User login
Hedgehog signaling offers prognostic, therapeutic potential in CLL
Activation of hedgehog (Hh) signaling could predict early disease progression in chronic lymphocytic leukemia (CLL) and offer a new therapeutic target, according to investigators.
Approximately 11% of treatment-naive patients had mutations associated with Hh signaling, reported lead author Emanuela M. Ghia, PhD, of the University of California, San Diego, and colleagues. In addition to early progression, Hh signaling was associated with expression of GLI1, which could be a future therapeutic target. In support of this possibility, in vitro experimentation with a GLI1 inhibitor showed high cytotoxicity for GLI1-positive CLL cells.
“Targeting GLI1 could block ligand-independent and ligand-dependent Hh pathway activation and perhaps overcome the apparent resistance to SMO inhibitors,” the investigators wrote in Blood.
Using the HALT Pan-Leukemia Gene Panel, which includes 103 genes, the investigators tested for mutations in cell samples from 841 patients with treatment-naive CLL. Specifically, the investigators focused on mutations that did not map to seven well-known signaling/metabolic pathways, such as Wnt and Notch.
This strategy revealed that 89 patients (11%) had nonsynonymous mutations in genes that drive Hh signaling. These mutations were highly associated with GLI1 expression (x2 test; P less than .0001), which stands to reason, as GLI1 is the main effector of the Hh signaling pathway. Of note, 62 of the 161 patients (38%) who did not test positive for an Hh pathway mutation still tested positive for GLI1, suggesting that they had Hh pathway activation, albeit without an identifiable mutational cause.
These findings are clinically significant, as GLI1 overexpression has been linked to numerous types of cancer and is an adverse prognostic indicator for acute myeloid leukemia and several carcinomas, the investigators wrote.
Considering these associations with other cancer types, the investigators looked for a relationship between GLI1 expression and outcomes in CLL. Comparing 103 patients with GLI1-positive disease with 107 GLI-negative patients revealed that GLI1 positivity was significantly associated with shorter median treatment-free survival (4.7 vs. 6.4 years; P = .002). Additional analysis showed that this prognostic relationship was present regardless of IgVH mutational status.
Based on these findings, the investigators concluded that “[a]ctivation of the Hh pathway can strongly influence disease progression in CLL.”
Two additional tests showed that GLI1-positivity was associated with cell survival. First, silencing GLI1 with a GLI1-specific small interfering RNA led to decreased cell viability. Second, GANT61, a small molecule inhibitor of GLI1, was highly cytotoxic for GLI1-positive cells. According to the investigators, these findings suggest that GLI1 could be a future therapeutic target.
“[T]his report shows that the Hh pathway frequently is activated in CLL and associated with relatively rapid disease progression, while identifying a new avenue for therapeutic intervention for patients with this disease,” they concluded.
The study was funded by the National Institutes of Health, the Cancer Stem Cell Consortium, the Canadian Institutes of Health Research, the Ontario Institute for Cancer Research, and other groups. The investigators reported having no competing financial interests.
SOURCE: Ghia EM et al. Blood. 2019 Jun 20;133(25):2651-63.
Activation of hedgehog (Hh) signaling could predict early disease progression in chronic lymphocytic leukemia (CLL) and offer a new therapeutic target, according to investigators.
Approximately 11% of treatment-naive patients had mutations associated with Hh signaling, reported lead author Emanuela M. Ghia, PhD, of the University of California, San Diego, and colleagues. In addition to early progression, Hh signaling was associated with expression of GLI1, which could be a future therapeutic target. In support of this possibility, in vitro experimentation with a GLI1 inhibitor showed high cytotoxicity for GLI1-positive CLL cells.
“Targeting GLI1 could block ligand-independent and ligand-dependent Hh pathway activation and perhaps overcome the apparent resistance to SMO inhibitors,” the investigators wrote in Blood.
Using the HALT Pan-Leukemia Gene Panel, which includes 103 genes, the investigators tested for mutations in cell samples from 841 patients with treatment-naive CLL. Specifically, the investigators focused on mutations that did not map to seven well-known signaling/metabolic pathways, such as Wnt and Notch.
This strategy revealed that 89 patients (11%) had nonsynonymous mutations in genes that drive Hh signaling. These mutations were highly associated with GLI1 expression (x2 test; P less than .0001), which stands to reason, as GLI1 is the main effector of the Hh signaling pathway. Of note, 62 of the 161 patients (38%) who did not test positive for an Hh pathway mutation still tested positive for GLI1, suggesting that they had Hh pathway activation, albeit without an identifiable mutational cause.
These findings are clinically significant, as GLI1 overexpression has been linked to numerous types of cancer and is an adverse prognostic indicator for acute myeloid leukemia and several carcinomas, the investigators wrote.
Considering these associations with other cancer types, the investigators looked for a relationship between GLI1 expression and outcomes in CLL. Comparing 103 patients with GLI1-positive disease with 107 GLI-negative patients revealed that GLI1 positivity was significantly associated with shorter median treatment-free survival (4.7 vs. 6.4 years; P = .002). Additional analysis showed that this prognostic relationship was present regardless of IgVH mutational status.
Based on these findings, the investigators concluded that “[a]ctivation of the Hh pathway can strongly influence disease progression in CLL.”
Two additional tests showed that GLI1-positivity was associated with cell survival. First, silencing GLI1 with a GLI1-specific small interfering RNA led to decreased cell viability. Second, GANT61, a small molecule inhibitor of GLI1, was highly cytotoxic for GLI1-positive cells. According to the investigators, these findings suggest that GLI1 could be a future therapeutic target.
“[T]his report shows that the Hh pathway frequently is activated in CLL and associated with relatively rapid disease progression, while identifying a new avenue for therapeutic intervention for patients with this disease,” they concluded.
The study was funded by the National Institutes of Health, the Cancer Stem Cell Consortium, the Canadian Institutes of Health Research, the Ontario Institute for Cancer Research, and other groups. The investigators reported having no competing financial interests.
SOURCE: Ghia EM et al. Blood. 2019 Jun 20;133(25):2651-63.
Activation of hedgehog (Hh) signaling could predict early disease progression in chronic lymphocytic leukemia (CLL) and offer a new therapeutic target, according to investigators.
Approximately 11% of treatment-naive patients had mutations associated with Hh signaling, reported lead author Emanuela M. Ghia, PhD, of the University of California, San Diego, and colleagues. In addition to early progression, Hh signaling was associated with expression of GLI1, which could be a future therapeutic target. In support of this possibility, in vitro experimentation with a GLI1 inhibitor showed high cytotoxicity for GLI1-positive CLL cells.
“Targeting GLI1 could block ligand-independent and ligand-dependent Hh pathway activation and perhaps overcome the apparent resistance to SMO inhibitors,” the investigators wrote in Blood.
Using the HALT Pan-Leukemia Gene Panel, which includes 103 genes, the investigators tested for mutations in cell samples from 841 patients with treatment-naive CLL. Specifically, the investigators focused on mutations that did not map to seven well-known signaling/metabolic pathways, such as Wnt and Notch.
This strategy revealed that 89 patients (11%) had nonsynonymous mutations in genes that drive Hh signaling. These mutations were highly associated with GLI1 expression (x2 test; P less than .0001), which stands to reason, as GLI1 is the main effector of the Hh signaling pathway. Of note, 62 of the 161 patients (38%) who did not test positive for an Hh pathway mutation still tested positive for GLI1, suggesting that they had Hh pathway activation, albeit without an identifiable mutational cause.
These findings are clinically significant, as GLI1 overexpression has been linked to numerous types of cancer and is an adverse prognostic indicator for acute myeloid leukemia and several carcinomas, the investigators wrote.
Considering these associations with other cancer types, the investigators looked for a relationship between GLI1 expression and outcomes in CLL. Comparing 103 patients with GLI1-positive disease with 107 GLI-negative patients revealed that GLI1 positivity was significantly associated with shorter median treatment-free survival (4.7 vs. 6.4 years; P = .002). Additional analysis showed that this prognostic relationship was present regardless of IgVH mutational status.
Based on these findings, the investigators concluded that “[a]ctivation of the Hh pathway can strongly influence disease progression in CLL.”
Two additional tests showed that GLI1-positivity was associated with cell survival. First, silencing GLI1 with a GLI1-specific small interfering RNA led to decreased cell viability. Second, GANT61, a small molecule inhibitor of GLI1, was highly cytotoxic for GLI1-positive cells. According to the investigators, these findings suggest that GLI1 could be a future therapeutic target.
“[T]his report shows that the Hh pathway frequently is activated in CLL and associated with relatively rapid disease progression, while identifying a new avenue for therapeutic intervention for patients with this disease,” they concluded.
The study was funded by the National Institutes of Health, the Cancer Stem Cell Consortium, the Canadian Institutes of Health Research, the Ontario Institute for Cancer Research, and other groups. The investigators reported having no competing financial interests.
SOURCE: Ghia EM et al. Blood. 2019 Jun 20;133(25):2651-63.
FROM BLOOD
Opioid use curbed with patient education and lower prescription quantities
Patients given lower prescription quantities of opioid tablets with and without opioid education used significantly less of the medication compared with those given more tablets and no education, according to data from 264 adults and adolescents who underwent anterior cruciate ligament (ACL) surgery.
Although lower default prescription programs have been shown to reduce the number of tablets prescribed, “the effect of reduced prescription quantities on actual patient opioid consumption remains undetermined,” wrote Kevin X. Farley, BS, of Emory University, Atlanta, and colleagues.
In a study published in JAMA, the researchers examined whether patients took fewer tablets if given fewer, and whether patient education about opioids further reduced the number of tablets taken.
The study population included adults and adolescents who underwent ACL surgery at a single center. The patients were divided into three groups: 109 patients received 50 opioid tablets after surgery, 78 received 30 tablets plus education prior to surgery about appropriate opioid use and alternative pain management, and 77 received 30 tablets but no education on opioid use.
Patients given 50 tablets consumed an average of 25 tablets for an average of 5.8 days. By contrast, patients given 30 tablets but no opioid education consumed an average of 16 tablets for an average of 4.5 days, and those given 30 tablets and preoperative education consumed an average of 12 tablets for an average of 3.5 days.
In addition, patients given 30 tablets reported lower levels of constipation and fatigue compared with patients given 50 tablets. No differences were seen in medication refills among the groups.
The findings were limited by several factors including the use of data from a single center, the lack of randomization, and the potential for recall bias, the researchers noted. However, the results suggest that prescribing fewer tablets may further reduce use, as each group consumed approximately half of the tablets given, the researchers added.
“Further investigation should evaluate whether similar opioid stewardship and education protocols would be successful in other patient populations,” they said.
Corresponding author John Xerogeanes, MD, disclosed personal fees from Arthrex and stock options from Trice. The other researchers had no financial conflicts to disclose.
Patients given lower prescription quantities of opioid tablets with and without opioid education used significantly less of the medication compared with those given more tablets and no education, according to data from 264 adults and adolescents who underwent anterior cruciate ligament (ACL) surgery.
Although lower default prescription programs have been shown to reduce the number of tablets prescribed, “the effect of reduced prescription quantities on actual patient opioid consumption remains undetermined,” wrote Kevin X. Farley, BS, of Emory University, Atlanta, and colleagues.
In a study published in JAMA, the researchers examined whether patients took fewer tablets if given fewer, and whether patient education about opioids further reduced the number of tablets taken.
The study population included adults and adolescents who underwent ACL surgery at a single center. The patients were divided into three groups: 109 patients received 50 opioid tablets after surgery, 78 received 30 tablets plus education prior to surgery about appropriate opioid use and alternative pain management, and 77 received 30 tablets but no education on opioid use.
Patients given 50 tablets consumed an average of 25 tablets for an average of 5.8 days. By contrast, patients given 30 tablets but no opioid education consumed an average of 16 tablets for an average of 4.5 days, and those given 30 tablets and preoperative education consumed an average of 12 tablets for an average of 3.5 days.
In addition, patients given 30 tablets reported lower levels of constipation and fatigue compared with patients given 50 tablets. No differences were seen in medication refills among the groups.
The findings were limited by several factors including the use of data from a single center, the lack of randomization, and the potential for recall bias, the researchers noted. However, the results suggest that prescribing fewer tablets may further reduce use, as each group consumed approximately half of the tablets given, the researchers added.
“Further investigation should evaluate whether similar opioid stewardship and education protocols would be successful in other patient populations,” they said.
Corresponding author John Xerogeanes, MD, disclosed personal fees from Arthrex and stock options from Trice. The other researchers had no financial conflicts to disclose.
Patients given lower prescription quantities of opioid tablets with and without opioid education used significantly less of the medication compared with those given more tablets and no education, according to data from 264 adults and adolescents who underwent anterior cruciate ligament (ACL) surgery.
Although lower default prescription programs have been shown to reduce the number of tablets prescribed, “the effect of reduced prescription quantities on actual patient opioid consumption remains undetermined,” wrote Kevin X. Farley, BS, of Emory University, Atlanta, and colleagues.
In a study published in JAMA, the researchers examined whether patients took fewer tablets if given fewer, and whether patient education about opioids further reduced the number of tablets taken.
The study population included adults and adolescents who underwent ACL surgery at a single center. The patients were divided into three groups: 109 patients received 50 opioid tablets after surgery, 78 received 30 tablets plus education prior to surgery about appropriate opioid use and alternative pain management, and 77 received 30 tablets but no education on opioid use.
Patients given 50 tablets consumed an average of 25 tablets for an average of 5.8 days. By contrast, patients given 30 tablets but no opioid education consumed an average of 16 tablets for an average of 4.5 days, and those given 30 tablets and preoperative education consumed an average of 12 tablets for an average of 3.5 days.
In addition, patients given 30 tablets reported lower levels of constipation and fatigue compared with patients given 50 tablets. No differences were seen in medication refills among the groups.
The findings were limited by several factors including the use of data from a single center, the lack of randomization, and the potential for recall bias, the researchers noted. However, the results suggest that prescribing fewer tablets may further reduce use, as each group consumed approximately half of the tablets given, the researchers added.
“Further investigation should evaluate whether similar opioid stewardship and education protocols would be successful in other patient populations,” they said.
Corresponding author John Xerogeanes, MD, disclosed personal fees from Arthrex and stock options from Trice. The other researchers had no financial conflicts to disclose.
FROM JAMA
Key clinical point: Patient education and fewer tablets prescribed significantly reduced the amount of opioid tablets taken compared with no education and more tablets prescribed.
Major finding: Patients given 50 tablets and no patient education, 30 tablets and no patient education, and 30 tablets plus education consumed an average of 25, 16, and 12 tablets, respectively.
Study details: The data come from 264 adolescents and adults who underwent ACL surgery at a single center.
Disclosures: Corresponding author John Xerogeanes, MD, disclosed personal fees from Arthrex and stock options from Trice. The other researchers had no financial conflicts to disclose.
Source: Farley KX et al. JAMA. 2019 June 25.321(24):2465-7.
Trump administration seeks more health care cost details for consumers
Anyone who has tried to “shop” for hospital services knows one thing: It’s hard to get prices.
President Donald Trump on Monday signed an executive order he said would make it easier.
The order directs agencies to draw up rules requiring hospitals and insurers to make public more information on the negotiated prices they hammer out in contract negotiations. Also, hospitals and insurers would have to give estimates to patients on out-of-pocket costs before they go in for nonemergency medical care.
The move, which officials said will help address skyrocketing health care costs, comes amid other efforts by the administration to elicit more price transparency for medical care and initiatives by Congress to limit so-called surprise bills. These are the often-expensive bills consumers get when they unwittingly receive care that is not covered by their insurers.
“This will put American patients in control and address fundamental drivers of health care costs in a way no president has done before,” said Health & Human Services Secretary Alex Azar during a press briefing on Monday.
The proposal is likely to run into opposition from some hospitals and insurers who say disclosing negotiated rates could instead drive up costs.
Just how useful the effort will prove for consumers is unclear.
Much depends on how the administration writes the rules governing what information must be provided, such as whether it will include hospital-specific prices, regional averages, or other measures. While the administration calls for a “consumer-friendly” format, it’s not clear how such a massive amount of data – potentially negotiated price information from thousands of hospitals and insurers for tens of thousands of services – will be presented to consumers.
“It’s well intended, but may grossly overestimate the ability of the average patient to decipher this information overload,” said Dan Ward, a vice president at Waystar, a health care payments service.
So, does this new development advance efforts to better arm consumers with pricing information? Some key point to consider:
Q: What does the order do?
It may expand on price information consumers receive.
The order directs agencies to develop rules to require hospitals and insurers to provide information “based on negotiated rates” to the public.
Currently, such rates are hard to get, even for patients, until after medical care is provided. That’s when insured patients get an “explanation of benefits [EOBs],” which shows how much the hospital charged, how much of a discount their insurer received, and the amount a patient may owe.
In addition to consumers being unable to get price information upfront in many cases, hospital list prices and negotiated discount rates vary widely by hospital and insurer, even in a region. Uninsured patients often are charged the full amounts.
“People are sick and tired of hospitals playing these games with prices,” said George Nation, a business professor at Lehigh University in Bethlehem, Pa. who studies hospital contract law. “That’s what’s driving all of this.”
Some insurers and hospitals do provide online tools or apps that can help individual patients estimate out-of-pocket costs for a service or procedure ahead of time, but research shows few patients use such tools. Also, many medical services are needed without much notice – think of a heart attack or a broken leg – so shopping simply isn’t possible.
Administration officials say they want patients to have access to more information, including “advance EOBs” outlining anticipated costs before patients get nonemergency medical care. In theory, that would allow consumers to shop around for lower-cost care.
Q: Isn’t this information already available?
Not exactly. In January, new rules took effect under the Affordable Care Act that require hospitals to post online their “list prices,” which hospitals set themselves and have little relation to actual costs or what insurers actually pay.
What resulted are often confusing spreadsheets that contain thousands of a la carte charges – ranging from the price of medicines and sutures to room costs, among other things – that patients have to piece together if they can to estimate their total bill. Also, those list charges don’t reflect the discounted rates insurers have negotiated, so they are of little use to insured patients who might want to compare prices hospital to hospital.
The information that would result from President Trump’s executive order would provide more detail based on negotiated, discounted rates.
A senior administration official at the press briefing said details about whether the rates would be aggregated or relate to individual hospitals would be spelled out only when the administration puts forward proposed rules to implement the order later this year. It also is unclear how the administration would enforce the rules.
Another limitation: The order applies only to hospitals and the medical staff they employ. Many hospitals, however, are staffed by doctors who are not directly employed, or laboratories that are also separate. That means negotiated prices for services provided by such laboratories or physicians would not have to be disclosed.
Q: How could consumers use this information?
In theory, consumers could get information allowing them to compare prices for, say, a hip replacement or knee surgery in advance.
But that could prove difficult if the rates were not fairly hospital specific, or if they were not lumped in with all the care needed for a specific procedure or surgery.
“They could take the top 20 common procedures the hospital does, for example, and put negotiated prices on them,” said Mr. Nation. “It makes sense to do an average for that particular hospital, so I can see how much it’s going to cost to have my knee replaced at St. Joe’s versus St. Anne’s.”
Having advance notice of out-of-pocket costs could also help patients who have high-deductible plans.
“Patients are increasingly subject to insurance deductibles and other forms of substantial cost sharing. For a subset of so-called shoppable services, patients would benefit from price estimates in advance that allow them to compare options and plan financially for their care,” said John Rother, president and CEO at the advocacy group National Coalition on Health Care.
Q: Will this push consumers to shop for health care?
The short answer is maybe. Right now, it’s difficult, even with some of the tools available, said Lovisa Gustafsson, assistant vice president at the Commonwealth Fund, which has looked at whether patients use existing tools or the list price information hospitals must post online.
“The evidence to date shows patients aren’t necessarily the best shoppers, but we haven’t given them the best tools to be shoppers,” she said.
Posting negotiated rates might be a step forward, she said, but only if it is easily understandable.
It’s possible that insurers, physician offices, consumer groups, or online businesses may find ways to help direct patients to the most cost-effective locations for surgeries, tests or other procedures based on the information.
“Institutions like Consumer Reports or Consumer Checkbook could do some kind of high-level comparison between facilities or doctors, giving some general information that might be useful for consumers,” said Tim Jost, a professor emeritus at the Washington and Lee University School of Law in Lexington, Va.
But some hospitals and insurers maintain that disclosing specific rates could backfire.
Hospitals charging lower rates, for example, might raise them if they see competitors are getting higher reimbursement from insurers, they say. Insurers say they might be hampered in their ability to negotiate if rivals all know what they each pay.
“We also agree that patients should have accurate, real-time information about costs so they can make the best, most informed decisions about their care,” said a statement from lobbying group America’s Health Insurance Plans. “But publicly disclosing competitively negotiated, proprietary rates will reduce competition and push prices higher – not lower – for consumers, patients, and taxpayers.”
Kaiser Health News is a nonprofit national health policy news service. It is an editorially independent program of the Henry J. Kaiser Family Foundation that is not affiliated with Kaiser Permanente.
Anyone who has tried to “shop” for hospital services knows one thing: It’s hard to get prices.
President Donald Trump on Monday signed an executive order he said would make it easier.
The order directs agencies to draw up rules requiring hospitals and insurers to make public more information on the negotiated prices they hammer out in contract negotiations. Also, hospitals and insurers would have to give estimates to patients on out-of-pocket costs before they go in for nonemergency medical care.
The move, which officials said will help address skyrocketing health care costs, comes amid other efforts by the administration to elicit more price transparency for medical care and initiatives by Congress to limit so-called surprise bills. These are the often-expensive bills consumers get when they unwittingly receive care that is not covered by their insurers.
“This will put American patients in control and address fundamental drivers of health care costs in a way no president has done before,” said Health & Human Services Secretary Alex Azar during a press briefing on Monday.
The proposal is likely to run into opposition from some hospitals and insurers who say disclosing negotiated rates could instead drive up costs.
Just how useful the effort will prove for consumers is unclear.
Much depends on how the administration writes the rules governing what information must be provided, such as whether it will include hospital-specific prices, regional averages, or other measures. While the administration calls for a “consumer-friendly” format, it’s not clear how such a massive amount of data – potentially negotiated price information from thousands of hospitals and insurers for tens of thousands of services – will be presented to consumers.
“It’s well intended, but may grossly overestimate the ability of the average patient to decipher this information overload,” said Dan Ward, a vice president at Waystar, a health care payments service.
So, does this new development advance efforts to better arm consumers with pricing information? Some key point to consider:
Q: What does the order do?
It may expand on price information consumers receive.
The order directs agencies to develop rules to require hospitals and insurers to provide information “based on negotiated rates” to the public.
Currently, such rates are hard to get, even for patients, until after medical care is provided. That’s when insured patients get an “explanation of benefits [EOBs],” which shows how much the hospital charged, how much of a discount their insurer received, and the amount a patient may owe.
In addition to consumers being unable to get price information upfront in many cases, hospital list prices and negotiated discount rates vary widely by hospital and insurer, even in a region. Uninsured patients often are charged the full amounts.
“People are sick and tired of hospitals playing these games with prices,” said George Nation, a business professor at Lehigh University in Bethlehem, Pa. who studies hospital contract law. “That’s what’s driving all of this.”
Some insurers and hospitals do provide online tools or apps that can help individual patients estimate out-of-pocket costs for a service or procedure ahead of time, but research shows few patients use such tools. Also, many medical services are needed without much notice – think of a heart attack or a broken leg – so shopping simply isn’t possible.
Administration officials say they want patients to have access to more information, including “advance EOBs” outlining anticipated costs before patients get nonemergency medical care. In theory, that would allow consumers to shop around for lower-cost care.
Q: Isn’t this information already available?
Not exactly. In January, new rules took effect under the Affordable Care Act that require hospitals to post online their “list prices,” which hospitals set themselves and have little relation to actual costs or what insurers actually pay.
What resulted are often confusing spreadsheets that contain thousands of a la carte charges – ranging from the price of medicines and sutures to room costs, among other things – that patients have to piece together if they can to estimate their total bill. Also, those list charges don’t reflect the discounted rates insurers have negotiated, so they are of little use to insured patients who might want to compare prices hospital to hospital.
The information that would result from President Trump’s executive order would provide more detail based on negotiated, discounted rates.
A senior administration official at the press briefing said details about whether the rates would be aggregated or relate to individual hospitals would be spelled out only when the administration puts forward proposed rules to implement the order later this year. It also is unclear how the administration would enforce the rules.
Another limitation: The order applies only to hospitals and the medical staff they employ. Many hospitals, however, are staffed by doctors who are not directly employed, or laboratories that are also separate. That means negotiated prices for services provided by such laboratories or physicians would not have to be disclosed.
Q: How could consumers use this information?
In theory, consumers could get information allowing them to compare prices for, say, a hip replacement or knee surgery in advance.
But that could prove difficult if the rates were not fairly hospital specific, or if they were not lumped in with all the care needed for a specific procedure or surgery.
“They could take the top 20 common procedures the hospital does, for example, and put negotiated prices on them,” said Mr. Nation. “It makes sense to do an average for that particular hospital, so I can see how much it’s going to cost to have my knee replaced at St. Joe’s versus St. Anne’s.”
Having advance notice of out-of-pocket costs could also help patients who have high-deductible plans.
“Patients are increasingly subject to insurance deductibles and other forms of substantial cost sharing. For a subset of so-called shoppable services, patients would benefit from price estimates in advance that allow them to compare options and plan financially for their care,” said John Rother, president and CEO at the advocacy group National Coalition on Health Care.
Q: Will this push consumers to shop for health care?
The short answer is maybe. Right now, it’s difficult, even with some of the tools available, said Lovisa Gustafsson, assistant vice president at the Commonwealth Fund, which has looked at whether patients use existing tools or the list price information hospitals must post online.
“The evidence to date shows patients aren’t necessarily the best shoppers, but we haven’t given them the best tools to be shoppers,” she said.
Posting negotiated rates might be a step forward, she said, but only if it is easily understandable.
It’s possible that insurers, physician offices, consumer groups, or online businesses may find ways to help direct patients to the most cost-effective locations for surgeries, tests or other procedures based on the information.
“Institutions like Consumer Reports or Consumer Checkbook could do some kind of high-level comparison between facilities or doctors, giving some general information that might be useful for consumers,” said Tim Jost, a professor emeritus at the Washington and Lee University School of Law in Lexington, Va.
But some hospitals and insurers maintain that disclosing specific rates could backfire.
Hospitals charging lower rates, for example, might raise them if they see competitors are getting higher reimbursement from insurers, they say. Insurers say they might be hampered in their ability to negotiate if rivals all know what they each pay.
“We also agree that patients should have accurate, real-time information about costs so they can make the best, most informed decisions about their care,” said a statement from lobbying group America’s Health Insurance Plans. “But publicly disclosing competitively negotiated, proprietary rates will reduce competition and push prices higher – not lower – for consumers, patients, and taxpayers.”
Kaiser Health News is a nonprofit national health policy news service. It is an editorially independent program of the Henry J. Kaiser Family Foundation that is not affiliated with Kaiser Permanente.
Anyone who has tried to “shop” for hospital services knows one thing: It’s hard to get prices.
President Donald Trump on Monday signed an executive order he said would make it easier.
The order directs agencies to draw up rules requiring hospitals and insurers to make public more information on the negotiated prices they hammer out in contract negotiations. Also, hospitals and insurers would have to give estimates to patients on out-of-pocket costs before they go in for nonemergency medical care.
The move, which officials said will help address skyrocketing health care costs, comes amid other efforts by the administration to elicit more price transparency for medical care and initiatives by Congress to limit so-called surprise bills. These are the often-expensive bills consumers get when they unwittingly receive care that is not covered by their insurers.
“This will put American patients in control and address fundamental drivers of health care costs in a way no president has done before,” said Health & Human Services Secretary Alex Azar during a press briefing on Monday.
The proposal is likely to run into opposition from some hospitals and insurers who say disclosing negotiated rates could instead drive up costs.
Just how useful the effort will prove for consumers is unclear.
Much depends on how the administration writes the rules governing what information must be provided, such as whether it will include hospital-specific prices, regional averages, or other measures. While the administration calls for a “consumer-friendly” format, it’s not clear how such a massive amount of data – potentially negotiated price information from thousands of hospitals and insurers for tens of thousands of services – will be presented to consumers.
“It’s well intended, but may grossly overestimate the ability of the average patient to decipher this information overload,” said Dan Ward, a vice president at Waystar, a health care payments service.
So, does this new development advance efforts to better arm consumers with pricing information? Some key point to consider:
Q: What does the order do?
It may expand on price information consumers receive.
The order directs agencies to develop rules to require hospitals and insurers to provide information “based on negotiated rates” to the public.
Currently, such rates are hard to get, even for patients, until after medical care is provided. That’s when insured patients get an “explanation of benefits [EOBs],” which shows how much the hospital charged, how much of a discount their insurer received, and the amount a patient may owe.
In addition to consumers being unable to get price information upfront in many cases, hospital list prices and negotiated discount rates vary widely by hospital and insurer, even in a region. Uninsured patients often are charged the full amounts.
“People are sick and tired of hospitals playing these games with prices,” said George Nation, a business professor at Lehigh University in Bethlehem, Pa. who studies hospital contract law. “That’s what’s driving all of this.”
Some insurers and hospitals do provide online tools or apps that can help individual patients estimate out-of-pocket costs for a service or procedure ahead of time, but research shows few patients use such tools. Also, many medical services are needed without much notice – think of a heart attack or a broken leg – so shopping simply isn’t possible.
Administration officials say they want patients to have access to more information, including “advance EOBs” outlining anticipated costs before patients get nonemergency medical care. In theory, that would allow consumers to shop around for lower-cost care.
Q: Isn’t this information already available?
Not exactly. In January, new rules took effect under the Affordable Care Act that require hospitals to post online their “list prices,” which hospitals set themselves and have little relation to actual costs or what insurers actually pay.
What resulted are often confusing spreadsheets that contain thousands of a la carte charges – ranging from the price of medicines and sutures to room costs, among other things – that patients have to piece together if they can to estimate their total bill. Also, those list charges don’t reflect the discounted rates insurers have negotiated, so they are of little use to insured patients who might want to compare prices hospital to hospital.
The information that would result from President Trump’s executive order would provide more detail based on negotiated, discounted rates.
A senior administration official at the press briefing said details about whether the rates would be aggregated or relate to individual hospitals would be spelled out only when the administration puts forward proposed rules to implement the order later this year. It also is unclear how the administration would enforce the rules.
Another limitation: The order applies only to hospitals and the medical staff they employ. Many hospitals, however, are staffed by doctors who are not directly employed, or laboratories that are also separate. That means negotiated prices for services provided by such laboratories or physicians would not have to be disclosed.
Q: How could consumers use this information?
In theory, consumers could get information allowing them to compare prices for, say, a hip replacement or knee surgery in advance.
But that could prove difficult if the rates were not fairly hospital specific, or if they were not lumped in with all the care needed for a specific procedure or surgery.
“They could take the top 20 common procedures the hospital does, for example, and put negotiated prices on them,” said Mr. Nation. “It makes sense to do an average for that particular hospital, so I can see how much it’s going to cost to have my knee replaced at St. Joe’s versus St. Anne’s.”
Having advance notice of out-of-pocket costs could also help patients who have high-deductible plans.
“Patients are increasingly subject to insurance deductibles and other forms of substantial cost sharing. For a subset of so-called shoppable services, patients would benefit from price estimates in advance that allow them to compare options and plan financially for their care,” said John Rother, president and CEO at the advocacy group National Coalition on Health Care.
Q: Will this push consumers to shop for health care?
The short answer is maybe. Right now, it’s difficult, even with some of the tools available, said Lovisa Gustafsson, assistant vice president at the Commonwealth Fund, which has looked at whether patients use existing tools or the list price information hospitals must post online.
“The evidence to date shows patients aren’t necessarily the best shoppers, but we haven’t given them the best tools to be shoppers,” she said.
Posting negotiated rates might be a step forward, she said, but only if it is easily understandable.
It’s possible that insurers, physician offices, consumer groups, or online businesses may find ways to help direct patients to the most cost-effective locations for surgeries, tests or other procedures based on the information.
“Institutions like Consumer Reports or Consumer Checkbook could do some kind of high-level comparison between facilities or doctors, giving some general information that might be useful for consumers,” said Tim Jost, a professor emeritus at the Washington and Lee University School of Law in Lexington, Va.
But some hospitals and insurers maintain that disclosing specific rates could backfire.
Hospitals charging lower rates, for example, might raise them if they see competitors are getting higher reimbursement from insurers, they say. Insurers say they might be hampered in their ability to negotiate if rivals all know what they each pay.
“We also agree that patients should have accurate, real-time information about costs so they can make the best, most informed decisions about their care,” said a statement from lobbying group America’s Health Insurance Plans. “But publicly disclosing competitively negotiated, proprietary rates will reduce competition and push prices higher – not lower – for consumers, patients, and taxpayers.”
Kaiser Health News is a nonprofit national health policy news service. It is an editorially independent program of the Henry J. Kaiser Family Foundation that is not affiliated with Kaiser Permanente.
Deep sedation did not improve polyp, adenoma detection
Deep sedation during colonoscopies did not confer any improvement in the detection rate for adenomas or polyps among average-risk patients, based on results from a retrospective analysis at a single institution that switched from moderate to deep sedation.
There remains a question as to whether moderate sedation, such as benzodiazepine plus opioids, might affect adenoma detection rate (ADR). The issue is important in part because of the recent push to use propofol in outpatient colonoscopy clinics, according to Erica Turse, DO, MPH, of the University of Missouri–Columbia, and colleagues.
Previous studies looking at moderate versus deep sedation have yielded mixed results, possibly as a result of confounding variables arising from mixed patient populations and conditions.
The current study, published in Gastrointestinal Endoscopy, aimed to eliminate potential confounders by focusing only on average-risk index colonoscopies, with similar patient populations in both groups.
The researchers examined data from a tertiary care outpatient center at the University of Missouri, which switched from moderate to deep sedation in the spring of 2016. Moderate sedation was achieved using midazolam and fentanyl, and propofol was later used for deep sedation. The study included a total of 585 colonoscopies, with 338 patients in the moderate-sedation group and 247 in the deep-sedation group. The overall polyp detection rate (PDR) was 70.1%, and the ADR was 41.7%.
The two groups did not significantly differ in PDR (71.9% moderate vs. 67.6% deep, P = .27) or ADR (44.1% vs. 38.5%; P = .18). Among women, there was no difference in PDR (69.3% vs. 64.8%; P = .41) or ADR (42.2% vs. 32.4%; P = .09). Among men, the results were the same (PDR, 75.3% vs. 71.4%; P = .56; ADR, 46.6% vs. 46.7%; P = 1.0).
A strength of the study was that the populations in both the moderate- and deep-sedation groups were similar. A weakness is that the study was conducted at a single center. The authors called for a randomized, controlled trial to gain more insight into the benefits of moderate versus deep sedation.
The study had no external funding. The authors reported having no financial conflicts of interest.
SOURCE: Turse E et al. Gastrointest Endosc. 2019 May 15. doi: 10.1016/j.gie.2019.05.011.
Deep sedation during colonoscopies did not confer any improvement in the detection rate for adenomas or polyps among average-risk patients, based on results from a retrospective analysis at a single institution that switched from moderate to deep sedation.
There remains a question as to whether moderate sedation, such as benzodiazepine plus opioids, might affect adenoma detection rate (ADR). The issue is important in part because of the recent push to use propofol in outpatient colonoscopy clinics, according to Erica Turse, DO, MPH, of the University of Missouri–Columbia, and colleagues.
Previous studies looking at moderate versus deep sedation have yielded mixed results, possibly as a result of confounding variables arising from mixed patient populations and conditions.
The current study, published in Gastrointestinal Endoscopy, aimed to eliminate potential confounders by focusing only on average-risk index colonoscopies, with similar patient populations in both groups.
The researchers examined data from a tertiary care outpatient center at the University of Missouri, which switched from moderate to deep sedation in the spring of 2016. Moderate sedation was achieved using midazolam and fentanyl, and propofol was later used for deep sedation. The study included a total of 585 colonoscopies, with 338 patients in the moderate-sedation group and 247 in the deep-sedation group. The overall polyp detection rate (PDR) was 70.1%, and the ADR was 41.7%.
The two groups did not significantly differ in PDR (71.9% moderate vs. 67.6% deep, P = .27) or ADR (44.1% vs. 38.5%; P = .18). Among women, there was no difference in PDR (69.3% vs. 64.8%; P = .41) or ADR (42.2% vs. 32.4%; P = .09). Among men, the results were the same (PDR, 75.3% vs. 71.4%; P = .56; ADR, 46.6% vs. 46.7%; P = 1.0).
A strength of the study was that the populations in both the moderate- and deep-sedation groups were similar. A weakness is that the study was conducted at a single center. The authors called for a randomized, controlled trial to gain more insight into the benefits of moderate versus deep sedation.
The study had no external funding. The authors reported having no financial conflicts of interest.
SOURCE: Turse E et al. Gastrointest Endosc. 2019 May 15. doi: 10.1016/j.gie.2019.05.011.
Deep sedation during colonoscopies did not confer any improvement in the detection rate for adenomas or polyps among average-risk patients, based on results from a retrospective analysis at a single institution that switched from moderate to deep sedation.
There remains a question as to whether moderate sedation, such as benzodiazepine plus opioids, might affect adenoma detection rate (ADR). The issue is important in part because of the recent push to use propofol in outpatient colonoscopy clinics, according to Erica Turse, DO, MPH, of the University of Missouri–Columbia, and colleagues.
Previous studies looking at moderate versus deep sedation have yielded mixed results, possibly as a result of confounding variables arising from mixed patient populations and conditions.
The current study, published in Gastrointestinal Endoscopy, aimed to eliminate potential confounders by focusing only on average-risk index colonoscopies, with similar patient populations in both groups.
The researchers examined data from a tertiary care outpatient center at the University of Missouri, which switched from moderate to deep sedation in the spring of 2016. Moderate sedation was achieved using midazolam and fentanyl, and propofol was later used for deep sedation. The study included a total of 585 colonoscopies, with 338 patients in the moderate-sedation group and 247 in the deep-sedation group. The overall polyp detection rate (PDR) was 70.1%, and the ADR was 41.7%.
The two groups did not significantly differ in PDR (71.9% moderate vs. 67.6% deep, P = .27) or ADR (44.1% vs. 38.5%; P = .18). Among women, there was no difference in PDR (69.3% vs. 64.8%; P = .41) or ADR (42.2% vs. 32.4%; P = .09). Among men, the results were the same (PDR, 75.3% vs. 71.4%; P = .56; ADR, 46.6% vs. 46.7%; P = 1.0).
A strength of the study was that the populations in both the moderate- and deep-sedation groups were similar. A weakness is that the study was conducted at a single center. The authors called for a randomized, controlled trial to gain more insight into the benefits of moderate versus deep sedation.
The study had no external funding. The authors reported having no financial conflicts of interest.
SOURCE: Turse E et al. Gastrointest Endosc. 2019 May 15. doi: 10.1016/j.gie.2019.05.011.
FROM GASTROINTESTINAL ENDOSCOPY
Revised CMS TAVR rules expected to widen access
The new National Coverage Determination by Medicare for transcatheter aortic valve replacement should produce a bump in the number of U.S. programs offering the procedure, especially with the Food and Drug Administration on the cusp of approving the procedure for low-risk patients.
In the revised National Coverage Determination (NCD) by the Centers for Medicare & Medicaid Services that went into effect on June 21, 2019, the agency allowed for Medicare coverage of transcatheter aortic valve (TAVR) procedures at hospitals that perform at least 20 of these procedures annually or at least 40 every 2 years, the same volume minimums that CMS first applied to TAVR in its prior 2012 NCD. Retention of this minimum ran against the 2018 proposal of the American College of Cardiology, the Society of Thoracic Surgeons, and two other collaborating societies that called for an annual TAVR volume minimum at a hospital program of 50 procedures annually or 100 every 2 years (J Am Coll Cardiol. 2019 Jan 29;73[3]:340-74).
That change, coupled with a cut in the minimum number of annual percutaneous coronary interventions a TAVR program needs to perform – newly revised to a minimum of 300 cases/year – will likely mean more U.S. sites performing TAVR, predicted James Vavricek, director of regulatory affairs for the ACC in Washington. TAVR volume is seen as a reasonable, approximate surrogate for a more rigorous, statistically adjusted assessment of program quality. The ACC and representatives from the other societies that collaborated on the 2018 statement used a 50 case/year minimum for a TAVR program because volume at that level generates enough outcomes data to allow for a meaningful, risk-adjusted measure of performance.
The ACC does not consider the minimum of 20 TAVR cases/year the “right decision,” Mr. Vavricek said in an interview, but the ACC sees it as a compromise that accommodated the interests of multiple TAVR stakeholders. “It will be interesting to see where new TAVR programs locate,” whether they will expand access in underserved regions or mostly cluster in regions already fairly replete with TAVR access, he added. Currently, over 600 U.S. TAVR programs are in operation.
In April 2019, the president of the ACC along with the presidents of three other U.S. societies with an interest in TAVR told the CMS in a comment letter that “we are extremely concerned that the proposed volume requirements will translate into a proliferation of low-volume TAVR programs at increased risk for having suboptimal outcomes.”
Another change to procedure volume requirements in the new NCD was setting a minimum of 100 total TAVR plus surgical aortic valve replacements in a 2-year period or 50 total procedures/year for each TAVR program. Setting a minimum that bundles TAVR plus surgical valve replacements is a “forward-looking” approach as wider application of TAVR gradually erodes the volume of surgical procedures, Mr. Vavricek said.
An additional notable change in the revised NCD was elimination of the “two-surgeon” rule, which the CMS had made mandatory for TAVR decisions until now, stipulating that a patient considered for TAVR needed independent assessment by two cardiac surgeons. The final 2019 NCD calls for the TAVR decision to come from one cardiac surgeon and one interventional cardiologist working together on a care team.
“The ACC is pleased to see CMS issue updated TAVR coverage criteria that emphasizes care by an interdisciplinary heart team for these complex patients, as well as continues to mandate the collection of TAVR patient data. With the new lowered minimum yearly volume criteria set by CMS in their efforts to improve patient access, the value of the STS/ACC TVT Registry, along with ACC’s Transcatheter Valve Certification, will be critical in assuring quality of care for our patients particularly in low-volume centers,” commented Richard J. Kovacs, MD, ACC’s president.
The new National Coverage Determination by Medicare for transcatheter aortic valve replacement should produce a bump in the number of U.S. programs offering the procedure, especially with the Food and Drug Administration on the cusp of approving the procedure for low-risk patients.
In the revised National Coverage Determination (NCD) by the Centers for Medicare & Medicaid Services that went into effect on June 21, 2019, the agency allowed for Medicare coverage of transcatheter aortic valve (TAVR) procedures at hospitals that perform at least 20 of these procedures annually or at least 40 every 2 years, the same volume minimums that CMS first applied to TAVR in its prior 2012 NCD. Retention of this minimum ran against the 2018 proposal of the American College of Cardiology, the Society of Thoracic Surgeons, and two other collaborating societies that called for an annual TAVR volume minimum at a hospital program of 50 procedures annually or 100 every 2 years (J Am Coll Cardiol. 2019 Jan 29;73[3]:340-74).
That change, coupled with a cut in the minimum number of annual percutaneous coronary interventions a TAVR program needs to perform – newly revised to a minimum of 300 cases/year – will likely mean more U.S. sites performing TAVR, predicted James Vavricek, director of regulatory affairs for the ACC in Washington. TAVR volume is seen as a reasonable, approximate surrogate for a more rigorous, statistically adjusted assessment of program quality. The ACC and representatives from the other societies that collaborated on the 2018 statement used a 50 case/year minimum for a TAVR program because volume at that level generates enough outcomes data to allow for a meaningful, risk-adjusted measure of performance.
The ACC does not consider the minimum of 20 TAVR cases/year the “right decision,” Mr. Vavricek said in an interview, but the ACC sees it as a compromise that accommodated the interests of multiple TAVR stakeholders. “It will be interesting to see where new TAVR programs locate,” whether they will expand access in underserved regions or mostly cluster in regions already fairly replete with TAVR access, he added. Currently, over 600 U.S. TAVR programs are in operation.
In April 2019, the president of the ACC along with the presidents of three other U.S. societies with an interest in TAVR told the CMS in a comment letter that “we are extremely concerned that the proposed volume requirements will translate into a proliferation of low-volume TAVR programs at increased risk for having suboptimal outcomes.”
Another change to procedure volume requirements in the new NCD was setting a minimum of 100 total TAVR plus surgical aortic valve replacements in a 2-year period or 50 total procedures/year for each TAVR program. Setting a minimum that bundles TAVR plus surgical valve replacements is a “forward-looking” approach as wider application of TAVR gradually erodes the volume of surgical procedures, Mr. Vavricek said.
An additional notable change in the revised NCD was elimination of the “two-surgeon” rule, which the CMS had made mandatory for TAVR decisions until now, stipulating that a patient considered for TAVR needed independent assessment by two cardiac surgeons. The final 2019 NCD calls for the TAVR decision to come from one cardiac surgeon and one interventional cardiologist working together on a care team.
“The ACC is pleased to see CMS issue updated TAVR coverage criteria that emphasizes care by an interdisciplinary heart team for these complex patients, as well as continues to mandate the collection of TAVR patient data. With the new lowered minimum yearly volume criteria set by CMS in their efforts to improve patient access, the value of the STS/ACC TVT Registry, along with ACC’s Transcatheter Valve Certification, will be critical in assuring quality of care for our patients particularly in low-volume centers,” commented Richard J. Kovacs, MD, ACC’s president.
The new National Coverage Determination by Medicare for transcatheter aortic valve replacement should produce a bump in the number of U.S. programs offering the procedure, especially with the Food and Drug Administration on the cusp of approving the procedure for low-risk patients.
In the revised National Coverage Determination (NCD) by the Centers for Medicare & Medicaid Services that went into effect on June 21, 2019, the agency allowed for Medicare coverage of transcatheter aortic valve (TAVR) procedures at hospitals that perform at least 20 of these procedures annually or at least 40 every 2 years, the same volume minimums that CMS first applied to TAVR in its prior 2012 NCD. Retention of this minimum ran against the 2018 proposal of the American College of Cardiology, the Society of Thoracic Surgeons, and two other collaborating societies that called for an annual TAVR volume minimum at a hospital program of 50 procedures annually or 100 every 2 years (J Am Coll Cardiol. 2019 Jan 29;73[3]:340-74).
That change, coupled with a cut in the minimum number of annual percutaneous coronary interventions a TAVR program needs to perform – newly revised to a minimum of 300 cases/year – will likely mean more U.S. sites performing TAVR, predicted James Vavricek, director of regulatory affairs for the ACC in Washington. TAVR volume is seen as a reasonable, approximate surrogate for a more rigorous, statistically adjusted assessment of program quality. The ACC and representatives from the other societies that collaborated on the 2018 statement used a 50 case/year minimum for a TAVR program because volume at that level generates enough outcomes data to allow for a meaningful, risk-adjusted measure of performance.
The ACC does not consider the minimum of 20 TAVR cases/year the “right decision,” Mr. Vavricek said in an interview, but the ACC sees it as a compromise that accommodated the interests of multiple TAVR stakeholders. “It will be interesting to see where new TAVR programs locate,” whether they will expand access in underserved regions or mostly cluster in regions already fairly replete with TAVR access, he added. Currently, over 600 U.S. TAVR programs are in operation.
In April 2019, the president of the ACC along with the presidents of three other U.S. societies with an interest in TAVR told the CMS in a comment letter that “we are extremely concerned that the proposed volume requirements will translate into a proliferation of low-volume TAVR programs at increased risk for having suboptimal outcomes.”
Another change to procedure volume requirements in the new NCD was setting a minimum of 100 total TAVR plus surgical aortic valve replacements in a 2-year period or 50 total procedures/year for each TAVR program. Setting a minimum that bundles TAVR plus surgical valve replacements is a “forward-looking” approach as wider application of TAVR gradually erodes the volume of surgical procedures, Mr. Vavricek said.
An additional notable change in the revised NCD was elimination of the “two-surgeon” rule, which the CMS had made mandatory for TAVR decisions until now, stipulating that a patient considered for TAVR needed independent assessment by two cardiac surgeons. The final 2019 NCD calls for the TAVR decision to come from one cardiac surgeon and one interventional cardiologist working together on a care team.
“The ACC is pleased to see CMS issue updated TAVR coverage criteria that emphasizes care by an interdisciplinary heart team for these complex patients, as well as continues to mandate the collection of TAVR patient data. With the new lowered minimum yearly volume criteria set by CMS in their efforts to improve patient access, the value of the STS/ACC TVT Registry, along with ACC’s Transcatheter Valve Certification, will be critical in assuring quality of care for our patients particularly in low-volume centers,” commented Richard J. Kovacs, MD, ACC’s president.
Recurrent Pruritic Multifocal Erythematous Rash
The Diagnosis: Wells Syndrome
Histopathologic examination of the biopsy demonstrated overlying acanthosis, focal spongiosis, and exocytosis. There also was proliferation and thickening of superficial capillaries and papillary fibrosis (Figure, A). There was a mixed interstitial and perivascular inflammatory infiltrate consisting of lymphocytes, histiocytes, plasma cells, and eosinophils (Figure, A and B). Occasional flame figures were identified (Figure, C).

Wells syndrome, also known as eosinophilic cellulitis, was first described in 1971 by Wells1 as a recurrent granulomatous dermatitis with eosinophilia. Rarely reported worldwide, this chronic relapsing condition is characterized by a pronounced eosinophilic infiltrate of the dermis resembling urticaria or cellulitis.2 The exact etiology has not been elucidated; however, links to certain medications, vaccines, exaggerated arthropod reactions, infections, and malignancies have been documented.3
Wells syndrome is a diagnosis of exclusion and lacks a predictable dermatologic presentation, thereby mandating focused clinical follow-up as well as correlation with histopathology findings. Although the classic histologic hallmark of Wells syndrome is scattered flame figures, this finding is not specific and can be found in other hypereosinophilic conditions.2 Clinical manifestations most often consist of 2 distinct phases: an initial painful burning or pruritic sensation, followed by the development of erythematous and edematous dermal plaques that may heal with slight hyperpigmentation over 4 to 8 weeks. A case series of 19 patients demonstrated variants of Wells syndrome, with an annular granuloma-like appearance found primarily in adults and the signature plaque-type appearance predominating in children.4
Acute urticaria is characterized by pruritic erythematous wheals secondary to a histamine-mediated response brought on by a variety of triggers, typically allergic and self-resolving within 24 hours. When such lesions last longer than 24 hours, biopsy should be performed to exclude urticarial vasculitis, which is characterized by a burning or painful sensation rather than pruritis, in addition to dermal neutrophilia and perivascular infiltrate on histology. Erythema migrans of Lyme disease begins at the site of a tick bite, evolving from a red macule to an expanding targetoid lesion and typically is not pruritic. Infectious cellulitis presents with warm, tender, and poorly defined erythematous patches; can progress rapidly; and is accompanied by systemic symptoms such as fevers, malaise, and lymphadenopathy.
Best evidence favors the use of moderate- to high-dose corticosteroids as first-line treatment.5 The use of tumor necrosis factor blockers, various immunomodulating agents, and combination therapy with levocetirizine and hydroxyzine have demonstrated variable levels of efficacy, albeit often followed by high rates of relapse with drug discontinuation.6
- Wells GC. Recurrent granulomatous dermatitis with eosinophilia. Trans St Johns Hosp Dermatol Soc. 1971;57:46-56.
- Aberer W, Konrad K, Wolff K. Wells' syndrome is a distinctive disease entity and not a histologic diagnosis. J Am Acad Dermatol. 1988;18:105-114.
- Kaufmann D, Pichler W, Beer JH. Severe episode of high fever with rash, lymphadenopathy, neutropenia, and eosinophilia after minocycline therapy for acne. Arch Intern Med. 1994;154:1983-1984.
- Caputo R, Marzano AV, Vezzoli P, et al. Wells syndrome in adults and children: a report of 19 cases. Arch Dermatol. 2006;142:1157-1161.
- Ferreli C, Pinna AL, Atzori L, et al. Eosinophilic cellulitis (Well's syndrome): a new case description. J Eur Acad Dermatol Venereol. 1999;13:41-45.
- Cormerais M, Poizeau F, Darrieux L, et al. Wells' syndrome mimicking facial cellulitis: a report of two cases. Case Rep Dermatol. 2015;7:117-122.
The Diagnosis: Wells Syndrome
Histopathologic examination of the biopsy demonstrated overlying acanthosis, focal spongiosis, and exocytosis. There also was proliferation and thickening of superficial capillaries and papillary fibrosis (Figure, A). There was a mixed interstitial and perivascular inflammatory infiltrate consisting of lymphocytes, histiocytes, plasma cells, and eosinophils (Figure, A and B). Occasional flame figures were identified (Figure, C).

Wells syndrome, also known as eosinophilic cellulitis, was first described in 1971 by Wells1 as a recurrent granulomatous dermatitis with eosinophilia. Rarely reported worldwide, this chronic relapsing condition is characterized by a pronounced eosinophilic infiltrate of the dermis resembling urticaria or cellulitis.2 The exact etiology has not been elucidated; however, links to certain medications, vaccines, exaggerated arthropod reactions, infections, and malignancies have been documented.3
Wells syndrome is a diagnosis of exclusion and lacks a predictable dermatologic presentation, thereby mandating focused clinical follow-up as well as correlation with histopathology findings. Although the classic histologic hallmark of Wells syndrome is scattered flame figures, this finding is not specific and can be found in other hypereosinophilic conditions.2 Clinical manifestations most often consist of 2 distinct phases: an initial painful burning or pruritic sensation, followed by the development of erythematous and edematous dermal plaques that may heal with slight hyperpigmentation over 4 to 8 weeks. A case series of 19 patients demonstrated variants of Wells syndrome, with an annular granuloma-like appearance found primarily in adults and the signature plaque-type appearance predominating in children.4
Acute urticaria is characterized by pruritic erythematous wheals secondary to a histamine-mediated response brought on by a variety of triggers, typically allergic and self-resolving within 24 hours. When such lesions last longer than 24 hours, biopsy should be performed to exclude urticarial vasculitis, which is characterized by a burning or painful sensation rather than pruritis, in addition to dermal neutrophilia and perivascular infiltrate on histology. Erythema migrans of Lyme disease begins at the site of a tick bite, evolving from a red macule to an expanding targetoid lesion and typically is not pruritic. Infectious cellulitis presents with warm, tender, and poorly defined erythematous patches; can progress rapidly; and is accompanied by systemic symptoms such as fevers, malaise, and lymphadenopathy.
Best evidence favors the use of moderate- to high-dose corticosteroids as first-line treatment.5 The use of tumor necrosis factor blockers, various immunomodulating agents, and combination therapy with levocetirizine and hydroxyzine have demonstrated variable levels of efficacy, albeit often followed by high rates of relapse with drug discontinuation.6
The Diagnosis: Wells Syndrome
Histopathologic examination of the biopsy demonstrated overlying acanthosis, focal spongiosis, and exocytosis. There also was proliferation and thickening of superficial capillaries and papillary fibrosis (Figure, A). There was a mixed interstitial and perivascular inflammatory infiltrate consisting of lymphocytes, histiocytes, plasma cells, and eosinophils (Figure, A and B). Occasional flame figures were identified (Figure, C).

Wells syndrome, also known as eosinophilic cellulitis, was first described in 1971 by Wells1 as a recurrent granulomatous dermatitis with eosinophilia. Rarely reported worldwide, this chronic relapsing condition is characterized by a pronounced eosinophilic infiltrate of the dermis resembling urticaria or cellulitis.2 The exact etiology has not been elucidated; however, links to certain medications, vaccines, exaggerated arthropod reactions, infections, and malignancies have been documented.3
Wells syndrome is a diagnosis of exclusion and lacks a predictable dermatologic presentation, thereby mandating focused clinical follow-up as well as correlation with histopathology findings. Although the classic histologic hallmark of Wells syndrome is scattered flame figures, this finding is not specific and can be found in other hypereosinophilic conditions.2 Clinical manifestations most often consist of 2 distinct phases: an initial painful burning or pruritic sensation, followed by the development of erythematous and edematous dermal plaques that may heal with slight hyperpigmentation over 4 to 8 weeks. A case series of 19 patients demonstrated variants of Wells syndrome, with an annular granuloma-like appearance found primarily in adults and the signature plaque-type appearance predominating in children.4
Acute urticaria is characterized by pruritic erythematous wheals secondary to a histamine-mediated response brought on by a variety of triggers, typically allergic and self-resolving within 24 hours. When such lesions last longer than 24 hours, biopsy should be performed to exclude urticarial vasculitis, which is characterized by a burning or painful sensation rather than pruritis, in addition to dermal neutrophilia and perivascular infiltrate on histology. Erythema migrans of Lyme disease begins at the site of a tick bite, evolving from a red macule to an expanding targetoid lesion and typically is not pruritic. Infectious cellulitis presents with warm, tender, and poorly defined erythematous patches; can progress rapidly; and is accompanied by systemic symptoms such as fevers, malaise, and lymphadenopathy.
Best evidence favors the use of moderate- to high-dose corticosteroids as first-line treatment.5 The use of tumor necrosis factor blockers, various immunomodulating agents, and combination therapy with levocetirizine and hydroxyzine have demonstrated variable levels of efficacy, albeit often followed by high rates of relapse with drug discontinuation.6
- Wells GC. Recurrent granulomatous dermatitis with eosinophilia. Trans St Johns Hosp Dermatol Soc. 1971;57:46-56.
- Aberer W, Konrad K, Wolff K. Wells' syndrome is a distinctive disease entity and not a histologic diagnosis. J Am Acad Dermatol. 1988;18:105-114.
- Kaufmann D, Pichler W, Beer JH. Severe episode of high fever with rash, lymphadenopathy, neutropenia, and eosinophilia after minocycline therapy for acne. Arch Intern Med. 1994;154:1983-1984.
- Caputo R, Marzano AV, Vezzoli P, et al. Wells syndrome in adults and children: a report of 19 cases. Arch Dermatol. 2006;142:1157-1161.
- Ferreli C, Pinna AL, Atzori L, et al. Eosinophilic cellulitis (Well's syndrome): a new case description. J Eur Acad Dermatol Venereol. 1999;13:41-45.
- Cormerais M, Poizeau F, Darrieux L, et al. Wells' syndrome mimicking facial cellulitis: a report of two cases. Case Rep Dermatol. 2015;7:117-122.
- Wells GC. Recurrent granulomatous dermatitis with eosinophilia. Trans St Johns Hosp Dermatol Soc. 1971;57:46-56.
- Aberer W, Konrad K, Wolff K. Wells' syndrome is a distinctive disease entity and not a histologic diagnosis. J Am Acad Dermatol. 1988;18:105-114.
- Kaufmann D, Pichler W, Beer JH. Severe episode of high fever with rash, lymphadenopathy, neutropenia, and eosinophilia after minocycline therapy for acne. Arch Intern Med. 1994;154:1983-1984.
- Caputo R, Marzano AV, Vezzoli P, et al. Wells syndrome in adults and children: a report of 19 cases. Arch Dermatol. 2006;142:1157-1161.
- Ferreli C, Pinna AL, Atzori L, et al. Eosinophilic cellulitis (Well's syndrome): a new case description. J Eur Acad Dermatol Venereol. 1999;13:41-45.
- Cormerais M, Poizeau F, Darrieux L, et al. Wells' syndrome mimicking facial cellulitis: a report of two cases. Case Rep Dermatol. 2015;7:117-122.
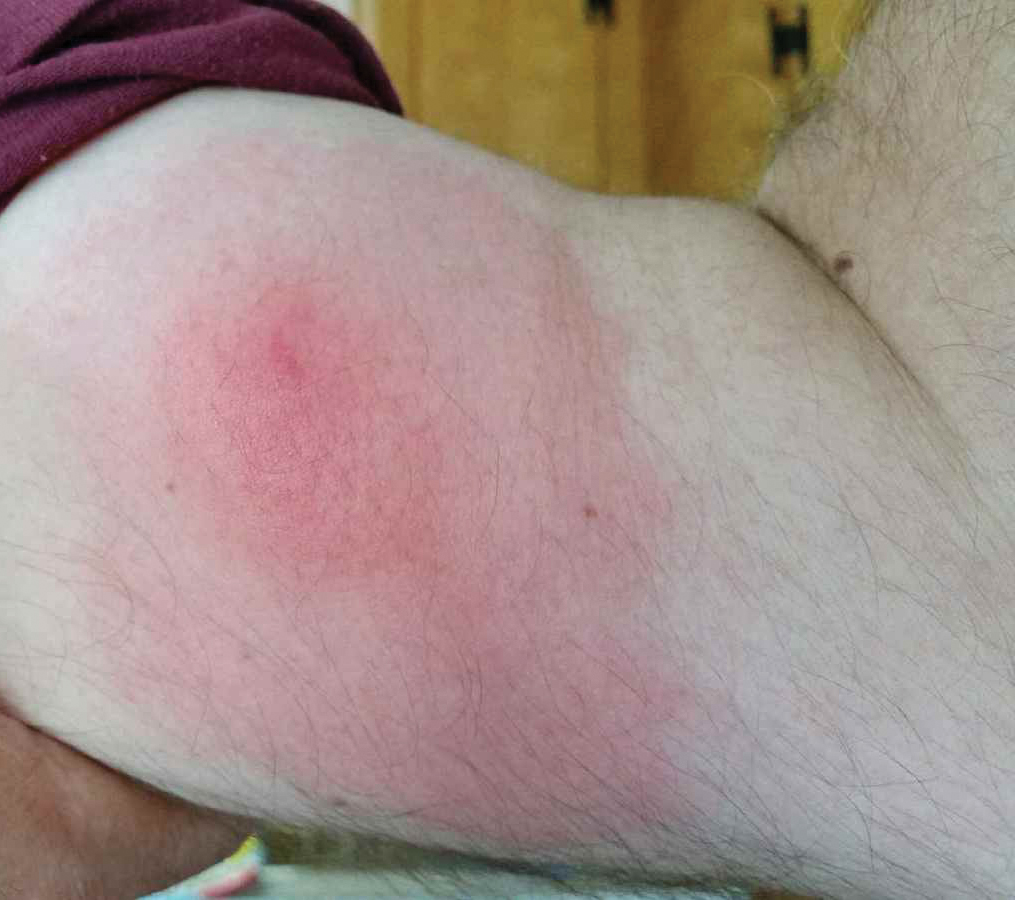
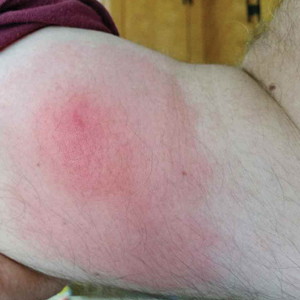
A 60-year-old man with a history of hyperlipidemia developed acute onset of an intensely pruritic and painful burning rash on the dorsal aspect of the left forearm of 8 days' duration. The patient described the rash as red and warm. It measured 2 cm at inception and peaked at 12 cm 6 months later when the patient presented. These symptoms resolved without therapeutic intervention.
Over the ensuing 6 months, he experienced 13 self-limited episodes of erythematous indurated cutaneous streaks, usually with proximal migration on the arms along with involvement of the posterior thorax and right leg. Five months prior to the onset of the initial rash, the patient had discontinued ezetimibe to treat hyperlipidemia due to swelling of the lips and tongue. He also reported that he regularly hunted in upstate Pennsylvania but reported no history of arthropod or animal bites. The patient did not take prescription or over-the-counter medications, and he denied the presence of fever, night sweats, fatigue, adenopathy, anorexia, weight loss, diarrhea, joint pain or swelling, or illicit drug use. Lyme titers, complete blood cell count, erythrocyte sedimentation rate, and comprehensive metabolic panel were within reference range. A punch biopsy was performed.
Rozanolixizumab may offer new treatment paradigm for ITP
AMSTERDAM – Rozanolixizumab, a subcutaneous antibody for the human neonatal Fc receptor, provides clinically meaningful improvements in platelet count for patients with primary immune thrombocytopenia, according to results from a recent phase 2 trial.
Rozanolixizumab was well tolerated across all dose groups, with higher doses delivering faster responses, reported lead author Tadeusz Robak, MD, PhD, of the Medical University of Lodz (Poland).
Targeting the Fc receptor interrupts recirculation of IgG, a key autoantibody in immune thrombocytopenia (ITP) pathogenesis, Dr. Robak explained during a presentation at the annual congress of the European Hematology Association. This approach represents an emerging treatment paradigm, he said, noting that rozanolixizumab is also being studied for the treatment of other IgG-driven autoimmune diseases, such as myasthenia gravis and chronic inflammatory demyelinating polyneuropathy.
The present open-label, dose-escalation study involved 54 adult patients with primary ITP of at least 3 months duration and platelet counts of less than 30 x 109/L at screening and 35 x 109/L at baseline. Eligibility required a previous response to ITP therapy. Enrolled patients were randomized into four dose groups: 4 mg/kg (five doses), 7 mg/kg (three doses), 10 mg/kg (two doses), or 15 mg/kg (one dose). After dosing, patients were followed for 8 weeks. Clinically relevant efficacy was defined as a platelet count of at least 50 x 109/L. Decreases in IgG were also reported.
A safety analysis showed that the regimen was well tolerated across all dose groups. In total, 20.4% of patients experienced at least one treatment-related adverse event. The most common adverse events were headache (31.5%), diarrhea (11.1%), and vomiting (3.7%); all of which were mild or moderate. Headache appeared to be dose related, as 42% of patients in the 15-mg/kg group reported headache, compared with 8% in the 10-mg/kg group, 7% in the 7-mg/kg group, and none in the 4-mg/kg group. Out of four reported serious adverse events, none were considered treatment related.
Concerning efficacy, higher doses were associated with higher response rates and faster response times. In the 4-mg/kg group, 33% of patients achieved a platelet count of at least 50 x 109/L, compared with 33% of the 7-mg/kg group, 50% of the 10-mg/kg group, and 67% of the 15-mg/kg group. Of the patients that achieved clinically meaningful responses, 20% of the 4-mg/kg group did so within 8 days, compared with 40% of 7-mg/kg responders, 50% of 10-mg/kg responders, and 87.5% of 15-mg/kg responders. Additional observations included dose-dependent decreases in IgG titer and longer response durations after multiple lower doses.
“Data from this study indicate that we can achieve effective increases in platelet levels, we can observe decreasing IgG levels, and the treatment was safe for the patients,” Dr. Robak said.
When asked about the intended clinical application of rozanolixizumab, Dr. Robak suggested that the agent may have a role in the postacute care setting. “We should develop a method of prolonged administration of [rozanolixizumab], as we saw that lower, multiple doses gave longer response durations.”
Still, he noted that more research is needed, since responses in diverse patient populations remain unknown. “We do not know how the drug will be active in truly refractory patients and we need this response before we establish the indication for the drug.”
The investigators reported financial relationships with Celgene, Roche, GlaxoSmithKline, Amgen, AbbVie, and other companies.
SOURCE: Robak T et al. EHA Congress, Abstract S850.
AMSTERDAM – Rozanolixizumab, a subcutaneous antibody for the human neonatal Fc receptor, provides clinically meaningful improvements in platelet count for patients with primary immune thrombocytopenia, according to results from a recent phase 2 trial.
Rozanolixizumab was well tolerated across all dose groups, with higher doses delivering faster responses, reported lead author Tadeusz Robak, MD, PhD, of the Medical University of Lodz (Poland).
Targeting the Fc receptor interrupts recirculation of IgG, a key autoantibody in immune thrombocytopenia (ITP) pathogenesis, Dr. Robak explained during a presentation at the annual congress of the European Hematology Association. This approach represents an emerging treatment paradigm, he said, noting that rozanolixizumab is also being studied for the treatment of other IgG-driven autoimmune diseases, such as myasthenia gravis and chronic inflammatory demyelinating polyneuropathy.
The present open-label, dose-escalation study involved 54 adult patients with primary ITP of at least 3 months duration and platelet counts of less than 30 x 109/L at screening and 35 x 109/L at baseline. Eligibility required a previous response to ITP therapy. Enrolled patients were randomized into four dose groups: 4 mg/kg (five doses), 7 mg/kg (three doses), 10 mg/kg (two doses), or 15 mg/kg (one dose). After dosing, patients were followed for 8 weeks. Clinically relevant efficacy was defined as a platelet count of at least 50 x 109/L. Decreases in IgG were also reported.
A safety analysis showed that the regimen was well tolerated across all dose groups. In total, 20.4% of patients experienced at least one treatment-related adverse event. The most common adverse events were headache (31.5%), diarrhea (11.1%), and vomiting (3.7%); all of which were mild or moderate. Headache appeared to be dose related, as 42% of patients in the 15-mg/kg group reported headache, compared with 8% in the 10-mg/kg group, 7% in the 7-mg/kg group, and none in the 4-mg/kg group. Out of four reported serious adverse events, none were considered treatment related.
Concerning efficacy, higher doses were associated with higher response rates and faster response times. In the 4-mg/kg group, 33% of patients achieved a platelet count of at least 50 x 109/L, compared with 33% of the 7-mg/kg group, 50% of the 10-mg/kg group, and 67% of the 15-mg/kg group. Of the patients that achieved clinically meaningful responses, 20% of the 4-mg/kg group did so within 8 days, compared with 40% of 7-mg/kg responders, 50% of 10-mg/kg responders, and 87.5% of 15-mg/kg responders. Additional observations included dose-dependent decreases in IgG titer and longer response durations after multiple lower doses.
“Data from this study indicate that we can achieve effective increases in platelet levels, we can observe decreasing IgG levels, and the treatment was safe for the patients,” Dr. Robak said.
When asked about the intended clinical application of rozanolixizumab, Dr. Robak suggested that the agent may have a role in the postacute care setting. “We should develop a method of prolonged administration of [rozanolixizumab], as we saw that lower, multiple doses gave longer response durations.”
Still, he noted that more research is needed, since responses in diverse patient populations remain unknown. “We do not know how the drug will be active in truly refractory patients and we need this response before we establish the indication for the drug.”
The investigators reported financial relationships with Celgene, Roche, GlaxoSmithKline, Amgen, AbbVie, and other companies.
SOURCE: Robak T et al. EHA Congress, Abstract S850.
AMSTERDAM – Rozanolixizumab, a subcutaneous antibody for the human neonatal Fc receptor, provides clinically meaningful improvements in platelet count for patients with primary immune thrombocytopenia, according to results from a recent phase 2 trial.
Rozanolixizumab was well tolerated across all dose groups, with higher doses delivering faster responses, reported lead author Tadeusz Robak, MD, PhD, of the Medical University of Lodz (Poland).
Targeting the Fc receptor interrupts recirculation of IgG, a key autoantibody in immune thrombocytopenia (ITP) pathogenesis, Dr. Robak explained during a presentation at the annual congress of the European Hematology Association. This approach represents an emerging treatment paradigm, he said, noting that rozanolixizumab is also being studied for the treatment of other IgG-driven autoimmune diseases, such as myasthenia gravis and chronic inflammatory demyelinating polyneuropathy.
The present open-label, dose-escalation study involved 54 adult patients with primary ITP of at least 3 months duration and platelet counts of less than 30 x 109/L at screening and 35 x 109/L at baseline. Eligibility required a previous response to ITP therapy. Enrolled patients were randomized into four dose groups: 4 mg/kg (five doses), 7 mg/kg (three doses), 10 mg/kg (two doses), or 15 mg/kg (one dose). After dosing, patients were followed for 8 weeks. Clinically relevant efficacy was defined as a platelet count of at least 50 x 109/L. Decreases in IgG were also reported.
A safety analysis showed that the regimen was well tolerated across all dose groups. In total, 20.4% of patients experienced at least one treatment-related adverse event. The most common adverse events were headache (31.5%), diarrhea (11.1%), and vomiting (3.7%); all of which were mild or moderate. Headache appeared to be dose related, as 42% of patients in the 15-mg/kg group reported headache, compared with 8% in the 10-mg/kg group, 7% in the 7-mg/kg group, and none in the 4-mg/kg group. Out of four reported serious adverse events, none were considered treatment related.
Concerning efficacy, higher doses were associated with higher response rates and faster response times. In the 4-mg/kg group, 33% of patients achieved a platelet count of at least 50 x 109/L, compared with 33% of the 7-mg/kg group, 50% of the 10-mg/kg group, and 67% of the 15-mg/kg group. Of the patients that achieved clinically meaningful responses, 20% of the 4-mg/kg group did so within 8 days, compared with 40% of 7-mg/kg responders, 50% of 10-mg/kg responders, and 87.5% of 15-mg/kg responders. Additional observations included dose-dependent decreases in IgG titer and longer response durations after multiple lower doses.
“Data from this study indicate that we can achieve effective increases in platelet levels, we can observe decreasing IgG levels, and the treatment was safe for the patients,” Dr. Robak said.
When asked about the intended clinical application of rozanolixizumab, Dr. Robak suggested that the agent may have a role in the postacute care setting. “We should develop a method of prolonged administration of [rozanolixizumab], as we saw that lower, multiple doses gave longer response durations.”
Still, he noted that more research is needed, since responses in diverse patient populations remain unknown. “We do not know how the drug will be active in truly refractory patients and we need this response before we establish the indication for the drug.”
The investigators reported financial relationships with Celgene, Roche, GlaxoSmithKline, Amgen, AbbVie, and other companies.
SOURCE: Robak T et al. EHA Congress, Abstract S850.
REPORTING FROM EHA CONGRESS
Budesonide tablets considerably outperformed placebo for active EoE
Budesonide orodispersible tablets (BOTs) are highly effective in inducing disease remission in adults with active eosinophilic esophagitis (EOE), according to a study of the tablets versus placebo in European patients.
“A 6-week treatment with 1 mg budesonide twice daily was highly superior over placebo with regard to all predefined primary and secondary outcomes,” wrote Alfredo J. Lucendo, MD, of Hospital General de Tomelloso in Real, Spain, and his coauthors. The study was published in Gastroenterology.
To assess the effectiveness and tolerability of BOT in adults with EOE, Dr. Lucendo and his fellow researchers launched a randomized, placebo-controlled trial made up of 88 European adults with active EoE. Patients were assigned to either a group that received BOT twice daily (n = 59) or a group that received placebo (n = 29). The primary endpoint was complete remission.
After 6 weeks, 34 of 59 patients (58%) receiving BOT had achieved complete remission, compared with 0 patients receiving placebo (P less than .0001). After 12 weeks, 50 of 59 patients (85%) in the BOT group had achieved complete remission. BOT was also well tolerated; no serious adverse event was reported, and no differences were observed between groups with regard to commonly reported adverse events.
The coauthors acknowledged their study’s limitations, including the fact that it was designed to demonstrate budesonide’s superiority to placebo at 6 weeks, not to identify the time of its maximal effect. In addition, the researchers did not identify a minimally effective dose; they did, however, note their belief that a lower dose could still achieve similar rates of remission and “a higher dose would not achieve a higher clinico-remission rate.”
The study was funded by Dr. Falk Pharma. The authors reported numerous conflicts of interest, including receiving research funding and speaker fees from various pharmaceutical companies and foundations.
SOURCE: Lucendo AJ et al. Gastroenterology. 2019 Mar 25. doi: 10.1053/j.gastro.2019.03.025.
Budesonide orodispersible tablets (BOTs) are highly effective in inducing disease remission in adults with active eosinophilic esophagitis (EOE), according to a study of the tablets versus placebo in European patients.
“A 6-week treatment with 1 mg budesonide twice daily was highly superior over placebo with regard to all predefined primary and secondary outcomes,” wrote Alfredo J. Lucendo, MD, of Hospital General de Tomelloso in Real, Spain, and his coauthors. The study was published in Gastroenterology.
To assess the effectiveness and tolerability of BOT in adults with EOE, Dr. Lucendo and his fellow researchers launched a randomized, placebo-controlled trial made up of 88 European adults with active EoE. Patients were assigned to either a group that received BOT twice daily (n = 59) or a group that received placebo (n = 29). The primary endpoint was complete remission.
After 6 weeks, 34 of 59 patients (58%) receiving BOT had achieved complete remission, compared with 0 patients receiving placebo (P less than .0001). After 12 weeks, 50 of 59 patients (85%) in the BOT group had achieved complete remission. BOT was also well tolerated; no serious adverse event was reported, and no differences were observed between groups with regard to commonly reported adverse events.
The coauthors acknowledged their study’s limitations, including the fact that it was designed to demonstrate budesonide’s superiority to placebo at 6 weeks, not to identify the time of its maximal effect. In addition, the researchers did not identify a minimally effective dose; they did, however, note their belief that a lower dose could still achieve similar rates of remission and “a higher dose would not achieve a higher clinico-remission rate.”
The study was funded by Dr. Falk Pharma. The authors reported numerous conflicts of interest, including receiving research funding and speaker fees from various pharmaceutical companies and foundations.
SOURCE: Lucendo AJ et al. Gastroenterology. 2019 Mar 25. doi: 10.1053/j.gastro.2019.03.025.
Budesonide orodispersible tablets (BOTs) are highly effective in inducing disease remission in adults with active eosinophilic esophagitis (EOE), according to a study of the tablets versus placebo in European patients.
“A 6-week treatment with 1 mg budesonide twice daily was highly superior over placebo with regard to all predefined primary and secondary outcomes,” wrote Alfredo J. Lucendo, MD, of Hospital General de Tomelloso in Real, Spain, and his coauthors. The study was published in Gastroenterology.
To assess the effectiveness and tolerability of BOT in adults with EOE, Dr. Lucendo and his fellow researchers launched a randomized, placebo-controlled trial made up of 88 European adults with active EoE. Patients were assigned to either a group that received BOT twice daily (n = 59) or a group that received placebo (n = 29). The primary endpoint was complete remission.
After 6 weeks, 34 of 59 patients (58%) receiving BOT had achieved complete remission, compared with 0 patients receiving placebo (P less than .0001). After 12 weeks, 50 of 59 patients (85%) in the BOT group had achieved complete remission. BOT was also well tolerated; no serious adverse event was reported, and no differences were observed between groups with regard to commonly reported adverse events.
The coauthors acknowledged their study’s limitations, including the fact that it was designed to demonstrate budesonide’s superiority to placebo at 6 weeks, not to identify the time of its maximal effect. In addition, the researchers did not identify a minimally effective dose; they did, however, note their belief that a lower dose could still achieve similar rates of remission and “a higher dose would not achieve a higher clinico-remission rate.”
The study was funded by Dr. Falk Pharma. The authors reported numerous conflicts of interest, including receiving research funding and speaker fees from various pharmaceutical companies and foundations.
SOURCE: Lucendo AJ et al. Gastroenterology. 2019 Mar 25. doi: 10.1053/j.gastro.2019.03.025.
FROM GASTROENTEROLOGY
Highlights of the 2019 Update to the Global Initiative for Chronic Obstructive Lung Disease (GOLD) Report and Their Application in Practice
Click here to read the supplement
Key Points
- The Global Initiative for Chronic Obstructive Lung Disease (GOLD) program releases consensus reports to provide evidence-based recommendations about the management and prevention of chronic obstructive pulmonary disease (COPD);
- The most recent major update occurred in November 2018.
- The 2019 GOLD strategy for COPD assessment now separates lung function measures from respiratory symptom scores because lung function is only weakly correlated to symptoms and health status impairment.
- The updated strategy now provides recommendations for initiation and maintenance of pharmacologic therapy in patients based on exacerbation risk and symptom scores, with consideration of blood eosinophil counts.
- Management of exacerbations now includes the prevention of future exacerbations, which may require adding another medication to the patient's maintenance regimen.
About the Authors:

Jennifer Banfield, APRN, FNP
Clinical Research Coordinator
Boys Town National Research Hospital
Boys Town, Nebraska

Kevin R. Murphy, MD
Director of Allergy, Asthma, and Pulmonary Research
Boys Town National Research Hospital
Boys Town, Nebraska
University of Nebraska Medical Center
Creighton University School of Medicine
Omaha, Nebraska
Click here to read the supplement
Key Points
- The Global Initiative for Chronic Obstructive Lung Disease (GOLD) program releases consensus reports to provide evidence-based recommendations about the management and prevention of chronic obstructive pulmonary disease (COPD);
- The most recent major update occurred in November 2018.
- The 2019 GOLD strategy for COPD assessment now separates lung function measures from respiratory symptom scores because lung function is only weakly correlated to symptoms and health status impairment.
- The updated strategy now provides recommendations for initiation and maintenance of pharmacologic therapy in patients based on exacerbation risk and symptom scores, with consideration of blood eosinophil counts.
- Management of exacerbations now includes the prevention of future exacerbations, which may require adding another medication to the patient's maintenance regimen.
About the Authors:

Jennifer Banfield, APRN, FNP
Clinical Research Coordinator
Boys Town National Research Hospital
Boys Town, Nebraska

Kevin R. Murphy, MD
Director of Allergy, Asthma, and Pulmonary Research
Boys Town National Research Hospital
Boys Town, Nebraska
University of Nebraska Medical Center
Creighton University School of Medicine
Omaha, Nebraska
Click here to read the supplement
Key Points
- The Global Initiative for Chronic Obstructive Lung Disease (GOLD) program releases consensus reports to provide evidence-based recommendations about the management and prevention of chronic obstructive pulmonary disease (COPD);
- The most recent major update occurred in November 2018.
- The 2019 GOLD strategy for COPD assessment now separates lung function measures from respiratory symptom scores because lung function is only weakly correlated to symptoms and health status impairment.
- The updated strategy now provides recommendations for initiation and maintenance of pharmacologic therapy in patients based on exacerbation risk and symptom scores, with consideration of blood eosinophil counts.
- Management of exacerbations now includes the prevention of future exacerbations, which may require adding another medication to the patient's maintenance regimen.
About the Authors:

Jennifer Banfield, APRN, FNP
Clinical Research Coordinator
Boys Town National Research Hospital
Boys Town, Nebraska

Kevin R. Murphy, MD
Director of Allergy, Asthma, and Pulmonary Research
Boys Town National Research Hospital
Boys Town, Nebraska
University of Nebraska Medical Center
Creighton University School of Medicine
Omaha, Nebraska