User login
MRI-guided revascularization noninferior to FFR
Myocardial perfusion cardiovascular MRI is as good as invasive angiography and measurement of fractional flow reserve to guide revascularization in patients with angina, research suggests.
In the New England Journal of Medicine, researchers present the outcomes of an unblinded, multicenter, clinical effectiveness trial comparing the two revascularization strategies in 918 patients who had typical angina and either two or more cardiovascular risk factors or a positive exercise treadmill test.
In the fractional flow reserve method, revascularization was recommended in all vessels with an FFR of 0.8 or less. In the MRI-guided method, all patients underwent myocardial perfusion cardiovascular MRI, and patients with clinically significant inducible ischemia then underwent invasive angiography, and revascularization if required.
Significantly fewer patients in the cardiovascular MRI group underwent index revascularization, compared with the fractional flow reserve group (36% vs. 45% respectively; P = .005), and only 48% in the cardiovascular MRI group underwent invasive angiography, compared with 97% of patients in the fractional flow reserve arm.
However, there was no significant difference between the two groups in the incidence of major cardiac adverse events after 1 year, signifying that the MRI approach met the criteria for noninferiority.
There was also no significant difference between the two groups in the percentage of patients who were free from angina after 12 months (49.2% in the MRI group and 43.8% in the FFR group).
“Current guidelines on the management of the care of patients with suspected coronary artery disease separate diagnostic strategies from therapeutic strategies owing to a lack of evidence comparing combined diagnostic and therapeutic pathways,” wrote Eike Nagel, MD, of the Goethe University Frankfurt Institute for Experimental and Translational Cardiovascular Imaging and coauthors. “The MR-INFORM trial closes this knowledge gap by comparing two frequently used, well-defined, standardized, and validated clinical management strategies.”
However, they pointed out that one limitation of their study was the lack of a third group of patients who received medical therapy without planned revascularization. They also noted that the incidence of the primary outcome of major adverse cardiac events was lower than expected at 1 year.
The study was supported by the Guy’s and St. Thomas’ Biomedical Research Centre of the National Institute for Health Research. Three authors declared support from study supporters related to the study, three declared grants, personal fees, and other support from the private sector unrelated to the study. No other conflicts of interest were declared.
SOURCE: Nagel E et al. New Engl J Med. 2019;380:2418-28. doi: 10.1056/NEJMoa1716734.
Myocardial perfusion cardiovascular MRI is as good as invasive angiography and measurement of fractional flow reserve to guide revascularization in patients with angina, research suggests.
In the New England Journal of Medicine, researchers present the outcomes of an unblinded, multicenter, clinical effectiveness trial comparing the two revascularization strategies in 918 patients who had typical angina and either two or more cardiovascular risk factors or a positive exercise treadmill test.
In the fractional flow reserve method, revascularization was recommended in all vessels with an FFR of 0.8 or less. In the MRI-guided method, all patients underwent myocardial perfusion cardiovascular MRI, and patients with clinically significant inducible ischemia then underwent invasive angiography, and revascularization if required.
Significantly fewer patients in the cardiovascular MRI group underwent index revascularization, compared with the fractional flow reserve group (36% vs. 45% respectively; P = .005), and only 48% in the cardiovascular MRI group underwent invasive angiography, compared with 97% of patients in the fractional flow reserve arm.
However, there was no significant difference between the two groups in the incidence of major cardiac adverse events after 1 year, signifying that the MRI approach met the criteria for noninferiority.
There was also no significant difference between the two groups in the percentage of patients who were free from angina after 12 months (49.2% in the MRI group and 43.8% in the FFR group).
“Current guidelines on the management of the care of patients with suspected coronary artery disease separate diagnostic strategies from therapeutic strategies owing to a lack of evidence comparing combined diagnostic and therapeutic pathways,” wrote Eike Nagel, MD, of the Goethe University Frankfurt Institute for Experimental and Translational Cardiovascular Imaging and coauthors. “The MR-INFORM trial closes this knowledge gap by comparing two frequently used, well-defined, standardized, and validated clinical management strategies.”
However, they pointed out that one limitation of their study was the lack of a third group of patients who received medical therapy without planned revascularization. They also noted that the incidence of the primary outcome of major adverse cardiac events was lower than expected at 1 year.
The study was supported by the Guy’s and St. Thomas’ Biomedical Research Centre of the National Institute for Health Research. Three authors declared support from study supporters related to the study, three declared grants, personal fees, and other support from the private sector unrelated to the study. No other conflicts of interest were declared.
SOURCE: Nagel E et al. New Engl J Med. 2019;380:2418-28. doi: 10.1056/NEJMoa1716734.
Myocardial perfusion cardiovascular MRI is as good as invasive angiography and measurement of fractional flow reserve to guide revascularization in patients with angina, research suggests.
In the New England Journal of Medicine, researchers present the outcomes of an unblinded, multicenter, clinical effectiveness trial comparing the two revascularization strategies in 918 patients who had typical angina and either two or more cardiovascular risk factors or a positive exercise treadmill test.
In the fractional flow reserve method, revascularization was recommended in all vessels with an FFR of 0.8 or less. In the MRI-guided method, all patients underwent myocardial perfusion cardiovascular MRI, and patients with clinically significant inducible ischemia then underwent invasive angiography, and revascularization if required.
Significantly fewer patients in the cardiovascular MRI group underwent index revascularization, compared with the fractional flow reserve group (36% vs. 45% respectively; P = .005), and only 48% in the cardiovascular MRI group underwent invasive angiography, compared with 97% of patients in the fractional flow reserve arm.
However, there was no significant difference between the two groups in the incidence of major cardiac adverse events after 1 year, signifying that the MRI approach met the criteria for noninferiority.
There was also no significant difference between the two groups in the percentage of patients who were free from angina after 12 months (49.2% in the MRI group and 43.8% in the FFR group).
“Current guidelines on the management of the care of patients with suspected coronary artery disease separate diagnostic strategies from therapeutic strategies owing to a lack of evidence comparing combined diagnostic and therapeutic pathways,” wrote Eike Nagel, MD, of the Goethe University Frankfurt Institute for Experimental and Translational Cardiovascular Imaging and coauthors. “The MR-INFORM trial closes this knowledge gap by comparing two frequently used, well-defined, standardized, and validated clinical management strategies.”
However, they pointed out that one limitation of their study was the lack of a third group of patients who received medical therapy without planned revascularization. They also noted that the incidence of the primary outcome of major adverse cardiac events was lower than expected at 1 year.
The study was supported by the Guy’s and St. Thomas’ Biomedical Research Centre of the National Institute for Health Research. Three authors declared support from study supporters related to the study, three declared grants, personal fees, and other support from the private sector unrelated to the study. No other conflicts of interest were declared.
SOURCE: Nagel E et al. New Engl J Med. 2019;380:2418-28. doi: 10.1056/NEJMoa1716734.
FROM NEW ENGLAND JOURNAL OF MEDICINE
Key clinical point: .
Major finding: The incidence of major cardiac adverse events similar at 1 year with cardiovascular MRI and invasive angiography.
Study details: MR-INFORM, an unblinded, multicenter, clinical effectiveness trial in 918 patients with angina.
Disclosures: The study was supported by the Guy’s and St. Thomas’ Biomedical Research Centre of the National Institute for Health Research. Three authors declared support from study supporters related to the study, three declared grants, personal fees and other support from the private sector unrelated to the study. No other conflicts of interest were declared.
Source: Nagel E et al. New Engl J Med. 2019;380:2418-28. doi: 10.1056/NEJMoa1716734.
Real-world data confirm nonacog alfa efficacy in hemophilia B
The recombinant factor IX product nonacog alfa appears safe and effective for patients with hemophilia B, according to results from a recent postmarketing study in Japan.
While nonacog alfa was approved in the United States and Europe in 1997, the product wasn’t approved in Japan until 2009. Since it was the first recombinant factor IX product available there, and only a small number of patients were enrolled in domestic clinical trials, the Japanese government required additional real-world studies.
“In the last couple of years, several extended half‐life blood coagulation factor products gained regulatory approval for the treatment of hemophilia B. However, access to this most advanced treatment option remains limited to developed countries, and the need for standard half‐life recombinant or plasma‐derived FIX products is still high,” wrote Katsuyuki Fukutake, MD, PhD, of Tokyo Medical University, Japan, and colleagues.
The researchers conducted an observational postmarketing surveillance study of 312 patients with hemophilia B who received nonacog alfa therapy from 2010 to 2014. The team evaluated the real-world safety, including the incidence of inhibitors and adverse events, and effectiveness of the recombinant product in Japan.
The findings were published in Haemophilia.
The study included both previously treated and untreated participants who were followed for 1 and 2 years, respectively, after starting recombinant factor IX therapy.
The primary safety outcome was the incidence and number of adverse drug reactions. Effectiveness was measured using clinical effectiveness rates and annualized bleeding rates (ABR).
After analysis, the researchers found that the effectiveness rates were 95.5% and 93.7% for patients who received routine prophylaxis and on-demand treatment, respectively.
The median ABR was lower during routine prophylaxis – 2.0 – versus the rest of the observation period – 8.3. “This difference was prominent among patients with severe haemophilia B or haemophilic arthropathy,” the researchers wrote.
With respect to safety, 11 adverse drug reactions were seen in seven previously treated patients. New inhibitor development was not observed in any participants, but recurrence was seen in one patient.
“Our results are consistent with those of previous studies where the incidence of inhibitor antibody development in hemophilia B has been reported as 1%-5%,” Dr. Fukutake and colleagues wrote.
The researchers acknowledged that one key limitation of the study was the observational design.
“The results suggest that nonacog alfa was well tolerated and appropriately used under routine clinical practice,” the authors concluded.
The study was funded and conducted by Pfizer Japan. The authors reported financial relationships with Pfizer and several other companies. One coauthor is an employee of Pfizer Japan.
SOURCE: Fukutake K et al. Haemophilia. 2019 Jun 6. doi: 10.1111/hae.13783.
The recombinant factor IX product nonacog alfa appears safe and effective for patients with hemophilia B, according to results from a recent postmarketing study in Japan.
While nonacog alfa was approved in the United States and Europe in 1997, the product wasn’t approved in Japan until 2009. Since it was the first recombinant factor IX product available there, and only a small number of patients were enrolled in domestic clinical trials, the Japanese government required additional real-world studies.
“In the last couple of years, several extended half‐life blood coagulation factor products gained regulatory approval for the treatment of hemophilia B. However, access to this most advanced treatment option remains limited to developed countries, and the need for standard half‐life recombinant or plasma‐derived FIX products is still high,” wrote Katsuyuki Fukutake, MD, PhD, of Tokyo Medical University, Japan, and colleagues.
The researchers conducted an observational postmarketing surveillance study of 312 patients with hemophilia B who received nonacog alfa therapy from 2010 to 2014. The team evaluated the real-world safety, including the incidence of inhibitors and adverse events, and effectiveness of the recombinant product in Japan.
The findings were published in Haemophilia.
The study included both previously treated and untreated participants who were followed for 1 and 2 years, respectively, after starting recombinant factor IX therapy.
The primary safety outcome was the incidence and number of adverse drug reactions. Effectiveness was measured using clinical effectiveness rates and annualized bleeding rates (ABR).
After analysis, the researchers found that the effectiveness rates were 95.5% and 93.7% for patients who received routine prophylaxis and on-demand treatment, respectively.
The median ABR was lower during routine prophylaxis – 2.0 – versus the rest of the observation period – 8.3. “This difference was prominent among patients with severe haemophilia B or haemophilic arthropathy,” the researchers wrote.
With respect to safety, 11 adverse drug reactions were seen in seven previously treated patients. New inhibitor development was not observed in any participants, but recurrence was seen in one patient.
“Our results are consistent with those of previous studies where the incidence of inhibitor antibody development in hemophilia B has been reported as 1%-5%,” Dr. Fukutake and colleagues wrote.
The researchers acknowledged that one key limitation of the study was the observational design.
“The results suggest that nonacog alfa was well tolerated and appropriately used under routine clinical practice,” the authors concluded.
The study was funded and conducted by Pfizer Japan. The authors reported financial relationships with Pfizer and several other companies. One coauthor is an employee of Pfizer Japan.
SOURCE: Fukutake K et al. Haemophilia. 2019 Jun 6. doi: 10.1111/hae.13783.
The recombinant factor IX product nonacog alfa appears safe and effective for patients with hemophilia B, according to results from a recent postmarketing study in Japan.
While nonacog alfa was approved in the United States and Europe in 1997, the product wasn’t approved in Japan until 2009. Since it was the first recombinant factor IX product available there, and only a small number of patients were enrolled in domestic clinical trials, the Japanese government required additional real-world studies.
“In the last couple of years, several extended half‐life blood coagulation factor products gained regulatory approval for the treatment of hemophilia B. However, access to this most advanced treatment option remains limited to developed countries, and the need for standard half‐life recombinant or plasma‐derived FIX products is still high,” wrote Katsuyuki Fukutake, MD, PhD, of Tokyo Medical University, Japan, and colleagues.
The researchers conducted an observational postmarketing surveillance study of 312 patients with hemophilia B who received nonacog alfa therapy from 2010 to 2014. The team evaluated the real-world safety, including the incidence of inhibitors and adverse events, and effectiveness of the recombinant product in Japan.
The findings were published in Haemophilia.
The study included both previously treated and untreated participants who were followed for 1 and 2 years, respectively, after starting recombinant factor IX therapy.
The primary safety outcome was the incidence and number of adverse drug reactions. Effectiveness was measured using clinical effectiveness rates and annualized bleeding rates (ABR).
After analysis, the researchers found that the effectiveness rates were 95.5% and 93.7% for patients who received routine prophylaxis and on-demand treatment, respectively.
The median ABR was lower during routine prophylaxis – 2.0 – versus the rest of the observation period – 8.3. “This difference was prominent among patients with severe haemophilia B or haemophilic arthropathy,” the researchers wrote.
With respect to safety, 11 adverse drug reactions were seen in seven previously treated patients. New inhibitor development was not observed in any participants, but recurrence was seen in one patient.
“Our results are consistent with those of previous studies where the incidence of inhibitor antibody development in hemophilia B has been reported as 1%-5%,” Dr. Fukutake and colleagues wrote.
The researchers acknowledged that one key limitation of the study was the observational design.
“The results suggest that nonacog alfa was well tolerated and appropriately used under routine clinical practice,” the authors concluded.
The study was funded and conducted by Pfizer Japan. The authors reported financial relationships with Pfizer and several other companies. One coauthor is an employee of Pfizer Japan.
SOURCE: Fukutake K et al. Haemophilia. 2019 Jun 6. doi: 10.1111/hae.13783.
FROM HAEMOPHILIA
BET inhibitors may target oncogene in ABC-like DLBCL
(ABC-like DLBCL).
Researchers found the TCF4 (E2-2) transcription factor is an oncogene in ABC-like DLBCL, and TCF4 can be targeted via BET inhibition.
The BET protein degrader ARV771 reduced TCF4 expression and exhibited activity against ABC-like DLBCL in vitro and in vivo.
Neeraj Jain, PhD, of the University of Texas MD Anderson Cancer Center in Houston and colleagues reported these findings in Science Translational Medicine.
The researchers performed a genomic analysis of 1,000 DLBCL tumors and discovered that gains of 18q21.2 were the most frequent genetic alteration in ABC-like DLBCL. In analyzing another 249 tumors, the researchers found that TCF4 was the target of the 18q21.2 gains.
Additional experiments with primary DLBCL tumors and DLBCL cell lines indicated that TCF4 regulates IGHM and MYC expression. The researchers also found that the TCF4 gene was “one of the most highly BRD4-loaded genes in DLBCL,” and knocking down BRD4 in DLBCL cell lines reduced TCF4 expression.
These findings prompted the team to test small-molecule BET inhibitors – JQ1 and OTX015 – and a BET protein degrader – ARV771 – in ABC-like DLBCL cell lines with a high TCF4 copy number.
Treatment with JQ1 and OTX015 resulted in upregulation of BRD4, but ARV771 treatment did not. As a result, subsequent experiments were performed with ARV771.
The researchers found that ARV771 induced apoptosis in the cell lines and reduced the expression of TCF4, IgM, and MYC. However, enforced TCF4 expression during ARV771 treatment rescued IgM and MYC expression. This suggests that “reduction of TCF4 is one of the mechanisms by which BET inhibition reduces IgM and MYC expression and induces apoptosis,” according to the researchers.
The team also tested ARV771 in mouse models of ABC-like DLBCL with high TCF4 expression. ARV771 significantly reduced tumor growth and prolonged survival (P less than .05 for both), and there were no signs of toxicity in ARV771-treated mice.
“Our data provide a clear functional rationale for BET inhibition in ABC-like DLBCL,” the researchers wrote. They did note, however, that BCL2 overexpression is associated with resistance to BET inhibitors, and most of the 18q DNA copy number gains the researchers observed in DLBCL encompass TCF4 and BCL2. The team therefore theorized that combining a BET inhibitor with a BCL2 inhibitor could be a promising treatment approach in ABC-like DLBCL and is worthy of further investigation.
This research was supported by the Nebraska Department of Health & Human Services, the Schweitzer Family Fund, the Fred & Pamela Buffet Cancer Center Support Grant, and the MD Anderson Cancer Center NCI CORE Grant. The authors reported having no conflicts of interest related to the study.
SOURCE: Jain N et al. Sci. Transl. Med. 2019 Jun 19. doi: 10.1126/scitranslmed.aav5599.
(ABC-like DLBCL).
Researchers found the TCF4 (E2-2) transcription factor is an oncogene in ABC-like DLBCL, and TCF4 can be targeted via BET inhibition.
The BET protein degrader ARV771 reduced TCF4 expression and exhibited activity against ABC-like DLBCL in vitro and in vivo.
Neeraj Jain, PhD, of the University of Texas MD Anderson Cancer Center in Houston and colleagues reported these findings in Science Translational Medicine.
The researchers performed a genomic analysis of 1,000 DLBCL tumors and discovered that gains of 18q21.2 were the most frequent genetic alteration in ABC-like DLBCL. In analyzing another 249 tumors, the researchers found that TCF4 was the target of the 18q21.2 gains.
Additional experiments with primary DLBCL tumors and DLBCL cell lines indicated that TCF4 regulates IGHM and MYC expression. The researchers also found that the TCF4 gene was “one of the most highly BRD4-loaded genes in DLBCL,” and knocking down BRD4 in DLBCL cell lines reduced TCF4 expression.
These findings prompted the team to test small-molecule BET inhibitors – JQ1 and OTX015 – and a BET protein degrader – ARV771 – in ABC-like DLBCL cell lines with a high TCF4 copy number.
Treatment with JQ1 and OTX015 resulted in upregulation of BRD4, but ARV771 treatment did not. As a result, subsequent experiments were performed with ARV771.
The researchers found that ARV771 induced apoptosis in the cell lines and reduced the expression of TCF4, IgM, and MYC. However, enforced TCF4 expression during ARV771 treatment rescued IgM and MYC expression. This suggests that “reduction of TCF4 is one of the mechanisms by which BET inhibition reduces IgM and MYC expression and induces apoptosis,” according to the researchers.
The team also tested ARV771 in mouse models of ABC-like DLBCL with high TCF4 expression. ARV771 significantly reduced tumor growth and prolonged survival (P less than .05 for both), and there were no signs of toxicity in ARV771-treated mice.
“Our data provide a clear functional rationale for BET inhibition in ABC-like DLBCL,” the researchers wrote. They did note, however, that BCL2 overexpression is associated with resistance to BET inhibitors, and most of the 18q DNA copy number gains the researchers observed in DLBCL encompass TCF4 and BCL2. The team therefore theorized that combining a BET inhibitor with a BCL2 inhibitor could be a promising treatment approach in ABC-like DLBCL and is worthy of further investigation.
This research was supported by the Nebraska Department of Health & Human Services, the Schweitzer Family Fund, the Fred & Pamela Buffet Cancer Center Support Grant, and the MD Anderson Cancer Center NCI CORE Grant. The authors reported having no conflicts of interest related to the study.
SOURCE: Jain N et al. Sci. Transl. Med. 2019 Jun 19. doi: 10.1126/scitranslmed.aav5599.
(ABC-like DLBCL).
Researchers found the TCF4 (E2-2) transcription factor is an oncogene in ABC-like DLBCL, and TCF4 can be targeted via BET inhibition.
The BET protein degrader ARV771 reduced TCF4 expression and exhibited activity against ABC-like DLBCL in vitro and in vivo.
Neeraj Jain, PhD, of the University of Texas MD Anderson Cancer Center in Houston and colleagues reported these findings in Science Translational Medicine.
The researchers performed a genomic analysis of 1,000 DLBCL tumors and discovered that gains of 18q21.2 were the most frequent genetic alteration in ABC-like DLBCL. In analyzing another 249 tumors, the researchers found that TCF4 was the target of the 18q21.2 gains.
Additional experiments with primary DLBCL tumors and DLBCL cell lines indicated that TCF4 regulates IGHM and MYC expression. The researchers also found that the TCF4 gene was “one of the most highly BRD4-loaded genes in DLBCL,” and knocking down BRD4 in DLBCL cell lines reduced TCF4 expression.
These findings prompted the team to test small-molecule BET inhibitors – JQ1 and OTX015 – and a BET protein degrader – ARV771 – in ABC-like DLBCL cell lines with a high TCF4 copy number.
Treatment with JQ1 and OTX015 resulted in upregulation of BRD4, but ARV771 treatment did not. As a result, subsequent experiments were performed with ARV771.
The researchers found that ARV771 induced apoptosis in the cell lines and reduced the expression of TCF4, IgM, and MYC. However, enforced TCF4 expression during ARV771 treatment rescued IgM and MYC expression. This suggests that “reduction of TCF4 is one of the mechanisms by which BET inhibition reduces IgM and MYC expression and induces apoptosis,” according to the researchers.
The team also tested ARV771 in mouse models of ABC-like DLBCL with high TCF4 expression. ARV771 significantly reduced tumor growth and prolonged survival (P less than .05 for both), and there were no signs of toxicity in ARV771-treated mice.
“Our data provide a clear functional rationale for BET inhibition in ABC-like DLBCL,” the researchers wrote. They did note, however, that BCL2 overexpression is associated with resistance to BET inhibitors, and most of the 18q DNA copy number gains the researchers observed in DLBCL encompass TCF4 and BCL2. The team therefore theorized that combining a BET inhibitor with a BCL2 inhibitor could be a promising treatment approach in ABC-like DLBCL and is worthy of further investigation.
This research was supported by the Nebraska Department of Health & Human Services, the Schweitzer Family Fund, the Fred & Pamela Buffet Cancer Center Support Grant, and the MD Anderson Cancer Center NCI CORE Grant. The authors reported having no conflicts of interest related to the study.
SOURCE: Jain N et al. Sci. Transl. Med. 2019 Jun 19. doi: 10.1126/scitranslmed.aav5599.
FROM SCIENCE TRANSLATIONAL MEDICINE
Appeals court allows Title X restrictions to take effect
The panel ruled that the Trump administration’s funding restrictions were a reasonable interpretation of the federal Title X statute and that the administration is likely to prevail in its argument that lower courts erroneously halted the rules from taking effect. The ruling means the restrictions can take effect in every state except for Maryland, which passed a 2019 measure approving the use of state funds to replace federal Title X money if the new rule is enacted.
Alex Azar, secretary of the Department of Health & Human Services, said agency officials were pleased the 9th Circuit recognized there was no need to hold up the new family planning rules that simply enforce laws already on the books.
“We are also pleased that the [9th] Circuit agreed that the three preliminary injunctions against the new rules, including two nationwide injunctions, were inappropriate,” Mr. Azar said in the statement. “This decision is a major step toward the Trump administration being able to ensure that all Title X projects comply with the Title X statute and do not support abortion as a method of family planning.”
Leana Wen, MD, president for the Planned Parenthood Federation of America called the court ruling “devastating” for the millions of patients who rely on Title X health centers for cancer screenings, HIV tests, affordable birth control, and other critical primary and preventive care.
“We will be immediately seeking emergency relief from the [U.S.] Court of Appeals,” Dr. Wen said in a statement. “Planned Parenthood will not let the government censor our doctors and nurses from informing patients where and how they can access health care. We will continue to fight the Trump administration in the courts and alongside champions in Congress to protect everyone’s fundamental right to health care.”
The changes to the Title X program – originally scheduled to take effect May 3 – make health clinics ineligible for Title X funding if they offer, promote, or support abortion as a method of family planning. Title X grants generally go to health centers that provide reproductive health care – such as STD testing, cancer screenings, and contraception – to low-income families. Under the rule, the government would withdraw financial assistance to clinics if they allow counseling or referrals associated with abortion, regardless of whether the money is used for other health care services.
More than 20 states and several abortion rights organizations sued over the rules in four separate states. District judges in Oregon, Washington, and California temporarily blocked the rules from taking effect. The 9th Circuit ruling overturns these injunctions.
The American College of Physicians, the American Academy of Family Physicians, the American College of Obstetricians and Gynecologists, the American Academy of Pediatrics, and other groups have voiced their opposition to the Title X restrictions.
In a joint court brief, the medical societies wrote that the Trump administration’s limitations to the Title X program will create cultural, geographic, and financial barriers to care; erode the physician-patient relationship; and cause extreme, immediate, and irreparable harm to millions of patients.
The panel ruled that the Trump administration’s funding restrictions were a reasonable interpretation of the federal Title X statute and that the administration is likely to prevail in its argument that lower courts erroneously halted the rules from taking effect. The ruling means the restrictions can take effect in every state except for Maryland, which passed a 2019 measure approving the use of state funds to replace federal Title X money if the new rule is enacted.
Alex Azar, secretary of the Department of Health & Human Services, said agency officials were pleased the 9th Circuit recognized there was no need to hold up the new family planning rules that simply enforce laws already on the books.
“We are also pleased that the [9th] Circuit agreed that the three preliminary injunctions against the new rules, including two nationwide injunctions, were inappropriate,” Mr. Azar said in the statement. “This decision is a major step toward the Trump administration being able to ensure that all Title X projects comply with the Title X statute and do not support abortion as a method of family planning.”
Leana Wen, MD, president for the Planned Parenthood Federation of America called the court ruling “devastating” for the millions of patients who rely on Title X health centers for cancer screenings, HIV tests, affordable birth control, and other critical primary and preventive care.
“We will be immediately seeking emergency relief from the [U.S.] Court of Appeals,” Dr. Wen said in a statement. “Planned Parenthood will not let the government censor our doctors and nurses from informing patients where and how they can access health care. We will continue to fight the Trump administration in the courts and alongside champions in Congress to protect everyone’s fundamental right to health care.”
The changes to the Title X program – originally scheduled to take effect May 3 – make health clinics ineligible for Title X funding if they offer, promote, or support abortion as a method of family planning. Title X grants generally go to health centers that provide reproductive health care – such as STD testing, cancer screenings, and contraception – to low-income families. Under the rule, the government would withdraw financial assistance to clinics if they allow counseling or referrals associated with abortion, regardless of whether the money is used for other health care services.
More than 20 states and several abortion rights organizations sued over the rules in four separate states. District judges in Oregon, Washington, and California temporarily blocked the rules from taking effect. The 9th Circuit ruling overturns these injunctions.
The American College of Physicians, the American Academy of Family Physicians, the American College of Obstetricians and Gynecologists, the American Academy of Pediatrics, and other groups have voiced their opposition to the Title X restrictions.
In a joint court brief, the medical societies wrote that the Trump administration’s limitations to the Title X program will create cultural, geographic, and financial barriers to care; erode the physician-patient relationship; and cause extreme, immediate, and irreparable harm to millions of patients.
The panel ruled that the Trump administration’s funding restrictions were a reasonable interpretation of the federal Title X statute and that the administration is likely to prevail in its argument that lower courts erroneously halted the rules from taking effect. The ruling means the restrictions can take effect in every state except for Maryland, which passed a 2019 measure approving the use of state funds to replace federal Title X money if the new rule is enacted.
Alex Azar, secretary of the Department of Health & Human Services, said agency officials were pleased the 9th Circuit recognized there was no need to hold up the new family planning rules that simply enforce laws already on the books.
“We are also pleased that the [9th] Circuit agreed that the three preliminary injunctions against the new rules, including two nationwide injunctions, were inappropriate,” Mr. Azar said in the statement. “This decision is a major step toward the Trump administration being able to ensure that all Title X projects comply with the Title X statute and do not support abortion as a method of family planning.”
Leana Wen, MD, president for the Planned Parenthood Federation of America called the court ruling “devastating” for the millions of patients who rely on Title X health centers for cancer screenings, HIV tests, affordable birth control, and other critical primary and preventive care.
“We will be immediately seeking emergency relief from the [U.S.] Court of Appeals,” Dr. Wen said in a statement. “Planned Parenthood will not let the government censor our doctors and nurses from informing patients where and how they can access health care. We will continue to fight the Trump administration in the courts and alongside champions in Congress to protect everyone’s fundamental right to health care.”
The changes to the Title X program – originally scheduled to take effect May 3 – make health clinics ineligible for Title X funding if they offer, promote, or support abortion as a method of family planning. Title X grants generally go to health centers that provide reproductive health care – such as STD testing, cancer screenings, and contraception – to low-income families. Under the rule, the government would withdraw financial assistance to clinics if they allow counseling or referrals associated with abortion, regardless of whether the money is used for other health care services.
More than 20 states and several abortion rights organizations sued over the rules in four separate states. District judges in Oregon, Washington, and California temporarily blocked the rules from taking effect. The 9th Circuit ruling overturns these injunctions.
The American College of Physicians, the American Academy of Family Physicians, the American College of Obstetricians and Gynecologists, the American Academy of Pediatrics, and other groups have voiced their opposition to the Title X restrictions.
In a joint court brief, the medical societies wrote that the Trump administration’s limitations to the Title X program will create cultural, geographic, and financial barriers to care; erode the physician-patient relationship; and cause extreme, immediate, and irreparable harm to millions of patients.
Many doubt Medicare-for-all would bring universal coverage
Does Medicare-for-all mean that everyone gets health insurance coverage?
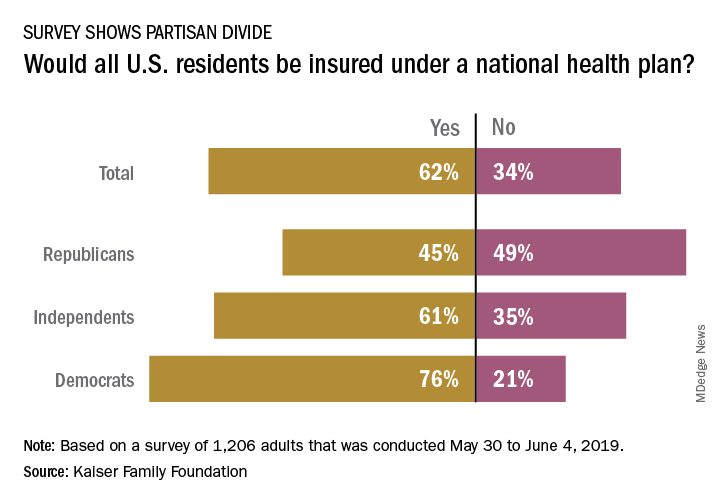
The public is not convinced that this would be the case, and Republicans are even more skeptical, according to a new survey from the Kaiser Family Foundation.
and there is a sizable split between Republicans and Democrats on the issue. Republicans, in fact, were more likely to say that all residents would not have coverage, with less than 49% of people identifying as Republican having answered “no” to the question and 45% having answered “yes”, Kaiser reported in its latest Health Tracking Poll.
Democrats were much more optimistic but not all were certain that all U.S. residents would have health insurance coverage. While 76% of Democrats responded yes when queried about whether all residents would get coverage, 21% answered no to the question. Responses to the question from independents – 61% said yes and 35% said no – very closely reflected the overall vote of 62% yes and 34% no, Kaiser said.
The partisan divide appeared again when respondents were asked if physicians and hospitals would be paid less under a national health plan: 64% of Republicans said payments would be reduced versus 42% of Democrats. Similarly, more than half of Republicans (53%) said that people who buy their own insurance would not be able to keep their current plans, compared with 24% of Democrats, the poll data show.
Those looking for common ground can point to responses regarding a couple of other potential effects of a national health plan. Republicans (57%) and Democrats (52%) largely agreed that private health insurance companies would not be the primary way Americans get health coverage, and they agreed that people would continue to pay deductibles and copays when they used health care services (68% for Republicans, 71% for Democrats), Kaiser said.
The poll was conducted from May 30 to June 4, 2019, and involved responses from 1,206 adults. The margin of sampling error was plus or minus 3 percentage points.
Does Medicare-for-all mean that everyone gets health insurance coverage?

The public is not convinced that this would be the case, and Republicans are even more skeptical, according to a new survey from the Kaiser Family Foundation.
and there is a sizable split between Republicans and Democrats on the issue. Republicans, in fact, were more likely to say that all residents would not have coverage, with less than 49% of people identifying as Republican having answered “no” to the question and 45% having answered “yes”, Kaiser reported in its latest Health Tracking Poll.
Democrats were much more optimistic but not all were certain that all U.S. residents would have health insurance coverage. While 76% of Democrats responded yes when queried about whether all residents would get coverage, 21% answered no to the question. Responses to the question from independents – 61% said yes and 35% said no – very closely reflected the overall vote of 62% yes and 34% no, Kaiser said.
The partisan divide appeared again when respondents were asked if physicians and hospitals would be paid less under a national health plan: 64% of Republicans said payments would be reduced versus 42% of Democrats. Similarly, more than half of Republicans (53%) said that people who buy their own insurance would not be able to keep their current plans, compared with 24% of Democrats, the poll data show.
Those looking for common ground can point to responses regarding a couple of other potential effects of a national health plan. Republicans (57%) and Democrats (52%) largely agreed that private health insurance companies would not be the primary way Americans get health coverage, and they agreed that people would continue to pay deductibles and copays when they used health care services (68% for Republicans, 71% for Democrats), Kaiser said.
The poll was conducted from May 30 to June 4, 2019, and involved responses from 1,206 adults. The margin of sampling error was plus or minus 3 percentage points.
Does Medicare-for-all mean that everyone gets health insurance coverage?

The public is not convinced that this would be the case, and Republicans are even more skeptical, according to a new survey from the Kaiser Family Foundation.
and there is a sizable split between Republicans and Democrats on the issue. Republicans, in fact, were more likely to say that all residents would not have coverage, with less than 49% of people identifying as Republican having answered “no” to the question and 45% having answered “yes”, Kaiser reported in its latest Health Tracking Poll.
Democrats were much more optimistic but not all were certain that all U.S. residents would have health insurance coverage. While 76% of Democrats responded yes when queried about whether all residents would get coverage, 21% answered no to the question. Responses to the question from independents – 61% said yes and 35% said no – very closely reflected the overall vote of 62% yes and 34% no, Kaiser said.
The partisan divide appeared again when respondents were asked if physicians and hospitals would be paid less under a national health plan: 64% of Republicans said payments would be reduced versus 42% of Democrats. Similarly, more than half of Republicans (53%) said that people who buy their own insurance would not be able to keep their current plans, compared with 24% of Democrats, the poll data show.
Those looking for common ground can point to responses regarding a couple of other potential effects of a national health plan. Republicans (57%) and Democrats (52%) largely agreed that private health insurance companies would not be the primary way Americans get health coverage, and they agreed that people would continue to pay deductibles and copays when they used health care services (68% for Republicans, 71% for Democrats), Kaiser said.
The poll was conducted from May 30 to June 4, 2019, and involved responses from 1,206 adults. The margin of sampling error was plus or minus 3 percentage points.
Case shows power of collaborative care for depression
Remission rate for Boeing employees climbed from 10% to 35%
SAN FRANCISCO – Under an accountable care contract with airplane maker Boeing, the University of Washington, Seattle, increased the rate of depression remission from about 10% to 35%, and the number of people in remission improved, based on Patient Health Questionnaire (PHQ-9) scores, from 20% to 70% – both in less than a year.
Boeing was particularly concerned about depression among its roughly 27,000 Puget Sound–area employees when it entered a contract with the University of Washington (UW) a few years ago for health services. Workers with depression are less likely to show up to work, and when they do, they are more likely to make mistakes and cause safety problems. To ensure that the university addressed the problem, Boeing tied payments to improved depression scores.
It didn’t take UW long to meet the PHQ-9 targets for improvement and remission, meaning a score below 5 points. Boeing also wanted its employees to be screened annually for depression and repeated testing of patients with depression to track how well they were doing. The university increased the number of patients rescreened within 8 weeks of their first PHQ-9 from about 45% to 75% – also in less than a year.
It simply scaled up the approach to meet Boeing’s targets.
“This has been an interesting journey,” said Jürgen Unützer, MD, MPH, who has been key to the efforts. “It’s required quite a bit of work, but it can be done. We’ve made a lot of progress,” he said at the American Psychiatric Association annual meeting.
Key components, besides the primary care provider, include evidence-based treatment, a mental health case manager, a system to track outcomes, and a psychiatrist to consult when patients do not improve. It’s a team approach.
Dr. Unützer and his colleagues have proved that it can work among older adults with depression and, in the end, save money (Am J Manag Care. 2008 Feb;14[2]:95-100). They’ve even published a how-to book, “Integrated Care: Creating Effective Mental and Primary Health Care Teams” (John Wiley & Sons, 2016).
A key challenge with Boeing was making sure that depressed patients returned for follow-up care and repeat PHQ-9s, and that they did not languish on ineffective treatments.
“We explain [to them that] this is not just a one-time thing,” said Dr. Unützer, chair of psychiatry and behavioral sciences at UW. “We [will] keep with them until they are well.”
Patients are enrolled in the patient portal on UW’s Epic records system to facilitate communication. The system sends out follow-up reminders, and sometimes it is used to send PHQ-9s directly to patients.
“We have automated this as much as possible.” When there’s no response, patients often are sent text messages or called by phone to make sure that they are doing OK and taking their medicine, he said.
Chart reviews are used to identify patients who are not improving. “We reach out to primary care and say, ‘We think you could use some help.’ It’s not always ”a comfortable conversation. “A lot of us like to assume our patients are getting better,” Dr. Unützer said.
Overall, “this notion of population-based care – the idea that ... you have a whole bucket of patients out there you might have seen at some point” but are still responsible for – “is a total change for most of us who are practicing clinicians,” he said.
Dr. Unützer did not report any disclosures.
Remission rate for Boeing employees climbed from 10% to 35%
Remission rate for Boeing employees climbed from 10% to 35%
SAN FRANCISCO – Under an accountable care contract with airplane maker Boeing, the University of Washington, Seattle, increased the rate of depression remission from about 10% to 35%, and the number of people in remission improved, based on Patient Health Questionnaire (PHQ-9) scores, from 20% to 70% – both in less than a year.
Boeing was particularly concerned about depression among its roughly 27,000 Puget Sound–area employees when it entered a contract with the University of Washington (UW) a few years ago for health services. Workers with depression are less likely to show up to work, and when they do, they are more likely to make mistakes and cause safety problems. To ensure that the university addressed the problem, Boeing tied payments to improved depression scores.
It didn’t take UW long to meet the PHQ-9 targets for improvement and remission, meaning a score below 5 points. Boeing also wanted its employees to be screened annually for depression and repeated testing of patients with depression to track how well they were doing. The university increased the number of patients rescreened within 8 weeks of their first PHQ-9 from about 45% to 75% – also in less than a year.
It simply scaled up the approach to meet Boeing’s targets.
“This has been an interesting journey,” said Jürgen Unützer, MD, MPH, who has been key to the efforts. “It’s required quite a bit of work, but it can be done. We’ve made a lot of progress,” he said at the American Psychiatric Association annual meeting.
Key components, besides the primary care provider, include evidence-based treatment, a mental health case manager, a system to track outcomes, and a psychiatrist to consult when patients do not improve. It’s a team approach.
Dr. Unützer and his colleagues have proved that it can work among older adults with depression and, in the end, save money (Am J Manag Care. 2008 Feb;14[2]:95-100). They’ve even published a how-to book, “Integrated Care: Creating Effective Mental and Primary Health Care Teams” (John Wiley & Sons, 2016).
A key challenge with Boeing was making sure that depressed patients returned for follow-up care and repeat PHQ-9s, and that they did not languish on ineffective treatments.
“We explain [to them that] this is not just a one-time thing,” said Dr. Unützer, chair of psychiatry and behavioral sciences at UW. “We [will] keep with them until they are well.”
Patients are enrolled in the patient portal on UW’s Epic records system to facilitate communication. The system sends out follow-up reminders, and sometimes it is used to send PHQ-9s directly to patients.
“We have automated this as much as possible.” When there’s no response, patients often are sent text messages or called by phone to make sure that they are doing OK and taking their medicine, he said.
Chart reviews are used to identify patients who are not improving. “We reach out to primary care and say, ‘We think you could use some help.’ It’s not always ”a comfortable conversation. “A lot of us like to assume our patients are getting better,” Dr. Unützer said.
Overall, “this notion of population-based care – the idea that ... you have a whole bucket of patients out there you might have seen at some point” but are still responsible for – “is a total change for most of us who are practicing clinicians,” he said.
Dr. Unützer did not report any disclosures.
SAN FRANCISCO – Under an accountable care contract with airplane maker Boeing, the University of Washington, Seattle, increased the rate of depression remission from about 10% to 35%, and the number of people in remission improved, based on Patient Health Questionnaire (PHQ-9) scores, from 20% to 70% – both in less than a year.
Boeing was particularly concerned about depression among its roughly 27,000 Puget Sound–area employees when it entered a contract with the University of Washington (UW) a few years ago for health services. Workers with depression are less likely to show up to work, and when they do, they are more likely to make mistakes and cause safety problems. To ensure that the university addressed the problem, Boeing tied payments to improved depression scores.
It didn’t take UW long to meet the PHQ-9 targets for improvement and remission, meaning a score below 5 points. Boeing also wanted its employees to be screened annually for depression and repeated testing of patients with depression to track how well they were doing. The university increased the number of patients rescreened within 8 weeks of their first PHQ-9 from about 45% to 75% – also in less than a year.
It simply scaled up the approach to meet Boeing’s targets.
“This has been an interesting journey,” said Jürgen Unützer, MD, MPH, who has been key to the efforts. “It’s required quite a bit of work, but it can be done. We’ve made a lot of progress,” he said at the American Psychiatric Association annual meeting.
Key components, besides the primary care provider, include evidence-based treatment, a mental health case manager, a system to track outcomes, and a psychiatrist to consult when patients do not improve. It’s a team approach.
Dr. Unützer and his colleagues have proved that it can work among older adults with depression and, in the end, save money (Am J Manag Care. 2008 Feb;14[2]:95-100). They’ve even published a how-to book, “Integrated Care: Creating Effective Mental and Primary Health Care Teams” (John Wiley & Sons, 2016).
A key challenge with Boeing was making sure that depressed patients returned for follow-up care and repeat PHQ-9s, and that they did not languish on ineffective treatments.
“We explain [to them that] this is not just a one-time thing,” said Dr. Unützer, chair of psychiatry and behavioral sciences at UW. “We [will] keep with them until they are well.”
Patients are enrolled in the patient portal on UW’s Epic records system to facilitate communication. The system sends out follow-up reminders, and sometimes it is used to send PHQ-9s directly to patients.
“We have automated this as much as possible.” When there’s no response, patients often are sent text messages or called by phone to make sure that they are doing OK and taking their medicine, he said.
Chart reviews are used to identify patients who are not improving. “We reach out to primary care and say, ‘We think you could use some help.’ It’s not always ”a comfortable conversation. “A lot of us like to assume our patients are getting better,” Dr. Unützer said.
Overall, “this notion of population-based care – the idea that ... you have a whole bucket of patients out there you might have seen at some point” but are still responsible for – “is a total change for most of us who are practicing clinicians,” he said.
Dr. Unützer did not report any disclosures.
REPORTING FROM APA 2019
No Pip/Tazo for patients with ESBL blood stream infections
Background: ESBL-producing gram-negative bacilli are becoming increasingly common. Carbapenems are considered the treatment of choice for these infections, but they may in turn select for carbapenem-resistant gram-negative bacilli.
Study design: Open-label, noninferiority, randomized clinical trial.
Setting: Adult inpatients from nine countries (not including the United States).
Synopsis: Patients with at least one positive blood culture for ESBL E. coli or K. pneumoniae were screened. Of the initial 1,646 patients assessed, only 391 were enrolled (866 met exclusion criteria, 218 patients declined, and 123 treating physicians declined). Patients were randomized within 72 hours of the positive blood culture collection to either piperacillin/tazobactam 4.5 g every 6 hours or meropenem 1 g every 8 hours. Patients were treated from 4 to 14 days, with the total duration of antibiotics left up to the treating physician.
The primary outcome was all-cause mortality at 30 days after randomization. The study was stopped early because of a significant mortality difference between the two groups (12.3% in the piperacillin/tazobactam group versus 3.7% in the meropenem group).
The overall mortality rate was lower than expected. The sickest patients may have been excluded because the treating physician needed to approve enrollment. Because of the necessity for empiric antibiotic therapy, there was substantial crossover in antibiotics between the groups, although this would have biased the study toward noninferiority.
Bottom line: For patients with ESBL E. coli or K. pneumoniae blood stream infections, treatment with piperacillin/tazobactam was inferior to meropenem for 30-day mortality.
Citation: Harris PNA et al. Effect of piperacillin-tazobactam vs meropenem on 30-day mortality for patients with E coli or Klebsiella pneumoniae bloodstream infection and ceftriaxone resistance: A randomized clinical trial. JAMA. 2018;320(10):984-94.
Dr. Gabriel is assistant professor of medicine and director of Preoperative Medicine and Medicine Consult Service in the division of hospital medicine at Mount Sinai Hospital, New York.
Background: ESBL-producing gram-negative bacilli are becoming increasingly common. Carbapenems are considered the treatment of choice for these infections, but they may in turn select for carbapenem-resistant gram-negative bacilli.
Study design: Open-label, noninferiority, randomized clinical trial.
Setting: Adult inpatients from nine countries (not including the United States).
Synopsis: Patients with at least one positive blood culture for ESBL E. coli or K. pneumoniae were screened. Of the initial 1,646 patients assessed, only 391 were enrolled (866 met exclusion criteria, 218 patients declined, and 123 treating physicians declined). Patients were randomized within 72 hours of the positive blood culture collection to either piperacillin/tazobactam 4.5 g every 6 hours or meropenem 1 g every 8 hours. Patients were treated from 4 to 14 days, with the total duration of antibiotics left up to the treating physician.
The primary outcome was all-cause mortality at 30 days after randomization. The study was stopped early because of a significant mortality difference between the two groups (12.3% in the piperacillin/tazobactam group versus 3.7% in the meropenem group).
The overall mortality rate was lower than expected. The sickest patients may have been excluded because the treating physician needed to approve enrollment. Because of the necessity for empiric antibiotic therapy, there was substantial crossover in antibiotics between the groups, although this would have biased the study toward noninferiority.
Bottom line: For patients with ESBL E. coli or K. pneumoniae blood stream infections, treatment with piperacillin/tazobactam was inferior to meropenem for 30-day mortality.
Citation: Harris PNA et al. Effect of piperacillin-tazobactam vs meropenem on 30-day mortality for patients with E coli or Klebsiella pneumoniae bloodstream infection and ceftriaxone resistance: A randomized clinical trial. JAMA. 2018;320(10):984-94.
Dr. Gabriel is assistant professor of medicine and director of Preoperative Medicine and Medicine Consult Service in the division of hospital medicine at Mount Sinai Hospital, New York.
Background: ESBL-producing gram-negative bacilli are becoming increasingly common. Carbapenems are considered the treatment of choice for these infections, but they may in turn select for carbapenem-resistant gram-negative bacilli.
Study design: Open-label, noninferiority, randomized clinical trial.
Setting: Adult inpatients from nine countries (not including the United States).
Synopsis: Patients with at least one positive blood culture for ESBL E. coli or K. pneumoniae were screened. Of the initial 1,646 patients assessed, only 391 were enrolled (866 met exclusion criteria, 218 patients declined, and 123 treating physicians declined). Patients were randomized within 72 hours of the positive blood culture collection to either piperacillin/tazobactam 4.5 g every 6 hours or meropenem 1 g every 8 hours. Patients were treated from 4 to 14 days, with the total duration of antibiotics left up to the treating physician.
The primary outcome was all-cause mortality at 30 days after randomization. The study was stopped early because of a significant mortality difference between the two groups (12.3% in the piperacillin/tazobactam group versus 3.7% in the meropenem group).
The overall mortality rate was lower than expected. The sickest patients may have been excluded because the treating physician needed to approve enrollment. Because of the necessity for empiric antibiotic therapy, there was substantial crossover in antibiotics between the groups, although this would have biased the study toward noninferiority.
Bottom line: For patients with ESBL E. coli or K. pneumoniae blood stream infections, treatment with piperacillin/tazobactam was inferior to meropenem for 30-day mortality.
Citation: Harris PNA et al. Effect of piperacillin-tazobactam vs meropenem on 30-day mortality for patients with E coli or Klebsiella pneumoniae bloodstream infection and ceftriaxone resistance: A randomized clinical trial. JAMA. 2018;320(10):984-94.
Dr. Gabriel is assistant professor of medicine and director of Preoperative Medicine and Medicine Consult Service in the division of hospital medicine at Mount Sinai Hospital, New York.
Antimalarial may be effective, safe for erosive oral lichen planus
MILAN – an investigator reported at the World Congress of Dermatology.
In a small retrospective study, 85% of patients treated with the oral antimalarial agent had marked improvement or full remission, according to Ziyad Khamaysi, MD, a dermatologist at Rambam Health Care Campus, Haifa, Israel. Adverse effects leading to discontinuation occurred in a minority of patients and included elevated kidney function tests, hyperpigmentation, and an abnormal eye exam, he said.
“It may be a useful and convenient alternative treatment either as a monotherapy or where a rapid symptomatic relief during periods of exacerbations is needed,” he said in an oral presentation at the meeting.
A variety of medications have been used for palliation of oral lichen planus, including corticosteroids, cyclosporine, calcineurin inhibitors, retinoids, and biologics, but few have been evaluated in larger series of patients, he pointed out.
In the retrospective, nonrandomized study, 15 women and 6 men with erosive, recalcitrant oral lichen planus were treated with hydroxychloroquine (Plaquenil) at a dose of 200 mg/day, which was increased to 400 mg/day at one month. The mean age of the patients was 55 years.
In one patient, treatment was stopped after a month because of side effects, Dr. Khamaysi said. Among the remaining patients, 5 (25%) had a complete remission, while 12 (60%) had moderate to marked improvement, and 3 (15%) had no improvement, he said. In the patients who did respond, improvement was noted within 2-4 months of treatment initiation, he added. “Hydroxychloroquine kept the disease under control, with either full remission or marked improvement as long as the patients took it.”
Treatment appeared to be more effective in male patients and those under age 65 years, the investigator commented.
These data corroborate the findings of a smaller study evaluating hydroxychloroquine for oral lichen planus, published in 1993, according to Dr. Khamaysi. In that report, 9 of 10 patients had an “excellent” response to treatment, according to the investigator (J Am Acad Dermatol. 1993 Apr;28[4]:609-12).
Of the 10 patients in that study, 6 had erosions at the start of treatment, and 3 of these patients had complete healing with 3-6 months of therapy, and the other 3 had reepithelialization and at least a 50% reduction in lesion size, according to the report.
Dr. Khamaysi had no disclosures relevant to his presentation.
MILAN – an investigator reported at the World Congress of Dermatology.
In a small retrospective study, 85% of patients treated with the oral antimalarial agent had marked improvement or full remission, according to Ziyad Khamaysi, MD, a dermatologist at Rambam Health Care Campus, Haifa, Israel. Adverse effects leading to discontinuation occurred in a minority of patients and included elevated kidney function tests, hyperpigmentation, and an abnormal eye exam, he said.
“It may be a useful and convenient alternative treatment either as a monotherapy or where a rapid symptomatic relief during periods of exacerbations is needed,” he said in an oral presentation at the meeting.
A variety of medications have been used for palliation of oral lichen planus, including corticosteroids, cyclosporine, calcineurin inhibitors, retinoids, and biologics, but few have been evaluated in larger series of patients, he pointed out.
In the retrospective, nonrandomized study, 15 women and 6 men with erosive, recalcitrant oral lichen planus were treated with hydroxychloroquine (Plaquenil) at a dose of 200 mg/day, which was increased to 400 mg/day at one month. The mean age of the patients was 55 years.
In one patient, treatment was stopped after a month because of side effects, Dr. Khamaysi said. Among the remaining patients, 5 (25%) had a complete remission, while 12 (60%) had moderate to marked improvement, and 3 (15%) had no improvement, he said. In the patients who did respond, improvement was noted within 2-4 months of treatment initiation, he added. “Hydroxychloroquine kept the disease under control, with either full remission or marked improvement as long as the patients took it.”
Treatment appeared to be more effective in male patients and those under age 65 years, the investigator commented.
These data corroborate the findings of a smaller study evaluating hydroxychloroquine for oral lichen planus, published in 1993, according to Dr. Khamaysi. In that report, 9 of 10 patients had an “excellent” response to treatment, according to the investigator (J Am Acad Dermatol. 1993 Apr;28[4]:609-12).
Of the 10 patients in that study, 6 had erosions at the start of treatment, and 3 of these patients had complete healing with 3-6 months of therapy, and the other 3 had reepithelialization and at least a 50% reduction in lesion size, according to the report.
Dr. Khamaysi had no disclosures relevant to his presentation.
MILAN – an investigator reported at the World Congress of Dermatology.
In a small retrospective study, 85% of patients treated with the oral antimalarial agent had marked improvement or full remission, according to Ziyad Khamaysi, MD, a dermatologist at Rambam Health Care Campus, Haifa, Israel. Adverse effects leading to discontinuation occurred in a minority of patients and included elevated kidney function tests, hyperpigmentation, and an abnormal eye exam, he said.
“It may be a useful and convenient alternative treatment either as a monotherapy or where a rapid symptomatic relief during periods of exacerbations is needed,” he said in an oral presentation at the meeting.
A variety of medications have been used for palliation of oral lichen planus, including corticosteroids, cyclosporine, calcineurin inhibitors, retinoids, and biologics, but few have been evaluated in larger series of patients, he pointed out.
In the retrospective, nonrandomized study, 15 women and 6 men with erosive, recalcitrant oral lichen planus were treated with hydroxychloroquine (Plaquenil) at a dose of 200 mg/day, which was increased to 400 mg/day at one month. The mean age of the patients was 55 years.
In one patient, treatment was stopped after a month because of side effects, Dr. Khamaysi said. Among the remaining patients, 5 (25%) had a complete remission, while 12 (60%) had moderate to marked improvement, and 3 (15%) had no improvement, he said. In the patients who did respond, improvement was noted within 2-4 months of treatment initiation, he added. “Hydroxychloroquine kept the disease under control, with either full remission or marked improvement as long as the patients took it.”
Treatment appeared to be more effective in male patients and those under age 65 years, the investigator commented.
These data corroborate the findings of a smaller study evaluating hydroxychloroquine for oral lichen planus, published in 1993, according to Dr. Khamaysi. In that report, 9 of 10 patients had an “excellent” response to treatment, according to the investigator (J Am Acad Dermatol. 1993 Apr;28[4]:609-12).
Of the 10 patients in that study, 6 had erosions at the start of treatment, and 3 of these patients had complete healing with 3-6 months of therapy, and the other 3 had reepithelialization and at least a 50% reduction in lesion size, according to the report.
Dr. Khamaysi had no disclosures relevant to his presentation.
REPORTING FROM WCD2019
AGA Editorial Fellowship: Three lasting lessons
As a first-year gastroenterology fellow, banding my first patient with a variceal bleed was an exciting – but also stress-provoking – event. What if I banded incorrectly and caused more bleeding? With a successful band, a patient’s hemorrhagic shock is now controlled, hemodynamics improved, and euphoria takes over. Now, in my third year of a gastroenterology fellowship but my first year of the American Gastroenterological Association (AGA) Editorial Fellowship, preparing to present the first manuscript that I handled to the Board of Editors at our weekly meeting has now induced the same excitement and need for the same level of dedication. Have I researched the foundational literature that this current manuscript was built on? What is the trajectory of this research and will this project be interesting to our readers and lead to breakthroughs in the field?
Gastroenterology is the premier flagship journal of the AGA and, in this Editorial Fellowship, I was selected to spend a fully immersive 1-year experience working on all aspects of this journal. In its second year of inception, I echo Dr. Eric Shah’s insight into the transformative and immersive nature of this fellowship.1 In this role, I have made three developments, and each one has left me with a valuable lesson.
Mentorship
My first development was as a direct mentee under the leadership of the two editors in chief Richard Peek, MD, and Douglas Corley, MD, and associate editor John Inadomi, MD. In this role, I reviewed submitted manuscripts regarding outcome data of oncologic studies in the fields of colon, esophageal, and gastric cancer. I served as a reviewer for submitted manuscripts and discussed the impact, novelty, and decision for publication with the Board of Editors. In our weekly meetings, the associate editors discussed manuscripts that needed further review prior to acceptance, revision, or rejection. A few themes underpinned the discussion of these manuscripts:
- Is this science reproducible and is there scientific rigor for study design, validity, and analysis?
- How does this manuscript add to the current state of the literature?
- What is the trajectory of this research field?
- How will this manuscript lead to breakthroughs in this field?
- Are the advancements in this manuscript likely to lead to paradigm shifts in the field in its approach, design, or findings?
I also was fortunate to meet leaders in the field, including working daily in person with multiple members of the Board of Editors at Vanderbilt University Medical Center, Nashville, Tenn., as well as visiting professors, including Dr. Corley, Linda Rabeneck, MD, and T. Jake Liang, MD, who not only spoke on their scientific inquiries but also about their transitional path from gastroenterology fellows to pioneers in their respective fields. From these lessons, I have learned the scientific rigor of manuscript review for Gastroenterology and how to approach modern challenges in our field to directly improve patient care.
AGA’s commitment to early-career investigators
The Editorial Fellowship allowed me to expand a traditional third-year gastroenterology fellowship to dive deep into the intense path to get a manuscript published in Gastroenterology. Whereas 1 year prior, I had found dilating a complete esophageal stricture difficult, I now found myself learning to master clinical trial design, applying modern techniques of artificial intelligence, understanding organoid development, and navigating the impact of the microbiome. I was fortunate to be selected for Vanderbilt’s Master’s in Science in Clinical Investigation, which allowed me to apply my education not only to my own research but also to synergistically understand and deconstruct new submissions ranging from modern statistics with Bayesian modeling to analysis of large genetic data. All of this was built in the supportive framework of my mentoring committee.
As a fellow, I am inspired to see the multicenter, international collaboration to answer important questions in our field. Leveraging large databases and the expertise of multiple investigators, breakthroughs were made because of the collaborative nature of the science. This also was felt in the review process, where experts generously reviewed manuscripts to enhance the quality of the submission in order to advance knowledge in the field. Reading hundreds of these reviews this year has allowed me to refocus my current research studies and improve the way I write my current reviews. In the spirit of reproducible science and challenging the precision of study design, I was impressed by the time, effort, and dedication reviewers from our field spent to help improve the literature. Dr. Peek and Dr. Corley, our editors in chief, committed their time in discussing my innovations and critiques and displayed their level of interest in the opinions of early-career investigators and fostering the next generation of scientists and practitioners. In this lesson, I was invigorated by the depth of AGA opportunities for fellows and junior faculty in education, research, and involvement.
Self-reflection
Having the honor and privilege to review manuscripts upon submission also increased my critical view of my current practices. I now question the level of evidence for which current patient care practices are based, which allows me to better understand the research areas that need increased attention to improve the quality of our guidelines and evidence. For motivated fellows interested in a path of academic medicine, I would strongly advise applying for this prestigious fellowship. In no other training process could I have learned such a breadth of scientific skills and directly apply them to my patient care, my research, and my role as an educator. Furthermore, I was able to contribute to the reviewing and editing process, which allowed me to directly contribute to the field at an early stage of my career. In this final lesson, I exit this impactful Editorial Fellowship in self-reflection. I leave this fellowship humbled – by you – the reader who continues to learn to improve your patient care, the scientist as she works tirelessly to answer questions and contribute to the literature, the gastroenterology community for their willingness to teach and mentor fellows and early-career investigators and practitioners, and the patients who remind us that we all have a shared mission to advance scientific knowledge to improve patient care.
Dr. Naik is a gastroenterology fellow in the department of gastroenterology and hepatology at Vanderbilt University Medical Center in Nashville, Tenn.
Reference
1. Shah ED. Skills acquired during my 1-year AGA Editorial Fellowship. Gastroenterology. 2018;154(6):1563.
As a first-year gastroenterology fellow, banding my first patient with a variceal bleed was an exciting – but also stress-provoking – event. What if I banded incorrectly and caused more bleeding? With a successful band, a patient’s hemorrhagic shock is now controlled, hemodynamics improved, and euphoria takes over. Now, in my third year of a gastroenterology fellowship but my first year of the American Gastroenterological Association (AGA) Editorial Fellowship, preparing to present the first manuscript that I handled to the Board of Editors at our weekly meeting has now induced the same excitement and need for the same level of dedication. Have I researched the foundational literature that this current manuscript was built on? What is the trajectory of this research and will this project be interesting to our readers and lead to breakthroughs in the field?
Gastroenterology is the premier flagship journal of the AGA and, in this Editorial Fellowship, I was selected to spend a fully immersive 1-year experience working on all aspects of this journal. In its second year of inception, I echo Dr. Eric Shah’s insight into the transformative and immersive nature of this fellowship.1 In this role, I have made three developments, and each one has left me with a valuable lesson.
Mentorship
My first development was as a direct mentee under the leadership of the two editors in chief Richard Peek, MD, and Douglas Corley, MD, and associate editor John Inadomi, MD. In this role, I reviewed submitted manuscripts regarding outcome data of oncologic studies in the fields of colon, esophageal, and gastric cancer. I served as a reviewer for submitted manuscripts and discussed the impact, novelty, and decision for publication with the Board of Editors. In our weekly meetings, the associate editors discussed manuscripts that needed further review prior to acceptance, revision, or rejection. A few themes underpinned the discussion of these manuscripts:
- Is this science reproducible and is there scientific rigor for study design, validity, and analysis?
- How does this manuscript add to the current state of the literature?
- What is the trajectory of this research field?
- How will this manuscript lead to breakthroughs in this field?
- Are the advancements in this manuscript likely to lead to paradigm shifts in the field in its approach, design, or findings?
I also was fortunate to meet leaders in the field, including working daily in person with multiple members of the Board of Editors at Vanderbilt University Medical Center, Nashville, Tenn., as well as visiting professors, including Dr. Corley, Linda Rabeneck, MD, and T. Jake Liang, MD, who not only spoke on their scientific inquiries but also about their transitional path from gastroenterology fellows to pioneers in their respective fields. From these lessons, I have learned the scientific rigor of manuscript review for Gastroenterology and how to approach modern challenges in our field to directly improve patient care.
AGA’s commitment to early-career investigators
The Editorial Fellowship allowed me to expand a traditional third-year gastroenterology fellowship to dive deep into the intense path to get a manuscript published in Gastroenterology. Whereas 1 year prior, I had found dilating a complete esophageal stricture difficult, I now found myself learning to master clinical trial design, applying modern techniques of artificial intelligence, understanding organoid development, and navigating the impact of the microbiome. I was fortunate to be selected for Vanderbilt’s Master’s in Science in Clinical Investigation, which allowed me to apply my education not only to my own research but also to synergistically understand and deconstruct new submissions ranging from modern statistics with Bayesian modeling to analysis of large genetic data. All of this was built in the supportive framework of my mentoring committee.
As a fellow, I am inspired to see the multicenter, international collaboration to answer important questions in our field. Leveraging large databases and the expertise of multiple investigators, breakthroughs were made because of the collaborative nature of the science. This also was felt in the review process, where experts generously reviewed manuscripts to enhance the quality of the submission in order to advance knowledge in the field. Reading hundreds of these reviews this year has allowed me to refocus my current research studies and improve the way I write my current reviews. In the spirit of reproducible science and challenging the precision of study design, I was impressed by the time, effort, and dedication reviewers from our field spent to help improve the literature. Dr. Peek and Dr. Corley, our editors in chief, committed their time in discussing my innovations and critiques and displayed their level of interest in the opinions of early-career investigators and fostering the next generation of scientists and practitioners. In this lesson, I was invigorated by the depth of AGA opportunities for fellows and junior faculty in education, research, and involvement.
Self-reflection
Having the honor and privilege to review manuscripts upon submission also increased my critical view of my current practices. I now question the level of evidence for which current patient care practices are based, which allows me to better understand the research areas that need increased attention to improve the quality of our guidelines and evidence. For motivated fellows interested in a path of academic medicine, I would strongly advise applying for this prestigious fellowship. In no other training process could I have learned such a breadth of scientific skills and directly apply them to my patient care, my research, and my role as an educator. Furthermore, I was able to contribute to the reviewing and editing process, which allowed me to directly contribute to the field at an early stage of my career. In this final lesson, I exit this impactful Editorial Fellowship in self-reflection. I leave this fellowship humbled – by you – the reader who continues to learn to improve your patient care, the scientist as she works tirelessly to answer questions and contribute to the literature, the gastroenterology community for their willingness to teach and mentor fellows and early-career investigators and practitioners, and the patients who remind us that we all have a shared mission to advance scientific knowledge to improve patient care.
Dr. Naik is a gastroenterology fellow in the department of gastroenterology and hepatology at Vanderbilt University Medical Center in Nashville, Tenn.
Reference
1. Shah ED. Skills acquired during my 1-year AGA Editorial Fellowship. Gastroenterology. 2018;154(6):1563.
As a first-year gastroenterology fellow, banding my first patient with a variceal bleed was an exciting – but also stress-provoking – event. What if I banded incorrectly and caused more bleeding? With a successful band, a patient’s hemorrhagic shock is now controlled, hemodynamics improved, and euphoria takes over. Now, in my third year of a gastroenterology fellowship but my first year of the American Gastroenterological Association (AGA) Editorial Fellowship, preparing to present the first manuscript that I handled to the Board of Editors at our weekly meeting has now induced the same excitement and need for the same level of dedication. Have I researched the foundational literature that this current manuscript was built on? What is the trajectory of this research and will this project be interesting to our readers and lead to breakthroughs in the field?
Gastroenterology is the premier flagship journal of the AGA and, in this Editorial Fellowship, I was selected to spend a fully immersive 1-year experience working on all aspects of this journal. In its second year of inception, I echo Dr. Eric Shah’s insight into the transformative and immersive nature of this fellowship.1 In this role, I have made three developments, and each one has left me with a valuable lesson.
Mentorship
My first development was as a direct mentee under the leadership of the two editors in chief Richard Peek, MD, and Douglas Corley, MD, and associate editor John Inadomi, MD. In this role, I reviewed submitted manuscripts regarding outcome data of oncologic studies in the fields of colon, esophageal, and gastric cancer. I served as a reviewer for submitted manuscripts and discussed the impact, novelty, and decision for publication with the Board of Editors. In our weekly meetings, the associate editors discussed manuscripts that needed further review prior to acceptance, revision, or rejection. A few themes underpinned the discussion of these manuscripts:
- Is this science reproducible and is there scientific rigor for study design, validity, and analysis?
- How does this manuscript add to the current state of the literature?
- What is the trajectory of this research field?
- How will this manuscript lead to breakthroughs in this field?
- Are the advancements in this manuscript likely to lead to paradigm shifts in the field in its approach, design, or findings?
I also was fortunate to meet leaders in the field, including working daily in person with multiple members of the Board of Editors at Vanderbilt University Medical Center, Nashville, Tenn., as well as visiting professors, including Dr. Corley, Linda Rabeneck, MD, and T. Jake Liang, MD, who not only spoke on their scientific inquiries but also about their transitional path from gastroenterology fellows to pioneers in their respective fields. From these lessons, I have learned the scientific rigor of manuscript review for Gastroenterology and how to approach modern challenges in our field to directly improve patient care.
AGA’s commitment to early-career investigators
The Editorial Fellowship allowed me to expand a traditional third-year gastroenterology fellowship to dive deep into the intense path to get a manuscript published in Gastroenterology. Whereas 1 year prior, I had found dilating a complete esophageal stricture difficult, I now found myself learning to master clinical trial design, applying modern techniques of artificial intelligence, understanding organoid development, and navigating the impact of the microbiome. I was fortunate to be selected for Vanderbilt’s Master’s in Science in Clinical Investigation, which allowed me to apply my education not only to my own research but also to synergistically understand and deconstruct new submissions ranging from modern statistics with Bayesian modeling to analysis of large genetic data. All of this was built in the supportive framework of my mentoring committee.
As a fellow, I am inspired to see the multicenter, international collaboration to answer important questions in our field. Leveraging large databases and the expertise of multiple investigators, breakthroughs were made because of the collaborative nature of the science. This also was felt in the review process, where experts generously reviewed manuscripts to enhance the quality of the submission in order to advance knowledge in the field. Reading hundreds of these reviews this year has allowed me to refocus my current research studies and improve the way I write my current reviews. In the spirit of reproducible science and challenging the precision of study design, I was impressed by the time, effort, and dedication reviewers from our field spent to help improve the literature. Dr. Peek and Dr. Corley, our editors in chief, committed their time in discussing my innovations and critiques and displayed their level of interest in the opinions of early-career investigators and fostering the next generation of scientists and practitioners. In this lesson, I was invigorated by the depth of AGA opportunities for fellows and junior faculty in education, research, and involvement.
Self-reflection
Having the honor and privilege to review manuscripts upon submission also increased my critical view of my current practices. I now question the level of evidence for which current patient care practices are based, which allows me to better understand the research areas that need increased attention to improve the quality of our guidelines and evidence. For motivated fellows interested in a path of academic medicine, I would strongly advise applying for this prestigious fellowship. In no other training process could I have learned such a breadth of scientific skills and directly apply them to my patient care, my research, and my role as an educator. Furthermore, I was able to contribute to the reviewing and editing process, which allowed me to directly contribute to the field at an early stage of my career. In this final lesson, I exit this impactful Editorial Fellowship in self-reflection. I leave this fellowship humbled – by you – the reader who continues to learn to improve your patient care, the scientist as she works tirelessly to answer questions and contribute to the literature, the gastroenterology community for their willingness to teach and mentor fellows and early-career investigators and practitioners, and the patients who remind us that we all have a shared mission to advance scientific knowledge to improve patient care.
Dr. Naik is a gastroenterology fellow in the department of gastroenterology and hepatology at Vanderbilt University Medical Center in Nashville, Tenn.
Reference
1. Shah ED. Skills acquired during my 1-year AGA Editorial Fellowship. Gastroenterology. 2018;154(6):1563.
Estate planning: A must-do for all medical professionals
As medical professionals, you may have encountered patients with serious illnesses and asked yourself the following questions: What if I was in that situation? Where will my assets go when I die? What will happen to my loved ones, and will they be taken care of? Who would handle my affairs if I became ill? These important questions can only be addressed through effective estate planning.
Everyone needs an estate plan regardless of age, health, and financial or family situation. An effective estate plan provides for the orderly management and disposition of your assets upon your death. In addition, as medical professionals may appreciate, an effective estate plan appoints individuals to manage your financial affairs and make health care decisions for you in the event that you become physically or mentally incapacitated.
The most common estate planning tool is a will, which dictates how your assets pass at death. In addition, a will identifies the personal representative of your estate (that is, the person who will see that your assets pass in accordance with your wishes) and, in many states, identifies the guardian of any minor children. Although a court will make the ultimate determination of who is appointed as the guardian, courts typically give significant weight to the person named in a will.
If you die “intestate,” meaning that you died without a valid will, your assets will be distributed in accordance with your state’s intestacy statutes, and any interested person (as opposed to the individual of your choice) may be appointed as the personal representative of your estate. Therefore, to ensure that your property goes to the individuals of your choice and that your final affairs are handled by the person you trust, a will is essential.
In many states, a revocable living trust can be equally beneficial. Like a will, a revocable living trust will dictate how your property passes at death and appoints a trustee to see that the property is distributed in accordance with your wishes. Revocable living trusts can be great tools for incapacity planning and, unlike a will, are not required to be recorded, so the trust agreement can remain private. The assets that are held in a revocable living trust also avoid the often lengthy and expensive probate process, which generally includes the preparation and filing of a petition to open the estate, an inventory identifying the assets of the estate, and an accounting that details all assets received and distributed, followed by the payment of fees based upon the value of the probate estate.
In many situations, leaving assets to young, disabled, or troubled children would result in catastrophic consequences, such as disqualification for government benefits, dissipation of assets for inappropriate uses, or attachment by creditors. Further, for wealthy individuals, outright distributions to spouses could lead to unnecessary estate tax. Wills and revocable trusts can protect against these issues by requiring that, at death, the decedent’s assets are held in further trust for these individuals.
There are various types of trusts that can help ensure that your assets are used for the benefit of your loved one while avoiding any unintended consequences, some of which include the following:
- Special needs trusts, which allow the trustee to use the trust funds for the benefit of the disabled beneficiary without disqualifying the beneficiary from important government benefits.
- Spendthrift trusts, which can protect the trust assets from claims of creditors or property division in a divorce action.
- Marital trusts, which can be used to reduce taxes and ensure that property will be distributed pursuant to your wishes upon the death of your spouse.
- Dynasty trusts, which can be used to protect assets for many generations and, in doing so, reduce the amount of federal and state transfer taxes.
Whether you use a will or revocable living trust, it is critical to coordinate the beneficiary designations of assets such as retirement accounts and life insurance policies, as well as any other account that passes by beneficiary designation. These beneficiary designations trump the provisions of your will and revocable living trust. Likewise, property owned jointly with another person as joint tenants with the right of survivorship, or with a spouse as tenants by the entirety, will pass directly to the joint owner and not pursuant to the terms of your will or revocable trust.
An effective estate plan involves not just planning for death, but also for your incapacity. A durable power of attorney allows you to select an agent or agents to manage your property during your lifetime. The power of attorney can become effective immediately so that the agent can act on your behalf upon execution of the document or the power of attorney can become effective only if and when you become incapacitated.
A durable power of attorney for health care (or advance health care directive) permits you to appoint an agent to make health care decisions on your behalf in the event that you cannot make your own decisions. In addition, should you become permanently unconscious or in a terminal condition, it permits you to appoint an agent who can withhold or withdraw life-sustaining treatment. With a living will, you can express in writing the circumstances under which you do or do not want artificial life-sustaining measures.
With respect to these powers of attorney, the persons that you appoint as your agents should be people that you trust. It is also important to have conversations with your designated agents to ensure that they understand their responsibilities and your wishes. Without these powers of attorney, in the event of your incapacitation, a court will appoint a guardian. The guardian may not be the person you would have appointed, and it will result in annual, and burdensome, court filings.
As busy medical professionals, it may be difficult to find time to develop an estate plan and you may believe that there is plenty of time to do it in the future. It is important to begin thinking about your estate-planning goals and to speak with an attorney to help develop and draft your estate-planning documents. If you already have estate-planning documents, it is important to review those documents periodically to ensure that your estate-planning objectives have remained the same and, if they have changed, to update your documents.
No one knows what the future will hold, so it is important to consult with a local attorney to establish or review your estate plan now. If you do, you will be comforted by the fact that you and your loved ones will be taken care of in accordance with your wishes if you are unable to do so in the future.
Mr. D’Emilio is a managing member and Mr. Riley is an associate at McCollom D’Emilio Smith Uebler, Wilmington, Del.
As medical professionals, you may have encountered patients with serious illnesses and asked yourself the following questions: What if I was in that situation? Where will my assets go when I die? What will happen to my loved ones, and will they be taken care of? Who would handle my affairs if I became ill? These important questions can only be addressed through effective estate planning.
Everyone needs an estate plan regardless of age, health, and financial or family situation. An effective estate plan provides for the orderly management and disposition of your assets upon your death. In addition, as medical professionals may appreciate, an effective estate plan appoints individuals to manage your financial affairs and make health care decisions for you in the event that you become physically or mentally incapacitated.
The most common estate planning tool is a will, which dictates how your assets pass at death. In addition, a will identifies the personal representative of your estate (that is, the person who will see that your assets pass in accordance with your wishes) and, in many states, identifies the guardian of any minor children. Although a court will make the ultimate determination of who is appointed as the guardian, courts typically give significant weight to the person named in a will.
If you die “intestate,” meaning that you died without a valid will, your assets will be distributed in accordance with your state’s intestacy statutes, and any interested person (as opposed to the individual of your choice) may be appointed as the personal representative of your estate. Therefore, to ensure that your property goes to the individuals of your choice and that your final affairs are handled by the person you trust, a will is essential.
In many states, a revocable living trust can be equally beneficial. Like a will, a revocable living trust will dictate how your property passes at death and appoints a trustee to see that the property is distributed in accordance with your wishes. Revocable living trusts can be great tools for incapacity planning and, unlike a will, are not required to be recorded, so the trust agreement can remain private. The assets that are held in a revocable living trust also avoid the often lengthy and expensive probate process, which generally includes the preparation and filing of a petition to open the estate, an inventory identifying the assets of the estate, and an accounting that details all assets received and distributed, followed by the payment of fees based upon the value of the probate estate.
In many situations, leaving assets to young, disabled, or troubled children would result in catastrophic consequences, such as disqualification for government benefits, dissipation of assets for inappropriate uses, or attachment by creditors. Further, for wealthy individuals, outright distributions to spouses could lead to unnecessary estate tax. Wills and revocable trusts can protect against these issues by requiring that, at death, the decedent’s assets are held in further trust for these individuals.
There are various types of trusts that can help ensure that your assets are used for the benefit of your loved one while avoiding any unintended consequences, some of which include the following:
- Special needs trusts, which allow the trustee to use the trust funds for the benefit of the disabled beneficiary without disqualifying the beneficiary from important government benefits.
- Spendthrift trusts, which can protect the trust assets from claims of creditors or property division in a divorce action.
- Marital trusts, which can be used to reduce taxes and ensure that property will be distributed pursuant to your wishes upon the death of your spouse.
- Dynasty trusts, which can be used to protect assets for many generations and, in doing so, reduce the amount of federal and state transfer taxes.
Whether you use a will or revocable living trust, it is critical to coordinate the beneficiary designations of assets such as retirement accounts and life insurance policies, as well as any other account that passes by beneficiary designation. These beneficiary designations trump the provisions of your will and revocable living trust. Likewise, property owned jointly with another person as joint tenants with the right of survivorship, or with a spouse as tenants by the entirety, will pass directly to the joint owner and not pursuant to the terms of your will or revocable trust.
An effective estate plan involves not just planning for death, but also for your incapacity. A durable power of attorney allows you to select an agent or agents to manage your property during your lifetime. The power of attorney can become effective immediately so that the agent can act on your behalf upon execution of the document or the power of attorney can become effective only if and when you become incapacitated.
A durable power of attorney for health care (or advance health care directive) permits you to appoint an agent to make health care decisions on your behalf in the event that you cannot make your own decisions. In addition, should you become permanently unconscious or in a terminal condition, it permits you to appoint an agent who can withhold or withdraw life-sustaining treatment. With a living will, you can express in writing the circumstances under which you do or do not want artificial life-sustaining measures.
With respect to these powers of attorney, the persons that you appoint as your agents should be people that you trust. It is also important to have conversations with your designated agents to ensure that they understand their responsibilities and your wishes. Without these powers of attorney, in the event of your incapacitation, a court will appoint a guardian. The guardian may not be the person you would have appointed, and it will result in annual, and burdensome, court filings.
As busy medical professionals, it may be difficult to find time to develop an estate plan and you may believe that there is plenty of time to do it in the future. It is important to begin thinking about your estate-planning goals and to speak with an attorney to help develop and draft your estate-planning documents. If you already have estate-planning documents, it is important to review those documents periodically to ensure that your estate-planning objectives have remained the same and, if they have changed, to update your documents.
No one knows what the future will hold, so it is important to consult with a local attorney to establish or review your estate plan now. If you do, you will be comforted by the fact that you and your loved ones will be taken care of in accordance with your wishes if you are unable to do so in the future.
Mr. D’Emilio is a managing member and Mr. Riley is an associate at McCollom D’Emilio Smith Uebler, Wilmington, Del.
As medical professionals, you may have encountered patients with serious illnesses and asked yourself the following questions: What if I was in that situation? Where will my assets go when I die? What will happen to my loved ones, and will they be taken care of? Who would handle my affairs if I became ill? These important questions can only be addressed through effective estate planning.
Everyone needs an estate plan regardless of age, health, and financial or family situation. An effective estate plan provides for the orderly management and disposition of your assets upon your death. In addition, as medical professionals may appreciate, an effective estate plan appoints individuals to manage your financial affairs and make health care decisions for you in the event that you become physically or mentally incapacitated.
The most common estate planning tool is a will, which dictates how your assets pass at death. In addition, a will identifies the personal representative of your estate (that is, the person who will see that your assets pass in accordance with your wishes) and, in many states, identifies the guardian of any minor children. Although a court will make the ultimate determination of who is appointed as the guardian, courts typically give significant weight to the person named in a will.
If you die “intestate,” meaning that you died without a valid will, your assets will be distributed in accordance with your state’s intestacy statutes, and any interested person (as opposed to the individual of your choice) may be appointed as the personal representative of your estate. Therefore, to ensure that your property goes to the individuals of your choice and that your final affairs are handled by the person you trust, a will is essential.
In many states, a revocable living trust can be equally beneficial. Like a will, a revocable living trust will dictate how your property passes at death and appoints a trustee to see that the property is distributed in accordance with your wishes. Revocable living trusts can be great tools for incapacity planning and, unlike a will, are not required to be recorded, so the trust agreement can remain private. The assets that are held in a revocable living trust also avoid the often lengthy and expensive probate process, which generally includes the preparation and filing of a petition to open the estate, an inventory identifying the assets of the estate, and an accounting that details all assets received and distributed, followed by the payment of fees based upon the value of the probate estate.
In many situations, leaving assets to young, disabled, or troubled children would result in catastrophic consequences, such as disqualification for government benefits, dissipation of assets for inappropriate uses, or attachment by creditors. Further, for wealthy individuals, outright distributions to spouses could lead to unnecessary estate tax. Wills and revocable trusts can protect against these issues by requiring that, at death, the decedent’s assets are held in further trust for these individuals.
There are various types of trusts that can help ensure that your assets are used for the benefit of your loved one while avoiding any unintended consequences, some of which include the following:
- Special needs trusts, which allow the trustee to use the trust funds for the benefit of the disabled beneficiary without disqualifying the beneficiary from important government benefits.
- Spendthrift trusts, which can protect the trust assets from claims of creditors or property division in a divorce action.
- Marital trusts, which can be used to reduce taxes and ensure that property will be distributed pursuant to your wishes upon the death of your spouse.
- Dynasty trusts, which can be used to protect assets for many generations and, in doing so, reduce the amount of federal and state transfer taxes.
Whether you use a will or revocable living trust, it is critical to coordinate the beneficiary designations of assets such as retirement accounts and life insurance policies, as well as any other account that passes by beneficiary designation. These beneficiary designations trump the provisions of your will and revocable living trust. Likewise, property owned jointly with another person as joint tenants with the right of survivorship, or with a spouse as tenants by the entirety, will pass directly to the joint owner and not pursuant to the terms of your will or revocable trust.
An effective estate plan involves not just planning for death, but also for your incapacity. A durable power of attorney allows you to select an agent or agents to manage your property during your lifetime. The power of attorney can become effective immediately so that the agent can act on your behalf upon execution of the document or the power of attorney can become effective only if and when you become incapacitated.
A durable power of attorney for health care (or advance health care directive) permits you to appoint an agent to make health care decisions on your behalf in the event that you cannot make your own decisions. In addition, should you become permanently unconscious or in a terminal condition, it permits you to appoint an agent who can withhold or withdraw life-sustaining treatment. With a living will, you can express in writing the circumstances under which you do or do not want artificial life-sustaining measures.
With respect to these powers of attorney, the persons that you appoint as your agents should be people that you trust. It is also important to have conversations with your designated agents to ensure that they understand their responsibilities and your wishes. Without these powers of attorney, in the event of your incapacitation, a court will appoint a guardian. The guardian may not be the person you would have appointed, and it will result in annual, and burdensome, court filings.
As busy medical professionals, it may be difficult to find time to develop an estate plan and you may believe that there is plenty of time to do it in the future. It is important to begin thinking about your estate-planning goals and to speak with an attorney to help develop and draft your estate-planning documents. If you already have estate-planning documents, it is important to review those documents periodically to ensure that your estate-planning objectives have remained the same and, if they have changed, to update your documents.
No one knows what the future will hold, so it is important to consult with a local attorney to establish or review your estate plan now. If you do, you will be comforted by the fact that you and your loved ones will be taken care of in accordance with your wishes if you are unable to do so in the future.
Mr. D’Emilio is a managing member and Mr. Riley is an associate at McCollom D’Emilio Smith Uebler, Wilmington, Del.












