User login
Good health goes beyond having a doctor and insurance, says AMA’s equity chief
Part of Dr. Aletha Maybank’s medical training left a sour taste in her mouth.
Her superiors told her not to worry about nonmedical issues affecting her patients’ quality of life, she said, because social workers would handle it. But she didn’t understand how physicians could divorce medical advice from the context of patients’ lives.
“How can you offer advice as recommendations that’s not even relevant to how their day to day plays out?” Dr. Maybank asked.
Today, Dr. Maybank is continuing to question that medical school philosophy. She was recently named the first chief health equity officer for the American Medical Association. In that job, she is responsible for implementing practices among doctors across the country to help end disparities in care. She has a full agenda, including launching the group’s Center for Health Equity and helping the Chicago-based doctors association reach out to people in poor neighborhoods in the city.
A pediatrician, Dr. Maybank previously worked for the New York City government as deputy commissioner for the health department and founding director of the city’s health equity center.
Carmen Heredia Rodriguez of Kaiser Health News recently spoke with Dr. Maybank about her new role and how health inequities affect Americans. This transcript has been edited for length and clarity.
Q: Can you tell me what health equity means to you, and what are some of the main drivers that are keeping health inequitable in this country?
The AMA policy around health equity is optimal health for all people.
But it’s not just an outcome; there’s a process to get there. How do we engage with people? How do we look at and collect our data to make sure our practices and processes are equitable? How do we hire differently to ensure diversity? All these things are processes to achieve health equity.
In order to understand what produces inequities, we have to understand what creates health. Health is created outside of the walls of the doctor’s office and at the hospital. What are patients’ jobs and employment like? The kind of education they have. Income. Their ability to build wealth. All of these are conditions that impact health.
Q: Is there anything along your career path that really surprised you about the state of health care in the United States?
There’s the perception that all of our health is really determined by whether you have a doctor or not, or if you have insurance. What creates health is much beyond that.
So if we really want to work on health and equity, we have to partner with people who are in the education space and the economic space and the housing spaces, because that’s where health inequities are produced. You could have insurance coverage. You could have a primary care doctor. But it doesn’t mean that you’re not going to experience health inequities.
Q: Discrimination based on racial lines is one obvious driver of health inequities. What are some of the other populations that are affected by health inequity?
I think structural racism is a system that affects us all.
It’s not just the black-white issue. So, whether it’s discrimination or inequities that exist among LGBT youth and transgender [or] nonconforming people, or if it’s folks who are immigrants or women, a lot of that is contextualized under the umbrella of white supremacy within the country.
Q: And what are some of your priorities?
A large part of my work will be how I build the organizational capacity to better understand health equity. The reality in this country is folks aren’t comfortable talking about those issues. So, we have to destigmatize talking about all of this.
Q: Are there any particular populations or relationships that you plan to focus on?
The AMA excluded black physicians until the 1960s. So one question is: How do we work to heal relationships as well as understand the impact of our past actions? The AMA definitely issued an apology in the early 2000s, and my new role is also a step in the right direction. However, there is more that we can and should do.
Another priority now is: How do we work, and who do we work with, in our own backyard of Chicago? What can we do to work directly with people experiencing the greatest burden of disease? How do we ensure that we acknowledge the power, assets and expertise of communities so that we have the process and solutions driven and led by communities? To that end, we’ve begun working with West Side United via a relationship at Rush Medical Center. West Side United is a community-driven, collective neighborhood planning, implementation and investment effort geared toward optimizing economic well-being and improved health outcomes.
Q: Is there anything else you feel is important to understand about health equity?
Health equity and social determinants of health have become jargon. But we are talking about people’s lives. We were all born equal. We are clearly not all treated equal, but we all deserve equity. I don’t live outside of it, and none of us really do. I am one of those women who were three to four times more likely to die at childbirth because I’m black. So I don’t live outside of this experience. I’m talking about my own life.
Kaiser Health News is a nonprofit national health policy news service. It is an editorially independent program of the Henry J. Kaiser Family Foundation that is not affiliated with Kaiser Permanente.
Part of Dr. Aletha Maybank’s medical training left a sour taste in her mouth.
Her superiors told her not to worry about nonmedical issues affecting her patients’ quality of life, she said, because social workers would handle it. But she didn’t understand how physicians could divorce medical advice from the context of patients’ lives.
“How can you offer advice as recommendations that’s not even relevant to how their day to day plays out?” Dr. Maybank asked.
Today, Dr. Maybank is continuing to question that medical school philosophy. She was recently named the first chief health equity officer for the American Medical Association. In that job, she is responsible for implementing practices among doctors across the country to help end disparities in care. She has a full agenda, including launching the group’s Center for Health Equity and helping the Chicago-based doctors association reach out to people in poor neighborhoods in the city.
A pediatrician, Dr. Maybank previously worked for the New York City government as deputy commissioner for the health department and founding director of the city’s health equity center.
Carmen Heredia Rodriguez of Kaiser Health News recently spoke with Dr. Maybank about her new role and how health inequities affect Americans. This transcript has been edited for length and clarity.
Q: Can you tell me what health equity means to you, and what are some of the main drivers that are keeping health inequitable in this country?
The AMA policy around health equity is optimal health for all people.
But it’s not just an outcome; there’s a process to get there. How do we engage with people? How do we look at and collect our data to make sure our practices and processes are equitable? How do we hire differently to ensure diversity? All these things are processes to achieve health equity.
In order to understand what produces inequities, we have to understand what creates health. Health is created outside of the walls of the doctor’s office and at the hospital. What are patients’ jobs and employment like? The kind of education they have. Income. Their ability to build wealth. All of these are conditions that impact health.
Q: Is there anything along your career path that really surprised you about the state of health care in the United States?
There’s the perception that all of our health is really determined by whether you have a doctor or not, or if you have insurance. What creates health is much beyond that.
So if we really want to work on health and equity, we have to partner with people who are in the education space and the economic space and the housing spaces, because that’s where health inequities are produced. You could have insurance coverage. You could have a primary care doctor. But it doesn’t mean that you’re not going to experience health inequities.
Q: Discrimination based on racial lines is one obvious driver of health inequities. What are some of the other populations that are affected by health inequity?
I think structural racism is a system that affects us all.
It’s not just the black-white issue. So, whether it’s discrimination or inequities that exist among LGBT youth and transgender [or] nonconforming people, or if it’s folks who are immigrants or women, a lot of that is contextualized under the umbrella of white supremacy within the country.
Q: And what are some of your priorities?
A large part of my work will be how I build the organizational capacity to better understand health equity. The reality in this country is folks aren’t comfortable talking about those issues. So, we have to destigmatize talking about all of this.
Q: Are there any particular populations or relationships that you plan to focus on?
The AMA excluded black physicians until the 1960s. So one question is: How do we work to heal relationships as well as understand the impact of our past actions? The AMA definitely issued an apology in the early 2000s, and my new role is also a step in the right direction. However, there is more that we can and should do.
Another priority now is: How do we work, and who do we work with, in our own backyard of Chicago? What can we do to work directly with people experiencing the greatest burden of disease? How do we ensure that we acknowledge the power, assets and expertise of communities so that we have the process and solutions driven and led by communities? To that end, we’ve begun working with West Side United via a relationship at Rush Medical Center. West Side United is a community-driven, collective neighborhood planning, implementation and investment effort geared toward optimizing economic well-being and improved health outcomes.
Q: Is there anything else you feel is important to understand about health equity?
Health equity and social determinants of health have become jargon. But we are talking about people’s lives. We were all born equal. We are clearly not all treated equal, but we all deserve equity. I don’t live outside of it, and none of us really do. I am one of those women who were three to four times more likely to die at childbirth because I’m black. So I don’t live outside of this experience. I’m talking about my own life.
Kaiser Health News is a nonprofit national health policy news service. It is an editorially independent program of the Henry J. Kaiser Family Foundation that is not affiliated with Kaiser Permanente.
Part of Dr. Aletha Maybank’s medical training left a sour taste in her mouth.
Her superiors told her not to worry about nonmedical issues affecting her patients’ quality of life, she said, because social workers would handle it. But she didn’t understand how physicians could divorce medical advice from the context of patients’ lives.
“How can you offer advice as recommendations that’s not even relevant to how their day to day plays out?” Dr. Maybank asked.
Today, Dr. Maybank is continuing to question that medical school philosophy. She was recently named the first chief health equity officer for the American Medical Association. In that job, she is responsible for implementing practices among doctors across the country to help end disparities in care. She has a full agenda, including launching the group’s Center for Health Equity and helping the Chicago-based doctors association reach out to people in poor neighborhoods in the city.
A pediatrician, Dr. Maybank previously worked for the New York City government as deputy commissioner for the health department and founding director of the city’s health equity center.
Carmen Heredia Rodriguez of Kaiser Health News recently spoke with Dr. Maybank about her new role and how health inequities affect Americans. This transcript has been edited for length and clarity.
Q: Can you tell me what health equity means to you, and what are some of the main drivers that are keeping health inequitable in this country?
The AMA policy around health equity is optimal health for all people.
But it’s not just an outcome; there’s a process to get there. How do we engage with people? How do we look at and collect our data to make sure our practices and processes are equitable? How do we hire differently to ensure diversity? All these things are processes to achieve health equity.
In order to understand what produces inequities, we have to understand what creates health. Health is created outside of the walls of the doctor’s office and at the hospital. What are patients’ jobs and employment like? The kind of education they have. Income. Their ability to build wealth. All of these are conditions that impact health.
Q: Is there anything along your career path that really surprised you about the state of health care in the United States?
There’s the perception that all of our health is really determined by whether you have a doctor or not, or if you have insurance. What creates health is much beyond that.
So if we really want to work on health and equity, we have to partner with people who are in the education space and the economic space and the housing spaces, because that’s where health inequities are produced. You could have insurance coverage. You could have a primary care doctor. But it doesn’t mean that you’re not going to experience health inequities.
Q: Discrimination based on racial lines is one obvious driver of health inequities. What are some of the other populations that are affected by health inequity?
I think structural racism is a system that affects us all.
It’s not just the black-white issue. So, whether it’s discrimination or inequities that exist among LGBT youth and transgender [or] nonconforming people, or if it’s folks who are immigrants or women, a lot of that is contextualized under the umbrella of white supremacy within the country.
Q: And what are some of your priorities?
A large part of my work will be how I build the organizational capacity to better understand health equity. The reality in this country is folks aren’t comfortable talking about those issues. So, we have to destigmatize talking about all of this.
Q: Are there any particular populations or relationships that you plan to focus on?
The AMA excluded black physicians until the 1960s. So one question is: How do we work to heal relationships as well as understand the impact of our past actions? The AMA definitely issued an apology in the early 2000s, and my new role is also a step in the right direction. However, there is more that we can and should do.
Another priority now is: How do we work, and who do we work with, in our own backyard of Chicago? What can we do to work directly with people experiencing the greatest burden of disease? How do we ensure that we acknowledge the power, assets and expertise of communities so that we have the process and solutions driven and led by communities? To that end, we’ve begun working with West Side United via a relationship at Rush Medical Center. West Side United is a community-driven, collective neighborhood planning, implementation and investment effort geared toward optimizing economic well-being and improved health outcomes.
Q: Is there anything else you feel is important to understand about health equity?
Health equity and social determinants of health have become jargon. But we are talking about people’s lives. We were all born equal. We are clearly not all treated equal, but we all deserve equity. I don’t live outside of it, and none of us really do. I am one of those women who were three to four times more likely to die at childbirth because I’m black. So I don’t live outside of this experience. I’m talking about my own life.
Kaiser Health News is a nonprofit national health policy news service. It is an editorially independent program of the Henry J. Kaiser Family Foundation that is not affiliated with Kaiser Permanente.
Aggressive lowering of LDL cholesterol: Is it a good idea?
SAN FRANCISCO – Powerful drugs now make it possible to lower LDL cholesterol levels to dramatically low levels. But is this a good idea? There are risks, and a cardiologist urged diabetes professionals to not overdo cholesterol reduction. But a colleague argued in favor of aggressively targeting “bad” cholesterol.
“We used to say you can’t be too rich or too thin. We now say you can’t be too rich or too thin or have a too-low LDL cholesterol,” said cardiologist Steven E. Nissen, MD, chairman of cardiovascular medicine at the Cleveland Clinic Foundation, who spoke at the annual scientific sessions of the American Diabetes Association about the wisdom of extreme LDL cholesterol lowering.
Dr. Nissen faced off in a debate with cardiologist Sanket Dhruva, MD, of the University of California, San Francisco, who doesn’t support aggressive LDL cholesterol lowering.
It is fine, Dr. Dhruva said, to treat patients so their LDL cholesterol levels drop below 100 mg/dL. “I don’t think there’s any argument there.”
But Dr. Dhruva questioned whether it’s a good idea to generally decrease LDL cholesterol well below 70 mg/dL, as is now possible with the use of proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibitors.
He pointed to a 2010 study that found aggressively lowering LDL cholesterol led to a mean net gain of 4.1 quality-adjusted life-years in high-risk patients, but less than 1 quality-adjusted life-year in low-risk patients. According to him, the study also found that the biggest benefits in both high- and low-risk patients came from the initial lower statin dose (Arch Intern Med. 2010 Jun 28;170[12]:1037-44).
“It’s really the statin initiation that provides the most benefit to our patients with diabetes,” Dr. Dhruva said.
Also, he added, a 2016 study questioned the value of aggressively lowering LDL cholesterol. It found that, although patients on statins with LDL cholesterol levels of 70-100 mg/dL had a lower risk of adverse cardiac outcomes than did those with levels between 100 and 130 mg/dL, no additional benefit was gained by achieving an LDL cholesterol level below 70 mg/dL (JAMA Intern Med. 2016 Aug 1;176[8]:1105-13)
As for risks, Dr. Dhruva highlighted a 2016 pooled analysis of 14 trials that linked the PCSK9 inhibitor alirocumab (Praluent) and LDL cholesterol levels below 25 mg/dL to significantly higher levels of cataracts, compared with levels of at least 25 mg/dL (hazard ratio, 3.4).
There are other reasons to be cautious of aggressive LDL cholesterol lowering. For one, many patients are not on statins when they’re prescribed PCSK-9 inhibitors. “We’re sometimes missing the building blocks before getting to expensive medications,” he said.
He added that PCSK-9 inhibitors are pricey, and some patients can’t get access to them. “Lipid control is incredibly important, but what about the stress or anxiety of our patients who are told this medication will reduce their cardiac risk but they can’t afford it? That’s not good for their cardiovascular risk.”
For his part, Dr. Nissen challenged Dr. Dhruva’s concerns about the cost of the drugs. “It’s not like they’re way out of line in terms of expense,” he said, noting that their cost – several thousand dollars a year – is similar to the cost of diabetes drugs known as glucagonlike peptide–1 receptor agonists and sodium-glucose transporter 2 inhibitors.
According to Dr. Nissen, multiple studies have supported aggressive LDL cholesterol lowering. “You’re going to see this over and over again in clinical trials: Every time we lower LDL by more, we get more reductions in morbidity and mortality.”
For example, he said, the FOURIER trial of the PCSK9 inhibitor evolocumab (Repatha) found that it lowered LDL cholesterol levels to a median 30 mg/dL “and reduced the risk of cardiovascular events. These findings show that patients with atherosclerotic cardiovascular disease benefit from lowering of LDL cholesterol levels below current targets [N Engl J Med 2017;376:1713-22].”
Dr. Nissen pointed to another study, this one also from 2017, that reported “in individuals with 5-year risk of major vascular events lower than 10%, each 1 mmol/L reduction in LDL cholesterol produced an absolute reduction in major vascular events of about 11 per 1,000 over 5 years. This benefit greatly exceeds any known hazards of statin therapy.”
In regard to adverse effects, he said, research has hinted at a slight uptick in blood sugar levels “that does not take away the major cardiovascular benefits of the drugs.”
Overall, he said, “compelling evidence from trials in hundreds of thousands of patients demonstrates that reducing LDL cholesterol to very low levels reduces cardiovascular events in broad populations and is extremely safe.”
Dr. Nissen reported consulting for many pharmaceutical companies and performing clinical trials for Amgen, AbbVie, AstraZeneca, Cerenis Therapeutics, Esperion Therapeutics, Lilly, Novartis, Novo Nordisk, the Medicines Company, Orexigen Therapeutics, Takeda, and Pfizer. He does not receive income for honoraria, speaking fees, or consulting fees as they are paid directly to charity.
SAN FRANCISCO – Powerful drugs now make it possible to lower LDL cholesterol levels to dramatically low levels. But is this a good idea? There are risks, and a cardiologist urged diabetes professionals to not overdo cholesterol reduction. But a colleague argued in favor of aggressively targeting “bad” cholesterol.
“We used to say you can’t be too rich or too thin. We now say you can’t be too rich or too thin or have a too-low LDL cholesterol,” said cardiologist Steven E. Nissen, MD, chairman of cardiovascular medicine at the Cleveland Clinic Foundation, who spoke at the annual scientific sessions of the American Diabetes Association about the wisdom of extreme LDL cholesterol lowering.
Dr. Nissen faced off in a debate with cardiologist Sanket Dhruva, MD, of the University of California, San Francisco, who doesn’t support aggressive LDL cholesterol lowering.
It is fine, Dr. Dhruva said, to treat patients so their LDL cholesterol levels drop below 100 mg/dL. “I don’t think there’s any argument there.”
But Dr. Dhruva questioned whether it’s a good idea to generally decrease LDL cholesterol well below 70 mg/dL, as is now possible with the use of proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibitors.
He pointed to a 2010 study that found aggressively lowering LDL cholesterol led to a mean net gain of 4.1 quality-adjusted life-years in high-risk patients, but less than 1 quality-adjusted life-year in low-risk patients. According to him, the study also found that the biggest benefits in both high- and low-risk patients came from the initial lower statin dose (Arch Intern Med. 2010 Jun 28;170[12]:1037-44).
“It’s really the statin initiation that provides the most benefit to our patients with diabetes,” Dr. Dhruva said.
Also, he added, a 2016 study questioned the value of aggressively lowering LDL cholesterol. It found that, although patients on statins with LDL cholesterol levels of 70-100 mg/dL had a lower risk of adverse cardiac outcomes than did those with levels between 100 and 130 mg/dL, no additional benefit was gained by achieving an LDL cholesterol level below 70 mg/dL (JAMA Intern Med. 2016 Aug 1;176[8]:1105-13)
As for risks, Dr. Dhruva highlighted a 2016 pooled analysis of 14 trials that linked the PCSK9 inhibitor alirocumab (Praluent) and LDL cholesterol levels below 25 mg/dL to significantly higher levels of cataracts, compared with levels of at least 25 mg/dL (hazard ratio, 3.4).
There are other reasons to be cautious of aggressive LDL cholesterol lowering. For one, many patients are not on statins when they’re prescribed PCSK-9 inhibitors. “We’re sometimes missing the building blocks before getting to expensive medications,” he said.
He added that PCSK-9 inhibitors are pricey, and some patients can’t get access to them. “Lipid control is incredibly important, but what about the stress or anxiety of our patients who are told this medication will reduce their cardiac risk but they can’t afford it? That’s not good for their cardiovascular risk.”
For his part, Dr. Nissen challenged Dr. Dhruva’s concerns about the cost of the drugs. “It’s not like they’re way out of line in terms of expense,” he said, noting that their cost – several thousand dollars a year – is similar to the cost of diabetes drugs known as glucagonlike peptide–1 receptor agonists and sodium-glucose transporter 2 inhibitors.
According to Dr. Nissen, multiple studies have supported aggressive LDL cholesterol lowering. “You’re going to see this over and over again in clinical trials: Every time we lower LDL by more, we get more reductions in morbidity and mortality.”
For example, he said, the FOURIER trial of the PCSK9 inhibitor evolocumab (Repatha) found that it lowered LDL cholesterol levels to a median 30 mg/dL “and reduced the risk of cardiovascular events. These findings show that patients with atherosclerotic cardiovascular disease benefit from lowering of LDL cholesterol levels below current targets [N Engl J Med 2017;376:1713-22].”
Dr. Nissen pointed to another study, this one also from 2017, that reported “in individuals with 5-year risk of major vascular events lower than 10%, each 1 mmol/L reduction in LDL cholesterol produced an absolute reduction in major vascular events of about 11 per 1,000 over 5 years. This benefit greatly exceeds any known hazards of statin therapy.”
In regard to adverse effects, he said, research has hinted at a slight uptick in blood sugar levels “that does not take away the major cardiovascular benefits of the drugs.”
Overall, he said, “compelling evidence from trials in hundreds of thousands of patients demonstrates that reducing LDL cholesterol to very low levels reduces cardiovascular events in broad populations and is extremely safe.”
Dr. Nissen reported consulting for many pharmaceutical companies and performing clinical trials for Amgen, AbbVie, AstraZeneca, Cerenis Therapeutics, Esperion Therapeutics, Lilly, Novartis, Novo Nordisk, the Medicines Company, Orexigen Therapeutics, Takeda, and Pfizer. He does not receive income for honoraria, speaking fees, or consulting fees as they are paid directly to charity.
SAN FRANCISCO – Powerful drugs now make it possible to lower LDL cholesterol levels to dramatically low levels. But is this a good idea? There are risks, and a cardiologist urged diabetes professionals to not overdo cholesterol reduction. But a colleague argued in favor of aggressively targeting “bad” cholesterol.
“We used to say you can’t be too rich or too thin. We now say you can’t be too rich or too thin or have a too-low LDL cholesterol,” said cardiologist Steven E. Nissen, MD, chairman of cardiovascular medicine at the Cleveland Clinic Foundation, who spoke at the annual scientific sessions of the American Diabetes Association about the wisdom of extreme LDL cholesterol lowering.
Dr. Nissen faced off in a debate with cardiologist Sanket Dhruva, MD, of the University of California, San Francisco, who doesn’t support aggressive LDL cholesterol lowering.
It is fine, Dr. Dhruva said, to treat patients so their LDL cholesterol levels drop below 100 mg/dL. “I don’t think there’s any argument there.”
But Dr. Dhruva questioned whether it’s a good idea to generally decrease LDL cholesterol well below 70 mg/dL, as is now possible with the use of proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibitors.
He pointed to a 2010 study that found aggressively lowering LDL cholesterol led to a mean net gain of 4.1 quality-adjusted life-years in high-risk patients, but less than 1 quality-adjusted life-year in low-risk patients. According to him, the study also found that the biggest benefits in both high- and low-risk patients came from the initial lower statin dose (Arch Intern Med. 2010 Jun 28;170[12]:1037-44).
“It’s really the statin initiation that provides the most benefit to our patients with diabetes,” Dr. Dhruva said.
Also, he added, a 2016 study questioned the value of aggressively lowering LDL cholesterol. It found that, although patients on statins with LDL cholesterol levels of 70-100 mg/dL had a lower risk of adverse cardiac outcomes than did those with levels between 100 and 130 mg/dL, no additional benefit was gained by achieving an LDL cholesterol level below 70 mg/dL (JAMA Intern Med. 2016 Aug 1;176[8]:1105-13)
As for risks, Dr. Dhruva highlighted a 2016 pooled analysis of 14 trials that linked the PCSK9 inhibitor alirocumab (Praluent) and LDL cholesterol levels below 25 mg/dL to significantly higher levels of cataracts, compared with levels of at least 25 mg/dL (hazard ratio, 3.4).
There are other reasons to be cautious of aggressive LDL cholesterol lowering. For one, many patients are not on statins when they’re prescribed PCSK-9 inhibitors. “We’re sometimes missing the building blocks before getting to expensive medications,” he said.
He added that PCSK-9 inhibitors are pricey, and some patients can’t get access to them. “Lipid control is incredibly important, but what about the stress or anxiety of our patients who are told this medication will reduce their cardiac risk but they can’t afford it? That’s not good for their cardiovascular risk.”
For his part, Dr. Nissen challenged Dr. Dhruva’s concerns about the cost of the drugs. “It’s not like they’re way out of line in terms of expense,” he said, noting that their cost – several thousand dollars a year – is similar to the cost of diabetes drugs known as glucagonlike peptide–1 receptor agonists and sodium-glucose transporter 2 inhibitors.
According to Dr. Nissen, multiple studies have supported aggressive LDL cholesterol lowering. “You’re going to see this over and over again in clinical trials: Every time we lower LDL by more, we get more reductions in morbidity and mortality.”
For example, he said, the FOURIER trial of the PCSK9 inhibitor evolocumab (Repatha) found that it lowered LDL cholesterol levels to a median 30 mg/dL “and reduced the risk of cardiovascular events. These findings show that patients with atherosclerotic cardiovascular disease benefit from lowering of LDL cholesterol levels below current targets [N Engl J Med 2017;376:1713-22].”
Dr. Nissen pointed to another study, this one also from 2017, that reported “in individuals with 5-year risk of major vascular events lower than 10%, each 1 mmol/L reduction in LDL cholesterol produced an absolute reduction in major vascular events of about 11 per 1,000 over 5 years. This benefit greatly exceeds any known hazards of statin therapy.”
In regard to adverse effects, he said, research has hinted at a slight uptick in blood sugar levels “that does not take away the major cardiovascular benefits of the drugs.”
Overall, he said, “compelling evidence from trials in hundreds of thousands of patients demonstrates that reducing LDL cholesterol to very low levels reduces cardiovascular events in broad populations and is extremely safe.”
Dr. Nissen reported consulting for many pharmaceutical companies and performing clinical trials for Amgen, AbbVie, AstraZeneca, Cerenis Therapeutics, Esperion Therapeutics, Lilly, Novartis, Novo Nordisk, the Medicines Company, Orexigen Therapeutics, Takeda, and Pfizer. He does not receive income for honoraria, speaking fees, or consulting fees as they are paid directly to charity.
EXPERT ANALYSIS FROM ADA 2019
Levothyroxine did not reduce fatigue in older patients with hypothyroidism
WASHINGTON – according to a new study.
The double-blind, randomized, placebo-controlled, parallel-group trial used subsets of data from the TRUST study. This new research, which was presented at the Annual Meeting of the Society of General Internal Medicine, provides further evidence that prescribing levothyroxine to older patients with subclinical hypothyroidism will not reduce fatigue.
Like the findings in the TRUST study, the new study, “Effect of Thyroid Hormone Replacement on Fatigability in Older Adults with Subclinical Hypothyroidism: A Randomized Placebo Controlled Trial,” showed that the use of levothyroxine in subclinical hypothyroidism was not effective in changing physical and mental fatigability in older adults.
“This study shows that levothyroxine treatment may not be necessary and results in high costs and side effects,” noted Mirah Stuber, MD, during her presentation of the findings at the meeting.
The new study examined the effect of levothyroxine on the tiredness of older adults but with a more detailed analysis of fatigue than the full TRUST study provided. In this new study, investigators used a new assessment tool, called the Pittsburgh Fatigability Scale (PFS), a 10-item self-administered questionnaire that can measure both physical and mental fatigability. Perceived fatigability anchors tiredness to a set of activities and has been shown to be a more sensitive measure than global fatigue, explained Dr. Stuber of the University of Chicago.
The scores range from 0-50, with higher scores indicating higher fatigability. The scales were divided between mental and physical activities and measured fatigue in relation to a defined activity of a specific duration and intensity. These activities included a leisurely 30-minute walk and participation in a 1-hour social activity.
This study involved 230 participants from Switzerland and Ireland, with a mean age of 73.4 years, who had persistent hypothyroidism. The population was randomized to 119 patients who were administered levothyroxine and 111 patients who received a placebo.
At baseline, the levothyroxine group had a mean physical PFS score of 14.7 ± 9.3, and the placebo group had a score of 11.1 ± 9.1. The baseline mean mental PFS score for the levothyroxine group was 7.4 ± 8.0, while it was 5.1 ± 6.9 for the placebo group.
After 12 months of the participants’ use of levothyroxine or placebo, the physical PFS scores increased for both the treatment and placebo groups. For the levothyroxine group, the mean physical PFS score was 14.8 ± 9.6, while it was 12.4 ± 9.3 for the placebo group (P = 0.88). The investigators found no significant differences between these scores for the levothyroxine and placebo groups.
The mean mental PFS score slightly decreased to 6.0 ± 7.8 for the levothyroxine group, while it slightly increased to 6.0 ± 8.0 for the placebo group (P = 0.26) at 12 months. The difference between the mental fatigability scores for the levothyroxine and placebo arms at 12 months was also not significant.
The physical fatigability between-group difference was 0.2 (95% confidence interval, –1.8 to 2.1; P = 0.88), while the mental fatigability difference was –1.0 (95% CI, –2.8 to 0.8; P = 0.26).
The study was funded by the European Union FP7 and the Swiss National Science Foundation. Merck KGaA, Darmstadt (Germany) provided levothyroxine and the placebo. Dr. Stuber has no conflicts of interest.
WASHINGTON – according to a new study.
The double-blind, randomized, placebo-controlled, parallel-group trial used subsets of data from the TRUST study. This new research, which was presented at the Annual Meeting of the Society of General Internal Medicine, provides further evidence that prescribing levothyroxine to older patients with subclinical hypothyroidism will not reduce fatigue.
Like the findings in the TRUST study, the new study, “Effect of Thyroid Hormone Replacement on Fatigability in Older Adults with Subclinical Hypothyroidism: A Randomized Placebo Controlled Trial,” showed that the use of levothyroxine in subclinical hypothyroidism was not effective in changing physical and mental fatigability in older adults.
“This study shows that levothyroxine treatment may not be necessary and results in high costs and side effects,” noted Mirah Stuber, MD, during her presentation of the findings at the meeting.
The new study examined the effect of levothyroxine on the tiredness of older adults but with a more detailed analysis of fatigue than the full TRUST study provided. In this new study, investigators used a new assessment tool, called the Pittsburgh Fatigability Scale (PFS), a 10-item self-administered questionnaire that can measure both physical and mental fatigability. Perceived fatigability anchors tiredness to a set of activities and has been shown to be a more sensitive measure than global fatigue, explained Dr. Stuber of the University of Chicago.
The scores range from 0-50, with higher scores indicating higher fatigability. The scales were divided between mental and physical activities and measured fatigue in relation to a defined activity of a specific duration and intensity. These activities included a leisurely 30-minute walk and participation in a 1-hour social activity.
This study involved 230 participants from Switzerland and Ireland, with a mean age of 73.4 years, who had persistent hypothyroidism. The population was randomized to 119 patients who were administered levothyroxine and 111 patients who received a placebo.
At baseline, the levothyroxine group had a mean physical PFS score of 14.7 ± 9.3, and the placebo group had a score of 11.1 ± 9.1. The baseline mean mental PFS score for the levothyroxine group was 7.4 ± 8.0, while it was 5.1 ± 6.9 for the placebo group.
After 12 months of the participants’ use of levothyroxine or placebo, the physical PFS scores increased for both the treatment and placebo groups. For the levothyroxine group, the mean physical PFS score was 14.8 ± 9.6, while it was 12.4 ± 9.3 for the placebo group (P = 0.88). The investigators found no significant differences between these scores for the levothyroxine and placebo groups.
The mean mental PFS score slightly decreased to 6.0 ± 7.8 for the levothyroxine group, while it slightly increased to 6.0 ± 8.0 for the placebo group (P = 0.26) at 12 months. The difference between the mental fatigability scores for the levothyroxine and placebo arms at 12 months was also not significant.
The physical fatigability between-group difference was 0.2 (95% confidence interval, –1.8 to 2.1; P = 0.88), while the mental fatigability difference was –1.0 (95% CI, –2.8 to 0.8; P = 0.26).
The study was funded by the European Union FP7 and the Swiss National Science Foundation. Merck KGaA, Darmstadt (Germany) provided levothyroxine and the placebo. Dr. Stuber has no conflicts of interest.
WASHINGTON – according to a new study.
The double-blind, randomized, placebo-controlled, parallel-group trial used subsets of data from the TRUST study. This new research, which was presented at the Annual Meeting of the Society of General Internal Medicine, provides further evidence that prescribing levothyroxine to older patients with subclinical hypothyroidism will not reduce fatigue.
Like the findings in the TRUST study, the new study, “Effect of Thyroid Hormone Replacement on Fatigability in Older Adults with Subclinical Hypothyroidism: A Randomized Placebo Controlled Trial,” showed that the use of levothyroxine in subclinical hypothyroidism was not effective in changing physical and mental fatigability in older adults.
“This study shows that levothyroxine treatment may not be necessary and results in high costs and side effects,” noted Mirah Stuber, MD, during her presentation of the findings at the meeting.
The new study examined the effect of levothyroxine on the tiredness of older adults but with a more detailed analysis of fatigue than the full TRUST study provided. In this new study, investigators used a new assessment tool, called the Pittsburgh Fatigability Scale (PFS), a 10-item self-administered questionnaire that can measure both physical and mental fatigability. Perceived fatigability anchors tiredness to a set of activities and has been shown to be a more sensitive measure than global fatigue, explained Dr. Stuber of the University of Chicago.
The scores range from 0-50, with higher scores indicating higher fatigability. The scales were divided between mental and physical activities and measured fatigue in relation to a defined activity of a specific duration and intensity. These activities included a leisurely 30-minute walk and participation in a 1-hour social activity.
This study involved 230 participants from Switzerland and Ireland, with a mean age of 73.4 years, who had persistent hypothyroidism. The population was randomized to 119 patients who were administered levothyroxine and 111 patients who received a placebo.
At baseline, the levothyroxine group had a mean physical PFS score of 14.7 ± 9.3, and the placebo group had a score of 11.1 ± 9.1. The baseline mean mental PFS score for the levothyroxine group was 7.4 ± 8.0, while it was 5.1 ± 6.9 for the placebo group.
After 12 months of the participants’ use of levothyroxine or placebo, the physical PFS scores increased for both the treatment and placebo groups. For the levothyroxine group, the mean physical PFS score was 14.8 ± 9.6, while it was 12.4 ± 9.3 for the placebo group (P = 0.88). The investigators found no significant differences between these scores for the levothyroxine and placebo groups.
The mean mental PFS score slightly decreased to 6.0 ± 7.8 for the levothyroxine group, while it slightly increased to 6.0 ± 8.0 for the placebo group (P = 0.26) at 12 months. The difference between the mental fatigability scores for the levothyroxine and placebo arms at 12 months was also not significant.
The physical fatigability between-group difference was 0.2 (95% confidence interval, –1.8 to 2.1; P = 0.88), while the mental fatigability difference was –1.0 (95% CI, –2.8 to 0.8; P = 0.26).
The study was funded by the European Union FP7 and the Swiss National Science Foundation. Merck KGaA, Darmstadt (Germany) provided levothyroxine and the placebo. Dr. Stuber has no conflicts of interest.
REPORTING FROM SGIM 2019
Lack of CBD oil regulations hearkens back to patent medicines
My vet sells cannabidiol (CBD) oil for dogs and cats.
So does the vape store down the street, the pharmacy around the corner ... and pretty much every place these days.
I probably have more patients ask me about CBD than any other drug, usually because a friend/cousin/in-law/child/parent/spouse/neighbor/coworker “said I should ask you about this.” That’s the power of the Internet: The most marginally proven treatments are portrayed as definitive cures, while some of the most effective treatments (like vaccines) are treated like a lethal-injection drug.
Nothing is, or ever will be, a miracle cure. There will always be nonresponders and those who have adverse effects. In that respect, what goes for one treatment goes for all of them.
But that doesn’t stop these things from being pushed in the most unreliable ways. On Yelp, Groupon, Facebook, and countless other nonmedical sites that aren’t required to back up their claims with hard evidence.
Anything I prescribe, and all over-the-counter medications, are subject to far more scrutiny. They have known risks and benefits. They’ve been through trials, and most have years of data to review when questions arise.
Granted, CBD oil has been approved, as Epidiolex, for different forms of epilepsy. That kind of regulation is a step forward, but the majority of people selling CBD oil are doing so with unregulated OTC forms.
These may work, but the lack of regulation means every one of these places can make their own formulations, purities, and strengths. In some respects it’s a throwback to the era of patent medicines, where each pharmacy was free to whip up their own concoctions, label them as treatments for whatever they wished, and advertise and sell them.
The Food and Drug Administration, however, continues to take an ostrich approach. At their level these OTC agents are illegal and cannot be sold or marketed. At the same time, though, the restrictions overall are not being enforced. This gives the impression that there is nothing wrong with selling them.
Let’s look at morphine, an effective pain reliever and controlled substance. It’s tightly regulated, as it should be. But what if those regulations were ignored? What if, in addition to it being available by prescription, it were sold OTC in perhaps weaker but unregulated strengths and forms, with a variety of unscientific claims made for its benefits?
I don’t see anyone getting away with doing that.
Like I said earlier, I have nothing against CBD oil. But I do think it should have to go through the same approval process as any other medication, with specific strengths, dosing, benefits, and side effects determined, and enforceable regulations around its manufacturing, sale, and use.
Anything less is snake oil.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
My vet sells cannabidiol (CBD) oil for dogs and cats.
So does the vape store down the street, the pharmacy around the corner ... and pretty much every place these days.
I probably have more patients ask me about CBD than any other drug, usually because a friend/cousin/in-law/child/parent/spouse/neighbor/coworker “said I should ask you about this.” That’s the power of the Internet: The most marginally proven treatments are portrayed as definitive cures, while some of the most effective treatments (like vaccines) are treated like a lethal-injection drug.
Nothing is, or ever will be, a miracle cure. There will always be nonresponders and those who have adverse effects. In that respect, what goes for one treatment goes for all of them.
But that doesn’t stop these things from being pushed in the most unreliable ways. On Yelp, Groupon, Facebook, and countless other nonmedical sites that aren’t required to back up their claims with hard evidence.
Anything I prescribe, and all over-the-counter medications, are subject to far more scrutiny. They have known risks and benefits. They’ve been through trials, and most have years of data to review when questions arise.
Granted, CBD oil has been approved, as Epidiolex, for different forms of epilepsy. That kind of regulation is a step forward, but the majority of people selling CBD oil are doing so with unregulated OTC forms.
These may work, but the lack of regulation means every one of these places can make their own formulations, purities, and strengths. In some respects it’s a throwback to the era of patent medicines, where each pharmacy was free to whip up their own concoctions, label them as treatments for whatever they wished, and advertise and sell them.
The Food and Drug Administration, however, continues to take an ostrich approach. At their level these OTC agents are illegal and cannot be sold or marketed. At the same time, though, the restrictions overall are not being enforced. This gives the impression that there is nothing wrong with selling them.
Let’s look at morphine, an effective pain reliever and controlled substance. It’s tightly regulated, as it should be. But what if those regulations were ignored? What if, in addition to it being available by prescription, it were sold OTC in perhaps weaker but unregulated strengths and forms, with a variety of unscientific claims made for its benefits?
I don’t see anyone getting away with doing that.
Like I said earlier, I have nothing against CBD oil. But I do think it should have to go through the same approval process as any other medication, with specific strengths, dosing, benefits, and side effects determined, and enforceable regulations around its manufacturing, sale, and use.
Anything less is snake oil.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
My vet sells cannabidiol (CBD) oil for dogs and cats.
So does the vape store down the street, the pharmacy around the corner ... and pretty much every place these days.
I probably have more patients ask me about CBD than any other drug, usually because a friend/cousin/in-law/child/parent/spouse/neighbor/coworker “said I should ask you about this.” That’s the power of the Internet: The most marginally proven treatments are portrayed as definitive cures, while some of the most effective treatments (like vaccines) are treated like a lethal-injection drug.
Nothing is, or ever will be, a miracle cure. There will always be nonresponders and those who have adverse effects. In that respect, what goes for one treatment goes for all of them.
But that doesn’t stop these things from being pushed in the most unreliable ways. On Yelp, Groupon, Facebook, and countless other nonmedical sites that aren’t required to back up their claims with hard evidence.
Anything I prescribe, and all over-the-counter medications, are subject to far more scrutiny. They have known risks and benefits. They’ve been through trials, and most have years of data to review when questions arise.
Granted, CBD oil has been approved, as Epidiolex, for different forms of epilepsy. That kind of regulation is a step forward, but the majority of people selling CBD oil are doing so with unregulated OTC forms.
These may work, but the lack of regulation means every one of these places can make their own formulations, purities, and strengths. In some respects it’s a throwback to the era of patent medicines, where each pharmacy was free to whip up their own concoctions, label them as treatments for whatever they wished, and advertise and sell them.
The Food and Drug Administration, however, continues to take an ostrich approach. At their level these OTC agents are illegal and cannot be sold or marketed. At the same time, though, the restrictions overall are not being enforced. This gives the impression that there is nothing wrong with selling them.
Let’s look at morphine, an effective pain reliever and controlled substance. It’s tightly regulated, as it should be. But what if those regulations were ignored? What if, in addition to it being available by prescription, it were sold OTC in perhaps weaker but unregulated strengths and forms, with a variety of unscientific claims made for its benefits?
I don’t see anyone getting away with doing that.
Like I said earlier, I have nothing against CBD oil. But I do think it should have to go through the same approval process as any other medication, with specific strengths, dosing, benefits, and side effects determined, and enforceable regulations around its manufacturing, sale, and use.
Anything less is snake oil.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
COPD rates reflect current smoking prevalence
, according to a Centers for Disease Control and Prevention analysis of respondents to a behavioral risk factor survey.
“Population-based strategies for smoking prevention and control have the potential to decrease the prevalence of COPD in the United States,” wrote Anne G. Wheaton, PhD, of the CDC’s National Center for Chronic Disease Prevention and Health Promotion and coauthors. The study was published in the Morbidity and Mortality Weekly Report.
Dr. Wheaton and her fellow researchers analyzed data from 418,378 adult respondents to the 2017 Behavioral Risk Factor Surveillance System survey. Responses came from all 50 states and Washington, D.C.; respondents who had smoked less than 100 lifetime cigarettes were categorized as “never smoked,” while those who had smoked at least 100 cigarettes but no longer smoked were categorized as “former smokers.” Anyone who had smoked at least 100 cigarettes and currently smoked was categorized as a “current smoker.”
The age-adjusted prevalence of COPD among U.S. adults was 6.2% (95% confidence interval, 6.0%-6.3%) in 2017. Current cigarette smokers had a prevalence of 15.2% (95% CI, 14.7%-15.7%); this dipped to 7.6% (95% CI, 7.3%-8.0%) among former smokers and 2.8% (95% CI, 2.7%-2.9%) among adults who had never smoked. Patterns were visible within states: Current smokers had a state-level prevalence of COPD that was strongly correlated with state-level current smoking prevalence (Pearson correlation coefficient, 0.69; P less than .001). State-level COPD prevalence among former smokers (Pearson correlation coefficient, 0.71; P less than .001) and those who never smoked (Pearson correlation coefficient, 0.64; P less than .001) were also strongly correlated with the current smoking prevalence, indicating secondhand smoke as a risk factor for COPD.
The coauthors acknowledged the study’s limitations, including relying on self-reporting for both COPD and smoking status. They also noted that there was no way to measure exposure to secondhand smoke, other indoor or outdoor air pollutants, or respiratory infection history, “all of which might contribute to COPD risk.”
No conflicts of interest were reported.
SOURCE: Wheaton AG et al. MMWR Morb Mortal Wkly Rep. 2019 Jun 21;68(24):533-8.
, according to a Centers for Disease Control and Prevention analysis of respondents to a behavioral risk factor survey.
“Population-based strategies for smoking prevention and control have the potential to decrease the prevalence of COPD in the United States,” wrote Anne G. Wheaton, PhD, of the CDC’s National Center for Chronic Disease Prevention and Health Promotion and coauthors. The study was published in the Morbidity and Mortality Weekly Report.
Dr. Wheaton and her fellow researchers analyzed data from 418,378 adult respondents to the 2017 Behavioral Risk Factor Surveillance System survey. Responses came from all 50 states and Washington, D.C.; respondents who had smoked less than 100 lifetime cigarettes were categorized as “never smoked,” while those who had smoked at least 100 cigarettes but no longer smoked were categorized as “former smokers.” Anyone who had smoked at least 100 cigarettes and currently smoked was categorized as a “current smoker.”
The age-adjusted prevalence of COPD among U.S. adults was 6.2% (95% confidence interval, 6.0%-6.3%) in 2017. Current cigarette smokers had a prevalence of 15.2% (95% CI, 14.7%-15.7%); this dipped to 7.6% (95% CI, 7.3%-8.0%) among former smokers and 2.8% (95% CI, 2.7%-2.9%) among adults who had never smoked. Patterns were visible within states: Current smokers had a state-level prevalence of COPD that was strongly correlated with state-level current smoking prevalence (Pearson correlation coefficient, 0.69; P less than .001). State-level COPD prevalence among former smokers (Pearson correlation coefficient, 0.71; P less than .001) and those who never smoked (Pearson correlation coefficient, 0.64; P less than .001) were also strongly correlated with the current smoking prevalence, indicating secondhand smoke as a risk factor for COPD.
The coauthors acknowledged the study’s limitations, including relying on self-reporting for both COPD and smoking status. They also noted that there was no way to measure exposure to secondhand smoke, other indoor or outdoor air pollutants, or respiratory infection history, “all of which might contribute to COPD risk.”
No conflicts of interest were reported.
SOURCE: Wheaton AG et al. MMWR Morb Mortal Wkly Rep. 2019 Jun 21;68(24):533-8.
, according to a Centers for Disease Control and Prevention analysis of respondents to a behavioral risk factor survey.
“Population-based strategies for smoking prevention and control have the potential to decrease the prevalence of COPD in the United States,” wrote Anne G. Wheaton, PhD, of the CDC’s National Center for Chronic Disease Prevention and Health Promotion and coauthors. The study was published in the Morbidity and Mortality Weekly Report.
Dr. Wheaton and her fellow researchers analyzed data from 418,378 adult respondents to the 2017 Behavioral Risk Factor Surveillance System survey. Responses came from all 50 states and Washington, D.C.; respondents who had smoked less than 100 lifetime cigarettes were categorized as “never smoked,” while those who had smoked at least 100 cigarettes but no longer smoked were categorized as “former smokers.” Anyone who had smoked at least 100 cigarettes and currently smoked was categorized as a “current smoker.”
The age-adjusted prevalence of COPD among U.S. adults was 6.2% (95% confidence interval, 6.0%-6.3%) in 2017. Current cigarette smokers had a prevalence of 15.2% (95% CI, 14.7%-15.7%); this dipped to 7.6% (95% CI, 7.3%-8.0%) among former smokers and 2.8% (95% CI, 2.7%-2.9%) among adults who had never smoked. Patterns were visible within states: Current smokers had a state-level prevalence of COPD that was strongly correlated with state-level current smoking prevalence (Pearson correlation coefficient, 0.69; P less than .001). State-level COPD prevalence among former smokers (Pearson correlation coefficient, 0.71; P less than .001) and those who never smoked (Pearson correlation coefficient, 0.64; P less than .001) were also strongly correlated with the current smoking prevalence, indicating secondhand smoke as a risk factor for COPD.
The coauthors acknowledged the study’s limitations, including relying on self-reporting for both COPD and smoking status. They also noted that there was no way to measure exposure to secondhand smoke, other indoor or outdoor air pollutants, or respiratory infection history, “all of which might contribute to COPD risk.”
No conflicts of interest were reported.
SOURCE: Wheaton AG et al. MMWR Morb Mortal Wkly Rep. 2019 Jun 21;68(24):533-8.
FROM MMWR
Physician burnout may be jeopardizing patient care
Clinical question: Is physician burnout associated with more patient safety issues, low professionalism, or poor patient satisfaction?
Background: Burnout is common among physicians and has a negative effect on their personal lives. It is unclear whether physician burnout is associated with poor outcomes for patients.
Study design: Meta-analysis.
Setting: Forty-seven published studies from 19 countries assessing inpatient and outpatient physicians and the relationship between physician burnout and patient care.
Synopsis: After a systematic review of the published literature, 47 studies were included to pool data from 42,473 physicians. Study subjects included residents, early-career and late-career physicians, and both hospital and outpatient physicians. All studies used validated measures of physician burnout.
Burnout was associated with a two-fold increased risk of physician-reported safety incidents (odds ratio, 1.96; 95% confidence interval, 1.59-2.40), low professionalism (OR, 2.31; 95% CI, 1.87-2.85), and likelihood of low patient-reported satisfaction (OR, 2.28; 95% CI, 1.42-3.68). There were no significant differences in these results based on country of origin of the study. Early-career physicians were more likely to have burnout associated with low professionalism than were late-career physicians.
Of the components of burnout, depersonalization was most strongly associated with these negative outcomes. Interestingly, the increased risk of patient safety incidents was associated with physician-reported, but not health care system–reported, patient safety outcomes. This raises concerns that the health care systems may not be capturing “near misses” in their metrics.
Bottom line: Physician burnout doubles the risk of being involved in a patient safety incident, low professionalism, and poor patient satisfaction.
Citation: Panagioti M et al. Association between physician burnout and patient safety, professionalism, and patient satisfaction. JAMA Intern Med. 2018;178(10):1317-30.
Dr. Gabriel is assistant professor of medicine and director of Pre-operative Medicine and Medicine Consult Service in the division of hospital medicine at Mount Sinai Hospital, New York.
Clinical question: Is physician burnout associated with more patient safety issues, low professionalism, or poor patient satisfaction?
Background: Burnout is common among physicians and has a negative effect on their personal lives. It is unclear whether physician burnout is associated with poor outcomes for patients.
Study design: Meta-analysis.
Setting: Forty-seven published studies from 19 countries assessing inpatient and outpatient physicians and the relationship between physician burnout and patient care.
Synopsis: After a systematic review of the published literature, 47 studies were included to pool data from 42,473 physicians. Study subjects included residents, early-career and late-career physicians, and both hospital and outpatient physicians. All studies used validated measures of physician burnout.
Burnout was associated with a two-fold increased risk of physician-reported safety incidents (odds ratio, 1.96; 95% confidence interval, 1.59-2.40), low professionalism (OR, 2.31; 95% CI, 1.87-2.85), and likelihood of low patient-reported satisfaction (OR, 2.28; 95% CI, 1.42-3.68). There were no significant differences in these results based on country of origin of the study. Early-career physicians were more likely to have burnout associated with low professionalism than were late-career physicians.
Of the components of burnout, depersonalization was most strongly associated with these negative outcomes. Interestingly, the increased risk of patient safety incidents was associated with physician-reported, but not health care system–reported, patient safety outcomes. This raises concerns that the health care systems may not be capturing “near misses” in their metrics.
Bottom line: Physician burnout doubles the risk of being involved in a patient safety incident, low professionalism, and poor patient satisfaction.
Citation: Panagioti M et al. Association between physician burnout and patient safety, professionalism, and patient satisfaction. JAMA Intern Med. 2018;178(10):1317-30.
Dr. Gabriel is assistant professor of medicine and director of Pre-operative Medicine and Medicine Consult Service in the division of hospital medicine at Mount Sinai Hospital, New York.
Clinical question: Is physician burnout associated with more patient safety issues, low professionalism, or poor patient satisfaction?
Background: Burnout is common among physicians and has a negative effect on their personal lives. It is unclear whether physician burnout is associated with poor outcomes for patients.
Study design: Meta-analysis.
Setting: Forty-seven published studies from 19 countries assessing inpatient and outpatient physicians and the relationship between physician burnout and patient care.
Synopsis: After a systematic review of the published literature, 47 studies were included to pool data from 42,473 physicians. Study subjects included residents, early-career and late-career physicians, and both hospital and outpatient physicians. All studies used validated measures of physician burnout.
Burnout was associated with a two-fold increased risk of physician-reported safety incidents (odds ratio, 1.96; 95% confidence interval, 1.59-2.40), low professionalism (OR, 2.31; 95% CI, 1.87-2.85), and likelihood of low patient-reported satisfaction (OR, 2.28; 95% CI, 1.42-3.68). There were no significant differences in these results based on country of origin of the study. Early-career physicians were more likely to have burnout associated with low professionalism than were late-career physicians.
Of the components of burnout, depersonalization was most strongly associated with these negative outcomes. Interestingly, the increased risk of patient safety incidents was associated with physician-reported, but not health care system–reported, patient safety outcomes. This raises concerns that the health care systems may not be capturing “near misses” in their metrics.
Bottom line: Physician burnout doubles the risk of being involved in a patient safety incident, low professionalism, and poor patient satisfaction.
Citation: Panagioti M et al. Association between physician burnout and patient safety, professionalism, and patient satisfaction. JAMA Intern Med. 2018;178(10):1317-30.
Dr. Gabriel is assistant professor of medicine and director of Pre-operative Medicine and Medicine Consult Service in the division of hospital medicine at Mount Sinai Hospital, New York.
Scoring below the cut but still depressed: What to do?
Depression is one of the most common mental health conditions in childhood, especially during socially turbulent adolescence when the brain is rapidly changing and parent-child relationships are strained by the teen’s striving for independence and identity. Often parents of teens call me worrying about possible depression, but in the next breath say “but maybe it is just puberty.” Because suicide is one of the most common causes of death among teens and is often associated with depression, we pediatricians have the scary job of sorting out symptoms and making a plan.
The Guidelines for Adolescent Depression in Primary Care (GLAD-PC)1,2 were revised in 2018 to help. This expert consensus document contains specific and practical guidance for all levels of depression. But for mild depression, GLAD-PC now advises pediatricians in Recommendation II to go beyond “watchful waiting.” It states, “After initial diagnosis,
Although a little vague, mild depression is diagnosed when there are “closer to 5” significant symptoms of depression, with “distressing but manageable” severity and only “mildly impaired” functioning. The most commonly used self-report adolescent depression screen, the Patient Health Questionnaire–Modified–9 (PHQ-9), has a recommended cut score of greater than 10, but 5-9 is considered mild depression symptoms. A clinical interview also is always required.
So what is this “active support” being recommended? After making an assessment of symptoms, severity, and impact – and ruling out significant suicide risk – the task is rather familiar to us from other medical conditions. We need to talk clearly and empathetically with the teen (and parents with consent) about depression and its neurological etiology, ask about contributing stress and genetic factors, and describe the typical course with optimism. This discussion is critical to pushing guilt or blame aside to rally family support. Substance use – (including alcohol) both a cause and attempted coping strategy for depression – must be addressed because it adds to risk for suicide or crashes and because it interacts with medicines.
Perhaps the biggest difference between active support for depression versus that for other conditions is that teens are likely reluctant, hopeless, and/or lacking energy to participate in the plan. The plan, therefore, needs to be approached in smaller steps and build on prior teen strengths, goals, or talents to motivate them and create reward to counteract general lethargy. You may know this teen used to play basketball, or sing at church, or love playing with a baby sister – all activities to try to reawaken. Parents can help recall these and are key to setting up opportunities.
GLAD-PC provides a “Self-Care Success!” worksheet of categories for goal setting for active support. These goals include:
- Stay physically active. Specified days/month, minutes/session, and dates and times.
- Engage spirituality and fun activities. Specify times/week, when, and with whom).
- Eat balanced meals. Specify number/day and names of foods.
- Spend time with people who can support you. Specify number/month, minutes/time, with whom, and doing what.
- Spend time relaxing. Specify days/week, minutes/time, and doing what.
- Determine small goals and simple steps. Establish these for a specified problem.
There is now evidence for these you can share with your teen patients and families.
Exercise
Exercise has a moderate effect size of 0.56 on depression, comparable to medications for mild to moderate depression and a useful adjunct to medications. The national Office of Disease Prevention and Health Promotion recommends that 6- to 17-year-olds get 60 minutes/day of moderate exercise or undertake vigorous “out of breath” exercise three times a week to maintain health. A meta-analysis of studies of yoga for people with depressive symptoms (not necessarily diagnosed depression) found reduced symptoms in 14 of 23 studies.
Pleasure
Advising fun has to include acknowledgment that a depressed teen is not motivated to do formerly fun things and may not get as much/any pleasure from it. You need to explain that “doing precedes feeling.” While what is fun is personal, new findings indicate that 2 hours/week “in nature” lowers stress, boosts mental health, and increases sense of well-being.
Nutrition
The MIND diet (Mediterranean-type diet high in leafy vegetables and berries but low in red meat) has evidence for lower odds of depression and psychological distress. Fatty acid supplements, specifically eicosapentaenoic acid at greater than 800 mg/day (930 mg), is better than placebo (P less than .001) for reducing mild depression within as little as 4 weeks. Natural S-Adenosyl-L-methionine (SAMe) has many studies showing benefit, according to National Center for Complementary and Alternative Medicine, a government-run website. NCCAM notes that St. John’s Wort has evidence for effectiveness equal to prescribed antidepressants for mild depression but with dangerous potential side effects, such as worsening of psychotic symptoms in bipolar disorder or schizophrenia, plus potentially life threatening drug interactions. While safe, valerian and probiotics have no evidence for reducing depression.
Social support
Family is usually the most important support for depressed teens even though they may be pushing family away, may refuse to come on outings, or may even refuse to come out of the bedroom. We should encourage parents and siblings to “hang out,” sitting quietly, available to listen rather than probing, cajoling, or nagging as they may have been doing. Parents also provide support by assuring adherence to visits, goals, and medications. Peer support helps a teen feel less alone and may increase social skills, but it can be difficult to sustain because friends may find depression threatening or give up when the teen avoids them and refuses activities. The National Association for Mental Illness has an online support group (www.strengthofus.org), as well as many excellent family resources. Sometimes medical efforts to be nonsectarian result in failure to recognize and remind teens and families of the value of religion, which is free and universally available, as a source of social support.
Relaxation
An evaluation of 15 studies concluded that relaxation techniques reduced depressive symptoms better than no treatment but not as much cognitive-behavior therapy (CBT). Yoga is another source of relaxation training. Mindfulness includes relaxation and specifies working to stay nonjudgmental about thoughts passing through one’s mind, recognizing and “arguing” with negative thinking, which is also part of CBT. Guided relaxation with a person, audiotape, or app (Calm or Headspace, among others) may be better for depressed teens because it inserts a voice to guide thoughts, which could potentially fend off ruminating on sad things.
Setting goals to address problems
In mild depression, compared with more endogenous moderate to severe major depressive disorder, a specific life stressor or relationship issue may be the precipitant. Identifying such factors (never forgetting possible trauma or abuse, which are harder to reveal), empathizing with the pain, and addressing them such as using Problem Solving Treatment for Primary Care (PST-PC) are within primary care skills. PST-PC involves four to six 30-minute sessions over 6-10 weeks during which you can provide perspective, help your patient set realistic goals and solutions to try out for situations that can be changed or coping strategies for emotion-focused unchangeable issues, iteratively check on progress via calls or televisits (the monitoring component), and renew problem-solving efforts as needed.
If mild depression fails to improve over several months or worsens, GLAD-PC describes evidence-based treatments. Even if it remits, your active support and monitoring should continue because depression tends to recur. You may not realize how valuable these seemingly simple active supports are to keeping mild depression in your teen patients at bay.
Dr. Howard is an assistant professor of pediatrics at Johns Hopkins University, Baltimore, and creator of CHADIS (www.CHADIS.com). She had no other relevant disclosures. Dr. Howard’s contribution to this publication was as a paid expert to MDedge News. E-mail her at [email protected].
References
1. Pediatrics. 2018 Mar 1. doi: 10.1542/peds.2017-4081.
2. Pediatrics. 2018 Mar 1. doi: 10.1542/peds.2017-4082.
Depression is one of the most common mental health conditions in childhood, especially during socially turbulent adolescence when the brain is rapidly changing and parent-child relationships are strained by the teen’s striving for independence and identity. Often parents of teens call me worrying about possible depression, but in the next breath say “but maybe it is just puberty.” Because suicide is one of the most common causes of death among teens and is often associated with depression, we pediatricians have the scary job of sorting out symptoms and making a plan.
The Guidelines for Adolescent Depression in Primary Care (GLAD-PC)1,2 were revised in 2018 to help. This expert consensus document contains specific and practical guidance for all levels of depression. But for mild depression, GLAD-PC now advises pediatricians in Recommendation II to go beyond “watchful waiting.” It states, “After initial diagnosis,
Although a little vague, mild depression is diagnosed when there are “closer to 5” significant symptoms of depression, with “distressing but manageable” severity and only “mildly impaired” functioning. The most commonly used self-report adolescent depression screen, the Patient Health Questionnaire–Modified–9 (PHQ-9), has a recommended cut score of greater than 10, but 5-9 is considered mild depression symptoms. A clinical interview also is always required.
So what is this “active support” being recommended? After making an assessment of symptoms, severity, and impact – and ruling out significant suicide risk – the task is rather familiar to us from other medical conditions. We need to talk clearly and empathetically with the teen (and parents with consent) about depression and its neurological etiology, ask about contributing stress and genetic factors, and describe the typical course with optimism. This discussion is critical to pushing guilt or blame aside to rally family support. Substance use – (including alcohol) both a cause and attempted coping strategy for depression – must be addressed because it adds to risk for suicide or crashes and because it interacts with medicines.
Perhaps the biggest difference between active support for depression versus that for other conditions is that teens are likely reluctant, hopeless, and/or lacking energy to participate in the plan. The plan, therefore, needs to be approached in smaller steps and build on prior teen strengths, goals, or talents to motivate them and create reward to counteract general lethargy. You may know this teen used to play basketball, or sing at church, or love playing with a baby sister – all activities to try to reawaken. Parents can help recall these and are key to setting up opportunities.
GLAD-PC provides a “Self-Care Success!” worksheet of categories for goal setting for active support. These goals include:
- Stay physically active. Specified days/month, minutes/session, and dates and times.
- Engage spirituality and fun activities. Specify times/week, when, and with whom).
- Eat balanced meals. Specify number/day and names of foods.
- Spend time with people who can support you. Specify number/month, minutes/time, with whom, and doing what.
- Spend time relaxing. Specify days/week, minutes/time, and doing what.
- Determine small goals and simple steps. Establish these for a specified problem.
There is now evidence for these you can share with your teen patients and families.
Exercise
Exercise has a moderate effect size of 0.56 on depression, comparable to medications for mild to moderate depression and a useful adjunct to medications. The national Office of Disease Prevention and Health Promotion recommends that 6- to 17-year-olds get 60 minutes/day of moderate exercise or undertake vigorous “out of breath” exercise three times a week to maintain health. A meta-analysis of studies of yoga for people with depressive symptoms (not necessarily diagnosed depression) found reduced symptoms in 14 of 23 studies.
Pleasure
Advising fun has to include acknowledgment that a depressed teen is not motivated to do formerly fun things and may not get as much/any pleasure from it. You need to explain that “doing precedes feeling.” While what is fun is personal, new findings indicate that 2 hours/week “in nature” lowers stress, boosts mental health, and increases sense of well-being.
Nutrition
The MIND diet (Mediterranean-type diet high in leafy vegetables and berries but low in red meat) has evidence for lower odds of depression and psychological distress. Fatty acid supplements, specifically eicosapentaenoic acid at greater than 800 mg/day (930 mg), is better than placebo (P less than .001) for reducing mild depression within as little as 4 weeks. Natural S-Adenosyl-L-methionine (SAMe) has many studies showing benefit, according to National Center for Complementary and Alternative Medicine, a government-run website. NCCAM notes that St. John’s Wort has evidence for effectiveness equal to prescribed antidepressants for mild depression but with dangerous potential side effects, such as worsening of psychotic symptoms in bipolar disorder or schizophrenia, plus potentially life threatening drug interactions. While safe, valerian and probiotics have no evidence for reducing depression.
Social support
Family is usually the most important support for depressed teens even though they may be pushing family away, may refuse to come on outings, or may even refuse to come out of the bedroom. We should encourage parents and siblings to “hang out,” sitting quietly, available to listen rather than probing, cajoling, or nagging as they may have been doing. Parents also provide support by assuring adherence to visits, goals, and medications. Peer support helps a teen feel less alone and may increase social skills, but it can be difficult to sustain because friends may find depression threatening or give up when the teen avoids them and refuses activities. The National Association for Mental Illness has an online support group (www.strengthofus.org), as well as many excellent family resources. Sometimes medical efforts to be nonsectarian result in failure to recognize and remind teens and families of the value of religion, which is free and universally available, as a source of social support.
Relaxation
An evaluation of 15 studies concluded that relaxation techniques reduced depressive symptoms better than no treatment but not as much cognitive-behavior therapy (CBT). Yoga is another source of relaxation training. Mindfulness includes relaxation and specifies working to stay nonjudgmental about thoughts passing through one’s mind, recognizing and “arguing” with negative thinking, which is also part of CBT. Guided relaxation with a person, audiotape, or app (Calm or Headspace, among others) may be better for depressed teens because it inserts a voice to guide thoughts, which could potentially fend off ruminating on sad things.
Setting goals to address problems
In mild depression, compared with more endogenous moderate to severe major depressive disorder, a specific life stressor or relationship issue may be the precipitant. Identifying such factors (never forgetting possible trauma or abuse, which are harder to reveal), empathizing with the pain, and addressing them such as using Problem Solving Treatment for Primary Care (PST-PC) are within primary care skills. PST-PC involves four to six 30-minute sessions over 6-10 weeks during which you can provide perspective, help your patient set realistic goals and solutions to try out for situations that can be changed or coping strategies for emotion-focused unchangeable issues, iteratively check on progress via calls or televisits (the monitoring component), and renew problem-solving efforts as needed.
If mild depression fails to improve over several months or worsens, GLAD-PC describes evidence-based treatments. Even if it remits, your active support and monitoring should continue because depression tends to recur. You may not realize how valuable these seemingly simple active supports are to keeping mild depression in your teen patients at bay.
Dr. Howard is an assistant professor of pediatrics at Johns Hopkins University, Baltimore, and creator of CHADIS (www.CHADIS.com). She had no other relevant disclosures. Dr. Howard’s contribution to this publication was as a paid expert to MDedge News. E-mail her at [email protected].
References
1. Pediatrics. 2018 Mar 1. doi: 10.1542/peds.2017-4081.
2. Pediatrics. 2018 Mar 1. doi: 10.1542/peds.2017-4082.
Depression is one of the most common mental health conditions in childhood, especially during socially turbulent adolescence when the brain is rapidly changing and parent-child relationships are strained by the teen’s striving for independence and identity. Often parents of teens call me worrying about possible depression, but in the next breath say “but maybe it is just puberty.” Because suicide is one of the most common causes of death among teens and is often associated with depression, we pediatricians have the scary job of sorting out symptoms and making a plan.
The Guidelines for Adolescent Depression in Primary Care (GLAD-PC)1,2 were revised in 2018 to help. This expert consensus document contains specific and practical guidance for all levels of depression. But for mild depression, GLAD-PC now advises pediatricians in Recommendation II to go beyond “watchful waiting.” It states, “After initial diagnosis,
Although a little vague, mild depression is diagnosed when there are “closer to 5” significant symptoms of depression, with “distressing but manageable” severity and only “mildly impaired” functioning. The most commonly used self-report adolescent depression screen, the Patient Health Questionnaire–Modified–9 (PHQ-9), has a recommended cut score of greater than 10, but 5-9 is considered mild depression symptoms. A clinical interview also is always required.
So what is this “active support” being recommended? After making an assessment of symptoms, severity, and impact – and ruling out significant suicide risk – the task is rather familiar to us from other medical conditions. We need to talk clearly and empathetically with the teen (and parents with consent) about depression and its neurological etiology, ask about contributing stress and genetic factors, and describe the typical course with optimism. This discussion is critical to pushing guilt or blame aside to rally family support. Substance use – (including alcohol) both a cause and attempted coping strategy for depression – must be addressed because it adds to risk for suicide or crashes and because it interacts with medicines.
Perhaps the biggest difference between active support for depression versus that for other conditions is that teens are likely reluctant, hopeless, and/or lacking energy to participate in the plan. The plan, therefore, needs to be approached in smaller steps and build on prior teen strengths, goals, or talents to motivate them and create reward to counteract general lethargy. You may know this teen used to play basketball, or sing at church, or love playing with a baby sister – all activities to try to reawaken. Parents can help recall these and are key to setting up opportunities.
GLAD-PC provides a “Self-Care Success!” worksheet of categories for goal setting for active support. These goals include:
- Stay physically active. Specified days/month, minutes/session, and dates and times.
- Engage spirituality and fun activities. Specify times/week, when, and with whom).
- Eat balanced meals. Specify number/day and names of foods.
- Spend time with people who can support you. Specify number/month, minutes/time, with whom, and doing what.
- Spend time relaxing. Specify days/week, minutes/time, and doing what.
- Determine small goals and simple steps. Establish these for a specified problem.
There is now evidence for these you can share with your teen patients and families.
Exercise
Exercise has a moderate effect size of 0.56 on depression, comparable to medications for mild to moderate depression and a useful adjunct to medications. The national Office of Disease Prevention and Health Promotion recommends that 6- to 17-year-olds get 60 minutes/day of moderate exercise or undertake vigorous “out of breath” exercise three times a week to maintain health. A meta-analysis of studies of yoga for people with depressive symptoms (not necessarily diagnosed depression) found reduced symptoms in 14 of 23 studies.
Pleasure
Advising fun has to include acknowledgment that a depressed teen is not motivated to do formerly fun things and may not get as much/any pleasure from it. You need to explain that “doing precedes feeling.” While what is fun is personal, new findings indicate that 2 hours/week “in nature” lowers stress, boosts mental health, and increases sense of well-being.
Nutrition
The MIND diet (Mediterranean-type diet high in leafy vegetables and berries but low in red meat) has evidence for lower odds of depression and psychological distress. Fatty acid supplements, specifically eicosapentaenoic acid at greater than 800 mg/day (930 mg), is better than placebo (P less than .001) for reducing mild depression within as little as 4 weeks. Natural S-Adenosyl-L-methionine (SAMe) has many studies showing benefit, according to National Center for Complementary and Alternative Medicine, a government-run website. NCCAM notes that St. John’s Wort has evidence for effectiveness equal to prescribed antidepressants for mild depression but with dangerous potential side effects, such as worsening of psychotic symptoms in bipolar disorder or schizophrenia, plus potentially life threatening drug interactions. While safe, valerian and probiotics have no evidence for reducing depression.
Social support
Family is usually the most important support for depressed teens even though they may be pushing family away, may refuse to come on outings, or may even refuse to come out of the bedroom. We should encourage parents and siblings to “hang out,” sitting quietly, available to listen rather than probing, cajoling, or nagging as they may have been doing. Parents also provide support by assuring adherence to visits, goals, and medications. Peer support helps a teen feel less alone and may increase social skills, but it can be difficult to sustain because friends may find depression threatening or give up when the teen avoids them and refuses activities. The National Association for Mental Illness has an online support group (www.strengthofus.org), as well as many excellent family resources. Sometimes medical efforts to be nonsectarian result in failure to recognize and remind teens and families of the value of religion, which is free and universally available, as a source of social support.
Relaxation
An evaluation of 15 studies concluded that relaxation techniques reduced depressive symptoms better than no treatment but not as much cognitive-behavior therapy (CBT). Yoga is another source of relaxation training. Mindfulness includes relaxation and specifies working to stay nonjudgmental about thoughts passing through one’s mind, recognizing and “arguing” with negative thinking, which is also part of CBT. Guided relaxation with a person, audiotape, or app (Calm or Headspace, among others) may be better for depressed teens because it inserts a voice to guide thoughts, which could potentially fend off ruminating on sad things.
Setting goals to address problems
In mild depression, compared with more endogenous moderate to severe major depressive disorder, a specific life stressor or relationship issue may be the precipitant. Identifying such factors (never forgetting possible trauma or abuse, which are harder to reveal), empathizing with the pain, and addressing them such as using Problem Solving Treatment for Primary Care (PST-PC) are within primary care skills. PST-PC involves four to six 30-minute sessions over 6-10 weeks during which you can provide perspective, help your patient set realistic goals and solutions to try out for situations that can be changed or coping strategies for emotion-focused unchangeable issues, iteratively check on progress via calls or televisits (the monitoring component), and renew problem-solving efforts as needed.
If mild depression fails to improve over several months or worsens, GLAD-PC describes evidence-based treatments. Even if it remits, your active support and monitoring should continue because depression tends to recur. You may not realize how valuable these seemingly simple active supports are to keeping mild depression in your teen patients at bay.
Dr. Howard is an assistant professor of pediatrics at Johns Hopkins University, Baltimore, and creator of CHADIS (www.CHADIS.com). She had no other relevant disclosures. Dr. Howard’s contribution to this publication was as a paid expert to MDedge News. E-mail her at [email protected].
References
1. Pediatrics. 2018 Mar 1. doi: 10.1542/peds.2017-4081.
2. Pediatrics. 2018 Mar 1. doi: 10.1542/peds.2017-4082.
Increased awareness needed of bipolar disorder in primary care
A significant number of patients with in primary care also have unrecognized bipolar disorder, according to researchers at the University of Manchester (England).
In a systematic review and meta-analysis published in General Hospital Psychiatry, the researchers searched Medline, Embase, Cochrane, and PsycINFO for that measured the rate of unrecognized bipolar disorder in primary care.
The overall prevalence of unrecognized bipolar disorder within the patient group was 17%; however, that rate varied significantly. Depending on the study, the range was 5%-28%. A subgroup analysis showed that studies that relied on clinical interviews for patients with confirmed bipolar disorder had lower rates than studies that relied on self-reporting. However, that difference did not reach statistical significance (14% vs. 22%; P = .121).
“There is ... an imperative need to improve the recognition of bipolar disorder in patients in primary care. A lack of effective training of primary care physicians, competing clinical demands, and reduced financial incentives ... are key reasons for the unrecognition of mental health conditions in primary care,” the investigators noted.
No conflicts of interest were reported.
SOURCE: Daveney J et al. Gen Hosp Psychiatry. 2019 Mar 27. doi: 10.1016/j.genhosppsych.2019.03.006.
A significant number of patients with in primary care also have unrecognized bipolar disorder, according to researchers at the University of Manchester (England).
In a systematic review and meta-analysis published in General Hospital Psychiatry, the researchers searched Medline, Embase, Cochrane, and PsycINFO for that measured the rate of unrecognized bipolar disorder in primary care.
The overall prevalence of unrecognized bipolar disorder within the patient group was 17%; however, that rate varied significantly. Depending on the study, the range was 5%-28%. A subgroup analysis showed that studies that relied on clinical interviews for patients with confirmed bipolar disorder had lower rates than studies that relied on self-reporting. However, that difference did not reach statistical significance (14% vs. 22%; P = .121).
“There is ... an imperative need to improve the recognition of bipolar disorder in patients in primary care. A lack of effective training of primary care physicians, competing clinical demands, and reduced financial incentives ... are key reasons for the unrecognition of mental health conditions in primary care,” the investigators noted.
No conflicts of interest were reported.
SOURCE: Daveney J et al. Gen Hosp Psychiatry. 2019 Mar 27. doi: 10.1016/j.genhosppsych.2019.03.006.
A significant number of patients with in primary care also have unrecognized bipolar disorder, according to researchers at the University of Manchester (England).
In a systematic review and meta-analysis published in General Hospital Psychiatry, the researchers searched Medline, Embase, Cochrane, and PsycINFO for that measured the rate of unrecognized bipolar disorder in primary care.
The overall prevalence of unrecognized bipolar disorder within the patient group was 17%; however, that rate varied significantly. Depending on the study, the range was 5%-28%. A subgroup analysis showed that studies that relied on clinical interviews for patients with confirmed bipolar disorder had lower rates than studies that relied on self-reporting. However, that difference did not reach statistical significance (14% vs. 22%; P = .121).
“There is ... an imperative need to improve the recognition of bipolar disorder in patients in primary care. A lack of effective training of primary care physicians, competing clinical demands, and reduced financial incentives ... are key reasons for the unrecognition of mental health conditions in primary care,” the investigators noted.
No conflicts of interest were reported.
SOURCE: Daveney J et al. Gen Hosp Psychiatry. 2019 Mar 27. doi: 10.1016/j.genhosppsych.2019.03.006.
FROM GENERAL HOSPITAL PSYCHIATRY
Residents are drowning in job offers – and debt
Physician search firm Merritt Hawkins did – actually, they heard from 391 residents – and 64% said that they had been contacted too many times by recruiters.
“Physicians coming out of training are being recruited like blue-chip athletes,” Travis Singleton, executive vice president of Merritt Hawkins, said in a statement. “There are simply not enough new doctors to go around.”
Merritt Hawkins asked physicians in their final year of residency about career choices, practice plans, and finances. Most said that they would prefer to be employed by a hospital or group practice, and a majority want to practice in a community with a population of 250,000 or more. More than half of the residents owed over $150,000 in student loans, but there were considerable debt differences between U.S. and international medical graduates.
The specialty distribution of respondents was 50% primary care, 30% internal medicine subspecialty/other, 15% surgical, and 5% diagnostic. About three-quarters were U.S. graduates and one-quarter of the residents were international medical graduates in this latest survey in a series that has been conducted periodically since 1991.
The survey was conducted in April 2018.
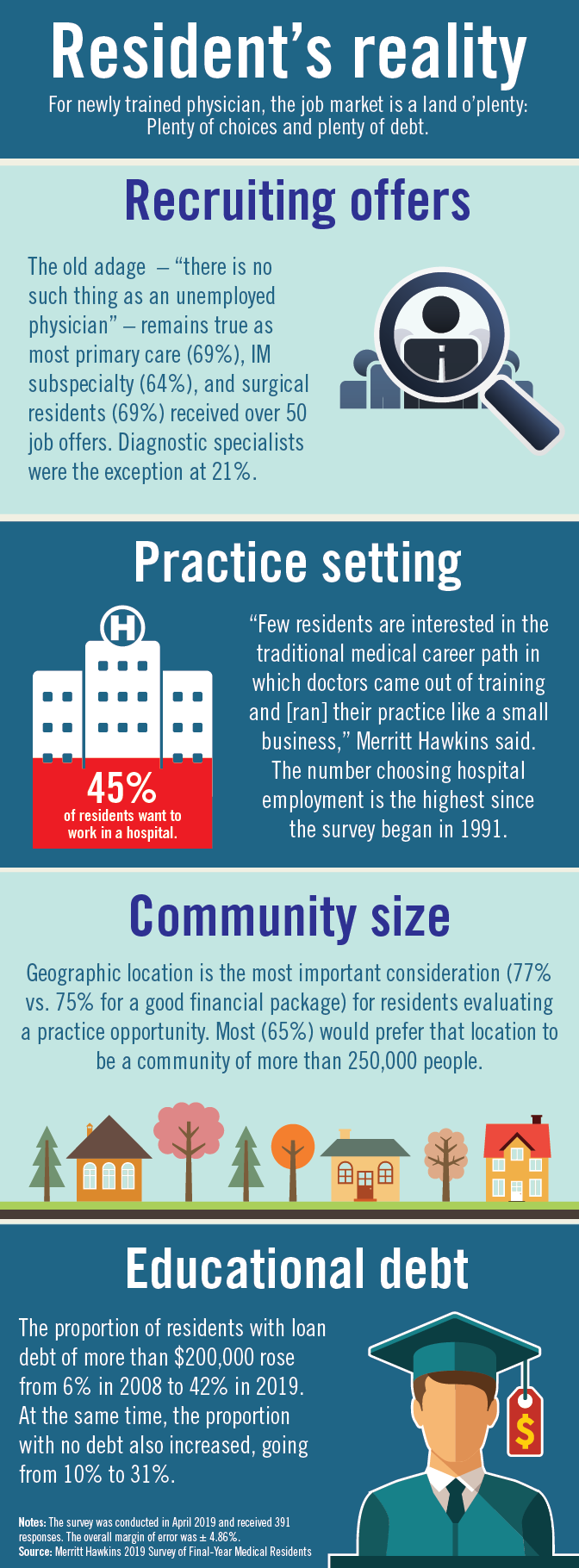
Physician search firm Merritt Hawkins did – actually, they heard from 391 residents – and 64% said that they had been contacted too many times by recruiters.
“Physicians coming out of training are being recruited like blue-chip athletes,” Travis Singleton, executive vice president of Merritt Hawkins, said in a statement. “There are simply not enough new doctors to go around.”
Merritt Hawkins asked physicians in their final year of residency about career choices, practice plans, and finances. Most said that they would prefer to be employed by a hospital or group practice, and a majority want to practice in a community with a population of 250,000 or more. More than half of the residents owed over $150,000 in student loans, but there were considerable debt differences between U.S. and international medical graduates.
The specialty distribution of respondents was 50% primary care, 30% internal medicine subspecialty/other, 15% surgical, and 5% diagnostic. About three-quarters were U.S. graduates and one-quarter of the residents were international medical graduates in this latest survey in a series that has been conducted periodically since 1991.
The survey was conducted in April 2018.

Physician search firm Merritt Hawkins did – actually, they heard from 391 residents – and 64% said that they had been contacted too many times by recruiters.
“Physicians coming out of training are being recruited like blue-chip athletes,” Travis Singleton, executive vice president of Merritt Hawkins, said in a statement. “There are simply not enough new doctors to go around.”
Merritt Hawkins asked physicians in their final year of residency about career choices, practice plans, and finances. Most said that they would prefer to be employed by a hospital or group practice, and a majority want to practice in a community with a population of 250,000 or more. More than half of the residents owed over $150,000 in student loans, but there were considerable debt differences between U.S. and international medical graduates.
The specialty distribution of respondents was 50% primary care, 30% internal medicine subspecialty/other, 15% surgical, and 5% diagnostic. About three-quarters were U.S. graduates and one-quarter of the residents were international medical graduates in this latest survey in a series that has been conducted periodically since 1991.
The survey was conducted in April 2018.

The right of conscientious objection
This particularly is true for those who consider medicine a vocation, or a calling, rather than just a job.
On May 2, 2019, the Department of Health and Human Services made public a 440-page document known as the Final Conscience Rule.1 It isn’t quite final. And the state of California already is suing to stop it.2 But the document represents the culmination of years of legal wrangling over whether physicians are allowed to have consciences or whether they must function as automatons providing any legally permitted care that a patient might demand. This comprehensive document provides a history of the issues, but was written in dense legalese, as if it expected to be answering challenges in court.
The short answer in the United States is that religious liberty continues to triumph over editorials in the New England Journal of Medicine. Consciences are allowed. The Final Conscience Rule begins with “The United States has a long history of providing protections in health care for individuals and entities on the basis of religious beliefs or moral convictions.” That history includes the Religious Freedom and Restoration Act of 1993.3 RFRA was introduced into the Senate by Sen. Ted Kennedy (D-Mass.), a bastion of liberal health care policies, and passed by a 97-3 vote. It was introduced into the House by then-Rep. Chuck Schumer (D-N.Y.) and passed by a unanimous voice vote. RFRA is not the invention of Republican fundamentalists.
For my colleagues in Canada, the Ontario Court of Appeals (ONCA, the highest provincial court) decided on May 15, 2019, that the opposite situation is the law in Canada. A recent Ontario law concerning medical assistance in dying (also known as physician-assisted suicide) requires Ontario physicians to either provide the assistance when requested or to make an effective referral, defined as “a referral made in good faith, to a non-objecting, available, and accessible physician, other health-care professional, or agency.” Some Canadian physicians objected to this requirement as a violation of their consciences and their Hippocratic Oaths. They lost. The ONCA decision is 74 readable, double-spaced pages and spells out the ethics and legal principles. In summary, the ONCA said the policies on requiring an effective referral “strike a reasonable balance between patients’ interests and physicians’ Charter-protected religious freedom. In short, they are reasonable limits prescribed by law that are demonstrably justified in a free and democratic society.”4
The California physician-assisted dying law, known as the End of Life Option Act, which became effective in 2016, has policies which are very different from the Ontario policies. The California law has clear protections for the consciences of physicians. The law empowers them to avoid being compelled or coerced into cooperating with these deaths. “Participation in activities authorized pursuant to this part shall be voluntary. … A person or entity that elects, for reasons of conscience, morality, or ethics, not to engage in activities authorized pursuant to this part is not required to take any action in support of an individual’s decision under this part.”5 If it seems strange that California would strongly protect conscience with its own statute but challenge the new federal regulations, welcome to tribal politics.
The point is that the role of physicians in abortion, physician aid in dying, and other controversial practices is not going to be decided by philosophical discussions about the ideal scope and purpose of medicine. Compromises are involved that reflect the values of society. Canada is more anticlerical than the United States, and Ontario chose a different path. French culture is even more extreme. Recently, mayors in two towns in France told their elementary schools to stop offering alternative entrées on days when pork was served for hot lunches. Secular schools were not to provide accommodation for students (Muslim and Jewish) who religiously objected to pork. Since the French Revolution, the emphasis is on assimilation and laïcité (France’s principle of secularism in public affairs). The cathedral Notre-Dame de Paris – recently damaged by fire – is owned by the state, not the Catholic Church. The United States has a different history and culture. It has supported religious liberty and reasonable accommodations. That is the loving thing to do. But as a reminder, the Peace of Westphalia in 1648, which ended European religious wars between Protestants and Catholics, was not a result of enlightened thinking and agapeic love. The fighting parties looked in the abyss of mutual annihilation and opted for coexistence instead.
Dr. Powell is a pediatric hospitalist and clinical ethics consultant living in St. Louis. Email him at [email protected].
References
1. Department of Health and Human Services, “HHS Announces Final Conscience Rule Protecting Health Care Entities and Individuals,” May 2, 2019.
2. “California sues Trump administration over ‘conscience rule’ that could limit abortions,” Los Angeles Times, May 21, 2019.
3. Wikipedia, “Religious Freedom Restoration Act of 1993”
4. Christian Medical and Dental Society of Canada v. College of Physicians and Surgeons of Ontario, 2019 ONCA 393.
5. California Assembly Bill No. 15, End of Life Option Act.
The article was updated on June 21, 2019.
This particularly is true for those who consider medicine a vocation, or a calling, rather than just a job.
On May 2, 2019, the Department of Health and Human Services made public a 440-page document known as the Final Conscience Rule.1 It isn’t quite final. And the state of California already is suing to stop it.2 But the document represents the culmination of years of legal wrangling over whether physicians are allowed to have consciences or whether they must function as automatons providing any legally permitted care that a patient might demand. This comprehensive document provides a history of the issues, but was written in dense legalese, as if it expected to be answering challenges in court.
The short answer in the United States is that religious liberty continues to triumph over editorials in the New England Journal of Medicine. Consciences are allowed. The Final Conscience Rule begins with “The United States has a long history of providing protections in health care for individuals and entities on the basis of religious beliefs or moral convictions.” That history includes the Religious Freedom and Restoration Act of 1993.3 RFRA was introduced into the Senate by Sen. Ted Kennedy (D-Mass.), a bastion of liberal health care policies, and passed by a 97-3 vote. It was introduced into the House by then-Rep. Chuck Schumer (D-N.Y.) and passed by a unanimous voice vote. RFRA is not the invention of Republican fundamentalists.
For my colleagues in Canada, the Ontario Court of Appeals (ONCA, the highest provincial court) decided on May 15, 2019, that the opposite situation is the law in Canada. A recent Ontario law concerning medical assistance in dying (also known as physician-assisted suicide) requires Ontario physicians to either provide the assistance when requested or to make an effective referral, defined as “a referral made in good faith, to a non-objecting, available, and accessible physician, other health-care professional, or agency.” Some Canadian physicians objected to this requirement as a violation of their consciences and their Hippocratic Oaths. They lost. The ONCA decision is 74 readable, double-spaced pages and spells out the ethics and legal principles. In summary, the ONCA said the policies on requiring an effective referral “strike a reasonable balance between patients’ interests and physicians’ Charter-protected religious freedom. In short, they are reasonable limits prescribed by law that are demonstrably justified in a free and democratic society.”4
The California physician-assisted dying law, known as the End of Life Option Act, which became effective in 2016, has policies which are very different from the Ontario policies. The California law has clear protections for the consciences of physicians. The law empowers them to avoid being compelled or coerced into cooperating with these deaths. “Participation in activities authorized pursuant to this part shall be voluntary. … A person or entity that elects, for reasons of conscience, morality, or ethics, not to engage in activities authorized pursuant to this part is not required to take any action in support of an individual’s decision under this part.”5 If it seems strange that California would strongly protect conscience with its own statute but challenge the new federal regulations, welcome to tribal politics.
The point is that the role of physicians in abortion, physician aid in dying, and other controversial practices is not going to be decided by philosophical discussions about the ideal scope and purpose of medicine. Compromises are involved that reflect the values of society. Canada is more anticlerical than the United States, and Ontario chose a different path. French culture is even more extreme. Recently, mayors in two towns in France told their elementary schools to stop offering alternative entrées on days when pork was served for hot lunches. Secular schools were not to provide accommodation for students (Muslim and Jewish) who religiously objected to pork. Since the French Revolution, the emphasis is on assimilation and laïcité (France’s principle of secularism in public affairs). The cathedral Notre-Dame de Paris – recently damaged by fire – is owned by the state, not the Catholic Church. The United States has a different history and culture. It has supported religious liberty and reasonable accommodations. That is the loving thing to do. But as a reminder, the Peace of Westphalia in 1648, which ended European religious wars between Protestants and Catholics, was not a result of enlightened thinking and agapeic love. The fighting parties looked in the abyss of mutual annihilation and opted for coexistence instead.
Dr. Powell is a pediatric hospitalist and clinical ethics consultant living in St. Louis. Email him at [email protected].
References
1. Department of Health and Human Services, “HHS Announces Final Conscience Rule Protecting Health Care Entities and Individuals,” May 2, 2019.
2. “California sues Trump administration over ‘conscience rule’ that could limit abortions,” Los Angeles Times, May 21, 2019.
3. Wikipedia, “Religious Freedom Restoration Act of 1993”
4. Christian Medical and Dental Society of Canada v. College of Physicians and Surgeons of Ontario, 2019 ONCA 393.
5. California Assembly Bill No. 15, End of Life Option Act.
The article was updated on June 21, 2019.
This particularly is true for those who consider medicine a vocation, or a calling, rather than just a job.
On May 2, 2019, the Department of Health and Human Services made public a 440-page document known as the Final Conscience Rule.1 It isn’t quite final. And the state of California already is suing to stop it.2 But the document represents the culmination of years of legal wrangling over whether physicians are allowed to have consciences or whether they must function as automatons providing any legally permitted care that a patient might demand. This comprehensive document provides a history of the issues, but was written in dense legalese, as if it expected to be answering challenges in court.
The short answer in the United States is that religious liberty continues to triumph over editorials in the New England Journal of Medicine. Consciences are allowed. The Final Conscience Rule begins with “The United States has a long history of providing protections in health care for individuals and entities on the basis of religious beliefs or moral convictions.” That history includes the Religious Freedom and Restoration Act of 1993.3 RFRA was introduced into the Senate by Sen. Ted Kennedy (D-Mass.), a bastion of liberal health care policies, and passed by a 97-3 vote. It was introduced into the House by then-Rep. Chuck Schumer (D-N.Y.) and passed by a unanimous voice vote. RFRA is not the invention of Republican fundamentalists.
For my colleagues in Canada, the Ontario Court of Appeals (ONCA, the highest provincial court) decided on May 15, 2019, that the opposite situation is the law in Canada. A recent Ontario law concerning medical assistance in dying (also known as physician-assisted suicide) requires Ontario physicians to either provide the assistance when requested or to make an effective referral, defined as “a referral made in good faith, to a non-objecting, available, and accessible physician, other health-care professional, or agency.” Some Canadian physicians objected to this requirement as a violation of their consciences and their Hippocratic Oaths. They lost. The ONCA decision is 74 readable, double-spaced pages and spells out the ethics and legal principles. In summary, the ONCA said the policies on requiring an effective referral “strike a reasonable balance between patients’ interests and physicians’ Charter-protected religious freedom. In short, they are reasonable limits prescribed by law that are demonstrably justified in a free and democratic society.”4
The California physician-assisted dying law, known as the End of Life Option Act, which became effective in 2016, has policies which are very different from the Ontario policies. The California law has clear protections for the consciences of physicians. The law empowers them to avoid being compelled or coerced into cooperating with these deaths. “Participation in activities authorized pursuant to this part shall be voluntary. … A person or entity that elects, for reasons of conscience, morality, or ethics, not to engage in activities authorized pursuant to this part is not required to take any action in support of an individual’s decision under this part.”5 If it seems strange that California would strongly protect conscience with its own statute but challenge the new federal regulations, welcome to tribal politics.
The point is that the role of physicians in abortion, physician aid in dying, and other controversial practices is not going to be decided by philosophical discussions about the ideal scope and purpose of medicine. Compromises are involved that reflect the values of society. Canada is more anticlerical than the United States, and Ontario chose a different path. French culture is even more extreme. Recently, mayors in two towns in France told their elementary schools to stop offering alternative entrées on days when pork was served for hot lunches. Secular schools were not to provide accommodation for students (Muslim and Jewish) who religiously objected to pork. Since the French Revolution, the emphasis is on assimilation and laïcité (France’s principle of secularism in public affairs). The cathedral Notre-Dame de Paris – recently damaged by fire – is owned by the state, not the Catholic Church. The United States has a different history and culture. It has supported religious liberty and reasonable accommodations. That is the loving thing to do. But as a reminder, the Peace of Westphalia in 1648, which ended European religious wars between Protestants and Catholics, was not a result of enlightened thinking and agapeic love. The fighting parties looked in the abyss of mutual annihilation and opted for coexistence instead.
Dr. Powell is a pediatric hospitalist and clinical ethics consultant living in St. Louis. Email him at [email protected].
References
1. Department of Health and Human Services, “HHS Announces Final Conscience Rule Protecting Health Care Entities and Individuals,” May 2, 2019.
2. “California sues Trump administration over ‘conscience rule’ that could limit abortions,” Los Angeles Times, May 21, 2019.
3. Wikipedia, “Religious Freedom Restoration Act of 1993”
4. Christian Medical and Dental Society of Canada v. College of Physicians and Surgeons of Ontario, 2019 ONCA 393.
5. California Assembly Bill No. 15, End of Life Option Act.
The article was updated on June 21, 2019.

















