User login
MRD negativity linked to survival in MM after auto-HCT
HOUSTON – Minimal residual disease (MRD) negativity by multiparameter flow cytometry was linked to survival benefit in multiple myeloma patients undergoing autologous transplantation, according to results of the first U.S.-based study evaluating this endpoint as part of a national randomized clinical trial.
MRD-negative status was prognostic for improved progression-free survival at all time points measured over the course of 1 year post transplant, in this ancillary study of patients in the randomized, 3-arm STAMiNA trial.
Moreover, there was an overall survival benefit for MRD-negative status at 1 year post transplant, investigator Theresa A. Hahn, PhD, of Roswell Park Comprehensive Cancer Center, Buffalo, N.Y., reported at the Transplantation & Cellular Therapy Meetings.
There was no significant difference in rate of conversion to MRD negativity in the arms of the trial, which evaluated several different upfront approaches to autologous hematopoietic stem cell transplantation (HCT).
Assessments of MRD beyond 1 year post transplant may be valuable in future trials, Dr. Hahn said.
“Trials are needed incorporating MRD as an endpoint for treatment decisions to augment, change, or discontinue therapy,” she added.
Results of the ancillary study known as PRIMeR (Prognostic Immunophenotyping for Myeloma Response) included 445 patients from STAMiNA who underwent MRD assessment at baseline, prior to maintenance, and at 1 year post transplantation.
As part of the overall STAMiNA trial, they were randomized to single autologous hematopoietic cell transplantation (HCT); autologous HCT followed by a second autologous HCT (tandem autologous HCT); or single autologous HCT followed by four cycles of consolidation with lenalidomide, bortezomib, and dexamethasone (RVD). All three arms continued on lenalidomide maintenance after those interventions.
Overall results of the STAMiNA trial, previously reported, showed no significant differences in progression-free survival or overall survival among the three transplant strategies (J Clin Oncol. 2019 Jan 17. doi: 10.1200/JCO.18.00685).
In this PRIMeR substudy, by contrast, progression-free survival was significantly increased for patients who were MRD negative at all three time points measured, Dr. Hahn reported, while overall survival was significantly improved based on MRD status measured at the 1-year time point.
The rate of MRD negativity did not differ significantly between arms at baseline or premaintenance time points, Dr. Hahn said. Those rates were 42%, 47%, and 40%, respectively, for the single transplant, tandem transplant, and single transplant plus consolidation arms, while the premaintenance MRD negativity rates were 77%, 83%, and 76%.
At 1 year, MRD negativity rates were significantly different between arms, but only in the intent-to-treat analysis.
Most of the difference was due to an increased rate of MRD negativity in the tandem-transplant arm, compared to a single auto-transplant. However, about 30% of patients in the tandem transplant arm did not receive the therapy, so in the analysis by actual treatment received, the rates of MRD negativity were 81% for single transplant, 90% for tandem transplant, and 85% for single transplant plus consolidation (P = 0.2).
Dr. Hahn said she and her colleagues will be updating their analysis of the PRIMeR study to assess the predictive value of MRD status in patients who were negative at all time points evaluated, versus those who converted to MRD negativity at the 1-year analysis.
The MRD assessments used in this trial have been incorporated into the recently completed BMT CTN 1401 trial and the ongoing BMT CTN 1302 study of allogeneic HCT plus ixazomib in high-risk myeloma, she added.
Dr. Hahn reported research funding from Celgene and the National Institutes of Health.
The meeting was held by the American Society for Blood and Marrow Transplantation and the Center for International Blood and Marrow Transplant Research. At its meeting, the American Society for Blood and Marrow Transplantation announced a new name for the society: American Society for Transplantation and Cellular Therapy (ASTCT).
SOURCE: Hahn TE et al. TCT 2019, Abstract 6.
HOUSTON – Minimal residual disease (MRD) negativity by multiparameter flow cytometry was linked to survival benefit in multiple myeloma patients undergoing autologous transplantation, according to results of the first U.S.-based study evaluating this endpoint as part of a national randomized clinical trial.
MRD-negative status was prognostic for improved progression-free survival at all time points measured over the course of 1 year post transplant, in this ancillary study of patients in the randomized, 3-arm STAMiNA trial.
Moreover, there was an overall survival benefit for MRD-negative status at 1 year post transplant, investigator Theresa A. Hahn, PhD, of Roswell Park Comprehensive Cancer Center, Buffalo, N.Y., reported at the Transplantation & Cellular Therapy Meetings.
There was no significant difference in rate of conversion to MRD negativity in the arms of the trial, which evaluated several different upfront approaches to autologous hematopoietic stem cell transplantation (HCT).
Assessments of MRD beyond 1 year post transplant may be valuable in future trials, Dr. Hahn said.
“Trials are needed incorporating MRD as an endpoint for treatment decisions to augment, change, or discontinue therapy,” she added.
Results of the ancillary study known as PRIMeR (Prognostic Immunophenotyping for Myeloma Response) included 445 patients from STAMiNA who underwent MRD assessment at baseline, prior to maintenance, and at 1 year post transplantation.
As part of the overall STAMiNA trial, they were randomized to single autologous hematopoietic cell transplantation (HCT); autologous HCT followed by a second autologous HCT (tandem autologous HCT); or single autologous HCT followed by four cycles of consolidation with lenalidomide, bortezomib, and dexamethasone (RVD). All three arms continued on lenalidomide maintenance after those interventions.
Overall results of the STAMiNA trial, previously reported, showed no significant differences in progression-free survival or overall survival among the three transplant strategies (J Clin Oncol. 2019 Jan 17. doi: 10.1200/JCO.18.00685).
In this PRIMeR substudy, by contrast, progression-free survival was significantly increased for patients who were MRD negative at all three time points measured, Dr. Hahn reported, while overall survival was significantly improved based on MRD status measured at the 1-year time point.
The rate of MRD negativity did not differ significantly between arms at baseline or premaintenance time points, Dr. Hahn said. Those rates were 42%, 47%, and 40%, respectively, for the single transplant, tandem transplant, and single transplant plus consolidation arms, while the premaintenance MRD negativity rates were 77%, 83%, and 76%.
At 1 year, MRD negativity rates were significantly different between arms, but only in the intent-to-treat analysis.
Most of the difference was due to an increased rate of MRD negativity in the tandem-transplant arm, compared to a single auto-transplant. However, about 30% of patients in the tandem transplant arm did not receive the therapy, so in the analysis by actual treatment received, the rates of MRD negativity were 81% for single transplant, 90% for tandem transplant, and 85% for single transplant plus consolidation (P = 0.2).
Dr. Hahn said she and her colleagues will be updating their analysis of the PRIMeR study to assess the predictive value of MRD status in patients who were negative at all time points evaluated, versus those who converted to MRD negativity at the 1-year analysis.
The MRD assessments used in this trial have been incorporated into the recently completed BMT CTN 1401 trial and the ongoing BMT CTN 1302 study of allogeneic HCT plus ixazomib in high-risk myeloma, she added.
Dr. Hahn reported research funding from Celgene and the National Institutes of Health.
The meeting was held by the American Society for Blood and Marrow Transplantation and the Center for International Blood and Marrow Transplant Research. At its meeting, the American Society for Blood and Marrow Transplantation announced a new name for the society: American Society for Transplantation and Cellular Therapy (ASTCT).
SOURCE: Hahn TE et al. TCT 2019, Abstract 6.
HOUSTON – Minimal residual disease (MRD) negativity by multiparameter flow cytometry was linked to survival benefit in multiple myeloma patients undergoing autologous transplantation, according to results of the first U.S.-based study evaluating this endpoint as part of a national randomized clinical trial.
MRD-negative status was prognostic for improved progression-free survival at all time points measured over the course of 1 year post transplant, in this ancillary study of patients in the randomized, 3-arm STAMiNA trial.
Moreover, there was an overall survival benefit for MRD-negative status at 1 year post transplant, investigator Theresa A. Hahn, PhD, of Roswell Park Comprehensive Cancer Center, Buffalo, N.Y., reported at the Transplantation & Cellular Therapy Meetings.
There was no significant difference in rate of conversion to MRD negativity in the arms of the trial, which evaluated several different upfront approaches to autologous hematopoietic stem cell transplantation (HCT).
Assessments of MRD beyond 1 year post transplant may be valuable in future trials, Dr. Hahn said.
“Trials are needed incorporating MRD as an endpoint for treatment decisions to augment, change, or discontinue therapy,” she added.
Results of the ancillary study known as PRIMeR (Prognostic Immunophenotyping for Myeloma Response) included 445 patients from STAMiNA who underwent MRD assessment at baseline, prior to maintenance, and at 1 year post transplantation.
As part of the overall STAMiNA trial, they were randomized to single autologous hematopoietic cell transplantation (HCT); autologous HCT followed by a second autologous HCT (tandem autologous HCT); or single autologous HCT followed by four cycles of consolidation with lenalidomide, bortezomib, and dexamethasone (RVD). All three arms continued on lenalidomide maintenance after those interventions.
Overall results of the STAMiNA trial, previously reported, showed no significant differences in progression-free survival or overall survival among the three transplant strategies (J Clin Oncol. 2019 Jan 17. doi: 10.1200/JCO.18.00685).
In this PRIMeR substudy, by contrast, progression-free survival was significantly increased for patients who were MRD negative at all three time points measured, Dr. Hahn reported, while overall survival was significantly improved based on MRD status measured at the 1-year time point.
The rate of MRD negativity did not differ significantly between arms at baseline or premaintenance time points, Dr. Hahn said. Those rates were 42%, 47%, and 40%, respectively, for the single transplant, tandem transplant, and single transplant plus consolidation arms, while the premaintenance MRD negativity rates were 77%, 83%, and 76%.
At 1 year, MRD negativity rates were significantly different between arms, but only in the intent-to-treat analysis.
Most of the difference was due to an increased rate of MRD negativity in the tandem-transplant arm, compared to a single auto-transplant. However, about 30% of patients in the tandem transplant arm did not receive the therapy, so in the analysis by actual treatment received, the rates of MRD negativity were 81% for single transplant, 90% for tandem transplant, and 85% for single transplant plus consolidation (P = 0.2).
Dr. Hahn said she and her colleagues will be updating their analysis of the PRIMeR study to assess the predictive value of MRD status in patients who were negative at all time points evaluated, versus those who converted to MRD negativity at the 1-year analysis.
The MRD assessments used in this trial have been incorporated into the recently completed BMT CTN 1401 trial and the ongoing BMT CTN 1302 study of allogeneic HCT plus ixazomib in high-risk myeloma, she added.
Dr. Hahn reported research funding from Celgene and the National Institutes of Health.
The meeting was held by the American Society for Blood and Marrow Transplantation and the Center for International Blood and Marrow Transplant Research. At its meeting, the American Society for Blood and Marrow Transplantation announced a new name for the society: American Society for Transplantation and Cellular Therapy (ASTCT).
SOURCE: Hahn TE et al. TCT 2019, Abstract 6.
REPORTING FROM TCT 2019
Anti-GM-CSF antibody reduced CAR T-cell toxicity
HOUSTON – Neutralizing granulocyte-macrophage colony-stimulating factor (GM-CSF) may be an effective strategy not only to manage toxicities associated with chimeric antigen receptor (CAR) T-cell therapy, but also to enhance CAR-T cell function, an investigator reported at the Transplantation & Cellular Therapy Meetings.
The GM-CSF targeted monoclonal antibody lenzilumab reduced neurotoxicity and cytokine release syndrome (CRS) related to CD19-targeted CAR T-cell therapy in a patient-derived xenograft model, said investigator Rosalie M. Sterner, an MD-PhD student in the department of immunology at Mayo Clinic, Rochester, Minn.
Other investigations showed that neutralizing or knocking out GM-CSF enhanced the antitumor functions of the CAR T cells, Ms. Sterner said in a podium presentation at the meeting held by the American Society for Blood and Marrow Transplantation and the Center for International Blood and Marrow Transplant Research. At its meeting, the American Society for Blood and Marrow Transplantation announced a new name for the society: American Society for Transplantation and Cellular Therapy (ASTCT).
“GM-CSF blockade does not impair CAR T-cell effector function, and in fact, enhances CAR T-cell effector functions in certain models, and actually can help to ameliorate CAR T-cell associated toxicities,” Ms. Sterner said.
Based on these early findings, the investigators have designed a phase 2 clinical trial to see if lenzilumab can prevent CAR T cell-related toxicities in patients with diffuse large B-cell lymphoma.
GM-CSF, a cytokine produced by T cells and myeloid cells, is the most statistically significantly elevated serum marker in patients with severe neurotoxicity related to CAR T-cell therapy, Ms. Sterner told attendees.
Investigations have shown that the combination of lenzilumab plus CD19-targeted T-cell therapy did not impair CAR T-cell function in vivo or in vitro, she said.
In other studies, they investigated the impact of GM-CSF neutralization in mice engrafted with primary acute lymphocytic leukemia (ALL) blasts and treated with high doses of CD19 CAR T-cells, lenzilumab, and a murine GM-CSF blocking antibody to neutralize the mouse GM-CSF. That strategy prevented weight loss, decreased myeloid cytokines, reduced cerebral edema, and enhanced disease control, Ms. Sterner said.
Investigators also reported on CD19 CAR T-cells with reduced GM-CSF secretion due to CRISPR/Cas9 gene editing of the GM-CSF gene during the CAR T-cell manufacturing process. Xenograft model results showed a slight enhancement of disease control for those GM-CSF knockout CAR T cells versus standard CAR T cells.
More details of the investigations were recently published in Blood (2019;133:697-709).
Taken together, the investigations highlight GM-CSF inhibition as a novel approach to reducing neurotoxicity and CRS that may also enhance CAR T-cell effector functions, Ms. Sterner said.
Ms. Sterner reported having no financial disclosures related to her presentation.
SOURCE: Sterner R et al. TCT 2019, Abstract 5.
HOUSTON – Neutralizing granulocyte-macrophage colony-stimulating factor (GM-CSF) may be an effective strategy not only to manage toxicities associated with chimeric antigen receptor (CAR) T-cell therapy, but also to enhance CAR-T cell function, an investigator reported at the Transplantation & Cellular Therapy Meetings.
The GM-CSF targeted monoclonal antibody lenzilumab reduced neurotoxicity and cytokine release syndrome (CRS) related to CD19-targeted CAR T-cell therapy in a patient-derived xenograft model, said investigator Rosalie M. Sterner, an MD-PhD student in the department of immunology at Mayo Clinic, Rochester, Minn.
Other investigations showed that neutralizing or knocking out GM-CSF enhanced the antitumor functions of the CAR T cells, Ms. Sterner said in a podium presentation at the meeting held by the American Society for Blood and Marrow Transplantation and the Center for International Blood and Marrow Transplant Research. At its meeting, the American Society for Blood and Marrow Transplantation announced a new name for the society: American Society for Transplantation and Cellular Therapy (ASTCT).
“GM-CSF blockade does not impair CAR T-cell effector function, and in fact, enhances CAR T-cell effector functions in certain models, and actually can help to ameliorate CAR T-cell associated toxicities,” Ms. Sterner said.
Based on these early findings, the investigators have designed a phase 2 clinical trial to see if lenzilumab can prevent CAR T cell-related toxicities in patients with diffuse large B-cell lymphoma.
GM-CSF, a cytokine produced by T cells and myeloid cells, is the most statistically significantly elevated serum marker in patients with severe neurotoxicity related to CAR T-cell therapy, Ms. Sterner told attendees.
Investigations have shown that the combination of lenzilumab plus CD19-targeted T-cell therapy did not impair CAR T-cell function in vivo or in vitro, she said.
In other studies, they investigated the impact of GM-CSF neutralization in mice engrafted with primary acute lymphocytic leukemia (ALL) blasts and treated with high doses of CD19 CAR T-cells, lenzilumab, and a murine GM-CSF blocking antibody to neutralize the mouse GM-CSF. That strategy prevented weight loss, decreased myeloid cytokines, reduced cerebral edema, and enhanced disease control, Ms. Sterner said.
Investigators also reported on CD19 CAR T-cells with reduced GM-CSF secretion due to CRISPR/Cas9 gene editing of the GM-CSF gene during the CAR T-cell manufacturing process. Xenograft model results showed a slight enhancement of disease control for those GM-CSF knockout CAR T cells versus standard CAR T cells.
More details of the investigations were recently published in Blood (2019;133:697-709).
Taken together, the investigations highlight GM-CSF inhibition as a novel approach to reducing neurotoxicity and CRS that may also enhance CAR T-cell effector functions, Ms. Sterner said.
Ms. Sterner reported having no financial disclosures related to her presentation.
SOURCE: Sterner R et al. TCT 2019, Abstract 5.
HOUSTON – Neutralizing granulocyte-macrophage colony-stimulating factor (GM-CSF) may be an effective strategy not only to manage toxicities associated with chimeric antigen receptor (CAR) T-cell therapy, but also to enhance CAR-T cell function, an investigator reported at the Transplantation & Cellular Therapy Meetings.
The GM-CSF targeted monoclonal antibody lenzilumab reduced neurotoxicity and cytokine release syndrome (CRS) related to CD19-targeted CAR T-cell therapy in a patient-derived xenograft model, said investigator Rosalie M. Sterner, an MD-PhD student in the department of immunology at Mayo Clinic, Rochester, Minn.
Other investigations showed that neutralizing or knocking out GM-CSF enhanced the antitumor functions of the CAR T cells, Ms. Sterner said in a podium presentation at the meeting held by the American Society for Blood and Marrow Transplantation and the Center for International Blood and Marrow Transplant Research. At its meeting, the American Society for Blood and Marrow Transplantation announced a new name for the society: American Society for Transplantation and Cellular Therapy (ASTCT).
“GM-CSF blockade does not impair CAR T-cell effector function, and in fact, enhances CAR T-cell effector functions in certain models, and actually can help to ameliorate CAR T-cell associated toxicities,” Ms. Sterner said.
Based on these early findings, the investigators have designed a phase 2 clinical trial to see if lenzilumab can prevent CAR T cell-related toxicities in patients with diffuse large B-cell lymphoma.
GM-CSF, a cytokine produced by T cells and myeloid cells, is the most statistically significantly elevated serum marker in patients with severe neurotoxicity related to CAR T-cell therapy, Ms. Sterner told attendees.
Investigations have shown that the combination of lenzilumab plus CD19-targeted T-cell therapy did not impair CAR T-cell function in vivo or in vitro, she said.
In other studies, they investigated the impact of GM-CSF neutralization in mice engrafted with primary acute lymphocytic leukemia (ALL) blasts and treated with high doses of CD19 CAR T-cells, lenzilumab, and a murine GM-CSF blocking antibody to neutralize the mouse GM-CSF. That strategy prevented weight loss, decreased myeloid cytokines, reduced cerebral edema, and enhanced disease control, Ms. Sterner said.
Investigators also reported on CD19 CAR T-cells with reduced GM-CSF secretion due to CRISPR/Cas9 gene editing of the GM-CSF gene during the CAR T-cell manufacturing process. Xenograft model results showed a slight enhancement of disease control for those GM-CSF knockout CAR T cells versus standard CAR T cells.
More details of the investigations were recently published in Blood (2019;133:697-709).
Taken together, the investigations highlight GM-CSF inhibition as a novel approach to reducing neurotoxicity and CRS that may also enhance CAR T-cell effector functions, Ms. Sterner said.
Ms. Sterner reported having no financial disclosures related to her presentation.
SOURCE: Sterner R et al. TCT 2019, Abstract 5.
REPORTING FROM TCT 2019
Female Sexual Dysfunction
Treosulfan may become standard in allo-HCT for AML/MDS
HOUSTON – A treosulfan-based conditioning regimen could become standard prior to allogeneic transplant in elderly or comorbid patients with acute myeloid leukemia or myelodysplastic syndromes, according to the lead investigator in a phase 3 trial.
The treosulfan/fludarabine myeloablative conditioning regimen had noninferior event-free survival, compared with a reduced-intensity busulfan-based regimen in the large, randomized trial that included elderly patients and those with multiple comorbidities, said researcher Dietrich Wilhelm Beelen, MD, PhD.
The experimental regimen was superior to busulfan in overall survival, nonrelapse mortality, and complete donor chimerism in the trial, added Dr. Beelen, who is with the department of bone marrow transplantation at the West German Cancer Center, University Hospital of Essen, Germany.
“The study results point to a potential benefit of the treosulfan/fludarabine regimen, while the early safety profile, engraftment kinetics, acute or chronic graft-versus-host-disease (GvHD), and the relapse risk of both regimens appear comparable,” Dr. Beelen said at the Transplantation & Cellular Therapy Meetings.
Allogeneic hematopoietic cell transplantation (HCT) is challenging in elderly and comorbid patients, who have an increased risk of nonrelapse mortality with standard myeloablative regimens, according to Dr. Beelen, who presented results on behalf of investigators from the international MC-FludT.14/L Study Group.
Their phase 3 randomized trial included patients who were 50-70 years of age, or who had a Hematopoietic Cell Transplantation Comorbidity Index of 2 or greater. The final analysis included 551 patients (352 with AML and 199 with MDS).
The primary endpoint of the study was event-free survival at 2 years. That endpoint comprised relapse/progression of disease, graft failure, or death.
Patient enrollment was terminated early the MC-FludT.14/L study following an interim analysis that investigators said “clearly demonstrated” the noninferiority of the treosulfan/fludarabine regimen versus the reduced intensity busulfan/fludarabine regimen.
In the final analysis, event-free survival at 2 years was about 14.5 percentage points higher in the treosulfan group, at 65.7% versus 51.2% (P = .0000001), Dr. Beelen reported at the meeting.
A number of other secondary endpoints also favored treosulfan/fludarabine over busulfan, including overall survival (P = .0037), nonrelapse mortality (P = .0343), and survival free of chronic GvHD or relapse (P = .0030).
These results help establish the new treosulfan/fludarabine regimen as a “relatively well-tolerable and effective preparative regimen” in elderly or comorbid AML/MDS patients, Dr. Beelen said.
However, treosulfan has not been authorized for use in allogeneic HCT conditioning regimens, and so should be considered experimental in this setting, he said at the meeting held by the American Society for Blood and Marrow Transplantation and the Center for International Blood and Marrow Transplant Research. At its meeting, the American Society for Blood and Marrow Transplantation announced a new name for the society: American Society for Transplantation and Cellular Therapy (ASTCT).
Dr. Beelen reported honoraria, travel support, and trial documentation support provided by medac GmbH, which sponsored the trial.
SOURCE: Beelen DW et al. TCT 2019, Abstract 4.
HOUSTON – A treosulfan-based conditioning regimen could become standard prior to allogeneic transplant in elderly or comorbid patients with acute myeloid leukemia or myelodysplastic syndromes, according to the lead investigator in a phase 3 trial.
The treosulfan/fludarabine myeloablative conditioning regimen had noninferior event-free survival, compared with a reduced-intensity busulfan-based regimen in the large, randomized trial that included elderly patients and those with multiple comorbidities, said researcher Dietrich Wilhelm Beelen, MD, PhD.
The experimental regimen was superior to busulfan in overall survival, nonrelapse mortality, and complete donor chimerism in the trial, added Dr. Beelen, who is with the department of bone marrow transplantation at the West German Cancer Center, University Hospital of Essen, Germany.
“The study results point to a potential benefit of the treosulfan/fludarabine regimen, while the early safety profile, engraftment kinetics, acute or chronic graft-versus-host-disease (GvHD), and the relapse risk of both regimens appear comparable,” Dr. Beelen said at the Transplantation & Cellular Therapy Meetings.
Allogeneic hematopoietic cell transplantation (HCT) is challenging in elderly and comorbid patients, who have an increased risk of nonrelapse mortality with standard myeloablative regimens, according to Dr. Beelen, who presented results on behalf of investigators from the international MC-FludT.14/L Study Group.
Their phase 3 randomized trial included patients who were 50-70 years of age, or who had a Hematopoietic Cell Transplantation Comorbidity Index of 2 or greater. The final analysis included 551 patients (352 with AML and 199 with MDS).
The primary endpoint of the study was event-free survival at 2 years. That endpoint comprised relapse/progression of disease, graft failure, or death.
Patient enrollment was terminated early the MC-FludT.14/L study following an interim analysis that investigators said “clearly demonstrated” the noninferiority of the treosulfan/fludarabine regimen versus the reduced intensity busulfan/fludarabine regimen.
In the final analysis, event-free survival at 2 years was about 14.5 percentage points higher in the treosulfan group, at 65.7% versus 51.2% (P = .0000001), Dr. Beelen reported at the meeting.
A number of other secondary endpoints also favored treosulfan/fludarabine over busulfan, including overall survival (P = .0037), nonrelapse mortality (P = .0343), and survival free of chronic GvHD or relapse (P = .0030).
These results help establish the new treosulfan/fludarabine regimen as a “relatively well-tolerable and effective preparative regimen” in elderly or comorbid AML/MDS patients, Dr. Beelen said.
However, treosulfan has not been authorized for use in allogeneic HCT conditioning regimens, and so should be considered experimental in this setting, he said at the meeting held by the American Society for Blood and Marrow Transplantation and the Center for International Blood and Marrow Transplant Research. At its meeting, the American Society for Blood and Marrow Transplantation announced a new name for the society: American Society for Transplantation and Cellular Therapy (ASTCT).
Dr. Beelen reported honoraria, travel support, and trial documentation support provided by medac GmbH, which sponsored the trial.
SOURCE: Beelen DW et al. TCT 2019, Abstract 4.
HOUSTON – A treosulfan-based conditioning regimen could become standard prior to allogeneic transplant in elderly or comorbid patients with acute myeloid leukemia or myelodysplastic syndromes, according to the lead investigator in a phase 3 trial.
The treosulfan/fludarabine myeloablative conditioning regimen had noninferior event-free survival, compared with a reduced-intensity busulfan-based regimen in the large, randomized trial that included elderly patients and those with multiple comorbidities, said researcher Dietrich Wilhelm Beelen, MD, PhD.
The experimental regimen was superior to busulfan in overall survival, nonrelapse mortality, and complete donor chimerism in the trial, added Dr. Beelen, who is with the department of bone marrow transplantation at the West German Cancer Center, University Hospital of Essen, Germany.
“The study results point to a potential benefit of the treosulfan/fludarabine regimen, while the early safety profile, engraftment kinetics, acute or chronic graft-versus-host-disease (GvHD), and the relapse risk of both regimens appear comparable,” Dr. Beelen said at the Transplantation & Cellular Therapy Meetings.
Allogeneic hematopoietic cell transplantation (HCT) is challenging in elderly and comorbid patients, who have an increased risk of nonrelapse mortality with standard myeloablative regimens, according to Dr. Beelen, who presented results on behalf of investigators from the international MC-FludT.14/L Study Group.
Their phase 3 randomized trial included patients who were 50-70 years of age, or who had a Hematopoietic Cell Transplantation Comorbidity Index of 2 or greater. The final analysis included 551 patients (352 with AML and 199 with MDS).
The primary endpoint of the study was event-free survival at 2 years. That endpoint comprised relapse/progression of disease, graft failure, or death.
Patient enrollment was terminated early the MC-FludT.14/L study following an interim analysis that investigators said “clearly demonstrated” the noninferiority of the treosulfan/fludarabine regimen versus the reduced intensity busulfan/fludarabine regimen.
In the final analysis, event-free survival at 2 years was about 14.5 percentage points higher in the treosulfan group, at 65.7% versus 51.2% (P = .0000001), Dr. Beelen reported at the meeting.
A number of other secondary endpoints also favored treosulfan/fludarabine over busulfan, including overall survival (P = .0037), nonrelapse mortality (P = .0343), and survival free of chronic GvHD or relapse (P = .0030).
These results help establish the new treosulfan/fludarabine regimen as a “relatively well-tolerable and effective preparative regimen” in elderly or comorbid AML/MDS patients, Dr. Beelen said.
However, treosulfan has not been authorized for use in allogeneic HCT conditioning regimens, and so should be considered experimental in this setting, he said at the meeting held by the American Society for Blood and Marrow Transplantation and the Center for International Blood and Marrow Transplant Research. At its meeting, the American Society for Blood and Marrow Transplantation announced a new name for the society: American Society for Transplantation and Cellular Therapy (ASTCT).
Dr. Beelen reported honoraria, travel support, and trial documentation support provided by medac GmbH, which sponsored the trial.
SOURCE: Beelen DW et al. TCT 2019, Abstract 4.
REPORTING FROM TCT 2019
Ixekizumab psoriasis outcomes, sliced and diced
WAIKOLOA, HAWAII – The highly selective interleukin-17A subunit inhibitor in the long-term extension phase of the randomized, controlled UNCOVER-3 (NCT01646177) trial, Craig L. Leonardi, MD, reported at the Hawaii Dermatology Seminar provided by the Global Academy for Medical Education/Skin Disease Education Foundation.
However, the strict inclusion and exclusion criteria employed in randomized trials such as this raise questions about the broader applicability of the results in real-world clinical practice. So separately at the Hawaii seminar, Dr. Leonardi presented a single-center retrospective observational cohort study of the rapidity and duration of response to ixekizumab in his own clinical practice after the biologic received Food and Drug Administration marketing approval. Those results, too, were impressive and, in his view, highly generalizable.
“It is expected that this study cohort is generally representative of patients who are routinely seen at dermatology referral practices in the U.S.,” commented Dr. Leonardi, of Saint Louis University.
UNCOVER-3 included 1,346 psoriasis patients initially randomized 2:2:2:1 to double-blind subcutaneous ixekizumab (Taltz) at 80 mg either every 2 weeks or every 4 weeks after a 160-mg loading dose; subcutaneous etanercept at 50 mg twice weekly; or placebo for 12 weeks, followed by a switch to ixekizumab at 80 mg every 4 weeks from week 12 out to 3 years. The long-term efficacy analysis was restricted to patients who received the biologic according to what ultimately became the approved dosing schedule: a 160-mg loading dose, followed by 80 mg every 2 weeks through week 12, then 80 mg every 4 weeks. The safety analysis, in contrast, included everybody.
Dr. Leonardi presented the efficacy data using several different statistical methodologies, thereby providing an instructive lesson regarding the importance of examining the fine print when viewing clinical trial results. At one extreme is the as-observed analysis. Under this methodology, if a patient dropped out of UNCOVER-3 at, for example, week 11, the last measurement of treatment response, recorded at week 8, is carried forward by investigators and assumed to be valid for the rest of the study. Since week 8 may have been the last time the patient was doing well on the drug, the as-observed analysis can create a distorted overly favorable picture of the drug’s performance.
“Patients fall out because the drug isn’t working well or they’re having a side effect, so over time, you tend to enrich for patients who are doing very well with the as-observed analysis,” the dermatologist explained.
Historically, many industry-sponsored clinical trials reported efficacy outcomes using the as-observed analysis; however, the FDA is increasingly unwilling to accept that approach as the sole analytic method.
At the other extreme is the nonresponder imputation method.
“This is the most stringent statistical package that exists. In fact, when a patient isn’t observed at one of the observation points – for example, at week 8 say the patient has a flat tire and can’t make it to the clinic – they’re counted as a treatment failure. So it’s a very tough statistical package,” according to Dr. Leonardi.
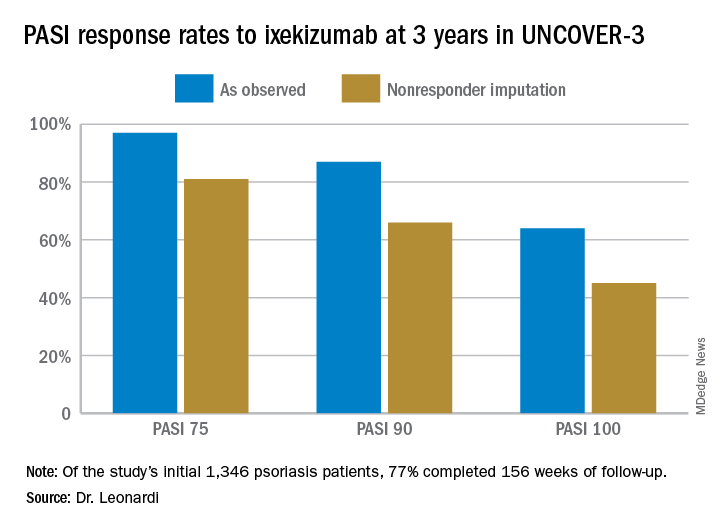
Seventy-seven percent of the initial 1,346 randomized patients in UNCOVER-3 completed 156 weeks of follow-up. To illustrate the importance of paying attention to the details of statistical methodology utilized in reporting efficacy outcomes, he noted that the PASI 75 rate at 156 weeks in study completers on the approved dosing regimen was 97% by the as-observed method, dropping to a still robust 81% by nonresponder imputation. The PASI 90 and -100 rates and static Physician’s Global Assessment (sPGA) results followed suit (see graphic).
Real-world performance
Dr. Leonard’s analysis of ixekizumab’s performance in his own practice included 106 patients placed on the drug following its FDA approval in March 2016, 74% of whom were still on the drug 12 months later. The cohort had a mean disease duration of 15 years. Three-quarters of them had previously received biologic therapy for their psoriasis, most often a tumor necrosis factor inhibitor. The study efficacy endpoints were the sPGA and Dermatology Life Quality Index (DLQI).
Already at 1 month, 30% of ixekizumab-treated patients had an sPGA score of 0, meaning their skin was totally clear. Another 29% had an sPGA of 1, meaning almost clear. At 3 months, 53% of patients had an sPGA of 0 and 21% had an sPGA of 1. Among patients on treatment at 12 months, the rates were 39% and 24% for sPGAs of 0 and 1, respectively. And in patients with an sPGA of 0/1 at 3 months, 73% maintained that score at 12 months, including 47% with an sPGA of 0.
A DLQI score of 0/1, indicative of little or no disease effect upon a patient’s life, was present in 63% of ixekizumab-treated patients at 1 month, 84% at 3 months, and 73% at 12 months.
The value in pushing for PASI 100
The ixekizumab experience in the phase-3 UNCOVER clinical trial program provided the first-ever evidence that incrementally improving psoriasis also provides stepwise improvement in DLQI, a key patient-reported outcome. At week 12 under double-blind conditions, only 4% of ixekizumab-treated patients with less than a PASI 50 response had a DLQI of 0/1. The rate rose to 18.8% in those with a PASI 50 to less than PASI 75 response. In patients with a week-12 PASI 75 to less than PASI 90 response, the DLQI 0/1 rate climbed to 52.3%. At a PASI 90 to less than PASI 100 response, the rate was 66.9%. And 82.9% of patients with a PASI 100 had a DLQI of 0/1. Every step of the way, those DLQI rates were significantly different from each other.
These data are “fascinating,” Dr. Leonardi commented. “If you ever get any inquiries from the friendly insurance carrier and they want to know if you’re improving your patient’s life, this is the kind of data that supports that they’re being improved dramatically.”
Dr. Leonardi noted that ixekizumab isn’t unique in its high rate of clinical effectiveness. That distinction is shared by the other approved IL-17 inhibitors, secukinumab (Cosentyx) and brodalumab (Siliq), as well as the IL-23 inhibitor guselkumab (Tremfya). He refers to these biologics collectively as “high-performance skin-clearance drugs.” He has calculated the number needed to treat (NNT) to achieve a PASI 100 response – complete clearance of the disease – based upon clinical trial data filed with the FDA and/or in the package inserts. The numbers are eye-opening: an NTT of 2.6 for ixekizumab based upon data from the UNCOVER-2 trial, 2.4 for brodalumab, 2.7 for guselkumab, and 3.6 for secukinumab. To help put that into perspective, the NNTs for methotrexate and etanercept (Enbrel) – not so long ago considered state of the art medications for moderate to severe psoriasis – are 25 and 23.3, respectively.
The UNCOVER trial portfolio and Dr. Leonardi’s single-center retrospective study were funded by Eli Lilly, which markets ixekizumab. He reported serving as a consultant to and receiving research funding from that company and more than a dozen others.
SDEF/Global Academy for Medical Education and this news organization are owned by the same parent company.
WAIKOLOA, HAWAII – The highly selective interleukin-17A subunit inhibitor in the long-term extension phase of the randomized, controlled UNCOVER-3 (NCT01646177) trial, Craig L. Leonardi, MD, reported at the Hawaii Dermatology Seminar provided by the Global Academy for Medical Education/Skin Disease Education Foundation.

However, the strict inclusion and exclusion criteria employed in randomized trials such as this raise questions about the broader applicability of the results in real-world clinical practice. So separately at the Hawaii seminar, Dr. Leonardi presented a single-center retrospective observational cohort study of the rapidity and duration of response to ixekizumab in his own clinical practice after the biologic received Food and Drug Administration marketing approval. Those results, too, were impressive and, in his view, highly generalizable.
“It is expected that this study cohort is generally representative of patients who are routinely seen at dermatology referral practices in the U.S.,” commented Dr. Leonardi, of Saint Louis University.
UNCOVER-3 included 1,346 psoriasis patients initially randomized 2:2:2:1 to double-blind subcutaneous ixekizumab (Taltz) at 80 mg either every 2 weeks or every 4 weeks after a 160-mg loading dose; subcutaneous etanercept at 50 mg twice weekly; or placebo for 12 weeks, followed by a switch to ixekizumab at 80 mg every 4 weeks from week 12 out to 3 years. The long-term efficacy analysis was restricted to patients who received the biologic according to what ultimately became the approved dosing schedule: a 160-mg loading dose, followed by 80 mg every 2 weeks through week 12, then 80 mg every 4 weeks. The safety analysis, in contrast, included everybody.
Dr. Leonardi presented the efficacy data using several different statistical methodologies, thereby providing an instructive lesson regarding the importance of examining the fine print when viewing clinical trial results. At one extreme is the as-observed analysis. Under this methodology, if a patient dropped out of UNCOVER-3 at, for example, week 11, the last measurement of treatment response, recorded at week 8, is carried forward by investigators and assumed to be valid for the rest of the study. Since week 8 may have been the last time the patient was doing well on the drug, the as-observed analysis can create a distorted overly favorable picture of the drug’s performance.
“Patients fall out because the drug isn’t working well or they’re having a side effect, so over time, you tend to enrich for patients who are doing very well with the as-observed analysis,” the dermatologist explained.
Historically, many industry-sponsored clinical trials reported efficacy outcomes using the as-observed analysis; however, the FDA is increasingly unwilling to accept that approach as the sole analytic method.
At the other extreme is the nonresponder imputation method.
“This is the most stringent statistical package that exists. In fact, when a patient isn’t observed at one of the observation points – for example, at week 8 say the patient has a flat tire and can’t make it to the clinic – they’re counted as a treatment failure. So it’s a very tough statistical package,” according to Dr. Leonardi.
Seventy-seven percent of the initial 1,346 randomized patients in UNCOVER-3 completed 156 weeks of follow-up. To illustrate the importance of paying attention to the details of statistical methodology utilized in reporting efficacy outcomes, he noted that the PASI 75 rate at 156 weeks in study completers on the approved dosing regimen was 97% by the as-observed method, dropping to a still robust 81% by nonresponder imputation. The PASI 90 and -100 rates and static Physician’s Global Assessment (sPGA) results followed suit (see graphic).
Real-world performance
Dr. Leonard’s analysis of ixekizumab’s performance in his own practice included 106 patients placed on the drug following its FDA approval in March 2016, 74% of whom were still on the drug 12 months later. The cohort had a mean disease duration of 15 years. Three-quarters of them had previously received biologic therapy for their psoriasis, most often a tumor necrosis factor inhibitor. The study efficacy endpoints were the sPGA and Dermatology Life Quality Index (DLQI).
Already at 1 month, 30% of ixekizumab-treated patients had an sPGA score of 0, meaning their skin was totally clear. Another 29% had an sPGA of 1, meaning almost clear. At 3 months, 53% of patients had an sPGA of 0 and 21% had an sPGA of 1. Among patients on treatment at 12 months, the rates were 39% and 24% for sPGAs of 0 and 1, respectively. And in patients with an sPGA of 0/1 at 3 months, 73% maintained that score at 12 months, including 47% with an sPGA of 0.
A DLQI score of 0/1, indicative of little or no disease effect upon a patient’s life, was present in 63% of ixekizumab-treated patients at 1 month, 84% at 3 months, and 73% at 12 months.
The value in pushing for PASI 100
The ixekizumab experience in the phase-3 UNCOVER clinical trial program provided the first-ever evidence that incrementally improving psoriasis also provides stepwise improvement in DLQI, a key patient-reported outcome. At week 12 under double-blind conditions, only 4% of ixekizumab-treated patients with less than a PASI 50 response had a DLQI of 0/1. The rate rose to 18.8% in those with a PASI 50 to less than PASI 75 response. In patients with a week-12 PASI 75 to less than PASI 90 response, the DLQI 0/1 rate climbed to 52.3%. At a PASI 90 to less than PASI 100 response, the rate was 66.9%. And 82.9% of patients with a PASI 100 had a DLQI of 0/1. Every step of the way, those DLQI rates were significantly different from each other.
These data are “fascinating,” Dr. Leonardi commented. “If you ever get any inquiries from the friendly insurance carrier and they want to know if you’re improving your patient’s life, this is the kind of data that supports that they’re being improved dramatically.”
Dr. Leonardi noted that ixekizumab isn’t unique in its high rate of clinical effectiveness. That distinction is shared by the other approved IL-17 inhibitors, secukinumab (Cosentyx) and brodalumab (Siliq), as well as the IL-23 inhibitor guselkumab (Tremfya). He refers to these biologics collectively as “high-performance skin-clearance drugs.” He has calculated the number needed to treat (NNT) to achieve a PASI 100 response – complete clearance of the disease – based upon clinical trial data filed with the FDA and/or in the package inserts. The numbers are eye-opening: an NTT of 2.6 for ixekizumab based upon data from the UNCOVER-2 trial, 2.4 for brodalumab, 2.7 for guselkumab, and 3.6 for secukinumab. To help put that into perspective, the NNTs for methotrexate and etanercept (Enbrel) – not so long ago considered state of the art medications for moderate to severe psoriasis – are 25 and 23.3, respectively.
The UNCOVER trial portfolio and Dr. Leonardi’s single-center retrospective study were funded by Eli Lilly, which markets ixekizumab. He reported serving as a consultant to and receiving research funding from that company and more than a dozen others.
SDEF/Global Academy for Medical Education and this news organization are owned by the same parent company.
WAIKOLOA, HAWAII – The highly selective interleukin-17A subunit inhibitor in the long-term extension phase of the randomized, controlled UNCOVER-3 (NCT01646177) trial, Craig L. Leonardi, MD, reported at the Hawaii Dermatology Seminar provided by the Global Academy for Medical Education/Skin Disease Education Foundation.

However, the strict inclusion and exclusion criteria employed in randomized trials such as this raise questions about the broader applicability of the results in real-world clinical practice. So separately at the Hawaii seminar, Dr. Leonardi presented a single-center retrospective observational cohort study of the rapidity and duration of response to ixekizumab in his own clinical practice after the biologic received Food and Drug Administration marketing approval. Those results, too, were impressive and, in his view, highly generalizable.
“It is expected that this study cohort is generally representative of patients who are routinely seen at dermatology referral practices in the U.S.,” commented Dr. Leonardi, of Saint Louis University.
UNCOVER-3 included 1,346 psoriasis patients initially randomized 2:2:2:1 to double-blind subcutaneous ixekizumab (Taltz) at 80 mg either every 2 weeks or every 4 weeks after a 160-mg loading dose; subcutaneous etanercept at 50 mg twice weekly; or placebo for 12 weeks, followed by a switch to ixekizumab at 80 mg every 4 weeks from week 12 out to 3 years. The long-term efficacy analysis was restricted to patients who received the biologic according to what ultimately became the approved dosing schedule: a 160-mg loading dose, followed by 80 mg every 2 weeks through week 12, then 80 mg every 4 weeks. The safety analysis, in contrast, included everybody.
Dr. Leonardi presented the efficacy data using several different statistical methodologies, thereby providing an instructive lesson regarding the importance of examining the fine print when viewing clinical trial results. At one extreme is the as-observed analysis. Under this methodology, if a patient dropped out of UNCOVER-3 at, for example, week 11, the last measurement of treatment response, recorded at week 8, is carried forward by investigators and assumed to be valid for the rest of the study. Since week 8 may have been the last time the patient was doing well on the drug, the as-observed analysis can create a distorted overly favorable picture of the drug’s performance.
“Patients fall out because the drug isn’t working well or they’re having a side effect, so over time, you tend to enrich for patients who are doing very well with the as-observed analysis,” the dermatologist explained.
Historically, many industry-sponsored clinical trials reported efficacy outcomes using the as-observed analysis; however, the FDA is increasingly unwilling to accept that approach as the sole analytic method.
At the other extreme is the nonresponder imputation method.
“This is the most stringent statistical package that exists. In fact, when a patient isn’t observed at one of the observation points – for example, at week 8 say the patient has a flat tire and can’t make it to the clinic – they’re counted as a treatment failure. So it’s a very tough statistical package,” according to Dr. Leonardi.
Seventy-seven percent of the initial 1,346 randomized patients in UNCOVER-3 completed 156 weeks of follow-up. To illustrate the importance of paying attention to the details of statistical methodology utilized in reporting efficacy outcomes, he noted that the PASI 75 rate at 156 weeks in study completers on the approved dosing regimen was 97% by the as-observed method, dropping to a still robust 81% by nonresponder imputation. The PASI 90 and -100 rates and static Physician’s Global Assessment (sPGA) results followed suit (see graphic).
Real-world performance
Dr. Leonard’s analysis of ixekizumab’s performance in his own practice included 106 patients placed on the drug following its FDA approval in March 2016, 74% of whom were still on the drug 12 months later. The cohort had a mean disease duration of 15 years. Three-quarters of them had previously received biologic therapy for their psoriasis, most often a tumor necrosis factor inhibitor. The study efficacy endpoints were the sPGA and Dermatology Life Quality Index (DLQI).
Already at 1 month, 30% of ixekizumab-treated patients had an sPGA score of 0, meaning their skin was totally clear. Another 29% had an sPGA of 1, meaning almost clear. At 3 months, 53% of patients had an sPGA of 0 and 21% had an sPGA of 1. Among patients on treatment at 12 months, the rates were 39% and 24% for sPGAs of 0 and 1, respectively. And in patients with an sPGA of 0/1 at 3 months, 73% maintained that score at 12 months, including 47% with an sPGA of 0.
A DLQI score of 0/1, indicative of little or no disease effect upon a patient’s life, was present in 63% of ixekizumab-treated patients at 1 month, 84% at 3 months, and 73% at 12 months.
The value in pushing for PASI 100
The ixekizumab experience in the phase-3 UNCOVER clinical trial program provided the first-ever evidence that incrementally improving psoriasis also provides stepwise improvement in DLQI, a key patient-reported outcome. At week 12 under double-blind conditions, only 4% of ixekizumab-treated patients with less than a PASI 50 response had a DLQI of 0/1. The rate rose to 18.8% in those with a PASI 50 to less than PASI 75 response. In patients with a week-12 PASI 75 to less than PASI 90 response, the DLQI 0/1 rate climbed to 52.3%. At a PASI 90 to less than PASI 100 response, the rate was 66.9%. And 82.9% of patients with a PASI 100 had a DLQI of 0/1. Every step of the way, those DLQI rates were significantly different from each other.
These data are “fascinating,” Dr. Leonardi commented. “If you ever get any inquiries from the friendly insurance carrier and they want to know if you’re improving your patient’s life, this is the kind of data that supports that they’re being improved dramatically.”
Dr. Leonardi noted that ixekizumab isn’t unique in its high rate of clinical effectiveness. That distinction is shared by the other approved IL-17 inhibitors, secukinumab (Cosentyx) and brodalumab (Siliq), as well as the IL-23 inhibitor guselkumab (Tremfya). He refers to these biologics collectively as “high-performance skin-clearance drugs.” He has calculated the number needed to treat (NNT) to achieve a PASI 100 response – complete clearance of the disease – based upon clinical trial data filed with the FDA and/or in the package inserts. The numbers are eye-opening: an NTT of 2.6 for ixekizumab based upon data from the UNCOVER-2 trial, 2.4 for brodalumab, 2.7 for guselkumab, and 3.6 for secukinumab. To help put that into perspective, the NNTs for methotrexate and etanercept (Enbrel) – not so long ago considered state of the art medications for moderate to severe psoriasis – are 25 and 23.3, respectively.
The UNCOVER trial portfolio and Dr. Leonardi’s single-center retrospective study were funded by Eli Lilly, which markets ixekizumab. He reported serving as a consultant to and receiving research funding from that company and more than a dozen others.
SDEF/Global Academy for Medical Education and this news organization are owned by the same parent company.
REPORTING FROM SDEF HAWAII DERMATOLOGY SEMINAR
Ultrasound method predicts liver complications in pediatric transplant
HOUSTON – An ultrasound method for assessing liver stiffness might be useful for predicting which pediatric patients will develop a life-threatening complication of hematopoietic stem cell transplantation.
Shear wave elastography values predicted severe hepatic sinusoidal obstruction syndrome/veno-occlusive disease (SOS/VOD) at least 4 days before standard diagnostic criteria in most patients treated in a small, prospective, two-center study, Sherwin S. Chan, MD, PhD, said at the Transplantation & Cellular Therapy Meetings.
Early identification of SOS/VOD using elastography could be beneficial in light of data showing that timing is critical in the administration of defibrotide, a treatment recommended for severe and very severe patients, according to Dr. Chan, vice chair of radiology for the University of Missouri at Kansas City.
“If you’re able to initiate it early, you can really increase day 100 survival,” Dr. Chan said in an oral presentation.
The data presented included 54 pediatric patients undergoing transplantation at one of two institutions.
At one site, the patients underwent shear wave elastography evaluation 10 days before the conditioning regimen began, and again at 5 and 14 days after the transplant. At the other site, patients with suspected SOS/VOD were enrolled and underwent elastography every other day for up to 10 exams.
Those are very different imaging protocols, Dr. Chan acknowledged in his presentation, noting that the studies started independently and data were pooled as investigators at the two institutions became aware of one another’s work.
A total of 16 patients, or 30%, developed SOS/VOD, Dr. Chan reported. Of those 16 cases, 12 (75%) were severe or very severe by the recent European Society for Blood and Marrow Transplantation (EBMT) criteria.
Increased shear wave elastography velocity was the best predictor of severe SOS/VOD, according to Dr. Chan, with a cutoff value of 1.65 m/s being 92% sensitive and 67% specific for severe SOS/VOD.
That threshold was passed at least 4 days before severe grading or death in 9 out of the 12 severe cases, he added.
Accordingly, a prospective, multicenter trial has been initiated at a number of U.S. centers to investigate whether the findings of this study are generalizable to other patient populations, Dr. Chan said at the meeting held by the American Society of Blood and Marrow Transplantation and the Center for International Blood and Marrow Transplant Research. At this meeting, the American Society for Blood and Marrow Transplantation announced a new name for the society: American Society for Transplantation and Cellular Therapy.
That prospective, multicenter trial is supported by Jazz Pharmaceuticals, according to Dr. Chan, who reported consulting with Jazz Pharmaceuticals in his disclosure statement.
SOURCE: Chan SS et al. TCT 2019, Abstract 55.
HOUSTON – An ultrasound method for assessing liver stiffness might be useful for predicting which pediatric patients will develop a life-threatening complication of hematopoietic stem cell transplantation.
Shear wave elastography values predicted severe hepatic sinusoidal obstruction syndrome/veno-occlusive disease (SOS/VOD) at least 4 days before standard diagnostic criteria in most patients treated in a small, prospective, two-center study, Sherwin S. Chan, MD, PhD, said at the Transplantation & Cellular Therapy Meetings.
Early identification of SOS/VOD using elastography could be beneficial in light of data showing that timing is critical in the administration of defibrotide, a treatment recommended for severe and very severe patients, according to Dr. Chan, vice chair of radiology for the University of Missouri at Kansas City.
“If you’re able to initiate it early, you can really increase day 100 survival,” Dr. Chan said in an oral presentation.
The data presented included 54 pediatric patients undergoing transplantation at one of two institutions.
At one site, the patients underwent shear wave elastography evaluation 10 days before the conditioning regimen began, and again at 5 and 14 days after the transplant. At the other site, patients with suspected SOS/VOD were enrolled and underwent elastography every other day for up to 10 exams.
Those are very different imaging protocols, Dr. Chan acknowledged in his presentation, noting that the studies started independently and data were pooled as investigators at the two institutions became aware of one another’s work.
A total of 16 patients, or 30%, developed SOS/VOD, Dr. Chan reported. Of those 16 cases, 12 (75%) were severe or very severe by the recent European Society for Blood and Marrow Transplantation (EBMT) criteria.
Increased shear wave elastography velocity was the best predictor of severe SOS/VOD, according to Dr. Chan, with a cutoff value of 1.65 m/s being 92% sensitive and 67% specific for severe SOS/VOD.
That threshold was passed at least 4 days before severe grading or death in 9 out of the 12 severe cases, he added.
Accordingly, a prospective, multicenter trial has been initiated at a number of U.S. centers to investigate whether the findings of this study are generalizable to other patient populations, Dr. Chan said at the meeting held by the American Society of Blood and Marrow Transplantation and the Center for International Blood and Marrow Transplant Research. At this meeting, the American Society for Blood and Marrow Transplantation announced a new name for the society: American Society for Transplantation and Cellular Therapy.
That prospective, multicenter trial is supported by Jazz Pharmaceuticals, according to Dr. Chan, who reported consulting with Jazz Pharmaceuticals in his disclosure statement.
SOURCE: Chan SS et al. TCT 2019, Abstract 55.
HOUSTON – An ultrasound method for assessing liver stiffness might be useful for predicting which pediatric patients will develop a life-threatening complication of hematopoietic stem cell transplantation.
Shear wave elastography values predicted severe hepatic sinusoidal obstruction syndrome/veno-occlusive disease (SOS/VOD) at least 4 days before standard diagnostic criteria in most patients treated in a small, prospective, two-center study, Sherwin S. Chan, MD, PhD, said at the Transplantation & Cellular Therapy Meetings.
Early identification of SOS/VOD using elastography could be beneficial in light of data showing that timing is critical in the administration of defibrotide, a treatment recommended for severe and very severe patients, according to Dr. Chan, vice chair of radiology for the University of Missouri at Kansas City.
“If you’re able to initiate it early, you can really increase day 100 survival,” Dr. Chan said in an oral presentation.
The data presented included 54 pediatric patients undergoing transplantation at one of two institutions.
At one site, the patients underwent shear wave elastography evaluation 10 days before the conditioning regimen began, and again at 5 and 14 days after the transplant. At the other site, patients with suspected SOS/VOD were enrolled and underwent elastography every other day for up to 10 exams.
Those are very different imaging protocols, Dr. Chan acknowledged in his presentation, noting that the studies started independently and data were pooled as investigators at the two institutions became aware of one another’s work.
A total of 16 patients, or 30%, developed SOS/VOD, Dr. Chan reported. Of those 16 cases, 12 (75%) were severe or very severe by the recent European Society for Blood and Marrow Transplantation (EBMT) criteria.
Increased shear wave elastography velocity was the best predictor of severe SOS/VOD, according to Dr. Chan, with a cutoff value of 1.65 m/s being 92% sensitive and 67% specific for severe SOS/VOD.
That threshold was passed at least 4 days before severe grading or death in 9 out of the 12 severe cases, he added.
Accordingly, a prospective, multicenter trial has been initiated at a number of U.S. centers to investigate whether the findings of this study are generalizable to other patient populations, Dr. Chan said at the meeting held by the American Society of Blood and Marrow Transplantation and the Center for International Blood and Marrow Transplant Research. At this meeting, the American Society for Blood and Marrow Transplantation announced a new name for the society: American Society for Transplantation and Cellular Therapy.
That prospective, multicenter trial is supported by Jazz Pharmaceuticals, according to Dr. Chan, who reported consulting with Jazz Pharmaceuticals in his disclosure statement.
SOURCE: Chan SS et al. TCT 2019, Abstract 55.
REPORTING FROM TCT 2019
Severe, uncontrolled asthma patients must avoid subcutaneous immunotherapy
SAN FRANCISCO – appears to be the “major factor” causing higher-grade systemic reactions or death from this treatment, David I. Bernstein, MD, said at the annual meeting of the American Academy of Allergy, Asthma, and Immunology.
While that was Dr. Bernstein’s top take-home message on how to optimize tolerability of subcutaneous immunotherapy (SCIT), a few other empiric rules have also emerged from his ongoing analysis of survey results from the AAAAI/American College of Allergy, Asthma, and Immunology SCIT surveillance study. The study began tracking the safety of SCIT in 2008 through annual surveys sent to members of either of these two allergy societies. By early 2019, the surveys had gathered data from more than 55 million office visits for SCIT, with responses from roughly 200-500 allergy practices annually, said Dr. Bernstein, professor of medicine at the University of Cincinnati.
The survey results identified seven SCIT-related fatalities over about a decade of surveillance. The most common risk factor among these cases was severe, uncontrolled asthma, prompting Dr. Bernstein to conclude that these patients should not receive SCIT. “If the asthma is well controlled, then SCIT is fine,” even if it had been severe before treatment, he said in an interview.
Other factors affecting SCIT safety based on the survey results included:
- Screening patients with an asthma history for current asthma symptoms and lung function before each injection. Survey results showed that while 86% of respondents screened for symptoms, only a third also checked lung function.
- Modifying the dose or stopping SCIT injections after a severe systemic reaction. Survey results showed that more than a quarter of all systemic reactions and more than a third of grade 3 systemic reactions (severe anaphylaxis) happened following a prior systemic reaction. Dr. Bernstein called this “an important, modifiable risk factor.”
- Administering SCIT only in a setting staffed to manage a possible anaphylaxis episode, and adhere to at least a 30-minute observation period. “A key step is observing for at least 30 minutes, and giving epinephrine promptly when needed; the sooner the better,” Dr. Bernstein said. Although the percentage of practices that observe patients for at least 30 minutes has steadily improved during the decade that the survey has run, in 2016 a quarter of responding practices continued to not observe patients for at least 30 minutes.
- Modifying the SCIT dose in high-risk patients during the peak season for aeroallergens like pollen. Survey results showed that practices that did not adjust their SCIT dosages during peak pollen seasons had about double the rate of grade 3 or 4 systemic reactions, compared with practices that dialed down their dosages.
- Reducing SCIT dosages during an accelerated cluster buildup, a treatment approach that in general increases the risk for systemic reactions.
Survey results also showed that sublingual immunotherapy, available in U.S. practice since 2014, has been very safe, with no reported associated deaths and only rare reports of anaphylactic episodes, Dr. Bernstein said. The most recent published report from the surveillance study appeared online a few days before Dr. Bernstein spoke (J Allergy Clin Immunol Pract. 2019 Feb 15. doi: 10.1016/j.jaip.2019.01.058).
Dr. Bernstein had no relevant disclosures.
SAN FRANCISCO – appears to be the “major factor” causing higher-grade systemic reactions or death from this treatment, David I. Bernstein, MD, said at the annual meeting of the American Academy of Allergy, Asthma, and Immunology.
While that was Dr. Bernstein’s top take-home message on how to optimize tolerability of subcutaneous immunotherapy (SCIT), a few other empiric rules have also emerged from his ongoing analysis of survey results from the AAAAI/American College of Allergy, Asthma, and Immunology SCIT surveillance study. The study began tracking the safety of SCIT in 2008 through annual surveys sent to members of either of these two allergy societies. By early 2019, the surveys had gathered data from more than 55 million office visits for SCIT, with responses from roughly 200-500 allergy practices annually, said Dr. Bernstein, professor of medicine at the University of Cincinnati.
The survey results identified seven SCIT-related fatalities over about a decade of surveillance. The most common risk factor among these cases was severe, uncontrolled asthma, prompting Dr. Bernstein to conclude that these patients should not receive SCIT. “If the asthma is well controlled, then SCIT is fine,” even if it had been severe before treatment, he said in an interview.
Other factors affecting SCIT safety based on the survey results included:
- Screening patients with an asthma history for current asthma symptoms and lung function before each injection. Survey results showed that while 86% of respondents screened for symptoms, only a third also checked lung function.
- Modifying the dose or stopping SCIT injections after a severe systemic reaction. Survey results showed that more than a quarter of all systemic reactions and more than a third of grade 3 systemic reactions (severe anaphylaxis) happened following a prior systemic reaction. Dr. Bernstein called this “an important, modifiable risk factor.”
- Administering SCIT only in a setting staffed to manage a possible anaphylaxis episode, and adhere to at least a 30-minute observation period. “A key step is observing for at least 30 minutes, and giving epinephrine promptly when needed; the sooner the better,” Dr. Bernstein said. Although the percentage of practices that observe patients for at least 30 minutes has steadily improved during the decade that the survey has run, in 2016 a quarter of responding practices continued to not observe patients for at least 30 minutes.
- Modifying the SCIT dose in high-risk patients during the peak season for aeroallergens like pollen. Survey results showed that practices that did not adjust their SCIT dosages during peak pollen seasons had about double the rate of grade 3 or 4 systemic reactions, compared with practices that dialed down their dosages.
- Reducing SCIT dosages during an accelerated cluster buildup, a treatment approach that in general increases the risk for systemic reactions.
Survey results also showed that sublingual immunotherapy, available in U.S. practice since 2014, has been very safe, with no reported associated deaths and only rare reports of anaphylactic episodes, Dr. Bernstein said. The most recent published report from the surveillance study appeared online a few days before Dr. Bernstein spoke (J Allergy Clin Immunol Pract. 2019 Feb 15. doi: 10.1016/j.jaip.2019.01.058).
Dr. Bernstein had no relevant disclosures.
SAN FRANCISCO – appears to be the “major factor” causing higher-grade systemic reactions or death from this treatment, David I. Bernstein, MD, said at the annual meeting of the American Academy of Allergy, Asthma, and Immunology.
While that was Dr. Bernstein’s top take-home message on how to optimize tolerability of subcutaneous immunotherapy (SCIT), a few other empiric rules have also emerged from his ongoing analysis of survey results from the AAAAI/American College of Allergy, Asthma, and Immunology SCIT surveillance study. The study began tracking the safety of SCIT in 2008 through annual surveys sent to members of either of these two allergy societies. By early 2019, the surveys had gathered data from more than 55 million office visits for SCIT, with responses from roughly 200-500 allergy practices annually, said Dr. Bernstein, professor of medicine at the University of Cincinnati.
The survey results identified seven SCIT-related fatalities over about a decade of surveillance. The most common risk factor among these cases was severe, uncontrolled asthma, prompting Dr. Bernstein to conclude that these patients should not receive SCIT. “If the asthma is well controlled, then SCIT is fine,” even if it had been severe before treatment, he said in an interview.
Other factors affecting SCIT safety based on the survey results included:
- Screening patients with an asthma history for current asthma symptoms and lung function before each injection. Survey results showed that while 86% of respondents screened for symptoms, only a third also checked lung function.
- Modifying the dose or stopping SCIT injections after a severe systemic reaction. Survey results showed that more than a quarter of all systemic reactions and more than a third of grade 3 systemic reactions (severe anaphylaxis) happened following a prior systemic reaction. Dr. Bernstein called this “an important, modifiable risk factor.”
- Administering SCIT only in a setting staffed to manage a possible anaphylaxis episode, and adhere to at least a 30-minute observation period. “A key step is observing for at least 30 minutes, and giving epinephrine promptly when needed; the sooner the better,” Dr. Bernstein said. Although the percentage of practices that observe patients for at least 30 minutes has steadily improved during the decade that the survey has run, in 2016 a quarter of responding practices continued to not observe patients for at least 30 minutes.
- Modifying the SCIT dose in high-risk patients during the peak season for aeroallergens like pollen. Survey results showed that practices that did not adjust their SCIT dosages during peak pollen seasons had about double the rate of grade 3 or 4 systemic reactions, compared with practices that dialed down their dosages.
- Reducing SCIT dosages during an accelerated cluster buildup, a treatment approach that in general increases the risk for systemic reactions.
Survey results also showed that sublingual immunotherapy, available in U.S. practice since 2014, has been very safe, with no reported associated deaths and only rare reports of anaphylactic episodes, Dr. Bernstein said. The most recent published report from the surveillance study appeared online a few days before Dr. Bernstein spoke (J Allergy Clin Immunol Pract. 2019 Feb 15. doi: 10.1016/j.jaip.2019.01.058).
Dr. Bernstein had no relevant disclosures.
REPORTING FROM AAAAI
Similar results for once- or twice-weekly carfilzomib in MM
Patients with newly diagnosed multiple myeloma have similar outcomes whether they receive carfilzomib once or twice a week, according to a pooled analysis of trial data.
Researchers found no significant difference in safety, progression-free survival (PFS), or overall survival (OS) whether patients received carfilzomib at 70 mg/m2 once a week or 36 mg/m2 twice a week.
Sara Bringhen, MD, PhD, of University of Turin, Italy, and her colleagues conducted this analysis and detailed the results in Haematologica.
The researchers pooled data from a phase 1/2 trial (NCT01857115) and a phase 2 trial (NCT01346787), both enrolling transplant-ineligible patients with newly diagnosed multiple myeloma.
In both studies, induction consisted of nine 4-week cycles of carfilzomib (given once or twice weekly), cyclophosphamide (300 mg on days 1, 8, and 15), and dexamethasone (40 mg on days 1, 8, 15, and 22). After induction, patients received carfilzomib maintenance (at either dose) until progression or intolerable toxicity.
The pooled analysis included 121 patients: 63 who received carfilzomib at 70 mg/m2 once weekly and 58 who received carfilzomib at 36 mg/m2 twice weekly.
There were no significant differences in baseline characteristics between the dosing groups. For the entire cohort, the median age at diagnosis was 72 years (range, 55-86), and the median follow-up was 39 months.
A total of 119 patients started induction (63 in the once-weekly group and 56 in the twice-weekly group), and 90 patients received maintenance (47 and 43, respectively). Patients received maintenance for a median of 17 months in the once-weekly group and 20 months in the twice-weekly group (P = .17).
There was no significant difference between the groups with regard to PFS or OS, either from enrollment or the start of maintenance.
From enrollment, the median PFS was 35.7 months in the once-weekly group and 35.5 months in the twice-weekly group (hazard ratio [HR] = 1.39; P = .26). The 3-year OS was 70% and 72%, respectively (HR = 1.27; P = .5).
From the start of maintenance, the 3-year PFS was 47% in the once-weekly group and 51% in the twice-weekly group (HR = 1.04; P = .92). The 3-year OS was 72% and 73%, respectively (HR = 0.82; P = .71).
There were no significant between-group differences in the rates of grade 3-5 adverse events (AEs) or the need for carfilzomib dose reduction or discontinuation.
Grade 3-5 hematologic AEs occurred in 24% of patients in the once-weekly group and 30% of those in the twice-weekly group. Grade 3-5 nonhematologic AEs occurred in 38% and 41%, respectively.
Twenty-nine percent of patients in the once-weekly group required a reduction in carfilzomib dose, as did 30% of patients in the twice-weekly group. Common AEs leading to dose reduction were acute kidney injury, infections, and hypertension.
AEs leading to carfilzomib discontinuation occurred in 27% of patients in the once-weekly group and 30% of those in the twice-weekly group. Common AEs leading to discontinuation were cardiac injury, infections, and thromboembolism.
Both trials were sponsored by Stichting Hemato-Oncologie voor Volwassenen Nederland in collaboration with Fondazione Neoplasie Sangue ONLUS and supported by funding from Amgen (Onyx Pharmaceuticals). Dr. Bringhen reported relationships with Amgen and other companies. Coauthor Antonio Palumbo, MD, is an employee of Takeda, and other authors reported relationships with a range of companies.
SOURCE: Bringhen S et al. Haematologica. 2019 Feb 7. doi: 10.3324/haematol.2018.208272.
Patients with newly diagnosed multiple myeloma have similar outcomes whether they receive carfilzomib once or twice a week, according to a pooled analysis of trial data.
Researchers found no significant difference in safety, progression-free survival (PFS), or overall survival (OS) whether patients received carfilzomib at 70 mg/m2 once a week or 36 mg/m2 twice a week.
Sara Bringhen, MD, PhD, of University of Turin, Italy, and her colleagues conducted this analysis and detailed the results in Haematologica.
The researchers pooled data from a phase 1/2 trial (NCT01857115) and a phase 2 trial (NCT01346787), both enrolling transplant-ineligible patients with newly diagnosed multiple myeloma.
In both studies, induction consisted of nine 4-week cycles of carfilzomib (given once or twice weekly), cyclophosphamide (300 mg on days 1, 8, and 15), and dexamethasone (40 mg on days 1, 8, 15, and 22). After induction, patients received carfilzomib maintenance (at either dose) until progression or intolerable toxicity.
The pooled analysis included 121 patients: 63 who received carfilzomib at 70 mg/m2 once weekly and 58 who received carfilzomib at 36 mg/m2 twice weekly.
There were no significant differences in baseline characteristics between the dosing groups. For the entire cohort, the median age at diagnosis was 72 years (range, 55-86), and the median follow-up was 39 months.
A total of 119 patients started induction (63 in the once-weekly group and 56 in the twice-weekly group), and 90 patients received maintenance (47 and 43, respectively). Patients received maintenance for a median of 17 months in the once-weekly group and 20 months in the twice-weekly group (P = .17).
There was no significant difference between the groups with regard to PFS or OS, either from enrollment or the start of maintenance.
From enrollment, the median PFS was 35.7 months in the once-weekly group and 35.5 months in the twice-weekly group (hazard ratio [HR] = 1.39; P = .26). The 3-year OS was 70% and 72%, respectively (HR = 1.27; P = .5).
From the start of maintenance, the 3-year PFS was 47% in the once-weekly group and 51% in the twice-weekly group (HR = 1.04; P = .92). The 3-year OS was 72% and 73%, respectively (HR = 0.82; P = .71).
There were no significant between-group differences in the rates of grade 3-5 adverse events (AEs) or the need for carfilzomib dose reduction or discontinuation.
Grade 3-5 hematologic AEs occurred in 24% of patients in the once-weekly group and 30% of those in the twice-weekly group. Grade 3-5 nonhematologic AEs occurred in 38% and 41%, respectively.
Twenty-nine percent of patients in the once-weekly group required a reduction in carfilzomib dose, as did 30% of patients in the twice-weekly group. Common AEs leading to dose reduction were acute kidney injury, infections, and hypertension.
AEs leading to carfilzomib discontinuation occurred in 27% of patients in the once-weekly group and 30% of those in the twice-weekly group. Common AEs leading to discontinuation were cardiac injury, infections, and thromboembolism.
Both trials were sponsored by Stichting Hemato-Oncologie voor Volwassenen Nederland in collaboration with Fondazione Neoplasie Sangue ONLUS and supported by funding from Amgen (Onyx Pharmaceuticals). Dr. Bringhen reported relationships with Amgen and other companies. Coauthor Antonio Palumbo, MD, is an employee of Takeda, and other authors reported relationships with a range of companies.
SOURCE: Bringhen S et al. Haematologica. 2019 Feb 7. doi: 10.3324/haematol.2018.208272.
Patients with newly diagnosed multiple myeloma have similar outcomes whether they receive carfilzomib once or twice a week, according to a pooled analysis of trial data.
Researchers found no significant difference in safety, progression-free survival (PFS), or overall survival (OS) whether patients received carfilzomib at 70 mg/m2 once a week or 36 mg/m2 twice a week.
Sara Bringhen, MD, PhD, of University of Turin, Italy, and her colleagues conducted this analysis and detailed the results in Haematologica.
The researchers pooled data from a phase 1/2 trial (NCT01857115) and a phase 2 trial (NCT01346787), both enrolling transplant-ineligible patients with newly diagnosed multiple myeloma.
In both studies, induction consisted of nine 4-week cycles of carfilzomib (given once or twice weekly), cyclophosphamide (300 mg on days 1, 8, and 15), and dexamethasone (40 mg on days 1, 8, 15, and 22). After induction, patients received carfilzomib maintenance (at either dose) until progression or intolerable toxicity.
The pooled analysis included 121 patients: 63 who received carfilzomib at 70 mg/m2 once weekly and 58 who received carfilzomib at 36 mg/m2 twice weekly.
There were no significant differences in baseline characteristics between the dosing groups. For the entire cohort, the median age at diagnosis was 72 years (range, 55-86), and the median follow-up was 39 months.
A total of 119 patients started induction (63 in the once-weekly group and 56 in the twice-weekly group), and 90 patients received maintenance (47 and 43, respectively). Patients received maintenance for a median of 17 months in the once-weekly group and 20 months in the twice-weekly group (P = .17).
There was no significant difference between the groups with regard to PFS or OS, either from enrollment or the start of maintenance.
From enrollment, the median PFS was 35.7 months in the once-weekly group and 35.5 months in the twice-weekly group (hazard ratio [HR] = 1.39; P = .26). The 3-year OS was 70% and 72%, respectively (HR = 1.27; P = .5).
From the start of maintenance, the 3-year PFS was 47% in the once-weekly group and 51% in the twice-weekly group (HR = 1.04; P = .92). The 3-year OS was 72% and 73%, respectively (HR = 0.82; P = .71).
There were no significant between-group differences in the rates of grade 3-5 adverse events (AEs) or the need for carfilzomib dose reduction or discontinuation.
Grade 3-5 hematologic AEs occurred in 24% of patients in the once-weekly group and 30% of those in the twice-weekly group. Grade 3-5 nonhematologic AEs occurred in 38% and 41%, respectively.
Twenty-nine percent of patients in the once-weekly group required a reduction in carfilzomib dose, as did 30% of patients in the twice-weekly group. Common AEs leading to dose reduction were acute kidney injury, infections, and hypertension.
AEs leading to carfilzomib discontinuation occurred in 27% of patients in the once-weekly group and 30% of those in the twice-weekly group. Common AEs leading to discontinuation were cardiac injury, infections, and thromboembolism.
Both trials were sponsored by Stichting Hemato-Oncologie voor Volwassenen Nederland in collaboration with Fondazione Neoplasie Sangue ONLUS and supported by funding from Amgen (Onyx Pharmaceuticals). Dr. Bringhen reported relationships with Amgen and other companies. Coauthor Antonio Palumbo, MD, is an employee of Takeda, and other authors reported relationships with a range of companies.
SOURCE: Bringhen S et al. Haematologica. 2019 Feb 7. doi: 10.3324/haematol.2018.208272.
FROM HAEMATOLOGICA
Physician PAC dollars support candidates against gun regulation
Despite many physician professional organizations endorsing policies that support firearm regulation, more of their political donations go to candidates who oppose those policies, according to a study of political action committee (PAC) campaign contributions during the 2016 election cycle.
“Our analysis indicates that most of the largest physician organizations’ PACs contribute more to candidates whose stances on firearm policy are in direct opposition to evidence-based firearm policies and to their organization’s stances,” wrote lead author Jeremiah D. Schuur, MD, of Brown University, Providence, R.I., and his coauthors.
The study was published in JAMA Network Open.
This retrospective, cross sectional study examined contributions from the 25 largest physician organization–affiliated PACs during the 2016 election cycle and compared them to federal candidate support for firearm regulation.
Support for regulation was measured by voting history on U.S. House and Senate legislation proposing firearm background checks and their rating from the National Rifle Association Political Victory Fund (NRA-PVF).
Health care professional–related PACs in general contributed $23.7 million during the 2016 election cycle; 57% of that sum ($13.6 million) came from the 25 largest physician-affiliated PACs.
Of the 29 Senate incumbents running for reelection who voted on S.A. 4750, an amendment that would have expanded background checks, those who voted against it (n = 21) received $500,000 more in contributions than did those who voted for it (n = 8).
The findings were similar with H.R. 1217, a bill in the House of Representatives to expand background checks; the PACs contributed $2,878,675 more to candidates who did not cosponsor it (n = 227) than to cosponsors (n = 166).
In regard to ratings from the NRA-PVF, the 25 PACs gave $5.6 million to candidates with an A rating and $4.1 million to candidates with a rating other than A.
But the trend was somewhat different when it came to the 2015 call to action on firearm-related injury and death, endorsed by several physician groups. Among the nine PACs with affiliated organizations that had endorsed the call to action, eight contributed to more candidates who did not support firearm safety policies. But after adjustment for political factors, those nine PACs had a lower likelihood of donating to NRA-PVF A-rated candidates, compared with nonendorsing PACs (odds ratio, 0.76; 95% confidence interval, 0.58-0.99; P = .04).
“Although endorsement of firearm safety policies may reflect a small difference in political giving, it does not mean that a physicians’ organization has elevated firearm policy to the level of a contribution criteria for the PAC,” the researchers wrote.
The researchers noted that it is “unlikely that physician organization–affiliated PACs contribute to candidates because they are opposed to firearm regulation.” Rather, they said, these PACs consider a number of factors, including a candidates’ stance on malpractice reform, physician payment policies, and the Patient Protection and Affordable Care Act, as well as their chance of winning.
The authors reported having no conflicts of interest.
SOURCE: Schuur JD et al. JAMA Netw Open. 2019 Feb 22. doi: 10.1001/jamanetworkopen.2018.7831.
Though national membership organizations have finally taken a lead in advocating for firearm safety, this study from Schuur et al. illustrates the disconnect between physician PACs and the physicians themselves, according to Rebecca M. Cunningham, MD, Marc A. Zimmerman, PhD, and Patrick M. Carter, MD, of the University of Michigan, Ann Arbor.
The study comes in the wake of the NRA admonishing physicians to “stay in their lane,” which was met by a very vocal response via social and mass media. “Health care professionals demonstrated that, contrary to the NRA position, they have an undeniably central role and authority in addressing this public health problem through the direct care that they provide to patients and their families, prevention-based research, and advocacy for policy-level changes that make patients safer,” they wrote.
The coauthors noted the parallels to the American Medical Association previously calling for tobacco regulation while financially supporting politicians who felt otherwise. It’s a comparison that is meant as a cautionary tale; as more focus is placed on this particular issue, “medical PACs must consider the increasing physician voice on the need to address firearm-associated morbidity and mortality in the policy arena to reduce their experience with this issue in emergency bays, operating rooms, and clinics.”
Rebecca M. Cunningham, MD, Marc A. Zimmerman, PhD, and Patrick M. Carter, MD, are with the University of Michigan, Ann Arbor. They reported having no conflicts of interest. Their comments are adapted from an accompanying editorial (JAMA Netw Open. 2019 Feb 22. doi: 10.1001/jamanetworkopen.2018.7823 ).
Though national membership organizations have finally taken a lead in advocating for firearm safety, this study from Schuur et al. illustrates the disconnect between physician PACs and the physicians themselves, according to Rebecca M. Cunningham, MD, Marc A. Zimmerman, PhD, and Patrick M. Carter, MD, of the University of Michigan, Ann Arbor.
The study comes in the wake of the NRA admonishing physicians to “stay in their lane,” which was met by a very vocal response via social and mass media. “Health care professionals demonstrated that, contrary to the NRA position, they have an undeniably central role and authority in addressing this public health problem through the direct care that they provide to patients and their families, prevention-based research, and advocacy for policy-level changes that make patients safer,” they wrote.
The coauthors noted the parallels to the American Medical Association previously calling for tobacco regulation while financially supporting politicians who felt otherwise. It’s a comparison that is meant as a cautionary tale; as more focus is placed on this particular issue, “medical PACs must consider the increasing physician voice on the need to address firearm-associated morbidity and mortality in the policy arena to reduce their experience with this issue in emergency bays, operating rooms, and clinics.”
Rebecca M. Cunningham, MD, Marc A. Zimmerman, PhD, and Patrick M. Carter, MD, are with the University of Michigan, Ann Arbor. They reported having no conflicts of interest. Their comments are adapted from an accompanying editorial (JAMA Netw Open. 2019 Feb 22. doi: 10.1001/jamanetworkopen.2018.7823 ).
Though national membership organizations have finally taken a lead in advocating for firearm safety, this study from Schuur et al. illustrates the disconnect between physician PACs and the physicians themselves, according to Rebecca M. Cunningham, MD, Marc A. Zimmerman, PhD, and Patrick M. Carter, MD, of the University of Michigan, Ann Arbor.
The study comes in the wake of the NRA admonishing physicians to “stay in their lane,” which was met by a very vocal response via social and mass media. “Health care professionals demonstrated that, contrary to the NRA position, they have an undeniably central role and authority in addressing this public health problem through the direct care that they provide to patients and their families, prevention-based research, and advocacy for policy-level changes that make patients safer,” they wrote.
The coauthors noted the parallels to the American Medical Association previously calling for tobacco regulation while financially supporting politicians who felt otherwise. It’s a comparison that is meant as a cautionary tale; as more focus is placed on this particular issue, “medical PACs must consider the increasing physician voice on the need to address firearm-associated morbidity and mortality in the policy arena to reduce their experience with this issue in emergency bays, operating rooms, and clinics.”
Rebecca M. Cunningham, MD, Marc A. Zimmerman, PhD, and Patrick M. Carter, MD, are with the University of Michigan, Ann Arbor. They reported having no conflicts of interest. Their comments are adapted from an accompanying editorial (JAMA Netw Open. 2019 Feb 22. doi: 10.1001/jamanetworkopen.2018.7823 ).
Despite many physician professional organizations endorsing policies that support firearm regulation, more of their political donations go to candidates who oppose those policies, according to a study of political action committee (PAC) campaign contributions during the 2016 election cycle.
“Our analysis indicates that most of the largest physician organizations’ PACs contribute more to candidates whose stances on firearm policy are in direct opposition to evidence-based firearm policies and to their organization’s stances,” wrote lead author Jeremiah D. Schuur, MD, of Brown University, Providence, R.I., and his coauthors.
The study was published in JAMA Network Open.
This retrospective, cross sectional study examined contributions from the 25 largest physician organization–affiliated PACs during the 2016 election cycle and compared them to federal candidate support for firearm regulation.
Support for regulation was measured by voting history on U.S. House and Senate legislation proposing firearm background checks and their rating from the National Rifle Association Political Victory Fund (NRA-PVF).
Health care professional–related PACs in general contributed $23.7 million during the 2016 election cycle; 57% of that sum ($13.6 million) came from the 25 largest physician-affiliated PACs.
Of the 29 Senate incumbents running for reelection who voted on S.A. 4750, an amendment that would have expanded background checks, those who voted against it (n = 21) received $500,000 more in contributions than did those who voted for it (n = 8).
The findings were similar with H.R. 1217, a bill in the House of Representatives to expand background checks; the PACs contributed $2,878,675 more to candidates who did not cosponsor it (n = 227) than to cosponsors (n = 166).
In regard to ratings from the NRA-PVF, the 25 PACs gave $5.6 million to candidates with an A rating and $4.1 million to candidates with a rating other than A.
But the trend was somewhat different when it came to the 2015 call to action on firearm-related injury and death, endorsed by several physician groups. Among the nine PACs with affiliated organizations that had endorsed the call to action, eight contributed to more candidates who did not support firearm safety policies. But after adjustment for political factors, those nine PACs had a lower likelihood of donating to NRA-PVF A-rated candidates, compared with nonendorsing PACs (odds ratio, 0.76; 95% confidence interval, 0.58-0.99; P = .04).
“Although endorsement of firearm safety policies may reflect a small difference in political giving, it does not mean that a physicians’ organization has elevated firearm policy to the level of a contribution criteria for the PAC,” the researchers wrote.
The researchers noted that it is “unlikely that physician organization–affiliated PACs contribute to candidates because they are opposed to firearm regulation.” Rather, they said, these PACs consider a number of factors, including a candidates’ stance on malpractice reform, physician payment policies, and the Patient Protection and Affordable Care Act, as well as their chance of winning.
The authors reported having no conflicts of interest.
SOURCE: Schuur JD et al. JAMA Netw Open. 2019 Feb 22. doi: 10.1001/jamanetworkopen.2018.7831.
Despite many physician professional organizations endorsing policies that support firearm regulation, more of their political donations go to candidates who oppose those policies, according to a study of political action committee (PAC) campaign contributions during the 2016 election cycle.
“Our analysis indicates that most of the largest physician organizations’ PACs contribute more to candidates whose stances on firearm policy are in direct opposition to evidence-based firearm policies and to their organization’s stances,” wrote lead author Jeremiah D. Schuur, MD, of Brown University, Providence, R.I., and his coauthors.
The study was published in JAMA Network Open.
This retrospective, cross sectional study examined contributions from the 25 largest physician organization–affiliated PACs during the 2016 election cycle and compared them to federal candidate support for firearm regulation.
Support for regulation was measured by voting history on U.S. House and Senate legislation proposing firearm background checks and their rating from the National Rifle Association Political Victory Fund (NRA-PVF).
Health care professional–related PACs in general contributed $23.7 million during the 2016 election cycle; 57% of that sum ($13.6 million) came from the 25 largest physician-affiliated PACs.
Of the 29 Senate incumbents running for reelection who voted on S.A. 4750, an amendment that would have expanded background checks, those who voted against it (n = 21) received $500,000 more in contributions than did those who voted for it (n = 8).
The findings were similar with H.R. 1217, a bill in the House of Representatives to expand background checks; the PACs contributed $2,878,675 more to candidates who did not cosponsor it (n = 227) than to cosponsors (n = 166).
In regard to ratings from the NRA-PVF, the 25 PACs gave $5.6 million to candidates with an A rating and $4.1 million to candidates with a rating other than A.
But the trend was somewhat different when it came to the 2015 call to action on firearm-related injury and death, endorsed by several physician groups. Among the nine PACs with affiliated organizations that had endorsed the call to action, eight contributed to more candidates who did not support firearm safety policies. But after adjustment for political factors, those nine PACs had a lower likelihood of donating to NRA-PVF A-rated candidates, compared with nonendorsing PACs (odds ratio, 0.76; 95% confidence interval, 0.58-0.99; P = .04).
“Although endorsement of firearm safety policies may reflect a small difference in political giving, it does not mean that a physicians’ organization has elevated firearm policy to the level of a contribution criteria for the PAC,” the researchers wrote.
The researchers noted that it is “unlikely that physician organization–affiliated PACs contribute to candidates because they are opposed to firearm regulation.” Rather, they said, these PACs consider a number of factors, including a candidates’ stance on malpractice reform, physician payment policies, and the Patient Protection and Affordable Care Act, as well as their chance of winning.
The authors reported having no conflicts of interest.
SOURCE: Schuur JD et al. JAMA Netw Open. 2019 Feb 22. doi: 10.1001/jamanetworkopen.2018.7831.
FROM JAMA NETWORK OPEN
What is the ‘microbiome’ and how may it influence gynecologic cancers?
Bacteria are everywhere, good and bad alike! It is well known in the scientific community that microbes significantly outnumber the cells in the human body by at least 10 times. Joshua Lederberg, PhD, gave meaning to the term “microbiome” in 2001 as the “ecological community of commensal, symbiotic, and pathogenic microorganisms that literally share our body space.”1 This community of microorganisms comprises bacteria, fungi, viruses, archaea, and protists.
In 2007, the National Institutes of Health Human Microbiome Project was established to study the human microbiome starting with five specific sites – the gastrointestinal tract, the mouth, the vagina, the skin, and nasal cavity. The goal was not only to identify the microbes inhabiting a specific body site but also to establish a range of “normal” for resident microbes as well as sequence the genomes of these microbes.2 Much of the research predating this era focused on microorganisms in terms of disease potential rather than a focus on the benefits of resident microorganisms.
The richness – the number of microorganisms in an area – and diversity – the relative proportion of microorganisms in an environment – can vary regionally. The microbiota that contribute to the class of resident microorganisms in a specific body habitat can be described broadly as commensals or mutualistic. With commensal microorganisms, one partner benefits and the other is unaffected. On the other hand, mutualistic microorganisms allow both parties to derive benefit. For example, resident microorganisms in the gut aid in the absorption of nutrients and in the production of vitamin K. On mucosal surfaces and the skin, it is possible that these resident microorganisms prevent colonization of pathogenic microbes, which could aid in prevention of disease.3
The microbiota composition can be influenced by multiple factors such as age, diet, medications, environment, early microbial exposure, and host genetics. The gut microbiota, for example, can be significantly altered by dietary intake or antibiotic use. Alterations in the diversity of microbes in certain body habitats has been linked to several human diseases such as obesity, inflammatory bowel disease, and bacterial vaginosis.4
In women, there are differences noted in the composition of resident microorganisms soon after birth as well as at prepubertal, postpubertal, and postmenopausal transitions. At puberty, anaerobic and aerobic lactobacilli aid in maintaining vaginal pH. If the normal microbiota is suppressed, it allows for yeast and other bacteria to grow causing vaginitis, and dramatic shifts in the makeup of the vaginal microbiota can lead to bacterial vaginosis. Interestingly, research has shown that the pH and microbiome of the vagina differs by ethnicity. These differences in composition of the vaginal microbiome likely contribute to known differences in the acquisition of sexually transmitted infections and development of bacterial vaginosis. The microbiome is believed to have a complex role in regulating human health and disease, including cancer.
There is growing evidence to suggest the gut microbiome may play an important role in the pathogenesis of both obesity and cancer. Two divisions of bacteria predominate in the gut in humans and mice, Bacteroidetes and Firmicutes, and the relative ratio of these two divisions is dramatically affected by obesity, such that Bacteroidetes levels decrease and Firmicutes levels increase.5 The change in the microbial environment leads to a greater ability to harvest dietary energy, which would be conducive to cancer development.
The microbiome and gynecologic cancers
The presence and relative abundance of bacterial species in the vagina are affected by unique factors such as hormonal contraception, pregnancy, and menopause. There are researchers investigating alterations in the microbiome of the vagina and implications in persistence of high-risk human papillomavirus infections and HPV-induced carcinogenesis. There were significant differences found in the composition of the vaginal microbiota in healthy women, compared with women with low-grade squamous intraepithelial neoplasm and high-grade squamous intraepithelial neoplasm.6
Conceivably, the subsequent clinical questions are: Can we apply this data to diagnose women at risk for dysplasia or can we alter the vaginal microbiome to impact the clearance rate of the HPV virus in susceptible or infected women to decrease the long-term risk of cervical dysplasia or malignancy?
The upper reproductive tract in women – the uterus, fallopian tubes, and ovaries – had been presumed to be a sterile environment. However, we know that bacteria have been isolated in the pre- and postmenopausal uterus of healthy women. Therefore, there also are investigators seeking to establish the microbiome of normal uteri to accurately compare it with malignant uteri. Notably, there also is interest in how treatments for cancer – chemotherapy and radiation – ultimately can affect a woman’s vaginal and gut microbiome.
Currently, microbiome research has an expansive range. Women will greatly benefit from research seeking to define improved prevention, diagnosis, and treatment based on alterations of the microbiome for common gynecologic premalignant and malignant conditions.
Dr. Hawkins is a fellow of gynecologic oncology and Dr. Rossi is an assistant professor in the division of gynecologic oncology at the University of North Carolina at Chapel Hill. They had no conflicts of interest to disclose.
References
1. “ ’Ome Sweet ’Omics – a genealogical treasury of words,” by Joshua Lederberg, The Scientist, Apr 2, 2001.
2. Genome Res. 2009 Dec;19(12):2317-23.
3. “Normal Human Microbiota,” Jawetz, Melnick & Adelberg’s Medical Microbiology, 27th edition (New York, NY: McGraw-Hill, 2016).
4. Nature. 2012 Jun 13;486(7402):207-14.
5. Nature. 2006 Dec 21;444(7122):1027-31.
6. Oncol Lett. 2018 Dec; 16(6): 7035-47.
Bacteria are everywhere, good and bad alike! It is well known in the scientific community that microbes significantly outnumber the cells in the human body by at least 10 times. Joshua Lederberg, PhD, gave meaning to the term “microbiome” in 2001 as the “ecological community of commensal, symbiotic, and pathogenic microorganisms that literally share our body space.”1 This community of microorganisms comprises bacteria, fungi, viruses, archaea, and protists.
In 2007, the National Institutes of Health Human Microbiome Project was established to study the human microbiome starting with five specific sites – the gastrointestinal tract, the mouth, the vagina, the skin, and nasal cavity. The goal was not only to identify the microbes inhabiting a specific body site but also to establish a range of “normal” for resident microbes as well as sequence the genomes of these microbes.2 Much of the research predating this era focused on microorganisms in terms of disease potential rather than a focus on the benefits of resident microorganisms.
The richness – the number of microorganisms in an area – and diversity – the relative proportion of microorganisms in an environment – can vary regionally. The microbiota that contribute to the class of resident microorganisms in a specific body habitat can be described broadly as commensals or mutualistic. With commensal microorganisms, one partner benefits and the other is unaffected. On the other hand, mutualistic microorganisms allow both parties to derive benefit. For example, resident microorganisms in the gut aid in the absorption of nutrients and in the production of vitamin K. On mucosal surfaces and the skin, it is possible that these resident microorganisms prevent colonization of pathogenic microbes, which could aid in prevention of disease.3
The microbiota composition can be influenced by multiple factors such as age, diet, medications, environment, early microbial exposure, and host genetics. The gut microbiota, for example, can be significantly altered by dietary intake or antibiotic use. Alterations in the diversity of microbes in certain body habitats has been linked to several human diseases such as obesity, inflammatory bowel disease, and bacterial vaginosis.4
In women, there are differences noted in the composition of resident microorganisms soon after birth as well as at prepubertal, postpubertal, and postmenopausal transitions. At puberty, anaerobic and aerobic lactobacilli aid in maintaining vaginal pH. If the normal microbiota is suppressed, it allows for yeast and other bacteria to grow causing vaginitis, and dramatic shifts in the makeup of the vaginal microbiota can lead to bacterial vaginosis. Interestingly, research has shown that the pH and microbiome of the vagina differs by ethnicity. These differences in composition of the vaginal microbiome likely contribute to known differences in the acquisition of sexually transmitted infections and development of bacterial vaginosis. The microbiome is believed to have a complex role in regulating human health and disease, including cancer.
There is growing evidence to suggest the gut microbiome may play an important role in the pathogenesis of both obesity and cancer. Two divisions of bacteria predominate in the gut in humans and mice, Bacteroidetes and Firmicutes, and the relative ratio of these two divisions is dramatically affected by obesity, such that Bacteroidetes levels decrease and Firmicutes levels increase.5 The change in the microbial environment leads to a greater ability to harvest dietary energy, which would be conducive to cancer development.
The microbiome and gynecologic cancers
The presence and relative abundance of bacterial species in the vagina are affected by unique factors such as hormonal contraception, pregnancy, and menopause. There are researchers investigating alterations in the microbiome of the vagina and implications in persistence of high-risk human papillomavirus infections and HPV-induced carcinogenesis. There were significant differences found in the composition of the vaginal microbiota in healthy women, compared with women with low-grade squamous intraepithelial neoplasm and high-grade squamous intraepithelial neoplasm.6
Conceivably, the subsequent clinical questions are: Can we apply this data to diagnose women at risk for dysplasia or can we alter the vaginal microbiome to impact the clearance rate of the HPV virus in susceptible or infected women to decrease the long-term risk of cervical dysplasia or malignancy?
The upper reproductive tract in women – the uterus, fallopian tubes, and ovaries – had been presumed to be a sterile environment. However, we know that bacteria have been isolated in the pre- and postmenopausal uterus of healthy women. Therefore, there also are investigators seeking to establish the microbiome of normal uteri to accurately compare it with malignant uteri. Notably, there also is interest in how treatments for cancer – chemotherapy and radiation – ultimately can affect a woman’s vaginal and gut microbiome.
Currently, microbiome research has an expansive range. Women will greatly benefit from research seeking to define improved prevention, diagnosis, and treatment based on alterations of the microbiome for common gynecologic premalignant and malignant conditions.
Dr. Hawkins is a fellow of gynecologic oncology and Dr. Rossi is an assistant professor in the division of gynecologic oncology at the University of North Carolina at Chapel Hill. They had no conflicts of interest to disclose.
References
1. “ ’Ome Sweet ’Omics – a genealogical treasury of words,” by Joshua Lederberg, The Scientist, Apr 2, 2001.
2. Genome Res. 2009 Dec;19(12):2317-23.
3. “Normal Human Microbiota,” Jawetz, Melnick & Adelberg’s Medical Microbiology, 27th edition (New York, NY: McGraw-Hill, 2016).
4. Nature. 2012 Jun 13;486(7402):207-14.
5. Nature. 2006 Dec 21;444(7122):1027-31.
6. Oncol Lett. 2018 Dec; 16(6): 7035-47.
Bacteria are everywhere, good and bad alike! It is well known in the scientific community that microbes significantly outnumber the cells in the human body by at least 10 times. Joshua Lederberg, PhD, gave meaning to the term “microbiome” in 2001 as the “ecological community of commensal, symbiotic, and pathogenic microorganisms that literally share our body space.”1 This community of microorganisms comprises bacteria, fungi, viruses, archaea, and protists.
In 2007, the National Institutes of Health Human Microbiome Project was established to study the human microbiome starting with five specific sites – the gastrointestinal tract, the mouth, the vagina, the skin, and nasal cavity. The goal was not only to identify the microbes inhabiting a specific body site but also to establish a range of “normal” for resident microbes as well as sequence the genomes of these microbes.2 Much of the research predating this era focused on microorganisms in terms of disease potential rather than a focus on the benefits of resident microorganisms.
The richness – the number of microorganisms in an area – and diversity – the relative proportion of microorganisms in an environment – can vary regionally. The microbiota that contribute to the class of resident microorganisms in a specific body habitat can be described broadly as commensals or mutualistic. With commensal microorganisms, one partner benefits and the other is unaffected. On the other hand, mutualistic microorganisms allow both parties to derive benefit. For example, resident microorganisms in the gut aid in the absorption of nutrients and in the production of vitamin K. On mucosal surfaces and the skin, it is possible that these resident microorganisms prevent colonization of pathogenic microbes, which could aid in prevention of disease.3
The microbiota composition can be influenced by multiple factors such as age, diet, medications, environment, early microbial exposure, and host genetics. The gut microbiota, for example, can be significantly altered by dietary intake or antibiotic use. Alterations in the diversity of microbes in certain body habitats has been linked to several human diseases such as obesity, inflammatory bowel disease, and bacterial vaginosis.4
In women, there are differences noted in the composition of resident microorganisms soon after birth as well as at prepubertal, postpubertal, and postmenopausal transitions. At puberty, anaerobic and aerobic lactobacilli aid in maintaining vaginal pH. If the normal microbiota is suppressed, it allows for yeast and other bacteria to grow causing vaginitis, and dramatic shifts in the makeup of the vaginal microbiota can lead to bacterial vaginosis. Interestingly, research has shown that the pH and microbiome of the vagina differs by ethnicity. These differences in composition of the vaginal microbiome likely contribute to known differences in the acquisition of sexually transmitted infections and development of bacterial vaginosis. The microbiome is believed to have a complex role in regulating human health and disease, including cancer.
There is growing evidence to suggest the gut microbiome may play an important role in the pathogenesis of both obesity and cancer. Two divisions of bacteria predominate in the gut in humans and mice, Bacteroidetes and Firmicutes, and the relative ratio of these two divisions is dramatically affected by obesity, such that Bacteroidetes levels decrease and Firmicutes levels increase.5 The change in the microbial environment leads to a greater ability to harvest dietary energy, which would be conducive to cancer development.
The microbiome and gynecologic cancers
The presence and relative abundance of bacterial species in the vagina are affected by unique factors such as hormonal contraception, pregnancy, and menopause. There are researchers investigating alterations in the microbiome of the vagina and implications in persistence of high-risk human papillomavirus infections and HPV-induced carcinogenesis. There were significant differences found in the composition of the vaginal microbiota in healthy women, compared with women with low-grade squamous intraepithelial neoplasm and high-grade squamous intraepithelial neoplasm.6
Conceivably, the subsequent clinical questions are: Can we apply this data to diagnose women at risk for dysplasia or can we alter the vaginal microbiome to impact the clearance rate of the HPV virus in susceptible or infected women to decrease the long-term risk of cervical dysplasia or malignancy?
The upper reproductive tract in women – the uterus, fallopian tubes, and ovaries – had been presumed to be a sterile environment. However, we know that bacteria have been isolated in the pre- and postmenopausal uterus of healthy women. Therefore, there also are investigators seeking to establish the microbiome of normal uteri to accurately compare it with malignant uteri. Notably, there also is interest in how treatments for cancer – chemotherapy and radiation – ultimately can affect a woman’s vaginal and gut microbiome.
Currently, microbiome research has an expansive range. Women will greatly benefit from research seeking to define improved prevention, diagnosis, and treatment based on alterations of the microbiome for common gynecologic premalignant and malignant conditions.
Dr. Hawkins is a fellow of gynecologic oncology and Dr. Rossi is an assistant professor in the division of gynecologic oncology at the University of North Carolina at Chapel Hill. They had no conflicts of interest to disclose.
References
1. “ ’Ome Sweet ’Omics – a genealogical treasury of words,” by Joshua Lederberg, The Scientist, Apr 2, 2001.
2. Genome Res. 2009 Dec;19(12):2317-23.
3. “Normal Human Microbiota,” Jawetz, Melnick & Adelberg’s Medical Microbiology, 27th edition (New York, NY: McGraw-Hill, 2016).
4. Nature. 2012 Jun 13;486(7402):207-14.
5. Nature. 2006 Dec 21;444(7122):1027-31.
6. Oncol Lett. 2018 Dec; 16(6): 7035-47.