User login
Ribociclib/fulvestrant boosts survival in advanced breast cancer
BARCELONA – In postmenopausal women with hormone receptor–positive, HER2-negative advanced breast cancer, the combination of ribociclib (Kisqali) and fulvestrant (Faslodex) was associated with a significant survival benefit, compared with fulvestrant alone, investigators reported.
Among 726 postmenopausal patients in MONALEESA-3 randomly assigned to receive either ribociclib or placebo plus fulvestrant who were followed for a median of 39.4 months, the median overall survival (OS) was not reached for patients assigned to ribociclib, compared with 40 months for patients in the control (fulvestrant-only) arm, reported Dennis J. Slamon, MD, PhD from the University of California, Los Angeles
“The combined data set of MONALEESA-3 and MONALEESA-7 represent some 1,400 patients, and the largest body of evidence for overall survival for any [cyclin-dependent kinases 4/6] inhibitor, and these data demonstrate a consistent, meaningful prolongation of survival irrespective of menopausal status, pre or post, and irrespective of whether the patient is treated in the first line or the second line,” he said at the European Society for Medical Oncology Congress.
Results of the MONALEESA-7 trial, which showed an overall survival advantage for adding ribociclib to endocrine therapy in premenopausal women with hormone receptor–positive, HER2-negative breast cancer, were reported at the 2019 annual meeting of the American Society of Clinical Oncology.
MONALEESA-3 investigators enrolled 726 postmenopausal women with hormone receptor–positive/HER2-negative advanced breast cancer who had received not more than one prior line of endocrine therapy for advanced disease, stratified them by the presence of liver and/or lung metastases and prior endocrine therapy, and then randomly assigned them to receive intramuscular fulvestrant 500 mg every 28 days, with an additional dose on day 15 of cycle one, plus either ribociclib 600 mg per day, 3 weeks on and 1 week off for every cycle, or placebo.
Dr. Slamon presented results of the primary endpoint of progression-free survival at the 2018 ASCO annual meeting, which showed a median PFS with ribociclib plus fulvestrant of 20.5 months versus 12.8 months for fulvestrant alone (hazard ratio, 5.93; P less than .0001). The overall survival data presented by Dr. Slamon at ESMO 2019 were not mature at that time.
As noted, at a median follow-up of 39.4 months, median overall survival was not reached in the combination arm, compared with 40 months in the fulvestrant/placebo arm, translating into a HR for risk of death with ribociclib of 0.724 (P = .00455).
Estimated OS rates at 36 months were 67% in the ribociclib arm versus 58.2% in the placebo arm, and at 42 months were 57.8% and 45.9%, respectively.
The survival benefit appeared consistent both for patients treated in the first line (median OS not reached vs. 45.1 months; HR, 0.70) and for those treated after early relapse of therapy for early breast or in the second line (median OS, 40.2 vs. 32.5 months, respectively; HR, 0.73). In both comparisons, however, the 95% confidence interval crossed 1, indicating that the results were not statistically significant.
An analysis of OS by subgroup indicated significant benefit or trends for most categories except Asian race, and treatment in Asia or Latin America, although the numbers of patients in each of those subgroups was less than 20, making it difficult to draw significant inferences from the findings, Dr. Slamon cautioned.
A descriptive analysis of updated PFS results were very similar to those previously reported, with a median of 20.6 months with ribociclib versus 12.8 months with placebo (HR, 0.587). Median PFS by line of therapy was 33.6 versus 19.2 months, respectively, in the first line, and 14.5 versus 9.1 months in the second line (HR, 0.546 and 0.571; P values not shown).
“CDK4/6 inhibitors not only improve progression-free survival in first- and second-line metastatic breast cancer, it also translates into an overall survival improvement. What more do we really want?” said invited discussant Sybil Loibl, MD, from Goethe University in Frankfurt, Germany.
“Outcome improves irrespective of pretreatment, menopausal status, endocrine sensitivity and site of metastases, which is good knowledge for our patients,” she said.
To date there are no biomarkers identified that can select subgroups that could experience particular benefit from CDK4/6 inhibitors. It may require a meta-analysis of all available clinical trial data on these agents in both the first and second line therapy to reveal potential differences among subgroups, she said.
MONALEESA-3 is supported by Novartis. Dr. Slamon disclosed a consulting or advisory role, research funding, honoraria, and travel expenses from Novartis and others. Dr. Loibl disclosed honoraria and research grants from Novartis and others.
SOURCE: Slamon DJ et al. ESMO 2019, Abstract LBA7_PR.
BARCELONA – In postmenopausal women with hormone receptor–positive, HER2-negative advanced breast cancer, the combination of ribociclib (Kisqali) and fulvestrant (Faslodex) was associated with a significant survival benefit, compared with fulvestrant alone, investigators reported.
Among 726 postmenopausal patients in MONALEESA-3 randomly assigned to receive either ribociclib or placebo plus fulvestrant who were followed for a median of 39.4 months, the median overall survival (OS) was not reached for patients assigned to ribociclib, compared with 40 months for patients in the control (fulvestrant-only) arm, reported Dennis J. Slamon, MD, PhD from the University of California, Los Angeles
“The combined data set of MONALEESA-3 and MONALEESA-7 represent some 1,400 patients, and the largest body of evidence for overall survival for any [cyclin-dependent kinases 4/6] inhibitor, and these data demonstrate a consistent, meaningful prolongation of survival irrespective of menopausal status, pre or post, and irrespective of whether the patient is treated in the first line or the second line,” he said at the European Society for Medical Oncology Congress.
Results of the MONALEESA-7 trial, which showed an overall survival advantage for adding ribociclib to endocrine therapy in premenopausal women with hormone receptor–positive, HER2-negative breast cancer, were reported at the 2019 annual meeting of the American Society of Clinical Oncology.
MONALEESA-3 investigators enrolled 726 postmenopausal women with hormone receptor–positive/HER2-negative advanced breast cancer who had received not more than one prior line of endocrine therapy for advanced disease, stratified them by the presence of liver and/or lung metastases and prior endocrine therapy, and then randomly assigned them to receive intramuscular fulvestrant 500 mg every 28 days, with an additional dose on day 15 of cycle one, plus either ribociclib 600 mg per day, 3 weeks on and 1 week off for every cycle, or placebo.
Dr. Slamon presented results of the primary endpoint of progression-free survival at the 2018 ASCO annual meeting, which showed a median PFS with ribociclib plus fulvestrant of 20.5 months versus 12.8 months for fulvestrant alone (hazard ratio, 5.93; P less than .0001). The overall survival data presented by Dr. Slamon at ESMO 2019 were not mature at that time.
As noted, at a median follow-up of 39.4 months, median overall survival was not reached in the combination arm, compared with 40 months in the fulvestrant/placebo arm, translating into a HR for risk of death with ribociclib of 0.724 (P = .00455).
Estimated OS rates at 36 months were 67% in the ribociclib arm versus 58.2% in the placebo arm, and at 42 months were 57.8% and 45.9%, respectively.
The survival benefit appeared consistent both for patients treated in the first line (median OS not reached vs. 45.1 months; HR, 0.70) and for those treated after early relapse of therapy for early breast or in the second line (median OS, 40.2 vs. 32.5 months, respectively; HR, 0.73). In both comparisons, however, the 95% confidence interval crossed 1, indicating that the results were not statistically significant.
An analysis of OS by subgroup indicated significant benefit or trends for most categories except Asian race, and treatment in Asia or Latin America, although the numbers of patients in each of those subgroups was less than 20, making it difficult to draw significant inferences from the findings, Dr. Slamon cautioned.
A descriptive analysis of updated PFS results were very similar to those previously reported, with a median of 20.6 months with ribociclib versus 12.8 months with placebo (HR, 0.587). Median PFS by line of therapy was 33.6 versus 19.2 months, respectively, in the first line, and 14.5 versus 9.1 months in the second line (HR, 0.546 and 0.571; P values not shown).
“CDK4/6 inhibitors not only improve progression-free survival in first- and second-line metastatic breast cancer, it also translates into an overall survival improvement. What more do we really want?” said invited discussant Sybil Loibl, MD, from Goethe University in Frankfurt, Germany.
“Outcome improves irrespective of pretreatment, menopausal status, endocrine sensitivity and site of metastases, which is good knowledge for our patients,” she said.
To date there are no biomarkers identified that can select subgroups that could experience particular benefit from CDK4/6 inhibitors. It may require a meta-analysis of all available clinical trial data on these agents in both the first and second line therapy to reveal potential differences among subgroups, she said.
MONALEESA-3 is supported by Novartis. Dr. Slamon disclosed a consulting or advisory role, research funding, honoraria, and travel expenses from Novartis and others. Dr. Loibl disclosed honoraria and research grants from Novartis and others.
SOURCE: Slamon DJ et al. ESMO 2019, Abstract LBA7_PR.
BARCELONA – In postmenopausal women with hormone receptor–positive, HER2-negative advanced breast cancer, the combination of ribociclib (Kisqali) and fulvestrant (Faslodex) was associated with a significant survival benefit, compared with fulvestrant alone, investigators reported.
Among 726 postmenopausal patients in MONALEESA-3 randomly assigned to receive either ribociclib or placebo plus fulvestrant who were followed for a median of 39.4 months, the median overall survival (OS) was not reached for patients assigned to ribociclib, compared with 40 months for patients in the control (fulvestrant-only) arm, reported Dennis J. Slamon, MD, PhD from the University of California, Los Angeles
“The combined data set of MONALEESA-3 and MONALEESA-7 represent some 1,400 patients, and the largest body of evidence for overall survival for any [cyclin-dependent kinases 4/6] inhibitor, and these data demonstrate a consistent, meaningful prolongation of survival irrespective of menopausal status, pre or post, and irrespective of whether the patient is treated in the first line or the second line,” he said at the European Society for Medical Oncology Congress.
Results of the MONALEESA-7 trial, which showed an overall survival advantage for adding ribociclib to endocrine therapy in premenopausal women with hormone receptor–positive, HER2-negative breast cancer, were reported at the 2019 annual meeting of the American Society of Clinical Oncology.
MONALEESA-3 investigators enrolled 726 postmenopausal women with hormone receptor–positive/HER2-negative advanced breast cancer who had received not more than one prior line of endocrine therapy for advanced disease, stratified them by the presence of liver and/or lung metastases and prior endocrine therapy, and then randomly assigned them to receive intramuscular fulvestrant 500 mg every 28 days, with an additional dose on day 15 of cycle one, plus either ribociclib 600 mg per day, 3 weeks on and 1 week off for every cycle, or placebo.
Dr. Slamon presented results of the primary endpoint of progression-free survival at the 2018 ASCO annual meeting, which showed a median PFS with ribociclib plus fulvestrant of 20.5 months versus 12.8 months for fulvestrant alone (hazard ratio, 5.93; P less than .0001). The overall survival data presented by Dr. Slamon at ESMO 2019 were not mature at that time.
As noted, at a median follow-up of 39.4 months, median overall survival was not reached in the combination arm, compared with 40 months in the fulvestrant/placebo arm, translating into a HR for risk of death with ribociclib of 0.724 (P = .00455).
Estimated OS rates at 36 months were 67% in the ribociclib arm versus 58.2% in the placebo arm, and at 42 months were 57.8% and 45.9%, respectively.
The survival benefit appeared consistent both for patients treated in the first line (median OS not reached vs. 45.1 months; HR, 0.70) and for those treated after early relapse of therapy for early breast or in the second line (median OS, 40.2 vs. 32.5 months, respectively; HR, 0.73). In both comparisons, however, the 95% confidence interval crossed 1, indicating that the results were not statistically significant.
An analysis of OS by subgroup indicated significant benefit or trends for most categories except Asian race, and treatment in Asia or Latin America, although the numbers of patients in each of those subgroups was less than 20, making it difficult to draw significant inferences from the findings, Dr. Slamon cautioned.
A descriptive analysis of updated PFS results were very similar to those previously reported, with a median of 20.6 months with ribociclib versus 12.8 months with placebo (HR, 0.587). Median PFS by line of therapy was 33.6 versus 19.2 months, respectively, in the first line, and 14.5 versus 9.1 months in the second line (HR, 0.546 and 0.571; P values not shown).
“CDK4/6 inhibitors not only improve progression-free survival in first- and second-line metastatic breast cancer, it also translates into an overall survival improvement. What more do we really want?” said invited discussant Sybil Loibl, MD, from Goethe University in Frankfurt, Germany.
“Outcome improves irrespective of pretreatment, menopausal status, endocrine sensitivity and site of metastases, which is good knowledge for our patients,” she said.
To date there are no biomarkers identified that can select subgroups that could experience particular benefit from CDK4/6 inhibitors. It may require a meta-analysis of all available clinical trial data on these agents in both the first and second line therapy to reveal potential differences among subgroups, she said.
MONALEESA-3 is supported by Novartis. Dr. Slamon disclosed a consulting or advisory role, research funding, honoraria, and travel expenses from Novartis and others. Dr. Loibl disclosed honoraria and research grants from Novartis and others.
SOURCE: Slamon DJ et al. ESMO 2019, Abstract LBA7_PR.
REPORTING FROM ESMO 2019
‘Bridging leaders’ link quality, medical education
A new community emerges
In June 2019, a 5-hour preconference seminar at the annual Integrating Quality Conference of the Association of American Medical Colleges (AAMC) in Minneapolis highlighted the emergence of a new concept, and a new community, within the larger field of hospital medicine.
“Bridging leaders” are clinician-educators with a foot in two worlds: leading quality and safety initiatives within their teaching hospitals – with the hospitalist’s customary participation in a broad spectrum of quality improvement (QI) efforts in the hospital – while helping to train future and current physicians. “Bridging” also extends to the third piece of the quality puzzle, the hospital and/or health system’s senior administrators.
“About 8 years ago, another hospitalist and I found ourselves in this role, bridging graduate medical education with hospital quality and safety,” said Jennifer S. Myers, MD, FHM, director of quality and safety education in the department of medicine at the University of Pennsylvania, Philadelphia. “The role has since begun to proliferate, in teaching settings large and small, and about 30-50 of us with somewhat similar job responsibilities have been trying to create a community.”
Following the lead of the American College of Graduate Medical Education1 and its standards for clinical learning environments that include integration of patient safety and quality improvement, these have become graduate medical education (GME) priorities. Students need to learn the theory and practice of safety and quality improvement on the job as part of their professional development. Residency program directors and other trainers thus need to find opportunities for them to practice these techniques in the clinical practice environment.
At the same time, mobilizing those eager medical learners to plan and conduct quality improvement projects can enhance a hospital’s ability to advance its mission in the new health care environment of accountable care and population health.
New concept arises
Is bridging leaders a real thing? The short answer is yes, said Thomas Ciesielski, MD, GME medical director for patient safety, quality education, and clinical learning environment review program development at Washington University in St. Louis. “This is a new trend, but it’s still in the process of defining itself. Every bridging leader has their own identity based on their institution. Some play a bridging role for the entire institution; others play similar roles but only within a specific department or division. There’s a lot of learning going on in our community,” he said.
The first Bridging Leaders track was held last year at AAMC’s 2018 Integrating Quality Conference, an event which has been held annually for the past decade. The concept was also highlighted in a 2017 article in the Journal of Graduate Medical Education2 by bridging leaders, including many of the faculty at the subsequent AAMC sessions, highlighting their roles and programs at six academic medical centers.
One of those coauthors, hospitalist Vineet Arora, MD, MAPP, MHM, was recently appointed to a new position at University of Chicago Medicine: associate chief medical officer for the clinical learning environment – which pulls together many of the threads of the bridging leaders movement into a single job title. Dr. Arora said her job builds on her prior work in GME and improves the clinical learning environment for residents and fellows by integrating them into the health system’s institutional quality, safety, and value missions. It also expands on that work to include faculty and allied health professionals. “I just happen to come from the health system side,” she said.
Natural bridges: From clinical to educational
As with the early days of the hospitalist movement, bridging leaders are trying to build a community of peers with common interests.
“We’re just at the beginning,” Dr. Arora said. “Hospitalists have been the natural torch bearers for quality and safety in their hospitals, and also play roles in the education of residents and medical students, working alongside residency program directors. They are well-versed in quality and in education. So, they are the natural bridges between education and clinical care,” she said. “We also know this is a young group that comes to our meetings. One-third of them have been doing this for only the past 2 years or less – so they are early in their career paths.”
Front-line clinical providers, such as residents, often have good ideas, and bridging leaders can bring these ideas to the health system’s leaders, Dr. Arora said. “Bridging at the leadership level also involves thinking about the larger priorities of the system.” There are trust issues that these leaders can help to bridge, as well as internal communication barriers. “We also realize that health systems have to move quickly in response to a rapidly changing environment,” she noted.
“You don’t want a hundred quality improvement projects being done by students that are unaligned with the organization’s priorities. That leads to waste, and highlights the need for greater alignment,” Dr. Arora added. “Think about using front-line staff as agents of change, of engaging with learners as a win/win – as a way to actually solve the problems we are facing.”
A bridging leader occupies a role in which they can influence and affect these two parts of the mission of health care, somebody whose leadership responsibilities sit at the intersection of these two areas, said Darlene Tad-y, MD, director of GME quality and safety programs at the University of Colorado, Aurora. “Once, these people were mostly in academic medical centers, but that’s not so true anymore. A director of quality for a hospital medicine group is responsible for developing the group’s quality strategy, but at the same time responsible for teaching members of the group – not only doing QI but teaching others how to do it,” she said.
“Hospitalists make terrific bridging leaders. We really are in that sweet spot, and we can and should step into these leadership roles,” Dr. Tad-y said. “Because of our role in the hospital, we know the ins and outs of how processes work or don’t work. We have an insider’s view of the system’s dysfunction, which puts us in a great place to lead these efforts.”
The bridging leaders movement has been a grass-roots development, Dr. Tad-y explained. “It’s not like people started with the job title. But because all of this work was needed, a few people started doing it – and they began seeking each other out. Then they found that there were more than a few of us. We just hadn’t known what it was called.”
What is being bridged?
There has long been a relationship between individuals who lead in the clinical environment and those who lead in education, such as the program directors of residency programs, said Janis Orlowski, MD, chief health care officer for AAMC, which represents 154 MD-granting medical schools and their associated teaching hospitals.
“Our association’s three missions of research, education, and patient care really come together around the bridging leaders concept. So, this movement is well aligned. And as bridging leaders started to develop as a group, they found a home in AAMC and at our Integrating Quality Conference,” she said.
“Where we see this integration is in the teaching of residents and medical students in the clinical environment,” Dr. Orlowski said. “It’s not just their knowledge of disease or treatments or procedural skills that needs to be taught. They also need to understand the safe and effective clinical environment, and the role of learners in patient safety, quality improvement, and efficient and cost-effective hospital care. They need to understand value.” A new field of health systems science is emerging and quality improvement is evolving to incorporate population health. But traditional medical faculty may not be that comfortable teaching it.
Any physician who sees that they have a role in the clinical, administrative, and educational worlds can do the bridging, Dr. Orlowski said. “It could be any environment in which care is provided and learning takes place. I mentioned QI and patient safety, but among the other essential skills for the doctor of tomorrow are teamwork, inter-professional training in how to work with, for example, the pharmacist and dietitian, and understanding the value they bring.”
Whenever quality improvement projects are undertaken as part of post-graduate medical education, they should be aligned with the institution’s quality improvement plan and with the priorities of the health system, said Rob Dressler, MD, MBA, quality and safety officer at Christiana Health Care System in Newark, Del., and president of the Alliance of Independent Academic Medical Centers (AIAMC), which represents 80 hospital and health systems active in the emerging movement for bridging leaders.
“GME needs to keep the C-suite aware of its front-line efforts to improve quality and safety, so the institution’s return on investment can be recognized,” he said. “The AIAMC has consistently advocated for the building of bridges between GME leaders and their C-suites at our member hospitals. If you are doing process improvement, you need to be aligned with the organization and its priorities, or you’ll be less successful.”
AIAMC convenes the National Initiative – a multi-institutional collaborative in which residents lead multi-disciplinary teams in quality improvement projects. A total of 64 hospitals and health systems have participated since the program started in 2007. “We need to train our clinicians to solve the problems of tomorrow,” Dr. Dressler said.
Bridging leaders in action
The leaders contacted for this article offered some examples of bridging in action. Dr. Arora has used “crowd sourcing” – a technique employed extensively in her work with Costs of Care, a global nonprofit trying to drive better health care at lower cost – to implement a local program for front-line clinicians to generate ideas on how to improve value and reduce unnecessary treatment.
“We created our local ‘Choosing Wisely’ challenge for residents and staff at the University of Chicago – with the understanding that the winner would get analytic and time support to pursue their project,” she said. A resident winner was a finalist in the RIV (Research, Innovations and Clinical Vignettes) competition at a recent SHM Annual Conference.
At the University of Colorado, there is an associate program director who is responsible for the quality improvement curriculum for residents, Dr. Tad-y said. Because teaching QI means doing QI, the associate program director had to start implementing QI in the hospital, learning how to choose appropriate QI projects for the residents. That meant looking at quality priorities for the hospital – including VTE prophylaxis, fall prevention, and rates of central line–associated bloodstream infections and catheter-associated urinary tract infections. “A critical priority was to align the learners’ QI projects with what the hospital is already working on,” she explained.
“In our practice, all fellows need education and training in patient safety, how to recognize medical errors and close calls, and how to use our errors reporting system,” Dr. Myers said. “They also need to participate in errors analysis discussions. But we have struggled to get residents to attend those meetings. There’s not enough time in their schedules, and here at Penn, we have 1,500 residents and fellows, and maybe only 20 of these formal medical errors conferences per year,” she said.
Dr. Myers worked with the hospital’s patient safety officer and head of GME to design a simulated approach to fill the gap, a simulation of the root cause analysis process – how it works, the various roles played by different individuals, and what happens after it is done. “In my role, I trained one faculty member in each large residency program in how to identify a case and how to use the simulation,” she said. “They can now teach their own learners and make it more relevant to their specialty.”
Penn also has a blueprint for quality – a road map for how the organization socializes health care quality, safety, and value, Dr. Myers said. “Every 3 or 4 years our CEO looks at the road map and tries to get feedback on its direction from payers and insurers, quality leaders, academic department heads – and residents. I was in a good position to organize a session for a representative group of residents to get together and talk about where they see the quality and safety gaps in their everyday work.”
The role of the bridging leader is a viable career path or target for many hospitalists, Dr. Arora said. “But even if it’s not a career path for you, knowing that hospitalists are at the forefront of the bridging leaders movement could help you energize your health system. If you are seeing gaps in quality and safety, this is an issue you can bring before the system.”
These days doctors are wearing a lot of hats and filling roles that weren’t seen as much before, said Dr. Orlowski. “Bridging leaders are not an exclusive group but open to anyone who finds their passion in teaching quality and safety. Maybe you’re doing quality and safety, but not education, but you recognize its importance, or vice versa. First of all, look to see what this bridging leaders thing really is, and how it might apply to you. You might say: ‘That accurately describes what I’m doing now. I have the interest; I want to learn more.’”
References
1. Accreditation Council for Graduate Medical Education. CLER pathways to excellence.
2. Myers JS et al. Bridging leadership roles in quality and patient safety: Experience of 6 U.S. Academic Medical Centers. J Grad Med Educ. 2017 Feb;9(1): 9-13.
A new community emerges
A new community emerges
In June 2019, a 5-hour preconference seminar at the annual Integrating Quality Conference of the Association of American Medical Colleges (AAMC) in Minneapolis highlighted the emergence of a new concept, and a new community, within the larger field of hospital medicine.
“Bridging leaders” are clinician-educators with a foot in two worlds: leading quality and safety initiatives within their teaching hospitals – with the hospitalist’s customary participation in a broad spectrum of quality improvement (QI) efforts in the hospital – while helping to train future and current physicians. “Bridging” also extends to the third piece of the quality puzzle, the hospital and/or health system’s senior administrators.
“About 8 years ago, another hospitalist and I found ourselves in this role, bridging graduate medical education with hospital quality and safety,” said Jennifer S. Myers, MD, FHM, director of quality and safety education in the department of medicine at the University of Pennsylvania, Philadelphia. “The role has since begun to proliferate, in teaching settings large and small, and about 30-50 of us with somewhat similar job responsibilities have been trying to create a community.”
Following the lead of the American College of Graduate Medical Education1 and its standards for clinical learning environments that include integration of patient safety and quality improvement, these have become graduate medical education (GME) priorities. Students need to learn the theory and practice of safety and quality improvement on the job as part of their professional development. Residency program directors and other trainers thus need to find opportunities for them to practice these techniques in the clinical practice environment.
At the same time, mobilizing those eager medical learners to plan and conduct quality improvement projects can enhance a hospital’s ability to advance its mission in the new health care environment of accountable care and population health.
New concept arises
Is bridging leaders a real thing? The short answer is yes, said Thomas Ciesielski, MD, GME medical director for patient safety, quality education, and clinical learning environment review program development at Washington University in St. Louis. “This is a new trend, but it’s still in the process of defining itself. Every bridging leader has their own identity based on their institution. Some play a bridging role for the entire institution; others play similar roles but only within a specific department or division. There’s a lot of learning going on in our community,” he said.
The first Bridging Leaders track was held last year at AAMC’s 2018 Integrating Quality Conference, an event which has been held annually for the past decade. The concept was also highlighted in a 2017 article in the Journal of Graduate Medical Education2 by bridging leaders, including many of the faculty at the subsequent AAMC sessions, highlighting their roles and programs at six academic medical centers.
One of those coauthors, hospitalist Vineet Arora, MD, MAPP, MHM, was recently appointed to a new position at University of Chicago Medicine: associate chief medical officer for the clinical learning environment – which pulls together many of the threads of the bridging leaders movement into a single job title. Dr. Arora said her job builds on her prior work in GME and improves the clinical learning environment for residents and fellows by integrating them into the health system’s institutional quality, safety, and value missions. It also expands on that work to include faculty and allied health professionals. “I just happen to come from the health system side,” she said.
Natural bridges: From clinical to educational
As with the early days of the hospitalist movement, bridging leaders are trying to build a community of peers with common interests.
“We’re just at the beginning,” Dr. Arora said. “Hospitalists have been the natural torch bearers for quality and safety in their hospitals, and also play roles in the education of residents and medical students, working alongside residency program directors. They are well-versed in quality and in education. So, they are the natural bridges between education and clinical care,” she said. “We also know this is a young group that comes to our meetings. One-third of them have been doing this for only the past 2 years or less – so they are early in their career paths.”
Front-line clinical providers, such as residents, often have good ideas, and bridging leaders can bring these ideas to the health system’s leaders, Dr. Arora said. “Bridging at the leadership level also involves thinking about the larger priorities of the system.” There are trust issues that these leaders can help to bridge, as well as internal communication barriers. “We also realize that health systems have to move quickly in response to a rapidly changing environment,” she noted.
“You don’t want a hundred quality improvement projects being done by students that are unaligned with the organization’s priorities. That leads to waste, and highlights the need for greater alignment,” Dr. Arora added. “Think about using front-line staff as agents of change, of engaging with learners as a win/win – as a way to actually solve the problems we are facing.”
A bridging leader occupies a role in which they can influence and affect these two parts of the mission of health care, somebody whose leadership responsibilities sit at the intersection of these two areas, said Darlene Tad-y, MD, director of GME quality and safety programs at the University of Colorado, Aurora. “Once, these people were mostly in academic medical centers, but that’s not so true anymore. A director of quality for a hospital medicine group is responsible for developing the group’s quality strategy, but at the same time responsible for teaching members of the group – not only doing QI but teaching others how to do it,” she said.
“Hospitalists make terrific bridging leaders. We really are in that sweet spot, and we can and should step into these leadership roles,” Dr. Tad-y said. “Because of our role in the hospital, we know the ins and outs of how processes work or don’t work. We have an insider’s view of the system’s dysfunction, which puts us in a great place to lead these efforts.”
The bridging leaders movement has been a grass-roots development, Dr. Tad-y explained. “It’s not like people started with the job title. But because all of this work was needed, a few people started doing it – and they began seeking each other out. Then they found that there were more than a few of us. We just hadn’t known what it was called.”
What is being bridged?
There has long been a relationship between individuals who lead in the clinical environment and those who lead in education, such as the program directors of residency programs, said Janis Orlowski, MD, chief health care officer for AAMC, which represents 154 MD-granting medical schools and their associated teaching hospitals.
“Our association’s three missions of research, education, and patient care really come together around the bridging leaders concept. So, this movement is well aligned. And as bridging leaders started to develop as a group, they found a home in AAMC and at our Integrating Quality Conference,” she said.
“Where we see this integration is in the teaching of residents and medical students in the clinical environment,” Dr. Orlowski said. “It’s not just their knowledge of disease or treatments or procedural skills that needs to be taught. They also need to understand the safe and effective clinical environment, and the role of learners in patient safety, quality improvement, and efficient and cost-effective hospital care. They need to understand value.” A new field of health systems science is emerging and quality improvement is evolving to incorporate population health. But traditional medical faculty may not be that comfortable teaching it.
Any physician who sees that they have a role in the clinical, administrative, and educational worlds can do the bridging, Dr. Orlowski said. “It could be any environment in which care is provided and learning takes place. I mentioned QI and patient safety, but among the other essential skills for the doctor of tomorrow are teamwork, inter-professional training in how to work with, for example, the pharmacist and dietitian, and understanding the value they bring.”
Whenever quality improvement projects are undertaken as part of post-graduate medical education, they should be aligned with the institution’s quality improvement plan and with the priorities of the health system, said Rob Dressler, MD, MBA, quality and safety officer at Christiana Health Care System in Newark, Del., and president of the Alliance of Independent Academic Medical Centers (AIAMC), which represents 80 hospital and health systems active in the emerging movement for bridging leaders.
“GME needs to keep the C-suite aware of its front-line efforts to improve quality and safety, so the institution’s return on investment can be recognized,” he said. “The AIAMC has consistently advocated for the building of bridges between GME leaders and their C-suites at our member hospitals. If you are doing process improvement, you need to be aligned with the organization and its priorities, or you’ll be less successful.”
AIAMC convenes the National Initiative – a multi-institutional collaborative in which residents lead multi-disciplinary teams in quality improvement projects. A total of 64 hospitals and health systems have participated since the program started in 2007. “We need to train our clinicians to solve the problems of tomorrow,” Dr. Dressler said.
Bridging leaders in action
The leaders contacted for this article offered some examples of bridging in action. Dr. Arora has used “crowd sourcing” – a technique employed extensively in her work with Costs of Care, a global nonprofit trying to drive better health care at lower cost – to implement a local program for front-line clinicians to generate ideas on how to improve value and reduce unnecessary treatment.
“We created our local ‘Choosing Wisely’ challenge for residents and staff at the University of Chicago – with the understanding that the winner would get analytic and time support to pursue their project,” she said. A resident winner was a finalist in the RIV (Research, Innovations and Clinical Vignettes) competition at a recent SHM Annual Conference.
At the University of Colorado, there is an associate program director who is responsible for the quality improvement curriculum for residents, Dr. Tad-y said. Because teaching QI means doing QI, the associate program director had to start implementing QI in the hospital, learning how to choose appropriate QI projects for the residents. That meant looking at quality priorities for the hospital – including VTE prophylaxis, fall prevention, and rates of central line–associated bloodstream infections and catheter-associated urinary tract infections. “A critical priority was to align the learners’ QI projects with what the hospital is already working on,” she explained.
“In our practice, all fellows need education and training in patient safety, how to recognize medical errors and close calls, and how to use our errors reporting system,” Dr. Myers said. “They also need to participate in errors analysis discussions. But we have struggled to get residents to attend those meetings. There’s not enough time in their schedules, and here at Penn, we have 1,500 residents and fellows, and maybe only 20 of these formal medical errors conferences per year,” she said.
Dr. Myers worked with the hospital’s patient safety officer and head of GME to design a simulated approach to fill the gap, a simulation of the root cause analysis process – how it works, the various roles played by different individuals, and what happens after it is done. “In my role, I trained one faculty member in each large residency program in how to identify a case and how to use the simulation,” she said. “They can now teach their own learners and make it more relevant to their specialty.”
Penn also has a blueprint for quality – a road map for how the organization socializes health care quality, safety, and value, Dr. Myers said. “Every 3 or 4 years our CEO looks at the road map and tries to get feedback on its direction from payers and insurers, quality leaders, academic department heads – and residents. I was in a good position to organize a session for a representative group of residents to get together and talk about where they see the quality and safety gaps in their everyday work.”
The role of the bridging leader is a viable career path or target for many hospitalists, Dr. Arora said. “But even if it’s not a career path for you, knowing that hospitalists are at the forefront of the bridging leaders movement could help you energize your health system. If you are seeing gaps in quality and safety, this is an issue you can bring before the system.”
These days doctors are wearing a lot of hats and filling roles that weren’t seen as much before, said Dr. Orlowski. “Bridging leaders are not an exclusive group but open to anyone who finds their passion in teaching quality and safety. Maybe you’re doing quality and safety, but not education, but you recognize its importance, or vice versa. First of all, look to see what this bridging leaders thing really is, and how it might apply to you. You might say: ‘That accurately describes what I’m doing now. I have the interest; I want to learn more.’”
References
1. Accreditation Council for Graduate Medical Education. CLER pathways to excellence.
2. Myers JS et al. Bridging leadership roles in quality and patient safety: Experience of 6 U.S. Academic Medical Centers. J Grad Med Educ. 2017 Feb;9(1): 9-13.
In June 2019, a 5-hour preconference seminar at the annual Integrating Quality Conference of the Association of American Medical Colleges (AAMC) in Minneapolis highlighted the emergence of a new concept, and a new community, within the larger field of hospital medicine.
“Bridging leaders” are clinician-educators with a foot in two worlds: leading quality and safety initiatives within their teaching hospitals – with the hospitalist’s customary participation in a broad spectrum of quality improvement (QI) efforts in the hospital – while helping to train future and current physicians. “Bridging” also extends to the third piece of the quality puzzle, the hospital and/or health system’s senior administrators.
“About 8 years ago, another hospitalist and I found ourselves in this role, bridging graduate medical education with hospital quality and safety,” said Jennifer S. Myers, MD, FHM, director of quality and safety education in the department of medicine at the University of Pennsylvania, Philadelphia. “The role has since begun to proliferate, in teaching settings large and small, and about 30-50 of us with somewhat similar job responsibilities have been trying to create a community.”
Following the lead of the American College of Graduate Medical Education1 and its standards for clinical learning environments that include integration of patient safety and quality improvement, these have become graduate medical education (GME) priorities. Students need to learn the theory and practice of safety and quality improvement on the job as part of their professional development. Residency program directors and other trainers thus need to find opportunities for them to practice these techniques in the clinical practice environment.
At the same time, mobilizing those eager medical learners to plan and conduct quality improvement projects can enhance a hospital’s ability to advance its mission in the new health care environment of accountable care and population health.
New concept arises
Is bridging leaders a real thing? The short answer is yes, said Thomas Ciesielski, MD, GME medical director for patient safety, quality education, and clinical learning environment review program development at Washington University in St. Louis. “This is a new trend, but it’s still in the process of defining itself. Every bridging leader has their own identity based on their institution. Some play a bridging role for the entire institution; others play similar roles but only within a specific department or division. There’s a lot of learning going on in our community,” he said.
The first Bridging Leaders track was held last year at AAMC’s 2018 Integrating Quality Conference, an event which has been held annually for the past decade. The concept was also highlighted in a 2017 article in the Journal of Graduate Medical Education2 by bridging leaders, including many of the faculty at the subsequent AAMC sessions, highlighting their roles and programs at six academic medical centers.
One of those coauthors, hospitalist Vineet Arora, MD, MAPP, MHM, was recently appointed to a new position at University of Chicago Medicine: associate chief medical officer for the clinical learning environment – which pulls together many of the threads of the bridging leaders movement into a single job title. Dr. Arora said her job builds on her prior work in GME and improves the clinical learning environment for residents and fellows by integrating them into the health system’s institutional quality, safety, and value missions. It also expands on that work to include faculty and allied health professionals. “I just happen to come from the health system side,” she said.
Natural bridges: From clinical to educational
As with the early days of the hospitalist movement, bridging leaders are trying to build a community of peers with common interests.
“We’re just at the beginning,” Dr. Arora said. “Hospitalists have been the natural torch bearers for quality and safety in their hospitals, and also play roles in the education of residents and medical students, working alongside residency program directors. They are well-versed in quality and in education. So, they are the natural bridges between education and clinical care,” she said. “We also know this is a young group that comes to our meetings. One-third of them have been doing this for only the past 2 years or less – so they are early in their career paths.”
Front-line clinical providers, such as residents, often have good ideas, and bridging leaders can bring these ideas to the health system’s leaders, Dr. Arora said. “Bridging at the leadership level also involves thinking about the larger priorities of the system.” There are trust issues that these leaders can help to bridge, as well as internal communication barriers. “We also realize that health systems have to move quickly in response to a rapidly changing environment,” she noted.
“You don’t want a hundred quality improvement projects being done by students that are unaligned with the organization’s priorities. That leads to waste, and highlights the need for greater alignment,” Dr. Arora added. “Think about using front-line staff as agents of change, of engaging with learners as a win/win – as a way to actually solve the problems we are facing.”
A bridging leader occupies a role in which they can influence and affect these two parts of the mission of health care, somebody whose leadership responsibilities sit at the intersection of these two areas, said Darlene Tad-y, MD, director of GME quality and safety programs at the University of Colorado, Aurora. “Once, these people were mostly in academic medical centers, but that’s not so true anymore. A director of quality for a hospital medicine group is responsible for developing the group’s quality strategy, but at the same time responsible for teaching members of the group – not only doing QI but teaching others how to do it,” she said.
“Hospitalists make terrific bridging leaders. We really are in that sweet spot, and we can and should step into these leadership roles,” Dr. Tad-y said. “Because of our role in the hospital, we know the ins and outs of how processes work or don’t work. We have an insider’s view of the system’s dysfunction, which puts us in a great place to lead these efforts.”
The bridging leaders movement has been a grass-roots development, Dr. Tad-y explained. “It’s not like people started with the job title. But because all of this work was needed, a few people started doing it – and they began seeking each other out. Then they found that there were more than a few of us. We just hadn’t known what it was called.”
What is being bridged?
There has long been a relationship between individuals who lead in the clinical environment and those who lead in education, such as the program directors of residency programs, said Janis Orlowski, MD, chief health care officer for AAMC, which represents 154 MD-granting medical schools and their associated teaching hospitals.
“Our association’s three missions of research, education, and patient care really come together around the bridging leaders concept. So, this movement is well aligned. And as bridging leaders started to develop as a group, they found a home in AAMC and at our Integrating Quality Conference,” she said.
“Where we see this integration is in the teaching of residents and medical students in the clinical environment,” Dr. Orlowski said. “It’s not just their knowledge of disease or treatments or procedural skills that needs to be taught. They also need to understand the safe and effective clinical environment, and the role of learners in patient safety, quality improvement, and efficient and cost-effective hospital care. They need to understand value.” A new field of health systems science is emerging and quality improvement is evolving to incorporate population health. But traditional medical faculty may not be that comfortable teaching it.
Any physician who sees that they have a role in the clinical, administrative, and educational worlds can do the bridging, Dr. Orlowski said. “It could be any environment in which care is provided and learning takes place. I mentioned QI and patient safety, but among the other essential skills for the doctor of tomorrow are teamwork, inter-professional training in how to work with, for example, the pharmacist and dietitian, and understanding the value they bring.”
Whenever quality improvement projects are undertaken as part of post-graduate medical education, they should be aligned with the institution’s quality improvement plan and with the priorities of the health system, said Rob Dressler, MD, MBA, quality and safety officer at Christiana Health Care System in Newark, Del., and president of the Alliance of Independent Academic Medical Centers (AIAMC), which represents 80 hospital and health systems active in the emerging movement for bridging leaders.
“GME needs to keep the C-suite aware of its front-line efforts to improve quality and safety, so the institution’s return on investment can be recognized,” he said. “The AIAMC has consistently advocated for the building of bridges between GME leaders and their C-suites at our member hospitals. If you are doing process improvement, you need to be aligned with the organization and its priorities, or you’ll be less successful.”
AIAMC convenes the National Initiative – a multi-institutional collaborative in which residents lead multi-disciplinary teams in quality improvement projects. A total of 64 hospitals and health systems have participated since the program started in 2007. “We need to train our clinicians to solve the problems of tomorrow,” Dr. Dressler said.
Bridging leaders in action
The leaders contacted for this article offered some examples of bridging in action. Dr. Arora has used “crowd sourcing” – a technique employed extensively in her work with Costs of Care, a global nonprofit trying to drive better health care at lower cost – to implement a local program for front-line clinicians to generate ideas on how to improve value and reduce unnecessary treatment.
“We created our local ‘Choosing Wisely’ challenge for residents and staff at the University of Chicago – with the understanding that the winner would get analytic and time support to pursue their project,” she said. A resident winner was a finalist in the RIV (Research, Innovations and Clinical Vignettes) competition at a recent SHM Annual Conference.
At the University of Colorado, there is an associate program director who is responsible for the quality improvement curriculum for residents, Dr. Tad-y said. Because teaching QI means doing QI, the associate program director had to start implementing QI in the hospital, learning how to choose appropriate QI projects for the residents. That meant looking at quality priorities for the hospital – including VTE prophylaxis, fall prevention, and rates of central line–associated bloodstream infections and catheter-associated urinary tract infections. “A critical priority was to align the learners’ QI projects with what the hospital is already working on,” she explained.
“In our practice, all fellows need education and training in patient safety, how to recognize medical errors and close calls, and how to use our errors reporting system,” Dr. Myers said. “They also need to participate in errors analysis discussions. But we have struggled to get residents to attend those meetings. There’s not enough time in their schedules, and here at Penn, we have 1,500 residents and fellows, and maybe only 20 of these formal medical errors conferences per year,” she said.
Dr. Myers worked with the hospital’s patient safety officer and head of GME to design a simulated approach to fill the gap, a simulation of the root cause analysis process – how it works, the various roles played by different individuals, and what happens after it is done. “In my role, I trained one faculty member in each large residency program in how to identify a case and how to use the simulation,” she said. “They can now teach their own learners and make it more relevant to their specialty.”
Penn also has a blueprint for quality – a road map for how the organization socializes health care quality, safety, and value, Dr. Myers said. “Every 3 or 4 years our CEO looks at the road map and tries to get feedback on its direction from payers and insurers, quality leaders, academic department heads – and residents. I was in a good position to organize a session for a representative group of residents to get together and talk about where they see the quality and safety gaps in their everyday work.”
The role of the bridging leader is a viable career path or target for many hospitalists, Dr. Arora said. “But even if it’s not a career path for you, knowing that hospitalists are at the forefront of the bridging leaders movement could help you energize your health system. If you are seeing gaps in quality and safety, this is an issue you can bring before the system.”
These days doctors are wearing a lot of hats and filling roles that weren’t seen as much before, said Dr. Orlowski. “Bridging leaders are not an exclusive group but open to anyone who finds their passion in teaching quality and safety. Maybe you’re doing quality and safety, but not education, but you recognize its importance, or vice versa. First of all, look to see what this bridging leaders thing really is, and how it might apply to you. You might say: ‘That accurately describes what I’m doing now. I have the interest; I want to learn more.’”
References
1. Accreditation Council for Graduate Medical Education. CLER pathways to excellence.
2. Myers JS et al. Bridging leadership roles in quality and patient safety: Experience of 6 U.S. Academic Medical Centers. J Grad Med Educ. 2017 Feb;9(1): 9-13.
Cancer burden: Multiple metrics needed to clarify the big picture
A new analysis of 40 years of U.S. cancer data underscores the importance of looking at multiple metrics to discern the complex interplay of factors influencing cancer burden in the population. Findings showed that the epidemiologic signature – a composite of two or three key metrics – differed across cancer types and was favorable in some cases and unfavorable in others.
“Epidemiologic signatures that illustrate trends in population-based data on cancer burden provide insight into true cancer occurrence, overdiagnosis, and treatment advances,” explain the analysts, led by H. Gilbert Welch, MD, MPH, Center for Surgery and Public Health, Brigham and Women’s Hospital, Boston. “They are important indicators of the potential contribution of environmental exposures, primary preventive interventions, new treatments, and changing diagnostic and screening practices.”
Dr. Welch and colleagues analyzed data for the years 1975 through 2015, assessing juxtaposed trends in incidence, mortality, and, when available, metastatic incidence (cancer already metastatic at diagnosis) for 11 cancers individually and for all cancers combined. Incidence data combining invasive and in situ cancers were obtained from the original nine Surveillance, Epidemiology, and End Results (SEER) registries, and mortality data were obtained from the National Vital Statistics System.
The analysts then explored implications of the epidemiologic signatures as they pertain to true cancer occurrence (the underlying incidence of clinically meaningful cancer), overdiagnosis (detection of cancers that will not cause symptoms or death), and treatment advances.
Individual cancers
Findings of the analysis, published in a special report in the New England Journal of Medicine, revealed three broad categories of epidemiologic signatures having different implications for the public health and oncology fields.
Desirable signatures showed, for example, declining mortality against a backdrop of stable incidence over the 40-year period, signaling improved treatment, as seen for chronic myeloid leukemia following introduction of imatinib (Gleevec), according to the analysts. Lung cancer incidence and mortality rose and fell in tandem, reflecting an increase in smoking followed by a decrease in response to prevention efforts. Stomach, cervical, and colorectal cancers had both falling incidence – likely reflecting a true decline in occurrence related to prevention and/or screening detection and subsequent treatment of precancerous lesions – and falling mortality.
Undesirable signatures showed a rising incidence juxtaposed with stable mortality and stable or rising metastatic incidence, signaling likely overdiagnosis, Dr. Welch and colleagues proposed. Three cancers—thyroid cancer, kidney cancer, and melanoma—fell into this category; for thyroid cancer and melanoma, fairly recent upticks in metastatic incidence may reflect upstaging.
Finally, some signatures showed mixed signals, with rising incidence and falling mortality. Breast cancer incidence rose and stabilized, coinciding with introduction of screening mammography, and possibly reflecting an increase in true cancer occurrence or overdiagnosis (with stable metastatic incidence favoring the latter), the analysts speculate. Declining mortality since the 1990s may be due to improved treatment or screening, or both. Prostate cancer incidence rose sharply with introduction of prostate-specific antigen screening but then fell to initial levels, suggesting sensitivity of this cancer to diagnostic scrutiny. Falling metastatic incidence indicates screening leads to earlier diagnosis in some cases, while declining mortality starting in the 1990s may again reflect improved treatment or screening, or both.
All cancers
The epidemiologic signature for all cancers combined differed somewhat by sex. Women had a rising incidence during the 1980s that was mainly driven by lung and breast cancers, according to Dr. Welch and colleagues; a continued rise since the mid-1990s was largely driven by melanoma, kidney cancer, and thyroid cancer. Declining mortality since 1990 has been primarily due to reductions in deaths from breast and colorectal cancers, and, more recently, lung cancer.
Men had a “volatile pattern” in the incidence of all cancers combined that was attributable to prostate cancer trends; drops in lung and colorectal cancer incidences were offset by rises in melanoma and kidney cancer incidences, the analysts proposed. Declining mortality since 1990 was more marked than that among women and reflects a longer period of decline in lung cancer mortality, plus reductions in deaths from prostate cancer and colorectal cancer.
“Falling mortality means that there has been real progress against cancer in the past 40 years – largely reflecting improved treatment and the decline of a uniquely powerful causal factor: cigarette smoking,” Dr. Welch and colleagues noted. “The lack of an accompanying fall in incidence is an unfortunate side effect of early cancer-detection efforts.”
Dr. Welch reported that he had no relevant disclosures. The analysis did not receive any specific funding.
SOURCE: Welch HG et al. N Engl J Med. 2019;381:1378-86. doi: 10.1056/NEJMsr1905447.
A new analysis of 40 years of U.S. cancer data underscores the importance of looking at multiple metrics to discern the complex interplay of factors influencing cancer burden in the population. Findings showed that the epidemiologic signature – a composite of two or three key metrics – differed across cancer types and was favorable in some cases and unfavorable in others.
“Epidemiologic signatures that illustrate trends in population-based data on cancer burden provide insight into true cancer occurrence, overdiagnosis, and treatment advances,” explain the analysts, led by H. Gilbert Welch, MD, MPH, Center for Surgery and Public Health, Brigham and Women’s Hospital, Boston. “They are important indicators of the potential contribution of environmental exposures, primary preventive interventions, new treatments, and changing diagnostic and screening practices.”
Dr. Welch and colleagues analyzed data for the years 1975 through 2015, assessing juxtaposed trends in incidence, mortality, and, when available, metastatic incidence (cancer already metastatic at diagnosis) for 11 cancers individually and for all cancers combined. Incidence data combining invasive and in situ cancers were obtained from the original nine Surveillance, Epidemiology, and End Results (SEER) registries, and mortality data were obtained from the National Vital Statistics System.
The analysts then explored implications of the epidemiologic signatures as they pertain to true cancer occurrence (the underlying incidence of clinically meaningful cancer), overdiagnosis (detection of cancers that will not cause symptoms or death), and treatment advances.
Individual cancers
Findings of the analysis, published in a special report in the New England Journal of Medicine, revealed three broad categories of epidemiologic signatures having different implications for the public health and oncology fields.
Desirable signatures showed, for example, declining mortality against a backdrop of stable incidence over the 40-year period, signaling improved treatment, as seen for chronic myeloid leukemia following introduction of imatinib (Gleevec), according to the analysts. Lung cancer incidence and mortality rose and fell in tandem, reflecting an increase in smoking followed by a decrease in response to prevention efforts. Stomach, cervical, and colorectal cancers had both falling incidence – likely reflecting a true decline in occurrence related to prevention and/or screening detection and subsequent treatment of precancerous lesions – and falling mortality.
Undesirable signatures showed a rising incidence juxtaposed with stable mortality and stable or rising metastatic incidence, signaling likely overdiagnosis, Dr. Welch and colleagues proposed. Three cancers—thyroid cancer, kidney cancer, and melanoma—fell into this category; for thyroid cancer and melanoma, fairly recent upticks in metastatic incidence may reflect upstaging.
Finally, some signatures showed mixed signals, with rising incidence and falling mortality. Breast cancer incidence rose and stabilized, coinciding with introduction of screening mammography, and possibly reflecting an increase in true cancer occurrence or overdiagnosis (with stable metastatic incidence favoring the latter), the analysts speculate. Declining mortality since the 1990s may be due to improved treatment or screening, or both. Prostate cancer incidence rose sharply with introduction of prostate-specific antigen screening but then fell to initial levels, suggesting sensitivity of this cancer to diagnostic scrutiny. Falling metastatic incidence indicates screening leads to earlier diagnosis in some cases, while declining mortality starting in the 1990s may again reflect improved treatment or screening, or both.
All cancers
The epidemiologic signature for all cancers combined differed somewhat by sex. Women had a rising incidence during the 1980s that was mainly driven by lung and breast cancers, according to Dr. Welch and colleagues; a continued rise since the mid-1990s was largely driven by melanoma, kidney cancer, and thyroid cancer. Declining mortality since 1990 has been primarily due to reductions in deaths from breast and colorectal cancers, and, more recently, lung cancer.
Men had a “volatile pattern” in the incidence of all cancers combined that was attributable to prostate cancer trends; drops in lung and colorectal cancer incidences were offset by rises in melanoma and kidney cancer incidences, the analysts proposed. Declining mortality since 1990 was more marked than that among women and reflects a longer period of decline in lung cancer mortality, plus reductions in deaths from prostate cancer and colorectal cancer.
“Falling mortality means that there has been real progress against cancer in the past 40 years – largely reflecting improved treatment and the decline of a uniquely powerful causal factor: cigarette smoking,” Dr. Welch and colleagues noted. “The lack of an accompanying fall in incidence is an unfortunate side effect of early cancer-detection efforts.”
Dr. Welch reported that he had no relevant disclosures. The analysis did not receive any specific funding.
SOURCE: Welch HG et al. N Engl J Med. 2019;381:1378-86. doi: 10.1056/NEJMsr1905447.
A new analysis of 40 years of U.S. cancer data underscores the importance of looking at multiple metrics to discern the complex interplay of factors influencing cancer burden in the population. Findings showed that the epidemiologic signature – a composite of two or three key metrics – differed across cancer types and was favorable in some cases and unfavorable in others.
“Epidemiologic signatures that illustrate trends in population-based data on cancer burden provide insight into true cancer occurrence, overdiagnosis, and treatment advances,” explain the analysts, led by H. Gilbert Welch, MD, MPH, Center for Surgery and Public Health, Brigham and Women’s Hospital, Boston. “They are important indicators of the potential contribution of environmental exposures, primary preventive interventions, new treatments, and changing diagnostic and screening practices.”
Dr. Welch and colleagues analyzed data for the years 1975 through 2015, assessing juxtaposed trends in incidence, mortality, and, when available, metastatic incidence (cancer already metastatic at diagnosis) for 11 cancers individually and for all cancers combined. Incidence data combining invasive and in situ cancers were obtained from the original nine Surveillance, Epidemiology, and End Results (SEER) registries, and mortality data were obtained from the National Vital Statistics System.
The analysts then explored implications of the epidemiologic signatures as they pertain to true cancer occurrence (the underlying incidence of clinically meaningful cancer), overdiagnosis (detection of cancers that will not cause symptoms or death), and treatment advances.
Individual cancers
Findings of the analysis, published in a special report in the New England Journal of Medicine, revealed three broad categories of epidemiologic signatures having different implications for the public health and oncology fields.
Desirable signatures showed, for example, declining mortality against a backdrop of stable incidence over the 40-year period, signaling improved treatment, as seen for chronic myeloid leukemia following introduction of imatinib (Gleevec), according to the analysts. Lung cancer incidence and mortality rose and fell in tandem, reflecting an increase in smoking followed by a decrease in response to prevention efforts. Stomach, cervical, and colorectal cancers had both falling incidence – likely reflecting a true decline in occurrence related to prevention and/or screening detection and subsequent treatment of precancerous lesions – and falling mortality.
Undesirable signatures showed a rising incidence juxtaposed with stable mortality and stable or rising metastatic incidence, signaling likely overdiagnosis, Dr. Welch and colleagues proposed. Three cancers—thyroid cancer, kidney cancer, and melanoma—fell into this category; for thyroid cancer and melanoma, fairly recent upticks in metastatic incidence may reflect upstaging.
Finally, some signatures showed mixed signals, with rising incidence and falling mortality. Breast cancer incidence rose and stabilized, coinciding with introduction of screening mammography, and possibly reflecting an increase in true cancer occurrence or overdiagnosis (with stable metastatic incidence favoring the latter), the analysts speculate. Declining mortality since the 1990s may be due to improved treatment or screening, or both. Prostate cancer incidence rose sharply with introduction of prostate-specific antigen screening but then fell to initial levels, suggesting sensitivity of this cancer to diagnostic scrutiny. Falling metastatic incidence indicates screening leads to earlier diagnosis in some cases, while declining mortality starting in the 1990s may again reflect improved treatment or screening, or both.
All cancers
The epidemiologic signature for all cancers combined differed somewhat by sex. Women had a rising incidence during the 1980s that was mainly driven by lung and breast cancers, according to Dr. Welch and colleagues; a continued rise since the mid-1990s was largely driven by melanoma, kidney cancer, and thyroid cancer. Declining mortality since 1990 has been primarily due to reductions in deaths from breast and colorectal cancers, and, more recently, lung cancer.
Men had a “volatile pattern” in the incidence of all cancers combined that was attributable to prostate cancer trends; drops in lung and colorectal cancer incidences were offset by rises in melanoma and kidney cancer incidences, the analysts proposed. Declining mortality since 1990 was more marked than that among women and reflects a longer period of decline in lung cancer mortality, plus reductions in deaths from prostate cancer and colorectal cancer.
“Falling mortality means that there has been real progress against cancer in the past 40 years – largely reflecting improved treatment and the decline of a uniquely powerful causal factor: cigarette smoking,” Dr. Welch and colleagues noted. “The lack of an accompanying fall in incidence is an unfortunate side effect of early cancer-detection efforts.”
Dr. Welch reported that he had no relevant disclosures. The analysis did not receive any specific funding.
SOURCE: Welch HG et al. N Engl J Med. 2019;381:1378-86. doi: 10.1056/NEJMsr1905447.
FROM NEW ENGLAND JOURNAL OF MEDICINE
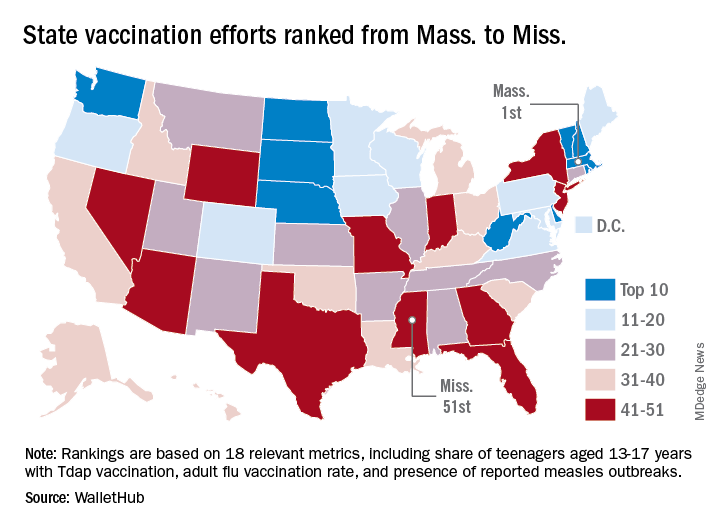
Massachusetts tops state vaccination rankings
according to a new analysis from personal finance website WalletHub.
The Bay State’s top finish in the “children and teenagers immunization rates” category moved it ahead of Vermont in the overall rankings, which had the highest score in each of the other two broad categories – “adult and elderly vaccination rates” and “immunization uptake disparities and influencing factors” – but only finished 15th in child/teen immunization, Wallethub reported.
The state that ranked 51st in child/teen immunization – Mississippi – also finished 51st overall, behind every other state and Washington, D.C. The rest of the bottom five consisted of Texas (50th); Florida (49th), which ranked last in the adult/elderly category; Georgia (48th); and Indiana (47th). New Mexico, however, managed to show that last is not always least by earning a mid-pack overall rank of 30 despite its last-place showing in the disparities/influencing factors category, the WalletHub analysis showed.
Scores for the three broad categories were determined using 18 relevant metrics, including influenza vaccination rate in children aged 6 months to 17 years (1st, Rhode Island; 51st, Wyoming), share of adults aged 60 years and older with zoster vaccination (1st, Vermont; 51st, Mississippi), and share of population without health insurance coverage (1st, Massachusetts; 51st, Texas), WalletHub said.
“Each state should tailor its vaccines policy to its need, with an understanding that those needs may change,” Dorit Rubinstein Reiss of the University of California Hastings College of the Law, San Francisco, told WalletHub. When parents refuse to have their children vaccinated, it’s important to remember that “the state is not denying these children schooling. It is requiring that they be protected from disease first.”
according to a new analysis from personal finance website WalletHub.
The Bay State’s top finish in the “children and teenagers immunization rates” category moved it ahead of Vermont in the overall rankings, which had the highest score in each of the other two broad categories – “adult and elderly vaccination rates” and “immunization uptake disparities and influencing factors” – but only finished 15th in child/teen immunization, Wallethub reported.
The state that ranked 51st in child/teen immunization – Mississippi – also finished 51st overall, behind every other state and Washington, D.C. The rest of the bottom five consisted of Texas (50th); Florida (49th), which ranked last in the adult/elderly category; Georgia (48th); and Indiana (47th). New Mexico, however, managed to show that last is not always least by earning a mid-pack overall rank of 30 despite its last-place showing in the disparities/influencing factors category, the WalletHub analysis showed.
Scores for the three broad categories were determined using 18 relevant metrics, including influenza vaccination rate in children aged 6 months to 17 years (1st, Rhode Island; 51st, Wyoming), share of adults aged 60 years and older with zoster vaccination (1st, Vermont; 51st, Mississippi), and share of population without health insurance coverage (1st, Massachusetts; 51st, Texas), WalletHub said.
“Each state should tailor its vaccines policy to its need, with an understanding that those needs may change,” Dorit Rubinstein Reiss of the University of California Hastings College of the Law, San Francisco, told WalletHub. When parents refuse to have their children vaccinated, it’s important to remember that “the state is not denying these children schooling. It is requiring that they be protected from disease first.”
according to a new analysis from personal finance website WalletHub.
The Bay State’s top finish in the “children and teenagers immunization rates” category moved it ahead of Vermont in the overall rankings, which had the highest score in each of the other two broad categories – “adult and elderly vaccination rates” and “immunization uptake disparities and influencing factors” – but only finished 15th in child/teen immunization, Wallethub reported.
The state that ranked 51st in child/teen immunization – Mississippi – also finished 51st overall, behind every other state and Washington, D.C. The rest of the bottom five consisted of Texas (50th); Florida (49th), which ranked last in the adult/elderly category; Georgia (48th); and Indiana (47th). New Mexico, however, managed to show that last is not always least by earning a mid-pack overall rank of 30 despite its last-place showing in the disparities/influencing factors category, the WalletHub analysis showed.
Scores for the three broad categories were determined using 18 relevant metrics, including influenza vaccination rate in children aged 6 months to 17 years (1st, Rhode Island; 51st, Wyoming), share of adults aged 60 years and older with zoster vaccination (1st, Vermont; 51st, Mississippi), and share of population without health insurance coverage (1st, Massachusetts; 51st, Texas), WalletHub said.
“Each state should tailor its vaccines policy to its need, with an understanding that those needs may change,” Dorit Rubinstein Reiss of the University of California Hastings College of the Law, San Francisco, told WalletHub. When parents refuse to have their children vaccinated, it’s important to remember that “the state is not denying these children schooling. It is requiring that they be protected from disease first.”
Photodermatoses: Differential diagnosis includes sunscreen allergy, connective tissue disease
SEATTLE – according to Vincent DeLeo, MD, of the department of dermatology at the University of Southern California, Los Angeles, who discussed common diagnoses at the annual Coastal Dermatology Symposium.
“They’re not common bread-and-butter dermatitis, like acne rosacea. Many people feel uncomfortable trying to work out the differential diagnosis when they see someone who comes in with what either the patient or the physician thinks is a reaction to the sun,” Dr. DeLeo said in an interview at the meeting.
“Usually, the best clue is the distribution of the rash or lesion,” which often are on the backs of the hands or on the arms from the sleeves down, he added. “When you see that, you should think of a photosensitive eruption,” and consider a differential diagnosis, using “certain tests, asking questions, and looking at the morphology and distribution of the reaction.”
The most common of the photodermatoses is polymorphous light eruption, which typically occurs in people on vacation, often among those who live in temperate climates and react violently to sun overexposure. “You usually have to rule out things like connective tissue disease by getting serology,” Dr. DeLeo noted in the interview.
In general, these patients either present with a reaction or describe a rash that has since cleared up. When a patient is describing a problem that has disappeared, he or she may have a photo of the reaction on their phone, which can be helpful, he said at the meeting jointly presented by the University of Louisville and Global Academy for Medical Education.
Some photodermatoses are characterized by tell-tale rashes indicative of a connective tissue disease like lupus or dermatomyositis, which can be followed up with biopsy and serology to confirm a diagnosis, said Dr. DeLeo, who is also professor emeritus of dermatology at the Icahn School of Medicine at Mount Sinai, New York. Other patterns, such as blisters on the hands, should prompt tests for porphyria cutanea tarda, which requires a 24-hour urine test to confirm.
But reactions in sun-exposed areas can also be caused by drug-induced photosensitivity and drugs such as NSAIDs, antibiotics, and diuretics, among others, Dr. DeLeo said.
Another common reaction can occur with exposure to photosensitizing plant materials, resulting in blisters combined with altered pigmentation. Most frequently, these reactions are caused by exposure to limes, which contain psoralens that absorb light and produce the reaction. “That is a clinical diagnosis and we can recognize that because, like poison ivy, it is usually a streaky kind of reaction in a distinct pattern. You don’t need [additional] tests for that,” he said. This can occur, for example, after making cocktails, he said, describing an example of parents who had been mixing drinks and then picked up their child. Later, they brought the child to the ED with a hand-shaped rash, and treating physicians contacted social services out of fears of child abuse.
In some cases, patients who present with no rash can elicit the problem. Solar urticaria can produce hives on demand – ask a patient to go out in the sun and return after a few minutes, and a rash will generally appear, Dr. DeLeo advised.
Patients might want to take steps to avoid such problems and there are several options available to do so, Dr. DeLeo noted. There is some evidence of efficacy for photoprotection for an extract from a fern plant found in Central and South America, a product called Heliocare, in a study sponsored by the manufacturer, Ferndale Healthcare (J Clin Aesthet Dermatol. 2015 Feb;8[2]:19-23). Patients can also undergo two or three treatments of phototherapy for 3-4 weeks in advance of a trip in order to “harden” the skin and reduce the chances of an overreaction.
Dr. DeLeo presented a simplified guide to use when considering differential diagnoses in patients with photodermatoses.
- Solar urticaria occurs quickly upon exposure and is short lived.
- Polymorphous light eruption usually occurs during a vacation and does not affect the face.
- A sun-related eczematous rash affecting the face, which recurs, is typically photoallergy to sunscreen.
- A chronic, severe dermatitis seen in a photodistribution pattern could be chronic actinic dermatitis. The patient should undergo phototesting. A biopsy of a severe case may resemble lymphoma.
- In photosensitive individuals with an uncertain diagnosis, serology testing for connective tissue disease should be performed to rule out lupus or dermatomyositis.
- When the patient has a rash in only sun-exposed areas, as well as a history of exposure to photosensitizing drugs, the drug should be discontinued to see if the rash disappears.
- Porphyria cutanea tarda is diagnosed with a 24-hour urine test that reveals at least five times normal uroporphyrin levels, along with hemochromatosis polymorphisms. Levels of uroporphyrin that are elevated but not five times normal are suggestive of pseudoporphyria.
- Phototoxic contact dermatitis caused by plants often results in unusual distributions.
- Hispanic individuals with a photodistributed rash, especially in the presence of cheilitis, may have actinic prurigo.
Dr. DeLeo is a consultant for Estee Lauder. The annual Coastal Dermatology Symposium is jointly presented by the University of Louisville and Global Academy for Medical Education. This publication and Global Academy for Medical Education are owned by the same parent company.
SEATTLE – according to Vincent DeLeo, MD, of the department of dermatology at the University of Southern California, Los Angeles, who discussed common diagnoses at the annual Coastal Dermatology Symposium.
“They’re not common bread-and-butter dermatitis, like acne rosacea. Many people feel uncomfortable trying to work out the differential diagnosis when they see someone who comes in with what either the patient or the physician thinks is a reaction to the sun,” Dr. DeLeo said in an interview at the meeting.
“Usually, the best clue is the distribution of the rash or lesion,” which often are on the backs of the hands or on the arms from the sleeves down, he added. “When you see that, you should think of a photosensitive eruption,” and consider a differential diagnosis, using “certain tests, asking questions, and looking at the morphology and distribution of the reaction.”
The most common of the photodermatoses is polymorphous light eruption, which typically occurs in people on vacation, often among those who live in temperate climates and react violently to sun overexposure. “You usually have to rule out things like connective tissue disease by getting serology,” Dr. DeLeo noted in the interview.
In general, these patients either present with a reaction or describe a rash that has since cleared up. When a patient is describing a problem that has disappeared, he or she may have a photo of the reaction on their phone, which can be helpful, he said at the meeting jointly presented by the University of Louisville and Global Academy for Medical Education.
Some photodermatoses are characterized by tell-tale rashes indicative of a connective tissue disease like lupus or dermatomyositis, which can be followed up with biopsy and serology to confirm a diagnosis, said Dr. DeLeo, who is also professor emeritus of dermatology at the Icahn School of Medicine at Mount Sinai, New York. Other patterns, such as blisters on the hands, should prompt tests for porphyria cutanea tarda, which requires a 24-hour urine test to confirm.
But reactions in sun-exposed areas can also be caused by drug-induced photosensitivity and drugs such as NSAIDs, antibiotics, and diuretics, among others, Dr. DeLeo said.
Another common reaction can occur with exposure to photosensitizing plant materials, resulting in blisters combined with altered pigmentation. Most frequently, these reactions are caused by exposure to limes, which contain psoralens that absorb light and produce the reaction. “That is a clinical diagnosis and we can recognize that because, like poison ivy, it is usually a streaky kind of reaction in a distinct pattern. You don’t need [additional] tests for that,” he said. This can occur, for example, after making cocktails, he said, describing an example of parents who had been mixing drinks and then picked up their child. Later, they brought the child to the ED with a hand-shaped rash, and treating physicians contacted social services out of fears of child abuse.
In some cases, patients who present with no rash can elicit the problem. Solar urticaria can produce hives on demand – ask a patient to go out in the sun and return after a few minutes, and a rash will generally appear, Dr. DeLeo advised.
Patients might want to take steps to avoid such problems and there are several options available to do so, Dr. DeLeo noted. There is some evidence of efficacy for photoprotection for an extract from a fern plant found in Central and South America, a product called Heliocare, in a study sponsored by the manufacturer, Ferndale Healthcare (J Clin Aesthet Dermatol. 2015 Feb;8[2]:19-23). Patients can also undergo two or three treatments of phototherapy for 3-4 weeks in advance of a trip in order to “harden” the skin and reduce the chances of an overreaction.
Dr. DeLeo presented a simplified guide to use when considering differential diagnoses in patients with photodermatoses.
- Solar urticaria occurs quickly upon exposure and is short lived.
- Polymorphous light eruption usually occurs during a vacation and does not affect the face.
- A sun-related eczematous rash affecting the face, which recurs, is typically photoallergy to sunscreen.
- A chronic, severe dermatitis seen in a photodistribution pattern could be chronic actinic dermatitis. The patient should undergo phototesting. A biopsy of a severe case may resemble lymphoma.
- In photosensitive individuals with an uncertain diagnosis, serology testing for connective tissue disease should be performed to rule out lupus or dermatomyositis.
- When the patient has a rash in only sun-exposed areas, as well as a history of exposure to photosensitizing drugs, the drug should be discontinued to see if the rash disappears.
- Porphyria cutanea tarda is diagnosed with a 24-hour urine test that reveals at least five times normal uroporphyrin levels, along with hemochromatosis polymorphisms. Levels of uroporphyrin that are elevated but not five times normal are suggestive of pseudoporphyria.
- Phototoxic contact dermatitis caused by plants often results in unusual distributions.
- Hispanic individuals with a photodistributed rash, especially in the presence of cheilitis, may have actinic prurigo.
Dr. DeLeo is a consultant for Estee Lauder. The annual Coastal Dermatology Symposium is jointly presented by the University of Louisville and Global Academy for Medical Education. This publication and Global Academy for Medical Education are owned by the same parent company.
SEATTLE – according to Vincent DeLeo, MD, of the department of dermatology at the University of Southern California, Los Angeles, who discussed common diagnoses at the annual Coastal Dermatology Symposium.
“They’re not common bread-and-butter dermatitis, like acne rosacea. Many people feel uncomfortable trying to work out the differential diagnosis when they see someone who comes in with what either the patient or the physician thinks is a reaction to the sun,” Dr. DeLeo said in an interview at the meeting.
“Usually, the best clue is the distribution of the rash or lesion,” which often are on the backs of the hands or on the arms from the sleeves down, he added. “When you see that, you should think of a photosensitive eruption,” and consider a differential diagnosis, using “certain tests, asking questions, and looking at the morphology and distribution of the reaction.”
The most common of the photodermatoses is polymorphous light eruption, which typically occurs in people on vacation, often among those who live in temperate climates and react violently to sun overexposure. “You usually have to rule out things like connective tissue disease by getting serology,” Dr. DeLeo noted in the interview.
In general, these patients either present with a reaction or describe a rash that has since cleared up. When a patient is describing a problem that has disappeared, he or she may have a photo of the reaction on their phone, which can be helpful, he said at the meeting jointly presented by the University of Louisville and Global Academy for Medical Education.
Some photodermatoses are characterized by tell-tale rashes indicative of a connective tissue disease like lupus or dermatomyositis, which can be followed up with biopsy and serology to confirm a diagnosis, said Dr. DeLeo, who is also professor emeritus of dermatology at the Icahn School of Medicine at Mount Sinai, New York. Other patterns, such as blisters on the hands, should prompt tests for porphyria cutanea tarda, which requires a 24-hour urine test to confirm.
But reactions in sun-exposed areas can also be caused by drug-induced photosensitivity and drugs such as NSAIDs, antibiotics, and diuretics, among others, Dr. DeLeo said.
Another common reaction can occur with exposure to photosensitizing plant materials, resulting in blisters combined with altered pigmentation. Most frequently, these reactions are caused by exposure to limes, which contain psoralens that absorb light and produce the reaction. “That is a clinical diagnosis and we can recognize that because, like poison ivy, it is usually a streaky kind of reaction in a distinct pattern. You don’t need [additional] tests for that,” he said. This can occur, for example, after making cocktails, he said, describing an example of parents who had been mixing drinks and then picked up their child. Later, they brought the child to the ED with a hand-shaped rash, and treating physicians contacted social services out of fears of child abuse.
In some cases, patients who present with no rash can elicit the problem. Solar urticaria can produce hives on demand – ask a patient to go out in the sun and return after a few minutes, and a rash will generally appear, Dr. DeLeo advised.
Patients might want to take steps to avoid such problems and there are several options available to do so, Dr. DeLeo noted. There is some evidence of efficacy for photoprotection for an extract from a fern plant found in Central and South America, a product called Heliocare, in a study sponsored by the manufacturer, Ferndale Healthcare (J Clin Aesthet Dermatol. 2015 Feb;8[2]:19-23). Patients can also undergo two or three treatments of phototherapy for 3-4 weeks in advance of a trip in order to “harden” the skin and reduce the chances of an overreaction.
Dr. DeLeo presented a simplified guide to use when considering differential diagnoses in patients with photodermatoses.
- Solar urticaria occurs quickly upon exposure and is short lived.
- Polymorphous light eruption usually occurs during a vacation and does not affect the face.
- A sun-related eczematous rash affecting the face, which recurs, is typically photoallergy to sunscreen.
- A chronic, severe dermatitis seen in a photodistribution pattern could be chronic actinic dermatitis. The patient should undergo phototesting. A biopsy of a severe case may resemble lymphoma.
- In photosensitive individuals with an uncertain diagnosis, serology testing for connective tissue disease should be performed to rule out lupus or dermatomyositis.
- When the patient has a rash in only sun-exposed areas, as well as a history of exposure to photosensitizing drugs, the drug should be discontinued to see if the rash disappears.
- Porphyria cutanea tarda is diagnosed with a 24-hour urine test that reveals at least five times normal uroporphyrin levels, along with hemochromatosis polymorphisms. Levels of uroporphyrin that are elevated but not five times normal are suggestive of pseudoporphyria.
- Phototoxic contact dermatitis caused by plants often results in unusual distributions.
- Hispanic individuals with a photodistributed rash, especially in the presence of cheilitis, may have actinic prurigo.
Dr. DeLeo is a consultant for Estee Lauder. The annual Coastal Dermatology Symposium is jointly presented by the University of Louisville and Global Academy for Medical Education. This publication and Global Academy for Medical Education are owned by the same parent company.
EXPERT ANALYSIS FROM COASTAL DERM
IHS Pediatrician Convicted on Sexual Abuse Counts
Stanley Patrick Weber, a former Indian Health Service (IHS) pediatrician, was convicted September 27 on 8 counts of sexual abuse of 4 Native American boys under his care at the Pine Ridge Indian Reservation, Oglala Lakota County, South Dakota.
Weber was an employee of the IHS from 1986 until he resigned in 2016. During that time, although suspicions were rampant that he was abusing young patients, he was moved from one reservation to another. An investigation by The Wall Street Journal and PBS/Frontline found officials in the IHS chain of command “ignored warning signs” and tried to silence whistleblowers.
Weber also was convicted last year and served time for abusing 2 boys at another government hospital in Montana before he arrived at Pine Ridge.
The Pine Ridge Reservation is home to some of the poorest communities in the US, with a dropout rate of > 70%, and a teen suicide rate 150% higher than America’s as a whole. Many of the youths testifying in the case described difficult childhoods, according to the Frontline program. In addition to assaulting them during health care visits, Weber lured them to his home with offers of alcohol and cash.
A White House task force was created to address the agency’s failures. In a statement, Rear Adm Michael Weahkee, IHS principal deputy director, said the IHS is “doing all we can throughout our agency to strengthen our protection for patients.” Steps taken include reinforcing with staff the prohibitions against inappropriate contact with children, reminding staff of the protections for whistleblowers, and hiring Integritas Creative Solutions to conduct a medical quality assurance review to examine whether laws, policies and procedures have been followed with regard to protecting patients from sexual abuse. The review complements work done by the Presidential Task Force on Protecting Native American Children in the Indian Health Service System and a separate review by the HHS Office of the Inspector General.
The IHS also is providing professional counseling for victims. Services do not have to be at an IHS facility or with an IHS provider. A confidential hot line has been established at 301-443-0658.
Weahkee calls the verdict “a long-awaited victory.” But, he adds, “we know that it will not completely heal the wounds inflicted on Dr. Weber’s victims.”
Stanley Patrick Weber, a former Indian Health Service (IHS) pediatrician, was convicted September 27 on 8 counts of sexual abuse of 4 Native American boys under his care at the Pine Ridge Indian Reservation, Oglala Lakota County, South Dakota.
Weber was an employee of the IHS from 1986 until he resigned in 2016. During that time, although suspicions were rampant that he was abusing young patients, he was moved from one reservation to another. An investigation by The Wall Street Journal and PBS/Frontline found officials in the IHS chain of command “ignored warning signs” and tried to silence whistleblowers.
Weber also was convicted last year and served time for abusing 2 boys at another government hospital in Montana before he arrived at Pine Ridge.
The Pine Ridge Reservation is home to some of the poorest communities in the US, with a dropout rate of > 70%, and a teen suicide rate 150% higher than America’s as a whole. Many of the youths testifying in the case described difficult childhoods, according to the Frontline program. In addition to assaulting them during health care visits, Weber lured them to his home with offers of alcohol and cash.
A White House task force was created to address the agency’s failures. In a statement, Rear Adm Michael Weahkee, IHS principal deputy director, said the IHS is “doing all we can throughout our agency to strengthen our protection for patients.” Steps taken include reinforcing with staff the prohibitions against inappropriate contact with children, reminding staff of the protections for whistleblowers, and hiring Integritas Creative Solutions to conduct a medical quality assurance review to examine whether laws, policies and procedures have been followed with regard to protecting patients from sexual abuse. The review complements work done by the Presidential Task Force on Protecting Native American Children in the Indian Health Service System and a separate review by the HHS Office of the Inspector General.
The IHS also is providing professional counseling for victims. Services do not have to be at an IHS facility or with an IHS provider. A confidential hot line has been established at 301-443-0658.
Weahkee calls the verdict “a long-awaited victory.” But, he adds, “we know that it will not completely heal the wounds inflicted on Dr. Weber’s victims.”
Stanley Patrick Weber, a former Indian Health Service (IHS) pediatrician, was convicted September 27 on 8 counts of sexual abuse of 4 Native American boys under his care at the Pine Ridge Indian Reservation, Oglala Lakota County, South Dakota.
Weber was an employee of the IHS from 1986 until he resigned in 2016. During that time, although suspicions were rampant that he was abusing young patients, he was moved from one reservation to another. An investigation by The Wall Street Journal and PBS/Frontline found officials in the IHS chain of command “ignored warning signs” and tried to silence whistleblowers.
Weber also was convicted last year and served time for abusing 2 boys at another government hospital in Montana before he arrived at Pine Ridge.
The Pine Ridge Reservation is home to some of the poorest communities in the US, with a dropout rate of > 70%, and a teen suicide rate 150% higher than America’s as a whole. Many of the youths testifying in the case described difficult childhoods, according to the Frontline program. In addition to assaulting them during health care visits, Weber lured them to his home with offers of alcohol and cash.
A White House task force was created to address the agency’s failures. In a statement, Rear Adm Michael Weahkee, IHS principal deputy director, said the IHS is “doing all we can throughout our agency to strengthen our protection for patients.” Steps taken include reinforcing with staff the prohibitions against inappropriate contact with children, reminding staff of the protections for whistleblowers, and hiring Integritas Creative Solutions to conduct a medical quality assurance review to examine whether laws, policies and procedures have been followed with regard to protecting patients from sexual abuse. The review complements work done by the Presidential Task Force on Protecting Native American Children in the Indian Health Service System and a separate review by the HHS Office of the Inspector General.
The IHS also is providing professional counseling for victims. Services do not have to be at an IHS facility or with an IHS provider. A confidential hot line has been established at 301-443-0658.
Weahkee calls the verdict “a long-awaited victory.” But, he adds, “we know that it will not completely heal the wounds inflicted on Dr. Weber’s victims.”
Vaping-associated lung injury cases exceed 1,000
More than 1,000 cases of vaping-associated lung injury have been reported in 48 states and the U.S. Virgin Islands, according to a telebriefing by the Centers for Disease Control and Prevention.
As of Oct. 1, there have been 1,080 confirmed and probable cases of lung injury associated with the use of e-cigarettes, or vaping, said Anne Schuchat, MD, principal deputy director of the CDC. The latest figures were also reported in a statement issued by the CDC.
Dr. Schuchat said 18 related deaths in 15 states have been confirmed, and additional deaths are under investigation.
“As we have continued to get data for additional cases, the trends we reported last week persist,” Dr. Schuchat said (MMWR. 2019 Sep 27;68[39];860-4).
“Most patients reported a history of using THC [tetrahydrocannabinol]-containing products, and most patients are male and young people.” Of the 1,080 cases identified, approximately 70% are male, roughly 80% are younger than 35 years of age, and 37% are under 21 years of age. The patients’ median age is 23 years (range, 13-75 years). Among patients who have died, the median age is 50 years (range, 27-71 years).
The CDC now has information from 578 patients on the substances used in vaping products in the 90 days before symptom onset. About 78% of these patients reported using THC-containing products, and 37% reported exclusive use of THC-containing products. Roughly 58% of patients reported using nicotine-containing products, and 17% reported exclusive use of nicotine-containing products.
“I wish we had more answers regarding the specific harmful products or components that are causing these illnesses,” Dr. Schuchat said. She noted that THC-containing products appear to be the most commonly used, but these products don’t appear to be the only culprit. Additionally, in a report released recently in the New England Journal of Medicine (2019 Sep 9. doi: 10.1056/NEJMoa1911614), THC-containing products bought “off the street” were commonly used by patients with lung injuries. However, the CDC can’t say for certain if it’s safer for consumers to buy THC-containing products from a licensed dispensary.
The CDC has deployed staff to several states to help investigate the lung injuries, reached out to the clinical community to increase awareness of the injuries, and worked with clinicians and medical examiners to review assessments of patients who have developed these injuries, including those who have died. The CDC has also convened clinical professional societies to “help strengthen the detection, reporting, and management of cases,” Dr. Schuchat said.
In addition, the CDC has joined with the Food and Drug Administration and other public health partners to develop a laboratory plan for “continued testing of products, aerosol testing of substances produced by the products, and clinical pathology lung specimens from patients,” Dr. Schuchat said.
The FDA is also working to gather more information about vaping-associated lung injuries. The FDA is trying to obtain “critical details” about the specific products or substances that may be involved, said Judy McMeekin, PharmD, deputy associate commissioner for regulatory affairs at the FDA.
“There does not currently appear to be one product or substance involved in all of the cases,” Dr. McMeekin said. “We are leaving no stone unturned and following all potential leads regarding any particular product, constituent, or compound that may be at issue.”
The FDA has collected more than 440 samples of vaping devices and products from 18 states. The agency is still analyzing these samples, but a preliminary analysis has shown that some products contain THC concentrations ranging from 14% to 76%, and some products contain a combination of THC and vitamin E acetate ranging from 31% to 88%.
For information about the collection of vaping products for possible testing by the FDA, email [email protected]. For information about collection and submission of clinical specimens for possible testing by the CDC, see the Healthcare Provider webpage.
Clinicians and health officials who have questions about this outbreak can email [email protected]. All others with questions about this outbreak can contact CDC-INFO at 800-232-4636 or submit information at the Contact CDC-INFO page.
More than 1,000 cases of vaping-associated lung injury have been reported in 48 states and the U.S. Virgin Islands, according to a telebriefing by the Centers for Disease Control and Prevention.
As of Oct. 1, there have been 1,080 confirmed and probable cases of lung injury associated with the use of e-cigarettes, or vaping, said Anne Schuchat, MD, principal deputy director of the CDC. The latest figures were also reported in a statement issued by the CDC.
Dr. Schuchat said 18 related deaths in 15 states have been confirmed, and additional deaths are under investigation.
“As we have continued to get data for additional cases, the trends we reported last week persist,” Dr. Schuchat said (MMWR. 2019 Sep 27;68[39];860-4).
“Most patients reported a history of using THC [tetrahydrocannabinol]-containing products, and most patients are male and young people.” Of the 1,080 cases identified, approximately 70% are male, roughly 80% are younger than 35 years of age, and 37% are under 21 years of age. The patients’ median age is 23 years (range, 13-75 years). Among patients who have died, the median age is 50 years (range, 27-71 years).
The CDC now has information from 578 patients on the substances used in vaping products in the 90 days before symptom onset. About 78% of these patients reported using THC-containing products, and 37% reported exclusive use of THC-containing products. Roughly 58% of patients reported using nicotine-containing products, and 17% reported exclusive use of nicotine-containing products.
“I wish we had more answers regarding the specific harmful products or components that are causing these illnesses,” Dr. Schuchat said. She noted that THC-containing products appear to be the most commonly used, but these products don’t appear to be the only culprit. Additionally, in a report released recently in the New England Journal of Medicine (2019 Sep 9. doi: 10.1056/NEJMoa1911614), THC-containing products bought “off the street” were commonly used by patients with lung injuries. However, the CDC can’t say for certain if it’s safer for consumers to buy THC-containing products from a licensed dispensary.
The CDC has deployed staff to several states to help investigate the lung injuries, reached out to the clinical community to increase awareness of the injuries, and worked with clinicians and medical examiners to review assessments of patients who have developed these injuries, including those who have died. The CDC has also convened clinical professional societies to “help strengthen the detection, reporting, and management of cases,” Dr. Schuchat said.
In addition, the CDC has joined with the Food and Drug Administration and other public health partners to develop a laboratory plan for “continued testing of products, aerosol testing of substances produced by the products, and clinical pathology lung specimens from patients,” Dr. Schuchat said.
The FDA is also working to gather more information about vaping-associated lung injuries. The FDA is trying to obtain “critical details” about the specific products or substances that may be involved, said Judy McMeekin, PharmD, deputy associate commissioner for regulatory affairs at the FDA.
“There does not currently appear to be one product or substance involved in all of the cases,” Dr. McMeekin said. “We are leaving no stone unturned and following all potential leads regarding any particular product, constituent, or compound that may be at issue.”
The FDA has collected more than 440 samples of vaping devices and products from 18 states. The agency is still analyzing these samples, but a preliminary analysis has shown that some products contain THC concentrations ranging from 14% to 76%, and some products contain a combination of THC and vitamin E acetate ranging from 31% to 88%.
For information about the collection of vaping products for possible testing by the FDA, email [email protected]. For information about collection and submission of clinical specimens for possible testing by the CDC, see the Healthcare Provider webpage.
Clinicians and health officials who have questions about this outbreak can email [email protected]. All others with questions about this outbreak can contact CDC-INFO at 800-232-4636 or submit information at the Contact CDC-INFO page.
More than 1,000 cases of vaping-associated lung injury have been reported in 48 states and the U.S. Virgin Islands, according to a telebriefing by the Centers for Disease Control and Prevention.
As of Oct. 1, there have been 1,080 confirmed and probable cases of lung injury associated with the use of e-cigarettes, or vaping, said Anne Schuchat, MD, principal deputy director of the CDC. The latest figures were also reported in a statement issued by the CDC.
Dr. Schuchat said 18 related deaths in 15 states have been confirmed, and additional deaths are under investigation.
“As we have continued to get data for additional cases, the trends we reported last week persist,” Dr. Schuchat said (MMWR. 2019 Sep 27;68[39];860-4).
“Most patients reported a history of using THC [tetrahydrocannabinol]-containing products, and most patients are male and young people.” Of the 1,080 cases identified, approximately 70% are male, roughly 80% are younger than 35 years of age, and 37% are under 21 years of age. The patients’ median age is 23 years (range, 13-75 years). Among patients who have died, the median age is 50 years (range, 27-71 years).
The CDC now has information from 578 patients on the substances used in vaping products in the 90 days before symptom onset. About 78% of these patients reported using THC-containing products, and 37% reported exclusive use of THC-containing products. Roughly 58% of patients reported using nicotine-containing products, and 17% reported exclusive use of nicotine-containing products.
“I wish we had more answers regarding the specific harmful products or components that are causing these illnesses,” Dr. Schuchat said. She noted that THC-containing products appear to be the most commonly used, but these products don’t appear to be the only culprit. Additionally, in a report released recently in the New England Journal of Medicine (2019 Sep 9. doi: 10.1056/NEJMoa1911614), THC-containing products bought “off the street” were commonly used by patients with lung injuries. However, the CDC can’t say for certain if it’s safer for consumers to buy THC-containing products from a licensed dispensary.
The CDC has deployed staff to several states to help investigate the lung injuries, reached out to the clinical community to increase awareness of the injuries, and worked with clinicians and medical examiners to review assessments of patients who have developed these injuries, including those who have died. The CDC has also convened clinical professional societies to “help strengthen the detection, reporting, and management of cases,” Dr. Schuchat said.
In addition, the CDC has joined with the Food and Drug Administration and other public health partners to develop a laboratory plan for “continued testing of products, aerosol testing of substances produced by the products, and clinical pathology lung specimens from patients,” Dr. Schuchat said.
The FDA is also working to gather more information about vaping-associated lung injuries. The FDA is trying to obtain “critical details” about the specific products or substances that may be involved, said Judy McMeekin, PharmD, deputy associate commissioner for regulatory affairs at the FDA.
“There does not currently appear to be one product or substance involved in all of the cases,” Dr. McMeekin said. “We are leaving no stone unturned and following all potential leads regarding any particular product, constituent, or compound that may be at issue.”
The FDA has collected more than 440 samples of vaping devices and products from 18 states. The agency is still analyzing these samples, but a preliminary analysis has shown that some products contain THC concentrations ranging from 14% to 76%, and some products contain a combination of THC and vitamin E acetate ranging from 31% to 88%.
For information about the collection of vaping products for possible testing by the FDA, email [email protected]. For information about collection and submission of clinical specimens for possible testing by the CDC, see the Healthcare Provider webpage.
Clinicians and health officials who have questions about this outbreak can email [email protected]. All others with questions about this outbreak can contact CDC-INFO at 800-232-4636 or submit information at the Contact CDC-INFO page.
AGA promotes workforce diversity in academic gastroenterology: The FORWARD program
“The American Gastroenterological Association (AGA) has worked diligently and effectively to expand the pool of underrepresented in medicine physicians who provide care for patients with digestive diseases,” remarks Byron Cryer, MD, FORWARD principal investigator and associate dean for the office of faculty diversity & development, University of Texas Southwestern Medical Center, Dallas.
This long history of promoting and encouraging diversity in the field is notable; however, the society recognizes that there is still more to be done to prepare and sponsor underrepresented in medicine physician-scientists to assume leadership positions in the field.
“As of 2017, only 3.2% of gastroenterology fellows were African American and 8.5% were Hispanic,” remarked Sandra Quezada, MD, AGA Chair, Diversity Committee. These statistics closely correspond to AGA’s membership demographics, where only 3.36% of AGA members are African American and 5.53% are Hispanic, among those reporting ethnicity.
Under the leadership of principal investigators Byron Cryer, MD, and Sheila Crowe, MD, and coinvestigators Juanita Merchant, MD, and Jesus Rivera-Nieves, MD, AGA developed the FORWARD (Fostering Opportunities Resulting in Workforce and Research Diversity) program.
This program was funded by the National Institute of Diabetes and Digestive and Kidney Diseases through an education project (R25) grant.
The overall objective of the grant is to enhance the diversity of the biomedical science research workforce in gastroenterology and to prepare individuals to assume leadership positions within the AGA and in academic medicine.
The FORWARD program has three aims:
1. Provide skill development in leadership – including general leadership development, executive coaching, and opportunities for leadership experience within the society.
2. Implement a diversity management plan focused on active mentoring approaches, using both one-on-one and networking approaches, to provide opportunities for broad support and sponsorship.
3. Provide training for skill development in research careers including training in research development, management of research groups, scientific manuscript, and grant writing.
The program targets fellows and early-career faculty and was promoted broadly to society membership and to past participants in AGA diversity programs. Through a rigorous selection process, 10 physician-scientists were selected among a qualified pool of applicants. Participants were matched with experienced academic gastroenterologists who committed to serve as their professional mentors throughout the course of their participation in the FORWARD program.
Leadership development
The first FORWARD cohort kicked off in March 2019 and will conclude May 2020. They convened at AGA’s inaugural 2½ day Leadership Development Conference. During the conference, the 10 FORWARD scholars joined with 18 AGA Future Leader program participants and 40 Women’s Leadership Conference attendees to participate in leadership development training that addressed such topics as building resilience, presentation skills, negotiation, career success in an ever-changing scientific environment, emotional intelligence, and other key topics designed to bolster their skills as emerging leaders in gastroenterology. With seven past and current AGA presidents in attendance, the FORWARD participants had the unique opportunity to learn about the career paths and gain advice from key leaders in the field while also networking with fellow emerging leaders in both the Future Leaders program and Women’s Leadership Conference.
To further prepare the FORWARD scholars to assume future leadership positions within the society, they’ve begun “internships” that included shadowing AGA committee meetings during Digestive Disease Week (DDW®) 2019 in San Diego and participating in networking events with AGA leaders at receptions and various events.
Mentoring and coaching
In addition to leadership development training at the conference, each FORWARD scholar identified a research topic that they would work on with their mentor and a grant-writing expert to develop into a full grant proposal by the conclusion of the program. The FORWARD mentors have committed to provide mentoring that addresses scientific research content as well as career guidance. The active mentoring provided to the FORWARD scholars is extensive and includes regular contact with their mentors as well as one-on-one executive coaching by a professional coach committed to learning about their needs and guiding them through an individual development plan.
To facilitate the coaching for their individual development plan, each FORWARD scholar completed a Self-Assessment for Physician Leaders and a 360° assessment, which informed the executive coaching that the scholars receive. For many of the scholars, this is the first time that they have benefited from individual executive coaching.
Academic research skills development
In addition to leadership development training and networking opportunities along with executive coaching and mentoring, the FORWARD scholars benefit from direct evaluation and consultation on grant writing. FORWARD scholars have access to a private learning management system where they participate in online grant-writing skills training designed by a grant-writing expert. Based on their training, they are developing various segments of an actual grant proposal and are receiving both virtual peer critiques as well as individualized critiques and guidance from the grant-writing consultant, their program mentor, and their home institution mentor. Future training modules will include lab management and manuscript writing.
Jesus Rivera-Nieves, MD, FORWARD coinvestigator, stated, “I have had the honor of monitoring the progress of our first class of scholars recently. I am incredibly proud of the caliber of these early-career professionals and have no doubt that they will emerge as leaders in our field, where more diversity is greatly needed.”
The scholars and their mentors will reconvene at DDW® 2020 for an opportunity to give a presentation and participate in a commencement ceremony concluding their involvement in the program. Following the graduation of this first cohort, there will be a period of evaluation prior to recruitment of the second cohort of FORWARD scholars.
The 2018-2020 FORWARD Cohort includes:
Yelina Alvarez, MD, PhD
|
Dominique Bailey, MD
|
|
Oriana M. Damas, MD
|
Patricia D. Jones, MD, MSCR
|
Folasade (Fola) May, MD, PhD, MPhil
|
Antonio Mendoza Ladd, MD, FACG, FASGE
|
Akinbowale Oyalowo, MD
|
|
Eric J. Vargas, MD |
The 2018-2020 – FORWARD Program Mentors
Maria Abreu, MD, AGAF
|
C. Rick Boland, MD, AGAF
|
John Carethers, MD, AGAF
|
Darwin Conwell, MD, MS
|
|
John Inadomi, MD, AGAF
|
|
|
|
Gary Wu, MD
|
Dr. Cryer is associate dean for faculty diversity and development, UT Southwestern Medical Center, Dallas; Dr. Rivera-Nieves, is professor of medicine, University of California, San Diego; and Ms. NuQuay, is senior director, member relations and constituency programs, American Gastroenterological Association, Bethesda, Md.
“The American Gastroenterological Association (AGA) has worked diligently and effectively to expand the pool of underrepresented in medicine physicians who provide care for patients with digestive diseases,” remarks Byron Cryer, MD, FORWARD principal investigator and associate dean for the office of faculty diversity & development, University of Texas Southwestern Medical Center, Dallas.
This long history of promoting and encouraging diversity in the field is notable; however, the society recognizes that there is still more to be done to prepare and sponsor underrepresented in medicine physician-scientists to assume leadership positions in the field.
“As of 2017, only 3.2% of gastroenterology fellows were African American and 8.5% were Hispanic,” remarked Sandra Quezada, MD, AGA Chair, Diversity Committee. These statistics closely correspond to AGA’s membership demographics, where only 3.36% of AGA members are African American and 5.53% are Hispanic, among those reporting ethnicity.
Under the leadership of principal investigators Byron Cryer, MD, and Sheila Crowe, MD, and coinvestigators Juanita Merchant, MD, and Jesus Rivera-Nieves, MD, AGA developed the FORWARD (Fostering Opportunities Resulting in Workforce and Research Diversity) program.
This program was funded by the National Institute of Diabetes and Digestive and Kidney Diseases through an education project (R25) grant.
The overall objective of the grant is to enhance the diversity of the biomedical science research workforce in gastroenterology and to prepare individuals to assume leadership positions within the AGA and in academic medicine.
The FORWARD program has three aims:
1. Provide skill development in leadership – including general leadership development, executive coaching, and opportunities for leadership experience within the society.
2. Implement a diversity management plan focused on active mentoring approaches, using both one-on-one and networking approaches, to provide opportunities for broad support and sponsorship.
3. Provide training for skill development in research careers including training in research development, management of research groups, scientific manuscript, and grant writing.
The program targets fellows and early-career faculty and was promoted broadly to society membership and to past participants in AGA diversity programs. Through a rigorous selection process, 10 physician-scientists were selected among a qualified pool of applicants. Participants were matched with experienced academic gastroenterologists who committed to serve as their professional mentors throughout the course of their participation in the FORWARD program.
Leadership development
The first FORWARD cohort kicked off in March 2019 and will conclude May 2020. They convened at AGA’s inaugural 2½ day Leadership Development Conference. During the conference, the 10 FORWARD scholars joined with 18 AGA Future Leader program participants and 40 Women’s Leadership Conference attendees to participate in leadership development training that addressed such topics as building resilience, presentation skills, negotiation, career success in an ever-changing scientific environment, emotional intelligence, and other key topics designed to bolster their skills as emerging leaders in gastroenterology. With seven past and current AGA presidents in attendance, the FORWARD participants had the unique opportunity to learn about the career paths and gain advice from key leaders in the field while also networking with fellow emerging leaders in both the Future Leaders program and Women’s Leadership Conference.
To further prepare the FORWARD scholars to assume future leadership positions within the society, they’ve begun “internships” that included shadowing AGA committee meetings during Digestive Disease Week (DDW®) 2019 in San Diego and participating in networking events with AGA leaders at receptions and various events.
Mentoring and coaching
In addition to leadership development training at the conference, each FORWARD scholar identified a research topic that they would work on with their mentor and a grant-writing expert to develop into a full grant proposal by the conclusion of the program. The FORWARD mentors have committed to provide mentoring that addresses scientific research content as well as career guidance. The active mentoring provided to the FORWARD scholars is extensive and includes regular contact with their mentors as well as one-on-one executive coaching by a professional coach committed to learning about their needs and guiding them through an individual development plan.
To facilitate the coaching for their individual development plan, each FORWARD scholar completed a Self-Assessment for Physician Leaders and a 360° assessment, which informed the executive coaching that the scholars receive. For many of the scholars, this is the first time that they have benefited from individual executive coaching.
Academic research skills development
In addition to leadership development training and networking opportunities along with executive coaching and mentoring, the FORWARD scholars benefit from direct evaluation and consultation on grant writing. FORWARD scholars have access to a private learning management system where they participate in online grant-writing skills training designed by a grant-writing expert. Based on their training, they are developing various segments of an actual grant proposal and are receiving both virtual peer critiques as well as individualized critiques and guidance from the grant-writing consultant, their program mentor, and their home institution mentor. Future training modules will include lab management and manuscript writing.
Jesus Rivera-Nieves, MD, FORWARD coinvestigator, stated, “I have had the honor of monitoring the progress of our first class of scholars recently. I am incredibly proud of the caliber of these early-career professionals and have no doubt that they will emerge as leaders in our field, where more diversity is greatly needed.”
The scholars and their mentors will reconvene at DDW® 2020 for an opportunity to give a presentation and participate in a commencement ceremony concluding their involvement in the program. Following the graduation of this first cohort, there will be a period of evaluation prior to recruitment of the second cohort of FORWARD scholars.
The 2018-2020 FORWARD Cohort includes:
Yelina Alvarez, MD, PhD
|
Dominique Bailey, MD
|
|
Oriana M. Damas, MD
|
Patricia D. Jones, MD, MSCR
|
Folasade (Fola) May, MD, PhD, MPhil
|
Antonio Mendoza Ladd, MD, FACG, FASGE
|
Akinbowale Oyalowo, MD
|
|
Eric J. Vargas, MD |
The 2018-2020 – FORWARD Program Mentors
Maria Abreu, MD, AGAF
|
C. Rick Boland, MD, AGAF
|
John Carethers, MD, AGAF
|
Darwin Conwell, MD, MS
|
|
John Inadomi, MD, AGAF
|
|
|
|
Gary Wu, MD
|
Dr. Cryer is associate dean for faculty diversity and development, UT Southwestern Medical Center, Dallas; Dr. Rivera-Nieves, is professor of medicine, University of California, San Diego; and Ms. NuQuay, is senior director, member relations and constituency programs, American Gastroenterological Association, Bethesda, Md.
“The American Gastroenterological Association (AGA) has worked diligently and effectively to expand the pool of underrepresented in medicine physicians who provide care for patients with digestive diseases,” remarks Byron Cryer, MD, FORWARD principal investigator and associate dean for the office of faculty diversity & development, University of Texas Southwestern Medical Center, Dallas.
This long history of promoting and encouraging diversity in the field is notable; however, the society recognizes that there is still more to be done to prepare and sponsor underrepresented in medicine physician-scientists to assume leadership positions in the field.
“As of 2017, only 3.2% of gastroenterology fellows were African American and 8.5% were Hispanic,” remarked Sandra Quezada, MD, AGA Chair, Diversity Committee. These statistics closely correspond to AGA’s membership demographics, where only 3.36% of AGA members are African American and 5.53% are Hispanic, among those reporting ethnicity.
Under the leadership of principal investigators Byron Cryer, MD, and Sheila Crowe, MD, and coinvestigators Juanita Merchant, MD, and Jesus Rivera-Nieves, MD, AGA developed the FORWARD (Fostering Opportunities Resulting in Workforce and Research Diversity) program.
This program was funded by the National Institute of Diabetes and Digestive and Kidney Diseases through an education project (R25) grant.
The overall objective of the grant is to enhance the diversity of the biomedical science research workforce in gastroenterology and to prepare individuals to assume leadership positions within the AGA and in academic medicine.
The FORWARD program has three aims:
1. Provide skill development in leadership – including general leadership development, executive coaching, and opportunities for leadership experience within the society.
2. Implement a diversity management plan focused on active mentoring approaches, using both one-on-one and networking approaches, to provide opportunities for broad support and sponsorship.
3. Provide training for skill development in research careers including training in research development, management of research groups, scientific manuscript, and grant writing.
The program targets fellows and early-career faculty and was promoted broadly to society membership and to past participants in AGA diversity programs. Through a rigorous selection process, 10 physician-scientists were selected among a qualified pool of applicants. Participants were matched with experienced academic gastroenterologists who committed to serve as their professional mentors throughout the course of their participation in the FORWARD program.
Leadership development
The first FORWARD cohort kicked off in March 2019 and will conclude May 2020. They convened at AGA’s inaugural 2½ day Leadership Development Conference. During the conference, the 10 FORWARD scholars joined with 18 AGA Future Leader program participants and 40 Women’s Leadership Conference attendees to participate in leadership development training that addressed such topics as building resilience, presentation skills, negotiation, career success in an ever-changing scientific environment, emotional intelligence, and other key topics designed to bolster their skills as emerging leaders in gastroenterology. With seven past and current AGA presidents in attendance, the FORWARD participants had the unique opportunity to learn about the career paths and gain advice from key leaders in the field while also networking with fellow emerging leaders in both the Future Leaders program and Women’s Leadership Conference.
To further prepare the FORWARD scholars to assume future leadership positions within the society, they’ve begun “internships” that included shadowing AGA committee meetings during Digestive Disease Week (DDW®) 2019 in San Diego and participating in networking events with AGA leaders at receptions and various events.
Mentoring and coaching
In addition to leadership development training at the conference, each FORWARD scholar identified a research topic that they would work on with their mentor and a grant-writing expert to develop into a full grant proposal by the conclusion of the program. The FORWARD mentors have committed to provide mentoring that addresses scientific research content as well as career guidance. The active mentoring provided to the FORWARD scholars is extensive and includes regular contact with their mentors as well as one-on-one executive coaching by a professional coach committed to learning about their needs and guiding them through an individual development plan.
To facilitate the coaching for their individual development plan, each FORWARD scholar completed a Self-Assessment for Physician Leaders and a 360° assessment, which informed the executive coaching that the scholars receive. For many of the scholars, this is the first time that they have benefited from individual executive coaching.
Academic research skills development
In addition to leadership development training and networking opportunities along with executive coaching and mentoring, the FORWARD scholars benefit from direct evaluation and consultation on grant writing. FORWARD scholars have access to a private learning management system where they participate in online grant-writing skills training designed by a grant-writing expert. Based on their training, they are developing various segments of an actual grant proposal and are receiving both virtual peer critiques as well as individualized critiques and guidance from the grant-writing consultant, their program mentor, and their home institution mentor. Future training modules will include lab management and manuscript writing.
Jesus Rivera-Nieves, MD, FORWARD coinvestigator, stated, “I have had the honor of monitoring the progress of our first class of scholars recently. I am incredibly proud of the caliber of these early-career professionals and have no doubt that they will emerge as leaders in our field, where more diversity is greatly needed.”
The scholars and their mentors will reconvene at DDW® 2020 for an opportunity to give a presentation and participate in a commencement ceremony concluding their involvement in the program. Following the graduation of this first cohort, there will be a period of evaluation prior to recruitment of the second cohort of FORWARD scholars.
The 2018-2020 FORWARD Cohort includes:
Yelina Alvarez, MD, PhD
|
Dominique Bailey, MD
|
|
Oriana M. Damas, MD
|
Patricia D. Jones, MD, MSCR
|
Folasade (Fola) May, MD, PhD, MPhil
|
Antonio Mendoza Ladd, MD, FACG, FASGE
|
Akinbowale Oyalowo, MD
|
|
Eric J. Vargas, MD |
The 2018-2020 – FORWARD Program Mentors
Maria Abreu, MD, AGAF
|
C. Rick Boland, MD, AGAF
|
John Carethers, MD, AGAF
|
Darwin Conwell, MD, MS
|
|
John Inadomi, MD, AGAF
|
|
|
|
Gary Wu, MD
|
Dr. Cryer is associate dean for faculty diversity and development, UT Southwestern Medical Center, Dallas; Dr. Rivera-Nieves, is professor of medicine, University of California, San Diego; and Ms. NuQuay, is senior director, member relations and constituency programs, American Gastroenterological Association, Bethesda, Md.
One-third of patients with severe asthma are overusing corticosteroids
MADRID – if data from a Dutch study presented at the annual congress of the European Respiratory Society are representative of practice elsewhere.
“The main message from our study is that OCS overuse is common and unnecessary in the majority of asthma patients,” reported Katrien A.B. Eger, MD, Amsterdam University Medical Centre.
In this study, 5,002 patients on high doses of inhaled corticosteroids (ICS), defined as at least 500 mcg/day, were identified in a pharmacy database in the Netherlands. These patients were asked to complete a questionnaire to determine how many had severe asthma and had received rescue or maintenance OCS in the past year.
Drawing from the pharmacy database, it could be determined that 29% of the 2,312 patients who responded to the questionnaire were taking harmfully high doses of OCS as well as high doses of ICS. For this study, harmful exposure was defined as a cumulative intake of 420 mg of prednisone-equivalent OCS over a 1-year period. The median cumulative 1-year exposure, according to Dr. Eger, was 750 mg of prednisone equivalent.
In this population, the investigators then calculated ICS medication adherence based on prescription refills. In addition, a subset of this population was evaluated for inhaler technique.
On the basis of these calculations, 47.4% of patients with harmful OCS exposure were found not to be adherent to their prescribed ICS. Of those who were adherent, 53.9% were found not be taking their inhaled steroids appropriately,
When these numbers are put together, the data suggest “78.1% of high OCS users are either nonadherent or using poor inhalation techniques, which means there is a big potential for treatment optimization,” Dr. Eger said.
Yet even among the 21.9% who were adherent and using good inhaler technique, identifying a group who presumably require OCS for exacerbations, the study found that only 46.1% had been prescribed a biologic, which Dr. Eger considers an important steroid-sparing option. She conceded that many of those not on a biologic might not be candidates, but she believes this is another missed opportunity for reducing OCS exposure.
“In the Netherlands, we have very good access to health care, and biologics are available to anyone who needs them,” said Dr. Eger, explaining that access to these drugs is not a barrier.
The evidence overall is that not enough is being done to ensure that asthma patients are being protected from the risks of OCS, according to Dr. Eger. Citing evidence that adverse events associated with OCS begin with a cumulative lifetime prednisone-equivalent exposure of only 500 mg, she believes that clinicians should be more aggressive in intervening.
“We know that there are both acute and chronic complications associated with OCS that involve a range of organ systems,” Dr. Eger said. She listed osteoporosis, diabetes mellitus, hypertension, and adrenal insufficiency as examples. Rescue OCS, even if used sparingly, can drive risk of OCS complications attributable to the importance of cumulative exposure.
In the session where these data were presented, the moderator, Guy Brusselle, MD, professor of asthma and immunology, Ghent (Belgium) University, labeled them “important.” However, he quibbled with Dr. Eger’s assertion that biologics represent a major opportunity to reduce OCS exposure.
“By suggesting that biologics are not being used often enough, there is an assumption that all of these patients have type 2 inflammatory asthma,” Dr. Brusselle said. “I think it makes more sense to emphasize steroid-sparing strategies, not just biologics.”
Dr. Eger did not disagree, but she emphasized that steroid-sparing alternatives are just one strategy to reduce OCS exposure, and ensuring that patients are adherent to prescribed ICS therapies and are using them correctly might have an even greater impact.
Dr. Eger reports no potential conflicts of interest.
MADRID – if data from a Dutch study presented at the annual congress of the European Respiratory Society are representative of practice elsewhere.
“The main message from our study is that OCS overuse is common and unnecessary in the majority of asthma patients,” reported Katrien A.B. Eger, MD, Amsterdam University Medical Centre.
In this study, 5,002 patients on high doses of inhaled corticosteroids (ICS), defined as at least 500 mcg/day, were identified in a pharmacy database in the Netherlands. These patients were asked to complete a questionnaire to determine how many had severe asthma and had received rescue or maintenance OCS in the past year.
Drawing from the pharmacy database, it could be determined that 29% of the 2,312 patients who responded to the questionnaire were taking harmfully high doses of OCS as well as high doses of ICS. For this study, harmful exposure was defined as a cumulative intake of 420 mg of prednisone-equivalent OCS over a 1-year period. The median cumulative 1-year exposure, according to Dr. Eger, was 750 mg of prednisone equivalent.
In this population, the investigators then calculated ICS medication adherence based on prescription refills. In addition, a subset of this population was evaluated for inhaler technique.
On the basis of these calculations, 47.4% of patients with harmful OCS exposure were found not to be adherent to their prescribed ICS. Of those who were adherent, 53.9% were found not be taking their inhaled steroids appropriately,
When these numbers are put together, the data suggest “78.1% of high OCS users are either nonadherent or using poor inhalation techniques, which means there is a big potential for treatment optimization,” Dr. Eger said.
Yet even among the 21.9% who were adherent and using good inhaler technique, identifying a group who presumably require OCS for exacerbations, the study found that only 46.1% had been prescribed a biologic, which Dr. Eger considers an important steroid-sparing option. She conceded that many of those not on a biologic might not be candidates, but she believes this is another missed opportunity for reducing OCS exposure.
“In the Netherlands, we have very good access to health care, and biologics are available to anyone who needs them,” said Dr. Eger, explaining that access to these drugs is not a barrier.
The evidence overall is that not enough is being done to ensure that asthma patients are being protected from the risks of OCS, according to Dr. Eger. Citing evidence that adverse events associated with OCS begin with a cumulative lifetime prednisone-equivalent exposure of only 500 mg, she believes that clinicians should be more aggressive in intervening.
“We know that there are both acute and chronic complications associated with OCS that involve a range of organ systems,” Dr. Eger said. She listed osteoporosis, diabetes mellitus, hypertension, and adrenal insufficiency as examples. Rescue OCS, even if used sparingly, can drive risk of OCS complications attributable to the importance of cumulative exposure.
In the session where these data were presented, the moderator, Guy Brusselle, MD, professor of asthma and immunology, Ghent (Belgium) University, labeled them “important.” However, he quibbled with Dr. Eger’s assertion that biologics represent a major opportunity to reduce OCS exposure.
“By suggesting that biologics are not being used often enough, there is an assumption that all of these patients have type 2 inflammatory asthma,” Dr. Brusselle said. “I think it makes more sense to emphasize steroid-sparing strategies, not just biologics.”
Dr. Eger did not disagree, but she emphasized that steroid-sparing alternatives are just one strategy to reduce OCS exposure, and ensuring that patients are adherent to prescribed ICS therapies and are using them correctly might have an even greater impact.
Dr. Eger reports no potential conflicts of interest.
MADRID – if data from a Dutch study presented at the annual congress of the European Respiratory Society are representative of practice elsewhere.
“The main message from our study is that OCS overuse is common and unnecessary in the majority of asthma patients,” reported Katrien A.B. Eger, MD, Amsterdam University Medical Centre.
In this study, 5,002 patients on high doses of inhaled corticosteroids (ICS), defined as at least 500 mcg/day, were identified in a pharmacy database in the Netherlands. These patients were asked to complete a questionnaire to determine how many had severe asthma and had received rescue or maintenance OCS in the past year.
Drawing from the pharmacy database, it could be determined that 29% of the 2,312 patients who responded to the questionnaire were taking harmfully high doses of OCS as well as high doses of ICS. For this study, harmful exposure was defined as a cumulative intake of 420 mg of prednisone-equivalent OCS over a 1-year period. The median cumulative 1-year exposure, according to Dr. Eger, was 750 mg of prednisone equivalent.
In this population, the investigators then calculated ICS medication adherence based on prescription refills. In addition, a subset of this population was evaluated for inhaler technique.
On the basis of these calculations, 47.4% of patients with harmful OCS exposure were found not to be adherent to their prescribed ICS. Of those who were adherent, 53.9% were found not be taking their inhaled steroids appropriately,
When these numbers are put together, the data suggest “78.1% of high OCS users are either nonadherent or using poor inhalation techniques, which means there is a big potential for treatment optimization,” Dr. Eger said.
Yet even among the 21.9% who were adherent and using good inhaler technique, identifying a group who presumably require OCS for exacerbations, the study found that only 46.1% had been prescribed a biologic, which Dr. Eger considers an important steroid-sparing option. She conceded that many of those not on a biologic might not be candidates, but she believes this is another missed opportunity for reducing OCS exposure.
“In the Netherlands, we have very good access to health care, and biologics are available to anyone who needs them,” said Dr. Eger, explaining that access to these drugs is not a barrier.
The evidence overall is that not enough is being done to ensure that asthma patients are being protected from the risks of OCS, according to Dr. Eger. Citing evidence that adverse events associated with OCS begin with a cumulative lifetime prednisone-equivalent exposure of only 500 mg, she believes that clinicians should be more aggressive in intervening.
“We know that there are both acute and chronic complications associated with OCS that involve a range of organ systems,” Dr. Eger said. She listed osteoporosis, diabetes mellitus, hypertension, and adrenal insufficiency as examples. Rescue OCS, even if used sparingly, can drive risk of OCS complications attributable to the importance of cumulative exposure.
In the session where these data were presented, the moderator, Guy Brusselle, MD, professor of asthma and immunology, Ghent (Belgium) University, labeled them “important.” However, he quibbled with Dr. Eger’s assertion that biologics represent a major opportunity to reduce OCS exposure.
“By suggesting that biologics are not being used often enough, there is an assumption that all of these patients have type 2 inflammatory asthma,” Dr. Brusselle said. “I think it makes more sense to emphasize steroid-sparing strategies, not just biologics.”
Dr. Eger did not disagree, but she emphasized that steroid-sparing alternatives are just one strategy to reduce OCS exposure, and ensuring that patients are adherent to prescribed ICS therapies and are using them correctly might have an even greater impact.
Dr. Eger reports no potential conflicts of interest.
REPORTING FROM ERS 2019
FDA approves Descovy as HIV PrEP for men and transgender women who have sex with men
The decision, backing the earlier recommendation of the FDA’s Antimicrobial Drugs Advisory Committee, was based upon results from DISCOVER, a pivotal, multiyear, global phase 3 clinical trial that evaluated the safety and efficacy of Descovy (emtricitabine 200 mg and tenofovir alafenamide 25-mg tablets for PrEP, compared with Truvada (emtricitabine 200 mg and tenofovir disoproxil fumarate 300-mg tablets).
DISCOVER included more than 5,300 adult cisgender men who have sex with men or transgender women who have sex with men.
In the trial, Descovy achieved noninferiority to Truvada.
Descovy has a Boxed Warning in its U.S. product label regarding the risk of posttreatment acute exacerbation of hepatitis B, according to the company.
The Descovy label also includes a Boxed Warning regarding the risk of drug resistance with PrEP use in undiagnosed early HIV-1 infection. The effectiveness of Descovy for PrEP in individuals at risk of HIV-1 from receptive vaginal sex was not tested, and thus cisgender women at risk for infection from vaginal sex were not included in the population for which the drug was approved.
The Descovy label and safety information is available here.
The FDA version of the announcement is available here.
The decision, backing the earlier recommendation of the FDA’s Antimicrobial Drugs Advisory Committee, was based upon results from DISCOVER, a pivotal, multiyear, global phase 3 clinical trial that evaluated the safety and efficacy of Descovy (emtricitabine 200 mg and tenofovir alafenamide 25-mg tablets for PrEP, compared with Truvada (emtricitabine 200 mg and tenofovir disoproxil fumarate 300-mg tablets).
DISCOVER included more than 5,300 adult cisgender men who have sex with men or transgender women who have sex with men.
In the trial, Descovy achieved noninferiority to Truvada.
Descovy has a Boxed Warning in its U.S. product label regarding the risk of posttreatment acute exacerbation of hepatitis B, according to the company.
The Descovy label also includes a Boxed Warning regarding the risk of drug resistance with PrEP use in undiagnosed early HIV-1 infection. The effectiveness of Descovy for PrEP in individuals at risk of HIV-1 from receptive vaginal sex was not tested, and thus cisgender women at risk for infection from vaginal sex were not included in the population for which the drug was approved.
The Descovy label and safety information is available here.
The FDA version of the announcement is available here.
The decision, backing the earlier recommendation of the FDA’s Antimicrobial Drugs Advisory Committee, was based upon results from DISCOVER, a pivotal, multiyear, global phase 3 clinical trial that evaluated the safety and efficacy of Descovy (emtricitabine 200 mg and tenofovir alafenamide 25-mg tablets for PrEP, compared with Truvada (emtricitabine 200 mg and tenofovir disoproxil fumarate 300-mg tablets).
DISCOVER included more than 5,300 adult cisgender men who have sex with men or transgender women who have sex with men.
In the trial, Descovy achieved noninferiority to Truvada.
Descovy has a Boxed Warning in its U.S. product label regarding the risk of posttreatment acute exacerbation of hepatitis B, according to the company.
The Descovy label also includes a Boxed Warning regarding the risk of drug resistance with PrEP use in undiagnosed early HIV-1 infection. The effectiveness of Descovy for PrEP in individuals at risk of HIV-1 from receptive vaginal sex was not tested, and thus cisgender women at risk for infection from vaginal sex were not included in the population for which the drug was approved.
The Descovy label and safety information is available here.
The FDA version of the announcement is available here.