User login
Primary care isn’t bouncing back
published by the Primary Care Collaborative (PCC) and Larry A. Green Center.
Since mid-March these organizations have issued short weekly and biweekly surveys to U.S. primary care physicians in an attempt to find the pulse of the county’s first line of care. “There is not a federal office for primary care, and it’s been anemically funded for decades,” Rebecca Etz, PhD, said in an interview. Yet these clinics represent the front lines of U.S. health care, and it’s where most Americans go for care and COVID-19 care, said Dr. Etz, director of the Virginia-based Larry A. Green Center, which is devoted to primary care research, development, and advocacy.
The latest survey responses, collected between Sept. 4 and 6, confirm what researchers had suspected: Primary care isn’t on solid footing. Eighty-one percent of respondents disagreed emphatically that primary care has bounced back, and another 13% said things were better than earlier this year but not normal.
Meanwhile, 35% of respondents said that revenue and pay are significantly lower than they were before the pandemic and net losses threaten their practices’ viability. Almost half (49%) said their mental exhaustion from work was at an all-time high.
“Because of how our system is set up – it’s a fee-for-service model – the more patients you see, the more money you get,” said Yalda Jabbarpour, MD, medical director at the Graham Center, a leading think tank on family medicine and health care policy. But the stay-at-home order, aversion to telehealth, and fear of in-person visits have been keeping patients away – and driving primary care revenue down. Even when practices transition to and expand their telehealth, payer reimbursement is not yet on parity with in-person visits.
Right now, primary care physicians are doing fewer procedures and spending more time on video visits. “So you may have the same overhead and time investment but you’re getting paid a fraction,” Dr. Etz said. In August, 50% of primary care physicians reported they were working the same or more hours per week as they did before the pandemic but for less money, according to an earlier survey from the Green Center and the PCC. That loss of revenue is compounded by the need for expensive personal protective equipment and preparation for the upcoming flu season, Dr. Etz said.
Ongoing surveys reveal stress
Over the last 20 weeks or so, the Green Center and PCC together have disseminated weekly (through June) or biweekly surveys to 100 professional organizations. Because there isn’t an entity that represents all of primary care and claims data take years to process, these surveys are intended to get real-time feedback from clinicians who are providing a lot of patient care during the pandemic.
The sample sizes are admittedly small, with the most recent survey including 489 clinicians. Dr. Jabbarpour noted, “489 – it’s a good number, but you would want more.” Generally, for a great survey response you’d want 20%-30% of the physician population because then you could assume you’re getting a good mix of geographies, practice sizes, and settings, she said.
Respondents to the most recent survey were from 49 different states; 70% identified their practice as family medicine. One-third had between one and three physicians in their practice and 40% had 10 or more clinicians. “It’s not perfect, but it sounds pretty representative of the primary care workforce,” Dr. Jabbarpour continued.
The latest Green Center-PCC survey, published last week, also found that one in five physicians surveyed said at least one clinician in their practice had opted for early retirement or left practice as a direct result of the pandemic. These declines in clinician staffing come as school reopenings threaten to cause a resurgence of COVID-19 cases and the 2021 flu season could complicate COVID-19 care and testing.
Shortfall could cascade to other specialties
News that primary care is both struggling and shrinking doesn’t come as a surprise to those who research this area, according to Dr. Jabbarpour. Yet it matters “because primary care is where the majority of Americans get their health care.” According to the Centers for Disease Control and Prevention, primary care accounts for 50% of all office visits. But the sector only encompasses 30% of the clinician workforce, according to a 2019 study of physician supply, and accounts for just 7% of national health expenditures, according to a separate 2019 study that measured primary care investment.
If primary care doesn’t bounce back, the shortfall could overwhelm the rest of the health care system, Dr. Jabbarpour said. “If primary care shortages increase, then urgent cares, ERs, and hospitals will become overwhelmed.”
Or public health could suffer as people don’t seek care at all. A study published earlier this summer found that up to 35% of excess deaths during the pandemic were not caused by COVID-19. Instead, they can be attributed to treatable causes, like heart disease, diabetes, and Alzheimers, the researchers concluded. In Dr. Etz’s estimation, this high cost is a glimpse of what happens when there isn’t adequate access to primary care.
When asked about the frequency of the PCC/Green Center surveys, Bianca Frogner, PhD, a health economist and deputy director at the University of Washington Primary Care Innovation Lab in Seattle, said it’s unusual to have this regularity. Also, it’s unique in that it offers a constant mix of physicians. “It’s a small sample, but it still gives a voice where there isn’t one.”
Smaller weekly surveys and secondary analysis projects from the Graham Center, an American Academy of Family Physicians affiliated research center, reinforce findings of the Green Center. The Graham Center surveys also found primary care is taking a financial hit, staff is being furloughed, and patient volume is down, according to Dr. Jabbarpour, who has been involved in most of the Graham Center’s work on COVID.
Dr. Frogner, Dr. Etz, and Dr. Jabbarpour agree that, as a nation, the United States has chronically underinvested in primary care, and now the system is in crisis. The hope is that the survey data gives policymakers, state leaders, and the federal government a better idea of what’s happening on the ground.
It’s also important for researchers “keeping an eye out for the available supply of primary care for certain populations,” Dr. Frogner said. The current conditions are especially a threat to rural and underserved areas, she added.
If primary health care isn’t near recovery that’s a problem for the entire population, Dr. Etz said. And what happens if there’s another surge of COVID-19 or even a second pandemic in our lifetime? Her recommendation: Treat it like disaster recovery. Step one is “Stop the hemorrhaging – they need immediate cash flow.”
A version of this article originally appeared on Medscape.com.
published by the Primary Care Collaborative (PCC) and Larry A. Green Center.
Since mid-March these organizations have issued short weekly and biweekly surveys to U.S. primary care physicians in an attempt to find the pulse of the county’s first line of care. “There is not a federal office for primary care, and it’s been anemically funded for decades,” Rebecca Etz, PhD, said in an interview. Yet these clinics represent the front lines of U.S. health care, and it’s where most Americans go for care and COVID-19 care, said Dr. Etz, director of the Virginia-based Larry A. Green Center, which is devoted to primary care research, development, and advocacy.
The latest survey responses, collected between Sept. 4 and 6, confirm what researchers had suspected: Primary care isn’t on solid footing. Eighty-one percent of respondents disagreed emphatically that primary care has bounced back, and another 13% said things were better than earlier this year but not normal.
Meanwhile, 35% of respondents said that revenue and pay are significantly lower than they were before the pandemic and net losses threaten their practices’ viability. Almost half (49%) said their mental exhaustion from work was at an all-time high.
“Because of how our system is set up – it’s a fee-for-service model – the more patients you see, the more money you get,” said Yalda Jabbarpour, MD, medical director at the Graham Center, a leading think tank on family medicine and health care policy. But the stay-at-home order, aversion to telehealth, and fear of in-person visits have been keeping patients away – and driving primary care revenue down. Even when practices transition to and expand their telehealth, payer reimbursement is not yet on parity with in-person visits.
Right now, primary care physicians are doing fewer procedures and spending more time on video visits. “So you may have the same overhead and time investment but you’re getting paid a fraction,” Dr. Etz said. In August, 50% of primary care physicians reported they were working the same or more hours per week as they did before the pandemic but for less money, according to an earlier survey from the Green Center and the PCC. That loss of revenue is compounded by the need for expensive personal protective equipment and preparation for the upcoming flu season, Dr. Etz said.
Ongoing surveys reveal stress
Over the last 20 weeks or so, the Green Center and PCC together have disseminated weekly (through June) or biweekly surveys to 100 professional organizations. Because there isn’t an entity that represents all of primary care and claims data take years to process, these surveys are intended to get real-time feedback from clinicians who are providing a lot of patient care during the pandemic.
The sample sizes are admittedly small, with the most recent survey including 489 clinicians. Dr. Jabbarpour noted, “489 – it’s a good number, but you would want more.” Generally, for a great survey response you’d want 20%-30% of the physician population because then you could assume you’re getting a good mix of geographies, practice sizes, and settings, she said.
Respondents to the most recent survey were from 49 different states; 70% identified their practice as family medicine. One-third had between one and three physicians in their practice and 40% had 10 or more clinicians. “It’s not perfect, but it sounds pretty representative of the primary care workforce,” Dr. Jabbarpour continued.
The latest Green Center-PCC survey, published last week, also found that one in five physicians surveyed said at least one clinician in their practice had opted for early retirement or left practice as a direct result of the pandemic. These declines in clinician staffing come as school reopenings threaten to cause a resurgence of COVID-19 cases and the 2021 flu season could complicate COVID-19 care and testing.
Shortfall could cascade to other specialties
News that primary care is both struggling and shrinking doesn’t come as a surprise to those who research this area, according to Dr. Jabbarpour. Yet it matters “because primary care is where the majority of Americans get their health care.” According to the Centers for Disease Control and Prevention, primary care accounts for 50% of all office visits. But the sector only encompasses 30% of the clinician workforce, according to a 2019 study of physician supply, and accounts for just 7% of national health expenditures, according to a separate 2019 study that measured primary care investment.
If primary care doesn’t bounce back, the shortfall could overwhelm the rest of the health care system, Dr. Jabbarpour said. “If primary care shortages increase, then urgent cares, ERs, and hospitals will become overwhelmed.”
Or public health could suffer as people don’t seek care at all. A study published earlier this summer found that up to 35% of excess deaths during the pandemic were not caused by COVID-19. Instead, they can be attributed to treatable causes, like heart disease, diabetes, and Alzheimers, the researchers concluded. In Dr. Etz’s estimation, this high cost is a glimpse of what happens when there isn’t adequate access to primary care.
When asked about the frequency of the PCC/Green Center surveys, Bianca Frogner, PhD, a health economist and deputy director at the University of Washington Primary Care Innovation Lab in Seattle, said it’s unusual to have this regularity. Also, it’s unique in that it offers a constant mix of physicians. “It’s a small sample, but it still gives a voice where there isn’t one.”
Smaller weekly surveys and secondary analysis projects from the Graham Center, an American Academy of Family Physicians affiliated research center, reinforce findings of the Green Center. The Graham Center surveys also found primary care is taking a financial hit, staff is being furloughed, and patient volume is down, according to Dr. Jabbarpour, who has been involved in most of the Graham Center’s work on COVID.
Dr. Frogner, Dr. Etz, and Dr. Jabbarpour agree that, as a nation, the United States has chronically underinvested in primary care, and now the system is in crisis. The hope is that the survey data gives policymakers, state leaders, and the federal government a better idea of what’s happening on the ground.
It’s also important for researchers “keeping an eye out for the available supply of primary care for certain populations,” Dr. Frogner said. The current conditions are especially a threat to rural and underserved areas, she added.
If primary health care isn’t near recovery that’s a problem for the entire population, Dr. Etz said. And what happens if there’s another surge of COVID-19 or even a second pandemic in our lifetime? Her recommendation: Treat it like disaster recovery. Step one is “Stop the hemorrhaging – they need immediate cash flow.”
A version of this article originally appeared on Medscape.com.
published by the Primary Care Collaborative (PCC) and Larry A. Green Center.
Since mid-March these organizations have issued short weekly and biweekly surveys to U.S. primary care physicians in an attempt to find the pulse of the county’s first line of care. “There is not a federal office for primary care, and it’s been anemically funded for decades,” Rebecca Etz, PhD, said in an interview. Yet these clinics represent the front lines of U.S. health care, and it’s where most Americans go for care and COVID-19 care, said Dr. Etz, director of the Virginia-based Larry A. Green Center, which is devoted to primary care research, development, and advocacy.
The latest survey responses, collected between Sept. 4 and 6, confirm what researchers had suspected: Primary care isn’t on solid footing. Eighty-one percent of respondents disagreed emphatically that primary care has bounced back, and another 13% said things were better than earlier this year but not normal.
Meanwhile, 35% of respondents said that revenue and pay are significantly lower than they were before the pandemic and net losses threaten their practices’ viability. Almost half (49%) said their mental exhaustion from work was at an all-time high.
“Because of how our system is set up – it’s a fee-for-service model – the more patients you see, the more money you get,” said Yalda Jabbarpour, MD, medical director at the Graham Center, a leading think tank on family medicine and health care policy. But the stay-at-home order, aversion to telehealth, and fear of in-person visits have been keeping patients away – and driving primary care revenue down. Even when practices transition to and expand their telehealth, payer reimbursement is not yet on parity with in-person visits.
Right now, primary care physicians are doing fewer procedures and spending more time on video visits. “So you may have the same overhead and time investment but you’re getting paid a fraction,” Dr. Etz said. In August, 50% of primary care physicians reported they were working the same or more hours per week as they did before the pandemic but for less money, according to an earlier survey from the Green Center and the PCC. That loss of revenue is compounded by the need for expensive personal protective equipment and preparation for the upcoming flu season, Dr. Etz said.
Ongoing surveys reveal stress
Over the last 20 weeks or so, the Green Center and PCC together have disseminated weekly (through June) or biweekly surveys to 100 professional organizations. Because there isn’t an entity that represents all of primary care and claims data take years to process, these surveys are intended to get real-time feedback from clinicians who are providing a lot of patient care during the pandemic.
The sample sizes are admittedly small, with the most recent survey including 489 clinicians. Dr. Jabbarpour noted, “489 – it’s a good number, but you would want more.” Generally, for a great survey response you’d want 20%-30% of the physician population because then you could assume you’re getting a good mix of geographies, practice sizes, and settings, she said.
Respondents to the most recent survey were from 49 different states; 70% identified their practice as family medicine. One-third had between one and three physicians in their practice and 40% had 10 or more clinicians. “It’s not perfect, but it sounds pretty representative of the primary care workforce,” Dr. Jabbarpour continued.
The latest Green Center-PCC survey, published last week, also found that one in five physicians surveyed said at least one clinician in their practice had opted for early retirement or left practice as a direct result of the pandemic. These declines in clinician staffing come as school reopenings threaten to cause a resurgence of COVID-19 cases and the 2021 flu season could complicate COVID-19 care and testing.
Shortfall could cascade to other specialties
News that primary care is both struggling and shrinking doesn’t come as a surprise to those who research this area, according to Dr. Jabbarpour. Yet it matters “because primary care is where the majority of Americans get their health care.” According to the Centers for Disease Control and Prevention, primary care accounts for 50% of all office visits. But the sector only encompasses 30% of the clinician workforce, according to a 2019 study of physician supply, and accounts for just 7% of national health expenditures, according to a separate 2019 study that measured primary care investment.
If primary care doesn’t bounce back, the shortfall could overwhelm the rest of the health care system, Dr. Jabbarpour said. “If primary care shortages increase, then urgent cares, ERs, and hospitals will become overwhelmed.”
Or public health could suffer as people don’t seek care at all. A study published earlier this summer found that up to 35% of excess deaths during the pandemic were not caused by COVID-19. Instead, they can be attributed to treatable causes, like heart disease, diabetes, and Alzheimers, the researchers concluded. In Dr. Etz’s estimation, this high cost is a glimpse of what happens when there isn’t adequate access to primary care.
When asked about the frequency of the PCC/Green Center surveys, Bianca Frogner, PhD, a health economist and deputy director at the University of Washington Primary Care Innovation Lab in Seattle, said it’s unusual to have this regularity. Also, it’s unique in that it offers a constant mix of physicians. “It’s a small sample, but it still gives a voice where there isn’t one.”
Smaller weekly surveys and secondary analysis projects from the Graham Center, an American Academy of Family Physicians affiliated research center, reinforce findings of the Green Center. The Graham Center surveys also found primary care is taking a financial hit, staff is being furloughed, and patient volume is down, according to Dr. Jabbarpour, who has been involved in most of the Graham Center’s work on COVID.
Dr. Frogner, Dr. Etz, and Dr. Jabbarpour agree that, as a nation, the United States has chronically underinvested in primary care, and now the system is in crisis. The hope is that the survey data gives policymakers, state leaders, and the federal government a better idea of what’s happening on the ground.
It’s also important for researchers “keeping an eye out for the available supply of primary care for certain populations,” Dr. Frogner said. The current conditions are especially a threat to rural and underserved areas, she added.
If primary health care isn’t near recovery that’s a problem for the entire population, Dr. Etz said. And what happens if there’s another surge of COVID-19 or even a second pandemic in our lifetime? Her recommendation: Treat it like disaster recovery. Step one is “Stop the hemorrhaging – they need immediate cash flow.”
A version of this article originally appeared on Medscape.com.
Stroke may be the first symptom of COVID-19 in younger patients
new research suggests. Investigators carried out a meta-analysis of data, including 160 patients with COVID-19 and stroke, and found that nearly half of patients under the age of 50 were asymptomatic at the time of stroke onset.
Although younger patients had the highest risk of stroke, the highest risk of death was in patients who were older, had other chronic conditions, and had more severe COVID-19–associated respiratory symptoms.
“One of the most eye-opening findings of this study is that, for patients under 50 years old, many were totally asymptomatic when they had a stroke related to COVID-19, [which] means that, for these patients, the stroke was their first symptom of the disease,” lead author Luciano Sposato, MD, MBA, associate professor and chair in stroke research at Western University, London, Ont.
The study was published online Sept. 15 in Neurology.
Anecdotal reports
“In early April of 2020, we realized that COVID-19 was a highly thrombogenic disease,” said Dr. Sposato. “Almost in parallel, I started to see anecdotal reports in social media of strokes occurring in patients with COVID-19, and there were also very few case reports.”
The investigators “thought it would be a good idea to put all the data together in one paper,” he said, and began by conducting a systematic review of 10 published studies of COVID-19 and stroke (n = 125 patients), which were then pooled with 35 unpublished cases from Canada, the United States, and Iran for a total of 160 cases.
The analysis examined in-hospital mortality rates of patients with stroke and COVID-19.
In addition, the researchers conducted a second review of 150 papers, encompassing a final cohort of 3,306 COVID-19 patients with stroke of any type and 5,322 with ischemic stroke.
“Some studies reported data for only ischemic stroke, and some reported data for all strokes considered together, which resulted in a different number of patients on each meta-analysis, with a lower number of ‘any stroke’ cases,” Dr. Sposato explained. “This review looked at the number of patients who developed a stroke during admission and included thousands of patients.”
Dr. Sposato noted that the first review was conducted on single case reports and small case series “to understand the clinical characteristics of strokes in patients with COVID-19 on an individual patient level,” since “large studies, including hundreds of thousands of patients, usually do not provide the level of detail for a descriptive analysis of the clinical characteristics of a disease.”
Cluster analyses were used to “identify specific clinical phenotypes and their relationship with death.” Patients were stratified into three age groups: <50, 50-70, and >70 years (“young,” “middle aged,” and “older,” respectively). The median age was 65 years and 43% were female.
Mortality ‘remarkably high’
The review showed that 1.8% (95% confidence interval, 0.9%-3.7%) of patients experienced a new stroke, while 1.5% (95% CI, 0.8%-2.8%) of these experienced an ischemic stroke. “These numbers are higher than historical data for other infectious diseases – for example, 0.75% in SARS-CoV-1, 0.78% in sepsis, and 0.2% in influenza,” Dr. Sposato commented.
Moreover, “this number may be an underestimate, given that many patients die without a confirmed diagnosis and that some patients did not come to the emergency department when experiencing mild symptoms during the first months of the pandemic,” he added.
Focusing on the review of 160 patients, the researchers described in-hospital mortality for strokes of all types and for ischemic strokes alone as “remarkably high” (34.4% [95% CI, 27.2%-42.4%] and 35.7% [95% CI, 27.5%-44.8%], respectively), with most deaths occurring among ischemic stroke patients.
“This high mortality rate is higher than the [roughly] 15% to 30% reported for stroke patients without COVID-19 admitted to intensive care units,” Dr. Sposato said.
High-risk phenotype
Many “young” COVID-19 patients (under age 50) who had a stroke (42.9%) had no previous risk factors or comorbidities. Moreover, in almost half of these patients (48.3%), stroke was more likely to occur before the onset of any COVID-19 respiratory symptoms.
Additionally, younger patients showed the highest frequency of elevated cardiac troponin compared with middle-aged and older patients (71.4% vs. 48.4% and 27.8%, respectively). On the other hand, mortality was 67% lower in younger versus older patients (odds ratio, 0.33; 95% CI, 0.12-0.94; P = .039).
Dr. Sposato noted that the proportion of ischemic stroke patients with large-vessel occlusion was “higher than previously reported” for patients with stroke without COVID-19 (47% compared with 29%, respectively).
“We should consider COVID-19 as a new cause or risk factor for stroke. At least, patients with stroke should probably be tested for SARS-CoV-2 infection if they are young and present with a large-vessel occlusion, even in the absence of typical COVID-19 respiratory symptoms,” he suggested.
The researchers identified a “high-risk phenotype” for death for all types of stroke considered together: older age, a higher burden of comorbidities, and severe COVID-19 respiratory symptoms. Patients with all three characteristics had the highest in-hospital mortality rate (58.6%) and a threefold risk of death, compared with the rest of the cohort (OR, 3.52; 95% CI, 1.53-8.09; P = .003).
“Several potential mechanisms can explain the increased risk of stroke among COVID-19 patients, but perhaps the most important one is increased thrombogenesis secondary to an exaggerated inflammatory response,” Dr. Sposato said.
Not just elders
Commenting on the study, Jodi Edwards, PhD, director of the Brain and Heart Nexus Research Program at the University of Ottawa Heart Institute, said the findings are “consistent with and underscore public health messaging emphasizing that COVID-19 does not only affect the elderly and those with underlying health conditions, but can have serious and even fatal consequences at any age.”
Dr. Edwards, who was not involved with the study, emphasized that “adherence to public health recommendations is critical to begin to reduce the rising incidence in younger adults.”
Dr. Sposato acknowledged that the study was small and that there “can be problems associated with a systematic review of case reports, such as publication bias, lack of completeness of data, etc, so more research is needed.”
Dr. Sposato is supported by the Kathleen & Dr. Henry Barnett Research Chair in Stroke Research at Western University, the Edward and Alma Saraydar Neurosciences Fund of the London Health Sciences Foundation, and the Opportunities Fund of the Academic Health Sciences Centre Alternative Funding Plan of the Academic Medical Organization of Southwestern Ontario. Dr. Sposato reported speaker honoraria from Boehringer Ingelheim, Pfizer, Gore, and Bayer and research/quality improvement grants from Boehringer Ingelheim and Bayer. The other authors’ disclosures are listed on the original article. Dr. Edwards has disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
new research suggests. Investigators carried out a meta-analysis of data, including 160 patients with COVID-19 and stroke, and found that nearly half of patients under the age of 50 were asymptomatic at the time of stroke onset.
Although younger patients had the highest risk of stroke, the highest risk of death was in patients who were older, had other chronic conditions, and had more severe COVID-19–associated respiratory symptoms.
“One of the most eye-opening findings of this study is that, for patients under 50 years old, many were totally asymptomatic when they had a stroke related to COVID-19, [which] means that, for these patients, the stroke was their first symptom of the disease,” lead author Luciano Sposato, MD, MBA, associate professor and chair in stroke research at Western University, London, Ont.
The study was published online Sept. 15 in Neurology.
Anecdotal reports
“In early April of 2020, we realized that COVID-19 was a highly thrombogenic disease,” said Dr. Sposato. “Almost in parallel, I started to see anecdotal reports in social media of strokes occurring in patients with COVID-19, and there were also very few case reports.”
The investigators “thought it would be a good idea to put all the data together in one paper,” he said, and began by conducting a systematic review of 10 published studies of COVID-19 and stroke (n = 125 patients), which were then pooled with 35 unpublished cases from Canada, the United States, and Iran for a total of 160 cases.
The analysis examined in-hospital mortality rates of patients with stroke and COVID-19.
In addition, the researchers conducted a second review of 150 papers, encompassing a final cohort of 3,306 COVID-19 patients with stroke of any type and 5,322 with ischemic stroke.
“Some studies reported data for only ischemic stroke, and some reported data for all strokes considered together, which resulted in a different number of patients on each meta-analysis, with a lower number of ‘any stroke’ cases,” Dr. Sposato explained. “This review looked at the number of patients who developed a stroke during admission and included thousands of patients.”
Dr. Sposato noted that the first review was conducted on single case reports and small case series “to understand the clinical characteristics of strokes in patients with COVID-19 on an individual patient level,” since “large studies, including hundreds of thousands of patients, usually do not provide the level of detail for a descriptive analysis of the clinical characteristics of a disease.”
Cluster analyses were used to “identify specific clinical phenotypes and their relationship with death.” Patients were stratified into three age groups: <50, 50-70, and >70 years (“young,” “middle aged,” and “older,” respectively). The median age was 65 years and 43% were female.
Mortality ‘remarkably high’
The review showed that 1.8% (95% confidence interval, 0.9%-3.7%) of patients experienced a new stroke, while 1.5% (95% CI, 0.8%-2.8%) of these experienced an ischemic stroke. “These numbers are higher than historical data for other infectious diseases – for example, 0.75% in SARS-CoV-1, 0.78% in sepsis, and 0.2% in influenza,” Dr. Sposato commented.
Moreover, “this number may be an underestimate, given that many patients die without a confirmed diagnosis and that some patients did not come to the emergency department when experiencing mild symptoms during the first months of the pandemic,” he added.
Focusing on the review of 160 patients, the researchers described in-hospital mortality for strokes of all types and for ischemic strokes alone as “remarkably high” (34.4% [95% CI, 27.2%-42.4%] and 35.7% [95% CI, 27.5%-44.8%], respectively), with most deaths occurring among ischemic stroke patients.
“This high mortality rate is higher than the [roughly] 15% to 30% reported for stroke patients without COVID-19 admitted to intensive care units,” Dr. Sposato said.
High-risk phenotype
Many “young” COVID-19 patients (under age 50) who had a stroke (42.9%) had no previous risk factors or comorbidities. Moreover, in almost half of these patients (48.3%), stroke was more likely to occur before the onset of any COVID-19 respiratory symptoms.
Additionally, younger patients showed the highest frequency of elevated cardiac troponin compared with middle-aged and older patients (71.4% vs. 48.4% and 27.8%, respectively). On the other hand, mortality was 67% lower in younger versus older patients (odds ratio, 0.33; 95% CI, 0.12-0.94; P = .039).
Dr. Sposato noted that the proportion of ischemic stroke patients with large-vessel occlusion was “higher than previously reported” for patients with stroke without COVID-19 (47% compared with 29%, respectively).
“We should consider COVID-19 as a new cause or risk factor for stroke. At least, patients with stroke should probably be tested for SARS-CoV-2 infection if they are young and present with a large-vessel occlusion, even in the absence of typical COVID-19 respiratory symptoms,” he suggested.
The researchers identified a “high-risk phenotype” for death for all types of stroke considered together: older age, a higher burden of comorbidities, and severe COVID-19 respiratory symptoms. Patients with all three characteristics had the highest in-hospital mortality rate (58.6%) and a threefold risk of death, compared with the rest of the cohort (OR, 3.52; 95% CI, 1.53-8.09; P = .003).
“Several potential mechanisms can explain the increased risk of stroke among COVID-19 patients, but perhaps the most important one is increased thrombogenesis secondary to an exaggerated inflammatory response,” Dr. Sposato said.
Not just elders
Commenting on the study, Jodi Edwards, PhD, director of the Brain and Heart Nexus Research Program at the University of Ottawa Heart Institute, said the findings are “consistent with and underscore public health messaging emphasizing that COVID-19 does not only affect the elderly and those with underlying health conditions, but can have serious and even fatal consequences at any age.”
Dr. Edwards, who was not involved with the study, emphasized that “adherence to public health recommendations is critical to begin to reduce the rising incidence in younger adults.”
Dr. Sposato acknowledged that the study was small and that there “can be problems associated with a systematic review of case reports, such as publication bias, lack of completeness of data, etc, so more research is needed.”
Dr. Sposato is supported by the Kathleen & Dr. Henry Barnett Research Chair in Stroke Research at Western University, the Edward and Alma Saraydar Neurosciences Fund of the London Health Sciences Foundation, and the Opportunities Fund of the Academic Health Sciences Centre Alternative Funding Plan of the Academic Medical Organization of Southwestern Ontario. Dr. Sposato reported speaker honoraria from Boehringer Ingelheim, Pfizer, Gore, and Bayer and research/quality improvement grants from Boehringer Ingelheim and Bayer. The other authors’ disclosures are listed on the original article. Dr. Edwards has disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
new research suggests. Investigators carried out a meta-analysis of data, including 160 patients with COVID-19 and stroke, and found that nearly half of patients under the age of 50 were asymptomatic at the time of stroke onset.
Although younger patients had the highest risk of stroke, the highest risk of death was in patients who were older, had other chronic conditions, and had more severe COVID-19–associated respiratory symptoms.
“One of the most eye-opening findings of this study is that, for patients under 50 years old, many were totally asymptomatic when they had a stroke related to COVID-19, [which] means that, for these patients, the stroke was their first symptom of the disease,” lead author Luciano Sposato, MD, MBA, associate professor and chair in stroke research at Western University, London, Ont.
The study was published online Sept. 15 in Neurology.
Anecdotal reports
“In early April of 2020, we realized that COVID-19 was a highly thrombogenic disease,” said Dr. Sposato. “Almost in parallel, I started to see anecdotal reports in social media of strokes occurring in patients with COVID-19, and there were also very few case reports.”
The investigators “thought it would be a good idea to put all the data together in one paper,” he said, and began by conducting a systematic review of 10 published studies of COVID-19 and stroke (n = 125 patients), which were then pooled with 35 unpublished cases from Canada, the United States, and Iran for a total of 160 cases.
The analysis examined in-hospital mortality rates of patients with stroke and COVID-19.
In addition, the researchers conducted a second review of 150 papers, encompassing a final cohort of 3,306 COVID-19 patients with stroke of any type and 5,322 with ischemic stroke.
“Some studies reported data for only ischemic stroke, and some reported data for all strokes considered together, which resulted in a different number of patients on each meta-analysis, with a lower number of ‘any stroke’ cases,” Dr. Sposato explained. “This review looked at the number of patients who developed a stroke during admission and included thousands of patients.”
Dr. Sposato noted that the first review was conducted on single case reports and small case series “to understand the clinical characteristics of strokes in patients with COVID-19 on an individual patient level,” since “large studies, including hundreds of thousands of patients, usually do not provide the level of detail for a descriptive analysis of the clinical characteristics of a disease.”
Cluster analyses were used to “identify specific clinical phenotypes and their relationship with death.” Patients were stratified into three age groups: <50, 50-70, and >70 years (“young,” “middle aged,” and “older,” respectively). The median age was 65 years and 43% were female.
Mortality ‘remarkably high’
The review showed that 1.8% (95% confidence interval, 0.9%-3.7%) of patients experienced a new stroke, while 1.5% (95% CI, 0.8%-2.8%) of these experienced an ischemic stroke. “These numbers are higher than historical data for other infectious diseases – for example, 0.75% in SARS-CoV-1, 0.78% in sepsis, and 0.2% in influenza,” Dr. Sposato commented.
Moreover, “this number may be an underestimate, given that many patients die without a confirmed diagnosis and that some patients did not come to the emergency department when experiencing mild symptoms during the first months of the pandemic,” he added.
Focusing on the review of 160 patients, the researchers described in-hospital mortality for strokes of all types and for ischemic strokes alone as “remarkably high” (34.4% [95% CI, 27.2%-42.4%] and 35.7% [95% CI, 27.5%-44.8%], respectively), with most deaths occurring among ischemic stroke patients.
“This high mortality rate is higher than the [roughly] 15% to 30% reported for stroke patients without COVID-19 admitted to intensive care units,” Dr. Sposato said.
High-risk phenotype
Many “young” COVID-19 patients (under age 50) who had a stroke (42.9%) had no previous risk factors or comorbidities. Moreover, in almost half of these patients (48.3%), stroke was more likely to occur before the onset of any COVID-19 respiratory symptoms.
Additionally, younger patients showed the highest frequency of elevated cardiac troponin compared with middle-aged and older patients (71.4% vs. 48.4% and 27.8%, respectively). On the other hand, mortality was 67% lower in younger versus older patients (odds ratio, 0.33; 95% CI, 0.12-0.94; P = .039).
Dr. Sposato noted that the proportion of ischemic stroke patients with large-vessel occlusion was “higher than previously reported” for patients with stroke without COVID-19 (47% compared with 29%, respectively).
“We should consider COVID-19 as a new cause or risk factor for stroke. At least, patients with stroke should probably be tested for SARS-CoV-2 infection if they are young and present with a large-vessel occlusion, even in the absence of typical COVID-19 respiratory symptoms,” he suggested.
The researchers identified a “high-risk phenotype” for death for all types of stroke considered together: older age, a higher burden of comorbidities, and severe COVID-19 respiratory symptoms. Patients with all three characteristics had the highest in-hospital mortality rate (58.6%) and a threefold risk of death, compared with the rest of the cohort (OR, 3.52; 95% CI, 1.53-8.09; P = .003).
“Several potential mechanisms can explain the increased risk of stroke among COVID-19 patients, but perhaps the most important one is increased thrombogenesis secondary to an exaggerated inflammatory response,” Dr. Sposato said.
Not just elders
Commenting on the study, Jodi Edwards, PhD, director of the Brain and Heart Nexus Research Program at the University of Ottawa Heart Institute, said the findings are “consistent with and underscore public health messaging emphasizing that COVID-19 does not only affect the elderly and those with underlying health conditions, but can have serious and even fatal consequences at any age.”
Dr. Edwards, who was not involved with the study, emphasized that “adherence to public health recommendations is critical to begin to reduce the rising incidence in younger adults.”
Dr. Sposato acknowledged that the study was small and that there “can be problems associated with a systematic review of case reports, such as publication bias, lack of completeness of data, etc, so more research is needed.”
Dr. Sposato is supported by the Kathleen & Dr. Henry Barnett Research Chair in Stroke Research at Western University, the Edward and Alma Saraydar Neurosciences Fund of the London Health Sciences Foundation, and the Opportunities Fund of the Academic Health Sciences Centre Alternative Funding Plan of the Academic Medical Organization of Southwestern Ontario. Dr. Sposato reported speaker honoraria from Boehringer Ingelheim, Pfizer, Gore, and Bayer and research/quality improvement grants from Boehringer Ingelheim and Bayer. The other authors’ disclosures are listed on the original article. Dr. Edwards has disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
From Neurology
CDC flips, acknowledges aerosol spread of COVID-19
The information reiterates, however, that “COVID-19 is thought to spread mainly through close contact from person to person, including between people who are physically near each other (within about 6 feet). People who are infected but do not show symptoms can also spread the virus to others.”
In a statement to the media, the CDC said, “Today’s update acknowledges the existence of some published reports showing limited, uncommon circumstances where people with COVID-19 infected others who were more than 6 feet away or shortly after the COVID-19–positive person left an area. In these instances, transmission occurred in poorly ventilated and enclosed spaces that often involved activities that caused heavier breathing, like singing or exercise. Such environments and activities may contribute to the buildup of virus-carrying particles.”
“This is HUGE and been long delayed. But glad it’s now CDC official,” tweeted Eric Feigl-Ding, MD, an epidemiologist and health economist at Harvard University, Boston on Oct. 5.
The CDC announcement follows an abrupt flip-flop on information last month surrounding the aerosol spread of the virus.
Information deleted from website last month
On September 18, the CDC had added to its existing guidance that the virus is spread “through respiratory droplets or small particles, such as those in aerosols, produced when an infected person coughs, sneezes, sings, talks, or breathes. These particles can be inhaled into the nose, mouth, airways, and lungs and cause infection.”
The CDC then deleted that guidance on Sept. 21, saying it was a draft update released in error.
A key element of the now-deleted guidance said, “this is thought to be the main way the virus spreads.”
The information updated today reverses the now-deleted guidance and says aerosol transmission is not the main way the virus spreads.
It states that people who are within 6 feet of a person with COVID-19 or have direct contact with that person have the greatest risk of infection.
The CDC reiterated in the statement to the media today, “People can protect themselves from the virus that causes COVID-19 by staying at least 6 feet away from others, wearing a mask that covers their nose and mouth, washing their hands frequently, cleaning touched surfaces often, and staying home when sick.”
Among the journals that have published evidence on aerosol spread is Clinical Infectious Diseases, which, on July 6, published the paper, “It Is Time to Address Airborne Transmission of Coronavirus Disease 2019,” which was supported by 239 scientists.
The authors wrote, “there is significant potential for inhalation exposure to viruses in microscopic respiratory droplets (microdroplets) at short to medium distances (up to several meters, or room scale).”
Aerosols and airborne transmission “are the only way to explain super-spreader events we are seeing,” said Kimberly Prather, PhD, an atmospheric chemist at the University of California at San Diego, in an interview Oct. 5 with the Washington Post.
Dr. Prather added that, once aerosolization is acknowledged, this becomes a “fixable” problem through proper ventilation.
“Wear masks at all times indoors when others are present,” Dr. Prather said. But when inside, she said, there’s no such thing as a completely safe social distance.
This article first appeared on Medscape.com.
The information reiterates, however, that “COVID-19 is thought to spread mainly through close contact from person to person, including between people who are physically near each other (within about 6 feet). People who are infected but do not show symptoms can also spread the virus to others.”
In a statement to the media, the CDC said, “Today’s update acknowledges the existence of some published reports showing limited, uncommon circumstances where people with COVID-19 infected others who were more than 6 feet away or shortly after the COVID-19–positive person left an area. In these instances, transmission occurred in poorly ventilated and enclosed spaces that often involved activities that caused heavier breathing, like singing or exercise. Such environments and activities may contribute to the buildup of virus-carrying particles.”
“This is HUGE and been long delayed. But glad it’s now CDC official,” tweeted Eric Feigl-Ding, MD, an epidemiologist and health economist at Harvard University, Boston on Oct. 5.
The CDC announcement follows an abrupt flip-flop on information last month surrounding the aerosol spread of the virus.
Information deleted from website last month
On September 18, the CDC had added to its existing guidance that the virus is spread “through respiratory droplets or small particles, such as those in aerosols, produced when an infected person coughs, sneezes, sings, talks, or breathes. These particles can be inhaled into the nose, mouth, airways, and lungs and cause infection.”
The CDC then deleted that guidance on Sept. 21, saying it was a draft update released in error.
A key element of the now-deleted guidance said, “this is thought to be the main way the virus spreads.”
The information updated today reverses the now-deleted guidance and says aerosol transmission is not the main way the virus spreads.
It states that people who are within 6 feet of a person with COVID-19 or have direct contact with that person have the greatest risk of infection.
The CDC reiterated in the statement to the media today, “People can protect themselves from the virus that causes COVID-19 by staying at least 6 feet away from others, wearing a mask that covers their nose and mouth, washing their hands frequently, cleaning touched surfaces often, and staying home when sick.”
Among the journals that have published evidence on aerosol spread is Clinical Infectious Diseases, which, on July 6, published the paper, “It Is Time to Address Airborne Transmission of Coronavirus Disease 2019,” which was supported by 239 scientists.
The authors wrote, “there is significant potential for inhalation exposure to viruses in microscopic respiratory droplets (microdroplets) at short to medium distances (up to several meters, or room scale).”
Aerosols and airborne transmission “are the only way to explain super-spreader events we are seeing,” said Kimberly Prather, PhD, an atmospheric chemist at the University of California at San Diego, in an interview Oct. 5 with the Washington Post.
Dr. Prather added that, once aerosolization is acknowledged, this becomes a “fixable” problem through proper ventilation.
“Wear masks at all times indoors when others are present,” Dr. Prather said. But when inside, she said, there’s no such thing as a completely safe social distance.
This article first appeared on Medscape.com.
The information reiterates, however, that “COVID-19 is thought to spread mainly through close contact from person to person, including between people who are physically near each other (within about 6 feet). People who are infected but do not show symptoms can also spread the virus to others.”
In a statement to the media, the CDC said, “Today’s update acknowledges the existence of some published reports showing limited, uncommon circumstances where people with COVID-19 infected others who were more than 6 feet away or shortly after the COVID-19–positive person left an area. In these instances, transmission occurred in poorly ventilated and enclosed spaces that often involved activities that caused heavier breathing, like singing or exercise. Such environments and activities may contribute to the buildup of virus-carrying particles.”
“This is HUGE and been long delayed. But glad it’s now CDC official,” tweeted Eric Feigl-Ding, MD, an epidemiologist and health economist at Harvard University, Boston on Oct. 5.
The CDC announcement follows an abrupt flip-flop on information last month surrounding the aerosol spread of the virus.
Information deleted from website last month
On September 18, the CDC had added to its existing guidance that the virus is spread “through respiratory droplets or small particles, such as those in aerosols, produced when an infected person coughs, sneezes, sings, talks, or breathes. These particles can be inhaled into the nose, mouth, airways, and lungs and cause infection.”
The CDC then deleted that guidance on Sept. 21, saying it was a draft update released in error.
A key element of the now-deleted guidance said, “this is thought to be the main way the virus spreads.”
The information updated today reverses the now-deleted guidance and says aerosol transmission is not the main way the virus spreads.
It states that people who are within 6 feet of a person with COVID-19 or have direct contact with that person have the greatest risk of infection.
The CDC reiterated in the statement to the media today, “People can protect themselves from the virus that causes COVID-19 by staying at least 6 feet away from others, wearing a mask that covers their nose and mouth, washing their hands frequently, cleaning touched surfaces often, and staying home when sick.”
Among the journals that have published evidence on aerosol spread is Clinical Infectious Diseases, which, on July 6, published the paper, “It Is Time to Address Airborne Transmission of Coronavirus Disease 2019,” which was supported by 239 scientists.
The authors wrote, “there is significant potential for inhalation exposure to viruses in microscopic respiratory droplets (microdroplets) at short to medium distances (up to several meters, or room scale).”
Aerosols and airborne transmission “are the only way to explain super-spreader events we are seeing,” said Kimberly Prather, PhD, an atmospheric chemist at the University of California at San Diego, in an interview Oct. 5 with the Washington Post.
Dr. Prather added that, once aerosolization is acknowledged, this becomes a “fixable” problem through proper ventilation.
“Wear masks at all times indoors when others are present,” Dr. Prather said. But when inside, she said, there’s no such thing as a completely safe social distance.
This article first appeared on Medscape.com.
Substance use tied to increased COVID-19 risk
Substance use disorders (SUD), particularly opioid addiction and smoking, are tied to an increased risk for COVID-19 and serious adverse outcomes including hospitalization and death, new research suggests.
A study funded by the National Institutes of Health assessed electronic health records of more than 73 million patients in the United States. Although only 10.3% of the participants had an SUD, “they represented 15.6% of the COVID-19 cases,” the investigators reported.
In addition, those with a recent diagnosis of SUD were eight times more likely to develop COVID-19 versus those without such a diagnosis. For specific SUDs, the greatest risk was for those with an opioid addiction followed by those who were addicted to cigarettes.
coinvestigator Nora Volkow, MD, director of the National Institute on Drug Abuse, said in a press release.
It may also be harder for individuals with addiction to access health care services for a variety of reasons, including low socioeconomic status or stigma, she said in an interview.
Dr. Volkow said she has encountered patients with medical emergencies who refuse to seek treatment at the emergency department because of previous experiences where they have been mistreated and encountered discrimination, and “that’s really very tragic.”
The findings were published online Sept. 14 in Molecular Psychiatry.
Is nicotine protective?
Dr. Volkow, her fellow senior author Rong Xu, PhD, Case Western Reserve University, Cleveland, and their team conducted the study because data released before the pandemic showed a significant increase in opioid overdose in 2019. “We were in an opioid crisis where we again saw an increase in mortality associated with overdose – and then COVID comes along. So the question was how are people who are already struggling faring? And if they were getting infected [with the coronavirus], what happened to them?”
Patients with SUDs have multiple medical comorbidities that are known risk factors for COVID-19, Dr. Volkow noted.
However, the only specific SUD that has been previously studied in this context is tobacco use disorder, she said. A report from Chinese investigators released early in the pandemic showed that smokers were more likely to be infected by coronavirus and more likely to die from COVID-19.
Interestingly, a cross-sectional study published in April suggested that smoking may be protective against COVID, and Dr. Volkow noted that a clinical study currently being conducted in France is assessing whether wearing a nicotine patch has the potential to prevent the virus.
“That’s very different from looking at a chronic smoker,” she pointed out. “It’s a potential that nicotine as a chemical [could be] a preventive measure as opposed to saying smoking will prevent you from getting COVID.”
Patients with SUDs, said Dr. Volkow, “are likely to be at greater risk because of the effects of drugs in the metabolic system and the interfering with oxygenation in the pulmonary vessels.”
The retrospective case-control study included EHR data from 73.1 million patients. In the study population, 54% were women, 55% were White, 10% Black, 2% Asian, 1% Hispanic/Latino, and the others were classified as other or unknown.
EHRs were collected through June 15 at 360 hospitals in all 50 states and were deidentified to ensure privacy. SUDs included alcohol, tobacco, cannabis, opioid, and cocaine.
Racial disparities
Results showed that about 7.5 million participants had a previous SUD diagnosis; of these, 722,370 had been diagnosed within the past year.
Tobacco use disorder was the most common diagnosis (n = 6,414,580), followed by alcohol (1,264,990), cannabis (490,420), opioid (471,520), and cocaine (222,680).
In addition, 12,030 (60% women) were diagnosed with COVID-19 and 1,880 had both COVID-19 and an SUD.
Adjusted analyses revealed that those who had a recent diagnosis of SUD were at a significantly greater increased risk for COVID-19 than individuals without an SUD (adjusted odds ratio, 8.7; 95% confidence interval, 8.4-9.0; P < 10–30).
This increased risk was greatest in participants with opioid use disorder (aOR, 10.2; 95% CI, 9.1-11.5; P < 10–30), followed by those with tobacco use disorder (aOR, 8.2; 95% CI, 7.9 - 8.5; P < 10–30).
Alcohol, cocaine, and cannabis had aORs of 7.7, 6.5, and 5.3, respectively. The aOR for lifetime SUD and COVID-19 was 1.5.
Among all patients with COVID-19, hospitalization rates were significantly greater in those with an SUD (43.8%) versus those without (30.1%), as were death rates at 9.6% versus 6.6%, respectively.
Race was a significant risk factor. Black patients with a recent SUD diagnosis were twice as likely as White patients to develop COVID-19 (aOR, 2.2; P < 10–30), and those specifically with opioid use disorder were four times more likely to develop the disease (aOR, 4.2 P < 10–25).
Black patients with both COVID-19 and lifetime SUD also had greater hospitalization and death rates versus their White peers (50.7% vs. 35.2% and 13% vs. 8.6%, respectively).
“This surprised me,” Dr. Volkow noted. “You can see the emergence of the racial disparities even under these conditions of really negative outcomes.”
Vulnerable populations
Cancer; obesity; HIV; diabetes; cardiovascular disease; and chronic kidney, liver, and lung diseases, which are all risk factors for COVID-19, were more prevalent in the group of patients with a recent SUD diagnosis versus those without.
In addition, asthma, type 2 diabetes, hypertension, obesity, and chronic kidney disease were more prevalent in the Black patents with a recent SUD than in the White patients.
Overall, the findings “identify individuals with SUD as a vulnerable population, especially African Americans with SUDs, who are at significantly increased risk for COVID-19 and its adverse outcomes,” the investigators wrote.
The results also highlight “the need to screen and treat individuals with SUD as part of the strategy to control the pandemic while ensuring no disparities in access to healthcare support,” they added.
Dr. Volkow noted that “marginalization” often occurs for individuals with addiction, making it more difficult for them to access health care services.
“It is incumbent upon clinicians to meet the unique challenges of caring for this vulnerable population, just as they would any other high-risk group,” she said.
“Patients should not just be treated for COVID, but should also be provided with treatment for their substance use disorder,” Dr. Volkow added.
‘Pretty convincing’
Andrew J. Saxon, MD, professor in the department of psychiatry and behavioral sciences at the University of Washington, Seattle, called the findings interesting.
“I found it pretty convincing that people who have substance use disorders are probably at higher risk for getting COVID-19 infection and more complications once they are infected,” he said.
Dr. Saxon, who was not involved with the research, is also director of the Center of Excellence in Substance Addiction Treatment and Education and is a member of the American Psychiatric Association’s Council on Addiction Psychiatry.
He noted that an important point from the study was not just about a patient having an SUD being at increased risk for COVID-19 “and a more severe disease trajectory.” Other factors associated with having an SUD, such as increased comorbidities, also likely play a part.
Dr. Saxon agreed that the ongoing opioid epidemic combined with the pandemic led to a “perfect storm” of problems.
“We were making slow but some progress getting more people the medications they need [to treat opioid use disorder], but the pandemic coming along disrupted those efforts. A lot of health care entities had to shut down for a while, seeing patients only remotely,” which led to barriers as many clinicians needed to learn how to proceed using telehealth options, said Dr. Saxon.
Universal screening?
Asked whether physicians should screen all patients for SUDs, Dr. Saxon said it’s a complicated question.
“Screening for tobacco and alcohol has a really good evidence base and practices should be doing that. The stigma is there but it’s a lot less than with illegal substances,” he said.
Screening for illegal substances or misuse of prescription substances may not be a good idea in health care settings “when it’s something they can’t do anything about. If you’re going to screen, you would have to have either referral processes in place or treatment available in your facility,” Dr. Saxon said.
Opioid use disorder is “especially amenable to treatment in a primary care or health care setting with prescribers,” he noted.
However, stimulant or cannabis use disorders “require fairly intensive behavioral interventions that are not easy to deliver in many health care settings. And we don›t have the workforce trained up to provide those treatments as widely as they should be,” said Dr. Saxon.
“Unless there’s some way to treat the issue, what’s the point of screening for it? That just creates frustration for patients and clinicians, as well,” he said. “It’s something we’re moving toward but we’re not quite there yet.”
The report authors and Dr. Saxon have disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Substance use disorders (SUD), particularly opioid addiction and smoking, are tied to an increased risk for COVID-19 and serious adverse outcomes including hospitalization and death, new research suggests.
A study funded by the National Institutes of Health assessed electronic health records of more than 73 million patients in the United States. Although only 10.3% of the participants had an SUD, “they represented 15.6% of the COVID-19 cases,” the investigators reported.
In addition, those with a recent diagnosis of SUD were eight times more likely to develop COVID-19 versus those without such a diagnosis. For specific SUDs, the greatest risk was for those with an opioid addiction followed by those who were addicted to cigarettes.
coinvestigator Nora Volkow, MD, director of the National Institute on Drug Abuse, said in a press release.
It may also be harder for individuals with addiction to access health care services for a variety of reasons, including low socioeconomic status or stigma, she said in an interview.
Dr. Volkow said she has encountered patients with medical emergencies who refuse to seek treatment at the emergency department because of previous experiences where they have been mistreated and encountered discrimination, and “that’s really very tragic.”
The findings were published online Sept. 14 in Molecular Psychiatry.
Is nicotine protective?
Dr. Volkow, her fellow senior author Rong Xu, PhD, Case Western Reserve University, Cleveland, and their team conducted the study because data released before the pandemic showed a significant increase in opioid overdose in 2019. “We were in an opioid crisis where we again saw an increase in mortality associated with overdose – and then COVID comes along. So the question was how are people who are already struggling faring? And if they were getting infected [with the coronavirus], what happened to them?”
Patients with SUDs have multiple medical comorbidities that are known risk factors for COVID-19, Dr. Volkow noted.
However, the only specific SUD that has been previously studied in this context is tobacco use disorder, she said. A report from Chinese investigators released early in the pandemic showed that smokers were more likely to be infected by coronavirus and more likely to die from COVID-19.
Interestingly, a cross-sectional study published in April suggested that smoking may be protective against COVID, and Dr. Volkow noted that a clinical study currently being conducted in France is assessing whether wearing a nicotine patch has the potential to prevent the virus.
“That’s very different from looking at a chronic smoker,” she pointed out. “It’s a potential that nicotine as a chemical [could be] a preventive measure as opposed to saying smoking will prevent you from getting COVID.”
Patients with SUDs, said Dr. Volkow, “are likely to be at greater risk because of the effects of drugs in the metabolic system and the interfering with oxygenation in the pulmonary vessels.”
The retrospective case-control study included EHR data from 73.1 million patients. In the study population, 54% were women, 55% were White, 10% Black, 2% Asian, 1% Hispanic/Latino, and the others were classified as other or unknown.
EHRs were collected through June 15 at 360 hospitals in all 50 states and were deidentified to ensure privacy. SUDs included alcohol, tobacco, cannabis, opioid, and cocaine.
Racial disparities
Results showed that about 7.5 million participants had a previous SUD diagnosis; of these, 722,370 had been diagnosed within the past year.
Tobacco use disorder was the most common diagnosis (n = 6,414,580), followed by alcohol (1,264,990), cannabis (490,420), opioid (471,520), and cocaine (222,680).
In addition, 12,030 (60% women) were diagnosed with COVID-19 and 1,880 had both COVID-19 and an SUD.
Adjusted analyses revealed that those who had a recent diagnosis of SUD were at a significantly greater increased risk for COVID-19 than individuals without an SUD (adjusted odds ratio, 8.7; 95% confidence interval, 8.4-9.0; P < 10–30).
This increased risk was greatest in participants with opioid use disorder (aOR, 10.2; 95% CI, 9.1-11.5; P < 10–30), followed by those with tobacco use disorder (aOR, 8.2; 95% CI, 7.9 - 8.5; P < 10–30).
Alcohol, cocaine, and cannabis had aORs of 7.7, 6.5, and 5.3, respectively. The aOR for lifetime SUD and COVID-19 was 1.5.
Among all patients with COVID-19, hospitalization rates were significantly greater in those with an SUD (43.8%) versus those without (30.1%), as were death rates at 9.6% versus 6.6%, respectively.
Race was a significant risk factor. Black patients with a recent SUD diagnosis were twice as likely as White patients to develop COVID-19 (aOR, 2.2; P < 10–30), and those specifically with opioid use disorder were four times more likely to develop the disease (aOR, 4.2 P < 10–25).
Black patients with both COVID-19 and lifetime SUD also had greater hospitalization and death rates versus their White peers (50.7% vs. 35.2% and 13% vs. 8.6%, respectively).
“This surprised me,” Dr. Volkow noted. “You can see the emergence of the racial disparities even under these conditions of really negative outcomes.”
Vulnerable populations
Cancer; obesity; HIV; diabetes; cardiovascular disease; and chronic kidney, liver, and lung diseases, which are all risk factors for COVID-19, were more prevalent in the group of patients with a recent SUD diagnosis versus those without.
In addition, asthma, type 2 diabetes, hypertension, obesity, and chronic kidney disease were more prevalent in the Black patents with a recent SUD than in the White patients.
Overall, the findings “identify individuals with SUD as a vulnerable population, especially African Americans with SUDs, who are at significantly increased risk for COVID-19 and its adverse outcomes,” the investigators wrote.
The results also highlight “the need to screen and treat individuals with SUD as part of the strategy to control the pandemic while ensuring no disparities in access to healthcare support,” they added.
Dr. Volkow noted that “marginalization” often occurs for individuals with addiction, making it more difficult for them to access health care services.
“It is incumbent upon clinicians to meet the unique challenges of caring for this vulnerable population, just as they would any other high-risk group,” she said.
“Patients should not just be treated for COVID, but should also be provided with treatment for their substance use disorder,” Dr. Volkow added.
‘Pretty convincing’
Andrew J. Saxon, MD, professor in the department of psychiatry and behavioral sciences at the University of Washington, Seattle, called the findings interesting.
“I found it pretty convincing that people who have substance use disorders are probably at higher risk for getting COVID-19 infection and more complications once they are infected,” he said.
Dr. Saxon, who was not involved with the research, is also director of the Center of Excellence in Substance Addiction Treatment and Education and is a member of the American Psychiatric Association’s Council on Addiction Psychiatry.
He noted that an important point from the study was not just about a patient having an SUD being at increased risk for COVID-19 “and a more severe disease trajectory.” Other factors associated with having an SUD, such as increased comorbidities, also likely play a part.
Dr. Saxon agreed that the ongoing opioid epidemic combined with the pandemic led to a “perfect storm” of problems.
“We were making slow but some progress getting more people the medications they need [to treat opioid use disorder], but the pandemic coming along disrupted those efforts. A lot of health care entities had to shut down for a while, seeing patients only remotely,” which led to barriers as many clinicians needed to learn how to proceed using telehealth options, said Dr. Saxon.
Universal screening?
Asked whether physicians should screen all patients for SUDs, Dr. Saxon said it’s a complicated question.
“Screening for tobacco and alcohol has a really good evidence base and practices should be doing that. The stigma is there but it’s a lot less than with illegal substances,” he said.
Screening for illegal substances or misuse of prescription substances may not be a good idea in health care settings “when it’s something they can’t do anything about. If you’re going to screen, you would have to have either referral processes in place or treatment available in your facility,” Dr. Saxon said.
Opioid use disorder is “especially amenable to treatment in a primary care or health care setting with prescribers,” he noted.
However, stimulant or cannabis use disorders “require fairly intensive behavioral interventions that are not easy to deliver in many health care settings. And we don›t have the workforce trained up to provide those treatments as widely as they should be,” said Dr. Saxon.
“Unless there’s some way to treat the issue, what’s the point of screening for it? That just creates frustration for patients and clinicians, as well,” he said. “It’s something we’re moving toward but we’re not quite there yet.”
The report authors and Dr. Saxon have disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Substance use disorders (SUD), particularly opioid addiction and smoking, are tied to an increased risk for COVID-19 and serious adverse outcomes including hospitalization and death, new research suggests.
A study funded by the National Institutes of Health assessed electronic health records of more than 73 million patients in the United States. Although only 10.3% of the participants had an SUD, “they represented 15.6% of the COVID-19 cases,” the investigators reported.
In addition, those with a recent diagnosis of SUD were eight times more likely to develop COVID-19 versus those without such a diagnosis. For specific SUDs, the greatest risk was for those with an opioid addiction followed by those who were addicted to cigarettes.
coinvestigator Nora Volkow, MD, director of the National Institute on Drug Abuse, said in a press release.
It may also be harder for individuals with addiction to access health care services for a variety of reasons, including low socioeconomic status or stigma, she said in an interview.
Dr. Volkow said she has encountered patients with medical emergencies who refuse to seek treatment at the emergency department because of previous experiences where they have been mistreated and encountered discrimination, and “that’s really very tragic.”
The findings were published online Sept. 14 in Molecular Psychiatry.
Is nicotine protective?
Dr. Volkow, her fellow senior author Rong Xu, PhD, Case Western Reserve University, Cleveland, and their team conducted the study because data released before the pandemic showed a significant increase in opioid overdose in 2019. “We were in an opioid crisis where we again saw an increase in mortality associated with overdose – and then COVID comes along. So the question was how are people who are already struggling faring? And if they were getting infected [with the coronavirus], what happened to them?”
Patients with SUDs have multiple medical comorbidities that are known risk factors for COVID-19, Dr. Volkow noted.
However, the only specific SUD that has been previously studied in this context is tobacco use disorder, she said. A report from Chinese investigators released early in the pandemic showed that smokers were more likely to be infected by coronavirus and more likely to die from COVID-19.
Interestingly, a cross-sectional study published in April suggested that smoking may be protective against COVID, and Dr. Volkow noted that a clinical study currently being conducted in France is assessing whether wearing a nicotine patch has the potential to prevent the virus.
“That’s very different from looking at a chronic smoker,” she pointed out. “It’s a potential that nicotine as a chemical [could be] a preventive measure as opposed to saying smoking will prevent you from getting COVID.”
Patients with SUDs, said Dr. Volkow, “are likely to be at greater risk because of the effects of drugs in the metabolic system and the interfering with oxygenation in the pulmonary vessels.”
The retrospective case-control study included EHR data from 73.1 million patients. In the study population, 54% were women, 55% were White, 10% Black, 2% Asian, 1% Hispanic/Latino, and the others were classified as other or unknown.
EHRs were collected through June 15 at 360 hospitals in all 50 states and were deidentified to ensure privacy. SUDs included alcohol, tobacco, cannabis, opioid, and cocaine.
Racial disparities
Results showed that about 7.5 million participants had a previous SUD diagnosis; of these, 722,370 had been diagnosed within the past year.
Tobacco use disorder was the most common diagnosis (n = 6,414,580), followed by alcohol (1,264,990), cannabis (490,420), opioid (471,520), and cocaine (222,680).
In addition, 12,030 (60% women) were diagnosed with COVID-19 and 1,880 had both COVID-19 and an SUD.
Adjusted analyses revealed that those who had a recent diagnosis of SUD were at a significantly greater increased risk for COVID-19 than individuals without an SUD (adjusted odds ratio, 8.7; 95% confidence interval, 8.4-9.0; P < 10–30).
This increased risk was greatest in participants with opioid use disorder (aOR, 10.2; 95% CI, 9.1-11.5; P < 10–30), followed by those with tobacco use disorder (aOR, 8.2; 95% CI, 7.9 - 8.5; P < 10–30).
Alcohol, cocaine, and cannabis had aORs of 7.7, 6.5, and 5.3, respectively. The aOR for lifetime SUD and COVID-19 was 1.5.
Among all patients with COVID-19, hospitalization rates were significantly greater in those with an SUD (43.8%) versus those without (30.1%), as were death rates at 9.6% versus 6.6%, respectively.
Race was a significant risk factor. Black patients with a recent SUD diagnosis were twice as likely as White patients to develop COVID-19 (aOR, 2.2; P < 10–30), and those specifically with opioid use disorder were four times more likely to develop the disease (aOR, 4.2 P < 10–25).
Black patients with both COVID-19 and lifetime SUD also had greater hospitalization and death rates versus their White peers (50.7% vs. 35.2% and 13% vs. 8.6%, respectively).
“This surprised me,” Dr. Volkow noted. “You can see the emergence of the racial disparities even under these conditions of really negative outcomes.”
Vulnerable populations
Cancer; obesity; HIV; diabetes; cardiovascular disease; and chronic kidney, liver, and lung diseases, which are all risk factors for COVID-19, were more prevalent in the group of patients with a recent SUD diagnosis versus those without.
In addition, asthma, type 2 diabetes, hypertension, obesity, and chronic kidney disease were more prevalent in the Black patents with a recent SUD than in the White patients.
Overall, the findings “identify individuals with SUD as a vulnerable population, especially African Americans with SUDs, who are at significantly increased risk for COVID-19 and its adverse outcomes,” the investigators wrote.
The results also highlight “the need to screen and treat individuals with SUD as part of the strategy to control the pandemic while ensuring no disparities in access to healthcare support,” they added.
Dr. Volkow noted that “marginalization” often occurs for individuals with addiction, making it more difficult for them to access health care services.
“It is incumbent upon clinicians to meet the unique challenges of caring for this vulnerable population, just as they would any other high-risk group,” she said.
“Patients should not just be treated for COVID, but should also be provided with treatment for their substance use disorder,” Dr. Volkow added.
‘Pretty convincing’
Andrew J. Saxon, MD, professor in the department of psychiatry and behavioral sciences at the University of Washington, Seattle, called the findings interesting.
“I found it pretty convincing that people who have substance use disorders are probably at higher risk for getting COVID-19 infection and more complications once they are infected,” he said.
Dr. Saxon, who was not involved with the research, is also director of the Center of Excellence in Substance Addiction Treatment and Education and is a member of the American Psychiatric Association’s Council on Addiction Psychiatry.
He noted that an important point from the study was not just about a patient having an SUD being at increased risk for COVID-19 “and a more severe disease trajectory.” Other factors associated with having an SUD, such as increased comorbidities, also likely play a part.
Dr. Saxon agreed that the ongoing opioid epidemic combined with the pandemic led to a “perfect storm” of problems.
“We were making slow but some progress getting more people the medications they need [to treat opioid use disorder], but the pandemic coming along disrupted those efforts. A lot of health care entities had to shut down for a while, seeing patients only remotely,” which led to barriers as many clinicians needed to learn how to proceed using telehealth options, said Dr. Saxon.
Universal screening?
Asked whether physicians should screen all patients for SUDs, Dr. Saxon said it’s a complicated question.
“Screening for tobacco and alcohol has a really good evidence base and practices should be doing that. The stigma is there but it’s a lot less than with illegal substances,” he said.
Screening for illegal substances or misuse of prescription substances may not be a good idea in health care settings “when it’s something they can’t do anything about. If you’re going to screen, you would have to have either referral processes in place or treatment available in your facility,” Dr. Saxon said.
Opioid use disorder is “especially amenable to treatment in a primary care or health care setting with prescribers,” he noted.
However, stimulant or cannabis use disorders “require fairly intensive behavioral interventions that are not easy to deliver in many health care settings. And we don›t have the workforce trained up to provide those treatments as widely as they should be,” said Dr. Saxon.
“Unless there’s some way to treat the issue, what’s the point of screening for it? That just creates frustration for patients and clinicians, as well,” he said. “It’s something we’re moving toward but we’re not quite there yet.”
The report authors and Dr. Saxon have disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Minorities bear brunt of pediatric COVID-19 cases
Black and Hispanic children comprised significantly more cases of COVID-19, compared with White children, based on data from a large, cross-sectional study of 1,000 cases.
“Data regarding disparities in SARS-CoV-2 infection and outcomes have been, thus far, mostly limited to adults,” wrote Monika K. Goyal, MD, of Children’s National Hospital, Washington, and colleagues. “Additional data further suggest that low socioeconomic status may further exacerbate health outcomes for racial and ethnic minorities.”
In a study published in Pediatrics, the researchers conducted a cross-sectional analysis of 1,000 children from a registry of non–acutely ill pediatric patients seen at a drive-through and walk-up COVID-19 test site.
Minority, socioeconomic status affect pediatric outcomes too
The median age of the study population was 8 years, and approximately half were male.
The researchers also examined the association of median family income (MFI) using census block group estimates data from the American Community Survey (2014–2018) to represent socioeconomic status.
Infection rates were significantly higher among children in the lowest three quartiles of MFI (24%, 27%, and 38% for quartiles 3, 2, and 1, respectively), compared with the highest quartile of MFI (9%).
After adjusting for age, sex, and MFI, Hispanic children were six times more likely and non-Hispanic Black children were twice as likely to test positive for COVID-19 than non-Hispanic White children (adjusted odds ratios, 6.3 and 2.3, respectively).
The study findings were limited by several factors including the use of clinician-reported ethnicity and thus potential for misclassification, the researchers noted. In addition, the socioeconomic and racial disparities may be underestimated because these groups have less access to primary care, and the study did not allow for confounding variables including housing conditions or occupancy.
“Although it was beyond the scope of this study to understand the causes for these differential rates of infection, the causes may be multifactorial, including, but not limited to, structural factors, poorer access to health care, limited resources, and bias and discrimination,” the researchers noted. In addition, the high infection rate among minority children may be impacted by parents who are less able to telework, find child care, or avoid public transportation, Dr. Goyal and associates wrote.
Future research should address “the modifiable reasons for these observed disparities as well as their differential impact in terms of SARS-CoV-2–related morbidity and mortality outcomes to mitigate the spread of infection and its health effects,” they concluded.
How to help
“This study is important because we need to understand which groups of children are at highest risk for SARS-CoV-2 infection in order to maximize efforts for screening, allocating resources, and prioritizing vaccine administration,” Karalyn Kinsella, MD, a pediatrician in private practice in Cheshire, Conn., said in an interview.
Dr. Kinsella said she was not surprised at the higher infection rates in general in minorities and low socioeconomic groups. “We already knew that adult COVID-19 rates were higher for people in certain racial/ethnic groups and those with socioeconomic disadvantages; however, I was shocked by the percentages. That is a huge burden for a population that already has disparities in health outcomes.”
“As the authors cite, this was not a research study of why these groups were more likely to be COVID-19 positive, but they speculated that crowded living conditions, multigenerational families living together, and many minorities being essential workers unable to work from home,” said Dr. Kinsella. Additional factors contributing to higher infection rates may include limited access to care, transportation issues, insurance coverage, schedule challenges, and fear of deportation. Some of these problems might be addressed by coming into communities in mobile vans, visiting community health centers and schools with free educational materials, using masks and hand sanitizer, and offering free access to testing.
“Future studies could confirm the cause of this discrepancy, as well as study community-based interventions and their outcomes,” Dr. Kinsella said. In the meantime, a take-home message for clinicians is the need to prioritize screening, resources, and vaccines to reflect the higher rates of SARS-CoV-2 infections in children from disadvantaged racial and socioeconomic backgrounds.
The study received no outside funding. The researchers had no financial conflicts to disclose, but lead author Dr. Goyal is a member of the Pediatrics editorial board. Dr. Kinsella had no financial conflicts to disclose, but serves on the Pediatric News editorial advisory board.
SOURCE: Goyal MK et al. Pediatrics. 2020 Sep 24. doi: 10.1542/peds.2020-009951.
Black and Hispanic children comprised significantly more cases of COVID-19, compared with White children, based on data from a large, cross-sectional study of 1,000 cases.
“Data regarding disparities in SARS-CoV-2 infection and outcomes have been, thus far, mostly limited to adults,” wrote Monika K. Goyal, MD, of Children’s National Hospital, Washington, and colleagues. “Additional data further suggest that low socioeconomic status may further exacerbate health outcomes for racial and ethnic minorities.”
In a study published in Pediatrics, the researchers conducted a cross-sectional analysis of 1,000 children from a registry of non–acutely ill pediatric patients seen at a drive-through and walk-up COVID-19 test site.
Minority, socioeconomic status affect pediatric outcomes too
The median age of the study population was 8 years, and approximately half were male.
The researchers also examined the association of median family income (MFI) using census block group estimates data from the American Community Survey (2014–2018) to represent socioeconomic status.
Infection rates were significantly higher among children in the lowest three quartiles of MFI (24%, 27%, and 38% for quartiles 3, 2, and 1, respectively), compared with the highest quartile of MFI (9%).
After adjusting for age, sex, and MFI, Hispanic children were six times more likely and non-Hispanic Black children were twice as likely to test positive for COVID-19 than non-Hispanic White children (adjusted odds ratios, 6.3 and 2.3, respectively).
The study findings were limited by several factors including the use of clinician-reported ethnicity and thus potential for misclassification, the researchers noted. In addition, the socioeconomic and racial disparities may be underestimated because these groups have less access to primary care, and the study did not allow for confounding variables including housing conditions or occupancy.
“Although it was beyond the scope of this study to understand the causes for these differential rates of infection, the causes may be multifactorial, including, but not limited to, structural factors, poorer access to health care, limited resources, and bias and discrimination,” the researchers noted. In addition, the high infection rate among minority children may be impacted by parents who are less able to telework, find child care, or avoid public transportation, Dr. Goyal and associates wrote.
Future research should address “the modifiable reasons for these observed disparities as well as their differential impact in terms of SARS-CoV-2–related morbidity and mortality outcomes to mitigate the spread of infection and its health effects,” they concluded.
How to help
“This study is important because we need to understand which groups of children are at highest risk for SARS-CoV-2 infection in order to maximize efforts for screening, allocating resources, and prioritizing vaccine administration,” Karalyn Kinsella, MD, a pediatrician in private practice in Cheshire, Conn., said in an interview.
Dr. Kinsella said she was not surprised at the higher infection rates in general in minorities and low socioeconomic groups. “We already knew that adult COVID-19 rates were higher for people in certain racial/ethnic groups and those with socioeconomic disadvantages; however, I was shocked by the percentages. That is a huge burden for a population that already has disparities in health outcomes.”
“As the authors cite, this was not a research study of why these groups were more likely to be COVID-19 positive, but they speculated that crowded living conditions, multigenerational families living together, and many minorities being essential workers unable to work from home,” said Dr. Kinsella. Additional factors contributing to higher infection rates may include limited access to care, transportation issues, insurance coverage, schedule challenges, and fear of deportation. Some of these problems might be addressed by coming into communities in mobile vans, visiting community health centers and schools with free educational materials, using masks and hand sanitizer, and offering free access to testing.
“Future studies could confirm the cause of this discrepancy, as well as study community-based interventions and their outcomes,” Dr. Kinsella said. In the meantime, a take-home message for clinicians is the need to prioritize screening, resources, and vaccines to reflect the higher rates of SARS-CoV-2 infections in children from disadvantaged racial and socioeconomic backgrounds.
The study received no outside funding. The researchers had no financial conflicts to disclose, but lead author Dr. Goyal is a member of the Pediatrics editorial board. Dr. Kinsella had no financial conflicts to disclose, but serves on the Pediatric News editorial advisory board.
SOURCE: Goyal MK et al. Pediatrics. 2020 Sep 24. doi: 10.1542/peds.2020-009951.
Black and Hispanic children comprised significantly more cases of COVID-19, compared with White children, based on data from a large, cross-sectional study of 1,000 cases.
“Data regarding disparities in SARS-CoV-2 infection and outcomes have been, thus far, mostly limited to adults,” wrote Monika K. Goyal, MD, of Children’s National Hospital, Washington, and colleagues. “Additional data further suggest that low socioeconomic status may further exacerbate health outcomes for racial and ethnic minorities.”
In a study published in Pediatrics, the researchers conducted a cross-sectional analysis of 1,000 children from a registry of non–acutely ill pediatric patients seen at a drive-through and walk-up COVID-19 test site.
Minority, socioeconomic status affect pediatric outcomes too
The median age of the study population was 8 years, and approximately half were male.
The researchers also examined the association of median family income (MFI) using census block group estimates data from the American Community Survey (2014–2018) to represent socioeconomic status.
Infection rates were significantly higher among children in the lowest three quartiles of MFI (24%, 27%, and 38% for quartiles 3, 2, and 1, respectively), compared with the highest quartile of MFI (9%).
After adjusting for age, sex, and MFI, Hispanic children were six times more likely and non-Hispanic Black children were twice as likely to test positive for COVID-19 than non-Hispanic White children (adjusted odds ratios, 6.3 and 2.3, respectively).
The study findings were limited by several factors including the use of clinician-reported ethnicity and thus potential for misclassification, the researchers noted. In addition, the socioeconomic and racial disparities may be underestimated because these groups have less access to primary care, and the study did not allow for confounding variables including housing conditions or occupancy.
“Although it was beyond the scope of this study to understand the causes for these differential rates of infection, the causes may be multifactorial, including, but not limited to, structural factors, poorer access to health care, limited resources, and bias and discrimination,” the researchers noted. In addition, the high infection rate among minority children may be impacted by parents who are less able to telework, find child care, or avoid public transportation, Dr. Goyal and associates wrote.
Future research should address “the modifiable reasons for these observed disparities as well as their differential impact in terms of SARS-CoV-2–related morbidity and mortality outcomes to mitigate the spread of infection and its health effects,” they concluded.
How to help
“This study is important because we need to understand which groups of children are at highest risk for SARS-CoV-2 infection in order to maximize efforts for screening, allocating resources, and prioritizing vaccine administration,” Karalyn Kinsella, MD, a pediatrician in private practice in Cheshire, Conn., said in an interview.
Dr. Kinsella said she was not surprised at the higher infection rates in general in minorities and low socioeconomic groups. “We already knew that adult COVID-19 rates were higher for people in certain racial/ethnic groups and those with socioeconomic disadvantages; however, I was shocked by the percentages. That is a huge burden for a population that already has disparities in health outcomes.”
“As the authors cite, this was not a research study of why these groups were more likely to be COVID-19 positive, but they speculated that crowded living conditions, multigenerational families living together, and many minorities being essential workers unable to work from home,” said Dr. Kinsella. Additional factors contributing to higher infection rates may include limited access to care, transportation issues, insurance coverage, schedule challenges, and fear of deportation. Some of these problems might be addressed by coming into communities in mobile vans, visiting community health centers and schools with free educational materials, using masks and hand sanitizer, and offering free access to testing.
“Future studies could confirm the cause of this discrepancy, as well as study community-based interventions and their outcomes,” Dr. Kinsella said. In the meantime, a take-home message for clinicians is the need to prioritize screening, resources, and vaccines to reflect the higher rates of SARS-CoV-2 infections in children from disadvantaged racial and socioeconomic backgrounds.
The study received no outside funding. The researchers had no financial conflicts to disclose, but lead author Dr. Goyal is a member of the Pediatrics editorial board. Dr. Kinsella had no financial conflicts to disclose, but serves on the Pediatric News editorial advisory board.
SOURCE: Goyal MK et al. Pediatrics. 2020 Sep 24. doi: 10.1542/peds.2020-009951.
FROM PEDIATRICS
COVID-19 may discourage pediatric flu vaccination
Parents who did not vaccinate their children against influenza last year were significantly less likely to do so this year than parents whose children were vaccinated last year, based on survey data from more than 2,000 parents with babies and young children.
“Pediatric vaccination will be an important component to mitigating a dual influenza/COVID-19 epidemic,” Rebeccah L. Sokol, PhD, of Wayne State University, Detroit, and Anna H. Grummon, PhD, of Harvard School of Public Health, Boston, reported in Pediatrics.
Although the pandemic has increased acceptance of some healthy behaviors including handwashing and social distancing, the impact on influenza vaccination rates remains unknown, they said.
To assess parents’ current intentions for flu vaccination of young children this season, the researchers conducted an online survey of 2,164 parents or guardians of children aged between 6 months and 5 years in the United States. The 15-minute online survey was conducted in May 2020 and participants received gift cards. The primary outcome was the impact of the COVID-19 pandemic on parental intentions for having their child vaccinated against seasonal flu this year.
“We measured change categorically, with response options ranging from 1 (I became much less likely to get my child the flu shot next year) to 5 (I became much more likely to get my child the flu shot next year),” the researchers said.
Pandemic changes some parents’ plans
Overall, 60% of parents said that the ongoing pandemic had altered their flu vaccination intentions for their children. About 34% percent of parents whose children did not receive flu vaccine last year said they would not seek the vaccine this year because of the pandemic, compared with 25% of parents whose children received last year’s flu vaccine, a statistically significant difference (P < .001).
Approximately 21% of parents whose children received no flu vaccine last year said the pandemic made them more likely to seek vaccination for the 2020-2021 season, compared with 38% of parents whose children received last year’s flu vaccine.
“These results suggest that overall seasonal influenza vaccination rates may not increase simply because of an ongoing infectious disease pandemic. Instead, a significant predictor of future behavior remains past behavior,” Dr. Sokol and Dr. Grummon said.
The study findings were limited by several factors including the use of a convenience sample and the timing of the survey in May 2020, meaning that survey results might not be generalizable this fall as the pandemic persists, they noted. “Additionally, we assessed intentions to vaccinate; future research will clarify the COVID-19 pandemic’s influence on actual vaccination behaviors.”
The challenge of how to increase uptake of the influenza vaccine during the era of COVID-19 remains, and targeted efforts could include social norms messaging through social media, mass media, or health care providers to increase parents’ intentions to vaccinate, as well as vaccination reminders and presumptive announcements from health care providers that present vaccination as the default option, the researchers added.
Potential for ‘twindemic’ is real
The uptake of flu vaccination is especially important this year, Christopher J. Harrison, MD, director of the vaccine and treatment evaluation unit and professor of pediatrics at the University of Missouri–Kansas City, said in an interview.
“This year we are entering a flu season where the certainty of the timing as well as the potential severity of the season are not known. That said, social distancing and wearing masks – to the extent that enough people conform to COVID-19 precautions – could delay or even blunt the usual influenza season,” he noted.
Unfortunately, the Centers for Disease Control and Prevention and the Food and Drug Administration have had their credibility damaged by the challenges of creating a successful response on the fly to a uniquely multifaceted virus to which previous rules do not apply, Dr. Harrison said. In addition, public confidence was eroded when information about testing and reopening policies were released by non-CDC nonscientists and labeled “CDC recommended,” with no opportunity for the scientific community to correct inaccuracies.
“The current study reveals that public trust in influenza vaccine and indirectly in health authorities has been affected by the pandemic,” said Dr. Harrison. “Vaccine hesitancy has increased somewhat even among previous vaccine accepters. One wonders if promises of a quick COVID-19 vaccine increased mistrust of the FDA because of safety concerns, even among the most ardent provaccine population, and whether these concerns are bleeding over into influenza vaccine concerns.
“This only adds to the anxiety that families feel about visiting any medical facility for routine vaccines while the pandemic rages, and we now are in a fall SARS-CoV-2 resurgence,” he added.
Although the current study data are concerning, “there could still be a net gain of pediatric influenza vaccine uptake this season because the 34% less likely to immunize among previously nonimmunizing families would be counterbalanced by 21% of the same group being more likely to immunize their children [theoretical net loss of 13%],” Dr. Harrison explained. “But the pandemic seems to have motivated previously influenza-immunizing families, i.e. while 24% were less likely, 39% are more likely to immunize [theoretical net gain of 15%]. That said, we would still be way short of the number needed to get to herd immunity.”
Dr. Harrison said he found the findings somewhat surprising, but perhaps he should not have. “I had hoped for more acceptance rather than most people staying in their prior vaccine ‘opinion lanes,’ ending up with likely little overall net change in plans to immunize despite increased health awareness caused by a pandemic.”
However, “the U.S. population has been polarized on vaccines and particularly influenza vaccines for more than 50 years, so why would a pandemic make us less polarized, particularly when the pandemic itself has been a polarizing event?” he questioned.
The greatest barriers to flu vaccination for children this year include a lack of motivation among families to visit immunization sites, given the ongoing need for social distancing and masks, Dr. Harrison said.
“Another barrier is the waning public confidence in our medical/scientific national leaders and organizations,” he emphasized. “This makes it crucial that primary care providers step up and be extra strong vaccine advocates, despite the fact that pandemic economics and necessary safety processes have stressed providers and devastated practices. Indeed, in times of medical stress, no one gets more trust from families than their own personal provider.”
Ultimately, avenues for future research include asking diverse groups of families what they feel they need to hear to be more engaged in immunizing children against influenza. But for now, the current study findings identify that “the public is not uniformly responding to the pandemic’s influence on their likelihood of immunizing their children against influenza,” Dr. Harrison said.
“We now know the size of the problem and hopefully governments, public health organizations, pediatric advocates and clinical care givers can find ways to magnify the message that a pandemic year is not a year to avoid seasonal influenza vaccine unless one has a true contraindication,” Dr. Harrison said.
In addition, “one wonders if the poll were taken today – post the president’s COVID-19 illness – would the answers be different?” he noted.
Dr. Sokol’s work was supported in part by the Eunice Kennedy Shriver National Institute of Child Health and Human Development but otherwise had no financial conflicts to disclose. Dr. Harrison disclosed that his institution receives grant funding from Merck, Pfizer, and GlaxoSmithKline for pediatric noninfluenza vaccine studies on which he is a subinvestigator, and support from the CDC for pediatric respiratory and gastrointestinal virus surveillance studies on which he is an investigator.
SOURCE: Sokol RL, Grummon AH. Pediatrics. 2020 Sep 30. doi: 10.1542/peds.2020-022871.
Parents who did not vaccinate their children against influenza last year were significantly less likely to do so this year than parents whose children were vaccinated last year, based on survey data from more than 2,000 parents with babies and young children.
“Pediatric vaccination will be an important component to mitigating a dual influenza/COVID-19 epidemic,” Rebeccah L. Sokol, PhD, of Wayne State University, Detroit, and Anna H. Grummon, PhD, of Harvard School of Public Health, Boston, reported in Pediatrics.
Although the pandemic has increased acceptance of some healthy behaviors including handwashing and social distancing, the impact on influenza vaccination rates remains unknown, they said.
To assess parents’ current intentions for flu vaccination of young children this season, the researchers conducted an online survey of 2,164 parents or guardians of children aged between 6 months and 5 years in the United States. The 15-minute online survey was conducted in May 2020 and participants received gift cards. The primary outcome was the impact of the COVID-19 pandemic on parental intentions for having their child vaccinated against seasonal flu this year.
“We measured change categorically, with response options ranging from 1 (I became much less likely to get my child the flu shot next year) to 5 (I became much more likely to get my child the flu shot next year),” the researchers said.
Pandemic changes some parents’ plans
Overall, 60% of parents said that the ongoing pandemic had altered their flu vaccination intentions for their children. About 34% percent of parents whose children did not receive flu vaccine last year said they would not seek the vaccine this year because of the pandemic, compared with 25% of parents whose children received last year’s flu vaccine, a statistically significant difference (P < .001).
Approximately 21% of parents whose children received no flu vaccine last year said the pandemic made them more likely to seek vaccination for the 2020-2021 season, compared with 38% of parents whose children received last year’s flu vaccine.
“These results suggest that overall seasonal influenza vaccination rates may not increase simply because of an ongoing infectious disease pandemic. Instead, a significant predictor of future behavior remains past behavior,” Dr. Sokol and Dr. Grummon said.
The study findings were limited by several factors including the use of a convenience sample and the timing of the survey in May 2020, meaning that survey results might not be generalizable this fall as the pandemic persists, they noted. “Additionally, we assessed intentions to vaccinate; future research will clarify the COVID-19 pandemic’s influence on actual vaccination behaviors.”
The challenge of how to increase uptake of the influenza vaccine during the era of COVID-19 remains, and targeted efforts could include social norms messaging through social media, mass media, or health care providers to increase parents’ intentions to vaccinate, as well as vaccination reminders and presumptive announcements from health care providers that present vaccination as the default option, the researchers added.
Potential for ‘twindemic’ is real
The uptake of flu vaccination is especially important this year, Christopher J. Harrison, MD, director of the vaccine and treatment evaluation unit and professor of pediatrics at the University of Missouri–Kansas City, said in an interview.
“This year we are entering a flu season where the certainty of the timing as well as the potential severity of the season are not known. That said, social distancing and wearing masks – to the extent that enough people conform to COVID-19 precautions – could delay or even blunt the usual influenza season,” he noted.
Unfortunately, the Centers for Disease Control and Prevention and the Food and Drug Administration have had their credibility damaged by the challenges of creating a successful response on the fly to a uniquely multifaceted virus to which previous rules do not apply, Dr. Harrison said. In addition, public confidence was eroded when information about testing and reopening policies were released by non-CDC nonscientists and labeled “CDC recommended,” with no opportunity for the scientific community to correct inaccuracies.
“The current study reveals that public trust in influenza vaccine and indirectly in health authorities has been affected by the pandemic,” said Dr. Harrison. “Vaccine hesitancy has increased somewhat even among previous vaccine accepters. One wonders if promises of a quick COVID-19 vaccine increased mistrust of the FDA because of safety concerns, even among the most ardent provaccine population, and whether these concerns are bleeding over into influenza vaccine concerns.
“This only adds to the anxiety that families feel about visiting any medical facility for routine vaccines while the pandemic rages, and we now are in a fall SARS-CoV-2 resurgence,” he added.
Although the current study data are concerning, “there could still be a net gain of pediatric influenza vaccine uptake this season because the 34% less likely to immunize among previously nonimmunizing families would be counterbalanced by 21% of the same group being more likely to immunize their children [theoretical net loss of 13%],” Dr. Harrison explained. “But the pandemic seems to have motivated previously influenza-immunizing families, i.e. while 24% were less likely, 39% are more likely to immunize [theoretical net gain of 15%]. That said, we would still be way short of the number needed to get to herd immunity.”
Dr. Harrison said he found the findings somewhat surprising, but perhaps he should not have. “I had hoped for more acceptance rather than most people staying in their prior vaccine ‘opinion lanes,’ ending up with likely little overall net change in plans to immunize despite increased health awareness caused by a pandemic.”
However, “the U.S. population has been polarized on vaccines and particularly influenza vaccines for more than 50 years, so why would a pandemic make us less polarized, particularly when the pandemic itself has been a polarizing event?” he questioned.
The greatest barriers to flu vaccination for children this year include a lack of motivation among families to visit immunization sites, given the ongoing need for social distancing and masks, Dr. Harrison said.
“Another barrier is the waning public confidence in our medical/scientific national leaders and organizations,” he emphasized. “This makes it crucial that primary care providers step up and be extra strong vaccine advocates, despite the fact that pandemic economics and necessary safety processes have stressed providers and devastated practices. Indeed, in times of medical stress, no one gets more trust from families than their own personal provider.”
Ultimately, avenues for future research include asking diverse groups of families what they feel they need to hear to be more engaged in immunizing children against influenza. But for now, the current study findings identify that “the public is not uniformly responding to the pandemic’s influence on their likelihood of immunizing their children against influenza,” Dr. Harrison said.
“We now know the size of the problem and hopefully governments, public health organizations, pediatric advocates and clinical care givers can find ways to magnify the message that a pandemic year is not a year to avoid seasonal influenza vaccine unless one has a true contraindication,” Dr. Harrison said.
In addition, “one wonders if the poll were taken today – post the president’s COVID-19 illness – would the answers be different?” he noted.
Dr. Sokol’s work was supported in part by the Eunice Kennedy Shriver National Institute of Child Health and Human Development but otherwise had no financial conflicts to disclose. Dr. Harrison disclosed that his institution receives grant funding from Merck, Pfizer, and GlaxoSmithKline for pediatric noninfluenza vaccine studies on which he is a subinvestigator, and support from the CDC for pediatric respiratory and gastrointestinal virus surveillance studies on which he is an investigator.
SOURCE: Sokol RL, Grummon AH. Pediatrics. 2020 Sep 30. doi: 10.1542/peds.2020-022871.
Parents who did not vaccinate their children against influenza last year were significantly less likely to do so this year than parents whose children were vaccinated last year, based on survey data from more than 2,000 parents with babies and young children.
“Pediatric vaccination will be an important component to mitigating a dual influenza/COVID-19 epidemic,” Rebeccah L. Sokol, PhD, of Wayne State University, Detroit, and Anna H. Grummon, PhD, of Harvard School of Public Health, Boston, reported in Pediatrics.
Although the pandemic has increased acceptance of some healthy behaviors including handwashing and social distancing, the impact on influenza vaccination rates remains unknown, they said.
To assess parents’ current intentions for flu vaccination of young children this season, the researchers conducted an online survey of 2,164 parents or guardians of children aged between 6 months and 5 years in the United States. The 15-minute online survey was conducted in May 2020 and participants received gift cards. The primary outcome was the impact of the COVID-19 pandemic on parental intentions for having their child vaccinated against seasonal flu this year.
“We measured change categorically, with response options ranging from 1 (I became much less likely to get my child the flu shot next year) to 5 (I became much more likely to get my child the flu shot next year),” the researchers said.
Pandemic changes some parents’ plans
Overall, 60% of parents said that the ongoing pandemic had altered their flu vaccination intentions for their children. About 34% percent of parents whose children did not receive flu vaccine last year said they would not seek the vaccine this year because of the pandemic, compared with 25% of parents whose children received last year’s flu vaccine, a statistically significant difference (P < .001).
Approximately 21% of parents whose children received no flu vaccine last year said the pandemic made them more likely to seek vaccination for the 2020-2021 season, compared with 38% of parents whose children received last year’s flu vaccine.
“These results suggest that overall seasonal influenza vaccination rates may not increase simply because of an ongoing infectious disease pandemic. Instead, a significant predictor of future behavior remains past behavior,” Dr. Sokol and Dr. Grummon said.
The study findings were limited by several factors including the use of a convenience sample and the timing of the survey in May 2020, meaning that survey results might not be generalizable this fall as the pandemic persists, they noted. “Additionally, we assessed intentions to vaccinate; future research will clarify the COVID-19 pandemic’s influence on actual vaccination behaviors.”
The challenge of how to increase uptake of the influenza vaccine during the era of COVID-19 remains, and targeted efforts could include social norms messaging through social media, mass media, or health care providers to increase parents’ intentions to vaccinate, as well as vaccination reminders and presumptive announcements from health care providers that present vaccination as the default option, the researchers added.
Potential for ‘twindemic’ is real
The uptake of flu vaccination is especially important this year, Christopher J. Harrison, MD, director of the vaccine and treatment evaluation unit and professor of pediatrics at the University of Missouri–Kansas City, said in an interview.
“This year we are entering a flu season where the certainty of the timing as well as the potential severity of the season are not known. That said, social distancing and wearing masks – to the extent that enough people conform to COVID-19 precautions – could delay or even blunt the usual influenza season,” he noted.
Unfortunately, the Centers for Disease Control and Prevention and the Food and Drug Administration have had their credibility damaged by the challenges of creating a successful response on the fly to a uniquely multifaceted virus to which previous rules do not apply, Dr. Harrison said. In addition, public confidence was eroded when information about testing and reopening policies were released by non-CDC nonscientists and labeled “CDC recommended,” with no opportunity for the scientific community to correct inaccuracies.
“The current study reveals that public trust in influenza vaccine and indirectly in health authorities has been affected by the pandemic,” said Dr. Harrison. “Vaccine hesitancy has increased somewhat even among previous vaccine accepters. One wonders if promises of a quick COVID-19 vaccine increased mistrust of the FDA because of safety concerns, even among the most ardent provaccine population, and whether these concerns are bleeding over into influenza vaccine concerns.
“This only adds to the anxiety that families feel about visiting any medical facility for routine vaccines while the pandemic rages, and we now are in a fall SARS-CoV-2 resurgence,” he added.
Although the current study data are concerning, “there could still be a net gain of pediatric influenza vaccine uptake this season because the 34% less likely to immunize among previously nonimmunizing families would be counterbalanced by 21% of the same group being more likely to immunize their children [theoretical net loss of 13%],” Dr. Harrison explained. “But the pandemic seems to have motivated previously influenza-immunizing families, i.e. while 24% were less likely, 39% are more likely to immunize [theoretical net gain of 15%]. That said, we would still be way short of the number needed to get to herd immunity.”
Dr. Harrison said he found the findings somewhat surprising, but perhaps he should not have. “I had hoped for more acceptance rather than most people staying in their prior vaccine ‘opinion lanes,’ ending up with likely little overall net change in plans to immunize despite increased health awareness caused by a pandemic.”
However, “the U.S. population has been polarized on vaccines and particularly influenza vaccines for more than 50 years, so why would a pandemic make us less polarized, particularly when the pandemic itself has been a polarizing event?” he questioned.
The greatest barriers to flu vaccination for children this year include a lack of motivation among families to visit immunization sites, given the ongoing need for social distancing and masks, Dr. Harrison said.
“Another barrier is the waning public confidence in our medical/scientific national leaders and organizations,” he emphasized. “This makes it crucial that primary care providers step up and be extra strong vaccine advocates, despite the fact that pandemic economics and necessary safety processes have stressed providers and devastated practices. Indeed, in times of medical stress, no one gets more trust from families than their own personal provider.”
Ultimately, avenues for future research include asking diverse groups of families what they feel they need to hear to be more engaged in immunizing children against influenza. But for now, the current study findings identify that “the public is not uniformly responding to the pandemic’s influence on their likelihood of immunizing their children against influenza,” Dr. Harrison said.
“We now know the size of the problem and hopefully governments, public health organizations, pediatric advocates and clinical care givers can find ways to magnify the message that a pandemic year is not a year to avoid seasonal influenza vaccine unless one has a true contraindication,” Dr. Harrison said.
In addition, “one wonders if the poll were taken today – post the president’s COVID-19 illness – would the answers be different?” he noted.
Dr. Sokol’s work was supported in part by the Eunice Kennedy Shriver National Institute of Child Health and Human Development but otherwise had no financial conflicts to disclose. Dr. Harrison disclosed that his institution receives grant funding from Merck, Pfizer, and GlaxoSmithKline for pediatric noninfluenza vaccine studies on which he is a subinvestigator, and support from the CDC for pediatric respiratory and gastrointestinal virus surveillance studies on which he is an investigator.
SOURCE: Sokol RL, Grummon AH. Pediatrics. 2020 Sep 30. doi: 10.1542/peds.2020-022871.
FROM PEDIATRICS
Stress tests before knee, hip replacement surgeries down, with no ill effects
Cardiac stress testing before hip and knee replacements has dropped steadily since 2006, according to results from a new study that also showed major cardiac complications to be low in the absence of stress testing – even among people with established risk factors.
Routine stress testing before noncardiac surgeries has come under fire in recent decades as an overuse of resources and a burden on patients. Practice guidelines issued in 2007 and 2014 by the American College of Cardiology and the American Heart Association sought to limit the use of preoperative testing to patients with specific cardiovascular risk factors who might have their management changed by the test results.
For their study, published online in JAMA Cardiology, Daniel S. Rubin, MD, of the University of Chicago and colleagues looked at employee-based insurance data, which included Medicare Advantage claims, for more than 800,000 total hip or knee arthroplasties (28% hip and 72% knee replacements) conducted between 2004 and 2017.
While some 10% of the cohort (mean age 62, 58% women) received a stress test in the 2 months before surgery, the investigators found that the frequency of preoperative stress testing dropped annually starting in late 2006, when it peaked at about 14%, to about 7% in 2017. Older age, male sex and a Revised Cardiac Risk Index score of 1 or greater were all associated with a higher likelihood of being tested.
The overall frequency of myocardial infarction or cardiac arrest was 0.24%, occurring in 1,677 of 686,067 patients. While the rate was higher in patients with at least one RCRI condition, this did not differ significantly between those who received a preoperative stress test and those who did not (0.60%; 221 of 36,554 vs. 0.57%; 694 of 122,466 patients.
The 2007 and 2014 ACC/AHA guidelines make clear that patients with zero RCRI conditions – which comprise a history of ischemic heart disease, heart failure, insulin therapy for diabetes, cerebrovascular disease, or chronic kidney disease – should not receive a stress test before an intermediate-risk surgery such as a hip or knee replacement. But in this study, Dr. Rubin and his colleagues found that almost half of patients who had no RCRI risk factors were stress tested anyway. This means, Dr. Rubin said in an interview, that “there’s still room for improvement” in reducing testing.
“I never want to question how a physician chooses to practice, but I have to applaud physicians for reining in the use of this test. We’re using less of this test and yet the incidence of myocardial infarction and cardiac arrest is also going down, which also calls into question whether we’re getting better at choosing the right patients for the test; or the test doesn’t impact outcomes; or overall health of these patients is improving,” he said.
One surprise finding in the study, Dr. Rubin noted, was a higher rate of complications among people without RCRI conditions who were stress tested, compared with those who were not, with a mean complication rate of 0.27%, compared with 0.14% among those who did not receive a test (P < .001). “The RCRI doesn’t capture certain things,” Dr. Rubin said. “And we know that no risk stratification tool is going to capture everything.”
The RCRI, he noted, is based on a clinical history. “If you haven’t been diagnosed yet, it won’t appear as a risk factor, even if you’re clearly at risk. The question then becomes for a physician, do you do the test or not? On a day-to-day basis it’s hard to make that decision because you want what’s best for the individual patient – and it’s hard to generalize from a study of 800,000 people what’s right for that one patient. That said, it doesn’t appear that stress testing improves outcomes and a decrease in testing appears appropriate.”
Dr. Rubin and his colleagues described as a weakness of their study that it did not capture the full scope of preoperative stress testing among Medicare patients, who are older and therefore more likely to be tested.
That the 2007 and 2014 practice guidelines bore on the drop in testing was not demonstrated by Dr. Rubin and colleagues’ study, which saw declines begin even before the guidelines were published. Nonetheless, the results appear to validate the approach advocated in the guidelines, said guideline coauthor Joshua Beckman, MD, of Vanderbilt University, whose recent research has focused on identifying risk factors for MI after noncardiac surgery.
“I hope that the guidelines have helped in changing the culture for the use of preoperative stress testing as a regular thing,” Dr. Beckman said in an interview. “In fact, the guidelines say you shouldn’t do anything before an operation that you wouldn’t do anyway. So these findings are certainly in agreement with what we’re suggesting and support the idea that unless you have something that is unstable or active, stress testing isn’t likely to help.”
Annemarie Thompson, MD, of Duke University in Durham, N.C., another coauthor on the 2014 guidelines, commented in an interview that Dr. Rubin and colleagues’ findings of a doubled rate of complications among people without RCRI conditions who were stress tested, compared with those who were not might mean something “other than just sheer overuse or overordering of tests inappropriately.”
Rather, she said, physicians might be seeing something in the clinic that cannot be captured by a screening tool reliant on existing diagnoses. “Maybe when they’re sitting in front of you in a clinic, they’re so immobile that you’re left wondering. Or maybe they haven’t been seen by a doctor in a long time,” Dr. Thompson said. “So they don’t have diagnoses if they haven’t been followed. I think what [this finding] shows is that clinicians are detecting something. They may not know what it is. But we have to give a little wiggle room to the clinician who is sitting there looking at a patient who looks like they may not make it through surgery.”
Dr. Thompson said it would be helpful, after a big-data study like this one, to go through the clinical histories of those patients – in this study fewer than 100 – who had no RCRI risk factors and yet were stress tested and ended up having complications. “Until then we’re not going to solve the mystery,” she said. “But it’s a very, very interesting study.”
Dr. Rubin is the president of DRDR Mobile Health, a company that creates mobile applications for health care and from which he has not received compensation. One of his coauthors on the study, Dr. Peter Nagele, reported fee income from Roche Diagnostics. Dr. Beckman disclosed personal fees from AstraZeneca, Boehringer Ingelheim, Bristol Myers Squibb, and other pharmaceutical manufacturers. Dr. Thompson has no disclosures.
SOURCE: Rubin et al. JAMA Cardiol. 2020 Sep 30. doi: 10.1001/jamacardio.2020.4311.
Cardiac stress testing before hip and knee replacements has dropped steadily since 2006, according to results from a new study that also showed major cardiac complications to be low in the absence of stress testing – even among people with established risk factors.
Routine stress testing before noncardiac surgeries has come under fire in recent decades as an overuse of resources and a burden on patients. Practice guidelines issued in 2007 and 2014 by the American College of Cardiology and the American Heart Association sought to limit the use of preoperative testing to patients with specific cardiovascular risk factors who might have their management changed by the test results.
For their study, published online in JAMA Cardiology, Daniel S. Rubin, MD, of the University of Chicago and colleagues looked at employee-based insurance data, which included Medicare Advantage claims, for more than 800,000 total hip or knee arthroplasties (28% hip and 72% knee replacements) conducted between 2004 and 2017.
While some 10% of the cohort (mean age 62, 58% women) received a stress test in the 2 months before surgery, the investigators found that the frequency of preoperative stress testing dropped annually starting in late 2006, when it peaked at about 14%, to about 7% in 2017. Older age, male sex and a Revised Cardiac Risk Index score of 1 or greater were all associated with a higher likelihood of being tested.
The overall frequency of myocardial infarction or cardiac arrest was 0.24%, occurring in 1,677 of 686,067 patients. While the rate was higher in patients with at least one RCRI condition, this did not differ significantly between those who received a preoperative stress test and those who did not (0.60%; 221 of 36,554 vs. 0.57%; 694 of 122,466 patients.
The 2007 and 2014 ACC/AHA guidelines make clear that patients with zero RCRI conditions – which comprise a history of ischemic heart disease, heart failure, insulin therapy for diabetes, cerebrovascular disease, or chronic kidney disease – should not receive a stress test before an intermediate-risk surgery such as a hip or knee replacement. But in this study, Dr. Rubin and his colleagues found that almost half of patients who had no RCRI risk factors were stress tested anyway. This means, Dr. Rubin said in an interview, that “there’s still room for improvement” in reducing testing.
“I never want to question how a physician chooses to practice, but I have to applaud physicians for reining in the use of this test. We’re using less of this test and yet the incidence of myocardial infarction and cardiac arrest is also going down, which also calls into question whether we’re getting better at choosing the right patients for the test; or the test doesn’t impact outcomes; or overall health of these patients is improving,” he said.
One surprise finding in the study, Dr. Rubin noted, was a higher rate of complications among people without RCRI conditions who were stress tested, compared with those who were not, with a mean complication rate of 0.27%, compared with 0.14% among those who did not receive a test (P < .001). “The RCRI doesn’t capture certain things,” Dr. Rubin said. “And we know that no risk stratification tool is going to capture everything.”
The RCRI, he noted, is based on a clinical history. “If you haven’t been diagnosed yet, it won’t appear as a risk factor, even if you’re clearly at risk. The question then becomes for a physician, do you do the test or not? On a day-to-day basis it’s hard to make that decision because you want what’s best for the individual patient – and it’s hard to generalize from a study of 800,000 people what’s right for that one patient. That said, it doesn’t appear that stress testing improves outcomes and a decrease in testing appears appropriate.”
Dr. Rubin and his colleagues described as a weakness of their study that it did not capture the full scope of preoperative stress testing among Medicare patients, who are older and therefore more likely to be tested.
That the 2007 and 2014 practice guidelines bore on the drop in testing was not demonstrated by Dr. Rubin and colleagues’ study, which saw declines begin even before the guidelines were published. Nonetheless, the results appear to validate the approach advocated in the guidelines, said guideline coauthor Joshua Beckman, MD, of Vanderbilt University, whose recent research has focused on identifying risk factors for MI after noncardiac surgery.
“I hope that the guidelines have helped in changing the culture for the use of preoperative stress testing as a regular thing,” Dr. Beckman said in an interview. “In fact, the guidelines say you shouldn’t do anything before an operation that you wouldn’t do anyway. So these findings are certainly in agreement with what we’re suggesting and support the idea that unless you have something that is unstable or active, stress testing isn’t likely to help.”
Annemarie Thompson, MD, of Duke University in Durham, N.C., another coauthor on the 2014 guidelines, commented in an interview that Dr. Rubin and colleagues’ findings of a doubled rate of complications among people without RCRI conditions who were stress tested, compared with those who were not might mean something “other than just sheer overuse or overordering of tests inappropriately.”
Rather, she said, physicians might be seeing something in the clinic that cannot be captured by a screening tool reliant on existing diagnoses. “Maybe when they’re sitting in front of you in a clinic, they’re so immobile that you’re left wondering. Or maybe they haven’t been seen by a doctor in a long time,” Dr. Thompson said. “So they don’t have diagnoses if they haven’t been followed. I think what [this finding] shows is that clinicians are detecting something. They may not know what it is. But we have to give a little wiggle room to the clinician who is sitting there looking at a patient who looks like they may not make it through surgery.”
Dr. Thompson said it would be helpful, after a big-data study like this one, to go through the clinical histories of those patients – in this study fewer than 100 – who had no RCRI risk factors and yet were stress tested and ended up having complications. “Until then we’re not going to solve the mystery,” she said. “But it’s a very, very interesting study.”
Dr. Rubin is the president of DRDR Mobile Health, a company that creates mobile applications for health care and from which he has not received compensation. One of his coauthors on the study, Dr. Peter Nagele, reported fee income from Roche Diagnostics. Dr. Beckman disclosed personal fees from AstraZeneca, Boehringer Ingelheim, Bristol Myers Squibb, and other pharmaceutical manufacturers. Dr. Thompson has no disclosures.
SOURCE: Rubin et al. JAMA Cardiol. 2020 Sep 30. doi: 10.1001/jamacardio.2020.4311.
Cardiac stress testing before hip and knee replacements has dropped steadily since 2006, according to results from a new study that also showed major cardiac complications to be low in the absence of stress testing – even among people with established risk factors.
Routine stress testing before noncardiac surgeries has come under fire in recent decades as an overuse of resources and a burden on patients. Practice guidelines issued in 2007 and 2014 by the American College of Cardiology and the American Heart Association sought to limit the use of preoperative testing to patients with specific cardiovascular risk factors who might have their management changed by the test results.
For their study, published online in JAMA Cardiology, Daniel S. Rubin, MD, of the University of Chicago and colleagues looked at employee-based insurance data, which included Medicare Advantage claims, for more than 800,000 total hip or knee arthroplasties (28% hip and 72% knee replacements) conducted between 2004 and 2017.
While some 10% of the cohort (mean age 62, 58% women) received a stress test in the 2 months before surgery, the investigators found that the frequency of preoperative stress testing dropped annually starting in late 2006, when it peaked at about 14%, to about 7% in 2017. Older age, male sex and a Revised Cardiac Risk Index score of 1 or greater were all associated with a higher likelihood of being tested.
The overall frequency of myocardial infarction or cardiac arrest was 0.24%, occurring in 1,677 of 686,067 patients. While the rate was higher in patients with at least one RCRI condition, this did not differ significantly between those who received a preoperative stress test and those who did not (0.60%; 221 of 36,554 vs. 0.57%; 694 of 122,466 patients.
The 2007 and 2014 ACC/AHA guidelines make clear that patients with zero RCRI conditions – which comprise a history of ischemic heart disease, heart failure, insulin therapy for diabetes, cerebrovascular disease, or chronic kidney disease – should not receive a stress test before an intermediate-risk surgery such as a hip or knee replacement. But in this study, Dr. Rubin and his colleagues found that almost half of patients who had no RCRI risk factors were stress tested anyway. This means, Dr. Rubin said in an interview, that “there’s still room for improvement” in reducing testing.
“I never want to question how a physician chooses to practice, but I have to applaud physicians for reining in the use of this test. We’re using less of this test and yet the incidence of myocardial infarction and cardiac arrest is also going down, which also calls into question whether we’re getting better at choosing the right patients for the test; or the test doesn’t impact outcomes; or overall health of these patients is improving,” he said.
One surprise finding in the study, Dr. Rubin noted, was a higher rate of complications among people without RCRI conditions who were stress tested, compared with those who were not, with a mean complication rate of 0.27%, compared with 0.14% among those who did not receive a test (P < .001). “The RCRI doesn’t capture certain things,” Dr. Rubin said. “And we know that no risk stratification tool is going to capture everything.”
The RCRI, he noted, is based on a clinical history. “If you haven’t been diagnosed yet, it won’t appear as a risk factor, even if you’re clearly at risk. The question then becomes for a physician, do you do the test or not? On a day-to-day basis it’s hard to make that decision because you want what’s best for the individual patient – and it’s hard to generalize from a study of 800,000 people what’s right for that one patient. That said, it doesn’t appear that stress testing improves outcomes and a decrease in testing appears appropriate.”
Dr. Rubin and his colleagues described as a weakness of their study that it did not capture the full scope of preoperative stress testing among Medicare patients, who are older and therefore more likely to be tested.
That the 2007 and 2014 practice guidelines bore on the drop in testing was not demonstrated by Dr. Rubin and colleagues’ study, which saw declines begin even before the guidelines were published. Nonetheless, the results appear to validate the approach advocated in the guidelines, said guideline coauthor Joshua Beckman, MD, of Vanderbilt University, whose recent research has focused on identifying risk factors for MI after noncardiac surgery.
“I hope that the guidelines have helped in changing the culture for the use of preoperative stress testing as a regular thing,” Dr. Beckman said in an interview. “In fact, the guidelines say you shouldn’t do anything before an operation that you wouldn’t do anyway. So these findings are certainly in agreement with what we’re suggesting and support the idea that unless you have something that is unstable or active, stress testing isn’t likely to help.”
Annemarie Thompson, MD, of Duke University in Durham, N.C., another coauthor on the 2014 guidelines, commented in an interview that Dr. Rubin and colleagues’ findings of a doubled rate of complications among people without RCRI conditions who were stress tested, compared with those who were not might mean something “other than just sheer overuse or overordering of tests inappropriately.”
Rather, she said, physicians might be seeing something in the clinic that cannot be captured by a screening tool reliant on existing diagnoses. “Maybe when they’re sitting in front of you in a clinic, they’re so immobile that you’re left wondering. Or maybe they haven’t been seen by a doctor in a long time,” Dr. Thompson said. “So they don’t have diagnoses if they haven’t been followed. I think what [this finding] shows is that clinicians are detecting something. They may not know what it is. But we have to give a little wiggle room to the clinician who is sitting there looking at a patient who looks like they may not make it through surgery.”
Dr. Thompson said it would be helpful, after a big-data study like this one, to go through the clinical histories of those patients – in this study fewer than 100 – who had no RCRI risk factors and yet were stress tested and ended up having complications. “Until then we’re not going to solve the mystery,” she said. “But it’s a very, very interesting study.”
Dr. Rubin is the president of DRDR Mobile Health, a company that creates mobile applications for health care and from which he has not received compensation. One of his coauthors on the study, Dr. Peter Nagele, reported fee income from Roche Diagnostics. Dr. Beckman disclosed personal fees from AstraZeneca, Boehringer Ingelheim, Bristol Myers Squibb, and other pharmaceutical manufacturers. Dr. Thompson has no disclosures.
SOURCE: Rubin et al. JAMA Cardiol. 2020 Sep 30. doi: 10.1001/jamacardio.2020.4311.
FROM JAMA CARDIOLOGY
FDA updates info on postmarketing surveillance study of Essure
The Food and Drug Administration has updated its page on Essure information for patients and health care providers to add additional information on adverse events reported by its manufacturer.
Essure was a permanent implantable birth control device approved by the FDA in 2002. FDA ordered Bayer in 2016 to conduct a postmarket surveillance study of Essure following reports of safety concerns, and expanded the study from 3 years to 5 years in 2018. Bayer voluntarily removed Essure from the market at the end of 2018, citing low sales after a “black box” warning was placed on the device. All devices were returned to the company by the end of 2019.
Bayer is required to report variances in Medical Device Reporting (MDR) requirements of Essure related to litigation to the FDA, which includes adverse events such death, serious injury, and “malfunction that would be likely to cause or contribute to a death or serious injury if the malfunction were to recur.” The reports are limited to events Bayer becomes aware of between November 2016 and November 2020. Bayer will continue to provide these reports until April 2021.
The FDA emphasized that the collected data are based on social media reports and already may be reported to the FDA, rather than being a collection of new events. “The limited information provided in the reports prevents the ability to draw any conclusions as to whether the device, or its removal, caused or contributed to any of the events in the reports,” Benjamin Fisher, PhD, director of the Reproductive, Gastro-Renal, Urological, General Hospital Device and Human Factors Office in the Center for Devices and Radiological Health, said in an FDA In Brief statement on Aug. 11.
The FDA first uploaded an Essure MDR variance spreadsheet in August 2020, listing 1,453 events, consisting of 53 reports of deaths, 1,376 reports of serious injury, and 24 reports of device malfunction that occurred as of June 2020. In September 2020, FDA uploaded a second variance spreadsheet, which added another 1,934 events that occurred as of July.
Interim analysis of postmarketing surveillance study
An interim analysis of 1,128 patients from 67 centers in the Essure postmarket surveillance study, which compared women who received Essure with those who received laparoscopic tubal sterilization, revealed that 94.6% (265 of 280 patients) in the Essure group had a successful implantation of the device, compared with 99.6% of women who achieved bilateral tubal occlusion from laparoscopic tubal sterilization.
Regarding safety, 9.1% of women in the Essure group and 4.5% in the laparoscopic tubal sterilization group reported chronic lower abdominal and/or pelvic pain, and 16.3% in the Essure group and 10.2% in the laparoscopic tubal sterilization group reported new or worsening abnormal uterine bleeding. In the Essure group, 22.3% of women said they experienced hypersensitivity, an allergic reaction, and new “autoimmune-like reactions” compared with 12.5% of women in the laparoscopic tubal sterilization group.
The interim analysis also showed 19.7% of women in the Essure group and 3.0% in the laparoscopic tubal sterilization group underwent gynecologic surgical procedures, which were “driven primarily by Essure removal and endometrial ablation procedures in Essure patients.” Device removal occurred in 6.8% of women with the Essure device.
Consistent data on Essure
An FDA search of the Manufacturer and User Facility Device Experience (MAUDE) database in January of 2020 revealed 47,856 medical device reports of Essure between November 2002 and December 2019. The most common adverse events observed during this period were:
- Pain or abdominal pain (32,901 cases).
- Heavy or irregular menses (14,573 cases). Headache (8,570 cases).
- Device fragment or foreign body in a patient (8,501 cases).
- Perforation (7,825 cases).
- Fatigue (7,083 cases).
- Gain or loss in weight (5,980 cases).
- Anxiety and/or depression (5,366 cases).
- Rash and/or hypersensitivity (5,077 cases)
- Hair loss (4,999 cases).
Problems with the device itself included reports of:
- Device incompatibility such as an allergy (7,515 cases).
- The device migrating (4,535 cases).
- The device breaking or fracturing (2,297 cases).
- The device dislodging or dislocating (1,797 cases).
- Improper operation including implant failure and pregnancy (1,058 cases).
In 2019, Essure received 15,083 medical device reports, an increase from 6,000 reports in 2018 and 11,854 reports in 2017.
To date, nearly 39,000 women in the United States have made claims to injuries related to the Essure device. In August, Bayer announced it would pay approximately $1.6 billion U.S. dollars to settle 90% of these cases in exchange for claimants to “dismiss their cases or not file.” Bayer also said in a press release that the settlement is not an admission of wrongdoing or liability on the part of the company.
In an interview, Catherine Cansino, MD, MPH, of the department of obstetrics and gynecology at the University of California, Davis, said the latest adverse event reports show “consistent info from [the] MAUDE database when comparing 2019 to previous years, highlighting most common problems related to pain and heavy or irregular bleeding.”
She emphasized ob.gyns with patients who have an Essure device should “consider Essure-related etiology that may necessitate device removal when evaluating patients with gynecological problems, especially with regard to abdominal/pelvic pain and heavy/irregular bleeding.”
Dr. Cansino reported no relevant financial disclosures. She is a member of the Ob.Gyn. News Editorial Advisory Board.
The Food and Drug Administration has updated its page on Essure information for patients and health care providers to add additional information on adverse events reported by its manufacturer.
Essure was a permanent implantable birth control device approved by the FDA in 2002. FDA ordered Bayer in 2016 to conduct a postmarket surveillance study of Essure following reports of safety concerns, and expanded the study from 3 years to 5 years in 2018. Bayer voluntarily removed Essure from the market at the end of 2018, citing low sales after a “black box” warning was placed on the device. All devices were returned to the company by the end of 2019.
Bayer is required to report variances in Medical Device Reporting (MDR) requirements of Essure related to litigation to the FDA, which includes adverse events such death, serious injury, and “malfunction that would be likely to cause or contribute to a death or serious injury if the malfunction were to recur.” The reports are limited to events Bayer becomes aware of between November 2016 and November 2020. Bayer will continue to provide these reports until April 2021.
The FDA emphasized that the collected data are based on social media reports and already may be reported to the FDA, rather than being a collection of new events. “The limited information provided in the reports prevents the ability to draw any conclusions as to whether the device, or its removal, caused or contributed to any of the events in the reports,” Benjamin Fisher, PhD, director of the Reproductive, Gastro-Renal, Urological, General Hospital Device and Human Factors Office in the Center for Devices and Radiological Health, said in an FDA In Brief statement on Aug. 11.
The FDA first uploaded an Essure MDR variance spreadsheet in August 2020, listing 1,453 events, consisting of 53 reports of deaths, 1,376 reports of serious injury, and 24 reports of device malfunction that occurred as of June 2020. In September 2020, FDA uploaded a second variance spreadsheet, which added another 1,934 events that occurred as of July.
Interim analysis of postmarketing surveillance study
An interim analysis of 1,128 patients from 67 centers in the Essure postmarket surveillance study, which compared women who received Essure with those who received laparoscopic tubal sterilization, revealed that 94.6% (265 of 280 patients) in the Essure group had a successful implantation of the device, compared with 99.6% of women who achieved bilateral tubal occlusion from laparoscopic tubal sterilization.
Regarding safety, 9.1% of women in the Essure group and 4.5% in the laparoscopic tubal sterilization group reported chronic lower abdominal and/or pelvic pain, and 16.3% in the Essure group and 10.2% in the laparoscopic tubal sterilization group reported new or worsening abnormal uterine bleeding. In the Essure group, 22.3% of women said they experienced hypersensitivity, an allergic reaction, and new “autoimmune-like reactions” compared with 12.5% of women in the laparoscopic tubal sterilization group.
The interim analysis also showed 19.7% of women in the Essure group and 3.0% in the laparoscopic tubal sterilization group underwent gynecologic surgical procedures, which were “driven primarily by Essure removal and endometrial ablation procedures in Essure patients.” Device removal occurred in 6.8% of women with the Essure device.
Consistent data on Essure
An FDA search of the Manufacturer and User Facility Device Experience (MAUDE) database in January of 2020 revealed 47,856 medical device reports of Essure between November 2002 and December 2019. The most common adverse events observed during this period were:
- Pain or abdominal pain (32,901 cases).
- Heavy or irregular menses (14,573 cases). Headache (8,570 cases).
- Device fragment or foreign body in a patient (8,501 cases).
- Perforation (7,825 cases).
- Fatigue (7,083 cases).
- Gain or loss in weight (5,980 cases).
- Anxiety and/or depression (5,366 cases).
- Rash and/or hypersensitivity (5,077 cases)
- Hair loss (4,999 cases).
Problems with the device itself included reports of:
- Device incompatibility such as an allergy (7,515 cases).
- The device migrating (4,535 cases).
- The device breaking or fracturing (2,297 cases).
- The device dislodging or dislocating (1,797 cases).
- Improper operation including implant failure and pregnancy (1,058 cases).
In 2019, Essure received 15,083 medical device reports, an increase from 6,000 reports in 2018 and 11,854 reports in 2017.
To date, nearly 39,000 women in the United States have made claims to injuries related to the Essure device. In August, Bayer announced it would pay approximately $1.6 billion U.S. dollars to settle 90% of these cases in exchange for claimants to “dismiss their cases or not file.” Bayer also said in a press release that the settlement is not an admission of wrongdoing or liability on the part of the company.
In an interview, Catherine Cansino, MD, MPH, of the department of obstetrics and gynecology at the University of California, Davis, said the latest adverse event reports show “consistent info from [the] MAUDE database when comparing 2019 to previous years, highlighting most common problems related to pain and heavy or irregular bleeding.”
She emphasized ob.gyns with patients who have an Essure device should “consider Essure-related etiology that may necessitate device removal when evaluating patients with gynecological problems, especially with regard to abdominal/pelvic pain and heavy/irregular bleeding.”
Dr. Cansino reported no relevant financial disclosures. She is a member of the Ob.Gyn. News Editorial Advisory Board.
The Food and Drug Administration has updated its page on Essure information for patients and health care providers to add additional information on adverse events reported by its manufacturer.
Essure was a permanent implantable birth control device approved by the FDA in 2002. FDA ordered Bayer in 2016 to conduct a postmarket surveillance study of Essure following reports of safety concerns, and expanded the study from 3 years to 5 years in 2018. Bayer voluntarily removed Essure from the market at the end of 2018, citing low sales after a “black box” warning was placed on the device. All devices were returned to the company by the end of 2019.
Bayer is required to report variances in Medical Device Reporting (MDR) requirements of Essure related to litigation to the FDA, which includes adverse events such death, serious injury, and “malfunction that would be likely to cause or contribute to a death or serious injury if the malfunction were to recur.” The reports are limited to events Bayer becomes aware of between November 2016 and November 2020. Bayer will continue to provide these reports until April 2021.
The FDA emphasized that the collected data are based on social media reports and already may be reported to the FDA, rather than being a collection of new events. “The limited information provided in the reports prevents the ability to draw any conclusions as to whether the device, or its removal, caused or contributed to any of the events in the reports,” Benjamin Fisher, PhD, director of the Reproductive, Gastro-Renal, Urological, General Hospital Device and Human Factors Office in the Center for Devices and Radiological Health, said in an FDA In Brief statement on Aug. 11.
The FDA first uploaded an Essure MDR variance spreadsheet in August 2020, listing 1,453 events, consisting of 53 reports of deaths, 1,376 reports of serious injury, and 24 reports of device malfunction that occurred as of June 2020. In September 2020, FDA uploaded a second variance spreadsheet, which added another 1,934 events that occurred as of July.
Interim analysis of postmarketing surveillance study
An interim analysis of 1,128 patients from 67 centers in the Essure postmarket surveillance study, which compared women who received Essure with those who received laparoscopic tubal sterilization, revealed that 94.6% (265 of 280 patients) in the Essure group had a successful implantation of the device, compared with 99.6% of women who achieved bilateral tubal occlusion from laparoscopic tubal sterilization.
Regarding safety, 9.1% of women in the Essure group and 4.5% in the laparoscopic tubal sterilization group reported chronic lower abdominal and/or pelvic pain, and 16.3% in the Essure group and 10.2% in the laparoscopic tubal sterilization group reported new or worsening abnormal uterine bleeding. In the Essure group, 22.3% of women said they experienced hypersensitivity, an allergic reaction, and new “autoimmune-like reactions” compared with 12.5% of women in the laparoscopic tubal sterilization group.
The interim analysis also showed 19.7% of women in the Essure group and 3.0% in the laparoscopic tubal sterilization group underwent gynecologic surgical procedures, which were “driven primarily by Essure removal and endometrial ablation procedures in Essure patients.” Device removal occurred in 6.8% of women with the Essure device.
Consistent data on Essure
An FDA search of the Manufacturer and User Facility Device Experience (MAUDE) database in January of 2020 revealed 47,856 medical device reports of Essure between November 2002 and December 2019. The most common adverse events observed during this period were:
- Pain or abdominal pain (32,901 cases).
- Heavy or irregular menses (14,573 cases). Headache (8,570 cases).
- Device fragment or foreign body in a patient (8,501 cases).
- Perforation (7,825 cases).
- Fatigue (7,083 cases).
- Gain or loss in weight (5,980 cases).
- Anxiety and/or depression (5,366 cases).
- Rash and/or hypersensitivity (5,077 cases)
- Hair loss (4,999 cases).
Problems with the device itself included reports of:
- Device incompatibility such as an allergy (7,515 cases).
- The device migrating (4,535 cases).
- The device breaking or fracturing (2,297 cases).
- The device dislodging or dislocating (1,797 cases).
- Improper operation including implant failure and pregnancy (1,058 cases).
In 2019, Essure received 15,083 medical device reports, an increase from 6,000 reports in 2018 and 11,854 reports in 2017.
To date, nearly 39,000 women in the United States have made claims to injuries related to the Essure device. In August, Bayer announced it would pay approximately $1.6 billion U.S. dollars to settle 90% of these cases in exchange for claimants to “dismiss their cases or not file.” Bayer also said in a press release that the settlement is not an admission of wrongdoing or liability on the part of the company.
In an interview, Catherine Cansino, MD, MPH, of the department of obstetrics and gynecology at the University of California, Davis, said the latest adverse event reports show “consistent info from [the] MAUDE database when comparing 2019 to previous years, highlighting most common problems related to pain and heavy or irregular bleeding.”
She emphasized ob.gyns with patients who have an Essure device should “consider Essure-related etiology that may necessitate device removal when evaluating patients with gynecological problems, especially with regard to abdominal/pelvic pain and heavy/irregular bleeding.”
Dr. Cansino reported no relevant financial disclosures. She is a member of the Ob.Gyn. News Editorial Advisory Board.
Children’s opioid harms vary by race, location
or dependence, compared with their White or rural/suburban counterparts, according to a study of 3.2 million Medicaid-enrolled children in North Carolina.
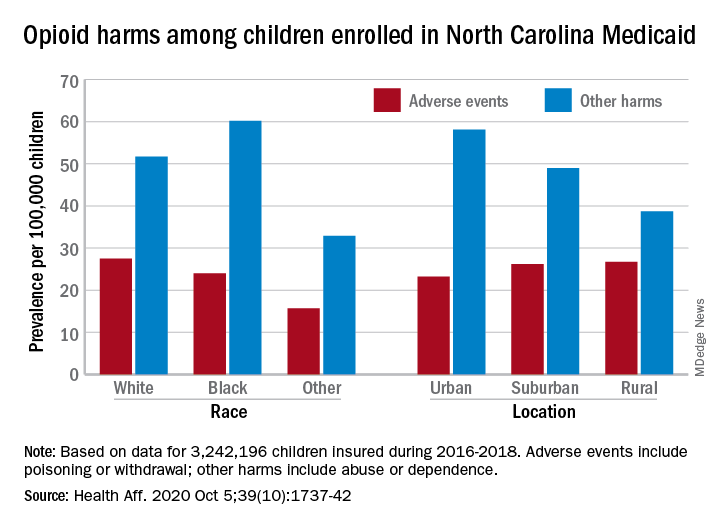
Analysis of the almost 138,000 prescription fills also showed that Black and urban children in North Carolina were less likely to fill a opioid prescription, suggesting a need “for future studies to explore racial and geographic opioid-related inequities in children,” Kelby W. Brown, MA, and associates at Duke University, Durham, N.C., said Oct. 5 in Health Affairs.
In 2016-2018, the prevalence of opioid-related adverse events, such as poisoning or withdrawal, was 24.0 per 100,000 children among Blacks aged 1-17 years, compared with 27.5 per 100,000 for whites. For other opioid-related harms such as abuse or dependence, the order was reversed: 60.2 for Blacks and 51.7 for Whites, the investigators reported. Children of all other races were lowest in both measures.
Geography also appears to play a part. The children in urban areas had the lowest rate of adverse events – 23.2 per 100,000 vs. 26.2 (suburban) and 26.7 (rural) – and the highest rate of other opioid-related harms – 58.1 vs. 49.0 (suburban) and 38.7 (rural), the Medicaid claims data showed.
Analysis of prescription fills revealed that black children aged 1-17 years had a significantly lower rate (2.7%) than Whites (3.1%) or those of other races (3.0%) and that urban children were significantly less likely to fill a prescription (2.7%) for opioids than the other two groups (suburban, 3.1%; rural, 3.4%), Mr. Brown and associates said.
The prescription data also showed that 48.4% of children aged 6-17 years who had an adverse event had filled a prescription for an opioid in the previous 6 months, compared with just 9.4% of those with other opioid-related harms. The median length of time since the last fill? Three days for children with an adverse event and 67 days for those with other harms, they said.
And those prescriptions, it turns out, were not coming just from the physicians of North Carolina. Physicians, with 35.5% of the prescription load, were the main source, but 33.3% of opioid fills in 2016-2018 came from dentists, and another 17.7% were written by advanced practice providers. Among physicians, the leading opioid-prescribing specialists were surgeons, with 17.3% of the total, the investigators reported.
“The distinct and separate groups of clinicians who prescribe opioids to children suggest the need for pediatric opioid prescribing guidelines, particularly for postprocedural pain,” Mr. Brown and associates wrote.
SOURCE: Brown KW et al. Health Aff. 2020;39(10):1737-42.
or dependence, compared with their White or rural/suburban counterparts, according to a study of 3.2 million Medicaid-enrolled children in North Carolina.

Analysis of the almost 138,000 prescription fills also showed that Black and urban children in North Carolina were less likely to fill a opioid prescription, suggesting a need “for future studies to explore racial and geographic opioid-related inequities in children,” Kelby W. Brown, MA, and associates at Duke University, Durham, N.C., said Oct. 5 in Health Affairs.
In 2016-2018, the prevalence of opioid-related adverse events, such as poisoning or withdrawal, was 24.0 per 100,000 children among Blacks aged 1-17 years, compared with 27.5 per 100,000 for whites. For other opioid-related harms such as abuse or dependence, the order was reversed: 60.2 for Blacks and 51.7 for Whites, the investigators reported. Children of all other races were lowest in both measures.
Geography also appears to play a part. The children in urban areas had the lowest rate of adverse events – 23.2 per 100,000 vs. 26.2 (suburban) and 26.7 (rural) – and the highest rate of other opioid-related harms – 58.1 vs. 49.0 (suburban) and 38.7 (rural), the Medicaid claims data showed.
Analysis of prescription fills revealed that black children aged 1-17 years had a significantly lower rate (2.7%) than Whites (3.1%) or those of other races (3.0%) and that urban children were significantly less likely to fill a prescription (2.7%) for opioids than the other two groups (suburban, 3.1%; rural, 3.4%), Mr. Brown and associates said.
The prescription data also showed that 48.4% of children aged 6-17 years who had an adverse event had filled a prescription for an opioid in the previous 6 months, compared with just 9.4% of those with other opioid-related harms. The median length of time since the last fill? Three days for children with an adverse event and 67 days for those with other harms, they said.
And those prescriptions, it turns out, were not coming just from the physicians of North Carolina. Physicians, with 35.5% of the prescription load, were the main source, but 33.3% of opioid fills in 2016-2018 came from dentists, and another 17.7% were written by advanced practice providers. Among physicians, the leading opioid-prescribing specialists were surgeons, with 17.3% of the total, the investigators reported.
“The distinct and separate groups of clinicians who prescribe opioids to children suggest the need for pediatric opioid prescribing guidelines, particularly for postprocedural pain,” Mr. Brown and associates wrote.
SOURCE: Brown KW et al. Health Aff. 2020;39(10):1737-42.
or dependence, compared with their White or rural/suburban counterparts, according to a study of 3.2 million Medicaid-enrolled children in North Carolina.

Analysis of the almost 138,000 prescription fills also showed that Black and urban children in North Carolina were less likely to fill a opioid prescription, suggesting a need “for future studies to explore racial and geographic opioid-related inequities in children,” Kelby W. Brown, MA, and associates at Duke University, Durham, N.C., said Oct. 5 in Health Affairs.
In 2016-2018, the prevalence of opioid-related adverse events, such as poisoning or withdrawal, was 24.0 per 100,000 children among Blacks aged 1-17 years, compared with 27.5 per 100,000 for whites. For other opioid-related harms such as abuse or dependence, the order was reversed: 60.2 for Blacks and 51.7 for Whites, the investigators reported. Children of all other races were lowest in both measures.
Geography also appears to play a part. The children in urban areas had the lowest rate of adverse events – 23.2 per 100,000 vs. 26.2 (suburban) and 26.7 (rural) – and the highest rate of other opioid-related harms – 58.1 vs. 49.0 (suburban) and 38.7 (rural), the Medicaid claims data showed.
Analysis of prescription fills revealed that black children aged 1-17 years had a significantly lower rate (2.7%) than Whites (3.1%) or those of other races (3.0%) and that urban children were significantly less likely to fill a prescription (2.7%) for opioids than the other two groups (suburban, 3.1%; rural, 3.4%), Mr. Brown and associates said.
The prescription data also showed that 48.4% of children aged 6-17 years who had an adverse event had filled a prescription for an opioid in the previous 6 months, compared with just 9.4% of those with other opioid-related harms. The median length of time since the last fill? Three days for children with an adverse event and 67 days for those with other harms, they said.
And those prescriptions, it turns out, were not coming just from the physicians of North Carolina. Physicians, with 35.5% of the prescription load, were the main source, but 33.3% of opioid fills in 2016-2018 came from dentists, and another 17.7% were written by advanced practice providers. Among physicians, the leading opioid-prescribing specialists were surgeons, with 17.3% of the total, the investigators reported.
“The distinct and separate groups of clinicians who prescribe opioids to children suggest the need for pediatric opioid prescribing guidelines, particularly for postprocedural pain,” Mr. Brown and associates wrote.
SOURCE: Brown KW et al. Health Aff. 2020;39(10):1737-42.
FROM HEALTH AFFAIRS
Music’s charms may soothe heart failure’s effects
Music listening and singing each showed early, promising evidence for producing cardiovascular benefits, part of a burgeoning area of research that is exploring and documenting ways to effectively use music to improve health.
A study run at four centers in Italy randomized 159 patients with heart failure, primarily New York Heart Association class I or II disease, to either a daily regimen of at least 30 minutes spent listening to music daily or to a control group that received usual care with no music prescription. After 3 months, the 82 patients in the daily music-listening group had a statistically significant improvement in their Minnesota Living with Heart Failure Questionnaire scores, compared with 77 controls for the study’s primary outcome measure. The results also showed significant benefits, compared with placebo, for other, secondary efficacy measures including improvements in anxiety, depression, sleep quality, and cognition.
Although the results are considered preliminary, they drew significant attention when published in July 2020 (J Card Fail. 2020 Jul 1;26[7]:541-9), where it was accompanied by two editorials in the same issue as well as an editor’s statement. All these commentators as well as other experts interested in music as medicine gathered to further discuss the topic during a panel session at the virtual annual meeting of the Heart Failure Society of America.
Music as a calming influence
The source of the primary benefits seen in this Italian study likely involved “emotional, psychological, and relaxation,” suggested Jerome L. Fleg, MD, program officer for clinical cardiovascular disease at the National Heart, Lung, and Blood Institute in Bethesda, Md. Researchers had used calming potential as a major criterion when selecting the 80 classical pieces that the heart failure patients in the intervention arm of the study could shuffle on their play lists.
“The tempo/rhythm was set up in a range between 60 and 80 beats per minute, because this range mirrors the human heart rate and facilitates relaxation,” the investigators said in their published report. Unfortunately, noted Dr. Fleg, the study lacked physiologic and biomarker measurements that could have provided objective evidence of effects from music. And the study failed to include a control arm of patients instructed to spend 30 minutes a day resting and relaxing without instruction to listen to music, he noted.
Dr. Fleg had authored one of the July editorials, where he said “It is hoped that findings from these studies and others can expand the scientific evidence for music-based interventions and bring these therapies into clinical practice. The current study from Burrai et al. is a positive step in this direction for patients with heart failure.” (J Card Fail. 2020 Jul 1;26[7]: 550-1). What’s needed now, he added during the virtual session, are “more objective data” to better and more comprehensively document the benefits from a music-based intervention in patients with heart failure.
An add-on to standard care
The findings in heart failure patients follows a growing literature that’s shown music can generate a restful state by doing things like activating autonomic parasympathetic outflow while dampening sympathetic outflow. This produces moderation in mood and emotion as well as depressed heart rate, lowered blood pressure, and slowed respiration, commented Emmeline Edwards, PhD director of the division of extramural research of the National Center for Complementary and Integrative Health in Bethesda, Md. Music also seems able to stimulate higher-order brain regions that can result in reduced psychological stress, anxiety, and depression.
“It’s a promising protective intervention to add to standard care for cardiac patients,” Dr. Edwards said during the virtual session. “Music is part of the toolbox for managing symptoms and improving health and well-being.”
“Music is not a substitute for standard therapy, but could add to it,” declared Dr. Fleg.
The already-established intervention known as music therapy has identified music’s ability to modulate breathing as an important mediator of music’s effect.
“Breathing is one of the few physiological processes that can be voluntarily controlled making it a viable target for intervention,” noted opera soprano Renée Fleming and Sheri L. Robb, PhD, in the second editorial that accompanied the Italian heart failure report (J Card Fail. 2020 Jul 1;26[7]:552-4). The music-listening intervention “may have had more effect if they had used compositional features [of the music] to teach patients how to structure their breathing,” said Dr. Robb, a music therapist at Indiana University–Purdue University Indianapolis, during the virtual session.
Another variable to consider is the type of music. “What is the emotional response to the music, and how does that affect heart rate,” wondered Dr. Robb, a professor at the Indiana University School of Nursing in Indianapolis.
Music as exercise
The division that Dr. Edwards directs recently funded a pilot study that assessed the feasibility of using music to stimulate activity and improve breathing another way, by repurposing singing as a novel form of rehabilitative exercise.
The pilot study enrolled patients with coronary disease into a randomized study that tested whether a 14-minute session of supervised singing could produce acute improvement in vascular function, “a biomarker for the risk of future cardiovascular disease events,” explained Jacqueline P. Kulinski, MD, a preventive cardiologist at the Medical College of Wisconsin in Milwaukee. Dr. Kulinski did not report details of her yet-unpublished study, but said that her initial findings held promise for developing musical activities such as singing as a novel way to stimulate therapeutic physical activity in patients with heart disease.
“It’s exciting to see this signal” of benefit. “I envision music therapy as a part of cardiac rehabilitation, or an alternative for patients who can’t participate in traditional rehab,” Dr. Kulinski said during the virtual session. “I think of singing as a physical activity, as exercise, and using this exercise as medicine.”
Harmonizing with the NIH
“Singing is like swimming: You need to hold your breath,” agreed Ms. Fleming, who participated on the virtual panel and has spearheaded a collaboration between the National Institutes of Health and the Kennedy Center for the Performing Arts, the Sound Health Initiative, that’s coordinating research into the connections between music and health. Ms. Fleming helped launch the Sound Health Initiative in 2017 by coauthoring a JAMA article with the NIH director that spelled out the rationale and goals of the project (JAMA. 2017 Jun 27;317[24]:2470-1), and by launching a lecture tour on the topic in a presentation she calls Music and the Mind.
Ms. Fleming has given her talk in more than 30 locations worldwide, and she’s found that “audiences love” the combination of neuroscience and music that her talks cover, she said. Her lectures highlight that, in addition to cardiovascular disease, the potential for music therapy and related interventions has been shown in patients with disorders that include autism, psychosis, pain, Parkinson’s disease, Alzheimer’s disease, and epilepsy.
The research highlighted in the session “opens new doors to prevention and treatment strategies using music for patients with heart failure and cardiovascular disease,” summed up Biykem Bozkurt, MD, professor of medicine at the Baylor College of Medicine in Houston and president of the Heart Failure Society of America, who helped organize the virtual session.
Dr. Fleg, Dr. Edwards, Dr. Robb, Dr Kulinski, Ms. Fleming, and Dr. Bozkurt had no relevant financial disclosures.
Music listening and singing each showed early, promising evidence for producing cardiovascular benefits, part of a burgeoning area of research that is exploring and documenting ways to effectively use music to improve health.
A study run at four centers in Italy randomized 159 patients with heart failure, primarily New York Heart Association class I or II disease, to either a daily regimen of at least 30 minutes spent listening to music daily or to a control group that received usual care with no music prescription. After 3 months, the 82 patients in the daily music-listening group had a statistically significant improvement in their Minnesota Living with Heart Failure Questionnaire scores, compared with 77 controls for the study’s primary outcome measure. The results also showed significant benefits, compared with placebo, for other, secondary efficacy measures including improvements in anxiety, depression, sleep quality, and cognition.
Although the results are considered preliminary, they drew significant attention when published in July 2020 (J Card Fail. 2020 Jul 1;26[7]:541-9), where it was accompanied by two editorials in the same issue as well as an editor’s statement. All these commentators as well as other experts interested in music as medicine gathered to further discuss the topic during a panel session at the virtual annual meeting of the Heart Failure Society of America.
Music as a calming influence
The source of the primary benefits seen in this Italian study likely involved “emotional, psychological, and relaxation,” suggested Jerome L. Fleg, MD, program officer for clinical cardiovascular disease at the National Heart, Lung, and Blood Institute in Bethesda, Md. Researchers had used calming potential as a major criterion when selecting the 80 classical pieces that the heart failure patients in the intervention arm of the study could shuffle on their play lists.
“The tempo/rhythm was set up in a range between 60 and 80 beats per minute, because this range mirrors the human heart rate and facilitates relaxation,” the investigators said in their published report. Unfortunately, noted Dr. Fleg, the study lacked physiologic and biomarker measurements that could have provided objective evidence of effects from music. And the study failed to include a control arm of patients instructed to spend 30 minutes a day resting and relaxing without instruction to listen to music, he noted.
Dr. Fleg had authored one of the July editorials, where he said “It is hoped that findings from these studies and others can expand the scientific evidence for music-based interventions and bring these therapies into clinical practice. The current study from Burrai et al. is a positive step in this direction for patients with heart failure.” (J Card Fail. 2020 Jul 1;26[7]: 550-1). What’s needed now, he added during the virtual session, are “more objective data” to better and more comprehensively document the benefits from a music-based intervention in patients with heart failure.
An add-on to standard care
The findings in heart failure patients follows a growing literature that’s shown music can generate a restful state by doing things like activating autonomic parasympathetic outflow while dampening sympathetic outflow. This produces moderation in mood and emotion as well as depressed heart rate, lowered blood pressure, and slowed respiration, commented Emmeline Edwards, PhD director of the division of extramural research of the National Center for Complementary and Integrative Health in Bethesda, Md. Music also seems able to stimulate higher-order brain regions that can result in reduced psychological stress, anxiety, and depression.
“It’s a promising protective intervention to add to standard care for cardiac patients,” Dr. Edwards said during the virtual session. “Music is part of the toolbox for managing symptoms and improving health and well-being.”
“Music is not a substitute for standard therapy, but could add to it,” declared Dr. Fleg.
The already-established intervention known as music therapy has identified music’s ability to modulate breathing as an important mediator of music’s effect.
“Breathing is one of the few physiological processes that can be voluntarily controlled making it a viable target for intervention,” noted opera soprano Renée Fleming and Sheri L. Robb, PhD, in the second editorial that accompanied the Italian heart failure report (J Card Fail. 2020 Jul 1;26[7]:552-4). The music-listening intervention “may have had more effect if they had used compositional features [of the music] to teach patients how to structure their breathing,” said Dr. Robb, a music therapist at Indiana University–Purdue University Indianapolis, during the virtual session.
Another variable to consider is the type of music. “What is the emotional response to the music, and how does that affect heart rate,” wondered Dr. Robb, a professor at the Indiana University School of Nursing in Indianapolis.
Music as exercise
The division that Dr. Edwards directs recently funded a pilot study that assessed the feasibility of using music to stimulate activity and improve breathing another way, by repurposing singing as a novel form of rehabilitative exercise.
The pilot study enrolled patients with coronary disease into a randomized study that tested whether a 14-minute session of supervised singing could produce acute improvement in vascular function, “a biomarker for the risk of future cardiovascular disease events,” explained Jacqueline P. Kulinski, MD, a preventive cardiologist at the Medical College of Wisconsin in Milwaukee. Dr. Kulinski did not report details of her yet-unpublished study, but said that her initial findings held promise for developing musical activities such as singing as a novel way to stimulate therapeutic physical activity in patients with heart disease.
“It’s exciting to see this signal” of benefit. “I envision music therapy as a part of cardiac rehabilitation, or an alternative for patients who can’t participate in traditional rehab,” Dr. Kulinski said during the virtual session. “I think of singing as a physical activity, as exercise, and using this exercise as medicine.”
Harmonizing with the NIH
“Singing is like swimming: You need to hold your breath,” agreed Ms. Fleming, who participated on the virtual panel and has spearheaded a collaboration between the National Institutes of Health and the Kennedy Center for the Performing Arts, the Sound Health Initiative, that’s coordinating research into the connections between music and health. Ms. Fleming helped launch the Sound Health Initiative in 2017 by coauthoring a JAMA article with the NIH director that spelled out the rationale and goals of the project (JAMA. 2017 Jun 27;317[24]:2470-1), and by launching a lecture tour on the topic in a presentation she calls Music and the Mind.
Ms. Fleming has given her talk in more than 30 locations worldwide, and she’s found that “audiences love” the combination of neuroscience and music that her talks cover, she said. Her lectures highlight that, in addition to cardiovascular disease, the potential for music therapy and related interventions has been shown in patients with disorders that include autism, psychosis, pain, Parkinson’s disease, Alzheimer’s disease, and epilepsy.
The research highlighted in the session “opens new doors to prevention and treatment strategies using music for patients with heart failure and cardiovascular disease,” summed up Biykem Bozkurt, MD, professor of medicine at the Baylor College of Medicine in Houston and president of the Heart Failure Society of America, who helped organize the virtual session.
Dr. Fleg, Dr. Edwards, Dr. Robb, Dr Kulinski, Ms. Fleming, and Dr. Bozkurt had no relevant financial disclosures.
Music listening and singing each showed early, promising evidence for producing cardiovascular benefits, part of a burgeoning area of research that is exploring and documenting ways to effectively use music to improve health.
A study run at four centers in Italy randomized 159 patients with heart failure, primarily New York Heart Association class I or II disease, to either a daily regimen of at least 30 minutes spent listening to music daily or to a control group that received usual care with no music prescription. After 3 months, the 82 patients in the daily music-listening group had a statistically significant improvement in their Minnesota Living with Heart Failure Questionnaire scores, compared with 77 controls for the study’s primary outcome measure. The results also showed significant benefits, compared with placebo, for other, secondary efficacy measures including improvements in anxiety, depression, sleep quality, and cognition.
Although the results are considered preliminary, they drew significant attention when published in July 2020 (J Card Fail. 2020 Jul 1;26[7]:541-9), where it was accompanied by two editorials in the same issue as well as an editor’s statement. All these commentators as well as other experts interested in music as medicine gathered to further discuss the topic during a panel session at the virtual annual meeting of the Heart Failure Society of America.
Music as a calming influence
The source of the primary benefits seen in this Italian study likely involved “emotional, psychological, and relaxation,” suggested Jerome L. Fleg, MD, program officer for clinical cardiovascular disease at the National Heart, Lung, and Blood Institute in Bethesda, Md. Researchers had used calming potential as a major criterion when selecting the 80 classical pieces that the heart failure patients in the intervention arm of the study could shuffle on their play lists.
“The tempo/rhythm was set up in a range between 60 and 80 beats per minute, because this range mirrors the human heart rate and facilitates relaxation,” the investigators said in their published report. Unfortunately, noted Dr. Fleg, the study lacked physiologic and biomarker measurements that could have provided objective evidence of effects from music. And the study failed to include a control arm of patients instructed to spend 30 minutes a day resting and relaxing without instruction to listen to music, he noted.
Dr. Fleg had authored one of the July editorials, where he said “It is hoped that findings from these studies and others can expand the scientific evidence for music-based interventions and bring these therapies into clinical practice. The current study from Burrai et al. is a positive step in this direction for patients with heart failure.” (J Card Fail. 2020 Jul 1;26[7]: 550-1). What’s needed now, he added during the virtual session, are “more objective data” to better and more comprehensively document the benefits from a music-based intervention in patients with heart failure.
An add-on to standard care
The findings in heart failure patients follows a growing literature that’s shown music can generate a restful state by doing things like activating autonomic parasympathetic outflow while dampening sympathetic outflow. This produces moderation in mood and emotion as well as depressed heart rate, lowered blood pressure, and slowed respiration, commented Emmeline Edwards, PhD director of the division of extramural research of the National Center for Complementary and Integrative Health in Bethesda, Md. Music also seems able to stimulate higher-order brain regions that can result in reduced psychological stress, anxiety, and depression.
“It’s a promising protective intervention to add to standard care for cardiac patients,” Dr. Edwards said during the virtual session. “Music is part of the toolbox for managing symptoms and improving health and well-being.”
“Music is not a substitute for standard therapy, but could add to it,” declared Dr. Fleg.
The already-established intervention known as music therapy has identified music’s ability to modulate breathing as an important mediator of music’s effect.
“Breathing is one of the few physiological processes that can be voluntarily controlled making it a viable target for intervention,” noted opera soprano Renée Fleming and Sheri L. Robb, PhD, in the second editorial that accompanied the Italian heart failure report (J Card Fail. 2020 Jul 1;26[7]:552-4). The music-listening intervention “may have had more effect if they had used compositional features [of the music] to teach patients how to structure their breathing,” said Dr. Robb, a music therapist at Indiana University–Purdue University Indianapolis, during the virtual session.
Another variable to consider is the type of music. “What is the emotional response to the music, and how does that affect heart rate,” wondered Dr. Robb, a professor at the Indiana University School of Nursing in Indianapolis.
Music as exercise
The division that Dr. Edwards directs recently funded a pilot study that assessed the feasibility of using music to stimulate activity and improve breathing another way, by repurposing singing as a novel form of rehabilitative exercise.
The pilot study enrolled patients with coronary disease into a randomized study that tested whether a 14-minute session of supervised singing could produce acute improvement in vascular function, “a biomarker for the risk of future cardiovascular disease events,” explained Jacqueline P. Kulinski, MD, a preventive cardiologist at the Medical College of Wisconsin in Milwaukee. Dr. Kulinski did not report details of her yet-unpublished study, but said that her initial findings held promise for developing musical activities such as singing as a novel way to stimulate therapeutic physical activity in patients with heart disease.
“It’s exciting to see this signal” of benefit. “I envision music therapy as a part of cardiac rehabilitation, or an alternative for patients who can’t participate in traditional rehab,” Dr. Kulinski said during the virtual session. “I think of singing as a physical activity, as exercise, and using this exercise as medicine.”
Harmonizing with the NIH
“Singing is like swimming: You need to hold your breath,” agreed Ms. Fleming, who participated on the virtual panel and has spearheaded a collaboration between the National Institutes of Health and the Kennedy Center for the Performing Arts, the Sound Health Initiative, that’s coordinating research into the connections between music and health. Ms. Fleming helped launch the Sound Health Initiative in 2017 by coauthoring a JAMA article with the NIH director that spelled out the rationale and goals of the project (JAMA. 2017 Jun 27;317[24]:2470-1), and by launching a lecture tour on the topic in a presentation she calls Music and the Mind.
Ms. Fleming has given her talk in more than 30 locations worldwide, and she’s found that “audiences love” the combination of neuroscience and music that her talks cover, she said. Her lectures highlight that, in addition to cardiovascular disease, the potential for music therapy and related interventions has been shown in patients with disorders that include autism, psychosis, pain, Parkinson’s disease, Alzheimer’s disease, and epilepsy.
The research highlighted in the session “opens new doors to prevention and treatment strategies using music for patients with heart failure and cardiovascular disease,” summed up Biykem Bozkurt, MD, professor of medicine at the Baylor College of Medicine in Houston and president of the Heart Failure Society of America, who helped organize the virtual session.
Dr. Fleg, Dr. Edwards, Dr. Robb, Dr Kulinski, Ms. Fleming, and Dr. Bozkurt had no relevant financial disclosures.











