User login
Primary care for the declining cancer survivor
As a family physician (FP), you are well positioned to optimize the quality of life of advanced cancer patients as they decline and approach death. You can help them understand their evolving prognosis so that treatment goals can be adjusted, and you can ensure that hospice is implemented early to improve the end-of-life experience. This practical review will help you to provide the best care possible for these patients.
Family physicians can fill a care gap
The term cancer survivor describes a patient who has completed initial cancer treatment. Within this population, many have declining health and ultimately succumb to their disease. There were 16.9 million cancer survivors in the United States as of January 1, 2019,1 with 53% likely to experience significant symptoms and disability.2 More than 600,000 American cancer survivors will die in 2019.3
In 2011, the Commission on Cancer mandated available outpatient palliative care services at certified cancer centers.4 Unfortunately, current palliative care resources fall far short of expected needs. A 2010 estimate of required hospice and palliative care physicians demonstrated a staffing gap of more than 50% among those providing outpatient services.5 The shortage continues,6 and many cancer patients will look to their FP for supportive care.
FPs, in addition to easing symptoms and adverse effects of medication, can educate patients and families about their disease and prognosis. By providing longitudinal care, FPs can identify critical health declines that oncologists, patients, and families often overlook. FPs can also readily appreciate decline, guide patients toward their care goals, and facilitate comfort care—including at the end of life.
Early outpatient palliative care improves quality of life and patient satisfaction. It also may improve survival time and ward off depression.7,8 Some patients and providers resist palliative care due to a misconception that it requires abandoning treatment.9 Actually, palliative care can be given in concert with all active treatments. Many experts recommend a name change from “palliative care” to “supportive care” to dispel this misconception.10
Estimate prognosis using the “surprise question”
Several algorithms are available—using between 2 and 13 patient parameters—to estimate advanced cancer survival. Most of these algorithms are designed to identify the last months or weeks of life, but their utility to predict death within these periods is limited.11
The “surprise question” may be the most valuable prognostic test for primary care. In this test, the physician asks him- or herself: Would I be surprised if this patient died in 1 year? Researchers found that when primary care physicians answered No, their patient was 4 times more likely to die within the year than when they answered Yes.12 This test has a positive predictive value of 20% and a negative predictive value of 95%, making it valuable in distinguishing patients with longer life expectancy.12 Although it overidentifies at-risk patients, the "surprise question" is a simple and sensitive tool for defining prognosis.
Continue to: Priorities for patients likely to live more than a year
Priorities for patients likely to live more than a year
For patients who likely have more than a year to live, the focus is on symptom management and preparation for future decline. Initiate and facilitate discussions about end-of-life topics. Cancer survivors are often open to discussions on these topics, which include advanced directives, home health aides, and hospice.13 Patients can set specific goals for their remaining time, such as engaging in travel, personal projects, or special events. Cancer patients have better end-of-life experiences and families have improved mental health after these discussions.14 Although cancer patients are more likely than other terminal patients to have end-of-life discussions, fewer than 40% ever do.15
Address distressing symptoms with a focus on maintaining function. More than 50% of advanced cancer patients experience fatigue, weakness, pain, weight loss, and anorexia,16 and up to 60% experience psychological distress.17 Deprescribing most preventive medications is recommended with transition to symptomatic treatment.18
Priorities for patients with less than a year to live
For patients who may have less than a year to live, focus shifts to their wishes for the time remaining and priorities for the dying process. Most patients start out with prognostic views more optimistic than those of their physicians, but this gap narrows after end-of-life discussions.19,20 Patients with incurable cancer are less likely to choose aggressive therapy if they believe their 6-month survival probability is less than 90%.21 Honest conversations, with best- and worst-case scenarios, are important to patients and families, and should occur while the patient is well enough to participate and set goals.22
In the last months of life, opioids become the primary treatment for pain and air hunger. As function declines, concerns about such adverse effects as falls and confusion decrease. Opioids have been shown to be most effective over the course of 4 weeks, and avoiding their use in earlier stages may increase their efficacy at the end of life.23
Hospice benefit—more comfort, with limitations
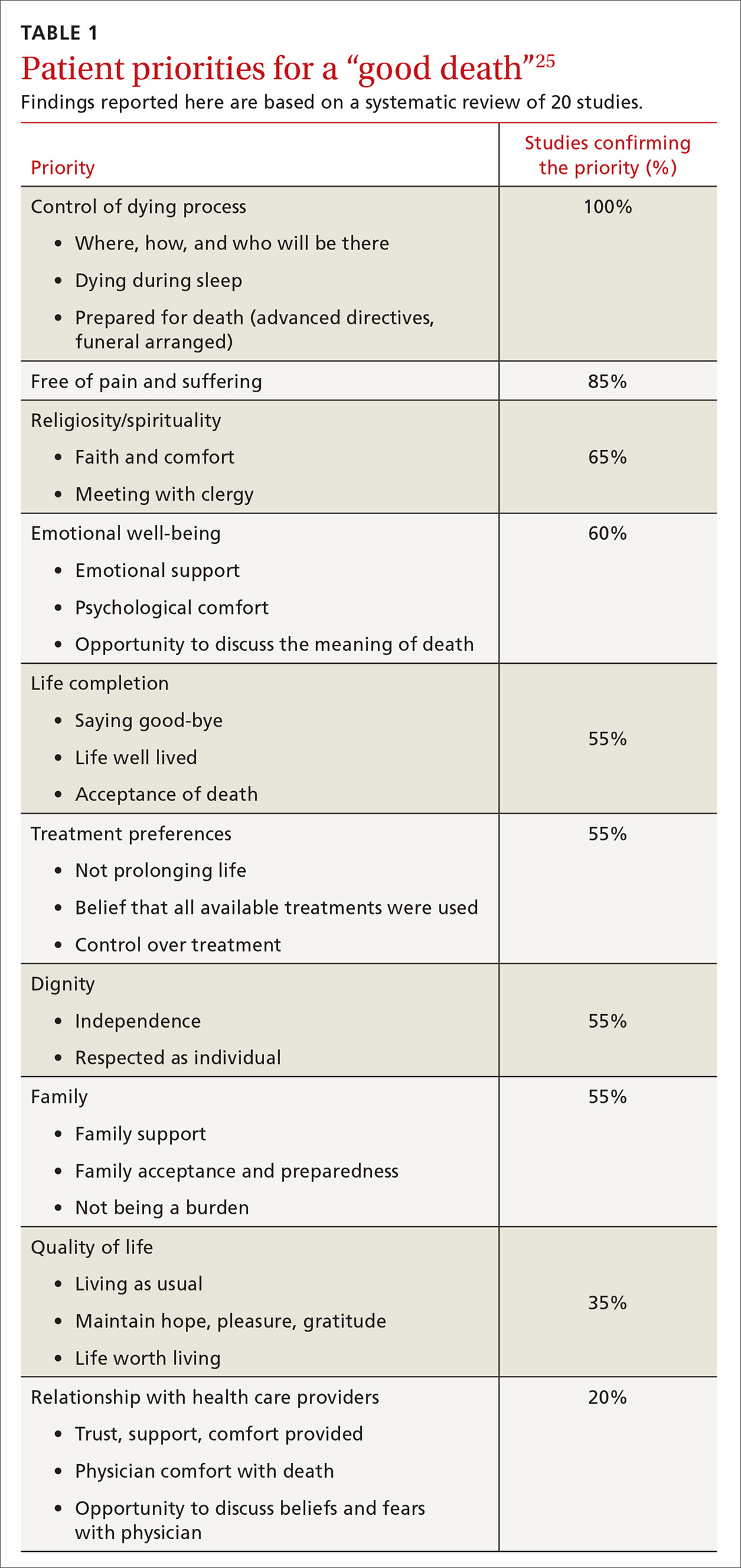
Hospice care consists of services administered by nonprofit and for-profit entities covered by Medicare, Medicaid, and many private insurers.24 Hospice strives to allow patients to approach death in comfort, meeting their goal of a “good death.” A recent literature review identified 4 aspects of a good death that terminally ill patients and their families considered most important: control of the dying process, relief of pain, spirituality, and emotional well-being (TABLE 1).25
Continue to: Hospice use is increasing...
Hospice use is increasing, yet many enroll too late to fully benefit. While cancer patients alone are not currently tracked, the use of hospice by Medicare beneficiaries increased from 44% in 2012 to 48% in 2019.24 In 2017, the median hospice stay was 19 days.24 Unfortunately, though, just 28% of hospice-eligible patients enrolled in hospice in their last week of life.24 Without hospice, patients often receive excessive care near death. More than 6% receive aggressive chemotherapy in their last 2 weeks of life, and nearly 10% receive a life-prolonging procedure in their last month.26
Hospice care replaces standard hospital care, although patients can elect to be followed by their primary care physician.9 Most hospice services are provided as needed or continuously at the patient’s home, including assisted living facilities. And it is also offered as part of hospital care. Hospice services are interdisciplinary, provided by physicians, nurses, social workers, chaplains, and health aides. Hospices have on-call staff to assess and treat complications, avoiding emergency hospital visits.9 And hospice includes up to 5 days respite care for family caregivers, although with a 5% copay.9 Most hospice entities run inpatient facilities for care that cannot be effectively provided at home.
Hospice care has limitations—many set by insurance. Medicare, for example, stipulates that a primary care or hospice physician must certify the patient has a reasonable prognosis of 6 months or less and is expected to have a declining course.27 Patients who survive longer than 6 months are recertified by the same criteria every 60 days.27
Hospice patients forgo treatments aimed at curing their terminal diagnosis.28 Some hospice entities allow noncurative therapies while others do not. Hospice covers prescription medications for symptom control only, although patients can receive care unrelated to the terminal diagnosis under regular benefits.28 Hospice care practices differ from standard care in ways that may surprise patients and families (TABLE 227,28). Patients can disenroll and re-enroll in hospice as they wish.28

Symptom control in advanced cancer
General symptoms
Pain affects 64% of patients with advanced cancer.29 Evidence shows that cancer pain is often undertreated, with a recent systematic review reporting undertreated pain in 32% of patients.30 State and national chronic opioid guidelines do not restrict use for cancer pain.31 Opioids are effective in 75% of cancer patients over 1 month, but there is no evidence of benefit after this period.23 In fact, increasing evidence demonstrates that pain is likely negatively responsive to opioids over longer periods.32 Opioid adverse effects can worsen other cancer symptoms, including depression, anxiety, fatigue, constipation, hypogonadism, and cognitive dysfunction.32 Delaying opioid therapy to end of life can limit adverse effects and may preserve pain-control efficacy for the dying process.
Continue to: Most cancer pain...
Most cancer pain is partially neuropathic, so anticonvulsant and antidepressant medications can help.33 Gabapentin, pregabalin, and duloxetine are recommended based on evidence not restricted to cancer.34 Cannabinoids have been evaluated in 2 trials of cancer pain with 440 patients and showed a borderline significant reduction of pain.35
Palliative radiation therapy can sometimes reduce pain. Bone metastases pain has been studied the most, and the literature suggests that palliative radiation provides improvement for 60% of patients and complete relief to 25% of patients.36 Palliative thoracic radiotherapy for primary or metastatic lung masses reduces pain by more than 70% while improving dyspnea, hemoptysis, and cough in a majority of patients.36
Other uses of palliative radiation have varied evidence. Palliative chemotherapy has less evidence of benefit. In a recent multicenter cohort trial, chemotherapy in end-stage cancer reduced quality of life in patients with good functional status, without affecting quality of life when function was limited.37 Palliative chemotherapy may be beneficial if combined with corticosteroids or radiation therapy.38
Treatment in the last weeks of life centers on opioids; dose increases do not shorten survival.39 Cancer patients are 4 times as likely as noncancer patients to have severe or excruciating pain during the last 3 days of life.40 Narcotics can be titrated aggressively near end of life with less concern for hypotension, respiratory depression, or level of consciousness. Palliative sedation remains an option for uncontrolled pain.41
Anorexia is only a problem if quality of life is affected. Cachexia is caused by increases in cytokines more than reduced calorie intake.42 Reversible causes of reduced eating may be found, including candidiasis, dental problems, depression, or constipation. Megestrol acetate improves weight (number needed to treat = 12), although it significantly increases mortality (number needed to harm = 23), making its use controversial.43 Limited study of cannabinoids has not shown effectiveness in treating anorexia.35
Continue to: Constipation...
Constipation in advanced cancer is often related to opioid therapy, although bowel obstruction must be considered. Opioid-induced constipation affects 40% to 90% of patients on long-term treatment,44 and 5 days of opioid treatment nearly doubles gastrointestinal transit time.45 Opioid-induced constipation can be treated by adding a stimulating laxative followed by a peripheral acting μ-opioid receptor antagonist, such as subcutaneous methylnaltrexone or oral naloxegol.46 These medications are contraindicated if ileus or bowel obstruction is suspected.46
Nausea and vomiting are common in advanced cancer and have numerous causes. Approximately half of reversible causes are medication adverse effects from either chemotherapy or pain medication.47 Opioid rotation may improve symptoms.47 A suspected bowel obstruction should be evaluated by specialists; surgery, palliative chemotherapy, radiation therapy, or stenting may be required. Oncologists can best manage adverse effects of chemotherapy. For nausea and vomiting unrelated to chemotherapy, consider treating constipation and pain. Medication can also be helpful; a systemic review suggests metoclopramide works best, with some evidence supporting other dopaminergic agonists, including haloperidol.47
Fatigue. Both methylphenidate and modafinil have been studied to treat cancer-related fatigue.48 A majority of patients treated with methylphenidate reported less cancer-related fatigue at 4 weeks and wished to continue treatment.49 Modafinil demonstrated minimal improvement in fatigue.50 Sleep disorders, often due to anxiety or sleep apnea, may be a correctable cause.
Later symptoms
Delirium occurs in up to 90% of cancer patients near the end of life, and can signal death.51 Up to half of the delirium seen in palliative care is reversible.51 Reversible causes include uncontrolled pain, medication adverse effects, and urinary and fecal retention (TABLE 348,51). Addressing these factors reduces delirium, based on studies in postoperative patients.52 Consider opioid rotation if neurotoxicity is suspected.51
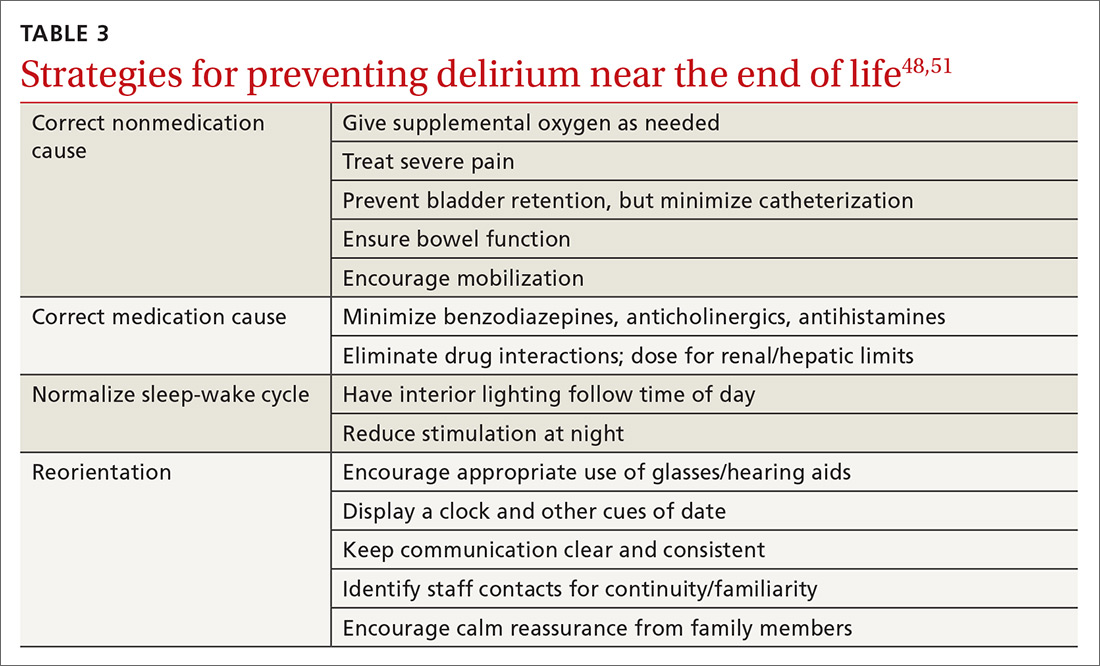
Delirium can be accompanied by agitation or decreased responsiveness.53 Agitated delirium commonly presents with moaning, facial grimacing, and purposeless repetitive movements, such as plucking bedsheets or removing clothes.51 Delirious patients without agitation have reported, following recovery, distress similar to that experienced by agitated patients.54 Caregivers are most likely to recognize delirium and often become upset. Educating family members about the frequency of delirium can lessen this distress.54
Continue to: Delirium can be treated with...
Delirium can be treated with antipsychotics; haloperidol has been most frequently studied.54 Antipsychotics are effective at reducing agitation but not at restoring cognition.55 Case reports suggest that use of atypical antipsychotics can be beneficial if adverse effects limit haloperidol dosing.56 Agitated delirium is the most frequent indication for palliative sedation.57
Dyspnea. In the last weeks, days, or hours of life, dyspnea is common and often distressing. Dyspnea appears to be multifactorial, worsened by poor control of secretions, airway hyperactivity, and lung pathologies.58 Intravenous hydration may unintentionally exacerbate dyspnea. Hospice providers generally discourage intravenous hydration because relative dehydration reduces terminal respiratory secretions (“death rattle”) and increases patient comfort.59
Some simple nonpharmacologic interventions have benefit. Oxygen is commonly employed, although multiple studies show no benefit over room air.59 Directing a handheld fan at the face does reduce dyspnea, likely by activation of the maxillary branch of the trigeminal nerve.60
Opioids effectively treat dyspnea near the end of life with oral and parenteral dosing, but the evidence does not support nebulized opioids.61 Opioid doses required to treat dyspnea are less than those for pain and do not cause significant respiratory depression.62 If a patient taking opioids experiences dyspnea, a 25% dose increase is recommended.63
Anticholinergic medications can improve excessive airway secretions associated with dyspnea. Glycopyrrolate causes less delirium because it does not cross the blood-brain barrier, while scopolamine patches have reduced anticholinergic adverse effects, but effects are delayed until 12 hours after patch placement.64 Atropine eye drops given sublingually were effective in a small study.65
Continue to: Palliative sedation
Palliative sedation
Palliative sedation can manage intractable symptoms near the end of life. A recent systematic review suggests that palliative sedation does not shorten life.57 Sedation is most often initiated by gradual increases in medication doses.57 Midazolam is most often employed, but antipsychotics are also used.57
CORRESPONDENCE
CDR Michael J. Arnold, MD, Uniformed Services University of the Health Sciences, 4501 Jones Bridge Road, Bethesda, MD 20814; [email protected].
ACKNOWLEDGEMENT
Kristian Sanchack, MD, and James Higgins, DO, assisted in the preparation of this manuscript.
1. American Cancer Society. Cancer Treatment & Survivorship Facts & Figures 2019-2021. www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-statistics/cancer-treatment-and-survivorship-facts-and-figures/cancer-treatment-and-survivorship-facts-and-figures-2019-2021.pdf. Accessed September 4, 2019.
2. Stein KD, Syrjala KL, Andrykowski MA. Physical and psychological long-term and late effects of cancer. Cancer. 2008;112(11 suppl):2577-2592.
3. National Comprehensive Cancer Network. NCCN Guidelines Version 2. 2019. Palliative Care. www.nccn.org/professionals/physician_gls/pdf/palliative.pdf. (Must register an account for access.) Accessed September 4, 2019.
4. American Cancer Society. New CoC accreditation standards gain strong support. www.facs.org/media/press-releases/2011/coc-standards0811. Accessed September 11, 2019.
5. Lupu D; American Academy of Hospice and Palliative Medicine Workforce Task Force. Estimate of current hospice and palliative medicine physician workforce shortage. J Pain Symptom Manage. 2010;40:899-911.
6. Lupu D, Quigley L, Mehfoud N, et al. The growing demand for hospice and palliative medicine physicians: will the supply keep up? J Pain Symptom Manage. 2018;55:1216-1223.
7. Rabow MW, Dahlin C, Calton B, et al. New frontiers in outpatient palliative care for patients with cancer. Cancer Control. 2015;22:465-474.
8. Haun MW, Estel S, Rücker G, et al. Early palliative care for adults with advanced cancer. Cochrane Database of Syst Rev. 2017:CD01129.
9. Buss MK, Rock LK, McCarthy EP. Understanding palliative care and hospice: a review for primary care providers. Mayo Clin Proc. 2017;92:280-286.
10. Hui D. Definition of supportive care: does the semantic matter? Curr Opin Oncol. 2014;26:372-379.
11. Simmons CPL, McMillan DC, McWilliams K, et al. Prognostic tools in patients with advanced cancer: a systematic review. J Pain Symptom Manage. 2017;53:962-970.
12. Lakin JR, Robinson MG, Bernacki RE, et al. Estimating 1-year mortality for high-risk primary care patients using the “surprise” question. JAMA Int Med. 2016;176:1863-1865.
13. Walczak A, Henselmans I, Tattersall MH, et al. A qualitative analysis of responses to a question prompt list and prognosis and end-of-life care discussion prompts delivered in a communication support program. Psychoonchology. 2015;24:287-293.
14. Yamaguchi T, Maeda I, Hatano Y, et al. Effects of end-of-life discussions on the mental health of bereaved family members and quality of patient death and care. J Pain Symptom Manage. 2017;54:17-26.
15. Wright AA, Zhang B, Ray A, et al. Associations between end-of-life discussions, patient mental health, medical care near death, caregiver bereavement adjustment. JAMA. 2008;300:1665-1673.
16. Teunissen SC, Wesker W, Kruitwagen C, et al. Symptom prevalence in patients with incurable cancer: a systematic review. J Pain Symptom Manage. 2007;34:94-104.
17. Gao W, Bennett MI, Stark D, et al. Psychological distress in cancer from survivorship to end of life: prevalence, associated factors and clinical implications. Eur J Cancer. 2010;46:2036-2044.
18. Scott IA, Gray LC, Martin JH, et al. Deciding when to stop: towards evidence-based deprescribing of drugs in older populations. Evid Based Med. 2013;18:121-124.
19. Gramling R, Fiscella K, Xing G, et al. Determinants of patient-oncologist prognostic discordance in advanced cancer. JAMA Oncol. 2016;2:1421-1426.
20. Epstein AS, Prigerson HG, O’Reilly EM, et al. Discussions of life expectancy and changes in illness understanding in patients with advanced cancer. J Clin Oncol. 2016;34:2398-2403.
21. Weeks JC, Cook EF, O’Day SJ, et al. Relationship between cancer patients’ predictions of prognosis and their treatment preferences. JAMA. 1998;279:1709-1714.
22. Myers J. Improving the quality of end-of-life discussions. Curr Opin Support Palliat Care. 2015;9:72-76.
23. Corli O, Floriani I, Roberto A, et al. Are strong opioids equally effective and safe in the treatment of chronic cancer pain? A multicenter randomized phase IV ‘real life’ trial on the variability of response to opioids. Ann Oncolog. 2016;27:1107-1115.
24. National Hospice and Palliative Care Organization. NHPCO Facts and Figures. 2018. www.nhpco.org/wp-content/uploads/2019/07/2018_NHPCO_Facts_Figures.pdf. Accessed September 24, 2019.
25. Meier EA, Gallegos JV, Thomas LP, et al. Defining a good death (successful dying): literature review and a call for research and public dialogue. Am J Geriatr Psychiatry. 2016;24:261-271.
26. Morden NE, Chang CH, Jacobson JO, et al. End-of-life care for Medicare beneficiaries with cancer is highly intensive overall and varies widely. Health Aff (Millwood). 2012;31:786-796.
27. Centers for Medicare & Medicaid Services. Medicare Hospice Benefit Facts. www.cgsmedicare.com/hhh/education/materials/pdf/Medicare_Hospice_Benefit_Facts.pdf. Accessed September 11, 2019.
28. Centers for Medicare & Medicaid Services. Medicare Hospice Benefits. www.medicare.gov/pubs/pdf/02154-medicare-hospice-benefits.pdf. Accessed September 11, 2019.
29. van den Beuken-van Everdingen MH, de Rijke JM, Kessels AG, et al. Prevalence of pain in patients with cancer: a systematic review of the past 40 years. Ann Oncol. 2007;18:1437-1449.
30. Greco MT, Roberto A, Corli O, et al. Quality of cancer pain management: an update of a systematic review of undertreatment of patients with cancer. J Clin Oncol. 2014;32:4149-4154.
31. Dowell D, Haegerich TM, Chou R. CDC guideline for prescribing opioids for chronic pain — United States, 2016. MMWR Recomm Rep. 2016;65:1-49.
32. Davis MP, Mehta Z. Opioids and chronic pain: where is the balance? Curr Oncol Rep. 2016;18:71.
33. Leppert W, Zajaczkowska R, Wordliczek J, et al. Pathophysiology and clinical characteristics of pain in most common locations in cancer patients. J Physiol Pharmacol. 2016;67:787-799.
34. Finnerup NB, Attal N, Haroutounian S, et al. Pharmacotherapy for neuropathic pain in adults: a systematic review and meta-analysis. Lancet Neurol. 2015;14:162-173.
35. Whiting PF, Wolff RF, Deshpande S, et al. Cannabinoids for medical use: a systematic review and meta-analysis. JAMA. 2015;313:2456-2473.
36. Jones JA, Lutz ST, Chow E. et al. Palliative radiotherapy at the end of life: a critical review. CA Cancer J Clin. 2014;64:296-310.
37. Prigerson HG, Bao Y, Shah MA, et al. Chemotherapy use, performance status, and quality of life at the end of life. JAMA Oncol. 2015;1:778-784.
38. Kongsgaard U, Kaasa S, Dale O, et al. Palliative treatment of cancer-related pain. 2005. www.ncbi.nlm.nih.gov/books/NBK464794/. Accessed September 24, 2019.
39. Sathornviriyapong A, Nagaviroj K, Anothaisintawee T. The association between different opioid doses and the survival of advanced cancer patients receiving palliative care. BMC Palliat Care. 2016;15:95.
40. Steindal SA, Bredal IS. Sørbye LW, et al. Pain control at the end of life: a comparative study of hospitalized cancer and noncancer patients. Scand J Caring Sci. 2011;25:771-779.
41. Maltoni M, Setola E. Palliative sedation in patients with cancer. Cancer Control. 2015;22:433-441.
42. Cooper C, Burden ST, Cheng H, et al. Understanding and managing cancer-related weight loss and anorexia: insights from a systematic review of qualitative research. J Cachexia Sarcopenia Muscle. 2015;6:99-111.
43. Ruiz Garcia V, LÓpez-Briz E, Carbonell Sanchis R, et al. Megesterol acetate for treatment of anorexia-cachexia syndrome. Cochrane Database Syst Rev. 2013;28:CD004310.
44. Chey WD, Webster L, Sostek M, et al. Naloxegol for opioid-induced constipation in patients with noncancer pain. N Engl J Med. 2014;370:2387-2396.
45. Poulsen JL, Nilsson M, Brock C, et al. The impact of opioid treatment on regional gastrointestinal transit. J Neurogastroenterol Motil. 2016;22:282-291.
46. Pergolizzi JV, Raffa RB, Pappagallo M, et al. Peripherally acting μ-opioid receptor antagonists as treatment options for constipation in noncancer pain patients on chronic opioid therapy. Patient Prefer Adherence. 2017;11:107-119.
47. Walsh D, Davis M, Ripamonti C, et al. 2016 updated MASCC/ESMO consensus recommendations: management of nausea and vomiting in advanced cancer. Support Care Cancer. 2017;25:333-340.
48. Mücke M, Mochamat, Cuhls H, et al. Pharmacological treatments for fatigue associated with palliative care. Cochrane Database Syst Rev. 2015(5):CD006788.
49. Escalante CP, Meyers C, Reuben JM, et al. A randomized, double-blind, 2-period, placebo-controlled crossover trial of a sustained-release methylphenidate in the treatment of fatigue in cancer patients. Cancer J. 2014;20:8-14.
50. Hovey E, de Souza P, Marx G, et al. Phase III, randomized, double-blind, placebo-controlled study of modafinil for fatigue in patients treated with docetaxel-based chemotherapy. Support Care Cancer. 2014;22:1233-1242.
51. Hosker CM, Bennett MI. Delirium and agitation at the end of life. BMJ. 2016;353:i3085.
52. Mercantonio ER, Flacker JM, Wright RJ, et al. Reducing delirium after hip fracture: a randomized trial. J Am Geriatr Soc. 2001;49:516-522.
53. Casarett DJ, Inouye SK. Diagnosis and management of delirium near the end of life. Ann Int Med. 2001;135:32-40.
54. Breitbart W, Alici Y. Agitation and delirium at the end of life: “We couldn’t manage him." JAMA. 2008;300:2898-2910.
55. Candy B, Jackson KC, Jones L, et al. Drug therapy for delirium in terminally ill patients. Cochrane Database Syst Rev. 2012;11:CD004770.
56. Bascom PB, Bordley JL, Lawton AJ. High-dose neuroleptics and neuroleptic rotation for agitated delirium near the end of life. Am J Hosp Palliat Med. 2014;31:808-811.
57. Maltoni M, Scarpi E, Rosati M, et al. Palliative sedation in end-of-life care and survival: a systematic review. J Clin Oncol. 2012;30:1378-1383.
58. Albert RH. End-of-life care: managing common symptoms. Am Fam Physician. 2017;95:356-361.
59. Arenella C. Artificial nutrition and hydration at the end of life: beneficial or harmful? https://americanhospice.org/caregiving/artificial-nutrition-and-hydration-at-the-end-of-life-beneficial-or-harmful/ Accessed September 11, 2019.
60. Booth S, Moffat C, Burkin J, et al. Nonpharmacological interventions for breathlessness. Curr Opinion Support Pall Care. 2011;5:77-86.
61. Barnes H, McDonald J, Smallwood N, et al. Opioids for the palliation of refractory breathlessness in adults with advanced disease and terminal illness. Cochrane Database Syst Rev. 2016(3)CD011008.
62. Lim RB. End-of-life care in patients with advanced lung cancer. Ther Adv Resp Dis. 2016;10:455-467.
63. Kreher M. Symptom control at the end of life. Med Clin North Am. 2016;100:1111-1122.
64. Baralatei FT, Ackerman RJ. Care of patients at the end of life: management of nonpain symptoms. FP Essent. 2016;447:18-24.
65. Protus BM, Grauer PA, Kimbrel JM. Evaluation of atropine 1% ophthalmic solution administered sublingual for the management of terminal respiratory secretions. Am J Hosp Palliat Med. 2013;30:388-392.
As a family physician (FP), you are well positioned to optimize the quality of life of advanced cancer patients as they decline and approach death. You can help them understand their evolving prognosis so that treatment goals can be adjusted, and you can ensure that hospice is implemented early to improve the end-of-life experience. This practical review will help you to provide the best care possible for these patients.
Family physicians can fill a care gap
The term cancer survivor describes a patient who has completed initial cancer treatment. Within this population, many have declining health and ultimately succumb to their disease. There were 16.9 million cancer survivors in the United States as of January 1, 2019,1 with 53% likely to experience significant symptoms and disability.2 More than 600,000 American cancer survivors will die in 2019.3
In 2011, the Commission on Cancer mandated available outpatient palliative care services at certified cancer centers.4 Unfortunately, current palliative care resources fall far short of expected needs. A 2010 estimate of required hospice and palliative care physicians demonstrated a staffing gap of more than 50% among those providing outpatient services.5 The shortage continues,6 and many cancer patients will look to their FP for supportive care.
FPs, in addition to easing symptoms and adverse effects of medication, can educate patients and families about their disease and prognosis. By providing longitudinal care, FPs can identify critical health declines that oncologists, patients, and families often overlook. FPs can also readily appreciate decline, guide patients toward their care goals, and facilitate comfort care—including at the end of life.
Early outpatient palliative care improves quality of life and patient satisfaction. It also may improve survival time and ward off depression.7,8 Some patients and providers resist palliative care due to a misconception that it requires abandoning treatment.9 Actually, palliative care can be given in concert with all active treatments. Many experts recommend a name change from “palliative care” to “supportive care” to dispel this misconception.10
Estimate prognosis using the “surprise question”
Several algorithms are available—using between 2 and 13 patient parameters—to estimate advanced cancer survival. Most of these algorithms are designed to identify the last months or weeks of life, but their utility to predict death within these periods is limited.11
The “surprise question” may be the most valuable prognostic test for primary care. In this test, the physician asks him- or herself: Would I be surprised if this patient died in 1 year? Researchers found that when primary care physicians answered No, their patient was 4 times more likely to die within the year than when they answered Yes.12 This test has a positive predictive value of 20% and a negative predictive value of 95%, making it valuable in distinguishing patients with longer life expectancy.12 Although it overidentifies at-risk patients, the "surprise question" is a simple and sensitive tool for defining prognosis.
Continue to: Priorities for patients likely to live more than a year
Priorities for patients likely to live more than a year
For patients who likely have more than a year to live, the focus is on symptom management and preparation for future decline. Initiate and facilitate discussions about end-of-life topics. Cancer survivors are often open to discussions on these topics, which include advanced directives, home health aides, and hospice.13 Patients can set specific goals for their remaining time, such as engaging in travel, personal projects, or special events. Cancer patients have better end-of-life experiences and families have improved mental health after these discussions.14 Although cancer patients are more likely than other terminal patients to have end-of-life discussions, fewer than 40% ever do.15
Address distressing symptoms with a focus on maintaining function. More than 50% of advanced cancer patients experience fatigue, weakness, pain, weight loss, and anorexia,16 and up to 60% experience psychological distress.17 Deprescribing most preventive medications is recommended with transition to symptomatic treatment.18
Priorities for patients with less than a year to live
For patients who may have less than a year to live, focus shifts to their wishes for the time remaining and priorities for the dying process. Most patients start out with prognostic views more optimistic than those of their physicians, but this gap narrows after end-of-life discussions.19,20 Patients with incurable cancer are less likely to choose aggressive therapy if they believe their 6-month survival probability is less than 90%.21 Honest conversations, with best- and worst-case scenarios, are important to patients and families, and should occur while the patient is well enough to participate and set goals.22
In the last months of life, opioids become the primary treatment for pain and air hunger. As function declines, concerns about such adverse effects as falls and confusion decrease. Opioids have been shown to be most effective over the course of 4 weeks, and avoiding their use in earlier stages may increase their efficacy at the end of life.23
Hospice benefit—more comfort, with limitations
Hospice care consists of services administered by nonprofit and for-profit entities covered by Medicare, Medicaid, and many private insurers.24 Hospice strives to allow patients to approach death in comfort, meeting their goal of a “good death.” A recent literature review identified 4 aspects of a good death that terminally ill patients and their families considered most important: control of the dying process, relief of pain, spirituality, and emotional well-being (TABLE 1).25

Continue to: Hospice use is increasing...
Hospice use is increasing, yet many enroll too late to fully benefit. While cancer patients alone are not currently tracked, the use of hospice by Medicare beneficiaries increased from 44% in 2012 to 48% in 2019.24 In 2017, the median hospice stay was 19 days.24 Unfortunately, though, just 28% of hospice-eligible patients enrolled in hospice in their last week of life.24 Without hospice, patients often receive excessive care near death. More than 6% receive aggressive chemotherapy in their last 2 weeks of life, and nearly 10% receive a life-prolonging procedure in their last month.26
Hospice care replaces standard hospital care, although patients can elect to be followed by their primary care physician.9 Most hospice services are provided as needed or continuously at the patient’s home, including assisted living facilities. And it is also offered as part of hospital care. Hospice services are interdisciplinary, provided by physicians, nurses, social workers, chaplains, and health aides. Hospices have on-call staff to assess and treat complications, avoiding emergency hospital visits.9 And hospice includes up to 5 days respite care for family caregivers, although with a 5% copay.9 Most hospice entities run inpatient facilities for care that cannot be effectively provided at home.
Hospice care has limitations—many set by insurance. Medicare, for example, stipulates that a primary care or hospice physician must certify the patient has a reasonable prognosis of 6 months or less and is expected to have a declining course.27 Patients who survive longer than 6 months are recertified by the same criteria every 60 days.27
Hospice patients forgo treatments aimed at curing their terminal diagnosis.28 Some hospice entities allow noncurative therapies while others do not. Hospice covers prescription medications for symptom control only, although patients can receive care unrelated to the terminal diagnosis under regular benefits.28 Hospice care practices differ from standard care in ways that may surprise patients and families (TABLE 227,28). Patients can disenroll and re-enroll in hospice as they wish.28

Symptom control in advanced cancer
General symptoms
Pain affects 64% of patients with advanced cancer.29 Evidence shows that cancer pain is often undertreated, with a recent systematic review reporting undertreated pain in 32% of patients.30 State and national chronic opioid guidelines do not restrict use for cancer pain.31 Opioids are effective in 75% of cancer patients over 1 month, but there is no evidence of benefit after this period.23 In fact, increasing evidence demonstrates that pain is likely negatively responsive to opioids over longer periods.32 Opioid adverse effects can worsen other cancer symptoms, including depression, anxiety, fatigue, constipation, hypogonadism, and cognitive dysfunction.32 Delaying opioid therapy to end of life can limit adverse effects and may preserve pain-control efficacy for the dying process.
Continue to: Most cancer pain...
Most cancer pain is partially neuropathic, so anticonvulsant and antidepressant medications can help.33 Gabapentin, pregabalin, and duloxetine are recommended based on evidence not restricted to cancer.34 Cannabinoids have been evaluated in 2 trials of cancer pain with 440 patients and showed a borderline significant reduction of pain.35
Palliative radiation therapy can sometimes reduce pain. Bone metastases pain has been studied the most, and the literature suggests that palliative radiation provides improvement for 60% of patients and complete relief to 25% of patients.36 Palliative thoracic radiotherapy for primary or metastatic lung masses reduces pain by more than 70% while improving dyspnea, hemoptysis, and cough in a majority of patients.36
Other uses of palliative radiation have varied evidence. Palliative chemotherapy has less evidence of benefit. In a recent multicenter cohort trial, chemotherapy in end-stage cancer reduced quality of life in patients with good functional status, without affecting quality of life when function was limited.37 Palliative chemotherapy may be beneficial if combined with corticosteroids or radiation therapy.38
Treatment in the last weeks of life centers on opioids; dose increases do not shorten survival.39 Cancer patients are 4 times as likely as noncancer patients to have severe or excruciating pain during the last 3 days of life.40 Narcotics can be titrated aggressively near end of life with less concern for hypotension, respiratory depression, or level of consciousness. Palliative sedation remains an option for uncontrolled pain.41
Anorexia is only a problem if quality of life is affected. Cachexia is caused by increases in cytokines more than reduced calorie intake.42 Reversible causes of reduced eating may be found, including candidiasis, dental problems, depression, or constipation. Megestrol acetate improves weight (number needed to treat = 12), although it significantly increases mortality (number needed to harm = 23), making its use controversial.43 Limited study of cannabinoids has not shown effectiveness in treating anorexia.35
Continue to: Constipation...
Constipation in advanced cancer is often related to opioid therapy, although bowel obstruction must be considered. Opioid-induced constipation affects 40% to 90% of patients on long-term treatment,44 and 5 days of opioid treatment nearly doubles gastrointestinal transit time.45 Opioid-induced constipation can be treated by adding a stimulating laxative followed by a peripheral acting μ-opioid receptor antagonist, such as subcutaneous methylnaltrexone or oral naloxegol.46 These medications are contraindicated if ileus or bowel obstruction is suspected.46
Nausea and vomiting are common in advanced cancer and have numerous causes. Approximately half of reversible causes are medication adverse effects from either chemotherapy or pain medication.47 Opioid rotation may improve symptoms.47 A suspected bowel obstruction should be evaluated by specialists; surgery, palliative chemotherapy, radiation therapy, or stenting may be required. Oncologists can best manage adverse effects of chemotherapy. For nausea and vomiting unrelated to chemotherapy, consider treating constipation and pain. Medication can also be helpful; a systemic review suggests metoclopramide works best, with some evidence supporting other dopaminergic agonists, including haloperidol.47
Fatigue. Both methylphenidate and modafinil have been studied to treat cancer-related fatigue.48 A majority of patients treated with methylphenidate reported less cancer-related fatigue at 4 weeks and wished to continue treatment.49 Modafinil demonstrated minimal improvement in fatigue.50 Sleep disorders, often due to anxiety or sleep apnea, may be a correctable cause.
Later symptoms
Delirium occurs in up to 90% of cancer patients near the end of life, and can signal death.51 Up to half of the delirium seen in palliative care is reversible.51 Reversible causes include uncontrolled pain, medication adverse effects, and urinary and fecal retention (TABLE 348,51). Addressing these factors reduces delirium, based on studies in postoperative patients.52 Consider opioid rotation if neurotoxicity is suspected.51

Delirium can be accompanied by agitation or decreased responsiveness.53 Agitated delirium commonly presents with moaning, facial grimacing, and purposeless repetitive movements, such as plucking bedsheets or removing clothes.51 Delirious patients without agitation have reported, following recovery, distress similar to that experienced by agitated patients.54 Caregivers are most likely to recognize delirium and often become upset. Educating family members about the frequency of delirium can lessen this distress.54
Continue to: Delirium can be treated with...
Delirium can be treated with antipsychotics; haloperidol has been most frequently studied.54 Antipsychotics are effective at reducing agitation but not at restoring cognition.55 Case reports suggest that use of atypical antipsychotics can be beneficial if adverse effects limit haloperidol dosing.56 Agitated delirium is the most frequent indication for palliative sedation.57
Dyspnea. In the last weeks, days, or hours of life, dyspnea is common and often distressing. Dyspnea appears to be multifactorial, worsened by poor control of secretions, airway hyperactivity, and lung pathologies.58 Intravenous hydration may unintentionally exacerbate dyspnea. Hospice providers generally discourage intravenous hydration because relative dehydration reduces terminal respiratory secretions (“death rattle”) and increases patient comfort.59
Some simple nonpharmacologic interventions have benefit. Oxygen is commonly employed, although multiple studies show no benefit over room air.59 Directing a handheld fan at the face does reduce dyspnea, likely by activation of the maxillary branch of the trigeminal nerve.60
Opioids effectively treat dyspnea near the end of life with oral and parenteral dosing, but the evidence does not support nebulized opioids.61 Opioid doses required to treat dyspnea are less than those for pain and do not cause significant respiratory depression.62 If a patient taking opioids experiences dyspnea, a 25% dose increase is recommended.63
Anticholinergic medications can improve excessive airway secretions associated with dyspnea. Glycopyrrolate causes less delirium because it does not cross the blood-brain barrier, while scopolamine patches have reduced anticholinergic adverse effects, but effects are delayed until 12 hours after patch placement.64 Atropine eye drops given sublingually were effective in a small study.65
Continue to: Palliative sedation
Palliative sedation
Palliative sedation can manage intractable symptoms near the end of life. A recent systematic review suggests that palliative sedation does not shorten life.57 Sedation is most often initiated by gradual increases in medication doses.57 Midazolam is most often employed, but antipsychotics are also used.57
CORRESPONDENCE
CDR Michael J. Arnold, MD, Uniformed Services University of the Health Sciences, 4501 Jones Bridge Road, Bethesda, MD 20814; [email protected].
ACKNOWLEDGEMENT
Kristian Sanchack, MD, and James Higgins, DO, assisted in the preparation of this manuscript.
As a family physician (FP), you are well positioned to optimize the quality of life of advanced cancer patients as they decline and approach death. You can help them understand their evolving prognosis so that treatment goals can be adjusted, and you can ensure that hospice is implemented early to improve the end-of-life experience. This practical review will help you to provide the best care possible for these patients.
Family physicians can fill a care gap
The term cancer survivor describes a patient who has completed initial cancer treatment. Within this population, many have declining health and ultimately succumb to their disease. There were 16.9 million cancer survivors in the United States as of January 1, 2019,1 with 53% likely to experience significant symptoms and disability.2 More than 600,000 American cancer survivors will die in 2019.3
In 2011, the Commission on Cancer mandated available outpatient palliative care services at certified cancer centers.4 Unfortunately, current palliative care resources fall far short of expected needs. A 2010 estimate of required hospice and palliative care physicians demonstrated a staffing gap of more than 50% among those providing outpatient services.5 The shortage continues,6 and many cancer patients will look to their FP for supportive care.
FPs, in addition to easing symptoms and adverse effects of medication, can educate patients and families about their disease and prognosis. By providing longitudinal care, FPs can identify critical health declines that oncologists, patients, and families often overlook. FPs can also readily appreciate decline, guide patients toward their care goals, and facilitate comfort care—including at the end of life.
Early outpatient palliative care improves quality of life and patient satisfaction. It also may improve survival time and ward off depression.7,8 Some patients and providers resist palliative care due to a misconception that it requires abandoning treatment.9 Actually, palliative care can be given in concert with all active treatments. Many experts recommend a name change from “palliative care” to “supportive care” to dispel this misconception.10
Estimate prognosis using the “surprise question”
Several algorithms are available—using between 2 and 13 patient parameters—to estimate advanced cancer survival. Most of these algorithms are designed to identify the last months or weeks of life, but their utility to predict death within these periods is limited.11
The “surprise question” may be the most valuable prognostic test for primary care. In this test, the physician asks him- or herself: Would I be surprised if this patient died in 1 year? Researchers found that when primary care physicians answered No, their patient was 4 times more likely to die within the year than when they answered Yes.12 This test has a positive predictive value of 20% and a negative predictive value of 95%, making it valuable in distinguishing patients with longer life expectancy.12 Although it overidentifies at-risk patients, the "surprise question" is a simple and sensitive tool for defining prognosis.
Continue to: Priorities for patients likely to live more than a year
Priorities for patients likely to live more than a year
For patients who likely have more than a year to live, the focus is on symptom management and preparation for future decline. Initiate and facilitate discussions about end-of-life topics. Cancer survivors are often open to discussions on these topics, which include advanced directives, home health aides, and hospice.13 Patients can set specific goals for their remaining time, such as engaging in travel, personal projects, or special events. Cancer patients have better end-of-life experiences and families have improved mental health after these discussions.14 Although cancer patients are more likely than other terminal patients to have end-of-life discussions, fewer than 40% ever do.15
Address distressing symptoms with a focus on maintaining function. More than 50% of advanced cancer patients experience fatigue, weakness, pain, weight loss, and anorexia,16 and up to 60% experience psychological distress.17 Deprescribing most preventive medications is recommended with transition to symptomatic treatment.18
Priorities for patients with less than a year to live
For patients who may have less than a year to live, focus shifts to their wishes for the time remaining and priorities for the dying process. Most patients start out with prognostic views more optimistic than those of their physicians, but this gap narrows after end-of-life discussions.19,20 Patients with incurable cancer are less likely to choose aggressive therapy if they believe their 6-month survival probability is less than 90%.21 Honest conversations, with best- and worst-case scenarios, are important to patients and families, and should occur while the patient is well enough to participate and set goals.22
In the last months of life, opioids become the primary treatment for pain and air hunger. As function declines, concerns about such adverse effects as falls and confusion decrease. Opioids have been shown to be most effective over the course of 4 weeks, and avoiding their use in earlier stages may increase their efficacy at the end of life.23
Hospice benefit—more comfort, with limitations
Hospice care consists of services administered by nonprofit and for-profit entities covered by Medicare, Medicaid, and many private insurers.24 Hospice strives to allow patients to approach death in comfort, meeting their goal of a “good death.” A recent literature review identified 4 aspects of a good death that terminally ill patients and their families considered most important: control of the dying process, relief of pain, spirituality, and emotional well-being (TABLE 1).25

Continue to: Hospice use is increasing...
Hospice use is increasing, yet many enroll too late to fully benefit. While cancer patients alone are not currently tracked, the use of hospice by Medicare beneficiaries increased from 44% in 2012 to 48% in 2019.24 In 2017, the median hospice stay was 19 days.24 Unfortunately, though, just 28% of hospice-eligible patients enrolled in hospice in their last week of life.24 Without hospice, patients often receive excessive care near death. More than 6% receive aggressive chemotherapy in their last 2 weeks of life, and nearly 10% receive a life-prolonging procedure in their last month.26
Hospice care replaces standard hospital care, although patients can elect to be followed by their primary care physician.9 Most hospice services are provided as needed or continuously at the patient’s home, including assisted living facilities. And it is also offered as part of hospital care. Hospice services are interdisciplinary, provided by physicians, nurses, social workers, chaplains, and health aides. Hospices have on-call staff to assess and treat complications, avoiding emergency hospital visits.9 And hospice includes up to 5 days respite care for family caregivers, although with a 5% copay.9 Most hospice entities run inpatient facilities for care that cannot be effectively provided at home.
Hospice care has limitations—many set by insurance. Medicare, for example, stipulates that a primary care or hospice physician must certify the patient has a reasonable prognosis of 6 months or less and is expected to have a declining course.27 Patients who survive longer than 6 months are recertified by the same criteria every 60 days.27
Hospice patients forgo treatments aimed at curing their terminal diagnosis.28 Some hospice entities allow noncurative therapies while others do not. Hospice covers prescription medications for symptom control only, although patients can receive care unrelated to the terminal diagnosis under regular benefits.28 Hospice care practices differ from standard care in ways that may surprise patients and families (TABLE 227,28). Patients can disenroll and re-enroll in hospice as they wish.28

Symptom control in advanced cancer
General symptoms
Pain affects 64% of patients with advanced cancer.29 Evidence shows that cancer pain is often undertreated, with a recent systematic review reporting undertreated pain in 32% of patients.30 State and national chronic opioid guidelines do not restrict use for cancer pain.31 Opioids are effective in 75% of cancer patients over 1 month, but there is no evidence of benefit after this period.23 In fact, increasing evidence demonstrates that pain is likely negatively responsive to opioids over longer periods.32 Opioid adverse effects can worsen other cancer symptoms, including depression, anxiety, fatigue, constipation, hypogonadism, and cognitive dysfunction.32 Delaying opioid therapy to end of life can limit adverse effects and may preserve pain-control efficacy for the dying process.
Continue to: Most cancer pain...
Most cancer pain is partially neuropathic, so anticonvulsant and antidepressant medications can help.33 Gabapentin, pregabalin, and duloxetine are recommended based on evidence not restricted to cancer.34 Cannabinoids have been evaluated in 2 trials of cancer pain with 440 patients and showed a borderline significant reduction of pain.35
Palliative radiation therapy can sometimes reduce pain. Bone metastases pain has been studied the most, and the literature suggests that palliative radiation provides improvement for 60% of patients and complete relief to 25% of patients.36 Palliative thoracic radiotherapy for primary or metastatic lung masses reduces pain by more than 70% while improving dyspnea, hemoptysis, and cough in a majority of patients.36
Other uses of palliative radiation have varied evidence. Palliative chemotherapy has less evidence of benefit. In a recent multicenter cohort trial, chemotherapy in end-stage cancer reduced quality of life in patients with good functional status, without affecting quality of life when function was limited.37 Palliative chemotherapy may be beneficial if combined with corticosteroids or radiation therapy.38
Treatment in the last weeks of life centers on opioids; dose increases do not shorten survival.39 Cancer patients are 4 times as likely as noncancer patients to have severe or excruciating pain during the last 3 days of life.40 Narcotics can be titrated aggressively near end of life with less concern for hypotension, respiratory depression, or level of consciousness. Palliative sedation remains an option for uncontrolled pain.41
Anorexia is only a problem if quality of life is affected. Cachexia is caused by increases in cytokines more than reduced calorie intake.42 Reversible causes of reduced eating may be found, including candidiasis, dental problems, depression, or constipation. Megestrol acetate improves weight (number needed to treat = 12), although it significantly increases mortality (number needed to harm = 23), making its use controversial.43 Limited study of cannabinoids has not shown effectiveness in treating anorexia.35
Continue to: Constipation...
Constipation in advanced cancer is often related to opioid therapy, although bowel obstruction must be considered. Opioid-induced constipation affects 40% to 90% of patients on long-term treatment,44 and 5 days of opioid treatment nearly doubles gastrointestinal transit time.45 Opioid-induced constipation can be treated by adding a stimulating laxative followed by a peripheral acting μ-opioid receptor antagonist, such as subcutaneous methylnaltrexone or oral naloxegol.46 These medications are contraindicated if ileus or bowel obstruction is suspected.46
Nausea and vomiting are common in advanced cancer and have numerous causes. Approximately half of reversible causes are medication adverse effects from either chemotherapy or pain medication.47 Opioid rotation may improve symptoms.47 A suspected bowel obstruction should be evaluated by specialists; surgery, palliative chemotherapy, radiation therapy, or stenting may be required. Oncologists can best manage adverse effects of chemotherapy. For nausea and vomiting unrelated to chemotherapy, consider treating constipation and pain. Medication can also be helpful; a systemic review suggests metoclopramide works best, with some evidence supporting other dopaminergic agonists, including haloperidol.47
Fatigue. Both methylphenidate and modafinil have been studied to treat cancer-related fatigue.48 A majority of patients treated with methylphenidate reported less cancer-related fatigue at 4 weeks and wished to continue treatment.49 Modafinil demonstrated minimal improvement in fatigue.50 Sleep disorders, often due to anxiety or sleep apnea, may be a correctable cause.
Later symptoms
Delirium occurs in up to 90% of cancer patients near the end of life, and can signal death.51 Up to half of the delirium seen in palliative care is reversible.51 Reversible causes include uncontrolled pain, medication adverse effects, and urinary and fecal retention (TABLE 348,51). Addressing these factors reduces delirium, based on studies in postoperative patients.52 Consider opioid rotation if neurotoxicity is suspected.51

Delirium can be accompanied by agitation or decreased responsiveness.53 Agitated delirium commonly presents with moaning, facial grimacing, and purposeless repetitive movements, such as plucking bedsheets or removing clothes.51 Delirious patients without agitation have reported, following recovery, distress similar to that experienced by agitated patients.54 Caregivers are most likely to recognize delirium and often become upset. Educating family members about the frequency of delirium can lessen this distress.54
Continue to: Delirium can be treated with...
Delirium can be treated with antipsychotics; haloperidol has been most frequently studied.54 Antipsychotics are effective at reducing agitation but not at restoring cognition.55 Case reports suggest that use of atypical antipsychotics can be beneficial if adverse effects limit haloperidol dosing.56 Agitated delirium is the most frequent indication for palliative sedation.57
Dyspnea. In the last weeks, days, or hours of life, dyspnea is common and often distressing. Dyspnea appears to be multifactorial, worsened by poor control of secretions, airway hyperactivity, and lung pathologies.58 Intravenous hydration may unintentionally exacerbate dyspnea. Hospice providers generally discourage intravenous hydration because relative dehydration reduces terminal respiratory secretions (“death rattle”) and increases patient comfort.59
Some simple nonpharmacologic interventions have benefit. Oxygen is commonly employed, although multiple studies show no benefit over room air.59 Directing a handheld fan at the face does reduce dyspnea, likely by activation of the maxillary branch of the trigeminal nerve.60
Opioids effectively treat dyspnea near the end of life with oral and parenteral dosing, but the evidence does not support nebulized opioids.61 Opioid doses required to treat dyspnea are less than those for pain and do not cause significant respiratory depression.62 If a patient taking opioids experiences dyspnea, a 25% dose increase is recommended.63
Anticholinergic medications can improve excessive airway secretions associated with dyspnea. Glycopyrrolate causes less delirium because it does not cross the blood-brain barrier, while scopolamine patches have reduced anticholinergic adverse effects, but effects are delayed until 12 hours after patch placement.64 Atropine eye drops given sublingually were effective in a small study.65
Continue to: Palliative sedation
Palliative sedation
Palliative sedation can manage intractable symptoms near the end of life. A recent systematic review suggests that palliative sedation does not shorten life.57 Sedation is most often initiated by gradual increases in medication doses.57 Midazolam is most often employed, but antipsychotics are also used.57
CORRESPONDENCE
CDR Michael J. Arnold, MD, Uniformed Services University of the Health Sciences, 4501 Jones Bridge Road, Bethesda, MD 20814; [email protected].
ACKNOWLEDGEMENT
Kristian Sanchack, MD, and James Higgins, DO, assisted in the preparation of this manuscript.
1. American Cancer Society. Cancer Treatment & Survivorship Facts & Figures 2019-2021. www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-statistics/cancer-treatment-and-survivorship-facts-and-figures/cancer-treatment-and-survivorship-facts-and-figures-2019-2021.pdf. Accessed September 4, 2019.
2. Stein KD, Syrjala KL, Andrykowski MA. Physical and psychological long-term and late effects of cancer. Cancer. 2008;112(11 suppl):2577-2592.
3. National Comprehensive Cancer Network. NCCN Guidelines Version 2. 2019. Palliative Care. www.nccn.org/professionals/physician_gls/pdf/palliative.pdf. (Must register an account for access.) Accessed September 4, 2019.
4. American Cancer Society. New CoC accreditation standards gain strong support. www.facs.org/media/press-releases/2011/coc-standards0811. Accessed September 11, 2019.
5. Lupu D; American Academy of Hospice and Palliative Medicine Workforce Task Force. Estimate of current hospice and palliative medicine physician workforce shortage. J Pain Symptom Manage. 2010;40:899-911.
6. Lupu D, Quigley L, Mehfoud N, et al. The growing demand for hospice and palliative medicine physicians: will the supply keep up? J Pain Symptom Manage. 2018;55:1216-1223.
7. Rabow MW, Dahlin C, Calton B, et al. New frontiers in outpatient palliative care for patients with cancer. Cancer Control. 2015;22:465-474.
8. Haun MW, Estel S, Rücker G, et al. Early palliative care for adults with advanced cancer. Cochrane Database of Syst Rev. 2017:CD01129.
9. Buss MK, Rock LK, McCarthy EP. Understanding palliative care and hospice: a review for primary care providers. Mayo Clin Proc. 2017;92:280-286.
10. Hui D. Definition of supportive care: does the semantic matter? Curr Opin Oncol. 2014;26:372-379.
11. Simmons CPL, McMillan DC, McWilliams K, et al. Prognostic tools in patients with advanced cancer: a systematic review. J Pain Symptom Manage. 2017;53:962-970.
12. Lakin JR, Robinson MG, Bernacki RE, et al. Estimating 1-year mortality for high-risk primary care patients using the “surprise” question. JAMA Int Med. 2016;176:1863-1865.
13. Walczak A, Henselmans I, Tattersall MH, et al. A qualitative analysis of responses to a question prompt list and prognosis and end-of-life care discussion prompts delivered in a communication support program. Psychoonchology. 2015;24:287-293.
14. Yamaguchi T, Maeda I, Hatano Y, et al. Effects of end-of-life discussions on the mental health of bereaved family members and quality of patient death and care. J Pain Symptom Manage. 2017;54:17-26.
15. Wright AA, Zhang B, Ray A, et al. Associations between end-of-life discussions, patient mental health, medical care near death, caregiver bereavement adjustment. JAMA. 2008;300:1665-1673.
16. Teunissen SC, Wesker W, Kruitwagen C, et al. Symptom prevalence in patients with incurable cancer: a systematic review. J Pain Symptom Manage. 2007;34:94-104.
17. Gao W, Bennett MI, Stark D, et al. Psychological distress in cancer from survivorship to end of life: prevalence, associated factors and clinical implications. Eur J Cancer. 2010;46:2036-2044.
18. Scott IA, Gray LC, Martin JH, et al. Deciding when to stop: towards evidence-based deprescribing of drugs in older populations. Evid Based Med. 2013;18:121-124.
19. Gramling R, Fiscella K, Xing G, et al. Determinants of patient-oncologist prognostic discordance in advanced cancer. JAMA Oncol. 2016;2:1421-1426.
20. Epstein AS, Prigerson HG, O’Reilly EM, et al. Discussions of life expectancy and changes in illness understanding in patients with advanced cancer. J Clin Oncol. 2016;34:2398-2403.
21. Weeks JC, Cook EF, O’Day SJ, et al. Relationship between cancer patients’ predictions of prognosis and their treatment preferences. JAMA. 1998;279:1709-1714.
22. Myers J. Improving the quality of end-of-life discussions. Curr Opin Support Palliat Care. 2015;9:72-76.
23. Corli O, Floriani I, Roberto A, et al. Are strong opioids equally effective and safe in the treatment of chronic cancer pain? A multicenter randomized phase IV ‘real life’ trial on the variability of response to opioids. Ann Oncolog. 2016;27:1107-1115.
24. National Hospice and Palliative Care Organization. NHPCO Facts and Figures. 2018. www.nhpco.org/wp-content/uploads/2019/07/2018_NHPCO_Facts_Figures.pdf. Accessed September 24, 2019.
25. Meier EA, Gallegos JV, Thomas LP, et al. Defining a good death (successful dying): literature review and a call for research and public dialogue. Am J Geriatr Psychiatry. 2016;24:261-271.
26. Morden NE, Chang CH, Jacobson JO, et al. End-of-life care for Medicare beneficiaries with cancer is highly intensive overall and varies widely. Health Aff (Millwood). 2012;31:786-796.
27. Centers for Medicare & Medicaid Services. Medicare Hospice Benefit Facts. www.cgsmedicare.com/hhh/education/materials/pdf/Medicare_Hospice_Benefit_Facts.pdf. Accessed September 11, 2019.
28. Centers for Medicare & Medicaid Services. Medicare Hospice Benefits. www.medicare.gov/pubs/pdf/02154-medicare-hospice-benefits.pdf. Accessed September 11, 2019.
29. van den Beuken-van Everdingen MH, de Rijke JM, Kessels AG, et al. Prevalence of pain in patients with cancer: a systematic review of the past 40 years. Ann Oncol. 2007;18:1437-1449.
30. Greco MT, Roberto A, Corli O, et al. Quality of cancer pain management: an update of a systematic review of undertreatment of patients with cancer. J Clin Oncol. 2014;32:4149-4154.
31. Dowell D, Haegerich TM, Chou R. CDC guideline for prescribing opioids for chronic pain — United States, 2016. MMWR Recomm Rep. 2016;65:1-49.
32. Davis MP, Mehta Z. Opioids and chronic pain: where is the balance? Curr Oncol Rep. 2016;18:71.
33. Leppert W, Zajaczkowska R, Wordliczek J, et al. Pathophysiology and clinical characteristics of pain in most common locations in cancer patients. J Physiol Pharmacol. 2016;67:787-799.
34. Finnerup NB, Attal N, Haroutounian S, et al. Pharmacotherapy for neuropathic pain in adults: a systematic review and meta-analysis. Lancet Neurol. 2015;14:162-173.
35. Whiting PF, Wolff RF, Deshpande S, et al. Cannabinoids for medical use: a systematic review and meta-analysis. JAMA. 2015;313:2456-2473.
36. Jones JA, Lutz ST, Chow E. et al. Palliative radiotherapy at the end of life: a critical review. CA Cancer J Clin. 2014;64:296-310.
37. Prigerson HG, Bao Y, Shah MA, et al. Chemotherapy use, performance status, and quality of life at the end of life. JAMA Oncol. 2015;1:778-784.
38. Kongsgaard U, Kaasa S, Dale O, et al. Palliative treatment of cancer-related pain. 2005. www.ncbi.nlm.nih.gov/books/NBK464794/. Accessed September 24, 2019.
39. Sathornviriyapong A, Nagaviroj K, Anothaisintawee T. The association between different opioid doses and the survival of advanced cancer patients receiving palliative care. BMC Palliat Care. 2016;15:95.
40. Steindal SA, Bredal IS. Sørbye LW, et al. Pain control at the end of life: a comparative study of hospitalized cancer and noncancer patients. Scand J Caring Sci. 2011;25:771-779.
41. Maltoni M, Setola E. Palliative sedation in patients with cancer. Cancer Control. 2015;22:433-441.
42. Cooper C, Burden ST, Cheng H, et al. Understanding and managing cancer-related weight loss and anorexia: insights from a systematic review of qualitative research. J Cachexia Sarcopenia Muscle. 2015;6:99-111.
43. Ruiz Garcia V, LÓpez-Briz E, Carbonell Sanchis R, et al. Megesterol acetate for treatment of anorexia-cachexia syndrome. Cochrane Database Syst Rev. 2013;28:CD004310.
44. Chey WD, Webster L, Sostek M, et al. Naloxegol for opioid-induced constipation in patients with noncancer pain. N Engl J Med. 2014;370:2387-2396.
45. Poulsen JL, Nilsson M, Brock C, et al. The impact of opioid treatment on regional gastrointestinal transit. J Neurogastroenterol Motil. 2016;22:282-291.
46. Pergolizzi JV, Raffa RB, Pappagallo M, et al. Peripherally acting μ-opioid receptor antagonists as treatment options for constipation in noncancer pain patients on chronic opioid therapy. Patient Prefer Adherence. 2017;11:107-119.
47. Walsh D, Davis M, Ripamonti C, et al. 2016 updated MASCC/ESMO consensus recommendations: management of nausea and vomiting in advanced cancer. Support Care Cancer. 2017;25:333-340.
48. Mücke M, Mochamat, Cuhls H, et al. Pharmacological treatments for fatigue associated with palliative care. Cochrane Database Syst Rev. 2015(5):CD006788.
49. Escalante CP, Meyers C, Reuben JM, et al. A randomized, double-blind, 2-period, placebo-controlled crossover trial of a sustained-release methylphenidate in the treatment of fatigue in cancer patients. Cancer J. 2014;20:8-14.
50. Hovey E, de Souza P, Marx G, et al. Phase III, randomized, double-blind, placebo-controlled study of modafinil for fatigue in patients treated with docetaxel-based chemotherapy. Support Care Cancer. 2014;22:1233-1242.
51. Hosker CM, Bennett MI. Delirium and agitation at the end of life. BMJ. 2016;353:i3085.
52. Mercantonio ER, Flacker JM, Wright RJ, et al. Reducing delirium after hip fracture: a randomized trial. J Am Geriatr Soc. 2001;49:516-522.
53. Casarett DJ, Inouye SK. Diagnosis and management of delirium near the end of life. Ann Int Med. 2001;135:32-40.
54. Breitbart W, Alici Y. Agitation and delirium at the end of life: “We couldn’t manage him." JAMA. 2008;300:2898-2910.
55. Candy B, Jackson KC, Jones L, et al. Drug therapy for delirium in terminally ill patients. Cochrane Database Syst Rev. 2012;11:CD004770.
56. Bascom PB, Bordley JL, Lawton AJ. High-dose neuroleptics and neuroleptic rotation for agitated delirium near the end of life. Am J Hosp Palliat Med. 2014;31:808-811.
57. Maltoni M, Scarpi E, Rosati M, et al. Palliative sedation in end-of-life care and survival: a systematic review. J Clin Oncol. 2012;30:1378-1383.
58. Albert RH. End-of-life care: managing common symptoms. Am Fam Physician. 2017;95:356-361.
59. Arenella C. Artificial nutrition and hydration at the end of life: beneficial or harmful? https://americanhospice.org/caregiving/artificial-nutrition-and-hydration-at-the-end-of-life-beneficial-or-harmful/ Accessed September 11, 2019.
60. Booth S, Moffat C, Burkin J, et al. Nonpharmacological interventions for breathlessness. Curr Opinion Support Pall Care. 2011;5:77-86.
61. Barnes H, McDonald J, Smallwood N, et al. Opioids for the palliation of refractory breathlessness in adults with advanced disease and terminal illness. Cochrane Database Syst Rev. 2016(3)CD011008.
62. Lim RB. End-of-life care in patients with advanced lung cancer. Ther Adv Resp Dis. 2016;10:455-467.
63. Kreher M. Symptom control at the end of life. Med Clin North Am. 2016;100:1111-1122.
64. Baralatei FT, Ackerman RJ. Care of patients at the end of life: management of nonpain symptoms. FP Essent. 2016;447:18-24.
65. Protus BM, Grauer PA, Kimbrel JM. Evaluation of atropine 1% ophthalmic solution administered sublingual for the management of terminal respiratory secretions. Am J Hosp Palliat Med. 2013;30:388-392.
1. American Cancer Society. Cancer Treatment & Survivorship Facts & Figures 2019-2021. www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-statistics/cancer-treatment-and-survivorship-facts-and-figures/cancer-treatment-and-survivorship-facts-and-figures-2019-2021.pdf. Accessed September 4, 2019.
2. Stein KD, Syrjala KL, Andrykowski MA. Physical and psychological long-term and late effects of cancer. Cancer. 2008;112(11 suppl):2577-2592.
3. National Comprehensive Cancer Network. NCCN Guidelines Version 2. 2019. Palliative Care. www.nccn.org/professionals/physician_gls/pdf/palliative.pdf. (Must register an account for access.) Accessed September 4, 2019.
4. American Cancer Society. New CoC accreditation standards gain strong support. www.facs.org/media/press-releases/2011/coc-standards0811. Accessed September 11, 2019.
5. Lupu D; American Academy of Hospice and Palliative Medicine Workforce Task Force. Estimate of current hospice and palliative medicine physician workforce shortage. J Pain Symptom Manage. 2010;40:899-911.
6. Lupu D, Quigley L, Mehfoud N, et al. The growing demand for hospice and palliative medicine physicians: will the supply keep up? J Pain Symptom Manage. 2018;55:1216-1223.
7. Rabow MW, Dahlin C, Calton B, et al. New frontiers in outpatient palliative care for patients with cancer. Cancer Control. 2015;22:465-474.
8. Haun MW, Estel S, Rücker G, et al. Early palliative care for adults with advanced cancer. Cochrane Database of Syst Rev. 2017:CD01129.
9. Buss MK, Rock LK, McCarthy EP. Understanding palliative care and hospice: a review for primary care providers. Mayo Clin Proc. 2017;92:280-286.
10. Hui D. Definition of supportive care: does the semantic matter? Curr Opin Oncol. 2014;26:372-379.
11. Simmons CPL, McMillan DC, McWilliams K, et al. Prognostic tools in patients with advanced cancer: a systematic review. J Pain Symptom Manage. 2017;53:962-970.
12. Lakin JR, Robinson MG, Bernacki RE, et al. Estimating 1-year mortality for high-risk primary care patients using the “surprise” question. JAMA Int Med. 2016;176:1863-1865.
13. Walczak A, Henselmans I, Tattersall MH, et al. A qualitative analysis of responses to a question prompt list and prognosis and end-of-life care discussion prompts delivered in a communication support program. Psychoonchology. 2015;24:287-293.
14. Yamaguchi T, Maeda I, Hatano Y, et al. Effects of end-of-life discussions on the mental health of bereaved family members and quality of patient death and care. J Pain Symptom Manage. 2017;54:17-26.
15. Wright AA, Zhang B, Ray A, et al. Associations between end-of-life discussions, patient mental health, medical care near death, caregiver bereavement adjustment. JAMA. 2008;300:1665-1673.
16. Teunissen SC, Wesker W, Kruitwagen C, et al. Symptom prevalence in patients with incurable cancer: a systematic review. J Pain Symptom Manage. 2007;34:94-104.
17. Gao W, Bennett MI, Stark D, et al. Psychological distress in cancer from survivorship to end of life: prevalence, associated factors and clinical implications. Eur J Cancer. 2010;46:2036-2044.
18. Scott IA, Gray LC, Martin JH, et al. Deciding when to stop: towards evidence-based deprescribing of drugs in older populations. Evid Based Med. 2013;18:121-124.
19. Gramling R, Fiscella K, Xing G, et al. Determinants of patient-oncologist prognostic discordance in advanced cancer. JAMA Oncol. 2016;2:1421-1426.
20. Epstein AS, Prigerson HG, O’Reilly EM, et al. Discussions of life expectancy and changes in illness understanding in patients with advanced cancer. J Clin Oncol. 2016;34:2398-2403.
21. Weeks JC, Cook EF, O’Day SJ, et al. Relationship between cancer patients’ predictions of prognosis and their treatment preferences. JAMA. 1998;279:1709-1714.
22. Myers J. Improving the quality of end-of-life discussions. Curr Opin Support Palliat Care. 2015;9:72-76.
23. Corli O, Floriani I, Roberto A, et al. Are strong opioids equally effective and safe in the treatment of chronic cancer pain? A multicenter randomized phase IV ‘real life’ trial on the variability of response to opioids. Ann Oncolog. 2016;27:1107-1115.
24. National Hospice and Palliative Care Organization. NHPCO Facts and Figures. 2018. www.nhpco.org/wp-content/uploads/2019/07/2018_NHPCO_Facts_Figures.pdf. Accessed September 24, 2019.
25. Meier EA, Gallegos JV, Thomas LP, et al. Defining a good death (successful dying): literature review and a call for research and public dialogue. Am J Geriatr Psychiatry. 2016;24:261-271.
26. Morden NE, Chang CH, Jacobson JO, et al. End-of-life care for Medicare beneficiaries with cancer is highly intensive overall and varies widely. Health Aff (Millwood). 2012;31:786-796.
27. Centers for Medicare & Medicaid Services. Medicare Hospice Benefit Facts. www.cgsmedicare.com/hhh/education/materials/pdf/Medicare_Hospice_Benefit_Facts.pdf. Accessed September 11, 2019.
28. Centers for Medicare & Medicaid Services. Medicare Hospice Benefits. www.medicare.gov/pubs/pdf/02154-medicare-hospice-benefits.pdf. Accessed September 11, 2019.
29. van den Beuken-van Everdingen MH, de Rijke JM, Kessels AG, et al. Prevalence of pain in patients with cancer: a systematic review of the past 40 years. Ann Oncol. 2007;18:1437-1449.
30. Greco MT, Roberto A, Corli O, et al. Quality of cancer pain management: an update of a systematic review of undertreatment of patients with cancer. J Clin Oncol. 2014;32:4149-4154.
31. Dowell D, Haegerich TM, Chou R. CDC guideline for prescribing opioids for chronic pain — United States, 2016. MMWR Recomm Rep. 2016;65:1-49.
32. Davis MP, Mehta Z. Opioids and chronic pain: where is the balance? Curr Oncol Rep. 2016;18:71.
33. Leppert W, Zajaczkowska R, Wordliczek J, et al. Pathophysiology and clinical characteristics of pain in most common locations in cancer patients. J Physiol Pharmacol. 2016;67:787-799.
34. Finnerup NB, Attal N, Haroutounian S, et al. Pharmacotherapy for neuropathic pain in adults: a systematic review and meta-analysis. Lancet Neurol. 2015;14:162-173.
35. Whiting PF, Wolff RF, Deshpande S, et al. Cannabinoids for medical use: a systematic review and meta-analysis. JAMA. 2015;313:2456-2473.
36. Jones JA, Lutz ST, Chow E. et al. Palliative radiotherapy at the end of life: a critical review. CA Cancer J Clin. 2014;64:296-310.
37. Prigerson HG, Bao Y, Shah MA, et al. Chemotherapy use, performance status, and quality of life at the end of life. JAMA Oncol. 2015;1:778-784.
38. Kongsgaard U, Kaasa S, Dale O, et al. Palliative treatment of cancer-related pain. 2005. www.ncbi.nlm.nih.gov/books/NBK464794/. Accessed September 24, 2019.
39. Sathornviriyapong A, Nagaviroj K, Anothaisintawee T. The association between different opioid doses and the survival of advanced cancer patients receiving palliative care. BMC Palliat Care. 2016;15:95.
40. Steindal SA, Bredal IS. Sørbye LW, et al. Pain control at the end of life: a comparative study of hospitalized cancer and noncancer patients. Scand J Caring Sci. 2011;25:771-779.
41. Maltoni M, Setola E. Palliative sedation in patients with cancer. Cancer Control. 2015;22:433-441.
42. Cooper C, Burden ST, Cheng H, et al. Understanding and managing cancer-related weight loss and anorexia: insights from a systematic review of qualitative research. J Cachexia Sarcopenia Muscle. 2015;6:99-111.
43. Ruiz Garcia V, LÓpez-Briz E, Carbonell Sanchis R, et al. Megesterol acetate for treatment of anorexia-cachexia syndrome. Cochrane Database Syst Rev. 2013;28:CD004310.
44. Chey WD, Webster L, Sostek M, et al. Naloxegol for opioid-induced constipation in patients with noncancer pain. N Engl J Med. 2014;370:2387-2396.
45. Poulsen JL, Nilsson M, Brock C, et al. The impact of opioid treatment on regional gastrointestinal transit. J Neurogastroenterol Motil. 2016;22:282-291.
46. Pergolizzi JV, Raffa RB, Pappagallo M, et al. Peripherally acting μ-opioid receptor antagonists as treatment options for constipation in noncancer pain patients on chronic opioid therapy. Patient Prefer Adherence. 2017;11:107-119.
47. Walsh D, Davis M, Ripamonti C, et al. 2016 updated MASCC/ESMO consensus recommendations: management of nausea and vomiting in advanced cancer. Support Care Cancer. 2017;25:333-340.
48. Mücke M, Mochamat, Cuhls H, et al. Pharmacological treatments for fatigue associated with palliative care. Cochrane Database Syst Rev. 2015(5):CD006788.
49. Escalante CP, Meyers C, Reuben JM, et al. A randomized, double-blind, 2-period, placebo-controlled crossover trial of a sustained-release methylphenidate in the treatment of fatigue in cancer patients. Cancer J. 2014;20:8-14.
50. Hovey E, de Souza P, Marx G, et al. Phase III, randomized, double-blind, placebo-controlled study of modafinil for fatigue in patients treated with docetaxel-based chemotherapy. Support Care Cancer. 2014;22:1233-1242.
51. Hosker CM, Bennett MI. Delirium and agitation at the end of life. BMJ. 2016;353:i3085.
52. Mercantonio ER, Flacker JM, Wright RJ, et al. Reducing delirium after hip fracture: a randomized trial. J Am Geriatr Soc. 2001;49:516-522.
53. Casarett DJ, Inouye SK. Diagnosis and management of delirium near the end of life. Ann Int Med. 2001;135:32-40.
54. Breitbart W, Alici Y. Agitation and delirium at the end of life: “We couldn’t manage him." JAMA. 2008;300:2898-2910.
55. Candy B, Jackson KC, Jones L, et al. Drug therapy for delirium in terminally ill patients. Cochrane Database Syst Rev. 2012;11:CD004770.
56. Bascom PB, Bordley JL, Lawton AJ. High-dose neuroleptics and neuroleptic rotation for agitated delirium near the end of life. Am J Hosp Palliat Med. 2014;31:808-811.
57. Maltoni M, Scarpi E, Rosati M, et al. Palliative sedation in end-of-life care and survival: a systematic review. J Clin Oncol. 2012;30:1378-1383.
58. Albert RH. End-of-life care: managing common symptoms. Am Fam Physician. 2017;95:356-361.
59. Arenella C. Artificial nutrition and hydration at the end of life: beneficial or harmful? https://americanhospice.org/caregiving/artificial-nutrition-and-hydration-at-the-end-of-life-beneficial-or-harmful/ Accessed September 11, 2019.
60. Booth S, Moffat C, Burkin J, et al. Nonpharmacological interventions for breathlessness. Curr Opinion Support Pall Care. 2011;5:77-86.
61. Barnes H, McDonald J, Smallwood N, et al. Opioids for the palliation of refractory breathlessness in adults with advanced disease and terminal illness. Cochrane Database Syst Rev. 2016(3)CD011008.
62. Lim RB. End-of-life care in patients with advanced lung cancer. Ther Adv Resp Dis. 2016;10:455-467.
63. Kreher M. Symptom control at the end of life. Med Clin North Am. 2016;100:1111-1122.
64. Baralatei FT, Ackerman RJ. Care of patients at the end of life: management of nonpain symptoms. FP Essent. 2016;447:18-24.
65. Protus BM, Grauer PA, Kimbrel JM. Evaluation of atropine 1% ophthalmic solution administered sublingual for the management of terminal respiratory secretions. Am J Hosp Palliat Med. 2013;30:388-392.
PRACTICE RECOMMENDATIONS
› Implement palliative/ supportive care shortly after the diagnosis of an incurable cancer. A
› Candidly communicate prognoses to patients and help them adjust their goals of care. B
› Recommend hospice care for patients who likely have less than 6 months to live, especially with treatmentrelated complications or significant caregiver stress. B
› Delay opioid therapy— if possible—to better control symptoms near the end of life. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Strategies to reduce and prevent polypharmacy in older patients
CASE
Ronald Wa is a 74-year old man with an extensive medical history: diabetes, hypertension, heart failure, atrial fibrillation, pancreatitis, hyperlipidemia, gout, depression, generalized anxiety, obstructive sleep apnea, and benign prostatic hypertrophy. He arrives at the emergency department (ED) of the hospital by nonemergent ambulance from home for evaluation of lethargy and confusion over the past week.
In the ED, Mr. W is afebrile, normotensive, and oxygenating on room air. Mucous membranes are dry. On physical examination, he appears pale, fatigued, and modestly confused but is able to state his name and birthday, although not the location or date.
Laboratory testing reveals: blood glucose, 107 mg/dL; serum creatinine, 2.3 mg/dL; sodium, 127 mEq/L; and hemoglobin level and hematocrit, within normal limits. Urinalysis is negative. Renal ultrasonography is unremarkable, without evidence of urinary tract obstruction.
Mr. W is admitted to the general medical unit with hyponatremia. The pharmacy admission specialist begins reconciliation of the long list of the patient’s home medications.
Overprescribing: Often, more is not better
Some experts consider prescribing medication to be the most common form of medical intervention; beyond that, polypharmacy—often defined as the use of more medications than are medically necessary (see the next section on terminology)—is recognized as an increasingly serious problem in many medical specialties.1 Here are specifics about the extent of, and harm caused by, the problem2,3:
- The US General Accounting Office reports that inappropriate polypharmacy is associated with significant morbidity and mortality.2 Research has established a strong relationship between polypharmacy and harmful clinical consequences,3 to which the older patient population is most susceptible.
- Polypharmacy is also recognized as an expensive practice; the US Center for Medicare and Medicaid Services estimates that polypharmacy cost US health insurers more than $50 billion annually.2
- Worldwide, with more and more people older than 65 years, polypharmacy is becoming more prevalent, and a growing concern, in older adults; approximately 50% of them take ≥ 1 medications that are medically unnecessary.3
Despite many programs to help with deprescribing, drug–drug interactions and the so-called prescribing cascade (ie, when signs and symptoms of an adverse drug effect are misdiagnosed as a new medical condition) continue to affect patients, leading to comorbidities. It is important, therefore, for physicians to be aware of commonly used tools to prevent polypharmacy and its consequences.
What is “polypharmacy” understood to mean?
Despite the compelling association of polypharmacy with the presence of multiple morbidities in the older patient population, there is no consensus on its definition:
- Starting with the dictionary, “polypharmacy” derives from 2 words in Ancient Greek: poly, “more than one,” and “pharmakon, “drug.”3
- The definition can vary based on the number of drugs a patient has been prescribed, their safety, and the appropriateness of their use.1
- Another definition is the use of more medications than are medically necessary; such a grouping includes agents that are not indicated, are ineffective, or constitute a therapeutic duplication. Although this definition is more clinically relevant than the others, it is premised on undertaking a clinical review of a medication regimen.3
- A numerical definition is the most commonly reported category, a number that varies from study to study—from ≥ 2 to ≥ 11 medications. When applied to health care settings, accepted definitions are ≥ 5 medications at hospital discharge and ≥ 10 during a hospital stay.4 Numerical definitions of polypharmacy do not ascertain the clinical appropriateness of therapy nor the process of rationalizing those medications.1
aA composite, hypothetical patient, based on the authors' clinical experience.
Continue to: Appropriateness
Appropriateness
Polypharmacy is classified as appropriate or inappropriate:
- Appropriate polypharmacy is the optimization of medications for patients with complex or multiple conditions, when the use of medicine is in agreement with best evidence.
- Inappropriate polypharmacy can increase the risk of adverse drug effects and drug–drug interactions and can be characterized by medication underuse and duplication.4
There are subdefinitions of “appropriateness,” but these are beyond the scope of this article.
What variables contribute to polypharmacy?
Multimorbidity is common in the older population. The presence of multiple chronic conditions increases the complexity of therapeutic management for health professionals and patients; such complexity can have a harmful impact on health outcomes. Combinations of medications to treat chronic diseases automatically push many patients into polypharmacy. Few treatment guidelines provide recommendations on when to stop medications.
Consequences of polypharmacy, some of which are masked as syndromes in the older patient, include delirium and dementia, urinary incontinence, dizziness, falls, adverse drug reactions, increased length of hospital stay, readmission soon after discharge, and death.3-5 Relatively high rates of drug consumption and other variables (eg, decreased renal and hepatic function, decreased total body water and lean body mass, cognitive impairment, age-related decline in vision and hearing, frequency of chronic diseases and medical comorbidities, communication barriers, prescribing cascades, and health care delivery involving multiple prescribers) can contribute to an increased prevalence of medication-associated morbidity and mortality as the result of polypharmacy.
In a descriptive study6 that examined these variables, researchers explored whether general practitioners experience barriers to medication review in multimorbid patients with polypharmacy. They concluded that the primary barriers were (1) lack of communication and teamwork with specialists and (2) the challenge of handling polypharmacy in a culture that encourages adding medications and inhibits conversations about medication withdrawal.6
Continue to: Reducing consequences of polypharmacy
Reducing consequences of polypharmacy
Collaborative medication review
Interventions to help physicians reduce polypharmacy include reviewing medications with older patients at every office visit and during transitions of care into and out of the hospital or other care facility. A 2016 Cochrane review of 5 randomized trials of inpatient medication reviews led by pharmacists, physicians, and other health care professionals showed a 36% reduction in ED visits 30 days to 1 year after discharge.7
Patients can collaborate in this effort by bringing all medications to each appointment or upon hospital admission—not just a list but the actual supply, to ensure that a correct medication list is compiled and a thorough review conducted.8 Explicitly ask open-ended questions of the patient about over-the-counter medications, herbal products, and other home remedies that have not been prescribed; many patients may have trouble with recall or are uncertain what fits the definition of a nonprescription medication.8,9
Compare the medication list with the patient’s current problem list; consider removing medications that do not have a pertinent indication. (Physicians can help in this regard when prescribing by making note in the medical record of the indication for each medication they prescribe.)
Evaluate the patient’s signs and symptoms as a possible drug-related adverse effect, thus making an effort to minimize the chance of a prescribing cascade.9
Use Beers criteria,10 which list potentially inappropriate medications to be avoided in older adults. The criteria serve as a filter when considering starting a new medication and aiding in the review process.8
Continue to: The NO TEARS tool...
The NO TEARS tool11 can be useful for simplifying the medication review process. Components of this tool are:
- Need and indication: Does the patient still require each of his medications? Was long-term treatment anticipated?
- Open questions: Ask the patient for his views about his medications; for example, “Do you think the drugs you take work?”
- Tests and monitoring: Are any of the patient’s conditions undertreated, based on laboratory and clinical findings?
- Evidence and guidelines: Has the base of evidence been updated for each of the patient’s medications since they were started?
- Adverse events: Is the patient experiencing adverse effects of medication? Have possible adverse drug interactions been noted?
- Risk reduction or prevention: Does the patient face risks of treatment (eg, loss of appetite, urinary incontinence) that can be reduced by optimizing the medication plan?
- Simplification and switches: Can treatment be simplified while maintaining effectiveness?
There are strategies to promote patient advocacy, as well. Encourage patients to use a holistic approach by asking you, their other physicians, and their pharmacist about how their condition is being treated:
- What other treatment options exist, including nonpharmacotherapeutic options?
- What are the possible benefits and harms of medical therapy?
- Under what circumstances would discontinuing a medication be appropriate?12
CASE
Medication reconciliation identifies > 20 medications that had been prescribed for the patient to take at home (TABLE 1). A clinical pharmacist then performs a home medication review as part of routine patient care upon transition of care into the hospital.

Identifying polypharmacy
Implementing polypharmacy identification tools is a necessary first step in the process of mitigating the risk of multiple concurrent medications (TABLE 22,10,12-18). In addition to tools that are used to identify polypharmacy, there are steps that physicians and pharmacists can take to decrease the risk of polypharmacy.
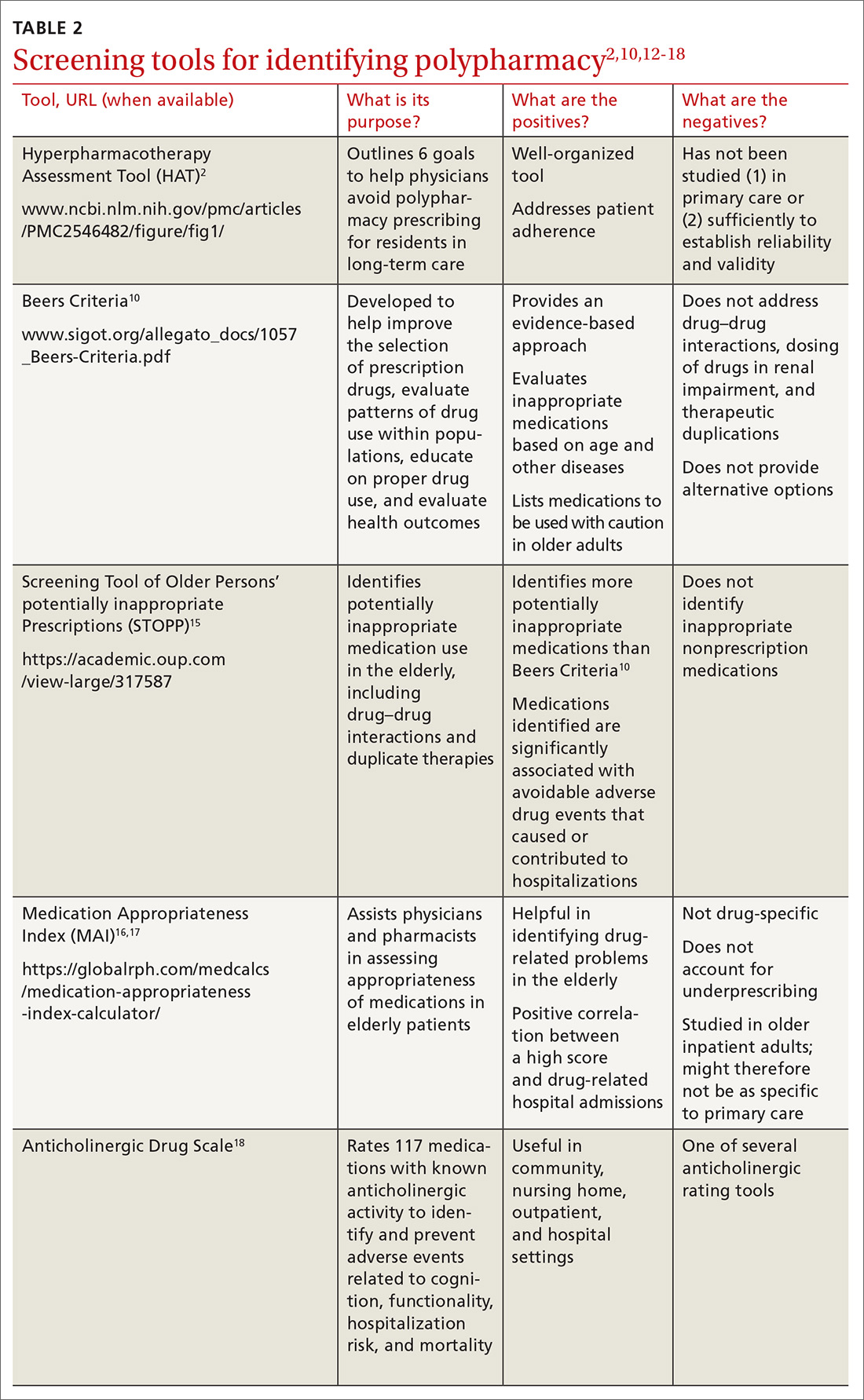
For example, in a longitudinal, time-series cohort study measuring polypharmacy events, a pharmacist intervention was used as the means to decrease polypharmacy.19 Pharmacists intervened twice (each intervention separated by 1 year) to identify and manage 5 categories of high-risk drugs in patients whose care was provided by a managed care plan.19 During that time, pharmacists provided drug therapy reviews, education to physicians and patients about drug safety, and information for physicians on ways to correct problems with polypharmacy.19
Continue to: Over the course of the 2 interventions...
Over the course of the 2 interventions, the overall rate of polypharmacy events decreased 67% after the first intervention and 39% after the second. The practice of having pharmacists spearhead this task was shown to reduce the cost and number of prescriptions in patients at risk for polypharmacy. (In fact, some general practitioners report that they deem multidisciplinary decision-making with pharmacists a necessary component of managing polypharmacy effectively.6)
Screening for medications as a cause of signs and symptoms
As noted earlier, a prescribing cascade arises when a drug administered to a patient causes an adverse event that is then mistakenly identified as a new condition, resulting in a new medication being prescribed.9 The pattern of a cascade then repeats itself, resulting in inappropriate polypharmacy.
Erroneous treatment of an adverse drug event as a medical condition is often the result of a lack of pharmacologic knowledge—which is why it is necessary to evaluate each new symptom with the mindset that a medication might, in fact, be causing the sign or symptom and with the aim of reducing the risk of a prescribing cascade.8,9 Routinely update a patient’s medication list in the event that a medication no longer has an indication aligned with the patient’s problem list; then, ideally, the initial therapy can be adjusted instead of starting additional medications.9
CASE
A review of Mr. W’s home medications reveals 1 therapeutic duplication and 2 drugs that lacked an indication. Application of the Screening Tool of Older Persons’ potentially inappropriate Prescriptions (STOPP)15 and Beers criteria10 helped the pharmacist identify additional elements of inappropriate polypharmacy, including inappropriate medication use, drug–disease interactions, contraindications, and recommendations for dosage adjustment based on kidney function. Specifically:
- Aripiprazole and quetiapine: Present an increased risk of falls. (General recommendation: Avoid using Frutiger LT Std≥ 3 drugs that act on the central nervous system [CNS], due to an increased risk of falls.)
- Fluoxetine: Can cause the syndrome of inappropriate secretion of antidiuretic hormone. Use with caution.
- Gabapentin: Presents an increased risk of CNS adverse effects. Reduce the dosage when the estimated creatinine clearance is < 60 mL/min.
- Hydrocodone–acetaminophen: Presents an increased risk of falls. (Again, avoid or minimize the number of drugs that act on the CNS.)
- Lorazepam: Indication is missing. Avoid use of this drug due to an increased risk of cognitive impairment and decreased metabolism of medication.
- Mirtazapine: Can cause the syndrome of inappropriate secretion of antidiuretic hormone. Use with caution.
- Pantoprazole: Avoid scheduled use for > 8 weeks, except in high-risk patients, due to the risk of Clostridium difficile infection and bone loss and fractures.
- Prazosin: Indication is missing. Avoid use of this drug as an antihypertensive due to the high risk of orthostatic hypotension.
- Ranitidine: Duplicates concurrent treatment with pantoprazole. Reduce the dosage when the estimated creatinine clearance is < 50 mL/min.
The value of deprescribing
Direct evidence of the efficacy and safety of deprescribing, and strategies for deprescribing, have been documented in the literature:
Observational study. Cessation of inappropriate antihypertensive agents was associated with fewer cardiovascular events and deaths over a 5-year follow-up period.20
Continue to: Deprescribing protocol
Deprescribing protocol. A method developed by Scott and co-workers21 is an additional resource to consider. Appropriate times to consider deprescribing are (1) when new symptoms suggest an adverse drug effect; (2) in the presence of end-stage disease, terminal illness, dementia, extreme frailty, or full dependence on others for all care; (3) upon receipt of high-risk medications or combinations; and (4) upon receipt of preventive medications for which risk outweighs benefit.21
This suggested method of deprescribing comprises several steps: (1) collecting all medications that the patient is taking and identifying the indication for each; (2) considering the overall risk of drug-induced harm to determine necessary intensity of deprescribing; (3) assessing each drug for its eligibility to be discontinued, such as no indication, part of a prescribing cascade, or lack of benefit; (4) prioritizing drugs for discontinuation; and (5) implementing and monitoring the drug discontinuation regimen.21
Drug-by-drug elimination trial. Reducing the dosage of, or stopping, only 1 medication at a time has been shown to be paramount to assessing development of medication-associated problems and then identifying a likely cause.14
Good Palliative-Geriatric Practice algorithm. This algorithm22 can be used to guide discontinuation of inappropriate medications and improve drug therapy in community-dwelling older adults. The algorithm has been shown to improve the overall well-being of patients studied; however, it has been tested only in patients in long-term care settings and community-dwelling palliative care patients, limiting its generalizability to a larger population. The algorithm is also difficult to apply to patients who have multiple comorbidities.
Risk vs. benefit of discontinuing chronic medical therapy. A systematic review of the effects of discontinuing chronic medication reveals that the risk of doing so might outweigh benefit14; this finding is thought to be due to potential relapse in the disease state being treated.11 The risks of discontinuation should be contemplated before removing the medication or reducing the dosage. Medications that can be considered to present a risk when discontinued include, but are not limited to, benzodiazepines, oral corticosteroids, antidepressants, acid suppressants, bisphosphonates, statins, and transdermal opioids.1
Continue to: CASE
CASE
After applying Beers criteria10 and STOPP15, the pharmacist makes several recommendations:
- Use aripiprazole and quetiapine with caution.
- Consider discontinuing fluoxetine, hydrocodone–acetaminophen, lorazepam, pantoprazole, and ranitidine.
- Reduce the dosage of gabapentin.
- Clarify the indication for prazosin. Consider discontinuing if being used as an antihypertensive.
In addition, the pharmacist recommends holding metformin because lactic acidosis can develop (however rarely) when a person taking metformin experiences acute kidney injury.
CORRESPONDENCE
Tracy Mahvan, PharmD, BCGP, University of Wyoming, School of Pharmacy, 1000 East University Avenue, Laramie, WY 82071; [email protected]
1. All Wales Medicines Strategy Group. Polypharmacy: Guidance for Prescribing. July 2014. http://awmsg.org/docs/awmsg/medman/Polypharmacy%20-%20Guidance%20for%20Prescribing.pdf. Accessed October 3, 2019.
2. Bushardt RL, Massey EB, Simpson TW, et al. Polypharmacy: misleading, but manageable. Clin Interv Aging. 2008;3:383-389.
3. Maher RL, Hanlon J, Hajjar ER. Clinical consequences of polypharmacy in elderly. Expert Opin Drug Saf. 2014;13:57-65.
4. Masnoon N, Shakib S, Kalisch-Ellett L, et al. What is polypharmacy? A systematic review of definitions. BMC Geriatr. 2017;17:230.
5. Milton JC, Hill-Smith I, Jackson SH. Prescribing for older people. BMJ. 2008;336:606-609.
6. Laursen J, Kornholt J, Betzer C, et al. General practitioners’ barriers toward medication reviews in polymedicated multimorbid patients: How can a focus on the pharmacotherapy in an outpatient clinic support GPs? Health Serv Res Manag Epidemiol. 2018;5:2333392818792169.
7. Christensen M, Lundh A. Medication review in hospitalized patients to reduce morbidity and mortality. Cochrane Database Syst Rev. 2016;2:CD008986.
8. Zurakowski T. The practicalities and pitfalls of polypharmacy. Nurse Pract. 2009;34:36-41.
9. Ponte ML, Wachs L, Wachs A, et al. Prescribing cascade. A proposed new way to evaluate it. Medicina (B Aires). 2017;77:13-16.
10. American Geriatrics Society 2015 Beers Criteria Update Expert Panel. American Geriatrics Society 2015 Updated Beers Criteria for Potentially Inappropriate Medication Use in Older Adults. J Am Geriatr Soc. 2015;63:2227-2246.
11. Lewis T. Using the NO TEARS tool for medication review. BMJ. 2004;329:434.
12. Hamilton HJ, Gallagher PF, O’Mahony D. Inappropriate prescribing and adverse events in older people. BMC Geriatr. 2009;9:5.
13. Skinner M. A literature review: polypharmacy protocol for primary care. Geriatr Nurs. 2015;36:367-371.
14. Salahudeen MS, Duffull SB, Nishtala PS. Anticholinergic burden quantified by anticholinergic risk scales and adverse outcomes in older people: a systematic review. BMC Geriatr. 2015;15:31.
15. Gallagher P, O’Mahony D. STOPP (Screening Tool of Older Persons’ potentially inappropriate Prescriptions): application to acutely ill elderly patients and comparison with Beers criteria. Age Ageing. 2008;37:673-679.
16. Hanlon JT, Schmader KE, Samsa GP, et al. A method for assessing drug therapy appropriateness. J Clin Epidemiol. 1992;45:1045-1051.
17. Samsa G, Hanlon JT, Schmader KE, et al. A summated score for the Medication Appropriateness Index: development and assessment of clinimetric properties including content validity. J Clin Epidemiol. 1994;47:891-896.
18. Carnahan RM, Lund BC, Perry PJ, et al. The Anticholinergic Drug Scale as a measure of drug-related anticholinergic burden: associations with serum anticholinergic activity. J Clin Pharmacol. 2006;46:1481-1486.
19. Zarowitz BJ, Stebelsky LA, Muma BK, et al. Reduction of high-risk polypharmacy drug combinations in patients in a managed care setting. Pharmacotherapy. 2005;25:1636-1645.
20. Thio SL, Nam J, van Driel ML, et al. Effects of discontinuation of chronic medication in primary care: a systematic review of deprescribing trials. Br J Gen Pract. 2018;68:e663-e672.
21. Scott IA, Hilmer SN, Reeve E, et al. Reducing inappropriate polypharmacy: the process of deprescribing. JAMA Intern Med. 2015;175:827-834.
22. Garfinkel D, Mangin D. Feasibility study of a systematic approach for discontinuation of multiple medications in older adults: addressing polypharmacy. Arch Intern Med. 2010;170:1648-1654.
CASE
Ronald Wa is a 74-year old man with an extensive medical history: diabetes, hypertension, heart failure, atrial fibrillation, pancreatitis, hyperlipidemia, gout, depression, generalized anxiety, obstructive sleep apnea, and benign prostatic hypertrophy. He arrives at the emergency department (ED) of the hospital by nonemergent ambulance from home for evaluation of lethargy and confusion over the past week.
In the ED, Mr. W is afebrile, normotensive, and oxygenating on room air. Mucous membranes are dry. On physical examination, he appears pale, fatigued, and modestly confused but is able to state his name and birthday, although not the location or date.
Laboratory testing reveals: blood glucose, 107 mg/dL; serum creatinine, 2.3 mg/dL; sodium, 127 mEq/L; and hemoglobin level and hematocrit, within normal limits. Urinalysis is negative. Renal ultrasonography is unremarkable, without evidence of urinary tract obstruction.
Mr. W is admitted to the general medical unit with hyponatremia. The pharmacy admission specialist begins reconciliation of the long list of the patient’s home medications.
Overprescribing: Often, more is not better
Some experts consider prescribing medication to be the most common form of medical intervention; beyond that, polypharmacy—often defined as the use of more medications than are medically necessary (see the next section on terminology)—is recognized as an increasingly serious problem in many medical specialties.1 Here are specifics about the extent of, and harm caused by, the problem2,3:
- The US General Accounting Office reports that inappropriate polypharmacy is associated with significant morbidity and mortality.2 Research has established a strong relationship between polypharmacy and harmful clinical consequences,3 to which the older patient population is most susceptible.
- Polypharmacy is also recognized as an expensive practice; the US Center for Medicare and Medicaid Services estimates that polypharmacy cost US health insurers more than $50 billion annually.2
- Worldwide, with more and more people older than 65 years, polypharmacy is becoming more prevalent, and a growing concern, in older adults; approximately 50% of them take ≥ 1 medications that are medically unnecessary.3
Despite many programs to help with deprescribing, drug–drug interactions and the so-called prescribing cascade (ie, when signs and symptoms of an adverse drug effect are misdiagnosed as a new medical condition) continue to affect patients, leading to comorbidities. It is important, therefore, for physicians to be aware of commonly used tools to prevent polypharmacy and its consequences.
What is “polypharmacy” understood to mean?
Despite the compelling association of polypharmacy with the presence of multiple morbidities in the older patient population, there is no consensus on its definition:
- Starting with the dictionary, “polypharmacy” derives from 2 words in Ancient Greek: poly, “more than one,” and “pharmakon, “drug.”3
- The definition can vary based on the number of drugs a patient has been prescribed, their safety, and the appropriateness of their use.1
- Another definition is the use of more medications than are medically necessary; such a grouping includes agents that are not indicated, are ineffective, or constitute a therapeutic duplication. Although this definition is more clinically relevant than the others, it is premised on undertaking a clinical review of a medication regimen.3
- A numerical definition is the most commonly reported category, a number that varies from study to study—from ≥ 2 to ≥ 11 medications. When applied to health care settings, accepted definitions are ≥ 5 medications at hospital discharge and ≥ 10 during a hospital stay.4 Numerical definitions of polypharmacy do not ascertain the clinical appropriateness of therapy nor the process of rationalizing those medications.1
aA composite, hypothetical patient, based on the authors' clinical experience.
Continue to: Appropriateness
Appropriateness
Polypharmacy is classified as appropriate or inappropriate:
- Appropriate polypharmacy is the optimization of medications for patients with complex or multiple conditions, when the use of medicine is in agreement with best evidence.
- Inappropriate polypharmacy can increase the risk of adverse drug effects and drug–drug interactions and can be characterized by medication underuse and duplication.4
There are subdefinitions of “appropriateness,” but these are beyond the scope of this article.
What variables contribute to polypharmacy?
Multimorbidity is common in the older population. The presence of multiple chronic conditions increases the complexity of therapeutic management for health professionals and patients; such complexity can have a harmful impact on health outcomes. Combinations of medications to treat chronic diseases automatically push many patients into polypharmacy. Few treatment guidelines provide recommendations on when to stop medications.
Consequences of polypharmacy, some of which are masked as syndromes in the older patient, include delirium and dementia, urinary incontinence, dizziness, falls, adverse drug reactions, increased length of hospital stay, readmission soon after discharge, and death.3-5 Relatively high rates of drug consumption and other variables (eg, decreased renal and hepatic function, decreased total body water and lean body mass, cognitive impairment, age-related decline in vision and hearing, frequency of chronic diseases and medical comorbidities, communication barriers, prescribing cascades, and health care delivery involving multiple prescribers) can contribute to an increased prevalence of medication-associated morbidity and mortality as the result of polypharmacy.
In a descriptive study6 that examined these variables, researchers explored whether general practitioners experience barriers to medication review in multimorbid patients with polypharmacy. They concluded that the primary barriers were (1) lack of communication and teamwork with specialists and (2) the challenge of handling polypharmacy in a culture that encourages adding medications and inhibits conversations about medication withdrawal.6
Continue to: Reducing consequences of polypharmacy
Reducing consequences of polypharmacy
Collaborative medication review
Interventions to help physicians reduce polypharmacy include reviewing medications with older patients at every office visit and during transitions of care into and out of the hospital or other care facility. A 2016 Cochrane review of 5 randomized trials of inpatient medication reviews led by pharmacists, physicians, and other health care professionals showed a 36% reduction in ED visits 30 days to 1 year after discharge.7
Patients can collaborate in this effort by bringing all medications to each appointment or upon hospital admission—not just a list but the actual supply, to ensure that a correct medication list is compiled and a thorough review conducted.8 Explicitly ask open-ended questions of the patient about over-the-counter medications, herbal products, and other home remedies that have not been prescribed; many patients may have trouble with recall or are uncertain what fits the definition of a nonprescription medication.8,9
Compare the medication list with the patient’s current problem list; consider removing medications that do not have a pertinent indication. (Physicians can help in this regard when prescribing by making note in the medical record of the indication for each medication they prescribe.)
Evaluate the patient’s signs and symptoms as a possible drug-related adverse effect, thus making an effort to minimize the chance of a prescribing cascade.9
Use Beers criteria,10 which list potentially inappropriate medications to be avoided in older adults. The criteria serve as a filter when considering starting a new medication and aiding in the review process.8
Continue to: The NO TEARS tool...
The NO TEARS tool11 can be useful for simplifying the medication review process. Components of this tool are:
- Need and indication: Does the patient still require each of his medications? Was long-term treatment anticipated?
- Open questions: Ask the patient for his views about his medications; for example, “Do you think the drugs you take work?”
- Tests and monitoring: Are any of the patient’s conditions undertreated, based on laboratory and clinical findings?
- Evidence and guidelines: Has the base of evidence been updated for each of the patient’s medications since they were started?
- Adverse events: Is the patient experiencing adverse effects of medication? Have possible adverse drug interactions been noted?
- Risk reduction or prevention: Does the patient face risks of treatment (eg, loss of appetite, urinary incontinence) that can be reduced by optimizing the medication plan?
- Simplification and switches: Can treatment be simplified while maintaining effectiveness?
There are strategies to promote patient advocacy, as well. Encourage patients to use a holistic approach by asking you, their other physicians, and their pharmacist about how their condition is being treated:
- What other treatment options exist, including nonpharmacotherapeutic options?
- What are the possible benefits and harms of medical therapy?
- Under what circumstances would discontinuing a medication be appropriate?12
CASE
Medication reconciliation identifies > 20 medications that had been prescribed for the patient to take at home (TABLE 1). A clinical pharmacist then performs a home medication review as part of routine patient care upon transition of care into the hospital.

Identifying polypharmacy
Implementing polypharmacy identification tools is a necessary first step in the process of mitigating the risk of multiple concurrent medications (TABLE 22,10,12-18). In addition to tools that are used to identify polypharmacy, there are steps that physicians and pharmacists can take to decrease the risk of polypharmacy.

For example, in a longitudinal, time-series cohort study measuring polypharmacy events, a pharmacist intervention was used as the means to decrease polypharmacy.19 Pharmacists intervened twice (each intervention separated by 1 year) to identify and manage 5 categories of high-risk drugs in patients whose care was provided by a managed care plan.19 During that time, pharmacists provided drug therapy reviews, education to physicians and patients about drug safety, and information for physicians on ways to correct problems with polypharmacy.19
Continue to: Over the course of the 2 interventions...
Over the course of the 2 interventions, the overall rate of polypharmacy events decreased 67% after the first intervention and 39% after the second. The practice of having pharmacists spearhead this task was shown to reduce the cost and number of prescriptions in patients at risk for polypharmacy. (In fact, some general practitioners report that they deem multidisciplinary decision-making with pharmacists a necessary component of managing polypharmacy effectively.6)
Screening for medications as a cause of signs and symptoms
As noted earlier, a prescribing cascade arises when a drug administered to a patient causes an adverse event that is then mistakenly identified as a new condition, resulting in a new medication being prescribed.9 The pattern of a cascade then repeats itself, resulting in inappropriate polypharmacy.
Erroneous treatment of an adverse drug event as a medical condition is often the result of a lack of pharmacologic knowledge—which is why it is necessary to evaluate each new symptom with the mindset that a medication might, in fact, be causing the sign or symptom and with the aim of reducing the risk of a prescribing cascade.8,9 Routinely update a patient’s medication list in the event that a medication no longer has an indication aligned with the patient’s problem list; then, ideally, the initial therapy can be adjusted instead of starting additional medications.9
CASE
A review of Mr. W’s home medications reveals 1 therapeutic duplication and 2 drugs that lacked an indication. Application of the Screening Tool of Older Persons’ potentially inappropriate Prescriptions (STOPP)15 and Beers criteria10 helped the pharmacist identify additional elements of inappropriate polypharmacy, including inappropriate medication use, drug–disease interactions, contraindications, and recommendations for dosage adjustment based on kidney function. Specifically:
- Aripiprazole and quetiapine: Present an increased risk of falls. (General recommendation: Avoid using Frutiger LT Std≥ 3 drugs that act on the central nervous system [CNS], due to an increased risk of falls.)
- Fluoxetine: Can cause the syndrome of inappropriate secretion of antidiuretic hormone. Use with caution.
- Gabapentin: Presents an increased risk of CNS adverse effects. Reduce the dosage when the estimated creatinine clearance is < 60 mL/min.
- Hydrocodone–acetaminophen: Presents an increased risk of falls. (Again, avoid or minimize the number of drugs that act on the CNS.)
- Lorazepam: Indication is missing. Avoid use of this drug due to an increased risk of cognitive impairment and decreased metabolism of medication.
- Mirtazapine: Can cause the syndrome of inappropriate secretion of antidiuretic hormone. Use with caution.
- Pantoprazole: Avoid scheduled use for > 8 weeks, except in high-risk patients, due to the risk of Clostridium difficile infection and bone loss and fractures.
- Prazosin: Indication is missing. Avoid use of this drug as an antihypertensive due to the high risk of orthostatic hypotension.
- Ranitidine: Duplicates concurrent treatment with pantoprazole. Reduce the dosage when the estimated creatinine clearance is < 50 mL/min.
The value of deprescribing
Direct evidence of the efficacy and safety of deprescribing, and strategies for deprescribing, have been documented in the literature:
Observational study. Cessation of inappropriate antihypertensive agents was associated with fewer cardiovascular events and deaths over a 5-year follow-up period.20
Continue to: Deprescribing protocol
Deprescribing protocol. A method developed by Scott and co-workers21 is an additional resource to consider. Appropriate times to consider deprescribing are (1) when new symptoms suggest an adverse drug effect; (2) in the presence of end-stage disease, terminal illness, dementia, extreme frailty, or full dependence on others for all care; (3) upon receipt of high-risk medications or combinations; and (4) upon receipt of preventive medications for which risk outweighs benefit.21
This suggested method of deprescribing comprises several steps: (1) collecting all medications that the patient is taking and identifying the indication for each; (2) considering the overall risk of drug-induced harm to determine necessary intensity of deprescribing; (3) assessing each drug for its eligibility to be discontinued, such as no indication, part of a prescribing cascade, or lack of benefit; (4) prioritizing drugs for discontinuation; and (5) implementing and monitoring the drug discontinuation regimen.21
Drug-by-drug elimination trial. Reducing the dosage of, or stopping, only 1 medication at a time has been shown to be paramount to assessing development of medication-associated problems and then identifying a likely cause.14
Good Palliative-Geriatric Practice algorithm. This algorithm22 can be used to guide discontinuation of inappropriate medications and improve drug therapy in community-dwelling older adults. The algorithm has been shown to improve the overall well-being of patients studied; however, it has been tested only in patients in long-term care settings and community-dwelling palliative care patients, limiting its generalizability to a larger population. The algorithm is also difficult to apply to patients who have multiple comorbidities.
Risk vs. benefit of discontinuing chronic medical therapy. A systematic review of the effects of discontinuing chronic medication reveals that the risk of doing so might outweigh benefit14; this finding is thought to be due to potential relapse in the disease state being treated.11 The risks of discontinuation should be contemplated before removing the medication or reducing the dosage. Medications that can be considered to present a risk when discontinued include, but are not limited to, benzodiazepines, oral corticosteroids, antidepressants, acid suppressants, bisphosphonates, statins, and transdermal opioids.1
Continue to: CASE
CASE
After applying Beers criteria10 and STOPP15, the pharmacist makes several recommendations:
- Use aripiprazole and quetiapine with caution.
- Consider discontinuing fluoxetine, hydrocodone–acetaminophen, lorazepam, pantoprazole, and ranitidine.
- Reduce the dosage of gabapentin.
- Clarify the indication for prazosin. Consider discontinuing if being used as an antihypertensive.
In addition, the pharmacist recommends holding metformin because lactic acidosis can develop (however rarely) when a person taking metformin experiences acute kidney injury.
CORRESPONDENCE
Tracy Mahvan, PharmD, BCGP, University of Wyoming, School of Pharmacy, 1000 East University Avenue, Laramie, WY 82071; [email protected]
CASE
Ronald Wa is a 74-year old man with an extensive medical history: diabetes, hypertension, heart failure, atrial fibrillation, pancreatitis, hyperlipidemia, gout, depression, generalized anxiety, obstructive sleep apnea, and benign prostatic hypertrophy. He arrives at the emergency department (ED) of the hospital by nonemergent ambulance from home for evaluation of lethargy and confusion over the past week.
In the ED, Mr. W is afebrile, normotensive, and oxygenating on room air. Mucous membranes are dry. On physical examination, he appears pale, fatigued, and modestly confused but is able to state his name and birthday, although not the location or date.
Laboratory testing reveals: blood glucose, 107 mg/dL; serum creatinine, 2.3 mg/dL; sodium, 127 mEq/L; and hemoglobin level and hematocrit, within normal limits. Urinalysis is negative. Renal ultrasonography is unremarkable, without evidence of urinary tract obstruction.
Mr. W is admitted to the general medical unit with hyponatremia. The pharmacy admission specialist begins reconciliation of the long list of the patient’s home medications.
Overprescribing: Often, more is not better
Some experts consider prescribing medication to be the most common form of medical intervention; beyond that, polypharmacy—often defined as the use of more medications than are medically necessary (see the next section on terminology)—is recognized as an increasingly serious problem in many medical specialties.1 Here are specifics about the extent of, and harm caused by, the problem2,3:
- The US General Accounting Office reports that inappropriate polypharmacy is associated with significant morbidity and mortality.2 Research has established a strong relationship between polypharmacy and harmful clinical consequences,3 to which the older patient population is most susceptible.
- Polypharmacy is also recognized as an expensive practice; the US Center for Medicare and Medicaid Services estimates that polypharmacy cost US health insurers more than $50 billion annually.2
- Worldwide, with more and more people older than 65 years, polypharmacy is becoming more prevalent, and a growing concern, in older adults; approximately 50% of them take ≥ 1 medications that are medically unnecessary.3
Despite many programs to help with deprescribing, drug–drug interactions and the so-called prescribing cascade (ie, when signs and symptoms of an adverse drug effect are misdiagnosed as a new medical condition) continue to affect patients, leading to comorbidities. It is important, therefore, for physicians to be aware of commonly used tools to prevent polypharmacy and its consequences.
What is “polypharmacy” understood to mean?
Despite the compelling association of polypharmacy with the presence of multiple morbidities in the older patient population, there is no consensus on its definition:
- Starting with the dictionary, “polypharmacy” derives from 2 words in Ancient Greek: poly, “more than one,” and “pharmakon, “drug.”3
- The definition can vary based on the number of drugs a patient has been prescribed, their safety, and the appropriateness of their use.1
- Another definition is the use of more medications than are medically necessary; such a grouping includes agents that are not indicated, are ineffective, or constitute a therapeutic duplication. Although this definition is more clinically relevant than the others, it is premised on undertaking a clinical review of a medication regimen.3
- A numerical definition is the most commonly reported category, a number that varies from study to study—from ≥ 2 to ≥ 11 medications. When applied to health care settings, accepted definitions are ≥ 5 medications at hospital discharge and ≥ 10 during a hospital stay.4 Numerical definitions of polypharmacy do not ascertain the clinical appropriateness of therapy nor the process of rationalizing those medications.1
aA composite, hypothetical patient, based on the authors' clinical experience.
Continue to: Appropriateness
Appropriateness
Polypharmacy is classified as appropriate or inappropriate:
- Appropriate polypharmacy is the optimization of medications for patients with complex or multiple conditions, when the use of medicine is in agreement with best evidence.
- Inappropriate polypharmacy can increase the risk of adverse drug effects and drug–drug interactions and can be characterized by medication underuse and duplication.4
There are subdefinitions of “appropriateness,” but these are beyond the scope of this article.
What variables contribute to polypharmacy?
Multimorbidity is common in the older population. The presence of multiple chronic conditions increases the complexity of therapeutic management for health professionals and patients; such complexity can have a harmful impact on health outcomes. Combinations of medications to treat chronic diseases automatically push many patients into polypharmacy. Few treatment guidelines provide recommendations on when to stop medications.
Consequences of polypharmacy, some of which are masked as syndromes in the older patient, include delirium and dementia, urinary incontinence, dizziness, falls, adverse drug reactions, increased length of hospital stay, readmission soon after discharge, and death.3-5 Relatively high rates of drug consumption and other variables (eg, decreased renal and hepatic function, decreased total body water and lean body mass, cognitive impairment, age-related decline in vision and hearing, frequency of chronic diseases and medical comorbidities, communication barriers, prescribing cascades, and health care delivery involving multiple prescribers) can contribute to an increased prevalence of medication-associated morbidity and mortality as the result of polypharmacy.
In a descriptive study6 that examined these variables, researchers explored whether general practitioners experience barriers to medication review in multimorbid patients with polypharmacy. They concluded that the primary barriers were (1) lack of communication and teamwork with specialists and (2) the challenge of handling polypharmacy in a culture that encourages adding medications and inhibits conversations about medication withdrawal.6
Continue to: Reducing consequences of polypharmacy
Reducing consequences of polypharmacy
Collaborative medication review
Interventions to help physicians reduce polypharmacy include reviewing medications with older patients at every office visit and during transitions of care into and out of the hospital or other care facility. A 2016 Cochrane review of 5 randomized trials of inpatient medication reviews led by pharmacists, physicians, and other health care professionals showed a 36% reduction in ED visits 30 days to 1 year after discharge.7
Patients can collaborate in this effort by bringing all medications to each appointment or upon hospital admission—not just a list but the actual supply, to ensure that a correct medication list is compiled and a thorough review conducted.8 Explicitly ask open-ended questions of the patient about over-the-counter medications, herbal products, and other home remedies that have not been prescribed; many patients may have trouble with recall or are uncertain what fits the definition of a nonprescription medication.8,9
Compare the medication list with the patient’s current problem list; consider removing medications that do not have a pertinent indication. (Physicians can help in this regard when prescribing by making note in the medical record of the indication for each medication they prescribe.)
Evaluate the patient’s signs and symptoms as a possible drug-related adverse effect, thus making an effort to minimize the chance of a prescribing cascade.9
Use Beers criteria,10 which list potentially inappropriate medications to be avoided in older adults. The criteria serve as a filter when considering starting a new medication and aiding in the review process.8
Continue to: The NO TEARS tool...
The NO TEARS tool11 can be useful for simplifying the medication review process. Components of this tool are:
- Need and indication: Does the patient still require each of his medications? Was long-term treatment anticipated?
- Open questions: Ask the patient for his views about his medications; for example, “Do you think the drugs you take work?”
- Tests and monitoring: Are any of the patient’s conditions undertreated, based on laboratory and clinical findings?
- Evidence and guidelines: Has the base of evidence been updated for each of the patient’s medications since they were started?
- Adverse events: Is the patient experiencing adverse effects of medication? Have possible adverse drug interactions been noted?
- Risk reduction or prevention: Does the patient face risks of treatment (eg, loss of appetite, urinary incontinence) that can be reduced by optimizing the medication plan?
- Simplification and switches: Can treatment be simplified while maintaining effectiveness?
There are strategies to promote patient advocacy, as well. Encourage patients to use a holistic approach by asking you, their other physicians, and their pharmacist about how their condition is being treated:
- What other treatment options exist, including nonpharmacotherapeutic options?
- What are the possible benefits and harms of medical therapy?
- Under what circumstances would discontinuing a medication be appropriate?12
CASE
Medication reconciliation identifies > 20 medications that had been prescribed for the patient to take at home (TABLE 1). A clinical pharmacist then performs a home medication review as part of routine patient care upon transition of care into the hospital.

Identifying polypharmacy
Implementing polypharmacy identification tools is a necessary first step in the process of mitigating the risk of multiple concurrent medications (TABLE 22,10,12-18). In addition to tools that are used to identify polypharmacy, there are steps that physicians and pharmacists can take to decrease the risk of polypharmacy.

For example, in a longitudinal, time-series cohort study measuring polypharmacy events, a pharmacist intervention was used as the means to decrease polypharmacy.19 Pharmacists intervened twice (each intervention separated by 1 year) to identify and manage 5 categories of high-risk drugs in patients whose care was provided by a managed care plan.19 During that time, pharmacists provided drug therapy reviews, education to physicians and patients about drug safety, and information for physicians on ways to correct problems with polypharmacy.19
Continue to: Over the course of the 2 interventions...
Over the course of the 2 interventions, the overall rate of polypharmacy events decreased 67% after the first intervention and 39% after the second. The practice of having pharmacists spearhead this task was shown to reduce the cost and number of prescriptions in patients at risk for polypharmacy. (In fact, some general practitioners report that they deem multidisciplinary decision-making with pharmacists a necessary component of managing polypharmacy effectively.6)
Screening for medications as a cause of signs and symptoms
As noted earlier, a prescribing cascade arises when a drug administered to a patient causes an adverse event that is then mistakenly identified as a new condition, resulting in a new medication being prescribed.9 The pattern of a cascade then repeats itself, resulting in inappropriate polypharmacy.
Erroneous treatment of an adverse drug event as a medical condition is often the result of a lack of pharmacologic knowledge—which is why it is necessary to evaluate each new symptom with the mindset that a medication might, in fact, be causing the sign or symptom and with the aim of reducing the risk of a prescribing cascade.8,9 Routinely update a patient’s medication list in the event that a medication no longer has an indication aligned with the patient’s problem list; then, ideally, the initial therapy can be adjusted instead of starting additional medications.9
CASE
A review of Mr. W’s home medications reveals 1 therapeutic duplication and 2 drugs that lacked an indication. Application of the Screening Tool of Older Persons’ potentially inappropriate Prescriptions (STOPP)15 and Beers criteria10 helped the pharmacist identify additional elements of inappropriate polypharmacy, including inappropriate medication use, drug–disease interactions, contraindications, and recommendations for dosage adjustment based on kidney function. Specifically:
- Aripiprazole and quetiapine: Present an increased risk of falls. (General recommendation: Avoid using Frutiger LT Std≥ 3 drugs that act on the central nervous system [CNS], due to an increased risk of falls.)
- Fluoxetine: Can cause the syndrome of inappropriate secretion of antidiuretic hormone. Use with caution.
- Gabapentin: Presents an increased risk of CNS adverse effects. Reduce the dosage when the estimated creatinine clearance is < 60 mL/min.
- Hydrocodone–acetaminophen: Presents an increased risk of falls. (Again, avoid or minimize the number of drugs that act on the CNS.)
- Lorazepam: Indication is missing. Avoid use of this drug due to an increased risk of cognitive impairment and decreased metabolism of medication.
- Mirtazapine: Can cause the syndrome of inappropriate secretion of antidiuretic hormone. Use with caution.
- Pantoprazole: Avoid scheduled use for > 8 weeks, except in high-risk patients, due to the risk of Clostridium difficile infection and bone loss and fractures.
- Prazosin: Indication is missing. Avoid use of this drug as an antihypertensive due to the high risk of orthostatic hypotension.
- Ranitidine: Duplicates concurrent treatment with pantoprazole. Reduce the dosage when the estimated creatinine clearance is < 50 mL/min.
The value of deprescribing
Direct evidence of the efficacy and safety of deprescribing, and strategies for deprescribing, have been documented in the literature:
Observational study. Cessation of inappropriate antihypertensive agents was associated with fewer cardiovascular events and deaths over a 5-year follow-up period.20
Continue to: Deprescribing protocol
Deprescribing protocol. A method developed by Scott and co-workers21 is an additional resource to consider. Appropriate times to consider deprescribing are (1) when new symptoms suggest an adverse drug effect; (2) in the presence of end-stage disease, terminal illness, dementia, extreme frailty, or full dependence on others for all care; (3) upon receipt of high-risk medications or combinations; and (4) upon receipt of preventive medications for which risk outweighs benefit.21
This suggested method of deprescribing comprises several steps: (1) collecting all medications that the patient is taking and identifying the indication for each; (2) considering the overall risk of drug-induced harm to determine necessary intensity of deprescribing; (3) assessing each drug for its eligibility to be discontinued, such as no indication, part of a prescribing cascade, or lack of benefit; (4) prioritizing drugs for discontinuation; and (5) implementing and monitoring the drug discontinuation regimen.21
Drug-by-drug elimination trial. Reducing the dosage of, or stopping, only 1 medication at a time has been shown to be paramount to assessing development of medication-associated problems and then identifying a likely cause.14
Good Palliative-Geriatric Practice algorithm. This algorithm22 can be used to guide discontinuation of inappropriate medications and improve drug therapy in community-dwelling older adults. The algorithm has been shown to improve the overall well-being of patients studied; however, it has been tested only in patients in long-term care settings and community-dwelling palliative care patients, limiting its generalizability to a larger population. The algorithm is also difficult to apply to patients who have multiple comorbidities.
Risk vs. benefit of discontinuing chronic medical therapy. A systematic review of the effects of discontinuing chronic medication reveals that the risk of doing so might outweigh benefit14; this finding is thought to be due to potential relapse in the disease state being treated.11 The risks of discontinuation should be contemplated before removing the medication or reducing the dosage. Medications that can be considered to present a risk when discontinued include, but are not limited to, benzodiazepines, oral corticosteroids, antidepressants, acid suppressants, bisphosphonates, statins, and transdermal opioids.1
Continue to: CASE
CASE
After applying Beers criteria10 and STOPP15, the pharmacist makes several recommendations:
- Use aripiprazole and quetiapine with caution.
- Consider discontinuing fluoxetine, hydrocodone–acetaminophen, lorazepam, pantoprazole, and ranitidine.
- Reduce the dosage of gabapentin.
- Clarify the indication for prazosin. Consider discontinuing if being used as an antihypertensive.
In addition, the pharmacist recommends holding metformin because lactic acidosis can develop (however rarely) when a person taking metformin experiences acute kidney injury.
CORRESPONDENCE
Tracy Mahvan, PharmD, BCGP, University of Wyoming, School of Pharmacy, 1000 East University Avenue, Laramie, WY 82071; [email protected]
1. All Wales Medicines Strategy Group. Polypharmacy: Guidance for Prescribing. July 2014. http://awmsg.org/docs/awmsg/medman/Polypharmacy%20-%20Guidance%20for%20Prescribing.pdf. Accessed October 3, 2019.
2. Bushardt RL, Massey EB, Simpson TW, et al. Polypharmacy: misleading, but manageable. Clin Interv Aging. 2008;3:383-389.
3. Maher RL, Hanlon J, Hajjar ER. Clinical consequences of polypharmacy in elderly. Expert Opin Drug Saf. 2014;13:57-65.
4. Masnoon N, Shakib S, Kalisch-Ellett L, et al. What is polypharmacy? A systematic review of definitions. BMC Geriatr. 2017;17:230.
5. Milton JC, Hill-Smith I, Jackson SH. Prescribing for older people. BMJ. 2008;336:606-609.
6. Laursen J, Kornholt J, Betzer C, et al. General practitioners’ barriers toward medication reviews in polymedicated multimorbid patients: How can a focus on the pharmacotherapy in an outpatient clinic support GPs? Health Serv Res Manag Epidemiol. 2018;5:2333392818792169.
7. Christensen M, Lundh A. Medication review in hospitalized patients to reduce morbidity and mortality. Cochrane Database Syst Rev. 2016;2:CD008986.
8. Zurakowski T. The practicalities and pitfalls of polypharmacy. Nurse Pract. 2009;34:36-41.
9. Ponte ML, Wachs L, Wachs A, et al. Prescribing cascade. A proposed new way to evaluate it. Medicina (B Aires). 2017;77:13-16.
10. American Geriatrics Society 2015 Beers Criteria Update Expert Panel. American Geriatrics Society 2015 Updated Beers Criteria for Potentially Inappropriate Medication Use in Older Adults. J Am Geriatr Soc. 2015;63:2227-2246.
11. Lewis T. Using the NO TEARS tool for medication review. BMJ. 2004;329:434.
12. Hamilton HJ, Gallagher PF, O’Mahony D. Inappropriate prescribing and adverse events in older people. BMC Geriatr. 2009;9:5.
13. Skinner M. A literature review: polypharmacy protocol for primary care. Geriatr Nurs. 2015;36:367-371.
14. Salahudeen MS, Duffull SB, Nishtala PS. Anticholinergic burden quantified by anticholinergic risk scales and adverse outcomes in older people: a systematic review. BMC Geriatr. 2015;15:31.
15. Gallagher P, O’Mahony D. STOPP (Screening Tool of Older Persons’ potentially inappropriate Prescriptions): application to acutely ill elderly patients and comparison with Beers criteria. Age Ageing. 2008;37:673-679.
16. Hanlon JT, Schmader KE, Samsa GP, et al. A method for assessing drug therapy appropriateness. J Clin Epidemiol. 1992;45:1045-1051.
17. Samsa G, Hanlon JT, Schmader KE, et al. A summated score for the Medication Appropriateness Index: development and assessment of clinimetric properties including content validity. J Clin Epidemiol. 1994;47:891-896.
18. Carnahan RM, Lund BC, Perry PJ, et al. The Anticholinergic Drug Scale as a measure of drug-related anticholinergic burden: associations with serum anticholinergic activity. J Clin Pharmacol. 2006;46:1481-1486.
19. Zarowitz BJ, Stebelsky LA, Muma BK, et al. Reduction of high-risk polypharmacy drug combinations in patients in a managed care setting. Pharmacotherapy. 2005;25:1636-1645.
20. Thio SL, Nam J, van Driel ML, et al. Effects of discontinuation of chronic medication in primary care: a systematic review of deprescribing trials. Br J Gen Pract. 2018;68:e663-e672.
21. Scott IA, Hilmer SN, Reeve E, et al. Reducing inappropriate polypharmacy: the process of deprescribing. JAMA Intern Med. 2015;175:827-834.
22. Garfinkel D, Mangin D. Feasibility study of a systematic approach for discontinuation of multiple medications in older adults: addressing polypharmacy. Arch Intern Med. 2010;170:1648-1654.
1. All Wales Medicines Strategy Group. Polypharmacy: Guidance for Prescribing. July 2014. http://awmsg.org/docs/awmsg/medman/Polypharmacy%20-%20Guidance%20for%20Prescribing.pdf. Accessed October 3, 2019.
2. Bushardt RL, Massey EB, Simpson TW, et al. Polypharmacy: misleading, but manageable. Clin Interv Aging. 2008;3:383-389.
3. Maher RL, Hanlon J, Hajjar ER. Clinical consequences of polypharmacy in elderly. Expert Opin Drug Saf. 2014;13:57-65.
4. Masnoon N, Shakib S, Kalisch-Ellett L, et al. What is polypharmacy? A systematic review of definitions. BMC Geriatr. 2017;17:230.
5. Milton JC, Hill-Smith I, Jackson SH. Prescribing for older people. BMJ. 2008;336:606-609.
6. Laursen J, Kornholt J, Betzer C, et al. General practitioners’ barriers toward medication reviews in polymedicated multimorbid patients: How can a focus on the pharmacotherapy in an outpatient clinic support GPs? Health Serv Res Manag Epidemiol. 2018;5:2333392818792169.
7. Christensen M, Lundh A. Medication review in hospitalized patients to reduce morbidity and mortality. Cochrane Database Syst Rev. 2016;2:CD008986.
8. Zurakowski T. The practicalities and pitfalls of polypharmacy. Nurse Pract. 2009;34:36-41.
9. Ponte ML, Wachs L, Wachs A, et al. Prescribing cascade. A proposed new way to evaluate it. Medicina (B Aires). 2017;77:13-16.
10. American Geriatrics Society 2015 Beers Criteria Update Expert Panel. American Geriatrics Society 2015 Updated Beers Criteria for Potentially Inappropriate Medication Use in Older Adults. J Am Geriatr Soc. 2015;63:2227-2246.
11. Lewis T. Using the NO TEARS tool for medication review. BMJ. 2004;329:434.
12. Hamilton HJ, Gallagher PF, O’Mahony D. Inappropriate prescribing and adverse events in older people. BMC Geriatr. 2009;9:5.
13. Skinner M. A literature review: polypharmacy protocol for primary care. Geriatr Nurs. 2015;36:367-371.
14. Salahudeen MS, Duffull SB, Nishtala PS. Anticholinergic burden quantified by anticholinergic risk scales and adverse outcomes in older people: a systematic review. BMC Geriatr. 2015;15:31.
15. Gallagher P, O’Mahony D. STOPP (Screening Tool of Older Persons’ potentially inappropriate Prescriptions): application to acutely ill elderly patients and comparison with Beers criteria. Age Ageing. 2008;37:673-679.
16. Hanlon JT, Schmader KE, Samsa GP, et al. A method for assessing drug therapy appropriateness. J Clin Epidemiol. 1992;45:1045-1051.
17. Samsa G, Hanlon JT, Schmader KE, et al. A summated score for the Medication Appropriateness Index: development and assessment of clinimetric properties including content validity. J Clin Epidemiol. 1994;47:891-896.
18. Carnahan RM, Lund BC, Perry PJ, et al. The Anticholinergic Drug Scale as a measure of drug-related anticholinergic burden: associations with serum anticholinergic activity. J Clin Pharmacol. 2006;46:1481-1486.
19. Zarowitz BJ, Stebelsky LA, Muma BK, et al. Reduction of high-risk polypharmacy drug combinations in patients in a managed care setting. Pharmacotherapy. 2005;25:1636-1645.
20. Thio SL, Nam J, van Driel ML, et al. Effects of discontinuation of chronic medication in primary care: a systematic review of deprescribing trials. Br J Gen Pract. 2018;68:e663-e672.
21. Scott IA, Hilmer SN, Reeve E, et al. Reducing inappropriate polypharmacy: the process of deprescribing. JAMA Intern Med. 2015;175:827-834.
22. Garfinkel D, Mangin D. Feasibility study of a systematic approach for discontinuation of multiple medications in older adults: addressing polypharmacy. Arch Intern Med. 2010;170:1648-1654.
PRACTICE RECOMMENDATIONS
› Use one of the available tested and recommended screening tools to identify polypharmacy. C
› Engage in collaborative medication review to reduce the incidence of polypharmacy. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Physician burnout: Signs and solutions
CASE
Dr. Peter D is a mid-career family physician in a group practice that recently adopted an electronic health record system. Although he realizes he is now competent at computerized medicine, he has far less of the one-on-one patient contact that he once found so gratifying about the field of medicine.
Others in the practice have similar concerns, but they suggest that everyone ought to “go along to get along.” To manage the increasing demands of his case load and the required documentation, Dr. D has begun staying late to finish charting, which is negatively impacting his family life.
Dr. D finds himself burdened by record keeping that is increasingly complicated and insurance company demands that are onerous. Pharmaceutical prior authorizations that previously had been mildly bothersome are now a full-on burden. More often than not, he finds himself becoming irritable over extra requests and administrative demands, impatient with some patients and staff, and extremely fatigued at the end of workdays. Simply put, he finds that practicing medicine is far less enjoyable than it once was. He takes the Maslach Burnout Inventory, and his score indicates that he has moderate burnout.

Physician burnout has been a growing concern in recent decades.1 Characterized by varying degrees of job dissatisfaction, cynicism, emotional exhaustion, clinical inefficiency, and depression, physician burnout can impede effective patient care, cause significant health issues among physicians, diminish professional gratification and feelings of accomplishment, and financially burden society as a whole. Here we present the information you need to recognize burnout in yourself and colleagues and address the problem on personal, organizational, and legislative levels.
A problem that affects physicians of all ages
Physician burnout has been recognized to present anywhere on a spectrum, manifesting as ineffectiveness, overextension, disengagement, and/or an inability to practice.2 Such features may lead to feelings of professional inadequacy among even the highest functioning physicians.
Burnout occurs in all stages of medical life—as students, residents, and practicing physicians.3-6 Due to pressures in excess of coping capacity, some physicians will suffer from alcohol or other drug abuse, depression, and/or suicidal thinking.7 Stress and burnout can also result in musculoskeletal disorders, immune system dysfunction, cardiac pathology, and a shorter lifespan.8
Not only do individual practitioners suffer consequences from burnout, but it also compromises health care delivery. In 2018, the Medscape National Physician Burnout and Depression Report surveyed 15,000 physicians from 29 specialties; 33% of the respondents said that they were more easily frustrated by patients, and 32% reported less personal engagement.9 Burnout adversely impacts care, patient satisfaction, productivity, physician retention, retirement, and income, as well.6 Safety during clinical practice deteriorates because of an increase in medical error rates.10 Resultant emotional distress for physicians creates a vicious cycle.10
[polldaddy:10427848]
Continue to: These issues negatively impact...
These issues negatively impact practice enthusiasm and may engender self-doubt.11 They may lead to absenteeism or, worse, to abandoning the profession, further contributing to physician shortages.12 The financial impact of physician burnout in lost revenue in 2018 was about $17 billion, according to the National Taskforce for Humanity in Medicine.13
How prevalent is physician burnout?
Between October 2012 and March 2013, the American Society of Clinical Oncology surveyed US oncologists and found that 45% had evidence of burnout.14 In another survey of US physicians from all specialties conducted in 2011, at least 1 symptom of burnout was documented in nearly 46% of respondents.15 By 2014, this percentage increased to 54%.16
In 2018, the Medscape National Physician Burnout and Depression Report indicated that 42% of physicians admitted to some burnout, while 12% said they were unhappy at work, and 3% reported being clinically depressed.9 About 48% of female practitioners reported burnout vs 38% of male peers.9 Work-related distress varies between specialties, with internists, family physicians, intensivists, neurologists, and gynecologists more affected than those from other specialties.9
Causes and contributing factors
Job stress generally increases with changes in the workplace. This can be heightened in the health care workplace, which demands perfection and leaves little room for emotional issues. Loss of autonomy, time constraints associated with clinical care, electronic health record (EHR) documentation, and disorganized workflow tend to contribute to provider dissatisfaction and stress, as do ethical disagreements about patient care between physicians and leadership.10,17 Fear of reprisal for speaking up about such issues can further exacerbate the problem. Some older physicians may have difficulty with technology and computerized record keeping. Reduced patient contact due to increasing reliance on computers can diminish physicians’ job satisfaction. And managing recurrent or difficult-to-treat ailments can result in compassion fatigue, diminished empathy, and emotional disengagement.
Burnout in the health care workplace is inconsistently addressed, despite negative professional and personal ramifications. The reasons include denial, uncertainty about monetary implications, and lack of corrective programs by decision-making organizations and/or employers.6 American medicine has lacked the political and financial will to implement strategies to mitigate burnout. Improvement requires changes on the part of government, physician groups, and the population at large.
The answer?
A multipronged approach
Identifying burnout is the first step in management. The 22-item Maslach Burnout Inventory (MBI) is a self-reporting questionnaire, reliable at detecting and assessing burnout severity.18 It screens 3 main domains: emotional exhaustion, depersonalization, and diminished feelings of accomplishment. The American Medical Association recommends the 10-item Zero Burnout Program—the “Mini Z Survey”—as being quicker and more convenient.19
Once the problem is recognized, experts suggest adopting a multipronged approach to prevention and intervention by using personal, organizational, and legislative strategies.20
Continue to: On a personal level...
On a personal level, it’s important to identify stressors and employ stress-reduction and coping skills, such as mindfulness and/or reflection.21 Mindfulness programs may help to minimize exhaustion, increase compassion, and improve understanding of other people’s feelings.22 Such programs are widely available and may be accessed through the Internet, mental health centers, or by contacting psychiatric or psychological services.
Other self-care methods include ensuring adequate sleep, nutrition, exercise, and enjoyable activities. If a physician who is suffering from burnout is taking any prescription or over-the-counter drugs or supplements, it is important to be self-aware of the potential for misuse of medications. Of course, one should never self-prescribe controlled drugs, such as opiates and sedatives. Consumption of alcohol must be well-controlled, without excesses, and drinking near bedtime is ill-advised. The use of illegal substances should be avoided.
Pursuing aspects of health care that are meaningful and that increase patient contact time can boost enthusiasm, as can focusing on the positives aspects of one’s career.23 Continuing medical education can enhance self-esteem and promote a sense of purpose.24
Peer support. Practice partners may assist their colleagues by alerting them to signs of burnout, offering timely intervention suggestions, and monitoring the effectiveness of strategies. Physicians should discuss stress and burnout with their peers; camaraderie within a practice group is helpful.
Professional coaches or counselors may be engaged to mitigate workplace distress. Coaching is best instituted collegially with pre-identified goals in order to minimize stigmatization.
Continue to: Professional societies and medical boards
Professional societies and medical boards. Reporting requirements by medical boards tend to stigmatize those seeking professional assistance. But that could change if all of us—through our participation in these organizations—pursue change.
Specifically, organizations and related societies could assist with better guidance and policy adjustment (see “Resources”). State medical boards could, for example, increase education of, and outreach to, physicians about mental health issues, while maintaining confidentiality.25 Medical organizations could regularly survey their membership to identify burnout early and identify personal, social, and institutional shortcomings that contribute to physician burnout. In addition, hospital quality improvement committees that monitor health care delivery appropriateness could take steps toward change as well.
SIDEBAR
Resources to help combat burnout
- National Academy of Medicine Action Collaborative on Clinician Well- Being and Resilience: https://nam.edu/initiatives/clinician-resilience-and-well-being/
- The Schwartz Center for Compassionate Healthcare: http://www.theschwartzcenter.org
- NEJM Catalyst: http://catalyst.nejm.org/posts?q=burnout
- The American Medical Association, Joy in Medicine: https://www.ama-assn.org/search?search=joy+in+medicine
- Agency for Healthcare Research and Quality: https://www.ahrq.gov/prevention/clinician/ahrq-works/burnout/index.html
- Accreditation Council for Graduate Medical Education Tools and Resources for Resident and Faculty Member Well-Being: https://www.acgme.org/What-We-Do/Initiatives/Physician-Well-Being/Resources
The American Medical Association (AMA) just recently announced that they are launching a new effort to fight the causes of physician burnout. The AMA’s Practice Transformation Inititative26 seeks to fill the knowledge gaps regarding effective interventions to reduce burnout. AMA’s leadership indicates that the initiative will focus on “improving joy in medicine by using validated assessment tools to measure burnout; field-testing interventions that are designed to improve workflows, applying practice science research methodology to evaluate impact, and sharing best practices within an AMA-facilitated learning community.”26
Stanford’s example. Stanford University instituted a ‘time bank’ program, to help their academic medical faculty balance work and life and reduce stress. They essentially offer services, such as home food delivery and house cleaning, in return for hours spent in the clinic.27
Reorganizing and reprioritizing. Prioritizing physician wellness as a quality indicator and instituting a committee to advocate for wellness can help attenuate burnout.28,29 Specific measures include minimizing rushed, overloaded scheduling and allowing more clinical contact time with patients. Using nursing and office staff to streamline workflow is also helpful.29 The University of Colorado’s “Ambulatory Process Excellence Model” strives to assist doctors by increasing the medical assistant-to-clinician ratio, yielding better productivity.23 Medical assistants are increasingly handling tasks such as data entry, medication reconciliation, and preventive care, to allow physicians more time to focus on medical decision-making.23
Continue to: The role of the EHR
The role of the EHR. One important way to boost professional morale is to simplify and shorten the EHR. The complexity of and reduced patient contact caused by today’s record-keeping systems is the source of great frustration among many physicians. In addition, many patients dislike the disproportionate attention paid by physicians to the computer during office visits, further compromising physician-patient relationships. Improving documentation methodology and/or employing medical assistant scribes can be helpful.30,31 (See “Advanced team-based care: How we made it work” at http://bit.ly/2lNaB5Q.)
Legislation with physician input can mandate policies for more appropriate work environments. A good way to initiate improvement and reform strategies is to contact local medical societies and political representatives. Federal and state collaboration to reduce physician shortages in selected specialties or geographic regions can improve work-related stress. This might be attained by expanding residency programs, using telemedicine in underserved regions, and employing more physician assistants.32
Health insurance. Enhancing universal access to affordable medical care, including pharmaceutical coverage, would alleviate stress for physicians and patients alike.33 Health insurance regulation to decrease paperwork and simplify coverage would decrease physician workload. Standardized policy requirements, fewer exclusionary rules, and simplified prescribing guidelines (including having less cumbersome prescription pre-authorizations and greater standardization of drug formularies by different payer sources or insurance plans) would facilitate better clinical management.
CASE
Dr. D begins by discussing his concerns with his colleagues in the group practice and finds he is not alone. Many of the concerns of the group center around brief, rushed appointments that diminish relationships with patients, a lack of autonomy, and the fear of medical malpractice. Several older physicians acknowledge that they just want to retire.
To address the patient contact and documentation issues, the group decides to hire scribes. They also decide to bring their concerns to the next county medical society meeting. The end result: They petitioned their state medical association to host presentations about mitigating burnout, to hold roundtable discussions, and to establish panels focused on remedying the situation.
Continue to: With this accomplished...
With this accomplished, Dr. D’s anxieties lessened. He surveyed relevant literature and shared tips for improving professional time management with his partners. In a hopeful mood, he volunteered to address burnout prevention at the next statewide medical meeting. He felt it was a good start.
CORRESPONDENCE
Steven Lippmann, MD, 401 E. Chestnut Street, Suite 610, Louisville, KY 40202; [email protected].
1. Ramirez AJ, Graham J, Richards MA, et al. Burnout and psychiatric disorder among cancer clinicians. Br J Cancer. 1995;71:1263-1269.
2. Leiter MP, Maslach C. Latent burnout profiles: a new approach to understanding the burnout experience. Burnout Research. 2016;3:89-100.
3. Dyrbye LN, Thomas MR, Massie FS, et al. Burnout and suicidal ideation among U.S. medical students. Ann Int Med. 2008;149:334-341.
4. West CP, Shanafelt TD, Kolars JC. Quality of life, burnout, educational debt, and medical knowledge among internal medicine residents. JAMA. 2011;306:952-960.
5. Shanafelt TD, Balch CM, Bechamps GJ, et al. Burnout and career satisfaction among American surgeons. Ann Surg. 2009;250:463-471.
6. Shanafelt TD, Goh J, Sinsky C. The business case for investing in physician well-being. JAMA Intern Med. 2017;177:1826-1832.
7. Cottler LB, Ajinkya S, Merlo LJ, et al. Lifetime psychiatric and substance use disorders among impaired physicians in a physicians health program. J Addict Med. 2013;7:108-112.
8. Consiglio C. Interpersonal strain at work: a new burnout facet relevant for the health of hospital staff. Burnout Res. 2014;1:69-75.
9. Peckham C. Medscape National Physician Burnout and Depression Report 2018. January 12, 2018. https://www.medscape.com/slideshow/2018-lifestyle-burnout-depression-6009235. Accessed October 4, 2019.
10. Shanafelt TD, Balch CM, Bechamps G, et al. Burnout and medical errors among American surgeons. Ann Surg. 2010;251:995-1000.
11. West CP, Dyrbye LN, Rabatin JT, et al. Intervention to promote physician well-being, job satisfaction, and professionalism: a randomized clinical trial. JAMA Intern Med. 2014;174:527-533.
12. Suñer-Soler R, Grau-Martin A, Flichtentrei D, et al. The consequences of burnout syndrome among healthcare professionals in Spain and Spanish speaking Latin American countries. Burnout Research. 2014;1:82-89.
13. National Taskforce for Humanity in Healthcare. Position paper: The business case for humanity in healthcare. April 2018. https://www.vocera.com/public/pdf/NTHBusinessCase_final003.pdf. Accessed October 4, 2019.
14. Shanafelt TD, Gradishar WJ, Kosty M, et al. Burnout and career satisfaction among US oncologists. J Clin Oncol. 2014;32:678-686.
15. Shanafelt TD, Boone S, Tan L, et al. Burnout and satisfaction with work-life balance among US physicians relative to the general US population. Arch Int Med. 2012;172:1377-1385.
16. Shanafelt TD, Hasan O, Dyrbye LN, et al. Changes in burnout and satisfaction with work-life balance in physicians and the general US working population between 2011 and 2014. Mayo Clin Proc. 2015;90;1600-1613.
17. Linzer M, Manwell LB, Williams ES, et al. Working conditions in primary care: physician reactions and care quality. Ann Intern Med. 2009;151:28-36.
18. Maslach C, Jackson SE. The measurement of experienced burnout. J Occcup Behav. 1981;2:99-113.
19. Linzer M, Guzman-Corrales L, Poplau S. Physician Burnout: improve physician satisfaction and patient outcomes. June 5, 2015. https://www.stepsforward.org/modules/physician-burnout. Accessed October 4, 2019.
20. West CP, Dyrbye LN, Erwin PJ, et al. Interventions to prevent and reduce physician burnout: a systematic review and meta-analysis. Lancet. 2016;388:2272-2281.
21. Nedrow A, Steckler NA, Hardman J. Physician resilience and burnout: can you make the switch? Fam Prac Manag. 2013;20:25-30.
22. Verweij H, van Ravesteijn H, van Hooff MLM, et al. Mindfulness-based stress reduction for residents: a randomized controlled trial. J Gen Intern Med. 2018;33:429-436.
23. Wright AA, Katz IT. Beyond burnout – redesigning care to restore meaning and sanity for physicians. N Eng J Med. 2018;378:309-311.
24. Shanafelt TD, Gorringe G, Menaker R, et. al. Impact of organizational leadership on physician burnout and satisfaction. Mayo Clin Proc. 2015;90:432-440.
25. Hengerer A, Kishore S. 2017. Breaking a culture of silence: the role of state medical boards. National Academy of Medicine, Washington DC. https://nam.edu/breaking-a-culture-of-silence-the-role-of-state-medical-boards/. Accessed October 4, 2019.
26. American Medical Association. AMA fights burnout with new practice transformation initiative. September 5, 2019. https://www.ama-assn.org/press-center/press-releases/ama-fights-burnout-new-practice-transformation-initiative. Accessed September 5, 2019.
27. Schulte B. Time in the bank: a Stanford plan to save doctors from burnout. The Washington Post. https://www.washingtonpost.com/news/inspired-life/wp/2015/08/20/the-innovative-stanford-program-thats-saving-emergency-room-doctors-from-burnout/?utm_term=.838c930e8de7. Accessed October 4, 2019.
28. Wallace JE, Lemaire JB, Ghali WA. Physician wellness: a missing quality indicator. Lancet. 2009;374:1714-1721.
29. Bodenheimer T, Sinsky C. From triple to quadruple aim: care of the patient requires care of the provider. Ann Fam Med. 2014;12:573-576.
30. Babbott S, Manwell LB, Brown R, et al. Electronic medical records and physician stress in primary care: results from the MEMO Study. J Am Med Info Assoc. 2014;21:E100-E106.
31. Bodenheimer T, Willard-Grace R, Ghorob A. Expanding the roles of medical assistants: who does what in primary care? JAMA Intern Med. 2014;174:1025-1026.
32. Mangiofico G. Physician shortage requires multi-prong solution. January 26, 2018. Am J Manag Care. https://www.ajmc.com/contributor/dr-gary-mangiofico/2018/01/physician-shortage-requires-multiprong-solution. Accessed October 4, 2019.
33. Reuben DB, Knudsen J, Senelick W, et al. The effect of a physician partner program on physician efficiency and patient satisfaction. JAMA Intern Med. 2014;174:1190-1193.
CASE
Dr. Peter D is a mid-career family physician in a group practice that recently adopted an electronic health record system. Although he realizes he is now competent at computerized medicine, he has far less of the one-on-one patient contact that he once found so gratifying about the field of medicine.
Others in the practice have similar concerns, but they suggest that everyone ought to “go along to get along.” To manage the increasing demands of his case load and the required documentation, Dr. D has begun staying late to finish charting, which is negatively impacting his family life.
Dr. D finds himself burdened by record keeping that is increasingly complicated and insurance company demands that are onerous. Pharmaceutical prior authorizations that previously had been mildly bothersome are now a full-on burden. More often than not, he finds himself becoming irritable over extra requests and administrative demands, impatient with some patients and staff, and extremely fatigued at the end of workdays. Simply put, he finds that practicing medicine is far less enjoyable than it once was. He takes the Maslach Burnout Inventory, and his score indicates that he has moderate burnout.

Physician burnout has been a growing concern in recent decades.1 Characterized by varying degrees of job dissatisfaction, cynicism, emotional exhaustion, clinical inefficiency, and depression, physician burnout can impede effective patient care, cause significant health issues among physicians, diminish professional gratification and feelings of accomplishment, and financially burden society as a whole. Here we present the information you need to recognize burnout in yourself and colleagues and address the problem on personal, organizational, and legislative levels.
A problem that affects physicians of all ages
Physician burnout has been recognized to present anywhere on a spectrum, manifesting as ineffectiveness, overextension, disengagement, and/or an inability to practice.2 Such features may lead to feelings of professional inadequacy among even the highest functioning physicians.
Burnout occurs in all stages of medical life—as students, residents, and practicing physicians.3-6 Due to pressures in excess of coping capacity, some physicians will suffer from alcohol or other drug abuse, depression, and/or suicidal thinking.7 Stress and burnout can also result in musculoskeletal disorders, immune system dysfunction, cardiac pathology, and a shorter lifespan.8
Not only do individual practitioners suffer consequences from burnout, but it also compromises health care delivery. In 2018, the Medscape National Physician Burnout and Depression Report surveyed 15,000 physicians from 29 specialties; 33% of the respondents said that they were more easily frustrated by patients, and 32% reported less personal engagement.9 Burnout adversely impacts care, patient satisfaction, productivity, physician retention, retirement, and income, as well.6 Safety during clinical practice deteriorates because of an increase in medical error rates.10 Resultant emotional distress for physicians creates a vicious cycle.10
[polldaddy:10427848]
Continue to: These issues negatively impact...
These issues negatively impact practice enthusiasm and may engender self-doubt.11 They may lead to absenteeism or, worse, to abandoning the profession, further contributing to physician shortages.12 The financial impact of physician burnout in lost revenue in 2018 was about $17 billion, according to the National Taskforce for Humanity in Medicine.13
How prevalent is physician burnout?
Between October 2012 and March 2013, the American Society of Clinical Oncology surveyed US oncologists and found that 45% had evidence of burnout.14 In another survey of US physicians from all specialties conducted in 2011, at least 1 symptom of burnout was documented in nearly 46% of respondents.15 By 2014, this percentage increased to 54%.16
In 2018, the Medscape National Physician Burnout and Depression Report indicated that 42% of physicians admitted to some burnout, while 12% said they were unhappy at work, and 3% reported being clinically depressed.9 About 48% of female practitioners reported burnout vs 38% of male peers.9 Work-related distress varies between specialties, with internists, family physicians, intensivists, neurologists, and gynecologists more affected than those from other specialties.9
Causes and contributing factors
Job stress generally increases with changes in the workplace. This can be heightened in the health care workplace, which demands perfection and leaves little room for emotional issues. Loss of autonomy, time constraints associated with clinical care, electronic health record (EHR) documentation, and disorganized workflow tend to contribute to provider dissatisfaction and stress, as do ethical disagreements about patient care between physicians and leadership.10,17 Fear of reprisal for speaking up about such issues can further exacerbate the problem. Some older physicians may have difficulty with technology and computerized record keeping. Reduced patient contact due to increasing reliance on computers can diminish physicians’ job satisfaction. And managing recurrent or difficult-to-treat ailments can result in compassion fatigue, diminished empathy, and emotional disengagement.
Burnout in the health care workplace is inconsistently addressed, despite negative professional and personal ramifications. The reasons include denial, uncertainty about monetary implications, and lack of corrective programs by decision-making organizations and/or employers.6 American medicine has lacked the political and financial will to implement strategies to mitigate burnout. Improvement requires changes on the part of government, physician groups, and the population at large.
The answer?
A multipronged approach
Identifying burnout is the first step in management. The 22-item Maslach Burnout Inventory (MBI) is a self-reporting questionnaire, reliable at detecting and assessing burnout severity.18 It screens 3 main domains: emotional exhaustion, depersonalization, and diminished feelings of accomplishment. The American Medical Association recommends the 10-item Zero Burnout Program—the “Mini Z Survey”—as being quicker and more convenient.19
Once the problem is recognized, experts suggest adopting a multipronged approach to prevention and intervention by using personal, organizational, and legislative strategies.20
Continue to: On a personal level...
On a personal level, it’s important to identify stressors and employ stress-reduction and coping skills, such as mindfulness and/or reflection.21 Mindfulness programs may help to minimize exhaustion, increase compassion, and improve understanding of other people’s feelings.22 Such programs are widely available and may be accessed through the Internet, mental health centers, or by contacting psychiatric or psychological services.
Other self-care methods include ensuring adequate sleep, nutrition, exercise, and enjoyable activities. If a physician who is suffering from burnout is taking any prescription or over-the-counter drugs or supplements, it is important to be self-aware of the potential for misuse of medications. Of course, one should never self-prescribe controlled drugs, such as opiates and sedatives. Consumption of alcohol must be well-controlled, without excesses, and drinking near bedtime is ill-advised. The use of illegal substances should be avoided.
Pursuing aspects of health care that are meaningful and that increase patient contact time can boost enthusiasm, as can focusing on the positives aspects of one’s career.23 Continuing medical education can enhance self-esteem and promote a sense of purpose.24
Peer support. Practice partners may assist their colleagues by alerting them to signs of burnout, offering timely intervention suggestions, and monitoring the effectiveness of strategies. Physicians should discuss stress and burnout with their peers; camaraderie within a practice group is helpful.
Professional coaches or counselors may be engaged to mitigate workplace distress. Coaching is best instituted collegially with pre-identified goals in order to minimize stigmatization.
Continue to: Professional societies and medical boards
Professional societies and medical boards. Reporting requirements by medical boards tend to stigmatize those seeking professional assistance. But that could change if all of us—through our participation in these organizations—pursue change.
Specifically, organizations and related societies could assist with better guidance and policy adjustment (see “Resources”). State medical boards could, for example, increase education of, and outreach to, physicians about mental health issues, while maintaining confidentiality.25 Medical organizations could regularly survey their membership to identify burnout early and identify personal, social, and institutional shortcomings that contribute to physician burnout. In addition, hospital quality improvement committees that monitor health care delivery appropriateness could take steps toward change as well.
SIDEBAR
Resources to help combat burnout
- National Academy of Medicine Action Collaborative on Clinician Well- Being and Resilience: https://nam.edu/initiatives/clinician-resilience-and-well-being/
- The Schwartz Center for Compassionate Healthcare: http://www.theschwartzcenter.org
- NEJM Catalyst: http://catalyst.nejm.org/posts?q=burnout
- The American Medical Association, Joy in Medicine: https://www.ama-assn.org/search?search=joy+in+medicine
- Agency for Healthcare Research and Quality: https://www.ahrq.gov/prevention/clinician/ahrq-works/burnout/index.html
- Accreditation Council for Graduate Medical Education Tools and Resources for Resident and Faculty Member Well-Being: https://www.acgme.org/What-We-Do/Initiatives/Physician-Well-Being/Resources
The American Medical Association (AMA) just recently announced that they are launching a new effort to fight the causes of physician burnout. The AMA’s Practice Transformation Inititative26 seeks to fill the knowledge gaps regarding effective interventions to reduce burnout. AMA’s leadership indicates that the initiative will focus on “improving joy in medicine by using validated assessment tools to measure burnout; field-testing interventions that are designed to improve workflows, applying practice science research methodology to evaluate impact, and sharing best practices within an AMA-facilitated learning community.”26
Stanford’s example. Stanford University instituted a ‘time bank’ program, to help their academic medical faculty balance work and life and reduce stress. They essentially offer services, such as home food delivery and house cleaning, in return for hours spent in the clinic.27
Reorganizing and reprioritizing. Prioritizing physician wellness as a quality indicator and instituting a committee to advocate for wellness can help attenuate burnout.28,29 Specific measures include minimizing rushed, overloaded scheduling and allowing more clinical contact time with patients. Using nursing and office staff to streamline workflow is also helpful.29 The University of Colorado’s “Ambulatory Process Excellence Model” strives to assist doctors by increasing the medical assistant-to-clinician ratio, yielding better productivity.23 Medical assistants are increasingly handling tasks such as data entry, medication reconciliation, and preventive care, to allow physicians more time to focus on medical decision-making.23
Continue to: The role of the EHR
The role of the EHR. One important way to boost professional morale is to simplify and shorten the EHR. The complexity of and reduced patient contact caused by today’s record-keeping systems is the source of great frustration among many physicians. In addition, many patients dislike the disproportionate attention paid by physicians to the computer during office visits, further compromising physician-patient relationships. Improving documentation methodology and/or employing medical assistant scribes can be helpful.30,31 (See “Advanced team-based care: How we made it work” at http://bit.ly/2lNaB5Q.)
Legislation with physician input can mandate policies for more appropriate work environments. A good way to initiate improvement and reform strategies is to contact local medical societies and political representatives. Federal and state collaboration to reduce physician shortages in selected specialties or geographic regions can improve work-related stress. This might be attained by expanding residency programs, using telemedicine in underserved regions, and employing more physician assistants.32
Health insurance. Enhancing universal access to affordable medical care, including pharmaceutical coverage, would alleviate stress for physicians and patients alike.33 Health insurance regulation to decrease paperwork and simplify coverage would decrease physician workload. Standardized policy requirements, fewer exclusionary rules, and simplified prescribing guidelines (including having less cumbersome prescription pre-authorizations and greater standardization of drug formularies by different payer sources or insurance plans) would facilitate better clinical management.
CASE
Dr. D begins by discussing his concerns with his colleagues in the group practice and finds he is not alone. Many of the concerns of the group center around brief, rushed appointments that diminish relationships with patients, a lack of autonomy, and the fear of medical malpractice. Several older physicians acknowledge that they just want to retire.
To address the patient contact and documentation issues, the group decides to hire scribes. They also decide to bring their concerns to the next county medical society meeting. The end result: They petitioned their state medical association to host presentations about mitigating burnout, to hold roundtable discussions, and to establish panels focused on remedying the situation.
Continue to: With this accomplished...
With this accomplished, Dr. D’s anxieties lessened. He surveyed relevant literature and shared tips for improving professional time management with his partners. In a hopeful mood, he volunteered to address burnout prevention at the next statewide medical meeting. He felt it was a good start.
CORRESPONDENCE
Steven Lippmann, MD, 401 E. Chestnut Street, Suite 610, Louisville, KY 40202; [email protected].
CASE
Dr. Peter D is a mid-career family physician in a group practice that recently adopted an electronic health record system. Although he realizes he is now competent at computerized medicine, he has far less of the one-on-one patient contact that he once found so gratifying about the field of medicine.
Others in the practice have similar concerns, but they suggest that everyone ought to “go along to get along.” To manage the increasing demands of his case load and the required documentation, Dr. D has begun staying late to finish charting, which is negatively impacting his family life.
Dr. D finds himself burdened by record keeping that is increasingly complicated and insurance company demands that are onerous. Pharmaceutical prior authorizations that previously had been mildly bothersome are now a full-on burden. More often than not, he finds himself becoming irritable over extra requests and administrative demands, impatient with some patients and staff, and extremely fatigued at the end of workdays. Simply put, he finds that practicing medicine is far less enjoyable than it once was. He takes the Maslach Burnout Inventory, and his score indicates that he has moderate burnout.

Physician burnout has been a growing concern in recent decades.1 Characterized by varying degrees of job dissatisfaction, cynicism, emotional exhaustion, clinical inefficiency, and depression, physician burnout can impede effective patient care, cause significant health issues among physicians, diminish professional gratification and feelings of accomplishment, and financially burden society as a whole. Here we present the information you need to recognize burnout in yourself and colleagues and address the problem on personal, organizational, and legislative levels.
A problem that affects physicians of all ages
Physician burnout has been recognized to present anywhere on a spectrum, manifesting as ineffectiveness, overextension, disengagement, and/or an inability to practice.2 Such features may lead to feelings of professional inadequacy among even the highest functioning physicians.
Burnout occurs in all stages of medical life—as students, residents, and practicing physicians.3-6 Due to pressures in excess of coping capacity, some physicians will suffer from alcohol or other drug abuse, depression, and/or suicidal thinking.7 Stress and burnout can also result in musculoskeletal disorders, immune system dysfunction, cardiac pathology, and a shorter lifespan.8
Not only do individual practitioners suffer consequences from burnout, but it also compromises health care delivery. In 2018, the Medscape National Physician Burnout and Depression Report surveyed 15,000 physicians from 29 specialties; 33% of the respondents said that they were more easily frustrated by patients, and 32% reported less personal engagement.9 Burnout adversely impacts care, patient satisfaction, productivity, physician retention, retirement, and income, as well.6 Safety during clinical practice deteriorates because of an increase in medical error rates.10 Resultant emotional distress for physicians creates a vicious cycle.10
[polldaddy:10427848]
Continue to: These issues negatively impact...
These issues negatively impact practice enthusiasm and may engender self-doubt.11 They may lead to absenteeism or, worse, to abandoning the profession, further contributing to physician shortages.12 The financial impact of physician burnout in lost revenue in 2018 was about $17 billion, according to the National Taskforce for Humanity in Medicine.13
How prevalent is physician burnout?
Between October 2012 and March 2013, the American Society of Clinical Oncology surveyed US oncologists and found that 45% had evidence of burnout.14 In another survey of US physicians from all specialties conducted in 2011, at least 1 symptom of burnout was documented in nearly 46% of respondents.15 By 2014, this percentage increased to 54%.16
In 2018, the Medscape National Physician Burnout and Depression Report indicated that 42% of physicians admitted to some burnout, while 12% said they were unhappy at work, and 3% reported being clinically depressed.9 About 48% of female practitioners reported burnout vs 38% of male peers.9 Work-related distress varies between specialties, with internists, family physicians, intensivists, neurologists, and gynecologists more affected than those from other specialties.9
Causes and contributing factors
Job stress generally increases with changes in the workplace. This can be heightened in the health care workplace, which demands perfection and leaves little room for emotional issues. Loss of autonomy, time constraints associated with clinical care, electronic health record (EHR) documentation, and disorganized workflow tend to contribute to provider dissatisfaction and stress, as do ethical disagreements about patient care between physicians and leadership.10,17 Fear of reprisal for speaking up about such issues can further exacerbate the problem. Some older physicians may have difficulty with technology and computerized record keeping. Reduced patient contact due to increasing reliance on computers can diminish physicians’ job satisfaction. And managing recurrent or difficult-to-treat ailments can result in compassion fatigue, diminished empathy, and emotional disengagement.
Burnout in the health care workplace is inconsistently addressed, despite negative professional and personal ramifications. The reasons include denial, uncertainty about monetary implications, and lack of corrective programs by decision-making organizations and/or employers.6 American medicine has lacked the political and financial will to implement strategies to mitigate burnout. Improvement requires changes on the part of government, physician groups, and the population at large.
The answer?
A multipronged approach
Identifying burnout is the first step in management. The 22-item Maslach Burnout Inventory (MBI) is a self-reporting questionnaire, reliable at detecting and assessing burnout severity.18 It screens 3 main domains: emotional exhaustion, depersonalization, and diminished feelings of accomplishment. The American Medical Association recommends the 10-item Zero Burnout Program—the “Mini Z Survey”—as being quicker and more convenient.19
Once the problem is recognized, experts suggest adopting a multipronged approach to prevention and intervention by using personal, organizational, and legislative strategies.20
Continue to: On a personal level...
On a personal level, it’s important to identify stressors and employ stress-reduction and coping skills, such as mindfulness and/or reflection.21 Mindfulness programs may help to minimize exhaustion, increase compassion, and improve understanding of other people’s feelings.22 Such programs are widely available and may be accessed through the Internet, mental health centers, or by contacting psychiatric or psychological services.
Other self-care methods include ensuring adequate sleep, nutrition, exercise, and enjoyable activities. If a physician who is suffering from burnout is taking any prescription or over-the-counter drugs or supplements, it is important to be self-aware of the potential for misuse of medications. Of course, one should never self-prescribe controlled drugs, such as opiates and sedatives. Consumption of alcohol must be well-controlled, without excesses, and drinking near bedtime is ill-advised. The use of illegal substances should be avoided.
Pursuing aspects of health care that are meaningful and that increase patient contact time can boost enthusiasm, as can focusing on the positives aspects of one’s career.23 Continuing medical education can enhance self-esteem and promote a sense of purpose.24
Peer support. Practice partners may assist their colleagues by alerting them to signs of burnout, offering timely intervention suggestions, and monitoring the effectiveness of strategies. Physicians should discuss stress and burnout with their peers; camaraderie within a practice group is helpful.
Professional coaches or counselors may be engaged to mitigate workplace distress. Coaching is best instituted collegially with pre-identified goals in order to minimize stigmatization.
Continue to: Professional societies and medical boards
Professional societies and medical boards. Reporting requirements by medical boards tend to stigmatize those seeking professional assistance. But that could change if all of us—through our participation in these organizations—pursue change.
Specifically, organizations and related societies could assist with better guidance and policy adjustment (see “Resources”). State medical boards could, for example, increase education of, and outreach to, physicians about mental health issues, while maintaining confidentiality.25 Medical organizations could regularly survey their membership to identify burnout early and identify personal, social, and institutional shortcomings that contribute to physician burnout. In addition, hospital quality improvement committees that monitor health care delivery appropriateness could take steps toward change as well.
SIDEBAR
Resources to help combat burnout
- National Academy of Medicine Action Collaborative on Clinician Well- Being and Resilience: https://nam.edu/initiatives/clinician-resilience-and-well-being/
- The Schwartz Center for Compassionate Healthcare: http://www.theschwartzcenter.org
- NEJM Catalyst: http://catalyst.nejm.org/posts?q=burnout
- The American Medical Association, Joy in Medicine: https://www.ama-assn.org/search?search=joy+in+medicine
- Agency for Healthcare Research and Quality: https://www.ahrq.gov/prevention/clinician/ahrq-works/burnout/index.html
- Accreditation Council for Graduate Medical Education Tools and Resources for Resident and Faculty Member Well-Being: https://www.acgme.org/What-We-Do/Initiatives/Physician-Well-Being/Resources
The American Medical Association (AMA) just recently announced that they are launching a new effort to fight the causes of physician burnout. The AMA’s Practice Transformation Inititative26 seeks to fill the knowledge gaps regarding effective interventions to reduce burnout. AMA’s leadership indicates that the initiative will focus on “improving joy in medicine by using validated assessment tools to measure burnout; field-testing interventions that are designed to improve workflows, applying practice science research methodology to evaluate impact, and sharing best practices within an AMA-facilitated learning community.”26
Stanford’s example. Stanford University instituted a ‘time bank’ program, to help their academic medical faculty balance work and life and reduce stress. They essentially offer services, such as home food delivery and house cleaning, in return for hours spent in the clinic.27
Reorganizing and reprioritizing. Prioritizing physician wellness as a quality indicator and instituting a committee to advocate for wellness can help attenuate burnout.28,29 Specific measures include minimizing rushed, overloaded scheduling and allowing more clinical contact time with patients. Using nursing and office staff to streamline workflow is also helpful.29 The University of Colorado’s “Ambulatory Process Excellence Model” strives to assist doctors by increasing the medical assistant-to-clinician ratio, yielding better productivity.23 Medical assistants are increasingly handling tasks such as data entry, medication reconciliation, and preventive care, to allow physicians more time to focus on medical decision-making.23
Continue to: The role of the EHR
The role of the EHR. One important way to boost professional morale is to simplify and shorten the EHR. The complexity of and reduced patient contact caused by today’s record-keeping systems is the source of great frustration among many physicians. In addition, many patients dislike the disproportionate attention paid by physicians to the computer during office visits, further compromising physician-patient relationships. Improving documentation methodology and/or employing medical assistant scribes can be helpful.30,31 (See “Advanced team-based care: How we made it work” at http://bit.ly/2lNaB5Q.)
Legislation with physician input can mandate policies for more appropriate work environments. A good way to initiate improvement and reform strategies is to contact local medical societies and political representatives. Federal and state collaboration to reduce physician shortages in selected specialties or geographic regions can improve work-related stress. This might be attained by expanding residency programs, using telemedicine in underserved regions, and employing more physician assistants.32
Health insurance. Enhancing universal access to affordable medical care, including pharmaceutical coverage, would alleviate stress for physicians and patients alike.33 Health insurance regulation to decrease paperwork and simplify coverage would decrease physician workload. Standardized policy requirements, fewer exclusionary rules, and simplified prescribing guidelines (including having less cumbersome prescription pre-authorizations and greater standardization of drug formularies by different payer sources or insurance plans) would facilitate better clinical management.
CASE
Dr. D begins by discussing his concerns with his colleagues in the group practice and finds he is not alone. Many of the concerns of the group center around brief, rushed appointments that diminish relationships with patients, a lack of autonomy, and the fear of medical malpractice. Several older physicians acknowledge that they just want to retire.
To address the patient contact and documentation issues, the group decides to hire scribes. They also decide to bring their concerns to the next county medical society meeting. The end result: They petitioned their state medical association to host presentations about mitigating burnout, to hold roundtable discussions, and to establish panels focused on remedying the situation.
Continue to: With this accomplished...
With this accomplished, Dr. D’s anxieties lessened. He surveyed relevant literature and shared tips for improving professional time management with his partners. In a hopeful mood, he volunteered to address burnout prevention at the next statewide medical meeting. He felt it was a good start.
CORRESPONDENCE
Steven Lippmann, MD, 401 E. Chestnut Street, Suite 610, Louisville, KY 40202; [email protected].
1. Ramirez AJ, Graham J, Richards MA, et al. Burnout and psychiatric disorder among cancer clinicians. Br J Cancer. 1995;71:1263-1269.
2. Leiter MP, Maslach C. Latent burnout profiles: a new approach to understanding the burnout experience. Burnout Research. 2016;3:89-100.
3. Dyrbye LN, Thomas MR, Massie FS, et al. Burnout and suicidal ideation among U.S. medical students. Ann Int Med. 2008;149:334-341.
4. West CP, Shanafelt TD, Kolars JC. Quality of life, burnout, educational debt, and medical knowledge among internal medicine residents. JAMA. 2011;306:952-960.
5. Shanafelt TD, Balch CM, Bechamps GJ, et al. Burnout and career satisfaction among American surgeons. Ann Surg. 2009;250:463-471.
6. Shanafelt TD, Goh J, Sinsky C. The business case for investing in physician well-being. JAMA Intern Med. 2017;177:1826-1832.
7. Cottler LB, Ajinkya S, Merlo LJ, et al. Lifetime psychiatric and substance use disorders among impaired physicians in a physicians health program. J Addict Med. 2013;7:108-112.
8. Consiglio C. Interpersonal strain at work: a new burnout facet relevant for the health of hospital staff. Burnout Res. 2014;1:69-75.
9. Peckham C. Medscape National Physician Burnout and Depression Report 2018. January 12, 2018. https://www.medscape.com/slideshow/2018-lifestyle-burnout-depression-6009235. Accessed October 4, 2019.
10. Shanafelt TD, Balch CM, Bechamps G, et al. Burnout and medical errors among American surgeons. Ann Surg. 2010;251:995-1000.
11. West CP, Dyrbye LN, Rabatin JT, et al. Intervention to promote physician well-being, job satisfaction, and professionalism: a randomized clinical trial. JAMA Intern Med. 2014;174:527-533.
12. Suñer-Soler R, Grau-Martin A, Flichtentrei D, et al. The consequences of burnout syndrome among healthcare professionals in Spain and Spanish speaking Latin American countries. Burnout Research. 2014;1:82-89.
13. National Taskforce for Humanity in Healthcare. Position paper: The business case for humanity in healthcare. April 2018. https://www.vocera.com/public/pdf/NTHBusinessCase_final003.pdf. Accessed October 4, 2019.
14. Shanafelt TD, Gradishar WJ, Kosty M, et al. Burnout and career satisfaction among US oncologists. J Clin Oncol. 2014;32:678-686.
15. Shanafelt TD, Boone S, Tan L, et al. Burnout and satisfaction with work-life balance among US physicians relative to the general US population. Arch Int Med. 2012;172:1377-1385.
16. Shanafelt TD, Hasan O, Dyrbye LN, et al. Changes in burnout and satisfaction with work-life balance in physicians and the general US working population between 2011 and 2014. Mayo Clin Proc. 2015;90;1600-1613.
17. Linzer M, Manwell LB, Williams ES, et al. Working conditions in primary care: physician reactions and care quality. Ann Intern Med. 2009;151:28-36.
18. Maslach C, Jackson SE. The measurement of experienced burnout. J Occcup Behav. 1981;2:99-113.
19. Linzer M, Guzman-Corrales L, Poplau S. Physician Burnout: improve physician satisfaction and patient outcomes. June 5, 2015. https://www.stepsforward.org/modules/physician-burnout. Accessed October 4, 2019.
20. West CP, Dyrbye LN, Erwin PJ, et al. Interventions to prevent and reduce physician burnout: a systematic review and meta-analysis. Lancet. 2016;388:2272-2281.
21. Nedrow A, Steckler NA, Hardman J. Physician resilience and burnout: can you make the switch? Fam Prac Manag. 2013;20:25-30.
22. Verweij H, van Ravesteijn H, van Hooff MLM, et al. Mindfulness-based stress reduction for residents: a randomized controlled trial. J Gen Intern Med. 2018;33:429-436.
23. Wright AA, Katz IT. Beyond burnout – redesigning care to restore meaning and sanity for physicians. N Eng J Med. 2018;378:309-311.
24. Shanafelt TD, Gorringe G, Menaker R, et. al. Impact of organizational leadership on physician burnout and satisfaction. Mayo Clin Proc. 2015;90:432-440.
25. Hengerer A, Kishore S. 2017. Breaking a culture of silence: the role of state medical boards. National Academy of Medicine, Washington DC. https://nam.edu/breaking-a-culture-of-silence-the-role-of-state-medical-boards/. Accessed October 4, 2019.
26. American Medical Association. AMA fights burnout with new practice transformation initiative. September 5, 2019. https://www.ama-assn.org/press-center/press-releases/ama-fights-burnout-new-practice-transformation-initiative. Accessed September 5, 2019.
27. Schulte B. Time in the bank: a Stanford plan to save doctors from burnout. The Washington Post. https://www.washingtonpost.com/news/inspired-life/wp/2015/08/20/the-innovative-stanford-program-thats-saving-emergency-room-doctors-from-burnout/?utm_term=.838c930e8de7. Accessed October 4, 2019.
28. Wallace JE, Lemaire JB, Ghali WA. Physician wellness: a missing quality indicator. Lancet. 2009;374:1714-1721.
29. Bodenheimer T, Sinsky C. From triple to quadruple aim: care of the patient requires care of the provider. Ann Fam Med. 2014;12:573-576.
30. Babbott S, Manwell LB, Brown R, et al. Electronic medical records and physician stress in primary care: results from the MEMO Study. J Am Med Info Assoc. 2014;21:E100-E106.
31. Bodenheimer T, Willard-Grace R, Ghorob A. Expanding the roles of medical assistants: who does what in primary care? JAMA Intern Med. 2014;174:1025-1026.
32. Mangiofico G. Physician shortage requires multi-prong solution. January 26, 2018. Am J Manag Care. https://www.ajmc.com/contributor/dr-gary-mangiofico/2018/01/physician-shortage-requires-multiprong-solution. Accessed October 4, 2019.
33. Reuben DB, Knudsen J, Senelick W, et al. The effect of a physician partner program on physician efficiency and patient satisfaction. JAMA Intern Med. 2014;174:1190-1193.
1. Ramirez AJ, Graham J, Richards MA, et al. Burnout and psychiatric disorder among cancer clinicians. Br J Cancer. 1995;71:1263-1269.
2. Leiter MP, Maslach C. Latent burnout profiles: a new approach to understanding the burnout experience. Burnout Research. 2016;3:89-100.
3. Dyrbye LN, Thomas MR, Massie FS, et al. Burnout and suicidal ideation among U.S. medical students. Ann Int Med. 2008;149:334-341.
4. West CP, Shanafelt TD, Kolars JC. Quality of life, burnout, educational debt, and medical knowledge among internal medicine residents. JAMA. 2011;306:952-960.
5. Shanafelt TD, Balch CM, Bechamps GJ, et al. Burnout and career satisfaction among American surgeons. Ann Surg. 2009;250:463-471.
6. Shanafelt TD, Goh J, Sinsky C. The business case for investing in physician well-being. JAMA Intern Med. 2017;177:1826-1832.
7. Cottler LB, Ajinkya S, Merlo LJ, et al. Lifetime psychiatric and substance use disorders among impaired physicians in a physicians health program. J Addict Med. 2013;7:108-112.
8. Consiglio C. Interpersonal strain at work: a new burnout facet relevant for the health of hospital staff. Burnout Res. 2014;1:69-75.
9. Peckham C. Medscape National Physician Burnout and Depression Report 2018. January 12, 2018. https://www.medscape.com/slideshow/2018-lifestyle-burnout-depression-6009235. Accessed October 4, 2019.
10. Shanafelt TD, Balch CM, Bechamps G, et al. Burnout and medical errors among American surgeons. Ann Surg. 2010;251:995-1000.
11. West CP, Dyrbye LN, Rabatin JT, et al. Intervention to promote physician well-being, job satisfaction, and professionalism: a randomized clinical trial. JAMA Intern Med. 2014;174:527-533.
12. Suñer-Soler R, Grau-Martin A, Flichtentrei D, et al. The consequences of burnout syndrome among healthcare professionals in Spain and Spanish speaking Latin American countries. Burnout Research. 2014;1:82-89.
13. National Taskforce for Humanity in Healthcare. Position paper: The business case for humanity in healthcare. April 2018. https://www.vocera.com/public/pdf/NTHBusinessCase_final003.pdf. Accessed October 4, 2019.
14. Shanafelt TD, Gradishar WJ, Kosty M, et al. Burnout and career satisfaction among US oncologists. J Clin Oncol. 2014;32:678-686.
15. Shanafelt TD, Boone S, Tan L, et al. Burnout and satisfaction with work-life balance among US physicians relative to the general US population. Arch Int Med. 2012;172:1377-1385.
16. Shanafelt TD, Hasan O, Dyrbye LN, et al. Changes in burnout and satisfaction with work-life balance in physicians and the general US working population between 2011 and 2014. Mayo Clin Proc. 2015;90;1600-1613.
17. Linzer M, Manwell LB, Williams ES, et al. Working conditions in primary care: physician reactions and care quality. Ann Intern Med. 2009;151:28-36.
18. Maslach C, Jackson SE. The measurement of experienced burnout. J Occcup Behav. 1981;2:99-113.
19. Linzer M, Guzman-Corrales L, Poplau S. Physician Burnout: improve physician satisfaction and patient outcomes. June 5, 2015. https://www.stepsforward.org/modules/physician-burnout. Accessed October 4, 2019.
20. West CP, Dyrbye LN, Erwin PJ, et al. Interventions to prevent and reduce physician burnout: a systematic review and meta-analysis. Lancet. 2016;388:2272-2281.
21. Nedrow A, Steckler NA, Hardman J. Physician resilience and burnout: can you make the switch? Fam Prac Manag. 2013;20:25-30.
22. Verweij H, van Ravesteijn H, van Hooff MLM, et al. Mindfulness-based stress reduction for residents: a randomized controlled trial. J Gen Intern Med. 2018;33:429-436.
23. Wright AA, Katz IT. Beyond burnout – redesigning care to restore meaning and sanity for physicians. N Eng J Med. 2018;378:309-311.
24. Shanafelt TD, Gorringe G, Menaker R, et. al. Impact of organizational leadership on physician burnout and satisfaction. Mayo Clin Proc. 2015;90:432-440.
25. Hengerer A, Kishore S. 2017. Breaking a culture of silence: the role of state medical boards. National Academy of Medicine, Washington DC. https://nam.edu/breaking-a-culture-of-silence-the-role-of-state-medical-boards/. Accessed October 4, 2019.
26. American Medical Association. AMA fights burnout with new practice transformation initiative. September 5, 2019. https://www.ama-assn.org/press-center/press-releases/ama-fights-burnout-new-practice-transformation-initiative. Accessed September 5, 2019.
27. Schulte B. Time in the bank: a Stanford plan to save doctors from burnout. The Washington Post. https://www.washingtonpost.com/news/inspired-life/wp/2015/08/20/the-innovative-stanford-program-thats-saving-emergency-room-doctors-from-burnout/?utm_term=.838c930e8de7. Accessed October 4, 2019.
28. Wallace JE, Lemaire JB, Ghali WA. Physician wellness: a missing quality indicator. Lancet. 2009;374:1714-1721.
29. Bodenheimer T, Sinsky C. From triple to quadruple aim: care of the patient requires care of the provider. Ann Fam Med. 2014;12:573-576.
30. Babbott S, Manwell LB, Brown R, et al. Electronic medical records and physician stress in primary care: results from the MEMO Study. J Am Med Info Assoc. 2014;21:E100-E106.
31. Bodenheimer T, Willard-Grace R, Ghorob A. Expanding the roles of medical assistants: who does what in primary care? JAMA Intern Med. 2014;174:1025-1026.
32. Mangiofico G. Physician shortage requires multi-prong solution. January 26, 2018. Am J Manag Care. https://www.ajmc.com/contributor/dr-gary-mangiofico/2018/01/physician-shortage-requires-multiprong-solution. Accessed October 4, 2019.
33. Reuben DB, Knudsen J, Senelick W, et al. The effect of a physician partner program on physician efficiency and patient satisfaction. JAMA Intern Med. 2014;174:1190-1193.
Serous and Hemorrhagic Bullae on the Leg
The Diagnosis: Fracture Blisters
The shave biopsy pathology demonstrated a subepidermal bulla with re-epithelialization that was clinically consistent with fracture blisters (also known as fracture bullae)(Figure). Fracture blisters are a complication of bone fractures, usually occurring 24 to 48 hours after the trauma but possibly up to 3 weeks later. The skin usually is edematous with tense bullae overlying the fracture (in this case it was distal to the fracture); most blisters contain clear fluid, but older blisters tend to be more flaccid with hemorrhagic fluid.1 The cause is thought to be the result of skin strain during fracture formation.2 Edema and hypoxia from injured vessels and lymphatics contribute to the formation of bullae, which are seen as a dermoepidermal junction split on histology.1
The bullae are histologically indistinguishable from edema blisters. A clinical history can help to differentiate. Edema blisters occur in the setting of an acute exacerbation of chronic edema, usually on the lower extremities in the setting of fluid overload.3 Bullous cellulitis is associated with skin erythema, warmth, and systemic symptoms. Bullous pemphigoid can be localized to the lower legs at times; however, biopsy would show a subepidermal bulla with eosinophils along the dermoepidermal junction. Linear IgA bullous dermatosis can be drug induced from vancomycin; however, pathology would show a subepidermal blister with a neutrophil predominant infiltrate. Nonsteroidal anti-inflammatory medications such as naproxen are a common culprit for bullous drug eruptions, which can be localized or generalized and include diagnoses such as fixed drug eruption, toxic epidermal necrolysis, and drug-induced pseudoporphyria. Naproxen-induced pseudoporphyria more commonly presents with blisters, erosions, and scarring with a predilection for the dorsal hands. Histology also will demonstrate subepidermal bullae. Clues to differentiate pseudoporphyria from fracture blisters include festooning of the dermal papilla and caterpillar bodies consisting of basement membrane material and colloid bodies in the basal layer of the epidermis, though they are not always present.4
Fracture blisters can be localized to the injury site or extend beyond the fracture site. They usually are found where there is minimal subcutaneous tissue, such as the tibia, ankles, and elbows. Fractures treated within 24 hours are much less likely to have bullae formation.1 The bullae are sterile but may lead to wound healing complications, such as infections or delay in surgical management. However, there are no major adverse effects of postoperative fracture blisters.1 Fracture blisters are self-healing, though silver sulfadiazine has been shown to minimize soft-tissue complications by promoting re-epithelialization.5
- Varela CD, Vaughan TK, Carr JB, et al. Fracture blisters: clinical and pathological aspects. J Orthop Trauma. 1993;7:417-427.
- Giordano CP, Scott D, Kummer F, et al. Fracture blister formation: a laboratory study. J Trauma. 1995;38:907-909.
- Mascaro JM. Other vesicobullous diseases. In: Bolognia JL, Schafer JV, Cerroni L, eds. Dermatology. Vol 1. Philadelphia, PA: Elsevier; 2018:554-561.
- Patterson JW. The vesicobullous reaction pattern. In: Patterson JW. Weedon's Skin Pathology. 4th ed. Oxford, UK: Churchill Livingstone/Elsevier; 2016:135-187.
- Strauss EJ, Petrucelli G, Bong M, et al. Blisters associated with lower-extremity fracture: results of a prospective treatment protocol. J Orthop Trauma. 2006;20:618-622.
The Diagnosis: Fracture Blisters
The shave biopsy pathology demonstrated a subepidermal bulla with re-epithelialization that was clinically consistent with fracture blisters (also known as fracture bullae)(Figure). Fracture blisters are a complication of bone fractures, usually occurring 24 to 48 hours after the trauma but possibly up to 3 weeks later. The skin usually is edematous with tense bullae overlying the fracture (in this case it was distal to the fracture); most blisters contain clear fluid, but older blisters tend to be more flaccid with hemorrhagic fluid.1 The cause is thought to be the result of skin strain during fracture formation.2 Edema and hypoxia from injured vessels and lymphatics contribute to the formation of bullae, which are seen as a dermoepidermal junction split on histology.1

The bullae are histologically indistinguishable from edema blisters. A clinical history can help to differentiate. Edema blisters occur in the setting of an acute exacerbation of chronic edema, usually on the lower extremities in the setting of fluid overload.3 Bullous cellulitis is associated with skin erythema, warmth, and systemic symptoms. Bullous pemphigoid can be localized to the lower legs at times; however, biopsy would show a subepidermal bulla with eosinophils along the dermoepidermal junction. Linear IgA bullous dermatosis can be drug induced from vancomycin; however, pathology would show a subepidermal blister with a neutrophil predominant infiltrate. Nonsteroidal anti-inflammatory medications such as naproxen are a common culprit for bullous drug eruptions, which can be localized or generalized and include diagnoses such as fixed drug eruption, toxic epidermal necrolysis, and drug-induced pseudoporphyria. Naproxen-induced pseudoporphyria more commonly presents with blisters, erosions, and scarring with a predilection for the dorsal hands. Histology also will demonstrate subepidermal bullae. Clues to differentiate pseudoporphyria from fracture blisters include festooning of the dermal papilla and caterpillar bodies consisting of basement membrane material and colloid bodies in the basal layer of the epidermis, though they are not always present.4
Fracture blisters can be localized to the injury site or extend beyond the fracture site. They usually are found where there is minimal subcutaneous tissue, such as the tibia, ankles, and elbows. Fractures treated within 24 hours are much less likely to have bullae formation.1 The bullae are sterile but may lead to wound healing complications, such as infections or delay in surgical management. However, there are no major adverse effects of postoperative fracture blisters.1 Fracture blisters are self-healing, though silver sulfadiazine has been shown to minimize soft-tissue complications by promoting re-epithelialization.5
The Diagnosis: Fracture Blisters
The shave biopsy pathology demonstrated a subepidermal bulla with re-epithelialization that was clinically consistent with fracture blisters (also known as fracture bullae)(Figure). Fracture blisters are a complication of bone fractures, usually occurring 24 to 48 hours after the trauma but possibly up to 3 weeks later. The skin usually is edematous with tense bullae overlying the fracture (in this case it was distal to the fracture); most blisters contain clear fluid, but older blisters tend to be more flaccid with hemorrhagic fluid.1 The cause is thought to be the result of skin strain during fracture formation.2 Edema and hypoxia from injured vessels and lymphatics contribute to the formation of bullae, which are seen as a dermoepidermal junction split on histology.1

The bullae are histologically indistinguishable from edema blisters. A clinical history can help to differentiate. Edema blisters occur in the setting of an acute exacerbation of chronic edema, usually on the lower extremities in the setting of fluid overload.3 Bullous cellulitis is associated with skin erythema, warmth, and systemic symptoms. Bullous pemphigoid can be localized to the lower legs at times; however, biopsy would show a subepidermal bulla with eosinophils along the dermoepidermal junction. Linear IgA bullous dermatosis can be drug induced from vancomycin; however, pathology would show a subepidermal blister with a neutrophil predominant infiltrate. Nonsteroidal anti-inflammatory medications such as naproxen are a common culprit for bullous drug eruptions, which can be localized or generalized and include diagnoses such as fixed drug eruption, toxic epidermal necrolysis, and drug-induced pseudoporphyria. Naproxen-induced pseudoporphyria more commonly presents with blisters, erosions, and scarring with a predilection for the dorsal hands. Histology also will demonstrate subepidermal bullae. Clues to differentiate pseudoporphyria from fracture blisters include festooning of the dermal papilla and caterpillar bodies consisting of basement membrane material and colloid bodies in the basal layer of the epidermis, though they are not always present.4
Fracture blisters can be localized to the injury site or extend beyond the fracture site. They usually are found where there is minimal subcutaneous tissue, such as the tibia, ankles, and elbows. Fractures treated within 24 hours are much less likely to have bullae formation.1 The bullae are sterile but may lead to wound healing complications, such as infections or delay in surgical management. However, there are no major adverse effects of postoperative fracture blisters.1 Fracture blisters are self-healing, though silver sulfadiazine has been shown to minimize soft-tissue complications by promoting re-epithelialization.5
- Varela CD, Vaughan TK, Carr JB, et al. Fracture blisters: clinical and pathological aspects. J Orthop Trauma. 1993;7:417-427.
- Giordano CP, Scott D, Kummer F, et al. Fracture blister formation: a laboratory study. J Trauma. 1995;38:907-909.
- Mascaro JM. Other vesicobullous diseases. In: Bolognia JL, Schafer JV, Cerroni L, eds. Dermatology. Vol 1. Philadelphia, PA: Elsevier; 2018:554-561.
- Patterson JW. The vesicobullous reaction pattern. In: Patterson JW. Weedon's Skin Pathology. 4th ed. Oxford, UK: Churchill Livingstone/Elsevier; 2016:135-187.
- Strauss EJ, Petrucelli G, Bong M, et al. Blisters associated with lower-extremity fracture: results of a prospective treatment protocol. J Orthop Trauma. 2006;20:618-622.
- Varela CD, Vaughan TK, Carr JB, et al. Fracture blisters: clinical and pathological aspects. J Orthop Trauma. 1993;7:417-427.
- Giordano CP, Scott D, Kummer F, et al. Fracture blister formation: a laboratory study. J Trauma. 1995;38:907-909.
- Mascaro JM. Other vesicobullous diseases. In: Bolognia JL, Schafer JV, Cerroni L, eds. Dermatology. Vol 1. Philadelphia, PA: Elsevier; 2018:554-561.
- Patterson JW. The vesicobullous reaction pattern. In: Patterson JW. Weedon's Skin Pathology. 4th ed. Oxford, UK: Churchill Livingstone/Elsevier; 2016:135-187.
- Strauss EJ, Petrucelli G, Bong M, et al. Blisters associated with lower-extremity fracture: results of a prospective treatment protocol. J Orthop Trauma. 2006;20:618-622.
A 61-year-old wheelchair-bound man presented to the emergency department with increased swelling, bruising, and blister formation on the right lower leg over the last week. He had history of alcoholism and heavy smoking. Two weeks prior to presentation he had an open reduction and internal fixation of a right hip fracture. He recently started taking naproxen for pain and had taken a course of ciprofloxacin for a urinary tract infection. Physical examination showed a well-healed surgical wound along the right upper lateral thigh with no purulence or erythema. His right lower leg had extensive ecchymosis and pitting edema, and there was a cluster of well-defined, variably sized, serous and hemorrhagic bullae over the right lower ankle and dorsal aspect of the foot. He was hemodynamically stable and afebrile. Due to initial concern of cellulitis, he was given a dose of vancomycin in the emergency department. Computed tomography of the right leg showed diffuse edematous changes consistent with the recent surgery, and duplex ultrasonography showed no evidence of deep vein thrombosis. A shave biopsy was performed.
Prepare for VAM 2020
Mark your calendars: the 2020 Vascular Annual Meeting will take place June 17-20 at the Toronto Convention Center in Toronto, Ontario, Canada. All U.S. residents entering Canada will be required to travel with a valid passport. Your passport expiration date may not be within six months of your travel dates. For additional information (including passport requirements for international travelers), please visit the Canada Border Services Agency’s website. Read all future VAM details here.
Mark your calendars: the 2020 Vascular Annual Meeting will take place June 17-20 at the Toronto Convention Center in Toronto, Ontario, Canada. All U.S. residents entering Canada will be required to travel with a valid passport. Your passport expiration date may not be within six months of your travel dates. For additional information (including passport requirements for international travelers), please visit the Canada Border Services Agency’s website. Read all future VAM details here.
Mark your calendars: the 2020 Vascular Annual Meeting will take place June 17-20 at the Toronto Convention Center in Toronto, Ontario, Canada. All U.S. residents entering Canada will be required to travel with a valid passport. Your passport expiration date may not be within six months of your travel dates. For additional information (including passport requirements for international travelers), please visit the Canada Border Services Agency’s website. Read all future VAM details here.
For Cancer Survivors, Nutrition Is Empowering
MINNEAPOLIS -- Ignore the big health claims about vitamin supplements, pork, and nitrate-free food products. Meet patients “where they are,” even if that means you focus first on helping a morbidly obese patient maintain her weight instead of losing pounds. And use nutrition to empower patients and reduce the risk of cancer recurrence.
Dianne Piepenburg, MS, RDN, CSO, a certified oncology nutritionist at the Malcolm Randall VA Medical Center in Gainesville, Florida, offered these tips and more in a presentation about nutrition for cancer survivors. She spoke at the annual meeting of the Association of VA Hematology/Oncology (AVAHO).
According to the National Institutes of Health, an estimated 17 million cancer survivors live in the US, accounting for 5% of the population. Nearly two-thirds are aged ≥ 65 years.1
Piepenburg highlighted the existence of certified specialists in oncology nutrition (CSOs). To be certified, registered dietitian nutritionists must have worked in that job for at least 2 years, have at least 2,000 hours of practice experience within the past 5 years and pass a board exam every 5 years.
Oncology nutritionists seek to empower cancer survivors to regain equilibrium in their lives, she said. “When a patient is told what scan to have next, what blood work they have to have, what treatment they need to be on, they feel they’re losing control,” she said. “Nutrition gives the power back to them, and they feel like there’s something they can do that’s in their control.”
Piepenburg urged colleagues to “meet patients where they are.” She gave the example of a patient with breast cancer whose body mass index is in the 50s, making her morbidly obese. “Our discussion wasn’t, ‘Let’s start [losing weight] today.’ Instead, I said, ‘Can we at least prevent you from gaining any more weight?’ She thought she could at least do that, try to recuperate a bit, and then start looking at a healthy weight loss. We’ll start there and circle back in a few months and see where we’re at.”
Piepenburg urged colleagues to bring exercise into the discussion. “We need people to be physically active no matter what phase of their survivorship journey they are in,” she said.
What about people who say, “I’ve never exercised a day in my life”? Her response: “I tell folks that we need them to move more. Maybe they’re walking to the mailbox or 3 laps around the house that day.”
Oncology patients should also watch sugar, meat, and processed foods. Refined sugar, fast food and processed food should be limited, Piepenburg said, along with red meats, such as beef, pork and lamb.
“Pork is not the ‘other white meat.’ How many of you grew up seeing and hearing that in the 1970s and 1980s? It’s a red meat, and it’s metabolized like a red meat.”
Advise patients to limit bacon, sausage, and lunch meat, she said, “even if they say, ‘I bought the nitrate-free and it’s really healthy for me.’”
It’s okay to eat some red meat, she said, “but there’s a tipping point. Tell them they can have some red meat but have it as a treat and please focus more on plant-based proteins—nuts, beans, legumes. But it’s tough for a lot of our veterans who grew up on meat and potatoes, and the only vegetable they eat is corn.”
It’s tough to limit grilling in a place like Minnesota, Piepenburg said, where the prime grilling season is short, and locals go a bit nuts when it’s nice enough outside. “I tell them to at least marinate the meat and put it on indirect heat.”
Finally, she encouraged oncology care providers to not fall for vitamin hype. Don’t rely on supplements for cancer prevention, she said. With some exceptions, she said, research has suggested they don’t work, and a 1990s study of beta-carotene and retinyl palmitate (vitamin A) in lung cancer was halted because patients actually fared worse on the regimen, although the effects didn’t seem to persist.2
1. US Department of Health and Human Services, National Institutes of Health, National Cancer Institute, Office of Cancer Survivorship. Statistics. Updated February 8, 2019. Accessed October 7, 2019.
2. Goodman GE, Thornquist MD, Balmes J, et al. The Beta-Carotene and Retinol Efficacy Trial: incidence of lung cancer and cardiovascular disease mortality during 6-year follow-up after stopping beta-carotene and retinol supplements. J Natl Cancer Inst. 2004;96(23):1743-1750.
MINNEAPOLIS -- Ignore the big health claims about vitamin supplements, pork, and nitrate-free food products. Meet patients “where they are,” even if that means you focus first on helping a morbidly obese patient maintain her weight instead of losing pounds. And use nutrition to empower patients and reduce the risk of cancer recurrence.
Dianne Piepenburg, MS, RDN, CSO, a certified oncology nutritionist at the Malcolm Randall VA Medical Center in Gainesville, Florida, offered these tips and more in a presentation about nutrition for cancer survivors. She spoke at the annual meeting of the Association of VA Hematology/Oncology (AVAHO).
According to the National Institutes of Health, an estimated 17 million cancer survivors live in the US, accounting for 5% of the population. Nearly two-thirds are aged ≥ 65 years.1
Piepenburg highlighted the existence of certified specialists in oncology nutrition (CSOs). To be certified, registered dietitian nutritionists must have worked in that job for at least 2 years, have at least 2,000 hours of practice experience within the past 5 years and pass a board exam every 5 years.
Oncology nutritionists seek to empower cancer survivors to regain equilibrium in their lives, she said. “When a patient is told what scan to have next, what blood work they have to have, what treatment they need to be on, they feel they’re losing control,” she said. “Nutrition gives the power back to them, and they feel like there’s something they can do that’s in their control.”
Piepenburg urged colleagues to “meet patients where they are.” She gave the example of a patient with breast cancer whose body mass index is in the 50s, making her morbidly obese. “Our discussion wasn’t, ‘Let’s start [losing weight] today.’ Instead, I said, ‘Can we at least prevent you from gaining any more weight?’ She thought she could at least do that, try to recuperate a bit, and then start looking at a healthy weight loss. We’ll start there and circle back in a few months and see where we’re at.”
Piepenburg urged colleagues to bring exercise into the discussion. “We need people to be physically active no matter what phase of their survivorship journey they are in,” she said.
What about people who say, “I’ve never exercised a day in my life”? Her response: “I tell folks that we need them to move more. Maybe they’re walking to the mailbox or 3 laps around the house that day.”
Oncology patients should also watch sugar, meat, and processed foods. Refined sugar, fast food and processed food should be limited, Piepenburg said, along with red meats, such as beef, pork and lamb.
“Pork is not the ‘other white meat.’ How many of you grew up seeing and hearing that in the 1970s and 1980s? It’s a red meat, and it’s metabolized like a red meat.”
Advise patients to limit bacon, sausage, and lunch meat, she said, “even if they say, ‘I bought the nitrate-free and it’s really healthy for me.’”
It’s okay to eat some red meat, she said, “but there’s a tipping point. Tell them they can have some red meat but have it as a treat and please focus more on plant-based proteins—nuts, beans, legumes. But it’s tough for a lot of our veterans who grew up on meat and potatoes, and the only vegetable they eat is corn.”
It’s tough to limit grilling in a place like Minnesota, Piepenburg said, where the prime grilling season is short, and locals go a bit nuts when it’s nice enough outside. “I tell them to at least marinate the meat and put it on indirect heat.”
Finally, she encouraged oncology care providers to not fall for vitamin hype. Don’t rely on supplements for cancer prevention, she said. With some exceptions, she said, research has suggested they don’t work, and a 1990s study of beta-carotene and retinyl palmitate (vitamin A) in lung cancer was halted because patients actually fared worse on the regimen, although the effects didn’t seem to persist.2
MINNEAPOLIS -- Ignore the big health claims about vitamin supplements, pork, and nitrate-free food products. Meet patients “where they are,” even if that means you focus first on helping a morbidly obese patient maintain her weight instead of losing pounds. And use nutrition to empower patients and reduce the risk of cancer recurrence.
Dianne Piepenburg, MS, RDN, CSO, a certified oncology nutritionist at the Malcolm Randall VA Medical Center in Gainesville, Florida, offered these tips and more in a presentation about nutrition for cancer survivors. She spoke at the annual meeting of the Association of VA Hematology/Oncology (AVAHO).
According to the National Institutes of Health, an estimated 17 million cancer survivors live in the US, accounting for 5% of the population. Nearly two-thirds are aged ≥ 65 years.1
Piepenburg highlighted the existence of certified specialists in oncology nutrition (CSOs). To be certified, registered dietitian nutritionists must have worked in that job for at least 2 years, have at least 2,000 hours of practice experience within the past 5 years and pass a board exam every 5 years.
Oncology nutritionists seek to empower cancer survivors to regain equilibrium in their lives, she said. “When a patient is told what scan to have next, what blood work they have to have, what treatment they need to be on, they feel they’re losing control,” she said. “Nutrition gives the power back to them, and they feel like there’s something they can do that’s in their control.”
Piepenburg urged colleagues to “meet patients where they are.” She gave the example of a patient with breast cancer whose body mass index is in the 50s, making her morbidly obese. “Our discussion wasn’t, ‘Let’s start [losing weight] today.’ Instead, I said, ‘Can we at least prevent you from gaining any more weight?’ She thought she could at least do that, try to recuperate a bit, and then start looking at a healthy weight loss. We’ll start there and circle back in a few months and see where we’re at.”
Piepenburg urged colleagues to bring exercise into the discussion. “We need people to be physically active no matter what phase of their survivorship journey they are in,” she said.
What about people who say, “I’ve never exercised a day in my life”? Her response: “I tell folks that we need them to move more. Maybe they’re walking to the mailbox or 3 laps around the house that day.”
Oncology patients should also watch sugar, meat, and processed foods. Refined sugar, fast food and processed food should be limited, Piepenburg said, along with red meats, such as beef, pork and lamb.
“Pork is not the ‘other white meat.’ How many of you grew up seeing and hearing that in the 1970s and 1980s? It’s a red meat, and it’s metabolized like a red meat.”
Advise patients to limit bacon, sausage, and lunch meat, she said, “even if they say, ‘I bought the nitrate-free and it’s really healthy for me.’”
It’s okay to eat some red meat, she said, “but there’s a tipping point. Tell them they can have some red meat but have it as a treat and please focus more on plant-based proteins—nuts, beans, legumes. But it’s tough for a lot of our veterans who grew up on meat and potatoes, and the only vegetable they eat is corn.”
It’s tough to limit grilling in a place like Minnesota, Piepenburg said, where the prime grilling season is short, and locals go a bit nuts when it’s nice enough outside. “I tell them to at least marinate the meat and put it on indirect heat.”
Finally, she encouraged oncology care providers to not fall for vitamin hype. Don’t rely on supplements for cancer prevention, she said. With some exceptions, she said, research has suggested they don’t work, and a 1990s study of beta-carotene and retinyl palmitate (vitamin A) in lung cancer was halted because patients actually fared worse on the regimen, although the effects didn’t seem to persist.2
1. US Department of Health and Human Services, National Institutes of Health, National Cancer Institute, Office of Cancer Survivorship. Statistics. Updated February 8, 2019. Accessed October 7, 2019.
2. Goodman GE, Thornquist MD, Balmes J, et al. The Beta-Carotene and Retinol Efficacy Trial: incidence of lung cancer and cardiovascular disease mortality during 6-year follow-up after stopping beta-carotene and retinol supplements. J Natl Cancer Inst. 2004;96(23):1743-1750.
1. US Department of Health and Human Services, National Institutes of Health, National Cancer Institute, Office of Cancer Survivorship. Statistics. Updated February 8, 2019. Accessed October 7, 2019.
2. Goodman GE, Thornquist MD, Balmes J, et al. The Beta-Carotene and Retinol Efficacy Trial: incidence of lung cancer and cardiovascular disease mortality during 6-year follow-up after stopping beta-carotene and retinol supplements. J Natl Cancer Inst. 2004;96(23):1743-1750.
Utilize SVS Patient Resources
Our website contains many resources that SVS members can use for help with managing a practice, continuing education, patient education materials and much more. The patient resource pages on the site cover a variety of vascular conditions, tests and treatments. Most recently, we’ve added a page for Transcarotid Artery Revascularization (TCAR). This, and most of our pages, can give patients and/or their loved ones a better understanding of their vascular condition, as well as how it’s being tested and treated. Take a look at our pages and share with your patients today.
Our website contains many resources that SVS members can use for help with managing a practice, continuing education, patient education materials and much more. The patient resource pages on the site cover a variety of vascular conditions, tests and treatments. Most recently, we’ve added a page for Transcarotid Artery Revascularization (TCAR). This, and most of our pages, can give patients and/or their loved ones a better understanding of their vascular condition, as well as how it’s being tested and treated. Take a look at our pages and share with your patients today.
Our website contains many resources that SVS members can use for help with managing a practice, continuing education, patient education materials and much more. The patient resource pages on the site cover a variety of vascular conditions, tests and treatments. Most recently, we’ve added a page for Transcarotid Artery Revascularization (TCAR). This, and most of our pages, can give patients and/or their loved ones a better understanding of their vascular condition, as well as how it’s being tested and treated. Take a look at our pages and share with your patients today.
Small-practice neurologists still have a role to play
Another solo-practice neurologist and I were talking last week. He’s understandably worried about the local hospital starting construction on a new “neuroscience center” down the street from us. They have ambitious plans for it, which apparently don’t include those of us who’ve served the community for 20-30 years.
Whatever. I’ve been in a large practice before, and don’t want to be a part of one again.
His concern, which I have, too, is that the hospital center will drive us little guys out of business. This seems to be a common medical practice model these days.
I hope not. I’ve been doing this for a long time, and am happy with my little world. I also believe, perhaps naively, that there’s still a place for a small practice.
My staff and I know my patients. We’re generally tuned in to who needs what, or how much time. We return all calls within a few hours (or less) and try be on top of getting medication refills and records requests done the same day they come in.
While a large practice has some advantages, based on my time with one I’d have to say we didn’t do those things as well there. Messages often weren’t relayed, or were sent to the wrong doctor. Here there’s only me.
I may not make as much, but my appointment times and intervals aren’t dictated by an accountant. This allows me to generally spend as much time as needed with each person and not feel rushed as the day goes on. I hope patients still desire that in a physician, as opposed to a place advertising “20 neurologists, no waiting!” on a sign that would fit in on the Vegas strip.
Obviously, I can’t control what the hospital will do. I can only manage my own little world. I’ll continue doing that as best I can, as long as I’m able.
Time spent worrying about things I can’t change isn’t productive and is bad for one’s blood pressure. So I’ll focus on what I can do, and try not to worry about the rest.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
Another solo-practice neurologist and I were talking last week. He’s understandably worried about the local hospital starting construction on a new “neuroscience center” down the street from us. They have ambitious plans for it, which apparently don’t include those of us who’ve served the community for 20-30 years.
Whatever. I’ve been in a large practice before, and don’t want to be a part of one again.
His concern, which I have, too, is that the hospital center will drive us little guys out of business. This seems to be a common medical practice model these days.
I hope not. I’ve been doing this for a long time, and am happy with my little world. I also believe, perhaps naively, that there’s still a place for a small practice.
My staff and I know my patients. We’re generally tuned in to who needs what, or how much time. We return all calls within a few hours (or less) and try be on top of getting medication refills and records requests done the same day they come in.
While a large practice has some advantages, based on my time with one I’d have to say we didn’t do those things as well there. Messages often weren’t relayed, or were sent to the wrong doctor. Here there’s only me.
I may not make as much, but my appointment times and intervals aren’t dictated by an accountant. This allows me to generally spend as much time as needed with each person and not feel rushed as the day goes on. I hope patients still desire that in a physician, as opposed to a place advertising “20 neurologists, no waiting!” on a sign that would fit in on the Vegas strip.
Obviously, I can’t control what the hospital will do. I can only manage my own little world. I’ll continue doing that as best I can, as long as I’m able.
Time spent worrying about things I can’t change isn’t productive and is bad for one’s blood pressure. So I’ll focus on what I can do, and try not to worry about the rest.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
Another solo-practice neurologist and I were talking last week. He’s understandably worried about the local hospital starting construction on a new “neuroscience center” down the street from us. They have ambitious plans for it, which apparently don’t include those of us who’ve served the community for 20-30 years.
Whatever. I’ve been in a large practice before, and don’t want to be a part of one again.
His concern, which I have, too, is that the hospital center will drive us little guys out of business. This seems to be a common medical practice model these days.
I hope not. I’ve been doing this for a long time, and am happy with my little world. I also believe, perhaps naively, that there’s still a place for a small practice.
My staff and I know my patients. We’re generally tuned in to who needs what, or how much time. We return all calls within a few hours (or less) and try be on top of getting medication refills and records requests done the same day they come in.
While a large practice has some advantages, based on my time with one I’d have to say we didn’t do those things as well there. Messages often weren’t relayed, or were sent to the wrong doctor. Here there’s only me.
I may not make as much, but my appointment times and intervals aren’t dictated by an accountant. This allows me to generally spend as much time as needed with each person and not feel rushed as the day goes on. I hope patients still desire that in a physician, as opposed to a place advertising “20 neurologists, no waiting!” on a sign that would fit in on the Vegas strip.
Obviously, I can’t control what the hospital will do. I can only manage my own little world. I’ll continue doing that as best I can, as long as I’m able.
Time spent worrying about things I can’t change isn’t productive and is bad for one’s blood pressure. So I’ll focus on what I can do, and try not to worry about the rest.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
Skin Scores: A Review of Clinical Scoring Systems in Dermatology
The practice of dermatology is rife with bedside tools: swabs, smears, and scoring systems. First popularized in specialties such as emergency medicine and internal medicine, clinical scoring systems are now emerging in dermatology. These evidence-based scores can be calculated quickly at the bedside—often through a free smartphone app—to help guide clinical decision-making regarding diagnosis, prognosis, and management. As with any medical tool, scoring systems have limitations and should be used as a supplement, not substitute, for one’s clinical judgement. This article reviews 4 clinical scoring systems practical for dermatology residents.
SCORTEN Prognosticates Cases of Stevens-Johnson Syndrome/Toxic Epidermal Necrolysis
Perhaps the best-known scoring system in dermatology, the SCORTEN is widely used to predict hospital mortality from Stevens-Johnson syndrome/toxic epidermal necrolysis. The SCORTEN includes 7 variables of equal weight—age of 40 years or older, heart rate of 120 beats per minute or more, cancer/hematologic malignancy, involved body surface area (BSA) greater than 10%, serum urea greater than 10 mmol/L, serum bicarbonate less than 20 mmol/L, and serum glucose greater than 14 mmol/L—each contributing 1 point to the overall score if present.1 The involved BSA is defined as the sum of detached and detachable epidermis.1
The SCORTEN was developed and prospectively validated to be calculated at the end of the first 24 hours of admission; for this calculation, use the BSA affected at that time, and use the most abnormal values during the first 24 hours of admission for the other variables.1 In addition, a follow-up study including some of the original coauthors recommends recalculating the SCORTEN at the end of hospital day 3, having found that the score’s predictive value was better on this day than hospital days 1, 2, 4, or 5.2 Based on the original study, a SCORTEN of 0 to 1 corresponds to a mortality rate of 3.2%, 2 to 12.1%, 3 to 35.3%, 4 to 58.3%, and 5 or greater to 90.0%.1
Limitations of the SCORTEN include its ability to overestimate or underestimate mortality as demonstrated by 2 multi-institutional cohorts.3,4 Recently, the ABCD-10 score was developed as an alternative to the SCORTEN and was found to predict mortality similarly when validated in an internal cohort.5
PEST Screens for Psoriatic Arthritis
Dermatologists play an important role in screening for psoriatic arthritis, as an estimated 1 in 5 patients with psoriasis have psoriatic arthritis.6 To this end, several screening tools have been developed to help differentiate psoriatic arthritis from other arthritides. Joint guidelines from the American Academy of Dermatology and the National Psoriasis Foundation acknowledge that “. . . these screening tools have tended to perform less well when tested in groups of people other than those for which they were originally developed. As such, their usefulness in routine clinical practice remains controversial.”7 Nevertheless, the guidelines state, “[b]ecause screening and early detection of inflammatory arthritis are essential to optimize patient [quality of life] and reduce morbidity, providers may consider using a formal screening tool of their choice.”7
With these limitations in mind, I have found the Psoriasis Epidemiology Screening Tool (PEST) to be the most useful psoriatic arthritis screening tool. One study determined that the PEST has the best trade-off between sensitivity and specificity compared to 2 other psoriatic arthritis screening tools, the Psoriatic Arthritis Screening and Evaluation (PASE) and the Early Arthritis for Psoriatic Patients (EARP).8
The PEST is comprised of 5 questions: (1) Have you ever had a swollen joint (or joints)? (2) Has a doctor ever told you that you have arthritis? (3) Do your fingernails or toenails have holes or pits? (4) Have you had pain in your heel? (5) Have you had a finger or toe that was completely swollen and painful for no apparent reason? According to the PEST, a referral to a rheumatologist should be considered for patients answering yes to 3 or more questions, which is 97% sensitive and 79% specific for psoriatic arthritis.9 Patients who answer yes to fewer than 3 questions should still be referred to a rheumatologist if there is a strong clinical suspicion of psoriatic arthritis.10
The PEST can be accessed for free in 13 languages via the GRAPPA (Group for Research and Assessment of Psoriasis and Psoriatic Arthritis) app as well as downloaded for free from the National Psoriasis Foundation’s website (https://www.psoriasis.org/psa-screening/providers).
ALT-70 Differentiates Cellulitis From Pseudocellulitis
Overdiagnosing cellulitis in the United States has been estimated to result in up to 130,000 unnecessary hospitalizations and up to $515 million in avoidable health care spending.11 Dermatologists are in a unique position to help fix this issue. In one retrospective study of 1430 inpatient dermatology consultations, 74.32% of inpatients evaluated for presumed cellulitis by a dermatologist were instead diagnosed with a cellulitis mimicker (ie, pseudocellulitis), such as stasis dermatitis or contact dermatitis.12
The ALT-70 score was developed and prospectively validated to help differentiate lower extremity cellulitis from pseudocellulitis in adult patients in the emergency department (ED).13 In addition, the score has retrospectively been shown to function similarly in the inpatient setting when calculated at 24 and 48 hours after ED presentation.14 Although the ALT-70 score was designed for use by frontline clinicians prior to dermatology consultation, I also have found it helpful to calculate as a consultant, as it provides an objective measure of risk to communicate to the primary team in support of one diagnosis or another.
ALT-70 is an acronym for the score’s 4 variables: asymmetry, leukocytosis, tachycardia, and age of 70 years or older.15 If present, each variable confers a certain number of points to the final score: 3 points for asymmetry (defined as unilateral leg involvement), 1 point for leukocytosis (white blood cell count ≥10,000/μL), 1 point for tachycardia (≥90 beats per minute), and 2 points for age of 70 years or older. An ALT-70 score of 0 to 2 corresponds to an 83.3% or greater chance of pseudocellulitis, suggesting that the diagnosis of cellulitis be reconsidered. A score of 3 to 4 is indeterminate, and additional information such as a dermatology consultation should be pursued. A score of 5 to 7 corresponds to an 82.2% or greater chance of cellulitis, signifying that empiric treatment with antibiotics be considered.15
The ALT-70 score does not apply to cases involving areas other than the lower extremities; intravenous antibiotic use within 48 hours before ED presentation; surgery within the last 30 days; abscess; penetrating trauma; burn; or known history of osteomyelitis, diabetic ulcer, or indwelling hardware at the site of infection.15 The ALT-70 score is available for free via the MDCalc app and website (https://www.mdcalc.com/alt-70-score-cellulitis).
Mohs AUC Determines the Appropriateness of Mohs Micrographic Surgery
In 2012, the American Academy of Dermatology, American College of Mohs Surgery, American Society for Dermatologic Surgery Association, and American Society for Mohs Surgery published appropriate use criteria (AUC) to guide the decision to pursue Mohs micrographic surgery (MMS) in the United States.16 Based on various tumor and patient characteristics, the Mohs AUC assign scores to 270 different clinical scenarios. A score of 1 to 3 signifies that MMS is inappropriate and generally not considered acceptable. A score 4 to 6 indicates that the appropriateness of MMS is uncertain. A score 7 to 9 means that MMS is appropriate and generally considered acceptable.16
Since publication, the Mohs AUC have been criticized for classifying most primary superficial basal cell carcinomas as appropriate for MMS17 (which an AUC coauthor18 and others19,20 have defended), excluding certain reasons for performing MMS (such as operating on multiple tumors on the same day),21 including counterintuitive scores,22 and omitting trials from Europe23 (which AUC coauthors also have defended24).
Final Thoughts
Scoring systems are emerging in dermatology as evidence-based bedside tools to help guide clinical decision-making. Despite their limitations, these scores have the potential to make a meaningful impact in dermatology as they have in other specialties.
- Bastuji-Garin S, Fouchard N, Bertocchi M, et al. SCORTEN: a severity-of-illness score for toxic epidermal necrolysis. J Invest Dermatol. 2000;115:149-153.
- Guegan S, Bastuji-Garin S, Poszepczynska-Guigne E, et al. Performance of the SCORTEN during the first five days of hospitalization to predict the prognosis of epidermal necrolysis. J Invest Dermatol. 2006;126:272-276.
- Micheletti RG, Chiesa-Fuxench Z, Noe MH, et al. Stevens-Johnson syndrome/toxic epidermal necrolysis: a multicenter retrospective study of 377 adult patients from the United States. J Invest Dermatol. 2018;138:2315-2321.
- Sekula P, Liss Y, Davidovici B, et al. Evaluation of SCORTEN on a cohort of patients with Stevens-Johnson syndrome and toxic epidermal necrolysis included in the RegiSCAR study. J Burn Care Res. 2011;32:237-245.
- Noe MH, Rosenbach M, Hubbard RA, et al. Development and validation of a risk prediction model for in-hospital mortality among patients with Stevens-Johnson syndrome/toxic epidermal necrolysis-ABCD-10. JAMA Dermatol. 2019;155:448-454.
- Alinaghi F, Calov M, Kristensen LE, et al. Prevalence of psoriatic arthritis in patients with psoriasis: a systematic review and meta-analysis of observational and clinical studies. J Am Acad Dermatol. 2019;80:251-265.e219.
- Elmets CA, Leonardi CL, Davis DMR, et al. Joint AAD-NPF guidelines of care for the management and treatment of psoriasis with awareness and attention to comorbidities. J Am Acad Dermatol. 2019;80:1073-1113.
- Karreman MC, Weel A, van der Ven M, et al. Performance of screening tools for psoriatic arthritis: a cross-sectional study in primary care. Rheumatology (Oxford). 2017;56:597-602.
- Ibrahim GH, Buch MH, Lawson C, et al. Evaluation of an existing screening tool for psoriatic arthritis in people with psoriasis and the development of a new instrument: the Psoriasis Epidemiology Screening Tool (PEST) questionnaire. Clin Exp Rheumatol. 2009;27:469-474.
- Zhang A, Kurtzman DJB, Perez-Chada LM, et al. Psoriatic arthritis and the dermatologist: an approach to screening and clinical evaluation. Clin Dermatol. 2018;36:551-560.
- Weng QY, Raff AB, Cohen JM, et al. Costs and consequences associated with misdiagnosed lower extremity cellulitis. JAMA Dermatol. 2017;153:141-146.
- Strazzula L, Cotliar J, Fox LP, et al. Inpatient dermatology consultation aids diagnosis of cellulitis among hospitalized patients: a multi-institutional analysis. J Am Acad Dermatol. 2015;73:70-75.
- Li DG, Dewan AK, Xia FD, et al. The ALT-70 predictive model outperforms thermal imaging for the diagnosis of lower extremity cellulitis: a prospective evaluation. J Am Acad Dermatol. 2018;79:1076-1080.e1071.
- Singer S, Li DG, Gunasekera N, et al. The ALT-70 predictive model maintains predictive value at 24 and 48 hours after presentation [published online March 23, 2019]. J Am Acad Dermatol. doi:10.1016/j.jaad.2019.03.050.
- Raff AB, Weng QY, Cohen JM, et al. A predictive model for diagnosis of lower extremity cellulitis: a cross-sectional study. J Am Acad Dermatol. 2017;76:618-625.e2.
- Connolly SM, Baker DR, Coldiron BM, et al. AAD/ACMS/ASDSA/ASMS 2012 appropriate use criteria for Mohs micrographic surgery: a report of the American Academy of Dermatology, American College of Mohs Surgery, American Society for Dermatologic Surgery Association, and the American Society for Mohs Surgery. J Am Acad Dermatol. 2012;67:531-550.
- Steinman HK, Dixon A, Zachary CB. Reevaluating Mohs surgery appropriate use criteria for primary superficial basal cell carcinoma. JAMA Dermatol. 2018;154:755-756.
- Montuno MA, Coldiron BM. Mohs appropriate use criteria for superficial basal cell carcinoma. JAMA Dermatol. 2019;155:394-395.
- MacFarlane DF, Perlis C. Mohs appropriate use criteria for superficial basal cell carcinoma. JAMA Dermatol. 2019;155:395-396.
- Kantor J. Mohs appropriate use criteria for superficial basal cell carcinoma. JAMA Dermatol. 2019;155:395.
- Ruiz ES, Karia PS, Morgan FC, et al. Multiple Mohs micrographic surgery is the most common reason for divergence from the appropriate use criteria: a single institution retrospective cohort study. J Am Acad Dermatol. 2016;75:830-831.
- Croley JA, Joseph AK, Wagner RF Jr. Discrepancies in the Mohs Micrographic Surgery appropriate use criteria [published online December 23, 2018]. J Am Acad Dermatol. doi:10.1016/j.jaad.2018.11.064.
- Kelleners-Smeets NW, Mosterd K. Comment on 2012 appropriate use criteria for Mohs micrographic surgery. J Am Acad Dermatol. 2013;69:317-318.
- Connolly S, Baker D, Coldiron B, et al. Reply to “comment on 2012 appropriate use criteria for Mohs micrographic surgery.” J Am Acad Dermatol. 2013;69:318.
The practice of dermatology is rife with bedside tools: swabs, smears, and scoring systems. First popularized in specialties such as emergency medicine and internal medicine, clinical scoring systems are now emerging in dermatology. These evidence-based scores can be calculated quickly at the bedside—often through a free smartphone app—to help guide clinical decision-making regarding diagnosis, prognosis, and management. As with any medical tool, scoring systems have limitations and should be used as a supplement, not substitute, for one’s clinical judgement. This article reviews 4 clinical scoring systems practical for dermatology residents.
SCORTEN Prognosticates Cases of Stevens-Johnson Syndrome/Toxic Epidermal Necrolysis
Perhaps the best-known scoring system in dermatology, the SCORTEN is widely used to predict hospital mortality from Stevens-Johnson syndrome/toxic epidermal necrolysis. The SCORTEN includes 7 variables of equal weight—age of 40 years or older, heart rate of 120 beats per minute or more, cancer/hematologic malignancy, involved body surface area (BSA) greater than 10%, serum urea greater than 10 mmol/L, serum bicarbonate less than 20 mmol/L, and serum glucose greater than 14 mmol/L—each contributing 1 point to the overall score if present.1 The involved BSA is defined as the sum of detached and detachable epidermis.1
The SCORTEN was developed and prospectively validated to be calculated at the end of the first 24 hours of admission; for this calculation, use the BSA affected at that time, and use the most abnormal values during the first 24 hours of admission for the other variables.1 In addition, a follow-up study including some of the original coauthors recommends recalculating the SCORTEN at the end of hospital day 3, having found that the score’s predictive value was better on this day than hospital days 1, 2, 4, or 5.2 Based on the original study, a SCORTEN of 0 to 1 corresponds to a mortality rate of 3.2%, 2 to 12.1%, 3 to 35.3%, 4 to 58.3%, and 5 or greater to 90.0%.1
Limitations of the SCORTEN include its ability to overestimate or underestimate mortality as demonstrated by 2 multi-institutional cohorts.3,4 Recently, the ABCD-10 score was developed as an alternative to the SCORTEN and was found to predict mortality similarly when validated in an internal cohort.5
PEST Screens for Psoriatic Arthritis
Dermatologists play an important role in screening for psoriatic arthritis, as an estimated 1 in 5 patients with psoriasis have psoriatic arthritis.6 To this end, several screening tools have been developed to help differentiate psoriatic arthritis from other arthritides. Joint guidelines from the American Academy of Dermatology and the National Psoriasis Foundation acknowledge that “. . . these screening tools have tended to perform less well when tested in groups of people other than those for which they were originally developed. As such, their usefulness in routine clinical practice remains controversial.”7 Nevertheless, the guidelines state, “[b]ecause screening and early detection of inflammatory arthritis are essential to optimize patient [quality of life] and reduce morbidity, providers may consider using a formal screening tool of their choice.”7
With these limitations in mind, I have found the Psoriasis Epidemiology Screening Tool (PEST) to be the most useful psoriatic arthritis screening tool. One study determined that the PEST has the best trade-off between sensitivity and specificity compared to 2 other psoriatic arthritis screening tools, the Psoriatic Arthritis Screening and Evaluation (PASE) and the Early Arthritis for Psoriatic Patients (EARP).8
The PEST is comprised of 5 questions: (1) Have you ever had a swollen joint (or joints)? (2) Has a doctor ever told you that you have arthritis? (3) Do your fingernails or toenails have holes or pits? (4) Have you had pain in your heel? (5) Have you had a finger or toe that was completely swollen and painful for no apparent reason? According to the PEST, a referral to a rheumatologist should be considered for patients answering yes to 3 or more questions, which is 97% sensitive and 79% specific for psoriatic arthritis.9 Patients who answer yes to fewer than 3 questions should still be referred to a rheumatologist if there is a strong clinical suspicion of psoriatic arthritis.10
The PEST can be accessed for free in 13 languages via the GRAPPA (Group for Research and Assessment of Psoriasis and Psoriatic Arthritis) app as well as downloaded for free from the National Psoriasis Foundation’s website (https://www.psoriasis.org/psa-screening/providers).
ALT-70 Differentiates Cellulitis From Pseudocellulitis
Overdiagnosing cellulitis in the United States has been estimated to result in up to 130,000 unnecessary hospitalizations and up to $515 million in avoidable health care spending.11 Dermatologists are in a unique position to help fix this issue. In one retrospective study of 1430 inpatient dermatology consultations, 74.32% of inpatients evaluated for presumed cellulitis by a dermatologist were instead diagnosed with a cellulitis mimicker (ie, pseudocellulitis), such as stasis dermatitis or contact dermatitis.12
The ALT-70 score was developed and prospectively validated to help differentiate lower extremity cellulitis from pseudocellulitis in adult patients in the emergency department (ED).13 In addition, the score has retrospectively been shown to function similarly in the inpatient setting when calculated at 24 and 48 hours after ED presentation.14 Although the ALT-70 score was designed for use by frontline clinicians prior to dermatology consultation, I also have found it helpful to calculate as a consultant, as it provides an objective measure of risk to communicate to the primary team in support of one diagnosis or another.
ALT-70 is an acronym for the score’s 4 variables: asymmetry, leukocytosis, tachycardia, and age of 70 years or older.15 If present, each variable confers a certain number of points to the final score: 3 points for asymmetry (defined as unilateral leg involvement), 1 point for leukocytosis (white blood cell count ≥10,000/μL), 1 point for tachycardia (≥90 beats per minute), and 2 points for age of 70 years or older. An ALT-70 score of 0 to 2 corresponds to an 83.3% or greater chance of pseudocellulitis, suggesting that the diagnosis of cellulitis be reconsidered. A score of 3 to 4 is indeterminate, and additional information such as a dermatology consultation should be pursued. A score of 5 to 7 corresponds to an 82.2% or greater chance of cellulitis, signifying that empiric treatment with antibiotics be considered.15
The ALT-70 score does not apply to cases involving areas other than the lower extremities; intravenous antibiotic use within 48 hours before ED presentation; surgery within the last 30 days; abscess; penetrating trauma; burn; or known history of osteomyelitis, diabetic ulcer, or indwelling hardware at the site of infection.15 The ALT-70 score is available for free via the MDCalc app and website (https://www.mdcalc.com/alt-70-score-cellulitis).
Mohs AUC Determines the Appropriateness of Mohs Micrographic Surgery
In 2012, the American Academy of Dermatology, American College of Mohs Surgery, American Society for Dermatologic Surgery Association, and American Society for Mohs Surgery published appropriate use criteria (AUC) to guide the decision to pursue Mohs micrographic surgery (MMS) in the United States.16 Based on various tumor and patient characteristics, the Mohs AUC assign scores to 270 different clinical scenarios. A score of 1 to 3 signifies that MMS is inappropriate and generally not considered acceptable. A score 4 to 6 indicates that the appropriateness of MMS is uncertain. A score 7 to 9 means that MMS is appropriate and generally considered acceptable.16
Since publication, the Mohs AUC have been criticized for classifying most primary superficial basal cell carcinomas as appropriate for MMS17 (which an AUC coauthor18 and others19,20 have defended), excluding certain reasons for performing MMS (such as operating on multiple tumors on the same day),21 including counterintuitive scores,22 and omitting trials from Europe23 (which AUC coauthors also have defended24).
Final Thoughts
Scoring systems are emerging in dermatology as evidence-based bedside tools to help guide clinical decision-making. Despite their limitations, these scores have the potential to make a meaningful impact in dermatology as they have in other specialties.
The practice of dermatology is rife with bedside tools: swabs, smears, and scoring systems. First popularized in specialties such as emergency medicine and internal medicine, clinical scoring systems are now emerging in dermatology. These evidence-based scores can be calculated quickly at the bedside—often through a free smartphone app—to help guide clinical decision-making regarding diagnosis, prognosis, and management. As with any medical tool, scoring systems have limitations and should be used as a supplement, not substitute, for one’s clinical judgement. This article reviews 4 clinical scoring systems practical for dermatology residents.
SCORTEN Prognosticates Cases of Stevens-Johnson Syndrome/Toxic Epidermal Necrolysis
Perhaps the best-known scoring system in dermatology, the SCORTEN is widely used to predict hospital mortality from Stevens-Johnson syndrome/toxic epidermal necrolysis. The SCORTEN includes 7 variables of equal weight—age of 40 years or older, heart rate of 120 beats per minute or more, cancer/hematologic malignancy, involved body surface area (BSA) greater than 10%, serum urea greater than 10 mmol/L, serum bicarbonate less than 20 mmol/L, and serum glucose greater than 14 mmol/L—each contributing 1 point to the overall score if present.1 The involved BSA is defined as the sum of detached and detachable epidermis.1
The SCORTEN was developed and prospectively validated to be calculated at the end of the first 24 hours of admission; for this calculation, use the BSA affected at that time, and use the most abnormal values during the first 24 hours of admission for the other variables.1 In addition, a follow-up study including some of the original coauthors recommends recalculating the SCORTEN at the end of hospital day 3, having found that the score’s predictive value was better on this day than hospital days 1, 2, 4, or 5.2 Based on the original study, a SCORTEN of 0 to 1 corresponds to a mortality rate of 3.2%, 2 to 12.1%, 3 to 35.3%, 4 to 58.3%, and 5 or greater to 90.0%.1
Limitations of the SCORTEN include its ability to overestimate or underestimate mortality as demonstrated by 2 multi-institutional cohorts.3,4 Recently, the ABCD-10 score was developed as an alternative to the SCORTEN and was found to predict mortality similarly when validated in an internal cohort.5
PEST Screens for Psoriatic Arthritis
Dermatologists play an important role in screening for psoriatic arthritis, as an estimated 1 in 5 patients with psoriasis have psoriatic arthritis.6 To this end, several screening tools have been developed to help differentiate psoriatic arthritis from other arthritides. Joint guidelines from the American Academy of Dermatology and the National Psoriasis Foundation acknowledge that “. . . these screening tools have tended to perform less well when tested in groups of people other than those for which they were originally developed. As such, their usefulness in routine clinical practice remains controversial.”7 Nevertheless, the guidelines state, “[b]ecause screening and early detection of inflammatory arthritis are essential to optimize patient [quality of life] and reduce morbidity, providers may consider using a formal screening tool of their choice.”7
With these limitations in mind, I have found the Psoriasis Epidemiology Screening Tool (PEST) to be the most useful psoriatic arthritis screening tool. One study determined that the PEST has the best trade-off between sensitivity and specificity compared to 2 other psoriatic arthritis screening tools, the Psoriatic Arthritis Screening and Evaluation (PASE) and the Early Arthritis for Psoriatic Patients (EARP).8
The PEST is comprised of 5 questions: (1) Have you ever had a swollen joint (or joints)? (2) Has a doctor ever told you that you have arthritis? (3) Do your fingernails or toenails have holes or pits? (4) Have you had pain in your heel? (5) Have you had a finger or toe that was completely swollen and painful for no apparent reason? According to the PEST, a referral to a rheumatologist should be considered for patients answering yes to 3 or more questions, which is 97% sensitive and 79% specific for psoriatic arthritis.9 Patients who answer yes to fewer than 3 questions should still be referred to a rheumatologist if there is a strong clinical suspicion of psoriatic arthritis.10
The PEST can be accessed for free in 13 languages via the GRAPPA (Group for Research and Assessment of Psoriasis and Psoriatic Arthritis) app as well as downloaded for free from the National Psoriasis Foundation’s website (https://www.psoriasis.org/psa-screening/providers).
ALT-70 Differentiates Cellulitis From Pseudocellulitis
Overdiagnosing cellulitis in the United States has been estimated to result in up to 130,000 unnecessary hospitalizations and up to $515 million in avoidable health care spending.11 Dermatologists are in a unique position to help fix this issue. In one retrospective study of 1430 inpatient dermatology consultations, 74.32% of inpatients evaluated for presumed cellulitis by a dermatologist were instead diagnosed with a cellulitis mimicker (ie, pseudocellulitis), such as stasis dermatitis or contact dermatitis.12
The ALT-70 score was developed and prospectively validated to help differentiate lower extremity cellulitis from pseudocellulitis in adult patients in the emergency department (ED).13 In addition, the score has retrospectively been shown to function similarly in the inpatient setting when calculated at 24 and 48 hours after ED presentation.14 Although the ALT-70 score was designed for use by frontline clinicians prior to dermatology consultation, I also have found it helpful to calculate as a consultant, as it provides an objective measure of risk to communicate to the primary team in support of one diagnosis or another.
ALT-70 is an acronym for the score’s 4 variables: asymmetry, leukocytosis, tachycardia, and age of 70 years or older.15 If present, each variable confers a certain number of points to the final score: 3 points for asymmetry (defined as unilateral leg involvement), 1 point for leukocytosis (white blood cell count ≥10,000/μL), 1 point for tachycardia (≥90 beats per minute), and 2 points for age of 70 years or older. An ALT-70 score of 0 to 2 corresponds to an 83.3% or greater chance of pseudocellulitis, suggesting that the diagnosis of cellulitis be reconsidered. A score of 3 to 4 is indeterminate, and additional information such as a dermatology consultation should be pursued. A score of 5 to 7 corresponds to an 82.2% or greater chance of cellulitis, signifying that empiric treatment with antibiotics be considered.15
The ALT-70 score does not apply to cases involving areas other than the lower extremities; intravenous antibiotic use within 48 hours before ED presentation; surgery within the last 30 days; abscess; penetrating trauma; burn; or known history of osteomyelitis, diabetic ulcer, or indwelling hardware at the site of infection.15 The ALT-70 score is available for free via the MDCalc app and website (https://www.mdcalc.com/alt-70-score-cellulitis).
Mohs AUC Determines the Appropriateness of Mohs Micrographic Surgery
In 2012, the American Academy of Dermatology, American College of Mohs Surgery, American Society for Dermatologic Surgery Association, and American Society for Mohs Surgery published appropriate use criteria (AUC) to guide the decision to pursue Mohs micrographic surgery (MMS) in the United States.16 Based on various tumor and patient characteristics, the Mohs AUC assign scores to 270 different clinical scenarios. A score of 1 to 3 signifies that MMS is inappropriate and generally not considered acceptable. A score 4 to 6 indicates that the appropriateness of MMS is uncertain. A score 7 to 9 means that MMS is appropriate and generally considered acceptable.16
Since publication, the Mohs AUC have been criticized for classifying most primary superficial basal cell carcinomas as appropriate for MMS17 (which an AUC coauthor18 and others19,20 have defended), excluding certain reasons for performing MMS (such as operating on multiple tumors on the same day),21 including counterintuitive scores,22 and omitting trials from Europe23 (which AUC coauthors also have defended24).
Final Thoughts
Scoring systems are emerging in dermatology as evidence-based bedside tools to help guide clinical decision-making. Despite their limitations, these scores have the potential to make a meaningful impact in dermatology as they have in other specialties.
- Bastuji-Garin S, Fouchard N, Bertocchi M, et al. SCORTEN: a severity-of-illness score for toxic epidermal necrolysis. J Invest Dermatol. 2000;115:149-153.
- Guegan S, Bastuji-Garin S, Poszepczynska-Guigne E, et al. Performance of the SCORTEN during the first five days of hospitalization to predict the prognosis of epidermal necrolysis. J Invest Dermatol. 2006;126:272-276.
- Micheletti RG, Chiesa-Fuxench Z, Noe MH, et al. Stevens-Johnson syndrome/toxic epidermal necrolysis: a multicenter retrospective study of 377 adult patients from the United States. J Invest Dermatol. 2018;138:2315-2321.
- Sekula P, Liss Y, Davidovici B, et al. Evaluation of SCORTEN on a cohort of patients with Stevens-Johnson syndrome and toxic epidermal necrolysis included in the RegiSCAR study. J Burn Care Res. 2011;32:237-245.
- Noe MH, Rosenbach M, Hubbard RA, et al. Development and validation of a risk prediction model for in-hospital mortality among patients with Stevens-Johnson syndrome/toxic epidermal necrolysis-ABCD-10. JAMA Dermatol. 2019;155:448-454.
- Alinaghi F, Calov M, Kristensen LE, et al. Prevalence of psoriatic arthritis in patients with psoriasis: a systematic review and meta-analysis of observational and clinical studies. J Am Acad Dermatol. 2019;80:251-265.e219.
- Elmets CA, Leonardi CL, Davis DMR, et al. Joint AAD-NPF guidelines of care for the management and treatment of psoriasis with awareness and attention to comorbidities. J Am Acad Dermatol. 2019;80:1073-1113.
- Karreman MC, Weel A, van der Ven M, et al. Performance of screening tools for psoriatic arthritis: a cross-sectional study in primary care. Rheumatology (Oxford). 2017;56:597-602.
- Ibrahim GH, Buch MH, Lawson C, et al. Evaluation of an existing screening tool for psoriatic arthritis in people with psoriasis and the development of a new instrument: the Psoriasis Epidemiology Screening Tool (PEST) questionnaire. Clin Exp Rheumatol. 2009;27:469-474.
- Zhang A, Kurtzman DJB, Perez-Chada LM, et al. Psoriatic arthritis and the dermatologist: an approach to screening and clinical evaluation. Clin Dermatol. 2018;36:551-560.
- Weng QY, Raff AB, Cohen JM, et al. Costs and consequences associated with misdiagnosed lower extremity cellulitis. JAMA Dermatol. 2017;153:141-146.
- Strazzula L, Cotliar J, Fox LP, et al. Inpatient dermatology consultation aids diagnosis of cellulitis among hospitalized patients: a multi-institutional analysis. J Am Acad Dermatol. 2015;73:70-75.
- Li DG, Dewan AK, Xia FD, et al. The ALT-70 predictive model outperforms thermal imaging for the diagnosis of lower extremity cellulitis: a prospective evaluation. J Am Acad Dermatol. 2018;79:1076-1080.e1071.
- Singer S, Li DG, Gunasekera N, et al. The ALT-70 predictive model maintains predictive value at 24 and 48 hours after presentation [published online March 23, 2019]. J Am Acad Dermatol. doi:10.1016/j.jaad.2019.03.050.
- Raff AB, Weng QY, Cohen JM, et al. A predictive model for diagnosis of lower extremity cellulitis: a cross-sectional study. J Am Acad Dermatol. 2017;76:618-625.e2.
- Connolly SM, Baker DR, Coldiron BM, et al. AAD/ACMS/ASDSA/ASMS 2012 appropriate use criteria for Mohs micrographic surgery: a report of the American Academy of Dermatology, American College of Mohs Surgery, American Society for Dermatologic Surgery Association, and the American Society for Mohs Surgery. J Am Acad Dermatol. 2012;67:531-550.
- Steinman HK, Dixon A, Zachary CB. Reevaluating Mohs surgery appropriate use criteria for primary superficial basal cell carcinoma. JAMA Dermatol. 2018;154:755-756.
- Montuno MA, Coldiron BM. Mohs appropriate use criteria for superficial basal cell carcinoma. JAMA Dermatol. 2019;155:394-395.
- MacFarlane DF, Perlis C. Mohs appropriate use criteria for superficial basal cell carcinoma. JAMA Dermatol. 2019;155:395-396.
- Kantor J. Mohs appropriate use criteria for superficial basal cell carcinoma. JAMA Dermatol. 2019;155:395.
- Ruiz ES, Karia PS, Morgan FC, et al. Multiple Mohs micrographic surgery is the most common reason for divergence from the appropriate use criteria: a single institution retrospective cohort study. J Am Acad Dermatol. 2016;75:830-831.
- Croley JA, Joseph AK, Wagner RF Jr. Discrepancies in the Mohs Micrographic Surgery appropriate use criteria [published online December 23, 2018]. J Am Acad Dermatol. doi:10.1016/j.jaad.2018.11.064.
- Kelleners-Smeets NW, Mosterd K. Comment on 2012 appropriate use criteria for Mohs micrographic surgery. J Am Acad Dermatol. 2013;69:317-318.
- Connolly S, Baker D, Coldiron B, et al. Reply to “comment on 2012 appropriate use criteria for Mohs micrographic surgery.” J Am Acad Dermatol. 2013;69:318.
- Bastuji-Garin S, Fouchard N, Bertocchi M, et al. SCORTEN: a severity-of-illness score for toxic epidermal necrolysis. J Invest Dermatol. 2000;115:149-153.
- Guegan S, Bastuji-Garin S, Poszepczynska-Guigne E, et al. Performance of the SCORTEN during the first five days of hospitalization to predict the prognosis of epidermal necrolysis. J Invest Dermatol. 2006;126:272-276.
- Micheletti RG, Chiesa-Fuxench Z, Noe MH, et al. Stevens-Johnson syndrome/toxic epidermal necrolysis: a multicenter retrospective study of 377 adult patients from the United States. J Invest Dermatol. 2018;138:2315-2321.
- Sekula P, Liss Y, Davidovici B, et al. Evaluation of SCORTEN on a cohort of patients with Stevens-Johnson syndrome and toxic epidermal necrolysis included in the RegiSCAR study. J Burn Care Res. 2011;32:237-245.
- Noe MH, Rosenbach M, Hubbard RA, et al. Development and validation of a risk prediction model for in-hospital mortality among patients with Stevens-Johnson syndrome/toxic epidermal necrolysis-ABCD-10. JAMA Dermatol. 2019;155:448-454.
- Alinaghi F, Calov M, Kristensen LE, et al. Prevalence of psoriatic arthritis in patients with psoriasis: a systematic review and meta-analysis of observational and clinical studies. J Am Acad Dermatol. 2019;80:251-265.e219.
- Elmets CA, Leonardi CL, Davis DMR, et al. Joint AAD-NPF guidelines of care for the management and treatment of psoriasis with awareness and attention to comorbidities. J Am Acad Dermatol. 2019;80:1073-1113.
- Karreman MC, Weel A, van der Ven M, et al. Performance of screening tools for psoriatic arthritis: a cross-sectional study in primary care. Rheumatology (Oxford). 2017;56:597-602.
- Ibrahim GH, Buch MH, Lawson C, et al. Evaluation of an existing screening tool for psoriatic arthritis in people with psoriasis and the development of a new instrument: the Psoriasis Epidemiology Screening Tool (PEST) questionnaire. Clin Exp Rheumatol. 2009;27:469-474.
- Zhang A, Kurtzman DJB, Perez-Chada LM, et al. Psoriatic arthritis and the dermatologist: an approach to screening and clinical evaluation. Clin Dermatol. 2018;36:551-560.
- Weng QY, Raff AB, Cohen JM, et al. Costs and consequences associated with misdiagnosed lower extremity cellulitis. JAMA Dermatol. 2017;153:141-146.
- Strazzula L, Cotliar J, Fox LP, et al. Inpatient dermatology consultation aids diagnosis of cellulitis among hospitalized patients: a multi-institutional analysis. J Am Acad Dermatol. 2015;73:70-75.
- Li DG, Dewan AK, Xia FD, et al. The ALT-70 predictive model outperforms thermal imaging for the diagnosis of lower extremity cellulitis: a prospective evaluation. J Am Acad Dermatol. 2018;79:1076-1080.e1071.
- Singer S, Li DG, Gunasekera N, et al. The ALT-70 predictive model maintains predictive value at 24 and 48 hours after presentation [published online March 23, 2019]. J Am Acad Dermatol. doi:10.1016/j.jaad.2019.03.050.
- Raff AB, Weng QY, Cohen JM, et al. A predictive model for diagnosis of lower extremity cellulitis: a cross-sectional study. J Am Acad Dermatol. 2017;76:618-625.e2.
- Connolly SM, Baker DR, Coldiron BM, et al. AAD/ACMS/ASDSA/ASMS 2012 appropriate use criteria for Mohs micrographic surgery: a report of the American Academy of Dermatology, American College of Mohs Surgery, American Society for Dermatologic Surgery Association, and the American Society for Mohs Surgery. J Am Acad Dermatol. 2012;67:531-550.
- Steinman HK, Dixon A, Zachary CB. Reevaluating Mohs surgery appropriate use criteria for primary superficial basal cell carcinoma. JAMA Dermatol. 2018;154:755-756.
- Montuno MA, Coldiron BM. Mohs appropriate use criteria for superficial basal cell carcinoma. JAMA Dermatol. 2019;155:394-395.
- MacFarlane DF, Perlis C. Mohs appropriate use criteria for superficial basal cell carcinoma. JAMA Dermatol. 2019;155:395-396.
- Kantor J. Mohs appropriate use criteria for superficial basal cell carcinoma. JAMA Dermatol. 2019;155:395.
- Ruiz ES, Karia PS, Morgan FC, et al. Multiple Mohs micrographic surgery is the most common reason for divergence from the appropriate use criteria: a single institution retrospective cohort study. J Am Acad Dermatol. 2016;75:830-831.
- Croley JA, Joseph AK, Wagner RF Jr. Discrepancies in the Mohs Micrographic Surgery appropriate use criteria [published online December 23, 2018]. J Am Acad Dermatol. doi:10.1016/j.jaad.2018.11.064.
- Kelleners-Smeets NW, Mosterd K. Comment on 2012 appropriate use criteria for Mohs micrographic surgery. J Am Acad Dermatol. 2013;69:317-318.
- Connolly S, Baker D, Coldiron B, et al. Reply to “comment on 2012 appropriate use criteria for Mohs micrographic surgery.” J Am Acad Dermatol. 2013;69:318.
Resident Pearls
- Mortality from Stevens-Johnson syndrome/toxic epidermal necrolysis can be estimated by calculating the SCORTEN at the end of days 1 and 3 of hospitalization.
- The Psoriasis Epidemiology Screening Tool (PEST) assists with triaging which patients with psoriasis should be evaluated for psoriatic arthritis by a rheumatologist.
- The ALT-70 score is helpful to support one’s diagnosis of cellulitis or pseudocellulitis.
- The Mohs appropriate use criteria (AUC) score 270 different clinical scenarios as appropriate, uncertain, or inappropriate for Mohs micrographic surgery.
Cleveland Clinic taps Abraham as chair
Jame Abraham, MD, has been appointed chair of the hematology/medical oncology department at Cleveland Clinic in Ohio. In this new role, Dr. Abraham will “recruit and develop staff and guide the department’s focus on patient access and a multidisciplinary approach to care,” according to a statement.
Dr. Abraham is also director of the breast oncology program at Taussig Cancer Institute, codirector of the Cleveland Clinic comprehensive breast cancer program, and a professor of medicine at Cleveland Clinic Lerner College of Medicine. He takes the helm from hematologist Matt Kalaycio, MD. Dr. Kalaycio also serves as editor-in-chief of Hematology News.
In other news, Zhe Ying, PhD, of the Fred Hutchinson Cancer Research Center in Seattle, has received a 5-year Pathway to Independence Award from the National Institute of Dental and Craniofacial Research.
With this award funding, Dr. Ying will investigate oncogene-induced differentiation in PI3K-mutant head and neck squamous cell carcinoma. Specifically, he aims to determine if genetic mutations and niche factors can overcome oncogene-induced differentiation to promote tumorigenesis.
Another grant winner is Gina Mantia-Smaldone, MD, of Fox Chase Cancer Center in Philadelphia. She will receive 3 years of funding from the Gynecologic Oncology Group Foundation and NRG Oncology to study gynecologic malignancies.
This award will also provide Dr. Mantia-Smaldone with research mentorship and opportunities to collaborate with other researchers. Her research is focused on developing targeted therapies for ovarian and endometrial cancers that will, ideally, improve patients’ quality of life.
Lastly, Edna (Eti) Cukierman, PhD, of Fox Chase Cancer Center, received a grant to conduct research with Ashani Weeraratna, PhD, of Johns Hopkins University in Baltimore, and Vivek Shenoy, PhD, and Arjun Raj, PhD, both of the University of Pennsylvania in Philadelphia.
The grant, from the National Cancer Institute, will be used to investigate the link between cell aging and melanoma. Dr. Cukierman, Dr. Weeraratna, Dr. Shenoy, and Dr. Raj will focus their research "on better understanding the deterioration of collagen integrity via cellular aging and its role in melanoma metastasis,” according to a statement.
Jame Abraham, MD, has been appointed chair of the hematology/medical oncology department at Cleveland Clinic in Ohio. In this new role, Dr. Abraham will “recruit and develop staff and guide the department’s focus on patient access and a multidisciplinary approach to care,” according to a statement.
Dr. Abraham is also director of the breast oncology program at Taussig Cancer Institute, codirector of the Cleveland Clinic comprehensive breast cancer program, and a professor of medicine at Cleveland Clinic Lerner College of Medicine. He takes the helm from hematologist Matt Kalaycio, MD. Dr. Kalaycio also serves as editor-in-chief of Hematology News.
In other news, Zhe Ying, PhD, of the Fred Hutchinson Cancer Research Center in Seattle, has received a 5-year Pathway to Independence Award from the National Institute of Dental and Craniofacial Research.
With this award funding, Dr. Ying will investigate oncogene-induced differentiation in PI3K-mutant head and neck squamous cell carcinoma. Specifically, he aims to determine if genetic mutations and niche factors can overcome oncogene-induced differentiation to promote tumorigenesis.
Another grant winner is Gina Mantia-Smaldone, MD, of Fox Chase Cancer Center in Philadelphia. She will receive 3 years of funding from the Gynecologic Oncology Group Foundation and NRG Oncology to study gynecologic malignancies.
This award will also provide Dr. Mantia-Smaldone with research mentorship and opportunities to collaborate with other researchers. Her research is focused on developing targeted therapies for ovarian and endometrial cancers that will, ideally, improve patients’ quality of life.
Lastly, Edna (Eti) Cukierman, PhD, of Fox Chase Cancer Center, received a grant to conduct research with Ashani Weeraratna, PhD, of Johns Hopkins University in Baltimore, and Vivek Shenoy, PhD, and Arjun Raj, PhD, both of the University of Pennsylvania in Philadelphia.
The grant, from the National Cancer Institute, will be used to investigate the link between cell aging and melanoma. Dr. Cukierman, Dr. Weeraratna, Dr. Shenoy, and Dr. Raj will focus their research "on better understanding the deterioration of collagen integrity via cellular aging and its role in melanoma metastasis,” according to a statement.
Jame Abraham, MD, has been appointed chair of the hematology/medical oncology department at Cleveland Clinic in Ohio. In this new role, Dr. Abraham will “recruit and develop staff and guide the department’s focus on patient access and a multidisciplinary approach to care,” according to a statement.
Dr. Abraham is also director of the breast oncology program at Taussig Cancer Institute, codirector of the Cleveland Clinic comprehensive breast cancer program, and a professor of medicine at Cleveland Clinic Lerner College of Medicine. He takes the helm from hematologist Matt Kalaycio, MD. Dr. Kalaycio also serves as editor-in-chief of Hematology News.
In other news, Zhe Ying, PhD, of the Fred Hutchinson Cancer Research Center in Seattle, has received a 5-year Pathway to Independence Award from the National Institute of Dental and Craniofacial Research.
With this award funding, Dr. Ying will investigate oncogene-induced differentiation in PI3K-mutant head and neck squamous cell carcinoma. Specifically, he aims to determine if genetic mutations and niche factors can overcome oncogene-induced differentiation to promote tumorigenesis.
Another grant winner is Gina Mantia-Smaldone, MD, of Fox Chase Cancer Center in Philadelphia. She will receive 3 years of funding from the Gynecologic Oncology Group Foundation and NRG Oncology to study gynecologic malignancies.
This award will also provide Dr. Mantia-Smaldone with research mentorship and opportunities to collaborate with other researchers. Her research is focused on developing targeted therapies for ovarian and endometrial cancers that will, ideally, improve patients’ quality of life.
Lastly, Edna (Eti) Cukierman, PhD, of Fox Chase Cancer Center, received a grant to conduct research with Ashani Weeraratna, PhD, of Johns Hopkins University in Baltimore, and Vivek Shenoy, PhD, and Arjun Raj, PhD, both of the University of Pennsylvania in Philadelphia.
The grant, from the National Cancer Institute, will be used to investigate the link between cell aging and melanoma. Dr. Cukierman, Dr. Weeraratna, Dr. Shenoy, and Dr. Raj will focus their research "on better understanding the deterioration of collagen integrity via cellular aging and its role in melanoma metastasis,” according to a statement.