User login
Seen or viewed: A black hematologist’s perspective
After a long day in hematology clinic, I skimmed the inpatient list to see if any of my patients had been admitted. Seeing Ms. Short’s name (changed for privacy), a delightful African American woman I met during my early days of fellowship, had me making the trek to the hospital. She was living with multiple myeloma complicated by extramedullary manifestations that had significantly impacted her quality of life.
During our first encounter, she showed me a growing left subscapular mass the size of an orange that was erythematous, hot, painful, and irritated. As an enthusiastic first-year fellow, I wanted to be aggressive in addressing her concerns in response to her obvious distress about this mass. Ultimately, she left clinic with antibiotics and an appointment with radiation oncology to see if they could use radiation to shrink the subscapular mass.
When I went back in to discuss the plan with her, she grabbed my hand, looked me in my eyes and said: “Thank you, I’ve been mentioning this for a while and you’re the first person to get something done about it.” In that moment I knew that she felt seen.
By the time I made it over to the hospital, she was getting settled in her room to start another cycle of cytoreductive chemotherapy.
“I told them I had a Black doctor!” she exclaimed as I walked into her hospital room. “I was looking for you today in clinic ... I kept telling them I had a Black doctor, but the nurses kept telling me no, that there were only Black nurse practitioners.” She had repeatedly told the staff that I, her “Black doctor,” did indeed exist, and she went on to describe me as “you know, the [heavy-chested] and short Black doctor I saw early this fall.” To this day, her description still makes me chuckle.
Though I laughed at her description, it hurt that I had worked in a clinic for 6 months yet was invisible. Initially disappointed, I left Ms. Short’s room with a smile on my face, energized and encouraged.
My time with Ms. Short prompted me to ruminate on my experience as a Black physician. To put it in perspective, 5% of all physicians are Black, 2% are Black women, and 2.3% are oncologists, even though African Americans make up 13% of the general U.S. population. I reside in a space where I am simultaneously scrutinized because I am one of the few (or the only) Black physicians in the building, and yet I am invisible because my colleagues and coworkers routinely ignore my presence.
Black physicians, let alone hematologists, are so rare that nurses often cannot fathom that a Black woman could be more than a nurse practitioner. Sadly, this is the tip of the iceberg of some of the negative experiences I, and other Black doctors, have had.
How I present myself must be carefully curated to make progress in my career. My peers and superiors seem to hear me better when my hair is straight and not in its naturally curly state. My introversion has been interpreted as being standoffish or disinterested. Any tone other than happy is interpreted as “aggressive” or “angry”. Talking “too much” to Black support staff was reported to my program, as it was viewed as suspicious, disruptive, and “appearances matter”.
I am also expected to be nurturing in ways that White physicians are not required to be. In my presence, White physicians have denigrated an entire patient population that is disproportionately Black by calling them “sicklers.” If there is an interpersonal conflict, I must think about the long-term consequences of voicing my perspective. My non-Black colleagues do not have to think about these things.
Imagine dealing with this at work, then on your commute home being worried about the reality that you may be pulled over and become the next name on the ever-growing list of Black women and men murdered at the hands of police. The cognitive and emotional impact of being invisible is immense and cumulative over the years.
My Blackness creates a bias of inferiority that cannot be overcome by respectability, compliance, professionalism, training, and expertise. This is glaringly apparent on both sides of the physician-patient relationship. Black patients’ concerns are routinely overlooked and dismissed, as seen with Ms. Short, and are reflected in the Black maternal death rate, pain control in Black versus White patients, and personal experience as a patient and an advocate for my family members.
Patients have looked me in the face and said, “all lives matter,” displaying their refusal to recognize that systematic racism and inequality exist. These facts and experiences are the antithesis of “primum non nocere.”
Sadly, my and Ms. Short’s experiences are not singular ones, and racial bias in medicine is a diagnosed, but untreated cancer. Like the malignancies I treat, ignoring the problem has not made it go away; therefore, it continues to fester and spread, causing more destruction. It is of great importance and concern that all physicians recognize, reflect, and correct their implicit biases not only toward their patients, but also colleagues and trainees.
It seems that health care professionals can talk the talk, as many statements have been made against racism and implicit bias in medicine, but can we take true and meaningful action to begin the journey to equity and justice?
I would like to thank Adrienne Glover, MD, MaKenzie Hodge, MD, Maranatha McLean, MD, and Darion Showell, MD, for our stimulating conversations that helped me put pen to paper. I’d also like to thank my family for being my editors.
Daphanie D. Taylor, MD, is a hematology/oncology fellow PGY-6 at Levine Cancer Institute, Charlotte, N.C.
References and further reading
Roy L. “‘It’s My Calling To Change The Statistics’: Why We Need More Black Female Physicians.” Forbes Magazine, 27 Feb. 2020.
“Diversity in Medicine: Facts and Figures 2019.” Association of American Medical Colleges, 2019.
“Facts & Figures: Diversity in Oncology.” American Society of Clinical Oncology. 2020 Jan 16.
After a long day in hematology clinic, I skimmed the inpatient list to see if any of my patients had been admitted. Seeing Ms. Short’s name (changed for privacy), a delightful African American woman I met during my early days of fellowship, had me making the trek to the hospital. She was living with multiple myeloma complicated by extramedullary manifestations that had significantly impacted her quality of life.
During our first encounter, she showed me a growing left subscapular mass the size of an orange that was erythematous, hot, painful, and irritated. As an enthusiastic first-year fellow, I wanted to be aggressive in addressing her concerns in response to her obvious distress about this mass. Ultimately, she left clinic with antibiotics and an appointment with radiation oncology to see if they could use radiation to shrink the subscapular mass.
When I went back in to discuss the plan with her, she grabbed my hand, looked me in my eyes and said: “Thank you, I’ve been mentioning this for a while and you’re the first person to get something done about it.” In that moment I knew that she felt seen.
By the time I made it over to the hospital, she was getting settled in her room to start another cycle of cytoreductive chemotherapy.
“I told them I had a Black doctor!” she exclaimed as I walked into her hospital room. “I was looking for you today in clinic ... I kept telling them I had a Black doctor, but the nurses kept telling me no, that there were only Black nurse practitioners.” She had repeatedly told the staff that I, her “Black doctor,” did indeed exist, and she went on to describe me as “you know, the [heavy-chested] and short Black doctor I saw early this fall.” To this day, her description still makes me chuckle.
Though I laughed at her description, it hurt that I had worked in a clinic for 6 months yet was invisible. Initially disappointed, I left Ms. Short’s room with a smile on my face, energized and encouraged.
My time with Ms. Short prompted me to ruminate on my experience as a Black physician. To put it in perspective, 5% of all physicians are Black, 2% are Black women, and 2.3% are oncologists, even though African Americans make up 13% of the general U.S. population. I reside in a space where I am simultaneously scrutinized because I am one of the few (or the only) Black physicians in the building, and yet I am invisible because my colleagues and coworkers routinely ignore my presence.
Black physicians, let alone hematologists, are so rare that nurses often cannot fathom that a Black woman could be more than a nurse practitioner. Sadly, this is the tip of the iceberg of some of the negative experiences I, and other Black doctors, have had.
How I present myself must be carefully curated to make progress in my career. My peers and superiors seem to hear me better when my hair is straight and not in its naturally curly state. My introversion has been interpreted as being standoffish or disinterested. Any tone other than happy is interpreted as “aggressive” or “angry”. Talking “too much” to Black support staff was reported to my program, as it was viewed as suspicious, disruptive, and “appearances matter”.
I am also expected to be nurturing in ways that White physicians are not required to be. In my presence, White physicians have denigrated an entire patient population that is disproportionately Black by calling them “sicklers.” If there is an interpersonal conflict, I must think about the long-term consequences of voicing my perspective. My non-Black colleagues do not have to think about these things.
Imagine dealing with this at work, then on your commute home being worried about the reality that you may be pulled over and become the next name on the ever-growing list of Black women and men murdered at the hands of police. The cognitive and emotional impact of being invisible is immense and cumulative over the years.
My Blackness creates a bias of inferiority that cannot be overcome by respectability, compliance, professionalism, training, and expertise. This is glaringly apparent on both sides of the physician-patient relationship. Black patients’ concerns are routinely overlooked and dismissed, as seen with Ms. Short, and are reflected in the Black maternal death rate, pain control in Black versus White patients, and personal experience as a patient and an advocate for my family members.
Patients have looked me in the face and said, “all lives matter,” displaying their refusal to recognize that systematic racism and inequality exist. These facts and experiences are the antithesis of “primum non nocere.”
Sadly, my and Ms. Short’s experiences are not singular ones, and racial bias in medicine is a diagnosed, but untreated cancer. Like the malignancies I treat, ignoring the problem has not made it go away; therefore, it continues to fester and spread, causing more destruction. It is of great importance and concern that all physicians recognize, reflect, and correct their implicit biases not only toward their patients, but also colleagues and trainees.
It seems that health care professionals can talk the talk, as many statements have been made against racism and implicit bias in medicine, but can we take true and meaningful action to begin the journey to equity and justice?
I would like to thank Adrienne Glover, MD, MaKenzie Hodge, MD, Maranatha McLean, MD, and Darion Showell, MD, for our stimulating conversations that helped me put pen to paper. I’d also like to thank my family for being my editors.
Daphanie D. Taylor, MD, is a hematology/oncology fellow PGY-6 at Levine Cancer Institute, Charlotte, N.C.
References and further reading
Roy L. “‘It’s My Calling To Change The Statistics’: Why We Need More Black Female Physicians.” Forbes Magazine, 27 Feb. 2020.
“Diversity in Medicine: Facts and Figures 2019.” Association of American Medical Colleges, 2019.
“Facts & Figures: Diversity in Oncology.” American Society of Clinical Oncology. 2020 Jan 16.
After a long day in hematology clinic, I skimmed the inpatient list to see if any of my patients had been admitted. Seeing Ms. Short’s name (changed for privacy), a delightful African American woman I met during my early days of fellowship, had me making the trek to the hospital. She was living with multiple myeloma complicated by extramedullary manifestations that had significantly impacted her quality of life.
During our first encounter, she showed me a growing left subscapular mass the size of an orange that was erythematous, hot, painful, and irritated. As an enthusiastic first-year fellow, I wanted to be aggressive in addressing her concerns in response to her obvious distress about this mass. Ultimately, she left clinic with antibiotics and an appointment with radiation oncology to see if they could use radiation to shrink the subscapular mass.
When I went back in to discuss the plan with her, she grabbed my hand, looked me in my eyes and said: “Thank you, I’ve been mentioning this for a while and you’re the first person to get something done about it.” In that moment I knew that she felt seen.
By the time I made it over to the hospital, she was getting settled in her room to start another cycle of cytoreductive chemotherapy.
“I told them I had a Black doctor!” she exclaimed as I walked into her hospital room. “I was looking for you today in clinic ... I kept telling them I had a Black doctor, but the nurses kept telling me no, that there were only Black nurse practitioners.” She had repeatedly told the staff that I, her “Black doctor,” did indeed exist, and she went on to describe me as “you know, the [heavy-chested] and short Black doctor I saw early this fall.” To this day, her description still makes me chuckle.
Though I laughed at her description, it hurt that I had worked in a clinic for 6 months yet was invisible. Initially disappointed, I left Ms. Short’s room with a smile on my face, energized and encouraged.
My time with Ms. Short prompted me to ruminate on my experience as a Black physician. To put it in perspective, 5% of all physicians are Black, 2% are Black women, and 2.3% are oncologists, even though African Americans make up 13% of the general U.S. population. I reside in a space where I am simultaneously scrutinized because I am one of the few (or the only) Black physicians in the building, and yet I am invisible because my colleagues and coworkers routinely ignore my presence.
Black physicians, let alone hematologists, are so rare that nurses often cannot fathom that a Black woman could be more than a nurse practitioner. Sadly, this is the tip of the iceberg of some of the negative experiences I, and other Black doctors, have had.
How I present myself must be carefully curated to make progress in my career. My peers and superiors seem to hear me better when my hair is straight and not in its naturally curly state. My introversion has been interpreted as being standoffish or disinterested. Any tone other than happy is interpreted as “aggressive” or “angry”. Talking “too much” to Black support staff was reported to my program, as it was viewed as suspicious, disruptive, and “appearances matter”.
I am also expected to be nurturing in ways that White physicians are not required to be. In my presence, White physicians have denigrated an entire patient population that is disproportionately Black by calling them “sicklers.” If there is an interpersonal conflict, I must think about the long-term consequences of voicing my perspective. My non-Black colleagues do not have to think about these things.
Imagine dealing with this at work, then on your commute home being worried about the reality that you may be pulled over and become the next name on the ever-growing list of Black women and men murdered at the hands of police. The cognitive and emotional impact of being invisible is immense and cumulative over the years.
My Blackness creates a bias of inferiority that cannot be overcome by respectability, compliance, professionalism, training, and expertise. This is glaringly apparent on both sides of the physician-patient relationship. Black patients’ concerns are routinely overlooked and dismissed, as seen with Ms. Short, and are reflected in the Black maternal death rate, pain control in Black versus White patients, and personal experience as a patient and an advocate for my family members.
Patients have looked me in the face and said, “all lives matter,” displaying their refusal to recognize that systematic racism and inequality exist. These facts and experiences are the antithesis of “primum non nocere.”
Sadly, my and Ms. Short’s experiences are not singular ones, and racial bias in medicine is a diagnosed, but untreated cancer. Like the malignancies I treat, ignoring the problem has not made it go away; therefore, it continues to fester and spread, causing more destruction. It is of great importance and concern that all physicians recognize, reflect, and correct their implicit biases not only toward their patients, but also colleagues and trainees.
It seems that health care professionals can talk the talk, as many statements have been made against racism and implicit bias in medicine, but can we take true and meaningful action to begin the journey to equity and justice?
I would like to thank Adrienne Glover, MD, MaKenzie Hodge, MD, Maranatha McLean, MD, and Darion Showell, MD, for our stimulating conversations that helped me put pen to paper. I’d also like to thank my family for being my editors.
Daphanie D. Taylor, MD, is a hematology/oncology fellow PGY-6 at Levine Cancer Institute, Charlotte, N.C.
References and further reading
Roy L. “‘It’s My Calling To Change The Statistics’: Why We Need More Black Female Physicians.” Forbes Magazine, 27 Feb. 2020.
“Diversity in Medicine: Facts and Figures 2019.” Association of American Medical Colleges, 2019.
“Facts & Figures: Diversity in Oncology.” American Society of Clinical Oncology. 2020 Jan 16.
What Tom Brady and Patrick Mahomes can teach us about physicians
Warning: This article will be about Tom Brady. If you love Tom Brady, hate Tom Brady, previously loved and now hate Tom Brady, I’m just warning you so you’ll be in the right frame of mind to continue. (If you don’t know who Tom Brady is, he’s Gisele’s husband).
Brady, who plays for the Tampa Bay Buccaneers, has played in the NFL for 21 seasons, an unbelievable number given the average career for a quarterback is 3 years. He’s 43 years old and was the oldest player in a Super Bowl, ever. He faced Patrick Mahomes, the quarterback for the opposing Kansas City Chiefs. Mahomes is one of the most athletic and talented quarterbacks of all time, and Mahomes is nearly 20 years younger than Brady. Yet, in a shot heard around the NFL world, Brady won.
But, was a Brady victory so shocking? Hot-shot residents may have a lot of moxie and talent, but experienced doctors often prevail by simply making sound decisions and avoiding mistakes. In our department, we’ve been discussing this lately: We’re hiring two dermatologists and we’re fortunate to have some amazing candidates apply. Some, like Mahomes, are young all-stars with outstanding ability and potential, right out of residency. Others, Brady-like, have been in practice for years and are ready to move to a new franchise.
Our medical group’s experiences are probably similar to many practices: New physicians out of residency often bring energy, inspiration, and ease with the latest therapies, devices, and surgical techniques. Yet, they sometimes struggle with efficiency and unforced errors. Experienced physicians might not know what’s hot, but they can often see where the best course of action lies, understanding not only the physiology but also the patient in ways that only experience can teach you. Fortunately, for those like me who’ve crossed midlife, there doesn’t seem to be an upper limit to experience – it is possible to keep getting better. Yes, I’m just like Tom Brady. (I wrote this article just to print that line.)
Some of the best doctors I’ve ever seen in action were emeritus physicians. In medical school at Wake Forest University, one of my professors was Dr. Eben Alexander. A retired neurosurgeon, he taught a case-based critical thinking skills class. I recall his brilliant insight and coaching, working through cases that had nothing to do with the brain or with surgery. He used his vast experience and wisdom to teach us how to practice medicine. He was, at that time, nearly 90 years old. Despite having been retired for decades, he was still writing articles and editing journals. He was inspiring. For a minute, he had me thinking I’d like to be a neurosurgeon, so I could be just like Eben Alexander. I did not, but I learned things from him that still impact my practice as a dermatologist today.
I’m sure you’ve had similar experiences of older colleagues or mentors who were the best doctor in the clinic or the O.R. They are the Dr. Anthony Faucis, not just practicing, but leading while in their 8th or 9th decade. We are all so fortunate that they keep playing.
We’ve not made our final choices on whom to hire, but with two positions, I expect we’ll choose both a young doctor and an experienced one to add to our team. It will be fun to watch and learn from them. Just like it will be fun to watch Tom Brady in the Super Bowl again next year.
Dr. Benabio is director of Healthcare Transformation and chief of dermatology at Kaiser Permanente San Diego. The opinions expressed in this column are his own and do not represent those of Kaiser Permanente. Dr. Benabio is @Dermdoc on Twitter. Write to him at [email protected].
Warning: This article will be about Tom Brady. If you love Tom Brady, hate Tom Brady, previously loved and now hate Tom Brady, I’m just warning you so you’ll be in the right frame of mind to continue. (If you don’t know who Tom Brady is, he’s Gisele’s husband).
Brady, who plays for the Tampa Bay Buccaneers, has played in the NFL for 21 seasons, an unbelievable number given the average career for a quarterback is 3 years. He’s 43 years old and was the oldest player in a Super Bowl, ever. He faced Patrick Mahomes, the quarterback for the opposing Kansas City Chiefs. Mahomes is one of the most athletic and talented quarterbacks of all time, and Mahomes is nearly 20 years younger than Brady. Yet, in a shot heard around the NFL world, Brady won.
But, was a Brady victory so shocking? Hot-shot residents may have a lot of moxie and talent, but experienced doctors often prevail by simply making sound decisions and avoiding mistakes. In our department, we’ve been discussing this lately: We’re hiring two dermatologists and we’re fortunate to have some amazing candidates apply. Some, like Mahomes, are young all-stars with outstanding ability and potential, right out of residency. Others, Brady-like, have been in practice for years and are ready to move to a new franchise.
Our medical group’s experiences are probably similar to many practices: New physicians out of residency often bring energy, inspiration, and ease with the latest therapies, devices, and surgical techniques. Yet, they sometimes struggle with efficiency and unforced errors. Experienced physicians might not know what’s hot, but they can often see where the best course of action lies, understanding not only the physiology but also the patient in ways that only experience can teach you. Fortunately, for those like me who’ve crossed midlife, there doesn’t seem to be an upper limit to experience – it is possible to keep getting better. Yes, I’m just like Tom Brady. (I wrote this article just to print that line.)
Some of the best doctors I’ve ever seen in action were emeritus physicians. In medical school at Wake Forest University, one of my professors was Dr. Eben Alexander. A retired neurosurgeon, he taught a case-based critical thinking skills class. I recall his brilliant insight and coaching, working through cases that had nothing to do with the brain or with surgery. He used his vast experience and wisdom to teach us how to practice medicine. He was, at that time, nearly 90 years old. Despite having been retired for decades, he was still writing articles and editing journals. He was inspiring. For a minute, he had me thinking I’d like to be a neurosurgeon, so I could be just like Eben Alexander. I did not, but I learned things from him that still impact my practice as a dermatologist today.
I’m sure you’ve had similar experiences of older colleagues or mentors who were the best doctor in the clinic or the O.R. They are the Dr. Anthony Faucis, not just practicing, but leading while in their 8th or 9th decade. We are all so fortunate that they keep playing.
We’ve not made our final choices on whom to hire, but with two positions, I expect we’ll choose both a young doctor and an experienced one to add to our team. It will be fun to watch and learn from them. Just like it will be fun to watch Tom Brady in the Super Bowl again next year.
Dr. Benabio is director of Healthcare Transformation and chief of dermatology at Kaiser Permanente San Diego. The opinions expressed in this column are his own and do not represent those of Kaiser Permanente. Dr. Benabio is @Dermdoc on Twitter. Write to him at [email protected].
Warning: This article will be about Tom Brady. If you love Tom Brady, hate Tom Brady, previously loved and now hate Tom Brady, I’m just warning you so you’ll be in the right frame of mind to continue. (If you don’t know who Tom Brady is, he’s Gisele’s husband).
Brady, who plays for the Tampa Bay Buccaneers, has played in the NFL for 21 seasons, an unbelievable number given the average career for a quarterback is 3 years. He’s 43 years old and was the oldest player in a Super Bowl, ever. He faced Patrick Mahomes, the quarterback for the opposing Kansas City Chiefs. Mahomes is one of the most athletic and talented quarterbacks of all time, and Mahomes is nearly 20 years younger than Brady. Yet, in a shot heard around the NFL world, Brady won.
But, was a Brady victory so shocking? Hot-shot residents may have a lot of moxie and talent, but experienced doctors often prevail by simply making sound decisions and avoiding mistakes. In our department, we’ve been discussing this lately: We’re hiring two dermatologists and we’re fortunate to have some amazing candidates apply. Some, like Mahomes, are young all-stars with outstanding ability and potential, right out of residency. Others, Brady-like, have been in practice for years and are ready to move to a new franchise.
Our medical group’s experiences are probably similar to many practices: New physicians out of residency often bring energy, inspiration, and ease with the latest therapies, devices, and surgical techniques. Yet, they sometimes struggle with efficiency and unforced errors. Experienced physicians might not know what’s hot, but they can often see where the best course of action lies, understanding not only the physiology but also the patient in ways that only experience can teach you. Fortunately, for those like me who’ve crossed midlife, there doesn’t seem to be an upper limit to experience – it is possible to keep getting better. Yes, I’m just like Tom Brady. (I wrote this article just to print that line.)
Some of the best doctors I’ve ever seen in action were emeritus physicians. In medical school at Wake Forest University, one of my professors was Dr. Eben Alexander. A retired neurosurgeon, he taught a case-based critical thinking skills class. I recall his brilliant insight and coaching, working through cases that had nothing to do with the brain or with surgery. He used his vast experience and wisdom to teach us how to practice medicine. He was, at that time, nearly 90 years old. Despite having been retired for decades, he was still writing articles and editing journals. He was inspiring. For a minute, he had me thinking I’d like to be a neurosurgeon, so I could be just like Eben Alexander. I did not, but I learned things from him that still impact my practice as a dermatologist today.
I’m sure you’ve had similar experiences of older colleagues or mentors who were the best doctor in the clinic or the O.R. They are the Dr. Anthony Faucis, not just practicing, but leading while in their 8th or 9th decade. We are all so fortunate that they keep playing.
We’ve not made our final choices on whom to hire, but with two positions, I expect we’ll choose both a young doctor and an experienced one to add to our team. It will be fun to watch and learn from them. Just like it will be fun to watch Tom Brady in the Super Bowl again next year.
Dr. Benabio is director of Healthcare Transformation and chief of dermatology at Kaiser Permanente San Diego. The opinions expressed in this column are his own and do not represent those of Kaiser Permanente. Dr. Benabio is @Dermdoc on Twitter. Write to him at [email protected].
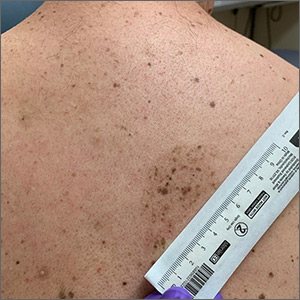
Cluster of hyperpigmented spots
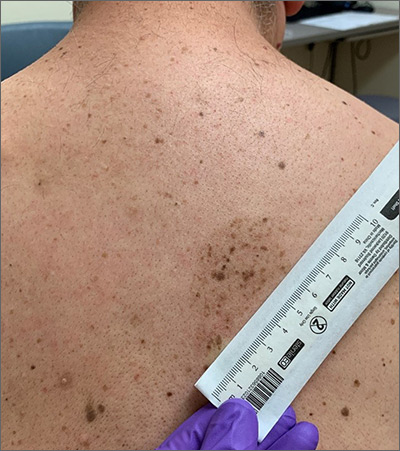
A large hyperpigmented patch with overlying darker macules and papules is characteristic of a speckled lentiginous nevus (SLN), also called a nevus spilus.
SLN is a cafe-au-lait˗like nevus that initially appears with a hyperpigmented background, usually at or around birth. Later, a speckled or polka-dot pattern of dark macules and papules appears over time. SLN is believed to be a form of congenital melanocytic nevus. There are believed to be 2 subtypes of SLN: nevus spilus maculosus and nevus spilus papulosus.
The maculosus subtype is characterized by flat and evenly distributed macules, that look like polka-dots. Histopathology reveals elongated interpapillary ridges containing increased numbers of melanocytes that form nests at the dermo-epidermal junction.
The papulosus subtype (which this patient had) is differentiated by superimposed speckles and papules whose size and distribution vary; this subype looks similar to a starry sky. Histopathology of the papulosus subtype shows melanocytic nevi of either the dermal or compound type—hence the papular appearance.
Given that SLN is a congenital melanocytic nevus, there is a small risk of transformation to malignant melanoma. The papulosus subtype is believed to have a more dynamic course with more lesions appearing over time. The maculosus subtype is considered to have a slightly higher risk of transformation into malignant melanoma compared to the papulosus subtype.
It is important to recognize that SLN is a distinct clinical entity, rather than a large irregular nevus. Mistaking it for a large suspicious nevus would require multiple biopsies of the most suspicious areas or excision of the entire lesion. Treatment for SLN includes serial surveillance with biopsy or excision of any suspicious areas that arise. In this case, the patient did not have any areas warranting biopsy, so the plan was to have him followed with annual clinical surveillance.
Photo and text courtesy of Erik Unruh, MD, MPH, and Daniel Stulberg, MD, FAAFP, Department of Family and Community Medicine, University of New Mexico School of Medicine, Albuquerque.
Happl, R. Speckled lentiginous naevus: which of the two disorders do you mean? Clin Exp Dermatol. 2009;34:133-135. doi: 10.1111/j.1365-2230.2008.02966.x.

A large hyperpigmented patch with overlying darker macules and papules is characteristic of a speckled lentiginous nevus (SLN), also called a nevus spilus.
SLN is a cafe-au-lait˗like nevus that initially appears with a hyperpigmented background, usually at or around birth. Later, a speckled or polka-dot pattern of dark macules and papules appears over time. SLN is believed to be a form of congenital melanocytic nevus. There are believed to be 2 subtypes of SLN: nevus spilus maculosus and nevus spilus papulosus.
The maculosus subtype is characterized by flat and evenly distributed macules, that look like polka-dots. Histopathology reveals elongated interpapillary ridges containing increased numbers of melanocytes that form nests at the dermo-epidermal junction.
The papulosus subtype (which this patient had) is differentiated by superimposed speckles and papules whose size and distribution vary; this subype looks similar to a starry sky. Histopathology of the papulosus subtype shows melanocytic nevi of either the dermal or compound type—hence the papular appearance.
Given that SLN is a congenital melanocytic nevus, there is a small risk of transformation to malignant melanoma. The papulosus subtype is believed to have a more dynamic course with more lesions appearing over time. The maculosus subtype is considered to have a slightly higher risk of transformation into malignant melanoma compared to the papulosus subtype.
It is important to recognize that SLN is a distinct clinical entity, rather than a large irregular nevus. Mistaking it for a large suspicious nevus would require multiple biopsies of the most suspicious areas or excision of the entire lesion. Treatment for SLN includes serial surveillance with biopsy or excision of any suspicious areas that arise. In this case, the patient did not have any areas warranting biopsy, so the plan was to have him followed with annual clinical surveillance.
Photo and text courtesy of Erik Unruh, MD, MPH, and Daniel Stulberg, MD, FAAFP, Department of Family and Community Medicine, University of New Mexico School of Medicine, Albuquerque.

A large hyperpigmented patch with overlying darker macules and papules is characteristic of a speckled lentiginous nevus (SLN), also called a nevus spilus.
SLN is a cafe-au-lait˗like nevus that initially appears with a hyperpigmented background, usually at or around birth. Later, a speckled or polka-dot pattern of dark macules and papules appears over time. SLN is believed to be a form of congenital melanocytic nevus. There are believed to be 2 subtypes of SLN: nevus spilus maculosus and nevus spilus papulosus.
The maculosus subtype is characterized by flat and evenly distributed macules, that look like polka-dots. Histopathology reveals elongated interpapillary ridges containing increased numbers of melanocytes that form nests at the dermo-epidermal junction.
The papulosus subtype (which this patient had) is differentiated by superimposed speckles and papules whose size and distribution vary; this subype looks similar to a starry sky. Histopathology of the papulosus subtype shows melanocytic nevi of either the dermal or compound type—hence the papular appearance.
Given that SLN is a congenital melanocytic nevus, there is a small risk of transformation to malignant melanoma. The papulosus subtype is believed to have a more dynamic course with more lesions appearing over time. The maculosus subtype is considered to have a slightly higher risk of transformation into malignant melanoma compared to the papulosus subtype.
It is important to recognize that SLN is a distinct clinical entity, rather than a large irregular nevus. Mistaking it for a large suspicious nevus would require multiple biopsies of the most suspicious areas or excision of the entire lesion. Treatment for SLN includes serial surveillance with biopsy or excision of any suspicious areas that arise. In this case, the patient did not have any areas warranting biopsy, so the plan was to have him followed with annual clinical surveillance.
Photo and text courtesy of Erik Unruh, MD, MPH, and Daniel Stulberg, MD, FAAFP, Department of Family and Community Medicine, University of New Mexico School of Medicine, Albuquerque.
Happl, R. Speckled lentiginous naevus: which of the two disorders do you mean? Clin Exp Dermatol. 2009;34:133-135. doi: 10.1111/j.1365-2230.2008.02966.x.
Happl, R. Speckled lentiginous naevus: which of the two disorders do you mean? Clin Exp Dermatol. 2009;34:133-135. doi: 10.1111/j.1365-2230.2008.02966.x.
When the X-Waiver gets X’ed: Implications for hospitalists
There are two pandemics permeating the United States: COVID-19 and addiction. To date, more than 468,000 people have died from COVID-19 in the U.S. In the 12-month period ending in May 2020, over 80,000 died from a drug related cause – the highest number ever recorded in a year. Many of these deaths involved opioids.
COVID-19 has worsened outcomes for people with addiction. There is less access to treatment, increased isolation, and worsening psychosocial and economic stressors. These factors may drive new, increased, or more risky substance use and return to use for people in recovery. As hospitalists, we have been responders in both COVID-19 and our country’s worsening overdose and addiction crisis.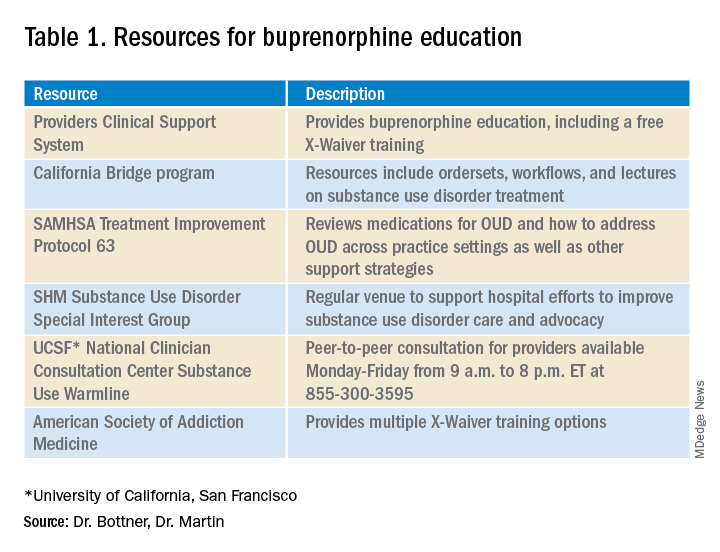
In December 2020’s Journal of Hospital Medicine article “Converging Crises: Caring for hospitalized adults with substance use disorder in the time of COVID-19”, Dr. Honora Englander and her coauthors called on hospitalists to actively engage patients with substance use disorders during hospitalization. The article highlights the colliding crises of addiction and COVID-19 and provides eight practical approaches for hospitalists to address substance use disorders during the pandemic, including initiating buprenorphine for opioid withdrawal and prescribing it for opioid use disorder (OUD) treatment.
Buprenorphine effectively treats opioid withdrawal, reduces OUD-related mortality, and decreases hospital readmissions related to OUD. To prescribe buprenorphine for OUD in the outpatient setting or on hospital discharge, providers need an X-Waiver. The X-Waiver is a result of the Drug Addiction Treatment Act 2000 (DATA 2000), which was enacted in 2000. It permits physicians to prescribe buprenorphine for OUD treatment after an 8-hour training. In 2016, the Comprehensive Addiction and Recovery Act extended buprenorphine prescribing to physician assistants (PAs) and advanced-practice nurses (APNs). However, PAs and APNs are required to complete a 24-hour training to receive the waiver.
On Jan. 14, 2021, the U.S. Department of Health and Human Services under the Trump administration announced it was removing the X-Waiver training previously required for physicians to prescribe this life-saving medication. However, on Jan. 20, 2021, the Biden administration froze the training requirement removal pending a 60-day review. The excitement about the waiver’s eradication further dampened on Jan. 25, when the plan was halted due to procedural factors coupled with the concern that HHS may not have the authority to void requirements mandated by Congress.
Many of us continue to be hopeful that the X-Waiver will soon be gone. The Substance Abuse and Mental Health Services Administration has committed to working with federal agencies to increase access to buprenorphine. The Biden administration also committed to addressing our country’s addiction crisis, including a plan to “make effective prevention, treatment, and recovery services available to all, including through a $125 billion federal investment.”
Despite the pause on HHS’s recent attempt to “X the X-Waiver,” we now have renewed attention and interest in this critical issue and an opportunity for greater and longer-lasting legislative impact. SHM supports that Congress repeal the legislative requirement for buprenorphine training dictated by DATA 2000 so that it cannot be rolled back by future administrations. To further increase access to buprenorphine treatment, the training requirement should be removed for all providers who care for individuals with OUD.
The X-Waiver has been a barrier to hospitalist adoption of this critical, life-saving medication. HHS’s stance to nix the waiver, though fleeting, should be interpreted as an urgent call to the medical community, including us as hospitalists, to learn about buprenorphine with the many resources available (see table 1). As hospital medicine providers, we can order buprenorphine for patients with OUD during hospitalization. It is discharge prescriptions that have been limited to providers with an X-Waiver.
What can we do now to prepare for the eventual X-Waiver training removal? We can start by educating ourselves with the resources listed in table 1. Those of us who are already buprenorphine champions could lead trainings in our home institutions. In a future without the waiver there will be more flexibility to develop hospitalist-focused buprenorphine trainings, as the previous ones were geared for outpatient providers. Hospitalist organizations could support hospitalist-specific buprenorphine trainings and extend the models to include additional medications for addiction.
There is a large body of evidence regarding buprenorphine’s safety and efficacy in OUD treatment. With a worsening overdose crisis, there have been increasing opioid-related hospitalizations. When new medications for diabetes, hypertension, or DVT treatment become available, as hospitalists we incorporate them into our toolbox. As buprenorphine becomes more accessible, we can be leaders in further adopting it (and other substance use disorder medications while we are at it) as our standard of care for people with OUD.
Dr. Bottner is a physician assistant in the Division of Hospital Medicine at Dell Medical School at The University of Texas at Austin and director of the hospital’s Buprenorphine Team. Dr. Martin is a board-certified addiction medicine physician and hospitalist at University of California, San Francisco, and director of the Addiction Care Team at San Francisco General Hospital. Dr. Bottner and Dr. Martin colead the SHM Substance Use Disorder Special Interest Group.
There are two pandemics permeating the United States: COVID-19 and addiction. To date, more than 468,000 people have died from COVID-19 in the U.S. In the 12-month period ending in May 2020, over 80,000 died from a drug related cause – the highest number ever recorded in a year. Many of these deaths involved opioids.
COVID-19 has worsened outcomes for people with addiction. There is less access to treatment, increased isolation, and worsening psychosocial and economic stressors. These factors may drive new, increased, or more risky substance use and return to use for people in recovery. As hospitalists, we have been responders in both COVID-19 and our country’s worsening overdose and addiction crisis.
In December 2020’s Journal of Hospital Medicine article “Converging Crises: Caring for hospitalized adults with substance use disorder in the time of COVID-19”, Dr. Honora Englander and her coauthors called on hospitalists to actively engage patients with substance use disorders during hospitalization. The article highlights the colliding crises of addiction and COVID-19 and provides eight practical approaches for hospitalists to address substance use disorders during the pandemic, including initiating buprenorphine for opioid withdrawal and prescribing it for opioid use disorder (OUD) treatment.
Buprenorphine effectively treats opioid withdrawal, reduces OUD-related mortality, and decreases hospital readmissions related to OUD. To prescribe buprenorphine for OUD in the outpatient setting or on hospital discharge, providers need an X-Waiver. The X-Waiver is a result of the Drug Addiction Treatment Act 2000 (DATA 2000), which was enacted in 2000. It permits physicians to prescribe buprenorphine for OUD treatment after an 8-hour training. In 2016, the Comprehensive Addiction and Recovery Act extended buprenorphine prescribing to physician assistants (PAs) and advanced-practice nurses (APNs). However, PAs and APNs are required to complete a 24-hour training to receive the waiver.
On Jan. 14, 2021, the U.S. Department of Health and Human Services under the Trump administration announced it was removing the X-Waiver training previously required for physicians to prescribe this life-saving medication. However, on Jan. 20, 2021, the Biden administration froze the training requirement removal pending a 60-day review. The excitement about the waiver’s eradication further dampened on Jan. 25, when the plan was halted due to procedural factors coupled with the concern that HHS may not have the authority to void requirements mandated by Congress.
Many of us continue to be hopeful that the X-Waiver will soon be gone. The Substance Abuse and Mental Health Services Administration has committed to working with federal agencies to increase access to buprenorphine. The Biden administration also committed to addressing our country’s addiction crisis, including a plan to “make effective prevention, treatment, and recovery services available to all, including through a $125 billion federal investment.”
Despite the pause on HHS’s recent attempt to “X the X-Waiver,” we now have renewed attention and interest in this critical issue and an opportunity for greater and longer-lasting legislative impact. SHM supports that Congress repeal the legislative requirement for buprenorphine training dictated by DATA 2000 so that it cannot be rolled back by future administrations. To further increase access to buprenorphine treatment, the training requirement should be removed for all providers who care for individuals with OUD.
The X-Waiver has been a barrier to hospitalist adoption of this critical, life-saving medication. HHS’s stance to nix the waiver, though fleeting, should be interpreted as an urgent call to the medical community, including us as hospitalists, to learn about buprenorphine with the many resources available (see table 1). As hospital medicine providers, we can order buprenorphine for patients with OUD during hospitalization. It is discharge prescriptions that have been limited to providers with an X-Waiver.
What can we do now to prepare for the eventual X-Waiver training removal? We can start by educating ourselves with the resources listed in table 1. Those of us who are already buprenorphine champions could lead trainings in our home institutions. In a future without the waiver there will be more flexibility to develop hospitalist-focused buprenorphine trainings, as the previous ones were geared for outpatient providers. Hospitalist organizations could support hospitalist-specific buprenorphine trainings and extend the models to include additional medications for addiction.
There is a large body of evidence regarding buprenorphine’s safety and efficacy in OUD treatment. With a worsening overdose crisis, there have been increasing opioid-related hospitalizations. When new medications for diabetes, hypertension, or DVT treatment become available, as hospitalists we incorporate them into our toolbox. As buprenorphine becomes more accessible, we can be leaders in further adopting it (and other substance use disorder medications while we are at it) as our standard of care for people with OUD.
Dr. Bottner is a physician assistant in the Division of Hospital Medicine at Dell Medical School at The University of Texas at Austin and director of the hospital’s Buprenorphine Team. Dr. Martin is a board-certified addiction medicine physician and hospitalist at University of California, San Francisco, and director of the Addiction Care Team at San Francisco General Hospital. Dr. Bottner and Dr. Martin colead the SHM Substance Use Disorder Special Interest Group.
There are two pandemics permeating the United States: COVID-19 and addiction. To date, more than 468,000 people have died from COVID-19 in the U.S. In the 12-month period ending in May 2020, over 80,000 died from a drug related cause – the highest number ever recorded in a year. Many of these deaths involved opioids.
COVID-19 has worsened outcomes for people with addiction. There is less access to treatment, increased isolation, and worsening psychosocial and economic stressors. These factors may drive new, increased, or more risky substance use and return to use for people in recovery. As hospitalists, we have been responders in both COVID-19 and our country’s worsening overdose and addiction crisis.
In December 2020’s Journal of Hospital Medicine article “Converging Crises: Caring for hospitalized adults with substance use disorder in the time of COVID-19”, Dr. Honora Englander and her coauthors called on hospitalists to actively engage patients with substance use disorders during hospitalization. The article highlights the colliding crises of addiction and COVID-19 and provides eight practical approaches for hospitalists to address substance use disorders during the pandemic, including initiating buprenorphine for opioid withdrawal and prescribing it for opioid use disorder (OUD) treatment.
Buprenorphine effectively treats opioid withdrawal, reduces OUD-related mortality, and decreases hospital readmissions related to OUD. To prescribe buprenorphine for OUD in the outpatient setting or on hospital discharge, providers need an X-Waiver. The X-Waiver is a result of the Drug Addiction Treatment Act 2000 (DATA 2000), which was enacted in 2000. It permits physicians to prescribe buprenorphine for OUD treatment after an 8-hour training. In 2016, the Comprehensive Addiction and Recovery Act extended buprenorphine prescribing to physician assistants (PAs) and advanced-practice nurses (APNs). However, PAs and APNs are required to complete a 24-hour training to receive the waiver.
On Jan. 14, 2021, the U.S. Department of Health and Human Services under the Trump administration announced it was removing the X-Waiver training previously required for physicians to prescribe this life-saving medication. However, on Jan. 20, 2021, the Biden administration froze the training requirement removal pending a 60-day review. The excitement about the waiver’s eradication further dampened on Jan. 25, when the plan was halted due to procedural factors coupled with the concern that HHS may not have the authority to void requirements mandated by Congress.
Many of us continue to be hopeful that the X-Waiver will soon be gone. The Substance Abuse and Mental Health Services Administration has committed to working with federal agencies to increase access to buprenorphine. The Biden administration also committed to addressing our country’s addiction crisis, including a plan to “make effective prevention, treatment, and recovery services available to all, including through a $125 billion federal investment.”
Despite the pause on HHS’s recent attempt to “X the X-Waiver,” we now have renewed attention and interest in this critical issue and an opportunity for greater and longer-lasting legislative impact. SHM supports that Congress repeal the legislative requirement for buprenorphine training dictated by DATA 2000 so that it cannot be rolled back by future administrations. To further increase access to buprenorphine treatment, the training requirement should be removed for all providers who care for individuals with OUD.
The X-Waiver has been a barrier to hospitalist adoption of this critical, life-saving medication. HHS’s stance to nix the waiver, though fleeting, should be interpreted as an urgent call to the medical community, including us as hospitalists, to learn about buprenorphine with the many resources available (see table 1). As hospital medicine providers, we can order buprenorphine for patients with OUD during hospitalization. It is discharge prescriptions that have been limited to providers with an X-Waiver.
What can we do now to prepare for the eventual X-Waiver training removal? We can start by educating ourselves with the resources listed in table 1. Those of us who are already buprenorphine champions could lead trainings in our home institutions. In a future without the waiver there will be more flexibility to develop hospitalist-focused buprenorphine trainings, as the previous ones were geared for outpatient providers. Hospitalist organizations could support hospitalist-specific buprenorphine trainings and extend the models to include additional medications for addiction.
There is a large body of evidence regarding buprenorphine’s safety and efficacy in OUD treatment. With a worsening overdose crisis, there have been increasing opioid-related hospitalizations. When new medications for diabetes, hypertension, or DVT treatment become available, as hospitalists we incorporate them into our toolbox. As buprenorphine becomes more accessible, we can be leaders in further adopting it (and other substance use disorder medications while we are at it) as our standard of care for people with OUD.
Dr. Bottner is a physician assistant in the Division of Hospital Medicine at Dell Medical School at The University of Texas at Austin and director of the hospital’s Buprenorphine Team. Dr. Martin is a board-certified addiction medicine physician and hospitalist at University of California, San Francisco, and director of the Addiction Care Team at San Francisco General Hospital. Dr. Bottner and Dr. Martin colead the SHM Substance Use Disorder Special Interest Group.
Chronic GVHD therapies offer hope for treating refractory disease
Despite improvements in prevention of graft-versus-host disease, chronic GVHD still occurs in 10%-50% of patients who undergo an allogeneic hematopoietic stem cell transplant, and these patients may require prolonged treatment with multiple lines of therapy, said a hematologist and transplant researcher.
“More effective, less toxic therapies for chronic GVHD are needed,” Stephanie Lee, MD, MPH, from the Fred Hutchinson Cancer Research Center in Seattle said at the Transplant & Cellular Therapies Meetings.
Dr. Lee reviewed clinical trials for chronic GVHD at the meeting held by the American Society for Blood and Marrow Transplantation and the Center for International Blood and Marrow Transplant Research.
Although the incidence of chronic GVHD has gradually declined over the last 40 years and both relapse-free and overall survival following a chronic GVHD diagnosis have improved, “for patients who are diagnosed with chronic GVHD, they still will see many lines of therapy and many years of therapy,” she said.
Among 148 patients with chronic GVHD treated at her center, for example, 66% went on to two lines of therapy, 50% went on to three lines, 37% required four lines of therapy, and 20% needed five lines or more.
Salvage therapies for patients with chronic GVHD have evolved away from immunomodulators and immunosuppressants in the early 1990s, toward monoclonal antibodies such as rituximab in the early 2000s, to interleukin-2 and to tyrosine kinase inhibitors such as ruxolitinib (Jakafi) and ibrutinib (Imbruvica).
There are currently 36 agents that are FDA approved for at least one indication and can also be prescribed for the treatment of chronic GVHD, Dr. Lee noted.
Treatment goals
Dr. Lee laid out six goals for treating patients with chronic GVHD. They include:
- Controlling current signs and symptoms, measured by response rates and patient-reported outcomes
- Preventing further tissue and organ damage
- Minimizing toxicity
- Maintaining graft-versus-tumor effect
- Achieving graft tolerance and stopping immunosuppression
- Decreasing nonrelapse mortality and improving survival
Active trials
Dr. Lee identified 33 trials with chronic GVHD as an indication that are currently recruiting, and an additional 13 trials that are active but closed to recruiting. The trials can be generally grouped by mechanism of action, and involve agents targeting T-regulatory cells, B cells and/or B-cell receptor (BCR) signaling, monocytes/macrophages, costimulatory blockage, a proteasome inhibition, Janus kinase (JAK) 1/2 inhibitors, ROCK2 inhibitors, hedgehog pathway inhibition, cellular therapy, and organ-targeted therapy.
Most of the trials have overall response rate as the primary endpoint, and all but five are currently in phase 1 or 2. The currently active phase 3 trials include two with ibrutinib, one with the investigational agent itacitinib, one with ruxolitinib, and one with mesenchymal stem cells.
“I’ll note that, when results are reported, the denominator really matters for the overall response rate, especially if you’re talking about small trials, because if you require the patient to be treated with an agent for a certain period of time, and you take out all the people who didn’t make it to that time point, then your overall response rate looks better,” she said.
BTK inhibitors
The first-in-class Bruton tyrosine kinase (BTK) inhibitor ibrutinib was the first and thus far only agent approved by the Food and Drug Administration for chronic GVHD. The approval was based on a single-arm, multicenter trial with 42 patients.
The ORR in this trial was 69%, consisting of 31% complete responses and 38% partial responses, with a duration of response longer than 10 months in slightly more than half of all patients. In all, 24% of patients had improvement of symptoms in two consecutive visits, and 29% continued on ibrutinib at the time of the primary analysis in 2017.
Based on these promising results, acalabrutinib, which is more potent and selective for BTK than ibrutinib, with no effect on either platelets or natural killer cells, is currently under investigation in a phase 2 trial in 50 patients at a dose of 100 mg orally twice daily.
JAK1/2 inhibition
The JAK1 inhibitor itacitinib failed to meet its primary ORR endpoint in the phase 3 GRAVITAS-301 study, according to a press release, but the manufacturer (Incyte) said that it is continuing its commitment to JAK inhibitors with ruxolitinib, which has shown activity against acute, steroid-refractory GVHD, and is being explored for prevention of chronic GVHD in the randomized, phase 3 REACH3 study.
The trial met its primary endpoint for a higher ORR at week 24 with ruxolitinib versus best available therapy, at 49.7% versus 25.6%, respectively, which translated into an odds ratio for response with the JAK inhibitor of 2.99 (P < .0001).
Selective T-cell expansion
Efavaleukin alfa is an IL-2-mutated protein (mutein), with a mutation in the IL-2RB-binding portion of IL-2 causing increased selectivity for regulatory T-cell expansion. It is bound to an IgG-Fc domain that is itself mutated, with reduced Fc receptor binding and IgG effector function to give it a longer half life. This agent is being studied in a phase 1/2 trial in a subcutaneous formulation delivered every 1 or 2 weeks to 68 patients.
Monocyte/macrophage depletion
Axatilimab is a high-affinity antibody targeting colony stimulating factor–1 receptor (CSF-1R) expressed on monocytes and macrophages. By blocking CSF-1R, it depletes circulation of nonclassical monocytes and prevents the differentiation and survival of M2 macrophages in tissue.
It is currently being investigated 30 patients in a phase 1/2 study in an intravenous formulation delivered over 30 minutes every 2-4 weeks.
Hedgehog pathway inhibition
There is evidence suggesting that hedgehog pathway inhibition can lessen fibrosis. Glasdegib (Daurismo) a potent selective oral inhibitor of the hedgehog signaling pathway, is approved for use with low-dose cytarabine for patients with newly diagnosed acute myeloid leukemia aged older than 75 years or have comorbidities precluding intensive chemotherapy.
This agent is associated with drug intolerance because of muscle spasms, dysgeusia, and alopecia, however.
The drug is currently in phase 1/2 at a dose of 50 mg orally per day in 20 patients.
ROCK2 inhibition
Belumosudil (formerly KD025) “appears to rebalance the immune system,” Dr. Lee said. Investigators think that the drug dampens an autoaggressive inflammatory response by selective inhibition of ROCK2.
This drug has been studied in a dose-escalation study and a phase 2 trial, in which 132 participants were randomized to receive belumosudil 200 mg either once or twice daily.
At a median follow-up of 8 months, the ORR with belumosudil 200 mg once and twice daily was 73% and 74%, respectively. Similar results were seen in patients who had previously received either ruxolitinib or ibrutinib. High response rates were seen in patients with severe chronic GVHD, involvement of four or more organs and a refractory response to their last line of therapy.
Hard-to-manage patients
“We’re very hopeful for many of these agents, but we have to acknowledge that there are still many management dilemmas, patients that we just don’t really know what to do with,” Dr. Lee said. “These include patients who have bad sclerosis and fasciitis, nonhealing skin ulcers, bronchiolitis obliterans, serositis that can be very difficult to manage, severe keratoconjunctivitis that can be eyesight threatening, nonhealing mouth ulcers, esophageal structures, and always patients who have frequent infections.
“We are hopeful that some these agents will be useful for our patients who have severe manifestations, but often the number of patients with these manifestations in the trials is too low to say something specific about them,” she added.
‘Exciting time’
“It’s an exciting time because there are a lot of different drugs that are being studied for chronic GVHD,” commented Betty Hamilton, MD, a hematologist/oncologist at the Cleveland Clinic.
“I think that where the field is going in terms of treatment is recognizing that chronic GVHD is a pretty heterogeneous disease, and we have to learn even more about the underlying biologic pathways to be able to determine which class of drugs to use and when,” she said in an interview.
She agreed with Dr. Lee that the goals of treating patients with chronic GVHD include improving symptoms and quality, preventing progression, ideally tapering patients off immunosuppression, and achieving a balance between preventing negative consequences of GVHD while maintain the benefits of a graft-versus-leukemia effect.
“In our center, drug choice is based on physician preference and comfort with how often they’ve used the drug, patients’ comorbidities, toxicities of the drug, and logistical considerations,” Dr. Hamilton said.
Dr. Lee disclosed consulting activities for Pfizer and Kadmon, travel and lodging from Amgen, and research funding from those companies and others. Dr. Hamilton disclosed consulting for Syndax and Incyte.
Despite improvements in prevention of graft-versus-host disease, chronic GVHD still occurs in 10%-50% of patients who undergo an allogeneic hematopoietic stem cell transplant, and these patients may require prolonged treatment with multiple lines of therapy, said a hematologist and transplant researcher.
“More effective, less toxic therapies for chronic GVHD are needed,” Stephanie Lee, MD, MPH, from the Fred Hutchinson Cancer Research Center in Seattle said at the Transplant & Cellular Therapies Meetings.
Dr. Lee reviewed clinical trials for chronic GVHD at the meeting held by the American Society for Blood and Marrow Transplantation and the Center for International Blood and Marrow Transplant Research.
Although the incidence of chronic GVHD has gradually declined over the last 40 years and both relapse-free and overall survival following a chronic GVHD diagnosis have improved, “for patients who are diagnosed with chronic GVHD, they still will see many lines of therapy and many years of therapy,” she said.
Among 148 patients with chronic GVHD treated at her center, for example, 66% went on to two lines of therapy, 50% went on to three lines, 37% required four lines of therapy, and 20% needed five lines or more.
Salvage therapies for patients with chronic GVHD have evolved away from immunomodulators and immunosuppressants in the early 1990s, toward monoclonal antibodies such as rituximab in the early 2000s, to interleukin-2 and to tyrosine kinase inhibitors such as ruxolitinib (Jakafi) and ibrutinib (Imbruvica).
There are currently 36 agents that are FDA approved for at least one indication and can also be prescribed for the treatment of chronic GVHD, Dr. Lee noted.
Treatment goals
Dr. Lee laid out six goals for treating patients with chronic GVHD. They include:
- Controlling current signs and symptoms, measured by response rates and patient-reported outcomes
- Preventing further tissue and organ damage
- Minimizing toxicity
- Maintaining graft-versus-tumor effect
- Achieving graft tolerance and stopping immunosuppression
- Decreasing nonrelapse mortality and improving survival
Active trials
Dr. Lee identified 33 trials with chronic GVHD as an indication that are currently recruiting, and an additional 13 trials that are active but closed to recruiting. The trials can be generally grouped by mechanism of action, and involve agents targeting T-regulatory cells, B cells and/or B-cell receptor (BCR) signaling, monocytes/macrophages, costimulatory blockage, a proteasome inhibition, Janus kinase (JAK) 1/2 inhibitors, ROCK2 inhibitors, hedgehog pathway inhibition, cellular therapy, and organ-targeted therapy.
Most of the trials have overall response rate as the primary endpoint, and all but five are currently in phase 1 or 2. The currently active phase 3 trials include two with ibrutinib, one with the investigational agent itacitinib, one with ruxolitinib, and one with mesenchymal stem cells.
“I’ll note that, when results are reported, the denominator really matters for the overall response rate, especially if you’re talking about small trials, because if you require the patient to be treated with an agent for a certain period of time, and you take out all the people who didn’t make it to that time point, then your overall response rate looks better,” she said.
BTK inhibitors
The first-in-class Bruton tyrosine kinase (BTK) inhibitor ibrutinib was the first and thus far only agent approved by the Food and Drug Administration for chronic GVHD. The approval was based on a single-arm, multicenter trial with 42 patients.
The ORR in this trial was 69%, consisting of 31% complete responses and 38% partial responses, with a duration of response longer than 10 months in slightly more than half of all patients. In all, 24% of patients had improvement of symptoms in two consecutive visits, and 29% continued on ibrutinib at the time of the primary analysis in 2017.
Based on these promising results, acalabrutinib, which is more potent and selective for BTK than ibrutinib, with no effect on either platelets or natural killer cells, is currently under investigation in a phase 2 trial in 50 patients at a dose of 100 mg orally twice daily.
JAK1/2 inhibition
The JAK1 inhibitor itacitinib failed to meet its primary ORR endpoint in the phase 3 GRAVITAS-301 study, according to a press release, but the manufacturer (Incyte) said that it is continuing its commitment to JAK inhibitors with ruxolitinib, which has shown activity against acute, steroid-refractory GVHD, and is being explored for prevention of chronic GVHD in the randomized, phase 3 REACH3 study.
The trial met its primary endpoint for a higher ORR at week 24 with ruxolitinib versus best available therapy, at 49.7% versus 25.6%, respectively, which translated into an odds ratio for response with the JAK inhibitor of 2.99 (P < .0001).
Selective T-cell expansion
Efavaleukin alfa is an IL-2-mutated protein (mutein), with a mutation in the IL-2RB-binding portion of IL-2 causing increased selectivity for regulatory T-cell expansion. It is bound to an IgG-Fc domain that is itself mutated, with reduced Fc receptor binding and IgG effector function to give it a longer half life. This agent is being studied in a phase 1/2 trial in a subcutaneous formulation delivered every 1 or 2 weeks to 68 patients.
Monocyte/macrophage depletion
Axatilimab is a high-affinity antibody targeting colony stimulating factor–1 receptor (CSF-1R) expressed on monocytes and macrophages. By blocking CSF-1R, it depletes circulation of nonclassical monocytes and prevents the differentiation and survival of M2 macrophages in tissue.
It is currently being investigated 30 patients in a phase 1/2 study in an intravenous formulation delivered over 30 minutes every 2-4 weeks.
Hedgehog pathway inhibition
There is evidence suggesting that hedgehog pathway inhibition can lessen fibrosis. Glasdegib (Daurismo) a potent selective oral inhibitor of the hedgehog signaling pathway, is approved for use with low-dose cytarabine for patients with newly diagnosed acute myeloid leukemia aged older than 75 years or have comorbidities precluding intensive chemotherapy.
This agent is associated with drug intolerance because of muscle spasms, dysgeusia, and alopecia, however.
The drug is currently in phase 1/2 at a dose of 50 mg orally per day in 20 patients.
ROCK2 inhibition
Belumosudil (formerly KD025) “appears to rebalance the immune system,” Dr. Lee said. Investigators think that the drug dampens an autoaggressive inflammatory response by selective inhibition of ROCK2.
This drug has been studied in a dose-escalation study and a phase 2 trial, in which 132 participants were randomized to receive belumosudil 200 mg either once or twice daily.
At a median follow-up of 8 months, the ORR with belumosudil 200 mg once and twice daily was 73% and 74%, respectively. Similar results were seen in patients who had previously received either ruxolitinib or ibrutinib. High response rates were seen in patients with severe chronic GVHD, involvement of four or more organs and a refractory response to their last line of therapy.
Hard-to-manage patients
“We’re very hopeful for many of these agents, but we have to acknowledge that there are still many management dilemmas, patients that we just don’t really know what to do with,” Dr. Lee said. “These include patients who have bad sclerosis and fasciitis, nonhealing skin ulcers, bronchiolitis obliterans, serositis that can be very difficult to manage, severe keratoconjunctivitis that can be eyesight threatening, nonhealing mouth ulcers, esophageal structures, and always patients who have frequent infections.
“We are hopeful that some these agents will be useful for our patients who have severe manifestations, but often the number of patients with these manifestations in the trials is too low to say something specific about them,” she added.
‘Exciting time’
“It’s an exciting time because there are a lot of different drugs that are being studied for chronic GVHD,” commented Betty Hamilton, MD, a hematologist/oncologist at the Cleveland Clinic.
“I think that where the field is going in terms of treatment is recognizing that chronic GVHD is a pretty heterogeneous disease, and we have to learn even more about the underlying biologic pathways to be able to determine which class of drugs to use and when,” she said in an interview.
She agreed with Dr. Lee that the goals of treating patients with chronic GVHD include improving symptoms and quality, preventing progression, ideally tapering patients off immunosuppression, and achieving a balance between preventing negative consequences of GVHD while maintain the benefits of a graft-versus-leukemia effect.
“In our center, drug choice is based on physician preference and comfort with how often they’ve used the drug, patients’ comorbidities, toxicities of the drug, and logistical considerations,” Dr. Hamilton said.
Dr. Lee disclosed consulting activities for Pfizer and Kadmon, travel and lodging from Amgen, and research funding from those companies and others. Dr. Hamilton disclosed consulting for Syndax and Incyte.
Despite improvements in prevention of graft-versus-host disease, chronic GVHD still occurs in 10%-50% of patients who undergo an allogeneic hematopoietic stem cell transplant, and these patients may require prolonged treatment with multiple lines of therapy, said a hematologist and transplant researcher.
“More effective, less toxic therapies for chronic GVHD are needed,” Stephanie Lee, MD, MPH, from the Fred Hutchinson Cancer Research Center in Seattle said at the Transplant & Cellular Therapies Meetings.
Dr. Lee reviewed clinical trials for chronic GVHD at the meeting held by the American Society for Blood and Marrow Transplantation and the Center for International Blood and Marrow Transplant Research.
Although the incidence of chronic GVHD has gradually declined over the last 40 years and both relapse-free and overall survival following a chronic GVHD diagnosis have improved, “for patients who are diagnosed with chronic GVHD, they still will see many lines of therapy and many years of therapy,” she said.
Among 148 patients with chronic GVHD treated at her center, for example, 66% went on to two lines of therapy, 50% went on to three lines, 37% required four lines of therapy, and 20% needed five lines or more.
Salvage therapies for patients with chronic GVHD have evolved away from immunomodulators and immunosuppressants in the early 1990s, toward monoclonal antibodies such as rituximab in the early 2000s, to interleukin-2 and to tyrosine kinase inhibitors such as ruxolitinib (Jakafi) and ibrutinib (Imbruvica).
There are currently 36 agents that are FDA approved for at least one indication and can also be prescribed for the treatment of chronic GVHD, Dr. Lee noted.
Treatment goals
Dr. Lee laid out six goals for treating patients with chronic GVHD. They include:
- Controlling current signs and symptoms, measured by response rates and patient-reported outcomes
- Preventing further tissue and organ damage
- Minimizing toxicity
- Maintaining graft-versus-tumor effect
- Achieving graft tolerance and stopping immunosuppression
- Decreasing nonrelapse mortality and improving survival
Active trials
Dr. Lee identified 33 trials with chronic GVHD as an indication that are currently recruiting, and an additional 13 trials that are active but closed to recruiting. The trials can be generally grouped by mechanism of action, and involve agents targeting T-regulatory cells, B cells and/or B-cell receptor (BCR) signaling, monocytes/macrophages, costimulatory blockage, a proteasome inhibition, Janus kinase (JAK) 1/2 inhibitors, ROCK2 inhibitors, hedgehog pathway inhibition, cellular therapy, and organ-targeted therapy.
Most of the trials have overall response rate as the primary endpoint, and all but five are currently in phase 1 or 2. The currently active phase 3 trials include two with ibrutinib, one with the investigational agent itacitinib, one with ruxolitinib, and one with mesenchymal stem cells.
“I’ll note that, when results are reported, the denominator really matters for the overall response rate, especially if you’re talking about small trials, because if you require the patient to be treated with an agent for a certain period of time, and you take out all the people who didn’t make it to that time point, then your overall response rate looks better,” she said.
BTK inhibitors
The first-in-class Bruton tyrosine kinase (BTK) inhibitor ibrutinib was the first and thus far only agent approved by the Food and Drug Administration for chronic GVHD. The approval was based on a single-arm, multicenter trial with 42 patients.
The ORR in this trial was 69%, consisting of 31% complete responses and 38% partial responses, with a duration of response longer than 10 months in slightly more than half of all patients. In all, 24% of patients had improvement of symptoms in two consecutive visits, and 29% continued on ibrutinib at the time of the primary analysis in 2017.
Based on these promising results, acalabrutinib, which is more potent and selective for BTK than ibrutinib, with no effect on either platelets or natural killer cells, is currently under investigation in a phase 2 trial in 50 patients at a dose of 100 mg orally twice daily.
JAK1/2 inhibition
The JAK1 inhibitor itacitinib failed to meet its primary ORR endpoint in the phase 3 GRAVITAS-301 study, according to a press release, but the manufacturer (Incyte) said that it is continuing its commitment to JAK inhibitors with ruxolitinib, which has shown activity against acute, steroid-refractory GVHD, and is being explored for prevention of chronic GVHD in the randomized, phase 3 REACH3 study.
The trial met its primary endpoint for a higher ORR at week 24 with ruxolitinib versus best available therapy, at 49.7% versus 25.6%, respectively, which translated into an odds ratio for response with the JAK inhibitor of 2.99 (P < .0001).
Selective T-cell expansion
Efavaleukin alfa is an IL-2-mutated protein (mutein), with a mutation in the IL-2RB-binding portion of IL-2 causing increased selectivity for regulatory T-cell expansion. It is bound to an IgG-Fc domain that is itself mutated, with reduced Fc receptor binding and IgG effector function to give it a longer half life. This agent is being studied in a phase 1/2 trial in a subcutaneous formulation delivered every 1 or 2 weeks to 68 patients.
Monocyte/macrophage depletion
Axatilimab is a high-affinity antibody targeting colony stimulating factor–1 receptor (CSF-1R) expressed on monocytes and macrophages. By blocking CSF-1R, it depletes circulation of nonclassical monocytes and prevents the differentiation and survival of M2 macrophages in tissue.
It is currently being investigated 30 patients in a phase 1/2 study in an intravenous formulation delivered over 30 minutes every 2-4 weeks.
Hedgehog pathway inhibition
There is evidence suggesting that hedgehog pathway inhibition can lessen fibrosis. Glasdegib (Daurismo) a potent selective oral inhibitor of the hedgehog signaling pathway, is approved for use with low-dose cytarabine for patients with newly diagnosed acute myeloid leukemia aged older than 75 years or have comorbidities precluding intensive chemotherapy.
This agent is associated with drug intolerance because of muscle spasms, dysgeusia, and alopecia, however.
The drug is currently in phase 1/2 at a dose of 50 mg orally per day in 20 patients.
ROCK2 inhibition
Belumosudil (formerly KD025) “appears to rebalance the immune system,” Dr. Lee said. Investigators think that the drug dampens an autoaggressive inflammatory response by selective inhibition of ROCK2.
This drug has been studied in a dose-escalation study and a phase 2 trial, in which 132 participants were randomized to receive belumosudil 200 mg either once or twice daily.
At a median follow-up of 8 months, the ORR with belumosudil 200 mg once and twice daily was 73% and 74%, respectively. Similar results were seen in patients who had previously received either ruxolitinib or ibrutinib. High response rates were seen in patients with severe chronic GVHD, involvement of four or more organs and a refractory response to their last line of therapy.
Hard-to-manage patients
“We’re very hopeful for many of these agents, but we have to acknowledge that there are still many management dilemmas, patients that we just don’t really know what to do with,” Dr. Lee said. “These include patients who have bad sclerosis and fasciitis, nonhealing skin ulcers, bronchiolitis obliterans, serositis that can be very difficult to manage, severe keratoconjunctivitis that can be eyesight threatening, nonhealing mouth ulcers, esophageal structures, and always patients who have frequent infections.
“We are hopeful that some these agents will be useful for our patients who have severe manifestations, but often the number of patients with these manifestations in the trials is too low to say something specific about them,” she added.
‘Exciting time’
“It’s an exciting time because there are a lot of different drugs that are being studied for chronic GVHD,” commented Betty Hamilton, MD, a hematologist/oncologist at the Cleveland Clinic.
“I think that where the field is going in terms of treatment is recognizing that chronic GVHD is a pretty heterogeneous disease, and we have to learn even more about the underlying biologic pathways to be able to determine which class of drugs to use and when,” she said in an interview.
She agreed with Dr. Lee that the goals of treating patients with chronic GVHD include improving symptoms and quality, preventing progression, ideally tapering patients off immunosuppression, and achieving a balance between preventing negative consequences of GVHD while maintain the benefits of a graft-versus-leukemia effect.
“In our center, drug choice is based on physician preference and comfort with how often they’ve used the drug, patients’ comorbidities, toxicities of the drug, and logistical considerations,” Dr. Hamilton said.
Dr. Lee disclosed consulting activities for Pfizer and Kadmon, travel and lodging from Amgen, and research funding from those companies and others. Dr. Hamilton disclosed consulting for Syndax and Incyte.
FROM TCT 2021
Opioids prescribed for diabetic neuropathy pain, against advice
Prescriptions for opioids as a first-line treatment for painful diabetic peripheral neuropathy (DPN) outnumbered those for other medications between 2014 and 2018, despite the fact that the former is not recommended, new research indicates.
“We know that for any kind of chronic pain, opioids are not ideal. They’re not very effective for chronic pain in general, and they’re definitely not safe,” senior author Rozalina G. McCoy, MD, an endocrinologist and primary care clinician at the Mayo Clinic in Rochester, Minn., told this news organization.
That’s true even for severe DPN pain or painful exacerbations, she added.
“There’s a myth that opioids are the strongest pain meds possible ... For painful neuropathic pain, duloxetine [Cymbalta], pregabalin [Lyrica], and gabapentin [Neurontin] are the most effective pain medications based on multiple studies and extensive experience using them,” she explained. “But I think the public perception is that opioids are the strongest. When a patient comes with severe pain, I think there’s that kind of gut feeling that if the pain is severe, I need to give opioids.”
What’s more, she noted, “evidence is emerging for other harms, not only the potential for dependency and potential overdose, but also the potential for opioid-induced hyperalgesia. Opioids themselves can cause chronic pain. When we think about using opioids for chronic pain, we are really shooting ourselves in the foot. We’re going to harm patients.”
The American Diabetes Association DPN guidelines essentially say as much, advising opioids only as a tertiary option for refractory pain, she observed.
The new findings, from a retrospective study of Mayo Clinic electronic health data, were published online in JAMA Network Open by Jungwei Fan, PhD, also of Mayo Clinic, and colleagues.
Are fewer patients with DPN receiving any treatment now?
The data also reveal that, while opioid prescribing dropped over the study period, there wasn’t a comparable rise in prescriptions of recommended pain medications, suggesting that recent efforts to minimize opioid prescribing may have resulted in less overall treatment of significant pain. (The study had to be stopped in 2018 when Mayo switched to a new electronic health record system, Dr. McCoy explained.)
“The proportion of opioids among new prescriptions has been decreasing. I’m hopeful that the rates are even lower now than they were 2 years ago. What was concerning to me was the proportion of people receiving treatment overall had gone down,” Dr. McCoy noted.
“So, while it’s great that opioids aren’t being used, it’s doubtful that people with DPN are any less symptomatic. So I worry that there’s a proportion of patients who have pain who aren’t getting the treatment they need just because we don’t want to give them opioids. There are other options,” Dr. McCoy said, including nonpharmacologic approaches.
Opioids dominated in new-onset DPN prescribing during 2014-2018
The study involved 3,495 adults with newly diagnosed DPN from all three Mayo Clinic locations in Rochester, Minn.; Phoenix, Ariz.; and Jacksonville, Fla. during the period 2014-2018. Of those, 40.2% (1,406) were prescribed a new pain medication after diagnosis. However, that proportion dropped from 45.6% in 2014 to 35.2% in 2018.
The odds of initiating any treatment were significantly greater among patients with depression (odds ratio, 1.61), arthritis (OR, 1.21), and back pain (OR, 1.34), but decreased over time among all patients.
Among those receiving drug treatment, opioids were prescribed to 43.8%, whereas guideline-recommended medications (gabapentin, pregabalin, and serotonin norepinephrine reuptake inhibitors including duloxetine) were prescribed to 42.9%.
Another 20.6% received medications deemed “acceptable” for treating neuropathic pain, including topical analgesics, tricyclic antidepressants, and other anticonvulsants.
Males were significantly more likely than females to receive opioids (OR, 1.26), while individuals diagnosed with comorbid fibromyalgia were less likely (OR, 0.67). Those with comorbid arthritis were less likely to receive recommended DPN medications (OR, 0.76).
Use of opioids was 29% less likely in 2018, compared with 2014, although this difference did not achieve significance. Similarly, use of recommended medications was 25% more likely in 2018, compared with 2014, also not a significant difference.
Dr. McCoy offers clinical pearls for treating pain in DPN
Clinically, Dr. McCoy said that she individualizes treatment for painful DPN.
“I tend to use duloxetine if the patient also has a mood disorder including depression or anxiety, because it can also help with that. Gabapentin can also be helpful for radiculopathy or for chronic low-back pain. It can even help with degenerative joint disease like arthritis of the knees. So, you maximize benefit if you use one drug to treat multiple things.”
All three recommended medications are generic now, although pregabalin still tends to be more expensive, she noted. Gabapentin can cause drowsiness, which makes it ideal for a patient with insomnia but much less so for a long-haul truck driver. Duloxetine doesn’t cause sleepiness. Pregabalin can, but less so than gabapentin.
“I think that’s why it’s so important to talk to your patient and ask how the neuropathy is affecting them. What other comorbidities do they have? What is their life like? I think you have to figure out what drug works for each individual person.”
Importantly, she advised, if one of the three doesn’t work, stop it and try another. “It doesn’t mean that none of these meds work. All three should be tried to see if they give relief.”
Nonpharmacologic measures such as cognitive behavioral therapy, acupuncture, or physical therapy may help some patients as well.
Supplements such as vitamin B12 – which can also help with metformin-induced B12 deficiency – or alpha-lipoic acid may also be worth a try as long as the patient is made aware of potential risks, she noted.
Dr. McCoy hopes to repeat this study using national data. “I don’t think this is isolated to Mayo ... I think it affects all practices,” she said.
Since the study, “we [Mayo Clinic] have implemented practice changes to limit use of opioids for chronic pain ... so I hope it’s getting better. It’s important to be aware of our patterns in prescribing.”
The study was funded by the National Institute of Diabetes and Digestive and Kidney Diseases. Dr. McCoy reported receiving grants from the AARP Quality Measure Innovation program through a collaboration with OptumLabs and the Mayo Clinic’s Robert D. and Patricia E. Kern Center for the Science of Health Care Delivery.
A version of this article first appeared on Medscape.com.
Prescriptions for opioids as a first-line treatment for painful diabetic peripheral neuropathy (DPN) outnumbered those for other medications between 2014 and 2018, despite the fact that the former is not recommended, new research indicates.
“We know that for any kind of chronic pain, opioids are not ideal. They’re not very effective for chronic pain in general, and they’re definitely not safe,” senior author Rozalina G. McCoy, MD, an endocrinologist and primary care clinician at the Mayo Clinic in Rochester, Minn., told this news organization.
That’s true even for severe DPN pain or painful exacerbations, she added.
“There’s a myth that opioids are the strongest pain meds possible ... For painful neuropathic pain, duloxetine [Cymbalta], pregabalin [Lyrica], and gabapentin [Neurontin] are the most effective pain medications based on multiple studies and extensive experience using them,” she explained. “But I think the public perception is that opioids are the strongest. When a patient comes with severe pain, I think there’s that kind of gut feeling that if the pain is severe, I need to give opioids.”
What’s more, she noted, “evidence is emerging for other harms, not only the potential for dependency and potential overdose, but also the potential for opioid-induced hyperalgesia. Opioids themselves can cause chronic pain. When we think about using opioids for chronic pain, we are really shooting ourselves in the foot. We’re going to harm patients.”
The American Diabetes Association DPN guidelines essentially say as much, advising opioids only as a tertiary option for refractory pain, she observed.
The new findings, from a retrospective study of Mayo Clinic electronic health data, were published online in JAMA Network Open by Jungwei Fan, PhD, also of Mayo Clinic, and colleagues.
Are fewer patients with DPN receiving any treatment now?
The data also reveal that, while opioid prescribing dropped over the study period, there wasn’t a comparable rise in prescriptions of recommended pain medications, suggesting that recent efforts to minimize opioid prescribing may have resulted in less overall treatment of significant pain. (The study had to be stopped in 2018 when Mayo switched to a new electronic health record system, Dr. McCoy explained.)
“The proportion of opioids among new prescriptions has been decreasing. I’m hopeful that the rates are even lower now than they were 2 years ago. What was concerning to me was the proportion of people receiving treatment overall had gone down,” Dr. McCoy noted.
“So, while it’s great that opioids aren’t being used, it’s doubtful that people with DPN are any less symptomatic. So I worry that there’s a proportion of patients who have pain who aren’t getting the treatment they need just because we don’t want to give them opioids. There are other options,” Dr. McCoy said, including nonpharmacologic approaches.
Opioids dominated in new-onset DPN prescribing during 2014-2018
The study involved 3,495 adults with newly diagnosed DPN from all three Mayo Clinic locations in Rochester, Minn.; Phoenix, Ariz.; and Jacksonville, Fla. during the period 2014-2018. Of those, 40.2% (1,406) were prescribed a new pain medication after diagnosis. However, that proportion dropped from 45.6% in 2014 to 35.2% in 2018.
The odds of initiating any treatment were significantly greater among patients with depression (odds ratio, 1.61), arthritis (OR, 1.21), and back pain (OR, 1.34), but decreased over time among all patients.
Among those receiving drug treatment, opioids were prescribed to 43.8%, whereas guideline-recommended medications (gabapentin, pregabalin, and serotonin norepinephrine reuptake inhibitors including duloxetine) were prescribed to 42.9%.
Another 20.6% received medications deemed “acceptable” for treating neuropathic pain, including topical analgesics, tricyclic antidepressants, and other anticonvulsants.
Males were significantly more likely than females to receive opioids (OR, 1.26), while individuals diagnosed with comorbid fibromyalgia were less likely (OR, 0.67). Those with comorbid arthritis were less likely to receive recommended DPN medications (OR, 0.76).
Use of opioids was 29% less likely in 2018, compared with 2014, although this difference did not achieve significance. Similarly, use of recommended medications was 25% more likely in 2018, compared with 2014, also not a significant difference.
Dr. McCoy offers clinical pearls for treating pain in DPN
Clinically, Dr. McCoy said that she individualizes treatment for painful DPN.
“I tend to use duloxetine if the patient also has a mood disorder including depression or anxiety, because it can also help with that. Gabapentin can also be helpful for radiculopathy or for chronic low-back pain. It can even help with degenerative joint disease like arthritis of the knees. So, you maximize benefit if you use one drug to treat multiple things.”
All three recommended medications are generic now, although pregabalin still tends to be more expensive, she noted. Gabapentin can cause drowsiness, which makes it ideal for a patient with insomnia but much less so for a long-haul truck driver. Duloxetine doesn’t cause sleepiness. Pregabalin can, but less so than gabapentin.
“I think that’s why it’s so important to talk to your patient and ask how the neuropathy is affecting them. What other comorbidities do they have? What is their life like? I think you have to figure out what drug works for each individual person.”
Importantly, she advised, if one of the three doesn’t work, stop it and try another. “It doesn’t mean that none of these meds work. All three should be tried to see if they give relief.”
Nonpharmacologic measures such as cognitive behavioral therapy, acupuncture, or physical therapy may help some patients as well.
Supplements such as vitamin B12 – which can also help with metformin-induced B12 deficiency – or alpha-lipoic acid may also be worth a try as long as the patient is made aware of potential risks, she noted.
Dr. McCoy hopes to repeat this study using national data. “I don’t think this is isolated to Mayo ... I think it affects all practices,” she said.
Since the study, “we [Mayo Clinic] have implemented practice changes to limit use of opioids for chronic pain ... so I hope it’s getting better. It’s important to be aware of our patterns in prescribing.”
The study was funded by the National Institute of Diabetes and Digestive and Kidney Diseases. Dr. McCoy reported receiving grants from the AARP Quality Measure Innovation program through a collaboration with OptumLabs and the Mayo Clinic’s Robert D. and Patricia E. Kern Center for the Science of Health Care Delivery.
A version of this article first appeared on Medscape.com.
Prescriptions for opioids as a first-line treatment for painful diabetic peripheral neuropathy (DPN) outnumbered those for other medications between 2014 and 2018, despite the fact that the former is not recommended, new research indicates.
“We know that for any kind of chronic pain, opioids are not ideal. They’re not very effective for chronic pain in general, and they’re definitely not safe,” senior author Rozalina G. McCoy, MD, an endocrinologist and primary care clinician at the Mayo Clinic in Rochester, Minn., told this news organization.
That’s true even for severe DPN pain or painful exacerbations, she added.
“There’s a myth that opioids are the strongest pain meds possible ... For painful neuropathic pain, duloxetine [Cymbalta], pregabalin [Lyrica], and gabapentin [Neurontin] are the most effective pain medications based on multiple studies and extensive experience using them,” she explained. “But I think the public perception is that opioids are the strongest. When a patient comes with severe pain, I think there’s that kind of gut feeling that if the pain is severe, I need to give opioids.”
What’s more, she noted, “evidence is emerging for other harms, not only the potential for dependency and potential overdose, but also the potential for opioid-induced hyperalgesia. Opioids themselves can cause chronic pain. When we think about using opioids for chronic pain, we are really shooting ourselves in the foot. We’re going to harm patients.”
The American Diabetes Association DPN guidelines essentially say as much, advising opioids only as a tertiary option for refractory pain, she observed.
The new findings, from a retrospective study of Mayo Clinic electronic health data, were published online in JAMA Network Open by Jungwei Fan, PhD, also of Mayo Clinic, and colleagues.
Are fewer patients with DPN receiving any treatment now?
The data also reveal that, while opioid prescribing dropped over the study period, there wasn’t a comparable rise in prescriptions of recommended pain medications, suggesting that recent efforts to minimize opioid prescribing may have resulted in less overall treatment of significant pain. (The study had to be stopped in 2018 when Mayo switched to a new electronic health record system, Dr. McCoy explained.)
“The proportion of opioids among new prescriptions has been decreasing. I’m hopeful that the rates are even lower now than they were 2 years ago. What was concerning to me was the proportion of people receiving treatment overall had gone down,” Dr. McCoy noted.
“So, while it’s great that opioids aren’t being used, it’s doubtful that people with DPN are any less symptomatic. So I worry that there’s a proportion of patients who have pain who aren’t getting the treatment they need just because we don’t want to give them opioids. There are other options,” Dr. McCoy said, including nonpharmacologic approaches.
Opioids dominated in new-onset DPN prescribing during 2014-2018
The study involved 3,495 adults with newly diagnosed DPN from all three Mayo Clinic locations in Rochester, Minn.; Phoenix, Ariz.; and Jacksonville, Fla. during the period 2014-2018. Of those, 40.2% (1,406) were prescribed a new pain medication after diagnosis. However, that proportion dropped from 45.6% in 2014 to 35.2% in 2018.
The odds of initiating any treatment were significantly greater among patients with depression (odds ratio, 1.61), arthritis (OR, 1.21), and back pain (OR, 1.34), but decreased over time among all patients.
Among those receiving drug treatment, opioids were prescribed to 43.8%, whereas guideline-recommended medications (gabapentin, pregabalin, and serotonin norepinephrine reuptake inhibitors including duloxetine) were prescribed to 42.9%.
Another 20.6% received medications deemed “acceptable” for treating neuropathic pain, including topical analgesics, tricyclic antidepressants, and other anticonvulsants.
Males were significantly more likely than females to receive opioids (OR, 1.26), while individuals diagnosed with comorbid fibromyalgia were less likely (OR, 0.67). Those with comorbid arthritis were less likely to receive recommended DPN medications (OR, 0.76).
Use of opioids was 29% less likely in 2018, compared with 2014, although this difference did not achieve significance. Similarly, use of recommended medications was 25% more likely in 2018, compared with 2014, also not a significant difference.
Dr. McCoy offers clinical pearls for treating pain in DPN
Clinically, Dr. McCoy said that she individualizes treatment for painful DPN.
“I tend to use duloxetine if the patient also has a mood disorder including depression or anxiety, because it can also help with that. Gabapentin can also be helpful for radiculopathy or for chronic low-back pain. It can even help with degenerative joint disease like arthritis of the knees. So, you maximize benefit if you use one drug to treat multiple things.”
All three recommended medications are generic now, although pregabalin still tends to be more expensive, she noted. Gabapentin can cause drowsiness, which makes it ideal for a patient with insomnia but much less so for a long-haul truck driver. Duloxetine doesn’t cause sleepiness. Pregabalin can, but less so than gabapentin.
“I think that’s why it’s so important to talk to your patient and ask how the neuropathy is affecting them. What other comorbidities do they have? What is their life like? I think you have to figure out what drug works for each individual person.”
Importantly, she advised, if one of the three doesn’t work, stop it and try another. “It doesn’t mean that none of these meds work. All three should be tried to see if they give relief.”
Nonpharmacologic measures such as cognitive behavioral therapy, acupuncture, or physical therapy may help some patients as well.
Supplements such as vitamin B12 – which can also help with metformin-induced B12 deficiency – or alpha-lipoic acid may also be worth a try as long as the patient is made aware of potential risks, she noted.
Dr. McCoy hopes to repeat this study using national data. “I don’t think this is isolated to Mayo ... I think it affects all practices,” she said.
Since the study, “we [Mayo Clinic] have implemented practice changes to limit use of opioids for chronic pain ... so I hope it’s getting better. It’s important to be aware of our patterns in prescribing.”
The study was funded by the National Institute of Diabetes and Digestive and Kidney Diseases. Dr. McCoy reported receiving grants from the AARP Quality Measure Innovation program through a collaboration with OptumLabs and the Mayo Clinic’s Robert D. and Patricia E. Kern Center for the Science of Health Care Delivery.
A version of this article first appeared on Medscape.com.
FDA clears novel daytime device for obstructive sleep apnea
eXciteOSA (Signifier Medical Technologies) is a prescription-only, neuromuscular stimulation device designed to improve tongue muscle function, which, over time, can help prevent the tongue from collapsing backwards and obstructing the airway during sleep, the FDA said.
The eXciteOSA mouthpiece has four electrodes that deliver a series of electrical pulses with rest periods in between. Two electrodes are located above the tongue and two are located below the tongue.
The patient uses the device for 20 minutes once a day while awake for 6 weeks, and once a week thereafter. It is indicated for adults aged 18 and older with snoring and mild OSA.
OSA is marked by the recurring collapse of the upper airways during sleep, intermittently reducing or completely blocking airflow. Common symptoms include snoring, restless sleep and daytime sleepiness. Untreated OSA can lead to serious complications such as cardiovascular disease and cognitive and behavioral disorders.
Continuous positive airway pressure therapy, administered through a face mask that is worn while asleep, is a first-line treatment for OSA.
The eXciteOSA device “offers a new option for the thousands of individuals who experience snoring or mild sleep apnea,” Malvina Eydelman, MD, director, FDA Office of Ophthalmic, Anesthesia, Respiratory, ENT, and Dental Devices, said in a news release.
The FDA reviewed data on the safety and effectiveness of the eXciteOSA device in 115 patients with snoring, including 48 patients with snoring and mild OSA. All patients used the device for 20 minutes once a day for 6 weeks, then stopped using it for 2 weeks before they were reassessed.
Overall, the percentage of time spent snoring at levels louder than 40 decibels was reduced by more than 20% in 87 out of the 115 patients.
In the subset of patients with snoring and mild OSA, the average apnea-hypopnea index score was reduced by 48%, from 10.21 to 5.27, in 41 of 48 patients. Mild OSA is defined as an AHI score greater than 5 but less than 15.
The most common adverse events were excessive salivation, tongue or tooth discomfort, tongue tingling, dental filling sensitivity, metallic taste, gagging, and tight jaw.
Before using the eXciteOSA device, patients should receive a comprehensive dental examination, the FDA said.
The device should not be used in patients with pacemakers or implanted pacing leads, or women who are pregnant. The device is also contraindicated in patients with temporary or permanent implants, dental braces, intraoral metal prosthesis/restorations, or ulcerations in or around the mouth.
The eXciteOSA device was approved under the de novo premarket review pathway for new low- to moderate-risk devices. More information on the device is available online.
A version of this article first appeared on Medscape.com.
eXciteOSA (Signifier Medical Technologies) is a prescription-only, neuromuscular stimulation device designed to improve tongue muscle function, which, over time, can help prevent the tongue from collapsing backwards and obstructing the airway during sleep, the FDA said.
The eXciteOSA mouthpiece has four electrodes that deliver a series of electrical pulses with rest periods in between. Two electrodes are located above the tongue and two are located below the tongue.
The patient uses the device for 20 minutes once a day while awake for 6 weeks, and once a week thereafter. It is indicated for adults aged 18 and older with snoring and mild OSA.
OSA is marked by the recurring collapse of the upper airways during sleep, intermittently reducing or completely blocking airflow. Common symptoms include snoring, restless sleep and daytime sleepiness. Untreated OSA can lead to serious complications such as cardiovascular disease and cognitive and behavioral disorders.
Continuous positive airway pressure therapy, administered through a face mask that is worn while asleep, is a first-line treatment for OSA.
The eXciteOSA device “offers a new option for the thousands of individuals who experience snoring or mild sleep apnea,” Malvina Eydelman, MD, director, FDA Office of Ophthalmic, Anesthesia, Respiratory, ENT, and Dental Devices, said in a news release.
The FDA reviewed data on the safety and effectiveness of the eXciteOSA device in 115 patients with snoring, including 48 patients with snoring and mild OSA. All patients used the device for 20 minutes once a day for 6 weeks, then stopped using it for 2 weeks before they were reassessed.
Overall, the percentage of time spent snoring at levels louder than 40 decibels was reduced by more than 20% in 87 out of the 115 patients.
In the subset of patients with snoring and mild OSA, the average apnea-hypopnea index score was reduced by 48%, from 10.21 to 5.27, in 41 of 48 patients. Mild OSA is defined as an AHI score greater than 5 but less than 15.
The most common adverse events were excessive salivation, tongue or tooth discomfort, tongue tingling, dental filling sensitivity, metallic taste, gagging, and tight jaw.
Before using the eXciteOSA device, patients should receive a comprehensive dental examination, the FDA said.
The device should not be used in patients with pacemakers or implanted pacing leads, or women who are pregnant. The device is also contraindicated in patients with temporary or permanent implants, dental braces, intraoral metal prosthesis/restorations, or ulcerations in or around the mouth.
The eXciteOSA device was approved under the de novo premarket review pathway for new low- to moderate-risk devices. More information on the device is available online.
A version of this article first appeared on Medscape.com.
eXciteOSA (Signifier Medical Technologies) is a prescription-only, neuromuscular stimulation device designed to improve tongue muscle function, which, over time, can help prevent the tongue from collapsing backwards and obstructing the airway during sleep, the FDA said.
The eXciteOSA mouthpiece has four electrodes that deliver a series of electrical pulses with rest periods in between. Two electrodes are located above the tongue and two are located below the tongue.
The patient uses the device for 20 minutes once a day while awake for 6 weeks, and once a week thereafter. It is indicated for adults aged 18 and older with snoring and mild OSA.
OSA is marked by the recurring collapse of the upper airways during sleep, intermittently reducing or completely blocking airflow. Common symptoms include snoring, restless sleep and daytime sleepiness. Untreated OSA can lead to serious complications such as cardiovascular disease and cognitive and behavioral disorders.
Continuous positive airway pressure therapy, administered through a face mask that is worn while asleep, is a first-line treatment for OSA.
The eXciteOSA device “offers a new option for the thousands of individuals who experience snoring or mild sleep apnea,” Malvina Eydelman, MD, director, FDA Office of Ophthalmic, Anesthesia, Respiratory, ENT, and Dental Devices, said in a news release.
The FDA reviewed data on the safety and effectiveness of the eXciteOSA device in 115 patients with snoring, including 48 patients with snoring and mild OSA. All patients used the device for 20 minutes once a day for 6 weeks, then stopped using it for 2 weeks before they were reassessed.
Overall, the percentage of time spent snoring at levels louder than 40 decibels was reduced by more than 20% in 87 out of the 115 patients.
In the subset of patients with snoring and mild OSA, the average apnea-hypopnea index score was reduced by 48%, from 10.21 to 5.27, in 41 of 48 patients. Mild OSA is defined as an AHI score greater than 5 but less than 15.
The most common adverse events were excessive salivation, tongue or tooth discomfort, tongue tingling, dental filling sensitivity, metallic taste, gagging, and tight jaw.
Before using the eXciteOSA device, patients should receive a comprehensive dental examination, the FDA said.
The device should not be used in patients with pacemakers or implanted pacing leads, or women who are pregnant. The device is also contraindicated in patients with temporary or permanent implants, dental braces, intraoral metal prosthesis/restorations, or ulcerations in or around the mouth.
The eXciteOSA device was approved under the de novo premarket review pathway for new low- to moderate-risk devices. More information on the device is available online.
A version of this article first appeared on Medscape.com.
Combo delivers ‘impressive’ survival results in first-line RCC setting
Results from the phase 3 trial were reported at the 2021 Genitourinary Cancers Symposium (Abstract 269) and simultaneously published in the New England Journal of Medicine.
Early-phase trials have shown the promise of targeting RCC from two angles, with both antiangiogenic therapy and immunotherapy, said presenter Robert J. Motzer, MD, of Memorial Sloan Kettering Cancer Center, New York.
The CLEAR trial was designed to compare monotherapy with sunitinib to treatment with lenvatinib plus either pembrolizumab or everolimus.
The risk of progression-free survival events was 61% lower with lenvatinib-pembrolizumab and 35% lower with lenvatinib-everolimus, compared with sunitinib. However, only the first combination significantly reduced the risk of death.
Treatment-related adverse events were more common with both combinations but manageable with dose modifications.
“These results support lenvatinib plus pembrolizumab as a potential first-line treatment for patients with advanced RCC,” Dr. Motzer said.
Oncologists will likely soon have a handful of first-line options from which to choose, he acknowledged.
“It is a great situation, that we have made such progress in RCC with IO [immuno-oncology] therapy in the first line with ipilimumab-nivolumab, and now with the IO-TKI [tyrosine kinase inhibitor] combinations,” Dr. Motzer said.
The choice will probably come down to personal preference, experience with the various combinations, and side effect profiles, he speculated.
“I will say, however, that the data with lenvatinib-pembrolizumab is very impressive in terms of the long progression-free survival, in terms of the doubling of response rate to over 70%, in terms of the 16% complete response rate,” he said.
Trial details
The CLEAR investigators evenly randomized 1,069 patients with advanced clear-cell RCC who had not received prior systemic therapy to treatment with lenvatinib-pembrolizumab, lenvatinib-everolimus, or sunitinib.
The primary analysis was conducted at a median follow-up of 27 months.
The median progression-free survival was 9.2 months with sunitinib, 23.9 months with lenvatinib-pembrolizumab (hazard ratio, 0.39; P < .001), and 14.7 months with lenvatinib-everolimus (HR for events, 0.65; P < .001).
Findings were similar across key subgroups, including International Metastatic RCC Database Consortium risk groups.
An interim analysis of overall survival showed that patients lived significantly longer with lenvatinib-pembrolizumab versus sunitinib (HR, 0.66; P = .005), with similar benefit across subgroups, except for the favorable risk group.
In contrast, lenvatinib-everolimus did not significantly improve overall survival (HR, 1.15; P = .3). The median overall survival was not reached in any treatment arm.
“To me, this emphasizes the role of IO therapy combinations in the first line. I think you need the IO in the first line to get the dramatic efficacy results that we saw in the CLEAR study,” Dr. Motzer said.
The confirmed objective response rate was 36.1% with sunitinib, 71.0% with lenvatinib-pembrolizumab (relative risk, 1.97; P < .001), and 53.5% with lenvatinib-everolimus (RR, 1.48; P <.001). The median duration of response was 14.6 months, 25.8 months, and 16.6 months, respectively.
Grade 3 or higher treatment-related adverse events occurred in 58.8% of patients in the sunitinib group, 71.6% of the lenvatinib-pembrolizumab group, and 73.0% of the lenvatinib-everolimus group. The higher rates with the combinations likely reflected longer treatment durations, according to Dr. Motzer.
The most common grade 3 or higher events with lenvatinib-pembrolizumab were hypertension (25.3%), diarrhea (8.2%), and proteinuria (7.4%). The most common grade 3 or higher events with lenvatinib-everolimus were hypertension (20.8%), hypertriglyceridemia (10.1%), and diarrhea (9.6%).
“The relatively low rates of hepatic toxicity, lack of myelosuppression, and low rate of high-grade hand-foot syndrome is an attractive feature for lenvatinib in combination,” Dr. Motzer said.
Which combination, which sequence?
“Lenvatinib plus pembrolizumab is another novel combination to have in our armamentarium now for first-line clear-cell RCC,” said invited discussant Stephanie A. Berg, DO, of Loyola University Medical Center in Maywood, Ill.
CLEAR is the fourth positive trial of combination tyrosine kinase inhibitor therapy and immunotherapy in this setting, although findings and study populations differ somewhat, and longer follow-up is needed, she said.
“None of these combinations have been directly compared to one another, and I don’t believe they will be compared head to head,” Dr. Berg said. “But other characteristics – for example, health-related quality of life, familiarity of the agents for clinicians, and high tumor burden versus slow-growing disease – may become important to choose the best first-line option for our patients.”
The emerging first-line options also raise some questions about the optimal sequencing of agents, according to Dr. Berg.
“If one starts with combination immunotherapy, it becomes an automatic choice to use a VEGF tyrosine kinase inhibitor second line,” she elaborated. “These trials establish that immuno-oncology–tyrosine kinase inhibitor combination therapy is now standard of care, but our second-line choice is less clear. Therefore, data is needed on the most suitable order of therapy for the entire population, as well as specific groups in the future.”
The CLEAR trial was sponsored by Eisai Inc. and Merck Sharp & Dohme Corp. Dr. Motzer disclosed relationships with Eisai, Merck, and many other companies. Dr. Berg disclosed a consulting or advisory role with Bristol-Myers Squibb.
Results from the phase 3 trial were reported at the 2021 Genitourinary Cancers Symposium (Abstract 269) and simultaneously published in the New England Journal of Medicine.
Early-phase trials have shown the promise of targeting RCC from two angles, with both antiangiogenic therapy and immunotherapy, said presenter Robert J. Motzer, MD, of Memorial Sloan Kettering Cancer Center, New York.
The CLEAR trial was designed to compare monotherapy with sunitinib to treatment with lenvatinib plus either pembrolizumab or everolimus.
The risk of progression-free survival events was 61% lower with lenvatinib-pembrolizumab and 35% lower with lenvatinib-everolimus, compared with sunitinib. However, only the first combination significantly reduced the risk of death.
Treatment-related adverse events were more common with both combinations but manageable with dose modifications.
“These results support lenvatinib plus pembrolizumab as a potential first-line treatment for patients with advanced RCC,” Dr. Motzer said.
Oncologists will likely soon have a handful of first-line options from which to choose, he acknowledged.
“It is a great situation, that we have made such progress in RCC with IO [immuno-oncology] therapy in the first line with ipilimumab-nivolumab, and now with the IO-TKI [tyrosine kinase inhibitor] combinations,” Dr. Motzer said.
The choice will probably come down to personal preference, experience with the various combinations, and side effect profiles, he speculated.
“I will say, however, that the data with lenvatinib-pembrolizumab is very impressive in terms of the long progression-free survival, in terms of the doubling of response rate to over 70%, in terms of the 16% complete response rate,” he said.
Trial details
The CLEAR investigators evenly randomized 1,069 patients with advanced clear-cell RCC who had not received prior systemic therapy to treatment with lenvatinib-pembrolizumab, lenvatinib-everolimus, or sunitinib.
The primary analysis was conducted at a median follow-up of 27 months.
The median progression-free survival was 9.2 months with sunitinib, 23.9 months with lenvatinib-pembrolizumab (hazard ratio, 0.39; P < .001), and 14.7 months with lenvatinib-everolimus (HR for events, 0.65; P < .001).
Findings were similar across key subgroups, including International Metastatic RCC Database Consortium risk groups.
An interim analysis of overall survival showed that patients lived significantly longer with lenvatinib-pembrolizumab versus sunitinib (HR, 0.66; P = .005), with similar benefit across subgroups, except for the favorable risk group.
In contrast, lenvatinib-everolimus did not significantly improve overall survival (HR, 1.15; P = .3). The median overall survival was not reached in any treatment arm.
“To me, this emphasizes the role of IO therapy combinations in the first line. I think you need the IO in the first line to get the dramatic efficacy results that we saw in the CLEAR study,” Dr. Motzer said.
The confirmed objective response rate was 36.1% with sunitinib, 71.0% with lenvatinib-pembrolizumab (relative risk, 1.97; P < .001), and 53.5% with lenvatinib-everolimus (RR, 1.48; P <.001). The median duration of response was 14.6 months, 25.8 months, and 16.6 months, respectively.
Grade 3 or higher treatment-related adverse events occurred in 58.8% of patients in the sunitinib group, 71.6% of the lenvatinib-pembrolizumab group, and 73.0% of the lenvatinib-everolimus group. The higher rates with the combinations likely reflected longer treatment durations, according to Dr. Motzer.
The most common grade 3 or higher events with lenvatinib-pembrolizumab were hypertension (25.3%), diarrhea (8.2%), and proteinuria (7.4%). The most common grade 3 or higher events with lenvatinib-everolimus were hypertension (20.8%), hypertriglyceridemia (10.1%), and diarrhea (9.6%).
“The relatively low rates of hepatic toxicity, lack of myelosuppression, and low rate of high-grade hand-foot syndrome is an attractive feature for lenvatinib in combination,” Dr. Motzer said.
Which combination, which sequence?
“Lenvatinib plus pembrolizumab is another novel combination to have in our armamentarium now for first-line clear-cell RCC,” said invited discussant Stephanie A. Berg, DO, of Loyola University Medical Center in Maywood, Ill.
CLEAR is the fourth positive trial of combination tyrosine kinase inhibitor therapy and immunotherapy in this setting, although findings and study populations differ somewhat, and longer follow-up is needed, she said.
“None of these combinations have been directly compared to one another, and I don’t believe they will be compared head to head,” Dr. Berg said. “But other characteristics – for example, health-related quality of life, familiarity of the agents for clinicians, and high tumor burden versus slow-growing disease – may become important to choose the best first-line option for our patients.”
The emerging first-line options also raise some questions about the optimal sequencing of agents, according to Dr. Berg.
“If one starts with combination immunotherapy, it becomes an automatic choice to use a VEGF tyrosine kinase inhibitor second line,” she elaborated. “These trials establish that immuno-oncology–tyrosine kinase inhibitor combination therapy is now standard of care, but our second-line choice is less clear. Therefore, data is needed on the most suitable order of therapy for the entire population, as well as specific groups in the future.”
The CLEAR trial was sponsored by Eisai Inc. and Merck Sharp & Dohme Corp. Dr. Motzer disclosed relationships with Eisai, Merck, and many other companies. Dr. Berg disclosed a consulting or advisory role with Bristol-Myers Squibb.
Results from the phase 3 trial were reported at the 2021 Genitourinary Cancers Symposium (Abstract 269) and simultaneously published in the New England Journal of Medicine.
Early-phase trials have shown the promise of targeting RCC from two angles, with both antiangiogenic therapy and immunotherapy, said presenter Robert J. Motzer, MD, of Memorial Sloan Kettering Cancer Center, New York.
The CLEAR trial was designed to compare monotherapy with sunitinib to treatment with lenvatinib plus either pembrolizumab or everolimus.
The risk of progression-free survival events was 61% lower with lenvatinib-pembrolizumab and 35% lower with lenvatinib-everolimus, compared with sunitinib. However, only the first combination significantly reduced the risk of death.
Treatment-related adverse events were more common with both combinations but manageable with dose modifications.
“These results support lenvatinib plus pembrolizumab as a potential first-line treatment for patients with advanced RCC,” Dr. Motzer said.
Oncologists will likely soon have a handful of first-line options from which to choose, he acknowledged.
“It is a great situation, that we have made such progress in RCC with IO [immuno-oncology] therapy in the first line with ipilimumab-nivolumab, and now with the IO-TKI [tyrosine kinase inhibitor] combinations,” Dr. Motzer said.
The choice will probably come down to personal preference, experience with the various combinations, and side effect profiles, he speculated.
“I will say, however, that the data with lenvatinib-pembrolizumab is very impressive in terms of the long progression-free survival, in terms of the doubling of response rate to over 70%, in terms of the 16% complete response rate,” he said.
Trial details
The CLEAR investigators evenly randomized 1,069 patients with advanced clear-cell RCC who had not received prior systemic therapy to treatment with lenvatinib-pembrolizumab, lenvatinib-everolimus, or sunitinib.
The primary analysis was conducted at a median follow-up of 27 months.
The median progression-free survival was 9.2 months with sunitinib, 23.9 months with lenvatinib-pembrolizumab (hazard ratio, 0.39; P < .001), and 14.7 months with lenvatinib-everolimus (HR for events, 0.65; P < .001).
Findings were similar across key subgroups, including International Metastatic RCC Database Consortium risk groups.
An interim analysis of overall survival showed that patients lived significantly longer with lenvatinib-pembrolizumab versus sunitinib (HR, 0.66; P = .005), with similar benefit across subgroups, except for the favorable risk group.
In contrast, lenvatinib-everolimus did not significantly improve overall survival (HR, 1.15; P = .3). The median overall survival was not reached in any treatment arm.
“To me, this emphasizes the role of IO therapy combinations in the first line. I think you need the IO in the first line to get the dramatic efficacy results that we saw in the CLEAR study,” Dr. Motzer said.
The confirmed objective response rate was 36.1% with sunitinib, 71.0% with lenvatinib-pembrolizumab (relative risk, 1.97; P < .001), and 53.5% with lenvatinib-everolimus (RR, 1.48; P <.001). The median duration of response was 14.6 months, 25.8 months, and 16.6 months, respectively.
Grade 3 or higher treatment-related adverse events occurred in 58.8% of patients in the sunitinib group, 71.6% of the lenvatinib-pembrolizumab group, and 73.0% of the lenvatinib-everolimus group. The higher rates with the combinations likely reflected longer treatment durations, according to Dr. Motzer.
The most common grade 3 or higher events with lenvatinib-pembrolizumab were hypertension (25.3%), diarrhea (8.2%), and proteinuria (7.4%). The most common grade 3 or higher events with lenvatinib-everolimus were hypertension (20.8%), hypertriglyceridemia (10.1%), and diarrhea (9.6%).
“The relatively low rates of hepatic toxicity, lack of myelosuppression, and low rate of high-grade hand-foot syndrome is an attractive feature for lenvatinib in combination,” Dr. Motzer said.
Which combination, which sequence?
“Lenvatinib plus pembrolizumab is another novel combination to have in our armamentarium now for first-line clear-cell RCC,” said invited discussant Stephanie A. Berg, DO, of Loyola University Medical Center in Maywood, Ill.
CLEAR is the fourth positive trial of combination tyrosine kinase inhibitor therapy and immunotherapy in this setting, although findings and study populations differ somewhat, and longer follow-up is needed, she said.
“None of these combinations have been directly compared to one another, and I don’t believe they will be compared head to head,” Dr. Berg said. “But other characteristics – for example, health-related quality of life, familiarity of the agents for clinicians, and high tumor burden versus slow-growing disease – may become important to choose the best first-line option for our patients.”
The emerging first-line options also raise some questions about the optimal sequencing of agents, according to Dr. Berg.
“If one starts with combination immunotherapy, it becomes an automatic choice to use a VEGF tyrosine kinase inhibitor second line,” she elaborated. “These trials establish that immuno-oncology–tyrosine kinase inhibitor combination therapy is now standard of care, but our second-line choice is less clear. Therefore, data is needed on the most suitable order of therapy for the entire population, as well as specific groups in the future.”
The CLEAR trial was sponsored by Eisai Inc. and Merck Sharp & Dohme Corp. Dr. Motzer disclosed relationships with Eisai, Merck, and many other companies. Dr. Berg disclosed a consulting or advisory role with Bristol-Myers Squibb.
FROM GUCS 2021
Family medicine: Who cares for the children?
according to new research.
This the latest sign of a long-term decline, and it “poses a broader concern for a specialty that defines itself by its comprehensive scope of practice,” said the study investigators of the Robert Graham Center in Washington, D.C., in a written statement. “This is consistent with previous Robert Graham Center research that reported a similar steady decline from 1992 to 2002.”
Self-reported data from family physicians indicate that 84.3% cared for children aged 18 years and under in 2017, compared with 83.0% in 2018, based on a cross-sectional analysis of data gathered from 11,674 family physicians who completed the practice demographic questionnaire attached to the American Board of Family Medicine’s certification exam in 2017 and 2018.
“This current trend is unsettling, because family physicians provide the majority of pediatric care in rural and pediatrically underserved areas of the United States,” study author Anuradha Jetty, MPH, and coauthors said in the statement.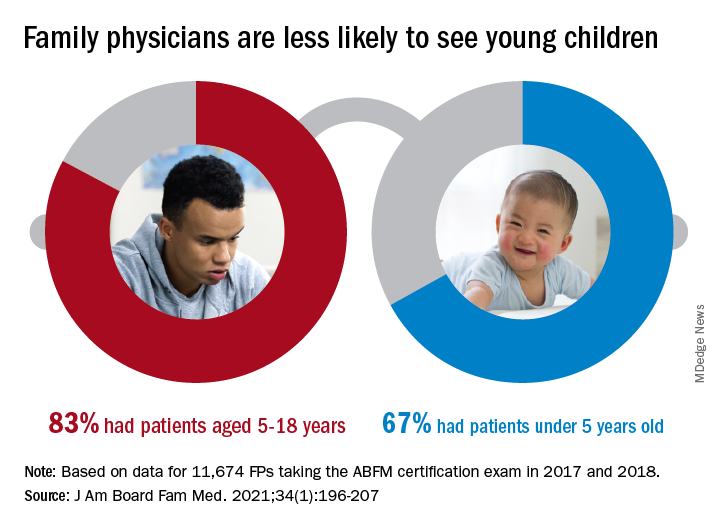
The analysis also offers a snapshot of the current state of pediatric care offered by family physicians. In 2017 and 2018, FPs were more likely to see patients aged 5-18 years than those under age 5 (83.0% vs. 67.0%), with variation by age, location, and race/ethnicity, said Ms. Jetty and colleagues, in their new paper.
FPs aged 60 years and older were much less likely to see pediatric patients, compared with those under age 40: odds ratios were 0.52 for children under 5 and 0.56 for children 5-18. Regional variation was even more pronounced: Compared with their colleagues in the Southern states, Midwestern FPs were 1.52 times as likely to treat children aged 5-18 and 2.52 times as likely to treat children under age 5, the investigators reported.
Non-Hispanic Asian and Hispanic family physicians had significantly lower odds of seeing pediatric patients, relative to non-Hispanic White family physicians, as did FPs who were international medical graduates (OR, 0.74), compared with those who trained in the United States, they said.
“Female gender was associated with seeing pediatric patients in a prior study using 2006-2009 [American Board of Family Medicine] data; however, we found no such association in 2017-2018,” Ms. Jetty and associates noted.
“Many diverse drivers likely influence the findings we observed, including organizational, personal, social, and economic factors,” they wrote, suggesting that the policies of some HMOs “may limit scope of practice for employed physicians,” while those who practice in areas of low pediatrician density might “capitalize on a market opportunity ... more than physicians in pediatrician-saturated areas with greater competition for young patients.”
The overall shortage of primary pediatric care may be a matter of debate, the investigators said, but “there is undoubtedly significant variability in the regional supply of pediatric primary care physicians and thus areas where family physicians are needed to meet current pediatric workforce demand.”
The authors reported no conflicts.
according to new research.

This the latest sign of a long-term decline, and it “poses a broader concern for a specialty that defines itself by its comprehensive scope of practice,” said the study investigators of the Robert Graham Center in Washington, D.C., in a written statement. “This is consistent with previous Robert Graham Center research that reported a similar steady decline from 1992 to 2002.”
Self-reported data from family physicians indicate that 84.3% cared for children aged 18 years and under in 2017, compared with 83.0% in 2018, based on a cross-sectional analysis of data gathered from 11,674 family physicians who completed the practice demographic questionnaire attached to the American Board of Family Medicine’s certification exam in 2017 and 2018.
“This current trend is unsettling, because family physicians provide the majority of pediatric care in rural and pediatrically underserved areas of the United States,” study author Anuradha Jetty, MPH, and coauthors said in the statement.
The analysis also offers a snapshot of the current state of pediatric care offered by family physicians. In 2017 and 2018, FPs were more likely to see patients aged 5-18 years than those under age 5 (83.0% vs. 67.0%), with variation by age, location, and race/ethnicity, said Ms. Jetty and colleagues, in their new paper.
FPs aged 60 years and older were much less likely to see pediatric patients, compared with those under age 40: odds ratios were 0.52 for children under 5 and 0.56 for children 5-18. Regional variation was even more pronounced: Compared with their colleagues in the Southern states, Midwestern FPs were 1.52 times as likely to treat children aged 5-18 and 2.52 times as likely to treat children under age 5, the investigators reported.
Non-Hispanic Asian and Hispanic family physicians had significantly lower odds of seeing pediatric patients, relative to non-Hispanic White family physicians, as did FPs who were international medical graduates (OR, 0.74), compared with those who trained in the United States, they said.
“Female gender was associated with seeing pediatric patients in a prior study using 2006-2009 [American Board of Family Medicine] data; however, we found no such association in 2017-2018,” Ms. Jetty and associates noted.
“Many diverse drivers likely influence the findings we observed, including organizational, personal, social, and economic factors,” they wrote, suggesting that the policies of some HMOs “may limit scope of practice for employed physicians,” while those who practice in areas of low pediatrician density might “capitalize on a market opportunity ... more than physicians in pediatrician-saturated areas with greater competition for young patients.”
The overall shortage of primary pediatric care may be a matter of debate, the investigators said, but “there is undoubtedly significant variability in the regional supply of pediatric primary care physicians and thus areas where family physicians are needed to meet current pediatric workforce demand.”
The authors reported no conflicts.
according to new research.

This the latest sign of a long-term decline, and it “poses a broader concern for a specialty that defines itself by its comprehensive scope of practice,” said the study investigators of the Robert Graham Center in Washington, D.C., in a written statement. “This is consistent with previous Robert Graham Center research that reported a similar steady decline from 1992 to 2002.”
Self-reported data from family physicians indicate that 84.3% cared for children aged 18 years and under in 2017, compared with 83.0% in 2018, based on a cross-sectional analysis of data gathered from 11,674 family physicians who completed the practice demographic questionnaire attached to the American Board of Family Medicine’s certification exam in 2017 and 2018.
“This current trend is unsettling, because family physicians provide the majority of pediatric care in rural and pediatrically underserved areas of the United States,” study author Anuradha Jetty, MPH, and coauthors said in the statement.
The analysis also offers a snapshot of the current state of pediatric care offered by family physicians. In 2017 and 2018, FPs were more likely to see patients aged 5-18 years than those under age 5 (83.0% vs. 67.0%), with variation by age, location, and race/ethnicity, said Ms. Jetty and colleagues, in their new paper.
FPs aged 60 years and older were much less likely to see pediatric patients, compared with those under age 40: odds ratios were 0.52 for children under 5 and 0.56 for children 5-18. Regional variation was even more pronounced: Compared with their colleagues in the Southern states, Midwestern FPs were 1.52 times as likely to treat children aged 5-18 and 2.52 times as likely to treat children under age 5, the investigators reported.
Non-Hispanic Asian and Hispanic family physicians had significantly lower odds of seeing pediatric patients, relative to non-Hispanic White family physicians, as did FPs who were international medical graduates (OR, 0.74), compared with those who trained in the United States, they said.
“Female gender was associated with seeing pediatric patients in a prior study using 2006-2009 [American Board of Family Medicine] data; however, we found no such association in 2017-2018,” Ms. Jetty and associates noted.
“Many diverse drivers likely influence the findings we observed, including organizational, personal, social, and economic factors,” they wrote, suggesting that the policies of some HMOs “may limit scope of practice for employed physicians,” while those who practice in areas of low pediatrician density might “capitalize on a market opportunity ... more than physicians in pediatrician-saturated areas with greater competition for young patients.”
The overall shortage of primary pediatric care may be a matter of debate, the investigators said, but “there is undoubtedly significant variability in the regional supply of pediatric primary care physicians and thus areas where family physicians are needed to meet current pediatric workforce demand.”
The authors reported no conflicts.
FROM THE JOURNAL OF THE AMERICAN BOARD OF FAMILY MEDICINE
New child COVID-19 cases decline as total passes 3 million
New COVID-19 cases in children continue to drop each week, but the total number of cases has now surpassed 3 million since the start of the pandemic, according to a report from the American Academy of Pediatrics and the Children’s Hospital Association.
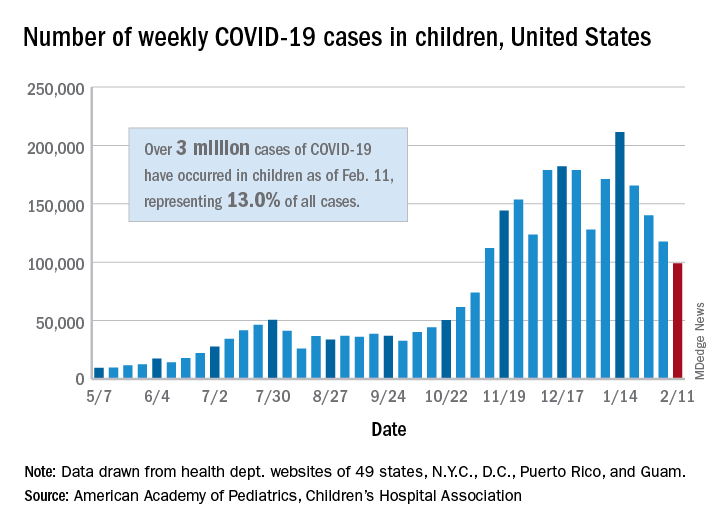
It was still enough, though, to bring the total to 3.03 million children infected with SARS-CoV-19 in the United States, the AAP and the CHA said in their weekly report.
The nation also hit a couple of other ignominious milestones. The cumulative rate of COVID-19 infection now stands at 4,030 per 100,000, so 4% of all children have been infected. Also, children represented 16.9% of all new cases for the week, which equals the highest proportion seen throughout the pandemic, based on data from health departments in 49 states (excluding New York), the District of Columbia, New York City, Puerto Rico, and Guam.
There have been 241 COVID-19–related deaths in children so far, with 14 reported during the week of Feb. 5-11. Kansas just recorded its first pediatric death, which leaves 10 states that have had no fatalities. Texas, with 39 deaths, has had more than any other state, among the 43 that are reporting mortality by age, the AAP/CHA report showed.
New COVID-19 cases in children continue to drop each week, but the total number of cases has now surpassed 3 million since the start of the pandemic, according to a report from the American Academy of Pediatrics and the Children’s Hospital Association.

It was still enough, though, to bring the total to 3.03 million children infected with SARS-CoV-19 in the United States, the AAP and the CHA said in their weekly report.
The nation also hit a couple of other ignominious milestones. The cumulative rate of COVID-19 infection now stands at 4,030 per 100,000, so 4% of all children have been infected. Also, children represented 16.9% of all new cases for the week, which equals the highest proportion seen throughout the pandemic, based on data from health departments in 49 states (excluding New York), the District of Columbia, New York City, Puerto Rico, and Guam.
There have been 241 COVID-19–related deaths in children so far, with 14 reported during the week of Feb. 5-11. Kansas just recorded its first pediatric death, which leaves 10 states that have had no fatalities. Texas, with 39 deaths, has had more than any other state, among the 43 that are reporting mortality by age, the AAP/CHA report showed.
New COVID-19 cases in children continue to drop each week, but the total number of cases has now surpassed 3 million since the start of the pandemic, according to a report from the American Academy of Pediatrics and the Children’s Hospital Association.

It was still enough, though, to bring the total to 3.03 million children infected with SARS-CoV-19 in the United States, the AAP and the CHA said in their weekly report.
The nation also hit a couple of other ignominious milestones. The cumulative rate of COVID-19 infection now stands at 4,030 per 100,000, so 4% of all children have been infected. Also, children represented 16.9% of all new cases for the week, which equals the highest proportion seen throughout the pandemic, based on data from health departments in 49 states (excluding New York), the District of Columbia, New York City, Puerto Rico, and Guam.
There have been 241 COVID-19–related deaths in children so far, with 14 reported during the week of Feb. 5-11. Kansas just recorded its first pediatric death, which leaves 10 states that have had no fatalities. Texas, with 39 deaths, has had more than any other state, among the 43 that are reporting mortality by age, the AAP/CHA report showed.