User login
New treatment options show promise for centrifugal cicatricial alopecia
according to a review of current strategies for this challenging disease, delivered at the virtual Skin of Color Update 2020.
Two case reports of favorable results with topical metformin were published earlier this year. A subsequent case in which metformin provided a major improvement in quality of life has provided further encouragement, according to Crystal Aguh, MD, director of the ethnic skin program at Johns Hopkins University, Baltimore.
In the subsequent case, there was complete scalp coverage, allowing the patient to no longer use a wig, which is “tough to achieve in patients with advanced disease,” Dr. Aguh said.
In the two published cases, 10% metformin compounded in Lipoderm (PCCA) produced notable hair growth within 6 months. Dr. Aguh said that the case studies were prompted by experimental evidence associating metformin with an antifibrotic effect.
This finding is potentially important. Most current treatments for CCCA are based on anti-inflammatory activity, according to Dr. Aguh, but fibrosis is strongly implicated in the pathogenesis of CCCA. Of several lines of evidence, one is the association between CCCA and other fibrosing conditions.
For example, women with CCCA “are several times more likely to have uterine fibroids than women without CCCA,” said Dr. Aguh, citing a study that she published in 2018. She suggested that there is an urgent need for new treatment options because of the “often-disappointing” responses to current standard therapies.
In the CCCA cases treated so far, topical metformin has been well tolerated, which is attributed to the low level of systemic absorption. No nausea or other gastrointestinal side effects common to oral metformin have been so far observed in Dr. Aguh’s cases.
“Some patients experience scalp dryness or irritation,” she said, but added that a light coating of emollient typically relieves this complaint.
Despite the promising results, topical metformin “is not a silver bullet,” Dr. Aguh cautioned. She estimated that only 10%-15% of patients respond, but this treatment can be considered “as an adjunctive option to avoid another round of intralesional steroids.”
Platelet-rich plasma (PRP) is another option that has demonstrated promise in a published case report for which Dr. Aguh served as a coauthor. In this series of two patients, only one had CCCA. The other had lichen planopilaris, but both patients experienced hair regrowth after failing standard therapies.
When treating alopecia with PRP, Dr. Aguh typically offers three or four sessions spaced 4 weeks apart. She does not start other treatments at the same time, but she does not discontinue topical treatments that patients are already taking, including topical minoxidil.
Again, like topical metformin, PRP is reasonably considered in patients who have failed standard therapies, according to Dr. Aguh. She cautioned that responses are not permanent. Patients who respond typically require retreatment a year or more later, but good responses have been seen after retreatment.
Appropriate hair care can help. Dr. Aguh recounted a case in which a patient with presumed CCCA was referred after failing intralesional triamcinolone injections. Ultimately, the patient was diagnosed with acquired trichorrhexis nodosa, but the large clinical improvements gained from better hair care practices, including avoidance of chemical relaxants and thermal styling, are relevant to CCCA, as well as other conditions resulting in hair loss.
In a book written by Dr. Aguh, titled “90 Days to Beautiful Hair,” strategies for better hair care practices include advice to reduce tension on hair follicles.
The role of increased traction is an issue in CCCA, agreed Amy McMichael, MD, chair of the department of dermatology, Wake Forest University, Winston-Salem, N.C. Although she provided data at the meeting suggesting that CCCA is a fibrosing disease linked to genetic susceptibility, she said there is also a “strong association” between the severity of CCCA and extensions, hair weaving, and other tension-associated hairstyles.
While there is an urgent need to develop therapies that address the underlying pathophysiology of CCCA, she concurred that patients with this or other conditions associated with hair loss, such as seborrheic dermatitis or frontal fibrosing alopecia, should not ignore appropriate hair care.
Dr. Aguh has financial relationships with LEO Pharma and UCB Pharma. Dr. McMichael’s disclosures included serving as an investigator and/or consultant for companies that included Allergan, Procter & Gamble, Nutrafol, Johnson & Johnson, and Aclaris.
according to a review of current strategies for this challenging disease, delivered at the virtual Skin of Color Update 2020.
Two case reports of favorable results with topical metformin were published earlier this year. A subsequent case in which metformin provided a major improvement in quality of life has provided further encouragement, according to Crystal Aguh, MD, director of the ethnic skin program at Johns Hopkins University, Baltimore.
In the subsequent case, there was complete scalp coverage, allowing the patient to no longer use a wig, which is “tough to achieve in patients with advanced disease,” Dr. Aguh said.
In the two published cases, 10% metformin compounded in Lipoderm (PCCA) produced notable hair growth within 6 months. Dr. Aguh said that the case studies were prompted by experimental evidence associating metformin with an antifibrotic effect.
This finding is potentially important. Most current treatments for CCCA are based on anti-inflammatory activity, according to Dr. Aguh, but fibrosis is strongly implicated in the pathogenesis of CCCA. Of several lines of evidence, one is the association between CCCA and other fibrosing conditions.
For example, women with CCCA “are several times more likely to have uterine fibroids than women without CCCA,” said Dr. Aguh, citing a study that she published in 2018. She suggested that there is an urgent need for new treatment options because of the “often-disappointing” responses to current standard therapies.
In the CCCA cases treated so far, topical metformin has been well tolerated, which is attributed to the low level of systemic absorption. No nausea or other gastrointestinal side effects common to oral metformin have been so far observed in Dr. Aguh’s cases.
“Some patients experience scalp dryness or irritation,” she said, but added that a light coating of emollient typically relieves this complaint.
Despite the promising results, topical metformin “is not a silver bullet,” Dr. Aguh cautioned. She estimated that only 10%-15% of patients respond, but this treatment can be considered “as an adjunctive option to avoid another round of intralesional steroids.”
Platelet-rich plasma (PRP) is another option that has demonstrated promise in a published case report for which Dr. Aguh served as a coauthor. In this series of two patients, only one had CCCA. The other had lichen planopilaris, but both patients experienced hair regrowth after failing standard therapies.
When treating alopecia with PRP, Dr. Aguh typically offers three or four sessions spaced 4 weeks apart. She does not start other treatments at the same time, but she does not discontinue topical treatments that patients are already taking, including topical minoxidil.
Again, like topical metformin, PRP is reasonably considered in patients who have failed standard therapies, according to Dr. Aguh. She cautioned that responses are not permanent. Patients who respond typically require retreatment a year or more later, but good responses have been seen after retreatment.
Appropriate hair care can help. Dr. Aguh recounted a case in which a patient with presumed CCCA was referred after failing intralesional triamcinolone injections. Ultimately, the patient was diagnosed with acquired trichorrhexis nodosa, but the large clinical improvements gained from better hair care practices, including avoidance of chemical relaxants and thermal styling, are relevant to CCCA, as well as other conditions resulting in hair loss.
In a book written by Dr. Aguh, titled “90 Days to Beautiful Hair,” strategies for better hair care practices include advice to reduce tension on hair follicles.
The role of increased traction is an issue in CCCA, agreed Amy McMichael, MD, chair of the department of dermatology, Wake Forest University, Winston-Salem, N.C. Although she provided data at the meeting suggesting that CCCA is a fibrosing disease linked to genetic susceptibility, she said there is also a “strong association” between the severity of CCCA and extensions, hair weaving, and other tension-associated hairstyles.
While there is an urgent need to develop therapies that address the underlying pathophysiology of CCCA, she concurred that patients with this or other conditions associated with hair loss, such as seborrheic dermatitis or frontal fibrosing alopecia, should not ignore appropriate hair care.
Dr. Aguh has financial relationships with LEO Pharma and UCB Pharma. Dr. McMichael’s disclosures included serving as an investigator and/or consultant for companies that included Allergan, Procter & Gamble, Nutrafol, Johnson & Johnson, and Aclaris.
according to a review of current strategies for this challenging disease, delivered at the virtual Skin of Color Update 2020.
Two case reports of favorable results with topical metformin were published earlier this year. A subsequent case in which metformin provided a major improvement in quality of life has provided further encouragement, according to Crystal Aguh, MD, director of the ethnic skin program at Johns Hopkins University, Baltimore.
In the subsequent case, there was complete scalp coverage, allowing the patient to no longer use a wig, which is “tough to achieve in patients with advanced disease,” Dr. Aguh said.
In the two published cases, 10% metformin compounded in Lipoderm (PCCA) produced notable hair growth within 6 months. Dr. Aguh said that the case studies were prompted by experimental evidence associating metformin with an antifibrotic effect.
This finding is potentially important. Most current treatments for CCCA are based on anti-inflammatory activity, according to Dr. Aguh, but fibrosis is strongly implicated in the pathogenesis of CCCA. Of several lines of evidence, one is the association between CCCA and other fibrosing conditions.
For example, women with CCCA “are several times more likely to have uterine fibroids than women without CCCA,” said Dr. Aguh, citing a study that she published in 2018. She suggested that there is an urgent need for new treatment options because of the “often-disappointing” responses to current standard therapies.
In the CCCA cases treated so far, topical metformin has been well tolerated, which is attributed to the low level of systemic absorption. No nausea or other gastrointestinal side effects common to oral metformin have been so far observed in Dr. Aguh’s cases.
“Some patients experience scalp dryness or irritation,” she said, but added that a light coating of emollient typically relieves this complaint.
Despite the promising results, topical metformin “is not a silver bullet,” Dr. Aguh cautioned. She estimated that only 10%-15% of patients respond, but this treatment can be considered “as an adjunctive option to avoid another round of intralesional steroids.”
Platelet-rich plasma (PRP) is another option that has demonstrated promise in a published case report for which Dr. Aguh served as a coauthor. In this series of two patients, only one had CCCA. The other had lichen planopilaris, but both patients experienced hair regrowth after failing standard therapies.
When treating alopecia with PRP, Dr. Aguh typically offers three or four sessions spaced 4 weeks apart. She does not start other treatments at the same time, but she does not discontinue topical treatments that patients are already taking, including topical minoxidil.
Again, like topical metformin, PRP is reasonably considered in patients who have failed standard therapies, according to Dr. Aguh. She cautioned that responses are not permanent. Patients who respond typically require retreatment a year or more later, but good responses have been seen after retreatment.
Appropriate hair care can help. Dr. Aguh recounted a case in which a patient with presumed CCCA was referred after failing intralesional triamcinolone injections. Ultimately, the patient was diagnosed with acquired trichorrhexis nodosa, but the large clinical improvements gained from better hair care practices, including avoidance of chemical relaxants and thermal styling, are relevant to CCCA, as well as other conditions resulting in hair loss.
In a book written by Dr. Aguh, titled “90 Days to Beautiful Hair,” strategies for better hair care practices include advice to reduce tension on hair follicles.
The role of increased traction is an issue in CCCA, agreed Amy McMichael, MD, chair of the department of dermatology, Wake Forest University, Winston-Salem, N.C. Although she provided data at the meeting suggesting that CCCA is a fibrosing disease linked to genetic susceptibility, she said there is also a “strong association” between the severity of CCCA and extensions, hair weaving, and other tension-associated hairstyles.
While there is an urgent need to develop therapies that address the underlying pathophysiology of CCCA, she concurred that patients with this or other conditions associated with hair loss, such as seborrheic dermatitis or frontal fibrosing alopecia, should not ignore appropriate hair care.
Dr. Aguh has financial relationships with LEO Pharma and UCB Pharma. Dr. McMichael’s disclosures included serving as an investigator and/or consultant for companies that included Allergan, Procter & Gamble, Nutrafol, Johnson & Johnson, and Aclaris.
FROM SOC 2020
How Kodak Gold contributed to bias in dermatology
A recent review looked at all articles describing skin manifestations associated with COVID-19 – 46 articles with a total of 130 clinical images – and found none that documented dermatologic conditions in people with dark skin. This was despite the disproportionate incidence of the disease in Black, Latino, and Native American/American Indian populations of color.
What’s going on? Temitayo Ogunleye, MD, an assistant professor of dermatology at the University of Pennsylvania, Philadelphia, spoke with lead investigator Jenna Lester, MD, a dermatologist and director of the Skin of Color Clinic at University of California, San Francisco, about the implications of her research.
Dr. Ogunleye: What prompted you to do this study in the first place?
Dr. Lester: It was actually driven by frustration. While we’re recognizing these COVID-related dermatologic manifestations, we’re still trying to figure out their clinical significance. We’ve learned what changes could be an early sign of infection and what might occur in people who are otherwise asymptomatic and should be tested. But in the process, we’re leaving out the group of people – dark-skinned populations – who are most heavily impacted by COVID. This is an injustice to the people who could benefit the most if found to have an early, visible sign of the disease on their skin. For example, pernio-like lesions and erythema, both of which have been seen in COVID patients, are harder to identify in darker skin.
As dermatologists, we know that the skin is the biggest organ and one that has the advantage of being visible. We partner with patients because they can see what we can see. We can explain what skin changes mean, and having examples to show them is really powerful. Because doctors typically respond best to numbers, I recognized that we needed data to prove that this lack of representation of persons of color in our COVID documentation was in fact true.
Can you tell us the key findings from your research?
We included any article that described the cutaneous manifestations of COVID – and also included a photograph – and was published over a period of 5 months. Then we categorized each image using the Fitzpatrick Skin Type Scale. We found that A handful, about 6%, depicted skin changes in patients with Fitzpatrick type IV skin.
Were you surprised by the findings?
No. I was not surprised at all, for a couple of reasons. First, many of the referrals that I had been getting to evaluate possible COVID manifestations were from primary care doctors. And as we both know, erythema, hyperpigmentation, and discoloration can be difficult for even a trained dermatologist to pick up on. So if these patients with skin changes potentially suggestive of COVID are presenting to their primary care doctor who could not determine what they were, perhaps because they had never seen them in someone with darker skin, it means those same clinicians are not likely to document these rashes. I suspect that a lot of these photos were sourced from primary care.
The other reason for my lack of surprise is that I have looked at this issue before. In a study I did in 2019 looking at images in dermatology textbooks, my coauthors and I found that there was a pretty dramatic underrepresentation of skin of color overall. Another analysis of images in core dermatology textbooks, published earlier this year by one of your colleagues at the University of Pennsylvania, Jules Lipoff, MD, showed that the percentage of images of dark skin ranged from as low as 4% to a high of about 18%.
So I wonder whether that makes us less likely to look for things in patients with darker skin, except for those conditions that we’re taught are more common in people with darker skin, like keloids, vitiligo, or certain types of hair loss. I wonder whether we just don’t think of other conditions because all the photos we ever see of psoriasis or rosacea, for example, are of people with lighter skin.
You bring up a good point about the cyclical nature of this process. If you don’t see skin conditions in darker skin, then you don’t know what to look for and so you never look for it.
Exactly. What if a Black patient says, for example: “This looks different on my skin; do you think this could be related to COVID?” And their doctor looks at their skin and says no. Not because it isn’t, but because they haven’t seen that before. Then they don’t take a picture of it and we’ll never know what that might have been.
The disturbing thing I have found is that there is an overrepresentation of dark skin in images of sexually transmitted infections. So based on what you are taught, you begin to create these powerful cognitive biases in your brain as a clinician and you start to put people in categories: “This person is more likely to get this because I’ve seen a lot of photos of it.”
Take the example of a 40ish Black woman with a cough who is presumed to have sarcoidosis, because we’ve all been taught that. But what we have not been taught, at least not as definitively, is that the highest prevalence of sarcoidosis is in Nordic countries, where there are not many Black people.
So these loops teach you things that don’t necessarily represent reality. We are taught to recognize patterns, but the patterns that we’ve created are not necessarily valid and carry the biases of the people who decided they were important for us to learn.
So the underrepresentation of persons of color in images depicting skin changes in COVID-19 is, in your perspective, a continuation of this pattern of bias?
I definitely think it is. It’s important to understand the history of photography and the development of color film in order to contextualize that question. Kodak Gold was one of the first color films made. To assist photographers, the company developed a Shirley card, which could be used for color balancing in developing this film. That card, which was distributed to film development labs across the country, depicted a fair-skinned White woman as the standard for how people should appear in the photograph. Film technology was built around this idea.
As a result, people with darker skin were never portrayed accurately and did not have a lot of detail to their features or their skin. A number of famous photographers boycotted by not using the film.
But it wasn’t until chocolate manufacturers and the furniture industries decided that Kodak Gold film was not showing their brown products in enough detail that Kodak was forced to change.
It took these big industries saying “we’re not going to use your film anymore” to spur the development of multicultural Shirley cards which included images of Asian, White, and Black women (a Latina was added later). That didn’t happen until 1995. By then, digital photography had already started to take off. Unfortunately, that advance built off of the original color film and still harbored some of the same issues.
So in addition to concerns about clinicians not recognizing a skin change in a darker-skinned person, if they do recognize it and do decide to photograph it, it just might not come out right. So then it’s possible that clinicians just decide to stop taking photos.
This is another example of structural racism – things that are just baked into the system about which people are unaware. I fear we’ll continue to perpetuate these unconscious biases with the development of augmented intelligence or various algorithms.
I think that this history – which I was unaware of – highlights what happens when White skin becomes the standard. It certainly explains the issue we’ve both seen of clinicians not recognizing erythema on dark skin.
That’s a big one. And I think it’s a big one because it’s a shared presenting sign of a lot of different rashes. It can also be a sign of a dermatologic emergency that requires rapid recognition and treatment. Erythema can be very subtle, especially in skin of color. It is one of the things that we use to grade severity of psoriasis, and as a result of it not being appreciated in people with darker skin, those patients have not been included in a lot of the clinical trials.
There has even been discussion of whether erythema is something we should deem to be an important finding in people with darker skin.
Maybe one of the problems is terminology. Erythema connotes pink or red coloration, and that does not really show up on dark skin as overtly.
Exactly. Emollient use is also different in people of color. So the “classic” scales of psoriasis often aren’t present in someone who uses a lot of lotion.
In addition to the different clinical appearance of many common skin conditions, cultural practices might be different in different groups, which may also alter your differential diagnoses and treatment recommendations – for instance, moisturizer use, shampooing frequency, or use of different hair styling products.
I totally agree. Hair is another big one. Identifying broken hairs, short hairs, texture changes, and variability in the size of the hair shaft is different in coiled, Black hair and is not really applicable to people with more textured hair patterns. People of different ethnicities and cultures do different things. Standards that we traditionally use, such as the hair-pull test, don’t work quite as well in different groups.
I think that speaks to the changes that we also need to make in educating both dermatologists and nondermatologists in diagnostic criteria and clinical findings.
Dermatology is the second least diverse specialty. And that means our experts – faculty, lecturers, mentors – are not likely to be people of color. You don’t know what you don’t know. You only have your own experiences. If you don’t have people who can explain why something may be different for a different group of people, you don’t have an opportunity to broaden your understanding. You and I noticed because we know what it’s like to have this type of hair. But it’s not something that other people who don’t share the same hair type would have the same perspective on.
It is even more reason to make sure that our dermatology workforce mirrors our population. About 3% of the dermatologists in the United States are Black; the numbers are equally bad for Latino and indigenous dermatologists. If you don’t have enough people in your immediate environment who can help broaden your perspective, that becomes a problem that can harm patients.
If we don’t have a diverse group of people in the field who are contributing to the literature, changing some of the ways that we think about practice, then everyone is just going to keep doing the wrong thing.
We need to build our evidence and pay more attention to the racial breakdown of patients included in studies. We are recognizing that we have blind spots but we don’t have people or data that can change that. If what we are publishing overrepresents a certain group, how will we ever learn to do things differently?
I recognize the fact that this is not a new idea. Others have worked on these issues for a long time. Your colleague, Susan Taylor, MD, a professor at University of Pennsylvania and a founder of the Skin of Color Society, used her energy and frustration about this issue and published a whole textbook on it: “Dermatology for Skin of Color.” When I tried to publish my first paper on this issue, I got a lot of pushback, but I’m happy that now it’s something that everyone is talking about. So just in the span of a couple of years, the acceptance of this idea has changed dramatically.
I hope that this is the start of sustainable, systemic changes that can have a real impact.
Let me ask a technical question. Is the excuse of not knowing how to photograph darker skin just that – an excuse? Or is that concern truly valid?
That’s a great question. There are certain techniques that one can employ. And yes, it can be more challenging if you are not used to taking photos of people with darker skin, but it certainly can be learned.
I think that an intelligent group of people who have faced things before that felt insurmountable could continue to push to figure out how to do it. But it is something that does require skills, and part of it is because there’s this bias built into the camera, so you have to make up for that.
I think every single dermatologist has that ability, and you just have to know that it’s worthwhile to do because your patient is that valuable.
A version of this article originally appeared on Medscape.com.
A recent review looked at all articles describing skin manifestations associated with COVID-19 – 46 articles with a total of 130 clinical images – and found none that documented dermatologic conditions in people with dark skin. This was despite the disproportionate incidence of the disease in Black, Latino, and Native American/American Indian populations of color.
What’s going on? Temitayo Ogunleye, MD, an assistant professor of dermatology at the University of Pennsylvania, Philadelphia, spoke with lead investigator Jenna Lester, MD, a dermatologist and director of the Skin of Color Clinic at University of California, San Francisco, about the implications of her research.
Dr. Ogunleye: What prompted you to do this study in the first place?
Dr. Lester: It was actually driven by frustration. While we’re recognizing these COVID-related dermatologic manifestations, we’re still trying to figure out their clinical significance. We’ve learned what changes could be an early sign of infection and what might occur in people who are otherwise asymptomatic and should be tested. But in the process, we’re leaving out the group of people – dark-skinned populations – who are most heavily impacted by COVID. This is an injustice to the people who could benefit the most if found to have an early, visible sign of the disease on their skin. For example, pernio-like lesions and erythema, both of which have been seen in COVID patients, are harder to identify in darker skin.
As dermatologists, we know that the skin is the biggest organ and one that has the advantage of being visible. We partner with patients because they can see what we can see. We can explain what skin changes mean, and having examples to show them is really powerful. Because doctors typically respond best to numbers, I recognized that we needed data to prove that this lack of representation of persons of color in our COVID documentation was in fact true.
Can you tell us the key findings from your research?
We included any article that described the cutaneous manifestations of COVID – and also included a photograph – and was published over a period of 5 months. Then we categorized each image using the Fitzpatrick Skin Type Scale. We found that A handful, about 6%, depicted skin changes in patients with Fitzpatrick type IV skin.
Were you surprised by the findings?
No. I was not surprised at all, for a couple of reasons. First, many of the referrals that I had been getting to evaluate possible COVID manifestations were from primary care doctors. And as we both know, erythema, hyperpigmentation, and discoloration can be difficult for even a trained dermatologist to pick up on. So if these patients with skin changes potentially suggestive of COVID are presenting to their primary care doctor who could not determine what they were, perhaps because they had never seen them in someone with darker skin, it means those same clinicians are not likely to document these rashes. I suspect that a lot of these photos were sourced from primary care.
The other reason for my lack of surprise is that I have looked at this issue before. In a study I did in 2019 looking at images in dermatology textbooks, my coauthors and I found that there was a pretty dramatic underrepresentation of skin of color overall. Another analysis of images in core dermatology textbooks, published earlier this year by one of your colleagues at the University of Pennsylvania, Jules Lipoff, MD, showed that the percentage of images of dark skin ranged from as low as 4% to a high of about 18%.
So I wonder whether that makes us less likely to look for things in patients with darker skin, except for those conditions that we’re taught are more common in people with darker skin, like keloids, vitiligo, or certain types of hair loss. I wonder whether we just don’t think of other conditions because all the photos we ever see of psoriasis or rosacea, for example, are of people with lighter skin.
You bring up a good point about the cyclical nature of this process. If you don’t see skin conditions in darker skin, then you don’t know what to look for and so you never look for it.
Exactly. What if a Black patient says, for example: “This looks different on my skin; do you think this could be related to COVID?” And their doctor looks at their skin and says no. Not because it isn’t, but because they haven’t seen that before. Then they don’t take a picture of it and we’ll never know what that might have been.
The disturbing thing I have found is that there is an overrepresentation of dark skin in images of sexually transmitted infections. So based on what you are taught, you begin to create these powerful cognitive biases in your brain as a clinician and you start to put people in categories: “This person is more likely to get this because I’ve seen a lot of photos of it.”
Take the example of a 40ish Black woman with a cough who is presumed to have sarcoidosis, because we’ve all been taught that. But what we have not been taught, at least not as definitively, is that the highest prevalence of sarcoidosis is in Nordic countries, where there are not many Black people.
So these loops teach you things that don’t necessarily represent reality. We are taught to recognize patterns, but the patterns that we’ve created are not necessarily valid and carry the biases of the people who decided they were important for us to learn.
So the underrepresentation of persons of color in images depicting skin changes in COVID-19 is, in your perspective, a continuation of this pattern of bias?
I definitely think it is. It’s important to understand the history of photography and the development of color film in order to contextualize that question. Kodak Gold was one of the first color films made. To assist photographers, the company developed a Shirley card, which could be used for color balancing in developing this film. That card, which was distributed to film development labs across the country, depicted a fair-skinned White woman as the standard for how people should appear in the photograph. Film technology was built around this idea.
As a result, people with darker skin were never portrayed accurately and did not have a lot of detail to their features or their skin. A number of famous photographers boycotted by not using the film.
But it wasn’t until chocolate manufacturers and the furniture industries decided that Kodak Gold film was not showing their brown products in enough detail that Kodak was forced to change.
It took these big industries saying “we’re not going to use your film anymore” to spur the development of multicultural Shirley cards which included images of Asian, White, and Black women (a Latina was added later). That didn’t happen until 1995. By then, digital photography had already started to take off. Unfortunately, that advance built off of the original color film and still harbored some of the same issues.
So in addition to concerns about clinicians not recognizing a skin change in a darker-skinned person, if they do recognize it and do decide to photograph it, it just might not come out right. So then it’s possible that clinicians just decide to stop taking photos.
This is another example of structural racism – things that are just baked into the system about which people are unaware. I fear we’ll continue to perpetuate these unconscious biases with the development of augmented intelligence or various algorithms.
I think that this history – which I was unaware of – highlights what happens when White skin becomes the standard. It certainly explains the issue we’ve both seen of clinicians not recognizing erythema on dark skin.
That’s a big one. And I think it’s a big one because it’s a shared presenting sign of a lot of different rashes. It can also be a sign of a dermatologic emergency that requires rapid recognition and treatment. Erythema can be very subtle, especially in skin of color. It is one of the things that we use to grade severity of psoriasis, and as a result of it not being appreciated in people with darker skin, those patients have not been included in a lot of the clinical trials.
There has even been discussion of whether erythema is something we should deem to be an important finding in people with darker skin.
Maybe one of the problems is terminology. Erythema connotes pink or red coloration, and that does not really show up on dark skin as overtly.
Exactly. Emollient use is also different in people of color. So the “classic” scales of psoriasis often aren’t present in someone who uses a lot of lotion.
In addition to the different clinical appearance of many common skin conditions, cultural practices might be different in different groups, which may also alter your differential diagnoses and treatment recommendations – for instance, moisturizer use, shampooing frequency, or use of different hair styling products.
I totally agree. Hair is another big one. Identifying broken hairs, short hairs, texture changes, and variability in the size of the hair shaft is different in coiled, Black hair and is not really applicable to people with more textured hair patterns. People of different ethnicities and cultures do different things. Standards that we traditionally use, such as the hair-pull test, don’t work quite as well in different groups.
I think that speaks to the changes that we also need to make in educating both dermatologists and nondermatologists in diagnostic criteria and clinical findings.
Dermatology is the second least diverse specialty. And that means our experts – faculty, lecturers, mentors – are not likely to be people of color. You don’t know what you don’t know. You only have your own experiences. If you don’t have people who can explain why something may be different for a different group of people, you don’t have an opportunity to broaden your understanding. You and I noticed because we know what it’s like to have this type of hair. But it’s not something that other people who don’t share the same hair type would have the same perspective on.
It is even more reason to make sure that our dermatology workforce mirrors our population. About 3% of the dermatologists in the United States are Black; the numbers are equally bad for Latino and indigenous dermatologists. If you don’t have enough people in your immediate environment who can help broaden your perspective, that becomes a problem that can harm patients.
If we don’t have a diverse group of people in the field who are contributing to the literature, changing some of the ways that we think about practice, then everyone is just going to keep doing the wrong thing.
We need to build our evidence and pay more attention to the racial breakdown of patients included in studies. We are recognizing that we have blind spots but we don’t have people or data that can change that. If what we are publishing overrepresents a certain group, how will we ever learn to do things differently?
I recognize the fact that this is not a new idea. Others have worked on these issues for a long time. Your colleague, Susan Taylor, MD, a professor at University of Pennsylvania and a founder of the Skin of Color Society, used her energy and frustration about this issue and published a whole textbook on it: “Dermatology for Skin of Color.” When I tried to publish my first paper on this issue, I got a lot of pushback, but I’m happy that now it’s something that everyone is talking about. So just in the span of a couple of years, the acceptance of this idea has changed dramatically.
I hope that this is the start of sustainable, systemic changes that can have a real impact.
Let me ask a technical question. Is the excuse of not knowing how to photograph darker skin just that – an excuse? Or is that concern truly valid?
That’s a great question. There are certain techniques that one can employ. And yes, it can be more challenging if you are not used to taking photos of people with darker skin, but it certainly can be learned.
I think that an intelligent group of people who have faced things before that felt insurmountable could continue to push to figure out how to do it. But it is something that does require skills, and part of it is because there’s this bias built into the camera, so you have to make up for that.
I think every single dermatologist has that ability, and you just have to know that it’s worthwhile to do because your patient is that valuable.
A version of this article originally appeared on Medscape.com.
A recent review looked at all articles describing skin manifestations associated with COVID-19 – 46 articles with a total of 130 clinical images – and found none that documented dermatologic conditions in people with dark skin. This was despite the disproportionate incidence of the disease in Black, Latino, and Native American/American Indian populations of color.
What’s going on? Temitayo Ogunleye, MD, an assistant professor of dermatology at the University of Pennsylvania, Philadelphia, spoke with lead investigator Jenna Lester, MD, a dermatologist and director of the Skin of Color Clinic at University of California, San Francisco, about the implications of her research.
Dr. Ogunleye: What prompted you to do this study in the first place?
Dr. Lester: It was actually driven by frustration. While we’re recognizing these COVID-related dermatologic manifestations, we’re still trying to figure out their clinical significance. We’ve learned what changes could be an early sign of infection and what might occur in people who are otherwise asymptomatic and should be tested. But in the process, we’re leaving out the group of people – dark-skinned populations – who are most heavily impacted by COVID. This is an injustice to the people who could benefit the most if found to have an early, visible sign of the disease on their skin. For example, pernio-like lesions and erythema, both of which have been seen in COVID patients, are harder to identify in darker skin.
As dermatologists, we know that the skin is the biggest organ and one that has the advantage of being visible. We partner with patients because they can see what we can see. We can explain what skin changes mean, and having examples to show them is really powerful. Because doctors typically respond best to numbers, I recognized that we needed data to prove that this lack of representation of persons of color in our COVID documentation was in fact true.
Can you tell us the key findings from your research?
We included any article that described the cutaneous manifestations of COVID – and also included a photograph – and was published over a period of 5 months. Then we categorized each image using the Fitzpatrick Skin Type Scale. We found that A handful, about 6%, depicted skin changes in patients with Fitzpatrick type IV skin.
Were you surprised by the findings?
No. I was not surprised at all, for a couple of reasons. First, many of the referrals that I had been getting to evaluate possible COVID manifestations were from primary care doctors. And as we both know, erythema, hyperpigmentation, and discoloration can be difficult for even a trained dermatologist to pick up on. So if these patients with skin changes potentially suggestive of COVID are presenting to their primary care doctor who could not determine what they were, perhaps because they had never seen them in someone with darker skin, it means those same clinicians are not likely to document these rashes. I suspect that a lot of these photos were sourced from primary care.
The other reason for my lack of surprise is that I have looked at this issue before. In a study I did in 2019 looking at images in dermatology textbooks, my coauthors and I found that there was a pretty dramatic underrepresentation of skin of color overall. Another analysis of images in core dermatology textbooks, published earlier this year by one of your colleagues at the University of Pennsylvania, Jules Lipoff, MD, showed that the percentage of images of dark skin ranged from as low as 4% to a high of about 18%.
So I wonder whether that makes us less likely to look for things in patients with darker skin, except for those conditions that we’re taught are more common in people with darker skin, like keloids, vitiligo, or certain types of hair loss. I wonder whether we just don’t think of other conditions because all the photos we ever see of psoriasis or rosacea, for example, are of people with lighter skin.
You bring up a good point about the cyclical nature of this process. If you don’t see skin conditions in darker skin, then you don’t know what to look for and so you never look for it.
Exactly. What if a Black patient says, for example: “This looks different on my skin; do you think this could be related to COVID?” And their doctor looks at their skin and says no. Not because it isn’t, but because they haven’t seen that before. Then they don’t take a picture of it and we’ll never know what that might have been.
The disturbing thing I have found is that there is an overrepresentation of dark skin in images of sexually transmitted infections. So based on what you are taught, you begin to create these powerful cognitive biases in your brain as a clinician and you start to put people in categories: “This person is more likely to get this because I’ve seen a lot of photos of it.”
Take the example of a 40ish Black woman with a cough who is presumed to have sarcoidosis, because we’ve all been taught that. But what we have not been taught, at least not as definitively, is that the highest prevalence of sarcoidosis is in Nordic countries, where there are not many Black people.
So these loops teach you things that don’t necessarily represent reality. We are taught to recognize patterns, but the patterns that we’ve created are not necessarily valid and carry the biases of the people who decided they were important for us to learn.
So the underrepresentation of persons of color in images depicting skin changes in COVID-19 is, in your perspective, a continuation of this pattern of bias?
I definitely think it is. It’s important to understand the history of photography and the development of color film in order to contextualize that question. Kodak Gold was one of the first color films made. To assist photographers, the company developed a Shirley card, which could be used for color balancing in developing this film. That card, which was distributed to film development labs across the country, depicted a fair-skinned White woman as the standard for how people should appear in the photograph. Film technology was built around this idea.
As a result, people with darker skin were never portrayed accurately and did not have a lot of detail to their features or their skin. A number of famous photographers boycotted by not using the film.
But it wasn’t until chocolate manufacturers and the furniture industries decided that Kodak Gold film was not showing their brown products in enough detail that Kodak was forced to change.
It took these big industries saying “we’re not going to use your film anymore” to spur the development of multicultural Shirley cards which included images of Asian, White, and Black women (a Latina was added later). That didn’t happen until 1995. By then, digital photography had already started to take off. Unfortunately, that advance built off of the original color film and still harbored some of the same issues.
So in addition to concerns about clinicians not recognizing a skin change in a darker-skinned person, if they do recognize it and do decide to photograph it, it just might not come out right. So then it’s possible that clinicians just decide to stop taking photos.
This is another example of structural racism – things that are just baked into the system about which people are unaware. I fear we’ll continue to perpetuate these unconscious biases with the development of augmented intelligence or various algorithms.
I think that this history – which I was unaware of – highlights what happens when White skin becomes the standard. It certainly explains the issue we’ve both seen of clinicians not recognizing erythema on dark skin.
That’s a big one. And I think it’s a big one because it’s a shared presenting sign of a lot of different rashes. It can also be a sign of a dermatologic emergency that requires rapid recognition and treatment. Erythema can be very subtle, especially in skin of color. It is one of the things that we use to grade severity of psoriasis, and as a result of it not being appreciated in people with darker skin, those patients have not been included in a lot of the clinical trials.
There has even been discussion of whether erythema is something we should deem to be an important finding in people with darker skin.
Maybe one of the problems is terminology. Erythema connotes pink or red coloration, and that does not really show up on dark skin as overtly.
Exactly. Emollient use is also different in people of color. So the “classic” scales of psoriasis often aren’t present in someone who uses a lot of lotion.
In addition to the different clinical appearance of many common skin conditions, cultural practices might be different in different groups, which may also alter your differential diagnoses and treatment recommendations – for instance, moisturizer use, shampooing frequency, or use of different hair styling products.
I totally agree. Hair is another big one. Identifying broken hairs, short hairs, texture changes, and variability in the size of the hair shaft is different in coiled, Black hair and is not really applicable to people with more textured hair patterns. People of different ethnicities and cultures do different things. Standards that we traditionally use, such as the hair-pull test, don’t work quite as well in different groups.
I think that speaks to the changes that we also need to make in educating both dermatologists and nondermatologists in diagnostic criteria and clinical findings.
Dermatology is the second least diverse specialty. And that means our experts – faculty, lecturers, mentors – are not likely to be people of color. You don’t know what you don’t know. You only have your own experiences. If you don’t have people who can explain why something may be different for a different group of people, you don’t have an opportunity to broaden your understanding. You and I noticed because we know what it’s like to have this type of hair. But it’s not something that other people who don’t share the same hair type would have the same perspective on.
It is even more reason to make sure that our dermatology workforce mirrors our population. About 3% of the dermatologists in the United States are Black; the numbers are equally bad for Latino and indigenous dermatologists. If you don’t have enough people in your immediate environment who can help broaden your perspective, that becomes a problem that can harm patients.
If we don’t have a diverse group of people in the field who are contributing to the literature, changing some of the ways that we think about practice, then everyone is just going to keep doing the wrong thing.
We need to build our evidence and pay more attention to the racial breakdown of patients included in studies. We are recognizing that we have blind spots but we don’t have people or data that can change that. If what we are publishing overrepresents a certain group, how will we ever learn to do things differently?
I recognize the fact that this is not a new idea. Others have worked on these issues for a long time. Your colleague, Susan Taylor, MD, a professor at University of Pennsylvania and a founder of the Skin of Color Society, used her energy and frustration about this issue and published a whole textbook on it: “Dermatology for Skin of Color.” When I tried to publish my first paper on this issue, I got a lot of pushback, but I’m happy that now it’s something that everyone is talking about. So just in the span of a couple of years, the acceptance of this idea has changed dramatically.
I hope that this is the start of sustainable, systemic changes that can have a real impact.
Let me ask a technical question. Is the excuse of not knowing how to photograph darker skin just that – an excuse? Or is that concern truly valid?
That’s a great question. There are certain techniques that one can employ. And yes, it can be more challenging if you are not used to taking photos of people with darker skin, but it certainly can be learned.
I think that an intelligent group of people who have faced things before that felt insurmountable could continue to push to figure out how to do it. But it is something that does require skills, and part of it is because there’s this bias built into the camera, so you have to make up for that.
I think every single dermatologist has that ability, and you just have to know that it’s worthwhile to do because your patient is that valuable.
A version of this article originally appeared on Medscape.com.
‘Celebration’ will be ‘short-lived’ if COVID vaccine rushed: Experts
on Wednesday.
The career staff of the Food and Drug Administration can be counted on to appropriately weigh whether a vaccine should be cleared for use in preventing COVID-19, witnesses, including Paul A. Offit, MD, of Children’s Hospital of Philadelphia, told the House Energy and Commerce Committee’s oversight and investigations panel.
FDA staffers would object to attempts by the Trump administration to rush a vaccine to the public without proper vetting, as would veteran federal researchers, including National Institutes of Health Director Francis S. Collins, MD, PhD, and Anthony S. Fauci, MD, director of the National Institute of Allergy and Infectious Diseases, Offit said.
“If COVID-19 vaccines are released before they’re ready to be released, you will hear from these people, and you will also hear from people like Dr. Francis Collins and Tony Fauci, both of whom are trusted by the American public, as well as many other academicians and researchers who wouldn’t stand for this,” he said.
“The public is already nervous about these vaccines,” said Offit, who serves on key FDA and Centers for Disease Control and Prevention committees overseeing vaccine policy. “If trusted health officials stand up and decry a premature release, the celebration by the administration will be short-lived.”
Overly optimistic estimates about a potential approval can only serve to erode the public’s trust in these crucial vaccines, said another witness, Ashish K. Jha, MD, MPH, the dean of Brown University’s School of Public Health, in Providence, Rhode Island.
“All political leaders need to stop talking about things like time lines,” Jha told the lawmakers.
President Donald Trump has several times suggested that a COVID vaccine might be approved ahead of the November 3 election, where he faces a significant challenge from his Democratic rival, former Vice President Joe Biden.
In a Tuesday night debate with Biden, Trump again raised the idea of a quick approval. “Now we’re weeks away from a vaccine,” Trump said during the debate.
Trump’s estimates, though, are not in line with those offered by most firms involved with making vaccines. The most optimistic projections have come from Pfizer Inc. The drugmaker’s chief executive, Albert Bourla, has spoken about his company possibly having data to present to the FDA as early as late October about the safety and effectiveness of a vaccine.
In a September 8 interview with the Today show, Bourla said there was a 60% chance his company would meet that goal. In response to a question, he made it clear his comments applied to a potential Pfizer application, not an approval or release of a vaccine by that time.
In response to concerns about political pressures, the FDA in June issued guidance outlining what its staff would require for approval of a COVID-19 vaccine.
Pushback on politics
Another witness at the Wednesday hearing, Mark McClellan, MD, PhD, a former FDA commissioner (2002 – 2004), pushed back on objections to a potential release of further guidance from the agency.
“Some recent statements from the White House have implied that FDA’s plan to release additional written guidance on its expectations for emergency use authorization of a vaccine is unnecessarily raising the bar on regulatory standards for authorization,” said McClellan in his testimony for the House panel. “That is not the case.”
Instead, further FDA guidance would be a welcome form of feedback for the firms trying to develop COVID-19 vaccines, according to McClellan, who also serves on the board of directors for Johnson & Johnson. Johnson & Johnson is among the firms that have advanced a COVID-19 vaccine candidate to phase 3 testing. In his role as a director, he serves on the board’s regulatory compliance committee.
Along with politics, recent stumbles at FDA with emergency use authorizations (EUAs) of treatments for COVID-19 have eroded the public’s confidence in the agency, Jha told the House panel. The FDA approved hydroxychloroquine, a medicine promoted by Trump for use in COVID, under an EUA in March and then revoked this clearance in June.
Jha said the FDA’s most serious misstep was its handling of convalescent plasma, which was approved through an EUA on August 23 “in a highly advertised and widely televised announcement including the president.
“The announcement solidified in the public conversation the impression that, increasingly with this administration, politics are taking over trusted, nonpartisan scientific institutions,” he said in his testimony.
Approving a COVID-19 vaccine on the limited evidence through an EUA would mark a serious departure from FDA policy, according to Jha.
“While we sometimes accept a certain level of potential harm in experimental treatments for those who are severely ill, vaccines are given to healthy people and therefore need to have a substantially higher measure of safety and effectiveness,” he explained.
Jha said the FDA has only once before used this EUA approach for a vaccine. That was for a vaccine against inhaled anthrax and was mostly distributed to high-risk soldiers and civilians in war zones.
COVID-19, in contrast, is an infection that has changed lives around the world. The virus has contributed to more than 1 million deaths, including more than 200,000 in the United States, according to the World Health Organization.
Scientists are hoping vaccines will help curb this infection, although much of the future success of vaccines depends on how widely they are used, witnesses told the House panel.
Debate on approaches for vaccine effectiveness
In his testimony, Jha also noted concerns about COVID-19 vaccine trials. He included a reference to a Sept. 22 opinion article titled, “These Coronavirus Trials Don›t Answer the One Question We Need to Know,” which was written by Peter Doshi, PhD, of the University of Maryland School of Pharmacy, in Baltimore, and Eric Topol, MD, a professor of molecular medicine at Scripps Research in La Jolla, Calif. Topol is also editor in chief of Medscape.
Topol and Doshi questioned why the firms Moderna, Pfizer, and AstraZeneca structured their competing trials such that “a vaccine could meet the companies’ benchmark for success if it lowered the risk of mild Covid-19, but was never shown to reduce moderate or severe forms of the disease, or the risk of hospitalization, admissions to the intensive care unit or death.”
“To say a vaccine works should mean that most people no longer run the risk of getting seriously sick,” Topol and Doshi wrote. “That’s not what these trials will determine.”
There was disagreement about this point at the hearing. U.S. Representative Morgan Griffith (R-Va.) read the section of the Doshi-Topol article quoted above and asked one witness, Offit, to weigh in.
“Do you agree with those concerns? And either way, tell me why,” Griffith asked.
“I don’t agree,” Offit responded.
“I think it’s actually much harder to prevent asymptomatic infection or mildly symptomatic infection,” he said. “If you can prevent that, you are much more likely to prevent moderate to severe disease. So I think they have it backwards.”
But other researchers also question the approaches used with the current crop of COVID-19 vaccines.
“With the current protocols, it is conceivable that a vaccine might be considered effective – and eventually approved – based primarily on its ability to prevent mild cases alone,” wrote William Haseltine, PhD, president of the nonprofit ACCESS Health International, in a September 22 opinion article in the Washington Post titled: “Beware of COVID-19 Vaccine Trials Designed to Succeed From the Start.”
In an interview with Medscape Medical News on Wednesday, Haseltine said he maintains these concerns about the tests. Earlier in his career, he was a leader in HIV research through his lab at Harvard University in Cambridge, Massachusetts, and he subsequently led a biotech company, Human Genome Sciences.
He fears consumers will not get what they might expect from the vaccines being tested.
“What people care about is if this is going to keep them out of the hospital and will it keep them alive. And that’s not even part of this protocol,” Haseltine said.
This article first appeared on Medscape.com.
on Wednesday.
The career staff of the Food and Drug Administration can be counted on to appropriately weigh whether a vaccine should be cleared for use in preventing COVID-19, witnesses, including Paul A. Offit, MD, of Children’s Hospital of Philadelphia, told the House Energy and Commerce Committee’s oversight and investigations panel.
FDA staffers would object to attempts by the Trump administration to rush a vaccine to the public without proper vetting, as would veteran federal researchers, including National Institutes of Health Director Francis S. Collins, MD, PhD, and Anthony S. Fauci, MD, director of the National Institute of Allergy and Infectious Diseases, Offit said.
“If COVID-19 vaccines are released before they’re ready to be released, you will hear from these people, and you will also hear from people like Dr. Francis Collins and Tony Fauci, both of whom are trusted by the American public, as well as many other academicians and researchers who wouldn’t stand for this,” he said.
“The public is already nervous about these vaccines,” said Offit, who serves on key FDA and Centers for Disease Control and Prevention committees overseeing vaccine policy. “If trusted health officials stand up and decry a premature release, the celebration by the administration will be short-lived.”
Overly optimistic estimates about a potential approval can only serve to erode the public’s trust in these crucial vaccines, said another witness, Ashish K. Jha, MD, MPH, the dean of Brown University’s School of Public Health, in Providence, Rhode Island.
“All political leaders need to stop talking about things like time lines,” Jha told the lawmakers.
President Donald Trump has several times suggested that a COVID vaccine might be approved ahead of the November 3 election, where he faces a significant challenge from his Democratic rival, former Vice President Joe Biden.
In a Tuesday night debate with Biden, Trump again raised the idea of a quick approval. “Now we’re weeks away from a vaccine,” Trump said during the debate.
Trump’s estimates, though, are not in line with those offered by most firms involved with making vaccines. The most optimistic projections have come from Pfizer Inc. The drugmaker’s chief executive, Albert Bourla, has spoken about his company possibly having data to present to the FDA as early as late October about the safety and effectiveness of a vaccine.
In a September 8 interview with the Today show, Bourla said there was a 60% chance his company would meet that goal. In response to a question, he made it clear his comments applied to a potential Pfizer application, not an approval or release of a vaccine by that time.
In response to concerns about political pressures, the FDA in June issued guidance outlining what its staff would require for approval of a COVID-19 vaccine.
Pushback on politics
Another witness at the Wednesday hearing, Mark McClellan, MD, PhD, a former FDA commissioner (2002 – 2004), pushed back on objections to a potential release of further guidance from the agency.
“Some recent statements from the White House have implied that FDA’s plan to release additional written guidance on its expectations for emergency use authorization of a vaccine is unnecessarily raising the bar on regulatory standards for authorization,” said McClellan in his testimony for the House panel. “That is not the case.”
Instead, further FDA guidance would be a welcome form of feedback for the firms trying to develop COVID-19 vaccines, according to McClellan, who also serves on the board of directors for Johnson & Johnson. Johnson & Johnson is among the firms that have advanced a COVID-19 vaccine candidate to phase 3 testing. In his role as a director, he serves on the board’s regulatory compliance committee.
Along with politics, recent stumbles at FDA with emergency use authorizations (EUAs) of treatments for COVID-19 have eroded the public’s confidence in the agency, Jha told the House panel. The FDA approved hydroxychloroquine, a medicine promoted by Trump for use in COVID, under an EUA in March and then revoked this clearance in June.
Jha said the FDA’s most serious misstep was its handling of convalescent plasma, which was approved through an EUA on August 23 “in a highly advertised and widely televised announcement including the president.
“The announcement solidified in the public conversation the impression that, increasingly with this administration, politics are taking over trusted, nonpartisan scientific institutions,” he said in his testimony.
Approving a COVID-19 vaccine on the limited evidence through an EUA would mark a serious departure from FDA policy, according to Jha.
“While we sometimes accept a certain level of potential harm in experimental treatments for those who are severely ill, vaccines are given to healthy people and therefore need to have a substantially higher measure of safety and effectiveness,” he explained.
Jha said the FDA has only once before used this EUA approach for a vaccine. That was for a vaccine against inhaled anthrax and was mostly distributed to high-risk soldiers and civilians in war zones.
COVID-19, in contrast, is an infection that has changed lives around the world. The virus has contributed to more than 1 million deaths, including more than 200,000 in the United States, according to the World Health Organization.
Scientists are hoping vaccines will help curb this infection, although much of the future success of vaccines depends on how widely they are used, witnesses told the House panel.
Debate on approaches for vaccine effectiveness
In his testimony, Jha also noted concerns about COVID-19 vaccine trials. He included a reference to a Sept. 22 opinion article titled, “These Coronavirus Trials Don›t Answer the One Question We Need to Know,” which was written by Peter Doshi, PhD, of the University of Maryland School of Pharmacy, in Baltimore, and Eric Topol, MD, a professor of molecular medicine at Scripps Research in La Jolla, Calif. Topol is also editor in chief of Medscape.
Topol and Doshi questioned why the firms Moderna, Pfizer, and AstraZeneca structured their competing trials such that “a vaccine could meet the companies’ benchmark for success if it lowered the risk of mild Covid-19, but was never shown to reduce moderate or severe forms of the disease, or the risk of hospitalization, admissions to the intensive care unit or death.”
“To say a vaccine works should mean that most people no longer run the risk of getting seriously sick,” Topol and Doshi wrote. “That’s not what these trials will determine.”
There was disagreement about this point at the hearing. U.S. Representative Morgan Griffith (R-Va.) read the section of the Doshi-Topol article quoted above and asked one witness, Offit, to weigh in.
“Do you agree with those concerns? And either way, tell me why,” Griffith asked.
“I don’t agree,” Offit responded.
“I think it’s actually much harder to prevent asymptomatic infection or mildly symptomatic infection,” he said. “If you can prevent that, you are much more likely to prevent moderate to severe disease. So I think they have it backwards.”
But other researchers also question the approaches used with the current crop of COVID-19 vaccines.
“With the current protocols, it is conceivable that a vaccine might be considered effective – and eventually approved – based primarily on its ability to prevent mild cases alone,” wrote William Haseltine, PhD, president of the nonprofit ACCESS Health International, in a September 22 opinion article in the Washington Post titled: “Beware of COVID-19 Vaccine Trials Designed to Succeed From the Start.”
In an interview with Medscape Medical News on Wednesday, Haseltine said he maintains these concerns about the tests. Earlier in his career, he was a leader in HIV research through his lab at Harvard University in Cambridge, Massachusetts, and he subsequently led a biotech company, Human Genome Sciences.
He fears consumers will not get what they might expect from the vaccines being tested.
“What people care about is if this is going to keep them out of the hospital and will it keep them alive. And that’s not even part of this protocol,” Haseltine said.
This article first appeared on Medscape.com.
on Wednesday.
The career staff of the Food and Drug Administration can be counted on to appropriately weigh whether a vaccine should be cleared for use in preventing COVID-19, witnesses, including Paul A. Offit, MD, of Children’s Hospital of Philadelphia, told the House Energy and Commerce Committee’s oversight and investigations panel.
FDA staffers would object to attempts by the Trump administration to rush a vaccine to the public without proper vetting, as would veteran federal researchers, including National Institutes of Health Director Francis S. Collins, MD, PhD, and Anthony S. Fauci, MD, director of the National Institute of Allergy and Infectious Diseases, Offit said.
“If COVID-19 vaccines are released before they’re ready to be released, you will hear from these people, and you will also hear from people like Dr. Francis Collins and Tony Fauci, both of whom are trusted by the American public, as well as many other academicians and researchers who wouldn’t stand for this,” he said.
“The public is already nervous about these vaccines,” said Offit, who serves on key FDA and Centers for Disease Control and Prevention committees overseeing vaccine policy. “If trusted health officials stand up and decry a premature release, the celebration by the administration will be short-lived.”
Overly optimistic estimates about a potential approval can only serve to erode the public’s trust in these crucial vaccines, said another witness, Ashish K. Jha, MD, MPH, the dean of Brown University’s School of Public Health, in Providence, Rhode Island.
“All political leaders need to stop talking about things like time lines,” Jha told the lawmakers.
President Donald Trump has several times suggested that a COVID vaccine might be approved ahead of the November 3 election, where he faces a significant challenge from his Democratic rival, former Vice President Joe Biden.
In a Tuesday night debate with Biden, Trump again raised the idea of a quick approval. “Now we’re weeks away from a vaccine,” Trump said during the debate.
Trump’s estimates, though, are not in line with those offered by most firms involved with making vaccines. The most optimistic projections have come from Pfizer Inc. The drugmaker’s chief executive, Albert Bourla, has spoken about his company possibly having data to present to the FDA as early as late October about the safety and effectiveness of a vaccine.
In a September 8 interview with the Today show, Bourla said there was a 60% chance his company would meet that goal. In response to a question, he made it clear his comments applied to a potential Pfizer application, not an approval or release of a vaccine by that time.
In response to concerns about political pressures, the FDA in June issued guidance outlining what its staff would require for approval of a COVID-19 vaccine.
Pushback on politics
Another witness at the Wednesday hearing, Mark McClellan, MD, PhD, a former FDA commissioner (2002 – 2004), pushed back on objections to a potential release of further guidance from the agency.
“Some recent statements from the White House have implied that FDA’s plan to release additional written guidance on its expectations for emergency use authorization of a vaccine is unnecessarily raising the bar on regulatory standards for authorization,” said McClellan in his testimony for the House panel. “That is not the case.”
Instead, further FDA guidance would be a welcome form of feedback for the firms trying to develop COVID-19 vaccines, according to McClellan, who also serves on the board of directors for Johnson & Johnson. Johnson & Johnson is among the firms that have advanced a COVID-19 vaccine candidate to phase 3 testing. In his role as a director, he serves on the board’s regulatory compliance committee.
Along with politics, recent stumbles at FDA with emergency use authorizations (EUAs) of treatments for COVID-19 have eroded the public’s confidence in the agency, Jha told the House panel. The FDA approved hydroxychloroquine, a medicine promoted by Trump for use in COVID, under an EUA in March and then revoked this clearance in June.
Jha said the FDA’s most serious misstep was its handling of convalescent plasma, which was approved through an EUA on August 23 “in a highly advertised and widely televised announcement including the president.
“The announcement solidified in the public conversation the impression that, increasingly with this administration, politics are taking over trusted, nonpartisan scientific institutions,” he said in his testimony.
Approving a COVID-19 vaccine on the limited evidence through an EUA would mark a serious departure from FDA policy, according to Jha.
“While we sometimes accept a certain level of potential harm in experimental treatments for those who are severely ill, vaccines are given to healthy people and therefore need to have a substantially higher measure of safety and effectiveness,” he explained.
Jha said the FDA has only once before used this EUA approach for a vaccine. That was for a vaccine against inhaled anthrax and was mostly distributed to high-risk soldiers and civilians in war zones.
COVID-19, in contrast, is an infection that has changed lives around the world. The virus has contributed to more than 1 million deaths, including more than 200,000 in the United States, according to the World Health Organization.
Scientists are hoping vaccines will help curb this infection, although much of the future success of vaccines depends on how widely they are used, witnesses told the House panel.
Debate on approaches for vaccine effectiveness
In his testimony, Jha also noted concerns about COVID-19 vaccine trials. He included a reference to a Sept. 22 opinion article titled, “These Coronavirus Trials Don›t Answer the One Question We Need to Know,” which was written by Peter Doshi, PhD, of the University of Maryland School of Pharmacy, in Baltimore, and Eric Topol, MD, a professor of molecular medicine at Scripps Research in La Jolla, Calif. Topol is also editor in chief of Medscape.
Topol and Doshi questioned why the firms Moderna, Pfizer, and AstraZeneca structured their competing trials such that “a vaccine could meet the companies’ benchmark for success if it lowered the risk of mild Covid-19, but was never shown to reduce moderate or severe forms of the disease, or the risk of hospitalization, admissions to the intensive care unit or death.”
“To say a vaccine works should mean that most people no longer run the risk of getting seriously sick,” Topol and Doshi wrote. “That’s not what these trials will determine.”
There was disagreement about this point at the hearing. U.S. Representative Morgan Griffith (R-Va.) read the section of the Doshi-Topol article quoted above and asked one witness, Offit, to weigh in.
“Do you agree with those concerns? And either way, tell me why,” Griffith asked.
“I don’t agree,” Offit responded.
“I think it’s actually much harder to prevent asymptomatic infection or mildly symptomatic infection,” he said. “If you can prevent that, you are much more likely to prevent moderate to severe disease. So I think they have it backwards.”
But other researchers also question the approaches used with the current crop of COVID-19 vaccines.
“With the current protocols, it is conceivable that a vaccine might be considered effective – and eventually approved – based primarily on its ability to prevent mild cases alone,” wrote William Haseltine, PhD, president of the nonprofit ACCESS Health International, in a September 22 opinion article in the Washington Post titled: “Beware of COVID-19 Vaccine Trials Designed to Succeed From the Start.”
In an interview with Medscape Medical News on Wednesday, Haseltine said he maintains these concerns about the tests. Earlier in his career, he was a leader in HIV research through his lab at Harvard University in Cambridge, Massachusetts, and he subsequently led a biotech company, Human Genome Sciences.
He fears consumers will not get what they might expect from the vaccines being tested.
“What people care about is if this is going to keep them out of the hospital and will it keep them alive. And that’s not even part of this protocol,” Haseltine said.
This article first appeared on Medscape.com.
Apatinib plus gefitinib: Better PFS but more toxicity
ACTIVE is the first phase 3 trial of an oral vascular epidermal growth factor receptor–2 (VEGFR2) tyrosine kinase inhibitor (TKI) added to an EGFR-TKI as first-line therapy in this population, according to Li Zhang, MD, of Sun Yat-sen University Cancer Center in Guangzhou, China.
Dr. Zhang presented results from ACTIVE at the European Society for Medical Oncology Virtual Congress 2020.
“This dual oral regimen will provide more convenient treatment for patients who require long-term administration,” Dr. Zhang said. He added that apatinib plus gefitinib “is expected to become a new first-line treatment option for EGFR-mutant NSCLC.”
A discussant for the ACTIVE study was less optimistic, however, noting that the regimen proved tough to tolerate for some patients, and the PFS benefit may not translate to overall survival.
Study rationale and details
Sensitizing EGFR mutations occur in about 10% of White patients and up to 50% of Asian patients, Dr. Zhang noted. Unfortunately, most patients progress after first-line treatment with EGFR-TKIs because of acquired resistance.
Blocking VEGF receptor pathways has been shown to enhance EGFR-TKIs in EGFR-mutated NSCLC, and pilot study results have shown apatinib – an oral VEGFR2–TKI – to be safe and well-tolerated with promising efficacy in combination with gefitinib, Dr. Zhang added.
To expand upon those results, he and his colleagues tested apatinib with gefitinib in the phase 3, double-blind, placebo-controlled ACTIVE trial (CTONG1706).
The trial included 313 patients (median age, 58.5 years) with locally advanced, metastatic, or recurrent nonsquamous NSCLC. All were chemotherapy-naive and EGFR mutation-positive (exon 19 deletion or exon 21 L858R).
Patients were randomized 1:1 to first-line apatinib at 500 mg daily plus gefitinib at 250 mg daily (n = 157) or placebo plus gefitinib at 250 mg daily (n = 156) until progressive disease or unacceptable toxicity.
Efficacy and safety
The primary endpoint was PFS by independent review. The median follow-up was 15.8 months.
The median PFS was 13.7 months in the apatinib group and 10.2 months in the placebo group (hazard ratio, 0.71; P = .0189).
Objective response rates were similar for both groups – 77.1% with apatinib and 73.7% with placebo. However, depth of response ≥30% and depth of response ≥50% both favored the apatinib arm – 89.2% versus 79.5% for ≥ 30% (P = .0209) and 64.3% versus 52.6% for ≥50% (P = .0238).
In addition, the median duration of response was longer for the apatinib group – 12.9 months versus 9.3 months (HR, 0.64; P = .005).
Exploratory biomarker analyses showed the benefit of apatinib was more common in patients with TP53 exon 8 mutations.
The rate of grade 3 or higher treatment-emergent adverse events was 84.1% in the apatinib arm and 37.7% in the placebo arm. Diarrhea (73.2%) and hypertension (68.2%) were the most common treatment-emergent adverse events in the apatinib group.
Dose interruptions were more common in the apatinib group (59.5% vs. 22.7%) as were dose reductions (48.4% vs. 4.5%). However, treatment discontinuations attributable to treatment-emergent adverse events were few in both arms (5.1% in the apatinib arm and 3.2% in the placebo arm).
Cause for hesitation
“VEGFR-TKIs have not yet found a solid home in lung cancer,” said study discussant Lecia V. Sequist, MD, of Massachusetts General Hospital in Boston.
Listing 10 VEGFR-TKIs, Dr. Sequist noted: “None of them have changed practice.”
She added that, while an all-oral regimen is appealing, the benefit of adding apatinib to gefitinib was modest, and the regimen was “fairly difficult” to tolerate. “The PFS with apatinib plus gefitinib is well below what we see with other EGFR/VEGF first-line studies,” she said.
Dr. Sequist also observed that most studies have shown a PFS benefit but no overall survival benefit. “That, in combination with the toxicity, makes me a little hesitant about this regimen. The role of VEGF remains unclear in EGFR mutation–positive lung cancer in 2020,” she concluded.
The ACTIVE study was funded by Jiangsu HengRui Medicine, the Chinese Thoracic Oncology Group, and grants from Sun Yat-sen University and the National Key R&D Program of China. Dr. Zhang disclosed relationships with AstraZeneca, Bristol-Myers Squibb, Eli Lilly, Pfizer, and Roche. Dr. Sequist disclosed relationships with AstraZeneca, Bristol-Myers Squibb, Blueprint Medicines, and many other companies.
SOURCE: Zhang L et al. ESMO 2020, Abstract LBA50.
ACTIVE is the first phase 3 trial of an oral vascular epidermal growth factor receptor–2 (VEGFR2) tyrosine kinase inhibitor (TKI) added to an EGFR-TKI as first-line therapy in this population, according to Li Zhang, MD, of Sun Yat-sen University Cancer Center in Guangzhou, China.
Dr. Zhang presented results from ACTIVE at the European Society for Medical Oncology Virtual Congress 2020.
“This dual oral regimen will provide more convenient treatment for patients who require long-term administration,” Dr. Zhang said. He added that apatinib plus gefitinib “is expected to become a new first-line treatment option for EGFR-mutant NSCLC.”
A discussant for the ACTIVE study was less optimistic, however, noting that the regimen proved tough to tolerate for some patients, and the PFS benefit may not translate to overall survival.
Study rationale and details
Sensitizing EGFR mutations occur in about 10% of White patients and up to 50% of Asian patients, Dr. Zhang noted. Unfortunately, most patients progress after first-line treatment with EGFR-TKIs because of acquired resistance.
Blocking VEGF receptor pathways has been shown to enhance EGFR-TKIs in EGFR-mutated NSCLC, and pilot study results have shown apatinib – an oral VEGFR2–TKI – to be safe and well-tolerated with promising efficacy in combination with gefitinib, Dr. Zhang added.
To expand upon those results, he and his colleagues tested apatinib with gefitinib in the phase 3, double-blind, placebo-controlled ACTIVE trial (CTONG1706).
The trial included 313 patients (median age, 58.5 years) with locally advanced, metastatic, or recurrent nonsquamous NSCLC. All were chemotherapy-naive and EGFR mutation-positive (exon 19 deletion or exon 21 L858R).
Patients were randomized 1:1 to first-line apatinib at 500 mg daily plus gefitinib at 250 mg daily (n = 157) or placebo plus gefitinib at 250 mg daily (n = 156) until progressive disease or unacceptable toxicity.
Efficacy and safety
The primary endpoint was PFS by independent review. The median follow-up was 15.8 months.
The median PFS was 13.7 months in the apatinib group and 10.2 months in the placebo group (hazard ratio, 0.71; P = .0189).
Objective response rates were similar for both groups – 77.1% with apatinib and 73.7% with placebo. However, depth of response ≥30% and depth of response ≥50% both favored the apatinib arm – 89.2% versus 79.5% for ≥ 30% (P = .0209) and 64.3% versus 52.6% for ≥50% (P = .0238).
In addition, the median duration of response was longer for the apatinib group – 12.9 months versus 9.3 months (HR, 0.64; P = .005).
Exploratory biomarker analyses showed the benefit of apatinib was more common in patients with TP53 exon 8 mutations.
The rate of grade 3 or higher treatment-emergent adverse events was 84.1% in the apatinib arm and 37.7% in the placebo arm. Diarrhea (73.2%) and hypertension (68.2%) were the most common treatment-emergent adverse events in the apatinib group.
Dose interruptions were more common in the apatinib group (59.5% vs. 22.7%) as were dose reductions (48.4% vs. 4.5%). However, treatment discontinuations attributable to treatment-emergent adverse events were few in both arms (5.1% in the apatinib arm and 3.2% in the placebo arm).
Cause for hesitation
“VEGFR-TKIs have not yet found a solid home in lung cancer,” said study discussant Lecia V. Sequist, MD, of Massachusetts General Hospital in Boston.
Listing 10 VEGFR-TKIs, Dr. Sequist noted: “None of them have changed practice.”
She added that, while an all-oral regimen is appealing, the benefit of adding apatinib to gefitinib was modest, and the regimen was “fairly difficult” to tolerate. “The PFS with apatinib plus gefitinib is well below what we see with other EGFR/VEGF first-line studies,” she said.
Dr. Sequist also observed that most studies have shown a PFS benefit but no overall survival benefit. “That, in combination with the toxicity, makes me a little hesitant about this regimen. The role of VEGF remains unclear in EGFR mutation–positive lung cancer in 2020,” she concluded.
The ACTIVE study was funded by Jiangsu HengRui Medicine, the Chinese Thoracic Oncology Group, and grants from Sun Yat-sen University and the National Key R&D Program of China. Dr. Zhang disclosed relationships with AstraZeneca, Bristol-Myers Squibb, Eli Lilly, Pfizer, and Roche. Dr. Sequist disclosed relationships with AstraZeneca, Bristol-Myers Squibb, Blueprint Medicines, and many other companies.
SOURCE: Zhang L et al. ESMO 2020, Abstract LBA50.
ACTIVE is the first phase 3 trial of an oral vascular epidermal growth factor receptor–2 (VEGFR2) tyrosine kinase inhibitor (TKI) added to an EGFR-TKI as first-line therapy in this population, according to Li Zhang, MD, of Sun Yat-sen University Cancer Center in Guangzhou, China.
Dr. Zhang presented results from ACTIVE at the European Society for Medical Oncology Virtual Congress 2020.
“This dual oral regimen will provide more convenient treatment for patients who require long-term administration,” Dr. Zhang said. He added that apatinib plus gefitinib “is expected to become a new first-line treatment option for EGFR-mutant NSCLC.”
A discussant for the ACTIVE study was less optimistic, however, noting that the regimen proved tough to tolerate for some patients, and the PFS benefit may not translate to overall survival.
Study rationale and details
Sensitizing EGFR mutations occur in about 10% of White patients and up to 50% of Asian patients, Dr. Zhang noted. Unfortunately, most patients progress after first-line treatment with EGFR-TKIs because of acquired resistance.
Blocking VEGF receptor pathways has been shown to enhance EGFR-TKIs in EGFR-mutated NSCLC, and pilot study results have shown apatinib – an oral VEGFR2–TKI – to be safe and well-tolerated with promising efficacy in combination with gefitinib, Dr. Zhang added.
To expand upon those results, he and his colleagues tested apatinib with gefitinib in the phase 3, double-blind, placebo-controlled ACTIVE trial (CTONG1706).
The trial included 313 patients (median age, 58.5 years) with locally advanced, metastatic, or recurrent nonsquamous NSCLC. All were chemotherapy-naive and EGFR mutation-positive (exon 19 deletion or exon 21 L858R).
Patients were randomized 1:1 to first-line apatinib at 500 mg daily plus gefitinib at 250 mg daily (n = 157) or placebo plus gefitinib at 250 mg daily (n = 156) until progressive disease or unacceptable toxicity.
Efficacy and safety
The primary endpoint was PFS by independent review. The median follow-up was 15.8 months.
The median PFS was 13.7 months in the apatinib group and 10.2 months in the placebo group (hazard ratio, 0.71; P = .0189).
Objective response rates were similar for both groups – 77.1% with apatinib and 73.7% with placebo. However, depth of response ≥30% and depth of response ≥50% both favored the apatinib arm – 89.2% versus 79.5% for ≥ 30% (P = .0209) and 64.3% versus 52.6% for ≥50% (P = .0238).
In addition, the median duration of response was longer for the apatinib group – 12.9 months versus 9.3 months (HR, 0.64; P = .005).
Exploratory biomarker analyses showed the benefit of apatinib was more common in patients with TP53 exon 8 mutations.
The rate of grade 3 or higher treatment-emergent adverse events was 84.1% in the apatinib arm and 37.7% in the placebo arm. Diarrhea (73.2%) and hypertension (68.2%) were the most common treatment-emergent adverse events in the apatinib group.
Dose interruptions were more common in the apatinib group (59.5% vs. 22.7%) as were dose reductions (48.4% vs. 4.5%). However, treatment discontinuations attributable to treatment-emergent adverse events were few in both arms (5.1% in the apatinib arm and 3.2% in the placebo arm).
Cause for hesitation
“VEGFR-TKIs have not yet found a solid home in lung cancer,” said study discussant Lecia V. Sequist, MD, of Massachusetts General Hospital in Boston.
Listing 10 VEGFR-TKIs, Dr. Sequist noted: “None of them have changed practice.”
She added that, while an all-oral regimen is appealing, the benefit of adding apatinib to gefitinib was modest, and the regimen was “fairly difficult” to tolerate. “The PFS with apatinib plus gefitinib is well below what we see with other EGFR/VEGF first-line studies,” she said.
Dr. Sequist also observed that most studies have shown a PFS benefit but no overall survival benefit. “That, in combination with the toxicity, makes me a little hesitant about this regimen. The role of VEGF remains unclear in EGFR mutation–positive lung cancer in 2020,” she concluded.
The ACTIVE study was funded by Jiangsu HengRui Medicine, the Chinese Thoracic Oncology Group, and grants from Sun Yat-sen University and the National Key R&D Program of China. Dr. Zhang disclosed relationships with AstraZeneca, Bristol-Myers Squibb, Eli Lilly, Pfizer, and Roche. Dr. Sequist disclosed relationships with AstraZeneca, Bristol-Myers Squibb, Blueprint Medicines, and many other companies.
SOURCE: Zhang L et al. ESMO 2020, Abstract LBA50.
FROM ESMO 2020
Post-COVID clinics get jump-start from patients with lingering illness
Clarence Troutman survived a 2-month hospital stay with COVID-19, then went home in early June. But he’s far from over the disease, still suffering from limited endurance, shortness of breath and hands that can be stiff and swollen.
“Before COVID, I was a 59-year-old, relatively healthy man,” said the broadband technician from Denver. “If I had to say where I’m at now, I’d say about 50% of where I was, but when I first went home, I was at 20%.”
He credits much of his progress to the “motivation and education” gleaned from a new program for post-COVID patients at the University of Colorado at Denver, Aurora, one of a small but growing number of clinics aimed at treating and studying those who have had the unpredictable coronavirus.
As the election nears, much attention is focused on daily infection numbers or the climbing death toll, but another measure matters: Patients who survive but continue to wrestle with a range of physical or mental effects, including lung damage, heart or neurologic concerns, anxiety, and depression.
“We need to think about how we’re going to provide care for patients who may be recovering for years after the virus,” said Sarah Jolley, MD, a pulmonologist with UCHealth University of Colorado Hospital and director of UCHealth’s Post-Covid Clinic, where Mr. Troutman is seen.
That need has jump-started post-COVID clinics, which bring together a range of specialists into a one-stop shop.
One of the first and largest such clinics is at Mount Sinai in New York City, but programs have also launched at the University of California,San Francisco; Stanford (Calif.) University Medical Center; and the University of Pennsylvania, Philadelphia. The Cleveland Clinic plans to open one early next year. And it’s not just academic medical centers: St. John’s Well Child and Family Center, part of a network of community clinics in south central Los Angeles, said this month it aims to test thousands of its patients who were diagnosed with COVID-19 since March for long-term effects.
Mental health specialists are also involved, along with social workers and pharmacists. Many of the centers also do research studies, aiming to better understand why the virus hits certain patients so hard.
“Some of our patients, even those on a ventilator on death’s door, will come out remarkably unscathed,” said Lekshmi Santhosh, MD, an assistant professor of pulmonary critical care and a leader of the post-COVID program at UCSF, called the OPTIMAL clinic. “Others, even those who were never hospitalized, have disabling fatigue, ongoing chest pain, and shortness of breath, and there’s a whole spectrum in between.”
‘Staggering’ medical need
It’s too early to know how long the persistent medical effects and symptoms will linger, or to make accurate estimates on the percentage of patients affected.
Some early studies are sobering. An Austrian report released this month found that 76 of the first 86 patients studied had evidence of lung damage 6 weeks after hospital discharge, but that dropped to 48 patients at 12 weeks.
Some researchers and clinics say about 10% of U.S. COVID patients they see may have longer-running effects, said Zijian Chen, MD, medical director of the Center for Post-COVID Care at Mount Sinai, which has enrolled 400 patients so far.
If that estimate is correct – and Dr. Chen emphasized that more research is needed to make sure – it translates to patients entering the medical system in droves, often with multiple issues.
How health systems and insurers respond will be key, he said. More than 6.5 million U.S. residents have tested positive for the disease. If fewer than 10% – say 500,000 – already have long-lasting symptoms, “that number is staggering,” Dr. Chen said. “How much medical care will be needed for that?”
Though start-up costs could be a hurdle, the clinics themselves may eventually draw much-needed revenue to medical centers by attracting patients, many of whom have insurance to cover some or all of the cost of repeated visits.
Dr. Chen said the specialized centers can help lower health spending by providing more cost effective, coordinated care that avoids duplicative testing a patient might otherwise undergo.
“We’ve seen patients that when they come in, they’ve already had four MRI or CT scans and a stack of bloodwork,” he said.
The program consolidates those earlier results and determines if any additional testing is needed. Sometimes the answer to what’s causing patients’ long-lasting symptoms remains elusive. One problem for patients seeking help outside of dedicated clinics is that when there is no clear cause for their condition, they may be told the symptoms are imagined.
“I believe in the patients,” said Dr. Chen.
About half the clinic’s patients have received test results showing damage, said Dr. Chen, an endocrinologist and internal medicine physician. For those patients, the clinic can develop a treatment plan. But, frustratingly, the other half have inconclusive test results yet exhibit a range of symptoms.
“That makes it more difficult to treat,” said Dr. Chen.
Experts see parallels to a push in the past decade to establish special clinics to treat patients released from ICU wards, who may have problems related to long-term bed rest or the delirium many experience while hospitalized. Some of the current post-COVID clinics are modeled after the post-ICU clinics or are expanded versions of them.
The ICU Recovery Center at Vanderbilt University Medical Center, Nashville, Tenn., for instance, which opened in 2012, is accepting post-COVID patients.
There are about a dozen post-ICU clinics nationally, some of which are also now working with COVID patients, said James Jackson, director of long-term outcomes at the Vanderbilt center. In addition, he’s heard of at least another dozen post-COVID centers in development.
The centers generally do an initial assessment a few weeks after a patient is diagnosed or discharged from the hospital, often by video call. Check-in and repeat visits are scheduled every month or so after that.
“In an ideal world, with these post-COVID clinics, you can identify the patients and get them into rehab,” he said. “Even if the primary thing these clinics did was to say to patients: ‘This is real, it is not all in your head,’ that impact would be important.”
A question of feasibility
Financing is the largest obstacle, program proponents said. Many hospitals lost substantial revenue to canceled elective procedures during stay-at-home periods.
“So, it’s not a great time to be pitching a new activity that requires a start-up subsidy,” said Glenn Melnick, PhD, a professor of health economics at the University of Southern California.
At UCSF, a select group of faculty members staff the post-COVID clinics and some mental health professionals volunteer their time, said Dr. Santhosh.
Dr. Chen said he was able to recruit team members and support staff from the ranks of those whose elective patient caseload had dropped.
Dr. Jackson said unfortunately there’s not been enough research into the cost-and-clinical effectiveness of post-ICU centers.
“In the early days, there may have been questions about how much value does this add,” he noted. “Now, the question is not so much is it a good idea, but is it feasible?”
Right now, the post-COVID centers are foremost a research effort, said Len Nichols, an economist and nonresident fellow at the Urban Institute. “If these guys get good at treating long-term symptoms, that’s good for all of us. There’s not enough patients to make it a business model yet, but if they become the place to go when you get it, it could become a business model for some of the elite institutions.”
Kaiser Health News is a nonprofit news service covering health issues. It is an editorially independent program of KFF (Kaiser Family Foundation), which is not affiliated with Kaiser Permanente.
Clarence Troutman survived a 2-month hospital stay with COVID-19, then went home in early June. But he’s far from over the disease, still suffering from limited endurance, shortness of breath and hands that can be stiff and swollen.
“Before COVID, I was a 59-year-old, relatively healthy man,” said the broadband technician from Denver. “If I had to say where I’m at now, I’d say about 50% of where I was, but when I first went home, I was at 20%.”
He credits much of his progress to the “motivation and education” gleaned from a new program for post-COVID patients at the University of Colorado at Denver, Aurora, one of a small but growing number of clinics aimed at treating and studying those who have had the unpredictable coronavirus.
As the election nears, much attention is focused on daily infection numbers or the climbing death toll, but another measure matters: Patients who survive but continue to wrestle with a range of physical or mental effects, including lung damage, heart or neurologic concerns, anxiety, and depression.
“We need to think about how we’re going to provide care for patients who may be recovering for years after the virus,” said Sarah Jolley, MD, a pulmonologist with UCHealth University of Colorado Hospital and director of UCHealth’s Post-Covid Clinic, where Mr. Troutman is seen.
That need has jump-started post-COVID clinics, which bring together a range of specialists into a one-stop shop.
One of the first and largest such clinics is at Mount Sinai in New York City, but programs have also launched at the University of California,San Francisco; Stanford (Calif.) University Medical Center; and the University of Pennsylvania, Philadelphia. The Cleveland Clinic plans to open one early next year. And it’s not just academic medical centers: St. John’s Well Child and Family Center, part of a network of community clinics in south central Los Angeles, said this month it aims to test thousands of its patients who were diagnosed with COVID-19 since March for long-term effects.
Mental health specialists are also involved, along with social workers and pharmacists. Many of the centers also do research studies, aiming to better understand why the virus hits certain patients so hard.
“Some of our patients, even those on a ventilator on death’s door, will come out remarkably unscathed,” said Lekshmi Santhosh, MD, an assistant professor of pulmonary critical care and a leader of the post-COVID program at UCSF, called the OPTIMAL clinic. “Others, even those who were never hospitalized, have disabling fatigue, ongoing chest pain, and shortness of breath, and there’s a whole spectrum in between.”
‘Staggering’ medical need
It’s too early to know how long the persistent medical effects and symptoms will linger, or to make accurate estimates on the percentage of patients affected.
Some early studies are sobering. An Austrian report released this month found that 76 of the first 86 patients studied had evidence of lung damage 6 weeks after hospital discharge, but that dropped to 48 patients at 12 weeks.
Some researchers and clinics say about 10% of U.S. COVID patients they see may have longer-running effects, said Zijian Chen, MD, medical director of the Center for Post-COVID Care at Mount Sinai, which has enrolled 400 patients so far.
If that estimate is correct – and Dr. Chen emphasized that more research is needed to make sure – it translates to patients entering the medical system in droves, often with multiple issues.
How health systems and insurers respond will be key, he said. More than 6.5 million U.S. residents have tested positive for the disease. If fewer than 10% – say 500,000 – already have long-lasting symptoms, “that number is staggering,” Dr. Chen said. “How much medical care will be needed for that?”
Though start-up costs could be a hurdle, the clinics themselves may eventually draw much-needed revenue to medical centers by attracting patients, many of whom have insurance to cover some or all of the cost of repeated visits.
Dr. Chen said the specialized centers can help lower health spending by providing more cost effective, coordinated care that avoids duplicative testing a patient might otherwise undergo.
“We’ve seen patients that when they come in, they’ve already had four MRI or CT scans and a stack of bloodwork,” he said.
The program consolidates those earlier results and determines if any additional testing is needed. Sometimes the answer to what’s causing patients’ long-lasting symptoms remains elusive. One problem for patients seeking help outside of dedicated clinics is that when there is no clear cause for their condition, they may be told the symptoms are imagined.
“I believe in the patients,” said Dr. Chen.
About half the clinic’s patients have received test results showing damage, said Dr. Chen, an endocrinologist and internal medicine physician. For those patients, the clinic can develop a treatment plan. But, frustratingly, the other half have inconclusive test results yet exhibit a range of symptoms.
“That makes it more difficult to treat,” said Dr. Chen.
Experts see parallels to a push in the past decade to establish special clinics to treat patients released from ICU wards, who may have problems related to long-term bed rest or the delirium many experience while hospitalized. Some of the current post-COVID clinics are modeled after the post-ICU clinics or are expanded versions of them.
The ICU Recovery Center at Vanderbilt University Medical Center, Nashville, Tenn., for instance, which opened in 2012, is accepting post-COVID patients.
There are about a dozen post-ICU clinics nationally, some of which are also now working with COVID patients, said James Jackson, director of long-term outcomes at the Vanderbilt center. In addition, he’s heard of at least another dozen post-COVID centers in development.
The centers generally do an initial assessment a few weeks after a patient is diagnosed or discharged from the hospital, often by video call. Check-in and repeat visits are scheduled every month or so after that.
“In an ideal world, with these post-COVID clinics, you can identify the patients and get them into rehab,” he said. “Even if the primary thing these clinics did was to say to patients: ‘This is real, it is not all in your head,’ that impact would be important.”
A question of feasibility
Financing is the largest obstacle, program proponents said. Many hospitals lost substantial revenue to canceled elective procedures during stay-at-home periods.
“So, it’s not a great time to be pitching a new activity that requires a start-up subsidy,” said Glenn Melnick, PhD, a professor of health economics at the University of Southern California.
At UCSF, a select group of faculty members staff the post-COVID clinics and some mental health professionals volunteer their time, said Dr. Santhosh.
Dr. Chen said he was able to recruit team members and support staff from the ranks of those whose elective patient caseload had dropped.
Dr. Jackson said unfortunately there’s not been enough research into the cost-and-clinical effectiveness of post-ICU centers.
“In the early days, there may have been questions about how much value does this add,” he noted. “Now, the question is not so much is it a good idea, but is it feasible?”
Right now, the post-COVID centers are foremost a research effort, said Len Nichols, an economist and nonresident fellow at the Urban Institute. “If these guys get good at treating long-term symptoms, that’s good for all of us. There’s not enough patients to make it a business model yet, but if they become the place to go when you get it, it could become a business model for some of the elite institutions.”
Kaiser Health News is a nonprofit news service covering health issues. It is an editorially independent program of KFF (Kaiser Family Foundation), which is not affiliated with Kaiser Permanente.
Clarence Troutman survived a 2-month hospital stay with COVID-19, then went home in early June. But he’s far from over the disease, still suffering from limited endurance, shortness of breath and hands that can be stiff and swollen.
“Before COVID, I was a 59-year-old, relatively healthy man,” said the broadband technician from Denver. “If I had to say where I’m at now, I’d say about 50% of where I was, but when I first went home, I was at 20%.”
He credits much of his progress to the “motivation and education” gleaned from a new program for post-COVID patients at the University of Colorado at Denver, Aurora, one of a small but growing number of clinics aimed at treating and studying those who have had the unpredictable coronavirus.
As the election nears, much attention is focused on daily infection numbers or the climbing death toll, but another measure matters: Patients who survive but continue to wrestle with a range of physical or mental effects, including lung damage, heart or neurologic concerns, anxiety, and depression.
“We need to think about how we’re going to provide care for patients who may be recovering for years after the virus,” said Sarah Jolley, MD, a pulmonologist with UCHealth University of Colorado Hospital and director of UCHealth’s Post-Covid Clinic, where Mr. Troutman is seen.
That need has jump-started post-COVID clinics, which bring together a range of specialists into a one-stop shop.
One of the first and largest such clinics is at Mount Sinai in New York City, but programs have also launched at the University of California,San Francisco; Stanford (Calif.) University Medical Center; and the University of Pennsylvania, Philadelphia. The Cleveland Clinic plans to open one early next year. And it’s not just academic medical centers: St. John’s Well Child and Family Center, part of a network of community clinics in south central Los Angeles, said this month it aims to test thousands of its patients who were diagnosed with COVID-19 since March for long-term effects.
Mental health specialists are also involved, along with social workers and pharmacists. Many of the centers also do research studies, aiming to better understand why the virus hits certain patients so hard.
“Some of our patients, even those on a ventilator on death’s door, will come out remarkably unscathed,” said Lekshmi Santhosh, MD, an assistant professor of pulmonary critical care and a leader of the post-COVID program at UCSF, called the OPTIMAL clinic. “Others, even those who were never hospitalized, have disabling fatigue, ongoing chest pain, and shortness of breath, and there’s a whole spectrum in between.”
‘Staggering’ medical need
It’s too early to know how long the persistent medical effects and symptoms will linger, or to make accurate estimates on the percentage of patients affected.
Some early studies are sobering. An Austrian report released this month found that 76 of the first 86 patients studied had evidence of lung damage 6 weeks after hospital discharge, but that dropped to 48 patients at 12 weeks.
Some researchers and clinics say about 10% of U.S. COVID patients they see may have longer-running effects, said Zijian Chen, MD, medical director of the Center for Post-COVID Care at Mount Sinai, which has enrolled 400 patients so far.
If that estimate is correct – and Dr. Chen emphasized that more research is needed to make sure – it translates to patients entering the medical system in droves, often with multiple issues.
How health systems and insurers respond will be key, he said. More than 6.5 million U.S. residents have tested positive for the disease. If fewer than 10% – say 500,000 – already have long-lasting symptoms, “that number is staggering,” Dr. Chen said. “How much medical care will be needed for that?”
Though start-up costs could be a hurdle, the clinics themselves may eventually draw much-needed revenue to medical centers by attracting patients, many of whom have insurance to cover some or all of the cost of repeated visits.
Dr. Chen said the specialized centers can help lower health spending by providing more cost effective, coordinated care that avoids duplicative testing a patient might otherwise undergo.
“We’ve seen patients that when they come in, they’ve already had four MRI or CT scans and a stack of bloodwork,” he said.
The program consolidates those earlier results and determines if any additional testing is needed. Sometimes the answer to what’s causing patients’ long-lasting symptoms remains elusive. One problem for patients seeking help outside of dedicated clinics is that when there is no clear cause for their condition, they may be told the symptoms are imagined.
“I believe in the patients,” said Dr. Chen.
About half the clinic’s patients have received test results showing damage, said Dr. Chen, an endocrinologist and internal medicine physician. For those patients, the clinic can develop a treatment plan. But, frustratingly, the other half have inconclusive test results yet exhibit a range of symptoms.
“That makes it more difficult to treat,” said Dr. Chen.
Experts see parallels to a push in the past decade to establish special clinics to treat patients released from ICU wards, who may have problems related to long-term bed rest or the delirium many experience while hospitalized. Some of the current post-COVID clinics are modeled after the post-ICU clinics or are expanded versions of them.
The ICU Recovery Center at Vanderbilt University Medical Center, Nashville, Tenn., for instance, which opened in 2012, is accepting post-COVID patients.
There are about a dozen post-ICU clinics nationally, some of which are also now working with COVID patients, said James Jackson, director of long-term outcomes at the Vanderbilt center. In addition, he’s heard of at least another dozen post-COVID centers in development.
The centers generally do an initial assessment a few weeks after a patient is diagnosed or discharged from the hospital, often by video call. Check-in and repeat visits are scheduled every month or so after that.
“In an ideal world, with these post-COVID clinics, you can identify the patients and get them into rehab,” he said. “Even if the primary thing these clinics did was to say to patients: ‘This is real, it is not all in your head,’ that impact would be important.”
A question of feasibility
Financing is the largest obstacle, program proponents said. Many hospitals lost substantial revenue to canceled elective procedures during stay-at-home periods.
“So, it’s not a great time to be pitching a new activity that requires a start-up subsidy,” said Glenn Melnick, PhD, a professor of health economics at the University of Southern California.
At UCSF, a select group of faculty members staff the post-COVID clinics and some mental health professionals volunteer their time, said Dr. Santhosh.
Dr. Chen said he was able to recruit team members and support staff from the ranks of those whose elective patient caseload had dropped.
Dr. Jackson said unfortunately there’s not been enough research into the cost-and-clinical effectiveness of post-ICU centers.
“In the early days, there may have been questions about how much value does this add,” he noted. “Now, the question is not so much is it a good idea, but is it feasible?”
Right now, the post-COVID centers are foremost a research effort, said Len Nichols, an economist and nonresident fellow at the Urban Institute. “If these guys get good at treating long-term symptoms, that’s good for all of us. There’s not enough patients to make it a business model yet, but if they become the place to go when you get it, it could become a business model for some of the elite institutions.”
Kaiser Health News is a nonprofit news service covering health issues. It is an editorially independent program of KFF (Kaiser Family Foundation), which is not affiliated with Kaiser Permanente.
Screening algorithm safely selects patients for OSA treatment before bariatric surgery
A novel algorithm for selecting patients who require treatment for obstructive sleep apnea (OSA) before undergoing bariatric surgery proved safe in a prospective cohort study of 1,103 patients.
Screening for OSA is recommended before bariatric surgery. OSA has been associated in several meta-analyses with increased risk for postoperative complications – not limited to bariatric surgery – and some studies have suggested that this increased risk may be limited to severe OSA, said Frédéric Series, MD, of Université Laval, Quebec City, at the virtual annual meeting of the Associated Sleep Societies.
The preoperative screening algorithm, which utilizes the results of nocturnal home oximetry and morning capillary gas measurements, effectively stratified patients for the risk of postoperative adverse events and “safely selected patients who don’t need [continuous positive airway pressure] before bariatric surgery,” he said. “The risk of postoperative adverse events following bariatric surgery was not increased in untreated OSA patients with low or moderate risk of severe OSA and hypoventilation.”
The study also demonstrated, he said, that patients with severe OSA with or without hypoventilation, even when correctly treated, remain at higher risk for complications.
The algorithm utilizes an oxygen desaturation index (ODI) corresponding to 3% drops in SaO2 and the percent of the total recording time with an SaO2 below 90%, as well as capillary gas measurements (PCO2). Treatment was initiated for those with severe OSA (ODI ≥ 25/hr, < 10% of recording time with a SaO2 below 90%) or OSA with hypoventilation (PCO2 ≥ 45).
“When the ODI was less than 25 per hour, and when the total recording time spent below 90% SaO2 was less than 10%, with PCO2 < 45 mmHg, we expected no need for CPAP treatment,” Dr. Series said. For analysis, the investigators considered part of the untreated group – those with an ODI < 10/hr (no or mild OSA) – as a control group.
Treated patients underwent CPAP/BiPAP for a mean duration of 1.5 months. Good treatment compliance was mandatory for surgery, and treatment was continued immediately after extubation, in the recovery room, in nearly all patients, Dr. Series reported.
The analysis covered 1,103 patients: 447 controls (40.8%), 358 untreated (32.7%), 289 treated for OSA (26.4%) and 9 (0.8%) treated for OSA + hypoventilation. Patients with OSA, particularly those with severe OSA and those with hypoventilation, were older and heavier and significantly more likely to have hypertension and diabetes than controls.
There were no differences between the four groups in 10-day reoperation or 30-day readmission occurrence, and postoperative complications were “particularly infrequent in the control and OSA-untreated groups, with no differences between these two groups,” Dr. Series said.
Cardiac arrhythmia (mainly atrial fibrillation) occurred more frequently in the OSA-treated group (2.4%) and the OSA/hypoventilation patients (11%) than in the other groups (0.5%-0.6%).
Respiratory failure occurred in about one-third of patients with hypoventilation, and admission to the ICU was “dramatically higher” in patients with hypoventilation (67%), because of respiratory failure, arrhythmia, or other unstable medical conditions, Dr. Series said.
There were no differences between the groups in the duration of surgery or the amount of anesthetic used, but the length of stay in the recovery room was significantly longer in the OSA-treated and hypoventilation groups. The length of hospital stay was also longer in these groups. Sleeve gastrectomy was the most frequent bariatric surgical procedure across all groups, including 100% of patients with hypoventilation, he noted.
Asked to comment on the study, Octavian C. Ioachimescu, MD, PhD, of Emory University in Atlanta and the Atlanta Veterans Affairs Medical Center in Decatur, said the algorithm “clearly deserves further validation in other clinical-based cohorts and longer-term outcome assessment.”
Dr. Series reported that he has no relevant disclosures. Dr. Ioachimescu also said he has no relevant disclosures.
A novel algorithm for selecting patients who require treatment for obstructive sleep apnea (OSA) before undergoing bariatric surgery proved safe in a prospective cohort study of 1,103 patients.
Screening for OSA is recommended before bariatric surgery. OSA has been associated in several meta-analyses with increased risk for postoperative complications – not limited to bariatric surgery – and some studies have suggested that this increased risk may be limited to severe OSA, said Frédéric Series, MD, of Université Laval, Quebec City, at the virtual annual meeting of the Associated Sleep Societies.
The preoperative screening algorithm, which utilizes the results of nocturnal home oximetry and morning capillary gas measurements, effectively stratified patients for the risk of postoperative adverse events and “safely selected patients who don’t need [continuous positive airway pressure] before bariatric surgery,” he said. “The risk of postoperative adverse events following bariatric surgery was not increased in untreated OSA patients with low or moderate risk of severe OSA and hypoventilation.”
The study also demonstrated, he said, that patients with severe OSA with or without hypoventilation, even when correctly treated, remain at higher risk for complications.
The algorithm utilizes an oxygen desaturation index (ODI) corresponding to 3% drops in SaO2 and the percent of the total recording time with an SaO2 below 90%, as well as capillary gas measurements (PCO2). Treatment was initiated for those with severe OSA (ODI ≥ 25/hr, < 10% of recording time with a SaO2 below 90%) or OSA with hypoventilation (PCO2 ≥ 45).
“When the ODI was less than 25 per hour, and when the total recording time spent below 90% SaO2 was less than 10%, with PCO2 < 45 mmHg, we expected no need for CPAP treatment,” Dr. Series said. For analysis, the investigators considered part of the untreated group – those with an ODI < 10/hr (no or mild OSA) – as a control group.
Treated patients underwent CPAP/BiPAP for a mean duration of 1.5 months. Good treatment compliance was mandatory for surgery, and treatment was continued immediately after extubation, in the recovery room, in nearly all patients, Dr. Series reported.
The analysis covered 1,103 patients: 447 controls (40.8%), 358 untreated (32.7%), 289 treated for OSA (26.4%) and 9 (0.8%) treated for OSA + hypoventilation. Patients with OSA, particularly those with severe OSA and those with hypoventilation, were older and heavier and significantly more likely to have hypertension and diabetes than controls.
There were no differences between the four groups in 10-day reoperation or 30-day readmission occurrence, and postoperative complications were “particularly infrequent in the control and OSA-untreated groups, with no differences between these two groups,” Dr. Series said.
Cardiac arrhythmia (mainly atrial fibrillation) occurred more frequently in the OSA-treated group (2.4%) and the OSA/hypoventilation patients (11%) than in the other groups (0.5%-0.6%).
Respiratory failure occurred in about one-third of patients with hypoventilation, and admission to the ICU was “dramatically higher” in patients with hypoventilation (67%), because of respiratory failure, arrhythmia, or other unstable medical conditions, Dr. Series said.
There were no differences between the groups in the duration of surgery or the amount of anesthetic used, but the length of stay in the recovery room was significantly longer in the OSA-treated and hypoventilation groups. The length of hospital stay was also longer in these groups. Sleeve gastrectomy was the most frequent bariatric surgical procedure across all groups, including 100% of patients with hypoventilation, he noted.
Asked to comment on the study, Octavian C. Ioachimescu, MD, PhD, of Emory University in Atlanta and the Atlanta Veterans Affairs Medical Center in Decatur, said the algorithm “clearly deserves further validation in other clinical-based cohorts and longer-term outcome assessment.”
Dr. Series reported that he has no relevant disclosures. Dr. Ioachimescu also said he has no relevant disclosures.
A novel algorithm for selecting patients who require treatment for obstructive sleep apnea (OSA) before undergoing bariatric surgery proved safe in a prospective cohort study of 1,103 patients.
Screening for OSA is recommended before bariatric surgery. OSA has been associated in several meta-analyses with increased risk for postoperative complications – not limited to bariatric surgery – and some studies have suggested that this increased risk may be limited to severe OSA, said Frédéric Series, MD, of Université Laval, Quebec City, at the virtual annual meeting of the Associated Sleep Societies.
The preoperative screening algorithm, which utilizes the results of nocturnal home oximetry and morning capillary gas measurements, effectively stratified patients for the risk of postoperative adverse events and “safely selected patients who don’t need [continuous positive airway pressure] before bariatric surgery,” he said. “The risk of postoperative adverse events following bariatric surgery was not increased in untreated OSA patients with low or moderate risk of severe OSA and hypoventilation.”
The study also demonstrated, he said, that patients with severe OSA with or without hypoventilation, even when correctly treated, remain at higher risk for complications.
The algorithm utilizes an oxygen desaturation index (ODI) corresponding to 3% drops in SaO2 and the percent of the total recording time with an SaO2 below 90%, as well as capillary gas measurements (PCO2). Treatment was initiated for those with severe OSA (ODI ≥ 25/hr, < 10% of recording time with a SaO2 below 90%) or OSA with hypoventilation (PCO2 ≥ 45).
“When the ODI was less than 25 per hour, and when the total recording time spent below 90% SaO2 was less than 10%, with PCO2 < 45 mmHg, we expected no need for CPAP treatment,” Dr. Series said. For analysis, the investigators considered part of the untreated group – those with an ODI < 10/hr (no or mild OSA) – as a control group.
Treated patients underwent CPAP/BiPAP for a mean duration of 1.5 months. Good treatment compliance was mandatory for surgery, and treatment was continued immediately after extubation, in the recovery room, in nearly all patients, Dr. Series reported.
The analysis covered 1,103 patients: 447 controls (40.8%), 358 untreated (32.7%), 289 treated for OSA (26.4%) and 9 (0.8%) treated for OSA + hypoventilation. Patients with OSA, particularly those with severe OSA and those with hypoventilation, were older and heavier and significantly more likely to have hypertension and diabetes than controls.
There were no differences between the four groups in 10-day reoperation or 30-day readmission occurrence, and postoperative complications were “particularly infrequent in the control and OSA-untreated groups, with no differences between these two groups,” Dr. Series said.
Cardiac arrhythmia (mainly atrial fibrillation) occurred more frequently in the OSA-treated group (2.4%) and the OSA/hypoventilation patients (11%) than in the other groups (0.5%-0.6%).
Respiratory failure occurred in about one-third of patients with hypoventilation, and admission to the ICU was “dramatically higher” in patients with hypoventilation (67%), because of respiratory failure, arrhythmia, or other unstable medical conditions, Dr. Series said.
There were no differences between the groups in the duration of surgery or the amount of anesthetic used, but the length of stay in the recovery room was significantly longer in the OSA-treated and hypoventilation groups. The length of hospital stay was also longer in these groups. Sleeve gastrectomy was the most frequent bariatric surgical procedure across all groups, including 100% of patients with hypoventilation, he noted.
Asked to comment on the study, Octavian C. Ioachimescu, MD, PhD, of Emory University in Atlanta and the Atlanta Veterans Affairs Medical Center in Decatur, said the algorithm “clearly deserves further validation in other clinical-based cohorts and longer-term outcome assessment.”
Dr. Series reported that he has no relevant disclosures. Dr. Ioachimescu also said he has no relevant disclosures.
REPORTING FROM SLEEP 2020
Gastroenterologists among the most likely to adopt telemedicine
It’s no secret that the COVID-19 pandemic has disrupted medical practice and led to a surge in telemedicine visits. A new report issued by the health care social network Doximity in September predicts that these changes will be permanent, and that the telehealth industry will more than triple from $29 billion at the end of this year to about $106 billion by 2023.
The report, titled “2020 State of Telemedicine,” follows a similar 2019 publication and captures the changes created by the pandemic. “Obviously, telemedicine has been around for many years, but the pandemic around COVID-19 has really changed the game. Something that had been getting gradual adoption really rocketed to the forefront,” said Peter Alparin, MD, who is an internist in San Francisco and vice president of product at Doximity, in an interview. The report predicts that 20% of medical visits will be conducted through telemedicine by the end of 2020.
Gastroenterology is one of the top specialties to adopt telemedicine, ranking third behind endocrinology and rheumatology, and that should come as no surprise. “Chronic disease patients lend themselves well to telemedicine because they have ongoing relationships with their physicians, so they can be seen more often and it’s more convenient for them. The specialties that take care of patients with those sorts of illnesses were the ones that adopted it the most readily,” said Dr. Alparin.
That’s probably in part because specialists dealing with chronic conditions have been triaging patients with telephone calls for years, making it easier to tell when a patient needs to come in for a physical visit. “It’s a skill you learn, to tell when something is just a little bit different for a patient. It’s really a clinical judgment that has been honed over years of experience,” said Dr. Alparin. The report backs up that idea, as it found that the physician age groups that most often adopted telemedicine were those in their 40s, 50s, and 60s.
Telemedicine is popular with patients once they try it, and it can greatly expand a physician’s reach, according to Dr. Alparin. “If you’re a specialist, you can perhaps see patients in areas where that specialty is underrepresented, whether that’s the inner city or a very rural area,” he said. The most important barrier is high-speed Internet access, which remains a problem in many areas.
Doximity researchers surveyed more than 2,000 U.S. adults to get their opinions on telemedicine, and analyzed telemedicine adoption data from the platform’s own set of telemedicine tools, and compared it to data from the 2019 report. They also reviewed studies looking at disparities in medicine and patient access to telemedicine.
Telemedicine use among patients grew from 14% before the pandemic, to 35% who reported at least one telemedicine visit after COVID-19. A total of 23% said they planned to continue use of telemedicine after the pandemic ends, and 27% said they had become more comfortable using telemedicine. Among patients, 28% said telemedicine provides the same or better benefit as an in-person visit, and this rose to 53% among those with chronic illnesses.
Among physicians, telemedicine adoption rose by 20% between 2015 and 2018, but increased by 38% between 2019 and 2020. The highest percentage of physician telemedicine adopters were in large metro areas and East Coast states, led by Massachusetts, North Carolina, and New Jersey. None of the top 10 adopter states were west of Illinois.
Equity concerns remain: 64.3% of households with annual incomes of $25,000 or lower have access to broadband internet, compared with 93.5% of those with incomes of $50,000 or lower. In nonmetropolitan areas, 78.1% of households have access, compared with 86.7% of metropolitan households. The good news is that many patients prefer cell phone use for telemedicine, and nearly as many Black and Hispanic Americans own cell phones as White Americans. “That has really democratized access,” said Dr. Alperin.
A key to successful telemedicine appointments is to make sure that the patient is prepared, according to Dr. Alparin. Make sure the patient is in a relatively quiet, well-lit place, and that they have thought about the questions they want to ask. It’s possible to replicate some aspects of a physical appointment with the right conditions. “You can visualize how they move their arms and legs; you can see how they’re breathing. You can gain a lot of information by just watching somebody,” said Dr. Alparin. A physician might also spot clues in the patient’s surroundings. “If a patient is asthmatic and you see cats walking all over the place, or a patient is allergic to gluten and they have loaves of bread everywhere,” he added.
A big concern for telemedicine has been reimbursement. In response to the pandemic, the Centers for Medicare & Medicaid Services created a number of waivers to requirements for billing for telemedicine services, and private insurers followed suit. In August, the agency announced it would make some of those waivers permanent, though others such as removal of restrictions on the site of care, eligible providers, and nonrural areas will likely require an act of Congress to enshrine, CMS administrator Seema Verma told reporters at an August press conference.
SOURCE: 2020 State of Telemedicine Report.
Yuval A. Patel MD, MHS, is assistant professor of medicine, division of gastroenterology, Duke University School of Medicine, Durham, N.C. He has no conflicts of interest.
Yuval A. Patel MD, MHS, is assistant professor of medicine, division of gastroenterology, Duke University School of Medicine, Durham, N.C. He has no conflicts of interest.
Yuval A. Patel MD, MHS, is assistant professor of medicine, division of gastroenterology, Duke University School of Medicine, Durham, N.C. He has no conflicts of interest.
It’s no secret that the COVID-19 pandemic has disrupted medical practice and led to a surge in telemedicine visits. A new report issued by the health care social network Doximity in September predicts that these changes will be permanent, and that the telehealth industry will more than triple from $29 billion at the end of this year to about $106 billion by 2023.
The report, titled “2020 State of Telemedicine,” follows a similar 2019 publication and captures the changes created by the pandemic. “Obviously, telemedicine has been around for many years, but the pandemic around COVID-19 has really changed the game. Something that had been getting gradual adoption really rocketed to the forefront,” said Peter Alparin, MD, who is an internist in San Francisco and vice president of product at Doximity, in an interview. The report predicts that 20% of medical visits will be conducted through telemedicine by the end of 2020.
Gastroenterology is one of the top specialties to adopt telemedicine, ranking third behind endocrinology and rheumatology, and that should come as no surprise. “Chronic disease patients lend themselves well to telemedicine because they have ongoing relationships with their physicians, so they can be seen more often and it’s more convenient for them. The specialties that take care of patients with those sorts of illnesses were the ones that adopted it the most readily,” said Dr. Alparin.
That’s probably in part because specialists dealing with chronic conditions have been triaging patients with telephone calls for years, making it easier to tell when a patient needs to come in for a physical visit. “It’s a skill you learn, to tell when something is just a little bit different for a patient. It’s really a clinical judgment that has been honed over years of experience,” said Dr. Alparin. The report backs up that idea, as it found that the physician age groups that most often adopted telemedicine were those in their 40s, 50s, and 60s.
Telemedicine is popular with patients once they try it, and it can greatly expand a physician’s reach, according to Dr. Alparin. “If you’re a specialist, you can perhaps see patients in areas where that specialty is underrepresented, whether that’s the inner city or a very rural area,” he said. The most important barrier is high-speed Internet access, which remains a problem in many areas.
Doximity researchers surveyed more than 2,000 U.S. adults to get their opinions on telemedicine, and analyzed telemedicine adoption data from the platform’s own set of telemedicine tools, and compared it to data from the 2019 report. They also reviewed studies looking at disparities in medicine and patient access to telemedicine.
Telemedicine use among patients grew from 14% before the pandemic, to 35% who reported at least one telemedicine visit after COVID-19. A total of 23% said they planned to continue use of telemedicine after the pandemic ends, and 27% said they had become more comfortable using telemedicine. Among patients, 28% said telemedicine provides the same or better benefit as an in-person visit, and this rose to 53% among those with chronic illnesses.
Among physicians, telemedicine adoption rose by 20% between 2015 and 2018, but increased by 38% between 2019 and 2020. The highest percentage of physician telemedicine adopters were in large metro areas and East Coast states, led by Massachusetts, North Carolina, and New Jersey. None of the top 10 adopter states were west of Illinois.
Equity concerns remain: 64.3% of households with annual incomes of $25,000 or lower have access to broadband internet, compared with 93.5% of those with incomes of $50,000 or lower. In nonmetropolitan areas, 78.1% of households have access, compared with 86.7% of metropolitan households. The good news is that many patients prefer cell phone use for telemedicine, and nearly as many Black and Hispanic Americans own cell phones as White Americans. “That has really democratized access,” said Dr. Alperin.
A key to successful telemedicine appointments is to make sure that the patient is prepared, according to Dr. Alparin. Make sure the patient is in a relatively quiet, well-lit place, and that they have thought about the questions they want to ask. It’s possible to replicate some aspects of a physical appointment with the right conditions. “You can visualize how they move their arms and legs; you can see how they’re breathing. You can gain a lot of information by just watching somebody,” said Dr. Alparin. A physician might also spot clues in the patient’s surroundings. “If a patient is asthmatic and you see cats walking all over the place, or a patient is allergic to gluten and they have loaves of bread everywhere,” he added.
A big concern for telemedicine has been reimbursement. In response to the pandemic, the Centers for Medicare & Medicaid Services created a number of waivers to requirements for billing for telemedicine services, and private insurers followed suit. In August, the agency announced it would make some of those waivers permanent, though others such as removal of restrictions on the site of care, eligible providers, and nonrural areas will likely require an act of Congress to enshrine, CMS administrator Seema Verma told reporters at an August press conference.
SOURCE: 2020 State of Telemedicine Report.
It’s no secret that the COVID-19 pandemic has disrupted medical practice and led to a surge in telemedicine visits. A new report issued by the health care social network Doximity in September predicts that these changes will be permanent, and that the telehealth industry will more than triple from $29 billion at the end of this year to about $106 billion by 2023.
The report, titled “2020 State of Telemedicine,” follows a similar 2019 publication and captures the changes created by the pandemic. “Obviously, telemedicine has been around for many years, but the pandemic around COVID-19 has really changed the game. Something that had been getting gradual adoption really rocketed to the forefront,” said Peter Alparin, MD, who is an internist in San Francisco and vice president of product at Doximity, in an interview. The report predicts that 20% of medical visits will be conducted through telemedicine by the end of 2020.
Gastroenterology is one of the top specialties to adopt telemedicine, ranking third behind endocrinology and rheumatology, and that should come as no surprise. “Chronic disease patients lend themselves well to telemedicine because they have ongoing relationships with their physicians, so they can be seen more often and it’s more convenient for them. The specialties that take care of patients with those sorts of illnesses were the ones that adopted it the most readily,” said Dr. Alparin.
That’s probably in part because specialists dealing with chronic conditions have been triaging patients with telephone calls for years, making it easier to tell when a patient needs to come in for a physical visit. “It’s a skill you learn, to tell when something is just a little bit different for a patient. It’s really a clinical judgment that has been honed over years of experience,” said Dr. Alparin. The report backs up that idea, as it found that the physician age groups that most often adopted telemedicine were those in their 40s, 50s, and 60s.
Telemedicine is popular with patients once they try it, and it can greatly expand a physician’s reach, according to Dr. Alparin. “If you’re a specialist, you can perhaps see patients in areas where that specialty is underrepresented, whether that’s the inner city or a very rural area,” he said. The most important barrier is high-speed Internet access, which remains a problem in many areas.
Doximity researchers surveyed more than 2,000 U.S. adults to get their opinions on telemedicine, and analyzed telemedicine adoption data from the platform’s own set of telemedicine tools, and compared it to data from the 2019 report. They also reviewed studies looking at disparities in medicine and patient access to telemedicine.
Telemedicine use among patients grew from 14% before the pandemic, to 35% who reported at least one telemedicine visit after COVID-19. A total of 23% said they planned to continue use of telemedicine after the pandemic ends, and 27% said they had become more comfortable using telemedicine. Among patients, 28% said telemedicine provides the same or better benefit as an in-person visit, and this rose to 53% among those with chronic illnesses.
Among physicians, telemedicine adoption rose by 20% between 2015 and 2018, but increased by 38% between 2019 and 2020. The highest percentage of physician telemedicine adopters were in large metro areas and East Coast states, led by Massachusetts, North Carolina, and New Jersey. None of the top 10 adopter states were west of Illinois.
Equity concerns remain: 64.3% of households with annual incomes of $25,000 or lower have access to broadband internet, compared with 93.5% of those with incomes of $50,000 or lower. In nonmetropolitan areas, 78.1% of households have access, compared with 86.7% of metropolitan households. The good news is that many patients prefer cell phone use for telemedicine, and nearly as many Black and Hispanic Americans own cell phones as White Americans. “That has really democratized access,” said Dr. Alperin.
A key to successful telemedicine appointments is to make sure that the patient is prepared, according to Dr. Alparin. Make sure the patient is in a relatively quiet, well-lit place, and that they have thought about the questions they want to ask. It’s possible to replicate some aspects of a physical appointment with the right conditions. “You can visualize how they move their arms and legs; you can see how they’re breathing. You can gain a lot of information by just watching somebody,” said Dr. Alparin. A physician might also spot clues in the patient’s surroundings. “If a patient is asthmatic and you see cats walking all over the place, or a patient is allergic to gluten and they have loaves of bread everywhere,” he added.
A big concern for telemedicine has been reimbursement. In response to the pandemic, the Centers for Medicare & Medicaid Services created a number of waivers to requirements for billing for telemedicine services, and private insurers followed suit. In August, the agency announced it would make some of those waivers permanent, though others such as removal of restrictions on the site of care, eligible providers, and nonrural areas will likely require an act of Congress to enshrine, CMS administrator Seema Verma told reporters at an August press conference.
SOURCE: 2020 State of Telemedicine Report.
Nerve damage linked to prone positioning in COVID-19
A new case series describes peripheral nerve injuries associated with this type of positioning and suggests ways to minimize the potential damage.

“Physicians should remain aware of increased susceptibility to peripheral nerve damage in patients with severe COVID-19 after prone positioning, since it is surprisingly common among these patients, and should refine standard protocols accordingly to reduce that risk,” said senior author Colin Franz, MD, PhD, director of the Electrodiagnostic Laboratory, Shirley Ryan AbilityLab, Chicago.
The article was published online Sept. 4 in the British Journal of Anaesthesiology.
Unique type of nerve injury
Many patients who are admitted to the intensive care unit with COVID-19 undergo invasive mechanical ventilation because of acute respiratory distress syndrome (ARDS). Clinical guidelines recommend that such patients lie in the prone position 12-16 hours per day.
“Prone positioning for up to 16 hours is a therapy we use for patients with more severe forms of ARDS, and high-level evidence points to mortality benefit in patients with moderate to severe ARDS if [mechanical] ventilation occurs,” said study coauthor James McCauley Walter, MD, of the pulmonary division at Northwestern University, Chicago.
With a “significant number of COVID-19 patients flooding the ICU, we quickly started to prone a lot of them, but if you are in a specific position for multiple hours a day, coupled with the neurotoxic effects of the SARS-CoV-2 virus itself, you may be exposed to a unique type of nerve injury,” he said.
Dr. Walter said that the “incidence of asymmetric neuropathies seems out of proportion to what has been reported in non–COVID-19 settings, which is what caught our attention.”
Many of these patients are discharged to rehabilitation hospitals, and “what we noticed, which was unique about COVID-19 patients coming to our rehab hospital, was that, compared with other patients who had been critically ill with a long hospital stay, there was a significantly higher percentage of COVID-19 patients who had peripheral nerve damage,” Dr. Franz said.
The authors described 12 of these patients who were admitted between April 24 and June 30, 2020 (mean age, 60.3 years; range, 23-80 years). The sample included White, Black, and Hispanic individuals. Eleven of the 12 post–COVID-19 patients with peripheral nerve damage had experienced prone positioning during acute management.
The average number of days patients received mechanical ventilation was 33.6 (range, 12-62 days). The average number of proning sessions was 4.5 (range, 1-16) with an average of 81.2 hours (range, 16-252 hours) spent prone.
A major contributor
Dr. Franz suggested that prone positioning is likely not the only cause of peripheral nerve damage but “may play a big role in these patients who are vulnerable because of viral infection and the critical illness that causes damage and nerve injuries.”
“The first component of lifesaving care for the critically ill in the ICU is intravenous fluids, mechanical ventilation, steroids, and antibiotics for infection,” said Dr. Walter.
“We are trying to come up with ways to place patients in prone position in safer ways, to pay attention to pressure points and areas of injury that we have seen and try to offload them, to see if we can decrease the rate of these injuries,” he added.
The researchers’ article includes a heat map diagram as a “template for where to focus the most efforts, in terms of decreasing pressure,” Dr. Walter said.
“The nerves are accepting too much force for gravely ill COVID-19 patients to handle, so we suggest using the template to determine where extra padding might be needed, or a protocol that might include changes in positioning,” he added.
Dr. Franz described the interventions used for COVID-19 patients with prone positioning–related peripheral nerve damage. “The first step is trying to address the problems one by one, either trying to solve them through exercise or teaching new skills, new ways to compensate, beginning with basic activities, such as getting out of bed and self-care,” he said.
Long-term recovery of nerve injuries depends on how severe the injuries are. Some nerves can slowly regenerate – possibly at the rate of 1 inch per month – which can be a long process, taking between a year and 18 months.
Dr. Franz said that therapies for this condition are “extrapolated from clinical trial work” on promoting nerve regeneration after surgery using electrical stimulation to enable nerves to regrow at a faster rate.
“Regeneration is not only slow, but it may not happen completely, leaving the patient with permanent nerve damage – in fact, based on our experience and what has been reported, the percentage of patients with full recovery is only 10%,” he said.
The most common symptomatic complaint other than lack of movement or feeling is neuropathic pain, “which may require medication to take the edge off the pain,” Dr. Franz added.
Irreversible damage?
Commenting on the study, Tae Chung, MD, of the departments of physical medicine, rehabilitation, and neurology, Johns Hopkins University, Baltimore, said the study “provides one of the first and the largest description of peripheral nerve injury associated with prone positioning for management of ARDS from COVID-19.”
Dr. Chung, who was not involved in the research, noted that “various neurological complications from COVID-19 have been reported, and some of them may result in irreversible neurological damage or delay the recovery from COVID-19 infection,” so “accurate and timely diagnosis of such neurological complications is critical for rehabilitation of the COVID-19 survivors.”
The study received no funding. Dr. Franz, Dr. Walter, study coauthors, and Dr. Chung report no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
A new case series describes peripheral nerve injuries associated with this type of positioning and suggests ways to minimize the potential damage.

“Physicians should remain aware of increased susceptibility to peripheral nerve damage in patients with severe COVID-19 after prone positioning, since it is surprisingly common among these patients, and should refine standard protocols accordingly to reduce that risk,” said senior author Colin Franz, MD, PhD, director of the Electrodiagnostic Laboratory, Shirley Ryan AbilityLab, Chicago.
The article was published online Sept. 4 in the British Journal of Anaesthesiology.
Unique type of nerve injury
Many patients who are admitted to the intensive care unit with COVID-19 undergo invasive mechanical ventilation because of acute respiratory distress syndrome (ARDS). Clinical guidelines recommend that such patients lie in the prone position 12-16 hours per day.
“Prone positioning for up to 16 hours is a therapy we use for patients with more severe forms of ARDS, and high-level evidence points to mortality benefit in patients with moderate to severe ARDS if [mechanical] ventilation occurs,” said study coauthor James McCauley Walter, MD, of the pulmonary division at Northwestern University, Chicago.
With a “significant number of COVID-19 patients flooding the ICU, we quickly started to prone a lot of them, but if you are in a specific position for multiple hours a day, coupled with the neurotoxic effects of the SARS-CoV-2 virus itself, you may be exposed to a unique type of nerve injury,” he said.
Dr. Walter said that the “incidence of asymmetric neuropathies seems out of proportion to what has been reported in non–COVID-19 settings, which is what caught our attention.”
Many of these patients are discharged to rehabilitation hospitals, and “what we noticed, which was unique about COVID-19 patients coming to our rehab hospital, was that, compared with other patients who had been critically ill with a long hospital stay, there was a significantly higher percentage of COVID-19 patients who had peripheral nerve damage,” Dr. Franz said.
The authors described 12 of these patients who were admitted between April 24 and June 30, 2020 (mean age, 60.3 years; range, 23-80 years). The sample included White, Black, and Hispanic individuals. Eleven of the 12 post–COVID-19 patients with peripheral nerve damage had experienced prone positioning during acute management.
The average number of days patients received mechanical ventilation was 33.6 (range, 12-62 days). The average number of proning sessions was 4.5 (range, 1-16) with an average of 81.2 hours (range, 16-252 hours) spent prone.
A major contributor
Dr. Franz suggested that prone positioning is likely not the only cause of peripheral nerve damage but “may play a big role in these patients who are vulnerable because of viral infection and the critical illness that causes damage and nerve injuries.”
“The first component of lifesaving care for the critically ill in the ICU is intravenous fluids, mechanical ventilation, steroids, and antibiotics for infection,” said Dr. Walter.
“We are trying to come up with ways to place patients in prone position in safer ways, to pay attention to pressure points and areas of injury that we have seen and try to offload them, to see if we can decrease the rate of these injuries,” he added.
The researchers’ article includes a heat map diagram as a “template for where to focus the most efforts, in terms of decreasing pressure,” Dr. Walter said.
“The nerves are accepting too much force for gravely ill COVID-19 patients to handle, so we suggest using the template to determine where extra padding might be needed, or a protocol that might include changes in positioning,” he added.
Dr. Franz described the interventions used for COVID-19 patients with prone positioning–related peripheral nerve damage. “The first step is trying to address the problems one by one, either trying to solve them through exercise or teaching new skills, new ways to compensate, beginning with basic activities, such as getting out of bed and self-care,” he said.
Long-term recovery of nerve injuries depends on how severe the injuries are. Some nerves can slowly regenerate – possibly at the rate of 1 inch per month – which can be a long process, taking between a year and 18 months.
Dr. Franz said that therapies for this condition are “extrapolated from clinical trial work” on promoting nerve regeneration after surgery using electrical stimulation to enable nerves to regrow at a faster rate.
“Regeneration is not only slow, but it may not happen completely, leaving the patient with permanent nerve damage – in fact, based on our experience and what has been reported, the percentage of patients with full recovery is only 10%,” he said.
The most common symptomatic complaint other than lack of movement or feeling is neuropathic pain, “which may require medication to take the edge off the pain,” Dr. Franz added.
Irreversible damage?
Commenting on the study, Tae Chung, MD, of the departments of physical medicine, rehabilitation, and neurology, Johns Hopkins University, Baltimore, said the study “provides one of the first and the largest description of peripheral nerve injury associated with prone positioning for management of ARDS from COVID-19.”
Dr. Chung, who was not involved in the research, noted that “various neurological complications from COVID-19 have been reported, and some of them may result in irreversible neurological damage or delay the recovery from COVID-19 infection,” so “accurate and timely diagnosis of such neurological complications is critical for rehabilitation of the COVID-19 survivors.”
The study received no funding. Dr. Franz, Dr. Walter, study coauthors, and Dr. Chung report no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
A new case series describes peripheral nerve injuries associated with this type of positioning and suggests ways to minimize the potential damage.

“Physicians should remain aware of increased susceptibility to peripheral nerve damage in patients with severe COVID-19 after prone positioning, since it is surprisingly common among these patients, and should refine standard protocols accordingly to reduce that risk,” said senior author Colin Franz, MD, PhD, director of the Electrodiagnostic Laboratory, Shirley Ryan AbilityLab, Chicago.
The article was published online Sept. 4 in the British Journal of Anaesthesiology.
Unique type of nerve injury
Many patients who are admitted to the intensive care unit with COVID-19 undergo invasive mechanical ventilation because of acute respiratory distress syndrome (ARDS). Clinical guidelines recommend that such patients lie in the prone position 12-16 hours per day.
“Prone positioning for up to 16 hours is a therapy we use for patients with more severe forms of ARDS, and high-level evidence points to mortality benefit in patients with moderate to severe ARDS if [mechanical] ventilation occurs,” said study coauthor James McCauley Walter, MD, of the pulmonary division at Northwestern University, Chicago.
With a “significant number of COVID-19 patients flooding the ICU, we quickly started to prone a lot of them, but if you are in a specific position for multiple hours a day, coupled with the neurotoxic effects of the SARS-CoV-2 virus itself, you may be exposed to a unique type of nerve injury,” he said.
Dr. Walter said that the “incidence of asymmetric neuropathies seems out of proportion to what has been reported in non–COVID-19 settings, which is what caught our attention.”
Many of these patients are discharged to rehabilitation hospitals, and “what we noticed, which was unique about COVID-19 patients coming to our rehab hospital, was that, compared with other patients who had been critically ill with a long hospital stay, there was a significantly higher percentage of COVID-19 patients who had peripheral nerve damage,” Dr. Franz said.
The authors described 12 of these patients who were admitted between April 24 and June 30, 2020 (mean age, 60.3 years; range, 23-80 years). The sample included White, Black, and Hispanic individuals. Eleven of the 12 post–COVID-19 patients with peripheral nerve damage had experienced prone positioning during acute management.
The average number of days patients received mechanical ventilation was 33.6 (range, 12-62 days). The average number of proning sessions was 4.5 (range, 1-16) with an average of 81.2 hours (range, 16-252 hours) spent prone.
A major contributor
Dr. Franz suggested that prone positioning is likely not the only cause of peripheral nerve damage but “may play a big role in these patients who are vulnerable because of viral infection and the critical illness that causes damage and nerve injuries.”
“The first component of lifesaving care for the critically ill in the ICU is intravenous fluids, mechanical ventilation, steroids, and antibiotics for infection,” said Dr. Walter.
“We are trying to come up with ways to place patients in prone position in safer ways, to pay attention to pressure points and areas of injury that we have seen and try to offload them, to see if we can decrease the rate of these injuries,” he added.
The researchers’ article includes a heat map diagram as a “template for where to focus the most efforts, in terms of decreasing pressure,” Dr. Walter said.
“The nerves are accepting too much force for gravely ill COVID-19 patients to handle, so we suggest using the template to determine where extra padding might be needed, or a protocol that might include changes in positioning,” he added.
Dr. Franz described the interventions used for COVID-19 patients with prone positioning–related peripheral nerve damage. “The first step is trying to address the problems one by one, either trying to solve them through exercise or teaching new skills, new ways to compensate, beginning with basic activities, such as getting out of bed and self-care,” he said.
Long-term recovery of nerve injuries depends on how severe the injuries are. Some nerves can slowly regenerate – possibly at the rate of 1 inch per month – which can be a long process, taking between a year and 18 months.
Dr. Franz said that therapies for this condition are “extrapolated from clinical trial work” on promoting nerve regeneration after surgery using electrical stimulation to enable nerves to regrow at a faster rate.
“Regeneration is not only slow, but it may not happen completely, leaving the patient with permanent nerve damage – in fact, based on our experience and what has been reported, the percentage of patients with full recovery is only 10%,” he said.
The most common symptomatic complaint other than lack of movement or feeling is neuropathic pain, “which may require medication to take the edge off the pain,” Dr. Franz added.
Irreversible damage?
Commenting on the study, Tae Chung, MD, of the departments of physical medicine, rehabilitation, and neurology, Johns Hopkins University, Baltimore, said the study “provides one of the first and the largest description of peripheral nerve injury associated with prone positioning for management of ARDS from COVID-19.”
Dr. Chung, who was not involved in the research, noted that “various neurological complications from COVID-19 have been reported, and some of them may result in irreversible neurological damage or delay the recovery from COVID-19 infection,” so “accurate and timely diagnosis of such neurological complications is critical for rehabilitation of the COVID-19 survivors.”
The study received no funding. Dr. Franz, Dr. Walter, study coauthors, and Dr. Chung report no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
FROM THE BRITISH JOURNAL OF ANAESTHESIOLOGY
Open Clinical Trials for Veterans With Suicidal Ideation (FULL)
Using Telehealth to Improve Outcomes in Veterans at Risk for Suicide
The investigators will randomize 120 veterans in this 3-site trial over 16 months. Eligible veterans will include those to be discharged for a hospitalization for suicidal ideation. Baseline data collection and randomization will occur at discharge. The 3-month intervention will have study assessments at 2, 4, 8, and 12 weeks postdischarge. The study’s primary outcome measure is suicidal ideation (measured with the Beck Scale for Suicidal Ideation (BSS) and secondarily with the Columbia Scale for Suicidality (C-SSRS).
ID: NCT03724370
Sponsor: VA Pittsburgh Healthcare System
Contact: Gretchen Haas, PhD, [email protected]; Crystal Spotts, MEd, [email protected]
Locations: James J. Peters Medical Center, Bronx, New York; VA NY Harbor Healthcare System, Manhattan Campus; VA Pittsburgh Healthcare System, Pennsylvania
Group (“Project Life Force”) vs. Individual Suicide Safety Planning RCT
The management of suicide risk is a pressing national public health issue especially among veterans. This grant consists of 2 arms: the novel treatment and treatment-as-usual. “Project Life Force” (PLF), a novel suicide safety planning group intervention has been developed to provide a mechanism to develop and enhance the Suicide Safety Plan (SSP) over time. PLF, a 10-session, group intervention, combines cognitive behavior therapy (CBT)/dialectical behavior therapy (DBT) skill-based, and psychoeducational approaches, to maximize suicide safety planning development and implementation. Veterans revise their plans over several weeks while learning coping, emotion regulation, and interpersonal skills to incorporate into their safety plans.
ID: NCT03653637
Sponsor: VA Office of Research and Development
Contact: Sarah R Sullivan, [email protected]
Locations: James J. Peters Medical Center, Bronx, New York; Corporal Michael J. Crescenz VA Medical Center, Philadelphia, PA
SAFER: A Brief Intervention Involving Family Members in Suicide Safety Planning (SAFER)
The management of suicide risk is a pressing national public health issue especially among veterans, and there exist no guidelines of how best to involve family members in this effort. This proposal will integrate family and couples communication skills training with suicide safety planning. The goal is for the sharing of veteran suicide safety plans with family members and the construction of a parallel family member safety plan, in efforts to mobilize and support family involvement.
ID: NCT03034863
Sponsor: VA Office of Research and Development
Contact: Marianne Goodman, MD, [email protected]
Contact: Sarah R Sullivan, [email protected]
Location: James J. Peters Medical Center, Bronx, New York
Suicide and Trauma Reduction Initiative Among Veterans (STRIVE)
The present study is a pragmatic clinical trial that will examine the effectiveness of Cognitive Processing Therapy (CPT) in reducing PTSD symptom severity, depression symptoms, and suicidal thoughts among military personnel and veterans with PTSD when delivered in 3 different formats: (1) 12 sessions delivered once per week in an office/clinic setting; (2) 12 sessions delivered once per day in an office/clinic setting; and (3) 12 sessions delivered once per day in a recreational setting.
ID: NCT03933059
Sponsor: University of Utah
Contact: Craig Bryan, PhD, ABPP, and Feea Leifker, PhD, MPH, [email protected]
Locations: University of Utah, Salt Lake City
CAMS-G Group Therapy for Suicidal Veterans
The primary aim of this pilot study is to determine the feasibility and acceptability of CAMS-G. Our aim is to determine if CAMS-G is an effective treatment and whether it has the potential to be tested in a large-scale setting.
ID: NCT03682406
Sponsor: Louisville VA Medical Center
Contact: Lora Johnson, PhD, [email protected]; Stephen O’Connor, PhD, [email protected]
Location: Robley Rex VA Medical Center, Louisville, Kentucky
RCT of Brief CBT-I in Primary Care Veterans With Suicidal Thoughts
There is a strong association between insomnia and suicidal thoughts and behaviors. Insomnia also frequently co-occurs with other common conditions associated with suicide such as depression and posttraumatic stress disorder. This project focuses on improving sleep as a novel suicide prevention strategy that can be delivered to a broad range of veterans. The study will examine how cognitive behavioral therapy for insomnia, an efficacious treatment for insomnia, may reduce suicidal thoughts in veterans who also suffer from co-occurring conditions when delivered by integrated primary care clinicians.
ID: NCT03603717
Sponsor: VA Office of Research and Development
Contact: Wilfred Pigeon, PhD, [email protected]; Jennifer Funderburk, PhD, [email protected]
Locations: VA Western New York Healthcare System, Buffalo; Canandaigua VA Medical Center, New York; Syracuse VA Medical Center, New York
Intranasal Ketamine for Suicidal Ideation in Veterans
To address the significant need for effective treatment of suicidal ideation in veterans, this trial is designed as an open label pilot study of intranasal ketamine in 15 people.
ID: NCT03788694
Sponsor: Bronx Veterans Medical Research Foundation, Inc
Contact: Rachel Harris, MA, [email protected]; Marianne Goodman, MD, [email protected]
Location: James J. Peters VA Medical Center, Bronx, New York
Couples Intervention to Improve Mental Health
Over the last decade, suicide rates have risen within the military and have remained high. Converging evidence suggests that suicide prevention efforts may be enhanced by explicitly including family members in treatment. The study’s objectives are to test the effect of the CCRP, a targeted single session couples intervention on suicide ideation among military service members and veterans, and to understand how the use of the CCRP impacts suicide risk during the 6 months immediately postdischarge from a psychiatric inpatient unit.
ID: NCT04084756
Sponsor: Wesleyan University
Contact: Alexis May, PhD, [email protected]
Location: Salt Lake Behavioral Health, Utah
Clinical and Imaging Trial of Uridine for Veterans With Suicidal Ideation
This is a randomized, double-blind, placebo-controlled study of the investigational drug uridine as a treatment for suicidal ideation in veterans. The investigators hypothesize that the administration of a naturally occurring dietary supplement, uridine, will rapidly reduce suicidal ideation in veterans. The purpose of this study is to determine whether 4 weeks of uridine supplementation is an effective treatment for suicidal ideation in veterans, when compared to a group taking a placebo.
ID: NCT03265964
Sponsor: VA Office of Research and Development
Contact: Douglas G Kondo, MD, [email protected]; Danielle Boxer, MS, [email protected]
Location: VA Salt Lake City Health Care System, Utah
Multisite RCT of STEP-Home: A Transdiagnostic Skill-based Community Reintegration Workshop (by invitation)
In this proposal, the investigators extend their previous SPiRE feasibility and preliminary effectiveness study to examine STEP-Home efficacy in a RCT design. This novel therapy will target the specific needs of a broad range of underserved post-9/11 veterans. It is designed to foster reintegration by facilitating meaningful improvement in the functional skills most central to community participation: emotional regulation (ER), problem solving (PS), and attention functioning (AT). The skills trained in the STEP-Home workshop are novel in their collective use and have not been systematically applied to a veteran population prior to the investigators’ SPiRE study. STEP-Home will equip veterans with skills to improve daily function, reduce anger and irritability, and assist reintegration to civilian life through return to work, family, and community, while simultaneously providing psychoeducation to promote future engagement in VA care.
ID: NCT03868930
Sponsor: VA Office of Research and Development
Locations: VA Boston Healthcare System, Jamaica Plain Campus, Massachusetts; Michael E. DeBakey VA Medical Center, Houston, Texas
The AIM Study: Investigating Whether Actigraphy and Ideation Measures Can Promote Patient Safety
This is a research project looking at whether measuring movements or responses to certain questions can help predict suicidal thoughts or actions. This project has 2 parts: The first part will occur while the participant is receiving hospitalized at the Bedford VA Hospital. It involves wearing a watch-like device on his/her wrist and answering questions or doing tasks to measure mood and other mental health symptoms, and suicidal thoughts. In the second phase, the investigators will call the participant around 12 months after s/he has left the hospital. The investigators will discuss how s/he is doing and if s/he has had suicidal thoughts or made suicidal acts.
ID: NCT03080168
Sponsor: VA Office of Research and Development
Contact: Eric G Smith, MD PhD MPH, [email protected]
Location: Edith Nourse Rogers Memorial Veterans Hospital, Bedford, Massachusetts
Using Telehealth to Improve Outcomes in Veterans at Risk for Suicide
The investigators will randomize 120 veterans in this 3-site trial over 16 months. Eligible veterans will include those to be discharged for a hospitalization for suicidal ideation. Baseline data collection and randomization will occur at discharge. The 3-month intervention will have study assessments at 2, 4, 8, and 12 weeks postdischarge. The study’s primary outcome measure is suicidal ideation (measured with the Beck Scale for Suicidal Ideation (BSS) and secondarily with the Columbia Scale for Suicidality (C-SSRS).
ID: NCT03724370
Sponsor: VA Pittsburgh Healthcare System
Contact: Gretchen Haas, PhD, [email protected]; Crystal Spotts, MEd, [email protected]
Locations: James J. Peters Medical Center, Bronx, New York; VA NY Harbor Healthcare System, Manhattan Campus; VA Pittsburgh Healthcare System, Pennsylvania
Group (“Project Life Force”) vs. Individual Suicide Safety Planning RCT
The management of suicide risk is a pressing national public health issue especially among veterans. This grant consists of 2 arms: the novel treatment and treatment-as-usual. “Project Life Force” (PLF), a novel suicide safety planning group intervention has been developed to provide a mechanism to develop and enhance the Suicide Safety Plan (SSP) over time. PLF, a 10-session, group intervention, combines cognitive behavior therapy (CBT)/dialectical behavior therapy (DBT) skill-based, and psychoeducational approaches, to maximize suicide safety planning development and implementation. Veterans revise their plans over several weeks while learning coping, emotion regulation, and interpersonal skills to incorporate into their safety plans.
ID: NCT03653637
Sponsor: VA Office of Research and Development
Contact: Sarah R Sullivan, [email protected]
Locations: James J. Peters Medical Center, Bronx, New York; Corporal Michael J. Crescenz VA Medical Center, Philadelphia, PA
SAFER: A Brief Intervention Involving Family Members in Suicide Safety Planning (SAFER)
The management of suicide risk is a pressing national public health issue especially among veterans, and there exist no guidelines of how best to involve family members in this effort. This proposal will integrate family and couples communication skills training with suicide safety planning. The goal is for the sharing of veteran suicide safety plans with family members and the construction of a parallel family member safety plan, in efforts to mobilize and support family involvement.
ID: NCT03034863
Sponsor: VA Office of Research and Development
Contact: Marianne Goodman, MD, [email protected]
Contact: Sarah R Sullivan, [email protected]
Location: James J. Peters Medical Center, Bronx, New York
Suicide and Trauma Reduction Initiative Among Veterans (STRIVE)
The present study is a pragmatic clinical trial that will examine the effectiveness of Cognitive Processing Therapy (CPT) in reducing PTSD symptom severity, depression symptoms, and suicidal thoughts among military personnel and veterans with PTSD when delivered in 3 different formats: (1) 12 sessions delivered once per week in an office/clinic setting; (2) 12 sessions delivered once per day in an office/clinic setting; and (3) 12 sessions delivered once per day in a recreational setting.
ID: NCT03933059
Sponsor: University of Utah
Contact: Craig Bryan, PhD, ABPP, and Feea Leifker, PhD, MPH, [email protected]
Locations: University of Utah, Salt Lake City
CAMS-G Group Therapy for Suicidal Veterans
The primary aim of this pilot study is to determine the feasibility and acceptability of CAMS-G. Our aim is to determine if CAMS-G is an effective treatment and whether it has the potential to be tested in a large-scale setting.
ID: NCT03682406
Sponsor: Louisville VA Medical Center
Contact: Lora Johnson, PhD, [email protected]; Stephen O’Connor, PhD, [email protected]
Location: Robley Rex VA Medical Center, Louisville, Kentucky
RCT of Brief CBT-I in Primary Care Veterans With Suicidal Thoughts
There is a strong association between insomnia and suicidal thoughts and behaviors. Insomnia also frequently co-occurs with other common conditions associated with suicide such as depression and posttraumatic stress disorder. This project focuses on improving sleep as a novel suicide prevention strategy that can be delivered to a broad range of veterans. The study will examine how cognitive behavioral therapy for insomnia, an efficacious treatment for insomnia, may reduce suicidal thoughts in veterans who also suffer from co-occurring conditions when delivered by integrated primary care clinicians.
ID: NCT03603717
Sponsor: VA Office of Research and Development
Contact: Wilfred Pigeon, PhD, [email protected]; Jennifer Funderburk, PhD, [email protected]
Locations: VA Western New York Healthcare System, Buffalo; Canandaigua VA Medical Center, New York; Syracuse VA Medical Center, New York
Intranasal Ketamine for Suicidal Ideation in Veterans
To address the significant need for effective treatment of suicidal ideation in veterans, this trial is designed as an open label pilot study of intranasal ketamine in 15 people.
ID: NCT03788694
Sponsor: Bronx Veterans Medical Research Foundation, Inc
Contact: Rachel Harris, MA, [email protected]; Marianne Goodman, MD, [email protected]
Location: James J. Peters VA Medical Center, Bronx, New York
Couples Intervention to Improve Mental Health
Over the last decade, suicide rates have risen within the military and have remained high. Converging evidence suggests that suicide prevention efforts may be enhanced by explicitly including family members in treatment. The study’s objectives are to test the effect of the CCRP, a targeted single session couples intervention on suicide ideation among military service members and veterans, and to understand how the use of the CCRP impacts suicide risk during the 6 months immediately postdischarge from a psychiatric inpatient unit.
ID: NCT04084756
Sponsor: Wesleyan University
Contact: Alexis May, PhD, [email protected]
Location: Salt Lake Behavioral Health, Utah
Clinical and Imaging Trial of Uridine for Veterans With Suicidal Ideation
This is a randomized, double-blind, placebo-controlled study of the investigational drug uridine as a treatment for suicidal ideation in veterans. The investigators hypothesize that the administration of a naturally occurring dietary supplement, uridine, will rapidly reduce suicidal ideation in veterans. The purpose of this study is to determine whether 4 weeks of uridine supplementation is an effective treatment for suicidal ideation in veterans, when compared to a group taking a placebo.
ID: NCT03265964
Sponsor: VA Office of Research and Development
Contact: Douglas G Kondo, MD, [email protected]; Danielle Boxer, MS, [email protected]
Location: VA Salt Lake City Health Care System, Utah
Multisite RCT of STEP-Home: A Transdiagnostic Skill-based Community Reintegration Workshop (by invitation)
In this proposal, the investigators extend their previous SPiRE feasibility and preliminary effectiveness study to examine STEP-Home efficacy in a RCT design. This novel therapy will target the specific needs of a broad range of underserved post-9/11 veterans. It is designed to foster reintegration by facilitating meaningful improvement in the functional skills most central to community participation: emotional regulation (ER), problem solving (PS), and attention functioning (AT). The skills trained in the STEP-Home workshop are novel in their collective use and have not been systematically applied to a veteran population prior to the investigators’ SPiRE study. STEP-Home will equip veterans with skills to improve daily function, reduce anger and irritability, and assist reintegration to civilian life through return to work, family, and community, while simultaneously providing psychoeducation to promote future engagement in VA care.
ID: NCT03868930
Sponsor: VA Office of Research and Development
Locations: VA Boston Healthcare System, Jamaica Plain Campus, Massachusetts; Michael E. DeBakey VA Medical Center, Houston, Texas
The AIM Study: Investigating Whether Actigraphy and Ideation Measures Can Promote Patient Safety
This is a research project looking at whether measuring movements or responses to certain questions can help predict suicidal thoughts or actions. This project has 2 parts: The first part will occur while the participant is receiving hospitalized at the Bedford VA Hospital. It involves wearing a watch-like device on his/her wrist and answering questions or doing tasks to measure mood and other mental health symptoms, and suicidal thoughts. In the second phase, the investigators will call the participant around 12 months after s/he has left the hospital. The investigators will discuss how s/he is doing and if s/he has had suicidal thoughts or made suicidal acts.
ID: NCT03080168
Sponsor: VA Office of Research and Development
Contact: Eric G Smith, MD PhD MPH, [email protected]
Location: Edith Nourse Rogers Memorial Veterans Hospital, Bedford, Massachusetts
Using Telehealth to Improve Outcomes in Veterans at Risk for Suicide
The investigators will randomize 120 veterans in this 3-site trial over 16 months. Eligible veterans will include those to be discharged for a hospitalization for suicidal ideation. Baseline data collection and randomization will occur at discharge. The 3-month intervention will have study assessments at 2, 4, 8, and 12 weeks postdischarge. The study’s primary outcome measure is suicidal ideation (measured with the Beck Scale for Suicidal Ideation (BSS) and secondarily with the Columbia Scale for Suicidality (C-SSRS).
ID: NCT03724370
Sponsor: VA Pittsburgh Healthcare System
Contact: Gretchen Haas, PhD, [email protected]; Crystal Spotts, MEd, [email protected]
Locations: James J. Peters Medical Center, Bronx, New York; VA NY Harbor Healthcare System, Manhattan Campus; VA Pittsburgh Healthcare System, Pennsylvania
Group (“Project Life Force”) vs. Individual Suicide Safety Planning RCT
The management of suicide risk is a pressing national public health issue especially among veterans. This grant consists of 2 arms: the novel treatment and treatment-as-usual. “Project Life Force” (PLF), a novel suicide safety planning group intervention has been developed to provide a mechanism to develop and enhance the Suicide Safety Plan (SSP) over time. PLF, a 10-session, group intervention, combines cognitive behavior therapy (CBT)/dialectical behavior therapy (DBT) skill-based, and psychoeducational approaches, to maximize suicide safety planning development and implementation. Veterans revise their plans over several weeks while learning coping, emotion regulation, and interpersonal skills to incorporate into their safety plans.
ID: NCT03653637
Sponsor: VA Office of Research and Development
Contact: Sarah R Sullivan, [email protected]
Locations: James J. Peters Medical Center, Bronx, New York; Corporal Michael J. Crescenz VA Medical Center, Philadelphia, PA
SAFER: A Brief Intervention Involving Family Members in Suicide Safety Planning (SAFER)
The management of suicide risk is a pressing national public health issue especially among veterans, and there exist no guidelines of how best to involve family members in this effort. This proposal will integrate family and couples communication skills training with suicide safety planning. The goal is for the sharing of veteran suicide safety plans with family members and the construction of a parallel family member safety plan, in efforts to mobilize and support family involvement.
ID: NCT03034863
Sponsor: VA Office of Research and Development
Contact: Marianne Goodman, MD, [email protected]
Contact: Sarah R Sullivan, [email protected]
Location: James J. Peters Medical Center, Bronx, New York
Suicide and Trauma Reduction Initiative Among Veterans (STRIVE)
The present study is a pragmatic clinical trial that will examine the effectiveness of Cognitive Processing Therapy (CPT) in reducing PTSD symptom severity, depression symptoms, and suicidal thoughts among military personnel and veterans with PTSD when delivered in 3 different formats: (1) 12 sessions delivered once per week in an office/clinic setting; (2) 12 sessions delivered once per day in an office/clinic setting; and (3) 12 sessions delivered once per day in a recreational setting.
ID: NCT03933059
Sponsor: University of Utah
Contact: Craig Bryan, PhD, ABPP, and Feea Leifker, PhD, MPH, [email protected]
Locations: University of Utah, Salt Lake City
CAMS-G Group Therapy for Suicidal Veterans
The primary aim of this pilot study is to determine the feasibility and acceptability of CAMS-G. Our aim is to determine if CAMS-G is an effective treatment and whether it has the potential to be tested in a large-scale setting.
ID: NCT03682406
Sponsor: Louisville VA Medical Center
Contact: Lora Johnson, PhD, [email protected]; Stephen O’Connor, PhD, [email protected]
Location: Robley Rex VA Medical Center, Louisville, Kentucky
RCT of Brief CBT-I in Primary Care Veterans With Suicidal Thoughts
There is a strong association between insomnia and suicidal thoughts and behaviors. Insomnia also frequently co-occurs with other common conditions associated with suicide such as depression and posttraumatic stress disorder. This project focuses on improving sleep as a novel suicide prevention strategy that can be delivered to a broad range of veterans. The study will examine how cognitive behavioral therapy for insomnia, an efficacious treatment for insomnia, may reduce suicidal thoughts in veterans who also suffer from co-occurring conditions when delivered by integrated primary care clinicians.
ID: NCT03603717
Sponsor: VA Office of Research and Development
Contact: Wilfred Pigeon, PhD, [email protected]; Jennifer Funderburk, PhD, [email protected]
Locations: VA Western New York Healthcare System, Buffalo; Canandaigua VA Medical Center, New York; Syracuse VA Medical Center, New York
Intranasal Ketamine for Suicidal Ideation in Veterans
To address the significant need for effective treatment of suicidal ideation in veterans, this trial is designed as an open label pilot study of intranasal ketamine in 15 people.
ID: NCT03788694
Sponsor: Bronx Veterans Medical Research Foundation, Inc
Contact: Rachel Harris, MA, [email protected]; Marianne Goodman, MD, [email protected]
Location: James J. Peters VA Medical Center, Bronx, New York
Couples Intervention to Improve Mental Health
Over the last decade, suicide rates have risen within the military and have remained high. Converging evidence suggests that suicide prevention efforts may be enhanced by explicitly including family members in treatment. The study’s objectives are to test the effect of the CCRP, a targeted single session couples intervention on suicide ideation among military service members and veterans, and to understand how the use of the CCRP impacts suicide risk during the 6 months immediately postdischarge from a psychiatric inpatient unit.
ID: NCT04084756
Sponsor: Wesleyan University
Contact: Alexis May, PhD, [email protected]
Location: Salt Lake Behavioral Health, Utah
Clinical and Imaging Trial of Uridine for Veterans With Suicidal Ideation
This is a randomized, double-blind, placebo-controlled study of the investigational drug uridine as a treatment for suicidal ideation in veterans. The investigators hypothesize that the administration of a naturally occurring dietary supplement, uridine, will rapidly reduce suicidal ideation in veterans. The purpose of this study is to determine whether 4 weeks of uridine supplementation is an effective treatment for suicidal ideation in veterans, when compared to a group taking a placebo.
ID: NCT03265964
Sponsor: VA Office of Research and Development
Contact: Douglas G Kondo, MD, [email protected]; Danielle Boxer, MS, [email protected]
Location: VA Salt Lake City Health Care System, Utah
Multisite RCT of STEP-Home: A Transdiagnostic Skill-based Community Reintegration Workshop (by invitation)
In this proposal, the investigators extend their previous SPiRE feasibility and preliminary effectiveness study to examine STEP-Home efficacy in a RCT design. This novel therapy will target the specific needs of a broad range of underserved post-9/11 veterans. It is designed to foster reintegration by facilitating meaningful improvement in the functional skills most central to community participation: emotional regulation (ER), problem solving (PS), and attention functioning (AT). The skills trained in the STEP-Home workshop are novel in their collective use and have not been systematically applied to a veteran population prior to the investigators’ SPiRE study. STEP-Home will equip veterans with skills to improve daily function, reduce anger and irritability, and assist reintegration to civilian life through return to work, family, and community, while simultaneously providing psychoeducation to promote future engagement in VA care.
ID: NCT03868930
Sponsor: VA Office of Research and Development
Locations: VA Boston Healthcare System, Jamaica Plain Campus, Massachusetts; Michael E. DeBakey VA Medical Center, Houston, Texas
The AIM Study: Investigating Whether Actigraphy and Ideation Measures Can Promote Patient Safety
This is a research project looking at whether measuring movements or responses to certain questions can help predict suicidal thoughts or actions. This project has 2 parts: The first part will occur while the participant is receiving hospitalized at the Bedford VA Hospital. It involves wearing a watch-like device on his/her wrist and answering questions or doing tasks to measure mood and other mental health symptoms, and suicidal thoughts. In the second phase, the investigators will call the participant around 12 months after s/he has left the hospital. The investigators will discuss how s/he is doing and if s/he has had suicidal thoughts or made suicidal acts.
ID: NCT03080168
Sponsor: VA Office of Research and Development
Contact: Eric G Smith, MD PhD MPH, [email protected]
Location: Edith Nourse Rogers Memorial Veterans Hospital, Bedford, Massachusetts
Validation of the Timberlawn Couple and Family Evaluation Scales–Self-Report in Veterans with PTSD
Although about 8.3% of the general adult civilian population will be diagnosed with posttraumatic stress disorder (PTSD) in their lifetime, rates of PTSD are even higher in the veteran population.1,2 PTSD is associated with a number of psychosocial consequences in veterans, including decreased intimate partner relationship functioning.3,4 For example, Cloitre and colleagues reported that PTSD is associated with difficulty with socializing, intimacy, responsibility, and control, all of which increase difficulties in intimate partner relationships.5 Similarly, researchers also have noted that traumatic experiences can affect an individual’s attachment style, resulting in progressive avoidance of interpersonal relationships, which can lead to marked difficulties in maintaining and beginning intimate partner relationships.6,7 Despite these known consequences of PTSD, as Dekel and Monson noted in a review,further research is still needed regarding the mechanisms by which trauma and PTSD result in decreased intimate partner relationship functioning among veterans.8 Nonetheless, as positive interpersonal relationships are associated with decreased PTSD symptom severity9,10 and increased engagement in PTSD treatment,11 determining methods of measuring intimate partner relationship functioning in veterans with PTSD is important to inform future research and aid the provision of care.
To date, limited research has examined the valid measurement of intimate partner relationship functioning among veterans with PTSD. Many existing measures that comprehensively assess intimate partner relationship functioning are time and resource intensive. One such measure, the Timberlawn Couple and Family Evaluation Scales (TCFES), comprehensively assesses multiple pertinent domains of intimate partner relationship functioning (ie, structure, autonomy, problem solving, affect regulation, and disagreement/conflict).12 By assessing multiple domains, the TCFES offers a method of understanding the specific components of an individual’s intimate partner relationship in need of increased clinical attention.12 However, the TCFES is a time- and labor-intensive observational measure that requires a couple to interact while a blinded, independent rater observes and rates their interactions using an intricate coding process. This survey structure precludes the ability to quickly and comprehensively assess a veteran’s intimate partner functioning in settings such as mental health outpatient clinics where mental health providers engage in brief, time-limited psychotherapy. As such, brief measures of intimate partner relationship functioning are needed to best inform clinical care among veterans with PTSD.
The primary aim of the current study was to create a psychometrically valid, yet brief, self-report version of the TCFES to assess multiple domains of intimate partner relationship functioning. The psychometric properties of this measure were assessed among a sample of US veterans with PTSD who were in an intimate partner relationship. We specifically examined factor structure, reliability, and associations to established measures of specific domains of relational functioning.
Methods
Ninety-four veterans were recruited via posted advertisements, promotion in PTSD therapy groups/staff meetings, and word of mouth at the Dallas Veterans Affairs Medical Center (VAMC). Participants were eligible if they had a documented diagnosis of PTSD as confirmed in the veteran’s electronic medical record and an affirmative response to currently being involved in an intimate partner relationship (ie, legally married, common-law spouse, involved in a relationship/partnership). There were no exclusion criteria.
Interested veterans were invited to complete several study-related self-report measures concerning their intimate partner relationships that would take about an hour. They were informed that the surveys were voluntary and confidential, and that they would be compensated for their participation. All veterans who participated provided written consent and the study was approved by the Dallas VAMC institutional review board.
Of the 94 veterans recruited, 3 veterans’ data were removed from current analyses after informed consent but before completing the surveys when they indicated they were not currently in a relationship or were divorced. After consent, the 91 participants were administered several study-related self-report measures. The measures took between 30 and 55 minutes to complete. Participants were then compensated $25 for their participation.
Intimate Partner Relationship Functioning
The 16-item TCFES self-report version (TCFES-SR) was developed to assess multiple domains of interpersonal functioning (Appendix). The observational TCFES assesses 5 intimate partner relationship characteristic domains (ie, structure, autonomy, problem solving, affect regulation, and disagreement/conflict) during a couple’s interaction by an independent trained rater.12 Each of the 16 TCFES-SR items were modeled after original constructs measured by the TCFES, including power, closeness, clarify, other’s views, responsibility, closure, negotiation, expressiveness, responsiveness, positive regard, negative regard, mood/tone, empathy, frequency, affective quality, and generalization and escalation. To maintain consistency with the TCFES, each item of the TCFES-SR was scored from 1 (severely dysfunctional) to 5 (highly functional). Additionally, all item wording for the TCFES-SR was based on wording in the TCFES manual after consultation with an expert who facilitated the development of the TCFES.12 On average, the TCFES-SR took 5 to 10 minutes to complete.
To measure concurrent validity of the modified TCFES-SR, several additional interpersonal measures were selected and administered based on prior research and established domains of the TCFES. The Positive and Negative Quality in Marriage Scale (PANQIMS) was administered to assess perceived attitudes toward a relationship.13,14 The PANQIMS generates 2 subscales: positive quality and negative quality in the relationship. Because the PANQIMS specifically assesses married relationships and our sample included married and nonmarried participants, wording was modified (eg, “spouse/partner”).
The relative power subscale of the Network Relationships Inventory–Relationship Qualities Version (NRI-RQV) measure was administered to assess the unequal/shared role romantic partners have in power equality (ie, relative power).15
The Revised Dyadic Adjustment Scale (RDAS) is a self-report measure that assesses multiple dimensions of marital adjustment and functioning.16 Six subscales of the RDAS were chosen based on items of the TCFES-SR: decision making, values, affection, conflict, activities, and discussion.
The Interpersonal Reactivity Index (IRI) empathetic concern subscale was administered to assess empathy across multiple contexts and situations17 and the Experiences in Close Relationships-Revised Questionnaire (ECR-R) was administered to assess relational functioning by determining attachment-related anxiety and avoidance.18
Sociodemographic Information
A sociodemographic questionnaire also was administered. The questionnaire assessed gender, age, education, service branch, length of interpersonal relationship, race, and ethnicity of the veteran as well as gender of the veteran’s partner.
Statistical Analysis
Factor structure of the TCFES-SR was determined by conducting an exploratory factor analysis. To allow for correlation between items, the Promax oblique rotation method was chosen.19 Number of factors was determined by agreement between number of eigenvalues ≥ 1, visual inspection of the scree plot, and a parallel analysis. Factor loadings of ≥ 0.3 were used to determine which items loaded on to which factors.
Convergent validity was assessed by conducting Pearson’s bivariate correlations between identified TCFES-SR factor(s) and other administered measures of interpersonal functioning (ie, PANQIMS positive and negative quality; NRI-RQV relative power subscale; RDAS decision making, values, affection, conflict, activities, and discussion subscales; IRI-empathetic concern subscale; and ECR-R attachment-related anxiety and avoidance subscales). Strength of relationship was determined based on the following guidelines: ± 0.3 to 0.49 = small, ± 0.5 to 0.69 = moderate, and ± 0.7 to 1.00 = large. Internal consistency was also determined for TCFES-SR factor(s) using Cronbach’s α. A standard level of significance (α=.05) was used for all statistical analyses.
Results
Eighty-six veterans provided complete data (Table 1). The Kaiser-Meyer-Olkin measure of sampling adequacy was indicative that sample size was adequate (.91), while Bartlett’s test of sphericity found the variables were suitable for structure detection, χ2 (120) = 800.00, P < .001. While 2 eigenvalues were ≥ 1, visual inspection of the scree plot and subsequent parallel analysis identified a unidimensional structure (ie, 1 factor) for the TCFES-SR. All items were found to load to this single factor, with all loadings being ≥ 0.5 (Table 2). Additionally, internal consistency was excellent for the scale (α = .93).
Pearson’s bivariate correlations were significant (P < .05) between TCFES-SR total score, and almost all administered interpersonal functioning measures (Table 3). Interestingly, no significant associations were found between any of the administered measures, including the TCFES-SR total score, and the IRI-empathetic concern subscale (P > .05).
Discussion
These findings provide initial support for the psychometric properties of the TCFES-SR, including excellent internal consistency and the adequate association of its total score to established measures of interpersonal functioning. Contrary to the TCFES, the TCFES-SR was shown to best fit a unidimensional factor rather than a multidimensional measure of relationship functioning. However, the TCFES-SR was also shown to have strong convergent validity with multiple domains of relationship functioning, indicating that the measure of overall intimate partner relationship functioning encompasses a number of relational domains (ie, structure, autonomy, problem solving, affect regulation, and disagreement/conflict). Critically, the TCFES-SR is brief and was administered easily in our sample, providing utility as clinical tool to be used in time-sensitive outpatient settings.
A unidimensional factor has particular strength in providing a global portrait of perceived intimate partner relationship functioning, and mental health providers can administer the TCFES-SR to assess for overall perceptions of intimate partner relationship functioning rather than administering a number of measures focusing on specific interpersonal domains (eg, decision making processes or positive/negative attitudes towards one’s relationship). This allows for the quick assessment (ie, 5-10 minutes) of overall intimate partner relationship functioning rather than administration of multiple self-report measures which can be time-intensive and expensive. However, the TCFES-SR also is limited by a lack of nuanced understanding of perceptions of functioning specific to particular domains. For example, the TCFES-SR score cannot describe intimate partner functioning in the domain of problem solving. Therefore, brief screening tools need to be developed that assess multiple intimate partner relationship domains.
Importantly, overall intimate partner relationship functioning as measured by the TCFES-SR may not incorporate perceptions of relationship empathy, as the total score did not correlate with a measure of empathetic concern (ie, the IRI-empathetic concern subscale). As empathy was based on one item in the TCFES-SR vs 7 in the IRI-empathetic concern subscale, it is unclear if the TCFES-SR only captures a portion of the construct of empathy (ie, sensitivity to partner) vs the comprehensive assessment of trait empathy that the IRI subscale measures. Additionally, the IRI-empathetic concern subscale did not significantly correlate with any of the other administered measures of relationship functioning. Given the role of empathy in positive, healthy intimate partner relationships, future research should explore the role of empathetic concern among veterans with PTSD as it relates to overall (eg, TCFES-SR) and specific aspects of intimate partner relationship functioning.20
While the clinical applicability of the TCFES-SR requires further examination, this measure has a number of potential uses. Information captured quickly by the TCFES-SR may help to inform appropriate referral for treatment. For instance, veterans reporting low total scores on the TCFES-SR may indicate a need for a referral for intervention focused on improving overall relationship functioning (eg, Integrative Behavioral Couple Therapy).21,22 Measurement-based care (ie, tracking and discussing changes in symptoms during treatment using validated self-report measures) is now required by the Joint Commission as a standard of care,and has been shown to improve outcomes in couples therapy.23,24 As a brief self-report measure, the TCFES-SR may be able to facilitate measurement-based care and assist providers in tracking changes in overall relationship functioning over the course of treatment. However, the purpose of the current study was to validate the TCFES-SR and not to examine the utility of the TCFES-SR in clinical care; additional research is needed to determine standardized cutoff scores to indicate a need for clinical intervention.
Limitations
Several limitations should be noted. The current study only assessed perceived intimate partner relationship functioning from the perspective of the veteran, thus limiting implications as it pertains to the spouse/partner of the veteran. PTSD diagnosis was based on chart review rather than a psychodiagnostic measure (eg, Clinician Administered PTSD Scale); therefore, whether this diagnosis was current or in remission was unclear. Although our sample was adequate to conduct an exploratory factor analysis,the overall sample size was modest, and results should be considered preliminary with need for further replication.25 The sample was also primarily male, white or black, and non-Hispanic; therefore, results may not generalize to a more sociodemographically diverse population. Finally, given the focus of the study to develop a self-report measure, we did not compare the TCFES-SR to the original TCFES. Thus, further research examining the relationship between the TCFES-SR and TCFES may be needed to better understand overlap and potential incongruence in these measures, and to ascertain any differences in their factor structures.
Conclusion
This study is novel in that it adapted a comprehensive observational measure of relationship functioning to a self-report measure piloted among a sample of veterans with PTSD in an intimate partner relationship, a clinical population that remains largely understudied. Although findings are preliminary, the TCFES-SR was found to be a reliable and valid measure of overall intimate partner relationship functioning. Given the rapid administration of this self-report measure, the TCFES-SR may hold clinical utility as a screen of intimate partner relationship deficits in need of clinical intervention. Replication in a larger, more diverse sample is needed to further examine the generalizability and confirm psychometric properties of the TCFES-SR. Additionally, further understanding of the clinical utility of the TCFES-SR in treatment settings remains critical to promote the development and maintenance of healthy intimate partner relationships among veterans with PTSD. Finally, development of effective self-report measures of intimate partner relationship functioning, such as the TCFES-SR, may help to facilitate needed research to understand the effect of PTSD on establishing and maintaining healthy intimate partner relationships among veterans.
Acknowledgments
The current study was funded by the Timberlawn Psychiatric Research Foundation. This material is the result of work supported in part by the US Department of Veterans Affairs; the Rocky Mountain Mental Illness Research, Education and Clinical Center (MIRECC) for Suicide Prevention; Sierra Pacific MIRECC; and the Office of Academic Affiliations, Advanced Fellowship Program in Mental Illness Research and Treatment, Department of Veterans Affairs.
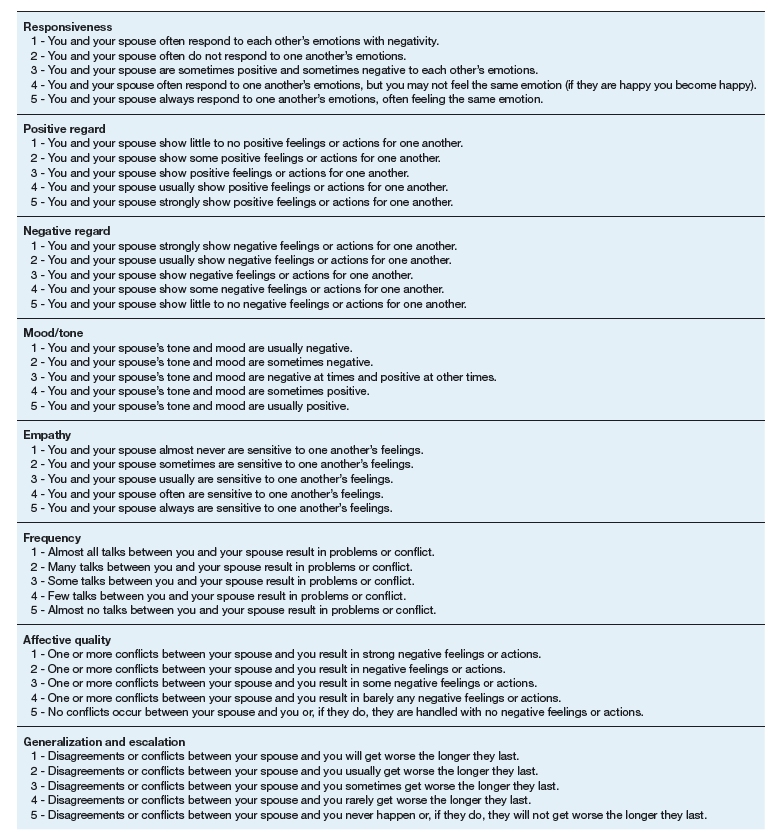
1. Kilpatrick DG, Resnick HS, Milanak ME, Miller MW, Keyes KM, Friedman MJ. National estimates of exposure to traumatic events and PTSD prevalence using DSM-IV and DSM-5 criteria. J Trauma Stress. 2013;26(5):537-547.
2. Lehavot K, Goldberg SB, Chen JA, et al. Do trauma type, stressful life events, and social support explain women veterans’ high prevalence of PTSD? Soc Psychiatry Psychiatr Epidemiol. 2018;53(9):943-953.
3. Galovski T, Lyons JA. Psychological sequelae of combat violence: a review of the impact of PTSD on the veteran’s family and possible interventions. Aggress Violent Behav. 2004;9(5):477-501.
4. Ray SL, Vanstone M. The impact of PTSD on veterans’ family relationships: an interpretative phenomenological inquiry. Int J Nurs Stud. 2009;46(6):838-847.
5. Cloitre M, Miranda R, Stovall-McClough KC, Han H. Beyond PTSD: emotion regulation and interpersonal problems as predictors of functional impairment in survivors of childhood abuse. Behav Ther. 2005;36(2):119-124.
6. McFarlane AC, Bookless C. The effect of PTSD on interpersonal relationships: issues for emergency service works. Sex Relation Ther. 2001;16(3):261-267.
7. Itzhaky L, Stein JY, Levin Y, Solomon Z. Posttraumatic stress symptoms and marital adjustment among Israeli combat veterans: the role of loneliness and attachment. Psychol Trauma. 2017;9(6):655-662.
8. Dekel R, Monson CM. Military-related post-traumatic stress disorder and family relations: current knowledge and future directions. Aggress Violent Behav. 2010;15(4):303-309.
9. Allen ES, Rhoades GK, Stanley SM, Markman HJ. Hitting home: relationships between recent deployment, posttraumatic stress symptoms, and marital functioning for Army couples. J Fam Psychol. 2010;24(3):280-288.
10. Laffaye C, Cavella S, Drescher K, Rosen C. Relationships among PTSD symptoms, social support, and support source in veterans with chronic PTSD. J Trauma Stress. 2008;21(4):394-401.
11. Meis LA, Noorbaloochi S, Hagel Campbell EM, et al. Sticking it out in trauma-focused treatment for PTSD: it takes a village. J Consult Clin Psychol. 2019;87(3):246-256.
12. Lewis JM, Gossett JT, Housson MM, Owen MT. Timberlawn Couple and Family Evaluation Scales. Dallas, TX: Timberlawn Psychiatric Research Foundation; 1999.
13. Fincham FD, Linfield KJ. A new look at marital quality: can spouses feel positive and negative about their marriage? J Fam Psychol. 1997;11(4):489-502.
14. Kaplan KJ. On the ambivalence-indifference problem in attitude theory and measurement: a suggested modification of the semantic differential technique. Psychol Bull. 1972;77(5):361-372.
15. Buhrmester D, Furman W. The Network of Relationship Inventory: Relationship Qualities Version [unpublished measure]. University of Texas at Dallas; 2008.
16. Busby DM, Christensen C, Crane DR, Larson JH. A revision of the Dyadic Adjustment Scale for use with distressed and nondistressed couples: construct hierarchy and multidimensional scales. J Marital Fam Ther. 1995;21(3):289-308.
17. Davis MH. A multidimensional approach to individual differences in empathy. JSAS Catalog Sel Doc Psychol. 1980;10:85.
18. Fraley RC, Waller NG, Brennan KA. An item-response theory analysis of self-report measures of adult attachment. J Pers Soc Psychol. 2000;78(2):350-365.
19. Tabachnick BG, Fidell L. Using Multivariate Statistics. 6th ed. Boston, MA: Pearson; 2013.
20. Sautter FJ, Armelie AP, Glynn SM, Wielt DB. The development of a couple-based treatment for PTSD in returning veterans. Prof Psychol Res Pr. 2011;42(1):63-69.
21. Jacobson NS, Christensen A, Prince SE, Cordova J, Eldridge K. Integrative behavioral couple therapy: an acceptance-based, promising new treatment of couple discord. J Consult Clin Psychol. 2000;9(2):351-355.
22. Makin-Byrd K, Gifford E, McCutcheon S, Glynn S. Family and couples treatment for newly returning veterans. Prof Psychol Res Pr. 2011;42(1):47-55.
23. Peterson K, Anderson J, Bourne D. Evidence Brief: Use of Patient Reported Outcome Measures for Measurement Based Care in Mental Health Shared Decision Making. Washington, DC: Department of Veterans Affairs; 2018. https://www.ncbi.nlm.nih.gov/books/NBK536143. Accessed September 13, 2019.
24. Fortney JC, Unützer J, Wrenn G, et al. A tipping point for measurement-based care. Psychiatr Serv. 2017;68(2):179-188.
25. Costello AB, Osborne JW. Best practices in exploratory factor analysis: four recommendations for getting the most from your analysis. Pract Assess Res Eval. 2005;10(7):1-9.
Although about 8.3% of the general adult civilian population will be diagnosed with posttraumatic stress disorder (PTSD) in their lifetime, rates of PTSD are even higher in the veteran population.1,2 PTSD is associated with a number of psychosocial consequences in veterans, including decreased intimate partner relationship functioning.3,4 For example, Cloitre and colleagues reported that PTSD is associated with difficulty with socializing, intimacy, responsibility, and control, all of which increase difficulties in intimate partner relationships.5 Similarly, researchers also have noted that traumatic experiences can affect an individual’s attachment style, resulting in progressive avoidance of interpersonal relationships, which can lead to marked difficulties in maintaining and beginning intimate partner relationships.6,7 Despite these known consequences of PTSD, as Dekel and Monson noted in a review,further research is still needed regarding the mechanisms by which trauma and PTSD result in decreased intimate partner relationship functioning among veterans.8 Nonetheless, as positive interpersonal relationships are associated with decreased PTSD symptom severity9,10 and increased engagement in PTSD treatment,11 determining methods of measuring intimate partner relationship functioning in veterans with PTSD is important to inform future research and aid the provision of care.
To date, limited research has examined the valid measurement of intimate partner relationship functioning among veterans with PTSD. Many existing measures that comprehensively assess intimate partner relationship functioning are time and resource intensive. One such measure, the Timberlawn Couple and Family Evaluation Scales (TCFES), comprehensively assesses multiple pertinent domains of intimate partner relationship functioning (ie, structure, autonomy, problem solving, affect regulation, and disagreement/conflict).12 By assessing multiple domains, the TCFES offers a method of understanding the specific components of an individual’s intimate partner relationship in need of increased clinical attention.12 However, the TCFES is a time- and labor-intensive observational measure that requires a couple to interact while a blinded, independent rater observes and rates their interactions using an intricate coding process. This survey structure precludes the ability to quickly and comprehensively assess a veteran’s intimate partner functioning in settings such as mental health outpatient clinics where mental health providers engage in brief, time-limited psychotherapy. As such, brief measures of intimate partner relationship functioning are needed to best inform clinical care among veterans with PTSD.
The primary aim of the current study was to create a psychometrically valid, yet brief, self-report version of the TCFES to assess multiple domains of intimate partner relationship functioning. The psychometric properties of this measure were assessed among a sample of US veterans with PTSD who were in an intimate partner relationship. We specifically examined factor structure, reliability, and associations to established measures of specific domains of relational functioning.
Methods
Ninety-four veterans were recruited via posted advertisements, promotion in PTSD therapy groups/staff meetings, and word of mouth at the Dallas Veterans Affairs Medical Center (VAMC). Participants were eligible if they had a documented diagnosis of PTSD as confirmed in the veteran’s electronic medical record and an affirmative response to currently being involved in an intimate partner relationship (ie, legally married, common-law spouse, involved in a relationship/partnership). There were no exclusion criteria.
Interested veterans were invited to complete several study-related self-report measures concerning their intimate partner relationships that would take about an hour. They were informed that the surveys were voluntary and confidential, and that they would be compensated for their participation. All veterans who participated provided written consent and the study was approved by the Dallas VAMC institutional review board.
Of the 94 veterans recruited, 3 veterans’ data were removed from current analyses after informed consent but before completing the surveys when they indicated they were not currently in a relationship or were divorced. After consent, the 91 participants were administered several study-related self-report measures. The measures took between 30 and 55 minutes to complete. Participants were then compensated $25 for their participation.
Intimate Partner Relationship Functioning
The 16-item TCFES self-report version (TCFES-SR) was developed to assess multiple domains of interpersonal functioning (Appendix). The observational TCFES assesses 5 intimate partner relationship characteristic domains (ie, structure, autonomy, problem solving, affect regulation, and disagreement/conflict) during a couple’s interaction by an independent trained rater.12 Each of the 16 TCFES-SR items were modeled after original constructs measured by the TCFES, including power, closeness, clarify, other’s views, responsibility, closure, negotiation, expressiveness, responsiveness, positive regard, negative regard, mood/tone, empathy, frequency, affective quality, and generalization and escalation. To maintain consistency with the TCFES, each item of the TCFES-SR was scored from 1 (severely dysfunctional) to 5 (highly functional). Additionally, all item wording for the TCFES-SR was based on wording in the TCFES manual after consultation with an expert who facilitated the development of the TCFES.12 On average, the TCFES-SR took 5 to 10 minutes to complete.
To measure concurrent validity of the modified TCFES-SR, several additional interpersonal measures were selected and administered based on prior research and established domains of the TCFES. The Positive and Negative Quality in Marriage Scale (PANQIMS) was administered to assess perceived attitudes toward a relationship.13,14 The PANQIMS generates 2 subscales: positive quality and negative quality in the relationship. Because the PANQIMS specifically assesses married relationships and our sample included married and nonmarried participants, wording was modified (eg, “spouse/partner”).
The relative power subscale of the Network Relationships Inventory–Relationship Qualities Version (NRI-RQV) measure was administered to assess the unequal/shared role romantic partners have in power equality (ie, relative power).15
The Revised Dyadic Adjustment Scale (RDAS) is a self-report measure that assesses multiple dimensions of marital adjustment and functioning.16 Six subscales of the RDAS were chosen based on items of the TCFES-SR: decision making, values, affection, conflict, activities, and discussion.
The Interpersonal Reactivity Index (IRI) empathetic concern subscale was administered to assess empathy across multiple contexts and situations17 and the Experiences in Close Relationships-Revised Questionnaire (ECR-R) was administered to assess relational functioning by determining attachment-related anxiety and avoidance.18
Sociodemographic Information
A sociodemographic questionnaire also was administered. The questionnaire assessed gender, age, education, service branch, length of interpersonal relationship, race, and ethnicity of the veteran as well as gender of the veteran’s partner.
Statistical Analysis
Factor structure of the TCFES-SR was determined by conducting an exploratory factor analysis. To allow for correlation between items, the Promax oblique rotation method was chosen.19 Number of factors was determined by agreement between number of eigenvalues ≥ 1, visual inspection of the scree plot, and a parallel analysis. Factor loadings of ≥ 0.3 were used to determine which items loaded on to which factors.
Convergent validity was assessed by conducting Pearson’s bivariate correlations between identified TCFES-SR factor(s) and other administered measures of interpersonal functioning (ie, PANQIMS positive and negative quality; NRI-RQV relative power subscale; RDAS decision making, values, affection, conflict, activities, and discussion subscales; IRI-empathetic concern subscale; and ECR-R attachment-related anxiety and avoidance subscales). Strength of relationship was determined based on the following guidelines: ± 0.3 to 0.49 = small, ± 0.5 to 0.69 = moderate, and ± 0.7 to 1.00 = large. Internal consistency was also determined for TCFES-SR factor(s) using Cronbach’s α. A standard level of significance (α=.05) was used for all statistical analyses.
Results
Eighty-six veterans provided complete data (Table 1). The Kaiser-Meyer-Olkin measure of sampling adequacy was indicative that sample size was adequate (.91), while Bartlett’s test of sphericity found the variables were suitable for structure detection, χ2 (120) = 800.00, P < .001. While 2 eigenvalues were ≥ 1, visual inspection of the scree plot and subsequent parallel analysis identified a unidimensional structure (ie, 1 factor) for the TCFES-SR. All items were found to load to this single factor, with all loadings being ≥ 0.5 (Table 2). Additionally, internal consistency was excellent for the scale (α = .93).
Pearson’s bivariate correlations were significant (P < .05) between TCFES-SR total score, and almost all administered interpersonal functioning measures (Table 3). Interestingly, no significant associations were found between any of the administered measures, including the TCFES-SR total score, and the IRI-empathetic concern subscale (P > .05).
Discussion
These findings provide initial support for the psychometric properties of the TCFES-SR, including excellent internal consistency and the adequate association of its total score to established measures of interpersonal functioning. Contrary to the TCFES, the TCFES-SR was shown to best fit a unidimensional factor rather than a multidimensional measure of relationship functioning. However, the TCFES-SR was also shown to have strong convergent validity with multiple domains of relationship functioning, indicating that the measure of overall intimate partner relationship functioning encompasses a number of relational domains (ie, structure, autonomy, problem solving, affect regulation, and disagreement/conflict). Critically, the TCFES-SR is brief and was administered easily in our sample, providing utility as clinical tool to be used in time-sensitive outpatient settings.
A unidimensional factor has particular strength in providing a global portrait of perceived intimate partner relationship functioning, and mental health providers can administer the TCFES-SR to assess for overall perceptions of intimate partner relationship functioning rather than administering a number of measures focusing on specific interpersonal domains (eg, decision making processes or positive/negative attitudes towards one’s relationship). This allows for the quick assessment (ie, 5-10 minutes) of overall intimate partner relationship functioning rather than administration of multiple self-report measures which can be time-intensive and expensive. However, the TCFES-SR also is limited by a lack of nuanced understanding of perceptions of functioning specific to particular domains. For example, the TCFES-SR score cannot describe intimate partner functioning in the domain of problem solving. Therefore, brief screening tools need to be developed that assess multiple intimate partner relationship domains.
Importantly, overall intimate partner relationship functioning as measured by the TCFES-SR may not incorporate perceptions of relationship empathy, as the total score did not correlate with a measure of empathetic concern (ie, the IRI-empathetic concern subscale). As empathy was based on one item in the TCFES-SR vs 7 in the IRI-empathetic concern subscale, it is unclear if the TCFES-SR only captures a portion of the construct of empathy (ie, sensitivity to partner) vs the comprehensive assessment of trait empathy that the IRI subscale measures. Additionally, the IRI-empathetic concern subscale did not significantly correlate with any of the other administered measures of relationship functioning. Given the role of empathy in positive, healthy intimate partner relationships, future research should explore the role of empathetic concern among veterans with PTSD as it relates to overall (eg, TCFES-SR) and specific aspects of intimate partner relationship functioning.20
While the clinical applicability of the TCFES-SR requires further examination, this measure has a number of potential uses. Information captured quickly by the TCFES-SR may help to inform appropriate referral for treatment. For instance, veterans reporting low total scores on the TCFES-SR may indicate a need for a referral for intervention focused on improving overall relationship functioning (eg, Integrative Behavioral Couple Therapy).21,22 Measurement-based care (ie, tracking and discussing changes in symptoms during treatment using validated self-report measures) is now required by the Joint Commission as a standard of care,and has been shown to improve outcomes in couples therapy.23,24 As a brief self-report measure, the TCFES-SR may be able to facilitate measurement-based care and assist providers in tracking changes in overall relationship functioning over the course of treatment. However, the purpose of the current study was to validate the TCFES-SR and not to examine the utility of the TCFES-SR in clinical care; additional research is needed to determine standardized cutoff scores to indicate a need for clinical intervention.
Limitations
Several limitations should be noted. The current study only assessed perceived intimate partner relationship functioning from the perspective of the veteran, thus limiting implications as it pertains to the spouse/partner of the veteran. PTSD diagnosis was based on chart review rather than a psychodiagnostic measure (eg, Clinician Administered PTSD Scale); therefore, whether this diagnosis was current or in remission was unclear. Although our sample was adequate to conduct an exploratory factor analysis,the overall sample size was modest, and results should be considered preliminary with need for further replication.25 The sample was also primarily male, white or black, and non-Hispanic; therefore, results may not generalize to a more sociodemographically diverse population. Finally, given the focus of the study to develop a self-report measure, we did not compare the TCFES-SR to the original TCFES. Thus, further research examining the relationship between the TCFES-SR and TCFES may be needed to better understand overlap and potential incongruence in these measures, and to ascertain any differences in their factor structures.
Conclusion
This study is novel in that it adapted a comprehensive observational measure of relationship functioning to a self-report measure piloted among a sample of veterans with PTSD in an intimate partner relationship, a clinical population that remains largely understudied. Although findings are preliminary, the TCFES-SR was found to be a reliable and valid measure of overall intimate partner relationship functioning. Given the rapid administration of this self-report measure, the TCFES-SR may hold clinical utility as a screen of intimate partner relationship deficits in need of clinical intervention. Replication in a larger, more diverse sample is needed to further examine the generalizability and confirm psychometric properties of the TCFES-SR. Additionally, further understanding of the clinical utility of the TCFES-SR in treatment settings remains critical to promote the development and maintenance of healthy intimate partner relationships among veterans with PTSD. Finally, development of effective self-report measures of intimate partner relationship functioning, such as the TCFES-SR, may help to facilitate needed research to understand the effect of PTSD on establishing and maintaining healthy intimate partner relationships among veterans.
Acknowledgments
The current study was funded by the Timberlawn Psychiatric Research Foundation. This material is the result of work supported in part by the US Department of Veterans Affairs; the Rocky Mountain Mental Illness Research, Education and Clinical Center (MIRECC) for Suicide Prevention; Sierra Pacific MIRECC; and the Office of Academic Affiliations, Advanced Fellowship Program in Mental Illness Research and Treatment, Department of Veterans Affairs.


Although about 8.3% of the general adult civilian population will be diagnosed with posttraumatic stress disorder (PTSD) in their lifetime, rates of PTSD are even higher in the veteran population.1,2 PTSD is associated with a number of psychosocial consequences in veterans, including decreased intimate partner relationship functioning.3,4 For example, Cloitre and colleagues reported that PTSD is associated with difficulty with socializing, intimacy, responsibility, and control, all of which increase difficulties in intimate partner relationships.5 Similarly, researchers also have noted that traumatic experiences can affect an individual’s attachment style, resulting in progressive avoidance of interpersonal relationships, which can lead to marked difficulties in maintaining and beginning intimate partner relationships.6,7 Despite these known consequences of PTSD, as Dekel and Monson noted in a review,further research is still needed regarding the mechanisms by which trauma and PTSD result in decreased intimate partner relationship functioning among veterans.8 Nonetheless, as positive interpersonal relationships are associated with decreased PTSD symptom severity9,10 and increased engagement in PTSD treatment,11 determining methods of measuring intimate partner relationship functioning in veterans with PTSD is important to inform future research and aid the provision of care.
To date, limited research has examined the valid measurement of intimate partner relationship functioning among veterans with PTSD. Many existing measures that comprehensively assess intimate partner relationship functioning are time and resource intensive. One such measure, the Timberlawn Couple and Family Evaluation Scales (TCFES), comprehensively assesses multiple pertinent domains of intimate partner relationship functioning (ie, structure, autonomy, problem solving, affect regulation, and disagreement/conflict).12 By assessing multiple domains, the TCFES offers a method of understanding the specific components of an individual’s intimate partner relationship in need of increased clinical attention.12 However, the TCFES is a time- and labor-intensive observational measure that requires a couple to interact while a blinded, independent rater observes and rates their interactions using an intricate coding process. This survey structure precludes the ability to quickly and comprehensively assess a veteran’s intimate partner functioning in settings such as mental health outpatient clinics where mental health providers engage in brief, time-limited psychotherapy. As such, brief measures of intimate partner relationship functioning are needed to best inform clinical care among veterans with PTSD.
The primary aim of the current study was to create a psychometrically valid, yet brief, self-report version of the TCFES to assess multiple domains of intimate partner relationship functioning. The psychometric properties of this measure were assessed among a sample of US veterans with PTSD who were in an intimate partner relationship. We specifically examined factor structure, reliability, and associations to established measures of specific domains of relational functioning.
Methods
Ninety-four veterans were recruited via posted advertisements, promotion in PTSD therapy groups/staff meetings, and word of mouth at the Dallas Veterans Affairs Medical Center (VAMC). Participants were eligible if they had a documented diagnosis of PTSD as confirmed in the veteran’s electronic medical record and an affirmative response to currently being involved in an intimate partner relationship (ie, legally married, common-law spouse, involved in a relationship/partnership). There were no exclusion criteria.
Interested veterans were invited to complete several study-related self-report measures concerning their intimate partner relationships that would take about an hour. They were informed that the surveys were voluntary and confidential, and that they would be compensated for their participation. All veterans who participated provided written consent and the study was approved by the Dallas VAMC institutional review board.
Of the 94 veterans recruited, 3 veterans’ data were removed from current analyses after informed consent but before completing the surveys when they indicated they were not currently in a relationship or were divorced. After consent, the 91 participants were administered several study-related self-report measures. The measures took between 30 and 55 minutes to complete. Participants were then compensated $25 for their participation.
Intimate Partner Relationship Functioning
The 16-item TCFES self-report version (TCFES-SR) was developed to assess multiple domains of interpersonal functioning (Appendix). The observational TCFES assesses 5 intimate partner relationship characteristic domains (ie, structure, autonomy, problem solving, affect regulation, and disagreement/conflict) during a couple’s interaction by an independent trained rater.12 Each of the 16 TCFES-SR items were modeled after original constructs measured by the TCFES, including power, closeness, clarify, other’s views, responsibility, closure, negotiation, expressiveness, responsiveness, positive regard, negative regard, mood/tone, empathy, frequency, affective quality, and generalization and escalation. To maintain consistency with the TCFES, each item of the TCFES-SR was scored from 1 (severely dysfunctional) to 5 (highly functional). Additionally, all item wording for the TCFES-SR was based on wording in the TCFES manual after consultation with an expert who facilitated the development of the TCFES.12 On average, the TCFES-SR took 5 to 10 minutes to complete.
To measure concurrent validity of the modified TCFES-SR, several additional interpersonal measures were selected and administered based on prior research and established domains of the TCFES. The Positive and Negative Quality in Marriage Scale (PANQIMS) was administered to assess perceived attitudes toward a relationship.13,14 The PANQIMS generates 2 subscales: positive quality and negative quality in the relationship. Because the PANQIMS specifically assesses married relationships and our sample included married and nonmarried participants, wording was modified (eg, “spouse/partner”).
The relative power subscale of the Network Relationships Inventory–Relationship Qualities Version (NRI-RQV) measure was administered to assess the unequal/shared role romantic partners have in power equality (ie, relative power).15
The Revised Dyadic Adjustment Scale (RDAS) is a self-report measure that assesses multiple dimensions of marital adjustment and functioning.16 Six subscales of the RDAS were chosen based on items of the TCFES-SR: decision making, values, affection, conflict, activities, and discussion.
The Interpersonal Reactivity Index (IRI) empathetic concern subscale was administered to assess empathy across multiple contexts and situations17 and the Experiences in Close Relationships-Revised Questionnaire (ECR-R) was administered to assess relational functioning by determining attachment-related anxiety and avoidance.18
Sociodemographic Information
A sociodemographic questionnaire also was administered. The questionnaire assessed gender, age, education, service branch, length of interpersonal relationship, race, and ethnicity of the veteran as well as gender of the veteran’s partner.
Statistical Analysis
Factor structure of the TCFES-SR was determined by conducting an exploratory factor analysis. To allow for correlation between items, the Promax oblique rotation method was chosen.19 Number of factors was determined by agreement between number of eigenvalues ≥ 1, visual inspection of the scree plot, and a parallel analysis. Factor loadings of ≥ 0.3 were used to determine which items loaded on to which factors.
Convergent validity was assessed by conducting Pearson’s bivariate correlations between identified TCFES-SR factor(s) and other administered measures of interpersonal functioning (ie, PANQIMS positive and negative quality; NRI-RQV relative power subscale; RDAS decision making, values, affection, conflict, activities, and discussion subscales; IRI-empathetic concern subscale; and ECR-R attachment-related anxiety and avoidance subscales). Strength of relationship was determined based on the following guidelines: ± 0.3 to 0.49 = small, ± 0.5 to 0.69 = moderate, and ± 0.7 to 1.00 = large. Internal consistency was also determined for TCFES-SR factor(s) using Cronbach’s α. A standard level of significance (α=.05) was used for all statistical analyses.
Results
Eighty-six veterans provided complete data (Table 1). The Kaiser-Meyer-Olkin measure of sampling adequacy was indicative that sample size was adequate (.91), while Bartlett’s test of sphericity found the variables were suitable for structure detection, χ2 (120) = 800.00, P < .001. While 2 eigenvalues were ≥ 1, visual inspection of the scree plot and subsequent parallel analysis identified a unidimensional structure (ie, 1 factor) for the TCFES-SR. All items were found to load to this single factor, with all loadings being ≥ 0.5 (Table 2). Additionally, internal consistency was excellent for the scale (α = .93).
Pearson’s bivariate correlations were significant (P < .05) between TCFES-SR total score, and almost all administered interpersonal functioning measures (Table 3). Interestingly, no significant associations were found between any of the administered measures, including the TCFES-SR total score, and the IRI-empathetic concern subscale (P > .05).
Discussion
These findings provide initial support for the psychometric properties of the TCFES-SR, including excellent internal consistency and the adequate association of its total score to established measures of interpersonal functioning. Contrary to the TCFES, the TCFES-SR was shown to best fit a unidimensional factor rather than a multidimensional measure of relationship functioning. However, the TCFES-SR was also shown to have strong convergent validity with multiple domains of relationship functioning, indicating that the measure of overall intimate partner relationship functioning encompasses a number of relational domains (ie, structure, autonomy, problem solving, affect regulation, and disagreement/conflict). Critically, the TCFES-SR is brief and was administered easily in our sample, providing utility as clinical tool to be used in time-sensitive outpatient settings.
A unidimensional factor has particular strength in providing a global portrait of perceived intimate partner relationship functioning, and mental health providers can administer the TCFES-SR to assess for overall perceptions of intimate partner relationship functioning rather than administering a number of measures focusing on specific interpersonal domains (eg, decision making processes or positive/negative attitudes towards one’s relationship). This allows for the quick assessment (ie, 5-10 minutes) of overall intimate partner relationship functioning rather than administration of multiple self-report measures which can be time-intensive and expensive. However, the TCFES-SR also is limited by a lack of nuanced understanding of perceptions of functioning specific to particular domains. For example, the TCFES-SR score cannot describe intimate partner functioning in the domain of problem solving. Therefore, brief screening tools need to be developed that assess multiple intimate partner relationship domains.
Importantly, overall intimate partner relationship functioning as measured by the TCFES-SR may not incorporate perceptions of relationship empathy, as the total score did not correlate with a measure of empathetic concern (ie, the IRI-empathetic concern subscale). As empathy was based on one item in the TCFES-SR vs 7 in the IRI-empathetic concern subscale, it is unclear if the TCFES-SR only captures a portion of the construct of empathy (ie, sensitivity to partner) vs the comprehensive assessment of trait empathy that the IRI subscale measures. Additionally, the IRI-empathetic concern subscale did not significantly correlate with any of the other administered measures of relationship functioning. Given the role of empathy in positive, healthy intimate partner relationships, future research should explore the role of empathetic concern among veterans with PTSD as it relates to overall (eg, TCFES-SR) and specific aspects of intimate partner relationship functioning.20
While the clinical applicability of the TCFES-SR requires further examination, this measure has a number of potential uses. Information captured quickly by the TCFES-SR may help to inform appropriate referral for treatment. For instance, veterans reporting low total scores on the TCFES-SR may indicate a need for a referral for intervention focused on improving overall relationship functioning (eg, Integrative Behavioral Couple Therapy).21,22 Measurement-based care (ie, tracking and discussing changes in symptoms during treatment using validated self-report measures) is now required by the Joint Commission as a standard of care,and has been shown to improve outcomes in couples therapy.23,24 As a brief self-report measure, the TCFES-SR may be able to facilitate measurement-based care and assist providers in tracking changes in overall relationship functioning over the course of treatment. However, the purpose of the current study was to validate the TCFES-SR and not to examine the utility of the TCFES-SR in clinical care; additional research is needed to determine standardized cutoff scores to indicate a need for clinical intervention.
Limitations
Several limitations should be noted. The current study only assessed perceived intimate partner relationship functioning from the perspective of the veteran, thus limiting implications as it pertains to the spouse/partner of the veteran. PTSD diagnosis was based on chart review rather than a psychodiagnostic measure (eg, Clinician Administered PTSD Scale); therefore, whether this diagnosis was current or in remission was unclear. Although our sample was adequate to conduct an exploratory factor analysis,the overall sample size was modest, and results should be considered preliminary with need for further replication.25 The sample was also primarily male, white or black, and non-Hispanic; therefore, results may not generalize to a more sociodemographically diverse population. Finally, given the focus of the study to develop a self-report measure, we did not compare the TCFES-SR to the original TCFES. Thus, further research examining the relationship between the TCFES-SR and TCFES may be needed to better understand overlap and potential incongruence in these measures, and to ascertain any differences in their factor structures.
Conclusion
This study is novel in that it adapted a comprehensive observational measure of relationship functioning to a self-report measure piloted among a sample of veterans with PTSD in an intimate partner relationship, a clinical population that remains largely understudied. Although findings are preliminary, the TCFES-SR was found to be a reliable and valid measure of overall intimate partner relationship functioning. Given the rapid administration of this self-report measure, the TCFES-SR may hold clinical utility as a screen of intimate partner relationship deficits in need of clinical intervention. Replication in a larger, more diverse sample is needed to further examine the generalizability and confirm psychometric properties of the TCFES-SR. Additionally, further understanding of the clinical utility of the TCFES-SR in treatment settings remains critical to promote the development and maintenance of healthy intimate partner relationships among veterans with PTSD. Finally, development of effective self-report measures of intimate partner relationship functioning, such as the TCFES-SR, may help to facilitate needed research to understand the effect of PTSD on establishing and maintaining healthy intimate partner relationships among veterans.
Acknowledgments
The current study was funded by the Timberlawn Psychiatric Research Foundation. This material is the result of work supported in part by the US Department of Veterans Affairs; the Rocky Mountain Mental Illness Research, Education and Clinical Center (MIRECC) for Suicide Prevention; Sierra Pacific MIRECC; and the Office of Academic Affiliations, Advanced Fellowship Program in Mental Illness Research and Treatment, Department of Veterans Affairs.


1. Kilpatrick DG, Resnick HS, Milanak ME, Miller MW, Keyes KM, Friedman MJ. National estimates of exposure to traumatic events and PTSD prevalence using DSM-IV and DSM-5 criteria. J Trauma Stress. 2013;26(5):537-547.
2. Lehavot K, Goldberg SB, Chen JA, et al. Do trauma type, stressful life events, and social support explain women veterans’ high prevalence of PTSD? Soc Psychiatry Psychiatr Epidemiol. 2018;53(9):943-953.
3. Galovski T, Lyons JA. Psychological sequelae of combat violence: a review of the impact of PTSD on the veteran’s family and possible interventions. Aggress Violent Behav. 2004;9(5):477-501.
4. Ray SL, Vanstone M. The impact of PTSD on veterans’ family relationships: an interpretative phenomenological inquiry. Int J Nurs Stud. 2009;46(6):838-847.
5. Cloitre M, Miranda R, Stovall-McClough KC, Han H. Beyond PTSD: emotion regulation and interpersonal problems as predictors of functional impairment in survivors of childhood abuse. Behav Ther. 2005;36(2):119-124.
6. McFarlane AC, Bookless C. The effect of PTSD on interpersonal relationships: issues for emergency service works. Sex Relation Ther. 2001;16(3):261-267.
7. Itzhaky L, Stein JY, Levin Y, Solomon Z. Posttraumatic stress symptoms and marital adjustment among Israeli combat veterans: the role of loneliness and attachment. Psychol Trauma. 2017;9(6):655-662.
8. Dekel R, Monson CM. Military-related post-traumatic stress disorder and family relations: current knowledge and future directions. Aggress Violent Behav. 2010;15(4):303-309.
9. Allen ES, Rhoades GK, Stanley SM, Markman HJ. Hitting home: relationships between recent deployment, posttraumatic stress symptoms, and marital functioning for Army couples. J Fam Psychol. 2010;24(3):280-288.
10. Laffaye C, Cavella S, Drescher K, Rosen C. Relationships among PTSD symptoms, social support, and support source in veterans with chronic PTSD. J Trauma Stress. 2008;21(4):394-401.
11. Meis LA, Noorbaloochi S, Hagel Campbell EM, et al. Sticking it out in trauma-focused treatment for PTSD: it takes a village. J Consult Clin Psychol. 2019;87(3):246-256.
12. Lewis JM, Gossett JT, Housson MM, Owen MT. Timberlawn Couple and Family Evaluation Scales. Dallas, TX: Timberlawn Psychiatric Research Foundation; 1999.
13. Fincham FD, Linfield KJ. A new look at marital quality: can spouses feel positive and negative about their marriage? J Fam Psychol. 1997;11(4):489-502.
14. Kaplan KJ. On the ambivalence-indifference problem in attitude theory and measurement: a suggested modification of the semantic differential technique. Psychol Bull. 1972;77(5):361-372.
15. Buhrmester D, Furman W. The Network of Relationship Inventory: Relationship Qualities Version [unpublished measure]. University of Texas at Dallas; 2008.
16. Busby DM, Christensen C, Crane DR, Larson JH. A revision of the Dyadic Adjustment Scale for use with distressed and nondistressed couples: construct hierarchy and multidimensional scales. J Marital Fam Ther. 1995;21(3):289-308.
17. Davis MH. A multidimensional approach to individual differences in empathy. JSAS Catalog Sel Doc Psychol. 1980;10:85.
18. Fraley RC, Waller NG, Brennan KA. An item-response theory analysis of self-report measures of adult attachment. J Pers Soc Psychol. 2000;78(2):350-365.
19. Tabachnick BG, Fidell L. Using Multivariate Statistics. 6th ed. Boston, MA: Pearson; 2013.
20. Sautter FJ, Armelie AP, Glynn SM, Wielt DB. The development of a couple-based treatment for PTSD in returning veterans. Prof Psychol Res Pr. 2011;42(1):63-69.
21. Jacobson NS, Christensen A, Prince SE, Cordova J, Eldridge K. Integrative behavioral couple therapy: an acceptance-based, promising new treatment of couple discord. J Consult Clin Psychol. 2000;9(2):351-355.
22. Makin-Byrd K, Gifford E, McCutcheon S, Glynn S. Family and couples treatment for newly returning veterans. Prof Psychol Res Pr. 2011;42(1):47-55.
23. Peterson K, Anderson J, Bourne D. Evidence Brief: Use of Patient Reported Outcome Measures for Measurement Based Care in Mental Health Shared Decision Making. Washington, DC: Department of Veterans Affairs; 2018. https://www.ncbi.nlm.nih.gov/books/NBK536143. Accessed September 13, 2019.
24. Fortney JC, Unützer J, Wrenn G, et al. A tipping point for measurement-based care. Psychiatr Serv. 2017;68(2):179-188.
25. Costello AB, Osborne JW. Best practices in exploratory factor analysis: four recommendations for getting the most from your analysis. Pract Assess Res Eval. 2005;10(7):1-9.
1. Kilpatrick DG, Resnick HS, Milanak ME, Miller MW, Keyes KM, Friedman MJ. National estimates of exposure to traumatic events and PTSD prevalence using DSM-IV and DSM-5 criteria. J Trauma Stress. 2013;26(5):537-547.
2. Lehavot K, Goldberg SB, Chen JA, et al. Do trauma type, stressful life events, and social support explain women veterans’ high prevalence of PTSD? Soc Psychiatry Psychiatr Epidemiol. 2018;53(9):943-953.
3. Galovski T, Lyons JA. Psychological sequelae of combat violence: a review of the impact of PTSD on the veteran’s family and possible interventions. Aggress Violent Behav. 2004;9(5):477-501.
4. Ray SL, Vanstone M. The impact of PTSD on veterans’ family relationships: an interpretative phenomenological inquiry. Int J Nurs Stud. 2009;46(6):838-847.
5. Cloitre M, Miranda R, Stovall-McClough KC, Han H. Beyond PTSD: emotion regulation and interpersonal problems as predictors of functional impairment in survivors of childhood abuse. Behav Ther. 2005;36(2):119-124.
6. McFarlane AC, Bookless C. The effect of PTSD on interpersonal relationships: issues for emergency service works. Sex Relation Ther. 2001;16(3):261-267.
7. Itzhaky L, Stein JY, Levin Y, Solomon Z. Posttraumatic stress symptoms and marital adjustment among Israeli combat veterans: the role of loneliness and attachment. Psychol Trauma. 2017;9(6):655-662.
8. Dekel R, Monson CM. Military-related post-traumatic stress disorder and family relations: current knowledge and future directions. Aggress Violent Behav. 2010;15(4):303-309.
9. Allen ES, Rhoades GK, Stanley SM, Markman HJ. Hitting home: relationships between recent deployment, posttraumatic stress symptoms, and marital functioning for Army couples. J Fam Psychol. 2010;24(3):280-288.
10. Laffaye C, Cavella S, Drescher K, Rosen C. Relationships among PTSD symptoms, social support, and support source in veterans with chronic PTSD. J Trauma Stress. 2008;21(4):394-401.
11. Meis LA, Noorbaloochi S, Hagel Campbell EM, et al. Sticking it out in trauma-focused treatment for PTSD: it takes a village. J Consult Clin Psychol. 2019;87(3):246-256.
12. Lewis JM, Gossett JT, Housson MM, Owen MT. Timberlawn Couple and Family Evaluation Scales. Dallas, TX: Timberlawn Psychiatric Research Foundation; 1999.
13. Fincham FD, Linfield KJ. A new look at marital quality: can spouses feel positive and negative about their marriage? J Fam Psychol. 1997;11(4):489-502.
14. Kaplan KJ. On the ambivalence-indifference problem in attitude theory and measurement: a suggested modification of the semantic differential technique. Psychol Bull. 1972;77(5):361-372.
15. Buhrmester D, Furman W. The Network of Relationship Inventory: Relationship Qualities Version [unpublished measure]. University of Texas at Dallas; 2008.
16. Busby DM, Christensen C, Crane DR, Larson JH. A revision of the Dyadic Adjustment Scale for use with distressed and nondistressed couples: construct hierarchy and multidimensional scales. J Marital Fam Ther. 1995;21(3):289-308.
17. Davis MH. A multidimensional approach to individual differences in empathy. JSAS Catalog Sel Doc Psychol. 1980;10:85.
18. Fraley RC, Waller NG, Brennan KA. An item-response theory analysis of self-report measures of adult attachment. J Pers Soc Psychol. 2000;78(2):350-365.
19. Tabachnick BG, Fidell L. Using Multivariate Statistics. 6th ed. Boston, MA: Pearson; 2013.
20. Sautter FJ, Armelie AP, Glynn SM, Wielt DB. The development of a couple-based treatment for PTSD in returning veterans. Prof Psychol Res Pr. 2011;42(1):63-69.
21. Jacobson NS, Christensen A, Prince SE, Cordova J, Eldridge K. Integrative behavioral couple therapy: an acceptance-based, promising new treatment of couple discord. J Consult Clin Psychol. 2000;9(2):351-355.
22. Makin-Byrd K, Gifford E, McCutcheon S, Glynn S. Family and couples treatment for newly returning veterans. Prof Psychol Res Pr. 2011;42(1):47-55.
23. Peterson K, Anderson J, Bourne D. Evidence Brief: Use of Patient Reported Outcome Measures for Measurement Based Care in Mental Health Shared Decision Making. Washington, DC: Department of Veterans Affairs; 2018. https://www.ncbi.nlm.nih.gov/books/NBK536143. Accessed September 13, 2019.
24. Fortney JC, Unützer J, Wrenn G, et al. A tipping point for measurement-based care. Psychiatr Serv. 2017;68(2):179-188.
25. Costello AB, Osborne JW. Best practices in exploratory factor analysis: four recommendations for getting the most from your analysis. Pract Assess Res Eval. 2005;10(7):1-9.