. Posted June 28, 2016. Updated April 14, 2020. Accessed September 23, 2020. https://clinicaltrials.gov/ct2/show/NCT02815670
© 2021 Society of Hospital Medicine
Policy in Clinical Practice: Choosing Post-Acute Care in the New Decade
CLINICAL SCENARIO
A 70-year-old woman with Medicare insurance and a history of mild dementia and chronic bronchiectasis was hospitalized for acute respiratory failure due to influenza. She was treated in the intensive care unit (ICU) for 2 days, received mechanical ventilation, and was subsequently extubated and weaned to high-flow nasal cannula (HFNC) at 8 liters of oxygen per minute and noninvasive ventilation at bedtime. She had otherwise stable cognition and required no other medical or nursing therapies. For recovery, she was referred to a
BACKGROUND AND HISTORY
In 2018, 44% of hospitalized patients with fee-for-service Medicare (herein referred to as Medicare) were discharged to PAC, accounting for nearly $60 billion in annual Medicare spending.1 PAC includes four levels of care—home health agencies (HHAs), SNFs, inpatient rehabilitation facilities (IRFs), and LTACHs—which vary in intensity and complexity of the medical, skilled nursing, and rehabilitative services they provide; use separate reimbursement systems; employ different quality metrics; and have different regulatory requirements (Table 1). Because hospitalists care for the majority of these patients and commonly serve in leadership roles for transitions of care and PAC use, PAC policy is important, as it has direct implications on discharge patterns and the quality and nature of patient care after discharge.
HHAs, the most commonly used PAC setting, provide skilled nursing or therapy to homebound beneficiaries.1 HHAs were historically reimbursed a standardized 60-day episode payment based on casemix, which was highly dependent on the number of therapy visits provided, with extremely little contribution from nontherapy services, such as skilled nursing and home health aide visits.2
SNFs, which comprise nearly half of PAC spending, provide short-term skilled nursing and rehabilitative services following hospitalization. SNFs are reimbursed on a per diem basis by Medicare, with reimbursement historically determined by the intensity of the dominant service furnished to the patient—either nursing, ancillary care (which includes medications, supplies/equipment, and diagnostic testing), or rehabilitation.3 Due to strong financial incentives, payment for more than 90% of SNF days was based solely on rehabilitation therapy furnished, with 33% of SNF patients receiving ultra-high rehabilitation (>720 minutes/week),3
IRFs provide intensive rehabilitation to patients who are able to participate in at least 3 hours of multidisciplinary therapy per day.1 IRF admissions are paid a bundled rate by Medicare based on the patient’s primary reason for rehabilitation, their age, and their level of functioning and cognition.
LTACHs, the most intensive and expensive PAC setting, care for patients with a range of complex hospital-level care needs, including intravenous (IV) infusions, complex wound care, and respiratory support. Since 2002, the only requirements for LTACHs have been to meet Medicare’s requirements for hospital accreditation and maintain an average length of stay of 25 days for their population.5 LTACH stays are paid a bundled rate by Medicare based on diagnosis.
POLICIES IN CLINICAL PRACTICE
Due to considerable variation in PAC use, with concerns that similar patients can be treated in different PAC settings,6,7 the
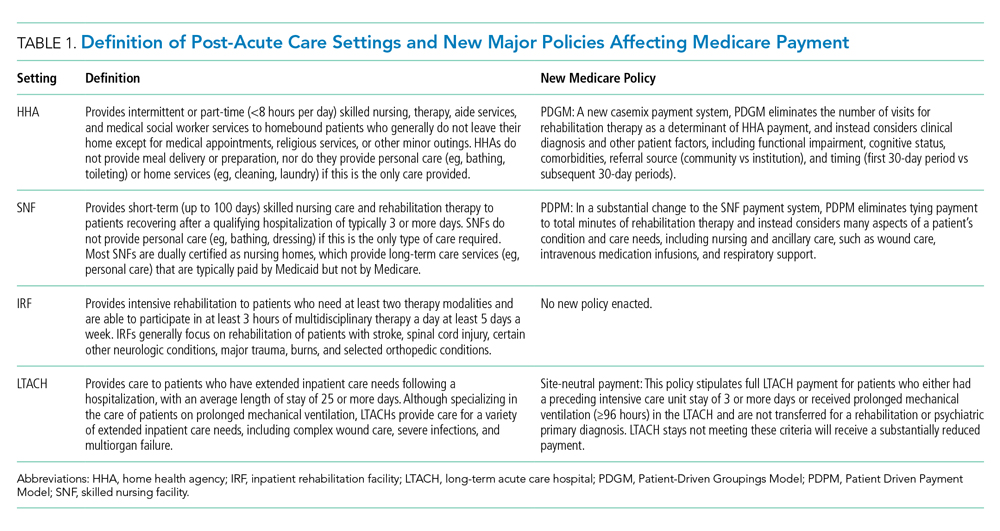
For HHAs and SNFs, CMS implemented new payment models to better align payment with patients’ care needs rather than the provision of rehabilitation therapy.1 For SNFs, the Patient
Driven Payment Model (PDPM) was implemented October 1, 2019, and for HHAs, the Patient-Driven Groupings Model (PDGM) was implemented January 1, 2020. These policies increase payment for patients who have nursing or ancillary care needs, such as IV medications, wound care, and respiratory support. For example, the per diem payment to SNFs is projected to increase 10% to 30% for patients needing dialysis, IV medications, wound care, and respiratory support, such as tracheostomy care.8 These policies also increase payment for patients with greater severity and complexity, such as patients with severe cognitive impairment and multimorbidity. Importantly, these policies pay HHAs and SNFs based on patients’ clinical needs and not solely based on the amount of rehabilitation therapy delivered, which could increase both the number and complexity of patients that SNFs accept.
To discourage LTACH use by patients who are unlikely to benefit from this level of care, CMS fully implemented the
COMMENTARY AND RECOMMENDATIONS
Historically, PAC payment policy has not properly incentivized the appropriate amount of care to be delivered in the appropriate setting.9 The recent HHA, SNF, and LTACH policy changes not only shift the discharge of patients across PAC settings, but also change the amount and type of care that occurs at each PAC site (Table 2). The potential benefit of these new policies is that they will help to align the right level of PAC with patients’ needs by discouraging inappropriate use and unnecessary services.
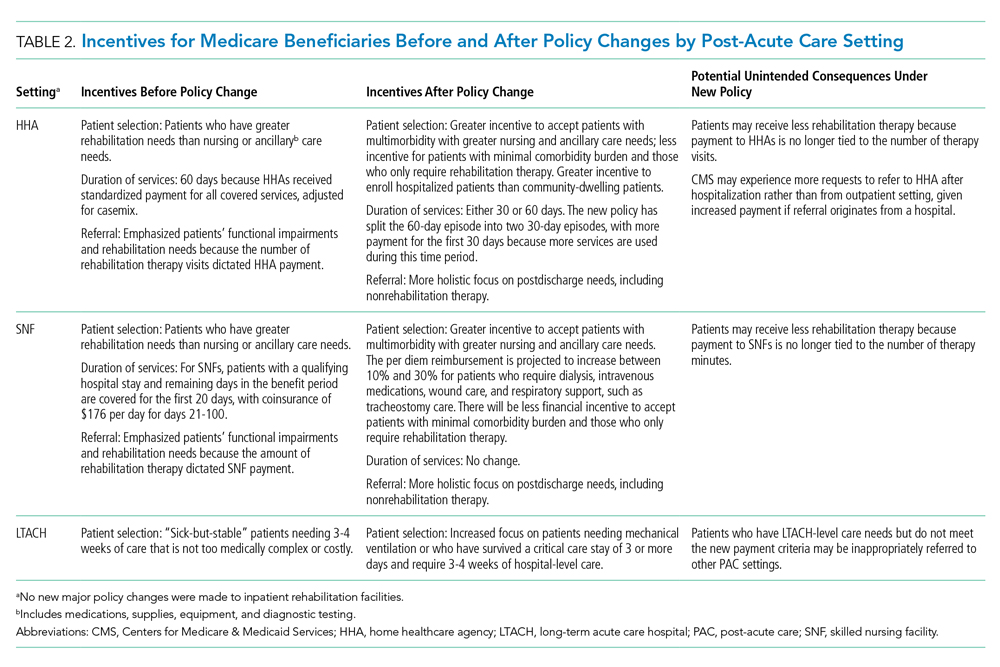
In terms of broader payment reform, the four PAC settings are still fragmented, with little effort to unify payment, regulation, and quality across the PAC continuum. As required by the Improving Medicare Post-Acute Care Transformation (IMPACT) Act of 2014, we would encourage the adoption of a unified PAC payment system that spans the four settings, with payments based on patient characteristics and needs rather than site of service.12 This type of reform would also harmonize regulation and quality measurement and reward payments across settings. Currently, CMS is standardizing patient assessment data and quality metrics across the four PAC settings. Given the COVID-19 pandemic, the transition to a unified PAC payment system is likely several years away.
WHAT SHOULD I TELL MY PATIENT?
For our patient who was transferred to an LTACH after referrals to SNFs were denied, PAC options now differ following these major PAC policy reforms, and SNF transfer would be an option. This is because SNFs will receive higher payment for providing respiratory support under the PDPM, and LTACHs will receive considerably lower reimbursement because the patient did not have a qualifying ICU stay or require prolonged mechanical ventilation. Furthermore, hospitals participating in accountable care organizations would achieve greater savings, given that LTACHs cost at least three times as much as SNFs for comparable diagnoses.
Instead of referring this patient to a LTACH, the care team (hospitalist, discharge navigator, and case manager) should inform and educate the patient about discharge options to SNFs for weaning from respiratory support. To help patients and caregivers choose a facility, the discharge planning team should provide data about the quality of SNFs (eg, CMS Star Ratings scores) instead of simply providing a list of names and locations.13,14
CONCLUSION
Recent major PAC policy changes will change where hospitals discharge medically complex patients and the services they will receive at these PAC settings. Historically, reduction in PAC use has been a key source for savings in alternative payment models that encourage value over volume, such as accountable care organizations and episode-based (“bundled”) payment models.15 We anticipate these PAC policy changes are a step in the right direction to further enable hospitals to achieve value by more closely aligning PAC incentives with patients’ needs.
1. Report to the Congress: Medicare Payment Policy. Medicare Payment Advisory Commision; 2020. http://www.medpac.gov/docs/default-source/reports/mar20_entirereport_sec.pdf?sfvrsn=0
2. Medicare and Medicaid Programs; CY 2020 Home Health Prospective Payment System Rate Update; Home Health Value-Based Purchasing Model; Home Heatlh Quality Reporting Requirements; and Home Infusion Therapy Requirements. Fed Regist. 2019;84(217):60478-60646. To be codified at 42 CFR Parts 409, 414, 484, and 486. https://www.govinfo.gov/content/pkg/FR-2019-11-08/pdf/2019-24026.pdf
3. Medicare Program; Prospective Payment System and Consolidated Billing for Skilled Nursing Facilities (SNF) Final Rule for FY 2019, SNF Value-Based Purchasing Program, and SNF Quality Reporting Program. Fed Regist. 2018;83(153):39162-39290. To be codified at 42 CFR Parts 411, 413, and 424. https://www.govinfo.gov/content/pkg/FR-2018-08-08/pdf/2018-16570.pdf
4. Weaver C, Mathews AW, McGinty T. How Medicare rewards copious nursing-home therapy. Wall Street Journal. Updated August 16, 2015. Accessed October 13, 2020. https://www.wsj.com/articles/how-medicare-rewards-copious-nursing-home-therapy-1439778701
5. Eskildsen MA. Long-term acute care: a review of the literature. J Am Geriatr Soc. 2007;55(5):775-779. https://doi.org/10.1111/j.1532-5415.2007.01162.x
6. Newhouse JP, Garber AM. Geographic variation in health care spending in the United States: insights from an Institute of Medicine report. JAMA. 2013;310(12):1227-1228. https://doi.org/10.1001/jama.2013.278139
7. Makam AN, Nguyen OK, Xuan L, Miller ME, Goodwin JS, Halm EA. Factors associated with variation in long-term acute care hospital vs skilled nursing facility use among hospitalized older adults. JAMA Intern Med. 2018;178(3):399-405. https://doi.org/10.1001/jamainternmed.2017.8467
8. Skilled Nursing Facilities Payment Models Research Technical Report. Acumen; 2017. https://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/SNFPPS/Downloads/SNF_Payment_Models_Research_Technical_Report201704.pdf
9. Ackerly DC, Grabowski DC. Post-acute care reform—beyond the ACA. New Engl J Med. 2014;370(8):689-691. https://doi.org/10.1056/NEJMp1315350
10. Span P. A change in Medicare has therapists alarmed. New York Times. November 29, 2019. Accessed September 16, 2020. https://www.nytimes.com/2019/11/29/health/new-old-age-medicare-physical-therapy.html
11. Graham J. Why home health care is suddenly harder to come by for Medicare patients. Kaiser Health News (KHN). February 3, 2020. Accessed September 16, 2020. https://khn.org/news/why-home-health-care-is-suddenly-harder-to-come-by-for-medicare-patients/
12. Medicare Payment Advisory Commision. Implementing a unified payment system for post-acute care. In: Report to the Congress: Medicare and the Health Care Delivery System. Medicare Payment Advisory Commision; 2017:chap 1. http://www.medpac.gov/docs/default-source/reports/jun17_ch1.pdf?sfvrsn=0
13. Nazir A, Little MO, Arling GW. More than just location: helping patients and families select an appropriate skilled nursing facility. Ann Long Term Care: Clin Care Aging. 2014;22(11):30-34. Published online August 12, 2014. https://www.managedhealthcareconnect.com/articles/more-just-location-helping-patients-and-families-select-appropriate-skilled-nursing
14. Tyler DA, Gadbois EA, McHugh JP, Shield RR, Winblad U, Mor V. Patients are not given quality-of-care data about skilled nursing facilities when discharged from hospitals. Health Aff (Millwood). 2017;36(8):1385-1391. https://doi.org/10.1377/hlthaff.2017.0155
15. Barnett ML, Mehrotra A, Grabowski DC. Postacute care—the piggy bank for savings in alternative payment models? New Engl J Med. 2019;381(4):302-303. https://doi.org/10.1056/NEJMp1901896
CLINICAL SCENARIO
A 70-year-old woman with Medicare insurance and a history of mild dementia and chronic bronchiectasis was hospitalized for acute respiratory failure due to influenza. She was treated in the intensive care unit (ICU) for 2 days, received mechanical ventilation, and was subsequently extubated and weaned to high-flow nasal cannula (HFNC) at 8 liters of oxygen per minute and noninvasive ventilation at bedtime. She had otherwise stable cognition and required no other medical or nursing therapies. For recovery, she was referred to a
BACKGROUND AND HISTORY
In 2018, 44% of hospitalized patients with fee-for-service Medicare (herein referred to as Medicare) were discharged to PAC, accounting for nearly $60 billion in annual Medicare spending.1 PAC includes four levels of care—home health agencies (HHAs), SNFs, inpatient rehabilitation facilities (IRFs), and LTACHs—which vary in intensity and complexity of the medical, skilled nursing, and rehabilitative services they provide; use separate reimbursement systems; employ different quality metrics; and have different regulatory requirements (Table 1). Because hospitalists care for the majority of these patients and commonly serve in leadership roles for transitions of care and PAC use, PAC policy is important, as it has direct implications on discharge patterns and the quality and nature of patient care after discharge.
HHAs, the most commonly used PAC setting, provide skilled nursing or therapy to homebound beneficiaries.1 HHAs were historically reimbursed a standardized 60-day episode payment based on casemix, which was highly dependent on the number of therapy visits provided, with extremely little contribution from nontherapy services, such as skilled nursing and home health aide visits.2
SNFs, which comprise nearly half of PAC spending, provide short-term skilled nursing and rehabilitative services following hospitalization. SNFs are reimbursed on a per diem basis by Medicare, with reimbursement historically determined by the intensity of the dominant service furnished to the patient—either nursing, ancillary care (which includes medications, supplies/equipment, and diagnostic testing), or rehabilitation.3 Due to strong financial incentives, payment for more than 90% of SNF days was based solely on rehabilitation therapy furnished, with 33% of SNF patients receiving ultra-high rehabilitation (>720 minutes/week),3
IRFs provide intensive rehabilitation to patients who are able to participate in at least 3 hours of multidisciplinary therapy per day.1 IRF admissions are paid a bundled rate by Medicare based on the patient’s primary reason for rehabilitation, their age, and their level of functioning and cognition.
LTACHs, the most intensive and expensive PAC setting, care for patients with a range of complex hospital-level care needs, including intravenous (IV) infusions, complex wound care, and respiratory support. Since 2002, the only requirements for LTACHs have been to meet Medicare’s requirements for hospital accreditation and maintain an average length of stay of 25 days for their population.5 LTACH stays are paid a bundled rate by Medicare based on diagnosis.
POLICIES IN CLINICAL PRACTICE
Due to considerable variation in PAC use, with concerns that similar patients can be treated in different PAC settings,6,7 the

For HHAs and SNFs, CMS implemented new payment models to better align payment with patients’ care needs rather than the provision of rehabilitation therapy.1 For SNFs, the Patient
Driven Payment Model (PDPM) was implemented October 1, 2019, and for HHAs, the Patient-Driven Groupings Model (PDGM) was implemented January 1, 2020. These policies increase payment for patients who have nursing or ancillary care needs, such as IV medications, wound care, and respiratory support. For example, the per diem payment to SNFs is projected to increase 10% to 30% for patients needing dialysis, IV medications, wound care, and respiratory support, such as tracheostomy care.8 These policies also increase payment for patients with greater severity and complexity, such as patients with severe cognitive impairment and multimorbidity. Importantly, these policies pay HHAs and SNFs based on patients’ clinical needs and not solely based on the amount of rehabilitation therapy delivered, which could increase both the number and complexity of patients that SNFs accept.
To discourage LTACH use by patients who are unlikely to benefit from this level of care, CMS fully implemented the
COMMENTARY AND RECOMMENDATIONS
Historically, PAC payment policy has not properly incentivized the appropriate amount of care to be delivered in the appropriate setting.9 The recent HHA, SNF, and LTACH policy changes not only shift the discharge of patients across PAC settings, but also change the amount and type of care that occurs at each PAC site (Table 2). The potential benefit of these new policies is that they will help to align the right level of PAC with patients’ needs by discouraging inappropriate use and unnecessary services.

In terms of broader payment reform, the four PAC settings are still fragmented, with little effort to unify payment, regulation, and quality across the PAC continuum. As required by the Improving Medicare Post-Acute Care Transformation (IMPACT) Act of 2014, we would encourage the adoption of a unified PAC payment system that spans the four settings, with payments based on patient characteristics and needs rather than site of service.12 This type of reform would also harmonize regulation and quality measurement and reward payments across settings. Currently, CMS is standardizing patient assessment data and quality metrics across the four PAC settings. Given the COVID-19 pandemic, the transition to a unified PAC payment system is likely several years away.
WHAT SHOULD I TELL MY PATIENT?
For our patient who was transferred to an LTACH after referrals to SNFs were denied, PAC options now differ following these major PAC policy reforms, and SNF transfer would be an option. This is because SNFs will receive higher payment for providing respiratory support under the PDPM, and LTACHs will receive considerably lower reimbursement because the patient did not have a qualifying ICU stay or require prolonged mechanical ventilation. Furthermore, hospitals participating in accountable care organizations would achieve greater savings, given that LTACHs cost at least three times as much as SNFs for comparable diagnoses.
Instead of referring this patient to a LTACH, the care team (hospitalist, discharge navigator, and case manager) should inform and educate the patient about discharge options to SNFs for weaning from respiratory support. To help patients and caregivers choose a facility, the discharge planning team should provide data about the quality of SNFs (eg, CMS Star Ratings scores) instead of simply providing a list of names and locations.13,14
CONCLUSION
Recent major PAC policy changes will change where hospitals discharge medically complex patients and the services they will receive at these PAC settings. Historically, reduction in PAC use has been a key source for savings in alternative payment models that encourage value over volume, such as accountable care organizations and episode-based (“bundled”) payment models.15 We anticipate these PAC policy changes are a step in the right direction to further enable hospitals to achieve value by more closely aligning PAC incentives with patients’ needs.
CLINICAL SCENARIO
A 70-year-old woman with Medicare insurance and a history of mild dementia and chronic bronchiectasis was hospitalized for acute respiratory failure due to influenza. She was treated in the intensive care unit (ICU) for 2 days, received mechanical ventilation, and was subsequently extubated and weaned to high-flow nasal cannula (HFNC) at 8 liters of oxygen per minute and noninvasive ventilation at bedtime. She had otherwise stable cognition and required no other medical or nursing therapies. For recovery, she was referred to a
BACKGROUND AND HISTORY
In 2018, 44% of hospitalized patients with fee-for-service Medicare (herein referred to as Medicare) were discharged to PAC, accounting for nearly $60 billion in annual Medicare spending.1 PAC includes four levels of care—home health agencies (HHAs), SNFs, inpatient rehabilitation facilities (IRFs), and LTACHs—which vary in intensity and complexity of the medical, skilled nursing, and rehabilitative services they provide; use separate reimbursement systems; employ different quality metrics; and have different regulatory requirements (Table 1). Because hospitalists care for the majority of these patients and commonly serve in leadership roles for transitions of care and PAC use, PAC policy is important, as it has direct implications on discharge patterns and the quality and nature of patient care after discharge.
HHAs, the most commonly used PAC setting, provide skilled nursing or therapy to homebound beneficiaries.1 HHAs were historically reimbursed a standardized 60-day episode payment based on casemix, which was highly dependent on the number of therapy visits provided, with extremely little contribution from nontherapy services, such as skilled nursing and home health aide visits.2
SNFs, which comprise nearly half of PAC spending, provide short-term skilled nursing and rehabilitative services following hospitalization. SNFs are reimbursed on a per diem basis by Medicare, with reimbursement historically determined by the intensity of the dominant service furnished to the patient—either nursing, ancillary care (which includes medications, supplies/equipment, and diagnostic testing), or rehabilitation.3 Due to strong financial incentives, payment for more than 90% of SNF days was based solely on rehabilitation therapy furnished, with 33% of SNF patients receiving ultra-high rehabilitation (>720 minutes/week),3
IRFs provide intensive rehabilitation to patients who are able to participate in at least 3 hours of multidisciplinary therapy per day.1 IRF admissions are paid a bundled rate by Medicare based on the patient’s primary reason for rehabilitation, their age, and their level of functioning and cognition.
LTACHs, the most intensive and expensive PAC setting, care for patients with a range of complex hospital-level care needs, including intravenous (IV) infusions, complex wound care, and respiratory support. Since 2002, the only requirements for LTACHs have been to meet Medicare’s requirements for hospital accreditation and maintain an average length of stay of 25 days for their population.5 LTACH stays are paid a bundled rate by Medicare based on diagnosis.
POLICIES IN CLINICAL PRACTICE
Due to considerable variation in PAC use, with concerns that similar patients can be treated in different PAC settings,6,7 the

For HHAs and SNFs, CMS implemented new payment models to better align payment with patients’ care needs rather than the provision of rehabilitation therapy.1 For SNFs, the Patient
Driven Payment Model (PDPM) was implemented October 1, 2019, and for HHAs, the Patient-Driven Groupings Model (PDGM) was implemented January 1, 2020. These policies increase payment for patients who have nursing or ancillary care needs, such as IV medications, wound care, and respiratory support. For example, the per diem payment to SNFs is projected to increase 10% to 30% for patients needing dialysis, IV medications, wound care, and respiratory support, such as tracheostomy care.8 These policies also increase payment for patients with greater severity and complexity, such as patients with severe cognitive impairment and multimorbidity. Importantly, these policies pay HHAs and SNFs based on patients’ clinical needs and not solely based on the amount of rehabilitation therapy delivered, which could increase both the number and complexity of patients that SNFs accept.
To discourage LTACH use by patients who are unlikely to benefit from this level of care, CMS fully implemented the
COMMENTARY AND RECOMMENDATIONS
Historically, PAC payment policy has not properly incentivized the appropriate amount of care to be delivered in the appropriate setting.9 The recent HHA, SNF, and LTACH policy changes not only shift the discharge of patients across PAC settings, but also change the amount and type of care that occurs at each PAC site (Table 2). The potential benefit of these new policies is that they will help to align the right level of PAC with patients’ needs by discouraging inappropriate use and unnecessary services.

In terms of broader payment reform, the four PAC settings are still fragmented, with little effort to unify payment, regulation, and quality across the PAC continuum. As required by the Improving Medicare Post-Acute Care Transformation (IMPACT) Act of 2014, we would encourage the adoption of a unified PAC payment system that spans the four settings, with payments based on patient characteristics and needs rather than site of service.12 This type of reform would also harmonize regulation and quality measurement and reward payments across settings. Currently, CMS is standardizing patient assessment data and quality metrics across the four PAC settings. Given the COVID-19 pandemic, the transition to a unified PAC payment system is likely several years away.
WHAT SHOULD I TELL MY PATIENT?
For our patient who was transferred to an LTACH after referrals to SNFs were denied, PAC options now differ following these major PAC policy reforms, and SNF transfer would be an option. This is because SNFs will receive higher payment for providing respiratory support under the PDPM, and LTACHs will receive considerably lower reimbursement because the patient did not have a qualifying ICU stay or require prolonged mechanical ventilation. Furthermore, hospitals participating in accountable care organizations would achieve greater savings, given that LTACHs cost at least three times as much as SNFs for comparable diagnoses.
Instead of referring this patient to a LTACH, the care team (hospitalist, discharge navigator, and case manager) should inform and educate the patient about discharge options to SNFs for weaning from respiratory support. To help patients and caregivers choose a facility, the discharge planning team should provide data about the quality of SNFs (eg, CMS Star Ratings scores) instead of simply providing a list of names and locations.13,14
CONCLUSION
Recent major PAC policy changes will change where hospitals discharge medically complex patients and the services they will receive at these PAC settings. Historically, reduction in PAC use has been a key source for savings in alternative payment models that encourage value over volume, such as accountable care organizations and episode-based (“bundled”) payment models.15 We anticipate these PAC policy changes are a step in the right direction to further enable hospitals to achieve value by more closely aligning PAC incentives with patients’ needs.
1. Report to the Congress: Medicare Payment Policy. Medicare Payment Advisory Commision; 2020. http://www.medpac.gov/docs/default-source/reports/mar20_entirereport_sec.pdf?sfvrsn=0
2. Medicare and Medicaid Programs; CY 2020 Home Health Prospective Payment System Rate Update; Home Health Value-Based Purchasing Model; Home Heatlh Quality Reporting Requirements; and Home Infusion Therapy Requirements. Fed Regist. 2019;84(217):60478-60646. To be codified at 42 CFR Parts 409, 414, 484, and 486. https://www.govinfo.gov/content/pkg/FR-2019-11-08/pdf/2019-24026.pdf
3. Medicare Program; Prospective Payment System and Consolidated Billing for Skilled Nursing Facilities (SNF) Final Rule for FY 2019, SNF Value-Based Purchasing Program, and SNF Quality Reporting Program. Fed Regist. 2018;83(153):39162-39290. To be codified at 42 CFR Parts 411, 413, and 424. https://www.govinfo.gov/content/pkg/FR-2018-08-08/pdf/2018-16570.pdf
4. Weaver C, Mathews AW, McGinty T. How Medicare rewards copious nursing-home therapy. Wall Street Journal. Updated August 16, 2015. Accessed October 13, 2020. https://www.wsj.com/articles/how-medicare-rewards-copious-nursing-home-therapy-1439778701
5. Eskildsen MA. Long-term acute care: a review of the literature. J Am Geriatr Soc. 2007;55(5):775-779. https://doi.org/10.1111/j.1532-5415.2007.01162.x
6. Newhouse JP, Garber AM. Geographic variation in health care spending in the United States: insights from an Institute of Medicine report. JAMA. 2013;310(12):1227-1228. https://doi.org/10.1001/jama.2013.278139
7. Makam AN, Nguyen OK, Xuan L, Miller ME, Goodwin JS, Halm EA. Factors associated with variation in long-term acute care hospital vs skilled nursing facility use among hospitalized older adults. JAMA Intern Med. 2018;178(3):399-405. https://doi.org/10.1001/jamainternmed.2017.8467
8. Skilled Nursing Facilities Payment Models Research Technical Report. Acumen; 2017. https://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/SNFPPS/Downloads/SNF_Payment_Models_Research_Technical_Report201704.pdf
9. Ackerly DC, Grabowski DC. Post-acute care reform—beyond the ACA. New Engl J Med. 2014;370(8):689-691. https://doi.org/10.1056/NEJMp1315350
10. Span P. A change in Medicare has therapists alarmed. New York Times. November 29, 2019. Accessed September 16, 2020. https://www.nytimes.com/2019/11/29/health/new-old-age-medicare-physical-therapy.html
11. Graham J. Why home health care is suddenly harder to come by for Medicare patients. Kaiser Health News (KHN). February 3, 2020. Accessed September 16, 2020. https://khn.org/news/why-home-health-care-is-suddenly-harder-to-come-by-for-medicare-patients/
12. Medicare Payment Advisory Commision. Implementing a unified payment system for post-acute care. In: Report to the Congress: Medicare and the Health Care Delivery System. Medicare Payment Advisory Commision; 2017:chap 1. http://www.medpac.gov/docs/default-source/reports/jun17_ch1.pdf?sfvrsn=0
13. Nazir A, Little MO, Arling GW. More than just location: helping patients and families select an appropriate skilled nursing facility. Ann Long Term Care: Clin Care Aging. 2014;22(11):30-34. Published online August 12, 2014. https://www.managedhealthcareconnect.com/articles/more-just-location-helping-patients-and-families-select-appropriate-skilled-nursing
14. Tyler DA, Gadbois EA, McHugh JP, Shield RR, Winblad U, Mor V. Patients are not given quality-of-care data about skilled nursing facilities when discharged from hospitals. Health Aff (Millwood). 2017;36(8):1385-1391. https://doi.org/10.1377/hlthaff.2017.0155
15. Barnett ML, Mehrotra A, Grabowski DC. Postacute care—the piggy bank for savings in alternative payment models? New Engl J Med. 2019;381(4):302-303. https://doi.org/10.1056/NEJMp1901896
1. Report to the Congress: Medicare Payment Policy. Medicare Payment Advisory Commision; 2020. http://www.medpac.gov/docs/default-source/reports/mar20_entirereport_sec.pdf?sfvrsn=0
2. Medicare and Medicaid Programs; CY 2020 Home Health Prospective Payment System Rate Update; Home Health Value-Based Purchasing Model; Home Heatlh Quality Reporting Requirements; and Home Infusion Therapy Requirements. Fed Regist. 2019;84(217):60478-60646. To be codified at 42 CFR Parts 409, 414, 484, and 486. https://www.govinfo.gov/content/pkg/FR-2019-11-08/pdf/2019-24026.pdf
3. Medicare Program; Prospective Payment System and Consolidated Billing for Skilled Nursing Facilities (SNF) Final Rule for FY 2019, SNF Value-Based Purchasing Program, and SNF Quality Reporting Program. Fed Regist. 2018;83(153):39162-39290. To be codified at 42 CFR Parts 411, 413, and 424. https://www.govinfo.gov/content/pkg/FR-2018-08-08/pdf/2018-16570.pdf
4. Weaver C, Mathews AW, McGinty T. How Medicare rewards copious nursing-home therapy. Wall Street Journal. Updated August 16, 2015. Accessed October 13, 2020. https://www.wsj.com/articles/how-medicare-rewards-copious-nursing-home-therapy-1439778701
5. Eskildsen MA. Long-term acute care: a review of the literature. J Am Geriatr Soc. 2007;55(5):775-779. https://doi.org/10.1111/j.1532-5415.2007.01162.x
6. Newhouse JP, Garber AM. Geographic variation in health care spending in the United States: insights from an Institute of Medicine report. JAMA. 2013;310(12):1227-1228. https://doi.org/10.1001/jama.2013.278139
7. Makam AN, Nguyen OK, Xuan L, Miller ME, Goodwin JS, Halm EA. Factors associated with variation in long-term acute care hospital vs skilled nursing facility use among hospitalized older adults. JAMA Intern Med. 2018;178(3):399-405. https://doi.org/10.1001/jamainternmed.2017.8467
8. Skilled Nursing Facilities Payment Models Research Technical Report. Acumen; 2017. https://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/SNFPPS/Downloads/SNF_Payment_Models_Research_Technical_Report201704.pdf
9. Ackerly DC, Grabowski DC. Post-acute care reform—beyond the ACA. New Engl J Med. 2014;370(8):689-691. https://doi.org/10.1056/NEJMp1315350
10. Span P. A change in Medicare has therapists alarmed. New York Times. November 29, 2019. Accessed September 16, 2020. https://www.nytimes.com/2019/11/29/health/new-old-age-medicare-physical-therapy.html
11. Graham J. Why home health care is suddenly harder to come by for Medicare patients. Kaiser Health News (KHN). February 3, 2020. Accessed September 16, 2020. https://khn.org/news/why-home-health-care-is-suddenly-harder-to-come-by-for-medicare-patients/
12. Medicare Payment Advisory Commision. Implementing a unified payment system for post-acute care. In: Report to the Congress: Medicare and the Health Care Delivery System. Medicare Payment Advisory Commision; 2017:chap 1. http://www.medpac.gov/docs/default-source/reports/jun17_ch1.pdf?sfvrsn=0
13. Nazir A, Little MO, Arling GW. More than just location: helping patients and families select an appropriate skilled nursing facility. Ann Long Term Care: Clin Care Aging. 2014;22(11):30-34. Published online August 12, 2014. https://www.managedhealthcareconnect.com/articles/more-just-location-helping-patients-and-families-select-appropriate-skilled-nursing
14. Tyler DA, Gadbois EA, McHugh JP, Shield RR, Winblad U, Mor V. Patients are not given quality-of-care data about skilled nursing facilities when discharged from hospitals. Health Aff (Millwood). 2017;36(8):1385-1391. https://doi.org/10.1377/hlthaff.2017.0155
15. Barnett ML, Mehrotra A, Grabowski DC. Postacute care—the piggy bank for savings in alternative payment models? New Engl J Med. 2019;381(4):302-303. https://doi.org/10.1056/NEJMp1901896
© 2021 Society of Hospital Medicine
A Smoky Dilemma
A 23-year-old woman presented to the emergency department complaining of “feeling terrible” for the past week. She described subjective fevers, chills, nonproductive cough, myalgias, and nausea. Her symptoms worsened on the day of presentation, with drenching night sweats, worsening myalgias, and generalized fatigue. She was unable to tolerate oral intake due to persistent nausea and had one episode of emesis.
While the initial constellation of symptoms suggests a viral syndrome, its progression over a week raises concern for something more ominous. Of her relatively nonspecific symptoms, prominent myalgias accompanied by a febrile illness may be most helpful. Fever, myalgias, and nonproductive cough are typical of seasonal influenza, although the presence of nausea and vomiting is atypical in adults. (Though this patient presented for care prior to the coronavirus disease 2019 [COVID-19] pandemic, depending on the timing of this presentation, COVID-19 should be considered.) Acute viral myositis can complicate many viral illnesses, such as influenza, coxsackie, and Epstein-Barr virus infections. Other infectious causes of myositis include systemic bacterial infections, spirochete diseases, and other viral infections, including dengue fever. Myalgias can also be a prominent feature of noninfectious systemic inflammatory conditions, such as systemic lupus erythematosus, rheumatoid arthritis, polymyositis, and systemic vasculitis. Night sweats, while concerning, can be present in myriad conditions, and are not usually a discriminating symptom.
Her past medical history included depression, nephrolithiasis, frequent urinary tract infections, bladder spasms, and recurrent genital herpes simplex virus infection. Her medications included bupropion, microgestin, mirabegron, and valacyclovir. Her father had emphysema.
The patient was employed as a physical therapy assistant in a geriatric care center. Two weeks prior to presentation, she traveled from her home in North Carolina to visit a friend in Atlanta, Georgia. Shortly after the patient returned home, her friend in Atlanta became ill and was treated empirically for Legionella infection because of a recent outbreak in the area. One week prior to presentation, the patient and her boyfriend went on a day hike in the Smoky Mountains in North Carolina, but the patient did not recall any insect or tick bites. Her boyfriend had not been ill.
This history elucidates several potentially relevant medication and environmental exposures. Although bupropion can cause myalgias, neither it nor the other medications she is taking are likely to cause her constellation of symptoms. Her travel history to Atlanta suggests possible, though unconfirmed, exposure to Legionella pneumophila. Notably, she would have had to be exposed to the same source as her friend, since transmission of Legionella occurs via contaminated water and soil, not by human-to-human contact. Legionella infection typically causes a pneumonic process as described here, but her prominent myalgias would not be typical.
Her hike in the Smoky Mountains could have exposed her to several vector-borne diseases. Mosquito-borne dengue in North Carolina is extremely rare, but West Nile virus and eastern equine virus are found within that region. West Nile virus could cause a similar illness, although the cough and lack of neurologic symptoms would be unusual. Eastern equine virus can also cause similar symptoms but is quite rare.
Tick-borne illnesses that should be considered for this region include Lyme disease, Rocky Mountain spotted fever (RMSF), ehrlichiosis, and babesiosis. These tend to present with nonspecific symptoms, but myalgias and fever are consistent features. Lyme disease this close to tick exposure usually presents with the characteristic erythema migrans rash, present in 80% of cases, with or without an influenza-like illness. Approximately 80% of patients do not recall a tick bite, even though a tick must be attached for 36 to 48 hours to transmit the spirochete. RMSF often presents with fever and myalgias, with arthralgias and headache, which are lacking in this case. The common, characteristic rash of blanching erythematous macules that convert to petechiae, starting at the ankles and wrists and spreading to the trunk, is often absent at presentation, showing up at days 3 to 5 in most patients.
Ehrlichiosis presents with an influenza-like illness, but up to half of patients also have nausea and cough. It can also present with a macular and petechial rash in a minority of patients. Lastly, babesiosis presents with an influenza-like illness and less often with cough or nausea. At this juncture, RMSF and ehrlichiosis are possibilities given the hiking history and symptoms, although the absence of a rash points more to ehrlichiosis.
The patient did not smoke cigarettes but had used a JUUL© vaporizer daily for the prior 2 years. Her last use was 1 week prior to admission. She used tetrahydrocannabinol (THC) pods purchased online in the vaporizer on a few occasions 1month prior but had not used THC since that time. She denied alcohol or other drug use.
Until recently, this important detail about vaping use would have been passed over without much consideration. Though reports of acute lung injury from vaping were published as early as 2017, it first came to national attention in August 2019 when the Centers for Disease Control and Prevention posted a Health Advisory about severe lung injury associated with e-cigarette use. Of note, this advisory and subsequent published case series outline that e-cigarette, or vaping, use-associated lung injury (EVALI) may present with more than just respiratory symptoms. Most patients have respiratory symptoms such as shortness of breath, cough, or pleurisy, but many have gastrointestinal symptoms which may include abdominal pain, nausea, vomiting, and diarrhea.1 Constitutional symptoms, including fever, chills, or weight loss, may also predominate.2 In some cases, the gastrointestinal symptoms precede the pulmonary symptoms. This patient’s symptoms warrant consideration of EVALI starting with a chest x-ray (CXR), which is usually abnormal in this disease.2
Physical examination revealed that the patient was alert, diaphoretic, and in mild respiratory distress. Temperature was 103.6 °F, blood pressure 129/75 mm Hg, pulse 130 beats per minute, respiratory rate 20 per minute, and oxygen saturation 97% while breathing ambient air. Cardiac examination revealed tachycardia without murmurs, rubs, or gallops. Lung exam revealed scattered rhonchi over the left posterior lower chest without egophony or dullness to percussion. Findings from abdominal, skin, neurologic, lymph node, and musculoskeletal exams were unremarkable.
Her fever, tachycardia, and respiratory distress point to a pulmonary process such as pneumonia or EVALI, even though she does not have definitive physical exam evidence of pneumonia. She presents with systemic inflammatory response syndrome without significant hypoxia and with borderline tachypnea, which could be related to sepsis or lactic acidosis from a systemic infection other than pneumonia. Her symptom complex could also be compatible with severe influenza infection. The absence of rash makes RMSF less likely.
Results of a complete blood count demonstrated a white blood cell count of 12,600/µL with 87% neutrophils. Results of a metabolic panel were normal, and a urine pregnancy test was negative. The electrocardiogram revealed sinus tachycardia without other abnormalities. A CXR showed no evidence of acute cardiopulmonary abnormalities.
Her lab studies lack thrombocytopenia, which is often found in ehrlichiosis and RMSF. Leukopenia is also absent, which can be seen in Lyme disease and ehrlichiosis. The mild leukocytosis could be consistent with pneumonia, influenza, and EVALI and is not discriminating. The normal CXR goes against pneumonia or EVALI; however, 9% of patients with EVALI in one case series had a normal CXR, while computed tomography (CT) of the chest demonstrated bilateral ground-glass opacities.3 Chest CT is indicated in this case given the poor correlation of the CXR findings and this patient’s pronounced respiratory symptoms.
CT of the chest with contrast did not show a pulmonary embolism but revealed diffuse ground-glass opacities, predominantly in the dependent lower lobes (Figure 1).
Acute conditions with diffuse ground-glass opacities include mycoplasma, Pneumocystis jiroveci and viral pneumonias, pulmonary hemorrhage and edema, acute interstitial pneumonia, eosinophilic lung diseases, and hypersensitivity pneumonitis. Diffuse ground-glass opacities are also seen in almost all patients with EVALI. Though less likely, RMSF, babesiosis, and ehrlichiosis are not ruled out by these chest CT findings, since these disease entities can sometimes cause pulmonary manifestations, including pneumonia, pulmonary edema, and acute respiratory distress syndrome (ARDS).4
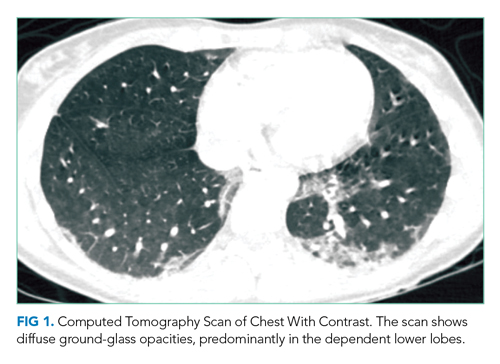
In addition to Legionella and pneumococcal urinary antigen tests, respiratory viral panel, and blood cultures, it would be judicious to obtain HIV, C-reactive protein, and erythrocyte sedimentation rate (ESR) testing; these last two tests are often markedly elevated in EVALI. The utility of bronchoalveolar lavage (BAL) in suspected EVALI cases is not clearly defined, but should be considered in this case to ensure that infectious etiologies are not missed.2 Because of her potential environmental exposures, serologic testing for RMSF and ehrlichiosis should be sent.
Given the overlap in signs and symptoms of EVALI with various, potentially life-threatening infections, she should be empirically treated with antibiotics to cover for community-acquired pneumonia. Adding or even substituting doxycycline for a macrolide antibiotic in this regimen should be considered given that it would treat both RMSF and ehrlichiosis pending further test results. Delay in treating RMSF is associated with worse outcomes. If she is presenting during influenza season, she should also be treated with a neuraminidase inhibitor while awaiting influenza test results. Though the pathophysiology of EVALI is not entirely known, it appears to be inflammatory in nature. Most presumed cases have responded to corticosteroids, with improvement in oxygenation.2 Therefore, treatment with corticosteroids may be warranted to improve oxygenation while ruling out infectious processes.
The patient was admitted to the general medicine wards and started on ceftriaxone and azithromycin for empiric treatment of community-acquired pneumonia. On hospital day 2, a respiratory viral panel returned negative. Procalcitonin, HIV, and blood cultures all returned negative. An ESR was elevated at 86 mm/h. The patient continued to have daily fevers and developed erythematous, blanching macules on the neck, chest, back, and arms, which were noted to occur during febrile periods. Ceftriaxone and azithromycin were discontinued, and doxycycline was started. By hospital day 4, the patient’s oxygen saturation worsened to 86% on ambient air. She continued to have fevers and her cough worsened, with occasional blood-streaked sputum. The patient was transferred to the intensive care unit for closer monitoring.
On hospital day 5, she required intubation for worsening hypoxia. Bronchoscopy was performed, which revealed small mucosal crypts along the left mainstem bronchus. A small amount of bleeding after transbronchial biopsy of the left lower lobe was noted, which resolved with occlusion using the bronchoscope. BAL was performed, which revealed red, cloudy aspirate with 1,100 white blood cells (85% neutrophils) and 22,400 red blood cells. No bacteria were identified.
The patient has developed hypoxic respiratory failure despite appropriate antibiotics and negative cultures, increasing the likelihood of a noninfectious etiology. Her rash is not typical for RMSF, which usually starts as a macular or petechial rash at the ankles and wrists, and spreads centrally to the trunk. Rash is not typically associated with EVALI, and in this case, may represent miliaria caused by her fever.
The mucosal crypts seen on bronchoscopy are nonspecific, likely indicating inflammation from vaping. The BAL otherwise suggests diffuse alveolar hemorrhage (DAH), although sequential BAL aliquots are needed to confirm this diagnosis. DAH is usually caused by pulmonary capillaritis from vasculitis, Goodpasture disease, rheumatic diseases, or diffuse alveolar damage from toxins, infections, rheumatic diseases, or interstitial or organizing pneumonias. Diffuse alveolar damage is the pathologic finding of ARDS, which can be seen in severe cases of many of the conditions discussed, including EVALI, ehrlichiosis, babesiosis, sepsis, and community-acquired pneumonia.4
The BAL is most consistent with EVALI, which often shows elevated neutrophils. DAH due to vaping has also been reported.5 In patients with EVALI, varied pathologic findings of acute lung injury have been reported, including diffuse alveolar damage.6 At this point, laboratory evaluation for rheumatologic diseases and vasculitis should be obtained, and lung biopsy results reviewed. Given her clinical deterioration, treatment with intravenous corticosteroids for presumed EVALI is warranted.
Urine Legionella and Streptococcal pneumoniae antigen tests were negative. The patient was started on methylprednisolone 40 mg intravenously every 8 hours. Further testing included antinuclear antibodies, which was positive at 1:320, with a dense, fine speckled pattern. Perinuclear antineutrophilic cytoplasmic autoantibody, cytoplasmic antineutrophilic cytoplasmic autoantibody, myeloperoxidase, proteinase 3, double-stranded DNA, and glomerular basement membrane IgG were all negative. Transbronchial lung biopsy revealed severe acute lung injury consistent with diffuse alveolar damage. The pulmonary interstitium was mildly expanded by edema, with a moderate number of eosinophilic hyaline membranes. There were no eosinophils or evidence of hemorrhage, granulomas, or giant cells. These changes, within this clinical context, were diagnostic for EVALI.
The patient was intubated for 4 days and completed a course of empiric antibiotics as well as a 10-day course of prednisone. She was discharged on hospital day 17 on 2 L continuous oxygen via nasal cannula. Two days after discharge, she developed worsening dyspnea and chest pain and was readmitted with worsening ground-glass opacities, left upper lobe and right- sided pneumothoraces, and subcutaneous emphysema (Figure 2). She was treated with continuous oxygen to maintain oxygen saturation at 100% and eventually discharged home 3 days later on 3 L continuous oxygen. She attended pulmonary rehabilitation and was weaned off oxygen 2 months later, with marked improvement in aeration of both lungs (Figure 3). She continued to abstain from tobacco and THC products.
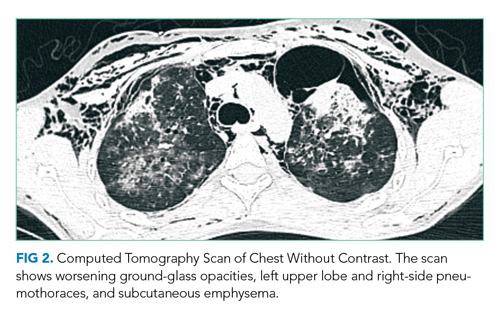
DISCUSSION
The first electronic cigarette (e-cigarette) device was developed in 2003 by a Chinese pharmacist and introduced to the American market in 2007.7 E-cigarettes produce an inhalable aerosol by heating a liquid containing a variety of chemicals, nicotine, and flavors, with or without other additives. Originally promoted as a safer nontobacco and cessation device by producers, e-cigarette sales grew at an annual rate of 115% between 2009 and 2012.8 E-cigarettes can also be used to deliver THC, the psychoactive component of cannabis.
Since the advent of e-cigarettes, their safety has been a topic of concern. In August 2019, the CDC announced 215 possible cases of severe pulmonary disease associated with the use of e-cigarette products that were reported by 25 state health departments.1 By February 2020, EVALI had affected more than 2,800 patients hospitalized across the United States.9
The presenting symptoms of EVALI are varied and nonspecific. The largest EVALI case series, published by Layden et al in 2020, included 98 patients who had a median duration of 6 days of symptoms prior to presentation.3 Respiratory symptoms occurred in 97% of patients, including shortness of breath, any chest pain, pleuritic chest pain, cough, and hemoptysis.3 Presentations also included a variety of gastrointestinal (77%) and constitutional (100%) symptoms, which most commonly included nausea, vomiting, and fever.3 Additional case series have supported a specific pattern of presentation, most commonly including pleuritic chest pain, nonproductive cough, or shortness of breath occurring days to weeks prior to presentation. Associated fatigue, fever, and tachycardia may be present, as well as nausea, vomiting, diarrhea and abdominal pain, and in some cases, these have preceded respiratory symptoms.3,10,11
The vital signs and physical examination, laboratory, and imaging results associated with EVALI are also fairly nonspecific. The most common reason for hospitalization in EVALI is hypoxia, which can progress to acute respiratory failure requiring supplemental oxygen or, as in this case, mechanical ventilation. The most common laboratory finding is leukocytosis greater than 11,000/µL, with more than 80% neutrophils and an ESR greater than 30 mm/hr. In the Layden et al case series, 83% of patients had an abnormal CXR. All patients who underwent CT scan of the chest had bilateral ground-glass opacities, often with subpleural sparing.3 A minority of patients were found to have a pneumothorax, generally a late finding.3,12 Accordingly, the CDC now defines confirmed EVALI as use of e-cigarettes during the 90 days before symptom onset with the presence of pulmonary infiltrates (opacities on CXR or ground-glass opacities on chest CT), negative results on testing for all clinically indicated respiratory infections including respiratory viral panel and influenza PCR, and no alternative plausible diagnoses.13
The presumed etiology of EVALI is chemical exposure because no consistent infectious etiology has been identified.6 No consistent e-cigarette product, substance, or additive has been identified in all cases, nor has one product been directly linked to EVALI. However, the CDC recently announced that vitamin E acetate in vaping products appears to be associated with EVALI.9 In December 2019, Blount et al identified vitamin E acetate in BAL fluid samples from 48 of 51 EVALI patients.14 Additionally, while no other toxins were identified, 94% of samples contained THC or its metabolites or patients had reported vaping THC within 90 days preceding illness.14
The most effective treatment strategy for EVALI is still unknown. It is recommended to treat with empiric antibiotics for at least 48 hours (and antivirals during influenza season) if the history is unclear or if the patient is intubated or has severe hypoxemia.2 If antibiotic and/or antiviral therapies do not lead to clinical improvement, corticosteroids should be added, as they lead to improved oxygenation in many patients.2 Kalininskiy et al recommend initial administration of methylprednisolone 40 mg every 8 hours, with transition to oral prednisone to complete a 2-week course.2 Given rates of rehospitalization (2.7%) and death (2%) in EVALI, the CDC advises that patients should be clinically stable for 24 to 48 hours prior to discharge; that follow-up visits should be arranged within 48 hours of discharge; and that cases of EVALI should be reported to the state and local health departments.15 As seen in the case presented here, with time and continued abstinence from e-cigarette use, the pulmonary effects of EVALI can improve, but long-term outcomes remain unclear. Clinicians must now consider EVALI in patients presenting with respiratory, constitutional, and gastrointestinal complaints when a history of e-cigarette use is present.
KEY TEACHING POINTS
- EVALI presents most commonly with a combination of respiratory, gastrointestinal, and constitutional symptoms. including shortness of breath, cough, nausea, vomiting, and fever.
- When considering EVALI, evaluate and treat for potential infectious causes of disease first.
- Corticosteroids are the mainstay of therapy in EVALI, leading to improvement in oxygenation in many patients.
- Most of the reported cases of EVALI have occurred in patients who have vaped THC-containing products.
1. Schier JG, Meiman JG, Layden J, et al. Severe pulmonary disease associated with electronic-cigarette-product use – Interim guidance. MMWR Morb Mortal Wkly Rep. 2019; 68(36):787-790. https://doi.org/10.15585/mmwr.mm6836e2
2. Kalininskiy A, Bach CT, Nacca NE, et al. E-cigarette, or vaping, product use associated lung injury (EVALI): case series and diagnostic approach. Lancet Respir Med. 2019;7(12):1017-1026. https://doi.org/10.1016/s2213-2600(19)30415-1
3. Layden JE, Ghinai I, Pray I, et al. Pulmonary illness related to e-cigarette use in Illiniois and Wisconsin – final report. N Engl J Med. 2020;382(10):903-916. https://doi.org/10.1056/nejmoa1911614
4. Faul JL, Doyle RL, Kao PN, Ruoss SJ. Tick-borne pulmonary disease: update on diagnosis and management. Chest. 1999;116(1):222-230. https://doi.org/10.1378/chest.116.1.222
5. Agustin M, Yamamoto M, Cabrera F, Eusebio R. Diffuse alveolar hemorrhage induced by vaping. Case Rep Pulmonol. 2018;2018:9724530. https://doi.org/10.1155/2018/9724530
6. Butt YM, Smith ML, Tazelaar HD, et al. Pathology of vaping-associated lung injury. N Engl J Med. 2019;381(18):1780-1781. https://doi.org/10.1056/nejmc1913069
7. Office of the Surgeon General. E-Cigarette Use Among Youth and Young Adults. Chapter 1. Public Health Service, U.S. Department of Health & Human Services; 2016. Accessed January 22, 2020. https://www.cdc.gov/tobacco/data_statistics/sgr/e-cigarettes/index.htm
8. Grana R, Benowitz N, Glantz SA. Background Paper on E-cigarettes (Electronic Nicotine Delivery Systems). UCSF: Center for Tobacco Control Research and Education; 2013. https://escholarship.org/uc/item/13p2b72n
9. Centers for Disease Control and Prevention. Outbreak of lung injury associated with e-cigarette use, or vaping. December 12, 2019. Updated February 25, 2020. Accessed January 22, 2020 and July 16, 2020. https://www.cdc.gov/tobacco/basic_information/e-cigarettes/severe-lung-disease.html
10. Davidson K, Brancato A, Heetkerks P, et al. Outbreak of e-cigarette-associated acute lipoid pneumonia—North Carolina, July-August 2019. MMWR Morb Mortal Wkly Rep. 2019;68(36);784-786. https://doi.org/10.15585/mmwr.mm6836e1
11. Maddock SD, Cirulis MM, Callahan SJ, et al. Pulmonary lipid-laden macrophages and vaping. N Engl J Med. 2019;381(15):1488-1489. https://doi.org/10.1056/nejmc1912038
12. Henry TS, Kanne JP, Klingerman SJ. Imaging of vaping-associated lung disease. N Engl J Med. 2019;381(15):1486-1487. https://doi.org/10.1056/nejmc1911995
13. Smoking and Tobacco Use: For State, Local, Territorial, and Tribal Health Departments. Centers for Disease Control and Prevention. Accessed Jan 24, 2020. https://www.cdc.gov/tobacco/basic_information/e-cigarettes/severe-lung-disease/health-departments/index.html
14. Blount BC, Karwowski MP, Shields PG, et al. Vitamin E acetate in bronchoalveolar-lavage fluid associated with EVALI. N Engl J Med. 2020;382(8):697-705. https://doi.org/10.1056/nejmoa1916433
15. Evans ME, Twentyman E, Click ES, et al. Update: Interim guidance for health care professionals evaluating and caring for patients with suspected e-cigarette, or vaping, product use–associated lung injury and for reducing the risk for rehospitalization and death following hospital discharge — United States, December 2019. MMWR Morb Mortal Wkly Rep. 2020;68(5152):1189-1194. https://doi.org/10.15585/mmwr.mm685152e2
A 23-year-old woman presented to the emergency department complaining of “feeling terrible” for the past week. She described subjective fevers, chills, nonproductive cough, myalgias, and nausea. Her symptoms worsened on the day of presentation, with drenching night sweats, worsening myalgias, and generalized fatigue. She was unable to tolerate oral intake due to persistent nausea and had one episode of emesis.
While the initial constellation of symptoms suggests a viral syndrome, its progression over a week raises concern for something more ominous. Of her relatively nonspecific symptoms, prominent myalgias accompanied by a febrile illness may be most helpful. Fever, myalgias, and nonproductive cough are typical of seasonal influenza, although the presence of nausea and vomiting is atypical in adults. (Though this patient presented for care prior to the coronavirus disease 2019 [COVID-19] pandemic, depending on the timing of this presentation, COVID-19 should be considered.) Acute viral myositis can complicate many viral illnesses, such as influenza, coxsackie, and Epstein-Barr virus infections. Other infectious causes of myositis include systemic bacterial infections, spirochete diseases, and other viral infections, including dengue fever. Myalgias can also be a prominent feature of noninfectious systemic inflammatory conditions, such as systemic lupus erythematosus, rheumatoid arthritis, polymyositis, and systemic vasculitis. Night sweats, while concerning, can be present in myriad conditions, and are not usually a discriminating symptom.
Her past medical history included depression, nephrolithiasis, frequent urinary tract infections, bladder spasms, and recurrent genital herpes simplex virus infection. Her medications included bupropion, microgestin, mirabegron, and valacyclovir. Her father had emphysema.
The patient was employed as a physical therapy assistant in a geriatric care center. Two weeks prior to presentation, she traveled from her home in North Carolina to visit a friend in Atlanta, Georgia. Shortly after the patient returned home, her friend in Atlanta became ill and was treated empirically for Legionella infection because of a recent outbreak in the area. One week prior to presentation, the patient and her boyfriend went on a day hike in the Smoky Mountains in North Carolina, but the patient did not recall any insect or tick bites. Her boyfriend had not been ill.
This history elucidates several potentially relevant medication and environmental exposures. Although bupropion can cause myalgias, neither it nor the other medications she is taking are likely to cause her constellation of symptoms. Her travel history to Atlanta suggests possible, though unconfirmed, exposure to Legionella pneumophila. Notably, she would have had to be exposed to the same source as her friend, since transmission of Legionella occurs via contaminated water and soil, not by human-to-human contact. Legionella infection typically causes a pneumonic process as described here, but her prominent myalgias would not be typical.
Her hike in the Smoky Mountains could have exposed her to several vector-borne diseases. Mosquito-borne dengue in North Carolina is extremely rare, but West Nile virus and eastern equine virus are found within that region. West Nile virus could cause a similar illness, although the cough and lack of neurologic symptoms would be unusual. Eastern equine virus can also cause similar symptoms but is quite rare.
Tick-borne illnesses that should be considered for this region include Lyme disease, Rocky Mountain spotted fever (RMSF), ehrlichiosis, and babesiosis. These tend to present with nonspecific symptoms, but myalgias and fever are consistent features. Lyme disease this close to tick exposure usually presents with the characteristic erythema migrans rash, present in 80% of cases, with or without an influenza-like illness. Approximately 80% of patients do not recall a tick bite, even though a tick must be attached for 36 to 48 hours to transmit the spirochete. RMSF often presents with fever and myalgias, with arthralgias and headache, which are lacking in this case. The common, characteristic rash of blanching erythematous macules that convert to petechiae, starting at the ankles and wrists and spreading to the trunk, is often absent at presentation, showing up at days 3 to 5 in most patients.
Ehrlichiosis presents with an influenza-like illness, but up to half of patients also have nausea and cough. It can also present with a macular and petechial rash in a minority of patients. Lastly, babesiosis presents with an influenza-like illness and less often with cough or nausea. At this juncture, RMSF and ehrlichiosis are possibilities given the hiking history and symptoms, although the absence of a rash points more to ehrlichiosis.
The patient did not smoke cigarettes but had used a JUUL© vaporizer daily for the prior 2 years. Her last use was 1 week prior to admission. She used tetrahydrocannabinol (THC) pods purchased online in the vaporizer on a few occasions 1month prior but had not used THC since that time. She denied alcohol or other drug use.
Until recently, this important detail about vaping use would have been passed over without much consideration. Though reports of acute lung injury from vaping were published as early as 2017, it first came to national attention in August 2019 when the Centers for Disease Control and Prevention posted a Health Advisory about severe lung injury associated with e-cigarette use. Of note, this advisory and subsequent published case series outline that e-cigarette, or vaping, use-associated lung injury (EVALI) may present with more than just respiratory symptoms. Most patients have respiratory symptoms such as shortness of breath, cough, or pleurisy, but many have gastrointestinal symptoms which may include abdominal pain, nausea, vomiting, and diarrhea.1 Constitutional symptoms, including fever, chills, or weight loss, may also predominate.2 In some cases, the gastrointestinal symptoms precede the pulmonary symptoms. This patient’s symptoms warrant consideration of EVALI starting with a chest x-ray (CXR), which is usually abnormal in this disease.2
Physical examination revealed that the patient was alert, diaphoretic, and in mild respiratory distress. Temperature was 103.6 °F, blood pressure 129/75 mm Hg, pulse 130 beats per minute, respiratory rate 20 per minute, and oxygen saturation 97% while breathing ambient air. Cardiac examination revealed tachycardia without murmurs, rubs, or gallops. Lung exam revealed scattered rhonchi over the left posterior lower chest without egophony or dullness to percussion. Findings from abdominal, skin, neurologic, lymph node, and musculoskeletal exams were unremarkable.
Her fever, tachycardia, and respiratory distress point to a pulmonary process such as pneumonia or EVALI, even though she does not have definitive physical exam evidence of pneumonia. She presents with systemic inflammatory response syndrome without significant hypoxia and with borderline tachypnea, which could be related to sepsis or lactic acidosis from a systemic infection other than pneumonia. Her symptom complex could also be compatible with severe influenza infection. The absence of rash makes RMSF less likely.
Results of a complete blood count demonstrated a white blood cell count of 12,600/µL with 87% neutrophils. Results of a metabolic panel were normal, and a urine pregnancy test was negative. The electrocardiogram revealed sinus tachycardia without other abnormalities. A CXR showed no evidence of acute cardiopulmonary abnormalities.
Her lab studies lack thrombocytopenia, which is often found in ehrlichiosis and RMSF. Leukopenia is also absent, which can be seen in Lyme disease and ehrlichiosis. The mild leukocytosis could be consistent with pneumonia, influenza, and EVALI and is not discriminating. The normal CXR goes against pneumonia or EVALI; however, 9% of patients with EVALI in one case series had a normal CXR, while computed tomography (CT) of the chest demonstrated bilateral ground-glass opacities.3 Chest CT is indicated in this case given the poor correlation of the CXR findings and this patient’s pronounced respiratory symptoms.
CT of the chest with contrast did not show a pulmonary embolism but revealed diffuse ground-glass opacities, predominantly in the dependent lower lobes (Figure 1).
Acute conditions with diffuse ground-glass opacities include mycoplasma, Pneumocystis jiroveci and viral pneumonias, pulmonary hemorrhage and edema, acute interstitial pneumonia, eosinophilic lung diseases, and hypersensitivity pneumonitis. Diffuse ground-glass opacities are also seen in almost all patients with EVALI. Though less likely, RMSF, babesiosis, and ehrlichiosis are not ruled out by these chest CT findings, since these disease entities can sometimes cause pulmonary manifestations, including pneumonia, pulmonary edema, and acute respiratory distress syndrome (ARDS).4

In addition to Legionella and pneumococcal urinary antigen tests, respiratory viral panel, and blood cultures, it would be judicious to obtain HIV, C-reactive protein, and erythrocyte sedimentation rate (ESR) testing; these last two tests are often markedly elevated in EVALI. The utility of bronchoalveolar lavage (BAL) in suspected EVALI cases is not clearly defined, but should be considered in this case to ensure that infectious etiologies are not missed.2 Because of her potential environmental exposures, serologic testing for RMSF and ehrlichiosis should be sent.
Given the overlap in signs and symptoms of EVALI with various, potentially life-threatening infections, she should be empirically treated with antibiotics to cover for community-acquired pneumonia. Adding or even substituting doxycycline for a macrolide antibiotic in this regimen should be considered given that it would treat both RMSF and ehrlichiosis pending further test results. Delay in treating RMSF is associated with worse outcomes. If she is presenting during influenza season, she should also be treated with a neuraminidase inhibitor while awaiting influenza test results. Though the pathophysiology of EVALI is not entirely known, it appears to be inflammatory in nature. Most presumed cases have responded to corticosteroids, with improvement in oxygenation.2 Therefore, treatment with corticosteroids may be warranted to improve oxygenation while ruling out infectious processes.
The patient was admitted to the general medicine wards and started on ceftriaxone and azithromycin for empiric treatment of community-acquired pneumonia. On hospital day 2, a respiratory viral panel returned negative. Procalcitonin, HIV, and blood cultures all returned negative. An ESR was elevated at 86 mm/h. The patient continued to have daily fevers and developed erythematous, blanching macules on the neck, chest, back, and arms, which were noted to occur during febrile periods. Ceftriaxone and azithromycin were discontinued, and doxycycline was started. By hospital day 4, the patient’s oxygen saturation worsened to 86% on ambient air. She continued to have fevers and her cough worsened, with occasional blood-streaked sputum. The patient was transferred to the intensive care unit for closer monitoring.
On hospital day 5, she required intubation for worsening hypoxia. Bronchoscopy was performed, which revealed small mucosal crypts along the left mainstem bronchus. A small amount of bleeding after transbronchial biopsy of the left lower lobe was noted, which resolved with occlusion using the bronchoscope. BAL was performed, which revealed red, cloudy aspirate with 1,100 white blood cells (85% neutrophils) and 22,400 red blood cells. No bacteria were identified.
The patient has developed hypoxic respiratory failure despite appropriate antibiotics and negative cultures, increasing the likelihood of a noninfectious etiology. Her rash is not typical for RMSF, which usually starts as a macular or petechial rash at the ankles and wrists, and spreads centrally to the trunk. Rash is not typically associated with EVALI, and in this case, may represent miliaria caused by her fever.
The mucosal crypts seen on bronchoscopy are nonspecific, likely indicating inflammation from vaping. The BAL otherwise suggests diffuse alveolar hemorrhage (DAH), although sequential BAL aliquots are needed to confirm this diagnosis. DAH is usually caused by pulmonary capillaritis from vasculitis, Goodpasture disease, rheumatic diseases, or diffuse alveolar damage from toxins, infections, rheumatic diseases, or interstitial or organizing pneumonias. Diffuse alveolar damage is the pathologic finding of ARDS, which can be seen in severe cases of many of the conditions discussed, including EVALI, ehrlichiosis, babesiosis, sepsis, and community-acquired pneumonia.4
The BAL is most consistent with EVALI, which often shows elevated neutrophils. DAH due to vaping has also been reported.5 In patients with EVALI, varied pathologic findings of acute lung injury have been reported, including diffuse alveolar damage.6 At this point, laboratory evaluation for rheumatologic diseases and vasculitis should be obtained, and lung biopsy results reviewed. Given her clinical deterioration, treatment with intravenous corticosteroids for presumed EVALI is warranted.
Urine Legionella and Streptococcal pneumoniae antigen tests were negative. The patient was started on methylprednisolone 40 mg intravenously every 8 hours. Further testing included antinuclear antibodies, which was positive at 1:320, with a dense, fine speckled pattern. Perinuclear antineutrophilic cytoplasmic autoantibody, cytoplasmic antineutrophilic cytoplasmic autoantibody, myeloperoxidase, proteinase 3, double-stranded DNA, and glomerular basement membrane IgG were all negative. Transbronchial lung biopsy revealed severe acute lung injury consistent with diffuse alveolar damage. The pulmonary interstitium was mildly expanded by edema, with a moderate number of eosinophilic hyaline membranes. There were no eosinophils or evidence of hemorrhage, granulomas, or giant cells. These changes, within this clinical context, were diagnostic for EVALI.
The patient was intubated for 4 days and completed a course of empiric antibiotics as well as a 10-day course of prednisone. She was discharged on hospital day 17 on 2 L continuous oxygen via nasal cannula. Two days after discharge, she developed worsening dyspnea and chest pain and was readmitted with worsening ground-glass opacities, left upper lobe and right- sided pneumothoraces, and subcutaneous emphysema (Figure 2). She was treated with continuous oxygen to maintain oxygen saturation at 100% and eventually discharged home 3 days later on 3 L continuous oxygen. She attended pulmonary rehabilitation and was weaned off oxygen 2 months later, with marked improvement in aeration of both lungs (Figure 3). She continued to abstain from tobacco and THC products.

DISCUSSION
The first electronic cigarette (e-cigarette) device was developed in 2003 by a Chinese pharmacist and introduced to the American market in 2007.7 E-cigarettes produce an inhalable aerosol by heating a liquid containing a variety of chemicals, nicotine, and flavors, with or without other additives. Originally promoted as a safer nontobacco and cessation device by producers, e-cigarette sales grew at an annual rate of 115% between 2009 and 2012.8 E-cigarettes can also be used to deliver THC, the psychoactive component of cannabis.

Since the advent of e-cigarettes, their safety has been a topic of concern. In August 2019, the CDC announced 215 possible cases of severe pulmonary disease associated with the use of e-cigarette products that were reported by 25 state health departments.1 By February 2020, EVALI had affected more than 2,800 patients hospitalized across the United States.9
The presenting symptoms of EVALI are varied and nonspecific. The largest EVALI case series, published by Layden et al in 2020, included 98 patients who had a median duration of 6 days of symptoms prior to presentation.3 Respiratory symptoms occurred in 97% of patients, including shortness of breath, any chest pain, pleuritic chest pain, cough, and hemoptysis.3 Presentations also included a variety of gastrointestinal (77%) and constitutional (100%) symptoms, which most commonly included nausea, vomiting, and fever.3 Additional case series have supported a specific pattern of presentation, most commonly including pleuritic chest pain, nonproductive cough, or shortness of breath occurring days to weeks prior to presentation. Associated fatigue, fever, and tachycardia may be present, as well as nausea, vomiting, diarrhea and abdominal pain, and in some cases, these have preceded respiratory symptoms.3,10,11
The vital signs and physical examination, laboratory, and imaging results associated with EVALI are also fairly nonspecific. The most common reason for hospitalization in EVALI is hypoxia, which can progress to acute respiratory failure requiring supplemental oxygen or, as in this case, mechanical ventilation. The most common laboratory finding is leukocytosis greater than 11,000/µL, with more than 80% neutrophils and an ESR greater than 30 mm/hr. In the Layden et al case series, 83% of patients had an abnormal CXR. All patients who underwent CT scan of the chest had bilateral ground-glass opacities, often with subpleural sparing.3 A minority of patients were found to have a pneumothorax, generally a late finding.3,12 Accordingly, the CDC now defines confirmed EVALI as use of e-cigarettes during the 90 days before symptom onset with the presence of pulmonary infiltrates (opacities on CXR or ground-glass opacities on chest CT), negative results on testing for all clinically indicated respiratory infections including respiratory viral panel and influenza PCR, and no alternative plausible diagnoses.13
The presumed etiology of EVALI is chemical exposure because no consistent infectious etiology has been identified.6 No consistent e-cigarette product, substance, or additive has been identified in all cases, nor has one product been directly linked to EVALI. However, the CDC recently announced that vitamin E acetate in vaping products appears to be associated with EVALI.9 In December 2019, Blount et al identified vitamin E acetate in BAL fluid samples from 48 of 51 EVALI patients.14 Additionally, while no other toxins were identified, 94% of samples contained THC or its metabolites or patients had reported vaping THC within 90 days preceding illness.14
The most effective treatment strategy for EVALI is still unknown. It is recommended to treat with empiric antibiotics for at least 48 hours (and antivirals during influenza season) if the history is unclear or if the patient is intubated or has severe hypoxemia.2 If antibiotic and/or antiviral therapies do not lead to clinical improvement, corticosteroids should be added, as they lead to improved oxygenation in many patients.2 Kalininskiy et al recommend initial administration of methylprednisolone 40 mg every 8 hours, with transition to oral prednisone to complete a 2-week course.2 Given rates of rehospitalization (2.7%) and death (2%) in EVALI, the CDC advises that patients should be clinically stable for 24 to 48 hours prior to discharge; that follow-up visits should be arranged within 48 hours of discharge; and that cases of EVALI should be reported to the state and local health departments.15 As seen in the case presented here, with time and continued abstinence from e-cigarette use, the pulmonary effects of EVALI can improve, but long-term outcomes remain unclear. Clinicians must now consider EVALI in patients presenting with respiratory, constitutional, and gastrointestinal complaints when a history of e-cigarette use is present.
KEY TEACHING POINTS
- EVALI presents most commonly with a combination of respiratory, gastrointestinal, and constitutional symptoms. including shortness of breath, cough, nausea, vomiting, and fever.
- When considering EVALI, evaluate and treat for potential infectious causes of disease first.
- Corticosteroids are the mainstay of therapy in EVALI, leading to improvement in oxygenation in many patients.
- Most of the reported cases of EVALI have occurred in patients who have vaped THC-containing products.
A 23-year-old woman presented to the emergency department complaining of “feeling terrible” for the past week. She described subjective fevers, chills, nonproductive cough, myalgias, and nausea. Her symptoms worsened on the day of presentation, with drenching night sweats, worsening myalgias, and generalized fatigue. She was unable to tolerate oral intake due to persistent nausea and had one episode of emesis.
While the initial constellation of symptoms suggests a viral syndrome, its progression over a week raises concern for something more ominous. Of her relatively nonspecific symptoms, prominent myalgias accompanied by a febrile illness may be most helpful. Fever, myalgias, and nonproductive cough are typical of seasonal influenza, although the presence of nausea and vomiting is atypical in adults. (Though this patient presented for care prior to the coronavirus disease 2019 [COVID-19] pandemic, depending on the timing of this presentation, COVID-19 should be considered.) Acute viral myositis can complicate many viral illnesses, such as influenza, coxsackie, and Epstein-Barr virus infections. Other infectious causes of myositis include systemic bacterial infections, spirochete diseases, and other viral infections, including dengue fever. Myalgias can also be a prominent feature of noninfectious systemic inflammatory conditions, such as systemic lupus erythematosus, rheumatoid arthritis, polymyositis, and systemic vasculitis. Night sweats, while concerning, can be present in myriad conditions, and are not usually a discriminating symptom.
Her past medical history included depression, nephrolithiasis, frequent urinary tract infections, bladder spasms, and recurrent genital herpes simplex virus infection. Her medications included bupropion, microgestin, mirabegron, and valacyclovir. Her father had emphysema.
The patient was employed as a physical therapy assistant in a geriatric care center. Two weeks prior to presentation, she traveled from her home in North Carolina to visit a friend in Atlanta, Georgia. Shortly after the patient returned home, her friend in Atlanta became ill and was treated empirically for Legionella infection because of a recent outbreak in the area. One week prior to presentation, the patient and her boyfriend went on a day hike in the Smoky Mountains in North Carolina, but the patient did not recall any insect or tick bites. Her boyfriend had not been ill.
This history elucidates several potentially relevant medication and environmental exposures. Although bupropion can cause myalgias, neither it nor the other medications she is taking are likely to cause her constellation of symptoms. Her travel history to Atlanta suggests possible, though unconfirmed, exposure to Legionella pneumophila. Notably, she would have had to be exposed to the same source as her friend, since transmission of Legionella occurs via contaminated water and soil, not by human-to-human contact. Legionella infection typically causes a pneumonic process as described here, but her prominent myalgias would not be typical.
Her hike in the Smoky Mountains could have exposed her to several vector-borne diseases. Mosquito-borne dengue in North Carolina is extremely rare, but West Nile virus and eastern equine virus are found within that region. West Nile virus could cause a similar illness, although the cough and lack of neurologic symptoms would be unusual. Eastern equine virus can also cause similar symptoms but is quite rare.
Tick-borne illnesses that should be considered for this region include Lyme disease, Rocky Mountain spotted fever (RMSF), ehrlichiosis, and babesiosis. These tend to present with nonspecific symptoms, but myalgias and fever are consistent features. Lyme disease this close to tick exposure usually presents with the characteristic erythema migrans rash, present in 80% of cases, with or without an influenza-like illness. Approximately 80% of patients do not recall a tick bite, even though a tick must be attached for 36 to 48 hours to transmit the spirochete. RMSF often presents with fever and myalgias, with arthralgias and headache, which are lacking in this case. The common, characteristic rash of blanching erythematous macules that convert to petechiae, starting at the ankles and wrists and spreading to the trunk, is often absent at presentation, showing up at days 3 to 5 in most patients.
Ehrlichiosis presents with an influenza-like illness, but up to half of patients also have nausea and cough. It can also present with a macular and petechial rash in a minority of patients. Lastly, babesiosis presents with an influenza-like illness and less often with cough or nausea. At this juncture, RMSF and ehrlichiosis are possibilities given the hiking history and symptoms, although the absence of a rash points more to ehrlichiosis.
The patient did not smoke cigarettes but had used a JUUL© vaporizer daily for the prior 2 years. Her last use was 1 week prior to admission. She used tetrahydrocannabinol (THC) pods purchased online in the vaporizer on a few occasions 1month prior but had not used THC since that time. She denied alcohol or other drug use.
Until recently, this important detail about vaping use would have been passed over without much consideration. Though reports of acute lung injury from vaping were published as early as 2017, it first came to national attention in August 2019 when the Centers for Disease Control and Prevention posted a Health Advisory about severe lung injury associated with e-cigarette use. Of note, this advisory and subsequent published case series outline that e-cigarette, or vaping, use-associated lung injury (EVALI) may present with more than just respiratory symptoms. Most patients have respiratory symptoms such as shortness of breath, cough, or pleurisy, but many have gastrointestinal symptoms which may include abdominal pain, nausea, vomiting, and diarrhea.1 Constitutional symptoms, including fever, chills, or weight loss, may also predominate.2 In some cases, the gastrointestinal symptoms precede the pulmonary symptoms. This patient’s symptoms warrant consideration of EVALI starting with a chest x-ray (CXR), which is usually abnormal in this disease.2
Physical examination revealed that the patient was alert, diaphoretic, and in mild respiratory distress. Temperature was 103.6 °F, blood pressure 129/75 mm Hg, pulse 130 beats per minute, respiratory rate 20 per minute, and oxygen saturation 97% while breathing ambient air. Cardiac examination revealed tachycardia without murmurs, rubs, or gallops. Lung exam revealed scattered rhonchi over the left posterior lower chest without egophony or dullness to percussion. Findings from abdominal, skin, neurologic, lymph node, and musculoskeletal exams were unremarkable.
Her fever, tachycardia, and respiratory distress point to a pulmonary process such as pneumonia or EVALI, even though she does not have definitive physical exam evidence of pneumonia. She presents with systemic inflammatory response syndrome without significant hypoxia and with borderline tachypnea, which could be related to sepsis or lactic acidosis from a systemic infection other than pneumonia. Her symptom complex could also be compatible with severe influenza infection. The absence of rash makes RMSF less likely.
Results of a complete blood count demonstrated a white blood cell count of 12,600/µL with 87% neutrophils. Results of a metabolic panel were normal, and a urine pregnancy test was negative. The electrocardiogram revealed sinus tachycardia without other abnormalities. A CXR showed no evidence of acute cardiopulmonary abnormalities.
Her lab studies lack thrombocytopenia, which is often found in ehrlichiosis and RMSF. Leukopenia is also absent, which can be seen in Lyme disease and ehrlichiosis. The mild leukocytosis could be consistent with pneumonia, influenza, and EVALI and is not discriminating. The normal CXR goes against pneumonia or EVALI; however, 9% of patients with EVALI in one case series had a normal CXR, while computed tomography (CT) of the chest demonstrated bilateral ground-glass opacities.3 Chest CT is indicated in this case given the poor correlation of the CXR findings and this patient’s pronounced respiratory symptoms.
CT of the chest with contrast did not show a pulmonary embolism but revealed diffuse ground-glass opacities, predominantly in the dependent lower lobes (Figure 1).
Acute conditions with diffuse ground-glass opacities include mycoplasma, Pneumocystis jiroveci and viral pneumonias, pulmonary hemorrhage and edema, acute interstitial pneumonia, eosinophilic lung diseases, and hypersensitivity pneumonitis. Diffuse ground-glass opacities are also seen in almost all patients with EVALI. Though less likely, RMSF, babesiosis, and ehrlichiosis are not ruled out by these chest CT findings, since these disease entities can sometimes cause pulmonary manifestations, including pneumonia, pulmonary edema, and acute respiratory distress syndrome (ARDS).4

In addition to Legionella and pneumococcal urinary antigen tests, respiratory viral panel, and blood cultures, it would be judicious to obtain HIV, C-reactive protein, and erythrocyte sedimentation rate (ESR) testing; these last two tests are often markedly elevated in EVALI. The utility of bronchoalveolar lavage (BAL) in suspected EVALI cases is not clearly defined, but should be considered in this case to ensure that infectious etiologies are not missed.2 Because of her potential environmental exposures, serologic testing for RMSF and ehrlichiosis should be sent.
Given the overlap in signs and symptoms of EVALI with various, potentially life-threatening infections, she should be empirically treated with antibiotics to cover for community-acquired pneumonia. Adding or even substituting doxycycline for a macrolide antibiotic in this regimen should be considered given that it would treat both RMSF and ehrlichiosis pending further test results. Delay in treating RMSF is associated with worse outcomes. If she is presenting during influenza season, she should also be treated with a neuraminidase inhibitor while awaiting influenza test results. Though the pathophysiology of EVALI is not entirely known, it appears to be inflammatory in nature. Most presumed cases have responded to corticosteroids, with improvement in oxygenation.2 Therefore, treatment with corticosteroids may be warranted to improve oxygenation while ruling out infectious processes.
The patient was admitted to the general medicine wards and started on ceftriaxone and azithromycin for empiric treatment of community-acquired pneumonia. On hospital day 2, a respiratory viral panel returned negative. Procalcitonin, HIV, and blood cultures all returned negative. An ESR was elevated at 86 mm/h. The patient continued to have daily fevers and developed erythematous, blanching macules on the neck, chest, back, and arms, which were noted to occur during febrile periods. Ceftriaxone and azithromycin were discontinued, and doxycycline was started. By hospital day 4, the patient’s oxygen saturation worsened to 86% on ambient air. She continued to have fevers and her cough worsened, with occasional blood-streaked sputum. The patient was transferred to the intensive care unit for closer monitoring.
On hospital day 5, she required intubation for worsening hypoxia. Bronchoscopy was performed, which revealed small mucosal crypts along the left mainstem bronchus. A small amount of bleeding after transbronchial biopsy of the left lower lobe was noted, which resolved with occlusion using the bronchoscope. BAL was performed, which revealed red, cloudy aspirate with 1,100 white blood cells (85% neutrophils) and 22,400 red blood cells. No bacteria were identified.
The patient has developed hypoxic respiratory failure despite appropriate antibiotics and negative cultures, increasing the likelihood of a noninfectious etiology. Her rash is not typical for RMSF, which usually starts as a macular or petechial rash at the ankles and wrists, and spreads centrally to the trunk. Rash is not typically associated with EVALI, and in this case, may represent miliaria caused by her fever.
The mucosal crypts seen on bronchoscopy are nonspecific, likely indicating inflammation from vaping. The BAL otherwise suggests diffuse alveolar hemorrhage (DAH), although sequential BAL aliquots are needed to confirm this diagnosis. DAH is usually caused by pulmonary capillaritis from vasculitis, Goodpasture disease, rheumatic diseases, or diffuse alveolar damage from toxins, infections, rheumatic diseases, or interstitial or organizing pneumonias. Diffuse alveolar damage is the pathologic finding of ARDS, which can be seen in severe cases of many of the conditions discussed, including EVALI, ehrlichiosis, babesiosis, sepsis, and community-acquired pneumonia.4
The BAL is most consistent with EVALI, which often shows elevated neutrophils. DAH due to vaping has also been reported.5 In patients with EVALI, varied pathologic findings of acute lung injury have been reported, including diffuse alveolar damage.6 At this point, laboratory evaluation for rheumatologic diseases and vasculitis should be obtained, and lung biopsy results reviewed. Given her clinical deterioration, treatment with intravenous corticosteroids for presumed EVALI is warranted.
Urine Legionella and Streptococcal pneumoniae antigen tests were negative. The patient was started on methylprednisolone 40 mg intravenously every 8 hours. Further testing included antinuclear antibodies, which was positive at 1:320, with a dense, fine speckled pattern. Perinuclear antineutrophilic cytoplasmic autoantibody, cytoplasmic antineutrophilic cytoplasmic autoantibody, myeloperoxidase, proteinase 3, double-stranded DNA, and glomerular basement membrane IgG were all negative. Transbronchial lung biopsy revealed severe acute lung injury consistent with diffuse alveolar damage. The pulmonary interstitium was mildly expanded by edema, with a moderate number of eosinophilic hyaline membranes. There were no eosinophils or evidence of hemorrhage, granulomas, or giant cells. These changes, within this clinical context, were diagnostic for EVALI.
The patient was intubated for 4 days and completed a course of empiric antibiotics as well as a 10-day course of prednisone. She was discharged on hospital day 17 on 2 L continuous oxygen via nasal cannula. Two days after discharge, she developed worsening dyspnea and chest pain and was readmitted with worsening ground-glass opacities, left upper lobe and right- sided pneumothoraces, and subcutaneous emphysema (Figure 2). She was treated with continuous oxygen to maintain oxygen saturation at 100% and eventually discharged home 3 days later on 3 L continuous oxygen. She attended pulmonary rehabilitation and was weaned off oxygen 2 months later, with marked improvement in aeration of both lungs (Figure 3). She continued to abstain from tobacco and THC products.

DISCUSSION
The first electronic cigarette (e-cigarette) device was developed in 2003 by a Chinese pharmacist and introduced to the American market in 2007.7 E-cigarettes produce an inhalable aerosol by heating a liquid containing a variety of chemicals, nicotine, and flavors, with or without other additives. Originally promoted as a safer nontobacco and cessation device by producers, e-cigarette sales grew at an annual rate of 115% between 2009 and 2012.8 E-cigarettes can also be used to deliver THC, the psychoactive component of cannabis.

Since the advent of e-cigarettes, their safety has been a topic of concern. In August 2019, the CDC announced 215 possible cases of severe pulmonary disease associated with the use of e-cigarette products that were reported by 25 state health departments.1 By February 2020, EVALI had affected more than 2,800 patients hospitalized across the United States.9
The presenting symptoms of EVALI are varied and nonspecific. The largest EVALI case series, published by Layden et al in 2020, included 98 patients who had a median duration of 6 days of symptoms prior to presentation.3 Respiratory symptoms occurred in 97% of patients, including shortness of breath, any chest pain, pleuritic chest pain, cough, and hemoptysis.3 Presentations also included a variety of gastrointestinal (77%) and constitutional (100%) symptoms, which most commonly included nausea, vomiting, and fever.3 Additional case series have supported a specific pattern of presentation, most commonly including pleuritic chest pain, nonproductive cough, or shortness of breath occurring days to weeks prior to presentation. Associated fatigue, fever, and tachycardia may be present, as well as nausea, vomiting, diarrhea and abdominal pain, and in some cases, these have preceded respiratory symptoms.3,10,11
The vital signs and physical examination, laboratory, and imaging results associated with EVALI are also fairly nonspecific. The most common reason for hospitalization in EVALI is hypoxia, which can progress to acute respiratory failure requiring supplemental oxygen or, as in this case, mechanical ventilation. The most common laboratory finding is leukocytosis greater than 11,000/µL, with more than 80% neutrophils and an ESR greater than 30 mm/hr. In the Layden et al case series, 83% of patients had an abnormal CXR. All patients who underwent CT scan of the chest had bilateral ground-glass opacities, often with subpleural sparing.3 A minority of patients were found to have a pneumothorax, generally a late finding.3,12 Accordingly, the CDC now defines confirmed EVALI as use of e-cigarettes during the 90 days before symptom onset with the presence of pulmonary infiltrates (opacities on CXR or ground-glass opacities on chest CT), negative results on testing for all clinically indicated respiratory infections including respiratory viral panel and influenza PCR, and no alternative plausible diagnoses.13
The presumed etiology of EVALI is chemical exposure because no consistent infectious etiology has been identified.6 No consistent e-cigarette product, substance, or additive has been identified in all cases, nor has one product been directly linked to EVALI. However, the CDC recently announced that vitamin E acetate in vaping products appears to be associated with EVALI.9 In December 2019, Blount et al identified vitamin E acetate in BAL fluid samples from 48 of 51 EVALI patients.14 Additionally, while no other toxins were identified, 94% of samples contained THC or its metabolites or patients had reported vaping THC within 90 days preceding illness.14
The most effective treatment strategy for EVALI is still unknown. It is recommended to treat with empiric antibiotics for at least 48 hours (and antivirals during influenza season) if the history is unclear or if the patient is intubated or has severe hypoxemia.2 If antibiotic and/or antiviral therapies do not lead to clinical improvement, corticosteroids should be added, as they lead to improved oxygenation in many patients.2 Kalininskiy et al recommend initial administration of methylprednisolone 40 mg every 8 hours, with transition to oral prednisone to complete a 2-week course.2 Given rates of rehospitalization (2.7%) and death (2%) in EVALI, the CDC advises that patients should be clinically stable for 24 to 48 hours prior to discharge; that follow-up visits should be arranged within 48 hours of discharge; and that cases of EVALI should be reported to the state and local health departments.15 As seen in the case presented here, with time and continued abstinence from e-cigarette use, the pulmonary effects of EVALI can improve, but long-term outcomes remain unclear. Clinicians must now consider EVALI in patients presenting with respiratory, constitutional, and gastrointestinal complaints when a history of e-cigarette use is present.
KEY TEACHING POINTS
- EVALI presents most commonly with a combination of respiratory, gastrointestinal, and constitutional symptoms. including shortness of breath, cough, nausea, vomiting, and fever.
- When considering EVALI, evaluate and treat for potential infectious causes of disease first.
- Corticosteroids are the mainstay of therapy in EVALI, leading to improvement in oxygenation in many patients.
- Most of the reported cases of EVALI have occurred in patients who have vaped THC-containing products.
1. Schier JG, Meiman JG, Layden J, et al. Severe pulmonary disease associated with electronic-cigarette-product use – Interim guidance. MMWR Morb Mortal Wkly Rep. 2019; 68(36):787-790. https://doi.org/10.15585/mmwr.mm6836e2
2. Kalininskiy A, Bach CT, Nacca NE, et al. E-cigarette, or vaping, product use associated lung injury (EVALI): case series and diagnostic approach. Lancet Respir Med. 2019;7(12):1017-1026. https://doi.org/10.1016/s2213-2600(19)30415-1
3. Layden JE, Ghinai I, Pray I, et al. Pulmonary illness related to e-cigarette use in Illiniois and Wisconsin – final report. N Engl J Med. 2020;382(10):903-916. https://doi.org/10.1056/nejmoa1911614
4. Faul JL, Doyle RL, Kao PN, Ruoss SJ. Tick-borne pulmonary disease: update on diagnosis and management. Chest. 1999;116(1):222-230. https://doi.org/10.1378/chest.116.1.222
5. Agustin M, Yamamoto M, Cabrera F, Eusebio R. Diffuse alveolar hemorrhage induced by vaping. Case Rep Pulmonol. 2018;2018:9724530. https://doi.org/10.1155/2018/9724530
6. Butt YM, Smith ML, Tazelaar HD, et al. Pathology of vaping-associated lung injury. N Engl J Med. 2019;381(18):1780-1781. https://doi.org/10.1056/nejmc1913069
7. Office of the Surgeon General. E-Cigarette Use Among Youth and Young Adults. Chapter 1. Public Health Service, U.S. Department of Health & Human Services; 2016. Accessed January 22, 2020. https://www.cdc.gov/tobacco/data_statistics/sgr/e-cigarettes/index.htm
8. Grana R, Benowitz N, Glantz SA. Background Paper on E-cigarettes (Electronic Nicotine Delivery Systems). UCSF: Center for Tobacco Control Research and Education; 2013. https://escholarship.org/uc/item/13p2b72n
9. Centers for Disease Control and Prevention. Outbreak of lung injury associated with e-cigarette use, or vaping. December 12, 2019. Updated February 25, 2020. Accessed January 22, 2020 and July 16, 2020. https://www.cdc.gov/tobacco/basic_information/e-cigarettes/severe-lung-disease.html
10. Davidson K, Brancato A, Heetkerks P, et al. Outbreak of e-cigarette-associated acute lipoid pneumonia—North Carolina, July-August 2019. MMWR Morb Mortal Wkly Rep. 2019;68(36);784-786. https://doi.org/10.15585/mmwr.mm6836e1
11. Maddock SD, Cirulis MM, Callahan SJ, et al. Pulmonary lipid-laden macrophages and vaping. N Engl J Med. 2019;381(15):1488-1489. https://doi.org/10.1056/nejmc1912038
12. Henry TS, Kanne JP, Klingerman SJ. Imaging of vaping-associated lung disease. N Engl J Med. 2019;381(15):1486-1487. https://doi.org/10.1056/nejmc1911995
13. Smoking and Tobacco Use: For State, Local, Territorial, and Tribal Health Departments. Centers for Disease Control and Prevention. Accessed Jan 24, 2020. https://www.cdc.gov/tobacco/basic_information/e-cigarettes/severe-lung-disease/health-departments/index.html
14. Blount BC, Karwowski MP, Shields PG, et al. Vitamin E acetate in bronchoalveolar-lavage fluid associated with EVALI. N Engl J Med. 2020;382(8):697-705. https://doi.org/10.1056/nejmoa1916433
15. Evans ME, Twentyman E, Click ES, et al. Update: Interim guidance for health care professionals evaluating and caring for patients with suspected e-cigarette, or vaping, product use–associated lung injury and for reducing the risk for rehospitalization and death following hospital discharge — United States, December 2019. MMWR Morb Mortal Wkly Rep. 2020;68(5152):1189-1194. https://doi.org/10.15585/mmwr.mm685152e2
1. Schier JG, Meiman JG, Layden J, et al. Severe pulmonary disease associated with electronic-cigarette-product use – Interim guidance. MMWR Morb Mortal Wkly Rep. 2019; 68(36):787-790. https://doi.org/10.15585/mmwr.mm6836e2
2. Kalininskiy A, Bach CT, Nacca NE, et al. E-cigarette, or vaping, product use associated lung injury (EVALI): case series and diagnostic approach. Lancet Respir Med. 2019;7(12):1017-1026. https://doi.org/10.1016/s2213-2600(19)30415-1
3. Layden JE, Ghinai I, Pray I, et al. Pulmonary illness related to e-cigarette use in Illiniois and Wisconsin – final report. N Engl J Med. 2020;382(10):903-916. https://doi.org/10.1056/nejmoa1911614
4. Faul JL, Doyle RL, Kao PN, Ruoss SJ. Tick-borne pulmonary disease: update on diagnosis and management. Chest. 1999;116(1):222-230. https://doi.org/10.1378/chest.116.1.222
5. Agustin M, Yamamoto M, Cabrera F, Eusebio R. Diffuse alveolar hemorrhage induced by vaping. Case Rep Pulmonol. 2018;2018:9724530. https://doi.org/10.1155/2018/9724530
6. Butt YM, Smith ML, Tazelaar HD, et al. Pathology of vaping-associated lung injury. N Engl J Med. 2019;381(18):1780-1781. https://doi.org/10.1056/nejmc1913069
7. Office of the Surgeon General. E-Cigarette Use Among Youth and Young Adults. Chapter 1. Public Health Service, U.S. Department of Health & Human Services; 2016. Accessed January 22, 2020. https://www.cdc.gov/tobacco/data_statistics/sgr/e-cigarettes/index.htm
8. Grana R, Benowitz N, Glantz SA. Background Paper on E-cigarettes (Electronic Nicotine Delivery Systems). UCSF: Center for Tobacco Control Research and Education; 2013. https://escholarship.org/uc/item/13p2b72n
9. Centers for Disease Control and Prevention. Outbreak of lung injury associated with e-cigarette use, or vaping. December 12, 2019. Updated February 25, 2020. Accessed January 22, 2020 and July 16, 2020. https://www.cdc.gov/tobacco/basic_information/e-cigarettes/severe-lung-disease.html
10. Davidson K, Brancato A, Heetkerks P, et al. Outbreak of e-cigarette-associated acute lipoid pneumonia—North Carolina, July-August 2019. MMWR Morb Mortal Wkly Rep. 2019;68(36);784-786. https://doi.org/10.15585/mmwr.mm6836e1
11. Maddock SD, Cirulis MM, Callahan SJ, et al. Pulmonary lipid-laden macrophages and vaping. N Engl J Med. 2019;381(15):1488-1489. https://doi.org/10.1056/nejmc1912038
12. Henry TS, Kanne JP, Klingerman SJ. Imaging of vaping-associated lung disease. N Engl J Med. 2019;381(15):1486-1487. https://doi.org/10.1056/nejmc1911995
13. Smoking and Tobacco Use: For State, Local, Territorial, and Tribal Health Departments. Centers for Disease Control and Prevention. Accessed Jan 24, 2020. https://www.cdc.gov/tobacco/basic_information/e-cigarettes/severe-lung-disease/health-departments/index.html
14. Blount BC, Karwowski MP, Shields PG, et al. Vitamin E acetate in bronchoalveolar-lavage fluid associated with EVALI. N Engl J Med. 2020;382(8):697-705. https://doi.org/10.1056/nejmoa1916433
15. Evans ME, Twentyman E, Click ES, et al. Update: Interim guidance for health care professionals evaluating and caring for patients with suspected e-cigarette, or vaping, product use–associated lung injury and for reducing the risk for rehospitalization and death following hospital discharge — United States, December 2019. MMWR Morb Mortal Wkly Rep. 2020;68(5152):1189-1194. https://doi.org/10.15585/mmwr.mm685152e2
© 2021 Society of Hospital Medicine
Point: Healthcare Providers Should Receive Treatment Priority During a Pandemic
Potential catastrophic surges in coronavirus disease 2019 (COVID-19) are leading to more patients requiring intensive care unit beds than are available, prompting hospitals to prepare to activate crisis standards of care (CSC).1,2 These guidelines manage the sobering process of determining which gravely ill patients will have access to limited ventilators, critical care specialists, and other essential hospital personnel. As a member of the CSC triage team at Brigham and Women’s Hospital, Boston, Massachusetts, during the initial surge,1 I was taught how to follow procedures that assign each patient a priority score that ranged from 1 to 8, with lower scores representing higher priority. Scoring decisions were largely based on current status of organ systems and major medical illnesses (predictive of short-term and longer-term survival, respectively), consistent with the objective of maximizing lives and life-years saved.1,3-7 Other parameters included improving the priority score of a pregnant woman with a viable fetus and breaking ties in favor of younger patients who had not lived through life’s major stages.4,7 One issue that elicited sharp disagreement among my colleagues was whether healthcare providers (HCPs; eg, physicians, nurses) should be treated any differently than other individuals.
I believe that HCPs should receive treatment priority during a pandemic because the community has a special obligation to those workers willing to risk serious illness by providing care to potentially infected patients.
THE UTILITARIAN CASE FOR TREATMENT PRIORITIZATION
The most common argument for prioritizing HCPs has been made on utilitarian grounds: save individuals who can save others.3,4,6 Such an approach is not founded on the claim that HCPs have higher intrinsic worth, but is based on the instrumental value of HCPs to keep others alive.4,6 An abiding concern for human life demands systems to ensure individuals with clinical expertise are protected so that they can use their skills to maximize the number of lives saved. A similar case has been made to justify prioritizing HCPs for early access to vaccines during a pandemic.5 To underscore these issues, imagine a scenario in which, because of serious illness among HCPs, there were not enough workers with requisite expertise to care for the rest of the community in which a virus was rapidly spreading. Prioritizing HCPs could mitigate this sequence of events by preventing them from becoming infected through early access to vaccinations or promoting their recovery from the illness, which might allow them to return to work caring for others.
THE ROLE OF SPECIAL OBLIGATIONS
Although the utilitarian argument has merit, my primary reason for advocating the prioritization of HCPs reflects a different ethical framework that emphasizes the reciprocal obligations between HCPs and the community. Obligations of physicians have been framed in terms of the commitments made to their self-chosen profession and the putative social contract that has been constructed with the community.8-10 These principles are well articulated in the American Medical Association’s (AMA’s) Code of Medical Ethics, which states, “Because of their commitment to care for the sick and injured, individual physicians have an obligation to provide urgent medical care during disasters…even in the face of greater than usual risks to their own safety, health, or life.”10,11 Although the AMA qualified its position by indicating that this obligation is not unconditional, it still formulated exceptions within the overarching structure of professional duty, allowing physicians to “balance immediate benefits to individual patients with ability to care for patients in the future.”11,12
If one accepts that HCPs have a professional obligation to take care of sick members of the community, even in perilous situations, what, if any, reciprocal obligation does the community have to its HCPs? Reciprocity is a fundamental ethical principle,13 serving as a foundation for the Golden Rule, which is a component of almost every ethical tradition.14 At its core, reciprocity asks us to treat other people as we would want to be treated. It requires endeavoring to take the perspective of others. Within this framework, a strategy for generating a just policy about treatment prioritization is to develop it under the assumption of not knowing which role one would end up playing in a situation. It is critical that if the positions of the individuals involved were reversed, the same rules and obligations would be accepted as fair.13 I suggest that if members of the community put themselves in the shoes of HCPs who are willing to risk exposure to a potentially deadly virus, they would acknowledge the legitimate expectation of HCPs to receive prioritized care if they became ill from the infection.
In most cases, reciprocity is not construed as requiring an identical exchange, but a fair one in which, for instance, sacrifice is returned in kind. Obligations can be viewed as debts that we either owe or are entitled to receive.15 In the current context, reciprocal obligations are derived from the relationship between HCPs and the community in which they serve. HCPs have a special set of obligations to carry out their work with a high degree of professionalism. If circumstances demand they take on substantial risk for their community, the community, in turn, has a special obligation to take care of them.
To highlight this perspective, imagine HCPs who become ill with COVID-19 and make claims for treatment priority despite having been unwilling to work with patients who are sick with COVID-19. We would consider such claims to be unjust because our moral intuition suggests that individuals are owed a debt for the actual risks they have taken, not for the potential ones they have avoided. A corollary of this view is that HCPs who have demonstrated a willingness to risk their lives contracting COVID-19 have a legitimate claim for prioritization.
Implementation
Acknowledgment of the community’s special obligation to HCPs does not negate competing claims for prioritization, such as trying to save the most lives or accounting for a patient’s pregnancy status and stage of life. Rather, there is a need for CSC guidelines to also include recognition of the special obligations owed HCPs by improving their priority score in the calculus used to triage care. Operationalizing the process would need to be worked out. One possibility would be for HCPs directly caring for patients ill from COVID-19 to have their priority score improve by 2 points, and HCPs directly caring for patients without known disease (but who could still be infectious) to benefit by 1 point. At a minimum, recognition of the risks taken should serve as a tiebreaker in favor of these workers.
To Whom Does the Community Have a Special Obligation?
If we acknowledge that during a pandemic, the community has a special obligation to HCPs because of the risks they are taking to serve others, by the same logic, this commitment should be extended to any personnel linked to the healthcare system (eg, employees in environmental services) or frontline workers providing essential services (eg, grocery store workers) who are taking similar risks that involve exposure to potentially infected individuals. Conversely, HCPs who are working exclusively from home via telemedicine should not receive treatment priority. An approach that extends treatment prioritization to other relevant workers mitigates concerns raised about prioritizing scarce critical care resources to an already advantaged class of individuals (ie, HCPs) as well as the negative optics of a committee of “deciders” in a hospital who are privileging care to their own members.12
CONCLUSION
Reciprocity, a critical component of our notion of justice, should be incorporated into CSC guidelines. The community’s reciprocity to HCPs and frontline workers needs to be commensurate with the sacrifice made by these groups. Although public demonstrations of gratitude may be much appreciated, such displays alone are not adequate for honoring the community’s special obligations. If, during a pandemic, HCPs or frontline workers deliver direct care or services to members of the community, despite serious risk to their own lives, the community has a reciprocal obligation to these individuals to prioritize their access to critical care. HCPs and frontline workers should be prioritized not because their lives have higher intrinsic worth or solely as a reflection of their instrumental value to the community, but out of recognition of the special debt owed them. This is not an unconditional obligation, but one that should be built into the complex, multifaceted decision-making process4,6,16 underlying the allocation of scarce medical resources in a pandemic.
Acknowledgments
The author deeply appreciates the thoughtful comments on the essay from William Snyder, PhD, Melissa Frumin, MD, Brittany McFeeley, BS, Lise Bliss, MBA, and especially Seth Gales, MD, and remains grateful for the guidance and support he received early in his academic career from his first mentors, Carol Gilligan, PhD, and Michael Walzer, PhD.
1. Milliken A, Jurchak M, Sadovnikoff N, et al. Addressing challenges associated with operationalizing a crisis standards of care protocol for the Covid-19 pandemic. NEJM Catalyst. 2020:1-14. https://doi.org/10.1056/CAT.20.0384
2. Paquette ET, Derrington S, Fry JT, et al. Shifting duties of children’s hospitals during the COVID-19 pandemic. J Hosp Med. 2020;15(10):631-633. https://doi.org/10.12788/jhm.3490
3. Emanuel EJ, Persad G, Upshur R, et al. Fair allocation of scarce medical resources in the time of Covid-19.N Engl J Med.2020;382(21):2049-2055. https://doi.org/10.1056/NEJMsb2005114
4. White DB, Lo B. A framework for rationing ventilators and critical care beds during the COVID-19 pandemic. JAMA. 2020;323(18):1773-1774. https://doi.org/10.1001/jama.2020.5046
5. Emanuel EJ, Wetheimer A. Who should get influenza vaccine when not all can? Science. 2006;312(5775):854-855. https://doi.org/10.1126/science.1125347
6. White DB, Katz MH, Luce JM, Lo B. Who should receive life support during a public health emergency? Using ethical principles to improve allocation decisions. Ann Intern Med. 2009;150(2):132-138. https://doi.org/10.7326/0003-4819-150-2-200901200-00011
7. The Commonwealth of Massachusetts Executive Office of Health and Human Services Department of Public Health. Crisis Standards of Care: Planning Guidance for the COVID-19 Pandemic. Accessed August 1, 2020. https://www.mass.gov/doc/statewide-advisory-committee-recommendations-forstandards-of-care/download?_ga=2.55928739.940920097.159299949-195847297.1590861397
8. Brody H, Avery EN. Medicine’s duty to treat pandemic illness: solidarity and vulnerability. Hastings Cent Rep. 2009;39(1):40-48. https://doi.org/10.1353/hcr.0.0104
9. Ruderman C, Tracy CS, Bensimon CM, et al. On pandemics and the duty to care: whose duty? who cares? BMC Med Ethics. 2006;7:E5. https://doi.org/10.1186/1472-6939-7-5
10. Huber SJ, Wynia MK. When pestilence prevails . . . physician responsibilities in epidemics. Am J Bioeth. 2004;4(1):W5-11. https://doi.org/10.1162/152651604773067497
11. AMA Council on Ethical and Judicial Affairs. Opinion 9.067 Physician Obligation in Disaster Preparedness and Response. Virtual Mentor. 2010;12(6):459. d10.1001/virtualmentor.2010.12.6.coet1-1006
12. Rothstein MA. Should health care providers get treatment priority in an influenza pandemic? J Law Med Ethics. 2010;38(2):412-419. https://doi.org/10.1111/j.1748-720X.2010.00499.x
13. Rawls J. A Theory of Justice. The Belknap Press: an imprint of Harvard University Press; 1971.
14. Green WS. Parsing reciprocity: questions for the Golden Rule. In: Neusner J, Chilton BD, eds. The Golden Rule: The Ethics of Reciprocity in World Religions. Continuum International Publishing Group; 2008:1-8.
15. Walzer M. Obligations: Essays on Disobedience, War and Citizenship. Harvard University Press; 1970.
16. Persad G, Wertheimer A, Emanuel EJ. Principles for allocation of scarce medical interventions. Lancet. 2009;373(9661):423-431. https://doi.org/10.1016/S0140-6736(09)60137-9
Potential catastrophic surges in coronavirus disease 2019 (COVID-19) are leading to more patients requiring intensive care unit beds than are available, prompting hospitals to prepare to activate crisis standards of care (CSC).1,2 These guidelines manage the sobering process of determining which gravely ill patients will have access to limited ventilators, critical care specialists, and other essential hospital personnel. As a member of the CSC triage team at Brigham and Women’s Hospital, Boston, Massachusetts, during the initial surge,1 I was taught how to follow procedures that assign each patient a priority score that ranged from 1 to 8, with lower scores representing higher priority. Scoring decisions were largely based on current status of organ systems and major medical illnesses (predictive of short-term and longer-term survival, respectively), consistent with the objective of maximizing lives and life-years saved.1,3-7 Other parameters included improving the priority score of a pregnant woman with a viable fetus and breaking ties in favor of younger patients who had not lived through life’s major stages.4,7 One issue that elicited sharp disagreement among my colleagues was whether healthcare providers (HCPs; eg, physicians, nurses) should be treated any differently than other individuals.
I believe that HCPs should receive treatment priority during a pandemic because the community has a special obligation to those workers willing to risk serious illness by providing care to potentially infected patients.
THE UTILITARIAN CASE FOR TREATMENT PRIORITIZATION
The most common argument for prioritizing HCPs has been made on utilitarian grounds: save individuals who can save others.3,4,6 Such an approach is not founded on the claim that HCPs have higher intrinsic worth, but is based on the instrumental value of HCPs to keep others alive.4,6 An abiding concern for human life demands systems to ensure individuals with clinical expertise are protected so that they can use their skills to maximize the number of lives saved. A similar case has been made to justify prioritizing HCPs for early access to vaccines during a pandemic.5 To underscore these issues, imagine a scenario in which, because of serious illness among HCPs, there were not enough workers with requisite expertise to care for the rest of the community in which a virus was rapidly spreading. Prioritizing HCPs could mitigate this sequence of events by preventing them from becoming infected through early access to vaccinations or promoting their recovery from the illness, which might allow them to return to work caring for others.
THE ROLE OF SPECIAL OBLIGATIONS
Although the utilitarian argument has merit, my primary reason for advocating the prioritization of HCPs reflects a different ethical framework that emphasizes the reciprocal obligations between HCPs and the community. Obligations of physicians have been framed in terms of the commitments made to their self-chosen profession and the putative social contract that has been constructed with the community.8-10 These principles are well articulated in the American Medical Association’s (AMA’s) Code of Medical Ethics, which states, “Because of their commitment to care for the sick and injured, individual physicians have an obligation to provide urgent medical care during disasters…even in the face of greater than usual risks to their own safety, health, or life.”10,11 Although the AMA qualified its position by indicating that this obligation is not unconditional, it still formulated exceptions within the overarching structure of professional duty, allowing physicians to “balance immediate benefits to individual patients with ability to care for patients in the future.”11,12
If one accepts that HCPs have a professional obligation to take care of sick members of the community, even in perilous situations, what, if any, reciprocal obligation does the community have to its HCPs? Reciprocity is a fundamental ethical principle,13 serving as a foundation for the Golden Rule, which is a component of almost every ethical tradition.14 At its core, reciprocity asks us to treat other people as we would want to be treated. It requires endeavoring to take the perspective of others. Within this framework, a strategy for generating a just policy about treatment prioritization is to develop it under the assumption of not knowing which role one would end up playing in a situation. It is critical that if the positions of the individuals involved were reversed, the same rules and obligations would be accepted as fair.13 I suggest that if members of the community put themselves in the shoes of HCPs who are willing to risk exposure to a potentially deadly virus, they would acknowledge the legitimate expectation of HCPs to receive prioritized care if they became ill from the infection.
In most cases, reciprocity is not construed as requiring an identical exchange, but a fair one in which, for instance, sacrifice is returned in kind. Obligations can be viewed as debts that we either owe or are entitled to receive.15 In the current context, reciprocal obligations are derived from the relationship between HCPs and the community in which they serve. HCPs have a special set of obligations to carry out their work with a high degree of professionalism. If circumstances demand they take on substantial risk for their community, the community, in turn, has a special obligation to take care of them.
To highlight this perspective, imagine HCPs who become ill with COVID-19 and make claims for treatment priority despite having been unwilling to work with patients who are sick with COVID-19. We would consider such claims to be unjust because our moral intuition suggests that individuals are owed a debt for the actual risks they have taken, not for the potential ones they have avoided. A corollary of this view is that HCPs who have demonstrated a willingness to risk their lives contracting COVID-19 have a legitimate claim for prioritization.
Implementation
Acknowledgment of the community’s special obligation to HCPs does not negate competing claims for prioritization, such as trying to save the most lives or accounting for a patient’s pregnancy status and stage of life. Rather, there is a need for CSC guidelines to also include recognition of the special obligations owed HCPs by improving their priority score in the calculus used to triage care. Operationalizing the process would need to be worked out. One possibility would be for HCPs directly caring for patients ill from COVID-19 to have their priority score improve by 2 points, and HCPs directly caring for patients without known disease (but who could still be infectious) to benefit by 1 point. At a minimum, recognition of the risks taken should serve as a tiebreaker in favor of these workers.
To Whom Does the Community Have a Special Obligation?
If we acknowledge that during a pandemic, the community has a special obligation to HCPs because of the risks they are taking to serve others, by the same logic, this commitment should be extended to any personnel linked to the healthcare system (eg, employees in environmental services) or frontline workers providing essential services (eg, grocery store workers) who are taking similar risks that involve exposure to potentially infected individuals. Conversely, HCPs who are working exclusively from home via telemedicine should not receive treatment priority. An approach that extends treatment prioritization to other relevant workers mitigates concerns raised about prioritizing scarce critical care resources to an already advantaged class of individuals (ie, HCPs) as well as the negative optics of a committee of “deciders” in a hospital who are privileging care to their own members.12
CONCLUSION
Reciprocity, a critical component of our notion of justice, should be incorporated into CSC guidelines. The community’s reciprocity to HCPs and frontline workers needs to be commensurate with the sacrifice made by these groups. Although public demonstrations of gratitude may be much appreciated, such displays alone are not adequate for honoring the community’s special obligations. If, during a pandemic, HCPs or frontline workers deliver direct care or services to members of the community, despite serious risk to their own lives, the community has a reciprocal obligation to these individuals to prioritize their access to critical care. HCPs and frontline workers should be prioritized not because their lives have higher intrinsic worth or solely as a reflection of their instrumental value to the community, but out of recognition of the special debt owed them. This is not an unconditional obligation, but one that should be built into the complex, multifaceted decision-making process4,6,16 underlying the allocation of scarce medical resources in a pandemic.
Acknowledgments
The author deeply appreciates the thoughtful comments on the essay from William Snyder, PhD, Melissa Frumin, MD, Brittany McFeeley, BS, Lise Bliss, MBA, and especially Seth Gales, MD, and remains grateful for the guidance and support he received early in his academic career from his first mentors, Carol Gilligan, PhD, and Michael Walzer, PhD.
Potential catastrophic surges in coronavirus disease 2019 (COVID-19) are leading to more patients requiring intensive care unit beds than are available, prompting hospitals to prepare to activate crisis standards of care (CSC).1,2 These guidelines manage the sobering process of determining which gravely ill patients will have access to limited ventilators, critical care specialists, and other essential hospital personnel. As a member of the CSC triage team at Brigham and Women’s Hospital, Boston, Massachusetts, during the initial surge,1 I was taught how to follow procedures that assign each patient a priority score that ranged from 1 to 8, with lower scores representing higher priority. Scoring decisions were largely based on current status of organ systems and major medical illnesses (predictive of short-term and longer-term survival, respectively), consistent with the objective of maximizing lives and life-years saved.1,3-7 Other parameters included improving the priority score of a pregnant woman with a viable fetus and breaking ties in favor of younger patients who had not lived through life’s major stages.4,7 One issue that elicited sharp disagreement among my colleagues was whether healthcare providers (HCPs; eg, physicians, nurses) should be treated any differently than other individuals.
I believe that HCPs should receive treatment priority during a pandemic because the community has a special obligation to those workers willing to risk serious illness by providing care to potentially infected patients.
THE UTILITARIAN CASE FOR TREATMENT PRIORITIZATION
The most common argument for prioritizing HCPs has been made on utilitarian grounds: save individuals who can save others.3,4,6 Such an approach is not founded on the claim that HCPs have higher intrinsic worth, but is based on the instrumental value of HCPs to keep others alive.4,6 An abiding concern for human life demands systems to ensure individuals with clinical expertise are protected so that they can use their skills to maximize the number of lives saved. A similar case has been made to justify prioritizing HCPs for early access to vaccines during a pandemic.5 To underscore these issues, imagine a scenario in which, because of serious illness among HCPs, there were not enough workers with requisite expertise to care for the rest of the community in which a virus was rapidly spreading. Prioritizing HCPs could mitigate this sequence of events by preventing them from becoming infected through early access to vaccinations or promoting their recovery from the illness, which might allow them to return to work caring for others.
THE ROLE OF SPECIAL OBLIGATIONS
Although the utilitarian argument has merit, my primary reason for advocating the prioritization of HCPs reflects a different ethical framework that emphasizes the reciprocal obligations between HCPs and the community. Obligations of physicians have been framed in terms of the commitments made to their self-chosen profession and the putative social contract that has been constructed with the community.8-10 These principles are well articulated in the American Medical Association’s (AMA’s) Code of Medical Ethics, which states, “Because of their commitment to care for the sick and injured, individual physicians have an obligation to provide urgent medical care during disasters…even in the face of greater than usual risks to their own safety, health, or life.”10,11 Although the AMA qualified its position by indicating that this obligation is not unconditional, it still formulated exceptions within the overarching structure of professional duty, allowing physicians to “balance immediate benefits to individual patients with ability to care for patients in the future.”11,12
If one accepts that HCPs have a professional obligation to take care of sick members of the community, even in perilous situations, what, if any, reciprocal obligation does the community have to its HCPs? Reciprocity is a fundamental ethical principle,13 serving as a foundation for the Golden Rule, which is a component of almost every ethical tradition.14 At its core, reciprocity asks us to treat other people as we would want to be treated. It requires endeavoring to take the perspective of others. Within this framework, a strategy for generating a just policy about treatment prioritization is to develop it under the assumption of not knowing which role one would end up playing in a situation. It is critical that if the positions of the individuals involved were reversed, the same rules and obligations would be accepted as fair.13 I suggest that if members of the community put themselves in the shoes of HCPs who are willing to risk exposure to a potentially deadly virus, they would acknowledge the legitimate expectation of HCPs to receive prioritized care if they became ill from the infection.
In most cases, reciprocity is not construed as requiring an identical exchange, but a fair one in which, for instance, sacrifice is returned in kind. Obligations can be viewed as debts that we either owe or are entitled to receive.15 In the current context, reciprocal obligations are derived from the relationship between HCPs and the community in which they serve. HCPs have a special set of obligations to carry out their work with a high degree of professionalism. If circumstances demand they take on substantial risk for their community, the community, in turn, has a special obligation to take care of them.
To highlight this perspective, imagine HCPs who become ill with COVID-19 and make claims for treatment priority despite having been unwilling to work with patients who are sick with COVID-19. We would consider such claims to be unjust because our moral intuition suggests that individuals are owed a debt for the actual risks they have taken, not for the potential ones they have avoided. A corollary of this view is that HCPs who have demonstrated a willingness to risk their lives contracting COVID-19 have a legitimate claim for prioritization.
Implementation
Acknowledgment of the community’s special obligation to HCPs does not negate competing claims for prioritization, such as trying to save the most lives or accounting for a patient’s pregnancy status and stage of life. Rather, there is a need for CSC guidelines to also include recognition of the special obligations owed HCPs by improving their priority score in the calculus used to triage care. Operationalizing the process would need to be worked out. One possibility would be for HCPs directly caring for patients ill from COVID-19 to have their priority score improve by 2 points, and HCPs directly caring for patients without known disease (but who could still be infectious) to benefit by 1 point. At a minimum, recognition of the risks taken should serve as a tiebreaker in favor of these workers.
To Whom Does the Community Have a Special Obligation?
If we acknowledge that during a pandemic, the community has a special obligation to HCPs because of the risks they are taking to serve others, by the same logic, this commitment should be extended to any personnel linked to the healthcare system (eg, employees in environmental services) or frontline workers providing essential services (eg, grocery store workers) who are taking similar risks that involve exposure to potentially infected individuals. Conversely, HCPs who are working exclusively from home via telemedicine should not receive treatment priority. An approach that extends treatment prioritization to other relevant workers mitigates concerns raised about prioritizing scarce critical care resources to an already advantaged class of individuals (ie, HCPs) as well as the negative optics of a committee of “deciders” in a hospital who are privileging care to their own members.12
CONCLUSION
Reciprocity, a critical component of our notion of justice, should be incorporated into CSC guidelines. The community’s reciprocity to HCPs and frontline workers needs to be commensurate with the sacrifice made by these groups. Although public demonstrations of gratitude may be much appreciated, such displays alone are not adequate for honoring the community’s special obligations. If, during a pandemic, HCPs or frontline workers deliver direct care or services to members of the community, despite serious risk to their own lives, the community has a reciprocal obligation to these individuals to prioritize their access to critical care. HCPs and frontline workers should be prioritized not because their lives have higher intrinsic worth or solely as a reflection of their instrumental value to the community, but out of recognition of the special debt owed them. This is not an unconditional obligation, but one that should be built into the complex, multifaceted decision-making process4,6,16 underlying the allocation of scarce medical resources in a pandemic.
Acknowledgments
The author deeply appreciates the thoughtful comments on the essay from William Snyder, PhD, Melissa Frumin, MD, Brittany McFeeley, BS, Lise Bliss, MBA, and especially Seth Gales, MD, and remains grateful for the guidance and support he received early in his academic career from his first mentors, Carol Gilligan, PhD, and Michael Walzer, PhD.
1. Milliken A, Jurchak M, Sadovnikoff N, et al. Addressing challenges associated with operationalizing a crisis standards of care protocol for the Covid-19 pandemic. NEJM Catalyst. 2020:1-14. https://doi.org/10.1056/CAT.20.0384
2. Paquette ET, Derrington S, Fry JT, et al. Shifting duties of children’s hospitals during the COVID-19 pandemic. J Hosp Med. 2020;15(10):631-633. https://doi.org/10.12788/jhm.3490
3. Emanuel EJ, Persad G, Upshur R, et al. Fair allocation of scarce medical resources in the time of Covid-19.N Engl J Med.2020;382(21):2049-2055. https://doi.org/10.1056/NEJMsb2005114
4. White DB, Lo B. A framework for rationing ventilators and critical care beds during the COVID-19 pandemic. JAMA. 2020;323(18):1773-1774. https://doi.org/10.1001/jama.2020.5046
5. Emanuel EJ, Wetheimer A. Who should get influenza vaccine when not all can? Science. 2006;312(5775):854-855. https://doi.org/10.1126/science.1125347
6. White DB, Katz MH, Luce JM, Lo B. Who should receive life support during a public health emergency? Using ethical principles to improve allocation decisions. Ann Intern Med. 2009;150(2):132-138. https://doi.org/10.7326/0003-4819-150-2-200901200-00011
7. The Commonwealth of Massachusetts Executive Office of Health and Human Services Department of Public Health. Crisis Standards of Care: Planning Guidance for the COVID-19 Pandemic. Accessed August 1, 2020. https://www.mass.gov/doc/statewide-advisory-committee-recommendations-forstandards-of-care/download?_ga=2.55928739.940920097.159299949-195847297.1590861397
8. Brody H, Avery EN. Medicine’s duty to treat pandemic illness: solidarity and vulnerability. Hastings Cent Rep. 2009;39(1):40-48. https://doi.org/10.1353/hcr.0.0104
9. Ruderman C, Tracy CS, Bensimon CM, et al. On pandemics and the duty to care: whose duty? who cares? BMC Med Ethics. 2006;7:E5. https://doi.org/10.1186/1472-6939-7-5
10. Huber SJ, Wynia MK. When pestilence prevails . . . physician responsibilities in epidemics. Am J Bioeth. 2004;4(1):W5-11. https://doi.org/10.1162/152651604773067497
11. AMA Council on Ethical and Judicial Affairs. Opinion 9.067 Physician Obligation in Disaster Preparedness and Response. Virtual Mentor. 2010;12(6):459. d10.1001/virtualmentor.2010.12.6.coet1-1006
12. Rothstein MA. Should health care providers get treatment priority in an influenza pandemic? J Law Med Ethics. 2010;38(2):412-419. https://doi.org/10.1111/j.1748-720X.2010.00499.x
13. Rawls J. A Theory of Justice. The Belknap Press: an imprint of Harvard University Press; 1971.
14. Green WS. Parsing reciprocity: questions for the Golden Rule. In: Neusner J, Chilton BD, eds. The Golden Rule: The Ethics of Reciprocity in World Religions. Continuum International Publishing Group; 2008:1-8.
15. Walzer M. Obligations: Essays on Disobedience, War and Citizenship. Harvard University Press; 1970.
16. Persad G, Wertheimer A, Emanuel EJ. Principles for allocation of scarce medical interventions. Lancet. 2009;373(9661):423-431. https://doi.org/10.1016/S0140-6736(09)60137-9
1. Milliken A, Jurchak M, Sadovnikoff N, et al. Addressing challenges associated with operationalizing a crisis standards of care protocol for the Covid-19 pandemic. NEJM Catalyst. 2020:1-14. https://doi.org/10.1056/CAT.20.0384
2. Paquette ET, Derrington S, Fry JT, et al. Shifting duties of children’s hospitals during the COVID-19 pandemic. J Hosp Med. 2020;15(10):631-633. https://doi.org/10.12788/jhm.3490
3. Emanuel EJ, Persad G, Upshur R, et al. Fair allocation of scarce medical resources in the time of Covid-19.N Engl J Med.2020;382(21):2049-2055. https://doi.org/10.1056/NEJMsb2005114
4. White DB, Lo B. A framework for rationing ventilators and critical care beds during the COVID-19 pandemic. JAMA. 2020;323(18):1773-1774. https://doi.org/10.1001/jama.2020.5046
5. Emanuel EJ, Wetheimer A. Who should get influenza vaccine when not all can? Science. 2006;312(5775):854-855. https://doi.org/10.1126/science.1125347
6. White DB, Katz MH, Luce JM, Lo B. Who should receive life support during a public health emergency? Using ethical principles to improve allocation decisions. Ann Intern Med. 2009;150(2):132-138. https://doi.org/10.7326/0003-4819-150-2-200901200-00011
7. The Commonwealth of Massachusetts Executive Office of Health and Human Services Department of Public Health. Crisis Standards of Care: Planning Guidance for the COVID-19 Pandemic. Accessed August 1, 2020. https://www.mass.gov/doc/statewide-advisory-committee-recommendations-forstandards-of-care/download?_ga=2.55928739.940920097.159299949-195847297.1590861397
8. Brody H, Avery EN. Medicine’s duty to treat pandemic illness: solidarity and vulnerability. Hastings Cent Rep. 2009;39(1):40-48. https://doi.org/10.1353/hcr.0.0104
9. Ruderman C, Tracy CS, Bensimon CM, et al. On pandemics and the duty to care: whose duty? who cares? BMC Med Ethics. 2006;7:E5. https://doi.org/10.1186/1472-6939-7-5
10. Huber SJ, Wynia MK. When pestilence prevails . . . physician responsibilities in epidemics. Am J Bioeth. 2004;4(1):W5-11. https://doi.org/10.1162/152651604773067497
11. AMA Council on Ethical and Judicial Affairs. Opinion 9.067 Physician Obligation in Disaster Preparedness and Response. Virtual Mentor. 2010;12(6):459. d10.1001/virtualmentor.2010.12.6.coet1-1006
12. Rothstein MA. Should health care providers get treatment priority in an influenza pandemic? J Law Med Ethics. 2010;38(2):412-419. https://doi.org/10.1111/j.1748-720X.2010.00499.x
13. Rawls J. A Theory of Justice. The Belknap Press: an imprint of Harvard University Press; 1971.
14. Green WS. Parsing reciprocity: questions for the Golden Rule. In: Neusner J, Chilton BD, eds. The Golden Rule: The Ethics of Reciprocity in World Religions. Continuum International Publishing Group; 2008:1-8.
15. Walzer M. Obligations: Essays on Disobedience, War and Citizenship. Harvard University Press; 1970.
16. Persad G, Wertheimer A, Emanuel EJ. Principles for allocation of scarce medical interventions. Lancet. 2009;373(9661):423-431. https://doi.org/10.1016/S0140-6736(09)60137-9
© 2021 Society of Hospital Medicine