User login
Retrospective Review on the Safety and Efficacy of Direct Oral Anticoagulants Compared With Warfarin in Patients With Cirrhosis
Coagulation in patients with cirrhosis is a complicated area of evolving research. Patients with cirrhosis were originally thought to be naturally anticoagulated due to the decreased production of clotting factors and platelets, combined with an increased international normalized ratio (INR).1 New data have shown that patients with cirrhosis are at a concomitant risk of bleeding and thrombosis due to increased platelet aggregation, decreased fibrinolysis, and decreased production of natural anticoagulants such as protein C and antithrombin.1 Traditionally, patients with cirrhosis needing anticoagulation therapy for comorbid conditions, such as nonvalvular atrial fibrillation (NVAF) or venous thromboembolism (VTE) were placed on warfarin therapy. Managing warfarin in patients with cirrhosis poses a challenge to clinicians due to the many food and drug interactions, narrow therapeutic index, and complications with maintaining a therapeutic INR.1
Direct oral anticoagulants (DOACs) have several benefits over warfarin therapy, including convenience, decreased monitoring, decreased drug and dietary restrictions, and faster onset of action.2 Conversely, DOACs undergo extensive hepatic metabolism giving rise to concerns about supratherapeutic drug levels and increased bleeding rates in patients with liver dysfunction.1 Consequently, patients with cirrhosis were excluded from the pivotal trials establishing DOACs for NVAF and VTE treatment. Exclusion of these patients in major clinical trials alongside the challenges of managing warfarin warrant an evaluation of the efficacy and safety of DOACs in patients with cirrhosis.
Recent retrospective studies have examined the use of DOACs in patients with cirrhosis and found favorable results. A retrospective chart review by Intagliata and colleagues consisting of 39 patients with cirrhosis using either a DOAC or warfarin found similar rates of all-cause bleeding and major bleeding between the 2 groups.3 A retrospective cohort study by Hum and colleagues consisting of 45 patients with cirrhosis compared the use of DOACs with warfarin or low-molecular weight heparin (LMWH).4 Hum and colleagues found patients prescribed a DOAC had significantly fewer major bleeding events than did patients using warfarin or LMWH.4 The largest retrospective cohort study consisted of 233 patients with chronic liver disease and found no differences among all-cause bleeding and major bleeding rates between patients using DOACs compared with those of patients using warfarin.5
The purpose of this research is to evaluate the safety and efficacy of DOACs in veteran patients with cirrhosis compared with patients using warfarin.
Methods
A retrospective single-center chart review was conducted at the Michael E. DeBakey Veterans Affairs Medical Center (MEDVAMC) in Houston, Texas, between October 31, 2014 and October 31, 2018. Patients included in the study were adults aged ≥ 18 years with a diagnosis of cirrhosis and prescribed any of the following oral anticoagulants: apixaban, dabigatran, edoxaban, rivaroxaban, or warfarin. Patients prescribed apixaban, dabigatran, edoxaban, or rivaroxaban were collectively grouped into the DOAC group, while patients prescribed warfarin were classified as the standard of care comparator group.
A diagnosis of cirrhosis was confirmed using a combination of the codes from the ninth and tenth editions of the International Classification of Diseases (ICD) for cirrhosis, documentation of diagnostic confirmation by clinicians from the gastroenterology or hepatology services, and positive liver biopsy result. Liver function tests, liver ultrasound results, and FibroSure biomarker assays were used to aid in confirming the diagnosis of cirrhosis but were not considered definitive. Patients were excluded from the trial if they had indications for anticoagulation other than NVAF and VTE and/or were prescribed triple antithrombotic therapy (dual antiplatelet therapy plus an anticoagulant). Patients who switched anticoagulant therapy during the trial period (ie, switched from warfarin to a DOAC) were also excluded from the analysis.
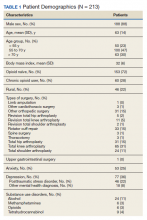
Patient demographic characteristics that were collected included weight; body mass index (BMI); etiology of cirrhosis; Child-Turcotte-Pugh, Model for End-Stage Liver Disease (MELD), and CHA2DS2-VASc score; concomitant antiplatelet, nonsteroidal anti-inflammatory drug (NSAID), proton pump inhibitor (PPI), and histamine-2 receptor antagonist
Two patient lists were used to identify patients for inclusion in the warfarin arm. The first patient list was generated using the US Department of Veterans Affairs (VA) Cirrhosis Tracker, which identified patients with an ICD-9/10 code for cirrhosis and an INR laboratory value. Patients generated from the VA Cirrhosis Tracker with an INR > 1.5 were screened for a warfarin prescription and then evaluated for full study inclusion. The second patient list was generated using the VA Advanced Liver Disease Dashboard which identified patients with ICD-9/10 codes for advanced liver disease and an active warfarin prescription. Patients with an active warfarin prescription were then evaluated for full study inclusion. A single patient list was generated to identify patients for inclusion in the DOAC arm. This patient list was generated using the VA DOAC dashboard, which identified patients with an active DOAC prescription and an ICD-9/10 code for cirrhosis. Patients with an ICD-9/10 code for cirrhosis and prescribed a DOAC were screened for full study inclusion. Patient data were collected from the MEDVAMC Computerized Patient Record System (CPRS) electronic health record (EHR). The research study was approved by the Baylor College of Medicine Institutional Review Board and the VA Office of Research and Development.
Outcomes
The primary endpoint for the study was all-cause bleeding. The secondary endpoints for the study were major bleeding and failed efficacy. Major bleeding was defined using the International Society on Thrombosis and Haemostasis (ISTH) 2005 definition: fatal bleeding, symptomatic bleeding in a critical organ area (ie, intracranial, intraspinal, intraocular, retroperitoneal, intraarticular, pericardial, or intramuscular with compartment syndrome), or bleeding causing a fall in hemoglobin level of > 2 g/dL or leading to the transfusion of ≥ 2 units of red cells.6 Failed efficacy was a combination endpoint that included development of VTE, stroke, myocardial infarction (MI), and/or death. A prespecified subgroup analysis was conducted at the end of the study period to analyze trends in the DOAC and warfarin groups with respect to all-cause bleeding. All-cause bleeding risk was stratified by weight, BMI, Child-Turcotte-Pugh score, MELD score, presence of gastric and/or esophageal varices, active malignancies, percentage of time within therapeutic INR range in the warfarin group, indications for anticoagulation, and antiplatelet, NSAID, PPI, and H2RA therapy.
Statistical Analysis
Data were analyzed using descriptive and inferential statistics. Continuous data were analyzed using the Student t test, and categorical data were analyzed using the Fisher exact test. Previous studies determined an all-cause bleeding rate of 10 to 17% for warfarin compared with 5% for DOACs.7,8 To detect a 12% difference in the all-cause bleeding rate between DOACs and warfarin, 212 patients would be needed to achieve 80% power at an α level of 0.05.
Results
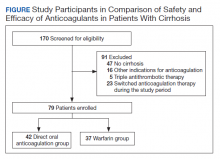
A total of 170 patients were screened, and after applying inclusion and exclusion criteria, 79 patients were enrolled in the study (Figure). The DOAC group included 42 patients, and the warfarin group included 37 patients. In the DOAC group, 69.1% (n = 29) of patients were taking apixaban, 21.4% (n = 9) rivaroxaban, and 9.5% (n = 4) dabigatran. There were no patients prescribed edoxaban during the study period.
Baseline characteristics were similar between the 2 groups except for Child-Turcotte-Pugh score, MELD score, mean INR, and number of days on anticoagulation therapy (Table 1). Most of the patients were male (98.7%), and the mean age was 71 years. The most common causes of cirrhosis were viral (29.1%), nonalcoholic fatty liver disease (NAFLD) (24.1%), multiple causes (22.8%), and alcohol (21.5%). Sixty-two patients (78.5%) had a NVAF indication for anticoagulation. The average CHA2DS2-VASc score was 3.7. Aspirin was prescribed in 51.9% (n = 41) of patients, and PPIs were prescribed in 48.1% (n = 38) of patients. At inclusion, esophageal varices were present in 13 patients and active malignancies were present in 6 patients.
Statistically significant differences in baseline characteristics were found between mean INR, Child-Turcotte-Pugh scores, MELD scores, and number of days on anticoagulant therapy. The mean INR was 1.3 in the DOAC group compared with 2.1 in the warfarin group (P = .0001). Eighty-one percent (n = 34) of patients in the DOAC group had a Child-Turcotte-Pugh score of A compared with 43.2% (n = 16) of patients in the warfarin group (P = .0009). Eight patients in the DOAC group had a Child-Turcotte-Pugh score of B compared with 19 patients in the warfarin group (P = .004). The mean MELD score was 9.4 in the DOAC group compared with 16.3 in the warfarin group (P = .0001). The mean days on anticoagulant therapy was 500.4 days for the DOAC group compared with 1,652.4 days for the warfarin group (P = .0001).
Safety Outcome
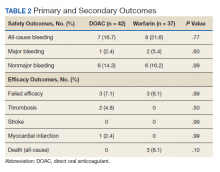
The primary outcome comparing all-cause bleeding rates between patients on DOACs compared with warfarin are listed in Table 2. With respect to the primary outcome, 7 (16.7%) patients on DOACs experienced a bleeding event compared with 8 (21.6%) patients on warfarin (P = .77). No statistically significant differences were detected between the DOAC and warfarin groups with respect to all-cause bleeding. Seven bleeding events occurred in the DOAC group; 1 met the qualification for major bleeding with a suspected gastrointestinal (GI) bleed.6 The other 6 bleeding episodes in the DOAC group consisted of hematoma, epistaxis, hematuria, and hematochezia. Eight bleeding events occurred in the warfarin group; 2 met the qualification for major bleeding with an intracranial hemorrhage and upper GI bleed.6 The other 6 bleeding episodes in the warfarin group consisted of epistaxis, bleeding gums, hematuria, and hematochezia. There were no statistically significant differences between the rates of major bleeding and nonmajor bleeding between the DOAC and warfarin groups.
Efficacy Outcomes
There were 3 events in the DOAC group and 3 events in the warfarin group (P = .99). In the DOAC group, 2 patients experienced a pulmonary embolism, and 1 patient experienced a MI. In the warfarin group, 3 patients died (end-stage heart failure, unknown cause due to death at an outside hospital, and sepsis/organ failure). There were no statistically significant differences between the composite endpoint of failed efficacy or the individual endpoints of VTE, stroke, MI, and death.
Subgroup Analysis
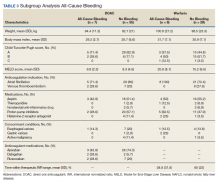
A prespecified subgroup analysis was conducted to determine risk factors for all-cause bleeding within each treatment group (Table 3). No significant trends were observed in the following risk factors: Child-Turcotte-Pugh score, indication for anticoagulation, use of NSAIDs, PPIs or H2RAs, presence of gastric or esophageal varices, active malignancies, and time within therapeutic INR range in the warfarin group. Patients with bleeding events had slightly increased weight and BMI vs patients without bleeding events. Within the warfarin group, patients with bleeding events had slightly elevated MELD scores compared to patients without bleeding events. There was an equal balance of patients prescribed aspirin therapy between the groups with and without bleeding events. Overall, no significant risk factors were identified for all-cause bleeding.
Discussion
Initially, patients with cirrhosis were excluded from DOAC trials due to concerns for increased bleeding risk with hepatically eliminated medications. New retrospective research has concluded that in patients with cirrhosis, DOACs have similar or lower bleeding rates when compared directly to warfarin.9,10
In this study, no statistically significant differences were detected between the primary and secondary outcomes of all-cause bleeding, major bleeding, or failed efficacy. Subgroup analysis did not identify any significant risk factors with respect to all-cause bleeding among patients in the DOAC and warfarin groups. To meet 80% power, 212 patients needed to be enrolled in the study; however, only 79 patients were enrolled, and power was not met. The results of this study should be interpreted cautiously as hypothesis-generating due to the small sample size. Strengths of this study include similar baseline characteristics between the DOAC and warfarin groups, 4-year length of retrospective data review, and availability of both inpatient and outpatient EHR limiting the amount of missing data points.
Baseline characteristics were similar between the groups except for mean INR, Child-Turcotte-Pugh score, MELD score, and number of days on anticoagulation therapy. The difference in mean INR between groups is expected as patients in the warfarin group have a goal INR of 2 to 3 to maintain therapeutic efficacy and safety. INR is not used as a marker of efficacy or safety with DOACs; therefore, a consistent elevation in INR is not expected. Child- Turcotte-Pugh scores are calculated using INR levels.11 When calculating the score, patients with an INR < 1.7 receive 1 point; patients with an INR between 1.7 and 2.3 receive 2 points.11 Therefore, patients in the warfarin group will have artificially inflated Child-Turcotte-Pugh scores as this group has goal INR levels of 2 to 3. This makes Child-Turcotte-Pugh scores unreliable markers of disease severity in patients using warfarin therapy. When the INR scores for patients prescribed warfarin were replaced with values < 1.7, the statistical difference disappeared between the warfarin and DOAC groups. The same effect is seen on MELD scores for patients prescribed warfarin therapy. The MELD score is calculated using INR levels.12 MELD scores also will be artificially elevated in patients prescribed warfarin therapy due to the INR elevation to between 2 and 3. When MELD scores for patients prescribed warfarin were replaced with values similar to those in the DOAC group, the statistical difference disappeared between the warfarin and DOAC groups.
The last statistically significant difference was found in number of days on anticoagulant therapy. This difference was expected as warfarin is the standard of care for anticoagulation treatment in patients with cirrhosis. The first DOAC, dabigatran, was not approved by the US Food and Drug Administration until 2010.13 DOACs have only recently been used in patients with cirrhosis accounting for the statistically significant difference in days on anticoagulation therapy between the warfarin and DOAC groups.
Limitations
The inability to meet power or evaluate adherence and appropriate renal dose adjustments for DOACs limited this study. This study was conducted at a single center in a predominantly male veteran population and therefore may not be generalizable to other populations. A majority of patients in the DOAC group were prescribed apixaban (69.1%), which may have affected the overall rate of major bleeding in the DOAC group. Pivotal trials of apixaban have shown a consistent decreased risk of major bleeding in patients with NVAF or VTE when compared with warfarin.14,15 Therefore, the results of this study may not be generalizable to all DOACs.
An inherent limitation of this study was the inability to collect data verifying adherence in the DOAC group. However, in the warfarin group, percentage of time within the therapeutic INR range of 2 to 3 was collected. While not a direct marker of adherence, this does allow for limited evaluation of therapeutic efficacy and safety within the warfarin group. Last, proper dosing of DOACs in patients with and without adequate renal function was not evaluated in this study.
Conclusions
The results of this study are consistent with other retrospective research and literature reviews. There were no statistically significant differences identified between the rates of all-cause bleeding, major bleeding, and failed efficacy between the DOAC and warfarin groups. DOACs may be a safe alternative to warfarin in patients with cirrhosis requiring anticoagulation for NVAF or VTE, but large randomized trials are required to confirm these results.
1. Qamar A, Vaduganathan M, Greenberger NJ, Giugliano RP. Oral anticoagulation in patients with liver disease. J Am Coll Cardiol. 2018;71(19):2162-2175. doi:10.1016/j.jacc.2018.03.023
2. Priyanka P, Kupec JT, Krafft M, Shah NA, Reynolds GJ. Newer oral anticoagulants in the treatment of acute portal vein thrombosis in patients with and without cirrhosis. Int J Hepatol. 2018;2018:8432781. Published 2018 Jun 5. doi:10.1155/2018/8432781
3. Intagliata NM, Henry ZH, Maitland H, et al. Direct oral anticoagulants in cirrhosis patients pose similar risks of bleeding when compared to traditional anticoagulation. Dig Dis Sci. 2016;61(6):1721-1727. doi:10.1007/s10620-015-4012-2
4. Hum J, Shatzel JJ, Jou JH, Deloughery TG. The efficacy and safety of direct oral anticoagulants vs traditional anticoagulants in cirrhosis. Eur J Haematol. 2017;98(4):393-397. doi:10.1111/ejh.12844
5. Goriacko P, Veltri KT. Safety of direct oral anticoagulants vs warfarin in patients with chronic liver disease and atrial fibrillation. Eur J Haematol. 2018;100(5):488-493. doi:10.1111/ejh.13045
6. Schulman S, Kearon C; Subcommittee on Control of Anticoagulation of the Scientific and Standardization Committee of the International Society on Thrombosis and Haemostasis. Definition of major bleeding in clinical investigations of antihemostatic medicinal products in non-surgical patients. J Thromb Haemost. 2005;3(4):692-694. doi:10.1111/j.1538-7836.2005.01204.x
7. Rubboli A, Becattini C, Verheugt FW. Incidence, clinical impact and risk of bleeding during oral anticoagulation therapy. World J Cardiol. 2011;3(11):351-358. doi:10.4330/wjc.v3.i11.351
8. Ruff CT, Giugliano RP, Braunwald E, et al. Comparison of the efficacy and safety of new oral anticoagulants with warfarin in patients with atrial fibrillation: a meta-analysis of randomised trials. Lancet. 2014;383(9921):955-962. doi:10.1016/S0140-6736(13)62343-0
9. Hoolwerf EW, Kraaijpoel N, Büller HR, van Es N. Direct oral anticoagulants in patients with liver cirrhosis: A systematic review. Thromb Res. 2018;170:102-108. doi:10.1016/j.thromres.2018.08.011
10. Steuber TD, Howard ML, Nisly SA. Direct oral anticoagulants in chronic liver disease. Ann Pharmacother. 2019;53(10):1042-1049. doi:10.1177/1060028019841582
11. Janevska D, Chaloska-Ivanova V, Janevski V. Hepatocellular carcinoma: risk factors, diagnosis and treatment. Open Access Maced J Med Sci. 2015;3(4):732-736. doi:10.3889/oamjms.2015.111
12. Singal AK, Kamath PS. Model for End-Stage Liver Disease. J Clin Exp Hepatol. 2013;3(1):50-60. doi:10.1016/j.jceh.2012.11.002
13. Joppa SA, Salciccioli J, Adamski J, et al. A practical review of the emerging direct anticoagulants, laboratory monitoring, and reversal agents. J Clin Med. 2018;7(2):29. Published 2018 Feb 11. doi:10.3390/jcm7020029
14. Granger CB, Alexander JH, McMurray JJ, et al. Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2011;365(11):981-992. doi:10.1056/NEJMoa1107039
15. Agnelli G, Buller HR, Cohen A, et al. Oral apixaban for the treatment of acute venous thromboembolism. N Engl J Med. 2013;369(9):799-808. doi:10.1056/NEJMoa1302507
Coagulation in patients with cirrhosis is a complicated area of evolving research. Patients with cirrhosis were originally thought to be naturally anticoagulated due to the decreased production of clotting factors and platelets, combined with an increased international normalized ratio (INR).1 New data have shown that patients with cirrhosis are at a concomitant risk of bleeding and thrombosis due to increased platelet aggregation, decreased fibrinolysis, and decreased production of natural anticoagulants such as protein C and antithrombin.1 Traditionally, patients with cirrhosis needing anticoagulation therapy for comorbid conditions, such as nonvalvular atrial fibrillation (NVAF) or venous thromboembolism (VTE) were placed on warfarin therapy. Managing warfarin in patients with cirrhosis poses a challenge to clinicians due to the many food and drug interactions, narrow therapeutic index, and complications with maintaining a therapeutic INR.1
Direct oral anticoagulants (DOACs) have several benefits over warfarin therapy, including convenience, decreased monitoring, decreased drug and dietary restrictions, and faster onset of action.2 Conversely, DOACs undergo extensive hepatic metabolism giving rise to concerns about supratherapeutic drug levels and increased bleeding rates in patients with liver dysfunction.1 Consequently, patients with cirrhosis were excluded from the pivotal trials establishing DOACs for NVAF and VTE treatment. Exclusion of these patients in major clinical trials alongside the challenges of managing warfarin warrant an evaluation of the efficacy and safety of DOACs in patients with cirrhosis.
Recent retrospective studies have examined the use of DOACs in patients with cirrhosis and found favorable results. A retrospective chart review by Intagliata and colleagues consisting of 39 patients with cirrhosis using either a DOAC or warfarin found similar rates of all-cause bleeding and major bleeding between the 2 groups.3 A retrospective cohort study by Hum and colleagues consisting of 45 patients with cirrhosis compared the use of DOACs with warfarin or low-molecular weight heparin (LMWH).4 Hum and colleagues found patients prescribed a DOAC had significantly fewer major bleeding events than did patients using warfarin or LMWH.4 The largest retrospective cohort study consisted of 233 patients with chronic liver disease and found no differences among all-cause bleeding and major bleeding rates between patients using DOACs compared with those of patients using warfarin.5
The purpose of this research is to evaluate the safety and efficacy of DOACs in veteran patients with cirrhosis compared with patients using warfarin.
Methods
A retrospective single-center chart review was conducted at the Michael E. DeBakey Veterans Affairs Medical Center (MEDVAMC) in Houston, Texas, between October 31, 2014 and October 31, 2018. Patients included in the study were adults aged ≥ 18 years with a diagnosis of cirrhosis and prescribed any of the following oral anticoagulants: apixaban, dabigatran, edoxaban, rivaroxaban, or warfarin. Patients prescribed apixaban, dabigatran, edoxaban, or rivaroxaban were collectively grouped into the DOAC group, while patients prescribed warfarin were classified as the standard of care comparator group.
A diagnosis of cirrhosis was confirmed using a combination of the codes from the ninth and tenth editions of the International Classification of Diseases (ICD) for cirrhosis, documentation of diagnostic confirmation by clinicians from the gastroenterology or hepatology services, and positive liver biopsy result. Liver function tests, liver ultrasound results, and FibroSure biomarker assays were used to aid in confirming the diagnosis of cirrhosis but were not considered definitive. Patients were excluded from the trial if they had indications for anticoagulation other than NVAF and VTE and/or were prescribed triple antithrombotic therapy (dual antiplatelet therapy plus an anticoagulant). Patients who switched anticoagulant therapy during the trial period (ie, switched from warfarin to a DOAC) were also excluded from the analysis.
Patient demographic characteristics that were collected included weight; body mass index (BMI); etiology of cirrhosis; Child-Turcotte-Pugh, Model for End-Stage Liver Disease (MELD), and CHA2DS2-VASc score; concomitant antiplatelet, nonsteroidal anti-inflammatory drug (NSAID), proton pump inhibitor (PPI), and histamine-2 receptor antagonist
Two patient lists were used to identify patients for inclusion in the warfarin arm. The first patient list was generated using the US Department of Veterans Affairs (VA) Cirrhosis Tracker, which identified patients with an ICD-9/10 code for cirrhosis and an INR laboratory value. Patients generated from the VA Cirrhosis Tracker with an INR > 1.5 were screened for a warfarin prescription and then evaluated for full study inclusion. The second patient list was generated using the VA Advanced Liver Disease Dashboard which identified patients with ICD-9/10 codes for advanced liver disease and an active warfarin prescription. Patients with an active warfarin prescription were then evaluated for full study inclusion. A single patient list was generated to identify patients for inclusion in the DOAC arm. This patient list was generated using the VA DOAC dashboard, which identified patients with an active DOAC prescription and an ICD-9/10 code for cirrhosis. Patients with an ICD-9/10 code for cirrhosis and prescribed a DOAC were screened for full study inclusion. Patient data were collected from the MEDVAMC Computerized Patient Record System (CPRS) electronic health record (EHR). The research study was approved by the Baylor College of Medicine Institutional Review Board and the VA Office of Research and Development.
Outcomes
The primary endpoint for the study was all-cause bleeding. The secondary endpoints for the study were major bleeding and failed efficacy. Major bleeding was defined using the International Society on Thrombosis and Haemostasis (ISTH) 2005 definition: fatal bleeding, symptomatic bleeding in a critical organ area (ie, intracranial, intraspinal, intraocular, retroperitoneal, intraarticular, pericardial, or intramuscular with compartment syndrome), or bleeding causing a fall in hemoglobin level of > 2 g/dL or leading to the transfusion of ≥ 2 units of red cells.6 Failed efficacy was a combination endpoint that included development of VTE, stroke, myocardial infarction (MI), and/or death. A prespecified subgroup analysis was conducted at the end of the study period to analyze trends in the DOAC and warfarin groups with respect to all-cause bleeding. All-cause bleeding risk was stratified by weight, BMI, Child-Turcotte-Pugh score, MELD score, presence of gastric and/or esophageal varices, active malignancies, percentage of time within therapeutic INR range in the warfarin group, indications for anticoagulation, and antiplatelet, NSAID, PPI, and H2RA therapy.
Statistical Analysis
Data were analyzed using descriptive and inferential statistics. Continuous data were analyzed using the Student t test, and categorical data were analyzed using the Fisher exact test. Previous studies determined an all-cause bleeding rate of 10 to 17% for warfarin compared with 5% for DOACs.7,8 To detect a 12% difference in the all-cause bleeding rate between DOACs and warfarin, 212 patients would be needed to achieve 80% power at an α level of 0.05.
Results
A total of 170 patients were screened, and after applying inclusion and exclusion criteria, 79 patients were enrolled in the study (Figure). The DOAC group included 42 patients, and the warfarin group included 37 patients. In the DOAC group, 69.1% (n = 29) of patients were taking apixaban, 21.4% (n = 9) rivaroxaban, and 9.5% (n = 4) dabigatran. There were no patients prescribed edoxaban during the study period.
Baseline characteristics were similar between the 2 groups except for Child-Turcotte-Pugh score, MELD score, mean INR, and number of days on anticoagulation therapy (Table 1). Most of the patients were male (98.7%), and the mean age was 71 years. The most common causes of cirrhosis were viral (29.1%), nonalcoholic fatty liver disease (NAFLD) (24.1%), multiple causes (22.8%), and alcohol (21.5%). Sixty-two patients (78.5%) had a NVAF indication for anticoagulation. The average CHA2DS2-VASc score was 3.7. Aspirin was prescribed in 51.9% (n = 41) of patients, and PPIs were prescribed in 48.1% (n = 38) of patients. At inclusion, esophageal varices were present in 13 patients and active malignancies were present in 6 patients.
Statistically significant differences in baseline characteristics were found between mean INR, Child-Turcotte-Pugh scores, MELD scores, and number of days on anticoagulant therapy. The mean INR was 1.3 in the DOAC group compared with 2.1 in the warfarin group (P = .0001). Eighty-one percent (n = 34) of patients in the DOAC group had a Child-Turcotte-Pugh score of A compared with 43.2% (n = 16) of patients in the warfarin group (P = .0009). Eight patients in the DOAC group had a Child-Turcotte-Pugh score of B compared with 19 patients in the warfarin group (P = .004). The mean MELD score was 9.4 in the DOAC group compared with 16.3 in the warfarin group (P = .0001). The mean days on anticoagulant therapy was 500.4 days for the DOAC group compared with 1,652.4 days for the warfarin group (P = .0001).
Safety Outcome
The primary outcome comparing all-cause bleeding rates between patients on DOACs compared with warfarin are listed in Table 2. With respect to the primary outcome, 7 (16.7%) patients on DOACs experienced a bleeding event compared with 8 (21.6%) patients on warfarin (P = .77). No statistically significant differences were detected between the DOAC and warfarin groups with respect to all-cause bleeding. Seven bleeding events occurred in the DOAC group; 1 met the qualification for major bleeding with a suspected gastrointestinal (GI) bleed.6 The other 6 bleeding episodes in the DOAC group consisted of hematoma, epistaxis, hematuria, and hematochezia. Eight bleeding events occurred in the warfarin group; 2 met the qualification for major bleeding with an intracranial hemorrhage and upper GI bleed.6 The other 6 bleeding episodes in the warfarin group consisted of epistaxis, bleeding gums, hematuria, and hematochezia. There were no statistically significant differences between the rates of major bleeding and nonmajor bleeding between the DOAC and warfarin groups.
Efficacy Outcomes
There were 3 events in the DOAC group and 3 events in the warfarin group (P = .99). In the DOAC group, 2 patients experienced a pulmonary embolism, and 1 patient experienced a MI. In the warfarin group, 3 patients died (end-stage heart failure, unknown cause due to death at an outside hospital, and sepsis/organ failure). There were no statistically significant differences between the composite endpoint of failed efficacy or the individual endpoints of VTE, stroke, MI, and death.
Subgroup Analysis
A prespecified subgroup analysis was conducted to determine risk factors for all-cause bleeding within each treatment group (Table 3). No significant trends were observed in the following risk factors: Child-Turcotte-Pugh score, indication for anticoagulation, use of NSAIDs, PPIs or H2RAs, presence of gastric or esophageal varices, active malignancies, and time within therapeutic INR range in the warfarin group. Patients with bleeding events had slightly increased weight and BMI vs patients without bleeding events. Within the warfarin group, patients with bleeding events had slightly elevated MELD scores compared to patients without bleeding events. There was an equal balance of patients prescribed aspirin therapy between the groups with and without bleeding events. Overall, no significant risk factors were identified for all-cause bleeding.
Discussion
Initially, patients with cirrhosis were excluded from DOAC trials due to concerns for increased bleeding risk with hepatically eliminated medications. New retrospective research has concluded that in patients with cirrhosis, DOACs have similar or lower bleeding rates when compared directly to warfarin.9,10
In this study, no statistically significant differences were detected between the primary and secondary outcomes of all-cause bleeding, major bleeding, or failed efficacy. Subgroup analysis did not identify any significant risk factors with respect to all-cause bleeding among patients in the DOAC and warfarin groups. To meet 80% power, 212 patients needed to be enrolled in the study; however, only 79 patients were enrolled, and power was not met. The results of this study should be interpreted cautiously as hypothesis-generating due to the small sample size. Strengths of this study include similar baseline characteristics between the DOAC and warfarin groups, 4-year length of retrospective data review, and availability of both inpatient and outpatient EHR limiting the amount of missing data points.
Baseline characteristics were similar between the groups except for mean INR, Child-Turcotte-Pugh score, MELD score, and number of days on anticoagulation therapy. The difference in mean INR between groups is expected as patients in the warfarin group have a goal INR of 2 to 3 to maintain therapeutic efficacy and safety. INR is not used as a marker of efficacy or safety with DOACs; therefore, a consistent elevation in INR is not expected. Child- Turcotte-Pugh scores are calculated using INR levels.11 When calculating the score, patients with an INR < 1.7 receive 1 point; patients with an INR between 1.7 and 2.3 receive 2 points.11 Therefore, patients in the warfarin group will have artificially inflated Child-Turcotte-Pugh scores as this group has goal INR levels of 2 to 3. This makes Child-Turcotte-Pugh scores unreliable markers of disease severity in patients using warfarin therapy. When the INR scores for patients prescribed warfarin were replaced with values < 1.7, the statistical difference disappeared between the warfarin and DOAC groups. The same effect is seen on MELD scores for patients prescribed warfarin therapy. The MELD score is calculated using INR levels.12 MELD scores also will be artificially elevated in patients prescribed warfarin therapy due to the INR elevation to between 2 and 3. When MELD scores for patients prescribed warfarin were replaced with values similar to those in the DOAC group, the statistical difference disappeared between the warfarin and DOAC groups.
The last statistically significant difference was found in number of days on anticoagulant therapy. This difference was expected as warfarin is the standard of care for anticoagulation treatment in patients with cirrhosis. The first DOAC, dabigatran, was not approved by the US Food and Drug Administration until 2010.13 DOACs have only recently been used in patients with cirrhosis accounting for the statistically significant difference in days on anticoagulation therapy between the warfarin and DOAC groups.
Limitations
The inability to meet power or evaluate adherence and appropriate renal dose adjustments for DOACs limited this study. This study was conducted at a single center in a predominantly male veteran population and therefore may not be generalizable to other populations. A majority of patients in the DOAC group were prescribed apixaban (69.1%), which may have affected the overall rate of major bleeding in the DOAC group. Pivotal trials of apixaban have shown a consistent decreased risk of major bleeding in patients with NVAF or VTE when compared with warfarin.14,15 Therefore, the results of this study may not be generalizable to all DOACs.
An inherent limitation of this study was the inability to collect data verifying adherence in the DOAC group. However, in the warfarin group, percentage of time within the therapeutic INR range of 2 to 3 was collected. While not a direct marker of adherence, this does allow for limited evaluation of therapeutic efficacy and safety within the warfarin group. Last, proper dosing of DOACs in patients with and without adequate renal function was not evaluated in this study.
Conclusions
The results of this study are consistent with other retrospective research and literature reviews. There were no statistically significant differences identified between the rates of all-cause bleeding, major bleeding, and failed efficacy between the DOAC and warfarin groups. DOACs may be a safe alternative to warfarin in patients with cirrhosis requiring anticoagulation for NVAF or VTE, but large randomized trials are required to confirm these results.
Coagulation in patients with cirrhosis is a complicated area of evolving research. Patients with cirrhosis were originally thought to be naturally anticoagulated due to the decreased production of clotting factors and platelets, combined with an increased international normalized ratio (INR).1 New data have shown that patients with cirrhosis are at a concomitant risk of bleeding and thrombosis due to increased platelet aggregation, decreased fibrinolysis, and decreased production of natural anticoagulants such as protein C and antithrombin.1 Traditionally, patients with cirrhosis needing anticoagulation therapy for comorbid conditions, such as nonvalvular atrial fibrillation (NVAF) or venous thromboembolism (VTE) were placed on warfarin therapy. Managing warfarin in patients with cirrhosis poses a challenge to clinicians due to the many food and drug interactions, narrow therapeutic index, and complications with maintaining a therapeutic INR.1
Direct oral anticoagulants (DOACs) have several benefits over warfarin therapy, including convenience, decreased monitoring, decreased drug and dietary restrictions, and faster onset of action.2 Conversely, DOACs undergo extensive hepatic metabolism giving rise to concerns about supratherapeutic drug levels and increased bleeding rates in patients with liver dysfunction.1 Consequently, patients with cirrhosis were excluded from the pivotal trials establishing DOACs for NVAF and VTE treatment. Exclusion of these patients in major clinical trials alongside the challenges of managing warfarin warrant an evaluation of the efficacy and safety of DOACs in patients with cirrhosis.
Recent retrospective studies have examined the use of DOACs in patients with cirrhosis and found favorable results. A retrospective chart review by Intagliata and colleagues consisting of 39 patients with cirrhosis using either a DOAC or warfarin found similar rates of all-cause bleeding and major bleeding between the 2 groups.3 A retrospective cohort study by Hum and colleagues consisting of 45 patients with cirrhosis compared the use of DOACs with warfarin or low-molecular weight heparin (LMWH).4 Hum and colleagues found patients prescribed a DOAC had significantly fewer major bleeding events than did patients using warfarin or LMWH.4 The largest retrospective cohort study consisted of 233 patients with chronic liver disease and found no differences among all-cause bleeding and major bleeding rates between patients using DOACs compared with those of patients using warfarin.5
The purpose of this research is to evaluate the safety and efficacy of DOACs in veteran patients with cirrhosis compared with patients using warfarin.
Methods
A retrospective single-center chart review was conducted at the Michael E. DeBakey Veterans Affairs Medical Center (MEDVAMC) in Houston, Texas, between October 31, 2014 and October 31, 2018. Patients included in the study were adults aged ≥ 18 years with a diagnosis of cirrhosis and prescribed any of the following oral anticoagulants: apixaban, dabigatran, edoxaban, rivaroxaban, or warfarin. Patients prescribed apixaban, dabigatran, edoxaban, or rivaroxaban were collectively grouped into the DOAC group, while patients prescribed warfarin were classified as the standard of care comparator group.
A diagnosis of cirrhosis was confirmed using a combination of the codes from the ninth and tenth editions of the International Classification of Diseases (ICD) for cirrhosis, documentation of diagnostic confirmation by clinicians from the gastroenterology or hepatology services, and positive liver biopsy result. Liver function tests, liver ultrasound results, and FibroSure biomarker assays were used to aid in confirming the diagnosis of cirrhosis but were not considered definitive. Patients were excluded from the trial if they had indications for anticoagulation other than NVAF and VTE and/or were prescribed triple antithrombotic therapy (dual antiplatelet therapy plus an anticoagulant). Patients who switched anticoagulant therapy during the trial period (ie, switched from warfarin to a DOAC) were also excluded from the analysis.
Patient demographic characteristics that were collected included weight; body mass index (BMI); etiology of cirrhosis; Child-Turcotte-Pugh, Model for End-Stage Liver Disease (MELD), and CHA2DS2-VASc score; concomitant antiplatelet, nonsteroidal anti-inflammatory drug (NSAID), proton pump inhibitor (PPI), and histamine-2 receptor antagonist
Two patient lists were used to identify patients for inclusion in the warfarin arm. The first patient list was generated using the US Department of Veterans Affairs (VA) Cirrhosis Tracker, which identified patients with an ICD-9/10 code for cirrhosis and an INR laboratory value. Patients generated from the VA Cirrhosis Tracker with an INR > 1.5 were screened for a warfarin prescription and then evaluated for full study inclusion. The second patient list was generated using the VA Advanced Liver Disease Dashboard which identified patients with ICD-9/10 codes for advanced liver disease and an active warfarin prescription. Patients with an active warfarin prescription were then evaluated for full study inclusion. A single patient list was generated to identify patients for inclusion in the DOAC arm. This patient list was generated using the VA DOAC dashboard, which identified patients with an active DOAC prescription and an ICD-9/10 code for cirrhosis. Patients with an ICD-9/10 code for cirrhosis and prescribed a DOAC were screened for full study inclusion. Patient data were collected from the MEDVAMC Computerized Patient Record System (CPRS) electronic health record (EHR). The research study was approved by the Baylor College of Medicine Institutional Review Board and the VA Office of Research and Development.
Outcomes
The primary endpoint for the study was all-cause bleeding. The secondary endpoints for the study were major bleeding and failed efficacy. Major bleeding was defined using the International Society on Thrombosis and Haemostasis (ISTH) 2005 definition: fatal bleeding, symptomatic bleeding in a critical organ area (ie, intracranial, intraspinal, intraocular, retroperitoneal, intraarticular, pericardial, or intramuscular with compartment syndrome), or bleeding causing a fall in hemoglobin level of > 2 g/dL or leading to the transfusion of ≥ 2 units of red cells.6 Failed efficacy was a combination endpoint that included development of VTE, stroke, myocardial infarction (MI), and/or death. A prespecified subgroup analysis was conducted at the end of the study period to analyze trends in the DOAC and warfarin groups with respect to all-cause bleeding. All-cause bleeding risk was stratified by weight, BMI, Child-Turcotte-Pugh score, MELD score, presence of gastric and/or esophageal varices, active malignancies, percentage of time within therapeutic INR range in the warfarin group, indications for anticoagulation, and antiplatelet, NSAID, PPI, and H2RA therapy.
Statistical Analysis
Data were analyzed using descriptive and inferential statistics. Continuous data were analyzed using the Student t test, and categorical data were analyzed using the Fisher exact test. Previous studies determined an all-cause bleeding rate of 10 to 17% for warfarin compared with 5% for DOACs.7,8 To detect a 12% difference in the all-cause bleeding rate between DOACs and warfarin, 212 patients would be needed to achieve 80% power at an α level of 0.05.
Results
A total of 170 patients were screened, and after applying inclusion and exclusion criteria, 79 patients were enrolled in the study (Figure). The DOAC group included 42 patients, and the warfarin group included 37 patients. In the DOAC group, 69.1% (n = 29) of patients were taking apixaban, 21.4% (n = 9) rivaroxaban, and 9.5% (n = 4) dabigatran. There were no patients prescribed edoxaban during the study period.
Baseline characteristics were similar between the 2 groups except for Child-Turcotte-Pugh score, MELD score, mean INR, and number of days on anticoagulation therapy (Table 1). Most of the patients were male (98.7%), and the mean age was 71 years. The most common causes of cirrhosis were viral (29.1%), nonalcoholic fatty liver disease (NAFLD) (24.1%), multiple causes (22.8%), and alcohol (21.5%). Sixty-two patients (78.5%) had a NVAF indication for anticoagulation. The average CHA2DS2-VASc score was 3.7. Aspirin was prescribed in 51.9% (n = 41) of patients, and PPIs were prescribed in 48.1% (n = 38) of patients. At inclusion, esophageal varices were present in 13 patients and active malignancies were present in 6 patients.
Statistically significant differences in baseline characteristics were found between mean INR, Child-Turcotte-Pugh scores, MELD scores, and number of days on anticoagulant therapy. The mean INR was 1.3 in the DOAC group compared with 2.1 in the warfarin group (P = .0001). Eighty-one percent (n = 34) of patients in the DOAC group had a Child-Turcotte-Pugh score of A compared with 43.2% (n = 16) of patients in the warfarin group (P = .0009). Eight patients in the DOAC group had a Child-Turcotte-Pugh score of B compared with 19 patients in the warfarin group (P = .004). The mean MELD score was 9.4 in the DOAC group compared with 16.3 in the warfarin group (P = .0001). The mean days on anticoagulant therapy was 500.4 days for the DOAC group compared with 1,652.4 days for the warfarin group (P = .0001).
Safety Outcome
The primary outcome comparing all-cause bleeding rates between patients on DOACs compared with warfarin are listed in Table 2. With respect to the primary outcome, 7 (16.7%) patients on DOACs experienced a bleeding event compared with 8 (21.6%) patients on warfarin (P = .77). No statistically significant differences were detected between the DOAC and warfarin groups with respect to all-cause bleeding. Seven bleeding events occurred in the DOAC group; 1 met the qualification for major bleeding with a suspected gastrointestinal (GI) bleed.6 The other 6 bleeding episodes in the DOAC group consisted of hematoma, epistaxis, hematuria, and hematochezia. Eight bleeding events occurred in the warfarin group; 2 met the qualification for major bleeding with an intracranial hemorrhage and upper GI bleed.6 The other 6 bleeding episodes in the warfarin group consisted of epistaxis, bleeding gums, hematuria, and hematochezia. There were no statistically significant differences between the rates of major bleeding and nonmajor bleeding between the DOAC and warfarin groups.
Efficacy Outcomes
There were 3 events in the DOAC group and 3 events in the warfarin group (P = .99). In the DOAC group, 2 patients experienced a pulmonary embolism, and 1 patient experienced a MI. In the warfarin group, 3 patients died (end-stage heart failure, unknown cause due to death at an outside hospital, and sepsis/organ failure). There were no statistically significant differences between the composite endpoint of failed efficacy or the individual endpoints of VTE, stroke, MI, and death.
Subgroup Analysis
A prespecified subgroup analysis was conducted to determine risk factors for all-cause bleeding within each treatment group (Table 3). No significant trends were observed in the following risk factors: Child-Turcotte-Pugh score, indication for anticoagulation, use of NSAIDs, PPIs or H2RAs, presence of gastric or esophageal varices, active malignancies, and time within therapeutic INR range in the warfarin group. Patients with bleeding events had slightly increased weight and BMI vs patients without bleeding events. Within the warfarin group, patients with bleeding events had slightly elevated MELD scores compared to patients without bleeding events. There was an equal balance of patients prescribed aspirin therapy between the groups with and without bleeding events. Overall, no significant risk factors were identified for all-cause bleeding.
Discussion
Initially, patients with cirrhosis were excluded from DOAC trials due to concerns for increased bleeding risk with hepatically eliminated medications. New retrospective research has concluded that in patients with cirrhosis, DOACs have similar or lower bleeding rates when compared directly to warfarin.9,10
In this study, no statistically significant differences were detected between the primary and secondary outcomes of all-cause bleeding, major bleeding, or failed efficacy. Subgroup analysis did not identify any significant risk factors with respect to all-cause bleeding among patients in the DOAC and warfarin groups. To meet 80% power, 212 patients needed to be enrolled in the study; however, only 79 patients were enrolled, and power was not met. The results of this study should be interpreted cautiously as hypothesis-generating due to the small sample size. Strengths of this study include similar baseline characteristics between the DOAC and warfarin groups, 4-year length of retrospective data review, and availability of both inpatient and outpatient EHR limiting the amount of missing data points.
Baseline characteristics were similar between the groups except for mean INR, Child-Turcotte-Pugh score, MELD score, and number of days on anticoagulation therapy. The difference in mean INR between groups is expected as patients in the warfarin group have a goal INR of 2 to 3 to maintain therapeutic efficacy and safety. INR is not used as a marker of efficacy or safety with DOACs; therefore, a consistent elevation in INR is not expected. Child- Turcotte-Pugh scores are calculated using INR levels.11 When calculating the score, patients with an INR < 1.7 receive 1 point; patients with an INR between 1.7 and 2.3 receive 2 points.11 Therefore, patients in the warfarin group will have artificially inflated Child-Turcotte-Pugh scores as this group has goal INR levels of 2 to 3. This makes Child-Turcotte-Pugh scores unreliable markers of disease severity in patients using warfarin therapy. When the INR scores for patients prescribed warfarin were replaced with values < 1.7, the statistical difference disappeared between the warfarin and DOAC groups. The same effect is seen on MELD scores for patients prescribed warfarin therapy. The MELD score is calculated using INR levels.12 MELD scores also will be artificially elevated in patients prescribed warfarin therapy due to the INR elevation to between 2 and 3. When MELD scores for patients prescribed warfarin were replaced with values similar to those in the DOAC group, the statistical difference disappeared between the warfarin and DOAC groups.
The last statistically significant difference was found in number of days on anticoagulant therapy. This difference was expected as warfarin is the standard of care for anticoagulation treatment in patients with cirrhosis. The first DOAC, dabigatran, was not approved by the US Food and Drug Administration until 2010.13 DOACs have only recently been used in patients with cirrhosis accounting for the statistically significant difference in days on anticoagulation therapy between the warfarin and DOAC groups.
Limitations
The inability to meet power or evaluate adherence and appropriate renal dose adjustments for DOACs limited this study. This study was conducted at a single center in a predominantly male veteran population and therefore may not be generalizable to other populations. A majority of patients in the DOAC group were prescribed apixaban (69.1%), which may have affected the overall rate of major bleeding in the DOAC group. Pivotal trials of apixaban have shown a consistent decreased risk of major bleeding in patients with NVAF or VTE when compared with warfarin.14,15 Therefore, the results of this study may not be generalizable to all DOACs.
An inherent limitation of this study was the inability to collect data verifying adherence in the DOAC group. However, in the warfarin group, percentage of time within the therapeutic INR range of 2 to 3 was collected. While not a direct marker of adherence, this does allow for limited evaluation of therapeutic efficacy and safety within the warfarin group. Last, proper dosing of DOACs in patients with and without adequate renal function was not evaluated in this study.
Conclusions
The results of this study are consistent with other retrospective research and literature reviews. There were no statistically significant differences identified between the rates of all-cause bleeding, major bleeding, and failed efficacy between the DOAC and warfarin groups. DOACs may be a safe alternative to warfarin in patients with cirrhosis requiring anticoagulation for NVAF or VTE, but large randomized trials are required to confirm these results.
1. Qamar A, Vaduganathan M, Greenberger NJ, Giugliano RP. Oral anticoagulation in patients with liver disease. J Am Coll Cardiol. 2018;71(19):2162-2175. doi:10.1016/j.jacc.2018.03.023
2. Priyanka P, Kupec JT, Krafft M, Shah NA, Reynolds GJ. Newer oral anticoagulants in the treatment of acute portal vein thrombosis in patients with and without cirrhosis. Int J Hepatol. 2018;2018:8432781. Published 2018 Jun 5. doi:10.1155/2018/8432781
3. Intagliata NM, Henry ZH, Maitland H, et al. Direct oral anticoagulants in cirrhosis patients pose similar risks of bleeding when compared to traditional anticoagulation. Dig Dis Sci. 2016;61(6):1721-1727. doi:10.1007/s10620-015-4012-2
4. Hum J, Shatzel JJ, Jou JH, Deloughery TG. The efficacy and safety of direct oral anticoagulants vs traditional anticoagulants in cirrhosis. Eur J Haematol. 2017;98(4):393-397. doi:10.1111/ejh.12844
5. Goriacko P, Veltri KT. Safety of direct oral anticoagulants vs warfarin in patients with chronic liver disease and atrial fibrillation. Eur J Haematol. 2018;100(5):488-493. doi:10.1111/ejh.13045
6. Schulman S, Kearon C; Subcommittee on Control of Anticoagulation of the Scientific and Standardization Committee of the International Society on Thrombosis and Haemostasis. Definition of major bleeding in clinical investigations of antihemostatic medicinal products in non-surgical patients. J Thromb Haemost. 2005;3(4):692-694. doi:10.1111/j.1538-7836.2005.01204.x
7. Rubboli A, Becattini C, Verheugt FW. Incidence, clinical impact and risk of bleeding during oral anticoagulation therapy. World J Cardiol. 2011;3(11):351-358. doi:10.4330/wjc.v3.i11.351
8. Ruff CT, Giugliano RP, Braunwald E, et al. Comparison of the efficacy and safety of new oral anticoagulants with warfarin in patients with atrial fibrillation: a meta-analysis of randomised trials. Lancet. 2014;383(9921):955-962. doi:10.1016/S0140-6736(13)62343-0
9. Hoolwerf EW, Kraaijpoel N, Büller HR, van Es N. Direct oral anticoagulants in patients with liver cirrhosis: A systematic review. Thromb Res. 2018;170:102-108. doi:10.1016/j.thromres.2018.08.011
10. Steuber TD, Howard ML, Nisly SA. Direct oral anticoagulants in chronic liver disease. Ann Pharmacother. 2019;53(10):1042-1049. doi:10.1177/1060028019841582
11. Janevska D, Chaloska-Ivanova V, Janevski V. Hepatocellular carcinoma: risk factors, diagnosis and treatment. Open Access Maced J Med Sci. 2015;3(4):732-736. doi:10.3889/oamjms.2015.111
12. Singal AK, Kamath PS. Model for End-Stage Liver Disease. J Clin Exp Hepatol. 2013;3(1):50-60. doi:10.1016/j.jceh.2012.11.002
13. Joppa SA, Salciccioli J, Adamski J, et al. A practical review of the emerging direct anticoagulants, laboratory monitoring, and reversal agents. J Clin Med. 2018;7(2):29. Published 2018 Feb 11. doi:10.3390/jcm7020029
14. Granger CB, Alexander JH, McMurray JJ, et al. Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2011;365(11):981-992. doi:10.1056/NEJMoa1107039
15. Agnelli G, Buller HR, Cohen A, et al. Oral apixaban for the treatment of acute venous thromboembolism. N Engl J Med. 2013;369(9):799-808. doi:10.1056/NEJMoa1302507
1. Qamar A, Vaduganathan M, Greenberger NJ, Giugliano RP. Oral anticoagulation in patients with liver disease. J Am Coll Cardiol. 2018;71(19):2162-2175. doi:10.1016/j.jacc.2018.03.023
2. Priyanka P, Kupec JT, Krafft M, Shah NA, Reynolds GJ. Newer oral anticoagulants in the treatment of acute portal vein thrombosis in patients with and without cirrhosis. Int J Hepatol. 2018;2018:8432781. Published 2018 Jun 5. doi:10.1155/2018/8432781
3. Intagliata NM, Henry ZH, Maitland H, et al. Direct oral anticoagulants in cirrhosis patients pose similar risks of bleeding when compared to traditional anticoagulation. Dig Dis Sci. 2016;61(6):1721-1727. doi:10.1007/s10620-015-4012-2
4. Hum J, Shatzel JJ, Jou JH, Deloughery TG. The efficacy and safety of direct oral anticoagulants vs traditional anticoagulants in cirrhosis. Eur J Haematol. 2017;98(4):393-397. doi:10.1111/ejh.12844
5. Goriacko P, Veltri KT. Safety of direct oral anticoagulants vs warfarin in patients with chronic liver disease and atrial fibrillation. Eur J Haematol. 2018;100(5):488-493. doi:10.1111/ejh.13045
6. Schulman S, Kearon C; Subcommittee on Control of Anticoagulation of the Scientific and Standardization Committee of the International Society on Thrombosis and Haemostasis. Definition of major bleeding in clinical investigations of antihemostatic medicinal products in non-surgical patients. J Thromb Haemost. 2005;3(4):692-694. doi:10.1111/j.1538-7836.2005.01204.x
7. Rubboli A, Becattini C, Verheugt FW. Incidence, clinical impact and risk of bleeding during oral anticoagulation therapy. World J Cardiol. 2011;3(11):351-358. doi:10.4330/wjc.v3.i11.351
8. Ruff CT, Giugliano RP, Braunwald E, et al. Comparison of the efficacy and safety of new oral anticoagulants with warfarin in patients with atrial fibrillation: a meta-analysis of randomised trials. Lancet. 2014;383(9921):955-962. doi:10.1016/S0140-6736(13)62343-0
9. Hoolwerf EW, Kraaijpoel N, Büller HR, van Es N. Direct oral anticoagulants in patients with liver cirrhosis: A systematic review. Thromb Res. 2018;170:102-108. doi:10.1016/j.thromres.2018.08.011
10. Steuber TD, Howard ML, Nisly SA. Direct oral anticoagulants in chronic liver disease. Ann Pharmacother. 2019;53(10):1042-1049. doi:10.1177/1060028019841582
11. Janevska D, Chaloska-Ivanova V, Janevski V. Hepatocellular carcinoma: risk factors, diagnosis and treatment. Open Access Maced J Med Sci. 2015;3(4):732-736. doi:10.3889/oamjms.2015.111
12. Singal AK, Kamath PS. Model for End-Stage Liver Disease. J Clin Exp Hepatol. 2013;3(1):50-60. doi:10.1016/j.jceh.2012.11.002
13. Joppa SA, Salciccioli J, Adamski J, et al. A practical review of the emerging direct anticoagulants, laboratory monitoring, and reversal agents. J Clin Med. 2018;7(2):29. Published 2018 Feb 11. doi:10.3390/jcm7020029
14. Granger CB, Alexander JH, McMurray JJ, et al. Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2011;365(11):981-992. doi:10.1056/NEJMoa1107039
15. Agnelli G, Buller HR, Cohen A, et al. Oral apixaban for the treatment of acute venous thromboembolism. N Engl J Med. 2013;369(9):799-808. doi:10.1056/NEJMoa1302507
Multidisciplinary Transitional Pain Service for the Veteran Population
Despite advancements in techniques, postsurgical pain continues to be a prominent part of the patient experience. Often this experience can lead to developing chronic postsurgical pain that interferes with quality of life after the expected time to recovery.1-3 As many as 14% of patients who undergo surgery without any history of opioid use develop chronic opioid use that persists after recovery from their operation.4-8 For patients with existing chronic opioid use or a history of substance use disorder (SUD), surgeons, primary care providers, or addiction providers often do not provide sufficient presurgical planning or postsurgical coordination of care. This lack of pain care coordination can increase the risk of inadequate pain control, opioid use escalation, or SUD relapse after surgery.
Convincing arguments have been made that a perioperative surgical home can improve significantly the quality of perioperative care.9-14 This report describes our experience implementing a perioperative surgical home at the US Department of Veterans Affairs (VA) Salt Lake City VA Medical Center (SLCVAMC), focusing on pain management extending from the preoperative period until 6 months or more after surgery. This type of Transitional Pain Service (TPS) has been described previously.15-17 Our service differs from those described previously by enrolling all patients before surgery rather than select postsurgical enrollment of only patients with a history of opioid use or SUD or patients who struggle with persistent postsurgical pain.
Methods
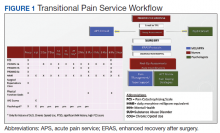
In January 2018, we developed and implemented a new TPS at the SLCVAMC. The transitional pain team consisted of an anesthesiologist with specialization in acute pain management, a nurse practitioner (NP) with experience in both acute and chronic pain management, 2 nurse care coordinators, and a psychologist (Figure 1). Before implementation, a needs assessment took place with these key stakeholders and others at SLCVAMC to identify the following specific goals of the TPS: (1) reduce pain through pharmacologic and nonpharmacologic interventions; (2) eliminate new chronic opioid use in previously nonopioid user (NOU) patients; (3) address chronic opioid use in previous chronic opioid users (COUs) by providing support for opioid taper and alternative analgesic therapies for their chronic pain conditions; and (4) improve continuity of care by close coordination with the surgical team, primary care providers (PCPs), and mental health or chronic pain providers as needed.
Once these TPS goals were defined, the Consolidated Framework for Implementation Research (CFIR) guided the implementation. CFIR is a theory-based implementation framework consisting of 5 domains: intervention characteristics, inner setting, outer setting, characteristics of individuals, and process. These domains were used to identify barriers and facilitators during the early implementation process and helped refine TPS as it was put into clinical practice.
Patient Selection
During the initial implementation of TPS, enrollment was limited to patients scheduled for elective primary or revision knee, hip, or shoulder replacement as well as rotator cuff repair surgery. But as the TPS workflow became established after iterative refinement, we expanded the program to enroll patients with established risk factors for OUD having other types of surgery (Table 1). The diagnosis of risk factors, such as history of SUD, chronic opioid use, or significant mental health disorders (ie, history of suicidal ideation or attempt, posttraumatic stress disorder, and inpatient psychiatric care) were confirmed through both in-person interviews and electronic health record (EHR) documentation. The overall goal was to identify all at-risk patients as soon as they were indicated for surgery, to allow time for evaluation, education, developing an individualized pain plan, and opioid taper prior to surgery if indicated.
Preoperative Procedures
Once identified, patients were contacted by a TPS team member and invited to attend a onetime 90-minute presurgical expectations class held at SLCVAMC. The education curriculum was developed by the whole team, and classes were taught primarily by the TPS psychologist. The class included education about expectations for postoperative pain, available analgesic therapies, opioid education, appropriate use of opioids, and the effect of psychological factors on pain. Pain coping strategies were introduced using a mindfulness-based intervention (MBI) and the Acceptance and Commitment Therapy (ACT) matrix. Classes were offered multiple times a week to help maximize convenience for patients and were separate from the anesthesia preoperative evaluation. Patients attended class only once. High-risk patients (patients with chronic opioid therapy, recent history of or current SUDs, significant comorbid mental health issues) were encouraged to attend this class one-on-one with the TPS psychologist rather than in the group setting, so individual attention to mental health and SUD issues could be addressed directly.
Baseline history, morphine equivalent daily dose (MEDD), and patient-reported outcomes using measures from the Patient-Reported Outcome Measurement System (PROMIS) for pain intensity (PROMIS 3a), pain interference (PROMIS 6b), and physical function (PROMIS 8b), and a pain-catastrophizing scale (PCS) score were obtained on all patients.18 PROMIS measures are validated questionnaires developed with the National Institutes of Health to standardize and quantify patient-reported outcomes in many domains.19 Patients with a history of SUD or COU met with the anesthesiologist and/or NP, and a personalized pain plan was developed that included preoperative opioid taper, buprenorphine use strategy, or opioid-free strategies.
Hospital Procedures
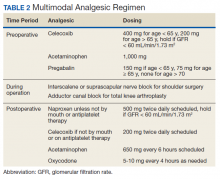
On the day of surgery, the TPS team met with the patient preoperatively and implemented an individualized pain plan that included multimodal analgesic techniques with nonsteroidal anti-inflammatory drugs, acetaminophen, gabapentinoids, and regional anesthesia, where appropriate (Table 2). Enhanced recovery after surgery protocols were developed in conjunction with the surgeons to include local infiltration analgesia by the surgeon, postoperative multimodal analgesic strategies, and intensive physical therapy starting the day of surgery for inpatient procedures.
After surgery, the TPS team followed up with patients daily and provided recommendations for analgesic therapies. Patients were offered daily sessions with the psychologist to reinforce and practice nonpharmacologic pain-coping strategies, such as meditation and relaxation. Prior to patient discharge, the TPS team provided recommendations for discharge medications and an opioid taper plan. For some patients taking buprenorphine before surgery who had stopped this therapy prior to or during their hospital stay, TPS providers transitioned them back to buprenorphine before discharge.
Postoperative Procedures
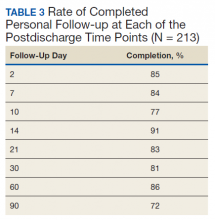
Patients were called by the nurse care coordinators at postdischarge days 2, 7, 10, 14, 21, 28, and then monthly for ≥ 6 months. For patients who had not stopped opioid use or returned to their preoperative baseline opioid dose, weekly calls were made until opioid taper goals were achieved. At each call, nurses collected PROMIS scores for the previous 24 hours, the most recent 24-hour MEDD, the date of last opioid use, and the number of remaining opioid tablets after opioid cessation. In addition, nurses provided active listening and supportive care and encouragement as well as care coordination for issues related to rehabilitation facilities, physical therapy, transportation, medication questions, and wound questions. Nurses notified the anesthesiologist or NP when patients were unable to taper opioid use or had poor pain control as indicated by their PROMIS scores, opioid use, or directly expressed by the patient.
The TPS team prescribed alternative analgesic therapies, opioid taper plans, and communicated with surgeons and primary care providers if limited continued opioid therapy was recommended. Individual sessions with the psychologist were available to patients after discharge with a focus on ACT-matrix therapy and consultation with long-term mental health and/or substance abuse providers as indicated. Frequent communication and care coordination were maintained with the surgical team, the PCP, and other providers on the mental health or chronic pain services. This care coordination often included postsurgical joint clinic appointments in which TPS providers and nurses would be present with the surgeon or the PCP.
For patients with inadequately treated chronic pain conditions or who required long-term opioid tapers, we developed a combined clinic with the TPS and Anesthesia Chronic Pain group. This clinic allows patients to be seen by both services in the same setting, allowing a warm handoff by TPS to the chronic pain team.
Heath and Decision Support Tools
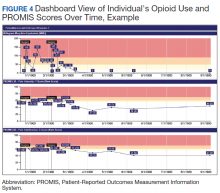
An electronic dashboard registry of surgical episodes managed by TPS was developed to achieve clinical, administrative, and quality improvement goals. The dashboard registry consists of surgical episode data, opioid doses, patient-reported outcomes, and clinical decision-making processes. Custom-built note templates capture pertinent data through embedded data labels, called health factors. Data are captured as part of routine clinical care, recorded in Computerized Patient Record System as health factors. They are available in the VA Corporate Data Warehouse as structured data. Workflows are executed daily to keep the dashboard registry current, clean, and able to process new data. Information displays direct daily clinical workflow and support point-of-care clinical decision making (Figures 2, 3, and 4). Data are aggregated across patient-care encounters and allow nurse care coordinators to concisely review pertinent patient data prior to delivering care. These data include surgical history, comorbidities, timeline of opioid use, and PROMIS scores during their course of recovery. This system allows TPS to optimize care delivery by providing longitudinal data across the surgical episode, thereby reducing the time needed to review records. Secondary purposes of captured data include measuring clinic performance and quality improvement to improve care delivery.
Results
The TPS intervention was implemented January 1, 2018. Two-hundred thirteen patients were enrolled between January and December 2018, which included 60 (28%) patients with a history of chronic opioid use and 153 (72%) patients who were considered opioid naïve. A total of 99% of patients had ≥ 1 successful follow-up within 14 days after discharge, 96% had ≥ 1 follow-up between 14 and 30 days after surgery, and 72% had completed personal follow-up 90 days after discharge (Table 3). For patients who TPS was unable to contact in person or by phone, 90-day MEDD was obtained using prescription and Controlled Substance Database reviews. The protocol for this retrospective analysis was approved by the University of Utah Institutional Review Board and the VA Research Review Committee.
By 90 days after surgery, 26 (43.3%) COUs were off opioids completely, 17 (28.3%) had decreased their opioid dose from their preoperative baseline MEDD (120 [SD, 108] vs 55 [SD, 45]), 14 (23.3%) returned to their baseline dose, and 3 (5%) increased from their baseline dose. Of the 153 patients who were NOUs before surgery, only 1 (0.7%) was taking opioids after 90 days. TPS continued to work closely with the patient and their PCP and that patient was finally able to stop opioid use 262 days after discharge. Ten patients had an additional surgery within 90 days of the initial surgery. Of these, 6 were COU, of whom 3 stopped all opioids by 90 days from their original surgery, 2 had no change in MEDD at 90 days, and 1 had a lower MEDD at 90 days. Of the 4 NOU who had additional surgery, all were off opioids by 90 days from the original surgery.
Although difficult to quantify, a meaningful outcome of TPS has been to improve satisfaction substantially among health care providers caring for complex patients at risk for chronic opioid abuse. This group includes the many members of the surgical team, PCPs, and addiction specialists who appreciate the close care coordination and assistance in caring for patients with difficult issues, especially with opioid tapers or SUDs. We also have noticed changes in prescribing practices among surgeons and PCPs for their patients who are not part of TPS.
Discussion
With any new clinical service, there are obstacles and challenges. TPS requires a considerable investment in personnel, and currently no mechanism is in place for obtaining payment for many of the provided services. We were fortunate the VA Whole Health Initiative, the VA Office of Rural Health, and the VA Centers of Innovation provided support for the development, implementation, and pilot evaluation of TPS. After we presented our initial results to hospital leadership, we also received hospital support to expand TPS service to include a total of 4 nurse care coordinators and 2 psychologists. We are currently performing a cost analysis of the service but recognize that this model may be difficult to reproduce at other institutions without a change in reimbursement standards.
Developing a working relationship with the surgical and primary care services required a concerted effort from the TPS team and a number of months to become effective. As most veterans receive primary care, mental health care, and surgical care within the VA system, this model lends itself to close care coordination. Initially there was skepticism about TPS recommendations to reduce opioid use, especially from PCPs who had cared for complex patients over many years. But this uncertainty went away as we showed evidence of close patient follow-up and detailed communication. TPS soon became the designated service for both primary care and surgical providers who were otherwise uncomfortable with how to approach opioid tapers and nonopioid pain strategies. In fact, a substantial portion of our referrals now come directly from the PCP who is referring a high-risk patient for evaluation for surgery rather than from the surgeons, and joint visits with TPS and primary care have become commonplace.
Challenges abound when working with patients with substance abuse history, opioid use history, high anxiety, significant pain catastrophizing, and those who have had previous negative experiences with surgery. We have found that the most important facet of our service comes from the amount of time and effort team members, especially the nurses, spend helping patients. Much of the nurses' work focuses on nonpain-related issues, such as assisting patients with finding transportation, housing issues, questions about medications, help scheduling appointments, etc. Through this concerted effort, patients gain trust in TPS providers and are willing to listen to and experiment with our recommendations. Many patients who were initially extremely unreceptive to the presurgery education asked for our support weeks after surgery to help with postsurgery pain.
Another challenge we continue to experience comes from the success of the program.
Conclusions
The multidisciplinary TPS supports greater preoperative to postoperative longitudinal care for surgical patients. This endeavor has resulted in better patient preparation before surgery and improved care coordination after surgery, with specific improvements in appropriate use of opioid medications and smooth transitions of care for patients with ongoing and complex needs. Development of sophisticated note templates and customized health information technology allows for accurate follow-through and data gathering for quality improvement, facilitating data-driven improvements and proving value to the facility.
Given that TPS is a multidisciplinary program with multiple interventions, it is difficult to pinpoint which specific aspects of TPS are most effective in achieving success. For example, although we have little doubt that the work our psychologists do with our patients is beneficial and even essential for the success we have had with some of our most difficult patients, it is less clear whether it matters if they use mindfulness, ACT matrix, or cognitive behavioral therapy. We think that an important part of TPS is the frequent human interaction with a caring individual. Therefore, as TPS continues to grow, maintaining the ability to provide frequent personal interaction is a priority.
The role of opioids in acute pain deserves further scrutiny. In 2018, with TPS use of opioids after orthopedic surgery decreased by > 40% from the previous year. Despite this more restricted use of opioids, pain interference and physical function scores indicated that surgical patients do not seem to experience increased pain or reduced physical function. In addition, stopping opioid use for COUs did not seem to affect the quality of recovery, pain, or physical function. Future prospective controlled studies of TPS are needed to confirm these findings and identify which aspects of TPS are most effective in improving functional recovery of patients. Also, more evidence is needed to determine the appropriateness or need for opioids in acute postsurgical pain.
TPS has expanded to include all surgical specialties. Given the high burden and limited resources, we have chosen to focus on patients at higher risk for chronic postsurgical pain by type of surgery (eg, thoracotomy, open abdominal, limb amputation, major joint surgery) and/or history of substance abuse or chronic opioid use. To better direct scarce resources where it would be of most benefit, we are now enrolling only NOUs without other risk factors postoperatively if they request a refill of opioids or are otherwise struggling with pain control after surgery. Whether this approach affects the success we had in the first year in preventing new COUs after surgery remains to be seen.
It is unlikely that any single model of a perioperative surgical home will fit the needs of the many different types of medical systems that exist. The TPS model fits well in large hospital systems, like the VA, where patients receive most of their care within the same system. However, it seems to us that the optimal TPS program in any health system will provide education, support, and care coordination beginning preoperatively to prepare the patient for surgery and then to facilitate care coordination to transition patients back to their PCPs or on to specialized chronic care.
Acknowledgments
We would like to acknowledge the contributions of Candice Harmon, RN; David Merrill, RN; Amy Beckstead, RN, who have provided invaluable assistance with establishing the TPS program at the VA Salt Lake City and helping with the evaluation process.
Funding for the implementation and evaluation of the TPS was received from the VA Whole Health Initiative, the VA Center of Innovation, the VA Office of Rural Health, and National Institutes of Health Grant UL1TR002538.
1. Ilfeld BM, Madison SJ, Suresh PJ. Persistent postmastectomy pain and pain-related physical and emotional functioning with and without a continuous paravertebral nerve block: a prospective 1-year follow-up assessment of a randomized, triple-masked, placebo-controlled study. Ann Surg Oncol. 2015;22(6):2017-2025. doi:10.1245/s10434-014-4248-7
2. Richebé P, Capdevila X, Rivat C. Persistent postsurgical pain. Anesthesiology. 2018;129(3):590-607. doi:10.1097/aln.0000000000002238
3. Glare P, Aubrey KR, Myles PS. Transition from acute to chronic pain after surgery. Lancet. 2019;393(10180):1537-1546. doi:10.1016/s0140-6736(19)30352-6
4. Brummett CM, Waljee JF, Goesling J, et al. New persistent opioid use after minor and major surgical procedures in US adults. JAMA Surgery. 2017;152(6):e170504-e170504. doi:10.1001/jamasurg.2017.0504
5. Swenson CW, Kamdar NS, Seiler K, Morgan DM, Lin P, As-Sanie S. Definition development and prevalence of new persistent opioid use following hysterectomy. Am J Obstet Gynecol. 2018;219(5):486.e1-486.e7. doi:10.1016/j.ajog.2018.06.010
6. Bartels K, Fernandez-Bustamante A, McWilliams SK, Hopfer CJ, Mikulich-Gilbertson SK. Long-term opioid use after inpatient surgery - a retrospective cohort study. Drug Alcohol Depend. 2018;187:61-65. doi:10.1016/j.drugalcdep.2018.02.013
7. Bedard N, DeMik D, Dowdle S, Callaghan J. Trends and risk factors for prolonged opioid use after unicompartmental knee arthroplasty. Bone Joint J. 2018;100-B(1)(suppl A):62-67. doi:10.1302/0301-620x.100b1.bjj-2017-0547.r1
8. Politzer CS, Kildow BJ, Goltz DE, Green CL, Bolognesi MP, Seyler T. Trends in opioid utilization before and after total knee arthroplasty. J Arthroplasty. 2018;33(7S):S147-S153.e1. doi:10.1016/j.arth.2017.10.060
9. Mariano ER, Walters TL, Kim ET, Kain ZN. Why the perioperative surgical home makes sense for Veterans Affairs health care. Anesth Analg. 2015;120(5):1163-1166. doi:10.1213/ane.0000000000000712
10. Walters TL, Howard SK, Kou A, et al. Design and implementation of a perioperative surgical home at a Veterans Affairs hospital. Semin Cardiothorac Vasc Anesth. 2016;20(2):133-140. doi:10.1177/1089253215607066
11. Walters TL, Mariano ER, Clark DJ. Perioperative surgical home and the integral role of pain medicine. Pain Med. 2015;16(9):1666-1672. doi:10.1111/pme.12796
12. Vetter TR, Kain ZN. Role of the perioperative surgical home in optimizing the perioperative use of opioids. Anesth Analg. 2017;125(5):1653-1657. doi:10.1213/ane.0000000000002280
13. Shafer SL. Anesthesia & Analgesia’s 2015 collection on the perioperative surgical home. Anesth Analg. 2015;120(5):966-967. doi:10.1213/ane.0000000000000696
14. Wenzel JT, Schwenk ES, Baratta JL, Viscusi ER. Managing opioid-tolerant patients in the perioperative surgical home. Anesthesiol Clin. 2016;34(2):287-301. doi:10.1016/j.anclin.2016.01.005
15. Katz J, Weinrib A, Fashler SR, et al. The Toronto General Hospital Transitional Pain Service: development and implementation of a multidisciplinary program to prevent chronic postsurgical pain. J Pain Res. 2015;8:695-702. doi:10.2147/jpr.s91924
16. Tiippana E, Hamunen K, Heiskanen T, Nieminen T, Kalso E, Kontinen VK. New approach for treatment of prolonged postoperative pain: APS Out-Patient Clinic. Scand J Pain. 2016;12(1):19-24. doi:10.1016/j.sjpain.2016.02.008
17. Katz J, Weinrib AZ, Clarke H. Chronic postsurgical pain: from risk factor identification to multidisciplinary management at the Toronto General Hospital Transitional Pain Service. Can J Pain. 2019;3(2):49-58. doi:10.1080/24740527.2019.1574537
18. Sullivan MJ, Bishop SR, Pivik J. The Pain Catastrophizing Scale: development and validation. Psychol Assess. 1995;7(4):524-532. doi:10.1037/1040-3590.7.4.524
19. HealthMeasures. Intro to PROMIS. https://www.healthmeasures.net/explore-measurement-systems/promis. Accessed September 28, 2020.
Despite advancements in techniques, postsurgical pain continues to be a prominent part of the patient experience. Often this experience can lead to developing chronic postsurgical pain that interferes with quality of life after the expected time to recovery.1-3 As many as 14% of patients who undergo surgery without any history of opioid use develop chronic opioid use that persists after recovery from their operation.4-8 For patients with existing chronic opioid use or a history of substance use disorder (SUD), surgeons, primary care providers, or addiction providers often do not provide sufficient presurgical planning or postsurgical coordination of care. This lack of pain care coordination can increase the risk of inadequate pain control, opioid use escalation, or SUD relapse after surgery.
Convincing arguments have been made that a perioperative surgical home can improve significantly the quality of perioperative care.9-14 This report describes our experience implementing a perioperative surgical home at the US Department of Veterans Affairs (VA) Salt Lake City VA Medical Center (SLCVAMC), focusing on pain management extending from the preoperative period until 6 months or more after surgery. This type of Transitional Pain Service (TPS) has been described previously.15-17 Our service differs from those described previously by enrolling all patients before surgery rather than select postsurgical enrollment of only patients with a history of opioid use or SUD or patients who struggle with persistent postsurgical pain.
Methods
In January 2018, we developed and implemented a new TPS at the SLCVAMC. The transitional pain team consisted of an anesthesiologist with specialization in acute pain management, a nurse practitioner (NP) with experience in both acute and chronic pain management, 2 nurse care coordinators, and a psychologist (Figure 1). Before implementation, a needs assessment took place with these key stakeholders and others at SLCVAMC to identify the following specific goals of the TPS: (1) reduce pain through pharmacologic and nonpharmacologic interventions; (2) eliminate new chronic opioid use in previously nonopioid user (NOU) patients; (3) address chronic opioid use in previous chronic opioid users (COUs) by providing support for opioid taper and alternative analgesic therapies for their chronic pain conditions; and (4) improve continuity of care by close coordination with the surgical team, primary care providers (PCPs), and mental health or chronic pain providers as needed.
Once these TPS goals were defined, the Consolidated Framework for Implementation Research (CFIR) guided the implementation. CFIR is a theory-based implementation framework consisting of 5 domains: intervention characteristics, inner setting, outer setting, characteristics of individuals, and process. These domains were used to identify barriers and facilitators during the early implementation process and helped refine TPS as it was put into clinical practice.
Patient Selection
During the initial implementation of TPS, enrollment was limited to patients scheduled for elective primary or revision knee, hip, or shoulder replacement as well as rotator cuff repair surgery. But as the TPS workflow became established after iterative refinement, we expanded the program to enroll patients with established risk factors for OUD having other types of surgery (Table 1). The diagnosis of risk factors, such as history of SUD, chronic opioid use, or significant mental health disorders (ie, history of suicidal ideation or attempt, posttraumatic stress disorder, and inpatient psychiatric care) were confirmed through both in-person interviews and electronic health record (EHR) documentation. The overall goal was to identify all at-risk patients as soon as they were indicated for surgery, to allow time for evaluation, education, developing an individualized pain plan, and opioid taper prior to surgery if indicated.
Preoperative Procedures
Once identified, patients were contacted by a TPS team member and invited to attend a onetime 90-minute presurgical expectations class held at SLCVAMC. The education curriculum was developed by the whole team, and classes were taught primarily by the TPS psychologist. The class included education about expectations for postoperative pain, available analgesic therapies, opioid education, appropriate use of opioids, and the effect of psychological factors on pain. Pain coping strategies were introduced using a mindfulness-based intervention (MBI) and the Acceptance and Commitment Therapy (ACT) matrix. Classes were offered multiple times a week to help maximize convenience for patients and were separate from the anesthesia preoperative evaluation. Patients attended class only once. High-risk patients (patients with chronic opioid therapy, recent history of or current SUDs, significant comorbid mental health issues) were encouraged to attend this class one-on-one with the TPS psychologist rather than in the group setting, so individual attention to mental health and SUD issues could be addressed directly.
Baseline history, morphine equivalent daily dose (MEDD), and patient-reported outcomes using measures from the Patient-Reported Outcome Measurement System (PROMIS) for pain intensity (PROMIS 3a), pain interference (PROMIS 6b), and physical function (PROMIS 8b), and a pain-catastrophizing scale (PCS) score were obtained on all patients.18 PROMIS measures are validated questionnaires developed with the National Institutes of Health to standardize and quantify patient-reported outcomes in many domains.19 Patients with a history of SUD or COU met with the anesthesiologist and/or NP, and a personalized pain plan was developed that included preoperative opioid taper, buprenorphine use strategy, or opioid-free strategies.
Hospital Procedures
On the day of surgery, the TPS team met with the patient preoperatively and implemented an individualized pain plan that included multimodal analgesic techniques with nonsteroidal anti-inflammatory drugs, acetaminophen, gabapentinoids, and regional anesthesia, where appropriate (Table 2). Enhanced recovery after surgery protocols were developed in conjunction with the surgeons to include local infiltration analgesia by the surgeon, postoperative multimodal analgesic strategies, and intensive physical therapy starting the day of surgery for inpatient procedures.
After surgery, the TPS team followed up with patients daily and provided recommendations for analgesic therapies. Patients were offered daily sessions with the psychologist to reinforce and practice nonpharmacologic pain-coping strategies, such as meditation and relaxation. Prior to patient discharge, the TPS team provided recommendations for discharge medications and an opioid taper plan. For some patients taking buprenorphine before surgery who had stopped this therapy prior to or during their hospital stay, TPS providers transitioned them back to buprenorphine before discharge.
Postoperative Procedures
Patients were called by the nurse care coordinators at postdischarge days 2, 7, 10, 14, 21, 28, and then monthly for ≥ 6 months. For patients who had not stopped opioid use or returned to their preoperative baseline opioid dose, weekly calls were made until opioid taper goals were achieved. At each call, nurses collected PROMIS scores for the previous 24 hours, the most recent 24-hour MEDD, the date of last opioid use, and the number of remaining opioid tablets after opioid cessation. In addition, nurses provided active listening and supportive care and encouragement as well as care coordination for issues related to rehabilitation facilities, physical therapy, transportation, medication questions, and wound questions. Nurses notified the anesthesiologist or NP when patients were unable to taper opioid use or had poor pain control as indicated by their PROMIS scores, opioid use, or directly expressed by the patient.
The TPS team prescribed alternative analgesic therapies, opioid taper plans, and communicated with surgeons and primary care providers if limited continued opioid therapy was recommended. Individual sessions with the psychologist were available to patients after discharge with a focus on ACT-matrix therapy and consultation with long-term mental health and/or substance abuse providers as indicated. Frequent communication and care coordination were maintained with the surgical team, the PCP, and other providers on the mental health or chronic pain services. This care coordination often included postsurgical joint clinic appointments in which TPS providers and nurses would be present with the surgeon or the PCP.
For patients with inadequately treated chronic pain conditions or who required long-term opioid tapers, we developed a combined clinic with the TPS and Anesthesia Chronic Pain group. This clinic allows patients to be seen by both services in the same setting, allowing a warm handoff by TPS to the chronic pain team.
Heath and Decision Support Tools
An electronic dashboard registry of surgical episodes managed by TPS was developed to achieve clinical, administrative, and quality improvement goals. The dashboard registry consists of surgical episode data, opioid doses, patient-reported outcomes, and clinical decision-making processes. Custom-built note templates capture pertinent data through embedded data labels, called health factors. Data are captured as part of routine clinical care, recorded in Computerized Patient Record System as health factors. They are available in the VA Corporate Data Warehouse as structured data. Workflows are executed daily to keep the dashboard registry current, clean, and able to process new data. Information displays direct daily clinical workflow and support point-of-care clinical decision making (Figures 2, 3, and 4). Data are aggregated across patient-care encounters and allow nurse care coordinators to concisely review pertinent patient data prior to delivering care. These data include surgical history, comorbidities, timeline of opioid use, and PROMIS scores during their course of recovery. This system allows TPS to optimize care delivery by providing longitudinal data across the surgical episode, thereby reducing the time needed to review records. Secondary purposes of captured data include measuring clinic performance and quality improvement to improve care delivery.
Results
The TPS intervention was implemented January 1, 2018. Two-hundred thirteen patients were enrolled between January and December 2018, which included 60 (28%) patients with a history of chronic opioid use and 153 (72%) patients who were considered opioid naïve. A total of 99% of patients had ≥ 1 successful follow-up within 14 days after discharge, 96% had ≥ 1 follow-up between 14 and 30 days after surgery, and 72% had completed personal follow-up 90 days after discharge (Table 3). For patients who TPS was unable to contact in person or by phone, 90-day MEDD was obtained using prescription and Controlled Substance Database reviews. The protocol for this retrospective analysis was approved by the University of Utah Institutional Review Board and the VA Research Review Committee.
By 90 days after surgery, 26 (43.3%) COUs were off opioids completely, 17 (28.3%) had decreased their opioid dose from their preoperative baseline MEDD (120 [SD, 108] vs 55 [SD, 45]), 14 (23.3%) returned to their baseline dose, and 3 (5%) increased from their baseline dose. Of the 153 patients who were NOUs before surgery, only 1 (0.7%) was taking opioids after 90 days. TPS continued to work closely with the patient and their PCP and that patient was finally able to stop opioid use 262 days after discharge. Ten patients had an additional surgery within 90 days of the initial surgery. Of these, 6 were COU, of whom 3 stopped all opioids by 90 days from their original surgery, 2 had no change in MEDD at 90 days, and 1 had a lower MEDD at 90 days. Of the 4 NOU who had additional surgery, all were off opioids by 90 days from the original surgery.
Although difficult to quantify, a meaningful outcome of TPS has been to improve satisfaction substantially among health care providers caring for complex patients at risk for chronic opioid abuse. This group includes the many members of the surgical team, PCPs, and addiction specialists who appreciate the close care coordination and assistance in caring for patients with difficult issues, especially with opioid tapers or SUDs. We also have noticed changes in prescribing practices among surgeons and PCPs for their patients who are not part of TPS.
Discussion
With any new clinical service, there are obstacles and challenges. TPS requires a considerable investment in personnel, and currently no mechanism is in place for obtaining payment for many of the provided services. We were fortunate the VA Whole Health Initiative, the VA Office of Rural Health, and the VA Centers of Innovation provided support for the development, implementation, and pilot evaluation of TPS. After we presented our initial results to hospital leadership, we also received hospital support to expand TPS service to include a total of 4 nurse care coordinators and 2 psychologists. We are currently performing a cost analysis of the service but recognize that this model may be difficult to reproduce at other institutions without a change in reimbursement standards.
Developing a working relationship with the surgical and primary care services required a concerted effort from the TPS team and a number of months to become effective. As most veterans receive primary care, mental health care, and surgical care within the VA system, this model lends itself to close care coordination. Initially there was skepticism about TPS recommendations to reduce opioid use, especially from PCPs who had cared for complex patients over many years. But this uncertainty went away as we showed evidence of close patient follow-up and detailed communication. TPS soon became the designated service for both primary care and surgical providers who were otherwise uncomfortable with how to approach opioid tapers and nonopioid pain strategies. In fact, a substantial portion of our referrals now come directly from the PCP who is referring a high-risk patient for evaluation for surgery rather than from the surgeons, and joint visits with TPS and primary care have become commonplace.
Challenges abound when working with patients with substance abuse history, opioid use history, high anxiety, significant pain catastrophizing, and those who have had previous negative experiences with surgery. We have found that the most important facet of our service comes from the amount of time and effort team members, especially the nurses, spend helping patients. Much of the nurses' work focuses on nonpain-related issues, such as assisting patients with finding transportation, housing issues, questions about medications, help scheduling appointments, etc. Through this concerted effort, patients gain trust in TPS providers and are willing to listen to and experiment with our recommendations. Many patients who were initially extremely unreceptive to the presurgery education asked for our support weeks after surgery to help with postsurgery pain.
Another challenge we continue to experience comes from the success of the program.
Conclusions
The multidisciplinary TPS supports greater preoperative to postoperative longitudinal care for surgical patients. This endeavor has resulted in better patient preparation before surgery and improved care coordination after surgery, with specific improvements in appropriate use of opioid medications and smooth transitions of care for patients with ongoing and complex needs. Development of sophisticated note templates and customized health information technology allows for accurate follow-through and data gathering for quality improvement, facilitating data-driven improvements and proving value to the facility.
Given that TPS is a multidisciplinary program with multiple interventions, it is difficult to pinpoint which specific aspects of TPS are most effective in achieving success. For example, although we have little doubt that the work our psychologists do with our patients is beneficial and even essential for the success we have had with some of our most difficult patients, it is less clear whether it matters if they use mindfulness, ACT matrix, or cognitive behavioral therapy. We think that an important part of TPS is the frequent human interaction with a caring individual. Therefore, as TPS continues to grow, maintaining the ability to provide frequent personal interaction is a priority.
The role of opioids in acute pain deserves further scrutiny. In 2018, with TPS use of opioids after orthopedic surgery decreased by > 40% from the previous year. Despite this more restricted use of opioids, pain interference and physical function scores indicated that surgical patients do not seem to experience increased pain or reduced physical function. In addition, stopping opioid use for COUs did not seem to affect the quality of recovery, pain, or physical function. Future prospective controlled studies of TPS are needed to confirm these findings and identify which aspects of TPS are most effective in improving functional recovery of patients. Also, more evidence is needed to determine the appropriateness or need for opioids in acute postsurgical pain.
TPS has expanded to include all surgical specialties. Given the high burden and limited resources, we have chosen to focus on patients at higher risk for chronic postsurgical pain by type of surgery (eg, thoracotomy, open abdominal, limb amputation, major joint surgery) and/or history of substance abuse or chronic opioid use. To better direct scarce resources where it would be of most benefit, we are now enrolling only NOUs without other risk factors postoperatively if they request a refill of opioids or are otherwise struggling with pain control after surgery. Whether this approach affects the success we had in the first year in preventing new COUs after surgery remains to be seen.
It is unlikely that any single model of a perioperative surgical home will fit the needs of the many different types of medical systems that exist. The TPS model fits well in large hospital systems, like the VA, where patients receive most of their care within the same system. However, it seems to us that the optimal TPS program in any health system will provide education, support, and care coordination beginning preoperatively to prepare the patient for surgery and then to facilitate care coordination to transition patients back to their PCPs or on to specialized chronic care.
Acknowledgments
We would like to acknowledge the contributions of Candice Harmon, RN; David Merrill, RN; Amy Beckstead, RN, who have provided invaluable assistance with establishing the TPS program at the VA Salt Lake City and helping with the evaluation process.
Funding for the implementation and evaluation of the TPS was received from the VA Whole Health Initiative, the VA Center of Innovation, the VA Office of Rural Health, and National Institutes of Health Grant UL1TR002538.
Despite advancements in techniques, postsurgical pain continues to be a prominent part of the patient experience. Often this experience can lead to developing chronic postsurgical pain that interferes with quality of life after the expected time to recovery.1-3 As many as 14% of patients who undergo surgery without any history of opioid use develop chronic opioid use that persists after recovery from their operation.4-8 For patients with existing chronic opioid use or a history of substance use disorder (SUD), surgeons, primary care providers, or addiction providers often do not provide sufficient presurgical planning or postsurgical coordination of care. This lack of pain care coordination can increase the risk of inadequate pain control, opioid use escalation, or SUD relapse after surgery.
Convincing arguments have been made that a perioperative surgical home can improve significantly the quality of perioperative care.9-14 This report describes our experience implementing a perioperative surgical home at the US Department of Veterans Affairs (VA) Salt Lake City VA Medical Center (SLCVAMC), focusing on pain management extending from the preoperative period until 6 months or more after surgery. This type of Transitional Pain Service (TPS) has been described previously.15-17 Our service differs from those described previously by enrolling all patients before surgery rather than select postsurgical enrollment of only patients with a history of opioid use or SUD or patients who struggle with persistent postsurgical pain.
Methods
In January 2018, we developed and implemented a new TPS at the SLCVAMC. The transitional pain team consisted of an anesthesiologist with specialization in acute pain management, a nurse practitioner (NP) with experience in both acute and chronic pain management, 2 nurse care coordinators, and a psychologist (Figure 1). Before implementation, a needs assessment took place with these key stakeholders and others at SLCVAMC to identify the following specific goals of the TPS: (1) reduce pain through pharmacologic and nonpharmacologic interventions; (2) eliminate new chronic opioid use in previously nonopioid user (NOU) patients; (3) address chronic opioid use in previous chronic opioid users (COUs) by providing support for opioid taper and alternative analgesic therapies for their chronic pain conditions; and (4) improve continuity of care by close coordination with the surgical team, primary care providers (PCPs), and mental health or chronic pain providers as needed.
Once these TPS goals were defined, the Consolidated Framework for Implementation Research (CFIR) guided the implementation. CFIR is a theory-based implementation framework consisting of 5 domains: intervention characteristics, inner setting, outer setting, characteristics of individuals, and process. These domains were used to identify barriers and facilitators during the early implementation process and helped refine TPS as it was put into clinical practice.
Patient Selection
During the initial implementation of TPS, enrollment was limited to patients scheduled for elective primary or revision knee, hip, or shoulder replacement as well as rotator cuff repair surgery. But as the TPS workflow became established after iterative refinement, we expanded the program to enroll patients with established risk factors for OUD having other types of surgery (Table 1). The diagnosis of risk factors, such as history of SUD, chronic opioid use, or significant mental health disorders (ie, history of suicidal ideation or attempt, posttraumatic stress disorder, and inpatient psychiatric care) were confirmed through both in-person interviews and electronic health record (EHR) documentation. The overall goal was to identify all at-risk patients as soon as they were indicated for surgery, to allow time for evaluation, education, developing an individualized pain plan, and opioid taper prior to surgery if indicated.
Preoperative Procedures
Once identified, patients were contacted by a TPS team member and invited to attend a onetime 90-minute presurgical expectations class held at SLCVAMC. The education curriculum was developed by the whole team, and classes were taught primarily by the TPS psychologist. The class included education about expectations for postoperative pain, available analgesic therapies, opioid education, appropriate use of opioids, and the effect of psychological factors on pain. Pain coping strategies were introduced using a mindfulness-based intervention (MBI) and the Acceptance and Commitment Therapy (ACT) matrix. Classes were offered multiple times a week to help maximize convenience for patients and were separate from the anesthesia preoperative evaluation. Patients attended class only once. High-risk patients (patients with chronic opioid therapy, recent history of or current SUDs, significant comorbid mental health issues) were encouraged to attend this class one-on-one with the TPS psychologist rather than in the group setting, so individual attention to mental health and SUD issues could be addressed directly.
Baseline history, morphine equivalent daily dose (MEDD), and patient-reported outcomes using measures from the Patient-Reported Outcome Measurement System (PROMIS) for pain intensity (PROMIS 3a), pain interference (PROMIS 6b), and physical function (PROMIS 8b), and a pain-catastrophizing scale (PCS) score were obtained on all patients.18 PROMIS measures are validated questionnaires developed with the National Institutes of Health to standardize and quantify patient-reported outcomes in many domains.19 Patients with a history of SUD or COU met with the anesthesiologist and/or NP, and a personalized pain plan was developed that included preoperative opioid taper, buprenorphine use strategy, or opioid-free strategies.
Hospital Procedures
On the day of surgery, the TPS team met with the patient preoperatively and implemented an individualized pain plan that included multimodal analgesic techniques with nonsteroidal anti-inflammatory drugs, acetaminophen, gabapentinoids, and regional anesthesia, where appropriate (Table 2). Enhanced recovery after surgery protocols were developed in conjunction with the surgeons to include local infiltration analgesia by the surgeon, postoperative multimodal analgesic strategies, and intensive physical therapy starting the day of surgery for inpatient procedures.
After surgery, the TPS team followed up with patients daily and provided recommendations for analgesic therapies. Patients were offered daily sessions with the psychologist to reinforce and practice nonpharmacologic pain-coping strategies, such as meditation and relaxation. Prior to patient discharge, the TPS team provided recommendations for discharge medications and an opioid taper plan. For some patients taking buprenorphine before surgery who had stopped this therapy prior to or during their hospital stay, TPS providers transitioned them back to buprenorphine before discharge.
Postoperative Procedures
Patients were called by the nurse care coordinators at postdischarge days 2, 7, 10, 14, 21, 28, and then monthly for ≥ 6 months. For patients who had not stopped opioid use or returned to their preoperative baseline opioid dose, weekly calls were made until opioid taper goals were achieved. At each call, nurses collected PROMIS scores for the previous 24 hours, the most recent 24-hour MEDD, the date of last opioid use, and the number of remaining opioid tablets after opioid cessation. In addition, nurses provided active listening and supportive care and encouragement as well as care coordination for issues related to rehabilitation facilities, physical therapy, transportation, medication questions, and wound questions. Nurses notified the anesthesiologist or NP when patients were unable to taper opioid use or had poor pain control as indicated by their PROMIS scores, opioid use, or directly expressed by the patient.
The TPS team prescribed alternative analgesic therapies, opioid taper plans, and communicated with surgeons and primary care providers if limited continued opioid therapy was recommended. Individual sessions with the psychologist were available to patients after discharge with a focus on ACT-matrix therapy and consultation with long-term mental health and/or substance abuse providers as indicated. Frequent communication and care coordination were maintained with the surgical team, the PCP, and other providers on the mental health or chronic pain services. This care coordination often included postsurgical joint clinic appointments in which TPS providers and nurses would be present with the surgeon or the PCP.
For patients with inadequately treated chronic pain conditions or who required long-term opioid tapers, we developed a combined clinic with the TPS and Anesthesia Chronic Pain group. This clinic allows patients to be seen by both services in the same setting, allowing a warm handoff by TPS to the chronic pain team.
Heath and Decision Support Tools
An electronic dashboard registry of surgical episodes managed by TPS was developed to achieve clinical, administrative, and quality improvement goals. The dashboard registry consists of surgical episode data, opioid doses, patient-reported outcomes, and clinical decision-making processes. Custom-built note templates capture pertinent data through embedded data labels, called health factors. Data are captured as part of routine clinical care, recorded in Computerized Patient Record System as health factors. They are available in the VA Corporate Data Warehouse as structured data. Workflows are executed daily to keep the dashboard registry current, clean, and able to process new data. Information displays direct daily clinical workflow and support point-of-care clinical decision making (Figures 2, 3, and 4). Data are aggregated across patient-care encounters and allow nurse care coordinators to concisely review pertinent patient data prior to delivering care. These data include surgical history, comorbidities, timeline of opioid use, and PROMIS scores during their course of recovery. This system allows TPS to optimize care delivery by providing longitudinal data across the surgical episode, thereby reducing the time needed to review records. Secondary purposes of captured data include measuring clinic performance and quality improvement to improve care delivery.
Results
The TPS intervention was implemented January 1, 2018. Two-hundred thirteen patients were enrolled between January and December 2018, which included 60 (28%) patients with a history of chronic opioid use and 153 (72%) patients who were considered opioid naïve. A total of 99% of patients had ≥ 1 successful follow-up within 14 days after discharge, 96% had ≥ 1 follow-up between 14 and 30 days after surgery, and 72% had completed personal follow-up 90 days after discharge (Table 3). For patients who TPS was unable to contact in person or by phone, 90-day MEDD was obtained using prescription and Controlled Substance Database reviews. The protocol for this retrospective analysis was approved by the University of Utah Institutional Review Board and the VA Research Review Committee.
By 90 days after surgery, 26 (43.3%) COUs were off opioids completely, 17 (28.3%) had decreased their opioid dose from their preoperative baseline MEDD (120 [SD, 108] vs 55 [SD, 45]), 14 (23.3%) returned to their baseline dose, and 3 (5%) increased from their baseline dose. Of the 153 patients who were NOUs before surgery, only 1 (0.7%) was taking opioids after 90 days. TPS continued to work closely with the patient and their PCP and that patient was finally able to stop opioid use 262 days after discharge. Ten patients had an additional surgery within 90 days of the initial surgery. Of these, 6 were COU, of whom 3 stopped all opioids by 90 days from their original surgery, 2 had no change in MEDD at 90 days, and 1 had a lower MEDD at 90 days. Of the 4 NOU who had additional surgery, all were off opioids by 90 days from the original surgery.
Although difficult to quantify, a meaningful outcome of TPS has been to improve satisfaction substantially among health care providers caring for complex patients at risk for chronic opioid abuse. This group includes the many members of the surgical team, PCPs, and addiction specialists who appreciate the close care coordination and assistance in caring for patients with difficult issues, especially with opioid tapers or SUDs. We also have noticed changes in prescribing practices among surgeons and PCPs for their patients who are not part of TPS.
Discussion
With any new clinical service, there are obstacles and challenges. TPS requires a considerable investment in personnel, and currently no mechanism is in place for obtaining payment for many of the provided services. We were fortunate the VA Whole Health Initiative, the VA Office of Rural Health, and the VA Centers of Innovation provided support for the development, implementation, and pilot evaluation of TPS. After we presented our initial results to hospital leadership, we also received hospital support to expand TPS service to include a total of 4 nurse care coordinators and 2 psychologists. We are currently performing a cost analysis of the service but recognize that this model may be difficult to reproduce at other institutions without a change in reimbursement standards.
Developing a working relationship with the surgical and primary care services required a concerted effort from the TPS team and a number of months to become effective. As most veterans receive primary care, mental health care, and surgical care within the VA system, this model lends itself to close care coordination. Initially there was skepticism about TPS recommendations to reduce opioid use, especially from PCPs who had cared for complex patients over many years. But this uncertainty went away as we showed evidence of close patient follow-up and detailed communication. TPS soon became the designated service for both primary care and surgical providers who were otherwise uncomfortable with how to approach opioid tapers and nonopioid pain strategies. In fact, a substantial portion of our referrals now come directly from the PCP who is referring a high-risk patient for evaluation for surgery rather than from the surgeons, and joint visits with TPS and primary care have become commonplace.
Challenges abound when working with patients with substance abuse history, opioid use history, high anxiety, significant pain catastrophizing, and those who have had previous negative experiences with surgery. We have found that the most important facet of our service comes from the amount of time and effort team members, especially the nurses, spend helping patients. Much of the nurses' work focuses on nonpain-related issues, such as assisting patients with finding transportation, housing issues, questions about medications, help scheduling appointments, etc. Through this concerted effort, patients gain trust in TPS providers and are willing to listen to and experiment with our recommendations. Many patients who were initially extremely unreceptive to the presurgery education asked for our support weeks after surgery to help with postsurgery pain.
Another challenge we continue to experience comes from the success of the program.
Conclusions
The multidisciplinary TPS supports greater preoperative to postoperative longitudinal care for surgical patients. This endeavor has resulted in better patient preparation before surgery and improved care coordination after surgery, with specific improvements in appropriate use of opioid medications and smooth transitions of care for patients with ongoing and complex needs. Development of sophisticated note templates and customized health information technology allows for accurate follow-through and data gathering for quality improvement, facilitating data-driven improvements and proving value to the facility.
Given that TPS is a multidisciplinary program with multiple interventions, it is difficult to pinpoint which specific aspects of TPS are most effective in achieving success. For example, although we have little doubt that the work our psychologists do with our patients is beneficial and even essential for the success we have had with some of our most difficult patients, it is less clear whether it matters if they use mindfulness, ACT matrix, or cognitive behavioral therapy. We think that an important part of TPS is the frequent human interaction with a caring individual. Therefore, as TPS continues to grow, maintaining the ability to provide frequent personal interaction is a priority.
The role of opioids in acute pain deserves further scrutiny. In 2018, with TPS use of opioids after orthopedic surgery decreased by > 40% from the previous year. Despite this more restricted use of opioids, pain interference and physical function scores indicated that surgical patients do not seem to experience increased pain or reduced physical function. In addition, stopping opioid use for COUs did not seem to affect the quality of recovery, pain, or physical function. Future prospective controlled studies of TPS are needed to confirm these findings and identify which aspects of TPS are most effective in improving functional recovery of patients. Also, more evidence is needed to determine the appropriateness or need for opioids in acute postsurgical pain.
TPS has expanded to include all surgical specialties. Given the high burden and limited resources, we have chosen to focus on patients at higher risk for chronic postsurgical pain by type of surgery (eg, thoracotomy, open abdominal, limb amputation, major joint surgery) and/or history of substance abuse or chronic opioid use. To better direct scarce resources where it would be of most benefit, we are now enrolling only NOUs without other risk factors postoperatively if they request a refill of opioids or are otherwise struggling with pain control after surgery. Whether this approach affects the success we had in the first year in preventing new COUs after surgery remains to be seen.
It is unlikely that any single model of a perioperative surgical home will fit the needs of the many different types of medical systems that exist. The TPS model fits well in large hospital systems, like the VA, where patients receive most of their care within the same system. However, it seems to us that the optimal TPS program in any health system will provide education, support, and care coordination beginning preoperatively to prepare the patient for surgery and then to facilitate care coordination to transition patients back to their PCPs or on to specialized chronic care.
Acknowledgments
We would like to acknowledge the contributions of Candice Harmon, RN; David Merrill, RN; Amy Beckstead, RN, who have provided invaluable assistance with establishing the TPS program at the VA Salt Lake City and helping with the evaluation process.
Funding for the implementation and evaluation of the TPS was received from the VA Whole Health Initiative, the VA Center of Innovation, the VA Office of Rural Health, and National Institutes of Health Grant UL1TR002538.
1. Ilfeld BM, Madison SJ, Suresh PJ. Persistent postmastectomy pain and pain-related physical and emotional functioning with and without a continuous paravertebral nerve block: a prospective 1-year follow-up assessment of a randomized, triple-masked, placebo-controlled study. Ann Surg Oncol. 2015;22(6):2017-2025. doi:10.1245/s10434-014-4248-7
2. Richebé P, Capdevila X, Rivat C. Persistent postsurgical pain. Anesthesiology. 2018;129(3):590-607. doi:10.1097/aln.0000000000002238
3. Glare P, Aubrey KR, Myles PS. Transition from acute to chronic pain after surgery. Lancet. 2019;393(10180):1537-1546. doi:10.1016/s0140-6736(19)30352-6
4. Brummett CM, Waljee JF, Goesling J, et al. New persistent opioid use after minor and major surgical procedures in US adults. JAMA Surgery. 2017;152(6):e170504-e170504. doi:10.1001/jamasurg.2017.0504
5. Swenson CW, Kamdar NS, Seiler K, Morgan DM, Lin P, As-Sanie S. Definition development and prevalence of new persistent opioid use following hysterectomy. Am J Obstet Gynecol. 2018;219(5):486.e1-486.e7. doi:10.1016/j.ajog.2018.06.010
6. Bartels K, Fernandez-Bustamante A, McWilliams SK, Hopfer CJ, Mikulich-Gilbertson SK. Long-term opioid use after inpatient surgery - a retrospective cohort study. Drug Alcohol Depend. 2018;187:61-65. doi:10.1016/j.drugalcdep.2018.02.013
7. Bedard N, DeMik D, Dowdle S, Callaghan J. Trends and risk factors for prolonged opioid use after unicompartmental knee arthroplasty. Bone Joint J. 2018;100-B(1)(suppl A):62-67. doi:10.1302/0301-620x.100b1.bjj-2017-0547.r1
8. Politzer CS, Kildow BJ, Goltz DE, Green CL, Bolognesi MP, Seyler T. Trends in opioid utilization before and after total knee arthroplasty. J Arthroplasty. 2018;33(7S):S147-S153.e1. doi:10.1016/j.arth.2017.10.060
9. Mariano ER, Walters TL, Kim ET, Kain ZN. Why the perioperative surgical home makes sense for Veterans Affairs health care. Anesth Analg. 2015;120(5):1163-1166. doi:10.1213/ane.0000000000000712
10. Walters TL, Howard SK, Kou A, et al. Design and implementation of a perioperative surgical home at a Veterans Affairs hospital. Semin Cardiothorac Vasc Anesth. 2016;20(2):133-140. doi:10.1177/1089253215607066
11. Walters TL, Mariano ER, Clark DJ. Perioperative surgical home and the integral role of pain medicine. Pain Med. 2015;16(9):1666-1672. doi:10.1111/pme.12796
12. Vetter TR, Kain ZN. Role of the perioperative surgical home in optimizing the perioperative use of opioids. Anesth Analg. 2017;125(5):1653-1657. doi:10.1213/ane.0000000000002280
13. Shafer SL. Anesthesia & Analgesia’s 2015 collection on the perioperative surgical home. Anesth Analg. 2015;120(5):966-967. doi:10.1213/ane.0000000000000696
14. Wenzel JT, Schwenk ES, Baratta JL, Viscusi ER. Managing opioid-tolerant patients in the perioperative surgical home. Anesthesiol Clin. 2016;34(2):287-301. doi:10.1016/j.anclin.2016.01.005
15. Katz J, Weinrib A, Fashler SR, et al. The Toronto General Hospital Transitional Pain Service: development and implementation of a multidisciplinary program to prevent chronic postsurgical pain. J Pain Res. 2015;8:695-702. doi:10.2147/jpr.s91924
16. Tiippana E, Hamunen K, Heiskanen T, Nieminen T, Kalso E, Kontinen VK. New approach for treatment of prolonged postoperative pain: APS Out-Patient Clinic. Scand J Pain. 2016;12(1):19-24. doi:10.1016/j.sjpain.2016.02.008
17. Katz J, Weinrib AZ, Clarke H. Chronic postsurgical pain: from risk factor identification to multidisciplinary management at the Toronto General Hospital Transitional Pain Service. Can J Pain. 2019;3(2):49-58. doi:10.1080/24740527.2019.1574537
18. Sullivan MJ, Bishop SR, Pivik J. The Pain Catastrophizing Scale: development and validation. Psychol Assess. 1995;7(4):524-532. doi:10.1037/1040-3590.7.4.524
19. HealthMeasures. Intro to PROMIS. https://www.healthmeasures.net/explore-measurement-systems/promis. Accessed September 28, 2020.
1. Ilfeld BM, Madison SJ, Suresh PJ. Persistent postmastectomy pain and pain-related physical and emotional functioning with and without a continuous paravertebral nerve block: a prospective 1-year follow-up assessment of a randomized, triple-masked, placebo-controlled study. Ann Surg Oncol. 2015;22(6):2017-2025. doi:10.1245/s10434-014-4248-7
2. Richebé P, Capdevila X, Rivat C. Persistent postsurgical pain. Anesthesiology. 2018;129(3):590-607. doi:10.1097/aln.0000000000002238
3. Glare P, Aubrey KR, Myles PS. Transition from acute to chronic pain after surgery. Lancet. 2019;393(10180):1537-1546. doi:10.1016/s0140-6736(19)30352-6
4. Brummett CM, Waljee JF, Goesling J, et al. New persistent opioid use after minor and major surgical procedures in US adults. JAMA Surgery. 2017;152(6):e170504-e170504. doi:10.1001/jamasurg.2017.0504
5. Swenson CW, Kamdar NS, Seiler K, Morgan DM, Lin P, As-Sanie S. Definition development and prevalence of new persistent opioid use following hysterectomy. Am J Obstet Gynecol. 2018;219(5):486.e1-486.e7. doi:10.1016/j.ajog.2018.06.010
6. Bartels K, Fernandez-Bustamante A, McWilliams SK, Hopfer CJ, Mikulich-Gilbertson SK. Long-term opioid use after inpatient surgery - a retrospective cohort study. Drug Alcohol Depend. 2018;187:61-65. doi:10.1016/j.drugalcdep.2018.02.013
7. Bedard N, DeMik D, Dowdle S, Callaghan J. Trends and risk factors for prolonged opioid use after unicompartmental knee arthroplasty. Bone Joint J. 2018;100-B(1)(suppl A):62-67. doi:10.1302/0301-620x.100b1.bjj-2017-0547.r1
8. Politzer CS, Kildow BJ, Goltz DE, Green CL, Bolognesi MP, Seyler T. Trends in opioid utilization before and after total knee arthroplasty. J Arthroplasty. 2018;33(7S):S147-S153.e1. doi:10.1016/j.arth.2017.10.060
9. Mariano ER, Walters TL, Kim ET, Kain ZN. Why the perioperative surgical home makes sense for Veterans Affairs health care. Anesth Analg. 2015;120(5):1163-1166. doi:10.1213/ane.0000000000000712
10. Walters TL, Howard SK, Kou A, et al. Design and implementation of a perioperative surgical home at a Veterans Affairs hospital. Semin Cardiothorac Vasc Anesth. 2016;20(2):133-140. doi:10.1177/1089253215607066
11. Walters TL, Mariano ER, Clark DJ. Perioperative surgical home and the integral role of pain medicine. Pain Med. 2015;16(9):1666-1672. doi:10.1111/pme.12796
12. Vetter TR, Kain ZN. Role of the perioperative surgical home in optimizing the perioperative use of opioids. Anesth Analg. 2017;125(5):1653-1657. doi:10.1213/ane.0000000000002280
13. Shafer SL. Anesthesia & Analgesia’s 2015 collection on the perioperative surgical home. Anesth Analg. 2015;120(5):966-967. doi:10.1213/ane.0000000000000696
14. Wenzel JT, Schwenk ES, Baratta JL, Viscusi ER. Managing opioid-tolerant patients in the perioperative surgical home. Anesthesiol Clin. 2016;34(2):287-301. doi:10.1016/j.anclin.2016.01.005
15. Katz J, Weinrib A, Fashler SR, et al. The Toronto General Hospital Transitional Pain Service: development and implementation of a multidisciplinary program to prevent chronic postsurgical pain. J Pain Res. 2015;8:695-702. doi:10.2147/jpr.s91924
16. Tiippana E, Hamunen K, Heiskanen T, Nieminen T, Kalso E, Kontinen VK. New approach for treatment of prolonged postoperative pain: APS Out-Patient Clinic. Scand J Pain. 2016;12(1):19-24. doi:10.1016/j.sjpain.2016.02.008
17. Katz J, Weinrib AZ, Clarke H. Chronic postsurgical pain: from risk factor identification to multidisciplinary management at the Toronto General Hospital Transitional Pain Service. Can J Pain. 2019;3(2):49-58. doi:10.1080/24740527.2019.1574537
18. Sullivan MJ, Bishop SR, Pivik J. The Pain Catastrophizing Scale: development and validation. Psychol Assess. 1995;7(4):524-532. doi:10.1037/1040-3590.7.4.524
19. HealthMeasures. Intro to PROMIS. https://www.healthmeasures.net/explore-measurement-systems/promis. Accessed September 28, 2020.
Penicillin Allergy Delabeling Can Decrease Antibiotic Resistance, Reduce Costs, and Optimize Patient Outcomes
Antibiotics are one of the most frequently prescribed medications in both inpatient and outpatient settings.1,2 More than 266 million courses of antibiotics are prescribed annually in the outpatient setting; 49.9% of hospitalized patients were prescribed ≥ 1 antibiotic during their hospitalization.1,2 Among all classes of antibiotics, penicillins are prescribed due to their clinical efficacy, cost-effectiveness, and general safety for all ages. Unfortunately, penicillins also are the most common drug allergy listed in medical records. Patients with this allergy are consistently treated with broad-spectrum antibiotics, have more antibiotic resistant infections, incur higher health care costs, and experience more adverse effects (AEs).3,4
Drug allergies are distinguished by different immune mechanisms, including IgE-mediated reaction, T-lymphocyte-mediated mild skin reactions, and severe cutaneous adverse reactions (SCAR), or other systemic immune syndromes, such as hemolytic anemia, nephritis, and rash with eosinophilia.3 Although drug allergies should be a concern, compelling evidence shows that > 90% of patients labeled with a penicillin allergy are not allergic to penicillins (and associated β-lactams).3,4 Although this evidence is growing, clinicians still hesitate to prescribe penicillin, and patients are similarly anxious to take them. This article reviews the health care consequences of penicillin allergy and the application of this information to military medicine and readiness.
Penicillin Allergy Prevalence
Since their approval for public use in 1945, penicillins have been one of the most often prescribed antibiotics due to their clinical efficacy for many types of infections.3 However, 8 to 10% of the US population and up to 15% of hospitalized patients have a documented penicillin allergy, which limits the ability to use these effective antibiotics.3,4 Once a patient is labeled with a penicillin allergy, many clinicians avoid prescribing all β-lactam antibiotics to patients. Clinicians also avoid prescribing cephalosporins due to the concern for potential cross-reactivity (at a rate of about 2%, which is lower than previously reported).3 These reported allergies are often not clear and range from patients avoiding penicillins because their parents exhibited allergies, they had a symptom that was not likely allergic (ie, nausea, headache, itching with no rash), being told by their parents that they had a rash as a child, or experiencing severe anaphylaxis or other systemic reaction.3,4 Despite the high rates of documented penicillin allergy, studies now show that most patients do not have a serious allergy; < 1% of the population has a true immune-mediated penicillin allergy.3,4
Broad-Spectrum Antibiotic Risks
Even though penicillin allergies are often not confirmed, many patients are treated with alternative antibiotics. Unfortunately, most alternative antibiotics are not as effective or as safe as penicillin.3,4 Twenty percent of hospitalized patients will experience an AE related to their antibiotic; 19.3% of emergency department visits for adverse drug reactions (ADRs) are from antibiotics.5,6 Sulfonamides, clindamycin, and quinolones were the antibiotics most commonly associated with AEs.6
In a large database study over a 3-year period, > 400,000 hospitalizations were analyzed in patients matched for admission type, with and without a penicillin allergy in their medical record.7 Those with a documented penicillin allergy had longer hospitalizations; were treated with broad-spectrum antibiotics; and had increased rates of Clostridium difficile (C difficile), methicillin-resistant Staphylococcus aureus (MRSA), and vancomycin-resistant Enterococcus (VRE).7,8 In addition to being first-line treatment for many common infections, penicillins often are used for dental, perinatal, and perioperative prophylaxis.1,3 Nearly 25 million antibiotics are prescribed annually by dentists.1 If a patient has a penicillin allergy listed in their medical record, they will inevitably receive a second- or third-line treatment that is less effective and has higher risks. Common alternative antibiotics include clindamycin, fluoroquinolones, macrolides, and vancomycin.3,7,8
Clindamycin and fluoroquinolones are associated with C difficile infections.9,10 Fluoroquinolones come with a boxed warning for known serious ADRs, including tendon rupture, peripheral neuropathy, central nervous system effects, and are known for causing cardiac reactions such as QT prolongation, life-threatening arrhythmias, and cardiovascular death.11,12 Fluoroquinolones are associated with an increased risk for VRE and MRSA, in more than any other antibiotic classes.3,7,12,13
Macrolides, such as azithromycin and clarithromycin, are another common class of antibiotics used as an alternative for penicillins. Both are used frequently for upper respiratory infections. Known ADRs to macrolides include gastrointestinal adverse effects (AEs) (ie, nausea, vomiting, diarrhea, and abdominal pain), liver toxicity (ie, abnormal liver function tests, hepatitis, and liver failure), and cardiac risks (ie, QT prolongation and sudden death). When compared with amoxicillin, there was an increased risk for cardiovascular mortality in those patients receiving macrolides.14,15
Vancomycin is known for its potential to cause “red man syndrome,” an infusion-related reaction causing redness and itching as well as nephrotoxic and hematologic effects requiring close monitoring.3 Vancomycin is less effective than methicillin in clearing MRSA or other sensitive pathogens; however, vancomycin is used in patients with a penicillin allergy label.16-18 Intrapartum antibiotic use of vancomycin for group B streptococcus infection was associated with clinically significant morbidity and ADRs.19,20 Perioperatively, patients with penicillin allergies developed more surgical site infections due to the use of second-line antibiotics, such as vancomycin or others.21
Cost of Penicillin Allergies
Penicillin allergy plays an important role in rising health care costs. In 2017, health care spending reached 17.9% of the gross domestic product.22 Macy and Contreras demonstrated the significantly higher costs associated with having a reported (and unverified) penicillin allergy in a matched cohort study. Inferred for the extra hospital use, the penicillin allergy group cost the health care system $64,626,630 more than for the group who did not have a penicillin allergy label.7 A subsequent study by Macy and Contreras of both inpatient and outpatient settings showed a potential savings of $2,000 per patient per year in health care expenses with the testing and delabeling of penicillin allergies.23 Use of newer and broad-spectrum antibiotics also are more costly and contribute to higher health care costs.24
When these potential savings are applied to the military insurance population of 9.4 million beneficiaries (TRICARE, including active duty, their dependents, and all retirees participating in the program), the results showed that this could impart a savings of nearly $1.7 billion annually, using the model by Macy and Contreras.23,25,26
Previously with colleagues, I reviewed penicillin’s role in military history, compiled data from relevant studies from military penicillin allergy rates and delabeling efforts, and calculated the potential economic impact of penicillin allergies along with the benefits of testing.26 Calculations were estimated using the TRICARE beneficiary population (9.4 million) × the estimated prevalence (10%) to get an estimate of 940,000 TRICARE patients with penicillin allergy in their medical record.25 If 90% of those patients were delabeled, this would equal 846,000 TRICARE patients. When multiplied by the potential savings of $2,000 per patient per year, the estimated savings would be $1,692,000,000 annually.23,26
Current literature provides compelling evidence that all health care plans should use penicillin allergy testing and delabeling programs.3,23,26 As most patients with a history of penicillin allergy in their medical records do not have a verified allergy, delabeling those who do not have a true allergy will have individual, public health, and cost benefits.3,7,23,26
Antibiotic Stewardship
Antibiotic stewardship programs are now mandated to combat antibiotic resistance.3,27 This program is supported by major medical organizations, including the Centers for Disease Control and Prevention, Society for Healthcare Epidemiology of America, Infectious Disease Society of America, and the American Academy of Allergy Asthma and Immunology.3 Given the role of broad-spectrum antibiotics in antibiotic resistance, penicillin allergy testing and delabeling is an important component of these programs.3
In the US, > 2 million people acquire antibiotic resistant infections annually; 23,000 people die of these infections.27 More than 250,000 illnesses and 14,000 deaths annually are due to C difficile.27 There are many factors contributing to the increase in antibiotic resistance; however, one established and consistent factor is the use of broad-spectrum antibiotics. Further, broad-spectrum antibiotics are often used when first-line agents, such as penicillins, cannot be used due to a reported “allergy.” In addition, there are fewer novel antibiotics being developed, and as they are introduced, pathogens develop resistance to these new agents.27
Military Relevance
Infectious diseases have always accompanied military activity.28-30 Despite preventive programs such as vaccinations, hygiene measures, and prophylactic antibiotics, military personnel are at increased risk for infections due to the military’s mobile nature and crowded living situations.28-30 This situation has operational relevance from basic training, deployments, and combat operations to peacetime activities.
Military recruits are treated routinely with penicillin G benzathine as standard prophylaxis against streptococcal infections.26,30 A recent study by the Marine Corps Recruiting Depot in San Diego, California showed that in a cohort of 402 young healthy male recruits, only 5 (1.5%) had a positive reaction to penicillin testing and challenge over a 21-month period.31 The delabeled other 397 (98.5%) marine recruits were able to receive benzathine penicillin prophylaxis successfully.31 Recruits with a penicillin allergy who had a positive test or were not tested received azithromycin (or erythromycin at some recruit training locations).26,31 Military members may need to operate in remote or austere locations; the ability to use penicillins is important for readiness.
Evaluation and Management of Reported Penicillin Allergy
Verifying penicillin allergies is an important first step in optimizing medical care and decreasing resistance and ADRs.3,4,32,33 Although allergists can provide specialized evaluation, due to the high prevalence of penicillin allergy in the US, all health care team members, including clinicians and pharmacists, should be educated about penicillin allergies and be able to implement evaluations in both inpatient and outpatient settings. Reactions to any of the penicillins should be considered, including the natural penicillins (penicillin V, etc), antistaphylococcal penicillins (dicloxacillin), aminopenicillins (amoxicillin and ampicillin), and extended-spectrum penicillins (piperacillin).3 A thorough history, including the prior reaction (age, type of reaction) and subsequent tolerance are helpful in stratifying patients.3,26
Patient Risk Levels
Based on the clinical history, patients would fall into 4 categories from low risk, medium risk, high risk, to do not test/use.3,32,33 Low-risk patients are those who report mild or nonallergic symptoms (ie, gastrointestinal symptoms, headache, yeast infection, etc), remote cutaneous reactions (> 10 years), or in those with a family history of penicillin allergy.3,32,33 Low-risk patients often can be safely tested with an oral challenge. Although there are different approaches to the oral challenge, a single amoxicillin dose of 250 mg followed by 1 hour of direct monitoring is usually sufficient.3,32,33
Medium-risk patients have a more recent (< 1 year) history of pruritic rashes, urticaria, and/or angioedema without a history of severe or systemic reactions. These patients benefit from negative skin testing prior to an oral challenge, which can be performed by trained clinicians or pharmacists or an allergist. However, due to limited availability of skin testing and the potential for false positive testing with skin tests, a single dose or graded challenge would be a reasonable approach as well.3,32,33
High-risk patients are those with severe symptoms (anaphylaxis), a history of reactions to other β-lactam antibiotics, and/or recurrent reactions to antibiotics. These patients benefit from a formal evaluation by an allergist and skin testing prior to challenge.3,32,33 Testing and/or challenge should not be performed in patients who report a history of severe cutaneous reactions (blistering rash, such as Stevens Johnson syndrome), hemolytic anemia, serum sickness, drug fever, and other organ dysfunction.3,4,31,32
The Figure describes a published questionnaire, personnel, resources, and procedures for penicillin delabeling.26 Although skin testing is reliable in revealing a immunoglobulin E-mediated penicillin allergy, there is potential for false positives.32,33 The oral amoxicillin challenge effectively clears the patient for future penicillin administration.3,32-34 In high-risk patients, desensitization should be considered if penicillins (or cephalosporins) are required as first-line treatment. A test dose (one-tenth dose, higher or lower depending on route of administration, historic reaction, clinical status, and level of certainty of prior reaction) may be considered in low- to moderate-risk patients, depending on the indication for the use of the antibiotics.32
Penicillin evaluation pathways can occur in both inpatient and outpatient settings where antibiotics will be prescribed.32-34 There are several proposed pathways, including a screening questionnaire to determine the penicillin allergy risk.26,32,33 Implementation of perioperative testing has been successful in decreasing the rates of vancomycin use and lessening the morbidity associated with use of second-line antibiotics.35 Many hospitals throughout the country have implemented standardized penicillin delabeling programs.3,32-34
Conclusions
Penicillin allergies are an important barrier to effective antibiotic treatments and are associated with worse outcomes and higher economic costs.3,7,23,26,34 Therefore, in addition to vaccinations, infection control measures, and public health education, penicillin allergy verification and delabeling programs should be a proactive component of military medical readiness and all antibiotic stewardship initiatives in all health care settings.29 Given the many issues and negative impact of having a penicillin allergy label, penicillin delabeling will allow service members to be treated with the necessary antibiotics with fewer adverse complications, and return them to health and readiness for operational duties. In the current standardization of the Defense Health Agency, implementing this program across all services would have significant clinical, public health, and cost benefits for patients, the health care team, taxpayers, and the community at large.
Many patients report an allergy to penicillin, but only a small portion have a current true immune-mediated allergy. Given the clinical, public health, and economic costs associated with a penicillin allergy label, evaluation and clearance of penicillin allergies is a simple method that would improve clinical outcomes, decrease AEs to high-risk alternative broad-spectrum antibiotics, and prevent the spread of antibiotic resistance. In the military, penicillin delabeling improves readiness with optimal antibiotic options and avoidance of unnecessary risks of using alternative antibiotics, expediting return to full duty for military personnel.
1. Centers for Disease Control and Prevention. Outpatient antibiotic prescriptions-United States, 2014. https://www.cdc.gov/antibiotic-use/community/pdfs/annual-reportsummary_2014.pdf. Accessed August 15, 2020.
2. Magill SS, Edwards JR, Beldavs ZG, et al. Prevalence of antimicrobial use in US acute care hospitals, May-September 2011. JAMA. 2014;312(14):1438-1446. doi:10.1001/jama.2014.12923
3. Shenoy ES, Macy E, Rowe T, Blumenthal KG. Evaluation and management of penicillin allergy. JAMA. 2019;321:188-199. doi:10.1001/jama.2018.19283
4. Har D, Solensky R. Penicillin and beta-lactam hypersensitivity. Immunol Allergy Clin North Am. 2017;37(4):643-662. doi:10.1016/j.iac.2017.07.001
5. Tamma PD, Avdic E, Li DX, Dzintars K, Cosgrove SE. Association of adverse events with antibiotic use in hospitalized patients. JAMA Intern Med. 2017;177(9):1308-1315. doi:10.1001/jamainternmed.2017.1938
6. Shebab N, Patel PR, Srinivasan A, Budnitz DS. Emergency department visits for antibiotic-associated adverse events. Clin Infect Dis. 2008;47(6):735-743. doi:10.1086/591126
7. Macy E, Contreras R. Healthcare use and serious infection prevalence associated with penicillin “allergy” in hospitalized patients: a cohort study. J Allergy Clin Immunol. 2014;133(3):790-796. doi:10.1016/j.jaci.2013.09.021
8. Blumenthal KG, Lu N, Zhang Y, Li Y, Walensky RP, Choi HK. Risk of methicillin resistant Staphylococcal aureus and Clostridium difficile in patients with a documented penicillin allergy: population-based matched cohort study. BMJ. 2018;361:k2400. doi:10.1136/bmj.k2400
9. Loo VG, Poirier L, Miller MA, et al. A predominantly clonal multi-institutional outbreak of Clostridium difficile associated diarrhea with high morbidity and mortality. N Engl J Med. 2005;353(23):2442-2449. doi:10.1056/NEJMoa051639
10. Pepin J, Saheb N, Coulombe MA, et al. Emergence of fluoroquinolone as the predominant risk factor for Clostridium difficile-associated diarrhea: a cohort study during an epidemic in Quebec. Clin Infect Dis. 2005;41(9):1254-1260. doi:10.1086/496986
11. Chou HW, Wang JL, Chang CH, et al. Risks of cardiac arrhythmia and mortality among patients using new-generation macrolides, fluoroquinolones, and β-lactam/β-lactamase inhibitors: a Taiwanese nationwide study. Clin Infec Dis. 2015;60(4):566-577. doi:10.1093/cid/ciu914
12. Rao GA, Mann JR, Shoaibi A, et al. Azithromycin and levofloxacin use and increased risk of cardiac arrhythmia and death. Ann Fam Med. 2014;12(2):121-127. doi:10.1370/afm.1601
13. LeBlanc L, Pepin J, Toulouse K, et al. Fluoroquinolone and risk for methicillin-resistant Staphylococcus aureus, Canada. Emerg Infect Dis. 2006;12(9):1398-1405. doi:10.3201/eid1209.060397
14. Schembri S, Williamson PA, Short PM, et al. Cardiovascular events after clarithromycin use in lower respiratory tract infections: analysis of two prospective cohort studies. BMJ. 2013;346:f1245. doi:10.1136/bmj.f1235
15. Ray WA, Murray KT, Hall K, Arbogast PG, Stein CM. Azithromycin and the risk of cardiovascular death. N Engl J Med. 2012;366(20):1881-1890. doi:10.1056/NEJMoa1003833
16. McDaniel JS, Perencevich EN, Diekema DJ, et al. Comparative effectiveness of beta-lactams versus vancomycin for treatment of methicillin-susceptible Staphylococcus aureus bloodstream infections among 122 hospitals. Clin Infect Dis. 2015;61(3):361-367. doi:10.1093/cid/civ308
17. Wong D, Wong T, Romney M, Leung V. Comparison of outcomes in patients with methicillin-susceptible Staphylococcus aureus (MSSA) bacteremia who are treated with β-lactam vs vancomycin empiric therapy: a retrospective cohort study. BMC Infect Dis. 2016;16:224. doi:10.1186/s12879-016-1564-5
18. Blumenthal KG, Shenoy ES, Huang M, et al. The impact of reporting a prior penicillin allergy on the treatment of methicillin-sensitivity Staphylococcus aureus bacteremia. PLoS One. 2016;11(7):e0159406. doi:10.1371/journal.pone.0159406
19. Verani JR, McGee L, Schrag SJ; . Prevention of perinatal group B streptococcal disease. MMWR Recomm Rep. 2010;59(RR-10):1-36.
20. Desai SH, Kaplan MS, Chen Q, Macy EM. Morbidity in pregnant women associated with unverified penicillin allergies, antibiotic use, and group B Streptococcus infections. Perm J. 2017;21:16-080. doi:10.7812/TPP/16-080
21. Blumenthal KG, Ryan EE, Li Y, Lee H, Kuhlen JL, Shenoy ES. The impact of a reported penicillin allergy on surgical site infection risk. Clin Infect Dis. 2018;66(3):329-336. doi:10.1093/cid/cix794
22. National Health Expenditures 2017 Highlights. Centers for Medicare & Medicaid services. https://www.cms.gov/Research-Statistics-Data-and-Systems/Statistics-Trends-and-Reports/NationalHealthExpendData/Downloads/highlights.pdf. Accessed August 25, 2020.
23. Macy E, Shu YH. The effect of penicillin allergy testing on future health care utilization: a matched cohort study. J Allergy Clin Immunol Pract. 2017;5(3):705-710. doi:10.1016/j.jaip.2017.02.012
24. Picard M, Begin P, Bouchard H, et al. Treatment of patients with a history of penicillin allergy in a large tertiary-care academic hospital. J Allergy Clin Immunol Pract. 2013;1(3):252-257. doi:10.1016/j.jaip.2013.01.006
25. US Department of Defense. Beneficiary population statistics. https://health.mil/I-Am-A/Media/Media-Center/Patient-Population-Statistics. Accessed August 25, 2020.
26. Lee RU, Banks TA, Waibel KH, Rodriguez RG. Penicillin allergy…maybe not? The military relevance for penicillin testing and de-labeling. Mil Med. 2019;184(3-4):e163-e168. doi:10.1093/milmed/usy194
27. Antibiotic resistance threats in the United States, 2013. Centers for Disease Control and Prevention. https://www.cdc.gov/drugresistance/threat-report-2013/index.html. Accessed May 10, 2019.
28. Gray GC, Callahan JD, Hawksworth AW, Fisher CA, Gaydos JC. Respiratory diseases among U.S. military personnel: countering emerging threats. Emerg Infect Dis. 1999;5(3):379-385. doi:10.3201/eid0503.990308
29. Beaumier CM, Gomez-Rubio AM, Hotez PJ, Weina PJ. United States military tropical medicine: extraordinary legacy, uncertain future. PLoS Negl Trop Dis. 2013;7(12):e2448. doi:10.1371/journal.pntd.0002448
30. Thomas RJ, Conwill DE, Morton DE, et al. Penicillin prophylaxis for streptococcal infections in the United States Navy and Marine Corps recruit camps, 1951-1985. Rev Infect Dis. 1988;10(1):125-130. doi:10.1093/clinids/10.1.125
31. Tucker MH, Lomas CM, Ramchandar N, Waldram JD. Amoxicillin challenge without penicillin skin testing in evaluation of penicillin allergy in a cohort of Marine recruits. J Allergy Clin Immunol Pract. 2017;5(3):813-815. doi:10.1016/j.jaip.2017.01.023
32. Blumenthal KG, Shenoy ES, Wolfson AR, et al. Addressing inpatient beta-lactam allergies: a multihospital implementation. J Allergy Clin Immunol Pract. 2017;5(3):616-625. doi:10.1016/j.jaip.2017.02.019
33. Kuruvilla M, Shih J, Patel K, Scanlon N. Direct oral amoxicillin challenge without preliminary skin testing in adult patients with allergy and at low risk with reported penicillin allergy. Allergy Asthma Proc. 2019;40(1):57-61. doi:10.2500/aap.2019.40.4184
34. Banks TA, Tucker M, Macy E. Evaluating penicillin allergies without skin testing. Curr Allergy Asthma Rep. 2019;19(5):27. doi:10.1007/s11882-019-0854-6
35. Park M, Markus P, Matesic
Antibiotics are one of the most frequently prescribed medications in both inpatient and outpatient settings.1,2 More than 266 million courses of antibiotics are prescribed annually in the outpatient setting; 49.9% of hospitalized patients were prescribed ≥ 1 antibiotic during their hospitalization.1,2 Among all classes of antibiotics, penicillins are prescribed due to their clinical efficacy, cost-effectiveness, and general safety for all ages. Unfortunately, penicillins also are the most common drug allergy listed in medical records. Patients with this allergy are consistently treated with broad-spectrum antibiotics, have more antibiotic resistant infections, incur higher health care costs, and experience more adverse effects (AEs).3,4
Drug allergies are distinguished by different immune mechanisms, including IgE-mediated reaction, T-lymphocyte-mediated mild skin reactions, and severe cutaneous adverse reactions (SCAR), or other systemic immune syndromes, such as hemolytic anemia, nephritis, and rash with eosinophilia.3 Although drug allergies should be a concern, compelling evidence shows that > 90% of patients labeled with a penicillin allergy are not allergic to penicillins (and associated β-lactams).3,4 Although this evidence is growing, clinicians still hesitate to prescribe penicillin, and patients are similarly anxious to take them. This article reviews the health care consequences of penicillin allergy and the application of this information to military medicine and readiness.
Penicillin Allergy Prevalence
Since their approval for public use in 1945, penicillins have been one of the most often prescribed antibiotics due to their clinical efficacy for many types of infections.3 However, 8 to 10% of the US population and up to 15% of hospitalized patients have a documented penicillin allergy, which limits the ability to use these effective antibiotics.3,4 Once a patient is labeled with a penicillin allergy, many clinicians avoid prescribing all β-lactam antibiotics to patients. Clinicians also avoid prescribing cephalosporins due to the concern for potential cross-reactivity (at a rate of about 2%, which is lower than previously reported).3 These reported allergies are often not clear and range from patients avoiding penicillins because their parents exhibited allergies, they had a symptom that was not likely allergic (ie, nausea, headache, itching with no rash), being told by their parents that they had a rash as a child, or experiencing severe anaphylaxis or other systemic reaction.3,4 Despite the high rates of documented penicillin allergy, studies now show that most patients do not have a serious allergy; < 1% of the population has a true immune-mediated penicillin allergy.3,4
Broad-Spectrum Antibiotic Risks
Even though penicillin allergies are often not confirmed, many patients are treated with alternative antibiotics. Unfortunately, most alternative antibiotics are not as effective or as safe as penicillin.3,4 Twenty percent of hospitalized patients will experience an AE related to their antibiotic; 19.3% of emergency department visits for adverse drug reactions (ADRs) are from antibiotics.5,6 Sulfonamides, clindamycin, and quinolones were the antibiotics most commonly associated with AEs.6
In a large database study over a 3-year period, > 400,000 hospitalizations were analyzed in patients matched for admission type, with and without a penicillin allergy in their medical record.7 Those with a documented penicillin allergy had longer hospitalizations; were treated with broad-spectrum antibiotics; and had increased rates of Clostridium difficile (C difficile), methicillin-resistant Staphylococcus aureus (MRSA), and vancomycin-resistant Enterococcus (VRE).7,8 In addition to being first-line treatment for many common infections, penicillins often are used for dental, perinatal, and perioperative prophylaxis.1,3 Nearly 25 million antibiotics are prescribed annually by dentists.1 If a patient has a penicillin allergy listed in their medical record, they will inevitably receive a second- or third-line treatment that is less effective and has higher risks. Common alternative antibiotics include clindamycin, fluoroquinolones, macrolides, and vancomycin.3,7,8
Clindamycin and fluoroquinolones are associated with C difficile infections.9,10 Fluoroquinolones come with a boxed warning for known serious ADRs, including tendon rupture, peripheral neuropathy, central nervous system effects, and are known for causing cardiac reactions such as QT prolongation, life-threatening arrhythmias, and cardiovascular death.11,12 Fluoroquinolones are associated with an increased risk for VRE and MRSA, in more than any other antibiotic classes.3,7,12,13
Macrolides, such as azithromycin and clarithromycin, are another common class of antibiotics used as an alternative for penicillins. Both are used frequently for upper respiratory infections. Known ADRs to macrolides include gastrointestinal adverse effects (AEs) (ie, nausea, vomiting, diarrhea, and abdominal pain), liver toxicity (ie, abnormal liver function tests, hepatitis, and liver failure), and cardiac risks (ie, QT prolongation and sudden death). When compared with amoxicillin, there was an increased risk for cardiovascular mortality in those patients receiving macrolides.14,15
Vancomycin is known for its potential to cause “red man syndrome,” an infusion-related reaction causing redness and itching as well as nephrotoxic and hematologic effects requiring close monitoring.3 Vancomycin is less effective than methicillin in clearing MRSA or other sensitive pathogens; however, vancomycin is used in patients with a penicillin allergy label.16-18 Intrapartum antibiotic use of vancomycin for group B streptococcus infection was associated with clinically significant morbidity and ADRs.19,20 Perioperatively, patients with penicillin allergies developed more surgical site infections due to the use of second-line antibiotics, such as vancomycin or others.21
Cost of Penicillin Allergies
Penicillin allergy plays an important role in rising health care costs. In 2017, health care spending reached 17.9% of the gross domestic product.22 Macy and Contreras demonstrated the significantly higher costs associated with having a reported (and unverified) penicillin allergy in a matched cohort study. Inferred for the extra hospital use, the penicillin allergy group cost the health care system $64,626,630 more than for the group who did not have a penicillin allergy label.7 A subsequent study by Macy and Contreras of both inpatient and outpatient settings showed a potential savings of $2,000 per patient per year in health care expenses with the testing and delabeling of penicillin allergies.23 Use of newer and broad-spectrum antibiotics also are more costly and contribute to higher health care costs.24
When these potential savings are applied to the military insurance population of 9.4 million beneficiaries (TRICARE, including active duty, their dependents, and all retirees participating in the program), the results showed that this could impart a savings of nearly $1.7 billion annually, using the model by Macy and Contreras.23,25,26
Previously with colleagues, I reviewed penicillin’s role in military history, compiled data from relevant studies from military penicillin allergy rates and delabeling efforts, and calculated the potential economic impact of penicillin allergies along with the benefits of testing.26 Calculations were estimated using the TRICARE beneficiary population (9.4 million) × the estimated prevalence (10%) to get an estimate of 940,000 TRICARE patients with penicillin allergy in their medical record.25 If 90% of those patients were delabeled, this would equal 846,000 TRICARE patients. When multiplied by the potential savings of $2,000 per patient per year, the estimated savings would be $1,692,000,000 annually.23,26
Current literature provides compelling evidence that all health care plans should use penicillin allergy testing and delabeling programs.3,23,26 As most patients with a history of penicillin allergy in their medical records do not have a verified allergy, delabeling those who do not have a true allergy will have individual, public health, and cost benefits.3,7,23,26
Antibiotic Stewardship
Antibiotic stewardship programs are now mandated to combat antibiotic resistance.3,27 This program is supported by major medical organizations, including the Centers for Disease Control and Prevention, Society for Healthcare Epidemiology of America, Infectious Disease Society of America, and the American Academy of Allergy Asthma and Immunology.3 Given the role of broad-spectrum antibiotics in antibiotic resistance, penicillin allergy testing and delabeling is an important component of these programs.3
In the US, > 2 million people acquire antibiotic resistant infections annually; 23,000 people die of these infections.27 More than 250,000 illnesses and 14,000 deaths annually are due to C difficile.27 There are many factors contributing to the increase in antibiotic resistance; however, one established and consistent factor is the use of broad-spectrum antibiotics. Further, broad-spectrum antibiotics are often used when first-line agents, such as penicillins, cannot be used due to a reported “allergy.” In addition, there are fewer novel antibiotics being developed, and as they are introduced, pathogens develop resistance to these new agents.27
Military Relevance
Infectious diseases have always accompanied military activity.28-30 Despite preventive programs such as vaccinations, hygiene measures, and prophylactic antibiotics, military personnel are at increased risk for infections due to the military’s mobile nature and crowded living situations.28-30 This situation has operational relevance from basic training, deployments, and combat operations to peacetime activities.
Military recruits are treated routinely with penicillin G benzathine as standard prophylaxis against streptococcal infections.26,30 A recent study by the Marine Corps Recruiting Depot in San Diego, California showed that in a cohort of 402 young healthy male recruits, only 5 (1.5%) had a positive reaction to penicillin testing and challenge over a 21-month period.31 The delabeled other 397 (98.5%) marine recruits were able to receive benzathine penicillin prophylaxis successfully.31 Recruits with a penicillin allergy who had a positive test or were not tested received azithromycin (or erythromycin at some recruit training locations).26,31 Military members may need to operate in remote or austere locations; the ability to use penicillins is important for readiness.
Evaluation and Management of Reported Penicillin Allergy
Verifying penicillin allergies is an important first step in optimizing medical care and decreasing resistance and ADRs.3,4,32,33 Although allergists can provide specialized evaluation, due to the high prevalence of penicillin allergy in the US, all health care team members, including clinicians and pharmacists, should be educated about penicillin allergies and be able to implement evaluations in both inpatient and outpatient settings. Reactions to any of the penicillins should be considered, including the natural penicillins (penicillin V, etc), antistaphylococcal penicillins (dicloxacillin), aminopenicillins (amoxicillin and ampicillin), and extended-spectrum penicillins (piperacillin).3 A thorough history, including the prior reaction (age, type of reaction) and subsequent tolerance are helpful in stratifying patients.3,26
Patient Risk Levels
Based on the clinical history, patients would fall into 4 categories from low risk, medium risk, high risk, to do not test/use.3,32,33 Low-risk patients are those who report mild or nonallergic symptoms (ie, gastrointestinal symptoms, headache, yeast infection, etc), remote cutaneous reactions (> 10 years), or in those with a family history of penicillin allergy.3,32,33 Low-risk patients often can be safely tested with an oral challenge. Although there are different approaches to the oral challenge, a single amoxicillin dose of 250 mg followed by 1 hour of direct monitoring is usually sufficient.3,32,33
Medium-risk patients have a more recent (< 1 year) history of pruritic rashes, urticaria, and/or angioedema without a history of severe or systemic reactions. These patients benefit from negative skin testing prior to an oral challenge, which can be performed by trained clinicians or pharmacists or an allergist. However, due to limited availability of skin testing and the potential for false positive testing with skin tests, a single dose or graded challenge would be a reasonable approach as well.3,32,33
High-risk patients are those with severe symptoms (anaphylaxis), a history of reactions to other β-lactam antibiotics, and/or recurrent reactions to antibiotics. These patients benefit from a formal evaluation by an allergist and skin testing prior to challenge.3,32,33 Testing and/or challenge should not be performed in patients who report a history of severe cutaneous reactions (blistering rash, such as Stevens Johnson syndrome), hemolytic anemia, serum sickness, drug fever, and other organ dysfunction.3,4,31,32
The Figure describes a published questionnaire, personnel, resources, and procedures for penicillin delabeling.26 Although skin testing is reliable in revealing a immunoglobulin E-mediated penicillin allergy, there is potential for false positives.32,33 The oral amoxicillin challenge effectively clears the patient for future penicillin administration.3,32-34 In high-risk patients, desensitization should be considered if penicillins (or cephalosporins) are required as first-line treatment. A test dose (one-tenth dose, higher or lower depending on route of administration, historic reaction, clinical status, and level of certainty of prior reaction) may be considered in low- to moderate-risk patients, depending on the indication for the use of the antibiotics.32
Penicillin evaluation pathways can occur in both inpatient and outpatient settings where antibiotics will be prescribed.32-34 There are several proposed pathways, including a screening questionnaire to determine the penicillin allergy risk.26,32,33 Implementation of perioperative testing has been successful in decreasing the rates of vancomycin use and lessening the morbidity associated with use of second-line antibiotics.35 Many hospitals throughout the country have implemented standardized penicillin delabeling programs.3,32-34
Conclusions
Penicillin allergies are an important barrier to effective antibiotic treatments and are associated with worse outcomes and higher economic costs.3,7,23,26,34 Therefore, in addition to vaccinations, infection control measures, and public health education, penicillin allergy verification and delabeling programs should be a proactive component of military medical readiness and all antibiotic stewardship initiatives in all health care settings.29 Given the many issues and negative impact of having a penicillin allergy label, penicillin delabeling will allow service members to be treated with the necessary antibiotics with fewer adverse complications, and return them to health and readiness for operational duties. In the current standardization of the Defense Health Agency, implementing this program across all services would have significant clinical, public health, and cost benefits for patients, the health care team, taxpayers, and the community at large.
Many patients report an allergy to penicillin, but only a small portion have a current true immune-mediated allergy. Given the clinical, public health, and economic costs associated with a penicillin allergy label, evaluation and clearance of penicillin allergies is a simple method that would improve clinical outcomes, decrease AEs to high-risk alternative broad-spectrum antibiotics, and prevent the spread of antibiotic resistance. In the military, penicillin delabeling improves readiness with optimal antibiotic options and avoidance of unnecessary risks of using alternative antibiotics, expediting return to full duty for military personnel.
Antibiotics are one of the most frequently prescribed medications in both inpatient and outpatient settings.1,2 More than 266 million courses of antibiotics are prescribed annually in the outpatient setting; 49.9% of hospitalized patients were prescribed ≥ 1 antibiotic during their hospitalization.1,2 Among all classes of antibiotics, penicillins are prescribed due to their clinical efficacy, cost-effectiveness, and general safety for all ages. Unfortunately, penicillins also are the most common drug allergy listed in medical records. Patients with this allergy are consistently treated with broad-spectrum antibiotics, have more antibiotic resistant infections, incur higher health care costs, and experience more adverse effects (AEs).3,4
Drug allergies are distinguished by different immune mechanisms, including IgE-mediated reaction, T-lymphocyte-mediated mild skin reactions, and severe cutaneous adverse reactions (SCAR), or other systemic immune syndromes, such as hemolytic anemia, nephritis, and rash with eosinophilia.3 Although drug allergies should be a concern, compelling evidence shows that > 90% of patients labeled with a penicillin allergy are not allergic to penicillins (and associated β-lactams).3,4 Although this evidence is growing, clinicians still hesitate to prescribe penicillin, and patients are similarly anxious to take them. This article reviews the health care consequences of penicillin allergy and the application of this information to military medicine and readiness.
Penicillin Allergy Prevalence
Since their approval for public use in 1945, penicillins have been one of the most often prescribed antibiotics due to their clinical efficacy for many types of infections.3 However, 8 to 10% of the US population and up to 15% of hospitalized patients have a documented penicillin allergy, which limits the ability to use these effective antibiotics.3,4 Once a patient is labeled with a penicillin allergy, many clinicians avoid prescribing all β-lactam antibiotics to patients. Clinicians also avoid prescribing cephalosporins due to the concern for potential cross-reactivity (at a rate of about 2%, which is lower than previously reported).3 These reported allergies are often not clear and range from patients avoiding penicillins because their parents exhibited allergies, they had a symptom that was not likely allergic (ie, nausea, headache, itching with no rash), being told by their parents that they had a rash as a child, or experiencing severe anaphylaxis or other systemic reaction.3,4 Despite the high rates of documented penicillin allergy, studies now show that most patients do not have a serious allergy; < 1% of the population has a true immune-mediated penicillin allergy.3,4
Broad-Spectrum Antibiotic Risks
Even though penicillin allergies are often not confirmed, many patients are treated with alternative antibiotics. Unfortunately, most alternative antibiotics are not as effective or as safe as penicillin.3,4 Twenty percent of hospitalized patients will experience an AE related to their antibiotic; 19.3% of emergency department visits for adverse drug reactions (ADRs) are from antibiotics.5,6 Sulfonamides, clindamycin, and quinolones were the antibiotics most commonly associated with AEs.6
In a large database study over a 3-year period, > 400,000 hospitalizations were analyzed in patients matched for admission type, with and without a penicillin allergy in their medical record.7 Those with a documented penicillin allergy had longer hospitalizations; were treated with broad-spectrum antibiotics; and had increased rates of Clostridium difficile (C difficile), methicillin-resistant Staphylococcus aureus (MRSA), and vancomycin-resistant Enterococcus (VRE).7,8 In addition to being first-line treatment for many common infections, penicillins often are used for dental, perinatal, and perioperative prophylaxis.1,3 Nearly 25 million antibiotics are prescribed annually by dentists.1 If a patient has a penicillin allergy listed in their medical record, they will inevitably receive a second- or third-line treatment that is less effective and has higher risks. Common alternative antibiotics include clindamycin, fluoroquinolones, macrolides, and vancomycin.3,7,8
Clindamycin and fluoroquinolones are associated with C difficile infections.9,10 Fluoroquinolones come with a boxed warning for known serious ADRs, including tendon rupture, peripheral neuropathy, central nervous system effects, and are known for causing cardiac reactions such as QT prolongation, life-threatening arrhythmias, and cardiovascular death.11,12 Fluoroquinolones are associated with an increased risk for VRE and MRSA, in more than any other antibiotic classes.3,7,12,13
Macrolides, such as azithromycin and clarithromycin, are another common class of antibiotics used as an alternative for penicillins. Both are used frequently for upper respiratory infections. Known ADRs to macrolides include gastrointestinal adverse effects (AEs) (ie, nausea, vomiting, diarrhea, and abdominal pain), liver toxicity (ie, abnormal liver function tests, hepatitis, and liver failure), and cardiac risks (ie, QT prolongation and sudden death). When compared with amoxicillin, there was an increased risk for cardiovascular mortality in those patients receiving macrolides.14,15
Vancomycin is known for its potential to cause “red man syndrome,” an infusion-related reaction causing redness and itching as well as nephrotoxic and hematologic effects requiring close monitoring.3 Vancomycin is less effective than methicillin in clearing MRSA or other sensitive pathogens; however, vancomycin is used in patients with a penicillin allergy label.16-18 Intrapartum antibiotic use of vancomycin for group B streptococcus infection was associated with clinically significant morbidity and ADRs.19,20 Perioperatively, patients with penicillin allergies developed more surgical site infections due to the use of second-line antibiotics, such as vancomycin or others.21
Cost of Penicillin Allergies
Penicillin allergy plays an important role in rising health care costs. In 2017, health care spending reached 17.9% of the gross domestic product.22 Macy and Contreras demonstrated the significantly higher costs associated with having a reported (and unverified) penicillin allergy in a matched cohort study. Inferred for the extra hospital use, the penicillin allergy group cost the health care system $64,626,630 more than for the group who did not have a penicillin allergy label.7 A subsequent study by Macy and Contreras of both inpatient and outpatient settings showed a potential savings of $2,000 per patient per year in health care expenses with the testing and delabeling of penicillin allergies.23 Use of newer and broad-spectrum antibiotics also are more costly and contribute to higher health care costs.24
When these potential savings are applied to the military insurance population of 9.4 million beneficiaries (TRICARE, including active duty, their dependents, and all retirees participating in the program), the results showed that this could impart a savings of nearly $1.7 billion annually, using the model by Macy and Contreras.23,25,26
Previously with colleagues, I reviewed penicillin’s role in military history, compiled data from relevant studies from military penicillin allergy rates and delabeling efforts, and calculated the potential economic impact of penicillin allergies along with the benefits of testing.26 Calculations were estimated using the TRICARE beneficiary population (9.4 million) × the estimated prevalence (10%) to get an estimate of 940,000 TRICARE patients with penicillin allergy in their medical record.25 If 90% of those patients were delabeled, this would equal 846,000 TRICARE patients. When multiplied by the potential savings of $2,000 per patient per year, the estimated savings would be $1,692,000,000 annually.23,26
Current literature provides compelling evidence that all health care plans should use penicillin allergy testing and delabeling programs.3,23,26 As most patients with a history of penicillin allergy in their medical records do not have a verified allergy, delabeling those who do not have a true allergy will have individual, public health, and cost benefits.3,7,23,26
Antibiotic Stewardship
Antibiotic stewardship programs are now mandated to combat antibiotic resistance.3,27 This program is supported by major medical organizations, including the Centers for Disease Control and Prevention, Society for Healthcare Epidemiology of America, Infectious Disease Society of America, and the American Academy of Allergy Asthma and Immunology.3 Given the role of broad-spectrum antibiotics in antibiotic resistance, penicillin allergy testing and delabeling is an important component of these programs.3
In the US, > 2 million people acquire antibiotic resistant infections annually; 23,000 people die of these infections.27 More than 250,000 illnesses and 14,000 deaths annually are due to C difficile.27 There are many factors contributing to the increase in antibiotic resistance; however, one established and consistent factor is the use of broad-spectrum antibiotics. Further, broad-spectrum antibiotics are often used when first-line agents, such as penicillins, cannot be used due to a reported “allergy.” In addition, there are fewer novel antibiotics being developed, and as they are introduced, pathogens develop resistance to these new agents.27
Military Relevance
Infectious diseases have always accompanied military activity.28-30 Despite preventive programs such as vaccinations, hygiene measures, and prophylactic antibiotics, military personnel are at increased risk for infections due to the military’s mobile nature and crowded living situations.28-30 This situation has operational relevance from basic training, deployments, and combat operations to peacetime activities.
Military recruits are treated routinely with penicillin G benzathine as standard prophylaxis against streptococcal infections.26,30 A recent study by the Marine Corps Recruiting Depot in San Diego, California showed that in a cohort of 402 young healthy male recruits, only 5 (1.5%) had a positive reaction to penicillin testing and challenge over a 21-month period.31 The delabeled other 397 (98.5%) marine recruits were able to receive benzathine penicillin prophylaxis successfully.31 Recruits with a penicillin allergy who had a positive test or were not tested received azithromycin (or erythromycin at some recruit training locations).26,31 Military members may need to operate in remote or austere locations; the ability to use penicillins is important for readiness.
Evaluation and Management of Reported Penicillin Allergy
Verifying penicillin allergies is an important first step in optimizing medical care and decreasing resistance and ADRs.3,4,32,33 Although allergists can provide specialized evaluation, due to the high prevalence of penicillin allergy in the US, all health care team members, including clinicians and pharmacists, should be educated about penicillin allergies and be able to implement evaluations in both inpatient and outpatient settings. Reactions to any of the penicillins should be considered, including the natural penicillins (penicillin V, etc), antistaphylococcal penicillins (dicloxacillin), aminopenicillins (amoxicillin and ampicillin), and extended-spectrum penicillins (piperacillin).3 A thorough history, including the prior reaction (age, type of reaction) and subsequent tolerance are helpful in stratifying patients.3,26
Patient Risk Levels
Based on the clinical history, patients would fall into 4 categories from low risk, medium risk, high risk, to do not test/use.3,32,33 Low-risk patients are those who report mild or nonallergic symptoms (ie, gastrointestinal symptoms, headache, yeast infection, etc), remote cutaneous reactions (> 10 years), or in those with a family history of penicillin allergy.3,32,33 Low-risk patients often can be safely tested with an oral challenge. Although there are different approaches to the oral challenge, a single amoxicillin dose of 250 mg followed by 1 hour of direct monitoring is usually sufficient.3,32,33
Medium-risk patients have a more recent (< 1 year) history of pruritic rashes, urticaria, and/or angioedema without a history of severe or systemic reactions. These patients benefit from negative skin testing prior to an oral challenge, which can be performed by trained clinicians or pharmacists or an allergist. However, due to limited availability of skin testing and the potential for false positive testing with skin tests, a single dose or graded challenge would be a reasonable approach as well.3,32,33
High-risk patients are those with severe symptoms (anaphylaxis), a history of reactions to other β-lactam antibiotics, and/or recurrent reactions to antibiotics. These patients benefit from a formal evaluation by an allergist and skin testing prior to challenge.3,32,33 Testing and/or challenge should not be performed in patients who report a history of severe cutaneous reactions (blistering rash, such as Stevens Johnson syndrome), hemolytic anemia, serum sickness, drug fever, and other organ dysfunction.3,4,31,32
The Figure describes a published questionnaire, personnel, resources, and procedures for penicillin delabeling.26 Although skin testing is reliable in revealing a immunoglobulin E-mediated penicillin allergy, there is potential for false positives.32,33 The oral amoxicillin challenge effectively clears the patient for future penicillin administration.3,32-34 In high-risk patients, desensitization should be considered if penicillins (or cephalosporins) are required as first-line treatment. A test dose (one-tenth dose, higher or lower depending on route of administration, historic reaction, clinical status, and level of certainty of prior reaction) may be considered in low- to moderate-risk patients, depending on the indication for the use of the antibiotics.32
Penicillin evaluation pathways can occur in both inpatient and outpatient settings where antibiotics will be prescribed.32-34 There are several proposed pathways, including a screening questionnaire to determine the penicillin allergy risk.26,32,33 Implementation of perioperative testing has been successful in decreasing the rates of vancomycin use and lessening the morbidity associated with use of second-line antibiotics.35 Many hospitals throughout the country have implemented standardized penicillin delabeling programs.3,32-34
Conclusions
Penicillin allergies are an important barrier to effective antibiotic treatments and are associated with worse outcomes and higher economic costs.3,7,23,26,34 Therefore, in addition to vaccinations, infection control measures, and public health education, penicillin allergy verification and delabeling programs should be a proactive component of military medical readiness and all antibiotic stewardship initiatives in all health care settings.29 Given the many issues and negative impact of having a penicillin allergy label, penicillin delabeling will allow service members to be treated with the necessary antibiotics with fewer adverse complications, and return them to health and readiness for operational duties. In the current standardization of the Defense Health Agency, implementing this program across all services would have significant clinical, public health, and cost benefits for patients, the health care team, taxpayers, and the community at large.
Many patients report an allergy to penicillin, but only a small portion have a current true immune-mediated allergy. Given the clinical, public health, and economic costs associated with a penicillin allergy label, evaluation and clearance of penicillin allergies is a simple method that would improve clinical outcomes, decrease AEs to high-risk alternative broad-spectrum antibiotics, and prevent the spread of antibiotic resistance. In the military, penicillin delabeling improves readiness with optimal antibiotic options and avoidance of unnecessary risks of using alternative antibiotics, expediting return to full duty for military personnel.
1. Centers for Disease Control and Prevention. Outpatient antibiotic prescriptions-United States, 2014. https://www.cdc.gov/antibiotic-use/community/pdfs/annual-reportsummary_2014.pdf. Accessed August 15, 2020.
2. Magill SS, Edwards JR, Beldavs ZG, et al. Prevalence of antimicrobial use in US acute care hospitals, May-September 2011. JAMA. 2014;312(14):1438-1446. doi:10.1001/jama.2014.12923
3. Shenoy ES, Macy E, Rowe T, Blumenthal KG. Evaluation and management of penicillin allergy. JAMA. 2019;321:188-199. doi:10.1001/jama.2018.19283
4. Har D, Solensky R. Penicillin and beta-lactam hypersensitivity. Immunol Allergy Clin North Am. 2017;37(4):643-662. doi:10.1016/j.iac.2017.07.001
5. Tamma PD, Avdic E, Li DX, Dzintars K, Cosgrove SE. Association of adverse events with antibiotic use in hospitalized patients. JAMA Intern Med. 2017;177(9):1308-1315. doi:10.1001/jamainternmed.2017.1938
6. Shebab N, Patel PR, Srinivasan A, Budnitz DS. Emergency department visits for antibiotic-associated adverse events. Clin Infect Dis. 2008;47(6):735-743. doi:10.1086/591126
7. Macy E, Contreras R. Healthcare use and serious infection prevalence associated with penicillin “allergy” in hospitalized patients: a cohort study. J Allergy Clin Immunol. 2014;133(3):790-796. doi:10.1016/j.jaci.2013.09.021
8. Blumenthal KG, Lu N, Zhang Y, Li Y, Walensky RP, Choi HK. Risk of methicillin resistant Staphylococcal aureus and Clostridium difficile in patients with a documented penicillin allergy: population-based matched cohort study. BMJ. 2018;361:k2400. doi:10.1136/bmj.k2400
9. Loo VG, Poirier L, Miller MA, et al. A predominantly clonal multi-institutional outbreak of Clostridium difficile associated diarrhea with high morbidity and mortality. N Engl J Med. 2005;353(23):2442-2449. doi:10.1056/NEJMoa051639
10. Pepin J, Saheb N, Coulombe MA, et al. Emergence of fluoroquinolone as the predominant risk factor for Clostridium difficile-associated diarrhea: a cohort study during an epidemic in Quebec. Clin Infect Dis. 2005;41(9):1254-1260. doi:10.1086/496986
11. Chou HW, Wang JL, Chang CH, et al. Risks of cardiac arrhythmia and mortality among patients using new-generation macrolides, fluoroquinolones, and β-lactam/β-lactamase inhibitors: a Taiwanese nationwide study. Clin Infec Dis. 2015;60(4):566-577. doi:10.1093/cid/ciu914
12. Rao GA, Mann JR, Shoaibi A, et al. Azithromycin and levofloxacin use and increased risk of cardiac arrhythmia and death. Ann Fam Med. 2014;12(2):121-127. doi:10.1370/afm.1601
13. LeBlanc L, Pepin J, Toulouse K, et al. Fluoroquinolone and risk for methicillin-resistant Staphylococcus aureus, Canada. Emerg Infect Dis. 2006;12(9):1398-1405. doi:10.3201/eid1209.060397
14. Schembri S, Williamson PA, Short PM, et al. Cardiovascular events after clarithromycin use in lower respiratory tract infections: analysis of two prospective cohort studies. BMJ. 2013;346:f1245. doi:10.1136/bmj.f1235
15. Ray WA, Murray KT, Hall K, Arbogast PG, Stein CM. Azithromycin and the risk of cardiovascular death. N Engl J Med. 2012;366(20):1881-1890. doi:10.1056/NEJMoa1003833
16. McDaniel JS, Perencevich EN, Diekema DJ, et al. Comparative effectiveness of beta-lactams versus vancomycin for treatment of methicillin-susceptible Staphylococcus aureus bloodstream infections among 122 hospitals. Clin Infect Dis. 2015;61(3):361-367. doi:10.1093/cid/civ308
17. Wong D, Wong T, Romney M, Leung V. Comparison of outcomes in patients with methicillin-susceptible Staphylococcus aureus (MSSA) bacteremia who are treated with β-lactam vs vancomycin empiric therapy: a retrospective cohort study. BMC Infect Dis. 2016;16:224. doi:10.1186/s12879-016-1564-5
18. Blumenthal KG, Shenoy ES, Huang M, et al. The impact of reporting a prior penicillin allergy on the treatment of methicillin-sensitivity Staphylococcus aureus bacteremia. PLoS One. 2016;11(7):e0159406. doi:10.1371/journal.pone.0159406
19. Verani JR, McGee L, Schrag SJ; . Prevention of perinatal group B streptococcal disease. MMWR Recomm Rep. 2010;59(RR-10):1-36.
20. Desai SH, Kaplan MS, Chen Q, Macy EM. Morbidity in pregnant women associated with unverified penicillin allergies, antibiotic use, and group B Streptococcus infections. Perm J. 2017;21:16-080. doi:10.7812/TPP/16-080
21. Blumenthal KG, Ryan EE, Li Y, Lee H, Kuhlen JL, Shenoy ES. The impact of a reported penicillin allergy on surgical site infection risk. Clin Infect Dis. 2018;66(3):329-336. doi:10.1093/cid/cix794
22. National Health Expenditures 2017 Highlights. Centers for Medicare & Medicaid services. https://www.cms.gov/Research-Statistics-Data-and-Systems/Statistics-Trends-and-Reports/NationalHealthExpendData/Downloads/highlights.pdf. Accessed August 25, 2020.
23. Macy E, Shu YH. The effect of penicillin allergy testing on future health care utilization: a matched cohort study. J Allergy Clin Immunol Pract. 2017;5(3):705-710. doi:10.1016/j.jaip.2017.02.012
24. Picard M, Begin P, Bouchard H, et al. Treatment of patients with a history of penicillin allergy in a large tertiary-care academic hospital. J Allergy Clin Immunol Pract. 2013;1(3):252-257. doi:10.1016/j.jaip.2013.01.006
25. US Department of Defense. Beneficiary population statistics. https://health.mil/I-Am-A/Media/Media-Center/Patient-Population-Statistics. Accessed August 25, 2020.
26. Lee RU, Banks TA, Waibel KH, Rodriguez RG. Penicillin allergy…maybe not? The military relevance for penicillin testing and de-labeling. Mil Med. 2019;184(3-4):e163-e168. doi:10.1093/milmed/usy194
27. Antibiotic resistance threats in the United States, 2013. Centers for Disease Control and Prevention. https://www.cdc.gov/drugresistance/threat-report-2013/index.html. Accessed May 10, 2019.
28. Gray GC, Callahan JD, Hawksworth AW, Fisher CA, Gaydos JC. Respiratory diseases among U.S. military personnel: countering emerging threats. Emerg Infect Dis. 1999;5(3):379-385. doi:10.3201/eid0503.990308
29. Beaumier CM, Gomez-Rubio AM, Hotez PJ, Weina PJ. United States military tropical medicine: extraordinary legacy, uncertain future. PLoS Negl Trop Dis. 2013;7(12):e2448. doi:10.1371/journal.pntd.0002448
30. Thomas RJ, Conwill DE, Morton DE, et al. Penicillin prophylaxis for streptococcal infections in the United States Navy and Marine Corps recruit camps, 1951-1985. Rev Infect Dis. 1988;10(1):125-130. doi:10.1093/clinids/10.1.125
31. Tucker MH, Lomas CM, Ramchandar N, Waldram JD. Amoxicillin challenge without penicillin skin testing in evaluation of penicillin allergy in a cohort of Marine recruits. J Allergy Clin Immunol Pract. 2017;5(3):813-815. doi:10.1016/j.jaip.2017.01.023
32. Blumenthal KG, Shenoy ES, Wolfson AR, et al. Addressing inpatient beta-lactam allergies: a multihospital implementation. J Allergy Clin Immunol Pract. 2017;5(3):616-625. doi:10.1016/j.jaip.2017.02.019
33. Kuruvilla M, Shih J, Patel K, Scanlon N. Direct oral amoxicillin challenge without preliminary skin testing in adult patients with allergy and at low risk with reported penicillin allergy. Allergy Asthma Proc. 2019;40(1):57-61. doi:10.2500/aap.2019.40.4184
34. Banks TA, Tucker M, Macy E. Evaluating penicillin allergies without skin testing. Curr Allergy Asthma Rep. 2019;19(5):27. doi:10.1007/s11882-019-0854-6
35. Park M, Markus P, Matesic
1. Centers for Disease Control and Prevention. Outpatient antibiotic prescriptions-United States, 2014. https://www.cdc.gov/antibiotic-use/community/pdfs/annual-reportsummary_2014.pdf. Accessed August 15, 2020.
2. Magill SS, Edwards JR, Beldavs ZG, et al. Prevalence of antimicrobial use in US acute care hospitals, May-September 2011. JAMA. 2014;312(14):1438-1446. doi:10.1001/jama.2014.12923
3. Shenoy ES, Macy E, Rowe T, Blumenthal KG. Evaluation and management of penicillin allergy. JAMA. 2019;321:188-199. doi:10.1001/jama.2018.19283
4. Har D, Solensky R. Penicillin and beta-lactam hypersensitivity. Immunol Allergy Clin North Am. 2017;37(4):643-662. doi:10.1016/j.iac.2017.07.001
5. Tamma PD, Avdic E, Li DX, Dzintars K, Cosgrove SE. Association of adverse events with antibiotic use in hospitalized patients. JAMA Intern Med. 2017;177(9):1308-1315. doi:10.1001/jamainternmed.2017.1938
6. Shebab N, Patel PR, Srinivasan A, Budnitz DS. Emergency department visits for antibiotic-associated adverse events. Clin Infect Dis. 2008;47(6):735-743. doi:10.1086/591126
7. Macy E, Contreras R. Healthcare use and serious infection prevalence associated with penicillin “allergy” in hospitalized patients: a cohort study. J Allergy Clin Immunol. 2014;133(3):790-796. doi:10.1016/j.jaci.2013.09.021
8. Blumenthal KG, Lu N, Zhang Y, Li Y, Walensky RP, Choi HK. Risk of methicillin resistant Staphylococcal aureus and Clostridium difficile in patients with a documented penicillin allergy: population-based matched cohort study. BMJ. 2018;361:k2400. doi:10.1136/bmj.k2400
9. Loo VG, Poirier L, Miller MA, et al. A predominantly clonal multi-institutional outbreak of Clostridium difficile associated diarrhea with high morbidity and mortality. N Engl J Med. 2005;353(23):2442-2449. doi:10.1056/NEJMoa051639
10. Pepin J, Saheb N, Coulombe MA, et al. Emergence of fluoroquinolone as the predominant risk factor for Clostridium difficile-associated diarrhea: a cohort study during an epidemic in Quebec. Clin Infect Dis. 2005;41(9):1254-1260. doi:10.1086/496986
11. Chou HW, Wang JL, Chang CH, et al. Risks of cardiac arrhythmia and mortality among patients using new-generation macrolides, fluoroquinolones, and β-lactam/β-lactamase inhibitors: a Taiwanese nationwide study. Clin Infec Dis. 2015;60(4):566-577. doi:10.1093/cid/ciu914
12. Rao GA, Mann JR, Shoaibi A, et al. Azithromycin and levofloxacin use and increased risk of cardiac arrhythmia and death. Ann Fam Med. 2014;12(2):121-127. doi:10.1370/afm.1601
13. LeBlanc L, Pepin J, Toulouse K, et al. Fluoroquinolone and risk for methicillin-resistant Staphylococcus aureus, Canada. Emerg Infect Dis. 2006;12(9):1398-1405. doi:10.3201/eid1209.060397
14. Schembri S, Williamson PA, Short PM, et al. Cardiovascular events after clarithromycin use in lower respiratory tract infections: analysis of two prospective cohort studies. BMJ. 2013;346:f1245. doi:10.1136/bmj.f1235
15. Ray WA, Murray KT, Hall K, Arbogast PG, Stein CM. Azithromycin and the risk of cardiovascular death. N Engl J Med. 2012;366(20):1881-1890. doi:10.1056/NEJMoa1003833
16. McDaniel JS, Perencevich EN, Diekema DJ, et al. Comparative effectiveness of beta-lactams versus vancomycin for treatment of methicillin-susceptible Staphylococcus aureus bloodstream infections among 122 hospitals. Clin Infect Dis. 2015;61(3):361-367. doi:10.1093/cid/civ308
17. Wong D, Wong T, Romney M, Leung V. Comparison of outcomes in patients with methicillin-susceptible Staphylococcus aureus (MSSA) bacteremia who are treated with β-lactam vs vancomycin empiric therapy: a retrospective cohort study. BMC Infect Dis. 2016;16:224. doi:10.1186/s12879-016-1564-5
18. Blumenthal KG, Shenoy ES, Huang M, et al. The impact of reporting a prior penicillin allergy on the treatment of methicillin-sensitivity Staphylococcus aureus bacteremia. PLoS One. 2016;11(7):e0159406. doi:10.1371/journal.pone.0159406
19. Verani JR, McGee L, Schrag SJ; . Prevention of perinatal group B streptococcal disease. MMWR Recomm Rep. 2010;59(RR-10):1-36.
20. Desai SH, Kaplan MS, Chen Q, Macy EM. Morbidity in pregnant women associated with unverified penicillin allergies, antibiotic use, and group B Streptococcus infections. Perm J. 2017;21:16-080. doi:10.7812/TPP/16-080
21. Blumenthal KG, Ryan EE, Li Y, Lee H, Kuhlen JL, Shenoy ES. The impact of a reported penicillin allergy on surgical site infection risk. Clin Infect Dis. 2018;66(3):329-336. doi:10.1093/cid/cix794
22. National Health Expenditures 2017 Highlights. Centers for Medicare & Medicaid services. https://www.cms.gov/Research-Statistics-Data-and-Systems/Statistics-Trends-and-Reports/NationalHealthExpendData/Downloads/highlights.pdf. Accessed August 25, 2020.
23. Macy E, Shu YH. The effect of penicillin allergy testing on future health care utilization: a matched cohort study. J Allergy Clin Immunol Pract. 2017;5(3):705-710. doi:10.1016/j.jaip.2017.02.012
24. Picard M, Begin P, Bouchard H, et al. Treatment of patients with a history of penicillin allergy in a large tertiary-care academic hospital. J Allergy Clin Immunol Pract. 2013;1(3):252-257. doi:10.1016/j.jaip.2013.01.006
25. US Department of Defense. Beneficiary population statistics. https://health.mil/I-Am-A/Media/Media-Center/Patient-Population-Statistics. Accessed August 25, 2020.
26. Lee RU, Banks TA, Waibel KH, Rodriguez RG. Penicillin allergy…maybe not? The military relevance for penicillin testing and de-labeling. Mil Med. 2019;184(3-4):e163-e168. doi:10.1093/milmed/usy194
27. Antibiotic resistance threats in the United States, 2013. Centers for Disease Control and Prevention. https://www.cdc.gov/drugresistance/threat-report-2013/index.html. Accessed May 10, 2019.
28. Gray GC, Callahan JD, Hawksworth AW, Fisher CA, Gaydos JC. Respiratory diseases among U.S. military personnel: countering emerging threats. Emerg Infect Dis. 1999;5(3):379-385. doi:10.3201/eid0503.990308
29. Beaumier CM, Gomez-Rubio AM, Hotez PJ, Weina PJ. United States military tropical medicine: extraordinary legacy, uncertain future. PLoS Negl Trop Dis. 2013;7(12):e2448. doi:10.1371/journal.pntd.0002448
30. Thomas RJ, Conwill DE, Morton DE, et al. Penicillin prophylaxis for streptococcal infections in the United States Navy and Marine Corps recruit camps, 1951-1985. Rev Infect Dis. 1988;10(1):125-130. doi:10.1093/clinids/10.1.125
31. Tucker MH, Lomas CM, Ramchandar N, Waldram JD. Amoxicillin challenge without penicillin skin testing in evaluation of penicillin allergy in a cohort of Marine recruits. J Allergy Clin Immunol Pract. 2017;5(3):813-815. doi:10.1016/j.jaip.2017.01.023
32. Blumenthal KG, Shenoy ES, Wolfson AR, et al. Addressing inpatient beta-lactam allergies: a multihospital implementation. J Allergy Clin Immunol Pract. 2017;5(3):616-625. doi:10.1016/j.jaip.2017.02.019
33. Kuruvilla M, Shih J, Patel K, Scanlon N. Direct oral amoxicillin challenge without preliminary skin testing in adult patients with allergy and at low risk with reported penicillin allergy. Allergy Asthma Proc. 2019;40(1):57-61. doi:10.2500/aap.2019.40.4184
34. Banks TA, Tucker M, Macy E. Evaluating penicillin allergies without skin testing. Curr Allergy Asthma Rep. 2019;19(5):27. doi:10.1007/s11882-019-0854-6
35. Park M, Markus P, Matesic
Serotonin Syndrome/Serotonin Toxicity
Serotonin, or 5-hydroxytryptamine (5-HT), is a chemical neurotransmitter in the central and peripheral nervous systems that was discovered in 1940s. 1 O ne of the most widely studied chemical messengers , serotonin influences many physiologic functions in humans, including regulation of mood, sleep-wake cycle, appetite suppression, memory, emesis, breathing, cognition, blood coagulation, libido, and many other functions. 2 In 1992, Insel and colleagues first document ed the toxic symptoms produced from too much serotonin in the central and peripheral nervous systems , naming it serotonin syndrome. 3,4
Serotonin Syndrome
Experts in the fields of psychiatry, pharmacy, and toxicology refer to these symptoms as serotonin toxicity, because the symptoms result from the toxic effects of too much serotonin.5-9 The term toxicity instead of syndrome “clarifies that it is a form of poisoning, just as lithium toxicity is a form of poisoning.”6 Therefore, serotonin toxicity (ST) can develop with administration of any serotonin-enhancing medication, including therapeutic use, polypharmacy, or accidental/intentional drug overdose.
The incidence of ST has increased over the past decade.5,6,10,11 Several reasons explain this increase: (1) ST mirrors the increase in depression in the US populations10,12,13; (2) There has been an increase in off-label antidepressant prescribing by both primary care and mental health providers14-16; (3) the increased use of illicit drugs13; (4) an increase in suicide attempts with antidepressants17; and (5) increased use of opioids for pain management, including both prescription and illicit use.11,14 This paper reviews the potential lethal combinations of commonly prescribed medications used to treat both veteran and nonveteran patients and includes the latest information on offending medications; a presentation of symptoms from in utero to adult; diagnostic criteria; and recommended treatments.
The Veterans Health Administration (VHA) and non-VHA health care providers can play a key role in identifying and preventing serotonin syndrome/ST by keeping abreast of the latest updates of potentially lethal drug combinations. Commonly prescribed medications with the potential for a reaction include antidepressants, anxiolytics, pain medications, antinausea medications, herbal medications, and over-the-counter (OTC) medications, such as cough suppressants. Patients may be at increased risk for ST due to the growth of polypharmacy management of comorbidities.
Antidepressants
Over the past decade, antidepressant use has increased substantially in the US,United Kingdom, and Canada.14 Also the types of antidepressants prescribed has changed and been replaced with the newer agents. The selective serotonin reuptake inhibitors (SSRIs) and selective norepinephrine reuptake inhibitors (SNRIs) have replaced the older tricyclics (TCAs) and monoamine oxidase inhibitors (MAOIs) as first-line treatments for depression due to their improved comparative efficacy, reduced mortality following overdose, adverse effects (AEs) that are more tolerable for most patients, and the SSRIs have no anticholinergic properties (except paroxetine) (Table 1).18
In 2017 the National Institute of Mental Health reported that about 17 million adults and 3 million adolescents (aged 11-18 years) experienced at least 1 episode of major depression.19 About 40% of US veterans will experience depression, which is 3 times higher than the rate of the general US population.12 A random sampling survey conducted of about 17,000 active-duty service members by the US Department of Defense (DoD) from November 2015 to April 2016 revealed 9.4% reported depression.20 Antidepressant usage in the US and among veterans continues to increase.12,16 In 2018, the list of top US prescribed drugs, included sertraline (14th), citalopram (21st), trazodone (24th), and escitalopram (26th).21 Antidepressant prescribing in the US increased 18% from 2012 to 2017.22 This trend also continues within the military with a 40% increase of antidepressant use in the past decade.16
One reason for the increase in antidepressant use is off-label prescribing.14,23 A sampling of about 2 billion psychiatric outpatient visits in a western portion of the US found 12.9% of the prescriptions filled were off-label.15 In Minnesota, off-label prescribing of antidepressants was found to contribute to an increase in drug interactions in elderly nursing home residents.24 An investigation by the Military Times of the military community revealed off-label prescribing occurs not only with antidepressant medications, but also with anticonvulsants, antipsychotics, anti-anxiety drugs, and antiepileptic medications.14
A case report that brought ST to the forefront occurred in the 1980s and involved a college student.25 She was initially diagnosed with the flu. Her symptoms progressed over a 24-hour period despite treatment, leading to seizures, hyperthermia, generalized clonus, muscle rigidity, respiratory failure, and death because of unrecognized ST. Her combination of serotonin-elevating drugs included meperidine, phenelzine, chlorpheniramine, and haldol. On autopsy, there were traces of cocaine found in some of her tissue samples.
Pathophysiology
Tryptophan is a precursor of serotonin and must be ingested from foods, including meats, dairy, fruits, and seeds. About 90% of all serotonin is made in the gastrointestinal epithelium and is the major component of the brain-gut axis.26 Serotonin cannot cross the blood-brain barrier; therefore, it is synthesized and stored in presynaptic terminals around the midline of the brainstem.1,26 Transport of serotonin is provided by serotonin transporter (SERT).1,26,27 Once released, serotonin can either stimulate postsynaptic neuron receptors or is taken up into the presynaptic terminals for reuse. SSRI antidepressants, such as citalopram and paroxetine inhibit the reuptake of serotonin by binding to 2 different sites on the SERT thus allowing more available serotonin to be accessible to other neurons.27 There are 7 families of serotonin receptors, 5-HT1 to 5-HT7 and at least 15 mammalian subtypes.28,29 The majority of these receptors have been implicated in depression or depressive-like behavior as evidenced by the efficacy of increasing extracellular serotonin for the treatment of depression with SSRIs, SNRIs, TCAs, and MAOIs.29 Three of the most studied receptors include 5-HTIA,5-HT1B,and 5-HT2A.
Etiology
Most serotonin-induced drug fatalities occur when combining serotonergic drugs that work through different pathways (Table 2).30 The most toxic combination of serotonin-enhancing drugs includes MAOIs taken with SSRIs or SNRIs, or a combination of 2 MAOIs.5-9
Other potentially lethal combinations may includepolypharmacy with antidepressants, pain medications, OTC medications, and illicit drugs. Linezolid, a new synthetic antimicrobial, is considered to be a weak MAOI. Therefore, prescribing it with other serotonin-elevating agents has been reported to precipitate ST.18
Most cases of ST do not require hospitalization and can be managed by stopping the medication or decreasing the dose. Therapeutic doses of a single drug are highly unlikely to cause toxicity, although there have been reported cases of patients who are sensitive or more susceptible and develop symptoms after administration of a single agent and/or a dosage increase.
Delayed ST reactions have occurred because of a prolonged half-life of a drug, iron deficiency anemia, and coingestion of shorter acting serotonin antagonists.31 Most antidepressants have a short half-life (< 24 hours)except for fluoxetine. A decrease in iron may contribute to ST because iron is needed to process serotonin from tryptophan. An example of 2 shorter-acting serotonin antagonists include cyproheptadine and olanzapine. Cyproheptadine is used in the treatment of ST, and olanzapine is an antipsychotic.
Symptoms
Symptoms of ST range from mild to severe and include a combination of neuromuscular, autonomic, and mental status changes (Table 3).5,10 Mild symptoms of ST can start within 1 to 2 hours after ingesting a medication that increases serotonin to a toxic state unless the drug has a long half-life (eg, fluoxetine). Sometimes mild symptoms of ST can be difficult to distinguish from common drug AEs, flu symptoms, or viruses. Patients taking therapeutic doses of SSRIs can experience serotonin symptoms, such as lower limb hyperreflexia or a few beats of ankle clonus without being toxic. One thing to remember is that not all patients will start with mild symptoms and may present in moderate or severe distress.
Moderate-to-severe ST symptoms require hospitalization, usually in the intensive care unit (ICU). At this stage, clonus progresses from the lower extremities to the upper body and becomes more generalized. Ocular clonus can be continuous, intermittent, or have a ping pong effect (short cycle, periodic, alternating lateral gaze).
Severe ST is life threatening and leads to multiorgan failure within hours if not treated. The patient is intubated to assist with breathing and sedated because excess agitation and muscular tremors can increase temperature, which is already elevated by the time the symptoms reach the severe state. Of note, hyperthermia is due to a noninfectious elevation of body temperature from hypertonicity, agitation, and muscle rigidity.A true core temperature > 105.8°F causes irreversible cell damage, cerebral injury, and death.32,33 The patient can develop seizures and a coma. Multiorgan failure occurs, including rhabdomyolysis, myoglobinuria, renal failure, metabolic acidosis, acute respiratory distress, and disseminated intravascular coagulation.
Diagnosis
The diagnosis of ST is clinical and based on a history of ingesting serotonin-elevating medications and physical findings as per Hunter Serotonin Toxicity Criteria34 (Table 4). An in-depth history needs to include previous and current prescriptions, indications of the prescriptions (eg, therapeutic, increase in dosage, suicide intent), OTC medications, and illicit drug use. Early recognition of symptoms, identification of serotonergic medications, and appropriate resuscitative measures lead to more successful outcomes. A serotonin drug level is ineffective and does not correlate with the dosage since serotonin does not cross the blood-brain barrier.
The type of drug determines the length and response of the episode. The drug(s) elimination half-lives need to be calculated along with the pharmacokinetic or pharmacodynamics; agonist, antagonist, reuptake inhibitor, etc. Many drugs have half-lives of < 24 hours; therefore, reducing or eliminating the offending drug(s) will result in a steady reduction of symptoms.Exceptions include medications with a longer activity, such as the irreversible MAOIs (eg, phenelzine, isocarboxazid) and drugs with a longer half-life, such as fluoxetine. These types of medications may have been stopped weeks earlier and may prolong reduction of symptoms.
When initiating or increasing SSRIs or SNRIs, there are common nontoxic AEs that are not consistent with ST, including anxiety, restlessness, and irritability that may last for 2 weeks. The difference in toxic vs nontoxic reactions are the timing and rapid progression of symptoms. The toxic symptoms will start within hours of ingesting the offending agents(s) and progress rapidly to severe symptoms within 24 hours. Therefore, it is imperative to review AEs with the patient and or caregiver, so they may act as their own advocate and seek immediate assistance.
Differentials
There are symptoms specific to ST that can be used to differentiate it from other conditions. These include hyperthermia, bilateral symmetric clonus (inducible, spontaneous, ocular), and hyperreflexia.These criteria form the basis for Hunter criteria.
Differential diagnoses to consider include neuroleptic malignant syndrome; antidepressant initiation AEs; antidepressant discontinuation syndrome; malignant hyperthermia; anticholinergic toxicity; meningitis/encephalitis; sepsis; drug overdose; alcohol/benzodiazepine withdrawal; and preeclampsia. Neuroleptic malignant syndrome (NMS) is the disorder most often misdiagnosed as ST.Key elements that distinguish ST from NMS include the timing of the clinical course (NMS develops over days to weeks); the medications ingested (NMS from dopaminergic drugs); and the symptoms of NMS (bradyreflexia, bradykinesia, bradyphrenia, and no clonus).According to Gillman, serotonin toxicity is a manifestation of toxicity that is predictable and common with specific drug combinations, while NMS is a “rare idiosyncratic reaction to essentially normal doses and very rarely occurs after overdoses.”35 Preeclampsia is a pregnancy complication that can mimic ST with symptoms of hypertension, clonus, and hyperreflexia. It has been estimated to complicate 2% to 8% of pregnancies and remains a principle cause of maternal and fetal morbidity and mortality.36,37
Treatment
Mild-to-moderate symptoms usually resolve on their own 1 to 3 days after decreasing or stopping the offending drug. The timing will depend on the half-life or active metabolites of the drug. Treatment is largely supportive and may require treatment for control of agitation with benzodiazepines and IV fluids for dehydration/hypotension.14 In cases not responding to supportive care, treatment with oral cyproheptadine is recommended.14
There are other medications that have been used in treatment such as olanzapine, chlorpromazine, propranolol, bromocriptine, dantrolene, droperidol, and haloperidol, but their efficacy is unproven and not recommended.10 Chlorpromazine can cause hypotension and increase hyperthermia. Propranolol has a long duration of action, may cause a prolonged hypotension, and can mask tachycardia that can be used to monitor the effectiveness of treatment.10 Bromocriptine is a serotonin agonist and may exacerbate symptoms. Dantrolene has no effect on survival in animal models.10 Droperidol and haloperidol can worsen hyperthermia by inhibiting sweating.38
Mechanical ventilation should be considered especially if muscle rigidity progresses and depressed respiratory function occurs. If the temperature starts to rise, immediate sedation, paralysis, mechanical ventilation, and cyproheptadine are administered.The overall goal is prevention of hyperthermia, which leads to multiorgan failure. A core temperature of ≥ 104°F is associated with neurologic cell death, and recovery is minimal.32 Consultation with an experienced toxicologist is strongly recommended.Antipyretics should not be used, because elevated temperature is centrally mediated from muscle rigidity. If presentation occurs within 1 hour, activated charcoal can be used for detoxification of potentially lethal amounts.
Warning Label Controversies
In 2006, the US Food and Drug Administration (FDA) issued an advisory warning against concurrently using a tryptan antimigraine drug and serotonin-mediated medications.39 In 2018, a research team conducted a 14-year retrospective analysis on 20,000 patients who were coprescribed a tryptan drug with SSRIs or SNRIs.40 The study reported that the risk of ST was rare and suggested that the FDA reconsider their advisory. There are several other controversial medications with a ST FDA warning label due to their mechanisms of action and inaccurate case reports.41
Human Poisonings
Consistent with the 2017 American Association of Poison Control Centers Toxic Exposure report, antidepressants continue to be in the top 5 substance classes most frequently involved in human exposures.42 Most accidental ingestions of antidepressants occur in toddlers, whereas intentional ingestions are usually done by adolesents.43 Over the past 10 years, antidepressants are the No. 1 fastest growing category of human exposures in all age groups.42
ST in the Pediatric Population
ST in the pediatric population mirrors that in adults.Differences include the inability of the child to report symptoms, lack of clinician awareness, and reluctance of adolescents to disclose recreational drug use.Management is the same as for adults, including discontinuing the offending drug, supportive care, adequate sedation, oxygen, IV fluids, and continuous cardiac monitoring. Sedation is weight based for benzodiazepines. Mild-to-moderate reactions require admission for observation. Severe reactions require admission to the ICU.
There have been at least 4 published case reports of children aged < 6 years with moderate-to-severe ST secondary to acute vilazodone ingestion.44 The dosages included 5.5 to 37 mg/kg. All 4 patients had altered mental status, seizures, hyperthermia, mild clonus, tachycardia, and hypertension. They all survived with intensive care treatment, including intubation, sedation, cyproheptadine in 2 cases, activated charcoal and IV lorazepam in the other cases.
Direk and colleagues reported a case of a 12-year-old girl who was brought to the emergency department by her stepmother for seizurelike activity and was diagnosed with epilepsy and status epilepticus.45 In the pediatric ICU she developed tachycardia, fever, agitation, dilated pupils, tremors, increased deep tendon reflexes, spontaneous clonus, and horizontal ocular movements. A detailed clinical history was retaken and revealed that the child had been prescribed risperidone 1 week before by the psychiatric clinic due to behavioral problems, including stealing money, lying, and running away from home and school. On further investigation, the stepmother was taking clomipramine and discovered 9 missing pills.
Pregnancy and Lactation
The American College of Obstetricians and Gynecologists recommends that clinicians screen patients at least once during the perinatal period for depression and anxiety symptoms, using a standardized, validated tool and complete a full assessment of mood and emotional well-being during the postpartum, including screening for postpartum depression and anxiety with a validated instrument.46 Treatment with antidepressants is controversial. “Current evidence is generally reassuring and indicates that the absolute risks of negative infant outcomes are small except for PNAS [poor neonatal adaptation syndrome], which largely appears to be self-limited.”47 Antidepressants cross the human placenta and fetal blood-brain barrier.48 Several cases of infant toxicity from SSRIs have been reported with citalopram and escitalopram.49,50 Symptoms included severe muscle rigidity, lethargy, tachycardia, QTc prolongation, altered consciousness, hypertonia, and seizures at birth. These mothers had taken an SSRI during pregnancy.
Conclusions
This article highlights some of the latest information on ST. Increased awareness of all clinicians and their patients may help decrease unnecessary comorbidities and death. Early identification of ST symptoms will increase the chances for survival, because of the rapid progression of symptoms within 24 hours. Most fatal reactions occur when combining MAOIs with SSRIs, SNRIs, or another MAOI. Overdose with an SSRI does not progress to the severe symptoms unless combined with another serotonin-elevating medication.
Education of all patients who are prescribed antidepressants must include awareness of the potential for serotonergic drug interactions, particularly from OTC medications, herbal medications, and illicit drugs. The diagnosis of ST is based on clinical findings and there must be a history of ingesting serotonin-elevating drug(s). Hunter Serotonin Toxicity Criteria is the gold standard for diagnosing symptoms along with consulting a toxicologist. Prevention of ST includes informed clinicians, patient education, careful prescribing and monitoring, and avoidance of multidrug regimens.
1. Rapport MM, Green AA, Page IH. Serum vasoconstrictor, serotonin; isolation and characterization. J Biol Chem. 1948;176(3):1243-1251.
2. McCorvy JD, Roth BL. Structure and function of serotonin g protein coupled receptors. Pharmacol Ther. 2015;150:129-142. doi:10.1016/j.pharmthera. 2015.01.009 3. Insel TR, Roy BF, Cohen RM, Murphy DL. Possible development of serotonin syndrome in man. Am J Psychiatry. 1982;139(7):954-955. doi:10.1176/ajp.139.7.954
4. Scotton WJ, Hill LJ, Williams AC, Barnes NM. Serotonin syndrome: pathophysiology, clinical features, management, and potential future directions. Int J Tryptophan Res. 2019;12:1-14. doi:10.1177/1178646919873925
5. Buckley N, Dawson AH, Isbister GK. Serotonin syndrome. BMJ. 2014;348:g1626. doi:10.1136/bmj.g1626
6. Gillman KP. Serotonin toxicity: introduction. https://psychotropical.com/serotonin-toxicity-introduction. Published November 13, 2014. Updated July 13, 2019. Accessed August 17, 2020.
7. Foong A-L, Patel T, Kellar J, Grindrod KA. The scoop on serotonin syndrome. Can Pharm J (OTT). 2018;151(4):233-239. doi:10.1177/1715163518779096
8. Foong A-L, Grindrod KA, Patel T, Kellar J. Demystifying serotonin syndrome (or serotonin toxicity). Can Fam Physician. 2018;64(10):720-727.
9. Gillman KP. Serotonin toxicity: summary. https://psychotropical.com/serotonin-toxicity-summary. Published November 13, 2014. Updated January 25, 2018. Accessed August 17, 2020.
10. Boyer EW. Serotonin syndrome (serotonin toxicity). https://www.uptodate.com/contents/serotonin-syndrome-serotonin-toxicity. Updated March 12, 2018. Access December 12, 2019.
11. Wang RZ, Vashistha V, Kaur S, Houchens NW. Serotonin syndrome: preventing, recognizing, and treating it. Cleve Clin J Med. 2016;83(11):810-817. doi:10.3949/ccjm.83a.15129
12. Walker T. The economic burden of depression among veterans. https://www.managedhealthcareexecutive.com/article/economic-burden-depression-among-veterans. Published November 9, 2018. Accessed August 17, 2020.
13. Substance Abuse and Mental Health Services Administration, Center for Behavioral Health Statistics and Quality. Key substance use and mental health indicators in the United States: results from the 2018 National Survey on Drug Use and Health (HHS Publication No. PEP19-5068, NSDUH Series H-54). Rockville, MD: Center for Behavioral Health Statistics and Quality, Substance Abuse and Mental Health Services Administration; 2019.
14. Wong J, Motulsky A, Abrahamowicz M, et al. Off label indications for antidepressants in primary care; descriptive study of prescriptions from an indication based electronic prescribing system. BMJ. 2017;356:j603. doi:org/10.1136/bmj.j603
15. Vijay A, Becker JE, Ross JS. Patterns and predictors of off-label prescription of psychiatric drugs. PLOS One. 2018;13(7):e0198363. doi:10.1371/journal.pone.0198363
16. Medicating the military—use of psychiatric drugs has spiked; concerns surface about suicide, other dangers. https://www.militarytimes.com/2013/03/29/medicating-the-military-use-of-psychiatric-drugs-has-spiked-concerns-surface-about-suicide-other-dangers. Published March 29, 2013. Accessed August 17, 2020.
17. Hengartner MP, Plöderl M. Newer-generation antidepressants and suicide risk in randomized controlled trials; a re-analysis of the FDA database [letter]. Psychother Psychosom. 2019:88:247-248. doi:10.1159/000501215
18. Rosebush PI. Serotonin syndrome. https://www.mhaus.org/nmsis/medical-education-programs/serotonin-syndrome. Accessed March 24, 2020.
19. National Institute of Mental Health. Major depression. https://www.nimh.nih.gov/health/statistics/major-depression.shtml. Updated February 2019. Accessed March 24, 2020.
20. Meadows SO, Engel RL, Beckman RL, et al. 2015 health related behaviors survey: mental and emotional health among U.S. active-duty service members. https://www.rand.org/pubs/research_briefs/RB9955z3.html. Published 2018. Accessed August 17, 2020.
21. ClinCalc. The top 300 drugs of 2020. https://clincalc.com/DrugStats/Top300Drugs.aspx. Update February 11, 2017. Accessed March 24, 2020.
22. Corrigan C. Revealed: massive rise in antidepressant prescribing. https://www.rte.ie/news/investigations-unit/2019/0218/1031271-massive-rise-antidepressant-prescribing. Published June 14, 2019. Accessed August 17, 2020.
23. Skånland SS, Cieślar-Pobuda A. Off-label uses of drugs for depression. Eur J Pharmacol. 2019;865: 172732. doi:10.1016/j.ejphar.2019.172732
24. Bobo WV, Grossardt BR, Lapid MI, et al. Frequency and predictors of the potential overprescribing of antidepressants in elderly residents of a geographically defined U.S. population. Pharmacol Res Perspect. 2019;7(1):e00461. doi:10.1002/prp2.461
25. Patel N. Learning lessons. The Libby Zion case revisited. J Am Coll Cardiol. 2014;64(25):2802-2804. doi:10.1016/j.jacc.2014.11.007
26. Jenkins TA, Nguyen JCD, Polglase KE, Bertrand PP. Influence of tryptophan and serotonin on mood and cognition with a possible role of the gut-brain axis. Nutrients. 2016;8(1):56. doi:10.3390/nu8010056
27. Coleman JA, Green EM, Gouaux E. X-ray structures and mechanism of the human serotonin transporter. Nature Int J Sci. 2016;532(7599):334-339. doi:10.1038/nature17629
28. Garcia-Garcia AL, Newman-Tancredi A, Leonardo ED. 5-HT(1A) [corrected] receptors in mood and anxiety: recent insights into autoreceptor versus heteroreceptor function. Psychopharmacology (Berl). 2013;231(4):623-636. doi:10.1007/s00213-013-3389-x
29. Nautiyal KM, Hen R. Serotonin receptors in depression: from A to B. F1000Res. 2017;6:123. doi:10.12688/f1000research.9736.1
30. Francescangeli J, Karamchandani K, Powell M, Bonavia A. The serotonin syndrome: from molecular mechanisms to clinical practice. Int J Mol Sci. 2019;20(9):2288. doi:10.3390/ijms20092288
31. Little K, Lin CM, Reynolds PM. Delayed serotonin syndrome in the setting of a mixed fluoxetine and serotonin antagonist overdose. Am J Case Rep. 2018;19:604-607. doi:10.12659/AJCR.909063
32. Walter EJ, Carraretto M. The neurological and cognitive consequences of hyperthermia. Crit Care. 2016;20:199. doi:10.1186/s13054-016-1376-4

33. Platt M, Price T. Heat illness. In: Walls, ed. Rosen’s Emergency Medicine: Concepts and Clinical Practice. Philadelphia, PA: Elsevier; 2018:1755-1764.
34. Dunkley EJC, Isbister GK, Sibbritt D, Dawson AH, Whyte IM. The Hunter Serotonin Toxicity Criteria: simple and accurate diagnostic decision rules for serotonin toxicity. QJM. 2003;96(9):635-642. doi:10.1093/qjmed/hcg109
35. Gillman KP. Serotonin toxicity contrasted with neuroleptic malignant syndrome. https://psychotropical.com/serotonin-syndrome-and-neuroleptic-malignant-syndrome. Published January 1, 2005. Updated November 6, 2017. Accessed August 17, 2020.
36. Asusta HB, Keyser E, Dominguez P, Miller M, Odedokun T. Serotonin syndrome in obstetrics: a case report and review of management. Mil Med. 2018;184(1-2):e284-e286. doi:10.1093/milmed/usy135
37. English FA, Kenny LC, McCarthy FP. Risk factors and effective management of preeclampsia. Integr Blood Press Control 2015;8:7-12. doi:10.2147/IBPC.S50641
38. Bruggeman C, O’Day CS. Selective serotonin reuptake inhibitor (SSRI) toxicity. https://pubmed.ncbi.nlm.nih.gov/30521236. Published December 3, 2019. Accessed August 17, 2020.
39. US Food and Drug Administration. Selective serotonin reuptake inhibitors (SSRIs) Information. https://www.fda.gov/drugs/information-drug-class/selective-serotonin-reuptake-inhibitors-ssris-information. Updated December 23, 2014. Accessed March 24, 2020.
40. Orlova Y, Rizzoli P, Loder E. Association of coprescription of triptan antimigraine drugs and selective serotonin reuptake inhibitor or selective norepinephrine reuptake inhibitor antidepressants with serotonin syndrome. JAMA Neurol. 2018;75(5):566-572. doi:10.1001/jamaneurol.2017.5144
41. Gillman KP. Regulatory agencies (WH0, FDA) offer ill-conceived advice about serotonin toxicity (serotonin syndrome) with 5–HT3 antagonist: a worldwide problem. https://psychotropical.com/serotonin-toxicity-and-5-ht3-antagonists. Published November 13, 2014. Updated March 23, 2019. Accessed August 17, 2020.
42. Gummin DD, Mowry JB, Spyker DA, Brooks DE, Osterthaler KM, Banner W. 2017 Annual Report of the American Association of Poison Control Centers’ National Poison Data System (NPDS): 35th Annual Report. Clin Toxicol (Phila). 2018;56(12):1213-1415. doi:10.1080/15563650.2018.1533727
43. Badawy MK, Maffei FA. Pediatric selective serotonin reuptake inhibitor toxicity. https://emedicine.medscape.com/article/1011436. Updated September 27, 2019. Accessed August 17, 2020.
44. Laliberte B, Kishk OA. Serotonin syndrome in a pediatric patient after vilazodone ingestion. Pediatr Emerg Care. 2018;34(12):e226-e228. doi:10.1097/PEC.0000000000001115
45. Direk MC, Yildirim V, Gϋnes S, Bozlu G, Okuyaz C. Serotonin syndrome after clomipramine overdose in a child. Clin Psychopharmacol Neurosci. 2016;14(4):388-390. doi:10.9758/cpn.2016.14.4.38846. ACOG Committee Opinion No. 757: Screening for perinatal depression. Obstet Gynecol. 2018;132(5):e208-e212. doi:10.1097/AOG.0000000000002927
47. Osborne LM, McEvoy K, Payne JL. Antidepressants in pregnancy: balancing needs and risks in clinical practice. Psychiatric Times. 2017;34(4).
48. Stewart D, Vigod S. Antenatal use of antidepressants and risk of teratogenicity and adverse pregnancy outcomes: selective serotonin reuptake inhibitors (SSRIs). https://www.uptodate.com/contents/antenatal-use-of-antidepressants-and-risk-of-teratogenicity-and-adverse-pregnancy-outcomes-selective-serotonin-reuptake-inhibitors-ssris. Accessed March 24, 2020.
49. Degiacomo J, Luedtke S. Neonatal toxicity from escitalopram use in utero: a case report. J Pediatr Pharmacol Ther. 2016;21(6):522-526. doi:10.5863/1551-6776-21.6.522
50. Eleftheriou G, Butera R, Cottini FC, Bonati M, Farina M. Neonatal toxicity following maternal citalopram treatment. Fetal Pediatr Pathol. 2013;32(5):362-356. doi:10.3109/15513815.2013.768743
Serotonin, or 5-hydroxytryptamine (5-HT), is a chemical neurotransmitter in the central and peripheral nervous systems that was discovered in 1940s. 1 O ne of the most widely studied chemical messengers , serotonin influences many physiologic functions in humans, including regulation of mood, sleep-wake cycle, appetite suppression, memory, emesis, breathing, cognition, blood coagulation, libido, and many other functions. 2 In 1992, Insel and colleagues first document ed the toxic symptoms produced from too much serotonin in the central and peripheral nervous systems , naming it serotonin syndrome. 3,4
Serotonin Syndrome
Experts in the fields of psychiatry, pharmacy, and toxicology refer to these symptoms as serotonin toxicity, because the symptoms result from the toxic effects of too much serotonin.5-9 The term toxicity instead of syndrome “clarifies that it is a form of poisoning, just as lithium toxicity is a form of poisoning.”6 Therefore, serotonin toxicity (ST) can develop with administration of any serotonin-enhancing medication, including therapeutic use, polypharmacy, or accidental/intentional drug overdose.
The incidence of ST has increased over the past decade.5,6,10,11 Several reasons explain this increase: (1) ST mirrors the increase in depression in the US populations10,12,13; (2) There has been an increase in off-label antidepressant prescribing by both primary care and mental health providers14-16; (3) the increased use of illicit drugs13; (4) an increase in suicide attempts with antidepressants17; and (5) increased use of opioids for pain management, including both prescription and illicit use.11,14 This paper reviews the potential lethal combinations of commonly prescribed medications used to treat both veteran and nonveteran patients and includes the latest information on offending medications; a presentation of symptoms from in utero to adult; diagnostic criteria; and recommended treatments.
The Veterans Health Administration (VHA) and non-VHA health care providers can play a key role in identifying and preventing serotonin syndrome/ST by keeping abreast of the latest updates of potentially lethal drug combinations. Commonly prescribed medications with the potential for a reaction include antidepressants, anxiolytics, pain medications, antinausea medications, herbal medications, and over-the-counter (OTC) medications, such as cough suppressants. Patients may be at increased risk for ST due to the growth of polypharmacy management of comorbidities.
Antidepressants
Over the past decade, antidepressant use has increased substantially in the US,United Kingdom, and Canada.14 Also the types of antidepressants prescribed has changed and been replaced with the newer agents. The selective serotonin reuptake inhibitors (SSRIs) and selective norepinephrine reuptake inhibitors (SNRIs) have replaced the older tricyclics (TCAs) and monoamine oxidase inhibitors (MAOIs) as first-line treatments for depression due to their improved comparative efficacy, reduced mortality following overdose, adverse effects (AEs) that are more tolerable for most patients, and the SSRIs have no anticholinergic properties (except paroxetine) (Table 1).18
In 2017 the National Institute of Mental Health reported that about 17 million adults and 3 million adolescents (aged 11-18 years) experienced at least 1 episode of major depression.19 About 40% of US veterans will experience depression, which is 3 times higher than the rate of the general US population.12 A random sampling survey conducted of about 17,000 active-duty service members by the US Department of Defense (DoD) from November 2015 to April 2016 revealed 9.4% reported depression.20 Antidepressant usage in the US and among veterans continues to increase.12,16 In 2018, the list of top US prescribed drugs, included sertraline (14th), citalopram (21st), trazodone (24th), and escitalopram (26th).21 Antidepressant prescribing in the US increased 18% from 2012 to 2017.22 This trend also continues within the military with a 40% increase of antidepressant use in the past decade.16
One reason for the increase in antidepressant use is off-label prescribing.14,23 A sampling of about 2 billion psychiatric outpatient visits in a western portion of the US found 12.9% of the prescriptions filled were off-label.15 In Minnesota, off-label prescribing of antidepressants was found to contribute to an increase in drug interactions in elderly nursing home residents.24 An investigation by the Military Times of the military community revealed off-label prescribing occurs not only with antidepressant medications, but also with anticonvulsants, antipsychotics, anti-anxiety drugs, and antiepileptic medications.14
A case report that brought ST to the forefront occurred in the 1980s and involved a college student.25 She was initially diagnosed with the flu. Her symptoms progressed over a 24-hour period despite treatment, leading to seizures, hyperthermia, generalized clonus, muscle rigidity, respiratory failure, and death because of unrecognized ST. Her combination of serotonin-elevating drugs included meperidine, phenelzine, chlorpheniramine, and haldol. On autopsy, there were traces of cocaine found in some of her tissue samples.
Pathophysiology
Tryptophan is a precursor of serotonin and must be ingested from foods, including meats, dairy, fruits, and seeds. About 90% of all serotonin is made in the gastrointestinal epithelium and is the major component of the brain-gut axis.26 Serotonin cannot cross the blood-brain barrier; therefore, it is synthesized and stored in presynaptic terminals around the midline of the brainstem.1,26 Transport of serotonin is provided by serotonin transporter (SERT).1,26,27 Once released, serotonin can either stimulate postsynaptic neuron receptors or is taken up into the presynaptic terminals for reuse. SSRI antidepressants, such as citalopram and paroxetine inhibit the reuptake of serotonin by binding to 2 different sites on the SERT thus allowing more available serotonin to be accessible to other neurons.27 There are 7 families of serotonin receptors, 5-HT1 to 5-HT7 and at least 15 mammalian subtypes.28,29 The majority of these receptors have been implicated in depression or depressive-like behavior as evidenced by the efficacy of increasing extracellular serotonin for the treatment of depression with SSRIs, SNRIs, TCAs, and MAOIs.29 Three of the most studied receptors include 5-HTIA,5-HT1B,and 5-HT2A.
Etiology
Most serotonin-induced drug fatalities occur when combining serotonergic drugs that work through different pathways (Table 2).30 The most toxic combination of serotonin-enhancing drugs includes MAOIs taken with SSRIs or SNRIs, or a combination of 2 MAOIs.5-9
Other potentially lethal combinations may includepolypharmacy with antidepressants, pain medications, OTC medications, and illicit drugs. Linezolid, a new synthetic antimicrobial, is considered to be a weak MAOI. Therefore, prescribing it with other serotonin-elevating agents has been reported to precipitate ST.18
Most cases of ST do not require hospitalization and can be managed by stopping the medication or decreasing the dose. Therapeutic doses of a single drug are highly unlikely to cause toxicity, although there have been reported cases of patients who are sensitive or more susceptible and develop symptoms after administration of a single agent and/or a dosage increase.
Delayed ST reactions have occurred because of a prolonged half-life of a drug, iron deficiency anemia, and coingestion of shorter acting serotonin antagonists.31 Most antidepressants have a short half-life (< 24 hours)except for fluoxetine. A decrease in iron may contribute to ST because iron is needed to process serotonin from tryptophan. An example of 2 shorter-acting serotonin antagonists include cyproheptadine and olanzapine. Cyproheptadine is used in the treatment of ST, and olanzapine is an antipsychotic.
Symptoms
Symptoms of ST range from mild to severe and include a combination of neuromuscular, autonomic, and mental status changes (Table 3).5,10 Mild symptoms of ST can start within 1 to 2 hours after ingesting a medication that increases serotonin to a toxic state unless the drug has a long half-life (eg, fluoxetine). Sometimes mild symptoms of ST can be difficult to distinguish from common drug AEs, flu symptoms, or viruses. Patients taking therapeutic doses of SSRIs can experience serotonin symptoms, such as lower limb hyperreflexia or a few beats of ankle clonus without being toxic. One thing to remember is that not all patients will start with mild symptoms and may present in moderate or severe distress.
Moderate-to-severe ST symptoms require hospitalization, usually in the intensive care unit (ICU). At this stage, clonus progresses from the lower extremities to the upper body and becomes more generalized. Ocular clonus can be continuous, intermittent, or have a ping pong effect (short cycle, periodic, alternating lateral gaze).
Severe ST is life threatening and leads to multiorgan failure within hours if not treated. The patient is intubated to assist with breathing and sedated because excess agitation and muscular tremors can increase temperature, which is already elevated by the time the symptoms reach the severe state. Of note, hyperthermia is due to a noninfectious elevation of body temperature from hypertonicity, agitation, and muscle rigidity.A true core temperature > 105.8°F causes irreversible cell damage, cerebral injury, and death.32,33 The patient can develop seizures and a coma. Multiorgan failure occurs, including rhabdomyolysis, myoglobinuria, renal failure, metabolic acidosis, acute respiratory distress, and disseminated intravascular coagulation.
Diagnosis
The diagnosis of ST is clinical and based on a history of ingesting serotonin-elevating medications and physical findings as per Hunter Serotonin Toxicity Criteria34 (Table 4). An in-depth history needs to include previous and current prescriptions, indications of the prescriptions (eg, therapeutic, increase in dosage, suicide intent), OTC medications, and illicit drug use. Early recognition of symptoms, identification of serotonergic medications, and appropriate resuscitative measures lead to more successful outcomes. A serotonin drug level is ineffective and does not correlate with the dosage since serotonin does not cross the blood-brain barrier.
The type of drug determines the length and response of the episode. The drug(s) elimination half-lives need to be calculated along with the pharmacokinetic or pharmacodynamics; agonist, antagonist, reuptake inhibitor, etc. Many drugs have half-lives of < 24 hours; therefore, reducing or eliminating the offending drug(s) will result in a steady reduction of symptoms.Exceptions include medications with a longer activity, such as the irreversible MAOIs (eg, phenelzine, isocarboxazid) and drugs with a longer half-life, such as fluoxetine. These types of medications may have been stopped weeks earlier and may prolong reduction of symptoms.
When initiating or increasing SSRIs or SNRIs, there are common nontoxic AEs that are not consistent with ST, including anxiety, restlessness, and irritability that may last for 2 weeks. The difference in toxic vs nontoxic reactions are the timing and rapid progression of symptoms. The toxic symptoms will start within hours of ingesting the offending agents(s) and progress rapidly to severe symptoms within 24 hours. Therefore, it is imperative to review AEs with the patient and or caregiver, so they may act as their own advocate and seek immediate assistance.
Differentials
There are symptoms specific to ST that can be used to differentiate it from other conditions. These include hyperthermia, bilateral symmetric clonus (inducible, spontaneous, ocular), and hyperreflexia.These criteria form the basis for Hunter criteria.
Differential diagnoses to consider include neuroleptic malignant syndrome; antidepressant initiation AEs; antidepressant discontinuation syndrome; malignant hyperthermia; anticholinergic toxicity; meningitis/encephalitis; sepsis; drug overdose; alcohol/benzodiazepine withdrawal; and preeclampsia. Neuroleptic malignant syndrome (NMS) is the disorder most often misdiagnosed as ST.Key elements that distinguish ST from NMS include the timing of the clinical course (NMS develops over days to weeks); the medications ingested (NMS from dopaminergic drugs); and the symptoms of NMS (bradyreflexia, bradykinesia, bradyphrenia, and no clonus).According to Gillman, serotonin toxicity is a manifestation of toxicity that is predictable and common with specific drug combinations, while NMS is a “rare idiosyncratic reaction to essentially normal doses and very rarely occurs after overdoses.”35 Preeclampsia is a pregnancy complication that can mimic ST with symptoms of hypertension, clonus, and hyperreflexia. It has been estimated to complicate 2% to 8% of pregnancies and remains a principle cause of maternal and fetal morbidity and mortality.36,37
Treatment
Mild-to-moderate symptoms usually resolve on their own 1 to 3 days after decreasing or stopping the offending drug. The timing will depend on the half-life or active metabolites of the drug. Treatment is largely supportive and may require treatment for control of agitation with benzodiazepines and IV fluids for dehydration/hypotension.14 In cases not responding to supportive care, treatment with oral cyproheptadine is recommended.14
There are other medications that have been used in treatment such as olanzapine, chlorpromazine, propranolol, bromocriptine, dantrolene, droperidol, and haloperidol, but their efficacy is unproven and not recommended.10 Chlorpromazine can cause hypotension and increase hyperthermia. Propranolol has a long duration of action, may cause a prolonged hypotension, and can mask tachycardia that can be used to monitor the effectiveness of treatment.10 Bromocriptine is a serotonin agonist and may exacerbate symptoms. Dantrolene has no effect on survival in animal models.10 Droperidol and haloperidol can worsen hyperthermia by inhibiting sweating.38
Mechanical ventilation should be considered especially if muscle rigidity progresses and depressed respiratory function occurs. If the temperature starts to rise, immediate sedation, paralysis, mechanical ventilation, and cyproheptadine are administered.The overall goal is prevention of hyperthermia, which leads to multiorgan failure. A core temperature of ≥ 104°F is associated with neurologic cell death, and recovery is minimal.32 Consultation with an experienced toxicologist is strongly recommended.Antipyretics should not be used, because elevated temperature is centrally mediated from muscle rigidity. If presentation occurs within 1 hour, activated charcoal can be used for detoxification of potentially lethal amounts.
Warning Label Controversies
In 2006, the US Food and Drug Administration (FDA) issued an advisory warning against concurrently using a tryptan antimigraine drug and serotonin-mediated medications.39 In 2018, a research team conducted a 14-year retrospective analysis on 20,000 patients who were coprescribed a tryptan drug with SSRIs or SNRIs.40 The study reported that the risk of ST was rare and suggested that the FDA reconsider their advisory. There are several other controversial medications with a ST FDA warning label due to their mechanisms of action and inaccurate case reports.41
Human Poisonings
Consistent with the 2017 American Association of Poison Control Centers Toxic Exposure report, antidepressants continue to be in the top 5 substance classes most frequently involved in human exposures.42 Most accidental ingestions of antidepressants occur in toddlers, whereas intentional ingestions are usually done by adolesents.43 Over the past 10 years, antidepressants are the No. 1 fastest growing category of human exposures in all age groups.42
ST in the Pediatric Population
ST in the pediatric population mirrors that in adults.Differences include the inability of the child to report symptoms, lack of clinician awareness, and reluctance of adolescents to disclose recreational drug use.Management is the same as for adults, including discontinuing the offending drug, supportive care, adequate sedation, oxygen, IV fluids, and continuous cardiac monitoring. Sedation is weight based for benzodiazepines. Mild-to-moderate reactions require admission for observation. Severe reactions require admission to the ICU.
There have been at least 4 published case reports of children aged < 6 years with moderate-to-severe ST secondary to acute vilazodone ingestion.44 The dosages included 5.5 to 37 mg/kg. All 4 patients had altered mental status, seizures, hyperthermia, mild clonus, tachycardia, and hypertension. They all survived with intensive care treatment, including intubation, sedation, cyproheptadine in 2 cases, activated charcoal and IV lorazepam in the other cases.
Direk and colleagues reported a case of a 12-year-old girl who was brought to the emergency department by her stepmother for seizurelike activity and was diagnosed with epilepsy and status epilepticus.45 In the pediatric ICU she developed tachycardia, fever, agitation, dilated pupils, tremors, increased deep tendon reflexes, spontaneous clonus, and horizontal ocular movements. A detailed clinical history was retaken and revealed that the child had been prescribed risperidone 1 week before by the psychiatric clinic due to behavioral problems, including stealing money, lying, and running away from home and school. On further investigation, the stepmother was taking clomipramine and discovered 9 missing pills.
Pregnancy and Lactation
The American College of Obstetricians and Gynecologists recommends that clinicians screen patients at least once during the perinatal period for depression and anxiety symptoms, using a standardized, validated tool and complete a full assessment of mood and emotional well-being during the postpartum, including screening for postpartum depression and anxiety with a validated instrument.46 Treatment with antidepressants is controversial. “Current evidence is generally reassuring and indicates that the absolute risks of negative infant outcomes are small except for PNAS [poor neonatal adaptation syndrome], which largely appears to be self-limited.”47 Antidepressants cross the human placenta and fetal blood-brain barrier.48 Several cases of infant toxicity from SSRIs have been reported with citalopram and escitalopram.49,50 Symptoms included severe muscle rigidity, lethargy, tachycardia, QTc prolongation, altered consciousness, hypertonia, and seizures at birth. These mothers had taken an SSRI during pregnancy.
Conclusions
This article highlights some of the latest information on ST. Increased awareness of all clinicians and their patients may help decrease unnecessary comorbidities and death. Early identification of ST symptoms will increase the chances for survival, because of the rapid progression of symptoms within 24 hours. Most fatal reactions occur when combining MAOIs with SSRIs, SNRIs, or another MAOI. Overdose with an SSRI does not progress to the severe symptoms unless combined with another serotonin-elevating medication.
Education of all patients who are prescribed antidepressants must include awareness of the potential for serotonergic drug interactions, particularly from OTC medications, herbal medications, and illicit drugs. The diagnosis of ST is based on clinical findings and there must be a history of ingesting serotonin-elevating drug(s). Hunter Serotonin Toxicity Criteria is the gold standard for diagnosing symptoms along with consulting a toxicologist. Prevention of ST includes informed clinicians, patient education, careful prescribing and monitoring, and avoidance of multidrug regimens.
Serotonin, or 5-hydroxytryptamine (5-HT), is a chemical neurotransmitter in the central and peripheral nervous systems that was discovered in 1940s. 1 O ne of the most widely studied chemical messengers , serotonin influences many physiologic functions in humans, including regulation of mood, sleep-wake cycle, appetite suppression, memory, emesis, breathing, cognition, blood coagulation, libido, and many other functions. 2 In 1992, Insel and colleagues first document ed the toxic symptoms produced from too much serotonin in the central and peripheral nervous systems , naming it serotonin syndrome. 3,4
Serotonin Syndrome
Experts in the fields of psychiatry, pharmacy, and toxicology refer to these symptoms as serotonin toxicity, because the symptoms result from the toxic effects of too much serotonin.5-9 The term toxicity instead of syndrome “clarifies that it is a form of poisoning, just as lithium toxicity is a form of poisoning.”6 Therefore, serotonin toxicity (ST) can develop with administration of any serotonin-enhancing medication, including therapeutic use, polypharmacy, or accidental/intentional drug overdose.
The incidence of ST has increased over the past decade.5,6,10,11 Several reasons explain this increase: (1) ST mirrors the increase in depression in the US populations10,12,13; (2) There has been an increase in off-label antidepressant prescribing by both primary care and mental health providers14-16; (3) the increased use of illicit drugs13; (4) an increase in suicide attempts with antidepressants17; and (5) increased use of opioids for pain management, including both prescription and illicit use.11,14 This paper reviews the potential lethal combinations of commonly prescribed medications used to treat both veteran and nonveteran patients and includes the latest information on offending medications; a presentation of symptoms from in utero to adult; diagnostic criteria; and recommended treatments.
The Veterans Health Administration (VHA) and non-VHA health care providers can play a key role in identifying and preventing serotonin syndrome/ST by keeping abreast of the latest updates of potentially lethal drug combinations. Commonly prescribed medications with the potential for a reaction include antidepressants, anxiolytics, pain medications, antinausea medications, herbal medications, and over-the-counter (OTC) medications, such as cough suppressants. Patients may be at increased risk for ST due to the growth of polypharmacy management of comorbidities.
Antidepressants
Over the past decade, antidepressant use has increased substantially in the US,United Kingdom, and Canada.14 Also the types of antidepressants prescribed has changed and been replaced with the newer agents. The selective serotonin reuptake inhibitors (SSRIs) and selective norepinephrine reuptake inhibitors (SNRIs) have replaced the older tricyclics (TCAs) and monoamine oxidase inhibitors (MAOIs) as first-line treatments for depression due to their improved comparative efficacy, reduced mortality following overdose, adverse effects (AEs) that are more tolerable for most patients, and the SSRIs have no anticholinergic properties (except paroxetine) (Table 1).18
In 2017 the National Institute of Mental Health reported that about 17 million adults and 3 million adolescents (aged 11-18 years) experienced at least 1 episode of major depression.19 About 40% of US veterans will experience depression, which is 3 times higher than the rate of the general US population.12 A random sampling survey conducted of about 17,000 active-duty service members by the US Department of Defense (DoD) from November 2015 to April 2016 revealed 9.4% reported depression.20 Antidepressant usage in the US and among veterans continues to increase.12,16 In 2018, the list of top US prescribed drugs, included sertraline (14th), citalopram (21st), trazodone (24th), and escitalopram (26th).21 Antidepressant prescribing in the US increased 18% from 2012 to 2017.22 This trend also continues within the military with a 40% increase of antidepressant use in the past decade.16
One reason for the increase in antidepressant use is off-label prescribing.14,23 A sampling of about 2 billion psychiatric outpatient visits in a western portion of the US found 12.9% of the prescriptions filled were off-label.15 In Minnesota, off-label prescribing of antidepressants was found to contribute to an increase in drug interactions in elderly nursing home residents.24 An investigation by the Military Times of the military community revealed off-label prescribing occurs not only with antidepressant medications, but also with anticonvulsants, antipsychotics, anti-anxiety drugs, and antiepileptic medications.14
A case report that brought ST to the forefront occurred in the 1980s and involved a college student.25 She was initially diagnosed with the flu. Her symptoms progressed over a 24-hour period despite treatment, leading to seizures, hyperthermia, generalized clonus, muscle rigidity, respiratory failure, and death because of unrecognized ST. Her combination of serotonin-elevating drugs included meperidine, phenelzine, chlorpheniramine, and haldol. On autopsy, there were traces of cocaine found in some of her tissue samples.
Pathophysiology
Tryptophan is a precursor of serotonin and must be ingested from foods, including meats, dairy, fruits, and seeds. About 90% of all serotonin is made in the gastrointestinal epithelium and is the major component of the brain-gut axis.26 Serotonin cannot cross the blood-brain barrier; therefore, it is synthesized and stored in presynaptic terminals around the midline of the brainstem.1,26 Transport of serotonin is provided by serotonin transporter (SERT).1,26,27 Once released, serotonin can either stimulate postsynaptic neuron receptors or is taken up into the presynaptic terminals for reuse. SSRI antidepressants, such as citalopram and paroxetine inhibit the reuptake of serotonin by binding to 2 different sites on the SERT thus allowing more available serotonin to be accessible to other neurons.27 There are 7 families of serotonin receptors, 5-HT1 to 5-HT7 and at least 15 mammalian subtypes.28,29 The majority of these receptors have been implicated in depression or depressive-like behavior as evidenced by the efficacy of increasing extracellular serotonin for the treatment of depression with SSRIs, SNRIs, TCAs, and MAOIs.29 Three of the most studied receptors include 5-HTIA,5-HT1B,and 5-HT2A.
Etiology
Most serotonin-induced drug fatalities occur when combining serotonergic drugs that work through different pathways (Table 2).30 The most toxic combination of serotonin-enhancing drugs includes MAOIs taken with SSRIs or SNRIs, or a combination of 2 MAOIs.5-9
Other potentially lethal combinations may includepolypharmacy with antidepressants, pain medications, OTC medications, and illicit drugs. Linezolid, a new synthetic antimicrobial, is considered to be a weak MAOI. Therefore, prescribing it with other serotonin-elevating agents has been reported to precipitate ST.18
Most cases of ST do not require hospitalization and can be managed by stopping the medication or decreasing the dose. Therapeutic doses of a single drug are highly unlikely to cause toxicity, although there have been reported cases of patients who are sensitive or more susceptible and develop symptoms after administration of a single agent and/or a dosage increase.
Delayed ST reactions have occurred because of a prolonged half-life of a drug, iron deficiency anemia, and coingestion of shorter acting serotonin antagonists.31 Most antidepressants have a short half-life (< 24 hours)except for fluoxetine. A decrease in iron may contribute to ST because iron is needed to process serotonin from tryptophan. An example of 2 shorter-acting serotonin antagonists include cyproheptadine and olanzapine. Cyproheptadine is used in the treatment of ST, and olanzapine is an antipsychotic.
Symptoms
Symptoms of ST range from mild to severe and include a combination of neuromuscular, autonomic, and mental status changes (Table 3).5,10 Mild symptoms of ST can start within 1 to 2 hours after ingesting a medication that increases serotonin to a toxic state unless the drug has a long half-life (eg, fluoxetine). Sometimes mild symptoms of ST can be difficult to distinguish from common drug AEs, flu symptoms, or viruses. Patients taking therapeutic doses of SSRIs can experience serotonin symptoms, such as lower limb hyperreflexia or a few beats of ankle clonus without being toxic. One thing to remember is that not all patients will start with mild symptoms and may present in moderate or severe distress.
Moderate-to-severe ST symptoms require hospitalization, usually in the intensive care unit (ICU). At this stage, clonus progresses from the lower extremities to the upper body and becomes more generalized. Ocular clonus can be continuous, intermittent, or have a ping pong effect (short cycle, periodic, alternating lateral gaze).
Severe ST is life threatening and leads to multiorgan failure within hours if not treated. The patient is intubated to assist with breathing and sedated because excess agitation and muscular tremors can increase temperature, which is already elevated by the time the symptoms reach the severe state. Of note, hyperthermia is due to a noninfectious elevation of body temperature from hypertonicity, agitation, and muscle rigidity.A true core temperature > 105.8°F causes irreversible cell damage, cerebral injury, and death.32,33 The patient can develop seizures and a coma. Multiorgan failure occurs, including rhabdomyolysis, myoglobinuria, renal failure, metabolic acidosis, acute respiratory distress, and disseminated intravascular coagulation.
Diagnosis
The diagnosis of ST is clinical and based on a history of ingesting serotonin-elevating medications and physical findings as per Hunter Serotonin Toxicity Criteria34 (Table 4). An in-depth history needs to include previous and current prescriptions, indications of the prescriptions (eg, therapeutic, increase in dosage, suicide intent), OTC medications, and illicit drug use. Early recognition of symptoms, identification of serotonergic medications, and appropriate resuscitative measures lead to more successful outcomes. A serotonin drug level is ineffective and does not correlate with the dosage since serotonin does not cross the blood-brain barrier.
The type of drug determines the length and response of the episode. The drug(s) elimination half-lives need to be calculated along with the pharmacokinetic or pharmacodynamics; agonist, antagonist, reuptake inhibitor, etc. Many drugs have half-lives of < 24 hours; therefore, reducing or eliminating the offending drug(s) will result in a steady reduction of symptoms.Exceptions include medications with a longer activity, such as the irreversible MAOIs (eg, phenelzine, isocarboxazid) and drugs with a longer half-life, such as fluoxetine. These types of medications may have been stopped weeks earlier and may prolong reduction of symptoms.
When initiating or increasing SSRIs or SNRIs, there are common nontoxic AEs that are not consistent with ST, including anxiety, restlessness, and irritability that may last for 2 weeks. The difference in toxic vs nontoxic reactions are the timing and rapid progression of symptoms. The toxic symptoms will start within hours of ingesting the offending agents(s) and progress rapidly to severe symptoms within 24 hours. Therefore, it is imperative to review AEs with the patient and or caregiver, so they may act as their own advocate and seek immediate assistance.
Differentials
There are symptoms specific to ST that can be used to differentiate it from other conditions. These include hyperthermia, bilateral symmetric clonus (inducible, spontaneous, ocular), and hyperreflexia.These criteria form the basis for Hunter criteria.
Differential diagnoses to consider include neuroleptic malignant syndrome; antidepressant initiation AEs; antidepressant discontinuation syndrome; malignant hyperthermia; anticholinergic toxicity; meningitis/encephalitis; sepsis; drug overdose; alcohol/benzodiazepine withdrawal; and preeclampsia. Neuroleptic malignant syndrome (NMS) is the disorder most often misdiagnosed as ST.Key elements that distinguish ST from NMS include the timing of the clinical course (NMS develops over days to weeks); the medications ingested (NMS from dopaminergic drugs); and the symptoms of NMS (bradyreflexia, bradykinesia, bradyphrenia, and no clonus).According to Gillman, serotonin toxicity is a manifestation of toxicity that is predictable and common with specific drug combinations, while NMS is a “rare idiosyncratic reaction to essentially normal doses and very rarely occurs after overdoses.”35 Preeclampsia is a pregnancy complication that can mimic ST with symptoms of hypertension, clonus, and hyperreflexia. It has been estimated to complicate 2% to 8% of pregnancies and remains a principle cause of maternal and fetal morbidity and mortality.36,37
Treatment
Mild-to-moderate symptoms usually resolve on their own 1 to 3 days after decreasing or stopping the offending drug. The timing will depend on the half-life or active metabolites of the drug. Treatment is largely supportive and may require treatment for control of agitation with benzodiazepines and IV fluids for dehydration/hypotension.14 In cases not responding to supportive care, treatment with oral cyproheptadine is recommended.14
There are other medications that have been used in treatment such as olanzapine, chlorpromazine, propranolol, bromocriptine, dantrolene, droperidol, and haloperidol, but their efficacy is unproven and not recommended.10 Chlorpromazine can cause hypotension and increase hyperthermia. Propranolol has a long duration of action, may cause a prolonged hypotension, and can mask tachycardia that can be used to monitor the effectiveness of treatment.10 Bromocriptine is a serotonin agonist and may exacerbate symptoms. Dantrolene has no effect on survival in animal models.10 Droperidol and haloperidol can worsen hyperthermia by inhibiting sweating.38
Mechanical ventilation should be considered especially if muscle rigidity progresses and depressed respiratory function occurs. If the temperature starts to rise, immediate sedation, paralysis, mechanical ventilation, and cyproheptadine are administered.The overall goal is prevention of hyperthermia, which leads to multiorgan failure. A core temperature of ≥ 104°F is associated with neurologic cell death, and recovery is minimal.32 Consultation with an experienced toxicologist is strongly recommended.Antipyretics should not be used, because elevated temperature is centrally mediated from muscle rigidity. If presentation occurs within 1 hour, activated charcoal can be used for detoxification of potentially lethal amounts.
Warning Label Controversies
In 2006, the US Food and Drug Administration (FDA) issued an advisory warning against concurrently using a tryptan antimigraine drug and serotonin-mediated medications.39 In 2018, a research team conducted a 14-year retrospective analysis on 20,000 patients who were coprescribed a tryptan drug with SSRIs or SNRIs.40 The study reported that the risk of ST was rare and suggested that the FDA reconsider their advisory. There are several other controversial medications with a ST FDA warning label due to their mechanisms of action and inaccurate case reports.41
Human Poisonings
Consistent with the 2017 American Association of Poison Control Centers Toxic Exposure report, antidepressants continue to be in the top 5 substance classes most frequently involved in human exposures.42 Most accidental ingestions of antidepressants occur in toddlers, whereas intentional ingestions are usually done by adolesents.43 Over the past 10 years, antidepressants are the No. 1 fastest growing category of human exposures in all age groups.42
ST in the Pediatric Population
ST in the pediatric population mirrors that in adults.Differences include the inability of the child to report symptoms, lack of clinician awareness, and reluctance of adolescents to disclose recreational drug use.Management is the same as for adults, including discontinuing the offending drug, supportive care, adequate sedation, oxygen, IV fluids, and continuous cardiac monitoring. Sedation is weight based for benzodiazepines. Mild-to-moderate reactions require admission for observation. Severe reactions require admission to the ICU.
There have been at least 4 published case reports of children aged < 6 years with moderate-to-severe ST secondary to acute vilazodone ingestion.44 The dosages included 5.5 to 37 mg/kg. All 4 patients had altered mental status, seizures, hyperthermia, mild clonus, tachycardia, and hypertension. They all survived with intensive care treatment, including intubation, sedation, cyproheptadine in 2 cases, activated charcoal and IV lorazepam in the other cases.
Direk and colleagues reported a case of a 12-year-old girl who was brought to the emergency department by her stepmother for seizurelike activity and was diagnosed with epilepsy and status epilepticus.45 In the pediatric ICU she developed tachycardia, fever, agitation, dilated pupils, tremors, increased deep tendon reflexes, spontaneous clonus, and horizontal ocular movements. A detailed clinical history was retaken and revealed that the child had been prescribed risperidone 1 week before by the psychiatric clinic due to behavioral problems, including stealing money, lying, and running away from home and school. On further investigation, the stepmother was taking clomipramine and discovered 9 missing pills.
Pregnancy and Lactation
The American College of Obstetricians and Gynecologists recommends that clinicians screen patients at least once during the perinatal period for depression and anxiety symptoms, using a standardized, validated tool and complete a full assessment of mood and emotional well-being during the postpartum, including screening for postpartum depression and anxiety with a validated instrument.46 Treatment with antidepressants is controversial. “Current evidence is generally reassuring and indicates that the absolute risks of negative infant outcomes are small except for PNAS [poor neonatal adaptation syndrome], which largely appears to be self-limited.”47 Antidepressants cross the human placenta and fetal blood-brain barrier.48 Several cases of infant toxicity from SSRIs have been reported with citalopram and escitalopram.49,50 Symptoms included severe muscle rigidity, lethargy, tachycardia, QTc prolongation, altered consciousness, hypertonia, and seizures at birth. These mothers had taken an SSRI during pregnancy.
Conclusions
This article highlights some of the latest information on ST. Increased awareness of all clinicians and their patients may help decrease unnecessary comorbidities and death. Early identification of ST symptoms will increase the chances for survival, because of the rapid progression of symptoms within 24 hours. Most fatal reactions occur when combining MAOIs with SSRIs, SNRIs, or another MAOI. Overdose with an SSRI does not progress to the severe symptoms unless combined with another serotonin-elevating medication.
Education of all patients who are prescribed antidepressants must include awareness of the potential for serotonergic drug interactions, particularly from OTC medications, herbal medications, and illicit drugs. The diagnosis of ST is based on clinical findings and there must be a history of ingesting serotonin-elevating drug(s). Hunter Serotonin Toxicity Criteria is the gold standard for diagnosing symptoms along with consulting a toxicologist. Prevention of ST includes informed clinicians, patient education, careful prescribing and monitoring, and avoidance of multidrug regimens.
1. Rapport MM, Green AA, Page IH. Serum vasoconstrictor, serotonin; isolation and characterization. J Biol Chem. 1948;176(3):1243-1251.
2. McCorvy JD, Roth BL. Structure and function of serotonin g protein coupled receptors. Pharmacol Ther. 2015;150:129-142. doi:10.1016/j.pharmthera. 2015.01.009 3. Insel TR, Roy BF, Cohen RM, Murphy DL. Possible development of serotonin syndrome in man. Am J Psychiatry. 1982;139(7):954-955. doi:10.1176/ajp.139.7.954
4. Scotton WJ, Hill LJ, Williams AC, Barnes NM. Serotonin syndrome: pathophysiology, clinical features, management, and potential future directions. Int J Tryptophan Res. 2019;12:1-14. doi:10.1177/1178646919873925
5. Buckley N, Dawson AH, Isbister GK. Serotonin syndrome. BMJ. 2014;348:g1626. doi:10.1136/bmj.g1626
6. Gillman KP. Serotonin toxicity: introduction. https://psychotropical.com/serotonin-toxicity-introduction. Published November 13, 2014. Updated July 13, 2019. Accessed August 17, 2020.
7. Foong A-L, Patel T, Kellar J, Grindrod KA. The scoop on serotonin syndrome. Can Pharm J (OTT). 2018;151(4):233-239. doi:10.1177/1715163518779096
8. Foong A-L, Grindrod KA, Patel T, Kellar J. Demystifying serotonin syndrome (or serotonin toxicity). Can Fam Physician. 2018;64(10):720-727.
9. Gillman KP. Serotonin toxicity: summary. https://psychotropical.com/serotonin-toxicity-summary. Published November 13, 2014. Updated January 25, 2018. Accessed August 17, 2020.
10. Boyer EW. Serotonin syndrome (serotonin toxicity). https://www.uptodate.com/contents/serotonin-syndrome-serotonin-toxicity. Updated March 12, 2018. Access December 12, 2019.
11. Wang RZ, Vashistha V, Kaur S, Houchens NW. Serotonin syndrome: preventing, recognizing, and treating it. Cleve Clin J Med. 2016;83(11):810-817. doi:10.3949/ccjm.83a.15129
12. Walker T. The economic burden of depression among veterans. https://www.managedhealthcareexecutive.com/article/economic-burden-depression-among-veterans. Published November 9, 2018. Accessed August 17, 2020.
13. Substance Abuse and Mental Health Services Administration, Center for Behavioral Health Statistics and Quality. Key substance use and mental health indicators in the United States: results from the 2018 National Survey on Drug Use and Health (HHS Publication No. PEP19-5068, NSDUH Series H-54). Rockville, MD: Center for Behavioral Health Statistics and Quality, Substance Abuse and Mental Health Services Administration; 2019.
14. Wong J, Motulsky A, Abrahamowicz M, et al. Off label indications for antidepressants in primary care; descriptive study of prescriptions from an indication based electronic prescribing system. BMJ. 2017;356:j603. doi:org/10.1136/bmj.j603
15. Vijay A, Becker JE, Ross JS. Patterns and predictors of off-label prescription of psychiatric drugs. PLOS One. 2018;13(7):e0198363. doi:10.1371/journal.pone.0198363
16. Medicating the military—use of psychiatric drugs has spiked; concerns surface about suicide, other dangers. https://www.militarytimes.com/2013/03/29/medicating-the-military-use-of-psychiatric-drugs-has-spiked-concerns-surface-about-suicide-other-dangers. Published March 29, 2013. Accessed August 17, 2020.
17. Hengartner MP, Plöderl M. Newer-generation antidepressants and suicide risk in randomized controlled trials; a re-analysis of the FDA database [letter]. Psychother Psychosom. 2019:88:247-248. doi:10.1159/000501215
18. Rosebush PI. Serotonin syndrome. https://www.mhaus.org/nmsis/medical-education-programs/serotonin-syndrome. Accessed March 24, 2020.
19. National Institute of Mental Health. Major depression. https://www.nimh.nih.gov/health/statistics/major-depression.shtml. Updated February 2019. Accessed March 24, 2020.
20. Meadows SO, Engel RL, Beckman RL, et al. 2015 health related behaviors survey: mental and emotional health among U.S. active-duty service members. https://www.rand.org/pubs/research_briefs/RB9955z3.html. Published 2018. Accessed August 17, 2020.
21. ClinCalc. The top 300 drugs of 2020. https://clincalc.com/DrugStats/Top300Drugs.aspx. Update February 11, 2017. Accessed March 24, 2020.
22. Corrigan C. Revealed: massive rise in antidepressant prescribing. https://www.rte.ie/news/investigations-unit/2019/0218/1031271-massive-rise-antidepressant-prescribing. Published June 14, 2019. Accessed August 17, 2020.
23. Skånland SS, Cieślar-Pobuda A. Off-label uses of drugs for depression. Eur J Pharmacol. 2019;865: 172732. doi:10.1016/j.ejphar.2019.172732
24. Bobo WV, Grossardt BR, Lapid MI, et al. Frequency and predictors of the potential overprescribing of antidepressants in elderly residents of a geographically defined U.S. population. Pharmacol Res Perspect. 2019;7(1):e00461. doi:10.1002/prp2.461
25. Patel N. Learning lessons. The Libby Zion case revisited. J Am Coll Cardiol. 2014;64(25):2802-2804. doi:10.1016/j.jacc.2014.11.007
26. Jenkins TA, Nguyen JCD, Polglase KE, Bertrand PP. Influence of tryptophan and serotonin on mood and cognition with a possible role of the gut-brain axis. Nutrients. 2016;8(1):56. doi:10.3390/nu8010056
27. Coleman JA, Green EM, Gouaux E. X-ray structures and mechanism of the human serotonin transporter. Nature Int J Sci. 2016;532(7599):334-339. doi:10.1038/nature17629
28. Garcia-Garcia AL, Newman-Tancredi A, Leonardo ED. 5-HT(1A) [corrected] receptors in mood and anxiety: recent insights into autoreceptor versus heteroreceptor function. Psychopharmacology (Berl). 2013;231(4):623-636. doi:10.1007/s00213-013-3389-x
29. Nautiyal KM, Hen R. Serotonin receptors in depression: from A to B. F1000Res. 2017;6:123. doi:10.12688/f1000research.9736.1
30. Francescangeli J, Karamchandani K, Powell M, Bonavia A. The serotonin syndrome: from molecular mechanisms to clinical practice. Int J Mol Sci. 2019;20(9):2288. doi:10.3390/ijms20092288
31. Little K, Lin CM, Reynolds PM. Delayed serotonin syndrome in the setting of a mixed fluoxetine and serotonin antagonist overdose. Am J Case Rep. 2018;19:604-607. doi:10.12659/AJCR.909063
32. Walter EJ, Carraretto M. The neurological and cognitive consequences of hyperthermia. Crit Care. 2016;20:199. doi:10.1186/s13054-016-1376-4

33. Platt M, Price T. Heat illness. In: Walls, ed. Rosen’s Emergency Medicine: Concepts and Clinical Practice. Philadelphia, PA: Elsevier; 2018:1755-1764.
34. Dunkley EJC, Isbister GK, Sibbritt D, Dawson AH, Whyte IM. The Hunter Serotonin Toxicity Criteria: simple and accurate diagnostic decision rules for serotonin toxicity. QJM. 2003;96(9):635-642. doi:10.1093/qjmed/hcg109
35. Gillman KP. Serotonin toxicity contrasted with neuroleptic malignant syndrome. https://psychotropical.com/serotonin-syndrome-and-neuroleptic-malignant-syndrome. Published January 1, 2005. Updated November 6, 2017. Accessed August 17, 2020.
36. Asusta HB, Keyser E, Dominguez P, Miller M, Odedokun T. Serotonin syndrome in obstetrics: a case report and review of management. Mil Med. 2018;184(1-2):e284-e286. doi:10.1093/milmed/usy135
37. English FA, Kenny LC, McCarthy FP. Risk factors and effective management of preeclampsia. Integr Blood Press Control 2015;8:7-12. doi:10.2147/IBPC.S50641
38. Bruggeman C, O’Day CS. Selective serotonin reuptake inhibitor (SSRI) toxicity. https://pubmed.ncbi.nlm.nih.gov/30521236. Published December 3, 2019. Accessed August 17, 2020.
39. US Food and Drug Administration. Selective serotonin reuptake inhibitors (SSRIs) Information. https://www.fda.gov/drugs/information-drug-class/selective-serotonin-reuptake-inhibitors-ssris-information. Updated December 23, 2014. Accessed March 24, 2020.
40. Orlova Y, Rizzoli P, Loder E. Association of coprescription of triptan antimigraine drugs and selective serotonin reuptake inhibitor or selective norepinephrine reuptake inhibitor antidepressants with serotonin syndrome. JAMA Neurol. 2018;75(5):566-572. doi:10.1001/jamaneurol.2017.5144
41. Gillman KP. Regulatory agencies (WH0, FDA) offer ill-conceived advice about serotonin toxicity (serotonin syndrome) with 5–HT3 antagonist: a worldwide problem. https://psychotropical.com/serotonin-toxicity-and-5-ht3-antagonists. Published November 13, 2014. Updated March 23, 2019. Accessed August 17, 2020.
42. Gummin DD, Mowry JB, Spyker DA, Brooks DE, Osterthaler KM, Banner W. 2017 Annual Report of the American Association of Poison Control Centers’ National Poison Data System (NPDS): 35th Annual Report. Clin Toxicol (Phila). 2018;56(12):1213-1415. doi:10.1080/15563650.2018.1533727
43. Badawy MK, Maffei FA. Pediatric selective serotonin reuptake inhibitor toxicity. https://emedicine.medscape.com/article/1011436. Updated September 27, 2019. Accessed August 17, 2020.
44. Laliberte B, Kishk OA. Serotonin syndrome in a pediatric patient after vilazodone ingestion. Pediatr Emerg Care. 2018;34(12):e226-e228. doi:10.1097/PEC.0000000000001115
45. Direk MC, Yildirim V, Gϋnes S, Bozlu G, Okuyaz C. Serotonin syndrome after clomipramine overdose in a child. Clin Psychopharmacol Neurosci. 2016;14(4):388-390. doi:10.9758/cpn.2016.14.4.38846. ACOG Committee Opinion No. 757: Screening for perinatal depression. Obstet Gynecol. 2018;132(5):e208-e212. doi:10.1097/AOG.0000000000002927
47. Osborne LM, McEvoy K, Payne JL. Antidepressants in pregnancy: balancing needs and risks in clinical practice. Psychiatric Times. 2017;34(4).
48. Stewart D, Vigod S. Antenatal use of antidepressants and risk of teratogenicity and adverse pregnancy outcomes: selective serotonin reuptake inhibitors (SSRIs). https://www.uptodate.com/contents/antenatal-use-of-antidepressants-and-risk-of-teratogenicity-and-adverse-pregnancy-outcomes-selective-serotonin-reuptake-inhibitors-ssris. Accessed March 24, 2020.
49. Degiacomo J, Luedtke S. Neonatal toxicity from escitalopram use in utero: a case report. J Pediatr Pharmacol Ther. 2016;21(6):522-526. doi:10.5863/1551-6776-21.6.522
50. Eleftheriou G, Butera R, Cottini FC, Bonati M, Farina M. Neonatal toxicity following maternal citalopram treatment. Fetal Pediatr Pathol. 2013;32(5):362-356. doi:10.3109/15513815.2013.768743
1. Rapport MM, Green AA, Page IH. Serum vasoconstrictor, serotonin; isolation and characterization. J Biol Chem. 1948;176(3):1243-1251.
2. McCorvy JD, Roth BL. Structure and function of serotonin g protein coupled receptors. Pharmacol Ther. 2015;150:129-142. doi:10.1016/j.pharmthera. 2015.01.009 3. Insel TR, Roy BF, Cohen RM, Murphy DL. Possible development of serotonin syndrome in man. Am J Psychiatry. 1982;139(7):954-955. doi:10.1176/ajp.139.7.954
4. Scotton WJ, Hill LJ, Williams AC, Barnes NM. Serotonin syndrome: pathophysiology, clinical features, management, and potential future directions. Int J Tryptophan Res. 2019;12:1-14. doi:10.1177/1178646919873925
5. Buckley N, Dawson AH, Isbister GK. Serotonin syndrome. BMJ. 2014;348:g1626. doi:10.1136/bmj.g1626
6. Gillman KP. Serotonin toxicity: introduction. https://psychotropical.com/serotonin-toxicity-introduction. Published November 13, 2014. Updated July 13, 2019. Accessed August 17, 2020.
7. Foong A-L, Patel T, Kellar J, Grindrod KA. The scoop on serotonin syndrome. Can Pharm J (OTT). 2018;151(4):233-239. doi:10.1177/1715163518779096
8. Foong A-L, Grindrod KA, Patel T, Kellar J. Demystifying serotonin syndrome (or serotonin toxicity). Can Fam Physician. 2018;64(10):720-727.
9. Gillman KP. Serotonin toxicity: summary. https://psychotropical.com/serotonin-toxicity-summary. Published November 13, 2014. Updated January 25, 2018. Accessed August 17, 2020.
10. Boyer EW. Serotonin syndrome (serotonin toxicity). https://www.uptodate.com/contents/serotonin-syndrome-serotonin-toxicity. Updated March 12, 2018. Access December 12, 2019.
11. Wang RZ, Vashistha V, Kaur S, Houchens NW. Serotonin syndrome: preventing, recognizing, and treating it. Cleve Clin J Med. 2016;83(11):810-817. doi:10.3949/ccjm.83a.15129
12. Walker T. The economic burden of depression among veterans. https://www.managedhealthcareexecutive.com/article/economic-burden-depression-among-veterans. Published November 9, 2018. Accessed August 17, 2020.
13. Substance Abuse and Mental Health Services Administration, Center for Behavioral Health Statistics and Quality. Key substance use and mental health indicators in the United States: results from the 2018 National Survey on Drug Use and Health (HHS Publication No. PEP19-5068, NSDUH Series H-54). Rockville, MD: Center for Behavioral Health Statistics and Quality, Substance Abuse and Mental Health Services Administration; 2019.
14. Wong J, Motulsky A, Abrahamowicz M, et al. Off label indications for antidepressants in primary care; descriptive study of prescriptions from an indication based electronic prescribing system. BMJ. 2017;356:j603. doi:org/10.1136/bmj.j603
15. Vijay A, Becker JE, Ross JS. Patterns and predictors of off-label prescription of psychiatric drugs. PLOS One. 2018;13(7):e0198363. doi:10.1371/journal.pone.0198363
16. Medicating the military—use of psychiatric drugs has spiked; concerns surface about suicide, other dangers. https://www.militarytimes.com/2013/03/29/medicating-the-military-use-of-psychiatric-drugs-has-spiked-concerns-surface-about-suicide-other-dangers. Published March 29, 2013. Accessed August 17, 2020.
17. Hengartner MP, Plöderl M. Newer-generation antidepressants and suicide risk in randomized controlled trials; a re-analysis of the FDA database [letter]. Psychother Psychosom. 2019:88:247-248. doi:10.1159/000501215
18. Rosebush PI. Serotonin syndrome. https://www.mhaus.org/nmsis/medical-education-programs/serotonin-syndrome. Accessed March 24, 2020.
19. National Institute of Mental Health. Major depression. https://www.nimh.nih.gov/health/statistics/major-depression.shtml. Updated February 2019. Accessed March 24, 2020.
20. Meadows SO, Engel RL, Beckman RL, et al. 2015 health related behaviors survey: mental and emotional health among U.S. active-duty service members. https://www.rand.org/pubs/research_briefs/RB9955z3.html. Published 2018. Accessed August 17, 2020.
21. ClinCalc. The top 300 drugs of 2020. https://clincalc.com/DrugStats/Top300Drugs.aspx. Update February 11, 2017. Accessed March 24, 2020.
22. Corrigan C. Revealed: massive rise in antidepressant prescribing. https://www.rte.ie/news/investigations-unit/2019/0218/1031271-massive-rise-antidepressant-prescribing. Published June 14, 2019. Accessed August 17, 2020.
23. Skånland SS, Cieślar-Pobuda A. Off-label uses of drugs for depression. Eur J Pharmacol. 2019;865: 172732. doi:10.1016/j.ejphar.2019.172732
24. Bobo WV, Grossardt BR, Lapid MI, et al. Frequency and predictors of the potential overprescribing of antidepressants in elderly residents of a geographically defined U.S. population. Pharmacol Res Perspect. 2019;7(1):e00461. doi:10.1002/prp2.461
25. Patel N. Learning lessons. The Libby Zion case revisited. J Am Coll Cardiol. 2014;64(25):2802-2804. doi:10.1016/j.jacc.2014.11.007
26. Jenkins TA, Nguyen JCD, Polglase KE, Bertrand PP. Influence of tryptophan and serotonin on mood and cognition with a possible role of the gut-brain axis. Nutrients. 2016;8(1):56. doi:10.3390/nu8010056
27. Coleman JA, Green EM, Gouaux E. X-ray structures and mechanism of the human serotonin transporter. Nature Int J Sci. 2016;532(7599):334-339. doi:10.1038/nature17629
28. Garcia-Garcia AL, Newman-Tancredi A, Leonardo ED. 5-HT(1A) [corrected] receptors in mood and anxiety: recent insights into autoreceptor versus heteroreceptor function. Psychopharmacology (Berl). 2013;231(4):623-636. doi:10.1007/s00213-013-3389-x
29. Nautiyal KM, Hen R. Serotonin receptors in depression: from A to B. F1000Res. 2017;6:123. doi:10.12688/f1000research.9736.1
30. Francescangeli J, Karamchandani K, Powell M, Bonavia A. The serotonin syndrome: from molecular mechanisms to clinical practice. Int J Mol Sci. 2019;20(9):2288. doi:10.3390/ijms20092288
31. Little K, Lin CM, Reynolds PM. Delayed serotonin syndrome in the setting of a mixed fluoxetine and serotonin antagonist overdose. Am J Case Rep. 2018;19:604-607. doi:10.12659/AJCR.909063
32. Walter EJ, Carraretto M. The neurological and cognitive consequences of hyperthermia. Crit Care. 2016;20:199. doi:10.1186/s13054-016-1376-4

33. Platt M, Price T. Heat illness. In: Walls, ed. Rosen’s Emergency Medicine: Concepts and Clinical Practice. Philadelphia, PA: Elsevier; 2018:1755-1764.
34. Dunkley EJC, Isbister GK, Sibbritt D, Dawson AH, Whyte IM. The Hunter Serotonin Toxicity Criteria: simple and accurate diagnostic decision rules for serotonin toxicity. QJM. 2003;96(9):635-642. doi:10.1093/qjmed/hcg109
35. Gillman KP. Serotonin toxicity contrasted with neuroleptic malignant syndrome. https://psychotropical.com/serotonin-syndrome-and-neuroleptic-malignant-syndrome. Published January 1, 2005. Updated November 6, 2017. Accessed August 17, 2020.
36. Asusta HB, Keyser E, Dominguez P, Miller M, Odedokun T. Serotonin syndrome in obstetrics: a case report and review of management. Mil Med. 2018;184(1-2):e284-e286. doi:10.1093/milmed/usy135
37. English FA, Kenny LC, McCarthy FP. Risk factors and effective management of preeclampsia. Integr Blood Press Control 2015;8:7-12. doi:10.2147/IBPC.S50641
38. Bruggeman C, O’Day CS. Selective serotonin reuptake inhibitor (SSRI) toxicity. https://pubmed.ncbi.nlm.nih.gov/30521236. Published December 3, 2019. Accessed August 17, 2020.
39. US Food and Drug Administration. Selective serotonin reuptake inhibitors (SSRIs) Information. https://www.fda.gov/drugs/information-drug-class/selective-serotonin-reuptake-inhibitors-ssris-information. Updated December 23, 2014. Accessed March 24, 2020.
40. Orlova Y, Rizzoli P, Loder E. Association of coprescription of triptan antimigraine drugs and selective serotonin reuptake inhibitor or selective norepinephrine reuptake inhibitor antidepressants with serotonin syndrome. JAMA Neurol. 2018;75(5):566-572. doi:10.1001/jamaneurol.2017.5144
41. Gillman KP. Regulatory agencies (WH0, FDA) offer ill-conceived advice about serotonin toxicity (serotonin syndrome) with 5–HT3 antagonist: a worldwide problem. https://psychotropical.com/serotonin-toxicity-and-5-ht3-antagonists. Published November 13, 2014. Updated March 23, 2019. Accessed August 17, 2020.
42. Gummin DD, Mowry JB, Spyker DA, Brooks DE, Osterthaler KM, Banner W. 2017 Annual Report of the American Association of Poison Control Centers’ National Poison Data System (NPDS): 35th Annual Report. Clin Toxicol (Phila). 2018;56(12):1213-1415. doi:10.1080/15563650.2018.1533727
43. Badawy MK, Maffei FA. Pediatric selective serotonin reuptake inhibitor toxicity. https://emedicine.medscape.com/article/1011436. Updated September 27, 2019. Accessed August 17, 2020.
44. Laliberte B, Kishk OA. Serotonin syndrome in a pediatric patient after vilazodone ingestion. Pediatr Emerg Care. 2018;34(12):e226-e228. doi:10.1097/PEC.0000000000001115
45. Direk MC, Yildirim V, Gϋnes S, Bozlu G, Okuyaz C. Serotonin syndrome after clomipramine overdose in a child. Clin Psychopharmacol Neurosci. 2016;14(4):388-390. doi:10.9758/cpn.2016.14.4.38846. ACOG Committee Opinion No. 757: Screening for perinatal depression. Obstet Gynecol. 2018;132(5):e208-e212. doi:10.1097/AOG.0000000000002927
47. Osborne LM, McEvoy K, Payne JL. Antidepressants in pregnancy: balancing needs and risks in clinical practice. Psychiatric Times. 2017;34(4).
48. Stewart D, Vigod S. Antenatal use of antidepressants and risk of teratogenicity and adverse pregnancy outcomes: selective serotonin reuptake inhibitors (SSRIs). https://www.uptodate.com/contents/antenatal-use-of-antidepressants-and-risk-of-teratogenicity-and-adverse-pregnancy-outcomes-selective-serotonin-reuptake-inhibitors-ssris. Accessed March 24, 2020.
49. Degiacomo J, Luedtke S. Neonatal toxicity from escitalopram use in utero: a case report. J Pediatr Pharmacol Ther. 2016;21(6):522-526. doi:10.5863/1551-6776-21.6.522
50. Eleftheriou G, Butera R, Cottini FC, Bonati M, Farina M. Neonatal toxicity following maternal citalopram treatment. Fetal Pediatr Pathol. 2013;32(5):362-356. doi:10.3109/15513815.2013.768743
Creeping fat in Crohn’s linked with microbial translocation
Creeping fat in Crohn’s disease is likely caused by microbial translocation from the gut to neighboring mesenteric adipose tissue (MAT), based on a recent study.
This finding may lead to early risk stratification for creeping fat, and nonsurgical interventions, according to principal author Suzanne Devkota, PhD, assistant professor at Cedars-Sinai Medical Center in Los Angeles.
Creeping fat, which is unique to Crohn’s disease, is characterized by hyperplastic MAT that grips areas of intestinal inflammation with invasive “fingerlike projections,” the investigators wrote in Cell. This phenomenon was first described by the eponymous Dr. Crohn in 1932; since then, despite associations with fibrotic strictures that may require surgical resection, underlying mechanisms have remained mysterious and largely unexplored.
That changed during a session of grand rounds at Cedars-Sinai in September 2016; Dr. Devkota was discussing adipose tissue when her surgeon colleague, Phillip Fleshner, MD, asked: “What about creeping fat?”
“Yeah, that’s cool,” Dr. Devkota replied, “but I don’t have access to creeping fat.”
“I see it all the time,” Dr. Fleshner said. “I can get you some.”
And so a partnership was born, allowing Dr. Devkota and colleagues to pursue the translocation hypothesis.
The present report involved tissue samples from 11 patients with Crohn’s disease and 13 patients with ulcerative colitis. Healthy tissue controls were taken from four subjects without inflammatory bowel disease who underwent ileostomy.
Microbial cultivation of Crohn’s disease and healthy patient samples revealed bacteria in the mesenteric tissue of both groups, suggesting that microbial translocation from the gut to MAT “may not be unusual;” however, Crohn’s disease samples were associated with an exclusive consortium of five species: Clostridium innocuum, Erysipeloclostridium ramosum, Parabacteroides distasonis, Clostridium symbiosum, and Bifidobacterium pseudolongum.
C. innocuum was isolated most frequently; and its unique characteristics increased suspicions that it was the creeping fat culprit.
“Core genomic features of C. innocuum include type IV pili and twitching motility, a preference for lipid-derived metabolic substrates, and multiple genes for lipid catabolism, as well as a functional substrate preference for b-hydroxybutyrate, a byproduct of fatty acid oxidation,” the investigators wrote. “This suggests that C. innocuum is well suited for, and perhaps prefers, a lipid-rich environment and seeks these out when the opportunity arises.”
To observe this opportunism firsthand, the investigators gavaged gnotobiotic mice with C. innocuum. Indeed, these mice demonstrated “dramatic mesenteric adiposity,” compared with controls.
Cotreatment with dextran sulfate sodium (DSS) was unnecessary to induce translocation of C. innocuum, which “suggests that overt inflammation is not a prerequisite for its translocation,” the investigators noted.
The profibrotic potential of C. innocuum was supported by in vitro experiments, in which adipose-derived stem cells and primary fibroblasts from Crohn’s disease MAT were exposed to either C. innocuum lysate or macrophage-conditioned media from C. innocuum–exposed macrophages. While the lysate alone did not alter genes involved in fibrosis and remodeling, the macrophage-conditioned media did, indicating that C. innocuum alters MAT indirectly via macrophage activity.
Although multiple signs suggest that C. innocuum causes creeping fat, Dr. Devkota noted that systematic testing is needed to confirm this likelihood.
“But I do think we’ve honed in on the consortium that is at play,” she said, referring to the five identified species.
According to Dr. Devkota, awareness of these microbes could lead to diagnostic and interventional benefits for patients with Crohn’s disease. For example, gut microbiota profiling could be used to measure levels of C. innocuum in newly diagnosed patients, thereby stratifying risk of creeping fat. And phage therapy, with its high specificity for bacterial species, could be an ideal intervention.
“I’m very eager to hear from the surgeons, and hear what their opinion is, and whether this will affect their treatment or how they approach [creeping fat],” Dr. Devkota said.
Beyond Crohn’s disease, the study findings could inform obesity research, as bacterial DNA has been found in obese adipose tissue, which is characteristically fibrotic.
“There are a lot of gene-expression patterns [in the present study], that are also seen in obesity literature,” Dr. Devkota said.
“Obviously there’s a lifestyle caloric aspect to [obesity],” she added. “I definitely don’t claim that microbes are the end-all and be-all of obesity – I want to make that clear. But it could be possible, and particularly related to abdominal fat. Expanded abdominal fat could be a sign that there’s underlying intestinal inflammation ... that there’s something deeper going on that may be unrelated to a metabolic defect.”
The study was funded by Leona M. and Harry B. Helmsley Charitable Trust and the National Institutes of Health. Dr. Devkota and Dr. Ha are inventors on U.S. patent application #62/679,624.
SOURCE: Ha CWY et al. Cell. 2020 Oct 29. doi: 10.1016/j.cell.2020.09.009.
Creeping fat in Crohn’s disease is likely caused by microbial translocation from the gut to neighboring mesenteric adipose tissue (MAT), based on a recent study.
This finding may lead to early risk stratification for creeping fat, and nonsurgical interventions, according to principal author Suzanne Devkota, PhD, assistant professor at Cedars-Sinai Medical Center in Los Angeles.
Creeping fat, which is unique to Crohn’s disease, is characterized by hyperplastic MAT that grips areas of intestinal inflammation with invasive “fingerlike projections,” the investigators wrote in Cell. This phenomenon was first described by the eponymous Dr. Crohn in 1932; since then, despite associations with fibrotic strictures that may require surgical resection, underlying mechanisms have remained mysterious and largely unexplored.
That changed during a session of grand rounds at Cedars-Sinai in September 2016; Dr. Devkota was discussing adipose tissue when her surgeon colleague, Phillip Fleshner, MD, asked: “What about creeping fat?”
“Yeah, that’s cool,” Dr. Devkota replied, “but I don’t have access to creeping fat.”
“I see it all the time,” Dr. Fleshner said. “I can get you some.”
And so a partnership was born, allowing Dr. Devkota and colleagues to pursue the translocation hypothesis.
The present report involved tissue samples from 11 patients with Crohn’s disease and 13 patients with ulcerative colitis. Healthy tissue controls were taken from four subjects without inflammatory bowel disease who underwent ileostomy.
Microbial cultivation of Crohn’s disease and healthy patient samples revealed bacteria in the mesenteric tissue of both groups, suggesting that microbial translocation from the gut to MAT “may not be unusual;” however, Crohn’s disease samples were associated with an exclusive consortium of five species: Clostridium innocuum, Erysipeloclostridium ramosum, Parabacteroides distasonis, Clostridium symbiosum, and Bifidobacterium pseudolongum.
C. innocuum was isolated most frequently; and its unique characteristics increased suspicions that it was the creeping fat culprit.
“Core genomic features of C. innocuum include type IV pili and twitching motility, a preference for lipid-derived metabolic substrates, and multiple genes for lipid catabolism, as well as a functional substrate preference for b-hydroxybutyrate, a byproduct of fatty acid oxidation,” the investigators wrote. “This suggests that C. innocuum is well suited for, and perhaps prefers, a lipid-rich environment and seeks these out when the opportunity arises.”
To observe this opportunism firsthand, the investigators gavaged gnotobiotic mice with C. innocuum. Indeed, these mice demonstrated “dramatic mesenteric adiposity,” compared with controls.
Cotreatment with dextran sulfate sodium (DSS) was unnecessary to induce translocation of C. innocuum, which “suggests that overt inflammation is not a prerequisite for its translocation,” the investigators noted.
The profibrotic potential of C. innocuum was supported by in vitro experiments, in which adipose-derived stem cells and primary fibroblasts from Crohn’s disease MAT were exposed to either C. innocuum lysate or macrophage-conditioned media from C. innocuum–exposed macrophages. While the lysate alone did not alter genes involved in fibrosis and remodeling, the macrophage-conditioned media did, indicating that C. innocuum alters MAT indirectly via macrophage activity.
Although multiple signs suggest that C. innocuum causes creeping fat, Dr. Devkota noted that systematic testing is needed to confirm this likelihood.
“But I do think we’ve honed in on the consortium that is at play,” she said, referring to the five identified species.
According to Dr. Devkota, awareness of these microbes could lead to diagnostic and interventional benefits for patients with Crohn’s disease. For example, gut microbiota profiling could be used to measure levels of C. innocuum in newly diagnosed patients, thereby stratifying risk of creeping fat. And phage therapy, with its high specificity for bacterial species, could be an ideal intervention.
“I’m very eager to hear from the surgeons, and hear what their opinion is, and whether this will affect their treatment or how they approach [creeping fat],” Dr. Devkota said.
Beyond Crohn’s disease, the study findings could inform obesity research, as bacterial DNA has been found in obese adipose tissue, which is characteristically fibrotic.
“There are a lot of gene-expression patterns [in the present study], that are also seen in obesity literature,” Dr. Devkota said.
“Obviously there’s a lifestyle caloric aspect to [obesity],” she added. “I definitely don’t claim that microbes are the end-all and be-all of obesity – I want to make that clear. But it could be possible, and particularly related to abdominal fat. Expanded abdominal fat could be a sign that there’s underlying intestinal inflammation ... that there’s something deeper going on that may be unrelated to a metabolic defect.”
The study was funded by Leona M. and Harry B. Helmsley Charitable Trust and the National Institutes of Health. Dr. Devkota and Dr. Ha are inventors on U.S. patent application #62/679,624.
SOURCE: Ha CWY et al. Cell. 2020 Oct 29. doi: 10.1016/j.cell.2020.09.009.
Creeping fat in Crohn’s disease is likely caused by microbial translocation from the gut to neighboring mesenteric adipose tissue (MAT), based on a recent study.
This finding may lead to early risk stratification for creeping fat, and nonsurgical interventions, according to principal author Suzanne Devkota, PhD, assistant professor at Cedars-Sinai Medical Center in Los Angeles.
Creeping fat, which is unique to Crohn’s disease, is characterized by hyperplastic MAT that grips areas of intestinal inflammation with invasive “fingerlike projections,” the investigators wrote in Cell. This phenomenon was first described by the eponymous Dr. Crohn in 1932; since then, despite associations with fibrotic strictures that may require surgical resection, underlying mechanisms have remained mysterious and largely unexplored.
That changed during a session of grand rounds at Cedars-Sinai in September 2016; Dr. Devkota was discussing adipose tissue when her surgeon colleague, Phillip Fleshner, MD, asked: “What about creeping fat?”
“Yeah, that’s cool,” Dr. Devkota replied, “but I don’t have access to creeping fat.”
“I see it all the time,” Dr. Fleshner said. “I can get you some.”
And so a partnership was born, allowing Dr. Devkota and colleagues to pursue the translocation hypothesis.
The present report involved tissue samples from 11 patients with Crohn’s disease and 13 patients with ulcerative colitis. Healthy tissue controls were taken from four subjects without inflammatory bowel disease who underwent ileostomy.
Microbial cultivation of Crohn’s disease and healthy patient samples revealed bacteria in the mesenteric tissue of both groups, suggesting that microbial translocation from the gut to MAT “may not be unusual;” however, Crohn’s disease samples were associated with an exclusive consortium of five species: Clostridium innocuum, Erysipeloclostridium ramosum, Parabacteroides distasonis, Clostridium symbiosum, and Bifidobacterium pseudolongum.
C. innocuum was isolated most frequently; and its unique characteristics increased suspicions that it was the creeping fat culprit.
“Core genomic features of C. innocuum include type IV pili and twitching motility, a preference for lipid-derived metabolic substrates, and multiple genes for lipid catabolism, as well as a functional substrate preference for b-hydroxybutyrate, a byproduct of fatty acid oxidation,” the investigators wrote. “This suggests that C. innocuum is well suited for, and perhaps prefers, a lipid-rich environment and seeks these out when the opportunity arises.”
To observe this opportunism firsthand, the investigators gavaged gnotobiotic mice with C. innocuum. Indeed, these mice demonstrated “dramatic mesenteric adiposity,” compared with controls.
Cotreatment with dextran sulfate sodium (DSS) was unnecessary to induce translocation of C. innocuum, which “suggests that overt inflammation is not a prerequisite for its translocation,” the investigators noted.
The profibrotic potential of C. innocuum was supported by in vitro experiments, in which adipose-derived stem cells and primary fibroblasts from Crohn’s disease MAT were exposed to either C. innocuum lysate or macrophage-conditioned media from C. innocuum–exposed macrophages. While the lysate alone did not alter genes involved in fibrosis and remodeling, the macrophage-conditioned media did, indicating that C. innocuum alters MAT indirectly via macrophage activity.
Although multiple signs suggest that C. innocuum causes creeping fat, Dr. Devkota noted that systematic testing is needed to confirm this likelihood.
“But I do think we’ve honed in on the consortium that is at play,” she said, referring to the five identified species.
According to Dr. Devkota, awareness of these microbes could lead to diagnostic and interventional benefits for patients with Crohn’s disease. For example, gut microbiota profiling could be used to measure levels of C. innocuum in newly diagnosed patients, thereby stratifying risk of creeping fat. And phage therapy, with its high specificity for bacterial species, could be an ideal intervention.
“I’m very eager to hear from the surgeons, and hear what their opinion is, and whether this will affect their treatment or how they approach [creeping fat],” Dr. Devkota said.
Beyond Crohn’s disease, the study findings could inform obesity research, as bacterial DNA has been found in obese adipose tissue, which is characteristically fibrotic.
“There are a lot of gene-expression patterns [in the present study], that are also seen in obesity literature,” Dr. Devkota said.
“Obviously there’s a lifestyle caloric aspect to [obesity],” she added. “I definitely don’t claim that microbes are the end-all and be-all of obesity – I want to make that clear. But it could be possible, and particularly related to abdominal fat. Expanded abdominal fat could be a sign that there’s underlying intestinal inflammation ... that there’s something deeper going on that may be unrelated to a metabolic defect.”
The study was funded by Leona M. and Harry B. Helmsley Charitable Trust and the National Institutes of Health. Dr. Devkota and Dr. Ha are inventors on U.S. patent application #62/679,624.
SOURCE: Ha CWY et al. Cell. 2020 Oct 29. doi: 10.1016/j.cell.2020.09.009.
FROM CELL
Vedolizumab shows long-term safety, efficacy
The gut-selective alpha4beta7 integrin antibody vedolizumab is safe and effective for long-term use in patients with inflammatory bowel disease (IBD), according to data from more than 2,200 patients in the GEMINI LTS trial.
With a median cumulative exposure of approximately 3 years and some patients taking vedolizumab for more than 9 years, the study revealed no new safety concerns and showed that responses were stable over time, reported lead author Edward V. Loftus Jr., MD, of the Mayo Clinic in Rochester, Minn., and colleagues.
“Interim analyses (based on 4 years of follow-up) demonstrated that long-term vedolizumab therapy was well-tolerated and also provided clinical and health-related quality of life (HRQOL) benefits,” the investigators wrote in Alimentary Pharmacology & Therapeutics. “In this final analysis ... we report the final safety outcomes, along with exploratory clinical and HRQOL outcomes.”
The phase 3 trial involved 1,822 patients with IBD from previous phase 2 and 3 trials, plus 421 vedolizumab-naive patients. Out of 2,243 participants in the final analysis, 894 had ulcerative colitis, and 1,349 had Crohn’s disease.
All patients received vedolizumab 300 mg IV every 4 weeks, which the investigators noted is more frequent than the dosing interval of 8 weeks that was approved after the trial was designed.
Median cumulative exposure times among patients with ulcerative colitis and Crohn’s disease were 42.4 months and 31.5 months, respectively. Adverse events of any grade occurred in 93% of patients with ulcerative colitis and 96% of patients with Crohn’s disease, approximately one-third of which were exacerbations of IBD. Serious adverse events occurred in 31% and 41% of patients with ulcerative colitis and Crohn’s disease, respectively. Adverse events led to discontinuation in 15% of ulcerative colitis patients and 17% of those with Crohn’s disease. Of the 10 deaths that occurred during the study period, the investigators categorized 2 of them as drug related (hepatocellular carcinoma and West Nile virus infection–related encephalitis). No increase in the rate of overall malignancies was observed.
Dr. Loftus and colleagues noted that rates of serious infection with vedolizumab treatment were superior to historical long-term data for adalimumab, at 18.0 (ulcerative colitis) and 33.6 (Crohn’s disease) per 1,000 person-years for vedolizumab, compared with 35 (ulcerative colitis) and 67 (Crohn’s disease) per 1,000 person-years for adalimumab, as reported by Colombel and colleagues.
“While these data suggest a decreased risk of systemic infections with vedolizumab, an increased risk of gastrointestinal infections is plausible given the gut-selective mechanism of action and evidence for reduced immune response in the gastrointestinal tract,” the investigators wrote, reporting rates of 34.9 and 39.6 per 1,000 person-years for ulcerative colitis and Crohn’s disease, respectively. Comparative, historical rates for adalimumab were not provided.
In the present trial, clinical response rates, clinical remission rates, and HRQOL estimates remained stable over time. At 400 treatment weeks, clinical remission was maintained in 33% and 28% of patients with ulcerative colitis and Crohn’s disease, respectively.
“[T]he final analysis of GEMINI LTS comprehensively demonstrates that vedolizumab therapy has a safety and tolerability profile suitable for long-term treatment of patients with moderately to severely active ulcerative colitis or Crohn’s disease,” the investigators concluded.
According to Randy Longman, MD, director of the Jill Roberts Center for Inflammatory Bowel Disease at Weill Cornell Medicine and New York-Presbyterian, “The results from the GEMINI LTS trial help to solidify the favorable safety profile of vedolizumab for the treatment of IBD. With large patient numbers and cumulative duration of vedolizumab exposure, these pivotal data convincingly reveal low rates of serious infections, identified no cases of progressive multifocal leukoencephalopathy, and showed no increased risk of malignancy. Arthralgias, or joint pain, was the most common treatment-emergent adverse event, but occurred largely in patients with a history of IBD-associated arthralgia and suggest that these symptoms should be monitored during treatment. Overall, these results provide meaningful reassurance to patients and providers using vedolizumab for the treatment of IBD.”
GEMINI LTS was funded by Takeda. The investigators reported additional relationships with AbbVie, Janssen, Amgen, and others. Dr. Longman reported no conflicts of interest.
SOURCE: Loftus Jr EV et al. AP&T. 2020 Sep 2. doi: 10.1111/apt.16060.
Help your patients better understand their IBD treatment options by sharing AGA’s patient education, “Living with IBD,” in the AGA GI Patient Center at http://ow.ly/szHz30rdKyx.
The gut-selective alpha4beta7 integrin antibody vedolizumab is safe and effective for long-term use in patients with inflammatory bowel disease (IBD), according to data from more than 2,200 patients in the GEMINI LTS trial.
With a median cumulative exposure of approximately 3 years and some patients taking vedolizumab for more than 9 years, the study revealed no new safety concerns and showed that responses were stable over time, reported lead author Edward V. Loftus Jr., MD, of the Mayo Clinic in Rochester, Minn., and colleagues.
“Interim analyses (based on 4 years of follow-up) demonstrated that long-term vedolizumab therapy was well-tolerated and also provided clinical and health-related quality of life (HRQOL) benefits,” the investigators wrote in Alimentary Pharmacology & Therapeutics. “In this final analysis ... we report the final safety outcomes, along with exploratory clinical and HRQOL outcomes.”
The phase 3 trial involved 1,822 patients with IBD from previous phase 2 and 3 trials, plus 421 vedolizumab-naive patients. Out of 2,243 participants in the final analysis, 894 had ulcerative colitis, and 1,349 had Crohn’s disease.
All patients received vedolizumab 300 mg IV every 4 weeks, which the investigators noted is more frequent than the dosing interval of 8 weeks that was approved after the trial was designed.
Median cumulative exposure times among patients with ulcerative colitis and Crohn’s disease were 42.4 months and 31.5 months, respectively. Adverse events of any grade occurred in 93% of patients with ulcerative colitis and 96% of patients with Crohn’s disease, approximately one-third of which were exacerbations of IBD. Serious adverse events occurred in 31% and 41% of patients with ulcerative colitis and Crohn’s disease, respectively. Adverse events led to discontinuation in 15% of ulcerative colitis patients and 17% of those with Crohn’s disease. Of the 10 deaths that occurred during the study period, the investigators categorized 2 of them as drug related (hepatocellular carcinoma and West Nile virus infection–related encephalitis). No increase in the rate of overall malignancies was observed.
Dr. Loftus and colleagues noted that rates of serious infection with vedolizumab treatment were superior to historical long-term data for adalimumab, at 18.0 (ulcerative colitis) and 33.6 (Crohn’s disease) per 1,000 person-years for vedolizumab, compared with 35 (ulcerative colitis) and 67 (Crohn’s disease) per 1,000 person-years for adalimumab, as reported by Colombel and colleagues.
“While these data suggest a decreased risk of systemic infections with vedolizumab, an increased risk of gastrointestinal infections is plausible given the gut-selective mechanism of action and evidence for reduced immune response in the gastrointestinal tract,” the investigators wrote, reporting rates of 34.9 and 39.6 per 1,000 person-years for ulcerative colitis and Crohn’s disease, respectively. Comparative, historical rates for adalimumab were not provided.
In the present trial, clinical response rates, clinical remission rates, and HRQOL estimates remained stable over time. At 400 treatment weeks, clinical remission was maintained in 33% and 28% of patients with ulcerative colitis and Crohn’s disease, respectively.
“[T]he final analysis of GEMINI LTS comprehensively demonstrates that vedolizumab therapy has a safety and tolerability profile suitable for long-term treatment of patients with moderately to severely active ulcerative colitis or Crohn’s disease,” the investigators concluded.
According to Randy Longman, MD, director of the Jill Roberts Center for Inflammatory Bowel Disease at Weill Cornell Medicine and New York-Presbyterian, “The results from the GEMINI LTS trial help to solidify the favorable safety profile of vedolizumab for the treatment of IBD. With large patient numbers and cumulative duration of vedolizumab exposure, these pivotal data convincingly reveal low rates of serious infections, identified no cases of progressive multifocal leukoencephalopathy, and showed no increased risk of malignancy. Arthralgias, or joint pain, was the most common treatment-emergent adverse event, but occurred largely in patients with a history of IBD-associated arthralgia and suggest that these symptoms should be monitored during treatment. Overall, these results provide meaningful reassurance to patients and providers using vedolizumab for the treatment of IBD.”
GEMINI LTS was funded by Takeda. The investigators reported additional relationships with AbbVie, Janssen, Amgen, and others. Dr. Longman reported no conflicts of interest.
SOURCE: Loftus Jr EV et al. AP&T. 2020 Sep 2. doi: 10.1111/apt.16060.
Help your patients better understand their IBD treatment options by sharing AGA’s patient education, “Living with IBD,” in the AGA GI Patient Center at http://ow.ly/szHz30rdKyx.
The gut-selective alpha4beta7 integrin antibody vedolizumab is safe and effective for long-term use in patients with inflammatory bowel disease (IBD), according to data from more than 2,200 patients in the GEMINI LTS trial.
With a median cumulative exposure of approximately 3 years and some patients taking vedolizumab for more than 9 years, the study revealed no new safety concerns and showed that responses were stable over time, reported lead author Edward V. Loftus Jr., MD, of the Mayo Clinic in Rochester, Minn., and colleagues.
“Interim analyses (based on 4 years of follow-up) demonstrated that long-term vedolizumab therapy was well-tolerated and also provided clinical and health-related quality of life (HRQOL) benefits,” the investigators wrote in Alimentary Pharmacology & Therapeutics. “In this final analysis ... we report the final safety outcomes, along with exploratory clinical and HRQOL outcomes.”
The phase 3 trial involved 1,822 patients with IBD from previous phase 2 and 3 trials, plus 421 vedolizumab-naive patients. Out of 2,243 participants in the final analysis, 894 had ulcerative colitis, and 1,349 had Crohn’s disease.
All patients received vedolizumab 300 mg IV every 4 weeks, which the investigators noted is more frequent than the dosing interval of 8 weeks that was approved after the trial was designed.
Median cumulative exposure times among patients with ulcerative colitis and Crohn’s disease were 42.4 months and 31.5 months, respectively. Adverse events of any grade occurred in 93% of patients with ulcerative colitis and 96% of patients with Crohn’s disease, approximately one-third of which were exacerbations of IBD. Serious adverse events occurred in 31% and 41% of patients with ulcerative colitis and Crohn’s disease, respectively. Adverse events led to discontinuation in 15% of ulcerative colitis patients and 17% of those with Crohn’s disease. Of the 10 deaths that occurred during the study period, the investigators categorized 2 of them as drug related (hepatocellular carcinoma and West Nile virus infection–related encephalitis). No increase in the rate of overall malignancies was observed.
Dr. Loftus and colleagues noted that rates of serious infection with vedolizumab treatment were superior to historical long-term data for adalimumab, at 18.0 (ulcerative colitis) and 33.6 (Crohn’s disease) per 1,000 person-years for vedolizumab, compared with 35 (ulcerative colitis) and 67 (Crohn’s disease) per 1,000 person-years for adalimumab, as reported by Colombel and colleagues.
“While these data suggest a decreased risk of systemic infections with vedolizumab, an increased risk of gastrointestinal infections is plausible given the gut-selective mechanism of action and evidence for reduced immune response in the gastrointestinal tract,” the investigators wrote, reporting rates of 34.9 and 39.6 per 1,000 person-years for ulcerative colitis and Crohn’s disease, respectively. Comparative, historical rates for adalimumab were not provided.
In the present trial, clinical response rates, clinical remission rates, and HRQOL estimates remained stable over time. At 400 treatment weeks, clinical remission was maintained in 33% and 28% of patients with ulcerative colitis and Crohn’s disease, respectively.
“[T]he final analysis of GEMINI LTS comprehensively demonstrates that vedolizumab therapy has a safety and tolerability profile suitable for long-term treatment of patients with moderately to severely active ulcerative colitis or Crohn’s disease,” the investigators concluded.
According to Randy Longman, MD, director of the Jill Roberts Center for Inflammatory Bowel Disease at Weill Cornell Medicine and New York-Presbyterian, “The results from the GEMINI LTS trial help to solidify the favorable safety profile of vedolizumab for the treatment of IBD. With large patient numbers and cumulative duration of vedolizumab exposure, these pivotal data convincingly reveal low rates of serious infections, identified no cases of progressive multifocal leukoencephalopathy, and showed no increased risk of malignancy. Arthralgias, or joint pain, was the most common treatment-emergent adverse event, but occurred largely in patients with a history of IBD-associated arthralgia and suggest that these symptoms should be monitored during treatment. Overall, these results provide meaningful reassurance to patients and providers using vedolizumab for the treatment of IBD.”
GEMINI LTS was funded by Takeda. The investigators reported additional relationships with AbbVie, Janssen, Amgen, and others. Dr. Longman reported no conflicts of interest.
SOURCE: Loftus Jr EV et al. AP&T. 2020 Sep 2. doi: 10.1111/apt.16060.
Help your patients better understand their IBD treatment options by sharing AGA’s patient education, “Living with IBD,” in the AGA GI Patient Center at http://ow.ly/szHz30rdKyx.
FROM ALIMENTARY PHARMACOLOGY & THERAPEUTICS
Vedolizumab shows long-term safety, efficacy
The gut-selective alpha4beta7 integrin antibody vedolizumab is safe and effective for long-term use in patients with inflammatory bowel disease (IBD), according to data from more than 2,200 patients in the GEMINI LTS trial.
With a median cumulative exposure of approximately 3 years and some patients taking vedolizumab for more than 9 years, the study revealed no new safety concerns and showed that responses were stable over time, reported lead author Edward V. Loftus Jr., MD, of the Mayo Clinic in Rochester, Minn., and colleagues.
“Interim analyses (based on 4 years of follow-up) demonstrated that long-term vedolizumab therapy was well-tolerated and also provided clinical and health-related quality of life (HRQOL) benefits,” the investigators wrote in Alimentary Pharmacology & Therapeutics. “In this final analysis ... we report the final safety outcomes, along with exploratory clinical and HRQOL outcomes.”
The phase 3 trial involved 1,822 patients with IBD from previous phase 2 and 3 trials, plus 421 vedolizumab-naive patients. Out of 2,243 participants in the final analysis, 894 had ulcerative colitis, and 1,349 had Crohn’s disease.
All patients received vedolizumab 300 mg IV every 4 weeks, which the investigators noted is more frequent than the dosing interval of 8 weeks that was approved after the trial was designed.
Median cumulative exposure times among patients with ulcerative colitis and Crohn’s disease were 42.4 months and 31.5 months, respectively. Adverse events of any grade occurred in 93% of patients with ulcerative colitis and 96% of patients with Crohn’s disease, approximately one-third of which were exacerbations of IBD. Serious adverse events occurred in 31% and 41% of patients with ulcerative colitis and Crohn’s disease, respectively. Adverse events led to discontinuation in 15% of ulcerative colitis patients and 17% of those with Crohn’s disease. Of the 10 deaths that occurred during the study period, the investigators categorized 2 of them as drug related (hepatocellular carcinoma and West Nile virus infection–related encephalitis). No increase in the rate of overall malignancies was observed.
Dr. Loftus and colleagues noted that rates of serious infection with vedolizumab treatment were superior to historical long-term data for adalimumab, at 18.0 (ulcerative colitis) and 33.6 (Crohn’s disease) per 1,000 person-years for vedolizumab, compared with 35 (ulcerative colitis) and 67 (Crohn’s disease) per 1,000 person-years for adalimumab, as reported by Colombel and colleagues.
“While these data suggest a decreased risk of systemic infections with vedolizumab, an increased risk of gastrointestinal infections is plausible given the gut-selective mechanism of action and evidence for reduced immune response in the gastrointestinal tract,” the investigators wrote, reporting rates of 34.9 and 39.6 per 1,000 person-years for ulcerative colitis and Crohn’s disease, respectively. Comparative, historical rates for adalimumab were not provided.
In the present trial, clinical response rates, clinical remission rates, and HRQOL estimates remained stable over time. At 400 treatment weeks, clinical remission was maintained in 33% and 28% of patients with ulcerative colitis and Crohn’s disease, respectively.
“[T]he final analysis of GEMINI LTS comprehensively demonstrates that vedolizumab therapy has a safety and tolerability profile suitable for long-term treatment of patients with moderately to severely active ulcerative colitis or Crohn’s disease,” the investigators concluded.
According to Randy Longman, MD, director of the Jill Roberts Center for Inflammatory Bowel Disease at Weill Cornell Medicine and New York-Presbyterian, “The results from the GEMINI LTS trial help to solidify the favorable safety profile of vedolizumab for the treatment of IBD. With large patient numbers and cumulative duration of vedolizumab exposure, these pivotal data convincingly reveal low rates of serious infections, identified no cases of progressive multifocal leukoencephalopathy, and showed no increased risk of malignancy. Arthralgias, or joint pain, was the most common treatment-emergent adverse event, but occurred largely in patients with a history of IBD-associated arthralgia and suggest that these symptoms should be monitored during treatment. Overall, these results provide meaningful reassurance to patients and providers using vedolizumab for the treatment of IBD.”
GEMINI LTS was funded by Takeda. The investigators reported additional relationships with AbbVie, Janssen, Amgen, and others. Dr. Longman reported no conflicts of interest.
SOURCE: Loftus Jr EV et al. AP&T. 2020 Sep 2. doi: 10.1111/apt.16060.
The gut-selective alpha4beta7 integrin antibody vedolizumab is safe and effective for long-term use in patients with inflammatory bowel disease (IBD), according to data from more than 2,200 patients in the GEMINI LTS trial.
With a median cumulative exposure of approximately 3 years and some patients taking vedolizumab for more than 9 years, the study revealed no new safety concerns and showed that responses were stable over time, reported lead author Edward V. Loftus Jr., MD, of the Mayo Clinic in Rochester, Minn., and colleagues.
“Interim analyses (based on 4 years of follow-up) demonstrated that long-term vedolizumab therapy was well-tolerated and also provided clinical and health-related quality of life (HRQOL) benefits,” the investigators wrote in Alimentary Pharmacology & Therapeutics. “In this final analysis ... we report the final safety outcomes, along with exploratory clinical and HRQOL outcomes.”
The phase 3 trial involved 1,822 patients with IBD from previous phase 2 and 3 trials, plus 421 vedolizumab-naive patients. Out of 2,243 participants in the final analysis, 894 had ulcerative colitis, and 1,349 had Crohn’s disease.
All patients received vedolizumab 300 mg IV every 4 weeks, which the investigators noted is more frequent than the dosing interval of 8 weeks that was approved after the trial was designed.
Median cumulative exposure times among patients with ulcerative colitis and Crohn’s disease were 42.4 months and 31.5 months, respectively. Adverse events of any grade occurred in 93% of patients with ulcerative colitis and 96% of patients with Crohn’s disease, approximately one-third of which were exacerbations of IBD. Serious adverse events occurred in 31% and 41% of patients with ulcerative colitis and Crohn’s disease, respectively. Adverse events led to discontinuation in 15% of ulcerative colitis patients and 17% of those with Crohn’s disease. Of the 10 deaths that occurred during the study period, the investigators categorized 2 of them as drug related (hepatocellular carcinoma and West Nile virus infection–related encephalitis). No increase in the rate of overall malignancies was observed.
Dr. Loftus and colleagues noted that rates of serious infection with vedolizumab treatment were superior to historical long-term data for adalimumab, at 18.0 (ulcerative colitis) and 33.6 (Crohn’s disease) per 1,000 person-years for vedolizumab, compared with 35 (ulcerative colitis) and 67 (Crohn’s disease) per 1,000 person-years for adalimumab, as reported by Colombel and colleagues.
“While these data suggest a decreased risk of systemic infections with vedolizumab, an increased risk of gastrointestinal infections is plausible given the gut-selective mechanism of action and evidence for reduced immune response in the gastrointestinal tract,” the investigators wrote, reporting rates of 34.9 and 39.6 per 1,000 person-years for ulcerative colitis and Crohn’s disease, respectively. Comparative, historical rates for adalimumab were not provided.
In the present trial, clinical response rates, clinical remission rates, and HRQOL estimates remained stable over time. At 400 treatment weeks, clinical remission was maintained in 33% and 28% of patients with ulcerative colitis and Crohn’s disease, respectively.
“[T]he final analysis of GEMINI LTS comprehensively demonstrates that vedolizumab therapy has a safety and tolerability profile suitable for long-term treatment of patients with moderately to severely active ulcerative colitis or Crohn’s disease,” the investigators concluded.
According to Randy Longman, MD, director of the Jill Roberts Center for Inflammatory Bowel Disease at Weill Cornell Medicine and New York-Presbyterian, “The results from the GEMINI LTS trial help to solidify the favorable safety profile of vedolizumab for the treatment of IBD. With large patient numbers and cumulative duration of vedolizumab exposure, these pivotal data convincingly reveal low rates of serious infections, identified no cases of progressive multifocal leukoencephalopathy, and showed no increased risk of malignancy. Arthralgias, or joint pain, was the most common treatment-emergent adverse event, but occurred largely in patients with a history of IBD-associated arthralgia and suggest that these symptoms should be monitored during treatment. Overall, these results provide meaningful reassurance to patients and providers using vedolizumab for the treatment of IBD.”
GEMINI LTS was funded by Takeda. The investigators reported additional relationships with AbbVie, Janssen, Amgen, and others. Dr. Longman reported no conflicts of interest.
SOURCE: Loftus Jr EV et al. AP&T. 2020 Sep 2. doi: 10.1111/apt.16060.
The gut-selective alpha4beta7 integrin antibody vedolizumab is safe and effective for long-term use in patients with inflammatory bowel disease (IBD), according to data from more than 2,200 patients in the GEMINI LTS trial.
With a median cumulative exposure of approximately 3 years and some patients taking vedolizumab for more than 9 years, the study revealed no new safety concerns and showed that responses were stable over time, reported lead author Edward V. Loftus Jr., MD, of the Mayo Clinic in Rochester, Minn., and colleagues.
“Interim analyses (based on 4 years of follow-up) demonstrated that long-term vedolizumab therapy was well-tolerated and also provided clinical and health-related quality of life (HRQOL) benefits,” the investigators wrote in Alimentary Pharmacology & Therapeutics. “In this final analysis ... we report the final safety outcomes, along with exploratory clinical and HRQOL outcomes.”
The phase 3 trial involved 1,822 patients with IBD from previous phase 2 and 3 trials, plus 421 vedolizumab-naive patients. Out of 2,243 participants in the final analysis, 894 had ulcerative colitis, and 1,349 had Crohn’s disease.
All patients received vedolizumab 300 mg IV every 4 weeks, which the investigators noted is more frequent than the dosing interval of 8 weeks that was approved after the trial was designed.
Median cumulative exposure times among patients with ulcerative colitis and Crohn’s disease were 42.4 months and 31.5 months, respectively. Adverse events of any grade occurred in 93% of patients with ulcerative colitis and 96% of patients with Crohn’s disease, approximately one-third of which were exacerbations of IBD. Serious adverse events occurred in 31% and 41% of patients with ulcerative colitis and Crohn’s disease, respectively. Adverse events led to discontinuation in 15% of ulcerative colitis patients and 17% of those with Crohn’s disease. Of the 10 deaths that occurred during the study period, the investigators categorized 2 of them as drug related (hepatocellular carcinoma and West Nile virus infection–related encephalitis). No increase in the rate of overall malignancies was observed.
Dr. Loftus and colleagues noted that rates of serious infection with vedolizumab treatment were superior to historical long-term data for adalimumab, at 18.0 (ulcerative colitis) and 33.6 (Crohn’s disease) per 1,000 person-years for vedolizumab, compared with 35 (ulcerative colitis) and 67 (Crohn’s disease) per 1,000 person-years for adalimumab, as reported by Colombel and colleagues.
“While these data suggest a decreased risk of systemic infections with vedolizumab, an increased risk of gastrointestinal infections is plausible given the gut-selective mechanism of action and evidence for reduced immune response in the gastrointestinal tract,” the investigators wrote, reporting rates of 34.9 and 39.6 per 1,000 person-years for ulcerative colitis and Crohn’s disease, respectively. Comparative, historical rates for adalimumab were not provided.
In the present trial, clinical response rates, clinical remission rates, and HRQOL estimates remained stable over time. At 400 treatment weeks, clinical remission was maintained in 33% and 28% of patients with ulcerative colitis and Crohn’s disease, respectively.
“[T]he final analysis of GEMINI LTS comprehensively demonstrates that vedolizumab therapy has a safety and tolerability profile suitable for long-term treatment of patients with moderately to severely active ulcerative colitis or Crohn’s disease,” the investigators concluded.
According to Randy Longman, MD, director of the Jill Roberts Center for Inflammatory Bowel Disease at Weill Cornell Medicine and New York-Presbyterian, “The results from the GEMINI LTS trial help to solidify the favorable safety profile of vedolizumab for the treatment of IBD. With large patient numbers and cumulative duration of vedolizumab exposure, these pivotal data convincingly reveal low rates of serious infections, identified no cases of progressive multifocal leukoencephalopathy, and showed no increased risk of malignancy. Arthralgias, or joint pain, was the most common treatment-emergent adverse event, but occurred largely in patients with a history of IBD-associated arthralgia and suggest that these symptoms should be monitored during treatment. Overall, these results provide meaningful reassurance to patients and providers using vedolizumab for the treatment of IBD.”
GEMINI LTS was funded by Takeda. The investigators reported additional relationships with AbbVie, Janssen, Amgen, and others. Dr. Longman reported no conflicts of interest.
SOURCE: Loftus Jr EV et al. AP&T. 2020 Sep 2. doi: 10.1111/apt.16060.
FROM ALIMENTARY PHARMACOLOGY & THERAPEUTICS
Bacteria may be associated with risk of MS relapse
No broad differences in gut bacterial composition, however, are associated with risk of relapse, according to the investigators. The findings were presented at the Joint European Committee for Treatment and Research in Multiple Sclerosis–Americas Committee for Treatment and Research in Multiple Sclerosis (ECTRIMS–ACTRIMS) 2020, this year known as MSVirtual2020.
Previous research has found an association between Blautia stercoris and disease activity in other immune-mediated diseases such as systemic lupus. Although the current study is the largest in patients with MS that includes data about the microbiome and relapses, its findings require replication, said Mary Horton, a doctoral candidate in epidemiology at the University of California, Berkeley.
Gut microbes digest food, produce vitamins (for example, B12 and K), create a barrier against pathogens, and regulate the immune system, among other tasks. Most current knowledge about the gut microbiome in MS comes from studies of patients with adult-onset MS. In 2016, Tremlett et al. found an increase in Desulfovibrionaceae and a decrease in Lachnospiraceae and Ruminococcaceae in patients with pediatric-onset MS. They also found that a decrease in Fusobacteria was associated with risk of relapse in this population.
Advanced analytical methods
Using a larger sample size and newer analytical methods than in the study by Tremlett and colleagues, Ms. Horton’s group sought to determine whether features of the gut microbiome are associated with relapse. From 2014 to 2018, the investigators recruited 53 patients with pediatric-onset MS from the University of California, San Francisco, and six centers in the U.S. Network of Pediatric MS Centers. At baseline, they collected stool samples, blood samples, information about past relapses, medication records, demographics, and environmental factors. At each relapse, the investigators collected information about the patient’s current and past medication use and about relapses that the patient had had since the previous visit.
Ms. Horton and colleagues analyzed the stool samples using 16S rRNA sequencing of the V4 region. They identified amplicon sequence variants (ASVs), which are used to define species of bacteria, with the Divisive Amplicon Denoising Algorithm-2 (DADA2). Taxonomies were assigned using the naive Bayesian classifier method, and the read count was normalized using multiple rarefaction.
The investigators identified ASV clusters using weighted genetic correlation network analysis (WGCNA). To evaluate whether individual ASVs were associated with relapse, they used a Prentice, Williams, and Peterson (PWP) recurrent event model, an extension of the Cox proportional hazards model.
The role of methanogenesis
Ms. Horton and colleagues included 53 patients (72% girls) in their study. The population’s mean age was 14.3 years at disease onset and 15.5 years at stool sample collection. About 70% of patients were White, and about 36% were Hispanic. Mean disease duration was 1.3 years, and median Expanded Disability Status Scale score was 1.0.
Approximately 45% of participants had one relapse, and 30% had more than one relapse during the subsequent mean follow-up of 2.5 years. About 91% of patients used a disease-modifying therapy during follow-up.
Gut bacterial abundance was broadly similar between patients who relapsed during the study period and those who did not. Of 270 ASVs included in the analyses, 20 were nominally associated with risk of relapse. Blautia stercoris had the most significant association with relapse risk (hazard ratio, 2.50). Blautia massiliensis also was among the 20 ASVs associated with risk of relapse.
WGCNA identified six ASV clusters. Higher values of one cluster’s eigengene were significantly associated with higher relapse risk (HR, 1.23). The following four ASVs nominally associated with higher relapse risk were in this cluster: Blautia massiliensis, Dorea longicatena, Coprococcus comes, and an unknown species in genus Subdoligranulum.
When Ms. Horton and colleagues examined the pathways from these bacterial species, they found 10 that were significantly associated with the risk of relapse. Four of these 10 pathways are involved in methane production, which suggests the involvement of methanogenesis pathways in relapse.
Although the investigators used advanced techniques for genetic and statistical analysis, the study’s sample size is small, Ms. Horton acknowledged. In addition, the conclusions that can be drawn from observational data are limited.
These suggest several avenues for future research. “There is a big question about how the different treatments that people are on when they are experiencing relapses might impact the microbiome,” said Ms. Horton. “Is the microbiome impacting your treatment response, or is it the reverse?” Investigators also could examine why the methane production pathway is overrepresented among people with MS who have relapses. “Which specific archaea might be leading to that increase in methane is a ripe future study question. Just what that means for health is really unknown.”
The National MS Society and the National Institute of Neurological Disorders and Stroke provided funding for the study. Ms. Horton had no disclosures.
SOURCE: Horton M et al. MSVirtual2020, Abstract LB01.05.
No broad differences in gut bacterial composition, however, are associated with risk of relapse, according to the investigators. The findings were presented at the Joint European Committee for Treatment and Research in Multiple Sclerosis–Americas Committee for Treatment and Research in Multiple Sclerosis (ECTRIMS–ACTRIMS) 2020, this year known as MSVirtual2020.
Previous research has found an association between Blautia stercoris and disease activity in other immune-mediated diseases such as systemic lupus. Although the current study is the largest in patients with MS that includes data about the microbiome and relapses, its findings require replication, said Mary Horton, a doctoral candidate in epidemiology at the University of California, Berkeley.
Gut microbes digest food, produce vitamins (for example, B12 and K), create a barrier against pathogens, and regulate the immune system, among other tasks. Most current knowledge about the gut microbiome in MS comes from studies of patients with adult-onset MS. In 2016, Tremlett et al. found an increase in Desulfovibrionaceae and a decrease in Lachnospiraceae and Ruminococcaceae in patients with pediatric-onset MS. They also found that a decrease in Fusobacteria was associated with risk of relapse in this population.
Advanced analytical methods
Using a larger sample size and newer analytical methods than in the study by Tremlett and colleagues, Ms. Horton’s group sought to determine whether features of the gut microbiome are associated with relapse. From 2014 to 2018, the investigators recruited 53 patients with pediatric-onset MS from the University of California, San Francisco, and six centers in the U.S. Network of Pediatric MS Centers. At baseline, they collected stool samples, blood samples, information about past relapses, medication records, demographics, and environmental factors. At each relapse, the investigators collected information about the patient’s current and past medication use and about relapses that the patient had had since the previous visit.
Ms. Horton and colleagues analyzed the stool samples using 16S rRNA sequencing of the V4 region. They identified amplicon sequence variants (ASVs), which are used to define species of bacteria, with the Divisive Amplicon Denoising Algorithm-2 (DADA2). Taxonomies were assigned using the naive Bayesian classifier method, and the read count was normalized using multiple rarefaction.
The investigators identified ASV clusters using weighted genetic correlation network analysis (WGCNA). To evaluate whether individual ASVs were associated with relapse, they used a Prentice, Williams, and Peterson (PWP) recurrent event model, an extension of the Cox proportional hazards model.
The role of methanogenesis
Ms. Horton and colleagues included 53 patients (72% girls) in their study. The population’s mean age was 14.3 years at disease onset and 15.5 years at stool sample collection. About 70% of patients were White, and about 36% were Hispanic. Mean disease duration was 1.3 years, and median Expanded Disability Status Scale score was 1.0.
Approximately 45% of participants had one relapse, and 30% had more than one relapse during the subsequent mean follow-up of 2.5 years. About 91% of patients used a disease-modifying therapy during follow-up.
Gut bacterial abundance was broadly similar between patients who relapsed during the study period and those who did not. Of 270 ASVs included in the analyses, 20 were nominally associated with risk of relapse. Blautia stercoris had the most significant association with relapse risk (hazard ratio, 2.50). Blautia massiliensis also was among the 20 ASVs associated with risk of relapse.
WGCNA identified six ASV clusters. Higher values of one cluster’s eigengene were significantly associated with higher relapse risk (HR, 1.23). The following four ASVs nominally associated with higher relapse risk were in this cluster: Blautia massiliensis, Dorea longicatena, Coprococcus comes, and an unknown species in genus Subdoligranulum.
When Ms. Horton and colleagues examined the pathways from these bacterial species, they found 10 that were significantly associated with the risk of relapse. Four of these 10 pathways are involved in methane production, which suggests the involvement of methanogenesis pathways in relapse.
Although the investigators used advanced techniques for genetic and statistical analysis, the study’s sample size is small, Ms. Horton acknowledged. In addition, the conclusions that can be drawn from observational data are limited.
These suggest several avenues for future research. “There is a big question about how the different treatments that people are on when they are experiencing relapses might impact the microbiome,” said Ms. Horton. “Is the microbiome impacting your treatment response, or is it the reverse?” Investigators also could examine why the methane production pathway is overrepresented among people with MS who have relapses. “Which specific archaea might be leading to that increase in methane is a ripe future study question. Just what that means for health is really unknown.”
The National MS Society and the National Institute of Neurological Disorders and Stroke provided funding for the study. Ms. Horton had no disclosures.
SOURCE: Horton M et al. MSVirtual2020, Abstract LB01.05.
No broad differences in gut bacterial composition, however, are associated with risk of relapse, according to the investigators. The findings were presented at the Joint European Committee for Treatment and Research in Multiple Sclerosis–Americas Committee for Treatment and Research in Multiple Sclerosis (ECTRIMS–ACTRIMS) 2020, this year known as MSVirtual2020.
Previous research has found an association between Blautia stercoris and disease activity in other immune-mediated diseases such as systemic lupus. Although the current study is the largest in patients with MS that includes data about the microbiome and relapses, its findings require replication, said Mary Horton, a doctoral candidate in epidemiology at the University of California, Berkeley.
Gut microbes digest food, produce vitamins (for example, B12 and K), create a barrier against pathogens, and regulate the immune system, among other tasks. Most current knowledge about the gut microbiome in MS comes from studies of patients with adult-onset MS. In 2016, Tremlett et al. found an increase in Desulfovibrionaceae and a decrease in Lachnospiraceae and Ruminococcaceae in patients with pediatric-onset MS. They also found that a decrease in Fusobacteria was associated with risk of relapse in this population.
Advanced analytical methods
Using a larger sample size and newer analytical methods than in the study by Tremlett and colleagues, Ms. Horton’s group sought to determine whether features of the gut microbiome are associated with relapse. From 2014 to 2018, the investigators recruited 53 patients with pediatric-onset MS from the University of California, San Francisco, and six centers in the U.S. Network of Pediatric MS Centers. At baseline, they collected stool samples, blood samples, information about past relapses, medication records, demographics, and environmental factors. At each relapse, the investigators collected information about the patient’s current and past medication use and about relapses that the patient had had since the previous visit.
Ms. Horton and colleagues analyzed the stool samples using 16S rRNA sequencing of the V4 region. They identified amplicon sequence variants (ASVs), which are used to define species of bacteria, with the Divisive Amplicon Denoising Algorithm-2 (DADA2). Taxonomies were assigned using the naive Bayesian classifier method, and the read count was normalized using multiple rarefaction.
The investigators identified ASV clusters using weighted genetic correlation network analysis (WGCNA). To evaluate whether individual ASVs were associated with relapse, they used a Prentice, Williams, and Peterson (PWP) recurrent event model, an extension of the Cox proportional hazards model.
The role of methanogenesis
Ms. Horton and colleagues included 53 patients (72% girls) in their study. The population’s mean age was 14.3 years at disease onset and 15.5 years at stool sample collection. About 70% of patients were White, and about 36% were Hispanic. Mean disease duration was 1.3 years, and median Expanded Disability Status Scale score was 1.0.
Approximately 45% of participants had one relapse, and 30% had more than one relapse during the subsequent mean follow-up of 2.5 years. About 91% of patients used a disease-modifying therapy during follow-up.
Gut bacterial abundance was broadly similar between patients who relapsed during the study period and those who did not. Of 270 ASVs included in the analyses, 20 were nominally associated with risk of relapse. Blautia stercoris had the most significant association with relapse risk (hazard ratio, 2.50). Blautia massiliensis also was among the 20 ASVs associated with risk of relapse.
WGCNA identified six ASV clusters. Higher values of one cluster’s eigengene were significantly associated with higher relapse risk (HR, 1.23). The following four ASVs nominally associated with higher relapse risk were in this cluster: Blautia massiliensis, Dorea longicatena, Coprococcus comes, and an unknown species in genus Subdoligranulum.
When Ms. Horton and colleagues examined the pathways from these bacterial species, they found 10 that were significantly associated with the risk of relapse. Four of these 10 pathways are involved in methane production, which suggests the involvement of methanogenesis pathways in relapse.
Although the investigators used advanced techniques for genetic and statistical analysis, the study’s sample size is small, Ms. Horton acknowledged. In addition, the conclusions that can be drawn from observational data are limited.
These suggest several avenues for future research. “There is a big question about how the different treatments that people are on when they are experiencing relapses might impact the microbiome,” said Ms. Horton. “Is the microbiome impacting your treatment response, or is it the reverse?” Investigators also could examine why the methane production pathway is overrepresented among people with MS who have relapses. “Which specific archaea might be leading to that increase in methane is a ripe future study question. Just what that means for health is really unknown.”
The National MS Society and the National Institute of Neurological Disorders and Stroke provided funding for the study. Ms. Horton had no disclosures.
SOURCE: Horton M et al. MSVirtual2020, Abstract LB01.05.
From MSVirtual2020
New nonhormonal hot flash treatments on the way
researchers told attendees at the virtual North American Menopause Society 2020 Annual Meeting.
“The KNDy [kisspeptin/neurokinin B/dynorphin] neuron manipulation is really exciting and holds great promise for rapid and highly effective amelioration of hot flashes, up to 80%, and improvement in other menopausal symptoms, though we’re still looking at the safety in phase 3 trials,” reported Susan D. Reed, MD, MPH, director of the Women’s Reproductive Health Research Program at the University of Washington, Seattle.
“If we continue to see good safety data, these are going to be the greatest things since sliced bread,” Dr. Reed said in an interview. “I don’t think we’ve seen anything like this in menopause therapeutics in a long time.”
While several nonhormonal drugs are already used to treat vasomotor symptoms in menopausal women with and without breast cancer, none are as effective as hormone treatments.
“For now, the SSRIs, SNRIs [serotonin norepinephrine reuptake inhibitors], and GABAergics are the best frontline nonhormonal options with a moderate effect, and clonidine and oxybutynin are effective, but we see more side effects with these,” Dr. Reed said. She noted the importance of considering patients’ mood, sleep, pain, sexual function, weight gain, overactive bladder, blood pressure, and individual quality of life (QOL) goals in tailoring those therapies.
But women still need more nonhormonal options that are at least as effective as hormonal options, Dr. Reed said. Some women are unable to take hormonal options because they are at risk for blood clots or breast cancer.
“Then there’s preference,” she said. “Sometimes people don’t like the way they feel when they take hormones, or they just don’t want hormones in their body. It’s absolutely critical to have these options available for women.”
Nanette F. Santoro, MD, a professor of ob.gyn. at the University of Colorado at Denver, Aurora, who was not involved in the presentation, said in an interview that physicians may not always realize the extent to which vasomotor symptoms interfere with women’s daily lives.
“They have an eroding effect on QOL that is not appreciated sometimes,” she said. Though hot flashes eventually subside in most women, others may continue to experience them into their 70s, when hormonal therapies can begin causing more harm than benefit.
“It goes underappreciated that, for a proportion of women, hot flashes will never go away, and they’re just as bad [as] when they were in their 50s,” Dr. Santoro said. “They need to be treated, and the nonhormonal treatments do not work for everybody.”
Promising KNDy therapeutics
Autopsy studies of postmenopausal women revealed that a complex of neurons in the hypothalamus was “massively hypertrophied” and sits right next to the thermoregulatory center of the brain, Dr. Reed explained.
The complex produces three types of molecules: kisspeptin (a neuropeptide), neurokinin B (a neuropeptide), and dynorphin (a kappa opioid), collectively referred to as the KNDy. The KNDy neural complex is located in the same place as the majority of hormone receptors in the arcuate nucleus, a collection of nerve cells in the hypothalamus.
The current hypothesis is that the KNDy neurons, which communicate with each other, become hyperactivated and cause hot flashes by spilling over to and triggering the thermoregulatory center next door. NKB (kisspeptin and neurokinin B) agonists activate KNDy neurons and dynorphin agonists inactivate KNDy, so the expectation is that NKB antagonists or dynorphin agonists would stop hot flashes.
Indeed, research published in 2015 showed that women taking kappa agonists experienced fewer hot flashes than women in the placebo group. However, no peripherally restricted kappa agonists are currently in clinical trials, so their future as therapeutics is unclear.
Right now, three different NK antagonists are in the pipeline for reducing vasomotor symptoms: MLE 4901 (pavinetant) and ESN364 (fezolinetant) are both NK3R antagonists, and NT-814 is a dual NK1R/NK3R antagonist. All three of these drugs were originally developed to treat schizophrenia.
Phase 2 clinical trials of pavinetant were discontinued in November 2017 by Millendo Therapeutics because 3 of 28 women experienced abnormal liver function, which normalized within 90 days. However, the study had shown an 80% decrease in hot flashes in women taking pavinetant, compared with a 30% decrease in the placebo group.
Fezolinetant, currently in phase 3 trials with Astellas, showed a dose response effect on reproductive hormones in phase 1 studies and a short half-life (4-6 hours) in women. It also showed no concerning side effects.
“There was, in fact, a decrease in the endometrial thickness, a delayed or impeded ovulation and a prolonged cycle duration,” Reed said.
The subsequent phase 2a study showed a reduction of five hot flashes a day (93% decrease), compared with placebo (54% decrease, P <.001) “with an abrupt return to baseline hot flash frequency after cessation,” she said. Improvements also occurred in sleep quality, quality of life, disability, and interference of hot flashes in daily life.
The phase 2b study found no difference in effects between once-daily versus twice-daily doses. However, two severe adverse events occurred: a drug-induced liver injury in one woman and cholelithiasis in another, both on the 60-mg, once-daily dose. Additionally, five women on varying doses had transient increases (above 1000 U/L) in creatinine kinase, though apparently without dose response.
A 52-week, three-arm, phase 3 trial of fezolinetant is currently under way with a goal of enrolling 1,740 participants, and plans to be completed by December 2021. Participants will undergo regular adverse event screening first biweekly, then monthly, with vital signs, blood, and urine monitoring.
Meanwhile, NT-814 from KaNDy Therapeutics, has completed phase 2a and phase 2b trials with phase 3 slated to begin in 2021. Adverse events in phase 1 included sleepiness and headache, and it had a long half-life (about 26 hours) and rapid absorption (an hour).
The phase 2a trial found a reduction of five hot flashes a day, compared with placebo, with main side effects again being sleepiness and headache. No events of abnormal liver function occurred. Phase 2b results have not been published.
So far, existing research suggests that KNDy interventions will involve a single daily oral dose that begins taking effect within 3 days and is fully in effect within 1-2 weeks. The reduction in hot flashes, about five fewer a day, is more effective than any other currently used nonhormonal medications for vasomotor symptoms. SSRIs and SNRIs tend to result in 1.5-2 fewer hot flashes a day, and gabapentin results in about 3 fewer per day. It will take longer-term studies, however, and paying attention to liver concerns for the NK3R antagonists to move into clinic.
“We want to keep our eye on the [luteinizing hormone] because if it decreases too much, it could adversely affect sexual function, and this does appear to be a dose-response finding,” Dr. Reed said. It would also be ideal, she said, to target only the KNDy neurons with NK3 antagonists without effects on the NK3 receptors in the liver.
Other nonhormonal options
Oxybutynin is another a nonhormonal agent under investigation for vasomotor symptoms. It’s an anticholinergic that resulted in 80% fewer hot flashes, compared with 30% with placebo in a 2016 trial, but 52% of women complained of dry mouth. A more recent study similarly found high efficacy – a 60%-80% drop in hot flashes, compared with 30% with placebo – but also side effects of dry mouth, difficulty urinating, and abdominal pain.
Finally, Dr. Reed mentioned three other agents under investigation as possible nonhormonal therapeutics, though she has little information about them. They include MT-8554 by Mitsubishi Tanabe; FP-101 by Fervent Pharmaceuticals; and Q-122 by QUE Oncology with Emory University, Atlanta, and the University of Queensland, Brisbane, Australia.
None of the currently available nonhormonal options provide as high efficacy as hormones, but they do reduce symptoms:
Clonidine is an off-label option some physicians already use as a nonhormonal treatment for vasomotor symptoms, but again, the side effects are problematic: dry mouth, constipation, drowsiness, postural hypotension, and poor sleep.
Paroxetine, at 7.5-10 mg, is the only FDA-approved nonhormonal treatment for vasomotor symptoms, but she listed other off-label options found effective in evidence reviews: gabapentin (100-2,400 mg), venlafaxine (37.5-75 mg), citalopram (10 mg), desvenlafaxine (150 mg), and escitalopram (10 mg).
“I want you to take note of the lower doses in all of these products that are efficacious above those doses that might be used for mood,” Dr. Reed added.
Dr. Reed receives royalties from UpToDate and research funding from Bayer. Dr. Santoro owns stock in MenoGeniX and serves as a consultant or advisor to Ansh Labs, MenoGeniX, and Ogeda/Astellas.
A version of this article originally appeared on Medscape.com.
researchers told attendees at the virtual North American Menopause Society 2020 Annual Meeting.
“The KNDy [kisspeptin/neurokinin B/dynorphin] neuron manipulation is really exciting and holds great promise for rapid and highly effective amelioration of hot flashes, up to 80%, and improvement in other menopausal symptoms, though we’re still looking at the safety in phase 3 trials,” reported Susan D. Reed, MD, MPH, director of the Women’s Reproductive Health Research Program at the University of Washington, Seattle.
“If we continue to see good safety data, these are going to be the greatest things since sliced bread,” Dr. Reed said in an interview. “I don’t think we’ve seen anything like this in menopause therapeutics in a long time.”
While several nonhormonal drugs are already used to treat vasomotor symptoms in menopausal women with and without breast cancer, none are as effective as hormone treatments.
“For now, the SSRIs, SNRIs [serotonin norepinephrine reuptake inhibitors], and GABAergics are the best frontline nonhormonal options with a moderate effect, and clonidine and oxybutynin are effective, but we see more side effects with these,” Dr. Reed said. She noted the importance of considering patients’ mood, sleep, pain, sexual function, weight gain, overactive bladder, blood pressure, and individual quality of life (QOL) goals in tailoring those therapies.
But women still need more nonhormonal options that are at least as effective as hormonal options, Dr. Reed said. Some women are unable to take hormonal options because they are at risk for blood clots or breast cancer.
“Then there’s preference,” she said. “Sometimes people don’t like the way they feel when they take hormones, or they just don’t want hormones in their body. It’s absolutely critical to have these options available for women.”
Nanette F. Santoro, MD, a professor of ob.gyn. at the University of Colorado at Denver, Aurora, who was not involved in the presentation, said in an interview that physicians may not always realize the extent to which vasomotor symptoms interfere with women’s daily lives.
“They have an eroding effect on QOL that is not appreciated sometimes,” she said. Though hot flashes eventually subside in most women, others may continue to experience them into their 70s, when hormonal therapies can begin causing more harm than benefit.
“It goes underappreciated that, for a proportion of women, hot flashes will never go away, and they’re just as bad [as] when they were in their 50s,” Dr. Santoro said. “They need to be treated, and the nonhormonal treatments do not work for everybody.”
Promising KNDy therapeutics
Autopsy studies of postmenopausal women revealed that a complex of neurons in the hypothalamus was “massively hypertrophied” and sits right next to the thermoregulatory center of the brain, Dr. Reed explained.
The complex produces three types of molecules: kisspeptin (a neuropeptide), neurokinin B (a neuropeptide), and dynorphin (a kappa opioid), collectively referred to as the KNDy. The KNDy neural complex is located in the same place as the majority of hormone receptors in the arcuate nucleus, a collection of nerve cells in the hypothalamus.
The current hypothesis is that the KNDy neurons, which communicate with each other, become hyperactivated and cause hot flashes by spilling over to and triggering the thermoregulatory center next door. NKB (kisspeptin and neurokinin B) agonists activate KNDy neurons and dynorphin agonists inactivate KNDy, so the expectation is that NKB antagonists or dynorphin agonists would stop hot flashes.
Indeed, research published in 2015 showed that women taking kappa agonists experienced fewer hot flashes than women in the placebo group. However, no peripherally restricted kappa agonists are currently in clinical trials, so their future as therapeutics is unclear.
Right now, three different NK antagonists are in the pipeline for reducing vasomotor symptoms: MLE 4901 (pavinetant) and ESN364 (fezolinetant) are both NK3R antagonists, and NT-814 is a dual NK1R/NK3R antagonist. All three of these drugs were originally developed to treat schizophrenia.
Phase 2 clinical trials of pavinetant were discontinued in November 2017 by Millendo Therapeutics because 3 of 28 women experienced abnormal liver function, which normalized within 90 days. However, the study had shown an 80% decrease in hot flashes in women taking pavinetant, compared with a 30% decrease in the placebo group.
Fezolinetant, currently in phase 3 trials with Astellas, showed a dose response effect on reproductive hormones in phase 1 studies and a short half-life (4-6 hours) in women. It also showed no concerning side effects.
“There was, in fact, a decrease in the endometrial thickness, a delayed or impeded ovulation and a prolonged cycle duration,” Reed said.
The subsequent phase 2a study showed a reduction of five hot flashes a day (93% decrease), compared with placebo (54% decrease, P <.001) “with an abrupt return to baseline hot flash frequency after cessation,” she said. Improvements also occurred in sleep quality, quality of life, disability, and interference of hot flashes in daily life.
The phase 2b study found no difference in effects between once-daily versus twice-daily doses. However, two severe adverse events occurred: a drug-induced liver injury in one woman and cholelithiasis in another, both on the 60-mg, once-daily dose. Additionally, five women on varying doses had transient increases (above 1000 U/L) in creatinine kinase, though apparently without dose response.
A 52-week, three-arm, phase 3 trial of fezolinetant is currently under way with a goal of enrolling 1,740 participants, and plans to be completed by December 2021. Participants will undergo regular adverse event screening first biweekly, then monthly, with vital signs, blood, and urine monitoring.
Meanwhile, NT-814 from KaNDy Therapeutics, has completed phase 2a and phase 2b trials with phase 3 slated to begin in 2021. Adverse events in phase 1 included sleepiness and headache, and it had a long half-life (about 26 hours) and rapid absorption (an hour).
The phase 2a trial found a reduction of five hot flashes a day, compared with placebo, with main side effects again being sleepiness and headache. No events of abnormal liver function occurred. Phase 2b results have not been published.
So far, existing research suggests that KNDy interventions will involve a single daily oral dose that begins taking effect within 3 days and is fully in effect within 1-2 weeks. The reduction in hot flashes, about five fewer a day, is more effective than any other currently used nonhormonal medications for vasomotor symptoms. SSRIs and SNRIs tend to result in 1.5-2 fewer hot flashes a day, and gabapentin results in about 3 fewer per day. It will take longer-term studies, however, and paying attention to liver concerns for the NK3R antagonists to move into clinic.
“We want to keep our eye on the [luteinizing hormone] because if it decreases too much, it could adversely affect sexual function, and this does appear to be a dose-response finding,” Dr. Reed said. It would also be ideal, she said, to target only the KNDy neurons with NK3 antagonists without effects on the NK3 receptors in the liver.
Other nonhormonal options
Oxybutynin is another a nonhormonal agent under investigation for vasomotor symptoms. It’s an anticholinergic that resulted in 80% fewer hot flashes, compared with 30% with placebo in a 2016 trial, but 52% of women complained of dry mouth. A more recent study similarly found high efficacy – a 60%-80% drop in hot flashes, compared with 30% with placebo – but also side effects of dry mouth, difficulty urinating, and abdominal pain.
Finally, Dr. Reed mentioned three other agents under investigation as possible nonhormonal therapeutics, though she has little information about them. They include MT-8554 by Mitsubishi Tanabe; FP-101 by Fervent Pharmaceuticals; and Q-122 by QUE Oncology with Emory University, Atlanta, and the University of Queensland, Brisbane, Australia.
None of the currently available nonhormonal options provide as high efficacy as hormones, but they do reduce symptoms:
Clonidine is an off-label option some physicians already use as a nonhormonal treatment for vasomotor symptoms, but again, the side effects are problematic: dry mouth, constipation, drowsiness, postural hypotension, and poor sleep.
Paroxetine, at 7.5-10 mg, is the only FDA-approved nonhormonal treatment for vasomotor symptoms, but she listed other off-label options found effective in evidence reviews: gabapentin (100-2,400 mg), venlafaxine (37.5-75 mg), citalopram (10 mg), desvenlafaxine (150 mg), and escitalopram (10 mg).
“I want you to take note of the lower doses in all of these products that are efficacious above those doses that might be used for mood,” Dr. Reed added.
Dr. Reed receives royalties from UpToDate and research funding from Bayer. Dr. Santoro owns stock in MenoGeniX and serves as a consultant or advisor to Ansh Labs, MenoGeniX, and Ogeda/Astellas.
A version of this article originally appeared on Medscape.com.
researchers told attendees at the virtual North American Menopause Society 2020 Annual Meeting.
“The KNDy [kisspeptin/neurokinin B/dynorphin] neuron manipulation is really exciting and holds great promise for rapid and highly effective amelioration of hot flashes, up to 80%, and improvement in other menopausal symptoms, though we’re still looking at the safety in phase 3 trials,” reported Susan D. Reed, MD, MPH, director of the Women’s Reproductive Health Research Program at the University of Washington, Seattle.
“If we continue to see good safety data, these are going to be the greatest things since sliced bread,” Dr. Reed said in an interview. “I don’t think we’ve seen anything like this in menopause therapeutics in a long time.”
While several nonhormonal drugs are already used to treat vasomotor symptoms in menopausal women with and without breast cancer, none are as effective as hormone treatments.
“For now, the SSRIs, SNRIs [serotonin norepinephrine reuptake inhibitors], and GABAergics are the best frontline nonhormonal options with a moderate effect, and clonidine and oxybutynin are effective, but we see more side effects with these,” Dr. Reed said. She noted the importance of considering patients’ mood, sleep, pain, sexual function, weight gain, overactive bladder, blood pressure, and individual quality of life (QOL) goals in tailoring those therapies.
But women still need more nonhormonal options that are at least as effective as hormonal options, Dr. Reed said. Some women are unable to take hormonal options because they are at risk for blood clots or breast cancer.
“Then there’s preference,” she said. “Sometimes people don’t like the way they feel when they take hormones, or they just don’t want hormones in their body. It’s absolutely critical to have these options available for women.”
Nanette F. Santoro, MD, a professor of ob.gyn. at the University of Colorado at Denver, Aurora, who was not involved in the presentation, said in an interview that physicians may not always realize the extent to which vasomotor symptoms interfere with women’s daily lives.
“They have an eroding effect on QOL that is not appreciated sometimes,” she said. Though hot flashes eventually subside in most women, others may continue to experience them into their 70s, when hormonal therapies can begin causing more harm than benefit.
“It goes underappreciated that, for a proportion of women, hot flashes will never go away, and they’re just as bad [as] when they were in their 50s,” Dr. Santoro said. “They need to be treated, and the nonhormonal treatments do not work for everybody.”
Promising KNDy therapeutics
Autopsy studies of postmenopausal women revealed that a complex of neurons in the hypothalamus was “massively hypertrophied” and sits right next to the thermoregulatory center of the brain, Dr. Reed explained.
The complex produces three types of molecules: kisspeptin (a neuropeptide), neurokinin B (a neuropeptide), and dynorphin (a kappa opioid), collectively referred to as the KNDy. The KNDy neural complex is located in the same place as the majority of hormone receptors in the arcuate nucleus, a collection of nerve cells in the hypothalamus.
The current hypothesis is that the KNDy neurons, which communicate with each other, become hyperactivated and cause hot flashes by spilling over to and triggering the thermoregulatory center next door. NKB (kisspeptin and neurokinin B) agonists activate KNDy neurons and dynorphin agonists inactivate KNDy, so the expectation is that NKB antagonists or dynorphin agonists would stop hot flashes.
Indeed, research published in 2015 showed that women taking kappa agonists experienced fewer hot flashes than women in the placebo group. However, no peripherally restricted kappa agonists are currently in clinical trials, so their future as therapeutics is unclear.
Right now, three different NK antagonists are in the pipeline for reducing vasomotor symptoms: MLE 4901 (pavinetant) and ESN364 (fezolinetant) are both NK3R antagonists, and NT-814 is a dual NK1R/NK3R antagonist. All three of these drugs were originally developed to treat schizophrenia.
Phase 2 clinical trials of pavinetant were discontinued in November 2017 by Millendo Therapeutics because 3 of 28 women experienced abnormal liver function, which normalized within 90 days. However, the study had shown an 80% decrease in hot flashes in women taking pavinetant, compared with a 30% decrease in the placebo group.
Fezolinetant, currently in phase 3 trials with Astellas, showed a dose response effect on reproductive hormones in phase 1 studies and a short half-life (4-6 hours) in women. It also showed no concerning side effects.
“There was, in fact, a decrease in the endometrial thickness, a delayed or impeded ovulation and a prolonged cycle duration,” Reed said.
The subsequent phase 2a study showed a reduction of five hot flashes a day (93% decrease), compared with placebo (54% decrease, P <.001) “with an abrupt return to baseline hot flash frequency after cessation,” she said. Improvements also occurred in sleep quality, quality of life, disability, and interference of hot flashes in daily life.
The phase 2b study found no difference in effects between once-daily versus twice-daily doses. However, two severe adverse events occurred: a drug-induced liver injury in one woman and cholelithiasis in another, both on the 60-mg, once-daily dose. Additionally, five women on varying doses had transient increases (above 1000 U/L) in creatinine kinase, though apparently without dose response.
A 52-week, three-arm, phase 3 trial of fezolinetant is currently under way with a goal of enrolling 1,740 participants, and plans to be completed by December 2021. Participants will undergo regular adverse event screening first biweekly, then monthly, with vital signs, blood, and urine monitoring.
Meanwhile, NT-814 from KaNDy Therapeutics, has completed phase 2a and phase 2b trials with phase 3 slated to begin in 2021. Adverse events in phase 1 included sleepiness and headache, and it had a long half-life (about 26 hours) and rapid absorption (an hour).
The phase 2a trial found a reduction of five hot flashes a day, compared with placebo, with main side effects again being sleepiness and headache. No events of abnormal liver function occurred. Phase 2b results have not been published.
So far, existing research suggests that KNDy interventions will involve a single daily oral dose that begins taking effect within 3 days and is fully in effect within 1-2 weeks. The reduction in hot flashes, about five fewer a day, is more effective than any other currently used nonhormonal medications for vasomotor symptoms. SSRIs and SNRIs tend to result in 1.5-2 fewer hot flashes a day, and gabapentin results in about 3 fewer per day. It will take longer-term studies, however, and paying attention to liver concerns for the NK3R antagonists to move into clinic.
“We want to keep our eye on the [luteinizing hormone] because if it decreases too much, it could adversely affect sexual function, and this does appear to be a dose-response finding,” Dr. Reed said. It would also be ideal, she said, to target only the KNDy neurons with NK3 antagonists without effects on the NK3 receptors in the liver.
Other nonhormonal options
Oxybutynin is another a nonhormonal agent under investigation for vasomotor symptoms. It’s an anticholinergic that resulted in 80% fewer hot flashes, compared with 30% with placebo in a 2016 trial, but 52% of women complained of dry mouth. A more recent study similarly found high efficacy – a 60%-80% drop in hot flashes, compared with 30% with placebo – but also side effects of dry mouth, difficulty urinating, and abdominal pain.
Finally, Dr. Reed mentioned three other agents under investigation as possible nonhormonal therapeutics, though she has little information about them. They include MT-8554 by Mitsubishi Tanabe; FP-101 by Fervent Pharmaceuticals; and Q-122 by QUE Oncology with Emory University, Atlanta, and the University of Queensland, Brisbane, Australia.
None of the currently available nonhormonal options provide as high efficacy as hormones, but they do reduce symptoms:
Clonidine is an off-label option some physicians already use as a nonhormonal treatment for vasomotor symptoms, but again, the side effects are problematic: dry mouth, constipation, drowsiness, postural hypotension, and poor sleep.
Paroxetine, at 7.5-10 mg, is the only FDA-approved nonhormonal treatment for vasomotor symptoms, but she listed other off-label options found effective in evidence reviews: gabapentin (100-2,400 mg), venlafaxine (37.5-75 mg), citalopram (10 mg), desvenlafaxine (150 mg), and escitalopram (10 mg).
“I want you to take note of the lower doses in all of these products that are efficacious above those doses that might be used for mood,” Dr. Reed added.
Dr. Reed receives royalties from UpToDate and research funding from Bayer. Dr. Santoro owns stock in MenoGeniX and serves as a consultant or advisor to Ansh Labs, MenoGeniX, and Ogeda/Astellas.
A version of this article originally appeared on Medscape.com.
FDA proposes withdrawing Makena’s approval
Makena should be withdrawn from the market because a postmarketing study did not show clinical benefit, according to a statement released today from the Center for Drug Evaluation and Research at the Food and Drug Administration.
The drug, hydroxyprogesterone caproate injection, was approved in 2011 to reduce the risk of preterm birth in women who with previous spontaneous preterm birth. The FDA approved the medication under an accelerated pathway that required another trial to confirm clinical benefit.
The required postmarketing study “not only failed to demonstrate Makena’s benefit to the neonate, but also failed to substantiate any effect of Makena on the surrogate endpoint of gestational age at delivery that was the basis of the initial approval,” Patrizia Cavazzoni, MD, acting director of the CDER, wrote in a letter to AMAG Pharma USA, which markets Makena. The letter also was sent to other companies developing products that use the drug.
Beyond the lack of efficacy, risks associated with the drug include thromboembolic disorders, allergic reactions, decreased glucose tolerance, and fluid retention. “The risk of exposing treated pregnant women to these harms, in addition to false hopes, costs, and additional healthcare utilization outweighs Makena’s unproven benefit,” Dr. Cavazzoni said.
The letter notifies companies about the opportunity for a hearing on the proposed withdrawal of marketing approval. Makena and its generic equivalents will remain on the market until the manufacturers remove the drugs or the FDA commissioner mandates their removal, the CDER said.
The FDA commissioner ultimately will decide whether to withdraw approval of the drug. An FDA panel previously voted to withdraw the drug from the market in October 2019, and the drug has remained in limbo since.
Health care professionals should discuss “Makena’s benefits, risks, and uncertainties with their patients to decide whether to use Makena while a final decision is being made about the drug’s marketing status,” the CDER announcement said.
A version of this article originally appeared on Medscape.com.
Makena should be withdrawn from the market because a postmarketing study did not show clinical benefit, according to a statement released today from the Center for Drug Evaluation and Research at the Food and Drug Administration.
The drug, hydroxyprogesterone caproate injection, was approved in 2011 to reduce the risk of preterm birth in women who with previous spontaneous preterm birth. The FDA approved the medication under an accelerated pathway that required another trial to confirm clinical benefit.
The required postmarketing study “not only failed to demonstrate Makena’s benefit to the neonate, but also failed to substantiate any effect of Makena on the surrogate endpoint of gestational age at delivery that was the basis of the initial approval,” Patrizia Cavazzoni, MD, acting director of the CDER, wrote in a letter to AMAG Pharma USA, which markets Makena. The letter also was sent to other companies developing products that use the drug.
Beyond the lack of efficacy, risks associated with the drug include thromboembolic disorders, allergic reactions, decreased glucose tolerance, and fluid retention. “The risk of exposing treated pregnant women to these harms, in addition to false hopes, costs, and additional healthcare utilization outweighs Makena’s unproven benefit,” Dr. Cavazzoni said.
The letter notifies companies about the opportunity for a hearing on the proposed withdrawal of marketing approval. Makena and its generic equivalents will remain on the market until the manufacturers remove the drugs or the FDA commissioner mandates their removal, the CDER said.
The FDA commissioner ultimately will decide whether to withdraw approval of the drug. An FDA panel previously voted to withdraw the drug from the market in October 2019, and the drug has remained in limbo since.
Health care professionals should discuss “Makena’s benefits, risks, and uncertainties with their patients to decide whether to use Makena while a final decision is being made about the drug’s marketing status,” the CDER announcement said.
A version of this article originally appeared on Medscape.com.
Makena should be withdrawn from the market because a postmarketing study did not show clinical benefit, according to a statement released today from the Center for Drug Evaluation and Research at the Food and Drug Administration.
The drug, hydroxyprogesterone caproate injection, was approved in 2011 to reduce the risk of preterm birth in women who with previous spontaneous preterm birth. The FDA approved the medication under an accelerated pathway that required another trial to confirm clinical benefit.
The required postmarketing study “not only failed to demonstrate Makena’s benefit to the neonate, but also failed to substantiate any effect of Makena on the surrogate endpoint of gestational age at delivery that was the basis of the initial approval,” Patrizia Cavazzoni, MD, acting director of the CDER, wrote in a letter to AMAG Pharma USA, which markets Makena. The letter also was sent to other companies developing products that use the drug.
Beyond the lack of efficacy, risks associated with the drug include thromboembolic disorders, allergic reactions, decreased glucose tolerance, and fluid retention. “The risk of exposing treated pregnant women to these harms, in addition to false hopes, costs, and additional healthcare utilization outweighs Makena’s unproven benefit,” Dr. Cavazzoni said.
The letter notifies companies about the opportunity for a hearing on the proposed withdrawal of marketing approval. Makena and its generic equivalents will remain on the market until the manufacturers remove the drugs or the FDA commissioner mandates their removal, the CDER said.
The FDA commissioner ultimately will decide whether to withdraw approval of the drug. An FDA panel previously voted to withdraw the drug from the market in October 2019, and the drug has remained in limbo since.
Health care professionals should discuss “Makena’s benefits, risks, and uncertainties with their patients to decide whether to use Makena while a final decision is being made about the drug’s marketing status,” the CDER announcement said.
A version of this article originally appeared on Medscape.com.