User login
COVID-19: Helping patients overcome what might feel like an existential crisis
Way back in the spring of last year, I wrote about a pandemic of posttraumatic stress disorder that would descend upon us because of COVID-19. At the time, we were told that, by summer – June or July 2020 – all the steps we needed to take to stay ahead of the virus, including remaining socially distant, and yes, even wearing masks, would be over. Life would get back to normal.
Little did we know that a national plan for our safety, including making sure that we had enough masks and PPE, would not be forthcoming, and that so many thousands of Americans would perish, leaving millions of distraught families and friends.
So many people are suffering. Mothers, for example, are struggling to balance remote schooling with additional child care and domestic work. More than 2 million women left the U.S. workforce last year between February 2020 and October 2020, according to a report by the National Women’s Law Center. Even before COVID-19, loneliness among young adults was considered a domestic epidemic – and the social isolation forced by the pandemic has worsened those trends, research shows. These trends are creating so much more anxiety, depression, despair, and yes, even PTSD. As mental health professionals, we have a lot of work to do in educating people about coping skills and in providing treatments when appropriate.
Experiences take on new meaning
One day a friend and professional colleague called me, and he sounded quite distraught. He had not been able to reach his primary care physician and thought that, as a physician, I might have some insights about his symptoms. He began telling me that something really strange was happening whenever he walked around outside with his mask on. He couldn’t breathe with it on, he told me. In addition, his eyes teared up, his nose started running, and his eyeglasses fogged up so much that he couldn’t see where he was going. He was really anxious, nervous, and felt a great sense of despair – and disorientation. He did not fully understand what was happening and didn’t know whether those disorienting symptoms were mask-related or whether he was incubating some yet undiagnosed illness.
I addressed his concerns in the moment by assuring him that I, too, had been experiencing similar challenges with fogged-up glasses and a runny nose; many people were experiencing some of the same things. I explained that even I had called an allergist to find out whether I might be allergic to some component in the mask and whether he had seen those symptoms in his practice.
Albeit, those issues tied to masks are relatively minor, compared with the enormous psychological toll this pandemic has taken on some people. But it’s clear that different people suffer different effects in light of the marked changes in life and lifestyles caused by the pandemic.
‘It’s something else’
Two people I know, both professionals, recently told me that in their social lives they constantly feel tired and anxious, and that their concentration has diminished. They worry more about their futures, they told me separately. (They don’t know each other.) They reported going through daily life “like being on automatic.” Both said they were far too irritable and reported feeling that social isolation had dulled their thinking.
They said they were not depressed; “it’s something else.” I reassured them both that this would pass with time and suggested that they work at not socially isolating – to the extent that they can – during the pandemic. I also encouraged them to get vaccinated and to talk with a professional if their malaise was altering their level of functioning.
So far, more than 475,000 people in the United States have died of COVID-19, and thousands continue to suffer. People are saying goodbye to loved ones on iPads, and watching news stories about hospitals at overcapacity and refrigeration units storing bodies. Meanwhile, health care workers, many of whom are putting their lives and those of their families at risk, are reporting increased levels of burnout – and moral injury.
Value of relaxation techniques
We know that the latest mitigation measures advised by the Centers for Disease Control and Prevention must continue during the COVID-19 vaccination process. The new CDC guidelines on the value of double masking make sense and should be followed. However, even as we learn more about the virus and how to stop its spread, we must recognize that social distancing is not the same as social isolation. We must continue to do what we can to maintain social relationships and keep open the lines of communication, including the use of virtual tools. I am pleased to see the growth of telemedicine during the pandemic. When applicable, telemedicine allows greater medical and mental health care without the stress of travel and the risk of exposure to more people than necessary.
, whether it’s hypnosis, mindfulness, transcendental meditation, or deep breathing exercises. For the more advanced therapies, guided imagery can help patients develop a sense of calm and equanimity.
For those who are not skilled in relaxation techniques, YouTube offers some excellent programs that teach relaxation and mindfulness. Another thing I do is talk regularly with people I know and sometimes with people I know I’ll disagree with – just to keep my brain active. I also try to learn new things in my spare time to establish new brain pathways and stay mentally active.
The pain and grief tied to the pandemic are unlike anything we’ve ever experienced. Our training as psychiatrists, psychologists, and other mental health care professionals makes us all uniquely suited to assist patients as they process these traumatic times. We must step forward and do so.
Dr. London is a practicing psychiatrist and has been a newspaper columnist for 35 years, specializing in and writing about short-term therapy, including cognitive-behavioral therapy and guided imagery. He is author of “Find Freedom Fast” (Kettlehole Publishing, 2019). He has no conflicts of interest.
Way back in the spring of last year, I wrote about a pandemic of posttraumatic stress disorder that would descend upon us because of COVID-19. At the time, we were told that, by summer – June or July 2020 – all the steps we needed to take to stay ahead of the virus, including remaining socially distant, and yes, even wearing masks, would be over. Life would get back to normal.
Little did we know that a national plan for our safety, including making sure that we had enough masks and PPE, would not be forthcoming, and that so many thousands of Americans would perish, leaving millions of distraught families and friends.
So many people are suffering. Mothers, for example, are struggling to balance remote schooling with additional child care and domestic work. More than 2 million women left the U.S. workforce last year between February 2020 and October 2020, according to a report by the National Women’s Law Center. Even before COVID-19, loneliness among young adults was considered a domestic epidemic – and the social isolation forced by the pandemic has worsened those trends, research shows. These trends are creating so much more anxiety, depression, despair, and yes, even PTSD. As mental health professionals, we have a lot of work to do in educating people about coping skills and in providing treatments when appropriate.
Experiences take on new meaning
One day a friend and professional colleague called me, and he sounded quite distraught. He had not been able to reach his primary care physician and thought that, as a physician, I might have some insights about his symptoms. He began telling me that something really strange was happening whenever he walked around outside with his mask on. He couldn’t breathe with it on, he told me. In addition, his eyes teared up, his nose started running, and his eyeglasses fogged up so much that he couldn’t see where he was going. He was really anxious, nervous, and felt a great sense of despair – and disorientation. He did not fully understand what was happening and didn’t know whether those disorienting symptoms were mask-related or whether he was incubating some yet undiagnosed illness.
I addressed his concerns in the moment by assuring him that I, too, had been experiencing similar challenges with fogged-up glasses and a runny nose; many people were experiencing some of the same things. I explained that even I had called an allergist to find out whether I might be allergic to some component in the mask and whether he had seen those symptoms in his practice.
Albeit, those issues tied to masks are relatively minor, compared with the enormous psychological toll this pandemic has taken on some people. But it’s clear that different people suffer different effects in light of the marked changes in life and lifestyles caused by the pandemic.
‘It’s something else’
Two people I know, both professionals, recently told me that in their social lives they constantly feel tired and anxious, and that their concentration has diminished. They worry more about their futures, they told me separately. (They don’t know each other.) They reported going through daily life “like being on automatic.” Both said they were far too irritable and reported feeling that social isolation had dulled their thinking.
They said they were not depressed; “it’s something else.” I reassured them both that this would pass with time and suggested that they work at not socially isolating – to the extent that they can – during the pandemic. I also encouraged them to get vaccinated and to talk with a professional if their malaise was altering their level of functioning.
So far, more than 475,000 people in the United States have died of COVID-19, and thousands continue to suffer. People are saying goodbye to loved ones on iPads, and watching news stories about hospitals at overcapacity and refrigeration units storing bodies. Meanwhile, health care workers, many of whom are putting their lives and those of their families at risk, are reporting increased levels of burnout – and moral injury.
Value of relaxation techniques
We know that the latest mitigation measures advised by the Centers for Disease Control and Prevention must continue during the COVID-19 vaccination process. The new CDC guidelines on the value of double masking make sense and should be followed. However, even as we learn more about the virus and how to stop its spread, we must recognize that social distancing is not the same as social isolation. We must continue to do what we can to maintain social relationships and keep open the lines of communication, including the use of virtual tools. I am pleased to see the growth of telemedicine during the pandemic. When applicable, telemedicine allows greater medical and mental health care without the stress of travel and the risk of exposure to more people than necessary.
, whether it’s hypnosis, mindfulness, transcendental meditation, or deep breathing exercises. For the more advanced therapies, guided imagery can help patients develop a sense of calm and equanimity.
For those who are not skilled in relaxation techniques, YouTube offers some excellent programs that teach relaxation and mindfulness. Another thing I do is talk regularly with people I know and sometimes with people I know I’ll disagree with – just to keep my brain active. I also try to learn new things in my spare time to establish new brain pathways and stay mentally active.
The pain and grief tied to the pandemic are unlike anything we’ve ever experienced. Our training as psychiatrists, psychologists, and other mental health care professionals makes us all uniquely suited to assist patients as they process these traumatic times. We must step forward and do so.
Dr. London is a practicing psychiatrist and has been a newspaper columnist for 35 years, specializing in and writing about short-term therapy, including cognitive-behavioral therapy and guided imagery. He is author of “Find Freedom Fast” (Kettlehole Publishing, 2019). He has no conflicts of interest.
Way back in the spring of last year, I wrote about a pandemic of posttraumatic stress disorder that would descend upon us because of COVID-19. At the time, we were told that, by summer – June or July 2020 – all the steps we needed to take to stay ahead of the virus, including remaining socially distant, and yes, even wearing masks, would be over. Life would get back to normal.
Little did we know that a national plan for our safety, including making sure that we had enough masks and PPE, would not be forthcoming, and that so many thousands of Americans would perish, leaving millions of distraught families and friends.
So many people are suffering. Mothers, for example, are struggling to balance remote schooling with additional child care and domestic work. More than 2 million women left the U.S. workforce last year between February 2020 and October 2020, according to a report by the National Women’s Law Center. Even before COVID-19, loneliness among young adults was considered a domestic epidemic – and the social isolation forced by the pandemic has worsened those trends, research shows. These trends are creating so much more anxiety, depression, despair, and yes, even PTSD. As mental health professionals, we have a lot of work to do in educating people about coping skills and in providing treatments when appropriate.
Experiences take on new meaning
One day a friend and professional colleague called me, and he sounded quite distraught. He had not been able to reach his primary care physician and thought that, as a physician, I might have some insights about his symptoms. He began telling me that something really strange was happening whenever he walked around outside with his mask on. He couldn’t breathe with it on, he told me. In addition, his eyes teared up, his nose started running, and his eyeglasses fogged up so much that he couldn’t see where he was going. He was really anxious, nervous, and felt a great sense of despair – and disorientation. He did not fully understand what was happening and didn’t know whether those disorienting symptoms were mask-related or whether he was incubating some yet undiagnosed illness.
I addressed his concerns in the moment by assuring him that I, too, had been experiencing similar challenges with fogged-up glasses and a runny nose; many people were experiencing some of the same things. I explained that even I had called an allergist to find out whether I might be allergic to some component in the mask and whether he had seen those symptoms in his practice.
Albeit, those issues tied to masks are relatively minor, compared with the enormous psychological toll this pandemic has taken on some people. But it’s clear that different people suffer different effects in light of the marked changes in life and lifestyles caused by the pandemic.
‘It’s something else’
Two people I know, both professionals, recently told me that in their social lives they constantly feel tired and anxious, and that their concentration has diminished. They worry more about their futures, they told me separately. (They don’t know each other.) They reported going through daily life “like being on automatic.” Both said they were far too irritable and reported feeling that social isolation had dulled their thinking.
They said they were not depressed; “it’s something else.” I reassured them both that this would pass with time and suggested that they work at not socially isolating – to the extent that they can – during the pandemic. I also encouraged them to get vaccinated and to talk with a professional if their malaise was altering their level of functioning.
So far, more than 475,000 people in the United States have died of COVID-19, and thousands continue to suffer. People are saying goodbye to loved ones on iPads, and watching news stories about hospitals at overcapacity and refrigeration units storing bodies. Meanwhile, health care workers, many of whom are putting their lives and those of their families at risk, are reporting increased levels of burnout – and moral injury.
Value of relaxation techniques
We know that the latest mitigation measures advised by the Centers for Disease Control and Prevention must continue during the COVID-19 vaccination process. The new CDC guidelines on the value of double masking make sense and should be followed. However, even as we learn more about the virus and how to stop its spread, we must recognize that social distancing is not the same as social isolation. We must continue to do what we can to maintain social relationships and keep open the lines of communication, including the use of virtual tools. I am pleased to see the growth of telemedicine during the pandemic. When applicable, telemedicine allows greater medical and mental health care without the stress of travel and the risk of exposure to more people than necessary.
, whether it’s hypnosis, mindfulness, transcendental meditation, or deep breathing exercises. For the more advanced therapies, guided imagery can help patients develop a sense of calm and equanimity.
For those who are not skilled in relaxation techniques, YouTube offers some excellent programs that teach relaxation and mindfulness. Another thing I do is talk regularly with people I know and sometimes with people I know I’ll disagree with – just to keep my brain active. I also try to learn new things in my spare time to establish new brain pathways and stay mentally active.
The pain and grief tied to the pandemic are unlike anything we’ve ever experienced. Our training as psychiatrists, psychologists, and other mental health care professionals makes us all uniquely suited to assist patients as they process these traumatic times. We must step forward and do so.
Dr. London is a practicing psychiatrist and has been a newspaper columnist for 35 years, specializing in and writing about short-term therapy, including cognitive-behavioral therapy and guided imagery. He is author of “Find Freedom Fast” (Kettlehole Publishing, 2019). He has no conflicts of interest.
The changing brain signature of HIV
“This shift in subcortical signatures may be contributing to the increasing range of neuropsychiatric and cognitive outcomes,” write Neda Jahanshad, PhD, University of Southern California, Los Angeles, and colleagues.
The study was published online Jan. 15 in JAMA Network Open.
Brain signature of HIV
The researchers with the HIV Working Group within the Enhancing Neuro Imaging Genetics Through Meta Analysis (ENIGMA) consortium examined structural brain associations with CD4+ T cell counts and HIV viral load.
These clinical markers are the most consistently available in studies of HIV and generalize across demographically and clinically diverse HIV-infected individuals, they point out. However, the degree to which they capture central nervous system injury is not fully understood.
In this cross-sectional study of 1,203 HIV-infected adults from 13 HIV neuroimaging studies, a lower CD4+ T-cell count was associated with smaller hippocampal and thalamic volume independent of treatment status. However, in a subset of adults not on cART, a lower CD4+ T-cell count was associated with smaller putamen volume.
Across all participants, detectable viral load was associated with smaller hippocampal volume, but in the subset on cART, detectable viral load was also associated with smaller amygdala volume.
The findings indicate that plasma markers universally used to monitor immune function and response to treatment in patients with HIV infection are associated with subcortical brain volume.
“Our findings,” they add, “extend beyond the classically implicated regions of the basal ganglia and may represent a generalizable brain signature of HIV infection in the cART era.”
A limitation of the analysis is that most of the participants were men (n = 880, 73%). “A more extensive international effort assessing the neurologic effects of HIV infection in women is needed,” they conclude.
This analysis, they add, demonstrates the feasibility and utility of a global collaborative initiative to understand the neurologic signatures of HIV infection. They invite other HIV researchers to join the ENIGMA-HIV consortium.
“With a greater collaborative effort, we will be able to assess factors that may modulate neurologic outcomes, including cART treatment regimens, comorbidities, coinfections, substance use, socioeconomic factors, and demographic factors, as well as the functional implications of such structural brain differences, in well-powered analyses,” the researchers say.
“Understanding the neurobiological changes that may contribute to neuropsychiatric and cognitive outcomes in HIV-positive individuals is critical for identifying individuals at risk for neurologic symptoms, driving novel treatments that may protect the CNS, and monitoring treatment response,” they add.
Support for this research was provided by grants from the National Institutes of Health, the SA Medical Research Council, the National Health and Medical Research Council, and the European Research Council. Dr. Jahanshad received partial research support from Biogen for work unrelated to the topic of this article. A complete list of author disclosures is in the original article.
A version of this article first appeared on Medscape.com.
“This shift in subcortical signatures may be contributing to the increasing range of neuropsychiatric and cognitive outcomes,” write Neda Jahanshad, PhD, University of Southern California, Los Angeles, and colleagues.
The study was published online Jan. 15 in JAMA Network Open.
Brain signature of HIV
The researchers with the HIV Working Group within the Enhancing Neuro Imaging Genetics Through Meta Analysis (ENIGMA) consortium examined structural brain associations with CD4+ T cell counts and HIV viral load.
These clinical markers are the most consistently available in studies of HIV and generalize across demographically and clinically diverse HIV-infected individuals, they point out. However, the degree to which they capture central nervous system injury is not fully understood.
In this cross-sectional study of 1,203 HIV-infected adults from 13 HIV neuroimaging studies, a lower CD4+ T-cell count was associated with smaller hippocampal and thalamic volume independent of treatment status. However, in a subset of adults not on cART, a lower CD4+ T-cell count was associated with smaller putamen volume.
Across all participants, detectable viral load was associated with smaller hippocampal volume, but in the subset on cART, detectable viral load was also associated with smaller amygdala volume.
The findings indicate that plasma markers universally used to monitor immune function and response to treatment in patients with HIV infection are associated with subcortical brain volume.
“Our findings,” they add, “extend beyond the classically implicated regions of the basal ganglia and may represent a generalizable brain signature of HIV infection in the cART era.”
A limitation of the analysis is that most of the participants were men (n = 880, 73%). “A more extensive international effort assessing the neurologic effects of HIV infection in women is needed,” they conclude.
This analysis, they add, demonstrates the feasibility and utility of a global collaborative initiative to understand the neurologic signatures of HIV infection. They invite other HIV researchers to join the ENIGMA-HIV consortium.
“With a greater collaborative effort, we will be able to assess factors that may modulate neurologic outcomes, including cART treatment regimens, comorbidities, coinfections, substance use, socioeconomic factors, and demographic factors, as well as the functional implications of such structural brain differences, in well-powered analyses,” the researchers say.
“Understanding the neurobiological changes that may contribute to neuropsychiatric and cognitive outcomes in HIV-positive individuals is critical for identifying individuals at risk for neurologic symptoms, driving novel treatments that may protect the CNS, and monitoring treatment response,” they add.
Support for this research was provided by grants from the National Institutes of Health, the SA Medical Research Council, the National Health and Medical Research Council, and the European Research Council. Dr. Jahanshad received partial research support from Biogen for work unrelated to the topic of this article. A complete list of author disclosures is in the original article.
A version of this article first appeared on Medscape.com.
“This shift in subcortical signatures may be contributing to the increasing range of neuropsychiatric and cognitive outcomes,” write Neda Jahanshad, PhD, University of Southern California, Los Angeles, and colleagues.
The study was published online Jan. 15 in JAMA Network Open.
Brain signature of HIV
The researchers with the HIV Working Group within the Enhancing Neuro Imaging Genetics Through Meta Analysis (ENIGMA) consortium examined structural brain associations with CD4+ T cell counts and HIV viral load.
These clinical markers are the most consistently available in studies of HIV and generalize across demographically and clinically diverse HIV-infected individuals, they point out. However, the degree to which they capture central nervous system injury is not fully understood.
In this cross-sectional study of 1,203 HIV-infected adults from 13 HIV neuroimaging studies, a lower CD4+ T-cell count was associated with smaller hippocampal and thalamic volume independent of treatment status. However, in a subset of adults not on cART, a lower CD4+ T-cell count was associated with smaller putamen volume.
Across all participants, detectable viral load was associated with smaller hippocampal volume, but in the subset on cART, detectable viral load was also associated with smaller amygdala volume.
The findings indicate that plasma markers universally used to monitor immune function and response to treatment in patients with HIV infection are associated with subcortical brain volume.
“Our findings,” they add, “extend beyond the classically implicated regions of the basal ganglia and may represent a generalizable brain signature of HIV infection in the cART era.”
A limitation of the analysis is that most of the participants were men (n = 880, 73%). “A more extensive international effort assessing the neurologic effects of HIV infection in women is needed,” they conclude.
This analysis, they add, demonstrates the feasibility and utility of a global collaborative initiative to understand the neurologic signatures of HIV infection. They invite other HIV researchers to join the ENIGMA-HIV consortium.
“With a greater collaborative effort, we will be able to assess factors that may modulate neurologic outcomes, including cART treatment regimens, comorbidities, coinfections, substance use, socioeconomic factors, and demographic factors, as well as the functional implications of such structural brain differences, in well-powered analyses,” the researchers say.
“Understanding the neurobiological changes that may contribute to neuropsychiatric and cognitive outcomes in HIV-positive individuals is critical for identifying individuals at risk for neurologic symptoms, driving novel treatments that may protect the CNS, and monitoring treatment response,” they add.
Support for this research was provided by grants from the National Institutes of Health, the SA Medical Research Council, the National Health and Medical Research Council, and the European Research Council. Dr. Jahanshad received partial research support from Biogen for work unrelated to the topic of this article. A complete list of author disclosures is in the original article.
A version of this article first appeared on Medscape.com.
Psoriasis registry study finds normal pregnancy outcomes
according to one of the largest studies to examine the issue to date.
However, “pregnancy-specific registries that include a larger number of pregnant women with psoriasis ... are needed to more fully characterize the association between psoriasis and treatment and birth outcomes,” acknowledged first author Alexa B. Kimball, MD, MPH, professor of dermatology, Harvard Medical School, Boston, and colleagues.
The cohort study, published in JAMA Dermatology, used data from the Psoriasis Longitudinal Assessment and Registry (PSOLAR), which “is not a pregnancy specific registry, and medical history is captured only at baseline,” they noted.
Their findings showed pregnancy outcomes such as spontaneous abortion, neonatal problems, and congenital anomalies among women with moderate to severe psoriasis were similar to rates in the general U.S. population, and are “consistent with previously reported data,” they reported. “And pregnancy outcomes for women exposed to biologics were similar to those for women with exposure to nonbiologics.”
The study “provides further reassurance that the biologics appear safe at least related to pregnancy outcomes,” commented Jenny Murase, MD, associate professor of dermatology at the University of California, San Francisco, who was not involved in the study. In an interview, she noted that the study “did not examine any potential immunosuppression of the fetus in the first 6 months of life,” which she described as “the heart of the concern, more than whether or not the psoriasis or the biologic affects the pregnancy itself.”
The study used data from the PSOLAR registry collected from June 20, 2007, to Aug.23, 2019, which included 2,224 women of childbearing age (18-45 years) who were collectively followed up for 12,929 patient-years. Among these women, 220 had 298 pregnancies, with 244 live births (81.9%).
“Birth outcomes among all 244 births included 231 healthy newborns (94.7%), 10 infants with a neonatal problem (4.1%), 1 stillbirth (0.4%), and 2 congenital anomalies (0.8%),” the authors reported.
There were also 41 spontaneous abortions (13.8%), and 13 elective terminations (4.4%). “No elective terminations were known to derive from a congenital anomaly or other medical issue,” they added.
Among the documented pregnancies, 252 occurred in women with exposure to biologic therapy either before or during pregnancy, including 168 (56.4%) during the prenatal period, while 46 pregnancies occurred in women with no exposure to biologic therapy.
Dr. Murase, director of medical consultative dermatology for the Palo Alto Foundation Medical Group in Mountain View, Calif., said that a more detailed comparison of the different psoriasis treatments, as well as the offspring outcomes during the first 6 months of life, might offer some further important insight,.
Infants born after exposure to infliximab “and potentially other anti–tumor necrosis factor–alpha agents during the third trimester may be unable to develop an appropriate immune response to live vaccines,” she and her coauthors cautioned in a letter published in 2011, which referred to a case of an infant with disseminated bacillus Calmette-Guérin infection, whose mother had received infliximab for Crohn’s disease throughout pregnancy.
Dr. Murase pointed out that, in the registry study, exposures to certolizumab, which is pegylated and does not cross the placental barrier, were not separated from other cases. It is important to consider “the cross over late in the second trimester and especially third trimester as the infant is getting the ‘antibody boost’ from the mother as it gets ready to set foot in this world and needs the maternal antibodies to prepare its immune system. If the IgG biologics cross third trimester and immunosuppress the infant ... then I think a medication that does not cross the placental barrier is important to consider.”
The study was sponsored by Janssen Scientific Affairs. Dr. Kimball’s disclosures included serving as a consultant and investigator for companies that included AbbVie, Bristol-Myers Squibb, and Janssen; several other authors also had disclosures related to multiple pharmaceutical companies. Dr. Murase’s disclosures included serving as a consultant for Dermira, UCB Pharma, Sanofi, Ferndale, and Regeneron.
according to one of the largest studies to examine the issue to date.
However, “pregnancy-specific registries that include a larger number of pregnant women with psoriasis ... are needed to more fully characterize the association between psoriasis and treatment and birth outcomes,” acknowledged first author Alexa B. Kimball, MD, MPH, professor of dermatology, Harvard Medical School, Boston, and colleagues.
The cohort study, published in JAMA Dermatology, used data from the Psoriasis Longitudinal Assessment and Registry (PSOLAR), which “is not a pregnancy specific registry, and medical history is captured only at baseline,” they noted.
Their findings showed pregnancy outcomes such as spontaneous abortion, neonatal problems, and congenital anomalies among women with moderate to severe psoriasis were similar to rates in the general U.S. population, and are “consistent with previously reported data,” they reported. “And pregnancy outcomes for women exposed to biologics were similar to those for women with exposure to nonbiologics.”
The study “provides further reassurance that the biologics appear safe at least related to pregnancy outcomes,” commented Jenny Murase, MD, associate professor of dermatology at the University of California, San Francisco, who was not involved in the study. In an interview, she noted that the study “did not examine any potential immunosuppression of the fetus in the first 6 months of life,” which she described as “the heart of the concern, more than whether or not the psoriasis or the biologic affects the pregnancy itself.”
The study used data from the PSOLAR registry collected from June 20, 2007, to Aug.23, 2019, which included 2,224 women of childbearing age (18-45 years) who were collectively followed up for 12,929 patient-years. Among these women, 220 had 298 pregnancies, with 244 live births (81.9%).
“Birth outcomes among all 244 births included 231 healthy newborns (94.7%), 10 infants with a neonatal problem (4.1%), 1 stillbirth (0.4%), and 2 congenital anomalies (0.8%),” the authors reported.
There were also 41 spontaneous abortions (13.8%), and 13 elective terminations (4.4%). “No elective terminations were known to derive from a congenital anomaly or other medical issue,” they added.
Among the documented pregnancies, 252 occurred in women with exposure to biologic therapy either before or during pregnancy, including 168 (56.4%) during the prenatal period, while 46 pregnancies occurred in women with no exposure to biologic therapy.
Dr. Murase, director of medical consultative dermatology for the Palo Alto Foundation Medical Group in Mountain View, Calif., said that a more detailed comparison of the different psoriasis treatments, as well as the offspring outcomes during the first 6 months of life, might offer some further important insight,.
Infants born after exposure to infliximab “and potentially other anti–tumor necrosis factor–alpha agents during the third trimester may be unable to develop an appropriate immune response to live vaccines,” she and her coauthors cautioned in a letter published in 2011, which referred to a case of an infant with disseminated bacillus Calmette-Guérin infection, whose mother had received infliximab for Crohn’s disease throughout pregnancy.
Dr. Murase pointed out that, in the registry study, exposures to certolizumab, which is pegylated and does not cross the placental barrier, were not separated from other cases. It is important to consider “the cross over late in the second trimester and especially third trimester as the infant is getting the ‘antibody boost’ from the mother as it gets ready to set foot in this world and needs the maternal antibodies to prepare its immune system. If the IgG biologics cross third trimester and immunosuppress the infant ... then I think a medication that does not cross the placental barrier is important to consider.”
The study was sponsored by Janssen Scientific Affairs. Dr. Kimball’s disclosures included serving as a consultant and investigator for companies that included AbbVie, Bristol-Myers Squibb, and Janssen; several other authors also had disclosures related to multiple pharmaceutical companies. Dr. Murase’s disclosures included serving as a consultant for Dermira, UCB Pharma, Sanofi, Ferndale, and Regeneron.
according to one of the largest studies to examine the issue to date.
However, “pregnancy-specific registries that include a larger number of pregnant women with psoriasis ... are needed to more fully characterize the association between psoriasis and treatment and birth outcomes,” acknowledged first author Alexa B. Kimball, MD, MPH, professor of dermatology, Harvard Medical School, Boston, and colleagues.
The cohort study, published in JAMA Dermatology, used data from the Psoriasis Longitudinal Assessment and Registry (PSOLAR), which “is not a pregnancy specific registry, and medical history is captured only at baseline,” they noted.
Their findings showed pregnancy outcomes such as spontaneous abortion, neonatal problems, and congenital anomalies among women with moderate to severe psoriasis were similar to rates in the general U.S. population, and are “consistent with previously reported data,” they reported. “And pregnancy outcomes for women exposed to biologics were similar to those for women with exposure to nonbiologics.”
The study “provides further reassurance that the biologics appear safe at least related to pregnancy outcomes,” commented Jenny Murase, MD, associate professor of dermatology at the University of California, San Francisco, who was not involved in the study. In an interview, she noted that the study “did not examine any potential immunosuppression of the fetus in the first 6 months of life,” which she described as “the heart of the concern, more than whether or not the psoriasis or the biologic affects the pregnancy itself.”
The study used data from the PSOLAR registry collected from June 20, 2007, to Aug.23, 2019, which included 2,224 women of childbearing age (18-45 years) who were collectively followed up for 12,929 patient-years. Among these women, 220 had 298 pregnancies, with 244 live births (81.9%).
“Birth outcomes among all 244 births included 231 healthy newborns (94.7%), 10 infants with a neonatal problem (4.1%), 1 stillbirth (0.4%), and 2 congenital anomalies (0.8%),” the authors reported.
There were also 41 spontaneous abortions (13.8%), and 13 elective terminations (4.4%). “No elective terminations were known to derive from a congenital anomaly or other medical issue,” they added.
Among the documented pregnancies, 252 occurred in women with exposure to biologic therapy either before or during pregnancy, including 168 (56.4%) during the prenatal period, while 46 pregnancies occurred in women with no exposure to biologic therapy.
Dr. Murase, director of medical consultative dermatology for the Palo Alto Foundation Medical Group in Mountain View, Calif., said that a more detailed comparison of the different psoriasis treatments, as well as the offspring outcomes during the first 6 months of life, might offer some further important insight,.
Infants born after exposure to infliximab “and potentially other anti–tumor necrosis factor–alpha agents during the third trimester may be unable to develop an appropriate immune response to live vaccines,” she and her coauthors cautioned in a letter published in 2011, which referred to a case of an infant with disseminated bacillus Calmette-Guérin infection, whose mother had received infliximab for Crohn’s disease throughout pregnancy.
Dr. Murase pointed out that, in the registry study, exposures to certolizumab, which is pegylated and does not cross the placental barrier, were not separated from other cases. It is important to consider “the cross over late in the second trimester and especially third trimester as the infant is getting the ‘antibody boost’ from the mother as it gets ready to set foot in this world and needs the maternal antibodies to prepare its immune system. If the IgG biologics cross third trimester and immunosuppress the infant ... then I think a medication that does not cross the placental barrier is important to consider.”
The study was sponsored by Janssen Scientific Affairs. Dr. Kimball’s disclosures included serving as a consultant and investigator for companies that included AbbVie, Bristol-Myers Squibb, and Janssen; several other authors also had disclosures related to multiple pharmaceutical companies. Dr. Murase’s disclosures included serving as a consultant for Dermira, UCB Pharma, Sanofi, Ferndale, and Regeneron.
FROM JAMA DERMATOLOGY
State of the evidence: Treatment-resistant depression in children and adolescents
Case
Max was a 17-year-old boy and avid video gamer who, predating COVID-19, was within a major depressive episode and continued to meet criteria through the duration of COVID-19 quarantine. He lives with his mother, who is a single mom and is working hard in a variety of jobs through the pandemic. Max had little motivation to engage in sports or other activities, and despite doing well enough in school, he spent much of his days escaping into video games and social media, where his friends communicated and bonded the most. He has had very little response to complete trials of two different selective serotonin reuptake inhibitors (SSRIs), and the off-label attempts at a serotonin-norepinephrine reuptake inhibitor and bupropion augmentation of an SSRI, as extrapolated by his pediatrician from adult data on treatment-resistant depression. He had ongoing supportive psychotherapy and his mother and pediatrician were wary of changing that relationship, as they were just happy he would engage at all. His shy nature made him very wary of attending any programs or groups. He had no other diagnosis including anxiety, substance abuse disorder, or learning disorder.
Case discussion
As a child and adolescent psychiatrist embedded in primary care practices, I (like you) am seeing more and more parents, children, and families struggling with depression through the course of this unprecedented and challenging year.
Max presented to me with his mother at the request of his primary care physician because within the course of many medication trials, it had been over 6 months of persistent symptoms without an end in sight for him, his family, or his primary care provider (PCP).
His diagnosis was treatment-resistant depression and his PCP was grasping at adult strategies to manage this all with additional psychopharmacology. As a consulting child-and-adolescent psychiatrist in primary care, how could I help the PCP? I too worry if there is anything that I can do to shift depression once standard treatments fail, and when the idea of engaging in behavioral activation or other pro-health activities is just too much for a depressed adolescent to bear. I weigh that with what is known about the evidence, and the good data driving us beyond medication solutions. I often find that it can be helpful to reiterate the following points to providers and families.
First, what to know about depression in kids
Depression looks different at different ages in children. In school-aged children, it’s widely known that irritability or psychosomatic symptoms (frequent headaches and stomach aches) can be the first preverbal signs of an emerging anxiety and depressive disorder in children. In adolescents, one would maybe expect more typical melancholic adult-like symptoms of depression; however, there is mounting evidence that adolescents actually present with more classically “atypical symptoms” of depression (low motivation, weight gain, escapism to sleep or video games, as with Max) with less persistence across setting (home, work, school) compared with adults (“Diagnosing Depression in Children and Adolescents” by Glen R. Elliott, PhD, MD, from The Carlat Child Psychiatry Report, November 2015, Antidepressant Use in Children).In addition, major life stressors (the breakup of a romantic relationship, failing a class, bullying) can be perceived as more permanent, and suicidal thinking can be acute and lethal in these contexts. With Max, it was accepted by all who were supporting him that he was struggling with depression, which is the first step in managing this well.
The idea of the designated patient
Often left out in a discussion of pediatric patients is the family. As a designated patient, much of our focus is on improving the symptoms of the patient in front of us. Parents direct their gaze at the child as the one with the condition who needs support. First following identification of depression, I find that a reframe of a diagnosis can be useful. Family systems theory approaches a child with a depression diagnosis, and says, that if one family member changes, even in a small way, a family as a system is forced to change. With a sense of an external locus of control, we often are left with a patient and parent feeling stuck. To provide a reorientation to the parents, ask how they are feeling. Can they get treatment for depression knowing the biology of the condition or consider making behavioral changes of their own and as a family? Can they consider family psychotherapy so they can cope better and break some cycles of maladaptive engagement shared across a family? These kinds of reorientations can be useful to shift the idea of treatment from the designated patient (the child) and medication options (limited data for kids who aren’t responding to them) to a family approach. Making the depression management strategy a family affair can help the entire family shift from seeing the only option as medications or interventions exclusively directed at a child. The Vermont Center for Children, Youth, and Families at the University of Vermont Medical Center has many pioneering developments in addressing family-based approaches to mental health concerns in the pediatric population, and can serve as a source of inspiration for this shift in discussing depression.
Practical strategies for the pediatrician
Medications can be useful for treating child and adolescent depression, but there is also strong evidence for psychotherapy, working with the child’s school and family, and different forms of behavioral activation (exercise, mindfulness, yoga, and other positive activities). Medications, if one is looking at standard of care treatment and Food and Drug Administration approval exclusively, is limited in scope and should not be the only intervention considered, as described in the case above.
In “The Use of Medication in Treating Childhood and Adolescent Depression: Information for Patients and Families,” which is a practical guide prepared by the American Psychiatric Association and the American Academy of Child and Adolescent Psychiatry, it is noted that Prozac and Lexapro are approved medications, as follows:
- Antidepressant medications can be effective in relieving the symptoms of depression for some children and adolescents. One antidepressant – fluoxetine, or Prozac – a medicine in the category of SSRIs, has been approved by the FDA for treating depression in children 8 years of age and older. Escitalopram, or Lexapro, has also been approved by the FDA for treating adolescents 12 years of age and older.
- About “60 percent of children and adolescents will respond to initial treatment with medication,” which leaves many children needing further interventions. “Of those who don’t [respond], a significant number may respond to another medication but also may respond to the addition of a form of psychotherapy called cognitive behavioral therapy (CBT).”
It is common, as in the case above, that a connection with a therapist or support is valued over the specific modality even if it’s not showing improvement or outcomes. It is important to consider CBT as a form of evidence-based treatment for children with depression and to cite the famous “Treatment for Adolescents with Depression Study (TADS)” funded by the National Institute of Mental Health, published in 2004, that shows the following findings: “After 12 weeks of treatment, 71 percent of the patients who received the combination of medication and CBT were much improved.” In looking at the group that does not improve with medications alone, adolescents in particular can have more gains with the addition of CBT.
Tracking progress, little by little
Often we reflexively ask parents of depressed children: Are they better? And we ask the child: How do you feel? It can be difficult for parents to reflect on that, or see progress or gains from appointment to appointment. I suggest trying to use structured measures and tools to frame a discussion with progress on medication and treatment such as what is available at the Mood Treatment Center website.I also suggest apps such as Mood Kit,which is for mood tracking with some CBT exercises in addition to behavioral activation strategies for children and parents. It can be useful to have families take some ownership of tracking their moods and what may be playing into them. In particular with the pandemic, we can reflect on how much isolation or socialization, activities, sleep, eating habits, and exercise can affect us and make corresponding behavioral changes as a family to improve our own coping. Depression itself can be like glasses clouding one’s vision in gray, and that can also cloud one’s review of progress. When we hear comments such as “nothing gets better” from a child or parent, it may be helpful to try to track any contributing factors to a persistent low mood and acknowledge any slow and steady progress.
In summary, we can strive as providers to maximize our approach to depression in children and adolescents beyond the limited FDA-approved medications, or extrapolating adult data to children. If we emphasize the evidence-based practice of CBT and other interventions in addition to encouraging a tracking and review of outcomes measures with parents and families, we can empower them to make meaningful change in both perspectives and behaviors that can perpetuate depressive states.
Dr. Pawlowski is an adult, adolescent, and child psychiatrist at the University of Vermont Medical Center and assistant professor of psychiatry at the Larner College of Medicine at UVM in Burlington.
Case
Max was a 17-year-old boy and avid video gamer who, predating COVID-19, was within a major depressive episode and continued to meet criteria through the duration of COVID-19 quarantine. He lives with his mother, who is a single mom and is working hard in a variety of jobs through the pandemic. Max had little motivation to engage in sports or other activities, and despite doing well enough in school, he spent much of his days escaping into video games and social media, where his friends communicated and bonded the most. He has had very little response to complete trials of two different selective serotonin reuptake inhibitors (SSRIs), and the off-label attempts at a serotonin-norepinephrine reuptake inhibitor and bupropion augmentation of an SSRI, as extrapolated by his pediatrician from adult data on treatment-resistant depression. He had ongoing supportive psychotherapy and his mother and pediatrician were wary of changing that relationship, as they were just happy he would engage at all. His shy nature made him very wary of attending any programs or groups. He had no other diagnosis including anxiety, substance abuse disorder, or learning disorder.
Case discussion
As a child and adolescent psychiatrist embedded in primary care practices, I (like you) am seeing more and more parents, children, and families struggling with depression through the course of this unprecedented and challenging year.
Max presented to me with his mother at the request of his primary care physician because within the course of many medication trials, it had been over 6 months of persistent symptoms without an end in sight for him, his family, or his primary care provider (PCP).
His diagnosis was treatment-resistant depression and his PCP was grasping at adult strategies to manage this all with additional psychopharmacology. As a consulting child-and-adolescent psychiatrist in primary care, how could I help the PCP? I too worry if there is anything that I can do to shift depression once standard treatments fail, and when the idea of engaging in behavioral activation or other pro-health activities is just too much for a depressed adolescent to bear. I weigh that with what is known about the evidence, and the good data driving us beyond medication solutions. I often find that it can be helpful to reiterate the following points to providers and families.
First, what to know about depression in kids
Depression looks different at different ages in children. In school-aged children, it’s widely known that irritability or psychosomatic symptoms (frequent headaches and stomach aches) can be the first preverbal signs of an emerging anxiety and depressive disorder in children. In adolescents, one would maybe expect more typical melancholic adult-like symptoms of depression; however, there is mounting evidence that adolescents actually present with more classically “atypical symptoms” of depression (low motivation, weight gain, escapism to sleep or video games, as with Max) with less persistence across setting (home, work, school) compared with adults (“Diagnosing Depression in Children and Adolescents” by Glen R. Elliott, PhD, MD, from The Carlat Child Psychiatry Report, November 2015, Antidepressant Use in Children).In addition, major life stressors (the breakup of a romantic relationship, failing a class, bullying) can be perceived as more permanent, and suicidal thinking can be acute and lethal in these contexts. With Max, it was accepted by all who were supporting him that he was struggling with depression, which is the first step in managing this well.
The idea of the designated patient
Often left out in a discussion of pediatric patients is the family. As a designated patient, much of our focus is on improving the symptoms of the patient in front of us. Parents direct their gaze at the child as the one with the condition who needs support. First following identification of depression, I find that a reframe of a diagnosis can be useful. Family systems theory approaches a child with a depression diagnosis, and says, that if one family member changes, even in a small way, a family as a system is forced to change. With a sense of an external locus of control, we often are left with a patient and parent feeling stuck. To provide a reorientation to the parents, ask how they are feeling. Can they get treatment for depression knowing the biology of the condition or consider making behavioral changes of their own and as a family? Can they consider family psychotherapy so they can cope better and break some cycles of maladaptive engagement shared across a family? These kinds of reorientations can be useful to shift the idea of treatment from the designated patient (the child) and medication options (limited data for kids who aren’t responding to them) to a family approach. Making the depression management strategy a family affair can help the entire family shift from seeing the only option as medications or interventions exclusively directed at a child. The Vermont Center for Children, Youth, and Families at the University of Vermont Medical Center has many pioneering developments in addressing family-based approaches to mental health concerns in the pediatric population, and can serve as a source of inspiration for this shift in discussing depression.
Practical strategies for the pediatrician
Medications can be useful for treating child and adolescent depression, but there is also strong evidence for psychotherapy, working with the child’s school and family, and different forms of behavioral activation (exercise, mindfulness, yoga, and other positive activities). Medications, if one is looking at standard of care treatment and Food and Drug Administration approval exclusively, is limited in scope and should not be the only intervention considered, as described in the case above.
In “The Use of Medication in Treating Childhood and Adolescent Depression: Information for Patients and Families,” which is a practical guide prepared by the American Psychiatric Association and the American Academy of Child and Adolescent Psychiatry, it is noted that Prozac and Lexapro are approved medications, as follows:
- Antidepressant medications can be effective in relieving the symptoms of depression for some children and adolescents. One antidepressant – fluoxetine, or Prozac – a medicine in the category of SSRIs, has been approved by the FDA for treating depression in children 8 years of age and older. Escitalopram, or Lexapro, has also been approved by the FDA for treating adolescents 12 years of age and older.
- About “60 percent of children and adolescents will respond to initial treatment with medication,” which leaves many children needing further interventions. “Of those who don’t [respond], a significant number may respond to another medication but also may respond to the addition of a form of psychotherapy called cognitive behavioral therapy (CBT).”
It is common, as in the case above, that a connection with a therapist or support is valued over the specific modality even if it’s not showing improvement or outcomes. It is important to consider CBT as a form of evidence-based treatment for children with depression and to cite the famous “Treatment for Adolescents with Depression Study (TADS)” funded by the National Institute of Mental Health, published in 2004, that shows the following findings: “After 12 weeks of treatment, 71 percent of the patients who received the combination of medication and CBT were much improved.” In looking at the group that does not improve with medications alone, adolescents in particular can have more gains with the addition of CBT.
Tracking progress, little by little
Often we reflexively ask parents of depressed children: Are they better? And we ask the child: How do you feel? It can be difficult for parents to reflect on that, or see progress or gains from appointment to appointment. I suggest trying to use structured measures and tools to frame a discussion with progress on medication and treatment such as what is available at the Mood Treatment Center website.I also suggest apps such as Mood Kit,which is for mood tracking with some CBT exercises in addition to behavioral activation strategies for children and parents. It can be useful to have families take some ownership of tracking their moods and what may be playing into them. In particular with the pandemic, we can reflect on how much isolation or socialization, activities, sleep, eating habits, and exercise can affect us and make corresponding behavioral changes as a family to improve our own coping. Depression itself can be like glasses clouding one’s vision in gray, and that can also cloud one’s review of progress. When we hear comments such as “nothing gets better” from a child or parent, it may be helpful to try to track any contributing factors to a persistent low mood and acknowledge any slow and steady progress.
In summary, we can strive as providers to maximize our approach to depression in children and adolescents beyond the limited FDA-approved medications, or extrapolating adult data to children. If we emphasize the evidence-based practice of CBT and other interventions in addition to encouraging a tracking and review of outcomes measures with parents and families, we can empower them to make meaningful change in both perspectives and behaviors that can perpetuate depressive states.
Dr. Pawlowski is an adult, adolescent, and child psychiatrist at the University of Vermont Medical Center and assistant professor of psychiatry at the Larner College of Medicine at UVM in Burlington.
Case
Max was a 17-year-old boy and avid video gamer who, predating COVID-19, was within a major depressive episode and continued to meet criteria through the duration of COVID-19 quarantine. He lives with his mother, who is a single mom and is working hard in a variety of jobs through the pandemic. Max had little motivation to engage in sports or other activities, and despite doing well enough in school, he spent much of his days escaping into video games and social media, where his friends communicated and bonded the most. He has had very little response to complete trials of two different selective serotonin reuptake inhibitors (SSRIs), and the off-label attempts at a serotonin-norepinephrine reuptake inhibitor and bupropion augmentation of an SSRI, as extrapolated by his pediatrician from adult data on treatment-resistant depression. He had ongoing supportive psychotherapy and his mother and pediatrician were wary of changing that relationship, as they were just happy he would engage at all. His shy nature made him very wary of attending any programs or groups. He had no other diagnosis including anxiety, substance abuse disorder, or learning disorder.
Case discussion
As a child and adolescent psychiatrist embedded in primary care practices, I (like you) am seeing more and more parents, children, and families struggling with depression through the course of this unprecedented and challenging year.
Max presented to me with his mother at the request of his primary care physician because within the course of many medication trials, it had been over 6 months of persistent symptoms without an end in sight for him, his family, or his primary care provider (PCP).
His diagnosis was treatment-resistant depression and his PCP was grasping at adult strategies to manage this all with additional psychopharmacology. As a consulting child-and-adolescent psychiatrist in primary care, how could I help the PCP? I too worry if there is anything that I can do to shift depression once standard treatments fail, and when the idea of engaging in behavioral activation or other pro-health activities is just too much for a depressed adolescent to bear. I weigh that with what is known about the evidence, and the good data driving us beyond medication solutions. I often find that it can be helpful to reiterate the following points to providers and families.
First, what to know about depression in kids
Depression looks different at different ages in children. In school-aged children, it’s widely known that irritability or psychosomatic symptoms (frequent headaches and stomach aches) can be the first preverbal signs of an emerging anxiety and depressive disorder in children. In adolescents, one would maybe expect more typical melancholic adult-like symptoms of depression; however, there is mounting evidence that adolescents actually present with more classically “atypical symptoms” of depression (low motivation, weight gain, escapism to sleep or video games, as with Max) with less persistence across setting (home, work, school) compared with adults (“Diagnosing Depression in Children and Adolescents” by Glen R. Elliott, PhD, MD, from The Carlat Child Psychiatry Report, November 2015, Antidepressant Use in Children).In addition, major life stressors (the breakup of a romantic relationship, failing a class, bullying) can be perceived as more permanent, and suicidal thinking can be acute and lethal in these contexts. With Max, it was accepted by all who were supporting him that he was struggling with depression, which is the first step in managing this well.
The idea of the designated patient
Often left out in a discussion of pediatric patients is the family. As a designated patient, much of our focus is on improving the symptoms of the patient in front of us. Parents direct their gaze at the child as the one with the condition who needs support. First following identification of depression, I find that a reframe of a diagnosis can be useful. Family systems theory approaches a child with a depression diagnosis, and says, that if one family member changes, even in a small way, a family as a system is forced to change. With a sense of an external locus of control, we often are left with a patient and parent feeling stuck. To provide a reorientation to the parents, ask how they are feeling. Can they get treatment for depression knowing the biology of the condition or consider making behavioral changes of their own and as a family? Can they consider family psychotherapy so they can cope better and break some cycles of maladaptive engagement shared across a family? These kinds of reorientations can be useful to shift the idea of treatment from the designated patient (the child) and medication options (limited data for kids who aren’t responding to them) to a family approach. Making the depression management strategy a family affair can help the entire family shift from seeing the only option as medications or interventions exclusively directed at a child. The Vermont Center for Children, Youth, and Families at the University of Vermont Medical Center has many pioneering developments in addressing family-based approaches to mental health concerns in the pediatric population, and can serve as a source of inspiration for this shift in discussing depression.
Practical strategies for the pediatrician
Medications can be useful for treating child and adolescent depression, but there is also strong evidence for psychotherapy, working with the child’s school and family, and different forms of behavioral activation (exercise, mindfulness, yoga, and other positive activities). Medications, if one is looking at standard of care treatment and Food and Drug Administration approval exclusively, is limited in scope and should not be the only intervention considered, as described in the case above.
In “The Use of Medication in Treating Childhood and Adolescent Depression: Information for Patients and Families,” which is a practical guide prepared by the American Psychiatric Association and the American Academy of Child and Adolescent Psychiatry, it is noted that Prozac and Lexapro are approved medications, as follows:
- Antidepressant medications can be effective in relieving the symptoms of depression for some children and adolescents. One antidepressant – fluoxetine, or Prozac – a medicine in the category of SSRIs, has been approved by the FDA for treating depression in children 8 years of age and older. Escitalopram, or Lexapro, has also been approved by the FDA for treating adolescents 12 years of age and older.
- About “60 percent of children and adolescents will respond to initial treatment with medication,” which leaves many children needing further interventions. “Of those who don’t [respond], a significant number may respond to another medication but also may respond to the addition of a form of psychotherapy called cognitive behavioral therapy (CBT).”
It is common, as in the case above, that a connection with a therapist or support is valued over the specific modality even if it’s not showing improvement or outcomes. It is important to consider CBT as a form of evidence-based treatment for children with depression and to cite the famous “Treatment for Adolescents with Depression Study (TADS)” funded by the National Institute of Mental Health, published in 2004, that shows the following findings: “After 12 weeks of treatment, 71 percent of the patients who received the combination of medication and CBT were much improved.” In looking at the group that does not improve with medications alone, adolescents in particular can have more gains with the addition of CBT.
Tracking progress, little by little
Often we reflexively ask parents of depressed children: Are they better? And we ask the child: How do you feel? It can be difficult for parents to reflect on that, or see progress or gains from appointment to appointment. I suggest trying to use structured measures and tools to frame a discussion with progress on medication and treatment such as what is available at the Mood Treatment Center website.I also suggest apps such as Mood Kit,which is for mood tracking with some CBT exercises in addition to behavioral activation strategies for children and parents. It can be useful to have families take some ownership of tracking their moods and what may be playing into them. In particular with the pandemic, we can reflect on how much isolation or socialization, activities, sleep, eating habits, and exercise can affect us and make corresponding behavioral changes as a family to improve our own coping. Depression itself can be like glasses clouding one’s vision in gray, and that can also cloud one’s review of progress. When we hear comments such as “nothing gets better” from a child or parent, it may be helpful to try to track any contributing factors to a persistent low mood and acknowledge any slow and steady progress.
In summary, we can strive as providers to maximize our approach to depression in children and adolescents beyond the limited FDA-approved medications, or extrapolating adult data to children. If we emphasize the evidence-based practice of CBT and other interventions in addition to encouraging a tracking and review of outcomes measures with parents and families, we can empower them to make meaningful change in both perspectives and behaviors that can perpetuate depressive states.
Dr. Pawlowski is an adult, adolescent, and child psychiatrist at the University of Vermont Medical Center and assistant professor of psychiatry at the Larner College of Medicine at UVM in Burlington.
Semaglutide for weight loss? A good first STEP, with caveats
The phase 3a STEP 1 trial that investigated the use of semaglutide (Novo Nordisk), a glucagonlike peptide–1 (GLP-1) agonist, for weight loss is aptly named, some say.
“In sum, we have a long way to go to control the obesity epidemic ... but on the face of it, the STEP 1 trial (like its name) is a good beginning,” wrote coeditorialists Julie R. Ingelfinger, MD, from Harvard Medical School, Boston, and a deputy editor of the New England Journal of Medicine, and Clifford J. Rosen, MD, from Tufts University School of Medicine, also in Boston.
The trial findings by John P.H. Wilding, DM, University of Liverpool (England), and colleagues and an accompanying editorial were published online Feb. 10, 2021, in the New England Journal of Medicine.
“The results are encouraging, with significantly more patients in the semaglutide group having clinically important weight loss,” Dr. Ingelfinger and Dr. Rosen stressed.
However, they also cautioned that “despite the positive results of this trial, the present study has some important limitations” and “there are concerns, including adverse events (mostly gastrointestinal – nausea, sometimes vomiting, and diarrhea) related primarily to the class of the agent.”
Two U.K. experts drew similar takeaways, speaking to the U.K. Science Media Centre.
“This was a well-designed study with unequivocal findings,” which showed that semaglutide “is indeed likely to be a game-changer in the fight against obesity,” according to Baptiste Leurent, PhD, London School of Hygiene and Tropical Medicine.
However, if the drug is approved at this dose for this use, patients would need close monitoring for gastrointestinal disorders, and “we also need to better understand what is happening once the treatment is stopped, and whether it could be taken for a shorter period of time.”
Sir Stephen O’Rahilly, MD, MRC Metabolic Diseases Unit, University of Cambridge (England), pointed out that “GLP-1 is made by cells in the intestine and levels increase in the blood after a meal, providing some of the signal to the brain that tells us we are ‘full,’ ” so GLP-1 agonists have been studied as appetite suppressants, in addition to their approved use to treat type 2 diabetes.
Only about 4.5% of participants in STEP 1 stopped taking semaglutide because of gastrointestinal issues, he noted, although more participants in that group reported problems with gallstones, which can follow rapid weight loss.
And “unlike some previous appetite suppressant drugs which caused significant psychological and psychiatric side effects, there is no evidence that semaglutide has any adverse effects of that nature,” Dr. O’Rahilly noted.
In sum, he said, “this is the start of a new era for obesity drug development with the future direction being to achieve levels of weight loss comparable to semaglutide, while having fewer side effects.”
‘Pressing need’ to address obesity; semaglutide filed for obesity
There is a “pressing need” to address the worldwide increase in obesity and weight-related coexisting conditions, Dr. Ingelfinger and Dr. Rosen noted.
Sustained long-term weight loss with diet and exercise is challenging; behavioral weight-loss strategies “fail more often than not,” bariatric surgery is invasive and often followed by eventual weight regain, they wrote.
In addition, said Dr. Wilding and colleagues, the “use of available [weight-loss] medications remains limited by modest efficacy, safety concerns, and cost.”
Subcutaneous semaglutide, approved for treating type 2 diabetes (as Ozempic) in adults at doses of up to 1 mg/week, induced weight loss at higher doses. The current study is part of the global Semaglutide Treatment Effect in People With Obesity program of four trials (STEP 1, 2, 3, and 4) that aimed to test the safety and efficacy of subcutaneous semaglutide 2.4 mg/week for weight loss.
Topline results from STEP 1 were presented June 4, 2020.
And as reported earlier, results from STEP 3 – a 68-week trial of semaglutide versus placebo in 611 participants who all received very intensive diet and exercise counseling – were presented at the virtual ObesityWeek 2020 meeting.
The four trials of semaglutide for weight loss have been completed and the data were submitted to the Food and Drug Administration on Dec. 4, 2020 (with a decision expected within 6 months) and to the European Medicines Agency on Dec. 18, 2020.
Most patients had 5% weight loss with semaglutide
The STEP 1 trial enrolled 1,961 adults with a body mass index (BMI) of at least 30 kg/m2 or at least 27 with at least one weight-related coexisting condition, but without type 2 diabetes, at 129 sites in 16 countries in Asia, Europe, North America, and South America.
Participants were a mean age of 47 and three-quarters were women. Most participants were White (76%), followed by Asian (13%), Black or African American (6%), or other (5%).
On average, they had a BMI of 38 and weighed 105 kg. Three-quarters had one or more coexisting conditions.
Participants were randomized to receive semaglutide (1,306 patients) or placebo (655 patients), added to lifestyle intervention.
Everyone received 17 monthly individual counseling sessions during which they learned about adhering to a diet with a 500-calorie/day deficit, were encouraged to build up to walking 150 minutes each week, and recorded their daily diet and exercise (in a diary or using an app).
Semaglutide was administered with a prefilled pen injector at a dose of 0.25 mg/week for the first 4 weeks, escalated to 2.4 mg/week by week 16 (or lower if the patient had unacceptable side effects).
At 68 weeks, participants in the semaglutide versus placebo group had greater mean weight loss (14.9% vs. 2.4%, or 15.3 kg vs. 2.6 kg).
Participants in the semaglutide versus placebo group were much more likely to have lost at least 5% of their initial weight (86% vs. 31.5%) or at least 10% of their initial weight (69.1% vs. 12.0%), or at least 15% of their initial weight (50.5% vs. 4.9%; P < .001 for all three comparisons).
About 80% of participants adhered to the study treatment. A third of participants in the semaglutide group who completed the study lost at least 20% of their initial weight, which approaches the 20%-30% reported weight loss 1-3 years after sleeve gastrectomy, the researchers noted.
Participants in the semaglutide group also had greater improvements in waist circumference and levels of hemoglobin A1c, C-reactive protein (a marker of inflammation), and fasting lipids, as well as in physical function scores on SF-36 and IWQOL-Lite-CT questionnaires.
In their editorial, Dr. Ingelfinger and Dr. Rosen noted that “daily oral semaglutide [already approved in 7-mg and 14-mg doses for the treatment of type 2 diabetes as Rybelsus] might be more appealing to many people,” as a weight-loss medication than a once-weekly subcutaneous dose. Semaglutide is the first GLP-1 agonist available as an oral agent.
The ongoing Semaglutide Effects on Heart Disease and Stroke in Patients With Overweight or Obesity (SELECT) trial (with expected completion in 2023) will shed light on cardiovascular outcomes after 2.5-5 years.
GI disorders and ‘important limitations’
More participants in the semaglutide than the placebo group reported gastrointestinal disorders (typically nausea, diarrhea, vomiting, and constipation; 74.2% vs. 47.9%), which were mostly transient and mild to moderate in severity, but also led to more treatment discontinuation (7.0% vs. 3.1%).
More patients in the semaglutide versus placebo group had a gall bladder–related disorder (2.6% vs. 1.2%, mostly cholelithiasis) and mild acute pancreatitis (3 vs. 0 participants), but there were no between-group differences in neoplasms.
Dr. Wilding and colleagues acknowledge the limitations of the study, including the fact that it enrolled mainly women, mainly non-White participants, was relatively short, and excluded patients with type 2 diabetes.
Mean placebo-corrected weight loss with 2.4 mg/weekly subcutaneous semaglutide was greater than with 3.0 mg once-daily subcutaneous liraglutide (Saxenda, Novo Nordisk) – the only GLP-1 agonist approved for weight management – in the 56-week SCALE trial (12.4% vs. 4.5%); however, the two studies had different populations.
The study was supported by Novo Nordisk. Dr. Ingelfinger is a deputy editor and Dr. Rosen is an associate editor of the New England Journal of Medicine. Dr. Ingelfinger, Dr. Rosen, and Dr. Leurent have reported no relevant financial relationships. Dr. O’Rahilly has a current research collaboration with Novo Nordisk scientists in an unrelated area and has been a consultant for the company.
A version of this article first appeared on Medscape.com.
The phase 3a STEP 1 trial that investigated the use of semaglutide (Novo Nordisk), a glucagonlike peptide–1 (GLP-1) agonist, for weight loss is aptly named, some say.
“In sum, we have a long way to go to control the obesity epidemic ... but on the face of it, the STEP 1 trial (like its name) is a good beginning,” wrote coeditorialists Julie R. Ingelfinger, MD, from Harvard Medical School, Boston, and a deputy editor of the New England Journal of Medicine, and Clifford J. Rosen, MD, from Tufts University School of Medicine, also in Boston.
The trial findings by John P.H. Wilding, DM, University of Liverpool (England), and colleagues and an accompanying editorial were published online Feb. 10, 2021, in the New England Journal of Medicine.
“The results are encouraging, with significantly more patients in the semaglutide group having clinically important weight loss,” Dr. Ingelfinger and Dr. Rosen stressed.
However, they also cautioned that “despite the positive results of this trial, the present study has some important limitations” and “there are concerns, including adverse events (mostly gastrointestinal – nausea, sometimes vomiting, and diarrhea) related primarily to the class of the agent.”
Two U.K. experts drew similar takeaways, speaking to the U.K. Science Media Centre.
“This was a well-designed study with unequivocal findings,” which showed that semaglutide “is indeed likely to be a game-changer in the fight against obesity,” according to Baptiste Leurent, PhD, London School of Hygiene and Tropical Medicine.
However, if the drug is approved at this dose for this use, patients would need close monitoring for gastrointestinal disorders, and “we also need to better understand what is happening once the treatment is stopped, and whether it could be taken for a shorter period of time.”
Sir Stephen O’Rahilly, MD, MRC Metabolic Diseases Unit, University of Cambridge (England), pointed out that “GLP-1 is made by cells in the intestine and levels increase in the blood after a meal, providing some of the signal to the brain that tells us we are ‘full,’ ” so GLP-1 agonists have been studied as appetite suppressants, in addition to their approved use to treat type 2 diabetes.
Only about 4.5% of participants in STEP 1 stopped taking semaglutide because of gastrointestinal issues, he noted, although more participants in that group reported problems with gallstones, which can follow rapid weight loss.
And “unlike some previous appetite suppressant drugs which caused significant psychological and psychiatric side effects, there is no evidence that semaglutide has any adverse effects of that nature,” Dr. O’Rahilly noted.
In sum, he said, “this is the start of a new era for obesity drug development with the future direction being to achieve levels of weight loss comparable to semaglutide, while having fewer side effects.”
‘Pressing need’ to address obesity; semaglutide filed for obesity
There is a “pressing need” to address the worldwide increase in obesity and weight-related coexisting conditions, Dr. Ingelfinger and Dr. Rosen noted.
Sustained long-term weight loss with diet and exercise is challenging; behavioral weight-loss strategies “fail more often than not,” bariatric surgery is invasive and often followed by eventual weight regain, they wrote.
In addition, said Dr. Wilding and colleagues, the “use of available [weight-loss] medications remains limited by modest efficacy, safety concerns, and cost.”
Subcutaneous semaglutide, approved for treating type 2 diabetes (as Ozempic) in adults at doses of up to 1 mg/week, induced weight loss at higher doses. The current study is part of the global Semaglutide Treatment Effect in People With Obesity program of four trials (STEP 1, 2, 3, and 4) that aimed to test the safety and efficacy of subcutaneous semaglutide 2.4 mg/week for weight loss.
Topline results from STEP 1 were presented June 4, 2020.
And as reported earlier, results from STEP 3 – a 68-week trial of semaglutide versus placebo in 611 participants who all received very intensive diet and exercise counseling – were presented at the virtual ObesityWeek 2020 meeting.
The four trials of semaglutide for weight loss have been completed and the data were submitted to the Food and Drug Administration on Dec. 4, 2020 (with a decision expected within 6 months) and to the European Medicines Agency on Dec. 18, 2020.
Most patients had 5% weight loss with semaglutide
The STEP 1 trial enrolled 1,961 adults with a body mass index (BMI) of at least 30 kg/m2 or at least 27 with at least one weight-related coexisting condition, but without type 2 diabetes, at 129 sites in 16 countries in Asia, Europe, North America, and South America.
Participants were a mean age of 47 and three-quarters were women. Most participants were White (76%), followed by Asian (13%), Black or African American (6%), or other (5%).
On average, they had a BMI of 38 and weighed 105 kg. Three-quarters had one or more coexisting conditions.
Participants were randomized to receive semaglutide (1,306 patients) or placebo (655 patients), added to lifestyle intervention.
Everyone received 17 monthly individual counseling sessions during which they learned about adhering to a diet with a 500-calorie/day deficit, were encouraged to build up to walking 150 minutes each week, and recorded their daily diet and exercise (in a diary or using an app).
Semaglutide was administered with a prefilled pen injector at a dose of 0.25 mg/week for the first 4 weeks, escalated to 2.4 mg/week by week 16 (or lower if the patient had unacceptable side effects).
At 68 weeks, participants in the semaglutide versus placebo group had greater mean weight loss (14.9% vs. 2.4%, or 15.3 kg vs. 2.6 kg).
Participants in the semaglutide versus placebo group were much more likely to have lost at least 5% of their initial weight (86% vs. 31.5%) or at least 10% of their initial weight (69.1% vs. 12.0%), or at least 15% of their initial weight (50.5% vs. 4.9%; P < .001 for all three comparisons).
About 80% of participants adhered to the study treatment. A third of participants in the semaglutide group who completed the study lost at least 20% of their initial weight, which approaches the 20%-30% reported weight loss 1-3 years after sleeve gastrectomy, the researchers noted.
Participants in the semaglutide group also had greater improvements in waist circumference and levels of hemoglobin A1c, C-reactive protein (a marker of inflammation), and fasting lipids, as well as in physical function scores on SF-36 and IWQOL-Lite-CT questionnaires.
In their editorial, Dr. Ingelfinger and Dr. Rosen noted that “daily oral semaglutide [already approved in 7-mg and 14-mg doses for the treatment of type 2 diabetes as Rybelsus] might be more appealing to many people,” as a weight-loss medication than a once-weekly subcutaneous dose. Semaglutide is the first GLP-1 agonist available as an oral agent.
The ongoing Semaglutide Effects on Heart Disease and Stroke in Patients With Overweight or Obesity (SELECT) trial (with expected completion in 2023) will shed light on cardiovascular outcomes after 2.5-5 years.
GI disorders and ‘important limitations’
More participants in the semaglutide than the placebo group reported gastrointestinal disorders (typically nausea, diarrhea, vomiting, and constipation; 74.2% vs. 47.9%), which were mostly transient and mild to moderate in severity, but also led to more treatment discontinuation (7.0% vs. 3.1%).
More patients in the semaglutide versus placebo group had a gall bladder–related disorder (2.6% vs. 1.2%, mostly cholelithiasis) and mild acute pancreatitis (3 vs. 0 participants), but there were no between-group differences in neoplasms.
Dr. Wilding and colleagues acknowledge the limitations of the study, including the fact that it enrolled mainly women, mainly non-White participants, was relatively short, and excluded patients with type 2 diabetes.
Mean placebo-corrected weight loss with 2.4 mg/weekly subcutaneous semaglutide was greater than with 3.0 mg once-daily subcutaneous liraglutide (Saxenda, Novo Nordisk) – the only GLP-1 agonist approved for weight management – in the 56-week SCALE trial (12.4% vs. 4.5%); however, the two studies had different populations.
The study was supported by Novo Nordisk. Dr. Ingelfinger is a deputy editor and Dr. Rosen is an associate editor of the New England Journal of Medicine. Dr. Ingelfinger, Dr. Rosen, and Dr. Leurent have reported no relevant financial relationships. Dr. O’Rahilly has a current research collaboration with Novo Nordisk scientists in an unrelated area and has been a consultant for the company.
A version of this article first appeared on Medscape.com.
The phase 3a STEP 1 trial that investigated the use of semaglutide (Novo Nordisk), a glucagonlike peptide–1 (GLP-1) agonist, for weight loss is aptly named, some say.
“In sum, we have a long way to go to control the obesity epidemic ... but on the face of it, the STEP 1 trial (like its name) is a good beginning,” wrote coeditorialists Julie R. Ingelfinger, MD, from Harvard Medical School, Boston, and a deputy editor of the New England Journal of Medicine, and Clifford J. Rosen, MD, from Tufts University School of Medicine, also in Boston.
The trial findings by John P.H. Wilding, DM, University of Liverpool (England), and colleagues and an accompanying editorial were published online Feb. 10, 2021, in the New England Journal of Medicine.
“The results are encouraging, with significantly more patients in the semaglutide group having clinically important weight loss,” Dr. Ingelfinger and Dr. Rosen stressed.
However, they also cautioned that “despite the positive results of this trial, the present study has some important limitations” and “there are concerns, including adverse events (mostly gastrointestinal – nausea, sometimes vomiting, and diarrhea) related primarily to the class of the agent.”
Two U.K. experts drew similar takeaways, speaking to the U.K. Science Media Centre.
“This was a well-designed study with unequivocal findings,” which showed that semaglutide “is indeed likely to be a game-changer in the fight against obesity,” according to Baptiste Leurent, PhD, London School of Hygiene and Tropical Medicine.
However, if the drug is approved at this dose for this use, patients would need close monitoring for gastrointestinal disorders, and “we also need to better understand what is happening once the treatment is stopped, and whether it could be taken for a shorter period of time.”
Sir Stephen O’Rahilly, MD, MRC Metabolic Diseases Unit, University of Cambridge (England), pointed out that “GLP-1 is made by cells in the intestine and levels increase in the blood after a meal, providing some of the signal to the brain that tells us we are ‘full,’ ” so GLP-1 agonists have been studied as appetite suppressants, in addition to their approved use to treat type 2 diabetes.
Only about 4.5% of participants in STEP 1 stopped taking semaglutide because of gastrointestinal issues, he noted, although more participants in that group reported problems with gallstones, which can follow rapid weight loss.
And “unlike some previous appetite suppressant drugs which caused significant psychological and psychiatric side effects, there is no evidence that semaglutide has any adverse effects of that nature,” Dr. O’Rahilly noted.
In sum, he said, “this is the start of a new era for obesity drug development with the future direction being to achieve levels of weight loss comparable to semaglutide, while having fewer side effects.”
‘Pressing need’ to address obesity; semaglutide filed for obesity
There is a “pressing need” to address the worldwide increase in obesity and weight-related coexisting conditions, Dr. Ingelfinger and Dr. Rosen noted.
Sustained long-term weight loss with diet and exercise is challenging; behavioral weight-loss strategies “fail more often than not,” bariatric surgery is invasive and often followed by eventual weight regain, they wrote.
In addition, said Dr. Wilding and colleagues, the “use of available [weight-loss] medications remains limited by modest efficacy, safety concerns, and cost.”
Subcutaneous semaglutide, approved for treating type 2 diabetes (as Ozempic) in adults at doses of up to 1 mg/week, induced weight loss at higher doses. The current study is part of the global Semaglutide Treatment Effect in People With Obesity program of four trials (STEP 1, 2, 3, and 4) that aimed to test the safety and efficacy of subcutaneous semaglutide 2.4 mg/week for weight loss.
Topline results from STEP 1 were presented June 4, 2020.
And as reported earlier, results from STEP 3 – a 68-week trial of semaglutide versus placebo in 611 participants who all received very intensive diet and exercise counseling – were presented at the virtual ObesityWeek 2020 meeting.
The four trials of semaglutide for weight loss have been completed and the data were submitted to the Food and Drug Administration on Dec. 4, 2020 (with a decision expected within 6 months) and to the European Medicines Agency on Dec. 18, 2020.
Most patients had 5% weight loss with semaglutide
The STEP 1 trial enrolled 1,961 adults with a body mass index (BMI) of at least 30 kg/m2 or at least 27 with at least one weight-related coexisting condition, but without type 2 diabetes, at 129 sites in 16 countries in Asia, Europe, North America, and South America.
Participants were a mean age of 47 and three-quarters were women. Most participants were White (76%), followed by Asian (13%), Black or African American (6%), or other (5%).
On average, they had a BMI of 38 and weighed 105 kg. Three-quarters had one or more coexisting conditions.
Participants were randomized to receive semaglutide (1,306 patients) or placebo (655 patients), added to lifestyle intervention.
Everyone received 17 monthly individual counseling sessions during which they learned about adhering to a diet with a 500-calorie/day deficit, were encouraged to build up to walking 150 minutes each week, and recorded their daily diet and exercise (in a diary or using an app).
Semaglutide was administered with a prefilled pen injector at a dose of 0.25 mg/week for the first 4 weeks, escalated to 2.4 mg/week by week 16 (or lower if the patient had unacceptable side effects).
At 68 weeks, participants in the semaglutide versus placebo group had greater mean weight loss (14.9% vs. 2.4%, or 15.3 kg vs. 2.6 kg).
Participants in the semaglutide versus placebo group were much more likely to have lost at least 5% of their initial weight (86% vs. 31.5%) or at least 10% of their initial weight (69.1% vs. 12.0%), or at least 15% of their initial weight (50.5% vs. 4.9%; P < .001 for all three comparisons).
About 80% of participants adhered to the study treatment. A third of participants in the semaglutide group who completed the study lost at least 20% of their initial weight, which approaches the 20%-30% reported weight loss 1-3 years after sleeve gastrectomy, the researchers noted.
Participants in the semaglutide group also had greater improvements in waist circumference and levels of hemoglobin A1c, C-reactive protein (a marker of inflammation), and fasting lipids, as well as in physical function scores on SF-36 and IWQOL-Lite-CT questionnaires.
In their editorial, Dr. Ingelfinger and Dr. Rosen noted that “daily oral semaglutide [already approved in 7-mg and 14-mg doses for the treatment of type 2 diabetes as Rybelsus] might be more appealing to many people,” as a weight-loss medication than a once-weekly subcutaneous dose. Semaglutide is the first GLP-1 agonist available as an oral agent.
The ongoing Semaglutide Effects on Heart Disease and Stroke in Patients With Overweight or Obesity (SELECT) trial (with expected completion in 2023) will shed light on cardiovascular outcomes after 2.5-5 years.
GI disorders and ‘important limitations’
More participants in the semaglutide than the placebo group reported gastrointestinal disorders (typically nausea, diarrhea, vomiting, and constipation; 74.2% vs. 47.9%), which were mostly transient and mild to moderate in severity, but also led to more treatment discontinuation (7.0% vs. 3.1%).
More patients in the semaglutide versus placebo group had a gall bladder–related disorder (2.6% vs. 1.2%, mostly cholelithiasis) and mild acute pancreatitis (3 vs. 0 participants), but there were no between-group differences in neoplasms.
Dr. Wilding and colleagues acknowledge the limitations of the study, including the fact that it enrolled mainly women, mainly non-White participants, was relatively short, and excluded patients with type 2 diabetes.
Mean placebo-corrected weight loss with 2.4 mg/weekly subcutaneous semaglutide was greater than with 3.0 mg once-daily subcutaneous liraglutide (Saxenda, Novo Nordisk) – the only GLP-1 agonist approved for weight management – in the 56-week SCALE trial (12.4% vs. 4.5%); however, the two studies had different populations.
The study was supported by Novo Nordisk. Dr. Ingelfinger is a deputy editor and Dr. Rosen is an associate editor of the New England Journal of Medicine. Dr. Ingelfinger, Dr. Rosen, and Dr. Leurent have reported no relevant financial relationships. Dr. O’Rahilly has a current research collaboration with Novo Nordisk scientists in an unrelated area and has been a consultant for the company.
A version of this article first appeared on Medscape.com.
Antidepressants may scupper efficacy of MDMA for PTSD
Pooled data from four phase 2 trials reveal that patients with recent SSRI exposure were significantly more likely to continue to meet PTSD diagnostic criteria after methylenedioxymethamphetamine (MDMA)-assisted psychotherapy than their peers who had not recently taken SSRIs.
Although preliminary, the findings have implications for clinical practice if MDMA-assisted psychotherapy is approved by the Food and Drug Administration, Allison Feduccia, PhD, study coauthor and founder of the education platform Psychedelic.Support, said in an interview.
“As psychedelic medicines become available, it’s going to be important that we try to understand what factors impact the response rate and if there are ways that we can improve the treatment outcomes. Allowing for a longer period for tapering completely off SSRIs before initiating MDMA sessions might increase the effectiveness of MDMA,” Dr. Feduccia said.
The study was published online Nov. 20, 2020, in Psychopharmacology (doi: 10.1007/s00213-020-05710-w).
Reduced response
The primary mechanism of action of MDMA involves the same reuptake transporters that are targeted by antidepressant medications commonly prescribed for PTSD. These medications include SSRIs, serotonin-norepinephrine reuptake inhibitors (SNRIs), NRIs, and norepinephrine-dopamine reuptake inhibitors (NDRIs).
Prior research shows that, when MDMA is coadministered with a reuptake inhibitor, subjective and psychological effects of the therapy are attenuated.
The researchers sought to determine whether or not recent tapering off of an antidepressant that targets the same primary binding sites as MDMA would affect treatment response. They analyzed data on 50 adults who underwent two sessions of MDMA-assisted psychotherapy in phase 2 clinical trials.
For 16 of these patients, SSRI therapy was tapered off prior to the MDMA sessions. For 34 patients, SSRI therapy was not tapered off, because the patients had not been taking the medication at the time of initial study screening (nontaper group).
The taper protocols specified that medications be tapered gradually over a period of weeks to minimize withdrawal symptoms and for them to be discontinued at least five half-lives of each drug prior to MDMA administration.
Demographics, baseline PTSD, and depression severity were similar between the taper and the nontaper groups. Participants in the studies had chronic PTSD (symptoms lasting >6 months). Severity scores on the Clinician-Administered PTSD Scale for DSM IV (CAPS-IV) were at least 50.
After MDMA-assisted psychotherapy, the nontaper group had significantly lower (better) CAPS-IV total scores, compared with the taper group (mean, 45.7 vs. 70.3; P = .009).
About two-thirds (63.6%) of the nontaper group no longer met PTSD criteria after MDMA-assisted therapy, compared with only 25% of those in the taper group.
The nontaper group also had lower depression symptom severity scores on the Beck Depression Inventory–II, compared with the taper group (mean, 12.7 vs. 22.6; P = .010).
“Another really interesting” observation, said Dr. Feduccia, is that the expected increases in systolic and diastolic blood pressure following MDMA administration were reduced in the taper group, compared with the nontaper group.
“This suggests that MDMA didn’t have the same physiological response in individuals who tapered SSRIs. This should be followed up,” she said.
The investigators offerred several potential mechanisms for the negative effect of recent SSRI use on MDMA-assisted psychotherapy for PTSD.
These include the down-regulation of binding sites (serotonin, dopamine, and/or norepinephrine) related to SSRI use, reduced MDMA treatment-relevant increases in blood pressure in patients with recent SSRI use, and the possibility that withdrawal symptoms from SSRIs may reduce the effectiveness of MDMA psychotherapy.
Important clinical implications
In a comment, Steven R. Thorp, PhD, professor at Alliant International University, San Diego, said the findings are “very interesting” and likely “not well known.”
“There has been great interest in MDMA-assisted psychotherapy in recent years, and if this finding is replicated, it will have important implications for that research,” Dr. Thorp said.
“Although psychotherapy is often preferred by clients with PTSD, compared to medications, and typically shows efficacy that is as strong or stronger (and longer lasting) than medications, many individuals with PTSD are provided with medication only,” Dr. Thorp noted.
“This study suggests that, in addition to the other potential disadvantages of medications (e.g., cost, side effects, potential for addiction), those who take SSRIs, SNRIs, NRIs, and NDRIs for PTSD may also benefit less from MDMA-assisted psychotherapy,” Dr. Thorp added.
The four phase 2 studies used in the analysis were sponsored by the Multidisciplinary Association for Psychedelic Studies, a nonprofit organization. Dr. Feduccia received salary support for full-time employment with MAPS Public Benefit Corporation. Dr. Thorp disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Pooled data from four phase 2 trials reveal that patients with recent SSRI exposure were significantly more likely to continue to meet PTSD diagnostic criteria after methylenedioxymethamphetamine (MDMA)-assisted psychotherapy than their peers who had not recently taken SSRIs.
Although preliminary, the findings have implications for clinical practice if MDMA-assisted psychotherapy is approved by the Food and Drug Administration, Allison Feduccia, PhD, study coauthor and founder of the education platform Psychedelic.Support, said in an interview.
“As psychedelic medicines become available, it’s going to be important that we try to understand what factors impact the response rate and if there are ways that we can improve the treatment outcomes. Allowing for a longer period for tapering completely off SSRIs before initiating MDMA sessions might increase the effectiveness of MDMA,” Dr. Feduccia said.
The study was published online Nov. 20, 2020, in Psychopharmacology (doi: 10.1007/s00213-020-05710-w).
Reduced response
The primary mechanism of action of MDMA involves the same reuptake transporters that are targeted by antidepressant medications commonly prescribed for PTSD. These medications include SSRIs, serotonin-norepinephrine reuptake inhibitors (SNRIs), NRIs, and norepinephrine-dopamine reuptake inhibitors (NDRIs).
Prior research shows that, when MDMA is coadministered with a reuptake inhibitor, subjective and psychological effects of the therapy are attenuated.
The researchers sought to determine whether or not recent tapering off of an antidepressant that targets the same primary binding sites as MDMA would affect treatment response. They analyzed data on 50 adults who underwent two sessions of MDMA-assisted psychotherapy in phase 2 clinical trials.
For 16 of these patients, SSRI therapy was tapered off prior to the MDMA sessions. For 34 patients, SSRI therapy was not tapered off, because the patients had not been taking the medication at the time of initial study screening (nontaper group).
The taper protocols specified that medications be tapered gradually over a period of weeks to minimize withdrawal symptoms and for them to be discontinued at least five half-lives of each drug prior to MDMA administration.
Demographics, baseline PTSD, and depression severity were similar between the taper and the nontaper groups. Participants in the studies had chronic PTSD (symptoms lasting >6 months). Severity scores on the Clinician-Administered PTSD Scale for DSM IV (CAPS-IV) were at least 50.
After MDMA-assisted psychotherapy, the nontaper group had significantly lower (better) CAPS-IV total scores, compared with the taper group (mean, 45.7 vs. 70.3; P = .009).
About two-thirds (63.6%) of the nontaper group no longer met PTSD criteria after MDMA-assisted therapy, compared with only 25% of those in the taper group.
The nontaper group also had lower depression symptom severity scores on the Beck Depression Inventory–II, compared with the taper group (mean, 12.7 vs. 22.6; P = .010).
“Another really interesting” observation, said Dr. Feduccia, is that the expected increases in systolic and diastolic blood pressure following MDMA administration were reduced in the taper group, compared with the nontaper group.
“This suggests that MDMA didn’t have the same physiological response in individuals who tapered SSRIs. This should be followed up,” she said.
The investigators offerred several potential mechanisms for the negative effect of recent SSRI use on MDMA-assisted psychotherapy for PTSD.
These include the down-regulation of binding sites (serotonin, dopamine, and/or norepinephrine) related to SSRI use, reduced MDMA treatment-relevant increases in blood pressure in patients with recent SSRI use, and the possibility that withdrawal symptoms from SSRIs may reduce the effectiveness of MDMA psychotherapy.
Important clinical implications
In a comment, Steven R. Thorp, PhD, professor at Alliant International University, San Diego, said the findings are “very interesting” and likely “not well known.”
“There has been great interest in MDMA-assisted psychotherapy in recent years, and if this finding is replicated, it will have important implications for that research,” Dr. Thorp said.
“Although psychotherapy is often preferred by clients with PTSD, compared to medications, and typically shows efficacy that is as strong or stronger (and longer lasting) than medications, many individuals with PTSD are provided with medication only,” Dr. Thorp noted.
“This study suggests that, in addition to the other potential disadvantages of medications (e.g., cost, side effects, potential for addiction), those who take SSRIs, SNRIs, NRIs, and NDRIs for PTSD may also benefit less from MDMA-assisted psychotherapy,” Dr. Thorp added.
The four phase 2 studies used in the analysis were sponsored by the Multidisciplinary Association for Psychedelic Studies, a nonprofit organization. Dr. Feduccia received salary support for full-time employment with MAPS Public Benefit Corporation. Dr. Thorp disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Pooled data from four phase 2 trials reveal that patients with recent SSRI exposure were significantly more likely to continue to meet PTSD diagnostic criteria after methylenedioxymethamphetamine (MDMA)-assisted psychotherapy than their peers who had not recently taken SSRIs.
Although preliminary, the findings have implications for clinical practice if MDMA-assisted psychotherapy is approved by the Food and Drug Administration, Allison Feduccia, PhD, study coauthor and founder of the education platform Psychedelic.Support, said in an interview.
“As psychedelic medicines become available, it’s going to be important that we try to understand what factors impact the response rate and if there are ways that we can improve the treatment outcomes. Allowing for a longer period for tapering completely off SSRIs before initiating MDMA sessions might increase the effectiveness of MDMA,” Dr. Feduccia said.
The study was published online Nov. 20, 2020, in Psychopharmacology (doi: 10.1007/s00213-020-05710-w).
Reduced response
The primary mechanism of action of MDMA involves the same reuptake transporters that are targeted by antidepressant medications commonly prescribed for PTSD. These medications include SSRIs, serotonin-norepinephrine reuptake inhibitors (SNRIs), NRIs, and norepinephrine-dopamine reuptake inhibitors (NDRIs).
Prior research shows that, when MDMA is coadministered with a reuptake inhibitor, subjective and psychological effects of the therapy are attenuated.
The researchers sought to determine whether or not recent tapering off of an antidepressant that targets the same primary binding sites as MDMA would affect treatment response. They analyzed data on 50 adults who underwent two sessions of MDMA-assisted psychotherapy in phase 2 clinical trials.
For 16 of these patients, SSRI therapy was tapered off prior to the MDMA sessions. For 34 patients, SSRI therapy was not tapered off, because the patients had not been taking the medication at the time of initial study screening (nontaper group).
The taper protocols specified that medications be tapered gradually over a period of weeks to minimize withdrawal symptoms and for them to be discontinued at least five half-lives of each drug prior to MDMA administration.
Demographics, baseline PTSD, and depression severity were similar between the taper and the nontaper groups. Participants in the studies had chronic PTSD (symptoms lasting >6 months). Severity scores on the Clinician-Administered PTSD Scale for DSM IV (CAPS-IV) were at least 50.
After MDMA-assisted psychotherapy, the nontaper group had significantly lower (better) CAPS-IV total scores, compared with the taper group (mean, 45.7 vs. 70.3; P = .009).
About two-thirds (63.6%) of the nontaper group no longer met PTSD criteria after MDMA-assisted therapy, compared with only 25% of those in the taper group.
The nontaper group also had lower depression symptom severity scores on the Beck Depression Inventory–II, compared with the taper group (mean, 12.7 vs. 22.6; P = .010).
“Another really interesting” observation, said Dr. Feduccia, is that the expected increases in systolic and diastolic blood pressure following MDMA administration were reduced in the taper group, compared with the nontaper group.
“This suggests that MDMA didn’t have the same physiological response in individuals who tapered SSRIs. This should be followed up,” she said.
The investigators offerred several potential mechanisms for the negative effect of recent SSRI use on MDMA-assisted psychotherapy for PTSD.
These include the down-regulation of binding sites (serotonin, dopamine, and/or norepinephrine) related to SSRI use, reduced MDMA treatment-relevant increases in blood pressure in patients with recent SSRI use, and the possibility that withdrawal symptoms from SSRIs may reduce the effectiveness of MDMA psychotherapy.
Important clinical implications
In a comment, Steven R. Thorp, PhD, professor at Alliant International University, San Diego, said the findings are “very interesting” and likely “not well known.”
“There has been great interest in MDMA-assisted psychotherapy in recent years, and if this finding is replicated, it will have important implications for that research,” Dr. Thorp said.
“Although psychotherapy is often preferred by clients with PTSD, compared to medications, and typically shows efficacy that is as strong or stronger (and longer lasting) than medications, many individuals with PTSD are provided with medication only,” Dr. Thorp noted.
“This study suggests that, in addition to the other potential disadvantages of medications (e.g., cost, side effects, potential for addiction), those who take SSRIs, SNRIs, NRIs, and NDRIs for PTSD may also benefit less from MDMA-assisted psychotherapy,” Dr. Thorp added.
The four phase 2 studies used in the analysis were sponsored by the Multidisciplinary Association for Psychedelic Studies, a nonprofit organization. Dr. Feduccia received salary support for full-time employment with MAPS Public Benefit Corporation. Dr. Thorp disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FDA approves orphan drug evinacumab-dgnb for homozygous FH
The Food and Drug Administration has approved the fully human monoclonal antibody evinacumab-dgnb (Evkeeza, Regeneron Pharmaceuticals) for use on top of other cholesterol-modifying medication in patients aged 12 years and older with homozygous familial hypercholesterolemia (HoFH), the agency and Regeneron have announced.
Evinacumab had received orphan drug designation and underwent priority regulatory review based primarily on the phase 3 ELIPSE trial, presented at a meeting in March 2020 and published in August 2020 in the New England Journal of Medicine (doi: 10.1056/NEJMoa2004215).
In the trial with 65 patients with HoFH on guideline-based lipid-modifying therapy, those who also received evinacumab 15 mg/kg intravenously every 4 weeks showed a nearly 50% drop in LDL cholesterol levels after 24 weeks, compared with patients given a placebo. Only 2% of patients in both groups discontinued therapy because of adverse reactions.
The drug blocks angiopoietin-like 3, itself an inhibitor of lipoprotein lipase and endothelial lipase. It therefore lowers LDL cholesterol levels by mechanisms that don’t directly involve the LDL receptor.
Regeneron estimates that about 1300 people in the United States have the homozygous genetic disorder, which can lead to LDL cholesterol levels of a 1,000 mg/dL or higher, advanced premature atherosclerosis, and extreme risk for cardiovascular events.
The drug’s average wholesale acquisition cost per patient in the United States is expected to be about $450,000 per year, the company said, adding that it has a financial support program to help qualified patients with out-of-pocket costs.
Regeneron’s announcement included a comment from dyslipidemia-therapy expert Daniel J. Rader, MD, University of Pennsylvania, Philadelphia, who called evinacumab “a potentially transformational new treatment for people with HoFH.”
The drug is currently under regulatory review for the same indication in Europe, the company said.
A version of this article first appeared on Medscape.com.
The Food and Drug Administration has approved the fully human monoclonal antibody evinacumab-dgnb (Evkeeza, Regeneron Pharmaceuticals) for use on top of other cholesterol-modifying medication in patients aged 12 years and older with homozygous familial hypercholesterolemia (HoFH), the agency and Regeneron have announced.
Evinacumab had received orphan drug designation and underwent priority regulatory review based primarily on the phase 3 ELIPSE trial, presented at a meeting in March 2020 and published in August 2020 in the New England Journal of Medicine (doi: 10.1056/NEJMoa2004215).
In the trial with 65 patients with HoFH on guideline-based lipid-modifying therapy, those who also received evinacumab 15 mg/kg intravenously every 4 weeks showed a nearly 50% drop in LDL cholesterol levels after 24 weeks, compared with patients given a placebo. Only 2% of patients in both groups discontinued therapy because of adverse reactions.
The drug blocks angiopoietin-like 3, itself an inhibitor of lipoprotein lipase and endothelial lipase. It therefore lowers LDL cholesterol levels by mechanisms that don’t directly involve the LDL receptor.
Regeneron estimates that about 1300 people in the United States have the homozygous genetic disorder, which can lead to LDL cholesterol levels of a 1,000 mg/dL or higher, advanced premature atherosclerosis, and extreme risk for cardiovascular events.
The drug’s average wholesale acquisition cost per patient in the United States is expected to be about $450,000 per year, the company said, adding that it has a financial support program to help qualified patients with out-of-pocket costs.
Regeneron’s announcement included a comment from dyslipidemia-therapy expert Daniel J. Rader, MD, University of Pennsylvania, Philadelphia, who called evinacumab “a potentially transformational new treatment for people with HoFH.”
The drug is currently under regulatory review for the same indication in Europe, the company said.
A version of this article first appeared on Medscape.com.
The Food and Drug Administration has approved the fully human monoclonal antibody evinacumab-dgnb (Evkeeza, Regeneron Pharmaceuticals) for use on top of other cholesterol-modifying medication in patients aged 12 years and older with homozygous familial hypercholesterolemia (HoFH), the agency and Regeneron have announced.
Evinacumab had received orphan drug designation and underwent priority regulatory review based primarily on the phase 3 ELIPSE trial, presented at a meeting in March 2020 and published in August 2020 in the New England Journal of Medicine (doi: 10.1056/NEJMoa2004215).
In the trial with 65 patients with HoFH on guideline-based lipid-modifying therapy, those who also received evinacumab 15 mg/kg intravenously every 4 weeks showed a nearly 50% drop in LDL cholesterol levels after 24 weeks, compared with patients given a placebo. Only 2% of patients in both groups discontinued therapy because of adverse reactions.
The drug blocks angiopoietin-like 3, itself an inhibitor of lipoprotein lipase and endothelial lipase. It therefore lowers LDL cholesterol levels by mechanisms that don’t directly involve the LDL receptor.
Regeneron estimates that about 1300 people in the United States have the homozygous genetic disorder, which can lead to LDL cholesterol levels of a 1,000 mg/dL or higher, advanced premature atherosclerosis, and extreme risk for cardiovascular events.
The drug’s average wholesale acquisition cost per patient in the United States is expected to be about $450,000 per year, the company said, adding that it has a financial support program to help qualified patients with out-of-pocket costs.
Regeneron’s announcement included a comment from dyslipidemia-therapy expert Daniel J. Rader, MD, University of Pennsylvania, Philadelphia, who called evinacumab “a potentially transformational new treatment for people with HoFH.”
The drug is currently under regulatory review for the same indication in Europe, the company said.
A version of this article first appeared on Medscape.com.
Key trends in hospitalist compensation from the 2020 SoHM Report
In a time of tremendous uncertainty, there is one trend that seems consistent year over year – the undisputed value of hospitalists. In the 2020 State of Hospital Medicine (SoHM) Report, the Society of Hospital Medicine partnered with the Medical Group Management Association (MGMA) to provide data on hospitalist compensation and productivity. The Report provides resounding evidence that hospitalists continue to be compensated at rising rates. This may be driven by both the continued demand-supply mismatch and a recognition of the overall value they generate rather than strictly the volume of their productivity.
In 2020, the median total compensation nationally for adult hospitalists (internal medicine and family medicine) was $307,633, representing an increase of over 6% from the 2018 Survey (see Figure 1). 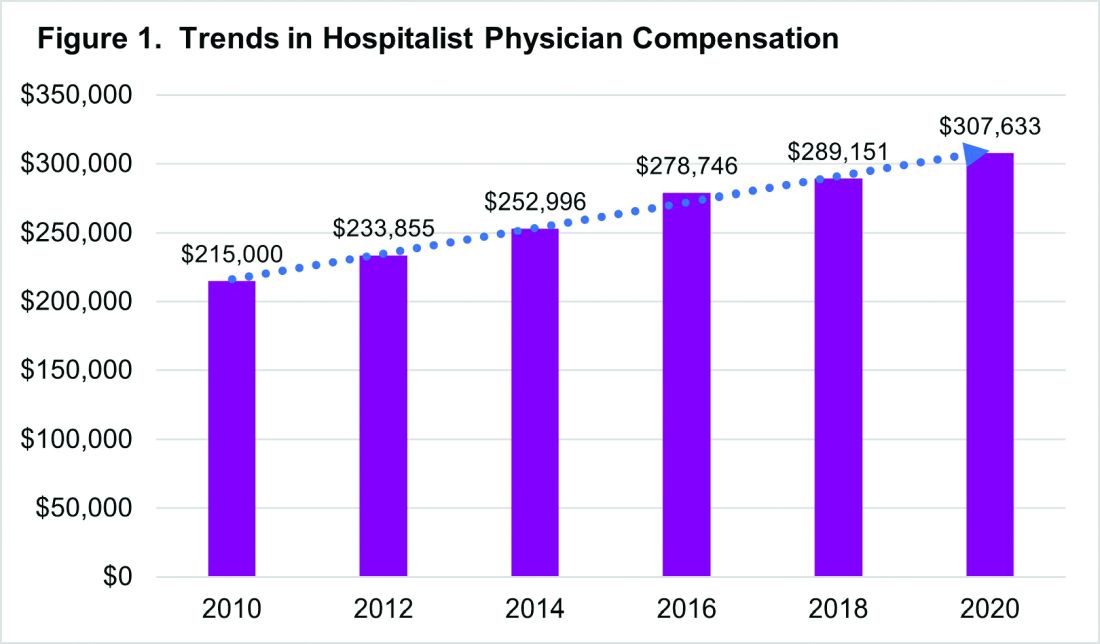
With significant regional variability in compensation across the country, hospitalists in the South continue to earn more than their colleagues in the East – a median compensation difference of about $33,000. However, absolute wage comparisons can be misleading without also considering regional variations in productivity as well.
Reviewing compensation per work relative value unit (wRVU) and per encounter offer additional insight for a more comprehensive assessment. When comparing regional compensation per wRVU, the 2020 Survey continues to show a trend toward hospitalists in the Midwest and West earning more per wRVU than their colleagues in other parts of the country ($78.13 per RVU in the Midwest, $78.95 per RVU in the West). More striking is how hospital medicine groups (HMGs) in the South garner lower compensation per RVU ($57.77) than those in the East ($67.54), even though their total compensation was much higher. This could reflect the gradual decline in compensation per wRVU that’s observed at higher productivity levels. While it’s typical for compensation to increase as productivity does, the rate of increase is generally to a lesser degree.
Like past SoHM Surveys, the 2020 Report revealed that academic and non-academic hospitalists are compensated similarly per wRVU (see Figure 2). 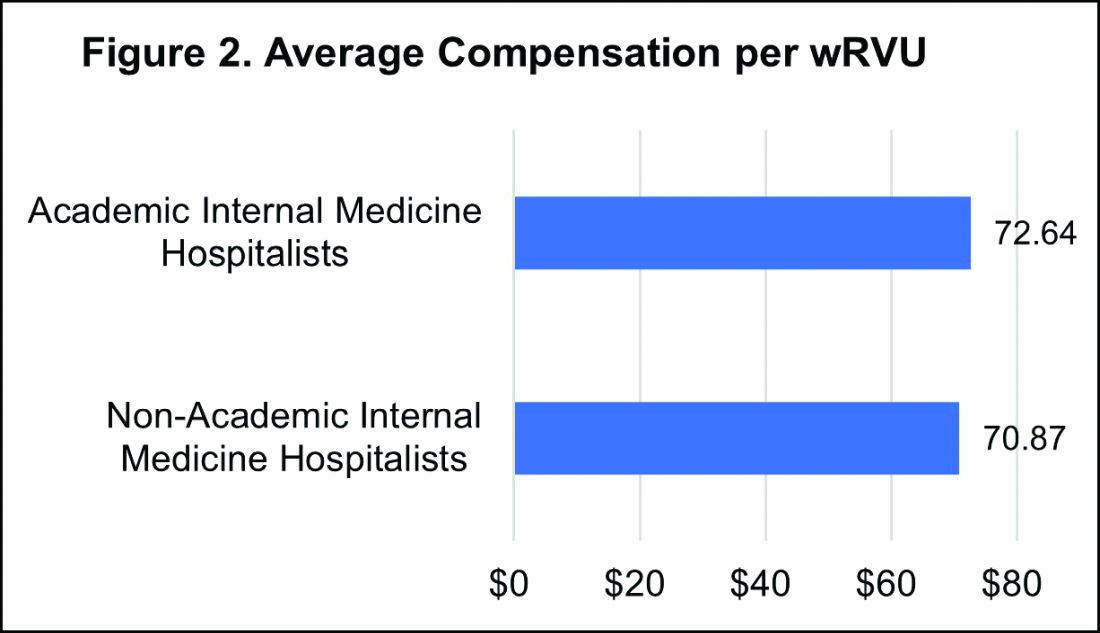
However, the total compensation continues to be lower for academic hospitalists, with a median compensation difference of approximately $70,000 compared to their non-academic colleagues. Some of this sizable variance is offset by the fact that academic HMGs receive more in employee benefits packages than non-academic groups – a difference in median value of $10,500. Ideally, academic hospitalist compensation models should appropriately reflect and value their work efforts toward the tripartite academic mission of clinical care, education, and research. It would be valuable for future surveys to define and measure academic production in order to develop national standards for compensation models that recognize these non-billable forms of productivity.
While it’s important to review compensation benchmarks to remain competitive, it’s difficult to put a price on some factors that many may consider more valuable – group culture, opportunities for professional growth, and schedules that afford better work-life integration. Indirect measures of such benefits include paid time off, paid sick days, CME allowances and time, and retirement benefits programs. In 2020, the median employer contribution to retirement plans was reported to be $13,955, with respondents in the Midwest receiving the highest retirement benefit of $33,771.
It’s encouraging to see that hospitalist compensation continues to rise compared to previous years, despite relatively flat trends in wRVUs and total patient encounters. Another continued trend over the past years is the rising amount of financial support per physician that hospitals or other organizations provide HMGs (see Figure 3). 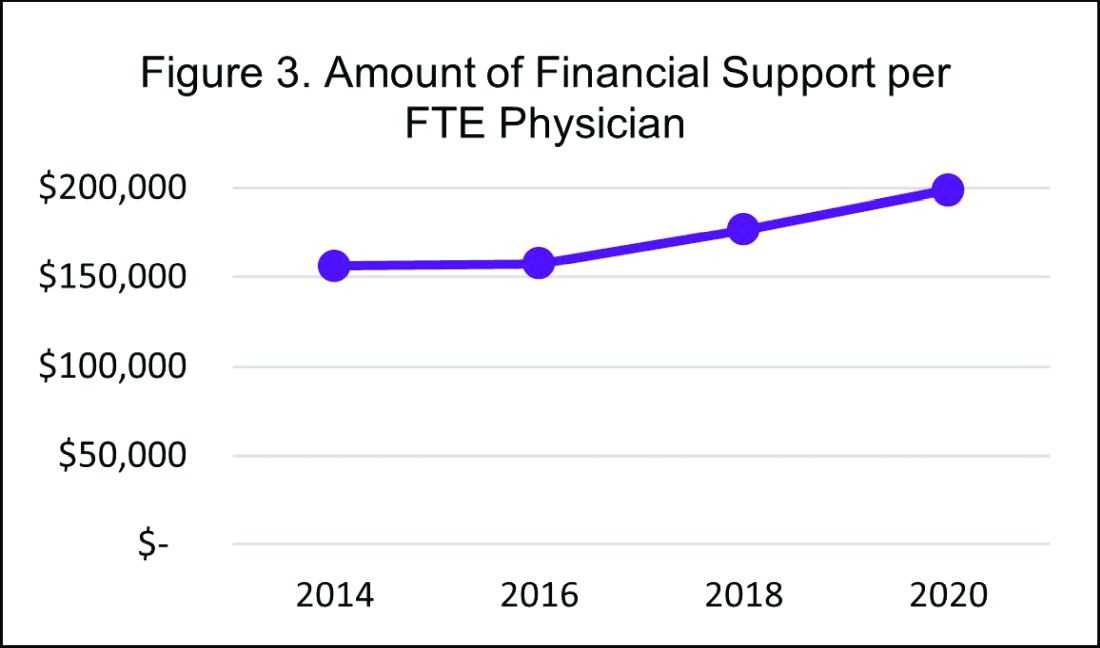
In 2020, the median financial support per FTE (full time equivalent) physician serving adult patients increased by 12% over 2018, to $198,750. Collectively these trends indicate hospitals are willing to compensate hospitalists for more than just their clinical volume.
There’s no doubt that the COVID-19 pandemic had some financial impact on hospital medicine groups in 2020. To assess this impact, SHM conducted a follow-up survey and compiled a COVID-19 Addendum to the SoHM Report. While 20.5% of HMG group respondents from the East reported providing hazard pay to clinicians caring for COVID-19 patients, nationally only 9.8% of groups said they offered this benefit. Of the 121 HMGs responding from across the country, 42% reported reductions in compensation, which included measures such as reductions in pay level and elimination or delays to bonus payments. The degree of reductions was not quantified, but fortunately the vast majority of these groups reported that these changes were likely to be temporary. To access all data in the 2020 SoHM Report and COVID-19 Addendum, visit hospitalmedicine.org/sohm to purchase your copy.
It’s certainly unclear what the future holds, but despite any transient changes observed during the COVID-19 pandemic, I believe that historical trends in hospitalist compensation will continue. If 2020 has taught us anything, it’s that hospitalists are essential, not only during an acute care crisis but for daily operations of any hospital.
Dr. Kurian is chief of the Division of Hospital Medicine at Northwell Health in New York. She is a member of SHM’s Practice Analysis Committee.
In a time of tremendous uncertainty, there is one trend that seems consistent year over year – the undisputed value of hospitalists. In the 2020 State of Hospital Medicine (SoHM) Report, the Society of Hospital Medicine partnered with the Medical Group Management Association (MGMA) to provide data on hospitalist compensation and productivity. The Report provides resounding evidence that hospitalists continue to be compensated at rising rates. This may be driven by both the continued demand-supply mismatch and a recognition of the overall value they generate rather than strictly the volume of their productivity.
In 2020, the median total compensation nationally for adult hospitalists (internal medicine and family medicine) was $307,633, representing an increase of over 6% from the 2018 Survey (see Figure 1). 
With significant regional variability in compensation across the country, hospitalists in the South continue to earn more than their colleagues in the East – a median compensation difference of about $33,000. However, absolute wage comparisons can be misleading without also considering regional variations in productivity as well.
Reviewing compensation per work relative value unit (wRVU) and per encounter offer additional insight for a more comprehensive assessment. When comparing regional compensation per wRVU, the 2020 Survey continues to show a trend toward hospitalists in the Midwest and West earning more per wRVU than their colleagues in other parts of the country ($78.13 per RVU in the Midwest, $78.95 per RVU in the West). More striking is how hospital medicine groups (HMGs) in the South garner lower compensation per RVU ($57.77) than those in the East ($67.54), even though their total compensation was much higher. This could reflect the gradual decline in compensation per wRVU that’s observed at higher productivity levels. While it’s typical for compensation to increase as productivity does, the rate of increase is generally to a lesser degree.
Like past SoHM Surveys, the 2020 Report revealed that academic and non-academic hospitalists are compensated similarly per wRVU (see Figure 2). 
However, the total compensation continues to be lower for academic hospitalists, with a median compensation difference of approximately $70,000 compared to their non-academic colleagues. Some of this sizable variance is offset by the fact that academic HMGs receive more in employee benefits packages than non-academic groups – a difference in median value of $10,500. Ideally, academic hospitalist compensation models should appropriately reflect and value their work efforts toward the tripartite academic mission of clinical care, education, and research. It would be valuable for future surveys to define and measure academic production in order to develop national standards for compensation models that recognize these non-billable forms of productivity.
While it’s important to review compensation benchmarks to remain competitive, it’s difficult to put a price on some factors that many may consider more valuable – group culture, opportunities for professional growth, and schedules that afford better work-life integration. Indirect measures of such benefits include paid time off, paid sick days, CME allowances and time, and retirement benefits programs. In 2020, the median employer contribution to retirement plans was reported to be $13,955, with respondents in the Midwest receiving the highest retirement benefit of $33,771.
It’s encouraging to see that hospitalist compensation continues to rise compared to previous years, despite relatively flat trends in wRVUs and total patient encounters. Another continued trend over the past years is the rising amount of financial support per physician that hospitals or other organizations provide HMGs (see Figure 3). 
In 2020, the median financial support per FTE (full time equivalent) physician serving adult patients increased by 12% over 2018, to $198,750. Collectively these trends indicate hospitals are willing to compensate hospitalists for more than just their clinical volume.
There’s no doubt that the COVID-19 pandemic had some financial impact on hospital medicine groups in 2020. To assess this impact, SHM conducted a follow-up survey and compiled a COVID-19 Addendum to the SoHM Report. While 20.5% of HMG group respondents from the East reported providing hazard pay to clinicians caring for COVID-19 patients, nationally only 9.8% of groups said they offered this benefit. Of the 121 HMGs responding from across the country, 42% reported reductions in compensation, which included measures such as reductions in pay level and elimination or delays to bonus payments. The degree of reductions was not quantified, but fortunately the vast majority of these groups reported that these changes were likely to be temporary. To access all data in the 2020 SoHM Report and COVID-19 Addendum, visit hospitalmedicine.org/sohm to purchase your copy.
It’s certainly unclear what the future holds, but despite any transient changes observed during the COVID-19 pandemic, I believe that historical trends in hospitalist compensation will continue. If 2020 has taught us anything, it’s that hospitalists are essential, not only during an acute care crisis but for daily operations of any hospital.
Dr. Kurian is chief of the Division of Hospital Medicine at Northwell Health in New York. She is a member of SHM’s Practice Analysis Committee.
In a time of tremendous uncertainty, there is one trend that seems consistent year over year – the undisputed value of hospitalists. In the 2020 State of Hospital Medicine (SoHM) Report, the Society of Hospital Medicine partnered with the Medical Group Management Association (MGMA) to provide data on hospitalist compensation and productivity. The Report provides resounding evidence that hospitalists continue to be compensated at rising rates. This may be driven by both the continued demand-supply mismatch and a recognition of the overall value they generate rather than strictly the volume of their productivity.
In 2020, the median total compensation nationally for adult hospitalists (internal medicine and family medicine) was $307,633, representing an increase of over 6% from the 2018 Survey (see Figure 1). 
With significant regional variability in compensation across the country, hospitalists in the South continue to earn more than their colleagues in the East – a median compensation difference of about $33,000. However, absolute wage comparisons can be misleading without also considering regional variations in productivity as well.
Reviewing compensation per work relative value unit (wRVU) and per encounter offer additional insight for a more comprehensive assessment. When comparing regional compensation per wRVU, the 2020 Survey continues to show a trend toward hospitalists in the Midwest and West earning more per wRVU than their colleagues in other parts of the country ($78.13 per RVU in the Midwest, $78.95 per RVU in the West). More striking is how hospital medicine groups (HMGs) in the South garner lower compensation per RVU ($57.77) than those in the East ($67.54), even though their total compensation was much higher. This could reflect the gradual decline in compensation per wRVU that’s observed at higher productivity levels. While it’s typical for compensation to increase as productivity does, the rate of increase is generally to a lesser degree.
Like past SoHM Surveys, the 2020 Report revealed that academic and non-academic hospitalists are compensated similarly per wRVU (see Figure 2). 
However, the total compensation continues to be lower for academic hospitalists, with a median compensation difference of approximately $70,000 compared to their non-academic colleagues. Some of this sizable variance is offset by the fact that academic HMGs receive more in employee benefits packages than non-academic groups – a difference in median value of $10,500. Ideally, academic hospitalist compensation models should appropriately reflect and value their work efforts toward the tripartite academic mission of clinical care, education, and research. It would be valuable for future surveys to define and measure academic production in order to develop national standards for compensation models that recognize these non-billable forms of productivity.
While it’s important to review compensation benchmarks to remain competitive, it’s difficult to put a price on some factors that many may consider more valuable – group culture, opportunities for professional growth, and schedules that afford better work-life integration. Indirect measures of such benefits include paid time off, paid sick days, CME allowances and time, and retirement benefits programs. In 2020, the median employer contribution to retirement plans was reported to be $13,955, with respondents in the Midwest receiving the highest retirement benefit of $33,771.
It’s encouraging to see that hospitalist compensation continues to rise compared to previous years, despite relatively flat trends in wRVUs and total patient encounters. Another continued trend over the past years is the rising amount of financial support per physician that hospitals or other organizations provide HMGs (see Figure 3). 
In 2020, the median financial support per FTE (full time equivalent) physician serving adult patients increased by 12% over 2018, to $198,750. Collectively these trends indicate hospitals are willing to compensate hospitalists for more than just their clinical volume.
There’s no doubt that the COVID-19 pandemic had some financial impact on hospital medicine groups in 2020. To assess this impact, SHM conducted a follow-up survey and compiled a COVID-19 Addendum to the SoHM Report. While 20.5% of HMG group respondents from the East reported providing hazard pay to clinicians caring for COVID-19 patients, nationally only 9.8% of groups said they offered this benefit. Of the 121 HMGs responding from across the country, 42% reported reductions in compensation, which included measures such as reductions in pay level and elimination or delays to bonus payments. The degree of reductions was not quantified, but fortunately the vast majority of these groups reported that these changes were likely to be temporary. To access all data in the 2020 SoHM Report and COVID-19 Addendum, visit hospitalmedicine.org/sohm to purchase your copy.
It’s certainly unclear what the future holds, but despite any transient changes observed during the COVID-19 pandemic, I believe that historical trends in hospitalist compensation will continue. If 2020 has taught us anything, it’s that hospitalists are essential, not only during an acute care crisis but for daily operations of any hospital.
Dr. Kurian is chief of the Division of Hospital Medicine at Northwell Health in New York. She is a member of SHM’s Practice Analysis Committee.
Exercise-Induced Vasculitis in a Patient With Negative Ultrasound Venous Reflux Study: A Mimic of Stasis Dermatitis
To the Editor:
The transient and generic appearance of exercise-induced vasculitis (EIV) makes it a commonly misdiagnosed condition. The lesion often is only encountered through photographs brought by the patient or by taking a thorough history. The lack of findings on clinical inspection and the generic appearance of EIV may lead to misdiagnosis as stasis dermatitis due to its presentation as erythematous lesions on the medial lower legs.
A 68-year-old woman with no notable medical history was referred to our clinic for suspected stasis dermatitis. At presentation, no lesions were identified on the legs, but she brought photographs of an erythematous urticarial eruption on the medial lower legs, extending from just above the sock line to the mid-calves (Figure). The eruptions had occurred over the last 16 years, typically presenting suddenly after playing tennis or an extended period of walking and spontaneously resolving in 4 days. The lesions were painless, restricted to the calves, and were not pruritic, though the initial presentation 16 years prior included pruritic pigmented patches on the anterior thighs. Because the condition spontaneously improved within days, no treatment was attempted. An ultrasound venous reflux study ruled out venous reflux and stasis dermatitis.
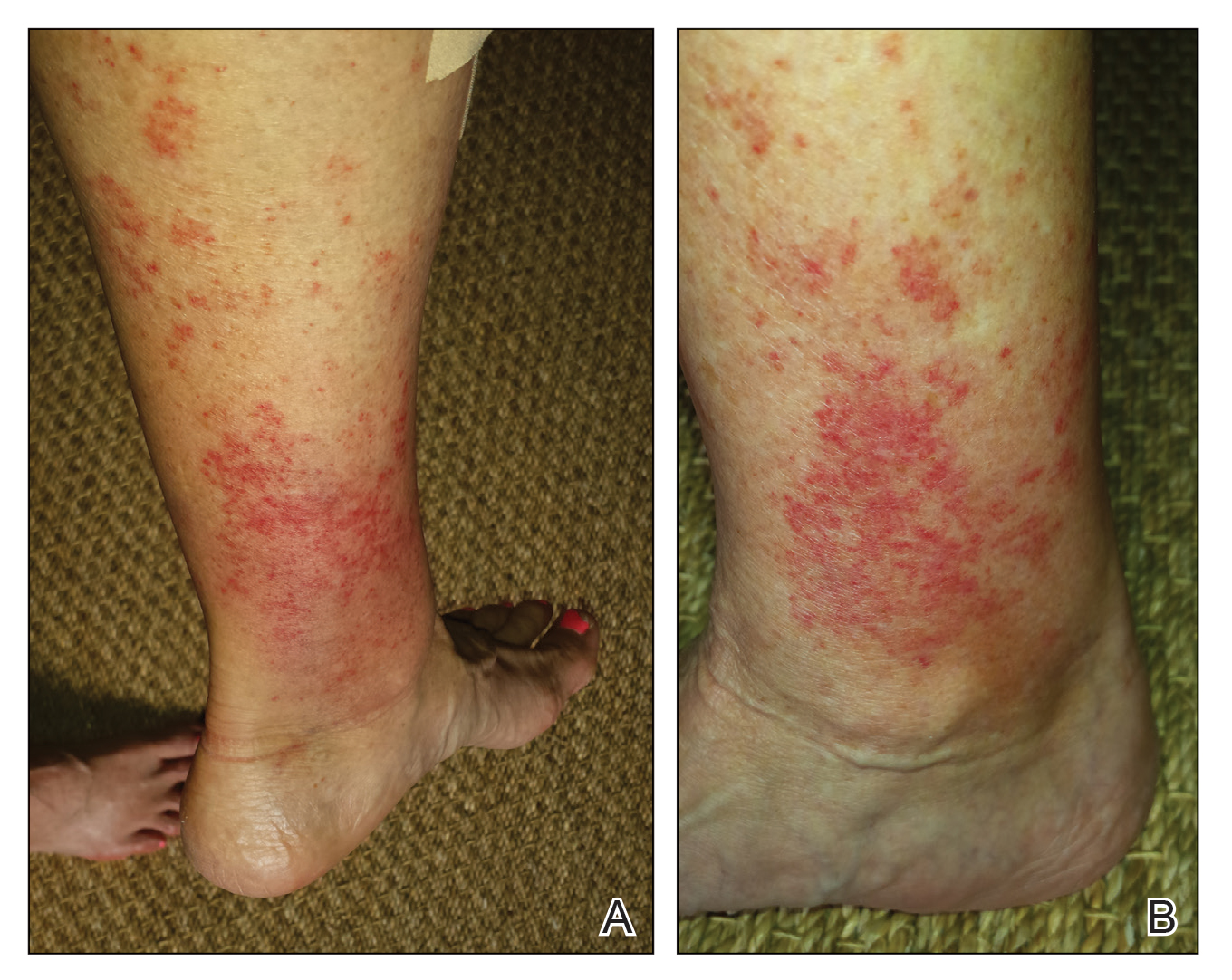
Our patient stated that her 64-year-old sister had reported the same presentation over the last 8 years. Her physical activity was limited strictly to walking, and the lesions occurred after walking for many hours during the day in the heat, involving the medial aspects of the lower legs extending from the ankles to the full length of the calves. Her eruption was warm but was not painful or pruritic. It resolved spontaneously after 5 days with no therapy.
Our patient was advised to wear compression stockings as a preventative measure, but she did not adhere to these recommendations, stating it was impractical to wear compression garments while playing tennis.
Exercise-induced vasculitis most commonly is seen in the medial aspects of the lower extremities as an erythematous urticarial eruption or pigmented purpuric plaque rapidly occurring after a period of exercise.1,2 Lesions often are symmetric and can be pruritic and painful with a lack of systemic symptoms.3 These generic clinical manifestations may lead to a misdiagnosis of stasis dermatitis. One case report included initial treatment of presumptive cellulitis.4 Important clinical findings include a sparing of skin compressed by tight clothing such as socks, a lack of systemic symptoms, rapid appearance after exercise, and spontaneous resolution within a few days. No correlation with chronic venous disease has been demonstrated, as EIV can occur in patients with or without chronic venous insufficiency.5 Duplex ultrasound evaluation showed no venous reflux in our patient.
The pathophysiology of EIV remains unknown, but the concept of exercise-altered microcirculation has been proposed. Heat generated from exercise is normally dissipated by thermoregulatory mechanisms such as cutaneous vasodilation and sweat.1,6 When exercise is extended, done concomitantly in the heat, or performed in legs with preexisting edema or substantial adipose tissue that limit heat attenuation, the thermoregulatory capacity is overloaded and heat-induced muscle fiber breakdown occurs.1,7 Atrophy impairs the skeletal muscle’s ability to pump the increased venous return demanded by exercise to the heart, leading to backflow of venous return and eventual venous stasis.1 Reduction of venous return together with cutaneous vasodilation is thought to induce erythrocyte extravasation.
Histologic examination demonstrates features of leukocytoclastic vasculitis with perivascular lymphocytic and neutrophilic infiltrates.2 Erythrocyte extravasation, IgM deposits, and identification of C3 also have been reported.8,9 The spontaneous resolution of EIV has led to treatment efforts being focused on preventative measures. Several cases have reported some degree of success in preventing EIV with compression therapy, venoactive drugs, systemic steroids, and application of topical steroids before exercise.3
The clinical morphology and lower leg location of EIV leads to a common misdiagnosis of stasis dermatitis. Clinical history of a transient nature is the mainstay in the diagnosis of EIV, and ultrasound venous reflux study may be required in some cases. Preventative measures are superior to treatment and mainly include compression therapy.
- Ramelet AA. Exercise-induced vasculitis. J Eur Acad Dermatol Venereol. 2006;20:423-427.
- Kelly RI, Opie J, Nixon R. Golfer’s vasculitis. Australas J Dermatol. 2005;46:11-14.
- Ramelet AA. Exercise-induced purpura. Dermatology. 2004;208:293-296.
- Cushman D, Rydberg L. A general rehabilitation inpatient with exercise-induced vasculitis. PM R. 2013;5:900-902.
- Veraart JC, Prins M, Hulsmans RF, et al. Influence of endurance exercise on the venous refilling time of the leg. Phlebology. 1994;23:120-123.
- Noakes T. Fluid replacement during marathon running. Clin J Sport Med. 2003;13:309-318.
- Armstrong RB. Muscle damage and endurance events. Sports Med. 1986;3:370-381.
- Prins M, Veraart JC, Vermeulen AH, et al. Leucocytoclastic vasculitis induced by prolonged exercise. Br J Dermatol. 1996;134:915-918.
- Sagdeo A, Gormley RH, Wanat KA, et al. Purpuric eruption on the feet of a healthy young woman. “flip-flop vasculitis” (exercise-induced vasculitis). JAMA Dermatol. 2013;149:751-756.
To the Editor:
The transient and generic appearance of exercise-induced vasculitis (EIV) makes it a commonly misdiagnosed condition. The lesion often is only encountered through photographs brought by the patient or by taking a thorough history. The lack of findings on clinical inspection and the generic appearance of EIV may lead to misdiagnosis as stasis dermatitis due to its presentation as erythematous lesions on the medial lower legs.
A 68-year-old woman with no notable medical history was referred to our clinic for suspected stasis dermatitis. At presentation, no lesions were identified on the legs, but she brought photographs of an erythematous urticarial eruption on the medial lower legs, extending from just above the sock line to the mid-calves (Figure). The eruptions had occurred over the last 16 years, typically presenting suddenly after playing tennis or an extended period of walking and spontaneously resolving in 4 days. The lesions were painless, restricted to the calves, and were not pruritic, though the initial presentation 16 years prior included pruritic pigmented patches on the anterior thighs. Because the condition spontaneously improved within days, no treatment was attempted. An ultrasound venous reflux study ruled out venous reflux and stasis dermatitis.

Our patient stated that her 64-year-old sister had reported the same presentation over the last 8 years. Her physical activity was limited strictly to walking, and the lesions occurred after walking for many hours during the day in the heat, involving the medial aspects of the lower legs extending from the ankles to the full length of the calves. Her eruption was warm but was not painful or pruritic. It resolved spontaneously after 5 days with no therapy.
Our patient was advised to wear compression stockings as a preventative measure, but she did not adhere to these recommendations, stating it was impractical to wear compression garments while playing tennis.
Exercise-induced vasculitis most commonly is seen in the medial aspects of the lower extremities as an erythematous urticarial eruption or pigmented purpuric plaque rapidly occurring after a period of exercise.1,2 Lesions often are symmetric and can be pruritic and painful with a lack of systemic symptoms.3 These generic clinical manifestations may lead to a misdiagnosis of stasis dermatitis. One case report included initial treatment of presumptive cellulitis.4 Important clinical findings include a sparing of skin compressed by tight clothing such as socks, a lack of systemic symptoms, rapid appearance after exercise, and spontaneous resolution within a few days. No correlation with chronic venous disease has been demonstrated, as EIV can occur in patients with or without chronic venous insufficiency.5 Duplex ultrasound evaluation showed no venous reflux in our patient.
The pathophysiology of EIV remains unknown, but the concept of exercise-altered microcirculation has been proposed. Heat generated from exercise is normally dissipated by thermoregulatory mechanisms such as cutaneous vasodilation and sweat.1,6 When exercise is extended, done concomitantly in the heat, or performed in legs with preexisting edema or substantial adipose tissue that limit heat attenuation, the thermoregulatory capacity is overloaded and heat-induced muscle fiber breakdown occurs.1,7 Atrophy impairs the skeletal muscle’s ability to pump the increased venous return demanded by exercise to the heart, leading to backflow of venous return and eventual venous stasis.1 Reduction of venous return together with cutaneous vasodilation is thought to induce erythrocyte extravasation.
Histologic examination demonstrates features of leukocytoclastic vasculitis with perivascular lymphocytic and neutrophilic infiltrates.2 Erythrocyte extravasation, IgM deposits, and identification of C3 also have been reported.8,9 The spontaneous resolution of EIV has led to treatment efforts being focused on preventative measures. Several cases have reported some degree of success in preventing EIV with compression therapy, venoactive drugs, systemic steroids, and application of topical steroids before exercise.3
The clinical morphology and lower leg location of EIV leads to a common misdiagnosis of stasis dermatitis. Clinical history of a transient nature is the mainstay in the diagnosis of EIV, and ultrasound venous reflux study may be required in some cases. Preventative measures are superior to treatment and mainly include compression therapy.
To the Editor:
The transient and generic appearance of exercise-induced vasculitis (EIV) makes it a commonly misdiagnosed condition. The lesion often is only encountered through photographs brought by the patient or by taking a thorough history. The lack of findings on clinical inspection and the generic appearance of EIV may lead to misdiagnosis as stasis dermatitis due to its presentation as erythematous lesions on the medial lower legs.
A 68-year-old woman with no notable medical history was referred to our clinic for suspected stasis dermatitis. At presentation, no lesions were identified on the legs, but she brought photographs of an erythematous urticarial eruption on the medial lower legs, extending from just above the sock line to the mid-calves (Figure). The eruptions had occurred over the last 16 years, typically presenting suddenly after playing tennis or an extended period of walking and spontaneously resolving in 4 days. The lesions were painless, restricted to the calves, and were not pruritic, though the initial presentation 16 years prior included pruritic pigmented patches on the anterior thighs. Because the condition spontaneously improved within days, no treatment was attempted. An ultrasound venous reflux study ruled out venous reflux and stasis dermatitis.

Our patient stated that her 64-year-old sister had reported the same presentation over the last 8 years. Her physical activity was limited strictly to walking, and the lesions occurred after walking for many hours during the day in the heat, involving the medial aspects of the lower legs extending from the ankles to the full length of the calves. Her eruption was warm but was not painful or pruritic. It resolved spontaneously after 5 days with no therapy.
Our patient was advised to wear compression stockings as a preventative measure, but she did not adhere to these recommendations, stating it was impractical to wear compression garments while playing tennis.
Exercise-induced vasculitis most commonly is seen in the medial aspects of the lower extremities as an erythematous urticarial eruption or pigmented purpuric plaque rapidly occurring after a period of exercise.1,2 Lesions often are symmetric and can be pruritic and painful with a lack of systemic symptoms.3 These generic clinical manifestations may lead to a misdiagnosis of stasis dermatitis. One case report included initial treatment of presumptive cellulitis.4 Important clinical findings include a sparing of skin compressed by tight clothing such as socks, a lack of systemic symptoms, rapid appearance after exercise, and spontaneous resolution within a few days. No correlation with chronic venous disease has been demonstrated, as EIV can occur in patients with or without chronic venous insufficiency.5 Duplex ultrasound evaluation showed no venous reflux in our patient.
The pathophysiology of EIV remains unknown, but the concept of exercise-altered microcirculation has been proposed. Heat generated from exercise is normally dissipated by thermoregulatory mechanisms such as cutaneous vasodilation and sweat.1,6 When exercise is extended, done concomitantly in the heat, or performed in legs with preexisting edema or substantial adipose tissue that limit heat attenuation, the thermoregulatory capacity is overloaded and heat-induced muscle fiber breakdown occurs.1,7 Atrophy impairs the skeletal muscle’s ability to pump the increased venous return demanded by exercise to the heart, leading to backflow of venous return and eventual venous stasis.1 Reduction of venous return together with cutaneous vasodilation is thought to induce erythrocyte extravasation.
Histologic examination demonstrates features of leukocytoclastic vasculitis with perivascular lymphocytic and neutrophilic infiltrates.2 Erythrocyte extravasation, IgM deposits, and identification of C3 also have been reported.8,9 The spontaneous resolution of EIV has led to treatment efforts being focused on preventative measures. Several cases have reported some degree of success in preventing EIV with compression therapy, venoactive drugs, systemic steroids, and application of topical steroids before exercise.3
The clinical morphology and lower leg location of EIV leads to a common misdiagnosis of stasis dermatitis. Clinical history of a transient nature is the mainstay in the diagnosis of EIV, and ultrasound venous reflux study may be required in some cases. Preventative measures are superior to treatment and mainly include compression therapy.
- Ramelet AA. Exercise-induced vasculitis. J Eur Acad Dermatol Venereol. 2006;20:423-427.
- Kelly RI, Opie J, Nixon R. Golfer’s vasculitis. Australas J Dermatol. 2005;46:11-14.
- Ramelet AA. Exercise-induced purpura. Dermatology. 2004;208:293-296.
- Cushman D, Rydberg L. A general rehabilitation inpatient with exercise-induced vasculitis. PM R. 2013;5:900-902.
- Veraart JC, Prins M, Hulsmans RF, et al. Influence of endurance exercise on the venous refilling time of the leg. Phlebology. 1994;23:120-123.
- Noakes T. Fluid replacement during marathon running. Clin J Sport Med. 2003;13:309-318.
- Armstrong RB. Muscle damage and endurance events. Sports Med. 1986;3:370-381.
- Prins M, Veraart JC, Vermeulen AH, et al. Leucocytoclastic vasculitis induced by prolonged exercise. Br J Dermatol. 1996;134:915-918.
- Sagdeo A, Gormley RH, Wanat KA, et al. Purpuric eruption on the feet of a healthy young woman. “flip-flop vasculitis” (exercise-induced vasculitis). JAMA Dermatol. 2013;149:751-756.
- Ramelet AA. Exercise-induced vasculitis. J Eur Acad Dermatol Venereol. 2006;20:423-427.
- Kelly RI, Opie J, Nixon R. Golfer’s vasculitis. Australas J Dermatol. 2005;46:11-14.
- Ramelet AA. Exercise-induced purpura. Dermatology. 2004;208:293-296.
- Cushman D, Rydberg L. A general rehabilitation inpatient with exercise-induced vasculitis. PM R. 2013;5:900-902.
- Veraart JC, Prins M, Hulsmans RF, et al. Influence of endurance exercise on the venous refilling time of the leg. Phlebology. 1994;23:120-123.
- Noakes T. Fluid replacement during marathon running. Clin J Sport Med. 2003;13:309-318.
- Armstrong RB. Muscle damage and endurance events. Sports Med. 1986;3:370-381.
- Prins M, Veraart JC, Vermeulen AH, et al. Leucocytoclastic vasculitis induced by prolonged exercise. Br J Dermatol. 1996;134:915-918.
- Sagdeo A, Gormley RH, Wanat KA, et al. Purpuric eruption on the feet of a healthy young woman. “flip-flop vasculitis” (exercise-induced vasculitis). JAMA Dermatol. 2013;149:751-756.
Practice Points
- Clinical history of a transient nature is the mainstay in the diagnosis of exercise-induced vasculitis.
- Exercise-induced vasculitis largely is documented in photographs or by history and may be misdiagnosed as stasis dermatitis due to its clinical morphology and lower leg location.
- Dermatologists should be aware of this disorder and consider performing further workup to rule out stasis dermatitis and diagnose this mimic.
- Preventative measures are superior to treatment and mainly include compression therapy.
Unilateral Nail Clubbing in a Hemiparetic Patient
To the Editor:
Few cases of unilateral nail changes affecting only the hemiplegic side after a stroke have been reported. We present a case of acquired unilateral nail clubbing and longitudinal melanonychia in a hemiparetic patient.
A 79-year-old Black man with a history of smoking and stroke presented with concerns of discoloration of the fingernails. His medical history was notable for congestive heart failure; hypertension; diabetes mellitus; hypercholesterolemia; and stroke 11 years prior, which resulted in right-sided hemiparesis. Physical examination revealed longitudinal, even hyperpigmentation of several fingernails on the hands, in addition to whitening of the nail beds, sparing the tips (Terry nails). Clubbing was noted only on the fingernails of the right hand; the fingernails of the left hand exhibited normal curvature (Figure). Pulse oximetry was conducted and demonstrated the following readings: unaffected left index finger, 98%; unaffected left middle finger, 100%; affected right index finger, 95%; and affected right middle finger, 97%. The patient was diagnosed with benign longitudinal melanonychia secondary to ethnic variation, Terry nails without underlying anemia or hypoalbuminemic state, and unilateral right-sided clubbing of the fingernails in the setting of right-sided hemiparesis.
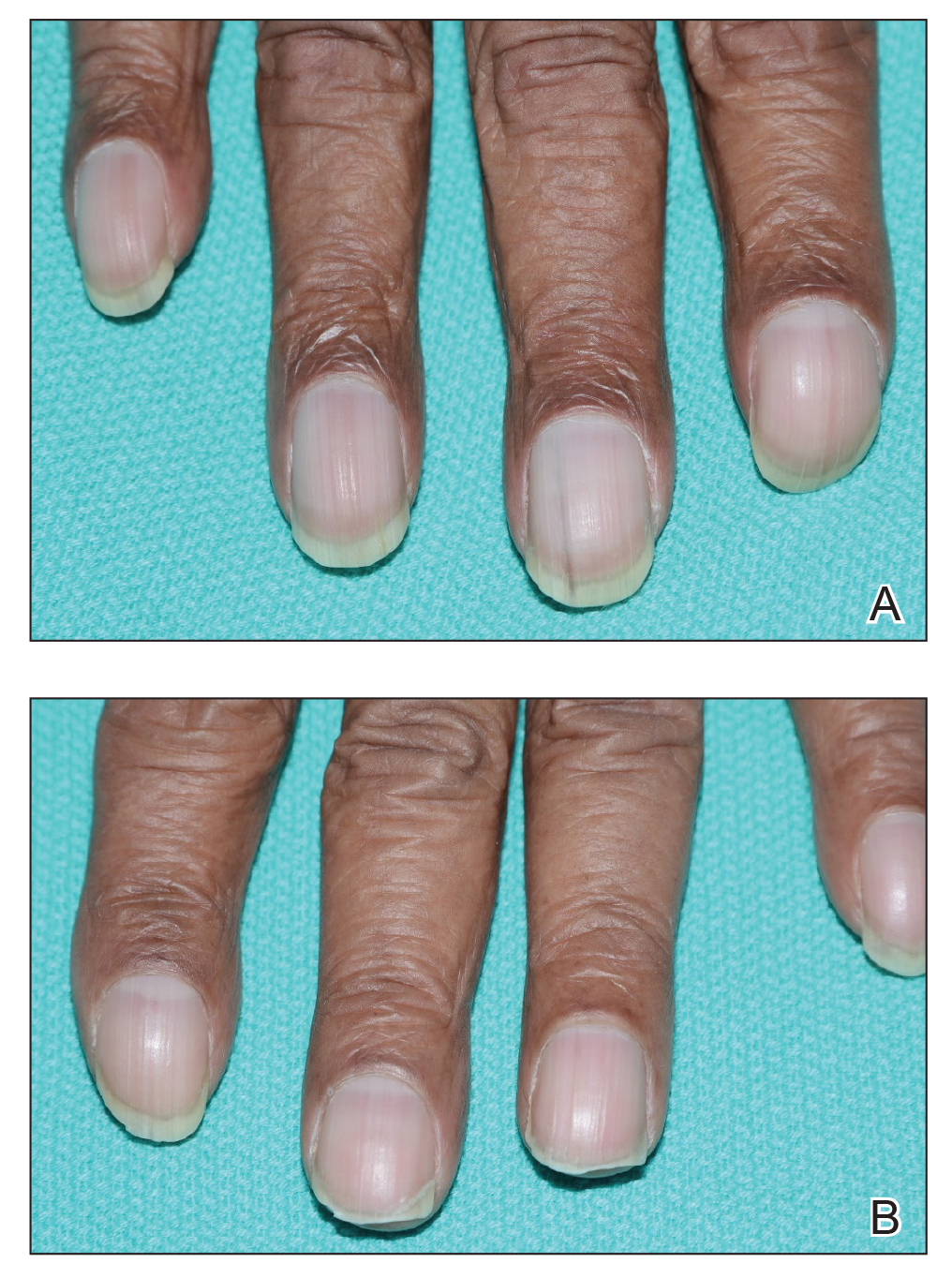
Prior reports have documented the occurrence of nail pathologies after stroke and affecting hemiplegic limbs. Unilateral digital nail clubbing following a stroke was first reported in 19751; 2 reports concluded clubbing developed in all digits affected by the stroke, and the severity of clubbing was associated with the duration of the stroke.1,2 One study noted longitudinal reddish striation, Neapolitan nails, and unilateral clubbing more commonly in hemiplegic patients.3 Longitudinal reddish striation was the most frequent condition observed in this population, always affecting the entire thumbnail of the hemiplegic limb.3 A similar report observed clubbing only on the fingernails of the hemiplegic side.4
Digital clubbing describes an exaggerated nail curvature and bulbous overgrowth of the fingertips due to an expansion of connective tissue between the nail plate and the nail bed.3,5 Clubbed fingers are found in various chronic conditions affecting the heart, lungs, and liver. Although the pathogenesis of clubbing remains unknown, many hypothesize that it is a state of proliferation in response to digital hypoxia.5 Fittingly, our patient exhibited a relative hypoperfusion of the clubbed fingers in comparison to the unaffected side.
This case provides additional support for the phenomenon of unilateral nail changes limited to hemiplegic or hemiparetic limbs. The unique presentation of longitudinal melanonychia, clubbing, and a lowered pulse oximetry reading only affecting the hemiparetic side demonstrates the possible connection between hypoxia and nail clubbing in this patient population.
- Denham M, Hodkinson H, Wright B. Unilateral clubbing in hemiplegia. Gerontology Clin (Basel). 1975;17:7-12.
- Alveraz A, McNair D, Wildman J, et al. Unilateral clubbing of the fingernails in patients with hemiplegia. Gerontology Clin (Basel). 1975;17:1-6.
- Siragusa M, Schepis C, Cosentino F, et al. Nail pathology in patients with hemiplegia. Br J Dermatol. 2001;144:557-560.
- Gül Ü, Çakmak S, Özel S, et al. Skin disorders in patients with hemiplegia and paraplegia. J Rehabil Med. 2009;41:681-683.
- Sarkar M, Mahesh D, Madabhavi I. Digital clubbing. Lung India. 2012;29:354-362.
To the Editor:
Few cases of unilateral nail changes affecting only the hemiplegic side after a stroke have been reported. We present a case of acquired unilateral nail clubbing and longitudinal melanonychia in a hemiparetic patient.
A 79-year-old Black man with a history of smoking and stroke presented with concerns of discoloration of the fingernails. His medical history was notable for congestive heart failure; hypertension; diabetes mellitus; hypercholesterolemia; and stroke 11 years prior, which resulted in right-sided hemiparesis. Physical examination revealed longitudinal, even hyperpigmentation of several fingernails on the hands, in addition to whitening of the nail beds, sparing the tips (Terry nails). Clubbing was noted only on the fingernails of the right hand; the fingernails of the left hand exhibited normal curvature (Figure). Pulse oximetry was conducted and demonstrated the following readings: unaffected left index finger, 98%; unaffected left middle finger, 100%; affected right index finger, 95%; and affected right middle finger, 97%. The patient was diagnosed with benign longitudinal melanonychia secondary to ethnic variation, Terry nails without underlying anemia or hypoalbuminemic state, and unilateral right-sided clubbing of the fingernails in the setting of right-sided hemiparesis.

Prior reports have documented the occurrence of nail pathologies after stroke and affecting hemiplegic limbs. Unilateral digital nail clubbing following a stroke was first reported in 19751; 2 reports concluded clubbing developed in all digits affected by the stroke, and the severity of clubbing was associated with the duration of the stroke.1,2 One study noted longitudinal reddish striation, Neapolitan nails, and unilateral clubbing more commonly in hemiplegic patients.3 Longitudinal reddish striation was the most frequent condition observed in this population, always affecting the entire thumbnail of the hemiplegic limb.3 A similar report observed clubbing only on the fingernails of the hemiplegic side.4
Digital clubbing describes an exaggerated nail curvature and bulbous overgrowth of the fingertips due to an expansion of connective tissue between the nail plate and the nail bed.3,5 Clubbed fingers are found in various chronic conditions affecting the heart, lungs, and liver. Although the pathogenesis of clubbing remains unknown, many hypothesize that it is a state of proliferation in response to digital hypoxia.5 Fittingly, our patient exhibited a relative hypoperfusion of the clubbed fingers in comparison to the unaffected side.
This case provides additional support for the phenomenon of unilateral nail changes limited to hemiplegic or hemiparetic limbs. The unique presentation of longitudinal melanonychia, clubbing, and a lowered pulse oximetry reading only affecting the hemiparetic side demonstrates the possible connection between hypoxia and nail clubbing in this patient population.
To the Editor:
Few cases of unilateral nail changes affecting only the hemiplegic side after a stroke have been reported. We present a case of acquired unilateral nail clubbing and longitudinal melanonychia in a hemiparetic patient.
A 79-year-old Black man with a history of smoking and stroke presented with concerns of discoloration of the fingernails. His medical history was notable for congestive heart failure; hypertension; diabetes mellitus; hypercholesterolemia; and stroke 11 years prior, which resulted in right-sided hemiparesis. Physical examination revealed longitudinal, even hyperpigmentation of several fingernails on the hands, in addition to whitening of the nail beds, sparing the tips (Terry nails). Clubbing was noted only on the fingernails of the right hand; the fingernails of the left hand exhibited normal curvature (Figure). Pulse oximetry was conducted and demonstrated the following readings: unaffected left index finger, 98%; unaffected left middle finger, 100%; affected right index finger, 95%; and affected right middle finger, 97%. The patient was diagnosed with benign longitudinal melanonychia secondary to ethnic variation, Terry nails without underlying anemia or hypoalbuminemic state, and unilateral right-sided clubbing of the fingernails in the setting of right-sided hemiparesis.

Prior reports have documented the occurrence of nail pathologies after stroke and affecting hemiplegic limbs. Unilateral digital nail clubbing following a stroke was first reported in 19751; 2 reports concluded clubbing developed in all digits affected by the stroke, and the severity of clubbing was associated with the duration of the stroke.1,2 One study noted longitudinal reddish striation, Neapolitan nails, and unilateral clubbing more commonly in hemiplegic patients.3 Longitudinal reddish striation was the most frequent condition observed in this population, always affecting the entire thumbnail of the hemiplegic limb.3 A similar report observed clubbing only on the fingernails of the hemiplegic side.4
Digital clubbing describes an exaggerated nail curvature and bulbous overgrowth of the fingertips due to an expansion of connective tissue between the nail plate and the nail bed.3,5 Clubbed fingers are found in various chronic conditions affecting the heart, lungs, and liver. Although the pathogenesis of clubbing remains unknown, many hypothesize that it is a state of proliferation in response to digital hypoxia.5 Fittingly, our patient exhibited a relative hypoperfusion of the clubbed fingers in comparison to the unaffected side.
This case provides additional support for the phenomenon of unilateral nail changes limited to hemiplegic or hemiparetic limbs. The unique presentation of longitudinal melanonychia, clubbing, and a lowered pulse oximetry reading only affecting the hemiparetic side demonstrates the possible connection between hypoxia and nail clubbing in this patient population.
- Denham M, Hodkinson H, Wright B. Unilateral clubbing in hemiplegia. Gerontology Clin (Basel). 1975;17:7-12.
- Alveraz A, McNair D, Wildman J, et al. Unilateral clubbing of the fingernails in patients with hemiplegia. Gerontology Clin (Basel). 1975;17:1-6.
- Siragusa M, Schepis C, Cosentino F, et al. Nail pathology in patients with hemiplegia. Br J Dermatol. 2001;144:557-560.
- Gül Ü, Çakmak S, Özel S, et al. Skin disorders in patients with hemiplegia and paraplegia. J Rehabil Med. 2009;41:681-683.
- Sarkar M, Mahesh D, Madabhavi I. Digital clubbing. Lung India. 2012;29:354-362.
- Denham M, Hodkinson H, Wright B. Unilateral clubbing in hemiplegia. Gerontology Clin (Basel). 1975;17:7-12.
- Alveraz A, McNair D, Wildman J, et al. Unilateral clubbing of the fingernails in patients with hemiplegia. Gerontology Clin (Basel). 1975;17:1-6.
- Siragusa M, Schepis C, Cosentino F, et al. Nail pathology in patients with hemiplegia. Br J Dermatol. 2001;144:557-560.
- Gül Ü, Çakmak S, Özel S, et al. Skin disorders in patients with hemiplegia and paraplegia. J Rehabil Med. 2009;41:681-683.
- Sarkar M, Mahesh D, Madabhavi I. Digital clubbing. Lung India. 2012;29:354-362.
Practice Points
- Unilateral nail changes can be limited to hemiplegic or hemiparetic limbs.
- Lowered pulse oximetry reading only affecting the hemiparetic side demonstrates the possible connection between hypoxia and nail clubbing in this patient population.