User login
Corticosteroid use in pregnancy linked to preterm birth
Pregnant women taking oral corticosteroids for rheumatoid arthritis may be at increased risk of preterm birth, according to research published online Sept. 30 in Rheumatology.
A study of 528 pregnant women with rheumatoid arthritis enrolled in the MotherToBaby Pregnancy Studies found that those taking a daily dose of 10 mg or more of prednisone equivalent – representing a mean cumulative dose of 2,208.6 mg over the first 139 days of pregnancy – had 4.77-fold higher odds of preterm birth, compared with those not taking oral corticosteroids. Women on medium doses – with a mean cumulative dose of 883 mg – had 81% higher odds of preterm birth, while those on low cumulative doses of 264.9 mg showed a nonsignificant 38% increase in preterm birth risk.
Women who did not use oral corticosteroids before day 140 of pregnancy had a 2.2% risk of early preterm birth. Among women with low use of oral corticosteroids, the risk was 3.4%, among those with medium use the risk was 3.3%, but among those with high use the risk was 26.7%.
After day 140 of gestation, there was a nonsignificant 64% increase in the risk for preterm birth with any use of oral corticosteroids, compared with no use. But among women taking 10 mg or more of prednisone equivalent per day, the risk was 2.45-fold higher, whereas those taking under 10 mg showed no significant increase in risk.
“Systemic corticosteroid use has been associated with serious infection in pregnant women and serious and nonserious infection in individuals with autoimmune diseases, independent of other immunosuppressive medications, especially for doses of 10 mg of prednisone equivalent per day and greater,” wrote Kristin Palmsten, ScD, a research investigator with HealthPartners Institute in Minneapolis, Minn., and coauthors.
Given that intrauterine infection is believed to contribute to preterm birth, some have suggested that the immunosuppressive effects of oral corticosteroids could be associated with an increased risk of preterm birth because of subclinical intra-amniotic infection, they wrote.
However, they noted that there was a lack of information on the effect of dose and timing of oral corticosteroids during pregnancy on the risk of preterm birth.
The authors acknowledged that dosage of oral corticosteroids during pregnancy was linked to disease activity, which was itself associated with preterm birth risk. They adjusted for self-assessed rheumatoid arthritis severity at enrollment, which was generally during the first trimester, and found that this did attenuate the association with preterm birth.
“Ideally, we would have measures of disease severity at the time of every medication start, stop, or dose change to account for time-varying confounding later in pregnancy,” they wrote.
The study did not find any effect of biologic or nonbiologic disease-modifying antirheumatic drugs, either before or after the first 140 days of gestation.
The authors also looked at pregnancy outcomes among women with inflammatory bowel disease and asthma who were taking corticosteroids for those conditions.
While noting that these estimates were “imprecise,” they did see the suggestion of an increase in preterm birth among women taking oral corticosteroids for asthma, especially when used in the first half of pregnancy. There was also a suggestion of increased preterm birth risk associated with high oral corticosteroid use for inflammatory bowel disease, but these estimates were unadjusted, they noted.
“Overall, IBD and asthma exploratory analyses align with the direction of the associations in the RA analysis despite limitations of precision and inability to adjust for IBD severity,” they wrote.
The conclusions to be drawn from the study are limited by its small size, the investigators noted, as well as a lack of information on the type of rheumatoid arthritis and longitudinal disease severity. They added that while the hypothesized mechanism of action linking oral corticosteroid use to preterm birth was subclinical intrauterine infection, they did not have access to placental pathology to confirm this.
The study was supported by the National Institutes of Health, and the MotherToBaby Pregnancy Studies are supported by research grants from a number of pharmaceutical companies. No other conflicts of interest were declared.
SOURCE: Palmsten K et al. Rheumatology 2019 Sep 30. doi: 10.1093/rheumatology/kez405.
Pregnant women taking oral corticosteroids for rheumatoid arthritis may be at increased risk of preterm birth, according to research published online Sept. 30 in Rheumatology.
A study of 528 pregnant women with rheumatoid arthritis enrolled in the MotherToBaby Pregnancy Studies found that those taking a daily dose of 10 mg or more of prednisone equivalent – representing a mean cumulative dose of 2,208.6 mg over the first 139 days of pregnancy – had 4.77-fold higher odds of preterm birth, compared with those not taking oral corticosteroids. Women on medium doses – with a mean cumulative dose of 883 mg – had 81% higher odds of preterm birth, while those on low cumulative doses of 264.9 mg showed a nonsignificant 38% increase in preterm birth risk.
Women who did not use oral corticosteroids before day 140 of pregnancy had a 2.2% risk of early preterm birth. Among women with low use of oral corticosteroids, the risk was 3.4%, among those with medium use the risk was 3.3%, but among those with high use the risk was 26.7%.
After day 140 of gestation, there was a nonsignificant 64% increase in the risk for preterm birth with any use of oral corticosteroids, compared with no use. But among women taking 10 mg or more of prednisone equivalent per day, the risk was 2.45-fold higher, whereas those taking under 10 mg showed no significant increase in risk.
“Systemic corticosteroid use has been associated with serious infection in pregnant women and serious and nonserious infection in individuals with autoimmune diseases, independent of other immunosuppressive medications, especially for doses of 10 mg of prednisone equivalent per day and greater,” wrote Kristin Palmsten, ScD, a research investigator with HealthPartners Institute in Minneapolis, Minn., and coauthors.
Given that intrauterine infection is believed to contribute to preterm birth, some have suggested that the immunosuppressive effects of oral corticosteroids could be associated with an increased risk of preterm birth because of subclinical intra-amniotic infection, they wrote.
However, they noted that there was a lack of information on the effect of dose and timing of oral corticosteroids during pregnancy on the risk of preterm birth.
The authors acknowledged that dosage of oral corticosteroids during pregnancy was linked to disease activity, which was itself associated with preterm birth risk. They adjusted for self-assessed rheumatoid arthritis severity at enrollment, which was generally during the first trimester, and found that this did attenuate the association with preterm birth.
“Ideally, we would have measures of disease severity at the time of every medication start, stop, or dose change to account for time-varying confounding later in pregnancy,” they wrote.
The study did not find any effect of biologic or nonbiologic disease-modifying antirheumatic drugs, either before or after the first 140 days of gestation.
The authors also looked at pregnancy outcomes among women with inflammatory bowel disease and asthma who were taking corticosteroids for those conditions.
While noting that these estimates were “imprecise,” they did see the suggestion of an increase in preterm birth among women taking oral corticosteroids for asthma, especially when used in the first half of pregnancy. There was also a suggestion of increased preterm birth risk associated with high oral corticosteroid use for inflammatory bowel disease, but these estimates were unadjusted, they noted.
“Overall, IBD and asthma exploratory analyses align with the direction of the associations in the RA analysis despite limitations of precision and inability to adjust for IBD severity,” they wrote.
The conclusions to be drawn from the study are limited by its small size, the investigators noted, as well as a lack of information on the type of rheumatoid arthritis and longitudinal disease severity. They added that while the hypothesized mechanism of action linking oral corticosteroid use to preterm birth was subclinical intrauterine infection, they did not have access to placental pathology to confirm this.
The study was supported by the National Institutes of Health, and the MotherToBaby Pregnancy Studies are supported by research grants from a number of pharmaceutical companies. No other conflicts of interest were declared.
SOURCE: Palmsten K et al. Rheumatology 2019 Sep 30. doi: 10.1093/rheumatology/kez405.
Pregnant women taking oral corticosteroids for rheumatoid arthritis may be at increased risk of preterm birth, according to research published online Sept. 30 in Rheumatology.
A study of 528 pregnant women with rheumatoid arthritis enrolled in the MotherToBaby Pregnancy Studies found that those taking a daily dose of 10 mg or more of prednisone equivalent – representing a mean cumulative dose of 2,208.6 mg over the first 139 days of pregnancy – had 4.77-fold higher odds of preterm birth, compared with those not taking oral corticosteroids. Women on medium doses – with a mean cumulative dose of 883 mg – had 81% higher odds of preterm birth, while those on low cumulative doses of 264.9 mg showed a nonsignificant 38% increase in preterm birth risk.
Women who did not use oral corticosteroids before day 140 of pregnancy had a 2.2% risk of early preterm birth. Among women with low use of oral corticosteroids, the risk was 3.4%, among those with medium use the risk was 3.3%, but among those with high use the risk was 26.7%.
After day 140 of gestation, there was a nonsignificant 64% increase in the risk for preterm birth with any use of oral corticosteroids, compared with no use. But among women taking 10 mg or more of prednisone equivalent per day, the risk was 2.45-fold higher, whereas those taking under 10 mg showed no significant increase in risk.
“Systemic corticosteroid use has been associated with serious infection in pregnant women and serious and nonserious infection in individuals with autoimmune diseases, independent of other immunosuppressive medications, especially for doses of 10 mg of prednisone equivalent per day and greater,” wrote Kristin Palmsten, ScD, a research investigator with HealthPartners Institute in Minneapolis, Minn., and coauthors.
Given that intrauterine infection is believed to contribute to preterm birth, some have suggested that the immunosuppressive effects of oral corticosteroids could be associated with an increased risk of preterm birth because of subclinical intra-amniotic infection, they wrote.
However, they noted that there was a lack of information on the effect of dose and timing of oral corticosteroids during pregnancy on the risk of preterm birth.
The authors acknowledged that dosage of oral corticosteroids during pregnancy was linked to disease activity, which was itself associated with preterm birth risk. They adjusted for self-assessed rheumatoid arthritis severity at enrollment, which was generally during the first trimester, and found that this did attenuate the association with preterm birth.
“Ideally, we would have measures of disease severity at the time of every medication start, stop, or dose change to account for time-varying confounding later in pregnancy,” they wrote.
The study did not find any effect of biologic or nonbiologic disease-modifying antirheumatic drugs, either before or after the first 140 days of gestation.
The authors also looked at pregnancy outcomes among women with inflammatory bowel disease and asthma who were taking corticosteroids for those conditions.
While noting that these estimates were “imprecise,” they did see the suggestion of an increase in preterm birth among women taking oral corticosteroids for asthma, especially when used in the first half of pregnancy. There was also a suggestion of increased preterm birth risk associated with high oral corticosteroid use for inflammatory bowel disease, but these estimates were unadjusted, they noted.
“Overall, IBD and asthma exploratory analyses align with the direction of the associations in the RA analysis despite limitations of precision and inability to adjust for IBD severity,” they wrote.
The conclusions to be drawn from the study are limited by its small size, the investigators noted, as well as a lack of information on the type of rheumatoid arthritis and longitudinal disease severity. They added that while the hypothesized mechanism of action linking oral corticosteroid use to preterm birth was subclinical intrauterine infection, they did not have access to placental pathology to confirm this.
The study was supported by the National Institutes of Health, and the MotherToBaby Pregnancy Studies are supported by research grants from a number of pharmaceutical companies. No other conflicts of interest were declared.
SOURCE: Palmsten K et al. Rheumatology 2019 Sep 30. doi: 10.1093/rheumatology/kez405.
FROM RHEUMATOLOGY
Baseline neurofilament light levels track with brain volume loss in MS
STOCKHOLM – according to a recent study. The correlation between CSF NfL at baseline and the percent of total brain volume change was statistically significant and persisted over time, said Alok Bhan, MD, in an interview at the annual congress of the European Committee for Treatment and Research in Multiple Sclerosis.
“We found a significant correlation between loss of total brain volume with baseline CSF NfL at the 5- and 10-year follow-up,” reported Dr. Bhan. At the 5-year mark, the correlation coefficient (r) was –0.430 (P = .003); at 10 years, the correlation coefficient was –0.395 (P = .042).
Higher CSF levels of the neuropeptide “thereby predicted steeper loss of brain volume over a 10-year disease course, and baseline CSF NfL could thus be a prognostic factor for later MRI-confirmed progression,” reported Dr. Bhan, of the department of neurology at Stavanger (Norway) University Hospital, and his coinvestigators.
Magnetic resonance imaging, the current standard in tracking MS lesions, has limitations, noted the investigators. “MRI pathology is only revealed after the injury has manifested itself, and as it is commonly being conducted no more frequent[ly] than annually, lesions may have developed up to a year” before they are evident on MRI scans, they said.
Concerns about long-term effects of the gadolinium deposition that results from frequent scans are rising as well, said Dr. Bhan. For these reasons, he said, MRI is an imperfect biomarker for MS progression.
NfL is a polypeptide in neurons that has been proposed as prognostic in MS, but “longitudinal data regarding its prognostic value” are still scarce, said Dr. Bhan.
To help fill that knowledge gap, the study aimed to assess how NfL obtained from CSF at the time of MS diagnosis correlated with MRI-determined loss of brain volume over a period of 10 years.
Participants’ brain atrophy was assessed using MRI performed at baseline and at 5 and 10 years using the same protocol on a 1.5 Tesla scanner. The protocol included dual spin echo T2-weighted imaging, 3-D T1-weighted imaging, and dual spin echo T1-weighted imaging. In addition to obtaining both global and regional changes in brain volume, the investigators also calculated regional volumes for subcortical deep gray matter.
Cerebrospinal fluid obtained from the lumbar puncture performed at the time of baseline assessment was the source for the single NfL value, obtained by use of a commercially available enzyme-linked immunosorbent assay.
Looking at volume loss in specific brain regions, significant correlations were seen between volume loss in the deep gray matter at both 5 and 10 years (r = –0.430 and –0.395; P = .006 and .042, respectively). At 10 years, pallidal nuclei and hippocampal volume losses also correlated significantly with baseline CSF NfL (r = –0.445 and –0.472; P = .020 and .013, respectively).
Attrition was substantial in the cohort, with the initial 44 patients dropping to 39 at 5 years and 27 by 10 years. Of those 44 initial enrollees, 30 were female, and the mean age was about 42 years at baseline. Duration of MS was a median 60 months at the time of enrollment, and the mean Extended Disability Status Scale (EDSS) score was 3.5 at baseline. Most patients (n = 35) had relapsing-remitting MS.
Just 16% of enrollees were on disease-modifying therapy at baseline, but this figure climbed to 52% by the end of the study period, said Dr. Bhan, a trend that in part reflected changes in practice patterns over the study period.
“Our study is yet another report that supports the use of CSF NfL in the routine work-up of newly diagnosed MS,” noted Dr. Bhan and his coauthors. Having a baseline CSF NfL level “would give patients an indication of future disease burden 10 years in advance,” thereby adding to the prognostic toolkit, they said.
Dr. Bhan reported receiving research funding from Novartis Norway, and two other coauthors reported several financial relationships with pharmaceutical companies.
SOURCE: Bhan A et al. ECTRIMS 2019, Abstract P592.
STOCKHOLM – according to a recent study. The correlation between CSF NfL at baseline and the percent of total brain volume change was statistically significant and persisted over time, said Alok Bhan, MD, in an interview at the annual congress of the European Committee for Treatment and Research in Multiple Sclerosis.
“We found a significant correlation between loss of total brain volume with baseline CSF NfL at the 5- and 10-year follow-up,” reported Dr. Bhan. At the 5-year mark, the correlation coefficient (r) was –0.430 (P = .003); at 10 years, the correlation coefficient was –0.395 (P = .042).
Higher CSF levels of the neuropeptide “thereby predicted steeper loss of brain volume over a 10-year disease course, and baseline CSF NfL could thus be a prognostic factor for later MRI-confirmed progression,” reported Dr. Bhan, of the department of neurology at Stavanger (Norway) University Hospital, and his coinvestigators.
Magnetic resonance imaging, the current standard in tracking MS lesions, has limitations, noted the investigators. “MRI pathology is only revealed after the injury has manifested itself, and as it is commonly being conducted no more frequent[ly] than annually, lesions may have developed up to a year” before they are evident on MRI scans, they said.
Concerns about long-term effects of the gadolinium deposition that results from frequent scans are rising as well, said Dr. Bhan. For these reasons, he said, MRI is an imperfect biomarker for MS progression.
NfL is a polypeptide in neurons that has been proposed as prognostic in MS, but “longitudinal data regarding its prognostic value” are still scarce, said Dr. Bhan.
To help fill that knowledge gap, the study aimed to assess how NfL obtained from CSF at the time of MS diagnosis correlated with MRI-determined loss of brain volume over a period of 10 years.
Participants’ brain atrophy was assessed using MRI performed at baseline and at 5 and 10 years using the same protocol on a 1.5 Tesla scanner. The protocol included dual spin echo T2-weighted imaging, 3-D T1-weighted imaging, and dual spin echo T1-weighted imaging. In addition to obtaining both global and regional changes in brain volume, the investigators also calculated regional volumes for subcortical deep gray matter.
Cerebrospinal fluid obtained from the lumbar puncture performed at the time of baseline assessment was the source for the single NfL value, obtained by use of a commercially available enzyme-linked immunosorbent assay.
Looking at volume loss in specific brain regions, significant correlations were seen between volume loss in the deep gray matter at both 5 and 10 years (r = –0.430 and –0.395; P = .006 and .042, respectively). At 10 years, pallidal nuclei and hippocampal volume losses also correlated significantly with baseline CSF NfL (r = –0.445 and –0.472; P = .020 and .013, respectively).
Attrition was substantial in the cohort, with the initial 44 patients dropping to 39 at 5 years and 27 by 10 years. Of those 44 initial enrollees, 30 were female, and the mean age was about 42 years at baseline. Duration of MS was a median 60 months at the time of enrollment, and the mean Extended Disability Status Scale (EDSS) score was 3.5 at baseline. Most patients (n = 35) had relapsing-remitting MS.
Just 16% of enrollees were on disease-modifying therapy at baseline, but this figure climbed to 52% by the end of the study period, said Dr. Bhan, a trend that in part reflected changes in practice patterns over the study period.
“Our study is yet another report that supports the use of CSF NfL in the routine work-up of newly diagnosed MS,” noted Dr. Bhan and his coauthors. Having a baseline CSF NfL level “would give patients an indication of future disease burden 10 years in advance,” thereby adding to the prognostic toolkit, they said.
Dr. Bhan reported receiving research funding from Novartis Norway, and two other coauthors reported several financial relationships with pharmaceutical companies.
SOURCE: Bhan A et al. ECTRIMS 2019, Abstract P592.
STOCKHOLM – according to a recent study. The correlation between CSF NfL at baseline and the percent of total brain volume change was statistically significant and persisted over time, said Alok Bhan, MD, in an interview at the annual congress of the European Committee for Treatment and Research in Multiple Sclerosis.
“We found a significant correlation between loss of total brain volume with baseline CSF NfL at the 5- and 10-year follow-up,” reported Dr. Bhan. At the 5-year mark, the correlation coefficient (r) was –0.430 (P = .003); at 10 years, the correlation coefficient was –0.395 (P = .042).
Higher CSF levels of the neuropeptide “thereby predicted steeper loss of brain volume over a 10-year disease course, and baseline CSF NfL could thus be a prognostic factor for later MRI-confirmed progression,” reported Dr. Bhan, of the department of neurology at Stavanger (Norway) University Hospital, and his coinvestigators.
Magnetic resonance imaging, the current standard in tracking MS lesions, has limitations, noted the investigators. “MRI pathology is only revealed after the injury has manifested itself, and as it is commonly being conducted no more frequent[ly] than annually, lesions may have developed up to a year” before they are evident on MRI scans, they said.
Concerns about long-term effects of the gadolinium deposition that results from frequent scans are rising as well, said Dr. Bhan. For these reasons, he said, MRI is an imperfect biomarker for MS progression.
NfL is a polypeptide in neurons that has been proposed as prognostic in MS, but “longitudinal data regarding its prognostic value” are still scarce, said Dr. Bhan.
To help fill that knowledge gap, the study aimed to assess how NfL obtained from CSF at the time of MS diagnosis correlated with MRI-determined loss of brain volume over a period of 10 years.
Participants’ brain atrophy was assessed using MRI performed at baseline and at 5 and 10 years using the same protocol on a 1.5 Tesla scanner. The protocol included dual spin echo T2-weighted imaging, 3-D T1-weighted imaging, and dual spin echo T1-weighted imaging. In addition to obtaining both global and regional changes in brain volume, the investigators also calculated regional volumes for subcortical deep gray matter.
Cerebrospinal fluid obtained from the lumbar puncture performed at the time of baseline assessment was the source for the single NfL value, obtained by use of a commercially available enzyme-linked immunosorbent assay.
Looking at volume loss in specific brain regions, significant correlations were seen between volume loss in the deep gray matter at both 5 and 10 years (r = –0.430 and –0.395; P = .006 and .042, respectively). At 10 years, pallidal nuclei and hippocampal volume losses also correlated significantly with baseline CSF NfL (r = –0.445 and –0.472; P = .020 and .013, respectively).
Attrition was substantial in the cohort, with the initial 44 patients dropping to 39 at 5 years and 27 by 10 years. Of those 44 initial enrollees, 30 were female, and the mean age was about 42 years at baseline. Duration of MS was a median 60 months at the time of enrollment, and the mean Extended Disability Status Scale (EDSS) score was 3.5 at baseline. Most patients (n = 35) had relapsing-remitting MS.
Just 16% of enrollees were on disease-modifying therapy at baseline, but this figure climbed to 52% by the end of the study period, said Dr. Bhan, a trend that in part reflected changes in practice patterns over the study period.
“Our study is yet another report that supports the use of CSF NfL in the routine work-up of newly diagnosed MS,” noted Dr. Bhan and his coauthors. Having a baseline CSF NfL level “would give patients an indication of future disease burden 10 years in advance,” thereby adding to the prognostic toolkit, they said.
Dr. Bhan reported receiving research funding from Novartis Norway, and two other coauthors reported several financial relationships with pharmaceutical companies.
SOURCE: Bhan A et al. ECTRIMS 2019, Abstract P592.
REPORTING FROM ECTRIMS 2019
Smoking Out the Truth About Pot and Cancer
MINNEAPOLIS -- Medical professionals within the US Department of Veterans Affairs (VA) can’t prescribe cannabis or certify patients to be able to get it. VA pharmacists can’t dispense it. Still, “we’re asked about it plenty,” a hospice and palliative care specialist told colleagues, at the annual meeting of the Association of VA Hematology/Oncology (AVAHO).
That brings up a big question, said Michael Stellini, MD, MS, FACP, FAAHPM, of Wayne State University, Karmanos Cancer Center, and the John D. Dingell VA Medical Center, in Detroit Michigan: “Should sick people be smoking pot?”
Even the question itself isn’t a simple one to answer since smoking isn’t the only way to consume cannabis for medical purposes. And figuring out the best advice is difficult. As Dr. Stellini said, there’s plenty of uncertainty about crucial cannabis topics like safety and benefits.
Dr. Stellini offered a number of facts and tips about cannabis in medicine.
Understand ‘qualifying conditions’ in your state
In states with legal medical marijuana, he said, physicians do not prescribe marijuana. However, they may certify that patients are eligible to get the drug for medical purposes if they meet certain qualifications.
A typical list of qualifying conditions includes diseases such as cancer, glaucoma, HIV/AIDS and Crohn’s disease. Qualifying conditions also tend to include treatments for severe diseases that produce wasting syndrome, severe and chronic pain, severe nausea, seizures and severe and persistent muscle spasm.
In Michigan, where Dr. Stellini practices, a panel in 2018 approved a long list of added qualifying conditions such as chronic pain, obsessive compulsive disorder and arthritis. But the panel rejected other conditions such as anxiety, asthma, panic attacks and schizophrenia.
Vaporizers are an alternative to joints, but...
Vaporizers are commonly used as an alternative to smoking marijuana joints, Dr. Stellini said, and they don’t significantly release tars or much if any carbon monoxide. While research is limited, he said, use of vaporizers hasn’t been linked to more lung cancer or chronic obstructive pulmonary disease.
“Vaping” is another option, but it’s been linked to dozens of deaths and hundreds of cases of illness in recent weeks. Many patients have reported using products that contain THC, a component of marijuana.
Other delivery methods exist
Marijuana can be ingested in liquid and solid food. “But edibles can have a slow onset of action compared to vaporizing or smoking,” Dr. Stellini said. “You might over-indulge. When users get to their steady state, they might have some adverse effects [AEs].”
Marijuana still has risks
Cannabis use has a long list of well-known AEs linked to the THC component. The most common are drowsiness, fatigue, dizziness, dry mouth, anxiety, cognitive effects, cough, and nausea, Dr. Stellini said. More serious AEs such as psychosis have been reported.
And, of course, users of cannabis with THC get high if they use enough.
A 2017 National Academies of Sciences, Engineering and Medicine report linked cannabis use to a higher risk of motor vehicle accidents. Still, Dr. Stellini said, “it’s relatively safe with respect to mortality, especially compared to opioids.”1
Risk of use in cancer may be low
Research suggest that patients with cancer use cannabis as much as other people and perhaps even more, Dr. Stellini said. But are they facing any extra risks? In general, he said, it doesn’t appear that way.
Cannabis seems to be safe when used with chemotherapy, he said, and drug-drug interactions in cancer appear to be rare. Some studies have suggested that cannabinoids—a component of marijuana—may be an effective treatment for chemotherapy-induced peripheral neuropathy.
However, he said, 1 study has raised a red flag about a possible interaction with cancer immunotherapy. Researchers found evidence that patients who used cannabis had lower tumor response rates to nivolomab for advanced melanoma, non-small cell lung cancer, and renal clear cell carcinoma. However, survival wasn’t affected.2
Meanwhile, he said, there’s no strong evidence that cannabis is a useful treatment for cancer, he said, although it’s worth investigating.
Cannabidiol is the hot new product
Cannabidiol, also known as CBD, has become hugely popular, Dr. Stellini said. It is derived from hemp and doesn’t cause a “buzz” like cannabis.
Due to lack of regulation, he said, buyers should beware. And, he said, CBD has multiple EAs. Standard doses can cause drowsiness, fatigue, dizziness, dry mouth, hypotension and lightheadedness.
Dr. Stellini reports no relevant disclosures.
1. National Academies of Sciences, Engineering, and Medicine. The Health Effects of Cannabis and Cannabinoids: The Current State of Evidence and Recommendations for Research. Washington, DC: The National Academies Press; 2017.
2. Taha T, Meiri D, Talhamy S, Wollner M, Peer A, Bar-Sela G. Cannabis impacts tumor response rate to nivolumab in patients with advanced malignancies. Oncologist. 2019;24(4):549-554.
MINNEAPOLIS -- Medical professionals within the US Department of Veterans Affairs (VA) can’t prescribe cannabis or certify patients to be able to get it. VA pharmacists can’t dispense it. Still, “we’re asked about it plenty,” a hospice and palliative care specialist told colleagues, at the annual meeting of the Association of VA Hematology/Oncology (AVAHO).
That brings up a big question, said Michael Stellini, MD, MS, FACP, FAAHPM, of Wayne State University, Karmanos Cancer Center, and the John D. Dingell VA Medical Center, in Detroit Michigan: “Should sick people be smoking pot?”
Even the question itself isn’t a simple one to answer since smoking isn’t the only way to consume cannabis for medical purposes. And figuring out the best advice is difficult. As Dr. Stellini said, there’s plenty of uncertainty about crucial cannabis topics like safety and benefits.
Dr. Stellini offered a number of facts and tips about cannabis in medicine.
Understand ‘qualifying conditions’ in your state
In states with legal medical marijuana, he said, physicians do not prescribe marijuana. However, they may certify that patients are eligible to get the drug for medical purposes if they meet certain qualifications.
A typical list of qualifying conditions includes diseases such as cancer, glaucoma, HIV/AIDS and Crohn’s disease. Qualifying conditions also tend to include treatments for severe diseases that produce wasting syndrome, severe and chronic pain, severe nausea, seizures and severe and persistent muscle spasm.
In Michigan, where Dr. Stellini practices, a panel in 2018 approved a long list of added qualifying conditions such as chronic pain, obsessive compulsive disorder and arthritis. But the panel rejected other conditions such as anxiety, asthma, panic attacks and schizophrenia.
Vaporizers are an alternative to joints, but...
Vaporizers are commonly used as an alternative to smoking marijuana joints, Dr. Stellini said, and they don’t significantly release tars or much if any carbon monoxide. While research is limited, he said, use of vaporizers hasn’t been linked to more lung cancer or chronic obstructive pulmonary disease.
“Vaping” is another option, but it’s been linked to dozens of deaths and hundreds of cases of illness in recent weeks. Many patients have reported using products that contain THC, a component of marijuana.
Other delivery methods exist
Marijuana can be ingested in liquid and solid food. “But edibles can have a slow onset of action compared to vaporizing or smoking,” Dr. Stellini said. “You might over-indulge. When users get to their steady state, they might have some adverse effects [AEs].”
Marijuana still has risks
Cannabis use has a long list of well-known AEs linked to the THC component. The most common are drowsiness, fatigue, dizziness, dry mouth, anxiety, cognitive effects, cough, and nausea, Dr. Stellini said. More serious AEs such as psychosis have been reported.
And, of course, users of cannabis with THC get high if they use enough.
A 2017 National Academies of Sciences, Engineering and Medicine report linked cannabis use to a higher risk of motor vehicle accidents. Still, Dr. Stellini said, “it’s relatively safe with respect to mortality, especially compared to opioids.”1
Risk of use in cancer may be low
Research suggest that patients with cancer use cannabis as much as other people and perhaps even more, Dr. Stellini said. But are they facing any extra risks? In general, he said, it doesn’t appear that way.
Cannabis seems to be safe when used with chemotherapy, he said, and drug-drug interactions in cancer appear to be rare. Some studies have suggested that cannabinoids—a component of marijuana—may be an effective treatment for chemotherapy-induced peripheral neuropathy.
However, he said, 1 study has raised a red flag about a possible interaction with cancer immunotherapy. Researchers found evidence that patients who used cannabis had lower tumor response rates to nivolomab for advanced melanoma, non-small cell lung cancer, and renal clear cell carcinoma. However, survival wasn’t affected.2
Meanwhile, he said, there’s no strong evidence that cannabis is a useful treatment for cancer, he said, although it’s worth investigating.
Cannabidiol is the hot new product
Cannabidiol, also known as CBD, has become hugely popular, Dr. Stellini said. It is derived from hemp and doesn’t cause a “buzz” like cannabis.
Due to lack of regulation, he said, buyers should beware. And, he said, CBD has multiple EAs. Standard doses can cause drowsiness, fatigue, dizziness, dry mouth, hypotension and lightheadedness.
Dr. Stellini reports no relevant disclosures.
MINNEAPOLIS -- Medical professionals within the US Department of Veterans Affairs (VA) can’t prescribe cannabis or certify patients to be able to get it. VA pharmacists can’t dispense it. Still, “we’re asked about it plenty,” a hospice and palliative care specialist told colleagues, at the annual meeting of the Association of VA Hematology/Oncology (AVAHO).
That brings up a big question, said Michael Stellini, MD, MS, FACP, FAAHPM, of Wayne State University, Karmanos Cancer Center, and the John D. Dingell VA Medical Center, in Detroit Michigan: “Should sick people be smoking pot?”
Even the question itself isn’t a simple one to answer since smoking isn’t the only way to consume cannabis for medical purposes. And figuring out the best advice is difficult. As Dr. Stellini said, there’s plenty of uncertainty about crucial cannabis topics like safety and benefits.
Dr. Stellini offered a number of facts and tips about cannabis in medicine.
Understand ‘qualifying conditions’ in your state
In states with legal medical marijuana, he said, physicians do not prescribe marijuana. However, they may certify that patients are eligible to get the drug for medical purposes if they meet certain qualifications.
A typical list of qualifying conditions includes diseases such as cancer, glaucoma, HIV/AIDS and Crohn’s disease. Qualifying conditions also tend to include treatments for severe diseases that produce wasting syndrome, severe and chronic pain, severe nausea, seizures and severe and persistent muscle spasm.
In Michigan, where Dr. Stellini practices, a panel in 2018 approved a long list of added qualifying conditions such as chronic pain, obsessive compulsive disorder and arthritis. But the panel rejected other conditions such as anxiety, asthma, panic attacks and schizophrenia.
Vaporizers are an alternative to joints, but...
Vaporizers are commonly used as an alternative to smoking marijuana joints, Dr. Stellini said, and they don’t significantly release tars or much if any carbon monoxide. While research is limited, he said, use of vaporizers hasn’t been linked to more lung cancer or chronic obstructive pulmonary disease.
“Vaping” is another option, but it’s been linked to dozens of deaths and hundreds of cases of illness in recent weeks. Many patients have reported using products that contain THC, a component of marijuana.
Other delivery methods exist
Marijuana can be ingested in liquid and solid food. “But edibles can have a slow onset of action compared to vaporizing or smoking,” Dr. Stellini said. “You might over-indulge. When users get to their steady state, they might have some adverse effects [AEs].”
Marijuana still has risks
Cannabis use has a long list of well-known AEs linked to the THC component. The most common are drowsiness, fatigue, dizziness, dry mouth, anxiety, cognitive effects, cough, and nausea, Dr. Stellini said. More serious AEs such as psychosis have been reported.
And, of course, users of cannabis with THC get high if they use enough.
A 2017 National Academies of Sciences, Engineering and Medicine report linked cannabis use to a higher risk of motor vehicle accidents. Still, Dr. Stellini said, “it’s relatively safe with respect to mortality, especially compared to opioids.”1
Risk of use in cancer may be low
Research suggest that patients with cancer use cannabis as much as other people and perhaps even more, Dr. Stellini said. But are they facing any extra risks? In general, he said, it doesn’t appear that way.
Cannabis seems to be safe when used with chemotherapy, he said, and drug-drug interactions in cancer appear to be rare. Some studies have suggested that cannabinoids—a component of marijuana—may be an effective treatment for chemotherapy-induced peripheral neuropathy.
However, he said, 1 study has raised a red flag about a possible interaction with cancer immunotherapy. Researchers found evidence that patients who used cannabis had lower tumor response rates to nivolomab for advanced melanoma, non-small cell lung cancer, and renal clear cell carcinoma. However, survival wasn’t affected.2
Meanwhile, he said, there’s no strong evidence that cannabis is a useful treatment for cancer, he said, although it’s worth investigating.
Cannabidiol is the hot new product
Cannabidiol, also known as CBD, has become hugely popular, Dr. Stellini said. It is derived from hemp and doesn’t cause a “buzz” like cannabis.
Due to lack of regulation, he said, buyers should beware. And, he said, CBD has multiple EAs. Standard doses can cause drowsiness, fatigue, dizziness, dry mouth, hypotension and lightheadedness.
Dr. Stellini reports no relevant disclosures.
1. National Academies of Sciences, Engineering, and Medicine. The Health Effects of Cannabis and Cannabinoids: The Current State of Evidence and Recommendations for Research. Washington, DC: The National Academies Press; 2017.
2. Taha T, Meiri D, Talhamy S, Wollner M, Peer A, Bar-Sela G. Cannabis impacts tumor response rate to nivolumab in patients with advanced malignancies. Oncologist. 2019;24(4):549-554.
1. National Academies of Sciences, Engineering, and Medicine. The Health Effects of Cannabis and Cannabinoids: The Current State of Evidence and Recommendations for Research. Washington, DC: The National Academies Press; 2017.
2. Taha T, Meiri D, Talhamy S, Wollner M, Peer A, Bar-Sela G. Cannabis impacts tumor response rate to nivolumab in patients with advanced malignancies. Oncologist. 2019;24(4):549-554.
Interventions significantly improve NICU immunization rates
according to a study in Pediatrics.
Investigators led by Raymond C. Stetson, MD, of the Mayo Clinic in Rochester, Minn., identified three root causes of underimmunization in a NICU at Mayo Clinic: providers’ lack of knowledge about recommended immunization schedules; immunizations not being ordered when they were due; and parental hesitancy toward vaccination. They addressed these causes with the following five phases of intervention: an intranet resource educating providers about vaccine schedules and dosing intervals; a spreadsheet-based checklist to track and flag immunization status; an intranet resource aimed at discussion with vaccine-hesitant parents; education about safety in providing immunization and review of material from the first three interventions; and education about documentation, including parental consent.
Over the project period, 1,242 infants were discharged or transferred from the NICU. The study included a 6-month “improve phase,” during which interventions were implemented, and a “control phase,” during which the ongoing effects after implementation were observed. At baseline, the rate of fully immunized infants in the NICU was only 56% by time of discharge or transfer, but during the combined improve and control phases, it was 93% with a P value of less than .001.
One of the limitations of the study is that the first three interventions were introduced simultaneously, which makes it hard to determine how much effect each might have had.
“Infants treated in NICUs represent a vulnerable population with the potential for high morbidity and mortality from vaccine-preventable infections,” the investigators wrote. “Our [quality improvement] effort, and others, demonstrate that this population is at risk for underimmunization and that immunization rates can be improved with a small number of interventions. Additionally, we were able to significantly decrease the number of days that immunizations were delayed compared to the routine infant vaccination schedule.”
There was no external funding for the study. One of the coauthors is on safety committees of vaccine studies for Merck. The other authors have no relevant financial disclosures.
SOURCE: Stetson R et al. Pediatr. 2019. doi: 10.1542/peds.2019-0337.
according to a study in Pediatrics.
Investigators led by Raymond C. Stetson, MD, of the Mayo Clinic in Rochester, Minn., identified three root causes of underimmunization in a NICU at Mayo Clinic: providers’ lack of knowledge about recommended immunization schedules; immunizations not being ordered when they were due; and parental hesitancy toward vaccination. They addressed these causes with the following five phases of intervention: an intranet resource educating providers about vaccine schedules and dosing intervals; a spreadsheet-based checklist to track and flag immunization status; an intranet resource aimed at discussion with vaccine-hesitant parents; education about safety in providing immunization and review of material from the first three interventions; and education about documentation, including parental consent.
Over the project period, 1,242 infants were discharged or transferred from the NICU. The study included a 6-month “improve phase,” during which interventions were implemented, and a “control phase,” during which the ongoing effects after implementation were observed. At baseline, the rate of fully immunized infants in the NICU was only 56% by time of discharge or transfer, but during the combined improve and control phases, it was 93% with a P value of less than .001.
One of the limitations of the study is that the first three interventions were introduced simultaneously, which makes it hard to determine how much effect each might have had.
“Infants treated in NICUs represent a vulnerable population with the potential for high morbidity and mortality from vaccine-preventable infections,” the investigators wrote. “Our [quality improvement] effort, and others, demonstrate that this population is at risk for underimmunization and that immunization rates can be improved with a small number of interventions. Additionally, we were able to significantly decrease the number of days that immunizations were delayed compared to the routine infant vaccination schedule.”
There was no external funding for the study. One of the coauthors is on safety committees of vaccine studies for Merck. The other authors have no relevant financial disclosures.
SOURCE: Stetson R et al. Pediatr. 2019. doi: 10.1542/peds.2019-0337.
according to a study in Pediatrics.
Investigators led by Raymond C. Stetson, MD, of the Mayo Clinic in Rochester, Minn., identified three root causes of underimmunization in a NICU at Mayo Clinic: providers’ lack of knowledge about recommended immunization schedules; immunizations not being ordered when they were due; and parental hesitancy toward vaccination. They addressed these causes with the following five phases of intervention: an intranet resource educating providers about vaccine schedules and dosing intervals; a spreadsheet-based checklist to track and flag immunization status; an intranet resource aimed at discussion with vaccine-hesitant parents; education about safety in providing immunization and review of material from the first three interventions; and education about documentation, including parental consent.
Over the project period, 1,242 infants were discharged or transferred from the NICU. The study included a 6-month “improve phase,” during which interventions were implemented, and a “control phase,” during which the ongoing effects after implementation were observed. At baseline, the rate of fully immunized infants in the NICU was only 56% by time of discharge or transfer, but during the combined improve and control phases, it was 93% with a P value of less than .001.
One of the limitations of the study is that the first three interventions were introduced simultaneously, which makes it hard to determine how much effect each might have had.
“Infants treated in NICUs represent a vulnerable population with the potential for high morbidity and mortality from vaccine-preventable infections,” the investigators wrote. “Our [quality improvement] effort, and others, demonstrate that this population is at risk for underimmunization and that immunization rates can be improved with a small number of interventions. Additionally, we were able to significantly decrease the number of days that immunizations were delayed compared to the routine infant vaccination schedule.”
There was no external funding for the study. One of the coauthors is on safety committees of vaccine studies for Merck. The other authors have no relevant financial disclosures.
SOURCE: Stetson R et al. Pediatr. 2019. doi: 10.1542/peds.2019-0337.
FROM PEDIATRICS
Judge rules for insurer in doctor’s allocation lawsuit
A judge has sided with a medical malpractice insurer in a legal challenge that accused the company of misallocating blame among physicians after a liability settlement.
In a Sept. 27 decision, Judge Debra Squires-Lee of the Commonwealth of Massachusetts Superior Court ruled that Medical Professional Mutual Insurance Company (ProMutual) acted reasonably when it settled a medical liability claim for $500,000 against several health providers and allocated responsibility for 30% of the settlement ($150,000) to internist Nataly Minkina, MD. ProMutual was well within its rights and obligations when it settled the underlying claim and did not act in bad faith when assigning responsibility in the case, Judge Squires-Lee wrote in her 49-page ruling.
“At its heart, this case is about a multiple defendant malpractice lawsuit with finger pointing by Dr. Minkina against her codefendants and others, and a disagreement about ProMutual’s ultimate determination about how to allocate a global settlement with the plaintiffs amongst ProMutual’s insureds,” Judge Squires-Lee wrote in the decision. “Dr. Minkina strongly believes that she did not fail [the patient], that she acted reasonably, and that her treatment of [the patient] satisfied the standard of care. She also questions why ProMutual failed to allocate liability in the [patient’s] suit to other physicians. However ... the question for this court is whether ProMutual committed unfair or deceptive acts or practices in its settlement and allocation of its settlement. I conclude that ProMutual did not.”
The case stems from a patient’s lawsuit against Dr. Minkina and several others at Blue Hills Medical Associates in Braintree, Mass. The patient alleged that the health care professionals were responsible for a missed breast cancer diagnosis. Dr. Minkina saw the patient just once in 2002 while covering for another doctor. During the visit, she confirmed some nodularity in the 55-year-old women’s breast and referred her for a mammogram and an ultrasound. A radiologist twice reported no abnormalities, which Dr. Minkina said she relayed to the patient. Dr. Minkina left the practice shortly after.
The patient visited the practice several more times and was referred for another mammogram in 2006, the results of which revealed some signs of malignancy, according to court documents. However, a nurse at the practice misread, misunderstood, or overlooked the signs and recorded that “the benign breast condition had no changes,” according to court transcripts. Later that year, the patient visited the practice complaining of headaches and a droopy eye at which time her primary care physician diagnosed sinusitis and prescribed antibiotics. In 2007, the patient underwent magnetic resonance imaging of the brain and the breast, which revealed widespread metastatic carcinoma. She and her family sued Dr. Minkina and several others in June 2007. The patient died in 2008.
ProMutual settled the case against the defendants for $500,000 in 2008, allocating 30% of the liability to Dr. Minkina, 10% of the nurse practitioner, 60% to the medical practice, and no liability to the other doctors named. ProMutual contended Dr. Minkina bore more responsibility than the other health care professionals named for the delayed diagnosis because of causation factors and standard of care violations, namely that Dr. Minkina should have pursued a biopsy for the patient.
Dr. Minkina sued the insurer in 2012, claiming chiefly that the insurer allocated an unjustifiably high percentage of liability to her because she was no longer insured and because the company had an economic incentive to allocate a disproportionate percentage of responsibility and damages.
A lower court initially dismissed Dr. Minkina’s suit, but the Commonwealth of Massachusetts Appeals Court in 2015 overturned that decision, ruling the case could move forward. In 2018, the superior court agreed Dr. Minkina had a valid bad faith claim, stating that she had provided information about ProMutual’s conduct from which “a reasonable juror could infer the defendant’s bad faith in connection with its settling the underlying malpractice suit, including the allocation of liability.
But Judge Squires-Lee ruled that trial evidence showed that ProMutual did not act for its own benefit or favor other insureds over Dr. Minkina. The judge wrote that the insurer satisfied its contractual and legal obligations when defending the underlying legal claim.
Dr. Minkina said she was disappointed with the ruling, but that she is considering her legal avenues.
“[The] judge’s decision in my case against ProMutual is obviously disappointing, but it is not the first time [the] trial court decided against me in this case,” Dr. Minkina said in an interview. “It is a battle between David and Goliath, [a] single physician against [a] $3.6 billion insurance company with unlimited resources. The decision has just been announced and it is voluminous. I am still reading it and evaluating my options with my attorneys, but it is not the end of the road yet.”
ProMutual declined to comment about the decision.
A judge has sided with a medical malpractice insurer in a legal challenge that accused the company of misallocating blame among physicians after a liability settlement.
In a Sept. 27 decision, Judge Debra Squires-Lee of the Commonwealth of Massachusetts Superior Court ruled that Medical Professional Mutual Insurance Company (ProMutual) acted reasonably when it settled a medical liability claim for $500,000 against several health providers and allocated responsibility for 30% of the settlement ($150,000) to internist Nataly Minkina, MD. ProMutual was well within its rights and obligations when it settled the underlying claim and did not act in bad faith when assigning responsibility in the case, Judge Squires-Lee wrote in her 49-page ruling.
“At its heart, this case is about a multiple defendant malpractice lawsuit with finger pointing by Dr. Minkina against her codefendants and others, and a disagreement about ProMutual’s ultimate determination about how to allocate a global settlement with the plaintiffs amongst ProMutual’s insureds,” Judge Squires-Lee wrote in the decision. “Dr. Minkina strongly believes that she did not fail [the patient], that she acted reasonably, and that her treatment of [the patient] satisfied the standard of care. She also questions why ProMutual failed to allocate liability in the [patient’s] suit to other physicians. However ... the question for this court is whether ProMutual committed unfair or deceptive acts or practices in its settlement and allocation of its settlement. I conclude that ProMutual did not.”
The case stems from a patient’s lawsuit against Dr. Minkina and several others at Blue Hills Medical Associates in Braintree, Mass. The patient alleged that the health care professionals were responsible for a missed breast cancer diagnosis. Dr. Minkina saw the patient just once in 2002 while covering for another doctor. During the visit, she confirmed some nodularity in the 55-year-old women’s breast and referred her for a mammogram and an ultrasound. A radiologist twice reported no abnormalities, which Dr. Minkina said she relayed to the patient. Dr. Minkina left the practice shortly after.
The patient visited the practice several more times and was referred for another mammogram in 2006, the results of which revealed some signs of malignancy, according to court documents. However, a nurse at the practice misread, misunderstood, or overlooked the signs and recorded that “the benign breast condition had no changes,” according to court transcripts. Later that year, the patient visited the practice complaining of headaches and a droopy eye at which time her primary care physician diagnosed sinusitis and prescribed antibiotics. In 2007, the patient underwent magnetic resonance imaging of the brain and the breast, which revealed widespread metastatic carcinoma. She and her family sued Dr. Minkina and several others in June 2007. The patient died in 2008.
ProMutual settled the case against the defendants for $500,000 in 2008, allocating 30% of the liability to Dr. Minkina, 10% of the nurse practitioner, 60% to the medical practice, and no liability to the other doctors named. ProMutual contended Dr. Minkina bore more responsibility than the other health care professionals named for the delayed diagnosis because of causation factors and standard of care violations, namely that Dr. Minkina should have pursued a biopsy for the patient.
Dr. Minkina sued the insurer in 2012, claiming chiefly that the insurer allocated an unjustifiably high percentage of liability to her because she was no longer insured and because the company had an economic incentive to allocate a disproportionate percentage of responsibility and damages.
A lower court initially dismissed Dr. Minkina’s suit, but the Commonwealth of Massachusetts Appeals Court in 2015 overturned that decision, ruling the case could move forward. In 2018, the superior court agreed Dr. Minkina had a valid bad faith claim, stating that she had provided information about ProMutual’s conduct from which “a reasonable juror could infer the defendant’s bad faith in connection with its settling the underlying malpractice suit, including the allocation of liability.
But Judge Squires-Lee ruled that trial evidence showed that ProMutual did not act for its own benefit or favor other insureds over Dr. Minkina. The judge wrote that the insurer satisfied its contractual and legal obligations when defending the underlying legal claim.
Dr. Minkina said she was disappointed with the ruling, but that she is considering her legal avenues.
“[The] judge’s decision in my case against ProMutual is obviously disappointing, but it is not the first time [the] trial court decided against me in this case,” Dr. Minkina said in an interview. “It is a battle between David and Goliath, [a] single physician against [a] $3.6 billion insurance company with unlimited resources. The decision has just been announced and it is voluminous. I am still reading it and evaluating my options with my attorneys, but it is not the end of the road yet.”
ProMutual declined to comment about the decision.
A judge has sided with a medical malpractice insurer in a legal challenge that accused the company of misallocating blame among physicians after a liability settlement.
In a Sept. 27 decision, Judge Debra Squires-Lee of the Commonwealth of Massachusetts Superior Court ruled that Medical Professional Mutual Insurance Company (ProMutual) acted reasonably when it settled a medical liability claim for $500,000 against several health providers and allocated responsibility for 30% of the settlement ($150,000) to internist Nataly Minkina, MD. ProMutual was well within its rights and obligations when it settled the underlying claim and did not act in bad faith when assigning responsibility in the case, Judge Squires-Lee wrote in her 49-page ruling.
“At its heart, this case is about a multiple defendant malpractice lawsuit with finger pointing by Dr. Minkina against her codefendants and others, and a disagreement about ProMutual’s ultimate determination about how to allocate a global settlement with the plaintiffs amongst ProMutual’s insureds,” Judge Squires-Lee wrote in the decision. “Dr. Minkina strongly believes that she did not fail [the patient], that she acted reasonably, and that her treatment of [the patient] satisfied the standard of care. She also questions why ProMutual failed to allocate liability in the [patient’s] suit to other physicians. However ... the question for this court is whether ProMutual committed unfair or deceptive acts or practices in its settlement and allocation of its settlement. I conclude that ProMutual did not.”
The case stems from a patient’s lawsuit against Dr. Minkina and several others at Blue Hills Medical Associates in Braintree, Mass. The patient alleged that the health care professionals were responsible for a missed breast cancer diagnosis. Dr. Minkina saw the patient just once in 2002 while covering for another doctor. During the visit, she confirmed some nodularity in the 55-year-old women’s breast and referred her for a mammogram and an ultrasound. A radiologist twice reported no abnormalities, which Dr. Minkina said she relayed to the patient. Dr. Minkina left the practice shortly after.
The patient visited the practice several more times and was referred for another mammogram in 2006, the results of which revealed some signs of malignancy, according to court documents. However, a nurse at the practice misread, misunderstood, or overlooked the signs and recorded that “the benign breast condition had no changes,” according to court transcripts. Later that year, the patient visited the practice complaining of headaches and a droopy eye at which time her primary care physician diagnosed sinusitis and prescribed antibiotics. In 2007, the patient underwent magnetic resonance imaging of the brain and the breast, which revealed widespread metastatic carcinoma. She and her family sued Dr. Minkina and several others in June 2007. The patient died in 2008.
ProMutual settled the case against the defendants for $500,000 in 2008, allocating 30% of the liability to Dr. Minkina, 10% of the nurse practitioner, 60% to the medical practice, and no liability to the other doctors named. ProMutual contended Dr. Minkina bore more responsibility than the other health care professionals named for the delayed diagnosis because of causation factors and standard of care violations, namely that Dr. Minkina should have pursued a biopsy for the patient.
Dr. Minkina sued the insurer in 2012, claiming chiefly that the insurer allocated an unjustifiably high percentage of liability to her because she was no longer insured and because the company had an economic incentive to allocate a disproportionate percentage of responsibility and damages.
A lower court initially dismissed Dr. Minkina’s suit, but the Commonwealth of Massachusetts Appeals Court in 2015 overturned that decision, ruling the case could move forward. In 2018, the superior court agreed Dr. Minkina had a valid bad faith claim, stating that she had provided information about ProMutual’s conduct from which “a reasonable juror could infer the defendant’s bad faith in connection with its settling the underlying malpractice suit, including the allocation of liability.
But Judge Squires-Lee ruled that trial evidence showed that ProMutual did not act for its own benefit or favor other insureds over Dr. Minkina. The judge wrote that the insurer satisfied its contractual and legal obligations when defending the underlying legal claim.
Dr. Minkina said she was disappointed with the ruling, but that she is considering her legal avenues.
“[The] judge’s decision in my case against ProMutual is obviously disappointing, but it is not the first time [the] trial court decided against me in this case,” Dr. Minkina said in an interview. “It is a battle between David and Goliath, [a] single physician against [a] $3.6 billion insurance company with unlimited resources. The decision has just been announced and it is voluminous. I am still reading it and evaluating my options with my attorneys, but it is not the end of the road yet.”
ProMutual declined to comment about the decision.
White AAV patients post highest mortality rates
Age-adjusted mortality from antineutrophil cytoplasmic autoantibody–associated vasculitides (AAV) in the United States declined by nearly 2% each year between 1999 and 2017, based on data from the Centers for Disease Control and Prevention.
Significant morbidity and mortality are associated with untreated AAV, wrote Alexander W. Steinberg, MD, of Saint Joseph Hospital, Denver, Colo., and colleagues.
“Although population data from the United Kingdom have shown decreased AAV-related mortality during the past 20 years, it is unknown whether this pattern has occurred in the United States,” they wrote.
In a study published in Annals of Internal Medicine, the researchers identified 11,316 AAV-related deaths from 1999 to 2017 in the CDC data.
Overall, age-adjusted mortality was 1.86 per 1,000,000 persons, with highest rates among non-Hispanic whites, men, and residents of the Midwest. Mortality from AAV declined by an average of 1.6% in each year of the study period, and changes in subgroups stratified by gender, race, and geographic region were similar.
Mortality increased with age and was highest among individuals aged 75-84 years, but a significant decline in mortality occurred among individuals aged 65-74 years. “The decrease in overall mortality and mortality among persons aged 65 to 74 years may reflect increased longevity due to improved treatment of AAV and common comorbid conditions,” the researchers said.
“Surprisingly, the authors found much lower age-adjusted mortality rates for non-Hispanic black persons (0.77) and moderately lower mortality rates for Hispanic persons (1.57) than for non-Hispanic white persons (2.03),” wrote John R. Stone, MD, PhD, of Creighton University, Omaha, Neb., in an accompanying editorial.
“Suppose the mortality rate differences reported by Steinberg and colleagues are statistically significant, accurately represent death certificate diagnoses, and match people’s racial/ethnic self-identification. The data then show neither that the vasculitides actually have lower mortality rates in blacks or Hispanics compared with whites, nor that the diseases are indeed less frequent in blacks and Hispanics,” he said. “Rather, these differences probably signify how social inequities, social structural violence, and inferior health care access adversely influence diagnosis of rare diseases and promote health inequity,” Dr. Stone added. The findings suggest that clinicians should remain alert to AAV in some ethnic groups to improve diagnostic accuracy, he said.
“Moreover, improved AAV diagnosis in such groups is key to recruiting participants for research investigating whether therapies should differ among populations,” he emphasized.
The study findings were limited by possible under- or overreporting of AAV on death certificates, but they were strengthened by the large sample size, the researchers noted. “We hope that the mortality patterns presented here can be used to direct future research on the driving forces behind these trends,” they said.
Dr. Steinberg had no financial conflicts to disclose. Dr. Stone had no financial conflicts to disclose.
SOURCES: Steinberg AW et al. Ann Intern Med. 2019 Oct 8. doi: 10.7326/M19-1564; and Stone JR. Ann Intern Med. 2019 Oct 8. doi: 10.7326/M19-2755.
Age-adjusted mortality from antineutrophil cytoplasmic autoantibody–associated vasculitides (AAV) in the United States declined by nearly 2% each year between 1999 and 2017, based on data from the Centers for Disease Control and Prevention.
Significant morbidity and mortality are associated with untreated AAV, wrote Alexander W. Steinberg, MD, of Saint Joseph Hospital, Denver, Colo., and colleagues.
“Although population data from the United Kingdom have shown decreased AAV-related mortality during the past 20 years, it is unknown whether this pattern has occurred in the United States,” they wrote.
In a study published in Annals of Internal Medicine, the researchers identified 11,316 AAV-related deaths from 1999 to 2017 in the CDC data.
Overall, age-adjusted mortality was 1.86 per 1,000,000 persons, with highest rates among non-Hispanic whites, men, and residents of the Midwest. Mortality from AAV declined by an average of 1.6% in each year of the study period, and changes in subgroups stratified by gender, race, and geographic region were similar.
Mortality increased with age and was highest among individuals aged 75-84 years, but a significant decline in mortality occurred among individuals aged 65-74 years. “The decrease in overall mortality and mortality among persons aged 65 to 74 years may reflect increased longevity due to improved treatment of AAV and common comorbid conditions,” the researchers said.
“Surprisingly, the authors found much lower age-adjusted mortality rates for non-Hispanic black persons (0.77) and moderately lower mortality rates for Hispanic persons (1.57) than for non-Hispanic white persons (2.03),” wrote John R. Stone, MD, PhD, of Creighton University, Omaha, Neb., in an accompanying editorial.
“Suppose the mortality rate differences reported by Steinberg and colleagues are statistically significant, accurately represent death certificate diagnoses, and match people’s racial/ethnic self-identification. The data then show neither that the vasculitides actually have lower mortality rates in blacks or Hispanics compared with whites, nor that the diseases are indeed less frequent in blacks and Hispanics,” he said. “Rather, these differences probably signify how social inequities, social structural violence, and inferior health care access adversely influence diagnosis of rare diseases and promote health inequity,” Dr. Stone added. The findings suggest that clinicians should remain alert to AAV in some ethnic groups to improve diagnostic accuracy, he said.
“Moreover, improved AAV diagnosis in such groups is key to recruiting participants for research investigating whether therapies should differ among populations,” he emphasized.
The study findings were limited by possible under- or overreporting of AAV on death certificates, but they were strengthened by the large sample size, the researchers noted. “We hope that the mortality patterns presented here can be used to direct future research on the driving forces behind these trends,” they said.
Dr. Steinberg had no financial conflicts to disclose. Dr. Stone had no financial conflicts to disclose.
SOURCES: Steinberg AW et al. Ann Intern Med. 2019 Oct 8. doi: 10.7326/M19-1564; and Stone JR. Ann Intern Med. 2019 Oct 8. doi: 10.7326/M19-2755.
Age-adjusted mortality from antineutrophil cytoplasmic autoantibody–associated vasculitides (AAV) in the United States declined by nearly 2% each year between 1999 and 2017, based on data from the Centers for Disease Control and Prevention.
Significant morbidity and mortality are associated with untreated AAV, wrote Alexander W. Steinberg, MD, of Saint Joseph Hospital, Denver, Colo., and colleagues.
“Although population data from the United Kingdom have shown decreased AAV-related mortality during the past 20 years, it is unknown whether this pattern has occurred in the United States,” they wrote.
In a study published in Annals of Internal Medicine, the researchers identified 11,316 AAV-related deaths from 1999 to 2017 in the CDC data.
Overall, age-adjusted mortality was 1.86 per 1,000,000 persons, with highest rates among non-Hispanic whites, men, and residents of the Midwest. Mortality from AAV declined by an average of 1.6% in each year of the study period, and changes in subgroups stratified by gender, race, and geographic region were similar.
Mortality increased with age and was highest among individuals aged 75-84 years, but a significant decline in mortality occurred among individuals aged 65-74 years. “The decrease in overall mortality and mortality among persons aged 65 to 74 years may reflect increased longevity due to improved treatment of AAV and common comorbid conditions,” the researchers said.
“Surprisingly, the authors found much lower age-adjusted mortality rates for non-Hispanic black persons (0.77) and moderately lower mortality rates for Hispanic persons (1.57) than for non-Hispanic white persons (2.03),” wrote John R. Stone, MD, PhD, of Creighton University, Omaha, Neb., in an accompanying editorial.
“Suppose the mortality rate differences reported by Steinberg and colleagues are statistically significant, accurately represent death certificate diagnoses, and match people’s racial/ethnic self-identification. The data then show neither that the vasculitides actually have lower mortality rates in blacks or Hispanics compared with whites, nor that the diseases are indeed less frequent in blacks and Hispanics,” he said. “Rather, these differences probably signify how social inequities, social structural violence, and inferior health care access adversely influence diagnosis of rare diseases and promote health inequity,” Dr. Stone added. The findings suggest that clinicians should remain alert to AAV in some ethnic groups to improve diagnostic accuracy, he said.
“Moreover, improved AAV diagnosis in such groups is key to recruiting participants for research investigating whether therapies should differ among populations,” he emphasized.
The study findings were limited by possible under- or overreporting of AAV on death certificates, but they were strengthened by the large sample size, the researchers noted. “We hope that the mortality patterns presented here can be used to direct future research on the driving forces behind these trends,” they said.
Dr. Steinberg had no financial conflicts to disclose. Dr. Stone had no financial conflicts to disclose.
SOURCES: Steinberg AW et al. Ann Intern Med. 2019 Oct 8. doi: 10.7326/M19-1564; and Stone JR. Ann Intern Med. 2019 Oct 8. doi: 10.7326/M19-2755.
FROM ANNALS OF INTERNAL MEDICINE
Key clinical point: The mortality rate from antineutrophil cytoplasmic autoantibody–associated vasculitides (AAV) in the United States was 1.86 per 1,000,000 individuals from 1999 to 2017.
Major finding: Age-adjusted mortality from AAV decreased by approximately 2% each year between 1999 and 2017.
Study details: The data come from a review of 11,316 AAV-related deaths.
Disclosures: Dr. Steinberg had no financial conflicts to disclose.
Source: Steinberg AW et al. Ann Intern Med. 2019 Oct 8. doi: 10.7326/M19-1564.
Clinician burnout can impact quality of care
Burnout among health care professionals has been associated with lower quality of care, but the effect may be smaller than it seems, based on data from a meta-analysis of more than 200,000 clinicians.
Previous studies have reported associations between burnout and lower quality of care, but a standardized approach to analyze bias in the studies is lacking, wrote Daniel S. Tawfik, MD, of Stanford (Calif.) University and colleagues.
In a study published in the Annals of Internal Medicine, the researchers identified 123 publications from 1994 to 2019 with 142 study populations that included 241,553 health care providers.
Emotional exhaustion was the primary predictor for lower quality of care in 75 study populations, and overall burnout and depersonalization were the primary predictors for 56 and 11 study populations, respectively.
In an analysis of 114 unique burnout-quality combinations, 58 showed effects of burnout related to poor-quality care, 6 showed burnout related to high-quality care, and 50 showed no significant effect. Approximately one-third (33%) of the burnout-quality combinations were reported at least three times. In a review of the 46 burnout-quality combinations with primary effect sizes, 24 showed a significant effect of burnout on poor quality of care, 1 showed a significant effect of burnout on high quality of care, and 21 showed no significant effect.
The researchers also tested study bias using the Ioannidis test and found “an excess of observed versus predicted statistically significant studies (73% observed vs. 62%).”
The findings were limited by several factors, including the use of many cross-sectional, observational studies that could not show causality, the researchers noted. However, the results suggest several implications for future research including the need to consider exaggerated effects and reduce bias.
“Although the effect sizes in the published literature are modestly strong, our finding of excess significance implies that the true magnitude may be smaller than reported, and the studies that attempted to lower the risk of bias demonstrate fewer significant associations than the full evidence base,” the researchers noted.
“Whether curtailing burnout improves quality of care, or whether improving quality of care reduces burnout, is not yet known, and adequately powered and designed randomized trials will be indispensable in answering these questions,” they concluded.
The study was supported by the Stanford Maternal and Child Health Research Institute. Dr. Tawfik disclosed grants from Stanford Maternal and Child Health Research Institute during the study period.
SOURCE: Tawfik DS et al. Ann Intern Med. 2019 Oct 8. doi: 10.7326/M19-1152.
The current meta-analysis is consistent with previous research, but offers nothing new on the relationship between clinician burnout and quality of care, wrote Carolyn S. Dewa, MPH, PhD, Karen Nieuwenhuijsen, PhD, and Jeffrey S. Hoch, PhD, in an accompanying editorial.
Some of the concerns they expressed included variability in the methods used to measure provider burnout, as well as variability in measuring and defining medical error. They suggested that the researchers could have conducted a subgroup analysis based on error definition. “Such analyses might shed light on the types of errors associated with burnout and suggest directions for the design of robust psychometric studies about the error metrics,” they wrote.
The editorialists also expressed concerns about the heterogeneity of the studies included in the review and the potential for confounding. Finally, they noted that the use of observational studies in a meta-analysis can be challenging because “the assessment of observational studies is not straightforward.” They added that knowing the limitations of the studies is important in allowing readers to be confident in the estimates from any meta-analyses.
“Considering the limitations of the available literature, prior reviews, and Tawfik and colleagues’ current meta-analysis, we conclude that higher burnout is associated with lower quality, but we are left without clear answers about the magnitude or clinical significance of the relationship,” they wrote (Ann Intern Med. 2019 Oct 8. doi: 10.7326/M19-2760).
Dr. Dewa and Dr. Hoch are affiliated with the University of California, Davis. Dr. Nieuwenhuijsen is affiliated with the University of Amsterdam. The editorialists had no financial conflicts to disclose.
The current meta-analysis is consistent with previous research, but offers nothing new on the relationship between clinician burnout and quality of care, wrote Carolyn S. Dewa, MPH, PhD, Karen Nieuwenhuijsen, PhD, and Jeffrey S. Hoch, PhD, in an accompanying editorial.
Some of the concerns they expressed included variability in the methods used to measure provider burnout, as well as variability in measuring and defining medical error. They suggested that the researchers could have conducted a subgroup analysis based on error definition. “Such analyses might shed light on the types of errors associated with burnout and suggest directions for the design of robust psychometric studies about the error metrics,” they wrote.
The editorialists also expressed concerns about the heterogeneity of the studies included in the review and the potential for confounding. Finally, they noted that the use of observational studies in a meta-analysis can be challenging because “the assessment of observational studies is not straightforward.” They added that knowing the limitations of the studies is important in allowing readers to be confident in the estimates from any meta-analyses.
“Considering the limitations of the available literature, prior reviews, and Tawfik and colleagues’ current meta-analysis, we conclude that higher burnout is associated with lower quality, but we are left without clear answers about the magnitude or clinical significance of the relationship,” they wrote (Ann Intern Med. 2019 Oct 8. doi: 10.7326/M19-2760).
Dr. Dewa and Dr. Hoch are affiliated with the University of California, Davis. Dr. Nieuwenhuijsen is affiliated with the University of Amsterdam. The editorialists had no financial conflicts to disclose.
The current meta-analysis is consistent with previous research, but offers nothing new on the relationship between clinician burnout and quality of care, wrote Carolyn S. Dewa, MPH, PhD, Karen Nieuwenhuijsen, PhD, and Jeffrey S. Hoch, PhD, in an accompanying editorial.
Some of the concerns they expressed included variability in the methods used to measure provider burnout, as well as variability in measuring and defining medical error. They suggested that the researchers could have conducted a subgroup analysis based on error definition. “Such analyses might shed light on the types of errors associated with burnout and suggest directions for the design of robust psychometric studies about the error metrics,” they wrote.
The editorialists also expressed concerns about the heterogeneity of the studies included in the review and the potential for confounding. Finally, they noted that the use of observational studies in a meta-analysis can be challenging because “the assessment of observational studies is not straightforward.” They added that knowing the limitations of the studies is important in allowing readers to be confident in the estimates from any meta-analyses.
“Considering the limitations of the available literature, prior reviews, and Tawfik and colleagues’ current meta-analysis, we conclude that higher burnout is associated with lower quality, but we are left without clear answers about the magnitude or clinical significance of the relationship,” they wrote (Ann Intern Med. 2019 Oct 8. doi: 10.7326/M19-2760).
Dr. Dewa and Dr. Hoch are affiliated with the University of California, Davis. Dr. Nieuwenhuijsen is affiliated with the University of Amsterdam. The editorialists had no financial conflicts to disclose.
Burnout among health care professionals has been associated with lower quality of care, but the effect may be smaller than it seems, based on data from a meta-analysis of more than 200,000 clinicians.
Previous studies have reported associations between burnout and lower quality of care, but a standardized approach to analyze bias in the studies is lacking, wrote Daniel S. Tawfik, MD, of Stanford (Calif.) University and colleagues.
In a study published in the Annals of Internal Medicine, the researchers identified 123 publications from 1994 to 2019 with 142 study populations that included 241,553 health care providers.
Emotional exhaustion was the primary predictor for lower quality of care in 75 study populations, and overall burnout and depersonalization were the primary predictors for 56 and 11 study populations, respectively.
In an analysis of 114 unique burnout-quality combinations, 58 showed effects of burnout related to poor-quality care, 6 showed burnout related to high-quality care, and 50 showed no significant effect. Approximately one-third (33%) of the burnout-quality combinations were reported at least three times. In a review of the 46 burnout-quality combinations with primary effect sizes, 24 showed a significant effect of burnout on poor quality of care, 1 showed a significant effect of burnout on high quality of care, and 21 showed no significant effect.
The researchers also tested study bias using the Ioannidis test and found “an excess of observed versus predicted statistically significant studies (73% observed vs. 62%).”
The findings were limited by several factors, including the use of many cross-sectional, observational studies that could not show causality, the researchers noted. However, the results suggest several implications for future research including the need to consider exaggerated effects and reduce bias.
“Although the effect sizes in the published literature are modestly strong, our finding of excess significance implies that the true magnitude may be smaller than reported, and the studies that attempted to lower the risk of bias demonstrate fewer significant associations than the full evidence base,” the researchers noted.
“Whether curtailing burnout improves quality of care, or whether improving quality of care reduces burnout, is not yet known, and adequately powered and designed randomized trials will be indispensable in answering these questions,” they concluded.
The study was supported by the Stanford Maternal and Child Health Research Institute. Dr. Tawfik disclosed grants from Stanford Maternal and Child Health Research Institute during the study period.
SOURCE: Tawfik DS et al. Ann Intern Med. 2019 Oct 8. doi: 10.7326/M19-1152.
Burnout among health care professionals has been associated with lower quality of care, but the effect may be smaller than it seems, based on data from a meta-analysis of more than 200,000 clinicians.
Previous studies have reported associations between burnout and lower quality of care, but a standardized approach to analyze bias in the studies is lacking, wrote Daniel S. Tawfik, MD, of Stanford (Calif.) University and colleagues.
In a study published in the Annals of Internal Medicine, the researchers identified 123 publications from 1994 to 2019 with 142 study populations that included 241,553 health care providers.
Emotional exhaustion was the primary predictor for lower quality of care in 75 study populations, and overall burnout and depersonalization were the primary predictors for 56 and 11 study populations, respectively.
In an analysis of 114 unique burnout-quality combinations, 58 showed effects of burnout related to poor-quality care, 6 showed burnout related to high-quality care, and 50 showed no significant effect. Approximately one-third (33%) of the burnout-quality combinations were reported at least three times. In a review of the 46 burnout-quality combinations with primary effect sizes, 24 showed a significant effect of burnout on poor quality of care, 1 showed a significant effect of burnout on high quality of care, and 21 showed no significant effect.
The researchers also tested study bias using the Ioannidis test and found “an excess of observed versus predicted statistically significant studies (73% observed vs. 62%).”
The findings were limited by several factors, including the use of many cross-sectional, observational studies that could not show causality, the researchers noted. However, the results suggest several implications for future research including the need to consider exaggerated effects and reduce bias.
“Although the effect sizes in the published literature are modestly strong, our finding of excess significance implies that the true magnitude may be smaller than reported, and the studies that attempted to lower the risk of bias demonstrate fewer significant associations than the full evidence base,” the researchers noted.
“Whether curtailing burnout improves quality of care, or whether improving quality of care reduces burnout, is not yet known, and adequately powered and designed randomized trials will be indispensable in answering these questions,” they concluded.
The study was supported by the Stanford Maternal and Child Health Research Institute. Dr. Tawfik disclosed grants from Stanford Maternal and Child Health Research Institute during the study period.
SOURCE: Tawfik DS et al. Ann Intern Med. 2019 Oct 8. doi: 10.7326/M19-1152.
FROM THE ANNALS OF INTERNAL MEDICINE
F-BEVAR safe in patients with one kidney
CHICAGO – Patients who have one kidney do as well after fenestrated-branched endovascular aneurysm repair (F-BEVAR) of pararenal or thoracoabdominal aortic aneurysm as patients with both kidneys, according to a study of almost 300 patients presented at the annual meeting of the Midwestern Vascular Surgery Society.
“Despite the worse baseline renal function associated with single functioning kidney patients, F-BEVAR is safe and effective with nearly identical outcomes in patients with a SFK [single functioning kidney] as compared to patients with two functioning kidneys,” said Keouna Pather of Mayo Clinic, Rochester, Minn.
The study evaluated 287 F-BEVAR patients enrolled in a physician-sponsored investigation device exemption study from November 2013 to October 2018. Thirty of those patients had one kidney, the remaining 257 were the control group. Ms. Pather noted that characteristics were similar between both patient groups with the exception that SFK patients were younger (age 70 vs. 74 years; P = .009) and had larger renal artery diameter (6 vs. 5.7 mm; P = .05). “Patients with a SFK had enlargement of their renal artery in a compensatory fashion,” she said.
Survival at 2 years was 92% for SFK patients and 84% for controls.
“The SFK patients did start at a worse baseline of CKD [chronic kidney disease] stages as compared to controls,” she noted. In the SFK group, 63% (n = 19) had Stage III CKD versus 40% (n = 104) of controls (P = .02). Likewise, rates of Stage IV CKD were 10% (n = 3) and 2% (n = 4), respectively (P = .03).
In terms of outcomes, two patients in the control group died within 30 days but none in the SFK group did, Ms. Pather said. Also, a higher percentage of SFK patients had estimated blood loss greater than 1 L, compared with controls (20% vs. 7%; P = .02). All other outcomes, including rates of acute kidney injury (20% vs. 12%; P = .26), were not statistically different, she said.
“Between the groups, there was no significant difference in CKD progression that needed stenting,” she added, with 27% (n = 8) and 26% (n = 67) of the SFK and controls progressing to CKD Stages III to V.
The study also identified predictors of acute kidney injury in SFK patients: total fluoroscopy time (hours), which raised the risk by 78.5%, and estimated blood loss greater than 1 L, which increased risk by 109%.
Predictors of renal function deterioration in SFK patients were renal artery occlusion or reintervention for branch stenosis or kink, which raised the risk threefold; a Crawford extent II, which more than doubled the risk; and acute kidney injury, which raised chances almost fivefold. “Development of postoperative AKI [acute kidney injury] is the most important predictor for renal function deterioration,” Pather said.
When freedom from renal function deterioration at 2 years was compared between the two groups, again the results were similar because of the small sample size of the SFK group: 100% for the SFK group and 84% for controls.
Ms. Pather had no financial relationships to disclose.
CHICAGO – Patients who have one kidney do as well after fenestrated-branched endovascular aneurysm repair (F-BEVAR) of pararenal or thoracoabdominal aortic aneurysm as patients with both kidneys, according to a study of almost 300 patients presented at the annual meeting of the Midwestern Vascular Surgery Society.
“Despite the worse baseline renal function associated with single functioning kidney patients, F-BEVAR is safe and effective with nearly identical outcomes in patients with a SFK [single functioning kidney] as compared to patients with two functioning kidneys,” said Keouna Pather of Mayo Clinic, Rochester, Minn.
The study evaluated 287 F-BEVAR patients enrolled in a physician-sponsored investigation device exemption study from November 2013 to October 2018. Thirty of those patients had one kidney, the remaining 257 were the control group. Ms. Pather noted that characteristics were similar between both patient groups with the exception that SFK patients were younger (age 70 vs. 74 years; P = .009) and had larger renal artery diameter (6 vs. 5.7 mm; P = .05). “Patients with a SFK had enlargement of their renal artery in a compensatory fashion,” she said.
Survival at 2 years was 92% for SFK patients and 84% for controls.
“The SFK patients did start at a worse baseline of CKD [chronic kidney disease] stages as compared to controls,” she noted. In the SFK group, 63% (n = 19) had Stage III CKD versus 40% (n = 104) of controls (P = .02). Likewise, rates of Stage IV CKD were 10% (n = 3) and 2% (n = 4), respectively (P = .03).
In terms of outcomes, two patients in the control group died within 30 days but none in the SFK group did, Ms. Pather said. Also, a higher percentage of SFK patients had estimated blood loss greater than 1 L, compared with controls (20% vs. 7%; P = .02). All other outcomes, including rates of acute kidney injury (20% vs. 12%; P = .26), were not statistically different, she said.
“Between the groups, there was no significant difference in CKD progression that needed stenting,” she added, with 27% (n = 8) and 26% (n = 67) of the SFK and controls progressing to CKD Stages III to V.
The study also identified predictors of acute kidney injury in SFK patients: total fluoroscopy time (hours), which raised the risk by 78.5%, and estimated blood loss greater than 1 L, which increased risk by 109%.
Predictors of renal function deterioration in SFK patients were renal artery occlusion or reintervention for branch stenosis or kink, which raised the risk threefold; a Crawford extent II, which more than doubled the risk; and acute kidney injury, which raised chances almost fivefold. “Development of postoperative AKI [acute kidney injury] is the most important predictor for renal function deterioration,” Pather said.
When freedom from renal function deterioration at 2 years was compared between the two groups, again the results were similar because of the small sample size of the SFK group: 100% for the SFK group and 84% for controls.
Ms. Pather had no financial relationships to disclose.
CHICAGO – Patients who have one kidney do as well after fenestrated-branched endovascular aneurysm repair (F-BEVAR) of pararenal or thoracoabdominal aortic aneurysm as patients with both kidneys, according to a study of almost 300 patients presented at the annual meeting of the Midwestern Vascular Surgery Society.
“Despite the worse baseline renal function associated with single functioning kidney patients, F-BEVAR is safe and effective with nearly identical outcomes in patients with a SFK [single functioning kidney] as compared to patients with two functioning kidneys,” said Keouna Pather of Mayo Clinic, Rochester, Minn.
The study evaluated 287 F-BEVAR patients enrolled in a physician-sponsored investigation device exemption study from November 2013 to October 2018. Thirty of those patients had one kidney, the remaining 257 were the control group. Ms. Pather noted that characteristics were similar between both patient groups with the exception that SFK patients were younger (age 70 vs. 74 years; P = .009) and had larger renal artery diameter (6 vs. 5.7 mm; P = .05). “Patients with a SFK had enlargement of their renal artery in a compensatory fashion,” she said.
Survival at 2 years was 92% for SFK patients and 84% for controls.
“The SFK patients did start at a worse baseline of CKD [chronic kidney disease] stages as compared to controls,” she noted. In the SFK group, 63% (n = 19) had Stage III CKD versus 40% (n = 104) of controls (P = .02). Likewise, rates of Stage IV CKD were 10% (n = 3) and 2% (n = 4), respectively (P = .03).
In terms of outcomes, two patients in the control group died within 30 days but none in the SFK group did, Ms. Pather said. Also, a higher percentage of SFK patients had estimated blood loss greater than 1 L, compared with controls (20% vs. 7%; P = .02). All other outcomes, including rates of acute kidney injury (20% vs. 12%; P = .26), were not statistically different, she said.
“Between the groups, there was no significant difference in CKD progression that needed stenting,” she added, with 27% (n = 8) and 26% (n = 67) of the SFK and controls progressing to CKD Stages III to V.
The study also identified predictors of acute kidney injury in SFK patients: total fluoroscopy time (hours), which raised the risk by 78.5%, and estimated blood loss greater than 1 L, which increased risk by 109%.
Predictors of renal function deterioration in SFK patients were renal artery occlusion or reintervention for branch stenosis or kink, which raised the risk threefold; a Crawford extent II, which more than doubled the risk; and acute kidney injury, which raised chances almost fivefold. “Development of postoperative AKI [acute kidney injury] is the most important predictor for renal function deterioration,” Pather said.
When freedom from renal function deterioration at 2 years was compared between the two groups, again the results were similar because of the small sample size of the SFK group: 100% for the SFK group and 84% for controls.
Ms. Pather had no financial relationships to disclose.
REPORTING FROM MIDWESTERN VASCULAR 2019
Key clinical point: Fenestrated-branched endovascular repair of abdominal aortic aneurysm is safe and effective.
Major finding: Two-year survival rates were 92% for one-kidney patients and 84% for those with two kidneys.
Study details: Retrospective review of a prospectively collected database of 287 patients who had F-BEVAR from 2013 to 2018.
Disclosures: Ms. Pather has no financial relationships to disclose.
Source: Pather K et al. Midwestern Vascular 2019, Abstract 2.
Secukinumab continues promising axial spondyloarthritis results in PREVENT
New results for secukinumab (Cosentyx) in the PREVENT trial continue its promising trend for treatment of the full spectrum of axial spondyloarthritis (axSpA), according to a release from Novartis. The PREVENT trial is assessing this therapy in nonradiographic axSpA (nr-axSpA); it’s already approved in ankylosing spondylitis.
Results of 16-week data were presented in mid-September, and the new results represent 52-week data. Full details and data will be revealed at an upcoming scientific congress.
The PREVENT trial is an ongoing, 2-year trial with a 2-year extension phase; it enrolled 550 men and women with nr-axSpA and assessed their response to secukinumab with an induction phase, to it without induction, or to placebo. The trial met its primary endpoint of 40% improvement in Assessment in Spondyloarthritis International Society (ASAS40) criteria at 16 and 52 weeks, which indicates meaningful and sustained improvement. ASAS40 is defined as an improvement of at least 40% and both 10 out of 100 points in three of four domains (patient global assessment, pain, function, and inflammation) and no worsening in the remaining one. The safety profile in this trial was consistent with those seen in previous trials.
Overall, the most common adverse reactions seen with this interleukin-17A antagonist are nasopharyngitis, diarrhea, and upper respiratory tract infections. Patients should be evaluated for tuberculosis before initiating treatment. Serious infections have been observed, so be mindful of patients with chronic infections and discontinue secukinumab in patients who develop infections. Caution should be exercised when prescribing to patients with inflammatory bowel disease. Full prescribing information can be found on the Novartis website.
New results for secukinumab (Cosentyx) in the PREVENT trial continue its promising trend for treatment of the full spectrum of axial spondyloarthritis (axSpA), according to a release from Novartis. The PREVENT trial is assessing this therapy in nonradiographic axSpA (nr-axSpA); it’s already approved in ankylosing spondylitis.
Results of 16-week data were presented in mid-September, and the new results represent 52-week data. Full details and data will be revealed at an upcoming scientific congress.
The PREVENT trial is an ongoing, 2-year trial with a 2-year extension phase; it enrolled 550 men and women with nr-axSpA and assessed their response to secukinumab with an induction phase, to it without induction, or to placebo. The trial met its primary endpoint of 40% improvement in Assessment in Spondyloarthritis International Society (ASAS40) criteria at 16 and 52 weeks, which indicates meaningful and sustained improvement. ASAS40 is defined as an improvement of at least 40% and both 10 out of 100 points in three of four domains (patient global assessment, pain, function, and inflammation) and no worsening in the remaining one. The safety profile in this trial was consistent with those seen in previous trials.
Overall, the most common adverse reactions seen with this interleukin-17A antagonist are nasopharyngitis, diarrhea, and upper respiratory tract infections. Patients should be evaluated for tuberculosis before initiating treatment. Serious infections have been observed, so be mindful of patients with chronic infections and discontinue secukinumab in patients who develop infections. Caution should be exercised when prescribing to patients with inflammatory bowel disease. Full prescribing information can be found on the Novartis website.
New results for secukinumab (Cosentyx) in the PREVENT trial continue its promising trend for treatment of the full spectrum of axial spondyloarthritis (axSpA), according to a release from Novartis. The PREVENT trial is assessing this therapy in nonradiographic axSpA (nr-axSpA); it’s already approved in ankylosing spondylitis.
Results of 16-week data were presented in mid-September, and the new results represent 52-week data. Full details and data will be revealed at an upcoming scientific congress.
The PREVENT trial is an ongoing, 2-year trial with a 2-year extension phase; it enrolled 550 men and women with nr-axSpA and assessed their response to secukinumab with an induction phase, to it without induction, or to placebo. The trial met its primary endpoint of 40% improvement in Assessment in Spondyloarthritis International Society (ASAS40) criteria at 16 and 52 weeks, which indicates meaningful and sustained improvement. ASAS40 is defined as an improvement of at least 40% and both 10 out of 100 points in three of four domains (patient global assessment, pain, function, and inflammation) and no worsening in the remaining one. The safety profile in this trial was consistent with those seen in previous trials.
Overall, the most common adverse reactions seen with this interleukin-17A antagonist are nasopharyngitis, diarrhea, and upper respiratory tract infections. Patients should be evaluated for tuberculosis before initiating treatment. Serious infections have been observed, so be mindful of patients with chronic infections and discontinue secukinumab in patients who develop infections. Caution should be exercised when prescribing to patients with inflammatory bowel disease. Full prescribing information can be found on the Novartis website.
New York declares end to 2018 measles outbreak
New York State has reported the end of all active measles cases related to the initial outbreak in 2018, but the state is now responding to new, unrelated cases in four counties, according to the Centers for Disease Control and Prevention.
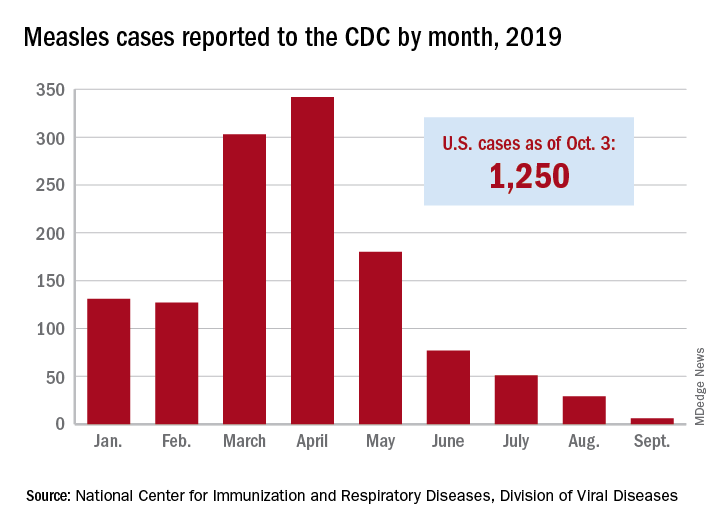
The new cases – two in Nassau County and one each in Monroe, Putnam, and Rockland counties – are “related to measles exposures from international travel but not affiliated with the 2018 outbreak,” the New York State Department of Health said in a written statement. Officials in Rockland County had declared its 2018 measles outbreak, which involved 312 cases in 2018 and 2019, over on Sept. 25.
. Of those cases, 1,163 (93%) were associated with 22 outbreaks, with the two largest occurring in New York City and Rockland County. “These two almost year-long outbreaks placed the United States at risk for losing measles elimination status,” the CDC said in a separate report, but “robust responses … ended transmission before the 1-year mark.”
New York State has reported the end of all active measles cases related to the initial outbreak in 2018, but the state is now responding to new, unrelated cases in four counties, according to the Centers for Disease Control and Prevention.

The new cases – two in Nassau County and one each in Monroe, Putnam, and Rockland counties – are “related to measles exposures from international travel but not affiliated with the 2018 outbreak,” the New York State Department of Health said in a written statement. Officials in Rockland County had declared its 2018 measles outbreak, which involved 312 cases in 2018 and 2019, over on Sept. 25.
. Of those cases, 1,163 (93%) were associated with 22 outbreaks, with the two largest occurring in New York City and Rockland County. “These two almost year-long outbreaks placed the United States at risk for losing measles elimination status,” the CDC said in a separate report, but “robust responses … ended transmission before the 1-year mark.”
New York State has reported the end of all active measles cases related to the initial outbreak in 2018, but the state is now responding to new, unrelated cases in four counties, according to the Centers for Disease Control and Prevention.

The new cases – two in Nassau County and one each in Monroe, Putnam, and Rockland counties – are “related to measles exposures from international travel but not affiliated with the 2018 outbreak,” the New York State Department of Health said in a written statement. Officials in Rockland County had declared its 2018 measles outbreak, which involved 312 cases in 2018 and 2019, over on Sept. 25.
. Of those cases, 1,163 (93%) were associated with 22 outbreaks, with the two largest occurring in New York City and Rockland County. “These two almost year-long outbreaks placed the United States at risk for losing measles elimination status,” the CDC said in a separate report, but “robust responses … ended transmission before the 1-year mark.”






